
Project Gutenberg's Scientific American Supplement No. 299, by Various This eBook is for the use of anyone anywhere at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org Title: Scientific American Supplement No. 299 Author: Various Posting Date: October 10, 2012 [EBook #8408] Release Date: July, 2005 First Posted: July 9, 2003 Language: English Character set encoding: ISO-8859-1 *** START OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN SUPPL., NO. 299 *** Produced by Olaf Voss, Don Kretz, Juliet Sutherland, and the Online Distributed Proofreading Team
The death of this distinguished man must be recorded. An interesting résumé of his labors by M. Daubree has appeared, from which we take the following facts. After a training in his native town at the Lyceum of Metz, which furnished so many scholars to the Polytechnic school, Delesse was admitted at the age of twenty to this school. In 1839 he left to enter the Corps des Mines. From the beginning of his career the student engineer applied himself with ardor to the sciences to which he was to devote his entire existence. The journeys which he undertook then, and continued later, in France, Germany, Poland, England, and Ireland, helped to confirm and develop the bent of his mind. He soon arrived at important scientific results, and was rewarded, in 1845, by having conferred to him by the university the course of mineralogy and geology in the Faculty at Besançon, where Delesse at the same time fulfilled the duties of engineer of mines. Five years later he returned to Paris, where he continued his university duties, at first as deputy of the course of geology at the Sorbonne, then as master of the conferences at the Superior Normal School. Besides this, he continued his profession of engineer of mines as inspector of the roads of Paris. The first original researches of the young savant concern pure mineralogy; he studied a certain number of species, of which the chemical nature was yet uncertain or altogether unknown, and his name was appended to one of the species which he defined. He studied also, and with success, the interesting modifications called pseudomorphism--the mode of association of minerals, as well as their magnetic properties. The attributes of a practical mineralogist aided him greatly in the culture of a branch of geology to which Delesse has rendered eminent services, in the recognition of rocks of igneous origin and of others allied to them. He studied in the field, as well as by investigations in the laboratory, for fifteen years, with an intelligent and indefatigable perseverance, and, aided by the results of hundreds of analyses, eruptive masses of the most varied kind, the knowledge derived from which threw light upon the principles of science, from granites and syenites to melaphyres and basalts. After thirty years of study and progress, other savants, without differing from him, progressed further in the intimate knowledge of rocks; but the historian of science will not forget that Delesse was the precursor of this order of research. His studies of metamorphism will long do him honor. The mineralogical modifications which the eruptive rocks have undergone in the mass are the permanent witnesses which attracted all his attention. The chemical comparison of the metamorphic with the normal rock pointed out distinctly the nature of the substances acquired or lost. One of the principal results of these analyses has been to lessen the importance attributed until then to heat alone, and to show in more than one case the intervention of thermal sources and of other emanations from below, to which the eruptive rocks have simply opened up tracks.
It is not only upon subjects relating to the history of rocks that Delesse has touched. Witness his work on the infiltration of water, as well as his volume relating to the materials of construction, published on the occasion of the Exhibition of 1855. The nature of the deposits which operate continually at the bottom of the sea offers points of interest which well repay the labor of the geologist. He finds there, indeed, a precious field to be compared with stratified deposits; for in spite of the enormous depth to which they form a part of continents, they are of analogous origin. Delesse laboriously studied the products of the innumerable soundings taken in most of the seas. He arranged the results in a work which has become classical with the beautiful atlas of submarine drawings which accompany it. Though he never slackened in his own especial work, he made much of the work of others. The "Revue des Progrès de la Géologie," with which he enriched the "Annales des Mines" for twenty years, would have been sufficient to engross the time of a less active scientific man, and one less ready to grasp the opening of a discovery. This indefatigable theorist never neglected the applications of science: the nature and the changes of the layers which form the under earth; the course and the depth of the subterraneous sheets of water; the mineralogical composition of the earth's vegetation, were represented by him on several charts and plans drawn out in proper form. His maps which follow the route of many of the great French lines of railway explain the kind of soil upon which they are laid, and are of daily use. In the pursuit of his numerous scientific works, Delesse never failed in discharging his duties in the Corps des Mines. Having in 1864 quitted the service of the Government of Paris, which he had occupied for eighteen years, he was made professor of agriculture, of drainage, and irrigation, at the School of Mines, where he established instruction in these before being called to found the course of geology at the Agricultural Institution. Promoted to be Inspector-General of Mines in 1878, and charged with the division of the south east of France, he preserved to the end of his life these new duties, for which, to the regret of the School of Mines, he gave up his excellent lessons there. During the year of 1870 Delesse fulfilled his duties as a citizen, as engineer in preparation of cartridges in the department.
His nomination to the Academy of Sciences, which took place on the 6th of January, 1879, satisfied the ambition of his life. He was for two years President of the Central Commission of the Geographical Society; he was also President of the Geological Society. He was not long to enjoy the noble position acquired by his intelligence and his work. He suffered from a serious malady, which, however, did not weaken his intellect, and he continued from his bed of suffering to prepare the reports for the Council-General of Mines, and that which recently he addressed to the Academy on the occasion of his election. The greatness and the rectitude of mind of Delesse, his astounding power of work, his profound knowledge of science, his sympathetic sweetness, which were associated with sterling modesty and loyalty of character, made him esteemed and cherished throughout his whole career. He died on the 24th of March.--The Engineer.
SUGGESTIONS IN DECOTATIVE ART.--SILVER EWER, BY
ODIOT, PARIS.
(From The Workshop)
On the afternoon of August 9, Earnock Colliery, near Hamilton, belonging to Mr. John Watson, of Earnock, was the scene of an interesting ceremonial which may well be said to mark a new era in mining annals. In proceeding to win the rich mineral wealth of his estate, Mr. Watson determined that, in respect of fittings, machinery, and general appointments, it should be a model, and he has been highly successful in giving practical effect to his aims. Among other things, he early resolved to, if at all practicable, substitute the electric light for the ordinary mode of illuminating the workings, and after investigating the various systems, he decided on giving that of Mr. Swan a trial. Accordingly, since April last, Messrs. D. & E. Graham, electrical engineers, Glasgow, have been engaged fitting up the Swan incandescent lamp, with modifications, to adapt it for safe use in the mine, and on Tuesday the inauguration of the new light took place in presence of a large company of leading gentlemen from Glasgow, Hamilton, and the West. Arrived at the colliery about half-past one o'clock, the visitors were received by Mr. Watson, and after a brief space spent in inspecting the three magnificent winding and fan engines, the Guibal fan, and the framework for screening the coal, they were conducted by Mr. James Gilchrist, manager, down into the workings in the ell seam at a depth of 118 fathoms. Here at the pit bottom, in the roads and at the face, twenty-one Swan lamps were burning, giving forth a brilliant, steady flame, the luminosity of which, while sufficient to supply the desired light, had none of the disagreeable intensity associated with most systems of electric lighting. Besides the pear-shaped Swan lamp, in which the glowing or incandescence is carried on in vacuo, there is an outer lantern, the invention of Mr. David Graham, consisting of a strong glass globe, air-tight, protected with steel guards. Each lamp was also connected with two different forms of Graham's patent safety air tight contacts and switches for cutting off and letting on the current, the effect of which, it is believed, would be to render the lamps quite safe, even in the presence of explosive gas. At first the intention was to employ the fan-engine to drive the dynamo-electric machine or generator, but this was departed from, and an engine of 12 horse-power was erected in the workshops on the surface for the purpose. From the generator the electric cables, two in number, are conducted along the roof of the workshops over ordinary telegraph poles to the pit-head at No. 2 shaft, and thence down into the workings. From the ridge of the workshops to the pithead, a distance of several hundred yards, the cables consist of ordinary copper wire, three-eighths of an inch in diameter; inside the workshop and below ground, to allow of their safe handling, they are composed of insulated wires, while on the way down the shaft they are inclosed in a galvanized tube. Near the bottom of the shaft, branches are taken off to supply light to the principal roadways and to the haulage engine-room, the main cables being carried into one of the sections of the mine a distance of half-a-mile. After a careful inspection of the lamps at the pit bottom, the party were photographed in three groups, with the aid of the electric light, by Mr. Annan, of Glasgow, who may well be credited with the distinction of being the first to exercise his skill in the bowels of the earth. They were then led to the haulage engine-room and into the workings, where they witnessed the effects of the light. At the latter point, while, of course, the visitors were at a safe distance, a shot was fired, bringing down a large mass of coal. Having spent fully an hour below ground, the party returned to the surface.--Colliery Guardian.
M. Bede, of Brussels, has an article in L'Ingénieur-Conseil on the above subject. He considers that a system of such wires forms the best and most complete security against lightning with which a town can be provided, because they protect not only the buildings in which they terminate, but also those over which they pass. At each end they communicate with the earth, and thus carry off safely any surplus of electricity with which they may become charged. It is, however, important that they should be provided with lightning conductors of their own, to carry off such surplus directly from the transmission wire to the earth wire, without allowing it to pass through the fine wires of the induction coils, which it might fuse.
Such lightning conductors usually consist of a toothed plate attached to one wire, close to another plate not toothed attached to the other wire. The copper even of such a conductor has been melted by the powerful current which it has carried away. In telephonic central offices, M. Bede has seen all the signals of one row of telephone wires fall at the same moment, proving that an electric discharge had fallen upon the wires, and been by them conveyed to earth.
This fact shows that wires, even without points, are capable of attracting the atmospheric electricity; but it must be remembered that there are two points at every join in the wire. M. Bede insists strongly upon the uselessness of terminating lightning conductors in wells, or even larger pieces of water. The experiments of MM. Becquerel and Pouillet proved that the resistance of water to the passage of electricity is one thousand million times greater than that of iron; consequently, if the current conveyed by a wire one square mm. thick were to be carried off by water without increased resistance, a surface of contact between the wire and the water of not less than 1,000 square meters must be established.
It is obvious that a wire let down into a well is simply useless. On the two-fluid theory, it offers no effectual way of escape to the terrestrial electricity; according to the older views, it would be absolutely dangerous, by attracting more electricity from the clouds than it could dispose of. The author advocates connecting lightning conductors with water or gas pipes, which have an immense surface of contact with the earth.
The experiments of the author have been principally directed to the alterations in shape and color produced in a flame when under the influence of positive or negative electricity. The flames were arranged so as to form one electrode of a frictional machine. When charged with positive electricity the flame became more blue, narrower, and pointed at the top, while little or nothing of the result was observed in negative flames.
A peculiar result is that the end of a negative flame returns to its own conductor, and that, according to the intensity of the electricity, and also depending on the width of the burner, this turning back of the flame is either intermittent or constant. Most noticeable are these results:
When the flame rises from a circular burner, or when burning round a metallic cylinder, in the latter case it returns to the metallic surface according to the intensity of electricity in an arc or angle, while the point of the flame divides into two branches, which separately perform more or less equal movements. If a body connected to the earth by a conducting wire is held opposite the flame at some distance, the flame will in all cases bend toward it; as the body is brought closer, the flame, if negative, will be repulsed, and, if positive, will be attracted, at least the upper luminous part of the flame, while the lower dark body of flame is also repulsed.
This phenomenon explains why a positive flame will burn through wire gauze, while a negative flame remains below the gauze. The positive flame becoming pointed explains the fact that this will drive a small fan wheel, while a negative flame will only just move it.
All these results are most prominently obtained with a pure gas flame, a stearine, wax, or tallow candle, very indifferently with a spirit flame, and least from a Bunsen flame rich in oxygen. They may not only be obtained with flames electrified direct, but also when placed under the influence of a long "Holtz" machine.
A flame placed between two small disks on the machine bends toward the negative pole, becomes widened, and, at a certain point of electric intensity, commences to vibrate and oscillate, exhibiting a peculiar stratification. Since these phenomena are also least observed in flames rich in oxygen, it appears to be a general law that carbon and hydrogen are more strongly attracted by the negative pole, while oxygen is more attracted by the positive pole, probably like in all polar differentially attractions, in consequence of a peculiar unipolar conductivity of the substances.
The return motion of the flame the author explains thus: The point of the flame loses more electricity by influence than it receives by conductivity. A paper strip fixed at one end to a large ball shows similar movements when its free end is pointed and made conductive. Why principally the negative flame returns may be explained in two ways--either the point of the flame loses much by radiation, or the base of the flame is a bad conductor. The former explanation would agree with the experiments made by Wiedemann and Ruhlmann, the latter with Erdman's theory of unipolar conductivity of flames. This theory is still further supported by the resistance on the negative electrodes noticed by Hittorf, which almost explains Erdman's experiments, because if negative electricity enters a flame with greater difficulty, then positive electricity must leave a flame with difficulty.--W. Holtz, in Wiedemanris Beiblätter to Poggendorfs Annalen.
The number of inventions for use as stop-motions in and about the various machines in the cotton mill has been to a certain extent something like the search after perpetual motion. Very available and quite satisfactory stop-motions have for a number of years been employed wherever the thread or sliver has been twisted so that strength was given it to resist a slight amount of friction, but the main trouble in the mill has been done after the sliver leaves the railway head and during its transit in the various processes employed between the railway head and the spinning frame or mule. Every carder or spinner knows that where an injury comes to the sliver because the sliver is soft, but partially condensed and very susceptible to injury, the injury is magnified and multiplied in every successive process. Virtually the field was long since abandoned for an accurate quick-working motion that should be applicable to any and all the machines and to every sliver or strand of the machine.
This invention was solved practically about two years since, and is now being employed as applied to drawing frames, doublers, speeder, intermediate, and slubber. It is a very cunning mechanical appliance, too, and has found favor to a great extent in England, where several thousand heads of drawing and speeders are already supplied.
This invention was exhibited at the Centennial in 1876, although in a somewhat crude state. Since that time it has been materially improved, and mechanically is very nearly perfect now. Many attempts have been made to apply a stop motion, which should be quick in its movement and accurate in its result, to carding engines or the card, not one of which, until the application of electricity, was worth the time spent in putting it on. With the electric motion, however, all this is changed, and the electric attachments are not of necessity so fragile as to be un-mechanical or to be not practical. The advantage has also been taken, in a mechanical way, of using cotton as one element, and, being non-conducting, so that no trouble shall arise from contact with the working parts of the electrical apparatus with the cotton itself.
To take into consideration all the possibilities that exist from the railway can to the front of the fine speeder is not needed by the practical reader, and would be useless to any other. The principle of this invention is the supplying of a magneto-electric current from a small magneto-electric machine attached to the card, speeder, or whatever machine it may be applied to which generates the current, and this machine is driven by a small belt from the main driving shaft. The machine in itself weighs but a few pounds, and can be driven by a half-inch or three quarter-inch belt, and requires a little more power than a light-running sewing machine.
One pole of the magneto-electric machine is connected by means of a rod or wire to the machine frame upon which it is to be used, and the other pole to the electromagnet in the ordinary way of conductivity of current, which means stretching the wire from one to the other. An armature is arranged so that when a thread is broken or a sliver or a strand of roving, the armature drops into a ratchet wheel; this ratchet wheel is made to revolve by the belt, and whenever it is impeded or stopped in its course it acts upon mechanism which throws the driving belt of the machine upon the loose pulley. Electrical contact is made by a very simple contrivance, and these attachments are only to act in the case of a breakage of a thread or strand.
As applied to a card, the calender rolls are both connected, one with the negative and one with the positive pole; when the sliver of cotton is between the calender rolls there is no connection, but if the sheet breaks down between the cone and the calender roll, the moment the calender rolls come in contact the electrical attachment operates and a stoppage ensues; and in the case, as with the American system, where a number of cards are used in a railway, this electric contact may be used for either one of two purposes-to stop the feeding of cotton into the card, or to ring a bell sharply and continue ringing it until the sliver is put between the calender rolls again and the card set to delivering cotton.
In drawing frames it may be attached so that, in the case of a breakage between the front roll and the calender roll, the electric machine acts; in the case of a lap upon one of the rolls or one end of the roll, or in case of breakage of the sliver at the back of the machine, in either case a stoppage would be instantly produced.
In being applied to the slubber a breakage either at the front or back can be arranged for. Upon intermediates the breakage of either one of the strands, if the machine was running two into one, from the creel to the roller, would cause the stoppage of the machine, or the breaking or tangling of ends between the front roll and the nose of the flier.
There are many other places where this motion can be applied. With mechanical means we require motion; with electricity we require simple contact of two differently arranged surfaces, and this can always be had by letting the cotton drop out from between the rollers; no radical changes are necessary, and we are glad to find that this electrical attachment is meeting with a very good success in England, France, and, so far, in the United States, and, undoubtedly, further and more extended opportunity will be found for this application.--Textile Record.
[Footnote: A paper recently read before the Society of Mechanical Engineers by F.C.Marshall.]
The author began by referring to a paper read at the Liverpool meeting in 1872, by Mr. F. J. Bramwell, F.R.S., on "The Progress effected in Economy of Fuel in Steam Navigation, considered in Relation to Compound Cylinder Engines and High-pressure Steam;" then proceeded to continue the subject from the date of that meeting, to trace out whether any, and if so what, progress had been made; further, to consider whether or no we have reached the finality so strongly deprecated by Sir Frederick Bramwell in the discussion referred to, and, if not, then in what direction we are to look for further development.
From a table it would seem that the steam pressures are now much higher, the boilers have less heating surface, and the cylinders are much smaller for the indicated horsepower developed than in 1872; and at the same time the average consumption of fuel is reduced from 2.11 lb. to 1.828 lb., or by 13.38 per cent.
The author then briefly described the modern marine engine and boiler. The three great types of compound engines may be placed as follows in the order of their general acceptance by the shipowning community: (1) The two-cylinder intermediate-receiver compound engine, having cranks at right angles. (2) The Woolf engine in the tandem form, having generally the high-pressure and low-pressure cylinders in line with each other, but occasionally alongside, and always communicating their power to one crank. Such a pair of engines is used sometimes singly, oftener two pairs together, working side by side to cranks at right angles; recently three pairs together, working to cranks placed 120 deg. apart. The system affords the opportunity of adding yet more engines to the same propeller to an indefinite extent. (3) The three cylinder intermediate-receiver compound engine, with one high and two low-pressure cylinders, the steam passing from the high-pressure cylinder into the receiver, and thence into the two low-pressure cylinders respectively. The cranks are placed at equal angles apart round the crank shaft, so as to balance the forces exerted upon the shaft.
These three types may be said to embrace all the engines now being manufactured in this country for the propulsion of steam vessels by the screw propeller. In their leading principles they also embrace nearly all paddle engines now being built, whether the cylinders be oscillating, fixed vertically, or inclined to the shaft.
The compound engine, in fact, in one of these three forms, may now be said to be universally adopted in this country; and the question of the relative value of simple expansion in one cylinder, and of compound expansion in two or more cylinders, which agitated the minds of some of our leading engineers ten years ago, is now practically solved in favor of the latter.
The marine boiler of to-day is in all its main features the same as it was ten years ago. The single-ended boiler, made with two, three, and sometimes four furnaces, is the simplest form, and for all powers under 500 indicated horse power is the most generally adopted. The double-ended form is largely used. It has been found more economically efficient than the single-ended form, by as much as ten per cent, in the writer's own experience. It is generally adopted for engines of large power, but for small power is inconvenient, owing to its occupying more room lengthwise in the vessel, and also involving two stokeholds and therefore more supervision. At one time great difficulty was found in keeping the bottoms of boilers of this kind tight. Owing to their length, the unequal expansion due to different temperatures at the top and bottom caused severe racking strains on the bottom seams and riveting--so severe in some cases as to rend the plating for a large part of the bottom circumference of the shell. This difficulty has now been to a large extent got over, in consequence of the greater attention given to the form and direction of the water spaces in the boiler itself, so as to induce circulation of water; the introduction of the feed-water at the top instead of near the bottom; the more careful management now usual on the part of engineers; and lastly, the use of larger plates, welded horizontal seams, drilled rivet holes, and more perfect workmanship throughout. A modification of double-ended boiler is that introduced by Mr. Alfred Holt. It has many decided advantages, but is costly to make. The formation of the two ends into separate fire-boxes leaves the bottom of the boiler free to adapt itself to the variations of temperature to which it is exposed. The separation of the furnaces from the combustion chamber, excepting through the opening afforded by a connecting tube, is an advantage in the same direction, and avoids almost entirely the racking strains due to irregular furnace action. The weight of water carried is less, and that of the boiler may also be made less; while the elliptical form of the two ends gives greater steam space.
A type of boiler largely used in her Majesty's Navy, somewhat like a locomotive boiler, is highly efficient in regard to weight and power developed. Many examples have yielded one indicated horse-power in the cylinders for every three square feet of heating surface, under natural draught and with a very moderate height of funnel; and this with a consumption of fuel not exceeding 2½ lb. per indicated horse-power per hour under a working pressure of 60 lb. With the aid of a steam jet in the funnel, the heating surface per indicated horse-power has fallen below 2½ square feet. The large water surface afforded for escape of steam secures almost entire freedom from priming, without the incumbrance of steam domes; and the large combustion chamber allows of the thorough combustion of the gases before their passage through the tubes. The locomotive type of boiler has lately occupied the writer's attention, with a view to its more definite introduction into marine work. The difficulties, however, which lie in the way of applying it to steamers going long voyages are very great. The principal difficulty lies in the necessity of burning a large quantity of fuel in a very limited space and time. This can only be done either by direct pressure or exhaust action applied at the furnace. In other words, we must either exhaust the funnel, which will absorb a large amount of power, but would be comparatively easy of application; or our stokers, as is the case with our miners, must work under a pressure of air.
The writer stated that his experience in the manufacture and working of steel boilers was satisfactory. Many steel boilers of sizes varying from six feet diameter to fourteen feet six inches diameter have left the works at St. Peter's since 1877, when the first was made; and in no case has there been a failure of a plate after being put into a boiler, either in the process of manufacture or in working at sea. The mode of working is as follows: For shell plates, from five-eighths inch to seven-eighths inch thick, to warm each to a dark red heat before rolling, having previously drilled a few holes to template for bolting the strakes together; the longitudinal seams are usually lap joints treble riveted, requiring the corners to be thinned, which is done after rolling. The furnace plates are generally welded two plates in length, and flanged to form Adamson rings, and at the back end to meet the tube plate; the back flame-box plates are flanged, also the tube plates and front and back plates; and wherever work is put on to the plate it is annealed before going into the place. The rivet holes are drilled throughout. In the putting together the longitudinal seams of the thicker plates of the shells, great care is always taken to set the upper and under plates for the lap to their proper angle before they are bolted together, a point generally overlooked by the practical boilersmith.
The question of corrosion is one which is gradually being answered as time goes on; and so far very satisfactorily for steel. Some steel boilers were examined a few weeks ago which were among the first made; and the superintending engineer reports: "There is no sign of pitting or corrosion in any part of the boiler; the boilers are washed out very carefully every voyage, and very carefully examined, and I cannot trace anything either leaking or eating away. No zinc is used, only care in washing out, drying out, and managing the water." This is the evidence of an engineer with a large number of vessels in his charge. On the other hand, some of the most prominent Liverpool engineers always use zinc, and take care to apply it most strictly. The evidence of one of them is as follows: "We always fix slabs of zinc to most boilers, exposing not less than a surface of one square foot for every twenty indicated horse-power, and distributed throughout the boiler. This zinc we find to be in a state of oxide and crumbling away in about three months. We then renew the whole, and find this will last twelve months or more, when it is renewed again. Meanwhile we have no pitting and no corrosion; but on the contrary, the interior surfaces appear to have taken a coating of oxide of zinc all over, and we have no trouble with them."
Then the writer considered our present marine engine as to its efficiency and capability of further improvement. The weight of machinery, water, and fuel carried for propelling ships has not had due attention in the general practice of engineers. By the best shipping authorities the writer is assured that every ton of dead weight capacity is worth on an average £10 per annum as earning freight. Assuming, therefore, the weight of the machinery and water of any ordinary vessel to be 300 tons, and that, by careful design and judicious use of materials, the engineer can reduce it by 100 tons, without increasing the cost of working, he makes the vessel worth £1,000 per annum more to her owners. That there is much room for improvement in this direction is shown by the following statement, giving, for various classes of ships, the average weight of machinery, including engines, boilers, water, and all fittings ready for sea, in pounds, per indicated horse power:
Lb. per I. H. P.
Merchant steamers.......................... 480
Royal Navy................................. 300
Engines specially designed for light draught
vessels...................................280
Royal Navy, Polyphemus class (given by Mr.
Wright).................................. 180
Modern locomotive.......................... 140
Torpedo vessels............................. 60
Ordinary marine boilers, including water... 196
Locomotive boilers, including water......... 60
The ordinary marine boiler, encumbered as it is by the regulations of the Board of Trade and of Lloyds' Committee, does not admit of much reduction in the weight of material or of water carried when working. The introduction of steel has reduced the weight by about one-tenth; but it will be the alteration of form to the locomotive, tubulous, or some other type, combined with some method of forced draught, to which we must look for such reductions in weight of material and water as will be of any great commercial value. The engine may be reduced in weight by reducing its size, and this can only be done by increasing the number of revolutions per minute.
It has hitherto been the practice to treat the propeller as dependent upon the size of engines, draught of water, and speed required. This process should be reversed. The propeller's diameter depends on the column of water behind necessary to overcome the resistance in front of it due to the properties of the vessel. This fixed, the speed will then fix the number of revolutions, which will be found much greater than is usual in practice, and from this the size of the engines and boilers will be determined. Great saving in weight can be effected by careful design and judicious selection and adaptation of materials, also by the substitution of trussed framing and a proper mode of securing the engine to the structure of the vessel, as worked out in H.M.S. Nelson, by Mr. A. C. Kirk, of Glasgow, and in the beautifully designed engines by Mr. Thornycroft, in place of the massive cast-iron bedplates and columns of the ordinary engines of commerce. The same may be said of the moving parts. In fine, the hull and engines should be as much as possible one structure; rigidity in one place and elasticity in others are the cause of most of the accidents so costly to the ship-owner; under such conditions mass and solidity cease to be virtues, and the sooner their place is taken by careful design, and the use of the smallest weight of material--of the very best kind for the purpose--consistent with thorough efficiency, the better for all concerned.
Coming to the question of the consumption of fuel, a considerable saving has been effected in nine years, as shown in the following table:
Item. 1872. 1881.
Working pressure, lb. per sq. in......... 52.5 77.4
Heating surface per I. H. P., sq. ft.... 4.64 3.919
Piston speed, feet per min.............. 376 467
Coal burnt per I. H. P., lb.............. 2.11 1.828
This shows a saving equal to 13.38 per cent, in quantity of fuel consumed. Mr. Marshall then read a letter from Mr. Alfred Holt, of Liverpool, bearing on this subject, in which Mr. Holt spoke favorably of the single-crank engine, and stated his belief that the compound system would ere long be abandoned for the simple engine. He is endeavoring to feel his way to using the steam in one cylinder only, and so far the results have been encouraging, and he is now fitting a 2,200-ton vessel on that system. He is also endeavoring to do without a crank shaft, the forward end of the screw shaft carrying an ordinary crank with overhung pin. This experiment also promises satisfactorily. In his opinion the great improvement of the immediate future is to increase the steam production of our boilers. A ton weight of a locomotive boiler produces as much steam as six tons of an ordinary steamboat boiler.
Mr. Holt speaks of the coal account as one of the minor disbursements of a steamer. He does not give the ratio which coals bear to the total disbursements, but from other reliable sources Mr. Marshall found that, according to the direction of the voyage, it varies from 16 to 20 percent.--or, say, an average of 18 per cent.--of the total disbursements, in a vessel carrying a cargo of 2,500 tons. This will represent to-day about £3,000 per annum, and in 1872, at equal prices, the cost would have been £3,750--showing a saving of £750, equal to a dividend of, say, 3 per cent. on the value of the ship. Again, the cost of coal per mile run for such a vessel, in 1872, would have been at least 16½d.; to-day it does not exceed 13d.
The marine boiler as now made is very efficient, but if the quantity of steam used be considered in relation to the increased pressure, it will be seen that the boiler of to-day is little if anymore efficient than that of ten years ago. The present boiler has an evaporative efficiency of about 75 per cent., and cannot be much improved so long as air is supplied to the furnace by the natural draught. To increase the efficiency from 75 to 82.5 per cent. would require about double the heating surface, the weight of boiler and water being also doubled, while the gain would be only 10 per cent. Mr. Blechynden's formula, used in Mr. Marshall's works for weights of cylindrical marine boilers of the ordinary type, and for pressures varying from 50 lb. to 150 lb., is as follows:
W = (P + 15) (S + D² L) / C
or W = 2S (P + 15) / C
when S = D² L, which is a common proportion.
Here W = weight in tons.
P = working pressure as on gauge.
S = heating surface, in square feet.
D = diameter, in feet.
L = length, in feet.
C = a constant divisor, depending on the class of
riveting, etc. For boilers to Lloyds' rules,
and with iron shells having 75 per cent.
strength of solid plate, C = 13,200.
This formula, if correct--and it is almost strictly so--would give the relative weight of boilers per sq. ft. of heating surface, for 105 lb. and 150 lb. total pressure, assuming we wish to increase the efficiency 10 per cent, as follows:
Weight at 105 lb. = 105 x 1 / C
Weight at 150 lb. = 150 x 1.75 / C = 263 / C
Hence the ratio of weight = 263 / 105 = 2.5
In other words, the boiler with the higher efficiency would weigh two and a half times that with the lower efficiency. In the case of a vessel of 3,000 tons, with engines and boilers of 1,500 indicated horse power, the introduction of locomotive boilers with forced draught would place at the disposal of the owner 150 tons of cargo space, representing £1,500 per annum in addition to the present earnings of such a vessel.
Mr. Thornycroft has for some years used the locomotive form of boiler for his steam launches, working them under an air pressure--produced by a fan discharging into a close stokehold--of from 1 in. to 6 in. of water, as may be required. The experiments made gave an evaporation of 7.61 lb. of water from 1 lb. of coal at 212° Fahr., with 2 in. of water pressure, and 6.41 lb. with 6 in. of pressure. These results are low, but it is to be remembered that the heating surface is necessarily small, in order to save weight, and the temperature of the funnel consequently high, ranging from 1,073° at the first pressure, and 1,444° at the 6 in. With the ordinary proportions of locomotive practice the efficiency can be made equal to the best marine boiler when working under the water pressure usual in locomotives, say from 3 in. to 4 in., including funnel draught.
It has fallen to the lot of the writer to fit three vessels recently with boilers worked under pressure in closed stokeholds. The results, even under unfavorable conditions, were very satisfactory. The pressure of air would be represented by 2 in. of water, and the indicated horse power given out by the engines was 2,800, as against 1,875 when working by natural draught, or exactly 50 per cent. gain in power developed.
Mr. Marshall then proceeded to refute the arguments which may be urged against the use of the locomotive boiler at sea, and which we need not reproduce. Coming to the engines, Mr. Marshall said that the total working pressure of to-day may be accepted as 105 lb., or equal to seven atmospheres. If it were boldly accepted that eleven atmospheres, or 165 lb., were to be the standard working pressure, the result would be a gain of 14.55 per cent., provided no counteracting influence came into play. Of course, there are forces which war against the attainment of the full extent of this advantage, viz., the greater condensation in the cylinders and loss in the receiver or passages.
In regard to the former, it may be questioned whether by steamjacketing the high pressure cylinder, correctly proportioning the steam passages, and giving a due amount of compression in both cylinders, this may not be reduced far below the generally received notion; and the latter cause of loss may be considerably reduced in its effect by a more carefully chosen cylinder ratio. The ratio usually adopted, between 3.5 and 4 to 1, whether the pressure be 70 lb. or 90 lb., may well be questioned. With a cylinder ratio of 2.95 to 1, the economic performance is very good, and equal to any with the higher ratio. A lower cylinder ratio has another advantage of considerable value, viz., that the working pressure can be much reduced as the boilers get older, while by giving a greater amount of steam the power may be maintained--at an extra cost of steam, of course, but not so great a cost as with higher ratios. The cut-off in the high-pressure cylinder usually takes place at about 0.6, and the ratio of expansion has decided the ratio of cylinders. The use of separate starting valves in both cylinders obviates that necessity.
The difficulties in the way of taking advantage of the higher economic properties of greater pressures than hitherto used on board ship, are, it is submitted, not insuperable, and it would be to the interest of all that they should be firmly and determinedly met. It may be accepted as an average result that the Woolf engine, as usually arranged, will use 10 per cent. more steam than the receiver engine for the same power.
Of the three-cylinder receiver type the data are insufficient to form a definite opinion upon; but so far the general working of the Arizona is stated to be as good, economically, as any of the two-cylinder receiver class. The surface condenser remains as it was ten years ago, with scarcely a detail altered. In most engines it remains a portion of the framing, and as such adds greatly to the weight of the engine.
It is a question seriously worth consideration whether or no the surface of tubes can be reduced. The practice at present is to make the surface one-half the boiler surface as a minimum, that is, equal to about 2 square feet per indicated horse power. In practice, the writer has found 1.4 square feet per indicated horse power to maintain a steady vacuum of 27½ inches.
Mr. Marshall has just completed six pairs of engines for three twin screw ships, having steel shafts of 10 inches diameter, and has in each case run the engines at 120 revolutions per minute, while indicating 1,380 horse power from each pair for ten to fifteen hours without stopping; and in no case has a single bearing or crank pin warmed or had water applied, the surfaces on examination being perfect. In these engines all working bolts, pins, and rods, except the piston and connecting rods, are of steel, all rods in tension being loaded to 8,000 lb. per square inch. The boilers are of the Navy type, made throughout of Siemens-Martin steel plates, riveted with steel rivets, all holes drilled. Furnaces are welded and flanged; the tubes are of brass. In comparison with an ordinary merchant steamer's iron boilers of the double ended type, they weigh, including water and all appurtenances, as follows:
Double ended Type. Navy Type.
Weight, tons............ 135 ........... 146
I. H. P................. 1,400 .......... 2,760
Draught................ Natural ......... Forced.
The screw propeller is still to a great extent an unsolved problem. We have no definite rule by which we can fix the most important factor of the whole, namely, the diameter. Mr. Froude has pointed out that by reducing the diameter, and thus the peripheral friction, we can increase the efficiency; and this is confirmed by cases--of Iris reduced 2 feet 3 inches, and the Arizona reduced 2 feet. This must, of course, be qualified by other considerations. The ship has by her form a definite resistance, and a certain speed is required; if the propeller be made too small in diameter, the ship will not be driven at the required speed, except at serious loss in other directions. This question was too large and complicated to be dealt with here, and should, in the first instance, be made the subject of careful and extended experiment, on which a separate paper should be written.
To sum up the whole. Progress has been made during the past nine years, and in the following particulars:
1. The power of the engines made and making show a great increase. 2. Speeds hitherto unattainable are now seen to be possible in vessels of all the various classes. 3. The consumption of fuel is reduced by 13.38 per cent. on the average; and numbers of vessels are now working on much less coal than that average, while the quality of the coal is in nearly all cases very inferior, so that it is not unfair to take credit for 20 per cent. reduction. 4. The working pressures of steam are much increased on the average, and are still increasing; many steamers now being built for 120 lb. per square inch, while 90 lb. is the standard pressure now required.
The small steam ferry boats represented in the accompanying cut are doing service in the port of Marseilles, and the following description of them has been given by Mr. Flecher in the Bulletin de la Société des Anciens Elèves d'Arts et Metiers:
All those who are acquainted with the Old Port of Marseilles know the inconvenience of communication between one shore and the other, and the high price of ferriage by row boats. To obviate this, Captain Advient has been struck with the happy idea of creating a cheap steam service (fare one cent), thus supplying a genuine want in the modes of locomotion of the city.
The building of these ferry boats, on a system providing for the use of separate hulls, was confided to Messrs. Stapfer, De Duclos & Co., of Marseilles, whose well-known reputation was a sufficient guarantee that the problem would be successfully solved.
There existed difficulties of two natures: The first of these related to the stability of boats such as this, having their engine, boiler, supply of coal, forty passengers who might all occupy one side of the vessel, a central superstructure, with roof; and, finally, all the weight centered on five feet of the deck, with nothing below to counterbalance it except the hollow hulls and two three-foot compartments, each placed toward the central portion of the hulls and designed as fresh-water reservoirs for the steam generator. The second difficulty was to obtain the best utilization possible of a screw placed in the current between the hulls and upon a shaft inclined toward the stern, that is, "stern" by analogy, for there is no distinction of fore and aft in ferry boats.
STEAM FERRYBOATS OF THE PORT OF MARSEILLES.
The conditions of the problem were finally fulfilled to the satisfaction of all concerned, and especially to that of the public.
The hulls, navicular in form and having a flat bottom, are constructed of one-tenth inch iron plate and 40x40 angle iron. Their dimensions are: Length, 33 feet; breadth, 3¼ feet; and depth, 5 feet. The internal distance between the two shells is 7¼ feet. These hulls, having absolutely water-tight decks, are connected below by tie bars of flat iron, and above by vertical stays 1 foot in length, which serve to support the floor-planks of the deck and boilerplate flooring of the engine-room. The engine-room, which is 19½ feet long by 5 feet wide, is constructed of varnished pitch-pine, with movable side-shutters of teak. The roof, of thin iron plate, is provided with a ventilator to allow of the escape of hot air.
The passengers, to the number of forty or fifty, can move about freely from larboard to starboard, or from stem to stern, or seat themselves on the benches running along the inside of the guard railing on the two sides of the vessel. They are protected from rain by a roof, and from the rays of the sun by a curtain extending along the sides.
Although the usual method of landing is fore and aft, gangways have been provided at the sides for side-landing should it become necessary.
The general appearance of one of these boats may be likened to that of a floating street-car. Finally, a small apartment, provided with benches, is provided for the use of those passengers who might be taken sick, or for office purposes, if need be.
The total weight of one of the boats is divided up as follows:
Forty passengers................ 6,200 pounds,
Engine and boiler............... 6,600 "
Ballast, water, and equipment... 9,900 "
Deck and superstructure......... 6,600 "
Hull and accessories............12,500 "
______
Total...........................41,800 "
or a displacement of about 700 cubic feet, corresponding to a maximum draught of 3.7 feet. The mean speed is 4 knots, or 4½ miles per hour, a great velocity being unnecessary, owing to the small distance to cross in a port often obstructed by the general movement of vessels taking place therein.
The engine is from 16 to 18 horse-power. Its frame is inclined perpendicularly to the direction of the screw-shaft, the extremity of which is supported near the screw by a strengthened cross-stay serving as a pillow-block. The cylinder is 8 inches in diameter, and the piston has a stroke of 6 inches, causing the screw (which is 3¼ feet diameter) to make 200 revolutions per minute. The screw, although it has a wide surface of thrust, gives, nevertheless, a recoil of about 30 per cent., because of its location between the hulls and its oblique action on the shaft.
The steam is furnished by a tubular boiler having an internal fireplace and a heating surface of sixteen square meters, the draught being effected by the exhaust of the engine. This boiler, which is tested up to 14 pounds, is fed by a steam pump, or by a pump actuated by the engine. The feed pumps take water successively from one or the other of the reservoirs in the hulls. The reservoirs are filled in the morning, and their level is ascertained by two small and ingenious Decondun indicators, the dials of which are placed against the walls of the engine-room.
Taken altogether, these little boats are well arranged and quite handsome; and, since they were put into service in June, 1880, they have proved a great convenience to the hard-working and active population for which they were built.
In July last, Admiral the Duke of Edinburgh, with the Naval Reserve Squadron under his command, arrived in the Firth of Forth and anchored in Leith Roads. His Royal Highness performed the ceremony of opening the new dock at Leith, which has been named after him. The "Edinburgh" Dock at Leith, which was commenced in 1874, consists of a center basin 500 ft. long and 650 ft. wide, and two basins 1,000 ft. long and 200 ft. wide, separated by a jetty having a width of 250 ft. The total amount of masonry in the wet docks is 100,000 cubic yards. The north and south quays are each 1,500 ft. long, and the two sides of the jetty 1,000 ft. long each, having a total quayage in connection with the dock of 6,775 ft. The walls are 15 ft. thick at the base, narrowing in two tiers to 8 ft. The new dock will cost altogether about £300,000. Leith now possesses five docks and a total quayage of three miles 808 yards, 1,234 yards of which is the old portion. These works have been constructed, at a cost of nearly one million sterling, by the Leith Dock Commissioners, whose chairman, Mr. James Currie, presented an address to the Duke of Edinburgh, on board the flag-ship H.M.S. Hercules, giving an account of their affairs. The other docks at Leith are named the "Old Dock," the "Queen's Dock," the "Victoria," the "Albert," and the "Prince of Wales Dock." The opening ceremony was arranged to consist of the steamer Berlin, with his Royal Highness and the Dock Commissioners on board, accompanied by Sir Donald Currie, M.P., and other gentlemen, passing through the entrance from the Albert Dock to the new dock, across which a blue ribbon had been stretched. At the moment when the ribbon snapped asunder, under the bow of the Berlin, the Duke of Edinburgh, stepping forward on the upper deck of the steamer, said, "I have now the gratification of declaring this dock open, and calling it the Edinburgh Dock." On this announcement being made, a signal was conveyed to a battery of guns, posted on the sea wall of the new dock, from which a party of the Royal Artillery fired a Royal salute. The steamer, having gone round the new dock, was brought up at the quay at the west. His Royal Highness the Duke of Edinburgh, with Prince Henry of Prussia, the officers of the fleet, and the Commissioners, disembarked and proceeded to the saloon in the new dock, where luncheon in honor of the occasion was given by the Leith Dock Commissioners.--Illustrated London News, Aug. 6.
OPENING OF A NEW ENGLISH DOCK.
The illustration shows the apparatus at work transferring a cargo of grain from the hold of a ship by means of an elevating band fitted with buckets. By a simple contrivance shown in the engraving by diamond-shaped squares, the elevating band can be shortened or lengthened at pleasure, so as to suit it to the position the grain to be elevated occupies in the ship or barge. When the grain is elevated to the point whence it is to be transferred to the granary, railway truck, or other destination, the band travels horizontally on suitable bearings, the buckets being so constructed that in traveling they retain their load intact. The contrivance for lengthening and shortening the bucket band is an application of the "lazytongs" device, which is well known. The float of the elevator is shown at the left hand of the engraving, and, as seen in the latter, there is an automatic weighing machine, by which the material may be weighed as it is delivered, before it goes to the bottom of the elevator, to be again transferred by its means to the barge or granary. Simplicity, efficiency, and adaptability to any position in which elevators of this class are desirable, are the claims the patentees, Messrs. Behrns & Unruth, Lubeck, make for the advantages of their apparatus.--London Miller.
IMPROVED FLOATING ELEVATOR.
We illustrate below a useful type of dredger made by Messrs. Rennie, of Blackfriars, England. The drawing almost explains itself. The machine consists of a double barge or pontoon, in which is erected a derrick. This derrick works a "spoon" dredge at the end of a lever. The spoon, as shown, is at its lowest position. It will make a forward stroke, through about one-sixth of a revolution, and will thus become filled with mud and be lifted above the surface of the water. The motion will be imparted to it by the chain and pulleys seen at outer end of the derrick jib. The jib will then be swung round over the bank on a hopper barge and its contents delivered. The requisite power is supplied by the steam engine at the end of the pontoon. Messrs. Rennie have made several of these little dredgers, which are found very useful and handy in shallow water.--The Engineer.
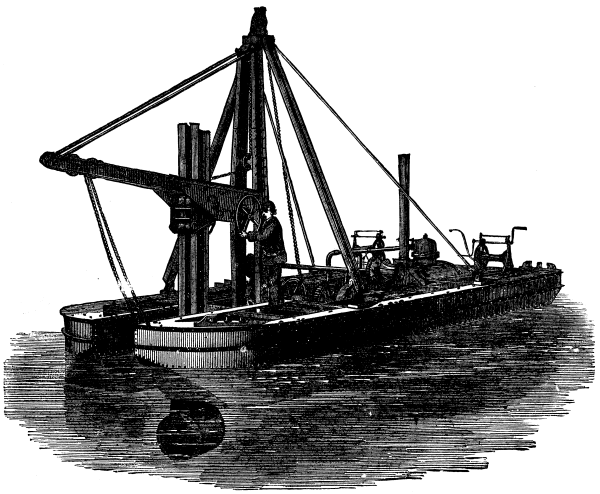
SINGLE BUCKET DIPPER DREDGER.
In order to prevent a train passing a danger signal during a fog or snowstorm without being seen by the engineer, the Southern Railway Company of France have attached to the locomotive a steam whistle, which is controlled by the signal. The whistle is connected with an insulated metallic brush placed under the engine. Between the rails there is a projecting contact bar, faced with copper, which is swept by the brush when the train passes. This contact piece is connected with the positive pole of a voltaic battery, the negative pole of which is in communication with a commutator on the signal post, from which a wire leads to the ground. When the signal is "line clear" the passage of the brush over the fixed contact produces no result; but when the signal marks "danger," the commutator brings the negative pole of the battery in direct communication with the ground, and when the brush passes over the contact the completion of the electric current causes the whistle to be sounded, so as to alarm the driver.--L'Ingen. Univ.
Sulphide of carbon (CS2) is prepared by passing the vapors of sulphur over charcoal heated to redness. In laboratories, charcoal and roll brimstone are employed so as to obtain as pure a product as possible; but sulphide of carbon having now become so important a commercial product, and being employed for so large a number of industrial purposes, it has been found more economical to substitute coke for charcoal and pyrites for brimstone.
The Messrs. Labois, in their system of furnace represented herewith, have had in view the manufacture of this product under as economical conditions as possible, by coupling over two connected fireplaces the retort in which the pyrites is distilled, and that in which the reaction of the sulphur and carbon takes place.
The pyrites is fed from the hopper, A, into a distributing box, B, furnished with a valve which is maneuvered by a lever. From thence it descends into the retort, G, where it is roasted by the heat of the fireplace, L. The sulphur converted into a state of vapor passes through the conduit, R, into the coke or charcoal retort, G', which is divided into two parts by the partition, g g', of refractory clay, and heated by the fireplace, L'.
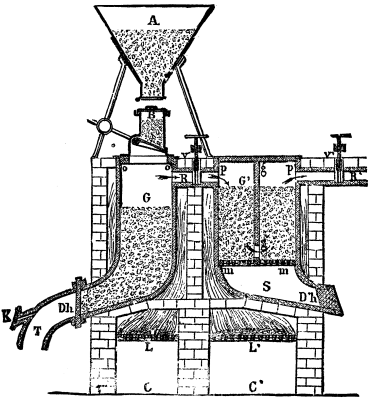
LABOIS'S SULPHIDE OF CARBON FURNACE.
The conduit, R', leads the sulphide of carbon in a state of vapor to the condensing apparatus. The uncombined sulphur which is carried along is deposited in the first part of the retort by the arrangement of the partition, which permits of passage only below. The registers, V and V', permit of the introduction of the sulphur vapor and the exit of the sulphide of carbon being regulated.
The apparatus is so easy of installation that it may be applied without much expense to pyrites furnaces already in operation.
Wherever a manufactory of the product is to be started, the system recommends itself by its simplicity, and by the facility with which the operation may be watched and conducted.
Brouardel's manometer, represented herewith, is designed for showing graphically variations in the pressure of gas, either at the works during the course of manufacture, or at any point whatever in the system of piping.
For this purpose water manometers have hitherto been employed; but, although the indications given by these are very accurate, their form and weight are such as to render them not easily transportable; and then, again, considerable care is necessary in putting them in place.
Mr. Brouardel's registering manometer does not give so accurate indications, perhaps, but it possesses, as an offset, the merit of being very portable and easily put in place; and, besides, it inscribes the hour at which the pressure is exerted.
The apparatus consists of a metallic cylinder, A B, which carries a circular shoulder, C, that rests on a plate, D--the latter being put in motion by a clock which is wound up by means of a button under the base, E, of the apparatus. The two standards, F F, carry a crosspiece which supports a disk that closes freely the aperture of the drum, A B, in such a manner as not to impede its rotation.
In the interior of the cylinder there is a metallic cup which is connected with the central reservoir by an impermeable membrane, I. These three parts form a closed chamber, into which the pressure comes through a tube, F, provided with a cock. A spring, M, which counteracts the pressure, is arranged between the crosspiece, G, and the bottom of the reservoir. The latter carries also a small rod, K, which is provided with a cord made of braided silk. This cord runs over a pulley, N, whose axle carries at its other end a still larger pulley, O. Toward the middle of the latter is fixed a silken cord which hangs down on each side, after making several turns around the pulley. To the front cord is attached a slide, Q, moving in a vertical direction, and to which is fixed an inscribing style, R. The other extremity of the thread enters the hollow upright, and carries a weight which is greater than the combined weights of the slide, the membrane, and the internal reservoir. The upright serves as a guide to this counterpoise.
In order to use the apparatus there is affixed to the cylinder, A B, a sheet of paper divided in a vertical direction into as many parts as the cylinder takes hours to make one revolution. The divisions running horizontally represent centimeters of water or of mercury, according to the strength of the spring, M, which should be so constructed as to be in relation with the pressure. The operation of the apparatus may be readily understood.
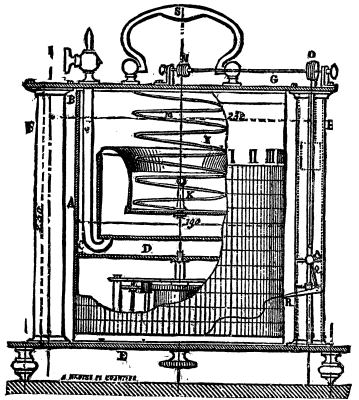
GAS INDICATOR OF MANOMETER.
When the gas reaches the pressure chamber, the spring, M, contracts, and consequently the counterpoise descends, and causes the cord, O, which carries the slide and writing style, to wind around the pulley. When the pressure diminishes, the movement takes place in an opposite direction.
The tracing is done by means of a special form of style giving indelible curves through the medium of colored glycerine. The position of the point is determined in such a way as to annul the friction of the pen, and consequently to give it greater sensitiveness.
It should be remarked that the course of the rod, K, is amplified in the tracing of the ordinates of the pressure according to the ratio of the diameters of the pulleys, N and O.
The apparatus may be carried by hand by means of the handle, S, either in or out of its case. To put it in operation, it is only necessary to connect the apparatus with a gas burner (located near the place where the variations of pressure are to be observed) by means of rubber tubing. The apparatus may be employed under the same circumstances as glass and U-shaped water manometers, with the further advantage that the results are registered, and consequently can be more easily compared.
The apparatus represented in Figs. 1, 2, 3, and 4 is the invention of Messrs. Taylor & Wailes, and is designed for casting metallic objects in annular form, its arrangement being slightly varied according to the nature of the objects to be cast. In all cases where a special form is to be given to the outer or inner circumference of the object, or where it is desired to exert a pressure on the circumference, such form or pressure is obtained by the introduction of a core which may be expanded or contracted as need may be. For this purpose an expansible, metallic core is employed, the arrangement of which is shown in Figs. 1 and 2, and which is so fashioned that the inner circumference of the ring to be cast may receive the desired form. This core is formed of the pieces, g, g', made of cast-iron or any other material which fuses with difficulty, and which are placed in the revolving mould in such a way that after the cooling of the pieces the parts, g, recede by the shrinkage of the piece and thus free the core. The parts, g, of the core are in the shape of circular segments, and are united at their external circumference by a flange, along with which they form a shoulder piece for the casting. As a consequence of the rapid revolution of the mould, these parts are pressed by centrifugal force against the molten metal which is run into the mould.
CENTRIFUGAL METAL MOULDING APPARATUS.
The plan, Fig. 2, shows the arrangement of the parts, g, g', and allows it to be seen that the pieces, g', act as wedges against the segments, g, and push these out so as to form a perfect circle. The molten metal cannot become oxidized in the mould, since it is shut off from contact with the external air by the cap, C, which covers it. Oxidation may, however, be further prevented by passing some deoxidizing or neutral gas into the mould. For this purpose the mould is filled before the casting is done with some such gas as illuminating gas, carbonic acid, nitrogen, or hydrogen.
This improved process of casting may also be employed for objects which do not possess an exactly annular section. The moulds are then arranged eccentrically in a frame which is made to revolve rapidly during the cooling of the metal In this way the pieces are less strongly compressed at the places where they are nearest the center of rotation than a the points where the radius is greater.
Figs. 3 and 4 show section and plan of an apparatus of this kind. The sand moulds are arranged in the frame, a b which revolves about the axle, c. In the moulds there are iron cores, h, which press the metal during rotation and thereby produce compact pieces.
For manufacturing wood pulp Mr. Dresel employs an apparatus such as represented in Figs. 1 and 2, consisting of an upright cylindrical reservoir, A, supported on a frame by means of trunnions, z. This reservoir, which is of boiler plate, is furnished with a cover, D, which has in its center a piece of tubing, with stop-cock, C. A series of tubes, R, whose diameter and length are proportioned to the volume of the boiler, A, is filled with the liquid which is contained in the boiler, so as always to be able to rapidly produce a pressure of nine atmospheres or more by direct heating. The flanges of the tubing are provided with a cut-off of angle iron identical with that of the tube, D. By means of this arrangement the cocks and the flanges, E, permit of communication between the serpentine tubing, R, and the boiler being interrupted; while the heat developed by the fire-place, F, causes an active circulation in both the tubing and boiler.
DRESEL'S WOOD PULP APPARATUS. Fig. 1
DRESEL'S WOOD PULP APPARATUS. Fig. 2
To put the apparatus in operation the cover, D, is first unscrewed, and there is put into the boiler a certain quantity of wood, which has been divided up by a cutting machine of special form. Then the boiler is filled to the proper height with the liquid necessary to dissolve the incrusting materials, the cocks, B, being closed. Afterwards there is fixed immediately beneath the angle-iron ring of the cover, D, a perforated iron plate upon which the contents of the boiler rest when the latter is turned up. Then the cover is fastened down and the boiler is put in communication with the heating apparatus. The cocks, E and B, are opened, so that the liquid may begin its movement in the tube, a, the boiler, A, and the tube, n. As soon as the proper temperature is reached for converting the wood into fiber and decomposing the incrusting matters, the heat is shut off in case the tubing, R, is not connected with another like boiler, and, after closing the cocks, E and B, and shut off communication between the tubing and the boiler, the latter is turned over and the cock, C, gradually opened in order to allow the steam to escape. When the temperature has descended to 100° in the boiler the cover, D, may be opened, after the liquid has been allowed to flow out through the cock, C. Next, lixiviation is effected by connecting the cock, C, with the steam pipe, P, and causing steam under pressure to enter the boiler, A. The action of the steam on the contents of the latter, which are now converted into cellulose, mixed with a large quantity of dissolved matters and of liquid, effects a complete washing and permits of the recovery of considerable quantities of useful chemical products. Moreover, the steam purifies, decolorizes, and completely separates the fibers, and renders them more easily susceptible of being bleached. Finally, the perforated bottom, S (which is formed of two parts), is removed and the boiler emptied.
In order to have the operations under control, and for the purpose of safety, there is riveted into the boiler, A, a tube, T, containing a thermometer: and there is fixed to the tube, a, a pressure-gauge, M, and a safety-valve. The level of the liquid is ascertained by means of a gauge-cock, H.
The thirty-fourth annual summer meeting of the Institution of Mechanical Engineers began on Aug. 2, at Newcastle-on-Tyne. The following is an abstract from the address of the president, Mr. E. A. Cowper.
He began by stating that as members of the Institution of Mechanical Engineers, on revisiting their brother members and friends here in Newcastle, after an interval of twelve years, they came as it were to one of their natural homes; certainly to the home of one of the greatest engineers that England has ever produced, and the birthplace of the locomotive, which has done more than any other improvement, of our age to lessen the cost of materials to the men who have to use them, and therefore to cheapen and extend production in the most wonderful manner. He then went on to say that it seems but a few years ago since George Stephenson, at a meeting in 1847, proposed the resolution that the Institution of Mechanical Engineers be formed. He was strongly supported by a large number of the mechanical engineers of the country, and the speaker had the honor of seconding the resolution that he be first president. The intention was that engineers from all parts of the country should join to form a compact body capable of discussing and judging of all mechanical subjects and appliances. In this the institution had been eminently successful, and it numbered among its members mechanical engineers in every large town in the country, and has increased in strength and importance.
The last twelve years have been marked by many very important changes, while low prices have generally ruled. Among other causes of fluctuations in demand and supply (and consequently in values) must be mentioned the occurrence and the threatening of foreign wars, which disturbed the course of commerce greatly for some years. Such causes must be considered as extraneous to the sphere of influence possessed by good or bad manufacturing or engineering. Mr. Cowper does not look upon the very great expense of improved war material and implements as an unmixed evil for this country; for it so happens that we can better meet such outlay than any other nation, and thus our wealth gives rise to greater power and security than our neighbors possess; while, seeing that we are not an aggressive nation, such power tends materially at once to the progress of this country, and to the peace of the world. Having referred briefly to one cause of disturbance to the progress of mechanical engineering, he named another, which at the present moment is occupying thoughtful men to a considerable extent, namely, the arbitrary imposition of duties and bounties for the professed object of protecting manufactures, while in fact they constitute taxes on a nation for the benefit of a few individuals. In some countries excessive duties have been imposed, as against our manufactures, and it is even proposed to increase them; while in other cases bounties are actually paid out of the public purse to men engaged in a particular manufacture, on their exporting to this county certain of their wares, as, for instance, beet-root sugar.
One extremely significant lesson, resulting from high duties--which it may be hoped will not be thrown away upon the American public--is, that whereas our cousins on the other side of the water used to build almost all the American "liners" of wood, they now find that, with their excessive duties against the importation of iron and steel from England, they cannot compete with English iron and steel ship-builders and marine engineers. This is one of those damaging effects naturally produced by excessive protective duties; which, while they enable American ironmasters quickly to realize enormous fortunes, drive the American merchants to purchase English ships, or intrust their merchandise in English bottoms, as it is impossible to maintain protective duties at sea.
Whatever fluctuations have occurred, it is now pretty clear that several foreign nations have settled down to cultivate and extend their manufactures, and we are brought face to face with the fact--which has now been for some years growing to its present importance--that many articles which in years gone by we thought it to be our especial province to supply, are now produced in the very countries requiring them. Even Spain is awakening to the advantage of producing hematite iron from her own excellent ores, with English and Welsh coke carried out in the same ships that bring Spanish ores to this country.
Now with regard to the possibility of any foreign nation eclipsing us in our manufactures, he would say at once that any such successful rivalry on their part is far worse than the effect of any duties, even if they be prohibitive; for it means rivalry in the markets of the world, and possibly in our own markets here at home. Therefore it behooves us to put our house in order, and see in what way we may be enabled to manufacture better and with greater economy. Mechanical engineering is of such extreme importance in advancing civilization, that it is most essential that its progress should be rapid and unimpeded.
Perhaps the very large increase in steam shipping, and the change from sailing ships and paddle steamers to screw steamers, has been one of the greatest improvements of recent times, and it is none the less real or important from having been gradual, while the result to this neighborhood has been most beneficial. This change has been due in great measure to the introduction of very economical marine engines, chiefly of the compound type, together with better boilers carrying a higher pressure.
The speed and regularity of ocean steamers has also greatly improved, and one small scientific improvement has added much to the safety of traversing such seas as the Atlantic at a high speed--namely, the careful and continual use of a good thermometer, to ascertain constantly the temperature of the sea-water at the surface. For if an iceberg is floating within a quarter of a mile--or even half a mile, if the sea is pretty smooth--the surface water will be several degrees colder than the rest of the sea; since the very cold fresh water, resulting from the melting iceberg, floats on the top of the sea water for some distance.
No doubt the use of iron, and now of steel, has contributed most largely to the increase of shipbuilding in this country. Good arrangements of water ballast have also proved very useful; and steam cranes and arrangements for loading and discharging cargo have greatly promoted the use of steam colliers, enabling them to make more voyages in the year.
Closely connected with marine engineering is the great improvement in the economy of stationary engines, which has become more fully developed during recent years, both in reference to waterworks engines and factory engines. In aid of stationary engines, "surface evaporator condensers" have been found very useful, particularly where the supply of water is very limited; and at waterworks it is now very common to pass the whole water pumped through a surface condenser, thus giving a good vacuum without the expenditure of any water, and with the result of only raising the temperature of the water a very few degrees, on account of its large volume.
Locomotives have shared to some extent in the general improvement in machinery. The boilers are better made, and are safer at the higher pressures now carried than they were formerly with a low pressure. Several new valve gears of great promise have been brought forward, both for locomotives and marine engines. Among them Joy's motion should be again noticed. Mr. Webb says: "The engine shown at Barrow has been at continuous work ever since the Barrow meeting, and has run 30,278 miles; we had it in for examination on the 18th inst., and found the motion practically as good as the day it went out of the shop, more especially the slides, about which so many of the people who spoke at the meeting seemed to have doubts. I do not think you could get a visiting card between the slides and the blocks; in fact, the engine has been sent out to work again, having had nothing whatever done to it. The first thing, of course, that will require doing will be the tires; as far as I can see nothing else will want doing for some time."
A very fine engineering work has now been accomplished in America in reference to navigation, namely, the deepening of the channel at the mouth of the Mississippi through the training of the river by jetties and banks. In consequence, ships of large size may now go up the river--there being plenty of deep water above the mouth--and bring down grain cargoes, without the expense and inconvenience of transshipment, thus reducing the freight of corn to this country. This great improvement is the work of Captain Eads. A somewhat similar improvement was the blowing up of about 50,000 tons of rock from the bed of the river at the narrow pass of Hell Gate, near New York. It is to be hoped that these good examples may spur on our friends on the Continent to improve their harbors, so that large channel boats may cross with comfort to the passengers, thus avoiding the excessive expense that a tunnel would involve.
Great improvements have been made in the illumination of lighthouses by oil lamps; a light equal to 1,300 candles has been produced by Mr. Douglass, of the Trinity House, and now two such lights will be placed one above the other, where required. The electric light has made such numerous and rapid strides that it is impossible even to notice its various applications; but on the one hand the lighting by Dr. Siemens of four miles of dock frontage at the Albert Dock of the London and St. Katherine Dock Company, together with the railway behind the warehouses, and the warehouses and ships themselves, and, on the other hand, the elegant and steady domestic light of Mr. Swan, are excellent examples of the two extremes in this department. I believe we shall have the pleasure of closely observing the Swan light during our visit here. The lighthouse electric light is also a noble application of the great power of a single electric light on the arc principle. The most powerful electric light in the world is situated near here on the coast, between the Tyne and the Wear. It is possible, and even probable, that one of the great uses to which electric force will be applied eventually, will be simple conveyance of power by means of large wires; and as a higher percentage of power is gradually being realized, this method will become more economical. I may mention that 60 per cent. has already been obtained.
The invention of Messrs. Thomas & Gilchrist, by which a very large field of ironstone is now, for the first time, made available for the purpose of making good steel by the Bessemer process, bids fair to make very considerable alterations in the steel-making trade, and in the hands of Mr. E. Windsor Richards it has been made a great success, while in Germany there are several works also using the process largely. Mild steel is now being used to a great extent for the construction of steam boilers as well as of ships, and in steel castings for a variety of purposes, such as spur wheels, frames of portable engines, manhole door frames, etc., etc. Among the uses to which steel may be put is the manufacture of steel sleepers in place of wood. It is a very encouraging fact that there are now, or rather there were already, at Dusseldorf, in 1880, 70,000 tons of iron or steel railway sleepers in use in Germany. Mr. Webb, of Crewe, has exhibited a very promising arrangement of sleepers and fastenings, to be made either of iron or steel. Steel sleepers should also be used for tramways.
If, now, some clever ironmaster could only accomplish the task of making a good "street pavement" of cast iron, the increased demand for pig metal would be enormous. It has nearly been accomplished already, by several different modes of construction; and there are very many streets where the luxury of wood pavement, which wears very rapidly, cannot be afforded, and where macadamizing will not stand the wear and tear of the heavy traffic. The use of ingot steel, or very mild steel, for making tin-plates is now an established thing, and manufacturers are now taking this metal for making large tinned sheets up to seven by three feet.
The making of casks by machinery, cheaper and better than those made by hand, is now an accomplished fact by Mr. Ransome's machines. There are twelve factories already established abroad, some turning out 2,000 or 3,000 casks a week. This is a good case of English invention taking the lead in a manufacture.
Among good mechanical appliances that have been proved to be highly valuable to the civil engineer may be mentioned the excavating machine, which answers well for certain soils and situations, though not for all; and the dredger of Messrs. Bruce & Batho, for excavating from the inside of piers in water.
In manufacturing chemistry, which, with its numerous mechanical appliances, is much indebted to mechanical science and engineering, great advances have been made during the last dozen or twenty years. Aluminum has been brought into practical use to a large extent, it being at once a very light metal and a very cleanly one. "Anthracine," obtained from coal tar, has been manufactured largely for the purpose of producing the various brilliant dyes now so common.
New materials for making candles have been manufactured, in some cases by purely mechanical means, such as boiling together for some hours, at a pressure of several hundred pounds per square inch, neutral grease and water, when the water takes up the base, viz., glycerine, and leaves the grease as an acid grease. This same effect has been noticed in some steam boilers, where the same water, without admixture of fresh, has been used over and over again with surface condensers. Then, again, large rotating chemical furnaces have been introduced; and improved glass furnaces--particularly tank glass furnaces, in which the batch is put in at one end, and the working holes are toward the other end--have cheapened the actual production of glass, and are being worked largely on the Continent, and to some extent in this neighborhood. Toughened glass has made some progress for certain purposes. Besides the improved and extended use of glass in lighthouse illumination, it has again been pressed into our service for other purposes, through our greatly extended knowledge of the laws of optics.
Spectrum analysis has become of practical use, and photographs of the various Fraunhofer lines in the spectrum have been taken as permanent records of each experiment. That such extended knowledge should have been developed by that one little instrument, the lens, is but natural; for the lens is at once the means by which we discover the extreme magnitude of some portion of the infinite works of the Almighty in the architecture of the heavens, and by which we appreciate to some extent the extremely minute markings of a diatom that one cannot see with the naked eye. At the same time we feel sure that there are other markings still smaller, as every increase in the power of the microscope has always rendered visible some markings still smaller than the last; and in like manner has every increase in the power of the telescope developed more worlds and suns far away from our system and beyond our Milky Way. An approach to the infinite in minuteness and to the infinite in magnitude and distance is thus furnished to us by one instrument alone.
There was but one further observation that he would venture to make, and it is this.
When one looks back upon the goodly list of clever men and benefactors of the human race, who have lived, say, during the last hundred years, one is sometimes tempted to wish that more of those scientific men, who have had the most brilliant ideas, and been our greatest discoverers, should have striven to carry out their discoveries into practice. For instance, take Faraday's beautiful discoveries in electricity. It was, in a manner, left to Sir Francis Ronalds, Professor Daniell, Professor Wheatstone, Fothergill Cooke, Dr. Siemens, and others, to develop from those discoveries the "intelligence wires," and "bands," that now encircle the earth, and unite nations, and do so much to prevent misunderstandings.
It is gratifying to know that the engineering profession has not been forgotten when honors have been conferred on distinguished men; and among others may be named Sir William Fairbairn, Sir John Rennie, Sir Peter Fairbairn, Sir Charles Fox, Sir William Armstrong, Sir Joseph Whitworth, Sir John Hawkshaw, Sir John Coode, Sir William Thomson, Sir Joseph Bazalgette, Sir Charles Hartley, Sir Charles Bright, Sir James Ramsden, Sir John Anderson, Sir George Elliot, Sir Daniel Gooch, Sir Henry Tyler, Sir Samuel Canning, Sir Edward Reed, and Sir Frederick Bramwell. With many noble examples before us, and with signs of an improvement in many branches of commerce, he trusted that the latter part of the present century will, with somewhat greater exertion of thought and enterprise on our parts, be marked, not only by numerous small improvements, but by many substantial inventions for the good of mankind.
Our thriving neighbor, Hoboken, just across the Hudson River, has a large and vitally important problem to solve. Of the 720 acres within the city limits, 270 acres lie at a considerable height above the river and constitute what are known as the knoll or uplands of Hoboken. Between this low ridge and Palisade Ridge lie 450 acres of marsh lands or meadows, 140 acres of which have already been built upon. The marsh is about half a mile wide, and something like a mile and a half long, extending southward into Jersey City. The surface is a network of matted vegetation and roots perhaps five feet deep, and under that lies a mass of blue clay or river silt 100 feet or more in depth. The original tidal flow over these marsh lands has been obstructed by viaducts for railroads and streets, leaving only two natural outlets, a sluice way at Fifteenth street on the north, and on the south a basin constructed by the D. L. & W. R. R., 100 feet wide, and 2,300 feet long. The average level of the marsh land is three feet above mean low water and a foot and a half below mean high water. In the part built upon the streets are but two feet above mean high water.
During long easterly and northerly storms, especially at times of high spring tides, the level of the water in the Hudson is often such as to cover the meadows even at low tide; and on several occasions the water at high tide has been 4½ feet above the level of the meadows, and a foot or more above the established grade of the streets.
The problem is to drain these marsh lands so as to make them properly habitable and to protect them from invasion by high tides and storm waters.
The first drainage map of the district was made about fifteen years ago; since then over $100,000 have been expended on tidal sewers and other devices, and several acts have been passed by the New Jersey Legislature in furtherance of the work. An extended review of the plans proposed and the experiments made thus far is given in a report presented to the Board of Health and Vital Statistics, last May, by Engineers Spielmann and Brush. Ten years ago Mr. Arthur Spielmann, on being directed by the City Council to prepare plans and estimates for a contemplated sewer in Ferry street to the western boundary of the city, reported adversely to the project, believing that such a sewer would fail to answer the purpose of its construction.
There were but two ways, he thought, of securing the end desired: First, by raising the grade sufficient to give a good drainage; second, by making reservoirs and forcing the drainage matter out into the river by steam pumps. The first method he found impracticable on account of the cost of filling in so large an area and of raising the large number of houses already on the low ground. The second plan was recommended as being much cheaper and entirely practicable. Substantially the same position is taken in the report of last May, wherein it is alleged that the superior economy of a pumping system has been sufficiently attested by several eminent hydraulic engineers who have since investigated the problems involved. On a small scale the efficacy of the pumping system has been practically tested, first, in Meadow street, between Ferry and First streets, and more recently in the southern part of the city, where a number of property owners have kept twenty-five acres free from water (except during storms) by means of a private pump.
The comparative economy of the pumping system is shown by estimates in detail of the cost of constructing and operating such a system in contrast with, the cost of raising the grade and introducing tidal sewers. Under both systems the cost of the ordinary sewers will be about the same. A proper system of tidal sewers, it is claimed, will necessitate the raising of the grade of the streets on the low lands to a height at least ten feet above mean high water. The extra cost of raising the streets is estimated at $3,000,000. The cost of the pumping system, with machinery and power sufficient to remove all storm water and sewage, is put at $150,000, while the running expenses, including interest on the first outlay, are put at $30,000 a year. The interest on the preliminary expenditure of the first plan considered is $180,000 a year, or six times as much as the pumping system would involve.
According to the estimates made by Engineer Kirkwood, in his report of 1874, a total pumping capacity of 134,500,000 gallons a day will ultimately have to be provided to meet the requirements during the heaviest storms, besides some six or seven million gallons a day of sewage proper, exclusive of storm waters. Not more than half that amount of pumping will be required at first, the increase to be made gradually as the marsh land is built upon.
Our plate illustrates the residence of Mr. J. E. Boehm, A.R.A., the sculptor. Bent's Brook is situated at Holmwood, not far south of Dorking, on the Mid-Sussex line, and commands some fine views of well-timbered country. The site itself is comparatively low, and the soil being clay it was advisable to keep the building well out of the ground, and in this way a rather unusually high elevation for such a house was obtained. The plan is very compactly arranged, with an ingenious approach to the well-centered hall and staircase, over which, by a mezzanine contrivance, a good store place is secured. The drawing-room has a belvedere bay, reached from the garden by an external stair, under which is a covered garden seat. A balcony overlooking the garden leads also from the drawing-room, and a billiard room is arranged on the basement level with a separate entrance from the porch. A tradesmen's entrance is provided elsewhere. The kitchen and offices are on the lower floor level, and a kitchen yard is conveniently placed at the rear. Red brick, with cut-brick dressings, is the material used throughout for the walls, the upper parts of which are hung with ornamental tiles. The gables are enriched with wide, massive barge boards, and the roof is surmounted with a white wooden cupola over the principal staircase. The terracotta panels along the entrance front, over the principal floor windows, were designed by Mr. Boehm himself. The work was executed by Mr. H. Batchelor, builder, of Betchworth, and the architect of the house was Mr. R. W. Edis, F.S.A., who superintended its erection.--Building News.
ARTISTS' HOMES No. 14 "BENT'S BROOK."
[Footnote: Lately read before the Institute of Mechanical Engineers.]
The author began by stating that probably in few trades have a smaller number of changes been made during recent years, in the processes employed, than in that of lead smelting and manufacturing. He then briefly noted what these changes are, and went on to describe the "steam desilverizing process," as used in the works of the writer's firm, and in other works licensed by them, which process is the invention of Messrs. Luce Fils et Rozan, of Marseilles. It is one which should commend itself especially to engineers, as in it mechanical means are employed, instead of the large amount of hand-labor used in the Pattinson process. It consists in using two pots only, of which the lower is placed at such a height that the bottom of it is about 12 in. to 15 in. above the floor level, while the upper is placed at a sufficiently high level to enable the lead to be run out of it into the lower pot. The capacity of the lower pot, in those most recently erected, is thirty-six tons--double that of the upper one. Round each pot is placed a platform, on which the workmen--of which there are two only to each apparatus--stand when skimming, slicing, and charging the pots. The upper pot is open at the top, but the lower one has a cover, with hinged doors; and from the top of the cover a funnel is carried to a set of condensers. At a convenient distance from the two pots is placed a steam or hydraulic crane, so arranged that it can plumb each pot, and also the large moulds which are placed at either side of the lower pot. The mode of working is as follows:
The silver lead is charged into the upper pot by means of the crane. When melted, the dross is removed, and the lead run into the lower, or working pot, among the crystals remaining from a previous operation.
When the whole charge is thoroughly melted, it is again drossed; and in order to keep the lead in a thoroughly uniform condition, and prevent it setting solid on the top and the outside, a jet of steam is introduced.
To enable this steam to rise regularly in the working pot, a disk-plate is placed above the nozzle, which acts as a baffle-plate; and uniform distribution of the steam is the result. To quicken the formation of crystals, and thus hasten the operation, small jets of water are allowed to play on the surface of the lead.
This, it might be thought, would make the lead set hard on the surface; but the violent action of the steam acts in the most effectual manner in causing the regular formation of crystals. Owing to the ebullition caused by this action of the steam, small quantities of lead are forced up, and set on the upper edges and cover of the pot. From time to time the valve controlling the thin stream of water playing on the top of the charge is closed, and the workman, opening the doors of the cover in rotation, breaks off this solidified lead, which falls among the rest of the charge, and instantly becomes uniformly mixed with it.
Very little practice enables an ordinary workman to judge when two-thirds of the contents of the big pot are in crystals, and one-third liquid; and when he sees this to be the case, instead of ladling out the crystals ladleful by ladleful, as in the old Pattinson process, he taps out the liquid lead by means of two pipes, controlled by valves, the crystals being retained in the pot by means of perforated plates.
The liquid lead is run into large cone-shaped moulds on either side of the pot; and a wrought iron ring being cast into the blocks thus formed, they are readily lifted, when set, by the crane. To give some idea of the rapidity of the process, it may be mentioned that from the time the lead is melted and fit to work in the big pot, to the time that it is crystallized and ready for tapping, is, in the case of a 36 ton pot, from thirty-five to forty-five minutes; and the time required for tapping the liquid lead into the large moulds is about eight minutes.
Before the lead begins to crystallize, the upper pot is charged with lead of half the richness of that in the lower pot. Thus, when the liquid lead has been tapped out of the lower pot, it is replaced by a similar amount of lead of the same richness as the remaining crystals, by simply tapping the upper or melting pot, and allowing the contents to run among the crystals.
The same operation is repeated from time to time, until the crystals are so poor in silver that they are fit to be melted, and run into pigs for market.
The large blocks of partially worked lead are placed by the crane in a semicircle round it, and pass successively through the subsequent operations. The advantages of the steam process, as compared to the old six-ton Pattinson pots formerly used by the writer's firm, are: (1) a saving of two-thirds amount of fuel used; (2) the saving of cost of calcination of the lead to the extent of at least four-fifths of all that is used; (3) above all, a saving in labor to the extent of two-thirds. The process has its disadvantages, and these are a larger original outlay for plant, and a constant expense in renewals and repairs. This is principally caused by the breakage of pots; but with increased experience this item has been very much reduced during the last two or three years.
The "zinc process" of desilverizing, which is largely used by Messrs. Locke, Blackett & Co., and was patented in the form adopted by them about fourteen years since. The action of this process is dependent on the affinity of zinc for silver. The following is a brief description of it:
A charge of silver lead, usually about fifteen tons, is heated to a point considerably above that which is used in either the Pattinson or the steam process. The quantity of zinc added is regulated by the amount of silver contained in the lead; but for lead containing 50 oz. to the ton, the quantity of zinc used is in most cases about 1½ per cent, of the charge of lead. The lead being melted as described, a portion of this zinc, usually about half of the total quantity required for the charge, is added to the melted lead, and thoroughly mixed with it by continued stirring. The lead is now allowed to cool, when the zinc is seen gradually to rise to the top, having incorporated with it a large proportion of the silver. The setting point of zinc being above that of lead, a zinc crust is gradually formed, and this is broken up and carefully lifted off into a small pot conveniently placed, care being taken to let as much lead drain off as possible. The fire is again applied strongly to the pot, and when the lead is sufficiently heated, a further quantity of zinc, about one-third of the whole quantity used, is added, when the same process of cooling and removing the zinc crust is repeated. This operation is gone through a third time with the remaining portion--¼ per cent.--of zinc; and if each of these operations has been carefully carried out, the lead will be found to be completely desilverized, and will only show a very small trace of zinc. In some works this trace of zinc is allowed to remain in the market lead, but at Messrs. Locke, Blackett & Co.'s works it is invariably removed by subjecting the lead to a high heat in a calcining furnace. The zinc crusts, rich in silver, are freed as far as possible from the lead by allowing this to sweat out in the small pot, after which the crusts are placed in a covered crucible, where the zinc is distilled off, and a portion of it recovered. The lead remaining, which is extremely rich in silver, is then taken to the refinery, and treated in the usual manner. The writer is given to understand that the quantity of zinc recovered is as high as from 50 to 60 per cent. of the total quantity used.
Although it was said that the rolling or milling of lead remains unchanged in its main features since the first mill was established, yet the writer's firm have introduced many important improvements. When lead is required for sheet making, instead of running out the market lead into the usual pigs of about one hundredweight each, it is run into large blocks of 3½ tons. These 3½ ton blocks are taken on a bogie to the mill-house, where the mill melting pot is charged with them by means of a double-powered hydraulic crane, lifting, however, with the single power only.
Three such blocks fill the pot, and when melted are tapped on to a large casting plate, 8 ft. 4 in. by 7 ft. 6 in., and about 7 in. thick. This block, weighing 10½ tons, is lifted on to the mill table by the same crane as fills the pot, but using the double power; and is moved along to the rolls in the usual manner by means of a rope working on a surging head. The mill itself, as regards the roll, is much the same as those of other firms; but instead of an engine with a heavy fly-wheel, always working in one direction, and connected to the rolls by double clutch and gearing, the work is done by a pair of horizontal reversing engines, in connection with which there is a very simple, and at the same time extremely effectual, system of hydraulic reversing. On the usual method there is no necessity for full or delicate control of lead mill engines; but with this system it is essential, and the hydraulic reversing gear contributes largely to such control. This may be explained as follows:
In all other mills with which the writer is acquainted, when the lead sheet, or the original block, has passed through the rolls, and before it can be sent back in the opposite direction, a man on either side of the mill must work it into the grip of the rolls with crowbars.
In the writer's system this labor is avoided, and the sheet or block is fed in automatically by means of subsidiary rolls, which are driven by power. When it is required to cut the block or sheet by the guillotine, or cross-cutting knife, instead of the block being moved to the desired point by hand-labor, the subsidiary driven rolls work it up to the knife; and such perfect control does the engine with its hydraulic reversing gear possess, that should the sheet overshoot the knife 1/8 in., or even less, the engine would bring it back to this extent exactly.
Another point, which the writer looks upon as one of the greatest improvements in this mill, is its being furnished with circular knives, which can be set to any desired width, and put in or out of gear at will; and which are used for dressing up the finished sheet in the longitudinal direction. This is a simple mechanical arrangement, but one which is found to be of immense benefit, and which, in the writer's opinion, is far superior to the usual practice of marking off the sheet with a chalk line, and then dressing off with hand knives. The last length of the mill table forms a weighbridge, and a hydraulic crane lifts the sheet from it either on to the warehouse floor or the tramway communicating with the shipping quay.
The mixing vessel--a porcelain kettle capable of containing twenty liters, made at the Royal Porcelain Factory at Berlin, whose products are unequaled for chemical purposes--is also the boiling vessel, and, therefore, fits tightly, by means of the tin ring with the wooden handles, on to a large water bath. The light-tight metal lid, which can be permanently affixed to the kettle, then supports a stirring arrangement of fine silver, which dips into the emulsion and has blades formed like a ship's screw.
The arrangements for injecting the silver vary. The simplest consists of a large glass vessel containing the silver solution, which is closed by a glass stopper, and terminates below in a funnel running to a fine point. This funnel-shaped bottle fits into an opening specially made for it in the lid of the kettle, and while revolving sends a fine stream into the gelatine. When it is wished to interrupt it, it is only necessary to raise the glass stopper in order to see the stream dry up after a short time.
Another arrangement consists of a contrivance constructed on the principle of the common India-rubber inhaling apparatus, and sends the silver solution into the gelatine in the form of the minutest air-bubbles. After the emulsion is boiled in such a kettle it is allowed to stand until cool, when the ammonia is added. With such a great quantity of emulsion and so large a water bath sufficient heat is retained as to allow the action of the ammonia to take place. As soon as the time set apart for that reaction has elapsed the water bath is emptied and filled with pieces of ice and iced water, and the kettle replaced in it.
If the stirring apparatus be now set in motion, even this large quantity of emulsion will stiffen in at least an hour and a half. It may be further remarked that, the outside of the kettle being black, the lid being light-tight, and all the apertures in it being firmly closed, nearly the whole process can be conducted by daylight, from the mixing to the stiffening, so that it is very convenient to be able to keep the emulsion in the same vessel during all these operations.
It is very desirable that those who do not prepare their emulsion by boiling, but by prolonged digestion, should possess a regulator which will keep the temperature at a given point. Such an apparatus would also be very useful for warming the emulsion for the preparation of plates, as then one would have no further occasion to pay attention to the thermometer and gas stove. In the accompanying diagram a simple contrivance is shown. The gas which feeds the stove passes through a narrow glass tube, a b, into the wider tube, c d e, which is made air-tight at e. This latter tube has an exit tube at f, by which the gas is supplied to the gas stove. At e it is hermetically closed, and at its deepest part it contains mercury, upon which a little sulphuric ether floats in the hermetically-closed limb, e.g. Lastly, there is a minute opening in the narrowest tube at i. The whole apparatus, or, at least, the under part of it, is dipped into the water bath warmed by the gas boiler. It acts thus: As the temperature rises the ethereal vapor in the shorter limb expands and drives the mercury up the longer tube until it closes the opening of the narrow tube, a b, and thereby impedes the power of the stream of gas. Still, the Bunsen burner does not go out, being always fed by the small opening, i, with sufficient gas to support a small flame until the water bath has so far cooled as to leave the opening at b free, when the burner again burns with a strong flame. By removing the cork, c, from the tube the temperature of the water bath is raised, while by pushing it in it is lowered. The apparatus never goes wrong, and is very cheap. It was first made by Herr C. Braun, of Berlin.
FIG. 1.
The apparatus hereafter described is in general use, and was invented by Herr Paul Grundner, of Berlin. It is particularly adapted for finely dividing large quantities of emulsion. It consists essentially of a wooden lid, a b, fitting upon a large stone pot, to the under side of which two strong trapezoid pieces of wood, e d and e f, are fixed, in the under part of which semicircular incisions are cut and held together by two leather straps, supporting a strong, easily-removable iron transverse bar, g h. Through the center of the lid, and turned by the crank, m, passes the axle i, which ends under the lid in the long ring, n.
The stiffened emulsion is then placed in the bag, o p q r, made of fine but strong canvas, with meshes about 0.5 mm. (such as is used for working upon with Berlin wool). The iron rod, g h, is then slipped through the four loops at the bottom of the bag, the open end is slung upon the ring, n, and bound tightly to it by the ribbons, r1. The loops upon the iron bar are then pushed as close together in the middle as possible, and the stone vessel is filled with water until o p q r is completely covered. The crank is then turned, by which the bag is wrung, and the emulsion squeezed through the meshes immediately into the water. When this process is continued until the purse between n and g h feels like a metal rod, the best part of the emulsion has been squeezed through, and if one now take out the bag and dissolve its contents, it will be found that the loss of emulsion is almost nil.
FIG. 2.
It may be remarked that the whole apparatus, with the exception of the crank, must be coated with asphalt varnish; also that the corners, r and q, must be separated off from the purse, as shown by the dotted line, s s s s, otherwise the emulsion would lodge there without being squeezed through. Instead of g h a strong glass rod may be used for small apparatus; but for large apparatus it is indispensable, as the power that requires to be exerted would be far too great for glass.
The fundamental idea of the apparatus shown in Fig. 3 first occurred to Herr Jos. Junk, of Berlin. In the present form all the subsequent improvements made by Herren Carl Such, Paul Grundner, and others are incorporated. It may be described as follows:
A tin vessel, the bottom of which sinks at e into the shape of a funnel, rests upon strong iron feet, f f, and is covered with a lid, having a double edge closing it light-tight. Through the center of the lid passes the tube, g h, by which the water enters. In the interior of the vessel upon iron hooks stands a wooden vessel saturated with paraffine, open at the ends, and over one end of which the finest hair cloth is stretched at o p. The water which enters the vessel runs off through the siphon. The proceedings are as follows: Turn the granulated gelatine and the water in which it is contained into the horsehair sieve, m n o p. Place the lid upon the apparatus and turn on the water. The whole apparatus fills with water until the siphon begins to act. If the diameter of the siphon be properly measured--one inch should be sufficient for the largest apparatus--and the cock by which the water is turned on properly adjusted, more water will run out by the siphon than runs in through the supply pipe, and the apparatus becomes completely empty.
The siphon has then performed its function, the apparatus fills again, and the play begins anew. The tube, g h, which reaches right down nearly to the bottom of the sieve, takes the water so deep into the vessel that, as long as the water in the apparatus stands high enough above o p, the gelatine nodules are in continuous motion. In order to prevent the finest particles of the emulsion from stopping up the pores of the sieve too much, and thereby incurring the danger of the water in the sieve overflowing its upper edge, thus occasioning loss of emulsion, the tube, g h, is now sometimes omitted and replaced by a supply pipe, represented in the diagram by the dotted lines, x y. In this way every possibility of loss is excluded, and yet a very careful washing provided. Then when, after being emptied by the siphon, the apparatus fills again, every particle of the emulsion which might have formerly been pressed down into the interstices of the sieve would now be driven up again by the upward pressure of the water entering from below, and thus the sieve would always be kept clear and open.
FIG. 3.
The great advantages of this apparatus are as follows: 1. From the moment the lid is closed one can work by daylight. 2. The method of washing in moving water is combined with that of complete change of water. 3. The emulsion never comes in contact with metal. 4. Whoever wishes to prepare dry gelatine only requires, when the washing is over and the vessel perfectly emptied, to leave the emulsion to drip for a time, and then to lift out the sieve and its contents and place it in a suitable vessel with absolute alcohol. The latter should be changed once, and when sufficient water has been extracted the sieve should be withdrawn from the vessel and the emulsion allowed to dry spontaneously. In this way all trouble occasioned by changing from vessel to vessel is avoided, and there is no loss of material.
This apparatus is principally valuable in dealing with large quantities, since it saves a great deal of labor, and affords perfect certainty of the emulsion being well washed. It may not be unnecessary to maintain that the difficulties of perfect washing--particularly if one do not wash with running water--increase at least in quadruple proportion to the quantity of emulsion manipulated.--Franz Stoke, Ph.D., in Br. Jour, of Photography.
Numerous complaints have reached me within the last few weeks of the difficulty experienced in preparing emulsion and coating plates; one is very likely to blame everything but the right, but doubtless the weather is the culprit.
I have always held that to boil gelatine is to spoil it, and, even when emulsification is made with a few grains to the ounce and cooled down before adding the bulk, the damage is done to the smaller quantity, so that when mixed it contaminates the whole mass; moreover, it is impossible to set and wash the gelatine without the aid of ice.
I have lately made several batches (with the thermometer at 92° in the shade, and the washing water at 78°) as follows:
Hard gelatine...............,...... ½ ounce. Water.............................. 2 ounces. Alcohol............................ 2 " Bromide ammonia....................150 grains. Liquor ammonia, 880................ 60 drops.
When all is thoroughly dissolved and of about 120° temperature, add, stirring all the time,
Nitrate silver..................... 60 grains, Water.............................. ¾ ounce. Alcohol............................ ¾ "
Then again add,
Nitrate silver.....................140 grains. Water.............................. 1 ounce. Alcohol............................ 1 "
Both solutions being warmed to about 120°.
My object is adding the silver in two quantities will be obvious to many--viz., when the first portion of silver is mixed, nitrate of ammonia is liberated (which is a powerful restrainer), and the bulk of the solution being increased, the remainder of the silver may be added in a much more concentrated state.
The alcohol, both in the gelatine and silver solutions, plays a most important part: (1) It prevents decomposition of the gelatine. (2) It allows the gelatine to be precipitated with a much smaller quantity of alcohol (say about 10 ounces).
After letting the emulsion stand for a few minutes to ripen, I pour in slowly about eight ounces of alcohol, stirring all the time, and keeping the emulsion warm; the emulsion will adhere to the stirring-rod and the bottom of the vessel in a soft mass, and all that is now required is to pour away the alcohol, allow the emulsion to cool, tear it into small pieces, wash in several changes of cold water, make up the quantity to ten ounces, and strain; it is then ready for coating.
By this formula I have no difficulties whatever; my plates set in about five minutes, and their quality is such that, "unless a better method is devised," I intend to adopt it in all weathers.
One word more as to the alcohol. It will prevent the decomposition of gelatine when boiling goes on, or when in the presence of foreign salts; no flocculent deposit is noticed in the alcohol after the emulsion has been precipitated.--Photographic News.
The industrial problem of the rectification of alcohols is based entirely upon the properties of volatile liquids, upon the laws of the maximum tensions of the vapors of these liquids, and upon the influence of temperature upon those different elements which find themselves in presence of each other in an alembic.
If we desire to follow, in their least details, all the phenomena which succeed one another in a rectifying column, and which are connected with one another by a continuous chain of reciprocal influences, the problem becomes exceedingly complex.
PICTET'S APPARATUS FOR THE RECTIFICATION OF ALCOHOL BY COLD.
In order that the new applications of the mechanical theory of heat may be readily understood, we shall divide this problem into a series of propositions, which we shall examine separately, and which collectively constitutes in its general features the methodical rectification of liquids.
I. Knowing the maximum tensions of pure water and pure alcohol, can we calculate directly the tensions of the vapors of any mixture whatever of alcohol and water?
Yes, we can calculate this tension by a general formula, provided we take into account the affinity of water for alcohol, which increases the value of the total latent heat of evaporation of the liquid. The results of the calculation are fully confirmed by experience. We thus establish the following laws:
a. For any temperature whatever, the maximum tension of the vapors of a mixture of water and alcohol is always comprised between that of pure water and that of pure alcohol.
b. The tension of the vapors of a mixture of water and alcohol approaches the tension of alcohol so much the nearer in proportion as the proof is higher; and, reciprocally, if water is in excess, the tension of the vapors approaches the tension of the vapors of water.
c. The curves of the maximum tensions of vapors formed by all mixtures of alcohol and water are represented by the same general formula, one factor only of which is a function of the richness of the alcoholic solution.
It results, then, from these laws that we may determine with the greatest exactness the richness of a solution containing alcohol and water, if we know the tension of the vapors that it gives off at a certain temperature. Such indications are confirmed by the centigrade alcoholmeter.
We see likewise that, for these solutions of alcohol and water, the laws of Dalton are completely at fault, since the total pressure of the vapors is never equal to the sum of the tensions of the two liquids, water and alcohol.
II. Being given a solution of water and alcohol, mixed in equal volumes, what will be the quality of the vapors emitted from it?
In other terms, do the vapors which escape from a definite mixture of water and alcohol also contain volumes of vapor of water and alcohol in the same proportion as the liquids?
We have discovered the following laws:
d. The quality of the vapors emitted by a mixture of water and alcohol varies according to the alcoholic richness of the solution, but is not in simple proportion thereto.
e. The quality of the vapors emitted by a definite mixture of water and alcohol varies according to the temperature.
f. In a same solution of water and alcohol, it is at low temperatures that the vapors emitted by the mixture contain the largest proportion of alcohol.
g. The more the temperature rises the more the tensions of the two liquids tend to become equalized.
We have been able to verify these different laws experimentally, and to find an interesting confirmation of our general formula of maximum tensions, in the following way:
Let us take a test tube containing a 50 per cent. solution of alcohol and water, plunge it into water of 20°C., and put its interior in hermetic communication with the receiver of a mercurial air-pump.
We vaporize at 20° a certain quantity of the liquid, and the vapors fill the known capacity of the pump. The pressure of the gases in the interior is ascertained by a pressure gauge, and this pressure should be constant if care is taken to act upon a sufficient mass of liquid and with moderate speed. When the receiver of the air-pump is full of vapors, communication between it and the test-tube is shut off, and communication is effected with a second test-tube, like the first, plunged into the same water at 20°. Care must be taken beforehand to create a perfect vacuum in this test-tube.
On causing the mercury to rise into the space that it previously occupied, the vapors are made to condense in the second test-tube at the same temperature as that at which they were formed.
We immediately ascertain that the pressure-gauge shows an elevation of pressure; moreover, the proof of the condensed alcohol has very perceptibly risen.
If, instead of causing these vapors to condense in the second test-tube, we leave the first communication open, the vapors recondense in the first test-tube without any elevation of pressure; and we do not see the least trace of liquid forming in the second test tube.
This difference of pressure in the two foregoing experiments must be attributed, then, to the specific action of the water on the vapors of alcohol. Now we can calculate the difference of the work of the pump, and put at 1 kilogramme of condensed liquid the difference of mechanical work represented in kilogrammeters. What is remarkable is that this difference is absolutely the equivalent of the heat disengaged when the condensed liquid and the old liquid are remixed; there is a complete identity. Thus the affinity of the water for the alcohol modifies the tension of the vapors which form or condense upon the free surface of the mixture. The two phenomena are closely connected by the law of equivalence.
It results from all the laws that we have cited that by properly regulating the tensions of the vapors of a mixture of alcohol and water, and the temperature of the liquid, we shall be able to obtain a liquid of a desired richness by the condensation of these vapors.
III. It was likewise indispensable to make sure of one important fact: When the temperature of a liquid like alcohol is considerably lowered, can the distillation of a given weight of this substance be effected with sufficient rapidity for industrial requirements? Repeated experiments with a host of volatile liquids have demonstrated the following laws:
If we introduce a volatile liquid into two spherical receivers connected by a wide tube, and if these be kept at different temperatures after driving out all the air from the apparatus, the liquid distills from the warmer into the cooler receiver, and we ascertain that:
h. The weight of the liquid which distills in the unit of time increases with the deviation of temperature between the two receivers.
i. The weight of the liquid which distills in the unit of time is constant for a same deviation of temperature between the receivers, whatever be, moreover, the absolute temperature of the receivers.
k. The weight of the liquid distilled in the unit of time is proportional to the active surfaces of the receivers; that is to say, to the surfaces which are the seat of passage of heat through their thickness.
l. The least trace of a foreign gas in the vapors left in the apparatus throws the preceding laws into confusion, and checks distillation to a considerable degree, especially at low temperatures.
Thus, water distilling between 100° and 60° will pass over as quickly as that which is distilling between 40° and 0°. Absolute temperature is without influence, provided every trace of air or foreign gas be got rid of.
The distillatory apparatus should be provided with an excellent air-pump, capable of preventing all those entrances of air which are inevitable in practice.
The following is the industrial application that we have endeavored to make of these theoretical views: The rectification of alcohols is one of the most complex of operations; it looks toward several results simultaneously. Alcohol derived from the fermentation of grain, sugar, and of all starchy matters in general, contains an innumerable host of different products, which may be grouped under four principal heads:
1. Empyreumatic essential oils, characteristic of the source of the alcohol, and having a powerful odor which infects the total mass of the crude spirits. 2. A considerable quantity of water. 3. A certain quantity of pure alcohol. 4. A variable proportion of volatile substances, composed in great part of ethers, different alcohols, and bodies as yet not well defined. These latter affect the quality of the alcohol by an odor which is entirely different from that of the essential oils.
The object of rectification is to bring out No. 3 all alone; that is to say, to extract the alcohol in a pure state by ridding it of oils, water, ether, and foreign alcohols.
The alcohol industry never realizes this operation in an absolutely complete manner. All the rectifying apparatus in operation at the present day are based on the use of high temperatures varying between 78.5° and 100°. The successive condensation and vaporization of the vapors issuing from the spirits effect in the rectifying columns a partial separation of these liquids, and there are received successively as products of rectification:
1. Bad tasting alcohols, containing the majority of the ethers and impure alcohols.
2. Fine alcohol.
3. Alcohols contaminated by notable proportions of empyreumatic oils.
Industry knows only one means of obtaining an excellent product, and that is to diminish the quantity of fine alcohol which comes from a same lot of spirits, and to make a large number of successive distillations. Hence the large expenses attending rectification, which produce fine alcohols necessarily at an elevated price. We may remark, in passing, that the toxic action of commercial alcohols is in great part caused by the presence of essential oils, amylic alcohol, and ethers, absolutely pure alcohol, as compared with these, being relatively innocent.
Why is it that our present apparatus cannot produce good results in rectifying alcohol? Because they are limited by the temperature at which they must operate. Between 78° and 100° the tension of the vapors of all the liquids mixed in the spirits is considerable for each of them; they all pass over, then, in certain proportions during the operation of rectification.
We have been led, by examining the theoretical question, to ascertain that the proportion of alcohol which evaporates from a mixture is maximum at low temperatures; consequently, we should seek to establish some arrangement which can realize the following conditions: (1) Render variable, at will, the temperature of the boiling liquid; and (2), render variable the pressure of the vapors which act on the liquid.
Thus, to effect the rectification of alcohol it suffices to cause its ebullition at very low temperatures, and to keep up the ebullition without changing such temperatures when once obtained.
It is exactly these two conditions that we have fulfilled in the apparatus that we have just installed in our factory in Rue Immeubles Industriels, at Paris.
By their arrangement, which is shown in the opposite figure, they form a mechanical system permitting of the rectification of alcohols at temperatures as low as -40° or even -50°. They verify experimentally, by their operation, the theoretical deductions which precede. The boilers, A, which, in an industrial application, may be more numerous, receive their supply of spirits from the country distilleries in the vicinity of the factory. There may even be introduced directly into them vinasses, or washes, that is to say, liquids, such as are obtained by alcoholic fermentation.
Above the boiler rises a rectifying column composed of superposed plates inclined one over the other, and surmounted by a tubular condenser, which serves to effect the retrogression of the first condensation by means of a current of water supplied by the reservoir placed above.
On leaving this condenser, the vapors which have escaped condensation pass into the refrigerator, C, where they are totally condensed by a current of water which goes to the reservoir above.
The first products obtained contain ethers and impure alcohols, which are collected in the reservoir, E.
When the first products have been thus introduced into the reservoir, and it is ascertained by tasting that good alcohol is passing over, the liquid produced is directed into the second boiler, F. The sliding valve, operated by a screw having a very fine pitch, establishes a communication between the refrigerator, C, and the second boiler, F. The office of this valve we shall learn further on. This first rectification is performed in a vacuum, for a system of metallic pipes connects the entire apparatus with an air-pump, O. The temperature at which the liquids shall enter into ebullition in the boilers, A A, may, then, be regulated in advance.
The operations will be carried on with a more or less complete vacuum, according to the nature of the products to be rectified. The distiller will have to be guided in this by practice alone.
The good tasted products are received in boiler No. 2, F, and there the liquids are submitted to the action of an almost absolute vacuum. As we have before said, their temperature falls immediately and spontaneously. The vapors which issue from this liquid contain almost solely pure alcohol. The other substances, which passed over in the first distillation, no longer emit vapors at temperatures ranging between -10° and +5°. Their temperature is shown by a thermometer running into the boiler, F.
These vapors, purified by ebullition at a low temperature, rise into a second rectifying column, G, which terminates in the refrigerator, H, filled with liquid sulphurous anhydride. This refrigerator is like those which we employ in our sulphurous anhydride frigorific apparatus. Under the action of a special pump, M, this liquid produces and maintains a constant temperature of -25° to -30° in the refrigerator. The vapors of alcohol condense therein at this low temperature, and the cold liquid alcohol flows into the lower part of the refrigerator.
By the action of a return cock, a portion of this liquid falls upon the plates of the column, G, and descends, while the vapors are rising therein. The other portion of the liquid obtained flows into the reservoir, K, at the beginning of the operation, and into the reservoir, L, during all the remainder of the rectification. The ice-making machine keeps up of itself alone the two operations.
In fact, the exhaust of the steam engine which actuates the sulphurous anhydride pump is directed into a worm which circulates through the first boiler, A, and the refrigerator, H, of the frigorific machine keeps up the second rectification, which was brought about below the surrounding temperature, and which for this reason takes place without necessitating any combustion of coal. It suffices to cause the current of water which issues from the condenser of the frigorific machine to pass into the worm of the boiler.
We have, then, two results, two like operations, both produced by the working of a single machine. Moreover, these two operations are performed in vacuo, and we know that under these conditions they are effected at lower temperatures. Owing to this fact, likewise, the weight of the water that must be evaporated diminishes just so much. Now, one kilogramme of water requires 636 heat units to cause it to pass from the liquid to the gaseous state, while one kilogramme of alcohol requires only 230 heat units to vaporize it. Thus every decrease of temperature in rectification has for an immediate corollary an important economy of fuel, which is proved by the diminution of radiation, and by the less quantity of water to be distilled.
Between the boilers, A, in which is maintained a temperature bordering on +50° to +60°, and the refrigerator, H, in which is easily obtained a temperature of -30° to -40°, there is at our disposal a range of temperature of nearly 100°, an immense difference compared with that which can be made use of in ordinary apparatus. Thanks to this powerful factor, which is manageable at will, we can take directly from the apparatus alcohols marking 98 and 99 degrees by the centigrade alcoholmeter. Such results are unobtainable by the usual methods.
We have likewise ascertained that at low temperatures the ebullition of alcohol is as active as at near 100°.
For a same range of temperature between the boiler and the refrigerator, the weight of alcohol which distills in an hour is constant. By the operation of the valve, D, it becomes easy to allow all the liquid condensed in the first refrigerator to pass into the second boiler; and thus the second rectification, which is effected in a more perfect vacuum, is supplied with exactness. The object of this valve, then, is to allow the liquid to pass, and yet to cut off the pressure in such a way as to have a double fall of temperature throughout the whole apparatus; from 60° to 20° in the first operation, and from 0° to -40° in the second. We may add that the regulation of the valve is extremely easy, because of the screw which actuates it.
To sum up the commercial advantages that our process procures, we may say that it realizes the following desiderata: 1. With the cost of a single distillation we have, at once, distillation and rectification, or a single expense for two results. 2. With one operation at a low temperature we obtain products which are almost impossible to get even by an indefinite number of rectifications at a high temperature, the temperature having an intrinsic value in the operation. 3. The alcohols obtained are wholesome, and can be put on the market without danger. 4. Their superior quality gives these alcohols an extra value difficult to calculate, but which is very notable. 5. The whole operation being performed in closed vessels, there is absolutely no waste. 6. For the same reason there is scarcely any danger of fire. 7. The management of the works and the service are performed by the pressure of the gases entirely; there are only a few cocks to be turned to perform all the interior maneuvers, empty and fill the vessels, etc. Hence economy in personnel.
[Footnote: NOTE.--Each of these determinations was accompanied by a series of results in which the practical determinations obtained from the method described were compared with the theoretical contents of the solutions of the various elements. These, however, would take up too much room for insertion in these columns.]
Ever since the electrolytic method for the estimation of copper came into general use, numerous chemists have endeavored to adapt this peculiarly simple and elegant method to the determination of other metals. According to the experiments which have been made up to the present time, it has been found that the separation of copper is best effected in a nitric acid solution, while that of nickel and cobalt takes place most readily in an ammoniacal solution, and for the precipitation of zinc and cadmium a potassium cyanide solution is the best. The accuracy of the results depend chiefly upon the following of certain fixed rules, such as, for instance, that the precipitation of copper only takes place when there is a definite amount of nitric acid in the solution; that of cobalt and nickel when a certain quantity of ammonium hydrate and ammonium sulphate is present. The electrolytic decomposition of the chlorides has not yet been successfully accomplished, so that prior to the operation it is necessary to convert them into sulphates. The experiments which have been made for the purpose of investigating the application of the electric current in quantitative analyses are very few, about the only exception being the separation of copper from the metals which are not precipitated from a nitric acid solution, or which are deposited as peroxides at the other electrode. We shall endeavor to show in that which follows, that copper, zinc, nickel, and cobalt, and even iron, manganese, cadmium, bismuth, and tin, whether they be present as sulphates, chlorides, or nitrates, may be precipitated and separated from each other by electrolytic methods much more rapidly than by any previously known process.
Neutral potassium oxalate is added in excess to the solution of a cobalt salt, and the clear solution of cobalt potassium oxalate submitted to electrolysis. The intense red color of this solution is soon changed into a dark green; the latter diminishing in intensity as the metal is deposited at the negative electrode. The electric current decomposes the potassium oxalate into the carbonate, so that a precipitate of cobalt carbonate is simultaneously formed with the separation of the metallic cobalt. This precipitate may be dissolved by adding oxalic acid or dilute sulphuric acid; the further action of the current will change the solution to an alkaline reaction, upon which the treatment with acid is repeated until all the cobalt has been separated out in its metallic condition. The electrolytic separation of cobalt is much more easily and rapidly effected when the potassium oxalate is substituted by the corresponding ammonium salt, as the latter forms a soluble double salt with the cobalt compounds. If the ammonium oxalate added is just sufficient to form the double salt, a red cobalt oxalate (which is only slowly reduced by the current) will separate out in addition to the cobalt. In order to obviate this difficulty, the solution to which the ammonium oxalate had been added in excess is heated, and then three or four grammes more of solid ammonium oxalate are added. The hot solution, when exposed to the action of the current, deposits the cobalt as a closely adhering gray film. By the aid of two Bunsen's elements, 0.2 gramme cobalt can be separated in an hour's time. When the reduction has been completed, and this is best determined by testing a small sample (removed by a pipette) with ammonium sulphide, the positive electrode[1] is removed from the solution, and the liquid poured off. The dish is immediately rinsed several times with water, and the excess of water removed at first with alcohol, and then with absolute ether. The cobalt in the dish is dried in the air bath at 100° C., and in the course of a few minutes a constant weight is obtained.
[Footnote 1: A piece of platinum foil, 4.5 cm. in diameter, is used for the positive electrode, and a deep platinum dish as the negative electrode.--Vide "Classen's Quantitative Analysis," 3d Edition, p. 46.]
This process is precisely identical with the previously described method for cobalt. The ammonium oxalate is added in excess to the solution, which is then heated, and four more grammes of the solid salt added. The separation of the nickel is as rapid as that of the cobalt. The nickel is precipitated as a gray, compact mass, tightly adhering to the electrode.
For this estimation, solutions of the chloride as well as those of the sulphate (ammonium, iron, alum) may be used in the manner previously described. The electrolysis is best effected in the presence of a sufficient quantity of ammonium oxalate; no separation of any iron compound takes place. The iron is deposited in the form of a bright, steel gray, firmly-adhering mass on the platinum dish. The iron may be exposed to the air for several days without any noticeable oxidation taking place.
Zinc may be separated from a solution of the double salt fully as easily and rapidly as the previously mentioned metals were. The reduced zinc has a dark gray color, and adheres very firmly to the electrode. The separated metal is dissolved by using dilute acids and heating. It is only removed with difficulty, and generally leaves a dark coating on the dish, which is separated by repeated ignitions and treatment with acid.
It is already known that manganese may be separated as the peroxide from its nitric acid solution. We find, however, that the precipitation is only completely effected when the quantity present is small; the amount of nitric acid must also be slight, and it is necessary to wash the dish without interrupting the current. If the manganese is converted into the soluble double salt, prepared by adding an excess of potassium, and submitted to the electric current, the whole of the manganese will be deposited at the positive electrode. When ammonium oxalate is used, the complete precipitation does not take place. As the separated peroxide does not adhere firmly to the electrode, it is necessary to filter it and convert it, by ignition, into the trimangano-tetroxide (Mn3O4).
This separation presents considerable difficulty, because the metal is not precipitated as a compact mass on the platinum. The bismuth is always obtained in the same form, no matter whether it is precipitated from an acid solution, or from the double ammonium oxalate, or, finally, from a solution to which potassium tartrate has been added. As large a surface as possible must be used, and the dish piled to the rim; then, if the quantity of bismuth is small, the washing with water, alcohol, and ether may be effected without any loss of the element. If small quantities of the metal separate from the dish, they must be collected on a tared filter, and determined separately. In our experiments, an excess of ammonium oxalate was added to a nitric acid solution of bismuth. During the electrolytic decomposition, a separation of the peroxide was observed at the positive electrode, which, however, slowly disappeared. In order to prevent the reduced metal from oxidation, the last traces of water are completely removed by repeated washings with alcohol and anhydrous ether.
The nitric solution of lead acts similarly to that of manganese. When the amount of peroxide separated is so large that it does not adhere firmly, and becomes mechanically precipitated on the negative electrode, it becomes impossible to complete the estimation without loss from the solution of the peroxide, and the results cannot be accepted.
If the double oxalate is submitted to electrolysis, the whole of the lead is separated out in its metallic state, but it is so rapidly oxidized by the air that it is very seldom that it can be dried without decomposition even when the operation is conducted in a current of illuminating gas. The electrolytic estimation of this element cannot be recommended.
The copper may be very easily and rapidly separated from the double ammonium oxalate salt, provided a sufficient excess of ammonium oxalate is present. Weak currents cannot be employed for the determination of this element when it is present in large quantities, for under such circumstances the metal does not adhere with sufficient firmness to the electrode. We employed a current which corresponded to an evolution of 330 c.c. of gas per hour, and we were able to precipitate 0.15 gramme metallic copper in about twenty-five minutes.
When the cadmium ammonium oxalate is submitted to the action of the electric current, the metal is thrown down in the form of a gray coating, which does not adhere very firmly to the electrode, but, however, sufficiently so as not to become separated on careful washing.
Tin may be easily estimated by electrolysis; it can be separated from its hydrochloric acid solution, or from its double salt with ammonium oxalate, as a beautiful silver gray coating on the platinum. When the ammonium oxalate is substituted by the potassium salt, the operation becomes more difficult, as a basic salt is formed at the opposite pole, and is not easily reduced. If the tin is separated from an acid solution, the current must not be interrupted while the washing takes place, a precaution which it is not necessary to follow when the ammonium oxalate is used. When the tin is dissolved from the platinum dish, it acts like the zinc; that is to say, a black coating is left on the electrode.
Antimony may be precipitated in its metallic state from a hydrochloric acid solution, but it does not adhere very firmly to the electrode. If potassium oxalate is added to a solution of the trichloride, the antimony may be readily reduced, but the metal adheres still less firmly to the electrode than it did in the first instance. An adherent coating may be obtained by adding an alkaline tartrate, but in that case the separation takes place too slowly. The precipitation of antimony may be very readily effected from solutions of its sulpho salts.
To a liquid, which may contain free hydrochloric acid, hydrogen sulphide is added, then neutralized with ammonium hydrate, and saturated with ammonium sulphide in excess. The reduction may be accelerated by the addition of some ammonium sulphate. The antimony separates out as a fine, light gray precipitate on the electrode, and which adheres very firmly, provided the precipitation has not been carried on too rapidly, i. e., if the current employed for the reduction was not too strong.
When the reduction has been completed, the supernatant liquid is poured off, and the residue washed in the ordinary manner.
Arsenic cannot be completely separated from either its aqueous hydrochloric acid, or from a solution to which ammonium oxalate has been added in excess. From its aqueous as well as from its oxalate solution, a portion of the metal may be separated, but if the current is passed through its hydrochloric acid solution for a sufficient length of time, all the arsenic will be volatilized as arsenious hydride (AsH3).
If a solution of ferric oxide and manganese ammonium oxalate is submitted to electrolysis, without the previous addition of ammonium oxalate, the characteristic color of permanganic acid immediately makes its appearance, and the peroxide gradually precipitates itself on the positive, while the iron is deposited on the negative electrode. When the examination is made in the above manner, it is impossible to separate the two metals, for the peroxide will bring down with it a considerable quantity of ferric hydrate. The separation of the two metals is only possible when the precipitation of the manganese peroxide is prevented, until the greater portion of the iron has been deposited. This result may be attained by adding sodium phosphate, or, better still, by the addition of ammonium oxalate in great excess. In both cases the characteristic coloration from permanganic acid is developed by the action of the current at the positive pole; this, however, disappears in the direction of the negative electrode. After the greater portion of the ammonium oxalate has been converted into carbonate, the coloration and necessarily the formation of manganese peroxide begins.
Ammonium oxalate is added to the solution, and heat applied; then three or four grammes more of ammonium oxalate are dissolved in the liquid, which is then immediately submitted to electrolysis. When the amount of manganese is small, the separation of the two elements takes place very rapidly, and the results are accurate. If the amount of manganese is more than double that of iron, the separation of the latter will take a much longer time. Then, in order to effect a complete separation of the two elements, it is necessary to redissolve the deposited manganese in oxalic acid (the acid is added, without interrupting the current, until the liquid becomes red), and the current is allowed to continue its action.
It was found desirable, in effecting this separation, not to employ too strong a current (two Bunsen elements will suffice), and only to increase the strength of the current when it is necessary, in consequence of a large amount of manganese being present, to redissolve the peroxide.
When the process is completed, it is not advisable to allow the current to act any longer, for otherwise some of the peroxide may adhere firmly to the iron, and the latter (after previously having poured off the liquid) must be redissolved in oxalic acid, that is to say, the electrolysis must be repeated. As has been already mentioned in the determination of manganese as peroxide, its precipitation from ammonium oxalate is not complete. The solution which contains the greater portion of manganese, suspended as peroxide, must first, therefore, be boiled to decompose the ammonium carbonate; the remainder of the ammonium oxalate is neutralized with nitric acid, and the manganese converted into the sulphide by ammonium sulphide. The manganese sulphide is then ignited in a current of hydrogen, and weighed as such.
The quantitative separation of iron from aluminum, which presented many difficulties according to the older methods, may be easily performed by electrolysis. If a solution of iron ammonium oxalate and aluminum oxalate, to which an excess of ammonium oxalate has been added, be submitted to the action of the electric current, the iron will be deposited as a firmly adhering coat on the negative electrode, while the aluminum oxide remains in solution, just so long as the quantity of ammonium oxalate is in excess of the quantity of ammonium carbonate produced. When, finally, a precipitation of aluminum oxide takes place the liquid is almost free from iron. From time to time, the solution, in which the aluminum oxide is suspended, is tested for iron by ammonium sulphide, and the current is interrupted when no further reaction is observed. The best method of procedure is to add ammonium oxalate in excess to a neutral, a slightly acid solution, or to one which has been neutralized by the addition of ammonium hydrate (a hydrochloric acid solution is not well adapted for this purpose); then as much more solid ammonium oxalate is added until for every 0.1 gramme there is 2 to 3 grammes of the oxalate present. The hot solution is then directly submitted to the action of the electric current. After the iron has been precipitated, it is best to stop the action of the current before all the aluminum oxide is thrown down, for otherwise a portion of the latter may adhere firmly to the iron, and be difficult to remove.
In such a case, as was mentioned previously in the separation of iron from manganese, it is necessary to redissolve the iron (after previously having poured off the liquid) in oxalic acid, and then the electrolysis is continued.
In order to effect the complete precipitation of the aluminum oxide from the solution which was poured from the iron, ammonium hydrate is added, and the solution boiled for some time, and then the aluminum oxide is determined in the usual manner. When the quantity of aluminum is less than that of iron, this method may be relied upon to give exact results. With the reverse (i. e., an excess of iron) the precipitate of aluminum oxide must be dissolved in oxalic acid (without the interruption of the current), and the electrolysis continued.--Berichte der Deutschen Chemischen Gesellschaft, 14, 1662.
In accordance with an announcement in the March number of the Naturalist, the editor of this department has sent out the seed of two species of pyrethrum, viz. P. roseum and P. cinerarioefolium, to a large number of correspondents in different parts of North America. Every mail brings us some inquiries for further particulars and directions to guide in the cultivation of the plant and preparation of the powder. We have concluded, therefore, that such information as is obtainable on these heads will prove of public interest, and we shall ask Professor Bessey's pardon for trenching somewhat on his domain.
There are very few data at hand concerning the discovery of the insecticide properties of pyrethrum. The powder has been in use for many years in Asiatic countries south of the Caucasus mountains. It was sold at a high price by the inhabitants, who successfully kept its nature a secret until the beginning of this century, when an Armenian merchant, Mr. Jumtikoff, learned that the powder was obtained from the dried and pulverized flower-heads of certain species of pyrethrum growing abundantly in the mountain region of what is now known as the Russian province of Transcaucasia. The son of Mr. Jumtikoff began the manufacture of the article on a large scale in 1828, after which year the pyrethrum industry steadily grew, until to-day the export of the dried flower-heads represents an important item in the revenue of those countries.
Still less seems to be known of the discovery and history of the Dalmatian species of pyrethrum (P. cinerarioefolum), but it is probable that its history is very similar to that of the Asiatic species. At the present time the pyrethrum flowers are considered by far the most valuable product of the soil of Dalmatia.
There is also very little information published regarding either the mode of growth or the cultivation of pyrethrum plants in their native home. As to the Caucasian species we have reasons to believe that they are not cultivated, at least not at the present time, statements to the contrary notwithstanding.[1]
[Footnote 1: Report Comm. of Patents, 1857, Agriculture, p. 130.]
The well-known Dr. Gustav Radde, director of the Imperial Museum of Natural History at Tiflis, Transcaucasia, who is the highest living authority on everything pertaining to the natural history of that region, wrote us recently as follows: "The only species of its genus Pyrethrum roseum, which gives a good, effective insect powder, is nowhere cultivated, but grows wild in the basal-alpine zone of our mountains at an altitude of from 6,000 to 8,000 feet." From this it appears that this species, at least, is not cultivated in its native home, and Dr. Radde's statement is corroborated by a communication of Mr. S. M. Hutton, Vice-Consul General of the U. S. at Moscow, Russia, to whom we applied for seed of this species. He writes that his agents were not able to get more than about half a pound of the seed from any one person. From this statement it may be inferred that the seeds have to be gathered from the wild and not from the cultivated plants.
As to the Dalmatian plant it is also said to be cultivated in its native home, but we can get no definite information on this score, owing to the fact that the inhabitants are very unwilling to give any information regarding a plant the product of which they wish to monopolize. For similar reasons we have found great difficulty in obtaining even small quantities of the seed of P. cinerarioefolium that was not baked or in other ways tampered with to prevent germination. Indeed, the people are so jealous of their plant that to send the seed out of the country becomes a serious matter, in which life is risked. The seed of Pyrethrum roseum is obtained with less difficulty, at least in small quantities, and it has even become an article of commerce, several nurserymen here, as well as in Europe, advertising it in their catalogues. The species has been successfully grown as a garden plant for its pale rose or bright pink flower-rays. Mr. Thomas Meehan, of Germantown, Pa., writes us: "I have had a plant of Pyrethrum roseum in my herbaceous garden for many years past, and it holds its own without any care much better than many other things. I should say from this experience that it was a plant which will very easily accommodate itself to culture anywhere in the United States." Peter Henderson, of New York, another well-known and experienced nurseryman, writes: "I have grown the plant and its varieties for ten years. It is of the easiest cultivation, either by seeds or divisions. It now ramifies into a great variety of all shades, from white to deep crimson, double and single, perfectly hardy here, and I think likely to be nearly everywhere on this continent." Dr. James C. Neal, of Archer, Fla., has also successfully grown P. roseum and many varieties thereof, and other correspondents report similar favorable experience. None of them have found a special mode of cultivation necessary. In 1856 Mr. C. Willemot made a serious attempt to introduce and cultivate the plant[1] on a large scale in France. As his account of the cultivation of pyrethrum is the best we know of we quote here his experience in full, with but few slight omissions: "The soil best adapted to its culture should be composed of pure ground, somewhat silicious and dry. Moisture and the presence of clay are injurious, the plant being extremely sensitive to an excess of water, and would in such case immediately perish. A southern exposure is the most favorable. The best time for putting the seeds in the ground is from March to April. It can be done even in the month of February if the weather will permit it. After the soil has been prepared and the seeds are sown they are covered by a stratum of ground mixed with some vegetable mould, when the roller is slightly applied to it. Every five or six days the watering is to be renewed, in order to facilitate the germination. At the end of about thirty or forty days the young plants make their appearance, and as soon as they have gained strength enough they are transplanted at a distance of about six inches from each other. Three months after this operation they are transplanted again at a distance of from fourteen to twenty inches, according to their strength. Each transplantation requires, of course, a new watering, which, however, should only be moderately applied. The blossoming of the pyrethrum commences the second year, toward the end of May, and continues to the end of September." Mr. Willemot also states that the plant is very little sensitive to cold, and needs no shelter, even during severe winters.
[Footnote 1: Mr. Willemot calls his plant Pyrèthre du Caucase (P. Willemoti. Duchartre), but it is more than probable that this is only a synonym of P. roseum. We have drawn liberally from Mr. Willemot's paper on the subject, a translation of which may be found in the Report of the Commissioner of Patents for the year 1861, Agriculture, pp. 223-331.]
The above quoted directions have reference to the climate of France, and as the cultivation of the plant in many parts of North America is yet an experiment, a great deal of independent judgment must be used. The plants should be treated in the same manner as the ordinary Asters of the garden or other perennial Compositae.
As to the Dalmatian plant, it is well known that Mr. G. N. Milco, a native of Dalmatia, has of late years successfully cultivated Pyrethrum cinerarioefolium near Stockton, Cal., and the powder from the California grown plants, to which Mr. Milco has given the name of "Buhach," retains all the insecticide qualities and is far superior to most of the imported powder, as we know from experience. Mr. Milco gives the following advice about planting--advice which applies more particularly to the Pacific coast: "Prepare a small bed of fine, loose, sandy, loamy soil, slightly mixed with fine manure. Mix the seed with dry sand and sow carefully on top of the bed. Then with a common rake disturb the surface of the ground half an inch in depth. Sprinkle the bed every evening until sprouted; too much water will cause injury. After it is well sprouted, watering twice a week is sufficient. When about a month old, weed carefully. They should be transplanted to loamy soil during the rainy season of winter or spring."
Our own experience with P. roseum as well as P. cinerarioefolium in Washington, D. C., has been so far quite satisfactory. Some that we planted last year in the fall came up quite well in the spring and will perhaps bloom the present year. The plants from sound seed which we planted this spring are also doing finely, and as the soil is a rather stiff clay and the rains have been many and heavy, we conclude that Mr. Willemot has overstated the delicacy of the plants.
In regard to manufacturing the powder, the flower heads should be gathered during fine weather when they are about to open, or at the time when fertilization takes place, as the essential oil that gives the insecticide qualities reaches, at this time, its greatest development. When the blossoming has ceased the stalks may be cut within about four inches from the ground and utilized, being ground and mixed with the flowers in the proportion of one-third of their weight. Great care must be taken not to expose the flowers to moisture, or the rays of the sun, or still less to artificial heat. They should be dried under cover and hermetically closed up in sacks or other vessels to prevent untimely pulverization. The finer the flower-heads are pulverized the more effectually the powder acts and the more economical in its use. Proper pulverization in large quantities is best done by those who make a business of it and have special mill facilities. Lehn & Fink, of New York, have furnished us with the most satisfactory powder. For his own use the farmer can pulverize smaller quantities by the simple method of pounding the flowers in a mortar. It is necessary that the mortar be closed, and a piece of leather through which the pestle moves, such as is generally used in pulverizing pharmaceutic substances in a laboratory, will answer. The quantity to be pulverized should not exceed one pound at a time, thus avoiding too high a degree of heat, which would be injurious to the quality of the powder. The pulverization being deemed sufficient, the substance is sifted through a silk sieve, and then the remainder, with a new addition of flowers, is put in the mortar and pulverized again.
The best vessels for keeping the powder are fruit jars with patent covers or any other perfectly tight glass vessel or tin box.--American Naturalist.
In a paper read before the Aix-la-Chapelle section of the Verein deutscher Ingenieure, Herr Robert Hasenclever presents a summary of the results obtained with various methods for the absorption of the sulphurous acid generated during the roasting of zinc-blende and other sulphurets. Though most of our own metallurgical works are not so located as to be forced to pay much attention to the removal of noxious vapors, the efforts made abroad possess some interest for American metallurgists. Besides containing sulphurous acid, the gases from the roasting furnaces hold varying quantities of sulphuric acid, and Dr. Bernoulli describes a process applied on a large scale in Silesian zinc works, where the gases were passed through towers filled with lime. It was found that there was no trouble on account of the absorption of carbonic acid by the lime, and that the latter acted very efficiently in reducing the quantity of sulphurous acid. Before entering the tower, they contained 0.258 per cent. by volume of sulphurous acid and 2.45 per cent. of carbonic acid; while, after their passage through it, they held 0.017 and 2.478 per cent, respectively. The process, however, is declared by Herr Hasenclever to be too costly for ordinary working, although he does not deny its value under special circumstances.
The removal of anhydrous sulphuric acid from the gases from roasting-furnaces has hitherto, as at the Waldmeister works, near Stolberg, been effected by means of water trickling down in a tower filled with coke, the gases entering below and moving upward. Herr Hasenclever tested the Freytag method, in which the water is replaced by sulphuric acid, and obtained favorable results, as shown by the following analyses of the gases before and after treatment. The figures given are grammes per 1,000 liters:
| BEFORE. | AFTER. | |||
| SO2. | SO3. | SO2. | SO3. | |
| 8.24 | 0.63 | 5.74 | 0.00 | |
| 8.29 | 0.37 | 6.74 | 0.07 | |
| 9.36 | 0.69 | 6.96 | 0.00 | |
| 9.46 | 0.63 | 7.38 | 0.05 | |
| 10.03 | 1.08 | 7.69 | 0.09 | |
| 16.52 | 2.97 | 14.39 | 0.23 | |
| 17.90 | 1.97 | 13.32 | 0.11 | |
| 17.80 | 2.46 | 16.18 | 0.69 | |
The average absorption for the first set of four analyses when three roasting-furnaces were discharging into the tower was 95 per cent. of the sulphuric acid, and that of the second set of four or five furnaces was 90 per cent. The amount of sulphuric acid charged per twenty-four hours was about 5,000 kilogrammes of 50 degrees Baumé, which flowed off with a density of from 56 to 58 degrees Baumé. The quantity of acid condensed varied according to the nature of the ores and the number of furnaces working. It ranged between 300 and 1,000 kilogrammes of 60 degrees Baumé per twenty-four hours. The condensation of anhydrous sulphuric acid would pay, according to estimates submitted by Herr Hasenclever; but to pass the gases through a tower filled with lime, in order to get rid of the remaining sulphurous acid, would prove too expensive at Stolberg. An attempt to use milk of lime proved partially successful; but it was not followed up, because it was decided to experiment with the process suggested by Prof. Cl. Winkler, of Freiberg, who proposes to pass the gases through a tower filled with iron in some suitable shape, over which water trickles. From the solution thus obtained, sulphurous acid pure enough to be used for the manufacture of sulphuric acid, sulphur, and a solution of green vitriol is made. Experiments with this process are making at Freiberg and at the Rhenania Works, near Stolberg. The trouble with the majority of methods thus far is, that the draught of the furnaces is so much impeded by the absorption towers that fans, blowers, or steam jets must be used to carry the gases through it.
The experience of Herr Hasenclever has proved how difficult it is to find a satisfactory means of removing the noxious vapors from furnace gases without incurring too serious an expense. Thus far the value of the products obtained by absorption of sulphurous acid has not been equal to the cost of producing them. Herr C. Landsberg, who is general manager of the Stolberg Company, has had similar experience, though his experiments were made to test methods suggested at various times by Dr. E. Jacob and Dr. Aarland. Both are very ingenious, and were successful on a small scale, but failed when tried in actual working.--Engineering and Mining Journal.
In common practice, the new exhauster at the Old Kent Road passes about five million cubic feet of gas per day of twenty-four hours, and requires the attention of two men and two boys for driving and stoking, at the following cost:
s. d.
Wages--2 men, at 5s. 6d 11 0
Wages--2 boys, at 3s. 6d 7 0
----£ 0 18 0
Oil, 1 gallon 0 3 6
Waste, 5 lb 0 1 0
--------
Total £ 1 2 6
for five million cubic feet, or 0.054d. per 1,000 feet. The boiler burns a mixture of coke and breeze, chiefly the latter, of small value, costing 0.0174d. per 1,000 feet of gas exhausted; therefore the total cost of exhausting gas by the new system is--
Fuel 0.0174d
Wages, oil, and waste 0.0540
--------
Total 0.07l4d.
per 1,000 cubic feet of gas, exclusive of repairs, which will be decidedly less for the new exhauster than for that on the older system, from the friction being so much less. The feed water evaporated is at the rate of about 7.4 lb. per pound of breeze, and 7.5 lb. per pound of coke.
IMPROVED GAS EXHAUSTER.
It will be seen that the exhausting arrangements at the Old Kent Road are extremely economical, the cost of fuel being reduced to a minimum; while a man and boy by day, and their reliefs for the night, attend to the machinery inside the exhauster-house, and also to the pumps outside, and stoke the boiler as well.--Journal of Gas Lighting.
The continued advance in the price of glycerine continues to excite comment among those who deal in or use it, and no one seems to know exactly where or when the advance is likely to stop, or by what means a retrograde movement will probably be brought about.
As we have heretofore stated, the rise has been brought about by a combination of two causes--a falling off in production and a great increase in the demand, owing to the discovery of new uses for it, and the extension of the branches of manufactures in which it has been heretofore employed.
In pharmacy, it is coming more and more into use daily, and in various other branches of manufacture the same tendency is observable. It has proved itself so elegant and so convenient a vehicle for the administration of various medicinal substances, is so easily miscible with both water and alcohol, and is so pleasant to the taste, that it seems almost a wonder that it should have been so long in attaining the rank among the articles of the Materia Medica which it now occupies. The two manufactures, however, which seem to lead in the demand for glycerine are of nitro-glycerine and of oleomargarine.
The uses to which it is put for the former are well known, but precisely what the latter could want of the article is not, at first glance, quite so obvious. We are informed, however, that it is valued for its antiseptic properties, and also for its softening effect on the quasi butter. Be this as it may, it seems that both here and in Europe the makers of these two articles are buying largely of both crude and refined glycerine.
So it appears that the willingness of the people to eat artificial butter, and the progress in schemes for internal improvement, such as the De Lesseps Canal, for instance, to say nothing of the European revolutionists, are responsible to a great extent for the scarcity of an important article of pharmaceutical use.
On the other hand, while there is a notable increase in the demand for the article, there is a gradual but very sure and noticeable falling off in the production.
At present the supply for the whole world comes from the candlemakers of Europe--chiefly France and Germany--and, as improved methods of illumination push candles out of the drawing rooms of the wealthier as well as the cabins of the poor, and consequently out of the markets, the production of glycerine naturally grows less. In France, for instance, candles are coming to be regarded among the wealthy chiefly as articles of luxury, and are lighted only for display at festivals of especial magnificence and ceremony, while among the poor the kerosene lamp is coming into almost as universal use as here.
To be sure, the inexorable inn-keeper still keeps up, we believe, the inevitable bougie, but even that is fast becoming more of a fiction than ever. Even in the churches, it is said, the use of candles is gradually falling off. To these causes must be attributed the decreasing supply of the crude material, but it may be doubted whether this decrease would be sufficient to materially affect the price for some time to come were it not for the increased demand for the two industries to which we have alluded. Obviously, there must be found eventually some substitute for glycerine, or else some new source from which it may be procured. The natural place to look for this would be in the waste lye from the soapmakers' boilers, but so far no one has succeeded in obtaining from this substance the glycerine it undoubtedly contains by any process sufficiently cheap to allow of its profitable employment.
We are assured by a veteran soap-boiler who has experimented much in this direction that it is impossible to recover a marketable article of glycerine from the lees of soap in which resin is an ingredient. In his words, it "kills the glycerine," and, as none but a few of the finest soaps are now made without resin, it would seem that the search for glycerine in this direction must be a hopeless one. It is a curious commentary in the present state of affairs that previous to about 1857, when candles were largely manufactured in this country, there was little or no demand for glycerine, and millions of pounds of it were run into the sewers. Even then, however, the use of it as a wholesome and pleasant article of diet was known to the workmen employed in the candle factories, who were accustomed to drink freely of the mingled glycerine and water which constituted the waste from the candles. Yet with this fact under their noses, as it were, it is only recently that members of the medical profession have begun to recommend the same use of glycerine as a substitute for cod liver oil.--Pharmacist.
Oils, fats, waxes, and bodies somewhat similar in nature, may--according to the substance of a paper recently read before the Chemical Society, by Mr. A. H. Allen, of Sheffield, and Mr. Thomson, of Manchester--be divided into two great classes, viz., those which combine with soda, potash, or other alkalies to form soaps, and those which do not; and as those two classes of bodies differ materially in their actions on substances such as iron, copper, etc., with which they come in contact, it often becomes a question of great importance to the users of oils for lubricating purposes to know what proportions of these different substances are contained in any oil or mixture of oils. The object of the authors was to give accurate methods for determining the percentages of these bodies contained in any sample. Hydrocarbon or mineral oils are now much used for lubricating the cylinders of engines, and especially of condensing engines, and that for two reasons--first, because they are neutral bodies, which have no action on metals; and, second, that they are not liable to deposit on the boilers, if they should happen to be introduced with the condensed water so as to produce burning of the ironwork over the flues.
Animal or vegetable oils or fats are composed of fatty acids in combination with glycerine, and these, under the influence of high-pressure steam, are decomposed or dissociated, the fatty acids being liberated from the glycerine, leaving the former to act upon or corrode the iron of the cylinder. But here their objectionable influence does not end. They form with the iron hard, insoluble compounds called iron soaps, which increase the friction between the cylinder and piston, and in some cases gradually collect into the form of hard balls inside the cylinder.
When the water is used over and over again a considerable proportion of the fatty acids of the oils used for lubricating the piston is carried over with the steam and is found in the condensed water which is introduced into the boiler along with the water. Here it commences action, which proves quite as injurious to the boiler as it does to the cylinder, but in a different way. It acts upon the iron of the boiler and on some of the lime salts which constitute the incrustation, forming greasy iron and lime soaps, which prevent the water from coming into absolute contact with it. Thus the heat cannot be drawn away quickly enough by the water, and the plates thus coated above the flues are liable to become burdened and weakened. This action has in many cases gone on to such an extent that the flues have collapsed under the pressure of the steam inside.
The authors give two different processes for the determination of animal or vegetable oils or fats and hydrocarbon or other neutral oils. They take a certain weight of the sample and boil it with twice its weight of an eight per cent, solution of caustic soda in alcohol. The soda combines with the fatty acids of the animal or vegetable oils forming soaps; bicarbonate of soda is then added to neutralize the excess of caustic soda; and, lastly, sand; and the whole is evaporated to dryness at the temperature of boiling water. The dry mixture is then transferred to a large glass tube, having a small hole in the bottom plugged with glass wool to act as a filter, and light petroleum spirit--which boils at about 150° to 180° Fahr.--is poured over it, till all the neutral or unsaponifiable oil is dissolved out. In the other process no sand is used, but the dry mixture is dissolved in water, and the soap solution which holds the neutral oils in solution is treated with ether, which dissolves out the neutral oil and then floats to the surface of the liquid. The ether solution is then drawn off, and the ether in the one case and petroleum spirit in the other are separated from the dissolved oils by distillation, the last traces of these volatile liquids being separated by blowing a current of filtered air through the flask containing the neutral oil, which is then weighed and its percentage on the original sample calculated.
All animal and vegetable oils yield a small quantity--about one per cent.--of unsaponifiable fatty matter, which must be deducted from the result obtained. Sperm oil, however, was found to be an exception, because from its peculiar chemical constitution it yields nearly half its weight of a greasy substance to the ether or to the petroleum spirit. The substance, however, dissolved from sperm oil after saponification has the appearance of jelly, when the ether or petroleum spirit solution is concentrated and allowed to cool, and the presence of sperm oil can thus be readily detected. Solid paraffin, heavy petroleum or paraffin oils, and rosin oil--which is produced by the destructive distillation of rosin--are not saponifiable, and yield about the whole of the amount employed to the petroleum spirit or ether. Japan wax is almost entirely saponifiable, while beeswax and spermaceti yield about half their weights to the petroleum spirit or ether.
Dr. Edgar Kurtz, of Florence, has found this medicament so useful in the various aches and pains of every-day life that he has persuaded many families of his acquaintance to keep it on hand as a domestic remedy. It is an excellent external application for stomach-ache, colic, tooth ache (whether nervous or arising from caries), neuralgia of the trigeminus, of the cervico-brachial plexus, etc. It is superior to anything else when inhaled in so-called angio-spastic hemicrania, giving rapid relief in the individual paroxysms and prolonging the intervals between the latter. No trial was made in cases of angio paralytic hemicrania, since in this affection the drug would be physiologically contraindicated. It has a very good effect in dysmenorrhoea, especially when occurring in chlorotic girls; in mild cases external applications suffice, otherwise the drug should be inhaled (when complicated with inflammatory conditions of the uterus or appendages the results were doubtful or negative). Its physiological action being that of a paralyzing agent of the muscular tissue of the blood vessels, with consequent dilatation of their caliber (most marked in the upper half of the body), nitrite of amyl is theoretically indicated in all conditions of cerebral anaemia. Practically it was found to be of much value in attacks of dizziness and faintness occurring in anæmic individuals, as also in a fainting-fit from renal colic, and in several cases of collapse during anaesthesia by chloroform.
It has been recommended in asphyxia from drowning, hanging, and in asphyxia of the new born, but the first indication in these cases is the induction of artificial respiration, after the successful initiation of which inhalations of nitrite of amyl doubtless assist in overcoming the concomitant spasm of the smaller arteries.
One of the most important indications for the use of the drug is threatening paralysis of the heart from insufficient compensation. In such cases it is necessary to gain time until digitalis and alcoholics can unfold their action, and here nitrite of amyl stands pre-eminent. A single case in point will suffice to illustrate this. The patient was suffering from mitral insufficiency, with irregular pulse, loss of appetite, enlargement of the liver, and mild jaundice. Temporary relief had been several times afforded by infusion of digitalis. In February, 1879, the condition of the patient suddenly became aggravated. The pulse became very irregular and intermittent. The condition described as delirium cordis presented itself, together with epigastric pulsation and vomiting. Vigorous counter-irritation, by means of hot bottles and sinapisms to the extremities, etc., proved useless. Digitalis and champagne, when administered, were immediately vomited. The pulse ran up from seventy until it could no longer be counted at the wrist, while the beats of the heart increased to one hundred and twenty and more per minute. The extremities grew cold, and the face became covered with perspiration. The urine was highly albuminous. Nitrite of amyl was then administered by inhalation: at first, three to five drops; then, ten to twenty; and finally, more or less was poured on the handkerchief without being measured. During each inhalation the condition of the patient rapidly improved, but as quickly grew worse, so that the drug was continued at short intervals all night, ten grammes in all having been used. In the morning the patient was better, and 0.5 gramme of digitalis was then given in infusion per rectum, and repeated on the following day, after which the patient remained comparatively well until a year and a half later, when a second attack of the kind just described was quickly cut short by similar treatment.
Another noteworthy case was that of a robust man of thirty years, who was attacked with acute gastro intestinal catarrh. The patient had as many as one hundred watery evacuations in forty-eight hours, with fainting fits, violent cramps in the calves of the legs, two attacks of general convulsions--in short, he presented the picture of a person attacked with cholera. Opium, champagne, hypodermic injections of sulphuric ether, counter-irritation, etc., proved useless. The doctor was on the point of injecting dilute liquor ammonii into the veins, but, none being obtainable, it occurred to him to try nitrite of amyl as a last resort. A considerable amount was poured on a handkerchief and held before the patient's mouth and nose, while the legs were also rubbed energetically with the same agent. Respiration soon became deeper and more regular, while the pulse gradually returned at the wrist. These procedures were repeated again and again, without regard to the quantity of the drug used, as soon as the radial pulse became weaker, and kept up until the patient complained of a sense of fullness in the head, and requested the discontinuance of the drug. The evacuations became less frequent, and in a week the patient was able to be up. Resuming then, Kurz concludes that nitrite of amyl is indicated in cardiac affections when the capillary circulation is obstructed and the cardiac muscle is threatened with paralysis from overwork; further, in cases of impeded circulation occasioned by cholera or severe diarrhea, particularly in the so-called hydrocephaloid (false hydrocephalus) of children. It is worthy of trial in tetanic and eclamptic seizures, and in tonic angiospasms such as occur during the chill of malarial fevers, although in the last-mentioned condition pilocarpine is perhaps more suitable, provided the energy of the heart be unimpaired.
As regards the dose, Kurz's experience demonstrates that we need not restrict ourselves to a few drops. The quantity may be increased, if necessary, until symptoms of cerebral congestion show themselves, when the drug should be momentarily or permanently discontinued. Usually from three to five or ten drops are sufficient, sometimes even less. Kurz has met with no unpleasant consequences, much less serious complications, from the application of nitrite of amyl. But the drug is contraindicated in cases associated with cerebral hyperaemia, in atheromatous conditions of the arteries, and in the so-called plethoric state--Beta's Memoabilien, March 24, 1881.
The treatment of simple acute articular rheumatism may be abandoned to palliatives and nature. Apart from complications, such cases nearly always recover under rest and careful nursing. Try and disabuse yourselves of the idea that their cure is dependent upon medicines alone; to help nature is often the best we can do. No treatment was ever invented which stopped a case of acute articular rheumatism. It cannot be stopped by bleeding, or sweating, or purging, by niter, by tartar emetic, by guaiacum, by alkalies, by salines, by salicylic acid, or by anything else. The physician can palliate the pain and perhaps shorten the attack, can control and perhaps prevent complications and stiffness of the joints, but he cannot arrest the disease. Where rest, proper diet, and warmth are enjoined, most cases will get well just as soon without as with the use of medicinal methods. Dr. Austin Flint, Sr., of New York, in support of this statement, subjected some patients, a number of years ago, to the expectant treatment, and found that they made just as rapid and just as complete recoveries as did those cases under the most active medication. Purgatives have been used in all ages in the treatment of this disease, because it was thought to be a fever. We are all but too ready to put our necks into the yoke of a theory. In old times they thought that the system ought to be reduced. Before the time of purgatives depletion was employed. This mode of treatment I will not even discuss. There is no evidence of which I am cognizant in favor of purgatives. There are very good reasons indeed why they should not be used: (1) Because they cannot possibly cure; (2) because they oblige the patient to make painful movements; and (3) because they expose him to the dangers of cold. A celebrated London physician had all his patients packed in blankets, and did not allow them to move a finger. This was going to the other extreme. There are certain cases in which purgatives are alleged to be of use, viz.: Those in which the bowels are constipated, and there is a bitter taste in the mouth. I have never seen such cases except in habitual drunkards, and in such cases a purgative does more harm than allowing the effete matter to remain in the system. Opium was once vaunted as a specific, and it was claimed that it diminished the tendency to complications in the course of the disease. Dr. Corrigan, of Dublin, said that large doses of opium were well borne--say from four to twelve grains in the course of twenty-four hours, or sometimes he advised giving as much as one grain every hour. Opium so employed does not produce narcotism, and does not constipate the bowels. More recent experience has shown that opium, of all remedies, is the most likely to cause heart complications. Some have recommended colchicum, arguing that because it does good in gout, it must, therefore, do good in rheumatism. But colchicum is not a remedy for rheumatism. Many years ago it was very much the custom to administer large doses of powdered Peruvian bark. The rationale of these large doses was founded upon their sedative effect. Haygrath, Morton, Heberden, and Fothergill were the first to employ this method. Later still, a number of noted French physicians, among them Briquet, Andral, Monerat, and Legroux, renewed the use of this medicine in the form of quinia, but gave it in smaller doses, seeking only its tonic effect, from five to fifteen grains being administered in the course of twenty-four hours, and then it was still continued in smaller doses. Still more recently, quinia taking the place of Peruvian bark, the old plan of administering large doses has been resumed. From thirty all the way up to one hundred grains have been administered in the course of twenty-four hours. Never was there a more profligate waste of a precious medicine. Even the physicians who so used it were obliged to acknowledge that it only did good in sub-acute and mild cases. I believe that it has also been fashionable in the so called cases of hyperpyrexia to immerse the patient in a bath varying in temperature from 60° to 98° Fahr. Although patients thus treated sometimes recovered, they also sometimes perished from congestion of the lungs and brain.
Among cardiac and nervous sedatives, digitalis, veratrum album and viride, veratria and aconite, have each, at one time or other, been employed indiscriminately. Such treatment, of course, has only proven itself to be a monument of rashness to those who employed it. Such sedatives may reduce the pulse, but do not shorten the disease. Indeed, if it is possible to prove the absurdity of anything more clearly by mere enumeration of these medicines as cures for rheumatism, I do not know of it. Do digitalis and aconite act in the same manner? This is just one expression of the folly which surrounded the use of digitalis at the time of its discovery. Then every affection of the heart was treated with digitalis.
Within the last few years new remedies have been proclaimed in the shape of salicylic acid and its sodium salt. I confess that I possess no personal knowledge of their use in this disease, for I was at first dissuaded from employing them by a prejudice against the grounds on which they were recommended, and more recently by the contradictory judgments respecting them, and the unquestionable mischief they have sometimes caused. According to their eulogists, the arrest of the disease is secured by them within four or five days, whether the attack be febrile or not; its mortality was diminished; relapses do not occur if the medicine is continued until full convalescence; it is without influence on the heart complications already existing, but it tends to prevent them as well as other serious inflammations. One of these gentlemen assures us that to say it far excels any other method of treatment would be to give it but scant praise. But, upon the other hand, it is accused of producing disorders, and even grave accidents in almost all the functions of the economy. In some cases it has produced ringing in the ears or deafness, or a rapid pulse, or an excessively high temperature, panting respiration, profuse perspiration, albuminuria, delirium, and imminent collapse. In one published case this anti-pyretic did not lower, but, on the contrary, seemed actually to raise the temperature so high that immediately after death it stood at 110° F. Many, very many, analogous cases have been published. I repeat, therefore, that I am personally unacquainted with the effects of this medicine in acute articular rheumatism, and that I have not thus far been tempted to employ it.
It may be difficult to see the connection between blisters and alkalies in their power to influence the course of acute articular rheumatism, and yet it is certain that they do so influence it, and in the same way, i. e., by altering the condition of the blood from acid to alkaline. If you ask me to explain to you how blisters act in this way I am obliged to confess my ignorance. To produce this result they must be applied over all the affected joints. Experience, if not science, has decided conclusively in their favor. They do effect a cessation of the local symptoms, render the urine alkaline, and diminish the amount of fibrin in the blood.
This brings us to a consideration of the use of alkalies. Alkalies neutralize the acids, act as diuretics, and eliminate the materies morbi. Alone, and in small doses, they are unable to influence the course of the disease; but when given in very large doses their effects are marvelous; the pulse falls, the urine is increased in quantity and becomes alkaline, and the inflammation subsides. The symptoms of the disease are moderated, the duration of the attack is shortened, and the cardiac complications are prevented. The dose of the alkalies must be increased until the acid secretions are neutralized. A very good combination of these remedies is the following:
Rx. Sodae bicarb 3 iss.
Potas. acet 3 ss.
Acid. cit f. 3 ss.
Aquae f. 3 ij. [1]
[Transcribers note 1: Could also be '2/3 ij.']
S. This dose should be repeated every three or four hours, until the urine becomes alkaline. On the subsidence of the active symptoms two grains of quinine may be added with advantage to each dose. The alkalies must be gradually discontinued, but the quinia continued. The diet should consist of beef tea or broth, with bread and milk; no solid food should be allowed. Woolen cloths, moistened with alkaline solutions, may with advantage be applied to the affected joints. To these laudanum may be added for its anodyne effect. The patient must be sedulously protected from vicissitudes of the temperature and be in bed between blankets. The alkaline treatment relieves the pain, abates the fever, and saves the heart by lessening the amount of fibrin in the blood. A long time ago Dr. Owen Rees, of London, introduced the use of lemon juice. This remedy was thought to convert uric acid into urea, and to so help elimination. Though the treatment is practically correct, the theory of it is all wrong. Lemon juice does good in mild cases, but cannot be relied upon in severe attacks. During the febrile stage of acute articular rheumatism the diet should consist mainly of farinaceous and mucilaginous preparations, with lemonade and carbonic acid water as drinks. The cloths applied to the joints should be changed when they become saturated with sweat, and in changing them the patient should be protected from the air. The sweating may be controlled by small doses of atropia, from the one-sixtieth to the one-thirtieth of a grain. To prevent subsequent stiffness the joints should be bathed with warm oil and chloroform, and wrapped in flannel cloths. In the proper season this condition is very well treated by sea-bathing. There is no specific plan of treatment in acute articular rheumatism. The treatment pursued must vary according to the intensity of the inflammation and the peculiarities of the patients.--Medical Gazette.
No psychologist has hitherto been able, and probably it is impossible, to define madness, or to give a clearly marked indication of the boundary line between sanity and insanity. Mental soundness is merged in unsoundness by degrees of decadence which are so small as to be practically inappreciable. It is with the mind-state which precedes the development of recognized form of insanity the therapeutist and the social philosopher are chiefly interested. Although in individual cases the subject of mental derangement may, as the phrase runs, "go mad" suddenly, speaking generally insanity is a symptom occurring in the course of disease, and, commonly, not until the malady of which it is the expression has made some progress. Those mental disturbances which consist in a temporary aberration of brain function, and which are the accidents of instability, rather than the effects of developed or even developing neuroses, can scarcely be classed as insanity; although it is true, and in an important sense, that these passing storms of excitement or spells of moody depression may--acting reflexly on the cerebral and nervous centers, as all mind-states and mind-movements react--exert a morbific influence and lay the physical bases of mental disease. The consideration most practical to the community and germane to the question of public safety is, that in any and every population there must exist a dangerously large proportion of persons who are always in a condition of mind to be injuriously influenced by any force which powerfully affects them. As a matter of history, it would seem that the majority of such persons are controlled rather than morbidly excited by the opportunity of throwing themselves into any popular movement. They may suffer afterward for the stimulation they receive at the time of public commotions, but while these are in progress they link their own consciousness with that of other minds, and the tendency to develop individual eccentricities of mental action is thereby for the moment repressed or exhausted. It is in the intervals of great public excitement the peace is disturbed by the vagaries of criminals who are more or less reasonably suspected of being "insane."
It would be premature to assume that the murderer of Mr. Gold, or the man who attempted to assassinate the President of the United States of America, is insane. There are circumstances in connection with each of these tragedies which must suggest the reflection that the assailants were possibly, or even probably, of unsound mind. We do not, however, propose to discuss these features of the respective cases at this juncture. The full facts are not, as yet, ascertained; but enough is known to warrant an endeavor to clear the way for future remark by disposing of the objection that the suspected perpetrator of the Brighton outrage and the would-be assassin of the President both showed "forethought" and "method." It is a common formula for the expression of doubt as to the irresponsibility of an alleged lunatic, that there is "method in his madness." Nothing can be farther from the truth than the inference to which this observation is intended to point. It is not in the least degree necessary that a madman should be unconscious of the act he performs, or of its nature as a violation of the law of God or man; nor is it necessary that he should do the deed under an ungovernable impulse, or at the supposed bidding of God or devil, angel or fiend. The forms of mental disease to which these presumptions apply are coarse developments of insanity. Dr. Prichard was among the first of English medico-psychologists to recognize the existence of a more subtle form of disease, which he termed "moral insanity." Herbert Spencer supplied the key-note to this mystery of madness when he propounded the doctrine of "dissolution;" and Dr. Hughlings Jackson has since applied that hypothesis to the elucidation of morbid mental states and their correlated phenomena. When disorganizing--or, if we may borrow an expression from the terminology of geological science, denuding--disease attacks the mental organism, it, so to say, strips off, layer by layer, the successive strata of "habit," "principle," and "nature," which compose the character. First in order go the higher moral qualities of the mind; next those which are the result of personally formed habits; then the inherited principles of personal and social life; at length the polish which civilization gives to humanity is lost, and in the process of denudation the evolutionary elements of man's nature are progressively destroyed, until he is reduced to the level of a creature inspired by purely animal passions, and obeying the lower brutish instincts. The term "moral insanity" is accurate as far as it goes, but it expresses only the first stage in a process of dissolution which is essentially the same throughout, but which has unfortunately received different designations as its several features have been recognized and studied apart. The difference between the subject of "moral insanity" and the general paralytic, who has lost all sense of decency and lives the life of a beast, is one of degree. The practical difficulty is to convince the mere observer that forms of insanity which seem to consist in the loss of moral qualities and principles only, may be as directly the effect of brain disease as any of those grosser varieties of mental disorder which he is perfectly well able to recognize, and fully prepared to ascribe to their proper cause.
To the professional mind, at least, it will follow from what we have said that the injury to mind properties or qualities inflicted by the invasion of disease may be partial, and must in every case be determined by laws or conditions governing the progress of disease, perhaps on the lines and in the directions which have been least well guarded by educationary influences. A man may lose his faculty of forming a wise judgment long before he is deprived of the power of distinguishing between right and wrong. This is so because it is a higher attainment in moral culture to do right advisedly, than simply to perceive the right thing to do. The application of principle to conduct is an advance on the mere recognition of virtue in the concrete, or even the possession of virtue in the abstract. The question whether any past act of wrongdoing was an act of insanity does not so much depend upon the great question whether the person doing it was insane as a whole being, or whether the deed done was the outcome of passion or error, the direct fruit of limited or special disease. In short, the insanity of the act must be inferred from the morbid condition of the brain from which it sprang, rather than from the act itself. A partially disorganized--or as we prefer to say "denuded"--brain may be fully capable of sane thought, except on some one topic, and able to exercise every intellectual function except of a particular order. Or there may be mental weakness and neurotic susceptibility in regard to a special class of impressions. It would be difficult to name any form of act or submission which may not be the outcome of incipient or limited disease. The practical difficulty is to avoid, on the other hand, treating the fruits of disease as willful offenses; while, on the other, we do not allow the supposition or presumption of disease to be employed as an excuse for wrongdoing. It is, of course, clear that there may be perfect method in such madness as springs from partial or commencing brain disease; for every element in the mental process which culminates in a mad act may be sane except the inception of the idea in which the act took its rise. Thus, in the case of the suspected murderer of Mr. Gold, there may have been perfect sanity in respect to every stage of the process by which the crime was planned and carried out, and yet insanity, the effect of brain disease, in the idea by which the deed was suggested. For example, when a man is suffering from morbid suspicion, and, fixing his distrust on some individual, purposes to murder him, the intellectual processes by which he lays his plans and fulfills his morbidly conceived intention, are performed with perfect sanity, as by a sane will. It is important to recognize this. There is no difference in nature between the mental operation by which a "sane" man contrives and executes a crime, and that by which a known "lunatic" will commit the like offense. There may be as much method in the one instance as in the other, and the faculties which exhibit this method may be as sound and effective, but in the one case the idea behind the act is sane, while in the other it is insane. The brain is not one large homogeneous organ to be healthy or diseased, orderly or deranged, throughout at any one period. Inflammations, and diseases generally, which affect the brain as a whole do not commonly cause insanity properly so called. The organ of the mind is a composite, or aggregate of cells, or molecules, any number or series of which may be affected with disease while the rest remain healthy. At present we are only on the threshold of investigation concerning the physical causes of insanity, and have scarcely done more than recognize the possibility of molecular disease of the brain. Hereafter science will, probably, succeed in unveiling the obscure facts of molecular brain pathology, and enable the medical psychologist to predicate disease of recognized classes of brain elements from the special phenomena of mind disturbance. This is the line of inquiry, and the result, to which the progress already made distinctly tends. For the present, the inferences we can surely draw from known facts are very few; but prominent among the number are certain which it is all-important to recognize in view of the judgment which must hereafter be formed on the two cases now engaging public attention on both sides of the Atlantic. The existence of method in madness is no marvel, and that characteristic cannot therefore be supposed, or alleged, to weigh as evidence against the "insanity" of the criminal. The perpetrators of these heinous offenses against common right and public safety may be more or less responsible for their acts, and, so far as these are concerned, more or less sane or insane. The measure of the morbid element in their individual cases will be the health or disease of the particular part or element of the brain from which the offense sprang. The ultimate judgment formed must be determined upon the basis of scientific tests to be applied to the action of the brain alleged to be the subject of partial or incipient disease. There is nothing in the facts as they stand to supply the materials for a judgment. Precise scientific inquiry can alone solve the enigma each case presents.
At first sight it seems almost superfluous to write or say a word about any method of arresting hemorrhage from wounds; for the practitioner, as a rule, is well acquainted with all the different manipulations and appliances for the purpose, and enough may be obtained from the text books. Nevertheless, to call attention to some useful, or old, or apparently forgotten matter occasionally, seems not to be amiss, for it refreshes our memory, stimulates us to think about and keeps before our eyes important subjects. A few hints on the above, I hope, will therefore be well received.
The treatment of hemorrhage, viz., the arresting of the same from open wounds, is not only important to the surgeon as the basis of surgery, but it is also of great importance to the laity, and especially to those workmen who are perpetually in danger of being injured. It is astonishing how unknowing the people seem to be, with any method to check bleeding from a wound temporarily; even the most simple method of pressure is in the majority of such accidents not resorted to. The sight of a little blood does not alone upset a timid, nervous woman, but many times the strongest of men; and why? because it naturally creates a feeling of awe and detestation. If a person is wounded by a machine, or otherwise, a crowd of all his fellow workmen gather around him, and look on the poor fellow bleeding; half a dozen or more will start out on a run in different directions to hunt a doctor, or some old woman who has a reputation for stopping bleeding by sympathy, either of whom they are likely to find "not at home." In the meantime the vital fluid trickles away; nobody knows what to do; everybody does something, but none the right thing. Now, it is true, it does not often happen that any one bleeds to death, wise mother nature, as a rule, coming to their assistance, especially in lacerated wounds; but the anemic condition produced by excessive loss of blood is followed by severe consequences, and is to be dreaded, for it retards recovery. To save all the blood possible ought to be apprehended as an important matter by every one.
Hardly a week passes that some unfortunate is not brought to my office, who has been badly injured in some way; he has been bleeding, perhaps, the distance of several blocks, and arrives almost faint. In the most of such cases they have something tied around their wounds, but hardly ever in any manner so as to be equal to stop the bleeding. In exceptional cases you find a tourniquet or the Spanish windlass applied. This, when applied by a surgeon, may answer very well, but when applied by a non-professional person it is invariably screwed up so tight that the pain produced thereby is so great and intolerable that the patient prefers rather to bleed to death. This is a great objection.
Therefore I will call attention to the method of forcible flexion; and though extreme flexion has been practiced by surgeons in isolated cases, still to Professor Adelman, of Dorpat, is due the credit of first having systematized the following method:
Bring the elbows of the patient as near as possible together upon the back, and fasten them with a bandage. From this point let a doppelt bandage pass down to and over the perineum; separate the bandages again in front, let one end run over the left, the other over the right groin back again to the elbows (see Fig. 1)
Fig. 1.
"The illustrations will explain at a glance."
Acute flexion of the elbow, simple bending of the forearm upon the upper arm, will suffice. But if there is bleeding from the arteries near the joint of the hand or from any part of the hand, then the hand must also be brought into flexion, and secured by a bandage. (See Fig. 2.) The bandage must always be wrapped around the wound first.
Fig. 2.
It needs no other explanation, as Fig. 3 shows the mode of stopping the hemorrhage from that region temporarily.
Bleeding from the front part of the leg (Art. Tibialis Ant.), same as Fig. 3.
FIG. 3.
Bleeding from the posterior part of the leg (Art. Tibiailis Post, et Peronea) same as above, with the addition of a tampon or compress under the knee joint, or like Fig. 4.
Flexion of the leg upon the thigh, and flexion of the foot upon the front of the tibia.
Objections might also be raised to the above method on account of the pain which it may produce; but the flexion never needs to be so forced as to be unendurable to the patient; the position may be a little uncomfortable to a very sensitive person, that is all. Furthermore, it has been proven that a limb can be kept in a flexed position for several days, "nine by some authors," without any injury, and with a complete closure of the arteries. We do not expect, however, that this method of arresting hemorrhage will ever be adopted as "the" method in surgery, neither will it be necessary here to point out any cases where the practitioner can have and under certain circumstances be obliged to have to resort to this simple method. Military surgeons may also profit by it, for it is certainly a valuable and admirable mode, and so easily applied in cases of emergency by any one, if the unfortunate should be distant from surgical aid. I also believe that it would be advisable and certainly humane, to instruct the people in general, by popular lectures or through the press, the manner of stopping hemorrhage temporarily.
Fig. 4.
The simplest of all methods, however, to arrest hemorrhage is the rubber bandage. It has displaced in surgery the old tourniquet almost completely, which required a certain skill and anatomical knowledge to apply it; not necessarily so with the rubber bandage. Any one can apply it, for the amount of pressure needed to arrest the hemorrhage from a wound suggests itself. The rubber bandage produces but little pain; the patient is comparatively comfortable and out of immediate danger and anxiety; while in the meantime the proper attention can be secured.
I think it would be well if our health officers would direct their attention a little to the accidental hemorrhages, and if they do not possess the power, to refer the matter to the proper tribunal to enact a law that would compel all owners and corporations of factories, saw, planing, and rolling mills, and, in fact, every establishment where the laborers are constantly in danger of accidents, to keep on hand a certain number of strong rubber bandages, according to the number of men employed, and that at least several of the men, if not all in every establishment of that kind, be instructed in the application of the bandage. Steamboats and other vessels should carry a supply, and railroad companies should be obliged to furnish all watchmen along their respective roads with rubber bandages, and see that the men know how to use them in case an accident should occur. Every train that goes out should have some bandages on board in care of some employe, who knows how to handle them when needed. Many pounds of precious blood may thus be saved, and danger to life from this cause be averted.--Indiana Medical Reporter.
Sporer has successfully treated cases of tetanus by merely applying to the nape of the neck and along the spine large pieces of flannel dipped in hot water, of a temperature just bearable to the hand (50-55° C.).--Allg. med. cent. Zeit., January 15, 1881.
After a week's postponement, rendered necessary by the unripe condition of the crops on the first of the month, the trials of sheaf-binding machines, using any other binding material than wire, instituted by the Royal Agricultural Society of England, began on Monday morning, the 8th of August. By nine o'clock, the time appointed for beginning operations, there was a very large number of gentlemen interested in these trials already collected on the farm of Mr. Hall, at Thulston, and the distances that many of them had come testified to the importance of the interests involved. The morning was perfect for reaping, though ominous clouds in the southwest led many to hazard conjectures, which unfortunately turned out too well founded, that the Royal Agricultural Society would not on this occasion escape the fate which had visited them so often. The corn stood ripe and upright in the various plots into which the fields had been divided, and the ground was level and dry. The published list of the competitors contained twenty entries, not by as many firms, however, for many names appeared more than once; but the rules of the society, which objects to different machines being used for different kinds of corn in these trials, together with non-attendance for unknown reasons, had reduced the actual list of competing machines to seven. These were as follows: Mr. W. A. Wood, the McCormick Harvesting Machine Company, the Johnston Harvester Company, Messrs. Samuelson & Company, Messrs. J. & F. Howard, Messrs. Aultman & Company, and Mr. H.J.H. King. All these machines were to be seen at the show, except the second named, which was delayed by the stranding of the steamship Britannic, and had only lately arrived in rather a weather-beaten condition. The trials were to be made upon oats, barley, and wheat, and the plots for the preliminary trials were about half an acre in extent. Shortly after half-past nine o'clock, the judges and engineers of the society having arrived upon the ground, a start was made upon the oats by the three machines belonging to Mr. Wood, Messrs. Samuelson & Co., and the Johnston Harvester Company. It should, perhaps, be mentioned that the strength of this crop of oats varied a good deal in different parts of the field. These three machines all belong to the class which has the automatic trip--that is, the binding gear is thrown into action by the pressure of the straw accumulated arriving at a certain value, independently of any special action on the part of the driver. The sheaves from Messrs. Samuelson's machines were extremely neat and well separated from each other, a point to which farmers attach great importance.
It would appear that it is impossible to secure the binding of every single sheaf. Here and there, even with the best binders, an occasional miss will occur, in which the corn is thrown out unbound. However, with Messrs Samuelson's machine this was extremely rare, and the neatness of the sheaves produced was remarkable. No doubt the shortness of the crop in the portion allotted to this machine may have had something to do with this, as a longer straw is more likely than a shorter one to connect two sheaves and produce that hanging together which in other machines is so often observed to precede a miss in the binding. Mr. Wood's machine had a stronger crop and longer straw to deal with, and the hanging together of the sheaves occurred far too frequently, and was almost always followed by a loose sheaf. The Johnston harvester went through a very fair performance; there was no hanging except at turning the corners, and the piece of work was finished in a shorter time than with the other machines. Notwithstanding the automatic character of the gear for binding, we believe it will be found that the sheaves produced in these machines vary very much in weight.
At about 10:20 the next lot of machines started. They were those of the McCormick Harvesting Machine Company, Messrs. Howard, and Messrs. Aultman & Co. Of these, the first-named only has the automatic trip. We believe it made no miss in binding during this trial, and the sheaves were neat, though, perhaps, rather too tightly bound. There was no hanging together or check in this run. The machine of Messrs. Aultman & Co. was not so successful in separating the sheaves, though this was not so often followed by an unbound sheaf as in some other machines. Sometimes as many as three sheaves, clinging closely together, were ejected at one time. To avoid this a man walked by the machine, and assisted the delivery of the sheaf. The tension of the string which binds the sheaves varies a good deal in this machine, some of the sheaves being rather too loosely held together, while at other times the fault is in the other direction. In Messrs. Howard's machine there is a tendency in the sheaves to cling together, but this is not accompanied to any extent with missing the binding. Mr. King attempted a run after the three last had finished their plots; but his machinery had not been fully adjusted, and after one course the trial stopped. As far as one could judge from this short performance, the chief fault in the sheaf produced was the uncertain position of the string upon it. Sometimes this was near the bottom of the straw, and sometimes among the corn. Unfortunately at 11:25 the rain began, and experiments were stopped till the afternoon. It was no light shower which could give a check to the ardor of the judges and other officers of the society, but a heavy downpour of some hours' duration, which soaked the crop through and through. Indeed, we think it a pity that the experiments should have been continued at all under circumstances in which practical harvesting would have been out of the question. However, after a short lull in the rain, the machines of Mr. Wood, Messrs. Samuelson, and the McCormick Harvesting Company went into the wet barley. The machine of Mr. Wood worked most rapidly, but the clinging of the sheaves and the failure to bind were again very apparent. The stubble left by this machine was the shortest and most even of the three. The machines of Messrs. Samuelson and the McCormick Company left a very ragged, long, and uneven stubble in this trial, though the delivery and binding of the sheaves seemed to be as good as in the oats trial. The binding in the former was rather too tight.
The remaining machines, with the exception of that of Mr. King, then attempted a trial; but Messrs. Howard's machine having too smooth a face to the driving wheel, was unable to drive all the gear in the wet condition of the ground. The damp weather had no doubt tightened up the canvas carriers, and thereby added to the work to be done; but this was the only machine that was found incapacitated through the action of the rain. Unfortunately the plots assigned to this machine and to the Johnston harvester were in juxtaposition, so that the latter machine was blocked by the former, and could not proceed, and that of Messrs. Aultman alone went through with its work. There was no improvement in the separation of the sheaves, and the misses were rather more frequent than in the trials among the oats. The sheaves, too, that issued singly were somewhat wanting in neatness. The whole of these barley trials must be looked upon as unsatisfactory, on account of the condition of the crop, and it is to be hoped that before the investigations are brought to a conclusion all these machines may have a more favorable opportunity of demonstrating the advantages which are claimed for them. It may be here said that throughout these trials there has been as yet no wind at all, which, as the investigations are in other respects to be so thoroughly carried out, is a matter of regret. Probably Messrs. Howard's machine was as well protected from the wind as any other of the seven competitors.
The following are the awards of the judges, which were made known on Wednesday evening: Gold medal--Messrs. McCormick & Co. Silver medals--Messrs. Samuelson, Messrs. Johnston & Co. Highly commended--Mr. H. J. King, for principle of tying and separating sheaves. The only gleaning binding machine which entered the field was that of Mr. J. G. Walker, made by the Notts Fork Company, but no official trials of this were made.--The Engineer.
Messrs. Ellwanger & Barry, of the Mount Hope Nurseries, at Rochester, give the following directions for setting out and cultivating strawberries, the result of long and successful experience, in their recently issued Strawberry Catalogue:
The Soil and Its Preparation.--The strawberry may be successfully grown in any soil adapted to the growth of ordinary field or garden crops. The ground should be well prepared, by trenching or plowing at least eighteen to twenty inches deep, and be properly enriched as for any garden crop. It is unnecessary to say that if the land is wet, it must be thoroughly drained.
Season for Transplanting.--In the Northern States, the season for planting in the spring is during the months of April and May. It may then be done with safety from the time the plants begin to grow until they are in blossom. This is the time we prefer for setting out large plantations.
During the months of August and September, when the weather is usually hot and dry, pot-grown plants may be planted to the best advantage. With the ball of earth attached to the roots, they can be transplanted without any failures, and the trouble and annoyance of watering, shading, etc., which are indispensable to the success of layer plants, are thus in a great measure avoided.
To Cultivate the Strawberry.--For family use, we recommend planting in beds four feet wide, with an alley two feet wide between. These beds will accommodate three rows of plants, which may stand fifteen inches apart each way, and the outside row nine inches from the alley. These beds can be kept clean, and the fruit can be gathered from them without setting the feet upon them.
Culture in Hills.--This is the best mode that can be adopted for the garden. If you desire fine, large, high-flavored fruit, pinch off the runners as fast as they appear, repeating the operation as often as may be necessary during the summer. Every runner thus removed produces a new crown at the center of the plant, and in the fall the plants will have formed large bushes or stools, on which the finest strawberries may be expected the following season. In the meantime, the ground among the plants should be kept clear of weeds, and frequently stirred with a hoe or fork.
Covering in Winter.--Where the winters are severe, with little snow for protection, a slight covering of leaves or litter, or the branches of evergreens, will be of great service. This covering should not be placed over the plants till after the ground is frozen, usually from the middle of November till the first of December in this locality. Fatal errors are often made by putting on too much and too early. Care must also be taken to remove the covering in spring just as soon as the plants begin to grow.
Mulching to Keep the Fruit Clean.--Before the fruit begins to ripen, mulch the ground among the plants with short hay or straw, or grass mowings from the lawn, or anything of that sort. This will not only keep the fruit clean, but will prevent the ground from drying and baking, and thus lengthen the fruiting season. Tan-bark can also be used as a mulch.
A bed managed in this way will give two full crops, and should then be spaded or plowed down, a new one having been in the meantime prepared to take its place.
The same directions with regard to soil, time of planting, protection, and mulching, as given above, are applicable when planting on a large scale.
The Matted Row System.--The mode of growing usually pursued has its advantages for field culture, but cannot be recommended for the garden. In the field we usually plant in rows three to four feet apart, and the plants a foot to a foot and a half apart in the row. In this case much of the labor is performed with the horse and cultivator.
How to Ascertain the Number of Plants Required for an Acre.--The number of plants required for an acre, at any given distance apart, may be ascertained by dividing the number of square feet in an acre (43,560) by the number of square feet given to each plant, which is obtained by multiplying the distance between rows by the distance between the plants. Thus strawberries planted three feet by one foot give each plant three square feet, or 14,520 plants to the acre.
Pretentious gardens are now gayly decorated with glowing masses of pelargoniums and vincas, belts of rich coleuses and fiery alternantheras, patchwork of feverfew and mesembryanthemum, and scroll-work of house leeks, but amid this gay checkering it is wonderful how few flowers there are for cutting for bouquets. As tender plants, except the few that may have been wintered in windows and cellars, are beyond the reach of most of our country folks, I will consider those only that are perfectly hardy and in full blossom now, July 21.
Koempfer's irises, blue, white, purple, streaked, marbled, and otherwise variegated, are in bloom; they are the grandest of their race, and as different varieties succeed one another, they may be had in bloom from June till August. They are easily raised from seed or by division--prefer rich, moist land, and if in a partly shaded place, their blossoms last longer than in full sunshine.
Trumpet lilies are bursting into bloom; the scarlet martagon is at its best; speciosum, tiger, and American Turk's cap lilies are yet to follow. I find the trumpet lilies have done better this year than any of the other sorts in open places. Most of the yellow day lilies are past, but the tawny one is at its best; they are all hardy, and seem to thrive alike in wild or cultivated land. Seibold's funkia (called also day lily) has pale bluish flowers, and large, handsome glaucous leaves: the undulated-leaved funkia has beautifully variegated leaves, and pale bluish blossoms; these, together with several others of their race, are in bloom. They like to grow in undisturbed clumps in rich and faintly-shaded nooks; if grown in full sunshine they bloom well enough, but their leaves get "scorched."
The European meadow sweet (Spiraea ulmaria), two feet high, and the Kamtchatka one, four feet high, are in bloom; the double varieties are far finer, whiter, and more lasting than the single ones. They will grow anywhere. There are many fine kinds of sedum or liveforever in season; some of them like album (white), pulchellum (pink), spurium splendens (pink), hispanicum (white), may more properly be called stonecups, but the stronger-growing sorts, as S. warscewiczii (yellow), should be regarded as liveforevers. They like open, sunny places, and dislike artificial waterings.
Dicentra eximia (pink-purple) is free, neat, copious, and a perpetual bloomer, as is also Corydalis lutea (yellow). The climbing fumitory comes up of itself from seed every year, and is now running over bushes, stakes, and strings, and is full of fern-like leaves and flesh-colored flowers. The long, scarlet wands of Pentstemon barbatus are conspicuous in the borders; this should be in every garden, it is so profuse and hardy. Many speedwells still remain in fine condition, notably Veronica longifolia; they are a hardy and a showy race of plants, and will grow anywhere. The main lot of perennial larkspurs are past, but by cutting them over now many flower spikes will be produced during the fall months. The yucca or bear-grass is in perfection; its massive flower scapes are very telling. It will grow anywhere, and once established it is hard to get rid of.
Many kinds of perennial bell-flowers are in fine condition, as the carpathian, peach-leaved (second crop), nettle-leaved, common harebell, and vase harebell. In the case of many of the tall-growing kinds, better results are obtained by treating them as biennials than perennials. No garden should be without the double white feverfew; the more you cut it the more it blooms. Anthemis tinctoria, yellow or white, the yellow is by far the best, and the lance-leaved, large-flowered, larkspur-leaved and eared coreopsises are fine, seasonable perennials, as are likewise the yellow, white, and pink yarrows, double sneezewort, the cone flowers, and large-flowered fleabanes, and all grow readily in any ordinary garden soil, and with little care. Hollyhocks are in perfection; feed them well and prevent many sprouts to each stool. Many kinds of meadow rue, as garden plants, have a bold, graceful appearance; they love moist soil.
In good soil and a partly shaded spot we have no handsomer plant in bloom than the tall bugbane (Cimicifuga racemosa); from a bunch of thrifty leaves arise a dozen scapes of racemes, creamy white, and six feet high. The scarlet lychnis and its many varieties are nearly past, but the large-flowered, Haag's, and others of that section, are in their prime, and showy plants they are. They are true and lasting perennials, bloom well the first season from seed, quite hardy, copious, and effective; any ordinary garden soil. The pyrenean prunella has large purple heads; the false dragonhead (Physostegia), pale rose-purple spikes; centranthuses, cymes of red and white; centaureas, heads of yellow, blue, and purple; pinks, divers shades of red and white; and monkshoods, hoods of blue or white; and all are very hardy, ready growers, and copious bloomers. The bee balm, one of our handsomest perennials, has bright red whorls; it spreads upon the surface of the ground like mint, and thus may be divided and increased to any extent. It loves rich, moist land, but is not fastidious. Among the evening primroses the Missouri one is the brightest and biggest; speciosa, white, from Texas, of blossoms the most prolific; glauca, riparia, fruticera, and linearis, all yellow; many others, though perennial, are best treated as annual or biennial. The spiked loosestrife planted by the water's edge of a pond is far finer than in the garden border. It has hundreds of red spikes.
Add to these, everlasting peas, musk mallows, spiderwort, globe thistles, bold senecios, the finer milkweeds, Scabiosa, Gallium, Chinese Astilbe, various kinds of loosestrife (Lysimachia), and many others as perennials, and Coreopsis, balsams, zinnias, marigolds, stocks, Swan river daisy, mignonnette, sweet peas, sweet alyssum, morning glories, larkspurs, canary flowers, cucumber-leaved sunflowers, verbenas, petunias, corn flower, Drummond phlox, double and single poppies, snapdragons, Phacelia, Gilia, Clarkia, candytuft, red flax, tassel flowers, blue Anchusa, Gaillardia, and a multitude besides of seasonable annuals, which can all be raised quite easily without a frame or green-house, and what excuse has any farmer for having a flowerless garden in midsummer?--William Falconer, in Country Gentleman.
Mr. Edward Prince, splint manufacturer, of Horseshoe Bay, Buckingham township, is authority for the statement that there are about twenty-two match factories in the United States and Canada, and that the daily production--and consequent daily consumption--is about twenty-five thousand gross per day. It may seem a queer statement to make that one hundred thousand hours of each successive day are spent by the people of the two countries in striking a light, but such is undoubtedly the case. In each gross of matches manufactured there are 144 boxes, so that the 25,000 gross produces 3,600,000 boxes. Each box, at least those made in the States, where a duty of one cent upon every box of matches is levied--contains 100 matches, so that the number of matches produced and used daily amounts to 360,000,000. Counting that it takes a second to light each match--and it is questionable whether it can be done in less time than that, while some men occupy several minutes sometimes in trying to strike a light, particularly when boozy--to light the 360,000,000 would take just that number of seconds. This gives 6,000,000 minutes, or 100,000 hours. In days of twenty-four hours each it figures up to 4,166 2-3, and gives eleven years and five months with a couple of days extra, as the time occupied during every twenty-four hours, by the people of North America--not figuring on the Mexicans--in striking matches. Figuring a little further it gives 4,159 years time in each year. The fact may seem amazing, but it is undoubtedly quite correct.--Ottawa Free Press.
A catalogue, containing brief notices of many important scientific papers heretofore published in the SUPPLEMENT, may be had gratis at this office.
Terms of Subscription, $5 a Year.
Sent by mail, postage prepaid, to subscribers in any part of the United States or Canada. Six dollars a year, sent, prepaid, to any foreign country.
All the back numbers of THE SUPPLEMENT, from the commencement, January 1, 1876, can be had. Price, 10 cents each.
All the back volumes of THE SUPPLEMENT can likewise be supplied. Two volumes are issued yearly. Price of each volume, $2.50, stitched in paper, or $3.50, bound in stiff covers.
COMBINED RATES--One copy of SCIENTIFIC AMERICAN and one copy of SCIENTIFIC AMERICAN SUPPLEMENT, one year, postpaid, $7.00.
A liberal discount to booksellers, news agents, and canvassers.
MUNN & CO., Publishers,
37 Park Row, New York, N. Y.
In connection with the Scientific American, Messrs. MUNN & Co. are Solicitors of American and Foreign Patents, have had 35 years' experience, and now have the largest establishment in the world. Patents are obtained on the best terms.
A special notice is made in the Scientific American of all Inventions patented through this Agency, with the name and residence of the Patentee. By the immense circulation thus given, public attention is directed to the merits of the new patent, and sales or introduction often easily effected.
Any person who has made a new discovery or invention can ascertain, free of charge, whether a patent can probably be obtained, by writing to MUNN & Co.
We also send free our Hand Book about the Patent Laws, Patents, Caveats. Trade Marks, their costs, and how procured, with hints for procuring advances on inventions. Address
MUNN & CO., 37 Park Row, New York.
Branch Office, cor. F and 7th Sts., Washington, D. C.
End of Project Gutenberg's Scientific American Supplement No. 299, by Various
*** END OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN SUPPL., NO. 299 ***
***** This file should be named 8408-h.htm or 8408-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/8/4/0/8408/
Produced by Olaf Voss, Don Kretz, Juliet Sutherland, and
the Online Distributed Proofreading Team
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation information page at www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at 809
North 1500 West, Salt Lake City, UT 84116, (801) 596-1887. Email
contact links and up to date contact information can be found at the
Foundation's web site and official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For forty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.