THE EVOLUTION OF MAN
Volume I
CHAPTER IX
THE GASTRULATION OF THE VERTEBRATE1
The remarkable processes of gastrulation,
ovum-segmentation, and formation of germinal layers present a most
conspicuous variety. There is to-day only the lowest of the
vertebrates, the amphioxus, that exhibits the original form of
those processes, or the palingenetic gastrulation which we have
considered in the preceding chapter, and which culminates in the
formation of the archigastrula (Fig.
38). In all other extant vertebrates these fundamental
processes have been more or less modified by adaptation to the
conditions of embryonic development (especially by changes in the
food-yelk); they exhibit various cenogenetic types of the formation
of germinal layers. However, the different classes vary
considerably from each other. In order to grasp the unity that
underlies the manifold differences in these phenomena and their
historical connection, it is necessary to bear in mind always the
unity of the vertebrate-stem. This “phylogenetic
unity,” which I developed in my General Morphology in
1866, is now generally admitted. All impartial zoologists agree
to-day that all the vertebrates, from the amphioxus and the fishes
to the ape and man, descend from a common ancestor, “the
primitive vertebrate.” Hence the embryonic processes, by
which each individual vertebrate is developed, must also be capable
of being reduced to one common type of embryonic development; and
this primitive type is most certainly exhibited to-day by the
amphioxus.
It must, therefore, be our next task to make a comparative study
of the various forms of vertebrate gastrulation, and trace them
backwards to that of the lancelet. Broadly speaking, they fall
first into two groups: the older cyclostoma, the earliest fishes,
most of the amphibia, and the viviparous mammals, have
holoblastic ova—that is to say, ova with total, unequal
segmentation; while the younger cyclostoma, most of the fishes, the
cephalopods, reptiles, birds, and monotremes, have
meroblastic ova, or ova with partial discoid segmentation. A
closer study of them shows, however, that these two groups do not
present a natural unity, and that the historical relations between
their several divisions are very complicated. In order to
understand them properly, we must first consider the various
modifications of gastrulation in these classes. We may begin with
that of the amphibia.
The most suitable and most available objects of study in this
class are the eggs of our indigenous amphibia, the tailless frogs
and toads, and the tailed salamander. In spring they are to be
found in clusters in every pond, and careful examination of the ova
with a lens is sufficient to show at least the external features of
the segmentation. In order to understand the whole process rightly
and follow the formation of the germinal layers and the gastrula,
the ova of the frog and salamander must be carefully hardened; then
the thinnest possible sections must be made of the hardened ova
with the microtome, and the tinted sections must be very closely
compared under a powerful microscope.
The ova of the frog or toad are globular in shape, about the
twelfth of an inch in diameter, and are clustered in jelly-like
masses, which are lumped together in the case of the frog, but form
long strings in the case of the toad. When we examine the opaque,
grey, brown, or blackish ova closely, we find that the upper half
is darker than the lower. The middle of the upper half is in many
species black, while the middle of the lower half is
white.2 In this way we get a definite axis of the ovum
with two poles. To give a clear
1. Cf. Balfour’s Manual of Comparative
Embryology, vol. ii; Theodore Morgan’s The Development
of the Frog’s Egg.
2. The colouring of the eggs of the amphibia is caused by the
accumulation of dark-colouring matter at the animal pole of the
ovum. In consequence of this, the animal cells of the ectoderm are
darker than the vegetal cells of the entoderm. We find the reverse
of this in the case of most animals, the protoplasm of the entoderm
cells being usually darker and coarser-grained.
[ 72 ]
idea of the segmentation of this ovum, it is best to
compare it with a globe, on the surface of which are marked the
various parallels of longitude and latitude. The superficial
dividing lines between the different cells, which come from the
repeated segmentation of the ovum, look like deep furrows on the
surface, and hence the whole process has been given the name of
furcation. In reality, however, this “furcation,” which
was formerly regarded as a very mysterious process, is nothing but
the familiar, repeated cell-segmentation. Hence also the
segmentation-cells which result from it are real cells.
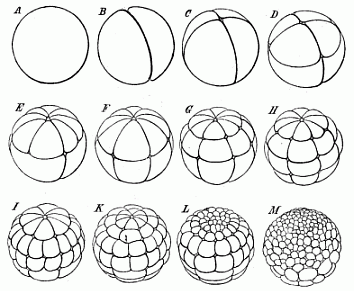
Fig. 40—The
cleavage of the frog’s ovum (magnified). A stem-cell.
B the first two segmentation-cells. C four cells.
D eight cells (4 animal and 4 vegetative). E twelve
cells (8 animal and 4 vegetative). F sixteen cells (8 animal
and 8 vegetative). G twenty-four cells (16 animal and 8
vegetative). H thirty-two cells. I forty-eight cells.
K sixty-four cells. L ninety-six cells. M 160
cells (128 animal and 32 vegetative). |
The unequal segmentation which we observe in the ovum of the
amphibia has the special feature of beginning at the upper and
darker pole (the north pole of the terrestrial globe in our
illustration), and slowly advancing towards the lower and brighter
pole (the south pole). Also the upper and darker hemisphere remains
in this position throughout the course of the segmentation, and its
cells multiply much more briskly. Hence the cells of the lower
hemisphere are found to be larger and less numerous. The cleavage
of the stem-cell (Fig. 40 A) begins with the formation of a
complete furrow, which starts from the north pole and reaches to
the south (B). An hour later a second furrow arises in the
same way, and this cuts the first at a right angle (Fig. 40
C). The ovum is thus divided into four equal parts. Each of
these four “segmentation cells” has an upper and darker
and a lower, brighter half. A few hours later a third furrow
appears, vertically to the first two (Fig. 40 D). The
globular germ now consists of eight cells, four smaller ones above
(northern) and four larger ones below (southern). Next, each of the
four upper ones divides into two halves by a cleavage beginning
from the north pole, so that we now have eight above and four below
(Fig. 40 E). Later, the
[ 73 ]
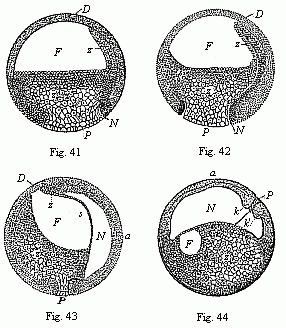
Figs.
41–44—Four vertical sections of the fertilised
ovum of the toad, in four successive stages of development. The
letters have the same meaning throughout: F
segmentation-cavity. D covering of same (D dorsal
half of the embryo, P ventral half). P yelk-stopper
(white round field at the lower pole). Z yelk-cells of the
entoderm (Remak’s “glandular embryo”). N
primitive gut cavity (progaster or Rusconian alimentary cavity).
The primitive mouth (prostoma) is closed by the yelk-stopper, P.
s partition between the primitive gut cavity (N) and the
segmentation cavity (F). k k', section of the large
circular lip-border of the primitive mouth (the Rusconian anus).
The line of dots between k and k' indicates the
earlier connection of the yelk-stopper (P) with the central
mass of the yelk-cells (Z). In Fig. 44 the ovum has turned
90°, so that the back of the embryo is uppermost and the
ventral side down. (From Stricker.). |
four new longitudinal divisions extend gradually to
the lower cells, and the number rises from twelve to sixteen
(F). Then a second circular furrow appears, parallel to the
first, and nearer to the north pole, so that we may compare it to
the north polar circle. In this way we get twenty-four
segmentation-cells—sixteen upper, smaller, and darker ones,
and eight smaller and brighter ones below (G). Soon,
however, the latter also sub-divide into sixteen, a third or
“meridian of latitude” appearing, this time in the
southern hemisphere: this makes thirty-two cells altogether
(H). Then eight new longitudinal lines are formed at the
north pole, and these proceed to divide, first the darker cells
above and afterwards the lighter southern cells, and finally reach
the south pole. In this way we get in succession forty,
forty-eight, fifty-six, and at last sixty-four cells (I, K).
In the meantime, the two hemispheres differ more and more from each
other. Whereas the sluggish lower hemisphere long remains at
thirty-two cells, the lively northern hemisphere briskly
sub-divides twice, producing first sixty-four and then 128 cells
(L, M). Thus we reach a stage in which we count on the
surface of the ovum 128 small cells in the upper half and
thirty-two large ones in the lower half, or 160 altogether. The
dissimilarity of the two halves increases: while the northern
breaks up into a great number of small cells, the southern consists
of a much smaller number of larger cells. Finally, the dark cells
of the upper half grow almost over the surface of the ovum, leaving
only a small circular spot
[ 74 ]
at the south pole, where the large and clear cells
of the lower half are visible. This white region at the south pole
corresponds, as we shall see afterwards, to the primitive mouth of
the gastrula. The whole mass of the inner and larger and clearer
cells (including the white polar region) belongs to the entoderm or
ventral layer. The outer envelope of dark smaller cells forms the
ectoderm or skin-layer.
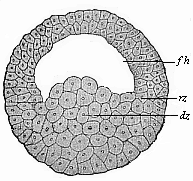 Fig.
45—Blastula of the water-salamander
(Triton). fh segmentation-cavity, dz yelk-cells, rz
border-zone.
(From Hertwig.)
Fig.
45—Blastula of the water-salamander
(Triton). fh segmentation-cavity, dz yelk-cells, rz
border-zone.
(From Hertwig.) |
In the meantime, a large cavity, full of fluid, has been formed
within the globular body—the segmentation-cavity or embryonic
cavity (blastocœl, Figs.
41–44 F). It extends considerably as the cleavage
proceeds, and afterwards assumes an almost semi-circular form (Fig.
41 F). The frog-embryo now represents a modified embryonic
vesicle or blastula, with hollow animal half and solid
vegetal half.
Now a second, narrower but longer, cavity arises by a process of
folding at the lower pole, and by the falling away from each other
of the white entoderm-cells (Figs. 41–44 N). This is
the primitive gut-cavity or the gastric cavity of the gastrula,
progaster or archenteron. It was first observed in the ovum of the
amphibia by Rusconi, and so called the Rusconian cavity. The reason
of its peculiar narrowness here is that it is, for the most part,
full of yelk-cells of the entoderm. These also stop up the whole of
the wide opening of the primitive mouth, and form what is known as
the “yelk-stopper,” which is seen freely at the white
round spot at the south pole (P). Around it the ectoderm is
much thicker, and forms the border of the primitive mouth, the most
important part of the embryo (Fig. 44 k, k'). Soon the
primitive gut-cavity stretches further and further at the expense
of the segmentation-cavity (F), until at last the latter
disappears altogether. The two cavities are only separated by a
thin partition (Fig. 43 s). With the formation of the
primitive gut our frog-embryo has reached the gastrula stage,
though it is clear that this cenogenetic amphibian gastrula is very
different from the real palingenetic gastrula we have considered
(Figs. 30–36).
In the growth of this hooded gastrula we cannot sharply mark off
the various stages which we distinguish successively in the
bell-gastrula as morula and gastrula. Nevertheless, it is not
difficult to reduce the whole cenogenetic or disturbed development
of this amphigastrula to the true palingenetic formation of the
archigastrula of the amphioxus.
 Fig.
46—Embryonic vesicle of triton (blastula),
outer view, with the transverse fold of the primitive mouth
(u). (From Hertwig.)
Fig.
46—Embryonic vesicle of triton (blastula),
outer view, with the transverse fold of the primitive mouth
(u). (From Hertwig.) |
This reduction becomes easier if, after considering the
gastrulation of the tailless amphibia (frogs and toads), we glance
for a moment at that of the tailed amphibia, the salamanders. In
some of the latter, that have only recently been carefully studied,
and that are phylogenetically older, the process is much simpler
and clearer than is the case with the former and longer known. Our
common water-salamander (Triton taeniatus) is a particularly
good subject for observation. Its nutritive yelk is much smaller
and its formative yelk less obscured with black pigment-cells than
in the case of the frog; and its gastrulation has better retained
the original palingenetic character. It was first described by
Scott and Osborn (1879), and Oscar Hertwig especially made a
careful study of it (1881), and rightly pointed out its great
importance in helping us to understand the vertebrate development.
Its globular blastula (Fig. 45) consists of loosely-aggregated,
[ 75 ]
yelk-filled entodermic cells or yelk-cells
(dz) in the lower vegetal half; the upper, animal half
encloses the hemispherical segmentation-cavity (fh), the
curved roof of which is formed of two or three strata of small
ectodermic cells. At the point where the latter pass into the
former (at the equator of the globular vesicle) we have the border
zone (rz). The folding which leads to the formation of the
gastrula takes place at a spot in this border zone, the primitive
mouth (Fig. 46 u).
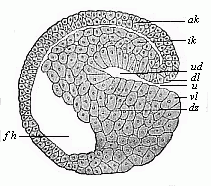 Fig.
47—Sagittal section of a hooded-embryo
(depula) of triton (blastula at the commencement of
gastrulation). ak outer germinal layer, ik inner
germinal layer, fh segmentation-cavity, ud primitive gut,
u primitive mouth, dl and vl dorsal and
ventral lips of the mouth, dz yelk-cells. (From
Hertwig.)
Fig.
47—Sagittal section of a hooded-embryo
(depula) of triton (blastula at the commencement of
gastrulation). ak outer germinal layer, ik inner
germinal layer, fh segmentation-cavity, ud primitive gut,
u primitive mouth, dl and vl dorsal and
ventral lips of the mouth, dz yelk-cells. (From
Hertwig.) |
Unequal segmentation takes place in some of the cyclostoma and
in the oldest fishes in just the same way as in most of the
amphibia. Among the cyclostoma (“round-mouthed”) the
familiar lampreys are particularly interesting. In respect of
organisation and development they are half-way between the acrania
(lancelet) and the lowest real fishes (Selachii); hence I
divided the group of the cyclostoma in 1886 from the real fishes
with which they were formerly associated, and formed of them a
special class of vertebrates. The ovum-segmentation in our common
river-lamprey (Petromyzon fluviatilis) was described by Max
Schultze in 1856, and afterwards by Scott (1882) and Goette
(1890).
Unequal total segmentation follows the same lines in the oldest
fishes, the selachii and ganoids, which are directly descended from
the cyclostoma. The primitive fishes (Selachii), which we
must regard as the ancestral group of the true fishes, were
generally considered, until a short time ago, to be discoblastic.
It was not until the beginning of the twentieth century that
Bashford Dean made the important discovery in Japan that one of the
oldest living fishes of the shark type (Cestracion
japonicus) has the same total unequal segmentation as the
amphiblastic plated fishes (ganoides).1 This is
particularly interesting in connection with our subject, because
the few remaining survivors of this division, which was so numerous
in paleozoic times, exhibit three different types of gastrulation.
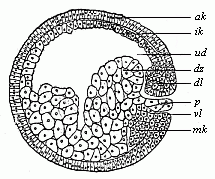 Fig.
48—Sagittal section of the gastrula of the
water-salamander (Triton). (From Hertwig.)
Letters as in Fig. 47; except—p yelk-stopper,
mk beginning of the middle germinal layer.)
Fig.
48—Sagittal section of the gastrula of the
water-salamander (Triton). (From Hertwig.)
Letters as in Fig. 47; except—p yelk-stopper,
mk beginning of the middle germinal layer.) |
The oldest and most conservative forms of the modern ganoids are
the scaly sturgeons (Sturiones), plated fishes of great
evolutionary importance, the eggs of which are eaten as caviar;
their cleavage is not essentially different from that of the
lampreys and the amphibia. On the other hand, the most modern of
the plated fishes, the beautifully scaled bony pike of the North
American rivers (Lepidosteus), approaches the osseous fishes, and
is discoblastic like them. A third genus (Amia) is midway between
the sturgeons and the latter.
The group of the lung-fishes (Dipneusta or Dipnoi)
is closely connected with the older ganoids. In respect of their
whole organisation they are midway between the gill-breathing
fishes and the lung-breathing amphibia; they share with the former
the shape of the body and limbs, and with the latter the form of
the heart
1. Bashford Dean, Holoblastic Cleavage in the
Egg of a Shark, Cestracion japonicus Macleay. Annotationes
zoologicae japonenses, vol. iv, Tokio, 1901.
[ 76 ]
and lungs. Of the older dipnoi
(Paladipneusta) we have now only one specimen, the
remarkable Ceratodus of East Australia; its amphiblastic
gastrulation has been recently explained by Richard Semon (cf.
Chapter XXI). That of the two modern dipneusta, of which
Protopterus is found in Africa and Lepidosiren in
America, is not materially different. (Cf. Fig. 51.)
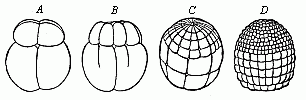
Fig.
49—Ovum-segmentation of the lamprey (Petromyzon
fluviatalis), in four successive stages. The small cells of the
upper (animal) hemisphere divide much more quickly than the cells
of the lower (vegetal) hemisphere. |
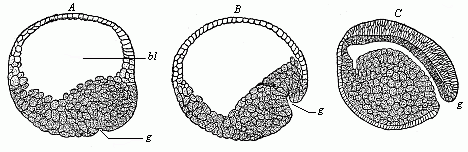
Fig.
50—Gastrulation of the lamprey (Petromyzon
fluviatilis). A blastula, with wide embryonic cavity
(blastocoel, bl), g incipient invagination. B
depula, with advanced invagination, from the primitive mouth
(g). C gastrula, with complete primitive gut: the
embryonic cavity has almost disappeared in consequence of
invagination. |
All these amphiblastic vertebrates, Petromyzon and
Cestracion, Accipenser and Ceratodus, and also the
salamanders and batrachia, belong to the old, conservative groups
of our stem. Their unequal ovum-segmentation and gastrulation have
many peculiarities in detail, but can always be reduced with
comparative ease to the original cleavage and gastrulation of the
lowest vertebrate, the amphioxus; and this is little removed, as we
have seen, from the very simple archigastrula of the Sagitta
and Monoxenia (see Fig.
29–36). All these and many other classes of animals
generally agree in the circumstance that in segmentation their
[ 77 ]
ovum divides into a large number of cells by
repeated cleavage. All such ova have been called, after Remak,
“whole-cleaving” (holoblasta), because their
division into cells is complete or total.
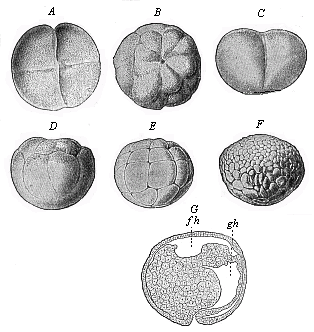
Fig.
51—Gastrulation of ceratodus (from Semon).
A and C stage with four cells, B and D
with sixteen cells. A and B are seen from above,
C and D sideways. E stage with thirty-two cells;
F blastula; G gastrula in longitudinal section.
fh segmentation-cavity. gh primitive gut or gastric
cavity. |
In a great many other classes of animals this is not the case,
as we find (in the vertebrate stem) among the birds, reptiles, and
most of the fishes; among the insects and most of the spiders and
crabs (of the articulates); and the cephalopods (of the molluscs).
In all these animals the mature ovum, and the stem-cell that arises
from it in fertilisation, consist of two different and separate
parts, which we have called formative yelk and nutritive yelk. The
formative yelk alone consists of living protoplasm, and is the
active, evolutionary, and nucleated part of the ovum; this alone
divides in segmentation, and produces the numerous cells which make
up the embryo. On the other hand, the nutritive yelk is merely a
passive part of the contents of the ovum, a subordinate element
which contains nutritive material (albumin, fat, etc.), and so
represents in a sense the provision-store of the developing embryo.
The latter takes a quantity of food out of this store, and finally
consumes it all. Hence the nutritive yelk is of great indirect
importance in embryonic development, though it has no direct share
in it. It either does not divide at all, or only later on, and does
not generally consist of cells. It is sometimes large and sometimes
small, but generally many times larger than the formative yelk; and
hence it is
[ 78 ]
that it was formerly thought the more important of
the two. As the respective significance of these two parts of the
ovum is often wrongly described, it must be borne in mind that the
nutritive yelk is only a secondary addition to the primary cell, it
is an inner enclosure, not an external appendage. All ova that have
this independent nutritive yelk are called, after Remak,
“partially-cleaving” (meroblasta). Their
segmentation is incomplete or partial.
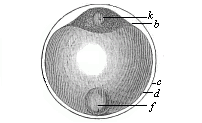 Fig.
52—Ovum of a deep-sea bony fish. b
protoplasm of the stem-cell, k nucleus of same, d
clear globule of albumin, the nutritive yelk, f fat-globule
of same, c outer membrane of the ovum, or ovolemma.)
Fig.
52—Ovum of a deep-sea bony fish. b
protoplasm of the stem-cell, k nucleus of same, d
clear globule of albumin, the nutritive yelk, f fat-globule
of same, c outer membrane of the ovum, or ovolemma.) |
There are many difficulties in the way of understanding this
partial segmentation and the gastrula that arises from it. We have
only recently succeeded, by means of comparative research, in
overcoming these difficulties, and reducing this cenogenetic form
of gastrulation to the original palingenetic type. This is
comparatively easy in the small meroblastic ova which contain
little nutritive yelk—for instance, in the marine ova of a
bony fish, the development of which I observed in 1875 at Ajaccio
in Corsica. I found them joined together in lumps of jelly,
floating on the surface of the sea; and, as the little ovula were
completely transparent, I could easily follow the development of
the germ step by step. These ovula are glossy and colourless
globules of little more than the 50th of an inch. Inside a
structureless, thin, but firm membrane (ovolemma, Fig. 52
c) we find a large, quite clear, and transparent globule of
albumin (d). At both poles of its axis this globule has a
pit-like depression. In the pit at the upper, animal pole (which is
turned downwards in the floating ovum) there is a bi-convex lens
composed of protoplasm, and this encloses the nucleus (k);
this is the formative yelk of the stem-cell, or the germinal disk
(b). The small fat-globule (f) and the large
albumin-globule (d) together form the nutritive yelk. Only
the formative yelk undergoes cleavage, the nutritive yelk not
dividing at all at first.
The segmentation of the lens-shaped formative yelk (b)
proceeds quite independently of the nutritive yelk, and in perfect
geometrical order.
When the mulberry-like cluster of cells has been formed, the
border-cells of the lens separate from the rest and travel into the
yelk and the border-layer. From this the blastula is developed; the
regular bi-convex lens being converted into a disk, like a
watch-glass, with thick borders. This lies on the upper and less
curved polar surface of the nutritive yelk like the watch glass on
the yelk. Fluid gathers between the outer layer and the border, and
the segmentation-cavity is formed. The gastrula is then formed by
invagination, or a kind of turning-up of the edge of the
blastoderm. In this process the segmentation-cavity disappears.
The space underneath the entoderm corresponds to the primitive
gut-cavity, and is filled with the decreasing food-yelk (n).
Thus the formation of the gastrula of our fish is complete. In
contrast to the two chief forms of gastrula we considered
previously, we give the name of discoid gastrula
(discogastrula, Fig. 54) to this
third principal type.
Very similar to the discoid gastrulation of the bony fishes is
that of the hags or myxinoida, the remarkable cyclostomes that live
parasitically in the body-cavity of fishes, and are distinguished
by several notable peculiarities from their nearest relatives, the
lampreys. While the amphiblastic ova of the latter are small and
develop like those of the amphibia, the cucumber-shaped ova of the
hag are about an inch long, and form a discoid gastrula. Up to the
present it has only been observed in one species (Bdellostoma
Stouti), by Dean and Doflein (1898).
It is clear that the important features which distinguish the
discoid gastrula from the other chief forms we have considered are
determined by the large food-yelk. This takes no direct part in the
building of the germinal layers, and completely fills the primitive
gut-cavity of the gastrula, even protruding at the mouth-opening.
If we imagine the original bell-gastrula (Figs. 30–36) trying
to swallow a
[ 79 ]
ball of food which is much bigger than itself, it
would spread out round it in discoid shape in the attempt, just as
we find to be the case here (Fig. 54). Hence we may derive the
discoid gastrula from the original bell-gastrula, through the
intermediate stage of the hooded gastrula. It has arisen through
the accumulation of a store of food-stuff at the vegetal pole, a
“nutritive yelk” being thus formed in contrast to the
“formative yelk.” Nevertheless, the gastrula is formed
here, as in the previous cases, by the folding or invagination of
the blastula. We can, therefore, reduce this cenogenetic form of
the discoid segmentation to the palingenetic form of the primitive
cleavage.
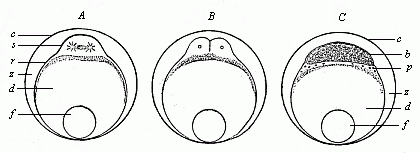
Fig.
53—Ovum-segmentation of a bony fish. A
first cleavage of the stem-cell (cytula), B division
of same into four segmentation-cells (only two visible), C
the germinal disk divides into the blastoderm (b) and the
periblast (p). d nutritive yelk, f
fat-globule, c ovolemma, z space between the ovolemma
and the ovum, filled with a clear fluid.) |
This reduction is tolerably easy and confident in the case of
the small ovum of our deep-sea bony fish, but it becomes difficult
and uncertain in the case of the large ova that we find in the
majority of the other fishes and in all the reptiles and birds. In
these cases the food-yelk is, in the first place, comparatively
colossal, the formative yelk being almost invisible beside it; and,
in the second place, the food-yelk contains a quantity of different
elements, which are known as “yelk-granules, yelk-globules,
yelk-plates, yelk-flakes, yelk-vesicles,” and so on.
Frequently these definite elements in the yelk have been described
as real cells, and it has been wrongly stated that a portion of the
embryonic body is built up from these cells. This is by no means
the case. In every case, however large it is—and even when
cell-nuclei travel into it during the cleavage of the
border—the nutritive yelk remains a dead accumulation of
food, which is taken into the gut during embryonic development and
consumed by the embryo. The latter develops solely from the living
formative yelk of the stem-cell. This is equally true of the ova of
our small bony fishes and of the colossal ova of the primitive
fishes, reptiles, and birds.
The gastrulation of the primitive fishes or selachii (sharks and
rays) has been carefully studied of late years by Ruckert, Rabl,
and H.E. Ziegler in particular, and is very important in the sense
that this group is the oldest among living fishes, and their
gastrulation can be derived directly from that of the cyclostoma by
the accumulation of a large quantity of food-yelk. The oldest
sharks (Cestracion) still have the unequal segmentation
inherited from the cyclostoma. But while in this case, as in the
case of the amphibia, the small ovum completely divides into cells
in segmentation, this is no longer so in the great majority of the
selachii (or Elasmobranchii). In these the contractility of
the active protoplasm no longer suffices to break up the huge mass
of the passive deutoplasm completely into cells; this is only
possible in the upper or dorsal part, but not in the lower or
ventral section. Hence we find in the primitive fishes a blastula
with a small eccentric segmentation-cavity (Fig.
55 b), the wall of which varies greatly in composition.
The circular border of the germinal disk which connects the roof
and floor of the segmentation-cavity corresponds to the border-zone
at the equator of the amphibian ovum. In the middle of its hinder
border we have the beginning of the invagination of the primitive
gut
[ 80 ]
(Fig. 56 ud); it
extends gradually from this spot (which corresponds to the
Rusconian anus of the amphibia) forward and around, so that the
primitive mouth becomes first crescent-shaped and then circular,
and, as it opens wider, surrounds the ball of the larger
food-yelk.
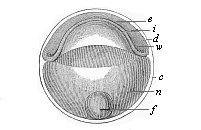 Fig.
54—Discoid gastrula (discogastrula) of a
bony fish. e ectoderm, i entoderm, w
border-swelling or primitive mouth, n albuminous globule of
the nutritive yelk, f fat-globule of same, c external
membrane (ovolemma), d partition between entoderm and
ectoderm (earlier the segmentation-cavity.)
Fig.
54—Discoid gastrula (discogastrula) of a
bony fish. e ectoderm, i entoderm, w
border-swelling or primitive mouth, n albuminous globule of
the nutritive yelk, f fat-globule of same, c external
membrane (ovolemma), d partition between entoderm and
ectoderm (earlier the segmentation-cavity.) |
Essentially different from the wide-mouthed discoid gastrula of
most of the selachii is the narrow-mouthed discoid gastrula (or
epigastrula) of the amniotes, the reptiles, birds, and
monotremes; between the two—as an intermediate stage—we
have the amphigastrula of the amphibia. The latter has
developed from the amphigastrula of the ganoids and dipneusts,
whereas the discoid amniote gastrula has been evolved from the
amphibian gastrula by the addition of food-yelk. This change of
gastrulation is still found in the remarkable ophidia
(Gymnophiona, Cœcilia, or Peromela),
serpent-like amphibia that live in moist soil in the tropics, and
in many respects represent the transition from the gill-breathing
amphibia to the lung-breathing reptiles. Their embryonic
development has been explained by the fine studies of the brothers
Sarasin of Ichthyophis glutinosa at Ceylon (1887), and those
of August Brauer of the Hypogeophis rostrata in the
Seychelles (1897). It is only by the historical and comparative
study of these that we can understand the difficult and obscure
gastrulation of the amniotes.
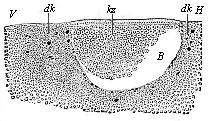 Fig.
55—Longitudinal section through the blastula of a
shark (Pristiuris). (From Ruckert.) (Looked at
from the left; to the right is the hinder end, H, to the
left the fore end, V.) B segmentation-cavity,
kz cells of the germinal membrane, dk yelk-nuclei.
Fig.
55—Longitudinal section through the blastula of a
shark (Pristiuris). (From Ruckert.) (Looked at
from the left; to the right is the hinder end, H, to the
left the fore end, V.) B segmentation-cavity,
kz cells of the germinal membrane, dk yelk-nuclei. |
The bird’s egg is particularly important for our purpose,
because most of the chief studies of the development of the
vertebrates are based on observations of the hen’s egg during
hatching. The mammal ovum is much more difficult to obtain and
study, and for this practical and obvious reason very rarely
thoroughly investigated. But we can get hens’ eggs in any
quantity at any time, and, by means of artificial incubation,
follow the development of the embryo step by step. The bird’s
egg differs considerably from the tiny mammal ovum in size, a large
quantity of food-yelk accumulating within the original yelk or the
protoplasm of the ovum. This is the yellow ball which we commonly
call the yolk of the egg. In order to understand the bird’s
egg aright—for it is very often quite wrongly
explained—we must examine it in its original condition, and
follow it from the very beginning of its development in the
bird’s ovary. We then see that the original ovum is a quite
small, naked, and simple cell with a nucleus, not differing in
either size or shape from the original ovum of the mammals and
other animals (cf. Fig. 13
E). As in the case of all the craniota (animals with a
skull), the original or primitive ovum (protovum) is covered
with a continuous layer of small cells. This membrane is the
follicle, from which the ovum afterwards issues. Immediately
underneath it the structureless yelk-membrane is secreted from the
yelk.
The small primitive ovum of the bird begins very early to take
up into itself a quantity of food-stuff through the yelk-membrane,
and work it up into the “yellow yelk.” In this way the
ovum
[ 81 ]
enters on its second stage (the metovum), which is
many times larger than the first, but still only a single enlarged
cell. Through the accumulation of the store of yellow yelk within
the ball of protoplasm the nucleus it contains (the germinal
vesicle) is forced to the surface of the ball. Here it is
surrounded by a small quantity of protoplasm, and with this forms
the lens-shaped formative yelk (Fig.
15 b). This is seen on the yellow yelk-ball, at a
certain point of the surface, as a small round white spot—the
“tread” (cicatricula). From this point a
thread-like column of white nutritive yelk (d), which
contains no yellow yelk-granules, and is softer than the yellow
food-yelk, proceeds to the middle of the yellow yelk-ball, and
forms there a small central globule of white yelk (Fig. 15
d). The whole of this white yelk is not sharply separated from
the yellow yelk, which shows a slight trace of concentric layers in
the hard-boiled egg (Fig. 15 c). We also find in the
hen’s egg, when we break the shell and take out the yelk, a
round small white disk at its surface which corresponds to the
tread. But this small white “germinal disk” is now
further developed, and is really the gastrula of the chick. The
body of the chick is formed from it alone. The whole white and
yellow yelk-mass is without any significance for the formation of
the embryo, it being merely used as food by the developing chick.
The clear, glarous mass of albumin that surrounds the yellow yelk
of the bird’s egg, and also the hard chalky shell, are only
formed within the oviduct round the impregnated ovum.
|
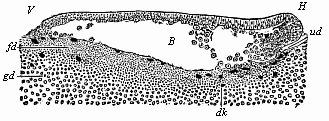
Fig.
56—Longitudinal section of the blastula of a shark
(Pristiurus) at the beginning of gastrulation. (From
Ruckert.) (Seen from the left.) V fore end, H
hind end, B segmentation-cavity, ud first trace of
the primitive gut, dk yelk-nuclei, fd fine-grained
yelk, gd coarse-grained yelk. |
When the fertilisation of the bird’s ovum has taken place
within the mother’s body, we find in the lens-shaped
stem-cell the progress of flat, discoid segmentation (Fig. 57). First two equal segmentation-cells
(A) are formed from the ovum. These divide into four
(B), then into eight, sixteen (C), thirty-two,
sixty-four, and so on. The cleavage of the cells is always preceded
by a division of their nuclei. The cleavage surfaces between the
segmentation-cells appear at the free surface of the tread as
clefts. The first two divisions are vertical to each other, in the
form of a cross (B). Then there are two more divisions,
which cut the former at an angle of forty-five degrees. The tread,
which thus becomes the germinal disk, now has the appearance of an
eight-rayed star. A circular cleavage next taking place round the
middle, the eight triangular cells divide into sixteen, of which
eight are in the middle and eight distributed around (C).
Afterwards circular clefts and radial clefts, directed towards the
centre, alternate more or less irregularly (D, E). In most
of the amniotes the formation of concentric and radial clefts is
irregular from the very first; and so also in the hen’s egg.
But the final outcome of the cleavage-process is once more the
formation of a large number of small cells of a similar nature. As
in the case of the fish-ovum, these segmentation-cells form a
round, lens-shaped disk, which corresponds to the morula, and is
embedded in a small depression of the white yelk. Between the
lens-shaped disk of the morula-cells and the underlying white yelk
a small cavity is now formed by the accumulation of fluid, as in
the fishes. Thus we get the peculiar and not easily recognisable
blastula of the bird (Fig. 58). The small
segmentation-cavity (fh) is very flat and much compressed.
The upper or dorsal wall (dw) is formed of a single layer of
clear, distinctly separated cells; this
[ 82 ]
corresponds to the upper or animal hemisphere of the
triton-blastula (Fig. 45). The lower or
ventral wall of the flat dividing space (vw) is made up of
larger and darker segmentation-cells; it corresponds to the lower
or vegetal hemisphere of the blastula of the water-salamander (Fig.
45 dz). The nuclei of the yelk-cells, which are in this case
especially numerous at the edge of the lens-shaped blastula, travel
into the white yelk, increase by cleavage, and contribute even to
the further growth of the germinal disk by furnishing it with
food-stuff.
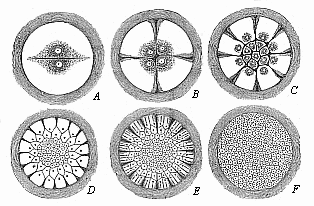
Fig. 57—Diagram
of discoid segmentation in the bird’s ovum (magnified).
Only the formative yelk (the tread) is shown in these six figures
(A to F), because cleavage only takes place in this.
The much larger food-yelk, which does not share in the cleavage, is
left out and merely indicated by the dark ring without. |
The invagination or the folding inwards of the bird-blastula
takes place in this case also at the hinder pole of the subsequent
chief axis, in the middle of the hind border of the round germinal
disk (Fig. 59 s). At this spot we
have the most brisk cleavage of the cells; hence the cells are more
numerous and smaller here than in the fore-half of the germinal
disk. The border-swelling or thick edge of the disk is less clear
but whiter behind, and is more sharply separated from contiguous
parts. In the middle of its hind border there is a white,
crescent-shaped groove—Koller’s sickle-groove (Fig. 59
s); a small projecting process in the centre of it is called
the sickle-knob (sk). This important cleft is the primitive
mouth, which was described for a long time as the “primitive
groove.” If we make a vertical section through this part, we
see that a flat and broad cleft stretches under the germinal disk
forwards from the primitive mouth; this is the primitive gut (Fig.
60 ud). Its roof or dorsal wall is formed by the folded
upper part of the blastula, and its floor or ventral wall by the
white yelk (wd), in which a number of yelk-nuclei
(dk) are distributed. There is a brisk multiplication of
these at the edge of the germinal disk, especially in the
neighbourhood of the sickle-shaped primitive mouth.
We learn from sections through later stages of this discoid
bird-gastrula that the primitive gut-cavity, extending forward from
the primitive mouth as a flat pouch, undermines the whole region of
the round flat lens-shaped blastula (Fig. 61
ud). At the same time, the segmentation-cavity gradually
disappears altogether, the folded inner germinal layer (ik)
placing itself from underneath on the overlying outer germinal
layer (ak). The typical process of invagination, though
greatly disguised, can thus be clearly seen in this case, as Goette
and Rauber, and more recently Duval (Fig. 61), have shown.
The older embryologists (Pander, Baer, Remak), and, in recent
times especially,
[ 83 ]
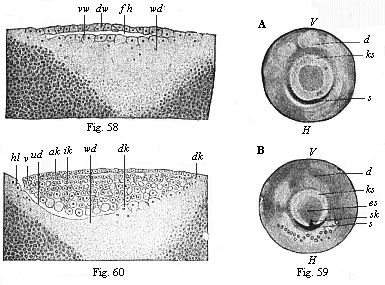
Fig. 58—Vertical
section of the blastula of a hen (discoblastula).
fh segmentation-cavity, dw dorsal wall of same,
vw ventral wall, passing directly into the white yelk
(wd). (From Duval.)
Fig. 59—The germinal disk of the hen’s ovum at the
beginning of gastrulation; A before incubation, B
in the first hour of incubation. (From Koller.) ks
germinal-disk, V its fore and H its hind border;
es embryonic shield, s sickle-groove, sk sickle
knob, d yelk.
Fig. 60—Longitudinal section of the germinal disk of a
siskin (discogastrula). (From Duval.) ud
primitive gut, vl, hl fore and hind lips of the primitive
mouth (or sickle-edge); ak outer germinal layer, ik
inner germinal layer, dk yelk-nuclei, wd white
yelk. |

Fig.
61—Longitudinal section of the discoid gastrula of the
nightingale. (From Duval.) ud primitive gut,
vl, hl fore and hind lips of the primitive mouth; ak, ik
outer and inner germinal layers; vr fore-border of the
discogastrula. |
His, Kölliker, and others, said that the two primary
germinal layers of the hen’s ovum—the oldest and most
frequent subject of observation!—arose by horizontal cleavage
of a simple germinal disk. In opposition to this accepted view, I
affirmed in my Gastræa Theory (1873) that the discoid
bird-gastrula, like that of all other vertebrates, is formed by
folding (or invagination), and that this typical process is merely
altered in a peculiar way and disguised by the immense accumulation
of food-yelk and the flat spreading of the discoid blastula at one
part of its surface. I endeavoured to establish this view by the
derivation of the vertebrates from one source, and especially by
proving that the birds descend from the reptiles, and these from
the amphibia. If this is correct, the discoid gastrula of the
amniotes must have been formed by the folding-in of a hollow
blastula, as has been shown by Remak and Rusconi of the discoid
gastrula of the amphibia, their direct ancestors. The accurate and
extremely careful observations of the authors I have mentioned
(Goette, Rauber, and Duval) have decisively proved this
[ 84 ]
recently for the birds; and the same has been done
for the reptiles by the fine studies of Kupffer, Beneke, Wenkebach,
and others. In the shield-shaped germinal disk of the lizard (Fig.
62), the crocodile, the tortoise, and other reptiles, we find in
the middle of the hind border (at the same spot as the sickle
groove in the bird) a transverse furrow (u), which leads
into a flat, pouch-like, blind sac, the primitive gut. The fore
(dorsal) and hind (ventral) lips of the transverse furrow
correspond exactly to the lips of the primitive mouth (or
sickle-groove) in the birds.
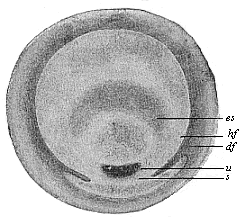 Fig.
62—Germinal disk of the lizard (Lacerta
agilis). (From Kupffer.) u primitive mouth,
s sickle, es embryonic shield, hf and df
light and dark germinative area.
Fig.
62—Germinal disk of the lizard (Lacerta
agilis). (From Kupffer.) u primitive mouth,
s sickle, es embryonic shield, hf and df
light and dark germinative area. |
The gastrulation of the mammals must be derived from this
special embryonic development of the reptiles and birds. This
latest and most advanced class of the vertebrates has, as we shall
see afterwards, evolved at a comparatively recent date from an
older group of reptiles; and all these amniotes must have come
originally from a common stem-form. Hence the distinctive embryonic
process of the mammal must have arisen by cenogenetic modifications
from the older form of gastrulation of the reptiles and birds.
Until we admit this thesis we cannot understand the formation of
the germinal layers in the mammal, and therefore in man.
I first advanced this fundamental principle in my essay On
the Gastrulation of Mammals (1877), and sought to show in this
way that I assumed a gradual degeneration of the food-yelk and the
yelk-sac on the way from the proreptiles to the mammals. “The
cenogenetic process of adaptation,” I said, “which has
occasioned the atrophy of the rudimentary yelk-sac of the mammal,
is perfectly clear. It is due to the fact that the young of the
mammal, whose ancestors were certainly oviparous, now remain a long
time in the womb. As the great store of food-yelk, which the
oviparous ancestors gave to the egg, became superfluous in their
descendants owing to the long carrying in the womb, and the
maternal blood in the wall of the uterus made itself the chief
source of nourishment, the now useless yelk-sac was bound to
atrophy by embryonic adaptation.”
My opinion met with little approval at the time; it was
vehemently attacked by Kölliker, Hensen, and His in
particular. However, it has been gradually accepted, and has
recently been firmly established by a large number of excellent
studies of mammal gastrulation, especially by Edward Van
Beneden’s studies of the rabbit and bat, Selenka’s on
the marsupials and rodents, Heape’s and
Lieberkühn’s on the mole, Kupffer and Keibel’s on
the rodents, Bonnet’s on the ruminants, etc. From the general
comparative point of view, Carl Rabl in his theory of the mesoderm,
Oscar Hertwig in the latest edition of his Manual (1902), and
Hubrecht in his Studies in Mammalian Embryology (1891), have
supported the opinion, and sought to derive the peculiarly modified
gastrulation of the mammal from that of the reptile.
In the meantime (1884) the studies of Wilhelm Haacke and
Caldwell provided a proof of the long-suspected and very
interesting fact, that the lowest mammals, the monotremes, lay
eggs, like the birds and reptiles, and are not viviparous like
the other mammals. Although the gastrulation of the monotremes was
not really known until studied by Richard
[ 85 ]
Semon in 1894, there could be little doubt, in view
of the great size of their food-yelk, that their ovum-segmentation
was discoid, and led to the formation of a sickle-mouthed
discogastrula, as in the case of the reptiles and birds. Hence I
had, in 1875 (in my essay on The Gastrula and Ovum-segmentation
of Animals), counted the monotremes among the discoblastic
vertebrates. This hypothesis was established as a fact nineteen
years afterwards by the careful observations of Semon; he gave in
the second volume of his great work, Zoological Journeys in
Australia (1894), the first description and correct explanation
of the discoid gastrulation of the monotremes. The fertilised ova
of the two living monotremes (Echidna and
Ornithorhynchus) are balls of one-fifth of an inch in diameter,
enclosed in a stiff shell; but they grow considerably during
development, so that when laid the egg is three times as large. The
structure of the plentiful yelk, and especially the relation of the
yellow and the white yelk, are just the same as in the reptiles and
birds. As with these, partial cleavage takes place at a spot on the
surface at which the small formative yelk and the nucleus it
encloses are found. First is formed a lens-shaped circular germinal
disk. This is made up of several strata of cells, but it spreads
over the yelk-ball, and thus becomes a one-layered blastula.
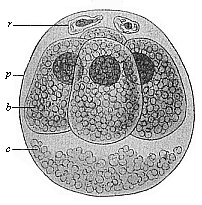 Fig.
63—Ovum of the opossum (Didelphys)
divided into four. (From Selenka.) b the four
segmentation-cells, r directive body, c unnucleated
coagulated matter, p, albumin-membrane.
Fig.
63—Ovum of the opossum (Didelphys)
divided into four. (From Selenka.) b the four
segmentation-cells, r directive body, c unnucleated
coagulated matter, p, albumin-membrane. |
If we then imagine the yelk it contains to be dissolved and replaced by a
clear liquid, we have the characteristic blastula of the higher
mammals. In these the gastrulation proceeds in two phases, as Semon
rightly observes: firstly, formation of the entoderm by cleavage at
the centre and further growth at the edge; secondly, invagination.
In the monotremes more primitive conditions have been retained
better than in the reptiles and birds. In the latter, before the
commencement of the gastrula-folding, we have, at least at the
periphery, a two-layered embryo forming from the cleavage. But in
the monotremes the formation of the cenogenetic entoderm does not
precede the invagination; hence in this case the construction of
the germinal layers is less modified than in the other amniota.
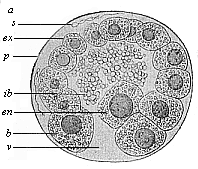 Fig.
64—Blastula of the opossum (Didelphys).
(From Selenka.) a animal pole of the blastula,
v vegetal pole, en mother-cell of the entoderm,
ex ectodermic cells, s spermia, ib unnucleated
yelk-balls (remainder of the food-yelk), p albumin
membrane.
Fig.
64—Blastula of the opossum (Didelphys).
(From Selenka.) a animal pole of the blastula,
v vegetal pole, en mother-cell of the entoderm,
ex ectodermic cells, s spermia, ib unnucleated
yelk-balls (remainder of the food-yelk), p albumin
membrane. |
The marsupials, a second sub-class, come next to the oviparous
monotremes, the oldest of the mammals. But as in their case the
food-yelk is already atrophied, and the little ovum develops within
the mother’s body, the partial cleavage has been reconverted
into total. One section of the marsupials still show points of
agreement with the monotremes, while another section of them,
according to the splendid investigations of Selenka, form a
connecting-link between these and the placentals.
The fertilised ovum of the opossum (Didelphys) divides,
according to Selenka, first into two, then four, then eight equal
cells; hence the segmentation is at first equal or homogeneous. But
in the course of the cleavage a larger cell, distinguished by its
less clear plasm and its containing more yelk-granules (the mother
cell of the entoderm, Fig. 64 en),
[ 86 ]
separates from the others; the latter multiply more
rapidly than the former. As, further, a quantity of fluid gathers
in the morula, we get a round blastula, the wall of which is of
varying thickness, like that of the amphioxus (Fig. 38 E) and the amphibia (Fig. 45). The upper or animal hemisphere is
formed of a large number of small cells; the lower or vegetal
hemisphere of a small number of large cells. One of the latter,
distinguished by its size (Fig. 64 en), lies at the vegetal
pole of the blastula-axis, at the point where the primitive mouth
afterwards appears. This is the mother-cell of the entoderm; it now
begins to multiply by cleavage, and the daughter-cells (Fig. 65
i) spread out from this spot over the inner surface of the
blastula, though at first only over the vegetal hemisphere. The
less clear entodermic cells (i) are distinguished at first
by their rounder shape and darker nuclei from the higher, clearer,
and longer entodermic cells (e), afterwards both are greatly
flattened, the inner blastodermic cells more than the outer.
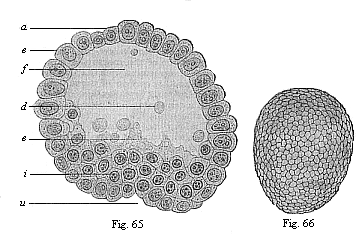
Fig. 65—Blastula
of the opossum (Didelphys) at the beginning of
gastrulation. (From Selenka.) e ectoderm, i
entoderm; a animal pole, u primitive mouth at the
vegetal pole, f segmentation-cavity, d unnucleated
yelk-balls (relics of the reduced food-yelk), c nucleated curd
(without yelk-granules)
Fig. 66—Oval gastrula of the opossum
(Didelphys), about eight hours old. (From Selenka)
(external view).) |
The unnucleated yelk-balls and curd (Fig. 65 d) that we
find in the fluid of the blastula in these marsupials are very
remarkable; they are the relics of the atrophied food-yelk, which
was developed in their ancestors, the monotremes, and in the
reptiles.
In the further course of the gastrulation of the opossum the
oval shape of the gastrula (Fig. 66) gradually changes into
globular, a larger quantity of fluid accumulating in the vesicle.
At the same time, the entoderm spreads further and further over the
inner surface of the ectoderm (e). A globular vesicle is
formed, the wall of which consists of two thin simple strata of
cells; the cells of the outer germinal layer are rounder, and those
of the inner layer flatter. In the region of the primitive mouth
(p) the cells are less flattened, and multiply briskly. From
this point—from the hind (ventral) lip of the primitive
mouth, which extends in a central cleft, the primitive
groove—the construction of the mesoderm proceeds.
Gastrulation is still more modified and curtailed
cenogenetically in the placentals than in the marsupials. It was
first accurately known to us by the distinguished investigations of
Edward Van Beneden in 1875, the first object of study being the
ovum of the rabbit. But as man also belongs to this sub-class, and
as his as yet unstudied gastrulation cannot be materially different
from that of the other placentals, it merits the closest attention.
We have, in the first place, the peculiar feature that the two
first segmentation-cells that proceed from the cleavage of the
fertilised ovum (Fig. 68) are of different
sizes and natures; the difference is sometimes greater, sometimes
less (Fig. 69). One of these first daughter-cells of the ovum is a
little
[ 87 ]
larger, clearer, and more transparent than the
other. Further, the smaller cell takes a colour in carmine, osmium,
etc., more strongly than the larger. By repeated cleavage of it a
morula is formed, and from this a blastula, which changes in a very
characteristic way into the greatly modified gastrula. When the
number of the segmentation-cells in the mammal embryo has reached
ninety-six (in the rabbit, about seventy hours after impregnation)
the fœtus assumes a form very like the archigastrula (Fig. 72). The spherical embryo consists of a
central mass of thirty-two soft, round cells with dark nuclei,
which are flattened into polygonal shape by mutual pressure, and
colour dark-brown with osmic acid (Fig. 72 i). This dark
central group of cells is surrounded by a lighter spherical
membrane, consisting of sixty-four cube-shaped, small, and
fine-grained cells which lie close together in a single stratum,
and only colour slightly in osmic acid (Fig. 72 e). The
authors who regard this embryonic form as the primary gastrula of
the placental conceive the outer layer as the ectoderm and the
inner as the entoderm. The entodermic membrane is only interrupted
at one spot, one, two, or three of the ectodermic cells being loose
there. These form the yelk-stopper, and fill up the mouth of the
gastrula (a). The central primitive gut-cavity (d) is
full of entodermic cells. The uni-axial type of the mammal gastrula
is accentuated in this way. However, opinions still differ
considerably as to the real nature of this “provisional
gastrula” of the placental and its relation to the blastula
into which it is converted.
As the gastrulation proceeds a large spherical blastula is
formed from this peculiar solid amphigastrula of the placental, as
we saw in the case of the marsupial. The accumulation of fluid in
the solid gastrula (Fig. 73 A) leads to the
formation of an eccentric cavity, the group of the darker
entodermic cells (hy) remaining directly attached at one
spot with the round enveloping stratum of the lighter ectodermic
cells (ep). This spot corresponds to the original primitive
mouth (prostoma or blastoporus). From this important spot the inner
germinal layer spreads all round on the inner surface of the outer
layer, the cell-stratum of which forms the wall of the hollow
sphere; the extension proceeds from the vegetal towards the animal
pole.
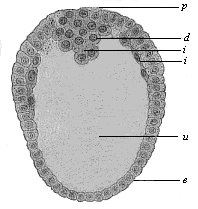 Fig.
67—Longitudinal section through the oval gastrula of
the opossum (Fig. 69). (From Selenka.) p
primitive mouth, e ectoderm, i entoderm, d
yelk remains in the primitive gut-cavity (u).
Fig.
67—Longitudinal section through the oval gastrula of
the opossum (Fig. 69). (From Selenka.) p
primitive mouth, e ectoderm, i entoderm, d
yelk remains in the primitive gut-cavity (u). |
The cenogenetic gastrulation of the placental has been greatly
modified by secondary adaptation in the various groups of this most
advanced and youngest sub-class of the mammals. Thus, for instance,
we find in many of the rodents (guinea-pigs, mice, etc.)
apparently a temporary inversion of the two germinal layers.
This is due to a folding of the blastodermic wall by what is called
the “girder,” a plug-shaped growth of Rauber’s
“roof-layer.” It is a thin layer of flat epithelial
cells, that is freed from the surface of the blastoderm in some of
the rodents; it has no more significance in connection with the
general course of placental gastrulation than the conspicuous
departure from the usual globular shape in the blastula of some of
the ungulates. In some pigs and ruminants it grows into a
thread-like, long and thin tube.
Thus the gastrulation of the placentals, which diverges most
from that of the amphioxus, the primitive form, is reduced to the
original type, the invagination of a modified blastula. Its chief
peculiarity is that the folded part of the blastoderm does not form
a completely closed (only open at the primitive mouth) blind sac,
as is usual; but this blind sac has a wide opening at the ventral
curve (opposite to the dorsal mouth); and through this opening the
primitive gut communicates from the first with the embryonic cavity
of the blastula. The folded crest-shaped
[ 88 ]
entoderm grows with a free circular border on the
inner surface of the entoderm towards the vegetal pole; when it has
reached this, and the inner surface of the blastula is completely
grown over, the primitive gut is closed. This remarkable direct
transition of the primitive gut-cavity into the segmentation-cavity
is explained simply by the assumption that in most of the mammals
the yelk-mass, which is still possessed by the oldest forms of the
class (the monotremes) and their ancestors (the reptiles), is
atrophied. This proves the essential unity of gastrulation in all
the vertebrates, in spite of the striking differences in the
various classes.
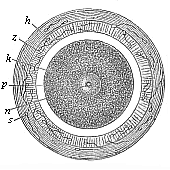 Fig. 68—Stem-cell of the mammal ovum
(from the rabbit). Fig. 68—Stem-cell of the mammal ovum
(from the rabbit).
k stem-nucleus, n nuclear corpuscle,
p protoplasm of the stem-cell,
z modified zona pellucida, h outer albuminous
membrane, s dead sperm-cells. |
|
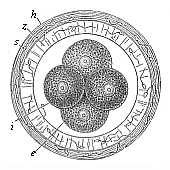
Fig. 70—The first four segmentation-cells of the mammal
ovum (from the rabbit).
e the two larger (and lighter) cells,
i the two smaller (and darker) cells,
z zona pellucida, h outer albuminous membrane. |
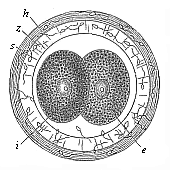
Fig.
69—Incipient cleavage of the mammal ovum (from the
rabbit). The stem-cell has divided into two unequal cells, one
lighter (e) and one darker (i). z zona
pellucida, h outer albuminous membrane, s dead
sperm-cell. |
|
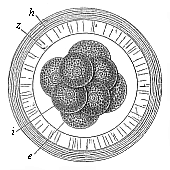
Fig.
71—Mammal ovum with eight segmentation-cells (from the
rabbit). e four larger and lighter cells, i four
smaller and darker cells, z zona pellucida, h outer
albuminous membrane. |
In order to complete our consideration of the important
processes of segmentation and gastrulation, we will, in conclusion,
cast a brief glance at the fourth chief type—superficial
segmentation. In the vertebrates this form is not found at all. But
it plays the chief part in the large stem of the
articulates—the insects,
[ 89 ]
spiders, myriapods, and crabs. The distinctive form
of gastrula that comes of it is the “vesicular
gastrula” (Perigastrula).
In the ova which undergo this superficial cleavage the formative
yelk is sharply divided from the nutritive yelk, as in the
preceding cases of the ova of birds, reptiles, fishes, etc.; the
formative yelk alone undergoes cleavage. But while in the ova with
discoid gastrulation the formative yelk is not in the centre, but
at one pole of the uni-axial ovum, and the food-yelk gathered at
the other pole, in the ova with superficial cleavage we find the
formative yelk spread over the whole surface of the ovum; it
encloses spherically the food-yelk, which is accumulated in the
middle of the ova. As the segmentation only affects the former and
not the latter, it is bound to be entirely
“superficial”; the store of food in the middle is quite
untouched by it. As a rule, it proceeds in regular geometrical
progression. In the end the whole of the formative yelk divides
into a number of small and homogeneous cells, which lie close
together in a single stratum on the entire surface of the ovum, and
form a superficial blastoderm. This blastoderm is a simple,
completely closed vesicle, the internal cavity of which is entirely
full of food-yelk. This real blastula only differs from that of the
primitive ova in its chemical composition. In the latter the
content is water or a watery jelly; in the former it is a thick
mixture, rich in food-yelk, of albuminous and fatty substances. As
this quantity of food-yelk fills the centre of the ovum before
cleavage begins, there is no difference in this respect between the
morula and the blastula. The two stages rather agree in this.
When the blastula is fully formed, we have again in this case
the important folding or invagination that determines gastrulation.
The space between the skin-layer and the gut-layer (the remainder
of the segmentation-cavity) remains full of food-yelk, which is
gradually used up. This is the only material difference between our
vesicular gastrula (perigastrula) and the original form of
the bell-gastrula (archigastrula). Clearly the one has been
developed from the other in the course of time, owing to the
accumulation of food-yelk in the centre of the
ovum.1
We must count it an important advance that we are thus in a
position to reduce all the various embryonic phenomena in the
different groups of animals to these four principal forms of
segmentation and gastrulation. Of these four forms we must regard
one only as the original palingenetic, and the other three as
cenogenetic and derivative. The unequal, the discoid, and the
superficial segmentation have all clearly arisen by secondary
adaptation from the primary segmentation; and the chief cause of
their development has been the gradual formation of the food-yelk,
and the increasing antithesis between animal and vegetal halves of
the ovum, or between ectoderm (skin-layer) and entoderm
(gut-layer).
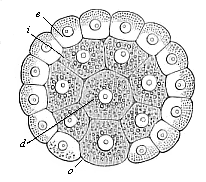 Fig.
72—Gastrula of the placental mammal (epigastrula
from the rabbit), longitudinal section through the axis. e ectodermic cells (sixty-four, lighter and smaller), i entodermic cells (thirty-two, darker and larger),
d central entodermic cell, filling the primitive gut-cavity,
o peripheral entodermic cell, stopping up the opening of the
primitive mouth (yelk-stopper in the Rusconian anus).
Fig.
72—Gastrula of the placental mammal (epigastrula
from the rabbit), longitudinal section through the axis. e ectodermic cells (sixty-four, lighter and smaller), i entodermic cells (thirty-two, darker and larger),
d central entodermic cell, filling the primitive gut-cavity,
o peripheral entodermic cell, stopping up the opening of the
primitive mouth (yelk-stopper in the Rusconian anus). |
The numbers of careful studies of animal gastrulation that have
been made in the last few decades have completely established the
views I have expounded, and which I first advanced in the years
1872–76. For a time they were greatly disputed by many
embryologists. Some said that the original embryonic form of the
metazoa was not the gastrula, but the “planula”—a
double-walled vesicle with closed cavity and without
mouth-aperture; the latter was supposed to pierce through
gradually. It was afterwards shown that this planula (found in
several sponges, etc.) was a later evolution from the gastrula.
1. On the reduction of all forms of gastrulation
to the original palingenetic form see especially the lucid
treatment of the subject in Arnold Lang’s Manual of
Comparative Anatomy (1888), Part I.
[ 90 ]
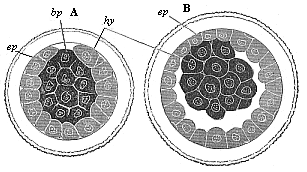
Fig. 73—Gastrula
of the rabbit. A as a solid, spherical cluster of cells, B
changing into the embryonic vesicle, bp primitive mouth,
ep ectoderm, hy entoderm. |
It was also shown that what is called
delamination—the rise of the two primary germinal layers by
the folding of the surface of the blastoderm (for instance, in the
Geryonidæ and other medusæ)—was a
secondary formation, due to cenogenetic variations from the
original invagination of the blastula. The same may be said of what
is called “immigration,” in which certain cells or
groups of cells are detached from the simple layer of the
blastoderm, and travel into the interior of the blastula; they
attach themselves to the inner wall of the blastula, and form a
second internal epithelial layer—that is to say, the
entoderm. In these and many other controversies of modern
embryology the first requisite for clear and natural explanation is
a careful and discriminative distinction between palingenetic
(hereditary) and cenogenetic (adaptive) processes. If this is
properly attended to, we find evidence everywhere of the biogenetic
law.
Title and Contents
Glossary
Chapter VIII
Chapter X
Figs. 1–209
Figs. 210–408