
The Project Gutenberg EBook of A Text-book of Tanning, by Henry R. Procter
This eBook is for the use of anyone anywhere in the United States and most
other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms of
the Project Gutenberg License included with this eBook or online at
www.gutenberg.org. If you are not located in the United States, you'll have
to check the laws of the country where you are located before using this ebook.
Title: A Text-book of Tanning
A treatise on the conversion of skins into leather both
practical and theoretical.
Author: Henry R. Procter
Release Date: February 19, 2018 [EBook #56601]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK A TEXT-BOOK OF TANNING ***
Produced by Chris Curnow, Tom Cosmas and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)

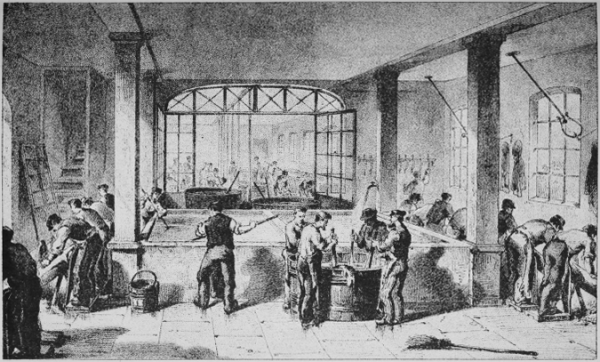
Pl. I.

LIME PITS AND RINSING TANKS.
A
TEXT-BOOK OF TANNING:
A TREATISE ON THE
CONVERSION OF SKINS INTO LEATHER,
BOTH PRACTICAL AND THEORETICAL.
BY
HENRY R. PROCTER, F.C.S.,
OF LOWLIGHTS TANNERY;
EXAMINER IN TANNING TO THE CITY AND GUILDS TECHNICAL INSTITUTE.
With 8 Plates and numerous Illustrations.
E. & F. N. SPON, 125, STRAND, LONDON.
NEW YORK: 35, MURRAY STREET.
1885.
The aim of the following handbook is two-fold; to give, in a compendious form, such a summary of our scientific knowledge as may be useful to the practical tanner; and such a sketch of manufacturing processes as may enable the chemist to apply his knowledge to their improvement. Each may, therefore, find some superfluous matter, for which his indulgence is asked. The book is an expansion of a short article which appeared in Spons' 'Encyclopædia of the Industrial Arts,' and to some extent still bears traces of its origin; and, having been written under stress of limited leisure, and defective eyesight, is very far from being so perfect as I should desire. For the sake of completeness it has been necessary to describe many processes which are outside the range of my own manufacturing experience, and in doing so I have generally referred to the sources of my information. Chapters III. and XXIV. are written by Mr. C. G. Warnford Lock, to whose kind assistance I am much indebted. It may be well to state in conclusion, that while the work is not intended for a cram-book for technical students, it is hoped that it may be an assistance to teachers of the subject.
HENRY R. PROCTER.
Tynemouth,
August 1885.
| PAGE | |
| INTRODUCTORY NOTE | 1 |
CHAPTER I. |
|
| Anatomical Structure of Hide | 2 |
CHAPTER II. |
|
| Chemical Composition of Hide | 17 |
CHAPTER III. |
|
| Commercial Tanning Materials | 23 |
CHAPTER IV. |
|
| The Chemistry of Tannins | 57 |
CHAPTER V. |
|
| Water as used in Tanning | 83 |
CHAPTER VI. |
|
| Methods of Chemical Analysis for the Tannery | 90 |
CHAPTER VII. |
|
| Sole-leather:—Preparing the Hides | 132 |
CHAPTER VIII.
« viii » |
|
| Sole-leather:—Unhairing Hides | 139 |
CHAPTER IX. |
|
| Sole-leather:—Tanning Materials | 157 |
CHAPTER X. |
|
| Sole-leather:—Treatment in the Tan-house | 169 |
CHAPTER XI. |
|
| Sole-leather:—Treatment in the Shed | 179 |
CHAPTER XII. |
|
| Dressing Leather | 184 |
CHAPTER XIII. |
|
| Currying | 193 |
CHAPTER XIV. |
|
| Enamelled, Patent, or Japanned Leather | 203 |
CHAPTER XV. |
|
| Morocco Leather | 206 |
CHAPTER XVI. |
|
| Russia Leather | 208 |
CHAPTER XVII. |
|
| Chamois or Wash-leather | 210 |
CHAPTER XVIII.
« ix » |
|
| Crown Leather, or Preller's Leather | 213 |
CHAPTER XIX. |
|
| Mineral-tanned Leather | 218 |
CHAPTER XX. |
|
| Calf-Kid | 223 |
CHAPTER XXI. |
|
| Glove-Kid | 225 |
CHAPTER XXII. |
|
| Construction and Maintenance of Tanneries | 231 |
CHAPTER XXIII. |
|
| Drying-sheds for Leather | 243 |
CHAPTER XXIV. |
|
| Commerce, Statistics, and Bibliography | 255 |
| Index | 275 |
Leather manufacture may be broadly divided into two stages: "tanning," in which the raw hide is converted into the imputrescible and more or less flexible material known as "leather"; and "currying," in which this leather is further manipulated, and treated with fatty matters, to soften and render it more waterproof, and to improve its appearance. Glove-kid, and certain other leathers, however, are not tanned at all, but "tawed," or prepared with a mixture in which alum and salt are the most active ingredients; chamois, "shammy," or "wash" leather, is produced by fulling with oil alone, and many leathers can scarcely be said to be curried, although more or less oil is used in the final processes of "finishing" or "dressing." The first subject to be treated of in this work will be the operation of tanning, properly so called, taking for example the tannage of sole- and belting-leather. This demands thorough explanation, in both its practical and theoretical aspects, not only because it is one of the most important branches of the trade, but because the principles involved are those which equally underlie all other tanning methods. The next to be dealt with will be the modifications of the process which are necessary in tanning the more flexible leathers used for boot-uppers, hose-pipes, and saddlery purposes; then the currying of these leathers; and finally, the manufacture of moroccos, Russian, and japanned leathers, calf- and glove-kid, &c.
ANATOMICAL STRUCTURE OF HIDE.
Before speaking of actual processes of manufacture, it is necessary to devote some attention to the structure and chemical constitution of hide or skin, which forms the raw material. Although a great variety of skins are employed in tanning, they are all constituted on the same general type, and an anatomical description of the hide of the ox will apply almost equally to those of the calf, sheep, and goat; but from differences in thickness and closeness of texture, their practical uses differ widely. Fig. 1 shows a section of ox-hide, cut parallel with the hair, magnified about 50 diam.: a, epithelial layer or epidermis, consisting of horny layer above, and rete malpighi below; b, pars papillaris, and c, pars reticularis of corium, derma, or true skin; d, hairs; e, sebaceous or fat-glands; f, sudoriferous or sweat-glands; g, opening of ducts of sweat-glands; h, erectores pili muscles, for erecting the hair.
The fresh hide consists of 2 layers: an outer, the epidermis; and an inner, the true skin. The epidermis is very thin as compared with the true skin which it covers, and is entirely removed preparatory to tanning; it nevertheless possesses important functions. It is shown in Fig. 1 at a, and more highly magnified in Fig. 2. Its inner mucous layer b, the rete malpighi, which rests upon the true skin c, is soft, and composed of living nucleated cells, which are elongated in the deeper layers, and gradually become flattened as they approach the surface, where they dry up, and form the horny layer a. This last is being constantly worn away, and thrown off as dead scales of skin; and as constantly renewed from below, by the continued multiplication of the cells. It is from « 3 » this epithelial layer that the hair, as well as the sweat- and fat-glands, are developed. It will be seen in Fig. 1 that each hair is surrounded by a sheath, which is continuous with the epidermis. In embryonic development, a small knob of cells forms on the under side of the epidermis, and this enlarges, and sinks deeper into the true skin, while the root of the young hair is formed within it; this is shown in Fig. 3, a b. Smaller projections also form on the stalk of the knob, and in due time produce the sebaceous glands. The process of development of the sudoriferous glands is very similar to that of the hairs. There is a great analogy between this process and that of the ordinary renewal of hair in the adult animal. « 4 » At d1 Fig. 1, is seen an old and worn-out hair. It is shrunken and elongated, and is almost ready to fall out. It will be noticed that its sheath or follicle projects somewhat below the hair to the right. This is the first production of a young hair, and is quite analogous to the knob of epithelium which has been described as forming the starting-point of a hair in embryo. At d2, the same process is seen further advanced, the young hair being already formed, and growing up into the old sheath. At d3, it is complete, the old hair having fallen out, and the young one having taken its place.
The hair itself is covered with a layer of overlapping scales, like the slates on a roof, but of irregular form. These give it a serrated outline at the sides, strongly developed in wool. Within these scales, which are sometimes called the "hair cuticle," is a fibrous substance, which forms the body of the hair; and sometimes, but not always, there is also a central and cellular pith, which is mostly transparent, though under the microscope it frequently appears black and opaque, from the optical effect of imprisoned air. On boiling or long soaking in water, alcohol, or turpentine, these air-spaces become saturated with the liquid, and then appear transparent.
The fibrous part of the hair is made up of long spindle-shaped cells, and contains the pigment which gives the hair its colour. The hair of the deer differs from that of most other animals in being almost wholly formed of polygonal cells, which, in white hairs, are usually filled with air. At its « 5 » base, the hair swells into a bulb, which is hollow, and rests on a sort of projecting knob of the corium, called the hair-papilla. This has blood-vessels and nerves, and supplies nourishment to the hair. The hair-bulb is composed of round, soft cells, which multiply rapidly; as they grow, they press upward through the hair-sheath, become elongated and hardened, and form the hair. In dark hairs, both the cells of the hair itself and those of its follicle or sheath are strongly pigmented, but the hair much the more so, and hence the bulb has usually a distinct dark form. The dark-haired portions of a hide from which the hair has been removed by liming still remain coloured, from the pigmented cells of the hair-sheaths, which can only be got out completely by bating and scudding. The cells outside the bulb, shown at f, in Fig. 4, pass upwards as they grow, and form a distinct coating around the hair, which is called the "inner root-sheath." This again consists of 2 separate layers, of which the inner is "Huxley's," the outer, "Henle's." They arise from the same cells in the base of the hair; but in the inner layer, these remain polygonal and nucleated, while in the outer, they become spindle-shaped and without nuclei. The inner root-sheath does not extend to the surface of the skin, but dies away below the sebaceous glands. This figure represents an ox-hair root, mag. 200 diam.: a, fibrous substance of hair; b, hair cuticle; c, inner root-sheath; d, outer root-sheath; e, dermic coat of hair-sheath; f, origin of inner sheath; g, bulb; h, papilla.
Outside the inner root-sheath is a layer of nucleated cells, continuous with those of the epidermis, and of the same « 6 » character. This is the "outer root-sheath," and is shown at d, Fig. 4. This, together with the whole of the epidermis, is covered next the corium with an exceedingly fine membrane, called the "hyaline" or glassy layer. It is possible that this forms the very thin buff-coloured "grain" of tanned leather, which evidently is of different structure from the rest of the corium, since, if it gets scraped off before tanning, the exposed portion of the pars papillaris remains nearly white, instead of colouring. The whole of the hair-sheath is enclosed in a coating of elastic and connective-tissue fibres, which are supplied with nerves and blood-vessels, and form part of the corium. Near the opening of the hair-sheaths to the surface of the skin, the ducts of the sebaceous or fat-glands (e, Fig. 1), pass into them, and secrete a sort of oil to lubricate the hair. The glands themselves are formed of large nucleated cells, arranged somewhat like a bunch of grapes; one is shown highly magnified in Fig. 5: a, sebaceous gland; b, hair-stem; c, part of erector pili muscle. The upper and more central cells are most highly charged with fat, which is shown by the darker shading.
As already remarked, the sudoriferous or sweat-glands are also derived from the epidermis layer. They are shown at f, Fig. 1, and on a larger scale (200 diam.) in Fig. 6: a, windings laid open in making section; they consist, in the ox and sheep, of a large wide tube, sometimes slightly twisted. In this, they differ considerably from those of man, which form a spherical knot of extremely convoluted tube. The walls of these glands are formed of longitudinal « 7 » fibres of connective tissue of the corium, lined with a single layer of large nucleated cells, which secrete the perspiration. The ducts, which are exceedingly narrow, and with walls of nucleated cells like those of the outer hair-sheaths, sometimes open directly through the epidermis, as shown at g, Fig. 1, but more frequently into the orifice of a hair-sheath, just at the surface of the skin. Each hair is provided with a slanting muscle (h, Fig. 1), called the arrector or erector pili, which is contracted by cold or fear, and causes the hair to "bristle," or stand on end; by forcing up the attached skin, it produces the effect known as "goose-skin." The muscle, which is of the unstriped or involuntary kind, passes from near the hair-bulb to the epidermis, and just under the sebaceous glands, which it compresses.
The corium or true skin is principally composed of interlacing bundles of white fibres, of the kind known as "connective tissue"; these are composed of fibrils of extreme fineness, cemented together by a substance of different composition from the fibres themselves. This may be demonstrated by steeping a small piece of hide for some days in a stoppered bottle in lime-, or baryta-water, in which the inter-fibrillar substance is soluble, and then teasing a small fragment of the fibre with needles on a glass microscope-slide, and examining with a power of at least 200-300 diam. In the middle portion of the skin, these bundles of fibre are closely interwoven; but next the body, they gradually become looser and more open, forming the pars reticularis (or netted part); and the innermost layer is a mere network of « 8 » loose membrane, generally loaded with masses of fat-cells, and hence called adipose tissue.
It is this adipose tissue which is removed in the "fleshing" process. On the other hand, the outermost layer, just beneath the epidermis, is exceedingly close and compact, the fibre-bundles that run into it being separated into their elementary fibrils, which are so interlaced that they can scarcely be recognised. This is the pars papillaris, and forms the lighter-coloured layer, called (together with its very fine outer coating) the "grain" of leather. It is in this part that the fat-glands are embedded, while the hair-roots and sweat-glands pass through it into the looser tissue beneath.
Besides the connective-tissue fibres, the skin contains a small proportion of fine yellow fibres, called "elastic" fibres. If a thin section of hide be soaked for a few minutes in strong acetic acid, and then examined under the microscope, the white connective-tissue fibres become swollen and transparent, and the yellow fibres may then be seen, as they are scarcely affected by the acid. The hair-bulbs and sweat- and fat-glands are also rendered distinctly visible.
The nerves of the skin are very numerous, each hair being supplied with fibres passing into both the papilla and sheath. They also pass into the skin papillæ. They cannot readily be seen, without special preparation, and, so far as is known, exercise no influence on the tanning process. "Breaking the nerve" is a technical term, which signifies a thorough stretching and softening of the skin, but has nothing to do with nerves properly so called. The blood- and lymph-vessels are, from the present point of view, somewhat more important. They may often be seen in sections, and are lined with nucleated cells, similar to those of the glands. These are surrounded by coatings of unstriped muscular fibre, running both around and lengthways, and also by connective-tissue fibres. In the arteries, the muscular coating is much stronger than in the veins.
It may be thought that the space devoted to a discussion of the anatomical structure of the skin is disproportionately « 9 » large; but there can be no doubt that, in order to make improvements, nothing is of more importance than a clear conception, even to the smallest details, of the materials and causes to be dealt with. The illustrations are from actual specimens, and enable the various parts of the hide to be identified under the microscope.
As this instrument is a most useful means of investigation in the tanning industry, and one likely to be of increasing importance, it will be well, before proceeding further, to say a few words, both on the selection of a suitable instrument, and on its manipulation in general.
To do useful work, it is not necessary to possess a very elaborate or expensive instrument, but it is essential that the microscope be well made and good of its kind. As high powers are often required in the examination, both of hide sections and of ferments, which are the principal objects of investigation in a tannery, it is of the first importance that the fine adjustment should be perfectly steady, without vibration or backlash. This, in the writer's experience, is never the case with cheap microscopes, in which the fine adjustment is made by a screw at the side of the tube moving the nose by means of a lever. A much more satisfactory arrangement is that in which the whole body of the microscope is raised or lowered by a screw in a pillar at the back of the stand on which it slides. A rack for the coarse adjustment is useful, but not essential. If a sliding tube only is provided, it must be tight enough not to slip, but must move easily up and down with a sort of screwing movement. A mechanical stage is not at all necessary, and for most purposes one of black glass is better as well as cheaper. The diaphragm for regulating the light should be as near level with the surface of the stage as possible, and when examined with a low power should appear in the centre of the field. For research work on the minuter ferments, an achromatic condenser and the finest oil- or water-immersion lenses are necessary, but directions for this are beyond the scope of the present work. It may, however, be mentioned that Prof. « 10 » Flügge,[A] a first-class authority on the subject, especially recommends Abbé's illuminating apparatus as made by Zeiss.
[A] "Fermente und Mikroparasiten," Leipzig, 1883.
A frequent defect in cheap English microscopes is that the mirror for substage illumination does not bring the rays of a lamp to a focus exactly on the slide, but frequently some inches above it. This may be to a great extent overcome by the use of a bulls'-eye condenser between the lamp and the microscope. Another defect is that sometimes the centre of the mirror is not in a line with that of the microscope body.
The objectives (or lenses at the lower end of the microscope) are the most important part of the instrument, and however good it may be in all other respects, if these are defective the whole is useless. The most useful lenses for our purpose, if only two are to be selected, are a 1-in., magnifying about 50 diam., and a 1/4-in., magnifying about 200 to 400, according to the eye-piece; a 1/8-in. giving, say, twice this magnification will be needed to see the smaller bacteria distinctly, but it is possible just to see even the small putrefaction bacteria with a really fine 1/4-in. In any case, the highest power should be as perfect and of as large an angle as attainable. A good 1/4-in. should resolve Pleurosigma angulatum with direct light, and should show the movement of the granules of protoplasm in the round corpuscles which are present in saliva. In using the latter test, it must be remembered that the motion only lasts a very short time on a cold slide.
About 5l. is the very least for which a microscope can be obtained which is suitable for tanners' use; where it can be afforded, a better one is advisable.
Without disparaging other makers, it may be mentioned that the writer has generally used both the eye-pieces and objectives of Dr. Hartnack of Potsdam; and that they are moderate in price, at least for the dry combinations, and perfectly satisfactory for all technical purposes. Numbers 2, 5, and 8 objectives with No. 3 eye-piece, are sufficient for all ordinary work. If only 2 objectives are to be obtained, « 11 » Nos. 3 and 7 would be perhaps the best selection. It is always better to use objectives on the stand, and with the eye-pieces for which they are intended, but in case Hartnack's objectives are used on an English stand (which is easily done by means of an adaptor ring), it is important to remember that they are constructed to work with a shorter tube than that customary on English microscopes, and that they will not perform well if its length is much more than 6 in.; these objectives are not provided with a movable adjustment for thickness of cover-glasses, which for technical purposes is not required, and in inexperienced hands is apt to prove troublesome. Extra-thin covers must therefore always be used. Where this adjustment is provided, the object must be accurately focused, and then, maintaining this focus with the fine focusing-screw, the collar must be cautiously turned till the best definition is obtained. Practically it will be best to make this adjustment accurately once for all, and to take care to use covers selected of a uniform thickness.
High-power objectives of wide angle (which condition is essential to good defining power) necessarily work extremely close to the object, and it is always best to use the thinnest cover-glasses which can be got. Even then, with such glasses as Hartnack's No. 8, unless the sections are very thin, it will be impossible to examine their lower parts; and one of the greatest difficulties of microscopic research is to obtain them thin enough. It will be obvious, from what has been said, that the greatest care is needed to avoid screwing the objective down on the cover, and so breaking one or both of them. One way to avoid this is to screw down as close as possible to begin with, and then focus upwards. Another plan, when the object on the slide is small, is to keep continuously moving the slide gently with the fingers, while looking into the tube. It is then easy to notice when the dust and small particles on the slide come into focus, and if the point should happen to be overstepped the contact will generally be felt before serious damage is done.
Illumination is one of the most important points in practical microscopy. With powers of not less than 1/2-in. focus, objects may generally be examined by light thrown upon them from above by a bulls'-eye condenser, or by good daylight. In this case they need not be transparent; and the plan is often convenient for a mere surface examination. In examining bodies illuminated in this way, prominences often appear as hollows and vice versâ, by a sort of optical illusion, which, once established, is very difficult to overcome. By remembering the direction of the light, and that this appears reversed in the microscope, it is easy to decide the truth.
For all finer work and higher powers, and most generally with the low powers also, it is necessary to render the object transparent, and to examine it by light transmitted from the mirror below the stage.
Good daylight is least trying to the eyes. Where artificial light must be used, that of a small paraffin lamp is best; and a blue chimney, or blue glass interposed between the stage and mirror, or lamp and microscope, spares the sight, and makes it easier to distinguish colours. The light should be sufficient, but not too dazzling. Work should never be prolonged after the least strain is felt, nor should the microscope be used for some little time after a meal. It is well to accustom oneself to keep both the eyes open while observing.
If it be required to see how far the cellular structures of the hide, such as hair-sheaths and fat-glands, are affected or destroyed in any stage of liming or bating, the following ready method may be employed. If a strip of hide be cut 2/3 through from the grain side, as shown at a in Fig. 7, and the flap be turned down, and held between the finger and thumb, the fibrous tissue will be put on the stretch, and will then allow a moderately thin shaving (including the grain and parts immediately below it) to be cut by a sharp razor. The hide should be held in the positions shown, and a steady drawing cut be made from flesh to grain, the razor being « 13 » steadied on the tip of the forefinger, and its hollow surface flooded with water. If the thin section be now placed on a glass slide, moistened with a drop of water, and examined on the microscope under a strong light from above, with a 1-in. objective, the fat-glands will be seen as yellow masses, embedded in the white fibrous tissue. If a drop of a mixture of equal vols. of strong acetic acid, glycerin, and water be used to moisten the section, the fibrous tissue will become quite transparent, and whatever remains of the cellular tissue will be easily visible, and may even be studied under tolerably high powers if covered with a thin glass, and lighted by the mirror from below. (The cover-glass must be carefully cleaned by rubbing with a linen handkerchief, and placed in position with a pair of tweezers, one side being supported by a needle, which is gradually withdrawn, so as to avoid air-bubbles.) Care must be taken that this mixture does not touch the brass-work of the microscope; even the vapour is apt to tarnish, so that the preparation must not remain longer than necessary on the microscope. The same method is applicable for ascertaining the completeness of the tannage of leather, and to decide whether the hide fibre is really tanned, or only dyed. Actually tanned leather is unaffected by the acetic acid, but raw or only stained hide swells and becomes transparent.
To prepare the very thin sections necessary for detailed study of the hide, more complicated methods are required. Small slips of hide, not exceeding 1/4 in. wide, and cut exactly « 14 » across the lie of the hair, are placed first in weak alcohol (equal parts methylated spirit and water), and, after a few hours, are removed into strong methylated spirit. It is then kept for some days in absolute alcohol, which must be repeatedly changed, until the hide is hard enough to give fine shavings, and may be cut either when held as above described, between cork or pith, or when embedded in paraffin wax. This is accomplished by placing the piece of hide in a little paper-box and covering it with melted paraffin (candle), which is just beginning to stiffen. The piece of hide may be fixed in position with a needle, which must of course be withdrawn before cutting. When hard, the paraffin is shaved away till the object is exposed, when it may be cut. The razor must be wet with alcohol, and the section be made exactly in the plane of the hair-roots, which may be seen with a hand-lens. (The use of a microtome for hide-sections is rarely successful, as it is almost impossible to fix the fragment of hide so that it is cut exactly with the hairs.) The slices may now be stained by placing them in a watch-glass with water and a few drops of the logwood or picrocarmine staining-mixtures sold by opticians, and afterwards either examined in glycerin, or, after soaking some hours in absolute alcohol, may be transferred to clove-oil, and afterwards to a slide, and covered with a drop of dammar varnish or Canada balsam dissolved in chloroform. The sections moistened with glycerin may also be mounted in Farrant's solution or glycerin jelly, under a cover-glass for permanent preservation. If picrocarmine be used, the connective-tissue fibres (gelatinous fibres) and the nuclei of the cells will be coloured red, and the cells themselves of both epidermis and glands, together with the muscles and elastic fibres, will be yellow.
Franz Kathreiner, who has made very elaborate researches on skin, and the changes which take place in it during the processes of tanning, employs a mixture of osmic and chromic acids for hardening, and at the same time staining the tissue. This mixture was first used by a German histologist « 15 » with whose name I am not acquainted, in a research on the internal organs of hearing, and was applied by Kathreiner in 1879 to the investigation of skin, and communicated by him to the writer in the autumn of that year. His method is briefly as follows. The pieces of hide to be examined must, if salted, be well washed, or if dry, be thoroughly softened. For the study of hide in its unaltered and natural condition, it is essential that it be quite fresh, and taken from the animal as soon as possible after death. In any case the Panniculus adiposus or fatty layer is, as far as possible, removed with scissors, the hair cut short, and the skin cut up into little pieces of 3-4 millimetres wide by 10-12 millimetres long (about 1/8 in. by 1/2 in.); the hair must lie exactly across these pieces.
They are then placed for 4-8 days, according to the thickness of the hide, in about 12 times their volume of a solution consisting of
| 0·2 | parts osmic acid.[B] |
| 0·5 | " chromic acid. |
| 200·0 | " water. |
[B] Solution of osmic acid is best preserved in sealed tubes in the dark. If obtained in solution it is rarely of full strength, for which allowance may have to be made. Care must be taken to avoid inhaling its fumes, which are very irritating to the eyes and to the respiratory organs, producing severe catarrh.
This solution must be kept from dust and light, in a glass stoppered bottle, and in a cool place. On removing the hide-pieces from this solution, they are placed in about 12 times their volume of absolute alcohol for 4-8 days, during which time the spirit must be at least 3 times renewed. The sections are cut with a razor flooded with absolute alcohol, so that the thin shavings float without friction upon it. The hide-pieces may be held either between soft cork, or, as is generally preferable, simply between the forefinger and thumb as shown in Fig. 7. The cut must be made exactly parallel with the direction of the hair roots, and from the grain towards the flesh; and the sections cannot possibly be too thin. After lying for 1/2-1 hour in « 16 » absolute alcohol, the sections are soaked till quite clear in clove oil (which must be pale and of the purest kind), and may then be mounted in dammar varnish, or solution of Canada balsam.
In these sections, fat and the oily contents of the fat glands are stained black, and the limits of the cells both of these glands and of other elements of the hide (rete malpighi, hair-bulb, &c.) are made very distinct, so as to be capable of the most delicate investigation under the highest powers; but the beginner will learn most easily to recognise the different tissues by studying at first some sections stained with picrocarmine as before described. The method is admirably adapted for the study of hide as affected by the limes and bates.
CHEMICAL COMPOSITION OF HIDE.
The chemical composition of skin is very imperfectly understood. The bulk of the skin is, as has long been known, converted by boiling into gelatin or glue. The yellow fibres and cellular tissue remain undissolved. Müntz, who made some interesting researches on the subject, found that completely dried hide contained—3·086 per cent. of cellular tissue insoluble in hot water, 1·058 of fat, 0·467 of mineral matter, and 95·395 of matters soluble in hot water. Müntz counts the whole of the tissue soluble in hot water as converted into glue; but this is not strictly the case. Gelatin is not identical with the fibre of the hide, which is only converted into it by boiling. The nature of the change is not well understood; but it is either simply molecular, or depends on the addition of one or more molecules of water. The gelatin of bones seems identical with that of skin and connective tissue, but that of cartilage differs slightly from it, and is called chondrin. Raw hide, unhaired and purified, contains, according to Müntz—carbon, 51·43 per cent.; hydrogen, 6·64; nitrogen, 18·16; oxygen, 23·06; ash, 0·71; while gelatin has—carbon, 50·1 per cent.; hydrogen, 6·6; nitrogen, 18·3 (Mulder); carbon, 50 per cent.; hydrogen, 6·5; nitrogen, 17·5 (Fremy). Probably, however, neither substance was quite pure.
Gelatin is insoluble in alcohol, ether, and cold water, but swells in the last, absorbing about 40 per cent. It is soluble in hot water, but is reprecipitated on the addition of a sufficient quantity of alcohol, resembling in this respect gum, dextrin, and many other substances. It is soluble in glycerin, with the aid of heat, and in concentrated sulphuric « 18 » acid in the cold. Moist gelatin exposed to the air rapidly putrefies. It first becomes very acid, from formation of butyric (and perhaps other) acids, but afterwards alkaline, from evolution of ammonia. Boiled with concentrated potash, it yields leucin (amidocaproic acid, C6H15NO2), glycocin (sugar of gelatin), and other substances. The same products are obtained by boiling with sulphuric acid, and probably also more gradually, and in greater or less proportions, by the prolonged action of lime or barium hydrate, by putrefaction, and by any other influence which tends to resolve the gelatin molecule into its simpler parts. Gelatin is precipitated by all tannins, even from very dilute solution. A solution containing 2/10000 parts is rendered turbid by infusion of gall-nuts or gallotannic acid. The precipitate is soluble in excess of gelatin. Solution of gelatin dissolves considerable quantities of lime phosphate, hence this is always largely present in common glue. Gelatin is precipitated by mercuric chloride, in this respect resembling peptones; but not by potassium ferrocyanide, by which it is distinguished from albuminoids; and it differs from albumen in not being coagulated by heat. On the contrary, by prolonged boiling glue loses the property of gelatinising, and becomes soluble in cold water, being split up into two peptones; semi-glutin, which is insoluble in alcohol, and precipitated by platinic chloride; and hemicollin, which is soluble in alcohol, and not precipitated by platinic chloride. Both are precipitated by mercuric chloride (see Hofmeister, abst. Chem. Soc. Jour. 1881, p. 294). Gelatin or glue with about 3 per cent. of potassium dichromate becomes insoluble when exposed to the light, from the formation of a chromium compound. This reaction is the base of several modern photographic processes, and has been used for waterproofing and for cementing glass, &c.
The connective-tissue fibres are partially converted into gelatin by the action of strong acids and alkalies, as well as by heat. By weak acids, they are swollen and gradually dissolved, and Reimer[C] has found that the material may « 19 » be reprecipitated by lime-water. It forms an irregular fibrous mass, which has not the sticky feel of gelatin, but is at once converted into that body by boiling. Rollet has demonstrated that when hide and other forms of connective tissue are soaked in lime- or baryta-water, the fibres become split up into finer fibrils, and as the action proceeds, these again separate into still finer, till the ultimate fibrils are as fine as can be distinguished under a powerful microscope. At the same time, the alkaline solution dissolves the substance which cemented the fibres together, and this may be recovered by neutralising the solution with acetic acid, when it comes down as a flocculent precipitate. This was considered by Rollet to be an albuminoid substance; but Reimer has shown that it is much more closely allied to the gelatigenous fibres, if indeed it is not actually produced from them by the action of the alkaline solution. Reimer used limed calf-skin for his experiments, and subjected it to prolonged cleansing with distilled water, so that all soluble parts must have been pretty thoroughly removed beforehand. He then digested it in closed glasses with lime-water for 7-8 days, and precipitated the clear solution with dilute acetic acid. He found that the same portion of hide might be used again and again, without becoming exhausted, which strongly supports the supposition that it is merely a product of the partial decomposition of the hide fibre. The substance, which he called "coriin," was purified by repeated solution in lime-water, and reprecipitation by acetic acid. It was readily soluble by alkalies, but insoluble in dilute acids, though in some cases it became so swollen and finely divided as to appear almost as if dissolved. It was, however, very soluble in common salt solution of about 10 per cent., though it was precipitated both by the addition of much water, and by saturating the solution with salt. Reimer found that a 10 per cent. salt solution was equally effective with lime-water in extracting it from the hide, and that it was partially precipitated on the addition of acid, and completely on saturating the acidified solution with salt. « 20 » Other salts of the alkalies and alkaline earths acted in a similar manner, so that Reimer was at first deceived when experimenting with baryta-water, because, being more concentrated than lime-water, the coriin remained dissolved in the baryta salt formed on neutralising with acid, and it was necessary to dilute before a precipitate could be obtained. The slightly acid solution of coriin gave no precipitate with potassium ferrocyanide, nor was it precipitated by boiling, being thus distinguished from albuminoids. The neutral or alkaline solution was not precipitated by iron or mercuric chloride, copper sulphate, nor by neutral lead acetate; but was precipitated by basic lead acetate, basic iron sulphate, and excess of tannin. Its elementary composition is—carbon, 45·91: hydrogen, 6·57; nitrogen, 17·82; oxygen, 29·60; and Reimer proposes the following equation as representing its relation to hide fibre:—
| Hide fibre. | Water. | Coriin. | ||
| C30H46N10O12 | + O + | 2H2O | = | C30H50N10O15. |
[C] Dingler's Polyt. Journal, vol. 220, p. 167.
Hide Albumen.—The fresh hide, besides this coriin (which, very possibly, is only evolved by the action of the lime), contains a portion of actual albumen, viz. that of the blood serum and of the lymph, which is not only contained in the abundant blood-vessels, but saturates the fibrous connective tissue, of which it forms the nourishment. This albumen is mostly removed by the liming and working on the beam, which is preparatory to tanning. Probably for sole-leather, the albumen itself would be rather advantageous if left in the hide, as it combines with tannin, and would assist in giving firmness and weight to the leather. It is, however, for reasons which will be seen hereafter, absolutely necessary to get rid of any lime which may be in combination with it. The blood also must be thoroughly cleansed from the hide before tanning, as its colouring matter contains iron, and, in combination with the tannin, would give a bad colour.
The reactions of blood and lymph albumen are very similar to those of ordinary white of egg. It is precipitated by « 21 » strong mineral acids, especially nitric, and also by boiling. The precipitate produced by strong hydrochloric acid redissolves by the aid of heat to a blue or purple solution. Tribasic phosphoric, tartaric, acetic, and most other organic acids, do not precipitate moderately dilute solutions of albumen, but convert it into a sort of jelly, which, like gelatin, does not coagulate, but liquefies on heating. It is precipitated by neutral salts of the alkali metals. Blood-albumen slightly acidified (with acetic acid) is precipitated by potassium ferrocyanide. It is not precipitated by dilute infusions of oak bark, but is rendered uncoagulable by heat, hence it cannot be employed to remove tannins from their solutions.
Elastic Fibres.—The elastic or yellow fibres of the hide are of a very stable character. They are not completely dissolved even by prolonged boiling, and acetic acid and hot solutions of caustic alkalies scarcely attack them. Probably they do not combine with tannin, and are very little changed in the tanning process.
Hair, Epidermis, and Glands.—These are, as has been seen, all derived from the epithelial layer, and hence, as might be inferred, have much in common in their chemical constitution. They are all classed by chemists under one name, "keratin," or horny tissue, and their ultimate analysis shows that in elementary composition they nearly agree. It is evident, however, that the horny tissues are rather a class than a single compound.
The keratins are gradually loosened by prolonged soaking in water, and, by continued boiling in a Papin's digester, are dissolved to an extract which does not gelatinise on cooling. Keratin is dissolved by caustic alkalies; the epidermis and the softer horny tissues are easily attacked, while hair and horn require strong solutions and the aid of heat to effect complete solution. The caustic alkaline earths act in the same manner as dilute alkaline solutions; hence lime easily attacks the epidermis, and loosens the hair, but does not readily destroy the latter. Alkaline sulphides, on the other hand, seem to attack the harder tissues with at least the same « 22 » facility as the soft ones, the hair being often completely disintegrated, while the epidermis is still almost intact; hence their applicability to unhairing by destruction of the hair. Keratins are dissolved by fuming hydrochloric acid, with the production of a blue or violet coloration, like the albuminoids. They also resemble albumen, in the fact that their solution in sulphuric acid is precipitated by potassium ferrocyanide. By fusion with potash, or prolonged boiling with dilute sulphuric acid, keratin is decomposed, yielding leucin, tyrosin, ammonia, &c. The alkaline solution of keratin (hair, horns, &c.) is precipitated by acids, and, mixed with oil and baryta sulphate, is employed under Dr. Putz's patent as a filling material for leather, for which purpose it acts in the same way as the egg-yolks and meal used in kid-leather manufacture. Eitner attempted to use it for the same purpose with bark-tanned leather, but without much success. Putz has also proposed to precipitate the material after working its solution into the pores of the leather.
COMMERCIAL TANNING MATERIALS.
Algarobilla.—The seed-pods of Prosopis pallida and P. Algarrobo are known as algarobilla, the two kinds being distinguished as negro and blanco. The trees are abundant in mountainous parts of South America, notably Chili and the Argentine Republic. The pods contain up to 50 per cent. of a bright-yellow tannin, somewhat resembling that of myrobalans. The friable tannin is readily soluble in cold water, and is so loosely held in the fibrous network of the pod, that great loss is sustained by careless handling. The commerce in algarobilla does not figure in the official trade returns; but J. Gordon & Co., Liverpool, obligingly state that they imported 50 tons, at an average value of 18l. 10s. a ton, in 1880. Widow Duranty & Son, also of Liverpool, are good enough to add that they received 160 tons in 1881, the first that had reached them for a long time. Havre imported 50 tons in 1881. The name algarrobo is also applied to Balsamocarpon brevifolium in Chili, and to Hymenæa Courbaril in Panama.
Chestnut-extract.—The wood of the chestnut (Castanea vesca) contains 14-20 per cent. of a dull-brown tannin. It is quite different from the bark and bark-extract of the American chestnut-oak (Quercus sessiliflora). Its extract is used largely to modify the colour produced by hemlock-extract, and for tanning and dyeing. The pulverised wood is also extensively employed in France. The imports are included in barks and extracts, p. 39.
Cork-bark. See Oak-barks.
Cutch, Catechu, or Terra Japonica (Fr., Cachou; Ger., Catechu).—The term kát, kut, or "cutch," is applied to « 24 » the dried extract, containing 45-55 per cent. of dark-coloured mimo-tannic acid, prepared chiefly from 2 trees:—(1) Acacia Catechu [Mimosa Catechu, M. sundra], a tree of 30-40 ft., common in most parts of India and Burma, growing also in the hotter and drier districts of Ceylon, and abundant in tropical East Africa—the Soudan, Sennar, Abyssinia, the Noer country and Mozambique, though the utilisation of its tannin is restricted to India; (2) A. [M.] Suma, a large tree inhabiting South India (Mysore), Bengal, and Gujerat.
The process for preparing cutch varies slightly in different districts. The trees are reckoned to be of proper age when their trunks are about 1 ft. diam. They are then cut down, and the whole of the woody part, with the exception of the smaller branches and the bark, is reduced to chips: some accounts state that only the darker heart-wood is thus used. The chips are placed with water in earthen jars, arranged in a series over a mud fire-place, usually in the open air. Here the water is made to boil, the liquor as it becomes thick and strong being decanted into another vessel, in which the evaporation is continued until the extract is sufficiently inspissated, when it is poured into moulds made of clay, or of leaves pinned together in the shape of cups, or in some districts on to a mat covered with the ashes of burnt cow-dung, the drying in each case being completed by exposure to the sun and air. The product is a dark-brown extract, which is the usual form in which cutch is known in Europe.
In Kumaon, North India, a slight modification of the process affords a drug of very different appearance. Instead of evaporating the decoction to the condition of an extract, the inspissation is stopped at a certain point, and the liquor is allowed to cool, coagulate, and crystallise over twigs and leaves thrown into the pots for the purpose. By this method is obtained from each pot about 2 lb. of kath or catechu, of an ashy-whitish appearance. In Burma, the manufacture and export of cutch form one of the most important items of forest revenue. The quantity of cutch exported from the province in 1869-70 was 10,782 tons, valued at 193,602l., of « 25 » which nearly half was the produce of manufactories situated in British territory. The article is imported in mats, bags, and boxes, often enveloped in the large leaf of Dipterocarpus tuberculatus. It is brought down from Berar and Nepal to Calcutta. That of Pegu has a high reputation.
Our imports of cutch in 1880 were 5155 tons, value 173,040l., from the British East Indies; 539 tons, 15,572l., from other countries; total, 5694 tons, 188,612l. Our exports in the same year were:—892 tons, 28,527l., to Germany; 676 tons, 24,562l., to the United States; 478 tons, 15,505l., to France; 303 tons, 10,537l., to Holland; 177 tons, 5859l., to Russia; 141 tons, 4835l., to Belgium; 245 tons, 8719l., to other countries; total, 2912 tons, 98,544l. The approximate London market value of Pegu cutch is 21-42s. a cwt.
An astringent extract prepared from the areca nut (Areca Catechu) is said to contribute to commercial cutch; if so, it is a totally distinct product from those just described.
Divi-divi, or Libi-dibi.—These names are applied to the seed-pods of Cæsalpinia coriaria, a tree of 20-30 ft., indigenous to several of the West Indies, Mexico, Venezuela, and North Brazil, and naturalised in Madras and Bombay Presidencies, and in the North-West Provinces. The pod may be known by its drying to the shape of a letter S; it contains 30-50 per cent. of a peculiar tannin, somewhat similar to that of valonia. It is cheap, and may be used in admixture with barks; but it is dangerously liable to undergo fermentation, suddenly staining the leather a dark-red colour, and is therefore not in extensive use. The imports of it are mainly from Maracaibo, Paraiba, and St. Domingo. Maracaibo, in 1880, exported 197,674 lb. of divi-divi, value 32221/4 dol. (4s. 2d.), to New York. Our imports of divi-divi into Liverpool, according to figures kindly furnished by Haw & Co., were 2200 tons in 1877, 1740 in 1878, 2132 in 1879, and 780 in 1880. The approximate market value is 12-17l. a ton.
Galls.—The generic term "gall" is applied to those excrescences on plants which are produced by the punctures « 26 » of insects, for the purpose of depositing their eggs. The excrescences are usually considered to be a diseased condition of vegetable tissue, resulting from the injection of some secretion of the insects. But this has been combated by A. S. Wilson, of Aberdeen, who considers that all insect galls are in reality leaf-buds, or fruit-buds, and not mere amorphous excrescences. The vascular lines which would form leaves can easily be followed up in the structure of the oak-leaf galls. And in cases where the egg has been deposited in the tissue of a young branch, the cap of the gall is sometimes surmounted by a leaf 2-3 in. long. But in the large blue Turkish galls, many lacunæ occur where the fleshified leaves have not filled up the spaces between them. If a dissection be made of one of the weevil-galls on the bulb of the turnip, the second or third slice will show the outer foliations, exactly similar to those of the root-buds. When the centre has been reached, where the maggot will be found, there will also be a vascular pencil running up from a medullary ray in the bulb, and bearing on its top a bud of the same description as that produced by a ray running out from a root. The insertion of the ovipositor brings a medullary ray into action, producing a tuberculated bud, and it is only the bud which the larva feeds upon. The growth of a bud is an intelligible cause of the growth of a gall, but nothing can be inferred from the injection of a fluid. The analogy to leaves is further shown by the fact that various microscopic fungi are matured in the interior of imperforate galls.
The principal commercial kinds of gall are oak-galls and Chinese galls.
Oak-galls, Nut-galls, Aleppo or Turkey-galls (Fr., Noix de Galle, Galle d'Alep; Ger., Levantische or Aleppische Gallen, Galläpfel).—These are formed by the punctures of Cynips [Diplolepis] Gallæ tinctoriæ on Quercus lusitanica var. infectoria [Q. infectoria], a shrubby tree of Greece, Cyprus, Asia Minor, and Syria, and probably other varieties and even species of oak. The female insect is furnished with a delicate ovipositor, by means of which she pierces the tender shoots of « 27 » the tree, and lays her eggs therein. In the centre of the full-grown gall, the larva is hatched and undergoes its transformations, finally (in 5-6 months) becoming a winged insect, and boring for itself a cylindrical exit-hole. The best commercial galls are those which have been gathered while the insect is still in the larval state. Such have a dark olive-green colour, and are comparatively heavy; but after the fly has escaped, they become yellowish-brown in hue, and lighter. Hence they are distinguished in the London market as "blue" or "green," and "white." In Smyrna, they are classified as "white," "green," and "black," the first two sorts generally fetching nearly the same price, while the black obtain considerably more, the approximate quotations being: white and green, per Turkish oke (of 2·83 lb.), 81/2-9 piastres (of 2d.); black, 131/2-14 piastres. The "nuts" come mostly from Melemen, Cassaba, and Magnesia, also from the Syrian coasts, being plentiful on the east of the river Jordan, and are chiefly forwarded to France, England, and Salonica. The triennial yield is said to be invariably the best. They begin to reach Smyrna from the interior towards the end of July. The crop of 1880 was estimated at over 50,000 okes. The province of Aleppo, which used to afford 10,000-12,000 quintals (of 2 cwt.) annually, only exported 3000 in 1871. The galls collected in the Kurdistan mountains are marketed at Diarbekir, and sent thence to Trebizonde for shipment. Bussora, Bagdad, and Bushire also export considerable quantities.
Knoppern, a species of gall formed from the immature acorns of Quercus pedunculata and Q. sessiliflora, are largely used for tanning throughout Austria.
The exports from Aleppo (including yellow berries) in 1880 were:—60 tons, 3600l., to Great Britain; 322 tons, 19,320l., France; 15 tons, 900l., Italy; 44 tons, 2640l., Austria; 55 tons, 3300l., Turkey; 30 tons, 1800l., Egypt; total, 526 tons, 31,560l. In 1878, the figures were 673 tons, 38,400l. Alexandretta exported in 1879 (including yellow berries):—41 tons, 2460l., to England; 299 tons, 17,940l., France; 20 tons, 1200l., Italy; 25 tons, 1500l., Austria; « 28 » 87 tons, 5220l., Turkey; 6 tons, 360l., Egypt; total 478 tons, 28,680l. The shipments from Trebizonde by steamer in 1880 were (from Turkey):—47 sacks (of 2 cwt.), 188l., to Turkey; 240 sacks, 960l., Great Britain; 264 sacks, 1056l., France; 103 sacks, 412l., Austria and Germany; 26 sacks, 104l., Greece; total, 680 sacks, 2720l.; (from Persia): 25 sacks, 100l., Great Britain; 31 sacks, 124l., France; 30 sacks, 120l., Austria and Germany; total, 86 sacks, 344l. Bushire despatched 5000r. worth to India in 1879. Syra sent 248l. worth to Great Britain in 1879. Venice exported 1745 tons of gall and bark, value 34,906l., in 1879.
The best oak-galls contain 60-70 per cent. of tannic or gallotannic acid, and 3 per cent. of gallic acid. "Rove" is a small crushed gall, containing 24-34 per cent. of gallotannic acid. There are many other varieties of non-commercial oak-gall.
Chinese or Japanese Galls.—These are vesicular protuberances formed on the leaf-stalks and branches of the Rhus semialata [Bucki-amela], a tree of 30-40 ft., common in North India, China, and Japan, ascending the outer Himálaya and the Khasia Hills to 2500-6000 ft., by punctures of the female of Aphis chinensis. The galls are collected when their green colour is changing to yellow, and are then scalded. They are light and hollow, 1-21/2 in. long, and of very varied and irregular form. The Japanese are the smaller and paler, and usually more esteemed. The galls contain about 70 per cent. of tannic or gallotannic acid, and 4 per cent. of another tannin. They are consumed mainly in Germany, for the manufacture of tannic acid.
Hankow exported 30,949 piculs (of 1331/3 lb.) in 1872; and 21,611 piculs, value 136,214 taels (of about 6s.), in 1874. In 1877, the total Chinese export did not exceed 17,515 piculs. Hankow exported 24,7421/2 piculs in 1878, and 28,392 piculs, 59,614l., in 1879; Pakhoi, 62l. worth in 1879; Canton, 31551/3 piculs in 1877, 1939 in 1878, 31631/2 in 1879; Ichang, 1001/2 piculs, 132l., in 1878, 4021/2 piculs, 586l., in 1879; Shanghai, 27,6591/2 piculs in 1879.
In China trade returns, they are always miscalled "nut-galls" or "gall-nuts": correctly, they are wu-pei-tze. Oak-galls are exported from China resembling those of Western Asia. Japanese galls, kifushi, are sent in increasing quantities from Hiogo.
Our imports of galls in 1880 were:—24,590 cwt., 68,697l., from China; 17,311 cwt., 60,648l., from Turkey; 9182 cwt., 9013l., from other countries: total, 51,083 cwt., 138,358l. Our re-exports in the same year were:—6260 cwt., 18,479l., to Holland; 6022 cwt., 18,147l., to Germany; 3214 cwt., 11,002l., to France; 3045 cwt., 8598l., to Belgium; 2651 cwt., 11,004l., to the United States; 1625 cwt., 5205l., to other countries; total, 22,817 cwt., 72,435l. The approximate London market values of galls are:—Bussora, blue, 82-102s. a cwt.; do., white and in sorts, 50-90s.; China, 50-70s.; Japan, 55-56s.
Gambier, Pale Catechu, or Terra Japonica (Fr., Gambir, Cachou jaune; Ger., Gambir).—These names are conferred upon an extract from the leaves of Uncaria Gambier [Nauclea Gambir] and U. acida, containing 36-40 per cent. of a brown tannin, which rapidly penetrates leather, and tends to swell it, but alone gives a soft porous tannage; it is largely used in conjunction with other materials for tanning both dressing- and sole-leather. The plants are stout climbing shrubs, the first-named being a native of the countries bordering the Straits of Malacca, and especially the islands at the eastern end, though apparently not indigenous to any of the islands of the volcanic band, growing also in Ceylon, where no use is made of it; while the second, probably a mere variety, flourishes in the Malay islands.
The shrubs are cultivated in plantations, often formed in jungle clearings; the soil is very rapidly exhausted, and further injured by excessive growth of the ineradicable lalang-grass (Andropogon caricosus). It is found advantageous to combine pepper-culture with that of gambier, the boiled leaves of the latter forming excellent manure for the former. The gambier-plants are allowed to grow 8-10 ft. high, and « 30 » as their foliage is always in season, each plant is stripped 3 or 4 times in the year. The tools and apparatus for the manufacture of the extract are of the most primitive description. A shallow cast-iron pan about 3 ft. across is built into an earthen fire-place. Water is poured into the pan, a fire is kindled, and the leaves and young shoots, freshly plucked, are scattered in, and boiled for about an hour. At the end of this time, they are thrown on to a capacious sloping trough, the lower end of which projects into the pan, and are squeezed with the hand so that the absorbed liquor may run back into the boiler. The decoction is then evaporated to the consistence of a thin syrup, and baled out into buckets. When sufficiently cool, it is subjected to curious treatment: instead of simply stirring it round, the workman pushes a stick of soft wood in a sloping direction into each bucket; and, placing two such buckets before him, he works a stick up and down in each. The liquid thickens round the stick, and, the thickened portion being constantly rubbed off, while at the same time the whole is in motion, it gradually sets into a mass, a result which, it is said, would never be produced by simple stirring: it is reasonable to suppose that this manner of treating the liquor favours the crystallisation of the catechin in a more concrete form than it might otherwise assume. The thickened mass, resembling soft yellowish clay, is now placed in shallow square boxes; when somewhat hardened, it is cut into cubes, and dried in the shade. The leaves are boiled a second time, and finally washed in water, which is saved for another operation.
A second plan is as follows:—The leaves are boiled, and bruised in a wooden mortar (lesong), from which they are put into a kind of basket of rattan open-work, which is pressed by a long piece of wood acting as a lever; the liquid is received into a trough, and there allowed to settle. When the sediment has acquired sufficient substance, it is put into a kulit-kayo, formed like a tub without a bottom, which lets the superfluous water drain off; when that is done, it is taken out, made into small cakes, and dried for use. A « 31 » plantation employing 5 labourers contains 70,000-80,000 shrubs, and yields 40-50 catties (of 11/3 lb.) of gambier daily.
Plantations were commenced in Singapore in 1829, and once numbered 800; but owing to scarcity of fuel, abundance of which is essential to the manufacture, and dearness of labour, the culture was fast declining in 1866. In 1872, it had much recovered. It is largely pursued on the mainland (Johore), and in the Rhio-Lingga Archipelago, S.-E. of Singapore; on Bintang, the most northerly of the group, there were 1250 plantations of it in 1854. None is cultivated in Sarawak, though found wild in many parts; the foreign export from Sarawak in 1879 had a total value of 88,148 dol. The best kind is brought largely from Sumatra, but is often adulterated with sago. The Rhio product is also thus sophisticated, and rendered heavier by the Chinese purposely packing it in baskets lined with wet cajangs, occasioning a loss to the purchaser of about 30 per cent.
Singapore is the great emporium for gambier, and exported 34,248 tons in 1871, 19,550 tons having been imported, chiefly from Rhio and the Malay Peninsula. In 1876, the export increased to over 50,000 tons of pressed block, and 2700 tons of cubes. In 1877, it fell to 39,117 tons, owing to differences with the Chinese dealers concerning adulteration; of this quantity, 21,607 tons were for London, 7572 for Liverpool, and 2345 for Marseilles. The United Kingdom imports in 1872 were 21,155 tons, 451,737l., almost all from the Straits Settlements; in 1880, they were 26,061 tons, 461,781l., from the Straits, and 352 tons, 6468l., from other countries; total, 26,413 tons, 468,249l. Our re-exports in 1880 were:—2487 tons, 48,507l., to Holland; 1591 tons, 31,542l. to Germany; 1137 tons, 23,694l., to Russia; 594 tons, 12,026l., to other countries; total, 5809 tons, 115,769l. The approximate London market values are 15s. 6d.-21s. 6d. a cwt. for block, 18-24s. for pressed cubes, and 23-27s. for free cubes.
Hemlock.—The bark of the hemlock or hemlock spruce (Abies canadensis), of Canada and the United States, contains nearly 14 per cent. of tannin. The stripping of the bark « 32 » commences in the southern parts of the United States in spring, and lasts during April-May; in New York, Michigan, and Wisconsin, the season is June-July; and farther north, it is still later. It is said that the best product is obtained farthest south. The destruction of the hemlock forests is fast approaching. Within the last 25 years, the preparation of an extract from the bark, containing 18-25 per cent. of a deep-red tannin, giving considerable weight and firmness to leather, has superseded the export of crude bark. One mode of preparing the extract is as follows:—The bark in pieces 1/2-1 in. thick, and several inches long, is soaked for about 15 minutes in water at 200° F. (93° C.); it is then fed into a hopper, which conducts it to a 3-roller machine, something like a sugar-cane mill, through which it passes, coming out lacerated and compressed; it next falls into a vat of hot water, where it is agitated by a wheel, that the tannin from the crushed cells may be dissolved in the water; hence it is raised by a series of buckets on an endless chain, somewhat in the manner of a grain-elevator, to another hopper, whence it is fed to another 3-roller mill; here it receives its final compression, and comes out in flakes or sheets, like coarse paper, and almost free from tannin. The buckets are made of coarse wire, that the water may drip through during the elevation. In order to avoid the blackening action of iron, wherever this metal will come into contact with the solutions it is thickly coated with zinc. The solution is evaporated to a solid consistency, generally by vacuum-pans. About 2 tons of bark are represented by 1 bar. (of less than 500 lb.) of extract. The chief makers are A. S. Thomas, Elmira, N.Y.; S. Brown & Co., New York; Canada Tanning Extract Co., St. Leonard and Bulstrode; J. Miller & Co., Millerton, New Brunswick. The total production is probably over 10,000 tons annually, ranging in value between 14l. and 20l. a ton. Our imports are included in barks and extracts.
Kino (Fr., Kino; Ger., Kino).—The term "gum kino" is applied to a class of astringent extracts of varied origin, none of which can accurately be called either resins or gums.
Pl. II.
REMOVING THE HAIR; SCRAPING AND CLEANING THE SKINS.
(1) East Indian or Amboyna Kino.—This is obtained from Pterocarpus Marsupium, a common tree in the central and southern parts of the Indian peninsula, and in Ceylon; and a liquid kind from P. indicus, of South India, Burma, Malacca, Penang, the Andamans, and Malaysia. The collection of the juice is effected in the following manner. A perpendicular incision, with lateral offshoots, is made in the stem of the tree when blossoming has set in, and a receptacle is placed at the foot of the incision. The exuding juice appears like red-currant jelly, but it soon thickens by exposure to the air, and when sufficiently dried, is packed into wooden boxes for exportation. It is one of the reserved timber-trees of the Government forests in Madras, and its juice is collected by natives, who pay a small fee for the permission. The hardened juice consists of blackish-red, angular, pea-like grains, partially soluble in water, almost entirely in spirit of wine of sp. gr. 0·838, readily in caustic alkaline solutions, and largely in a saturated solution of sugar. The liquid kino produces a very inferior article on drying. The annual collection of kino in Madras probably does not exceed 1-2 tons. Its approximate London market value is 60-150s. a cwt. It is employed medicinally, and in the manufacture of wines, and might be employed as a source of tannin in dyeing and tanning, if sufficiently cheap.
(2) Butea, Bengal, Palas or Dhak Kino.—This variety is afforded by the palas or dhak tree (Butea frondosa), common throughout India and Burma, and affording a dyestuff, and a fibre, as well as by B. superba and B. parviflora. During the hot season, there issues from natural fissures and from wounds made in the bark of the stem, a red juice, which quickly hardens to a ruby-coloured, brittle, astringent mass. It occurs in small drops or tears, and in flat pieces which have been dried on leaves, and is almost always mixed with bark-fragments. It is transparent, freely soluble in cold water, and does not soften in the mouth. It is unknown in European commerce, but is employed in India as a substitute for the kind first described.
(3) African or Gambia Kino.—This is derived from Pterocarpus erinaceus, a native of Tropical West Africa, from Senegambia to Angola. The juice exudes naturally from fissures in the bark, but more abundantly from incisions, and soon coagulates to a blood-red and very brittle mass, known to the Portuguese of Angola as sangue del drago ("dragon's-blood"). It is practically undistinguishable from the officinal kind first described, but is not a regular article of commerce.
(4) Australian, Botany Bay, or Eucalyptus Kino.—Several species of Eucalyptus afford astringent extracts, those from the "red," "white," or "flooded" gum (E. rostrata), the "blood-wood" (E. corymbosa), and E. citriodora, being quite suitable for replacing the officinal kind. It is chiefly obtained by woodcutters, being found in a viscid state in flattened cavities in the wood, and soon becoming inspissated, hard, and brittle. Minor quantities are procured in a liquid state by incising the bark of living trees, forming a treacly fluid yielding 35 per cent. of solid kino on evaporation. It is imported from Australia, but there are no statistics to show in what quantity.
Mimosa- or Wattle-bark.—The bark of numerous species of Acacia, natives of Australia, contains considerable percentages of deep-red mimo-tannic acid, which forms a hard and heavy tannage if used strong, though soft upper-leathers may be tanned with it in weak liquors. The chief kinds are as follows:—The common wattle (Acacia decurrens), including its variety A. mollissima, is known also under the names of green, black, and feathery, but must not be confounded with the silver wattle (A. dealbata), though but doubtfully a distinct species. The bark is obtainable in vast abundance, and is much used by tanners. The trees are stripped in September and the 2 or 3 months following, and the bark, being allowed to dry, is then in a marketable condition. This tree, which grows in the uplands, affords a larger percentage of tannin than the silver wattle.
Blackwood or lightwood (A. melanoxylon) yields tanners' « 35 » bark, which, is inferior, however, to that from A. decurrens. The bark of A. penninervis yields of tannic acid 17·9 per cent., and of gallic acid 3·8 per cent. The bark of the native hickory (A. suppurosa) yields of tannic acid 6·6 per cent., and of gallic acid 1·2 per cent.
The bark of A. saligna, of South-Western Australia, is much used by tanners, as it contains nearly 30 per cent. of mimo-tannin. A. harpophylla, of South Queensland, furnishes a considerable share of the mercantile wattle-bark for tanning purposes. The bark of A. lophantha contains only about 8 per cent. of tannin.
The broad-leaved or golden wattle (A. pycnantha), of Victoria and South Australia, deserves extensive cultivation. It is of rapid growth, will succeed even in sandy tracts, and yields seed copiously, which germinates with the greatest ease. The perfectly-dried bark contains about 25 per cent. of tannin. The aqueous infusion of the bark can be reduced by boiling to a dry extract, which in medicinal and other respects is equal to the best Indian cutch. It yields approximately 30 per cent. of tannin, about half of which, or more, is mimo-tannic acid. Probably no other tanning plants give so quick a return in cultivation as the A. pycnantha and A. decurrens of Australia. The latter varies in its proportions of tannin from 8 to 33 per cent. In the mercantile bark, the percentage is somewhat less, according to the state of its dryness, it retaining about 10 per cent. of moisture. The bark of the silver wattle (A. dealbata) is of less value, often even fetching only half the price of that of the black wattle. The bark improves by age and desiccation, and yields 40 per cent. of tannin, rather more than half of which is tannic acid.
Amongst all the kinds, the bark of the broad-leaved wattle is considered the most valuable, containing the greatest quantity of tannin; that of the silver wattle is not so valuable, being deficient in tannin; the black wattle is considered the most productive species; it can be barked at 8 years of age, and will produce 40-60 lb. dried bark, and full-grown trees will yield 100-150 lb. per tree.
The cultivation of wattles for commercial purposes has till now remained undeveloped; but no doubt, as soon as it is understood, the utilisation of many acres of land lying waste, or which have already been exhausted and rendered unfit for the growth of cereals, will be effected by the cultivation of the wattle. It requires so little attention as to make it very profitable, and wattle-growing and grazing can be combined satisfactorily. After the first year, when the young trees in the plantation have reached the height of 3-4 ft., sheep can be turned in.
Wattles grow in almost any soil, even the poorest, but their growth is most rapid on loose sandy patches, or where the surface has been broken for agricultural purposes. When the soil is hard and firm, plough furrows should be made at a regular distance of 6-8 ft. apart, into which the seeds are dropped. The seed should be sown in May, having been previously soaked in hot water, a little below boiling temperature, in which they may be allowed to remain for a few hours. The seed should be dropped at an average distance of 1 ft. apart along the furrow, in which case, about 7200 seeds would suffice for one acre of land. The seed should not be covered with more than about 1/4 in. of soil.
On loose sandy soil, it might even be unnecessary to break up the soil in any way; the furrows may be dispensed with, and the seed sown broadcast after the land is harrowed. After the plants have come up, they should be thinned so that they stand 6-8 ft. apart. When the young trees have attained the height of 3-4 ft., the lower branches should be pruned off, and every effort afterwards made to keep the stem straight and clear, in order to facilitate the stripping, and induce an increased yield of bark. It is advisable that the black and broad-leaved should be grown separately, as the black wattle, being of much larger and quicker growth, would oppress the slower-growing broad-leaved one. Care should be taken to replace every tree stripped by re-sowing, in order that there should be as little variation in the yield as possible. The months of September-December, in Victoria, « 37 » are those in which the sap rises without intermission, and the bark is charged with tannin. Analysis proves that the bark from trees growing on limestone is greatly inferior in tannin to that obtained from other formations, differing 10-25 per cent.
The estimated expenditure on a wattle-bark plantation of 100 acres during 8 years is:—
| £ | s. | d. | |
| Rent of 100 acres for 8 years at 6s. per acre per annum | 240 | 0 | 0 |
| Ploughing 100 acres in drills 10 ft. apart | 25 | 0 | 0 |
| Sowing wattles and actual cultivation, including cost of seed | 37 | 10 | 0 |
| Supervision for 8 years (nominal), say 10l. per annum | 80 | 0 | 0 |
| Pruning the trees, taking off useless wood (necessary for 2 years), 10s. per annum | 50 | 0 | 0 |
| Incidental and unforeseen expenses | 27 | 10 | 0 |
| Interest on the whole amount expended during 8 years | 240 | 0 | 0 |
| 700 | 0 | 0 | |
| Actual cost of stripping and carting, as shown below | 1515 | 0 | 0 |
| £2215 | 0 | 0 |
The receipts derivable from a wattle plantation of 100 acres, planted in the manner proposed, would be:—
| £ | s. | d. | |
| Each acre planted with wattles, 10 ft. apart, would carry 400 trees, and at end of 5th year trees would yield say 56 lb. matured bark: stripping only every 3rd tree, 332 trees would be obtained off 100 acres: this, at 4l. per ton, would give for 1st stripping | 1332 | 0 | 0 |
| In the 6th or following year, a similar number of trees would be stripped: the bark having increased in weight (say 14 lb.), the increased yield of 2nd stripping would be 400 tons at 4l. per ton | 1600 | 0 | 0 |
| In the 7th year, the remaining trees would be stripped, from which a still greater increase would be obtained, say 480 tons at 4l. per ton | 1920 | 0 | 0 |
| Total yield of bark | 4852 | 0 | 0 |
| The cost of stripping would not exceed 15s. per ton, on account of the facilities presented by the regularity of the trees, while carting would represent another 10s. per ton: these combined charges would be 25s. per ton, and on 1215 tons, would be | 1515 | 0 | 0 |
| Leaving a clear profit on the 100 acres of | £2637 | 0 | 0 |
The exports of mimosa-bark in 1876 were 11,899 tons from Victoria, 4758 from South Australia, and 1735 from Tasmania. Later returns are included in barks, p. 39. Shanghai imported 7038 piculs (of 1331/3 lb.) in 1879. The approximate London market values of mimosa-bark are:—Ground, 6-13l. a ton; chopped, 5-12l.; long, 5l.-9l. 10s. A very superior extract has been made from this bark.
Myrobalans or Myrabolams.—The fruits of several species of Terminalia constitute the myrobalans of commerce; they are chiefly T. Chebula and T. Bellerica, natives of India, the former being a tree 40-50 ft. high, and esteemed for its timber also. The fruits contain 30-35 per cent. of gallotannic and ellagitannic acids, producing a soft and porous tannage, and good samples giving a bright-yellow colour. The tannin exists in the pulp, and is absent from the very hard "stone." The dried fruits are known locally as har, harra, or bahera, and are used commonly for dyeing, but not for tanning.
Our imports of myrobalans in 1880 were:—238,151 cwt., 121,465l., from Bombay and Sind; 115,670 cwt., 51,339l., from Madras; 11,020 cwt., 4717l., from Bengal and Burma; 3520 cwt., 1402l., from other countries; total, 368,361 cwt., 178,923l. Our re-exports in 1880 were 8015 cwt., 4328l., to Germany; 16,127 cwt., 8515l., to other countries; total, 24,142 cwt., 12,843l. The approximate London market values of myrobalans are 7-14s. a cwt. for good, and 5-10s. for common. Shanghai imported 4403 piculs (of 1331/3 lb.) in 1879.
Oak-barks (Fr., Écorces de Chêne; Ger., Eichenrinden).—The barks of several species of oak have valuable tanning properties. They are chiefly:—The common oak (Quercus Robur, varieties: sessiliflora, Ger. Traubeneiche; pedunculata, Ger. Stieleiche), which is of even greater importance as a timber-tree; the cork-oak (Q. Suber); the evergreen oak (Q. Ilex); and the American chestnut-oak (Q. Castanea). These barks are among the most esteemed tannins as regards quality of leather, but are incapable of giving much weight, and from their bulk are costly to handle, containing only 10-12 per « 39 » cent. of tannin (quercitannic acid). They give a reddish fawn-coloured leather, and deposit a good deal of bloom, but yield little or no gallic acid. The barks of the cork-oak and evergreen oak from Southern Europe, are stronger and darker-coloured than English bark. The American chestnut-oak contains a peculiar fluorescent principle like æsculin.
Our imports of unspecified barks for tanners' and dyers' use in 1880 were:—189,399 cwt., 101,108l., from Australia; 123,302 cwt., 32,974l., Belgium; 57,232 cwt., 20,988l., United States; 22,100 cwt., 6030l., Holland; 18,648 cwt., 3676l., Italy; 16,151 cwt., 6972l., Algeria; 22,669 cwt., 8838l., other countries; total, 449,501 cwt., 180,586l. Our imports of unenumerated bark-extracts in the same year were valued at:—516,578l. from Holland, 92,654l. France, 30,187l. United States, 16,315l. British North America, 12,796l. Belgium, 13,769l. other countries; total, 682,299l. Our re-exports of barks in 1880 were:—19,548 cwt., 10,348l., to Germany; 14,627 cwt., 7425l., France; 4555 cwt., 3041l., Holland; 10,304 cwt., 6080l., other countries; total, 49,034 cwt., 26,894l.
With regard to cork-tree bark, James Gordon & Co., Liverpool, obligingly write that very little comes to England, the great bulk going direct to Ireland, where the consumption is large. The imports at Liverpool in 1880 were 186 tons, average value 8l. per ton. Of oak-bark, Hungary, in 1877, produced 25,000 tons, of which, 20,000 were exported to Germany for tanning purposes. The approximate London market values of oak-bark are:—English, 12-16l. per load of 45 cwt.; Foreign, tree, 5-8l. a ton; ditto, coppice, 6-8l. In 1879, Algiers exported 12,660,047 kilo. (of 2·2 lb.) of tanning bark.
Quebracho.—The local name quebracho, contracted from quebra-hacho ("axe-breaker"), is applied to several South American trees possessing hard wood, belonging to distinct genera. They are chiefly as follows:—(1) Aspidosperma Quebracho, the quebracho blanco, a tree growing in the province of Catamarca, Argentine Republic; (2) Loxopterygium« 40 » [Quebrachia] Lorentzii, the quebracho colorado, most prevalent in the province of Corrientes, the wood and bark of which come largely into commerce as tanning materials; (3) Iodina rhombifolia, the quebracho flojo, whose wood and bark are mixed with those of No. 2; (4) Machærium fertile [Tipuana speciosa], the tipa, which affords both wood and bark of less tanning value than No. 2. It would seem that the wood and bark of No. 2 are by far the most largely employed, containing 15-23 per cent. of a bright-red tannin. The wood and an extract from it are imported into Europe.
From information kindly furnished by James Gordon & Co., and Haw & Co., of Liverpool, it appears that the imports of quebracho-wood into Liverpool in 1880 were 200 tons, value about 4l. 10s. a ton; and of quebracho-bark, about 20 tons, none of which had been sold.
Sumach or Shumac (Fr., Sumac; Ger., Gerbersumach, Schmack).—The commercial term "sumach" is applied to the dried leaves of a number of South European and American tannin-yielding plants. These are chiefly as follows:—In Sicily, the European or tanning-sumach (Rhus Coriaria); in Tuscany, R. Coriaria, often adulterated with leaves of Pistacia lentiscus; in Spain, several Rhus spp., the products being divided into 3 kinds—Malaga or Priego, Malina, and Valladolid; in the Tyrol, the smoke-tree or fragrant or Venetian sumach (R. Cotinus); in France, Coriaria myrtifolia, divided into 4 sorts—fauvis, douzère, redoul or redon, and pudis; in Algeria, Tezera sumach (R. pentaphylla), used by the Arabs for making morocco-leather; in North America, the smooth or white sumach (R. glabra), the Canadian sumach (R. canadensis), the staghorn sumach (R. typhina), and the dwarf or black sumach (R. copallina). These are found growing wild in the countries indicated, and are further subjected to cultivation in some districts, notably in Sicily. R. glabra and R. copallina are recommended chiefly for extended cultivation in the United States.
The soil usually chosen for cultivation of the plants is poor and light; but a much larger crop of leaves can be « 41 » secured from strong, rich, deep soils, and it is generally admitted that the product in the latter case is also better. In Italy, limestone soils are considered to be especially suited to this culture, but the American varieties appear to be well adapted to sandy and clay soils as well. The primary requisite in a soil is that it shall be well drained, the presence of stagnant water about the roots being exceedingly prejudicial. To prepare the soil for planting, it is ploughed as deeply as possible, and laid out in rows about 2 ft. apart. In Italy, small holes are made about 2 ft. long, 7 in. wide, and 5 in. deep, and a plant is inserted at each end. A more convenient method would consist in marking the field in shallow furrows in one direction 2 ft. apart, and then, with a heavy plough, tolerably deep furrows the same distance apart as, and at right angles to, the first. A plant may then be placed in the deep furrows at each intersection, the furrow again filled with the plough, and the earth pressed about the plant with the foot. If this were done in early spring-time, as soon as the earth is sufficiently dry to be conveniently worked, there can be no doubt that it would be successful, while it would certainly involve little cost. Plants are generally propagated from the young shoots which form each year about the base of an older plant, but may also be produced from cuttings made from young well-ripened wood, rooted by setting in a nursery or in frames, as in the propagation of grape-vines from cuttings. This latter method is scarcely ever required, however, when the cultivation has been started. Plants are also raised from seed, and seedlings are always found to be strong, vigorous, and thoroughly hardy; but on account of the greater time and labour involved in their production, this method of propagation has not received extended application. The first-mentioned generally gives the quickest, and probably most satisfactory results.
In selecting plants from any source, there are certain points to be observed:—(1) The shoots should come from young vigorous plants; (2) they should be over 1 ft. long; « 42 » (3) those with large roots and few rootlets should be rejected; (4) those having white roots, covered with a fibrous, white, silky down, are also to be rejected, this being an indication of the presence of a very injurious subterranean parasitic fungus, capable of destroying the entire crop; (5) a good shoot is straight, at least 1/2 in. diam., 18 in. long, furnished with numerous buds close to each other, root short, but covered with rootlets. Shoots for planting may be collected in autumn, after the leaves have fallen, and be preserved in a nursery until spring; or this may be done in early spring, when the ground is very moist and soft. In either case, care should be observed that the rootlets are not injured by drying, or from any other cause.
The culture to be given the plant is somewhat similar to that required by Indian corn: the earth about it should be kept tolerably mellow and free from weeds, and such conditions can probably be maintained to a degree sufficient for sumach, by working several times during the growing season with a cultivator, and passing through the rows occasionally with a plough. All this work is not absolutely necessary to the life of the plant, but its vigour, and consequently its yield in leaves, may be considerably increased and strengthened thereby. After the first year, the number of operations may be diminished, but they should always be sufficient to keep the ground free from weeds and grass.
Shortly after planting, and when the plant is well set, the stock is pruned to a length of 6-8 in., when the plant is left to assume any form, and is no further pruned except by the process of collecting the leaves, unless hand-picking is resorted to; in such case, after the 2nd year, pruning takes place each year in the fall or winter, the plant being reduced to a height of 6-10 in. After the 3rd year, the plant begins to produce the shoots from about its base, already mentioned; these, if not needed for new plantations, should be removed each year, for if left to develop, they weaken the plant. If not removed during the summer, the operation should without fail be effected during the fall or winter.
The 1st crop of leaves may be secured during the year following that of planting. This develops and matures somewhat later than that from older plants, and in Italy it is not collected until the end of August or the 1st of September; but there are reasons for believing that in the United States, especially in the Northern States, the collection of leaves from native varieties should be made much earlier, because the summer is much shorter, and the habits of the varieties grown differ from the Sicilian. Macagno has shown (Chem. Soc. Journ., xxxviii., p. 733) that the leaves from the upper side of the branches contain much more tannin than those below, and that especially in the lower leaves the percentage of tannin is much higher in June than in August. All the leaves, except the young and tender ones of the extremities of the branches, are stripped off and placed in baskets, in which they are carried to a threshing-floor, where they are spread out in thin layers to dry. Here they must be frequently stirred and turned over, for which purpose a fork with wooden prongs is employed. In the fall, when growth is finished, and before the leaves have had time to become red, those remaining on the extremities are collected. To this end, the branches are broken just below the tuft of leaves, and the latter are allowed to remain suspended from the branch by a piece of bark not detached, and left in this condition until nearly or quite dry. They are then collected and treated in the same manner as other leaves, but the product obtained in this way is always of inferior quality.
After the 2nd year, crops of larger quantity and superior quality are obtained, and the collection is made in a different way, and much more frequently. The two methods followed in Sicily are (1) pruning, and (2) defoliation. The first, which is the more ancient, but much less costly, requires less care, and is simple and rapid; but it is injurious to the future condition of the plant, and the quantity of subsequent crops. The second, though slower, serves to better maintain the vigour of the plant, and the uniform quantity of the crop « 44 » from year to year; in consequence, it reduces the necessity for frequent renewal of stocks.
Harvest by pruning is carried on in Italy as follows. During May, the lower leaves, which, from greater age, appear to have attained full maturity, and may be in danger of loss from falling, are removed in the same manner as described for collecting the leaves from yearling plants. Toward the end of June, and during the course of July, all branches bearing leaves are cut away, reducing the plant to the principal stock: by this means, the crop is harvested and the plant is pruned at the same time. But even in Sicily, the time for this operation is limited to no absolute period, and varies with the development of the leaf, as indicated by cessation of growth and increase in size. In this condition, also, the leaves will have acquired their deepest green colour, and attained their maximum weight and best quality. It is further stated that while this time varies according to locality, about Palermo it is never earlier than June nor later than July. The harvest by pruning must always be made by men accustomed to the work, and equal to the exertion required. Provided with a pruning-bill, they cut off all leaf-bearing branches, collecting them an the left arm, until each has cut as much as he can conveniently carry, when he places the armful on the ground with the butts in the direction of the prevailing wind, which, if tolerably strong, might carry away some of the leaves if turned in the opposite direction; finally, he presses down the branches with his foot, to make the heap more compact, and leave less surface exposed to the wind and sun. Another labourer deposits a second armful in the same place, presses it with his foot in like manner, and the two deposits constitute a bundle. At the close of the operation, there remain the young shoots which are formed about the base of the plant, the leaves of which are not fully developed, and consequently not fit for collection until at least 20 days later. After this time, they are removed by hand, care being observed not to injure the buds, especially if the shoots are « 45 » to be used for stocks in the formation of plantations in the following year.
Defoliation, or collection by hand, is carried on whenever the leaf may be fully developed and ripe, beginning at first with the lower leaves, and continuing eventually to the ends of the branches. It takes place at 3 different times during the season: the 1st in May, the 2nd late in July or early August, and the 3rd in September. At the last collection, the extremities of the branches are broken down, and the leaves are allowed to dry before removal from the plant, as described under collections of the 2nd year. In the application of this method, the regular pruning is effected during the fall or winter, when the plant is dormant, and under such conditions the operation becomes a regenerative one, giving in this particular an advantage over the other method, in which the pruning is effected in the summer when the plant is in full vegetative activity, and so has a strongly deteriorating influence. In both methods of pruning, care should be observed to leave a long slanting section, upon which water will be less likely to settle and promote decay.
The leaves collected by either method are dried in the open field where they have grown, and when dried, are carried to a threshing-floor to be beaten, or at once to the threshing-floor and dried there. In the former, the operation is rather more rapid, but there is greater danger of injury by rain, the effect of which is very deleterious, especially if it fall upon the leaves when they are partially dried. The damage resulting from this cause is less if the leaves are not lying upon the ground, and are so arranged that the air may circulate freely about and under them. In the pruning method, the leaves are dried upon the branches and in the heaps where they are first deposited. Sometimes they are turned, but generally it is considered better not to disturb them until completely dried, and ready for transportation to the threshing-floor. In this way, they are protected to a greater extent from the action of direct sunlight, which is said to be injurious to the quality of the product. When « 46 » the leaves are collected by hand, they are dried upon the threshing-floor, where they are spread in thin layers, and stirred 3-4 times a day. They are then beaten with a flail to separate the leaves from the branches and stems. If this be done during the middle of the day, when the leaves are most thoroughly dry and consequently brittle, they are reduced to small particles, producing what is called "sumach for grinding." But if it be done in the morning, or on damp days, when the air is charged with moisture and the leaves are tough, they are separated from the stems more nearly entire and less broken, and the product obtained is called "sumach for baling." The stems remaining after the separation of sumach for baling still retain small particles of leaves attached to them, and they are therefore again beaten when perfectly dry for the production of a low-grade sumach, called by the Italians gammuzza. The products are classed as follows:—
| Relative Market Value. |
|
| Sumach for baling | 2·5 |
| " " grinding | 2·3 |
| " from yearling plants | 1·5 |
| " " ends of branches collected in autumn |
1·0 |
To prepare these different grades for ultimate consumption, they are ground in mills similar to those employed for crushing olives, that is, in which two large stone wheels follow each other, revolving upon a circular bed, the whole construction being about the same as the Spanish or Mexican arrastre. The sumach thus pulverised is passed through bolting-screens, to separate the finer from the coarser particles.
In Virginia, the leaves are collected and cured by the country people, and sold and delivered to owners of mills for grinding. Their particular object being to secure the largest possible quantity of product at the lowest cost, little attention is given to the quality obtained, or the manner of collecting. The most intelligent dealers in the raw material urge upon collectors to observe the following particulars:—The leaf « 47 » should be taken when full of sap, before it has turned red, has begun to wither, or has been affected by frost, to ensure a maximum value for tanning purposes. Either the leaf-bearing stems may be stripped off, or the entire stalk may be cut away, and the leaves upon it allowed to wither before being carried to the drying-shed; but care must be observed that they are neither scorched nor bleached by the sun. When wilted, they are carried to a covered place, and spread upon open shelving or racks to dry, avoiding the deposit in any one place of a quantity so great as to endanger the quality of the product by overheating and fermentation. Sumach should be allowed to remain within the drying-house at least one month before sending to the market; in case of bad weather, a longer period may be required. When ready for packing for shipment, it should be perfectly dry and very brittle, otherwise it is likely to suffer injury in warehouses from heating and fermentation.
Buyers of sumach leaves for grinding depend largely upon colour for the determination of the value; the leaves should, therefore, when ready for market, present a bright-green colour, which is evidence that they have suffered neither from rain after being gathered, nor from heating during the process of drying. Leaves having a mouldy odour or appearance are rejected. The Virginian crop reaches 7000-8000 tons, and is collected at any time between July 1 and the appearance of frost.
There is an important difference in the value of the European and American products. The proportion of tannic acid in the latter exceeds that found in the former by 6-8 per cent., yet the former is much preferred by tanners and dyers. By using Sicilian sumach it is possible to make the finer white leathers, in great demand for gloves and fancy shoes; while by the employment of the American product, the leather has a disagreeable yellow or dark colour, apparently due to a colouring matter, which, according to Loewe, consists of quercitrin and quercetin, and exists in larger quantity in the American than in the Sicilian.
The experimental results obtained by collecting sumach at different seasons were:—
| Per Cent. of Tannic Acid. |
||
| Virginia, mixed, collected in | June, gave | 22·75 |
| " " " | July, " | 27·38 |
| " R. glabra " | August, " | 23·56 |
| " B. copallina " | " " | 16·99 |
| Sicilian, B. Coriaria " | " " | 24·27 |
It is evident, therefore, that in order to secure the maximum amount of tannic acid, the sumach should be collected in July, but the colouring matter of the leaves has an important influence upon the value of the product. The leaves of the upper extremities of the stalks are always richer in tannic acid than those of the base; and the increase of age of the plant is accompanied by a general diminution of this acid. Yet the collection of the crop should be delayed as long as possible, because the diminution of tannin in the leaves will be abundantly compensated for by the quality of the product.
Experiments upon the presence of colouring matters were made by treating gelatine solutions, and gave the following results:—
| Virginia, mixed, collected in | June, gave | A nearly white precipitate. |
| " " " | July, " | A decidedly yellowish-white precipitate. |
| " R. copallina, " | August " | A dirty-yellow precipitate. |
| " B. glabra, " | " " | A very dirty-white precipitate. |
| Fredericksburg mixed, " | " " | A dirty-yellow precipitate. |
| Sicilian " | " " | A slightly yellowish-white precipitate. |
It is therefore advised that for the purpose of tanning white and delicately-coloured leathers, the collection should be made in June; while for tanning dark-coloured leathers, and for dyeing and calico-printing in dark colours, where the slightly yellow colour will have no injurious effect, the collection be made in July. It appears that for all purposes, the sumach collected after the 1st of August is inferior in quality.
Fig. 8 shows a mill for grinding sumach-leaves; it consists of a heavy solid circular wooden bed a, 15 ft. diam., with a depression around the edge b, a few inches deep and 1 ft. wide, for the reception of the ground sumach from the bed, and 2 edge-rollers c, weighing about 2500 lb. each, 5-6 ft. diam., and provided with numerous teeth of iron or wood, thickly inserted. Most mills have to be stopped to allow the unloading of the bed, but this delay is obviated by an apparatus consisting of an angular arm d, attached to a scraper e, and worked by a lever f, which passes through the hollow shaft g and extends to the room above, where it terminates « 50 » in a handle h. The scraper carries the ground sumach to the opening i, whence it is taken by an elevator to a revolving sieve or screen in a room above. After screening, the sumach is packed in bags, 15 to the ton, being always sold by that weight. The chasers and beds are inclosed in a case or drum, and the grinding is done by the application of power to the upright shaft g. The mills are fed from above. The packing is sometimes done by machinery alone. The best mills cost about 600l. In Europe, and in some parts of the Southern States, sumach is still ground by stones revolving on a stone bed, and the sifting is often done by hand.
E. Coez & Co., St. Denis, near Paris, make a sumach extract. It is concentrated to a syrupy consistence in a vacuum-pan, and keeps well, exhibiting none of the acidity which is manifested by a simple decoction of sumach leaves. Sumach contains 16-24 per cent. of gallotannic acid, and is somewhat similar in tanning properties to myrobalans, but paler in colour. It is principally used for tanning morocco and other fancy leathers.
The district of Ancona yields 200 tons per annum of sumach, said to be equal to and cheaper than the Sicilian, but mostly consumed locally. Palermo exported of "ventilated" sumach to the United States 120,043 bags (14 = 1 ton) in 1877, and 50,085 in 1878, the average value being 14l. a ton. Trieste exported 7800 cwt. by land in 1877; in 1878, the shipments to England were 16,600 kilo. (of 2·2 lb.), value 1328 fl. (of 2s.), and in 1880, 91,800 kilo. 7344 fl. Rustchuk in 1880 exported 1400 tons, chiefly to Roumania and Austria. Our imports in 1880 were 10,573 tons, 133,249l. from Italy, and 1047 tons, 12,416l., from other countries; total, 11,620 tons, 145,665l. The approximate London market value is 15s.-16s. 6d. a cwt. for Sicilian, 10-11s. for Spanish.
Valonia (Fr., Vélanèdes; Ger., Valonia). This is the commercial name for the large pericarps or acorn-cups of several species or varieties of oak, chiefly Quercus Ægilops and Q. macrolepis. The former is found growing in the highlands of the Morea, Roumelia, the Greek Archipelago, Asia « 51 » Minor, and Palestine; the latter constitutes vast forests in many parts of Greece, and especially on the lower slopes of Taygetos, towards Ætylon and Mani (Laconia). Prof. Orphanides, of Athens, alludes to a third species or variety called porto galussa, which yields a superior kind of valonia, and named by him Q. stenophylla. The chief localities of production in Asia Minor are Ushak, Borlo, Demirdji, Ghiördes, Adala, Nazlü, Buldur, Sokia, Balat, Troja, Aivalik, and Mytilene. The annual exports, mainly from Smyrna, reach 600,000 quintals (of 2 cwt.), value about 400,000l. In Greece, the production is chiefly centred in the following districts: (1) The province of Lacedemonia, which afforded 10,000 cwt. in 1872; (2) the province of Gythium, in the lower part of Mount Taygetos, which gave 60,000 cwt. in 1872; (3) the island of Zea, which formerly yielded 30,000-40,000 cwt., lately reduced to 15,000 cwt. yearly; (4) Attica, especially the neighbourhood of Cacossalessi, grows 3000-5000 cwt., shipped from Oropos, in the Strait of Chalcis; (5) the island of Eubœa, whence about 1000 cwt. are shipped annually at Bouffalo; (6) the province of Triphyllia raises 3000 cwt., which go to Trieste, viâ Cyparissie; (7) the province of Pulos, especially the commune of Ligudista, grows over 2000 cwt., despatched from Navarino to Trieste; (8) the province of Achaia has a yearly crop of 30,000-40,000 cwt., shipped to Trieste from Courupeli and Caravostassi, between Patras and Cape Papa; (9) the small towns of Anatolico and Astakos (Dragomestre) collect the valonia of the eastern parts of Ætylon, Acarnania, and Cravassaras (a port in the Gulf of Arta), and of all the other western parts, to be sent to Trieste for shipment to England and Italy. Ætolia and Acarnania furnish abundant crops, that of 1872 exceeding 100,000 cwt. The total area of the Greek valonia-yielding forests is said to be about 13,000 stremme (of 1191/2 sq. yd.). The total production in 1877 was estimated at 2,601,000 quintals (of 2 cwt.); the greater part is exported, about 2/3 going to Austria, and the rest to Italy and England. The proportions of tannic acid in the valonia from different districts « 52 » of Greece are said to vary as follows: Patras, 19-281/2 per cent.; Gythium, 271/4-351/2; Zea, 121/4-251/4; Vonitza, 18-20.
In Turkey, the fruit ripens in July-August, when the trees are beaten, and the fallen acorns left on the ground to dry. The natives afterwards gather them, and transport them on camel-back to stores in the towns, whence they go by camel and train to Smyrna, and are there placed in heaps 5-6 ft. deep in large airy stores for some weeks, during which the mass heats, and the acorn itself, which contains but little tannin, and is used for feeding pigs, contracts and falls from the cup. This incipient fermentation is attended with considerable risk; if carried too far, a large proportion of the valonia becomes dark-coloured and otherwise damaged. When ready for shipment, the heaps are hand-picked, the best being reserved for the Austrian market (Trieste), and the rest going to England. In some cases, the rubbish having been removed, the remainder is known as "natural," and is thus exported to England.
In Greek commerce, three qualities are distinguished, chamada, rhabdisto, and charcala. The chamada (camata and camatina of Asia Minor) is the best; it is collected in April, before the acorn is matured, hence the cup which encloses the acorn is small and incompletely developed. The rhabdisto is the second quality; it is collected in September-October, and is distinguished by the fruit being larger and riper; the name means "beaten," the fruits being beaten down from the trees with sticks. After mid-October the collection ceases, because the first rains cause the fallen fruit to ferment or turn black, and they then take the name of charchala. They are distinguished by the cups being completely open, and containing no acorns. They are considered much inferior, possessing little tannin.
Pl. IV.
REVOLVING, WASHING AND PRESSING MACHINES.
Sometimes the acorn cup is attacked by a kind of honey-dew, which deposits on the cup, and makes it very liable to heat when gathered, the cup becoming very dark and deficient in tannin. The Turkish crop of 1875 was much damaged from this cause, many parcels reaching England in an unsaleable condition. The cause of the disease is yet unknown; it seems specially prevalent when the crop is large and the acorn fully developed. A good sample of valonia should be composed of medium-sized cups, with the rim or wall very thick, and the exterior spines small and uniform. The cut or broken cup should show a bright-drab fractured surface. Valonia contains 25-35 per cent. of a tannin somewhat resembling that of oak-bark, but giving a browner colour and heavier bloom. It makes a hard and heavy leather, and is generally used in admixture with oak-bark, myrobalans, or mimosa-bark.
The Greek crop in 1880 was much damaged by the cold spring: It gave 600 tons in Acarnania and Ætolia, 650 in Cape Papa, and 1400 in Mania; total, 2650 tons. Calamata and Messenia produced 115 tons, 1700l. Syra exported in 1879, 1174l. worth to Great Britain, 348l. Austria, 259l. Russia, 250l. Turkey, 178l. Egypt. Hungary exported 942 tons in 1880. Adana shipped 9450l. worth in 1878; and Dedeagatch, in the same year, 1,500,000 lb., 9000l. Musyna [Mersineh] sent 670 tons, 3350l., to Italy, and 450 tons, 2250l. to Austria, in 1879; and 480 tons, 2240l., to Italy, and 128 tons, 640l., to Greece, in 1880. Our imports in 1880 were:—From Turkey, 30,391 tons, 471,637l.; Greece, 2916 tons, 41,312l.; other countries, 466 tons, 7105l.; total, 33,773 tons, 520,054l. The approximate London market values are:—Smyrna, 12s. 6d.-20s. 6d. a cwt.; Camata, 15s.-19s.; Morea, 10s. 6d.-18s.
Miscellaneous.—Besides the foregoing tannins, which already occupy prominent places in European and American commerce, there are many others as yet of minor importance, but possessing qualities which may bring them into note in the near future. They are as follows:—
Abies Larix bark, the larch, contains 6-8 per cent. of a red tannin.
Acacia albicans fruits, the hiusache of Mexico, are used as substitutes for gall-nuts, costing locally about 5d. a lb. A. arabica, the babul of India, yields a tannin which gives a « 54 » nearly pure-white precipitate with gelatine: the proportions are 12·55 per cent, in trunk-bark, 18·95 in branch-bark, 15·45 in twig-bark. The supply is unlimited. It works well with myrobalans. A. Cebil, the red cebil of the Argentine Republic, contains 10-15 per cent. of tannin in the bark, and 6-7 per cent. in the leaves; another variety, the white cebil, contains 8-12 per cent. in the bark, and 7-8 per cent. in the leaves. A. Cavenia, the espinillo of the Argentine Republic, has 33-34 per cent. of tannin in the fruit-husks. A. penninervis bark, the "hardy" acacia of Australia, contains 18 per cent. of tannic acid and 3-4 of gallic.
Alnus glutinosa bark, the common alder, contains about 16 per cent. of tannin.
Cœsalpinia Cacalaco fruits, the cascalote of Mexico, are very rich in tannic and gallic acids, and are locally used for tanning.
Comptonia asplenifolia leaves, the sweet-fern of the United States, contain 9-10 per cent. of tannin.
Coriaria ruscifolia bark, the tutu of New Zealand, contains 16-17 per cent. of tannin.
Elæocarpus dentatus bark, the kiri-hinau of New Zealand, contains 21-22 per cent. of tannin. E. Hookerianus bark, the pokako of New Zealand, contains 9-10 per cent. of tannin.
Ephedra antisyphilitica, on the tablelands of Arizona and Utah, gives 11-12 per cent. of tannin.
Eucalyptus longifolia bark, the "woolly-butt" of Australia, contains 8·3 per cent. of tannic acid, and 2·8 of gallic. The "peppermint"-tree contains 20 per cent. of tannic acid in its bark. The "stringy-bark" (E. obliqua) gives 131/2 per cent. of kinotannic acid. The Victorian "iron-bark" (E. leucoxylon) contains 22 per cent. of kinotannic acid, but is available only for inferior leather.
Eugenia Maire bark, the whawhako of New Zealand, contains 16-17 per cent. of tannin. E. Smithii bark, the "myrtle"-tree of Australia, contains 17 per cent. of tannic acid and 3-4 of gallic.
Fuchsia macrostemma root-bark is thin, brittle, and easily exhausted; it contains about 25 per cent. of a bright-red « 55 » tannin, which has been successfully tried. It is the churco bark of Chili, which, however, is attributed by the Kew authorities to Oxalis gigantea.
Inga Feuillei pods, the pay-pay of Peru, contain 24 per cent. of an almost colourless tannin.
Laurus Peumo rind is used in Chili for tanning uppers.
Malpighia punicifolia bark, the naucite, or manquitta bark of Nicaragua, contains 20-30 per cent. of a very light-coloured tannin.
Persea Lingue bark is red-brown, soft, and easily exhausted by water; it contains 20-24 per cent. of tannin, and much slimy matter which promotes the swelling of the hides. It serves in South America, especially in the Chilian province of Valdivia, for tanning Valdivia leather. In Southern Chili are enormous forests of the tree. The imported bark has given good results with heavy leathers.
Phyllocladus tricomanoides bark, the kiri-toa-toa of New Zealand, contains 23 per cent. of tannin.
Polygonum amphibium leaves, an annual plant abundant in the Missouri Valley, contain 18 per cent. of tannin, and can be mown and stacked like hay. It is largely used in Chicago tanneries, and said to give a leather which is tougher, more durable, of finer texture, and capable of higher polish, than that tanned with oak-bark.
Punica Granatum fruit-rind, the pomegranate, contains about 13·6 per cent. of a tannin like myrobalans, and a considerable quantity of starch; the tannin is greatest in the bitter kind, which is used for preparing morocco leather; the root-bark also is rich in tannin.
Rhizophora Mangle bark, the mangrove, of Venezuela, contains 24-30 per cent. of deep-red tannin, if obtained from young stems; samples from the West Indies have given 11·94 per cent., probably by the gelatine process; two samples from Shanghai, by Löwenthal's improved method, gave respectively 9·8 and 9·5 per cent. calculated as oak tannin, and 71·96 and 78·52 of woody fibre. Guayaquil exported 9328 cwt. of the bark to Peru in 1879.
Tecoma pentaphylla bark, the roble colorado of Venezuela, contains 27 per cent. of tannin, accompanied by a soluble orange-red colouring matter.
Wagatea spicata pods contain 15 per cent. of tannic acid. The plant, a scrambling shrub, is a native of the Concans.
Weinmannia racemosa bark, the tawhero towai, or kamai of New Zealand, contains 12-13 per cent. of tannin.
THE CHEMISTRY OF TANNINS.
The essential constituents of tanning materials are various members of a large group of organic compounds called tannins or tannic acids.[D]
[D] Ger. gerbsäure; in German the word tannin denotes usually gallotannic acid only.
These bodies often differ widely both in chemical constitution and reaction, but have the common property of precipitating gelatin from solution, and forming insoluble compounds with gelatin-yielding tissues. By virtue of this power, they convert animal hide into the insoluble and imputrescible material called "leather." They are mostly uncrystallisable; and all form blackish-blue or blackish-green compounds with ferric salts, and in common with many other organic substances are precipitated by lead and copper acetates, stannous chloride, and many other metallic salts, and those of organic bases, such as quinine. In some cases, the tannin combines with the base only, liberating the acid; but frequently the salt as a whole enters into combination. This is the case with the precipitates formed with lead and copper acetates. With alkalies, the tannins and many of their derivatives give solutions which oxidise and darken rapidly, usually becoming successively orange, brown, and black. A. H. Allen has shown that these bodies also give instantaneously a deep-red coloration with a solution of potassium ferricyanide and ammonia. The reaction is one of considerable delicacy.
Tannins are more or less soluble in water; and freely so in alcohol, mixtures of alcohol and ether, and ethyl acetate, but scarcely in dry ether alone, nor in dilute sulphuric « 58 » acid; and insoluble in carbon disulphide, petroleum spirit, benzene, and chloroform.
From their amorphous character, tannins are extremely difficult to purify; and when, as is frequently the case, two or more tannins occur in the same plant, it is often quite impossible completely to separate them. Owing to their considerable differences in character, no general method of purification can be given, but the following processes will be found in many cases to give good results. For the special methods adopted by different investigators, the original memoirs must be consulted, references to many of which will be found in the following pages.
Preparation and Purification of Tannins.
The oldest method of separating tannins from other constituents is that applied by Pelouze to the preparation of commercial gallotannic acid from gall-nuts. The finely pulverised material is placed in a percolator and exhausted with commercial ether containing water and alcohol. The liquid separates, on standing, into 2 layers of which the lower contains most of the tannin in a tolerably pure form, dissolved in water and alcohol with a little ether, while the upper mainly ethereous layer contains the gallic acid. Gall-nuts thus treated yield 35-40 per cent. of tannin. If equal parts of ether and 90 per cent. alcohol are used, a larger yield is obtained, but the liquid does not separate into 2 layers, and it is questionable if the product is so pure. For Chinese galls, washed ether acts better than ether alcohol. The tannin may be still further purified by dissolving in a mixture of 1 part water with 2 of ether, when 3 layers are formed, of which the lowest contains nearly pure tannin.
These methods are applicable to the dried or highly concentrated extracts of many tanning materials. Many tannins may be separated from their strong aqueous solution in a state of considerable purity by first agitating with ether to remove gallic acid, and then saturating with common salt, « 59 » and shaking well with acetic ether, which takes up the tannin. Another method is to extract with alcohol, evaporate to a small bulk at as low a temperature as possible, and treat at once with a considerable quantity of cold water. The infusion is then precipitated with successive small quantities of lead acetate; the first and last portions of the precipitate are filtered off and rejected as contaminated with colouring matters and other impurities, while the remainder, after rapid washing, is suspended in water and decomposed with sulphuretted hydrogen. The filtrate is shaken with ether to remove gallic acid, and the aqueous portion is evaporated at a low temperature in a partial vacuum to a thin syrup, and the drying completed over sulphuric acid in vacuo.
General Chemistry.
The natural tannins are all compounds of carbon, hydrogen and oxygen only. They all contain the benzene group of carbon atoms, but their ultimate structure is, except in the case of gallotannic acid, very imperfectly understood, and probably differs considerably in type in different members of the family.
In order to make clear to those readers who have not studied modern organic chemistry, what we do know on the subject, a few words of introduction will be necessary. All organic compounds contain carbon, in combination with hydrogen, and very frequently also with oxygen, nitrogen, and other elements. A single atom of carbon is able to combine with 4 atoms of hydrogen, as it does to form marsh gas, or methyl hydride, CH4. Other elements may be substituted for the hydrogen; for instance, if we replace 3 of the hydrogen atoms with chlorine, we obtain chloroform, CHCl3. Again an atom of oxygen may be inserted between the carbon atom and one of the hydrogen atoms, producing methyl hydroxide or wood spirit. The group CH3 is called methyl, and we may substitute in wood spirit this entire methyl group for one of the atoms of hydrogen, when we shall have « 60 » ordinary alcohol, C2H5OH. This building-up process may be repeated almost ad infinitum, producing a whole series of alcohols of higher and higher boiling point as the atoms of carbon become more numerous. Again, if in wood spirit we substitute an atom of oxygen for 2 of the remaining atoms of hydrogen we obtain formic acid, CHO.OH, the first of a long series of acids, of which the second, corresponding to ordinary alcohol, is acetic acid, and the highest members, such as stearic acid, C18H35O.OH, are solid fats. Hence the whole series are commonly called the fatty acids. A few structural formulæ will serve to make these points clearer, but it may be well to say that such formulæ must be taken simply as indicating the order in which the different atoms are united, and in no sense their actual position in space. The atoms in a molecule are held together by attractions and are in continual motion, so that they are more comparable to the planets of the solar system than to a rigid shape.
| Methyl Hydride. | Methyl Alcohol. | Common Alcohol. |
| H | H──C──H | H |
H | H──C──O──H | H |
H H | | H──C──C──O──H | | H H |
| Chloroform. | Formic Acid. | Acetic Acid. |
| Cl | H──C──Cl | Cl |
O ║ H──C──O──H |
H O | ║ H──C──C──O──H | H |
In benzene, C6H6 we have a compound of another type. There is reason to think that the carbon atoms in this case are united in a ring, as shown,
This benzene group forms the foundation of an immense number of bodies known as the aromatic series, to which belong aniline, carbolic acid, picric acid, gallic acid, and a host of other compounds important alike in a scientific and commercial sense, and among which we may pretty safely group the whole of the tannins. Commencing with benzene, we may, by inserting atoms of oxygen, produce a series of alcohols or phenols, of which common phenol (usually but incorrectly called carbolic acid) is the first.
The following table gives a general view of some of these, so far as they are known, with their corresponding acids:—
| C6H6 | C6H5OH | C6H4(OH)2 | C6H3(OH)3 | |||||||||||||||||||
| Benzene. | Phenol. | Pyrocatechol (or catechol), Hydroquinol, Resorcinol. |
Pyrogallol, Phloroglucol. |
|||||||||||||||||||
|
|
|
|
|||||||||||||||||||
| Benzoic acid. | Salycylic acid, Oxybenzoic acid. |
Protocatechuic acid (and 5 other isomeric acids). |
Gallic acid, &c. |
It will be noticed that a large proportion of the formulæ given above represent several compounds identical in composition, but frequently very distinct in their properties. The explanation of these differences lies in the different relative position of the OH and CO.OH groups round the benzene ring. Thus the following diagram represents the relative positions of the pyrocatechol series. It may be noted that each phenol yields two isomeric[E] acids. Miller (C. S. Jour., xli. 398), who has investigated these acids, remarks, "Of the 3 phenols C6H4OH2, catechol alone gives a « 62 »precipitate with lead acetate, and of the 6 acids, C6N5OH2, CO.OH, none yields precipitates with lead acetate, except the 2 which are obtained from catechol."
[E] Isomeric, of similar composition but different structure and properties.
| Pyrocatechol 1-2. | Resorcinol 1-3. | Hydroquinol 1-4. |
| H──C══C──O──H | | H──C C──O──H ║ ║ H──C──C──H |
H──C══C──O──H | | H──C C──O──H ║ ║ H──C──C──O──H |
H──C══C──O──H | | H──C C──H ║ ║ H──O──C──C──H |
All the natural tannins with which we are acquainted, are derived from, and yield on decomposition either catechol, phloroglucol, or pyrogallol, and sometimes more than one of these. Artificial products, however, with many of the reactions of tannins have been obtained from other members of the group, and most phenols and their derived acids give either purplish or greenish black with ferric salts.
Several classifications of the tannins have been suggested. The division most obvious to the tanner is into those tannins which yield the whitish deposit in the surface of the leather, called "bloom," and those which do not. Stenhouse, some years since, divided tannins into 2 classes, one of which gives a bluish, and the other a greenish-black with ferric salts. In the main these 2 classes correspond to the 2 former, as most tannins which yield a blue-black with iron acetate also give bloom to the leather. In some cases, however, the difference of tint is due to accidental impurities, and even gallotannic acid will give a decided green with strongly acid ferric chloride. These classifications both correspond to well-defined differences of constitution, and it is obviously more scientific to arrange tannins according to the products which they yield on decomposition, and which indicate their ultimate structure, rather than on any less essential point.
If those tannins which give bloom to leather are cautiously heated to about 392° F. (200° C.), they are decomposed, and a substance is volatilised which condenses in feathery crystals, and which on examination turns out to be pyrogallol. Those tannins, on the other « 63 » hand, which yield no bloom, but red deposits, produce a somewhat similar sublimate of catechol. From oak-bark and valonia, which yield both bloom and red colouring matters, both catechol and pyrogallol have been obtained. We may, therefore, divide tannins broadly into derivatives of catechol, which yield no bloom, and usually give greenish-blacks with iron acetate, and which include hemlock, mimosa, cutch, gambier, quebracho, &c; derivatives of pyrogallol, which give bluish-blacks with iron, deposit bloom in leather, and embrace galls, sumach, divi-divi, myrobalans, pomegranate rind, &c., and tannins which contain both pyrogallol and catechol, such as oak-bark and valonia, and which, as is well known, yield bloom, and give blue-blacks with iron.
If tannins are boiled with dilute sulphuric or hydrochloric acids, and allowed to ferment under the influence of pectose and other natural ferments, which are always present in vegetable tanning materials, a different series of decompositions takes place. Many tannins yield glucose, or starch sugar, as one of their products, or as that of closely associated impurities. Of this more must be said later. In addition it will be found that the catechol tannins invariably yield insoluble reddish-brown bodies which have been called phlobaphenes, and which differ from the original tannins in containing one or more molecules less water, and which, in chemical language, are anhydrides of their respective tannic acids. The pyrogallol tannins, on the other hand, yield gallic acid, or ellagic acid (the deposit forming bloom) either alone or in mixture. Oak-bark and valonia give both bloom and insoluble reds, and by digestion with acids in sealed tubes also gallic acid.
If the red anhydrides, which are produced from the catechol tannins, be fused with caustic potash, or in many cases, if they be simply boiled with concentrated potash solution, they are broken up still further, and from the fused mass, protocatechuic acid (which bears the same relation to catechol that gallic acid does to pyrogallol) may always « 64 » be obtained. This is in many cases accompanied by phloroglucol, a phenol isomeric with pyrogallol, as may be seen by the table on p. 61, but which tastes sweet like a sugar. Cutch, gambier, mimosa, quebracho, and probably many others, are phloroglucide tannins. The tannins which do not yield phloroglucol frequently give acetic acid, and other acids of the "fatty" group, along with protocatechuic acid. We may summarise this classification in the following table:—
| Tannins boiled with dilute sulphuric acid yield (frequently glucose, and) Insoluble Reds, which fused with potash yield protocatechuic acid, and,── Phloroglucol, as chestnut, gambier, kino, cutch, quebracho, rhatany, fustic, horse-chestnut, tormentil. Acetic acid, coffee, Peruvian bark, male-fern. |
||
| Reds and gallic and ellagic acids; no glucose |
Oak-bark and valonia tannins. | |
| No reds, but gallic and ellagic acids |
Galls, myrobalans, sumach, divi-divi, pomegranate rind. These are probably mixtures of two tannins which yield |
|
| Gallic acid only | Digallic, or pure gallotannic acid. | |
| Ellagic acid only | Pure ellagitannic acid. | |
This classification is as yet very incomplete, and there are many tannins of which the decomposition products have not been examined, while our knowledge of the differences between the tannins which are classed together is extremely limited. In order to make the information which has been given practically available for further research, the characteristics and mode of recognition of the different products will be given, and as simple a scheme as possible of treatment of the tannin to be examined will be described; but the recognition of such products in a state of mixture presents great practical difficulties, and the tanner will usually be compelled to confine his attention to simpler, though less conclusive tests, based on the work of chemical specialists. Such tests will be described later (p. 111).
Pl. III.
ARTIFICIAL FERMENTATION.
General Methods of Examination of Tannins.
Decomposition by Heat.—The ordinary method is to distil the tannin or dried extract in a small retort, and examine the distillate for catechol and pyrogallol. Unless the heat be very carefully regulated, much loss is caused by the destruction of the catechol and pyrogallol with formation of metagallic acid, &c., and their detection is greatly complicated by the presence of secondary products. This difficulty is somewhat lessened by passing a stream of carbon dioxide through the retort, which carries the products quickly out of the heated portion. A better method is to heat the tannin in glycerin (Thorpe, Chem. Soc. Abstr., 1881, 663; Allen, 'Commercial Organic Analysis,' 2nd ed.). About 1 grm. of the sample is heated with 5 c.c. of pure glycerin to 392°-410° F. (200°-210° C.) for 20 minutes. After cooling, about 20 c.c. of water is added, and the liquid is shaken with an equal volume of ether, without previous filtration. The ethereous layer, which contains the pyrogallol and catechol, is separated from the aqueous portion, evaporated to dryness, and dissolved in 50 c.c. of water. The filtered solution is divided into several portions and tested with lime-water, ferric chloride, and ferric acetate (see pp. 66-7); by these means it is easy to distinguish between catechol and pyrogallol; and either may be detected in presence of a small portion of the other; but if in nearly equal quantities, their recognition is difficult. Catechol may be derived from catechin, &c., and pyrogallol from gallic acid, and it is therefore necessary in some cases to remove these bodies from the tannin before treatment. As a general rule, however, catechins and catechol derivatives are only present in any quantity with catechol-tannins, and the same is true of gallic acid with regard to pyrogallol. (For methods of separation see pp. 69, 71, 80). Catechol has been formed by long continued heating of cellulose, starch, and other carbohydrates with water under pressure (see p. 67).
Products of the Decomposition of Tannins by Heat.—Pyrogallol, « 66 » pyrogallic acid, C6H6O3, has a bitter, but not sour taste, and feebly reddens litmus, but the addition of the smallest trace of alkali gives it an alkaline reaction. It is poisonous, 2 gr. having killed a dog. It is soluble in less than 3 parts of cold water, and still more freely in hot. It is also soluble in alcohol, ether, acetone, ethyl acetate, and glycerin, but not in absolute chloroform, or petroleum spirit. It fuses at 268° F. (131° C.) (Etti), and sublimes at about 410° F. (210° C.).
With pure ferrous sulphate it gives a white precipitate, which redissolves to a fine blue liquid in presence of the least trace of ferric salt. Mineral acids change this to red, and the blue tint is restored by cautious neutralisation with ammonia, and is not destroyed, but sometimes rendered greenish by excess of acetic, and other organic acids. Any excess of ammonia produces an amethyst-red, and acetic acid restores the blue. Its solution is turned brown by traces of nitrous acid. With lime-water it produces a beautiful but evanescent purple, rapidly turning brown. In presence of alkalies it absorbs oxygen from the air with great avidity, turning orange, brown, and black. Pyrogallol does not precipitate gelatin. Its solution rapidly reduces permanganate, Fehling's solution, and salts of gold, silver,[F] mercury, and platinum. It precipitates copper and lead acetates, and with ammoniacal cupric sulphate it gives an intense purple-brown coloration. Gum arabic, saliva, and various other organic matters cause solutions of pyrogallol exposed to the air to absorb oxygen, by which purpurogallin is formed and separates in small yellow capillary crystals. If 0·2 per cent. of pyrogallol be added to a 1 per cent. solution of gum arabic, it becomes yellow in a few hours, and purpurogallin separates in hairlike crystals, which continue to increase for some months. It these crystals are freed from pyrogallol by washing with water, and a trace of alkali is added, they dissolve with an intense blue colour. Purpurogallol is also formed by oxidation with silver nitrate, potash permanganate, « 67 » and many other reagents. Pyrogallol forms compounds with aldehydes; with formaldehyde, a body which reacts like a tannin and precipitates gelatin is produced. If pyrogallol be heated with hydrochloric acid, and aldehyde, chloral, or acetone, a red substance is produced. The less volatile portions of crude beech-tar creasote contains ethers of pyrogallol, methyl-pyrogallol, and propyl-pyrogallol, from which these bodies may be obtained by the action of hydrochloric acid under pressure. Methyl- and propyl-pyrogallols differ from ordinary pyrogallol in having an atom of hydrogen replaced by the groups CH3 or C3H7 respectively; and it is very probable that some tannins are derivatives of such modified pyrogallols.
[F] Hence its use as a "developer" in photography.
If pyrogallol be heated rapidly to 482° F. (250° C.) it parts with the elements of water, and is converted into metagallic acid, C6H4O2, a black amorphous body, insoluble in water, soluble in alkalies. When pyrogallol is made in the ordinary way by heating gallic or tannic acids to 410° F. (210° C.), much of this body is formed, even if the process be conducted in a stream of carbonic acid, and the yield of pyrogallol usually amounts to only about 5 per cent. of the gallic acid employed (see p. 65).
Catechol.—Pyrocatechol, pyrocatechin, oxyphenic acid, C6H4(OH)2.
Sources.—Beside that of the decomposition of certain tannins by heat (see p. 63), catechol is produced by the dry distillation of catechin and some allied bodies which frequently accompany the tannins. It is also formed together with pyrogallol and its homologues (see above) by the dry distillation of wood, wood tar creasote consisting largely of ethers of pyrocatechol and its homologues, methyl and pyrocatechol, &c., and hence it is also found in crude pyroligneous acid. It has also been produced by heating carbohydrates with water under pressure, and is found ready formed in Virginia-creeper (Ampelopsis hæderacea), and probably in other plants. It has also been formed synthetically.
Reactions.—Catechol melts at 232° F. (111° C.) and sublimes « 68 » at about the same temperature, condensing in brilliant laminæ like benzoic acid. It is readily soluble in water, alcohol, and ether, and is extracted from its aqueous solution when shaken with the latter. Its aqueous solution precipitates lead acetate but not gelatin or alkaloids. With lime-water or caustic soda solution it becomes reddish, but remains clear for some time. It does not colour ferrous salts, but gives a dark green with ferric (avoiding excess); and after some time a black precipitate. The green is changed to a fine violet-red by alkalies and hydric sodic carbonate, and restored by acids. To fir-wood moistened with hydrochloric acid it gives, like phloroglucol, a violet coloration by combination with the trace of vanillin which this wood contains. This reaction does not seem to be given by pyrogallol or by common phenol. Catechol gives a red coloration with citric acid, and after standing, ceases to react with iron.
Decomposition of Tannins by Dilute Acids.—It has been stated that tannins when heated with dilute sulphuric or hydrochloric acids are decomposed, yielding frequently glucose, and either gallic or ellagic acids, or red anhydrides. To determine whether glucose is produced, the tannin must first be carefully purified from glucose, gums, or other bodies likely to interfere, by the methods mentioned on p. 58. Either the tannin itself or its washed lead-salt may be used, and must be heated to 212° F. (100° C.) for some hours in a sealed tube, or tightly closed bottle with dilute hydrochloric acid. After cooling, the mixture must be allowed to stand for some time to separate any sparingly soluble products, which must be filtered off. The filtrate must be shaken with ether and acetic ether to remove gallic acid (p. 59), the aqueous solution must be boiled, neutralised with soda, precipitated with basic lead acetate to remove any traces of tannin or colouring matters, the liquid again filtered, and excess of lead removed with dilute sulphuric acid, the mixture again neutralised with soda, and heated to boiling with Fehling's copper-solution, when a yellow or red precipitate of cuprous oxide will prove the formation of glucose. The « 69 » precipitate produced by cooling may consist (of lead chloride, if the lead salt has been used,) of ellagic acid, or of red anhydrides or phlobaphenes of the tannin. The lead chloride may be removed by washing with boiling water. If the remaining precipitate has a pale yellow or fawn colour it probably consists of ellagic acid (see p. 71), soluble in ammonia and hot alcohol and dissolving freely in strong nitric acid, forming an intense crimson liquid.
The ethereous layer will contain the gallic acid, if any has been formed, and must be evaporated to dryness, and the residue taken up with cold water, and filtered. Addition of a few drops of solution of potassium cyanide will produce a fine red coloration if gallic acid be present, which rapidly fades, but is restored by shaking. A solution of picric acid, to which excess of ammonia has been added, gives a red coloration rapidly changing to a fine green, even in very dilute solutions of gallic acid.
It is not, however, generally necessary to resort to so elaborate a process merely to distinguish the class to which tannins belong. The tannin, or its infusion, may be simply boiled with dilute hydrochloric acid for some time, replacing the acid lost by evaporation. The solution is diluted to 50 c.c. and allowed to cool. Ellagic acid and phlobaphenes may separate, and must be filtered off. If the precipitate is pale, it is probably ellagic acid, and maybe recognised by the nitric acid test. If red, it probably consists of phlobaphenes, and may be treated with cold alcohol, in which phlobaphenes are freely soluble, but ellagic acid very little. The ellagic acid will therefore be left on the filter if present in any quantity, while the alcoholic solution may be precipitated by the addition of water, and the phlobaphenes further examined by treatment with potash.
Gallic Acid.—Dioxysalicylic acid, C6H2(OH)3CO.OH, exists ready formed in some plants, and is a product of the fermentation of gallotannic acid under the influence of the nitrogenous ferment, pectase, or of its decomposition by boiling with acids or alkalies. It crystallises in white, or yellowish « 70 » white needles, containing 1 mol. (9·5 per cent.) of water, which it loses at 212° F. (100° C.). It is soluble in 100 parts of cold or 3 of boiling water, in alcohol or glycerin, and slightly so in ether, by agitation with which it may however be removed from its aqueous solution. Gallic acid fuses at a temperature of about 449° F. (232° C.) (Etti, Chem. Soc. Jour., xxxvi. 160), but at about 410° F. (210° C.) begins to lose carbonic dioxide, and yields a crystalline sublimate of pyrogallol (see p. 66). If the heat be raised suddenly to 482° F. (250° C.) a considerable quantity of black shining metagallic acid is formed.
Aqueous solution of gallic acid gives the following reactions:—Solution of ferric chloride gives a deep blue coloration which is destroyed by boiling. Ferrous sulphate, if free from ferric salt, gives no reaction in dilute solutions, but a white precipitate in strong ones. The mixture rapidly darkens by oxidation. In alkaline solution gallic acid absorbs oxygen from the air and darkens from the formation of tannomelanic acid. Lime-water produces a white precipitate which rapidly becomes blue from oxidation. The same reaction is produced by baryta-water, or by the chlorides of barium or calcium on addition of ammonia (distinction from pyrogallol). It is distinguished from gallotannic acid by the following:—It does not precipitate gelatin, except in the presence of gum. It does not precipitate tartar emetic in presence of ammonic chloride, though both tannin and gallic acid are precipitated by tartar emetic alone. It precipitates lead acetate but not lead nitrate, while tannin precipitates both. A dilute solution of potassium cyanide gives a red coloration which disappears on standing, but is restored by shaking with air. If to even a very dilute solution of gallic acid, sodic arsenate, or some other faintly alkaline salts be added, the mixture absorbs oxygen and becomes a deep green. Aqueous solution of picric acid to which excess of ammonia has previously been added gives a red coloration, changing to green. Tannic and pyrogallic acid produce no reaction with cyanide, and with ammonic « 71 » picrate a reddish coloration only. Gallic acid reduces silver nitrate and gold chloride rapidly when hot, but not Fehling's solution, and decolorises acidified potassic permanganate. If tannin and other oxidisable bodies be removed from its solution it may be estimated quantitatively by titration with permanganate in presence of indigo (see p. 118). It may be separated from tannin by gelatin or hide raspings (see pp. 121, 124). Gallotannic and quercitannic acids may also be removed by precipitation with ammoniacal solution of cupric sulphate, or by cupric acetate, in presence of excess of ammonic carbonate (see also p. 125). Many other tannins, however, give precipitates with cupric salts which are soluble in ammonia and ammonic carbonate. In absence of such tannins it may be estimated gravimetrically by precipitation with cupric acetate. The precipitate is rapidly washed with water and digested with a solution of ammonic carbonate, in which it dissolves; any insoluble cupric tannate is filtered off, the solution is evaporated to dryness and the residue moistened with nitric acid and ignited. The weight of the remaining cupric oxide multiplied by 0·9 gives the weight of the gallic acid plus a little tannin dissolved by the ammonic solution.
Gallic acid may also be separated from tannin by lead acetate strongly acidified with acetic acid, by which tannic acid is precipitated, while lead gallate is dissolved.
Ellagic acid C14H8O9, when pure, is a sulphur-yellow crystalline body almost insoluble even in boiling water, and only slightly so in alcohol and ether, though by agitation with the latter, small quantities may be completely removed from aqueous solution. In hot alcohol it dissolves with a yellow colour, and crystallises on cooling. Solid ellagic acid gives with ferric chloride at first a greenish, and then a black coloration. In strong nitric acid it is soluble with a deep crimson coloration: that from divi-divi gives a crimson liquid on dilution with water, but from other sources it is rather orange.
Ellagic acid may be obtained in considerable quantity by « 72 » pouring a concentrated alcoholic extract of divi-divi into water, when it separates and may be filtered off and recrystallised from hot alcohol. It may also be obtained by boiling the aqueous extracts of divi, myrabolans, pomegranate rind, &c., with dilute hydrochloric acid, and purified by the same means. It may be prepared from gallic acid by heating the latter with dry arsenic acid to 320° F. (160° C.), but is difficult to purify from traces of arsenic. Ellagic acid has not been reconverted into gallic acid. Its constitutional formula is, according to Schiff,
| C6H2 | CO.OH | |||
| OH | ||||
| O─── | ||||
| O | ||||
| C6H2 | CO | |||
| O─── | ||||
| OH | ||||
| OH | ||||
differing from gallotannic acid only by the loss of two atoms of hydrogen.
Air-dried ellagic acid, C14H8O9 + OH2, contains 1 mol. of water, which it loses at 212° F. (100° C.) but reabsorbs in moist air. When heated to 392°-410° F. (200°-210° C.) it forms an anhydride, C14H6O8, losing another molecule of water, which it does not recover from moist air, but is slowly reconverted to ellagic acid by boiling with water.
The phlobaphenes or reds are chemically the anhydrides of the different tannic acids from which they are derived, or in other words they are formed from the tannins by the loss of one or more molecules of water. It is in this way that they are produced by the action of acids, and similarly they are often formed when alcoholic or highly concentrated aqueous extracts are poured into cold water, under which circumstances a part of the tannin seems unable to take up water again, and separates as a red precipitate. They exist ready formed in most tanning materials capable of producing them. They are soluble in alcohol, by which they may be extracted from tanning materials or dried residues containing them. « 73 » They are also dissolved by dilute alkalies and alkaline carbonates, and by borax, which is said to be used in the preparation of some extracts, and was suggested by Sadlon as a means of making phlobaphenes available for tanning. Many of them are scarcely soluble in water even at a boiling temperature, though they become more so in presence of sugar, tannic acid, and some other substances. Their solubility in water depends on their degree of hydration, many tannins giving a series of anhydrides of which those containing only one molecule of water less than the original tannin are quite soluble in water, while the higher members of the series become less and less soluble as they lose water. Those which are soluble form the colouring matters of tanning materials, and generally are practically tannins, precipitating gelatin and combining with hide to form leather. Hemlock bark yields a series of such bodies, of which the lower members are deep red soluble tannins, while the higher form the red sediment, so well known to extract-tanners. Thus it is chemically impossible to decolorise hemlock extract without at the same time greatly lessening its tanning power, though by careful manufacturing and concentration at low temperature, the proportion of the higher anhydrides formed may be kept at a minimum. In many cases it is known, as in gambier, and in others it is probable that the tannin itself is merely the first anhydride of the series, and derived from a catechin which itself is a white crystalline body destitute of tanning properties (see p. 79).
Decomposition of the Phlobaphenes by Fusion with Caustic Alkalies.—It has been mentioned (p. 64) that the reds of different tannins yielded, in addition to protocatechuic acid, either phloroglucol, or acetic acid, or some other member of the fatty acid series. Some tannins, as those of alder and hop, give both phloroglucol and acetic acid, but it is very possible that this arises from the presence of two distinct tannins in these materials. It is stated that all those tannins which yield acetic acid on fusion with potash, also yield considerable quantities of glucose to dilute acids, while the « 74 » phloroglucide tannins do not do so. Gallic acid fused with caustic soda has been found by Barth and Schroeder to produce a small quantity of phloroglucol, and it is similarly formed by resorcinol and common phenol (Chem. Soc. Jour., xliv. 60). It is therefore possible that in some cases where phloroglucol is detected, it may have been formed by the action of the alkali, and not have been originally a constituent of the tannin.
The following is the best method in which to proceed to investigate the products of the action of potash. 20 grm. of the red, or of the tannin from which it is derived, or its lead salt, is boiled with 150 c.c. of solution of caustic potash of 1·20 sp. gr. for 3 hours, and the liquid is then concentrated with constant stirring till it becomes pasty. It is then cooled and treated with a volume of dilute sulphuric acid slightly more than enough to neutralise the alkali employed. After cooling it is filtered from potassium sulphate and other solid matters, and the filtrate treated with sodium bicarbonate till its wine-red reaction with litmus shows that the sulphuric acid is neutralised. The liquid is then shaken with an equal measure of ether, the ethereous layer drawn off and the treatment repeated several times. On distilling off the ether, phloroglucol is left and may be purified by solution in water, when protocatechuic acid and other products may be precipitated by neutral lead acetate, and filtered off, and the phloroglucol again extracted with ether, and recognised by its reaction with ferric chloride and bromine water, and by its sweet taste.
Phloroglucol.—Phloroglucin C6H6O3 is a phenol isomeric with pyrogallol. It crystallises with 2 molecules of water, which it loses at 212° F. (100° C.). It melts at about 428° F. (220° C.), sublimes without odour, and solidifies again on cooling. It is soluble in water, alcohol, and ether, and by agitation with the latter it may be removed from its aqueous solution. It is not precipitated by any metallic salt but basic lead acetate. It is coloured deep violet red by ferric chloride. If bromine be added to its concentrated solution in water, it « 75 » absorbs 3 atoms, forming tribromophloroglucol, C6H6Br3O3, which separates in crystalline needles, with evolution of heat and a very irritating odour. If a deal shaving be moistened with solution of phloroglucol, and then with strong hydrochloric acid, it soon takes a deep violet colour, from the formation of phloroglucol-vanillin with the trace of vanillin contained in all coniferous woods. Pyrocatechol gives a similar reaction, and it is stated by Etti (Chem. Soc. Jour., xliv. 60) that pyrogallol forms a similar compound; it does not, however, give the colour reaction on deal. It is extremely probable that this reaction may be used to detect phloroglucide tannins without the troublesome fusion with potash, in cases where pyrocatechol is absent. The reaction is strongly given by gambier, which is known to contain phloroglucol, but not by oak bark and valonia, though these contain protocatechuic acid. If to a dilute solution of phloroglucol a solution of aniline or toluidine nitrate be added, and then a trace of potassic nitrite, the liquid gradually becomes yellow or orange, then turbid, and finally deposits a cinnabar-red precipitate. This reaction is given by gambier, but is also produced by oak bark infusion, which is not supposed to contain phloroglucol; and gall tannin, pyrogallol, and other substances give similar but browner precipitates.
Protocatechuic Acid.—C6H3(OH)2CO.OH, one of the six isomeric dihydroxybenzoic acids of this formula (see Miller, Chem. Soc. Jour., xli. 198), crystallises in needles and plates with 1 mol. water, which it loses at 212° F. (100° C.). It melts at 228° F. (109° C.) and on further heating is decomposed into pyrocatechol and carbonic acid. It is somewhat soluble in cold water, and readily so in hot water, alcohol, and ether. It is coloured bluish green by ferric chloride, which is changed to red by alkalies. Solutions of protocatechuates give a violet coloration with ferric salts. It is precipitated by lead acetate, and reduces silver ammonio-nitrate, but not Fehling's solution (see also p. 107).
Constitution of Tannins.
Having described the products of decomposition, something must be said of the way in which these constituents are combined to form the unaltered tannins. The only tannin of which we have as yet anything approaching complete knowledge is that obtained from galls, sumach, and myrabolans, and which is thence called gallotannic acid.
Gallotannic, or digallic acid exists as the principal tannic acid of the galls of oak, tamarisk, &c.; and, in mixture with more or less ellagitannic acid, in myrabolans, divi-divi, sumach, pomegranate rind, and many other plant-products. It has also been formed by Schiff from gallic acid, by mixing it, after drying at 230° F. (110° C.), with phosphorus oxychloride to a thin paste, and heating, first to 212° F. (100° C.) and then to 248° F. (120° C ). Much hydrochloric acid was evolved, and the gallic acid was converted into a yellow powder, which after purification by washing with ether, dissolving in water, allowing the gallic acid to crystallise out, saturating with salt, washing the precipitated tannin in salt solution, and redissolving in alcohol and ether, had all the reactions of purified gall tannin, but was perfectly reconverted into gallic acid on boiling with hydrochloric acid, without the formation of any trace of ellagic acid, or glucose. By analysis of the tannin and its acetyl compounds it was shown to be digallic acid, and its constitutional formula is almost certainly as follows:—
| C6H2 | CO.OH | |||
| OH | ||||
| OH | ||||
| O | ||||
| C6H2 | CO | |||
| OH | ||||
| OH | ||||
| OH | ||||
By boiling gallic acid with solution of arsenic acid, Schiff obtained a product which precipitated gelatin, and otherwise reacted like tannin, and he regards this as digallic acid, but « 77 » other experimenters have failed to obtain digallic acid by this means, and have found that on complete removal of arsenic, the compound was reconverted to gallic acid. It therefore remains a moot point whether digallic acid is really formed, and reconverted into gallic acid by the prolonged action of hydric sulphide, which is necessary to remove the arsenic, or whether, as seems more probable, the supposed tannin is merely an arsenical compound of gallic acid.
Gallotannic acid as obtained from plants invariably yields traces of glucose, as well as of ellagic acid, when boiled with dilute acids. It is still an open question whether the glucose exists in the plant as a glucoside of tannic acid or is always the product of some impurity (as is shown by Etti to be the case with oak bark, where lævulin is always present). It seems most probable however that natural gallotannic acid is really a glucoside of digallic acid, or possibly, according to the theory of Hlasiwetz, a gummide, or compound of dextrin, which, by the action of acids, is easily converted into glucose. What has been said of gallotannic acid in this respect, applies to many other tannins, which like it give glucose by treatment with acids.
Gallotannic acid is met with, in commerce, in the form of light buff-coloured scales, with a faint peculiar odour and a powerfully astringent taste. It is soluble in 6 parts of cold water or glycerin, and very readily in hot. It is also very soluble in alcohol containing water, but much less so in absolute. It is moderately soluble in washed, but scarcely at all in anhydrous ether, chloroform, benzene, or petroleum spirit.
The commercial acid usually contains more or less of gallic acid, which may be detected by dissolving in water, shaking with ether, and after decanting and evaporating the ether, applying the tests described under gallic acid (p. 70). It may also be frequently distinguished in the simple aqueous solution of the tannic acid by the tests given. Its quantity may be determined (in the absence of other impurities) by the Löwenthal method (p. 121), the gallic acid forming the « 78 » "not-tannin," by comparison with a solution of pure gallic acid.
Commercial tannic acid is sometimes adulterated with starch, which is left undissolved on treating the sample with ordinary alcohol.
For the estimation of gallotannic acid see pp. 118 et seq. For its principal reactions, Table, p. 113.
Ellagitannic acid, C14H10O10, is contained in divi-divi, myrabolans, and as a glucoside in pomegranate rind. When boiled with dilute acids, or treated with water at 230° F. (110° C.) in a sealed tube, it yields its anhydride, ellagic acid (see p. 71), C14H8O9. In its reactions ellagitannic acid closely resembles gallotannic acid.
Quercitannic acid. Oak-bark tannin.—This tannin may be prepared from oak bark by agitating an alcoholic extract with ethyl acetate, and separating and evaporating the ethereous layer. It is still contaminated with a brownish-green terpene resin, and with some of the higher anhydrides of the tannin. The resin may be removed by treating the dried extract with ether or benzene, in which it is readily soluble; and the phlobaphenes, or higher anhydrides, by dissolving the tannin in ether-alcohol, or probably to a great extent, by simple solution in cold water in which the phlobaphenes are scarcely soluble.
It may also be prepared by evaporating the alcoholic extract, and extracting with water, which leaves the phlobaphenes, or higher anhydrides undissolved. The first anhydride, which is partially soluble, may be precipitated by the addition of salt, and the quercitannic acid extracted by shaking the filtered solution with acetic ether. In very dilute alcohol it yields a pure yellow precipitate with lead acetate. In aqueous solution the precipitate is light-brown. It gives a blue-black with ferric salts. When pure, quercitannic acid yields nothing to pure ether or to benzene.
If quercitannic acid be heated to 266°-284° F. (130°-140° C.) it loses water, and yields a red anhydride slightly soluble in water, which constitutes the red colouring matter of oak « 79 » bark, and which has also been called "difficultly soluble tannin." It precipitates gelatin and is one of the class which Eitner well designates "tanning colouring matters." It gives a brownish red with lead acetate, and a blue-black with ferric salts; it is difficultly soluble in water and ether, but readily so in alcohol of all strengths. Together with other anhydrides, it exists naturally formed in the bark. At higher temperatures, or by boiling with acids, a series of higher anhydrides may be obtained which are quite insoluble in water, but are soluble in alcohol and caustic alkalies. No glucose is produced by treatment of pure quercitannic acid with acids, that formed by so treating oak-bark extract being due to the alteration of the lævulose present. If oak-reds are fused with potash they yield, according to Johansen (Chem. Soc. Jour., xxxii. 721), protocatechuic and butyric acids. If heated in sealed tubes with dilute hydrochloric acid, gallic acid only is formed, with evolution of methyl chloride. The constitutional formula of quercitannic acid is as yet very uncertain: it is probably a methyl derivative of digallic acid. Its formula is variously given as C17H16O9 (Etti, Chem. Soc. Jour., xliv. 994), C28H26O15 (Ibid. xl. 901, Löwe), C19H16O10 (Böttinger, Berichte, xvi. 2710). For further details the original memoirs must be consulted.
For the reactions of oak-bark and valonia infusions the Table, p. 113, may be consulted.
Before describing the catechol-tannins it will be necessary to speak of a group of compounds of which it is probable that these tannins are decomposition products. These are the catechins. It is as yet by no means certain how far they should be considered a group, some chemists holding the opinion that there is only one catechin, of which the rest are merely impure preparations.
Catechin is a white crystalline substance, contained to the extent of some 30 per cent. in cube gambier, and in smaller proportion in block gambier and cutch, and very probably in all tanning materials yielding catechol-tannins. It melts at 4221/2° F. (217° C.) and yields a sublimate of catechol on « 80 » further heating. It is readily soluble in alcohol and in boiling water, but requires 1133 parts of cold water for its solution. Hence it separates on cooling from a hot solution of gambier, and may be purified by redissolving in hot water and treatment with animal charcoal, and subsequent crystallisation. It may also be extracted from its aqueous solution by agitation with ether. It possesses no acid properties, though some writers have incorrectly called it catechuic acid. The aqueous solution gives precipitates with lead acetate and mercuric chloride, and reduces ammonia-nitrate of silver; but, unlike tannins, it does not precipitate gelatin, alkaloids, or tartar emetic. It is oxidised by permanganate in presence of free acid, and hence, when in solution, is estimated by the Löwenthal method as "not tannin." It dissolves in concentrated sulphuric acid with a deep purple coloration. By fusion with caustic alkalies it yields protocatechuic acid and phloroglucol together with hydrogen. Its constitution is very uncertain, but it is probable that the phloroglucol stands in a somewhat similar relation to the protocatechuic acid that glycerin does to the fatty acids in natural fats; and that its decomposition by fused alkalies is a process much akin to saponification. This constitution is represented by the following formula:—
| C19H18O8 or C7H8O2 | O.C6H3(OH)2 O.C6H3(OH)2 |
When acted on by heat and dilute acids the following anhydrides are produced—the formulæ given must be taken as to some extent provisional.
| Catechin 2C19H18O8 | = | C38H36O16 | Not acid; does not precipitate gelatin. | |
| Catechutannic acid | C38H34O15 | Acid, precipitate gelatin. | ||
| Dianydride | C38H32O14 | |||
| Trianydride | C38H30O13 | Insoluble in water. | ||
| Catechuretin | C38H30O12 | Soluble in alcohol and alkalies. |
The white deposit which occurs on pit sides and in the interior of leather where gambier is largely used, and which is sometimes called "the whites," consists of catechin. It « 81 » may be decomposed by warm sulphuric acid. This deposit is favoured by the use of hot gambier liquors. It is probable that by exposure to the air and by boiling, catechin is gradually converted into catechutannic acid during the tanning process, and hence the practical tanning value of cube gambier is probably greater than analysis indicates. Hunt has, however, shown (Jour. Soc. Chem. Ind., iv. 266) that, as estimated by the Löwenthal process, the tannin is to some extent lessened by boiling.
Kinoin, C14H12O6 (Etti, Berl. Ber., xi. 1879), obtained from green or malabar kino, a product very similar to cutch, by boiling with dilute hydrochloric acid and extraction by agitation with ether, is very similar in its properties to catechin. It does not itself precipitate gelatin, but like catechin, yields a series of anhydrides or reds, which do so. On dry distillation it yields catechol and common phenol; and when heated with hydrochloric acid at 248°-266° F. (120°-130° C.) methyl chloride, catechol and gallic acid. Hence its constitution is probably that of methyl-catechol gallate.
Quebracho-catechin was found by P. N. Arata (Chem. Soc. Jour., xl. 1152) in the wood of quebracho colorado (p. 40), but in too small quantity for detailed investigation. It probably bears a similar relation to quebrachitannic acid that ordinary catechin does to catechutannic acid. It is insoluble in cold and only slightly soluble in hot water, but very soluble in alcohol and ether. Its solution is clouded by normal lead acetate, and gives rose-coloured precipitates with basic lead acetate and mercurous nitrate, and blackish with ferric acetate; it reduces silver-nitrate and gold chloride, and is coloured yellow by nitric acid, red by sulphuric acid, yellowish by sodium hypochlorite, and green by Fehling's solution. It does not precipitate gelatin, or alkaloids.
Catechutannic acid has been pretty fully described under catechin, of which it is the first anhydride (p. 80). It is possibly identical with mimo-tannic acid, the tannin of cutch and mimosa bark, which is chemically very similar, but « 82 » greatly differs in its practical effect in tanning. For some further reactions of cutch and gambier infusions see p. 113. It gives a greyish-green precipitate with ferric salts, and (distinction from gallotannic acid) precipitates cupric sulphate but not tartar emetic.
Quebrachitannic acid is got from the wood of the quebracho colorado, Quebrachia lorentzii (formerly Loxopterygium) which must not be confounded with the bark of the Aspidospermum quebrachia, which is valuable, not for its tannin, but for an alkaloid, aspidospermin, which is used for medical purposes. It has been pretty thoroughly investigated by P. N. Arata (Chem. Soc. Jour., xxxiv. 986 and xl. 1152). It seems, however, a little doubtful to the writer whether the substance investigated by Arata was not the anhydride of the tannin, rather than the tannin itself, as it presents many points of analogy to catechu-red and was less soluble in water than quebracho tannin appears in practice to be.
According to Arata, quebrachitannic acid is a pale red amorphous mass, having an astringent taste and yielding a light cinnamon coloured powder. It is insoluble in carbon-bisulphide, turpentine oil, and benzene. Its aqueous solution gives a white precipitate with both normal and basic lead acetate, which when heated, acquires first a rose, and then a chocolate colour; with ferric chloride a green liquid is produced, which changes after a time to red and becomes black on addition of sodium acetate. It forms white precipitates with gelatin, albumen, and alkaloids. By dry distillation it yields catechol. By fusion with potash or the action of sulphuric acid, phloroglucol and protocatechuic acid, while nitric acid converts it into oxalic and picric acids. While it shows great similarity in its reactions to catechutannic acid, it differs materially in percentage composition, containing only 52·5 as compared with 62·0 per cent. of carbon.
WATER AS USED IN TANNING.
Water, as obtained from rivers, wells, or water companies, contains a variety of impurities which affect its use in tanning, but of which in most cases the precise influence is very imperfectly known. These may be classified into (1) merely suspended matters, such as clay and mud, and sometimes animal or vegetable organisms such as infusoria; (2) dissolved mineral matters, which consist mostly of lime and magnesia salts and which make the water hard; (3) and organic dissolved impurities, such as the brown colour of peat water and the putrefying animal matters of sewage contamination.
Mud is always objectionable. It frequently contains organic slime and organisms which encourage the putrefaction of hides put in it to wash or soften. It also almost invariably contains iron as one of its constituents, and hence stains leather, and gives bad coloured liquors. It is not easily got rid of by filtration, as large filter-beds are expensive and difficult to keep in order, and much space is required to clear water by subsidence. Some filter easily cleaned offers the best chance of success. The Pulsometer Company supply such a filter, consisting of sponge tightly packed below a perforated piston. To cleanse the filter a stream of water is passed the reverse way, and the piston raised, and worked up and down, either by hand or power, so as to loosen and knead the sponge. The Atkins "water scrubber," in which sand may be used as a filtering medium, seems also well adapted for the purpose. If lime be precipitated by Clark's, or other process, it usually carries down the mud with it.
Rain water and the water of streams in mountain districts of hard igneous rock are generally nearly free from mineral constituents. This is the case with the Glasgow water from Loch Katrine, and the Thirlmere water which is to supply Manchester. Such water, if cold enough, and free from mud and organic impurity, is the best for almost every purpose in tanning. Most river water, however, and all spring water, is contaminated with mineral matter which it has dissolved out of the soil and rocks through which it has flowed. The principal of these mineral constituents are lime and magnesia. These occur both as sulphates and chlorides, and as hydric carbonates, or "bicarbonates." The sulphates and chlorides constitute "permanent" hardness, while that due to bicarbonates is called "temporary," from the fact that on boiling, half the carbonic acid is driven off, and the lime or magnesia is deposited as an insoluble neutral carbonate, thus softening the water. Any water which can be softened in this way by boiling may also be softened by the addition of a suitable quantity of lime, thus:—
| Calcic hydric carbonate. |
Lime. | Chalk. | Water. | |||
| (CO3)2CaH2 | + | Ca.(OH)2 | = | 2CaCO3 | + | 2OH2 |
This is Clark's process, and the chalk may either be separated by subsidence, which quickly takes place, or by a special filter (Porter-Clark). Thus the Bristol water, which from determinations by Mr. W. N. Evans, contains considerable temporary hardness and but little of permanent, may be almost completely softened by Clark's method. (For method of determining hardness and quantity of lime required, see p. 97).
The lime and magnesia constituting permanent hardness may be removed by the addition of sodic carbonate (soda ash or crystals); but this is expensive on a large scale, and as an equivalent quantity of sodic sulphate or chloride is left in the water, it is for most purposes of questionable advantage, though in some cases useful for the feed water of boilers. When employed for this purpose, the water should if possible « 85 » be softened and settled before using, instead of adding the soda in the boiler itself, as is generally done. Soda is the active ingredient of many boiler compositions. For preventing furring, most tanning materials or even waste tan-liquors are very effective, and the danger of any corrosive action is lessened by the addition of a portion of soda ash. So far as is yet known, from the tanning point of view, it is hardly necessary to make any distinction between lime and magnesia, which may be considered simply as "hardness." A hard water probably softens dried hides more slowly, though it is possible that the observed difference may be due in many cases to the lower temperature of wells from which hard water is generally derived. In the actual limes, the hardness of the water can have no appreciable influence, though if sodium sulphide be used alone, a certain waste occurs from temporary hardness, which may render it advisable to add a little lime. It is in the washing of the hides from lime that the influence is first distinctly felt. If limey goods, after unhairing, are placed in a water with much temporary hardness, the same action occurs as in Clark's water softening process, and chalk is deposited in the surface of the hides, making them harsh and apt to "frize" or roughen the grain in scudding, and causing bad colour by combining with the organic acids of the liquors. The common, but not wholly satisfactory, expedient is to add a little lime, or better, a few pailfuls of lime liquor to the water before putting in the hides. The best plan is to use a properly softened water. Permanent hardness is not injurious in this way.
The hardness of water, and the dissolved carbonic acid which it contains, are, together with its temperature, the principal factors which determine whether a hide will plump or fall in it. Almost the only accurate investigation of this point has been made by W. Eitner ('Der Gerber,' iii. 183). He placed pieces of hide, unhaired by sweating, and quite flat and fallen, in water for 4 days at a temperature of 46° F. (8° C.), with the following results:—
| 1. | In distilled water | Scarcely at all plumped. | |
| 2. | " water saturated with CO2 | Well plumped. | |
| 3. | " " with lime bicarbonate, 20° German scale of hardness |
} | Tolerably plump. |
| 4. | " " " magnesia bicarbonate, 20° do. | do. do. | |
| 5. | " " " lime sulphate, 20° do. | Well plumped. | |
| 6. | " " " magnesia sulphate, 20° do. | Best plumped. | |
| 7. | " " " magnesium chloride, 20° do. | Not at all plumped. | |
| 8. | " " " common salt, 20° do. | do. do. | |
| (1 German degree of hardness corresponds to 1 of lime in 100,000.) | |||
The peculiarities which were shown by the hide pieces on removal from the water were maintained throughout the tanning, which was conducted in imitation of the German method, the hide being swollen and coloured through in weak birch-bark liquors, made with distilled water and acidified in each case with equal quantities of lactic acid, and finally laid away till tanned in a mixture of oak bark and valonia. No. 6, from magnesia sulphate, was the best; then No. 2; No. 3 was less good, but all the pieces from 1 to 6 were firm, close, and of good substance and texture, No. 1 having swelled well in the sour liquor. On the other hand, 7 and 8 scarcely swelled in liquor, but remained flat throughout, and were looser, thinner, and of finer fibre. From this experiment it is clear that while sulphates and carbonates exert a favourable influence on plumping, chlorides do the reverse, not only not plumping themselves, but placing the hides in an unfavourable condition for the plumping action of acids in the liquors. These experiments are quite borne out by the writer's experience in practice. The water at the Lowlights Tannery, which in dry weather is mostly obtained from beds of what was originally sea-sand, and which consequently contains a very abnormal proportion of chlorides (up to 68 pts. NaCl per 100,000), requires special and very careful management to make thick leather, notwithstanding its containing a considerable quantity of calcium and magnesium sulphates. These facts also indicate the importance of the thorough removal of salt from hides intended for sole-leather. Plumping is not a desirable thing in leather intended for « 87 » dressing purposes, and it is possible that the use of a small percentage of salt in the liquors or wash waters might enable bating to be dispensed with. Like a bate, salt would dissolve a small proportion of hide substance (see p. 19). There is no practicable means of removing chlorides from water, but Eitner suggests the addition of a small quantity of sulphuric acid to water containing much temporary hardness (bicarbonates), by which it is converted into permanent (sulphates), which, as we have seen, plumps better. For this purpose about 2·8 oz. of ordinary English vitriol (sp. gr. 1·490) per 100 cub. ft. of water is required for each part of lime Ca(OH)2 per 100,000 (see p. 97 for testing of water). A simpler guide is to add enough to purple, but not to redden litmus paper, even after moving it about in the water for some minutes. The acid must of course be well mixed by plunging. It must be borne in mind that Eitner's experiment was on sweated hides, and that with limed hide, which is kept plump by the dissolved lime retained in the hide, the conditions are different, and different results as regards carbonic acid and bicarbonates would probably be obtained. Both these would convert the lime in the hide into chalk, which is both insoluble and inert, and the hide would probably fall, at any rate till the lime was completely carbonated, while hides would remain plumpest in waters most free from substances capable of neutralising lime. One of the waters most effective in plumping limed hides is that of the river at Lincoln. Its hardness and contents in chlorine is, as compared with Lowlights water in dry weather,
| Per 100,000. | |||||||||
| Lincoln, | permanent hardness | 8·43, | temporary | 8·32, | chlorine | 2·60 pt. | |||
| Lowlights, | " " | 60·5, | " | 45·0 | " | 41·7 " | |||
Both waters have a considerable quantity of organic matter, and both owe their hardness in part to magnesia. From this we might conclude, what may be à priori expected, that the softer the water, the plumper limed hides remain in it. I am informed, however, by Mr. S. L. Evans, that in the Dartmoor water, which is very soft, but peaty, hides « 88 » fall rapidly. In this case the colouring matters of the peat, which are of the nature of very weak acids, probably neutralise the lime. It may also be remarked, that wherever the conditions of putrefaction or decaying organic matter is present, hides rapidly fall, for the same reasons as they do in a bate.
While the injurious effect of bicarbonates on limed hide is matter of common experience, their influence on liquors and tanning is not so well understood. It is certain that they neutralise and combine with the organic acids of the liquors, and probably with some species of tannin, and as 1 part per 100,000 amounts to 1 oz. per 100 cub. ft., the acid required to neutralise a very hard water amounts to something considerable. It is well known that hard waters make bad tea, and the influence of hardness on the extraction of tannin is a subject well worthy of investigation, and which the writer hopes to examine.
On dyeing, at least as regards dye-woods, the influence of bicarbonates is distinctly favourable, and this is also stated to be true of woad, cochineal, and indigo-carmine.
Beside lime and magnesia salts, water may contain sulphates and chlorides of soda and potash; but not carbonates of these bases in presence of permanent hardness. In soft waters carbonates are sometimes present, and form carbonate of lime in limed hides. Hides are said to soften rapidly in such water. Alkaline sulphates are not known to have any injurious action, and chlorides have already been spoken of. Iron may be present in solution as bicarbonate, but not in any other form in presence of bicarbonate of lime. It is removed completely with the temporary hardness by Clark's process, or boiling. Iron is much more common merely in suspension, as mud, but is always objectionable. Most waters contain a little silicic acid and alumina, and some few considerable quantities. Such waters are said to harden leather, but the writer knows of no case where they are in use in England; and their occurrence is comparatively rare.
For comparison, analyses of a few spring and river waters are given on p. 89.
Analyses of various Waters.
| Thames, at Kew. | Thames, at London Bridge. | Severn, Wales. | Thirlmere. | Rhine, Basle. | Spring, Witley, Surrey. | Spring, Watford, Herts. | Artesian, Well Trafalgar Square. | Ripley's, Well Holbeck, Yorks. | Well, Council Acad., Vienna. | River Witham, Lincoln. | Beamhouse well, Lowlights. | |||||
| Total solids | 31·0 | 40·8 | 3·87 | 5·15 | 16·9 | 7·6 | 33·8 | 84·9 | 150·4 | 212·2 | 33·0 | .. | ||||
| Ca | 7·6 | 8·21 | ·3 | ·43 | 5·55 | ·81 | 11·0 | 1·56 | 1·22 | 19·6 | 6·08 | .. | ||||
| Mg | ·47 | ·2 | ·12 | ·48 | ·18 | .. | ·84 | ·42 | 10·4 | .. | .. | |||||
| Na | ·87 | 1·43 | ·6 | ·49 | ·06 | ·64 | 1·1 | 29·4 | 58·1 | 41·1 | .. | .. | ||||
| K | ·39 | ·17 | ·1 | .. | .. | ·23 | .. | ·85 | ·83 | 10·5 | .. | .. | ||||
| CO3 | 10·53 | 6·94 | ·2 | 1·09 | 8·62 | trace | 15·6 | 11·3 | 39·8 | 97·6 | .. | .. | ||||
| SO4 | 3·95 | 3·22 | 1·3 | ·75 | 1·54 | 1·33 | ·68 | 20·6 | 1·03 | 26·7 | 7·59 | .. | ||||
| Cl | 1·21 | 6·36 | ·8 | 1·1 | ·15 | 1·28 | 1·21 | 16·5 | 45·2 | 3·5 | 2·60 | 21·8 | ||||
| SiO2 | ·63 | ·18 | ·2 | ·07 | ·21 | 1·23 | 1·16 | ·57 | 2·63 | ·3 | .. | .. | ||||
| Temporary Hardness | 20·0 | .. | ·9 | ·7 | .. | 2·8 | .. | .. | .. | .. | 8·0 | 39·7 | ||||
| Permanent hardness | 8·4 | 48·0 | ||||||||||||||
METHODS OF CHEMICAL ANALYSIS FOR THE TANNERY.
It is assumed that the reader has an elementary knowledge of chemistry, and of the common manipulations of the laboratory; but at the risk of giving information which to many is already familiar, the principles that underlie those methods of testing which are most applicable to technical purposes must be briefly explained.
Standard Solutions.—If 40 grm. of pure caustic soda (NaHO) be dissolved in water, and a little tincture of litmus added, it will be coloured a bright blue. If hydrochloric acid be now added, drop by drop, the litmus will at last become purple, and a single drop more would turn it a bright red. At this point the liquid is neither acid nor alkaline, and if it be evaporated to dryness, nothing will be left but 58·5 grm. of common salt (NaCl), while 18 grm. of water will be formed and have escaped. We have therefore used exactly 36·5 grm. of pure HCl, and if we dissolve 40 grm. of caustic soda in 1 litre of water, and 36·5 grm. of pure HCl in another, equal parts of these liquids will always exactly neutralise each other, forming nothing but common salt and water. It will be obvious that if we have a soda solution of the strength named, we can find the amount of hydrochloric acid in any solution of unknown strength, by seeing how much of it is required to neutralise, say, 10 c.c. (= 0·4 grm. soda) of the known solution. Instead of 40 grm. of caustic soda, we may take 56 grm. of potash to the litre, and it will exactly neutralise an equal volume of the hydrochloric solution containing 36·5 grm. If, again, « 91 » we make a solution containing 49 grm. of pure sulphuric acid (SO4H2) per litre, it will neutralise an exactly equal volume of either the soda or the potash solution, thus being precisely equivalent to the HCl solution. Such solutions are called normal, and any normal acid solution will neutralise an equal volume of any normal alkali, and vice versâ. For many purposes normal solutions are too strong, and solutions containing 1/10 of the quantities required for normal solution are preferable; such solutions are called decinormal. All solutions containing known quantities of chemicals, and intended for use in volumetric analysis, are called Standard solutions.
Indicators.—The tincture of litmus used to show when the solution is exactly neutral is called an indicator, and many materials are used in a similar way in different analytical processes. Thus the indigo solution in Löwenthal's process is an indicator. A more useful indicator than litmus for tannery purposes is Dr. Lunge's "methyl orange," which is indifferent to carbonic acid, and may therefore be used in the cold with solutions of alkaline carbonates; which are much more easily made and preserved than those of the caustic alkalies necessary with litmus. It is very sensitive to mineral acids, but not equally so to organic. It may be obtained of Messrs. Mawson and Swan, of Newcastle; and as a minute quantity only must be used for each test, it is really cheaper than litmus, and a few grm. will last a lifetime. It must be dissolved in water, and not more than 2 or 3 drops taken for each titration. (Titration signifies an estimation by means of a standard solution.) Other indicators will be named in connection with the analytical methods in which they are used.
Instruments.—To practically carry out analysis by standard solutions, measuring glasses are required. One or more flasks marked in the neck to hold exact quantities (Fig. 9), one at least, holding 1 litre, are indispensable. One or two graduated cylinders (Fig. 10), holding 100 c.c., and divided into « 92 » tenths of c.c., are very useful, and it is well also to have one holding a litre, and provided with a stopper (Fig. 11). This is called a "test mixer," but is not absolutely essential.
Pipettes (Fig. 12) are tubes with a mark on the stem by which exact quantities of liquid can be taken. Several holding 5, 10, 20, and 25 c.c. are necessary, and one holding 10 c.c. and divided into tenths is advisable. Most important of all is the burette (Fig. 13). If only one is to be had, it must be a Mohr's burette with a glass tap, but as alkaline solutions are apt to set glass taps fast, it is well to have one with a tap, and another with a pinchcock (Fig. 14). They should hold 50 or 25 c.c. and be divided into tenths. The burette in use is fixed in a stand (Fig. 15) and filled up to the top of the graduation, and the quantity of solution delivered is then « 93 » shown by the scale. It is usual to read by the under side of the hollow of the liquid, keeping the eye carefully level with it.
A chemical balance suitable for the preparation of standard solutions and general analytical use, is shown in Fig. 16. The beam is provided with steel or rock-crystal knife-edges at the centre, which are supported on agate planes, and similar edges a support the pans. Except at the moment of weighing, the beam, and in good balances the pans also (at b), are steadied by supports raised by turning the milled head c. The long pointer d moving over a scale, shows when the beam is horizontal; but the weighing is performed, not by waiting till the balance comes to rest, but by noting when the oscillations are equal on each side of the zero point. The weights, which should run from 50 grm. downwards, are usually of brass (preferably gilded) down to 1 grm., while the fractions to 0·01 grm. are of platinum foil. Milligrammes and fractions are weighed by a "rider" of wire weighing 0·01 grm., and moved along the beam (which is graduated for the purpose like a steelyard) by the arms e. A fair balance should turn distinctly with 0·001 grm., and a « 94 » good one with 0·0001 grm. If equal weights are placed on each pan, they should of course balance, and if changed side for side the balance should be maintained. If not, the arms of the beam are unequal. Weights always have trifling errors, but if by a really good maker, these are generally so small that they may be disregarded except in very delicate researches. The weights should always be placed on the scale in regular order, beginning with the heaviest, and it is well to accustom oneself to reading the weight by the vacant places in the box as well as by the weights on the scale.
While of course it is most important, and for accurate work essential, to have as good a balance as possible, « 95 » much may be done in technical work, even with a good pair of druggists' scales; and most standard solutions may be bought ready made; while from two or three accurately adjusted solutions many others may be made volumetrically.
Preparation of Standard Acid and Alkaline Solutions.—In practice it is very difficult to obtain perfectly pure caustic soda, free from water and carbonic acid, both of which are greedily absorbed by it from the air, so that a standard solution cannot practically be made by directly weighing out the substance as suggested in the introductory paragraph. In sodic carbonate, however, we have a substance which is easily obtained pure and dry, and which may be used for almost all the purposes to which a caustic solution could be applied. A decinormal solution is strong enough for most of the work in a tannery, though it is a convenience to have both normal and decinormal, and a stock of the stronger solution will last a longer time and is readily diluted to decinormal strength by adding 1 part to 9 parts of distilled water. To make a normal solution, about 60 grm. of the purest sodic carbonate are placed in a porcelain basin or platinum crucible and heated over a Bunsen gas-burner or spirit-lamp, nearly to redness, and allowed to cool closely covered up. Of the salt thus dried 53 grm. are accurately weighed into a beaker and dissolved in distilled water. The solution is then poured into a gauged litre flask, and carefully filled up with water at a temperature of 59° F. (15° C.) to the mark on the neck. The whole is then poured into a good-sized stoppered bottle (40 oz.) and vigorously shaken for 5-10 minutes. This thorough shaking is important with all standard solutions, and without experience no one would believe how much shaking is required uniformly to mix a solution. Probably more difficulty to beginners in analysis arises from neglect of this matter than from any other cause. To make a decinormal solution, proceed in precisely the same way, using 5·3 grm. instead of 53; or dilute as above.
Standard Acid Solution.—For this purpose any one of several acids may be used, each of which has its special advantages.
Oxalic acid is the easiest to make of any. A sufficient quantity of pure crystallised oxalic acid is powdered and pressed between filter paper, so as to absorb the moisture which occasionally is retained in cavities of the crystals. 6·3 grm. is then weighed out and dissolved in water, exactly as was done with sodic carbonate, forming a decinormal solution. It is used in Löwenthal's tannin estimation process and may also be employed to determine alkalies, but forms insoluble calcium oxalate with lime salts, and does not give a sharp reaction with methyl orange indicator. Hence litmus must be used, or a few drops of a neutral solution of calcium chloride added to the methyl orange, when hydrochloric acid will be liberated as soon as there is excess of the acid, and the indicator will be promptly reddened. Sulphuric acid is the most permanent of any acid solution, and may be generally employed. It forms insoluble sulphates with lime, baryta, and strontia. To make a normal solution, 35 c.c. of the pure concentrated acid are poured into at least 3 or 4 times as much distilled water, and allowed to cool, and are then made up to about 1 litre and well shaken. The burette is filled with the mixture, 10 c.c. of the standard sodic carbonate are measured into a beaker, 2 or 3 drops of methyl orange solution are added, and the acid is run in with constant stirring till the indicator is just beginning to redden. This must be repeated, and the two titrations should exactly agree. Suppose that 9.5 c.c. are required, then 950 c.c. of the trial acid are equal to 1 litre of the soda. If therefore 950 c.c. be measured into a test mixer, and made up to 1 litre, the solution should be accurately decinormal. Of course great care must be used in the whole process. If a gauged flask only is at hand it will be easier to measure into it the water required to make up the litre, and then fill to the mark with the trial acid. Normal hydrochloric acid may be made exactly as described for sulphuric acid, but using about 100 c.c. of the « 97 » strongest acid. Decinormal solutions of both these acids may be made by the same methods; using 1 tenth the quantities, or by dilution of the normal solution.
Beside comparison with sodic carbonate solution, hydrochloric acid may also be checked by determining the amount of chlorine present, with silver nitrate (see p. 98) 10 c.c. of decinormal acid should of course be equal to 10 c.c. of decinormal silver nitrate.
Table giving the Quantity of the Following Substances contained in or equivalent to 1 litre of Normal or 10 litres of Decinormal Standard Solution.
| Sulphuric acid | 49 | grm. | SO4H2 = 40 grm. SO3 | |
| Hydrochloric acid | 36·5 | " | ClH = 35·5 grm. Cl. | |
| [G] | Oxalic acid | 63·0 | " | C2O4H2 + 2 Aq. |
| Acetic " | 60·0 | " | C2H3O2H. | |
| Soda | 40·0 | " | NaHO. | |
| Sodic carbonate | 53·0 | " | Na2CO3. | |
| [G] | Lime | 28·0 | " | CaO = 37·0 grm. CaH2O2. |
| [G] | Calcic carbonate | 50·0 | " | CaCO3. |
| Ammonia | 17·0 | " | NH3. | |
| [G] | Barium hydrate | 76·5 | " | BaO = 85·5 grm. BaH2O2. |
| Barium chloride | 104·0 | " | BaCl2. | |
| Zinc chloride or sulphate | 32·6 | " | Zn = 16·0 grm. S. as sulphide. | |
| Silver nitrate | 170·0 | " | AgNO3 = 35·5 grm. Cl. | |
| Potassic permanganate | 31·6 | " | K2MnO4. |
[G] Insufficiently soluble in water to form a normal solution.
EXAMINATION OF WATER.
Hardness (Hehner's process). (a) Temporary Hardness.—As has been stated (p. 84), this consists of lime and magnesia carbonates. As methyl orange is not affected by carbonic acid, bicarbonates of alkaline earths have an alkaline reaction, and may be estimated in solution by standard acid like the alkalies themselves. 100 c.c., or in soft waters 200 c.c., of the water is measured into a beaker, a drop or two of solution of methyl orange added, and decinormal hydrochloric or sulphuric acid run in from the burette with constant stirring till the colour just changes to pink. This is repeated, and the average taken. The two determinations should not at the most differ more than 1/10 c.c. Each c.c.« 98 » represents 5 parts per 100,000 of CaCO3 or 2·8 parts of CaO; or corresponding quantities of magnesia (4·2 parts of MgCO3 or 2 parts MgO), when 100 c.c. of water are used.
(b) Permanent Hardness.—200 c.c. are measured into a beaker and boiled for 15 minutes with 40 c.c. decinormal sodic carbonate. The mixture is then allowed to cool and made up to 250 c.c.; or the flask and its contents may be weighed before boiling and made up again to the same weight. It is then filtered, and 60 c.c. representing 50 c.c. of the original water, is twice titrated with decinormal acid and the result added. If the water were pure, exactly 10 c.c. should be required to neutralise the 10 c.c. of sodic carbonate, but if there be permanent hardness a part of the sodic carbonate will be already neutralised with the acids of the lime and magnesia salts, which have been precipitated as carbonates together with the carbonates of these bases originally present in the water. The hardness will therefore be represented by the loss, i. e. the number of c.c. of acid used for 100 c.c. of the original water must be subtracted from 20 and the remainder calculated as before, or if calculated as sulphates, each c.c. represents 6·8 parts of CaSO4 or 6 parts of MgSO4 per 100,000. If, as is sometimes the case, more acid is required than is needed for the sodic carbonate used, the excess corresponds to sodic carbonate originally present in the water. In this case there can be no permanent hardness.
Chlorine in Water.—If silver nitrate be added to a solution of any chloride, the silver is precipitated as white curdy insoluble silver chloride. As indicator, a few drops of neutral potassic chromate are used. So long as any chloride is present the red silver chromate which forms is at once decomposed, and the silver converted into white chloride. But as soon as all the chloride is exhausted, the red chromate becomes permanent. To prepare a standard decinormal solution of silver, 17 grm. of pure recrystallised silver nitrate are dissolved in 1 litre of distilled water. To perform the estimation 50 c.c. of water are measured into a beaker, 2 or 3 drops of strong solution of pure yellow potassic chromate are added, and then « 99 » silver nitrate from the burette till a permanent red is formed. This is repeated, and the results are added together, representing 100 c.c. of water. Each c.c. of silver nitrate used represents 3·55 parts of chlorine, or 5·85 parts of sodic chloride per 100,000. If more than 10 c.c. of silver solution are required to 50 c.c., it is advisable to use a smaller quantity of water. If the process be applied to other liquids than natural water, it must be borne in mind that the solution must not contain free acids or alkalies except carbonic acid. If this is not the case the liquid may be rendered faintly alkaline, with lime-water free from chlorides, and the excess of lime removed by passing carbonic acid through it; or it may be slightly acidified with sulphuric acid, and shaken with a little pure precipitated calcic or baric carbonate.
Detection of other Impurities.—Sulphuric acid (as sulphates) is seldom wholly absent, but its presence may be proved, by adding excess of barium chloride to the water slightly acidified with hydrochloric acid (2-3 c.c. of saturated solution of BaCl2 are sufficient for any ordinary water); if the mixture be allowed to stand overnight in a 100 c.c. cylinder beside a solution containing a known, and not very different quantity of decinormal sulphuric acid, the quantity present may be roughly compared by measuring the bulk of the precipitates.
Lime may be similarly detected and roughly measured by precipitation with excess of ammonic oxalate in presence of ammonium chloride, to hinder precipitation of magnesia. Lime-water, which may be used as a standard, contains about 128 parts of lime per 100,000.
Magnesia is detected by adding ammonium phosphate to the filtrate from the precipitated oxalate of lime. If the mixture be allowed to stand in a warm place for 24 hours all the magnesia will be precipitated as ammonio-magnesic phosphate.
Silica, &c.—100 c.c. of the water is acidified with a little HCl evaporated to dryness, moistened with HCl, and treated with a little hot water. The silica or silicic acid is left undissolved. The solution from which the silicic acid has been filtered off is evaporated to small bulk and ammonia « 100 » added, when iron will be precipitated as brown ferric oxide, which is coloured black by tannin or tanning liquor. If copper be present it will give a blue solution with the ammonia. Iron may also be recognised by evaporating the water to small bulk with a trace of HCl, and adding a little sodium acetate, when if iron be present it will be coloured black by tannin, red by ammonium sulphocyanide, and blue by potassium ferrocyanide (prussiate of potash). Its quantity may be estimated (Thomson, Chem. Soc. Abstracts, May 1885) by measuring 100 c.c. of the water to be tested and 100 c.c. distilled water into two similar cylinders, adding to each 5 c.c. of dilute hydrochloric acid (1:5) and 15 c.c. of a solution of potassium sulphocyanide (40 grm. per litre), and then adding to the distilled water cylinder a very dilute standard solution of ferric salt, till its colour matches the other. If the iron contained in the water is in the ferrous condition, it must be oxidised with potassic permanganate before testing.
A suitable ferric standard solution may be made by dissolving 0·1 grm. of clean, bright, soft iron wire in a little hydrochloric acid in a long-necked flask, adding nitric acid so long as red fumes are produced, evaporating nearly to dryness, and making up to 1 litre (more accurately 996 c.c.). Each c.c. will then equal 0·0001 grm. Fe.
Lead (and copper) may be detected by passing sulphuretted hydrogen through the water acidified with HCl, or by adding a drop of fresh ammonium or sodium sulphide to the slightly acidified water, when a brownish coloration clearly visible in a deep beaker set on a sheet of white paper will be produced. Iron also gives a black with sulphides in alkaline solution. Copper may be distinguished from lead by the blue given with ammonia, and by a reddish-brown precipitate with potassium ferrocyanide.
For accurate quantitative estimation of these impurities, the regular works on the subject, such as Thorpe's 'Quantitative Analysis,' Sutton's 'Volumetric Analysis,' or Fresenius' 'Quantitative Analysis,' must be consulted.
EXAMINATION OF COMMERCIAL ACIDS.
Sulphuric acid 10 grm. may be made up to 100 c.c. and well mixed, and of this 10 c.c. may be tested with normal sodic carbonate in presence of methyl orange. Each 1 c.c. of soda solution used corresponds to 0·049 grm. or 4·9 per cent. of H2SO4. For most purposes, the strength may be ascertained from the specific gravity, as measured by a hydrometer or weighed in a specific gravity bottle. The following table gives the strength at 59° F. (15° C.):—
|
|
[H] Degrees of Twaddell's hydrometer may be reduced to specific gravity by multiplying by ·005 and adding 1·, thus 10° Tw. = 1·050 sp. gr.
The impurities of sulphuric acid most common and injurious for tanning purposes are iron and nitrous acid. Iron is detected on neutralising with soda or ammonia, when it falls as a yellowish precipitate, which may be recognised by the ordinary tests (p. 100). Nitric and nitrous acids are detected by pouring a strong solution of ferrous sulphate cautiously on to the top of the strong cold acid, when a dark ring is formed at the junction of the two liquids.
Hydrochloric acid may be tested with soda solution like sulphuric. 1 c.c. of normal soda = 0·0365 grm. or 3·65 « 102 » per cent. HCl. It may also be calculated from specific gravity.
| Specific Gravity, 15° C. |
Per cent. HCl. |
| 1·200 | 40 |
| 1·177 | 35 |
| 1·151 | 30 |
| 1·126 | 25 |
| 1·100 | 20 |
| 1·075 | 15 |
| 1·050 | 10 |
| 1·025 | 5 |
The presence of iron is indicated by a yellow colour, and may be confirmed by the usual tests as in sulphuric acid.
Oxalic acid should be pure white and soluble in distilled or rain-water. 6·3 grm. may be weighed out, and made up to 200 c.c. If 20 c.c. of the solution for a test be used, each c.c. of normal soda solution equals 10 per cent. of pure crystallised acid, C2O4H2 + 2 Aq. The end-reaction with methyl orange is rendered sharper by the addition of a few drops of neutral calcic chloride towards the end of the titration.
Acetic acid may be similarly determined, each c.c. of normal alkali being equivalent to 0·06 grm. of C2H4O2. Caustic soda, or lime-water and litmus, give sharper results than sodic carbonate and methyl orange. Brown pyroligneous acid is difficult to test from the dark compounds formed with soda, but may be indirectly determined by the quantity of marble, baric carbonate, or magnesia which it will dissolve (compare p. 100), or very possibly by lime-water like tan-liquors with a little tannin as indicator.
EXAMINATION OF LIME AND LIME-LIQUORS.
The quantity of caustic lime in either quicklime or lime-bottoms may be determined by weighing a quantity of the finely powdered material containing not more than 1 grm. of caustic lime, and shaking it thoroughly with 1 litre of distilled water and filtering. 100 c.c. should be taken, and decinormal acid, sulphuric or hydrochloric (or if oxalic, with addition of neutral calcic chloride, or with litmus « 103 » instead of methyl orange as indicator). Each c.c. of decinormal acid corresponds to 0·0028 grm. of CaO. If the filter and residue be treated with sufficient normal acid to dissolve the whole of the carbonates, and then titrated back with normal sodic carbonate and methyl orange, the loss (less soda solution required than acid was originally employed) is equal to the carbonate of lime and carbonate and hydrate of magnesia present. 1 c.c. of normal acid = 0·05 grm. of CaCO3.
Lime-water and lime-liquors may be titrated as above, with sulphuric or hydrochloric acid and methyl orange; but in the latter case ammonia (and if soda ash or "Inoffensive" is used, soda and potash also), and the lime salts of weak organic acids will be estimated with it. It is difficult to get a sharp end-reaction in old liquors from the organic acids (caproic, amidocaproic, &c.) present. To determine the ammonia, 50-100 c.c. of the liquor may be distilled in a small retort or flask, and the escaping NH3 collected in a U-tube or "nitrogen bulb" (Fig. 17), containing 20-50 c.c. of normal acid, which is afterwards titrated back with sodic carbonate and methyl orange. Kathreiner employs the arrangement shown in Fig. 18. 30 c.c. of the liquor to be examined is placed in a shallow vessel on a piece of ground-glass, and 10 c.c. of normal acid in a second cup, which is supported over the other by a glass or wire triangle. The whole is covered with a small bell-glass, of which the « 104 » edges are smeared with, vaseline. At the end of 24 hours, all the ammonia will have been absorbed by the acid, which is titrated back. The lime-liquor sample should be drawn after well plunging the lime, and rapidly filtered into a flask from a funnel covered with a clock-glass.
Determination of Gelatin and Coriin in Lime-liquors.—This cannot be done directly, though considerable quantities of dissolved hide-substance are precipitated on acidification of the liquor with hydrochloric acid and saturation with common salt. If the liquor be neutralised with hydrochloric acid, and evaporated to dryness on the water-bath, nitrogen may be determined in the residue by combustion, and the hide-substance calculated from it (compare p. 108). This method is serviceable in determining the amount of hide dissolved by different solutions, or under different conditions.
The total solids of lime-liquors are estimated by evaporating 20-30 c.c. in a porcelain crucible at 212° F. (100° C.). The organic matter is then found by igniting and determining loss (using ammonia nitrate if necessary to complete the combustion of the carbon). The ash is mostly lime carbonate. Soda, potash, and other bases may be determined in it by the usual methods, if required.
ESTIMATION OF SULPHUR AS SULPHIDE IN SODIUM SULPHIDE, &c.
32·6 grm. of chemically pure zinc is dissolved in dilute sulphuric or hydrochloric acid. This is readily accomplished in a flask, if a piece of platinum foil, or a few drops of platinic chloride are added to form a galvanic couple with the zinc. After solution, sufficient ammonia is added to redissolve the precipitate at first formed,[I] and the whole is made up to 1 litre. Each c.c. = 0·016 grm. sulphur or 0·242 grm. of sodic sulphide. This solution is added drop by drop from a burette to the solution of sulphide, and forms a white precipitate of zincic sulphide. The end of the reaction is « 105 » known by placing a drop (with a glass rod) side by side on a piece of white filter paper, with a drop of solution of lead acetate. So long as sulphide remains in solution, it will form a black margin of lead sulphide where the drops touch. The drops must not be placed too close, as the solid zinc sulphide is always darkened if it comes in contact with lead acetate. It must be noted that tank-waste liquors, and many other sulphur solutions, contain polysulphides which are estimated by zinc, but which do not unhair, at any rate in an unaltered state.
[I] If any brown residue remains, the zinc is contaminated with iron.
CHEMICAL EXAMINATION OF LEATHER.
Estimation of Grease.—To determine oil and grease, a weighed quantity (5-10 grm.) of the leather in fine shavings or raspings is exhausted with petroleum-ether (gasoline) in a fat-extraction apparatus, of which a convenient form is represented in Fig. 19. The leather is placed in the upper vessel, of which the lower opening is loosely plugged with cotton-wool, and the petroleum-ether in the flask, which is gently heated in a water-bath. The petroleum-ether boils and condenses in the inclined condenser through the casing of which a stream of cold water is passed, whence it drops back into the flask through the material to be exhausted. When the exhaustion « 106 » is complete (when a drop of petroleum-ether from the leather leaves no grease when allowed to evaporate on a clean glass), the upper part of the apparatus is removed, and the ether is distilled off. If the flask has been previously weighed, it is maintained in an air-bath at 212°-248° F. (100°-120° C.) for some hours, allowed to cool, and weighed, when the gain of weight is the grease and oil. Paraffin would also be extracted and reckoned, and probably traces of resin if present. Ordinary ethylic ether cannot be used, since tannins and many of their products are soluble in it. Probably carbon disulphide might be substituted. Care must be taken to avoid explosion, as the vapours of petroleum are very combustible. The residue left in the percolator may be examined for matters soluble in water, by extracting again with hot distilled water, or for resins (and phlobaphenes) by extraction with alcohol.
Estimation of matters soluble in water.—This is important both to detect weighting, and to draw conclusions as to the materials used in tanning. Fine raspings or shavings may be exhausted with warm water in a percolator, or roughly a weighed piece (20 grm.) of leather, air-dry, may be well kneaded and worked in 100 c.c. of warm water in a basin. 50 c.c. of this may be evaporated to dryness in a light basin over the water-bath (or under a paper hood on a steam boiler), and the gain of weight will give the amount dissolved from 10 grm. This is more accurate and quicker than redrying the leather and weighing loss. The residue will contain tannins and their products, often in considerable quantities, and may be examined by the table of reactions, p. 112, though these are as yet very imperfect. It will also contain glucose, dextrin, and soluble salts, if these have been used to give weight and firmness. The absolute proof of weighting with glucose or dextrin is difficult, since tanning materials naturally contain these and analogous principles. The residue may be powdered and exhausted with cold water, and the tannins and colouring matter removed by shaking with magnesia (p. 108) or lead carbonate. « 107 » Fehling's solution[J] is then added and the mixture is heated nearly to boiling. A rapidly formed and considerable precipitate of red cuprous oxide indicates weighting with glucose or dextrin. Leather extracts, however, invariably reduce Fehling's solution more or less, and a conclusion can only be drawn after some experience and comparative tests. Gallotannic acid and pyrogallol reduce it when heated, but not cane sugar or gum arabic. If a solution of cane sugar be heated to 68° C. for 1/4 hour with 10 per cent. of fuming hydrochloric acid, it is "inverted," and then after neutralising the acid with potash or soda, will reduce Fehling's solution when heated.
[J] 4 grm. cryst. cupric sulphate are dissolved in 20 c.c. of water; and 16 grm. of neutral potassic tartrate and 13 grm. of fused sodic hydrate are dissolved in 60 c.c. The two are mixed, made up to 100 c.c., and boiled for some minutes. It should always be tested before use by boiling a portion, which should remain perfectly clear.
The soluble mineral salts are detected by igniting the residue left after evaporation of a separate portion in a porcelain crucible.[K] From unweighted leather, the quantity is very small. The ash is exhausted with a few c.c. of distilled water, which will dissolve most sulphates and chlorides, which may be detected in small portions of the solution by baric chloride and silver nitrate respectively. Baric chloride and lead acetate are precipitated by a drop of sulphuric acid, and the latter is blackened with ammonic or sodic sulphide. Lime is precipitated by addition of ammonic chloride, ammonia, and ammonic oxalate; magnesia by the subsequent addition of sodic phosphate (see p. 109). The carbonates in the insoluble part (mostly derived from salts of organic acids) may be taken up by dilute hydrochloric acid and tested separately, or the acid may be used at first. Any residue undissolved by the acid is probably lead chloride, and will be dissolved by hot water.
[K] A platinum crucible must not be used for fear of its destruction by lead, unless this metal has been proved absent.
Estimation of ash.—The leather in small pieces (either after or before extraction with water) is incinerated in a porcelain « 108 » crucible. The ash is extracted with hydrochloric acid. The insoluble portion may contain barium sulphate (barytes), lead sulphate, sand, clay, &c. For further examination, ordinary chemical text-books must be consulted. Any large amount of ash indicates weighting. Müntz found only about 0·5 per cent. of ash from bark-tanned leather.
Determination of hide substance.—It is sometimes of interest to determine the proportion of dry hide-substance in a sample of leather, but there is no known means of doing this directly. If, however, the leather be dried, finely powdered by rasping, and the nitrogen determined by combustion, either with soda-lime (Will and Varrentrapp's method), or with copper oxide (Dumas), the hide-substance may be calculated, since tannin contains no nitrogen. Müntz found unhaired skin dried at 230° F. (110° C.) to contain 51·43 per cent. of nitrogen (compare also p. 20).
DETERMINATION OF FREE ACIDS IN TAN-LIQUOR.
The lime-water method mentioned on p. 172 is, from its simplicity, well suited for daily use in the tannery as a control method for ordinary working; but where it is necessary to make very exact estimations, or to determine the various acids separately, it is not so satisfactory as one recently published by Kohnstein and Simand (Dingl. Polyt. Jour., 1885, cclvi. 38).
The acids usually present in liquor consist of several members of the fatty or acetic group, which distil over with boiling water, of other non-volatile organic acids, and sometimes sulphuric acid, which is added to assist the swelling of the leather.
To determine the acids of the acetic group, Kohnstein and Simand proceed as follows:—100 c.c. of the liquor are distilled, in a flask or retort with a good condenser, to about 30 c.c., allowed to cool a little, made up again to 100 c.c., and again distilled; and this is repeated till about 300 c.c.« 109 » have passed over. The distillate is then made up to 300 c.c., well mixed by shaking, and the acid is determined with standard soda. Methyl orange and sodic carbonate is not so suitable for this titration, as caustic soda and litmus, since methyl orange is not very sensitive to vegetable acids. If it be desired to ascertain what quantity of acids of the acetic group exist in combination with lime and other bases in the liquor, small excess of sulphuric acid may be added to the residue in the retort, and the distillation repeated, when the organic salts will be decomposed and the volatile acids come over.
To determine the total free organic acids, Kohnstein and Simand shake about 80 c.c. of the liquor with 3-4 grm. of freshly ignited magnesia, quite free from carbonate and from lime, and allow to stand for some hours with frequent vigorous shaking, till the liquor, which at first is brown or dirty green, becomes almost colourless and gives no reaction of either acid or tannin. The mixture is then filtered, and the tannin and colouring matter are retained on the filter in combination with magnesia, while the organic salts of magnesia, which are mostly soluble, pass through with the filtrate. 10-30 c.c. of the filtrate, according to the amount of acid present, is evaporated to dryness, and gently ignited so as not to decompose any magnesic sulphate present. The residue is moistened with water saturated with carbonic acid, to convert any magnesic oxide into carbonate, and then dried, in order to make the mass powdery, and easier to wash, It is next taken up with hot distilled water, filtered and well washed. Any sulphate which is present passes into the filtrate, while the carbonate, which corresponds to the organic salts present before ignition, remains on the filter, and after solution in hydrochloric acid, is estimated as magnesic pyrophosphate. To the hydrochloric solution is added excess of ammonia and sufficient ammonic chloride to redissolve the precipitate formed, and prevent the precipitation of the magnesia; the solution is heated and then ammonic oxalate solution, first dilute, and then concentrated, is added to precipitate « 110 » any lime which may be derived from lime salts present in the liquor. After filtering out and washing the precipitate, 10-15 c.c. of 10 per cent. sodic phosphate solution is added, and the liquid is stirred with a glass rod without touching the sides of the beaker, and allowed to stand 12 hours. The crystalline precipitate is then rinsed on to a filter, and washed with a mixture of 1 of ammonia and 3 of water, till the washings no longer give any milkiness with silver nitrate. The filter is then dried and the precipitate is placed in a platinum crucible and first gently, and then strongly ignited with the cover on; the filter paper, freed as much as possible from the precipitate, is burnt in the usual way on the crucible lid, the ashes are added to the precipitate in the crucible, and the whole is again ignited and allowed to cool in the desiccator, and finally weighed. 111 parts of magnesia pyrophosphate correspond to 120 parts of acetic, or 180 parts of lactic acid. Kohnstein and Simand calculate the pyrophosphate corresponding to the acetic acid already found by distillation, and after deducting it reckon out the remainder as lactic acid. Of course the volatile acids are really a mixture consisting of acetic, propionic, butyric and other members of the fatty group; but it would be difficult if not impossible to separate them. Similarly other fixed acids exist in mixture beside the lactic acid, but as their action is similar and lactic acid is always the most abundant, these acids are to be reckoned as lactic.
It has been mentioned that when sulphuric acid is present in the liquor it is found in the filtrate from the magnesia carbonate as sulphate. After removal of the lime as oxalate, as previously described, the magnesia may be similarly determined as pyrophosphate, and reckoned out as sulphuric acid (111 parts of pyrophosphate being equal to 98 parts sulphuric acid, H2SO4). It may also be estimated with barium chloride, but in this case regard must be had to the sulphates originally present in the liquor.
Since waters invariably contain both lime and magnesia salts, a portion (50 or 100 c.c.) must be evaporated, ignited, « 111 » and after precipitation of the lime, the magnesia must be estimated as already described, and deducted from the amount found in a similar amount of liquor after saturating with magnesia. If, together with the organic acids, the liquor contains sulphuric acid, the correction may be divided equally between the two.
The method is not applicable in presence of phosphoric, tartaric, or oxalic acids. To overcome this difficulty, Messrs. Kohnstein and Simand are at present investigating a method dependent on decolorisation of the liquor with bone charcoal, completely free from mineral salts, and subsequent titration with soda.
It may be interesting to add the determinations of a complete set of handlers in a Continental upper-leather tannery, in which larch bark is used. 100 c.c. of liquor contained as follows, in grm.:—
| No. of Handler. |
Total Acids reckoned as Acetic. |
Volatile Acids reckoned as Acetic. |
Fixed Organic Acids reckoned as Lactic. |
| 1 | 0·205 | 0·050 | 0·232 |
| 2 | 0·628 | 0·237 | 0·586 |
| 3 | .. | 0·372 | .. |
| 4 | 0·688 | 0·426 | 0·393 |
| 5 | 0·569 | 0·432 | 0·206 |
| 6 | 0·509 | 0·453 | 0·084 |
| 7 | 0·487 | 0·456 | 0·047 |
QUALITATIVE DETECTION OF TANNINS.
It is often desirable to determine from what tanning materials an extract or liquor is made, or with what a sample of leather is tanned. The following table gives reactions of the principal tanning materials, which will enable any one of them to be recognised with certainty, and in many cases will determine the constituents in a mixture of several, though this is naturally far more difficult. In such cases, colour reactions are apt to mislead, that of one tannin being modified by another, and it is safest to rely on the categorical test of precipitate or no precipitate, coloration or no coloration, without regard to the tint. The infusions must be very weak, not exceeding 1-2° Bktr., or precipitates will be formed where mere coloration or clouding is noted. In some cases only negative peculiarities can be given, and the material cannot be positively determined in mixture with materials where these peculiarities are present. Thus myrobalans could not be distinguished from divi with certainty, where any other material, such as gambier, was present, which gave a deep coloration with concentrated sulphuric acid. The writer will feel greatly obliged by the communication of more distinctive reactions.
CHEMICAL ANALYSIS FOR THE TANNERY
| Reagent. | Myrabolanes. | Divi-divi. | Valonia. | Oak Bark. | Chestnut wood |
| Boiled with equal volume of sulphuric acid (1 vol. to 9 vol. water). | Pale deposit (eliagic acid) on cooling. | Pale deposit (eliagic acid) on cooling. | Slight pale deposit. | Slight pale deposit or turbidity on cooling. | Slight red deposit on cooling. |
| Bromine water. | No pp. | No pp. | No pp. | Pale pp. | No pp. |
| Dilute ferric chloride. | Blue-black pp. | Dark blue pp. | Blue-black pp. | Bluish black pp. | Blue-black pp. |
| Add ammonia. | Brown pp. | Dark red pp. | Red brown pp. | Red brown pp. | Dull red pp. |
| Sol. tartar emetic. | No pp. | Faint clouding. | No pp. | No pp. | Slight clouding. |
| Add ammonic chloride. | Light pp. | Dense pp. | Pale pp. | Whitish pp. | Pale pp. |
| Copper sulphate. | Faint clouding. | Slight green pp. | No pp. | Slight pp. | No pp. |
| Add ammonia. | Dense dark pp. | Dense dark pp. | Dark reddish pp. | Brown pp. | Dark brown pp. |
| Lime-water. | Yellow pp. turning greenish. | Yellow pp. turning purple. | Yellow pp. turning red-purple. | Brown pp. | Purplish brown pp. |
| Ammon. molybdate in nitric acid. | Dirty yellow pp. | Dark greenish pp. | Dark greenish pp. | Greenish pp. | Dirty green pp. |
| With sodic sulphide exposed to air on a tile. | Yellow colour. | Yellow colour. | Turns purpulish red. | Turns red. | Reddish pp. |
| Add concentrated sulphuric acid to 1 drop infusion. | Yellow colour. | Intense crimson. | Deep yellow. | Deep red pp. on dilution. | Dark brown. |
| Lead Nitrate | Light yellow pp. | Dark yellow pp. | Pale pp. | Brown pp. | Brown pp. |
| Cobalt Acetate | Buff pp. | Buff pink pp. | Dirty pink pp. | Ditto. | Dirty yellow pp. |
| Manganese acetate. | Yellow pp. | Yellow pp. | Dirty yellow pp. | Ditto. | Grey pp. |
| Uranium acetate. | Dark red colour. | Dark red colour. | Dark red colour. | Dark brown colour. | Dark red colour. |
| Ammoniacal picric acid sol. | No pp. | No pp. | Brown pp. | No pp. | No pp. |
| Potassic dichromate. | Brown pp. | Brown pp. | Brown pp. | Brown pp. | Brown pp. |
CHEMICAL ANALYSIS FOR THE TANNERY — Continued.
| Hungarian Larch (Extract). | Hemlock (Extract). | Mimosa bark. | Cutch (Pegu). | Gambier (Cuba). | Gallotannic Acid, 1 per cent. |
| Yellow Flocculent deposit separates quickly. | Abundant red flocculent deposit. | Heavy red depositon cooling. | Light red depositon cooling. | Reddish depositon cooling. | Usually some pale deposit. |
| Yellow pp. | Yellow pp. | Yellow pp. | Yellow pp. | Yellow pp. | No pp. |
| Dull brown pp. | Dirty green pp. | Full brown pp. | Green-black pp. | Intense green colour. | Blue-black pp. |
| Dull red pp. | Reddened pp. | Purple colour. | Dark red pp. | Reddened pp. | Reddened pp. |
| No pp. | No pp. | White pp. | No pp. | No pp. | No pp. |
| Pale pp. | Slight pale pp. | Dense white pp. | Pale pp. | Faint clouding. | White pp. |
| Slight cloud. | Pale pp. | Slight pp. | Dense pp. | No pp. | No pp. |
| Deep blue coloration. | Dark green coloration. | Deep red pp. | Deep violet coloration. | Dark green coloration. | Brown pp. |
| Dirty brown pp. | Brown pp. | Slight reddish pp. | Slight cloud soluble in excess. | No pp. | Pale pp. turns blue. |
| Slight clouding. | Slight pp. | Brown pp. | Ditto. | Ditto. | Yellow colour. |
| No change. | No change. | Turns red. | Slight reddening. | No change. | No change. |
| Dark brown or crimson. | Intense crimson. | Intense purple-red. | Deep red no pp. on dilution. | Dark brown or crimson. | Yellow. |
| Pale pp. | Pale pp. | Clouding. | No pp. | Faint clouding. | White pp. |
| Purplish pp. | Purple pp. | Brown pp. | Brown pp. | No pp. | Purple pp. |
| Slight clouding. | Slight pp. | No pp. | No pp. | Ditto. | White pp. |
| Slight darkening. | Light brown pp. | Dark red colour. | Dark red colour. | Dark red colour. | Crimson colour. Brown pp. |
| No pp. | Clouding. | No pp. | No pp. | No pp. | No pp. |
| Ditto. | Brown pp. slowly formed. | Brown pp. | Brown colour. | Brown pp. slowly formed. | Brown pp. |
QUANTITATIVE DETERMINATION.
Many processes have been proposed for the quantitative estimation of tannins, but it cannot be said that any method yet known is wholly satisfactory. The oldest, that of Sir H. Davy, recently improved by Stoddart and others, consists in precipitating with gelatin, and drying and weighing the precipitate. This is almost impossible to filter off as directed by Davy; but by the use of a little alum, and by pouring hot water on the precipitate, it becomes curdled into a mass which may be washed by decantation. As the precipitate contains varying quantities of tannin, according to the strength of solution employed; as it is soluble in excess of gelatin solution, and as it is almost if not quite impossible to wash it free from gelatin and alum, the method can hardly lay claim to much accuracy. A somewhat better one consists in the employment of a standard solution of gelatin with a little alum, determining the end of the reaction by filtering off a portion and ascertaining if another drop of the reagent produces a further precipitate. This method is very tedious, the end reaction is difficult to hit, the standard solution is very unstable, it is inapplicable to gambier and cutch because the « 115 » mixture will not filter clear, and its results are irregular, probably from the power of tannin to combine with various proportions of gelatin. A plan, which has a seductive appearance of simplicity, is that of Hammer; he takes the sp. gr. of the infusion, then absorbs the tannin with slightly moistened hide-raspings, again takes the sp. gr., and from the difference calculates the percentage of tannin, a difference of 5 per cent. of tannin corresponding to one of 1·020 sp. gr. (20° barkometer). Unfortunately the hide is more or less soluble in the liquor, and absorbs acids other than tannic with considerable energy; the moistening of the raspings introduces an error, and the smallness of the quantity to be measured makes a slight error completely vitiate the results. With extreme care, due corrections for temperature, for the water introduced with the raspings, and for their solubility, and by substituting evaporation of the infusions to dryness for mere calculation from their sp. gr., the method is useful as giving almost the only information obtainable as to the actual weight of tannin in any material capable of being absorbed by hide. It is, however, only suitable for use as a check on easier and more rapid methods, such as Löwenthal's, which give accurate relative results, but no information as to absolute weight of unknown tannins. A modification of Hammer's method has been introduced by Müntz and Ramspacher, in which the liquor whence the tannin is to be removed is forced through a piece of raw hide by pressure. This method, except that it is more rapid, has all the evils of Hammer's in an intensified form, and gives such variable results as to be quite useless in practice. A set of very careful determinations of one sample of sumach gave results ranging from 18 to 28 per cent., and similar variations occurred when the experiment was repeated with valonia. Wagner's method by precipitation with a standard solution of cinchonine and magenta has proved wholly unreliable.
Gerland's method with a volumetric solution of tartar emetic, used in presence of ammonic chloride, gives constant results with sumachs, 2/3 of those given by permanganate and « 116 » Neubauer's equivalent. Tartar emetic does not precipitate the tannins of cutch and gambier. Fleck's, by precipitation with copper acetate, and subsequent washing with ammonic carbonate and gravimetric estimation, either of the tannate dried at 212° F. (100° C.), or of the copper oxide left on ignition; and Carpene's, by precipitation with ammoniacal zinc acetate, and subsequent estimation with permanganate and indigo, though giving fairly accurate results on some tannins, are only of limited application. They may therefore be passed over, as well as Jean's method with a volumetric solution of iodine in presence of sodic carbonate, and Allen's method with lead acetate, which are tedious and difficult, and present no advantage over Löwenthal's improved process. This last is easy of execution, constant in results, and universally applicable. Before proceeding to describe it in detail, it may be well to give some hints as to the best modes of sampling and preparing tanning materials for analysis, since this is often more difficult and tedious than the actual analysis.
Sampling.—Samples should always be drawn from at least 10 sacks or separate parts of the bulk, and, in the case of valonia, special care should be taken to have a fair average quantity of "beard." No attention is usually paid to this point by merchants, and the proportion varies greatly in different parts of the same cargo. If several sacks are spread in layers on a level floor, and then portions going quite to the ground are taken from several parts of the floor, this will be accomplished. Where samples must be dealt with which have not been specially drawn, it might be safest to weigh out from each the same proportion of beard and whole cups, bearing in mind that the beard is always the richest part of the valonia. In sampling myrobalans, it should be remembered that the poor and light nuts will rise to the top, and hence the hand should be plunged well into the sack. Grinding of valonia and myrobalans when practicable is probably best done in a small disintegrator, fitted with gratings. The material, of which some pounds must be « 117 » used, is screened over a sieve of say 15 wires per in., and all coarser parts are returned to the mill till they will pass. The mill must grind into a close box, that no dust may be lost. Bark may be reduced to fine saw-dust by cutting a portion of each piece in the sample with a circular saw or rasp driven by a lathe. The advantage of these methods is that samples can be ground without previous drying, and thus in many cases time may be saved and separate determination of moisture avoided. When this is not practicable, the sample of some lbs. at least is ground in an ordinary bark-mill, well mixed, spread out flat on a floor or table, and several portions are taken as already described, say 50-100 grm. in all, and dried in a water- or air-oven at 212° F. (100° C.). The moisture is best determined, to save time, in a small separate portion of 10 grm., which must be dried till it ceases to lose weight, and the loss taken as moisture. It must be weighed in a covered capsule, as it is very hygroscopic. When the larger portion of the sample has been dried some hours, it is passed twice through a good coffee-mill, and then returned to the oven till thoroughly dried, for which, 12-24 hours is generally sufficient. Another method sometimes convenient is to take each acorn, or each piece of bark of the sample to be tested, and snip a piece from it with a pair of tinners' shears, taking care that in the case of valonia the section runs right to the centre of the cup; and in bark, that fair shares of the outer and inner layers are taken. The reason for drying before grinding is, that unless hard dried, tanning materials cannot be passed through a small mill. Bark and valonia usually contain 12-16 per cent. of moisture.
Exhaustion.—10 grm. of valonia, 20-30 grm. of bark, or corresponding quantities of other material, are boiled briskly for half an hour with 1 litre of distilled water, a funnel being placed in the neck of the flask, and great care being taken at first to avoid frothing and boiling over. The flasks used should have a capacity of at least 11/2 litre. The whole contents are finally rinsed into a gauged flask, allowed to cool to 59° F. (15° C.), and made up to 1 litre. In the case of « 118 » sumach, a little more boiling even than this is desirable. This method has been found by the writer to give better results than boiling with successive portions of water. Another method is to boil for 1/2 hour with 250 c.c. of water, then pour the whole on a filter, wash with boiling water so long as a drop of the filtrate blackens paper moistened with a dilute solution of ferric acetate, and finally make up to 1 litre. Many materials, however, clog the filter to such an extent that washing is almost impossible. Kathreiner has used 15 litres of water, and corresponding quantities of material, in a large steam-jacketed copper pan, for each exhaustion, making the weight up finally to 15 kilos., with very uniform and excellent results. (See also p. 130.) With all materials which deposit ellagic acid or other insoluble derivatives, on cooling and standing, considerably higher results will be obtained if the titration be made as soon as the liquor is cold, than if it be allowed to stand 24 hours; in this respect, a uniform practice should be adhered to. Addition of 1/2 c.c. of glacial acetic acid renders the infusions less liable to change.
Analysis.—Of all the methods which have been proposed for the estimation of tannins, the only one which has met with any general acceptance is that of Löwenthal, and indeed it is the only one which in rapidity of execution and constancy of results is fitted for general use. The method, as originally proposed, depends on the oxidation of the astringent solution by permanganate in presence of indigo, which not only serves as an indicator, but controls the oxidation, limiting it to those bodies which are more oxidisable than indigo. As, however, these include gallic acid and other substances which are useless to either tanner or dyer, it is necessary to remove the tannin, and by a second titration to obtain its value by difference. This Löwenthal (Zeitschrift f. Anal. Chemie, 1877, p. 33) accomplished by a solution of gelatin and common salt, to which, after mixture with the tannin infusion, a small quantity of sulphuric or hydrochloric acid was added. It was necessary to let this stand « 119 » at least some hours before a clear filtrate could be obtained, and the gelatin remaining in solution had a slight though generally negligible effect on the permanganate. In some cases, even after long standing, perfect filtration was extremely difficult and tedious, and it was also clearly proved by Simand (Ding. Polyt. Jour., ccxliv. 400) that a certain proportion of the tanno-gelatin precipitate, varying with the acid present, and with the species of tannin, remained in solution, and thus gave too low a result. He therefore proposed to revert to the old method of separating tannin with hide raspings, or, as an improved substitute, with the gelatinous tissue of bones, and this is probably the most accurate method, but has the disadvantage of requiring considerable time for its execution. (See also p. 130.) The writer has therefore tried, and he thinks successfully, so to modify Löwenthal's original method as to increase its accuracy, and at the same time to make it more rapid and easy of execution. It was found that by saturating the clear filtrate with salt, a further precipitate containing tannin was formed, but unfortunately, it was so finely divided that no amount of standing, or even of warming, and repeated passing through the paper, would obtain a clear filtrate. Finally, he hit on the device of mixing with the liquid, before filtration, a portion of the pure kaolin used by photographers. The effect was instantaneous and complete. A perfectly clear filtrate was obtained without any of the tedious waiting which before was necessary, and it was not only free from tannin, but also nearly so from gelatin, so that it only gave the faintest cloudiness with tannin solution. Gelatin gives a more considerable precipitate, but this is simply due to its insolubility in the saturated salt solution, and it is redissolved on dilution with water.[L]
[L] Hunt (Jour. Soc. Chem. Industry, April 1885) states, that saturation with salt causes partial precipitation of gallic acid when present, and that results agreeing more closely with those obtained by absorption with hide are obtained by employing a mixture of 50 c.c. liquor, 25 c.c. 2 per cent. gelatin solution, and 25 c.c. saturated solution of salt containing 50 c.c. of concentrated sulphuric acid per litre and a teaspoonful of kaolin. This approaches very nearly to Löwenthal's original method, but with the addition of the kaolin, and as in it, it is to be feared that a portion of the tannate of gelatin will remain in solution. For accurate work, therefore, absorption by hide-raspings is preferable, though even that has been shown by the writer to remove gallic acid and other matters beside tannin. Hunt states that raw hide also absorbs catechin.
A slight error is introduced by the presence of a trace of oxidisable matter in the gelatin, and when very great accuracy is required, it is well to make a blank estimation of "not-tannin" without tannin infusion, and deduct 1/2 of the permanganate consumed as a correction from the not-tannin; but this may usually be disregarded. Each titration should be made twice, and successive tests should not differ by more than 0·1 c.c. of permanganate.
Reagents.—Solutions are required of (1) Pure potash permanganate, 1 grm. per litre. (2) Pure soda or potash sulphindigotate, 5 grm., and concentrated sulphuric acid, 50 grm. per litre. (3) Pure oxalic acid, 6·3 grm. per litre (decinormal). The sulphindigotate (indigo carmine), must be filtered, and when oxidised by permanganate, should give a pure clear yellow, free from any trace of brown or orange. Any contamination with indigo-purple, which gives brown oxidation-products, is quite fatal to the accuracy of the analysis. The permanganate solution is standardised by measuring 10 c.c. of the (decinormal) oxalic acid solution, adding a little pure sulphuric acid and distilled water, warming to 136° F. (58° C.), and running in the permanganate till a faint permanent pink is produced, for which about 32-33 c.c. should be required. The indigo-carmine solution should be of such strength that 14-16 c.c. of permanganate are required to bleach the quantity employed, which may be 20-25 c.c., as convenient. (4) Gelatin solution: 2 grm. of Nelson's or other good gelatin are allowed to swell in distilled water for two hours, melted by setting the glass in a pan of boiling water, and made up to 100 c.c. This will not keep. (5) Dilute sulphuric acid: 10 c.c. of pure concentrated acid « 121 » are added to 90 c.c. of distilled water. (6) Good table salt. (7) Purified kaolin.
The analysis is performed in the following manner:—20 c.c. of indigo solution, and 5 c.c. of the infusion of tanning material is added, in a white basin as recommended by Kathreiner, to about 3/4 litre good water, which it is best to measure approximately, so that if it contains any impurity which affects the permanganate it should be constant, and thus be eliminated with the indigo. Permanganate solution is then allowed to drop in, with constant stirring till the pure yellow liquid shows a faint pinkish rim, most clearly seen on the shaded side. This end-reaction, which is of extraordinary delicacy, is due to Kathreiner, and is quite different to the pink caused by excess of permanganate, being an effect common to all pure yellow liquids. It is not needful to make the titration so slowly as has been advised—the permanganate may be dropped in steadily with vigorous stirring, so long as there is large excess of indigo, but as soon as the bottom of the basin can be seen through the solution, it must be added very cautiously, one or two drops at a time, and with occasional pauses, to allow time for its complete mixture through so large a mass of fluid. The titration is repeated twice, and the results added together and denoted by a. Then take 50 c.c. of the infusion, and add 28·6 c.c. of the gelatin solution of Nelson's gelatin of 2 grm. to 100 c.c. After shaking, the mixture is saturated with salt, which brings the volume up to 90 c.c., and 10 c.c. of the dilute sulphuric acid (containing 1 vol. of concentrated acid in 10) and a teaspoonful of pure kaolin are added. It is best to do this in a flask in which it can be well shaken, after which, filtration may be at once proceeded with, although it is safer to let it stand an hour or two: (the flask may be cleansed with caustic soda solution). 10 c.c. of this filtrate (= 5 c.c. of the original infusion) are employed for a second pair of titrations, which are added as before, and the result denoted b. If, further, c be the quantity of permanganate required to oxidise 10 c.c. of decinormal oxalic « 122 » acid, and 10 grm. of the tanning material have been employed to make 1 litre of infusion, c : (a - b) :: 6·3 : x, where x is the percentage of tannin expressed in terms of crystallised oxalic acid. If it be desired to calculate the gallic acid and non-tannin substances contained in the infusion, the value in permanganate of the indigo alone must be determined. Calling this d, as c is to (b - d), so is 6·3 to the percentage of non-tannins in terms of oxalic acid, and for the present it is best invariably to calculate results in this way, since we do not actually know the relation of any single tannin to permanganate, even Neubauer's number for gallotannic acid being probably too high, according to the recent investigations of Councler and Schroeder,[M] and Oser's for quercitannic being at most only approximate. It happens, moreover, that this last equivalent (62·36 grm. of quercitannic acid = 63 grm. of crystallised oxalic acid) does not differ from that of oxalic acid more than the ordinary limits of error of such estimation, and the substitution is therefore of no commercial importance, while it is surely better to employ a standard which is easily and exactly verified than one which is certain to be modified by further research, and so to run the risk either of having our results made useless for future comparison, or of establishing a false or arbitrary equivalent. What is wanted for practical purposes is not the absolute weight of tannins in the various materials, but only a means for the relative comparison of two samples of the same material; cross comparisons of different tannins being simply delusive. If, however, it is necessary at any time to give actual percentages of gallotannic acid, it is probably best to stick to Neubauer's number for the present, as it is in general use. Neubauer states that 63 grm. of oxalic acid consume as much permanganate as 41·37 grm. of gallotannic acid. Tshekawa found 41·688 as the equivalent for tannin from Japanese gall nuts (Chem. News, xlii. 274). Councler and « 123 » Schroeder on the other hand give only 34·3 grm. Simand gives 61·1 grm. as the equivalent of quercitannic acid. Commercial "pure tannin" always gives results higher than the truth, as the gallic acid which it contains consumes more permanganate than an equal weight of tannin, or even than the tannin which would yield it if boiled with acid. When this is done the equivalent used should be definitely stated, or it will certainly lead to confusion. Neubauer's equivalent is only properly applicable to gall nuts, and possibly to sumach and myrabolans. For oak bark Oser's number or that of oxalic acid is most likely nearly correct; and this may also be approximately true of oak wood and valonia, but as respects all other materials we have no information whatever, and the oxalic equivalent is as likely to be right as any other. (Compare note, p. 128.)
[M] From researches by von Schroeder, published since the above was penned, it seems that the permanganate consumed by tannin is largely influenced by the way in which the titration is conducted, see p. 128.
A few results are given below, not as showing the relative values of the materials, which, of course, cannot be directly compared by any analytical process, but for comparison with those obtained by other methods and modes of calculation:—
| Tannin (as Oxalic Acid). |
Other Bodies Oxidised (as Oxalic Acid). |
|
| Spent Liquor | 0·12 | 11·0 |
| Valonia (good Smyrna). Sample 1 | 29·1 | 2·3 |
| " " Sample 2 | 30·7 | 2·1 |
| " " Sample 3 | 30·5 | 1·9 |
| Hungarian Larch Extract. Sample 1 | 14·78 | 1·95 |
| " " Sample 2 | 18·08 | 2·33 |
| Chestnut-wood Extract, 25° B. | 25·53 | 3·68 |
| Pegu Cutch | 63·59 | 2·45 |
It is proved by experiment that kaolin removes nothing which is oxidised by permanganate, but simply facilitates the precipitation and filtration; and it is often found useful to clarify the original infusions and liquors before the first titration. On the other hand, there is no doubt that the salt and acid of Löwenthal's method precipitate of themselves « 124 » a large proportion of certain tannins. In the case of cutch this amounted, in the analysis given, to 67 per cent. of the whole. There is, however, good reason to believe that this would also have been absorbed, or at least removed from solution by hide in the process of tanning. This is shown by the analysis of the spent liquor above given, which originally contained the tannins of oak bark, valonia, myrabolans, gambier, hemlock, and oak wood extracts, &c., to the extent of 10 to 15 per cent., but which was reduced by contact with hide to 0·12 per cent. That a portion had not been absorbed but decomposed is proved by the large accumulation of oxidisable impurities (equal to 11 per cent. of oxalic acid); at the same time this example shows that the method is capable of estimating a very small portion of tannin in presence of much gallic acid and other analogous substances. It is worth remark that such spent liquors become very pale in colour, and also that the filtrates, freed from tannin by precipitation, are nearly colourless, thus proving that the colouring matters present in tanning materials are of the nature of tannins, at least as regards their precipitability by hide and gelatin.
Simand (Dingl. Polyt. Jour., ccxlvi. 133) has recommended instead of precipitation with gelatin, the use of the gelatinous tissue of bones to remove the tannin. For this purpose porous bones, such as horn piths, are coarsely powdered, and after treatment with dilute soda solution to remove the fat, are steeped in weak hydrochloric acid till all the calcareous matter is dissolved. They are then thoroughly washed, ground wet through a steel mill, washed again and dried at a low temperature; the tannin is removed more quickly than by raw hide, and the amount of gelatinous matter dissolved by cold water is a very trifling one. This method, or that with purified hide-powder, is to be recommended for scientific research, since no element capable of precipitating substances other than those absorbed by the hide is introduced, while it is not certain in all cases that saturation with salt and acidification may not remove other constituents of the liquor « 125 » besides tannins. It has, however, for technical purposes the great disadvantage of requiring a much longer time for absorption of the tannin than is the case with gelatin solution, and of the process being much more difficult of execution. If hide-powder be employed, it must be moistened with a small quantity of water before adding to the infusion, and this water must be taken into account in the quantity of the filtrate employed for the titration of the "non-tannin." The digestion with the hide- or bone-powder must be continued till the filtered liquid does not give the faintest clouding with a drop of clear gelatin solution, and it is always very difficult to be sure that the tannin is so completely removed as with gelatin and salt. Hide- or bone-powder may be employed to determine the actual weight of any unknown tannin absorbable by hide, by evaporating equal quantities of the original infusion and of that freed from tannin by digestion with the powder; the difference giving the tannin absorbed. The evaporation must be conducted as far as possible in absence of air, for instance in vacuo, or in a current of carbonic dioxide, and the residues both dried at 212° F. (100° C.) so long as they lose weight. The amount of matter dissolved from an equal quantity of the hide- or bone-powder by water must also be ascertained and taken into the calculation.
Ammoniacal solution of cupric acetate or sulphate has been employed by several chemists to remove tannin from solutions. N. H. Darton of New York, who has a large practice in tannin analysis, employs cuprammonic sulphate in the following manner.
The infusion, for which 20 grm. of hemlock bark or a corresponding quantity of other material must be used, is made by exhausting with 2 or 3 quantities of water successively, first cold, and then with heat (by placing the flask in a pan of boiling water), each portion of water being poured off into a litre flask. The last should be almost colourless. The liquor is thus made up to nearly 1 litre, 25 c.c. of dilute sulphuric acid (about 1 vol. concentrated in « 126 » 10) is added, and the liquor is filtered through a small filter, which is finally rinsed with a small quantity of water. Liquid ammonia is now added till the liquor slightly smells of it, and, if any precipitate is formed, it is filtered off as before; 25 c.c. of dilute sulphuric acid is again added (which should give the liquid an acid reaction), and it is made up to 1 litre. The titration is done as described under Löwenthal's method, but instead of precipitating with gelatin, 100 c.c. is mixed with 100 c.c. of a solution of copper sulphate to which sufficient ammonia has been added to redissolve the precipitate first formed, and containing 11/4 per cent. of copper sulphate. This is well shaken and filtered, and the "not-tannin" is determined in the filtrate just as with gelatin; a little dilute sulphuric acid being added in the basin to neutralise the ammonia. The writer has examined this method with regard to a few tanning materials. With valonia (and therefore probably with oak bark) the preliminary treatment is unnecessary, and copper precipitation gives results practically identical with the improved gelatin, while it is less troublesome. On the other hand, a sample of Miller's Hungarian Larch Extract which gave tannin equal to 18·08 per cent. (by the gelatin method) gave no precipitate with cuprammonic sulphate, and hence a result in tannin of nil by Darton's method. It is worth remark that by the copper method it is therefore possible to estimate the valonia tannin alone in a mixture of larch and valonia tannin. Probably this mode of analysis may also be utilised to separate other tannins. With chestnut extract the results seem satisfactory, as regards the precipitation of the tannin by copper, the figures agreeing very closely with those by gelatin, but the preliminary treatment with sulphuric acid and ammonia precipitates about 75 per cent. of what is usually reckoned as tannin, leaving 7·53 per cent. of tannin only instead of 25·53 per cent. as reckoned by the gelatin method; which, judging by practical results in tanning, can hardly be accepted as correct. The results of the gelatin method are found to agree fairly with those of direct absorption by hide-powder, which is « 127 » strong confirmation that what is estimated as tannin is what is absorbed by the hide. It is well known that sulphuric acid precipitates many tannins, and in an experiment with cutch it was found by the writer that saturation with salt and the addition of dilute sulphuric acid as for Löwenthal's process, but without the gelatin, precipitated 67 per cent. of the total tannin as usually reckoned.
It is obvious that it is impossible by analysis to compare the relative value of different tannins, such as those of myrobalans and gambier, or hemlock and valonia. All that analysis can reasonably be expected to do is to give the relative values of different samples of the same substance, or at the most, of materials of the same class. All other comparisons are misleading; and would be so, even if the exact percentage of each tannin could be calculated; since the commercial and practical value of different materials does not depend on the quantity of tannin only, but on the character of the leather it produces, hard or soft, dark- or light-coloured and heavy- or light-weighing.
A Commission of German technical chemists, under the presidency of Dr. Councler of Eberswalde, and including Messrs. Eberz, Kathreiner, Schaun, von Schroeder, and Simand, have recently reported on methods of tannin estimation ('Bericht über die Verhandlungen der Commission zur Feststellung einer einheitlichen Methode der Gerbstoffbestimmung,' Cassel, 1885). After reviewing earlier methods, they recommend the following modifications of the Löwenthal method, for general adoption.
Chemicals employed.
(1) Permanganate solution. 10 grm. of the purest potash permanganate are dissolved in 6 litres of distilled water.
(2) Indigo solution. 30 grm. dry sulphindigotate of soda (Carminum cærul. opt., "pure Indigotin I" of Gehe & Co., Dresden), air-dry, are dissolved in 3 litres of dilute sulphuric « 128 »acid (1 vol. H2SO4 to 3 vols, water), 3 litres of distilled water are added, the whole is shaken till dissolved, and filtered. In each titration, 20 c.c. are used in 3/4 litre of water, and reduce about 10·7 c.c. of permanganate.
(3) Hide-powder must be white and in a fine woolly state of division, and should yield to cold water no substance capable of reducing permanganate. Such a powder is prepared by Dr. Both of Berlin,[N] and by the Vienna Research Station.
[N] Messrs. Mawson and Swan, of Newcastle, have kindly undertaken to keep these, and the other reagents mentioned in this book, in stock for the convenience of English tanners and chemists.
Mode of Titration.
Instead of adding the permanganate solution drop by drop, to the mixture of indigo, water, and liquor (as described, p. 121), it is recommended to add it 1 c.c. at a time,[O] vigorously stirring 5-10 seconds after each addition. When the liquid has become bright green, 2-3 drops at a time are cautiously added with stirring, till the liquid is pure yellow. Either a beaker on a white tile or a white basin may be used (compare p. 121). It is advantageous in strong sunlight to shade the window with white tissue-paper.
[O] It has been noted by several chemists, and especially by Kathreiner, and later by Prof. von Schroeder, that the quantity of permanganate reduced by a given amount of tannin varies within rather wide limits, according to the rate at which the permanganate is added; and the "1 c.c. method" was suggested by Prof. von Schroeder, to secure uniformity in this particular. It has, however, been found by the writer, in the course of experiments not yet completed, that the quantity of permanganate required, was a function not simply of time, but of the rapidity of diffusion through so large a bulk of liquid; and by the alternate use of a simple glass rod, and of a specially constructed perforated stirrer, he was able, while adhering strictly to Prof. von Schroeder's directions, to obtain results even more divergent by the "1 c.c. method" than could be obtained by the drop method previously recommended, when properly carried out. Employed in conjunction with the use of tannin for standardising, as recommended by the Commission, either method gives perfectly dependable results.
The explanation of the variation is a simple one. The oxidation in the Löwenthal process should be limited to indigo, and bodies more oxidisable than indigo, but there exist both ready formed in liquor, and among these oxidation products many substances which in the absence of indigo will readily reduce permanganate. When the latter is added rapidly, and with insufficient stirring, it destroys the indigo and tannin in contact with it, and proceeds also to oxidise the other matters present, although in other parts of the beaker indigo and tannin still exist. Thus more permanganate is reduced than corresponds to the indigo and tannin, and this is especially so towards the end of the process, when very little of either remains. The more slowly the permanganate is added, and the more vigorously it is stirred, the more closely it will approximate to the theoretical quantity required merely to oxidise the indigo and tannin. It seems to the writer more scientific to approach this as nearly as possible, than to attempt to establish a purely arbitrary standard such as the "1 c.c. method;" but he would rather refrain from committing himself to a definite opinion till his experiments are complete.
Standardisation of the permanganate.
To avoid the uncertainty involved in comparing tannin (which reduces different quantities of permanganate according to the method of titration) with so dissimilar a reducing agent as oxalic acid, it is recommended to employ tannin, titrated under precisely the same conditions as the tanning material, so that whatever method be employed, the differences will be common to both, and will so be eliminated. Prof. von Schroeder has shown (Report, p. 74) by careful experiment, that with the purest samples of tannin the permanganate value estimated on the total dry substance of the tannin varied by very little from that of the part of the tannin absorbed by hide as determined by Hammer's process, but on the average bore the proportion of 1 : 1·05. The percentage of water in an air-dried tannin must be estimated by drying a portion at 201°-212° F. (94°-100° C.) and determining the loss, and a quantity equivalent to 2 grm. must be dissolved in 1 litre of water and 10 c.c. titrated with indigo in the usual way. If the permanganate value thus obtained be multiplied by 1·05, it will be equivalent to that of 2 grm. of chemically pure tannin. It is only necessary to determine the moisture occasionally, if the tannin be kept in a well-closed box or bottle.
To ascertain if a tannin is pure enough for this, use a solution made as above described (it is not necessary to determine the moisture) and 10 c.c. are titrated with permanganate « 130 » in the usual way. 50 c.c. are then digested in the cold with 3 grm. hide-powder (previously moistened with distilled water and well squeezed in linen) for 18-20 hours, with frequent shaking, filtered, and 10 c.c. again titrated. If the second titration ("not-tannin") does not exceed 5 per cent. of the total, it is good, but it may be used so long as the "not-tannin" does not exceed 10 per cent.[P] The purest tannin examined by Prof. von Schroeder was Schering's Phar. Ger., which may be obtained of Messrs. Mawson and Swan.
[P] Gallic acid suggests itself to the writer as being a good standard, since it behaves with permanganate like tannin, and being crystalline is easily purified and of definite composition.
The course of analysis is as follows:—
Preparation of the infusions.—Extracts are dissolved in hot water, and if necessary, filtered. Barks and other solid materials are treated in Prof. von Schroeder's extraction-apparatus (Fig. 20) (which seems very well adapted for its purpose). This consists of a perfectly cylindrical vessel of cast-tin, about 12·5 c.m. deep and 7 c.m. diameter. A strainer covered with fine muslin fits it like a piston.[Q] The powdered material is placed in the cylinder, and stirred up with 200 c.c. of cold water. At the end of an hour, the piston is inserted and pressed down gently, the clear liquor is poured off, and the process is 4 times repeated with hot water, at intervals of 1/2 hour, placing the cylinder in a water-bath. The liquid is made up to 1 litre, and, if necessary, filtered (Report, p. 66). The quantity of material used should be such « 131 » as to give an infusion of which 10 c.c. do not reduce more than 8 c.c. permanganate. If it is desired to determine separately the "easily soluble tannin" (viz. that extracted by cold water), Real's Press (Fig. 21) is employed, which consists of a cylinder a, through which water may be forced by the pressure of a column of liquid. The small sieve d, covered with a disc of linen, is placed in a, next the tanning material previously thoroughly moistened with water, and the tap is closed. The press is then filled with water and left 15 hours under a pressure of 11/2 metres. The tap is then opened and 1 litre is allowed to run through in the course of about 2 hours, and mixed by shaking. The material is finally exhausted like a new material in von Schroeder's apparatus to extract the difficultly soluble tannin.
[Q] Both this apparatus, and the Real's press, may be obtained from C. Focke, Zinngiesser, Grosse Kirchgasse 3, Dresden.
The titration is carried out as before described; in each infusion separately to determine the "not-tannin" 50 c.c. are treated with 3 grm. hide-powder, and 10 c.c. are titrated.
It may be well in conclusion for the writer to state for the information of the non-chemical reader, that though for purposes of comparison of the results of different chemists, it is most desirable to have a standard method of the highest possible perfection; any of the accepted modifications of the Löwenthal method will give excellent practical results in careful hands.
SOLE-LEATHER:—Preparing the Hides.
The principal sources of hides for sole-leather are:—
(I.) Market hides, from the cattle slaughtered for food in the United Kingdom. These are received by the tanner, fresh, or slightly salted, and are either bought directly from the butcher, or, now more commonly, through the auction markets established in all large towns. The latter system, while it perhaps slightly enhances the price of the hides to the tanner, ensures him a better classification according to weight, and, in some cases, as notably in that of Glasgow, a better flaying, through an organised system of inspection and sorting. The Scotch hides, being mostly from Highland cattle, are many of them small and very plump, for, as a rule, the hides are thickest on those animals which are exposed to cold and the hardships of out-door life. On the other hand, the hides of highly-bred cattle are apt to be thin and spreading; and, if they have been kept much indoors, and negligently managed, the grain of the hide is injured by the dung which adheres to it. The Irish hides are usually somewhat roughly flayed.
(II.) South American hides are from the River Plate, Uruguay, and Rio Grande. Those from the River Plate are considered the best, as being stoutest and finest in texture. They are usually cured by salting, and are known as "saladeros," "estancias," and "mataderos," according to the slaughter and cure. The saladeros are the best, and are from cattle killed at large slaughtering establishments on the coast. The estancias are from cattle killed in the interior, and are worse in flaying than the saladeros, but free from the objectionable dark cure of the mataderos, which are « 133 » killed by the city butchers. Many hides are brought from Brazil, and are generally both salted and sun-dried, or simply stretched out and dried. Hides are also imported from Valparaiso, both dry and wet-salted.
Chinese and West Indian hides are mostly dried. Chinese hides are occasionally infected with Bacillus anthracis, which produces the dangerous "malignant-pustule," or "wool-sorters' disease." Hence any pimple appearing after working with such hides should have immediate medical attention. French market hides have been of recent years largely imported; they are mostly well flayed, and some of them very heavy, but are sold at original butchers' weight, and, in the experience of some tanners, the result in leather is 5-6 per cent. less than from English market hides. They usually lose about 25 per cent. in skulling and salting. Lisbon hides are often well flayed, but are frequently branded, and the grain is injured by insects. They yield considerably more leather than market hides in proportion to weight. Hambro' hides are salted, but mostly wet and ill-flayed. Very heavy hides are produced in the Rhine district and in Switzerland.
For further information about hides, see the Commercial Section.
Preparation for Tanning.—Market hides should be well washed in fresh water, to remove blood and dirt, before unhairing; but prolonged soaking dissolves a portion of hide-substance, and probably reduces weight, though it facilitates the action of the lime. It is very advantageous if grease and flesh, and also dung can be removed before liming, and if hand-labour is too costly machinery might be employed. Salted hides should be soaked somewhat longer, and in clean water, so as to remove the salt before liming. This water should be frequently changed, since 10 per cent. brine dissolves coriin freely (see p. 19). Dried hides require more lengthened treatment. Before they are prepared for tanning, they must be brought back as far as possible to the condition of fresh hides, and, for this purpose, must be « 134 » thoroughly soaked and softened in water. There are many ways of doing this: sometimes hides are suspended in running water; sometimes laid in soaks, which may be either renewed, or allowed to putrefy; sometimes in water to which salt, borax, or carbolic acid has been added, to prevent putrefaction.
The first of these methods, were it desirable, is rarely possible in these days of River Pollution Acts; of the others, it is difficult to say which is better, since the treatment desirable varies with the hardness of the hide and the temperature at which it has been dried. The great object is to thoroughly soften the hide, without allowing putrefaction to injure it. As dried hides are often damaged already from this cause, either before drying, or from becoming moist and heated on ship-board, it is frequently no easy matter to accomplish this. The fresh hide, as has been seen, contains considerable portions of albumen, and if the hide is dried at a high temperature, this becomes wholly or partially coagulated and insoluble. The gelatinous fibre and the coriin (if indeed the latter exists ready formed in the fresh hide) do not coagulate by heat, but also become less readily soluble. Gelatin dried at 266° F. (130° C.) can only be redissolved by acids, or water at 248° F. (120° C.). Eitner experimented with pieces of green calf-skin of equal thickness, which were dried at different temperatures, with results given in the following table:—
| Sample. | Temperature of Drying. | Remarks. | Time of Softening in Water. |
Remarks. | Coriin Dissolved by Salt Solution. |
||||
| I. | 59° F. (15° C.) | In vacuo | 24 hours | Without mechanical work |
1·68 per cent. | ||||
| II. | 711/2° F. (22° C.) | In sun | 2 days | 1·62 " | |||||
| III. | 95° F. (35° C.) | In drying-closet | 5 " | twice worked | 0·15 " | ||||
| IV. | 140° F. (60° C.) | " | Refused to soften sufficiently for tanning |
Traces. | |||||
Hence it is evident that, for hides dried at low temperatures, short soaking in fresh and cold water is sufficient, and, « 135 » except in warm weather, there would be little danger of putrefaction. With harder drying, longer time is required, and it may be necessary to use brine instead of water. A well-known tanner recommends a solution of 30°-35° barkometer (sp. gr. 1·035, or about 5 per cent. of NaCl). This will have a double action, not only preserving from putrefaction, but dissolving a portion of the hide-substance in the form of coriin. Although this is undoubtedly a loss to the tanner, it is questionable if there is any process which will soften overdried hides without loss of weight: since even prolonged soaking in cold water at too low a temperature to allow of putrefaction will dissolve a serious amount of hide-substance. Water containing a small quantity of carbolic acid has been recommended for the purpose, and will prevent putrefaction, while it has no solvent power on the hide, but, on the contrary, will coagulate and render insoluble albuminous matters. Concentrated carbolic acid, however, tans the grain and renders it incapable of colouring in the liquors. Borax has been proposed for the same purpose, and, in strong solution, certainly prevents putrefaction, but is probably too costly. Sodium sulphide and other sulphides seem to have considerable effect in softening dried hides, from their property of attacking hard albuminous matters, without injuring the true hide-fibre.
For some descriptions of hides, however, and notably for India kips, putrid soaks seem actually to be an advantage, the putrefactive action softening and rendering soluble the hardened tissue. In India the native tanners soften their hides in very few hours by plunging them in putrid pools, into which every description of tannery refuse is allowed to run. Putrefactive processes are always dangerous, as the action, through changes of temperature, or variation in the previous state of the liquor, is apt to be irregular, and either to attack one portion of the hide before another, or to proceed faster than was expected. Hence hides in the soaks require constant and careful watching, and the goods must be withdrawn as soon as they are thoroughly softened, for the « 136 » putrefaction is constantly destroying as well as softening the hides. It is possible that putrefactive softening is less injurious to kips, and such goods as are intended for upper-leather, than to those for sole purposes, as it is generally considered necessary in the former case that the albumen and interfibrillary matter be removed, and that the fibre be well divided into its constituent fibrils for the sake of softness and pliability; so that the putrid soak, if acting rightly, only accomplishes a part of the work which would afterwards have to be done by the lime and the bate. The actual fibre of the hide seems less readily putrescible than the albuminoid parts; hence the putrefaction may soften the latter better, and even at less expense of valuable hide-substance, because more rapidly, than fresh water. On this point, there is room for investigation. Putrefaction is a general name for a class of decompositions which are caused by a great variety of living organisms, each of which has its own special products and modes of action. It is quite possible that, if we knew what precise form of putrefaction was most advantageous, we might by appropriate conditions be able to encourage it to the exclusion of others, and obtain better results than at present. It will be necessary to revert to this subject when speaking of the bates used in preparing dressing-leather, which also owe their activity to putrid fermentation.
Beside merely soaking the hides, it is necessary to work them mechanically, to promote their softening, which was formerly accomplished by "breaking over" the hides on the beam with a blunt knife. This process is now usually superseded or supplemented by the use of the "stocks"; these consist of a wooden or metallic box, of peculiar shape, wherein work 2 very heavy hammers, raised alternately by pins in a wheel, and let fall upon the hides, which they force up against the side of the box with a sort of kneading action. The ordinary form of this machine is shown in Fig. 22. A more modern form, which seems to possess some advantages, is the American double-shover, seen in Fig. 23.
The number of hides which can be stocked at once naturally varies with the size of both hides and stocks, but should be such that the hides work regularly and steadily over and over. The whole number should not be put in at « 138 » once, but should be added one after another, as they get into regular work. The duration of stocking is 10-30 min., according to the condition and character of the hides. Hides should not be stocked till they are so far softened that they can be doubled sharply, without breaking or straining the fibre. After stocking, they must be soaked again for a short time, and then be brought into an old lime. A small quantity of sodium sulphide added to the soaks or in the stocks has been recommended as of great value in softening obstinate hides, and probably with justice, from its well-known softening action upon cellular and horny tissues.
In Continental yards, another machine is in use for softening hides, and which seems to present some advantages over stocks, as being less severe on the thinner portions of the hide. It consists of a pair of rollers, arranged like those of a wringing machine, and pressed together by springs, but not allowed to come into actual contact. One of them is studded with rounded pegs, which correspond in position to grooves round the other, and the hide when passed between them is thus subjected to a very thorough kneading and stretching. Tumbler drums of various forms may also be used with good effect for softening purposes, especially for skins.
SOLE-LEATHER:—Unhairing Hides.
In England, lime is the agent almost universally employed for loosening the hair, though every tanner admits its deficiencies and disadvantages. It is hard, however, to recommend a substitute which is free from the same or greater evils, and lime has one or two valuable qualities which will make it very difficult to supersede. One of these is that, though it inevitably causes loss of substance and weight, it is also impossible, with any reasonable care, totally to destroy a pack of hides by its use; which is by no means the case with some of its rivals. Another advantage is that, owing to its very limited solubility in water, it is a matter of comparatively small consequence whether much or little is used; and even if the hides are left in a few days longer than necessary, the mischief, though certain, is only to be detected by careful and accurate observation. With all other methods, exact time and quantity are of primary importance, and it is not easy to get ordinary workmen to pay the necessary attention to such details. Again, the qualities of lime, its virtues and failings, have been matter of experience for hundreds of years, and so far as such experience can teach, we know exactly how to deal with it. A new method, on the other hand, brings new and unlooked-for difficulties, and often requires changes in other parts of the process, as well as in the mere unhairing, to make it successful. As our knowledge of the chemical and physical changes involved becomes greater, we may look to overcoming these obstacles more readily; for the power of dealing successfully with new difficulties constitutes one of the « 140 » main advantages of a really scientific knowledge over an empirical one.
Slaked lime is soluble in water at 60° F. (15° C.), to the extent of 1 part in 778. Unlike most substances, it decreases in solubility at higher temperatures, requiring 972 parts of water at 130° F. (54° C.), and 1270 parts at 212° F. (100° C.). Its action upon animal tissues increases rapidly, however, with temperature, though no doubt it is moderated to some extent by the lessened solubility. Calculating from Dalton's numbers, pure lime-water at 60° F. (15° C.) contains 1·285 grm.[R] of CaO per litre, and should require 459 c.c. of decinormal acid to neutralise it. This estimate in some cases appears to be slightly too high; e. g. a saturated lime-water from Carboniferous limestone at 56.5° F. (13° C.) required only 433 c.c. of decinormal acid, which equals 1·211 grm. of CaO per litre, and this lime-water, kept with excess of lime, gave nearly constant results for many months together. A magnesian limestone lime-water tested at the same time required 472 c.c. of decinormal acid, confirming the old observation of tanners, that such lime is stronger than that made either from chalk or carboniferous limestone. This increased strength must arise from the presence of some soluble base other than lime, and may be due to the magnesia, which, however, is very slightly soluble. Magnesian limestone contains a very large amount of magnesia, and hence would not go so far as a purer limestone; but as a very large proportion of the lime ordinarily used is thrown away undissolved, this is perhaps of little practical moment. (For the chemical examination of limes, see p. 102).
[R] 1 grm. per litre is very approximately equal to 1 oz. per cub. ft.
The action of lime on the hide has already been spoken of to some extent. It is throughout a solvent one. The hardened cells of the epidermis swell up and soften, the rete malpighi and the hair-sheaths are loosened and dissolved, so that, on scraping with a blunt knife, both come away more or less completely with the hair (constituting "scud," as some English tanners name it, Ger. gneist or grund). The hair « 141 » itself is very slightly altered, except at its soft and growing root-bulb, but the true skin is vigorously acted on. The fibres swell and absorb water, so that the hides become plump and swollen, and, at the same time, the "cement-substance" (coriin) is dissolved, the fibres become differentiated into finer fibrils, and the fibrils themselves become first swollen and transparent, and finally corroded, and even dissolved. This swelling of the fibres is produced both by alkalies and acids, and is probably due to weak combinations formed with the fibre-substance, which have greater affinities for water than the unaltered hide. It is useful to the tanner, since it renders the hide easier to "flesh" (i. e. to free from the adhering flesh), on account of the greater firmness which it gives to the true skin. It also assists the tanning, by opening up the fibre, and so exposing a greater surface. This is advantageous in dressing leather which is afterwards tanned in sweet liquors, and must have the cement-substance dissolved and removed for the sake of flexibility; and, in the case of sole-leather, it is necessary for the sake of weight and firmness that the hide be plumped; but it is probable that the effect is produced with less loss of substance and solidity by suitable acidity of the liquors. A more certain advantage of lime is that it acts on the fat of the hide, converting it more or less completely into an insoluble soap, and so hindering its injurious effects on the after tanning process, and on the finished leather. If strong acids are used later on, this lime soap is decomposed, and the grease is again set free. In sweated or very low-limed hides this grease is a formidable evil.
The customary method of liming is simply to lay the hides flat in milk of lime in large pits. Every day, or even twice a day, the hides are drawn out ("hauled"), and the pit is well plunged up, to distribute the undissolved lime through the liquor. The hides are then drawn in again ("set"), care being taken that they are fully spread out. How much lime is required is doubtful, but owing to its limited solubility, an excess, if well slaked, is rather wasteful than « 142 » injurious. Great differences exist in the quantity of the lime used, the time given, and the method of working. Lime, as we have seen (p. 140), is only soluble to the extent of about 1·25 grm. per litre, or (as 1 cub. ft. of water weighs about 1000 oz.) say 11/4 oz. per cub. ft., or, in an ordinary lime-pit, not more than 1/4 lb. per hide. Only the lime in solution acts on the hide, but it is necessary to provide a surplus of solid lime which dissolves as that in the liquor is consumed. Jackson Schultz prescribes 1 bush. (56 lb.) of fresh lime to 60-70 hides, and 3-4 days as sufficient time to unhair and plump them; while a well-known English tanner states that, after working for 6-10 days through a series of old limes, the hides (presumably wet-salted South Americans) should have 4 days in a fresh lime, made with 3-12 lb. of lime per hide. It is obvious that if the American authority is right, the English process is wasteful in the extreme, both in hide-substance and lime. Much depends on the amount of hauling which the hides receive, and the more frequently they are moved the better. It is probable, however, that it would be found impossible to unhair and flesh hides, to suit the English market, in cold limes with the quantity and time mentioned, and if the limes are steamed, it is quite likely that the destructive action on the pelt may be even greater than by the longer and slower process in the cold. Most likely a compromise between the two is the most desirable, but about 2-4 lb. of lime per hide, according to weight, should be amply sufficient; while a week for market hides, and 14 days for heavy salted, will loosen the hair and plump the pelt as much as is requisite. This is on the supposition that the limes are kept at a uniform average temperature of about 60° F. (15° C.) in winter and summer. If they are heated to 80°-90° F. (27°-32° C.), of course much less time is required; but there are no published experiments showing the relative weights made by the two processes, and, from the fact that warmed limes are principally used for descriptions of leather where weight and solidity are not of primary importance, it may be concluded that, in this direction, the « 143 » results are unsatisfactory. Hides do not plump in warm limes.
Another undecided point is whether the best results are obtained by making fresh limes for every pack, or by strengthening up the old ones. An old lime becomes charged with decomposing animal matter and with ammonia, and, within limits, loosens the hair more effectually than a new one. An experienced tanner states that, by using old limes, better weights are obtained, but that the leather is thinner than when a fresh portion of lime is used; and this is quite possible. If, however, the old lime-liquor be retained too long, it ceases to swell the hides as it should, and, in warm weather, the liming proper is complicated by a putrefactive process allied in principle to sweating.
Beside considerable quantities of ammonia, old limes contain tyrosin, leucin or amidocaproic acid, and some caproic acid, the disagreeable goaty odour of which is very obvious on acidifying an old lime-liquor with sulphuric acid, by which considerable quantities of a partially altered gelatin are at the same time precipitated. Very old limes, especially in hot weather, often contain active bacteria, which may be seen in the microscope under a good 1/4-in. objective. Their presence is always an indication that putrefaction is going forward, and leather out of such limes will generally prove loose and hollow-grained. Spherical concretions of calcium carbonate may also be seen under the microscope, resembling on a smaller scale those found in Permian limestone, and caused perhaps in both cases by crystallisation from a liquid containing much organic matter. It is probable that in many tanneries the ammonia would pay for recovery from the lime-liquors, which would be easily done by steaming the old limes in suitable vessels, and condensing the ammoniacal vapours in dilute sulphuric acid. (Some appliances suitable for this purpose are described in the Journal of the Soc. of Chem. Industry, iii. 630.) For methods of estimation of ammonia, see p. 103.
Several variations in the above-described method of liming « 144 » have been proposed. The hides may be suspended on laths, or by strings attached to pegs or notches, and the liquor agitated by plunging in place of hauling. Probably this is an actual improvement, especially if some mechanical agitating contrivance be substituted for hand plunging. It has, however, the drawback that much room is required, though this may be, to some extent, compensated by the hides liming more quickly. The method has been long in use in America, and had been tried in several places in England before the patent of Messrs. Conyers and Pullein was obtained. Two other American labour-saving methods in connection with liming may be mentioned here. One is to have the liming-vat double the ordinary size, and, instead of hauling the hides, to simply draw them from one side to the other by two strings, which are attached to the fore and hind shank of each hide, either by sharp iron hooks or by loops. The strings are looped over iron rods at the four corners of the pit, or have simple knots, which are placed in notches sawn in wood. Of course, while the hides are at one side of the pit, the other side may be plunged or warmed. The other method (Fig. 24) is to have a spindle sunk below the surface of the liquor, and with discs A, at each end, to which the hides or sides are attached by hooks set round the edges. The hides are turned over by revolving the spindle with a handspike inserted in the holes C, at the ends of the cross-arms B, and the whole spindle is also capable of being raised and lowered in the liquor, in the slot D. In Germany, hides are frequently suspended on laths radiating from a central upright revolving spindle in a round vat (Drehkalk).
An American plan, sometimes known as the "Buffalo method," is described by Jackson Schultz. The hide is prepared in the usual way, and is then thrown into a strong lime for 8-10 hours, when it is taken out and immersed in water heated up to 110° F. (43° C.), in which it remains 24-48 hours. The warm water soaks, softens, and swells the roots of the hair, and much the same result is obtained as in "scalding" pigs. So little lime really permeates the inner fibre that, after « 145 » a slight wheeling, the hides may be thrown into cold water, and allowed to cool and plump, preparatory to taking their places in the handlers. The process is strongly recommended for sole-leather, particularly where great firmness of fibre is desired. The tanner who tries it must be satisfied if he gets 20-30 sides a man unhaired and fully ready for the liquor per diem. Of course this process may be varied to any extent by giving more liming, and less hot water, and this is frequently done in America. About 3-4 days' cold liming in good limes, and with hauling if possible twice daily, followed by 12-24 hours in water at 86°-95° F. (30°-35° C.), which should be changed at least once, will give good results. The hides are of course less plump than usual, but if properly managed in the handlers will swell well in the tan-house. Grease is obviously less thoroughly "killed" than in the ordinary method, and especial care must be used that the hides are well worked on the beam, both on grain and flesh. In this method, and indeed in all liming processes, much is gained if the fat can be fleshed off green.
On the Continent and in America, the prevalent mode of loosening the hair, at least for sole-leather purposes, is called « 146 » "sweating," and consists in inducing an incipient putrefaction, which attacks the soft parts of the epidermis and root-sheaths, before materially injuring the hide-substance proper. The old European method of "warm-sweating" consisted simply in laying the hides in pile, and, if necessary, in supplying heat by covering them with fermenting tan; but as this crude and dangerous process is everywhere being supplanted by the American plan, where sweating at all is adhered to, it is not necessary to do more than describe the latter. This is called "cold sweating," but really consists in hanging the hides in a moist chamber, kept at a uniform temperature of 60°-70° F. (15°-21° C.); or in some cases slightly warmer.
The "sweating-pit" now in use is sometimes of wood, but usually consists of a building of brick or stone, protected from changes of temperature, both above, and at the sides, by thick banks of soil or spent tan. If soil be used, it will form an excellent bed for vines, &c., which are fertilised by the ammonia penetrating from below, which is evolved in large quantities and which assists the unhairing process by its action on the epidermis.[S] Though called a "pit," it is undesirable that it should be actually below the level of the ground, but should be arranged so that the hides can be wheeled in and out in barrows. It is lighted and ventilated by a lantern roof above a central passage, and should be divided into chambers, each capable of suspending a pack of hides. By means of sprinklers above and steam-pipes below, the chambers may be cooled or warmed, as required, and the air kept so moist that globules of condensed water collect on all parts of the hides, which are suspended from tenterhooks.
[S] Hides have been unhaired by the action of gaseous ammonia alone, but the method does not seem suited for technical use.
The process is principally used in America for dried hides, but may be employed either for wet or dry salted, after complete removal of the salt. It is imperatively necessary that dried hides should be completely softened before sweating. As the sweating process advances more rapidly in the upper « 147 » than in the lower part of the pit, and as the thick portions are more resistant than the thin ones, the hides, after about 3 days' sweating, require constant attention in changing their positions, and in checking the forward ones by taking down and laying in piles on the bottom of the pit.
The usual treatment for sweated hides, when the hair is sufficiently loosened, is to throw them into the stocks, and work out in this way the slime and most of the hair. This has the disadvantage of working out too much of the dissolved gelatin, and of fulling the hair so firmly into the flesh, that it is difficult again to remove it. To overcome these evils, some American tanners now pass the hides, after sweating, through a weak lime. This, to a great extent, prevents the hair fixing itself in the flesh, and tends to counteract the injurious effect of the vitriol (which is almost invariably used in plumping sweat stock) on the colour of the leather. By this process, 10,000 Texas and New Orleans wet-salted hides gave an average yield of leather of 73 per cent. on their green weight, and the leather was excellent in quality (Schultz). If sweated or very lightly limed hides are imperfectly worked on the grain, greasy spots are apt to remain, which will not colour in the liquors ("white spots"). These may be made to colour by scraping and working the grain with a knife, or by the application of a solution of soda or soda ash, and would probably be avoided by the use of soda ash in the soaks on greasy parcels of hides.
It must be clearly understood that all sweating depends on partial putrefaction. This is proved both by the plentiful production of ammonia in the pits, and by the fact that antiseptics, such as salt or carbolic acid, entirely prevent sweating till they are removed. Although the process undoubtedly has advantages, and especially so in the treatment of dried hides, it is an open question whether it gives the extreme gains over liming in weight and firmness, which are claimed by some of its advocates.
An unhairing process, largely coming into use on the Continent, depends on the action of alkaline sulphides, and « 148 » particularly sodium sulphide, upon the hair. While all the methods already spoken of involve the softening and destruction of the hair-sheaths, either by lime or by putrefaction, the sulphides are peculiar in attacking the hair itself; when strong, they disintegrate it rapidly and completely into a sort of paste. From very early times to the present day, arsenic sulphide ("rusma") mixed with lime has been used in unhairing skins for glove-leather and similar purposes. About 1840, Böttger concluded that the efficacy of arsenic sulphide was due simply to the sulphydrate of lime formed by combination of the sulphur with the lime, and proposed lime sulphydrate, formed by passing sulphuretted hydrogen into milk of lime, as a substitute for the poisonous and expensive arsenic compound. It proved a most effective depilatory, but has never obtained much hold in practice. This is probably due to the fact that it will not keep, oxidising rapidly on exposure to the air; hence it must be prepared as it is required, which is both troublesome and expensive. A minor objection is the unpleasant smell of sulphuretted hydrogen, which is inseparable from its use.
It was proposed to replace it by sodium sulphide, which, though at first said to be only effective when mixed with lime, so as to produce calcic sulphide, has since proved a powerful depilatory alone. Its use has been greatly extended on the one hand by its production on a large scale, and in the crystallised form (at first by reduction of sulphate by heating with small coal), and on the other, by the great interest which Wilhelm Eitner, the able director of the Austrian Imperial Research Station for the Leather Trades, has taken in its introduction. The substance, as manufactured by De Haen, of List, Hanover, is in small crystals, coloured deep greenish-black, by iron sulphide, which must have been held in suspension at the time of crystallisation. If the salt be dissolved in water, and the solution be allowed to stand, this is gradually deposited as a black sediment, leaving the supernatant liquor perfectly clear and colourless. Sodium sulphide is now manufactured « 149 » from tank waste in a much purer form by Schaffner and Helbig's process, of which Messrs. Gamble of St. Helens are sole licencees. The crystallised salt is SNa210Aq, and therefore contains 69·8 per cent. of water.
For sole-leather, the method recommended by Eitner is to dissolve 4-5 lb. of sulphide per gal. of water, making the solution into a thin paste (of soupy consistence) with lime or pipe-clay. This is spread liberally on the hair side of the hides, one man pouring it down the middle of the hide from a pail, while another, with a mop or cane broom, rubs it into every part. The hide is then folded into a cushion, and in 15-20 hours will be ready for unhairing, the hair being reduced to a paste. In the writer's experience, the concentrated solution here prescribed will completely destroy all hair wetted with it in 2-3 hours, and if left on longer, will produce bluish patches, and render the grain very tender. The hides should be thrown into water before unhairing, to enable them to plump, and to wash off the sulphide, which is very caustic, attacking the skin and nails of the workmen. There is no doubt that this process gives good weight, and tough and solid leather; but there are several difficulties attending its use. Unless the mopping is done with great care, it will fail to completely destroy the hair, and the patches of short hair left are very difficult to remove. The expense of the material and the loss of hair are also important considerations. The hides are rather difficult to flesh, unless previously plumped by a light liming, and it is necessary to swell them with acid or sour liquor in the tanhouse, as the sulphide has but little plumping effect.
Another method, which is much cheaper in labour and easier in execution, is to suspend in a solution of sodium sulphide, containing 3/4 lb. a hide or upwards; the hide should unhair in 24 hours. Very weak solutions loosen the hair, without destroying it; but it is always weakened, as the specific action of the sulphides is on the hair itself. After or before unhairing, the hides may receive a light liming, to plump them, or lime may be added to the solution of sulphide, which « 150 » by forming calcium sulphide, and liberating caustic soda, considerably increases the unhairing and plumping effect. The pit may be several times strengthened for successive packs, but the loosened hair must be fished out, or it will quickly spoil the solution. When hides have been suspended in sodium sulphide solution, the hair is very quickly loosened by a short liming. Squire, Claus, and J. Palmer have all taken out patents for the use of tank-waste as a depilatory. It consists of impure calcium sulphides, and when brought into the form of soluble sulphydrate, either by boiling in water, or by the oxidising action of the air, it will unhair hides. The conversion is, however, very imperfect in either case, and its action is uncertain and slow; while the iron present is apt to cause unsightly stains. It is probable that the weights obtained may somewhat exceed those by liming. Palmer employs sulphuric acid to plump the hide and remove stains, and then reduces it by a bate of whiting and water. He claims that this prepares the hide for rapid and heavy tanning, but the swelling and subsequent reduction almost certainly entail loss of weight and quality, and to get good results the bate should at most only be allowed to have a superficial effect. Professor Lufkin proposed the use of a mixture of various sulphides of lime and soda, formed by mixing 10 lb. each of soda ash and sulphur, kneading to a paste with a little moist slaked and then mixing warm in a cask with 80 lb. stone lime slaked to a paste. This quantity will unhair 50 hides in the same way and in about the same time as an ordinary lime. The pelt is not much plumped and is easily reduced by a few minutes' wheeling in warm water. (J. S. Schultz.)
Various other depilatories have been proposed, but as they have not come into general use, brief mention of the most important will suffice. Anderson, in 1871, patented the use of wood-charcoal, applied in a similar manner to lime in the ordinary process. The hair is probably loosened simply by putrefaction, as in sweating, while the charcoal acts as a deodoriser, very little smell being produced, and the action « 151 » proceeding with considerable uniformity. John Palmer has patented a process for unhairing, in which the hides are alternately steeped in water and exposed to the air till the hair loosens. In this, very similar principles to those of the charcoal method are involved. Caustic potash and soda will loosen hair, but seem to have no decided advantage over lime, though it is quite possible that in skilful hands good results might be obtained. They are more costly, and their corroding action on the hide-substance is more powerful, but they form soluble soaps with the grease of the hide. Unless used in very dilute solution, the pelt is so swollen as to fix the hair, and the leather is dark-coloured and spongy. Soda-ash or crystals (sodic carbonate) may be used to strengthen ordinary limes, in which caustic soda is formed. The time of liming is shortened, the hides are more swollen, and the grease is better "killed" than when lime alone is used. The patent for Moret's "Inoffensive" claimed the use of the carbonate or caustic potash formed from calcined wool-washings, for unhairing. This is more costly than, and has no advantage over soda. I am not aware whether "Inoffensive," as now sold, has other constituents.
Whatever method of loosening the hair may be adopted, the next step is to remove it by mechanical means. This is usually accomplished by throwing the hide over a sloping beam, and scraping it with a blunt two-handled knife (Fig. 25), the workman pushing the hair downwards and away from him. The beam is now usually made of metal. The knife employed is also shown at C, Fig. 26.
When a hide is lightly limed, it is often easy to remove the long hair, but excessively difficult to get rid of the short under-coat of young hairs, which are found in spring, and which can sometimes only be removed by the dangerous expedient of shaving with a sharp knife. The reason of this difficulty is obvious: not only do the short hairs offer very little hold to the unhairing knife, but, as has been explained in describing the anatomical structure of the skin, their roots are actually deeper seated than those of the old hairs they « 152 » replace. Several attempts have been made to unhair by machinery, but so far without such success as to lead to their general adoption. The fleshing-machine invented by Garric and Terson, and manufactured in this country by T. Haley and Co., of Bramley (Fig. 27), is furnished with a special wheel for unhairing. An American machine for the purpose, invented by J. W. Macdonald, and said to be capable of unhairing 800 sides a day, is shown in Fig. 28.
When the hair is very thoroughly loosened, as by sweating, or destroyed, as by sodium sulphide, it is not uncommon to work it off by friction in the stocks; but it is very doubtful whether the saving of labour is not more than compensated by the loss of weight, consequent upon submitting the hide while its gelatin is in a partially dissolved condition, to such rough usage.
After unhairing, the loose flesh and fat are removed from the inner side of the hide by a sharp-edged knife E (Fig. 26), partly by brushing or scraping, partly by paring. It is necessary not only to cut off the visible adhering fat, but to work the hide well, so as to force out that contained in the loose areolar tissue, which would not only impede tanning, but is liable to soak completely through the hide, producing most unsightly blotches. Several machines have been introduced to supersede hand-fleshing, but with only partial success. One of the best is Garric and Terson's machine (Fig. 27), which gives a very level flesh, free from galls, and without so much loss of weight, but scarcely so clean as desirable, while the saving in labour is not great. Molinier's machine (Fig. 29), and that of Jones and Rocke, are well adapted for skins, but hardly capable of fleshing an entire « 155 » hide. All these machines are very similar in principle, the working parts consisting of drums with oblique or spiral knives.
When unhaired and fleshed, the hides intended for sole-leather are, in England, almost invariably "rounded," or separated into (1) "butts," which are the best and thickest parts, and receive the most solid tannage, and (2) "offal," which is thinner, and for which a cheaper and more rapid tannage is sufficient. Fig. 30 shows the customary division. Frequently the butt is divided down the centre, and the halves are then called "bends." A piece called a "middle" is sometimes taken between the butt and the shoulder.
After rounding, it is necessary to get rid of the lime, as completely as possible, before taking into the tan-house. For this purpose, the butts are usually suspended in fresh water for 12-24 hours, and frequently shaken up in it to remove adhering lime and dirt. If the water is hard, it is best to add to it, before putting in the butts, a few pailfuls « 156 » of clear lime-water, to precipitate the lime bicarbonate,[T] which would otherwise cause a deposit of chalk on the surface of the butts; this would not only make the grain harsh, but afterwards, by combining with the tannin of the liquors, would cause bad colour. For the same reasons, it is important that limey hides should be as little exposed to the air as possible, as the latter always contains a small amount of carbonic acid, which renders the lime insoluble.
[T] Lime softens water containing lime bicarbonate in solution by combining with half the carbonic acid, when the whole is precipitated as normal carbonate or chalk. CaO + CaCO3 . H2CO3 = 2CaCO3 + OH2. This is Clark's process. See also p. 84.
This suspension in water is frequently considered sufficient for sole-leather, but it removes the lime very imperfectly. In olden days, it was customary not only to wash the hides much more thoroughly in water, but to "scud" them (i.e. work them over with a blunt knife), to remove lime, and the detritus of hair-roots and fat-glands, and this should never be omitted from sole-leather treatment where bright colour and clean buff are desired. Some tanners go so far as to bate best butts slightly with hen-dung, but with such treatment firmness and weight are lost. Washing in weak solution of sugar, or ammonic chloride or sulphate, or of sulphuric, or hydrochloric acid, may be adopted. It is essential to use acids nearly free from iron, as it may be precipitated on the butts and give a bluish colour in the liquors, and the acid must be of such a strength as neither to allow the iron to be precipitated, nor, on the other hand, perceptibly to plump the butts, which in this stage would endanger buff and colour. 100 cc. may neutralise 15-20 cc. of lime-water for this purpose. Hydrochloric acid and chlorides have a tendency to prevent plumping, and are therefore better adapted for dressing than for sole leather. Great care must also be taken to prevent putrefaction, or the use of putrid solutions, if firmness and plumpness are desired.
SOLE-LEATHER.—Tanning Materials.
Before describing the management of the hides in the tan-house, it is necessary to say a few words about one or two of the principal materials used, and the methods of preparing them for use. Further details of their nature and origin have been given in the section on Tannins, p. 23.
Oak-bark is one of the oldest of tanning materials, and the leather produced by its aid is still considered for many purposes the best. For sole-leather, its weakness in tannin (8-12 per cent.), the slowness of its action, and the light weight of the leather produced, render it unavailable alone except for the very finest class of work. It is, however, generally used in admixture with stronger and cheaper materials, such as valonia.
Valonia, the acorn-cup of an evergreen oak growing in Greece and the Levant, is perhaps the most important of materials to the English sole-leather tanner. It contains 25-35 per cent. of a tannin somewhat similar to oak-bark, and, like it, communicating a light-coloured bloom to the leather, but giving much greater firmness and weight, and a browner colour.
Myrabolanes or myrobalans, the fruit of an Indian shrub, contains about as large a percentage of tannin as valonia, and gives a similar bloom, and excellent colour; but it can only be used very sparingly on butts, since it produces a soft and porous leather.
Divi-divi is a South American bean, which contains much of a brown tannin in the pod, being considerably stronger than valonia. It makes a heavy and solid, but somewhat horny leather. Its great danger arises from a tendency to sudden « 158 » fermentation in thundery weather, which, produces brown or red stains on the leather. At all times it is liable to give a bluish or violet colour, which is most obvious in the interior of the leather, and which resists both acids and alkalies.
Mimosa-bark is the product of several Australian acacias, and is probably nearly as strong as valonia. It gives a hard and heavy leather, but of a dark-red colour.
Hemlock-extract is a deep-red syrupy extract of the bark of the hemlock pine of America.
Chestnut-extract is a similar product from the rasped wood of the Spanish chestnut. Its colour is paler and yellower than that of the hemlock, and hence it is often employed to correct the red tone produced by the latter.
Oakwood extract is an analogous preparation from oak saw-dust.
Grinding and Exhaustion of Tanning Materials.
Before tanning materials can be exhausted, it is almost invariably necessary to crush or grind them, so as to enable the water to get freely at the tannin, which, in most cases, is enclosed in the cellular tissue of the plant. It may be thought that for this purpose it would scarcely be possible to crush too finely, but in practice, a very fine powder is extremely difficult to spend, as it cakes into compact and clay-like masses, through which liquor will not percolate. The object, therefore, is to grind finely enough to allow the liquor ready access to the interior, but not so finely as to prevent liquids running through the mass. The mill most usually employed for this purpose consists of a toothed cone, working inside another cone, also toothed on its interior, precisely like those of a coffee-mill. As bark is frequently delivered "unhatched," or in long pieces, it is necessary to crush it preparatory to grinding, and this is usually accomplished by rollers composed of toothed discs, called breakers. In Fig. 31 is illustrated such a mill, as made by Newall and Barker, of Warrington, combining both utensils. Fig. 32 « 159 » shows a section of the well-known American "keystone" mill, in which the preliminary breaking is accomplished by the arms A; the bark is then finely ground by the toothed cones N, and discharged at the spout R by the revolving shover M. Fig. 33 shows a somewhat similar mill, made by Gläser of Vienna, in which the axis is horizontal, and driven directly by a belt. It is better to drive bark-mills by a belt than by toothed gearing, as in event of iron getting into them there is less danger of breakage. In America, a cheap cast-iron coupling is frequently used, weak enough to give way before serious damage is done. Safety "friction" clutches are generally ineffective. American bark-mills are run faster than English, up to about 80 rev. per minute, and where the bark is to be used immediately it is frequently damped by a small « 160 » jet of steam below the mill, which lays dust, and prevents danger of fire. Bark which is damp before grinding can scarcely be ground in these toothed mills, but must be dried, or a disintegrator used.
Now that a large variety of other materials besides bark are required by tanners, the mills just described are not always sufficient for the purpose. Myrobalans and mimosa-bark have proved specially troublesome, the former from its very hard stones and clogging character, and the latter from its combined hardness and toughness. "Disintegrators" of various makes have proved admirably adapted for grinding both of these materials, their advantage being the universality of their reducing powers, ranging from oak-bark to bones or brick-dust, and their disadvantages, the somewhat considerable power they consume, and the rather large portion of fine dust they make. Their principle is that of knocking the « 161 » material to powder by rapidly revolving beaters, which, in the smaller mills, are driven at so high a speed as 2500-3000 rev. a minute. Wilson's is shown in Fig. 34, as an example. It is one of the oldest tanners' disintegrators, and probably still one of the best. In the figure, it is opened, showing the disc with its steel beaters attached. When myrobalans are only required roughly crushed, a machine with fluted or toothed rollers (Fig. 35) acts better than a disintegrator, making less dust, and requiring less power. Such a machine also crushes valonia very satisfactorily.
In England, the tanning material is generally carried
from the mill, to the pits where it is exhausted, in baskets or
barrows; in America, this is frequently accomplished by a
« 162 »
"conductor," or horizontal spout, in which a double belt, or
malleable iron "drive chain,"[U] with wooden cross-pieces,
carries the bark forward, on the same principle as the
elevators of corn-mills. Fig. 36 shows the conveyors used
in a Chicago tannery. Another American plan is to use
circular tubs for extraction. These are mounted on wheels,
and are worked on a railway, coming up to the mill to be
filled, and thence under a series of sprinklers like those used
by brewers, and finally "dumping" their contents before the
boilers, which are heated solely by wet bark, burnt in a
peculiar furnace with brick chambers. This furnace for
burning wet bark seems worthy of extended adoption in
Europe, as spent tan is frequently not only valueless, but
costly to get rid of. Full details and scale drawings may be
found in Jackson S. Schultz's book on 'Leather Manufacture'
and in Fig. 37 is shown a modification of it, patented by
Huxham and Brown, which has been very successfully used
in burning wet tan, either alone or with a portion of coal.
In American sole-leather tanneries, where the bark is resinous
and almost unlimited in quantity, sufficient steam may be
« 163 »
« 164 »
raised with tan wet from the leaches; but in England, where
material is more sparingly used, it is advisable partially to
dry it before burning. This is accomplished by powerful
roller-presses, as shown in Fig. 38. Gläser, of Vienna, constructs
tan-burning furnaces
on a different principle from
the American, the essential
point being the use of a
"ladder-grate" (Treppenrost),
on which the burning tan is
exposed to a draught of air
playing over its surface.
Fig. 39 shows a portable stove
of this construction. Gläser
also makes furnaces of larger
size for heating air for drying-rooms,
and for boiler
purposes. The essentials of
successful tan-burning are
good draught, a large grate-surface,
and a high temperature
of the combustion-chamber,
and hence the
ordinary Cornish or Lancashire
boiler, with its limited
grate-area, surrounded by the
comparatively cool boiler-tube,
is peculiarly ill-adapted
for the purpose. The writer
has profitably burnt a mixture
of wet tan and very
small coal in such a boiler by
the aid of a steam-jet under-grate
blower, but such a
method can only be regarded as a makeshift in default of
better appliances.
[U] Such chains with attachments for elevators and conveyers, are manufactured in this country by Ley's Malleable Casting Company, in Derby.
In England, the tanning material is usually exhausted in pits called "leaches," "latches," or "taps." These, in large « 165 » yards, are made capable of holding about 50 cwt. of material. The new material is first flooded with a pretty strong liquor. When this has gained as much strength as possible, it is pumped off, and is followed by a weaker one, and so on « 166 » till the material is exhausted. Much of the economy of a tan-yard depends on the way, systematic or otherwise, in which this is done. It is customary to complete the exhaustion with hot liquors, or water, but opinions differ on the expediency of the practice. By the use of heat, however, stronger liquors and more rapid spending are attained; and with some materials, such as mimosa, complete exhaustion is impossible in the cold.
The worst tap is frequently boiled by inserting a steam-pipe; but if heat is used at all, it would probably be better to heat a strong liquor by a steam-coil, and run it on the new material, which would be softened and swollen, and yield a much larger proportion of its strength to the first liquor; while it is stated by Eitner that the colouring matters of tanning materials are much less soluble in strong than in weak infusions. Boiling weak old liquors containing lime is specially prejudicial, causing great darkening and discoloration.
Careful tanners also cast their material over from one pit into another, before throwing away, so as to lighten it up, and allow the liquor to penetrate to every part. In bark-yards, latches are frequently worked in series, which are connected by pipes, so that the liquor flows from the bottom of one upon the top of the next stronger. This is an excellent plan for bark, which is open and porous, but is scarcely adapted to such materials as valonia or myrabolans, which have a tendency to form compact masses, through which the liquor does not circulate. The same objection, in an almost higher degree, must be urged against the Allen and Warren, or sprinkler leach, in which the liquor, distributed on the surface by a rotary sprinkler, is allowed to percolate downwards, and run freely away at the bottom. In this case, it is almost sure to form channels, instead of flowing uniformly, and, in addition, the material is constantly exposed to the action of the air, which causes oxidation, with its attendant discoloration and loss of tannin. Various attempts have been made to exhaust tanning materials in closed vessels. « 167 » Dr. Kohlrausch applied the diffuseur used in extracting beet-root sugar, and which consists of a series of closed copper vessels in which the coarsely ground material is placed, of which the bottom of one is connected with the top of the next « 168 » by a pipe, through which the liquor is forced by steam pressure. This apparatus is in use at the large tannery of Gerhardus, Flesch, and Co., of Vienna, and is said to give satisfaction, though it is very costly, and the liquors produced are not of great strength. Gläser, of Vienna, has patented an apparatus of which a model is illustrated in Fig. 40, in which the materials are used finely powdered, and very rapidly exhausted by the combined action of heat and mechanical agitation. Of its mechanism I have not been able to obtain any detailed description, but it is said to be capable of exhausting 9 tons of valonia per diem, to 2 per cent., giving only 70° liquors, clear and of good colour, while good bark is exhausted to 0·5 per cent. giving 30° liquors. The cost of the apparatus is very heavy, but if the results claimed are realised in practice it would pay well for an extensive tannery. I have not been able to ascertain where it is to be seen in use.
It is one of the great attractions of extracts that they avoid almost all the expense and labour inseparable from the exhaustion of other tanning materials. It is usually necessary to dissolve the fluid extracts in water or liquor of as high a temperature as has been employed in their preparation, as otherwise, from some unexplained chemical change, a large portion of the tannin is precipitated, probably as an anhydride of the tannin. Gambier is usually dissolved by boiling or steaming, but is said to give a better colour when dissolved cold. This may be accomplished in a rotating latticed drum, sunk in a pit of liquor.
Where circumstances permit, it is a great advantage to place the taps either on a higher or a lower level than the layers and handlers, so that liquors may be run one way without pumping.
SOLE-LEATHER.—Treatment in the Tan-house.
On first coming into the yard, the butts are usually suspended by the shoulder or butt ends from sticks placed across the pits. They should be kept in almost constant movement, either by raising and shaking them by hand, or by supporting them on frames, which are rocked, or otherwise worked. Perhaps the best device for this purpose is the "travelling handler" of W. N. Evans, which consists of a frame supported on wheels, and worked slowly backwards and forwards by power. This frame should extend the length of a range of pits sufficient to take in at least a 3 days' stock of butts, which should be tied to sticks resting crossways upon it. It should have a stroke of 1-2 ft., repeated, say 6 times a minute. The power required is very small.
The American rocker consists of a wooden frame balanced on its centre, and made to oscillate by power. It is a cheap and efficient machine, its defects being that the butts at the ends are much more moved than those in the centre, and that their upper parts, being lifted out of the liquor, are liable to become blackened.
The suspender pits should be supplied with old handler liquors, which, if the tannage is a mixed one, may range from 12° to 20° barkometer, as a large proportion of the weight consists only of lime-salts, gallic acid, and other worthless products. It must here be explained that the barkometer (also called "barkrometer" or "barktrometer") is a hydrometer, graduated to show the sp. gr. thus—20° Bark. = 1·020 sp. gr. In using it the temperature of the liquor must be at or near 60° F. (15° C.). It is, of course, affected by any other matters in solution, precisely the same as by « 170 » tannins. In the Lowlights Tannery the waste liquors are constantly about 12° Bark., and contain tannin equal to less than 0·2 per cent. (expressed as crystal oxalic acid), and gallic acid and similar matters equal to O·6 to 0·7 per cent. If the tannage is pure bark, it may perhaps be advisable to let the strength be somewhat less, but something depends on whether the exhausted liquors are returned with all their impurities to the "taps" or liquor-brewing pits, or whether the liquors are made with water, and hence purer. In any case, the free acid in the suspenders should always be sufficient in quantity to neutralise the lime brought in by the butts, or bad colour will certainly result, making itself visible in the shed, or as the tanning proceeds. If the butts, when first brought into liquor, take a lemon-yellow colour, especially in places that have been imperfectly exposed to it, this is an indication of danger which must not be disregarded. It may be met either by cleansing the butts more thoroughly before bringing into the yard, or by adding acid (acetic, hydrochloric, or sulphuric) to the liquor. If this be done, great care must be taken not to over-do it, and an acid free from iron must be used. The use of sulphurous acid for the purpose has been patented, and presents some advantages. Sulphites have been observed by the writer to give a pink or purple reaction even with very dilute infusions of valonia (see p. 112); but any coloration from this cause would probably disappear as the tannage proceeds. The difficulty can, however, often be remedied, either by altering the way of working the liquors, so as to bring more sour liquor down to the suspenders, or by using a larger proportion of materials capable of yielding acetic acid by fermentation, such as myrobalans. It is a common error to call all the free acid of sour liquors "gallic," as this is scarcely present in pure bark-yards, and at the best is a very feeble acid. The most abundant acid is usually acetic, though butyric, lactic, and other acids are frequently present in varying proportions, according to the tanning materials employed. In the English process, with its comparatively short layers, « 171 » in which the butts almost float in strong liquors, but little souring takes place, and we have nothing comparable to the German "sour bark" and "sour liquor" from long layers with weak liquor, and much dusty material. These contain large quantities of acetic and lactic acids, and plump almost like vitriol. Though the American tanners generally use the latter, their hemlock liquors sour much more intensely than those of English yards. It must always be borne in mind, in comparing English with American and Continental tanning, that, in the first, the opening up of the fibre is effected by lime, and the swelling is maintained in the liquors, not so much by acids, which are only present in very small proportion, as by the careful and gradual working forward into infusions stronger and stronger in tannin; while in the two latter, lime, if used at all, is simply employed to loosen the hair, and the swelling and differentiation of the fibre is first accomplished in the liquors either by vegetable or mineral acids. Hence good results cannot be expected in English yards from such processes as sweating or painting with sodium sulphide, which does not plump, without a radical modification of the whole tanning process. This point has been ably treated by Eitner in a series of papers on Extract-gerberei, published during the last few years in 'Der Gerber,' which will well repay attentive perusal by English as well as German tanners.
The butts should at first be brought into the weakest liquor; a circulation system, by which the liquors are all pumped in at one end of a set of suspenders, and run out at the other, the butts being moved forward in the opposite direction, seems to have much to recommend it. In this case, the top of one pit should be connected by a wooden box with the bottom of the next.
It is usually advisable to run away the first liquor into which butts are brought from the lime-yard, as it is very completely spent, and highly charged with lime salts and impurities. Whether other exhausted liquors are to be retained or rejected is largely a question of climate, and mode « 172 » of working. In hot weather, such liquors, charged with organised ferments (moulds, bacilli, and bacteria), are apt to cause ropiness, and other fermentive diseases of the liquors. This danger may be lessened by boiling all spent liquors, so as to kill the ferments, before running on the taps, or prevented by the free use of antiseptics, such as carbolic acid. Small doses of carbolic acid, however, are useless; at least 1/10 per cent. must be employed; and it must be borne in mind that antiseptics prevent souring as well as other fermentations, and hence, where they are employed, other means must be adopted to maintain the necessary acidity. Such liquors are very liable to darken if boiled.
The suspender liquors should be acid enough freely to redden litmus-paper. The present author has published a simple volumetric method for the determination of the free acid; 10 cc. of the carefully filtered liquor is placed in a beaker, and clear lime-water is run in from a burette till permanent cloudiness is produced. The quantity of lime-water employed is that which the acid is capable of neutralising, without producing discoloration of the leather, and care must be taken that the lime introduced with the butts does not exceed this proportion. The explanation of the reaction is that dark-coloured tannates of lime are formed, which are dissolved by the free acid so long as it remains in excess. It must be remembered that this process estimates all acids capable of retaining tannates of lime in solution, including some so feeble as to have practically no plumping effect. A liquor may have acidity equal to several cc. of lime-water, and yet react absolutely alkaline to methyl-orange (see p. 9), a colour which is distinctly reddened by small excess of acids, even so weak as gallic, which is barely acid to the taste. Hence, the acidity of a liquor available for plumping may be taken as represented by the lime-water required to change the red of methyl-orange to yellow, and if the liquor does not redden methyl-orange it is incapable of plumping. If 5 or 10 drops of orange solution be added to the pale filtered liquor from suspenders, there is no difficulty in approximately hitting the point of change, but great « 173 » accuracy is not to be expected. If the liquor will not filter clear, kaolin (see p. 119) may be used to clear it. It is well to test the lime-water occasionally on 10 cc. of decinormal sulphuric or oxalic acid (p. 96), to make certain of its constancy. Lime-water should be kept in a bottle with excess of lime, shaken occasionally, and a small quantity filtered off as required. Liquors are frequently miscalled "sour" which are not acid, but putrid. Such liquors will not plump, but reduce and soften hides placed in them. (Compare also p. 185). Suspender liquors usually consist mainly of liquors from the handler shift. If liquors be used direct from the leaches, they generally produce harsh grain and bad colour.
From the suspenders, the butts are transferred to the "handlers," where they are laid flat in the liquor. They are usually pulled over by hooks, which are very apt to scratch the grain. Sometimes strings are used, attached to the corners and held in notches or on pegs at the edge of the pit. Other tanners place a frame below the pack, with ropes at the four corners, by which it is raised sufficiently for the men to grasp the top butts with their hands. This is only practicable in pits of ample size. In American yards, the handling is almost universally performed by tying the sides with strings or fastening them in a long band by drawing the slit tail of one side through a hole in the nose of the next, and inserting a wooden "key." The string of the sides is then wound from one pit to another over a skeleton reel (Fig. 41). This method is also used in the lime-yard, and is frequently employed in England to handle offal, but it is not well adapted for butts. Fig. 42 shows the application of mechanical power in a Chicago yard for the same purpose, by means of Ewart's drive-chain, which is manufactured in this country by Ley's Malleable Castings Co., at Derby, to whom I am indebted for the block.
The handlers are generally worked in sets, to each of which
a fresh liquor is daily run, and the most forward pack is pulled
over into it, and is often also dusted down with a little fine
bark or myrabolans. The second pack follows into the
liquor out of which the first has been taken; the third into
« 174 »
that of the second, and so on. Frequently the greenest
packs are handled up a second time in the course of the day,
and put down again in the same liquor. The strength of
liquors, and the length of time for which butts are retained
in the handlers, are varied; but a time of 1-2 months, and
« 175 »
« 176 »
liquors of 20°-35° Bark. are usual. It is well to divide the
handlers into at least two sets. Gambier is very useful,
especially to the greener goods, and if hemlock and other
extracts are employed, their appropriate place is in the
forward handlers or earlier layers. New valonia liquors
must be avoided, but old layer liquors of considerable
strength (up to 40° Bark. where the handling is long
continued) may be employed.
At the end of this period, the butts are taken to the "layers" or "bloomers," in which they are laid down with stronger liquors and much larger quantities of "dust"; the latter is usually bark or valonia, though mimosa is occasionally used. The liquors vary from 40° to 60° or 70° Bark. in strength in mixed tannage, and the duration of each layer from 10 days in the earlier stages to a month in the later ones. For the best heavy tannages, 6-8 layers are required. Each time the butts are raised, they should be mopped on the grain, to remove dirt and loose bloom. Strong valonia liquors, or heavy valonia dusting, causes a brown sandy crust to form on the freely exposed parts of the butts. This is removed in striking, but is sometimes very troublesome on rough dried dressing leather. In pure bark tannage, which, however, is gradually becoming extinct, the liquors used are of necessity much weaker, as it is extremely difficult to obtain liquors of more than 25°-30° Bark. from this material. The last layer, however, should always have liquors of the greatest strength which can possibly be obtained, or the leather will be deficient in firmness.
After receiving their last layer, the butts are well mopped or brushed and washed up in a clear liquor, and thrown over a horse to drain before going into the shed. In America, the Howard scrubber (Fig. 43) is generally employed instead of hand labour at this stage. It consists of 2 rotating wooden frames at the top of a pit, provided with brushes or birch-brooms, and, when in use, enclosed by a cover A, through a slit G in which the sides are inserted and drawn back, while water is supplied by the pump B. Sometimes the brush-drums are placed one above another, and the leather is passed in at the side.
Pl. VI.

OPENING, STRETCHING AND STORING WHITE SKINS.
In mixed tannages, where the colour is dark, the leather is frequently handled or suspended in a warm sumach or myrobalanes liquor, and occasionally in dilute sulphuric or oxalic acids. If these acids are not effectually removed before drying, the toughness of the leather will be destroyed, and in extreme cases the leather will become brittle and refuse to take black. In any case, strong acids are prejudicial to the durability of the leather. In America, alternate baths of vitriol and sugar of lead are frequently used for bleaching and weighting the leather, but the colour given is not durable.
The great point to aim at, in arranging the mode of work of a tannery, is to contrive that butts should always receive the strongest liquors they can bear with safety, and that the strength should constantly increase in a regular and systematic way. To attain this end, very frequent handling and change of liquor are requisite in the early stages, when the butts « 178 » rapidly absorb the tannin presented to them. As the process advances, the exterior part of the butt becomes thoroughly tanned, and the liquor only slowly reaches the interior, which is yet susceptible of its action, and hence longer layers in stronger liquors are permissible.
The varied requirements of the trade render it difficult to give any practical information as to the selection of tanning materials. As a general rule, it is important at the outset to give the required colour; and if materials undesirable in this respect are to be used for the sake of cheapness, they should be introduced in the form of liquors in the middle stages of the process, i. e. in the later handlers or earlier layers. Materials used as dust generally have more effect in producing bloom and colouring the leather, than those used in liquors at this stage. Some information as to the respective qualities of the different tanning materials will be found in the chapter on Tannins; but even practical men are very deficient in accurate information on these points, since many materials are never used alone, but invariably in connection with others which mask their effects.
The use of extracts, and the demand for low-priced leathers, to compete with the American tannages, has introduced still more rapid methods than those described, and very fair-looking heavy leather has been tanned in 5-10 weeks. These tannages are very various, but their main feature is the free use of hot liquors, composed principally of extracts and gambier. This treatment imparts great firmness, or more properly speaking, hardness; but the leather is deficient in toughness, and the grain usually cracks on bending sharply. Extract properly used is, however, capable of making excellent leather; it is employed in at least one of the highest priced tannages in the country.
It may be noted here, that when Continental writers speak of extracts and extract tannage, what we should call liquor tannage only is meant, and not specially the use of the concentrated extracts, to which alone in England the term is applied.
SOLE-LEATHER.—Treatment in the Shed.
The butts, after being treated as above described, are frequently oiled lightly on the grain, and are taken into the drying-lofts, where they are hung on poles till about half dry. They are then laid on the floor in piles, and covered up till they heat or "sweat" a little, which facilitates the succeeding operation of "striking." This is performed by laying the butt over a horizontal "beam" or "horse," and scraping its surface with a triangular pin, shown at D in Fig. 25. This pin has an even, though tolerably sharp, edge, and is so used that it stretches and smooths out the grain, without breaking it; and at the same time « 180 » it removes a portion of the white deposit called "bloom," which has been mentioned. Common goods are frequently struck by the machine introduced by Priestman, of Preston Brook, shown in Fig. 44; but the work is not very uniform, and the leather is much compressed and stretched. For offal, the machine is a very useful one, and perfectly satisfactory.
Butts are now generally struck by the very ingenious machine of Wilson, whose name has also been mentioned in connection with the disintegrator, and which is shown in Fig. 45. The arms carry blunt brass or steel knives or sleekers, and work outwards from the centre, while the butt is carried backwards and forwards over the drum. Stones may be substituted for the sleekers, when it is required to remove the bloom. The machine requires a firm foundation, as its reciprocating motion causes considerable vibration.
After a light oiling and a little further drying, the butt is laid on a flat "bed" of wood or zinc, and is rolled with a brass roller loaded with heavy weights. Various machines are also in use for this purpose. In Fig. 46, is shown Wilson's spring butt-roller, in which the pressure is produced by « 182 » springs immediately above the roller, which works backward and forward over a flat table, beneath a fixed girder. In the later patterns of this machine the roller is automatically reversed by a mechanical finger before coming to the edge of the butt. Fig. 47 shows an adaptation of the American pendulum roller, which is specially suited for refinishing Singapore kip sides and the commoner class of goods, giving great firmness and a high gloss. Fig. 48 represents a machine in which the roller is fixed, and works over a brass drum; it is specially adapted for offal, and, when used for butts, is apt to make them "baggy." In this machine, the reversing motion is obtained by using two belts, one being crossed.
The leather is now frequently coloured on the grain with a mixture, for which each tanner has a recipe of his own, but usually consisting mainly of yellow ochre with size or « 183 » liquor and oil in order to give a gloss, and to hide uneven or dull colour, and, when sufficiently dry, is well brushed by hand or power, rolled a second time, and dried-off in a room gently heated by steam. This is the Bristol method of finishing. In the Lancashire district, butts are generally struck out much wetter, and "stoned," so as to remove the whole of the bloom, and show the natural brown "bottom" of the grain. When sufficiently dry, they are struck a second time, to set the grain, and rolled as described, the painting being omitted. This method has the disadvantage of requiring more labour, and causing a loss of weight; but leather so got up brings a higher price, as the finish is only applicable to such tannages as make a fair colour. The usual London plan is a compromise between the Bristol and Lancashire methods; the leather is sammed, or tempered by partial drying and piling before striking; stoning is not resorted to, but the bloom is thoroughly removed from the surface with the pin and scrubbing-brush. Colour is not generally used.
It is very important, and especially so with heavy mixed tannages, that the drying should be conducted in the dark, and not too rapidly. No artificial heat should be used, except in frosty weather, to wet leather; and it should be carefully protected from harsh drying winds. After the leather is finished, it should be dried off in a well-ventilated drying-shed, heated to about 70° F. (21° C.). The same observations apply to the drying of rough dressing-leather, except that artificial heat should be avoided. Frost makes dressing-leather porous, and prevents it carrying a proper quantity of grease in currying. On the construction of drying-sheds, see pp. 243-54.
DRESSING LEATHER.
Hides which are intended for purposes where softness and flexibility are required, as for instance, for the upper-leathers of boots, and for saddlery purposes, are called "dressing" or "common" hides, or, if they are shaved down to reduce their thickness before tanning, they are denominated "shaved" hides. Hides for this purpose are limed much in the same way as has been described for butts; but if they are required very soft and flexible, a somewhat longer liming is permissible. After unhairing, fleshing, and washing in water, they are usually transferred to a "bate," composed of pigeon- or hen-dung, in the proportion of about 1 peck to 25-30 hides.
In this they are retained for some days, being handled frequently. They completely lose their plumpness, and become soft and slippery; the caustic lime is entirely removed; and the remaining portions of hair-sheaths and fat-glands are so loosened that they are easily worked out by a blunt knife on the beam. This final cleansing process is called "scudding." The theory of the action of the "bate," or "pure," as it is sometimes called, is somewhat imperfect. It is frequently attributed to the action of ammonia salts, and phosphates, contained in the fermenting dung. Ammonia salts certainly will remove caustic lime, free ammonia being liberated in its place, and weak solutions of ammonia sulphate or chloride will rapidly reduce hides, and remove or neutralise the lime. The phosphates in dung are mostly, if not entirely, in the form of lime phosphate, which is quite inert. In point of fact, the process seems to be a fermentive one, the active bate swarming « 185 » with bacteria; to this, rather than to its chemical constituents, its action must be attributed. The bacteria act not only on the organic constituents of the dung, but on those of the hide, producing sulphuretted hydrogen, together with tyrosin and leucin, and other weak organic acids, which neutralise and remove the lime, and, at the same time, soften the hide by dissolving out the coriin, and probably also portions of the gelatinous fibre. The truth of this theory is supported by the fact that, in warm weather, the activity of the bate is greatly increased, and that, if one pack of hides is over-bated, the next following is much more severely affected, the hides having in fact themselves furnished food for the multiplication of the bacterian ferment from the destruction of their own tissues. It also explains the effective use (as a substitute) of warm water with a very small portion of glucose, which, in itself, would be insufficient to dissolve the lime, but with a small quantity of nitrogenous matter, forms an excellent nidus for the multiplication of these organisms. An American invention for bating is the use of old lime-liquor neutralised with sulphuric acid, an idea which is much more scientific than would at first sight appear. Old lime-liquors, as we have seen (p. 143) contain much ammonia and weak organic acids, such as caproic, amidocaproic (leucin), and tyrosin. On adding sulphuric acid, the lime forms an inert sulphate, and the sulphate of ammonia and the weak organic acids which remain dissolved are just what are required in a chemical bate. The lime-liquor should of course be filtered or settled clear before using, and enough acid added barely to neutralise the lime, and the liquor again settled or filtered. By this means both the dissolved gelatin and the iron of the acid will be got rid of. The liquor might then be slightly acidified before use. The writer has no experience of the method, but imagines that used as described it might be worth trying, although it would have a very unpleasant smell. In this connection may be mentioned the fact that, when bran « 186 » drenches are used, in which lactic acid is developed, the butyric fermentation is liable, in hot weather, to take its place, and as butyric acid is a powerful solvent of gelatinous tissue, and the dissolved tissue itself feeds the fermentation, rapid destruction of the skins is the result. Cleanliness, scalding out of the drench vats, and washing the bran before using with cold water to remove adhering flour, are useful precautions.
If the removal of the lime be the only object aimed at in bating, the ordinary process is most wasteful, as well as disgusting, from the loss of pelt it entails. It is easy to find chemical reagents which will remove the lime; but the resultant leather has been found wanting in softness, and it is probable that the solution of the inter-fibrillar matter is in many cases advantageous. Probably one reason for the non-use of such chemicals is their expense. Maynard has patented the use of sulphurous acid for the purpose. If sugar, glucose, or ammonia salts be used, and the alkalinity of the solution nearly neutralised after each lot of hides by common vitriol, the same liquor may be used again and again. In this case, if iron is contained in the acid it will be precipitated by the ammonia and must be settled out. The writer is convinced, from his own experience, that with suitable tannage such bating would yield better weights and quite as satisfactory leather for many purposes as the ordinary mode. French tanners, by the free use of water, and careful working at the beam, and the employment of very weak liquors at the commencement of tanning, make excellent dressing leather without bating and this is also true of the celebrated French calf.
The bating required may be shortened, and probably with advantage, by washing the hides with warm water in a "tumbler," or rotating drum, Fig. 49, prior to putting them into the bate, or the whole bating may be done in the tumbler. After a short bating, also, the hides may be softened and cleansed by stocking for 15-20 minutes. Warm bates act much more rapidly than cold ones.
Various machines have been proposed to take the place of hand-labour in the beam work, and, at least as regards the smaller skins, with considerable success. As a type of these, may be mentioned Molinier's hide-working machine, Fig. 29, which consists of a drum covered with helical knives, rotating at a speed of about 500 rev. a minute, over a cylinder coated with india-rubber. The skin is allowed to be drawn in between these drums, and the two being pressed together by a treadle, it is drawn out by a mechanical arrangement in a direction contrary to the rotation of the knives, which scrape off the flesh, or work off the hair.
After bating, "shaved" hides are reduced in thickness in the stronger parts by a shaving-knife, on an almost perpendicular beam. The workman stands behind the beam, and works downwards. The knife is represented at A, Fig. 26, and is a somewhat peculiar instrument. The blade is of softish steel, and after sharpening, the edge is turned completely over by pressure with a blunt tool, so as to cut at « 188 » right angles to the blade. There is an obvious economy in shaving before tanning, since the raw shavings are valuable for glue-making, while, if taken off by the currier, they are useless for this purpose. The hide also tans faster.
Instead of shaving, the untanned hide is frequently split, by drawing it against a rapidly vibrating knife. The piece removed is tanned for some inferior purpose, if sufficiently perfect. In sheep-skins, which are split by a special machine, the grain-side is tanned for French morocco or basil, while the flesh-side is dressed with oil, and forms the ordinary chamois or wash-leather (see p. 210). Such a machine is shown in Fig. 50.
Tanned leather is frequently split by forcing it against a fixed knife, as in the American "Union" machine, Fig. 51. This is however being gradually superseded by the band-knife splitting machine, Fig. 52, in which an endless steel blade travels over two pulleys like a belt, and is kept constantly sharpened by a pair of emery-wheels seen below the machine. I am indebted for the block to Messrs. Haley and Co., who have made great numbers of these machines.
After bating, scudding, and shaving, the hides are taken into the tan-house, where they are grained, either by frequent handling, or by working in a paddle-tumbler (a vat agitated with a paddle-wheel, and known in America as an "England wheel"), with a liquor of suitable strength. What this strength should be depends on whether a well-marked grain is required or not. The stronger the liquor, the more it contracts the hide, wrinkling the surface into a network of numberless crossing furrows, which form the well-known marking of "grain-leather." In bark tannage, the after management is much like that described with sole-leather, except that weaker infusions are employed, and acid liquors, which would swell the hide and produce a harsh leather, are avoided. In old-fashioned country yards, which produce some of the best bark-tanned shaved hides, the liquors rarely range above 10°-15° of the barkometer, and the time employed is 3-6 months. The hides, after passing through a set of handlers, of gradually increasing strength, in which they are at first moved every day, are laid away with bark liquor and a good dusting of bark, receiving perhaps 4-5 layers of 2-4 weeks each. Unfortunately, these tannages are so unprofitable that they are rapidly being supplanted by quicker and cheaper methods.
These more rapid and cheap tannages mostly depend on the use of "terra" (block or cube gambier) in combination with bark, valonia, mimosa, and myrobalanes. Liquors warmed to 110° or even 140° F. (43°-60° C.) are frequently employed, and a bright colour is finally imparted by handling in a warm sumach or myrobalanes liquor, which dissolves out much of the colour imparted by terra or extracts. The tannage is helped forward by frequent handling, by working in tumblers, or sometimes by suspension on rocking or travelling frames, after the American fashion.
To this class of tannage belongs that of East India kips, which is largely carried on in the neighbourhood of Leeds. These kips are the hides of the small cattle of India, and are imported in a dried condition, and with their flesh-side protected (and loaded) with a coat of salt and whitewash or plaster. They are usually softened in putrid soaks, and unhaired with lime, and are used in England for many of the purposes for which calf-skins were formerly employed. A variety of East India kips, called "arsenic kips," are treated (instead of plastering) with a small quantity of arsenic before drying, to prevent the ravages of insects, which are often very destructive to these goods. Many kips tanned in India have also been imported of late years, and have greatly interfered with the profits of English tanners.
In yards where the leather is intended to be sold uncurried, it is taken up into the drying-sheds, well oiled on the grain with cod-liver oil, and either simply hung on the poles to dry, or stretched with a "righter," a tool shaped somewhat like a spade-handle, and finally set out with it to a smooth and rounded form. As in the case of sole-leather, too much light or wind must be avoided, and it is very difficult to use artificial heat successfully in the early stages of the process. It is, however, now very common for the tanner who produces such leather also to curry it, and, as this effects a « 192 » considerable economy, both in labour and material, it is likely to become universal. When leather is to be sold rough, it is necessary to tan it in such a way as to give it a white appearance, from the deposit of "bloom" already mentioned; this being regarded by curriers as an essential mark of a good tannage, although the first step in the currying process is to completely scour it out. When the tanner curries his own leather, he of course aims at putting in as little bloom as possible, thus economising both tanning material and labour. In addition, the leather goes direct from the tan-house to the currying-shops, thus saving both drying and soaking again, and, it is said, giving better weight and quality. The tanner, too, is enabled to shave his hides or skins more completely, utilising the material for glue-stuff, which, had the leather been for sale in the rough, must have been left on to obtain a profitable weight.
CURRYING.
In general terms, the process of currying consists in softening, levelling, and stretching the hides and skins which are required for the upper-leathers of boots, and other purposes demanding flexibility and softness, and in saturating or "stuffing" them with fatty matters, not only in order to soften them, but to make them watertight, and to give them an attractive appearance.
It is obvious that great differences must be made in the currying process, according to the character of the skin and the purpose for which it is intended, since the preparation of French calf for a light boot, and of the heaviest leather for machine belting, equally lie within the domain of currying. In this case, however, as in that of tanning, the clearest idea of the general principles involved will be gained by taking a typical case, and afterwards pointing out the different modifications needed for other varieties. The French method of currying waxed calf is selected as an example, since the well-known excellence of this leather makes it interesting to compare the details with the methods ordinarily in use in this country.
After raising the skins from the pits, and beating off the loose tan, they are hung in the sheds till partially dry (essorage), great care being taken that the drying is uniform over the whole skin. In modern shops, this drying is usually accomplished at once, and in a very satisfactory manner, by means of a hydraulic press. If dried in the air, they must be laid in pile for a short time to equalise the moisture, and then brushed over on flesh and grain. The next process consists in paring off loose flesh and inequalities (dérayage).« 194 » This is done on a beam, and with a knife similar to that used in bate-shaving, and shown in A, Fig. 26. This knife has the edge turned by rubbing with a strong steel, and is called couteau à revers.
Next follows the mise au vent. The skins are first placed in a tub with water or weak tan-liquor for 24 hours; they are then folded and placed in a tub with enough water to cover them, and beaten with wooden pestles for 1/4 hour. At the present day, stocks (foulon vertical), or a "drum-tumbler" (tonneau à fouler), a machine on the principle of the barrel-churn, usually take the place of this hand-labour. The skin is next placed on a marble table, flesh upwards, and with one flank hanging somewhat over the edge, and is worked with a "sleeker" or stretching-iron (étire), B, Fig. 26. The first 2 strokes are given down and up the back, to make the skin adhere to the table, and it is then worked out regularly all round the side on the table, so as to stretch and level it. The flesh is then washed over with a grass-brush (brosse à chien-dent), the skin is turned, and the other flank is treated in the same way. It is lastly folded in 4, and steeped again in water. The next process is the cleansing of the grain. The skin is spread again on the table, as before, but grain upwards, and is worked over with a stone (cœurse), set in handles, and ground to a very obtuse edge. This scours out the bloom; after washing the grain with the grass-brush, it is followed by the sleeking-iron, as on the flesh.
The next step is resetting (retenage). For this, except in summer, the skins must be dried again, either by press or in the shed. This is another setting out with the sleeker, and, the skin being dried, it now retains the smoothness and extension which is thus given to it. The skins are now ready for oiling in the grain, for which whale-oil or cod-liver oil is generally employed. Olive-oil, castor-oil, and even linseed-oil may, however, be used, and are sometimes made into an emulsion with neutral soap and water. After oiling the grain, the skins are folded and allowed to lie for 2-3 days before oiling the flesh.
The oiling on the flesh is done with a mixture of dégras and tallow, in such proportions as not to run off during the drying. Dégras is the surplus oil from the chamois-leather manufacture, which in France is effected by daily stocking the skins with oil, and hanging in the air for oxidation. The dégras (toise, moëllon) is obtained, not by washing the skins in an alkaline lye, as in the English and German method, but by simple pressing or wringing. This oil, altered by oxidation, is so valuable for currying purposes that skins are frequently worked simply for its production, being oiled and squeezed again and again till not a rag is left. It is generally mixed in commerce with more or less of ordinary fish-oil. Eitner recommends, where the dégras is of indifferent quality, a mixture of 65 parts dégras, 20 of neutral soap (i. e. soap without the usual excess of alkali), and 15 of soft tallow. After oiling the flesh, which is accomplished by extending the skin on the marble table with the sleeker, and applying grease with a sheep-skin pad, it is hung to dry at a temperature of 65°-70° F. (18°-21° C.). After drying, the surplus oil is removed by a fine sleeker from both flesh and grain, and the skins are ready for "whitening" (blanchissage). This consists in taking a thin shaving off the flesh, and was originally accomplished by the shaving-knife on the currier's beam, and some curriers are still in favour of this method. It is now, however, usually done by a sleeker with a turned edge. The grain then undergoes a final stoning and sleeking, to remove the last traces of adhering oil, and the skin is grained by rubbing it in a peculiar way under a pommel covered with cork. It is then coated on the flesh with a mixture, of which the following is a specimen:—5 parts of lamp-black are rubbed with 4 of linseed-oil, and 35 parts of fish-oil are added; 15 parts of tallow and 3 of wax are melted together and added to the mixture; and, after cooling, 3 parts of treacle. This compound is put on with a brush, and allowed to dry for some days. Finally, the skins are sized over with a glue-size, which is sometimes darkened by the addition of aniline-black.
The preceding account will give some idea of the care and labour expended on these goods in France. In England, cheaper productions are more in vogue, and almost every process is accomplished by machinery. An illustration of the Fitzhenry or Jackson scouring-machine, which is largely employed both for scouring and setting out, is given in Fig. 53. This is a simple and efficient machine, and has been largely used, both here and in America.
Fig. 54 shows the improved tool-carriage introduced by C. Holmes of Boston, in which the brush and sleekers or stones are controlled by handles which are stationary instead of moving rapidly with the slide, as in the older form. Spiral springs are also substituted for the older elliptical ones.
The Fitzhenry machine has also been constructed so as to
work in any direction over a fixed table, being driven by a
small direct-acting steam-cylinder supplied by jointed pipes.
But probably the most perfect scouring and setting machine
which has yet been introduced is the Lockwood Automatic
Scourer, which may also be regarded as a development of
« 198 »
the Fitzhenry machine. This has been some years in use in
America with great success, and has received considerable
improvements, but has only very recently been introduced
into England by Messrs. Schrader and Mitchell of Glasgow,
who have kindly furnished the annexed illustration (Fig. 56).
In this machine the table is fixed, and the tool-carriage can
be moved over it in every direction. The large projecting
carriage, or cross-head, which supports it, travels on a
horizontal rail, which may be observed below and behind the
table. Motion is given to it by a screw which is driven in
either direction by the pulleys at each side of the cross-head.
In a similar way the tool-carriage is traversed forwards or
backwards by a second screw at right angles to the first, and
by a most ingenious interlocking arrangement both screws
are controlled by a single handle. The tool-carriage or
"trundle frame" can also be turned like a turntable, so as
to deliver its stroke in any direction, the tool-holder being
« 199 »
« 200 »
driven by a horizontal crank in the centre of the frame, and
immediately above the tools. Though the machine is complicated,
and necessarily expensive, it has not been found
either in America or Scotland difficult to work or liable to
get out of order, while both the quantity and quality of its
work are all that can be desired. Fig. 55 is Gläser's scouring
machine. Fig. 57 illustrates the latest English scouring
machine, Messrs. Haley and Co.'s Climax Scourer, which is
also ingenious and effective. In it the table instead of the
tool-holder is movable by screws driven by belts thrown into
gear by a handle, and it is provided with two tables of which
one is in work while the hides are being changed and spread
on the other. The oscillating tool-holder, instead of being
actuated by the rise and fall of the connecting-rod, is moved
by an adjustable eccentric.
In the case of strap-butts, the currying is, of course, far less elaborate. They are well scoured out, heavily stuffed, and stretched in screw-frames, to prevent their giving afterwards when in use.
In Germany, Switzerland, and Austria, a method of stuffing strap-butts is frequently employed, which, so far as I am aware, is not in use in England. It is called Einbrennen or « 201 » "burning in," and consists in applying very hot tallow to the dry leather. The butts are washed free from liquor in a tumbler, boarded to soften them thoroughly, scoured, set out with a sleeker, nailed on laths, and air-dried. They are then very completely dried in a room heated to 104°-113° F. (40°-45° C.), as if any moisture remains in the hide, the fibre will be destroyed by the heat of the melted tallow. The tallowing generally takes place in the same room, as a high temperature is required to allow it to soak in, and the leather would greedily reabsorb moisture if exposed to damp air. The tallow is heated, generally by steam in a jacketed pan, to 167°-212° F. (75°-100° C.). There are two ways of applying it. The melted tallow may be applied on a table to the flesh side of the butt with a ladle, and rubbed on with a brush or rag. In this case, as soon as the tallow has sufficiently soaked in, the butts are placed in water to prevent its striking through to the grain. The second way is to have the pan of sufficient size and suitable shape, and for two men to draw the butt through the melted tallow with tongs, and more or less rapidly according to the quantity it is desired that the leather should absorb; and in some cases the process is repeated once or more. In this case, it is useless to wet in water, and the butts are allowed to cool gradually in pile.
The leather is now impregnated with grease, but it is far from being properly stuffed. Instead of the grease being spread over the finest fibres in a minute state of division, it simply fills the spaces between the larger fibres. To remedy this, the butts are well softened in water (which, if they have been drawn through the tallow and allowed to cool, must be tepid), and are then worked in a damp condition in a drum tumbler, by which they brighten in colour and become uniformly stuffed. They are then allowed to lie in a pile a day or two, are stoned and worked out with the sleeker, and hung up to dry. When in right temper they receive a final setting out with the sleeker, and when dry are either rolled or glassed. For further details, Nos. 256 and 257 of 'Der « 202 » Gerber,' 1885, must be consulted, where the matter has been exhaustively treated by Eitner, in his papers on "Extract-Gerberei."
In England, curried leathers are generally sold by weight, which leads to the use of glucose and other materials to add to the weight. In America, all upper leathers are sold by measure, and this is now ascertained by a very ingenious machine (Fig. 58). The skin is laid on a latticed table, and a frame, from which rows of bullets are suspended, is let down upon it. The total weight of the frame is indicated by a spring balance, and as the bullets which are over the skin are supported by it, the diminution of weight indicates the measurement. Several modified forms of this machine are now made.
ENAMELLED, PATENT, OR JAPANNED LEATHER.
These are terms used to designate those leathers, whether of the ox, the horse, the calf, or the seal, which are finished with a waterproof and bright varnished surface, similar to the lacquered wood-work of the Japanese. The name "enamelled" is generally applied when the leathers are finished with a roughened or grained surface, and "patent" or "japanned" are the terms used when the finish is smooth. Though generally black, yet a small quantity of this leather is made in a variety of colours.
In America, large thin hides are principally used for the purpose. They are limed and bated in the usual way, stoned after bating, and tanned with hemlock and oak barks in a paddle tumbler, which is run for 10-15 minutes in each hour. When one-third tanned, they are levelled on the flesh, and split with the belt-knife splitter, Fig. 52. After splitting, the portions are drummed with strong gambier liquor for 1/4 hour, and then tanned out with bark. The grains are scoured with the Fitzhenry or Lockwood machine (Figs. 53 and 56). They are then lightly oiled and stretched on frames which can be enlarged by screws or a sort of knuckle-joint at the corners. When quite dry, they are grounded with a mixture of linseed-oil with white lead and litharge, boiled together and thickened with chalk and ochre. This is dried in closets heated by steam, into which the frames are slid face downwards, the heat being gradually increased from 80° to 160° F. (27° to 71° C.). If it be desired to employ a higher temperature, the leather is first saturated with a solution of 2 oz. each of borax and alum in 1 gal. water, when temperatures of 230°-250° F. (110°-120° C.) may « 204 » be used. The remaining treatment is much as above described, but a little turpentine is used to make the paint work freely. The final varnish is composed of 20 parts spirit of turpentine, 20 linseed oil, 10 thick copal varnish, and 1 of asphaltum or other colouring material. This must be mixed 2-3 weeks before use, and applied with a brush.
The splits are also often enamelled, and as a preparation receive a dressing of linseed-oil boiled to a jelly and thinned with turpentine or naphtha. This is applied with a stiff brush after the splits are stretched on the frames and are still damp, so that it does not penetrate the leather, but forms a sort of artificial grain.
Leather destined to be finished in this way requires to be curried without the use of much dubbing, and to be well softened. The English practice is to nail the skins thus prepared, and quite dry, on large smooth boards, fitted to slide in and out of stoves maintained at a temperature of 160°-170° F. (71°-77° C.), coating them repeatedly with a sort of paint composed (for black) of linseed-oil, lamp-black, and Prussian blue, well ground together. Each coating is allowed to dry in the stoves, before the next is applied. The number of coatings varies with the kind of skin under treatment, and the purpose for which it is intended. The surface of every coat must be rubbed smooth with pumice; finally, a finishing coat of oil-varnish is applied, and, like the preceding coats, is dried in the stove. The exact degrees of dryness and flexibility, the composition of the paint, and the thickness and number of the coats, are nice points, difficult to describe in writing.
This branch of the leather industry, so far as it relates to calf-skins, is carried on to a larger extent, and has been brought to greater perfection in Germany and France than in England. In the former countries, the heat of the sun is employed to dry some of the coatings. The United States have also brought this style to a high degree of excellence, especially in ox-hides. There, use is said to be made of the « 205 » oils and spirits obtained from petroleum, and without doubt, French and German emigrant workmen have materially assisted in attaining this high standard.
Leather finished in these styles is used for slippers, parts of shoes, harness, ladies' waist-belts, hand-bags, &c., and has now maintained a place among the varieties of leather for a long period of years.
MOROCCO LEATHER.
Morocco leather is produced from goat-skins. Rough-haired or "blue-back" seal-skins are also used, and produce an excellent article; while an inferior description, called "French morocco," is produced from sheep-skins. The skins are unhaired by liming in the usual way, and are then baited with a mixture of dogs' dung and water. The tanning is done chiefly with sumach, at first in paddle-tumblers, and then in handlers, lasting about a month in all. Sheep-skins are usually tanned through in about 24 hours, by being sewn up into bags, grain-side outwards, and nearly filled with strong sumach infusion. A little air is then blown in, to completely distend the skin, and they are floated in a sumach bath, and kept moving by means of a paddle. After the first day's immersion, they are thrown up on a shelf, and allowed to drain; they are then again filled with sumach liquor; when this has a second time exuded through the skin, they are sufficiently tanned, and the sewing being ripped open, they are washed and scraped clean, and hung up to dry, making what are called "crust-roans." The dyeing is sometimes done by brushing on a table, grain-side upwards, but more usually the skins are folded closely down the back, flesh-side inwards, so as to protect it as much as possible from the influence of the colour, and then passed through the dye-bath, which is now generally of aniline colours. The original oriental method of manufacture for red morocco was to dye with cochineal before tanning, and this is still customary in the East, but is quite obsolete in this country. A grain or « 207 » polish is given to the leather, either by boarding, or by working under small pendulum rollers, called "jiggers," which are engraved either with grooves or with an imitation of grain. A well-cleaned sumach-tanned skin is capable of being dyed in the finest shades of colour; and this branch of the manufacture of leather has been brought to great perfection.
RUSSIA LEATHER (Ger., Juchtenleder).
This is tanned in Russia with, the bark of various species of willow, poplar and larch, either by laying away in pits, or handling in liquors, much like other light leathers, the lime being first removed by bating, either in a drench of rye- and oat-meal and salt, by dogs' dung, or by sour liquors. After tanning, the hides are again softened and cleansed by a weak drench of rye- and oat-meal. They are then shaved down, carefully sleeked and scoured out, and dried. The peculiar odour is given by saturating them with birch-bark oil, which is rubbed into the flesh-side with cloths. This oil is produced by dry distillation of the bark and twigs of the birch. The red colour is given by dyeing with Brazilwood; and the diamond-shaped marking by rolling with grooved rollers.
Much of the leather now sold as "Russia" is produced in Germany, France, and England. It is tanned in the customary way, occasionally with willow, but more generally with oak-bark, and probably other materials. Economy would suggest the use of such materials as, from their red colour, are objectionable for other purposes, and therefore cheap. The currying is in the usual manner, care being taken that the oil used does not strike through to the grain, which would prevent it taking the dye. The colour is given by grounding with a solution of chloride of tin (100 parts tin perchloride, 30 parts nitric acid, 25 parts hydrochloric acid, allowed to stand some days, and the clear solution poured off, and mixed with 12 volumes of water). The dye-liquor may be composed of 70 parts rasped Brazilwood, 3 parts tartar, and 420 parts water, boiled together, strained, and allowed to settle clear. The grounding and dyeing are done on a table with a brush or sponge (see Glove-kid dyeing, p. 229). The odour is communicated by rubbing the flesh-side with a mixture of fish-oil and birch-bark oil, which sometimes contains no more than 5 per cent. of the latter.
Pl. VII.
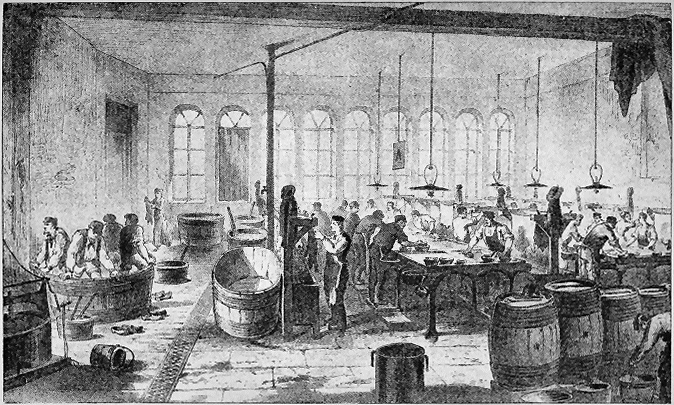
TREADING AND DYEING THE SKINS.
CHAMOIS OR WASH-LEATHER.
This leather, which is remarkable for its soft felty texture, which it retains even after wetting, although perfectly porous and free from greasiness in its finished state, is prepared by the action of oil on the raw skin. Wash-leather was formerly manufactured from sheep- and calf-skins, and from those of the chamois, and various deer (hence the name), from which, after liming, the grain was removed (frized) with a sharp knife, either with the hair, or after unhairing. The flesh-splits of sheep-skins are now generally employed for ordinary wash-leather, and of course no such process is needed, though buff-leather for belts and military purposes is still so manufactured. The skins receive a thorough liming, which, where softness is desired, is so conducted as very thoroughly to remove the cement-substance (coriin) from between the fibres; and this removal is frequently carried still further by a short bran-drench, which also secures the complete absence of lime. After the usual beam-work, the skins are pressed or wrung out to remove surplus water, and while still moist are oiled on a table and folded in cushions. Fish-, seal-, or whale-oil is generally used, and vegetable oils do not seem to answer even in mixture, with the exception perhaps of olive-oil. The skins are next stocked for 2-3 hours, shaken out, and hung up for 1/2-1 hour to cool and partially dry. They are then again folded in bundles, and stocked for a short time, taken out, oiled again, and returned to the stocks; and this process is repeated, until the skins lose their original smell of limed hide, and acquire a peculiar mustard-like odour, and the water at first present has been entirely replaced by oil. The later dryings « 211 » are frequently conducted in a heated room, and when the oiling is complete, the skins are piled on the floor, and the oxidation of the oil, which has already commenced during the fullings and dryings above described, is completed by a sort of fermentation, in which the skins heat very considerably. During this process, they are carefully watched, and if the heat rises so high as to endanger the quality of the leather, the pile must be turned over, so as to cool the skins, and bring those which were originally outside to the centre. When the fermentation comes to an end, the skins are no longer susceptible of heating, and are of the well-known yellow or chamois colour. Where this colour is objectionable, the oxidation is sometimes completed by hanging the leather in a heated room instead of by piling. It is now necessary to remove the surplus oil, and this in France is done by oiling with any sort of oil, throwing into hot water, and wringing or squeezing. The oil obtained in this way forms the moëllon or dégras so much prized for currying purposes. The unoxidised oil still retained by the skins is removed by washing with soda or potash lye. In England and Germany, the whole of the uncombined oil is removed in this way, and is recovered from the lye, in which it exists in a partially saponified state, by neutralisation with sulphuric acid. It forms the "sod" oil of commerce. About half the oil employed is obstinately retained by the skin, and cannot be removed even by boiling with alkalies, while no gelatin is obtained by boiling water, to which the chamoised skin is much more resistant than ordinary leather. The nature of the tanning process does not seem to be well understood. It is generally stated that the fibres of the skin are unaltered, but are merely coated with the oxidised products of the oil. It is hard, however, on this hypothesis to understand their extraordinary indifference to water, even at a boiling temperature, which speedily converts kid and other tawed leathers into a solution which gelatinises on cooling; and it seems more probable to the present writer that some actual chemical combination is « 212 » formed. Lietzman ('Herstellung der Leder,' p. 164) supposes that the whole of the gelatigenous tissue has been removed by liming and bating, and that only the very indifferent yellow elastic fibres (see p. 21) remain. This view, however, is quite untenable, in consideration of the very small proportion of these fibres originally present in the skin. Müntz, in his researches (see p. 17), showed that the fibres insoluble in boiling water scarcely exceeded 3 per cent, of the dried pelt. Dry gelatigenous fibre has a considerable resistance to heat, and it is possible that the action of the oil may consist in preventing the absorption of water. This, however, will not explain its resistance to alkalies. Cotton or other vegetable fibres moistened with oil, readily undergo oxidation, with so much evolution of heat as sometimes to cause spontaneous combustion; but the oxidation products are easily and completely removed by alkaline solutions, leaving the fibre in its original state, as indeed is noted by Lietzman (loc. cit.).
The finishing processes consist in staking during drying to retain softness, and in whitening and smoothing the flesh (or sometimes both sides) on the fluffing wheel. Skins for gloves, &c., are bleached like linen, by sprinkling and exposure to the sun; or more rapidly by treatment with a weak solution of potash permanganate, and subsequently with sulphurous, or very dilute sulphuric acid, to remove the brown manganous oxide formed (Barreswil, Dingl. Polyt. Jour., 161, 312). Gaseous sulphurous acid from burning sulphur may also be used for bleaching. The "dyeing" of chamois leather is generally done with ochres and similar colouring matters, and may be removed by washing. Treatment with egg-yolk in water, or with an emulsion of olive-oil with a little soap, and rubbing, or stretching, will restore softness to chamois leather which has become stiff by washing.
CROWN LEATHER, OR PRELLER'S LEATHER.
The process of manufacture of this leather, which has obtained a firm position as the most suitable material for certain classes of belting, picker-straps, &c., was discovered about 35 years since by Theodor Klemm, a cabinet-maker in Wurtemburg and founder of the present well-known firm of leather manufacturers, Gebrüder Klemm of Pfullingen. Klemm, at that time in poor circumstances, sold his patent in Paris to an Englishman, Preller, who started a manufacture of it in Southwark and adopted a crown as his trade-mark. Since this time the manufacture has spread, first to Switzerland and then through Germany; but in England, to the writer's knowledge, it is confined to one or two firms.
The process of manufacture of crown leather is in principle intermediate between that of calf-kid (see p. 223), and the pure oil-tanning, if we may call it so, of which the chamois leather (see p. 210) is typical. It depends on impregnating the raw hide with a mixture of fats and albumens, to which salt or saltpetre is added to prevent putrefaction. The process as described in the original patent was as follows,—The hides were unhaired by liming or painting (with sulphides), and cleansed as usual, no plumping lime being given. After unhairing they were allowed to dry some little time in the air till no longer plump, and were then worked in a tumbler drum, without water, till uniformly soft. They were then spread on a table and brushed over on the flesh-sides with a mixture of 23 parts of ox-brain, 61/2 of butter, 28 of soft fat, and 4 of salt or saltpetre, with 26 of barley-flour and 121/2 of milk, of which the leading 4 ingredients « 214 » were first to be mixed and the flour stirred in, the milk being last added. The hides were then returned to the tumbler, which was provided with tubular axes, through which a portion of exhaust steam was admitted to warm the drum. After tumbling some hours, the drum was opened, and the hides were examined. If the tanning was not complete, the hides were hung in the air for a time to dry, and the process was repeated till a cut showed that the mixture had completely penetrated the hide.
From Eitner's researches it appears that the essential tanning ingredients of the mixture above described are the fat (and butter which acts simply as fat) and the albuminous matter of the milk (casein), brains (albumen, &c.), and flour (gluten); the starch serving at most to assist in the emulsification of the fats. Eitner treated crown leather with dilute potash solution to remove the albumen and fats, and after washing and drying obtained a material like an insufficiently stocked chamois leather. On again stuffing with a quantity of fat equal to that removed, but without the albumen, the leather became dark and quite greasy, so that by sharp bending oil could be pressed out. Good results may be obtained in crown leather manufactured with fats and flour only, without the use of milk or brains, so that it is obvious that the same purpose is served by either vegetable or animal albumenoids. The most important point in the purposes for which crown leather is employed is toughness, and this is given by the unaltered hide-fibres, which are merely preserved by the coating of oily matter with which, like those of chamois leather, they are surrounded. The albumen serves the purpose of filling the spaces between the fibres, and giving solidity and firmness, so that the belts may keep their shape, and not stretch inordinately. It also serves to make the leather waterproof, and fit it for water-bags for military purposes (as it gives no taste to the water) and for hose-pipes. The albumen, which much resembles the hide-fibres in composition, is like them preserved by the fats.
For the modern process of manufacture, good, even and « 215 » well-flayed hides are selected, and unhaired either by sweating, or by a very short liming, which must be assisted by rockers or some mechanical mode of moving the hides, so as to get them unhaired in the shortest possible time and with the least injury to the fibre. Sodium sulphide (see p. 147) may be employed with great advantage. The fleshing and scudding are performed as usual, according to the mode of unhairing adopted. The hides are then very commonly rounded, and the bellies tanned in the usual manner; but sometimes the whole hide is made into crown leather.
As crown leather is naturally almost white, it is usual at this stage to colour the hide with bark or other liquors. As in this case simply colouring and not tanning of the grain is required, high-coloured liquors, made by steaming materials with much colour and little tannin, are preferable. For this purpose wood extracts, such as chestnut, quebracho, or oak-wood are said to be very suitable, and beech, pine or alder bark may also be used. In practice, chestnut and hemlock extracts, and occasionally cutch are employed; but the last named is not to be recommended. A chestnut liquor of 71/2° Tw. or 5° B. (34° Bark.), with constant handling or in a paddle-tumbler, will give a satisfactory colour and grain in 1-2 hours. This rapid colouring is preferable to the slower process, which occupies 24 hours in weaker liquors. If sweated, the hides are now plumped with sulphuric acid, but only to a very moderate extent. This process is best performed in a paddle-tumbler; about 31/2 oz. of sulphuric acid are required per hide, and a time of 6-12 hours according to the water employed. The liquor may be several times used, strengthened with the necessary quantity of acid. Limed hides do not require further swelling. The hides are washed through clean water, and hung up to dry somewhat.
The hides are next spread on a table, flesh-side uppermost, and covered with a layer of the tanning paste nearly 1/4 in. thick. The composition of this paste may be varied according to the relative prices of different materials, and the amount of hard fats must be regulated according to « 216 » whether or not appliances are provided for heating the tumbler. A good mixture is 7 parts common wheat-flour, 7 of horse-grease, 1 of salt, and 1-2 of tallow. If too soft, more tallow may be employed. The salt is first added to the horse-grease, then the melted tallow, These fats are added little by little to the flour till a uniform paste is obtained. Another good mixture is 27 parts wheat-flour, 25 of bone-grease, 4 of tallow, and 4 of salt. Another recipe gives 28 lb. fine white flour made to a paste with 13-14 pints water and then worked up to a uniform mass with a tepid mixture of 28 lb. beef tallow and 28 lb. hard horse-fat (Pferdekammfett). These mixtures are all for use in warmed drums; a specimen of one used in a factory where the mixture was simply trodden in cold into the leather in open tubs is as follows:—7 parts flour, 9·4 of horse-fat, 2·8 of fish-oil, 7 of ox-brains and 0·7 of salt. The hides are next folded in bundles and placed in the drum; or in stocks, which are occasionally used for the purpose. If a drum be used, it must be of large diameter, 8-9 ft., provided with pegs inside, and should make about 25 revolutions per minute, so as to work the hides with considerable force. Much more care is needed in warming the drum, than is required in ordinary stuffing, and this is best accomplished by warmed damp air. This may be arranged by the use of an air-pump, which draws air through water warmed by exhaust steam, and forces it through the hollow axles of the drum (or drums); or a simple aspirator consisting of a cask filled with water may be connected to one axle, so that as the water runs out it will draw air through the drum from the opposite axle, which is connected with a cask half filled with hot water through which air is allowed to bubble. Probably the same effect could be reached in a still simpler and cheaper manner by the use of a steam-jet blower, such as Körting's. In any case the drum must be warmed to a temperature of 82°-104° F. (28°-40° C.). Warm dry air may also be used, but is not so suitable, as it dries the hides too much. The hides are tumbled 8-12 hours, hung up till half-dry, and the « 217 » process is repeated. For very heavy hides, 4 tumblings may be required. In the later tumblings, a lower temperature, 95° F. (35° C.), may be employed, and the time extended to 15 hours.
The currying of crown leather is very simple. It is set out on flesh and grain, and boarded to raise the grain. Mossner, before currying, washes 2 hours in water and brushes with tepid soda solution (1 in 60). The yield of weight is small, only amounting to about 30-40 per cent. of the raw hide employed, and hence the price per lb. must be considerably higher than that of tanned leather to yield a profit. The above information is mostly drawn from articles by W. Eitner ('Der Gerber,' iv. 1 et seq.) and Franz Kathreiner ('Gerber Zeitung,' 21st December, 1875).
MINERAL-TANNED LEATHER.
The invention of the earliest form of mineral tanning, that with alum and salt, dates from remote antiquity; but as it is in large measure the type of all that has been since done, it deserves examination in some detail, at least as regards principles. In practice it is used alone in curing skins with the hair on, and for making white leather for laces and other purposes; and, in combination with oil and albumen, which, as we have seen, are the tanning agents in the case of "crown leather," it forms the process for producing calf and glove kids, as will be described under those headings (pp. 223, 225).
Careful researches by Reimer (Ding. Polyt. Jour., 205, p. 143 et seq.) show (what has long been known in practice) that alum alone is not capable of making a pliable leather. The salt, nevertheless, does not enter into combination with the alum, or even with the hide. Its function is partially physical, increasing the diffusion of the solution, and partially chemical, as in the presence of acids (and salts of acid reaction) it precipitates the coriin, and prevents it from gluing the fibres into a horny mass as it dries. Prof. Knapp has shown that this is the first essential in producing leather, and that raw hide may be converted into a pliable material with all the properties of white leather by simply withdrawing the water with alcohol, in which coriin is not soluble, and by which it is therefore precipitated. This leather, containing when dried no added constituent, is of course at once reconverted into raw hide by soaking in water. Both the salt and a portion of the alumina is removed from tawed leather by soaking in water, and it then dries hard and « 219 » horny, and by boiling in water will yield a considerable percentage of gelatin. The alum is not absorbed as a whole. It is a double salt (alumina and potash sulphate or alumina and ammonia sulphate), and only the alumina sulphate is absorbed, potash (or ammonia) sulphate accumulating in the liquor. The alumina salt retained by the hide, especially in presence of much salt, contains slightly more than its normal proportion of alumina to acid, or in chemical language is to some extent basic. This is caused partly by the lime remaining in the skin from the unhairing process, which neutralises a portion of sulphuric acid, but in part is the result of the affinity of the hide-fibres for alumina, a certain small proportion of free sulphuric acid being left in the liquor. The accumulation of this and of potash sulphate is the reason why such liquors cannot be used perpetually by mere strengthening with alum, but must be frequently renewed. The attraction of hide-fibre for alumina sulphate is so strong, that in presence of a sufficient excess of hide it may be completely removed even from dilute solutions. Alumina acetate or sulphate may be substituted for alum with equally good results in practice, the only advantage of the latter being its easier preparation. Ferric and chromic salts and iron or chrome alum, may be substituted for common alum, and are absorbed in a similar manner, and in presence of common salt give equally pliable leathers, of a buff and pale greenish tint respectively. Without salt, the leathers are hard and brittle. In all these cases, the tanning agent may be to a large extent removed by simple washing with water. The tannage may be rendered more durable by passing the leather before drying through a weak bath of sodic carbonate or even lime-water, which precipitates the alumina, iron, or chrome in a basic form on the hide-fibres. Soap baths may also be used, by which aluminic, ferric, or chromic stearates and oleates are formed, possessing considerable toughness and resistance to water. So far as the writer is aware, no mineral tannage has yet been produced which will not yield gelatin when treated, first with dilute acid and then with boiling « 220 » water; but this is rather a gain than otherwise, as leather scraps might be utilised for glue. There seems no reason why good and durable leather, for boot-uppers and for many mechanical purposes, should not be fabricated with salts of iron and chromium in conjunction with salt. If eggs and flour were also used, products similar to calf-kid would be obtained. Iron-leathers may of course be blacked with infusions of galls or many tanning materials, or with logwood. Ferrous salts have no tanning properties.
If, instead of using neutral iron salts, basic ferric salts (which may be obtained by dissolving ferric oxide in solution of neutral ferric salts, or by oxidising ferrous sulphate with manganese black oxide, or nitric acid) be employed, much larger quantities are absorbed by the hide, and if this be fixed with soap baths and finished with a moderate quantity of oil, a gain of weight—approaching 50 per cent. of the finished leather, or about the same as that given by bark, may be obtained. The leather, however, has by no means the same resistance to wet and decay as bark-tanned leather, and invariably has a tendency to crack when sharply bent. The process has been most carefully worked out by Professor Knapp, and was patented and worked commercially for a short time in Brunswick, but apparently without financial success. Professor Knapp's method is as follows:—The iron solution is prepared by adding nitric acid to a boiling solution of ferrous sulphate (green vitriol) till the iron is completely oxidised to the ferric condition. To this, ferrous sulphate is again added so long as it continues to cause effervescence. The resulting solution is a clear orange, and of more or less syrupy consistence, and may be evaporated without decomposition or crystallisation to a transparent varnish. The hides are unhaired and prepared for tanning in the usual way, and are then handled in solutions of the iron salt, which are at first weak, and are gradually strengthened. Skins are tanned in 2-3 days, and the heaviest hides in a week. After tanning, the hides are stuffed in a drum ventilated through the axes, very similar to that « 221 » described under "crown leather," p. 213, with an insoluble iron-soap made by precipitating soap solution with the iron-liquor; or the iron-soaps may be formed in the hide by the alternate use of iron and soap solutions, as already described. The leather is finally saturated with a solution of stearin and paraffin, to render it waterproof.
A process which has been worked on a larger scale, is that of Dr. Heinzerling, introduced about 1878, with the usual promise of "complete revolution" in the leather trade; but which, in spite of the most determined and persevering efforts of the Eglinton Chemical Company, who own the English patent, has failed to take any very prominent position in commerce. The tanning materials employed are alum and salt, with a varying proportion of potash, soda, or magnesia bichromate. These salts have a very marked hardening effect on animal tissues, and, when mixed with gelatin and exposed to light or acted on by acids, become reduced, and at the same time render the gelatin insoluble even in hot water, a property which is made useful in many photographic processes. This is probably due to the formation of salts of chromium, which, as has been stated (p. 219) have a similar tanning effect, but perhaps more powerful, than those of alumina. However this may be, the effect of potash bichromate when exposed to light with gelatin, differs from that of the addition of chrome salts ready formed, the gelatin in the first case becoming incapable of even swelling in hot water, while in the second, though rendered insoluble, it becomes soft and swollen. The use of potash bichromate in tanning had been previously patented by Cavalin, who used it in conjunction with alum and salt, and with the addition of a portion of green vitriol, to give the leather a colour more similar to that of bark-tanned.
Dr. Heinzerling uses metallic zinc in the salt and alum solution to assist in the precipitation of amorphous alumina on the hide-fibres. The same material was used in a similar way by Jennings (No. 2295, 1861), but with the object of whitening the goods. Yellow or red prussiates of potash « 222 » (potassic ferrocyanide or ferricyanide), are also sometimes mixed with the solution, in order to enable the leather to be blacked with iron-liquor, with which they produce prussian blue. To fix the tannage on the fibre, and prevent its washing out, the use of barium chloride, lead acetate, and of soap solution is claimed; the latter having been also patented for similar purposes by Knapp, and subsequently by Jennings and others.
In order to render the leather waterproof, it is finally saturated with solutions of paraffin, stearin, and other fats and hydrocarbons (resin is employed, though not named in the patent), in petroleum spirit and similar solvents.
Such is the original patent, which, it will be seen, is rather a combination of older processes than an original discovery. Whether it is still worked on the same lines the writer is unable to say, but he is aware that considerable improvements have been made in the finish and appearance of the goods. The leather in its present form possesses considerable resistance to water, is free from the brittleness so common in mineral tannages, and like other alumed leathers, considerably exceeds bark-tanned leather in toughness and elasticity. These make it valuable for many purposes, and among others, for machine-belting, although it has the disadvantage of elongating considerably while in use.
CALF-KID.
Calf-kid is used for light upper-leather, and belongs to a different class from any yet described, being "tawed" instead of tanned. In this respect, and in most details of its manufacture, it resembles glove-kid.
The process is as follows. Selected calf-skins, dried or salted, are the raw material, and after a suitable softening in fresh water, are limed for 2-3 weeks, or till the hair goes easily. They are then unhaired and fleshed in the usual manner, pured with a bate of dogs' dung, scudded, and again cleansed with a bran drench. In Germany, the bran drench is used alone, and is composed of 33 lb. bran to 100 medium skins. Before use, the bran should, especially in summer, be well washed, to free it from adhering meal. The temperature of the drench should not exceed 100° F. (38° C.), and the skins should remain in for 8-10 hours. Lactic acid is produced by fermentation; this removes lime, and is itself neutralised by the products of putrid fermentation which succeeds it.
The tanning is accomplished in a drum with a mixture of alum and salt; and after drying, the skins are again moistened, and worked in the drum with a mixture of oil, flour, and egg-yolk. In the German method, these two operations are combined. Eitner, who has written a series of articles on the process, gives 40 lb. flour, 20 lb. alum, 9 lb. salt, 250 eggs, or about 11/3 gal. of egg-yolk, 7/8 pint (1/2 litre) of olive-oil, and 12-16 gal. water, as a suitable mixture. The skins are worked in a drum-tumbler (preferably a square one, see Plate 5) for 20 minutes, then allowed to rest 10 minutes, and this process is twice repeated. The temperature must not exceed 100° F. (38° C.), and it is said « 224 » to be important that the drum should be ventilated by holes at the axis.
The skins are allowed to drain, are then rapidly dried at a temperature of 140°-160° F. (60°-71° C.), and, after "samming," or damping with cold water, are staked by drawing them to and fro over a blunt knife fixed on the top of a post (see Plate 6). They are then wetted down and shaved, either with the moon-knife or ordinary curriers' shaving-knife, and sometimes receive a second dressing of oil, flour, and egg, to soften them still further.
Dyeing black is accomplished either by brushing on a table, or by "ridging" or folding, grain-side outwards, and drawing quickly through baths of the mordant and colour. To prepare them for the colour, stale urine is generally employed. A deeper colour, and one less liable to strike through the skin, is obtained by adding 1/4 lb. potash bichromate to 4 gal. of urine, or the following mixture may be substituted with advantage, viz. 1/2 lb. Marseilles soap dissolved in boiling water, 5 or 6 egg-yolks added, and the whole made up to 4 gal. with water and 1/4 lb. potash bichromate. The colour used is infusion of logwood or its extract, or two-thirds logwood, which is best extracted by stale urine or old soak-liquor, with addition of a small quantity of soda (1 lb. to 25 lb. dye-wood). It is fixed and darkened by a wash of iron-liquor (1 of iron protosulphate in 75 of cold water). After being again dried, the skins are grounded with the moon-knife, and rubbed over on the grain with a composition containing oil, wax, &c., and are finally ironed with a flat-iron, to give them a fine and smooth surface. Eitner gives a recipe for the gloss:—1 lb. gum arabic, 1/2 lb. yellow wax, 1/2 lb. beef-tallow, 3/4 lb. Marseilles soap, 2 lb. strong logwood infusion, and 1 gal. water. The water is brought to a boil in an earthen pot, and then the soap, wax, gum, and tallow are added successively, each being stirred till dissolved before adding the next, and lastly the logwood. After boiling for an hour, it is allowed to completely cool, being incessantly stirred during the whole process.
GLOVE-KID.
This branch of leather manufacture is mainly carried on in Germany, Austria, and France. In Germany and Austria, lamb-skins are principally employed; in France, kid-skins. For fine gloves, the skins of very young animals only can be used. The ordinary style of manufacture is as follows:—The soaking of the dried skins is effected in large wooden tubs (Kufen, Bottichen), and occupies on the average 3-4 days, according to the character of the soak-water, the size of the skins, and the time they have been stored. The skins, when thoroughly and uniformly softened, are unhaired, either by painting the flesh-side with a thin paste of lime, or in lime-pits. In unhairing by painting (Schwöden), the skins, after coating the flesh-side with lime, are folded together, so that the lime comes as little as possible into contact with the wool, and these bundles or "cushions" are placed in a tub, in which they are most frequently covered with water. After unhairing on the beam with a blunt knife, the skins must be limed for some days, in order that the leather may stretch well, a quality which the Germans denominate Zug. By this method of unhairing, the wool is preserved uninjured, but it is not suitable for the finer sorts of leather. The unhairing in lime-pits is done either with gas-lime (Grünkalk), or, as is now almost exclusively the practice, with the so-called "poison-limes" (Giftäscher). These are prepared by mixing red arsenic (arsenic sulphide) with lime, while it is being slaked, and is at its hottest. The calcic sulphydrate (and perhaps sulpharsenite) thus formed hastens the unhairing, and gives the grain a higher gloss. Well-conducted establishments now avoid as much as possible the use of old limes, which « 226 » produce a loose, porous leather, with a rough, dull grain. The liming lasts on the average 10 days, and is of the greatest importance. It is essential that the interfibrillary substance shall be dissolved, that the leather may have the quality known as Stand, that is to say, may be strongly stretched in either length or breadth without springing back. It also depends upon the liming (and this is of special importance in the case of lamb-skins), whether the tissue of the fat-glands is well loosened, so that the fat, either as such, or as lime- or ammonia-soap, may be readily and completely worked out. Skins in which this is neglected can never be properly dyed.
When the hair (or wool) is well loosened, the skins are rinsed in water, and then unhaired on the beam with a blunt knife. The water employed in washing should not be much colder than the limes, or it will prevent the hair from coming away readily. The wool or hair is washed and dried for sale. The skins are thrown into water, to which a little lime-liquor has been added, to prevent precipitation of the lime in the skins by the free carbonic acid of the water, which would have the effect of making them rough-grained.
Next comes the first fleshing (Vergleichen) or "levelling." By this, the loose cellular tissue on the flesh-side is removed, together with the head, ears, and shanks, and the flanks are trimmed. The skins are then again thrown into water, softened with lime-liquor as above described, and then into a bate of dogs' dung. This is prepared by stirring up white and putrid dogs' dung with boiling water, and straining it through a sieve or wicker basket. The bate must be used tepid, and not too strong. The skins "fall" (lose their plumpness) in it rapidly, and become extremely soft and fine to the touch; and the fat-glands, remaining hairs, and other dirt, can now be very readily scudded out. So far no completely satisfactory substitute has been found for this somewhat disgusting mixture, but it has been noted that guano will produce similar effects. With regard to the mode of action of the dung bate, much has been speculated without « 227 » proof, and exact analytical evidence is wanting; but, no doubt, a weak putrefactive action goes on, as may be deduced from the presence of bacteria; further, the ammonia and weak organic acids present in the putrefying dung are capable of acting on fat and lime; and finally, a direct mechanical effect seems to be produced, difficult to describe, but favourable to the succeeding manipulation. Too strong bates, or too long continuance in them, produces evident putrefactive effects on the skins. (See also p. 184.)
When the skins come out of the bate, they are stretched and worked (abgezogen) on the flesh with a sharp knife, and any remaining subcutaneous tissue is removed. This constitutes the second fleshing. They are then rinsed in warm water, and beaten with clubs (Stoss-keule), see Plates 3 and 4, in a tub, or worked in a tumbler-drum (Walkfass), in either case with a very little water only; and finally brought into a tank of water, not too cold, and kept in constant motion with a paddle-wheel.
The skins are next cleansed on the grain-side by working on the beam with plates of vulcanite with wooden handles, so as to remove fat, lime- and ammonia-soaps, and other lime compounds, together with all remaining hair or wool. The skins are now a second time washed in the "paddle-tumbler," first in cold, and then in tepid water; and after allowing the water to drain from them, they are transferred to the bran drench.
This is prepared by soaking wheaten bran in cold water, diluting with warm water, and straining the extract through a fine hair-sieve. Sufficient of the liquid must be employed to well cover the skins, and the temperature may range from 50° F. (10° C.) to 68° F. (20° C.). These conditions are favourable to bacterial activity, which comes into play, and, on the one hand, evolves formic, acetic, lactic, and butyric acids, which dissolve any remaining traces of lime, and on the other, loosens and differentiates the hide tissue, so as to fit it to absorb the tawing solution (Gare). Much care is required in the management of the bran drench, especially « 228 » in summer, since the lactic readily passes into the butyric fermentation (see also p. 186). The tawing mixture is composed (like that employed in the fabrication of calf-kid, q. v.) of alum, salt, flour, and egg-yolks, in a quite thin paste. The skins are either trodden in it with the feet, or put into a tumbler-drum with it (Fig. 48). Kathreiner pointed out, some years since (in vol. i. of 'Der Gerber'), that a mixture of olive-oil and glycerine might be partially substituted for the egg-yolks, in both the tanning and dyeing of glove-kid leather.
The tawed skins are now dried by hanging on poles, grain inwards. Rapid drying in well-ventilated, but only moderately-heated, rooms is essential to the manufacture of a satisfactory product.
The dry leather is rapidly passed through tepid water, and after being hung for a very short time, to allow the water to drain off, is trodden tightly into chests, and allowed to remain in them for about 12 hours, so that the moisture may be uniformly distributed. It is then trodden on hurdles (Horden), composed of square bars of wood, joined corner to corner, so as to make a floor of sharply angular ridges, Fig. 59. The next operation is stretching over a circular knife, called the Stollmond (stollen, Eng. "staking"), shown in Fig. 60; then the leather is dried nearly completely, and staked again.
Dyeing.
The dyeing of glove-kids is done in 2 ways:—a. The skins are plunged into the dye-bath (Tunkfarben). In this way, all light colours are ordinarily produced, such as gris-perle (pearl-grey), paillé (straw-yellow), chamois (reddish yellow), silver-grey, aquamarine, &c. b. The skins are spread on an inclined or rounded table of stone or metal, and brushed over, on the grain side, first with a mordant (Beize), then with the dye-liquor, and lastly, with a solution of a mineral salt (Plate 7). The mordant serves to fix the colour on the surface of the skin, to prevent its striking through, to produce certain modifications of colour, and to enable any parts of the skin which yet contain fat to take the colour evenly with the rest. To satisfy these conditions, the composition of the mordants is very varied. Potash bichromate, ammonia, potash, soda, and stale urine are among the most frequently employed, seldom separately, but usually in a mixture containing 2 or more.
Dye-stuffs of vegetable origin have always held the first place. Those most in use are logwood (Blauholz), Brazilwood (Rothholz), the two fustics—Cuba Gelbholz (Morus tinctoria) and Ungarisches Gelbholz (Rhus cotinus), several species of willow-bark and of berries, indigo-carmine, and indigo dissolved in sulphuric acid.
Aniline colours used alone remained in fashion for a short time only, but are now usefully employed as top-colours (Ueberfarben), viz. brushed in very dilute solution over vegetable colours. In this way, particularly tasteful shades of green, violet, and marine-blue may be produced.
After the mordant has been applied once or twice, and the colour 3-6 times, a wash (Ueberstrich) containing some metallic salt is generally applied, with the object either of bringing out the special tone required, or of making the colour more lively and permanent. The so-called "vitriols" are mostly employed: "white vitriol" (zinc sulphate),« 230 » "blue vitriol" (copper sulphate), "green vitriol" (iron sulphate), and occasionally other salts.
Before dyeing, the greater part of the flour, salt, and alum must be removed from the skins by washing with tepid water; and they therefore require a second feeding (Nahrung) of egg-yolk and salt. In the case of the skins which are dyed by plunging into the dye-vat (Tunkfarben), this is done after the dyeing is completed; in that of brush-dyeing, before the dyeing process.
After the dyeing, the skins, if dipped, are wrung out; if brush-dyed, sleeked out with a brass plate, to get rid of superfluous water. They are then dried in an airy room. Before staking (stretching), the skins are laid or hung in a damp cellar, or in moist saw-dust. They are staked twice: once damp, and once nearly dry.
Skins which are much damaged on the grain, or otherwise faulty, are smoothed with lump pumice on the flesh-side, either by hand or machine. They are then dyed on this side, mostly by dipping, but occasionally with the brush, in which case, the method described is slightly modified.
Indebtedness is acknowledged to F. Kathreiner, of Worms, and David Richardson, of Newcastle, for much information on the production of light leathers. The Plates 1 to 8 represent the works of Messrs. Tréfousse et Cie., at Chaumont (Haute-Marne).
CONSTRUCTION AND MAINTENANCE OF TANNERIES.
As few architects have specially studied the construction of tanneries, and in most cases much of the arrangement depends on the knowledge of the tanner himself, a short chapter on the subject will not be out of place.
In the selection of a site, a clay or loamy soil is to be preferred to a gravelly or sandy one, as lessening the liability to leakage, and waste of liquor. Perhaps, however, the first consideration of all is the water supply, since for manufacturing purposes town water is generally very expensive. With regard to quality and impurities of water, information may be found on p. 83; but, as a general rule, the softer and purer the supply the better. It is also of great advantage when the source is at such a level as to flow into the tan-yard, or at least into the beam-house, without pumping. Filtration too, when needed, is much facilitated by a sufficient head of water.
Of scarcely less importance than the water supply is the drainage of the yard. It not unfrequently happens that tanneries are prohibited from discharging their refuse liquors, limes, and soaks into rivers and watercourses, and it is sometimes a matter of extreme difficulty to find any other way of getting rid of them. In default of an outlet, recourse must be had to precipitation and filtration, but this is a costly expedient, and in fixing a site for a new yard it is far better to provide against such a possible contingency. Should, however, such means become necessary, it may be borne in mind that limes and liquors in great measure mutually precipitate each other, and that if all the various « 232 » refuse is run into one tank, mixed, and settled, much is accomplished in the direction of purification. The further treatment of the effluent water must be determined by its nature and composition.
The site chosen, the next question is the arrangement of the buildings. It is very doubtful, where ground is not inordinately expensive, whether it is wise to erect drying-sheds over the pits. In case of fire, very serious damage is done to liquor and leather by the heat and burning timber. If the turret form of drier be decided on, strong foundations are required, and the ground-floor or basement is occupied with heating apparatus; and, on the other hand, the tan-house may be easily and cheaply covered with slated roofs, with sections of glass, to the north, if possible, like a weaving-shed, through which sufficient light for convenient work and cleanliness is admitted. The direct rays of the sun should be avoided, but in the writer's opinion the balance of advantage is largely in favour of a liberal supply of light. Iron roofs are unsuitable, since the moisture condenses on, and rusts them; and particles of oxide fall into the liquors, and cause iron-stains.
Good ventilation along the ridge of the roof should be provided, wherever there is any steam or hot liquor used; or the condensed moisture soon leads to decay.
As regards the general plan of the buildings, much depends on local circumstances; but as far as possible, they must be so arranged that the hides and leather work straight forward from one department to another with as little wheeling or carrying as possible; that the buildings where power is used be near to the engine, so as to avoid long transmissions, which are very wasteful of power; and that the different buildings be so isolated as to diminish the risk of the whole being destroyed in case of fire.
As regards the first of these conditions, if the various soaks, limes, bates, and handlers are well arranged, it is hardly necessary to do more than draw the goods from one pit into the next throughout the whole of the process. To, and from « 233 » the layers, the goods must generally be carried or wheeled. In the sheds, if it be a sole-leather tannery, the butts should first come into turrets or open sheds for the rough drying; then into a room sheltered from draughts to temper for striking. The striking machines or beams should be in an adjoining room, or immediately below; then a small shed-space for drying before rolling; next the roller room; and then the warm stove for drying off. If two of these can be provided to be used alternately, it will allow the goods to be aired off without taking down, and they may then be immediately handed or lowered into the warehouse, without fear of over-drying, which is sometimes difficult to avoid where leather must be taken direct out of the hot drying-room.
To fulfil the second condition named, the engine should be at the centre of the main range of buildings, with perhaps the grinding machinery on one side, and the leather-finishing on the other; but this would be rather contrary to the third requirement. A very good plan would be to have the engine-house in the centre as suggested, but separated from the buildings on each side by brick gables; and with the boiler-house behind it, and under a separate roof, say of corrugated iron. Figs. 61, 62, from Eitner's book on American Tanning, show the arrangement of a sole-leather tannery in the United States. If it be impossible to have the engine near its work, it is in most cases better to employ a separate high-pressure engine, which may be within a glass partition, and will work all day with scarcely any attention. The loss of power in carrying steam for moderate distances through sufficiently large and well-clothed pipes is much smaller than that of long lines of shafting. The writer has known cases where fully half the indicated power of the engine was consumed in friction of the engine, shafting, and belts. High-pressure engines are as a rule to be preferred to condensing for tannery use, since the waste steam can generally be employed for heating, and both the first cost and that of maintenance are smaller. Where much fuel is used, it is quite worth « 234 » while to have the cylinders indicated occasionally, both running light, and driving the machinery; much information is gained in this way as to the power spent on the various machines, and very frequently large economy is effected by proper adjustment of the valves. To work economically, an engine should be of ample power for all it has to do; and adjusted to its work, not by lowering the pressure of steam, or by checking it at the throttle-valve, but by setting the slide-valves to cut off as early in the stroke as may be. As to how early this is possible, an indicator-diagram will at once give information. In arranging shafting, moderate speeds, say 100-150 rev. per min., should be chosen for main lines, and when higher speeds are necessary, they should be got up by light and well-balanced counter-shafts, with wrought-iron pulleys. In calculating speeds, it must be remembered that they vary inversely as the size of the pulleys. Thus a 3 ft. pulley running at 100 rev., will drive a 2 ft. one at 150 rev., and a 12 in. one at 300. Of course the higher its speed, the more power any shaft will transmit, but increased friction and wear and tear soon limit this advantage. The velocity of a belt in feet per min. is obtained by multiplying the number of revolutions per « 235 » minute by the girth of the pulley in feet or by its diameter multiplied by 31/7, or more accurately, 3·1416.
Pulleys should always be of ample breadth for the power they have to transmit; and it is more economical both in power and cost, to use broad single belting than the same « 236 » strength in double. If the pulley will not take a belt broad enough for the work it has to do, a second belt may be made to run on the top of the first, and will do its share of the work. Belts should be washed occasionally with soap and tepid water, and oiled with cod-oil; but if of sufficient breadth, should not require the use of rosin, or adhesive materials, to make them grip the pulley. Makers of machines often err in constructing their driving pulleys too small both in breadth and diameter.
The horse-power which a belt is capable of transmitting obviously varies extremely with circumstances, but may be approximately calculated by the formula
| a · v | , |
| 66000 |
where a is the area of contact of the belt with the smallest pulley, and v its velocity in feet per minute. Another rule is, that at a velocity of 1000 ft. per min. each inch of breadth of belt should transmit 21/2 horse-power on metal pulleys, or 5 on wooden ones, on which the adhesion is greater. Adhesion may also be increased by covering the pulleys with leather or india-rubber. Both rules assume that the belt is of ample strength. One horse-power would be transmitted by a belt running 1000 ft. per min. with a pull of 33 lb. A good single belt should not break with a much less strain than 1000 lb. per inch of breadth, and should stand about 1/10 as much as a working strain.
Countershafting and high-speed machinery, such as disintegrators, striking machines of the Priestman type, &c., should run without material jar or vibration. If this occurs, it is generally a sign that the running part is not equally balanced. In this case, the shaft must be taken out of its bearings, and supported on two exactly horizontal straight-edges, when it will roll till the heaviest part is downwards; and weight must be taken off or added till it will lie in any position. In this way, the writer had recently to add fully 2 lb. of iron to the drum of a striking machine before equilibrium was secured, and a most troublesome vibration prevented. Of course all machinery should be supported as solidly as possible; and if circumstances permit, most « 237 » machines are better on a ground-floor. In placing bark mills, however, it is frequently convenient to fix them in the top of a building, so that the ground material may be sent down shoots by its own weight to the required places. An alternative plan is to set the mill on the ground-floor, and to raise the ground material with a bucket-elevator. This may be done successfully by letting the material fall directly from the mill into the buckets; but otherwise it must be thrown in with a shovel, as buckets will not pick up ground bark, even from a hopper; and in any case such elevators are often troublesome. In a grinding plant designed by the writer, the unground material is filled on the basement floor into an iron barrow, which may be wheeled into an iron bow working between upright guide-rails. On pulling a brake-line, the barrow is raised to the top of the building, and its contents are tipped into a large hopper, after which the barrow rights itself, and descends for another load. In the bottom of the hopper is a sliding shover, which forces the material on to vibrating screens, by which it is guided either into a disintegrator, or crusher rolls, at pleasure. Both these discharge through iron spouts into large hoppers on the outside of a brick gable, from which, powdery materials like myrabolanes and valonia, can be run direct into barrows or trucks. It is very desirable that such hoppers should be separated from the main building by a fireproof partition. The writer is glad to say, he does not know of a case of fire from disintegrators grinding tanning materials, but he is informed that a Carter's disintegrator employed in grinding bones in a manure works has repeatedly set fire to the flannel bag into which the dust was allowed to escape. If this were to occur with a dry and dusty tanning material, it is not unlikely that it might result in an explosion such as sometimes happens in flour-mills from a similar cause. On the whole, however, mills of the coffee-mill type are probably more dangerous than disintegrators; since if they become partially choked, the heat caused by friction is very great.
For lubricating purposes, mineral oils of high density are « 238 » not more dangerous than animal or vegetable, but rather the reverse; as, though they are possibly more inflammable, their mixture with cotton-waste and other porous vegetable materials is not spontaneously combustible, while vegetable and animal oils occasionally are. Heavy mineral oils should always be used as cylinder oils in high-pressure engines, in preference to other oils or tallow, since they are not decomposed by steam, and do no harm if blown into the feed-water, but serve to loosen and prevent scale and deposit. Ordinary oils and tallow, on the other hand, when submitted to the action of high-pressure steam, are separated into glycerin and fatty acids (see p. 60), and the latter corrode the valve faces and seatings, and in combination with temporary hardness in the boilers form a very dangerous porous deposit, which often leads to overheating of the tubes.
Next to the machinery, the pits demand special consideration. The chapter on the subject in Mr. Schultz's book on 'Leather Manufacture,' is well worth attentive study as giving American practice on the subject.
The old-fashioned method of sinking pits is to make them of wood, and carefully puddle them round with clay, which should be well worked up before use. Such pits, if made of good pine and kept in constant use, are very durable, some of the original pits at Lowlights Tannery, constructed in 1765, being still in use. Loam mixed with water to the consistence of thin mortar may also be employed, the pits being filled up with water, to keep them steady, at the same rate as the loam is run in. Probably the best materials for pit-sides are the large Yorkshire flagstones. Where these are not attainable, very durable pits may be made of brick, either built with Lias lime, and pointed with Portland cement, or built entirely with the latter. Common lime cannot be used, as it spoils both liquors and leather; and even cements with too large a percentage of lime are unsatisfactory. Brick and common mortar are, however, suitable for lime-pits.
The writer has constructed wooden pits in two ways. In « 239 » the one case, after making the excavation, beams were laid in a well-puddled bed of clay; on these a floor of strong tongued and grooved deals was laid, and on this the pits were constructed of similar wood to the floor, and puddled round with clay. In the second case the pits were built like large boxes above ground, and when finished, lowered on to a bed of clay prepared for them, and then puddled both around and between. It may have been from defective workmanship in the first case, but those made on the last-named plan, which is that adopted from very early times, have certainly proved the tightest and most satisfactory. Mr. Schultz describes a plan as the Buffalo method, in which a floor is laid as just described, and grooves cut with a plane for the reception of the sides, which are formed of perpendicular planks, each end and side being finally tightened up by the insertion of a "wedge plank."
If bricks be used, great care must be taken that the cement is not merely laid so as to fill the joints towards the two surfaces of the wall, as is the habit of modern bricklayers, but actually floated into all the joints so as to make the wall a solid mass; or leaks can hardly be avoided. Cement pits are very good, and, though not particularly cheap in material, which must be of the best, are readily made by intelligent labourers under good supervision. The first step is to lay a level floor of good concrete, in which glazed pipes for emptying the pits may be embedded; care being also taken that all joints in these are thoroughly tight, since future repairs are impossible. The next step is to make frames, the exact length and breadth of the pits required, and perhaps 15 in. deep. These are arranged on the floor where the pits are to be, and the intervening spaces are filled with concrete of perhaps 1 of cement to 3 or 4 of crushed stone or brick. Rough stones and bricks may also be bedded in the concrete as the work goes on, to help to fill up. After the first layer has set, the frames may be raised and a second added, and so on. The work is generally finished by floating over it, while still damp, a little pure cement, to give a smooth surface. « 240 » Before using, the cement should be tried on a small scale, to be sure that it does not discolour leather or liquors, and the pits should always be seasoned with old or cheap liquor before actual use.
If possible, both latches and handler-pits should be provided with plugs and underground pipes, communicating with a liquor-well some feet below their levels. Glazed fire-clay is very suitable both for pipes and plug-holes, which should be in the pit corners. Some means should also be provided for the ready clearing of the pipes when choked with tanning materials. A good plan is to let each line of pipes end in a liquor-well large enough for a man to go down. As it is almost impossible to make plugs fit without occasional leakage, it is not well to run pits with very different strengths of liquors to one well, but the layers, handlers, and different sets of leaches should each have their own, so as to avoid mixture. A good means of clearing pipes consists in a series of iron rods 3-4 ft. long, connected by hooks fitting into double eyes, as shown in Fig. 63. It is obvious that in a narrow pipe or drain, these cannot become disconnected.
It is, as Schultz points out, of questionable advantage to lay wooden troughs for supplying liquor to each pit under the alleys, since it is almost impossible to preserve them from decay; but the same objection would not apply to glazed pipes, well clayed or cemented. A very good and cheap plan in practice, is to let the liquor-pump, or a raised liquor-cistern, discharge into a large and quite horizontal trough raised 5 or 6 feet above the level of the yard, and provided with plug-holes at intervals, under which short troughs may be set to run the liquor into the various pits.
Pl. VIII.
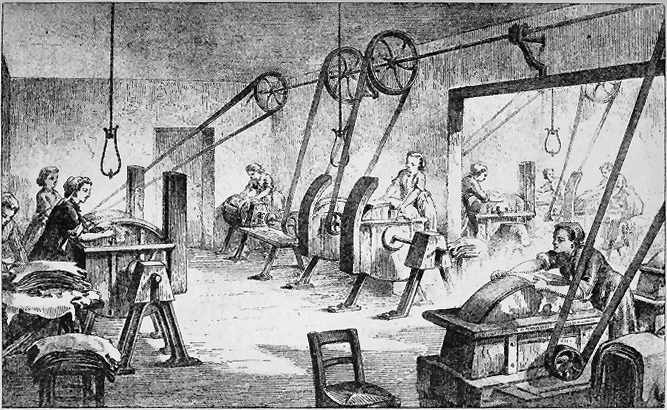
RUBBING-DOWN DYED SKINS.
In tan-yard construction, iron should, as far as possible, be avoided wherever it can come into contact with liquor, as it discolours the leather. In default of underground pipes, india-rubber suction hose may be employed. Direct-acting steam pumps without fly-wheels are not suitable for tanneries, as they "hammer" when the pit is nearly sucked up. Steam-jet elevators and the pulsometer are very useful for some purposes, but slightly warm, and dilute the liquors with condensed steam.
Much that has been said about pits applies also to leaches. They may be constructed either of wood, or brick and cement, and where heat is employed the latter is the better. They are also to be provided with plugs and pipes leading to a liquor-well. About 6 in. from the bottom of the pit is a false bottom B made of boards, perforated with holes or set a little distance apart; and in the corner is an "eye" C (Ger. Pfaff) consisting of 2 boards set at right angles, so as to preserve a vertical channel communicating with the space under the false bottom. This serves, in pits provided with pipes, for the insertion of the plug; and where this is absent, for that of a suction hose to pump off the liquor. In the American Press-leck System, the eye of one pit communicates by a horizontal spout with the top of the next (see D, Fig. 64). The Allen and Warren Sprinkler Leck (Fig. 65) has very much superseded this arrangement in America, though it is doubtful if it spends the bark so completely. The round tubs, however, have several advantages and « 242 » may well be used for many purposes in English yards. Their construction is described in some detail in Mr. Schultz's book above cited. Some details will also be found on p. 209 of the 'Manufacture of Leather' by Davis. The rule for finding the capacity of a round tub with perpendicular sides in cubic feet is to square the diameter and multiply by ·7854, and by the depth in feet; or roughly, to square half the diameter and multiply by the depth and by 31/7.
Leaches and liquors are generally heated by blowing in steam direct. In this case, the condensed water mixes with the liquor, and in heating a liquor to boiling point it may be taken that about 20 per cent. of water will be thus added. Where strong liquors are to be heated, it is therefore obviously much better to pass the steam into a closed copper coil in the liquor. Such a coil, with steam at 30 lb. pressure, will heat about 271/2 gal. per hour per square foot of surface from 46° F. to boiling, and evaporate about half that quantity of liquor already at boiling temperature. (See Box, 'Treatise on Heat,' p. 176.) Heating coils must of course be provided with steam traps to carry off condensed water; and in boiling by open steam it is very desirable to let the steam pass through such a trap before use, to stop water condensed in the pipes, which usually contains iron, and discolours the liquors.
DRYING-SHEDS FOR LEATHER.
The primitive way of drying leather was to hang it on poles in the open air, but this in our uncertain climate has become quite obsolete. The oldest plan now actually in use is to hang on poles in a shed generally raised some height above the ground, so as to catch the wind, and provided on all sides with louvre boards arranged so as to open and shut as required. These sheds, to give good results (especially on mixed tannages, which need much more care in drying than bark), demand very watchful management. In windy weather, and with wet leather at all times, the louvres must be kept nearly or quite closed, and on the sunny side of the shed the same precaution is generally necessary. Again, in very damp weather the leather does not dry at all, and in frosty seasons it is apt to freeze, by which sole leather is made soft and spongy, and dressing leather, though whitened, is said to be less capable of carrying grease. To prevent freezing, and to enable leather to be dried in damp or cold weather, it became customary to provide sheds with ranges of steam-pipes on the floor; this, though decidedly a valuable addition, has not proved by any means an entirely satisfactory solution of the problem of leather drying. No sufficient means are provided for controlling the ventilation, and the upward currents of hot air dry the leather irregularly, and produce bad colour. A much more satisfactory shed is the American turret drier.
This consists of a lofty building, 3 to 8 stories high, without louvres, but with latticed floors. J. S. Schultz recommends 5 stories, of 7 ft. clear between beams, as a convenient height, and the building should be divided by partitions « 244 » from top to bottom into 4 or more series of chambers one above another, each of which is capable of having the heat and ventilation separately regulated. The Americans usually fill one of these series at once, and dry off the whole in about 10 days, so that as many will be required for a tannery as will hold a 10 days' production. For ventilation, each of these sets of chambers is provided with a lantern ventilator at the top for the exit, and shutters or dampers on the bottom floor for the admission of air. The bottom floor is also provided with steam-pipes, of which those for each set of compartments are controlled by a separate cock. When warmth is applied at the bottom, the tall building acts like a chimney, and a continuous current of air passes from the ventilators at the base up to those at the top. The usual American practice is, after filling one of these ranges of compartments, to apply no steam-heat for the first 3 or 4 days, and, if the weather be dry or windy to keep the ventilators also closed. After the third or fourth day, a moderate degree of heat is given, and this is increased so that at the end of about 10 days the stock is fully dry.
This is in accordance with a common American practice, in which the leather is fully dried before rolling, in order to fix the soluble colour, and prevent it striking out to the surface in the finishing. The wet leather is raised by an elevator, consisting of an endless chain provided with hooks, to which the leather is attached at the bottom, and from which it is taken at the top. Various ways are adopted to lower the leather from these tall turrets to the room where it is stored prior to damping down for rolling. In some cases, the lattice floors are made movable, and the whole contents of the room, including the sticks from which the leather is hung, are allowed to fall into the lowest room. This method is of very questionable advantage, if we take into account the labour of separating the sticks and carrying them back to their places. Another plan is to have shoots from each loft, down which the sides are slid to the rolling-room. The floors should have what light is necessary supplied « 245 » through glass windows, so arranged as not to admit direct sunlight.
To adapt the turret drier for English requirements, some slight modification is needed, since we do not dry our leather right off, and then damp back, but, when it is suitably dry, lay it in a pile to "sammy" for striking; then, perhaps, after striking, hang up again for a short time to temper for rolling, possibly again between rollings, and finally to dry off at a temperature of, say, 68-77° F. (20-25° C.). Perhaps on this account, the writer has seen no complete turret-driers in use in England, though a portion of one of the large sheds at Dartford belonging to Messrs. Hepburn was converted by them some years since into a very good turret, which gave excellent results both for sole leather and kip butts in stuff. This turret is represented in section in Fig. 66, and is about 56 ft. × 24 ft. in area, and 50 ft. high from the ground-line to the top of the roof, which is ventilated by a dormer, a, with fixed louvres at the top, while air is admitted at the bottom through ventilators with sliding flaps, b b. It is heated by 10 rows of 4-in. steam-pipe, c c, each 54 ft. long, making a total of 540 ft. run, or about 640 ft. superficial (a 4-in. pipe being about 45/8 in. diameter outside). I am informed by Mr. J. G. Hepburn that he considers 4-in. pipes inferior for the purpose to smaller ones, giving too much heat in one place, and without sufficiently distributing it, and were he constructing a new turret he would replace them by 11/2 in. wrought-iron, using about 3 of 11/2 in. to replace 2 of 4 in., small pipes being much more effective (as will be seen by table, p. 250) than larger ones, in proportion to their surface. He considers, however, that the best way of heating drying-sheds, though more expensive in first cost, is by means of hot water, which is much more constant in temperature than steam. Mr. Hepburn, to whom I am much indebted for the above information, informs me that the turret still acts very well, drying kip butts on the upper floor a good colour in all weathers in about a week. He finds, « 246 » however, that the steam-pipes as described are hardly sufficient in very cold weather, and intends to increase them, or replace with 1300-1400 ft. of hot water pipe heated by a saddle boiler. At Lowlights tannery, a shed arranged on the turret principle (though much less completely carried out from want of height in the buildings) has been for many years in operation, principally for drying off sole-leather, with the most satisfactory results.
It is noted by Box ('Practical Treatise on Heat,' p. 166) that an exit for the moist air should not be placed at the top of a drying-chamber, but at the bottom, since in the first case, the hot dry air tends to rise at once to the opening, and pass away unsaturated with moisture, while that cooled by evaporating water from the goods, being heavier, tends to form downward currents and remain in the chamber. To this it may be objected that aqueous vapour is much lighter « 247 » than air; this is true, other things being equal, but in practice the evaporation of a given quantity of water cools the air and makes it heavier in a materially greater degree than the admixture of aqueous vapour lightens it. This source of waste of heat exists in the turret drier, but is there, from its great height, reduced to a minimum. In lower sheds it becomes very material, and the air currents formed are productive of much harm by causing irregular drying. This difficulty has been met by Mr. Edward Wilson, of Exeter, to whom the leather trade owes several very useful inventions, by an ingenious drying-room constructed on the lines indicated by Box, though I do not know that he was in any way indebted to that writer for the idea. In this Mr. Wilson arranges the steam-pipes, instead of on the floor, in a vertical compartment partitioned from the chamber, through which air is admitted and heated. This hot air fills the top of the chamber and from its lightness floats in a horizontal layer, only descending and escaping by apertures in the floor as it becomes cooled by evaporating the moisture of the hides. Mr. Wilson states that the method answers well in practice, and it is certainly the most scientific in conception, but it might be feared that, as applied to a single floor, the upper parts of the butts, suspended near the ceiling, would dry more rapidly than those near the floor. If applied to a double-floored building, this disadvantage would, from the stronger draught, and consequent larger supply of air, be less likely to show itself, and the upper floor with its uniform warm air would be well adapted for drying off finished sole-leather, while the cooler and milder drying of the ground floor would be fitted both in character and situation for that wet out of the yard. Special precaution would be needed to prevent the heated air escaping by doors opening into the upper floor. There is little doubt that as regards heat this is the most economical system which has yet been invented.
A method has been introduced in the United States of drying wet and finished leather all together, in drying-rooms heated to a considerable temperature, and closely shut up. « 248 » This is found to answer fairly on leather from sour liquors, but that from strong and sweet liquors is darkened, as might be expected. The drying is accomplished in much shorter time than by the turret drier. The mixture of wet and dry leather, and the lack of ventilation produce an atmosphere nearly saturated with moisture, and hence the drying is not nearly so harsh as might be supposed from the considerable temperatures made use of. There does not, however, seem anything in the principle to recommend its general adoption.
Another invention, of which we have as yet heard little definite in England, consists in drying at a low temperature by air artificially deprived of its moisture. This may be accomplished in several ways. Experiments have been made in drying in a closed chamber provided with trays of calcium chloride to absorb the moisture evaporated. Air when artificially cooled by compression and subsequent expansion, as in the case of ice-making machines, parts with a large portion of its moisture, which is condensed in the form of ice in the tubes of the machine. Such air, if subsequently warmed, would dry powerfully and rapidly.
Before leaving the subject of drying-sheds, a few words on the mechanics of drying in general may not be out of place. Air-drying is dependent on the condition that the air must be capable of taking up more moisture than it already contains. It is a matter of common experience that there are warm days when the air is so saturated with moisture in the form of invisible vapour, that scarcely any drying takes place; and similarly, cool dry days, when leather dries rapidly. The relative amount of moisture in the air is easily ascertained by the simple instrument known as the wet and dry bulb hygrometer; an instrument which ought to be in every drying-shed, especially where steam heat is used. It consists of two similar thermometers, side by side, of which one has the bulb covered with muslin and kept wet by a piece of lamp-cotton attached to it, and dipping in a cup or bottle of water. This water evaporates more or less rapidly, according to the dryness of the air; and as heat is « 249 » consumed by it in passing into the gaseous condition, the wet thermometer falls more or less below the dry in proportion to the rapidity of the evaporation. On a summer's day, the difference may amount to 9°-12° F. (5°-7° C.), and this is about the extreme dryness permissible in a drying-room for finished leather. Wet leather should of course be dried much more slowly. The influence of heat on drying is two-fold. It increases the capacity of the air for moisture, and it replaces the heat consumed by evaporation. The following tables give the capacity of air for moisture at different temperatures, and the percentage of saturation as shown by the wet and dry thermometer. At Greenwich, the mean humidity for the year is 82 per cent.; or for the day-time only 76 per cent., varying from 62 in summer to 86 in winter:—
Table I.—Capacity of Air for Moisture.
| Temp. Fahr. | Weight in Pounds of a Cub. Ft. of Dry Air. |
Weight in Pounds of Moisture contained in a Cub. Ft. of Saturated Air. |
| 32° | ·0807 | ·000304 |
| 42 | ·0791 | ·000440 |
| 52 | ·0776 | ·000627 |
| 62 | ·0761 | ·000881 |
| 72 | ·0747 | ·001221 |
| 82 | ·0733 | ·001667 |
| 92 | ·0720 | ·002250 |
| 102 | ·0707 | ·002997 |
Table II.—Hygrometer Table.
| Temperature of Air. |
Degrees between Wet and Dry Thermometers. | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 32° F. | 87 | 75 | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. |
| 42 | 92 | 85 | 78 | 72 | 66 | 60 | 54 | 49 | 44 | 40 | 36 | 33 |
| 62 | 94 | 88 | 82 | 77 | 72 | 67 | 62 | 58 | 54 | 50 | 47 | 44 |
| 82 | 95 | 90 | 85 | 80 | 76 | 72 | 68 | 64 | 60 | 57 | 54 | 51 |
| Per cent. of moisture, saturation being 100. | ||||||||||||
As regards the heat consumed in evaporation; it requires about 1000 times as much heat to convert 1 lb. of water into vapour, as it does to raise the temperature of the same quantity 1° F. At least as much heat as this must be supplied if the air which has been used in drying is to retain the same temperature it had at the outset, and therefore if a turret is to keep at a higher temperature than the air, which is necessary to create a draught, this is the minimum amount of heat which must be supplied per pound of water to be evaporated. In practice much more will be needed.
The following table shows the heat given out by different sizes of pipes at different temperatures, and steam pressures, in units equal to the heat required to raise 1 lb. of water 1° F., and the cubic feet of air which they will heat.[V]
[V] To illustrate the use of such tables, the following example may be given. To dry 100 butts in a turret, each containing 20 lb. of moisture, at least 20 × 1000 × 100 = 2,000,000 units of heat will be required to replace the loss by evaporation alone. As a 4-in. pipe at 300° gives off 669 units per foot per hour (see Table III.), about 125 ft. would give off 2,000,000 units per day. If we compare this with Mr. Hepburn's practical experience, supposing the 4 working floors of his turret to hold 100 butts each (a low estimate), and to dry in 10 days; we have 540 ft. for 40 butts or 1350 ft. for 100 butts a day; showing that more than 10 times the minimum is required in practice. Of course this allows for weather in which the air must be heated considerably before it will dry at all, for heat that escapes uselessly at the top and sides of the building, and for the fact that the pipes are not heated the whole time, and probably, on the average, to a much lower temperature.
Table III.—Heating Effect of Pipes freely exposed to Air at 60° F.
| Temp. of Pipe. | Pressure of Steam per In. |
Units of Heat per Ft.-run of Pipe per Hour. |
Cub. Ft. of Air at 60° F. (151/2° C.) heated 1° per Ft.-run of Pipe per Hour. |
||||||
| 2 in. | 3 in. | 4 in. | 6 in. | 2 in. | 3 in. | 4 in. | 6 in. | ||
| ° F. | lb. | ||||||||
| 300 | 53 | 403 | 545 | 669 | 938 | 22235 | 28713 | 36919 | 51760 |
| 280 | 35 | 355 | 480 | 587 | 825 | 19582 | 26490 | 32387 | 45521 |
| 260 | 21 | 312 | 421 | 515 | 723 | 17218 | 23233 | 28421 | 39952 |
| 240 | 10 | 271 | 366 | 448 | 627 | 14946 | 20199 | 24717 | 34594 |
| 220 | 2·5 | 233 | 313 | 384 | 537 | 12858 | 17271 | 21184 | 29629 |
| 200 | .. | 195 | 263 | 322 | 452 | 10775 | 14507 | 17780 | 24967 |
| 180 | .. | 160 | 216 | 264 | 369 | 8830 | 11920 | 14573 | 20368 |
| 160 | .. | 128 | 172 | 210 | 295 | 7070 | 9487 | 11590 | 16300 |
It may be taken that 1/20 of the above volumes may be heated 20°, from 50° F. to 70° F., and so on; but if the average temperature is higher than 60° F., the duty will be less, and to obtain the same effect the pipe must be heated so much hotter as to keep the same difference as before between the pipe and air. Thus a pipe at 300° F. will only heat as much air at 80° F. as one of 280° F. will of air at 60° F.
It will be noted that the efficiency of small pipes is much greater than that of larger ones, and in these days of high-pressure steam, much may be said in favour of the use of comparatively small wrought-iron steam-pipes instead of the larger cast metal ones. The first cost is small, the pipes are easily obtained ready screwed, and in the lengths required, and may be put together by any intelligent workman. The risk of fracture by the concussion of condensed water is very trifling, as compared to that of metal, and much lighter pipes are safe for high pressures. Steam-pipes must always be laid with an incline of say 1 in. in 10 ft. from the end where the steam is admitted, so that the condensed water may get away, and at the lowest point a steam-trap must be provided for its escape. In the writer's experience, the best form is that of Holman, made by Tangye of Birmingham, of which the principle will readily be understood from Fig. 67. The cup-shaped vessel a floats on the water in the outer casing, and so closes the valve b until a gets full, when it sinks and allows the water to escape until it floats up again. It is important that this trap should be set level, or the valve « 252 » will not close properly. Each pound of condensed water is equivalent to about 1000 units of heat given off (see Table III.). In planning steam-pipes, it is not necessary that they should be arranged in a single line. Even if in gridiron form the steam will still reach every part, in proportion to the condensation which takes place. A series of large pipes may be supplied by small pipes from a common main, and discharge their condensed water into a common waste-pipe with branch from each. A 1/2-in. pipe from a high-pressure boiler will supply a considerable range, say 100 ft. of 4-in. pipe, though a larger size is advisable. At the farther end of a range of steam-pipes a small tap must be provided to let out the air which accumulates in them. In employing the exhaust steam of an engine for heating purposes, the pipes must be of ample size and freely open at the ends to avoid back-pressure. For this purpose the gridiron form is a very good one.
The planning of hot-water pipes is much more difficult than that of steam-pipes, but the general principle is that the pipes must rise all the way from the boiler to the farther end, where there must be an expansion-box or supply-cistern to allow the water to rise and fall and dissolved air to escape. From this the pipes must fall more or less, throughout the distance, back to the boiler, entering it at the bottom. If at any point the pipe has to fall, leaving an upward bend, a tap must be provided for the escape of air, but such upward bends are a fertile source of difficulty and failure of action. With long runs of either steam or water pipes, arrangements must be made to allow of expansion and contraction, which will amount to 1-2 in. per 100 ft., according to the temperature employed. If one end of the system can be left free, all that is needed is to support the pipes on rollers (pieces of old pipe may be used); if not, stuffing-boxes must be provided.
The air heated by boilers, and other sources of waste heat, may often be utilised for heating purposes, but generally requires to be driven by a fan, unless the drying-room can be arranged directly above the source of heat. If air has to « 253 » be conveyed, the air-ways must be of ample size, and if the ascending force of heated air be relied on, passages less than 2 ft. sq. are seldom of much use. This ascending force is generally much overrated where the differences of temperature are so small as those employed in a drying-room. In a boiler chimney, where the temperature of the escaping gases is 552° F. (289° C.), the specific gravity of the air is about half that outside, and a chimney of 50 ft. in height gives a draught equal to the pressure of a column of about 1/3 in. of water, and the hot gases theoretically have a velocity of about 80 ft. per second; whereas the same chimney with a difference of temperature of 30° F. would have a draught equal to 1/300 in. of water only, and a velocity of 8 ft. per second.
The following table will enable the reader to calculate approximately loss in friction in air-passages and the pressure required to pass a given volume of air. The pressure needed increases in proportion to the length of the pipe and the square of the velocity of the current of air to be passed. Thus if we double the length of the pipe we must double the pressure to pass the same quantity; and in order to double the quantity of air through a given pipe, the pressure must be quadrupled.
Table IV.
Head, or Difference of Pressure at the two ends of a Circular Pipe 1 yd. long in inches of water required to pass 1000 cub. ft. of air per minute.
| Velocity in Ft. per Sec. | Diameter of Pipe. | Head. | ||
| in. | ||||
| 84·8 | 6 | ·186 | To pass 100 ft. per min. these figures must be divided by 100. To pass 10,000 ft. they must be multiplied by 100. | |
| 37·7 | 9 | ·02442 | ||
| 21·2 | 12 | ·00579 | ||
| 9·4 | 18 | ·000763 | ||
| 5·3 | 24 | ·000181 | ||
| 3·4 | 30 | ·0000593 | ||
To calculate the head required for a long pipe, multiply the head given by the table by the length in yards. The air « 254 » passed by square pipes of the same diameters will be 1·273 times greater with the same heads.
To be added to the pressure required to overcome friction is that needed to force the air out at the end of the pipe. This varies with the shape of the tube, &c., but for our purpose may be taken as given in the following table:—
Table V.
Approximate Pressure needed to force Air out of a Pipe with a Velocity of—
| Ft. per Sec. | Head in Inches, Water. |
|
| 84·8 | 1·8 | |
| 37·7 | 0·36 | |
| 21·2 | 0·13 | |
| 9·4 | 0·02 | |
| 5·3 | under | 0·01 |
| 3·4 | under | 0·005 |
Air-passages should be, as far as practicable, of uniform area throughout their length, as much velocity is lost in passing even from a smaller to a larger tube. Of course sharp bends must be avoided.
COMMERCE, STATISTICS, AND BIBLIOGRAPHY.
Skins.—The trade in skins possesses no small importance. Many of the statistics relating to skins are collective, and not specific; these will be grouped under the heads of the respective countries, after all accessible details have been given upon each kind of skin.
Alligator.—In the Southern United States, notably Florida, the supply of alligator-skins amounts to many thousands annually, and the "farming" of the reptiles for their skins is even spoken of. The principal market for them is Europe, but no statistics of the trade are published. The alligators often attain a length of 18-20 ft. The hides are stripped off, and the belly and sides, the only portions fit for use, are packed in barrels in a strong brine, and shipped to the Northern tanner, who keeps them under treatment for 6-8 months, when they are ready to be cut up. So far the leather has been principally used in the manufacture of boots and shoes, for which it is especially adapted.
Armadillo.—The skins of this animal were exported from Brunei (Borneo) to Singapore to the value of 121 dol. (of 4s. 2d.) in 1879.
Ass.—Hankow exported 24021/2 piculs (of 1331/2 lb.) of asses' skins in 1878, and 1068 piculs in 1879.
Buffalo.—Manilla (Philippines), in 1878, exported 379 tons of buffalo-skins, value 12,130l., and 274 tons of cuttings, 6579l. Hankow exported 1091 piculs in 1878, and 1238 in 1879. Brunei (Borneo) sent 1362 dol. (of 4s. 2d.) worth to Singapore in 1879. The approximate London market values of buffalo-skins are:—Batavia, 4d.-7d. a lb.; Bengal, 3d.-6d.; other sorts, 21/2d.-61/2d.
Calf.—Hamburg exported to Great Britain of calf and other skins in 1876, 20,731 cwt.; in 1877, 27,550; in 1878, 14,583; and in 1879, 19,287 cwt. The Hawaiian Islands sent 168 pieces to Germany in 1879. Christiania shipped 31,000 kroner (of 1s. 11/2d.) worth to Great Britain in 1878, and 300 kr. in 1879. The exports from Archangel (including seal) in 1878 were 335 pieces to Holland, and 23,108 to Germany: total value, 2343l. Honolulu, in 1878, exported 651 pieces, being 500 to Germany, 135 to China, and 16 to the United States. Memel, in 1879, sent landwise over the Russian frontier for German markets, 34,400 pieces, value 5450l. The approximate London market value of calf-skins is 15d.-34d. a lb.
Deer.—San José (Costa Rica) exported 12,121 lb. in 1878. Kiungchow (China) exported 17,544 pieces, value 541l., in 1879. Ciudad Bolivar (Venezuela), in 1879, sent 77,305 pieces (168,1761/2 lb.) to New York, and 14,695 pieces to Germany. Guatemala, in 1879, exported 2353 pieces to Germany, 693 to New York, and 100 to Belize. Panama shipped 765l. worth of deer and other skins to the United States in 1879. Costa Rica exported 82,168 lb. in the year ended April 30, 1879. Puerto Cabello (Venezuela), in 1879, shipped 2466 kilo. (of 2·2 lb.) to Great Britain, 11,619 to Germany, 6182 to the United States, and 1281 to Holland. The Commercial Society of Mozambique sold 41 deer, 391 buck, 2168 blesbok, and 3071 other antelope skins at Rotterdam in June 1876. The approximate London market values of deer-skins are: Blesbok, Cape, 6-17d. a lb.; Deer, East Indian, 22-50s. a doz.
Dog.—Dog-skin makes a nice, thin, tough leather, but most of the gloves sold as dog-skin are made of lamb-skin.
Dugong and Manatee.—The skins of these animals, more important perhaps as oil-yielders, are smooth, bluish-black in colour, and nearly 1 in. thick. They are well adapted for machine-belting. About 50 are shipped annually from Queensland.
Fish.—Although the skins of fish are chiefly gelatinous, « 257 » and easily soluble in water, some are of a firm, strong texture, and of a useful character. Up to within a few years, however, their employment for practical purposes has been rather limited, and it is only comparatively recently that attention has been more generally directed to their utilisation on an extended scale. At a Maritime Exhibition held at the Westminster Aquarium in 1876, a Norway exhibitor showed a variety of tanned fish-skins, among which were:—Tanned whale-skins; upper leather, made from the white whale, the source of the so-called porpoise hide used for laces; skins of flatfish, prepared for gloves; skins of soles, tanned and dressed for purses; skins of thornbacks, prepared as a substitute for sandpaper; and skins of eels, dressed and dyed, suitable for braces, &c. Shoes have been made at Gloucester, Mass., from the skins of the cusk or torsk (Brosmus vulgaris), the use of which has been patented, and an industry is said to be carried on at Colborn, Canada, with the skins of species of siluroids for glove making. In Egypt, fish-skins from the Red Sea are used for soles of shoes. The skin of the losh or burbot (Lota maculata) is used by the people in many parts of Russia and Siberia to trim their dresses. It is also utilised by some of the Tartar tribes as material for their summer dresses, and the bags in which they pack their animal skins. The spiny and tuberculous skins of many sharks and allied fishes are largely employed, under various trade names, for polishing woods, and for covering boxes, cases, &c. From a certain portion of the skin of the angel shark (Squalina angelus) the Turks make the most beautiful sea-green watch cases. Turners, ebonists, and carpenters in Europe use the rough skin of the blue dog-fish (Squalus glaucus), like emery paper, for smoothing their work and preparing it for polishing. This shark-skin is also made into shagreen. That most used at present appears to be the skin of the ray (Hypolophus sephen), which is very common on the Malabar coast. The house of Giraudon, Paris, makes excellent use of them for morocco and tabletterie. At the recent Paris Exhibition this establishment exhibited numerous illustrations of the ornamental « 258 » application of the prepared skin in large office-table inkstands, candlesticks, boxes and caskets, paper-knives, reticules, card-cases, photograph frames, bracelets, scent bottles, &c. The fish called chat (Squalus catulus) at Marseilles is smaller than the angel fish, and furnishes a product known as peau de rousette. This skin is reddish, and without spots, and of a uniform grain, flat, and only used to make cases and other articles known as shagreen. Peau de chien de mer is another name given to some species of Squalus. That found on the French coasts is known under the names of chien marin, rousette tigrée, &c. Turners, cabinet makers, and carpenters use the skin for scraping and smoothing their work, and it is also used for like purposes by metal workers. This skin, when worked up with the tubercles with which it is studded, takes the name of galuchat, and is usually dyed green, to cover cases, sheaths, and boxes. Under the name of chagrin, these skins used to be much employed in Turkey, Syria, Tunis, and Tripoli—that made in Tripoli being considered the best. It was coloured black, green, white, and red. France imported 18,000 lb. of ray-skins in 1863, chiefly from Portugal.
Goat and Kid.—Our imports of undressed goat-skins in 1883 were:—From Russia 18,355, 2523l.; Sweden 1296, 229l.; Norway 19,391, 3316l.; Denmark 11,012, 1856l.; Germany 52,571, 5856l.; Holland 13,336, 1858l.; Belgium 40,518, 4632l.; France 81,798, 14,121l.; Italy, 5708, 987l.; Austrian territories 37,827, 3844l.; Turkey 38,166, 4580l.; Egypt 16,228, 933l.; British Possessions in South Africa 1,176,535, 139,632l.; Aden 39,800, 4797l.; British India: Bombay 122,242, 10,487l.; Madras 169,642, 17,895l.; Bengal 2,568,526, 203,256l.; China 93,738, 5864l.; Australasia 44,340, 5518l.; United States of America 6822, 845l.; Chile 16,756, 2553l.; Brazil 159,949, 16,189l.; Argentine Republic 12,000, 952l.; other countries 3239, 229l.; total 4,749,795, 452,952l. Ciudad Bolivar (Venezuela) sent 317 pieces (284 lb.) to New York in 1879. Tripoli exported 7000l. worth in 1879, and 3000l. in 1880. In 1880, a number of raw goat-skins were sent from « 259 » the Marche and Romagna to the United States, weighing about 11/2 kilo. (of 2·2 lb.) each, and to be used chiefly for ladies' shoes and pocket-books. Shanghai, in 1878, exported 164,285 pieces. Tangier, in 1879, sent 12 cwt., 60l., to Great Britain; 3637 cwt., 18,185l., to France and Algiers; 10 cwt., 50l., to Spain; total, 14,636 doz., 18,295l.; and 3046 cwt., 13,707l., in 1880. The Hawaiian Islands, in 1879, shipped 24,940 pieces to the United States (Pacific ports). In 1879, Christiania exported 65,000 kroner (of 1s. 11/2d.) worth of goat and sheep skins to Great Britain. The shipments of goat and kid skins from the French East Indies fell from 5500 in 1876, to 4894 in 1877, and 300 in 1879, with none since. The shipments from the Cape to Great Britain were 794,637 in 1878, 657,509 in 1879, and 934,810 in 1880. Cadiz, in 1877, sent 404 kilo. (of 2·2 lb.) of kid skins, value 84l., to Great Britain, and 3866 kilo. 805l., to France. Puerto Cabello (Venezuela), in 1879, despatched 28,684 kilo. to Germany, 124,964 to the United States, 14,295 to France, and 18,536 to Holland. Honolulu sent 64,525 pieces to the United States in 1878. Samsoun (Turkey) exported 130,700 kilo., 6796l., to France in 1878. The Cape exports fell from 1,478,761 pieces in 1874, to 687,570 in 1879. Memel sent by sea 7 cwt., 73l., in 1879. Tientsin (China) exported 38,107 piculs (of 1331/3 lb.) in 1879. Mogador (Morocco) forwarded 112,974 doz., 59,243l., to Marseilles in 1878, and 8407 bales, 48,000l., in 1880; these skins are used for the manufacture of morocco leather, for which they are peculiarly suitable, owing to their fineness of grain, caused, it is said, by the rich diet, consisting of the fruits of the argan tree. The approximate London market values of goat-skins are:—East Indian, 4-15d. a lb.; best tanned, 2s. 4d.-3s. 8d.; inferior to good tanned, 9d.-2s. 5d.; Cape, best, 11-18d.; Cape, inferior to good, 8-14d. Turkey is one of the largest rearers of goats, and consequently the manufacture of morocco leather is extensively carried on in that country. Formerly, nearly all the buck-skins found their way to London, but they were displaced by Indian goat-skins; « 260 » and, for a time, the exportation of Turkish buck-skins experienced a check, the result being the establishment of a large number of manufactories in Vienna and the different Austro-Hungarian provinces. These establishments have prospered and been enlarged, and get the major portion of their goat-skins from the London market. It is, however, proposed in Austria to do without the London market in future, and to institute at Trieste periodical sales of goat-skins, which will be, especially for Vienna, of great advantage from the point of view of cost of transport. Notwithstanding the exportation of buck-, goat-, and sheep-skins from Turkey, there are still sufficient remaining in the country to form the basis of a very flourishing and entirely indigenous industry. The Turk is very unskilful in the manufacture of sole leather; but the article in which he excels is morocco leather for slippers, tanned exclusively with sumach. The production of tanned buck-skins reaches yearly a total of nearly a million skins, and of sheep half a million; the best kinds are those of Philippopolis, Samakof, and Peristra. The Bulgarian skins are not so well tanned as those mentioned, although the quality of the raw skins is superior. The best at the present day are those of Sophia.
Horse.—Shanghai exported 4581/2 piculs in 1878. Rio Grande do Sul exported 10,714 pieces salted, and 601 dried, in 1879. The approximate London market values of horse-hides are:—English, 9-14d. a lb.; River Plate, 6-21s. a hide.
Kangaroo.—The skins of this animal are largely exported from Australia and Tasmania, forming some of the most pliable leather known. To prepare them for market, they should be carefully taken off, pegged out, and dried slowly in the shade.
Lamb.—The exports from Asterabad (Persia) viâ Gez in 1879 were 788 bales Bokharan, 60,613l. Calamata and Messenia (Greece) produced in 1880, 137,500 lb., 2680l. Dedeagatch (Turkey), in 1878, exported 500 bales of lamb- and kid-skins, value 4000l. The exports from Ancona (Italy), including kid and rabbit, in 1878, were 609,826 kilo. (of « 261 » 2·2 lb.) to Italy, 41,480 to Austria, 2714 to Germany, 2655 to Greece, 19,486 to England, 3180 to Turkey; total, 679 tons, 50,321l. Tientsin (China), in 1879, shipped 35,008 piculs (of 1331/3 lb.).
Llama.—The skin of the llama is growing in importance in Parisian shoemaking. It weighs on an average 6 lb., and contains 18 sq. ft. of leather, costing about 1l. The source of supply is the Peruvian Andes.
Ox and Cow.—Coquimbo (Chili) exported 4709 ox-hides in 1879. Santos (Brazil) in the year ending Sept. 30, 1879, exported 316,940 kilo. salted, valued 5800l., and 1282, 25l. The shipments from Christiania to Great Britain fell from 47,500 kroner (of 1s. 11/2d.) worth in 1877, to 3500 kr. in 1879. San José (Costa Rica) despatched 449,870 lb. in 1878. The exports from the Cape, including cow, fell from 150,875 pieces in 1878, to 104,281 in 1879. Rio Grande do Sul, in 1879, shipped 455,315 pieces salted, and 499,960 dried. Of cow-hides, Hankow exported 35,265 piculs (of 1331/3 lb.) in 1878, and 21,063 in 1879. The Kiungchow exports (including buffalo) in 1879 were 490 piculs, 818l. From Shanghai (including buffalo) went 26,070 piculs in 1879. Chinkiang fell from 7262 piculs in 1877, to 3974 in 1878, and none in 1879. Memel, in 1879, sent away by sea, 75 cwt., 136l.; and over the Russian frontier for German markets, 3000 pieces, 3000l. The approximate London market values of ox and cow hides are:—Buenos Ayres and Monte Video, 1st dry, 9-101/2d. a lb.; 2nd dry, 7-81/2d.; best light, 8-91/2d.; salted, 51/4-73/4d.; Brazil, dry, 7-101/2d.; dry salted, 41/2-9d.; West Indies, salted, 31/2-7d.; United States, salted, 31/2-61/2d.; East India, best, 4-13d.; 2nd, 13/4-111/4d.; 3rd and 4th, 11/4-9d.; Australian, salted, 23/4-6d.; Cape, wet salted, 21/2-71/2d.; Continental, salted, 33/4-5d.; English, 23/4-7d.
Seal.—Our imports of undressed seal-skins in 1883 were:—From Norway 112,809, 35,267l.; Denmark 866, 138l.; Germany 28,669, 8428l.; Channel Islands 1048, 305l.; France 2798, 2002l.; British Possessions in South Africa 7020, 5635l.; British India: Bombay 830, 1850l.; China « 262 » 2083, 4000l.; Japan 11,943, 17,369l.; Australasia 1487, 890l.; British North America 341,778, 88,413l.; United States of America 98,566, 256,018l.; Central America 563, 563l.; Chile 1974, 1803l.; Uruguay 13,950, 4884l.; Whale Fisheries: Northern 44,474, 15,208l.; Other Countries 426, 253l. Total, 671,284, 443,026l. The exports from Christiania in 1879 were 74,090 pieces; to Great Britain, the value was 254,400 kroner (of 131/2d.) in 1878, and 172,900 kr. in 1879. Our total imports from Norway rose from 29,912 pieces in 1877, to 63,540 in 1878, and receded to 54,005 in 1880. From the Cape, they were 11,065 in 1877, 15,128 in 1879, and 7731 in 1880. And from Newfoundland, 413,057 in 1879, and 253,656 in 1880. The approximate London market values of seal-skins (not fur seals) are 1s. 9d.-10s. 6d. each for Newfoundland, and 2-11s. for Greenland.
Sheep.—Our imports of undressed sheep-skins in 1883 were:—From Russia 7374, 820l.; Sweden 16,780, 1446l.; Norway 23,756, 2469l.; Denmark 80,226, 7803l.; Germany 126,867, 12,476l.; Holland 34,213, 4182l.; Belgium 94,966, 14,479l.; Channel Islands 9579, 2900l.; France 644,080, 66,510l.; Spain 147,480, 19,730l.; Italy 41,743, 3790l.; Austrian Territories 41,011, 4031l.; Turkey 244,579, 23,593l.; Egypt 8870, 517l.; British Possessions in South Africa 2,521,109, 339,374l.; Aden 29,780, 2720l.; British India 190,202, 17,745l.; Australasia 2,693,064, 267,289l.; United States of America 45,692, 4367l.; Bermudas 2342, 365l.; Peru 7220, 1692l.; Chile 3582, 681l.; Brazil 18,616, 2453l.; Uruguay 92,499, 21,303l.; Argentine Republic 985,268, 176,900l.; Falkland Islands 26,747, 3105l.; Other Countries 7086, 747l. Total, 8,145,431, 1,003,487l.
Bosnia Serai, in 1879, exported about 10 tons. Shanghai, in 1878, 50,285 pieces (including lamb). Coquimbo (Chili), in 1879, 45 tons (including goat). Bagdag, in 1878, 86,351 pieces, 4071l., to India and Europe (including lamb). Falkland Islands, 1940l. worth in 1879. Cape, 1,480,875 pieces in 1879. Hankow, 7606 pieces, 9276l., in 1879. Tientsin, 206,777 piculs (of 1331/3 lb.) in 1878, 8737 in 1879. Mollendo (Peru) 79 quintals (of 2 cwt,) in 1878. Mogador, in « 263 » 1880, 15 bales, 80l., to Great Britain; 345, 1700l., to France; 2, 3l., to Spain. Our imports from the French East Indies have fallen from 5600 pieces in 1876, to 3762 in 1877, 410 in 1879, and none since; from Italy, from 339,973 in 1876, to 39,751 in 1880; from European Turkey, from 230,922 in 1876, to 63,236 in 1880; from Asiatic Turkey, they have risen from 93,965 in 1876, to 185,543 in 1880; from Brazil, 41,604 in 1876, 2623 in 1877, 5730 in 1880, and none in the intermediate years; from the Argentine Republic, 3,539,589 in 1876, 898,155 in 1879, 1,248,553 in 1880; from the Cape, 1,496,039 in 1877, 1,819,772 in 1880; from India, 3,927,934 in 1876, 2,911,974 in 1880; from Victoria, 1,667,330 in 1876, 1,158,686 in 1880; from New South Wales, 83,167 in 1878, 36,995 in 1880; New Zealand, 168,984 in 1878, 334,792 in 1880. The approximate London market values of sheep-skins are:—Cape, 10-34s. a doz.; fine wool, 28-59s.; superior, 40-82s.; Mogador, 14-27s.; Buenos Ayres, 4-13d. a lb.; Australian, 4-16d.; tanned East Indian, best, 2-4s.; ordinary to good, 1s.-2s. 9d.
Walrus.—Our imports of walrus skins from Christiania in 1879 were valued at 7900 kroner (of 131/2d).
Unenumerated.—Our imports of unenumerated skins and hides in 1883 were as follows:—
Dressed skins, not leather.—From Russia 410, 645l.; Germany 5249, 559l.; Holland 5917, 795l.; Belgium 16,524, 2658l.; France 41,069, 2680l.; British India 2005, 218l.; Australasia 2925, 795l.; British North America 300, 313l.; United States of America 502, 225l.; Other Countries 94, 40l. Total 74,995, 8928l.
Undressed skins:—From Denmark 15,950, 2418l.; Germany 10,815, 3391l.; Holland 7700, 765l.; Belgium 10,400, 800l.; France 3162, 309l.; British Possessions in South Africa 4699, 422l.; British India 21,846, 3260l.; China 86, 410l.; Australasia 65,305, 3274l.; British North America 1698, 255l.; United States of America 3575, 1295l.; Brazil 12,853, 1097l.; Other Countries 7457, 559l. Total 165,546, 18,255l.
Wet hides.—From Sweden 1945 cwt., 6133l.; Norway « 264 » 1561 cwt., 4197l.; Denmark 4757 cwt., 10,873l.; Germany 33,617 cwt., 86,210l.; Holland 19,006 cwt., 47,859l.; Belgium 74,288 cwt., 210,698l.; Channel Islands 2478 cwt., 4724l.; France 64,212 cwt., 178,941l.; Portugal 18,031 cwt., 52,328l.; Gibraltar 888 cwt., 2616l.; Italy 13,411 cwt., 37,431l.; Austrian Territories 940 cwt., 2260l.; British Possessions in South Africa 23,881 cwt., 66,779l.; Japan 806 cwt., 2300l.; Australasia 93,891 cwt., 209,158l.; United States of America 11,590 cwt., 31,610l.; Bermudas 923 cwt., 2257l.; British West India Islands 3329 cwt., 8105l.; Brazil 64,406 cwt., 191,051l.; Uruguay 99,391 cwt., 308,940l.; Argentine Republic 25,142 cwt., 73,518l.; Falkland Islands 1434 cwt., 4100l.; Whale Fisheries: Northern 782 cwt., 4985l.; Other Countries 2024 cwt., 4660l. Total 562,733 cwt., 1,551,733l.
Dry raw hides and pieces.—From Russia 10,829 cwt., 79,940l.; Sweden 193 cwt., 1233l.; Denmark 1641 cwt., 10,677l.; Germany 13,022 cwt., 62,248l.; Holland 10,874 cwt., 37,716l.; Belgium 3791 cwt., 15,355l.; France 3393 cwt., 12,262l.; Gibraltar 225 cwt., 1000l.; Italy 451 cwt., 1366l.; Austrian Territories 555 cwt., 2813l.; Turkey 925 cwt., 3327l.; Egypt 468 cwt., 1493l.; West Coast of Africa, not particularly designated 673 cwt., 1398l.; British Possessions in South Africa 39,501 cwt., 160,716l.; East Coast of Africa (Native States) 2990 cwt., 8808l.; Madagascar 2850 cwt., 8773l.; Mauritius 2669 cwt., 8433l.; Aden 6745 cwt., 22,282l.; British India: Bombay 33,548 cwt., 105,081l.; Madras 3860 cwt., 13,248l.; Bengal and Burmah 370,369 cwt., 1,329,822l.; Straits Settlements 51,456 cwt., 130,244l.; Ceylon 2314 cwt., 6468l.; Java 3288 cwt., 10,670l.; Cochin China, Camboja, and Tonquin 2236 cwt., 5153l.; China 18,892 cwt., 63,192l.; Australasia 7009 cwt., 15,506l.; United States of America 17,842 cwt., 56,536l.; British West India Islands 953 cwt., 3713l.; United States of Colombia 1053 cwt., 4955l.; Peru 1120 cwt., 3577l.; Chile 1118 cwt., 4089l.; Brazil 9125 cwt., 31,089l.; Uruguay 2937 cwt., 9924l.; Argentine Republic 3556 cwt., 12,426l.; Other Countries 1645 cwt., 5596l. Total 634,116 cwt., 2,251,129l..
Undressed leather.—From Germany 89,073 lb., 5563l.; Holland 86,048 lb., 6239l.; Belgium 25,650 lb., 1943l.; France 62,799 lb., 4655l.; Spain 42,773 lb., 3555l.; Aden 21,280 lb., 1853l.; British India: Bombay 3,663,452 lb., 274,625l.; Madras 17,859,652 lb., 1,375,484l.; Bengal and Burmah 1,821,925 lb., 124,193l.; Straits Settlements 3,957,651 lb., 147,962l.; China 37,923 lb., 2068l.; Australasia: West Australia 12,750 lb., 487l.; South Australia 238,249 lb., 11,283l.; Victoria 7,175,550 lb., 357,032l.; New South Wales 2,428,147 lb., 120,675l.; Queensland 11,980 lb., 621l.; Tasmania 40,863 lb., 1986l.; New Zealand 1,573,289 lb., 74,776l.; British North America 155,500 lb., 7405l.; United States of America 17,329,692 lb., 778,392l.; Other Countries 186,333 lb., 11,121l. Total 56,820,579 lb., 3,311,918l.
Dressed leather.—From Russia 1669 lb., 252l.; Germany 1,397,928 lb., 369,719l.; Holland 1,809,262 lb., 293,196l.; Belgium 260,657lb., 41,146l.; France 5,187,323 lb., 725,485l.; Turkey 6226 lb., 339l.; British Possessions in South Africa 700 lb., 250l.; British India 156,802 lb., 15,720l.; Australasia 2812 lb., 423l.; British North America 779,321 lb., 57,326l.; United States of America 7,858,956 lb., 533,419l.; Other Countries 5183 lb., 543l. Total 17,466,839 lb., 2,037,818l.
Varnished, japanned, or enamelled leather.—From Russia 44,088 lb., 9725l.; Germany 18,236 lb., 3895l.; Holland 209,707 lb., 56,667l.; Belgium 648 lb., 200l.; France 67,724 lb., 25,598l.; Turkey 190 lb., 50l.; British North America 6868 lb., 1151l.; United States of America 83,387 lb., 19,377l. Total, 430,848 lb., 116,663l.
Boots and Shoes.—From Germany 3766 dzn. pairs, 10,413l.; Holland 23,321 dzn. pairs, 85,585l.; Belgium 38,203 dzn. pairs, 81,253l.; Channel Islands 464 dzn. pairs, 2327l.; France 53,437 dzn. pairs, 233,038l.; Turkey 350 dzn. pairs, 149l.; Australasia: New South Wales 29 dzn. pairs, 100l.; British North America 3080 dzn. pairs, 6706l.; United States of America 331 dzn. pairs, 1400l.; Other Countries 77 dzn. pairs, 243l. Total, 123,058 dzn. pairs, 421,214l.
Gloves.—From Sweden 350 dzn. pairs, 237l.; Norway 50 dzn. pairs, 58l.; Denmark 19,320 dzn. pairs, 16,093l.; Germany 3090 dzn. pairs, 2693l.; Holland 309,416 dzn. pairs, 321,080l.; Belgium 197,444 dzn. pairs, 222,946l.; Channel Islands 10 dzn. pairs, 16l.; France 1,138,343 dzn. pairs, 1,375,988l.; Italy 232 dzn. pairs, 280l.; Australasia: Victoria 43 dzn. pairs, 96l. Total, 1,668,298 dzn. pairs, 1,939,487l.
Unenumerated leather manufactures.—From Sweden 108l.; Norway 109l.; Denmark 236l.; Germany 22,705l.; Holland 119,462l.; Belgium 35,762l.; France 48,308l.; British Possessions in South Africa 198l.; British North America 931l.; United States of America 19,388l.; Other Countries 571l. Total, 247,778l.
Our imports of hides from the undermentioned countries have fluctuated as shown:—
Abyssinia.—Undressed, 7289 cwt. in 1876, 327 in 1878, 2159 in 1879, and 324 in 1880.
Aden.—Undressed, 8190 cwt. in 1876, 113 in 1879, 8294 in 1880.
Algiers.—Raw, 2,051,701 kilo. (of 2·2 lb.) in 1879.
Argentine Republic.—Undressed, 94,479 cwt. in 1877, 32,961 in 1879, 34,905 in 1880.
Austro-Hungary.—Vienna, 24,672 metrical centners in 1878, 48,950 in 1879; Fiume, raw, 1400 kilo. in 1879.
Bahamas.—167l. worth in 1879.
Barbados.—363l. worth in 1877, 913l. in 1878.
Belgium.—Undressed, 51,069 cwt. in 1877, 82,021 in 1878, 68,123 in 1880. Dressed, 176,635 lb. in 1878, 418,906 in 1880.
Brazil.—Undressed, 137,351 cwt. in 1878, 115,137 in 1880. Pernambuco in 1878-9 exported, dried, 31,717 kilo. to Great Britain, 28,077 France, 25,606 Portugal, total value, 3002l.; salted, 383,691 kilo. Great Britain, 937,976 United States, 585,868 France, 40,770 Spain, 463,269 Portugal, total value 75,523l.; in 1880, 61 tons, 2267l. Maceio exported in 1877, 4728 pieces (average 28 lb. each) to Great Britain, 1440 New York and Lisbon; in 1879, 36,775; in 1880, 11,405. Bahia exported 1,432,864 kilo. in 1877-8, and 1,773,965 in 1878-9, « 267 » principally to the United States and Germany. Santos exported 397,000 kilo. in 1879. Ceara exported in 1878, 372,808 kilo. to England, 31,966 Havre, 775,863 Hamburg, 7800 New York.
British India.—Undressed, 281,198 cwt. in 1876, 463,764 in 1880; dressed, 14,835,979 lb. in 1878, 6,178,370 in 1880.
Bulgaria.—Rustchuk, in 1879, exported 254,196 kilo. (250 tons) to Austria.
Canada.—Dressed, 939,759 lb. in 1876, 372,359 in 1879, 1,066,043 in 1880.
Cape.—Undressed, 15,370 cwt. in 1876, 44,503 in 1878, 29,442 in 1880.
Central America.—Undressed, 72 cwt. in 1876, 1113 in 1878, 356 in 1880.
Chili.—Undressed, 318 cwt. in 1876, 17,042 in 1879, 1566 in 1880; dressed, 33,026 lb. in 1876, 3929 in 1877, 199,965 in 1878, 224 in 1879, 2930 in 1880.
China.—Undressed, 5671 cwt. in 1876, 60,871 in 1878, 2705 in 1880. Hankow exported in 1879, 7797 pieces, 1656l.; Kiungchow, 490 piculs (of 1331/3 lb.), 818l.; Newchwang, 17,665 pieces; Tientsin, 4354 piculs; Canton, in 1878, 653 pieces of skins, 8733/4 piculs of hides.
Costa Rica.—San José exported 308,794 lb. in 1879.
Denmark.—Undressed, 20,806 cwt. in 1877, 5632 in 1880; Copenhagen exported 1,166,172 lb. to Great Britain in 1878.
Ecuador.—Undressed, 680 cwt. in 1876, 18 in 1877, 115 in 1879, 89 in 1880. Guayaquil exported in 1878, 5711 quintals raw, 17,133l., to the United States, and 12,504 halves tanned, 8752l., to South America; and in 1880, 8859 quintals raw, 22,148l., and 4861 tanned, 2916l. Manabi, in 1878, exported 1321 quintals, 3963l.
Egypt.—Undressed, 1250 cwt. in 1877, 718 in 1878, 1286 in 1880. In 1879, the values were 620l. to Austria, 380l. France, 1950l. Great Britain, 45,500l. Greece, 280l. Italy, 62,500l. Turkey.
Falklands.—Undressed, 4315 cwt. in 1878, 2679 in 1880. The value of the exports was 5020l. in 1879.
France.—Undressed, 26,866 cwt. in 1876, 57,305 in 1880; dressed, 2,727,190 lb. in 1876, 4,338,485 in 1880. Calais in 1878 sent 2188 kilo. prepared to Great Britain, and 76,811 kilo. in 1879.
French East Indies.—Dressed, 24,600 lb. in 1876, 12,713 in 1877, none since.
Gambia.—Exported 15,380 pieces in 1878.
Germany.—Undressed, 45,002 cwt. in 1876, 21,143 in 1878, 44,383 in 1880; dressed, 1,269,143 lb. in 1876, 954,578 in 1878, 1,318,659 in 1880. Hamburg sent to Great Britain, 33,458 cwt. dry and salted in 1877, 13,972 in 1879. Königsberg exported 1535 cwt. raw in 1878, 424 in 1879.
Greece.—Dressed: Syra in 1877 sent 60,217l. worth to Turkey, 23,259l. to the Danubian Principalities, 2748l. to Austria; in 1879, 492l. Turkey, 251l. Austria, 200l. Russia.
Guatemala.—Exports in 1877, 62,343 dol. worth; in 1878, 8441/2 quintals to England, 1293 France, 2476 Germany, 822 New York, 149 California; 1879, 412,605 Germany, 12,360 New York.
Hawaiian Islands.—Exports 1880, 24,885 pieces.
Holland.—Undressed, 55,705 cwt. in 1876, 53,568 in 1880; dressed, 941,372 lb. in 1876, 896,734 in 1880.
Java.—Exports 1878-9, 357,353 pieces and 1240 piculs to Holland, 7212 pieces to the Channel for orders, 1200 pieces to France, 7369 pieces to Italy, 5695 pieces and 872 piculs to Singapore.
Madagascar.—Undressed, 252 cwt. in 1877, 3088 in 1879, none since.
Mauritius.—Undressed, 5341 cwt. in 1876, 2945 in 1880.
Morocco.—Undressed, 0 in 1877, 5445 cwt. in 1878, 1014 in 1880. Tangiers exported in 1879, 2727 cwt., 6000l., to Great Britain; 1818 cwt., 4365l., France; 21 cwt., 42l., Spain. Mogador, in 1880, sent 44 bales, 150l., to Great Britain; 667, 2250l., France; 243, 770l., Portugal.
Natal.—Undressed, 32,555 cwt. in 1876, 17,496 in 1878, 23,908 in 1880.
New Granada.—Undressed, 12,217 cwt. in 1878, 574 in 1879, 6059 in 1880.
New South Wales.—Undressed, 9386 cwt. in 1878, 79,972 in 1880; dressed, 2,257,041 lb. in 1877, 1,694,015 in 1880.
New Zealand.—Undressed, 39 cwt. in 1878, 6335 in 1880; dressed, 140,448 lb. in 1878, 446,102 in 1880.
Persia.—Bushire exported in 1879, 4000 rupees' worth to England, 5000 r. India; Lingah, 2800 r. India, 1950 r. Persian coast; Bahrein, 6000 r. Koweit, Bussora, and Bagdad.
Peru.—Undressed, 2859 cwt. in 1876, 622 in 1878, 1235 in 1880. Mollendo exported 538 quintals in 1878, and 1307 q. dry in 1879.
Philippines.—Undressed, 1024 cwt. in 1876, 102 in 1880. Manilla, in 1879, exported 7976 piculs, 12,761l., to China and Japan.
Portugal.—Undressed, 17,456 cwt. in 1877, 10,983 in 1880.
Queensland.—Undressed, 1315 cwt. in 1879, 5019 in 1880.
Roumania.—Galatz exported 341 bales in 1879.
Russia.—Undressed, 482 cwt. in 1876, 6020 in 1880; dressed, 88,225 lb. in 1876, 46,694 in 1880. Riga shipped 14,839 poods (of 36 lb.) in 1877, 11,311 in 1879. Poti, in 1877-8, sent away 5654 poods, and 2149 from Persia.
Saigon.—Exports in 1879, 10,582 piculs.
San Domingo.—Exports in 1878, 630 pieces to Great Britain, 490 France, 3100 Italy, 3980 Spain, 460 United States, 560 West Indies; in 1880, 1340 Italy, 2541 Spain, 7142 United States, 97 West Indies.
South Australia.—Dressed, 38,108 lb. in 1878, 303,143 in 1880.
Spanish West Indies.—Puerto Rico exported in 1878, 167 quintals United States, 5673 Spain, 637 Germany.
Straits Settlements.—Undressed, 28,444 cwt. in 1876, 48,213 in 1880; dressed, 603,389 lb. in 1876, 2,778,159 in 1880.
Surinam.—Exports in 1878, 9221 kilo.
Sweden and Norway.—Christiania exported 95,200 kroner worth in 1875, 4200 kr. in 1878. Gothenburg exported 10,960 cwt. in 1879.
Tasmania.—Dressed, 65,803 lb. in 1878, 38,141 in 1880.
Tripoli.—Bengazi, in 1878, sent 50,000 pieces, 4000l., to « 270 » Malta. The value of the exports was 2000l. in 1879, and 4500l. in 1880.
Turkey.—Aleppo exported in 1878, 181 tons, 10,824l., to France; 5, 320l., Italy; 11, 704l., Austria; 52, 3328l., Turkey; 12, 768l., Egypt. Thessaly exported 15,000l. worth in 1880. Samos sent 19,300l. worth tanned to Turkey and Egypt in 1879. Van exported 1500l. worth in 1879. Kerasund shipped by steamer in 1879, 557 bales, 3899l. Trebizond in 1879 sent 940 bales (of 12 and 60 pieces), 6580l., to Turkey; 1567, 10,969l., France; 501, 3507l., Russia; 80, 560l., Greece. Dedeagatch, in 1879, exported 1300 bales, 40,000l. Alexandretta, in 1879, sent 280 tons, 16,800l., to France; 3, 180l., Austria; 10, 600l., Russia; 96, 6720l., Turkey; 29, 2030l., Egypt. Adana, in 1879, sent 250 tons, 7500l., to France; 140, 4200l., Turkey; 27, 810l., Greece. Jaffa exported 18,000 okes (49,500 lb.), 666l., for Turkey in 1879.
United States.—Undressed, 115,767 cwt. in 1876, 7888 in 1879; 14,358 in 1880; dressed, 16,716,711 lb. in 1879, 22,543,033 in 1880. Savannah exported 8758 bundles in 1880. Galveston exported in 1879-80, 9878 bales and 7510 single, dry; and 6905 bundles wet-salted. Texas State in 1878-9 exported 28,104,065 lb., 562,081l.
Uruguay.—Undressed, 116,738 cwt. in 1876, 65,846 in 1879, 104,691 in 1880.
Venezuela.—Puerto Cabello exported in 1879, 10,126 kilo. to Great Britain, 8817 Germany, 75,794 United States, 5756 France, 696 Holland, 1023 Spain. Ciudad Bolivar sent 35,562 pieces, 762,234 lb., to New York in 1879.
Victoria.—Undressed, 0 in 1878, 2710 in 1879, 8705 in 1880; dressed, 3,506,562 lb. in 1876, 5,096,696 in 1880. The values of the exports in 1878 were 9417l. hides, and 19,706l. skins and pelts.
Tanning Materials.—Our imports of bark in 1883 were:—From Sweden 6410 cwt., 1281l.; Norway 8858 cwt., 1687l.; Holland 12,855 cwt., 3246l.; Belgium 59,936 cwt., 15,987l.; France 3323 cwt., 2138l.; Algeria 46,052 cwt., « 271 » 19,577l.; British East Indies 4605 cwt., 3321l.; Australasia 183,777 cwt., 119,292l.; United States of America 36,203 cwt., 12,368l.; Other Countries 3087 cwt., 1852l. Total, 365,106 cwt., 180,749l.
Our imports of cutch and gambier in 1883 were:—From British India: Bombay 277 tons, 7682l.; Bengal and Burmah 8115 tons, 221,651l.; Straits Settlements 17,477 tons, 453,804l.; Philippine Islands 47 tons, 1227l.; United States of America 877 tons, 25,149l.; Other Countries 44 tons, 1208l. Total, 26,837 tons, 710,721l.
Our imports of myrobalans in 1883 were:—From Germany 1133 cwt., 503l.; British India:—Bombay 349,275 cwt., 184,983l.; Madras 120,262 cwt., 54,997l.; Bengal 23,579 cwt., 9663l.; Straits Settlements 214 cwt., 107l.; Ceylon 1105 cwt., 483l.; Japan 306 cwt., 146l. Total, 495,874 cwt., 250,882l.
Our imports of extracts in 1883 were:—From Denmark 2773l.; Germany 36,638l.; Holland 54,600l.; Belgium 7353l.; France 261,690l.; Spain 1000l.; Italy 4571l.; British North America 33,311l., United States of America 66,114l.; Mexico 3937l.; Other Countries 1625l. Total, 473,612l.
Our imports of galls in 1883 were:—From Austrian Territories 474 cwt., 1022l.; Turkey 9056 cwt., 23,388l.; Egypt 2435 cwt., 6210l.; Persia 269 cwt., 770l.; British India 1572 cwt., 1062l.; China 22,625 cwt., 66,731l.; Japan 2936 cwt., 9276l.; Other Countries 185 cwt., 486l. Total, 39,552 cwt., 108,945l.
Our imports of valonia in 1883 were:—From Holland 100 tons, 1600l.; France 59 tons, 947l.; Austrian Territories 178 tons, 2732l.; Greece 3101 tons, 46,526l.; Turkey 27,030 tons, 432,423l. Total, 30,468 tons, 484,228l.
Our imports of sumach in 1883 were:—From France 688 tons, 8979l.; Spain 54 tons, 616l.; Italy 12,395 tons, 184,152l.; Austrian Territories 1707 tons, 21,121l.; Other Countries 32 tons, 430l. Total, 14,876 tons, 215,298l.
Literature.
The chief works relating to the tanning, currying, and dressing of leather, and the tanning materials employed, are as follows:—
| Abridgments of Specifications: Skin, Hides, and Leather, 1627-1866. | London: 1872. |
| Bernardin (R. J.). | |
| Classification de 350 Matières Tannantes. | Gand: 1880. |
| Brüggemann (A.). | |
| Weissgerberei. | Quedlinburg. |
| Brüggemann (A.). | |
| Saffian Fabrikation. | Quedlinburg. |
| Brüggemann (A.). | |
| Glacéleder Färberei. | Quedlinburg. |
| Councler (Dr. C.). | |
| Bericht über die Verhandlungen der Commission zur Feststellung einer einheitlichen Methode der Gerbstoffbestimmung. | Cassel: 1885. |
| Das Ganze der Lederbereitung. | Quedlinburg. |
| Davis (C. T.). | |
| Manufacture of Leather. | Philadelphia: 1885. |
| Dussauce (F.). | |
| Tanning, Currying, and Leather-dressing. | Philadelphia: 1865. |
| Eitner (W.). | |
| Leder-Industrie: Bericht über die Welt-Ausstellung in Philadelphia, 1876. | Vienna: 1877. |
| Hansen (A.). | |
| Die Quebracho Rinde. | Berlin: 1880. |
| Höhnel (F. R. von). | |
| Die Gerberinden. | Berlin: 1880. |
| Knapp (Fr.). | |
| Natur und Wesen der Gerberei und des Leders. | München: 1858. |
| Lange (J. C.). | |
| Lederbereitung. | Quedlinburg. |
| Lietzmann (J. C. H.). « 273 » | |
| Herstellung des Leder in ihren Chemischen und Physikalischen Vorgängen. | Berlin: 1875. |
| McMurtrie (W.). | |
| Culture of Sumac, and preparation for market: Department of Agriculture Special Report, No. 26. | Washington: 1880. |
| Morfit (C.). | |
| The Arts of Tanning, Currying, and Leather-dressing. | Philadelphia: 1852. |
| Neubrand (J. G.). | |
| Die Gerbrinde. | Frankfurt-on-Maine: 1869. |
| Olivet (P.). | |
| Lederfärberei. | Quedlinburg. |
| Schultz (J. S.) | |
| Leather Manufacture: a Dissertation on the Methods and Economics of Tanning. | New York: 1876. |
| Sonnenfeldt (Dr.). | |
| Färben der Pelzwaaren. | Quedlinburg. |
| Villain (H.). | |
| Cuirs et Peaux: Tannage, Corroyage, et Mégisserie. | Paris: 1867. |
| Vincent (C.). | |
| Fabrication et Commerce des Cuirs et Peaux. | Paris: 1872. |
| Wattle Bark: Report of the Board of Inquiry. | Melbourne: 1878. |
| Wiener (F.). | |
| Die Lohgerberei, oder die Fabrikation des Lohgaren Leders. | Leipzig: 1879. |
| Wiesner (J.). | |
| Die Rohstoffe des Pflanzenreiches. | Leipzig: 1873. |
| Wittmack (L.). | |
| Die Nutzpflanzen aller Zonen. | Berlin: 1879. |
| Leather Trades Circular and Review. | London. |
| Tanners' and Curriers' Journal. | London. |
| Scottish Leather Trader. | Glasgow. « 274 » |
| Shoe and Leather Reporter. | New York. |
| La Halle aux Cuirs. | Paris. |
| Der Gerber. | Vienna. |
| Gerber Zeitung. | Berlin. |
| Deutsche Gerber Zeitung. | Berlin. |
| Gerber Courier. | Vienna. |
| Gazetta dei Pellami. | Milan. |
LONDON:
PRINTED BY WILLIAM CLOWES AND SONS, LIMITED, STAMFORD STREET AND CHARING CROSS.
Transcriber Note
Illustrations were moved to avoid splitting paragraphs. Hyphenization was standardized to the most commonly used form. Several minor typos were corrected. This file and all images was produced from images generously made available by The Internet Archive. The cover image was composed from the TIA images and is placed in the Public Domain.
End of Project Gutenberg's A Text-book of Tanning, by Henry R. Procter
*** END OF THIS PROJECT GUTENBERG EBOOK A TEXT-BOOK OF TANNING ***
***** This file should be named 56601-h.htm or 56601-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/5/6/6/0/56601/
Produced by Chris Curnow, Tom Cosmas and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.