


Containing Over One Hundred Highly
Amusing and Instructive Tricks
With Chemicals.
By A. ANDERSON.
HANDSOMELY ILLUSTRATED.
New York:
FRANK TOUSEY, Publisher,
24 Union Square.
Entered according to Act of Congress, in the year 1898, by
FRANK TOUSEY,
in the Office of the Librarian of Congress at Washington, D.C.
HOW TO DO
CHEMICAL TRICKS.

From the remotest ages chemistry has exercised the strongest fascination on the minds of the curious, nor is it a matter of surprise that boys should feel themselves drawn strongly by its mystery and seeming magic. This attraction is undoubtedly caused by what the ancients called the elements, earth, air, fire and water. There is something so weird about the manifestation of air and fire, that it is not difficult to understand how the alchemists believed them to be forces able to be used at the bidding of spirits, who might be conjured up by incantations and spells.
Now it is known that these uncanny beings existed only in the imagination of the forerunners of modern chemists. Yet what boy can look on the brilliantly colored fires of a Fourth of July display, or the burnished gold of the setting sun, or the fantastic pictures in the glowing coals in a grate, and not feel that there is still something of magic and mystery in fire still? What the boy feels, the scientist cannot explain. Nobody knows actually what fire is. All that can be said is that fire is produced by certain substances, such as coals, wood, or paper, that give out heat, while passing from one state to another.
Now the word “element” was and is used to mean that[4] simplest form of matter, which, with other simplest forms goes to make up the whole world of everything in it. The earth, animals, plants, the sea, the atmosphere, are all made up of one or more of some seventy substances called elements. Hence it is clear that the earth, air and water are not, as the ancients thought, elements at all. As will be seen in this little book, both air and water consist of mixtures of elements. In chemistry such mixtures are called compounds. This word occurs again and again, so its explanation should be remembered.
One great fact must be remembered, which is at the very root of chemistry. Nothing is really lost, however much its form may be changed, or however many changes it may pass through. For instance, it may seem that when a block of wood be burned that a very large amount of it is lost. If, however, the ashes, the smoke, and the carbon that is burned by the air be all weighed, the result would be exactly the same as the weight of the original block of wood.
Again take an instance of a different nature. A lump of sugar is placed in a small glass of water. Gradually the solid is dissolved, and in time disappears. It is not lost, however. By boiling the mixture until all the water has evaporated the sugar will be found adhering as crystals on the sides of the glass. If these be carefully collected, they will be found to weigh precisely as much as the original lump of sugar.
Once more, take a block of ice weighing an ounce. Having removed it into a room, the solid will in an hour or two have disappeared entirely, but the water that has replaced the block of ice will weigh neither more nor less than an ounce. If again heat be applied to the water it will all disappear, but if weighed in a jam jar, the steam, although invisible to the eye, will still weigh one ounce exactly.
From the above-given experiments it may be seen that, however matter may change its form it cannot really be destroyed. This truth will appear in every experiment that can be performed, whether those given in this little book or in the most learned treatise on chemistry.
This high-sounding term means that substances have a power of uniting together that can be better explained by an experiment. Allow a few drops of water to fall on a perfectly clean piece of iron. In a short time a reddish-brown substance will appear on the iron that in ordinary language is called rust. What does this mean? Water is a compound substance composed of oxygen and hydrogen, but when brought into contact with iron the oxygen prefers to unite with the iron and sets the hydrogen free. Hence, would the chemist say, oxygen has a “stronger affinity” for iron than for hydrogen. In this case the rust is composed of rust, a combination of iron and oxygen called oxide of iron. What has taken place may be shown by the following, which will be easily understood:
So all that the chemical combination in the above means is that the iron has taken the place of the hydrogen in the water used for the experiment. If weighed it would be found as always, that the water and the iron weighed precisely the same as the oxide of iron and the hydrogen.
It is to this same principle of chemical affinity that the curious experiments of magic writing with sympathetic inks are possible.
By means of these may be carried on a correspondence which is beyond the discovery of all not in the secret. With one class of these inks the writing becomes visible only when moistened with a particular solution. Thus, if we write to you with a solution of sulphate of iron the letters are invisible. On the receipt of our letter, you rub over the sheet a feather or sponge, wet with a solution of nut-galls, and the letters burst forth into sensible being at once, and are permanent.
2. If we write with a solution of sugar of lead and you moisten with a sponge or pencil dipped in water impregnated with sulphuretted hydrogen, the letters will appear with metallic brilliancy.
3. If we write with a weak solution of sulphate of copper, and you apply ammonia, the letters assume a beautiful blue. When the ammonia evaporates as it does on exposure to the sun or fire, the writing disappears, but may be revived again as before.
4. If you write with oil of vitriol very much diluted, so as to prevent its destroying the paper, the manuscript will be invisible except when held to the fire, when the letters will appear black.
5. Write with cobalt dissolved in diluted muriatic acid; the letters will be invisible when cold, but when warmed they will appear a bluish green.
Secrets thus written will not be brought to the knowledge of a stranger, because he does not know the solution which was used in writing, and therefore knows not what to apply to bring out the letters.
Other forms of elective affinity produce equally novel results. Thus, two invisible gases, when combined, form sometimes a visible solid. Muriatic acid and ammonia are examples, also ammonia and carbonic acid.
On the other hand, if a solution of sulphate of soda be mixed with a solution of muriate of lime the whole becomes solid.
Some gases when united form liquids, as oxygen and hydrogen, which unite and form water. Some solids when combined form liquids.
Chemical affinity is sometimes called elective, or the effect of choice, as if one substance exerted a kind of preference for another, and chose to be united to it rather than to that with which it was previously combined; thus, if you pour some vinegar, which is a weak acetic acid, upon some pearlash (a combination of potash and carbonic acid), or some carbonate of soda (a combination of the same acid with soda), a violent effervescence will take place, occasioned by the escape of the carbonic acid, displaced in consequence of the potash or soda preferring the acetic acid, and forming a compound called an acetate.
Then, if some sulphuric acid be poured on this new compound, the acetic acid will, in its turn, be displaced by the greater attachment of either of the bases, as they are termed, for the sulphuric acid. Again, if into a solution[7] of blue vitriol (a combination of sulphuric acid with copper), the bright blade of a knife be introduced, the knife will speedily be covered with a coat of copper, deposited in consequence of the acid preferring the iron of which the knife is made, a quantity of it being dissolved in exact proportion to the quantity of copper deposited.
It is on the same principle that a very beautiful preparation called a silver-tree, or a lead-tree, may be formed, thus: Fill a wide bottle, capable of holding from half a pint to a pint, with a tolerably strong solution of nitrate of silver (lunar caustic), or acetate of lead, in pure distilled water. Then attach a small piece of zinc by a string to the cork or stopper of the bottle, so that the zinc shall hang about the middle of the bottle, and set it by where it may be quite undisturbed. In a short time brilliant plates of silver or lead, as the case may be, will be seen to collect around the piece of zinc, assuming more or less of the crystalline form. This is a case of elective affinity; the acid with which the silver or lead was united prefers the zinc to either of those metals, and in consequence discards them in order to attach the zinc to itself; and this process will continue until the whole of the zinc is taken up, or the whole of the silver or lead deposited.
Form a small basket about the size of the hand, of iron wire or split willow; then take some cotton, such as ladies use for running into flounces; untwist it and wind it round every limb of the basket. Boil eighteen ounces of alum in a quart of water, or quantities in that proportion; stir the mixture while boiling until the alum is completely dissolved. Pour the solution into a deep pan, or other convenient vessel, and suspend the basket in the liquor, so that no part of the basket shall touch the vessel, or be exposed to the air. Let the whole remain perfectly at rest for twenty-four hours. When you then remove the basket the alum will be found very prettily crystallized over all the limbs of the cottoned frame.
Saturate water kept boiling with alum; then set the solution in a cool place, suspending in it, by a hair, or fine silk thread, a cinder, a sprig of a plant, or any other trifle. As the solution cools, a beautiful crystallization of the salt takes place upon the cinders, etc., which are made to resemble specimens of mineralogical spars.
Dilute a saturated solution of chloride of gold with five times its bulk of water; place a thin strip of fresh burned charcoal into it, and apply heat, gradually increasing it until the solution gently boils. The heat will make the charcoal precipitate the metal on the charcoal, in the form of brilliant spangles.
Lay a crystal of nitrate of silver upon a piece of burning charcoal; the metallic salt will catch fire, and throw out the most beautiful scintillations that can be imagined. The silver is reduced, and, in the end, produces upon the charcoal a very brilliant appearance.
Many animal and vegetable substances, consist, for the most part, of carbon, or charcoal, united with oxygen and hydrogen, which remember, together combined, form water. Now oil of vitriol or strong sulphuric acid, has so powerful an affinity or so great a thirst for water, that it will abstract it from almost any body in which it exists. If you pour some of this acid on a lump of sugar, or place a chip of wood in a small quantity of it, the sugar or wood will become speedily blackened, that is charred, in consequence of the oxygen and hydrogen being removed by the sulphuric acid, and only the carbon or charcoal left.
When Cleopatra dissolved pearls of wondrous value in vinegar, she was unwittingly giving an example of chemical affinity. The pearl is simply carbonate of lime stored up by the oyster in layers. Consequently the precious jewels were decomposed by the greater affinity or fondness of lime for the acetic acid in the vinegar, than for the carbonic[9] acid with which it had been before united. This was an example of inconstancy in strong contrast with the conduct of their owner, who chose death rather than become the wife of her lover’s conqueror.
It is necessary to distinguish between burning and the mere appearance of it. A gas flame is gas in a state of combustion, whereas the electric light is no example of it, although the wire within the glassen cylinder is red hot, and to all appearance burning. Combustion generally takes place through the strong affinity of some element, such as carbon in a substance for the oxygen in the atmosphere. In coal gas, for instance, the carbon contained in it unites with the oxygen in the air to form a colorless substance called carbonic acid gas. The latter is unable to support life, and may be called, therefore, poisonous. It is the presence of this gas which makes it unhealthy to burn many jets without proper ventilation.
Also, carbonic acid gas is given off by the lungs. It may seem curious, but it is none the less true, that breathing is a process of combustion. The blood brings to the surface of the lungs the carbon, which has resulted from the waste of the internal organs of the body. When drawing in a breath the oxygen present in the atmosphere meets the impure blood at the surface of the lungs, and purifies it by uniting with the carbon in it. Then, though oxygen has been breathed in, carbonic acid gas has been breathed out.
To prove this will be interesting: Obtain from a chemist a little lime water—two cents worth will do. It looks like ordinary water, being perfectly transparent and colorless. Pour some into a clean glass, and through a glass tube blow steadily into the water. In half a minute the hitherto colorless liquid will become milky and opaque. If allowed to stand there will fall down at the bottom of the glass a white powder.
What has happened in this case? The carbonic acid gas from the lungs has formed with the lime in the lime water a substance called carbonate of lime, which, being insoluble in water, falls to the bottom of the glass as a white powder.
If carbonic acid gas were not present in the air blown from the lungs, this milkiness would not appear, for no other gas, except this, would alter the lime water’s clearness.
Before proceeding further, it will be well to perform one or two experiments, to prove that the air we breathe is by no means the simple substance it is generally supposed to be. Although it is invisible, it must be remembered that it presses with a force of over fifteen pounds to the square inch, over the whole surface of the earth. It extends, too, to a height of some forty miles above the earth, and though it cannot be seen, it can be felt in the rush of the hurricane, and heard in the roar of the tempest. It is chiefly composed of a mixture of two gases, oxygen and nitrogen.
Did the air consist entirely of the former, people would breathe too quickly, and die in a very short time in a high fever, burned up, in fact. If only consisting of nitrogen, the human race would also die, because this element is incapable of supporting life; people would be suffocated, in fact.
Therefore, a judicious mixture of the two is essential to the life of animals. Generally, in a hundred parts of air by weight there are seventy-six parts of nitrogen to twenty-three of oxygen.
Besides these two gases, there is also a quantity of carbonic acid gas in the air, given off by all the fires and animals in the world. Of course, its amount is much greater in the great towns and manufacturing centers than in country districts.
Now herein must be recorded one of these charming arrangements which Nature has designed for the benefit of her children. Carbonic acid gas is much heavier than the air, and, therefore, sinks towards the ground, where, if allowed to accumulate, would cause the death of every animal. Fortunately, however, plants breathe in through their leaves carbonic acid gas during sunshine, and break it up into carbon and oxygen. The former, they use for[11] building up their trunks, leaves, and flowers, while during the night they give off oxygen into the air.
This is the reason why plants and trees planted in the streets so largely help to sweeten and purify the foul air of a great city.
An experiment to prove that the atmosphere does consist of nitrogen and oxygen, may be prettily proved in the following simple manner: A glass marmalade jar, or a soup-plate filled with water, and a piece of phosphorus as large as a pea, are the only things necessary. Take very great care not to touch the phosphorus, for the heat of the hand is sufficient to set it on fire, and a terrible wound would be caused.
Place the phosphorus in a match-box on the surface of the water, touch it with a lighted match, and put the jar-mouth downwards over it to the bottom of the plate. The phosphorus burns with a dazzling brilliancy, and gives off dense white fumes. At the same time the water rises a third of the way up the jar, but not to the top, thus showing that all the invisible matter has not been consumed. The white soon settles into the water and is dissolved. The phosphorus has combined with the oxygen in the jar and forms phosphoric oxide, which dissolves in water. There is then only the nitrogen left. The disappearance of the oxygen allows the water to fill up the space it formerly occupied.
This may be followed by another experiment.
To show that oxygen is necessary for the support of combustion, fix two or three pieces of wax taper on flat pieces of cork, and set them floating on water in a soup-plate, light them, and invert over them a glass jar.
As they burn, the heat produced may perhaps at first expand the air, so as to force a small quantity out of the jar, but the water will soon rise in the jar, and continue to do so until the tapers expire, when you will find that a considerable portion of the air has disappeared, and what remains will no longer support flame.
The oxygen has been converted partly into water, and partly into carbonic acid gas, by uniting with the carbon and hydrogen of which the taper consists, and the remaining air is principally nitrogen, with some carbonic acid.[12] The presence of the latter may be proved by decanting some of the remaining air into a bottle, and then shaking some lime water with it, which will absorb the carbonic acid and form chalk.
Into an ale glass, two thirds full of water at about 140 degrees, drop one or two pieces of phosphorus about the size of peas, and they will remain unaltered. Then take a bladder containing oxygen gas, to which is attached a stop cock and a long fine tube. Pass the end of the tube to the bottom of the water, turn the stop cock, and press the bladder gently. As the gas reaches the phosphorus it will take fire, and burn under the water with a brilliant flame, filling the glass with brilliant flashes of light dashing through the water.
Into another glass put some cold water; introduce carefully some of the salt called chlorate of potash; upon that drop a piece of phosphorus; then let some strong sulphuric acid (oil of vitriol) trickle slowly down the side of the glass, or introduce it by means of a dropping bottle.
As soon as the acid touches the salt the latter is decomposed, and liberates a gas which ignites the phosphorus, producing much the same appearance as in the last experiment.
Into the half of a broken phial put some chlorate of potash, and pour in some oil of vitriol. The phial will soon be filled with a heavy gas of a deep yellow color. Tie a small test tube at right angles to the end of a stick not less than a yard long, put a little ether into the tube, and pour it gently into the phial of gas, when an instantaneous explosion will take place, and the ether will be set on fire. This experiment should be performed in a place where there are no articles of furniture to be damaged, as the ingredients are often scattered by the explosion, and the oil of vitriol destroys all animal and vegetable substances.
Into a jar containing oxygen gas introduce a coil of soft iron wire, suspended to a cork that fits the neck of the jar and having attached a small piece of charcoal to the lower part of the wire, ignite the charcoal. The iron will take fire and burn with a brilliant light, throwing out bright scintillations, which are oxide of iron, formed by the union of the gas with the iron; and they are so intensely hot[13] that some of them will probably melt their way into the sides of the jar, if not through them.
But by far the most intense heat, and most brilliant light, may be produced by introducing a piece of phosphorus into a jar of oxygen. The phosphorus may be placed in a small copper cup, with a long handle of thick wire passing through a hole in a cork that fits the jar. The phosphorus must first be ignited; and as soon as it is introduced into the oxygen, it gives out a light so brilliant that no eye can bear it, and the whole jar appears filled with an intensely luminous atmosphere. It is well to dilute the oxygen with about one-fourth part of common air, to moderate the intense heat, which is nearly certain to break the jar if pure oxygen is used.
The following experiment shows the production of heat by chemical action alone: Bruise some fresh-prepared crystals of nitrate of copper, spread them over a piece of tin foil, sprinkle them with a little water; then fold up the foil tightly, as rapidly as possible, and in a minute or two it will become red hot, the tin apparently burning away. This heat is produced by the energetic action of the tin on the nitrate of copper, taking away its oxygen in order to unite with the nitric acid, for which, as well as for the oxygen the tin has a much greater affinity than the copper has.

Combustion without flame may be shown in a very elegant and agreeable manner, by taking a coil of platinum wire and twisting it round the stem of a tobacco pipe, or any cylindrical body for a dozen times or so, leaving about an inch straight, which should be inserted into the wick of a spirit lamp. Light the lamp, and after it has burned for a minute or two, extinguish the flame quickly; the wire will soon become red hot, and, if kept from draughts[14] of air, will continue to burn until all the spirit is consumed.
Spongy platinum, as it is called, answers rather better than wire, and has been employed in the formation of fumigators for the drawing-room, in which, instead of pure spirit, some perfume, such as lavender water, is used; by its combustion an agreeable odor is diffused through the apartment. These little lamps were much in vogue a few years ago, but are now nearly out of fashion. Finally, all the readers of this little book should be very careful in performing all experiments. If possible, he should use a room with a stone floor and no curtains, while an outhouse with an earthen floor is still less dangerous.
A most interesting class of experiments can be made with an air pump, a piece of apparatus unfortunately beyond the pocket-money supply of the average boy. Nevertheless, if the following instructions are exactly followed and carefully carried out, a very excellent air pump can be made at a comparatively small cost. Some pretty, as well as interesting results will amply repay you for the trouble you take to make the pump. Although the air seems so light in comparison with water or a heavy metal like iron, you must remember that it really presses upon every square inch of the earth’s surface, aye, on every square inch of your own bodies, with a force of fourteen and a half pounds. In other words, the weight of the air at the sea level resting on each square inch of surface weighs fourteen and a half pounds.
Don’t be frightened, boys, at the explanation of one word that must be used in connection with air experiments. The word is vacuum. Vacuum really means an empty space, devoid of all matter, even of air. Although it seems easy to think of an empty space, it is quite impossible to exhaust a space of all matter, even of air. For this reason, the alchemists of the middle ages used to say: “Nature abhors a vacuum.” This was only their way of saying how impossible it was to make a space, such as the inside of a vessel, quite empty. Yet it is possible to reduce the amount of air in a vessel almost to nothing.
Now for the pump. In the first place obtain three pieces of gutta-percha tubing of the following lengths:
No. 1.—A tube twelve and a half inches long, measuring outside two and a half, inside one and a half inches in circumference.
No. 2.—This must be seven and a half inches long, one and a half inches outside, and an inch inside.
No. 3.—This is a length of tubing about sixty inches long, two and a half inches in outside circumference, and at least an inch thick. If an inch and a half thick all the better, as it will be more air-tight.
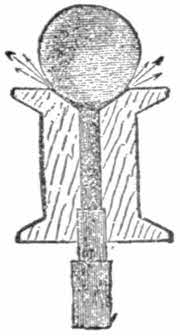
Divide tube No. 2 into two equal parts, cutting from right to left at an angle of 45 degrees. Into one of the parts fit a plug of hard wood pierced lengthwise by a red hot wire (fig. 1); the figure shows the shape of it sufficiently. In the hollow side cut a small opening, and over this tie very tightly a band of flexible india-rubber (fig. 3). This band will serve as the valve of the piston of the pump. Figs. 3 and 4 give a side and front view of this valve. Great care must be taken neither to split the plug in boring the hole nor to cut the tube.
This valve must now be inserted in the large tube No. 1, as seen in fig. 2.
At the other end of the large tube, which will serve as the body of the pump, at B fig. 2, fix a similar valve to the above, but the india-rubber band must be fixed on the other side of the valve as at B fig. 2. The fitting A will serve for escape, the second for withdrawing the air from the space to be exhausted. Finally fix tube No. 3 on valves A or B, fig. 2, according to your wish to produce a vacuum or to compress the air.
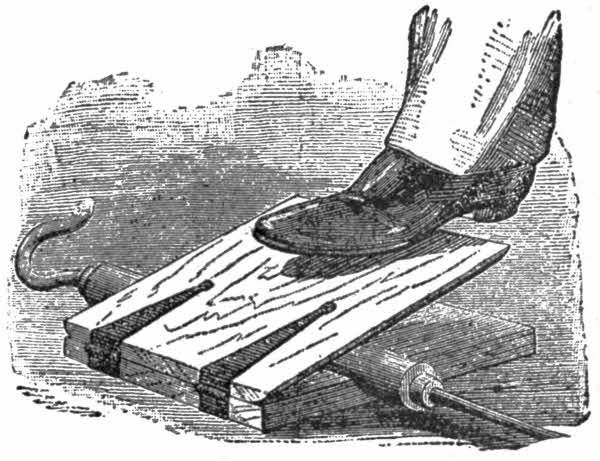
Fig. 5.
By means of a pedal made simply with two boards put together on hinges (fig. 5), one pressed with the foot, the air contained in the body of the pump (fig. 2) tends to escape. It therefore lifts the valve of the fitting fixed at A, and escapes through the flexible elastic band tied over the hole in the hollow side of tube No. 2. If the pressure ceases the big tube, on account of its own elasticity, takes its former form and sucks in the air. This time it is the valve at B which is lifted and lets pass the air which fills the body of the pump. If one has fixed on to the fitting at B, the long india-rubber tube No. 3, which is plunged in a receiver—a receiver is any vessel in which the air is exhausted, or into which it is forced—it is easily understood[18] that after a few moves of the pedal, the air is drawn out, and a vacuum is obtained.

Fig. 6.
If one wishes to have a force-pump one has only to modify slightly the construction of the valve. Instead of a band of india-rubber fixed as shown in fig. 3, it is altered as in fig. 4, that is to say the valve is formed by a band of supple india-rubber fastened by two tacks only on one side of the opening in the side of the plug. For this object it is also necessary to take stronger tubes.
Let us now review the few experiments that can be made with this machine.
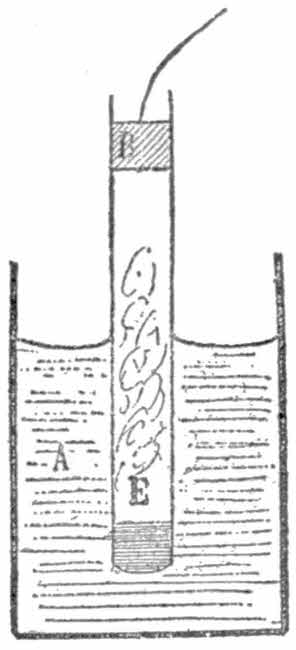
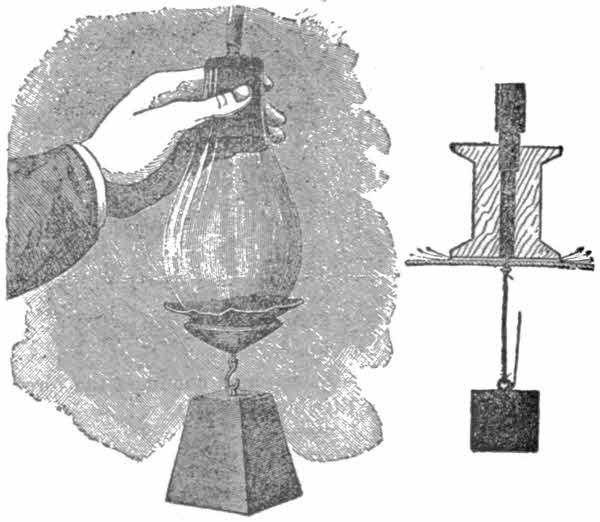
In order to conduct experiments a receiver must be obtained. The best vessel for your purpose is a large bell-jar with a ground glass stopper and neck to insure absolute tightness. Such a jar may be cheaply obtained at a[19] scientific instrument maker’s for about seventy-five cents. If you cannot get a bell-jar procure a 4-lb. jam pot and a tightly-fitting bung. In the middle of the latter bore a hole to admit a glass tube, some six inches long and an inch in diameter, and then sealing-wax the whole of the upper surface of the bung so that air cannot enter. Over the projecting end of the glass tube, bind very tightly the free end of the long tubing affixed to the pump. To ensure tight binding, waxed thread should be used.
Put a mouse—it is necessary to catch him first—into the receiver, and work the pump. Soon the animal will show all the signs of being choked, and eventually will die. This is proof sufficient that animals cannot live without air.
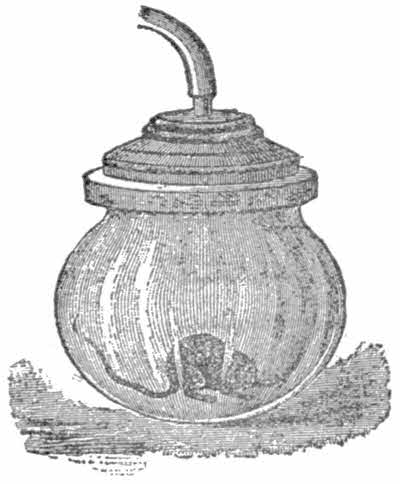
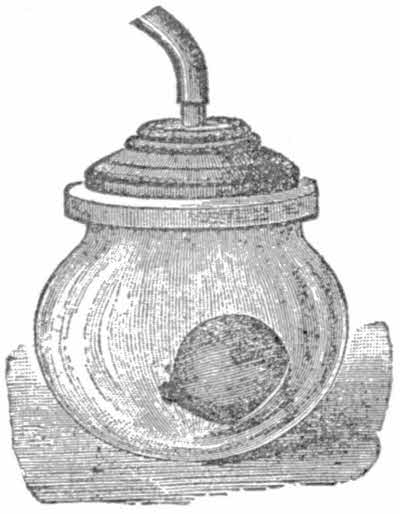
Place in the receiver a small bladder, such as are sold in the streets for a few cents. Wet it a little to make it more supple. Now, in the ordinary way the air inside the bladder[20] exerts the same pressure on the skin of the bladder as does the air on the outside. Now work the pedal so that the air in the receiver is gradually exhausted. The bladder will be seen to gradually swell and finally burst. It bursts because as the air in the receiver is exhausted by the pump, the air outside the bladder exerts a less force than the air inside. But the air inside is confined by the bladder skin, a not very strong material, as you know, so as soon as the difference between the inside and outside pressures is greater than the strength of the bladder, the latter bursts. This experiment also shows the expansible power of air.
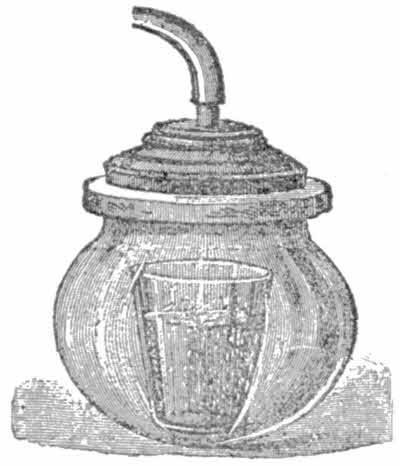
Place in the receiver a tumbler of cold water and work the pump as before. In a few minutes, as soon as the air is sufficiently exhausted, the water will apparently boil. Yet you know the water does not boil in a kettle unless heated to 212 degrees. This phenomenon is thus explained: The[21] vacuum causes the air-bubbles contained in the water to escape. They easily do so, because there is scarcely any reserve on the surface of the liquid (see fig.).
This force, the pressure of the air which you have just ascertained, supplies various experiments in its illustration.
Take a tin tube, for example, the tin holder of a penny pen, which you may procure at any stationer’s. Put a little water in it and make it boil so that the steam takes the place of the air.
When steaming furiously stop the mouth of the tube with a small cork, sealing the opening hermetically. Oil it a little, so it may glide with ease. If you cool the tube by plunging it in a basin of cold water, for example, the steam is condensed, forming a vacuum in the interior, and under the atmospheric pressure the cork will glide down. Fasten a string to the cork and you can withdraw it and begin the operation again. As the water gets hot, steam is reformed; you will see the cork come up again.
A capital way of making this cork is to stick the tube in a piece of potato, cutting out of the latter a perfectly-fitting cork.
Instead of a jar-receiver, take a long-necked bottle open at both ends. If you place the hand on one of the open ends and exhaust the air, by attaching the long tube of the pump to the other you cannot remove the hand easily. Do not try to pump the air out entirely, as the suction may be too strong and draw blood. It is by the rarefaction of the air that the cupping-glass is applied to people who require bleeding. In the antiquated surgical operation of cupping, the doctor burned a few pieces of paper in small[23] glass cups, which are then applied to the skin; the air, in getting cold, contracted and produced a partial vacuum, thus acting as the bottle did in the above experiment.

Now you shall learn something about the pressure exercised by the atmospheric layer which surrounds the earth to the height of about forty miles. This is done with the aid of a very well-known instrument called the barometer.
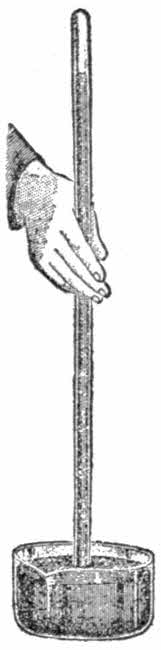
You may construct one yourselves. Procure a glass tube closed at one end, about a yard long and one tenth of an inch in diameter. Fill it with mercury, then turn it upside down into a bowl filled with the same metal, taking care that the air does not enter the tube. The column will stop at a height between 29 and 30 inches.
This, therefore is the measure of the force of the air’s pressure, for in the upper part of the tube there is an absolute vacuum and nothing would prevent the mercury from going higher up. The weight of the air layer corresponds, therefore, to a height of nearly 30 inches of mercury.
This weight has been before stated, viz., fourteen and a half pounds, such a weight being supported by every single square inch of the globe’s surface. A marvelous pressure is thus exerted on the whole earth. In other words, the weight of the air that surrounds the earth on all sides[25] is no less than the following enormous number of 5,184,740,000,000,000 tons.
A man of average height, himself supports the enormous pressure of 34,171 pounds, or over 15 tons, and yet does not feel the least inconvenience in his movements. It is because this pressure is exercised in all directions, and a human body carries within it elastic fluids that counterbalance that tremendous weight.
So accustomed do people become to this weight that when the weather is stormy, a feeling of heaviness comes on.
However, it is just the contrary which takes place when the barometer is lower; that is to say, the atmospheric pressure has diminished. Consequently there is less weight to be carried.
You would experience the same sensation when going up in a balloon. As you rise higher and higher the weight of the air is less felt, and this makes people so uncomfortable that at a height of about 9,000 or 10,000 yards the liquids in our body—the blood, the water, the bile—tend to escape outwards. Why? Because they are no longer balanced by an outside pressure equal in force to them. In fact, if you continued to ascend, your fate would be that of the bladder in the first experiment—you would burst. Thus are you and all creatures attached to the face of the earth, and it seems as if great heights were forbidden to our curiosity.
Construct a toy house of cardboard, painted, and let there be two open doorways in the front, and let it stand on a wooden platform to represent the ground. The two sides and back may come right down to the ground, but there must be a slight space between the front of the house and the ground upon which it stands.
Next make a flat wheel or disc of wood about the thickness of a penny, its diameter or measurement across the center to measure the same as the distance between the two doorways of the house. The wheel disc or turn-table must have a shaft or spindle in the middle, so that it will revolve easily in a hole made for it in the floor or ground which[26] your cardboard house stands on; this pivot-hole should be just within the house and exactly half way between the two doors.
In the next place get two small dolls of such size that they will pass easily through the doorways, or you may cut them out of cork or some light substance. Dress one to represent an old man and the other as his wife, and fix them opposite each other at the edge of the disc or wheel in such a manner, that when it turns on its axle, the figures move in and out of the two doorways provided for their accommodation, for it appears that, although residing in the same house, they are not on very good terms. When the husband goes out the wife remains at home, and as she only ventures abroad in fine weather, her spouse is obliged to look out when rain may be expected.
The motive power has now to be provided and this takes the form of a piece of catgut, such as violin strings are made of; this is a substance very susceptible of atmospheric influences, for dry weather contracts or tightens it, while a damp atmosphere causes it to relax. Double your catgut and twist it, fasten one end of the rope so formed near the back of the house inside and fasten the other to the pivot or axle, with two or three turns round it. As the weather changes the tightening or relaxing of the rope will cause the figures to move in and out of the house. Of course, the figures must be arranged so that the lady comes out when the rope is tightened by the dryness of the atmosphere.
To make experiments with compressed air, you must put your wits together to make a reservoir. Air, you know, is a gas consisting of particles called atoms. These atoms are at a certain distance from one another. They can be pushed further from one another as when you heat them, or closer together by cold and compression. So compressed air only means air whose atoms are pressed more closely together than as the case with the air around us.
Now you have heard that a column of air on a square inch weighs fourteen and a half pounds. Also, you know that air in a receiver or any other vessel presses on the vessel[27] inside and out with a force (or weight) of fourteen and a half pounds.
If now into the vessel you push another quantity of air, equal to the vessel’s capacity, you simply push the atoms of air closer together. In fact, they are now only half as far apart as the atoms of an open vessel. But the pressure is doubled and the compressed air, therefore, will press on the inside of the vessel with a force of twenty-nine pounds.
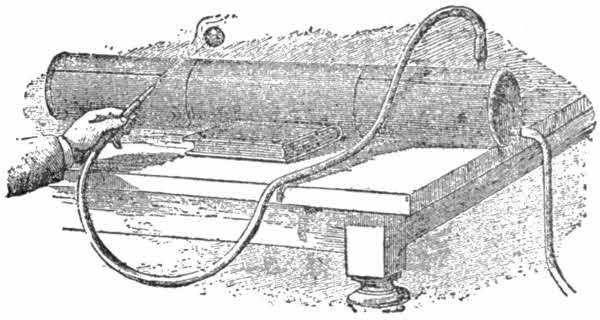
Now to make the reservoir. Get a tin tube about 40[28] inches long and four in diameter, closed at both ends. Take care that the soldering is well done. Two openings must be made, and a small tube inserted in each. To each of these attach an indiarubber tube, one four feet long, and the other six. (See fig.).
To fill this reservoir with compressed air, apply the air-pump fitted with the valve shown in fig. 4, in the description of an air-pump. Squeeze tightly the upper tube of the reservoir before beginning to pump, and then it will be easy to judge the amount of compression of the air. For the first experiment place a light ball or sheet of paper over the mouth of the tube, and loosen your hold on it. The object will immediately be blown away with considerable force.
We know that sound is a succession of vibrations which must be transmitted in a medium with weight, as air or water; in other words, in a vacuum there can be no sound at all. To prove this, introduce into the receiver a small bell, and as the air is extracted the sounds become weaker[29] and weaker, and cease altogether when the air is completely rarified.
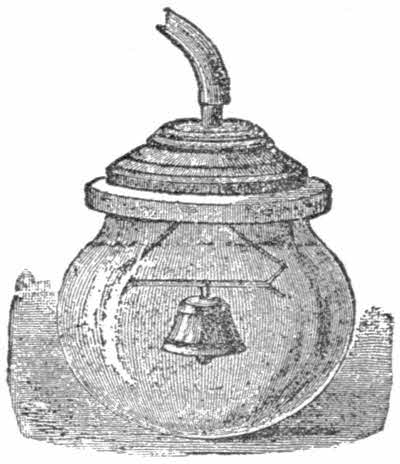
Tie a thin piece of light indiarubber round the top of the bottle, and you will notice that as the air is withdrawn, the indiarubber will stretch, and at length form a round small balloon in the interior of the bottle. (Fig. 1).
If a piece of bladder is tightly stretched and tied round the vessel (fig. 2.) it will burst under the force of the atmospheric pressure which acts upon it, through a vacuum having been made underneath. This is another case of the first experiment with the air pump described above.
Another experiment will still better make you appreciate the value of this factor: the weight of the air.
Put a piece of supple leather in which a ring is attached under the bottle; pump the air out of the latter and you will be astonished at the weight you may hang on this leather without dragging it off.
Should you not have at hand a glass receiver, a wooden reel may serve instead (see fig.). On one of its faces place a piece of strong cardboard, in the middle of which a hook has been fastened; when the rarefaction is made, rather heavy weights must be hooked on before the cardboard is detached from the face of the rest.
Fuse together in a crucible, eight parts of bismuth, five of lead and three of tin; these metals will combine and form an alloy, of which spoons may be made, possessed of the remarkable property of melting in boiled water.
Take a wooden reel and hollow out either the top or bottom,[31] beginning at the hole in the center and working towards the edge. In the hollow place a ball. Apply to the other end the indiarubber tube which conducts the forced air, and the ball will be lifted up (see fig.).
If you are about to perform a conjuring trick, you will, of course take great care that your apparatus is ready. Therefore, clean your piece of iron or steel from dirt. Dip a piece of polished iron—the blade of your knife, for instance—into a solution, either of nitrate or sulphate of copper, when it will assume the appearance of a piece of pure copper.
Before entering into the next series of experiments the young chemist must know that all the substances of which the world and everything in it are made up—i.e., the elements are arranged in two classes, the metals and the non-metals. The former are by far the more numerous, altogether numbering more than fifty. Among the better known are such well known substances as iron, mercury, copper, tin, potassium, antimony, strontium, and nickel. The non-metals are more widely distributed and together made up of the bulk of the universe.
They comprise the gases—oxygen, hydrogen, nitrogen, and chlorine, and such substances as sulphur, carbon, phosphorus and iodine. To the latter class also belongs a peculiar element called fluorine, which, when combined with hydrogen, destroys glass. It is the only liquid known which cannot be contained in a glassen or earthenware vessel, and when used for experimental purposes must be kept in a leaden bottle.
Of course it will be understood that the above is not a complete list by any means, but is sufficient to give a clear idea of the difference between the two classes. The metals generally speaking are of a more or less sparkling, lustrous appearance. The metals, too, are good conductors of heat and electricity, and generally heavy. These characteristics are almost entirely wanting in the non-metals. We shall now give some tricks with the metals.
Potassium was discovered by Sir H. Davy, in the beginning of the present century, while acting upon potash with the enormous galvanic battery of the Royal Institution, consisting of two thousand pairs of four inch plates. It is a brilliant metal, so soft as to be easily cut with a penknife, and so light as to swim upon water, on which it acts with great energy, uniting with the oxygen and liberating the hydrogen, which takes fire as it escapes.
Trace some continuous lines on paper with a camel’s-hair brush dipped in water, and place a piece of potassium about the size of a pea on one of the lines, and it will follow the course of the pencil, taking fire as it runs, and burning with a purplish light.
The paper will be found covered with a solution of ordinary potash. If turmeric paper be used, the course of the potassium will be marked with a deep brown color. Hence if you touch potassium with wet fingers you will burn them.
If a small piece of the metal be placed on a piece of ice, it will instantly take fire, and form a deep hole which will be found to contain a solution of potash.
In consequence of its great affinity for oxygen, potassium must be kept in some fluid destitute of it, such as naphtha[33] acid, which has been displaced by the great affinity or liking of the oxygen and acid for the copper.
2. When the copper is no longer coated, but remains clean and bright when immersed in the fluid, all the silver has been deposited, and the glass now contains a solution of copper.
Nearly all the colors used in the arts are produced by metals and their combinations; indeed, one is named chromium, from a Greek word signifying color, on account of the beautiful tints obtained from its various combinations with oxygen and the other metals. All the various tints, of green, orange, yellow and red are obtained from this metal.
Solutions of most of the metallic salts give precipitates with solutions of alkalies and their salts, as well as with many other substances, such as what are usually called prussiate of potash, hydrosulphret of ammonia, etc. The colors differ according to the metal employed; and so small a quantity is required to produce the color, that the solutions before mixing may be nearly colorless.
To a solution of sulphate of iron add a drop or two of a solution of prussiate of potash, and a blue color will be produced.
2. Substitute sulphate of copper for iron, and the color will be a rich brown.
3. Another blue, of quite a different tint, may be produced by letting a few drops of a solution of ammonia fall into one of sulphate of copper, when a precipitate of a light blue falls down, which is dissolved by an additional quantity of the ammonia, and forms a transparent solution of the most splendid rich blue color.
4. Into a solution of sulphate of iron, drop a few drops of strong infusion of galls, and the color will become a bluish black—in fact ink. A little tea will answer as well as the infusion of galls. This is the reason why certain stuffs formerly in general use for dressing-gowns for gentlemen were so objectionable; for as they were indebted to a salt of iron for their color, buff as it was called, a drop of tea accidentally spilled produced all the effect of a drop of ink.
5. Put into a largish test tube two or three small pieces of granulated zinc, fill it about one-third full of water, put in a few grains of iodine, and boil the water, which will at first acquire a dark purple color, gradually fading as the iodine combines with the zinc. Add a little more iodine from time to time, until the zinc is nearly all dissolved. If a few drops of this solution be added to an equally colorless solution of corrosive sublimate (a salt of mercury), a precipitate will take place of a splendid scarlet color, brighter, if possible, than vermilion, which is also a preparation of mercury.
Some of the metals assume certain definite forms in return from the fluid to the solid state. Bismuth shows this property more readily than most others.
Melt a pound or two of bismuth in an iron ladle over the fire; remove it as soon as the whole is fluid; and when the surface has become solid break a hole in it and pour out the still fluid metal from the interior; what remains will exhibit beautifully formed crystals of a cubic shape.
Sulphur may be crystallized in the same manner, but its fumes, when heated, are so very unpleasant that few would wish to encounter them.
One of the most remarkable facts in chemistry—a science abounding in wonders—is the circumstance that the mere contact of hydrogen, the lightest body known, with the metal platinum, the heaviest when in a state of minute division called spongy platinum, produces an intense heat sufficient to inflame the hydrogen; of course this experiment must be made in the presence of atmospheric air or oxygen. If a small piece of the metal in the state above named be introduced into a mixture of oxygen and hydrogen, it will cause them to explode. A very small quantity of gas should be employed and placed in a jar lightly covered with a card, or the explosion would be dangerous.
Nearly all the metals are characterized by the crystals, which are formed as they pass from a state of intense heat[35] to that of comparative coldness. It is by this process they have been formed when in the mine or vein in the rocks. The earth was once a fiery mass of molten matter, as seen even now when a volcano is in a state of eruption. And it was only by the cooling of the outside shell of the earth, or crust, as it is called, that it became habitable.
When the crust was cooling down the metals crystallized among the cooling rocks and gradually formed the crude arts. You may represent by a very pretty experiment the manner in which this cooling off of the earth took place. Obtain a little flour of sulphur and put it in a red earthenware unglazed jar. Thrust it well into the fire and watch the rust. As soon as the heat has penetrated the vessel the yellow powdery sulphur becomes first of all brown, and then assumes the consistency of thick birdlime. Take out a little of this on the end of a stick and plunge it into cold water. It can then be pulled backwards and forwards like cobblers’ wax. This well represents the state of the half-cooled crust of the earth.
Meanwhile the sulphur on the fire begins to boil, and looks very much like bubbling treacle. Remove it from the fire and allow it to cool. When quite cool the surface will be a flat, yellow mass, like ordinary roll sulphur, which, when ground, give the ordinary flour of sulphur.
With a sharp knife separate the mass from the vessel and look at the under-surface. There it will be found to have assumed a very different form, owing to the exclusion of the air, and consequent slower cooling. Large six-sided crystals, transparent, and of a most exquisitely delicate yellow, will be seen, piled on one another as appear the masses of ore in rocks.
Nature always works in such cases on such a gigantic scale that it seems at first difficult to believe that such huge piles as the Giant’s Causeway in Ireland, or Fingals in Scotland, or the lodes of tin ore in Cornwall, worked by the Phœnicians three thousand years ago, and still being worked, were all formed by the same process.
The time that the earth must have taken to cool fairly staggers the imagination, yet it is only from guessing, by means of such a study as this, that geologists are able to form any idea of how long ago it was that the earth’s crust[36] became cool enough to allow animal and plant life to exist upon it.
The most beautiful crystalline form is perhaps the diamond, and yet this precious gem is but the same thing, chemically, as charcoal. Charcoal is pure carbon in the uncrystallized state, which the magic of crystallization has transformed into the symbol of all that is brilliant and beautiful.
Dissolve alum in hot water until no more can be dissolved in it; place in it a smooth glass rod and a stick of the same size. Next day the stick will be found covered with crystals, but the glass rod will be free from them. In this case the crystals cling to the rough surface of the stick, but have no hold upon the smooth surface of the glass rod.
But if the rod be roughened with a file at certain intervals, and then placed in the alum and water, the crystals will adhere to the rough surfaces, and leave the smooth bright and clear.
Tie some threads of lamp-cotton irregularly around a copper wire or glass rod. Place it in a hot solution of blue vitriol, strong as above, and the threads will be covered with beautiful blue crystals, while the glass rod will be bare.
Bore a hole through a piece of coke, and suspend it by a string from a stick placed across a hot solution of alum. It will float. But as it becomes loaded with crystals it will sink in the solution according to the length of the string. Gas-coke has mostly a smooth, shining, and almost metallic surface, which the crystals will avoid, while they will cling only to the most irregular and porous parts.
If powdered turmeric be added to the hot solution of alum the crystals will be of a bright yellow. Litmus will cause them to be of a bright red. Logwood will yield purple; and common writing ink, black. And the more muddy the solution the finer will be the crystals.
To keep colored alum crystals from breaking or losing their color, place them under a glass shade with a saucer of water.
This will preserve the atmosphere moist, and prevent the crystals getting too dry.
If crystals be formed on wire they will be liable to break off, from the expansion and contraction of the wire by changes of temperature.
Dissolve camphor in spirit of wine, moderately heated, until the spirit will not dissolve any more; pour some of the solution into a cold glass, and the camphor will instantly crystallize in beautiful tree-like forms, such as we see in the show-glasses of camphor in druggists’ windows.
Heat some blue vitriol (sulphate of copper) in an iron ladle till all the water contained in the crystals is driven off, and the color changes to a gray. Take the lumps out without breaking them, and lay the dried blue vitriol on a plate. If this be moistened with water steam is produced; and if a slice of phosphorus is then laid on the sulphate of copper it ignites, demonstrating again that the condensation of a liquid produces heat. The addition of the water restores the blue color, thus proving that water was necessary to the composition of blue vitriol.
Mix five parts by weight of powdered sal ammoniac, five parts of nitre in powder, and sixteen parts of water. A temperature of twenty-two degrees below the freezing point of water is produced; and if a phial of water, or any convenient metallic cylinder containing water, be surrounded with a sufficient quantity of the freezing mixture, ice is formed. The ice clings to the interior of the tube, but may easily be removed by dipping it in tepid water.
This experiment is the reverse of the last and proves that the sudden reduction of a solid to the liquid condition always affords cold.
An amusing combination of two experiments may be made by putting some fresh-burned lime into one tea pot and this freezing mixture into another. When water is poured on the one containing lime, it gives out steam[38] from the spout, while the addition of water to the other produces so much cold that it can hardly be kept in the hand. Thus heat and cold are afforded through the same medium, water.
Melt a small quantity of the sulphate of potash and copper in a spoon over a spirit lamp. It will be fused at a heat just below redness, and produce a liquid of a dark-green color. Remove the spoon from the flame, when the liquid will become a solid of a brilliant emerald green color, and so remain until its heat sinks nearly to that of boiling water, when suddenly a commotion will take place throughout the mass, beginning from the surface, and each atom, as if animated, will start up and separate itself from the rest, till in a few moments the whole will become a heap of powder.
Provide two small pieces of glass; sprinkle a minute portion of sulphur upon one piece, lay thin slips of wood around it, and place upon it the other piece of glass. Move them slowly over the flame of a lamp or candle, and the sulphur will become sublimed, and form gray, nebulous patches, which are very curious microscopic objects. Each cluster consists of thousands of transparent globules, imitating in miniature the nebulæ which we see figured in treatises on astronomy. By observing the largest particles we shall find them to be flattened on one side. Being very transparent, each of them acts the part of a little lens, and forms in its focus the image of a distant light, which can be perceived even in the smaller globules, until it vanishes from minuteness. If they are examined again after a certain number of hours, the smaller globules will generally be found to have retained their transparency, while the larger ones will have become opaque, in consequence of the sulphur having undergone some internal spontaneous change. But the most remarkable circumstance attending this experiment is that the globules are found adhering to the upper glass only; the reason of which is that the upper glass is somewhat cooler than the lower one, by which[39] means we see that the vapor of sulphur is very powerfully repelled by heated glass. The flattened form of the particles is owing to the force with which they endeavor to recede from the lower glass, and their consequent pressure against the surface of the upper one. This experiment is considered by its originator, Mr. H. F. Talbot, to be a satisfactory argument in favor of the repulsive power of heat.
Although glass is a bad conductor it yet allows heat to pass through it, and the purer the glass the more easy is this done. Heat a poker red hot, and having opened a window, apply the poker very near to the outside of the pane, and the hand to the inside. A strong heat will be felt at the instant, which will cease as soon as the poker is withdrawn, and may be again renewed and made to cease as quickly as before. It is well known that if a piece of glass be so much warmed as to convey the impression of heat to the hand, it will retain some part of that heat for a minute or more; but in this experiment the heat will vanish in a moment. It will not, therefore, be the heated pane of glass that we shall feel, but heat which has come through the glass in a free or radiant state.
All metals do not conduct heat at the same rate as may be proved by holding in the flame of a candle at the same time a piece of silver wire and a piece of platina wire, when the silver wire will become too hot to hold, much sooner than the platina. Or cut a cone of each wire, tip it with wax, and place it upon a heated plate (as a fire-shovel), when the wax will melt at different periods.
Mix a small quantity of chlorate of potash with spirit of wine in a strong saucer; add a little sulphuric acid, and an orange vapor will arise and burst into flame with a loud crackling sound.
Place before a fire a set of polished fire-irons, and beside them a rough, unpolished poker, such as is used in the[40] kitchen, instead of a bright poker. The polished irons will remain for a long time without becoming warmer than the temperature of the room, because the heat radiated from the fire is all reflected, or thrown off, by the polished surface of the irons, and none of it is absorbed. The rough poker will, however, become speedily hot, so as not to be used without inconvenience. Hence, the polish of fire-irons is not merely ornamental, but useful.
Provide an iron rod, and fit it exactly into a metal ring; heat the rod red hot, and it will no longer enter the ring.
Observe an iron gate on a warm day, when it will shut with difficulty; whereas it will shut loosely and easily on a cold day.
Dissolve in water a small quantity, about as much as will lay on a ten-cent piece, of chloride of cobalt, which is of a bluish-green color, and the solution will be pink; write with it and the characters will scarcely be visible; but if gently heated they will appear in brilliant green, which will disappear as the paper cools.
Dissolve in water a few grains of prussiate of potash; write with this liquid, which is invisible when dry; wash over with a dilute solution of iron, made by dissolving a nail in a little aqua fortis; a blue and legible writing is immediately apparent.
Put a small portion of the compound called mineral chameleon into several glasses. Pour upon each water at different temperatures and the contents of each glass will exhibit a different shade of color. A very hot solution will be of a beautiful green color; a cold one a deep purple.
Make a colorless solution of sulphate of copper; add to it a little ammonia equally colorless, and the mixture will be of an intense blue color; add to it a little sulphuric acid, and the blue color will disappear; pour in a little solution of caustic ammonia, and the blue color will be restored. Thus may the liquor be changed at pleasure.
Dissolve indigo in diluted sulphuric acid, and add to it an equal quantity of solution of carbonate of potash. If a piece of white cloth be dipped in the mixture it will be changed to blue; yellow cloth, in the same mixture, may be changed to green; red to purple; and blue litmus paper to red.
Nearly fill a wine glass with the juice of beet-root, which is of a deep red color; add a little lime water and the mixture will be colorless; dip into it a piece of white cloth, dry it rapidly, and in a few hours the cloth will become red.
Mix a little solution of subacetate of lead with port wine; filter the mixture through blotting-paper, and a colorless liquid will pass through; to this add a small quantity of dry salt of tartar; distill in a retort, when a spirit will arise, which may be inflamed.
More than two-thirds of the earth’s surface is water, so that in mere quantity alone it is the most important substance with which we are acquainted. Without it life would be impossible, for, owing to its quality of dissolving other bodies, it may be regarded as the great purifier, as well as the vehicle which brings nourishment to plants and animals alike.
Not only is water useful, but is among the most beautiful of Nature’s products. It has carved the valleys between mountain ranges by its slow dropping for ages, and has made the fairy glens by rushing down their sides in torrents. The stately rivers and the roaring oceans are but forms of its might.
In another state it works out those fantastic grottoes, mountains and fields of glittering white, that make the Polar seas the very head center of dreamland.
In still another form it paints the rainbow in the sky, and hangs like a veil over the landscape, passing from the most delicate blue over the plain to the deep purple clinging to distant hills.
To it the golden and red hues of sunrise and sunset are due. The light fleecy clouds that speak the beauty of spring, and the great thunder stocks that gleam, with lightning flashes are all composed of water, and water alone.
It drives our engines and machinery, and speeds our ships across the sea. Neither is it confined to this earth alone, for astronomers tell us that vast seas and even clouds can be seen on the next great planet to the earth, Mars.
Surely, then, as this wondrous substance is examined, the ancients can be excused for worshiping the ocean as a god, and the old alchemists for believing it to be an element.
Nevertheless, water is not a simple substance. It is composed of two gases, which must be combined before water is produced. These gases are oxygen and hydrogen. Every atom of water consists of one part of the former gas and two parts by volume of the latter. This you may prove in the following way:
Buy a piece of sodium, a metal that must not be touched with the fingers, and thrust it into a small one-ounce jar half full of water; cork the jar tightly.
Through a hole in the cork pass a glass tube, the outer end being drawn in a flame to a fine point. Apply a light at the end of the tube. The escaping gas will catch fire and burn with a light blue flame. This gas is hydrogen.
Next empty the jar and fill with warm water, and place by means of another cork a small glass jar on to the tube. Into the lower jar drop a piece of blazing hot platinum. Repeat this again and again with the same piece of platinum, being careful not to uncork the upper jar, so that every time the metal is dropped into the lower jar, you remove the upper jar with the tube and two corks. After doing this a dozen times or more take a match that is still glowing after having been extinguished, and plunge it into the upper jar. It will burst into flame immediately, and the gas in the upper jar is oxygen.
It has been discovered that a mixture of nitrate of silver[43] with hyposulphite of soda, both of which are remarkably bitter, will produce the sweetest known substance.
Write with French chalk on a looking-glass; wipe it with a handkerchief and the lines will disappear; breathe on it and they will reappear. This alteration will take place for a great number of times, and after the lapse of a considerable period.
Rub together in a mortar a small quantity of sulphate of soda and acetate of lead, and as they mix they will become liquid.
Carbonate of ammonia and sulphate of copper, previously reduced to powder separately, will also, when mixed, become liquid, and acquire a most splendid blue color.
The greater number of salts have a tendency to assume regular forms, or become crystallized, when passing from the fluid to the solid state; and the size and regularity of the crystals depends in a great measure on the slow or rapid escape of the fluid in which they were dissolved.
Sugar is a capital example of this property; the ordinary loaf-sugar being rapidly boiled down, as it is called; while to make rock-candy, which is nothing but sugar in a crystallized form, the solution is allowed to evaporate slowly, and as it cools it forms into those beautiful crystals termed rock-candy. The threads found in the center of some of the crystals are merely placed for the purpose of hastening the formation of the crystals.
Water being a colorous fluid ought, one would imagine when mixed with other substances of no decided color, to produce a colorless compound. Nevertheless, it is to water only that blue vitriol or sulphate of copper owes its vivid blueness, as will be plainly evinced by the following simple experiment. Heat a few crystals of the vitriol in a fire-shovel, pulverize them, and the powder will be of a dull and dirty white appearance. Pour a little water upon this[44] when a slight hissing noise will be heard, and at the same moment the blue color will instantly reappear.
Under the microscope the beauty of this experiment will be increased, for the instant that a drop of water is placed in contact with the vitriol, the powder may be seen to shoot into blue prisms. If a crystal of prussiate of potash be similarly heated its yellow color will vanish, but reappear on being dropped into water.
Dissolve chloride of lime in water until it will dissolve no more; measure out an equal quantity of oil of vitriol; both will be transparent fluids; but if equal quantities of each be slowly mixed and stirred together, they will become a solid mass, with the evolution of smoke or fumes.
Rub together in a mortar equal quantities of the crystals of Glauber salts and nitrate of ammonia, and the two salts will slowly become a liquid.
Take the solid mixture of the solutions of muriate of lime and carbonate of potash, pour upon it a very little nitric acid, and the solid opaque mass will be changed to a transparent liquid.
Mix four drams of sulphuric acid (oil of vitriol) with one dram of cold water, suddenly, in a cup, and the mixture will be nearly half as hot again as boiling water.
Although this trick is performed by means of chemicals, yet its product is obtained really by the use of mechanical laws. We must remember that ice is exactly the same thing as water so far as its composition is concerned, differing only in its state of density.
Ice, water, and steam differ in density through the possession of a greater or less quantity of heat. Hence, the[45] turning of water into ice really is a case of the operation of mechanical laws.
Now for the experiment. Put into a wide-mouthed jam-jar a smaller glass vessel containing the water to be frozen. Around the latter put a mixture of sulphate of soda (Glauber’s salt) and hydrochloric acid (spirits of salts). The proportions must be eight parts of the former to five of the latter.
The action of these two chemicals on one another is to cause a cold of fifteen to seventeen degrees below zero, or forty-seven degrees below freezing point.
The same result may be obtained by mixing equal parts of nitrate of ammonia and water. In winter-time when the snow is on the ground, with a mixture of one part snow and one part common table salt an intense cold of twenty degrees below zero is obtained.
From this last fact we see how stupid are those people who sprinkle the salt on the pavements to get rid of the snow. True, the latter melts, but only after the production of intense cold, which is the cause of many diseases, not only slight ones like colds and chilblains, but too often the forerunners of consumption and other lung troubles.
Let there be no other light than a taper in the room; then put on a pair of dark-green spectacles, and having closed one eye view the taper with the other. Suddenly remove the spectacles and the taper will assume a bright red appearance; but if the spectacles be instantly replaced, the eye will be unable to distinguish anything for a second or two. The order of colors will therefore be as follows: green, red, green, black.
Soak a cotton wick in a strong solution of salt and water, dry it, place it in a spirit lamp, and when lit it will give a bright yellow light for a long time. If you look through a piece of blue glass at the flame, it will lose all its yellow light and you will only perceive feeble violet rays. If before the blue glass you place a pale yellow glass, the[46] lamp will be absolutely invisible, though a candle may be distinctly seen through the same glasses.
Hold over a lighted match a purple columbine or a blue larkspur, and it will change first to pink and then to black. The yellow of other flowers held as above will continue unchanged.
Thus, the purple tint will instantly disappear from a heart’s-ease, but the yellow will remain; and the yellow of a wall-flower will continue the same, though the brown streak will be discharged. If a scarlet, crimson, or maroon dahlia be tried, the color will change to yellow, a fact known to gardeners, who by this mode variegate their growing dahlias.
Some flowers which are red, become blue by merely bruising them. Thus, if the petals of the common corn-poppy be rubbed upon white paper, they will stain it purple, which may be made green by washing it over with a strong solution of potash in water. Put poppy petals into very dilute muriatic acid, and the infusion will be of a florid red color; by adding a little chalk, it will become the color of port wine; and this tint, by the addition of potash may be changed to green or yellow.
Hold a red rose over the blue flame of a common match and the color will be discharged wherever the fume touches the leaves of the flower, so as to render it beautifully variegated, or entirely white. If it be then dipped into water, the redness, after a time, will be restored.
Write upon linen with permanent ink (which is a strong solution of nitrate of silver), and the characters will be scarcely visible; remove the linen to a dark room, and they will not change; but expose them to a strong light, and they will be of an indelible black.
Cut a circular piece of card to fit the top of a hyacinth glass, so as to rest upon the ledge, and exclude the air. Pierce a hole through the center of the card, and pass through it a strong thread, having a small piece of wood tied to one end, which, resting transversely on the card, prevents it being drawn through. To the other end of the thread attach an acorn; and having half filled the glass with water, suspend the acorn at a short distance from the surface.
The glass must be kept in a warm room, and in a few days the steam will hang from the acorn in a drop, the skin will burst, and the root will protrude and thrust itself in the water, and in a few days more a stem will shoot out at the other end, and rising upwards, will press against the card, in which an orifice must be made to allow it to pass through. From this stem small leaves will soon be observed to sprout; and in the course of a few weeks you will have a handsome oak plant, several inches in height.
A variety of rays of light are exhibited by colored flames, which are not to be seen in white light. Thus pure hydrogen gas will burn with a blue flame, in which many of the rays of light are wanting.
The flame of an oil lamp contains most of the rays which are wanting in the sunlight. Alcohol mixed with water, when heated or burned, affords a flame with no other rays but yellow. The following salts, if finely powdered, and introduced into the exterior flame of a candle, or into the wick of a spirit lamp, will communicate to the flame their peculiar colors:
| Chloride of Soda (common salt) | Yellow. |
| “ of Potash | Pale violet. |
| “ of Lime | Brick red. |
| “ of Strontia | Bright crimson. |
| “ of Lithia | Red. |
| “ of Baryta | Apple green. |
| “ of Copper | Bluish green. |
| Borax | Yellow. |
Or either of the above salts may be mixed with spirit of wine, as directed, for Red Fire.
Burn spirit of wine on chloride of calcium, a substance obtained by evaporating muriate of lime to dryness.
Burn spirit of wine on a little powdered nitrate of silver.
Heat together potassium and sulphur, and they will instantly burn very vividly.
Heat a little nitre on a fire-shovel, sprinkle on it flour of sulphur, and it will instantly burn. If iron filings be thrown upon red hot nitre, they will detonate and burn.
Of heat and cold, as of wit and madness, it may be said that “thin partitions do their bounds divide.” Thus, paint one half of the surface of a tin pot with a mixture of lamp black and size, and leave the other half or side bright; fill the vessel with boiling water, and by dipping a thermometer, or even the finger, into it shortly after, it will be found to cool much more rapidly upon the blackened than the bright side of the pot.
Place upon the surface of snow, as upon the window-sill, in bright daylight or sunshine, pieces of cloth of the same size and quality, but of different colors, black, blue, green, yellow and white; the black cloth will soon melt the snow beneath it, and sink downwards; next the blue, and then the green; the yellow but slightly; but the snow beneath the white cloth will be as firm as at first.
The above fanciful appellation has been given to nitrous oxide, from the very agreeable sensations excited by inhaling it. In its pure state it destroys animal life, but loses this noxious quality when inhaled, because it becomes blended with the atmospheric air which it meets in the lungs. This gas is made by putting three or four drams of nitrate of ammonia in crystals into a small glass retort,[49] which being held over a spirit lamp, the crystals will melt, and the gas be evolved.
Having thus produced the gas, it is to be passed into a large bladder having a stop-cock; and when you are desirous of exhibiting its effects you cause the person who wishes to experience them to first exhale the atmospheric air from the lungs, and then quickly placing the cock in his mouth you turn it, and bid him inhale the gas. Immediately a sense of extraordinary cheerfulness, fanciful flights of imagination, an uncontrollable propensity to laughter, and a consciousness of being capable of great muscular exertion, supervene. It does not operate in exactly the same manner on all persons; but in most cases the sensations are agreeable, and have this important difference from those produced by wine or spirituous liquors, that they are not succeeded by any depression of mind.
Provide a glass tube about three feet long and half an inch in diameter; nearly fill it with water, upon the surface of which pour a little colored ether; then close the open end of the tube carefully with the palm of the hand, invert it in a basin of water, and rest the tube against the wall. The ether will rise through the water to the upper end of the tube; pour a little hot water over the tube, and it will soon cause the ether to boil within, and its vapor may thus be made to drive nearly all of the water out of the tube into the basin. If, however, you then cool the tube by pouring cold water over it, the vaporized ether will again become a liquid, and float upon the water as before.
Nearly fill a wine glass with diluted sulphuric acid, and place in it a wire of silver and another of zinc, taking care that they do not touch each other, when the zinc will be changed by the acid, but the silver will remain inert. But cause the upper ends of the wires to touch each other, and a stream of gas will issue from them.
A beautiful green fire may be thus made: Take of flour of sulphur thirteen parts, nitrate of baryta seventy-seven, chlorate of potash five, metallic arsenic two, and charcoal three. Let the nitrate of baryta be well dried and powdered; then add to it the other ingredients, all finely pulverized, and exceedingly well mixed and rubbed together. Place a portion of the composition in a small tin pan, having a polished reflector fitted to one side, and set light to it, when a splendid green illumination will be the result. By adding a little calamine it will burn more slowly.
Mix a grain or two of potassium with an equal quantity of sodium; add a globule of quicksilver, and the three metals, when shaken, will take fire and burn vividly.
Take a smooth cylindrical piece of metal, about one inch and a half in diameter, and eight inches long. Wrap very closely round it a piece of clean writing paper, then hold the paper in the flame of a spirit lamp, and it will not take fire. But it may be held there for a considerable time without being in the least affected by the flame. If the paper be strained over a cylinder of wood it is quickly scorched.
Hold both hands in water which causes the thermometer to rise to ninety degrees, and when the liquid has become still, you will be insensible to the heat, and that the hand is touching anybody. Then remove one hand to water that causes the thermometer to rise to two hundred degrees, and the other in water at thirty-two degrees.
After holding the hands thus for some time remove them, and again immerse them in the water at ninety degrees. Then you will find warmth in one hand and cold in the other. To the hand which had been immersed in the water at thirty-two degrees, the water at ninety degrees will feel hot; and to the hand which had been immersed in the water at two hundred degrees, the water at ninety[51] degrees will feel cool. If, therefore, the touch in this case be trusted, the same water will be judged to be hot and cold at the same time.
Fill a wine glass with cold water, pour lightly upon its surface a little ether; light it by a slip of paper, and it will burn for some time.
Drop a globule of potassium, about the size of a large pea, into a small cup nearly full of water containing a drop or two of strong nitric acid; the moment that the metal touches the liquid it will float upon its surface, enveloped with a beautiful rose-colored flame, and entirely dissolve.
Fill a large glass tube with water, and throw into it a few particles of bruised amber or shreds of litmus; then hold the tube by a handle for the purpose, upright in the flame of a lamp, and as the water becomes warm it will be seen that currents, carrying with them the pieces of amber will begin to ascend in the center, and to descend towards the circumference of the tube. These currents will soon become rapid in their motions, and continue till the water boils.
Pour into a glass tube, about ten inches long and one inch in diameter, a little water colored with pink or other dye; then fill it up gradually and carefully with colorless water, so as not to mix them; apply heat at the bottom of the tube, and the colored water will ascend and be diffused throughout the whole.
All fluids except water diminish in bulk till they freeze. Thus, fill a large thermometer tube with water, say of the temperature of eighty degrees, and then plunge the bulb into pounded ice and salt, or any other freezing mixture; the water will go on shrinking in the tube till it has attained[52] the temperature of about forty degrees, and then, instead of continuing to contract till it freezes, it will be seen slowly to expand, and consequently to rise in the tube until it congeals.
In this case the expansion below forty degrees and above forty degrees seem to be equal, so that the water will be of the same bulk at thirty-two degrees as at forty-eight degrees, that is, at eight degrees above or below forty degrees.
This pretty toy may be purchased at any optician’s for seventy-five cents. It consists of a cup in which is placed a human standing figure concealing a syphon or bent tube, with one end longer than the other. This rises in one leg of the figure to reach the chin, and descends through the other leg, through the bottom of the cup to a reservoir beneath. If you pour water in the cup it will rise in the shorter leg by its upward pressure, driving out the air before it through the longer leg; and when the cup is filled above the bend of the syphon, that is, level with the chin of the figure, the pressure of the water will force it over into the longer leg of the syphon, and the cup will be emptied, the toy thus imitating Tantalus, of mythology, who is represented by the poets as punished in Erebus with an insatiable thirst, and placed up to the chin in a pool of water, which, however, flowed away as soon as he attempted to taste it.
Fill a glass tumbler with water, throw upon its surface a few fragments or thin shavings of camphor, and they will instantly begin to move, and acquire a motion both progressive and rotary, which will continue for a considerable time. During these rotations if the water be touched by any substance which is at all greasy, the floating particles will quickly dart back, and, as if by a stroke of magic, be instantly deprived of their motion and vivacity.
In like manner, if thin slices of cork be steeped in sulphuric ether in a closed bottle for two or three days, and then placed upon the water, they will rotate for several[53] minutes, like the camphor, until the slices of cork, having discharged all their ether, and become soaked with water, they will keep at rest.
If the water be made hot the motion of the camphor will be more rapid than in cold water, but it will cease in proportionately less time. Thus, provide two glasses, one containing water at fifty-eight degrees, and the other at two hundred and ten degrees; place raspings of camphor upon each at the same time; the camphor in the first glass will rotate for about five hours, until all but a very minute portion has evaporated, while the rotation of the camphor in the hot water will last only nineteen minutes. About half the camphor will pass off and the remaining pieces, instead of being dull, white and opaque, will be vitreous and transparent, and evidently soaked with water. The gyrations, too, which at first will be very rapid, will gradually decline in velocity until they become quite sluggish.
The stilling influence of oil upon waves has become proverbial. The extraordinary manner in which a small quantity of oil instantly spreads over a very large surface of troubled water, and the stealthy manner in which even a rough wind glides over it must have excited the admiration of all who have witnessed it.
By the same principle a drop of oil may be made to stop the motion of the camphor, as follows: Throw some camphor, both in slices and in small particles, upon the surface of water, and while they are rotating dip a glass rod into oil of turpentine. Then allow a single drop thereof to trickle down the inner side of the glass to the surface of the water. The camphor will instantly dart to the opposite point of the liquid surface, and cease to rotate.
If a few drops of sulphuric or muriatic acid be let fall into the water, they will gradually stop the motion of the camphor, but if camphor be dropped into nitric acid, diluted with its own bulk of water, it will rotate rapidly for a few seconds and then stop.
If a piece of the rotating camphor be attentively examined with a lens, the currents of the water can be well distinguished, jetting out, chiefly from the corners of the camphor, and bearing it round with irregular force.
The currents, as given out by the camphor, may also be seen by means of the microscope; a drop or two of pure water being placed upon a slip of glass, with a particle of camphor floating upon it. By this means the current may be detected, and it will be seen that they cause the rotations.
A flat watch-glass may be employed, raised a few inches and supported on a wire ring, kept steady by thrusting one end into an upright piece of wood like a retort stand. Then put the camphor and water in the watch-glass, and place under the frame a sheet of white paper, so that it may receive the shadow of the glass, camphor, etc., to be cast by a steady light, placed above, and somewhat on one side of the watch-glass.
On observing the shadow, which may be considered a magnified representation of the object itself, the rotations and currents can be distinguished.
Put thirty grains of phosphorus into a bottle which contains three or four ounces of water. Place the vessel over a lamp and give it a boiling heat. Balls of fire will soon be seen to issue from the water after the manner of an artificial firework, attended with the most beautiful coruscations.
Make a piece of steel red in the fire, then hold it with a pair of pincers or tongs; take in the other hand a stick of brimstone and touch the piece of steel with it. Immediately after their contact you will see the steel melt and drop like a liquid.
Put into a phial some sulphuric ether, color it red with alkanet, then saturate the tincture with spermaceti. This preparation is solid ten degrees above freezing point, and melts and boils at twenty degrees. Place the phial which contains it in a lady’s hand and tell her that if in love, the solid mass will dissolve. In a few minutes the substance will become fluid.
To accomplish this seemingly incredible act requires the following preparation: You must take an egg and soak it in strong vinegar, and in process of time its shell will become quite soft so that it may be extended lengthways without breaking; then insert it into the neck of a small bottle, and by pouring cold water upon it, it will reassume its former figure and hardness. This is really a complete curiosity, and baffles those who are not in the secret to find out how it is accomplished. If the vinegar used to saturate the egg is not sufficiently strong to produce the required softness of shell, add one teaspoonful of strong acetic acid to every two tablespoonfuls of vinegar. This will render the egg perfectly flexible, and of easy insertion into the bottle, which must then be filled with cold water.
Take two small glass jars and close them with corks. In each of these pierce two holes and introduce a glass tube curved in the form of a lengthened V. The two extremities of this tube must not reach further than just a little below the inner surface of the corks. In one jar pour water until it is three-quarters full, and pass through the second hole of the cork a straight glass tube, open at both ends and reaching nearly the bottom. This jar must be hermetically corked. (If necessary, seal the top.) In the other jar put some chalk, and in the second hole of the cork, left free, pass the extremity of a paper funnel in which you place a pellet of wax or putty.
Your apparatus thus being ready, through the funnel pour some vinegar, or better still, some sulphuric acid. The latter ingredient coming in contact with the chalk, forms carbonic acid, which, not being able to escape through the funnel closed by the pellet, passes through the curved tube into the other jar and is dissolved in the water.
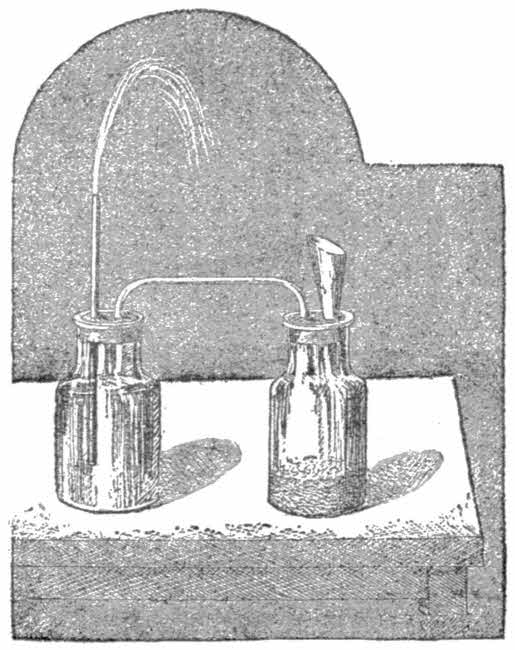
After some time a strong pressure will be exercised on the liquid, and the water rising rapidly up through the vertical tube, will spout out as from a fountain.
This experiment may be varied and reduced to a simpler one. Take one jar, fill it up two-thirds with water, and fit it with a cork with two holes, through which pass two[56] tubes; the one going to the bottom, the other resting just over the surface of the liquid. The latter should be fitted with a receiver.
Seal the cork so as to render it air-tight. In the top receiver pour water, which will go down into the jar and raise the level of the water already contained in it.
The air, being compressed, will act upon the liquid mass in the lower jar, and the water will escape through the free tube in a jet with more or less force according to the pressure exercised.
Do not be cast down because you see another term to be[57] explained. A gas is, you may have already guessed, simply a fluid. Matter exists in three states, solid, liquid and gaseous. Everything can exist in these three states under different conditions of heat and pressure.
For instance, ice, water, and steam are precisely the same thing, a mixture of oxygen and hydrogen, though in different states. Hence steam is simply the gaseous form[58] of ice or water. Now some gases are heavier than air, and among them is carbonic acid, a gas given off from the lungs in breathing.
By means of a very simply-constructed balance, you can prove this gas to be heavier than air. Sounds queer, doesn’t it? to talk of weighing something that you cannot handle or see.
It is not difficult to do. Bend some wire, minding that the beams of the balance are curved as in the figure.
For one side of the scales a strong cardboard box will answer admirably; for the other the lid of a round box will serve. Hang the whole on a string and adjust it by putting some grains of sand in the round scale on which the weights are placed, to make each side balance one another and the scales are ready for use.
The production of carbonic acid is easy. Pour a little sulphuric acid and water over some chalk. Collect the gas given off in a bottle or jar. In doing so you need not be afraid that it will escape, since it is heavier than the air.
In pouring it in the box of the scale, you will see the box sink down, which is clearly an indication that the gas, which has just been poured into the scale is heavier than the air, whose place it has taken. This experiment may be tried in other curious ways.
With some lycopodium, powder the surface of a large or small vessel of water; you may then challenge any one to drop a piece of money into the water, and that you will get it with the hand without wetting your skin. The lycopodium adheres to the hand, and prevents its contact with the water. A little shake of the hand after the feat is over will dislodge the powder.
This is another experiment on the density of liquids. In a small jar put some wine or colored alcohol, and close it with a cork, through which you have passed a small tube, a quill or a hollow straw. In lowering this jar gently in a pail full of water, you will soon see the liquid escape and rise to the surface of the water, describing spirals which[59] resemble smoke, and give a pretty good image, considerably diminished, of a volcano.
Make two holes in the wainscot of a room, each a foot high and ten inches wide, and about a foot distant from each other. Let these apertures be about the height of a man’s head, and in each of them place a transparent glass in a frame like a common mirror.
Behind the partition, and directly facing each aperture, place two mirrors inclosed in the wainscot, in an angle of forty-five degrees. These mirrors are each to be eighteen inches square, and all the space between must be inclosed with pasteboard painted black, and well closed that no light can enter; let there be also two curtains to cover them, which you may draw aside at pleasure.
When a person looks into one of these fictitious mirrors, instead of seeing his own face, he will see the object that is in front of the other; thus, if two persons stand at the same time before these mirrors, instead of each seeing himself, they will reciprocally see each other.
There should be a sconce with a lighted candle placed on each side of the two glasses in the wainscot, to enlighten the faces of the persons who look in them, or the experiment will not have so remarkable an effect.
To accomplish this metamorphosis, it is necessary to have earthen vases which have little edges or rims near their mouths, and should be of a size sufficiently large to hold suspended the bird or flower which you intend placing in them. You should likewise be provided with stoppers of cork, of a diameter equal to that of their mouths. To make an experiment upon some bird, it is necessary to commence by making a hole in the stopper, sufficiently large to contain the neck of the bird without strangling it. This done, you divide the diameter of the stopper into two equal parts so as to facilitate the placing of it around the neck without doing injury to the bird. The two parts being brought together, you place at the bottom of the vase an ounce of quicklime, and beneath that a quarter of an ounce of sal ammoniac.[60] When you perceive the effervescence commence to take place, you promptly insert the stopper, to which the bird is attached, leaving the neck outside. The plumage of the body, exposed to this effervescent vapor, will become impregnated with the various colors produced by this chemical combination.
Fix three pins in the table and lay the piece of money upon them; then place a heap of the flour of sulphur below the piece of money, and another above it, and set fire to them. When the flame is extinct, you will find on the upper part of the piece a thin plate of metal, which has been detached from it.
Mix two drachms of the filings of iron with one ounce of concentrated spirit of vitriol in a strong bottle that holds about a quarter of a pint; stop it close, and in a few moments shake the bottle; then taking out the cork, put a lighted candle near its mouth which should be a little inclined, and you will soon observe an inflammation arise from the bottle, attended with a loud explosion.
To guard against the danger of the bottle bursting, the best way would be to bury it in the ground and apply the light to the mouth by means of a taper fastened to the end of a long stick.
Ether poured upon a glass tube in a thin stream will evaporate and cool it to such a degree that water contained in it may be frozen.
Fill a quill with quicksilver; seal it at both ends with good hard wax; then have an egg boiled; take a small piece of the shell off the small end and thrust in the quill with the quicksilver; lay it on the ground and it will not cease tumbling about as long as any heat remains in it; or if you put quicksilver into a small bladder and blow it up,[61] then warm the bladder, it will skip about as long as heat remains in it.
Dissolve camphor in spirits of wine and deposit the vessel containing the solution in a very close room, where the spirits of wine must be made to evaporate by strong and speedy boiling. If any one then enters the room with a lighted candle the air will inflame, while the combustion will be so sudden and of so short a duration as to occasion no danger.
Bring a bar of iron to a white heat and then apply to it a roll of sulphur. The iron will immediately melt and run into drops.
The experiment should be performed over a basin of water, in which the drops that fall down will be quenched. These drops will be found reduced into a sort of cast iron.
Take a crystal or two of the nitrate of copper and bruise them; then moisten them with water and roll them up quickly in a piece of tinfoil, and in half a minute or little more, the tinfoil will begin to smoke and soon after take fire and explode with a slight noise. Unless the crystals of the nitrate of copper are moistened, no heat will be produced.
Put three ounces of rock alum and one ounce of honey or sugar into a new earthen dish, glazed, and which is capable of standing a strong heat; keep the mixture over the fire, stirring it continually until it becomes very dry and hard; then remove it from the fire and pound it to a coarse powder. Put this powder into a long-necked bottle, leaving a part of the vessel empty; and having placed it in the crucible, fill up the crucible with fine sand and surround it with burning coals. When the bottle has been kept at a red heat for about seven or eight minutes, and no more vapor issues from it, remove it from the fire, then stop it with[62] a piece of cork; and, having suffered it to cool, preserve the mixture in small bottles, well closed.
If you unclose one of these bottles and let fall a few grains of this powder on a bit of paper, or any other very dry substance it will first become blue, then brown, and will at last burn the paper or other substance on which it has fallen.
A very pleasing exhibition may be made, with very little trouble or expense, in the following manner: Provide a box, which you can fit up with architectural designs cut on pasteboard; prick small holes into those parts of the building where you wish the illuminations to appear, observing that, in proportion to the perspective, the holes are to be made smaller, and on the near objects the holes are to be made larger. Behind these designs thus perforated you fix a lamp or candle, but in such a manner that the reflection of the light shall only shine through the hole: then placing a light of just sufficient brilliancy to show the design of the buildings before it, and making a hole for the sight at the front end of the box, you will have a tolerable representation of illuminated buildings.
The best way of throwing the light in front is to place an oiled paper before it, which will cast a mellow gleam over the scenery, and not diminish the effect of the illumination. This can be very easily planned, both not to obstruct the sight, nor be seen to disadvantage. The lights behind the picture should be very strong, and if a magnifying glass were placed in the sight hole it would tend greatly to increase the effect. The box must be covered in, leaving an aperture for the smoke of the lights to pass through.
The above exhibition can only be shown at candle light; but there is another way, by fixing small pieces of gold on the building, instead of drilling the holes, which gives something like the appearance of illumination, but by no means equal to the foregoing experiment.
N. B.—It would be an improvement if paper of various colors, rendered transparent by oil, were placed between the lights behind the aperture in the buildings, as they would then resemble lamps of different colors.
Put a small quantity of spirits of wine into a glass, and put a cent or coin in with it; then direct the rays of the sun by means of a burning glass upon the coin, and in a short time it will become so hot as to inflame the spirits.
Put half a drachm of solid phosphorus into a large pint flask—holding it slanting that the phosphorus may not break the glass. Pour upon it a gill and a half of water and place the whole over a tea-kettle lamp, or any common tin lamp filled with spirits of wine. Light the wick which should be almost half an inch from the flask; and as soon as the water is heated, streams of fire will issue from the water by starts, resembling sky-rockets; some particles will adhere to the sides of the glass, representing stars, and will frequently display brilliant rays. These appearances will continue at times till the water begins to simmer, when immediately a curious aurora borealis begins, and gradually ascends till it collects to a pointed flame; when it has continued half a minute, blow out the flame of the lamp and the point that was formed will rush down, forming beautiful illuminated clouds of fire, rolling over each other for some time, which, disappearing, a splendid hemisphere of stars presents itself; after waiting a minute or two, light the lamp again, and nearly the same phenomenon will be displayed as from the beginning. Let the repetition of lighting and blowing out the lamp be made for three or four times at least, that the stars may be increased. After the third or fourth time of blowing out the lamp, in a few minutes after the internal surface of the flask is dry, many of the stars will shoot with great splendor from side to side, and some of them will fire off with brilliant rays; these appearances will continue several minutes. What remains in the flask will serve for the same experiment several times, and without adding any more water. Care should be taken after the operation is over, to lay the flask and water in a cool, secure place.
It is an interesting and amusing experiment to inflate a[64] balloon made of gold-beater’s skin (using a little gum arabic to close any holes or fissures), filling it from a bladder or jar, and tying a thread around the mouth of it, to prevent the escape of the gas. When fully blown, attach a fanciful car of colored paper, or very thin pasteboard, to it, and let it float in a large room; it will soon gain the ceiling, where it will remain for any length of time; if it be let off in the open air it will soon ascend out of sight. This experiment may be varied by putting small grains of shot into the car, in order to ascertain the difference between the weight of hydrogen gas and atmospheric air.
Wrap up a very smooth ball of lead in a piece of paper, taking care that there be no wrinkles in it, and that it be everywhere in contact with the ball; if it be held in this state over the flame of a taper, the lead will be melted without the paper being burnt. The lead, indeed, when once fused will not fail in a short time to pierce the paper, and run through.
Infuse a few shavings of logwood in common water, and when the liquid is sufficiently red pour it into a bottle. Then take three drinking glasses and rinse one of them with strong vinegar; throw into the second a small quantity of pounded alum, which will not be observed if the glass has been washed, and leave the third without any preparation. If the red liquor in the bottle be poured into the first glass, it will appear of a straw color; if the second it will pass gradually from a bluish gray to black, when stirred with a key or any piece of iron which has been previously dipped in strong vinegar. In the third glass the red liquor will assume a violet tint.

OUR TEN CENT HAND BOOKS.
USEFUL, INSTRUCTIVE AND AMUSING.
Containing valuable information on almost every subject, such as Writing, Speaking, Dancing, Cooking; also Rules of Etiquette, The Art of Ventriloquism, Gymnastic Exercises, and The Science of Self-Defense, etc., etc.
1 Napoleon’s Oraculum and Dream Book.
2 How to Do Tricks.
3 How to Flirt.
4 How to Dance.
5 How to Make Love.
6 How to Become an Athlete.
7 How to Keep Birds.
8 How to Become a Scientist.
9 How to Become a Ventriloquist.
10 How to Box.
11 How to Write Love Letters.
12 How to Write Letters to Ladies.
13 How to Do It; or, Book of Etiquette.
14 How to Make Candy.
15 How to Become Rich.
16 How to Keep a Window Garden.
17 How to Dress.
18 How to Become Beautiful.
19 Frank Tousey’s U. S. Distance Tables, Pocket Companion and Guide.
20 How to Entertain an Evening Party.
21 How to Hunt and Fish.
22 How to Do Second Sight.
23 How to Explain Dreams.
24 How to Write Letters to Gentlemen.
25 How to Become a Gymnast.
26 How to Row, Sail and Build a Boat.
27 How to Recite and Book of Recitations.
28 How to Tell Fortunes.
29 How to Become an Inventor.
30 How to Cook.
31 How to Become a Speaker.
32 How to Ride a Bicycle.
33 How to Behave.
34 How to Fence.
35 How to Play Games.
36 How to Solve Conundrums.
37 How to Keep House.
38 How to Become Your Own Doctor.
39 How to Raise Dogs, Poultry, Pigeons and Rabbits.
40 How to Make and Set Traps.
41 The Boys of New York End Men’s Joke Book.
42 The Boys of New York Stump Speaker.
43 How to Become a Magician.
44 How to Write in an Album.
45 The Boys of New York Minstrel Guide and Joke Book.
46 How to Make and Use Electricity.
47 How to Break, Ride and Drive a Horse.
48 How to Build and Sail Canoes.
49 How to Debate.
50 How to Stuff Birds and Animals.
51 How to Do Tricks with Cards.
52 How to Play Cards.
53 How to Write Letters.
54 How to Keep and Manage Pets.
55 How to Collect Stamps and Coins.
56 How to Become an Engineer.
57 How to Make Musical Instruments.
58 How to Become a Detective.
59 How to Make a Magic Lantern.
60 How to Become a Photographer.
61 How to Become a Bowler.
62 How to Become a West Point Military Cadet.
63 How to Become a Naval Cadet.
64 How to Make Electrical Machines.
65 Muldoon’s Jokes.
66 How to Do Puzzles.
67 How to Do Electrical Tricks.
68 How to Do Chemical Tricks.
69 How to Do Sleight of Hand.
70 How to Make Magic Toys.
71 How to Do Mechanical Tricks.
72 How to Do Sixty Tricks with Cards.
73 How to Do Tricks with Numbers.
74 How to Write Letters Correctly.
75 How to Become a Conjuror.
76 How to Tell Fortunes by the Hand.
77 How to Do Forty Tricks with Cards.
78 How to Do the Black Art.
79 How to Become an Actor.
80 Gus Williams’ Joke Book.
All the above books are for sale by newsdealers throughout the United States and Canada, or they will be sent, post-paid, to your address, on receipt of 10c. each.
Send Your Name and Address for Our Latest Illustrated Catalogue.
FRANK TOUSEY, Publisher,
24 UNION SQUARE, NEW YORK.
Illustrations have been moved to paragraph breaks near where they are mentioned.
Punctuation has been made consistent.
Variations in spelling and hyphenation were retained as they appear in the original publication, except that obvious typos have been corrected.
Additional notes:
p. 3: Inserted “that” (paper, that give)
p. 6: “choose” changed to “chose” (and chose to)
p. 11: “jar, or a soup-plate” should be “jar, and a soup-plate”
p. 18: “altered as in fig. 4” should be “altered as in fig. 6”
p. 18: “lightness” changed to “tightness” (absolute tightness. Such)
p. 22: “entirely. As” changed to “entirely, as” (out entirely, as)
p. 28: “valve shown in fig. 4” should be “valve shown in fig. 6”
p. 45: “with” inserted (ground, with a)