THE EVOLUTION OF MAN
Volume I
CHAPTER XII
EMBRYONIC SHIELD AND GERMINATIVE AREA
The three higher classes of vertebrates which we
call the amniotes—the mammals, birds, and reptiles—are
notably distinguished by a number of peculiarities of their
development from the five lower classes of the stem—the
animals without an amnion (the anamnia). All the amniotes
have a distinctive embryonic membrane known as the amnion (or
“water-membrane”), and a special embryonic
appendage—the allantois. They have, further, a large
yelk-sac, which is filled with food-yelk in the reptiles and birds,
and with a corresponding clear fluid in the mammals. In consequence
of these later-acquired structures, the original features of the
development of the amniotes are so much altered that it is very
difficult to reduce them to the palingenetic embryonic processes of
the lower amnion-less vertebrates. The gastræa theory shows
us how to do this, by representing the embryology of the lowest
vertebrate, the skull-less amphioxus, as the original form, and
deducing from it, through a series of gradual modifications, the
gastrulation and cœlomation of the craniota.
It was somewhat fatal to the true conception of the chief
embryonic processes of the vertebrate that all the older
embryologists, from Malpighi (1687) and Wolff (1750) to Baer (1828)
and Remak (1850), always started from the investigation of the
hen’s egg, and transferred to man and the other vertebrates
the impressions they gathered from this. This classical object of
embryological research is, as we have seen, a source of dangerous
errors. The large round food-yelk of the bird’s egg causes,
in the first place, a flat discoid expansion of the small gastrula,
and then so distinctive a development of this thin round embryonic
disk that the controversy as to its significance occupies a large
part of embryological literature.
One of the most unfortunate errors that this led to was the idea
of an original
[ 116 ]
antithesis of germ and yelk. The latter was regarded
as a foreign body, extrinsic to the real germ, whereas it is
properly a part of it, an embryonic organ of nutrition. Many
authors said there was no trace of the embryo until a later stage,
and outside the yelk; sometimes the two-layered embryonic disk
itself, at other times only the central portion of it (as
distinguished from the germinative area, which we will describe
presently), was taken to be the first outline of the embryo. In the
light of the gastræa theory it is hardly necessary to dwell
on the defects of this earlier view and the erroneous conclusions
drawn from it. In reality, the first segmentation-cell, and even
the stem-cell itself and all that issues therefrom, belong to the
embryo. As the large original yelk-mass in the undivided egg of the
bird only represents an inclosure in the greatly enlarged ovum, so
the later contents of its embryonic yelk-sac (whether yet segmented
or not) are only a part of the entoderm which forms the primitive
gut. This is clearly shown by the ova of the amphibia and
cyclostoma, which explain the transition from the yelk-less ova of
the amphioxus to the large yelk-filled ova of the reptiles and
birds.
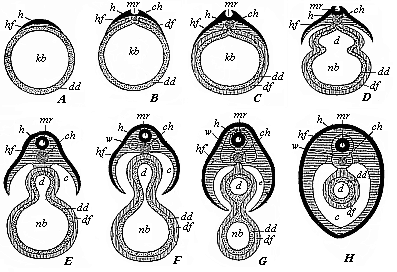
Fig.
105—Severance of the discoid mammal embryo from the
yelk-sac, in transverse section (diagrammatic). A The
germinal disk (h, hf) lies flat on one side of the
branchial-gut vesicle (kb). B In the middle of the
germinal disk we find the medullary groove (mr), and
underneath it the chorda (ch). C The gut-fibre-layer
(df) has been enclosed by the gut-gland-layer (dd).
D The skin-fibre-layer (hf) and gut-fibre-layer
(df) divide at the periphery; the gut (d) begins to
separate from the yelk-sac or umbilical vesicle (nb).
E The medullary tube (mr) is closed; the body-cavity
(c) begins to form. F The provertebræ
(w) begin to grow round the medullary tube (mr) and
the chorda (ch): the gut (d) is cut off from the
umbilical vesicle (nb). H The vertebræ
(w) have grown round the medullary tube (mr) and
chorda; the body-cavity is closed, and the umbilical vesicle has
disappeared. The amnion and serous membrane are omitted. The
letters have the same meaning throughout: h horn-plate,
mr medullary tube, hf skin-fibre-layer, w
provertebræ, ch chorda, c body-cavity or
cœloma, df gut-fibre-layer, dd gut-gland-layer,
d gut-cavity, nb umbilical vesicle. |
It is precisely in the study of these difficult features that we
see the incalculable value of phylogenetic considerations in
explaining complex ontogenetic facts, and the need of separating
cenogenetic phenomena from palingenetic. This is particularly clear
as regards the comparative embryology of the vertebrates, because
here the phylogenetic unity of the stem has been already
established by the well-known facts of paleontology and comparative
anatomy. If this unity of the stem, on the basis of the amphioxus,
were always borne in mind, we should not have these errors
constantly recurring.
In many cases the cenogenetic relation of the embryo to the
food-yelk has until now given rise to a quite wrong idea of
[ 117 ]
the first and most important embryonic processes in
the higher vertebrates, and has occasioned a number of false
theories in connection with them. Until thirty years ago the
embryology of the higher vertebrates always started from the
position that the first structure of the embryo is a flat,
leaf-shaped disk; it was for this reason that the cell-layers that
compose this germinal disk (also called germinative area) are
called “germinal layers.” This flat germinal disk,
which is round at first and then oval, and which is often described
as the tread or cicatricula in the laid hen’s egg, is found
at a certain part of the surface of the large globular food-yelk. I
am convinced that it is nothing else than the discoid, flattened
gastrula of the birds. At the beginning of germination the flat
embryonic disk curves outwards, and separates on the inner side
from the underlying large yelk-ball. In this way the flat layers
are converted into tubes, their edges folding and joining together
(Fig. 105). As the embryo grows at the expense of the food-yelk,
the latter becomes smaller and smaller; it is completely surrounded
by the germinal layers. Later still, the remainder of the food-yelk
only forms a small round sac, the yelk-sac or umbilical vesicle
(Fig. 105 nb). This is enclosed by the visceral layer, is
connected by a thin stalk, the yelk-duct, with the central part of
the gut-tube, and is finally, in most of the vertebrates, entirely
absorbed by this (H). The point at which this takes place,
and where the gut finally closes, is the visceral navel. In the
mammals, in which the remainder of the yelk-sac remains without and
atrophies, the yelk-duct at length penetrates the outer ventral
wall. At birth the umbilical cord proceeds from here, and the point
of closure remains throughout life in the skin as the navel.
As the older embryology of the higher vertebrates was mainly
based on the chick, and regarded the antithesis of embryo (or
formative-yelk) and food-yelk (or yelk-sac) as original, it had
also to look upon the flat leaf-shaped structure of the germinal
disk as the primitive embryonic form, and emphasise the fact that
hollow grooves were formed of these flat layers by folding, and
closed tubes by the joining together of their edges.
This idea, which dominated the whole treatment of the embryology
of the higher vertebrates until thirty years ago, was totally
false. The gastræa theory, which has its chief application
here, teaches us that it is the very reverse of the truth. The
cup-shaped gastrula, in the body-wall of which the two primary
germinal layers appear from the first as closed tubes, is the
original embryonic form of all the vertebrates, and all the
multicellular invertebrates; and the flat germinal disk with its
superficially expanded germinal layers is a later, secondary form,
due to the cenogenetic formation of the large food-yelk and the
gradual spread of the germ-layers over its surface. Hence the
actual folding of the germinal layers and their conversion into
tubes is not an original and primary, but a much later and
tertiary, evolutionary process. In the phylogeny of the vertebrate
embryonic process we may distinguish the following three
stages:—
A. First stage:
Primary
(palingenic)
embryonic process. |
B. Second stage:
Secondary
(cenogenetic)
embryonic process. |
C. Third stage:
Tertiary
(cenogenetic)
embryonic process. |
The germinal layers form from the
first closed tubes, the one-layered blastula being converted into
the two-layered gastrula by invagination.
No food-yelk.
(Amphioxus.) |
The germinal layers spread out
leaf-wise, food-yelk gathering in the ventral entoderm, and a large
yelk-sac being formed from the middle of the gut-tube.
(Amphibia.) |
The germinal layers form a flat
germinal disk, the borders of which join together and form closed
tubes, separating from the central yelk-sac.
(Amniotes.) |
As this theory, a logical conclusion from the gastræa
theory, has been fully substantiated by the comparative study of
gastrulation in the last few decades, we must exactly reverse the
hitherto prevalent mode of treatment. The yelk-sac is not to be
treated, as was done formerly, as if it were originally antithetic
to the embryo, but as an essential part of it, a part of its
visceral tube. The primitive gut of the gastrula has, on this view,
been divided into two parts in the higher animals as a result of
the cenogenetic formation of the food-yelk—the permanent gut
(metagaster), or permanent alimentary canal, and the
yelk-sac (lecithoma), or umbilical vesicle. This is very
clearly shown by the comparative ontogeny of the fishes and
amphibia. In these cases the whole yelk undergoes cleavage at
first, and forms a yelk-gland, composed of yelk-cells, in the
ventral wall
[ 118 ]
of the primitive gut. But it afterwards becomes so
large that a part of the yelk does not divide, and is used up in
the yelk-sac that is cut off outside.
When we make a comparative study of the embryology of the
amphioxus, the frog, the chick, and the rabbit, there cannot, in my
opinion, be any further doubt as to the truth of this position,
which I have held for thirty years. Hence in the light of the
gastræa theory we must regard the features of the amphioxus
as the only and real primitive structure among all the vertebrates,
departing very little from the palingenetic embryonic form. In the
cyclostoma and the frog these features are, on the whole, not much
altered cenogenetically, but they are very much so in the chick,
and most of all in the rabbit. In the bell-gastrula of the
amphioxus and in the hooded gastrula of the lamprey and the frog
the germinal layers are found to be closed tubes or vesicles from
the first. On the other hand, the chick-embryo (in the new laid,
but not yet hatched, egg) is a flat circular disk, and it was not
easy to recognise this as a real gastrula. Rauber and Goette have,
however, achieved this. As the discoid gastrula grows round the
large globular yelk, and the permanent gut then separates from the
outlying yelk-sac, we find all the processes which we have shown
(diagrammatically) in Figure 1.108—processes that were
hitherto regarded as principal acts, whereas they are merely
secondary.
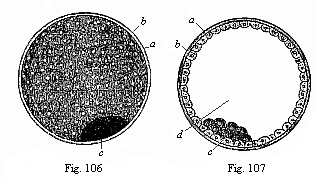
|
Fig.
106—The visceral embryonic vesicle
(blastocystis or gastrocystis) of a rabbit (the
“blastula” or vesicula blastodermica of other
writers), a outer envelope (ovolemma), b skin-layer
or ectoderm, forming the entire wall of the yelk-vesicle, c
groups of dark cells, representing the visceral layer or
entoderm.
Fig. 107—The same in section. Letters as above.
d cavity of the vesicle. (From Bischoff.) |
The oldest, oviparous mammals, the monotremes, behave in the
same way as the reptiles and birds. But the corresponding embryonic
processes in the viviparous mammals, the marsupials and placentals,
are very elaborate and distinctive. They were formerly quite
misinterpreted; it was not until the publication of the studies of
Edward van Beneden (1875) and the later research of Selenka,
Kuppfer, Rabl, and others, that light was thrown on them, and we
were in a position to bring them into line with the principles of
the gastræa theory and trace them to the embryonic forms of
the lower vertebrates. Although there is no independent food-yelk,
apart from the formative yelk, in the mammal ovum, and although its
segmentation is total on that account, nevertheless a large
yelk-sac is formed in their embryos, and the “embryo
proper” spreads leaf-wise over its surface, as in the
reptiles and birds, which have a large food-yelk and partial
segmentation. In the mammals, as well as in the latter, the flat,
leaf-shaped germinal disk separates from the yelk-sac, and its
edges join together and form tubes.
How can we explain this curious anomaly? Only as a result of
very characteristic and peculiar cenogenetic modifications of the
embryonic process, the real causes of which must be sought in the
change in the rearing of the young on the part of the viviparous
mammals. These are clearly connected with the fact that the
ancestors of the viviparous mammals were oviparous amniotes like
the present monotremes, and only gradually became viviparous. This
can no longer be questioned now that it has been shown (1884) that
the monotremes, the lowest and oldest of the mammals, still lay
eggs, and that these develop like the ova of the reptiles and
birds. Their nearest descendants, the marsupials, formed the habit
of retaining the eggs, and developing them in the
[ 119 ]
oviduct; the latter was thus converted into a womb
(uterus). A nutritive fluid that was secreted from its wall, and
passed through the wall of the blastula, now served to feed the
embryo, and took the place of the food-yelk. In this way the
original food-yelk of the monotremes gradually atrophied, and at
last disappeared so completely that the partial ovum-segmentation
of their descendants, the rest of the mammals, once more became
total. From the discogastrula of the former was evolved the
distinctive epigastrula of the latter.
It is only by this phylogenetic explanation that we can
understand the formation and development of the peculiar, and
hitherto totally misunderstood, blastula of the mammal. The
vesicular condition of the mammal embryo was discovered 200 years
ago (1677) by Regner de Graaf. He found in the uterus of a rabbit
four days after impregnation small, round, loose, transparent
vesicles, with a double envelope. However, Graaf’s discovery
passed without recognition. It was not until 1827 that these
vesicles were rediscovered by Baer, and then more closely studied
in 1842 by Bischoff in the rabbit (Figs. 106, 107). They are found
in the womb of the rabbit, the dog, and other small mammals, a few
days after copulation. The mature ova of the mammal, when they have
left the ovary, are fertilised either here or in the oviduct
immediately afterwards by the invading sperm-cells.1 (As
to the womb and oviduct see Chapter XXIX) The cleavage and
formation of the gastrula take place in the oviduct. Either here in
the oviduct or after the mammal gastrula has passed into the uterus
it is converted into the globular vesicle which is shown externally
in Fig. 106, and in section in Fig. 107. The thick, outer,
structureless envelope that encloses it is the original
ovolemma or zona pellucida, modified, and clothed with a
layer of albumin that has been deposited on the outside. From this
stage the envelope is called the external membrane, the primary
chorion or prochorion (a). The real wall of the vesicle
enclosed by it consists of a simple layer of ectodermic cells
(b), which are flattened by mutual pressure, and generally
hexagonal; a light nucleus shines through their fine-grained
protoplasm (Fig. 108). At one part (c) inside this hollow
ball we find a circular disc, formed of darker, softer, and rounder
cells, the dark-grained entodermic cells (Fig. 109).
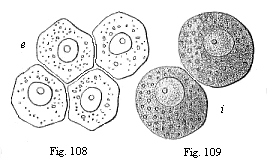
|
Fig.
108—Four entodermic cells from the embryonic
vesicle of the rabbit.
Fig. 109—Two entodermic cells from the embryonic
vesicle of the rabbit. |
The characteristic embryonic form that the developing mammal now
exhibits has up to the present usually been called the
“blastula” (Bischoff), “sac-shaped embryo”
(Baer), “vesicular embryo” (vesicula
blastodermica, or, briefly, blastosphæra). The
wall of the hollow vesicle, which consists of a single layer of
cells, was called the “blastoderm,” and was supposed to
be equivalent to the cell-layer of the same name that forms the
wall of the real blastula of the amphioxus and many of the
invertebrates (such as Monoxenia, Fig. 29 F, G). Formerly this real
blastula was generally believed to be equivalent to the embryonic
vesicle of the mammal. However, this is by no means the case. What
is called the “blastula” of the mammal and the real
blastula of the amphioxus and many of the invertebrates are totally
different embryonic structures. The latter (blastula) is
palingenetic, and precedes the formation of the gastrula. The
former (blastodermic vesicle) is cenogenetic, and follows
gastrulation. The globular wall of the blastula is a real
blastoderm, and consists of homogeneous (blastodermic) cells; it is
not yet differentiated into the two primary germinal layers. But
the globular wall of the mammal vesicle is the differentiated
ectoderm, and at one point in it we find a circular disk of quite
different cells—the entoderm. The round
1. In man and the other mammals the fertilisation
of the ova probably takes place, as a rule, in the oviduct; here
the ova, which issue from the female ovary in the shape of the
Graafian follicle, and enter the inner aperture of the oviduct,
encounter the mobile sperm-cells of the male seed, which pass into
the uterus at copulation, and from this into the external aperture
of the oviduct. Impregnation rarely takes place in the ovary or in
the womb.
[ 120 ]
cavity, filled with fluid, inside the real blastula
is the segmentation-cavity. But the similar cavity within the
mammal vesicle is the yelk-sac cavity, which is connected with the
incipient gut-cavity. This primitive gut-cavity passes directly
into the segmentation-cavity in the mammals, in consequence of the
peculiar cenogenetic changes in their gastrulation, which we have
considered previously (Chapter IX). For these reasons it is very
necessary to recognise the secondary embryonic vesicle in the
mammal (gastrocystis or blastocystis) as a
characteristic structure peculiar to this class, and distinguish it
carefully from the primary blastula of the amphioxus and the
invertebrates.
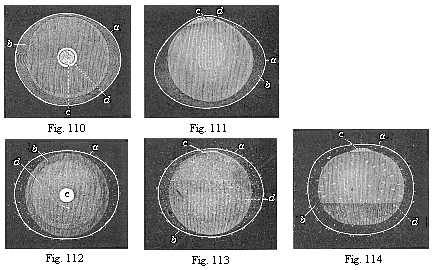
Fig. 110—Ovum
of a rabbit from the uterus, one sixth of an inch in diameter.
The embryonic vesicle (b) has withdrawn a little from the
smooth ovolemma (a). In the middle of the ovolemma we see
the round germinal disk (blastodiscus, c), at the edge of
which (at d) the inner layer of the embryonic vesicle is
already beginning to expand. (Figs. 110–114 from
Bischoff.
Fig. 111—The same ovum, seen in profile. Letters as in
Fig. 110.
Fig. 112—Ovum of a rabbit from the uterus, one-fourth
of an inch in diameter. The blastoderm is already for the most part
two-layered (b). The ovolemma, or outer envelope, is tufted
(a).
Fig. 113—The same ovum, seen in profile. Letters as in
Fig. 112.
Fig. 114—Ovum of a rabbit from the uterus, one-third
of an inch in diameter. The embryonic vesicle is now nearly
everywhere two-layered (k) only remaining one-layered below
(at d). |
[ 121 ]
The small, circular, whitish, and opaque spot which the gastric
disk (Fig. 106) forms at a certain part of
the surface of the clear and transparent embryonic vesicle has long
been known to science, and compared to the germinal disk of the
birds and reptiles. Sometimes it has been called the germinal disk,
sometimes the germinal spot, and usually the germinative area. From
the area the further development of the embryo proceeds. However,
the larger part of the embryonic vesicle of the mammal is not
directly used for building up the later body, but for the
construction of the temporary umbilical vesicle. The embryo
separates from this in proportion as it grows at its expense; the
two are only connected by the yelk-duct (the stalk of the
yelk-sac), and this maintains the direct communication between the
cavity of the umbilical vesicle and the forming visceral cavity (Fig. 105).
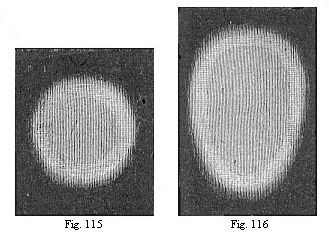
Fig. 115—Round
germinative area of the rabbit, divided into the central light
area (area pellucida) and the peripheral dark area (area
opaca). The light area seems darker on account of the dark
ground appearing through it.
Fig. 116—Oval area, with the opaque whitish border of
the dark area without. |
The germinative area or gastric disk of the animal consists at
first (like the germinal disk of birds and reptiles) merely of the
two primary germinal layers, the ectoderm and entoderm. But soon
there appears in the middle of the circular disk between the two a
third stratum of cells, the rudiment of the middle layer or fibrous
layer (mesoderm). This middle germinal layer consists from
the first, as we have seen in Chapter X, of two separate epithelial
plates, the two layers of the cœlom-pouches (parietal and
visceral). However, in all the amniotes (on account of the large
formation of yelk) these thin middle plates are so firmly pressed
together that they seem to represent a single layer. It is thus
peculiar to the amniotes that the middle of the germinative area is
composed of four germinal layers, the two limiting (or primary)
layers and the middle layers between them (Figs. 96, 97). These four secondary
germinal layers can be clearly distinguished as soon as what is
called the sickle-groove (or “embryonic sickle”) is
seen at the hind border of the germinative area. At the borders,
however, the germinative area of the mammal only consists of two
layers. The rest of the wall of the embryonic vesicle consists at
first (but only for a short time in most of the mammals) of a
single layer, the outer germinal layer.
From this stage, however, the whole wall of the embryonic
vesicle becomes two-layered. The middle of the germinative area is
much thickened by the growth of the cells of the middle layers, and
the inner layer expands at the same time, and increases at the
border of the disk all round. Lying close on the outer layer
throughout, it grows over its inner surface at all points, covers
first the upper and then the lower hemisphere, and at last closes
in the middle of the inner layer (Figs. 110–114). The wall of
the embryonic vesicle now consists throughout of two layers of
cells, the ectoderm without and the entoderm within. It is only in
the centre of the circular area, which becomes thicker and thicker
through the growth of the middle layers, that it is made up of all
four layers. At the same time, small structureless tufts or warts
are deposited on the surface of the outer
[ 122 ]
ovolemma or prochorion, which has been raised above
the embryonic vesicle (Figs. 112–114 a).
We may now disregard both the outer ovolemma and the greater
part of the vesicle, and concentrate our attention on the
germinative area and the four-layered embryonic disk. It is here
alone that we find the important changes which lead to the
differentiation of the first organs. It is immaterial whether we
examine the germinative area of the mammal (the rabbit, for
instance) or the germinal disk of a bird or a reptile (such as a
lizard or tortoise). The embryonic processes we are now going to
consider are essentially the same in all members of the three
higher classes of vertebrates which we call the amniotes. Man is
found to agree in this respect with the rabbit, dog, ox, etc.; and
in all these animals the germinative area undergoes essentially the
same changes as in the birds and reptiles. They are most frequently
and accurately studied in the chick, because we can have incubated
hens’ eggs in any quantity at any stage of development.
Moreover, the round germinal disk of the chick passes immediately
after the beginning of incubation (within a few hours) from the
two-layered to the four-layered stage, the two-layered mesoderm
developing from the median primitive groove between the ectoderm
and entoderm (Figs.
82–95).
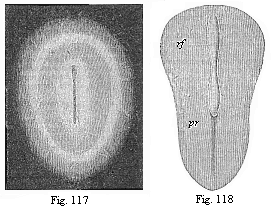
Fig. 117—Oval
germinal disk of the rabbit, magnified. As the delicate,
half-transparent disk lies on a black ground, the pellucid area
looks like a dark ring, and the opaque area (lying outside it) like
a white ring. The oval shield in the centre also looks whitish, and
in its axis we see the dark medullary groove. (From
Bischoff.)
Fig. 118—Pear-shaped germinal shield of the rabbit
(eight days old), magnified. rf medullary groove. pr
primitive groove (primitive mouth). (From
Kölliker. |
The first change in the round germinal disk of the chick is that
the cells at its edges multiply more briskly, and form darker
nuclei in their protoplasm. This gives rise to a dark ring, more or
less sharply set off from the lighter centre of the germinal disk
(Fig. 115). From this point the latter takes the name of the
“light area” (area pellucida), and the darker
ring is called the “dark area” (area opaca). (In
a strong light, as in Figs. 115–117, the light area seems
dark, because the dark ground is seen through it; and the dark area
seems whiter). The circular shape of the area now changes into
elliptic, and then immediately into oval (Figs. 116, 117). One end
seems to be broader and blunter, the other narrower and more
pointed; the former corresponds to the anterior and the latter to
the posterior section of the subsequent body. At the same time, we
can already trace the characteristic bilateral form of the body,
the antithesis of right and left, before and behind. This will be
made clearer by the “primitive streak,” which appears
at the posterior end.
At an early stage an opaque spot is seen in the middle of the
clear germinative
[ 123 ]
area, and this also passes from a circular to an
oval shape. At first this shield-shaped marking is very delicate
and barely perceptible; but it soon becomes clearer, and now stands
out as an oval shield, surrounded by two rings or areas (Fig. 117).
The inner and brighter ring is the remainder of the pellucid area,
and the dark outer ring the remainder of the opaque area; the
opaque shield-like spot itself is the first rudiment of the dorsal
part of the embryo. We give it briefly the name of embryonic shield
or dorsal shield. In most works this embryonic shield is described
as “the first rudiment or trace of the embryo,” or
“primitive embryo.” But this is wrong, though it rests
on the authority of Baer and Bischoff.
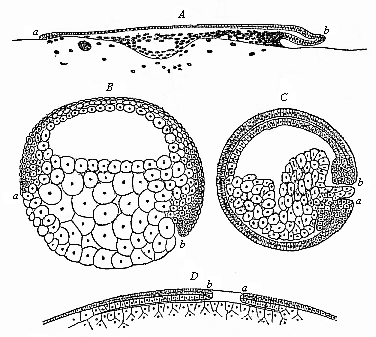
Fig. 119—Median
longitudinal section of the gastrula of four vertebrates. (From
Rabl.) A discogastrula of a shark
(Pristiurus). B amphigastrula of a sturgeon
(Accipenser). C amphigastrula of an amphibium
(Triton). D epigastrula of an amniote (diagram).
a ventral, b dorsal lip of the primitive mouth. |
As a matter of fact, we already have the embryo in the
stem-cell, the gastrula, and all the subsequent stages. The
embryonic shield is simply the first rudiment of the dorsal part,
which is the earliest to develop. As the older names of
“embryonic rudiment” and “germinative area”
are used in many different senses—and this has led to a fatal
confusion in embryonic literature—we must explain very
clearly the real significance of these important embryonic parts of
the amniote. It will be useful to do so in a series of formal
principles:—
1. The so-called ”first trace of the embryo” in the
amniotes, or the embryonic shield, in the centre of the pellucid
area, consists merely of an early differentiation and formation of
the middle dorsal parts.
2. Hence the best name for it is ”the dorsal
shield,” as I proposed long ago.
3. The germinative area, in which the first embryonic
blood-vessels appear at an early stage, is not opposed as an
external area to the ”embryo proper,” but is a part of
it.
4. In the same way, the yelk-sac or the umbilical vesicle is not
a foreign external
[ 124 ]
appendage of the embryo, but an outlying part of its
primitive gut.
5. The dorsal shield gradually separates from the germinative
area and the yelk-sac, its edges growing downwards and folding
together to form ventral plates.
6. The yelk-sac and vessels of the germinative area, which soon
spread over its whole surface, are, therefore, real embryonic
organs, or temporary parts of the embryo, and have a transitory
importance in connection with the nutrition of the growing later
body; the latter may be called the ”permanent body” in
contrast to them.
The relation of these cenogenetic features of the amniotes to
the palingenetic structures of the older non-amniotic vertebrates
may be expressed in the following theses: The original gastrula,
which completely passes into the embryonic body in the acrania,
cyclostoma, and amphibia, is early divided into two parts in the
amniotes—the embryonic shield, which represents the dorsal
outline of the permanent body; and the temporary embryonic organs
of the germinative area and its blood-vessels, which soon grow over
the whole of the yelk-sac. The differences which we find in the
various classes of the vertebrate stem in these important
particulars can only be fully understood when we bear in mind their
phylogenetic relations on the one hand, and, on the other, the
cenogenetic modifications of structure that have been brought about
by changes in the rearing of the young and the variation in the
mass of the food-yelk.
We have already described in Chapter IX the changes which this
increase and decrease of the nutritive yelk causes in the form of
the gastrula, and especially in the situation and shape of the
primitive mouth. The primitive mouth or prostoma is originally a
simple round aperture at the lower pole of the long axis; its
dorsal lip is above and ventral lip below. In the amphioxus this
primitive mouth is a little eccentric, or shifted to the dorsal
side (Fig. 39). The aperture
increases with the growth of the food-yelk in the cyclostoma and
ganoids; in the sturgeon it lies almost on the equator of the round
ovum, the ventral lip (a) in front and the dorsal lip
(b) behind (Fig. 119 b). In the wide-mouthed,
circular discoid gastrula of the selachii or primitive fishes,
which spreads quite flat on the large food-yelk, the anterior
semi-circle of the border of the disk is the ventral, and the
posterior semicircle the dorsal lip (Fig. 119 A). The
amphiblastic amphibia are directly connected with their earlier
fish-ancestors, the dipneusts and ganoids, and further the oldest
selachii (Cestracion); they have retained their total
unequal segmentation, and their small primitive mouth (Fig. 119
C, ab), blocked up by the yelk-stopper, lies at the limit of
the dorsal and ventral surface of the embryo (at the lower pole of
its equatorial axis), and there again has an upper dorsal and a
lower ventral lip (a, b). The formation of a large food-yelk
followed again in the stem-forms of the amniotes, the protamniotes
or proreptilia, descended from the amphibia (Fig. 119 D).
But here the accumulation of the food-yelk took place only in the
ventral wall of the primitive-gut, so that the narrow primitive
mouth lying behind was forced upwards, and came to lie on the back
of the discoid ”epigastrula” in the shape of the
”primitive groove”; thus (in contrast to the case of
the selachii, Fig. 119 A) the dorsal lip (b) had to
be in front, and the ventral lip (a) behind (Fig. 119
D). This feature was transmitted to all the amniotes, whether
they retained the large food-yelk (reptiles, birds, and
monotremes), or lost it by atrophy (the viviparous mammals).
This phylogenetic explanation of gastrulation and
cœlomation, and the comparative study of them in the various
vertebrates, throw a clear and full light on many ontogenetic
phenomena, as to which the most obscure and confused opinions were
prevalent thirty years ago. In this we see especially the high
scientific value of the biogenetic law and the careful separation
of palingenetic from cenogenetic processes. To the opponents of
this law the real explanation of these remarkable phenomena is
impossible. Here, and in every other part of embryology, the true
key to the solution lies in phylogeny.
Title and Contents
Glossary
Chapter XI
Chapter XIII
Figs. 1–209
Figs. 210–408