
The cover image was created by the transcriber and is placed in the public domain.
WILDLIFE RESEARCH REPORTS
This series comprises reports of research relating to birds, mammals, and other wildlife and their ecology, and specialized bibliographies on these, issued for wildlife research and management specialists. The Service distributes these reports to official agencies, to libraries, and to researchers in fields related to the Service's work.
Library of Congress Cataloging in Publication Data
Conservation of marine birds of northern North America.
(Wildlife research report: 11)
Supt. of Docs. no.: I 49.47/4:11
1. Sea birds—North America—Congresses. 2. Sea birds—Northwest, Pacific—Congresses. 3. Birds, Protection of—North America—Congresses. 4. Birds, Protection of—Northwest, Pacific—Congresses. 5. Birds—North America—Congresses. 6. Birds—Northwest, Pacific—Congresses. I. Bartonek, James C. II. Natural Resources Council of America. III. United States. Fish and Wildlife Service. IV. Series.
QL681.C59333.9'5 79-607005
Use of trade names does not imply U.S. Government endorsement of commercial products.
CONSERVATION OF MARINE BIRDS OF NORTHERN NORTH AMERICA

Edited by
James C. Bartonek and David N. Nettleship
UNITED STATES DEPARTMENT OF THE INTERIOR
FISH AND WILDLIFE SERVICE
Wildlife Research Report 11
Washington, D.C. • 1979
Dedicated to the Memory
of
Robert D. Bergman, Leonard A. Boughton, and J. Larry Haddock, Wildlife Biologists of the Fish and Wildlife Service, and Robert Johnson, Pilot of the Office of Aircraft Services, all of the U.S. Department of the Interior, who perished in the Gulf of Alaska on 30 September 1974 while conducting aerial surveys of marine birds,
and to
Einar Brun, Professor of Zoology in Tromsø University and a contributor to these proceedings, who perished in the Vega Sea on 13 July 1976 when returning from making aerial surveys of marine birds.
| Page | |
|---|---|
| Foreword, by Harvey K. Nelson | vii |
| Introduction, by Lynn A. Greenwalt | ix |
| Marine Environment of Birds | 1 |
| Long-term Climatic and Oceanographic Cycles Regulating Seabird Distributions and Numbers, by M. T. Myres | 3 |
| Sea Ice as a Factor in Seabird Distribution and Ecology in the Beaufort, Chukchi, and Bering Seas, by George J. Divoky | 9 |
| Status of Marine Bird Populations | 19 |
| Distribution and Status of Marine Birds Breeding Along the Coasts of the Chukchi and Bering Seas, by James C. Bartonek and Spencer G. Sealy | 21 |
| Breeding Distribution and Status of Marine Birds in the Aleutian Islands, Alaska, by Palmer C. Sekora, G. Vernon Byrd, and Daniel D. Gibson | 33 |
| The Historical Status of Nesting Seabirds of the Northern and Western Gulf of Alaska, by LeRoy W. Sowl | 47 |
| Status and Distribution of Breeding Seabirds of Southeastern Alaska, British Columbia, and Washington, by David A. Manuwal and R. Wayne Campbell | 73 |
| The Biology and Ecology of Marine Birds in the North | 93 |
| Trophic Relations of Seabirds in the Northeastern Pacific Ocean and Bering Sea, by David G. Ainley and Gerald A. Sanger | 95 |
| Population Dynamics in Northern Marine Birds, by William H. Drury | 123 |
| Time-energy Use and Life History Strategies of Northern Seabirds, by Erica H. Dunn | 141 |
| Zoogeography and Taxonomic Relationships of Seabirds in Northern North America, by M. D. F. Udvardy | 167 |
| Conflicts Between the Conservation of Marine Birds and Uses of Other Resources | 171 |
| Social and Economic Values of Marine Birds, by David R. Cline, Cynthia Wentworth, and Thomas W. Barry | 173 |
| Resource Development Along Coasts and on the Ocean Floor: Potential Conflicts with Marine Bird Conservation, by Donald E. McKnight and C. Eugene Knoder | 183 |
| Mortality to Marine Birds Through Commercial Fishing, by Warren B. King, R. G. B. Brown, and Gerald A. Sanger | 195 |
| Interactions Among Marine Birds and Commercial Fish in the Eastern Bering Sea, by Richard R. Straty and Richard E. Haight | 201 |
| Interrelations Between Seabirds and Introduced Animals, by Robert D. Jones, Jr., and G. Vernon Byrd | 221 |
| Oil Vulnerability Index for Marine Oriented Birds, by James G. King and Gerald A. Sanger | 227 |
| Programs and Authorities Related to Marine Bird Conservation | 241 |
| Programs and Authorities Related to Marine Bird Conservation in Washington State, by Ralph W. Larson | 243 |
| Programs and Authorities of the Province of British Columbia Related to Marine Bird Conservation, by W. T. Munro and R. Wayne Campbell | 247 |
| Petroleum Industry's Role in Marine Bird Conservation, by Keith G. Hay [Pg vi] | 251 |
| Conservation of Marine Birds in Other Lands | 259 |
| Conservation of Marine Birds in New Zealand, by Gordon R. Williams | 261 |
| Marine Birds in the Danish Monarchy and Their Conservation, by Finn Salomonsen | 267 |
| Present Status and Trends in Population of Seabirds in Norway, by Einar Brun | 289 |
| Symposium Summary | 303 |
| Conservation of Marine Birds of Northern North America—a Summary, by Ian C. T. Nisbet | 305 |
| Appendix. Papers and oral summaries presented at the symposium but which do not appear in this publication | 319 |
The international symposium "Conservation of Marine Birds of Northern North America" was convened because of a growing awareness that not all was well with our marine birds. The symposium provided a forum for scientists, governmental administrators, conservationists, and laypeople to discuss the diverse topics and issues that we must all understand if we are to act both responsively and responsibly to assure that marine birds will not be lost through our neglect.
The symposium was cosponsored by the Natural Resources Council of America, National Audubon Society, National Wildlife Federation, and the U.S. Department of the Interior, Fish and Wildlife Service; additional support was provided by the Canadian Wildlife Service, the International Association of Game, Fish, and Conservation Commissioners, the Pacific Seabird Group, the Sierra Club, the Smithsonian Institution, the Wildlife Management Institute, and the Wildlife Society.
Persons interested and knowledgeable in the many and varied aspects of marine bird conservation were invited to participate in this symposium. There were 139 registered and several score of unregistered participants in attendance. Major topics treated were: (1) socioeconomic considerations and conservation of marine birds; (2) the marine environment of birds; (3) status of marine bird populations on land and sea; (4) the biology and ecology of marine birds in the North; (5) conflicts between the conservation of marine birds and uses of other resources; (6) programs and authorities related to the conservation of marine birds; and (7) conservation of marine birds in other lands.
The objective of the symposium was to identify problems and the needed information and programs necessary for the conservation of marine birds of northern North America. For the purpose of this symposium the term "northern North America" referred to the coasts of Washington, British Columbia, Alaska, Yukon Territory, and Northwest Territories and the adjacent North Pacific and Arctic Oceans. "Marine bird" was defined as being any bird using marine or estuarine waters. Speakers were asked to describe the status of information or the state of the art as it pertained to their topic within the limitations set by the objective of the symposium. Examples from other regions and of bird species not found in the regions of concern were to be used for comparative purposes when little pertinent information was known for regions or species of concern. Speakers were asked to identify the gaps in the knowledge and methodology that are most critical to their topic.
I believe that this symposium was particularly successful in that it provided a timely forum for many scientists who were about to embark on studies of marine birds in those areas of Alaska and California being considered for outer continental shelf oil and gas exploration and development. These published proceedings may be of lesser importance from that standpoint because some data, particularly those on populations, are out of date. However, I believe that the proceedings will long be of importance to biologists and administrators alike in charting their respective courses to ultimately assure conservation of this valuable avian resource.
Many people from many organizations and agencies worked hard to put together the symposium in the relatively short time of about 8 months. Nathaniel P. Reed was the person primarily responsible for bringing this symposium to fruition. The Steering Committee was composed of Daniel A. Poole, John S. Gottschalk, David N. Nettleship, Amos S. Eno, C. Eugene Knoder, Warren G. King, Louis Clapper, Robert Hughes, Fred G. Evenden, James C. Bartonek, and me. James C. Bartonek, Warren G. King, David N. Nettleship (Co-chairmen), C. Eugene Knoder, David A. Manuwal, William H. Drury, and Spencer G. Sealy served on the Program Committee. David A. Manuwal and Terence R. Wahl arranged trips for persons to observe pelagic birds off the Washington coast and other birds on Skagit Flats. C. Eugene Knoder handled financial matters. John A. Sayre and Richard Bauer made arrangements for facilities and entertainment. Elaine Rhode prepared the program and abstracts for printing. John Pitcher kindly contributed the artwork used in this publication as well as that used in the program and abstracts.
George Reiger made general introductions to the symposium; Spencer G. Sealy, Daniel W. Anderson, and I served as Session Chairmen; and James C. Bartonek served as General Chairman. Elvis J. Stahr was guest speaker at the symposium banquet.
Most credit for the success of this symposium goes to the 52 persons who as authors, coauthors, or summarizers of sessions presented much meaningful information in their presentations, during recorded discussions, and during many informal occasions. I wish to make special recognition of Ian C. T. Nisbet for his skillful summary of the symposium.
Editorial assistance in preparing the proceedings was provided by Judith Brogan.
Harvey K. Nelson
Chairman of Symposium and
Director of Wildlife Resources
Migratory birds make up a resource that is shared by many people of many nations. Public awareness of marine birds—their manifold values, ecological requirements, and problems—is prerequisite to their protection. I am proud that the Fish and Wildlife Service can further this needed awareness by publishing these proceedings of the international symposium "Conservation of Marine Birds of Northern North America."
Lynn A. Greenwalt, Director
Fish and Wildlife Service
by
M. T. Myres
Department of Biology, University of Calgary
Calgary, Alberta, Canada T2N 1N4
Seabird ornithologists have generally paid little attention to the possible roles played by long-term climatic cycles or air-ocean interactions on population changes at established colonies or on the processes of colony establishment or extinction. Yet, a rapidly expanding literature in the physical sciences suggests that seabird numbers are not naturally stable at particular colonies for any great length of time. It is suggested that the establishment of new colonies at one end of the range may counter the decline of colonies at the other end. Perhaps these changes in small marginal colonies are important, and they may be more indicative and significant (when detected and explained) than are much larger changes in numbers in bigger reproductive units in the center of a species' range. Fluctuations in seabird numbers must in future be first considered as possible responses either to short-term, or turnarounds in longer term, natural climatic or oceanographic cycles, or to trends ranging in length from a few years to at least several decades.
During the last 30 years extensive literature in the fields of physical and biological oceanography has accumulated that is not readily accessible to the nonprofessional student of seabirds and not as widely understood by career seabird ornithologists as it should be. This literature in oceanography and marine fisheries is as extensive in Russian and Japanese together as in the main languages of Western Europe combined; this abundance compounds the problem of becoming familiar with it if, as a student of seabirds, one's interest in the literature is initially somewhat marginal. Nevertheless, to achieve the best possible appreciation of the oceanographic influences affecting seabirds, particularly in the north Pacific Ocean and its adjacent embayment seas, it is necessary to make the effort.
Because of the rigor of carrying out their primary duties while at sea, only a very few North American and European oceanographers or fishery biologists have found time to interest themselves in seabirds and then, with a few notable individual exceptions, only as an off-duty pastime. The reason is not far to seek. It is far less important to examine the ecology of organisms at the next highest level of the food chain to the ones that are the primary concern than it is to examine the next lowest level (the food of the fishes or, in the case of phytoplankton, the physical and chemical environment in which the organisms grow best).
Seabirds are at the very top of the marine food chain, and they are not wholly aquatic in any case since they mainly travel through the air rather than the water and reproduce on land rather than in the sea. Only with the relatively recent recognition that seabirds contribute to the recycling of nutrients back into the ocean to an important degree, have seabirds gained a new scientific constituency.
At about the same time, governments have begun to recognize that seabirds are relatively easily examined indicators of the presence of unseen chemical pollutants in coastal seas, perhaps primarily for the very same reasons that they were previously so largely ignored; namely, that they are at the top of the food chains (and so accumulate the most-persistent and least-degradable pollutants) and that the on-land failures in their reproductive biology are readily visible.
During the last 10 years, it has become evident that yet another fundamental science is even more basic to the achievement of a balanced and in-depth understanding of the influence of the environment upon seabirds—the combined field of astrophysics, geophysics, and climatology. New developments in this field (when they are not published in Nature or Science) appear in journals that are less familiar to seabird ornithologists than those in which the fishery biologists and biological oceanographers publish their findings.
Unfortunately, important advances in understanding the dynamics and energy transport mechanisms of both the atmosphere and the water masses of the oceans are not being picked up by students of seabirds because of the natural lag in communication that occurs between disparate disciplines. Only in the last few years have oceanographers and climatologists been invited to address gatherings of ornithologists, and the modesty with which they have sometimes done so has limited the impact of their offerings.
At this symposium, it was left to a biologist with no pretentions in either physics or mathematics to demonstrate the need for seabird ornithologists to understand basic environmental processes well beyond their usual range of interests. I did so with a series of slides taken from this "other" literature, and I had intended to include in the published version of this paper an extensive bibliography, subdivided into category groupings, so that seabird ornithologists could make their own selection of the points in the spectrum at which they most needed information.
Unfortunately, limitations upon space in this volume, daily additions to the exploding literature, and my own inability to keep up with understanding this have forced me to omit any references and not to attempt to expound detailed specific physical mechanisms.
Thus unencumbered here, I shall briefly outline instead what I perceive to be some of the significance for seabird ornithology and conservation of the rapidly expanding understanding of the oceans, the air-sea interface, atmospheric dynamics, and influences upon the world's climate of extraterrestrial events.
There is no need to dwell on the well-known events that could be mentioned under this heading. Seabird ornithologists are familiar with the fact that the atmosphere is the medium of seabirds both when searching the ocean for feeding areas and when on migration, and also a violent enemy, as when particular storms cause occasional "wrecks" of seabirds inland from coastlines. As a refinement of the former, Manikowski of Poland suggests that seabirds respond to the passage of weather systems, so that their distribution over the open ocean may be constantly changing. Whereas some species may attempt to avoid the stormy conditions of low-pressure areas (cyclonic conditions), others more highly specialized for exploiting the aerodynamic properties of wind over a moving water surface may possibly, instead, try to avoid large high-pressure regions (anticyclonic conditions with little or no wind). My student, Juan Guzman, is attempting to determine whether this may be so; if it is, it might be possible, for example, to predict some things about the distribution patterns and population structure of southern hemisphere shearwaters while they are visiting the oceans of the northern hemisphere during the nonbreeding season.
In comparison with the "wrecks" brought about by storms, which are of short duration and not usually very serious, seabird ornithologists are also familiar with relatively brief and localized disasters caused by changes in the ocean itself. The best-known example is a slight change in the boundary of an ocean current (or other shift in the position of a distinctive water mass) that results in the failure of food fishes to appear as they normally would, close to breeding sites of conspicuous colonial seabirds, such as the periodic shift in the El Niño off the west coast of South America. A scarcely studied refinement of this type of event would be the effects of less-pronounced oceanic changes that might reduce the planktonic food supply of nocturnally active, burrow-nesting seabirds. In such instances, the effects might also be a breeding failure for only one or two seasons; in all probability such events occur, but whether they are as likely to be detected by us is problematical. However, the populations of most seabirds are probably already adapted to survive short-term crises of this type because, having long adult life spans, reproductive adults that fail to raise young one year may mostly live to succeed in doing so in the next or succeeding year, when the oceanic "anomaly" has disappeared. What constitutes an "anomaly" will be considered again shortly.
A third critical condition for seabirds may be local or widespread, temporary or final, or some combination of these. A single local spill, or outfall, of a chemical pollutant will be short term if we can take steps to alleviate the consequences or stem the flow. Alternately, we may consider it to be long term if we take the view that it is one additional act of violence resulting from the "progress" of Industrial Man, and that it is never going to shift into reverse gear. We may say that the effect on seabird populations of spills of oil products or chemical pollutants in coastal waters of a region will be a "final solution" for any that become wholly extinct before the oil wells go dry or the industries fail. On the other hand, the effect will have been merely a perturbation of the population if the species survives and outlives these activities. Recent upturns in populations of peregrine falcons (Falco peregrinus) and pelicans (Pelecanus sp.) in certain places where environmental controls have been enacted give us hope that crises of several years' duration can be withstood by at least those species that once were common in relation to their respective food sources or available safe breeding habitats. The really critical features to document are the means whereby abandoned breeding sites are reoccupied and the time it takes.
It must never be forgotten that we know almost nothing about the ecology of subadult or nonreproductive adult seabirds during the years they are at sea unconfined by membership in a breeding unit and that we know almost nothing about the activities of pelagic seabirds in the nonbreeding season. These birds may be far from land and hard to study, but what happens during those phases of their lives is basic to the composition of the colony and condition of the birds when breeding. A start would be to learn everything that is known and is being discovered about the oceans by oceanographers and, thus forearmed, go looking for the seabirds with certain questions clearly in mind.
A scientist's working life lasts only a few decades, and few studies of seabirds by a single author or agency have been continued for longer than 5-10 years on any one problem. Further, while we as individuals may live to be equally active in a certain field of research 20 years hence, our collective conscience and collective muscle consist of several levels of government that tend to exhibit 4- or 5-year changes of direction and priorities. Certainly, the civil service may live on as an inertial recorder of collective experience. Certainly, too, those who live under one form or another of dictatorship or, as in some Canadian provinces, where conservative patterns of voting occur, may experience a continuity of research and development and conservation policies that exceed the 4- to 5-year turnaround pattern that is most common. Yet, even these more continuous systems may come to an end quite suddenly because of economic or political happenstance.
The point of this digression is to show that seabird ornithologists must not rely on government programs to provide continuous data over a long period of years—not, at least, in most countries. Monitoring the biological circumstances of seabirds is not the same as recording the temperature regularly by machine at a weather station, since this activity is unlikely to be terminated unless the society collapses altogether. We may know that in some countries the amateur naturalist exists in such numbers that records of seabirds will continue to be made whatever the circumstances. Nevertheless, planning of censuses that will be repeated every 10 years is best assured if government and career biologists combine with the amateur element, so that any one of them can continue the work if any other element should be incapacitated. At any one time, either the amateur or the government or the university personnel may be the prime mover, and each of these forms now exists in various countries.
What the scientific literature in the fields of the geophysical, atmospheric, and oceanographic disciplines demonstrates is that natural climatic oscillations probably range in length from the 11-year sunspot cycle through several decades (or a human lifetime) to several hundred years. So, when our children are the new trustees of seabird colonies 20 or 40 years hence, they must interpret their data using the full range of physical as well as biological data that we can leave for them. Indeed, the information is, I believe, already available over a long enough period (since 1940 at least) to allow some speculative interpretations of what may have been happening to our seabird populations, whether or not we knew or had any evidence of it.
I have already suggested that extraterrestrial events, particularly the 11-year sunspot cycle, are increasingly believed to influence the atmosphere of this planet. The Chinese and Japanese have remarkably precise records of the northern limits of certain agricultural crops at particular times, the phenology of flowering, and the freezing of lakes. These demonstrate long-term trends in overall climate in eastern Asia that extend over hundreds of years. The climate of Japan is influenced by the high-pressure area in winter over mainland East Asia. There is evidence that severe ice conditions in the Bering Sea during the early 1970's may have been due to an eastward shifting of this high-pressure area. Again, the water mass of the Kuroshio Extension and the West Wind Drift takes several years to travel across the Pacific Ocean, and there is an established temperature variation that travels like a slow wave with it. Off Japan, the Kuroshio Current periodically develops meanders which slow the speed of the eastward flow. Cold and warm "pools" of water approach the west coast of Canada and the western United States from time to time.
Ocean currents are driven by the atmospheric motion above them, which consists of several convective cells between the equator and each pole. The outcome is zonal winds, such as the trade winds and the westerlies. However, as the influence of the sun on the atmosphere is variable, the input of heat and the extent of the major high-pressure areas vary, as does the path of the jet stream. The recent droughts in northern Africa and unusually heavy rains in Australia are both linked to a southward shift of the Intertropical Convergence Zone in the atmosphere and a "corrugation" of the wind circulation from a more normal zonal (latitudinal) path. These shifts in the atmospheric circulation are almost certainly transmitted also to the ocean currents and the marine ecosystem, with the influence being felt for a long period of years.
One of the oceanic domains of the North Pacific is the transitional domain, which lies east-west where the West Wind Drift impinges upon the coasts of British Columbia and Washington State. It is precisely in this sector that there was a well-documented "temperature anomaly" in 1957-58. Since an anomaly implies something completely out of the ordinary, I seriously question the appropriateness of the term for an event that may or may not be recurrent (at the time it was a pronounced variation from the oceanographic records accumulated up to that time, but the period had not been a very long one). It is no coincidence that the numbers of albatrosses recorded at Ocean Weather Station "Papa" was higher during this warm-water "anomaly" than subsequently (indeed, an 18-year record of the seabirds recorded at "Papa" also exhibits other interesting fluctuations from the base-line data in certain years).
Recent analyses of sediments from off the coast of California have demonstrated long-term fluctuations in sardine populations extending back at least 1,800 years, with increases lasting 20-150 years and spaced 20-200 years apart. The number of anchovies declined steadily. Yet until now, El Niño events have been treated as anomalies in that region as well as off the coast of Peru. Just as we recognize that different species of fish follow the warm water north on such occasions, we must also recognize the rather distinct seabird species assemblage that is trapped, as it were, in the Gulf of California. Clearly, like the termination point of the West Wind Drift at about the 45-55° parallel, the coast of Baja California and southern California State, from the 25-35° parallel where the California Current begins to swing away from the coast to the west as the North Equatorial Current, is another zone of instability.
I think that it is no accident that the southern limit of several northern species of North Pacific seabirds ends in southeastern Alaska or northern British Columbia, and that the northern limit of the ranges of several other species occurs in Washington State or southern British Columbia. Indeed, the west coast of Vancouver Island is not rich in species, and several of those that exist are not present in great numbers. This is a region of rather more variable conditions than elsewhere, and species evidently find that it is difficult to colonize and it quickly becomes unsuitable again. Since 1940, indeed, there has been a parallel decline in the annual mean sea-surface temperature at a number of coastal recording stations in British Columbia, and this seems to have been a rebound from a less well-documented rise in sea-surface temperatures during the 20 years before that, which culminated in a peak around 1940. Salinity has likewise trended downwards during the last 30 years. The seabird colony size data before 1960 are so nonquantitative that it is impossible to be sure what changes in seabird populations and breeding sites may have taken place in response to these physical changes.
The lesson is that we must now examine all future census and distribution data with trends in sea-surface temperature and salinity in mind as two of several likely factors influencing them. We must no more ignore data outside our own field than a salmon ecologist might.
We know little of the accuracy of censuses of seabird numbers made between 1850 and 1950. There has been a tendency to assume that numbers of seabirds at long-established colonies have been relatively unchanging, even though the expansion of some species into previously unrecorded breeding sites in low numbers is well documented. Contraction of breeding ranges, likewise, has most commonly been attributed to the influence of man. Recent literature from the physical sciences, on the contrary, suggests that seabird numbers at particular colonies are most unlikely to have been stable for any great length of time, at least at high or middle latitudes and particularly at points where boundaries between currents impinge on continental coasts. Indeed, some early estimates of colony sizes may not have been as much in error as we may have assumed, neither when apparently too large nor when apparently unlocated by previous visitors.
The halving of a large colony over a period of 20 to 50 years in the middle of the range of a species and the establishment and disappearance of smaller breeding groups at opposite extremes of the range (both latitudinally and longitudinally), may equally reflect natural long-term climatic or oceanographic changes and may naturally be reversed at some time in the future, perhaps within half a century. The implication for conservation of seabird colonies that are at the contracting end of a species' range is that cultural rather than biological criteria may be the best determinants.
by
George J. Divoky[1]
U.S. Fish and Wildlife Service
Fairbanks, Alaska
Arctic sea ice has a variety of effects on seabirds. Although the decrease in surface area available for feeding and roosting is probably the major restrictive effect, also important are productivity of water covered by ice and the reduced prey abundance in nearshore areas due to ice scour. The most important benefit that sea ice provides to seabirds is the plankton bloom that occurs in the ice in the spring. In the Beaufort and Chukchi seas this bloom supports an under-ice fauna that is an important food source for seabirds.
Sea ice is a major factor in the distribution and ecology of many of the birds treated in this symposium. Sea ice is defined here as ice formed by the freezing of seawater and includes both free floating pack ice and the more stable shorefast ice. Since icebergs are composed of ice of land origin, they are not discussed.
Before discussing the specific relationship of birds and sea ice in the Beaufort, Chukchi, and Bering seas, I list the general effects that arctic ice can have on seabirds. For purposes of discussion these effects can be divided into negative effects, or disadvantages, and positive effects, or advantages.
The decrease in the surface area of water is the simplest and most immediate effect that sea ice has on birds. Ice acts as a barrier that restricts the availability of food in the water. Surface feeders are the most severely affected since, in general, ice cover of 50% reduces the possible feeding area by half. The effect on diving species is not as severe since, if open water is scattered throughout the ice, diving species still have access to much of the prey in the water column and benthos. When open water is scarce, however, diving species can become concentrated in the available water, resulting in intense competition for available prey. In certain situations the open water is used only as a migratory pathway, but open water is necessary for birds that must roost or feed.
Ice inhibits phytoplankton blooms in the water column, thus decreasing the biological productivity of ice-covered waters. This inhibition occurs in two ways:
• By decreasing light penetration of the water column.—Much of the sunlight reaching the ice is reflected by the ice and by snow on the ice. The amount of light reaching the water depends on the angle of the light, thickness of ice, and amount of snow cover. When the layer of under-ice algae forms, it absorbs light and further reduces the amount of light reaching the water (Bunt 1963). This reduction in light reduces the depth of the euphotic zone.
• By increasing the stability of the water column.—Increased stability of the water column reduces the upwelling of nutrient-rich waters into the euphotic zone. Ice stabilizes the water column primarily by preventing wind-driven movement of surface waters and by forming a layer of meltwater at the surface in the spring and summer (Dunbar 1968).
Benthic flora and fauna can be reduced by the presence of ice in two ways: In shallow water ice can freeze to the bottom for much of the year and prevent the establishment of plant and animal populations; and when ice floes are pushed together, they form underwater ice keels that can scour the bottom when the ice moves. Both of these events not only act directly to decrease benthic populations but also disturb the sediment, making it less suitable for colonization. In areas with heavy ice scour, sessile benthic populations can be greatly reduced, although motile species may move into scoured areas during the ice-free period in summer. In addition to preventing the establishment of sessile benthic animal populations, ice scour also prevents the establishment of beds of kelp and eelgrass (Zostera marina), thus decreasing the diversity and productivity of arctic inshore waters. Both kelp and eelgrass beds are important feeding sites for birds in areas south of the region affected by ice scour.
The formation of ice between the mainland and offshore islands allows the arctic fox (Alopex lagopus) and other predators access to the islands used by breeding birds. Foxes can become permanently established on islands that have food sources during the period when birds are absent from the island. Often, however, there is little to attract foxes to the islands other than breeding birds. Because moats form around many islands before the breeding birds arrive, foxes are primarily a problem when moat formation is incomplete or when the breakup of ice is late. Arctic foxes are found on the pack ice throughout the summer and thus can visit islands that are separated from the mainland by open water but are adjacent to the pack ice.
The first detailed studies on the blooms of diatoms that occur in the lower levels of ice were done by Appollonio (1961). The importance of this bloom in the energy budgets of arctic and subarctic seas has only recently been realized (Alexander 1974; McRoy and Goering 1974). In areas where ice is present throughout the year, the plankton bloom supports a population of under-ice invertebrates. These populations have been little studied but apparently consist primarily of copepods and amphipods (Mohr and Geiger 1968). Feeding on the invertebrates associated with the ice are two species of fish, polar cod (Arctogadus glacialis) and arctic cod (Boreogadus saida). Andriashev (1968) used the term cryopelagic to describe such fish, which are found in the midwater zone but also are associated with ice during some part of their life cycle.
The underside of multi-year ice has numerous ridges and pockets that provide a heterogeneous environment for the under-ice fauna. This environment is protected from disturbance from currents and wave action by ice keels acting as barriers, which also provide shelter from predators in the same manner as a coral reef. The overall effect of the under-ice flora and fauna is to increase the diversity of surface waters in arctic seas by creating an inverted benthic biota.
The mammals that inhabit the ice in the Chukchi and Bering seas and their adaptations to the pack ice environment were discussed by Fay (1974). Many of these species frequently haul out on the ice, where they provide food in the form of feces, placentas, and carcasses.
Ice provides a hard substrate that allows seabirds to leave the water to roost. This allows such species as the Larus gulls, which typically roost on hard substrates, to occur in large numbers well offshore.
The unevenness of the upper surface of the ice reduces the speed of winds directly over the ice, thus providing a microhabitat and reducing the amount of wind chill for birds sitting on and next to the ice.
Ice floating on the water reduces the surface disturbance of the water. Although swells pass through areas with much ice cover, waves do not. In addition, surface waters on the lee side of ice floes and cakes usually have little surface disturbance. Surface feeders may be able to locate prey more easily because of these reductions in surface disturbance.
The retreat of the pack ice each spring and the formation of new ice each fall greatly affect a large area of the Arctic Ocean off the coast of Alaska and much of the Bering Sea. Specific ways in which birds are affected by ice in the western Arctic are discussed on a seasonal basis. All observations are my own, unless otherwise stated.
From late November to mid-April, ice cover of the Chukchi and Beaufort seas is almost complete. The only areas where birds can be expected to winter in these seas are the chronic lead systems. Such lead systems are found off Wainwright and Point Barrow and south of the Point Hope-Cape Thompson area (Shapiro and Burns 1975). Only the black guillemot (Cepphus grylle) is known to regularly winter offshore from Wainwright and Point Barrow (Gabrielson and Lincoln 1959; Nelson 1969). In the Point Hope-Cape Thompson area, glaucous gulls (Larus hyperboreus), the common murre (Uria aalge), and the thick-billed murre (U. lomvia) occur throughout the winter (Swartz 1967). It is likely that black guillemots are also found in this area.
The lack of chronic lead systems in the Beaufort Sea precludes the presence of wintering seabirds. The one species that may be found wintering in the Beaufort is the Ross' gull (Rhodostethia rosea). Ross' gull is believed to winter primarily in the Arctic Ocean (Bailey 1948). The number of sightings that have been obtained in both the eastern and western Arctic indicate that the species may winter over much of the Arctic Ocean. It may thus be expected to occur in both the Chukchi and Beaufort seas during winter.
Ice cover—not prey abundance—plays the major role in severely limiting bird numbers in the Arctic Ocean in winter. Prey is known to be abundant in parts of the Arctic Ocean during the period of ice cover. In the Chukchi Sea, Eskimos fishing through the ice can catch 23 kg of arctic cod per person per day (D.C. Foote, unpublished data). Eskimos jig for the fish at considerable depths, and the cod do not appear to be as common directly below the ice as they are in summer. The effects of new ice (which forms on the underside of the ice during the winter) on the under-ice fauna are not known. The abundance of amphipods in ice-covered waters in winter is demonstrated by the experience of the Greeley Expedition in the eastern arctic. They discovered that any scrap of food thrown into a lead was quickly consumed by amphipods. Nets were made to catch the amphipods and the availability of this food source played a major part in the survival of the expedition (Schmitt 1965).
Aside from the food found in leads in the ice, the only food available to birds in the Beaufort and Chukchi seas in winter is carrion and the feces of mammals found on the pack ice. The presence of the arctic foxes on the pack ice during the winter demonstrates the availability of scavenging opportunities on the ice. Arctic foxes on the pack ice live on feces and the remains of seals killed by polar bears (Ursus maritimus). Polar bear and seals are both common in the Beaufort and Chukchi seas in winter, but no scavenging seabirds are found there in the winter. It was thought that the ivory gull (Pagaphila eburnea) was associated with marine mammals during the winter, but they are now known to winter at the Bering Sea ice edge, where they feed on fish and crustaceans (Divoky 1976). The only birds associated with polar bear kills in the Chukchi Sea in March are ravens, Corvus corax (T. J. Ely, Jr., personal communication).
Ice begins to cover the northern Bering Sea in November and reaches its maximum by February, when it usually extends as far south as the edge of the continental shelf, and covers nearly 75% of the surface of the Bering Sea (Lisityn 1969). Coverage can vary greatly from year to year. In certain years Bristol Bay may be completely covered and in others ice is found only in the northern part of the Bay. Almost all ice in the Bering Sea is first-year ice. This ice tends to be flat on the top and underside and in general lacks the extensive keels and pressure ridges found on multi-year ice.
The Bering Sea ice has a number of large-scale features of importance to birds. The "front" is a zone of ice south of the consolidated pack that is composed of small floes, ice pans, and brash ice. This zone is prevented from forming large floes by the action of swells from the open water to the south. The front continually changes in width. When winds are from the south, it is compressed into a narrow band; when winds are from the north, it is a broad zone composed of bands of ice interspersed with open water.
Polynias (areas of open water) are found immediately south of the large islands in the northern Bering Sea. They are formed by the southward movement of ice caused by the prevailing winds. This movement causes ice to be pushed away from the south side of islands, leaving areas of open water. Large polynias are associated with St. Lawrence, St. Matthew, and Nunivak islands and with the south side of the Seward Peninsula (Shapiro and Burns 1975).
The most biologically active area of the Bering Sea in winter is the ice front. Studies of primary productivity in April show that production at the surface in the ice front is high (1.98 mg C/m3 per h). Surface waters directly under the pack ice have much lower production (0.29 mg C/m3 per h), and that in the water south of the ice is lower yet. At this time production within the ice is very high (more than 5 mg C/m3 per h) (McRoy and Goering 1974). Because this phytoplankton bloom is trapped in the ice, it is not available to grazers. Thus, before the spring melt the ice front is the only area where a large quantity of phytoplankton is available to higher levels of the marine food chain.
The winter distribution of birds in the Bering Sea correlates well with the findings on primary productivity. Densities south of the ice and the continental shelf average less than 10 birds/km2. At the ice front during one cruise in March, densities exceeded 500 birds/km2. Densities at the ice front increase from south to north; they drop in the region where the ice front grades into more consolidated pack ice, and are less than 0.1 bird/km2 in the consolidated pack.
The most numerous species at the ice front are common and thick-billed murres, which constitute more than 90% of all birds seen. Irving et al. (1970) were the first to report on the large number of murres at the ice front. Feeding flocks of 25,000 individuals have been observed at the front, in which densities were as high as 10,000 birds/km2. No other diving species is common at the ice front. The parakeet auklet (Cyclorhynchus psittaculus) is seen on most cruises, but only during a small percentage of observation periods and always in low numbers. Black guillemots are common north of the ice front and stragglers are occasionally seen at the front. Pigeon guillemots (Cepphus columbus), least auklets (Aethia pusilla), and crested auklets (A. cristatella) are irregular visitors to the front.
Surface feeding species commonly found at the ice front include the northern fulmar (Fulmarus glacialis) and five species of gulls. The fulmar is common south of the ice and is found only in the southern portion of the front. Three species of Larus are found at the ice front. The most common is the glaucous-winged gull (Larus glaucescens); the glaucous gull is less frequently seen. The slaty-backed gull (L. schistisagus), a species that breeds in Asia, is most common west of St. Matthew Island (McRoy et al. 1971). The black-legged kittiwake (Rissa tridactyla) is common in open water south of the ice but is also found throughout the entire width of the front. The ivory gull is unique in that it is found only at the ice front in winter. In addition to these species, the fork-tailed storm-petrel (Oceanodroma furcata) is a regular but uncommon visitor to the ice front in winter. Densities of surface feeding species at the ice front are low when compared to the high densities of murres, and do not regularly exceed [Pg 13]10 birds/km2.
The primary food consumed by birds at the ice front is pollock (Theragra chalcogramma). An amphipod (Parathemisto libellula) and the euphausiids are less important. Examination of the stomach contents of birds and fish show that large feeding flocks are usually associated with schools of pollock feeding on P. libellula and euphausiids.
The habitat of the consolidated pack in the Bering Sea is markedly different from that at the ice front. Whereas the front is characterized by bands of ice interspersed with open water and ice coverage rarely exceeding 4 oktas (4/8), the consolidated pack consists primarily of large expanses of unbroken ice. Small leads are formed by the shifting of the ice caused by currents and wind. Ice coverage is usually 7 to 8 oktas. The southern part of the consolidated pack, which grades into the ice front, has frequent leads. Most of the species found at the ice front can be found in the southern part of the consolidated pack, but murres are most common. Their numbers decrease, however, in the more northerly pack, where leads are less frequent. Black guillemots, in contrast, increase with increasing ice cover, and reach their greatest abundance in the small leads constantly forming and refreezing deep within the ice. Because they exploit this habitat, they are dependent on the formation of lead systems. I have often seen leads a quarter mile wide refrozen to the point where new ice covered all but a small patch of open water; black guillemots were frequently crowded into this open water. Before the lead closes completely the guillemots must fly to an open lead. When winds are light and temperatures low, lead systems fail to form as rapidly as usual, and when they do they refreeze quickly, causing a loss of the preferred habitat of wintering black guillemots. A severe winter in the White Sea in 1965-66 decreased the amount of open water and caused an increased black guillemot mortality (Bianchi and Karpovitsch 1969). On a windless day in March I conducted bird observations in the Bering Sea ice where no leads or open water were encountered. The only bird seen was a black guillemot flying over the ice. In situations such as this, where black guillemots are prospecting for open water, they may use the "water sky" and steam fog associated with leads as visual aids. "Water sky" is the reflection of the dark water in the clouds over the lead, and contrasts sharply with the "ice sky." The presence of "water sky" allows birds to detect open water from a distance of many miles.
Aside from birds found in and near island-associated polynias, only murres and black guillemots are regularly found on the consolidated pack ice in winter.
The polynia associated with islands in the consolidated pack provide refuge(s) for seabirds. Fay and Cade (1959) found the polynias south of St. Lawrence to be most important to oldsquaws (Clangula hyemalis). King eiders (Somateria spectabilis), common eiders (S. mollisima), and oldsquaws are common in the St. Matthew Island polynias (McRoy et al. 1971). Because these polynias are in shallow-water areas, they provide feeding opportunities for benthic feeding species.
In April and May a lead system develops from the Bering Strait north to Cape Lisburne and then northeast to Point Barrow. The lead is a flaw lead that occurs between the shorefast ice and the free-floating pack. It is a major migration route for a number of species of birds, primarily eiders. East of Point Barrow in the Beaufort Sea, no similar well-defined large lead exists. Consequently, there is a greater chance of bird mortality occurring in the Beaufort Sea than in the Chukchi Sea because the early migrants are unable to find open water. In 1960, 10% of all the king eiders that migrate through the Beaufort Sea died during a late freeze (Barry 1968). Additional records of eider mortality due to late breakup or sudden freezes were presented by Palmer (1976).
In late May, rivers that empty into the northern Chukchi and Beaufort seas begin to flow. The shorefast ice is still present at this time and the rivers flow over the ice. For large rivers, such as the Colville and the Sagavanirktok, the area of ice covered by water is considerable. Openings in the ice develop sometime after the river runoff starts and the river water drains through the ice.
This river overflow plays an important role in the breeding biology of certain island nesting species, since the overflow surrounds islands and prevents arctic foxes from reaching the islands. The overflow also allows birds to sit in the water near breeding sites. It is not known whether river overflow contains prey items available to birds. After the overflow drains through the ice, the shorefast ice that has been covered with river overflow decomposes quickly, and patches of open water occur early in areas just seaward of major river deltas. For this reason the largest breeding colonies on barrier islands in the northern Chukchi and Beaufort seas are all found near the mouths of large rivers. Islands away from rivers become isolated from the pack ice by moats, which are caused by the absorption of solar radiation by the islands and the melting of the ice immediately adjacent to them. Moat formation is not as predictable and uniform as river overflow.
When the ice in the Bering Sea begins to melt in April, the edge of the pack does not recede northward as is frequently thought. Rather, there is a general decomposition of ice throughout the pack. The leads that are constantly forming in the ice no longer freeze. As melt continues and ice becomes rotten, leads form with increasing frequency. This manner of ice decomposition is important to birds. The leads that form deep in the pack ice provide feeding and roosting areas near the large seabird colonies found north of the ice edge, and are used by certain tundra-nesting ocean migrants such as eiders, red phalaropes (Phalaropus fulicarius), and jaegers (Stercorarius spp.). If ice decomposition is retarded by persistent low temperatures, the initiation of breeding may be delayed at northern Bering Sea colonies and for some tundra species.
At the time of decomposition the large standing stock of phytoplankton present in the pack ice is released into the water. No information is available on fish and invertebrate populations that are associated with the decomposing ice. The quantity of organic carbon released is considerable, although it is not known what fish or invertebrate populations are supported by this plankton as soon as it is released. For birds breeding in areas where ice is present in the initial stages of breeding, the phytoplankton released by the disintegrating ice could play an important part in the birds' energy budgets.
In the northern Chukchi and Beaufort seas the nearshore marine environment is dominated by sea ice in June and July. In June the coastal areas are characterized by a snow-free tundra teeming with nesting waterfowl and shorebirds next to an expanse of sea ice almost completely devoid of bird life. In areas where river outflow does not occur, the use of nearshore waters usually begins when a moat forms along the shoreline. Amphipods and other invertebrates are found in this moat, especially at stream mouths. Limited but regular use of the moat occurs, primarily by loons (Gavia spp.), oldsquaws, and arctic terns (Sterna paradisaea).
As the snow on top of the shorefast ice begins to melt, ponds form on top of the ice. As melt proceeds, these melt ponds merge into long, parallel channels and may cover well over 50% of the ice surface. Only when thaw holes form and the melt ponds are connected to the water under the ice is food present in the channels. Amphipods are then seen swimming in these channels. Bird use of these channels is not extensive.
It is usually late July before the nearshore ice begins its rapid decomposition. Ice in the lagoons is the first to melt. Ice seaward of the barrier islands decomposes more slowly because of the presence of keels and pressure ridges. As the ice melts, the in-ice algal bloom is released into the water. These algae are important because they provide at least 25 to 30% of the productivity in coastal waters and allow the biological growing season to begin before the open-water plankton bloom occurs (Alexander 1974). In nearshore areas close to Barrow, large populations of mysids and amphipods are associated with the decomposing ice. At least in certain areas, these ice-associated zooplankton populations are a major food source for nearshore migrants, especially red phalaropes, arctic terns, and Sabine's gulls (Xema sabini).
The effects of ice scour on the shoreline and the nearshore bottom of the Chukchi and Beaufort seas is demonstrated by the absence of sessile benthic fauna and flora. The effect this absence has on birds is seen in the feeding habits of nearshore birds. Oldsquaws and eiders, which frequently feed on molluscs, feed instead on motile benthos species such as mysids, amphipods, and isopods. The emperor goose (Philacte canagica) is absent from the northern Chukchi and Beaufort seas, apparently due to the absence of eelgrass beds. Ice scour is the major cause of the absence of eelgrass in northern Alaska (C. P. McRoy, personal communication).
The offshore ice in the Chukchi decomposes more rapidly than that in the Beaufort, largely because Bering Sea water enters the Chukchi through the Bering Strait (Coachman and Barnes 1961). By late July the Chukchi is usually ice free as far north as Icy Cape. In the Beaufort, however, ice decomposition occurs slowly through June and July, and only in August does a definite strip of open water develop between the shore and the edge of the pack ice. The amount of open water varies greatly from year to year. In certain years the Beaufort is not navigable due to the lack of open water.
Aerial censusing in June and July shows that bird densities on the offshore ice are extremely low. In August and September, when shipboard censusing can be conducted, densities on the pack ice in both seas are about 10 birds/km2. Unlike the Bering Sea, where densities south of the ice are much less than on the ice, bird densities south of the ice in the Beaufort and Chukchi seas are slightly higher in the open water south of the ice, averaging about 20 birds/km2. In the Chukchi the principal species encountered on the ice are the black-legged kittiwake and the thick-billed murre. In the Beaufort, red phalaropes, oldsquaws, and glaucous gulls are the most common species.
Numerous arctic cod are associated with the underside of the summer pack ice. Shipboard censusing in the ice is complicated when cod are stranded on ice floes, as the ice shifts under the weight of the ship. Gulls, arctic terns, and jaegers gather behind the ship to feed on these fish; mixed flocks of more than 100 birds are common. In the absence of a ship to provide the disturbance needed to make large numbers of cod available, these birds are dependent on locating the fish in the surface waters next to ice floes. Because cod frequently swim over underwater ice shelves they are highly visible from above and should be easily accessible to aerial feeders.
By the time ice formation begins in late September or early October, most seabirds have left the Arctic on their southward migration. The principal exception is the oldsquaw, which does not begin its migration until September. Some oldsquaws remain in nearshore waters until they are driven out by the formation of new ice. In contrast to the spring mortality, there are few records of extensive bird mortality in the fall due to lack of open water. One instance was reported for 1975, when nearshore waters froze early and flightless eiders were seen sitting on the ice near Pt. Lay in the Chukchi Sea. The birds were in a weakened condition, apparently due to their inability to obtain food (W. J. Wiseman, personal communication).
In the offshore waters the species associated with the pack ice in September are the same as those in August. In late September, however, ivory and Ross' gulls become the most common species at the ice edge in the Chukchi. Glaucous gulls and black guillemots are also associated with the advancing ice edge (Watson and Divoky 1972). Except for the Ross' gull, which apparently winters in the arctic basin, these species remain with the ice as it advances into the Bering Sea.
Little is known about bird distribution in the Bering Sea during ice formation because cruises in rapidly forming ice are potentially hazardous. It is not known if the large numbers of birds found at the ice edge in March are present in December and January.
The principal effect of the arctic pack ice is to lower biological productivity and bird densities in the areas it covers. Unlike the antarctic pack ice, which supports a large biomass of pagophilic species, the number of pagophilic species supported by the arctic pack ice is small. Only the ivory gull, Ross' gull, and black guillemot have specific adaptations to the ice environment. The Ross' gull and guillemot winter in the pack ice, and the ivory gull is associated with ice throughout the year. The total biomass of these species is low. Other species which are regularly associated with the arctic pack, such as murres and black-legged kittiwakes, are also found in large numbers away from the ice. In addition, these species are usually associated with ice for limited periods during the year—murres primarily in winter and spring and kittiwakes primarily in summer.
The difference in the antarctic and arctic pack ice systems is largely due to the antarctic pack ice being surrounded by ocean, whereas the arctic pack ice is, in general, surrounded by land. The high productivity associated with the antarctic pack ice is due primarily to the mixing that occurs at the edge of the pack ice. There is little opportunity for mixing to occur next to the arctic pack ice, except where it is next to large expanses of boreal waters. This occurs in the Bering Sea in winter and spring, in the North Atlantic, and to a minor extent in the Chukchi Sea in summer and fall (Dunbar 1968). The limited geographic range and seasonal nature of high productivity at the arctic pack ice edge has been a major factor in preventing a well-developed pagophilic avifauna.
The importance of the in-ice algal bloom and its associated under-ice fauna is not yet clear. It is probably most important in areas such as the Beaufort Sea, where productivity in the water column is low. Although considerable numbers of seabirds are regularly found in the summer pack ice feeding on arctic cod and zooplankton associated with the ice, bird densities south of the ice are usually greater than those in the ice. The only species that appear to depend on the ice-associated fauna for much of their food are the three pagophilic species mentioned above.
Alexander, V. 1974. Primary productivity regimes of the nearshore Beaufort Sea, with reference to potential roles of ice biota. Pages 609-632 in J. C. Reed and J. E. Sater, eds. The coast and shelf of the Beaufort Sea. Arctic Institute of North America, Arlington, Virginia.
Andriashev, A. P. 1968. The problem of the life community associated with the antarctic fast ice. Pages 147-155 in R. I. Currie, ed. Symposium on antarctic oceanography. Scott Polar Research Institute, Cambridge.
Appollonio, S. 1961. The chlorophyll content of arctic sea ice. Arctic 14:197-200.
Bailey, A. M. 1948. Birds of arctic Alaska. Colo. Mus. Nat. Hist. Popular Ser. 8. 317 pp.
Barry, T. W. 1968. Observations on natural mortality and native use of eider ducks along the Beaufort Sea coast. Can. Field-Nat. 82(2): 140-144.
Bianchi, V. V., and V. N. Karpovitsch. 1969. The influence of abnormal ice-cover of the White Sea and Murman in 1966 upon birds and mammals. (In Russian, English summary.) Zool. Zh. 48(6):871-875.
Bunt, J. S. 1963. Diatoms of antarctic sea ice as agents of primary production. Nature (Lond.) 199:1255-1257.
Coachman, L. K., and C. A. Barnes. 1961. The contribution of Bering Sea water to the Arctic Ocean. Arctic 15:147-161.
Divoky, G. J. 1976. The pelagic feeding habits of Ivory and Ross' Gulls. Condor 78:85-90.
Dunbar, M. J. 1968. Ecological development in polar regions: a study in evolution. Prentice-Hall, Englewood Cliffs, N.J. 119 pp.
Fay, F. H. 1974. The role of ice in the ecology of marine mammals of the Bering Sea. Pages 383-399 in D. W. Hood and E. J. Kelly, eds. Oceanography of the Bering Sea. Univ. Alaska Inst. Mar. Sci. Occas. Publ. 2.
Fay, F. H., and T. J. Cade. 1959. An ecological analysis of the avifauna of St. Lawrence Island, Alaska. Univ. Calif. Publ. Zool. 63:73-150.
Gabrielson, I. N., and F. C. Lincoln. 1959. The birds of Alaska. The Stackpole Company, Harrisburg, Pennsylvania, and Wildlife Management Institute, Washington, D.C. 922 pp.
Irving, L., C. P. McRoy, and J. J. Burns. 1970. Birds observed during a cruise in the ice-covered Bering Sea in March 1968. Condor 72:110-112.
Lisityn, A. P. 1969. Recent sedimentation in the Bering Sea (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 614 pp.
McRoy, C. P., and S. R. Goering. 1974. The influence of ice on the primary productivity of the Bering Sea. Pages 403-421 in D. W. Hood and E. J. Kelly, eds. Oceanography of the Bering Sea. Univ. Alaska Inst. Mar. Sci. Occas. Publ. 2.
McRoy, C. P., S. W. Stoker, G. E. Hall, and E. Muktoyuk. 1971. Winter observations of mammals and birds, St. Matthew Island. Arctic 24:63-65.
Mohr, J. L., and S. R. Geiger. 1968. Arctic Basin faunal precis-animals taken mainly from arctic drifting stations and their significance for biogeography and water-mass recognition. Pages 297-313 in J. E. Sater, coordinator. Arctic drifting stations. Arctic Institute of North America.
Nelson, R. K. 1969. Hunters of the northern ice. University of Chicago Press, Chicago, Ill. 429 pp.
Palmer, R. S. 1976. Handbook of North American birds. Vol. 3. Yale University Press, New Haven, Conn. 560 pp.
Schmitt, W. L. 1965. Crustaceans. University of Michigan Press, Ann Arbor. 204 pp.
Shapiro, L. H., and J. J. Burns. 1975. Major late-winter features of ice in northern Bering and Chukchi seas as determined from satellite imagery. Univ. Alaska Geophys. Inst. Rep. 75-78.
Swartz, L. G. 1967. Distribution and movements of birds in the Bering and Chukchi seas. Pacific Sci. 21:332-347.
Watson, G. E., and G. J. Divoky. 1972. Pelagic bird and mammal observations in the eastern Chukchi Sea, early fall 1970. U.S. Coast Guard Oceanogr. Rep. 50:111-172.
by
James C. Bartonek[2]
U.S. Fish and Wildlife Service
Fairbanks, Alaska
and
Spencer G. Sealy
University of Manitoba
Winnipeg, Manitoba, Canada
The Alaska coast fronting on the Chukchi and Bering seas, exclusive of the Aleutian Islands, supports seven complexes of marine bird colonies numbering more than 1 million birds each, nine colonies of 100,000 to almost 1 million birds, and many smaller colonies. Colonies are found on most headlands and islands and are dominated numerically by alcids and kittiwakes (Rissa sp.). Estuarine habitats (mainly the lowlands of northern Seward Peninsula, Yukon-Kuskokwim delta, and the north side of the Alaska Peninsula) are extremely important for breeding and migrating marine waterfowl, shorebirds, gulls (Larus sp.), and terns (Sterna sp.). Information on population size and distribution of breeding marine birds within this area is extensive for only a few of the more heavily hunted species of waterfowl. Except for the intensive and systematic censusing of a few colonies in this region, population data on cliff-, burrow-, and crevice-nesting birds are such that all but gross changes in numbers may go unnoticed, and if noticed they could not be measured.
Habitats for breeding marine birds are found along much of the 4,100-km coastline of Alaska that fronts on the Chukchi and Bering seas. Seasonal sea ice and an extensive outer continental shelf are dominant features that contribute to the productivity of these marine waters, which sustain populations of fishes, birds, and mammals that are of considerable and diverse values to man (Kelley and Hood 1974).
Our purpose in this paper is to describe the distribution, abundance, and relative status of some of the nearly 100 species of marine birds breeding within this region and the information base from which the descriptions are derived. Although the selection is admittedly arbitrary, we discuss mainly the colonial nesting species because they are generally in greater jeopardy from lost breeding habitat and from catastrophes than are the species that are widely dispersed or solitary in nesting. Because we believe matters affecting the conservation of marine birds will be geographically oriented, we discuss the status and distribution of breeding birds on that basis, rather than by the more traditional taxonomic approach. We use the terms "colony" and "colonies" somewhat loosely and interchangeably to include any aggregation of birds of the same or different species nesting in proximity to each other, even those on the same island or headland, although populations may be miles apart and occupy different kinds of habitats. The nature of this paper and the scale of our maps do not allow for detailed resolution of each colony's location [Pg 22](for the most part this information is not available), but rather facilitates a general impression of status.
Most place-names used by us are shown in Fig. 1; the others may be located by referring to Orth's (1967) gazetteer on Alaska.
There is no adequate catalog of marine bird colonies and other avian habitats for the Bering-Chukchi region or for Alaska as a whole. King and Lensink (1971) described the waterfowl populations and major lowland habitats of the State and listed only a few of the many colonies of cliff-nesting birds. LeResche and Hinman (1973) identified a few additional colonies, provided fragmentary information on composition and abundance at some of these sites, and delineated areas of wetland habitats on maps in their statewide atlas on wildlife. General and occasionally site-specific information on the location, but rarely on population size and composition, of colonies can be gleaned from the 321 species accounts presented by Gabrielson and Lincoln (1959) and from the general works by Bent (1919, 1921, 1922, 1923, 1925, 1927, 1929), Dement'ev and Gladkov (1951), Dement'ev et al. (1951, 1952), Palmer (1962), Fisher (1952), Tuck (1960), and others. The birds on the Asiatic side of these waters, which are not treated in this paper, were described by Portenko (1973).
Information on the status of waterfowl in the region is generally more detailed than that for most other groups of birds because waterfowl have been the object of systematic surveys since the late 1940's as part of the continent-wide effort to manage populations for sport hunting. Because the emphasis of these surveys has been directed toward the species of ducks important to hunters in the "lower 48" States, data are not adequate to measure changes in populations for most sea ducks and marine geese nesting in this region. These surveys have, however, enabled biologists to delineate waterfowl habitats and make reasonable estimates of populations for some of the more abundant and conspicuous species (King and Lensink 1971; U.S. Fish and Wildlife Service [FWS] 1973c; U.S. National Park Service [NPS] 1973).
A disproportionate percentage of ornithological investigations in arctic Alaska have centered about Barrow, where ornithologists were attracted because of the propensity of vagrant birds to collect there and because of the above average facilities, conveniences, and transportation afforded first by the whaling station, then by the military, and later by a research laboratory. Recent petroleum development near Prudhoe Bay has resulted in a somewhat commensal eastward shift in ornithological studies.
Bailey (1948), Gabrielson and Lincoln (1959), and Pitelka (1974) reviewed much of the published information on arctic avifauna, including that of the Chukchi coast. Selkregg [1975] mapped various avian habitats, ascribed either relative or absolute values for the population size of certain groups of birds, and included a selected bibliography that did not entirely duplicate those provided by the other reviewers. Watson and Divoky (1975) described the avifauna of Alaska's Beaufort Sea coast, which is much the same as that of the Chukchi coast from Point Barrow south to Cape Lisburne (both coasts are of low relief).
Intensive studies near Barrow have done much to characterize the behavior, productivity, and ecological requirements of calidridine sandpipers (Pitelka 1959; Pitelka et al. 1974; Holmes 1970, 1971) and, to partly explain the cyclical relationships between jaegers (Stercorarius spp.) and their prey (e.g., Pitelka et al. 1955; Maher 1974). Quantitative estimates of certain bird populations at Cape Thompson (Swartz 1966; Williamson et al. 1966), Little Diomede (Kenyon and Brooks 1960), and on the coastal lowlands of the Seward Peninsula (King and Lensink 1971; U.S. NPS 1973), and for black guillemots (Cepphus grylle) throughout the region (Divoky et al. 1974) are among the best data on status of marine birds for any locality in Alaska. Grinnell (1900a) described the birds he observed in the Kotzebue Sound area.
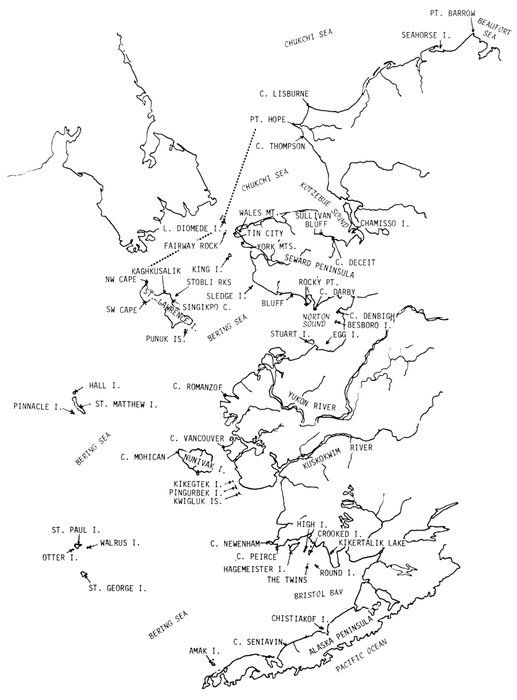
Fig. 1. Place-names in the region of the Chukchi and Bering seas.
Cursory aerial surveys conducted by J. C. Bartonek, J. G. King, and D. R. Cline (U.S. FWS 1973a; U.S. NPS 1973; this paper) in 1972 and 1973 provided information on the location and relative size of most, if not all, colonies of cliff-nesting marine birds between Point Barrow and the Bering Strait, including those at Cape Lisburne, at Motherhood Point, Nine-mile Point, Cape Deceit, Towalevic Point, Sullivan Bluff, all on the northern base of the Seward Peninsula, and at Fairway Rock. The relative size of populations of most species was probably underestimated because the burrow-and crevice-nesting species were largely unseen.
Aside from work by Gabrielson and Lincoln (1959) and the early but understandably incomplete accounts by Nelson (1883, 1887) and Turner (1886), no comprehensive description of the avifauna of the Alaskan coast of the Bering Sea exists. Many studies adequately describe local avifauna, and some of them are exemplary assessments of the status of populations.
Most of the coastline suitable for cliff-nesting marine birds and most of the smaller nearshore islands from the Bering Strait south to the tip of the Alaska Peninsula were reconnoitered piecemeal from aircraft between 1970 and 1973 by J. C. Bartonek, J. G. King, D. R. Cline, C. D. Evans, and M. L. Plenert (U.S. FWS 1973a, 1973b; this paper). In late June 1973 Bartonek, Cline, and Plenert made brief reconnaissances on foot of King, Besboro, and Shaiak islands. Bartonek and J. G. Divoky, traveling by boat and occasionally on foot, reconnoitered colonies at Cape Seniavin, a portion of the Walrus Islands group, Shaiak Island, and the coastline from Cape Peirce around Cape Newenham to Security Cove (U.S. FWS 1973a, 1973b; this paper). Although these cursory surveys (especially those from aircraft) tended to identify nesting sites of cliff-nesting birds while missing sites used by burrow-and crevice-nesting species, information was obtained on the location and relative size of many previously unreported colonies.
The mainland and island colonies in Norton Sound have received little notice in the published literature. Bailey (1943, 1948), although working mainly at Little Diomede and in Arctic and Lopp lagoons on the north side of the Seward Peninsula, mentioned the birds at Wales Mountain and Tin City. Nelson (1883, 1887) traveled throughout the region studying the avifauna and the anthropology of Eskimos. Grinnell (1900b) at Nome, McGregor (1902) along the Koyuk River, Hersey (1917) and Turner (1886) near St. Michael, and Cade (1952) at Sledge Island provide fragmentary examples of the area's marine bird populations. Colonies at King, Besboro, Egg, and Sledge islands, near York Mountains, and at Bluff were described in proposals for new National Wildlife Refuges (U.S. FWS 1973a).
Sealy et al. (1971) reviewed the literature and discussed the various zoogeographic relationships among the avifauna of St. Lawrence Island. Fay and Cade (1959) estimated numbers and biomass of all birds on St. Lawrence Island but did not identify locations and sizes of particular populations; consequently, replication of their estimates is precluded. An exemplary study by Bédard (1969) identified the locations and sizes of all populations of crested auklets (Aethia cristatella), least auklets (A. pusilla), and parakeet auklets (Cyclorrhynchus psittacula) on the island. Sealy (1973) identified breeding sites of horned puffins (Fratercula corniculata) there and throughout the species' range. Thompson (1967) listed the birds observed at Northeast Cape and on nearby Punuk Islands.
Annotated accounts have been published on the breeding avifauna of St. Matthew, Hall, and Pinnacle islands by Elliott (1882), Hanna (1917), Bent (1919), and Gabrielson and Lincoln (1959). Klein (1959) presented quantitative data on the birds he observed incidental to his study of reindeer (Rangifer tarandus).
The avifauna of the Yukon-Kuskokwim delta, which is rich both in numbers and diversity, has been treated extensively in the literature. Nelson (1883, 1887), Turner (1886), Conover (1926), Brandt (1943), Gabrielson and Lincoln (1959), Williamson (1957), Kessel et al. (1964), Harris (1966), Dau (1972), and Holmes and Black (1973) all described the avifauna in the same general area of the delta, i.e., the eroding portion in the general vicinity of Hooper and Hazen bays. The avifauna of the aggrading portion of the Yukon delta and of the Kuskokwim's mouth have not been accorded similar attention. Populations of waterfowl nesting on the delta and their wintering affinities were described by King and Lensink (1971) and U.S. FWS (1973c).
Studies of particular species of marine birds on the delta (again, all in the general vicinity of Hooper and Hazen bays) were reported by Hansen and Nelson (1957) and Shepherd (1960) for black brant (Branta bernicla), by Headley (1967) and Eisenhauer and Kirkpatrick (1977) for emperor geese (Anser canagica), by Dau (1974) and Mickelson (1975) for spectacled eiders (Somateria fischeri), by Petersen (1976) for red-throated loons (Gavia stellata), and by Holmes (1970, 1971, 1972) for dunlins (Calidris alpina) and western sandpipers (C. mauri).
Birds of Nunivak Island were reported by Swarth (1934), but the importance of the island to marine birds was not put into proper perspective until the Nunivak National Wildlife Refuge was evaluated for designation as a wilderness area (U.S. FWS 1972).
The Pribilof Islands have served as a focal point for ornithological investigations of the Bering Sea in much the same way that Barrow has for the Arctic. The avifauna of the Pribilofs has been described by Coues (1874), Elliott (1882), Palmer (1899), Hanna (1918), Preble and McAtee (1923), Gabrielson and Lincoln (1959), Kenyon and Phillips (1965), and a host of others that mainly added new species to the record list. Although most of these ornithologists marveled at the numbers of birds, information is lacking from which most changes in populations can be noted. (An exception is the record of common and thick-billed murres, Uria aalge and U. lomvia, which formerly nested in such abundance on Walrus Island that annually several tons of eggs were gathered for consumption by residents of the islands [Palmer 1899], but were greatly reduced in numbers by the summer of 1973, when J. C. Bartonek, J. G. King, G. J. Divoky, and D. T. Montgomery observed only a few thousand murres on a small portion of the island. Most of the suitable nesting sites, especially the flat areas often used by common murres, were occupied by Steller's sea lions, Eumetopias jubata, which, apparently because of reduced hunting pressure, occupied the island and displaced the murres.)
For some unexplained reason the numerous and large marine bird colonies along the north side of Bristol Bay appear to have been largely overlooked until recent years (Bartonek and Gibson 1972). Gabrielson and Lincoln (1959) summarized the few observations by Osgood (1904) and Turner (1886) in this area, but obviously were unaware that, in aggregate, these colonies rival those of the Pribilofs. Dick and Dick (1971) made an exemplary study of marine birds and their numbers at Cape Peirce and on nearby Shaiak Island. Murie (1959) provided annotated remarks on marine birds of Amak Island, but not of nearby Sealion Rocks.
Seven groups of colonies of cliff-, burrow-, and crevice-nesting birds are found on the headlands and islands in the coastal region, each numbering more than 1 million birds; nine colonies range downward to 100,000 birds; and a host of others range downward to 1,000 birds (Fig. 2). Un-estimated numbers of other marine birds nest on the lowlands about Kotzebue Sound, the Yukon-Kuskokwim delta, and Bristol Bay, but are not shown in Fig. 2. The occurrence at colonies of 20 of the nearly 100 species of marine birds is shown in Fig. 3; their relative numbers at these sites are not shown because data are generally lacking.
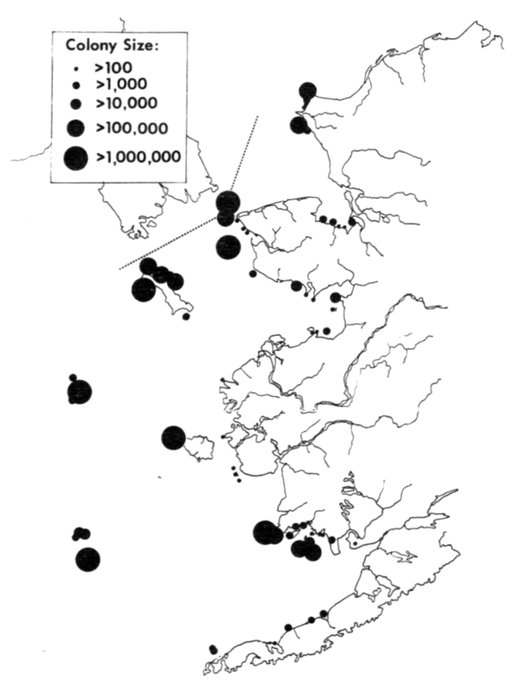
Fig. 2. Relative numbers of marine birds at colonies in different localities, without regard to species composition or breeding status.
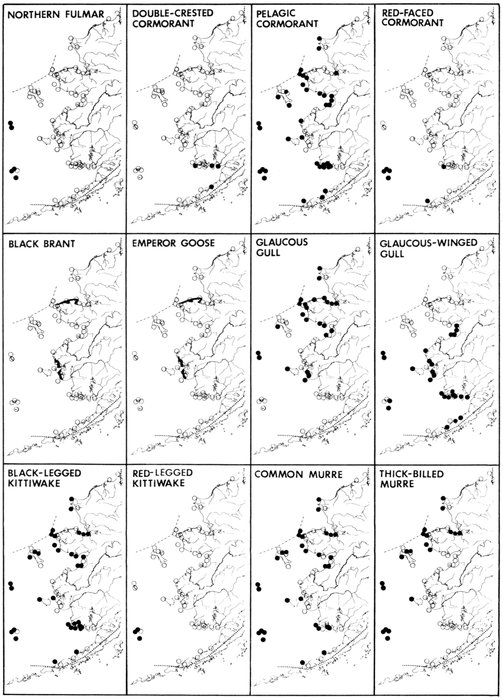
Fig. 3. Location of known breeding populations of some marine bird species without regard to size of population.
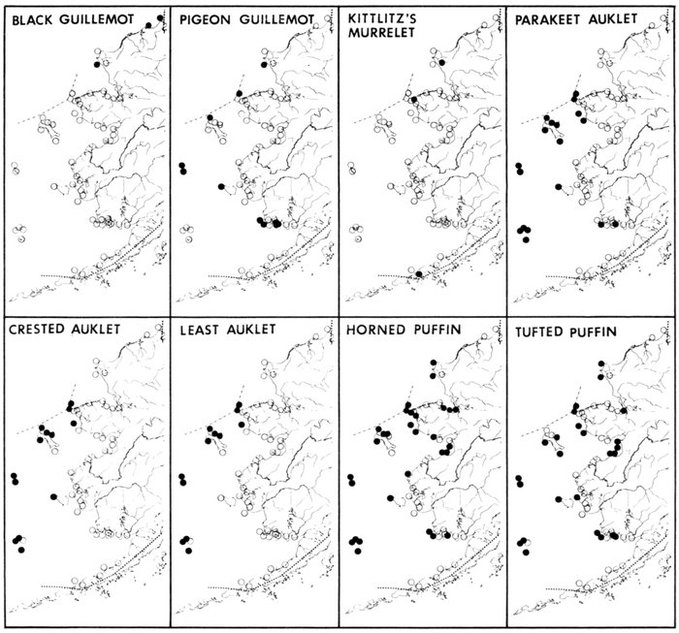
The largest colonies of seabirds in the Chukchi Sea are those on Little Diomede Island, Cape Lisburne, Cape Thompson, and Fairway Rock. Smaller colonies are in Kotzebue Sound along the northern base of the Seward Peninsula. These colonies are largely dominated by thick-billed and common murres and black-legged kittiwakes (Rissa tridactyla) and on the islands in the Bering Strait also the crested, least, and parakeet auklets. Horned puffins, tufted puffins (Lunda cirrhata), pelagic cormorants (Phalacrocorax pelagicus), and glaucous gulls (Larus hyperboreus) make up the remaining majority. For the whole area there are probably fewer than a hundred birds each of black guillemots and pigeon guillemots (Cepphus columba) occupying colonies. Dovekies (Alle alle) are occasionally sighted in this area, but only as stragglers from their normal range.
Part of the mystery surrounding the nesting location of Kittlitz's murrelet (Brachyramphus brevirostris) was solved when Thompson et al. (1966) discovered a downy chick in the Kukpuk River drainage nearly 45 km by river from salt water. Other nesting sites of the Kittlitz's murrelets in this region[Pg 28] were reported for Wales Mountain (Ford 1936; Bailey 1943, 1948) and the Cold Bay area (Bailey 1973) (Fig. 3).
Only the colonies at Cape Thompson have been censused systematically throughout a breeding season. During one of three years of varying census efforts, Swartz (1966) estimated that about 400,000 birds of nine species occupied the cliffs. Whereas the Cape Thompson colonies received considerable attention because of Swartz's efforts, the colonies that extend along nearly 35 km of headlands southward from, but mainly at, Cape Lisburne have received little if any attention by either early or recent ornithologists in the Arctic, even though they support perhaps twice the number of birds. Also perplexing is why Chamisso and Puffin islands with their several thousand nesting horned puffins and lesser numbers of other seabirds were designated as the Chamisso National Wildlife Refuge in the early 1900's when none of the many larger and more species-diverse colonies in the area received comparable recognition by and protection through refuge designation.
The lowlands on the north side of the Seward Peninsula produce fall flights of sea ducks that average 49,200 oldsquaws (Clangula hyemalis), 51,000 eiders (mostly common eiders, Somateria mollissima), and 26,700 scoters (mostly black scoters, Melanitta nigra) (King and Lensink 1971). Small populations of black brant and emperor geese breed in what outwardly appears to be excellent habitat, and King and Lensink (1971) speculated that subsistence hunting by local Eskimos is responsible for suppressing these populations.
The largest concentration of nesting seabirds in the Bering Sea and perhaps in the entire North Pacific is that on St. George Island. Colonies that rank somewhere below that at St. George are along the coast from Cape Newenham to Cape Peirce, in the Walrus Islands (Round, High, Crooked, and Summit islands, The Twins, and Black Rock), at Cape Mohican on Nunivak Island, St. Matthew Island, Southwest Cape of St. Lawrence Island, and King Island.
The Pribilofs have the unique distinction of being the primary nesting site of red-legged kittiwakes (Rissa brevirostris). They are also interesting from the zoogeographic standpoint in that they are the northernmost stronghold of red-faced cormorants (Phalacrocorax urile); guillemots are conspicuous by their absence, and larid gulls are conspicuously scarce nesters.
St. Matthew Island and associated Hall and Pinnacle islands, and all but Walrus Island of the Pribilofs, are sites of nesting northern fulmars (Fulmarus glacialis). Nesting fork-tailed or Leach's storm-petrels (Oceanodroma furcata and O. leucorhoa) have been found nowhere in this region, although both are commonly observed at sea and both nest throughout the Aleutians.
Most colony sites identified in Fig. 2 are dominated by common or thick-billed murres (or both) and black-legged kittiwakes. Glaucous gulls (generally north of the Yukon-Kuskokwim delta), glaucous-winged gulls (Larus glaucescens) (generally to the south of the delta), and pelagic cormorants occupy almost every rocky prominence along the entire coast (most of these sites are not shown in Figs. 2 and 3). Double-crested cormorants (Phalacrocorax auritus) nest at a few island and inland locations in the Bristol Bay area. The small auklets are largely restricted to islands in the Bering Sea; the parakeet auklet is the only one occasionally found in mainland colonies.
The marine birds of the Yukon-Kuskokwim delta lowlands, although largely uncounted, in their aggregate probably exceed the numbers at any individual site identified in Fig. 2. This is not particularly surprising since the delta has nearly 70,000 km2 of habitat (King and Lensink 1971) in contrast to the generally small parcels of habitat occupied at the sea-cliff and island sites.
King and Lensink (1971) estimated that fall flights of sea ducks originating on the delta averaged 292,300 oldsquaws, 51,000 eiders (mostly common and spectacled eiders with lesser numbers of Steller's eiders, Polysticta stelleri), and 157,000 scoters (primarily black scoters). They also estimated that half of the 150,000 black brant and most of the 150,000 emperor geese in Alaska's fall flight originate there. Although no counts have been made, we believe that the delta's lowlands support easily more than half of Alaska's nesting dun[Pg 29]lins, black turnstones (Arenaria melanocephala), rock sandpipers (Calidris ptilocnemis), western sandpipers, and substantial percentages of red phalaropes (Phalaropus fulicarius), northern phalaropes (Lobipes lobatus), and red-throated loons.
The north side of the Alaska Peninsula (including the wetlands, uplands, and estuaries) is perhaps more important to marine birds as a staging, feeding, and resting area than as a nesting habitat. The importance of Izembek Lagoon to black brant and emperor geese during fall and spring is a classic example. King and Lensink (1971) estimated that the fall flight of sea ducks originating from the Peninsula averages 53,400 oldsquaws, 1,700 eiders, and 74,400 scoters. Breeding geese are scarce throughout the area.
Most of the major breeding habitats of marine birds in the Chukchi and Bering seas are known, but imprecisely identified as to location and size. With few exceptions, the populations of birds using these habitats are described only by the subjective and ambiguous descriptors of abundance such as "abundant, common, occasional, and rare," which makes measurement of change impossible.
We recommend that first and foremost a catalog of habitats used by birds be developed to aid resource administrators, developers, and biologists (all of whom should be "conservationists") in identifying critical habitats. We believe that such a catalog would preclude many problems because birds and their habitats could be considered at the planning stage rather than only at the operational stage. Such a catalog would also be useful to students of ornithology who are seeking locations suitable for particular studies.
Nowhere in this region have studies of marine birds been of sufficient duration to enable changes in populations (from whatever cause) to be characterized. Since some species of marine bird are known not to breed before at least 3 or more years of age, meaningful information on survival and recruitment in populations cannot be obtained by studies of less than 10 years. We therefore recommend that long-term studies be initiated at as many places as possible, but at least at one site on the Yukon-Kuskokwim delta; at a mainland colony site that has predominantly murres, kittiwakes, puffins, and cormorants; and at an island site that also has small auklets. Although the nesting distribution of the Kittlitz's murrelet remains an enigma, we regard it less of a conservation issue and more of an ornithological challenge. Consideration of logistics and support facilities must, of course, be included in the site selection process. Most of the areas suggested for these studies also merit recognition and protection by being designated as a National Wildlife Refuge, a National Park or Monument, or a State Game Sanctuary.
Bailey, A. M. 1943. The birds of Cape Prince of Wales, Alaska. Proc. Colo. Mus. Nat. Hist. 18. 113 pp.
Bailey, A. M. 1948. Birds of arctic Alaska. Colo. Mus. Nat. Hist. Popular Ser. 8. 317 pp.
Bailey, E. P. 1973. Discovery of a Kittlitz's murrelet nest. Condor 75(4):457.
Bartonek, J. C., and D. D Gibson. 1972. Summer distribution of pelagic birds in Bristol Bay, Alaska. Condor 74(4):416-422.
Bédard, J. 1969. The nesting of the least, crested, and parakeet auklets around St. Lawrence Island, Alaska. Can. J. Zool. 47(5):1025-1050.
Bent, A. C. 1919. Life histories of North American diving birds. U.S. Natl. Mus. Bull. 107. 245 pp.
Bent, A. C. 1921. Life histories of North American gulls and terns. U.S. Natl. Mus. Bull. 113. 345 pp.
Bent, A. C. 1922. Life histories of North American petrels, pelicans, and their allies. U.S. Natl. Mus. Bull. 121. 343 pp.
Bent, A. C. 1923. Life histories of North American wild fowl. Part I. U.S. Natl. Mus. Bull. 126. 250 pp.
Bent, A. C. 1925. Life histories of North American wild fowl. Part II. U.S. Natl. Mus. Bull. 130. 311 pp.
Bent, A. C. 1927. Life histories of North American shore birds. Part I. U.S. Natl. Mus. Bull. 142. 359 pp.
Bent, A. C. 1929. Life histories of North American shore birds. Part II. U.S. Natl. Mus. Bull. 146. 340 pp.
Brandt, H. 1943. Alaska bird trails. Bird Research Foundation, Cleveland, Ohio. 464 pp.
Cade, T. J. 1952. Note on the birds of Sledge Island, Bering Sea, Alaska. Condor 52(1):51-54.
Conover, H. B. 1926. Game birds of the Hooper Bay region, Alaska. Auk 43(2):162-180, 43(3):308-318.
Coues, E. 1874. Ornithology of the Prybilov Islands. Pages 168-212 in H. W. Elliott, Report on the Prybilov Group, or Seal Islands of Alaska. U.S. Treasury Department, Washington, D.C.
Dau, C. 1972. Observations on the spring migration of birds at Old Kashunuk Village, Alaska. Univ. Alaska, Dep. Wildl. Fish., Fairbanks. 54 pp. (Unpublished report)
Dau, C. 1974. Nesting biology of the spectacled eider Somateria fischeri (Brandt) on the Yukon-Kuskokwim delta, Alaska. M. S. Thesis. Univ. of Alaska, Fairbanks. 72 pp.
Dement'ev, G. P., and N. A. Gladkov. 1951. Birds of the Soviet Union. Vol. 1. (Transl. from Russian.) Israel Progr. Sci. Transl., 1966. 705 pp.
Dement'ev, G. P., N. A. Gladkov, Yu. A. Isakov, N. N. Kartashev, S. V. Kirikov, A. V. Mikheev, and E. S. Prushenko. 1952. Birds of the Soviet Union. Vol. 4. (Transl. from Russian.) Israel Progr. Sci. Transl., 1967. 683 pp.
Dement'ev, G. P., N. A. Gladkov, and E. P. Spageburg. 1951. Birds of the Soviet Union. Vol. 2. (Transl. from Russian.) Israel Progr. Sci. Transl., 1968. 553 pp.
Dick, M. H., and L. S. Dick. 1971. The natural history of Cape Peirce and Nanvak Bay, Cape Newenham Natl. Wildl. Refuge, Alaska. U.S. Bureau Sport Fisheries and Wildlife, Bethel, Alaska. 78 pp.
Divoky, G. J., G. E. Watson, and J. C. Bartonek. 1974. Breeding of the black guillemot in northern Alaska. Condor 76(3):339-343.
Eisenhauer, D. I., and C. M. Kirkpatrick. 1977. Ecology of the emperor goose in Alaska. Wildl. Monogr. 57. 62 pp.
Elliott, H. W. 1882. Seal-islands of Alaska. U.S. Comm. Fish Fish. Spec. Bull. 176, Sect. IX, Monogr. A. 176 pp.
Fay, F. H., and T. J. Cade. 1959. An ecological analysis of the avifauna of St. Lawrence Island, Alaska. Univ. Calif. Publ. Zool. 63:73-150.
Fisher, J. 1952. The fulmar. N. M. N. Collins, London. 496 pp.
Ford, E. R. 1936. Kittlitz's murrelet breeding at Wales, Alaska. Auk 51:214-215.
Gabrielson, I. N., and F. C. Lincoln. 1959. The birds of Alaska. The Stackpole Co., Harrisburg, Pennsylvania, and Wildlife Management Institute of North America, Washington, D.C. 992 pp.
Grinnell, J. 1900a. Birds of the Kotzebue Sound region, Alaska. Pac. Coast Avifauna 1. 80 pp.
Grinnell, J. 1900b. Notes on some birds of Cape Nome, Alaska. Condor 2(5):112-115.
Hanna, G. D. 1917. The summer birds of the St. Matthew Island Bird Reservation. Auk 34(4):403-410.
Hanna, G. D. 1918. Check list of birds of the Pribilof Islands, Alaska, with names of persons first recording the species from the islands. Pages 105-107 in W. T. Bower, ed., Report of U.S. Commissioner of Fisheries. Bureau of Fisheries Document 872.
Hansen, H. A., and U. C. Nelson. 1957. Brant of the Bering Sea—migration and mortality. Trans. N. Am. Wildl. Nat. Resour. Conf. 22:237-256.
Harris, S. W. 1966. Summer birds of the lower Kashunuk River, Yukon-Kuskokwim delta, Alaska. Murrelet 47(3):57-65.
Headley, P. C. 1967. Waterfowl. Ecology of the emperor goose. Alaska Dep. Fish Game Small Furbearer Invest. 8:1-25.
Hersey, F. S. 1917. The present abundance of birds in the vicinity of Fort St. Michael, Alaska. Auk 34(2):147-159.
Holmes, R. T. 1970. Differences in population density, territoriality, and food supply of dunlin on arctic and subarctic tundra. Pages 303-319 in A. Watson, ed., Animal populations in relation to their food resources. Br. Ecol. Soc. Symp. 10.
Holmes, R. T. 1971. Density habitat and mating system of the western sandpiper (Calidris mauri). Oecologia 7(2):191-208.
Holmes, R. T. 1972. Ecological factors influencing the breeding season schedule of western sandpipers (Calidris mauri) in sub-arctic Alaska. Am. Midl. Nat. 87(2):472-491.
Holmes, R. T., and C. P. Black. 1973. Ecological distribution of birds in the Kolomak River-Askinuk Mountain region, Yukon-Kuskokwim delta, Alaska. Condor 75(2):150-163.
Kelley, E. J., and D. W. Hood, eds. 1974. PROBES: a prospectus on processes and resources of the Bering Sea shelf, 1975-1985. Univ. Alaska, Inst. Mar. Sci. Publ. Inf. Bull. 75-1. 71 pp.
Kenyon, K. W., and J. W. Brooks. 1960. Birds of Little Diomede Island, Alaska. Condor 62(6):457-463.
Kenyon, K. W., and R. E. Phillips. 1965. Birds from the Pribilof Islands and vicinity. Auk 82(4):624-635.
Kessel, B., H. K. Springer, and C. M. White. 1964. June birds of the Kolomak River, Yukon-Kuskokwim delta, Alaska. Murrelet 45(3):37-47.
King, J. G., and C. J. Lensink. 1971. An evaluation of Alaskan habitat for migratory birds. U.S. Bur. Sport Fish. Wildl., Washington, D.C. 46 pp. (Unpublished administrative report)
Klein, D. R. 1959. Saint Matthew Island reindeer-range study. U.S. Fish Wildl. Serv., Spec. Sci. Rep.—Wildl. 43. 48 pp.
LeResche, R. E., and R. A. Hinman, eds. 1973. Alaska's wildlife and habitat. Alaska Department of Fish Game, Juneau. 144 pp. + 564 maps.
Maher, W. J. 1974. Ecology of pomarine, parasitic, and long-tailed jaegers in northern Alaska. Pac. Coast Avivauna 37. 148 pp.
McGregor, R. C. 1902. A list of birds collected in Norton Sound, Alaska. Condor 4(6):135-144.
Mickelson, P. G. 1975. Breeding biology of cackling geese and associated species on the Yukon-Kuskokwim delta, Alaska. Wildl. Monogr. 45. 35 pp.
Murie, O. J. 1959. Fauna of the Aleutian Islands and Alaska Peninsula. U.S. Fish Wildl. Serv., N. Am. Fauna 61:1-364.
Nelson, E. W. 1883. Birds of the Bering Sea and Arctic Ocean. Pages 44-120 in Cruise of the Revenue-steamer Corwin in Alaska and the N. W. Arctic Ocean. U.S. Treas. Dep. Doc. 429.
Nelson, E. W. 1887. Report on natural history collections made in Alaska between the years 1877 and 1881. U.S. Army, Signal Serv. Arct. Ser. Publ. 3. 337 pp.
Orth, D. J. 1967. Dictionary of Alaska place names. U.S. Geol. Surv. Prof. Pap. 567. 1084 pp.
Osgood, W. H. 1904. A biological reconnaissance of the base of the Alaska Peninsula. U.S. Dep. Agric., Div. Biol. Surv., N. Am. Fauna 24:9-86.
Palmer, R. S., ed. 1962. Handbook of North American birds. Vol. 1. Loons through flamingos. Yale Univ. Press, New Haven, Conn. 567 pp.
Palmer, W. 1899. The avifauna of the Pribilof Islands. Pages 355-431 in D. S. Jordan, ed. The fur seals and Fur Seal Islands of the North Pacific, Part III. U.S. Treasury Department, Commercial Fur-seal Investigations, Washington, D.C.
Petersen, M. R. 1976. Breeding biology of arctic and red-throated loons. M. S. Thesis. Univ. of California, Davis. 55 pp.
Pitelka, F. A. 1959. Numbers, breeding schedules, and territoriality in pectoral sandpipers of northern Alaska. Condor 61(4):233-264.
Pitelka, F. A. 1974. An avifaunal review for the Barrow region and North Slope of Alaska. Arct. Alp. Res. 6(2):161-184.
Pitelka, F. A., R. T. Holmes, and S. F. MacLean, Jr. 1974. Ecology and evolution of social organization in arctic sandpipers. Am. Zool. 14:185-204.
Pitelka, F. A., P. Q. Tomich, and G. W. Treichel. 1955. Ecological relations of jaegers and owls near Barrow, Alaska. Condor 57(1): 3-18.
Portenko, L. A. 1973. The birds of the Chukotsk Peninsula and Wrangel Island. Acad. Sci. S.S.S.R., Zool. Inst., Sci. Publishers, Leningrad. 2 vols. [In Russian.]
Preble, E. A., and W. L. McAtee. 1923. A biological survey of the Pribilof Islands, Alaska. Part I. Birds and mammals. U.S. Bur. Biol. Surv., N. Am. Fauna 46:1-128.
Sealy, S. G. 1973. Breeding biology of the horned puffin on St. Lawrence Island, Bering Sea, with zoogeographical notes on the North Pacific puffins. Pac. Sci. 27:99-119.
Sealy, S. G., F. H. Fay, J. Bédard, and M. D. F. Udvardy. 1971. New records and zoogeographical notes on the birds of St. Lawrence Island, Bering Sea. Condor 73(3):322-336.
Selkregg, L. L., ed. [1975]. Alaska regional profiles: Arctic region. Univ. Alaska, Arct. Environ. Inf. Data Cent., Anchorage. 218 pp.
Shepherd, P. E. K. 1960. Production and abundance of the black brant in Alaska. Alaska Dep. Fish Game, Game Div. Pittman-Robertson Proj. Rep. 2(8):58-77.
Swarth, H. S. 1934. Birds on Nunivak Island, Alaska. Pac. Coast Avifauna 22. 64 pp.
Swartz, L. G. 1966. Sea-cliff birds. Pages 611-678 in N. J. Wilimovsky and J. N. Wolfe, eds. Environment of the Cape Thompson region, Alaska. U.S. AEC, Div. Tech. Inf., Oak Ridge, Tennessee.
Thompson, C. F. 1967. Notes on the birds of the Northeast Cape of St. Lawrence Island and of the Punuk Islands, Alaska. Condor 69(4):411-419.
Thompson, M. C., J. Q. Hines, and F. S. L. Williamson. 1966. Discovery of the downy young of Kittlitz's murrelet. Auk 83(3):349-351.
Tuck, L. 1960. The murres. Can. Wildl. Serv. Ser. 1. 260 pp.
Turner, L. M. 1886. Contributions to the natural history of Alaska. U.S. Army, Signal Serv. Arct. Ser. Publ. 2. 226 pp.
U.S. Fish and Wildlife Service. 1972. Nunivak National Wildlife Refuge wilderness study report. Agency, Anchorage, Alaska. 200 pp.
U.S. Fish and Wildlife Service, Alaska Planning Group. 1973a. Proposed Alaska Coastal Refuges, Alaska. Agency Draft Environ. Statement 73-94. 247 pp. + appendixes.
U.S. Fish and Wildlife Service, Alaska Planning Group. 1973b. Proposed Togiak National Wildlife Refuge. Agency Draft Environ. Statement 73-100. 183 pp. + appendixes.
U.S. Fish and Wildlife Service, Alaska Planning Group. 1973c. Proposed Yukon Delta National Wildlife Refuge, Alaska. Agency Draft Environ. Statement 73-101. 205 pp. + appendixes.
U.S. National Park Service, Alaska Planning Group. 1973. Proposed Chukchi-Imuruk National Wildlands, Alaska. Agency Draft Environ. Statement 73-84. 306 pp. + appendixes.
Watson, G. E., and G. J. Divoky. 1975. Marine birds in the Beaufort Sea. Pages 681-695 in J. C. Reed and J. E. Sater, eds. The coast and shelf of the Beaufort Sea. Arctic Institute of North America, Washington, D.C.
Williamson, F. S. L. 1957. Ecological distribution of birds in the Napaskiak area of the Kuskokwim River delta, Alaska. Condor 59(5):317-338.
Williamson, F. S. L., M. C. Thompson, and J. Q. Hines. 1966. Avifaunal investigations. Pages 437-480 in N. J. Wilimovsky and J. N. Wolfe, eds. Environment of the Cape Thompson region, Alaska. U.S. AEC, Div. Tech. Inf., Oak Ridge, Tennessee. 1250 pp.
by
Palmer C. Sekora[3]
U.S. Fish and Wildlife Service
Kailua, Hawaii
G. Vernon Byrd[4]
U.S. Fish and Wildlife Service
Adak, Alaska
and
Daniel D. Gibson
University of Alaska
Fairbanks, Alaska
Seabird population estimates are generally lacking for the 1,800-km-long Aleutian Islands. Only the locations of the larger colonies are known, and for these there are only imprecise estimates of colony sizes and often even of species composition. Changes in the status of several species and populations resulting from geologic and marine actions and from human intrusions are evident. Accounts are given for 25 species of marine birds breeding in these islands.
The 1,800-km-long chain of islands known as the Aleutians provides nesting habitat for various species of marine birds, including three species of Procellariiformes and three of cormorants (Phalacrocorax spp.), one species of gull (Larus glaucescens), both kittiwake species (Rissa spp.), two species of terns (Sterna spp.), and at least 13 species of alcids.
Seabird population estimates of known accuracy are lacking for this isolated area. Locations of larger colonies of breeding seabirds are known, however, and sufficient data are available to place colonies in broad size ranges. Published information on the breeding biology of marine birds is also lacking from the Aleutians, but some studies are under way. The distribution of nesting marine birds away from the nesting cliffs is totally unknown.
Introduced predators, primarily arctic foxes (Alopex lagopus), are now found on nearly every island. Breeding marine bird populations have suffered drastic reductions as a result. They have probably also changed because of natural habitat modifications caused by earthquakes, volcanic eruptions, tidal waves, and marine erosion.
The purpose of this paper is to summarize the known present distribution and status of breeding marine birds in the Aleutian Islands.
Description of the Aleutian Islands
The Aleutian Islands form an arc that separates the Bering Sea and the north Pacific Ocean (Fig. 1). The island chain extends from the tip of the Alaska Peninsula to within 483 km of the Commander Islands of Siberia. The chain contains more than 200 islands—the peaks of a submarine volcanic mountain range. Volcanic activity and earthquakes occur regularly.
Weather is characterized by perpetual overcast, dense summer fog, high-velocity winds, and mild temperatures with low annual and diurnal variations. The sea is ice-free year-round except in extremely cold winters, when the arctic ice pack may reach the extreme northern islands.
The Aleutians are treeless except for a few introduced, stunted spruces. Woody shrubs are restricted to the most northern islands on each end of the Chain. Mosses, lichens, club mosses, and heaths are common ground-cover plants, and taller grasses, sedges, and umbellifers constitute the overstory. Hulten (1960) provided a list of terrestrial plants found in the Aleutians. Amundsen and Clebsch (1971) discussed terrestrial plant ecology at Amchitka, central Aleutians. The marine plant communities around the islands are fairly diverse. Lebednik et al. (1971) described marine algal communities at Amchitka.
The easternmost Aleutian island, Unimak, has a mammalian fauna like that of the Alaska Peninsula, including brown bear (Ursus arctos), caribou (Rangifer tarandus), wolf (Canis lupus), and wolverine (Gulo gulo). West of Unimak, red foxes (Vulpes fulva) occurred historically as far as Umnak, and arctic foxes were apparently on Attu when the Russians came in 1741 (Murie 1959). Except for man and dog, no land mammals occurred between Umnak and Aggatu islands. Arctic foxes, introduced before 1930 for fur farming, still roam almost every island. Norway rats (Rattus norvegicus) were introduced on many islands when ships were wrecked or as a result of military activities during World War II.
Sea otters (Enhydra lutris) have repopulated most of the Aleutians after being nearly extirpated by 1900. Rookeries of Steller's sea lion (Eumetopias jubata) are scattered throughout the Aleutians during summer, and numerous harbor seals (Phoca vitulina) haul out on beaches and offshore rocks.
All five species of Pacific salmon (Oncorhynchus spp.) occur near the islands, and at least four of them (all but O. tshawytscha) spawn in Aleutian streams. Dolly Varden (Salvelinus malma) and three-spine sticklebacks (Gasterosteus aculeatus) are found nearly everywhere there is fresh water. The marine environment provides habitat used by at least 77 species of fish (Isakson et al. 1971). O'Clair and Chew (1971) furnished a recent reference to littoral macrofauna at Amchitka.
About 200 species of birds have been recorded in the Aleutians (Aleutian Islands National Wildlife Refuge, unpublished data). Many of these are windblown stragglers from both North America and Asia; only 59 species breed on the islands. Although seabirds make up less than half (26 species or 44%) of the breeding birds, they may compose more than 90% of the breeding avian biomass.
Published ornithological information from the Aleutian Islands is relatively scarce. G. W. Steller, naturalist on Vitus Bering's 1741 expedition to Alaska, was the first person to record ornithological information in the islands (Stejneger 1936). More than a century passed before W. H. Dall (1873, 1874) published the next papers dealing with birds in the Aleutians. In 1878, the U.S. Army Signal Corps sent L. M. Turner to the Aleutians to set up weather stations at several locations. Turner kept notes on birds at various locations in the Aleutians and published two papers (1885, 1886) on his observations. Turner's data (1886) provided the first report based on extended and widespread observations in the area. E. W. Nelson, who replaced Turner, also provided data on birds (Nelson 1887).
In 1906, A. C. Bent came to the Aleutians specifically to look for birds, and he and Alexander Wetmore recorded birds throughout the island chain (Bent 1912). A. H. Clark[Pg 35] (1910) provided a valuable record of his observations in the Near Islands. All these workers recorded birds in several locations, but none provided data on more than a very few seabird colonies.
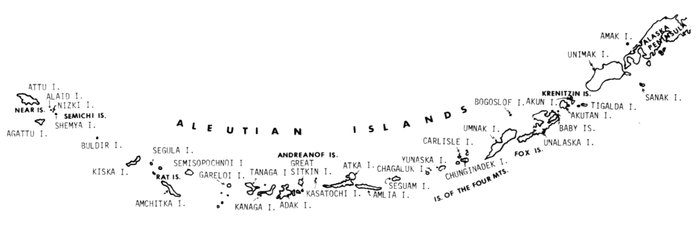
Fig. 1. The Aleutian Islands.
O. J. Murie, U.S. Biological Survey, made the most complete survey of the Aleutians (Murie 1959). He specifically recorded seabird colonies, spending parts of four summers in the area. Murie visited every large Aleutian island and most small ones. He recorded nearly every major colony of cliff-nesting or talus-nesting seabirds known in the Aleutians, but seldom gave sizes of colonies, and separate colonies on a particular island were often not differentiated.
World War II brought several ornithologists to the Aleutians. Cahn (1947), Sutton and Wilson (1946), Taber (1946), and Wilson (1948) provided accounts of birds observed at specific locations. After the war, Fish and Wildlife Service personnel—including I. N. Gabrielson (Gabrielson and Lincoln 1959), K. W. Kenyon (Kenyon 1961), and R. D. Jones (Refuge Narrative Reports 1949-1970)—recorded observations of breeding seabirds at several locations in the Aleutians. Investigations associated with Atomic Energy Commission nuclear testing at Amchitka Island provided the first ecological study of avifauna of an Aleutian island (White et al. 1977). Byrd et al. (1974) provided a list of birds at Adak.
In 1971, the Near Islands were surveyed by U.S. Fish and Wildlife personnel in a Cape Cod dory. In 1972, the Aleutian Islands National Wildlife Refuge obtained a vessel, the Aleutian Tern, which allowed visits to all parts of the island chain. That year, nearly every large island as far west as Buldir was visited, and seabird colonies were mapped. Every island has been visited at least once since 1972.
In estimating the current status of seabirds in the Aleutians, all available data were considered. Most of the information used, however, is from surveys conducted by the U.S. Fish and Wildlife Service (1970-75, unpublished data). Because these surveys only incidentally included Unimak, Akun, Akutan, Unalaska, and Umnak islands, data for these areas are almost totally lacking. Data for Bogoslof, Adak, Amchitka, Buldir, Agattu, Nizki, Alaid, and Attu are most accurate because fairly intensive investigations have been conducted there since 1970.
The available data are of unknown accuracy. The method used by most investigators who have surveyed areas in the Aleutians for seabird colonies has been to circle islands in a ship or small boat; when a colony was encountered, they simply estimated the number of birds they saw at the time. The accuracy of the estimates is affected by weather, distance from the colony, density of birds, ability and experience of the observer, and other variables. Estimates of kittiwakes and cormorants should be the most accurate, since nests were actually counted. Murres (Uria spp.) are readily visible on the cliffs, but the percentage of breeders on the cliffs at a particular time of day during a particular part of the breeding season is not known. Auklet numbers are perhaps hardest to estimate,[Pg 36] since swirling "clouds" of birds are encountered.
Even when the estimates of birds seen are assumed to be accurate, data interpretation is complex. Lack of information on diurnal rhythms adds difficulty to data interpretation. Counts of burrow-nesting birds (e.g., puffins) have been inaccurately interpreted because of the lack of understanding of their nesting ecology. Gulls (Larus spp.), terns, and jaegers (Stercorarius spp.) are not well known since shore parties have seldom investigated island interiors. Nocturnal species (e.g., ancient murrelet, Synthliboramphus antiquus, and storm-petrels, Oceanodroma spp.) are perhaps the least known. Since only crude estimates of colony sizes are available, broad limits are used in this paper to describe known colonies.
Even from the sparse literature available, it is apparent that some seabird populations are now drastically different from those in the Aleutians around 1900. Changes in nesting habitat due to volcanic eruptions, tidal waves, marine erosion, and earthquakes have occurred for centuries, and colonial nesting bird populations have fluctuated accordingly. In addition, native Aleuts used marine birds and their eggs for food and their skins for clothing, but the Aleuts were so diminished in numbers by 1900 that they have had little recent effect on the bird populations.
From about 1900 to 1936, arctic foxes were introduced to most of the Aleutians for fur farming. The foxes lived on birds in summer, and some species (e.g., Aleutian Canada geese, Branta canadensis leucopareia) were wiped out wherever foxes were introduced. Ground-nesting and some burrow-nesting seabirds were also drastically reduced or extirpated on many islands.
During World War II the thousands of troops in the Aleutians brought dogs and cats to some of the islands as pets, and many of the animals were set free when the men departed. The military also accidentally introduced Norway rats to some of the islands. Their role in seabird population reductions is unknown.
Figures 2-15 (pages 40-46) present data on the distribution of populations of birds that have survived the foxes and other introduced predators. An annotated list of seabirds breeding in the Aleutians follows.
Northern fulmars breed on only three islands: Buldir (200 pairs), Gareloi (1,500 pairs), and Chagulak (more than 100,000 pairs). Fulmars were apparently much more widespread formerly (Murie 1959; Turner 1886). Introduced foxes were probably involved in the decline.
The distribution of storm-petrels is poorly known due to their nocturnal behavior near the nesting colonies. The presence of birds has generally been noted by finding them aboard ships anchored near islands after darkness. Population estimates are not available for any colonies, so symbols used in Fig. 3 indicate probable numbers of breeding birds. In few cases have active burrows or crevices been discovered. Storm-petrels were formerly much more common. Murie (1959) and John L. Trapp (personal communication) found large numbers of storm-petrel remains in fox dens. Most present breeding colonies are probably confined to offshore islets and fox-free islands.
Double-crested cormorants breed as far west as the Islands of Four Mountains. The colonies vary in size from a few to 25 pairs. Pelagic and red-faced cormorants nest from Amak to Attu on nearly every island. Relative abundance of the two in mixed colonies varies between areas as well as from year to year. Red-faced cormorants tend to nest in colonies mixed with kittiwakes and murres, but pure colonies also occur. Pelagic cormorants occupy isolated, small colonies, but they also[Pg 37] nest with kittiwakes and murres and are often found with red-faced cormorants. By far the densest concentration of cormorants occurs in the Near Islands, especially at Attu, where an estimated 77,000 birds were seen in 1970. In the Aleutians as a whole, red-faced cormorants outnumber pelagic cormorants, and double-crested cormorants make up only a very small percentage of the breeding population.
The distribution of jaegers is poorly known because investigators have spent little time ashore on most islands. Murie (1959) found jaegers on a number of islands, and most of the data in Fig. 5 are his. Population estimates are available only for Amchitka (25 pairs; White et al. 1977) and Buldir (30-40 pairs; G. V. Byrd, unpublished data).
Glaucous-winged gulls no longer nest on islands where foxes occur except where islands in lakes are available. Most colonies are on offshore rocks or islets and range in size from a few pairs to over 200 pairs, and occasionally more. They are found throughout the Aleutians, but the largest known colonies are at Bogoslof (500 pairs) and Buldir (250 pairs).
Black-legged kittiwakes breed locally in every major island group, usually mixed with murres and cormorants. The large colonies contain over 25,000 birds, but colonies of less than 50 pairs also occur. Red-legged kittiwakes breed only on Buldir and Bogoslof. They are remnants of a previously more widespread population.
Terns breed locally in each island group. Both species occur at Attu, Amchitka, Adak, and Umnak, but only arctic terns are found at Nizki. Factors limiting distribution are unknown. Colonies vary in size from less than 10 pairs to 100 pairs.
Like kittiwakes, murres are abundant locally. A pure colony of either species is almost unknown, although one species often makes up more than 90% of a colony. Common murres may have been reduced by foxes, since they tend to use sites with less slope than those used by thick-billed murres. At Bogoslof and the Baby islands, the birds use inland, gently sloping areas because there are no foxes. The presence of the lichen (Caloplaca spp.), which according to Tuck (1960) is indicative of bird roosts, on several extensive cliff areas suggests that either murres or kittiwakes, or both, formerly used areas they do not use now.
This species has been noted near almost every island that has been visited. Nesting under beach boulders and driftwood, the birds only occasionally are found in large concentrations (near Great Sitkin more than 4,000 birds were seen in 1971). Murie et al. (1937) summed up the distribution of pigeon guillemot accurately: "Each island has its meager quota of these birds, nesting unobtrusively among the rocks but never assembled in any really large groups." Estimates of populations may be extremely inaccurate because the diurnal rhythm of the pigeon guillemot is unknown.
Nests of neither species have been located in the Aleutians, but nesting of both is suspected at Adak, Unalaska, and Unimak, where specimens of Kittlitz's with brood patches or eggs in the oviduct have been collected in nearshore waters. Courtship has been recorded in marbled murrelets (Byrd et al. 1974).
The distribution of this species is very poorly known, since it is nocturnal near nesting colonies. Murie (1959) wrote, "This is one of the species that undoubtedly has greatly declined in recent years, as a result of increase[Pg 38] of the blue-fox industry." The leading of downy young to sea by the adults is a very noisy process and foxes could easily take large numbers. Also, these murrelets nest in fairly shallow burrows which foxes could dig out easily. Birds were recorded near islands in every group during surveys from 1972 to 1975, but workers seldom went ashore to determine if they were nesting. In Fig. 12, the only basis for designating most of the areas marked as colonies is the presence of birds during breeding season (15 May-1 July).
This is another species that was more common before the fox was introduced. Cassin's auklet now seems to occur only locally, but these nocturnal birds are probably often overlooked. They are known only from Buldir, Umnak, and the vicinity of Oglodak.
This auklet, which nests under beach boulders, in burrows, and in rock crevices, seems to use a greater variety of breeding sites than do the other auklets. The largest known colony is at Chagulak, where an estimated 10,000 were seen in 1972. Smaller colonies are found as far west as Buldir.
Aethia nest primarily in rock crevices of talus slides. Such habitat occurs locally in each major island group except the Near Islands. Least auklets outnumber crested auklets in the Aleutians, and whiskered auklets are far less common than either. Estimates of populations are probably grossly inaccurate because of the difficulty both in estimating the number of birds in the milling flocks observed and in interpreting the estimates after they are obtained.
Horned puffins favor rock crevices in talus slides and cliff faces for nesting, whereas tufted puffins are primarily burrow nesters. The historical distribution of the two species was probably based on availability of nesting sites, so tufted puffins were more widespread and numerous. However, in areas where extensive talus slopes are available, horned puffins reached high densities. Predation by introduced foxes may have altered the distribution of tufted puffins, which now nest primarily on fox-free islets just offshore from the larger islands where foxes occur. The distribution of horned puffins may not have been altered significantly, since they are relatively free from fox predation in their rock crevices.
A complete survey of the Aleutian Islands has not been done. This should be done, by methods that will provide accurate population estimates. Life history information is needed on almost all species, and data should be gathered on selected populations to determine trends. Information on winter distribution should also be compiled. The effects of introduced predators should be evaluated quantitatively, and if control measures are needed, effective, humane methods should be devised and implemented.
The authors are grateful to the following Fish and Wildlife Service personnel who helped collect previously unpublished data used in this paper: E. P. Bailey, C. S. Craighead, C. P. Dau, M. H. Dick, G. J. Divoky, R. Martin, J. L. Trapp, G. W. Watson, and C. M. White. Most of the data were collected from the deck of the Aleutian Islands National Wildlife Refuge research vessel Aleutian Tern. Captain George Putney is acknowledged for his peerless seamanship and constant encouragement; he also contributed observations of birds.
The maps were adapted from a master supplied by Elaine Rhode, Public Affairs Office, U.S. Fish and Wildlife Service, Anchorage; she also suggested the use of squares to display data. C. M. White graciously made his in-press manuscript available. W. B. Emison, R. J. Gordon, and J. L. Trapp kindly made their field notes available, and Trapp helped compile data. Most of the funds for the surveys in 1971-75 were provided by the U.S. Fish and Wildlife Service.
Amundsen, C. C., and E. E. C. Clebsch. 1971. Dynamics of the terrestrial ecosystem vegetation of Amchitka Island, Alaska. Bioscience 21:619-623.
Bent, A. C. 1912. Notes on birds observed during a brief visit to the Aleutian Islands and Bering Sea in 1911. Smithsonian Misc. Coll. 56(2):1-29.
Byrd, G. V., D. D. Gibson, and D. L. Johnson. 1974. The birds of Adak Island, Alaska. Condor 76:288-300.
Cahn, A. R. 1947. Notes on the birds of the Dutch Harbor area of the Aleutian Islands. Condor 49:78-82.
Clark, A. H. 1910. The birds collected and observed during the cruise of the United States Fisheries steamer "Albatross" in the north Pacific Ocean and in the Bering, Okhotsk, Japan, and Eastern seas from April to December 1906. Proc. U.S. Natl. Mus. 38:25-74.
Dall, W. H. 1873. Notes on the avifauna of the Aleutian Islands, from Unalaska eastward. Proc. Calif. Acad. Sci. 5:25-35.
Dall, W. H. 1874. Notes on the avifauna of the Aleutian Islands, especially those west of Unalaska. Proc. Calif. Acad. Sci. 5:270-281.
Gabrielson, I. N., and F. C. Lincoln. 1959. The birds of Alaska. The Stackpole Co., Harrisburg, Penn., and Wildlife Management Institute, Washington, D.C. 922 pp.
Hulten, E. 1960. Flora of the Aleutian Islands. J. Kramer, Weinham/Bergstr., Sweden. 376 pp.
Isakson, J. S., C. A. Sinensted, and R. L. Burgner. 1971. Fish communities and food chains in the Amchitka area. Bioscience 21:666-670.
Jones, R. D. 1949-1970. Annual refuge narrative reports, Aleutian Islands N.W.R. Cold Bay, Alaska. (Unpublished administrative report.)
Kenyon, K. W. 1961. Birds of Amchitka Island, Alaska. Auk 78:305-326.
Lebednik, P. A., F. C. Weinmann, and R. E. Norris. 1971. Spatial and seasonal distributions of marine algal communities at Amchitka Island, Alaska. Bioscience 21:656-660.
Murie, O. J. 1959. Fauna of the Aleutian Islands and Alaska Peninsula. U.S. Fish Wildl. Serv., N. Am. Fauna 61. 364 pp.
Nelson, E. W. 1887. Report upon natural history collections made in Alaska between the years 1877 and 1881. U.S. Army, Signal Serv. Arct. Ser. Publ. 3. 337 pp.
O'Clair, C. E., and K. K. Chew. 1971. Transect studies of littoral macrofauna, Amchitka Island, Alaska. Bioscience 21:661-664.
Stejneger, L. 1936. George Wilhelm Steller—the pioneer of Alaska natural history. Harvard University Press, Cambridge, Mass. 623 pp.
Sutton, G. M., and R. S. Wilson. 1946. Notes on the winter birds of Attu. Condor 48:83-91.
Taber, R. D. 1946. The winter birds of Adak, Alaska. Condor 48:272-277.
Tuck, L. M. 1960. The murres. Ottawa. Canadian Wildlife Series 1. 260 pp.
Turner, L. M. 1885. Notes on the birds of the Near Islands, Alaska. Auk 2:154-159.
Turner, L. M. 1886. Contributions to the natural history of Alaska. U.S. Army, Signal Serv. Arct. Ser. Publ. 2. 226 pp.
White, C. M., F. S. L. Williamson, and W. B. Emison. 1977. Avifaunal investigations. Pages 227-260 in M. L. Merritt and R. G. Fuller, eds. Environments of Amchitka Island, Alaska. U.S. Energy Research and Development Association, Oak Ridge, Tenn. 682 pp.
Wilson, R. S. 1948. The summer bird life of Attu. Condor 50:124-129.
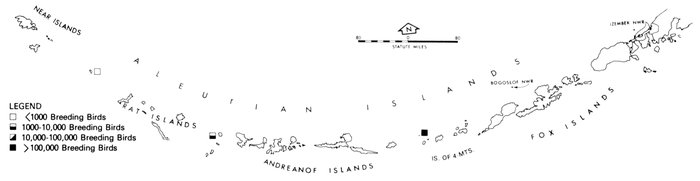
Fig. 2. Breeding distribution of northern fulmar.
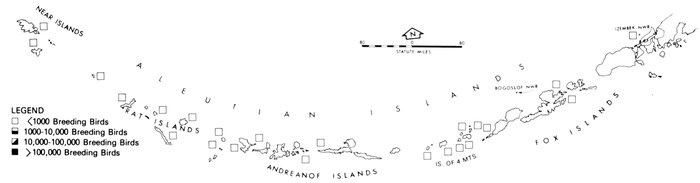
Fig. 3. Breeding distribution of storm-petrels.
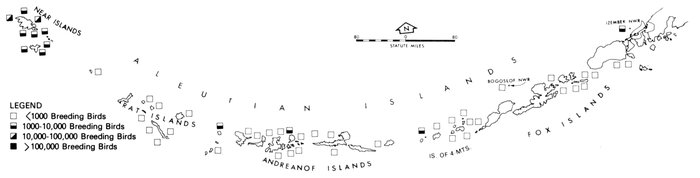
Fig. 4. Breeding distribution of cormorants.
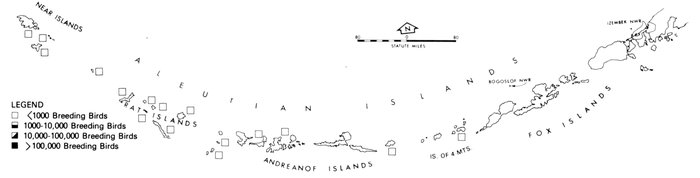
Fig. 5. Breeding distribution of parasitic jaeger.
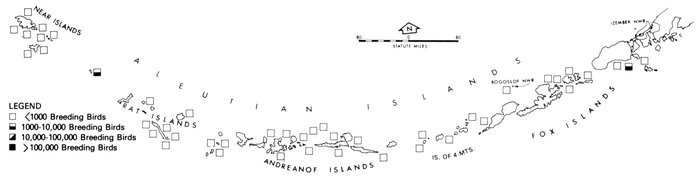
Fig. 6. Breeding distribution of glaucous-winged gull.
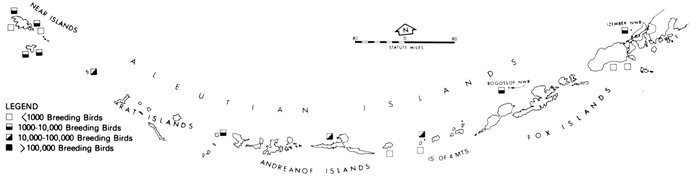
Fig. 7. Breeding distribution of kittiwakes.
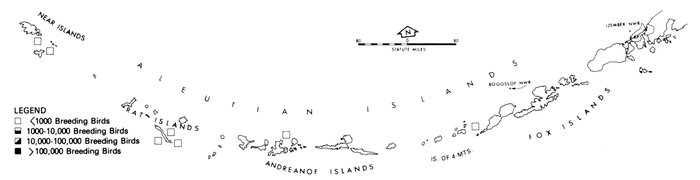
Fig. 8. Breeding distribution of terns.
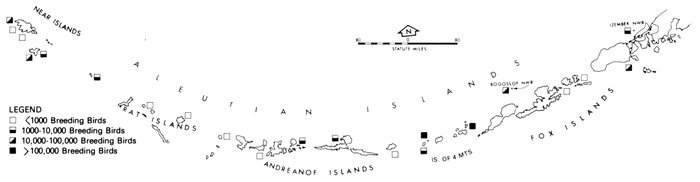
Fig. 9. Breeding distribution of murres.
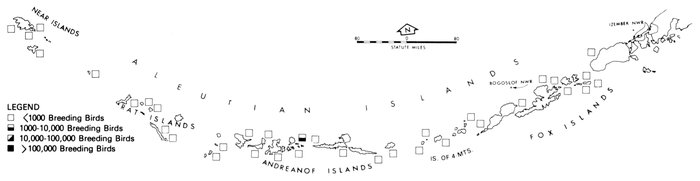
Fig. 10. Breeding distribution of pigeon guillemot.
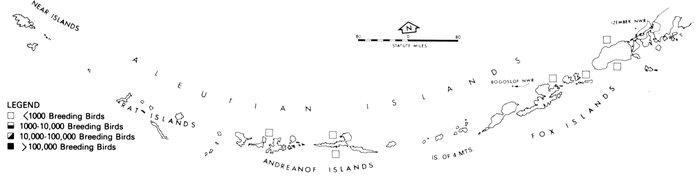
Fig. 11. Breeding distribution of marbled and Kittlitz's murrelet.
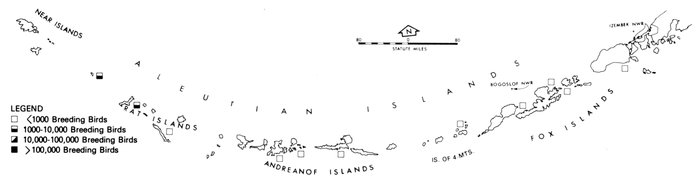
Fig. 12. Breeding distribution of ancient murrelet.
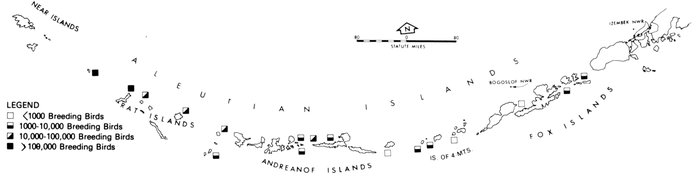
Fig. 13. Breeding distribution of auklets.
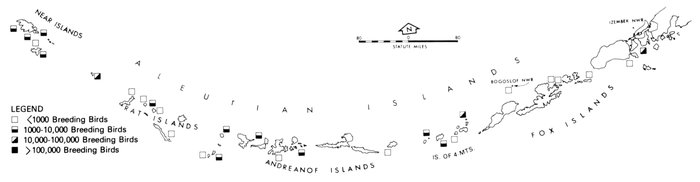
Fig. 14. Breeding distribution of horned puffin.
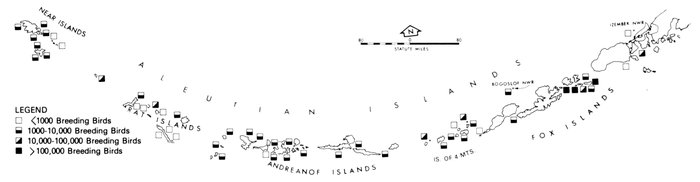
Fig. 15. Breeding distribution of tufted puffin.
[3] Present address: U.S. Fish and Wildlife Service, William L. Finley National Wildlife Refuge, Route 2, Box 208, Corvallis, Oregon 97330.
by
LeRoy W. Sowl
U.S. Fish and Wildlife Service
1011 East Tudor Road
Anchorage, Alaska 99507
The history of ornithological field work in the Gulf of Alaska dates back to 20 July 1741 and Bering's discovery of Alaska. In spite of this long history, the record is fragmentary and often seemingly contradictory. The coming of the tanker terminal at Valdez and the pending development of oil and gas resources on the outer continental shelf threaten massive change for seabirds in the Gulf of Alaska. Often overlooked, however, is the fact that man has already effected a change in status for many of these birds. In this paper I examine the scanty, general record from the exploratory period, roughly 1741 to 1935, and the somewhat more comprehensive record of the reconnaissance period, 1936-74, and attempt to develop a basis for better understanding of the change in seabird status that has already taken place. This paper should be treated as a verbal model which can be improved as our knowledge of seabirds in the Gulf of Alaska is expanded.
From the perspective of history, 1970 should prove to have been a momentous year for Alaska and its seabirds. Two events, the construction of the Trans-Alaska Pipeline and the passage of the National Environmental Policy Act (NEPA) merged head on in 1970 with the decision that Section 2c of NEPA applied to the proposed pipeline. The systematic appraisal of potential environmental impacts required by Section 2c quickly exposed the inadequacy of the existing data base in many areas. With respect to seabirds in the Gulf of Alaska, it was apparent that there had never been any effort to develop a synthesis of the information accumulated over 230 years. The data gaps which were uncovered were appalling.
While the Trans-Alaska Pipeline impact statement had provided shock therapy, it was not the only influential event on the horizon. Two local disturbances had already preceded the pipeline. These were Project Chariot at Cape Thompson and the Amchitka Island test program. Now in quick succession the Wilderness Act and native land claims added new urgency to the need for solid resource information. More recently, the outer continental shelf minerals leasing program has made the quick development of base-line information even more essential.
All of the new activity in Alaska's coastal waters has the potential to affect seabirds in one way or another. We must remember, however, that man's activities have been affecting seabirds for a long time. We cannot accurately assess the effect of a tanker terminal at Valdez or offshore oil activity without first developing some understanding of the current status of seabirds in the context of the historical record.
Seabird work in Alaska can be divided roughly into three periods. The first is the early historical or exploratory period; it extended from Georg Steller's 1741 visit to Kayak Island to 1935. This was literally a period of exploration and the collection of in[Pg 48]formation was dependent upon interest and opportunity. The second is the reconnaissance period; during this period investigators were dispatched to a particular area to gather general information for management application. This period begins with Murie's extensive investigations of the Alaska Peninsula and the Aleutian Islands; I see it extending from 1936 to 1975. In 1975 the need for data became so acute that it was necessary to enter the third period, one of intensive data gathering. Knowing where the big seabird colonies were located and knowing their general species composition was no longer adequate. The current intensive data-gathering effort in the waters over oil and gas leasing areas is a partial response to the recognition of this inadequacy.
In this paper I draw some tentative conclusions relative to the status of the 26 species of primary seabirds (Fisher and Lockley 1954) breeding in, or which may have bred in, the northern and western Gulf of Alaska area. This area extends from Cape Fairweather, 59°N 138°W, westerly along the coast to Ikatan Bay, 55°N 163°W, at the end of the Alaska Peninsula. These bird species tend to be colonial, but not exclusively so. Two birds which are primary seabirds, the mew gull (Larus canus) and Bonaparte's gull (L. philadelphia), have not been included because they tend to be more riverine than marine in habit. Several marine ducks have been excluded because they are secondary seabirds.
Information from the early exploratory period is summarized under the next section. The more detailed information from the reconnaissance period is discussed in the species accounts.
The history of ornithological field work in the Gulf of Alaska goes back 235 years to 20 July 1741. On that day Bering's surgeon/naturalist, Georg W. Steller, spent a scant 10 h ashore on Kayak Island. He collected a single bird. This bird, later named for Steller, reminded him of a plate of the blue jay by Make Catesby, the colonial-era predecessor of Audubon, in Volume 1 of the Natural History of Carolina, Florida, and the Bahama Islands (Stejneger's annotated translation of Steller's journal in Golder 1925). Collection of the bird confirmed for Steller that the first Russian Expedition had reached America.
Steller was an accomplished naturalist, but his overbearing and superior manner had apparently sorely irritated Bering and his officers long before the expedition reached Kayak Island. The seamen made little effort to go ashore anywhere in Alaska and Steller was blocked from doing so as well. In addition to Kayak Island, he was able to go ashore only on Nagai Island, first with a water party on 30 August and again the next day. He noted that "all sorts of waterbirds in abundance were seen." These included two kinds of cormorants, auks, ducks, gulls, divers, pigeon guillemots (Cepphus columba), tufted puffins (Lunda cirrhata), and horned puffins (Fratercula corniculata).
Stejneger's comment on the identity of the cormorants is interesting because, based on his experience, he assumed them to be pelagic and double-crested cormorants (Phalacrocorax pelagicus and P. auritus). He gave no thought to red-faced cormorants (P. urile) which are now common there.
Steller noted on 6 September off Bird Island in the Shumagin Islands, that "when we were out to sea about half a mile we were especially astonished at the untold numbers of seabirds which we saw on the northern side of the island." These birds were listed as cormorants, auks, horned puffins, fulmars (Fulmarus glacialis), pigeon guillemots, black oystercatchers (Haematopus backmani), and a pied diver which Stejneger assumed was an ancient murrelet (Synthliboramphus antiquus).
On 15 September when Bering's vessel, the St. Peter, was south of Amukta Pass, Steller recorded observing "river gulls." The observation is not as interesting as Stejneger's comment (Golder 1925) concerning it. Stejneger stated that no true river gulls lived in the Aleutians and these must, therefore, have been another small gull with red feet. He thought they must have been the red-legged kittiwake (Rissa brevirostris), which "inhabits the Aleutian Islands from Bering Island to Sannak."
Thirty-seven years after Bering's voyage, Captain James Cook sailed into the Gulf of[Pg 49] Alaska, arriving off Kayak Island on 11 May 1778. Cook was not accompanied by an able naturalist. His surgeon, William Anderson, did have some experience gained on earlier voyages in preparing skins and taking notes, but he had contracted tuberculosis and became so ill that even his notes ceased after 8 June, while the expedition was in Cook Inlet.
Cook was under orders to keep a careful record of everything he saw. One of the results was that he had birds collected even though he had no naturalist to do the work. Several birds were collected in Prince William Sound while Cook's vessels were at anchor in Port Etches. These included two marbled murrelets (Brachyramphus marmoratus—type specimens), a black oystercatcher, a surfbird (Aphriza virgata), a surf scoter (Melanitta perspicillata), and a red-breasted merganser (Mergus serrator—type specimen), along with several forest birds (Stresemann 1949).
The watch journals of Cook and his officers provide some additional information. Captain Charles Clerke (Beaglehole 1974) remarked in his log on the passage out of Prince William Sound through Montague Strait on 20 May that "it had almost become tautology to mention whales and seals and innumerable sea fowl that so confoundingly kept their distance."
Between the Trinity Islands and Chirikof Island on 18 June, Cook's men collected a single tufted puffin. Later Cook passed close to the Semidi Islands and the Shumagin Islands and directly through the Sandman Reefs. Beaglehole's version of this part of the voyage makes no mention of seabirds.
There is a gap of 87 years during which there is almost no hint of published material bearing on the status of seabirds in the Gulf of Alaska. In 1865 the Russo-American Telegraph Expedition touched this area. Dall and Bannister (1869) provide us with a few scraps garnered during that expedition, primarily by Bischoff. The glaucous-winged gull (Larus glaucescens) was described as the most common species from California northward. Bischoff's collections at Kodiak indicate that the horned and tufted puffins were collected with ease. He was able also to collect an Aleutian tern (Sterna aleutica—type specimen) along with an egg.
Dall (1873) noted in 1872 that the black-legged kittiwake (Rissa tridactyla) was common at Round Island and Delarof Harbor, Unga Island, in the Shumagins. The inference is that it was more common at these two places than elsewhere. The Arctic tern (Sterna paradisaea) was abundant in the Shumagin Islands and particularly at Range Island in Popoff Strait. Dall expressed the opinion that the horned puffin was very abundant in the Shumagins and appeared to fill the niche of the tufted puffin, which he did not see there. The only other bird which he thought to be very common was the pigeon guillemot. He did not note the common murre (Uria aalge) at all.
In 1908 the second of three Alexander Expeditions conducted field work in the Prince William Sound area. From Dixon (1908) and Grinnell (1910) we can derive some basis for assessing status in a very general way. The most common seabird noted was the marbled murrelet. Glaucous-winged gulls and black-legged kittiwakes were common; the glaucous-winged gull was the more common. Horned puffins were judged to be slightly more common than tufted puffins by both authors. The northern end of Montague Strait appears to have been the center of abundance for puffins. Dixon noted that on 16 July 1908 there were swarms of puffins in the channel along Green Island. Pigeon guillemots were common along the rocky coasts. Parakeet auklets (Cyclorrhyncus psittacula), common murres, and ancient murrelets were noted only in very small numbers.
After the Alexander Expeditions there was another doldrum in which little was done. During this lull in activity, a note by Townsend (1913) appeared which compared the numbers of crested auklets (Aethia cristatella) at Yukon Harbor, Big Koniuji Island, to the least auklets (A. pusilla) of St. George Island, stating that the crested auklets were more numerous. He sailed into the Yukon Harbor anchorage on the evening of 1 August and observed that crested auklets "were present in myriads. The surface of the water was covered with them, and the air was filled with them."
The formal record available to researchers is very shallow for this exploratory period. With a few exceptions it was compiled by non-scientists, primarily explorers and egg and skin collectors.
This paper should be viewed as a conceptual model. While I attempted to be as objective as possible, subjectivity was unavoidable. Many of the tentative conclusions are based on very little data. Each improvement will make it a better management tool. Because of the space limitations, it is not possible to go into a detailed tracking of my reasoning for each species. In an attempt to overcome this handicap, I am including some examples of the sorts of reasoning that went into the process.
In 1973 I led a Fish and Wildlife Service (FWS) reconnaissance survey team that was delineating seabird colonies along the Alaska Peninsula. In the Shumagin Islands we entered or crossed Koniuji Strait twice (on 11 and 12 June) without even suspecting the presence of a horned puffin colony. A third passage through the strait (13 June) was not so uneventful. The water and the air were filled with horned puffins. This led to the discovery that the 430-m mountain on the southeastern corner of Big Koniuji was also covered with horned puffins, clear to its top. The minimum estimate of the birds that were visible was 140,000. Even this number of birds would make this the largest horned puffin colony ever discovered. David Spencer (personal communication) had noted similar swarms of horned puffins in this strait in 1956 while flying sea otter surveys in the area. In 1975 a field camp was established at Yukon Harbor, with study of this colony as one of the prime objectives of the investigators. As far as these investigators could tell no such large colony existed there, even though the nesting habitat was still there, unaltered. This sort of event, one of the banes and vagaries of estimating seabird numbers, is not rare.
In 1973, when FWS personnel delineated the colony on the southwestern end of Bird Island in the Shumagins, there were estimated to be 43,000 kittiwakes, 24,000 murres, and 6,000 cormorants present; no tufted puffins were seen about the colony. The last time (in 1970) one of the observers, Edgar Bailey, had visited the colony with Robert Jones, there was an extremely large colony of tufted puffins which Jones (E. Bailey, personal communication) estimated at more than 1 million birds. We made a particular effort to visit Jude Island, between the Shumagin Islands and the Pavlof Islands, because David Spencer (personal communication) had reported once having seen the air over the island filled with an extremely large number of tufted puffins. However, there were no puffins at this colony either.
Let us examine the facts in context. On 8 June we had visited High Island where we had attempted to collect puffin eggs for pesticide analysis, but had been able to find only one egg. Also, there were only 6,000 tufted puffins where George Putney, master/engineer of the Aleutian Tern, had seen much larger numbers in 1972. These two facts could easily be related to explain the current situation because it was still early in the breeding season. The horned puffin observations in Koniuji Strait (11-13 June) were in keeping with this conclusion also—an indication that these birds had not yet settled down to a full breeding effort. The erratic comings and goings of common puffins (Fratercula arctica) early in the season have been well documented (Lockley 1962). It is an easy step to extend this reasoning to the absence of birds at Bird Island on 11 June, even though fresh signs of the characteristic evidence of tufted puffin occupancy were missing. Jude Island provides a different clue, however. There were 3,000 pigeon guillemots, an unheard-of concentration, apparently occupying abandoned tufted puffin burrows on 15 June. Also, on 7 June we had made a very interesting observation that had no special significance at the time: murres on Spitz Island were occupying little parapets created by mashing down the mouths of puffin burrows which filled the slope above the cliff portion of their colony.
After looking at all of the observations cited above, I conclude that tufted puffins were greatly reduced in numbers on these sites in 1973 and that they had been absent from the burrows used by the murres and pigeon guillemots for more than the current breeding season. What causes these sorts of changes? I do not know.
One reason for year-to-year change may be local movements of colonies. Black-legged kittiwakes nest at several places in lower Orca Inlet, Prince William Sound. Counts made at these sites in 1972 and 1974 yielded almost[Pg 51] identical totals but the numbers of birds varied between individual sites. This may be an indication that all of these sites are part of one large composite colony and that, at least in this colony and for this species, the birds shift at will.
The best record of population flux involving two species has been summarized by Peterson and Fisher (1955). In 1872 and 1873 the murres observed on Walrus Island in the Pribilofs were almost entirely common murres. In 1890 common and thick-billed murres (Uria lomvia) were evenly matched in number. By 1901 the colony was almost exclusively dominated by thick-billed murres. In 1911 and 1914 the few thick-billed murres present were almost lost among the then dominant common murres. In 1940 thick-billed murres dominated again. When Peterson and Fisher visited the island in 1953, the situation was again reversed and common murres had almost completely replaced the thick-billed murres. These changes are even more impressive because of the number of birds involved, between 1 and 2 million in 1953. There are more tenuous indications that somewhat the same thing may occur between two other congener pairs, the pelagic and red-faced cormorants and the black-legged and red-legged kittiwakes. The causative factor, or factors, is not readily apparent. One possibility is long-term climatic fluctuation.
Dement'ev and Gladkov (1966) provide an example of abrupt and massive change. Before 1876, the pelagic cormorant abounded on the Commander Islands. During the winter of 1876-77, the birds were decimated by an unknown epizootic disease. By spring only a few individuals remained alive. The record shows that by 1882 they were already becoming common again. Red-faced cormorants were apparently not reduced in number because Dement'ev and Gladkov (1966) state that they were common in "the second half of the last century and the beginning of this." Did they flourish only while the pelagic cormorants were reduced in number?
Bowles (1908) gives another indication of naturally induced population impact. He noted large numbers of dead seabirds on Washington beaches and the ocean "rather plentifully dotted with sick birds ..." He examined some birds and found "many hundreds" of tapeworms in every bird. His conclusion was that their intestines were so solidly packed with tapeworms that starvation was "an absolute certainty."
Some apparent disruptions are long term. In the Gulf of Alaska there is a hiatus in the distributions of a number of small seabirds that are active around their colonies only at night. Repeatedly, the northern Gulf of Alaska shows up as an area of reduced population, as a boundary between subspecies, or as a limit to a range. This same area has a noticeable lack of total darkness during a substantial portion of the breeding season.
The nocturnal habit no doubt evolved because it was advantageous to concentrate on the breeding grounds only under the cover of darkness, when diurnal predators were at a great disadvantage. Cody (1973) states that Cassin's auklet (Ptychoramphus aleuticus), which is strictly nocturnal around its colonies, avoids these colonies on brightly moonlit nights. He sees this as an apparent response to gull predation. At higher latitudes the small alcids have overcome this disadvantage by swamping predators through their sheer numbers. In the Gulf of Alaska I suspect that few of the small seabirds, except possibly the fork-tailed storm-petrel (Oceanodroma furcata), have ever achieved great enough numbers to offset the impact of extended daylight.
Past disruptions of seabird populations are both natural and man-induced; however, the documentary record is much too fragmentary to allow us to fully appreciate what has occurred or what the long-term effect has been. To give some perspective to the problems associated with assessing change and attempting to understand it, some of the indicators of natural and unnatural change and flux in seabird populations are reviewed here.
The flux in bird numbers can be related to the time of day, season of the year, and atmospheric conditions on a short-term basis. This sort of flux or apparent flux can easily be explained. The underlying cause of some of the longer term flux is not so easily arrived at. Murie (1959), Gabrielson and Lincoln (1959), and Sowl and Bartonek (1974) have noted some of the man-induced changes. These are also explored to some extent in the species accounts as they are found to apply.
I sometimes refer to a colony size class[Pg 52] when discussing the existing data rather than to an actual population estimate. The size classes used are defined as follows:
The Dictionary of Alaska Place Names (Orth 1967) is the reference for those who wish to locate some of the less obvious sites. The Coast Pilot, No. 9 (U.S. Department of Commerce 1964) is another useful reference.
Petrels of a number of species can be found in the Gulf of Alaska, some of them in great numbers. Only the northern fulmar breeds there.
The fulmar is common in the offshore waters of the northern Gulf of Alaska throughout most of the year (Isleib and Kessel 1973). Most authors, including Clark (1911), one of the earlier ones, who commented on the distribution of fulmars farther out in the Gulf, have considered them to be abundant. Nichols (1927) raised one of the few voices of apparent dissent; he noted that in 1926 he encountered the largest number of fulmars (about 800) on 11 July in Shelikof Strait after he had left the Gulf. During the summer, fulmars are very common seaward of Montague Island, particularly to the northeast of Patton Bay and in the approaches to Montague Strait. Data derived from FWS surveys in July and August 1972 showed an estimated 10,000 fulmars in a stretch of waters 19 km wide along the east side of Montague Island (Isleib and Kessel 1973).
Over the Portlock Banks and in Stevenson Entrance, fulmars sometimes concentrate in very large numbers, either by themselves or in company with sooty shearwaters (Puffinus griseus). In August 1973, FWS observers crossing Perenosa Bay saw large numbers of tube-nosed birds moving northeastward across the Bay. Although these appeared to be predominantly shearwaters, there were also many fulmars. There was a general movement of birds through Shuyak Strait from Shelikof Strait into the Gulf of Alaska. It was not determined whether the fulmars were moving with the shearwaters or on a regular feeding flight. Fulmars are often found close to Afognak Island in the area between Sea Lion Rocks and Sea Otter Island. Gabrielson and Lincoln (1959) reported seeing swarms of fulmars in Marmot Strait and around the small islands on the north side of Afognak in early August. Murie (1959) noted fulmars in Shelikof Strait and again around the Shumagin Islands. There is nothing in this record to indicate any change in their distribution at sea recently.
The Semidi Islands support the Gulf of Alaska's largest fulmar breeding population, a Class V colony (U.S. Bureau of Sport Fisheries and Wildlife 1973). Gabrielson and Lincoln (1959) considered it to be one of the four largest colonies in Alaska.
Gabrielson (1940) was told by Captain Sellevold of the marine vessel Brown Bear that he thought the birds nested on Sea Otter Island in Perenosa Bay. Gabrielson also learned that they probably nested on Sea Lion Rock at the head of Marmot Strait. In August 1973 I observed fulmars in close proximity to Sea Lion Rock. More recently, small numbers of apparently breeding fulmars have been found in the Barren Islands (L. W. Sowl, personal observation and Edgar Bailey, unpublished FWS report, Anchorage, Alaska). Although no other colonies are known or suspected, the evidence suggests the possible existence of some.
Peterson and Fisher (1955), on noting dark fulmars between St. Paul and St. George when only the light morph was present on any of the colonies in the Pribilofs, expressed no surprise. They offered the opinion that a round trip of 960 km to one of the dark morph colonies in the Aleutians just might be within the operating range of a fulmar on a 4-day vacation from nest-tending duties. Using this as a general yardstick, it appears that the rich foraging grounds over the Portlock Banks might also be within the range of breeding fulmars from the Semidis. The trip up Shelikof Strait and on to Portlock Bank by way of Shuyak Strait is only slightly longer than the one from Chagulak to St. Paul. The feeding grounds off Montague Island would require a[Pg 53] 1,600-km round trip from the colonies in the Semidi Islands. Birds from the Barren Islands and any colonies around Shuyak Island could easily reach the Montague Island grounds, but why would they cross the Portlock Banks to do so?
Fulmar colonies may be found in the Chiswell Islands. It is also a possibility that the existence of colonies on islands along the north coast of Afognak Island will be verified and that others will be found in the vicinity of Shuyak Island. Gabrielson and Lincoln (1959) expressed the opinion that there is almost certainly a colony on Sutwik Island. If there is one, however, I did not see it on one quick trip around the island in 1973.
Gabrielson (1940) expressed surprise at the size of the Semidi Island breeding colony. Gabrielson and Lincoln (1959) considered 1911 to be the first time breeding fulmars were found in the Shumagins. They apparently based this on two eggs collected there that year and documented in a plate in Bent (1964). Other than Gabrielson's opinion, there is nothing to indicate a major change in fulmar status during this century. If there has been a change in status, it has probably been in the direction of increasing populations.
The fork-tailed storm-petrel probably breeds throughout the Gulf of Alaska. It is abundant at sea during the summer in most offshore waters. Murie (1959) described it as the dominant petrel in the Bering Sea and the North Pacific.
In view of its wide distribution and apparent abundance very little is known about the fork-tailed storm-petrel's breeding colonies. Friedmann (1935) recorded specimens and eggs from Kodiak dating back to 1843. Murie (1959) noted them as nesting on Sanak Island and stated that they almost certainly nested in the Shumagins and on other islands along the Alaska Peninsula. David Roseneau (Isleib and Kessel 1973) found this storm-petrel "breeding by the 10,000's" on East Amatuli Island in the Barren Islands in June 1965. This was subsequently verified in 1974 by Edgar P. Bailey (unpublished report, FWS, Anchorage, Alaska).
On 2 July 1972, responding to a tip by James W. Brooks (personal communication), M. E. Isleib and I anchored at Fish Island in the Wooded Islands. We did not locate any storm-petrel burrows, but a steady flow of storm-petrels passed over the boat throughout the darkest part of the night. Surveys conducted at about that time provided an estimate of 19,000 fork-tailed storm-petrels in Prince William Sound, primarily in or close to Montague Strait, and in coastal waters on the east side of the Sound's outer islands. In this area Isleib (personal communication) has noted a general movement of fork-tailed storm-petrels westward around Montague Island and into Prince William Sound through Montague Strait each morning and a corresponding countermovement each evening. I conclude that in 1972 there was a Class IV colony in the Wooded Islands, numbering between 19,000 and 38,000 birds. Additional colonies will be discovered in a similar manner as more systematic searches are made.
No colonies were discovered during the 1973 reconnaissance survey of the islands south of Alaska Peninsula. Working primarily inshore, FWS investigators encountered very few storm-petrels during the day. On the night of 14 June, the FWS vessel, Aleutian Tern, responded to a Mayday call and was either in transit or participating in rescue operations from 2245 to 0420 h on the morning of 15 June. During this period numerous fork-tailed storm-petrels were encountered, particularly off Cape Wedge on Nagai Island. After we anchored in Eagle Harbor on Nagai, more storm-petrels were heard about the vessel.
At about this same date, National Marine Fisheries Service enforcement officers flying fisheries patrols observed storm-petrels in abundance south of the Shumagin Islands (James Branson, personal communication). These observations support the belief that there are probably substantial undiscovered colonies in the Shumagin Islands.
Fork-tailed storm-petrels are abundant summer residents in the northern Gulf of Alaska and the estimate by Isleib and Kessel (1973) is that populations using the waters off the North Gulf Coast probably number in the millions. Certainly the same estimate is valid for the rest of the Gulf area west of the Chugach Islands.
The status of these birds relative to their[Pg 54] historical abundance cannot be derived from the existing information. There is strong suspicion that the introduction of fox on many of the islands in the area during the early part of this century probably caused a reduction in their numbers. Murie (1959) said that experience taught him that wings left from fox kills or remains of storm-petrels in fox droppings could be accepted as evidence of the presence of a colony. Gabrielson and Lincoln (1959) reported that E. P. Walker visited the Wooded Islands in 1922 searching for a storm-petrel colony that had been reported to exist there in 1918. He could not find it even though he searched diligently. This apparent disappearance was attributed to the introduction of fox.
There is another factor to consider, however. The limited number of specimens now available from the Gulf of Alaska indicates that separate subspecies occupy the eastern and western Gulf of Alaska. The accepted boundary is somewhere in the vicinity of Prince William Sound. This is an indication that there has been a hiatus in this area of rather long duration. I have speculated that this sort of break may be in some way related to the length of day and a period during the summer when there is little darkness to cover activities near the colony. Thoresen (1964) and Cody (1973) have both reported that western gulls (Larus occidentalis) assemble in Cassin's auklet colonies on moonlit nights to prey on arriving adults. It is likely that other nocturnal species would provoke the same sort of hunting tactic. A light-related predation factor implies that the predators rely on sight. Avian predators are indicated.
Even less well understood than the breeding distribution of the fork-tailed storm-petrel is that of Leach's storm-petrel.
Bendire (1895) quotes notes from Chase Littlejohn, who found Leach's storm-petrel to be an abundant breeder on unspecified small islands near Sanak in 1894. It greatly outnumbered the fork-tailed storm-petrel. On his visit in 1937 Murie (1959) learned that all of the large colonies of seabirds that had once existed there were gone. He attributed this to overfishing and associated perturbation and to the introduction of fox. No systematic assessment of seabirds on Sanak has been attempted since Littlejohn's time.
No Leach's storm-petrel colonies have been encountered during reconnaissance surveys of the Gulf of Alaska. Small numbers have been reported from time to time and while it is very much less abundant than the fork-tailed storm-petrel, I expect that it will be found in small numbers at various places in the Gulf of Alaska when it becomes possible to make more thorough searches. It may occur in remote areas like the smaller islands scattered throughout the Sandman Reefs—possibly even in large numbers. On the basis of the Sanak record, we must assume that this storm-petrel has been greatly reduced in numbers, at least in the western portion of the Gulf.
The white-crested cormorant, the race of the double-crested cormorant residing in the Gulf of Alaska, is principally an inhabitant of the marine environment. This cormorant is a common, but apparently patchily distributed, resident throughout the northern and western Gulf of Alaska.
Gabrielson and Lincoln (1959) thought that it nested only from Kodiak Island westward into the Aleutians. However, it probably breeds from Yakutat Bay westward. Isleib and Kessel (1973) estimated the abundance of the double-crested cormorant along the North Gulf Coast as several thousands, about one-tenth as common as the pelagic cormorant. It is the third most abundant of the four cormorant species nesting in the area. It occurs as scattered inclusions in many colonies throughout the area, and at least in the Shumagin Islands, even occurs in some colonies by itself.
There are no data on which to base an estimate of any change in status. It probably is not much affected by many of the naturally occurring perturbations.
On 22 July 1972, 13 Brandt's cormorants (4 sitting on nests) were found at Seal Rocks in[Pg 55] Hinchinbrook Entrance, Prince William Sound (Isleib and Kessel 1973). Two years later I positively identified two individuals in breeding plumage among a mixed group of cormorants in the Chiswell Islands west of Seward. Are these recent range extensions? Possibly, but I propose an alternative explanation.
Palmer (1962) showed the distribution of this cormorant as breeding north to Puget Sound and as a straggler north to Forrester Island, Alaska. This viewpoint is shared by the American Ornithologists' Union (1957), which regards the bird as casual as far north as Forrester Island, where this species was collected by Willet (1918).
Let us look at the other record, the one that is not supported by specimens. Bent (1964) thought of Brandt's cormorant as a breeding resident of Forrester Island. Gabrielson and Lincoln (1959) admonished bird observers to be on the lookout for this particular cormorant in the vicinity of Ketchikan and Prince of Wales Island. Brandt's cormorant also appears on the bird list for the Kodiak National Wildlife Refuge as an accidental visitor.
Early observers like Bent were explorers. They carefully examined and made notes on all the birds they saw because there was always a chance of a new discovery. It is also very probable that Bent paid particular attention to the cormorants when he was at a place like Forrester Island. He would have undoubtedly been very interested in trying to confirm the presence of the now extinct Palla's cormorant (P. perspiculatus), as he must have been aware of Schlegel's (1862-64) list of the birds in the Dresden Museum since Willet (1914) had recently referred to it. The staffs for the Kodiak and Aleutian Islands National Wildlife refuges have included some very careful observers, such as Frank Beals. These men would have noticed the difference if a new bird such as Brandt's cormorant was seen, verified the sighting visually, and then noted it in their field diaries. They would not have bothered to develop the type of proof needed for an undisputable record, but the bird would have appeared in the refuge bird list (as it does).
The outside coasts of the Alexander Archipelago, Kenai Peninsula, and the Islands of the Kodiak Archipelago impose some logistical requirements which discourage all but the most determined birders. Not many have been able to reach more than very limited segments of the entire coast. Given the vast distances involved, few of the FWS vessels passing through the area have had the time to thoroughly examine any cormorant colonies or roosts bird by bird. Even for those who pause, the ever present swells and the constant chop of the summer westerlies make positive identification difficult.
It is possible that Brandt's cormorant has been in the area in small numbers for a long time, either regularly or intermittently. It could have escaped observation because of the conditions described above. This species may be there as a relict, as a pioneer, or only because surplus birds are being pushed into marginal habitat by population pressures on their main range to the south.
The pelagic cormorant is the most abundant of the four cormorants residing in the Gulf of Alaska. It is found throughout coastal Alaska south of the Bering Strait and even in some colonies in the southern Chukchi Sea.
Cormorants have a certain invisibility which is brought about by their universal presence. This blindness appears to have affected everyone, even the earliest observers.
The earliest accounts provide a composite picture of the distribution and abundance of the pelagic cormorant which is very similar to that encountered today. In southeastern Alaska, beginning at the eastern edge of the area under discussion, the pelagic cormorant was pictured as the sole resident cormorant. However, we know from Willet's collection of a Brandt's cormorant at Forrester Island that this might not be quite true. From Yakutat Bay westward into the Aleutians this species coexisted with the double-crested cormorant. In the Western Aleutians there is some disagreement, but in general it appears to have been accepted that the red-faced cormorant occurred there along with pelagic and possibly double-crested cormorants. In the Bering Sea this species coexisted with the red-faced cormorant.
A number of recent authors (Gabrielson[Pg 56] 1940, 1944; Murie 1959; and others) have considered the pelagic cormorant to be the most widely distributed and abundant of the four species found in Alaska. Since the modern picture fits, in a general way at least, it would be easy to conclude that the species enjoys an unchanged status. There is just a faint suggestion that this may not be true.
Dement'ev and Gladkov (1966) refer to a great die-off of pelagic cormorants referred to earlier, in the Commander Islands. Stejneger (1885) enlarges on this disaster. It is true that Stejneger visited these islands a relatively short time after the die-off, but he reported that even though the pelagic cormorants were increasing, "people having seen their former multitude think that there is no comparison between the past and the present." Murie (1959) thought that the pelagic cormorant, while numerous, was outnumbered by the red-faced cormorant in the Aleutians. More recently there has been the rapid eastward expansion of the red-faced cormorant. Although it is not possible to determine what the real status of the pelagic cormorant is relative to its past status, I conclude that during this century its status relative to that of the red-faced cormorant has declined.
The red-faced cormorant, in spite of superficial similarities to the pelagic cormorant, just does not look the same to an experienced observer. However, it would have been possible for inexperienced observers in the days before modern optics to overlook the differences. The problem was further compounded by the "invisibility" of the ubiquitous cormorants referred to earlier. Apparent absences or blank spots in their range may not have been real.
Dement'ev and Gladkov (1966), reporting on the Russian record, stated that the red-faced cormorant was common in the Commander Islands during the last part of the 19th century and into the early part of the 20th. Older authors had also reported it from Kamchatka and the Kurile Islands. Now, according to Dement'ev and Gladkov, it is an uncommon breeder on Mednyi Island in the Commander Islands and occurs only as an autumn visitor to some of the southern Kurile Islands.
Turner (1885) reported that the double-crested cormorant was abundant in the Near Islands and that the pelagic cormorant was common, but makes no reference to the red-faced cormorant. One specimen of the latter in the Leningrad Academy of Science was taken at Attu on 16 September 1844 (Gabrielson and Lincoln 1959), which indicates that they were probably present during the period reported on by Turner and, therefore, relatively uncommon. Clark (1911) identified red-faced cormorants only a few times and in the Aleutians only once, near Agattu. Dall (1874) noted two red-faced cormorants collected at Amchitka but he (Dall 1873) apparently did not see any east of Unalaska.
Nelson (1887) apparently found red-faced cormorants breeding on the Siberian and Alaskan mainlands at either side of Bering Strait, but Bailey (1948) searched for some sign of their presence and found none. Nelson (1887) also reported the red-faced cormorant from St. Matthew and St. Lawrence islands in the northern Bering Sea and from St. Michael and Nelson Island on the Alaskan coast. Gabrielson and Lincoln (1959) pointed out that it has not been found breeding north of the Pribilofs since then. Friedmann (1934) provides support for Nelson by reporting red-faced cormorant bones from archeological sites on St. Lawrence. Gabrielson and Lincoln (1959) cited two red-faced cormorants in the Leningrad Academy of Science which were collected in the Pribilofs in 1843. Dall and Bannister (1869) reported them to be plentiful on St. George Island. Baird (1869) also noted their presence in the Pribilofs.
Bent (1964) makes no mention of seeing the red-faced cormorant in the Aleutians. He gives their breeding range as the Bering Sea region, the Pribilof Islands, and perhaps the western Aleutians, the Commander Islands, and the coast of Siberia north of North Cape. The American Ornithologists' Union (1931) gave their breeding range as the Pribilof Islands, the Commander Islands, and Siberia north to North Cape.
Murie (1959) found a colony of between 4,000 and 5,000 red-faced cormorants nesting on Amak Island in 1925. In 1936 he was surprised to find that the red-faced cormorant was the most abundant breeding cormorant in the Aleutian Islands. Pelagic cormorants still[Pg 57] appeared to be most numerous, but there were large numbers of nonbreeding birds. In 1936 he located "a good sized colony" of red-faced cormorants at Unga in the Shumagin Islands. He found about 300 birds starting their nests on 16 May.
In August 1946 Gabrielson (Gabrielson and Lincoln 1959) visited the colony at Delarof Harbor, Unga, where several thousand cormorants were observed. From a number of small samples he estimated that the red-faced cormorants outnumbered pelagic cormorants five to two. In 1973 I observed about 2,000 cormorants, mostly red-faced, in this colony. Gabrielson also located them at two other sites in the Shumagins and at Aghiyuk Island in the Semidi Islands.
Howell (1948) noted only double-crested cormorants at Double Island, Kodiak. Shortly after that the leaflet, Birds of the Kodiak Island National Wildlife Refuge (first issued in 1955), listed red-faced cormorants as common summer residents. The red-faced cormorant was next found at Katchemak Bay about 1963. Isleib (Isleib and Kessel 1973) first noticed red-faced cormorants wintering in Prince William Sound in 1969. In July 1972 Isleib and Sowl had found a colony containing 75 nests at Point Elrington at the western approach to Prince William Sound. By 1974 Isleib and Haddock (unpublished data, FWS, Anchorage, Alaska) found them east of the Copper River Delta at Wingham Island.
The relatively rapid expansion of the range and apparent population size of the red-faced cormorant is remarkable. But has this been a real expansion into vast stretches of new territory? The record in the literature which I have summarized shows, I think, something else. We can demonstrate a historical range for the red-faced cormorant that extends on the Asiatic Coast from North Cape, Siberia, south to the Kurile Islands, the entire Aleutian Arc including the Commander Islands, all the Bering Sea islands north to Bering Strait, Norton Sound, Nelson Island, and the islands south of the Alaska Peninsula at least as far east as Kodiak Island. The recently occupied coast from Cook Inlet to the Copper River may represent a real range extension. The breeding range of this species at the present time does not include parts of its historical range west of the Commander Islands or north of the Pribilof Islands.
The fragmentary record appears to show a long-term perturbation in the range and populations of the red-faced cormorant that covers at least 100 years. I believe that we are probably seeing a recovery of lost range and a return to something resembling a former distribution and abundance.
What caused the perturbation? I am not prepared to answer this question, but there are two occurrences which I find suggestive.
It is interesting to note (Dement'ev and Gladkov 1966) that on the Commander Islands the red-faced cormorant was most abundant during the first 50-odd years after the pelagic cormorants had been wiped out in the winter of 1876-77. Perhaps some clues are to be found in the interactions between these similar species.
It does not appear that the introduction of fox could have been a causative factor. The first observations of population expansion were noted almost concurrently with the heyday of the fox-farming industry. Because of its choice of nesting habitat (very steep cliffs), this cormorant would not have been affected by predators except for the one that went into a very rapid population decline at a time that would fit—the Aleut.
Jochelson (1968) and Hrdlicka (1945) summarized references to Aleut clothing in the diaries and reports of early Russian visitors to the Aleutian Islands. Evidently Aleut women sometimes wore a long, robe-like parka made of harbor seal (Phoca vitulina) skins or, for women of high rank, parkas made of sea otter (Enhydra lutra). The men in almost all reports were said to have worn bird-skin parkas; puffins and guillemots appear to have been preferred, but cormorants were sometimes used. It took about 40 puffin skins to fabricate a parka and a man evidently needed from one to three of these garments each year.
Sea otter populations were drastically reduced by Russian hunters. Rats were introduced to the Aleutians very early during the Russian period and must have had a substantial impact on populations of tufted puffins and guillemots. The introduction of fox would have had a further impact on burrow-nesting birds. Turner (1885) noted that Aleuts in the Near Islands kept the fox confined to Attu so[Pg 58] that they could keep the fox away from the birds on Agattu. This is evidence of an Aleut recognition of serious competition. Could cormorants, particularly red-faced cormorants, have been preferred sources of fiber? Were Aleuts forced to rely more heavily on cormorant skins as puffin and guillemot numbers were reduced by rats and fox and sea otters by men?
Whatever the cause and effect, the status of red-faced cormorants now appears to be better in the Gulf of Alaska than for at least the last 100 years.
The glaucous-winged gull is apparently one of the more successful seabirds breeding in the Gulf of Alaska. While it is outnumbered (both locally and in total abundance) by the black-legged kittiwake, it is generally the most commonly seen and most uniformly distributed gull in the Gulf of Alaska. Murie (1959) called it the common breeding gull about the Alaska Peninsula. Cahalane (1943, 1944) considered it to be numerous to abundant around Kodiak and in the Shelikoff Strait area. Gabrielson (1944) reported that it could be seen in small numbers everywhere. Most recently, Isleib and Kessel (1973) reported it to be an abundant resident in the north Gulf Coast area. My own experience would confirm these observations.
This gull appears to use a wider variety of nesting sites than some others (Gabrielson and Lincoln 1959). Except where man's activities have created new food sources, there appears to be a close link between the location of glaucous-winged gull colonies and those of murres, kittiwakes, and cormorants. Swartz (1966) found that during the breeding season glaucous-winged gulls at Cape Thompson derived almost all of their food from murre eggs and chicks. I have noted small numbers of these gulls nesting, usually on turf near the tops of cliffs, in most colonies of favored prey species.
The glaucous-winged gull is the principal scavenger throughout much of coastal south-central Alaska. This has sometimes resulted in the development of large concentrations near canneries and, more recently, near dumps.
Two glaucous-winged gull concentrations stand out in the northern Gulf of Alaska. One of these is on Egg Island at the western end of the Copper River Delta. Patten (1976) estimated that this colony contained 10,000-12,000 gulls in 1975. At times it appears to spread onto nearby Hinchinbrook Island. M. E. Isleib (personal communication) has estimated its size as high as 25,000 gulls. The other large concentration is on the Susitna Flats across Cook Inlet from Anchorage. This colony, or colony cluster, may be larger than the one at Egg Island. There are no other known colonies even approaching these in size. Most colonies range between a few pairs and 2,000-3,000.
Glaucous-winged gulls do not appear to have had any great changes in population that can be detected from the literature. There have almost certainly been local fluctuations in the number of breeding birds as food supplies, such as canneries and dumps, have appeared or disappeared in an area. Long-term changes in salmon runs have undoubtedly had an impact as well. One other change, the reduced level of egging, has undoubtedly had an effect also. Along the Alaska Peninsula and in the Shumagin Islands, cannery workers of Filipino heritage and fishermen who have a strong Aleut heritage still harvest gull eggs for food. However, this activity is much reduced from what it must have been.
The herring gull is a resident of Upper Cook Inlet and is found up and down the coast from Prince William Sound to the Alaska Peninsula. Not too much was learned about it during the recent FWS reconnaissance. Williamson and Peyton (1963) reported the interbreeding of herring gulls and glaucous-winged gulls in this area. This interbreeding has resulted in a situation in which assignment of these gulls to one group or another in the field can be rather arbitrary. The result has most often been that field observers tend to lump them with glaucous-winged gulls unless their herring gull characteristics are obvious. Specimens collected by Williamson and Peyton (1963) indicate that herring gulls have the edge in numbers in Upper Cook Inlet.
The black-legged kittiwake is the most abundant gull in the northern and western Gulf of Alaska. Colonies of this species can be found throughout the entire area, and range in size from a few pairs (Class I) to more than 100,000 birds (Class V). They may be found in essentially pure colonies, but are often found sharing colonies with murres.
The center of abundance for breeding black-legged kittiwakes in the Gulf of Alaska is in the Semidi Islands, where Palmer Sekora (U.S. Bureau of Sport Fisheries and Wildlife 1973) estimated that there were 426,000 breeding kittiwakes in 1972. He located kittiwake colonies at eight sites, ranging in size from 1,000 to 109,000 nesting birds. The size of the average colonial site was 27,000 birds. Ten sites were Class IV in size and one was a solid Class V.
The easternmost known colony in the northern Gulf of Alaska is at Wingham Island. Up to 1973, 22 colonies had been located in Prince William Sound. The largest of these contained only 5,636 nests in 1972 (Isleib and Kessel 1973). Class IV or larger colonies are found at Cape Resurrection, the Barren Islands, Chisik Island, Boulder Bay and Cape Chiniak on Kodiak Island, and at Delarof Harbor and the Haystacks in the Shumagin Islands. It is interesting to note that Gabrielson (1940) considered Whale Island to be one of the largest known kittiwake colonies in Alaska. He stated that there were many thousands of pairs extending over a mile or more of cliff. He saw a second site which he did not visit but looked equally large. A photograph in an article by East (1943) also indicated the presence of a large colony. C. J. Lensink (personal communication) estimated that there were about 100,000 kittiwakes in the colony in 1956. When last visited by Vernon Berns (personal communication), this colony contained only 3,000 birds. It is also of interest that Gabrielson (1940, 1944) did not notice either the kittiwakes or the murres now breeding on Nord Island in the Barren Islands or the kittiwakes on East Amatuli Island.
Whale Island and possibly the colonies in the Barren Islands give evidence of local population fluctuations, but for the most part I have not found an indication of a major perturbation over the past 40 years. Before 1936, the record is too fragmentary to allow an assessment.
One of the interesting aspects of kittiwake ecology in the Gulf of Alaska is the common occurrence of breeding failure. David Snarski (December 1943 Quarterly Progress Report, Alaska Cooperative Wildlife Research Unit, University of Alaska) observed breeding failure on colonies in the Tuxedni National Wildlife Refuge in 1970 and 1971 and obtained circumstantial evidence of another failure in 1972. In 1973 all of the breeding cliffs were occupied and nesting was successful. Whatever the cause of these periodic failures, they do not yet appear to have had a permanent impact that we are able to measure.
Red-legged kittiwakes are not now known to breed in the western Gulf of Alaska. Turner (1886) stated that he saw a few at Sanak in 1878. We also have Stejneger's (1885) statement, that "red-legged" kittiwakes nest from Bering Island to Sanak. Friedmann (1937) reported two humeri from Kodiak Island middens. During the summer of 1976, two birds were observed off Kodiak Island by Irving M. Warner (personal communication), and one at 158°W and 54°30'-54°20'N south and east of the Shumagin Islands (Patrick J. Gould, personal communication).
Turner (1885) listed the red-legged kittiwake as abundant and breeding in the Near Islands. Turner (1886) also stated that he had seen quite a number about a cliff back of the village on Akutan Island in 1878. He added that to the westward this kittiwake was more abundant than the black-legged kittiwake. Murie (1959) expressed the opinion that Turner had confused the short-billed gull with the "short-billed" kittiwake. Clark (1911) also reported that he had seen the red-legged kittiwake in small numbers near Unalaska and that they became progressively more common west to the Near Islands. Nelson (1887) reported seeing large numbers of red-legged kittiwakes at Unalaska. Murie (1959) and Gabrielson (1940, 1944) did not see any red-legged kittiwakes in the Aleutian Islands. The species has recently been discovered breeding at Buldir and Bogoslof islands (G. Vernon[Pg 60] Byrd, personal communication).
Is it possible that we have here another species which is exhibiting a response to some unknown long-term perturbation? The suggestion that such an event has occurred is faint, but it is there. Do we have in the red-legged and black-legged kittiwakes an example of yet another congener pair that has been affected by some perturbation in which one was affected positively and the other negatively? Clark (1911) reported small numbers of black-legged kittiwakes to go with large numbers of red-legged kittiwakes in the Near Islands, which is the reverse of the current situation.
Gabrielson and Lincoln (1959) attribute to the Arctic tern the most extensive range of any Alaskan water bird. It is found in suitable habitat everywhere north of Tracy Arm in Southeastern Alaska. Murie (1959) stated that he found it nesting at suitable sites everywhere he went. Isleib and Kessel (1973) considered it to be an abundant breeder in Prince William Sound and along the northern Gulf Coast.
The Arctic tern was observed in FWS aerial surveys in Prince William Sound, and surveys in July and August 1972 provided an estimate of 45,000 terns in the Sound (Isleib and Kessel 1973). On the other hand, tern colonies were located only rarely in the FWS colony surveys before 1975. This is, however, a reflection of the equipment and methods used and not of the abundance of terns.
From the fragmentary data available, it is not possible to detect changes in Arctic tern status at the present time. We have to assume that the widespread introduction of fox had at least local impact. Although this tern uses a wide variety of nesting sites, it tends to nest on flat sites where access by mammalian predators is easy.
No Aleutian tern colonies were discovered in the Gulf of Alaska area during FWS colony surveys in the early 1970's. This is again a reflection of the fact that surveys were not designed to locate tern colonies. Aleutian terns were encountered at least twice, once during late March 1972 in Hawkins Cutoff, Prince William Sound, and again when two birds were noted offshore from the Katmai National Monument on 30 May 1973 (L. W. Sowl, personal observations).
The type specimen of the Aleutian tern and a single egg were collected at Kodiak Island on 12 June 1868 by Bischoff (Coues 1874). Fisher (Gabrielson and Lincoln 1959) collected four more eggs in 1882. The bird was not found breeding there until Howell (1948) found a colony of 50 pairs at Bell's Flats in 1944. Walker (1923) found them nesting on the Situk River, Yakutat, in 1917 and shortly thereafter saw them at the Alsek River Flats. He also reported that D. H. Stevenson of the Bureau of Biological Survey had told him that they nested on the Isanotski Islands at the end of the Alaska Peninsula. This latter report was the only one from the Aleutian Island chain for many years. Isleib and Kessel (1973) considered it an uncommon local breeder in the northern Gulf of Alaska. Isleib estimated its population at a few hundred pairs on the Copper River Delta in May 1973 and 300-500 birds in June 1970. He also reported that they appeared more or less regularly near Controller Bay and off the Situk River.
In recent years Aleutian terns have been seen with increasing frequency in many places in western Alaska and the Aleutian Islands. This is probably partly due to the increasing level of field work. At Amchitka Island the several colonies that have been found in recent years are almost certainly exhibiting a response to the removal of fox from the island.
Although there is no way of determining what the past status of the Aleutian tern has been in the Gulf of Alaska area, it has been there in small numbers since it was first discovered on Kodiak. It has probably not been abundant at any time and may have suffered a long-term decline brought about by the introduction of fox.
The common murre is resident in the northern and western Gulf of Alaska from Pinnacle Rock, Kayak Island, westward. East of Cook Inlet colonies are located at Wingham Island, the Martin Islands, Middleton Island, Por[Pg 61]poise Rock in Hinchinbrook Entrance, Barwell Island/Cape Resurrection, the Chiswell Islands, the Barren Islands, and Chisik Island.
For some reason, the islands of the Kodiak-Afognak Archipelago do not host any known major murre colonies. There is also a rather large gap between the Chisik Island colony and the next major colony at Oil Creek west of Puale Bay. Directly west of Oil Creek is another colony at Cape Unalishagvak. Both of these latter colonies are Class V and they are the first colonies of this size to be encountered in the Gulf of Alaska. West of these colonies the next large colony is at Atkulik Island. To the south, midway between the last-named colonies, lies the major composite murre colony in the Semidi Islands. These sites make up the only Class VI colony in the Gulf of Alaska. Westward, the next major colony, a Class V, is at Spitz Island south of Mitrofania Island. In the Shumagin Islands one Class V colony is at Karpa Island, and lesser colonies with large murre components are found at the Haystacks, Castle Rock, and Bird Island. Only minor murre colonies are found between the Shumagin Islands and the end of the Alaska Peninsula.
Gabrielson and Lincoln (1959) were aware only of the colonies at Cape Resurrection (which Gabrielson considered to be large), at the Chiswell Islands, and at Chisik Island for the area from Cook Inlet east. Gabrielson visited the Barren Islands on 13 June 1940 and apparently did not notice the present murre colonies, both Class IV, at East Amutuli (an island which he visited) and Nord Island.
Gabrielson (Gabrielson and Lincoln 1959) found a few small colonies at Kodiak, mostly on small offshore islands. Gabrielson found common murres to be abundant in the Semidi Islands and stated that there were no notable colonies in the Shumagins, although on his return to the Shumagins in 1949 he did find a fairly large colony at the Haystacks. That size description would fit the colony that is there now. He obviously did not see the other colonies. Rausch (1958) reported murres from Middleton Island.
There is quite a difference between the distribution of murres as we know it today and the way Gabrielson and Lincoln pictured it. Why does this difference exist? There are two possible answers: either the number of colonies has increased, or the coverage of colony locations has improved. The latter case, at least, is established. I must confess to being puzzled by the way Gabrielson was able to move about close to what are now known to be sizeable colonies without seeing them, those in the Barren Islands and the Shumagin Islands in particular. Perhaps this represents the vague outlines of yet another population change.
The center of abundance for murre distribution in the Gulf of Alaska today is from Paule Bay west to eastern Shumagin Islands. The Semidi Islands are the heartland of this area of maximum abundance. We have no definitive data on species composition of these colonies. Common murres undoubtedly dominate in most of the colonies; the only ones where we know of a sizeable thick-billed murre component are in the Shumagin Islands.
Thick-billed murre population information cannot be separated from that of the common murre on the basis of existing data. A direct assessment of present-day status is not possible. After reviewing what we know about their distribution, I suggest a way to examine the question indirectly.
The thick-billed murre is found in colonies with the common murre from Middleton Island westward; Rausch (1958) noted about 400 murres at Middleton Island and observed that the thick-billed murre outnumbered the common murre by several times. Isleib and Sowl (FWS, unpublished data) saw a thick-billed murre mixed with common murres at Porpoise Rock in July 1972. Isleib and Kessel (1973) expressed the opinion that small numbers of thick-billed murres will be found in most common murre colonies in the northern Gulf of Alaska when it is possible to survey these colonies in detail. Karpa Island had a significant component of thick-billed murres in June 1973, and they constituted 40% of the colony at the Haystacks (L. W. Sowl, unpublished data).
Bent (1963) reported that many thick-billed murre eggs have been taken by collectors at Round Island in the Shumagin Islands. Dall and Bannister (1869) reported a thick-billed[Pg 62] murre that was taken at Kodiak in 1867.
The Gulf of Alaska is at the periphery of the breeding range of the thick-billed murre. While it probably occurs in mixed colonies with the common murre throughout this area, the thick-billed murre is much less abundant. Occasionally in the Gulf of Alaska, a colony will be occupied predominantly by the thick-billed murre. Gabrielson and Lincoln (1959) noted that the thick-billed murre outnumbered the common murre in many colonies in the Aleutians and that it became progressively more common at higher latitudes.
We have almost no data relative to the species composition of murre colonies in the Gulf of Alaska. Until we do it will not be possible to fully understand the population status of the thick-billed murre. It appears that changes in the species composition of murre colonies in the Bering Sea may be an indicator of perturbation. The data for the Gulf of Alaska are still too fragmentary to provide any indication of whether or not the same indicator would work there. Close monitoring of the Shumagin Islands colonies over a number of years might produce the answer.
Earlier in this paper I noted the dramatic changes in species composition of murre colonies on Walrus Island. Gabrielson and Lincoln (1959) also commented on this well-documented and anything but static situation. Investigators who visited this island during 1976 reported seeing no murres on the island and only small numbers on offshore rocks. James Bartonek (personal communication) said that this situation has prevailed for several years.
There is an indication that a similar population fluctuation and change in species composition of murre colonies have also occurred on St. Matthew Island. Bent (1963) found mostly common murres and few thick-billed murres at St. Matthew. Hanna (1916) saw only thick-billed murres. Later, Gabrielson (1941) found this to be true in 1940.
Dramatic fluctuation in murre populations may be common and, at least in some cases, the two species may be affected differently. Perhaps this phenomenon has potential for providing us with an indicator of some natural perturbations.
Peterson and Fisher (1955) expressed the opinion that thick-billed murres arrived at the nesting ledges later than the common murre and had to take the sites that were left. Tuck (1960) reported data from the western Atlantic showing that thick-billed murres arrive later than common murres. On the other hand, Belopol'skii (1961) reported data showing that the two species arrive on breeding colonies in East Murman simultaneously. At Cape Thompson, Swartz (1966) found that thick-billed murres arrived about a week before common murres. The date of arrival, while perhaps a contributing factor, is probably not decisive. Interspecific competition of another sort is indicated.
In mixed murre colonies where there are large numbers of common murres, this species occupies the choice nesting sites. Thick-billed murres are usually left with the narrower ledges while the common murres occupy the longer, broader ledges (Belopol'skii 1961). The broader ledges have lower chick and egg mortality (Spring 1971). Spring also noted that thick-billed murres are excluded from the centers of mixed colonies. Johnson (1938) found that this contributes to higher losses of eggs to predators and to the loss of other social benefits of occupying the colony center (Johnson 1941).
Kozlova (1961) said that during the occupation of a colony there is a sharp competitive struggle between the two species. In the end thick-billed murres are pushed out to the periphery of the colonies or left with narrow ledges or other equally unfavorable sites. Spring (1971) studied the functional anatomy of both species and concluded that the common murre is more successful in these encounters because it has a more upright gait and greater agility than the thick-billed murre.
It follows that in a portion of their respective ranges, where the two species overlap and where there is an equal chance that either common murres or thick-billed murres will dominate a given colony, the common murre dominates. I conclude from this that where there are dramatic changes in species composition of murre colonies, such as at Walrus Island, it is probably because the common murre has been greatly reduced in numbers at the colony.
Spring (1971) concluded that the common murre is well adapted to pursuit and capture of pelagic fishes and that the thick-billed[Pg 63] murre is better adapted for deep diving and the capture of benthic fishes and pelagic and benthic invertebrates. Having greater latitude for food selection, the thick-billed murre would have a greater tolerance for ecological perturbations affecting the available food supply. The common murre has an advantage when pelagic fishes are available but cannot switch to the other foods as readily as can the thick-billed murre. The low density of pelagic fishes in high arctic areas probably also accounts for the greater success of the thick-billed murre at higher latitudes relative to common murres.
Belopol'skii (1961) presented data from East Murman which indicates that the common murre restricts its diet almost entirely to a small number of fish species. Swartz (1966) found strong indications that there were significant differences in the food preferences of the two species of murres. Thick-billed murres made much greater use of invertebrates. Bédard (1976) asserted that it is well known that the common murre is quite partial to zooplankton. So again the issue is not clear-cut.
The situation is, of course, much more complex than I have portrayed it. Nonetheless, I think that it offers potential for use as a tool in assessing population change and perturbations in the food supply which should be studied quite closely.
Gabrielson and Lincoln (1959) noted that the pigeon guillemot was one of the most regularly observed birds in Alaskan waters. It is found everywhere throughout the northern and western Gulf of Alaska area, with only a few understandable and relatively small blanks, such as in the silty waters of Upper Cook Inlet. Because it obviously lacks the breeding murres' need for close contact with its nearest neighbors, it is able to exploit the available nesting habitat to the fullest. It seems that literally every bit of suitable nesting habitat is normally occupied.
Because of the dispersed way in which it breeds and because it does much of its feeding in the onshore zone (which is hazardous for boats) the pigeon guillemot is an almost impossible species to inventory by standard methods.
There is no evidence that the pigeon guillemot has been greatly affected by any major perturbation. Because of its choice of nesting habitat, it is probably subject to the attack of only one egg predator, the rat. Because of its loose social structure and the way it selects nesting sites, eggs and young do not sustain loss from panic flights. Its dispersed distribution should insure that man-made impacts such as oil spills will have limited impact.
The population levels of the pigeon guillemot are probably relatively very stable. The widespread introduction of the rat to most of its nesting range undoubtedly had impact, but this impact has gone undocumented. It would be interesting to follow the response of guillemot populations on islands where rats had been totally removed, if that ever becomes more than a dream.
The marbled murrelet apparently breeds throughout most of the northern and western Gulf of Alaska. This apparently is a necessary condition because to date, at least in this part of Alaska, we can only guess where and under what conditions this murrelet breeds.
In some relatively sheltered waters like Prince William Sound, where marbled murrelets were estimated to number about 250,000 in 1972 (Isleib and Kessel 1973), they are the most abundant seabirds. We know from Dixon (1908) and Grinnell (1910) that this has been so in Prince William Sound since the beginning of the century. We know also that the type specimens came from there as well (Stresemann 1949), which is not necessarily an indication of abundance but is suggestive of their abundance relative to species not collected.
Gabrielson (Gabrielson and Lincoln 1959) found marbled murrelets common near Yakutat, in Prince William Sound, in Resurrection Bay, and at Kodiak, and reported seeing them at the Chiswell Islands and at Chignik and Pavlof Bay on the Alaska Peninsula. Cahalane (1943, 1944) found them to be common in Kupreanof Strait, and along the Alaska Peninsula north of Katmai Bay. Murie (1959) found them all along the Alaska Peninsula. My own field notes from 1973 indicate that the only place where they were common along the Alaska Peninsula was at Wide Bay.
We can sample marbled murrelet numbers by using standard transect methodology; however, I have some very serious reservations about our ability to convert these data into a population estimate. This is not an unusual assessment for Alaskan seabirds in general, but I think it is particularly apropos to this species.
We are still able only to guess at where the marbled murrelet nests and we have not a clue as to what sort of nesting strategy they pursue. I am not prepared to accept, on the basis of one North American record (Binford et al. 1975), that tree nesting is its habit throughout its range. What has been proved is that the marbled murrelet nests in trees and not, as these authors would have us believe, that it does not nest on the ground. It has become rather fashionable to ignore the Chichagof Island record (a ground nest), but it has not been discredited. The color of the Chichagof egg differs from that of the Big Basin egg, but does agree with the one taken from an oviduct by Cantwell (Gabrielson and Lincoln 1959). My own experience leads me to believe that tree nesting, if it occurs, is not the common habit of marbled murrelets nesting in the Prince William Sound region.
After many hours of observing marbled murrelets over a period of several years, I am intrigued by a number of things. These birds, as often as not, appear to be clustered in "pairs" as they feed. This occurs even at what should be the height of the breeding season. On several occasions I have noted a very pronounced evening flight of these birds from gathering areas on the water up into the surrounding mountains at sunset. This has moved me to wonder if their nesting strategy includes incubating at night but less than full-time attendance on days when the eggs can be warmed by the sun. We know that periodic egg-neglect is an aspect of storm-petrel behavior (Pefaur 1974). Is this behavior also possible on a more regular basis in an alcid? If so, it would certainly help explain why nests are hard to find.
It is apparent that more needs to be known about the population dynamics and life history of the marbled murrelet before we can make a proper estimate of its abundance. In spite of the fragmentary record, I conclude that the marbled murrelet probably enjoys the same relative abundance and distribution that it did at the beginning of the century.
The Kittlitz's murrelet is not as abundant as the marbled murrelet, but locally it is sometimes found in large numbers. FWS surveys conducted during July-August 1972 provide an estimate of 57,000 murrelets of this species in Prince William Sound. Almost a fifth of these were concentrated in Unakwik Inlet above Unakwik Reef. Even more interesting, about 2,500 of these birds were concentrated in one loose flock.
In addition to Unakwik Inlet, Kittlitz's murrelets concentrate in College Fjord in Prince William Sound and in the waters fronting the Bering-Malaspina ice-fields (Isleib and Kessel 1973). Common as they are in these waters, this species is supposed to be even more abundant at Glacier Bay. The common feature of these waters is the amount of ice that can be found below their tributary glaciers.
The Kittlitz's murrelet is apparently distributed from LeConte Bay, east of Petersburg, Alaska, north to Point Barrow and west across the Aleutians to Attu, where Murie collected a pair (Gabrielson and Lincoln 1959). I once flushed a murrelet from an area of tread and riser topography near the top of the highest point on Kiska Island in heavy cloud cover, and although I could not see this bird well, I thought it to be of this species. From the range description in Gabrielson and Lincoln (1959) and Udvardy's (1963) range map, it is apparent that the distribution of this species is rather patchy, but I suspect that for the more mountainous part of its range this is more apparent than accurate. The record is too fragmentary to allow an assessment of any change in status during the historical period.
Chase Littlejohn (Bendire 1895) spent the spring and summer of 1894 collecting eggs on islands south of the Alaska Peninsula. He has left us a detailed record of what he saw but not where he saw it. Bent (1963) stated flatly[Pg 65] that the site of his collecting was Sanak Island and this has common acceptance. Several things in his account point to a site which was a small island with several peers close by, but this could not have been Sanak. It could have been an island in the Sanak Island group or it could equally well have been somewhere in the Sandman Reefs. Unfortunately, because of this the record is clouded. There has never been anything approaching a survey of the southern half of the Sandman Reefs. We do not know what breeding colonies are there.
At any rate, Littlejohn told of the large numbers of Leach's storm-petrels, fork-tailed storm-petrels, auklets (of which only Cassin's is specifically identified), and ancient murrelets which occupied a large number of small islands. He could not calculate the number of breeding murrelets on his small island, the size of which I interpret to have been of the same order of magnitude as two others which he estimated were about 2 acres. He does say that the murrelets must have numbered several thousand and could, if left alone by the Aleuts, have quickly grown too numerous for the island to accommodate.
Murie (1959) made a brief visit to Sanak in 1937 and learned that there were no longer any large colonies of seabirds. He attributes this to exploitation of the fisheries and to the fox-farming industry. Littlejohn told of the repeated visits of Aleuts to his small islands, where they took hundreds of birds each time and all of the eggs they could find. This kind of activity could not help but disrupt the breeding on these islands.
Littlejohn's description of the ancient murrelet's nest leaves little doubt that the birds could be reached by fox or rats with ease. The birds showed no particular care in selecting a nest site and often worked their way back no more than about a meter into the dead vegetative cover from preceding years, where they scratched out a shallow nest.
There are few records of the ancient murrelet from the northern and western Gulf of Alaska. Friedmann (1935) reported the collection of a series of eggs in 1884 on Kodiak Island. Chase Littlejohn (Bendire 1895) collected eggs from somewhere in the Sanak Group in 1894. In 1908 Dixon (Grinnell 1910) saw a bird in Port Nellie Juan. Several were seen by Jaques (1930) near Belkofski in May 1928. Gabrielson collected one bird at Cordova in September 1941 and another at the Chiswell Islands in July 1945 (Gabrielson and Lincoln 1959). He saw numerous flocks in the Gulf of Alaska on 30 July of an unnamed year. In 1943, he would have been near Cape Spencer on that date. In 1945 he would have been near the Chiswell Islands. In either case, he was probably somewhere in Blying Sound.
The ancient murrelet is relatively uncommon but regularly observed in the inshore waters along the outer coasts of the islands fronting Prince William Sound. FWS surveys in July-August 1972 provided an estimate of almost 1,000 birds, mostly in nonbreeding plumage, along the outer coast of Prince William Sound (Isleib and Kessel 1973). Small numbers were found feeding close to the Wooded Islands on 24 July (my personal observation). Rausch (1958) saw a few off Middleton Island in 1956. Isleib (Isleib and Kessel 1973) saw 400-500 widely distributed at the mouth of Yakutat Bay in July and August 1968. The only large numbers of ancient murrelets encountered on the FWS survey of the Alaskan Peninsula in 1973 were in the Shumagin Islands. They were very common in East Nagai Strait on 9 June and more than half of the 1,300 seabirds per square nautical mile encountered between Little Koniuji and Chernabura Islands on 11 June were ancient murrelets. At Nagai Island an estimated 5,000 ancient murrelets were observed in the west bay at Pirate Shake, and later (on 19 June) several were observed in the vicinity of Midun Island (FWS, Anchorage, Alaska, unpublished data).
On the basis of the observations recounted above, I have to conclude that ancient murrelets are fairly regularly, if patchily, distributed throughout the northern and western Gulf of Alaska. I do not believe that the void in their range shown for the northern Gulf of Alaska by Udvardy (1963) is correct. Several colonies are there, awaiting discovery.
Ancient murrelets are not abundant in the Gulf of Alaska but they are certainly more numerous than we have been able to prove. It is not possible to tell from the existing data whether they were once more abundant than they are now. I suspect, on the basis of the Sanak Island experience, that we can conclude that this species has been reduced in number by various of man's activities.
Cassin's auklet is a very uncommon bird in the northern Gulf of Alaska. In the western Gulf it is more common, particularly from the Shumagins west.
This auklet apparently once bred in great numbers on islands in or near the Sanak Group where Chase Littlejohn (Bendire 1895) found them to be twice as numerous as the ancient murrelets. Murie (1959) did not find them there.
Littlejohn began encountering Cassin's auklets at sea some 290 km southeast of Unga, Shumagin Islands. Murie (1959) encountered them near the Shumagins in May 1937. During the FWS 1973 reconnaissance survey of the Alaska Peninsula, these auklets were not encountered (or at least not identified) until we reached the vicinity of Unga Strait where we saw a few in mixed flocks with other murrelets and auklets. They were most numerous in East Nagai Strait. We encountered them only twice in a situation which indicated they might be breeding—on Hall and Herendeen islands on the north end of Little Koniuji Island.
Murie (1959) considered Cassin's auklet to be no longer common west of Kodiak. In Gabrielson's many voyages through the northern and western Gulf of Alaska he encountered them only twice, once off Cape Spencer and once in the Chiswell Islands.
Thoresen (1964) commented that throughout the northern part of its range the Cassin's auklet has become gradually less frequent. Although there are no data to dispute this, I believe, as do Isleib and Kessel (1973), that they are more numerous than observations would indicate, and I would apply this to the entire area. There are certainly colonies remaining in the Shumagin Islands, and quite probably along the south coast of the Kenai Peninsula. When it is possible to fully explore the Sandman Reefs there is a good probability that they will be found there.
We can only guess at the reasons for their decline. Bendire (1895) and Murie (1959) have described some contributing factors.
Gabrielson and Lincoln (1959) described the parakeet auklet as the least colonial of any of the Alaskan auklets. They also considered the Aleutian Islands to be its principal nesting grounds. There are old records of breeding parakeet auklets from Kodiak (Friedmann 1935) and Little Koniuji (Bean 1882). Grinnell (1910) reported two that were seen on Green Island, Prince William Sound, and several more that were seen near Knight Island.
Murie (1959) did not see any parakeet auklets near Kodiak and Afognak islands which he considered to be the eastern part of their range. He did not think they were abundant anywhere along the Alaska Peninsula. He found a few near Sutwik Island in May 1936 and then noted that they were fairly common near the Shumagins in May 1937.
Gabrielson found this species to be quite numerous on the north side of Chowiet Island in the Semidi Islands in 1945 (Gabrielson and Lincoln 1959). He also saw numerous individuals in Marmot Strait and saw one in the Chiswell Islands during the same year. David Roseneau (Isleib and Kessel 1973) found hundreds close to East Amatuli Island in the Barren Islands in 1965.
During FWS colony surveys, parakeet auklets have been found in close proximity to six seabird colonies in Prince William Sound. During the July-August 1972 surveys, they were estimated to number about 3,000 in the Sound. They have also been found closely associated with Chisik Island (David Snarski, personal communication), the Chiswell Islands, Nord and Sud islands in the Barrens, Sea Otter Island, and Central and Long islands along the Alaska Peninsula. They were most numerous in the Shumagin Islands, where they were found near Castle Rock, Hall (9,000), Herendeen (3,000), Atkins (more than 5,000), and Little Koniuju islands. They were again encountered south and west of Cold Bay at High, Fawn, Let, Amagat, Umga, and Patton islands. Many of these islands are in the north half of the Sandman Reefs, the only portion where any attempt has been made to survey seabird colonies.
The parakeet auklet may not be abundant anywhere in the Gulf of Alaska but, based on the numbers of places it has been seen in recent years, its population appears to be well dispersed and probably doing very well. This auklet is most abundant from the Shumagin Islands westward. It is almost certainly more[Pg 67] numerous than has been thought. Its habits are secretive enough so that it could easily escape notice.
Because the parakeet auklet nests predominantly under boulders, it probably was not much affected by fox. Rats would certainly have reduced its numbers wherever these were introduced into its breeding habitat. We have no data to tell us whether there may have been population fluctuations in the past, but there undoubtedly were at least minor ones locally after rats were introduced.
Udvardy (1963) shows the breeding range of the crested auklet as extending from southern Kodiak Island westward. Within the northern and western Gulf of Alaska, it is certainly most abundant in the eastern Shumagin Islands.
Isleib saw this auklet in Prince William Sound 3 times during the winter of 1972-73. These are the only records he was aware of for that area (Isleib and Kessel 1973). David Roseneau (Isleib and Kessel 1973) saw several in Amatuli Cove, Barren Islands, in June 1965. I observed one in the vicinity of Cape Spencer in August 1973.
Friedmann (1935) listed the crested auklet as a breeding bird at Kodiak, but considered it to be much more abundant as a wintering bird. Townsend (1913) has provided us with a vivid description of the myriads of crested auklets he encountered at Yukon Harbor, Little Koniuji Island. Gabrielson and Lincoln (1959) noted large numbers of crested auklet around Simeonof and Bird islands in the Shumagin Islands in 1946 and stated that the Yukon Harbor colony was still thriving.
Crested auklets were not encountered on the 1973 FWS reconnaissance survey until we reached the Shumagin Islands. They were abundant only at the southeastern end of Little Koniuji, where we encountered perhaps 10,000 in Yukon Harbor and more than 50,000 in a small cove directly south of Yukon Harbor on the opposite side of the island. As numerous as they were, they did not match Townsend's myriads or even come close to his assessment that they "were here more numerous than the 'choochkies' at St. George." St. George Island in the Pribilofs is famous for its least auklets which, in the past, have been estimated to number as high as 36 million (Peterson and Fisher 1955). The numbers there today do not even approach this level and we have no way of knowing how abundant they were when Townsend visited the Pribilofs, but I think it is safe to say that they probably numbered in the millions. There are probably more crested auklets than we observed on Little Koniuji, but there is certainly no longer anything approaching millions of birds. Properly pronounced, Koniuji is the Aleut name for the crested auklet, so we can assume that the original inhabitants were impressed by its numbers.
During the 1973 FWS survey we did not see crested auklets at either Simeonof or Bird islands. On the overgrazed and cattle-trampled Simeonof it does not seem possible that any could still exist.
I suspect that a cattleman's greed has been the undoing of any crested auklets that may have nested on Simeonof Island. This would not account for the loss of any colonies that may have been on Bird Island, but the decaying fox-trapper's cabin on that island undoubtedly tells the story. Churnabura, with its feral cattle, presents much the same problem as Simeonof. As for Little Koniuji, have horned puffins been partly responsible for the decrease in crested auklets? The puffin colony at the south end of Little Koniuji must be exactly where Townsend's millions of crested auklets once nested.
No least auklets were encountered in FWS surveys in the Gulf of Alaska in the early 1970's. Udvardy (1963) shows their breeding range as starting well west in the Aleutians. Gabrielson and Lincoln (1959) give the eastern limit of their breeding range as the Shumagin Islands. Bent (1963) listed their breeding range as extending east to Kodiak Island, and Friedmann (1935) knew of only a few specimens taken in the winter from Kodiak. Perhaps least auklets nested somewhere in the western Gulf of Alaska, and they may still, but at the moment we have no evidence to prove that they do.
Udvardy (1963) would have us believe that the rhinoceros auklet did not nest between[Pg 68] southeastern Alaska and the southern Kurile Islands. Bent (1963b), on the other hand, lists their breeding range as extending from Washington to Agattu. Clark (1910) noted this species in small numbers at Atka and Agattu. Because of the lack of proof, Udvardy probably had no options. I believe that Bent was probably closer to describing their original range. I base this assumption on recent observations and on the additional fragments of information reported by Gabrielson and Lincoln (1959). Murie (1959) failed to find this species anywhere in the Aleutians, but his primary reason for being there, the fox-farming industry, may have had a lot to do with his not being able to find any.
The FWS surveys in Prince William Sound in July-August 1972 located small numbers of rhinoceros auklets in breeding plumage at the Wooded Islands and at Stoney Island and Channel Island in Montague Strait. These birds gave every impression of being local breeders. David Roseneau (Isleib and Kessel 1973) encountered two at the Barren Islands in June 1965. Isleib and Kessel (1973) list a few other records from this area.
My own experience leads me to believe that there is a large colony somewhere on Afognak Island, probably on or near Tonki Cape. On 30 May 1973 I noted a lone bird north of Afognak Island. Later, on 8 and 9 August, I saw several in the same area. On 13 August in Marmot Strait I observed a number of rhinoceros auklets, either singly or in groups of up to 12. Some of these had small fish in their beaks. As they flushed, they all flew off toward Tonki Cape. This observation was made just at last light, and I believe that there were many others about that could not be seen in the dying light. We did not encounter this species along the Alaska Peninsula during the FWS survey in 1973 until we reached the end. There I had one quick glimpse of what I was certain was a rhinoceros auklet at Amagat Island.
The horned puffin is one of the most abundant breeding birds in the Gulf of Alaska. There are only a few really large colonies but these birds breed just about anywhere there is a cliff (even a low one) with suitable fractures and crevices. During the Alaska Peninsula surveys in 1973, I estimated that the frequency with which these birds were seen on the water was about half that of the tufted puffin. They have been recorded in so many places that there is nothing to be gained by a reiteration of the record in the literature.
The horned puffins reach their greatest density in the Gulf of Alaska west of Kodiak Island. Murie (1959) estimated that the colony at Amagat Island, Morzhovi Bay, contained 15,000 birds, one of the largest he had seen. It contained at least 50,000 in 1973. Even at that, it was no match for the colony on Little Koniuji Island with its minimum 140,000 horned puffins. Other colonies with large horned puffin components were at High Island (40,000), Castle Rock (20,000), Mitrofani Island (35,000), and Sosbee Bay (15,000).
Earlier in this paper, I commented at length on the great and often rapid fluctuations in populations of tufted puffins. The same phenomenon affects horned puffins. In 1975 there were relatively small numbers of horned puffins at Little Koniuji where they had flourished 2 years earlier (James Bartonek, personal communication). Because they are apparently subject to erratically oscillating populations, it is hard to tell how they have fared over the years.
The tufted puffin, as previously indicated, is also a bird with widely fluctuating populations. Until we develop an understanding of their population dynamics and can understand the underlying cause of these fluctuations it will not be possible to assess trends in their populations or understand the implications of such trends.
Tufted puffins are abundant throughout the Gulf of Alaska. Small colonies can be located almost anywhere. Along the Alaska Peninsula there are a number of colonies with an estimated breeding population in 1973 of more than 15,000 birds. These are: Ashiiak Island (20,000), Central Island (90,000), the Brother Islands (45,000), The Haystacks (19,000), Castle Rock (85,000), Bird Island (none, but may contain 500,000-1,000,000 at times), Peninsula Islands (35,000), the Twins (18,000), Amagat Island (40,000), and Umga Island (22,000). These colonies correspond to the area where colonies were listed for the[Pg 69] horned puffin.
Tufted puffin populations respond readily to some undetermined short-term perturbations. This is clearly demonstrated by their rapid population fluctuations. Because of their numbers and because of the apparent rapidity with which their numbers rebound, it is not so apparent that they have been affected by long-term perturbations, as so many other seabirds apparently have.
There is much unused or underused nesting habitat suitable for this species. In some cases there are very strong clues pointing to why this habitat is vacant. On many islands along the Alaska Peninsula, which have very good-looking tufted puffin nesting habitat and no puffins, there are visible signs of the presence of fox—either fox trails or abandoned trappers' cabins. I also suspect that the brown bear (Ursa arctos) is another possible contributing factor to population declines of burrow nesters along this coast. I have seen brown bears swimming from island to island on foraging expeditions. George J. Divoky (personal communication) has found brown bears visiting Ugaiushak Island, which is 13 km from shore. There are other islands between Ugaiushak and the mainland but the shortest route from shore would require one swim of 7 km. The motivation must be strong.
Tufted puffins may shift from colony to colony. This could be an explanation for apparent local population fluctuation, but if so, I am puzzled by the apparent tenacity with which puffins cling to some sites. Their constant occupancy of sites where the vegetative mat is breakaway tundra (Amundsen 1972) or is underlain by sand results in the destruction of these sites. Tufted puffins often cling to them in spite of the fact that they have been reduced to "slums."
My conclusion is that in spite of their large numbers it appears that tufted puffin populations in the Gulf of Alaska probably have been reduced to a level below that of their undisturbed state.
Seabird numbers in the Gulf of Alaska are not static. Generally, they are probably much less abundant than they were when Bering made his voyage of discovery. There are, nonetheless, considerable numbers of seabirds breeding along the coasts of these waters. Some species show signs of recovery from past insults by man. With enlightened management there is still time to preserve the vast natural heritage that they represent and, in many cases, to improve their status.
In attempting to address a complicated subject in short space and a relatively narrow frame of reference, I have certainly erred a number of times. I would like to see the wealth of new data that will be derived from current work applied to this concept. An understanding of past population fluctuations and the underlying perturbations that they reflect is essential for managers faced with the problem of making good decisions on measures to mitigate the potential adverse impact of development.
American Ornithologists' Union. 1931. Check-list of North American birds, 4th ed. American Ornithologists' Union, Baltimore, Md. 530 pp.
American Ornithologists' Union. 1957. Check-list of North American birds, 5th ed. American Ornithologists' Union, Baltimore, Md. 691 pp.
Amundsen, C. C. 1972. Plant ecology of Amchitka Island. Amchitka bio-environmental program. Final Report, Battelle Columbus Lab., Columbus, Ohio. 27 pp.
Bailey, A. M. 1948. Birds of arctic Alaska. Colo. Mus. Nat. Hist. Pop. Publ. 8. 316 pp.
Baird, S. F. 1869. On additions to the bird fauna of North America, made by the scientific corps of the Russo-American Telegraph Expedition. Trans. Chic. Acad. Sci. 1(2):311-325.
Beaglehole, J. C. 1974. The life of Captain James Cook. Stanford Univ. Press, Stanford, Calif. 734 pp.
Bean, T. H. 1882. Notes on birds collected during the summer of 1880 in Alaska and Siberia. Proc. U.S. Natl. Mus. 5:144-173.
Bédard, J. 1976. Coexistence, coevolution and convergent evolution in seabirds: a comment. Ecology 57(1):177-184.
Belopol'skii, L. O. 1961. Ecology of sea colony birds of the Barents Sea. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 346 pp.
Bendire, C. 1895. Notes on the ancient murrelet (Synthliboramphus antiquus) by Chase Littlejohn with annotations. Auk 12(3):270-278.
Bent, A. C. 1963. Life histories of North American diving birds. Dover Publications, Inc., New York. 239 pp.
Bent, A. C. 1964. Life histories of North American petrels, pelicans and their allies. Dover Publications, Inc., New York. 335 pp.
Binford, L. C., B. G. Elliot, and S. W. Singer. 1975. Discovery of the nest and downy young of the marbled murrelet. Wilson Bull. 87(3):303-319.
Bowles, J. H. 1908. Tapeworm epidemic among Washington seabirds. Condor 11:33.
Cahalane, V. H. 1943. Notes on the Kodiak-Afognak Island group. Auk 60(4):536-541.
Cahalane, V. H. 1944. Birds of the Katmai region, Alaska. Auk 61(3):351-375.
Clark, A. H. 1910. The birds collected and observed during the cruise of the United States Fisheries Steamer "Albatross" in the North Pacific Ocean and in the Bering, Okhotsk, Japan and Eastern seas from April to December 1906. Proc. U.S. Natl. Mus. 38:25-74.
Cody, M. L. 1973. Coexistence, coevolution and convergent evolution in seabirds. Ecology 51(1):31-44.
Coues, E. 1874. Birds of the Northwest. U.S. Geological Survey of the Territories, Misc. Publ. 3. 791 pp.
Dall, W. H. 1873. Notes on the avifauna of the Aleutian Islands, from Unalaska eastward. Proc. Calif. Acad. Sci. 5(1):25-35.
Dall, W. H. 1874. Notes on the avifauna of the Aleutian Islands, especially those west of Unalaska. Proc. Calif. Acad. Sci. 5:270-281.
Dall, W. H., and H. M. Bannister. 1869. List of the birds of Alaska, with biographical notes. Trans. Chic. Acad. Sci. 1(2):267-310.
Dement'ev, G. P., and N. A. Gladkov, eds. 1966. Birds of the Soviet Vol. I. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 553 pp.
Dixon, J. S. 1908. Field notes from Alaska. Condor 10:139-143.
East, B. 1943. Seabird cities of the Aleutians. Nat. Hist. 51:64-71.
Fisher, J., and R. M. Lockley. 1954. Seabirds. William Collins and Sons, London. 320 pp.
Friedmann, H. 1934. Bird bones from Eskimo ruins on St. Lawrence Island. J. Wash. Acad. Sci. 24:83-96.
Friedmann, H. 1935. The birds of Kodiak Island, Alaska. Bull. Chic. Acad. Sci. 5(3):13-54.
Friedmann, H. 1937. Bird bones from archeological sites in Alaska. J. Wash. Acad. Sci. 27:431-438.
Gabrielson, I. N. 1940. America's greatest bird concentrations, Part I. Bird Lore 42:497-506.
Gabrielson, I. N. 1941. America's greatest bird concentrations, Part II. Audubon Mag. 43:15-23.
Gabrielson, I. N. 1944. Some Alaskan notes. Auk 61:105-130, 207-287.
Gabrielson, I. N., and F. C. Lincoln. 1959. The birds of Alaska. The Stackpole Company, Harrisburg, Penn. 922 pp.
Golder, F. A. 1925. Bering's voyages, Vol. II. American Geographical Society No. 2. 290 pp.
Grinnell, J. 1910. Birds of the 1908 Alexander Alaskan Expedition with a note on the avifaunal relationships of the Prince William Sound district. Univ. Calif. Publ. Zool. 5:361-428.
Hanna, G. D. 1916. Records of birds new to the Pribilof Islands, including two new to North America. Auk 33:400-403.
Howell, J. C. 1948. Observations on certain birds of the region of Kodiak Island. Auk 65:352-358.
Hrdlicka, A. 1945. The Aleutian and Commander Islands and their inhabitants. Wistar Institute Anatomical Biology, Philadelphia. 630 pp.
Isleib, M. E., and B. Kessel. 1973. Birds of the North Gulf Coast-Prince William Sound region, Alaska. Biol. Pap. Univ. Alaska No. 14. 148 pp.
Jaques, F. L. 1930. Water birds observed on the Arctic Ocean and the Bering Sea in 1928. Auk 47:353-366.
Jochelson, W. 1968. History, ethnology and anthropology of the Aleut. Carnegie Inst. Wash. Publ. 432. 91 pp.
Johnson, R. A. 1938. Predation of gulls in murre colonies. Wilson Bull. 50(3):161-176.
Johnson, R. A. 1941. Nesting behavior of the Atlantic murre. Auk 58(2):152-163.
Kozlova, E. V. 1961. Charadriiformes, Suborder Alcae. Fauna of USSR: Birds 2:1-140. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 140 pp.
Lockley, R. M. 1962. Puffins. The Natural History Library, Anchor Books, Doubleday and Company, Inc., Garden City, N. Y. 222 pp.
Murie, O. J. 1959. Fauna of the Aleutian Islands and the Alaska Peninsula. U.S. Fish Wildl. Serv., N. Am. Fauna 61. 406 pp.
Nelson, E. W. 1887. Report upon natural history collections made in Alaska between the years 1877 and 1881. U.S. Army Signal Corps. Arct. Ser. Pub. 3.
Nichols, J. T. 1927. Tubinares of the Northwest Coast. Auk 44(3):326-328.
Orth, D. J. 1967. Dictionary of Alaska place names. Geol. Surv. Prof. Pap. 567. 1084 pp.
Palmer, R. S. 1962. Handbook of North American Birds. Vol. I. Yale University Press, New Haven, Conn. 567 pp.
Patten, S. M., Jr. 1976. Sympatry and interbreeding of herring and glaucous-winged gulls in southern Alaska. Pac. Seabird Group Bull. 3(1):25-26.
Pefaur, J. E. 1974. Egg-neglect in the Wilson's storm petrel. Wilson Bull. 87(1):16-22.
Peterson, R. T., and J. Fisher. 1955. Wild America. Weather Vane Books, New York. 434 pp.
Rausch, R. L. 1958. The occurrence and distribution of birds on Middleton Island, Alaska. Condor 60:227-242.
Schlegel, H. 1862-64. A catalogue of birds in the Dresden Museum of Natural History, Leyden. Dresden Museum of Natural History, 7 Vols.
Sowl, L. W., and J. C. Bartonek. 1974. Seabirds—Alaska's most neglected resource. Trans. N. Am. Wildl. Natl. Resour. Conf. 39:117-125.
Spring, L. 1971. A comparison of functional and morphological adaptions in the common murre (Uria aalge) and thick-billed murre (Uria lomvia). Condor 73:1-27.
Stejneger, L. 1885. Results of ornithological explorations in the Commander Islands and in Kamtschatka. Bull. U.S. Natl. Mus. 29:1-382.
Stresemann, E. 1949. Birds collected in the North Pacific area during Captain James Cook's last voyage (1778 and 1779). Ibis 91:244-255.
Swartz, L. G. 1966. Sea-cliff birds. Pages 611-678 in N. J. Wilimovsky and J. N. Wolf, eds. Environment of the Cape Thomson Region, Alaska. U.S. Atomic Energy Commission, Oak Ridge, Tenn.
Thoresen, A. C. 1964. The breeding behavior of the Cassin's auklet. Condor 66:456-476.
Townsend, C. H. 1913. The crested auklet. Bird-Lore 15:133-136.
Tuck, L. M. 1960. The Murres. Queen's Printer, Ottawa. 260 pp.
Turner, L. M. 1885. Notes on the birds of the Near Islands, Alaska. Auk 2:154-159.
Turner, L. M. 1886. Contributions to the natural history of Alaska. Part V, Birds. U.S. Army, Signal Corps, Washington, D.C. Pages 115-196.
Udvardy, M. D. F. 1963. Zoogeographic study of the Alcidae. Pages 85-111 in J. L. Gressitt, ed. Pacific Basin biogeography, a symposium. Pac. Sci. Congr. Proc. 10.
U.S. Bureau of Sport Fisheries and Wildlife. 1973. Semidi Islands wilderness report. Semidi Islands, National Wildlife Refuge. (Unpublished report)
U.S. Department of Commerce. 1964. United States Coast Pilot, No. 9, Pacific and Arctic Coasts, Cape Spencer to the Beaufort Sea. Coast and Geodetic Survey, Washington, D.C. 330 pp.
Walker, E. P. 1923. Definite breeding record for the Aleutian tern in southern Alaska. Condor 25:113-117.
Willet, G. 1914. Birds of Sitka and vicinity, Southeastern Alaska. Condor 16:71-91.
Willet, G. 1918. Bird notes from Forrester Island, Alaska. Condor 20:85.
Williamson, F. S. L., and L. S. Peyton. 1963. Interbreeding of glaucous-winged herring gulls in the Cook Inlet Region, Alaska. Condor 65:28.
by
David A. Manuwal
College of Forest Resources
University of Washington
Seattle, Washington 98195
and
R. Wayne Campbell
Provincial Museum
Victoria, British Columbia, Canada
Current breeding seabird population estimates, nest-site preferences, and population changes are reviewed for southeastern Alaska, British Columbia, and Washington. There are 19 species of seabirds and a minimum of 216,566 pairs breeding in British Columbia and Washington. There are limited data on breeding populations for southeastern Alaska. Species diversity ranges from 17 species in Alaska to 15 species in British Columbia and 14 species in Washington. Eighty percent of all British Columbia seabirds breed on the east coast of Queen Charlotte Islands and the northwest coast of Vancouver Island. The three most numerous species in British Columbia are the fork-tailed storm-petrel, Oceanodroma furcata (31.3%); Cassin's auklet, Ptychoramphus aleuticus (24.6%); and ancient murrelet, Synthliboramphus antiquus (12.5%). In Washington, 74% (43,274 pairs) of the seabird population resides on the Olympic coast; the remaining 26% are in the San Juan Island area. About 54% of this population consists of the common murre (Uria aalge) and rhinoceros auklet (Cerorhinca monocerata). The rhinoceros auklet and glaucous-winged gull (Larus glaucescens) make up 97% of the total seabird population of the San Juan Islands. About 68% of all seabirds on the northeastern Pacific coast are nocturnal, burrow or rock crevice-nesting species. Currently available population data are inadequate to determine significant changes in population density for most species. Suggested topics for future research are presented.
The purpose of this paper is to discuss the known distribution, habitat, abundance, and status of breeding seabirds of the Alexander Archipelago in southeastern Alaska, the Province of British Columbia, Canada, and the State of Washington.
Even though several studies of the breeding biology of several seabird species in this area have been published, there have been few published surveys of known breeding colonies. In British Columbia the most extensive work has been done by the British Columbia Provincial Museum and the University of British Columbia (Drent and Guiguet 1961). Gabrielson and Lincoln (1959) summarized the available literature on Alaskan birds up to about 1958. Since then, no extensive surveys have been conducted in southeastern Alaska. The U.S. Department of the Interior (1972), in its environmental impact statement for the Trans-Alaska Pipeline, presented additional information on the seabirds of other parts of Alaska. In Washington, there are no published comprehensive surveys except those of Kenyon and Scheffer (1961) and unpublished surveys by the U.S. Fish and Wildlife Service and the University of Washington.
| Family | Common name | Regions | |||
|---|---|---|---|---|---|
| British Columbia | Washington | Southeastern Alaska | Total forms | ||
| Hydrobatidae | Storm-petrels | 2 | 2 | 2 | 2 |
| Phalacrocoracidae | Cormorants | 3 | 3 | 1 | 3 |
| Haematopodidae | Oystercatchers | 1 | 1 | 1 | 1 |
| Laridae | Gulls and terns | 2 | 2 | 3 | 4 |
| Alcidae | Auks, murres, puffins | 6 | 7 | 9 | 9 |
| Total | 14 | 15 | 16 | 19 | |
There are 19 species of seabirds that breed along the Pacific coast of southeastern Alaska, British Columbia, and Washington (Table 1). Southeastern Alaska has the largest number (17) of species. Errors in species identification are most likely with the Larus gulls, particularly in southeastern Alaska where the herring gull (L. argentatus) and glaucous-winged gull (L. glaucescens) breed in mixed colonies (Patten and Weisbrod 1974). A similar situation exists in Washington where the western (L. occidentalis) and glaucous-winged gulls intergrade (Scott 1971). Brandt's (Phalacrocorax penicillatus) and double-crested cormorants (P. auritus) are often difficult to identify from the air. This would be a problem in Washington and the southwest coast of Vancouver Island, where the two species are locally sympatric.
The area under consideration is the 400-km-long Alexander Archipelago (Fig. 1). This complex pattern of islands, bays, and inlets is characterized by extremely high precipitation and typical cool marine temperatures. Average annual precipitation in the Sitka area is 245.4 cm (1931-60), and the average annual temperature is 6.3°C (U.S. Weather Bureau 1974). As a consequence of this cool, humid environment, most of the islands are densely covered with conifers, chiefly Sitka spruce (Picea sitkensis) and hemlock (Tsuga heterophylla), and an almost impenetrable shrub cover composed of salmonberry (Rubus spectabilis), elderberry (Sambucus callicaipa), devil's club (Echinopanax horridus), and three species of Vaccinium (Heath 1915).
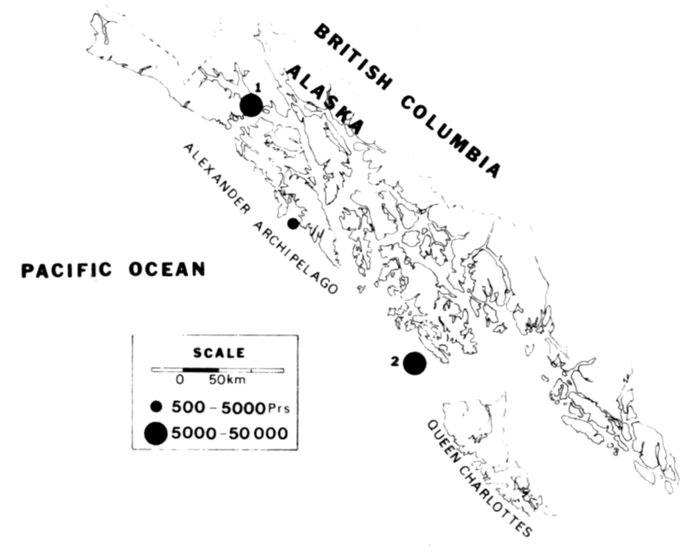
Fig. 1. Map of southeastern Alaska showing major seabird breeding colonies: 1—North Marble Island; 2—Forrester Island.
There are 16 species of marine birds breeding in the Alexander Archipelago. The major seabird breeding colonies are located at Glacier Bay and at St. Lazaria, Hazy, and Forrester islands (Fig. 1; Table 2). Published surveys of these colonies are available only for St. Lazaria (Willett 1912) and Forrester islands (Heath 1915; Willett 1915). Several authors have reported on seabirds from surrounding areas (Grinnell 1897, 1898, 1909; Swarth 1911, 1922, 1936; Patten 1974). There have been no surveys of seabirds of southeastern Alaska since before the 1940's (J. G. King, Jr., personal communication). However, since census data are available for only two colonies, we discuss them in more detail.
| Bird species | St. Lazaria Island | Forrester Island | ||
|---|---|---|---|---|
| Number of pairs | Percent of total | Number of pairs | Percent of total | |
| Fork-tailed storm-petrel | 2,000 | 8.0 | 10,000 | 6.0 |
| Leach's storm-petrel | 20,000 | 80.0 | 50,000 | 30.0 |
| Pelagic cormorant | 150 | 0.6 | 150 | 0.0 |
| Black oystercatcher | 4 | 0.0 | 50 | 0.0 |
| Glaucous-winged gull | 300 | 1.2 | 8,000 | 4.8 |
| Herring gull | 220 | 0.0 | ||
| Common murre | 300 | 1.2 | 20,000 | 12.0 |
| Pigeon guillemot | 150 | 0.6 | 300 | 0.0 |
| Ancient murrelet | 20,000 | 12.0 | ||
| Cassin's auklet | 2,000 | 1.2 | ||
| Rhinoceros auklet | 75 | 0.0 | 20,000 | 12.0 |
| Horned puffin | 12 | 0.0 | 1,100 | 0.7 |
| Tufted puffin | 2,000 | 8.0 | 35,000 | 21.0 |
| Total | 24,991 | 166,820 | ||
The studies by Willett (1912, 1915) and Heath (1915) provide some base-line information on species composition and abundance with which future studies on St. Lazaria and Forrester islands can be compared (Table 2). The somewhat greater species diversity on Forrester Island is primarily due to its greater size and more suitable soil type for ancient murrelets (Synthliboramphus antiquus) and Cassin's auklets (Ptychoramphus aleutica), species that are absent on St. Lazaria. Storm-petrels (Oceanodroma spp.) are the most numerous species on both islands, but there are proportionately more storm-petrels (88%) on St. Lazaria than on Forrester (36%). On the other hand, there are many large, burrowing alcids on Forrester Island. Nearly a third of the birds on Forrester are rhinoceros auklets (Cerorhinca monocerata), tufted puffins (Lunda cirrhata), and horned puffins (Fratercula corniculata).
The species composition of seabirds breeding on other islands is similar to that found on Forrester and St. Lazaria islands but less abundant. In Glacier Bay, for example, the only population data available are those provided by Patten (1974) for North Marble Island: pelagic cormorants, Phalacrocorax pelagicus (30 pairs); black oystercatchers, Haematopus bachmani (8); herring gulls (7); glaucous-winged gulls (500); common murres, Uria aalge (18); pigeon guillemots, Cepphus columba (60); horned puffins (4); and tufted puffins (30).
At the present time, it is impossible to draw any conclusions about changes in population density and distribution for most of the seabirds breeding in southeastern Alaska. Adequate data are available only for St. Lazaria and Forrester islands where Willet and Heath provided the only early extensive census data for this part of Alaska.
The rugged British Columbia coastline is characterized by 930 km of islands and inlets (Figs. 2, 3). With the exception of the inner southern portion, this coast is mostly uninhabited. The physical characteristics of the offshore islands are similar to those found off the Washington coast. Descriptions of some of these islands and the 15 species of breeding seabirds on them have been given by Drent and Guiguet (1961), Guiguet (1971), and Summers (1974).
A detailed analysis of British Columbia seabirds is not presented here since a more thorough analysis is in preparation by R. W. Campbell and R. H. Drent (manuscript). Instead, we present seabird population estimates available for the Province up to the summer of 1975; Tables 3 and 4 summarize these estimates for the five major portions of coastal British Columbia. The coast of British Columbia contains a myriad of small islands where there may be small numbers of breed[Pg 76]ing seabirds. Many of these have not been censused and are too numerous to include in Tables 3 and 4.
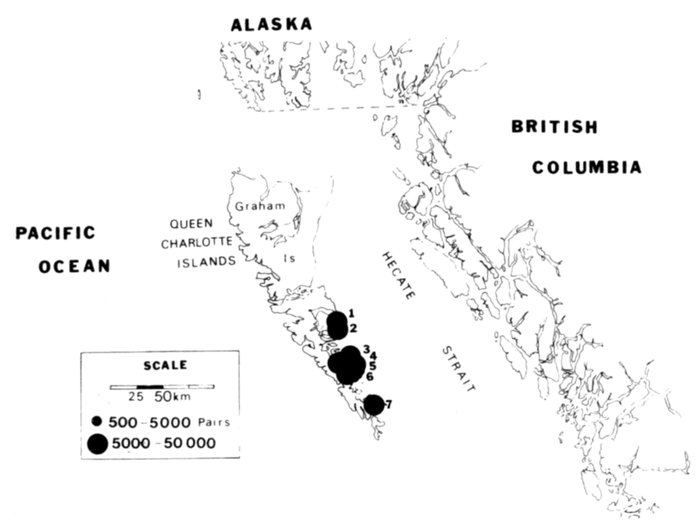
Fig. 2. Map of northern British Columbia showing sites of major seabird breeding colonies: 1—Skedans Island; 2—Limestone Island; 3—Agglomerate Island; 4—Bischoff Island; 5—Ramsey Island; 6—Alder Island; 7—Rankins Island.
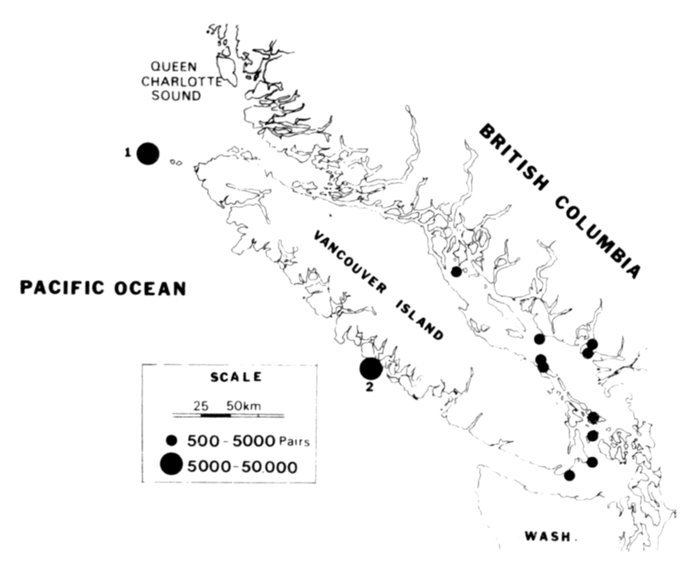
Fig. 3. Map of southern British Columbia showing sites of major seabird breeding colonies: 1—Triangle Island; 2—Cleland Island.
More than half of the breeding seabirds in British Columbia are found on the east coast of the Queen Charlotte Islands, and the fork-tailed storm-petrel (Oceanodroma furcata) comprises more than half of that total. However, new unpublished data (K. Vermeer) for Triangle Island and the northwest coast of Vancouver Island indicate that the population figures in Table 3 for this area are underestimates. Nevertheless, these two regions have nearly 80% of all the breeding seabirds in the Province. This results from the very large populations of the rhinoceros auklet and tufted puffin on Triangle Island and the fork-tailed storm-petrel, ancient murrelet, and Cassin's auklet on various islands on the east coast of the Queen Charlotte Islands (Table 3).
Continuing surveys of breeding seabirds are being conducted by personnel of the British Columbia Provincial Museum and the Canadian Wildlife Service.
For this report, we have distinguished two major geographical areas in Washington where breeding seabirds are found—the western coast of the Olympic Peninsula and the San Juan Islands, including the Strait of Juan de Fuca.
On the Olympic Peninsula, seabirds breed on the offshore rocks, islands, and precipitous cliffs from Copalis Beach to Cape Flattery (Fig. 4). The offshore rocks and islands throughout this area (except Tatoosh Island) are now included in the Washington Islands National Wildlife Refuge. Most of the larger rocks and islands have dense stands of salmonberry, salal, and grasses, and a few support stands of stunted conifers (Fig. 5); most are inaccessible to man. The adjacent coast is dominated by the Olympic rain forest where the mean annual precipitation is about 337.1 cm (U.S. Weather Bureau 1956, 1965a, 1965b).
Because the San Juan Islands lie northeast of the Olympic Peninsula and east of Vancouver Island (Fig. 6) they are in a rain shadow; however, because of highly variable topography and aspect, most islands have a diverse assemblage of plant communities (Franklin and Dyrness 1973). Exposed south-facing slopes are occupied by grassland vegetation and frequently by scattered trees, usually Pseudotsuga menziesii and Arbutus menziesii. Most of the seabird colonies are located on rather small exposed islands with[Pg 77] short, grassy, shrubby vegetation. In general, these islands are not suitable for burrowing species.
| Bird species | Straits of Georgia and Juan de Fuca | Southwest coast of Vancouver Island | Northwest coast of Vancouver Island | West coast of Queen Charlotte Island | East coast of Queen Charlotte Island | Prince Rupert to Queen Charlotte Island | Total birds | Percent of total |
|---|---|---|---|---|---|---|---|---|
| Fork-tailed storm-petrel | + | + | 1,050 | 97,100 | + | 98,160 | 31.3 | |
| Leach's storm-petrel | 10,000 | + | 1,800 | 180 | 750 | 12,730 | 4.1 | |
| Double-crested cormorant | 1,058 | 0 | 0 | 0 | 0 | 0 | 2,116 | >1.0 |
| Brandt's cormorant | 370 | 0 | 0 | 0 | 0 | 370 | >1.0 | |
| Pelagic cormorant | 2,174 | 336 | 3,350 | 1,456 | 496 | 12 | 9,998 | 3.2 |
| Glaucous-winged gull | 10,123 | 6,870 | 600 | 412 | 866 | 540 | 29,534 | 9.4 |
| Common murre | 16 | 3,000 | 0 | 0 | 0 | 3,016 | 1.0 | |
| Pigeon guillemot | 1,029 | 204 | 250 | 358 | 1,458 | 1,650 | 5,978 | 1.9 |
| Ancient murrelet | 0 | 0 | 200 | 42,150 | 4 | 42,354 | 13.5 | |
| Cassin's auklet | + | 50,000 | + | 26,500 | 450 | 76,950 | 4.6 | |
| Rhinoceros auklet | 1,200 | + | 10,000 | 300 | 200 | 11,700 | 3.7 | |
| Tufted puffin | 1 | 154 | 20,000 | 190 | 0 | 42 | 20,388 | 6.5 |
| Total | 14,385 | 19,150 | 77,200 | 15,466 | 169,050 | 3,648 | 313,294 | 81.2 |
| Geographic location | Population estimate | Percent of total |
|---|---|---|
| Straits of Georgia and Juan de Fuca | 14,385 | 9.2 |
| Southwest Coast of Vancouver Island | 9,575 | 6.1 |
| Northwest Coast of Vancouver Island | 38,600 | 24.6 |
| West Coast of Queen Charlotte Island | 7,733 | 4.9 |
| East Coast of Queen Charlotte Island | 84,530 | 54.0 |
| Prince Rupert to Queen Charlotte Strait | 1,824 | 1.2 |
| Total | 156,647 | 100.0 |
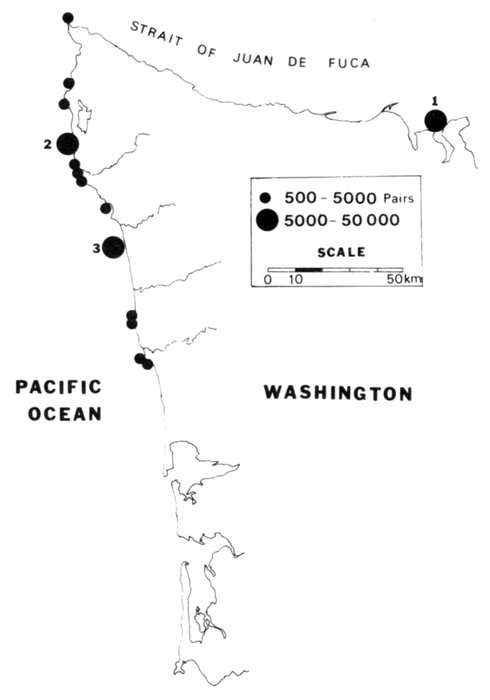
Fig. 4. Map of the Olympic Peninsula of Washington State showing sites of major seabird breeding colonies: 1—Protection Island; 2—Carroll Island; 3—Destruction Island.
In the Strait of Juan de Fuca, the two most important sites are Smith and Protection islands. Both are composed of glacial deposits and heavy sod that has developed under dense grassy vegetation (Fig. 7). Consequently, these two islands support most of the burrowing seabirds in the region. Unfortunately, both islands have historically been subjected to much human disturbance (Richardson 1961; Manuwal 1974).
The existing information on seabird colonies in both the coastal and San Juan Island areas has been largely derived from aerial surveys by the U.S. Fish and Wildlife Service. These surveys are inherently biased toward surface-nesting species such as gulls and cormorants. Population estimates for guillemots, auklets, storm-petrels, and puffins are less accurate. Some additional information obtained by direct island visitation has been provided by Kenyon and Scheffer (1961), Richardson (1961), Thoresen and Galusha (1971), G. Eddy (unpublished data), and D. A. Manuwal (unpublished data). Although other accounts of Washington seabirds are available, the references listed above are specifically oriented toward population assessment.
Despite the large number of offshore rocks, islets, and islands along the Pacific coast of Washington, significant seabird colonies are present only on about 30 islands. Since Table 5 summarizes the population estimates for 12 species of seabirds breeding on 24 major sites, it represents only the majority and not the total number of breeding seabirds on the Pacific coast of Washington. About 74% of the entire Washington seabird population resides on the coastal rocks and islands.
Major colony sites with more than 2,500 breeding pairs are Grenville Arch,[Pg 79] Willoughby Rock, Destruction Island, Cake Rock, Carroll Island, and Bodelteh Island. More intensive censusing, especially of nocturnal burrowing species will undoubtedly raise the population estimates for these and other islands off the coast. About 54% of the total coastal population is composed of the common murre and rhinoceros auklet.

Fig. 5. Photograph of Destruction Island off the coast of Washington.
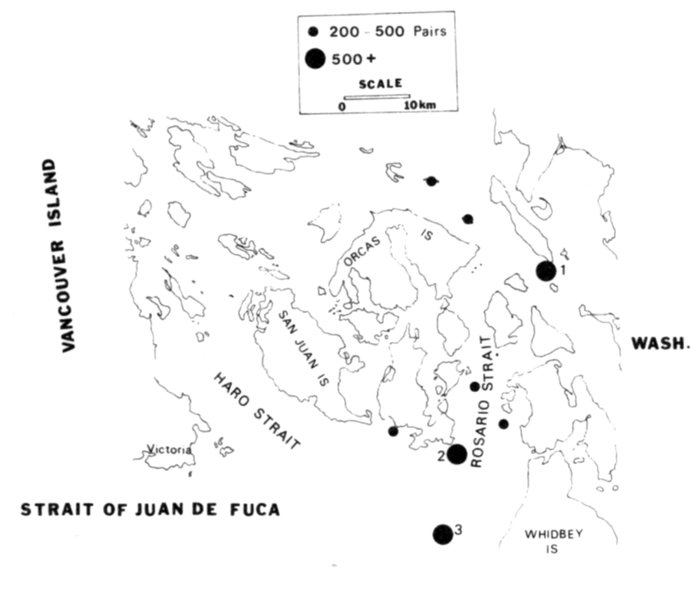
Fig. 6. Map of the San Juan Archipelago showing sites of major seabird breeding colonies: 1—Viti Rocks; 2—Colville Island; 3—Smith Island.
There are about 86 actual or potential seabird colony sites in this area; 25 (30%) are now considered important. Eleven islands are under Federal protection as National Wildlife Refuges. Part of Protection Island is owned by the Washington State Game Department to protect the largest rhinoceros auklet colony in the State. Most colony sites are on small islands with poorly developed soil which prevents burrowing species from using them. Consequently, the dominant species are surface nesters (such as gulls and cormorants) and rock-crevice nesters (like the pigeon guillemot). In all, about 31,000 seabirds of 7 species breed in the San Juan Island area. Breeding seabird population estimates for 49 of the 86 nesting sites are given in Table 6. Even though this does not represent all the colonies, it covers the most important islands and those islands where there appears to be potential for seabird breeding.

Fig. 7. Photograph of Smith Island in the Strait of Juan de Fuca, Washington. The glacial deposits are evident from the composition of the cliff faces.
| Breeding site | Species | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Storm-petrels | Cormorants | Black oyster-catcher | Gulls | Common murre | Pigeon guillemot | Auklets | Tufted puffin | Total | |||||||
| Fork-tailed | Leach's | Unidentified | Double-crested | Brandt's | Pelagic | Western | Glaucous-winged western | Cassin's | Rhinoceros | ||||||
| Copalis Rock | — | — | 15 | — | — | — | — | 30 | — | — | — | — | — | — | 45 |
| Point Grenville | — | — | — | 60 | 30 | 80 | — | 165 | 40 | 1,100 | — | — | — | — | 1,475 |
| Grenville Arch | — | — | — | 30 | 20 | — | 1 | 60 | — | 3,000 | 4 | — | — | 3 | 3,118 |
| Flat Rock | — | — | 30 | — | — | — | — | — | 60 | 300 | — | — | — | — | 390 |
| Split Rock | — | — | — | 100 | — | — | 1 | 150 | — | 2,100 | 4 | — | — | — | 2,355 |
| Willoughby Rock | — | — | — | 80 | 40 | 15 | — | 150 | — | 3,000 | — | — | — | 25 | 3,310 |
| South Rock | — | — | — | — | — | 40 | — | — | 50 | — | — | — | — | — | 90 |
| Abbey Islet | — | — | — | — | — | 30 | 3 | — | 50 | — | — | — | — | 10 | 93 |
| Destruction Island | — | — | — | — | — | — | 12 | 350 | — | — | 25 | — | 10,940 | 350 | 11,677 |
| Middle Rock | — | — | — | — | — | 25 | — | — | 25 | — | 50 | — | — | — | 100 |
| North Rock | — | — | — | — | — | — | — | — | 25 | — | — | — | — | — | 25 |
| Alexander Island | — | — | — | — | — | 50 | 5 | — | 225 | — | — | — | — | 1,550 | 1,830 |
| Rounded Island | — | — | — | — | — | 25 | — | 25 | — | — | 1 | — | — | — | 51 |
| Giant's Graveyard | — | — | — | — | — | 10 | — | — | — | 150 | — | 50 | 150 | — | 360 |
| Quillayute Needles | — | — | — | 50 | 50 | 50 | — | — | 150 | 900 | — | — | — | 350 | 1,550 |
| James Island | — | 30 | — | — | — | 40 | — | — | 150 | 750 | 40 | — | — | 20 | 1,030 |
| Cake Rock | — | 500 | — | — | — | 150 | — | — | 600 | 300 | 12 | — | 50 | 1,000 | 2,612 |
| Sealion Rock | — | — | — | 70 | — | 30 | — | — | 250 | — | — | — | — | 5 | 355 |
| Carroll Island | — | 3,100 | — | — | — | 100 | 3 | — | 550 | — | — | 25 | 250 | 2,400 | 6,428 |
| Ball Rock | — | — | — | — | — | 50 | 7 | — | 150 | — | — | — | — | 750 | 957 |
| White Rock | — | — | — | — | — | 100 | — | — | 75 | 250 | — | — | — | 100 | 525 |
| Ozette Island | — | — | — | — | — | — | 1 | — | 15 | — | — | — | — | — | 16 |
| Bodelteh Island | 1,900 | — | — | — | — | 100 | 2 | — | 300 | — | 5 | — | — | 750 | 3,057 |
| Tatoosh Island | — | 25 | — | — | — | 100 | — | — | 1,500+ | 100 | 20 | 25? | 25? | 30 | 1,825 |
| Total | 1,900 | 3,655 | 45 | 390 | 140 | 995 | 35 | 930 | 4,215 | 11,950 | 161 | 100 | 11,415 | 7,343 | 43,274 |
| Breeding site | Species | |||||||
|---|---|---|---|---|---|---|---|---|
| Cormorants | Black oyster-catcher | Glaucous-winged gull | Pigeon guillemot | Tufted puffin | Rhinoceros Auklet | Total | ||
| Double-crested | Pelagic | |||||||
| Bare Island | — | 50 | 1 | 120 | + | — | 2 | 173 |
| Barren Island | — | — | — | — | — | — | — | 0 |
| Battleship Island | — | — | — | — | — | — | — | 0 |
| Bird Rocks | 30 | — | + | 320 | — | — | — | 350 |
| Cactus Island | — | — | 1 | — | — | — | — | 1 |
| Castle Island | — | — | — | — | 30 | — | — | 30 |
| Colville Island | — | 40 | 1 | 1,000 | — | — | — | 1,041 |
| Danger Island | — | — | — | 125 | 7 | — | — | 132 |
| Decatur Island | — | — | — | — | — | — | — | 0 |
| Eliza Island | — | — | — | 3 | 1 | — | — | 4 |
| Eliza Rock | — | — | — | 1 | — | — | — | 1 |
| Flat Top Island | — | — | — | — | + | — | — | + |
| Flower Island | — | 17 | — | 90 | — | — | — | 107 |
| Goose Island | — | — | — | 60 | — | — | — | 60 |
| Gull Rock | — | — | + | 125 | 7 | — | — | 132 |
| Gull Reef | — | — | — | — | — | — | — | 0 |
| Hall Island | — | — | 1 | 275 | — | — | — | 276 |
| Harbor Rock | — | — | — | — | — | — | — | 0 |
| Iceberg Island | — | — | — | — | — | — | — | 0 |
| Johns Island | — | — | 1 | — | — | — | — | 1 |
| Long Island | — | — | 8 | 80 | — | — | — | 88 |
| Low Island | — | — | 1 | 75 | 17 | — | — | 93 |
| Lummi Rocks | — | — | — | 4 | — | — | — | 4 |
| Matia Island | — | — | — | — | + | — | — | + |
| Mummy Rocks | — | — | — | 55 | — | — | — | 55 |
| Minor Island | — | — | — | 100 | — | — | — | 100 |
| O'Neal Island | — | — | — | — | — | — | — | 0 |
| Patos Island | — | — | — | 20 | + | — | — | 20 |
| North Peapod Island | — | — | 1 | 220 | 2 | — | — | 223 |
| South Peapod Island | — | — | 1 | 75 | 2 | — | — | 78 |
| Pearl Island | — | — | — | — | — | — | — | 0 |
| Pointer Island | — | — | — | 58 | 2 | — | — | 60 |
| Protection Island | 3 | 110 | 3 | 1,500 | 30 | 9,200 | 35 | 10,881 |
| Puffin Island | — | — | 1 | 350 | 15 | — | — | 366 |
| Ripple Island | — | — | — | — | — | — | — | 0 |
| Sentinel Island | — | — | — | — | 10 | — | — | 10 |
| Sentinel Rock | — | — | 1 | — | — | — | — | 1 |
| Skip Jack Island | — | — | — | 75 | 20 | — | — | 95 |
| Smith Island | — | 20 | 6 | 10 | 30 | 600 | — | 666 |
| Speiden Island | — | — | — | — | — | — | — | 0 |
| South Sister Island | 2 | 11 | 1 | 131 | — | — | — | 145 |
| Middle Sister Island | — | — | 1 | 22 | — | — | — | 23 |
| North Sister Island | — | — | 2 | 412 | 3 | — | — | 417 |
| Viti Rocks | 29 | 80 | 1 | 387 | 1 | — | — | 498 |
| Waldron Island | — | — | — | — | 2 | — | — | 2 |
| Williamson Rocks | — | 67 | 1 | 346 | 2 | — | — | 416 |
| Whale Island | — | — | 1 | 70 | — | — | — | 71 |
| White Rock | — | — | + | 125 | 13 | — | — | 138 |
| Yellow Island | — | — | — | — | — | — | — | 0 |
| Total per species | 64 | 395 | 34 | 6,234 | 194 | 9,800 | 37 | 16,758 |
| Percent of total population | 0.4 | 2.3 | 0.2 | 37.2 | 1.2 | 58.5 | 0.2 | 100.0 |
The major colony sites with more than 250 breeding pairs are located at Protection and Smith islands, Bird Rocks, Colville Island, Hall Island, North and South Peapod rocks, Puffin Island, North Sisters, Viti Rocks, and Williamson Rocks (Fig. 6). Glaucous-winged gulls are the predominant species on all these islands except Protection and Smith islands, where there are large colonies of rhinoceros auklets. Rhinoceros auklets (65%) and glaucous-winged gulls (32%) make up 97% of the total San Juan Islands seabird population.
Food supply and availability of nest sites are two critically important factors influencing the distribution and abundance of seabirds. Whereas information on general diet composition is known for most seabird species, we know little about the availability of favored seabird prey. The dynamics of seabird food chains is reviewed elsewhere in these proceedings.
The nest-site preferences for seabirds of the northeast Pacific Ocean are given in Table 7, and Table 8 indicates the proportion of seabirds that belong to specific nest-site categories. These preferences, in conjunction with knowledge of the physical characteristics of seabird habitat, permit a partial explanation of the present distribution and abundance of seabirds. For example, if we compare the San Juan Island habitats with those of the Washington coast, it is apparent that there are more cliff-nesting species on the coast. This reflects the physical characteristics of the two habitats. There are few cliffs in the San Juan Islands, and those that exist are very unstable. Colony sites in the San Juan Islands are typically on low, flat islands. Glaucous-winged gulls are the most abundant nesting species there. Coastal islands, on the other hand, are either covered by dense vegetation or are large monolithic chunks of rock with few available flat areas. Population estimates for the Washington coast are heavily biased toward surface nesters, since most of the data have been gathered by aerial surveys. Consequently, the burrow and rock crevice categories are underestimated. The aerial survey is appropriate for only about 43% of the birds nesting on the Washington coast.
| Nest-site type | Bird species |
|---|---|
| Burrow-rock crevice | |
| Diurnal | Pigeon guillemot |
| Horned puffin | |
| Tufted puffin | |
| Nocturnal | Fork-tailed storm-petrel |
| Leach's storm-petrel | |
| Kittlitz's murrelet | |
| Ancient murrelet | |
| Cassin's auklet | |
| Rhinoceros auklet | |
| Open nests | |
| Flat or slope | Double-crested cormorant |
| Brandt's cormorant | |
| Glaucous-winged gull | |
| Herring gull | |
| Western gull | |
| Black oystercatcher | |
| Cliff face | Pelagic cormorant |
| Common murre | |
| Black-legged kittiwake | |
| Tree branch | Marbled murrelet |
Northern and southern British Columbia provide another good example of habitat availability as revealed through seabird population estimates. The population data are more comprehensive and have largely been gathered by island visitations. The islands in the northern portion are heavily vegetated and many have well-developed soil into which storm-petrels, auklets, and murrelets can burrow. Indeed, 96% of the seabird population consists of nocturnal, burrow-nesting species. In southern British Columbia, however, there are more open-nest species, particularly glaucous-winged gulls and cormorants.
Overall, 68% of the breeding seabirds found along the northeastern Pacific coast are nocturnal and nest in burrows or rock crevices (Table 8). The most conspicuous nesting birds such as gulls, cormorants, and murres, comprise only 22% of the total population. Consequently, our current estimates of breeding seabirds still underestimate the more secretive, nocturnal, burrow-nesting species.
| Site | Estimated number of pairs | Percent of population | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| British Columbia | San Juan Islands | Washington coast | British Columbia | San Juan Islands | Washington coast | |||||
| Northern | Southern | Northern | Southern | Population | Percent | |||||
| Burrow-rock crevice | ||||||||||
| Diurnal | 1,849 | 11,334 | 231 | 7,504 | 2.0 | 18.1 | 1.4 | 17.3 | 20,918 | 9.7 |
| Nocturnal | 90,347 | 30,600 | 9,800 | 17,070 | 96.0 | 48.9 | 58.6 | 39.4 | 147,817 | 68.1 |
| Open nests | ||||||||||
| Flat or slope | 909 | 15,101 | 6,298 | 5,755 | 1.0 | 24.2 | 37.6 | 13.3 | 28,063 | 13.0 |
| Cliff face | 982 | 5,525 | 395 | 12,945 | 1.0 | 8.8 | 2.4 | 30.0 | 19,847 | 9.2 |
| Total | 94,087 | 62,560 | 16,724 | 43,274 | 216,645 | |||||
The available data are inadequate to detect changes in population distribution and density for most species (Table 9). In Washington, for instance, limited unsubstantiated information suggests an overall decline of the double-crested cormorant and tufted puffin in the San Juan Island area. Likewise, there seems to be an increase in glaucous-winged gulls there. In British Columbia, Drent and Guiguet (1961) were able to detect changes in some species. For example, they noted increases in the double-crested cormorant, pelagic cormorant, and glaucous-winged gull. No change was observed in the tufted puffin. Since then, the Brandt's cormorant has established a colony in Barkley Sound (Guiguet 1971). The data in southeastern Alaska are inadequate for all species except, perhaps, the Cassin's auklet which Gabrielson and Lincoln (1959) reported to be declining throughout Alaska. In short, no definitive statements can now be made concerning changes in seabird population numbers.
Storm-petrels are especially difficult to census because they are nocturnal, and the burrows and rock crevices where they breed are often difficult to locate, especially in mixed-species colonies. The census data are inadequate to determine whether there have been changes in population density and distribution. Indeed, the biology of this species is perhaps the least known of the North Pacific colonial seabirds. In southeastern Alaska, this species is outnumbered by at least 5 to 1 by the Leach's storm-petrel (Oceanodroma leucorhoa). The reasons for this are poorly understood. There is some evidence that the numbers of breeding fork-tailed storm-petrels on Forrester Island may fluctuate drastically from one year to the next (Gabrielson and Lincoln 1959).
Of the two subspecies of this petrel (O. l. leucorhoa and O. l. beali), only O. l. beali is found in southeastern Alaska. The leucorhoa subspecies is more northerly in distribution. Where both fork-tailed and Leach's storm-petrels are sympatric, Leach's predominates; however, this relationship becomes more unpredictable in British Columbia and Washington. This species is undoubtedly widespread in the forested islands of the Alexander Archipelago.
The double-crested cormorant apparently does not breed in southeastern Alaska since Willett (1912), Gabrielson and Lincoln (1959), and S. Patten (personal communication) do not report breeding colonies for the area. The largest populations occur in southern British Columbia principally in the Gulf Islands, where 71% of all breeding double-crested cormorants are found (Table 10). According to Jewett et al. (1953), this species was less common in Puget Sound than was Brandt's cormorant, but is certainly not the case today (D. A. Manuwal, unpublished data). The only common cormorants in the San Juan Islands are the pelagic and double-crested species. The double-crested cormorant seems to have declined in numbers on both coastal and inland waters. On the basis of his observations, R. W. Campbell believes that this species is increasing in British Columbia.
| Family and species | Common name | Washington | British Columbia | Southeastern Alaska | |||
|---|---|---|---|---|---|---|---|
| Presence | Status | Presence | Status | Presence | Status | ||
| Hydrobatidae | |||||||
| Oceanodroma furcata | Fork-tailed storm-petrel | X | ? | X | ? | X | ? |
| O. leucorhoa | Leach's storm-petrel | X | ? | X | - | X | ? |
| Phalacrocoracidae | |||||||
| Phalacrocorax auritus | Double-crested cormorant | X | - | X | - | ||
| P. penicillatus | Brandt's cormorant | X | ? | X | 0 | ? | |
| P. pelagicus | Pelagic cormorant | X | ? | X | + | X | ? |
| Haematopodidae | |||||||
| Haematopus bachmani | Black oystercatcher | X | ? | X | + | X | ? |
| Laridae | |||||||
| Larus glaucescens | Glaucous-winged gull | X | + | X | + | X | ? |
| L. occidentalis | Western gull | X | ? | X | ? | ||
| L. argentatus | Herring gull | X | ? | ||||
| Rissa tridactyla | Black-legged kittiwake | X | ? | ||||
| Alcidae | |||||||
| Uria aalge | Common murre | X | ? | X | - | X | ? |
| Cepphus columba | Pigeon guillemot | X | ? | X | + | X | ? |
| Brachyramphus marmoratus | Marbled murrelet | X | ? | X | ? | X | ? |
| B. brevirostris | Kittlitz's murrelet | X | ? | ||||
| Synthliboramphus antiquus | Ancient murrelet | X | ? | X | ? | ||
| Ptychoramphus aleuticus | Cassin's auklet | X | ? | X | ? | X | - |
| Cerorhinca monocerata | Rhinoceros auklet | X | ? | X | + | X | ? |
| Fratercula corniculata | Horned puffin | X | ? | ||||
| Lunda cirrhata | Tufted puffin | X | - | X | 0 | X | ? |
| Total species | 14 | 15 | 16 | ||||
Brandt's cormorant is the least abundant of the three cormorant species that nest in the study area. Washington is at the northernmost edge of the breeding distribution of this species. Only one more northerly colony exists, on Sartine Island off Vancouver Island (Vermeer et al. 1976). Brandt's cormorant comprises about 85% of the cormorant population in Oregon (U.S. Fish and Wildlife Service, unpublished data). However, in Washington it is only about 9% and in British Columbia 3% of the total cormorant population.
| Bird species | Northern British Columbia | Southern British Columbia | San Juan Islands | Washington coast | Total all regions | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population | Percent | Population | Percent | Population | Percent | Population | Percent | Population | Percent | |
| Fork-tailed storm-petrel | 49,080 | 52.2 | ? | - | 0 | - | 1,900 | 4.4 | 50,980 | 23.5 |
| Leach's storm-petrel | 1,365 | 1.5 | 5,000 | 8.0 | 0 | - | 3,655 | 8.5 | 10,020 | 4.6 |
| Double-crested cormorant | 0 | - | 1,058 | 1.7 | 64 | >0.1 | 390 | >0.1 | 1,512 | >0.1 |
| Brandt's cormorant | 0 | - | 185 | >0.1 | 0 | - | 140 | >0.1 | 325 | >0.1 |
| Pelagic cormorant | 982 | 1.0 | 4,017 | 6.4 | 395 | 2.4 | 995 | 2.3 | 6,389 | 3.0 |
| Glaucous-winged gull | 909 | 1.0 | 13,858 | 22.2 | 6,234 | 37.3 | 4,215 | 9.8 | 25,216 | 11.6 |
| Western gull | 0 | - | ? | - | 0 | - | 930 | 2.2 | 930 | >0.1 |
| Common murre | 0 | - | 1,508 | 2.4 | 0 | - | 11,950 | 27.7 | 13,458 | 6.2 |
| Pigeon guillemot | 1,733 | 1.8 | 1,256 | 2.0 | 194 | 1.2 | 161 | >0.1 | 3,345 | 1.5 |
| Ancient murrelet | 21,177 | 22.5 | 0 | - | 0 | - | 0 | - | 21,177 | 9.8 |
| Cassin's auklet | 13,475 | 14.3 | 25,000 | 40.0 | 0 | - | 100 | >0.1 | 38,575 | 17.8 |
| Rhinoceros auklet | 5,250 | 5.6 | 6,000 | >0.1 | 9,800 | 58.6 | 11,415 | 26.4 | 27,065 | 12.5 |
| Horned puffin | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - |
| Tufted puffin | 116 | >0.1 | 10,078 | 16.1 | 37 | >0.1 | 7,343 | 17.0 | 17,574 | 8.1 |
| Total | 94,087 | 67,960 | 16,724 | 43,194 | 216,566 | |||||
Comparing information in Jewett et al. (1953) with the current situation, it is apparent that there has been a drastic change in the distribution and probably in the numbers of this species in Washington. Today, there are no Brandt's cormorant colonies in the San Juan Islands or Strait of Juan de Fuca. Yet Jewett et al. (1953) reported colonies at Bellingham Bay and on Lopez and Matia islands. We have observed juvenile Brandt's cormorants in the San Juan Islands during the summer. This species may be particularly susceptible to human disturbance, since all three areas listed above are heavily used in the summer for recreation.
The distribution of breeding colonies of the pelagic cormorant is strongly determined by the availability of the steep cliffs on which it constructs its nest. This is the only common cormorant in southeastern Alaska. Throughout its extensive range, this species is generally found breeding in small numbers. Nothing is known about fluctuations in its numbers in Alaska.
This species is common in both British Columbia and Washington; nesting sites are of the same type as those in Alaska except in the San Juan Islands, where 200-300 birds nest on cliff faces composed of glacial deposits. Here, there is frequent nest loss due to slippage off the cliff face; this loss is especially severe on Smith and Protection islands. There do not appear to be any changes in the distribution of pelagic cormorants, but an accurate assessment of abundance is impossible from the data currently available.
The glaucous-winged gull is the characteristic gull of southeastern Alaska and British Columbia. In Washington, it is the dominant gull in the San Juan Island area but interbreeds with the western gull on the Washington outer coast from Tatoosh to Copalis Beach (Scott 1971). In Alaska, it is widely distributed and locally abundant on Forrester Island, St. Lazaria, and throughout Glacier Bay (S. Patten, personal communication). The biology of this species has been extensively studied in the southern part of its range, especially by Vermeer (1963) and James-Veitch and Booth (1954). The only study of the breeding biology of this species in southeastern Alaska is by Patten (1974) for Glacier Bay. Glaucous-winged gulls are apparently increasing in British Columbia (R. W. Campbell, unpublished data) and in Washington (T. R. Wahl, personal communication). This increase is undoubtedly a result of the proximity of breeding colonies to garbage dumps and commercial fishing fleets in both Canada and the United States. Little is known about changes in populations of gulls in southeastern Alaska.
The western gull is the common breeding gull of the Washington outer coast; however, there is increased interbreeding with glaucous-winged gulls northward from Destruction Island to Tatoosh Island. The percentage of glaucous-winged gulls steadily increases until Vancouver Island and the Strait of Juan de Fuca, where western gulls are rare. Population estimates of gulls on the outer coast of Washington are derived primarily from aerial flights. This makes identification of gulls difficult, and in view of the amount of interbreeding, it is probably impossible to classify many of the breeding gulls as to species. Western gulls appear to be increasing in the Grays Harbor area (G. D. Alcorn, personal communication).
The herring gull is typically found in inland Alaska but can be found uncommonly along the coast of southeastern Alaska, where it often forms mixed colonies with glaucous-winged gulls. These two species apparently hybridize where they are sympatric (Williamson and Peyton 1963; Patten and Weisbrod 1974; Patten 1974).
The black-legged kittiwake is found only in the northern portions of southeastern Alaska. It apparently is a common breeding bird in Glacier Bay National Monument (S. M. Patten, Jr., personal communication). No population estimates are available for this species other than that it is locally abundant.
Common murres are common in southeastern Alaska and the coast of Washington but breed only in small numbers in British Columbia and are absent in the San Juan Islands. Since this species usually prefers cliffs or the tops of inaccessible rocks, they are probably limited by island topography in British Columbia, and are most certainly so limited in the San Juan and Gulf Island groups.
In Alaska, common murres breed in unknown numbers in Glacier Bay and in large numbers on St. Lazaria, Forrester, and the Hazy islands. No data on population changes are available for any of the three regions.
The pigeon guillemot is common throughout the region from Cape Fairweather to Washington. Even though it is not truly colonial, it may be locally abundant where there are suitable nest sites. Since these nest sites are usually difficult to find, population estimates are seldom accurate, usually being conservative. It is evident that guillemots appear to be small in number when compared with other seabirds nesting at major colony sites in the north Pacific region (Table 10). This disparity may be exaggerated by the difficulty of censusing guillemots.
Since the marbled murrelet has been found to nest in coniferous forests (Binford et al. 1975), traditional census techniques are unsuitable. This species is common in southeastern Alaska (Gabrielson and Lincoln 1959), in British Columbia (Drent and Guiguet 1961), and in Washington (Jewett et al. 1953).
The difficulties in assessing breeding populations of Kittlitz's murrelet are the same as those for the marbled murrelet. This species nests on the ground at high elevation near the coast (Bailey 1973). The largest concentrations are in the vicinity of Glacier Bay National Monument (Gabrielson and Lincoln 1959). They are not found breeding in Washington or British Columbia.
Ancient murrelets appear to be locally common throughout southeastern Alaska. Their presence is probably strongly dependent upon a suitable soil in which to excavate burrows. The only available population estimates are those by Willett (1915) for Forrester Island (Table 1). Censusing this species is especially difficult because its burrows are easily confused with those of Cassin's auklet. There are no studies of this species in southeastern Alaska; however, it has been well studied in the Queen Charlotte Islands to the south by Sealy (1975).
A synthesis of literature and unpublished observations led Gabrielson and Lincoln (1959) to conclude that Cassin's auklet has greatly decreased in numbers and is not abundant anywhere in Alaska. They also concluded that the colony on Forrester Island (Table 1) was the only well-documented colony in southeastern Alaska. Fishermen in the southeastern Alaska area occasionally see this species (M. E. Isleib, personal communication), but it is apparently still uncommon though more widespread than just Forrester Island. The nocturnal habits and burrowing in dense vegetation makes censusing this species very difficult. Nothing is known about the ecology of this species in Alaska.
Rhinoceros auklets seem to be found breeding only on islands where there is a well-developed soil in which to excavate their extensive burrows. From the limited evidence available, it appears that the largest rhinoceros auklet populations probably are to be found in southeastern Alaska. Willett (1912) found a very large population on Forrester Island (Table 2), and the species has been found in the summer in the Barren Islands east of Kodiak Island (E. P. Bailey, personal communication). More intensive surveys of the Alexander Archipelago will probably reveal other populations of this species.
This species is less common in British Columbia than either Alaska or Washington. A possible reason for this is lack of suitable nesting areas. In Washington, the two largest colonies are at Protection Island in the Strait of Juan de Fuca and Destruction Island on the outer coast. Smaller numbers exist on other coastal islands and on Smith Island in the Strait of Juan de Fuca. The Smith Island colony is an interesting one since it appears that early human disturbance in the late 19th or early 20th century eliminated the species from the island. In their discussion of Smith Island, Jewett et al. (1953) made no mention of auklets, only of puffins and guillemots. Couch (1929) did not record the species in 1925. The colony now numbers about 600 pairs.
Although the horned puffin is one of the most abundant seabirds in other parts of Alaska, it is much less abundant in the southeastern portion. In addition to the information discussed by Sealy (1973), it now appears that this species may breed as far south as Triangle Island, British Columbia (K. Vermeer, personal communication; D. A. Manuwal, personal observation). Here, as on Forrester Island, it is greatly outnumbered by the tufted puffin. No data are available on the breeding or status of this species in the study area.
The tufted puffin is found breeding on scattered islands throughout the region. The largest known colonies are on Forrester Island, Alaska, Triangle Island, British Columbia, and Carroll Island, Washington. It is notably absent from most of the gulf and San Juan Islands. Even though puffins have apparently never been numerous in the San Juan Islands, their population has noticeably declined during the past 35 years. For example, Jewett et al. (1953) reported a colony of 50 pairs on Bare Island in 1937, but in 1973 only 2 pairs were counted (D. A. Manuwal, unpublished data). Likewise, in 1915 there were more than 250 pairs on Smith Island, but by 1916 there were only 75 pairs (Jewett et al. 1953). The decline is attributed to rapid erosion of the glacial-deposit cliffs. There are no puffins on Smith Island today, and the largest colony in the Puget Sound area is the 35 pairs on Protection Island (D. A. Manuwal, unpublished data).
The total minimum estimate of the breeding seabird populations of British Columbia and Washington is 216,500 pairs (Table 10). No comprehensive estimates are available for breeding seabirds of southeastern Alaska. It is likely, however, that the number of breeding seabirds in the Alexander Archipelago may be equal to (or exceed) the populations of both British Columbia and Washington. Data are desperately needed from that area. Of the total seabird population in the study area (Table 10) 43% reside in northern British Columbia. The Washington State population represents 28% of the total. Fork-tailed storm-petrels comprise almost 25% of all the breeding seabirds in the area under consideration. The Cassin's auklet is the next most numerous species (18% of the total).
It is apparent that current data are, for the most part, inadequate for assessing anything but catastrophic changes in seabird breeding colonies. This inadequacy is due to inadequate censusing because of excessive reliance upon aerial surveys; in the past, this has often been a result of insufficient funding.
Of the several threats facing seabird populations, none may be as important as oil pollution. A general review of this subject is presented elsewhere by Vermeer and Vermeer (1975). It is apparent from this review that the most vulnerable species are those that dive beneath the sea surface, including all the alcids and cormorants breeding along the coast that are discussed in this paper. This group makes up almost 60% of all the breeding seabirds in this area. Unfortunately, our knowledge of several of these species is scanty and our current census techniques are unsuitable for most of these birds.
Studies of the changes in seabird numbers have been made in other oceans. For example, in Great Britain (Bourne 1972a, 1972b; Harris 1970), eastern Canada (Nettleship 1973), and the Atlantic coast of the United States (Kadlec and Drury 1968), two major trends seem apparent. First, there is an overall decline in[Pg 90] alcid and tern numbers. The decline in auks may be due to their extreme vulnerability to oil pollution (Bourne 1972a, 1972b; Vermeer and Vermeer 1975). The Atlantic puffin, however, may be suffering the additional effects of gull cleptoparasitism (Nettleship 1972). Secondly, there seems to be an increase in gull populations on both sides of the Atlantic, particularly the herring gull and black-legged kittiwake.
Compared with the Atlantic coast of North America and northern Europe, the data base for seabird populations of the Pacific coast is poor. The fragmentary evidence now available indicates that there may be small population increases in the western and glaucous-winged gulls and range extensions of the Brandt's and double-crested cormorants and of the rhinoceros auklet (Scott et al. 1974). Whether these changes represent actual population increases or displacements remains unclear. The remote locations of most of the large Pacific seabird colonies may provide unofficial protection from human interference. Intensive surveys are needed to establish base-line inventories in these areas.
As a consequence of this first comprehensive review of the status of breeding marine birds of the northeast Pacific coast of North America, we recommend the following future research topics as necessary for the conservation of this great international resource.
• Seabird colony census techniques should be refined since almost 68% of the seabirds in this area are nocturnal and nest in burrows. The present reliance on aerial censusing, although economical, is inadequate to census most breeding seabird populations; more on-site surveys are needed. For surface-nesting species and diurnal, burrowing species, studies on species specific activity cycles are needed so that census data can be corrected for birds not observed at the colony. For nocturnal, burrowing species seasonal burrow occupancy rates must be determined so that burrow counts can be corrected for inactive burrows.
• Comprehensive surveys should be made every 3-5 years.
• In 1980 a coordinated breeding bird survey of the entire Pacific coasts of Mexico, Canada, and the United States should be conducted.
• Specific islands where key populations exist should be carefully monitored for subtle changes in population density or species composition.
• Increased study of the breeding biology of seabirds should be carried out so that base-line reproductive characteristics can be determined.
• Detailed studies of the effects of human disturbance should be made, especially for species that breed near large coastal cities or marine recreation areas.
Bailey, E. P. 1973. Discovery of a Kittlitz's murrelet nest. Condor 75:457.
Binford, L., B. Elliott, and S. Singer. 1975. Discovery of a nest and the downy young of the marbled murrelet. Wilson Bull. 87:303-319.
Bourne, W. 1972a. The decline of auks in Great Britain. Biol. Conserv. 4:144-146.
Bourne, W. 1972b. Threats to seabirds. Int. Counc. Bird Preserv. Bull. XI:200-218.
Couch, L. 1929. Introduced European rabbits in the San Juan Islands, Washington. J. Mammal. 10:334-336.
Drent, R. H., and C. Guiguet. 1961. A catalogue of British Columbia seabird colonies. Occas. Pap. B. C. Prov. Mus. 12. 173 pp.
Franklin, J., and C. Dyrness. 1973. Natural vegetation of Oregon and Washington. U.S. For. Serv. Gen. Tech. Rep. PNW-8. 417 pp.
Gabrielson, I., and F. Lincoln. 1959. The birds of Alaska. The Stackpole Company, Harrisburg, Penn., and Wildlife Management Institute, Washington, D.C. 922 pp.
Grinnell, J. 1897. Petrels of Sitka, Alaska. Nidologist 4:76-78.
Grinnell, J. 1898. Summer birds of Sitka, Alaska. Auk 15:122-131.
Grinnell, J. 1909. Birds and mammals of the 1907 Alexander Expedition to southeastern Alaska. Univ. Calif. Publ. Zool. 5:171-264.
Guiguet, C. 1971. A list of seabird nesting sites in Barkley Sound, British Columbia. Syesis 4:253-259.
Harris, M. P. 1970. Rates and causes of increases of some British gull populations. Bird Study 17:325-335.
Heath, H. 1915. Birds observed on Forrester Island, Alaska during the summer of 1913. Condor 17:20-41.
James-Veitch, E., and E. Booth. 1954. Behavior and life history of the glaucous-winged gull. Walla Walla Coll., Publ. Biol. 12.
Jewett, S., W. Taylor, W. Shaw, and J. Aldrich. 1953. Birds of Washington State. Univ. of Washington Press, Seattle. 767 pp.
Kadlec, J., and W. Drury. 1968. Structure of the New England herring gull population. Ecology 49:644-676.
Kenyon, K., and V. Scheffer. 1961. Wildlife surveys along the northwest coast of Washington. Murrelet 42:29-37.
Manuwal, D. A. 1974. Conservation notes (Protection Island). Pac. Seabird Group Bull. 1.
Nettleship, D. 1972. Breeding success of the common puffin (Fratercula arctica L.) on different habitats at Great Island, Newfoundland. Ecol. Monogr. 42:239-268.
Nettleship, D. 1973. Tenth census of seabirds in the sanctuaries of the north shore of the Gulf of St. Lawrence. Can. Field-Nat. 87:395-402.
Patten, S. 1974. Breeding ecology of the glaucous-winged gull (Larus glaucescens) in Glacier Bay, Alaska. M.S. Thesis. Univ. of Washington, Seattle. 78 pp.
Patten, S., and A. R. Weisbrod. 1974. Sympatry and interbreeding of herring and glaucous-winged gulls in southeastern Alaska. Condor 76:343.
Richardson, F. 1961. Breeding biology of the rhinoceros auklet on Protection Island, Washington. Condor 63:456-473.
Scott, J. M. 1971. Interbreeding of the glaucous-winged gull and western gull in the Pacific Northwest. Calif. Birds 2:129-133.
Scott, M., W. Hoffman, D. Ainley, and C. Zeillemaker. 1974. Range expansion and activity patterns in rhinoceros auklets. West. Birds 5:13-20.
Sealy, S. 1973. Breeding biology of the horned puffin on St. Lawrence Island, Bering Sea, with zoogeographical notes on the north Pacific puffins Pac. Sci. 27:99-199.
Sealy, S. 1975. Feeding ecology of the ancient and marbled murrelets near Langara Island, British Columbia. Can. J. Zool. 53:418-433.
Summers, K. 1974. Seabirds breeding along the east coast of Moresby Island, Queen Charlotte Islands, British Columbia. Syesis 7:1-12.
Swarth, H. 1911. Birds and mammals of the 1909 Alexander Alaska Expedition. Univ. Calif. Publ. Zool. 7:9-172.
Swarth, H. 1922. Birds and mammals of the Stikine River Region of northern British Columbia and southeastern Alaska. Univ. Calif. Publ. Zool. 24:194-314.
Swarth, H. 1936. Origins of the fauna of the Sitkan District. Alaska. Proc. Calif. Acad. Sci. Ser. 4, 15:59-78.
Thoresen, A., and J. Galusha. 1971. A nesting population study of some islands in the Puget Sound area. Murrelet 52:20-23.
U.S. Department of the Interior, Interagency Task Force. 1972. Environmental setting between Port Valdez, Alaska, and west coast ports. Final Environmental Impact Statement, Vol. 3.
U.S. Weather Bureau. 1956. Climatic summary of the United States—supplement for 1931 through 1952, Washington. Climatography of the United States 11-39. 79 pp.
U.S. Weather Bureau, Environmental Data Service. 1965a. Climatic summary of the United States—supplement for 1951 through 1960, Oregon. Climatography of the United States 86-31. 96 pp.
U.S. Weather Bureau, Environmental Data Service. 1965b. Climatic summary of the United States—supplement for 1951 through 1960, Washington. Climatography of the United States 86-39. 92 pp.
U.S. Weather Bureau, Environmental Data Service. 1974. Climatological Data, Alaska Annual Summary 1974, 60:13.
Vermeer, K. 1963. The breeding ecology of the glaucous winged gull (Larus glaucescens) on Mandarte Island. B.C. Occas. Pap. B.C. Prov. Mus. 13. 104 pp.
Vermeer, K., D. A. Manuwal, and D. S. Bingham. 1976. Seabirds and pinnipeds of Sartine Island, Scott Island group, British Columbia. Murrelet 57(1):14-16.
Vermeer, K., and R. Vermeer. 1975. Oil threat to birds on the Canadian west coast. Can. Field-Nat. 89:278-298.
Willett, G. 1912. Report of George Willett, Agent and Warden stationed on St. Lazaria Bird Reservation, Alaska. Bird-lore 14:419-426.
Willett, G. 1915. Summer birds of Forrester Island, Alaska. Condor 19:15-17.
Williamson, F. S. L., and L. Peyton. 1963. Interbreeding of glaucous-winged and herring gulls in the Cook Inlet region, Alaska. Condor 65:24-28.
[5] Data are minimum estimates of pairs and do not include breeding sites with less than 100 birds.
[6] Does not include the black oystercatcher, marbled murrelet, and western gull.
[7] Estimates only for colonies of 100 or more birds.
[8] Estimates are in number of pairs.
[9] Estimates are number of pairs.
[10] Estimates are numbers of pairs.
[11] Data for southeastern Alaska were inadequate to enable estimates of breeding pairs.
[12] Population estimates are minimum and represent numbers of pairs.
[13] Does not include the following species for which population estimates are lacking: black oystercatcher, herring gull, black-legged kittiwake, marbled murrelet, Kittlitz's murrelet.
by
David G. Ainley
Point Reyes Bird Observatory
Stinson Beach, California 94970
and
Gerald A. Sanger[15]
National Marine Fisheries Service
Marine Mammal Division
Seattle, Washington
Literature on the diets of seabirds is reviewed for 70 species found in five subarctic oceanographic regions of the northeastern North Pacific Ocean and Bering Sea. Species inhabiting estuaries and sheltered bays are not included. The diets of cormorants, marine ducks, alcids, and marine raptors are best known; less information is available for loons, grebes, petrels, and gulls. Enough is known, however, to broadly characterize the diet of each species. Less than 7% of all species feed on one type of prey, about 60% feed on two or three types, and the rest feed on four or more types. Only 12% of all species feed on eight or more types of prey. Most seabirds (77%) feed as secondary and tertiary carnivores. Where overlap in diet exists, seabirds partition resources through use of different feeding methods, selection of different-sized prey, and zonation of habitat. Species that have specialized diets are probably more susceptible than others to local environmental catastrophes. Species whose feeding methods are highly adapted for exploitation of resources in polar and subpolar habitats are not adapted for coping with oil pollution. Competition between birds and man for marine resources can sometimes benefit seabirds and at other times harm them. More research is needed on seabird feeding relations so that the ecological roles played by marine birds can be defined and placed in perspective. Such work should be conducted at the community level, year-round, and should be so conducted as to facilitate comparison with biological oceanographic data.
The ecology, morphology, and much of the behavior of a seabird species are definable in terms of the food resources it exploits year-round and the spatial and temporal relations between food and breeding sites. This general point unifies such important reports as those by Kuroda (1954), Bédard (1969a), Ashmole and Ashmole (1967), Ashmole (1971), Spring (1971), and Sealy (1972). More concretely, information on trophic relations of seabirds is useful in several ways. In conjunction with biological oceanographic data, it can provide insight into geographic location, marine habitat, depth, time of day, and general method of food capture by seabirds. Collected over several years, it can provide a basis for understanding annual differences in seabird breeding phenology and success. Finally, supplemented with data on how much seabirds eat and excrete, it is necessary for an understanding of the energetic and ecological roles played by the birds in the functioning of marine ecosystems.
Several studies that describe trophic relations within seabird communities have helped to define the principals of community organization pertaining to the exploitation of available food resources and have given clues to food-chain pathways. Trophic relations have been described for breeding communities in the Barents Sea (Uspenski 1958; Belopol'skii 1961), in the tropical Pacific Ocean (Ashmole and Ashmole 1967; Ashmole 1968), in the North Sea (Pearson 1968), and in the Chukchi Sea (Swartz 1966). The last-named study pertained most directly to the geographic region discussed in this paper, but several other studies have provided sound information on segments of communities in the northeastern North Pacific and Bering Sea. These include the work on three species of auklets (Aethia, Cyclorrhynchus) in the Bering Sea (Bédard 1969a); investigations on cormorants and other fish predators in British Columbia by Munro (1941), Munro and Clemens (1931), and Robertson (1974); studies of murres in Bristol Bay by Ogi and Tsujita (1973); observations on several species near the Pribilof Islands by Preble and McAtee (1923); work on diving species off Oregon by Scott (1973); and studies of murrelets by Sealy (1975).
A review of available reports reveals three obvious gaps in the emphasis placed in seabird food studies. First, few studies have ever considered in detail the trophic relations of seabird communities during the winter or nonbreeding season. Partial exceptions are the works by Cottam (1939) and others on marine diving ducks, species that are seabirds only during the winter, and by several researchers (Munro and Clemens 1931; Munro 1941; Robertson 1974) on seabirds in British Columbia. Divoky (1976) studied diets of pack-ice gulls during the nonbreeding season, but those species are not included in the present analysis because they rarely are found south of the Bering Strait. Second, no study has considered the trophic relationships of an entire seabird community, i.e., not just breeding species but also nonbreeding species. In the rather broad communities considered here, 50-70% or more of the birds breed in another part of the world. To say that these nonbreeding species have no significant impact on resource exploitation or on organization and evolution among breeding members would be naive. Finally, few investigators have attempted to fit birds into an entire ecosystem, including lower trophic level origins as well as fish, marine mammals, and man.
The reasons for these gaps in study emphasis are readily apparent: the inconvenience of marine research during the winter when weather is stormy, the need for costly study platforms (boats), and the difficulties in organizing the specialized community of biologists required for such tasks. A less obvious but important reason is that oceanographers and fishery biologists have overlooked seabirds as important members of marine ecosystems.
Relatively good information exists for most pelecaniformes of the region. A notable exception is the brown pelican (Pelecanus occidentalis), an endangered species. This is unfortunate because dietary information is important for understanding the species' ecology. Observations in eastern North America (Palmer 1962) and Peru (Murphy 1936) indicated that their diet consisted of fish that occur at the surface. The larger cormorants are piscivorous, particularly on schooling fishes that occur at moderate to great depths (Table 1). The smaller cormorants feed more heavily on benthic fish and decapod crustaceans. Cormorants apparently feed only during daylight and then only for short periods because their wettable plumage loses its buoyancy. Thus they remain relatively close (50 km) to nesting and loafing areas.
| Location | Diet | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRUSTACEAN | FISH | ||||||||||||||||||||||||
| A m p h i p o d |
I s o p o d |
D e c a p o d |
C l u p e a |
E n g r a u l i s |
S a l m o n i d |
A r g e n t i n i d |
P o r i c h t h y s |
O t o p h i d i u m |
B o r e o g a d u s |
M i c r o g a d u s |
G a s t e r o s t e u s |
S e b a s t e s |
H e x a g r a m m i d |
C o t t i d |
A g o n i d |
E m b i o t o c i d |
C h r o m i s |
O x y j u l i s |
S t i c h a c i d |
P h o l i d |
G o b i i d |
A m m o d y t e s |
P l e u r o n e c t i d |
B o t h i d |
|
| Double-crested cormorant (Phalacrocorax auritus)[16] | |||||||||||||||||||||||||
| Alaska Peninsula (Palmer 1962) | o | o | o | x | x | x | o | x | |||||||||||||||||
| SE Alaska (Bailey 1927) | x | ||||||||||||||||||||||||
| Mandarte Island (Robertson 1974) | * | * | * | * | * | * | o | x | x | ||||||||||||||||
| Vancouver Island (Munro and Clemens 1931) | x | ||||||||||||||||||||||||
| Oregon (Palmer 1962) | x | * | * | x | * | x | * | * | |||||||||||||||||
| Farallon Island (PRBO, unpublished data) | * | * | * | x | * | * | * | ||||||||||||||||||
| Brandt's cormorant (P. penicillatus)[17] | |||||||||||||||||||||||||
| Vancouver Island (Robertson, unpublished data) | x | o | x | ||||||||||||||||||||||
| Vancouver Island (Munro and Clemens 1931) | x | ||||||||||||||||||||||||
| Washington (Jewett et al. 1953) | x | ||||||||||||||||||||||||
| Yaquina Head (Scott 1973) | x | o | o | * | o | o | o | ||||||||||||||||||
| Farallon Island (PRBO, unpublished data) | * | o | * | * | x | x | x | * | o | * | * | * | |||||||||||||
| San Diego (Hubbs et al. 1970) | * | * | o | o | * | x | |||||||||||||||||||
| Pelagic cormorant (P. pelagicus)[18] | |||||||||||||||||||||||||
| Cape Thompson (Swartz 1966) | x | x | x | x | |||||||||||||||||||||
| Pribilof Island (Preble and McAtee 1923) | x | x | x | ||||||||||||||||||||||
| Alaska (Palmer 1962) | x | x | x | x | x | x | x | x | x | ||||||||||||||||
| SE Alaska (Heath 1915) | x | x | x | ||||||||||||||||||||||
| Mandarte Island (Robertson 1974) | x | * | * | x | x | ||||||||||||||||||||
| Vancouver Island (Munro and Clemens 1921) | x | x | x | x | x | x | |||||||||||||||||||
| Washington (Jewett et al. 1953) | x | x | x | x | |||||||||||||||||||||
| Netarts, Oregon (Gabrielson and Jewett 1940) | * | * | x | x | |||||||||||||||||||||
| Yaquina Head (Scott 1973) | o | o | x | ||||||||||||||||||||||
| Farallon Island (PRBO, unpublished data) | x | x | x | x | |||||||||||||||||||||
| Red-faced cormorant (P. urile) | |||||||||||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | x | x | x | x | x | x | |||||||||||||||||||
| Location | Diet[19] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLANTS | CRUSTACEANS | MOLLUSCS | ECHINODERMS | FISH | FISH EGGS | ||||||||
| Amphipods | Decapods | Barnacles | Mussels | Rock clams | Razor clams | Oysters, Scallops | Littorinids | Chitons | |||||
| Geese | x | ||||||||||||
| (Branta spp.) | |||||||||||||
| Emperor goose | x | ||||||||||||
| (Philacte canagica) | |||||||||||||
| Oldsquaw | o | * | * | * | * | * | * | * | o | ||||
| (Clangula hyemalis) | |||||||||||||
| Harlequin duck | * | x | x | o | * | * | o | o | * | * | |||
| (Histrionicus histrionicus) | |||||||||||||
| Steller's eider | o | x | * | * | * | * | o | * | * | * | * | ||
| (Polysticta stelleri) | |||||||||||||
| Common eider | * | * | x | * | o | * | o | o | * | o | * | ||
| (Somateria mollissima) | |||||||||||||
| King eider | * | * | o | * | x | * | o | o | * | x | * | ||
| (S. spectabilis) | |||||||||||||
| Spectacled eider | x | * | x | o | * | * | * | * | |||||
| (S. fischeri) | |||||||||||||
| White-winged scoter | * | * | * | * | o | x | * | x | o | * | * | o | o |
| (Melanitta deglandi) | |||||||||||||
| Surf scoter | o | * | * | * | x | * | * | x | o | * | * | * | o |
| (M. perspicillata) | |||||||||||||
| Black scoter | o | * | * | o | x | * | * | x | * | * | * | * | |
| (M. nigra) | |||||||||||||
| Red-breasted merganser | x | ||||||||||||
| (Mergus serrator) | |||||||||||||
Information on diets of marine ducks (Table 2) is more nearly complete than for most other seabirds. These birds fall into four groups with some overlap: species feeding on plants (Branta, Philacte, Anas-type, and Somateria fischeri); those feeding on benthic crustaceans (Clangula hyemalis, Histrionicus histrionicus, Polysticta stelleri, S. mollissima); those feeding on benthic molluscs (Somateria spp. and Melanitta spp.); and those feeding on fish (Mergus serrator, Clangula hyemalis, and Melanitta deglandi). A study by Perthon (1968), one of the few on a seabird's diet during most of a year, showed a seasonal change in diet for S. mollissima in Norway. In general, waterfowl seem to specialize in their diets much more than other seabirds and, for that reason, are perhaps more restricted in their distributions. Some marine ducks are known to dive to considerable depths (reviewed by Kooyman 1974), but usually they occur in shallow waters where plants and sessile invertebrates are readily available.
The summer diet of the pigeon guillemot (Cepphus columba) is the best known among seabirds in the region being considered here (Table 3). Only in the extreme southern part of its range (i.e., the California Channel Islands) is there no information available on its diet. The species feeds on organisms, mostly fish, from rocky habitat and apparently can dive to considerable depths (Follett and Ainley 1976). Because so much is known about guillemot diets during summer, a study of the winter diet would be valuable.
The diets of other alcids are known well enough to at least characterize them broadly. The larger species, murres, tufted and horned puffins (Lunda cirrhata, Fratercula corniculata), and the rhinoceros auklet (Cerorhinca monocerata), feed heavily on fish, mainly species that school in midwater (Table 4). To a great degree, these birds are opportunistic, feeding rather heavily at times on cephalopods and crustaceans, particularly nektonic forms. Morphological differences between the two murre species suggest that thick-billed murres (Uria lomvia) feed on benthic organisms much more than do common murres (U. aalge), and that the latter species is more piscivorous (Spring 1971); however, field data on diets are barely adequate to confirm this. Ogi and Tsujita (1973) analyzed the stomach contents of murres drowned in salmon gill nets but did not separate the two species. For the present paper we considered them to be mostly U. aalge, since this species predominates in the region of the food study (Bartonek and Gibson 1972). Adult murres sometimes eat different items than they feed to their chicks (Spring 1971; Scott 1973). The smaller alcids, ancient and marbled murrelets—Synthliboramphus antiquus and Brachyramphus marmoratus—(Table 5) and auklets (Table 6), feed on macrozooplankton: crustaceans, and fish and squid larvae. Little is known about the food or feeding ecology of Kittlitz's murrelet (B. brevirostris). Its diet is probably similar to that of the other murrelets, especially the marbled murrelet, its allopatric congener, but the diets of the other murrelets differ somewhat (Bédard 1969b; Sealy 1975). The Kittlitz's murrelet's shorter bill suggests that it feeds more on invertebrates. Alcids feed in deep or shallow water, depending on food distribution. Some alcid species can be found at great distances from land, particularly in winter (Hamilton 1958; Scott et al. 1971).
Information on the diets of other seabirds in the region is fragmentary and sometimes rather anecdotal. A little is known about the feeding habits of loons (Gavia spp.) and grebes (Podiceps spp. and Aechmophorus occidentalis), especially off British Columbia (Table 7). The larger of these birds feed mainly on inshore fish, but as species become progressively smaller, there is a tendency toward eating crustaceans. Work by Madsen (1957) in Denmark, indicated that loons and grebes tend to take prey near or on the bottom. Much more information is available on these birds' diets at their freshwater breeding sites but this provides only partial insight into what they might eat in marine habitats.
Information is especially poor for albatrosses and petrels (order Procellariiformes) (Table 8). Yet, based on sheer numbers alone, members of this diverse group are easily among the most ecologically dominant of the region (Sanger 1972; Ainley 1977). The Laysan albatross (Diomedea immutabilis) seems to be a squid specialist; the black-footed albatross (D. nigripes), northern fulmar (Fulmarus glacialis), scaled petrel (Pterodroma inexpectata), and the fork-tailed and Leach's storm-petrels (Oceanodroma furcata and O. leucorhoa) appear to be large, medium, small, and tiny versions, respectively, of surface-feeding generalists that eat whatever they can find, including live and dead fish, squid, coelenterates, crustaceans, and other organisms. The shearwaters (Puffinus spp.) feed to an unknown degree on schooling fish, squid, and crustaceans that occur near the surface. For these very abundant shearwaters, that, unfortunately, is close to the extent of our knowledge both for the North Pacific, where they winter, and the South Pacific, where they breed. Most petrels remain in oceanic habitats, but shearwaters, particularly the sooty shearwater (Puffinus griseus), and sometimes fulmars feed close to, if not within, the inshore neritic habitat. A much better understanding of the diets of this group is sorely needed.
| Location | Diet | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRUSTACEAN | O C T O P U S |
FISH | |||||||||||||||||||||
| A m p h i p o d |
I s o p o d |
D e c a p o d |
P e t r o m y z o n t i d |
C h i m a e r i d |
C l u p e i d |
O s m e r i d |
G a d i d |
G a s t e r o s t e i d |
S c o r p a e n i d |
C o t t i d |
A g o n i d |
E m b i o t o c i d |
B a t h y m a s t e r i d |
C l i n i d |
C r y p t a c a n t h o d i d |
C e b i d i c h t h y i d |
S t i c h a e i d |
P h o l i d |
A m m o n d y t i d |
B o t h i d |
P l e u r o n e c t i d |
||
| Cape Thompson (Swartz 1966) | o | o | |||||||||||||||||||||
| Pribilof Island (Preble and McAttee 1923) | o | o | o | ||||||||||||||||||||
| Mandarte Island (Drent 1965; Koelink 1972) | o | o | * | o | x | o | o | * | o | * | o | o | o | o | o | o | |||||||
| Vancouver Island (Munro and Clemens 1931) | o | o | o | ||||||||||||||||||||
| Olympic Peninsula (Thoresen and Booth 1958) | o | o | o | o | |||||||||||||||||||
| Yaquina Head (Scott 1973) | o | o | o | o | o | ||||||||||||||||||
| Farallon Island (Follett and Ainley 1976) | o | o | * | x | x | * | o | o | o | o | |||||||||||||
Knowledge on the food of gulls, shorebirds, and related species is surprisingly scanty in view of all that is known about their breeding biology and social behavior. Little is known about the marine food of phalaropes, but by inference from their association with storm-petrels, plankton-feeding whales, and convergence lines (Martin and Myers 1969), their tiny size, and their method of feeding (picking at minuscule items on the water surface), one can guess that they feed on zooplankton and detritus. Skuas (Catharacta skua) and jaegers (Stercorarius spp.) apparently eat what they can find at the surface, as well as whatever they can steal from gulls and terns. Almost all the literature on their feeding (Bent 1946) dwells on accounts of their stealing from other birds. That spectacular behavior would seem to be so energetically costly, though, that it is probably less important than we have been led to believe. Rather surprisingly, the question of what foods the gulls and terns eat in the eastern North Pacific is difficult to answer from the literature (Tables 9 and 10). Some information exists for five of the larger larids at isolated places, but little is known about food elsewhere in their respective ranges, and the diets of the seven smaller gulls and the terns are practically unknown. Studies on gull diets in the Atlantic region (e.g., Spaans 1971; Harris 1965) provide information on what to expect from the same species in the Pacific, but that information must be considered only in general terms because, the birds being somewhat opportunistic, their diets differ greatly from one locality to another (Ingolfsson 1967). A few observations are available for arctic terns (Sterna paradisaea) in Alaska, but little information exists for other terns (Table 10). Bent (1921) noted that Aleutian terns (S. aleutica) sometimes associate with arctic terns during feeding.
Finally, we must include raptors, particularly the peregrine (Falco peregrinus) and bald eagle (Haliaeetus leucocephalus), because they are important predators on the smaller seabirds (White et al. 1971, 1973). Peregrines have, in fact, been observed feeding on storm-petrels far at sea (Craddock and Carlson 1970).
We have compared and summarized in general terms the food partitioning by species in five rather broad oceanographic regions and their subdivisions in the northeastern North Pacific and Bering Sea, based on the specific details on diets presented in Tables 1 through 10. The five broad regions, defined oceanographically by Dodimead et al. (1963) and Favorite et al. (1976) and modified by Sanger (1972), are shown in Fig. 1. The five oceanographic regions (domains) were divided further into inshore neritic, offshore neritic, and oceanic habitats (Sanger and King, this volume). We did not include estuarine habitats or sheltered bays in the analysis.
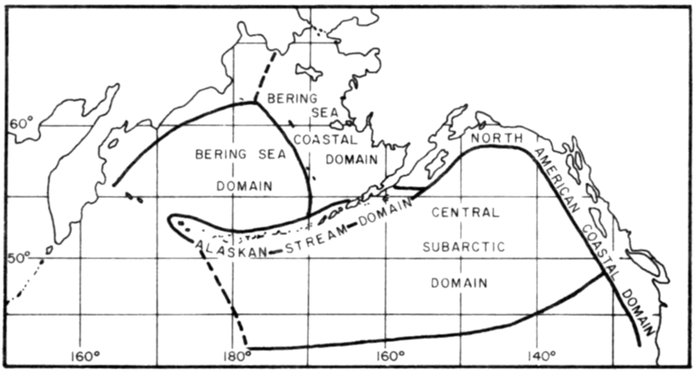
Fig. 1. Schematic oceanographic domains of the subarctic Pacific regions (defined by Dodimead et al. (1963) and Favorite et al. (1976) and modified by Sanger (1972).)
| LOCATION | Diet | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRUSTACEAN | P O L Y C H A E T E |
C E P H A L O P O D |
FISH | |||||||||||||||||||||||||||
| E u p h a u s i i d |
A m p h i p o d |
I s o p o d |
D e c a p o d |
C l u p e a |
S a r d i n o p s |
E n g r a u l i s |
S a l m o |
O n c h o r h y n c h u s |
H y p o m e s u s |
T h a l e i c h t h y s |
M a l l o t u s |
B o r e o g a d u s |
M i c r o g a d u s |
T h e r a g r a |
L y c o d e s |
G a s t e r o s t e u s |
S e b a s t e |
T r i g l o p s |
M y o x o c e p h a l u s |
C o t t i d |
C y m a t o g a s t e r |
E m b i o t o c i d |
C h i r o l o p h i s |
S t i c h a e i d |
A m m o d y t e s |
P l e u r o n e c t i d |
L i p a r i d |
|||
| Common murre(Uria aalge)[20] | ||||||||||||||||||||||||||||||
| Cape Thompson (Swartz 1966) | o | o | o | o | o | x | o | o | o | x | ||||||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | x | o | o | o | ||||||||||||||||||||||||||
| E. Bering Sea (Ogi and Tsujita 1973) | x | o | x | x | x | |||||||||||||||||||||||||
| Forrester Island (Heath 1915) | o | o | x | |||||||||||||||||||||||||||
| Vancouver Island | o | o | x | o | ||||||||||||||||||||||||||
| (Robertson, unpublished data) | x | x | o | o | ||||||||||||||||||||||||||
| Olympic Peninsula (Cody 1973) | x | x | o | x | o | o | o | |||||||||||||||||||||||
| Yaquina Head | x | o | ||||||||||||||||||||||||||||
| (Scott 1973) | x | o | o | * | x | o | o | |||||||||||||||||||||||
| Farallon Islands (PRBO, unpublished data) | x | o | ||||||||||||||||||||||||||||
| Thick-billed murre (U. lomvia)[21] |
x | o | o | o | o | o | x | o | ||||||||||||||||||||||
| Cape Thompson (Swartz 1966) | o | o | o | |||||||||||||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | x | x | ||||||||||||||||||||||||||||
| Hooker Island | x | o | ||||||||||||||||||||||||||||
| (Demme 1934, in Dement'ev et al. 1968) | o | x | * | x | * | o | x | o | o | o | ||||||||||||||||||||
| NE Canada (Tuck and Squires 1937) | x | o | o | o | ||||||||||||||||||||||||||
| Tufted puffin | x | |||||||||||||||||||||||||||||
| (Lunda cirrhata)[22] | * | |||||||||||||||||||||||||||||
| Cape Thompson | * | |||||||||||||||||||||||||||||
| (Swartz 1966) | x | |||||||||||||||||||||||||||||
| Forrester Island (Heath 1915) | x | |||||||||||||||||||||||||||||
| Langara Island | x | |||||||||||||||||||||||||||||
| (Sealy 1973a) | x | x | x | x | ||||||||||||||||||||||||||
| Washington | o | |||||||||||||||||||||||||||||
| (Jewett et al. 1953) | x | o | x | |||||||||||||||||||||||||||
| Olympic Peninsula | x | |||||||||||||||||||||||||||||
| (Cody 1973) | x | o | ||||||||||||||||||||||||||||
| Farallon Island (PRBO, unpublished data) | x | |||||||||||||||||||||||||||||
| Horned puffin | x | o | ||||||||||||||||||||||||||||
| (Fratercula corniculata) | o | x | ||||||||||||||||||||||||||||
| Cape Thompson (Swartz 1966) | o | |||||||||||||||||||||||||||||
| Alaska (Bent 1946) | x | |||||||||||||||||||||||||||||
| Forrester Island (Heath 1915) | ||||||||||||||||||||||||||||||
| Rhinoceros auklet (Cerorhinca monocerata) | x | o | x | x | ||||||||||||||||||||||||||
| NW Pacific (Kozlova 1961; | x | |||||||||||||||||||||||||||||
| Komaki 1967) | x | |||||||||||||||||||||||||||||
| Forrester Island (Heath 1915) | x | |||||||||||||||||||||||||||||
| Langara Island (Sealy 1973a) | o | x | ||||||||||||||||||||||||||||
| Destruction Island | o | |||||||||||||||||||||||||||||
| (Richardson 1961) | x | x | o | |||||||||||||||||||||||||||
| Olympic Peninsula (Cody 1973) | x | |||||||||||||||||||||||||||||
| So. California (Linton 1908; Grinnell 1899) | x | |||||||||||||||||||||||||||||
| Location | DIET | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRUSTACEAN | SQUID | FISH | ||||||||||||||||
| Euphausiid | Thysanoessa | Euphausia | Mysid | Acanthomysis | Amphipod | Gammarid | Carid shrimp | Decapod | Larvae | Larvae | Engraulis | Osmerid | Scorpaenid | Cymatogaster | Stichaeid | Ammodytes | Larvae | |
| Ancient murrelet (Synthliboramphus antiquus)[23] | ||||||||||||||||||
| Commander Islands (Dement'ev et al. 1968) | x | x | ||||||||||||||||
| Amchitka Island (White et al. 1971, 1973) | x | x | x | x | x | |||||||||||||
| Langara Island (Sealy 1975) | x | x | x | * | * | * | o | o | x | |||||||||
| Marbled murrelet (Brachyramphus marmoratus)[24] | ||||||||||||||||||
| SE Alaska (Grinnell 1897) | o | |||||||||||||||||
| Langara Island (Sealy 1975) | x | x | * | * | * | * | x | * | ||||||||||
| Vancouver Island (Munro and Clemens 1931) | x | x | x | |||||||||||||||
| Olympic Peninsula (Cody 1973) | x | x | ||||||||||||||||
| Location | Diet | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRUSTACEAN | POLYCHAETE | SQUID | FISH | ||||||||||||
| Euphausiid | Thysanoessa | Mysid | Stylomysis | Amphipod | Parathemisto | Phronema | Gammarid | Copepod | Calanus | Carid shrimp | Larvae | Cottid | Larvae | ||
| Cassin's auklet (Ptychoramphus aleuticus) | |||||||||||||||
| Forrester Island (Heath 1915) | x | x | x | ||||||||||||
| Olympic Peninsula (Cody 1973) | x | x | |||||||||||||
| Farallon Islands (Manuwal 1974) | x | x | x | x | x | o | x | ||||||||
| Parakeet auklet (Cyclorrhynchus psittaculus) | |||||||||||||||
| Chukhotsk Peninsula (Portenko 1934, in Dement'ev et al. 1968) | x | x | x x | x | |||||||||||
| Aleutian Islands (Bent 1946) | x | ||||||||||||||
| St. Lawrence Island (Bédard 1969a) | x | x | o | x | x | o | o | * | o | o | o | ||||
| Crested auklet (Aethia cristatella)[25] | |||||||||||||||
| W. Bering Sea (Portenko 1934, in Dement'ev et al. 1968) | x | x | |||||||||||||
| Commander Islands (Stejneger 1885) | x | ||||||||||||||
| Amchitka (White et al. 1973) | x | x | x | x | x | ||||||||||
| St. Lawrence Island. (Bédard 1969a) | x | x | x | o o | x | x | * | ||||||||
| Pribilof Islands (Preble and McAtee 1923) | x | x | |||||||||||||
| Least auklet (A. pusilla) | |||||||||||||||
| Commander Islands (Stejneger 1885) | x | ||||||||||||||
| Aleutian Islands (Bent 1946) | x | ||||||||||||||
| St. Lawrence Island (Bédard 1969a) | o | o | * | o | o | o | x | x | x | ||||||
| Whiskered auklet (A. pygmaea) | |||||||||||||||
| Commander Islands (Stejneger 1885) | x | x | * | ||||||||||||
| Location | Diet | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRUSTACEANS | POLYCHAETE | FISH | |||||||||||||||||||
| Euphausid | Amphipod | Mysid | Decapod | Anguilla | Clapea | Sardinops | Salmo | Thaleichthys | Atherinops | Zoarchid | Gadid | Fundulus | Gasterosteus | Sebastes | Cattid | Cymatogaster | Stichaeid | Ammodytes | Gobiid | ||
| Common loon (Gavia immer) | |||||||||||||||||||||
| Alaska (Palmer 1962) | * | * | o | o | o | o | o | ||||||||||||||
| Vancouver Island (Munro and Clemens 1931) | x | ||||||||||||||||||||
| Denmark (Madsen 1957) | * | o | x | * | o | ||||||||||||||||
| Yellow-billed loon (G. adamsii)[26] | |||||||||||||||||||||
| Alaska (Cottam and Knappen 1939) | * | * | o | x | |||||||||||||||||
| Alaska (Bailey 1922) | x | ||||||||||||||||||||
| Arctic loon (G. arctica)[27] | |||||||||||||||||||||
| Vancouver Island (Palmer 1962) | x | ||||||||||||||||||||
| Vancouver Island (Robertson, unpublished data) | x | ||||||||||||||||||||
| California (Palmer 1962) | x | ||||||||||||||||||||
| Denmark (Madsen 1957) | * | o | * | x | x | * | x | * | |||||||||||||
| Red-throated loon (G. stellata)[28] | |||||||||||||||||||||
| Oregon (Palmer 1962) | x | ||||||||||||||||||||
| No. Atlantic (Palmer 1962) | x | x | o | ||||||||||||||||||
| Denmark (Madsen 1957) | * | o | * | x | o | * | * | o | |||||||||||||
| Western grebe (Aechmophorus occidentalis) | |||||||||||||||||||||
| Vancouver Island (Munro 1941) | o | * | x | x | |||||||||||||||||
| Vancouver Island (Robertson, unpublished data) | x | x | |||||||||||||||||||
| Puget Sound (Phillips and Carter 1957) | x | * | o | o | o | ||||||||||||||||
| Washington (Chatwin 1956) | * | x | |||||||||||||||||||
| California (Palmer 1962) | o | * | x | x | x | * | |||||||||||||||
| Red-necked grebe (Podiceps grisegena) | |||||||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | o | ||||||||||||||||||||
| Vancouver Island (Wetmore 1924) | x | ||||||||||||||||||||
| Vancouver Island (Munro 1941) | x | o | x | ||||||||||||||||||
| E. No. America (Wetmore 1924) | o | * | o | o | x | ||||||||||||||||
| Horned grebe (P. auritus)[29] | |||||||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | x | o | |||||||||||||||||||
| W. No. America (Wetmore 1924) | x | x | * | o | o | ||||||||||||||||
| Vancouver Island (Munro 1941) | x | x | o | o | * | ||||||||||||||||
| Denmark (Madsen 1957) | o | o | o | o | |||||||||||||||||
| Eared grebe (P. nigricollis)[30] | |||||||||||||||||||||
| W. No. America (Wetmore 1924) | * | x | * | o | |||||||||||||||||
| Vancouver Island (Munro 1941) | x | x | o | ||||||||||||||||||
| Denmark (Madsen 1957) | x | * | o | ||||||||||||||||||
| Location | Diet | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRUSTACEAN | COELENTERATE | ECHINODERM | CEPHALOPOD | FISH | ||||||||||||
| Euphausiid | Amphipod | Copepod | Decapod | Larvae | Barnacle | "Fish" | Engraulis | Myctophid | Sebastes | Ammodytes | Carrion, fish offal | Fish eggs | ||||
| Black-footed albatross (Diomedea nigripes) | ||||||||||||||||
| No. Pacific (Palmer 1962) | x | x | x | |||||||||||||
| Aleutian Islands (Cottam and Knappen 1939) | o | x | o | x | x | |||||||||||
| California (Miller 1936, 1940) | o | o | x | x | x | x | ||||||||||
| Laysan albatross (D. immutabilis) | ||||||||||||||||
| No. Pacific (Palmer 1962; Bartsch 1922; Fisher 1904) | x | |||||||||||||||
| Northern fulmar (Fulmarus glacialis) | ||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | o | x | ||||||||||||||
| Alaska (Gabrielson and Lincoln 1959) | x | x | x | |||||||||||||
| Oregon (Gabrielson and Jewett 1940) | x | |||||||||||||||
| No. Atlantic (Hartley and Fisher 1936; Einarsson 1945; Fisher 1952) | x | x | ||||||||||||||
| Flesh-footed shearwater (Puffinus carneipes) | ||||||||||||||||
| Australia (Oliver 1955; Serventy et al. 1971) | x | x | x | |||||||||||||
| Pink-footed shearwater (P. creatopus) | ||||||||||||||||
| California (Murphy 1936; Ainley, personal observation) | x | x | ||||||||||||||
| E. Pacific (Cottam and Knappen 1939) | x | x | ||||||||||||||
| Buller's shearwater (P. bulleri) | ||||||||||||||||
| SW Pacific (Falla 1934; Serventy et al. 1971) | x | x | x | |||||||||||||
| Peru (Murphy 1936) | x | |||||||||||||||
| Sooty shearwater (P. griseus) | ||||||||||||||||
| Aleutian Islands (Sanger, personal observation) | x | x | x | |||||||||||||
| British Columbia (Martin 1942; Sealy 1973a) | x | x | x | |||||||||||||
| Oregon (Gabrielson and Jewett 1940) | x | |||||||||||||||
| California (Ainley, personal observation) | x | x | ||||||||||||||
| Peru (Murphy 1936) | x | x | x | |||||||||||||
| SW Pacific (Oliver 1955; Serventy et al. 1971) | x | x | x | |||||||||||||
| Short-tailed shearwater (P. tenuirostris) | ||||||||||||||||
| Bristol Bay (Bartonek, personal communication) | x | |||||||||||||||
| Alaska (Cottam and Knappen 1939) | x | x | x | o | ||||||||||||
| No. Pacific (Palmer 1962; Kuroda 1955) | x | x | x | |||||||||||||
| Australia (Serventy et al. 1971) | x | x | x | |||||||||||||
| Bass Strait (Sheard 1953) | x | |||||||||||||||
| Mottled petrel (Pterodroma inexpectata) | ||||||||||||||||
| Pacific Ocean (Imber 1973) | x | |||||||||||||||
| E. No. Pacific (Kuroda 1955) | x | |||||||||||||||
| Fork-tailed storm-petrel (Oceanodroma furcata) | ||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | x | |||||||||||||||
| SE Alaska (Heath 1915) | x | |||||||||||||||
| British Columbia (Martin 1942) | x | |||||||||||||||
| California (Ainley, personal observation) | x | |||||||||||||||
| Leach's storm-petrel (O. leucorhoa) | ||||||||||||||||
| SE Alaska (Heath 1915) | x | |||||||||||||||
| California (PRBO, unpublished data) | x | x | x | x | x | |||||||||||
| So. California (Palmer 1962) | x | x | ||||||||||||||
| No. Atlantic[31] (Palmer 1962) | x | x | x | x | x | |||||||||||
| Location | Diet | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRUSTACEAN | P O L Y C H A E T E |
MOLLUSC | E C H I N O D E R M |
C O E L E N T E R A T E |
FISH | C A R R I O N - O F F A L |
BIRD | F I S H E G G S |
|||||||||||||||||||||
| E u p h a u s i i d |
B a r n a c l e |
D e c a p o d |
S h e l l f i s h |
C e p h a l o p o d |
" F i s h " |
C l u p e a |
E n g r a u l i s |
O s m e r u s |
P o r i c h i h y s |
O t a p h i d i u m |
M a l l o t u s |
B o r r o g a d u s |
M i c r o g a d u s |
G a d u s |
L y c o d e s |
S e b a s t e s |
M y x o c e p h a l u s |
G e n y o n e m u s |
E m b i o t o c i d |
A m m o d y t e s |
E g g s |
C h i c k s |
A d u l t s |
||||||
| Glaucous gull (Larus hyperboreus) | |||||||||||||||||||||||||||||
| St. Lawrence Island (Fay and Cade 1959) | x | x | x | ||||||||||||||||||||||||||
| Chukchi Sea (Swartz 1966) | x | x | x | x | x | x | x | ||||||||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | x | x | x | x | x | ||||||||||||||||||||||||
| Vancouver Island (Munro and Clemens 1931) | x | x | |||||||||||||||||||||||||||
| Glaucous-winged gull (L. glaucescens)[32] | |||||||||||||||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | x | x | x | x | x | x | x | ||||||||||||||||||||||
| Alaska (Bent 1921) | x | ||||||||||||||||||||||||||||
| No. Pacific (Sanger 1973) | x | * | * | ||||||||||||||||||||||||||
| Mandarte Island (Ward 1973) | x | x | x | x | x | ||||||||||||||||||||||||
| Vancouver Island (Munro and Clemens 1931; Robertson, unpublished data) | x | x | x | ||||||||||||||||||||||||||
| Western gull (L. occidentalis)[33] | |||||||||||||||||||||||||||||
| Farallon Islands (PRBO, unpublished data) | x | x | o | * | x | * | * | x | x | o | o | o | x | o | o | x | o | o | o | ||||||||||
| Herring gull (L. argentatus) | |||||||||||||||||||||||||||||
| No. Atlantic (Zelikman 1961) | x | ||||||||||||||||||||||||||||
| E. No. America (Bent 1946; Ainley, personal observation) | x | x | x | x | x | x | * | o | x | ||||||||||||||||||||
| Vancouver Island (Munro and Clemens 1931) | x | x | x | ||||||||||||||||||||||||||
| Mew gull (L. canus) | |||||||||||||||||||||||||||||
| Alaska (Bent 1921) | x | x | x | x | |||||||||||||||||||||||||
| Vancouver Island (Munro and Clemens 1931) | x | x | x | ||||||||||||||||||||||||||
| Heermann's gull (L. heermanni) | |||||||||||||||||||||||||||||
| California (Bent 1921) | x | x | x | x | |||||||||||||||||||||||||
| Bonaparte's gull (L. philadelphia) | |||||||||||||||||||||||||||||
| E. No. America (Bent 1921) | x | x | x | ||||||||||||||||||||||||||
| Black-legged kittiwake (Rissa tridactyla)[34] | |||||||||||||||||||||||||||||
| Chukchi Sea (Swartz 1966) | o | * | o | o | x | o | o | x | |||||||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | o | x | o | ||||||||||||||||||||||||||
| Alaska (Bent 1921) | x | o | |||||||||||||||||||||||||||
| Cook Inlet[35] (Snarski, personal communication) | o | o | o | x | o | x | |||||||||||||||||||||||
| No. Atlantic (Hartley and Fisher 1936; Zelikman 1961) | x | ||||||||||||||||||||||||||||
| Red-legged kittiwake (R. breuirostris) | |||||||||||||||||||||||||||||
| Pribilof Islands (Preble and McAtee 1923) | x | x | x | ||||||||||||||||||||||||||
| Sabine's gull (Xenia sabini) | |||||||||||||||||||||||||||||
| Pt. Barrow (Banner 1954) | x | ||||||||||||||||||||||||||||
| Location | Diet | |||||
|---|---|---|---|---|---|---|
| CRUSTACEAN | FISH | |||||
| "Crustacean" | Mallotus | Euphausiid | Cottid | Amphipod | Ammodytes larvae |
|
| Arctic tern (Sterna paradisaea) | ||||||
| Pribilof Islands (Preble and McAtee 1923) | x | x | ||||
| Alaska (Bent 1921) | x | x | x | |||
| No. Atlantic (Hartley and Fisher 1936) | x | |||||
| Common tern (S. hirundo) | ||||||
| E. No. America (Bent 1921) | x | x | ||||
The oceanic habitat includes waters of the photic zone overlying the deep ocean and continental slopes beyond the continental or insular shelves. The Bering Sea and central subarctic domains are largely made up of oceanic habitat. The other three domains include both inshore and offshore neritic as well as some oceanic habitat. The boundary between the inshore and offshore neritic has yet to be defined in terms of bird life, but it lies at that line beyond which the bottom is too deep for a diving bird to exploit. A depth contour thus defines the boundary. In the antarctic South Pacific, emperor penguins (Aptenodytes fosteri) dive to depths of 275 m, but so far as is known, no comparable bird exists in the North Pacific. Some marine ducks and loons reportedly dive to 50-60 m (Kooyman 1974). The inshore-offshore neritic boundary for seabirds may lie near the 70-m depth contour.
Food resource partitioning by seabirds in the five oceanographic domains are shown in Tables 11-15. Within each domain, the common and usual members of the seabird community are listed, and the major and minor categories in each of their diets are shown (on the basis of available literature, Tables 1-10). The categories are grouped further, and rather tenuously, according to the trophic level at which a bird is presumably feeding: I = herbivore, II = secondary carnivore, III = tertiary carnivore, IV = final carnivore, and Sc = scavengers (carnivorous) feeding at many levels. Birds at level I feed on large algae and seed plants and are not directly part of the same food webs involving other species. These food webs originate with phytoplankton (Fig. 2). So far as is known, no bird feeds on phytoplankton and few, if any, feed on microzooplankton; hence birds do not generally feed as primary carnivores. An exception at times might be the least auklet (Aethia pusilla) when it feeds on small copepods (see Bédard 1969b).
The above groupings are "tenuous" because prey in each category may represent more than one trophic level, and a single prey species could occur at one level one day or place and at another level the next day or place, depending upon what it happened to be eating. This is shown in Fig. 2, where the parakeet auklet (Cyclorrhynchus psittaculus) can occur in the food web at different levels, depending both on the prey it is eating and on what its prey is eating. Even without this complication, many seabirds feed at more than one level in the food web. For instance, murres eating euphausiids would be feeding at a different level than murres feeding on larger fish. It might be "safer" to regard prey organisms in level II as macrozooplankton, prey organisms in level III as micronekton, and prey organisms (seabirds themselves) in level IV as macronekton (after Sverdrup et al. 1942).
Fig. 2. Schematic food web of the parakeet auklet in the eastern Bering Sea (based on Bédard 1969a and Dunbar 1946). Arrow sizes indicate relative importance of prey and Roman numerals refer to prey sizes (see text).
| Seabirds | Habitat, bird trophic levels (I-IV. Sc), and food categories | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oceanic and offshore neritic | Inshore neritic | |||||||||||||||||||
| II | III | IV | Sc | I | II | III | IV | Sc | ||||||||||||
| C r u s t a c e a n |
P o l y c h a e t e |
C o e l e n t e r a t e |
F i s h / s q u i d e g g s & l a r v a e |
F i s h |
C e p h a l o p o d |
B i r d s |
C a r r i o n / o f f a l / d e t r i t u s |
P l a n t |
C r u s t a c e a n , m i d w a t e r |
C r u s t a c e a n , b e n t h i c |
C o e l e n t e r a t e |
E c h i n o d e r m |
M o l l u s c |
F i s h / s q u i d e g g s & l a r v a e |
F i s h , m i d w a t e r |
F i s h , b e n t h i c |
C e p h a l o p o d |
B i r d s |
C a r r i o n / o f f a l / d e t r i t u s |
|
| Gavia adamsii | * | o | x | |||||||||||||||||
| G. arctica | o | x | ||||||||||||||||||
| Podiceps grisegena | o | o | x | |||||||||||||||||
| Diomedea nigripes | x | o | o | o | x | x | x | |||||||||||||
| Fulmarus glacialis | x | o | x | o | x | x | x | |||||||||||||
| Puffinus griseus | x | x | x | o | o | |||||||||||||||
| P. tenuirostris | x | o | x | o | o | |||||||||||||||
| Oceanodroma furcata | x | o | o | x | x | x | x | |||||||||||||
| Phalacrocorax auritus | o | x | o | |||||||||||||||||
| P. pelagicus | x | x | ||||||||||||||||||
| P. urile | x | x | ||||||||||||||||||
| Branta bernicla | x | |||||||||||||||||||
| Philacte canagica | x | |||||||||||||||||||
| Clangula hyemalis | o | x | o | o | ||||||||||||||||
| Histrionicus histrionicus | o | x | o | |||||||||||||||||
| Polysticta stelleri | o | x | o | o | ||||||||||||||||
| Samateria mollissima | x | o | x | |||||||||||||||||
| S. spectabilis | o | o | o | x | ||||||||||||||||
| S. fischeri | x | x | ||||||||||||||||||
| Melanitta deglandi | x | o | ||||||||||||||||||
| M. nigra | o | o | x | |||||||||||||||||
| Haliaeetus leucocephalus | x | x | x | |||||||||||||||||
| Falco peregrinus | x | |||||||||||||||||||
| Phalaropus fulicarius | x | x | o | x | x | |||||||||||||||
| Lobipes lobatus | x | x | o | x | x | |||||||||||||||
| Stercorarius spp. | o | x | x | ? | x | x | x | x | ||||||||||||
| Larus hyperboreus | o | o | o | o | o | o | o | o | o | o | x | x | x | |||||||
| L. glaucescens | o | o | o | o | o | o | o | o | o | o | x | x | x | |||||||
| L. argentatus | o | o | o | o | o | o | o | o | o | o | x | o | x | |||||||
| L. canus | x | o | o | o | x | x | ||||||||||||||
| Rissa tridactyla | x | x | x | o | ||||||||||||||||
| Xema sabini | x | x | o | o | ||||||||||||||||
| Sterna paradisaea | x | x | o | o | ||||||||||||||||
| Uria aalge | x | o | x | x | o | o | x | o | ||||||||||||
| U. lomvia | x | o | x | x | o | x | o | x | ||||||||||||
| Lunda cirrhata | ? | x | x | |||||||||||||||||
| Fratercula corniculata | * | x | x | |||||||||||||||||
| Cepphus columba | o | x | o | |||||||||||||||||
| Synthliboramphus antiquus | x | x | o | |||||||||||||||||
| Brachyramphus brevirostris | x | o | ||||||||||||||||||
| Cyclorrhynchus psittaculus | x | o | * | * | ||||||||||||||||
| Aethia cristatella | x | x | ||||||||||||||||||
| A. pusilla | x | o | ||||||||||||||||||
| Seabirds | Bird trophic levels and food categories | |||||||
|---|---|---|---|---|---|---|---|---|
| II | III | IV | Sc | |||||
| Crustacean | Polychaete | Coelenterate | Fish/Squid eggs & Larvae | Fish | Cephalopod | Birds | Carrion/offal/detritus | |
| Diomedea nigripes | x | o | o | o | x | x | x | |
| D. immutabilis | x | |||||||
| Fulmarus glacialis | x | o | x | o | x | x | x | |
| Puffinus griseus | x | x | x | |||||
| P. tenuirostris | x | o | x | |||||
| Pterodroma inexpectata | x | x | ||||||
| Oceanodroma furcata | x | o | o | x | x | x | x | |
| Phalaropus fulicarius | x | x | o | |||||
| Lobipes lobatus | x | x | o | |||||
| Stercorarius spp. | o | x | x | ? | x | |||
| Larus hyperboreus | x | o | o | o | x | x | ? | x |
| L. glaucescens | x | o | o | o | x | x | ? | x |
| Rissa tridactyla | x | x | x | o | ||||
| R. brevirostris | x | x | x | o | ||||
| Xema sabini | x | x | o | o | ||||
| Sterna paradisaea | x | x | o | o | ||||
| Uria aalge | x | o | x | x | ||||
| U. lomvia | x | o | x | x | ||||
| Lunda cirrhata | ? | x | x | |||||
| Fratercula corniculata | * | x | x | |||||
| Synthliboramphus antiquus | x | x | ||||||
| Cyclorrhynchus psittaculus | x | o | * | * | ||||
| Aethia cristatella | x | x | ||||||
| A. pusilla | x | o | ||||||
| A. pygmaea | x | |||||||
Information contained in Tables 11-15 can be summarized to show characteristics of seabird trophic relations. One such characteristic is the range of diet breadth or diet complexity (Table 16). Few species (about 6%) feed on only one type of prey and might, therefore, be referred to as "specialists." Included are eared grebe (Podiceps caspicus), Laysan albatross, brown pelican, emperor goose (Philacte canagica), black brant (Bernicia bernicla), peregrine falcon, and whiskered auklet (Aethia pygmaea). Consideration of these species as specialists may require revision when more data become available. Except for the albatross and auklet, these species are members of the inshore neritic cohort. Food specialization does not seem to be characteristic of oceanic birds in particular or of most seabirds in general.
| Seabirds | Habitat, bird trophic levels (I-IV. Sc), and food categories | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oceanic and offshore neritic | Inshore neritic Inshore neritic | |||||||||||||||||||
| II | III | IV | Sc | I | II | III | IV | Sc | ||||||||||||
| C r u s t a c e a n |
P o l y c h a e t e |
C o e l e n t e r a t e |
F i s h / s q u i d e g g s & l a r v a e |
F i s h |
C e p h a l o p o d |
B i r d s |
C a r r i o n / o f f a l / d e t r i t u s |
P l a n t |
C r u s t a c e a n , m i d w a t e r |
C r u s t a c e a n , b e n t h i c |
C o e l e n t e r a t e |
E c h i n o d e r m |
M o l l u s c |
F i s h , m i d w a t e r |
F i s h , b e n t h i c |
C e p h a l o p o d |
B i r d s |
F i s h / s q u i d e g g s & l a r v a e |
C a r r i o n / o f f a l / d e t r i t u s |
|
| Gavia immer | * | x | x | |||||||||||||||||
| G. adamsii | * | o | x | |||||||||||||||||
| G. stellata | * | o | x | |||||||||||||||||
| Podiceps grisegena | o | x | o | |||||||||||||||||
| Diomedea nigripes | x | o | o | o | x | x | x | |||||||||||||
| Fulmarus glacialis | x | o | x | o | x | x | x | |||||||||||||
| Puffinus griseus | x | x | x | o | ||||||||||||||||
| P. tenuirostris | x | o | x | o | ||||||||||||||||
| Pterodroma inexpectata | x | x | ||||||||||||||||||
| Oceanodroma furcata | x | o | o | x | x | x | x | |||||||||||||
| Phalacrocorax auritus | o | x | o | |||||||||||||||||
| P. pelagicus | x | x | ||||||||||||||||||
| P. urile | x | x | ||||||||||||||||||
| Philacte canagica | x | |||||||||||||||||||
| Clangula hyemalis | o | x | o | |||||||||||||||||
| Histrionicus histrionicus | x | x | o | |||||||||||||||||
| Polysticta stelleri | o | x | o | o | ||||||||||||||||
| Somateria mollissima | x | o | x | |||||||||||||||||
| S. spectabilis | o | o | x | |||||||||||||||||
| S. fischeri | x | x | ||||||||||||||||||
| Melanitta deglandi | x | o | ||||||||||||||||||
| M. perspicillata | o | x | o | |||||||||||||||||
| M. nigra | o | o | x | |||||||||||||||||
| Mergus serrator | o | x | x | |||||||||||||||||
| Haliaeetus leucocephalus | x | x | x | |||||||||||||||||
| Falco peregrinus | x | |||||||||||||||||||
| Phalaropus fulicarius | x | x | o | x | x | |||||||||||||||
| Lobipes lobatus | x | x | o | x | x | |||||||||||||||
| Stercorarius spp. | o | x | x | ? | x | x | x | x | ||||||||||||
| Larus hyperboreus | o | o | o | o | o | o | o | o | o | o | x | x | x | |||||||
| L. glaucescens | o | o | o | o | o | o | o | o | o | o | x | x | x | |||||||
| L. argentatus | o | o | o | o | o | o | o | o | o | o | x | o | x | |||||||
| L. canus | x | o | o | o | x | x | ||||||||||||||
| Rissa tridactyla | x | x | x | o | ||||||||||||||||
| R. brevirostris | x | x | x | o | ||||||||||||||||
| Sterna paradisaea | x | x | o | o | x | o | ||||||||||||||
| S. aleutica | x | o | ||||||||||||||||||
| Uria aalge | x | o | x | x | o | o | x | o | ||||||||||||
| U. lomvia | x | o | x | x | o | x | o | x | ||||||||||||
| Lunda cirrhata | ? | x | x | |||||||||||||||||
| Fratercula corniculata | * | x | x | |||||||||||||||||
| Cepphus columba | o | x | o | |||||||||||||||||
| Brachyramphus marmoratus | x | o | x | |||||||||||||||||
| B. brevirostris | x | o | ||||||||||||||||||
| Synthliboramphus antiquus | x | x | x | |||||||||||||||||
| Cyclorrhynchus psittaculus | x | o | * | * | ||||||||||||||||
| Aethia cristatella | x | x | ||||||||||||||||||
| A. pusilla | x | o | ||||||||||||||||||
| A. pygmaea | x | |||||||||||||||||||
| Seabirds | Bird trophic levels and food categories | |||||||
|---|---|---|---|---|---|---|---|---|
| II | III | IV | Sc | |||||
| Crustacean | Polychaete | Coelenterate | Fish/squid eggs & larvae | Fish | Cephalopod | Birds | Carrion/offal/detritus | |
| Diomedea nigripes | x | o | o | o | x | x | x | |
| D. immutabilis | x | |||||||
| Fulmarus glacialis | x | o | x | o | x | x | x | |
| Puffinus carneipes | o | x | x | |||||
| P. griseus | x | x | x | |||||
| P. tenuirostris | x | o | x | |||||
| Pterodroma inexpectata | x | x | ||||||
| Oceanodroma furcata | x | o | o | o | x | x | x | |
| O. leucorhoa | x | o | o | o | x | x | x | |
| Phalaropus fulicarius | x | x | o | |||||
| Lobipes lobatus | x | x | o | |||||
| Stercorarius spp. | o | x | x | ? | x | |||
| Larus hyperboreus | x | o | o | o | x | x | ? | x |
| L. glaucescens | x | o | o | o | x | x | ? | x |
| L. argentatus | x | o | o | o | x | x | x | |
| Rissa tridactyla | x | x | x | o | ||||
| Xema sabini | x | x | o | o | ||||
| Sterna paradisaea | x | x | o | o | ||||
| Uria aalge | x | * | x | x | ||||
| U. lomvia | x | * | x | x | ||||
| Lunda cirrhata | o | x | x | |||||
| Fratercula corniculata | * | x | x | |||||
| Cerorhinca monocerata | x | x | ||||||
| Synthliboramphus antiquus | x | x | ||||||
| Cyclorrhynchus psittaculus | x | o | * | * | ||||
| Ptychoramphus aleuticus | x | o | ||||||
Most species (roughly 53% in any community) include two or three prey categories in their diets—usually midwater schooling fish, squid, and crustaceans. These birds include the most numerous in the communities—the shearwaters and some alcids—which feed largely on three prey types, and also include some of the less abundant birds, the marine ducks, which feed mostly on two prey categories.
The remaining seabirds are more general in their feeding. Many have large populations, but are not as abundant as shearwaters or most alcids. The true "generalists" are the species that feed on as many as eight or more types of prey, and relatively few (12%) such species exist in each avian community. These birds, the scavengers, include black-footed albatross, fulmar, storm-petrels, and large gulls. The petrels are the scavengers of the oceanic habitat and the gulls are their counterparts in the neritic habitat (but see Sanger 1973).
Another comparison is shown in Table 17, where the species in each community are categorized according to the number feeding at each trophic level. If a species feeds at more than one level, it is tallied once in each level. Most seabirds (66-77%) feed at the second and third levels as secondary and tertiary carnivores. Few feed as terminal carnivores, and relatively few are scavengers. Actually, most scavenging occurs at levels II and III, so about 90% of the seabirds in each community feed at levels II and III. Communities including an inshore neritic feeding element are the only ones that include herbivores, and even then, few of these species exist in significant numbers in the marine environment (discounting estuaries and sheltered bays).
| Seabirds | Habitat, bird trophic levels (I-IV, Sc), and food categories | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oceanic and offshore neritic | Inshore neritic | |||||||||||||||||||
| II | III | IV | Sc | I | II | III | IV | Sc | ||||||||||||
| C r u s t a c e a n |
P o l y c h a e t e |
C o e l e n t e r a t e |
F i s h - s q u i d e g g s & l a r v a e |
F i s h |
C e p h a l o p o d |
B i r d s |
C a r r i o n / o f f a l / d e t r i t u s |
P l a n t |
C r u s t a c e a n , m i d w a t e r |
C r u s t a c e a n , b e n t h i c |
C o e l e n t e r a t e |
E c h i n o d e r m |
M o l l u s c |
F i s h / s q u i d e g g s & l a r v a e |
F i s h , m i d w a t e r |
F i s h , b e n t h i c |
C e p h a l o p o d |
B i r d s |
C a r r i o n / o f f a l / d e t r i t u s |
|
| Gavia immer | * | x | x | |||||||||||||||||
| G. adamsii | * | o | x | |||||||||||||||||
| G. arctica | o | x | ||||||||||||||||||
| G. stellata | o | x | ||||||||||||||||||
| Podiceps grisegena | o | x | o | |||||||||||||||||
| P. nigricollis | x | o | ||||||||||||||||||
| P. auritus | x | x | o | |||||||||||||||||
| Aechmophorus occidentalis | o | x | x | |||||||||||||||||
| Diomedea nigripes | x | o | o | o | x | x | x | |||||||||||||
| Fulmarus glacialis | x | o | x | o | x | x | x | |||||||||||||
| Puffinus creatopus | o | x | x | |||||||||||||||||
| P. carneipes | o | x | x | |||||||||||||||||
| P. bulleri | x | x | x | |||||||||||||||||
| P. griseus | x | x | x | o | o | o | ||||||||||||||
| P. tenuirostris | x | o | x | o | o | o | ||||||||||||||
| Oceanodroma furcata | x | o | o | o | x | x | x | |||||||||||||
| Pelecanus occidentalis | x | |||||||||||||||||||
| Phalacrocorax auritus | o | x | o | |||||||||||||||||
| P. penicillatus | o | x | ||||||||||||||||||
| P. pelagicus | x | x | ||||||||||||||||||
| Branta bernicla | x | |||||||||||||||||||
| Clangula hyemalis | o | x | o | o | ||||||||||||||||
| Histrionicus histrionicus | o | x | o | |||||||||||||||||
| Melanitta deglandi | x | o | o | |||||||||||||||||
| M. perspicillata | o | x | o | |||||||||||||||||
| M. nigra | * | o | o | x | ||||||||||||||||
| Mergus serrator | * | x | x | |||||||||||||||||
| Haliaeetus leucocephalus | x | x | x | |||||||||||||||||
| Falco peregrinus | x | |||||||||||||||||||
| Phalaropus fulicarius | x | x | o | x | x | o | ||||||||||||||
| Lobipes lobatus | x | x | o | x | x | o | ||||||||||||||
| Stercorarius spp. | o | x | x | ? | x | x | x | o | x | |||||||||||
| Larus hyperboreus | o | o | o | o | ? | o | o | o | o | o | o | x | x | x | ||||||
| L. glaucescens | o | o | o | o | ? | o | o | o | o | o | x | x | x | x | ||||||
| L. occidentalis | x | x | x | x | o | x | o | o | o | * | * | x | x | o | x | |||||
| L. argentatus | o | o | o | o | o | x | o | o | o | o | o | x | o | x | ||||||
| L. heermanni | x | x | x | |||||||||||||||||
| L. canus | x | o | o | o | x | x | x | |||||||||||||
| L. philadelphia | x | x | o | |||||||||||||||||
| Rissa tridactyla | x | x | x | o | ||||||||||||||||
| Xema sabini | x | x | o | o | ||||||||||||||||
| Sterna paradisaea | x | x | o | o | ||||||||||||||||
| S. hirundo | o | x | ||||||||||||||||||
| Uria aalge | x | o | x | x | o | x | o | |||||||||||||
| U. lomvia | x | o | x | x | o | x | o | x | ||||||||||||
| Lunda cirrhata | ? | x | x | |||||||||||||||||
| Fratercula corniculata | * | x | x | |||||||||||||||||
| Cerorhinca monocerata | x | x | ||||||||||||||||||
| Cepphus columba | o | x | o | |||||||||||||||||
| Brachyramphus marmoratus | x | o | x | |||||||||||||||||
| B. brevirostris | x | o | ||||||||||||||||||
| Synthliboramphus antiquus | x | x | o | |||||||||||||||||
| Ptychoramphus aleuticus | x | x | ||||||||||||||||||
| Oceanographic region (domain) | Number of categories in the diets[36] | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5-7 | 7 | 8+ | |
| Bering Sea coastal | 3 | 11 | 9 | 6 | 5 | 4 | 5 |
| Bering Sea | 2 | 6 | 5 | 7 | 0 | 5 | 0 |
| Alaskan Stream | 3 | 14 | 14 | 5 | 4 | 4 | 5 |
| Central Subarctic | 1 | 6 | 8 | 4 | 0 | 7 | 0 |
| North American Coastal | 3 | 14 | 17 | 6 | 3 | 4 | 6 |
| Total | 12 | 51 | 53 | 28 | 12 | 24 | 16 |
| Percent total species (196) | 6 | 26 | 27 | 14 | 6 | 12 | 8 |
It is readily apparent from the foregoing comparisons that much overlap exists in the prey eaten by seabirds within each community. The question whether real competition ever exists is academic. Competition perhaps exists only rarely because seabirds partition resources through use of different feeding methods, selection of different-sized prey, and habitat zonation. Table 18 lists feeding methods (after Ashmole 1971 and Ainley 1977) and the body size and bill length of each species considered in this review. Bill length is usually related directly to body size (Ashmole 1968; Bédard 1969b), but note, for instance, that the longer species of the two kittiwakes has the shorter bill. Body weight would be a better measure of relative size than body size, but few reliable weight data are available for seabirds.
The use of different feeding methods by species in each community grossly assigns birds to feeding at different depths. Thus, whereas shearwaters, puffins, and small gulls (Xema sp., Rissa spp.) overlap almost entirely in prey categories and even prey species, the gulls can capture these organisms only at the surface; the shearwaters capture them at shallow depths; and the puffins capture them at much deeper depths. Direct field observations of this phenomenon are few but Gould (1971) and Sealy (1973a) compared the diets of birds feeding in mixed-species flocks. An example of how even finer divergence in feeding methods helps to partition food resources has been provided by Spring (1971) in his comparison of the two murres. Both species feed by diving to great depths, but the thick-billed murre is able to hover over the bottom and thereby is better able to capture benthic organisms.
| Domain | Oceanic/offshore neritic | Inshore neritic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| II | III | IV | Sc | I | II | III | IV | Sc | |
| Bering Sea Coastal | 11 | 17 | 1? | 10 | 6 | 23 | 18 | 6 | 6 |
| Bering Sea | 22 | 21 | 3? | 11 | — | — | — | — | — |
| Alaska Stream | 21 | 19 | 1? | 12 | 5 | 28 | 21 | 6 | 6 |
| Central Subarctic | 23 | 22 | 3? | 12 | — | — | — | — | — |
| North American Coastal | 25 | 24 | 3? | 11 | 3 | 28 | 35 | 7 | 10 |
| Total | 102 | 103 | 11? | 56 | 14 | 79 | 74 | 19 | 22 |
| Proportion | 0.38 | 0.39 | 0.02[37] | 0.21 | 0.07 | 0.38 | 0.28 | 0.09 | 0.10 |
| Species | Body length[38] (cm) | Bill length[39] (mm) | Feeding[40] method |
|---|---|---|---|
| Gavia adamsii | 63.5 | 90-91 | D |
| G. immer | 61.0 | 80-82 | D |
| G. arctica | 45.7 | 51-52 | D |
| G. stellata | 43.5 | 51-52 | D |
| Podiceps grisegena | 33.0 | 48-50 | D |
| P. nigricollis | 22.9 | 24-26 | D |
| P. auritus | 24.1 | 23-24 | D |
| Aechmophorus occidentalis | 45.7 | 65-76 | D |
| Diomedea nigripes | 71.1 | 141-144 | SS |
| D. immutabilis | 71.1 | 102-112 | SS |
| Fulmarus glacialis | 45.7 | 36-37 | SS |
| Puffinus carneipes | 45.7 | 41-46 | PP |
| P. creatopus | 45.7 | 41-46 | PP |
| P. bulleri | 38.1 | 38-45 | PP |
| P. griseus | 40.3 | 41-42 | PP |
| P. tenuirostris | 38.1 | 31-32 | PP |
| Oceanodroma furcata | 19.0 | 15 | Di,SS |
| O. leucorhoa | 19.0 | 16 | Di,SS |
| Pterodroma inexpectata | 29.2 | 26-27 | SS |
| Phalacrocorax auritus | 68.6 | 55-57 | D |
| P. penicillatus | 73.7 | 66-71 | D |
| P. urile | 71.1 | 54-55 | D |
| P. pelagicus | 55.9 | 47-50 | D |
| Pelecanus occidentalis | 104.0 | 294-319 | P |
| Branta spp. (bernicla) | 43.5 | 33-36 | T |
| Philacte canagica | 45.7 | 37-42 | T |
| Anas spp. | 40.0 | 32-35 | T |
| Clangula hyemalis | 38.1 | 25-27 | D |
| Histrionicus histrionicus | 30.5 | 25-28 | D |
| Polysticta stelleri | 30.5 | 37-43 | D |
| Somateria mollisima | 43.5 | 45-55 | D |
| S. spectabilis | 40.3 | 31-33 | D |
| S. fischeri | 38.1 | 22-26 | D |
| Melanitta deglandi | 35.6 | 41-44 | D |
| M. perspicillata | 40.3 | ca. 40 | D |
| M. nigra | 35.6 | 42-47 | D |
| Mergus serrator | 40.3 | 45-54 | D |
| Haliaeetus leucocephalus | 80.0 | 52-54 | X |
| Falco peregrinus | 37.5 | 21-25 | X |
| Phalaropus fulicarius | 16.5 | 22 | SS |
| Lobipes lobatus | 15.2 | 22 | SS |
| Stercorarius pomarinus | 43.5 | 40 | SS,A |
| S. parasiticus | 40.3 | 32 | SS,A |
| S. longicaudus | 38.1 | 29 | SS,A |
| Larus hyperboreus | 61.0 | 55-60 | SS |
| L. glaucescens | 55.9 | 54-58 | SS |
| L. occidentalis | 53.0 | 54-57 | SS,Di |
| L. argentatus | 50.8 | 48-54 | SS,Di |
| L. californicus | 43.5 | 45-50 | SS,Di |
| L. heermanni | 38.1 | 42-46 | SS,Di |
| L. canus | 35.6 | 34-36 | SS,Di |
| L. philadelphia | 27.9 | 30-31 | Di |
| Rissa tridactyla | 34.2 | 39-40 | Di |
| R. brevirostris | 38.1 | 29-30 | Di |
| Xema sabini | 27.9 | 26-27 | Di |
| Sterna paradisaea | 38.1 | 31-33 | Di,SP |
| S. hirundo/forsteri | 35.6 | 36-39 | Di,SP |
| S. aleutica | 33.0 | 33 | Di,SP |
| Uria aalge | 35.6 | 43-47 | D |
| U. lomvia | 35.6 | 39-42 | D |
| Lunda cirrhata | 31.8 | 57-60 | D |
| Fratercula corniculata | 29.2 | 49-51 | D |
| Cerorhinca monocerata | 29.2 | 34-35 | D |
| Cepphus columba | 26.7 | 32-33 | D |
| Brachyramphus marmoratus | 20.3 | 15 | D |
| B. brevirostris | 19.0 | 10 | D |
| Synthliboramphus antiquus | 20.3 | 13 | D |
| Ptychoramphus aleuticus | 17.8 | 19 | D |
| Aethia pygmaea | 16.5 | 8-9 | D |
| A. pusilla | 13.3 | 8 | D |
| A. cristatella | 17.8 | 11 | D |
| Cyclorrhynchus psittaculus | 18.4 | 15 | D |
The scavengers (generalists) offer a good example of how a range of bird and bill sizes is usually represented among species having similar diets and feeding methods. The progression of oceanic scavenger sizes is graded rather evenly from the black-footed albatross down to the northern fulmar, to the scaled petrel, to the storm-petrel. All these species capture prey that occur only at or near the water surface. Recently Sanger (1973) reported appreciable numbers of glaucous-winged gulls (Larus glaucescens) and herring gulls (L. argentatus), noted neritic scavengers, out in the oceanic realm of the petrel. He presented limited data that suggested an overlap between the diet of these gulls and that of black-footed albatrosses, as noted by Miller (1940). It would not be surprising if these gulls were as much generalists in the oceanic habitat as they are in the neritic. Interestingly, their bill and body sizes fall between those of the albatross and the fulmar, thus in theory enabling them to invade the oceanic habitat without great competition. It is likely that their invasion occurred during historical times and is related to their habit of following fishing boats from shore out to sea (Sanger 1973). If so, the gulls might be assuming from other species part of a previously uncontested resource.
Another interesting group of species that shows close similarities in diet consists of the piscivorous loons, grebes, and mergansers. All these birds, including seven or eight species, apparently feed on fish occurring on or near the bottom in the inshore neritic habitat. Again, however, an even progression in size exists: yellow-billed loon (Gavia adamsii), common loon (G. immer), arctic loon (G. arctica), red-throated loon (G. stellata), western grebe (Aechmophorus occidentalis), red-necked grebe (Podiceps grisegena), and com[Pg 118]mon merganser (Mergus merganser). Most likely then, they select different-sized fish. Another example of this phenomenon is provided by the eight neritic gulls, which are largely scavengers and show a remarkably even progression in bill and body size. Finally, as shown clearly by Bédard (1969a, 1969b) and Harris (1970), alcids of different sizes select different-sized prey, often of the same species.
A final important way in which seabirds partition available resources is by inhabiting different zones. Zonation is especially evident during the breeding season when species common to the same breeding site sort themselves out according to the distances they range for food. This phenomenon was discussed by Murphy (1936), Shuntov (1974), Sealy (1972), Cody (1973), and Scott (1973).
The species that appear to have specialized food habits (if further research confirms that indeed they do) are probably very sensitive to vagaries in food availability or are, at least, much more sensitive than other species. Some specialists which also have very restricted distributions would, therefore, be susceptible to localized catastrophes occurring where specialists are concentrated around the food resource. This is proved in the case of the scoters, which are both specialized and rather restricted to nearshore beds of molluscs and have fallen victim to local oil slicks (Smail et al. 1972). An example of another potentially critical situation is that of the black brant, which at certain times of the year concentrate their entire population around eelgrass beds in Bristol Bay, Alaska, where much offshore oil drilling may soon occur.
Birds adapted to feed by diving, with the exception of cormorants, spend most of their time in the water. These species are therefore most susceptible to oiling (Smail et al. 1972), but pursuit plungers (the shearwaters) are also highly susceptible (Point Reyes Bird Observatory, unpublished data). A characteristic of polar and subpolar seabird communities is the high percentage of birds that feed by diving and pursuit plunging. These birds are mostly absent from tropical and subtropical communities because feeding by these methods is not adaptive there (Ainley 1977). Hence, oil pollution has all the potential of rendering maladaptive the principal feeding methods of many polar seabirds.
Another way in which seabird feeding relates to conservation problems concerns competition between birds and man for commercially valuable fishes. A related problem is the mass mortality of seabirds due to man's fishing gear. An acute situation is the drowning of seabirds caught in salmon gill nets (Bartonek et al. 1974; Pacific Seabird Group 1975; Ripley 1975; King et al., this volume). Immediate action is definitely required.
Further, competition between birds and man for the same resource has the potential for disastrous effects on bird populations if humans out-compete the birds and overfish the resource. A classic example, reviewed by Idyll (1973), is the possible collapse of the Peruvian anchovy (Engraulis ringens) fishery; if overfishing and an El Niño should coincide, the Peruvian seabird populations could collapse as well. The California fisheries and apparently the double-crested cormorants that nest on the Farallon Islands have both suffered from the demise of the Pacific sardine (Sardinops caerulea) in the California current (Ainley and Lewis 1974). In regulating fish harvests, fishery organizations should include in their calculations the harvest by creatures other than man (Schaefer 1970), rather than evading the issue by referring to a vague "natural mortality."
Finally, fishing by humans can benefit seabirds by removing fish (or whales) that compete with birds for food (Laws 1977). A potential example is that of northern California, where salmon and seabirds both feed heavily on juvenile rockfishes (Fitch and Lavenberg 1971; Point Reyes Bird Observatory, unpublished data). Harvest of salmon should theoretically leave more rockfish available for birds to eat. This sort of situation has not yet been fully documented and definitely warrants further study, especially in such areas as the Bering Sea, where some fish stocks have become depressed due to overfishing (Gulland 1970).
Many people realize intuitively that seabirds are important members of marine ecosystems. Although the supporting evidence is not now available, it will be needed if seabirds are to be protected. Emotion alone will not justify the protection of seabirds in an age when the human race moves steadily toward global famine. The job at hand is, in part, to sell seabirds, not just to the public, government officials, executives of oil companies, or fish-packing concerns, but also to marine biologists and oceanographers, for the scientists have the best means to study organisms at sea. We must move away from the concept that seabirds are merely yo-yos of various sizes, shapes, and colors on strings of various lengths that venture forth to sea from the land, grab a quick lunch, and then return to the safety of terra firma. Seabirds are marine organisms and deserve at least as much research attention as that currently given marine mammals.
The information now available on seabird diets is largely presented in terms of the number and volume of various prey species taken. Whereas these data provide the relative importance of prey, fishery data on prey stocks are usually measured in terms of biomass. Thus, it is difficult to relate seabird data to the immense wealth of information on biological oceanography. If we are to recognize the importance of seabirds in the nutrient and energy cycling of marine ecosystems, rather than considering them merely as "yo-yo predators," we must relate them to the total marine community.
The goal of marine ornithologists should be to refine and broaden considerably in detail such studies as those by Sanger (1972), Shuntov (1974), and Laws (1977), who attempted to assess the relations between seabird populations and stocks of other marine organisms for the northern North Pacific, the world oceans, and the Antarctic, respectively. The trophic roles played by seabirds must be studied in detail at the community level year-round before those analyses can be properly refined. Another exemplary work is that done by Brownell (1974), who studied trophic relations of higher vertebrates off Uruguay, including dolphins, pinnipeds, seabirds, and some large fish. In a review study, Sanger (1974) considered the food-chain relations of similar vertebrates in the Bering Sea. These sorts of studies will serve to bring the role of seabirds into perspective with other upper trophic level feeders.
We much appreciate the opportunity to participate in the symposium at which this paper was presented. The encouragement and help given by J. C. Bartonek was indispensable. D. G. Ainley's participation in the symposium was supported by the Point Reyes Bird Observatory. This is contribution No. 124 of the Point Reyes Bird Observatory.
Ainley, D. G. 1977. Feeding methods of seabirds: a comparison of polar and tropical communities. Pages 669-685 in G. A. Llano, ed. Adaptations in Antarctic ecosystems. Gulf Publishing Co., Houston, Texas.
Ainley, D. G., and T. J. Lewis. 1974. The history of Farallon Island marine bird populations, 1854-1972. Condor 76:432-446.
Ashmole, N. P. 1968. Body size, prey size and ecological segregation in five sympatric tropical terns (Ayes; Laridae). Syst. Zool. 17:292-304.
Ashmole, N. P. 1971. Sea bird ecology and the marine environment. Pages 223-286 in D. S. Farner and J. R. King, eds. Avian Biology, Vol. 1. Academic Press, N. Y.
Ashmole, N. P., and M. J. Ashmole. 1967. Comparative feeding ecology of sea birds of a tropical oceanic island. Peabody Mus. Nat. Hist., Yale Univ., Bull. 24:1-131.
Bailey, A. 1922. Notes on the yellow-billed loon. Condor 24:204-205.
Bailey, A. 1927. Notes on the birds of southeastern Alaska. Auk 44:1-23.
Banner, A. H. 1954. New records of Mysidacea and Euphausiacea from the northeastern Pacific and adjacent areas. Pac. Sci. 8:125-139.
Bartonek, J. C., R. Elsner, and F. H. Fay. 1974. Mammals and birds. Pages 23-28 in E. J. Kelly and D. W. Hood, eds. Probes: a prospectus on processes and resources of the Bearing Sea shelf. Inst. Mar. Sci., Univ. Alaska, Fairbanks, Publ. Inf. Bull. 74-1.
Bartonek, J. C., and D. D. Gibson. 1972. Summer distribution of pelagic birds in Bristol Bay, Alaska. Condor 74:416-422.
Bartsch, P. 1922. A visit to Midway Island. Auk 39:481-488.
Bédard, J. 1969a. Feeding of the least, crested, and parakeet auklets around St. Lawrence Island, Alaska. Can. J. Zool. 47:1025-1050.
Bédard, J. 1969b. Adaptive radiation in Alcidae. Ibis 111:189-198.
Belopol'skii, L. O. 1961. Ecology of sea colony birds of the Barents Sea. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 346 pp.
Bent, A. C. 1921. Life histories of North American gulls and terns. U.S. Natl. Mus. Bull. 113. 345 pp.
Bent, A. C. 1922. Life histories of North American petrels and pelicans and their allies. U.S. Natl. Mus. Bull. 121. 343 pp.
Bent, A. C. 1925. Life histories of North American wild fowl. U.S. Natl. Mus. Bull. 130. 311 pp.
Bent, A. C. 1946. Life histories of North American diving birds. (Reprint of U.S. Natl. Mus. Bull. 107.) Dodd, Mead and Co., N. Y. 237 pp.
Brownell, R. L., Jr. 1974. Feeding ecology of the Franciscana Dolphin, Pontoporia blainvillei, and associated top trophic vertebrates in Uruguayan waters. Ph.D. Thesis. Univ. Tokyo, Tokyo, Japan.
Chatwin, S. L. 1956. The western grebe taken on hook and line. Condor 58:73-74.
Cleaver, F. C., and D. M. Franett. 1945. The predation by seabirds upon the eggs of the Pacific herring (Clupea pallasii) at Holmes Harbor during 1945. State of Washington, Department of Fisheries, Biological Report 46B. 18 pp.
Cody, M. L. 1973. Coexistence, coevolution and convergent evolution in seabird communities. Ecology 54:31-44.
Cottam, C. 1939. Food habits of North American diving ducks. U.S. Dep. Agric., Tech. Bull. 643. 140 pp.
Cottam, C., and P. Knappen. 1939. Food of some uncommon North American birds. Auk 56:138-169.
Craddock, D. R., and R. D. Carlson. 1970. Peregrine falcon observed feeding far at sea. Condor 72:375-376.
Dement'ev, G. P., R. N. Meklenburtsev, A. M. Sudilovskaya, and E. P. Sangenberg. 1968. Birds of the Soviet Union. Vol. 2. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 553 pp.
Divoky, G. J. 1976. The pelagic feeding habits of the Ivory and Ross' gulls. Condor 78:85-90.
Dodimead, A. J., F. Favorite, and T. Hirano. 1963. Review of oceanography of the subarctic Pacific region. Int. North Pac. Fish. Comm. Bull. 13. 195 pp.
Drent, R. H. 1965. Breeding biology of the pigeon guillemot, Cepphus columba. Ardea 53:99-160.
Dunbar, M. J. 1946. On Themisto libellula in Baffin Island coastal waters. J. Fish. Res. Board Can. 6:419-434.
Einarrson, H. 1945. Euphausiacea. I. North Atlantic species. Dana-Rep. Carlsberg Found. 27. 185 pp.
Falla, R. A. 1934. The distribution and breeding habits of petrels in northern New Zealand. Rec. Aukl. Inst. Mus. I:245-260.
Favorite, F., A. J. Dodimead, and K. Nasu. 1976. Oceanography of the subarctic Pacific region, 1960-71. Int. North Pac. Fish. Comm. Bull. 33:1-187.
Fay, F. H., and T. J. Cade. 1959. An ecological analysis of the avifauna of St. Lawrence Island, Alaska. Univ. Calif. Publ. Zool. 63:73-150.
Fisher, J. 1952. The fulmar. Collins, London. 496 pp.
Fisher, W. K. 1904. On the habits of the Laysan albatross. Auk 21:8-19.
Fitch, J. E., and R. J. Lavenberg. 1971. Marine food and game fishes of California. Univ. Calif. Press, Berkeley. 179 pp.
Follett, W. I., and D. G. Ainley. 1976. Fishes collected by pigeon guillemots, Cepphus columba Pallas, nesting on Southeast Farallon Island, California. Calif. Fish Game 62:28-31.
Friedmann, H. 1950. Birds of North and middle America. Part XI. U.S. Natl. Mus. Bull. 50. 793 pp.
Gabrielson, I. N., and S. G. Jewett. 1940. Birds of Oregon. Oregon State College, Corvallis. 650 pp.
Gabrielson, I. N., and F. C. Lincoln. 1959. Birds of Alaska. Stackpole Co., Harrisburg, Pennsylvania. 922 pp.
Gould, P. J. 1971. Interactions of seabirds over the open ocean. Ph.D. Thesis. Univ. of Arizona, Tucson. 110 pp.
Grinnell, J. 1897. Notes on the marbled murrelet. Osprey 1:115-117.
Grinnell, J. 1899. The rhinoceros auklet at Catalina Island. Bull. Cooper Ornithol. Club 1:87-88.
Gulland, J. A. 1970. The fish resources of the ocean. FAO, Rome. 255 pp.
Hamilton, W. J. 1958. Pelagic birds observed on a north Pacific crossing. Condor 60:159-164.
Harris, M. P. 1965. The food of some Larus gulls. Ibis 107:43-53.
Harris, M. P. 1970. Differences in the diet of British auks. Ibis 112:540-541.
Hart, J. L. 1973. Pacific fishes of Canada. Fish. Res. Board Can. Bull. 180. 740 pp.
Hartley, C. H., and J. Fisher. 1936. The marine foods of birds in an inland fjord region of West Spitsbergen. Part 2. Birds. J. Anim. Ecol. 5:370-389.
Heath, H. 1915. Birds observed on Forrester Island, Alaska, during the summer of 1913. Condor 17:20-41.
Hubbs, C. L., A. L. Kelly, and C. Limbaugh. 1970. Diversity in feeding by Brandt's cormorant near San Diego. Calif. Fish Game 53:156-165.
Idyll, C. P. 1973. The anchovy crisis. Sci. Am. 228:22-29.
Imber, M. J. 1973. The food of grey-faced petrels (Pterodroma macroptera gouldi Hutton), with special reference to diurnal migration of their prey. J. Anim. Ecol. 42:645-662.
Ingolfsson, A. 1967. The feeding ecology of five species of large gulls (Larus) in Iceland. Ph.D. Thesis. Univ. of Michigan, Ann Arbor. 186 pp.
Jewett, S. G., W. P. Taylor, W. T. Shaw, and J. W. Aldrich. 1953. Birds of Washington State. Univ. of Washington Press, Seattle. 767 pp.
Koelink, A. F. 1972. Bioenergetics of growth in the pigeon guillemot, Cepphus columba. M. S. Thesis. Univ. of British Columbia, Vancouver, Canada. 71 pp.
Komaki, Y. 1967. On the surface swarming of euphausiid crustaceans. Pac. Sci. 21:433-448.
Kooyman, G. L. 1974. Behaviour and physiology of diving. Pages 115-138 in B. Stonehouse, ed. The biology of penguins. Macmillan, London.
Kortright, F. H. 1942. The ducks, geese and swans of North America. Wildlife Management Institute, Washington, D.C., and Stackpole Co., Harrisburg, Pennsylvania. 476 pp.
Kozlova, E. V. 1961. Fauna of the U.S.S.R., Birds: Alcae. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 140 pp.
Kuroda, N. 1954. On the classification and phylogeny of the order Tubinares, particularly the shearwaters (Puffinus). Herald Co., Tokyo. 179 pp.
Kuroda, N. 1955. Observations on pelagic birds of the Northwest Pacific. Condor 57:290-300.
Laws, R. M. 1977. The significance of vertebrates in the Antarctic marine ecosystem. Pages 411-438 in G. A. Llano, ed. Adaptations in Antarctic ecosystems. Gulf Publishing Co., Houston, Texas.
Linton, C. B. 1908. Notes from Santa Cruz Island. Condor 10:124.
Mabbot, D.C. 1920. Food habits of seven species of American shoal-water ducks. U.S. Dep. Agric., Bull. 862. 68 pp.
Madsen, F. J. 1957. On the food habits of some fish-eating birds in Denmark. Dan. Rev. Game Biol. 3(2):19-83.
Manuwal, D. A. 1974. The natural history of Cassin's auklet (Ptychoramphus aleuticus). Condor 76:421-431.
Martin, P. W. 1942. Notes on some pelagic birds off the coast of British Columbia. Condor 44:27-29.
Martin, P. W., and M. T. Myers. 1969. Observations on the distribution and migration of some seabirds off the outer coasts of British Columbia and Washington State, 1946-1949. Syesis 2:241-256.
McGilvrey, E. B. 1967. Food habits of sea ducks from the northeastern United States. Pages 142-145 in Wildfowl Trust 18th annual report.
Miller, L. 1936. Some maritime birds observed off San Diego, California. Condor 38:9-16.
Miller, L. 1940. Observations on the black-footed albatross. Condor 42:229-238.
Munro, J. A. 1941. Studies of waterfowl in British Columbia: the grebes. B. C. Prov. Mus. Occas. Pap. 3. 71 pp.
Munro, J. A., and W. A. Clemens. 1931. Waterfowl in relation to the spawning of herring in British Columbia. Biol. Board Can. Bull. 17. 46 pp.
Munro, J. A., and W. A. Clemens. 1939. The food and feeding habits of the red-breasted merganser in British Columbia. J. Wildl. Manage. 3:46-53.
Murphy, R. C. 1936. Oceanic birds of South America, Vol. 2. Macmillan, N. Y. 604 pp.
Ogi, H., and T. Tsujita. 1973. Preliminary examination of stomach contents of murres (Uria spp.) from the eastern Bering Sea and Bristol Bay, June-August, 1970 and 1971. Jpn. J. Ecol. 23:201-209.
Oliver, W. R. B. 1955. New Zealand birds, 2nd ed. A. H. and A. W. Reed, Wellington, New Zealand.
Pacific Seabird Group. 1975. Policy statement: Incidental seabird kills from salmon gillnet fisheries. Pac. Seabird Group Bull. 2:19-20.
Palmer, R. S. 1962. Handbook of North American birds. Vol. 1. Yale Univ. Press, New Haven, Connecticut.
Pearson, T. H. 1968. The feeding biology of sea bird species breeding on the Farne Islands, Northumberland. J. Anim. Ecol. 37:521-551.
Perthon, P. 1968. Food and feeding habits of the common eider (Somateria mollissima). Nytt. Mag. Zool. 15:97-111.
Phillips, R. E., and G. D. Carter. 1957. Winter food of western grebes. Murrelet 38:5-6.
Preble, E. A., and W. L. McAtee. 1923. A biological survey of the Pribilof Islands, Alaska. U.S. Bur. Biol. Surv. N. Am. Fauna 46. 255 pp.
Richardson, F. 1961. Breeding biology of the rhinoceros auklet on Protection Island, Washington. Condor 63:456-473.
Ripley, S. D. 1975. The view from the castle. Smithsonian 6:6.
Robbins, C. S., B. Brunn, and H. S. Zim. 1966. Birds of North America. Golden Press, N. Y. 340 pp.
Roberts, A. A., and E. H. Huntington. 1959. Food habits of the merganser in New Mexico. N. M. Dep. Fish Game, Bull. 9. 36 pp.
Robertson, I. 1974. The food of nesting double-crested and pelagic cormorants at Mandarte Island, British Columbia, with notes on feeding ecology. Condor 76:346-348.
Robertson, I. 1972. Studies on fish-eating birds and their influence on stocks of the Pacific herring, Clupea harengus, in the Gulf Islands of British Columbia. Herring Investigations, Pac. Biol. Stn., Naimo, B.C. 28 pp. (Unpublished report)
Sanger, G. A. 1972. Preliminary standing stock and biomass estimates of seabirds in the subarctic Pacific region. Pages 589-611 in A. Y. Takenouti et al., eds. Biological oceanography of the northern north Pacific Ocean. Idemitsu Shoten, Tokyo. 626 pp.
Sanger, G. A. 1973. Pelagic records of glaucous-winged and herring gulls in the north Pacific Ocean. Auk 90:384-393.
Sanger, G. A. 1974. A preliminary look at marine mammal food-chain relationships in Alaskan waters. U.S. Natl. Mar. Fish. Serv., Mar. Mammal Div., Seattle. 29 pp. (Unpublished report)
Schaefer, M. S. 1970. Men, birds and anchovies in the Peru current—dynamic interactions. Trans. Am. Fish. Soc. 99:461-467.
Scott, J. M. 1973. Resource allocation in four synoptic species of marine diving birds. Ph.D. Thesis. Oregon State Univ., Corvallis. 97 pp.
Scott, J. M., J. Butler, W. G. Pearcy, and G. A. Bertrand. 1971. Occurrence of the Xantus' murrelet off the Oregon coast. Condor 73:254.
Sealy, S. G. 1972. Adaptive differences in breeding biology in the marine bird family Alcidae. Ph.D. Thesis. Univ. of Michigan, Ann Arbor. 283 pp.
Sealy, S. G. 1973a. Interspecific feeding assemblages of marine birds off British Columbia. Auk 90:796-802.
Sealy, S. G. 1973b. Breeding biology of the horned puffin on St. Lawrence Island, Bering Sea, with zoogeographical notes on the North Pacific puffins. Pac. Sci. 27:99-119.
Sealy, S. G. 1975. Feeding ecology of the ancient and marbled murrelets near Langara Island, British Columbia. Can. J. Zool. 53:418-433.
Serventy, D. L., V. Serventy, and J. Warham. 1971. The handbook of Australian sea birds. A. H. and A. W. Reed, Wellington, New Zealand. 254 pp.
Sheard, K. 1953. Taxonomy, distribution and development of the Euphausiacea (Crustacea). Rep. B.A.N.Z. Antarct. Res. Exped., Ser. B (Zoology and Botany), 8:1-72.
Shuntov, V. P. 1972. Sea birds and the biological structure of the ocean. (Transl. from Russian.) Bur. Sport Fish. Wildl. and Natl. Sci. Found., Washington, D.C. Nat. Tech. Inf. Serv. TT 7455032. 566 pp.
Smail, J., D. G. Ainley, and H. Strong. 1972. Notes on birds killed in the 1971 San Francisco oil spill. Calif. Birds 3:25-32.
Spaans, A. L. 1971. On the feeding ecology of the herring gull Larus argentatus Pont. in the northern part of the Netherlands. Ardea 59:73-188.
Spring, L. 1971. A comparison of functional and morphological adaptations in the common murre (Uria aalge) and thick-billed murre (Uria lomvia). Condor 73:1-27.
Stejneger, L. 1885. Results of ornithological explorations in the Commander Islands and Kamchatka. U.S. Natl. Mus. Bull. 29:7-362.
Sverdrup, H. V., M. W. Johnson, and R. H. Fleming. 1942. The oceans: their physics, chemistry and general biology. Prentice-Hall, Englewood Cliffs, N. J. 1087 pp.
Swartz, L. G. 1966. Sea-cliff birds. Pages 611-678 in N. J. Wilimovsky and J. N. Wolfe, eds. Environment of the Cape Thompson region, Alaska. U.S. Atomic Energy Commission, Div. Tech. Inf., Oak Ridge, Tennessee. 1250 pp.
Thoresen, A. C., and E. S. Booth. 1958. Breeding activities of the pigeon guillemot, Cepphus columba columba (Pallas). Dep. Biol. Sci., Walla Walla College, Washington, Publ. 23. 34 pp.
Tuck, L. M., and H. J. Squires. 1955. Food and feeding habits of Brunnich's murre (Uria lomvia lomvia) on Akpatok Island. J. Fish. Res. Board Can. 12:781-792.
Uspenski, S. M. 1958. The bird bazaars of Novaya Zemlya. (Transl. from Russian.) Transl. by Department of Northern Affairs and Natural Resources, Canada. 159 pp.
Ward, J. G. 1973. Reproductive success, food supply, and the evolution of clutch-size in the glaucous-winged gull. Ph.D. Thesis. Univ. of British Columbia, Vancouver, Canada. 119 pp.
Wetmore, A. 1924. Food and economic relations of North American grebes. U.S. Dep. Agric., Bull. 1196. 23 pp.
White, C. M., W. B. Emison, and F. S. L. Williamson. 1971. Dynamics of raptor predation on Amchitka Island, Alaska. Bioscience 21:623-627.
White, C. M., W. B. Emison, and F. S. L. Williamson. 1973. DDE in a resident Aleutian Island peregrine population. Condor 75:306-311.
Zelikman, E. A. 1961. The behavior pattern of the Barents Sea Euphausiacea and possible causes of seasonal vertical migrations. Int. Rev. Ges. Hydrobiol. Hydrogr. 46:276-281.
[15] Present address: U.S. Fish and Wildlife Service, Office of Biological Services, 1011 East Tudor Road, Anchorage, Alaska 99503.
[16] Other incidental prey were squid and atherinid fishes, both at the Farallon Islands.
[17] Other incidental prey were squid and such fishes as atherinids, Zaniolepis, Genyonemus and Peprilus at the Farallones, and atherinids, Trachurus and Heterostichus at San Diego.
[18] Other incidental prey were polychaetes at Netarts and the Farallon Islands.
[19] Principal sources: Bent 1925; Cleaver and Franett 1945; Cottam 1939; Cottam and Knappen 1939; Kortright 1942; Mabbot 1920; McGilvrey 1967; Munro and Clemens 1939; Roberts and Huntington 1959.
[20] Other incidental items were the fish Cololabis and Peprilus at the Farallon Islands.
[21] Other incidental items were myctophid fish in northeastern Canada.
[22] Other incidental items included the lamprey (Lampetra) at the Farallon Islands.
[23] Bent (1946) listed "fish" as prey.
[24] Grinnell (1897) listed "fish" as the major dietary component.
[25] Bédard (1969a) also listed "fish" as an incidental item.
[26] Other incidental prey were copepods and isopods.
[27] Other incidental prey were pholids in Denmark.
[28] Other incidental prey were copepods and cephalopods in North Atlantic areas.
[29] Other incidental prey were isopods in western North America and fish eggs near Vancouver Island.
[30] Other incidental prey were fish eggs in Denmark.
[31] Offal from wounded whales and seals, and bits of food, primarily crustaceans and fish, from feeding whales are important scavenger foods (Bent 1922).
[32] Other incidental prey were isopods in the North Pacific.
[33] Other incidental prey were the fish Merluccius at the Farallon Islands.
[34] Other incidental prey were isopods near the Pribilofs and in the Chukchi Sea, and amphipods in the latter area; Bent (1921) considered "crustaceans" to be major prey.
[35] Study conducted during period of breeding failure.
[36] These are the "food categories" of Tables 11-15. Items included in diets are not included here.
[37] Proportion based on the arbitrary assumption that half (5) of the 11 species in question catch and eat birds at sea.
[38] Information on body sizes (length) is from Robbins et al. (1966).
[39] Information on bill lengths is from Palmer (1962), Dement'ev et al. (1968), and Friedmann (1950).
by
William H. Drury
College of the Atlantic
Bar Harbor, Maine 04609
It seems only reasonable to assume that populations of marine birds fluctuate even when not disturbed by man; such fluctuations would result both from the secondary effects of species adaptive tactics and from changes in the marine environment. I briefly review some human activities and some other natural processes that have resulted in changes in numbers and distribution of seabirds and present a short discussion of theoretical models which emphasizes that conclusions drawn or predictions made from models of the dynamics of populations depend upon the assumptions about stability that were used in preparing the models. I then review those special characteristics of seabirds which are directly relevant to planning programs intended to protect seabirds or encourage their increase and identify several goals for improving our understanding of the population dynamics and biology of marine birds. My general conclusion is that enough is already known to undertake effective conservation programs, and that time is pressing.
Seabirds have been categorized as renewable resources in only a few places, although their symbolic value has been recognized for centuries (for example, the medieval poem "The Seafarer" and the designs on Saint Cuthbert's tunic). With the exception of the Russians (Belopol'skii 1961; Uspenski 1956), the Australians (Serventy 1967), and the Icelanders, industrialized peoples have not considered seabirds to be salable and therefore worth managing. Yet during many centuries the seabirds of the northern seas were a major food for coastal and island villages (Bent 1919, 1921, 1922; Fisher and Lockley 1954).
Some biological principles that affect the dynamics of seabird populations are identified in this paper. I believe these principles must form the basis of plans to maintain and increase seabird numbers.
I describe some observations of population changes, review briefly the conflicting theoretical frameworks for population dynamics, and identify some of the biological characteristics of marine birds that affect the way in which population changes occur. The terms "seabirds" and "marine birds" are used interchangeably for those bird species which depend upon salt water for some part of their annual cycle (c.f., the Pacific Seabird Group).
Broadly stated, the populations of northern seabirds have shown marked short-and long-term fluctuations. Most authors have assumed that all such fluctuations reflect human disturbance of the natural system, because of the obvious effects of human predation during the last 200 years.
In the centuries before people traveled extensively between islands, seabirds were taken in ways that we judge must have allowed the survival of the colonies (e.g., those at the Faroes or Saint Kilda, those in Iceland and Greenland, or those in the Aleutian Islands and the Bering Strait). We presume either that the populations of island peoples were regulated by shortage of resources other[Pg 124] than seabirds or that those who overcropped and eliminated the seabirds suffered the consequences.
When a sea-going, commodity-oriented way of life evolved, seabirds were killed in huge numbers for such uses as the plumage trade, fish bait, or rendering into oil (Tuck 1960; Fisher and Lockley 1954). Even the elimination of several colonies—e.g., Funk Island, Newfoundland (Tuck 1960); Seal Island, Eastern Egg Rock, Maine (Norton 1921); Muskeget, Massachusetts (Forbush 1929)—may have had little effect on the rate of cropping because those who killed off one source could probably seek out another. As the colonial seabirds became scarce they became more valuable, which stimulated more intensive pursuit of the remnants (Dutcher 1901, 1904).
In some places where seabird colonies did not supply a croppable economic resource, the islands were used for alternative crops with at least temporary commodity value (e.g., foxes were introduced in the Aleutian Islands; Bent 1919). Large herbivores were introduced to supply meat for island residents (e.g., Saint Matthew Island; Klein 1959), as well as pigs, cattle, sheep, goats, and rabbits on islands in the North Atlantic and southern oceans (many authors). Increases in many seabird populations over the last 75 years have been generally associated with relief from predation by humans such as the fowlers, eggers, and plume hunters of the 19th century. Such relief may have been partly responsible for the increase of North Atlantic gannets, Sula bassana, and common murres or guillemots, Uria aalge (Fisher and Vevers 1943, 1944; Cramp et al. 1974). On a smaller scale, several population increases along the coast of New England have been recorded following the enactment of protective legislation (Dutcher 1901, 1904; Norton 1921, 1924; Palmer 1949; Drury 1973).
Coulson (1974) argued that in addition to relief from predation, the explosion of the population of kittiwakes (Rissa tridactyla) in this century resulted from access to previously un-occupiable breeding sites. Nesting cliffs and buildings suitable for kittiwake nesting are abundant and now protected from egging or fowling.
There can be little doubt that human activities have also had marked positive effects in some cases. For example, Fisher (1952) suggested that the North Atlantic fulmar (Fulmarus glacialis) was provided food first by whaling, then by commercial fishing, and that this food allowed the species to increase steadily over the last 3 centuries.
The worldwide increase of gulls (Larus argentatus, L. fuscus, L. dominicanus, L. ridibundus, L. novae-hollandii) has been credited to availability of food from wasteful human garbage disposal (Murray and Carrick 1964; Fordham 1968, 1970; Harris 1964; Harris and Plumb 1965; Kadlec and Drury 1968; Brown 1967; Mills 1973; Vermeer 1963).
It is hard to dismiss the evidence pointing to the impact of human activities on seabird populations during the last 3 centuries. Yet it would be misleading to assume that without man's interference seabird populations would have remained stable. Success in designing programs of protection and population enhancement must allow for the realities—that seabird populations fluctuate inherently, and that secular changes occur regularly in their environment.
Some population changes appear to result from sudden impacts; other changes are gradual.
Gromme (1927) reported windrows of dead murres in the Unimak Pass and Alaska Peninsula; die-offs of murres in winter storms in the Atlantic and Arctic Oceans were reported by Tuck (1960) and Dement'ev et al. (1968).
Recently some mass mortalities have been associated with specific causes. Bailey and Davenport (1972) reported that starvation caused the die-off of common murres in the southern Bering Sea—Bristol Bay area. Foul weather, which apparently inhibited feeding between 19 and 23 April 1970, culminated in an intense storm. Similarly in late winter 1969 bad weather in the Irish Sea, combined with strains of molt and perhaps contamination[Pg 125] with industrial chemicals, seems to have contributed to mass mortality of the same species (called common guillemot in Britain; Holdgate 1971). The seabird victims of this event had metabolized their body fat and as a result, polychlorinated biphenyls (PCB) and other industrial chemicals passed into livers, kidneys, and brains. Again, a storm at the end of a period of stress seems to have been more than the birds could tolerate.
A further example of a die-off of waterfowl apparently brought on by starvation was given by Barry (1968), who estimated that about 100,000 king eiders (Somateria spectabilis) died when they arrived before the ice broke up in the Beaufort Sea in spring 1964.
Diseases have produced massive die-offs in marine birds. Fowl cholera caused high mortality in nesting common eiders (Somateria mollissima) in the Gulf of St. Lawrence in Quebec (Reed and Cousineau 1967) and in Penobscot Bay, Maine, in the early 1960's (H. Mendall, personal communication). Poisoning from a "red tide" (a bloom of the dinoflagellate Gonyaulax tamerensis) caused a die-off of black ducks (Anas rubripes) and herring gulls on the coast of New England in 1972. Similarly a die-off of shags (Phalacrocorax aristotelis) on the east coast of England was caused by a "red tide" (Coulson et al. 1968). During a period of 1 week 90% of the shag nests on the Farne Islands in Northumberland were deserted and about 80% of the breeding population died.
When the new volcanic island of Bogoslov emerged in the western Aleutians, Preble and McAtee (1923) reported that it was colonized by large numbers of pigeon guillemots (Cepphus columba), but in the following decades the guillemots have steadily decreased (G. J. Divoky, personal communication). As a further example, the nesting population of Atlantic puffins (Fratercula arctica) in the Atlantic has declined over the past several years, especially those nesting on the Outer Hebrides (Flegg 1972; Harris 1976).
It is difficult to find seabird species whose nesting grounds have not been affected by humans but whose numbers have been censused. The best illustrations of secular changes in relatively constant habitats are probably those available in the British Trust for Ornithology's breeding censuses of songbirds. Songbirds are short-lived and their populations change on relatively short time scales. The northwestern European landscape has remained relatively constant for the last 75 years, yet there are observable decade-long trends—for example, of willow warblers (Phylloscopus trochilus) and dunnock (Prunella modularis). There are detailed data on population changes in great tits (Parus major) through the work of Kluyver (1951), Lack (1964), and Perrins (1965).
Nelson (1966) argued that the increase of gannets in the North Atlantic during this century has been related to increasing temperatures rather than (as usually ascribed) to increased food from fish damaged or escaped during commercial fishing.
Ainley and Lewis (1974) described a particularly interesting example of the effects of environmental change on seabird populations. The events begin with the decrease of seabirds on the Farallon Islands off California as a result of human depredations. Even after fowling was made illegal, the populations of murres, double-crested cormorants (Phalacrocorax auritus), and especially of tufted puffins (Lunda cirrhata) and pigeon guillemots continued to decline as a result of oil pollution. During the last 3 decades the smaller species of seabirds nesting on the Farallons, such as rhinoceros auklets (Cerorhinca monocerata), have increased rapidly and the authors suggest that their increase was abetted by an increase in the small prey fish, northern anchovy (Engraulis mordax). One of course expects predators to be affected by changes in the abundance of their prey. During this same period, larger species of seabirds such as double-crested cormorants and tufted puffins have failed to recover their numbers, and the authors speculate that this failure is related to a decrease of the larger prey fish, Pacific sardine (Sardinops caerulea).
A widely publicized impact of environmental fluctuation upon seabird populations is that of the northeast wind, El Niño, off the Peruvian coast. This wind pushes the upwell[Pg 126]ing Humboldt Current water offshore and causes mass mortality in the Peruvian anchovies (Engraulis ringens) and, as a consequence, a die-off among the millions of seabirds such as Peruvian guanay cormorants (Phalacrocorax bougainvillii) and Peruvian boobies or piquero (Sula variegata) which feed upon them (Murphy 1936).
Can useful generalizations be drawn from these observations on population changes? Can a model be constructed of the forces which drive population changes or of population-habitat interactions which keep populations from extinction? Some conflicting theories and assumptions of population dynamics are examined and discussed below.
During the 5 decades before 1970, it was widely accepted that most animal populations were generally stable and saturated before the arrival of the white man. Although a few field biologists vigorously dissented, "establishment" ecologists regarded fluctuations as a departure from the norm, and as such, a hazard to the population. Many theorists of both evolution and ecology argued that adaptations were required to damp fluctuations or the fluctuations would become "random walks" and the population would rapidly become extinct. As a consequence, relatively all theoretical models included stability as a central assumption.
• The basic element of this theoretical complex has been the Lotka-Volterra formula for a logistic curve of population growth and stabilization. According to this formula it has been reasoned that by establishing the inherent rate of increase of a population (i.e., its average natality relative to mortality, or r) and by measuring the carrying capacity of the environment (which is the density of the population at saturation, or K), one can predict the maximally productive population size, and maximum rate of production of new individuals (or maximum sustained yield). These assumptions have supplied the theoretical framework for virtually all game management and many fisheries practices.
Once stability was assumed, a mechanism for maintaining stability was necessary. This mechanism was found in an interaction between the population and the environment, called density-dependent mortality (Nicholson 1933). The impact of this feedback has been assumed to cause the point of inflection of the "sigmoid curve" and to regulate the density "at equilibrium."
Populations growing in relatively isolated or closed systems have been observed to follow a sigmoid curve toward a steady state. We have data on the growth of several Massachusetts gull colonies which show this type of short-period rapid increase followed by a long sequence of shallow oscillations (Drury and Nisbet 1972). But usually observations have been terminated at about the time the population passed through the point of inflection.
• Lack (1954) accepted the principles formulated by Lotka-Volterra and hence viewed Nicholson's (1933) density-dependence as logically necessary. Lack (1948, 1954) argued that reproductive effort (clutch size or litter size times the number of broods) must be as large as the parents can successfully raise to independence because these biological characteristics are directly subject to natural selection. He argued that because reproductive potential is excessive (Darwin 1859), mortality must be density-dependent if a population is to avoid fluctuations. The only adequately density-dependent regulating process he accepted was the population's response to its food supply (Lack 1954). In fact, for many years Lack rejected Kluyver and Tinbergen's (1953) hypothesis that territory could act as a control on population size in birds because, he argued, territories were compressible and therefore allowed wide fluctuations. To his credit, however, Lack eventually acknowledged this mistake.
The first defect in the concept of "carrying capacity" is the idea that populations have "mechanisms" or "institutions" (Wynne-Edwards 1959) by which the population is kept stable at the carrying capacity in a stable habitat.
The second defect in the concept of carrying capacity is that it presupposes a stable environment. During the early decades of the 20th century most climatologists believed that a departure from the norms of a regional climate set processes in motion which would return the climate to normal. During the last decades, however, climatologists and oceanographers have shown clearly that environments are continuously in flux.
Some theorists rejected the concept of carrying capacity as soon as it was formulated. Andrewartha and Birch (1954) predicted fluctuations would be undamped by inherent population mechanisms but rather would be controlled by external forces indifferent to the density. Their supporting data were drawn from field studies of insects in arid climates. Some of their ideas are directly relevant to seabirds; for example, their assertion that in many cases limits to carrying capacity of the habitat are not set in a way responsive to the density of the population. The number of occupiable ledges on a seabird cliff are fixed and when they are full no more birds can breed there regardless of the amount of food available. For another example, some biological processes act in a way that reinforces fluctuations. Predation can act in this way in the relatively closed system of a seabird colony; i.e., the smaller the prey population the larger the percentage taken by the predators. The importance of predation as a selecting factor is shown by the adaptations marine birds and waterfowl make to avoid it. The fact that large colonies of seafowl are usually concentrated on isolated, predator-free islands is one obvious case (Lack 1966).
Although their ideas are useful in understanding changes in many species, primarily insect populations, the generality of Andrewartha and Birch's (1954) hypothesis is weakened because it conflicts with detailed studies of seabirds which show that in many cases local food resources do limit breeding success. Ashmole (1963) showed this for tropical terns, and Hunt (1972) for some colonies of herring gulls on the New England coast. Nettleship (1972), studying the effects of herring gulls on Atlantic puffins, showed that the effect of harassment and stealing food from the parents was to reduce the amount of food brought to the young and thus reproductive success. In those parts of the colony where gulls were numerous or where the puffins were at a disadvantage in escaping from gulls (i.e., flat rather than steep slopes) the reproductive success of puffins was significantly lower than in areas away from the gulls.
The literal application of Andrewartha and Birch's general ideas also conflicts with observations on subtle adaptations some waterfowl have made to counter predation.
Barry (1967) described the density-avoiding adaptations of arctic-nesting geese to evade predation—specifically by foxes. Black brant (Branta nigricans) nest on low coastal or delta islands seeking to escape by remoteness. Snow geese (Chen caerulescens) are colonial on large, flat areas, seeking protection in numbers. White-fronted geese (Anser albifrons) are solitary nesters on inland swamps, seeking to be "over-dispersed" among scrub willow.
Common eiders, black scoters (Melanitta nigra), tufted ducks (Aythya fuligula), and other ducks select gull colonies as nesting habitat. Although there is little doubt that the ducks choose gull colonies for nesting, there is some doubt as to the reasons. Finnish biologists (summarized by Bergman 1957; Hildén 1965) have concluded generally that gulls protect the duck nests from predation by hooded crows (Corvus corone).
Recently theorists have built models based on assumptions that fluctuations are a general characteristic of population dynamics, such as Gilpin's (1975) model describing multi-phased oscillations. He took account of the fact that fluctuations (and models) become more complex as more species and nonlinear effects are included. May and Leonard (1975) emphasized that the effect of nonlinearities is to make it impossible to speak even in principle of the equilibrium point of a[Pg 128] community. They pointed out that even though the model is deterministic (i.e., assumes that the system will come to equilibrium) the oscillations are so complex that they may appear to be random, and it may be a very long time before the system returns to a position near its starting point. "On the other hand a truly random ecological system could always be fitted by a suitably ingenious limit cycle. This suggests that ecological analysis which does not consider component processes must be viewed with great suspicion" (Gilpin 1975). May and Leonard (1975) and Gilpin are both making a familiar point—that neither the logic nor the interactions described in a formula will describe biological reality unless the assumptions are correct. They are also making a different point—that an ingenious mathematician can create a formula to describe almost any operation (whether its workings are systematic or random), and the formula may seem to work.
Gilpin's moral is that one cannot learn very much that is helpful by studying fluctuations as such. One must study the factors controlling populations. This is a very old idea.
It would appear that defining carrying capacity and inherent rate of increase will not be very instructive in managing seabird populations other than in speculating upon what might be ideal upper limits. It can also encourage the musty sophistry that when a population increases beyond this abstract carrying capacity it "needs" to be hunted to prevent overcropping resources and damage to itself through a population decline. But we will not have the time to carry out detailed studies of life histories seeking for critical population-habitat interactions over several fluctuations for each species involved in a disaster before designing programs to help seabird populations to build up their numbers.
Because general theory does not seem to work and because detailed studies take too much time, I conclude that it is necessary to identify certain general principles upon which to base applied programs. These categories of knowledge include: (1) how vulnerable certain categories of seabirds, waterfowl, and shorebirds are to specific types of disasters, (2) how quickly their numbers build up after they have been reduced, and (3) at what stages we can help them best (i.e., at the breeding grounds, at the winter gathering grounds, or on migration). I believe that we already know enough to design effective programs and to begin work. To this end some characteristics of seabirds are identified which determine the population structures and ways in which their numbers respond to changes in the environment.
Although the shallow oceans, islands, and seashores are among the most permanent features of the earth in general, the details of their numbers and distribution change rapidly. Sandy shores are obviously being reworked even in the short span of a single lifetime. Distribution of islands and the sediment load, extent, and strengths of currents vary constantly in space and change with time.
The food that seabirds use is patchy and subject to both short-and long-term fluctuations in numbers and shifts in geography. Suitable breeding habitat is scattered, and in many places where oceanic conditions provide a good food supply there are no nesting sites. Consequently, seabirds aggregate in colonies, often dense, and the colonies are clumped for geographical as well as biological reasons.
Lack (1966) discussed some general features of how the breeding adaptations of seabirds are adjusted to the distances the birds must go to find food. The species which feed close to the nest characteristically establish isolated territories or nest in small groups, and they accept many different kinds of nesting substrate. Their clutch sizes are large, individuals move nesting sites readily, and their young grow rapidly compared to the species which feed far at sea. Species which feed far at sea aggregate in large colonies. These species are often rigid in their requirements for suitable nesting sites, their clutches are usually limited to one egg per season, their young grow slowly, and there seems to be strong attachment to traditional colony sites.
Ashmole (1963) suggested that the clutch size of some oceanic birds is small and colonies occupy only part of the available habitat because food resources within efficient commuting distance of the breeding site are limited. We can see this effect in the usual failure of common terns to raise a third chick, even in the colonies that are surrounded by favorable habitat (Nisbet 1973). Herring gulls whose colonies are close to sources of human refuse raise more young than do those whose colonies are at some distance (Drury 1963; Kadlec and Drury 1968; Hunt 1972).
Ashmole (1963) suggested that during the course of the breeding season the birds exhaust the available food supply. The validity of this suggestion is reflected in the long distances some species (petrels, boobies, murres, dovekies) go for food to feed their young. One would therefore expect that early nesting pairs would be more successful, and this seems to be the case in herring gulls (Nisbet and Drury 1972), kittiwakes (Coulson 1966), and red-billed gulls, Larus novaehollandiae (Mills 1973).
If food is in short supply and parents have to seek over a wide area for food so that they can bring back only a little food at long time intervals, one would expect these birds to have a small clutch and their young to grow slowly, as is the case. One would also expect seabird colonies situated near oceanic currents to be larger and more successful because food is continuously renewed. Conversely, one would expect colonies next to still waters to be smaller and less successful.
The small clutch size of seabirds means that when a population has been reduced, it will grow slowly toward its former abundance. The growth rates of seabird populations on the New England coast since their release from human predation reflects this. Species such as black guillemots with only two eggs per clutch and herring gulls with three eggs per clutch have increased more slowly than have the populations of common eiders or double-crested cormorants both with three to six eggs per clutch (Drury 1973).
If the species that nest in colonies show a high degree of site tenacity, they are not likely to reestablish a colony after it has been eliminated. An exception to this is the food subsidy provided by man, which seems to have been important in creating a nonbreeding population of herring gulls large enough to form a "critical mass" for the formation of a new gullery.
Because the main element of population size—the number of breeding adults—is limited by the number of breeding colonies and the food available to those colonies, one assumes that the total numbers of seabirds is much less than could be supported by the larger areas of productive oceans. Hence one suspects that there is lessened competition for food outside the breeding season and that lack of competition for food is a major reason for seabirds being long-lived, often to extremes little suspected until recently. Mortalities of 10-12% per year are common, and some as low as 4% (wandering albatross, Diomedea exulans; Tickell 1968) have been recorded.
In contrast, songbirds with large clutches, such as the titmice studied by Kluyver (1951), produce a large number of young with whom they and other adults must compete for food during the winter period of food shortage. Because the titmice are permanent residents, they occupy all of the available habitat throughout the year. Hence titmice suffer intense intraspecific competition, which shortens the survival of adults. Kluyver's experiments (1966) with nest boxes used by a closed population of great tits on Vlieland, The Netherlands, showed that by artificially reducing clutch size the survival of adults was increased.
Similar competition for the few territories available on marshes and consequent shortened life expectancy, can be expected in waterfowl with large broods. The effect should be less marked for geese with smaller clutches that nest in less confined habitats.
The long life span of seabirds means that a population will have a large component of older age categories; this characteristic has several implications:
• It means that the population can survive years of reproductive failure without the observable immediate effects that would be[Pg 130] manifest in titmice, grouse, or rabbits. Near failure of reproduction during a breeding season among arctic seabirds at Bear Island was reported by Bertram et al. (1934). Many similar observations have been made since then: Pitelka et al. (1955) reported such a case among skuas and gulls at Point Barrow, Drury (1961) for greater snow geese (Chen cerulescens atlantica) at Bylot Island, Jones (1970) for black brant gathering at Isambek Lagoon on the Alaska Peninsula, and D. A. Snarski (personal communication) for kittiwakes at Cook Inlet. Reproductive failure can sometimes be chronic, as observed by Nisbet (1972) for terns at Cape Cod, Massachusetts, or by Drury (1963) and Hunt (1972) for herring gulls on the outer islands on the coast of Maine.
When reproductive failure becomes chronic as observed on peregrine falcons (Falco peregrinus) by Hickey (1969) and in ospreys (Pandion haliaetus) by Ames and Mersereau (1964), the population of adults may hold on for a number of years without evident decline. Damage to the structure of the whole population may be serious before any numerical results are evident.
• Although there may not be intensive competition for food in the habitat away from breeding colonies, there is intense competition for food and breeding sites at and around the colonies. Hence age and previous experience in seabirds assume importance in establishing territory and in breeding success. Associated with this is the tendency for immature birds to delay breeding until they are several years old and for the immatures to remain on feeding grounds at some distance from the colonies. In some cases young birds may "hang around" breeding colonies and even feed some of the young. When young birds do first breed they usually lay smaller clutches and raise fewer young than do older birds. The importance of age and experience upon breeding success has been well documented for kittiwakes (Coulson 1966) and red-billed gulls (Mills 1973).
The fundamental biological importance of this delayed maturity seems to be emphasized by the persistence for several years of immature plumages, so clearly identifiable that even a human observer can recognize the age of an individual. One assumes such an evident feature must have adaptive significance.
When colonial nesting seabirds leave their breeding islands for their wintering grounds, their identification with that island is lost as far as population effects are concerned, because birds from many colonies mingle on the wintering grounds. Major mortality takes place on the wintering grounds and must therefore act on the species population as a whole rather than differentially on individuals associated with especially dense colonies. Such a direct relation between colony density and mortality would be necessary for density-dependent mortality to regulate the number of birds on a breeding colony. Conversely, one cannot expect that all colonies will decrease equally because mortality should be equally distributed if all the population gathers on a common wintering ground. Thus density-dependence acts only in a very general way upon the sum of animals considered as an abstract entity—the population.
In fact, on the wintering grounds, as shown by a graph of numbers of gulls reported on Christmas Counts on Cape Cod, Massachusetts (Kadlec and Drury 1968), herring gulls are very responsive to local conditions and move several tens of miles to gather at favorable feeding sites. An aerial survey of the gulls on the East Coast of the United States (Kadlec and Drury 1968) showed that more than half of the gulls were gathered near major food sources in large metropolitan districts. Most of the remainder were gathered near small fishing ports. Very few were scattered along the shoreline in what one assumes is the traditional gull habitat. Later analyses of the relation between the distribution of banding recoveries of birds in their first winter and the distribution of immatures as found on this winter census (Drury and Nisbet 1972) suggested that proportionately more first-year gulls died in those areas where the birds were sparsely distributed than died in the crowded metropolitan areas.
These results suggest both that there is not a direct feedback between reproductive rate and mortality, and that mortality may even be inversely density-dependent on wintering grounds. This last runs counter to traditional[Pg 131] ecological ideas that density causes a change in mortality rate. The idea that individuals gather where "living is easy" and mortalities are low is consistent with the theory of natural selection. One would not expect the food of the gulls to be evenly distributed, and one would expect individuals to move away from areas where food is scarce and mortality is high.
Breeding success has been shown to vary among individual pairs of gulls (Drost et al. 1961). Certain groups of individuals nesting in patches within a single colony have greater breeding success than do others (Coulson 1968; Drury and Nisbet, in preparation). Differences in breeding success also occur between colonies (Frazer-Darling 1938; Kadlec and Drury 1968; Drury and Nisbet 1972). Some colonies reproduce consistently better than others—for example, the gull colonies close to fishing ports and metropolitan areas. Other colonies produce consistently fewer young, such as the colonies on the outer islands in the Gulf of Maine (Drury 1963; Kadlec and Drury 1968; Hunt 1972). The populations of successful colonies grow while the numbers of unsuccessful colonies decline, even during a period of general population increase (Kadlec and Drury 1968).
The difference between success and failure, growth and decline, appears to lie in the food available. Colonies increase where breeding success is high and decrease where breeding success is low. One important reason seems to be that adult gulls may move to a more productive colony even after they have nested with another colony (Drury and Nisbet 1972; Kadlec 1971). Such adaptations can be viewed as adjustments by which individuals meet the requirements of an environment in which the availability of food and other necessities is patchy and shifting.
In general terms, the willingness of some individuals to disperse while the majority of individuals remain loyal to a colony can be considered a major mechanism of population maintenance. If conditions deteriorate seriously at one place so that the local populations decline or disappear, dispersal from other centers can be expected to repopulate the area as soon as local conditions again become suitable. This subject has been treated in more detail by Drury and Nisbet (1972) and Drury (1974b).
Occupation of new, or return to former, nesting sites has been recorded in detail for fulmars (Fulmaris glacialis) by Fisher (1952) and for herring gulls by Kadlec and Drury (1968). Dispersal is also known for waterfowl. Hansen and Nelson (1957) reported that of some 8,000 brant banded in midsummer on the Yukon delta 8 were recovered in northern Siberia and 28 in northern Alaska and arctic Canada. They suspected that pairing on the wintering grounds was responsible for the change in breeding areas, a change that would not be expected among other North American species of geese. Similarly, wide dispersal seems to occur in pintails (Anas acuta), mallards (Anas platyrhynchos), and wood ducks (Aix sponsa).
The general tendency for some individuals to disperse and the frequency of "extra limital" breeding attempts is especially well established in the Bering Sea region, in part at least because vagrants from Siberia or North America are readily identified as such. In the Aleutian Islands, Emison et al. (1971) and Byrd et al. (1974) have enumerated the nesting vagrants. For the Pribilof Islands, Kenyon and Phillips (1965), Sladen (1966), and Thompson and DeLong (1969) have recorded the repeated appearance of birds of Siberian distribution, and Fay and Cade (1959) and Sealy et al. (1971) did the same for St. Lawrence Island.
One can conclude that a few individuals are constantly trying to settle in new geographical areas. As climatic and habitat conditions change, some populations are able to become established; for example, southern species such as mockingbirds (Mimus polyglottus), cardinals (Cardinalis cardinalis), and tufted titmice (Parus bicolor) have settled in southeastern New England during the last 2 decades. These southern species have received much publicity. But at the same time, a less publicized dispersal of white-throated spar[Pg 132]rows (Zonotrichia albicollis), hermit thrushes (Catharus guttatus), and dark-eyed juncos (Junco hyemalis) has resulted in new nesting records of more northerly species, also in southeastern New England.
The ability (or lack of ability) of some organisms to expand their ranges over time has been a subject of consideration for a number of years by plant and animal geographers. An important botanical paper on this subject in the Bering Sea region was presented by Hultén (1937), who analyzed the ranges of plants of the area of Kamchatka, eastern Siberia, Alaska, and northwest Canada, showing that diverse floras occur in some restricted geographic areas. He called these areas "refugia," and postulated that many species had survived Pleistocene glaciations in them because these refugia remained ice-free. He, like Fernald (1925), was puzzled as to why only certain species had been able to expand their ranges outward from these "areas of persistence," while other apparently more "conservative" species were unable to do so. Similarly, there appear to be conservative endemic bird species of the Bering Sea region: the extinct Commander Islands cormorant (Phalacrocorax perspicillatus), Steller's eider (Polysticta stelleri), spectacled eider (Lampronetta fisheri), emperor goose (Philacte canagica), whiskered auklet (Aethia pygmaea), least auklet (A. pusilla), parakeet auklet (Cyclorrhynchus psittacula), Aleutian tern (Sterna aleutica), red-legged kittiwake (Rissa brevirostris), bristle-thighed curlew (Numenius tahitiensis), long-billed dowitcher (Limnodromus scolopaceus), surfbird (Aphriza virgata), black turnstone (Arenaria melanocephala), rock sandpiper (Calidris ptilocnemis), and western sandpiper (C. mauri).
The ranges of horned puffins (Fratercula corniculata), Kittlitz's murrelet (Brachyramphus brevirostris) and, perhaps, crested auklet (Aethia cristatella) suggest that some species of "Beringian" seabirds have expanded their ranges from Hultén's (1937) "refugia."
The presence of several sub-elements of a species population and, therefore, the opportunity for dispersion among alternative breeding sites may be an important factor in the regional persistence of a species on the margin of its range, as illustrated by the history of laughing gulls (Larus atricilla) in New England.
Between 1875 and 1900 there were fewer than 50 laughing gulls in Massachusetts (Mackay 1893) and about 35 in Maine (Norton 1924). In Massachusetts the laughing gulls all settled on one large island, Muskeget, where by 1940 there were about 20,000 pairs (Noble and Würm 1943). Meanwhile, in Maine the population had been disturbed by sheep and men and had shifted about among seven islands. The Maine population grew to only about 350 pairs by 1940 (Palmer 1949).
The laughing gull population in both States has decreased since 1940. In Massachusetts, where all pairs occupied one island, the population had fallen to about 250 pairs by 1972, but the Maine population, still divided into five colonies, stabilized at 250 pairs (i.e., the same as instead of only 1% of the Massachusetts population).
Game biologists have successfully maintained the populations of hunted animals by using a number of classical principles of game management. They have controlled mortality by regulating kill and have increased standing stock by improving habitat on a local scale. This seems to have worked in species which are short-lived, have large clutch sizes or litters, and which occupy mosaics of highly productive "successional habitat." Seabirds, however, contrast with these species in a number of important biological characteristics. They have small clutches, postpone breeding until they are several years old, and are subject to periodic or chronic reproductive failures. Therefore, their populations are skewed toward older animals and replacement of lost individuals is slow. Many seabirds, like some geese, have a high level of site tenacity and thus may resist recolonization or fail in the attempt to recolonize a breeding site once eliminated from it. In those species studied it appears that the breeding birds at a small per[Pg 133]centage of colonies are responsible for a large proportion of the annual crop of young. It is probably dangerous, therefore, to risk either damage to or elimination of well-established colonies.
Studies of kittiwakes by Coulson and White (1958, 1961), sooty terns (Sterna fuscata) by Ashmole (1963) and Harrington (1974), Atlantic puffins by Nettleship (1972), and Cassin's auklets (Ptychoramphus aleutica) by Manuwal (1974), and the practice of eider "farming" in Iceland indicate that the number of available territories or breeding sites may limit the size of a population and that populations can be increased by increasing the number of sites available. This suggests one way in which direct steps can be taken to encourage the numbers of breeding seabirds. Other studies indicate that seabirds will move into synthetic habitat such as created by the window ledges on buildings (Coulson and White 1958) or the islands created by dumping spoil from channel dredging operations (Buckley and Buckley 1971, 1975; Soots and Parnell 1975).
Most generalizations of population biology have been derived from the study of insects, songbirds, or game species. It seems inadvisable to assume that those principles will apply to seabirds without modification. For example, predation by gulls and ravens may have a disastrous effect on a seabird colony at low colony density but have progressively less impact as the colony size and density increase. Fox predation may have important effects over most ranges of prey density because the presence of foxes has important psychological effects.
The habitats of seabirds include elements in which birds are widely dispersed (feeding areas) and others in which birds are crowded and narrowly localized (nesting sites). Thus effective programs of conservation should include guarantees that a number of colony sites be available in as widely dispersed a pattern as possible. Each productive feeding ground should, if possible, have several colony sites available.
We have argued elsewhere (Drury and Nisbet 1972; Drury 1974a) that one of the chief defenses any population has against extinction is the combination of being divided into a number of population centers with having some movement of individuals between the centers, but not too much. Because it is highly improbable that a single catastrophe will affect more than a part of a species' range at any time, the more numerous and widely scattered the partially independent segments of a population are, the better the species is insured against extinction. This, of course, suggests that the size of each colony may be less important for long-term survival than is the total number of colonies.
One intuitively concludes that "conservative" species, such as those endemic to the Bering Sea region (whose dispersal and colonizing mechanisms seem to be poorly developed), are especially vulnerable to the effects of local population crashes. These "local" species therefore deserve special consideration.
I would like to emphasize two points to be included in designing a "management" program:
• It seems that the most promising management techniques will be built upon ensuring the health of colonies and the associated feeding areas at which reproductive success is high enough to "export" young. Thus it is useful to identify those colonies which are exporting young and to give special care to their preservation. As populations of prey species change locally, so will the success of the local nesting birds. A colony which is thriving at one time may be barely maintaining itself at another (Ainley and Lewis 1974), or it may decrease, as in the case of "guano birds" during El Niño years in the Peru Current.
• Because centers of abundance of marine birds shift (Fisher 1952; Drury 1963, 1974a), it will be prudent to plan for large areas and over long periods of time. Harrison Lewis, a pioneer in seabird management in eastern Canada, said (personal communication) that just as soon as he got approval of a new seabird sanctuary through the long corridors of the distant government bureaucracies in Ottawa, the birds would move to a new island and he had to start the process all over again.
The objective is to maintain a variety of colony sites for populations to move among as local patterns of productivity in the shallow sea shift.
1. To learn the distribution and relative importance of seabird colonies, the number of pairs nesting and nonbreeding individuals at each colony, and the timing of breeding activities for each geographical region. The most important step toward conserving marine birds is to get public ownership and protection for their breeding grounds.
2. To understand the life cycle of key species. Three needs are clear:
a. To identify key species whose biological characteristics can conveniently be studied and measured. Studies of these species may be useful in monitoring the "health" of seabird breeding areas.
If it is established that the reproductive success of certain species varies similarly in response to changes in their marine habitat (such as black-legged kittiwakes and horned puffins), one could use key species (black-legged kittiwake) to assess the performance of those species in a colony whose breeding success is difficult to measure (horned puffin).
b. To develop efficient and practical ways of censusing and measuring productivity of crevice-, scree-, and hole-nesting species such as puffins and auklets.
c. To establish annual differences in reproductive success and mortality rates by age classes of the key species, and from these to identify rates of population turnover so as to be able to predict the effects of mass mortalities.
3. To learn enough about the differences in behavior and productivity among colonies to establish which colonies produce surplus young and which have low productivity. At first, maximum efforts for conservation should be concentrated at those sites which produce surplus young.
4. To learn about colonial behavior. Two needs are apparent:
a. To know enough about the lives of individually marked birds of known age so as to be able to infer the behavior of population elements at all stages of their life cycle.
b. To know enough about the lives of subadult birds to understand what proportion of subadults visit and become established at breeding sites, why the subadults visit the breeding sites and what effect their presence has on the territories and breeding success of their neighbors and biological relatives.
5. To know enough about places where seabirds, waterfowl, and shorebirds gather on migration and during the winter to identify those areas which need special protection from effects of economic development.
a. It is important to determine the areas where marine birds gather at sea when they are away from their breeding grounds. What factors of habitat and food supply make certain places preferable to others? What is the relation between gathering grounds and underwater topography (banks and edges of the continental shelf)? What are the seasonal and annual differences in preferred gathering grounds? What special hazards exist, such as unusual extent of sea ice or exceptional storms?
b. It is important to plot coastal areas where waterfowl and shorebirds gather on migration, for molting, and during the winter. Which open leads in the ice and patches of open water at the mouths of rivers are of especial importance in spring? What shorelines and beaches act as "leading lines" during migration? Which capes and points result in concentrated overflights of migrating waterfowl, and hence are locations of unusually high kills by hunters? What wetlands, bogs, coastal ponds, lakes, and lagoons are used as gathering grounds and to what extent do waterfowl and shorebirds exchange between gathering grounds? How much redundancy of wetlands is needed to make the wetlands system maximally productive for waterfowl and shorebirds?
Answers to these questions will identify which geographic areas deserve special protection during development. The answers will also identify the kinds of influences which might lower the contribution of each critical area to the survival of seabirds, waterfowl, and shorebirds. Areas identified as important under these categories must be included in policy decisions related to land-use planning and management.
6. To learn more about the effects of varying quantities of food on breeding behavior and performance:
a. What are the effects of food abundance in early spring on date of laying, clutch size, and egg size?
b. What effects do storms have at different stages of the reproductive schedule?
c. What effects do quantitative and qualitative (species composition of prey) changes in food supply have on the survival of chicks?
d. What are the similarities and differences between what parents eat and what they feed their chicks?
Although this is important basic biological knowledge, it contributes little to conservation efforts because food differences result from changes in the ocean over which humans can have little effect.
7. To learn more about prey species and their availability to marine birds:
a. To know more about the breeding areas, reproductive rates, growth rates, and routes of dispersal of the major prey food species. In most areas a few species of teleost fish (e.g., Ammodytes) or Crustacea (e.g., copepods, euphausids, mycids, or amphipods) make up most of the food of marine birds. Yet, the barest minimum is known about the biology of such species. A good first estimate of the "condition" of the marine environment can probably be made by measuring reproductive rates and growth rates of these key prey species. Hence an efficient (though indirect) way to measure those rates may be by monitoring reproduction of birds.
b. To know more about the density and distribution of key prey items season by season, and to learn more about the relation of their abundance and distribution to their availability to birds, as Bédard (1969) showed for Calanus to least auklets, and Thysanoessa to crested auklets.
There are some indications that the population size of prey items can vary widely without having a marked effect on the numbers of their predators. Does commercial fishing for the large, predatory fish have a measurable effect on the food available to marine fish? Do the large pollock and salmon fisheries (high seas) make zooplankton available to smaller alcids? Do marine birds affect a fishery?
c. To know more about the oceanography of continental shelf waters, more specifically the waters between 6 and 60 m deep. The shallow waters of continental shelves are some of the most productive of sea waters, but are among the least studied. Although some species (black-legged kittiwakes, tufted puffins, and fulmars) move into deep waters, many species of marine birds of northern waters gather in large numbers on preferred feeding grounds at or near the edges of continental shelves during their winter season (Fisher 1952; Tuck 1960).
8. To know more about the potential effects of proposed developments on seabirds and waterfowl.
a. To prepare models which will predict probabilities of contamination of breeding and feeding areas (summer, winter, and during migration) using existing knowledge of
(1) areas of proposed mineral development;
(2) areas that will be influenced by secondary development such as dredging new harbors, laying subsurface pipelines;
(3) tidal and oceanic currents;
(4) numbers of marine birds or waterfowl using specific geographic areas and habitats (e.g., waters below nesting cliffs, feeding grounds, wintering grounds, and gatherings during migration);
(5) the distribution and patchiness of habitats (i.e., the redundancy among and within habitats and the degree to which populations exchange between alternative habitats);
(6) the biological importance of species in local ecosystems (Are they predators whose effects increase diversity?);
(7) the human importance of the species (Are they endemics? Do they have unusual "charisma" for the public?);
(8) the vulnerability of the species (Is its distribution restricted? Is it subject to oil pollution? Are their preferred grounds near areas of high development potential?);
(9) the types of biological effects (e.g., oil contamination of plumage, PCB contamination of food chains); and
(10) whether the potential impacts are reversible or irreversible and to what degree.
b. To understand more of the effects of hunting on the behavior of marine birds and waterfowl on their breeding grounds, and to assess the effects on breeding performance of changes in behavior which result from human activities (such as hunting or studying the[Pg 136] birds).
c. To understand the effects of the presence of predators (whether introduced or native) on breeding colonies in order to assess the importance of removing the predators or preventing their access to breeding grounds.
Although peaceful coexistence of wildlife populations and economic development are here assumed to be practical, some new social institutions are needed to control damaging activities of people during economic development. Human activities and industrial products which damage wildlife or their habitat must be identified, as must the space and resources which wildlife require for survival and health.
1. What seabird cliffs, islands, lagoons, wetlands, river mouths, and other habitat features are of first importance for breeding or for maintaining the populations? Some small areas of habitat are critical for the survival of some species during periods of stress. Those habitats need official recognition. Steps are needed to ensure that the habitats are maintained.
2. What physical expressions of economic development are of little, modest, or serious impact on wildlife and its habitat? These activities and constructions include harbors, storage sites, transshipment facilities, roads, pipelines, summer camps, and suburban or vacation developments.
3. What kinds of human activities will disturb, damage, or change the behavior or accessibility of wildlife? Many activities of one group of people have secondary effects which affect the enjoyment of resources for other groups. These include
a. gill netting for salmon, which may kill large numbers of murres and diving ducks;
b. release of predators on seabird nesting islands, which may kill adults or inhibit their feeding their young;
c. free running of pets (such as dogs and cats) over wetlands or wildlife habitat, because pets are predators and harass the wildlife which may be feeding;
d. flights of aircraft, especially helicopters, near or over seabird cliffs because such flights may cause serious damage to eggs and young;
e. hunting, because the game becomes timid and flees from those who might enjoy watching wildlife;
f. snow machines, because their presence is disagreeable to many and they provide easy access by which disturbing activities may reach into areas where wildlife would otherwise be undisturbed.
4. What limitations or alterations are needed in the existing legal institutions, such as the Marine Mammals Protection Act, the instruments implementing native land claims, the process of Alaska State lands withdrawal, the conditions for leasing State and Federal lands for development of mineral resources, and traditional rights of private property? All of these legal institutions are relevant to problems of wildlife survival and restoration, and within most of these institutions there exist conflicts between rights and benefits of special political interests and the husbanding of renewable common property resources.
Experience in Europe and in New England suggests that if reasonable limitations are set on human activities and that if adequate money charge is made against those who profit by economic development to defray full social costs, wildlife can continue to do well. In most cases where damage has occurred it is because those who administer the public institutions have failed to include consideration of the common property resources.
Ainley, D. G., and T. J. Lewis. 1974. The history of Farallon Island marine bird populations. Condor 76(4):432-446.
Ames, P. L., and G. S. Mersereau. 1964. Some factors in the decline of the osprey in Connecticut. Auk 81(2):173-185.
Andrewartha, H. G., and L. C. Birch. 1954. The distribution and abundance of animals. Univ. Chicago Press, Chicago, Ill. 782 pp.
Ashmole, N. P. 1963. The regulation of numbers of tropical oceanic birds. Ibis 103b:458-473.
Bailey, E. P., and G. H. Davenport. 1972. Die-off of common murres on the Alaska Peninsula and Unimak Island. Condor 74(2):215-219.
Barry, T. W. 1967. Geese of the Anderson River delta, Northwest Territories. Ph.D. Thesis. Univ. of Alberta, Edmonton, Alberta, Canada. 176 pp.
Barry, T. W. 1968. Observations on natural mortality and native use of eider ducks along the Beaufort Sea Coast. Can. Field-Nat. 82(2):140-144.
Bédard, J. 1969. Feeding of the least, crested, and parakeet auklets around St. Lawrence Island, Alaska. Can. J. Zool. 47:1025-1050.
Belopol'skii, L. O. 1961. Ecology of sea bird colonies of the Barents Sea. (Translated from Russian.) Israel Program for Scientific Translations, Jerusalem. 346 pp.
Bent, A. C. 1919. Life histories of North American diving birds. U.S. Natl. Mus. Bull. 107. 245 pp.
Bent, A. C. 1921. Life histories of North American gulls and terns. U.S. Natl. Mus. Bull. 113. 345 pp.
Bent, A. C. 1922. Life histories of North American petrels and pelicans and their allies. U.S. Natl. Mus. Bull. 121. 343 pp.
Bergman, G. 1957. Concerning the problem of mixed colonies: the tufted duck and gulls. Die Vogelwarte 19(1):15-25.
Bertram, G. C. L., D. Lack, and B. B. Roberts. 1934. Notes on East Greenland birds, with a discussion of the periodic non-breeding among arctic birds. Ibis 13(4):816-831.
Brown, R. G. B. 1967. Breeding success and population growth in a colony of herring and lesser black-backed gulls Larus argentatus and L. fuscus. Ibis 109(4):502-515.
Buckley, F. G., and P. A. Buckley. 1971. The breeding ecology of royal terns (Sterna [thalasseus] maxima maxima). Ibis 114(3):344-359.
Buckley, P. A., and F. G. Buckley. 1975. The significance of dredge spoil islands to colonially nesting waterbirds in certain national parks. Proc. Conf. on Management of Dredge Islands in North Carolina Estuaries, May 1974. N.C. Sea Grant Publ. UNC-SG-75-01, Raleigh.
Byrd, G. V., D. D. Gibson, and D. L. Johnson. 1974. The birds of Adak, Alaska. Condor 76(3):288-300.
Coulson, J. C. 1966. The influence of the pair-bond and age on the breeding biology of the kittiwake gull Rissa tridactyla. J. Anim. Ecol. 35:269-279.
Coulson, J. C. 1968. Differences in the quality of birds nesting in the centre and on the edges of a colony. Nature 217 (Feb. 3):478-479.
Coulson, J. C. 1974. Kittiwakes Rissa tridactyla. Pages 134-141 in S. Cramp, W. R. P. Bourne, and D. Saunders, The Seabirds of Britain and Ireland. Taplinger Publ. Co., Inc., New York. 287 pp.
Coulson, J. C., G. A. Potts, I. R. Deans, and S. M. Fraser. 1968. Exceptional mortality of shags and other seabirds caused by paralytic shellfish poison. Brit. Birds 61:381-404.
Coulson, J. C., and E. White. 1958. The effect of age on the breeding biology of the kittiwake Rissa tridactyla. Ibis 100(1):40-51.
Coulson, J. C., and E. White. 1961. An analysis of the factors influencing the clutch-size of the kittiwake. Proc. Zool. Soc. Lond. 136:207-217.
Cramp, S., W. R. P. Bourne, and D. Saunders. 1974. The seabirds of Britain and Ireland. Taplinger Publ. Co., Inc., New York. 287 pp.
Darwin, C. 1859. On the origin of species by means of natural selection. John Murray, London. 490 pp.
Dement'ev, G. P., R. N. Meklenburtsev, A. M. Sudilovskya, and E. P. Spangeberg. 1968. Birds of the Soviet Union. Vol. 2. (Translated from Russian.) Israel Program for Scientific Translations, Jerusalem. 553 pp.
Drost, R., E. Focke, and G. Freytag. 1961. Entwicklung und aufbau einer population der Silbermöwe, Larus argentatus argentatus. J. Ornithol. 102(4):404-429.
Drury, W. H. 1961. Observations on some breeding water birds on Bylot Island. Can. Field-Nat. 75(2):84-101.
Drury, W. H. 1963. Results of a study of herring gull populations and movements in southeastern New England. Pages 207-217 in Colloque: Le problème des oiseaux sur les aérodromes (Nice, 1963). Inst. Nat. Rech. Agron., Paris.
Drury, W. H. 1973. Population changes in New England seabirds. Bird-Banding 44(4):267-313.
Drury, W. H. 1974a. Population changes in New England seabirds. Bird-Banding 45(1):1-15.
Drury, W. H. 1974b. Rare species. Biol. Conserv. 6(3):162-169.
Drury, W. H., and I. C. T. Nisbet. 1972. The importance of movements in the biology of herring gulls in New England. Pages 173-212 in Population Ecology of Migratory Birds: a Symposium. U.S. Fish Wildl. Serv. Wildl. Res. Rep. 2. 278 pp.
Dutcher, W. 1901. Results of special protection to gulls and terns obtained through the Thayer Fund. Auk 18(2):76-103.
Dutcher, W. 1904. Report of the A.O.U. Committee on the Protection of North American Birds for the year 1903. Auk 21(suppl.):97-208.
Emison, W. B., F. S. L. Williamson, and C. M. White. 1971. Geographical affinities and migrations of the avifauna on Amchitka Island, Alaska. BioScience 21(12):627-631.
Fay, F. H., and T. J. Cade. 1959. An ecological analysis of the avifauna of St. Lawrence Island, Alaska. Univ. Calif. Publ. Zool. 63:73-150.
Fernald, M. L. 1925. Persistence of plants in unglaciated areas of boreal America. Mem. Am. Acad. Arts Sci. 15:239-342.
Fisher, J. 1952. The fulmar. Collins, London. 496 pp.
Fisher, J., and R. M. Lockley. 1954. Seabirds: An introduction to the natural history of the seabirds of the North Atlantic. Houghton Mifflin Co., Boston. 320 pp.
Fisher, J., and H. G. Vevers. 1943. The breeding distribution, history and population of the North Atlantic gannet (Sula bassana). Part I. A history of the gannet's colonies, and the census in 1939. J. Anim. Ecol. 12:173-213.
Fisher, J., and H. G. Vevers. 1944. The breeding distribution, history and population of the North[Pg 138] Atlantic gannet (Sula bassana). Part II. The changes in the world numbers of the gannet in a century. J. Anim. Ecol. 13:49-62.
Flegg, J. J. M. 1972. The puffin on St. Kilda 1969-71. Bird Study 19:7-17.
Forbush, E. H. 1929. Birds of Massachusetts and other New England States. Vol. I. Massachusetts Department of Agriculture, Boston. 481 pp.
Fordham, R. A. 1968. Dispersion and dispersal of the Dominican gull in Wellington, New Zealand. Proc. New Zeal. Ecol. Soc. 15:40-50.
Fordham, R. A. 1970. Mortality and population change of Dominican gulls in Wellington, New Zealand, with a statistical appendix by R. M. Cormack. J. Anim. Ecol. 39(1):13-27.
Frazer-Darling, F. 1938. Bird flocks and the breeding cycle. Cambridge Univ. Press, Cambridge, England. 124 pp.
Gilpin, M. E. 1975. Limit cycles in competition communities. Am. Nat. 109:51-60.
Gromme, O. J. 1927. Some highlights on the faunal life of the Alaskan Peninsula. Milw. Public Mus. Yearb. 7:30-45.
Hansen, H. A., and U. C. Nelson. 1957. Brant of the Bering Sea—migration and mortality. Trans. N. Am. Wildl. Nat. Resour. Conf. 22:237-256.
Harrington, B. A. 1974. Colony visitation behavior and breeding ages of sooty terns (Sterna fuscata). Bird-Banding 45(2):115-144.
Harris, M. P. 1964. Aspects of the breeding biology of the gulls Larus argentatus, L. fuscus and L. marinus. Ibis 106(4):432-456.
Harris, M. P. 1976. The present status of the puffin in Britain and Ireland. Brit. Birds 69(7):239-264.
Harris, M. P., and W. J. Plumb. 1965. Experiments on the ability of herring gulls Larus argentatus and lesser black-backed gulls L. fuscus to raise larger than normal broods. Ibis 107(2):256-257.
Hickey, J. J., editor. 1969. Peregrine falcon populations—their biology and decline. Univ. Wisconsin Press, Madison. 596 pp.
Hildén, O. 1965. Habitat selection in birds: a review. Ann. Zool. Fenn. 2(1):53-75.
Holdgate, M. W., editor. 1971. The sea bird wreck in the Irish Sea, autumn 1969. Nat. Environ. Res. Council Publ. Ser. C, No. 4. 17 pp.
Hultén, E. 1937. Outline of the history of arctic and boreal biota during the Quaternary period. Bokforlags Aktiebolaget Thule, Stockholm. 168 pp.
Hunt, G. L. 1972. Influence of food distribution and human disturbance on the reproductive success of herring gulls. Ecology 53(6):1051-1061.
Jones, R. D. 1970. Reproductive success and age distribution of black brant. J. Wildl. Manage. 34(2):328-333.
Kadlec, J. A. 1971. Effects of introducing foxes and raccoons on herring gull colonies. J. Wildl. Manage. 35(4):625-636.
Kadlec, J. A., and W. H. Drury. 1968. Structure of the New England herring gull population. Ecology 49(4):644-676.
Kenyon, K. W., and R. E. Phillips. 1965. Birds from the Pribilof Islands and vicinity. Auk 82(4):624-635.
Klein, D. R. 1959. Saint Matthew Island reindeer-range study. U.S. Fish Wildl. Serv., Spec. Sci. Rep.—Wildl. 43. 48 pp.
Kluyver, H. N. 1951. The population ecology of the great tit, Parus major L. Ardea 39:1-135.
Kluyver, H. N. 1966. Regulation of a bird population. Ostrich 38 (Suppl. 6):389-396.
Kluyver, H. N., and L. Tinbergen. 1953. Territory and the regulation of density in titmice. Arch. Neér. Zool. 10:265-289.
Lack, D. 1948. Natural selection and family size in the starling. Evolution 2:95-110.
Lack, D. 1954. The natural regulation of animal numbers. Clarendon Press, Oxford. 343 pp.
Lack, D. 1964. A long-term study of the great tit (Parus major). J. Anim. Ecol. 33(Suppl.):159-173.
Lack, D. 1966. Population studies of birds. Clarendon Press, Oxford. 341 pp.
Mackay, G. H. 1893. Observations on the breeding habits of Larus atricilla in Massachusetts. Auk 10(4):333-336.
Manuwal, D. A. 1974. Effects of territoriality on breeding in a population of Cassin's auklet. Ecology 55(6):1399-1406.
May, R. M., and W. J. Leonard. 1975. Nonlinear aspects of competition between species. SIAM J. Appl. Math. 29(2):243-253.
Mills, J. A. 1973. The influence of age and pair bond on the breeding biology of the red-billed gull Larus novaehollandiae scopulinus. J. Anim. Ecol. 42:147-162.
Murphy, R. C. 1936. Oceanic birds of South America, Vols. I and II. Am. Mus. Nat. Hist., New York. 1245 pp.
Murray, M. D., and R. Carrick. 1964. Seasonal movements and habitats of the silver gull Larus novaehollandiae Stephens in southeastern Australia. CSIRO Wildl. Res. 9:160-188.
Nelson, J. B. 1966. Population dynamics of the gannet at Bass Rock, with comparative information for other Sulidae. J. Anim. Ecol. 35:443-470.
Nettleship, D. 1972. Breeding success of the common puffin (Fratercula arctica L.) on different habitats at Great Island, Newfoundland. Ecol. Monogr. 42:239-268.
Nicholson, A. J. 1933. The balance of animal populations. J. Anim. Ecol. 2:132-178.
Nisbet, I. C. T. 1972. Disaster year for terns. Man and Nature, Dec. 1972:16-21.
Nisbet, I. C. T. 1973. Courtship-feeding, egg-size and breeding success in common terns. Nature 241:141-142.
Nisbet, I. C. T., and W. H. Drury. 1972. Post-fledging survival in herring gulls in relation to brood-size and date of hatching. Bird-Banding 43(3):161-172.
Noble, G. K., and M. Würm. 1943. The social behavior of the laughing gull. Ann. N. Y. Acad. Sci. 45:179-200.
Norton, A. H. 1921. Report of the field agent for Maine. Bird-Lore 23:355-356.
Norton, A. H. 1924. Notes on birds of the Knox County region. Maine Nat. 4:35-39.
Palmer, R. S. 1949. Marine birds. Harvard Bull. Mus. Comp. Zool. 102. 656 pp.
Perrins, C. 1965. Population fluctuations and clutch-size in the great tit, Parus major L. J. Anim. Ecol. 34:601-647.
Pitelka, F. A., P. Q. Tomich, and G. W. Treichel. 1955. Ecological relations of jaegers and owls as lemming predators near Barrow, Alaska. Ecol. Monogr. 25:85-117.
Preble, E. A., and W. L. McAtee. 1923. A biological survey of the Pribilof Islands, Alaska. U.S. Fish. Wildl. Serv., N. Am. Fauna 46:1-255.
Reed, A., and J. G. Cousineau. 1967. Epidemics involving the common eider (Somateria mollissima) at Ile Blanche, Quebec. Nat. Can. (Que.) 94:327-334.
Sealy, S. G., F. H. Fay, J. Bédard, and M. D. F. Udvardy. 1971. New records and zoogeographical notes on the birds of St. Lawrence Island, Bering Sea. Condor 73(3):322-336.
Serventy, D. L. 1967. Aspects of the population ecology of the short-tailed shearwater Puffinus tenuirostris. Proc. Int. Ornithol. Congr. xiv:165-190.
Sladen, W. J. L. 1966. Additions to the avifauna of the Pribilof Islands, Alaska, including five species new to North America. Auk 83:130-135.
Soots, R. F., Jr., and J. F. Parnell. 1975. Ecological succession of breeding birds in relation to plant succession on dredge islands in North Carolina estuaries. N.C. Sea Grant Publ. UNC-SG-75-27, Raleigh.
Thompson, M. C., and R. L. DeLong. 1969. Birds new to North America and the Pribilof Islands, Alaska. Auk 86(4):747-749.
Tickell, W. L. N. 1968. The biology of the great albatrosses, Diomedea exulans and Diomedea epomorpha. Antarct. Res. Ser. 12:1-55.
Tuck, L. M. 1960. The murres: their distribution, populations and biology. Can. Wildl. Serv., Rep. Ser. No. 1. 260 pp.
Uspenski, A. M. 1956. The bird bazaars of Novaya Zemlya. U.S.S.R. Acad. Sci., Moscow. (Translated from Russian.) Canad. Wildl. Serv. Transl. Russian Game Rep. 4. 159 pp.
Vermeer, K. 1963. The breeding ecology of the glaucous-winged gull (Larus glaucescens) on Mandarte Island, B.C. Occas. Pap. B. C. Prov. Mus. 13:1-104.
Wynne-Edwards, V. C. 1959. The control of population-density through social behaviour: a hypothesis. Ibis 101:436-441.
by
Erica H. Dunn
Long Point Bird Observatory
Point Rowan, Ontario, Canada
Time and energy budgets can be compared among species of birds with very different ecology as a way of summarizing differences and as an approach to determining selective pressures on each species. This paper reviews time-energy use of northern seabirds. Energetic cost of maintenance (basal metabolism, thermoregulation, and procurement and processing of food) depends largely on the following factors: (1) small birds have higher metabolic costs per unit size than do larger ones; (2) body structure affects the cost of locomotion as well as of food procurement; (3) climate affects metabolic costs; and (4) food availability and nutrition vary among food types, and throughout the year within a food type. Little is known of maintenance energetics in seabirds. Time and energy allocations to items beyond basic maintenance are also compared. Patterns and costs of molt and migration are known only in a general way, and the variety of possible patterns suggests that more research would be of value. Almost nothing is known of location and daily activities of seabirds outside the breeding season. The review of breeding season activities is more comprehensive, and stresses the variety of factors known to affect timing, and the total time devoted to and the energetic costs of various aspects of reproduction. Some of these factors are weather, year, geographic location, feeding conditions, age, sex, and distance of food source from the breeding colony. Species characteristics such as clutch size, egg and yolk size, developmental type, growth rate, food type, and behavior combine with environmental variables to make seabirds a very diverse group in time and energy budgeting. Time-energy studies and determination of productive energy (energy remaining after maintenance needs have been met) can be useful in pinpointing those groups of birds and the times of year when birds are most vulnerable to environmental stress. Life history considerations suggest that most seabirds are adapted to low population turnover and would not be able to recover quickly from sudden increases in mortality.
Effective management of a population requires manipulation of the factors most critical in causing population increase or decrease. Deciding what these factors are and which are most suitable for effective manipulation is very difficult due to the complexity of life cycles and possible factors affecting demography. It takes a thorough knowledge of a species and of its relationships with the biotic and abiotic environment to make effective management decisions. The following review of seabird time and energy use is meant to emphasize the wide variation of species ecology within this avian group.
Time and energy patterning is being used as the basis of ecological comparison for the following reason. Any activity of an animal requires time and energy use; therefore, the patterning of use makes a common thread to which allocation to all activities in a bird's life cycle can be related. Time-energy patterns can be compared among birds with diverse food types, habitats, life cycles, and life expectancy, and therefore offer an opportunity for[Pg 142] comparison not available through other kinds of analysis (King 1974).
The amounts of time and energy allocated by an organism to different aspects of survival and reproduction should be regarded as being molded by natural selection to optimize (not necessarily to maximize) lifetime reproductive output (Fisher 1958; Williams 1966; Schoener 1971). Thus, differences in time and energy use between species should reflect adaptation to different biotic and abiotic environments. By comparing time and energy use, one can gain insight into the selective pressures on each species and have a basis on which to compare complex ecology more meaningfully than if one listed other types of differences.
This review of time-energy use in northern seabirds cannot be comprehensive, largely because many of the necessary data are lacking. It stresses major areas of difference, however, and points out aspects about which little is yet known.
Every animal must expend a basic amount of energy on normal maintenance, excluding activities normally allocated to a relatively narrow time span, such as reproduction. This "existence energy" expenditure consists of basal metabolism, thermoregulation, and the costs of gathering and processing food, and could also be referred to as the animal's basic "cost of living." In discussing the components of the cost of living, energy use is emphasized and time largely ignored—partly because metabolism occurs irrespective of time (it is not something the animal can turn off for a period) and partly because time use in normal maintenance and foraging has been little studied.
Basal metabolic rate (BMR) depends greatly on body size (Lasiewski and Dawson 1967; Zar 1968), and the costs per unit size are higher for a small bird than for a large one (Fig. 1). The BMR is somewhat lower in seabirds and other nonpasserines than in passerines of similar size (Dawson and Hudson 1970).
Fig. 1. Energy cost of various metabolic functions in relation to body size in birds. "0° Existence" refers to total metabolic costs of caged birds held at 0°C. From Calder (1974).
The relationship between BMR and body size is paralleled by that between size and other metabolic costs, such as for thermoregulation at a given temperature and for activity (Fig. 1; Kendeigh 1970; Tucker 1970; Schmidt-Nielsen 1972; Berger and Hart 1974; Calder 1974). Basal metabolic rate can therefore be used as an index of the overall cost of living as far as metabolic functions are concerned. Small birds must allocate a greater proportion of their energy resources than larger ones to merely staying alive, and have a higher cost of living.
The suggestion in Fig. 1 that it is easy to measure activity costs in a straightforward manner is misleading, because the figure represents measures taken under standard conditions. Factors known to affect the cost of flight, for example, include anatomical adaptations (such as wing loading and wing shape), the type of flight (ascending, descending, glid[Pg 143]ing), and the speed of flight (Fig. 2; Tucker 1969, 1974; Hainsworth and Wolf 1975). The cost of a series of short flights may be higher than that for a long one because of the extra energy required for takeoff and landing. A few estimates have been made for the cost of flight, mostly in birds moving almost constantly (Lasiewski 1963; Nisbet 1963; Tucker 1972, 1974; Utter and LeFebvre 1970; Berger and Hart 1974), but the methods may be inadequate for birds that fly short distances frequently.
Fig. 2. Energy cost of flying at different speeds and angles as compared with basal metabolic rate (BMR). Solid lines and solid circle refer to flight cost and BMR for budgerigar (Melopsitticus undulatus). Dashed line and open circle refer to flight cost and BMR of the laughing gull. From Tucker (1969).
Little work has been done on the cost of locomotion in seabirds: that of Eliassen (1963) on great black-backed gulls (Larus marinus), Berger et al. (1970) on ring-billed gulls (L. delawarensis), and Tucker (1972) on the laughing gull (L. atricilla), and indirect calculations of soaring flight characteristics in albatrosses, Diomedea spp. (Cone 1964), and the fulmar, Fulmarus glacialis (Pennycuick 1960). Swimming has been shown to be more costly than flying in ducks (Schmidt-Nielsen 1972) and may be for seabirds as well. More energy is also probably used in underwater swimming than in flying.
The energetic costs of thermoregulation under natural conditions are not easy to estimate. Thermal energy is gained from and lost to the environment, and the degree of exchange depends not only on air temperature but also on metabolic rate, insulation, body temperature, posture, humidity, convection, and radiation. Radiation, in turn, depends on cloud cover, shade, and reflective and absorptive characteristics of the organism and of the environment (Porter and Gates 1969; Calder and King 1974). Most of these quantities are changing constantly, and insulation and metabolic rate may vary on a seasonal basis with acclimation (Dawson and Hudson 1970).
At present, no direct measurement technique exists for determining natural thermoregulatory costs, although a few estimates have been made (King 1974), including several for seabird nestlings (Dunn 1976a, 1976b for double-crested cormorants, Phalacrocorax auritus, and for herring gulls, Larus argentatus). For most birds, the temperature environment actually faced over a year's time has never been measured, and for no bird has a full description of the complete thermal environment been made. It is clear that climate and degree of exposure are important elements in the basic cost of living, and that thermoregulatory costs average higher in small birds than in larger ones, but beyond that little information is available. Work on thermoregulatory costs of free-living chicks of two species of seabirds suggests that insulative properties can lead to marked differences in the metabolic costs of different species in an essentially identical environment (Dunn 1976a, 1976b).
Gathering and processing food is another major component of the cost of living. Both the nutritional value of food and its availability (a rather vague term covering both abundance and ease of capture) are extremely diverse and variable, making estimations of foraging cost and benefit difficult (Ashmole 1971; Fisher 1972; Sealy 1975a).
Availability of food varies throughout the year, particularly in marine invertebrates that form the diet of many seabirds (e.g., Spaans 1971; Bédard 1969a). High arctic[Pg 144] oceans have a very high peak of productivity in the summer, whereas the low arctic has a lower, but longer-lasting, peak (Ashmole 1971). Fish stocks increase in summer as well (Snow 1960; Pearson 1968; Sealy 1975a), and decline or disperse in autumn (Potts 1968). Catch-ability may also differ widely from year to year (e.g., E. K. Dunn 1973).
Marine foods are likely to have a patchy distribution, which may make food stocks difficult to locate, even in times of abundance (Ashmole 1971; Sealy 1975a). Birds in localities with low food abundance frequently show alterations in time and pattern of foraging, sometimes even changing diets (Cramp 1972; Henderson 1972; Hunt 1972; Lemmetyinen 1972). The time and energy expended in finding and capturing food by different seabird species must vary widely according to the form of foraging used: plunge-diving, beach scavenging, aerial robbing, underwater pursuit, and so on. Even when different species have traveled the same distance to an identical food stock, therefore, the costs of procurement differ.
Time and energy spent foraging depends not only on abundance and ease of capture, but also on nutritional return, and on the age and size of the bird. Fig. 3 shows that the smaller species in a seabird community may spend the most time foraging. Even though this illustration is taken from the breeding season when food demands of the young must be taken into account, it suggests a difference based on cost of living according to size.
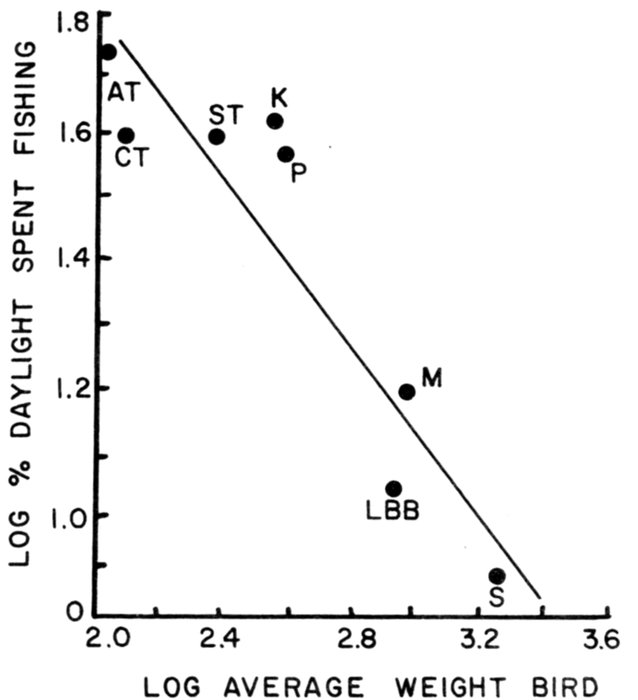
Fig. 3. Time spent foraging in the breeding season as a function of body size. From Pearson (1968). AT = arctic tern (Sterna paradisaea), CT = common tern (S. hirundo), ST = sandwich tern (Thalasseus sandvicensis), K = black-legged kittiwake, P = common puffin, M = common murre, LBB = lesser black-backed gull, S = shag.
Age of the bird affects time and energy commitment to foraging because younger birds are often less skilled at capturing food. This has been noted particularly in long-lived seabird species (Orians 1969; Dunn 1972; LeCroy 1972; Buckley and Buckley 1974; Barash et al. 1975). Older juveniles may be excluded from feeding areas by more experienced, territorial adults (Moyle 1966), whereas immatures are not (Drury and Smith 1968; Ingolfsson 1969).
Nutritional and energetic return obtained from food is a very important factor in foraging strategy that has not received the attention it deserves. Table 1 lists the caloric value of various foodstuffs and illustrates how little is known about foods eaten by seabirds. Although caloric content and abundance of food have often been accepted as the most important determinants of foraging strategies (Bookhout 1958; Emlen 1966; West 1967; Bryant 1973), they may frequently be less important than nutritional value and digestibility, also shown in Table 1 (Pulliam 1974).
Since fish seem to be highly digestible, most of the energy contained in them is available to the consumer. There are, unfortunately, no data on the digestibility of marine invertebrates, but those for insects suggest that digestibility, at least of crustaceans with exoskeletons, is somewhat lower than that for fish. A bird would therefore have to eat a larger biomass of invertebrates than of fish to satisfy the same energetic needs (although cost of procurement might not be as high as for fish).
| Food type | Kcal/g fresh wt. | Percent fresh wt. composed of: | Digestive efficiency | Kcal metabolizable energy/g fresh wt. | ||
|---|---|---|---|---|---|---|
| H2O | Fat | Protein | ||||
| Vegetable | 1.2-5.2 | 59-86 | 0.4-3 | 3-5 | 30-32 | 0.3-2.3 |
| Tropical fruits | 1.2 | 75 | 8 | 1 | ||
| Various seeds | 4.0-7.3 | 3-13 | 1-40 | 10-29 | 76-80 | 3.0-5.2 |
| Various insects | 1.4-5.2 | 65-75 | 1-3 | 9-18 | 66-69 | 0.9-3.5 |
| Whiting (fish) | 1.1 | 81 | 79 | 0.9 | ||
| Various freshwater fishes | 1.2 | 75 | 5 | 18 | 81 | |
| Mix of fish eaten by double-crested cormorants on NE coast | 1.1 | 74 | 1 | 16 | 82 | 0.9 |
| Fresh herring and mackerel | 1.9 | 67 | 13 | 19 | ||
| Garbage ("average" mix) | 1.5 | 67 | 8 | 19 | ||
| Crab with eggs | 1.0 | 68 | 5 | 1 | ||
| Euphausid shrimp | 0.8 | 80 | 2 | 1 | ||
| Clam (edible part only) | 0.8 | 80 | 1 | 13 | ||
A bird must satisfy not only energetic needs, but also nutritional requirements. Fish are high in protein (Table 1), but what little is known of marine invertebrates suggests a low proportion of protein in relation to total bulk. Protein is vital to growth of nestlings (Fisher 1972; Lemmetyinen 1972), and 4-8% protein in the diet seems to be required for minimal maintenance of adults (Martin 1968; Fisher 1972). Some seabirds (such as puffins, Fratercula) that eat a varied diet raise their young exclusively on fish (Bédard 1969b; Nettleship 1972; Sealy 1973a).
Other aspects of nutrition, such as vitamins and minerals, are also important to avian health (Brisbin 1965; Fisher 1972). To further complicate matters, nutritional values vary with season, as do birds' requirements for them (Myton and Ficken 1967; Moss 1972). Adults must adjust their time and energy allocation to foraging to optimize not only energetic, but also nutritional return.
Optimal time and energy allocation has been studied in theory (Orians 1971; Schoener 1971; Pulliam 1974; Katz 1974) and some direct observations have been carried out, largely on seedeaters (Bookhout 1958; Myton and Ficken 1967; Royama 1970; Moss 1972; Willson 1971; Willson and Harmeson 1973). The direct studies, in particular, point out the basic importance of studying cost-benefit ratios by interrelating complex factors of food availability, searching patterns, and type, size, and caloric and nutritional value of foods.
This section concerns variation in time and energy allocated by seabirds to activities above and beyond the cost of living—particularly to migration, molt, and reproduction. Allocation to such items as avoidance of predation and competition is not considered here, because they are not readily analyzed as activities to which time and energy are devoted in a specific part of the annual cycle.
The previous discussion dwelt on energy considerations and could have referred to almost any group of birds. The following treatment centers on time use of northern seabirds. Little is known of energetics beyond the cost of living, although estimates have been made for certain aspects of migration, molt, and reproduction (Nisbet 1963; Hussell 1969; Hart and Berger 1972; Payne 1972; Ricklefs 1974). Essentially nothing is known, however, of the relationship of such costs to the amount of energy available to the bird once basic maintenance costs have been met (productive energy). Because such complete data are not available, the following account dwells largely on variation in timing and total time devoted to activities beyond basic maintenance.
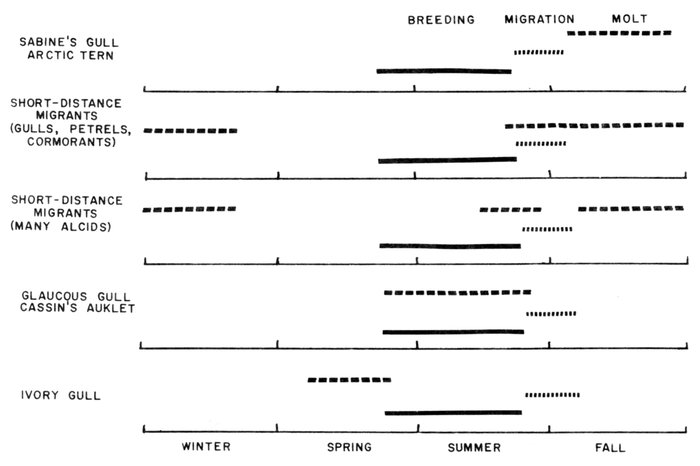
Fig. 4. Typical patterns of generalized annual cycles in reproduction, migration, and wing molt in northern seabirds. Solid line shows reproductive season, dotted line the period of migration or dispersion, and dashed line the period of annual primary molt. Data from Dorst (1961), Stresemann and Stresemann (1966), and Ashmole (1971).
Among northwestern North American seabirds, most coastal feeders, such as gulls, cormorants, and many alcids and petrels, have only a short southward migratory movement, and many others are more or less resident (Dorst 1961; Ashmole 1971). Terns, on the other hand, migrate long distances in a short time to places where small fish are available near shore in the winter. Other long-distance migrants—Sabine's gull (Xema sabini), jaegers (Stercorarius spp.), pelagic phalaropes (Phalaropus, Lobipes), and kittiwakes (Rissa spp.)—tend to scatter widely over the southern ocean, concentrating near areas of upwelling (Dorst 1961; Ashmole 1971). Groups such as murres (Uria spp.), eiders (Somateria spp.), and grebes (Podiceps spp.) may move considerable distances by swimming (Dorst 1961; Tuck 1960). True migration tends to take place directly before and after reproduction, whereas dispersal or nomadism takes place over a long period of the winter (Fig. 4).
Among species remaining in the northern hemisphere, younger birds frequently disperse greater distances than do breeding adults (Coulson 1961; Kadlec and Drury 1968; Southern 1967), and the degree of dispersal can vary among colonies of the same species (Coulson and Brazendale 1968).
Energy costs of migration must vary according to distances covered and amount of time allocated to migration. Aside from the references to cost of flight mentioned earlier, however, migratory costs have scarcely been studied. Dolnik (1971) has estimated that chaffinches (Fringilla coelebs) expend about as much energy migrating south as they would on thermoregulation if they overwintered on their breeding grounds. Long-distance migration is presumably selected because the birds are able to collect food more efficiently, because the risks of death or injury in migrating are less than in residency, and so on. Interspecific and intraspecific competition may also be involved (Cox 1968). In other words, migratory patterns are selected to optimize survival and reproduction in alternating environments (Cohen 1967; Drury and Nisbet 1972).
| Rapidity of wing molt and (indented) flight capability in molt | Timing of start of molt | Species |
|---|---|---|
| Slow Retained |
During care of young | Cassin's auklet, parakeet auklet (Cyclorhynchus psittacula), whiskered auklet (Aethia pygmaea) |
| Retained | After young become independent | Least auklet (A. pusilla), crested auklet (A. cristatella) |
| Rapid Poor |
After arrival in winter quarters | Marbled murrelet, Kittlitz's murrelet (Brachyramphus brevirostris) |
| Almost synchronous None |
After end of breeding | Xantus' murrelet (Endomychura hypoleuca) |
| Synchronous None |
As soon as young go to sea | Guillemots (Cepphus spp.), murres, razorbill (Alca torda), dovekie (Alle alle) |
| None | In winter, after body molt | Puffins |
Although one may suspect that location of winter food supply is the main environmental factor affecting migratory patterns, there is little direct evidence on the reasons for, or the benefits accruing from, the different patterns seen in seabirds. Study of cost-benefit ratios of foraging in different stages of migration might help clarify the question.
Patterns of molt vary widely among seabirds. The commonest pattern is for a prenuptial body molt to occur in spring, and for an extended wing molt to begin after the breeding season and continue well into the winter (Fig. 4). In short-distance migrants, molt may overlap slightly with the end of breeding and can last up to 6 months, as in most gulls, terns, alcids, nonmigratory jaegers, and cormorants (Stresemann and Stresemann 1966).
Long-distance migrants frequently delay molt until in the winter quarters (lesser black-backed gull, Larus fuscus; Sabine's gull; jaegers; arctic tern, Sterna paradisaea; and marbled murrelet, Brachyramphus marmoratus) and molt there may occur rapidly (3.5 months in the arctic tern). Certain other long-distance migrants begin molt before leaving the breeding grounds (herring gull; skua, Catharacta skua; Leach's petrel, Oceanodroma leucorhoa; and fulmar), although molt may be interrupted during migration, as in Larus argentatus heuglini (Stresemann and Stresemann 1966). Duration, timing, and rapidity of molt are particularly varied among the alcids (Table 2).
A few unusual molt patterns are found in northern seabirds. The ivory gull (Pagophila eburnea) has its major annual wing and body molt immediately before it breeds. In several other species such as the glaucous gull (Larus hyperboreus) and Cassin's auklet (Ptychoramphus aleuticus) the molt almost completely overlaps the reproductive cycle (Johnston 1961; Payne 1965). Potts (1971) documented a molt pattern in shags (Phalacrocorax aristotelis) which is more typical of tropical seabirds. Several cycles of wing molt take place simultaneously, each lasting more than a year, and molt ceases in winter. By the time breeding age is reached, each flight feather is replaced once a year.
Within these broad categories of molt pattern there are sometimes variations according to age, sex, and even subspecies (Stresemann and Stresemann 1966). Male common eiders (Somateria mollissima) molt directly after mating, when their reproductive role is completed, whereas females molt only after they[Pg 148] have taken their young to sea. Nonbreeders and failed breeders frequently begin molt while other adults are still raising young and not molting—e.g., many alcids, gulls, storm-petrels, and fulmars (Stresemann and Stresemann 1966; Ingolfsson 1970; Harris 1971; Harris 1974; Sealy 1975b). In ivory gulls, which molt just before reproduction, and in Sabine's gulls, which complete molt just before breeding, nonbreeders may extend wing feather growth into the breeding season (Stresemann and Stresemann 1966).
There is little information on the energetic cost of molt, although there are indications of at least some expense. Belopol'skii (1961) showed that nonmolting seabird species tended to gain weight after reproduction, whereas those that immediately started molt tended to lose weight. Among other birds, however, it is common for individuals to gain weight just before, and even during molt (Payne 1972). The BMR is known to rise in molting birds (Blackmore 1969; Lustick 1970; Payne 1972), from as little as 5% to as much as 34% above nonmolting levels. In one study, about 35-40% of the increased BMR represented extra thermoregulatory costs incurred by lessening of insulation and increase in heat loss from well-vascularized new feathers; the rest of the increase represented the energetic cost of growing feathers (Gavrilov 1974). The fact that molt rarely overlaps with breeding suggests that the energetic cost, even if slight, may be incompatible with the already high costs of reproduction (Payne 1972). Cassin's auklets, which do molt while breeding, may cease molt while feeding large young (Payne 1965), and certain species interrupt molt during migration (Stresemann and Stresemann 1966). Doubtless a rapid simultaneous molt is more costly than a long gradual one.
Rapid molt appears to occur at a time in the annual cycle when food resources are abundant (spring or late summer), whereas extended molt generally occurs over winter (e.g., Bédard 1969a). If one speculates that energy availability is the main determinant of molt patterns, one can also speculate on the cause behind some of the more unusual patterns. Possibly birds in which molt and breeding overlap either have extraordinary available energy at that time or else face shortages in other periods. For example, ivory gulls, which breed in the high Arctic, molt when food resources have become abundant in the low Arctic but before the high Arctic breeding grounds have thawed sufficiently for reoccupation.
Speed of molt may also reflect availability of energy resources or of nutrients needed for feather growth (Payne 1972), but must also be influenced by the need for full flight capabilities to obtain food. The eider duck and many alcids that shed wing feathers almost simultaneously do not need their wings for flight after the young have left the breeding colony. Hydrodynamic considerations suggest that their fishing capabilities may even be improved (Storer 1960). This is not true for the smaller species—e.g., Aethia molts only one feather at a time and retains full flight capabilities (Table 2). Climate may also influence simultaneity of molt if heat loss in rapid molt is particularly severe.
Time use of seabirds is best known for the reproductive period, when the birds are on relatively accessible breeding grounds, the weather is most suitable for observation, and academic researchers are freed from their jobs. Even so, the details of timing are known for only a few of the species and localities on the northwest North American coast (e.g., Drent and Guiguet 1961; Drent et al. 1964; Cody 1973; Sealy 1973a, 1975b, 1975c). The following discussion emphasizes the multitude of environmental factors known to influence timing and total length of time devoted to various aspects of the reproductive cycle.
Each species of seabird returns to the colony site when weather conditions have ameliorated sufficiently to meet its particular needs. For example, the early arrivals to islands in the Barents Sea are murres, kittiwakes, and herring gulls, which need only small cracks in the sea ice to meet their feeding requirements (Belopol'skii 1961). Eiders in North America also return early, when a few ice leads have formed (Schamel 1974). Common puffins (Fratercula arctica) and mew[Pg 149] gulls (Larus canus) are somewhat later arrivals, and terns and a few parasitic jaegers (Stercorarius parasiticus) are the latecomers to Barents Sea colonies (Belopol'skii 1961).
The timing of the season (as illustrated in Fig. 5) varies widely among localities, and because of local weather patterns and ocean currents, this variation can be unrelated to latitude (Belopol'skii 1961). Examples of such variation are also known in North America: for instance, Leach's storm-petrels in Alaska lay eggs 2 to 3 weeks later than do those in California (Harris 1974); however, the details of timing are largely unknown for many species in this region. Progression of thaw, which also varies from year to year, causes variation in the timing of the breeding season (Belopol'skii 1961; Evans and McNicholl 1972). Fig. 6 shows the diversity in start of the breeding season for different species on the same island in the Barents Sea as well as variation in time devoted to various components of the reproductive cycle.
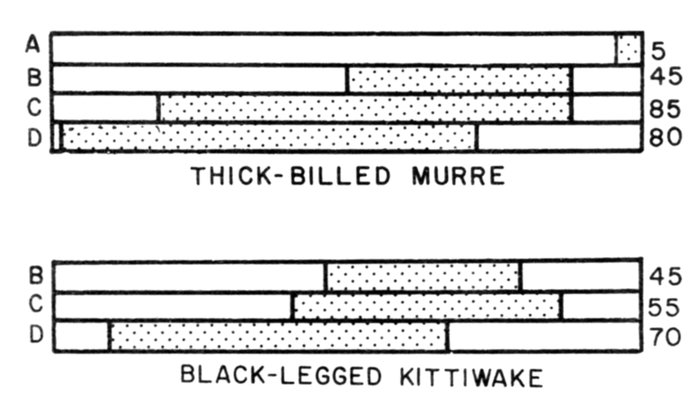
Fig. 5. Differential average arrival on breeding grounds and average duration of prenesting period of thick-billed murres (Uria lomvia) and black-legged kittiwakes on various colonies in the Barents Sea. From Belopol'skii (1961). Length of prenesting period in days (shaded bars) indicated on right. Letters represent locations as follows: A = Novaya Zemlya, Kara Straits; B = Novaya Zemlya, Karmakuly Bay; C = Franz Josef Land; and D = East Murman.
Some species are apparently able to delay maturity of sexual organs until environmental conditions are suitable for nesting—e.g., burrow and crevice nesters in the Barents Sea do not become sexually mature until snowmelt (Belopol'skii 1961). Many others, however, reach sexual maturity soon after arrival on the breeding grounds, and a few (such as jaegers and kittiwakes) mature in migration or on the wintering grounds (Belopol'skii 1961). Northern phalaropes (Lobipes lobatus) sometimes lay eggs as early as 1 week after arrival (Hilden and Vuolanto 1972). This factor, in combination with timing of arrival, affects the amount of time spent in prenesting activities (Fig. 6). Most species gain weight during this period (Belopol'skii 1961), and the time required for each species to reach full breeding condition must also depend on feeding conditions and the state of the bird on its arrival at the nesting site. These factors help explain why early arriving species are not necessarily early nesters (Fig. 6).
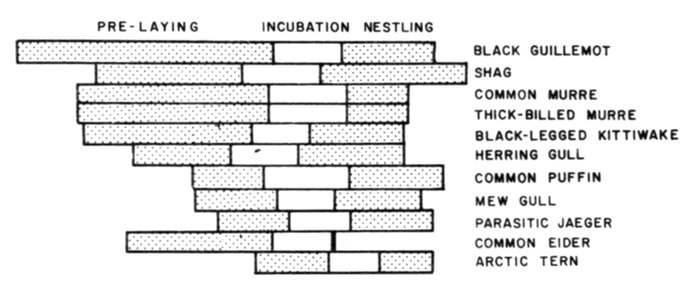
Fig. 6. Variation in timing of events in the reproductive cycle of Barents Sea seabirds nesting on the same island. Data from Belopol'skii (1961). Shaded bars at left indicate the prelaying periods, open bars the incubation periods, and shaded bars at right the portion of the growth period in which the chick remains at the nest site. Total length of time indicated is about 6 months.
Aside from nest building, most prenesting activity consists of courtship and territorial behavior. These activities have been well described for representative seabird species, but because assessments of time and energy devoted to them have been almost completely neglected, they are not discussed further here. For examples, see accounts in Gross (1935) for Leach's storm-petrel; Tinbergen (1935), Bengtson (1968), Höhn (1971), and Howe (1975) for phalaropes; Storer (1952) for common murre, Uria aalge, and black guillemot, Cepphus grylle; Tschanz (1959) for common murre; Brown et al. (1967) for Sabine's gull; Tinbergen (1960) for herring gull; McKinney[Pg 150] (1961) for eiders; Snow (1963) for shag; Thoresen (1964) for Cassin's auklet; Vermeer (1963) and James-Veitch and Booth (1974) for glaucous-winged gull (Larus glaucescens); and Andersson (1973) for jaegers.
Although many northern seabirds have essentially no nest, they may spend considerable time working or displaying at the site (Belopol'skii 1961). Black-legged kittiwakes (Rissa tridactyla) have substantial nests, but they are built in a comparatively short time (about a week) soon after the birds arrive (Fig. 6). Shags also have substantial nests, but they are not completed until about 1 or 2 weeks before the first egg is laid (Snow 1963). Herring gulls build smaller nests, 5 to 10 days before laying, although in the Far North they and glaucous gulls may not start building the nest until the first egg is laid (Belopol'skii 1961). The eider always begins preparing the nest when the first egg is laid (Belopol'skii 1961; Schamel 1974), and terns and skuas, which build no nests, choose their sites at that time. Murres, which frequently lay their eggs directly on snow, choose a site somewhat earlier and spend considerable time protecting it (Belopol'skii 1961). Burrows may be dug within a period as short as 3 days for Leach's storm-petrel (Gross 1935) to one as long as several weeks in Cassin's auklets (Manuwal 1974a). Overall, the prelaying period is longer for burrow nesters than for those using crevices (Sealy 1973a).
The amount of time and energy spent by the male and female in nest building differs among species. In Leach's storm-petrel, the male digs the burrow (Gross 1935), whereas in eiders, the nest is built entirely by the female. In most seabird species, the sexes share in nest construction, but roles may still be separated. For example, in shags the male collects the nest material and the female builds the nest (Snow 1963).
Timing of egg laying is influenced not only by weather (Erskine 1972; Sealy 1975c), but also by numerous biotic factors. Smith (1966) showed that where glaucous gulls, herring gulls, and Thayer's gulls (Larus thayeri) breed in mixed colonies, the peak of sexual activity and egg laying in Thayer's gull is about midway between the peaks for the other two species (Fig. 7). In nearby colonies where herring gulls are absent, however, the peak of sexual activity in Thayer's gulls is delayed about a week, and activity continues for a significantly longer period (Fig. 7).
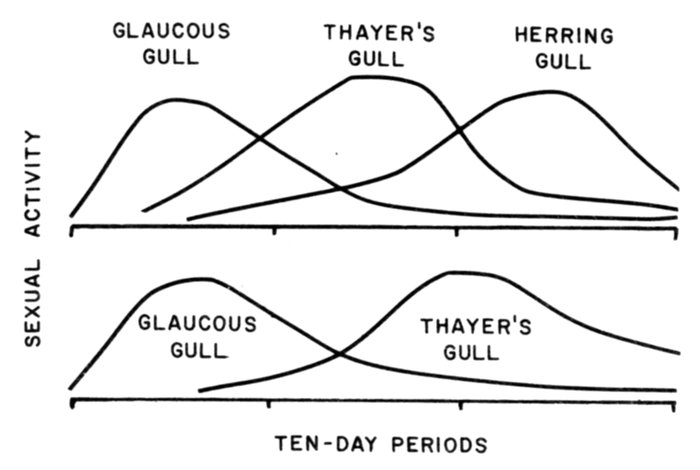
Fig. 7. Timing of peak sexual activity (a combined measure of egg laying and testes size) in colonies of arctic gulls of different species composition. From Smith (1966).
Annual variations in food supply also will affect the start of the egg-laying season. Belopol'skii (1961) cited an example from the Barents Sea in 1940 when a series of storms made it difficult for certain seabirds to find food. Murres and kittiwakes, which were able to catch fish, started reproductive activities on schedule. Gull breeding was delayed, however, and egg laying began in force only after fishing boats arrived and started discarding offal. Onset of egg laying in great cormorants (Phalacrocorax carbo) is correlated to April air temperatures (Erskine 1972), and this may also be related to variations in spring increase of food availability. In certain birds the breeding season has been shown to start particularly early when food supplies are unusually abundant (Högstedt 1974; Källender 1974), but this has not yet been demonstrated in seabirds.
Lastly, age and sex of seabirds are known to affect the timing of egg laying (e.g., Coulson and White 1960; Lack 1966); older, more experienced birds tend to lay earlier than do younger ones. In shags, males tend to breed progressively earlier as they increase in age, but females do not (Snow 1963).
Contrary to the situation in passerines, seabirds tend to lay their clutches with relatively large time intervals between eggs. Eggs may be laid every 2nd or 3rd day in alcids, larids, sternids, stercorariids, and phalacrocoracids (Lack 1968), but every day in phalaropes (Howe 1975). Inasmuch as clutch size in northern seabirds varies from one to five or six, the length of the laying period varies widely among species.
Energetic costs of egg laying depend on the actual caloric content of the egg and the speed with which the ova are developed (Ricklefs 1974). The energy in the egg is contained mainly in the yolk, and yolk size depends largely on the developmental pattern shown by the young after hatching (Table 3). Precocial chicks are hatched at a relatively advanced stage, are covered with down, and have open eyes, can maintain reasonably homeothermic body temperature, and leave the nest site to feed themselves after a few hours or days. At hatching, semiprecocial chicks appear similar to precocial chicks, although they are slightly less well developed (Ricklefs 1974; Dunn 1975a). In contrast to precocial chicks, they remain at the nest site for some time, are fed by their parents, and tend to grow rather rapidly (Ricklefs 1968). Altricial nestlings hatch at a much less advanced stage of development. They are naked, blind, helpless, essentially poikilothermic, and depend completely on their parents for food and shelter. They usually remain at the nest until full grown. Semialtricial chicks show somewhat intermediate characteristics (Nice 1962).
| Developmental type | Percent yolk (by weight) |
|---|---|
| Precocial | 30-60 |
| Semiprecocial | 25-30 |
| Altricial | 15-25 |
The amount of yolk (and therefore energy) in an egg is positively correlated to the degree of development at hatching (Table 3). The same is true for egg size: altricial and semialtricial birds have smaller eggs relative to adult body weight than do semiprecocial and precocial birds (Fig. 8). Clutch size, however, is unrelated to energy content of the eggs. For example, shags (which are altricial) and eiders (precocial) have among the largest clutches of northern seabirds (four to six eggs).
Fig. 8. Egg weight as a function of body weight in various northern seabirds. Solid symbols represent precocial and semiprecocial species, and open symbols altricial and semialtricial species: solid circles, alcids; solid triangles, gulls, terns, and jaegers; solid squares, eiders; open squares, cormorants and Morus bassanus; and open circles, petrels. Data from Belopol'skii (1961); Drent (1965); Schönwetter (1967); Lack (1968); Bédard (1969a); Cody (1973); Sealy (1973b); Harris (1974); and Manuwal (1974a).
The energetic cost of egg laying depends not only on caloric content of the egg and clutch size, but also on speed of development. Ricklefs (1974) has shown that the energetic cost per day of egg laying can be calculated from the energy content of the yolk and white, clutch size, the amount of follicular growth per day, and the laying interval between eggs. The energy content of a single egg (expressed as percentage of BMR) has been estimated as follows: 45 (altricial passerines), 103 (semial[Pg 152]tricial raptors), 126 (precocial galliformes), 180 (precocial ducks), 226 (precocial shorebirds), and 320 (semiprecocial gulls and terns). Gulls and terns thus have very costly eggs, as well as a moderately high clutch size (three). However, the development time for a single ovum in the herring gull is unusually long—9 to 10 days (King 1973). Ricklefs (1974), who calculated the energetic cost per day (expressed as percentage BMR), estimated the cost of a clutch in gulls and terns (120% BMR per day) to be similar to that for various groups of precocial birds (about 125-180% BMR per day). Unfortunately, the data required for calculation of the average energetic cost of a clutch are not available for other northern seabirds.
For no species have all the additional factors influencing the energetic cost of a clutch been taken into account. For example, eggs in a clutch may vary in size (and caloric content) according to sequence in the clutch (Preston and Preston 1953; Snow 1960; Coulson 1963; Coulson et al. 1969). Age has a definite effect on laying energetics, as older birds lay larger eggs (Coulson and White 1958; Snow 1960; Coulson 1963; Coulson et al. 1969) and lay larger clutches (Coulson and White 1960; Snow 1960). They also lay, on average, earlier in the season (Coulson and White 1958; Snow 1960; Coulson et al. 1969), and eggs laid late in the season (whether by young birds or older ones in re-nesting attempts) tend to be smaller and contain less energy. In addition, egg quality can vary with food supply: Snow (1960) found eggs to have more yolk in years when food was abundant than in years when food was scarce.
Egg-laying costs are, of course, borne entirely by the female, although males may contribute some time and energy toward egg laying through courtship feeding (Ashmole 1971; Henderson 1972; Nisbet 1973). Courtship feeding takes place in most lariforms but not in eiders, phalaropes, or cormorants.
The time and energy expended on egg laying can be profoundly influenced by the degree of nest destruction, since females usually lay a replacement clutch if the loss of the first does not occur too late in the season. Factors causing egg destruction are numerous, but among the most important in the north is predation. As is shown in Table 4, the degree of egg predation in common murres is correlated to degree of exposure of the nest—so even such an unlikely sounding factor as physical characteristics of the nest site can affect the average time and energy expended on egg laying by a given species or population. Genuine second clutches are occasionally laid by phalaropes (Hilden and Vuolanto 1972) and Cassin's auklets (Manuwal 1974a).
| Nest exposure | Nests destroyed by predators (%) |
|---|---|
| Completely hidden | 3.2 |
| Partly exposed | 5.8 |
| Largely exposed | 13.6 |
| Completely exposed | 18.2 |
In short, time and energy devoted to egg laying depend not only on the species, but also on a multitude of other biotic and abiotic factors, such as age, sex, degree of nest destruction, weather, other species present, and feeding conditions.
The total time devoted to incubation does not depend directly on developmental type or egg size but differs markedly among families (Lack 1968). Since incubation period seems to be closely linked to fledging period, factors affecting growth rate (discussed later) apparently affect incubation period as well.
Each species has a different incubation rhythm. In birds in which the sexes share in incubation, the sexes exchange places at intervals that differ widely among different birds: several hours in lariforms and some alcids (Drent 1965; Lack 1968; Preston 1968; Drent 1970); about 4 h in shags (Snow 1963); up to 11 h in the ivory gull (Bateson and Plowright 1959); up to 24 h in certain other alcids (Manuwal 1974a; Sealy 1975a); 33 h (on the average) in common puffins (Myrberget 1962); 72 h in ancient murrelets, Synthliboramphus antiquus (Sealy 1975a); and 96 h in Leach's storm-petrel (Gross 1935). Degree of attentiveness once a bird is on the nest also varies. Petrels may leave the egg for several days[Pg 153] (Gross 1935), whereas herring gulls cover their eggs 98% of the time (Drent 1970).
The sexes share in incubation in most seabirds (Snow 1960; Drent 1965, 1970; Bédard 1969a), although females frequently take on the greater role (Belopol'skii 1961). Only male phalaropes incubate the eggs, and only female eiders. Eider hens do not feed during the entire incubation period (25 days) and leave the nest only for short periods of about 10 min (Belopol'skii 1961; Schamel 1974).
Several methods exist for calculating the amount of heat input necessary for normal development of a clutch of eggs (Ricklefs 1974). There is controversy, however, as to whether an adult can provide this warmth from excess body heat lost during the course of normal metabolism or whether the adult must raise its metabolic level to produce extra heat (Kendeigh 1973; King 1973; Ricklefs 1974). Several studies of incubating birds suggest that, in at least some situations, adults need not raise metabolic levels, but in others (large clutch, severe weather), they probably do (Ricklefs 1974). Drent (1972) estimated that herring gulls raise metabolic levels to a significant degree during incubation.
In spite of the lack of quantitative data, one can surmise that the cost of incubation varies among seabirds. Precocial and semiprecocial birds tend to have a larger clutch weight relative to body weight than do altricial birds (Fig. 8; Lack 1968), and therefore require greater heat input to the eggs. These costs may be reduced by heavily insulating the nest (e.g., eiders), or by nesting in burrows, which have much more moderate and even climates than do external nests (Richardson 1961; Manuwal 1974a). Other semiprecocial species, however, such as the murre, may sometimes lay eggs directly on snow or ice (Belopol'skii 1961)—presumably at increased incubation costs. Lastly, certain species incubate eggs with their feet (e.g., cormorants), rather than develop featherless brood patches. There are no measurements of comparative heat flow from feet versus brood patches.
The length of the nestling period (hatching until departure from the nest) varies greatly among northern seabirds (Fig. 6). Nestling period depends on the stage of growth at which the young leave the nest and the rate at which they attain that stage. Growth rate in turn depends largely on body size and developmental type.
The stage of growth attained when birds leave the nest varies considerably (Fig. 9). Precocial eiders leave the nest within a day of hatching, whereas altricial shags remain until completely grown. The young of semiprecocial species, on the other hand, leave the nest at all stages between these extremes. Larids normally remain at the nest until 75-90% grown, but certain alcids leave much sooner—well before the young can fly.
Fig. 9. Percentage of total growth completed in the egg (shaded bar at left), at the nest site (open bar), and after nest-leaving (shaded bar at right) in various northern seabirds. From Belopol'skii (1961).
Growth rate depends both on body size and developmental type (Fig. 10). The length of stay at the nest for precocial young is unaffected by growth rate (which is typically very slow), since they leave soon after hatching. The nestling period of semiprecocial and altricial seabirds is, however, affected by the rate at which the young grow to the nest-leaving stage. This depends mainly on body size (Fig. 10) and to a certain degree on developmental type, as some semiprecocial species grow rather slowly. Certain seabirds with clutches of one egg grow particularly slowly (petrels, some alcids, sulids). Several other alcids with single-egg clutches, however, grow[Pg 154] at rates normal for semiprecocial chicks (Fig. 10). Very slow growth may be related to food stress (Lack 1968; Ricklefs 1968) or to reduction of reproductive effort in the adults (discussed later). Contrary to Cody (1973), slow growth in alcids does not correlate to the distance adults must commute for food. (Cody tried to directly compare growth in birds of different sizes.) Chicks in nocturnal species, however, tend to have slow growth rates (Sealy 1973b).
Fig. 10. Growth rate as a function of body weight. Growth rate T10-90 represents the number of days to grow from 10% to 90% of asymptotic weight (Ricklefs 1968). Data from Ricklefs (1968, 1973), E. H. Dunn (1973), and Sealy (1973b). Solid circles and regression line, altricial birds; solid triangles, semiprecocial birds except for seabirds with one-egg clutches; open circles, precocial shorebirds; open triangles, precocial ducks, rails, and gallinaceous birds; solid squares, alcids with one-egg clutches; and open squares, northern petrels, gannet, and Manx shearwater (Puffinus puffinus).
Daily time budgets of adults raising nestlings also vary widely, depending on the amount of brooding required, food requirements of the young, and foraging costs (which differ in the breeding season from those at other times of the year).
Nestlings have imperfect control of body temperature at hatching (Fig. 11) and develop this capacity only gradually. Altricial birds are hatched at a particularly undeveloped stage; e.g., double-crested cormorants attain reasonable control of body temperature in moderate ambient temperatures only after about 14 days (Fig. 11; Table 5). Semiprecocial seabirds, which are more fully developed physically at hatching, attain control of body temperature much sooner, in a matter of several days, and precocial eiders can thermoregulate within a few hours after hatching (Table 5).
Until the age of temperature control, nestlings must be brooded almost constantly, and occasional brooding takes place for some time afterward, especially in severe weather, in all species studied (Tinbergen 1960; Belopol'skii 1961; Weaver 1970; Dunn 1976a, 1976b). Thermoregulatory capabilities in cold weather are better in ducklings of species nesting at high latitudes than at lower ones (Koskimies and Lahti 1964), and the same may be true of gull species (Dawson et al. 1972). The cooling mechanisms of double-crested cormorants are better than in the more northerly distributed pelagic cormorant, Phalacrocorax pelagicus (Lasiewski and Snyder 1969). Thus, variation in cost of thermoregulation due to different environments may be reduced through adaptation.
Food requirements of the chick depend on[Pg 155] growth rate, amount of fat deposition, cost of thermoregulation, degree of activity and other factors (E. H. Dunn 1973). Estimated energy budgets for nestling double-crested cormorants and herring gulls in the same year and locality (Fig. 12) indicate that these factors vary according to developmental type, and comparison with budgets for nonseabird species suggests wide variation within developmental types according to the particular adaptations of each species to its own environment (E. H. Dunn 1973).
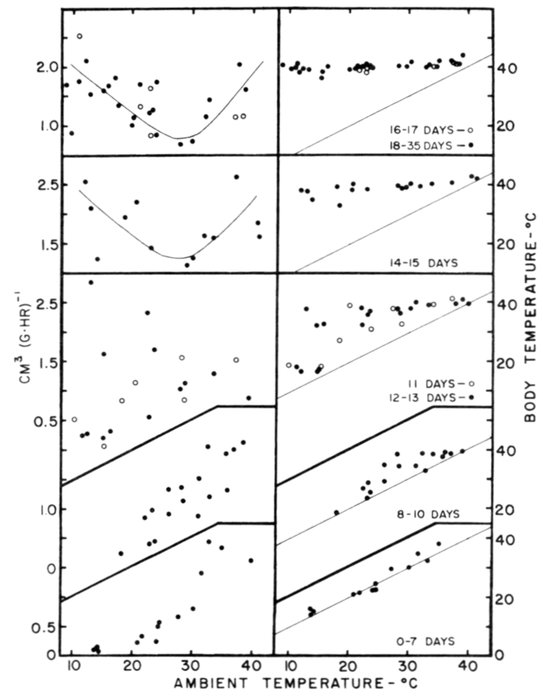
Fig. 11. Development of thermoregulatory capabilities in nestling double-crested cormorants. From Dunn (1976a). Ages at right refer also to corresponding oxygen consumption data on the left. Thin diagonal lines show equality between body and air temperature. All data taken after 2 h of exposure.
Thus, the energy demands of nestlings are not easy to predict. Brood size differences multiply variation in food demand on adults (except in precocial birds whose young feed themselves). Energy demands are labile, however, particularly in requirements for activity and growth, and adults can frequently raise young successfully without providing optimum amounts of food (Spaans 1971; Kadlec et al. 1969; LeCroy and Collins 1972; Lemmetyinen 1972; Cody 1973; E. H. Dunn and I. L. Brisbin, manuscript in preparation). Studies of double-crested cormorants by Dunn (1975b) and pigeon guillemots (Cepphus columba) by Koelink (1972) have suggested that each adult providing optimum amounts of food to a normal-sized brood would have to approximately double the amount of food gathered each day over the amount gathered by nonbreeders. This relation does not imply, however, that the time and energy allocation of the adults would be the same for the two species.
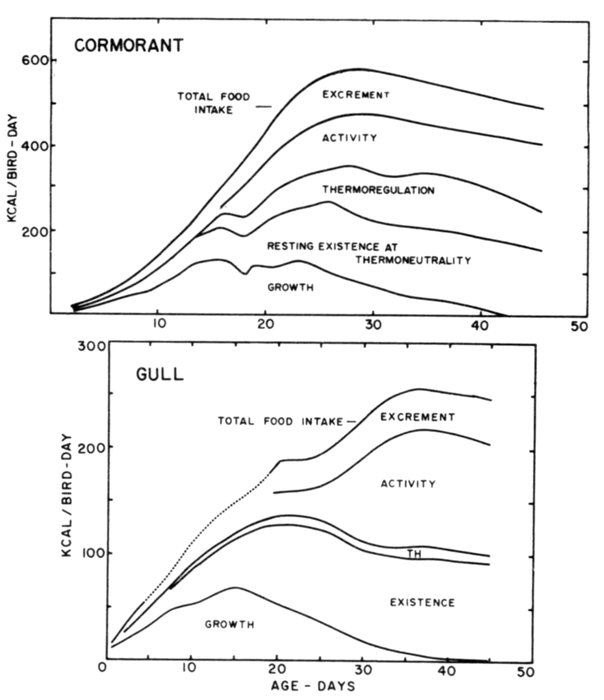
Fig. 12. Energy budgets of nestling double-crested cormorants and herring gulls. Data from E. H. Dunn (1973) and Brisbin (1965).
Cost-benefit ratios of food gathering in the nestling period differ from those at other times. Besides facing increased food demands, costs of delivery to the nest, and changes in food availability, the parents' choice of foods is constrained by the need to forage within reasonable commuting distance of the nest and perhaps by concentrated competition with conspecifics and other seabird species. In addition, small nestlings are frequently unable to eat foods normally eaten by adults (Drent 1965; personal observation). In the face of these constraints, adults often shift[Pg 156] food preferences while raising nestlings (Belopol'skii 1961). For example, female mew gulls in the Barents Sea forage in the tidal zone, eating more small invertebrates than at other times of the year, while males continue to forage at sea and consume larger quantities of fish (Fig. 13).
| Species | Age when moderate temperature control is attained (days) |
Source |
|---|---|---|
| Common eider | 0.1-0.3[41] | V. V. Rolnik, in Belopol'skii (1961) |
| Herring gull | 1.5-2 | V. V. Rolnik, in Belopol'skii (1961) |
| Herring gull | 2-3 | E. H. Dunn (1976b) |
| Leach's storm-petrel | [2] | Ricklefs (1974) |
| Mew gull | 2-3 | V. V. Rolnik, in Belopol'skii (1961) |
| Lesser black-backed gull | 2-3 | E. K. Barth (in Farner and Serventy 1959) |
| Greater black-backed gull | 2-3 | E. K. Barth (in Farner and Serventy 1959) |
| Pigeon guillemot | 2-4 | Drent (1965) |
| Common tern | 3 | LeCroy and Collins (1972) |
| Roseate tern (Sterna dougallii) | 3 | LeCroy and Collins (1972) |
| Common murre | 3 | V. V. Rolnik and Yu. M. Kaftonowski (in Sealy 1973b) |
| Razorbill (Alca torda) | 3 | V. V. Rolnik and Yu. M. Kaftonowski (in Sealy 1973b) |
| Black guillemot | 3-4 | V. V. Rolnik, in Belopol'skii (1961) |
| Tufted puffin | 3.5[42] | Cody (1973) |
| Northern phalarope | 4-5[43] | Hilden and Vuolanto (1972) |
| Cassin's auklet | 5-6 | Manuwal (1974a) |
| Horned puffin (Fratercula corniculata) |
2-6 | Sealy (1973a) |
| Common puffin | 6-7 | V. V. Rolnik and Yu. M. Kaftonowski (in Sealy 1973b) |
| Black-legged kittiwake | 6-7 | V. V. Rolnik, in Belopol'skii (1961) |
| Double-crested cormorant | 14 | Dunn (1976a) |
| Shag | 12-15 | V. V. Rolnik, in Belopol'skii (1961) |
Commuting distances vary tremendously among species (Fig. 14), but the number of feeding trips to the nest per day does not correlate with foraging distance (Cody 1973; Sealy 1973a, 1973b). There is not, therefore, a simple relationship between time and energy expenditures of the adults and foraging distances. Nocturnality, on the other hand, correlates with reduced feeding rates (usually one visit to the nest each night). Seabirds feeding far from the colony tend to show adaptations for bringing larger amounts of food per visit, such as carrying more than one fish at a time, as in tufted puffins, Lunda cirrhata, and rhinoceros auklets, Cerorhinca monocerata, vs. guillemots and murres (Richardson 1961; Cody 1973; Sealy 1973a, 1973b); developing a sublingual storage pouch, as in Cassin's auklets (Speich and Manuwal 1974); or concentration of food into stomach oil, as in petrels and albatrosses (Ashmole 1971). Commuting costs are largely eliminated when the young leave the nest, but only in the alcids does nest leaving occur long before attainment of full growth. Early nest leaving may allow adults and young to disperse to better feeding areas than are exploitable from the colony site (Sealy 1973b) and probably involves a major change in optimal food size and type as well (Lind 1965).
Patterning of adult time budgets may differ[Pg 157] between geographical regions. For example, rhinoceros auklets are nocturnal in the far north (where the summer night is particularly short), crepuscular in the Olympic Peninsula, and mainly diurnal in the Farallon Islands (Manuwal 1974a).
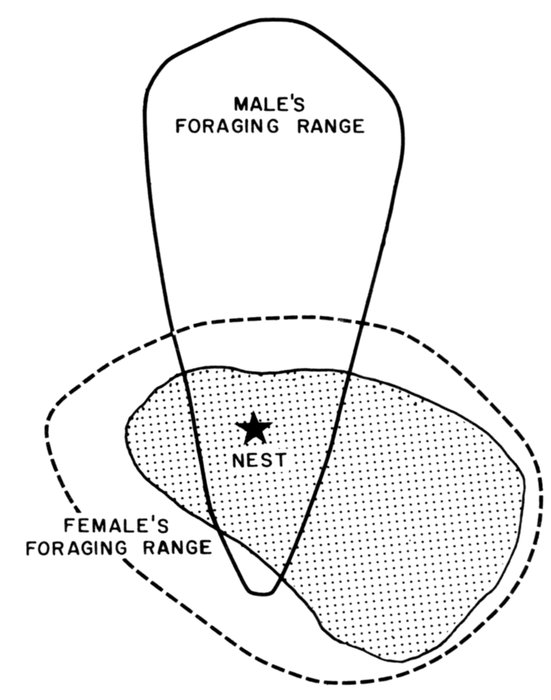
Fig. 13. Foraging ranges of a pair of mew gulls during the breeding season, on a Barents Sea colony. From Belopol'skii (1961).
Food demands of nestlings have a great influence on the time and energy allocation of breeding over nonbreeding seabirds. Because food is particularly abundant in the reproductive season, however, one cannot ascertain whether the vulnerability of breeding birds to time or energy crises is far different from that at other times of the year.
Little is known about the amount of care provided by adults to young after they are fully grown. At least some species, such as gannets and procellariiformes (Ashmole 1971), are known to desert their young, whereas others are known to feed their young, at least occasionally, for some weeks or months after they can fly—e.g., terns and gulls, many alcids, and shags (Snow 1963; Vermeer 1963; Drury and Smith 1968; Ashmole and Tovar S. 1968; Potts 1968; Ashmole 1971; LeCroy 1972).
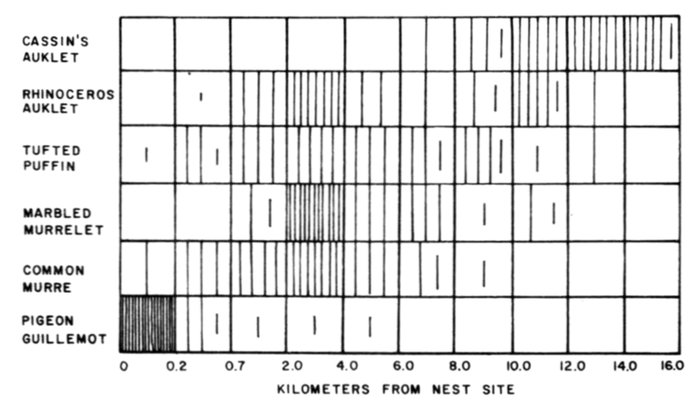
Fig. 14. Percentage observations of foraging seabirds at different distances from the nest site. After Cody (1973). Each vertical bar represents 5% of total observations. Note nonlinear horizontal scale.
The discussion of time and energy allocation during reproduction was complex and detailed because so much more is known about the influences altering budgeting during this period than during other times of the year. It is likely that influences on molt and migration will prove to be equally complicated, once more is learned about them.
If all data on time and energy allocation for a single species were known, it would be possible to make up detailed budgets for birds of different age, sex, and experience throughout the year. However, such detailed data have not been collected for any species. An annual time budget for male and female yellow-billed magpie, Pica nuttalli (Verbeek 1972), points out the great amount of difference between the sexes (Fig. 15). A time and energy budget for the reproductive season only (Fig. 16) shows large differences between two closely related species, as well as between sexes; it also indicates the wide difference between the budgeting of energy as opposed to budgeting of time. All other time-energy budgets to date are for nonseabird species and for only a portion of the annual cycle (Verbeek 1964; Verner[Pg 158] 1965; Schartz and Zimmerman 1971; Stiles 1971; Wolf and Hainsworth 1971; Smith 1973; Utter and LeFebvre 1973).
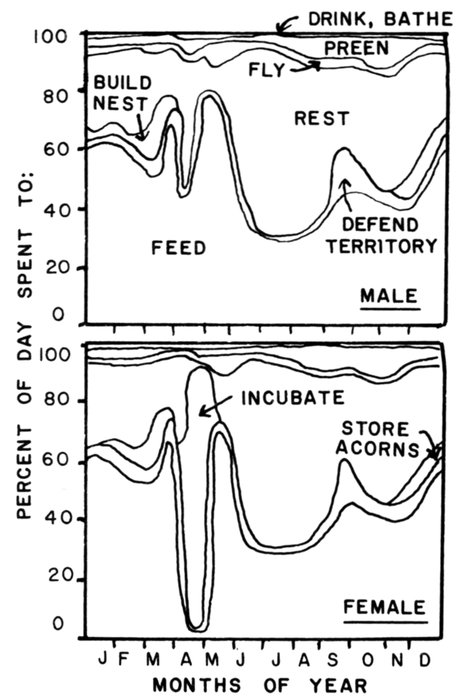
Fig. 15. Time budget of male (upper panel) and female (lower panel) yellow-billed magpies throughout the year. From Verbeek (1972). Non-labeled portions in each graph correspond to labeled sections in the other.
Time-energy budget analysis can be useful in determining the leeway a bird has in surviving unusual stress at different times of the year. For example, a study by Feare et al. (1974) showed that rooks (Corvus frugilegus) in the dry part of the summer spent 90% of 15 h of daylight to collect 150 kcal of food energy. In winter, foraging in snow, the same birds were able to collect 240 kcal of food in only 30% of a 10-h day. This suggests that rooks would be far more vulnerable to unexpected periods of stress in late summer than in winter. Such information would clearly be useful in making management decisions.
A more precise measure of vulnerability, although much more difficult to determine, is that of productive energy—the amount of caloric intake left over after the birds' cost of living (metabolic functions and procurement and processing of food) have been accounted for. Costs are highest when temperatures are extremely hot or cold or when food is most difficult to obtain. Productive energy is highest in summer (Kendeigh 1972), and that is presumably why reproduction normally takes place then. It is unknown whether birds are more vulnerable to time and energy shortages in the harder nonbreeding season or in the breeding season after the extra demands of reproduction have been accounted for. Vulnerability may also differ between sexes and among age groups.
Time-energy studies, although useful in comparing ecology, determining vulnerability, and cataloging location of birds, do have limitations. Careful studies are time-consuming and are not the best approach to determining key factors influencing population increase or decrease. Even when different kinds of data are being sought, however, it is worthwhile keeping the time-energy framework in mind as a "big picture" into which other facts can be fitted and their significance considered.
The study of life history strategies is largely theoretical, and in the following discussion I do not comment on current theoretical arguments. On the other hand, life history strategies can be regarded as time and energy allocation on a grand scale, and it therefore seems appropriate to look briefly at their implications for seabird management.
Annual reproduction evidently has a negative effect on resources remaining for other functions, and may reduce the chances for an organism to reproduce again in a later season (Cody 1966, 1971; Williams 1966; Gadgil and Bossert 1970; Gadgil and Solbrig 1972; Hussell 1972; Trivers 1972; Calow 1973). If the chances of survival to another breeding season are small, the selective advantage lies with the bird putting the most effort into early reproduction, in spite of its negative effects on survival, because future chances of reproduction are small. If chances of survival[Pg 159] are good, however, it may be more advantageous to reduce annual reproductive effort and allocate resources to other functions.
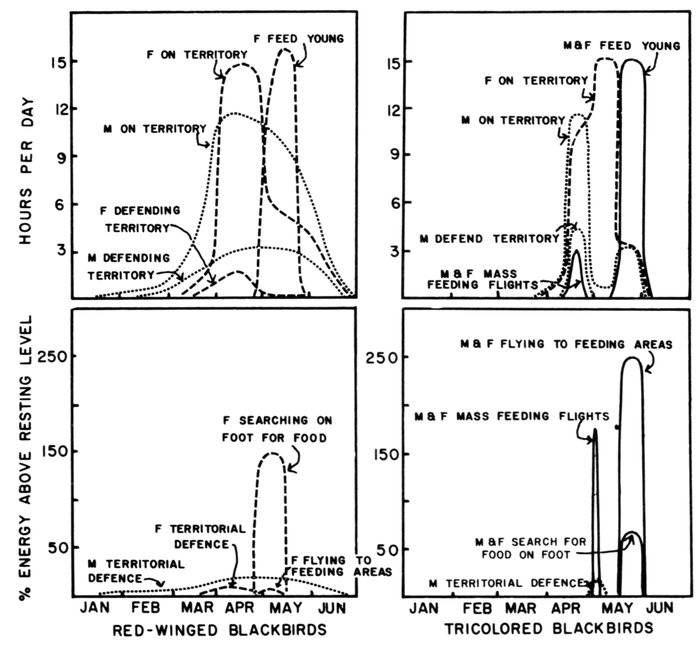
Fig. 16. Time and energy budgets of male and female red-winged (Agelaius phoeniceus) and tricolored (A. tricolor) blackbirds in the breeding season. From Orians (1961). Dotted lines show male (M) activity, dashed lines show female (F) activity, and solid lines show shared activities.
Seabirds are generally long-lived, have small clutches, and generally delay first breeding until at least the 2nd year, and usually longer (Table 6). Phalaropes seem to differ from this pattern (Hilden and Vuolanto 1972; Howe 1975). Several ecological factors (not entirely independent) are believed to contribute to the evolution of the long life and low reproductive effort pattern favored by seabirds.
First, if population size is determined largely by density-dependent mortality, individuals may be favored that allocate resources to attaining longer life (and more chances to reproduce) or insuring greater chances of survival of their offspring (Murphy 1968; Hairston et al. 1970). Density-independent mortality, on the other hand, is so unpredictable that there is no advantage in allocating resources toward protection against it (Gadgil and Solbrig 1972).
Two factors closely linked with density-dependence are high levels of competition, and perennial difficulties in obtaining food. In adapting to these difficulties, a bird may be selected which develops more efficient foraging techniques, wider dispersal, or better abili[Pg 160]ties to defend nesting territory—all of which may reduce resources available for reproduction. As mentioned earlier, marine foods tend to be patchily distributed, and a long learning period seems to be necessary before seabirds become proficient at foraging. In addition, there is evidence that food availability is low, at least in the tropics, and perhaps in the winter in other regions (Ashmole 1971). If nesting places are in short supply, long life may be favored so that the bird can live long enough for a place to become vacant. Several authors feel that competition is a serious factor in the life of seabirds, both for food (Lack 1966; Cody 1973) and for nesting space (Snow 1960; Belopol'skii 1961; Lack 1966; Manuwal 1974b). Others, however, disagree, at least for the breeding season (e.g., Pearson 1968).
| Species | Annual adult survival (%) |
Age at first breeding (years) |
Clutch size |
|---|---|---|---|
| Fulmar | 94 | 7+ | 1 |
| Gannet (Morus bassanus) | 94 | (4)-5+ | 1 |
| Manx shearwater | 93-96 | (4)-5+ | 1 |
| Shag | 85 (♂) | (2)-3 | 3-4 |
| 80 (♀) | |||
| Herring gull | 91-96 | 3.5 (♂) | (2)-3 |
| 5 (♀) | |||
| Black-legged kittiwake | 88 | 4-5 (♂) | 3 |
| 3-4 (♀) | |||
| Arctic tern | 89-91 | (2)-3+ | 2 |
| 75 | |||
| 82[45] | |||
| Common murre | 87 | 3+? | 1 |
| Black guillemot | 88+[46] | 3?[46] | 2[46] |
| Cassin's auklet | 83[47] | 3[47] | 1 |
There is some evidence of density-dependent population size control in seabirds, although much of it is circumstantial. For example, there are large nonbreeding populations in such diverse species as shags, herring gulls, and Cassin's auklets, which move into a breeding area when established adults are removed or colonize new breeding areas (J. C. Coulson, personal communication; Kadlec and Drury 1968; Drury and Nisbet 1972; Manuwal 1974b). Lack (1966) and Ashmole (1971) presented other arguments for density-dependence. Density-dependent mortality is difficult to demonstrate, at best, and may be obscured by interpopulation movements (Drury and Nisbet 1972).
If long life is a life history option, a low annual reproductive effort could be favored in several ways. First, it may be necessary for insuring long life, if breeding has a serious negative feedback on life expectancy (Calow 1973). Second, if survival of offspring is more unpredictable than that of adults, low annual effort may be selected so that reproductive effort will not be wasted in years when young have poor chances of survival. Unpredictable and variable first-year survival in seabirds has been documented (Potts 1968; Drury and Nisbet 1972). In addition, some seabirds show adaptations that allow high reproductive success in any given year but which do not drain off resources if the season turns out to be poor (e.g., small last eggs in the clutch or asynchronous hatching, both of which lead to elimination of the smallest chicks when conditions are poor [Parsons 1970; E. H. Dunn 1973]).
It should be emphasized that the factors involved in the evolution of life histories are[Pg 161] complex and poorly understood, and simple formulas should not be expected to apply to all situations (Wilbur et al. 1974).
In the framework of life-history strategies, small clutch sizes and slow growth rates exhibited by some seabirds can be explained as adaptive reductions in annual reproductive effort, rather than as responses to immediate food shortages. Arguments for this view are presented on theoretical grounds (Dunn 1973) and by the fact that many seabirds are able to raise larger than normal broods in certain situations (Vermeer 1963; Nelson 1964; Harris 1970; Hussell 1972; Ward 1972; Corkhill 1973). In addition, seabirds with particularly slow growth rates all grow at about the same rate, regardless of body size (contrary to the situation in other birds). This suggests that low growth rates do not reflect variations in feeding abilities among species (Ricklefs 1968).
Several conclusions relating to management of seabird populations can be drawn from the above discussion. First, if population size is determined largely by density-dependent factors, the birds are not adapted to precipitous and unexpected declines in population levels. Because there is low annual reproductive effort geared to a world in which there is slow turnover in population, seabirds are not able to rebound quickly from disasters. Provision of excess food should not be expected to improve breeding performance, at least in experienced birds.
Second, because seabirds are able to reproduce in many different seasons and are adapted to a low reproductive effort within a given season, one should expect them to be easily disturbed and to fail to complete the reproductive cycle during any given breeding attempt. A few indications of such failures have already been observed (Erskine 1972; Manuwal 1974a; Nettleship 1975).
Again, the tentative nature of this discussion should be emphasized, and conclusions drawn from it may not apply equally to all seabird species.
In this discussion I have tried to emphasize the variety of factors affecting seabird life cycles and the diverse responses among different species to their environment. The main conclusion I stress is that each species (and age group and sex within that species) has a different vulnerability to stress, which may be most severe at different times of the year for each group. To determine these periods of stress, researchers may find a time-energy approach to be useful.
As for northwestern North American seabirds in particular, ignorance is vast. Twelve years ago, Bourne (1963:846) noted the following needs in seabird research (among others): "The investigation of seabird biology has been reduced to a routine, but there is a great need for more study of some other aspects of the life or annual cycle, including events in the period immediately after fledging, and behaviour and survival in the immature period and outside the breeding season. Much more accurate information is needed about breeding distribution and seasons in many parts of the world, about molting seasons and ranges in most parts, and the distribution of birds of different age groups during these periods in practically all areas."
Since the time of Bourne's remarks, a number of excellent studies have provided data on the breeding biology of certain northwestern seabird species. Scientists remain largely ignorant, however, about where birds of different age groups are located throughout the year. Such knowledge is necessary for effective protection and is basic to understanding population dynamics, even if it does not elucidate causes. Studies of timing of annual cycles and movements should be carried out hand in hand with resource analysis—not just finding what birds eat, but discovering where the food is at what times, how hard it is to catch, and what the nutritional return is. Much careful field work must be done before effective management of most of our northwestern seabirds can become a reality.
Andersson, M. 1973. Behaviour of the pomarine skua Stercorarius pomarinus Temm. with comparative remarks on Stercorariinae. Ornis Scand. 4:1-16.
Ashmole, N. P. 1971. Seabird ecology and the marine environment. Pages 223-286 in D. S.[Pg 162] Farner and J. R. King, eds. Avian biology. Vol. I. Academic Press, New York.
Ashmole, N. P., and H. Tovar S. 1968. Prolonged parental care in royal terns and other birds. Auk 85:90-100.
Barash, D. P., P. Donovan, and R. Myrick. 1975. Clam dropping behavior of the glaucous-winged gull (Larus glaucescens). Wilson Bull. 87:60-64.
Bateson, P. P. G., and R. C. Plowright. 1959. The breeding biology of the ivory gull in Spitsbergen. Br. Birds 52:105-114.
Bédard, J. 1969a. Feeding of the least, crested and parakeet auklets around St. Lawrence Island, Alaska. Can. J. Zool. 47:1025-1050.
Bédard, J. 1969b. Adaptive radiation in alcidae. Ibis 111:189-198.
Belopol'skii, L. O. 1961. Ecology of sea colony birds of the Barents Sea. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 346 pp.
Bengtson, S. 1968. Breeding behavior of the grey phalarope in west Spitzbergen. Var Fågelvärld 27:1-13.
Berger, M., and J. S. Hart. 1974. Physiology and energetics of flight. Pages 415-477 in D. S. Farner and J. R. King, eds. Avian biology. Vol. IV. Academic Press, New York.
Berger, M., J. S. Hart, and O. Z. Roy. 1970. Respiration, oxygen consumption and heart rate in some birds during rest and flight. Z. Vgl. Physiol. 66:201-214.
Birkhead, T. R. 1974. Movement and mortality rates of British guillemots. Bird Study 21:241-254.
Blackmore, F. H., Jr. 1969. The effect of the temperature, photoperiod and molt on the energy requirements of the house sparrow, Passer domesticus. Comp. Biochem. Physiol. 30:433-444.
Bookhout, T. A. 1958. The availability of plant seeds to bobwhite quail in southern Illinois. Ecology 39:671-681.
Bourne, W. R. P. 1963. A review of oceanic studies of the biology of seabirds. Proc. Int. Ornithol. Congr. 13:831-854.
Brisbin, I. L., Jr. 1965. A quantitative analysis of ecological growth efficiency in the herring gull. M.S. Thesis. Univ. of Georgia, Athens. 28 pp.
Brown, R. G. B., N. G. Blurton-Jones, and D. J. T. Hussell. 1967. The breeding behaviour of Sabine's gull, Xema sabini. Behaviour 28:110-140.
Bryant, D. M. 1973. The factors influencing the selection of food by the house martin (Delichon urbica (L.)). J. Anim. Ecol. 42:539-564.
Buckley, F. G., and P. A. Buckley. 1974. Comparative feeding ecology of wintering adult and juvenile royal terns (Aves: Laridae, Sterninae). Ecology 55:1053-1063.
Calder, W. A. 1974. Consequences of body size for avian energetics. Pages 86-144 in R. A. Paynter, ed. Avian energetics. Publ. Nuttall Ornithol. Club 15.
Calder, W. A., and J. R. King. 1974. Thermal and caloric relations of birds. Pages 259-413 in D. S. Farner and J. R. King, eds. Avian biology. Vol. IV. Academic Press, New York.
Calow, P. 1973. The relationship between fecundity, phenology, and longevity: a systems approach. Am. Nat. 107:559-574.
Cody, M. L. 1966. A general theory of clutch size. Evolution 20:174-184.
Cody, M. L. 1971. Ecological aspects of reproduction. Pages 461-512 in D. S. Farner and J. R. King, eds. Avian biology. Vol. I. Academic Press, New York.
Cody, M. L. 1973. Coexistence, coevolution and convergent evolution in seabird communities. Ecology 54:31-44.
Cohen, D. 1967. Optimization of seasonal migratory behavior. Am. Nat. 101:5-17.
Cone, C. D., Jr. 1964. A mathematical analysis of the dynamic soaring flight of the albatross with ecological interpretations. Va. Inst. Mar. Sci., Spec. Sci. Rep. 50:1-104.
Corkhill, P. 1973. Food and feeding ecology of puffins. Bird Study 20:207-220.
Coulson, J. C. 1961. Movements and seasonal variation in mortality of shags and cormorants ringed on the Farne Islands, Northumberland. Br. Birds 54:225-235.
Coulson, J. C. 1963. Egg size and shape in the kittiwake (Rissa tridactyla) and their use in estimating age composition in populations. Proc. Zool. Soc. Lond. 140(2):211-227.
Coulson, J. C., and M. G. Brazendale. 1968. Movements of cormorants ringed in the British Isles and evidence of colony-specific dispersal. Br. Birds 61:1-21.
Coulson, J. C., G. R. Potts, and J. Horobin. 1969. Variation in the eggs of the shag (Phalacrocorax aristotelis). Auk 86:232-245.
Coulson, J. C., and E. White. 1958. The effect of age on the breeding biology of the kittiwake, Rissa tridactyla. Ibis 100:40-51.
Coulson, J. C., and E. White. 1960. The effect of age and density of breeding birds on the time of breeding of the kittiwake, Rissa tridactyla. Ibis 102:71-80.
Cox, G. W. 1968. The role of competition in the evolution of migration. Evolution 22:180-192.
Cramp, S. 1972. The breeding of urban wood pigeons. Ibis 114:163-171.
Cullen, J. M. 1957. Plumage, age and mortality in the arctic tern. Bird Study 4:197-207.
Dawson, W. R., and J. W. Hudson. 1970. Birds. Pages 223-310 in G. C. Whittow, ed. Comparative physiology of thermoregulation. Vol. I. Academic Press, New York.
Dawson, W. R., J. W. Hudson, and R. W. Hill. 1972. Temperature regulation in newly hatched laughing gulls (Larus atricilla). Condor 74:177-184.
Dolnik, V. R. 1971. Comparative energy expenditure in migration and wintering birds. Ekologiya 2(3):88-89. In Russian.
Dorst, J. 1961. The migrations of birds. William Heinimann Ltd., London.
Drent, R. H. 1965. Breeding biology of the pigeon guillemot Cepphus columba. Ardea 53:99-160.
Drent, R. H. 1970. Functional aspects of incubation in the herring gull (Larus argentatus Pont.). Behaviour Suppl. 17:1-132.
Drent, R. 1972. Adaptive aspects of the physiology of incubation. Proc. Int. Ornithol. Congr. 15:255-280.
Drent, R. H., and C. J. Guiguet. 1961. A catalogue of British Columbia seabird colonies. B. C. Prov. Mus., Occas. Pap. 12:1-173.
Drent, R., G. F. van Tets, F. Tompa, and K. Vermeer. 1964. The breeding birds of Mandarte Island, British Columbia. Can. Field-Nat. 78:208-263.
Drury, W. H., and I. C. T. Nisbet. 1972. The importance of movements in the biology of herring gulls in New England. Pages 173-212 in Population ecology of migratory birds: a symposium. U.S. Fish Wildl. Serv., Wildl. Res. Rep. 2.
Drury, W. H., Jr., and W. J. Smith. 1968. Defense of feeding areas by adult herring gulls and intrusion by young. Evolution 22:193-201.
Dunn, E. H. 1973. Energy allocation of nestling double-crested cormorants. Ph.D. Thesis. Univ. of Michigan, Ann Arbor. 153 pp.
Dunn, E. H. 1975a. Growth, body components and caloric value of nestling double-crested cormorants. Condor 77:431-438.
Dunn, E. H. 1975b. Caloric intake of nestling double-crested cormorants. Auk 92:553-565.
Dunn, E. H. 1976a. Development of endothermy and existence energy expenditure of nestling double-crested cormorants. Condor 78:350-356.
Dunn, E. H. 1976b. Development of endothermy and existence energy expenditure in herring gull chicks. Condor 78:493-498.
Dunn, E. K. 1972. Effect of age on the fishing ability of sandwich terns Sterna sandvicensis. Ibis 114:360-366.
Dunn, E. K. 1973. Changes in fishing ability of terns associated with wind speed and sea surface conditions. Nature (Lond.) 244:520-521.
Eliassen, E. 1963. Preliminary results from new methods of investigating the physiology of birds during flight. Ibis 105:234-237.
Emlen, J. M. 1966. The role of time and energy in food preference. Am. Nat. 100:611-617.
Erskine, A. J. 1972. The great cormorants of eastern Canada. Can. Wildl. Serv. Occas. Pap. 14:1-23.
Evans, R. M., and M. K. McNicholl. 1972. Variations in reproductive activities of arctic terns at Churchill, Manitoba. Arctic 25:131-141.
Farner, D. S., and D. L. Serventy. 1959. Body temperature and the ontogeny of thermoregulation in the slender-billed shearwater. Condor 61:426-433.
Feare, C. J., G. M. Dunnet, and I. J. Patterson. 1974. Ecological studies of the rook (Corvus frugilegus L.) in north-east Scotland: food intake and feeding behaviour. J. Appl. Ecol. 11:867-896.
Fisher, H. 1972. The nutrition of birds. Pages 431-469 in D. S. Farner and J. R. King, eds. Avian biology. Vol. II. Academic Press, New York.
Fisher, R. A. 1958. The genetical theory of natural selection. 2nd rev. ed. Dover Publishers, Inc., New York.
Gadgil, M., and W. H. Bossert. 1970. Life historical consequences of natural selection. Am. Nat. 104:1-24.
Gadgil, M., and O. T. Solbrig. 1972. The concept of r-and K-selection: evidence from wild flowers and some theoretical considerations. Am. Nat. 106:14-31.
Gavrilov, V. 1974. Metabolism of molting birds. Zool. Zh. 53(9):1363-1374. In Russian, English summary.
Gross, A. O. 1935. The life history cycle of Leach's petrel (Oceanodroma leucorhoa leucorhoa) on the outer sea islands of the Bay of Fundy. Auk 52:382-399.
Hainsworth, F. R., and L. L. Wolf. 1975. Wing disc loading: implications and importance for hummingbird energetics. Am. Nat. 109:229-233.
Hairston, N. G., D. W. Tinkle, and H. M. Wilbur. 1970. Natural selection and the parameters of population growth. J. Wildl. Manage. 34:681-690.
Harris, M. P. 1970. Breeding ecology of the swallow-tailed gull, Creagus furcatus. Auk 87:215-243.
Harris, M. P. 1971. Ecological adaptations of moult in some British gulls. Bird Study 18:113-118.
Harris, S. W. 1974. Status, chronology, and ecology of nesting storm petrels in northwestern California. Condor 76:249-261.
Hart, J. S., and M. Berger. 1972. Energetics, water economy and temperature regulation during flight. Proc. Int. Ornithol. Congr. 15:189-199.
Henderson, B. A. 1972. The control and organization of parental feeding and its relationships to the food supply for the glaucous-winged gull, Larus glaucescens. M.S. Thesis. Univ. of British Columbia, Vancouver.
Hilden, O., and S. Vuolanto. 1972. Breeding biology of the red-necked phalarope Phalaropus lobatus in Finland. Ornis Fenn. 49:57-81.
Högstedt, G. 1974. Length of the pre-laying period in the lapwing Vanellus vanellus L. in relation to its food resources. Ornis Scand. 5:1-4.
Höhn, E. O. 1971. Observation on the breeding behavior of grey and red-necked phalaropes. Ibis 113:335-348.
Howe, M. A. 1975. Behavioral aspects of the pair bond in Wilson's phalarope. Wilson Bull. 87:248-270.
Hunt, G. L., Jr. 1972. Influence of food distribution and human disturbance on the reproductive success of herring gulls. Ecology 53:1051-1061.
Hussell, D. J. T. 1969. Weight loss of birds during nocturnal migration. Auk 86:75-83.
Hussell, D. J. T. 1972. Factors affecting clutch size in arctic passerines. Ecol. Monogr. 42:317-364.
Ingolfsson, A. 1969. Behaviour of gulls robbing eiders. Bird Study 16:45-52.
Ingolfsson, A. 1970. The moult of remiges and rectrices in great black-backed gulls Larus marinus and glaucous gulls L. hyperboreus in Iceland. Ibis 112:83-92.
James-Veitch, E., and E. S. Booth. 1974. Behavior and life history of the glaucous-winged gull. Walla Walla Coll. Publ. 12:1-39.
Johnston, D. W. 1961. Timing of annual molt in the glaucous gulls of northern Alaska. Condor 63:474-478.
Kadlec, J. A., and W. H. Drury. 1968. Structure of the New England herring gull population. Ecology 49:644-676.
Kadlec, J. A., W. H. Drury, Jr., and D. K. Onion. 1969. Growth and mortality of herring gull chicks. Bird-Banding 40:222-233.
Källender, H. 1974. Advancement of laying of great tits by the provision of food. Ibis 116:365-367.
Katz, P. L. 1974. A long-term approach to foraging optimization. Am. Nat. 108:758-782.
Kendeigh, S. C. 1970. Energy requirements for existence in relation to size of bird. Condor 72:60-65.
Kendeigh, S. C. 1972. Monthly variations in the energy budget of the house sparrow throughout the year. Pages 17-43 in S. C. Kendeigh and J. Pinowski, eds. Productivity, population dynamics and systematics of granivorous birds. Institute of Ecology, Polish Academy of Science, Warsaw.
Kendeigh, S. C. 1973. [Discussion.] Pages 311-320 in D. S. Farner, ed. Breeding biology of birds. National Academy of Sciences, Washington, D.C.
King, J. R. 1973. Energetics of reproduction in birds. Pages 78-107 in D. S. Farner, ed. Breeding biology of birds. National Academy of Science, Washington, D.C.
King, J. R. 1974. Seasonal allocation of time and energy resources in birds. Pages 4-70 in R. A. Paynter, ed. Avian energetics. Publ. Nuttall Ornithol. Club 15.
Koelink, A. F. 1972. Bioenergetics of growth in the pigeon guillemot, Cepphus columba. M.S. Thesis. Univ. of British Columbia, Vancouver.
Koskimies, J., and L. Lahti. 1964. Cold-hardiness of the newly hatched young in relation to ecology and distribution in ten species of European ducks. Auk 81:281-307.
Lack, D. 1966. Population studies of birds. Clarendon Press, Oxford.
Lack, D. 1968. Ecological adaptations for breeding in birds. Methuen, London.
Lasiewski, R. C. 1963. Oxygen consumption of torpid, resting, active and flying hummingbirds. Physiol. Zool. 36:122-140.
Lasiewski, R. C., and W. R. Dawson. 1967. A reexamination of the relation between standard metabolic rate and body weight in birds. Condor 69:13-23.
Lasiewski, R. C., and G. K. Snyder. 1969. Responses to high temperature in nestling double-crested cormorants and pelagic cormorants. Auk 86:529-540.
LeCroy, M. 1972. Young common and roseate terns learning to fish. Wilson Bull. 84:201-202.
LeCroy, M., and C. T. Collins. 1972. Growth and survival of roseate and common tern chicks. Auk 89:595-611.
Lemmetyinen, R. 1972. Growth and mortality in the chicks of arctic terns in the Kongsfjord area, Spitzbergen in 1970. Ornis Fenn. 49:45-53.
Lind, H. 1965. Parental feeding in the oystercatcher Haematopus o. ostralegus (L.). Dan. Ornithol. Foren. Tidsskr. 59:1-31.
Lustick, S. 1970. Energy requirements of molt in cowbirds. Auk 87:742-746.
Manuwal, D. A. 1974a. The natural history of Cassin's auklet (Ptychoramphus aleuticus). Condor 76:421-431.
Manuwal, D. A. 1974b. Effects of territoriality on breeding in a population of Cassin's auklet. Ecology 55:1399-1406.
Martin, E. W. 1968. The effects of dietary protein on the energy and nitrogen balance of the tree sparrow (Spizella a. arborea). Physiol. Zool. 41:313-331.
McKinney, F. 1961. An analysis of the displays of the European eider Somateria mollissima mollissima (Linnaeus) and the Pacific eider Somateria mollissima v. nigra Bonaparte. Behaviour Suppl. 7:1-124.
Moss, R. 1972. Food selection by red grouse (Logopus logopus scoticus (Lath.)) in relation to chemical composition. J. Anim. Ecol. 4:411-428.
Moyle, P. 1966. Feeding behavior of the glaucous-winged gull on the Alaskan salmon stream. Wilson Bull. 78:175-190.
Murphy, G. I. 1968. Patterns in life history and the environment. Am. Nat. 102:391-403.
Myrberget, S. 1962. Contribution to the breeding biology of the puffin Fratercula arctica (L.). Eggs, incubation and young. Pap. Norw. State Game Res. Inst. Ser. 2, 11:1-49.
Myton, B. A., and R. W. Ficken. 1967. Seed size preference in chickadees and tits in relation to ambient temperatures. Wilson Bull. 79:319-321.
Nelson, J. B. 1964. Factors influencing clutch-size and chick growth in the North Atlantic gannet Sula bassana. Ibis 106:63-77.
Nettleship, D. N. 1972. Breeding success of the common puffin (Fratercula arctica L.) on different habitats at Great Island, Newfoundland. Ecol. Monogr. 42:239-268.
Nettleship, D. N. 1975. A recent decline of gannets, Morus bassanus, on Bonaventure Island, Quebec. Can. Field-Nat. 89:125-133.
Nice, M. M. 1962. Development of behavior in precocial birds. Trans. Linn. Soc. N. Y. 8:1-211.
Nisbet, I. C. T. 1963. Weight-loss during migration. Part II: Review of other estimates. Bird-Banding 34:139-159.
Nisbet, I. C. T. 1973. Courtship feeding, egg-size and breeding success in common terns. Nature (Lond.) 241:141-142.
Orians, G. H. 1961. The ecology of blackbird (Agelaius) social systems. Ecol. Monogr. 31:285-312.
Orians, G. H. 1969. Age and hunting success in the brown pelican (Pelecanus occidentalis). Anim. Behav. 17:316-319.
Orians, G. 1971. Ecological aspects of behavior. Pages 513-546 in D. S. Farner and J. R. King, eds. Avian biology. Vol. I. Academic Press, New York.
Parsons, J. 1970. Relationship between egg size and post-hatching chick mortality in the herring gull[Pg 165] (Larus argentatus). Nature (Lond.) 228:1221-1222.
Payne, R. B. 1965. The molt of breeding Cassin auklets. Condor 67:220-228.
Payne, R. B. 1972. Mechanisms and control of molt. Pages 103-155 in D. S. Farner and J. R. King, eds. Avian biology. Vol. II. Academic Press, New York.
Pearson, T. H. 1968. The feeding biology of sea-bird species breeding on the Farne Islands, Northumberland. J. Anim. Ecol. 37:521-552.
Pennycuick, C. J. 1960. Gliding flight of the fulmar petrel. J. Exp. Biol. 37:330-338.
Porter, W. P., and D. M. Gates. 1969. Thermodynamic equilibria of animals with environment. Ecol. Monogr. 39:227-244.
Potts, G. R. 1968. Influence of eruptive movements, age, population size and other factors on the survival of the shag. J. Anim. Ecol. 37:53-102.
Potts, G. R. 1971. Moult in the shag Phalacrocorax aristotelis, and the ontogeny of the "Staffelmauser." Ibis 113:298-305.
Preston, F. W., and E. J. Preston. 1953. Variation of the shapes of birds eggs within the clutch. Ann. Carnegie Mus. 33:129-139.
Preston, W. C. 1968. Breeding ecology and social behavior of the black guillemot, Cepphus grylle. Ph.D. Thesis. Univ. of Michigan, Ann Arbor.
Pulliam, H. R. 1974. On the theory of optimal diets. Am. Nat. 108:59-74.
Richardson, F. 1961. Breeding biology of the rhinoceros auklet on Protection Island, Washington. Condor 63:456-473.
Ricklefs, R. E. 1968. Patterns of growth in birds. Ibis 110:419-451.
Ricklefs, R. E. 1973. Patterns of growth in birds. II. Growth rate and mode of development. Ibis 115:177-201.
Ricklefs, R. E. 1974. Energetics of reproduction in birds. Pages 152-292 in R. A. Paynter, ed. Avian energetics. Publ. Nuttall Ornithol. Club 15.
Royama, T. 1970. Factors governing the hunting behavior and selection of food by the great tit (Parus major L.). J. Anim. Ecol. 39:619-668.
Schamel, D. L. 1974. The breeding biology of the Pacific eider (Somateria mollissima v-nigra Bonaparte) on Barrier Island in the Beaufort Sea, Alaska. M.S. Thesis. Univ. of Alaska, Fairbanks.
Schartz, R. L., and J. L. Zimmerman. 1971. The time and energy budget of the male dickcissel (Spiza americana). Condor 73:65-76.
Schmidt-Nielsen, K. 1972. Locomotion: energy cost of swimming, flying, and running. Science 177:222-228.
Schoener, T. 1971. Theory of feeding strategies. Annu. Rev. Ecol. Syst. 2:369-404.
Schönwetter, M. 1967. Handbuch der oologie. Vol. I. Akademie-Verlag, Berlin.
Sealy, S. G. 1973a. Breeding biology of the horned puffin on St. Lawrence Island, Bering Sea, with zoogeographical notes on the North Pacific puffins. Pac. Sci. 27:99-119.
Sealy, S. G. 1973b. Adaptive significance of post-hatching developmental patterns and growth rates in alcidae. Ornis Scand. 4:113-121.
Sealy, S. G. 1975a. Feeding ecology of the ancient and marbled murrelets near Langara Island, British Columbia. Can. J. Zool. 53:418-433.
Sealy, S. G. 1975b. Aspects of the breeding biology of the marbled murrelet in British Columbia. Bird-Banding 46:141-154.
Sealy, S. G. 1975c. Influence of snow on egg-laying in auklets. Auk 92:528-538.
Smith, C. R. 1973. Time budget of the loggerhead shrike in southwest Florida. Abstracts of papers presented to the Wilson Society meeting, Cornell University.
Smith, N. G. 1966. Evolution of some arctic gulls (Larus): an experimental study of isolating mechanisms. Am. Ornithol. Union Monogr. 4. 99 pp.
Snow, B. K. 1960. The breeding biology of the shag Phalacrocorax aristotelis on the Island of Lundy, Bristol Channel. Ibis 102:554-575.
Snow, B. K. 1963. The behaviour of the shag. Br. Birds 56:77-103; 164-186.
Southern, W. E. 1967. Dispersal and migration of ring-billed gulls from a Michigan population. Jack-Pine Warbler 45:102-111.
Spaans, A. L. 1971. On the feeding ecology of the herring gull Larus argentatus Pont. in the northern part of the Netherlands. Ardea 59:73-189.
Speich, S., and D. A. Manuwal. 1974. Gular pouch development and population structure of Cassin's auklet. Auk 91:291-306.
Stiles, F. G. 1971. Time, energy and territoriality of the Anna hummingbird (Calypte anna). Science 173:818-821.
Storer, R. W. 1952. A comparison of variation, behaviour and evolution in the seabird genera Uria and Cepphus. Univ. Calif. Publ. Zool. 52:121-222.
Storer, R. W. 1960. Evolution in the diving birds. Proc. Int. Ornithol. Congr. 12(Vol. II):694-707.
Stresemann, E., and V. Stresemann. 1966. Die Mauser der Vögel. J. Ornithol. 107 (suppl.):1-448.
Thoresen, A. C. 1964. The breeding behavior of the Cassin auklet. Condor 66:456-476.
Tinbergen, N. 1935. Field observations of east Greenland birds. I. The behaviour of the red-necked phalarope (Phalaropus lobatus L.) in spring. Ardea 24:1-42.
Tinbergen, N. 1960. The herring gull's world. Basic Books, Inc., New York.
Trivers, R. L. 1972. Parental investment and sexual selection. Pages 136-179 in B. Campbell, ed. Sexual selection and the descent of man, 1871-1971. Aldine-Atherton, Chicago, Illinois.
Tschanz, B. 1959. Zur brutbiologie der trottellumme (Uria aalge aalge Pont.). Behaviour 14:1-100.
Tuck, L. 1960. The murres. Canadian Wildlife Service Series 1. 260 pp.
Tucker, V. A. 1969. The energetics of bird flight. Sci. Am. 220(5):70-78.
Tucker, V. A. 1970. Energetic cost of locomotion in animals. Comp. Biochem. Physiol. 34:841-846.
Tucker, V. A. 1972. Metabolism during flight in the laughing gull, Larus atricilla. Am. J. Physiol. 222:237-245.
Tucker, V. A. 1974. Energetics of natural flight. Pages 298-328 in R. A. Paynter, Jr., ed. Avian energetics. Publ. Nuttall Ornithol. Club 15.
Utter, J. M., and E. A. LeFebvre. 1970. Energy expenditure for free flight by the purple martin (Progne subis). Comp. Biochem. Physiol. 35:713-719.
Utter, J. M., and E. A. LeFebvre. 1973. Daily energy expenditure of purple martins (Progne subis) during the breeding season: Estimates using D_{2}O18 and time budget methods. Ecology 54:597-604.
Verbeek, N. A. M. 1964. A time and energy budget of the Brewer blackbird. Condor 66:70-74.
Verbeek, N. A. 1972. Daily and annual time budget of the yellow-billed magpie. Auk 89:567-582.
Vermeer, K. 1963. The breeding ecology of the glaucous-winged gull (Larus glaucescens) on Mandarte Island, B. C. B. C. Prov. Mus. Occas. Pap. 13:1-104.
Verner, J. 1965. Time budget of the male long-billed marsh wren during the breeding season. Condor 67:125-139.
Ward, J. G. 1972. Studies on the feeding ecology and breeding success of the glaucous-winged gull (Larus glaucescens) on Mandarte and Cleland Islands. Ph.D. Thesis. Univ. of British Columbia, Vancouver.
Weaver, D. K. 1970. Parental behavior of the herring gull (Larus argentatus smithsonianus) and its effect on reproductive success. Ph.D. Thesis. Univ. of Michigan, Ann Arbor.
West, G. C. 1967. Nutrition of tree sparrows during winter in central Illinois. Ecology 48:58-67.
Wilbur, H. M., D. W. Tinkle, and J. P. Collins. 1974. Environmental certainty, trophic level, and resource availability in life history evolution. Am. Nat. 108:805-817.
Williams, G. C. 1966. Adaptation and natural selection. Princeton Univ. Press, Princeton, N. J.
Willson, M. F. 1971. Seed selection in some North American finches. Condor 73:415-429.
Willson, M. F., and J. C. Harmeson. 1973. Seed preferences and digestive efficiency of cardinals and song sparrows. Condor 75:225-233.
Wolf, L., and F. R. Hainsworth. 1971. Time and energy budgets of territorial hummingbirds. Ecology 52:980-988.
Zar, J. H. 1968. Standard metabolism comparisons between orders of birds. Condor 70:278.
[41] Common eider, 2 to 7 h.
[42] No data given.
[43] Indirect evidence that young are brooded this long.
[44] Data from Lack (1968) and Ashmole (1971) unless otherwise noted.
[45] Cullen (1957).
[46] Birkhead (1974).
by
M. D. F. Udvardy
California State University
Sacramento, California 95819
The zoogeography and taxonomic relationships among 42 living and 1 extinct species of marine birds from the northern and northwestern coasts of North America are described. Seventeen species are circumpolar in distribution; 17 are endemic to Beringia, and 8 have origins in the North Pacific.
This discussion concerns the northern and western coasts of the continent, from about the Mackenzie Delta westward and southward to the mouth of the Columbia River. Besides bona fide seabirds, I include marine birds that predominantly breed and feed on or around the marine littoral, but exclude two groups: shorebirds, jaegers, and phalaropes, which breed inland and move out from the Arctic after an undetermined postbreeding period; and Anseriformes which become "marine birds" in their southern winter quarters. What remains is 42 living species (Table 1).
The Procellariiformes, or tube-nosed seabirds, have a predominantly southern hemispheric, Gondwanan distribution. The North Pacific basin is an important feeding ground of several shearwaters (Puffinus spp.) that breed in the South Pacific and subantarctic. Only three species breed in the area under consideration: the fulmar (Fulmarus glacialis) and two storm-petrels (Oceanodroma spp.), all of which are still relatively widespread.
Of the Pelecaniformes, the very successful, worldwide cormorants (Phalacrocorax spp.)—inland water as well as coastal and "amphibious" species are on every continent—are ancient Pacific dwellers, with a high grade of endemism here: Of the two subarctic species, one (P. perspicillatus) became extinct long ago, and the other, the red-faced cormorant (P. urile), is very restricted, and deserves our greatest attention. The pelagic cormorant (P. pelagicus), Brandt's cormorant (P. penicillatus), and the double-crested cormorant (P. auritus) are widespread and successful, extending south of the area here considered; double-crested cormorants also breed inland and across toward the North Atlantic coast. As fish-eaters they are often persecuted where coastal fishermen possess firearms, and thus are sensitive to increasing human influence on the coasts.
Two species of arctic geese need special attention. The emperor goose (Philacte canagica) is a Beringean endemic and lives in a very restricted area of both sides of this sea; its status (endangered?) is unknown to me. Since the black brant (Branta bernicla) is a long-range migrant, it is hunted as a game bird at its winter grounds, and subject to management measures. Whereas the emperor goose is a unique offshoot of the genus Anser, the Pacific brant is considered a subspecies; its general distribution is circumpolar.
Five arctic ducks, and one other, constitute the sea ducks of the area. The common eider (Somateria mollissima), king eider (S. spectabilis), and the oldsquaw (Clangula hyemalis) are widespread, and circumpolar or nearly so; hunting and down-robbing in other parts of the Arctic may provide clues as to their relative tolerance of primitive or advanced civilization. The spectacled eider (S. fischeri) and Steller's eider (Polysticta stelleri) are restricted to the Bering Sea coasts and neighboring High Arctic coasts, respectively; their status is precarious.
| Species | Distribution | ||||||
|---|---|---|---|---|---|---|---|
| Circumpolar | Widespread in North Pacific | North coast of Alaska | Beringia[48] | Aleutian Islands | South coast of Alaska[49] | Temperate northeast Pacific coast[50] | |
| Fulmarus glacialis | x | w | x | x | x | w | |
| Oceanodroma furcata | x | x | x | x | |||
| O. leucorhoa | x | x | x | x | x | ||
| Phalacrocorax auritus | x | x | x | ||||
| P. penicillatus | (x) | x | |||||
| P. pelagicus | x | x | x | x | x | ||
| P. urile | x | x | x | ||||
| Branta bernicla | x | x | x | (w) | (w) | w | |
| Anser canagicus | x | w | w | ||||
| Clangula hyemalis | x | w | x | x | w | w | w |
| Histrionicus histrionicus | x | w | w | w | w | w | |
| Polysticta stelleri | x | x | (w) | ||||
| Somateria mollissima | x | x | x | x | x | ||
| S. spectabilis | x | x | x | w | (w) | ||
| S. fischeri | x | x | |||||
| Larus hyperboreus | x | w | x | x | w | w | w |
| L. glaucescens | x | x | x | x | |||
| L. occidentalis | (x) | ||||||
| L. argentatus | x | w | x | (x)w | |||
| L. thayeri | w | x | w | w | w | w | |
| L. canus | x | w | x | x | (x)w | ||
| Rissa tridactyla | x | w | x | x | x | x | w |
| R. brevirostris | x | x | (x) | ||||
| Xema sabini | x | x | x | w | |||
| Sterna paradisaea | x | w | x | x | x | w | |
| S. aleutica | x | x | |||||
| Uria aalge | x | x | (x) | x | x | x | x |
| U. lomvia | x | x | x | x | x | x | |
| Alle alle | x | * | |||||
| Cepphus grylle | x | x | w | ||||
| C. columba | x | x | x | x | x | ||
| Brachyramphus marmoratus | x | (x) | x | x | |||
| B. brevirostris | x | x | x | ||||
| Synthliboramphus antiquus | x | x | x | x | x | ||
| Ptychoramphus aleuticus | x | x | x | ||||
| Cyclorrhynchus psittacula | x | x | |||||
| Aethia cristatella | x | x | |||||
| A. pusilla | x | x | |||||
| A. pygmaea | x | ||||||
| Cerorhinca monocerata | x | x | x | ||||
| Fratercula corniculata | x | x | x | x | x | ||
| Lunda cirrhata | x | x | x | x | x | ||
| Total number of nesting species | 17 | 11 | 15 | 27 | 25 | 24 | 17 |
| Total number of wintering species | 9 | 4 | 7 | 9 | 9 | ||
| Grand total | 17 | 20 | 15 | 31 | 32 | 33 | 26 |
The harlequin duck (Histrionicus histrionicus) stands alone without close relatives. It often breeds far from the sea, but spends the shortest time—only a few weeks—away from the rocky coast. There is a year-round population of yearlings in the sea. The drakes of the nearest breeding pairs at lower latitudes are back to the sea, abandoning their mates at the breeding stream when the alpine stream-dwellers are still at sea awaiting the thawing of their breeding grounds. Harlequin ducks live in large parts of Siberia, from arctic Alaska to central California and Colorado, and also in the eastern Arctic. They do not seem to me to be in immediate danger globally, though perhaps they are locally.
Gulls are a highly successful group of seabirds, and of the eight species on our coasts the four more southern ones—the western gull (Larus occidentalis), glaucous-winged gull (L. glaucescens), common gull (L. canus), and herring gull (L. argentatus)—are expanding wherever civilization creates new scavenging opportunities. Nothing is said about the populations of the kittiwake (Rissa tridactyla), black-legged kittiwake (R. brevirostris) and Sabine's gull (Xema sabini), or of the other two high arctic species (Pagophila eburnea, Rhodostethia rosea) which do not nest regularly in the area considered here.
The arctic tern (Sterna paradisaea) is circumpolarly widespread and successful, whereas the Aleutian tern (S. aleutica) is a very restricted Beringean endemic, and its status needs to be exactly known.
Almost one-third of the seabirds in this area are alcids, a family centered in the North Pacific and, more specifically, in the Bering Sea. Most species breed in enormous rookeries. Any impact of civilization is highly detrimental under such circumstances. Of the four circumpolar species the two Uria guillemots (murres) are important. The dovekie (Alle alle) is a sparse pioneer of Bering Strait, as is the black guillemot (Cepphus grylle) on our side of the Arctic Sea. Its congener, the pigeon guillemot (C. columba), is common and successful all the way to coastal central California. Of the remaining 11 species, special attention should be paid to the whiskered auklet (Aethia pygmaea) of the Aleutian chain; the Kittlitz's murrelet (Brachyramphus brevirostris) of the eastern Beringean and southern Alaska coast; and to the widespread, but very sporadic rhinoceros auklet, or puffin (Cerorhinca monocerata).
To sum up, I have tabulated these 42 species, and indicated whether modern life-history and population studies are extant:
| No. species | No. studied | |
|---|---|---|
| Procellariiformes | 3 | 2 |
| Phalacrocorax | 4 | 2 |
| Anseres | 2 | 1 |
| Anates | 6 | — |
| Lari | 9 | 2 |
| Sterni | 2 | — |
| Alcidae | 16 | 7 |
| Total | 42 | 14 |
Thus, 28 species await studies preliminary to, and highly necessary for, conservation measures.
Seventeen species of marine birds are spread either circumpolarly around the northern perimeter or along the north-south coasts of the Laurasian continents. Four of these are of the High Arctic (Branta bernicla, Somateria spectabilis, Xema sabini, Alle alle); another seven penetrate the Bering Sea as well (Fulmarus glacialis, Somateria mollissima, Clangula hyemalis, Larus hyperboreus, Rissa tridactyla, Sterna paradisaea, Uria lomvia); and six are panboreal-subboreal, widespread in their distribution—Oceanodroma leucorhoa (extends far south), Histrionicus histrionicus, Larus argentatus (widespread latitudinally), L. canus (also inland), Uria aalge, and Cepphus grylle.
Seventeen species of marine birds are endemic to Beringia: Anser canagicus, Polysticta stelleri, Somateria fischeri, Rissa brevirostris, and Aethia pusilla (and the extinct Phalacrocorax perspicillatus); P. urile, Sterna aleutica, Aethia pygmaea, A. cristatella, and Cyclorrhynchus extend westward to the Sea of Okhotsk, as do Brachyramphus brevirostris and Larus glaucescens, which also extend eastward; and Phalacrocorax pelagicus, Cepphus columba, Fratercula corniculata, and[Pg 170] Lunda cirrhata are amphipacific species in Beringia.
Eight species of marine birds are associated with the North Pacific. Four are found on both sides of the ocean—Oceanodroma furcata, Brachyramphus marmoratus, Synthliboramphus antiquus, and Cerorhinca monocerata (very disjunct). The four others occur on only the North American side—Phalacrocorax auritus (also inland), P. penicillatus, Larus occidentalis (albeit barely), and Ptychoramphus aleuticus.
Finally, one species, Larus thayeri, is endemic at the central Canadian Arctic, extending westward into the area here considered.
[48] Beringia comprises the islands and coasts of the Bering Sea.
[49] South coast of Alaska extends from the tip of the Alaska Peninsula to Glacier Bay.
by
David R. Cline[51] and Cynthia Wentworth
U.S. Fish and Wildlife Service
Anchorage, Alaska
and
Thomas W. Barry
Canadian Wildlife Service
Edmonton, Alberta, Canada
Throughout history, marine birds have provided tangible and intangible benefits to human societies. Unregulated exploitation of some species by explorers, mariners, and colonists led to the extinction of the great auk (Pinguinus impennis) and near extinction of others, including the Bermuda petrel (Pterodroma cahow) and the North Pacific albatrosses (Diomedea spp.). Marine birds continue to provide commercial, subsistence, recreational, scientific, and educational values to people of many nations, while playing critical roles in the economies of the world's oceans.
Annual harvest of slender-billed shearwaters (Puffinus tenuirostris) known as "muttonbirds" in Australia, sooty tern (Sterna fuscata) eggs in the Caribbean, murres (Uria spp.) and eiders (Somateria spp.) in Greenland and the Soviet Union, and guano in Peru and Africa represent the principal commercial uses of marine birds and their products. Residents of the Faeroes Islands and thousands of native people in Greenland and arctic Canada and Alaska use various species for subsistence. The annual rituals of bird hunting and egg gathering are deeply ingrained in the sociocultural traditions of these peoples and continue to be important to their social welfare.
Most countries of the world are currently providing at least some protection to their marine bird resources. However, the destruction of bird habitats by man's developments and the contamination of marine environments by industrial pollutants are posing increasingly serious threats to many species. If managed and used in accordance with scientific principles of sustained yield, some of the more abundant species of marine birds can continue to provide long-term social and economic benefits to man.
Increasing numbers of people are expending considerable sums of money to reach marine bird viewing areas off the coasts of North American States and Provinces. Preliminary evidence indicates such nonconsumptive pursuits are contributing significant amounts of money to regional economies and helping businessmen earn a living. An accurate evaluation of both biological and economic impacts resulting from these nonconsumptive activities is urgently needed.
The possibility of establishing an excise tax on designated outdoor recreational equipment appears to hold considerable potential for more adequately funding marine bird programs, as well as those for other nongame wildlife.
Greater citizen involvement in sociopolitical processes will, to a large extent, determine the success of marine bird conservation programs. Sound conservation legislation that insures adequate protection of habitat and provides for enlightened and innovative thrusts in conservation, education, research, management, and law enforcement will help insure the survival of all species of marine birds and, in turn, provide social and economic benefits to people across generations.
In its 17 March 1975 issue, Time magazine reported battalions of observers from all over the country flocking to Salisbury, Massachusetts, armed with telescopes, cameras dwarfed by huge telephoto lenses, sketch pads, and binoculars. There, 1,500 strong the first weekend alone, they took up vigil along the seawall of the Merrimack River. A local businessman circulated among the chilly bird-watchers with free coffee and hot chocolate, while handing out a pamphlet advertising his restaurant.
The cause of the commotion was the appearance of a single, unassuming, pigeon-like seabird called a Ross' gull (Rhodostethia rosea), almost never seen south of the Arctic Circle and never before in the contiguous 48 States. Time stated that "for those who care about such matters the event was as electrifying as the descent of a Martian spaceship."
Meanwhile, far above the Arctic Circle at Point Barrow on the Arctic Ocean, Eskimo hunters probably puzzled at the strange ways of the white "birdmen," as they recalled the savory dishes Ross' gulls provided many of them during the previous fall hunting season. This particular gull is considered a delicacy by the Eskimos, and the birds are actively sought each year as they fly near shore during their fall wanderings from Asian breeding grounds.
Perhaps this dichotomy of people's interests in a single species is indicative of the broad spectrum of social and economic values man derives from marine birds. Perhaps, too, it represents the challenge that wildlife professionals, administrators, and citizen conservation leaders face in today's complex world in striving to sort out priorities in allocation of such common property (amenity) resources among beneficial users.
As with the Ross' gull, socioeconomic values of marine birds involve both consumptive and nonconsumptive uses. Consumptive uses may provide socioeconomic values in the form of meat, eggs, oil, feathers, down, and guano. Cultural and recreational benefits may also be involved. Nonconsumptive uses benefit the tourist and recreation industries as well as providing less tangible social values, such as esthetic appreciation and environmental education and scientific study opportunities.
In this paper we examine some social and economic indicators that are believed to demonstrate people's growing awareness and interest in marine birds. These indicators involve a broad spectrum of values and illustrate the critical need for adoption of a strong North American marine bird conservation program.
Since earliest times, marine birds have accompanied the evolution of human societies in coastal and insular environments of the world. Their social value is in part recorded in kitchen middens of ancient campsites and villages. From the time man first inhabited the seacoasts and ventured out in ships, the company of seabirds has added life and inspiration to what otherwise would be a bleak and desolate landscape. Fishermen long ago learned to use seabirds to show them where the rich fishing grounds were located, and the cries of birds were often used to guide mariners away from dangerous cliffs during foggy weather.
At the time of the first contact with Europeans, native peoples of arctic Canada and Alaska reportedly took birds with bolas, snares, spears, arrows, and nets; they herded flightless waterfowl and gathered eggs as well. Brandt (1943) said that Alaskan Eskimos would have been destitute if eiders (Somateria spp.) had not been available for food and clothing, and Ekblaw (1928) believed the dovekie (Plautus alle) saved the polar Eskimo from extinction.
Marine birds have often served as an emergency food supply for explorers, sailors, and others: according to Tuck (1960) "The accounts of early arctic explorers and marooned whalers describe many instances in which starvation was averted by eating murres" (Uria spp.). One burrowing petrel of Australia was given the title "the bird of providence" because it saved the lives of shipwrecked mariners and convicts when supply ships from Sydney failed to reach them between March and August of 1790 (Serventy 1958).
Marine birds have also been taken because of the economic values of their feathers and oil. When economic overutilization has oc[Pg 175]curred, entire species were sometimes totally destroyed. This in fact happened to the great auk (Pinguinus impennis). When Jacques Cartier visited the Funk Islands off Newfoundland in May 1534, he and his crew filled several barrels with great auks and salted them down for future consumption. So severe was the slaughter in the next 3 centuries that the species became extinct in its known breeding haunts, which originally extended from Newfoundland through Greenland and Iceland, to the Hebrides. The last one was killed at a stack rock off Iceland in 1884 (Lockley 1973).
Other species have been almost totally destroyed. Colonization of Bermuda by Spain in the 17th century resulted in the near annihilation of the Bermuda petrel (Pterodroma cahow) there. Ships' crews found the birds to be fat and delicious, and they dried and salted those that could not be eaten fresh. Today, only about 20 breeding pairs remain, and are under strict protection by the Bermudan government (Lockley 1973).
The North Pacific albatrosses (Diomedea spp.) were nearly exterminated by Japanese feather hunters near the end of the 18th century. The short-tailed albatross (D. albatrus) was also nearly wiped out at its breeding colonies west of the Hawaiian Islands (Bourne 1972).
Other species that were carelessly exploited for their meat and plumage in the past, but which have since regained their numbers, include the fulmar (Fulmarus glacialis) on St. Kilda Island in the North Atlantic; and the North Atlantic, South African, and Australian gannets (Morus bassanus, M. capensis, and M. serrator) (Bourne 1972; Lockley 1973). In some instances entire breeding colonies of a species have been destroyed while others have survived. On the Abrothos Islands in western Australia, for example, large nesting colonies of sooty terns (Sterna fuscata) and common noddies (Anous stolidus) appear to have been wiped out on Rat Island by indiscriminate "egging" for food, whereas similar-sized colonies survive on other islands, where they are now controlled by the Fisheries and Fauna Department (Serventy et al. 1971).
Historically, it has probably been man's unregulated harvest of marine birds that has been the primary cause of their destruction. Generally, the loss of a species because of unregulated harvest is no longer a matter of major concern, because most countries of the world are providing at least some protection for their marine birds. However, other factors such as habitat destruction and contamination of the marine environment by industrial pollutants are posing increasingly serious threats to many.
Economic indicators concerning consumptive uses of wildlife, including marine birds, are frequently misunderstood. In a dollar-oriented and over-consumptive society like ours, economic values are usually seen as being in conflict with esthetic values. "Economic use" usually conjures up images of man's overutilization and, hence, long-term depletion of wildlife resources. However, when speaking of economic use, it is important to distinguish between such overuse and sustained-yield management.
Although both types of use have provided economic benefits over the years, overharvest that results in long-term resource depletion is not usually the most or best economic use in the long run; obviously a "harvest" cannot be sustained at a given level when the resource base is constantly being depleted. On the other hand, when certain species of marine birds are used in accordance with principles of sustained yield, they can provide long-term economic values to society in conjunction with the social, esthetic, and intangible values that their preservation insures. Of course, for many species esthetic values far outweigh economic ones derived through commercialization.
The muttonbird industry of Australia is an excellent example of the commercial use of marine birds on a sustained-yield basis. Fledgling Tasmanian muttonbirds, or slender-billed shearwaters (Puffinus tenuirostris), are commercially harvested each year from their colonies on islands of Bass Strait, mainly in the[Pg 176] Flinders Island group.
These muttonbirds are marketed as fresh or salted "Tasmanian squab." Various by-products, including oil, body fat, and feathers, are also sold. In 1968, a total of just under one-half million young birds were taken. Prices to the producers varied from $12 to $14 (Australian dollars) per hundred salted birds and $16 per hundred fresh birds. Stomach oil brought 75¢ per gallon. Assuming the average price per hundred birds to be $14, the meat alone was worth about $70,000 per year to the producers. The retail value was of course much higher. Although the muttonbird harvest is no longer the mainstay of the Flinders Island economy, according to Serventy (1969) it is still a picturesque and important annual social event.
Serventy et al. (1971) believed the commercialization of the muttonbird preserved its numbers: "Had there been no vested interests to preserve the 'birding islands' as such, many of them would in the course of time have been 'improved' as sheep stations and the shearwater populations would have declined and vanished."
The Caribbean is the home of the world's most important wild egg producer—the sooty tern. In some years about 2 million sooty tern eggs from the Seychelles and 0.6 million from Morant and Pedro bays have reached Caribbean markets (Tuck 1960).
Although the shooting of birds is not as important economically to Greenland's approximately 50,000 residents as are sealing, whaling, and fox hunting, the harvest of seabirds is an ancient tradition that still means production of an important food source that the many Greenlanders could not exist without. About 30 species of marine birds are harvested for human consumption, eider ducks and murres being by far the most important. In west Greenland about 750,000 birds (equivalent to about 825 tons of meat) and 10,000 eggs are harvested annually. Murres constitute the main dish in summer at small coastal outposts with access to rookeries. Great quantities are also dried and salted for use in winter. Murre canneries at Upernavik have supplied southern cities with the frozen meat of about 25,000 to 30,000 murres annually. However, this commercial activity would be prohibited by a proposed new Greenland game law (Salomonsen 1970).
Banding has shown that about 22% of Greenland's eider population, or about 150,000 birds, is shot annually. Collecting of eider eggs is now prohibited except in the Thule District, where 10,000 are taken annually. Eider down is still collected from nests for sale to a trading company for the manufacture of much demanded eider-down coverlets (Salomonsen 1970).
A growing human population, the widespread use of modern firearms, and the increasing use of speedboats in hunting have resulted in serious declines in many of Greenland's marine bird populations. The Greenland government has demonstrated its concern by instituting protective measures in response to Danish expert advice. For example, the common puffin (Fratercula arctica) was given 10 years of total protection in 1961 after bird numbers had seriously declined as a result of over-harvesting of the birds and their eggs (Lockley 1973). This protection was extended in 1970. Also, it is now illegal to discharge firearms at most marine bird rookeries in Greenland.
With protection of bird habitats from human intrusion and toxic environmental pollutants, adequate enforcement of sound conservation laws, greater efforts in conservation education, and scientific regulation of harvests, Greenland's valuable marine bird resource could probably withstand intensive utilization indefinitely (F. Salomonsen, personal communication). Salomonsen has been quick to point out, however, that people should not be encouraged to believe that the value of seabirds for food is the only reason they should be saved.
Although several species of marine birds serve as sources of food in the Soviet Union, down of eider ducks and eggs of murres are considered to be the most important to the economy. These birds are referred to as trade birds due to their commercial importance (Belopol'skii 1961).
Peruvian guano beds are currently being managed on a sustained-yield basis; the harvest, as in the days of the Incas, depends entirely on the amount of guano deposited each year. Conservation and management policies have resulted in a steady increase in the amount extracted, from around 20,000 tons in 1900 to over 200,000 tons in 1971 (Lockley 1973).
The islands off south and southwest Africa are also commercial producers of guano. The annual yield from these breeding colonies averaged 3,971 tons in the 12-year period, 1961-72. In 1969, guano brought 4.75 Rands (equivalent to $7.11) per 200-pound bag. South African gannets are apparently depositing guano that is worth twice as much as the fish they consume to produce it (Jarvis 1971).
Marine birds also play significant roles in the economies of the world's oceans, where algae, invertebrates, fish, seabirds, mammals, and man interact in complex ways. The bioenergetics and nutrient cycling in ocean ecosystems is even less well understood than the contributions seabirds make to man's dollar economies.
Sanger (1972) has conservatively estimated that in the subarctic Pacific region alone, birds consume from 0.6 to 1.2 million tons of food and return from 0.12 million to 0.24 million tons of feces each year.
Marine bird excrement is especially rich in nitrates and phosphates, which phytoplankton, the basis of ocean food pyramids, requires. Marine birds then, at least to some extent, help to sustain the northern commercial, recreational, and subsistence fishing industries. The fisheries in turn sustain seals and certain other mammals which are also essential elements of northern subsistence and recreational economies. Thus, marine birds contribute economic benefits indirectly as well as directly by serving as critical links in ecosystem food chains (Tuck 1960).
The use of marine birds and their products does not have to be commercial to be economic. Economics is the science of the allocation of scarce resources. Any resource, regardless of whether it is bought or sold, has value to people and is therefore an economic commodity. Thus, any society has an economy whether or not it uses cash, and when the meat, feathers, or oil of marine birds are used, the birds have economic value. The problem, of course, is that of trying to determine just what this value is when a cash medium does not exist.
One of the ways to estimate this value is to assign implicit gross dollar values to seabirds, based on what it would cost to replace products derived from them with store-bought items of a similar, or substitutable, nature (this is a gross rather than a net value because it does not include the cost of guns, ammunition, transportation, etc., required to harvest and process the resource).
There have been many occasions in the past when it would have been physically impossible to find substitutes for seabird products. In such cases, and where seabirds may well have meant the difference between life and death, the economic value of the resource could be considered a plus infinity.
There are probably few, if any, places in the world today where people would starve if they could not obtain marine birds. However, there are still many situations where available substitutes are poor, or very expensive. And there are others where, even though the birds are no longer necessary for economic survival, they are still very important in terms of sociocultural traditions. According to Tuck (1960), "Wherever a wild animal is important to the economy of a people, its capture and use become part of the tradition of that people." Thus, while economic values can be measured in terms of substitutable store-bought foods, social and cultural values cannot be. To force complete dependence on a people by flying in foods from "Outside" is often socially intolerable because it tends to remove pride, a sense of worth, and therefore the reasons for living.
Marine birds have served as important sources of food in the Faeroes Islands for cen[Pg 178]turies, the puffin being unquestionably the most valuable. Williamson (1945) reported that in a good year the total puffin catch may be between 400,000 and 500,000. In addition, as many as 120,000 murres are snared or shot annually by the Faeroese, and at least twice that many eggs are taken and Tuck (1960) stated, "The economic necessity of 'fowling' in the Faeroes has by virtue of long centuries of usage become part of the national life, affecting folklore and customs, and providing outlets for the sporting instinct inherent in the people." A Faeroese guidebook even suggests that its importance to the Faeroese culture has been in no way diminished by the influence of modern civilization. Current Faroese game laws appear to be effective in assuring a sustained yield of marine birds while guaranteeing their long-term survival.
Seabirds and their eggs constitute a small, but still very important, part of the total diet of the Eskimos and Indians living along the Arctic coast of the Northwest Territories and Alaska. In spite of the many changes occurring in the North, there is, even for the wage earner, a strong psychological attachment to the land and sea and the free life it represents. In spring, the release from the long monotonous winter is marked by the rites of ratting, fishing, sealing, whaling, or marine bird hunting and egg gathering, according to village tradition.
For those living off the land in such remote coastal outposts as Sachs Harbor on Banks Island, Holman Island on the Mackenzie Delta, Point Hope and Point Barrow in northern Alaska, Inalik on Diomede Island in the Bering Strait, or Hooper Bay on the Yukon-Kuskokwim Delta, the spring marine bird hunt represents a change of diet and activity. It offers opportunity to renew age-old traditions and continues a cultural bond among those confined to jobs in the settlements—vacationing and absenteeism from jobs and schools are always highest during late May and early June.
Marine birds yield between a few grams and 2 kg of meat, depending on the species. Usually the birds are either consumed soon after they are taken or stored in an icehouse for use throughout the summer. Most often the meat is cooked into a soup or stew with rice, noodles, and onions. A few birds may be dried or salted so that they can be used for special holiday feasts during the winter. Sometimes feathers are saved for the manufacture of parkas, ceremonial fans, and masks. In some areas of the Yukon Delta, goose and duck down is still saved and used in quilts that can be found in nearly every home. In the spring 1975 issue of the catalog of a Seattle, Washington, outfitter, down quilts for single beds were listed at $95. Thus, there is a substantial cash savings by home manufacture of such items.
The Yukon Delta in western Alaska is the area where the use of marine birds is most extensive and significant. Klein (1966) provided harvest data by village for the entire area and showed that, in general, geese were more important than ducks, representing about two thirds of the take in both the spring and the fall. The average numbers of ducks (mostly pintails, Anas acutus) and geese (primarily white-fronted geese, Anser albifrons); emperor geese, Philacta canagica; cackling Canada geese, Branta canadensis minima; and black brant, Branta nigricans, taken per household were 77 by the Yukon River villages, 69 by the Kuskokwim River and tundra villages, and 94 by the Bering Sea coastal villages. Although eggs gathered by Yukon River villagers averaged less than a dozen per household, Kuskokwim people took about 3 dozen and coastal people about 6.5 dozen on the average. Eggs of black brant and cackling Canada geese were especially favored, but even those of small passerines were acceptable. The average size of households for all areas was believed to be between 5.5 and 6.5 persons.
A 1968 survey of waterfowl taken in the Mackenzie Delta region, made by the Canadian Wildlife Service, showed an average take per household of about 70 birds, a figure comparable to that for the Yukon Delta. In the Mackenzie region, however, ducks were more important than geese, representing about 60% of the harvest.
More recent data on Alaska waterfowl harvest per household is available for other coastal regions. Data provided by two regional native corporations for the Joint Federal-State Land Use Planning Commission for Alaska in 1973 showed an average per-household waterfowl harvest of 33 ducks and[Pg 179] geese for Kotzebue area villages, 68 for Norton Sound villages, 24 for northwest Seward Peninsula villages, and 37 for St. Lawrence, Diomede, and King Island villages.
A 1974 subsistence survey carried out jointly by the University of Alaska and the Bristol Bay Native Corporation showed that, in 20 Bristol Bay villages, 57% of the households harvested waterfowl. The average kill was 32 birds per household.
Eider ducks are the most important marine birds taken by residents of Barrow, Alaska. Johnson (1971) interviewed 31 adult hunters with average kills of 88 birds per hunter. Barrow people also take substantial numbers of geese at Atkasook, a summer camp on the Meade River 80 miles southeast of Barrow.
Point Hope, Alaska, villagers also favor eider ducks above all others. Pederson (1971) indicated that each household that hunted took about 150 eiders in the summer of 1971. Each summer, Point Hope and Kivalina residents travel to the Cape Thompson and Cape Lisburne cliffs to gather murre eggs. Both Pederson (1971) and Kessel and Saario (1966) showed an average harvest of 5 to 10 dozen eggs per household (equivalent in weight to 10 to 20 dozen chicken eggs).
To our knowledge, there is no available evidence to indicate that the number of migratory birds taken in the North in spring and fall is a significant factor in the survival of a particular species. The birds are, however, a significant factor in the economy and culture of the people of the Mackenzie Delta region and much of coastal Alaska. This may not always be true, for their social and economic conditions are changing rapidly.
With the native birthrate twice the national average and with hunting technology improving yearly, the day will undoubtedly come when marine birds and other wildlife resources are not able to withstand intensified harvest pressures without more regulation and control. An obvious need exists for government conservation agencies to work more closely with the native people of northern regions in conservation education and development of sound harvest regulations.
No attempt was made in this evaluation to affix dollar values to every marine bird enjoyed by recreationists. Goldstein (1971), in his economic study of wetlands, found it impossible to fix the value of the production and harvest of migratory waterfowl in Minnesota.
The amount of money spent by recreationists in seeking enjoyment from marine birds does not measure the values they derive; it measures only their costs to participate in such ventures. The analogy that could be made is that the value of a diamond is equal to the cost of mining it. Nevertheless, expenditure data for services and goods provided by air-taxi and charter boat operators and merchants selling bird guides, binoculars, and other outdoor recreational equipment are useful indicators in establishing the secondary or indirect benefits of recreational activities associated with marine birds.
The normal economic concept of net benefits from marine bird recreation would include only those accruing to individuals who provide goods and services to the recreationists, gross revenues minus the costs (Wollman 1962; Pearse and Bowden 1969). This economic return, however, in no way measures direct benefits of marine bird resources to the recreationists.
Another important consideration in evaluating recreational use of marine birds is to recognize that many of the nonparticipants either value the option of being able to take advantage of them in the future, or simply believe that the availability of such resources benefits society (Stegner 1968). Such benefits are difficult, if not impossible, to quantify yet may be exceedingly important due to the uniqueness of the marine bird resource and because many decisions affecting it may prove irreversible.
Increasing numbers of bird enthusiasts throughout North America are discovering the excitement and pleasures derived from visiting marine bird rookeries. As pointed out by Sowl and Bartonek (1974), and as anyone can attest who has ever had the privilege of watching the antics of tufted puffins (Lunda cirrhata) near their colonies on a day when the sun is obscured and the air buoyant, watching seabirds is fun.
We have found that organizations and businesses in practically every North American coastal State and Province, from Nova Scotia[Pg 180] to Florida and Alaska to California, are busy scheduling boat or airplane excursions to marine-bird viewing areas off their shores. The Alaska and Washington State ferry systems have for years been providing passengers opportunity to enjoy seabirds of the North Pacific coast. Audubon chapters in San Diego, Los Angeles, Monterey, Seattle, Anchorage, and other cities sponsor annual excursions to seabird colonies.
In 1975 a charter airline service in Anchorage, Alaska, booked 530 people in 51 tours to fly to the Pribilof Islands in the Bering Sea to view the outstanding seabird and fur seal colonies there. Included in the bookings were three National Audubon Society International Ecology Workshops, the Massachusetts Audubon Society, the National Wildlife Federation, and Canadian Nature Federation. Participants paid from $1,500 to $2,000 for these tour packages to Alaska. At $300 to $380 per person, depending on the length of the excursion, the air charter service grossed about $160,000 from these tours (Reeve Aleutian Airways, personal communication).
Fairweather Outings, a small cruise business based in Sitka, Alaska, takes people on wilderness excursions in the west Chichagof-Glacier Bay area of the southeastern part of the State. The seabird rookeries are one of the principal attractions for the 90 people taking these trips each year. Over one-third of the clientele has been from outside Alaska; thus their dollars are new dollars to the State's economy. Fairweather Outings grossed about $11,000 in 1974 (Charles Johnstone, personal communication).
These examples illustrate how seabirds, both directly and indirectly, help small coastal businessmen earn a living. It is also important to recognize that the multiplier effects generated by the expenditures in all of the above examples ripple through the regional and State economies.
Despite the great social and economic significance of such activities along our coasts, apparently no attempt is being made to determine the number of people involved in such pursuits and how much they are spending. A study of the phenomenon would undoubtedly produce startling results.
The Wildlife Management Institute (1975) revealed that the national estimated value of manufacturers' shipments in 1972 was $157 million for camping equipment, $5 million for binoculars, and $19.9 million for bird feed. Sales of wild bird feed have been increasing 5 to 10% per year recently. These are all economic indicators of recreation trends of which enjoyment of marine birds is a part.
A major use of photographic equipment and related products and services is in the natural and scenic areas of the nation. Manufacturers' shipments of photographic equipment, and photofinishing, were valued at $2.3 billion in 1972. A 5% excise tax on these items would have generated nearly $118 million (Wildlife Management Institute 1975).
Since inadequate funding plagues most nongame management initiatives, the Wildlife Management Institute (1975) recommended that Congress authorize a matching grant-in-aid program to benefit nongame fish and wildlife. Funds would be obtained from new manufacturers' excise taxes on designated outdoor recreational equipment to initially yield at least $40 million annually.
The Executive Committee of the International Association of Game, Fish and Conservation Commissioners and the Council of the Wildlife Society have already endorsed model legislation for a State program for nongame wildlife conservation (Madson and Kozicky 1972). We urge that these proposals be given serious consideration in terms of future funding of marine bird conservation programs in North America.
It is encouraging to note that several States, including Washington, Oregon, and California, have recently initiated nongame wildlife programs that have resulted in substantial benefits to their citizens. The California legislature, for example, enacted a law in 1974 to provide a means for individuals and organizations to donate funds for supporting nongame species management. The California Department of Fish and Game has increased its nongame staff and appointed a citizen Nongame Advisory Committee to help develop and implement nongame programs.
Because most species of marine birds are not hunted by sportsmen in North America, this increased emphasis on nongame species may eventually benefit research and management programs for seabirds substantially.
Even now, marine-bird research studies and inventories require the expenditure of several million dollars annually along our coasts. In Alaska a multimillion dollar Federal effort has been initiated to assess the environmental risks of developing offshore petroleum potential in the Gulf of Alaska and five other key areas of the State. These areas represent 60% of the nation's total continental shelf and support some of the largest marine-bird populations in the world. The program to examine life-forms and the physical environment of the petroleum lease areas will require 4 to 5 years to complete. Approximately $1.5 million has been allocated to conduct an environmental assessment of marine bird resources in the first 18 months alone.
The U.S. Fish and Wildlife Service is spending about $40,000 to determine the seasonal occurrence, density, and distribution of marine birds in coastal waters adjacent to new national wildlife refuges in Alaska being proposed pursuant to the Alaska Native Claims Settlement Act of 1971, and almost $200,000 to study and manage migratory birds—including marine birds—on existing refuges.
Although generated by external events (including requirements pursuant to the National Environmental Policy Act of 1969) rather than by the resources themselves, these expenditures at least indirectly reflect a social concern for the welfare of marine birds.
Another encouraging aspect of seabird conservation and its meaning to society is the increasing involvement of citizens in the issue. Although agencies have not been as responsive as many would like, administration of government at all levels has been shaken and stimulated by citizen participation. As Russell W. Peterson, Chairman of the Council on Environmental Quality, has stated, "Citizen action is the essence of democracy. Citizen movements should be encouraged and expanded. The involvement of people is necessary to counterbalance the disproportionate influence of the professional lobbyists and public relations operators hired to further the special interests of their clients." Mr. Peterson further emphasized that government thrives much better on citizen concern and attention than on indifference and neglect.
Therefore, it is highly significant that the Pacific Seabird Group has many nonprofessional, as well as professional, members and that the 1975 International Symposium on Conservation of Marine Birds of Northern North America had strong citizen involvement and participation. As everyone recognizes, nothing works in government unless people, be they doctors, lawyers, college professors, students, environmentalists, or Indian chiefs, make it work.
Educators must upgrade training in environmental sciences so that an environmental awareness (conservation ethic) is instilled in young people. In this regard, an Alaskan bird study program proposed for Alaska schools by J. G. King, Jr., of the U.S. Fish and Wildlife Service in 1962 deserves close scrutiny. This highly innovative and practical environmental education proposal apparently arrived before its time, for nothing was ever done to institute it. Possibly, now would be a good time to give it a closer look.
Success in more adequately recognizing and using social and economic indicators to strengthen and broaden seabird programs will depend on the ability of the resource management agencies to blend the old with the new. It is obvious to most that new alignments, programs, authorities, and sources of funds are needed, but by themselves, they will not be enough to overcome the continuing massive losses of wildlife habitat due to population growth and technological impacts resulting from various developmental programs.
No marine bird programs will be successful without a strong political base. If this is to be assured, resource agencies must be more responsive to the needs of both consumptive and nonconsumptive users and involve them in their programs from early in the planning process. Because marine birds and the natural environments they inhabit are jointly valued[Pg 182] over time and are jointly owned, it is important to ask not only what is efficient from the point of view of the present generation but also what is equitable across generations.
Belopol'skii, L. O. 1961. Ecology of sea colony birds of the Barents Sea. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 346 pp.
Bourne, W. R. P. 1972. Threats to seabirds. Int. Counc. Bird Preserv. Bull. II:200-218.
Brandt, H. 1943. Alaskan bird trails: adventures of an expedition by dogsled to the delta of the Yukon River at Hooper Bay. Bird Research Foundation, Cleveland, Ohio. 464 pp.
Ekblaw, W. E. 1928. The material response of the polar Eskimo to their far arctic environment. Ann. Assoc. Am. Geog. 17(4):147-195.
Goldstein, J. H. 1971. Competition for wetlands in the Midwest: an economic analysis. Resources for the Future, Inc., Washington, D.C. 105 pp.
Jarvis, M. J. F. 1971. Interactions between man and the South African gannet. Biol. Conserv. 3(4):269-273.
Johnson, L. L. 1971. The migration, harvest and importance of waterfowl at Barrow, Alaska. M.S. Thesis. Univ. of Alaska, Fairbanks. 87 pp.
Kessel, B., and D. Saario. 1966. Human ecological investigations at Kivalena. Pages 969-1039 in N. J. Wilimovsky and J. N. Wolfe, eds. Environment of the Cape Thompson region, Alaska. U.S.A.E.C., Div. Tech. Inf., Oak Ridge, Tennessee.
Klein, D. R. 1966. Waterfowl in the economy of the Eskimos on the Yukon-Kuskokwim Delta, Alaska. Arctic 19(4):319-335.
Lockley, R. M. 1973. Man and seabirds. Pages 74-90 in R. M. Lockley. Ocean wanders: the migratory seabirds of the world. Stackpole Books, Harrisburg, Pennsylvania.
Madson, J., and E. Kozicky. 1972. A law for wildlife: model legislation for a State nongame wildlife conservation program. Winchester-Western Division, Conservation Department, East Alton, Illinois. 20 pp.
Pearse, P. H., and G. K. Bowden. 1969. Economic evaluation of recreational resources: problems and prospects. Trans. N. Am. Wildl. Nat. Resour. Conf. 34:283-293.
Pederson, S. 1971. Status and trends of subsistence resource use at Point Hope. Pages 37-89 in B. MacLean, ed. Point Hope project report. Univ. of Alaska, Fairbanks.
Salomonsen, F. 1970. Birds useful to man in Greenland. Pages 169-175 in Productivity and conservation in northern circumpolar lands. International Union for Conservation of Nature and Natural Resources, Morges, Switzerland.
Sanger, G. A. 1972. Preliminary standing stock and biomass estimates of seabirds on the subarctic Pacific region. Pages 589-611 in A. Y. Yakenouti et al., eds. Biological oceanography of the North Pacific. Idemitsy Shoten, Tokyo.
Serventy, D. L. 1958. Mutton-birding. Pages 233-234 in A. H. Chisholm, ed. The Australian Encyclopedia, Vol. 6. Angus and Robertson, Sydney.
Serventy, D. L. 1969. Mutton-birding. Pages 53-60 in K. Taylor, ed. Bass Strait Australia's last frontier. Australian Broadcasting Company, Sydney.
Serventy, D. L., U. Serventy, and J. Warham. 1971. Seabird conservation problems in Australia. Pages 40-44 in D. L. Serventy, U. Serventy, and J. Warham. The handbook of Australian birds. A. H. and A. W. Reed, Sydney.
Sowl, L. W., and J. C. Bartonek. 1974. Seabirds: Alaska's most neglected resource. Trans. N. Am. Wildl. Nat. Resour. Conf. 39:117-126.
Stegner, W. 1968. The meaning of wilderness in American civilization. Pages 192-197 in R. Nash, ed. The American environment: readings in the history of conservation. Addison-Wesley Publishing Co., Reading, Massachusetts.
Tuck, L. M. 1960. The murres. Ottawa, Can. Wildl. Ser. 1. 260 pp.
Wildlife Management Institute. 1975. Current investments, projected needs, and potential new sources of income for nongame fish and wildlife programs in the United States. Wildlife Management Institute, Washington, D.C. 55 pp.
Williamson, K. 1945. The economic importance of seafowl in the Faeroes Islands. Ibis 87:249-269.
Wollman, N., chairman. 1962. The value of water in alternative uses, with special application to water use in the San Juan and Rio Grande basins of New Mexico. Univ. of New Mexico Press, Albuquerque. 426 pp.
by
Donald E. McKnight
Alaska Department of Fish and Game
Subport Building
Juneau, Alaska 99801
and
C. Eugene Knoder
National Audubon Society
Lakewood, Colorado
Although development of hard mineral resources, expansion of the timber industry, and resultant increases in human pressures along the North Pacific and Arctic coasts will ultimately adversely affect northern marine bird populations, current and proposed activities of the petroleum industry are the most immediate threat to marine birds. The Federal Government's recently announced plans for oil and gas leasing on the Pacific outer continental shelf eclipse the significance of North Slope and Cook Inlet oil developments. Within a few years, onshore storage facilities and supertankers plying these waters will undoubtedly result in widespread chronic and localized catastrophic contamination of northern marine ecosystems.
Coastal and offshore waters south of the reaches of the seasonal ice pack are tremendously productive, supporting a diverse wealth of bird life throughout the year. Because these ecosystems are relatively stable and the impact of temporal oscillations on the physical environment is not as great as in the Arctic, birds in these areas are probably least susceptible to man's influence on a long-term basis.
Avifaunal associations of the Arctic are less diverse and have shorter food chains than more southerly ones; consequently they are more susceptible to environmental perturbations. Slow growth and maturation rates of arctic species and resultant prolonged population recovery periods further aggravate this situation.
Available knowledge of northern seabirds and their environmental requirements is in inverse relation to the latitude at which they are found and to the ecological stability of the ecosystems involved. Arctic bird associations and their fragile environments are least understood, but are doubtless the most vulnerable to the detrimental effects of man-caused environmental degradation. The paucity of knowledge about them limits the possibility of predicting the consequences of petrochemical exploitation and thereby safeguarding against potential problems. Existing technology and support system capabilities of the oil industry are more poorly defined for arctic areas, further compounding this problem. Regardless of information amassed in the future and precautionary measures taken during exploitation of arctic petroleum reserves, the potential for disastrous and perhaps irrecoverable losses to northern marine bird species and populations is great. Losses of major magnitude could appreciably alter the productivity of northern marine ecosystems.
Although the coastal waters of the northwestern United States and western Canada support a plenitude of marine life, including marine birds, relatively little is known about these ecosystems. Sustained interest in quantitative aspects of this area's marine bird populations has developed only within the past few years. As Sowl and Bartonek (1974) indicated, seabirds are the most visible component of a marine ecosystem and, at the same time, they are the least understood. Management information has been haphazardly gathered, and because seabirds occur in incredibly large numbers in north Pacific and arctic waters, it has been convenient to assume that, in the absence of problems, systematized data gathering and analysis were unnecessary.
The sudden emergence in the late 1960's of Alaska and portions of northwest Canada as potential major oil production areas has changed this situation dramatically. Ongoing and planned petroleum development in the North and the concurrent expansion of hard mineral extraction and logging activities now threaten to adversely affect these marine bird resources. Alaska's human population, which numbered only slightly over 400,000 in 1975, will probably double within the present decade. Doubtless, increased numbers of people, oriented toward mineral and other resource exploitation rather than toward traditional wildland values, will compound these problems. Pressures on State and local governments for increased services necessitated by increasing populations will require additional expenditures. In Alaska, at least, these demands are being imposed before revenues from minerals become available. This necessitates additional oil leases, timber sales, and other means for obtaining immediate funding, thereby adding to the acceleration and irreversibility of industrial expansion into the North.
This atmosphere of change has spawned major government-and industry-supported programs to broaden knowledge of northern marine ecosystems, including their avifauna. There has been a recent flurry of publications on seabird populations and biology and a proliferation of papers stressing the need to learn more about the biota of this area. Nevertheless, environmental impact statements on proposed developmental programs in the North still raise more questions than are being answered. Attempts are being made to apply available information on oil spills, human disturbance, and other aspects of environmental degradation gathered from experiences in other areas to expected problems in northern environments, but one must realize that much of the information gained from experience elsewhere is not applicable to these areas. It is realistic to assume that, until development-related problems occur in the North, biologists cannot estimate the magnitude or ecological dimensions of their effects. However, existing knowledge of ecological "laws" and of the biology of some species provides the base for limited predictive efforts.
It is the purpose of this paper to describe significant current and proposed resource development along the coasts and the ocean floors, to summarize existing knowledge of the ecology of marine birds in these areas, and to identify potential conflicts with marine bird conservation. We hope that identification of these problems will provide impetus to data gathering and management programs necessary for conservation of these valuable resources.
The region discussed here encompasses nearly half of the United States and Canadian coastlines, extending from Washington to the eastern edge of the Northwest Territories. Alaska alone has two-thirds of the United States' continental shelf (Bartonek et al. 1971). This region's marine and estuarine waters are some of the most productive in the world and support a diverse wealth of bird life throughout the year. Sanger (1972), for example, estimated total summer standing stocks of some 21 million birds in an area approximating the outer continental shelf from the Bering Strait south along the coasts of the Aleutian Islands and North America to central California. Sanger and King (this volume), to whom more data were available, revised this estimate upward to 45 million. Bartonek et al. (1974) provided estimates of year-round standing stocks of 27 million birds[Pg 185] in the Bering Sea alone.
North and east of the Bering Strait, population estimates of the bird fauna are less complete. Swartz (1966) estimated, however, that seabird populations of five colonies in the vicinity of Cape Thompson in the Chukchi Sea exceeded a total of 420,000 breeding birds in 1960. Information provided by Bartonek and Sealy (this volume) indicates that large colony complexes at Cape Lisburne and Little Diomede Island each number, in aggregate, over 1 million breeding birds, mainly alcids, kittiwakes (Rissa spp.), gulls (Larus spp.), fulmars (Fulmarus glacialis), and cormorants (Phalacrocorax spp.). Although the Chukchi Sea coast north of Cape Lisburne has no rocks suitable for cliff-nesting seabirds, large numbers of tundra-nesting species use the inshore waters as a migratory pathway, and many nonbreeding cliff nesters summer in these waters (J. M. Scott, comments by Pacific Seabird Group on U.S. Department of the Interior Draft Environmental Statement 74-90). According to Scott, sea ducks and gulls are the most numerous birds in the Beaufort Sea. Observations by Thompson and Person (1963) of an estimated 1 million eiders, mostly king eiders (Somateria spectabilis) and Pacific eiders (S. mollissima), passing over Point Barrow en route to molting areas, reflect the numbers involved. Oldsquaws (Clangula hyemalis) use coastal waters of the Beaufort Sea for postbreeding wing molts; Bartels (1973) estimated their numbers at nearly 400,000 in the fall and perhaps more during the molting period. Shorebirds, jaegers (Stercorarius spp.), gulls, and terns, most of which use coastal waters at some time during the summer season, swell bird numbers by several millions in this area (Arctic Institute of North America 1974).
As indicated by Sanger (1972), the seabirds inhabiting coastal areas south of Bering Strait are mainly members of the Procellariidae in summer and Alcidae in winter. Sooty shearwaters (Puffinus griseus) are the prevalent summer species and ancient murrelets (Synthliboramphus antiquus) and marbled murrelets (Brachyramphus marmoratus) are the most abundant winter species. Sanger's central subarctic domain (offshore waters including the Gulf of Alaska) had a different species composition. During the summer, procellariids—mostly slender-billed shearwaters (Puffinus tenuirostris) and sooty shearwaters—made up 94% of the biomass. Procellariids, including fulmars, larids (largely glaucous-winged gulls, Larus glaucescens), black-legged kittiwakes (Rissa tridactyla), and large alcids, including the tufted puffin (Lunda cirrhata), made up 87% of the winter biomass in this domain (Sanger 1972).
Although most of the arctic waters, including the Bering, Chukchi, and Beaufort seas, are unavailable to birds during the winter because of pack ice, they seasonally host an avifauna dominated by colony nesters, such as common and thick-billed murres (Uria aalge and U. lomvia), and tundra nesters, such as oldsquaws and eiders. In far northern waters, sea ducks (mainly eiders and oldsquaws), red phalaropes (Phalaropus fulicarius), and gulls are the predominant species.
Intertidal areas throughout the Alaska, British Columbia, and Washington coasts support characteristic assemblages of shorebirds, including the black oystercatcher (Haematopus bachmani), rock sandpiper (Erolia ptilocnemis), wandering tattler (Heteroscelus incanum), surfbird (Aphriza virgata), and black turnstone (Arenaria melanocephala) as reported by J. M. Scott (comments by Pacific Seabird Group to U.S. Department of the Interior Draft Environmental Statement 74-90). Perhaps the greatest concentrations of shorebirds in this whole region occur during spring and fall migrations in Prince William Sound. The tremendous numbers of migrating birds using these tidal and marsh areas are hard to imagine, but densities of up to 250,000 shorebirds per 259 hectares (ha) on portions of the more than 51,820-ha tidal flats of the Copper River Delta have been recorded (Isleib and Kessel 1973).
Although this region's avifauna is remarkable from the numerical standpoint, it is important to remember also that some of its species are limited in distribution to this area. According to Bartonek et al. (1971), Alaska is the only known breeding area for black turnstones, bristle-thighed curlews (Numenius tahitiensis), surfbirds, western sandpipers (Ereunetes mauri), and Kittlitz's murrelets (Brachyramphus brevirostris). Several waterfowl species, including the dusky Canada goose (Branta canadensis occidentalis), cack[Pg 186]ling Canada goose (B. c. minima), Aleutian Canada goose (B. c. leucopareia), and Aleutian common teal (Anas crecca nimia) nest only in Alaska coastal areas (Bartonek et al. 1971). Izembek Lagoon on the Alaska Peninsula annually hosts the entire population of black brant, Branta nigricans (Hansen and Nelson 1957), and many other waterfowl, seabird, and shorebird species nest or live in this region in numbers important to their worldwide welfare.
The immense nonrenewable resource wealth of Alaska and other arctic regions has remained virtually unrecognized or unexploited until recently because of the availability of these resources in more accessible locations. As supplies have diminished or been exhausted elsewhere and demands have increased, however, it has become economically feasible or necessary to tap supplies in less-accessible regions. For this reason, the petroleum industry has recently expanded its exploratory efforts in the far North with well-known success. Deposits of metallic ores, coal, and other raw materials to feed industry have likewise been discovered and plans devised for their extraction and sale. Pressed with decreased availability of commercial timber elsewhere, the logging industry has similarly begun to broaden its efforts into Alaska. Expansion of industrial activities into the North is proceeding at a rapidly accelerating pace, and these industries, their associated support industries, and expanded human populations are having and will continue to have unprecedented impact on these marine ecosystems, including their avifauna.
The existence of potentially marketable oil and gas deposits in Alaska has been recognized since the early 1900's, but it was not until the Swanson River, Alaska, oil field was discovered in 1957 and later developed that the Arctic entered the modern era of oil development (McKnight and Hiliker 1970). This field and offshore fields in the Upper Cook Inlet basin have been producing oil for nearly a decade. The discovery of petroleum reserves on Alaska's North Slope and Canada's Mackenzie River Delta is common knowledge, and a pipeline has been constructed to transport Alaska oil to a tanker facility at Valdez in Prince William Sound. Alternative proposals to pipe North Slope natural gas along the existing corridor to a facility in Prince William Sound or to build a new pipeline to take this gas to existing fields, and a planned pipeline on the Mackenzie River Delta and south through Canada, are being considered. Construction of a gas liquefaction facility in Prince William Sound and tanker traffic through the Sound and the Gulf of Alaska are potential ramifications of an Alaska gas pipeline.
As McKnight and Hiliker (1970) and Bartonek et al. (1971) pointed out, the greatest potential problem for marine bird populations from North Slope oil will be associated with the operations of the Alyeska Pipeline system's terminal at Valdez. Oil storage and ship-loading facilities at this port and heavy tanker traffic through Prince William Sound represent a pollution source that could result in significant seabird and waterfowl mortalities. Certainly, development of gas liquefaction facilities in the Sound, with inherent increases in human populations and tanker traffic, would compound this potential problem.
Although future impacts from existing petrochemical developments are cause for concern, the Federal Government's recently announced plans for oil and gas leasing on the Pacific outer continental shelf (Fig. I) eclipse the significance of North Slope and Cook Inlet oil developments. It now appears the Gulf of Alaska is the most favorable area of the outer continental shelf for oil and gas production (Council on Environmental Quality 1974). This area, covering more than 10.3 million ha, has already been subjected to extensive seismic investigations, and estimates of its undiscovered, economically recoverable crude oil and natural gas resources range from 3 to 25 billion barrels and 15 to 30 trillion cubic feet, respectively (Council on Environmental Quality 1974).
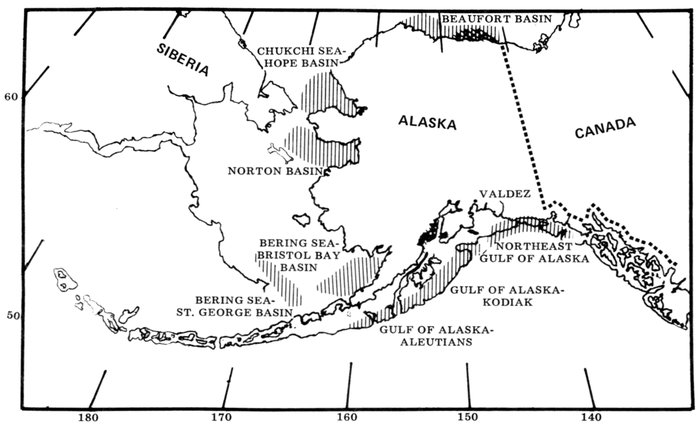
Fig. 1. North Pacific, showing portions of the outer continental shelf being considered for gas and oil leasing by the Federal Government (vertical hatching) and areas leased or proposed for leasing by the State of Alaska (cross hatching).
Kinney et al. (1970) reported that in Cook Inlet, Alaska, an estimated 0.3% of the oil produced and handled in offshore platform wells is spilled. Several routine offshore operations result in discharges of oil and other materials into water, and, unlike accidental spills, the probability of their occurrence is 100% (Council on Environmental Quality 1974). During drilling operations, cleaned drilling mud and drill cuttings are discharged overboard. Drilling mud may consist of such substances as bentonite clay, caustic soda, organic polymer, proprietary defoamer, and ferrochrome lignosulfate. Waters from geological formations are often produced and discharged into the sea while the wells are in production. These waters may be fresh or saline, and often contain small amounts of oil. All of these pollutants increase the adverse effects of offshore oil production, and when potential spills are also considered, the ultimate impact on the marine ecosystem may be substantial.
The State of Alaska has already leased offshore sites in Kachemak Bay, and present considerations for future leases in the lower Cook Inlet and Beaufort Sea further reflect the widespread and massive nature of petrochemical developments in the Arctic planned for the next 2 decades (Fig. 1). Proved crude oil reserves are less than 1 billion barrels and natural gas reserves are less than 2 trillion cubic feet in Cook Inlet, but it appears that undiscovered recoverable oil and gas resources may be much greater (Council on Environmental Quality 1974). There are also indications that known onshore oil reserves along Alaska's northwest coast will soon be opened for development by the Arctic Slope Regional Corporation, landowners in the area as a result of the Native Land Claims Act of 1971. This group is at least considering the transportation of these petroleum products to market in tankers, from an open-water port in the Chukchi Sea—thereby adding to the tanker traffic in northern waters.
As indicated by Bartonek et al. (1971), there has been renewed interest in opening up Alaska's hard mineral resources to economic development as new transportation routes[Pg 188] and modes have been developed. Plans are being completed to develop the Bering River coal field, with the eventual goal of exporting coking coal to Japan. Although mining operations might ultimately affect freshwater environments to the detriment of several waterfowl species, including the trumpeter swan (Olor buccinator), the chief cause for concern will be additional freighter traffic through Prince William Sound. Similar plans to develop Klukwan and Snettisham iron deposits in southeastern Alaska for the use of Japanese industry (Bartonek et al. 1971) may result in the imposition of further traffic in Alaska shipping lanes.
Plans are under way to strip-mine coal deposits in the Beluga field near the west side of Cook Inlet and transport a coal slurry via pipeline to a thermal electric generation plant opposite Anchorage on the Inlet. Impact on tidal areas may be minor, but thermal pollution of the waters is a possibility.
Development plans for tin and tungsten deposits in the Lost River area of Alaska's Seward Peninsula are under way after several years of faltering starts and stops. These activities and possible extraction of gold lying offshore from Nome may ultimately have some effect on these coastal areas. Methods for recovering gold, regardless of the type, would disrupt marine and estuarine environments used by marine birds (Bartonek et al. 1971), and transportation of ores would also increase freighter traffic in the Bering Sea.
Although the timber industry has long been established along the coast from Washington north through southeastern Alaska, timber harvests are rapidly expanding on U.S. Forest Service lands in Alaska. The impact of this industry is principally on terrestrial ecosystems, but certainly log rafting in estuarine areas, disposal of wastes from pulp mills, and freighter traffic transporting wood pulp or logs to Japan and west coast markets contribute to the chronic degradation of marine bird environments. Recent meager studies on the Vancouver Canada goose (Branta canadensis fulva) in southeastern Alaska have pointed out the importance to this species of coastal timber stands for nesting and estuarine environments for brood rearing and wintering. This essentially nonmigratory goose (Hansen 1962) may be particularly vulnerable to logging activities in these areas. Similarly, recent evidence indicates that marbled murrelets may nest in large conifer trees adjacent to the coast, from northwestern California to northern southeastern Alaska (Harris 1971; Savile 1972). If this is true, logging may eventually greatly restrict the breeding of this numerically important inhabitant of northern coastal waters.
Although extraction of hard mineral resources, expansion of the timber industry, and resultant increases in human pressures along North Pacific and Arctic coasts will ultimately affect northern marine bird populations, current and proposed activities of the petroleum industry pose the most immediate threat to marine birds. Chronic degradation of estuarine and marine coastal waters by logging wastes, pulp mill and sewage effluents, and bilge oils is an insidious process, the impacts of which will be difficult, at best, to quantify. Results of a major oil spill or even low-level contamination of marine ecosystems with oil will be more apparent, however. For this reason, and the fact that the industry is expanding rapidly into the North, most of this discussion will be directed at the impacts of oil development on northern marine birds.
Potential sources of adverse environmental degradation affecting these birds resulting from oil and gas exploration, development, and production include: (1) oil discharges into marine waters, both chronic and catastrophic, (2) gravel excavation and dumping in coastal areas, (3) seismic activities, (4) discharge of drilling mud and drill cuttings into marine waters, including toxic heavy metal constituents of drilling mud, (5) disturbance resulting from petrochemical activities, and (6) increased human populations resulting in interference with critical life processes and increased hunting of game species. Each source of environmental change will vary by latitu[Pg 189]dinal and seasonal factors in their effects upon the birds. We consider herein only coastal and ocean floor developments and their anticipated generalized impacts on populations.
Although this is a discussion of "northern" marine birds, it is important to remember that we are considering a diverse avifauna existing in an environmental gradient from temperate to polar regions. In general, the more southerly portions of this marine environment are characterized by a greater diversity of species, more complex food chains, and a resultant greater stability (Dunbar 1968). Arctic marine ecosystems, on the other hand, are characterized by numerical dominance by a few species, relatively simple food chains, and an inherent instability or fragility (Dunbar 1968). According to Dunbar, arctic systems are regulated primarily by temporal oscillations in the physical environment, whereas biological interactions (e.g., competition, predation) are considered more significant in the maintenance of temperate and tropical ecosystems.
Because of their relative instability, arctic ecosystems are more susceptible to alteration by extreme environmental perturbation, either natural or man-imposed (Burns and Morrow 1973). Slow growth and maturation rates of the avian constituents of these ecosystems and resultant long recovery periods (Ashmole 1971) further aggravate this situation.
Regardless of their seasonal availability, these arctic waters constitute some of the most productive areas for seabirds in the western hemisphere (Bartonek et al. 1974). Upwelling, nutrient-rich waters, combined with intense and prolonged incident radiation, result in lush phytoplankton "blooms" that form the foundation of relatively simple but numerically strong plant and animal communities (Ashmole 1971). A relatively small number of avian species have evolved to take advantage of this seasonally available food supply, and the ability to migrate to lower latitudes in winter is a characteristic of most arctic-nesting species. Because summers are short in arctic regions, early arrival and a synchronous breeding schedule are necessary to enable the young to leave the breeding grounds before severe weather conditions prevail (Ashmole 1971). Arrival of these birds generally coincides closely with the earliest availability of nesting habitat and food (Williamson et al. 1966). Migration, molting, and reproduction place tremendous stresses on these birds, and as a result, arctic-nesting species tend to reproduce less often and at older ages than do those of more temperate regions (Ashmole 1971).
In spite of these adaptations, arctic bird species tread a thin line between extinction and survival, and natural disasters take a heavy toll. Bailey and Davenport (1972) reported a massive mortality in a pelagic population of common murres in Bristol Bay, Alaska, during April 1970. They felt that this disaster, resulting in the death of probably 100,000 or more birds, most likely resulted from starvation precipitated by severe weather. Barry (1968) reported a similar loss to starvation of about 100,000 eiders along the Beaufort Sea coast during the extremely cold spring of 1964. Observers along Alaska's Beaufort Sea reported finding eiders and oldsquaws dead and dying from the effects of cold weather in 1970 (Bartonek et al. 1971). It is readily apparent that the tenuous existence into which these birds have evolved leaves them particularly vulnerable to the man-induced stress of developments during the arctic summer.
The most obvious, and perhaps the most disastrous consequence of petrochemical development on northern marine bird populations is that of a major oil spill or a well blowout into marine waters. Although temperate and tropical waters are apparently able to assimilate oil spills and chronic pollution from petroleum and its products (Nelson-Smith 1972), this has not been demonstrated to be true for arctic waters. In fact, studies in the Beaufort Sea have shown that the bacteria that degrade oil do not use hydrocarbons at the ambient temperatures of the Arctic (Glaeser and Vance 1971). Therefore, a large oil spill in the Arctic could persist for many years. As demonstrated by Campbell and Martin (1973), the diffusion and transport mechanisms generated by the pack-ice dynamics of the Beaufort Sea and the slow rate[Pg 190] of oil biodegradation under arctic conditions would combine to diffuse an oil spill over the sea and eventually deposit oil on the ice surface. This, in turn, would lower the natural albedo over a large area and melt the ice in the area of the spill. This pack ice supports an under-ice community which is an important food source for phalaropes, jaegers, gulls, terns, and other seabirds (Watson and Divoky 1972).
As indicated by Nelson-Smith (1972) many investigators have stated that a spot of oil "no bigger than a dollar" on the breast of a bird is enough to bring about death by exposure, at least in the colder seas. It is easy to see the relative vulnerability of already stressed birds in arctic areas to a spill, and because of the concentration of these birds in available open-water areas, possibilities for catastrophic mortalities are evident.
Such disasters already have occurred in north Pacific waters. Dickason (1970) reported an incident in which diesel oil reaching the Alaska coast, probably from the sinking of two Japanese freighters some distance offshore, affected an estimated 90,000 murres. J. G. King, Jr. (cited in Bartonek et al. 1971) estimated that at least 100,000 birds, mostly alcids and waterfowl, died in the vicinity of Kodiak Island during winter 1970 as a result of oil pollution (probably ballast dumped by tankers entering Cook Inlet). It must not be forgotten that chronic pollution in similar areas where oil development and transport activities are taking place probably kills more birds every year than die after a single catastrophic spill. Total annual losses due to oil in the North Sea and North Atlantic, excluding disasters, amount to 150,000 to 450,000 seabirds (Nelson-Smith 1972).
That oil pollution, both chronic and catastrophic, can dramatically affect populations of marine birds has already been demonstrated elsewhere. Uspenskii (1964) reported that more than 30,000 wintering oldsquaws perished from oil pollution near Botland Island in the Baltic and that in later years this species had almost disappeared from Swedish Lapland. Jackass penguins (Spheniscus demersus), found only in South Africa, have suffered losses from pollution caused by oil traffic around the Cape of Good Hope (Stander and Venter 1968). Their total population was estimated at 100,000 in 1960, and in two separate but not isolated incidents 1 to 2% of this number were known to have been killed by oil. Unknown but considerable numbers were uncounted or were lost at sea. Colony nesters, including puffins (Fratercula arctica), razorbills (Alca torda), and murres in the southerly portions of the North Sea are declining rapidly (Nelson-Smith 1972). Puffins, which numbered 100,000 on Annet in the Scilly Isles in 1907, were reduced to 100 birds by 1967; by then, colonies farther east on the Great Britain coast were already extinct. Pollution from the Torrey Canyon disaster alone killed five-sixths of the puffins in the main French colony on the Sept Isles in Brittany and reduced the razorbills to a mere 50 birds, one-ninth of previous numbers (Bourne 1970).
There is every reason to believe that similar reductions in numbers could occur along the tanker route from Valdez to Puget Sound, with localized extirpation of colonies. Even more disastrous, however, would be an inopportune well blowout or other major spill in arctic waters. Massed concentrations of birds, already stressed by severe weather and food shortages, would be extremely vulnerable to this type of situation.
As pointed out by Nelson-Smith (1972), peculiarities of bird behavior determine, to some extent, the vulnerability of a species to oil spills. Auks, murrelets, and puffins (all Alcidae), loons (Gavia spp.), grebes (Podiceps spp.), and diving ducks may be most susceptible to oiling. Auks and loons, because they float low in the water, may more readily become completely covered by oil. Diving species that become flightless during their molt, such as alcids and waterfowl, or which do not fly because of social bonds between adults and flightless young (common murre) and spend most of their lives on the water, would be particularly vulnerable (J. M. Scott, comments by Pacific Seabird Group on U.S. Department of the Interior Draft Environmental Statement 74-90). All divers can easily surface into oil, and their reaction is to dive again, which in a large spill could result in surfacing into more oil. Phalaropes (Phalaropus spp.), which flock to feed in eddies which concentrate drift, may similarly be vulnerable to adverse effects of oil that would also concen[Pg 191]trate in these areas. On the other hand, gulls swimming along the surface are likely to take wing before becoming seriously contaminated.
Nelson-Smith (1972) reported that gannets (Morus bassana), which collected oiled sea-weed for building nest mounds, contaminated themselves and their eggs. Behavioral problems associated with oil spills can be more subtle, however, and Darling's (1938) conclusions that the display of adjacent males contributes to stimulation of the female during courtship in seabirds breeding in massed colonies, is a good example. If Darling was correct, this behavioral characteristic could further impede the recovery of a population of auks, for example, from mortalities resulting from catastrophic losses to spills.
On the basis of this information it is possible to predict that alcids, which make up the bulk of the birds inhabiting the coastal areas during winter (Sanger 1972), would be very susceptible to oil spills from future tanker traffic in these waters. The potential exists, therefore, for a tremendous impact (from a single inopportune oil spill) upon these species and upon the entire ecosystem. Sea ducks too, because of their diving behavior, propensity for flocking, and flightless molt period, would be very vulnerable to oil spills. Wintering flocks of oldsquaws and several species of scoters along the coasts of Alaska, British Columbia, and Washington can be expected to dwindle as North Slope oil begins to be transported to Puget Sound ports.
It is recognized now that seabirds transfer and recycle nutrients and energy between trophic levels and between regions of an ocean (Sowl and Bartonek 1974). Although the significance of this role in the marine ecosystem can only be surmised at present, conservative estimates by Sanger (1972) indicated that birds consume from 0.6 to 1.2 million tons of food and return from 0.12 to 0.24 million tons of feces into the subarctic Pacific region annually. G. A. Sanger's (personal communication) revised estimates of these bird populations indicated that his 1972 estimates should be doubled. Regardless, it appears that the disastrous effects of such a spill would extend beyond the bird populations involved.
By no means would direct losses attributable to contamination by oil be the only threat to marine bird populations as a result of petrochemical expansions into these waters. Some water birds that become contaminated with nonlethal doses of petroleum during the breeding season are not likely to breed (J. M. Scott, comments by Pacific Seabird Group on U.S. Department of the Interior Draft Environmental Statement 74-90). Viability of embryos is greatly reduced when the eggshell becomes smeared with oil from the contaminated plumage of the female (Hartung 1965). Degradation of habitat, particularly to nesting areas and food supplies, will certainly occur, and its most pronounced effects will be felt in the Arctic. Gravel removal for construction of offshore drilling pads, causeways, and onshore production facilities would displace nesting birds and, combined with subsequent discharge of drill cuttings, perhaps have an adverse impact on bottom food organisms. Nesting habitat loss through destruction or the inability of birds to accept disturbance could be substantial, particularly along the Beaufort Sea coasts of Alaska and Canada, where offshore barrier islands and tundra-covered islands provide protection from mammalian predators for nesting by Pacific eiders, Sabine's gulls (Xemia sabini), Arctic terns (Sterna paradisaea), black guillemots (Cepphus grylle), and other species (Arctic Institute of North America 1974). Flaxman Island near the mouth of the Canning River is a tundra island supporting a nesting population of whistling swans (Olor columbianus), and the only nesting colony of the Alaska snow goose (Chen caerulescens) is on Howe Island in the Sagavanirktok River Delta (Arctic Institute of North America 1974).
Although there would probably be little actual nesting habitat loss for cliff-nesting species, human disturbance to colonies during the nesting period, particularly from helicopter and fixed-wing aircraft flybys, could have considerable impact (Sowl and Bartonek 1974). The "living waterfall" effect of thou[Pg 192]sands of seabirds pouring off a rookery is truly spectacular, but each such occurrence during incubation and brooding periods causes a rain of eggs or young to fall from the cliffs (Sowl and Bartonek 1974). Temporarily abandoned chicks and eggs are susceptible to predation by gulls or jaegers.
Even for species nesting on level ground, aircraft overflights close to breeding colonies may cause major losses to young and eggs. Sladen and LeResche (1970) reported that flights by an LH-34 helicopter (at 305 m altitude) over an Adelie penguin (Pygoscelis adeliae) colony caused some egg loss. Landing this aircraft 183 m from the colony caused 50 to 80% of the birds to flee territories, resulting in egg and chick loss. Disturbance caused by visitors walking through or near nesting areas of the South African gannet (Sula capensis) on Bird Island, Lamberts Bay, South Africa, caused desertion of nesting sites (Jarvis and Cram 1971). Studies of disturbance on breeding black brant, Pacific eiders, glaucous gulls (Larus hyperboreus), and arctic terns at Nunaluk Spit and Phillips Bay, Yukon, in July 1972 indicated that human presence was the most critical form of disturbance affecting incubating behavior of these species (LGL Limited 1972a). Disturbance by aircraft—especially helicopters—affected the normal incubating behavior of all species except Pacific eiders. Nesting success of black brant and arctic terns was reduced by this disturbance.
Disturbance can adversely affect molting birds. The process of molting places heavy energy demands on birds, and particularly on waterfowl whose molt results in a flightless period; few areas provide adequate protection from predators necessary during this period. Prime molting areas are scarce along the arctic coast, yet are vital to the welfare of thousands of sea ducks and seabirds. Studies conducted by LGL Limited (1972b) indicated that aircraft traffic over sea duck molting areas altered normal behavior, and therefore had a detrimental effect. Recommendations resulting from these studies were that air traffic be suspended over these areas during the molting season.
For some arctic-nesting waterfowl, premigration staging activity, during which fat reserves to sustain southward migration are stored, is a very important component of the annual cycle (Delacour 1964). Snow geese, breeding mainly in arctic Canada, concentrate in large numbers on staging grounds along the Beaufort Sea coast of eastern Alaska and the Yukon. Because gas compressor stations would be required along the proposed arctic gas pipeline route, experimental studies were conducted in September 1972 to determine the effect of disturbance from sounds generated by compressors (LGL Limited 1972c). These studies indicated that compressor noise was disruptive to staging geese.
Indirect effects on marine bird resources resulting from development activities may ultimately prove to be more detrimental than the aforementioned direct factors. It is conceivable that the impact of these industries, mainly on the benthic and demersal fauna of the coastal areas, could greatly lower the carrying capacity of this habitat for marine birds (Bartonek et al. 1974). Because of the simplified and short arctic food chains and the lack of alternative food sources in these areas, arctic ecosystems would be particularly vulnerable to this type of problem (Burns and Morrow 1973).
Ecological or toxic influences on several food species could result in substantial declines in bird populations. In the Arctic, where temperatures are low, and bacterial and other decompositional activities are consequently slow, spilled oil would persist for many years, with concomitant deleterious effects on the marine organisms of the area (Burns and Morrow 1973). Reduced recruitment of young would no longer balance inevitable or density-independent population mortality (Ashmole 1971). Although indications are that arctic species are the most vulnerable to this type of impact, the lack of knowledge of the feeding niches of most seabirds discourages further evaluation of this potential problem. It is obvious, however, that ecology of arctic birds is least understood, and these species are the most vulnerable to the detrimental effects of man-caused environmental degradation.
Predictability of the impact of resource development on marine birds in northern waters[Pg 193] is limited by our relative ignorance of these birds and their ecology. Just as there exists a latitudinal gradient in the ecological stability of the ecosystems involved, available knowledge of these ecosystems is in inverse relationship to the latitude at which they occur. Arctic bird associations and their fragile environments are least understood but are doubtless the most vulnerable to the detrimental effects of man-caused environmental degradation. Existing technology and support system capabilities of the oil industry are poorly defined for Arctic areas, further compounding this problem (Arctic Institute of North America 1974).
Although activities associated with the extraction of hard minerals and the timber industry will ultimately affect northern seabirds, petrochemical developments pose the most immediate threat to this resource. Exploration and development of many coastal and offshore sedimentary basins with a potential for oil or gas production are proceeding rapidly. Within a few years, oil storage and loading facilities at Valdez, Alaska, and supertankers plying northern waters will probably result in widespread chronic and localized catastrophic contamination of northern marine environments. Experience in other areas has demonstrated that oil spills are a considerable potential threat to these bird populations, directly through widespread mortality and indirectly through effects on the environment. This threat is of such magnitude that entire populations or species could be lost to a single spill if it occurred at the wrong place at the wrong time of year. Because many of these species require 3 to 4 years for maturation and may rear only one or two young per year, recovery time for their populations is great (Ashmole 1971). For these and other reasons, the Council on Environmental Quality (1974) concluded that the Gulf of Alaska appeared more vulnerable to major environmental damage from outer continental shelf oil and gas development than sites off the Atlantic coast.
As Bartonek et al. (1971) pointed out, it would be a national tragedy if the great nongame bird populations along Alaska's coast were decimated during the "Environmental Decade" without even being properly described. Regardless of information amassed in the future and precautionary measures taken during exploitation of arctic petroleum reserves, the potential for disastrous and perhaps irrecoverable losses to northern marine bird species and populations is great. Losses of major magnitude could appreciably alter the productivity of northern marine ecosystems, to the detriment of other renewable resources.
Knowledge of northern marine birds, their environments, and their ecology must be greatly expanded if the consequences of petrochemical exploitation are to be predicted and safeguards established against potential problems. To the extent possible, oil exploration and development activities should be limited to temperate, more stable, marine ecosystems, at least until more northerly areas are better understood. Similarly, these activities must be conducted in such places and at such times that impact on the environment will be minimized. State and federal governments and the petroleum industry are ultimately answerable for this responsibility.
The Nation must be aware of the potential costs of energy independence set forth as a goal of proposed oil and gas leasing of Alaska's outer continental shelf. We must ask ourselves if we are willing to risk extermination of species to reach this goal, or if we can afford the luxury of reducing the biological productivity of these waters.
Arctic Institute of North America. 1974. The Alaskan arctic coast—a background study of available knowledge. 551 pp.
Ashmole, N. P. 1971. Seabird ecology and the marine environment. Pages 223-286 in D. S. Farner and J. R. King, eds. Avian biology. Vol. I. Academic Press, New York.
Bailey, E. P., and G. H. Davenport. 1972. Die-off of common murres on the Alaska Peninsula and Unimak Island. Condor 74(2):215-219.
Barry, T. W. 1968. Observations on natural mortality and native use of eider ducks along the Beaufort Sea coast. Can. Field-Nat. 82(2):140-144.
Bartels, F. B. 1973. Bird survey techniques on Alaska's north coast. M.S. Thesis. Iowa State Univ., Ames. 47 pp.
Bartonek, J. C., J. G. King, and H. K. Nelson. 1971. Problems confronting migratory birds in Alaska. Trans. N. Am. Wildl. Nat. Resour. Conf. 36:345-361.
Bartonek, J. C., R. Elsner, and F. H. Fay. 1974. Mammals and birds. Pages 23-28 in E. J. Kelley and D. W. Hood, eds. Probes: a prospectus on processes and resources of the Bering Sea shelf, 1975-1985. Univ. Alaska, Inst. Mar. Sci. Publ. Inf. Bull. 74-1.
Bourne, W. R. P. 1970. Special review after the "Torrey Canyon" disaster. Ibis 112:120-125.
Burns, J. J., and J. E. Morrow. 1973. The Alaskan Arctic marine mammals and fisheries. Arctic oil and gas problems and possibilities, 5th International Congress. 24 pp.
Campbell, W. J., and S. Martin. 1973. Oil and ice in the Arctic Ocean: possible large-scale interactions. Science 181:56-58.
Council on Environmental Quality. 1974. OCS oil and gas—an environmental assessment. Vol. I. Washington, D.C. 214 pp.
Darling, F. F. 1938. Bird flocks and the breeding cycle; a contribution to the study of avian sociability. Cambridge Univ. Press, N.Y.
Delacour, J. 1964. The waterfowl of the world. Vol. IV. Country Life Ltd., London. 364 pp.
Dickason, O. E. 1970. Center for short-lived phenomena event report. Smithsonian Institution, Cambridge, Massachusetts, 36(1970):926.
Dunbar, M. J. 1968. Ecological development in polar regions. A study in evolution. Prentice-Hall, Inc., Englewood Cliffs, N.J. 119 pp.
Glaeser, J. L., and G. P. Vance. 1971. A study of the behavior of oil spills in the Arctic. U.S. Coast Guard, Office of Research and Development, Washington, D.C. 60 pp.
Hansen, H. A. 1962. Canada geese of coastal Alaska. Trans. N. Am. Wildl. Nat. Resour. Conf. 27:301-320.
Hansen, H. A., and U. C. Nelson. 1957. Brant of the Bering Sea—migration and mortality. Trans. N. Am. Wildl. Nat. Resour. Conf. 22:237-256.
Harris, R. D. 1971. Further evidence of tree nesting in the marbled murrelet. Can. Field-Nat. 85(1):67-68.
Hartung, R. 1965. Some effects of oiling on reproduction of ducks. J. Wildl. Manage. 29(4):872-874.
Isleib, M. E., and B. Kessel. 1973. Birds of the north gulf coast—Prince William Sound region, Alaska. Biol. Pap. Univ. Alaska 14. 149 pp.
Jarvis, M. J. F., and D. L. Cram. 1971. Bird Island, Lamberts Bay, South Africa: an attempt at conservation. Biol. Conserv. 3(4):269-272.
Kinney, P. J., D. K. Button, D. M. Schell, B. R. Robertson, and J. Groves. 1970. Quantitative assessment of oil pollution problems in Alaska's Cook Inlet. Univ. Alaska Inst. Mar. Sci. Rep. R-69-16. 43 pp.
LGL Limited. 1972a. Disturbance studies of breeding black brant, common eiders, glaucous gulls and Arctic terns at Nunaluk Spit and Phillips Bay, Yukon Territory. Report prepared for Northern Engineering Service, Ltd. 50 pp.
LGL Limited. 1972b. Aircraft disturbance to molting sea ducks, Herschel Island, Yukon Territory. Report prepared for Northern Engineering Service, Ltd. 31 pp.
LGL Limited. 1972c. Gas compressor noise simulator disturbance to snow geese, Kamakuk Beach, Yukon Territory. Report prepared for Northern Engineering Service, Ltd.
McKnight, D. E., and B. L. Hiliker. 1970. The impact of oil development on waterfowl populations in Alaska. Proc. Western Assoc. State Game Fish Comm. 50:286-297.
Nelson-Smith, A. 1972. Oil pollution and marine ecology. Paul Elek, Ltd., London. 260 pp.
Sanger, G. A. 1972. Preliminary standing stock and biomass estimates of seabirds on the subarctic Pacific region. Pages 589-611 in A. Y. Takenouti et al., eds. Biological oceanography of the North Pacific. Idemitsu Shoten, Tokyo.
Savile, D. B. O. 1972. Evidence of tree nesting by the marbled murrelet in the Queen Charlotte Islands. Can. Field-Nat. 86(4):389-390.
Sladen, W. J. L., and R. E. LeResche. 1970. New and developing techniques in antarctic ornithology. Pages 585-616 in M. W. Holdgate, ed. Antarctic Ecology. Vol. I. Academic Press.
Sowl, L. W., and J. C. Bartonek. 1974. Seabirds—Alaska's most neglected resource. Trans. N. Am. Wildl. Nat. Resour. Conf. 39:117-126.
Stander, G. H., and J. A. V. Venter. 1968. Oil pollution in South Africa. Pages 251-259 in Proceedings of the International Conference on Oil Pollution of the Sea.
Swartz, L. G. 1966. Sea-cliff birds. Pages 611-678 in N. J. Wilimovsky and J. N. Wolfe, eds. Environment of the Cape Thompson region, Alaska. U.S.A.E.C., Div. Tech. Inf., Oak Ridge, Tennessee.
Thompson, D. Q., and R. A. Person. 1963. The eider pass at Point Barrow, Alaska. J. Wildl. Manage. 27(3):348-356.
Uspenskii, S. M. 1964. Present day problems of nature conservation in the Arctic. Problems of the North 7:171-178.
Watson, G. E., and G. J. Divoky. 1972. Pelagic bird and mammal observations in the eastern Chukchi Sea, early fall 1970. Pages 111-172 in C. I. Merton et al., eds. An ecological survey in the eastern Chukchi Sea. U.S. Coast Guard Oceanogr. Rep. 50.
Williamson, F. S. L., M. C. Thompson, and J. Q. Hines. 1966. Pages 437-480 in N. J. Wilimovsky and J. N. Wolfe, eds. Environment of the Cape Thompson region, Alaska. U.S.A.E.C., Div. Tech. Inf., Oak Ridge, Tennessee.
by
Warren B. King
International Council for Bird Preservation
Smithsonian Institution, Washington, D.C.
R. G. B. Brown
Canadian Wildlife Service
Dartmouth, Nova Scotia, Canada
and
Gerald A. Sanger[52]
U.S. National Marine Fisheries Service
Seattle, Washington
Commercial fishing has been responsible for incidental mortality of seabirds for centuries, but with the advent of offshore salmon gill-net fishing in the North Pacific in 1952 and in the North Atlantic in 1965, the magnitude of this kill has increased, and there is strong indication that populations of some seabirds are being adversely affected. Murres (Uria spp.) are most frequently killed, although several other species are caught in lesser numbers. The seabird resources of several nations are involved in this mortality. Longline fishing and inshore gill-net fishing for salmon and cod also are responsible for mortality of seabirds, although usually not in significant numbers.
That the activities of commercial fishermen have caused mortality of marine birds surprises no one nowadays. Traditions of exploitation of marine birds by fishermen date from previous centuries, and fishing has contributed to the extinction of some species. For example, great auks (Pinguinus impennis) and other birds were used as food by fishermen fishing for Atlantic cod (Gadus morhua) on the Grand Banks of Newfoundland since the beginning of that fishery in the early 16th century (Collins 1884; Lucas 1890). The last great auk died in 1844, but smaller species, such as storm-petrels (Hydrobatidae), greater shearwaters (Puffinus gravis), and black-legged kittiwakes (Rissa tridactyla), were used for food until rather recently (Templeman 1945). This practice has now lapsed, however.
Until the advent of the offshore salmon gill-net fisheries in the North Pacific in 1952 and the North Atlantic in 1965, most seabird mortality in these areas was the result of local fishing close to shore. Several records of such bird mortality have been published. For example, 8,000-10,000 seabirds—presumably mostly alcids—were reported caught annually off Hammerfest in northern Norway (Holgersen 1961). E. Brun (personal communication) reported that the longline fishery off the coast of Norway is having serious consequences on Norwegian populations of murres.
Numbers of alcids are caught in nets set for Atlantic salmon (Salmo salar) around the coasts of Ireland and Scotland (Biddy 1971). A similar situation exists along the west Greenland coast, although it is overshadowed there by the direct exploitation of huge numbers of alcids by hunting. Nonetheless, in 1967 for example, 15,000 alcids were recovered from fish nets in southwestern Greenland, where they were sold as food (Evans and Waterston 1976). The annual salmon catch of the west Greenland inshore fishery has fluctuated between 60 and 1,500 metric tons and has averaged about 1,000 tons. There are no data comparing the relative catch of birds and fish in this fishery.
Atlantic cod follow the spawning capelin (Mallotus villosus) inshore along the east coast of Newfoundland in late June and early July. They are traditionally fished with traps and handlines along this coast, but there has been a recent trend toward using drift nets set on the bottom. Since alcids feed extensively on capelin at this time, many are caught in the cod nets set in areas close to the large colonies off Witless Bay (D. N. Nettleship, personal communication). Additionally, gill nets are set at the surface for salmon in the same area. Common murres (Uria aalge) are most affected, but Atlantic puffins (Fratercula arctica) are also taken.
There are as yet no estimates of the total alcid mortality from this fishery, although the annual catch of birds is believed to be smaller during the present than during the last decade because the fishing effort is reduced, and fishermen in the area now avoid setting nets near alcid concentrations because of the annoyance of having to remove the birds from their nets. The Witless Bay colonies contain over 77,000 pairs of common murres, or 11% of the total eastern North American population, and over 235,000 pairs of Atlantic puffins, or 71% of the North American population outside of Greenland (Brown et al. 1975). The potential danger is obvious.
There are few data on mortality of seabirds from inshore commercial fisheries in the North Pacific. Some mortality of alcids has been shown to take place in Cook Inlet, Alaska, from beach-netting for Pacific salmon (Oncorhynchus spp.) adjacent to seabird rookeries and from drift-netting in the inlet (D. A. Snarski, personal communication), but this mortality has not been quantified.
Bilateral agreements between the United States and Japan, the U.S.S.R. and the Republic of Korea, concerning the use of inshore waters adjacent to some of the Aleutian Islands, Kodiak, Nunivak, St. Matthew, St. George, Kayak, and Forrester Islands permit trawling, longlining, and loading fish and fuel in some of these areas and at certain periods. Although these activities may affect the seabirds of these areas, the extent of the effects are not known (U.S. Department of the Interior, Alaska Planning Group 1974). Murie (1959) indicated, however, that the disappearance of the ancient murrelet (Synthliboramphus antiquus) from Sanak Island, Gulf of Alaska, was probably due as much to fisheries as to the blue fox industry. It has been suggested that the Japanese murrelet (Synthliboramphus wumizusumi) may have declined as the result of fishing activities near breeding sites off the coast of Japan (Bourne 1971).
In 1965, Denmark began an offshore gill-net fishery for Atlantic salmon in the Davis Strait off the coast of west Greenland. The offshore fishery catch increased from 36 metric tons in 1965 to more than 1,200 metric tons in 1969, and then gradually decreased.
The fact that large numbers of seabirds—almost entirely thick-billed murres (Uria lomvia)—were being drowned in the salmon gill nets was brought to the attention of the International Council for Bird Preservation at its 15th World Conference in 1970. The Council's recommendation was submitted to the Danish government and stated: "... having noted that during the 1969 fishing season about 250,000 individuals of Brunnich's guillemot or thick-billed murre (Uria lomvia), a pelagic diving bird, were caught in these drift nets and drowned, which number represents no less than 25 percent of the Greenland population and exceeds its annual reproductive capacity; urges the Danish Government, and the national governments of all other countries involved in this fishing, to take all possible measures to eliminate this very serious problem."
The figures in the recommendation were not supported by research; they appeared instead to have been derived from the observed mortality on an offshore fishery vessel in 1965, which was then related to the salmon catch on that vessel and applied to the total catch of the inshore fishery in 1964 (Anonymous 1969). Studies in 1969 and 1970 by the Fisheries Research Board of Canada finally gave a firm basis for the earlier, though poorly substantiated concern. On the basis of the assumption that the ratio of salmon to murres caught in experimental fishing applied to the commercial fishery, an estimate of an annual mortality of 0.5 million murres (±50%) was made on the basis of a salmon catch of 1,200 metric tons (Tull et al. 1972).
The birds being killed were from colonies in west Greenland, the eastern Canadian Arctic, and possibly east Greenland and Spitzbergen. Coupled with other known causes of mortality (particularly hunting on the Greenland and Newfoundland coasts, an unknown but definitely substantial kill from oil pollution, a calculated mortality of pre-fledging young, and an unknown natural post-fledging mortality) there is no doubt that the estimated annual production of 1.5 million chicks from west Greenland and the Canadian Arctic was less than the estimated total annual mortality (Tull et al. 1972). Thus, it comes as no surprise that recent surveys of murre populations of west Greenland and the Canadian Arctic have revealed massive declines in numbers (Evans and Waterston 1976; D. N. Nettleship, personal communication). It is therefore encouraging news that, as a result of an agreement between the United States and Denmark, the offshore salmon gill-net fishery was terminated at the end of the 1975 season. The inshore fishery remained in operation, however, but was restricted to a total annual salmon catch of 1,100 metric tons.
In the north Pacific Ocean, the Japanese gill-net fisheries for salmon (Oncorhynchus spp.), which have operated since 1952, might be expected to have an even more destructive effect on seabirds, since the annual salmon catch by the three Japanese salmon drift-net fisheries was about one hundred times that in west Greenland in recent years. The first, the mothership fishery, comprising about 369 catcher-boats[53] serviced by 11 mother-ships, operates west of 175°W and generally north of 46°N during the summer. The second, the land-based fishery of about 325 ocean-going vessels, operates west of 175°W and south of 46°N; and the third, the coastal fishery, made up of about 1,380 short-haul vessels, operates off Hokkaido. The relative salmon catches of these three fisheries is on the order of 1:1.34:0.65.
Data collected on U.S. National Marine Fisheries Service research vessels in 1974 (obtained through the cooperation of Francis M. Fukuhara and Richard Bakkala, Northwest Fisheries Center, Seattle, Washington) give, for the first time, an estimate of the magnitude of the incidental seabird kill of the Japanese salmon gill-net fishery. The kill data are available only from the mothership area and from an area east of it to 165°W. The Japanese salmon fishery is restricted to waters west of 175°W by agreement with the United States. Bird kills from the other two areas may be estimated by the relative salmon catch figures for the areas, assuming that seabird densities, species composition, and catch effort are similar.
An estimate of the total kill of seabirds in the mothership area may be made by calculating the bird mortality per length of gill-net set by research vessels, multiplied by the total length of gill nets set by the 369 catcher-boats of the Japanese mothership fishery. About 4,666 km of nets are set and retrieved daily during the approximately 65-day fishing season. The estimated annual mortality in the mothership area is about 75,000 to 250,000 birds. The lower number is based on data from 10 cruises (450 km of nets set) west of 175°W, within the area of the mothership fishery. The higher number is based on data from 20 cruises, including those in the first figure, west of 165°W, and covering the period 18 April to 3 September 1974 (956 km of nets [Pg 198]set), whereas the mothership fishery usually operates between mid-May and late July. Assuming similar seabird densities and catch per unit of effort in the areas of the land-based and coastal fisheries, the estimated annual mortality is between 214,500 and 715,000 birds. Since 1952, as many as 4.7 million birds may have been killed by the Japanese salmon gill-net fishery. It must be stressed that seabird densities and catch per unit of effort are not known to be similar for the areas in question; consequently the projection of bird kill figures from one area to all three is speculative.
In the mothership area and adjacent seas to the east, in addition to murres (48% of birds killed), significant numbers of shearwaters, Puffinus spp. (27%); puffins (9%); and fulmars, Fulmarus glacialis (5%) are killed, as are lesser numbers of small alcids, albatrosses (Diomedea spp.), and storm-petrels. The murres and puffins taken in the mothership area are of U.S. and U.S.S.R. origin, and the shearwaters come from New Zealand, Australia, and Chile. In the coastal fishery area, Japanese and U.S.S.R. alcids are taken. Available knowledge of the populations of the species making up the bulk of the kill, which has been taking place for 20 years, is insufficient to suggest whether their annual reproduction can tolerate such losses. Prohibition of fishing within 160 km of North Pacific seabird breeding islands would help to decrease losses of alcids of U.S. origin, but would not help the shearwaters from the southern hemisphere.
Comparison of statistics of the salmon fisheries and associated bird kills from the North Atlantic and the North Pacific shows that the North Atlantic salmon fishery is concentrated in a relatively small area which is also along a major migration pathway of murres. Virtually all seabird mortality is confined to one species. Enough information is at hand to indicate that this cause of mortality, in conjunction with others known to be significant, is causing a drastic decline in the thick-billed murre population.
In the North Pacific, on the other hand, the fishery is more widely dispersed and the ratio of seabirds to salmon caught is much lower. Furthermore, several species are subject to mortality. No information is available to indicate whether alcid populations (which make up two-thirds of the kill) are stable or decreasing. The shearwaters, primarily sooty (Puffinus griseus) and slender-billed (P. tenuirostris), appear to be able to sustain not only these losses but also a sizable harvest of birds of the year (the so-called muttonbirds) on their New Zealand and Australian breeding grounds. Thus, although the latest estimates of the total standing stock of seabirds in the North Pacific in summer may be as high as 100 million (Sanger and King, this volume), and thus only about 1 of every 200 birds in the North Pacific region may be caught, the fact that a few species, particularly murres, are selectively caught raises questions about the impact of this fishery on populations of these species.
The U.S.-Japan Migratory Bird Convention of 1973 specifically protects all of the species thought to be subject to gill-net mortality in the Pacific. Thus, the Japanese salmon fleet apparently operates in constant violation of this convention.
A recent analysis of recoveries of Laysan albatrosses (Diomedea immutabilis) and black-footed albatrosses (D. nigripes) banded on the northwest Hawaiian chain from 1937 to 1969 showed that of a sample of 532 recovered birds, 57.4% of the Laysan species and 49.5% of the black-footed species were caught on fishhooks or in nets, and the means of recovery of many additional birds was thought to have been the same (Robbins and Rice 1974). It is likely that the large majority are taken on Japanese and U.S.S.R. longline tuna fishing gear. Although this cause of mortality is insignificant in terms of the total population of either species (only 0.2% of banded Laysan and 0.8% of banded black-footed albatrosses have been recovered by any means away from their breeding grounds), these species are protected by the U.S.-Japan Migratory Bird Convention. Furthermore, the possibility exists that individuals of the endangered short-tailed albatross (Diomedea albatrus) might be killed in this manner.
Capelin are important food fish for many seabirds in the northwest Atlantic, and the development and expansion of this fishery off eastern Canada must be carefully monitored. In theory, the capelin fishery ought not to seriously affect the birds because it is designed to exploit a surplus of capelin artificially created by the overfishing of Atlantic cod, the capelin's most important predator. It is hoped that there is no prospect of the overfishing that may have contributed to the recent drastic decline of the Peruvian anchovy (Engraulis ringens) and the seabird species dependent on it (Paulik 1971). However, the relative influence of overfishing and "El Niño" oceanographic conditions on the decline remains unclear. North Atlantic seabirds are, in any case, more versatile in their feeding habits (Belopol'skii 1961). But, the threat may be a subtle one. The important point to the seabirds may well be not merely the survival of a reasonably large capelin stock, but the presence of capelin schools in high densities in certain areas or at certain seasons. Lower densities might, for example, reduce the foraging efficiency of breeding birds, and hence their nesting success. The very large common murre colony on Funk Island, Newfoundland (500,000 pairs: Tuck 1960), might be particularly vulnerable. It lies close to an area where capelin are especially abundant and one which is already being exploited by the developing fishery.
Anonymous. 1969. Seabird slaughter. Sports Fish. Inst. Bull. 203:5.
Belopol'skii, L. O. 1961. Ecology of sea colony birds of the Barents Sea. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem. 346 pp.
Biddy, C. J. 1971. Auks drowned by fishnets. Seabird Rep. No. 2.
Bourne, W. R. P. 1971. General threats to seabirds. ICBP [Int. Counc. Bird Preservation] Bull. 11:200-219.
Brown, R. G. B., D. N. Nettleship, P. Germain, C. E. Tull, and T. Davis. 1975. Atlas of eastern Canadian seabirds. Canadian Wildlife Service, Ottawa. 220 pp.
Collins, J. W. 1884. Notes on the habits and methods of capture of various species of seabirds that occur on the fishing banks off the east coast of North America and which are used as bait for catching codfish by New England fishermen. U.S. Comm. Fish Fish. Rep. 1882:311-335.
Evans, P., and G. Waterston. 1976. The decline of the thick-billed murre in Greenland. Polar Rec. 18:283-286.
Holgersen, H. 1961. On the movements of Norwegian Uria aalge. (In Norwegian, English summary.) Sterna 4:229-240.
Lucas, F. A. 1890. Expedition to the Funk Island, with observations on the history and anatomy of the Great Auk. Rep. U.S. Natl. Mus., 1887-1888:493-529.
Murie, O. J. 1959. Fauna of the Aleutian Islands and Alaska Peninsula. U.S. Fish Wildl. Serv., N. Am. Fauna 61. 406 pp.
Paulik, A. J. 1971. Anchovies, birds, and fishermen in the Peru Current. Pages 156-185 in W. W. Murdoch, ed. Environmental Resources and Society. Sinauer Associates, Inc., Stamford, Conn.
Robbins, C. S., and D. W. Rice. 1974. Recoveries of banded Laysan albatrosses (Diomedea immutabilis) and black-footed albatrosses (D. nigripes). Pages 232-271 in W. B. King, ed. Pelagic studies of seabirds in the Central and Eastern Pacific Ocean. Smithson. Contrib. Zool. 158.
Templeman, W. 1945. Observations on some Newfoundland seabirds. Can. Field-Nat. 59:136-147.
Tuck, L. M. 1960. The murres. Canadian Wildlife Service, Ottawa. 260 pp.
Tull, C. E., P. Germain, and A. W. May. 1972. Mortality of thick-billed murres in the west Greenland salmon fishery. Nature (Lond.) 237 (5349):42-44.
U.S. Department of the Interior, Alaska Planning Group. 1974. Final environmental impact statement, proposed Alaska Coastal National Wildlife Refuges. 678 pp.
[52] Present address: U.S. Fish and Wildlife Service, Office of Biological Services—Coastal Ecosystems. 1011 E. Tudor Road, Anchorage, Alaska 99503.
by
Richard R. Straty and Richard E. Haight
National Marine Fisheries Service
Auke Bay Fisheries Laboratory
Auke Bay, Alaska 99821
The high primary and secondary productivity of the eastern Bering Sea makes it one of the greatest producers of commercial fish and largest congregating areas of marine birds in the world. The fish and birds are so interrelated that fluctuations in the abundance of one may well be responsible for changes in the abundance of the other. The seasonal and annual variation in the impact of birds on fish is a function of the life history, food habits, growth rate, and final size of the fish species of concern and of the distribution, abundance, and feeding habits of bird populations—plus the effects of the environment on these factors. Stages in the life history of some of the important commercial fish and shellfish of the Bering Sea directly or indirectly influenced by marine birds are identified.
The eastern Bering Sea is one of the world's richest fish-producing areas and is also one of the world's major congregating areas for marine birds. The large extent of the continental shelf and the climatic and oceanographic characteristics of the eastern Bering Sea combine to make this region extremely productive biologically. The distribution and abundance of plankton, benthos, and fish determine the distribution, time, and character of the migration of marine birds in the eastern Bering Sea (Shuntov 1961). Several studies have illustrated the close relation between marine birds and the biological properties of surface waters (Tuck 1960; Bourne 1963; Solomensen 1965). Spatial and temporal variations in the abundance of the fish families Clupeidae (herring), Gadidae (codfish), Osmeridae (capelin), and Ammodytidae (sand lance) are thought to be major determinants of the breeding seasons, breeding places, and movements of boreal seabirds (Ashmole 1971). The timing of breeding among larids and alcids is related to the seasonal changes in the surface waters inhabited by Ammodytidae and Clupeidae in the North Sea (Pearson 1968).
The eastern Bering Sea contains members of these and other fish families that are extensively exploited by man; the fish are also important as forage for other species of commercial fish, marine mammals, and marine birds. During some part of their life cycles, all fish species feed on plankton, nekton, benthos, or other fishes.
The incidental use or dependence of marine birds on commercial fish and the items on which the fish feed account for the major interaction between man and these two groups of animals.
In this paper, we consider how marine birds and fish interact. Although some of what we present is only speculative, we identify certain areas that have received little or no scientific study, areas in which further research is needed for a better understanding of the role of commercial fish in the ecology and dynamics of marine birds in the eastern Bering Sea.
Most of the fishing in the eastern Bering Sea is done by Japan and the Soviet Union. Japan resumed fishing in the Bering Sea in 1953 (7 years after World War II), the Soviet Union started fishing in the region in 1959, and since the early 1960's both nations have accelerated their exploitation of Bering Sea fish stocks (Chitwood 1969).
Species of major concern to Japan and the Soviet Union include fish—walleye pollock (Theragra chalcogramma), yellowfin sole (Limanda aspera), Pacific cod (Gadus macrocephalus), Pacific ocean perch (Sebastes alutus), Pacific herring (Clupea harengus pallasi), and sablefish (Anoplopoma fimbria)—and snow crabs (Chionoecetes spp.). The distribution of the principal species being harvested in Bristol Bay and the eastern Bering Sea are shown in Figs. 1, 2, and 3. The weight of each of the major species in the total catches made by foreign and domestic fishermen in 1973 is shown in Table 1. In 1972, the catch of commercial finfish in the eastern Bering Sea alone amounted to 5% of the total world catch of marine fishes (H. Larkins, personal communication).
Most species of commercial fish in the Bering Sea are in a state of decline or in a depressed condition from overexploitation (Table 1). This is indicated by a reduction in the catch per unit of effort and in the mean size of fish in the commercial catch (H. Larkins, personal communication). The notable exception is the king crab (Paralithodes sp.), which has increased in abundance in recent years as a result of reduced foreign fishing.
| Species | Catch (metric tons) |
|---|---|
| Fish | |
| Pollock | 1,500,000 |
| Flatfish | 125,000 |
| Pacific cod | 45,000 |
| Herring | 35,033 |
| Salmon | 11,785 |
| Sablefish | 7,000 |
| Pacific halibut | 222 |
| Other | 40,000 |
| Shellfish | |
| King crabs | 26,798 |
| Snow crabs | 17,694 |
| Shrimp | Minor |
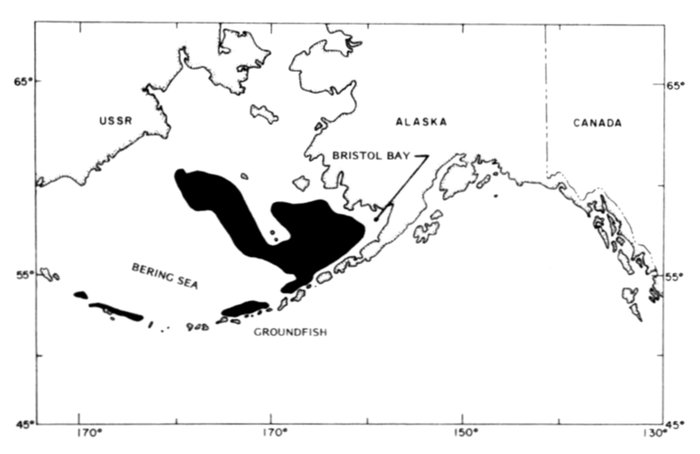
Fig. 1. Areas of major concentrations of ground fish (Pacific pollock, halibut, yellowfin sole, rock sole, flathead sole, Pacific ocean perch, and Pacific cod) in Bristol Bay and the Bering Sea.
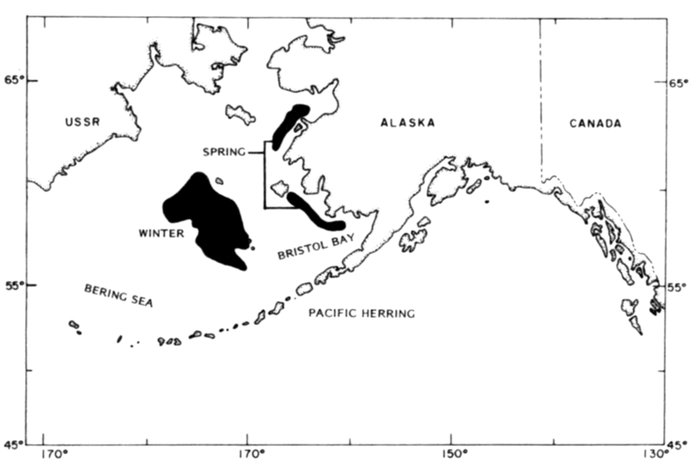
Fig. 2. Areas of major winter and spring concentrations of Pacific herring in Bristol Bay and the Bering Sea.
Fig. 3. Areas of major concentrations of king and snow crab in Bristol Bay and the Bering Sea.
The obvious ways in which marine birds and fish of commercial importance interact in the eastern Bering Sea are illustrated by the simplified food web diagram in Fig. 4. The major animal groups and species included in two of the categories in this figure—secondary producers (invertebrate forage) and intermediate carnivores (commercial and forage marine fish and shellfish)—are as follows:
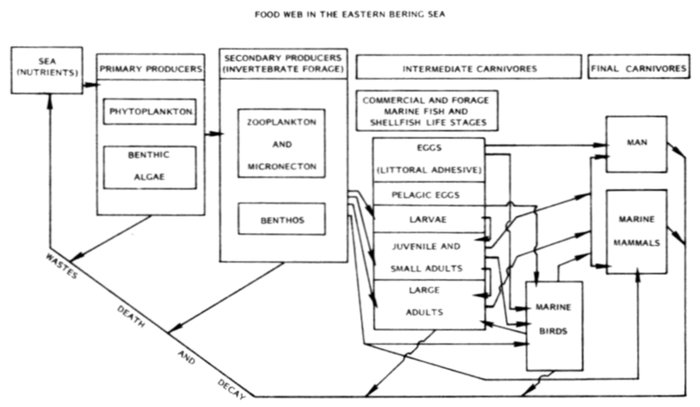
Fig. 4. Food web in the eastern Bering Sea, showing routes of interaction between marine birds and the various life history stages of commercial fish and shellfish.
In our discussion, we mainly consider predation by birds on commercial fish and competition between birds and commercial fish for food. The extent of these interactions determines the potential for birds and fish to influence each other's abundance. The extent of the interactions also determines the impact of man's commercial harvest of fish on the abundance of birds or of the bird's harvest on the abundance of fish.
The extent of the interaction between marine birds and commercial fish depends on the abundance, distribution, feeding habits, and life history of the fish species of concern. We have limited our discussion to examples of the major commercial pelagic and demersal fish and shellfish of the eastern Bering Sea. We also use as examples those species of marine birds whose abundance in the eastern Bering Sea and feeding habits give them the greatest potential for influence on, or being influenced by, fish abundance.
Information on the general abundance and distribution of the most important marine birds in the eastern Bering Sea in the summer and winter is scattered among many published and unpublished reports: Shuntov (1961, 1966), Sanger (1972), Bartonek and Gibson (1972), and Ogi and Tsujita (1973); and surveys by D. T. Montgomery and W. E. Oien ("Bristol Bay waterbird survey, 1972," unpublished report of the U.S. Bureau of Sport Fisheries and Wildlife, Alaska area) and by J. G. King and D. E. McKnight (1969, "A waterbird survey in Bristol Bay and proposals for future studies," unpublished report of the U.S. Bureau of Sport Fisheries and Wildlife and the Alaska Department of Fish and Game, Juneau, Alaska).
In summer, the most abundant birds appear to be the procellariids, mainly the slender-billed shearwater (Puffinus tenuirostris) and Pacific fulmar (Fulmarus glacialis); the alcids, mainly the common murre (Uria aalge), thick-billed murre (U. lomvia), tufted puffin (Lunda cirrhata), horned puffin (Fratercula corniculata), and the ancient murrelet (Synthliboramphus antiquus); and the larids, mainly the glaucous-winged gull (Larus glaucescens) and the black-legged kittiwake (Rissa tridactyla).
In winter, the alcids and larids appear to be the most abundant groups, the procellariids having been reduced by the departure of the slender-billed shearwaters for breeding grounds in the southern hemisphere. The selection of the types of food to be consumed by these marine birds is a function of their morphological and physiological adaptations and of the resultant feeding behavior. Ashmole (1971) classified the feeding behavior of various genera of marine birds and the relative importance of the kinds of food eaten by each group; this information for some of the Bering Sea bird species occurring in the genera listed by Ashmole (1971) is summarized in Fig. 5.
Fish and invertebrates are evidently of moderate to major importance in the diet of these marine birds (Fig. 5). The extent to which a given fish species is fed upon by or is in competition with marine birds for food is determined by the life history of the fish. Most pelagic and some demersal fish and shellfish are more subject to predation by pursuit diving birds than by birds restricted to the near-surface waters. Invertebrates appear to be equal to or more important than fish in the diets of birds feeding in near-surface waters (Fig. 5).
The literature contains numerous accounts of marine birds feeding on marine fish and shellfish of commercial importance. Some studies quantify the impact of some bird species on certain species of commercial fish (Outram 1958; Shaefer 1970; Wiens and Scott 1976) and shellfish (Glude 1967). Other studies have shown that in some regions the value of guano produced by birds may exceed the value of the commercial fish they consume (Jarvis 1970). Some fish of worldwide commercial importance that are important in the diets of marine birds are listed in Table 2.
| Fish | Shearwaters | Murres | Puffins | Fulmars | Gulls |
|---|---|---|---|---|---|
| Anchovy | X | — | — | — | — |
| Sardines | X | — | — | — | — |
| Herring | X | X | X | X | X |
| Sprat | X | — | — | — | — |
| Pilchard | X | — | — | — | — |
| Capelin | — | X | X | — | X |
| Salmon | — | X | — | — | — |
| Mackerel | — | X | — | — | — |
| Pollock | — | X | — | X | — |
| Haddock | — | X | — | — | — |
| Cod | — | X | — | — | — |
The significance of bird predation on pelagic or demersal fish and shellfish (Fig. 5) depends on the feeding behavior of the birds and on the life history of the fish (e.g., distribution, abundance, growth, and adult size). Pursuit diving birds, such as murres and puffins, can consume fish at greater depths than can birds that feed near the surface, such as shearwaters, kittiwakes, fulmars, and gulls.
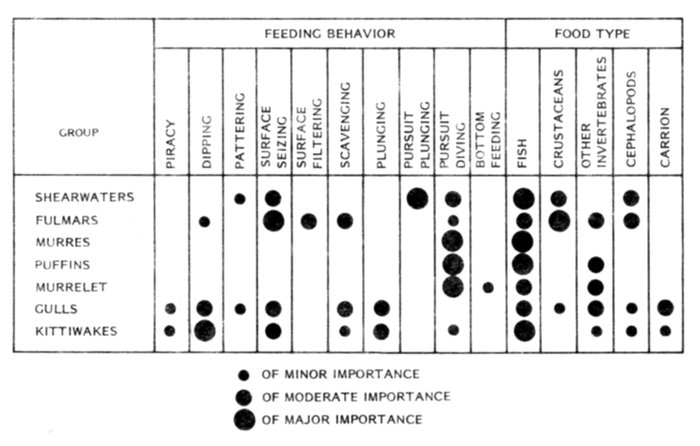
Fig. 5. Feeding behavior and relative importance of food of some groups of marine birds that occur in the eastern Bering Sea.
Fish that are pelagic during part of their lives, such as salmon and herring, and forage fish like smelt, capelin, and sand lance, are vulnerable to greater predation by a wider variety of marine birds than are bottom-dwelling demersal fish, such as pollock, cod, sole, ocean perch, and halibut, as well as king and snow crabs. Some species that live on the bottom as adults have pelagic stages during which they are vulnerable to predation by marine birds. Juveniles of some demersal species (pollock, cod, halibut, some species of sole, and king crabs) are sometimes found in shallow water where they might be subject to predation by birds.
The early life histories of the commercially important demersal fish of the eastern Bering Sea are quite different (Table 3). For example, the eggs and larvae of Pacific halibut (Hippoglossus stenolepis) generally occur at depths greater than 100 m (Hart 1973), whereas those of pollock and yellowfin sole are found at or near the surface (Musienko 1963, 1970). The eggs of Pacific cod are demersal, but the larvae are oceanic (pelagic) and occur from 25-150 m (Mukhacheva and Zviagina 1960).
In their juvenile stages, many demersal fish frequent the near-surface waters (Table 3), where they become vulnerable to predation by piscivorous marine birds. Juvenile pollock, for example, form into small schools that usually move about close to the bottom but sometimes move into areas as shallow as 3 m. Juvenile Pacific cod prefer the warmer water close to shore and may be found within 10 m of the surface (Moiseev 1953). The young of many species of flatfish, such as yellowfin sole, rock sole (Lepidopsetta bilineata), and flathead sole (Hippoglosoides elassodon), remain for a time in shallow warm water after assuming a demersal existence. Yellowfin sole 2-2.5 cm in total length may be found in abundance in areas as shallow as 5 m (Fadeev 1965; Moiseev 1953).
| Fecundity | Spawning season | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Length of female (cm)[54] | Mean no. of eggs | Total period | Peak period | Life stage | Total length (cm)[56] |
Depth from surface (m) |
Seasonal period of pelagic life | Duration of life stages (days) |
Source of data |
| Walleye pollock (Theragra chalcogramma Pallas) | |||||||||
| 31-35 | 95,700 | Feb.- June | April-May | Egg | 0.1-0.2 | 0-10 | Feb.-June | 12 at 6-7°C | Yusa 1954; Tanino et al. 1959; Kobayashi 1963; Musienko 1963, 1970; Serobaba 1968; Hart 1973[55] |
| 20.5 at 3.4°C[57] | |||||||||
| Larval | 0.4-0.9 | 10-25 | March-? | > 25 at 6-7°C | |||||
| 46-50 | 324,400 | — | — | Larval | 0.9-? | 25-? | ?-Sept. | ? | |
| — | — | — | — | Juvenile | 2.2-4.1 | 0-?[58] | Summer | — | |
| — | — | — | — | Juvenile | 6.0-30.0 | 4-37 | Summer | — | |
| — | — | — | — | Adult | 30.0-70.0 | 0-386 | — | — | |
| Pacific cod (Gadus macrocephalus Tilesius) | Egg | 0.1-0.11 | 100-250 | Demersal | 8-9 at 11°C | Moiseev 1953; Mukhacheva and Zviagina 1960; Musienko 1970; Hart 1973[55] | |||
| 60 | 1,200,000 | Jan.-March | ? | 17 at 5°C | |||||
| 28 at 2°C | |||||||||
| Larval | 0.5-3.2 | 25-150 | Feb.-Aug. | ? | |||||
| 78 | 3,300,000 | — | — | Juvenile | ? | 10-? | Summer | — | |
| — | — | — | — | Adult | 40.0-99.0 | 0-900 | — | — | |
| Pacific herring (Clupea harengus pallasi Valenciennes) | Egg | 0.1-0.2 | 0-12 | Demersal | 10-20[57] | Stevenson 1962; Musienko 1970; Rumyantsev and Darda 1970; Reid 1972; Hart 1973[55] | |||
| 20.5-22.0 | 26,600 | May-June | Varies | Larval | 0.9 | 0.5-8 | May-June | 42-56 | |
| 28.0-31.0 | 77,800 | — | — | Larval | 1.3 | 0.5-8 | June-July | ||
| — | — | — | — | Larval | 2.5 | 1-6 | July-Aug. | ||
| — | — | — | — | Juvenile | 2.5-20.5 | 0-? | March-Nov. | — | |
| — | — | — | — | Adult | 20.5-31.0 | 0-140 | March-Nov. | — | |
| Capelin (Mallotus villosus (Muller)) | Egg | 0.1 | <20 | Demersal | 14-? | Clemens and Wilby 1961; Musienko 1970; Hart 1973 | |||
| ? | 3,000 | June-July | ? | Larval | 0.5-? | ? | June-? | ? | |
| ? | 6,000 | — | — | Juvenile | ? | ? | March-Nov.(est.) | — | |
| 10.3 | 6,670 | — | — | ||||||
| ? | 60,000 | — | — | Adult | ? | 0-? | March-Nov. | —[Pg 207] | |
| Pacific sand lance (Ammodytes hexapterus Pallas) | |||||||||
| — | ? | June- Aug. | [59] | Egg | ? | ? | Demersal | ? | Musienko 1963, 1970; Kashkina 1970; Hart 1973 |
| — | — | — | — | Larval | 0.7-3.4 | 0-? | June-Sept. | ? | |
| — | — | — | — | Juvenile | 3.6-9.6 | 0-? | ? | — | |
| — | — | — | — | Adult | 26 | 0-? | ? | — | |
| Pacific ocean perch (Sebastes alutus (Gilbert)) | |||||||||
| 26 | 10,000 | March-May | ? | Egg[61] | — | — | — | — | Paraketsov 1963; Lisovenko 1965; Lyubimova 1965; Kashkina 1970[60] |
| 44 | 180,000 | — | — | Larval[62] | 0.6-? | [62] | March-Aug. | ? | |
| — | — | — | — | Juvenile | 6.2 | 37-128 | — | — | |
| — | — | — | — | Juvenile | 10.4 | 37-154 | — | — | |
| — | — | — | — | Juvenile | 14.7-21.3 | 37-230 | — | — | |
| — | — | — | — | Adult | 21.3-51.0 | 37-420 | — | — | |
| Pacific halibut (Hippoglossus stenolepis Schmidt) | |||||||||
| 75 | 101,723 | Oct.-March | ? | Egg | 0.3-0.4 | 40-935 | Oct.-March | 48 at ? | Novikov 1964; Hart 1973 |
| 135 | 2,800,837 | — | — | Larval | 0.8-1.5 | >200 | Nov.-May | 70-98 | |
| — | — | — | — | Larval | 1.5-2.9 | <100 | May-Sept. | ||
| — | — | — | — | Juvenile | 3.4-4.2 | 7-43 | — | — | |
| — | — | — | — | Juvenile | 19-25 | 7-45 | — | — | |
| Yellowfin sole (Limanda aspera (Pallas)) | |||||||||
| 26.1-28.0 | 1,295,000 | June-Aug. | July | Egg | 0.07-0.09 | >0 | June-Aug. | 9.4 at 13.1°C[57] | Moiseev 1953; Pertseva-Ostraumova 1954; Musienko 1963; Fadeev 1965; Kashkina 1965a, 1965b[55] |
| 40.1-42.0 | 3,319,500 | — | — | Larval | 0.2-1.2 | >0 | July-Oct. | ? | |
| — | — | — | — | Juvenile | 2.1-2.5 | 5-15 | — | — | |
| King crabs (Paralithodes camtschatica (Tilesius)) | |||||||||
| 9.4 | 55,408 | April-June | ? | Egg | — | 100-200[63] | — | ? | Kurata 1960, 1964; Korolev 1964; Rodin 1970 |
| 17.1 | 444,651 | — | — | Zoeal | 0.55-0.65 | ? | April-July | 33 at 7-10°C | |
| — | — | — | — | Zoeal | 23 at 12.3-12.5°C | ||||
| — | — | — | — | Glaucothoeal | 0.38x0.18 | ? | May-? | ? | |
| — | — | — | — | Juvenile | ? | 1-? | — | ? | |
| Snow crabs (Chionoecetes bairdi Rathbun) | |||||||||
| ? | ? | ?[65] | ? | Egg | — | 100[63] | — | ? | Haynes 1973[55] Jewett and Haight[64] |
| — | — | — | — | Prezoeal | 0.22-0.28 | ? | May-? | 1-2 at 2.5°C | |
| — | — | — | — | 1st zoeal | 0.50-0.56 | ? | Summer | ? | |
| — | — | — | — | 2d zoeal | ? | 0-10 | Summer | ? | |
| — | — | — | — | Megalopal | 0.30-0.35x | ? | Summer | — | |
| 0.18-0.21 | |||||||||
| — | — | — | — | Juvenile | 0.44-0.48x | ? | — | — | |
| 0.32-0.35[Pg 208] | |||||||||
| Snow crabs (Chionoecetesopilio (O. Fabricius)) | Egg | ? | 93[60] | — | ? | Ito 1968; Kon 1970; Haynes 1973; Motoh 1973; Jewett and Haight[64] | |||
| ? | ? | ?[65] | ? | Prezoeal | — | ? | May-? | 63-66 at 11-13°C | |
| — | — | — | — | 1st zoeal | 0.48-0.54 | ? | Summer | ||
| — | — | — | — | 2d zoeal | 0.62-0.71 | ? | Summer | ||
| — | — | — | — | Megalopal | 0.29-0.33 | ? | Summer | ||
| — | — | — | — | 0.19 | |||||
| — | — | — | — | Juvenile | 4.4-4.8x | ? | — | — | |
| — | — | — | — | 3.2-3.5 | |||||
The commercially important king and snow crabs of the eastern Bering Sea also have larval stages that are pelagic (Table 3). Zoeae and megalopa of snow crabs are found near the surface where they are vulnerable to plankton-feeding marine birds. The eggs of king crabs are attached to the abdomen of the female, but after hatching, the larvae become pelagic and occur near the surface. They are planktonic through five larval stages before settling to the bottom to take up demersal residence (Kurata 1960, 1964). These larvae attain a length of 5.5-6.5 mm and spend 33 days or more in the plankton (Kurata 1960). Even after the young king crabs have settled to the bottom, they may still frequent water shallow enough to make them vulnerable to predation by some marine birds. Juvenile king crabs 1 and 2 years of age appear to prefer shallower water than do older crabs. In southeastern Alaska, during the spring, small juvenile crabs have been observed in pods at depths as little as 1 m below the low tide level.
The available life stages of king and snow crabs and commercially important demersal fish (Table 3) represent an enormous food supply for other fishes and marine birds. Predation by marine birds on pelagic eggs and on the larval and juvenile stages of demersal fish is not well documented, probably because the rapid digestion rate of birds makes species identification of these stages difficult. Investigators must often depend on the presence of the hard parts of fish (such as scales and otoliths) in the stomachs of birds to identify the species eaten. Because these hard parts have not yet formed in the larvae and most juveniles, predation by marine birds on older fish is more apparent on examination of stomach contents. Full understanding of predation by marine birds on demersal fish and shellfish requires additional data on when and where the egg, larval, and juvenile stages are present.
Many fish, such as herring, capelin, smelt, and salmon, are pelagic for part of their lives, particularly during the spring and summer feeding periods. The extent of predation by marine birds on these species depends primarily on the location of their spawning grounds, their growth rates, and the size of the adults. The spawning location determines the extent of predation on eggs, whereas growth rate and adult size determine during how much of its lifetime a given fish species is vulnerable to the wide variety of marine birds.
Herring spawn in intertidal and subtidal zones and spend most of their post-larval lives in bays or estuaries near the coast. They deposit their adhesive eggs primarily on vegetation, and the eggs are particularly vulnerable to predation by a wide variety of marine and terrestrial birds. Outram (1958) estimated that gulls alone accounted for 39% of the egg loss on the spawning grounds at Vancouver Island, British Columbia. When herring larvae hatch, they are between 0.7 and 0.8 cm long; when they metamorphose about 6-8 weeks later, they are between 2.6 and 3.5 cm long. Thereafter, juvenile herring grow rapidly and reach a length of about 7-10 cm before winter. Although herring as old as 13 years and up to 38 cm long have been reported in Alaska, they seldom exceed 30 cm and 11 years of age (Rounsefell 1929). During spring and summer, herring are commonly within 10 m of the surface, but in winter, they are in water 100-140 m deep. Although herring are particularly vulnerable to predation in spring and summer, they are available to marine birds during most of their life.
The life history of capelin is somewhat different than that of herring—they live in the open sea near the surface and throughout the water column most of their lives. Sometime in June or early July, they migrate in large schools toward shore to spawn (Musienko 1970). In British Columbia, capelin bury their eggs in coarse sand and gravel in the intertidal and subtidal zones. The larvae are 0.5-0.7 cm long at hatching and are carried by currents to the open sea where they develop in the plankton. Capelin attain an age of 5 years and a maximum length of about 22 cm; their small size makes them vulnerable to predation by marine birds most of their lives, and they are an important pelagic food fish for other commercial fish in the Bering Sea.
The sand lance reaches a maximum size of 20-26 cm and is vulnerable to bird predation during most of its life. Little information is available on the maximum age attained by[Pg 210] this species in the Bering Sea, but because of its size, it is an important forage fish for many commercial fish species.
The five species of Pacific salmon of the eastern Bering Sea spawn in fresh water, unlike herring, capelin, and sand lance. Their eggs are not vulnerable to extensive predation by marine birds; gulls take mainly salmon eggs which have been dislodged from the gravel and are drifting or being rolled along the stream bottom by the current (Moyle 1966). After a few months to several years in fresh water, the juvenile salmon (5-14 cm long) enter the Bering Sea during late spring or early summer and migrate through these waters to feeding grounds, primarily in the north Pacific Ocean. At maturity, the survivors return to their home streams and rivers to spawn. It is during the seaward migratory phase of their life cycle that salmon are most vulnerable to predation by marine birds.
The sockeye salmon (Oncorhynchus nerka) is the most abundant and valuable species harvested by American fishermen in the waters adjacent to the Bering Sea and, as a result, the one that has been most extensively studied during early marine life. Juvenile sockeye salmon are between 8 and 14 cm long when they enter the Bering Sea between late May and early July. They are most abundant in the upper 1 m of water at night and the upper 2 m during the day (Straty 1974)—well within the regime that can be exploited by many species of marine birds.
The numbers of juvenile sockeye salmon migrating seaward from the Bristol Bay region of the Bering Sea in a single year has ranged between 46.3 and 370.4 million (H. Jaenicke, personal communication). This is equivalent to between 409 and 3,267 metric tons (on the basis of the mean weight of the juveniles when they enter the Bering Sea). These large numbers of juvenile sockeye salmon, plus juvenile chinook salmon (O. tshawytscha), coho salmon (O. kisutch), chum salmon (O. keta), and pink salmon (O. gorbuscha) from all other rivers entering the Bering Sea, represent a considerable input of energy from fresh water in the form of prime forage fish for other fishes, marine birds, and mammals. Young salmon enter the Bering Sea each year over a period of only 6 to 8 weeks and may follow rather discrete coastal migration routes through the Bering Sea (Fig. 6), with the result that predators have access to an abundant but transient food supply.
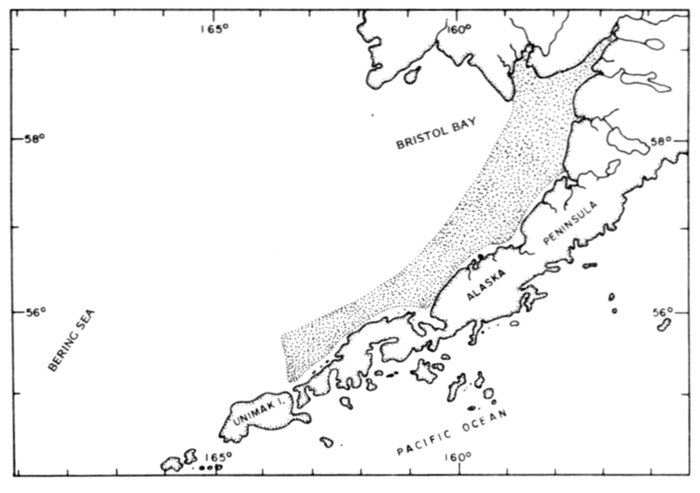
Fig. 6. Distribution of juvenile sockeye salmon in Bristol Bay and the eastern Bering Sea (adapted from Straty 1974).
The only published account of predation by marine birds on juvenile salmon in the Bering Sea is that of Ogi and Tsujita (1973). They found juvenile sockeye salmon in the stomachs of murres captured in gill nets in the eastern Bering Sea. The predation did not appear extensive, but most of the birds were captured outside or on the fringes of the main seaward migration route of the salmon. The foods of marine birds should be studied in conjunction with studies of the migrations of juvenile salmon.
Incubation time for fish eggs, the length of the pelagic larval period (Table 3), and the growth rate of juvenile fish are species-specific and temperature-dependent. The extent to which a fish species is subjected to predation by marine birds is directly related to the rate at which development and growth occur. For example, the less time it takes the pelagic eggs of demersal fish and shellfish to hatch and complete pelagic larval life, the less is the time they will be preyed on by marine birds. For fish species that are pelagic during their entire life, the rate of growth will deter[Pg 211]mine how long they remain small enough for birds to eat. Some of the smaller pelagic fish, such as herring, capelin, and smelt, are vulnerable to bird predation most of their lives; larger pelagic species like salmon may be preyed on for only a very short time. The maximum size fish that can be eaten by marine birds is, therefore, important in evaluating predation on a given species of fish.
The literature on the food habits of marine birds contains little on the sizes of fish consumed. Tuck (1960) stated that murres probably will take fish up to 18 cm long. Ogi and Tsujita (1973) estimated the lengths of Pacific pollock in the stomachs of murres taken in the eastern Bering Sea at 24 cm.
Herring in the eastern Bering Sea reach an age of 11 years and grow to about 33 cm. Herring could, therefore, be taken during most of their lives by murres but during only the first few years by smaller birds such as fulmars and shearwaters. Capelin and some species of smelt would be vulnerable to birds during all their lives. Although the size of adult Pacific salmon varies with the species, they are all so large that they are not preyed upon by marine birds. Once in the ocean, juvenile salmon grow at such a rapid rate that they are probably not very vulnerable to marine birds after their first 4 to 6 months at sea. Limited studies on the growth of juvenile sockeye salmon in the eastern Bering Sea (Straty 1974) indicate they may double their size in their first 8 weeks at sea. A sockeye salmon that entered the Bering Sea at 12 cm in mid-June would be 24 cm long in August—the maximum size that a murre could eat; the fish could be eaten by smaller marine birds for much less time. Pink and chum salmon enter the sea at a smaller size than sockeye salmon and would be vulnerable to predation both by a greater variety of marine birds and for a longer period of time.
We do not know the importance of competition between marine birds and commercial fish in the eastern Bering Sea. Only a few investigators have even alluded to competition between marine birds and fish for food. Ogi and Tsujita (1973) mentioned that competition seemed to exist between murres and juvenile sockeye salmon for euphausiids in the eastern Bering Sea. We have listed some of the types of forage fish and invertebrates eaten by commercial fish (Table 4) and marine birds (Table 5) in the eastern Bering Sea; comparison of these two tables clearly indicates that competition could occur.
The principal factors determining the extent of competition between marine birds and fish are the numbers of birds and fish, the length of time that various life history stages of the fish are in association with the birds, and the abundance of the preferred foods at these times. The impact of competition depends on the adaptability of the birds and fish to alternative types of food.
The types and sizes of food eaten by fish vary with the life history stage—especially with size at each stage. For instance, very young herring eat the eggs and nauplii of copepods or small copepodite stages and barnacles. As herring grow, their diet includes small fish and larger zooplankton, such as mature copepods, amphipods, euphausiids, and pteropods. Pacific cod shorter than 9 cm feed on small crustaceans (Moiseev 1953), whereas larger cod eat young crabs, shrimp, and fish. Small juvenile sockeye salmon feed mainly on larval stages of euphausiids (Straty 1974), but larger juveniles also eat the more adult forms, which eventually make up a significant part of their diet (Nishiyama 1974).
The change in the diet of fishes with growth results in competition with a changing variety of marine birds. For example, deep-diving birds may replace surface feeders as the major bird competitors of the Pacific cod and pollock as these fish increase in size and seek deeper waters. The diet of cod changes from small crustaceans in shallow water to progressively larger food that eventually includes herring, sand lance, shrimp, and crabs. The change to herring and sand lance, and quite possibly small crabs, places the adult cod in competition with both the surface feeders and pursuit diving birds, but adult cod do not compete with birds for zooplankton.
| Food item | Herring | Salmon | Walleye pollock | Pacific cod | Pacific ocean perch | Yellowfin sole | Pacific halibut |
|---|---|---|---|---|---|---|---|
| Invertebrates | |||||||
| Pteropods | X | X | — | — | X | — | — |
| Squid | — | X | — | X | X | — | X |
| Polychaetes | X | X | X | X | — | X | X |
| Copepods | X | X | X | — | — | — | — |
| Amphipods | X | X | X | X | X | X | — |
| Euphausiids | X | X | X | — | X | X | — |
| Decapods | X | X | X | X | X | X | X |
| Fish | |||||||
| Capelin | X | X | X | X | — | X | — |
| Sand lance | — | X | X | X | — | — | X |
| Food item | Shearwaters | Murres | Puffins | Murrelets | Fulmars | Kittiwakes | Gulls |
|---|---|---|---|---|---|---|---|
| Forage fish | |||||||
| Sand lance | X | X | X | — | — | X | X |
| Capelin | — | — | X | — | — | — | — |
| Invertebrates | |||||||
| Copepods | — | — | — | — | — | X | — |
| Euphausiids | X | X | — | — | — | X | — |
| Amphipods | X | X | — | — | — | X | — |
| Decapods | X | X | — | — | — | X | — |
| Pteropods | — | X | — | — | — | — | — |
| Chaetognaths | — | — | — | — | — | — | — |
| Polychaetes | — | X | X | — | — | X | — |
| Squid | X | X | — | — | X | — | — |
As pollock increase in size, they continue to feed mainly on zooplankton, but they change from copepods near the surface to euphausiids at mid-depths and near the bottom. Euphausiids are large and abundant zooplankters which, for the most part, are available only to deep-diving birds. Adult pollock also consume herring, sand lance, capelin, and other small fish.
Both marine birds and fish are capable of exploiting a wide variety of food, and often their stomach contents reflect the relative abundance of food items in the area. Ogi and Tsujita (1973) illustrated the differences in the food taken by murres captured at different locations in the eastern Bering Sea. Carlson (1977) and Ogi and Tsujita (1973) reported on differences in the diet of juvenile sockeye salmon captured at various locations in Bristol Bay and the eastern Bering Sea. The diets of many species of birds and fish, however, seem to be largely determined by their physiological and morphological adaptations and resultant feeding behavior. For instance, adult sockeye and pink salmon have well-developed gill rakers and feed largely on zooplankton, whereas chinook and coho salmon have poorly developed gill rakers and feed almost entirely on fish. In the eastern Bering Sea, murres appear to prefer the Pacific sand lance, whereas the slender-billed shearwater consumes mainly euphausiids (Ogi and Tsujita 1973). Thus, murres may be greater competitors with piscivorous fish than are shearwaters. Shearwaters are probably more important as competitors with zoo[Pg 213]plankton-eating fish that inhabit shallow water in juvenile stages and with pelagic fish species (such as pollock, herring, salmon, and capelin) that are heavily dependent on euphausiids.
Some species of marine birds may interact with fish as predators and competitors. As an example, pursuit diving birds, such as murres and puffins, may be important predators on juvenile salmon in the eastern Bering Sea, but these same birds may compete for food with adult salmon. Surface-feeding birds, such as fulmars, shearwaters, kittiwakes, and gulls, may be important as both predators and competitors with herring and capelin and some demersal fish.
The interactions of commercial fish and marine birds of the Bering Sea can be determined only if we know their distribution, abundance, and food habits, especially while they are associated with one another. Information is particularly lacking for all life history stages of commercial fish species and the seasonal movements of birds. We have some knowledge of the distribution and abundance of the various life history stages and the food habits of commercial fish in the Bering Sea. Little is known of the abundance, seasonal movements, and food habits of marine birds in this region, however, probably because marine birds have had little direct commercial value in the northern hemisphere. Food studies on marine birds are particularly difficult because their rapid digestion soon destroys the identity of the food.
We can make a reasonable guess as to some bird-fish associations for two regions of the Bering Sea where we have information on the distribution of marine birds and the various life history stages of commercial fish. For example, piscivorous birds, such as murres, puffins, black-legged kittiwakes, and slender-billed shearwaters, are extremely abundant in the summer along the seaward migration route of juvenile sockeye salmon (Fig. 7); the juvenile salmon, kittiwakes, and shearwaters all feed on plankton. Shuntov (1961) showed that kittiwakes are most abundant along the edge of the continental shelf in the Bering Sea in the summertime. This distribution coincides with the distribution of the eggs and larvae of pollock, certain flatfish, rockfish, sablefish, and several other species. These birds both exploit the fish directly (predation) and compete with them for plankton. Not enough information is available on the food habits of birds at the time fish eggs and larvae are present to evaluate this interaction.
Because fish are cold-blooded animals, temperature, through its influence on the rate of metabolism, is a major variable in determining the amount of energy needed for maintenance and for performing such essential activities as swimming and feeding—fish are less active, feed less, and grow more slowly in cold waters. For example, growth in young sockeye salmon is very slow at temperatures lower than 4°C (Donaldson and Foster 1941), and temperature profoundly affects their swimming speed (Brett et al. 1958). The rates of development of the eggs of some flatfish are closely correlated with water temperature (Ketchen 1956)—flatfish developed more rapidly at higher temperatures (Fig. 8). At lower temperatures, the rate of growth is also slower and, therefore, the duration of pelagic larval life is longer for demersal fish and shellfish.
Variations in sea temperature should, therefore, influence the extent to which fish are vulnerable to predation and competition. For example, eggs would take a longer time to hatch in colder than in warmer sea water. In both pelagic fish such as herring, whose eggs are laid in the intertidal zone, and in demersal fish with pelagic eggs such as the sole, the period of vulnerability of eggs to bird predation would be extended. At lower temperatures the length of the pelagic life of demersal fish and shellfish and their vulnerability to predation would also be greater than at higher temperatures. For example, the number of[Pg 214] days between molts of the zoeal stages of snow crabs is temperature-dependent—the warmer the water, the less the time between molts (Kon 1970).
Fig. 7. Distribution and numbers of birds observed in Bristol Bay along seaward migration route of sockeye salmon (from Bartonek and Gibson 1972).
Temperature, through its effects on swimming speed, feeding activity, and growth of juvenile fish, might influence the magnitude of predation by birds on pelagic fish in the following ways: (1) lower sea temperatures would increase the vulnerability of juvenile fish to bird predation because swimming speed would decrease, and the time the fish are of a size that could be eaten by would-be predators would increase; (2) lower sea temperatures would reduce the feeding by fish and decrease the competition by fish for food exploited by birds; and (3) higher sea temperatures would have the opposite effect—the feeding by fish would increase consumption of the foods that birds feed on.
In the eastern Bering Sea, water temperatures may vary greatly between years for the same month (Fig. 9). Such variation should result in variation in the temperature-de[Pg 215]pendent activities of fish and, in turn, in magnitude of marine bird predation and competition.
Fig. 8. The relation of temperature to the rate of development to hatching of lemon sole, as compared with two European flatfishes (Ketchen 1956).
We have noted that the abundance and age and size composition of major stocks of fish in the Bering Sea have been drastically reduced by commercial fishing. This has resulted in the reduction in numbers of fish at all life history stages, including those on which marine birds and other fishes depend for food. What effect this reduction has had on the abundance and distribution of marine birds in the Bering Sea is unknown. It depends in part on the ability of birds to eat other fish or increase their use of zooplankton or nekton.
We can hypothesize on probable changes in bird and fish abundance that resulted from the heavy commercial harvest of fish but any such changes cannot be documented or quantified. A reduction in stocks of a fish species could result in a reduced supply of food for a species of bird and cause a shift in the diet of this bird to other species of fish or to more zooplankton. For a bird species with specific food preferences, this could mean a reduction in its abundance to a level supportable by the available food supply. For bird species with less specific food requirements, a reduction in a species of fish could mean a reduction in competition for food with that fish—which could increase survival of the birds.
Man's intentional harvest of marine birds, such as the shearwater in parts of the southern hemisphere, and his inadvertent harvest of other bird species which are entangled or caught in fishing gear reduce predation and competition by marine birds. This, in turn, may aid the survival of the fish stocks in the Bering Sea.
The status of most stocks of commercial fish and shellfish in the Bering Sea is such that reductions in harvest are warranted, have been proposed, or are in effect. If the 200-mile (61-km) limit of jurisdiction over the marine resources by adjacent coastal States is implemented, either as a result of the Law of the Sea Conferences or unilaterally by the United States, we can expect commercial fishing in the eastern Bering Sea to be more tightly regulated. Such action should result in a reduction in harvest of those fish species now in a depleted condition, which, in turn, could influence the abundance of marine birds. Now is an opportune time to implement the studies required to increase our knowledge of the abundance, distribution, and seasonal movements of marine birds and their relationship to commercial fish resources of the eastern Bering Sea.
• The eastern Bering Sea is a region of high biological productivity; it is one of the world's great producers of commercial fish and major congregating areas for marine birds.
• The vulnerability of fish to predation by marine birds depends on life history features, such as place of spawning, duration of larval stages, growth rate, sea temperature, and adult size of fish, and on the distribution, feeding behavior, and food habits of marine birds.
Fig. 9. Sea temperatures in Bristol Bay and southeastern Bering Sea in mid-June and early July of 1967 and 1971 (from Straty 1974).
• The most apparent predation by marine birds on fish is on fish large or mature enough that some hard body parts persist and can be found in the stomach samples of birds.
• Little is known of the extent of bird predation on the pelagic eggs and larvae of demersal fish and shellfish in the Bering Sea because of lack of investigation and the rapid digestion of eggs and larvae by birds.
• Predation by marine birds on juvenile salmon is not well documented because of the lack of investigation in areas where both birds and fish are present.
• Marine birds and commercial fish eat similar zooplankton and fish in the eastern Bering Sea. The food exploited by both generally reflects the relative abundance of the types of food in the area, but food preference is displayed by some species of fish and birds.
• More is known about the food habits of the commercial fish than of the marine birds of the Bering Sea.
• Sea water temperature may be a major environmental factor in the Bering Sea since it influences both the extent to which fish are vulnerable to predation and the amount of competition with marine birds. Sea temperatures may vary greatly from year to year in the Bering Sea, and this may result in variations in the magnitude of predation and competition between birds and fish.
• The distribution of marine birds and the various stages in the life history of commercial fish are not well known for the eastern Bering Sea. Where these have been studied, they are intimately related. Such knowledge is required to gain some insight into even the potential for predation and competition in the dynamics of the marine bird and commercial fish populations of this region. In two instances, it is known that the occurrence of marine birds and the early life history stages of fish coincide so as to result in both potential predation on the fish by the birds and competition for food between the fish and the birds.
• The possibility exists that the commercial fish resources of the eastern Bering Sea will eventually come under the jurisdiction of the United States. This could mean reduced harvests of fish to restore depleted stocks. Such action could result in changes in the abundance of the marine birds of this region by creating an increased food supply for some and decreased supply for others.
We thank J. C. Bartonek and H. R. Carlson, H. Jaenicke, H. Larkins, and B. L. Wing for supplying various materials presented in this paper.
Ahlstrom, E. H. 1959. Vertical distribution of pelagic fish eggs and larvae off California and Baja California. U.S. Fish Wildl. Serv. Fish. Bull. 60(161):107-146.
Ahlstrom, E. H. 1961. Distribution and relative abundance of rockfish (Sebastodes spp.) larvae of California and Baja California. Int. Comm. Northwest. Atl. Fish. Spec. Publ. 3:169-176.
Ashmole, N. P. 1971. Sea bird ecology and the marine environment. Pages 223-286 in D. S. Farner, J. R. King, and K. C. Parkes, eds. Avian Biology. Vol. 1. Academic Press, New York.
Bartonek, J. C., and D. D. Gibson. 1972. Summer distribution of pelagic birds in Bristol Bay, Alaska. Condor 74(4):416-422.
Bourne, W. R. P. 1963. A review of oceanic studies of the biology of seabirds. Proc. Int. Ornithol. Congr. 13:831-854.
Brett, J. R., M. Hollands, and D. F. Alderdice. 1958. The effect or temperature on the cruising speed of young sockeye and coho salmon. J. Fish. Res. Board Can. 15(4):587-605.
Carlson, H. R. 1977. Food habits of juvenile sockeye salmon, Oncorhynchus nerka, in the inshore coastal waters of Bristol Bay, Alaska. Fish. Bull. 74(2):458-462.
Chitwood, P. E. 1969. Japanese, Soviet, and South Korean Fisheries off Alaska, development and history through 1966. U.S. Fish Wildl. Serv. Circ. 310. 34 pp.
Clemens, W. A., and G. V. Wilby. 1961. Fishes of the Pacific Coast of Canada. Fish. Res. Board Can., Bull. 68, 2d ed. 443 pp.
Donaldson, L. R., and F. J. Foster. 1941. Experimental study of the effect of various water temperatures on the growth, food utilization, and mortality rates of fingerling sockeye salmon. Trans. Am. Fish. Soc. 70:339-346.
Fadeev, N. S. 1965. Comparative outline of the biology of flatfishes in the southeastern part of the Bering Sea and the condition of their resources. Part 4, pages 112-119 in Soviet fisheries investigations in the northeast Pacific Ocean. (Trans. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Glude, J. B. 1967. The effect of scoter duck predation on a clam population in Dabob Bay, Washington. Proc. Natl. Shellfish Assoc. 55:73-86.
Hart, J. L. 1973. Pacific fishes of Canada. Fish. Res. Board Can., Bull. 180. 740 pp.
Haynes, E. 1973. Descriptions of prezoeae and stage 1 zoeae of Chionoecetes bairdi and C. opilio (Oxyrhyncha Oregoniinae). U.S. Natl. Mar. Fish. Serv. Fish. Bull. 73:769-775.
Ito, K. 1968. Ecological studies on the edible crab, Chionoecetes opilio (O. Fabricius) in the Japan Sea. II. Description of young crabs, with note on their distribution. Bull. Jpn. Sea Reg. Fish. Res. Lab. 19:43-50. (Transl. from Japanese.) Fish. Res. Board Can. Transl. Ser. 1184.
Jarvis, M. J. F. 1970. Interactions between man and the South African gannet Sula capensis. Ostrich Suppl. 8:497-513.
Kashkina, A. A. 1965a. Winter ichthyoplankton of the Commander Islands region. Part 4, pages 170-181 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Kashkina, A. A. 1965b. Reproduction of yellowfin sole (Limanda aspera Pallas) and changes in its spawning stocks in the eastern Bering Sea. Part 4, pages 182-190 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Kashkina, A. A. 1970. Summer ichthyoplankton of the Bering Sea. Part 5, pages 225-247 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Ketchen, K. S. 1956. Factors influencing the survival of the lemon sole (Parophrys vetulus) in Hecate Strait, British Columbia. J. Fish. Res. Board Can. 13(5):647-694.
Kobayashi, K. 1963. Larvae and young of the whiting, Theragra chalcogramma (Pallas), from the north Pacific. Bull. Fac. Fish. Hokkaido Univ. 14(2):55-63.
Kon, T. 1970. Fisheries biology of the tanner crab. IV. The duration of planktonic stages estimated by rearing experiments of larvae. Bull., Jpn. Soc. Sci. Fish. 36(3):219-224. (Transl. from Japanese.) Fish. Res. Board Can. Transl. Ser. 1603.
Korolev, N. G. 1964. The biology and commercial exploitation of the king crab, Paralithodes camtschatica (Tilesius), in the southeastern Bering Sea. Part 2, pages 102-108 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Kurata, H. 1960. Studies on the larva and post-larva of Paralithodes camtschatica. III. The influence of temperature and salinity on the survival and growth of the larva. Bull. Hokkaido Reg. Fish. Res. Lab. 21:9-14.
Kurata, H. 1964. Larvae of the decapod crustacea of Hokkaido. 6. Lithodidae (Anomwia). Bull. Hokkaido Reg. Fish. Res. Lab. 28:49-65.
Lisovenko, L. A. 1965. Fecundity of Sebastodes alutus Gilbert in the Gulf of Alaska. Part 4, pages 162-169 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Lyubimova, T. G. 1965. Main stages in the life cycle of the rockfish Sebastodes alutus Gilbert in the Gulf of Alaska. Part 4, pages 85-111 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Moiseev, P. A. 1953. Cod and flounders of far-eastern waters. (Transl. from Russian.) Fish. Res. Board Can. Transl. Ser. 119.
Motoh, H. 1973. Laboratory-reared zoeae and megalopa of zuwai crab from the Sea of Japan. Bull. Jpn. Soc. Sci. Fish. 39(12):1223-1230.
Moyle, P. 1966. Feeding behavior of the glaucous-winged gull on an Alaskan salmon stream. Wilson Bull. 78(2):175-190.
Mukhacheva, V. A., and O. A. Zviagina. 1960. Development of the Pacific Ocean cod Gadus morhua macrocephalus Tilesius. (Transl. from Russian.) Fish. Res. Board Can., Transl. Ser. 393.
Musienko, L. N. 1963. Ichthyoplankton of the Bering Sea (data of the Bering Sea Expedition of 1958-59). Part 1, pages 251-286 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Musienko, L. N. 1970. Reproduction and development of Bering Sea fishes. Part 5, pages 161-224 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Nishiyama, T. 1974. Energy requirement of Bristol Bay sockeye salmon in the central Bering Sea and Bristol Bay. Inst. Mar. Sci., Univ. Alaska 2:321-343.
Novikov, N. P. 1964. Basic elements of the biology of the Pacific halibut (Hippoglossus hippoglossus stenolepis Schmidt) in the Bering Sea. Part 2, pages 175-219 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Ogi, H., and T. Tsujita. 1973. Preliminary examination of stomach contents of murres (Uria spp.) from the eastern Bering Sea and Bristol Bay, June-August 1970 and 1971. Jpn. J. Ecol. 23(5):201-209.
Outram, D. N. 1958. The magnitude of herring spawn losses due to bird predation on the west coast of Vancouver Island. Fish. Res. Board Can., Prog. Rep. Pac. Coast Stn. 111:9-13.
Paraketsov, I. A. 1963. On the biology of Sebastodes alutus of the Bering Sea. Part 1, pages 319-327 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Pearson, T. H. 1968. The feeding biology of sea-bird species breeding on the Farne Islands, Northumberland. J. Anim. Ecol. 37:521-552.
Pertseva-Ostraumova, T. A. 1954. Material on the development of the far-eastern flatfish. 1. Development of the yellowfin sole. Tr. Inst. Okeanol. Akad. Nauk., SSSR-11.
Reid, G. J. 1972. Alaska's fishery resources, the Pacific herring. U.S. Natl. Mar. Fish. Serv., Fish. Facts 2, 20 pp.
Rodin, V. E. 1970. An estimation of the state of the king crab (Paralithodes camtschatica Tilesius) stock in the southeastern Bering Sea. Part 5, pages 149-156 in Soviet fisheries investigations in the northeast Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Rounsefell, G. A. 1929. Contribution to the biology of the Pacific herring, Clupea pallasii, and the condition of the fishery in Alaska. Bull. U.S. Bur. Fish. 45:227-320.
Rumyantsev, A. I., and M. A. Darda. 1970. Summer herring in the eastern Bering Sea. Part 5, pages 409-441 in Soviet fisheries investigations in the northeastern Pacific Ocean. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Sanger, G. A. 1972. Preliminary standing stock and biomass estimates of seabirds in the subarctic Pacific region. Pages 499-611 in A. Y. Takenouti, ed. Biological oceanography of the northern north Pacific Ocean.
Serobaba, I. I. 1968. On the spawning of Alaska pollock Theragra chalcogramma (Pallas) in the northeastern part of the Bering Sea. Vopr. Ikhtiol. 6(53):992-1003. (Transl. from Russian.) Israel Program for Scientific Translations, Jerusalem.
Shaefer, M. B. 1970. Men, birds, and anchovies in the Peru current—dynamic interactions. Trans. Am. Fish. Soc. 99(3):461-467.
Shuntov, V. P. 1961. Migration and distribution of marine birds in the southeastern Bering Sea during spring-summer season. Zool. Zh. 40(7):1058-1069. (In Russian, summary in English.)
Shuntov, V. P. 1966. Concerning wintering of birds in the far eastern seas and in the northern part of the Pacific Ocean. Zool. Zh. 45(11):698-711. (Transl. from Russian.) Can. Wildl. Serv.
Solomensen, F. 1965. The geographical variation of the fulmar (Fulmarus glacealis) and the zones of marine environment in the North Atlantic. Auk 82:327-355.
Stevenson, J. C. 1962. Distribution and survival of herring larvae (Clupea pallasi Valenciennes) in British Columbia waters. J. Fish. Res. Board Can. 19(5):735-810.
Straty, R. R. 1974. Ecology and behavior of juvenile sockeye salmon (Oncorhynchus nerka) in Bristol Bay and the eastern Bering Sea. Inst. Mar. Sci., Univ. Alaska, Occas. Publ. 2:285-320.
Tanino, Y., H. Tsujisaki, K. Nakamichi, and K. Kyushin. 1959. On the maturity of Alaska pollock, Theragra chalcogramma (Pallas). Bull. Hokkaido Reg. Fish. Res. Lab. 20:145-164.
Taylor, F. H. C. 1967. The relationship of midwater trawl catches to sound scattering layers off the coast of northern British Columbia. J. Fish. Res. Board Can. 25(3):457-472.
Tuck, L. M. 1960. The murres: their distribution, populations, and biology, a study of the genus Uria. Can. Wildl. Ser. 1. 260 pp.
Wiens, J. A., and J. M. Scott. 1976. Model estimation of energy flow in Oregon coastal seabird populations. Condor 77(4):439-452.
Yusa, T. 1954. On the normal development of the fish, Theragra chalcogramma (Pallas), Alaska pollock. Bull. Hokkaido Reg. Fish. Res. Lab. 10. 15 pp.
[54] For crabs, this measurement is carapace width.
[55] Authors' data.
[56] For crabs, the measurements are total length for zoeal stages and carapace length and width for postzoeal stages.
[57] The incubation period for an egg is temperature dependent. Embryo development is faster at higher temperatures.
[58] Juvenile pollock have diurnal migrations.
[59] The peak period varies with latitude: to 55°N—June; to 55-60°N—July; to north of 60°N—August.
[60] H. R. Carlson and R. E. Haight (in preparation), Juvenile life of Pacific ocean perch, Sebastes alutus, in coastal fiords of southeastern Alaska: their environment, growth, food habits, and schooling behavior.
[61] The genus Sebastes is a live bearer.
[62] Rockfish larvae resemble each other quite closely, and complete descriptions for the 10 species in the Bering Sea do not exist. The following depth distribution for rockfish larvae may or may not include S. alutus: 45-365 m (Taylor 1967) off British Columbia; 0-88 m (Ahlstrom 1959, 1961) off California and Baja California.
[63] In crabs, the eggs are attached to the female.
[64] S. C. Jewett and R. E. Haight (in preparation), A description of megalopa of the snow crab, Chionoecetes bairdi Rathbun (Majidae, subfamily Oregoniinae).
by
Robert D. Jones, Jr.
U.S. Fish and Wildlife Service
1011 East Tudor Road
Anchorage, Alaska 99507
and
G. Vernon Byrd[66]
U.S. Fish and Wildlife Service
Aleutian Islands National Wildlife Refuge
Adak, Alaska
Animals introduced to insular seabird habitats are of both intentional and accidental origin. The results of the introductions—particularly of herbivores—cannot be predicted, but may range from severely destructive to beneficial. Herbivores are of both domestic and wild stocks of ungulates, hares, and rabbits. Rats are the most commonly introduced omnivore on a worldwide basis. In Alaska the commonest carnivore introduction has been the red fox (Vulpes fulva) and arctic fox (Alopex lagopus), and the first of these were made in the early 19th century by the Russian-American Company. These foxes nearly extirpated the Aleutian Canada goose (Branta canadensis leucopareia) from its nesting grounds. Black flies (Simuliidae), which are vectors of avian blood parasites, have been introduced to three of the Aleutian Islands.
The purpose of this paper is to discuss some influences of introduced animals, primarily mammals, on seabirds and their nesting habitat, with emphasis on the coasts of Washington, British Columbia, and Alaska. Our discussion focuses on island introductions partly because a large proportion of seabirds choose island nesting sites, and because islands present ecosystems vulnerable to such introductions.
Flightless animals have no means of immigration, hence little probability of colonizing islands. In these circumstances marine birds evolve populations in relatively simple ecosystems (Carlquist 1965; MacArthur and Wilson 1967), though the degree of simplicity depends on several variables, including the island's size and its distance from a source of immigrants. These systems have achieved ecological homeostasis through reciprocal adaptation over an extended period. Experience has shown that introductions to such systems result in severe perturbations (Odum 1971:221).
The introductions can be categorized as being either intentional or accidental events. Effects of such introductions have varied widely, depending on the type of animal introduced, the types of birds present and the habitat they occupy, the size and shape of the island, the type of nesting area used by the birds, and the status of their populations before the introduction. An example drawn from our Aleutian experience with gallinaceous birds illustrates the interaction of these variables. The dark phase of the arctic fox (Alopex lagopus) was introduced to Adak and Amchitka islands, both of which had native populations of the rock ptarmigan, Lagopus mutus [Pg 222](Gabrielson and Lincoln 1959). Foxes were released on Adak in 1924, and on Amchitka in 1921. Adak has an area of 751 km2 and Amchitka 350 km2. Adak is irregular in shape with extensive precipitous shorelines, relatively few beaches, and a large, central mountainous hinterland which foxes rarely penetrated. Amchitka, on the other hand, presents a zone of marine planation on its eastern two thirds, low mountains on the rest, shelving beaches around most of the island, and a long, linear, narrow shape that foxes explored completely. By 1949 ptarmigan were difficult to find on Amchitka, and then only in the highest, steepest section of the mountains. They were extirpated from the low, eastern two thirds of the island. The foxes flourished on Amchitka, but did much less well on Adak, where the ptarmigan population fluctuated in a normal cyclic manner, apparently uninfluenced by the foxes. Then the foxes were eradicated on Amchitka in the 1950's, and by 1962 the ptarmigan had spread over the whole of the island and become one of the most conspicuous avian features of the landscape.
Man has taken ungulates with him to many islands. Although numerous records of livestock introductions are available, few provide information relating to the effects of these animals on the habitat and their fauna unless the impact has been severe.
A most noteworthy example of destruction by ungulates occurred on Guadalupe Island off the coast of Baja California. Domestic goats (Capra hircus) were introduced in the unrecorded past with the result that little of the once abundant vegetation remains. In its place introduced species capable of withstanding heavy grazing are abundant over most of the island. Several endemic avian species are now considered extinct, including the Guadalupe storm-petrel, Oceanodroma macrodactyla (Howell and Cade 1954; Jehl 1972).
Sheep (Ovis aries) have been introduced to seabird nesting islands with varying results. In Bass Strait, Australia, Norman (1970) studied the effects of introduced sheep on vegetation and birds. He cited various papers attributing destruction of colonies of shearwaters (Puffinus sp.) to the activities of sheep, primarily their treading on the burrows. He found, however, that on Big Green Island and Phillip Island, sheep were not responsible for declines in shearwater breeding success, nor did they prevent expansion of colonies.
Other authors have reported damage to seabird nesting areas by sheep. One such example in the eastern North Pacific region concerns Protection Island, Washington. According to Richardson (1961), 100 to 300 sheep grazed freely on the island since 1958. He reported damage by grazing and frequent trampling of nesting areas of rhinoceros auklets (Cerorhinca monocerata). Landslides were initiated by these activities, rendering the slopes unusable by auklets. Of the burrows in his study area, 46% were buried by slides. He did not determine mortality.
Other avian consequences may flow from sheep introductions. Husbandry of these ungulates has been practiced with varying success for many years in the Aleutian Islands, most notably on Umnak and Unalaska islands, both of which have large native populations of bald eagles, Haliaeetus leucocephalus (Gabrielson and Lincoln 1959). Before the introduction of sheep, these raptors were oriented to the sea, hunting fish and seabirds. Sheep presented a new resource and presently the industry found itself confronted by a formidable predator, and demanded that eagles be destroyed (letter to William Egan, Governor of Alaska, from James S. Bynum, Secretary-treasurer, Umnak Company, Inc.).
Other ungulates introduced on Alaska islands include cattle (Bos taurus) on Chernofski and Chernabura islands; caribou (Rangifer tarandus) on Adak; reindeer on St. Matthew, Nunivak, Atka, Umnak, St. Paul, St. Lawrence, Hagemeister, and Kodiak as well as many interior locations; deer (Odocoileus hemionus) on Kodiak and Afognak; elk (Cervus canadensis) on Afognak; and musk oxen (Ovibos moschatus) on Nunivak. All these animals have maintained populations on islands for a time, and some appear likely to do so into the distant future. Specific effects on seabirds is generally not known, but trampling of grassy slopes such as that re[Pg 223]ported for sheep develops in some cases. Bailey et al. (1933) speculated that nests of the snow goose (Anser caerulescens) were destroyed by reindeer or their herdsmen in the Point Barrow area.
The destruction of vegetation by introduced rabbits and hares has been documented for many areas in the world. This destruction has often extended to seabirds. Perhaps the most dramatic example occurred on Laysan Island in the Hawaiian archipelago, where rabbits of unknown species were introduced in 1903. According to Warner (1963) it took less than 20 years for the rabbits to remove every green plant but three patches of Sesuvium portulacastrum. The Laysan duck (Anas laysanensis) was brought perilously close to extinction. The rabbits were eliminated in the 1920's, and the population of ducks increased to over 600 by 1963, a figure thought to approximate the pre-disturbance population.
European hares (Lepus europaeus) were introduced on Smith, San Juan, and Long islands, in Washington. On Smith Island, these burrowing animals apparently grazed nearly all the succulent vegetation close to the ground. By 1924, their burrows riddled the bluffs, causing them to cave into the ocean (Couch 1929). Couch found no seabirds nesting on the island, but found numerous tufted puffins (Lunda cirrhata) present on the bluffs, but not nesting. A removal campaign was directed against the hares in 1924 and in a few years they were gone. Smith Island now supports nesting pelagic birds (D. Manuwal, personal communication).
Accounts of hare and rabbit introductions to islands are legion, but not all such introductions have drastically affected seabirds. Manana Island, Hawaii, is such a case. Tomich et al. (1968) believed that introduced rabbits (Oryctolagus cuniculas) were not even indirectly detrimental to the nesting noddies (Anous tolidus) and sooty terns (Sterna fuscata). In some situations, introduced lagomorphs have been credited with benefiting seabirds. Lockley (1942) suggested that rabbits helped to open new breeding colonies of manx shearwaters (Puffinus puffinus) at Skomer and in west Wales in general. In Alaska rabbits were introduced to Middleton Island in 1952 (Rausch 1958) and to Ananiuliak Island at an earlier unrecorded date. Both have developed sustaining populations in the presence of large seabird populations without measurable effect on the birds. On Ananiuliak glaucous-winged gulls (Larus glaucescens) have been observed feeding on rabbits (W. S. Laughlin, personal communication).
Invertebrates have been introduced on three islands in the Aleutians. The black fly (Simulium sp.) reached Adak by 1958, Shemya by 1964, and Amchitka in connection with activities of the Atomic Energy Commission in 1968. Apparently the insects were transported on jet aircraft. The pest appears well established on Adak, but its status on the other two islands is uncertain. Like the mosquito, the female black fly sucks blood from warm-blooded animals, and in the process becomes the vector of a Leucocytozoan blood parasite of birds. In years of black fly abundance at Seney (Michigan) National Wildlife Refuge the blood parasite has been responsible for reproductive failure in Canada geese (Branta canadensis; Sherwood 1968). If black fly problems reach such a scale in the Aleutians, the parasites might prove limiting to pelagic birds as well as to waterfowl. Winds, for which the Aleutian region is famous, constitute a limiting factor for obligate blood-feeding Simuliids and may control the severity of this problem.
The list of introduced animals that prey on seabirds is extensive. Often several animals have been introduced to the same island. For example, in 1951 Amchitka Island in the Aleutians supported populations of feral dogs (Canis familiaris) and cats (Felis catus), rats (Rattus norvegicus), and arctic fox. Their presence resulted from three of the usual sources of predator introductions: escape of pets, escape from visiting ships (and aircraft), and commercial introductions. Add introductions to control pests, such as that of the mongoose (Herpestes auropunctatus) to the Hawaiian Islands, and only one source remains—the desire of man to improve on nature. In the Aleutians this impulse has taken the more innocuous form of fish and plant introductions, such as rainbow trout (Salmo gairdneri) on Adak and Shemya, and trees[Pg 224] (mostly Sitka spruce, Picea sitkensis) on every military base in the "Chain."
Rats appear to be the most commonly introduced predators on a worldwide scale. Ships furnish the traditional source of their introduction, but one of us (R.D.J.) has observed them disembarking from a military aircraft at Cold Bay on the Alaska Peninsula. These animals probably entered the plane at Adak, which received rats from military ships early in World War II.
Rats may be able to exploit a larger percentage of the seabird species on a given island than other introduced predators because they can enter crevices and burrows in search of the birds and their eggs and young. They also destroy ground-nesters, and cliff-nesters may not be altogether safe from them. Clayton M. White (personal communication) found that Rattus norvegicus had ravaged every one of 16 eyries of the peregrine falcon (Falco peregrinus) that he checked in 1971 at Amchitka Island, Alaska. Only one egg had tooth marks, however. Kenyon (1961) ascribed the disappearance of the song sparrow (Melospiza melodia maxima) and the winter wren (Troglodytes troglodytes kiskensis) from Amchitka to predation by rats.
Many authors have mentioned potential rat damage, but few have quantitatively documented it. Imber (1974), however, provided data concerning the magnitude of rat predation on diving petrels and storm-petrels on some New Zealand islands. He found that rats were taking between 10 and 35% of the chicks of gray-faced petrels (Pterodroma macroptera gouldi) on Whale Island in the parts of the colonies where burrows were dense. On those parts of the island with a very low density of petrel burrows, rats were believed to have killed virtually every chick. Imber revealed that where Polynesian rats (Rattus exulans) occur, diving petrels and storm-petrels are rare or absent, though they are widespread on neighboring islands. Imber concluded from his studies of the ecology of petrels and Polynesian and Norway rats that a petrel colony is endangered if invaded by a species of rat whose maximum weight approaches or exceeds the mean adult weight of the petrel. Harris (1970), who worked with dark-rumped petrels (Pterodroma phacopygia) on Santa Cruz in the Galapagos Islands, indicated that black rats (Rattus rattus) were responsible for the extremely low nesting success of the petrels there.
In British Columbia, Campbell (1968) recorded predation by the Alexandrian rat (R. rattus) on ancient murrelets (Synthliboramphus antiquus) at Langara Island. The extent of damage to the murrelet population is not known.
The animals most widely introduced in Alaska seabird habitat are the red fox (Vulpes fulva) and the arctic fox. The red fox is native to the Alaska Peninsula and to the easternmost group of islands in the Aleutians, known as the Lissii or Fox Islands (Berkh 1823; Murie 1959). At the other end of the archipelago, in the group known as the Near Islands, Attu Island has a native population of the arctic fox (Tikhmenev 1861; Bancroft 1886). Between Umnak Island, the westernmost island of the Fox group, and Attu there are no native terrestrial mammals, and substantial avian populations evolved an ecology in the absence of mammalian predation (Murie 1959).
At the time of Russian contact with the Aleutians, both fox species were dominantly dark phase, and the early introductions (about 1836) by the Russian-American Company were of both species (Tikhmenev 1861). Initially both species were successful in developing insular populations, but in the long run the arctic fox proved the more successful. At Great Sitkin, Adak, and Kanaga, introduced red foxes maintained populations that were eliminated in the 1920's to be replaced by arctic foxes (unpublished records of the Aleutian Islands National Wildlife Refuge). Differential harvest of the preferred dark phase had in the meantime altered the genetic makeup of the population, and the light phase had become dominant. In the arctic fox populations, the dark phase remained generally dominant at about 95%, but in some small islands with limited genetic stock (e.g., the Semichis) the proportion approached one to one (unpublished records of the Aleutian Islands National Wildlife Refuge).
By 1936, the Aleutian archipelago constituted a large-scale fox farm, which in its 23 years of existence as a refuge had produced 25,641 fox pelts with a value of $1,162,826. During the same period, and perhaps earlier,[Pg 225] arctic foxes were introduced on almost every island from the Aleutians to Prince William Sound, and on some of the islands in southeastern Alaska. The Aleutian Islands National Wildlife Refuge maintained records from which the above figures are quoted, but though records of other islands' use for fur farms exist in the archives of the Alaska Game Commission, no record of the fur values was kept.
Murie (1959) assessed the influence of the foxes by examining 2,501 fox droppings collected in 1936 and 1937 from 22 of the Aleutian Islands. He reported 57.8% of the food items in these droppings was avian—48.9% seabirds. The result of his investigations was the adoption of new policies governing issuance of permits for fox farming in the Refuge. The essential feature of these policies was the revocation of certain existing permits, with a view to reserving the islands concerned for wildlife use. The decision proved academic, for fur prices declined until no market for Aleutian arctic fox pelts could be found. But the foxes remained.
The most obvious damage has been the nearly complete extermination of the Aleutian Canada goose (Branta canadensis leucopareia). It has vanished from its former nesting range in the Aleutian and Kuril Islands, except for Buldir Island in the western Aleutians (Jones 1963). Clark (1910) described this goose as extremely abundant on Agattu Island in 1909; however, foxes from Attu were introduced there in 1923, 1925, and 1929. Murie (1959) found "probably less than six pairs" in 4 days of traveling over the island in 1937.
In our main area of interest, cats appear to have been widely introduced, but we found no record of extensive predation on marine birds. Jehl (1972) attributed the extinction of the Guadalupe petrel to predation by cats, in combination with the destruction of vegetation by goats. Imber (1974) reported that "serious predation by cats upon a colony of gray-faced petrels on Little Barrier Island, New Zealand was observed in 1950. Since that time, the colony has become extinct."
Though feral dogs are reported present on islands in our area of interest, they do not appear to have significant influence on seabirds. On Attu Island, the pet dogs of personnel of the Coast Guard LORAN station are reported to take common eiders (Somateria mollissima).
Ecological consequences of animal introductions to islands are rarely well documented. Usually no thought is devoted to such consequences until redress becomes difficult or quite impossible. Many of the introductions stem from a period before ecological understanding, and the introduced animal has acquired the status of a native. The arctic fox in the Aleutians fits all of these conditions. Until we conducted a thorough search of the literature, some of it difficult to secure and written in several languages, the original status of this animal was not known. Its elimination, now under way on selected islands, is difficult and expensive. Rapid recovery of some avian species, including certain passerines, has been observed. However, ecological homeostasis is the product of evolution, and restoration in the Aleutians must follow that course. It is not likely to proceed rapidly to a point thought desirable by man. The accidental introductions of animals such as rats and black flies in the Aleutians constitute particularly irksome events because they cannot be reversed. The new ecology of Amchitka, from which the foxes have been removed, must evolve in the presence of these species. Its face will look very different than if they were not there. We would like to suggest a means by which such introductions may be prevented, but it seems likely that more, not less, can be expected.
Preventing the introduction of ungulates seems more likely to be successful, especially if the islands lie within a National Wildlife Refuge. Even this, however, becomes less certain with an expanding human population and, with it, demands for more land on which to produce food.
Legal restrictions have been suggested as a means to control or prevent introductions, but in the northern islands, little enforcement is likely. There is a phrase bearing on this, said to have governed human behavior in the early years of Caucasoid occupation of the Aleutian Islands, "Heaven is too high and the Czar too far away."
Bailey, A. M., C. D. Brower, and L. B. Bishop. 1933. Birds of the region of Point Barrow, Alaska. Prog. Act. Chic. Acad. Sci. 4(2):14-40.
Bancroft, H. H. 1886. History of Alaska, 1730-1885. San Francisco.
Berkh, V. 1823. The chronological history of the discovery of the Aleutian Islands or the exploits of the Russian merchants. N. Grech., St. Petersburg.
Campbell, R. W. 1968. Alexandrian rat predation on ancient murrelet eggs. Murrelet 49:38.
Carlquist, S. 1965. Island life: a natural history of the islands of the world. Natural History Press, New York.
Clark, A. H. 1910. The birds collected and observed during the cruises of the United States Fisheries Steamer "Albatross" in the North Pacific Ocean and in the Bering, Okhotsk, Japan, and Eastern seas from April to December 1906. Proc. U.S. Natl. Mus. 38:25-74.
Couch, L. K. 1929. Introduced European rabbits in the San Juan Islands, Washington. J. Mammal. 10:334-336.
Gabrielson, I. N., and F. C. Lincoln. 1959. The birds of Alaska. Stackpole Co. and Wildlife Management Institute, Washington, D.C.
Harris, M. P. 1970. The biology of an endangered species, the dark-rumped petrel (Pterodroma phacopygia) in the Galapagos Islands. Condor 72:76-84.
Howell, T. R., and T. J. Cade. 1954. The birds of Guadalupe Island in 1953. Condor 56:283-291.
Imber, M. J. 1974. The rare and endangered species of the New Zealand region and the policies that exist for their management: petrels and predators. Paper presented to the International Council for Bird Preservation (XVI World Conference), Canberra.
Jehl, J. R. 1972. On the cold trail of an extinct petrel. Pac. Discovery 25:24-28.
Jones, R. D. 1963. Buldir Island, site of a remnant breeding population of Aleutian Canada geese. Wildfowl Trust Annu. Rep. 14:80-84.
Kenyon, K. W. 1961. Birds of Amchitka Island, Alaska. Auk 78(3):305-326.
Lockley, R. M. 1942. Shearwaters. Devin-Adair, New York.
MacArthur, R. H., and E. O. Wilson. 1967. The theory of island biogeography. Princeton Univ. Press, Princeton, N.J.
Murie, O. J. 1959. Fauna of the Aleutian Islands and Alaska Peninsula. U.S. Fish Wildl. Serv., N. Am. Fauna 61:1-364.
Norman, F. I. 1970. The effects of sheep on the breeding success and habitat of the short-tailed shearwater (Puffinus tenuirostris) (Temminch). Aust. J. Zool. 18:215-242.
Odum, E. P. 1971. Fundamentals of ecology. W. B. Saunders Co., Philadelphia, Pa. 574 pp.
Rausch, R. 1958. The occurrence and distribution of birds on Middleton Island, Alaska. Condor 60(4):227-242.
Richardson, F. 1961. Breeding biology of the rhinoceros auklet on Protection Island. Condor 63(6):456-473.
Sherwood, G. A. 1968. Factors limiting production and expansion of local populations of Canada geese. Pages 73-85 in R. L. Hine and C. Schoenfeld, eds. Canada goose management. Dembar Educational Research Service, Madison, Wis.
Tikhmenev, P. 1861. Historical review of the origin of the Russian-American Co. and its activity up to and the present time. Edward Weimar, St. Petersburg.
Tomich, P. Q., N. Wilson, and C. H. Lamoureux. 1968. Ecological factors on Manana Island, Hawaii. Pac. Sci. 12:352-368.
Warner, R. E. 1963. Recent history and ecology of the Laysan duck. Condor 65(1):2-23.
by
James G. King
U.S. Fish and Wildlife Service
P. O. Box 1287
Juneau, Alaska 99802
and
Gerald A. Sanger
U.S. Fish and Wildlife Service
Anchorage, Alaska
The 176 species of birds using marine habitats of the Northeast Pacific are graded on the basis of 20 factors that affect their survival. A score of 0, 1, 3, or 5, respectively, representing no, low, medium, or high significance is assigned for each factor. The total score is the Oil Vulnerability Index (OVI). The OVI's range from 1 to 100, an index of 100 indicating the greatest vulnerability. Using this system, one can rank the avifauna of different areas according to their vulnerability to environmental hazards as an aid in making management decisions.
Today's decision makers require an ever-increasing array of information and planning documents. The Federal Government's requirement for environmental impact statements under the National Environmental Protection Act of 1969 is but one example of this trend. These documents generally consider the effects of proposed actions on waterfowl and a few other species of birds, but the bulk of the avifauna is usually only listed, or sometimes ignored completely. A simple system for evaluating and presenting avian data is badly needed so that those interested in birds, whether technically trained or not, can easily grasp the implications of proposed actions. It is incumbent on biologists to devise new ways of presenting their knowledge so that it can be easily and effectively used by decision makers, who are often less informed. In short, biologists must do for the environmental impact statement assessors what Roger Tory Peterson did for the bird watchers by giving them a simple and comprehensible system.
The need for a system to evaluate relative vulnerabilities of bird populations is particularly great for birds that are being increasingly affected by marine oil pollution. The system needs to allow comparisons of potential impacts to birds resulting from various oil development projects in different locations and served by various modes of transport. The Oil Vulnerability Index (OVI) is our attempt to fulfill this informational need on the avifauna of the Northeast Pacific. Insofar as we know, this approach to assessing a wildlife management problem has been attempted only for ranking endangered species in a numeric ranking system that identified where restoration efforts could best be directed (Sparrowe and Wight 1975).
We are indebted to Gene Ruhr and Keith Schreiner for ideas generated in their work with endangered species. Frank Pitelka, James Bartonek, Kent Wohl, and Mary Lou King reviewed portions of the manuscript and offered helpful suggestions. Jack Hodges helped prepare the OVI tables.
A list of 176 species of birds using marine habitats in or near the States of Washington and Alaska and the Province of British Columbia (Table 1, left column) was compiled from checklists by the American Ornithologists' Union (AOU 1957) and Gibson (1970). Nomenclature is from AOU (1957). The scientific names of three species of shorebirds recently identified in the Aleutian Islands that were not listed by the AOU (1957) came from Peterson et al. (1967).
Each bird was scored on 20 factors that affect its survival (Table 1). Point scores for most birds were either 0, 1, 3, or 5, indicating no, low, medium, or high importance, respectively, in their biology or habits as related to Northeast Pacific oil development. Rare or accidental species were given only one point for occurrence, and endangered species 99 points for population size plus 1 point for occurrence. Thus the potential range of the OVI's is from 1 to 100.
The factors in Table are largely self-explanatory. The items under "range" apply to the entire world population of the species. "Productivity" is derived from a combination of clutch size and age at first nesting. Specialization is used in the biological sense to compare a versatile species like mallards (Anas platyrhynchos) with a less versatile species such as the trumpeter swan (Olor buccinator). Mortality under "history of oiling" is based on our knowledge that some species (e.g., alcids) have been more involved than others such as gulls. Exposure relates to the level of exposure within the Pacific area in any season.
Information on many of the factors for many species is scanty at best, and subjective appraisals were made by us when information was lacking. Opinions as to appropriate scores will vary among experts. References used, in part, in preparing Table 1 were: AOU 1957; Fay and Cade 1959; Gabrielson and Lincoln 1959; Isleib and Kessel 1973; Kortright 1942; Murie 1959; Palmer 1962; Robbins et al. 1966; Sanger 1972; and Stout et al. 1967.
The OVI for each of 176 bird species is listed in Table 1. The average OVI for 22 avian families comprising 128 species that are neither rare stragglers nor endangered ranged from 19 to 88, with a mean of 51 (Table 3).
Tables 4 and 5 show a possible use for the OVI by comparing impacts in two large, widely separated areas. A species list from Southeast Alaska (U.S. Forest Service and Alaska Department of Fish and Game 1970) is compared with a list from the Aleutian Islands (U.S. Fish and Wildlife Service 1974). Only commonly occurring species are included. These tables graphically display rather strong differences in the vulnerability of the avifauna of each area. A person explaining comparative impacts of projects might use the tables in the following way:
• Column 1, with scores from 1 to 20 points, indicates birds with a low level of project involvement, where damage or future costs would not be expected. As this will normally be the longest list, as in Tables 4 and 5, one would expect an immediate rise of interest on the part of the planning agency, which is probably eager to learn where problems will be fewest.
• Column 2 (21 to 40 points) indicates birds for which there is a low level of concern. Perhaps all that is needed is a review to determine if special characteristics of the project might be detrimental to these species.
• Column 3 (41 to 60 points) might be called "trial and error" species. If some birds are adversely affected, it will not be catastrophic. As the project develops it will be merely necessary to monitor these to make sure their status is not adversely affected. If it is, there will be time to develop conservation measures.
• Columns 4 and 5 (61 to 80 points and 81 to 100 points, respectively) include the species where concern is high. It is for these species that research money will be needed, where project modifications may be required, where a contingency plan in case of disaster is needed, where a conservation technology will be needed, and where periodic project shutdown could be called for.
| Family, common (AOU) name and scientific name | Range | Population | Habits | Mortality | Annual exposure | OVI | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B r e e d i n g r a n g e s i z e |
M i g r a t i o n l e n g t h |
W i n t e r r a n g e s i z e |
M a r i n e o r i e n t a t i o n |
P o p u l a t i o n s i z e |
P r o d u c t i v i t y |
R o o s t i n g |
F o r a g i n g |
E s c a p e |
F l o c k i n g o n w a t e r |
N e s t i n g d e n s i t y |
S p e c i a l i z a t i o n |
H u n t e d b y m a n |
A n i m a l d e p r e d a t i o n s |
N o n - o i l p o l l u t i o n |
H i s t o r y o f o i l i n g |
S p r i n g |
S u m m e r |
F a l l |
W i n t e r |
T o t a l P o i n t s |
|
| Gaviidae | |||||||||||||||||||||
| Common loon (Gavia immer) | 1 | 3 | 3 | 3 | 1 | 5 | 5 | 5 | 5 | 1 | 1 | 3 | 1 | 1 | 3 | 3 | 1 | 0 | 1 | 1 | 47 |
| Yellow-billed loon (G. adamsii) | 3 | 3 | 5 | 3 | 5 | 5 | 5 | 5 | 5 | 1 | 1 | 3 | 1 | 1 | 0 | 3 | 5 | 1 | 5 | 5 | 65 |
| Arctic loon (G. arctica) | 3 | 3 | 3 | 3 | 3 | 5 | 5 | 5 | 5 | 1 | 1 | 3 | 1 | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 58 |
| Red-throated loon (G. stellata) | 1 | 3 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 1 | 1 | 3 | 1 | 1 | 3 | 3 | 1 | 0 | 1 | 1 | 49 |
| Podicipedidae | |||||||||||||||||||||
| Red-necked grebe (Podiceps grisegena) | 1 | 3 | 3 | 3 | 1 | 3 | 5 | 5 | 5 | 1 | 1 | 3 | 0 | 1 | 3 | 3 | 1 | 0 | 1 | 1 | 44 |
| Horned grebe (P. auritus) | 1 | 3 | 3 | 3 | 1 | 3 | 5 | 5 | 5 | 3 | 1 | 3 | 0 | 3 | 3 | 3 | 1 | 0 | 1 | 1 | 48 |
| Western grebe (Aechmophorus occidentalis) | 3 | 3 | 3 | 5 | 1 | 3 | 5 | 5 | 5 | 5 | 1 | 3 | 0 | 1 | 3 | 5 | 1 | 0 | 1 | 3 | 56 |
| Diomedeidae | |||||||||||||||||||||
| Short-tailed albatross (Diomedea albatrus) | 99 | 1 | 100 | ||||||||||||||||||
| Black-footed albatross (D. nigripes) | 5 | 1 | 1 | 5 | 3 | 5 | 5 | 3 | 3 | 1 | 5 | 5 | 0 | 0 | 1 | 3 | 1 | 1 | 1 | 1 | 50 |
| Laysan albatross (D. immutabilis) | 5 | 1 | 1 | 5 | 3 | 5 | 5 | 3 | 3 | 1 | 5 | 5 | 0 | 0 | 1 | 3 | 1 | 1 | 1 | 3 | 52 |
| Procellaridae | |||||||||||||||||||||
| Fulmar (Fulmarus glacialis) | 3 | 3 | 1 | 5 | 1 | 5 | 5 | 3 | 3 | 3 | 5 | 3 | 0 | 1 | 1 | 3 | 3 | 3 | 3 | 3 | 57 |
| Pink-footed shearwater (Puffinus creatopus) | 3 | 1 | 1 | 5 | 1 | 5 | 5 | 3 | 3 | 3 | 5 | 3 | 0 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 47 |
| Pale-footed shearwater (P. carneipes) | 1 | 1 | |||||||||||||||||||
| New Zealand shearwater (P. bulleri) | 1 | 1 | |||||||||||||||||||
| Sooty shearwater (P. griseus) | 1 | 1 | 1 | 5 | 1 | 5 | 5 | 3 | 3 | 5 | 5 | 3 | 1 | 1 | 1 | 3 | 1 | 5 | 1 | 0 | 51 |
| Slender-billed shearwater (P. tenuirostris) | 1 | 1 | 3 | 5 | 1 | 5 | 5 | 3 | 3 | 5 | 5 | 3 | 1 | 1 | 1 | 3 | 1 | 5 | 1 | 0 | 53 |
| Scaled petrel (Pterodroma inexpectata) | 1 | 1 | |||||||||||||||||||
| Cooks petrel (P. cookii) | 1 | 1 | |||||||||||||||||||
| Hydrobatidae | |||||||||||||||||||||
| Fork-tailed storm-petrel (Oceanodroma furcata) | 3 | 3 | 3 | 5 | 1 | 5 | 5 | 3 | 3 | 3 | 5 | 3 | 0 | 1 | 1 | 3 | 5 | 5 | 5 | 5 | 67 |
| Leach's storm-petrel (O. leucorhoa) | 1 | 3 | 1 | 5 | 1 | 5 | 5 | 3 | 3 | 3 | 5 | 3 | 0 | 1 | 1 | 3 | 5 | 5 | 5 | 5 | 63 [Pg 230] |
| Pelecanidae | |||||||||||||||||||||
| Brown pelican (Pelecanus occidentalis) | 1 | 1 | |||||||||||||||||||
| Phalacrocoracidae | |||||||||||||||||||||
| Double-crested cormorant (Phalacrocorax auritus) | 1 | 3 | 3 | 3 | 3 | 3 | 1 | 5 | 3 | 1 | 3 | 3 | 0 | 1 | 3 | 5 | 3 | 3 | 5 | 52 | |
| Brandt's cormorant (P. penicillatus) | 3 | 3 | 3 | 5 | 3 | 3 | 1 | 5 | 3 | 1 | 3 | 3 | 0 | 1 | 3 | 5 | 3 | 3 | 3 | 3 | 57 |
| Pelagic cormorant (P. pelagicus) | 3 | 3 | 3 | 5 | 3 | 3 | 1 | 5 | 3 | 3 | 3 | 3 | 0 | 1 | 3 | 5 | 5 | 1 | 5 | 5 | 63 |
| Red-faced cormorant (P. urile) | 5 | 3 | 3 | 5 | 3 | 3 | 1 | 5 | 3 | 3 | 3 | 3 | 0 | 1 | 1 | 5 | 5 | 5 | 3 | 3 | 63 |
| Ardeidae | |||||||||||||||||||||
| Great blue heron (Ardea herodias) | 1 | 3 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 3 | 3 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 29 |
| Anatidae | |||||||||||||||||||||
| Whooper swan (Olor cygnus) | 1 | 1 | |||||||||||||||||||
| Whistling swan (O. columbianus) | 3 | 3 | 3 | 3 | 3 | 3 | 5 | 3 | 1 | 5 | 1 | 3 | 3 | 1 | 3 | 1 | 3 | 0 | 3 | 0 | 50 |
| Trumpeter swan (O. buccinator) | 5 | 5 | 3 | 3 | 5 | 3 | 5 | 5 | 1 | 5 | 1 | 5 | 1 | 1 | 3 | 3 | 3 | 0 | 3 | 3 | 63 |
| Canada goose (Branta canadensis) | 1 | 3 | 1 | 1 | 5 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 34 |
| Black Brant (B. nigricans) | 3 | 3 | 3 | 5 | 3 | 3 | 5 | 5 | 3 | 5 | 3 | 3 | 5 | 1 | 3 | 5 | 3 | 1 | 5 | 3 | 70 |
| Emperor goose (Philacte canagica) | 3 | 5 | 5 | 5 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 5 | 5 | 3 | 5 | 5 | 70 |
| White-fronted goose (Anser albifrons) | 3 | 3 | 3 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 36 |
| Snow goose (Chen hyperborea) | 1 | 3 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 32 |
| Mallard (Anas platyrhynchos) | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 36 |
| Gadwall (A. strepera) | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 38 |
| Pintail (A. acuta) | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 36 |
| Common teal (A. crecca) | 1 | 1 | |||||||||||||||||||
| Green-winged teal (A. carolinensis) | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 34 |
| Blue-winged teal (A. discors) | 1 | 1 | |||||||||||||||||||
| Cinnamon teal (A. cyanoptera) | 1 | 1 | |||||||||||||||||||
| European wigeon (Mareca penelope) | 1 | 1 | |||||||||||||||||||
| American wigeon (M. americana) | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 36 |
| Shoveler (Spatula clypeata) | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 34 |
| Redhead (Aythya americana) | 1 | 3 | 1 | 1 | 5 | 3 | 5 | 5 | 5 | 3 | 1 | 3 | 5 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 52 |
| Ring-necked duck (A. collaris) | 1 | 1 | |||||||||||||||||||
| Canvasback (A. valisineria) | 1 | 3 | 1 | 1 | 5 | 3 | 5 | 5 | 5 | 3 | 1 | 3 | 5 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 52 |
| Greater scaup (A. marila) | 1 | 3 | 1 | 5 | 1 | 3 | 5 | 5 | 5 | 3 | 1 | 3 | 5 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 52 |
| Lesser scaup (A. affinis) | 1 | 3 | 1 | 3 | 1 | 3 | 5 | 5 | 5 | 3 | 1 | 3 | 5 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 50 [Pg 231] |
| Common goldeneye (Bucephala clangula) | 1 | 3 | 1 | 3 | 1 | 3 | 5 | 5 | 5 | 3 | 1 | 3 | 3 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 48 |
| Barrow's goldeneye (B. islandica) | 3 | 3 | 1 | 3 | 1 | 3 | 5 | 5 | 5 | 3 | 1 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 56 |
| Bufflehead (B. albeola) | 1 | 3 | 1 | 3 | 1 | 3 | 5 | 5 | 5 | 3 | 1 | 3 | 1 | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 52 |
| Oldsquaw (Clangula hyemalis) | 1 | 3 | 1 | 5 | 1 | 3 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 1 | 1 | 5 | 5 | 3 | 5 | 5 | 66 |
| Harlequin duck (Histrionicus histrionicus) | 3 | 5 | 1 | 5 | 1 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 1 | 5 | 5 | 5 | 5 | 5 | 60 |
| Steller's eider (Polysticta stelleri) | 3 | 3 | 5 | 5 | 1 | 3 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 1 | 1 | 5 | 5 | 3 | 5 | 5 | 72 |
| Common eider (Somateria mollissima) | 3 | 5 | 3 | 5 | 1 | 3 | 5 | 5 | 5 | 3 | 1 | 3 | 1 | 1 | 1 | 5 | 5 | 3 | 5 | 5 | 68 |
| King eider (S. spectabilis) | 3 | 5 | 3 | 5 | 1 | 3 | 5 | 5 | 5 | 5 | 1 | 3 | 1 | 1 | 1 | 5 | 5 | 3 | 5 | 5 | 70 |
| Spectacled eider (Lampronetta fisheri) | 5 | 5 | 5 | 5 | 3 | 3 | 5 | 5 | 5 | 5 | 3 | 3 | 1 | 1 | 1 | 5 | 5 | 3 | 5 | 5 | 78 |
| White-winged scoter (Melanitta deglandi) | 3 | 3 | 3 | 3 | 1 | 3 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 1 | 3 | 5 | 5 | 5 | 5 | 5 | 72 |
| Surf scoter (M. perspicillata) | 3 | 3 | 3 | 3 | 1 | 3 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 1 | 3 | 5 | 5 | 5 | 5 | 5 | 72 |
| Common scoter (Oidemia nigra) | 3 | 3 | 3 | 3 | 1 | 3 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 1 | 3 | 5 | 5 | 5 | 5 | 5 | 72 |
| Ruddy duck (Oxyura jamaicensis) | 1 | 3 | 1 | 3 | 1 | 1 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 3 | 3 | 3 | 1 | 0 | 1 | 3 | 55 |
| Hooded merganser (Laphodytes cucullatus) | 1 | 3 | 1 | 1 | 3 | 3 | 3 | 5 | 3 | 1 | 1 | 3 | 1 | 1 | 3 | 1 | 1 | 0 | 1 | 1 | 37 |
| Common merganser (Mergus merganser) | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 5 | 5 | 3 | 1 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 56 |
| Red-breasted merganser (M. serrator) | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 5 | 5 | 3 | 1 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 56 |
| Accipitridae | |||||||||||||||||||||
| Bald eagle (Haliaeetus leucocephalus) | 1 | 5 | 3 | 3 | 5 | 5 | 0 | 1 | 1 | 0 | 1 | 5 | 0 | 0 | 5 | 3 | 5 | 5 | 5 | 5 | 58 |
| Steller's sea eagle (H. pelagicus) | 1 | 1 | |||||||||||||||||||
| Marsh hawk (Circus cyaneus) | 1 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 19 |
| Pandionidae | |||||||||||||||||||||
| Osprey (Pandion haliaetus) | 1 | 3 | 1 | 1 | 5 | 5 | 0 | 1 | 1 | 0 | 1 | 5 | 3 | 1 | 5 | 1 | 1 | 1 | 1 | 0 | 37 |
| Falconidae | |||||||||||||||||||||
| Peregrine falcon (Falco peregrinus) | 1 | 3 | 1 | 1 | 5 | 5 | 0 | 1 | 1 | 0 | 1 | 3 | 3 | 0 | 5 | 1 | 3 | 3 | 3 | 1 | 41 |
| Gruidae | |||||||||||||||||||||
| Sandhill crane (Grus canadensis) | 1 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 24 |
| Rallidae | |||||||||||||||||||||
| American coot (Fulica americana) | 1 | 3 | 1 | 1 | 1 | 1 | 3 | 3 | 1 | 3 | 1 | 1 | 3 | 3 | 3 | 1 | 1 | 0 | 1 | 1 | 33 |
| Haematopodidae | |||||||||||||||||||||
| Black oystercatcher (Haematopus bachmani) | 5 | 5 | 5 | 5 | 3 | 5 | 1 | 1 | 1 | 1 | 1 | 5 | 0 | 1 | 3 | 3 | 5 | 5 | 5 | 5 | 65 |
| Charadriidae | |||||||||||||||||||||
| Ringed plover (Charadrius hiaticula) | 1 | 1 | |||||||||||||||||||
| Semipalmated plover (C. semipalmatus) | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 5 | 1 | 1 | 3 | 1 | 3 | 0 | 28 |
| Mongolian plover (C. mongolus) | 1 | 1 | |||||||||||||||||||
| Killdeer (C. vociferus) | 1 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 5 | 1 | 1 | 1 | 1 | 1 | 0 | 26 [Pg 232] |
| Dotterel (Eudromias morinellus) | 1 | 1 | |||||||||||||||||||
| American golden plover (Pluvialis dominica) | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 1 | 1 | 3 | 1 | 3 | 3 | 5 | 0 | 1 | 1 | 0 | 1 | 0 | 35 |
| Black-bellied plover (Squatarola squatarola) | 1 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 3 | 5 | 1 | 3 | 3 | 1 | 3 | 0 | 43 |
| Surfbird (Aphriza virgata) | 5 | 1 | 5 | 5 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 0 | 5 | 1 | 3 | 5 | 0 | 5 | 3 | 54 |
| Ruddy turnstone (Arenaria interpres) | 1 | 1 | 3 | 5 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 0 | 5 | 1 | 3 | 3 | 3 | 3 | 0 | 44 |
| Black turnstone (A. melanocephala) | 5 | 3 | 3 | 5 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 0 | 5 | 1 | 3 | 5 | 3 | 5 | 3 | 57 |
| Scolopacidae | |||||||||||||||||||||
| Common snipe (Capella gallinago) | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 5 | 1 | 1 | 1 | 1 | 1 | 0 | 29 |
| Eurasian curlew (Numenius arquata) | 1 | 1 | |||||||||||||||||||
| Whimbrel (N. phaeopus) | 1 | 1 | 1 | 3 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | 3 | 1 | 3 | 0 | 37 |
| Bristle-thighed curlew (N. tahitiensis) | 5 | 1 | 1 | 5 | 5 | 3 | 3 | 1 | 1 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 3 | 1 | 3 | 0 | 45 |
| Eskimo curlew (N. borealis) | 99 | 1 | 100 | ||||||||||||||||||
| Upland plover (Bartramia longicauda) | 1 | 1 | 1 | 0 | 5 | 3 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 3 | 1 | 0 | 1 | 1 | 1 | 0 | 26 |
| Spotted sandpiper (Actitis macularia) | 1 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 1 | 1 | 1 | 1 | 1 | 0 | 24 |
| Common sandpiper (Tringa hypoleucos) | 1 | 1 | |||||||||||||||||||
| Solitary sandpiper (T. solitaria) | 1 | 1 | |||||||||||||||||||
| Wood sandpiper (T. glareola) | 1 | 1 | |||||||||||||||||||
| Wandering tattler (Heteroscelus incanum) | 5 | 1 | 1 | 5 | 5 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | 5 | 0 | 5 | 0 | 48 |
| Polynesian tattler (H. brevipes) | 1 | 1 | |||||||||||||||||||
| Willet (Catoptrophorus semipalmatus) | 1 | 1 | |||||||||||||||||||
| Greater yellowlegs (Totanus melanoleucus) | 1 | 5 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 0 | 1 | 0 | 30 |
| Lesser yellowlegs (T. flavipes) | 1 | 5 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 0 | 1 | 0 | 30 |
| Spotted redshank (T. totanus) | 1 | 1 | |||||||||||||||||||
| Greenshank (Tringa nebularia) | 1 | 1 | |||||||||||||||||||
| Knot (Calidris canutus) | 1 | 1 | 1 | 5 | 5 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 3 | 3 | 1 | 0 | 39 |
| Great knot (C. tenuirostris) | 1 | 1 | |||||||||||||||||||
| Rock sandpiper (Erolia ptilocnemis) | 5 | 3 | 3 | 5 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 0 | 3 | 1 | 3 | 5 | 5 | 5 | 5 | 59 |
| Sharp-tailed sandpiper (E. acuminata) | 3 | 1 | 3 | 5 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 0 | 3 | 3 | 3 | 3 | 0 | 3 | 3 | 46 |
| Pectoral sandpiper (E. melanotos) | 1 | 1 | 3 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 1 | 1 | 3 | 3 | 3 | 0 | 32 |
| White-rumped sandpiper (E. fuscicollis) | 1 | 1 | |||||||||||||||||||
| Baird sandpiper (E. bairdii) | 1 | 3 | 3 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 1 | 1 | 3 | 3 | 3 | 0 | 34 |
| Least sandpiper (E. minutilla) | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 1 | 1 | 3 | 3 | 3 | 0 | 34 |
| Long-toed stint (E. subminuta) | 1 | 1 | |||||||||||||||||||
| Temminck's stint (Calidrus temminckii) | 1 | 1 | |||||||||||||||||||
| Rufous-necked sandpiper (E. ruficollis) | 3 | 1 | 3 | 5 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 0 | 3 | 1 | 3 | 1 | 1 | 1 | 0 | 36 |
| Curlew sandpiper (E. ferruginea) | 1 | 1 [Pg 233] | |||||||||||||||||||
| Dunlin (E. alpina) | 1 | 3 | 1 | 5 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 41 |
| Short-billed dowitcher (Limnodromus griseus) | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 0 | 45 |
| Long-billed dowitcher (L. scolopaceus) | 5 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 0 | 47 |
| Stilt sandpiper (Micropalama himantopus) | 1 | 1 | |||||||||||||||||||
| Semipalmated sandpiper (Ereunetes pusillus) | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 1 | 1 | 3 | 5 | 3 | 0 | 34 |
| Western sandpiper (E. mauri) | 5 | 3 | 3 | 5 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 3 | 3 | 3 | 5 | 3 | 1 | 47 |
| Buff-breasted sandpiper (Tryngites subruficollis) | 1 | 1 | |||||||||||||||||||
| Marbled godwit (Limosa fedoa) | 1 | 1 | |||||||||||||||||||
| Bar-tailed godwit (L. lapponica) | 3 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 5 | 5 | 3 | 0 | 49 |
| Hudsonian godwit (L. haemastica) | 1 | 1 | |||||||||||||||||||
| Black-tailed godwit (L. limosa) | 1 | 1 | |||||||||||||||||||
| Ruff (Philomachus pugnax) | 1 | 1 | |||||||||||||||||||
| Sanderling (Crocethia alba) | 3 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 0 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 45 |
| Spoon-billed sandpiper (Eurynorhynchus pygmeum) | 1 | 1 | |||||||||||||||||||
| Phalaropodidae | |||||||||||||||||||||
| Red phalarope (Phalaropus fulicarius) | 3 | 1 | 1 | 5 | 1 | 3 | 5 | 5 | 1 | 5 | 1 | 5 | 0 | 3 | 1 | 5 | 5 | 3 | 5 | 0 | 58 |
| Wilson's phalarope (Steganopus tricolor) | 1 | 1 | |||||||||||||||||||
| Northern phalarope (Lobipes lobatus) | 3 | 1 | 3 | 5 | 1 | 3 | 5 | 5 | 1 | 5 | 1 | 5 | 0 | 3 | 3 | 5 | 5 | 3 | 5 | 0 | 62 |
| Stercorariidae | |||||||||||||||||||||
| Pomarine jaeger (Stercorarius pomarinus) | 1 | 1 | 1 | 5 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 0 | 41 |
| Parasitic jaeger (S. parasiticus) | 1 | 1 | 1 | 5 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 1 | 1 | 3 | 3 | 3 | 3 | 3 | 0 | 43 |
| Long-tailed jaeger (S. longicaudus) | 1 | 1 | 1 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 0 | 39 |
| Skua (Catharacta skua) | 1 | 1 | |||||||||||||||||||
| Laridae | |||||||||||||||||||||
| Glaucous gull (Larus hyperboreus) | 1 | 5 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 1 | 0 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 45 |
| Glaucous-winged gull (L. glaucescens) | 5 | 1 | 3 | 5 | 1 | 3 | 3 | 3 | 1 | 3 | 5 | 1 | 1 | 1 | 1 | 1 | 5 | 5 | 5 | 3 | 56 |
| Slaty-backed gull (L. schistisagus) | 1 | 1 | |||||||||||||||||||
| Western gull (L. occidentalis) | 3 | 1 | 3 | 5 | 1 | 3 | 3 | 3 | 1 | 3 | 5 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 48 |
| Herring gull (L. argentatus) | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 38 |
| Thayer's gull (L. thayeri) | 3 | 3 | 5 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 3 | 3 | 42 |
| California gull (L. californicus) | 3 | 5 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 38 |
| Ring-billed gull (L. delawarensis) | 1 | 5 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 36 |
| Mew gull (L. canus) | 1 | 5 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 44 |
| Black-headed gull (L. ridibundus) | 1 | 1 | |||||||||||||||||||
| Franklin's gull (L. pipixcan) | 1 | 1[Pg 234] | |||||||||||||||||||
| Bonaparte's gull (L. philadelphia) | 1 | 5 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 3 | 1 | 40 |
| Heerman's gull (L. heermanni) | 1 | 1 | |||||||||||||||||||
| Ivory gull (Pagophila eburnea) | 1 | 5 | 3 | 5 | 3 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 43 |
| Black-legged kittiwake (Rissa tridactyla) | 1 | 3 | 3 | 5 | 1 | 3 | 3 | 3 | 1 | 3 | 5 | 3 | 0 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 49 |
| Red-legged kittiwake (R. brevirostris) | 5 | 5 | 5 | 5 | 3 | 3 | 3 | 3 | 1 | 3 | 5 | 3 | 0 | 1 | 0 | 1 | 5 | 5 | 5 | 5 | 66 |
| Ross' gull (Rhodostethia rosea) | 5 | 5 | 3 | 5 | 3 | 3 | 3 | 3 | 1 | 3 | 5 | 5 | 0 | 1 | 0 | 1 | 3 | 1 | 3 | 3 | 56 |
| Sabine's gull (Xema sabini) | 3 | 3 | 3 | 5 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 0 | 1 | 1 | 1 | 3 | 3 | 3 | 0 | 44 |
| Common tern (Sterna hirundo) | 1 | 1 | |||||||||||||||||||
| Arctic tern (S. paradisaea) | 1 | 1 | 1 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 0 | 1 | 1 | 1 | 3 | 1 | 3 | 0 | 32 |
| Aleutian tern (S. aleutica) | 5 | 3 | 3 | 5 | 3 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 0 | 1 | 1 | 1 | 5 | 5 | 5 | 1 | 53 |
| Caspian tern (Hydroprogne caspia) | 1 | 1 | |||||||||||||||||||
| Black tern (Chlidonias niger) | 1 | 1 | |||||||||||||||||||
| Alcidae | |||||||||||||||||||||
| Common murre (Uria aalge) | 1 | 5 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 1 | 1 | 3 | 5 | 3 | 3 | 3 | 3 | 70 |
| Thick-billed murre (U. lomvia) | 1 | 5 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 1 | 1 | 3 | 5 | 3 | 3 | 3 | 3 | 70 |
| Dovekie (Plautus alle) | 1 | 1 | |||||||||||||||||||
| Black guillemot (Cepphus grylle) | 1 | 5 | 3 | 5 | 3 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 1 | 1 | 1 | 5 | 3 | 3 | 3 | 3 | 70 |
| Pigeon guillemot (C. columba) | 5 | 5 | 3 | 5 | 3 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 1 | 1 | 3 | 5 | 5 | 5 | 5 | 3 | 82 |
| Marbled murrelet (Brachyramphus marmoratus) | 5 | 5 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 1 | 3 | 3 | 5 | 5 | 5 | 5 | 5 | 84 |
| Kittlitz's murrelet (B. brevirostris) | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 5 | 5 | 5 | 5 | 5 | 88 |
| Xantus' murrelet (Endomychura hypoleuca) | 1 | 1 | |||||||||||||||||||
| Ancient murrelet (Synthliboramphus antiquus) | 3 | 3 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 5 | 3 | 3 | 3 | 3 | 74 |
| Cassin's auklet (Ptychoramphus aleutica) | 5 | 3 | 5 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 5 | 5 | 5 | 5 | 3 | 84 |
| Parakeet auklet (Cyclorrhynchus psittacula) | 3 | 3 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 5 | 5 | 5 | 5 | 3 | 80 |
| Crested auklet (Aethia cristatella) | 3 | 3 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 3 | 1 | 5 | 5 | 3 | 5 | 3 | 76 |
| Least auklet (A. pusilla) | 3 | 3 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 5 | 5 | 5 | 5 | 3 | 80 |
| Whiskered auklet (A. pygmaea) | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 5 | 5 | 5 | 5 | 5 | 88 |
| Rhinoceros auklet (Cerorhinca monocerata) | 3 | 3 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 3 | 3 | 5 | 3 | 3 | 3 | 3 | 74 |
| Horned puffin (Fratercula corniculata) | 3 | 5 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 1 | 3 | 1 | 5 | 3 | 3 | 3 | 3 | 72 |
| Tufted puffin (Lunda cirrhata) | 3 | 5 | 3 | 5 | 1 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 1 | 3 | 1 | 5 | 3 | 3 | 3 | 3 | 72 |
| Alcedinidae | |||||||||||||||||||||
| Belted kingfisher (Megaceryle alcyon) | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 5 | 1 | 0 | 1 | 3 | 0 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 28 |
| Corvidae | |||||||||||||||||||||
| Common raven (Corvus corax) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 21 |
| Northwestern crow (C. caurinus) | 3 | 5 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 3 | 1 | 1 | 1 | 1 | 5 | 5 | 5 | 5 | 47 |
| Point assignment | |||
|---|---|---|---|
| 1 | 3 | 5 | |
| Range | |||
| Breeding | Large | Medium | Small |
| Migration | Long | Medium | Short |
| Winter | Large | Medium | Small |
| Marine orientation | Coastal zone | Intertidal | Open water |
| Population | |||
| Size | Large | Medium | Small |
| Productivity | Large | Medium | Small |
| Habits | |||
| Roosting | Shore | Drift | Water |
| Foraging | Walking | Flying | Swimming |
| Escape | Leave area | Fly | Dive |
| Flocking | Small | Medium | Large |
| Nesting density | Low | Medium | High |
| Specialization | Low | Medium | High |
| Mortality | |||
| Hunted by man | Low | Medium | High |
| Animal depredations | Low | Medium | High |
| Non-oil pollution | Low | Medium | High |
| History of oiling | Low | Medium | High |
| Exposure | |||
| Spring | Low | Medium | High |
| Summer | Low | Medium | High |
| Fall | Low | Medium | High |
| Winter | Low | Medium | High |
With these points in mind it is immediately obvious that Southeast Alaska (Table 4), which has only 9 high-score birds, offers far less potential for bird problems than does the Aleutian area (Table 5), which has 24 high-score species. The planning agency could make some immediate decisions on site priorities and research funding based on such information.
We are convinced that the OVI principle expressed here will become a useful management tool with all sorts of possible applications. We recognize some difficulties with the present version, but believe it is timely to present the system so that a broader range of thought, improvements, and application can be applied to it.
Of prime importance is the system's simplicity. The use of four levels of value for each factor, instead of five or more, is an attempt to simplify. Ian McHarg (1969) has shown that extremely complex land-use values can be graphically compared and displayed by using three levels in a way that is useful to decision makers. The difficulty of using more levels of value was indicated by Sparrowe and Wight (1975) who used up to 10 levels, enormously complicating the problem of dealing with low-quality information, which is often all that is available. The use of scores of 0, 1, 3, 5 instead of 0, 1, 2, 3 for 20 factors enabled us to use the convenient 100 points instead of 60 points as the maximum potential total score for any species.
The 20 factors that were evaluated are admittedly arbitrary; with refinement and more detailed data they could be adjusted to show better separation between affected species. The decision to use 20 factors instead of more or less again relates to simplicity. This appears to be the minimum number that will assure species separation and that can be neatly displayed.
| Family | Number of species | Total OVI | OVI per species | |
|---|---|---|---|---|
| Average | Range | |||
| Loons—Gaviidae | 4 | 219 | 55 | 47-65 |
| Grebes—Podicipedidae | 3 | 148 | 49 | 44-56 |
| Albatrosses—Diomedeidae | 2 | 102 | 51 | 50-52 |
| Shearwaters—Procellaridae | 4 | 208 | 52 | 47-57 |
| Storm-petrels—Hydrobatidae | 2 | 130 | 65 | 63-67 |
| Cormorants—Phalacrocoracid | 4 | 235 | 59 | 52-63 |
| Herons—Ardeidae | 1 | 29 | 29 | 29 |
| Waterfowl—Anatidae | 33 | 1,765 | 53 | 32-78 |
| Eagles and Hawks—Accipitridae | 2 | 77 | 39 | 19-58 |
| Ospreys—Pandionidae | 1 | 37 | 37 | 37 |
| Falcons—Falconidae | 1 | 41 | 41 | 41 |
| Cranes—Gruidae | 1 | 24 | 24 | 24 |
| Rails and Coots—Rallidae | 1 | 33 | 33 | 33 |
| Oystercatchers—Haematopodidae | 1 | 65 | 65 | 65 |
| Plovers—Charadriidae | 7 | 287 | 41 | 26-57 |
| Sandpipers—Scolopacidae | 22 | 857 | 39 | 24-59 |
| Phalaropes—Phalaropodidae | 2 | 120 | 60 | 58-62 |
| Jaegers and Skuas—Stercorariidae | 3 | 123 | 41 | 39-43 |
| Gulls and Terns—Laridae | 16 | 730 | 46 | 32-66 |
| Auks—Alcidae | 15 | 1,164 | 78 | 70-88 |
| Kingfishers—Alcedinidae | 1 | 28 | 28 | 28 |
| Crows—Corvidae | 2 | 68 | 34 | 21-47 |
| Total and Mean | 128 | 6,490 | 51 | 19-88 |
The system will be much more useful when it is expanded to the subspecific level. Many Holarctic species are represented in the Northeast Pacific by a single race that would have a much higher OVI than the species as a whole. For example, the OVI for the Peale's peregrine falcon (Falco peregrinus pealei) confined to marine habitats within the Pacific region would be high; and the endangered Aleutian Canada goose (Branta canadensis leucopareia) would score 100 points instead of the 34 we show for Canada geese (B. c.). If Tables 4 and 5 showed subspecies, the differences in value would be more marked.
Tables 4 and 5 are for broad geographical areas. A comparison between smaller areas would probably show more dramatic differences.
Because the dearth of easily available, applicable information poses a problem in evaluating the various factors, our scoring was conservative. Experts on the various avian families can doubtless refine the scoring. If this system proves useful, investigators will begin to acquire the information needed for more precise evaluations. Ultimate perfection may never be achieved; however, as with the field guides, the fact of minor professional disagreement should not destroy the system's utility.
We believe re-scoring of all birds on the basis of various projects should be avoided because a standard against which individual projects can be measured is needed. If everyone did their own scoring, there would be no standard, and projects evaluated by different investigators would not be comparable. If a species list for the project area and standard point scores are used, the level of involvement for many species and perhaps for most species will be properly identified. As with any system, there will be exceptions and the assessor will need to deal with these as appropriate. The result will still be to focus attention on those species and impacting factors where it is most needed.
| OVI 1-20 | OVI 21-40 | OVI 41-60 | OVI 61-80 | OVI 81-100 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Marsh hawk | 19 | Great blue heron | 29 | Common loon | 47 | Pelagic cormorant | 63 | Pigeon guillemot | 82 |
| 52 species, rare or occasional (one point each) | 52 | Canada goose | 34 | Arctic loon | 58 | Oldsquaw | 66 | Marbled murrelet | 84 |
| White-fronted goose | 36 | Red-throated loon | 49 | White-winged scoter | 72 | ||||
| Snow goose | 32 | Red-necked grebe | 44 | Surf scoter | 72 | ||||
| Mallard | 36 | Horned grebe | 48 | Black oystercatcher | 65 | ||||
| Pintail | 36 | Whistling swan | 50 | Northern phalarope | 62 | ||||
| Green-winged teal | 34 | Trumpeter swan | 63 | Common murre | 70 | ||||
| American wigeon | 36 | Greater scaup | 52 | ||||||
| Semipalmated plover | 28 | Lesser scaup | 52 | ||||||
| Killdeer | 26 | Common goldeneye | 48 | ||||||
| Common snipe | 29 | Barrow's goldeneye | 56 | ||||||
| Spotted sandpiper | 24 | Bufflehead | 52 | ||||||
| Greater yellowlegs | 30 | Harlequin duck | 60 | ||||||
| Lesser yellowlegs | 30 | Common merganser | 56 | ||||||
| Pectoral sandpiper | 32 | Red-breasted merganser | 56 | ||||||
| Least sandpiper | 34 | Bald eagle | 58 | ||||||
| Herring gull | 38 | Peregrine falcon | 41 | ||||||
| Bonaparte's gull | 40 | Black turnstone | 57 | ||||||
| Arctic tern | 32 | Rock sandpiper | 59 | ||||||
| Belted kingfisher | 28 | Dunlin | 41 | ||||||
| Common raven | 21 | Short-billed dowitcher | 41 | ||||||
| Western sandpiper | 47 | ||||||||
| Glaucous-winged gull | 56 | ||||||||
| Thayer's gull | 42 | ||||||||
| Mew gull | 44 | ||||||||
| Northwestern crow | 47 | ||||||||
| Totals | 71 | 665 | 1,324 | 470 | 166 | ||||
| OVI 1-20 | OVI 21-40 | OVI 41-60 | OVI 61-80 | OVI 81-100 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 80 species, rare or occasional (one point each) | 80 | Canada goose | 34 | Fulmar | 57 | Fork-tailed storm-petrel | 67 | Pigeon guillemot | 82 |
| Least sandpiper | 34 | Slender-billed shearwater | 53 | Leach's storm-petrel | 63 | Whiskered auklet | 88 | ||
| Arctic tern | 32 | Greater scaup | 52 | Pelagic cormorant | 63 | ||||
| Common raven | 21 | Common goldeneye | 48 | Red-faced cormorant | 63 | ||||
| Bufflehead | 52 | Black Brant | 70 | ||||||
| Harlequin duck | 60 | Emperor goose | 70 | ||||||
| Bald eagle | 58 | Oldsquaw | 66 | ||||||
| Peregrine falcon | 41 | Steller's eider | 72 | ||||||
| Ruddy turnstone | 44 | Common eider | 68 | ||||||
| Rock sandpiper | 59 | King eider | 70 | ||||||
| Western sandpiper | 47 | White-winged scoter | 72 | ||||||
| Red phalarope | 58 | Common scoter | 72 | ||||||
| Parasitic jaeger | 43 | Black oystercatcher | 65 | ||||||
| Glaucous-winged gull | 56 | Red-legged kittiwake | 66 | ||||||
| Black-legged kittiwake | 49 | Common murre | 70 | ||||||
| Thick-billed murre | 70 | ||||||||
| Ancient murrelet | 74 | ||||||||
| Parakeet auklet | 80 | ||||||||
| Crested auklet | 76 | ||||||||
| Least auklet | 80 | ||||||||
| Horned puffin | 72 | ||||||||
| Tufted puffin | 72 | ||||||||
| Totals | 80 | 121 | 777 | 1,541 | 170 | ||||
We have used our OVI system to show the vulnerability of birds to oil, but it seems likely that the vulnerability index could be applied on a much broader scale to help make decisions in other areas of human activity and resource development. The vulnerability index system could be applied to terrestrial as well as aquatic species by adding or subtracting impacting factors, as appropriate. Indexes relating the impact of man upon each North American species could have broad uses in the field of conservation. Population explosions, as well as declines, might be predictable. Human activity could be better adjusted to favor or depress wildlife populations, as appropriate.
We believe that this vulnerability index system has promise for aiding in the decision-making processes upon which future bird conservation will depend.
American Ornithologists' Union. 1957. Check-list of North American birds. 5th ed. Lord Baltimore Press, Baltimore, Maryland.
Fay, F. H., and T. J. Cade. 1959. An ecological analysis of the avifauna of St. Lawrence Island, Alaska. Univ. Calif. Publ. Zool. 63(2):73-150.
Gabrielson, J. N., and F. C. Lincoln. 1959. The birds of Alaska. The Stackpole Company, Harrisburg, Pa., and Wildlife Management Institute, Washington, D.C. 922 pp.
Gibson, D. D. 1970. Check-list of the birds of Alaska. Univ. of Alaska, Fairbanks. 2 pp.
Isleib, M. E., and B. Kessel. 1973. Birds of the North Gulf Coast-Prince William Sound region, Alaska. Univ. of Alaska Biol. Pap. 14. 149 pp.
Kortright, F. H. 1942. The ducks, geese and swans of North America. Wildlife Management Institute, Washington, D.C.
McHarg, I. L. 1969. Design with nature. Natural History Press, Garden City, N. Y. 197 pp.
Murie, O. J. 1959. Fauna of the Aleutian Islands and Alaska Peninsula. U.S. Fish Wildl. Serv., N. Am. Fauna 61:1-364.
Palmer, R. S. 1962. Handbook of North American birds. Vol. 1. Yale University Press, New Haven, Conn. 567 pp.
Peterson, R. T., G. Montfort, and P. H. D. Hallom. 1967. A field guide to the birds of Britain and Europe. Houghton Mifflin Co., New York.
Robbins, D. S., B. Bruun, and H. S. Zimm. 1966. Birds of North America, a guide to field identification. Golden Press, New York.
Sanger, G. A. 1972. Preliminary standing stock and biomass estimates of seabirds in the subarctic Pacific region. Pages 589-611 in A. Y. Takenouti et al., eds. Biological oceanography of the North Pacific Ocean. Idemitsy Shoten, Tokyo.
Sparrowe, R. D., and H. M. Wight. 1975. Setting priorities for the endangered species program. Proc. N. Am. Wildl. Nat. Resourc. Conf. 40:142-156.
Stout, G. D., P. Matthiessen, V. R. Clem, and R. S. Palmer. 1967. The shorebirds of North America. Viking Press, New York. 270 pp.
U.S. Fish and Wildlife Service. 1974. Birds of the Aleutian Islands National Wildlife Refuge. Washington, D.C.
U.S. Forest Service and Alaska Department of Fish and Game. 1970. Birds of Southeast Alaska, a check-list. The agencies, Juneau, Alaska. 12 pp.
by
Ralph W. Larson
Washington Department of Game
600 North Capitol Way
Olympia, Washington 98504
Seabirds are one of the most visible biological components of ecosystems, and yet little is known about them. They could readily be used as an index of marine environmental quality if adequate studies were conducted to determine populations, habitat needs, and causes of fluctuations in abundance. The lack of adequate funding at the State level has precluded necessary studies to make these determinations and to provide habitat protection and preservation.
The State of Washington has developed a funding source for protection, preservation, and enhancement of nongame wildlife, which includes seabirds. The sale of personalized license plates for vehicles is now providing some funds for nongame wildlife management—funds which should increase as the popularity of the licensing program increases. Outdoor Recreation Bonds are providing funding for habitat preservation. Authorities provided the Washington Game Department are adequate to manage and protect seabird species. Other State laws offer additional protection to their habitat—specifically the Shoreline Management Act of 1971 and the State Environmental Act.
It has been often stated that seabirds are one of the most visible biological components of ecosystems, and yet little is known about them. Most studies to date have been on fish, and because of their recreational and commercial value, the concern for maintaining the marine environment has been primarily a result of the concern for maintaining the fishery resource. The visible knowledge of the fishery resource, however, becomes an "after-the-fact" knowledge since the status of the stocks relates to the value and amount of the fishery—a fishery resulting from survival under the surface in the marine environment that can be very secretive about its quality until it is too late to do something about it. Seabirds, however, are visible above the surface, in numbers that can reflect changes in the marine environment that occur below the surface, since many depend on the subsurface quality that reflects populations of fish.
Studies in Oregon have indicated that consumption of pelagic fish by murres (Uria spp.), cormorants (Phalacrocorax spp.), storm-petrels (Oceanodroma spp.), and shearwaters (Puffinus spp.) account for about 22% of the annual production of various species of these fish. A decline in this food source will reflect a decline in the seabird population. Why then should it be necessary to use only fish populations as an index of marine environmental quality, when seabirds can more readily be observed and can reflect the same changes that occur?
As a public wildlife agency, the Washington Department of Game is often attempting to justify the value of seabirds, and sometimes that is not easy. When fishermen complain that the seabirds are eating all of the food of our mighty salmon, and hunters indicate little compassion because the birds have no value to sport hunting, one has to think a little to[Pg 244] explain their value. However, rhinoceros auklets (Cerorhinca monocerata) do drive herring into ball-shaped schools, which attracts salmon in search of food—which in turn provides a signal to fishermen that salmon may soon be in the area. Explaining value to the hunter is a bit more difficult, but anyone who has taken the time to go out on our marine waters and observe the many species of seabirds and watch them flying and feeding cannot help but be fascinated by them. The flight of thousands of murres skimming over the water surface and somehow managing not to dash headlong into a wave is a fascinating sight.
We who are in fish and wildlife work have had to readjust our thinking and values during recent years. Our primary programs and concerns for many years were with the fish, birds, and animals that were of value to fishermen and hunters. Species of wildlife that we now classify as nongame received incidental benefit from programs related to game fish, game birds, and game animals, but we did not do badly in maintaining and enhancing these incidental wildlife species, mostly by indirection. However, in the last few years our Department, at least, has taken on a new responsibility and a new look as related to nongame wildlife.
Our first positive step in this direction was to develop a funding source for nongame wildlife programs. Our funding attempt charted its way through stormy waters, but finally ended up being voted on by the citizens of the State. Our citizens passed Referendum 33, which provided funds to the Department for nongame wildlife programs from the sale of personalized license plates. Although the funds have not been adequate, they are a step in the right direction and have permitted the Department to engage in a modest program of research and management. We have placed one person in charge of our program to do the planning and programming so necessary for developing an effective, growing program. During the 1st year of operation, we contracted studies on the rhinoceros auklet, the tufted puffin (Lunda cirrhata), and the black oystercatcher (Haematopus bachmani). These studies have provided a basic knowledge of some of the problems facing these seabird species. As funds increase, additional studies will be made to provide more information on these birds and others.
During the 1975 legislative session we were successful in amending the personalized license program to include automobiles other than passenger cars—a step which should further enhance our funding. We anticipate that funding will increase from the sale of these license plates each year. They serve as their own advertisement, and as more plates are sold, the exposure to the public increases. We anticipate that within the next few years the funding should reach $150,000 per year—a modest sum to be sure, but nevertheless adequate to establish a viable program.
We have been involved in studies funded through other agencies that involve seabirds. The principal reasons for the studies are not seabirds, but they become an integral part of any analysis that must be made of our saltwater environs. One such study involves a comprehensive status survey of the marine shoreline fauna of Washington. The Department of Ecology has provided the funding as a part of their analysis of resources that may be adversely affected by oil spills and economic development of our shorelines. This study will be the first one designed to comprehensively identify wildlife species associated with our shorelines and will determine the species, their status, location, and habitat. This study will provide a basis for readily identifying visually the results of oil spills and of the economic development of critical habitat areas, and provide sound basic data for use in combating destructive projects in the marine environment.
We are finding that you cannot separate functions of other governmental agencies that deal with marine waters from seabird analysis. Pollution responsibilities, shoreline management, coastal zone management, clam dredging, channel dredging, erosion control, housing development, industrial expansion, shipping port development—to name a few—all must have some effect on our seabird species. Therefore, we must concentrate on obtaining an adequate data base to insure the perpetuation of these valuable marine species.
As I indicated earlier, the Department of Game has not had a special program to manage seabirds in the past, but this should not indicate that we have not assisted in maintaining the seabird resource. Our basic land[Pg 245] acquisition program designed for waterfowl enhancement has benefited seabirds. We now own some 15,500 acres of lands, tideland, and marshes bordering the marine waters (including our Skagit and Nisqually holdings) which provide habitat and protection for many seabirds. We also recently acquired 48 acres on Protection Island, designed to protect the nesting area of the rhinoceros auklet. This purchase was an excellent example of how combined efforts of several groups accomplished a nearly impossible goal.
Protection Island had been subdivided for summer home development and many lots had been sold. The developer, however, got caught in the requirements of our Shoreline Management Act with his last subdivision. The uproar caused due to the use of this subdivision by auklets created an atmosphere that made subdivision a real conservation issue. The outspoken critics of the project from the Audubon Society, Fish and Wildlife Service, independent conservationists, and our Department enlisted the aid of Nature Conservancy to negotiate for purchase of this subdivision, and after lengthy negotiations the option was obtained, and the Department purchased the land from the Nature Conservancy with funds provided by the Interagency Committee for Outdoor Recreation. The area now is destined to be a seabird sanctuary, with limited public viewing and incidental recreation use. This project is an excellent example of the power of cooperative efforts by conservationists to protect a resource.
The State of Washington now has a reasonably good legislative base to insure constructive programs for management of our seabird resource. Our legislative authority lies in State statutes under Title 77. These authorities first provide that the wild birds, wild animals, and game fish of the State are the property of the State and that they shall be preserved, protected, and perpetuated. Any regulations for taking shall be designed so as to not "impair the supply thereof."
The commission also has the authority to classify wild birds. Seabirds, other than hunted species, fall into the category of nongame birds. We also have the authority to regulate the propagation and protection of wild birds, develop rules and regulations for taking them (or to prohibit taking them), and to create game reserves and closed areas where necessary to protect various species. Our authorities also include the obligation to enforce the laws, rules, and regulations pertaining to the protection of all wild birds.
The Department may also acquire land for habitat and for sanctuaries for nongame birds and may exchange lands for these purposes. We may also enter into agreements with the Federal Government, persons, and municipal subdivisions of the State for all matters relating to propagation, protection, and conservation of all wild birds, and may lease State lands for this purpose.
We believe our authorities are now totally adequate to satisfactorily manage the State's marine bird resources.
In addition to our personalized license plate legislation, which earmarks funds for nongame wildlife, other State laws and programs assist in protection of this resource. One program that has assisted materially in providing funds for habitat acquisition is a bond issue passed by citizens of the State designed to acquire and develop recreational land in the State for public use. Our Interagency Committee for Outdoor Recreation provides the necessary mechanism for funding of projects, using these bond monies to match Federal funds. Although recreation is a key factor in obtaining funding, it is still possible to acquire key habitat for wildlife and develop a people-use program around the primary purpose for acquisition.
The purchase of a portion of Protection Island was accomplished by use of these funds, as I indicated earlier, and we are now working again with Nature Conservancy to acquire key bald eagle habitat on the Skagit River in northwestern Washington. The bond issues total some $50 million, of which this Department receives about 15%. The State now is in its third bond issue, and we hope the citizens will continue to support this program.
One of the newer laws is the Shoreline Management Act of 1971. This act provides for development of comprehensive shoreline management programs designed to control the development of these areas to insure protection of the public interest, while still recognizing and protecting private property rights consistent with this public interest. These plans[Pg 246] must be developed with citizen involvement. Shoreline classification generally falls into four categories—natural, conservancy, rural, and urban. The natural classification can accomplish the most substantial benefit for marine birds. Provisions are also made for protection of "shorelines of statewide significance." Plans for these areas must give preference to uses favoring the public and long-range goals. These shorelines cover the areas between low and high tide levels on inland waters and high water and the western boundary of the State on our Pacific Ocean coast.
Our State Environmental Policy Act, which requires that environmental impact statements be prepared for various programs and developments, gives our Department an opportunity to insure that our valuable wildlife resources are given consideration during the planning phase of the proposed project.
The Department feels that our authorities at this time are adequate to protect marine bird populations and their habitat. The one lacking factor, as usual, is the funding for both adequate management programs and habitat protection. Our marine habitat is rapidly being developed for recreational homesites and public use which can eliminate key habitat use. A greater public awareness of the needs of marine birds can be a help in preventing destruction of their habitat; however, money talks the loudest. The acquisition of these key habitats is the most positive means of insuring their retention. We have no solution at this time to the funding problem and only hope that someone smarter than we are can provide an acceptable solution before all of our efforts become too little and too late.
by
W. T. Munro
British Columbia Fish and Wildlife Branch
300-1019 Wharf Street
Victoria, British Columbia, Canada V8W 2Z1
and
R. Wayne Campbell
British Columbia Provincial Museum
Victoria, British Columbia, Canada
British Columbia Provincial agencies are given authority for protecting marine birds and their habitats by the Provincial Wildlife Act, the Parks Act, and the Ecological Reserves Act. The Provincial Museum Act accommodates research on marine birds. The Fish and Wildlife branch has protected over 30,000 ha of intertidal estuarine habitat in the form of reserves and has conducted limited inventories of birds on the Queen Charlotte Islands and northern mainland coast. The Provincial Museum has conducted inventories and life-history studies of marine birds and maintains a repository for information on seabirds, including a catalog of colonies. Pollution from oil and chemicals, improper logging practices, and disturbance by boating recreationists are the most apparent threats to the well-being of birds. Additional inventories and the determination of seasonal distribution are among the information needed to better protect the marine birds of British Columbia.
Most marine-associated birds in Canada are covered by the Migratory Birds Convention Act and are therefore federally protected. In British Columbia additional protection is provided by the Provincial Wildlife Act. Several other provincial acts provide authorities related to seabirds. The Provincial Museum Act permits research related to natural history; the Parks Act and Ecological Reserves Act provide for the protection of habitat and prohibit harassment of wildlife within park and reserve boundaries; and the Firearms Act permits the closure of areas frequented by selected wildlife to the discharge of firearms. The fact that several authorities for the protection and conservation of marine birds are available does not mean that they have been used to full advantage.
British Columbia's irregular shores provide thousands of kilometers of coastline, much of which is used by marine birds for nesting and wintering as well as during migration. Through legislation of different types, some of the more ecologically important and unique sites have been protected. Twelve "ecological reserves," which are basically inviolate preserve areas, provide habitat for and protection to a number of major breeding colonies. Over 30,000 ha of intertidal estuarine habitat has been protected by the provincial Fish and Wildlife Branch in the form of reserves. Less than half of the total area is in Order-in-Council reserves (passed by the Provincial Cabinet), which afford strong protection; the rest is in departmental map reserves, which merely means other agencies must inform the[Pg 248] branch before they disturb them; they are hardly secure. Provincial Parks Branch protects other areas used by marine birds by incorporating them within parks.
Research and conservation of seabirds in British Columbia have not been a high priority in the Fish and Wildlife Branch, basically because seabirds are not consumed by people. Our primary interest in seabirds has been in their role as a life support system for the peregrine falcon (Falco peregrinus). Most Fish and Wildlife Branch reserves have been established to protect estuarine habitat for fishes, waterfowl, and shorebirds rather than for true seabirds. That situation is not likely to change in the near future unless additional funds become available to the Branch. About the most we can expect to do is designate key areas as sanctuaries or wildlife management reserves. Under the folio and referral systems now operational among resource agencies in British Columbia, we have the opportunity to advise other disciplines against approving practices that would adversely affect wildlife. By those methods we are attempting to protect critical seabird habitat. It must be stressed, however, that we can only advise; we cannot force other agencies to follow procedures we suggest.
The only significant work relating to seabirds in which the Fish and Wildlife Branch is presently engaged involves inventory of specific sites on the Queen Charlotte Islands and the northwest mainland coast. Those areas are ones on which we expect to find seabird colonies and where applications for logging are pending. To enable us to advise the Forest Service on the wildlife values of those sites, we began field work in the summer of 1975.
The Federal Government, in comparison to what it has done on the east coast and in the north of Canada, has been negligent in its support of seabird conservation on the west coast. By far the most seabird research by a government agency in British Columbia has been accomplished by the staff at the Provincial Museum in Victoria. In the past, beginning in the 1940's, museum personnel (mainly C. J. Guiguet) explored and inventoried seabird colonies along the British Columbia coast. Most work then was exploratory, and little quantitative information was gathered. More recently, precise counts have been obtained of seabirds nesting in the Strait of Georgia, Juan de Fuca Strait, the central west coast of Vancouver Island, the northern mainland coast, and the east coast of the Queen Charlotte Islands. That information, along with quantitative data gathered in the summer of 1975, will be used to update the "Catalogue of British Columbia Seabird Colonies" published in 1961 by the museum.
The museum has a number of programs under way.
• A cooperative survey with Washington State of colonies of the double-crested cormorant (Phalacrocorax auritus) in the Pacific Northwest. To limit disturbance, that survey is to be conducted at 5-year intervals beginning in the summer of 1975.
• A survey of all islands, whether or not they are supporting seabirds, in the Strait of Georgia and Juan de Fuca Strait in 1980, to detect changes in populations after 1974.
• Monitoring changes in seabird populations along the west coast of Vancouver Island, gathering data for all islands there. Permanent quadrats will be established on ecological reserves in the area to help detect such changes. As a result of such quadrats having been set up in 1967 on Cleland Island and being re-examined in 1974, we can document a significant decrease in Leach's storm-petrel (Oceanodroma leucorhoa) and a corresponding increase in rhinoceros auklet (Cerorhinca monocerata).
• Mapping vegetation substrate as it relates to seabird populations on selected islands in the Province.
• Investigating differences in eggshell thickness between eggs within clutches of glaucous-winged gulls (Larus glaucescens) near Victoria.
• A saturation banding program for cormorants (Phalacrocorax penicillatus, P. pelagicus, and P. auritus) on south-coast colonies.
• Continued banding of select colonies of glaucous-winged gulls which began in the 1960's. Life tables, survivorship curves, and dispersal patterns should result.
The museum also acts as a repository for information on seabirds in British Columbia and maintains files on the history of seabird islands as well as references to literature published on all seabirds in the Province. The ref[Pg 249]erences include unpublished theses and reports. This information is easily retrievable—not a small contribution in today's paper-producing society.
Future programs planned by the Provincial Museum, in addition to the continuance of some of those already mentioned, include a system of monitoring colonies every 5 to 10 years, depending on the sensitivity of the species involved, to detect changes in population numbers and distribution. It is also hoped that the first complete provincial census, with cooperation from Federal and provincial agencies, naturalist groups, and the like, can be budgeted and arranged for in the summer of 1980. That census could conceivably be expanded to include the entire Pacific coast of North America.
Some research on the breeding biology of seabirds has been conducted by universities, notably the University of British Columbia under the guidance of R. H. Drent and M. Udvardy. We expect that graduates returning to coastal universities will continue that work. The section of government dealing with ecological reserves has just recently received funding to permit field studies on reserves harboring marine birds. J. B. Foster, Coordinator of Ecological Reserves, emphasizes that research by other agencies is encouraged under permit on ecological reserves.
There are a number of threats to seabirds in British Columbia. Along with the chemical pollutants in their environment and food, logging, and the specter of huge oil tankers plying the west coast, we are greatly concerned by the potential threat of boating enthusiasts and recreationists. Well-meaning but uninformed vacationers and boaters stopping to visit or picnic at seabird islands can do serious damage to nesting seabirds. The possibility of loss of habitat to seabirds from people searching for island summer homes poses a threat, and indeed some seabird islands have already been lost to speculators. With increased leisure time and travel the potential of unintentionally introducing predators, such as rats (Rattus spp.) and snakes, to seabird islands is great. Intentional or accidental introduction of mammals, such as mink (Mustella vison), rabbit (Sylvilagus spp.), fox (Vulpes fulva), and raccoon (Procyon lotor), to islands is another serious threat to the future existence of seabird populations. The recent unauthorized and apparently unsuccessful introduction of mink on the Queen Charlotte Islands could have resulted in the eventual devastation of seabird colonies there and on adjacent islands. The destruction of habitat by logging near colonies on large islands and complete logging on small offshore islands will no doubt adversely affect some seabird populations. Competition between increasing numbers of gulls (Larus spp.) and certain species of seabirds (e.g., storm-petrels and cormorants) may result in reduced numbers of the seabirds.
What types of programs are needed? About 80% of all known seabird colonies in British Columbia have been investigated to date, and a modest program to monitor changes has been established. We do, however, require exploratory work along the west coast of the Queen Charlotte Islands and northern mainland coast. We need to know more about the breeding biology and reproductive potential of each of the species nesting in the Province, as well as about their adaptability to different habitats. Will some burrow-nesting alcids use man-made tubes erected in otherwise marginal habitat? Can and should more man-made habitat be created for cormorants that have been displaced from ancestral breeding grounds?
Of immediate urgency is exploratory work involving seasonal distribution, abundance, and flight lanes of pelagic seabirds along the coast of British Columbia—especially the northern portion. We lack the base-line data which could help influence routes of oil tankers to lessen the potential danger of spills to marine birds. We know little about the winter distribution of marine birds, especially alcids.
As a general rule, offshore islands of less than 100 ha should be protected completely from logging, and the larger ones supporting major seabird colonies should have some protection from development. We must also consider the possibility of preserving some islands which may act as buffer areas and provide potential alternate habitat to seabirds.
Another concern is the effect of commercial and sport fishing in the Province on food supplies for seabirds, and what damage, if any, gillnetting may have on diving seabirds. Per[Pg 250]haps we should discourage fishing by nets in areas where large numbers of seabirds aggregate to feed.
We also need to know more about the effects of chemical pollutants on individual species and on their reproduction. Of paramount importance, and one which biologists tend to neglect, is communication among all disciplines interested in seabirds. For example, a comprehensive file of the history of seabird colonies in British Columbia is established at the Provincial Museum. It would be a waste of time and money to duplicate that file and have three or four scattered across the country. We would be better advised to tackle another phase of work yet to be accomplished. Communication assures that seabirds benefit and are not unduly harassed.
Annual meetings, both local and international, of persons interested in marine birds should be arranged so that problems relating to seabirds can be discussed. For example, populations of glaucous-winged gulls in British Columbia have increased exponentially in the past 10 years. If they are a threat to the existence of other seabirds (e.g., Leach's storm-petrel, double-crested cormorants), should they be controlled, and, if so, how? Such meetings would also help develop a pattern of universal census methods and techniques that could be put to use along the Pacific Coast to provide comparable data from different areas.
Finally, in today's world, natural resource agencies must operate on limited funding. How can one convince administrators to divert a significant portion of those funds to the investigation of species that are widely regarded as having little social importance?
A detailed bibliography of seabirds of British Columbia is available from either of us.
We thank D. F. Hatler, J. B. Foster, and A. L. Allen for comments on the manuscript.
by
Keith G. Hay
American Petroleum Institute
2101 L Street NW
Washington, D.C. 20037
Despite improved safety practices, engineering, and navigational skills, marine tanker transportation will not be 100% accident free. The industry seeks to mitigate wildlife losses through improved technology, research in the rehabilitation of species exposed to oil, and the development of oil spill/wildlife contingency plans.
Oil spills and marine birds not only constitute a deadly mix but have proved to be one of our toughest environmental problems to solve. The rehabilitation of these tragic victims is plagued with controversy, emotion, apathy, and biological unknowns. The costs have been high and the survival rates low. During the last 10 years, a few dedicated people working here and in Europe have reversed this trend. They have, in addition, taken steps to develop contingency plans and conducted research to reduce seabird mortalities from oil spills. I present a brief status report on their progress and the melange of problems involved.
The unfortunate encounter between spilled oil and marine birds is not new. It goes back at least to the turn of the century, when coal-burning steamships and sailing clippers were replaced by oil-fueled vessels. Since then thousands of marine birds have succumbed to floating oil, especially during World Wars I and II (Blanks 1942) and in recent spills here and off the coast of Europe (Clark 1969).
With the current and projected demands for energy in the United States and with expanded tanker traffic and accelerated development of offshore petroleum reserves, the oil-contaminated ("oiled") bird is not going to go away. Periodically, this ugly problem will arise, despite the efforts of the petroleum industry to improve its safety practices, engineering, and navigational skills. Unfortunately, the problem is the product of the inherent fallibility of man and his imperfect machines.
We cannot ignore the situation. We must here, as elsewhere, improve our technology and mitigate the impact.
A study of more than 100 spills that occurred throughout the world between 1960 and 1971 revealed that about 1 in 5 spills (20%) involved 50 or more birds (Ottway 1971). Nearshore spills have a far greater effect on waterfowl than do spills occurring several miles or more offshore.
In the 1967 Torrey Canyon tanker spill, some 8,000 oiled birds were rescued. About 6,000 were picked up alive in England and about 2,000 in France, at a cost estimated at $160,000 (Clark 1969; Bourne 1970). Less than 5% of those treated by British authorities survived for release some months later. The survival rate of those rescued in France is unknown.
In 1969 the Santa Barbara spill resulted in the treatment of 1,575 marine birds, of which 169 were eventually released. Many of those released were found dead within a short time (Smail 1971).
In 1970 the tanker Delian Apollon was responsible for a spill in Tampa Bay, Florida. Thousands of seabirds were lost. No exact count was taken, but hundreds of birds were[Pg 252] cleaned and farmed out for rehabilitation. Reports show that many of the birds were returned dead within a few days (Smithsonian Institution 1971).
In 1971, when two tankers collided under the Golden Gate Bridge at the mouth of San Francisco Bay, the resulting spill involved some 4,686 oiled birds taken to cleaning centers (Lassen 1972). Eight months later the last of 200 survivors (less than 5%) were released at a cost estimated at $900 per bird (Smith 1975).
The most vulnerable species involved in spills have been the oceanic birds such as the alcids—murres (Uria spp.), auks (Pinguinus spp., Alca spp.), puffins (Fratercula spp., Lunda spp.), and guillemots (Cepphus spp.). Other species less affected included ruddy ducks (Oxyura jamaicensis), scaup (Aythya marila, A. affinis), scoters (Melanitta spp.), mergansers (Lophodytes spp.), oldsquaws (Clangula spp.), and goldeneyes (Bucephala spp.). Grebes (Podiceps spp.), eiders (Polysticta spp.), loons (Gavia spp.), and cormorants (Phalacrocorax spp.) are also frequently involved. Ruddy ducks and scaup are particularly vulnerable during winter on large river systems with heavy oil transport traffic. Fortunately, none of the above species have been reported in jeopardy as a result of spills in American waters.
In Europe and South Africa, however, it is believed that oil pollution is responsible for a steady decline in seabird colonies. For example, in known oil-dumping areas in the Baltic Sea, where some mortality of oldsquaws has been associated with surface oil, their population has dropped to about one-tenth of the pre-World War II level (Bergman 1961). Other authors report that oil spills have reduced the number of scoters in the Baltic and off southeast England (Atkinson-Willes 1963). The auk populations off the coast of England have been reported to be substantially decreased by oil pollution (Parslow 1967). Tankers traversing South Africa's Cape of Good Hope are said to be responsible for the reduction of jackass penguins, Spheniscus demersus (Rowan 1968). Oil pollution, especially sustained pollution, has thus been cited as a limiting factor on certain seabird populations.
Estimates of seabird mortalities from an oil spill are imprecise; they may differ by thousands of birds. It is believed that only a small fraction of the birds killed in a spill wash up on the shore. Some authors have even speculated that the death rate at sea could range from 6 to 25 times the number washed ashore (Tanis and Mörzer-Bruyns 1968).
In contrast to terrestrial birds and semiaquatic species (e.g., ducks; geese; coots, Fulica spp.; or gulls, Larus spp.), totally seaborne species have a restricted reproductive potential. Many, such as the alcids, do not breed until they are 3 or more years old, and lay only one egg per year. Only one in five survives to go to sea.
Until about 5 years ago we knew little about seabirds. They are not game species (they taste fishy) and thus do not constitute an important economic resource. They have never been the subject of intensive waterfowl management or research by either State or Federal governments.
During the last 5 years a small group of people here and in England have been studying marine birds—their distribution, population status, physiology, diseases, and husbandry in captivity. Four organizations have primarily been involved: The American Petroleum Institute (API); the Wildlife Rehabilitation Center at Upton, Massachusetts; England's Advisory Committee on Oil Pollution of the Sea; and the International Bird Rescue Research Center in Berkeley, California. They have encountered many common biological and people problems, some of which I discuss here.
The recuperation record for oiled seabirds in the past has admittedly been dismal. A few birds have been returned to nature, but only after a long and costly period of care. In the process, semidomestication often takes place. The percentage of cleaned birds that actually survive after release is even smaller. One should not infer from this small percentage that rehabilitated birds cannot readjust to life in the wild. Several successful reintroductions have been documented. U.S. Fish and Wildlife Service bands were returned from two western grebes that were cleaned and released[Pg 253] after the 1971 San Francisco spill. One bird was picked up a year later near Treasure Island, California, and the second after almost 2 years, in the State of Washington (Fletcher 1973).
Survival rates have zoomed with recent strides in cleaning technology and husbandry. The International Bird Rescue Research Center reported a survival rate of 41%, based on hundreds of birds and about 20 different species over a 2-year period (Smith 1975). In South Africa, where powdered clay was used as a cleaning agent on jackass penguins, nearly 50% survived, although exact percentages have not been published (Edwards 1963; Holmes 1973). Rapid retrieval, the relatively small groups of birds treated, and expert cleaning and husbandry techniques are largely responsible for high success ratios. Rehabilitation success is measured not only in terms of percent survival but also in terms of median length of captivity and average cost per bird.
Rescued oiled birds arrive at cleaning centers under a wide range of physical conditions. Before capture they may have spent hours or days in water, during which their energy has been continuously drained. The oil destroys the bird's protective insulation, and metabolic rate must be increased to sustain body temperature. Constant preening also takes energy. Food demands increase, but feeding attempts, especially for diving birds, are thwarted by oil-fouled plumage. A bird may arrive at the cleaning center under stress, chilled, exhausted, dehydrated, starved, and ill from ingested oil. Cold weather accentuates these conditions. Often such birds are jammed together with other species, hauled long distances, and immediately put through a series of cleaning processes that would leave even a healthy bird weak and in a state of shock. One marvels at the stamina of the survivors.
In most past spills, every bird found was routinely cleaned regardless of its condition. Instead of attempting to reclaim all birds, a selective judgment should be made. If a bird's physical condition makes its chances of survival nearly impossible, it should be humanely killed (except for rare or endangered species). This would enable workers to devote more time and care to birds having a reasonable chance at survival.
Fletcher (1973) stated that many variables affect bird survival: weather conditions, the type and amount of oil in and on the bird, the species, the distance of the spill from the shore, the time lag from initial fouling until initial treatment, the degree of stress a bird is subjected to, the husbandry techniques used, the time of release (the sooner released, the higher the apparent survival), the number of birds being cared for (the fewer birds being handled, the higher the survival rate), the quality of the facilities available, and the training and experience of the people handling the birds.
Many of the above biological problems are under study here and in Europe, including the following.
• The effect of ingested oil on the mucosal transport mechanism of marine birds. To use seawater, birds must be able to transport sodium ions through the gut and expel the excess salt through the nasal passages. Oil can block the mucosal ion transport mechanism, resulting in dehydration and eventual death.
• The development of a successful program of hormonal and electrolyte therapy to restore osmotic balance and the functioning of the salt glands in contaminated seabirds.
• Treatment and prevention of aspergillosis (fungus infection); septic arthritis or "bumble-foot" (joint capsule infections); breast sores (especially in seabirds confined on hard surfaces); eye lesions (caused by ammonia fumes from unsanitary pens); dehydration and hypoglycemia; lipid pneumonia; and bacterial infections.
• Treatment of stress after capture, including perfection of handling and cleaning techniques, administration of proper steroids, crowding, light, temperature, noise levels, and so on.
• Development of proper nutritional regimes for certain species and feeding techniques to eliminate forced feeding.
• The establishment of criteria for confident recognition of terminal pathological conditions in oiled birds.
• Determination of optimum density of confined birds to insure healthy conditions and adequate room for preening.
• Determination of proper time and conditions for reintroduction of the birds into their native habitat.
Handling an over-responsive and emotional army of bird-cleaning volunteers and training them to play constructive roles is a major undertaking. Planning, cooperation, understanding, patience, and clear direction must be developed. In the absence of these virtues, chaos can and has prevailed.
The San Francisco Bay oil spill of 1971 was a classic example. There was virtually no State or Federal coordination. Splinter groups of volunteers established their own "treatment centers" and jealously guarded their patients. Some actually absconded with their pet patients to seek better care elsewhere. Long hours, fatigue, and frustrations led to dissension and bitter quarrels. Antiestablishment sentiment was rampant.
Instant experts on bird cleaning, avian medicine, and nutrition appeared or developed overnight. Veterinarians volunteered their services, but their knowledge of oiled-bird treatment was limited. A wide variety of food (from canned dog food to live shrimp) was given the birds. Forced feeding was routine. Medications and vitamins of all kinds were also administered. Needless to say, the states of the art in treating oiled birds and handling volunteers were both in their infancy. For both, the success ratio was near zero.
To prevent such fruitless efforts and the frantic, unorganized response that prevailed, a well-designed contingency plan for wildlife involved in an oil spill is needed.
It is only prudent to take reasonable measures to prepare for oiled-bird emergencies. This is especially true in regions where bird concentrations and oil shipment traffic converge. Almost equal attention must be devoted to handling volunteers as to handling birds. Safety is a major consideration. The sharp beaks of birds can be very dangerous.
A model State contingency plan should include the following:
• A list of State and Federal agencies to be alerted, including 24-h, 7-day-a-week telephone numbers, and names of individuals to contact.
• Clarification of the roles of State and Federal agencies under the Regional Response Plan of the National Oil and Hazardous Substances Pollution Contingency Plan.
• A list of State and Federal laws pertaining to possession of birds and mammals.
• An updated roster should be maintained of team members, assignments, and responsibilities for inland and marine spills, including discovery and notification, record keeping, public information, containment and counter-measures, wildlife protection, and cleanup, restoration and evaluation of effects on the biota.
• A list of individuals or organizations that possess skills and experience in treatment of oiled birds (locally and nationally).
• Location of emergency wildlife reception and treatment centers.
• A list of the necessary supplies, equipment, and holding facilities for cleaning, treating, drying, and post-care operations. Such information can be obtained from:
—California Department of Fish and Game, Oil and Hazardous Materials Contingency Plan (July 1974)
—International Bird Rescue Research Center, Aquatic Park, Berkeley, California 94710
—American Petroleum Institute, 2101 L Street, Northwest, Washington, D.C. 20037
—Wildlife Rehabilitation Center, 84 Grove Street, Upton, Massachusetts 01568
• An organizational plan which includes assignments of duties and responsibilities for personnel manning a bird-cleaning center. In addition to bird cleaning and husbandry, assignments must be made for record keeping, internal communications, public relations, logistics (supplies), security, sanitation, safety, and meals.
• A slide lecture or film to instruct volunteers in the correct techniques for handling, cleaning, and post-care of oiled birds.
• A selected bibliography of key references on oiled-bird cleaning and care.
• Appendices to the plan should include maps of the major coastal oil terminals, bays, and estuarine areas with heavy oil transport[Pg 255] traffic. Map overlays would depict the location of resident species and the migratory patterns, species composition, relative abundance, and winter concentration areas of migrants. Additional overlays would locate commercially important demersal seafood areas (e.g., oyster and abalone beds, lobster and crabbing locales) and marine mammal habitats. Further refinement of an atlas could include information on tides, prevailing winds, ocean currents, and water mass movements to assist in predicting the path of spilled oil.
The petroleum industry, through the API, took prompt steps to mitigate the problem after the first seabird mortalities were reported from Santa Barbara in 1969. They commissioned a young aviculturist, Philip Stanton, who has extensive experience working with wild waterfowl, to start a research program on cleaning and caring for oiled birds. At his Wildlife Rehabilitation Center at Upton, Massachusetts. Stanton, with the help of API, has been conducting research on oiled birds for 7 years. He is also an assistant professor of biology at nearby Framingham State College. Stanton's studies (unpublished) include investigations on food shape and color preferences in wild ducks, the effects of lengthened photoperiods on breeding of arctic geese, and the effects of diets of varying protein concentrations on growth and development of the common eider duck.
As a result of his research on cleaning techniques and agents, Stanton has recommended a nontoxic liquid cleaner called Polycomplex A-11. Although not perfect, it is one of several cleaning agents being successfully used today. He has authored a "how to" guide for oiled-bird treatment entitled "Operation Rescue" and prepared a companion bibliography (Stanton 1972). These booklets have been distributed throughout the United States to State and Federal agencies and conservation organizations. He has provided consulting services at numerous spills and has worked to establish oiled-wildlife treatment centers in coastal States.
Since 1972 the API has sponsored an avian physiology study at the University of California at Santa Barbara. Under the direction of W. N. Holmes, the studies are directed at the effects of ingested crude oil and petroleum products on marine birds. Holmes has revealed that small quantities of crude oil introduced into the gut of a saltwater-adapted bird can affect the mucosal transport and extra-renal excretory mechanisms, resulting in acute dehydration and eventual death. Dr. Holmes is also examining the effects of the various distillation fractions derived from crude oil and the long-term effects of ingested oil in mature birds. Incidentally, Alaska North Slope oil was found to be almost innocuous when administered to ducklings in amounts similar to the effective doses of other oils (Holmes and Cronshaw 1975).
Refined products (diesel oil, No. 2 fuel oil, and Bunker "C") are known to be more toxic than crude oil. For example, the relatively small spills of Bunker "C" at Tampa, Florida, in 1970 and in San Francisco in 1971 caused approximate mortalities of 90 and 20 birds per ton of spilled product, respectively. The crude oil spills of the Torrey Canyon and at Santa Barbara, however, resulted in mortalities of only 0.5 and 0.6 bird per ton of oil (Clark 1973).
Dr. Holmes is now testing measured amounts of the above refined oils on adult birds. He is determining the degree of dehydration incurred, the resulting pathological changes, and the replacement (hormonal and electrolyte) therapy necessary to rehabilitate the birds.
It is obviously important to keep as many birds away from an oil slick as possible. This was the objective of an API contract with the Av-Alarm Corporation of Santa Maria, California. Their objective was to determine the feasibility of repelling aquatic birds from an area by using an acoustical jamming device as the stimulus.
The flocking instinct in birds provides mutual protection through their almost constant communication with one another. When this (audio) communication is prevented by jamming with high-frequency sounds, the birds immediately leave the area to seek relief. This harmless technique has been used successfully for years to repel agricultural pest birds.
The Av-Alarm device was tested on waterfowl at the Grizzly Island Game Refuge some 48 km north of San Francisco Bay and in the bay itself over a 2-year period (1972-73). Using a single, fixed-location system covering a three-quarter square mile (1.21 km2) area Crummett (1973) repelled 82% of the ducks and 92% of the shorebirds on the Refuge. The intrepid coot, however, was found to be relatively indifferent to the sounds. Immediately upon activation, there was a sudden drop in the bird count, which was followed by a continual decline in numbers.
In tests of the device from a cruising boat in ocean and bay waters, the degree of effectiveness varied by species. Ducks were repelled 100%; pelicans (Pelecanus spp.) 92%; great egrets (Casmerodius albus) 85%; gulls 42%; cormorants 75%; shearwaters (Adamastor spp.) 29%; and murres, 51%.
Grebes and murres dived away from the stimulus, then surfaced and dived again if the threat was still present. To prevent driving the diving species deeper into the center of a slick, investigators recommended that buoyed repelling equipment be placed within the spill area. When the alarm system was used in conjunction with the occasional firing of a rocket or shellcracker, an even greater percentage of birds was repelled.
The International Bird Rescue Research Center, a nonprofit corporation in Berkeley, California, was an outgrowth of the Richmond Bird Care Center that played an active role in the 1971 San Francisco Bay spill. Since that time, a small group of individuals has continued research on bird-cleaning techniques, testing cleaning agents, perfecting husbandry methods, and alleviating stress. Their 41% survival rate speaks for itself. A paper describing their work is being presented at this conference (Smith 1975).
Under a grant from the API, the center is currently evaluating various cleaning agents, and testing the pressurized jet versus serial baths and the re-establishment of feather waterproofing. The center is also perfecting an audio-visual slide presentation that will illustrate how to select the proper cleaning agent, together with the latest bird-cleaning and care procedures.
About 5 years ago, England's Advisory Committee on Oil Pollution of the Sea established a research unit in the Department of Zoology at the University of Newcastle-Upon-Tyne. It was funded by a grant from the Royal Society for Prevention of Cruelty to Animals, the Royal Society for the Preservation of Birds, the World Wildlife Fund Seabird Appeal, and the British Institute of Petroleum.
Their efforts have also led to high survival rates. Focusing primarily on the efficiency of various detergents, they have found that the loss of waterproofing is largely due to soap and oil residues and the disturbance of the feather structure in the cleaning process. Consequently, they have devoted their efforts to selecting detergents that can be completely removed with a minimum disturbance of plumage (Seabird Research Unit 1971).
In May 1974, the API in cooperation with the U.S. Fish and Wildlife Service convened a seminar on Oil Spill Wildlife Response Planning. The 2-day workshop was held at the Patuxent Wildlife Research Center at Laurel, Maryland. Some 70 State and Federal government personnel in charge of oil spill response plans involving wildlife participated. The program addressed itself to fish and wildlife considerations and the role of regional response teams under the National Oil and Hazardous Substances Pollution Contingency Plan. The actions of State wildlife departments, U.S. Fish and Wildlife Service, Environmental Protection Agency, U.S. Coast Guard, and the oil industry in handling spills involving wildlife were examined. The latest oil spill cleanup technology was reviewed, and the workshop ended with demonstrations of the cleaning of oiled waterfowl. Similar seminars were planned for the Gulf of Mexico and the West Coast.
It was obvious from this seminar that the most comprehensive wildlife oil spill contingency plan had been developed by the State of California. Copies of this plan (Oil and Hazardous Materials Contingency Plan, California Department of Fish and Game, July 1974) were later distributed to all coastal States as a prototype or model plan by API.
The U.S. Fish and Wildlife Service has been conducting experiments on various bird-cleaning agents and techniques at its Migratory[Pg 257] Bird and Habitat Research Laboratory near Laurel, Maryland. The Fish and Wildlife Service is also working with the API in developing information on migratory patterns and winter waterfowl concentration areas on the East Coast as they relate to petroleum transport traffic and oil terminals.
In Canada, the Petroleum Association for Conservation of the Canadian Environment (PACCE) employed the services of a consulting firm to make a comprehensive review of dispersal and rehabilitation of waterfowl associated with oil spills. The resulting PACCE report (LGL Ltd. 1974) codified what was known about the problem, identified research needs, and developed effective wildlife oil-spill contingency plans for critical areas on Canada's east and west coasts, the Great Lakes, and the Arctic.
The Florida Game and Fresh Water Fish Commission has initiated a program for the rehabilitation and treatment of oiled birds. It is being organized by veterinarian Harold F. Albers of St. Petersburg. He is working in cooperation with the Florida Associated Marine Institutes, the Shell Oil Company, Clean Gulf Associates, and the API.
The Standard Oil Company of California provided a grant to James Naviaux of Pleasant Hill, California, to develop bird-cleaning technology, including the testing of various cleaners. Dr. Naviaux had treated birds from the 1971 San Francisco spill. A publication on the after-care of oil-covered birds (Naviaux 1972) resulted from the collaboration with Alan Pittman, research chemist of the U.S. Department of Agriculture's Western Research Laboratory.
In 1971, the API in cooperation with the National Wildlife Federation (NWF) initiated an NWF/API Fellowship program. One of the first grants under this program was to Charles W. Kirkpatrick, Professor of Wildlife Management at Purdue University. He and assistants studied for 4 years the nesting ecology and productivity of the emperor goose (Philacte canagica) in the Igiak Bay area of the Yukon Delta in Alaska (Eisenhauer and Kirkpatrick 1977).
An extensive program of marine bird research was initiated on the North Slope of Alaska by the Atlantic Richfield Company in 1969. It has been continued ever since and includes the acquisition of extensive base-line data on all waterfowl, including June surveys of breeding pair counts and August surveys for brood counts. The results of these surveys for 1969-73 are presented by Gavin (1975).
Base-line data on marine birds of the Gulf of Alaska are currently being collected and compiled through grants to various universities and institutions by the American petroleum industry. These data will constitute elements of a report on the environmental status of the Gulf of Alaska. Such information is essential prior to development of the Gulf's offshore petroleum resources.
Most sea mammals are relatively resistant to oil slicks and tend to avoid contaminated waters. As a result, little research has been conducted on cleaning and treatment techniques except for experiments on live beavers and on the carcasses and pelts of sea otters and beavers.
No sea otter or seal has ever been oiled and subsequently cleaned in an oil spill situation. It is possible, however, that a spill could have significant adverse effects on sea otters and fur seals, especially at a rookery during the pupping season. These animals depend on an air blanket trapped in their dense underfur for warmth and buoyancy. Any form of pollutant, especially oil, could penetrate the outer guard hairs and underfur and allow water to reach the skin, with disastrous effects.
Seals and otters are powerful animals, and the larger males and females can be quite aggressive and dangerous. Only professional wildlife specialists and consulting veterinarians should be permitted to handle and treat them. A guide to cleaning and care of oiled sea otters can be found in the California Oil and Hazardous Materials Contingency Plan.
This status report has revealed that substantial efforts and progress have been made in oiled-wildlife research. New techniques being developed are leading to higher survival rates. Preventive measures are being devised to keep birds from entering a spill area. Wild[Pg 258] life contingency plans are being developed and materials to handle future emergencies are being stockpiled. Basic research is being continued on the difficult problems inherent in achieving high survival levels and a rapid return to the wild, at a reasonable cost.
Much more must be done, but these pioneering efforts both within and outside of industry reflect a difficult problem yielding to the time and attention of dedicated men and women.
Advisory Committee on Oil Pollution of the Sea. 1972. Research unit on the rehabilitation of oiled seabirds. Committee, Dep. Zool., Univ. of Newcastle-Upon-Tyne, England, Annu. Rep. 2. 33 pp.
Atkinson-Willes, C. 1963. Wildfowl in Great Britain. Nat. Conserv. Monogr. 3. 368 pp.
Bergman, G. 1961. The migrating populations of the long-tailed duck (Clangula hyemalis) and the common scoter (Melanitta nigra) in the spring, 1960. Suomen Riista 14:69-74.
Blanks, D. W. 1942. Birds in the war zone. Gull 24(4):11.
Bourne, W. R. P. 1970. Special review—after the Torrey Canyon disaster. Ibis 112(1):124.
Clark, R. B. 1969. Oil pollution and the conservation of seabirds. Proc. Int. Conf. on Oil Pollut. of the Sea 1968:76-112.
Clark, R. B. 1973. Impact of chronic and acute oil pollution of seabirds. Page 634 in Background papers for a workshop on inputs, fates and effects of petroleum in the marine environment, Vol. 11. Ocean Board National Academy of Science, Washington, D.C.
Crummett, J. G. 1973. Bird dispersal techniques for use in oil spills. Final report. American Petroleum Institute, Washington, D.C.
Edwards, R. A. 1963. Treatment of washed up penguins. Bokmakierie 15(1):8.
Eisenhauer, D. I., and C. M. Kirkpatrick. 1977. Ecology of the emperor goose in Alaska. Wildl. Monogr. 57. 62 pp.
Fletcher, A. 1973. Why save oiled birds? University of California, College of Forestry, Berkeley. 26 pp. (Unpublished report)
Gavin, A. 1975. Wildlife of the North Slope, a five year study, 1969-73. Atlantic Richfield Co., Anchorage, Alaska. 63 pp.
Holmes, M. 1973. Oil and penguins don't mix. Natl. Geogr. Mag. 143(3):384-397.
Holmes, W. N., and J. Cronshaw. 1975. Final progress report on studies completed, 1972-75, on the effects of petroleum on marine birds. American Petroleum Institute, Washington, D.C. 77 pp. (Unpublished report)
LGL Limited. 1974. Review of current knowledge on reducing bird mortalities associated with oil spills. Petroleum Association for Conservation of the Canadian Environment Rep. 75-4. 50 pp.
Lassen, R. W. 1972. Waterbirds and the San Francisco oil spill. Proc. Cal-Nev Wildl. 1972:20-24.
Naviaux, J. L. 1972. Aftercare of oil covered birds. National Wildlife Health Foundation, Pleasant Hill, Calif. 52 pp.
Ottway, S. M. 1971. A review of world oil spillages, 1960-1971. Oil Pollut. Res. Unit, Orielton Field Centre, Pembroke, Wales.
Parslow, J. L. F. 1967. Changes in status among breeding birds in Britain and Ireland. Br. Birds 60:2-47, 97-122, 177-202.
Rowan, M. K. 1968. Oiling of marine birds in South Africa. Pages 121-124 in Proc. Int. Conf. on Oil Pollut. of the Sea.
Seabird Research Unit. 1972. Advisory Committee on Oil Pollution of the Sea Research Unit on the Rehabilitation of Oiled Seabirds. Second annual report. Dep. Zool., Univ. of Newcastle-Upon-Tyne, England. 33 pp.
Smail, John. 1971. The oil spill in retrospect. Point Reyes Bird Observatory News 18:1-2.
Smith, D.C. 1975. Rehabilitating oiled aquatic birds. Pages 241-247 in Proc. 1975 Conf. Prevention and Control of Oil Pollution.
Smithsonian Institution. 1971. Annual report of the Center for Short-lived Phenomena, 1970. Cambridge, Massachusetts.
Stanton, P. B. 1972. Operation rescue. American Petroleum Institute, Washington, D.C. 32 pp.
Tanis, J. J. C., and M. F. Mörzer-Bruyns. 1968. The impact of oil-pollution on seabirds in Europe. Proc. Int. Conf. on Oil Pollut. of the Sea. 1968:67-76.
by
Gordon R. Williams
New Zealand Wildlife Service
Department of Internal Affairs
Wellington, New Zealand
Marine species (pelagic birds and those of exposed coasts) make up about 48% of New Zealand's native avifauna, excluding stragglers and antarctic species. The biological history that has led to the present status of marine birds in this archipelago of some 700 islands is outlined, methods of conservation are briefly described, and some illustrative case histories of management programs are given. In spite of the major environmental changes that have occurred in New Zealand during 200 years of European occupation, only one marine species has become extinct, although five such endemic species are currently regarded as threatened as are a few subspecies of widely distributed forms.
New Zealand, which lies some 2,000 km southeast of Australia, has been a changing archipelago for many millions of years. It has been separated from any major landmass (first, Gondwanaland and later, Australia) for at least 80 million years.
Before the arrival of man, probably between 1,000 and 1,500 years ago, New Zealand was free of any land mammals except two species of bats, and there were few avian predators. These, among a number of other biological peculiarities, reflect the archipelago's considerable and long-standing isolation.
There are nearly 700 islands 0.5 ha or more in area in the New Zealand region; and, if North, South, and Stewart islands are regarded collectively as the mainland, about 650 of these islands lie within 50 km of the coast and 30 beyond that limit, to about 850 km offshore (Atkinson and Bell 1973). The archipelago extends from about 30° to 52°S lat. (over a distance of about 2,400 km)—that is, from the subtropical to the subAntarctic—and from about 166° to 176°W long. (Fig. 1).
Pelagic and coastal birds must obviously be an important part of the avifauna and, in fact, aside from stragglers, antarctic species, and established introduced species, they make up about 48% of the 173 in the New Zealand Checklist (Kinsky 1970). Of the 83 species I have regarded as marine, 48 (28%) are pelagic and 35 (20%) shorebirds of exposed coasts. Ten of the 48 pelagics (21%) and 12 (34%) of the 35 shorebirds are endemic.
More than a thousand years of occupation by Polynesian man with his commensal Polynesian rats (Rattus exulans) and a peculiar breed of domesticated and feral dog (now extinct), did little damage to pelagic and open coast species, even though many, if not most, were used as food—especially the petrels, and particularly those belonging to the genera Puffinus, Procellaria, and Pterodroma. However, the Europeans, who arrived about 200 years ago, brought with them a menagerie of mammals and birds, and 33 species of each have become established and are now feral (Gibb and Flux 1973; Williams 1973). They also put into practice, on a large scale, European methods of land use that had unfortunate effects on almost the entire native avifauna. Although terrestrial, freshwater, and estuarine species suffered most, marine species suffered also. However, reduction in numbers and range rather than extinction was the rule, except locally.
Apart from habitat destruction by man and[Pg 262] the various mammalian browsers and grazers, the most inimical agents have been black rats (Rattus rattus), Norway rats (R. norvegicus), feral cats, and feral pigs. One would expect the inhospitality or inaccessibility of an island to be a marine species' best protection, and so it has generally proved-the greatest losses have occurred on the two major mainland islands (North Island and South Island). Bourne (1967) suggested that Polynesians in pre-European times may have caused the extinction of numerous petrels in the Chatham Islands. There are still a few islands on which no exotic mammals occur, but modern transport, allied with human curiosity and cupidity, are stripping all but the most wild and remote of these of the protection against invasion they have had so far. Cruises by nature-hungry but sometimes environmentally illiterate tourists are beginning to be a local problem.
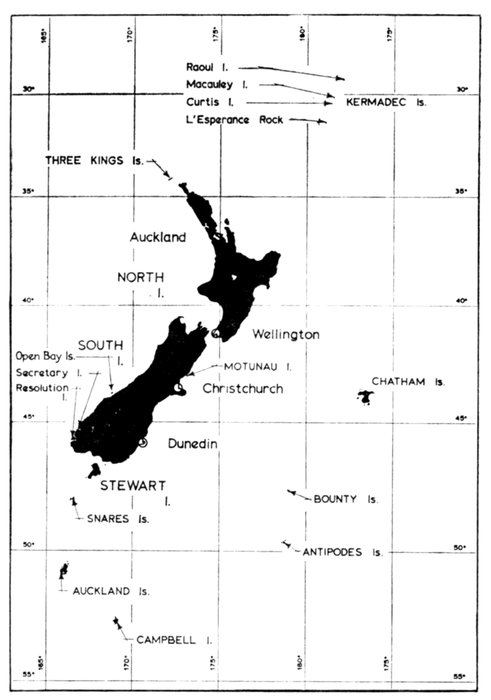
Fig. 1. New Zealand and its main offshore and outlying islands (from Atkinson and Bell 1973).
The matter of conservation of marine species in New Zealand has stemmed mainly from the recognition of the value of certain islands as refuges for whole ecosystems, as convenient areas for study, and as arks for the rescue of the threatened species that can be successfully established on them—an often highly hazardous and uncomfortable procedure for men as well as birds.
By statute, all feral species of birds in New Zealand are automatically protected unless specifically legislated for otherwise. (About 50 of our grand total of 285 species have been so legislated for.) One fortunate consequence of this provision is that all new arrivals—vagrants or new discoveries—are also fully protected. The legislation also states that it is illegal to have in one's possession the nests, eggs, feathers, skins, or bones of any fully protected species unless one has been issued a permit for this purpose. This restriction may apply to institutions as well as to persons.
After this good start and the setting aside of conservation reserves of various kinds, active conservation measures depend on making careful and comprehensive surveys of the species and its ecosystem—often none too easy a task in the New Zealand region because of the rough seas, the relative inaccessibility of many of the important islands and their ruggedness, and the near-impenetrability of some of the vegetation types they support. Having decided that positive action is necessary, the next step is to use all available media to inform the public (local as well as national, if the island is inhabited) of the situation and the proposals for remedying it. As in most other countries, uninformed emotionalism is one of the most pervasive and serious obstacles to effective conservation because of the political pressure it can generate.
Apart from formal ecological studies, the New Zealand Wildlife Service uses three main methods to support threatened species (other than the attempts we are making to breed certain freshwater and terrestrial species in captivity):
• The translocation and founding of new colonies in promising or unmodified habitat. Such habitats are not common in New Zealand because of the ubiquity of the introduced[Pg 263] mammalian browsers, grazers, and predators (Williams 1977).
• The destruction, or at least the reduction, of such browsers, grazers, and predators by physical, chemical, or biological methods, or combinations of these.
• The exertion of social influences to promote changes in methods of land use or in traditional harvest for food (the latter can be particularly important as far as the Polynesian [Maori] population is concerned, as nowadays the taking of birds for food is predominantly a cultural rather than an economic matter).
Translocation has been a valuable technique for increasing the numbers and ranges of a few threatened terrestrial species. The very nature of most marine species, however, limits its application as far as they are concerned. Nevertheless, we have considered it worth trying for one nonmigrant wader; and no doubt it could be tried under similar circumstances elsewhere.
Convincing local experiences have shown that predator or competitor destruction is likely to be practical only on small, not-too-rugged islands, usually no larger than about 500 ha. However, special circumstances have prompted us to attempt destruction, or at least control, on much larger and more difficult islands. It is implicit that the predators or competitors are exotic, not indigenous. Recently, on those rare islands that are inhabited but still free of either black or Norway rats, we have set up permanent bait stations (at which sodium fluoroacetate—"1080"—is used as the poison) on wharves and jetties in the hope that such a precaution will, with the addition of a propaganda campaign calling for the regular fumigation of visiting vessels, prolong the charmed lives that these fortunate islands have so far enjoyed. It goes without saying that we ask that the greatest care be taken when expeditions land stores on uninhabited, rat-free islands which, if by "rat-free" we mean also free of R. exulans, are even rarer in our seas.
The sociolegal approach is effective only when ecosystems or communities have not been seriously modified, otherwise it is no substitute for either of the other two measures discussed.
Last century, an endemic monotypic genus of wader—the New Zealand shore plover (Thinornis novaeseelandiae)—was widespread and occasionally very common around the coasts of the North and South islands and the Chatham Islands. As a result of European settlement and the accompanying predation by feral cats and rats, the species now occurs only on South East Island in the Chatham group (860 km east of the mainland), where it at present seems safe, since there are no rats on the island and it is now a reserve. However, the population numbers only about 120 individuals. Because calamities can always occur (for example, ship rats recently reached shore on three important islets off the southwest coast of Stewart Island), the Wildlife Service is anxious to spread the shore plover to other suitable islands, if they can be found. The species is not a migrant and is rather sedentary. The first translocation attempts failed, probably because mainly adult birds were used, and we are now continuing our studies of the species with the thought in mind, among others, that success may come if young birds are used instead; the question is—how young?
As is widely known, the New Zealand Wildlife Service has been remarkably successful in recent years in translocating one species of the endemic wattlebird family—the forest-dwelling saddleback (Philesturnus carunculatus)—to other islands than the four small ones it had been reduced to by the early 1960's; three of these islands were the ones recently invaded by ship rats, referred to above.
Some 25 km off the North Island's east coast lies the 3,000-ha, very rugged and forested Little Barrier Island, which has now been a reserve for the protection of flora and fauna for about 80 years. Before that, it had been almost continually occupied by Maoris since their arrival in New Zealand, and about one-third of its forest was felled or burnt, especially after European settlement of the adjoining New Zealand mainland began.
Most unusually, Little Barrier is now free of any grazing or browsing mammals, and has only the Polynesian rat (a reminder of the Maori occupation) and feral cats (a European legacy) to impair its extreme importance as a reserve. The rats have been unmolested by man because, rightly or wrongly, they are considered ineffective predators generally; however, their impact has probably been under-rated. More than half a century of trapping and hunting of cats by successive caretakers on the island has not effectively reduced that population.
Among its other important attributes, Little Barrier supports two birds endemic to New Zealand—the rare black petrel (Procellaria parkinsoni), and one endemic honey-eater, the stitchbird (Notiomystis cincta), which was once widespread on the North Island but is now found only on Little Barrier in moderate numbers, and apparently in no immediate danger. The impact of feral cats on stitchbirds has not been determined, but it is known that cats are seriously affecting the black petrel especially: they kill at least 90% of the chicks and some adults annually. Their impact on a locally remnant population of Cook's petrels (P. cookii) is apparently less severe.
In 1968-69 the Wildlife Service, with veterinary advice and assistance, added an attempt at biological control to the campaign of poisoning ("1080" in fish was the poison and bait used), trapping, and shooting. The very specific viral disease—feline enteritis—was introduced by trapping island cats, infecting them, and then releasing them. Some estimates of the resulting mortality from the combined techniques were as high as 90%; but there has been a recovery since, and the campaign is expensive in both time and man-power. And, oddly enough, the control effort has met with some opposition. Nevertheless, another campaign is planned.
The Kermadecs are a group of small islands about 800 km north-northeast of the North Island. Their biological significance, insofar as this symposium is concerned, is that they are the southernmost breeding area in New Zealand seas for many elements of the Pacific tropic and subtropical marine avifauna. Unfortunately, goats were liberated on the two largest islands—Raoul (3,000 ha) and Macauley (300 ha)—almost 150 years ago and Macauley Island was burnt over; such forest cover as it had was severely damaged or destroyed, probably at about the same time. The goats were to be an emergency food supply for whalers and shipwrecked mariners. Cats, too, became feral on Raoul Island during one of its fitful periods of occupation. The New Zealand Wildlife Service, in spite of the distance and difficulties involved, has undertaken pest destruction campaigns on both islands, but I offer here only an account of the simpler, and more successful, Macauley operation.
In 1966, a 5-week expedition to this waterless and almost treeless island resulted in the shooting of what was then thought to be all of its 3,000-odd goats (a density of about 15/ha). Four years later, a follow-up expedition found and destroyed another 17 goats (a later brief inspection suggested that these were indeed the last), and rehabilitation of the island is well under way. Now that the short turf is disappearing, erosion of the soft volcanic soils is reduced. With compaction no longer occurring, it will be interesting to see what the effect will be on birds breeding on the island—six breeding species of petrels, three breeding species of terns, and other marine species.
The taking of petrels and other procellariiform birds for food has always been part of the Polynesian economy and culture throughout the Pacific. In New Zealand, the practice now has only minor economic importance, but it is still an essential part of Maori culture and tradition. The most commonly taken species are the sooty shearwater (Puffinus griseus) and, until recently, the gray-faced petrel (Pterodroma macroptera). Although no formal study of the impact of the annual harvest of chicks on the population has yet been made, all the indications are that it is not significant. Nevertheless, the Maoris willingly accepted the limited amount of legislation that has been passed to afford the two principal exploited species at least token protection. However, on the Chatham Islands, where there is a strong tradition of taking some of the albatrosses, this tradition has persisted, even[Pg 265] though all albatrosses are fully protected throughout New Zealand.
Enforcement of legislation in small and isolated communities is not always easy and sometimes may not be wholly politic. However, the Maoris of the Chathams have been specially informed of the conservation issues at stake, and a "gentleman's agreement" has been reached: If a planned survey shows that full protection of albatrosses in the Chathams is indeed essential, the Maoris will honor the legislation to the letter; on the other hand, if limited exploitation seems justified, the Wildlife Service has agreed that it will be allowed.
Insofar as conservation measures of a passive type are concerned, it is fortunate that the offshore and outlying islands not yet occupied, farmed, or set aside as reserves, are likely to remain unexploited, either because they are too remote for exploitation to be economical or because they are too inhospitable, or both. In any event, public opinion is now such that unmodified or otherwise biologically important islands not already reserved would be proclaimed as reserves if threat of exploitation arose unexpectedly, unless they were found to be major sites for oil or minerals. Even so, legislation exists that offers the possibility of protection even from this threat, and has already been used to exempt some important mainland areas from prospecting and the granting of mining rights.
It is gratifying to realize that, although some endemic marine subspecies (generally not very different from neighboring subspecies) are endangered to varying degrees, there are very few whose disappearance would result in the disappearance of the species itself from the New Zealand area. Only one endemic marine species has become extinct in recent times, the Auckland Island merganser (Mergus australis) in about 1905, and only six are currently in any real danger: the Chatham Island taiko (Pterodroma magentae), the black petrel, Hutton's shearwater (Puffinus huttoni), the Westland black petrel (Procellaria westlandica), the shore plover, and the Chatham Island oystercatcher (Haematopus chathamensis). However, a list of this kind is often a matter of some controversy. Something is at present being done to help all but the first and last of these. The Chatham Island taiko had not been positively identified for about 50 years, until 1977 when this species was "rediscovered" on the main island of the Chatham group; though its numerical status is unknown, it is rare. The Chatham Island oystercatcher, although certainly "threatened" (only about 50 are known to exist), does occur on four islands, two of which are reserves. Although this species has not been actively studied until now, it is soon to be the subject of a full ecological survey.
A few words about the hunting of marine species: Mutton-birding aside—that is, apart from the taking by Maoris of the young of the sooty shearwater and the gray-faced petrel—there has been no legal hunting of any marine birds in New Zealand for 35 years now, nor is there likely to be. This situation reflects the consistently increasing weight of informed public opinion in favor of, let alone scientific concern for, transoceanic migrants. The pro-hunting lobby for some species of waders, in particular the eastern bar-tailed godwit (Limosa lapponica baueri), is a small one, the numbers of which decrease yearly. However, small-scale poaching occasionally occurs; it is punished when discovered.
Protection for marine species extends only to the 3-mile limit of New Zealand's territorial waters, but it would be extended further should New Zealand follow the present trend of including as territorial waters all those that cover the continental shelf or beyond. [This extension occurred in 1977; the marine fishing zone for New Zealand waters has been extended to 200 miles (360 km) around all coasts.]
Only three marine species are not afforded full protection under the Wildlife Act: two, the black-backed or Dominican gull (Larus dominicanus) and the black shag (Phalacrocorax carbo), are totally unprotected—the first because of its predation on some rare shorebirds during the breeding season and for its attacks on sheep and lambs at a similar time, and the second because of its depredations (seldom serious) on the introduced trout and salmon, mainly in fresh waters—the third[Pg 266] species, the southern skua (Stercorarius skua lonnbergi), may be destroyed only when it is actually attacking sheep or lambs, an occasional event confined to the Chatham Islands. Destruction of these three common species is not encouraged by the Wildlife Service except when black-backed gulls become too active among colonies of, say, the fairy tern (Sterna nereis), which is very rare in New Zealand but not elsewhere in its range. Otherwise, control of the species is left in the hands of those most affected by their depredations but whose judgment is usually reasonable.
Marine birds, therefore, are generally satisfactorily protected by law or managed for conservation in New Zealand—especially when one considers the remarkable changes that have occurred in the New Zealand archipelago over the last 200 years. Although the situation could be better, it would certainly have been worse if the Wildlife Service (and other conservation organizations) had not been untiring in keeping the general public and the legislature aware of the issues at stake and seen to it that as much as possible of the necessary conservation work was done—and done before it was too late.
I thank my Wildlife Service colleagues, B. D. Bell, M. J. Imber, D. V. Merton, and C. J. R. Robertson, for valuable comments and advice on the preparation of this paper.
Atkinson, I. A. E., and B. D. Bell. 1973. Offshore and outlying islands. Pages 372-392 in G. R. Williams, ed. The natural history of New Zealand. A. H. & A. W. Reed, Wellington.
Bourne, W. R. P. 1967. Subfossil petrel bones from the Chatham Islands. Ibis 109:1-7.
Kinsky, F. C. 1970. Annotated checklist of the birds of New Zealand. A. H. & A. W. Reed, Wellington.
Gibb, J. A., and J. E. C. Flux. 1973. Mammals. Pages 334-371 in G. R. Williams, ed. The natural history of New Zealand. A. H. & A. W. Reed, Wellington.
Williams, G. R. 1973. Birds. Pages 304-333 in G. R. Williams, ed. The natural history of New Zealand. A. H. & A. W. Reed, Wellington.
Williams, G. R. 1977. Marooning—a technique for saving threatened species from extinction. Int. Zoo Yearb. 17:102-106.
by
Finn Salomonsen
Chief Curator of Birds
The Zoological Museum
University of Copenhagen
Copenhagen, Denmark
Most species of seabirds that regularly breed in Denmark are declining, for a variety of reasons: shooting; oil pollution; toxic chemicals; reclamation of land; collecting of eggs; disturbance at breeding sites by visitors, motorboats, camping, etc.; destruction by predators; and others. On the other hand, the numbers of certain other species are increasing as a result of climatic changes (six species), protection (three species), and increase in food supply (three species of gulls). In addition to breeding birds, a total of about 3 million birds occur in Danish waters as passage migrants or winter visitors. More than half of the European winter populations of a number of marine waterfowl species winter in Denmark. Large numbers of seabirds spend the summer in Danish waters, including several hundred thousand immature gulls and just as many molting waterfowl.
The seabird fauna of the Faroe Islands is very rich, the immense number of birds being attracted by the local abundance of macroplankton and fish. The seabirds are harvested by man, formerly by fowling (capturing and shooting), now primarily by shooting. Until about 1910, more than 400,000 birds were taken annually by fowling. The Faroese game act is now very restrictive, and most seabird populations appear to be almost stable. However, a census in 1972 indicated that common murres (Uria aalge) have declined by about 20% to a population of about 600,000. Shooting and snaring appear to be the primary causes of the decline; oil pollution and toxic chemicals do not seem to be contributing to the population decrease.
In Greenland seabirds provide an important source of human food; however, because of the increase in human population and in the use of guns and speedboats for hunting, and the absence of a game act, serious overshooting of seabirds is taking place. A new game act passed in 1977 should largely alleviate this overharvest. Oil pollution and toxic chemicals do not yet play an important part in influencing the number of seabirds, though offshore oil drilling is being initiated in West Greenland. A recently established gigantic national park, covering 200,000 km2 of ice-free land, is the largest nature reserve in the world.
The Danish Monarchy consists of three parts far removed from each other, scattered in the North Atlantic—namely Denmark proper, the Faroe Islands, and Greenland. They differ so much from each other in climate and in bird life that they must be treated separately in this paper. The Faroes possess a provincial government and also a sort of home rule. Greenland also has a provincial government, but all statutory provisions, including acts concerning hunting or wildlife protection, must be passed by Danish authorities, usually by the Ministry of Greenland.
Insofar as seabirds are concerned, it is important that Greenland is an arctic country, whereas the Faroes and Denmark are boreal. In both Greenland and the Faroes the breeding birds are most significant, from an ecological point of view, whereas in Denmark the passage migrants and winter visitors are far more important.
There are other differences as well. In Greenland and the Faroes the seabirds mostly breed in colonies on high and steep cliffs, and the structure of these breeding places is not disturbed by man. In Denmark, on the other hand, the seabirds usually breed on glacial deposits, now forming meadows, low islets, salt marshes, etc., and these habitats have unfortunately been largely changed in the last hundred years by draining and reclamation. This practice has taken place in Denmark on a much larger scale than in most other countries and has, therefore, to a high degree diminished the life conditions of seabirds.
Denmark is situated on the continental shelf of western Europe; all seas surrounding the country are shallow (less than 100 m deep), apart from the Skagerrak, north of Jutland, which is much deeper. The shallow depth, combined with the rapid flow of water between the Baltic and the North seas causes much upwelling, which forms excellent life conditions for plants and animals. It is well known that the fishery in Danish waters, especially in the North Sea, is very rich. This richness of the seas provides suitable conditions for a high diversity of seabirds and ecological types.
Seabirds regularly breeding in Denmark include five species of terns (common tern, Sterna hirundo; arctic tern, S. paradisaea; least tern, S. albifrons; Sandwich tern, S. sandvicensis; and gull-billed tern, Gelochelidon nilota); seven species of gulls (black-headed gull, Larus ridibundus; herring gull, L. argentatus; lesser black-backed gull, L. fuscus; great black-backed gull, L. marinus; mew gull, L. canus; little gull, L. minutus; and black-legged kittiwake, Rissa tridactyla); four species of geese, swans, and ducks (mute swan, Cygnus olor; greylag goose, Anser anser; common eider, Somateria mollissima; common merganser, Mergus merganser; and red-breasted merganser, M. serrator); three species of auks (black guillemot, Cepphus grylle; common murre, Uria aalge; and razorbill, Alca torda); and one species of cormorant (great cormorant, Phalacrocorax carbo). Shorebirds have not been included in this review. Some of the species mentioned are partly freshwater birds—for example, the black-headed gull, little gull, mute swan, greylag goose, and the two species of mergansers. The gull-billed tern forages in terrestrial habitats, but nests along the coast with the other seabirds. It is often difficult, therefore, to make a clear-cut distinction between seabirds and freshwater birds.
Among the auks, the black guillemot breeds in the Cattegat area in the huge heaps of boulders on small raised islets, or in holes (mostly formed by starlings, Sturnus vulgaris) on steep clayey slopes or promontories. The common murre and razorbill are restricted to the islet Graesholm in the Christiansø Archipelago, about 24 km east of Bornholm Island in the Baltic, where they breed on small cliffs of Precambrian granite rock.
The estimated number of seabirds of different species that breed in Denmark is shown in Table 1. Species like the mergansers, mute swan, and greylag goose, which breed partly or mostly in freshwater localities, are not included. Overall, the number of breeding seabirds is slowly declining, probably due to many factors which are discussed below. There are two exceptions, however, to this general decrease—the herring gull (and to a lesser degree the other big gull species) and common eider. Both species have increased during the last 50 years. Since they breed in the same habitat, usually mixed together, the eider is probably dependent on herring gulls for protection against predators. When the ducklings are fledged, the herring gull acts as a successful predator itself, but the eider nevertheless maintains a close association with herring gulls.
| Species | Number of breeding pairs |
|---|---|
| Sterna paradisaea | 5,750 |
| S. hirundo | 900 |
| S. sandvicensis | 4,000 |
| S. albifrons | 600 |
| Gelochelidon nilotica | 105 |
| Larus marinus | 300 |
| L. argentatus | 60,000 |
| L. fuscus | 2,000 |
| L. canus | 28,500 |
| L. ridibundus | 135,000 |
| L. minutus | 25 |
| Rissa tridactyla | 125 |
| Phalacrocorax carbo | 600 |
| Somateria mollissima | 3,800 |
| Cepphus grylle | 325 |
| Alca torda | 400 |
| Uria aalge | 1,100 |
| Total | 243,530 |
More than 90% of the herring gull population breeds on small islands, and a large proportion occurs in a few large colonies. It never breeds in freshwater localities, but is exclusively found as a breeding bird in coastal habitats. The population has particularly increased in the last 5 decades, some colonies reaching their maximum size in the 1960's. Others are still expanding and occupying new breeding grounds. Today the largest colonies are found on the following islands: Saltholm, 20,000-40,000 pairs; Christiansø 9,000 pairs; Hirsholmene, 2,500 pairs; Jordsand, 1,800 pairs; Samsø, 2,000 pairs; Hjelm, 1,500 pairs; and the archipelago south of Funen, a total of 3,500 pairs in several colonies.
Attempts have been made to reduce the breeding population of herring gulls at Hirsholmene and Christiansø sanctuaries (in 1973 and 1974, respectively), to improve conditions for other nesting seabirds. In 1969 the Bird Strike Committee of the Royal Danish Airforce also initiated a program to reduce the number of herring gulls breeding on Saltholm Island, which is near the Kastrup airport in Copenhagen. Nests were sprayed with a formaldehyde oil dye, which resulted in a 33% reduction in population. In Christiansø and Hirsholmene, where the adult breeding birds were poisoned, the effect is not yet known.
The total number of seabirds occurring in the Danish waters as passage migrants and winter visitors is substantially larger than the breeding population, because Denmark is situated on a very important fall migration route for seabirds from Scandinavia, the Baltic countries, northern Russia, and northwestern Siberia. Furthermore, the shallow waters of the Danish seas (less than 10 m deep) that occupy extensive regions bordering the coasts are important feeding grounds for diving ducks. Birds frequenting the seas outside the breeding season include hundreds of thousands, or probably millions, of gulls; numerous ducks (especially diving ducks); swans and brants, Branta bernicla; jaegers, Stercorarius spp. (four species); loons, Gavia spp. (four species); grebes, Podiceps spp. (four or five species); gannet, Morus bassanus; great cormorant; northern fulmar, Fulmarus glacialis; common murre; razorbill; and other species of alcids. To these should be added a number of species of various seabirds, especially gulls, tubenoses, phalaropes, and others which appear as casual or accidental visitors and which are not further mentioned in this paper.
Table 2. Total numbers of ducks, swans, and coots recorded in Denmark during a winter census in January 1973 (based on ground counts and aerial surveys), compared with estimated flyway populations wintering in western Europe and annual bird harvest in Denmark (after Joensen 1974:23, 155, 168).
| Species | Census, January 1973 | Estimated winter populations of the Western Europe Flyway | Average annual bag in Denmark |
|---|---|---|---|
| Anas platyrhynchos | 127,000 | 1,550,000 | 380,000 |
| A. crecca | 500 | 260,000 | 76,000 |
| A. querquedula | 11 | [67] | [68] |
| A. acuta | 100 | 70,000 | 13,000 |
| A. strepera | 5 | [67] | [69] |
| A. penelope | 3,000 | 485,000 | 44,000 |
| A. clypeata | 17 | 63,000 | 9,000 |
| Tadorna tadorna | 13,000 | 105,000 | [69] |
| Aythya ferina | 7,100 | 235,000 | 5,000 |
| A. fuligula | 94,700 | 530,000 | 35,000 |
| A. marila | 80,900 | 145,000 | 8,000 |
| Clangula hyemalis | 11,000 | [67] | 11,000 |
| Melanitta nigra | 148,100 | [67] | 18,000 |
| M. fusca | 6,700 | [67] | 9,000 |
| Somateria mollissima | 450,800 | [67] | 136,000 |
| Bucephala clangula | 67,000 | 142,000 | 25,000 |
| Mergus serrator | 11,700 | 40,000 | 8,000 |
| M. merganser | 23,200 | 75,000 | 6,000 |
| M. albellus | 206 | 5,000 | [69] |
| Cygnus olor | 48,900 | 120,000 | [69] |
| C. cygnus | 5,700 | 17,000 | [69] |
| C. bewickii | 1,113 | 6,000 | [69] |
| Fulica atra | 142,500 | [67] | 70,000 |
| Totals | 1,243,252 | 3,848,000 | 853,000 |
A comprehensive investigation of the nonbreeding waterfowl in Danish waters was recently undertaken by the Game Biology Station Kalø (Joensen 1974). Aerial surveys of marine ducks indicate that a large percentage of the ducks that winter in European waters do so in the shallow areas of the Danish seas. A census in January 1973 indicated a total of more than 1.2 million birds (Table 2). In a number of other countrywide surveys, undertaken in all winters since 1967, usually 1.0-1.5 million birds have been recorded. Since such censuses usually give minimum numbers, and certain species-especially marine ducks—generally go unrecorded, the normal winter population (November to February) of ducks, swans, and coots in Danish waters can scarcely be less than 2 million birds (Joensen 1974:156). In Table 2, bird numbers in Denmark are compared with the estimated winter populations in western Europe, based on the investigation of Atkinson-Willes (1972). When all the winter censuses in Denmark are compared with those for Europe, as was done by Joensen (1974:156), it is evident that Danish waters support about half of all greater scaup (Aythya marila), common goldeneye (Bucephala clangula), red-breasted merganser, mute, whooper (Cygnus cygnus), and tundra swans (C. bewickii) wintering in Europe; about one-third of the population of tufted duck (Aythya fuligula) and common merganser; and probably also one-third of the population of common eider and coot (Fulica atra).
The wintering population of common eider is very large. According to banding records it makes up the greater part of Baltic breeding birds; however, it is not possible to calculate its percentage contribution to the total European winter population since its size is unknown in most European countries. Although most of the surface-feeding ducks disappear from Denmark waters in winter, extremely large numbers occur there during the fall migration period. For example, it has been estimated that for species like common teal (Anas crecca) and wigeon (A. penelope) about one[Pg 271]-third of the West European Flyway population passes Denmark in the fall. Possibly some of the surface-feeding ducks listed in Table 2 for January 1973 were recorded in fresh water and not from the seas, but at the time the census was taken most freshwater lakes were frozen and, therefore, unavailable for water birds.
These breeding seabirds and the off-season visitors do not constitute the total population in Danish waters. Large numbers also occur in summer as nonbreeding birds; most are in two categories: (1) several hundred thousand pre-adult (up to 4-5 years of age) gulls (mostly great black-backed, herring, and lesser black-backed gulls), which feed inshore or at the coast, and (2) large concentrations of waterfowl that carry out a molt migration in Danish waters, particularly in shallow areas. Black scoter (Melanitta nigra), velvet scoter (M. fusca), common eider, and whooper swan are especially numerous, totaling hundreds of thousands of individuals, and probably constituting the majority of the European molting populations of these species. Less numerous, but still totaling thousands of molting birds, are sheld-duck (Tadorna tadorna), common goldeneye, red-breasted merganser, and possibly some other diving ducks. About 3,000 surface-feeding ducks of various species, most of which undoubtedly are local breeding birds undergo wing molt in Danish waters. Comprehensive descriptions of the molt migration, particularly in Denmark, were published by Salomonsen (1968) and Joensen (1973a, 1974).
It may then be concluded that very large numbers of seabirds are found in Danish waters in all periods of the year; most feed in the inshore zone and some offshore, but none in the pelagic zone.
Seabirds are affected by several factors related to human activities, most of which pose a threat to them and will eventually reduce their numbers. Some factors, however, tend to increase bird numbers, like climatic changes which, as reported by Salomonsen (1963), have given rise to the immigration to Denmark of great cormorant (in 1938); eared grebe, Podiceps nigricollis (about 1870); red-crested pochard, Netta rufina (1940); common pochard, Aythya ferina (about 1860); tufted duck (about 1900); and common murre (1929). They all still breed in Denmark, having more or less increased in number.
Another reason for increases of certain species is legal protection. Among protected seabirds are the sheld-duck, which has been completely protected since 1931, and particularly the mute swan, of which only 2 or 3 pairs were breeding in Denmark when the species was completely protected in 1926. Since then, mute swans have increased enormously, reaching at least 2,740 pairs in 1966 (Bloch 1971:43), of which large numbers were breeding colonially on small islets of boulders or on sand reefs off the coast (Bloch 1970:152). The gannet has also increased considerably as a fall visitor since about 1945, apparently due to protection in England and other countries.
Finally, some gull populations have increased in size because of an increase in the food supply, consisting especially of wastes from commercial fisheries and garbage dumps. In Denmark, this unnatural food source has caused an enormous increase since about 1925 in herring gulls (from less than 500 pairs to 60,000 pairs), lesser black-backed gulls (all three subspecies, fuscus, intermedius, and graelsii have immigrated to Denmark), and great black-backed gulls (immigrated to Denmark in 1930). Improved waste disposal practices in recent years have not yet offset the rate of growth of these gull populations. The increase of common eiders, which also started in about 1925, is probably related to the increases in the larger gulls.
A variety of factors tend to reduce the numbers of seabirds. The most important ones are outlined below, with comments on what has been done or what is expected to be done to reduce the impact of these activities on seabirds and protect this endangered resource.
The shooting of seabirds in Denmark is considerable, because the seabirds are extraordinarily numerous, and the number of sports[Pg 272]men is very large, amounting to about 135,000 (a larger number per capita than in any other country).
The Danish game statistics are excellent—well known to be much more accurate than in most other countries (see Salomonsen 1954; Strandgaard 1964). According to Danish bag records, almost one million ducks, geese, and coots (Joensen 1974:31) and about 100,000-200,000 gulls (Salomonsen 1954:125) are shot each year. The average annual bag of each species of wildfowl is given in Table 2 and the open season for each species of seabirds in Table 3. The open season for dabbling ducks is long, extending from 16 August to 31 December, which means that local birds are persecuted almost as soon as birds-of-the-year are able to fly. This has resulted in a dabbling duck breeding population that is much smaller than what the available food supply could support, and in the large-scale development of artificial rearing of mallards for later shooting. A 5-month hunting season on specialized birds like loons, grebes, and various auks is not good management practice and should be carefully reviewed.
Four other important facts about the shooting of seabirds in Denmark merit inclusion here: (1) there is no bag-limit for any species; (2) in general, all marine areas within territorial limits are open to all Danish sportsmen, and the admission is free; (3) motorboats with a maximum speed of 10 knots are allowed for shooting in the period 1 October-30 April; and (4) the shooting of seabirds is permissible from 1.5 h before sunrise to 1.5 h (in December 1 h) after sunset, whereas for most other birds shooting is prohibited between sunset and sunrise.
Shooting is a national tradition in Denmark, and the large number of sportsmen has considerable political power. Too much influence is given to the representatives of the hunters' organizations, which have the decisive force in game committees dealing with protective measures. It is difficult, therefore, to change the existing system.
Shooting of seabirds, especially various waterfowl, is popular and intensive. The number of ducks taken by Danish sportsmen is probably in the order of 10-15% of the total kill on the West European Flyway (Joensen 1974:171). Excessive duck shooting can, in some cases, be controlled by banding in the breeding areas; the ensuing results then give rise to strong protests from the Scandinavian countries against the extensive persecution. As stated above, Denmark has (in relation to its size) the largest number of sportsmen of any nation in the world and the most intensive shooting. The number of sportsmen shooting ducks and shorebirds per 100 km2 is 278 in Denmark, 28 in Sweden, 37 in Finland, 10 in Poland, 83 in Holland, 164 in Britain, and 129 in Western Germany; the number of ducks shot per 100 km2 is 1,856 in Denmark, 39 in Sweden, 68 in Finland, and 129 in Western Germany (Nowak 1973). This shooting is undoubtedly of importance to dabbling duck populations, which are popular as shooting objects everywhere in Europe.
Insofar as marine ducks are concerned, it can be seen in Table 2 that appreciable numbers are shot in Denmark. The same is true for other Scandinavian countries, whereas shooting on the high seas is rather modest in most other European countries. The Danish bag undoubtedly makes up a significant proportion of the total number of marine ducks killed each year, but when the total number of ducks in European waters is considered, the shooting pressure in Denmark appears to be of only minor importance. However, the shooting, particularly when undertaken from motorboats, is so noisy and makes such a disturbance over large areas that the time for seabirds to rest and forage is significantly reduced. It must also be noted that the number of pleasure craft is steadily increasing in the present period of prosperity, and that increasing numbers of sportsmen will probably make use of the free shooting in territorial waters, since it is becoming more and more expensive to lease hunting areas.
To restrict seabird shooting, the Danish Ornithological Society has recently (1975) submitted a proposal to the Danish Government, of which the following points are relevant:
• The open season for dabbling ducks and geese should begin 15 September except for pintail (Anas strepera), shoveler (A. clypeata), wigeon, and pochard—species which should not be hunted until 1 October;
• the open season for all diving ducks, as well as for coot, should end 31 December;
• the open season for the great cormorant should be restricted to the period between 15 September and 31 October;
• murres, razorbill, great-crested grebe (Podiceps cristatus), and all species of loon should be fully protected;
• it should be prohibited to shoot from motorboats less than 1 km from the shoreline, as well as in certain narrow sounds and fjords;
• it should be prohibited to shoot from shooting-punts less than 100 m from the shoreline;
• it should be prohibited to sell waterfowl and shorebirds shot, except for eider ducks and mallards (Anas platyrhynchos); and
• no shooting should be allowed between sunset and one hour before sunrise.
Oil Pollution
Oil pollution incidents constitute one of the greatest dangers to seabird populations in Danish waters. The enormous masses of seabirds present in these waters throughout the year, combined with the fact that Danish waters contain some of the heaviest shipping traffic in the world would give rise to anxiety for oil disasters. The majority of all tanker traffic from the Atlantic and the North Sea to the Baltic passes through the Cattegat and the narrow straits of the Sound, the Great Belt, and the Little Belt, to supply a population of about 100 million people. Up to 100,000 ships pass through these waters each year, half through the Sound.
There have been severe oil pollution disasters every year since about 1935, accompanied by enormous mortalities of seabirds, particularly marine ducks. The Danish Game Biology Station, which has studied these disasters (Joensen 1972a, 1972b, 1973b), has noticed that the number of seabirds involved has increased in recent years, in spite of increased control by Danish authorities.
Unfortunately, it appears that small amounts of oil in the sea, originating from cleaning the tanks of vessels, or from the release of a few tons of oil, are enough to create mass mortality of seabirds when large concentrations of birds are present in the vicinity. Such incidents have passed unnoticed in spite of control measures. In no case has the source of the pollution been traced (Joensen 1972b:27). There has not yet been a real "oil disaster" in the Danish waters similar to the Torrey Canyon catastrophe. If such a disaster takes place, the destruction of seabirds will be enormous and immeasurable.
| Species | Oil incident no. | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Totals | |
| Gavia stellata | 1 | 9 | 1 | 4 | 15 | |
| G. arctica | 2 | 2 | 4 | 8 | 16 | |
| Gavia sp. | 4 | 1 | 5 | |||
| Podiceps grisegena | 4 | 1 | 8 | 8 | 21 | |
| P. cristatus | 1 | 1 | ||||
| Phalacrocorax carbo | 20 | 20 | ||||
| Anas platyrhynchos | 2 | 2 | 4 | |||
| A. clypeata | 2 | 2 | ||||
| Aythya marila | 6 | 2 | 8 | |||
| Clangula hyemalis | 35 | 2 | 26 | 6 | 4 | 73 |
| Melanitta nigra | 387 | 241 | 521 | 262 | 77 | 1,488 |
| M. fusca | 197 | 33 | 417 | 223 | 119 | 989 |
| Somateria mollissima | 1,683 | 1,081 | 947 | 1,713 | 19 | 5,443 |
| Bucephala clangula | 3 | 3 | 13 | 9 | 28 | |
| Mergus serrator | 48 | 28 | 28 | 2 | 106 | |
| Cygnus olor | 10 | 17 | 1 | 28 | ||
| C. Cygnus | 1 | 1 | ||||
| Fulica atra | 1 | 1 | 2 | 5 | 9 | |
| Larus sp. | 13 | 13 | ||||
| Alca torda | 1 | 12 | 1 | 14 | ||
| Uria aalge | 1 | 1 | ||||
| Cepphus grylle | 1 | 2 | 16 | 19 | ||
| Total birds examined | 2,380 | 1,362 | 1,996 | 2,324 | 242 | 8,304 |
| Estimated minimum number of birds killed | 10,000 | 5,000 | 12,000 | 15,000 | 1,500 | 43,500 |
| Percent of total birds contributed by three species[71] | 95.3 | 99.5 | 94.4 | 94.6 | 88.8 | 95.4 |
As a result of five of the major oil pollution incidents in the Cattegat from 1969-71, a total of 43,500 birds were killed, of which 8,304 were examined and enumerated (Table 4). Altogether, 21 or 22 species were involved, but 95% of all birds examined were diving ducks: common eider and black and velvet scoters. At present, it has not been possible to identify any decrease in the number of these ducks in Danish waters due to oil pollution. However, if these disasters continue, it can be expected that duck populations of northern Europe and the Baltic area will be severely reduced, and that an overall decline will take place from which the birds may not be able to recover.
A particularly disastrous year was 1972, when large numbers of ducks were killed as a result of rather small oil spills. A tanker disaster in March 1972 off the eastern coast of Jutland, in the northern Cattegat, and another in December 1972 in the Danish Waddensea, both took place in areas critical to major concentrations of sea ducks. A total of more than 60,000 birds were killed, of which about 95% consisted of the same three species of diving ducks mentioned above. These tragic events represent a further increase in the annual mortality of birds caused by oil, and there is reason to believe that a critical upper limit is rapidly being approached.
It appears, however, that the measures taken by pollution control and naval authorities have greatly improved in recent years. In January 1973, when a Polish merchant vessel collided with a Swedish tanker in the Sound, about 300 tons of heavy fuel oil were released into the sea. Several Danish and Swedish ships working in cooperation succeeded in dis[Pg 275]persing the oil, and no serious effect on seabird populations took place (Joensen 1973b:118). It seems that the best way of cleaning up such oil disasters is through a mechanical removal of the oil, but this is a very expensive and difficult procedure.
Chemical pollution is probably the most ominous threat to seabirds at present. Since all toxic chemicals used in agriculture ultimately end up in the sea, and many large factories release their industrial wastes directly into the sea, the effects of this pollution on marine organisms is attracting a growing interest. Many students have worked on these problems, and the results that concern birds were summarized by Bourne (1972:205). It is known that organochlorine residues have been found in seabirds in all the oceans of the world, including Antarctic waters and Arctic seas (Bogan and Bourne 1972:358). The chemicals most often found in birds are DDE (a metabolite of DDT) and PCB's (polychlorinated biphenyls), a mixture of related chemical compounds often originating from industrial wastes. In addition, some mercury will always be found, sometimes in increased concentrations. The present restrictions on the use of DDT and PCB in Denmark have not yet resulted in a corresponding decrease in the amount of these pesticides in birds.
It is well known that marine pollution reaches a peak in the Baltic. This high level of pollution is reflected in seabirds. For example, analyses have shown that eggs from the colony of common murres on Christiansø in the Baltic contain about 100 times as much DDE and 50 times as much PCB as eggs of murres from the Faroe Islands in the Atlantic Ocean (Dyck 1975).
A similar difference exists in the mercury content in birds examined in the two areas. Feathers of a large sample of black guillemots and murres from the Cattegat and the Baltic had higher mercury levels than those from the Faroe Islands and Greenland. It is interesting that this difference existed over a hundred years ago, as evidenced by the analysis of feathers in museum specimens. The Baltic populations of both species show very significant increases in the mercury content in 1965-70, as compared with the values earlier in this century. Since 1970 there has been a sharp decrease in mercury content, and in 1973 the level was almost as low as it was early in the century. These results indicate that the strict control of mercury discharges enforced in Sweden has resulted in a quick recovery of nearly normal conditions in the Baltic (Somer and Appelquist 1974). However, recent studies by Koeman et al. (1975:286) appear to show that mercury does not accumulate to the same extent in seabirds as it does in seals.
High concentrations of chlorinated hydrocarbon residues accumulate in carnivorous birds and upset the normal breeding behavior by making the eggshells too thin and fragile to survive (Peakall 1970:73; Mueller and Leach 1974:289). In Denmark, shells of herring gull eggs from the Baltic population were thinner, lighter, and more heavily contaminated with DDE and PCB than were shells of eggs from other colonies (Jørgensen and Kraul 1974:173). This further emphasizes the pollution of the Baltic Sea.
Massive mortalities of common murres, such as the one reported in the Irish Sea in the fall of 1969 which was apparently caused partly by malnutrition and PCB poisoning (Parslow and Jefferies 1973:87), are unknown in Danish waters.
It should be added that the pollution of seawater with cadmium, so very dangerous for man, has been high in recent years owing to the increased use of this element in industry, but no analysis of its importance for seabirds in Danish waters has yet been made.
It should also be mentioned that pollution of fresh water in lagoons or lakes near the sea can often cause serious declines in numbers of certain seabirds. This is well illustrated by recent events in the sanctuary Nakskov Indrefjord on the island of Lolland. This landlocked fjord once supported numerous breeding populations of ducks, grebes, and terns, but in recent years a number of species (e.g., eared grebe; common teal; garganey, Anas querquedula; pintail; and black tern, Chlidonias nigra) have failed to breed and practically all other species have declined in numbers. The main reason for these changes is a severe pollution from the admission of raw sewage from tributaries (Bloch et al. 1972). After several out[Pg 276]breaks of botulism in recent years, procedures to improve conditions are now being developed.
The most dangerous threats to seabirds are those discussed above. Authorities are aware of these dangers and attempts are being made to improve conditions. Some results have been achieved in the combat against oil pollution, and the control of shooting is reaching an acceptable level. Game management agencies in Denmark and other Scandinavian countries (Norway, Sweden, and Finland) are cooperating on the request of the parliamentary body of the Nordic Council. If game biologists in these countries could agree on proposed changes in the game acts, owing to the marked decline of a number of bird species, the parliamentary basis for such a legal step would be absolutely certain.
However, it must be admitted that the impact of man on the environment is enormous, especially in a country like Denmark, which possesses no raw materials, and where agriculture has transformed the whole country. In such a country, the birds have to "face the music," and by this sharing of resources with man, they will inevitably decrease in number. It is the responsibility of biologists and politicians, without emotional biases, to find the balance between the requirements of the two spheres of interest.
Many other dangers that threaten seabirds, some of which are unrelated to human activities, are listed here.
• Land reclamation.—Reclamation of land has reduced extensive areas of shallow water, lagoons, marsh land, etc., from seabirds for foraging or breeding places. Draining and diking of coastlands, estuaries, and saltings have had the same effect. This activity is now almost stopped, as these projects are no longer subsidized by the government.
• Egg-collecting.—According to the present game act, collecting gull eggs is permitted until 24 May. This creates much disturbance on the breeding grounds, and eggs of terns and shorebirds are also taken. This practice should be halted. The "Bird Island Group" of the Danish Ornithological Society, in a symposium in 1972, prepared some rules for the protection of seabirds, among which is a proposal to stop egg-collecting.
• Common property.—The Nature Conservancy Act regards all land not fenced in, even small uninhabited islets, as common property. People have free access to such areas with the result that seabirds breeding in colonies, or separately on islands, are disturbed by visitors arriving by boat. At the same time, noisy motorboats, bathing parties, or camping visitors frighten the birds, making successful breeding almost impossible. Even ornithologists, bird-banding teams, and bird photographers add to the destruction. The "Bird Island Group" of the Danish Ornithological Society has proposed a general prohibition against visitors on important bird islands from 1 March to 15 July to protect the breeding seabirds.
• Destruction by predators.—Fox, ermine, and stone-marten do not play an essential role. Rats are more important, even on small islands, and have caused destruction of tern and gull colonies. Rat numbers do not decline until a severe winter with much ice occurs, or until high tide kills them all. Large gulls also cause a great deal of destruction, but crows and magpies are unimportant as predators in seabird colonies. Numbers of nonbreeding mute swans or greylag geese may sometimes be a nuisance, trampling eggs and nestlings in seabird colonies.
• Forestry practices.—The prevailing practice of the forestry industry in Denmark of not preserving old trees with holes has considerably diminished the breeding habitat of hole-nesting species like the common merganser. Artificial nest-boxes have now been established in several areas.
• Sea conditions.—During high water, or rough sea, salt water may flood colonies of breeding seabirds nesting on low islets, often reducing the production of young.
• Aircraft disturbance.—Disturbances are also caused by noise from jet aircraft flying low, especially in military training areas where air traffic may be heavy.
• Commercial fisheries.—Modern commercial fisheries are depleting so-called industrially important fish stocks such as sand eels (Ammodytes), herrings, and other small fish over large areas of the sea for the production[Pg 277] of fish meal. This fishing has undoubtedly been the main reason for the decline in the number of terns—especially sandwich terns which depend on these small fish species for food.
• Unknown factors at sea.—Large numbers of pelagic seabirds, particularly fulmars, kittiwakes, and gannets, are washed up on the western coast of Jutland in certain years (e.g., 1959, Joensen 1961:212). These birds died at sea, for unknown reasons, and apparently as a result of food shortages or oil pollution.
The threats to seabirds mentioned above are all well known to conservationists, who are attempting to reduce the impact of these factors on seabirds where possible. Insofar as legal protection is concerned, it must be admitted that there are no marine sanctuaries in Denmark, although several discussions have taken place reviewing the possibility of establishing some in critical areas. There are, however, a number of sanctuaries on islands where seabirds breed. In the Sanctuary Act of 1936 these areas were called "Scientific Reserves" because they were the site of scientific investigations of bird life. All admission was forbidden, at least during the breeding season, and all shooting was prohibited, with few exceptions. These sanctuaries were administered by the government's Nature Conservancy.
The following Scientific Reserves are important for seabirds: Hirsholmene Islands (in Cattegat off Frederikshavn), Knotterne Islands (small islets east of Laesø Island), Vejlerne (diked in, landlocked fjords, densely covered with vegetation, at the Lim Fjord), Tipperne Peninsula and Klaegbanken Island (in Ringkøbing Fjord, western Jutland), Varsø Island (Horsens Fjord, eastern Jutland), and Græholm Island (Christiansø Archipelago, in the Baltic off Bornholm). A detailed description of these sites and their erection, bird life, and ornithological value was given by Salomonsen (1945). More recently, two additional Scientific Reserves have been established: Aegholm Islet (south of Sealand), and Hesselø Island in the southern part of Cattegat.
In addition to these scientific sanctuaries, there are game reserves and governmental forest reserves in Denmark. The game reserves are administered by the Ministry of Agriculture, which is also responsible for hunting legislation. The purpose of game reserves is to support and protect the stock of game, which includes migrating birds. Shooting is usually prohibited, but a restricted shooting season is allowed at some reserves. More than 50 game reserves are now present and functioning. Regulations differ widely from reserve to reserve, but entry to some of them is not allowed in the breeding season. Many reserves are important for breeding or migrating waterfowl and some seabirds. In fact, a total of 26 game reserves contain seabirds, the most important of which are the following: Ulvedybet (landlocked fjord at the Lim Fjord), Hjarbaek Fjord (landlocked fjord with brackish water at the Lim Fjord), Felsted Kog (landlocked fjord at Nissum Fjord), Jordsand (large stretches, almost 11,000 ha, of the Danish Waddensea), Stavns Fjord (at Samsø Island), Esrum Lake (in northern Sealand), and Kalvebod Beach (at Amager Island, near Copenhagen).
In the Nature Conservancy Act of 1969, differences between scientific and game reserves were abolished, although regulatory provisions that were in force for the scientific sanctuaries were maintained. Unfortunately, the amalgamation of the two types of reserve has given more power to the hunters' associations, which constitute the majority of the administrative body of the reserves, the so-called Game Commission ("Vildtnævnet"). However, any change in status of the original scientific reserves will not be tolerated by conservationists and other environmental groups in Denmark.
The number of seabirds in the Faroe Islands is greater than in any other region of the North Atlantic, and is closely related to the extraordinary richness of the plankton. The high phytoplankton production is due to a strong vertical mixing of the water in the northeast Atlantic, especially at the slopes of the submarine ridges, where both tidal currents and oceanic currents are usually strong. The resulting upwelling enriches the upper layers of water with large quantities of nutrient salts for the phytoplankton, and this, in turn, produces a teeming life of macroplankton and fish on which the seabirds are dependent (Salomonsen 1955).
The enormous seabird population of the Faroes is apparent from the first description of the islands, "De mensura orbis terrae," a document written in the year 825 by the Irish monk Dicuilus, who described the most characteristic feature of the Faroes as being the fact that "the islands were full of various kinds of marine birds." This richness has remained to the present, and has provided an important source of food for the resident human population, particularly in former times. There are few, if any, countries in the world in which wild-fowling and other exploitations of birdlife have played such a major role as in the Faroes. A number of elaborate and varied bird-catching methods were invented, and these have remained essentially the same for at least the last 500 years. Bird-fowling at great heights on precipitous sea-cliffs was a dangerous venture, and each year lives were lost. The main thing, however, was that food obtained from fowling meant life and death for local inhabitants and so was undertaken in such a well-balanced way that the seabird populations did not decrease or disappear. Some fowling still takes place, but on a reduced scale, since most men are now engaged in the fishery during the summer. Shooting is now of much greater importance than in former times.
The Faroese game acts (from 1897, 1928, and 1954) are very severe and show a broad consideration for birdlife. Practically all terrestrial birds, including shorebirds, are protected, and existing regulations permit people to catch or shoot only common murres, razorbills, puffins, shags (Phalacrocorax aristotelis), fulmars, gannets, parasitic jaegers (Stercorarius parasiticus), and gulls, as well as a few "pest" species like crows (Corvus corone) and ravens (C. corax). The legal right of fowling on a "fowling cliff" belongs to the registered owner of the land on which the cliff is situated. There are some sound restrictive laws for these cliffs. For example, shooting within 3.2 km of any seabird colony is prohibited.
| Species | Number of birds caught per year |
|---|---|
| Uria aalge | 60,000 |
| Fratercula arctica | 270,000 |
| Puffinus puffinus | 1,500 |
| Fulmarus glacialis | 80,000 |
| Morus bassanus | 1,300 |
| Total | 412,800 |
The annual number of seabirds caught by fowling in the early 1900's (summarized in Table 5) were reported in Salomonsen (1935). This large harvest of birds, taken by fowling year after year for centuries, did not appear to influence the seabird populations, as bird numbers remained stable. However, in recent years, shooting and a special form of snaring of murres have increased dramatically and seem to have endangered the murre population. The annual number of murres killed is estimated to be about 120,000, of which 70,000 are snared and at least 50,000 shot (estimates of birds shot range from 50,000 to 100,000). This total is almost double the number of birds caught during fowling, and because of an apparent decline in murre numbers the provincial government decided to investigate the matter, and in 1972 the Danish Ornithological Society agreed to conduct the study. Figures from the 1972 census of murres (Table 6) show that almost 600,000 birds were counted, from which an estimate of more than 393,000 breeding pairs was calculated (Dyck and Meltofte 1975). In spite of this large number, Dyck and Meltofte (1975) concluded that the Faroese murre population has declined by about 20% during the last 10-15 years. Investigations are under way to monitor further changes in murre numbers, and to determine the trend, and whether reductions in shooting and snaring are necessary to maintain the population.
Oil pollution is practically unknown in Faroese waters, but since drilling for oil will probably take place in the near future, the importance of oil to birds in this region may change. Toxic chemicals do not appear to be[Pg 279] involved in the decline in murres. Investigations of concentrations of chemical pollutants in their eggs show that levels of DDE (mean 1.1 ppm), PCB (mean 2.0 ppm), and mercury (mean 0.2 ppm) (Dyck and Meltofte 1975) are relatively low and unlikely to affect reproduction (Dyck and Meltofte 1975). Levels are much smaller than those found in seabirds in Britain, the Baltic, or in albatrosses in the Pacific (Fisher 1973).
| Colony | Number of birds observed | Number of pairs[72] |
|---|---|---|
| Suderoy | 73,945 | 49,500 |
| Lítla Dímun | 13,220 | 8,800 |
| Stóra Dímun | 68,050 | 45,600 |
| Sandoy | 101,710 | 68,100 |
| Hestur | 17,290 | 11,600 |
| Mykines | 14,500 | 9,700 |
| Vágar | 4,224 | 2,800 |
| Streymoy | 27,214 | 18,200 |
| Eysturoy | 10,520 | 7,000 |
| Kalsoy | 14,150 | 9,500 |
| Vidoy | 5,980 | 4,000 |
| Fugloy | 22,730 | 15,200 |
| Totals | 587,333 | 393,200[72] |
Greenland, which has an area of 2,175,600 km2 and extends for a distance of 2,670 km from the northernmost to the southernmost point of the country, is almost a continent by itself. The range of the different species of seabirds, therefore, is greatly varied, and it is necessary to classify them according to the relation between their distributions and the marine zones. A description of the zones of the marine environment in the North Atlantic was given by Salomonsen (1965), and the breeding distributions of seabirds in Greenland based on this system are given in Table 7. The terrestrial area of southernmost West Greenland belongs to the subarctic zone of the boreal province, and one boreal bird species, the black-headed gull, has bred there in recent years. It is, however, as much a freshwater bird as a marine one.
Fig. 1. Breeding range in Greenland of four boreo-panarctic seabirds, Fulmarus glacialis, Somateria mollissima, Rissa tridactyla, and Fratercula arctica.
Fig. 2. Breeding range in Greenland of three boreo-panarctic seabirds, Sterna paradisaea, Cepphus grylle, and Stercorarius parasiticus, and one low arctic species, Phalaropus lobatus.
Fig. 3. Breeding range in Greenland of three panarctic seabirds, Uria lomvia, Larus hyperboreus, and Clangula hyemalis, and one high arctic species, Stercorarius longicaudus.
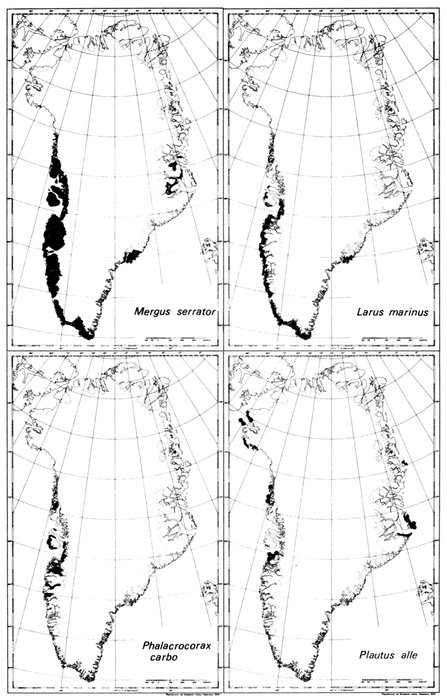
Fig. 4. Breeding range in Greenland of three boreo-low arctic seabirds, Mergus serrator, Larus marinus, and Phalacrocorax carbo, and one high arctic species, Plautus alle.
The widely differing ranges of Greenland seabirds are shown in Figs. 1-4 and are based on my new and previously unpublished data. The borderline between the high arctic and low arctic zones is situated in Melville Bay on the west coast, and just south of Scoresby Sound on the east coast; the innermost parts of Scoresby Sound belong to the low arctic zone.
In the low arctic Pacific region the number of seabirds is said to be about 51 million in summer and 8 million in winter (Sowl and Bartonek 1974). No similar estimate is available for low arctic West Greenland, but I suggest that it is much lower in summer and slightly higher in winter.
The human population of Greenland, now numbering about 50,000 individuals, is restricted to the seashore, where all cities and minor outposts are situated. Although shooting seabirds is an ancient tradition in Greenland, the true land-birds, which are few in number, are usually left alone. Seabirds collected by shooting provide an important source of food that the Greenlanders could not do without. Since special shooting and hunting regulations have not been developed in Greenland, these activities often resemble a sort of slaughter rather than true hunting. There is no game act in Greenland, and practically all birds can be shot. This condition is similar to that in Canada, where according to Section 5(7) of the Migratory Birds Regulations (Canadian Wildlife Service, Ottawa 1973) "an Indian or Inuk may at any time, without a permit, take auks, auklets, guillemots, murres, puffins and scoters and their eggs for human food and clothing." Much the same sort of hunting privileges exist for native peoples of Alaska. What is still worse, however, is the enormous illegal shooting of ducks, geese, swans, and cranes that is known to take place in arctic North America, but is largely ignored by police and game authorities. Bartonek et al. (1971) described this situation very well for Alaska. In Greenland, it is not possible any more to distinguish between "native Eskimos" and Greenlanders (including Danes working in the country), but the attitude toward animals among the inhabitants is the same as it has always been—a food source to hunt and kill.
With a rapidly growing human population, and a readily available supply of guns and speedboats for hunting, the whole natural ecosystem is beginning to break down, and it cannot be permitted to continue. The provincial government is aware of this fact, and various legal enactments have been issued from both the government and the local magistrates. However, since the size of the police force (mostly Greenlanders) is small, it is of little help for the preservation of wildlife, and sometimes even the policemen themselves do not know the local ordinances. The result has been that seabirds, previously profusely flourishing, have considerably decreased in number in West Greenland.
I have previously described the shooting and hunting of seabirds in Greenland and the statutory provisions issued to protect them (see Salomonsen 1970). At present, the following seabirds and their eggs are totally protected: whooper swan; common puffin, Fratercula arctica; and harlequin duck, Histrionicus histrionicus. Some other species have a closed season or are protected in certain parts of the country: snow goose, Anser caerulescens; common eider; king eider, Somateria spectabilis; great cormorant; dovekie, Plautus alle; black guillemot; and thick-billed murre, Uria lomvia. Furthermore, all catching and hunting of birds within 2 km of breeding colonies of murres and kittiwakes is prohibited. Bird sanctuaries where hunting, catching, and collecting of eggs and down are prohibited are Avsigsut, Nunatsiaq, and Satuarssunguit islands, which are scattered in Disko Bay, and Tasiussarssuaq Fjord (the inner part of Arfersiorfik Fjord, south of Egedesminde).
However, the Greenland Provincial Council has been alarmed by the serious decline in the numbers of seabirds due to increases in human persecution, and it has decided to introduce a game law similar to those in Denmark and other European countries. The preparation of this legislation was left to me, and a draft of this Greenland game act has been issued (Salomonsen 1974); the new law was passed in parliament in 1977 and went into force on 1 January 1978.
It is not possible to review in detail the different parts of the new law, but certain important points should be mentioned. In northern parts of West Greenland (north of Egedesminde) the sea is ice-covered for 7-8 months a year, and seabird hunting is therefore not pos[Pg 285]sible outside the breeding season. Because of this, it was necessary to allow some hunting of murres, eiders, and immature gulls during the breeding period, but away from nesting locations. Consumption of seabirds is to be limited to local residents, and sales to canneries for shipment to other cities is to cease. Previously, canneries in northwest Greenland exported large numbers of thick-billed murres to South Greenland—e.g., 25,606 birds in 1971; and 30,029 in 1972 (Anonymous 1974:64). This marketing of murres will end.
Other parts of the proposal important for seabirds include:
• A general closed season extending from 15 June to 15 August.
• Prohibition of shooting at breeding colonies of seabirds, as is in force at present (cf. above).
• Eggs of terns and gulls can be collected for food in southwest Greenland to 1 July, and in northwest Greenland to 10 July; fulmar and murre eggs can also be collected in northwest Greenland.
• Each hunter is allowed to shoot or catch 50 birds per day, but the entire bag must be used for human consumption.
• All shooting from speedboats, aircraft, and motor vehicles is prohibited.
• Catching flightless common eiders, king eiders, and oldsquaws (Clangula hyemalis) is prohibited.
• Practically all seabirds and shorebirds can be shot; all other birds (except rock ptarmigan and raven) are totally protected.
The principles of this radical new act must be taught to the population by all possible means of communication, including radio, public meetings, schools, etc.
Another matter of great concern to seabirds in Greenland is the Atlantic salmon fishery off the west coast by Danish, Greenlandic and foreign fishermen. It is well known that many birds are killed in the fishing gear, and a serious political controversy has arisen, especially between the governments of the United States and Denmark. The fact that a large number of thick-billed murres were drowned in salmon gill nets during their southward swimming migration along the Greenland coast was significant. In a resolution sent by the XV World Conference of the International Council for Bird Preservation in Texel to the Danish Government, it was stated that the annual incidental drowning of murres probably involved about 250,000 individuals—a figure exceeding the reproductive capacity of the species. This estimate was doubted by Danish fishery biologists, but recent investigations carried out by the Canadian Wildlife Service and the Fisheries Research Board of Canada have shown that the figure is even greater, and that the total kill amounts to about half a million murres annually (Tull et al. 1972).
Because of this mortality of murres, an agreement was reached between the American and Danish governments, namely that:
From 1 January 1976, all salmon fisheries outside the 12-mile boundary shall totally stop. In the years 1972-75 the fishery carried out by Danish and Faroese fishermen shall be reduced gradually from 800 to 300 tons of fish, and shall terminate on 31 December 1975. The fish quota by Greenland fishermen must amount to no more than 1,100 tons annually, but from 1976 onwards, the fishery shall be restricted to areas within the 12-mile limit.
This agreement, which has drastically reduced the number of murres caught, was discussed at a meeting of the International Committee of North Atlantic Fisheries in May 1972, and was ratified by the countries involved in July 1972.
Oil pollution has never occurred in Greenland, but concessions for offshore oil drilling along the West Greenland coast have just been granted by the Danish Government, and this new development gives rise for concern. However, it is clearly stated in the concession that the Ministry for Greenland can lay down rules for protection against oil pollution and other damage to human or animal life, and can adopt measures to fight pollution which has already taken place (section 5(9)). It is up to the concessionary to oversee industrial developments in the area and see that marine pollution is avoided (section 11).
Toxic chemicals have been found in Greenland seabirds, as everywhere else in the world, but it must be emphasized that no pesticides whatsoever are in use in Greenland itself. Investigations by Somer and Appelquist (1974) indicated that the mercury content in black[Pg 286] guillemots in Greenland has doubled over the last 20 years, and has now reached 2 ppm, which is, however, a relatively low figure. Levels of DDE, PCB, and aldrin in Greenland birds were investigated by Braestrup et al. (1974). Common eider, king eider, harlequin duck, and oldsquaw, as well as thick-billed murre and great cormorant, were examined; all were found to be contaminated with pesticides, although to varying degrees. Highest concentrations occurred in the cormorant, which contained 6.5-15 ppm of DDE and 14.1-46.7 ppm of PCB. These specific differences appear to show that the pesticide level in the different species of seabirds is influenced more by the position of the bird in the food chain than by its migratory habits.
And finally, I wish to mention a more happy event. On 9 May 1974 a new law of nature protection in Greenland was passed by the Danish Parliament. According to this law, a National Park is to be established covering almost the entire northeast and north regions of Greenland, from the Thule District in northern West Greenland around the entire north coast of Greenland and south along the east coast to the northern inner parts of Scoresby Sound. All hunting, fishing, egg-collecting, and disturbances to the environment are forbidden in this enormous area. This is by far the greatest National Park in the world, covering about 800,000 km2. Of this total area, the greater part is a lifeless icecap, to be sure, but about 200,000 km2 is ice-free land and suitable habitat for numerous high-arctic birds.
Anonymous. 1974. Grønland 1973, Arsberetning. Vol. 4. Ministeriet for Grønland, Copenhagen. 106 pp.
Atkinson-Willes, G. L. 1972. The international wildfowl censuses as a basis for wetland evaluation and hunting rationalization. Pages 87-110 in Proc. Int. Conf. Conserv. Wetlands Waterfowl, Iran 1971.
Bartonek, J. C., J. G. King, and H. K. Nelson. 1971. Problems confronting migratory birds in Alaska. Trans. N. Am. Wildl. Nat. Resour. Conf. 36:345-361.
Bloch, D. 1970. The mute swan (Cygnus olor) breeding in colony in Denmark. [Danish with English summary.] Dansk Ornith. Foren. Tidsskr. 64:152-162.
Bloch, D. 1971. Ynglebestanden af knopsvane (Cygnus olor) i Danmark 1966. Danske Vildtundersøgelser 16. 47 pp.
Bloch, D., C. H. Ovesen, B. H. Fenger, J. Jensen, and J. Pedersen. 1972. Vildtreservatet Nakskov Indrefjord. Resume af biologiske, kemiske og fysiske underseøgelser i 1970-1971. Vildtbiologisk Station, Kalø, Rønde. 15 pp.
Bogan, J. A., and W. R. P. Bourne. 1972. Organochlorine levels in Atlantic seabirds. Nature (Lond.) 240(5380):358.
Bourne, W. R. P. 1972. Threats to seabirds. Int. Counc. Bird Preserv. Bull. 9:200-218.
Braestrup, L., J. Clausen, and O. Berg. 1974. DDE, PCB and aldrin levels in arctic birds of Greenland. Bull. Environ. Contam. Toxicol. 11(4):326-332.
Dyck, J. 1975. Miljøgifte i danske fugle. Panda-Nyt. Verdensnaturfonden 1975(1):11-13.
Dyck, J., and H. Meltofte. 1975. The guillemot Uria aalge population of the Faeroes 1972. Dansk Ornith. Foren. Tidsskr. 69:55-64.
Fisher, H. I. 1973. Pollutants in North Pacific albatrosses. Pacific Sci. 27(3):220-225.
Joensen, A. H. 1961. Disaster among fulmars (Fulmarus glacialis (L.)) and kittiwakes (Rissa tridactyle (L.)) in Danish waters. [Danish with English summary.] Dansk Ornith. Foren. Tidsskr. 55:212-218.
Joensen, A. H. 1972a. Oil pollution and seabirds in Denmark 1935-1968. Dan. Rev. Game Biol. 6(8):1-24.
Joensen, A. H. 1972b. Studies on oil pollution and seabirds in Denmark 1968-1971. Dan. Rev. Game Biol. 6(9):1-32.
Joensen, A. H. 1973a. Moult migration and wing-feather moult of seaducks in Denmark. Dan. Rev. Game Biol. 8(4):1-42.
Joensen, A. H. 1973b. Danish seabird disasters in 1972. Mar. Pollut. Bull. 4(8):117-118.
Joensen, A. H. 1974. Waterfowl populations in Denmark 1965-1973. A survey of the non-breeding populations of ducks, swans and coot and their shooting utilization. Dan. Rev. Game Biol. 9(1):1-206.
Jørgensen, O. H., and I. Kraul. 1974. Eggshell parameters, and residues of PCB and DDE in eggs from Danish herring gulls, Larus a. argentatus. Ornis Scand. 5(2):173-179.
Koeman, J. H., W. S. M. van de Ven, J. J. M. de Goejj, P. S. Tjioe, and J. L. van Haaften. 1975. Mercury and selenium in marine mammals and birds. Sci. Total Environ. 3:279-287.
Mardal, W. 1974. Ternegruppen. Feltornithologen 16:4-7.
Mueller, W. J., and R. M. Leach, Jr. 1974. Effects of chemicals on egg shell formation. Annu. Rev. Pharmacol. 14:289-303.
Nowak, E. 1973. Hunting kill statistics investigation. Int. Waterfowl Res. Bur. Bull. 35:26-30.
Parslow, J. L. F., and D. J. Jefferies. 1973. Relationship between organochlorine residues in livers and whole bodies of guillemots. Environ. Pollut. 5:87-101.
Peakall, D. B. 1970. Pesticides and the reproduction of birds. Sci. Am. 222(4):72-78.
Salomonsen, F. 1935. Ayes. In R. Spärck, ed. Zoology of the Faroes 3(pt. 2) (lxiv):269 + 6 suppl. A. F. Høst, Copenhagen.
Salomonsen, F. 1945. De videnskabelige reservater i Danmark og deres Fugleliv. Fauna och Flora 40:250-261.
Salomonsen, F. 1954. The Danish game-statistics. [Danish with English summary.] Dansk Ornith. Foren. Tidsskr. 48:123-126.
Salomonsen, F. 1955. The food production in the sea and the annual cycle of Faeroese marine birds. Oikos 6(1):92-100.
Salomonsen, F. 1963. Oversight over Danmarks fugle. [With explanatory notes in English.] Munksgaard, Copenhagen. 156 pp.
Salomonsen, F. 1965. The geographical variation of the fulmar (Fulmarus glacialis) and the zones of marine environment in the North Atlantic. Auk 82:327-355.
Salomonsen, F. 1968. The moult migration. Wildfowl 19:5-24.
Salomonsen, F. 1970. Birds useful to man in Greenland. Pages 169-175 in Productivity and conservation in northern circumpolar lands. International Union for Conservation of Nature and Natural Resources, Morges, Switzerland.
Salomonsen, F. 1974. Forslag til vedtægt om jagt på fuglene i Grønland. Diskussionsoplæg til kommunalbestyrelserne udarbejdet på opfordring af Det grønlandske Landsråd. Tidsskr. Grønland 1974(5):155-172.
Somer, E., and H. Appelquist. 1974. Changes and differences in mercury level in the Baltic and Kattegat compared to the North Atlantic using Uria sp. (guillemot sp.) and Cepphus grylle (black guillemot) as indicators. 9th Conf. of the Baltic Oceanographers, Kiel. 12 pp. Danish Isotope Centre, Copenhagen.
Sowl, L. W., and J. C. Bartonek. 1974. Seabirds—Alaska's most neglected resource. Trans. N. Am. Wildl. Nat. Resour. Conf. 39:117-126.
Strandgaard, H. 1964. The Danish bag record. I. Studies in game geography based on the Danish bag record for the years 1956-57 and 1957-58. Dan. Rev. Game Biol. 4(2):1-116.
Tull, C. E., P. Germain, and A. W. May. 1972. Mortality of thick-billed murres in the West Greenland salmon fishery. Nature (Lond.) 237(5349):42-44.
[67] Not counted.
[68] No estimate, but number insignificant.
[69] Species totally protected.
[70] Branta bernicla is fully protected since 1972.
[71] Somateria mollissima, Melanitta nigra, and M. fusca.
[72] The "number of pairs" is calculated by multiplying the number of birds observed by 0.67 (Dyck and Meltofte 1975).
by
Einar Brun[74]
University of Tromsø
Tromsø, Norway
The most numerous seabird in Norway is the puffin (Fratercula arctica), but its current breeding population of 1.25 million pairs is slowly declining. The kittiwake (Rissa tridactyla), however, is increasing and establishing new colonies; its population now stands at 510,000 pairs. The population of the common murre (Uria aalge), the seabird species most vulnerable to human activity, was about 160,000 breeding pairs in 1964 but is now decreasing at a rate of nearly 5% per year. Of the other alcids, the razorbill (Alca torda) and thick-billed murre (Uria lomvia) show similar declines, and the black guillemot (Cepphus grylle) is maintaining a stable population. The fulmar (Fulmarus glacialis) and the gannet (Sula bassana) have both spread from the British Isles and have established a number of breeding colonies in Norway during this century. Evidently immigration of gannets is still occurring, since the observed rate of increase far exceeds the population's intrinsic rate of increase. The impact of human activity on bird mortality varies from species to species. The two most serious factors are coastal oil pollution and the use of fishing gear; direct hunting pressure accelerates the decline of murres and razorbills. Persistent toxic chemicals are not yet a serious problem in Norway.
Norway, with a coastline of more than 20,000 km, an abundance of islands, and areas of offshore upwelling, provides good conditions for a rich seabird fauna. A regional study of this seabird fauna has been undertaken as a sideline of basic marine research. Although the ultimate aim has been to evaluate the importance of seabirds in the energy flow of a marine ecosystem, a more realistic problem (given priority so far) has been to study yearly production and the dynamics behind changes in the breeding populations.
Good population estimates are of fundamental importance to studies of population dynamics. Because the available censuses of seabirds in Norway were few and largely inadequate, a long-term program was started in 1961. In the beginning, resources and assistance were very limited, and the work was concentrated on cliff-breeding seabirds, particularly the gannet (Sula bassana), fulmar (Fulmarus glacialis), kittiwake (Rissa tridactyla), razorbill (Alca torda), common murre (Uria aalge), thick-billed murre (U. lomvia), and puffin (Fratercula arctica). Until 1970, the study involved making annual censuses in the approximately 20 major colonies of cliff-breeding seabirds and mapping the distribution of the quantitatively less important colonies.
Since 1970, the Norwegian seabird program has also involved more detailed studies in some selected colonies. In these colonies, emphasis has been on investigation of yearly production and of the factors limiting this production, and evaluation of the effects of human activity on the population growth.
The logistics of census operations have gradually improved from the use of slow, local transportation to the use of fast pneumatic boats and, in more recent years, seaplanes. Various census methods have been used, depending on species and circumstances.
For puffins, a method based on measurement of feeding frequency and on the number of puffins per time unit that pass a particular observation post when they return from the feeding ground was used (Brun 1971a). Kittiwakes and gannets were readily censused by a combination of photographic methods and detailed counts in sample areas (Brun 1971b). Direct counting is by far the most accurate method for razorbills, murres, and fulmars; but in the larger colonies of common murre, lack of time permitted accurate counts for only a limited proportion of the cliff. Direct counts of individuals, the egg/chick ratio, and estimates of the relative size of the censused population were used to estimate the total population of the colony.
In a colony of kittiwakes near Tromsø, environmental factors that limit breeding success, such as temperature and wind exposure, were monitored throughout the breeding season on a data recorder, and detailed measurements of temperatures on and inside the eggs have been recorded. For further information about the influence of environmental parameters on incubation rhythm and nest attendance, the presence of the male and female at a particular nest was recorded by using radioactive bands and a Geiger-Muller tube connected to a pen recorder.
In a study of the effects of human activity, egg samples of selected species were analyzed for mercury, PCB, and DDT derivates. An effort was also made to obtain figures for the mortality caused by oil pollution and fishing gear as well as by direct hunting pressure.
By far the most numerous seabird in Norway is the puffin (Fig. 1), which is the only species with a breeding population of more than 1 million breeding pairs (Tables 1, 2). In a 1964 census (Brun 1966) the total breeding population was put at 1.5 million pairs. The current figure of 1.25 million pairs includes several newly discovered colonies and some not censused in 1964; it is more accurate than the previous census for most of the 15 largest colonies which make up 99.9% of the total population. The puffin population is concentrated in Troms and Nordland (94%), with only about 3% in Finnmark.
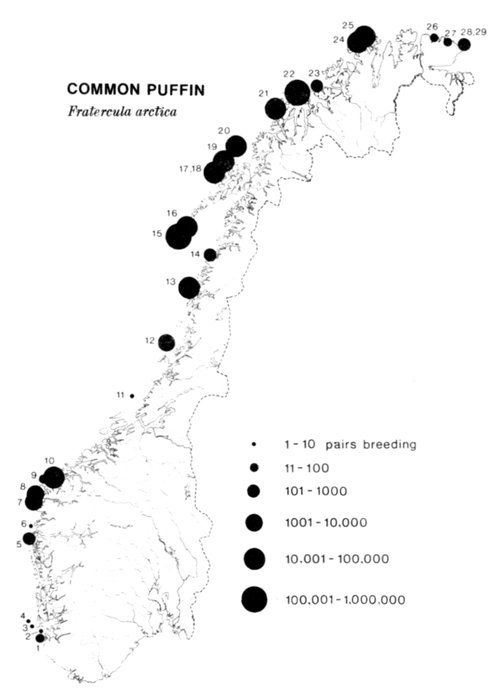
Fig. 1. Distribution of the puffin (Fratercula arctica) in Norway. Numbers refer to localities listed in Table 2.
The second most numerous seabird species in Norway is the kittiwake, which dominates in a number of the larger cliff colonies. Its distribution pattern differs from that of the puffin—the main occurrence of the kittiwake population (about 63%) is in Finnmark (Table 3).
| Species | Thousands of pairs[75] | Increase (+) or decline (-) |
|---|---|---|
| Fratercula arctica | 1250 | - |
| Rissa tridactyla | 510 | + |
| Larus argentatus | (260)[76] | + |
| L. canus | (150)[76] | + |
| Uria aalge | 100 | - |
| L. marinus | (40)[76] | + |
| Phalacrocorax aristotelis | 33 | + |
| Alca torda | 30 | - |
| Cepphus grylle | 22 | 0 |
| Sterna paradisaea | (21)[76] | - |
| S. hirundo | (13)[76] | - |
| Phalacrocorax carbo | 12 | + |
| L. fuscus | 9[76] | + |
| Stercorarius parasiticus | (8) | 0 |
| L. ridibundus | 4[76] | + |
| Fulmarus glacialis | 1.1 | + |
| U. lomvia | 1.0 | - |
| Hydrobates pelagicus | ? | ? |
| Sula bassana | 0.76 | + |
| Oceanodroma leucorrhoa | ? | ? |
The annual production of kittiwakes shows enormous variation, both throughout the coastline and in different years; however, at our sample stations in north Norway, the mean production in 1974 (Table 4) was more stable and was near the minimum value necessary to maintain zero population growth.
This minimum production, mx (number of females produced per breeding female), can be computed from survival rates
mx = (1-P)/1x = 0.13/0.57 = 0.23
where P is annual adult survival and 1x is survival of fledged chicks up to first breeding. Data on survival are taken from Coulson and White (1959) and from Norwegian banding recoveries.
The kittiwake has, however, established a number of new colonies, and although the local increase in some of these is spectacular, the long-term change during the last 15 years is only about 1% increase per year in northern Norwegian colonies (E. Brun, unpublished data). In southern Norway, the population has increased much more rapidly (Brun 1971c).
The common murre (Fig. 2) has shown a considerable decrease. The most spectacular decrease is at Sør-Fugløy, where a colony of 10,000 pairs in 1940 was reduced to 4,000 pairs in 1961, to 1,100 pairs in 1966, and to only about 10 breeding pairs in 1974 (Table 5). Most of the census work was done in 1964 and 1974. The general trend in population change, as expressed by the yearly decrease or increase, has been extrapolated forward to 1974 or back to 1964 for those colonies where censuses were missing for either of these years, to enable a better comparison (Table 6). The overall decrease in Norwegian colonies of the common murre is, thus, near 5% per year; the few cases with a positive trend are based either on very small figures or on extrapolation from old, inadequate censuses.
The thick-billed murre (Fig. 3) was first proved to breed in Norwegian colonies in 1964; it was then found at three localities and has since been found breeding at eight localities (Table 7). It is now fairly certain that the thick-billed murre is not a newcomer but has remained unnoticed among the common murre for generations, possibly since the original immigration of the Uria species after the last glacial period. Data are not sufficient to show whether this small population of thick-billed murres is decreasing at the same rate as the common murre.
| Locality | Year of census | Number of pairs | Percent of population | |
|---|---|---|---|---|
| 1. | Kjør | 1975 | 80 | <0.1 |
| 2. | Heglane | 1970 | 4 | <0.1 |
| 3. | Ferkingstadøyene | 1970 | 5 | <0.1 |
| 4. | Utsira | 1970 | 2 | <0.1 |
| 5. | Utvær | 1970 | 200 | <0.1 |
| 6. | Ryggsteinen | 1970 | 2 | <0.1 |
| 7. | Veststeinen | 1970 | 1,500 | 0.1 |
| 8. | Einevarden | 1970 | 1,500 | 0.1 |
| 9. | Svinøy | 1970 | 100 | <0.1 |
| 10. | Runde | 1974 | 30,000 | 2.4 |
| 11. | Saløy | 1970 | 2 | 0.1 |
| 12. | Sklinna | 1974 | 2,000 | 0.2 |
| 13. | Lovunden | 1968 | 60,000 | 4.8 |
| 14. | Fugløy i Gildeskål | 1968 | 800 | 0.1 |
| 15. | Røst | 1964 | 700,000 | 55.7 |
| 16. | Værøy | 1974 | 70,000 | 5.6 |
| 17. | Nykvåg | 1967 | 40,000 | 3.2 |
| 18. | Frugga | 1975 | 5,000 | 0.4 |
| 19. | Anda | 1970 | 10,000 | 0.8 |
| 20. | Bleik | 1968 | 40,000 | 3.2 |
| 21. | Sør-Fugløy | 1968 | 40,000 | 3.2 |
| 22. | Nord-Fugløy | 1967 | 218,000 | 17.3 |
| 23. | Loppa | 1968 | 180 | <0.1 |
| 24. | Hjelmsøy | 1964 | 20,000 | 1.6 |
| 25. | Gjesværstappen | 1973 | 18,000 | 1.4 |
| 26. | Kongsøy | 1966 | 30 | <0.1 |
| 27. | Syltefjord | 1966 | 100 | <0.1 |
| 28. | Hornøy | 1967 | 160 | <0.1 |
| 29. | Reinøy | 1967 | 40 | <0.1 |
| Total | 1,257,705 | 100.0 |
Another colonial cliff-breeding alcid, the razorbill (Fig. 4), has a distribution pattern very similar to that of the common murre, but the individual colonies (Table 8) are, with one exception, smaller. The total breeding population was estimated at 36,000 pairs in 1966-69 (Brun 1969b); some more recent censuses show a definite decline, but data are not sufficient to estimate the overall decline in the Norwegian population. At most, the current breeding population is 30,000 pairs.
The fulmar is one of two species of seabirds that have spread from colonies in the British Isles and established themselves as breeding birds in Norway during this century (the other is the gannet).
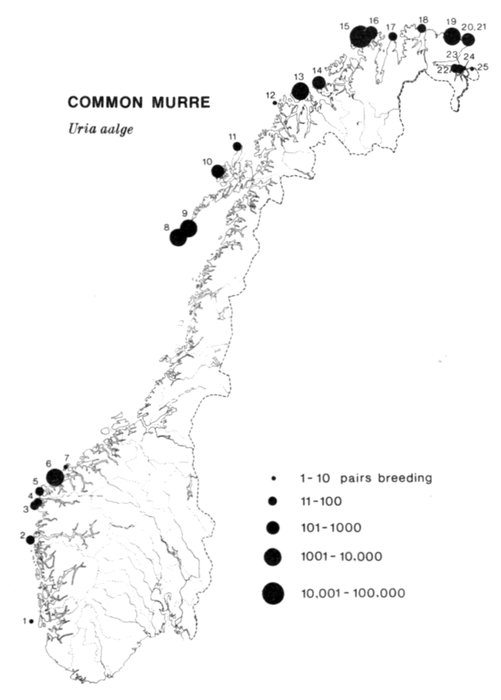
Fig. 2. Distribution of the common murre (Uria aalge) in Norway. Numbers refer to localities listed in Tables 5 and 6.
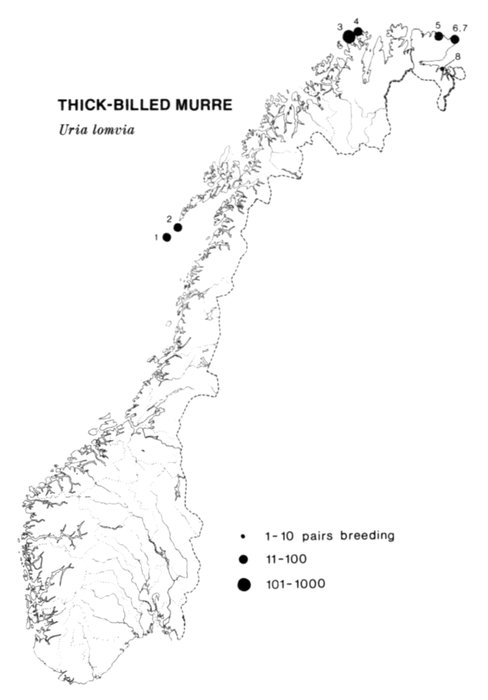
Fig. 3. Distribution of the thick-billed murre (Uria lomvia) in Norway. Numbers refer to localities listed in Table 7.
| County | Breeding pairs | |||
|---|---|---|---|---|
| Rissa | Fratercula | |||
| Number (thousands) |
Percent | Number (thousands) |
Percent | |
| Finnmark | 321 | 62.9 | 38 | 3.0 |
| Troms | 9 | 1.8 | 258 | 20.5 |
| Nordland | 72 | 14.1 | 928 | 73.7 |
| Trøndelag (S, N) | 1 | 0.2 | 2 | 0.2 |
| Møre and Romsdal | 105 | 20.6 | 30 | 2.4 |
| Sogn and Fjordane | 1.9 | 0.4 | 3 | 0.2 |
| Rogaland | 0.1 | — | <0.1 | — |
| Total | 510 | 100.0 | 1,259 | 100.0 |
The fulmar began nesting in the early 1920's on Runde, the only sizeable seabird colony in south Norway, off Alesund. Further immigration of birds from the British Isles probably occurred in the first 25 years, when the population increased about 10% annually to about 350 pairs in 1947 (Valeur 1947). Since then the population increase has slowed down to about 3% annually, and the population on Runde in 1971 was about 700 pairs (Table 9). From Runde, fulmars have spread not only to a number of islands in the same region, but also much farther afield—south to Utsira[Pg 294] (59°18'N, 4°55'E) and north to Bleik (69°3'N, 15°42'E). The total Norwegian population of fulmars in 1971 was estimated at 1,100 pairs.
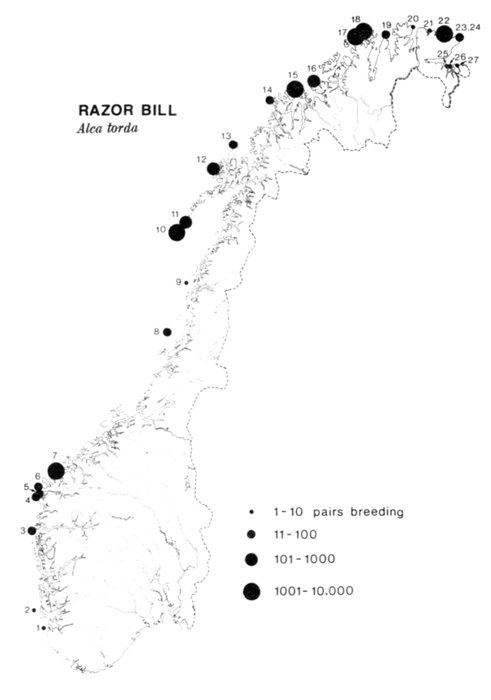
Fig. 4. Distribution of the razorbill (Alta torda) in Norway. Numbers refer to localities listed in Table 8.
Fig. 5. Distribution of the gannet (Sula bassana) in Norway. Numbers refer to localities listed in Table 10.
| Locality | Year | Sample size (number) | mx |
|---|---|---|---|
| Vedøy, Røst | 1972 | 852 | 0.21 |
| Hekkingen, Troms | 1974 | 264 | 0.46 |
| Hjelmsøy | 1974 | 357 | 0.18 |
| Jarfjord | 1974 | 146 | 0.31 |
| Total | 1,619 | 0.25 |
The gannet (Fig. 5), the most recently established and least numerous of the cliff-breeding seabirds, has the best-known population change. Like the fulmar, it was established in 1946 on Runde, and the first individuals were undoubtedly of British origin. During its entire breeding history on Runde, and also in two of the three new colonies in northern Norway established in the 1960's, the yearly increase has far exceeded the intrinsic rate of increase (Table 10); for gannets with a 50% breeding success, adult mortality of 6%, and 35% survival up to first breeding, the intrinsic rate of increase is about 2% per year. The Runde and Syltefjord colonies are naturally protected by their inaccessibility, but the colonies at Mosken and Nordmjele, which are on small islets, are both easily accessible. The Nordmjele colony, however, has been effectively protected from its start, whereas the Mosken colony has been open to visitors; this difference is probably reflected in their different breeding success and annual growth rate (Table 11). The breeding success necessary to maintain a stable population with the[Pg 295] mortality figures given above is 34%:
mx = (1-P)/1x = 0.66/0.35 = 0.17
For equal sex ratio, breeding success is 2 times mx = 0.34.
A British ringed gannet from Ailsa Craig (55°12'N, 5°07'W) was found nesting when 4 years old in the Nordmjele colony in 1970 (Brun 1972), giving direct evidence that immigration from colonies in Great Britain (Scotland) still takes place.
| Locality | Last census | Previous census | Reference | |||
|---|---|---|---|---|---|---|
| Year | No. of breeding pairs | Year | No. of breeding pairs | |||
| 1. | Utsira | 1970 | 1 | 1950 | 10 | Holgersen 1951 |
| 2. | Utvær | 1970 | 17 | 1948 | 55 | Willgohs 1952 |
| 3. | Veststeinen | 1970 | 29 | 1950 | 40 | Willgohs 1952 |
| 4. | Klovningen | 1970 | 35 | 1950 | 20 | Willgohs 1952 |
| 5. | Einevarden | 1970 | 30 | 1952 | 25 | Willgohs 1955 |
| 6. | Runde | 1974 | 6,000 | 1963 | 7,600 | Brun 1969a |
| 7. | Storholmen | 1970 | 8 | — | — | |
| 8. | Røst | 1974 | 6,800 | 1964 | 9,700 | Brun 1969a |
| 9. | Værøy | 1974 | 1,750 | 1964 | 2,400 | Brun 1969a |
| 10. | Nykvåg | 1974 | 350 | 1966 | 430 | Brun 1969a |
| 11. | Bleik | 1974 | 60 | 1952 | 90 | Regnell 1957 |
| 1964 | 75 | Brun 1969a | ||||
| 12. | Sør-Fugløy | 1974 | 10 | 1940 | 10,000 | Soot-Ryen 1941 |
| 1961 | 4,000 | Brun 1963 | ||||
| 1966 | 1,100 | Brun 1969a | ||||
| 13. | Nord-Fugløy | 1967 | 9,000 | 1963 | 15,000 | Lütken 1965 |
| 14. | Loppa | 1974 | 500 | 1966 | 800 | Brun 1969a |
| 15. | Hjelmsøy | 1974 | 70,000 | 1964 | 110,000 | Brun 1965 |
| 1967 | 95,000 | Brun 1969a | ||||
| 16. | Gjesværstappene | 1973 | 580 | 1967 | 750 | Brun 1969a |
| 17. | Sværholtklubben | 1973 | 20 | 1966 | 25 | Brun 1969a |
| 18. | Omgangsstauran | 1973 | 70 | 1967 | 85 | Brun 1969a |
| 19. | Syltefjorden | 1974 | 9,000 | 1966 | 12,300 | Brun 1969a |
| 20. | Hornøy | 1974 | 500 | 1964 | 730 | Brun 1969a |
| 21. | Reinøy | 1974 | 110 | 1964 | 160 | Brun 1969a |
| 22. | Kjøfjord | 1970 | 21 | — | — | |
| 23. | Skogerøy | 1970 | 8 | 1967 | 6 | Brun 1969a |
| 24. | Sagfjord | 1970 | 9 | 1967 | 12 | Brun 1969a |
| 25. | Kobbholmfjorden | 1970 | 2 | 1967 | 1 | Brun 1969a |
In addition to the more detailed censuses of the cliff-breeding species dealt with so far, notes have been made on all seabirds observed during numerous flights along the Norwegian coast. Although a first attempt at putting a figure to all seabird species in Norway may be somewhat premature, it is believed that even an extrapolation combined with an educated guess is of some value until more accurate censuses covering the whole coast can be made. Although the data (Table 1) are arranged in the same way as the results from "Operation Seafarer" in the British Isles (Cramp et al. 1974), it must be stressed that the accuracy of the Norwegian figures, at least for the non-cliff-breeding birds, is far inferior to the very fine British data. The table includes data for two petrels (Hydrobates pelagicus, Oceanodroma leucorrhoa), which in Norway breed on Røst (well north of the Arctic Circle), where they have adapted to a delayed breeding season with egg laying in August because of the conflict of their nocturnal habits with the continuous daylight due to the midnight sun. Of the present population trends that are given for each species in Table 1, all auks except the black guillemot (Cepphus grylle) are decreasing, whereas the gulls, the gannets, and the fulmars are increasing.
| Locality | Number of breeding pairs[77] | Percentage yearly decrease (-) or increase (+) | ||
|---|---|---|---|---|
| 1964 | 1974 | |||
| 1. | Utsira | 2 | 1 | -12.2 |
| 2. | Utvær | 23 | 14 | -5.5 |
| 3. | Veststeinen | 32 | 27 | -1.6 |
| 4. | Klovningen | 30 | 39 | +2.8 |
| 5. | Einevarden | 28 | 31 | +1.0 |
| 6. | Runde | 7,438 | 6,000 | -2.2 |
| 7. | Storholmen | 9 | 7 | (-2.2)[78],[79] |
| 8. | Røst | 9,700 | 6,800 | -3.6 |
| 9. | Værøy | 2,400 | 1,750 | -3.2 |
| 10. | Nykvåg | 453 | 350 | -2.6 |
| 11. | Bleik | 75 | 60 | -2.3 |
| 12. | Sør-Fugløy | 1,844 | 10 | -68.5 |
| 13. | Nord-Fugløy | 13,201 | 3,681 | -13.6 |
| 14. | Loppa | 900 | 500 | -6.1 |
| 15. | Hjelmsøy | 110,000 | 70,000 | -4.6 |
| 16. | Gjesværstappen | 853 | 556 | -4.4 |
| 17. | Sværholtklubben | 27 | 19 | -3.2 |
| 18. | Omgangsstauran | 94 | 68 | -3.3 |
| 19. | Syltefjorden | 13,299 | 9,000 | -4.0 |
| 20. | Hornøy | 730 | 500 | -3.9 |
| 21. | Reinøy | 160 | 110 | -3.8 |
| 22. | Kjøfjord | 21 | 20 | (-0.4)[78],[79] |
| 23. | Skogerøy | 5 | 12 | +10.1 |
| 24. | Sagfjord | 16 | 6 | -10.1 |
| 25. | Kobbholmfjord | 1 | 5 | +26.0 |
| Total | 161,341 | 99,566 | -4.9 | |
| Locality | Year | No. of breeding pairs | Percentage of total Uria population | |
|---|---|---|---|---|
| 1. | Vedøy, Røst | 1974 | 15 | 0.3 |
| 2. | Værøy | 1966 | 20 | 0.9 |
| 3. | Hjelmsøy | 1974 | 850 | 1.2 |
| 4. | Gjesværstappene | 1973 | 25 | 4.3 |
| 5. | Syltefjord | 1970 | 90 | 0.9 |
| 6. | Hornøy | 1966 | 55 | 8.1 |
| 7. | Reinøy | 1964 | 1 | 0.6 |
| 8. | Kjøfjord | 1970 | 1 | 4.8 |
| Estimated number, Norway, 1974 | >1,000 | ca. 1.0 | ||
| Locality | Year | No. of breeding pairs | Percent | |
|---|---|---|---|---|
| 1. | Kjør | 1970 | 1 | <0.1 |
| 2. | Utsira | 1970 | 25 | 0.1 |
| 3. | Utvær | 1970 | 16 | 0.1 |
| 4. | Veststeinen | 1970 | 22 | 0.1 |
| 5. | Klovningen | 1970 | 12 | <0.1 |
| 6. | Einevarden | 1970 | 45 | 0.2 |
| 7. | Runde | 1974 | 2,800 | 9.5 |
| 8. | Sklinna | 1974 | 15 | 0.1 |
| 9. | Lovunden | 1968 | 8 | <0.1 |
| 10. | Røst | 1974 | 3,900 | 13.2 |
| 11. | Værøy | 1974 | 800 | 2.7 |
| 12. | Nykvåg | 1966 | 250 | 0.8 |
| 13. | Bleik | 1968 | 28 | 0.1 |
| 14. | Sør-Fugløy | 1974 | 15 | 0.1 |
| 15. | Nord-Fugløy | 1967 | 10,000 | 33.8 |
| 16. | Loppa | 1969 | 750 | 2.5 |
| 17. | Hjelmsøy | 1974 | 7,000 | 23.7 |
| 18. | Gjesvær | 1973 | 2,500 | 8.5 |
| 19. | Sværholtklubben | 1973 | 18 | 0.1 |
| 20. | Omgangsstauran | 1973 | 6 | <0.1 |
| 21. | Kongsøy | 1966 | 8 | <0.1 |
| 22. | Syltefjorden | 1966 | 1,200 | 4.1 |
| 23. | Hornøy | 1967 | 65 | 0.2 |
| 24. | Reinøy | 1967 | 55 | 0.2 |
| 25. | Kjøfjord | 1970 | 9 | <0.1 |
| 26. | Skogerøy | 1970 | 4 | <0.1 |
| 27. | Jarfjordnes | 1970 | 3 | <0.1 |
| Total | ca. 30,000 | |||
Since the coastline of Norway is about the same length as the coastline of Great Britain and Ireland, it is interesting to compare the population figures (Table 12), although the accuracy is very different. Populations of auks and gulls are similar in both areas, but the species composition is different. There are more terns in the British Isles, but skuas (Catharacta skua), shags (Phalacrocorax aristotelis), and great cormorants (P. carbo) are present in similar numbers. The most striking difference is the very small number of procelli-forms and gannets in Norway compared to Britain and Ireland, where they are almost as numerous as the gulls and the auks.
| County | Number of localities | Number of breeding pairs |
|---|---|---|
| Nordland | 6 | 140 |
| Møre and Romsdal | 7 | 945 |
| Sogn and Fjordane | 2 | 11 |
| Rogaland | 2 | 2 |
| Total | 17 | 1,098 |
| Colony | Year established | Mean yearly growth rate 1969-1974 (%) |
No. of breeding pairs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1969 | 1970 | 1971 | 1972 | 1973 | 1974 | ||||
| 1. | Runde | 1946 | 8.4 | 330 | 331 | 383 | 422 | 450 | 494 |
| 2. | Mosken | ca. 1960 | 5.4 | 50 | 83 | 77 | 60 | 62 | 65 |
| 3. | Nordmjele | 1967 | 83.3 | 7 | 36 | 65 | 103 | 127 | 145 |
| 4. | Syltefjord | 1961 | 14.5 | 28 | 29 | 44 | 48 | 51 | 55 |
| Total | 12.8 | 415 | 479 | 569 | 633 | 690 | 759 | ||
| Yearly growth rate (%) | 15.4 | 18.8 | 11.2 | 9.0 | 10.0 | ||||
According to Norwegian laws, all seabirds, with the exception (for some odd reason) of the gannet and fulmar, can be hunted from 21 August to 1 March. However, only the two species of murre and the razorbill are still regularly hunted and, although no statistics support it, an estimate based on interviews with some of the hunters reveals that murres and razorbills are shot in the ratio of about 50:1. One man can shoot as many as 380 murres and razorbills during a winter season as a sideline to fishing. Although not many hunt on this scale, an absolute minimum of 5,000 murres and razorbills are killed this way each season.
A new law based on modern principles of conservation has been under consideration for several years, and this will mean an improvement. However, the speed of the decline of the auks, particularly the murres, makes it imperative to stop this hunting immediately, and it is of very little economic importance to the few who take part. Some illegal "fishing" for auks still takes place at Røst and Vaerøy, where fishnets are anchored over wooden frames outside the auk colonies at the beginning of the nesting season. At Vedøy on Røst in 1972, up to 80 murres were taken daily. Thus an estimated total of 500-700 murres were taken that year—about 5% of the breeding population on this island.
Egg collecting was important during World War II, but in these more affluent times and because of the relative inaccessibility of the auks' nests, egg collecting is now both less attractive and less important. Human disturbance of the breeding colonies, however, is gradually becoming a more serious factor.
| Mosken | Nordmjele | |||
|---|---|---|---|---|
| Annual growth rate | Breeding success (%) | Annual growth rate | Breeding success (%) | |
| 1969 | 62 | — | ||
| 1.66 | 5.14 | |||
| 1970 | 51 | 61 | ||
| 0.93 | 1.81 | |||
| 1971 | 36 | 46 | ||
| 0.78 | 1.58 | |||
| 1972 | 33 | 62 | ||
| 1.03 | 1.23 | |||
| 1973 | 50 | 35 | ||
| 1.05 | 1.14 | |||
| 1974 | 12 | 39 | ||
| 1969-1974 | 1.05 | 40 | 1.83 | 46 |
Although on a scale different from that in western Greenland, drift-net and longline fishing for Atlantic salmon (Salmo salar) outside the 19-km (12-mile) limit off the northern Norwegian coast present a serious mortality hazard to some seabirds. Reliable data exist only for the longline fisheries. In the 1969 season (with 75 effective days from mid-March to mid-June), one boat using 1,040 hooks per day caught 294 birds: 52 fulmars, 3 gannets, 43 kittiwakes, 107 murres, and 89 puffins. No razorbills were identified, but they may have been included in the murre figure. If this sample is representative, the 100 or so Norwegian boats using longlines plus about 20 Danish boats (which used 4,000-6,000 hooks per day and consequently caught more birds) would have caught roughly 10,000 fulmars, 600 gannets, 9,000 kittiwakes, 21,000 murres, and 18,000 puffins in the 1969 season. The drift-nets in Norwegian waters are reported to be less damaging to seabirds than are the longlines, but even without adding the figures from the drift-nets, the numbers are substantial in view of the size of the Norwegian breeding populations.
| Species | Number of breeding pairs[80] | |
|---|---|---|
| Great Britain and Ireland | Norway | |
| Fulmarus glacialis | 306,000 | 1,100 |
| Puffinus puffinus | > 175,000 | — |
| Hydrobates pelagicus | 105 or 106 | 103 or 104 |
| Oceanodroma leucorrhoa | 104 | 102 |
| Sula bassana | 138,000 | 760 |
| Phalacrocorax carbo | 8,100 | 12,000 |
| P. aristotelis | 31,000 | 33,000 |
| Stercorarius skua | 3,100 | 1[81] |
| S. parasiticus | 1,100 | 8,000 |
| Larus ridibundus | 74,000 | 4,000[82] |
| L. canus | 12,000 | (150,000)[82] |
| L. fuscus | 47,000 | 9,000[82] |
| L. argentatus | 333,000 | (260,000)[82] |
| L. marinus | 22,000 | (40,000)[82] |
| Rissa tridactyla | 470,000 | 510,000 |
| Sterna sandvicensis | 12,000 | — |
| S. dougalli | 2,300 | — |
| S. hirundo | 14,000 | (13,000)[82] |
| S. paradisaea | (31,000) | (21,000)[82] |
| S. albifrons | 1,800 | — |
| Alca torda | (144,000) | 30,000 |
| Uria aalge | (577,000) | 100,000 |
| U. lomvia | — | 1,000 |
| Cepphus grylle | 8,300 | 22,000 |
| Fratercula arctica | (490,000) | 1,250,000 |
| Total | ca. 3,000,000 | ca. 2,500,000 |
Use of fishing gear close inshore, especially pound nets set near colonies of diving seabirds, can take a heavy toll under special weather conditions. In 1969 at Runde, 85 birds, mainly auks, shags, and some diving ducks, were caught in one net in 24 hours; this is an exceptionally high figure. The total loss of diving seabirds in pound nets per year in Norway (about 6,000 nets fishing for 40 days) was estimated to be at least 40,000 birds in 1969. The data are too unreliable to give species composition, however, since fishermen rarely make note of this.
Amounts of fish offal from offshore trawlers, drift-netters, and longline fishing boats have increased in recent years, and some seabirds, particularly kittiwakes, fulmars, and gannets make use of this new and readily available food source. Thus, although the use of fishing gear is a serious threat to seabird survival, fish waste from the same boats provides an abundant food supply for the more pelagic species.
No quantitative investigation similar to those made in Great Britain, Netherlands, and Belgium (Tanis and Bruyns 1968) has been carried out on the impact of oil pollution on seabirds in Norway. The northern Norwegian population of the most threatened species, murres and razorbills, winter in North Sea coastal areas where oil pollution and oiled birds have most frequently been found. It is possible that whole populations winter every year in the same area, and if they happen to be in a heavily polluted area, a particular population may be seriously affected. Such an occurrence is believed to have caused the dramatic decline in the Sør Fugløy population (cf. Table 5).
Although not yet serious, pollution by persistent toxic chemicals such as organochlorines and mercury is a problem even in northern Norway, because the northbound coastal current brings water masses, plankton, and nekton from areas with industrial wastes. Analysis of the eggs of herring gull (Larus argentatus), murre, razorbill, and kittiwake in 1972 showed relatively low levels of mercury; the only species with a relatively high level of mercury (mean 0.58 ppm) was the gannet (Fimreite et al. 1974). This elevated toxic burden may have caused a reduced breeding success for the gannet. Analysis of concentrations of PCB's and DDT/DDE showed that the levels of these organochlorines were generally also lower in Norwegian seabirds than in those of Britain (Fimreite et al. 1977).
Total protection of some of the important seabird colonies (including the surrounding nearshore waters) has proven very effective, especially when the protection is so strict that landing is prohibited for a specified period during incubation and fledging. However, to reduce the rapid decrease of some species, a total hunting prohibition of those species must be instigated, oil pollution must be reduced, and the fisheries must be regulated to reduce the mortality caused by fishing gear.
The factors discussed so far are all results of human activities which directly or indirectly influence seabird mortality. Yearly production or breeding success is, however, also influenced by a number of natural factors such as food supply, availability of suitable nest sites, predation, climate (weather), and population-dependent factors (age, breeding experience, population density). For the gannet, whose breeding success has been studied in some detail (Brun 1974), it was concluded that the differences in exposure (to severe weather) and in breeding experience were the most important factors responsible for annual fluctuation in breeding success. For such species as murres, razorbills, and puffins, food supply is an important limiting factor. If the spawning of the fish species that constitute their main food items fails 1 year for some reason, it may be very difficult for the seabirds to find an adequate alternative food supply and most of the chicks starve to death. To a lesser degree, food supply is limiting for the kittiwake, which seems to be more influenced by bad weather (Norderhaug et al. 1977).
Two opposite population trends have been observed—the decline of the coastal-bound murres and razorbills and the increase and spread of the more pelagic gannets, fulmars, and kittiwakes. These changes are attributed to a number of factors, which include the following:
• The diving murres and razorbills spend a major part of their time swimming on the surface and are thus more susceptible to surface oil pollution than are the pelagic species.
• The coastal-bound murres and razorbills are quite heavily hunted, whereas there is no regular hunting of the pelagic species.
• The pelagic species are mainly surface feeders and do not swim under water, and are thus less affected by the drift-nets than are diving birds.
• The pelagic species are the principal beneficiaries of recently increased supply of fish offal from trawlers.
The program was originally sponsored by Tromsø Museum, later University of Tromsø, and has been financially supported by the Norwegian Research Council for Science and Humanities and the Norwegian Game Fund. The research grants are gratefully acknowledged. I also thank my field assistants and the many local people at the breeding sites who have been most helpful.
Brun, E. 1963. Ornithological features of Nord-Fugløy and Sør-Fugløy. Astarte 1(22):1-13.
Brun, E. 1965. Brunnich's Guillemot, Uria lomvia (L.), as a breeding bird in Norway. (In Norwegian, English summary.) Sterna 6:229-250.
Brun, E. 1966. The breeding population of puffins (Fratercula arctica) in Norway. (In Norwegian, English summary.) Sterna 7:1-17.
Brun, E. 1969a. The breeding distribution and population of guillemots (Uria aalge) in Norway. (In Norwegian, English summary.) Sterna 8:209-224.
Brun, E. 1969b. The breeding distribution and population of razorbills (Alca torda) in Norway. (In Norwegian, English summary.) Sterna 8:345-359.
Brun, E. 1971a. Census of Puffins (Fratercula arctica) on Nord-Fugløy, Troms. Astarte 4:41-45.
Brun, E. 1971b. Breeding distribution and population of cliff-breeding seabirds in Sør-Varanger, north Norway. Astarte 4:53-60.
Brun, E. 1971c. Population changes of some seabirds in south Norway. (In Norwegian, English summary.) Sterna 10:35-56.
Brun, E. 1972. Establishment and population increase of the gannet Sula bassana in Norway. Ornis Scand. 3:27-38.
Brun, E. 1974. Breeding success of gannets Sula bassana at Nordmjele, Andøya, north Norway. Astarte 7:77-89.
Coulson, J. C., and E. White. 1959. The post-fledging mortality of the kittiwake. Bird Study 6:97-102.
Cramp, S., W. R. P. Bourne, and D. Saunders. 1974. The seabirds of Britain and Ireland. Collins, London. 287 pp.
Fimreite, N., J. E. Bjerk, N. Kveseth, and E. Brun. 1977. DDE and PCBs in eggs of Norwegian seabirds. Astarte 10:15-20.
Fimreite, N., E. Brun, A. Frøslie, P. Frederichsen, and N. Gundersen. 1974. Mercury in eggs of Norwegian seabirds. Astarte 7:71-75.
Holgersen, H. 1951. Investigations of seabirds in Rogaland 1949-1950. (In Norwegian, English summary.) Stavanger Mus. Arb. 1950:61-76.
Lütken, E. 1965. The breeding birds on Nord-Fugløy, north Norway, their distribution and numbers. (In Danish, English summary.) Dansk Orn. For. Tidsskr. 58:166-193.
Norderhaug, M., E. Brun, and G. U. Møllen. 1977. Barentshavets sjøfuglressurser. Forhold i tilknytning til status, miljøproblemer of forshningsoppgave. Medd. Norsk Polar Inst. 104:1-119.
Regnell, S. 1957. Fran det nordnorska fåggelberget Bleiksøya. Fauna Flora, Upps. 52:199-202.
Soot-Ryen, T. 1941. The seabird rookeries of Troms county. (In Norwegian, English summary.) Tromsø Mus. Aarsh. 62(1):1-112.
Tanis, J. J. C., and M. F. M. Bruyns. 1968. The impact of oil pollution on seabirds in Europe. Proc. Int. Conf. Oil Pollution of the Sea 1968:67-74.
Valeur, P. 1947. Havhesten og havsula på Rundøy. Naturen 70:370-379.
Willgohs, J. F. 1952. On the distribution of some seabirds in western Norway. Univ. Bergen Årb. 1951 Naturvit. Rekke (9):1-20.
Willgohs, J. F. 1955. Om forekomsten av endel kyst-og sjøfugl på Vestlandet. Fauna, Oslo 8:16-27.
[74] Deceased.
[75] Numbers in parentheses are not based on a complete census of the coast.
[76] In addition, an unknown number of pairs breeding inland.
[77] Numbers in italics were censused from 1964 and 1974.
[78] Estimated values from trends in neighboring colonies.
[79] Numbers in parentheses are not based on a complete census of the whole coast.
[80] Numbers in parentheses are not based on a complete census of the whole coast.
[81] New in 1975 (Wim Vader, personal communication).
by
Ian C. T. Nisbet
Massachusetts Audubon Society
Lincoln, Massachusetts 01773
This is not going to be a straightforward summary of the conference because it is my view that a number of important topics have not been addressed. In particular, what was supposed to be the main theme of the conference—the need for conservation of marine birds of northern North America—has been taken for granted by many speakers and has been treated by others in what may be a misleadingly brief way. So instead of simply summarizing the information that has been presented in the papers, I want to give my own views about how we should use this information to make a case for the conservation of marine birds. I feel strongly that we can make a good case for conserving them, and that we know enough to start doing so. The task of making a case for conservation and of proposing priorities for action has been left to me as the conference summarizer.
Particularly in the first half of this conference, we heard a long series of accounts of the birds of the area which stressed our ignorance—large amounts of information that was not known and large amounts of research that needed to be done. Now, I have an unexpected advantage over most of these speakers in that I have very little direct experience in the area. What I learned from their papers, not having any very clear picture of the islands, the birds, their habits, or the food that they eat, is that we already know quite a lot about the marine birds of northern North America. We certainly know enough to decide what we ought to do next and how to take the basic steps in conserving them.
After listening to the presentations, reading the abstracts, and studying the maps posted in the conference hall, I drew up a list of 10 points that I will first list and then elaborate on.
• We know that we are discussing a very important biological resource which has been neglected for a long time.
• We know roughly what this resource consists of and which aspects of it are biologically important.
• We know why this resource is in its present condition, and we know something about the ways in which it is related to other resources.
• We know a certain number of things that the birds do which make them vulnerable to changes in the environment.
• We know that the resource has already been disturbed in the past, both by human-induced and by natural changes, and we know that it has already been damaged.
• We can identify at least some of the major threats that the resource will face in the next few years.
• We know that the resource can be conserved, at least to a modest and partial extent.
• We have a fairly good idea of what we ought to do now to start conserving the resource.
• We have some ideas—so far rather rough and ill-formulated—about why we should conserve the resource.
• We know—or so I believe—that it is practicable and economically feasible to conserve the resource.
I am sure that there will be some disagreements with some of these assertions, especially with the last two, so I will give reasons why I believe that we should conserve these birds and that we can afford to do so.
The papers in the first half of the conference which reviewed the abundance and distribution of the birds in the northern North Pacific Ocean, the Bering Sea, and adjacent seas suggested that we are dealing with numbers of birds of the order of 100 million. That is 100 million birds at sea plus some unknown number of millions of birds along the shore. We do not have to take these numbers literally—I am sure that the persons who produced them did not mean them to be taken literally—but certainly we are talking about something on the order of tens of millions and not much more than some hundreds of millions. At least, it is on the order of a hundred million rather than ten million or a billion. I do not think it an exaggeration to say that this is one of the great neglected biological resources of the world.
Three important aspects of this resource have not been identified clearly in the papers delivered at the conference, in part because the papers summarizing the biological surveys did not include much of the detail that was available in the maps posted in the conference hall. [Maps in this volume do not show the detail of those posted.] These are the numerical abundance of the birds, their diversity, and their unique characteristics.
As to abundance, figures have been mentioned on the order of 50 million for shearwaters (Puffinus spp.) and 25 million for murres (Uria spp.). For other species the quoted numbers have been less specific, but I would estimate from what I have read and heard that the total population must run into millions for eiders (Somateria spp.), kittiwakes (Rissea brevirostris), and fulmars (Fulmarus glacialis), and doubtless for other species. The numbers of the smaller alcids, in particular, must be very great.
As to diversity, there is an impressive number of species and a wide variety of habitats. We have been shown in the photographs some spectacular island colonies, particularly in the Bering Sea and the Aleutian Islands, some of which have a remarkable variety of species. Several different definitions of "seabird" have been used at this conference, but certainly there are dozens, and probably scores, of genuine marine species that either breed in the area or use it as a major nonbreeding area. The collection of birds in the area of the North Pacific and the Bering seas seems more impressive in terms of both abundance and diversity than anything in the north Atlantic Ocean, which has been so much more fully studied.
As to the uniqueness, there has been almost no mention of the endemic species at the conference. It is therefore important to emphasize in this summary that a significant group of marine or coastal birds is endemic to this area. These birds include the red-legged kittiwake (Rissa spp.), the Aleutian tern (Sterna aleutica), the spectacled eider (Somateria fischeri), the emperor goose (Philacte canagica), and the red-faced cormorant (Phalacrocorax urile); a number of alcids, including the whiskered (Aethia pygmaea), parakeet (Cyclorrhynchus psittacula), crested (A. cristatella), and least auklets (A. pusilla); the horned puffin (Fratercula corniculata); and Kittlitz's murrelet (Brachyramphus brevirostris). In addition, we should not forget some migrants that make exclusive use of this area in their nonbreeding season. These include the short-tailed albatross (Diomedea albatrus), the scaled petrel (Pterodroma inexpectata), and I believe also Cook's petrel (P. cookii), which has not previously been mentioned. From the little we know about its off-season distribution, the short-tailed albatross appears to use these waters exclusively; hence it has as much claim to be regarded as an endangered species of the United States as the whooping crane (Grus americana).
Perusal of the lists of species presented at the conference brings out one important point. Although we are meeting in the United States and have been looking at the birds from a United States-Canadian viewpoint, this is truly an international resource in almost every respect that I have mentioned. The most abundant species, in terms of both numbers and biomass, is probably the short[Pg 307]-tailed shearwater, a migrant from the southern hemisphere. The rarest species, and the most endangered, is the short-tailed albatross, which breeds only on one island in Japan. There are migrants in large numbers from Chile, Australia, New Zealand, and especially the Soviet Union. All of these use the area of ocean and shallow sea that we have been considering as a major area for a substantial part of their annual cycle.
What more do we need to know about the extent of this resource? In my opinion we should not place high priority on determining the exact numbers of the birds—whether there are 25 million or 26 million murres, for example. It would be difficult, if not impossible, to determine such numbers in the kind of geographical and climatic area we are considering. Moreover, even if we were to measure the populations with great accuracy and to determine in a few years that they had changed by 10%, we would not be able to draw any conclusions about the reasons for the change or what should be done about it.
To set priorities for further exploration, I think it is more important to survey in greater detail the general distribution of the breeding colonies. So far, we know the location of only the largest colonies; we know almost nothing about the colonies of a mere 10,000 pairs or less. So I think future surveys should concentrate on locating the medium-sized colonies and getting some impression of roughly how many smaller colonies there are. It is important to locate and be sure that we know of all the major colonies that have a considerable number of species; these large, diverse colonies should be given priority for conservation. Most important of all, we need to locate and survey the endemic species with some precision. This need is especially great for the species that we suspect are limited to small areas or that may otherwise be particularly vulnerable.
If we are to measure population changes over the next few decades, it is of course essential to have a good base-line survey. However, I do not think it is either practicable or desirable to try to inventory the entire population of breeding seabirds with great accuracy. A more realistic and worthwhile program would be to select some sample colonies and to catalogue these sample areas in some detail, preferably with a photographic record, so that they can be resurveyed in later years to determine whether substantial population changes have taken place. Criteria for selection of sample colonies for inclusion in this base-line survey should include not only numerical size and species diversity but also ease of access, ease of observation, and the practicability of obtaining good photographic records.
In the opening session of this conference, several speakers reviewed our general knowledge of the ecology of seabirds; others summarized our specific knowledge of the birds of the North Pacific, Bering, and adjacent seas, and their relation to physical and biological factors in the environment. There is no need to summarize these reviews again here except to point out that information on the relation between the birds and the marine environment is being generated very rapidly. We are beginning to understand the factors that control the breeding distribution of the individual species, their foraging strategies, and their dispersion at sea, at least in summer. However, it is clear from what has been said at this conference that we know much less about their ecology and distribution in winter. This lack of information is important because conflicting opinions have been expressed as to whether factors operating in the winter range or at the breeding colonies are more critical in limiting population size.
It is evident from what was said in the opening session that the distribution of the birds is very closely related to the distribution of marine resources. It is clearly no accident that the distribution of large numbers of many species of birds coincides with that of the major fisheries. Similarly, it is no accident that there is a relation between the distribution of birds and the extent of the continental shelf. These coincidences, which reflect the fundamental dependence of both birds and fish upon marine productivity, set the stage for existing and further conflicts between conservation of the birds and human exploitation of other resources of the area.
Perhaps the most significant gap in our knowledge of North Pacific seabirds is in the area of productivity and demography. As far as I can judge, we know almost nothing about the breeding success of these birds, their post-fledging survival, their longevity, their age at first breeding, the age structure of their populations, the fluctuations in their breeding performance, or their survival from year to year. For most species, we lack even the most basic life history and life table information.
If we can argue by analogy from studies made in other parts of the world, including the North Atlantic, we can make some basic generalizations that we would expect to apply to the birds of our area. We know that as a class seabirds have some peculiar characteristics which make them difficult to manage and cause some of the problems we have in conserving them. In general, they are long-lived and breed slowly, most lay small clutches, and the historical experience is that they take a very long time to recover from depletion of population. Many have an irregular breeding performance; some have long series of bad years interspersed with occasional years of good breeding success. Many seabird populations have traditionally fluctuated, as exemplified by those of the North Atlantic, whose fluctuations were described by W. H. Drury and W. R. P. Bourne.
Some species of seabirds are conservative, staying in the same colonies for many years or generations. Others are volatile, dispersing freely from one site to another and forming new colonies in an unpredictable way. Seabirds exhibit a wide range of ecological adaptations; some are highly specialized, others are highly generalized and adaptable. These differences can be very important when their environment changes, as D. N. Nettleship's film "Puffins, predators, and pirates" graphically illustrated.
As M. T. Myres pointed out on the 1st day of the conference, seabird populations exhibit both short-and long-term fluctuations. Long-term fluctuations are those that take place over times comparable to the generation time of the species, which may be many years or even decades for some seabirds. By surveying populations and measuring changes in them, we usually obtain information only about long-term population trends, reflecting long-term changes in the environment. Short-term perturbations in the environment are usually not reflected quickly by changes in total population—certainly not by changes that we can measure with the accuracy of our present-day census techniques. Many of the man-made changes we are concerned about are short-term. To identify their effects we should look not for changes in total population but rather for changes in biological parameters, such as the first-year survival rate or the number of young raised. I therefore suggest that some of the most critical parameters to be measured are changes in age structure of populations. We should therefore select as biological monitors species that can readily be aged—for example, gulls, which have a sequence of distinguishable immature plumages.
In specifying gaps in our knowledge of the ecology of birds, we should set clear priorities rather than compile a long "shopping list" of research projects. On the basis of the foregoing survey, I would suggest the following as priority items for further study. First, we need to know a lot more about winter distribution, not only of the marine birds, but also of inshore and coastal species. Second, we need to study in greater detail the relation between the day-to-day distribution of birds and the local patchiness of the resources on which they depend. Evidence that seabirds are able to locate and use fluctuating and shifting food sources has been given by several speakers at the conference. We need to understand how birds locate these resources and what relation this has to their survival and vulnerability to human activities. There is a special need to study the ecology of endemic species because their conservation is of special importance. We need to learn more about the relation of the birds to the commercial fisheries, both to resolve existing or alleged conflicts and to avert future problems.
However, I believe that the highest research priority should be given to obtaining basic information on reproductive success and life table data for some representative species. Clearly, we cannot study many species in detail, but in selecting key species for such studies we should pick a variety of ecological types—for example, at least one generalist species and one specialist, one sedentary species and one migrant, one species at a high[Pg 309] trophic level and one at a low trophic level.
For the purpose for which we convened this symposium—conservation—I do not think that we need detailed knowledge of the factors which regulate populations. Such knowledge is, of course, of immense biological interest and will ultimately be needed for effective long-term management. However, it does not have immediate or even medium-term relevance to the urgent problems of conservation that we now face. What we do need to do is to set up some long-term studies of a few carefully selected species—preferably long-lived species—so that we can trace the effect of environmental fluctuations on their performance for a long period.
We already understand a number of factors that make some of these bird populations particularly vulnerable to the kind of human activities which we can envisage in the next decade or two. Most of the breeding birds concentrate on islands where they are vulnerable to predators and to human disturbance. Many of them concentrate in flocks on human fishing grounds and over other areas of the continental shelf which are likely to be the focus of human activity in the near future. In particular, some of the birds are known to concentrate in the passes through the Aleutian Islands, where they will be particularly vulnerable to future oil spills. In all these ways the birds are concentrated in areas where they are likely to receive disproportionate impacts from human activity and exploitation.
One point that has been barely mentioned in this symposium is the effect of molting on the vulnerability of some of these populations. The eiders, for example, concentrate on molting grounds in the Arctic. The exact location of these molting grounds may not be fully known, but we certainly know that the birds molt somewhere in an area where they will be vulnerable to oil spills (and also to human hunting if the people who move to the Arctic choose to hunt them). Nor are eiders the only species that are flightless when they molt. Some alcids and loons are also flightless for short periods and, hence, particularly vulnerable to oil spills during molt.
In the speech opening the symposium, Assistant Secretary Reed referred to this biological resource as still relatively unspoiled. While "relatively" may be an appropriate word, we do have spectacular evidence of changes and damage to these bird populations. The use of the Aleutian Islands for fox farming seems to me a quite horrifying situation. We know also that the early whalers and sealers exploited seabird populations. Although I know of little specific information about the effects of such exploitation on birds in the northern North Pacific, D. G. Ainley in his survey of historical records from the Farallon Islands has shown very clearly the massive effects of human exploitation of birds, starting early in the 19th century. In our area of discussion alone, one species (the spectacled cormorant) is extinct and another (the short-tailed albatross) became virtually extinct and is still very rare. I believe that one or two southern hemisphere species, which must have been substantial elements in the northern summer bird population, have also been seriously depleted as a result of human activity on their breeding grounds.
Several speakers emphasized the importance of long-term fluctuations in bird populations resulting from natural causes, including some examples from the North Pacific. Other types of human activity must also have had some indirect effects on the birds. For example, whaling and sealing in the 19th century must have provided large amounts of food for scavenging birds and eliminated important competitors for the larger fish-eating birds. A similar experiment is now in progress as the predatory fish are being overfished.
We now know enough about the distribution and ecology of the seabirds to identify the major threats to them that are likely to be posed by the projected increase in human activity in the coming decades. The relative importance of these threats clearly varies from species to species and from area to area. How[Pg 310]ever, I think that few of us would disagree that the largest single threat in the area as a whole is posed by oil, not only by the prospect of large-scale drilling for oil on the Alaskan continental shelf but also by prospective spills during transportation and deliberate dumping from ships.
My guess is that the second most important threat to the seabirds of the northern North Pacific is the presence of introduced predators, especially foxes and rats, at the breeding colonies. Much of the damage inflicted by these predators may already have been done, but I think their continuing presence is likely to have as great a negative effect on the bird populations as anything else discussed at the conference.
The relative importance of the other identifiable threats to the birds is even more conjectural. Drowning of diving birds in fishnets is obviously of great potential impact, but its importance depends greatly on the rapidly changing practices of fishermen. This problem must be kept under close surveillance, and the establishment and enforcement of international agreements will be critical.
Mineral development has not been mentioned much. It is my understanding that there are prospects for substantial onshore, and perhaps offshore, developments of heavy metal minerals. These are likely to lead to local disturbance in the coastal zone, and the tailings in particular may well pose a threat to coastal and inshore birds.
Ocean dumping has not been mentioned. I do not expect that there will be much dumping of toxic chemicals from Alaskan industries, but we must remember that this area is downstream from Japan and the Soviet Union. I do not know the current practices of these countries, but the unregulated dumping of toxic substances from some European countries apparently has led to large-scale pollution problems in the North Atlantic.
On present evidence, persistent pesticides and polychlorinated biphenyls (PCB's) do not seem to pose a significant threat to north Pacific seabirds, although high levels of PCB's have been reported in shearwaters off the California coast. In my judgment, we have probably turned the corner in regulating these chemicals, at least in the northern hemisphere, and their impact will probably not be allowed to get worse.
Human disturbance is obviously going to get very much worse, both from the influx of new human populations who will be involved in more industrialized activities in Alaska and from the likely increase in tourism. A matter of particular concern is the prospective influx of natural history tours, which can have major adverse effects if not carefully regulated.
Finally, we should not forget the impact of natural phenomena, including climatic changes and vulcanism. Bearing in mind the experience of Katmai, we might expect a natural disaster to strike a major bird colony at any moment.
Experience from other countries, as related in various papers at this conference, has shown that conservation of seabirds is possible and practicable, even in remote and inaccessible areas. We have heard today particularly about conservation programs and achievements in Europe and New Zealand. W. H. Drury spoke briefly about experience in eastern North America and F. Salomonsen told us how the bird populations of the Faeroes Islands have been managed for sustained yield.
At least in the North Atlantic, where the history of the bird populations is much better known, the conservation situation has been, and probably still is, very much worse than that now prevailing in the North Pacific. Looking back on 200 years in the North Atlantic, we find that two major marine species have been extinguished, at least one and probably two or three others became endangered, and almost all the seabirds were drastically reduced in numbers (at least in temperate latitudes). Starting in the late 19th century when many species first received effective protection, most showed impressive recoveries, but some have declined again in the last 30 years.
We can learn several lessons from that experience. One is that we can do great damage to seabird populations in a very short time if[Pg 311] we do things that cause substantial adult mortality. A second is that seabird populations can recover well with protection and modest management—although most of them, being slow breeders, recover slowly. A third lesson is that in the last 30 years we have caused substantial damage through oil spills, human disturbance at the breeding colonies, chemical pollution, and indirectly by promoting the spread of gulls. Much has been said at the conference about these present-day human impacts. However, with the sole exception of the oil spills which have affected alcids and sea ducks in parts of northwest Europe, it seems to me that the damage caused by human activity in the past 30 years is considerably less than that in the last 30 years of the 19th century.
Another lesson we can learn from the recent experience in other areas is that it is possible to ameliorate some of these adverse human impacts with local, small-scale, and even rather amateurish management activities—for example, protecting seabird colonies from gulls, regulating human visits, and controlling the use of the most toxic chemicals. Our most conspicuous failure is in controlling oil pollution. Although safety precautions imposed on offshore drilling rigs and at shipping terminals have proved reasonably effective in averting major damage to seabirds, attempts to control oil pollution during transportation have been essentially fruitless. Tanker accidents and deliberate discharges from vessels remain the major threat to seabird populations.
Another lesson from other areas is that public education has been very effective in putting pressure behind conservation measures, and is doing so increasingly. At the same time, however, it is resulting in an increase of the disturbances that the birds suffer at their breeding grounds from casual visitors, photographers, and sometimes, well-meaning naturalists.
Finally, in very recent years, there have been encouraging developments in rehabilitating oiled birds, captive breeding, and reintroduction into areas from which they have been depleted. Restoration of seabird populations no longer seems an impossible goal.
We now know enough about the seabirds of the northern North Pacific to specify in principle what should be done immediately to conserve them. I will not address the institutional arrangements needed for conservation; R. E. LeResche's paper presented a very clear picture of the institutional problems involved in protecting and managing seabirds on an interregional and international basis. I will simply endorse his principal recommendation: that we should try to bring the various responsible agencies together to formulate comprehensive management plans.
On the level at which we as individuals and as a group of biologists can work, we can already make some positive recommendations. The most important is that since prevention of damage is a lot better than cure, measures to avert damage should have the highest priority. We have heard a great deal from the oil industry about the "inevitability" of accidents. One speaker mentioned the "inherent fallibility of man." Well, we are all fallible, but the experience of the last 50 years is that some people are more fallible than others. No oil company has a perfect record, but some have 10 times as many accidents as the best, and some, I believe, have considerably more than 10 times as many. This means, very simply, that it is possible to eliminate most—not all, but most—of the major threats to the seabirds, merely by upgrading the safety performance of the entire industry to that already achieved by its best segments. I suggest that our major challenge in the coming years is to work for effective regulation of the industry: to achieve regulations which will decisively penalize bad performance and as decisively reward care.
Perhaps the second priority in conservation is to protect and manage the existing breeding colonies. In most cases protection is legally feasible if we have the will. Most of the major colonies are in remote areas or in public ownership where development and disturbance can be controlled. Management of the breeding populations is less straightforward, however, because we do not know[Pg 312] enough about the functioning of this complex biological resource. Seabird populations fluctuate and they have a very long response time, the environment is not constant, we do not understand the dynamics of multispecies communities, and we do not know how they respond either to external changes or to our attempts to manage them. Management will have to be improvisatory for a very long time. We must recognize that effective conservation of a bird population with a 20-year generation time will take at least 20 years to show results.
Another priority task is to control predators. I have been impressed by the evidence we have for major effects of predators on the seabird populations here. I would regard control of predators and management of habitats on some of the major seabird islands as an extremely urgent task.
A longer-term but no less important program is public education. This program has several important aspects: one is to increase public support for political actions and effective regulations to protect seabirds; another is to educate the public about the vulnerability of seabirds and to prevent disturbance or deliberate human destruction.
Another aspect of public education is to develop public interest by making some of the birds more visible. The great problem with this biological resource we have been talking about is that no one knows it is there. Probably half of us did not know how substantial and important a resource it is even 5 years ago. In setting up a large-scale conservation program, we should not make the mistake of basing it only on the most remote and inaccessible colonies, even if these are the most important numerically. Many of the smaller colonies are locally very important, both biologically and for human interest and education. One example given at this conference was the State of Washington's program for conserving what are, by northern Pacific standards, quite small colonies. This program is important and impressive because it is conserving bird populations near people who want to see the birds. We have the same sort of situation in Massachusetts and Maine, where effective protection programs have been established for extremely small seabird colonies. We have learned from these programs that a few hundred birds, or even a few dozen if properly managed, can be of immense educational importance. If human access is carefully managed so that people can see the birds without disturbing them, these programs can generate support for conservation of larger bird populations that may be thousands of miles away where people may never see them.
As I have tried to show, we know something about the importance of this biological resource, and we know in outline what we should do to conserve it. But why should we? Almost no one knows the birds are there. We ourselves do not know whether there are 50 million or 250 million birds in the north Pacific Ocean. Who cares if 10 million disappear? If we cannot give a good answer to this question, we might as well go home and study chickadees instead.
To justify spending money on conserving marine birds—or any other natural resources—we must establish their value. Some of the arguments made in this conference for assigning economic values to seabirds have been dangerously weak. The annual value of "muttonbirds" (Puffinus tenuirostris) in the New Zealand markets is about $70,000. Some speakers have tried to argue that seabirds might play some subtle role that we do not yet understand in regulating marine communities—perhaps they weed out the sick fish. The direct economic values that we have specified for seabirds are really not very impressive, even in terms of the costs involved in conserving and studying them. The biggest number we have heard for the value of these seabirds is the amount of money we are spending on surveys.
However, this is not the real issue. In judging the costs and benefits of a conservation program, we should not look just at the value of the birds as meat, or oil, or indicators of pollution. The real issue here, as in all economic problems, is the rational allocation of resources. H. Boyd posed the rhetorical question: "Why should we waste public money on conserving birds when there are so many[Pg 313] other things to spend it on?" The question is more properly posed in reverse: "Why should the government waste so much public money on unproductive projects when only a small amount of money can achieve conservation of these birds which some people think are important?"
The fact is that we already know why we should allocate resources to conservation. I believe that we have just been evading the answer. We ought to conserve these birds because many people want them to be conserved.
This is not, as one speaker said, an elite interest. The public, as we well know, is already willing to spend money to conserve natural resources and is increasingly demonstrating that willingness. The public, in fact, is ahead of the administrators and bureaucrats. To appreciate this, we need only look at some of the laws already on the books. The Congress of the United States, in the National Environmental Policy Act of 1969, declared that it was the national policy to "create and maintain conditions under which man and nature can exist in productive harmony, and fulfill the social, economic, and other requirements of present and future generations of Americans." The Marine Mammal Protection Act of 1972 found that "marine mammals have proven themselves to be resources of great international significance, esthetic and recreational as well as economic, and it is the sense of Congress that they should be protected and encouraged to the greatest extent feasible commensurate with sound policies of resource management and that the primary object of their management should be to maintain the health and stability of the marine ecosystem." As these laws have been enacted, their language has become progressively stronger. The Endangered Species Act of 1973 declares as the policy of Congress "that all federal departments and agencies shall seek to conserve endangered species and threatened species and shall use their authorities in furtherance of the purposes of this Act" (P.L. 93-205). It further directs all Federal departments and agencies to carry out conservation programs for the conservation of endangered or threatened species and to insure that their actions do not jeopardize the continued existence of these species or destroy or modify critical habitat.
These references are not only to Federal laws passed by remote politicians who can vote with only a modest responsibility to their constituents. As we have heard, there are many State laws and local ordinances which specify the same kind of thing. All these laws are on the books for a powerful reason: public opinion was behind them. The fact that they have not been enforced and implemented fully means that we have not been doing our job.
In fact, there is no philosophical problem in justifying conservation. What we face is an institutional problem. There is both a public determination that natural resources should be conserved and a public apathy and bureaucratic resistance toward doing it. As concerned biologists, we should be combating this apathy by pointing out that conservation represents a rational allocation of public resources.
Those who do not learn the lessons of history are destined to repeat it. If we study the history of conservation, we find that it developed most rapidly in those countries which mismanaged their natural resources earliest. Within the developed countries there has been a progressive historical trend toward rational use and conservation of natural resources. Conservation of natural resources, in fact, represents the future and, as biologists, it is our duty to promote it.
Conservation is cheap. Most of us are accustomed to working on what are essentially shoestring budgets—on the order of $100,000, $10,000, or even $1,000 per year. When we hear of a million dollars as the cost of doing something, we tend to think of it as a lot of money. H. Boyd mentioned a situation on Baffin Island, where it would cost about a million dollars to dispose of mine tailings on shore instead of dumping them into the ocean under a fulmar colony. I do not think a million dollars is very much—certainly not in comparison with the cost of restoring a colony of half a million fulmars.
We heard this morning about the acquisition of Protection Island at a cost of several hundred thousand dollars. It was pointed out[Pg 314] that it could have been acquired much more cheaply only a few years ago. Acquisition of habitat is cheap if we do it now compared with what it will cost in a few years or a few decades. Management is cheap. None of us gets paid very much, but each of us could manage several colonies with a couple of students to help us. Wardens are cheap. Surveys are cheap. The cost of conserving seabirds is minuscule in comparison with the amount spent on the exploitation of resources that threatens them, and it is minuscule in relation to the cost of restoring a seabird population after it has been depleted.
It is far cheaper to avoid oiling birds than it is to rehabilitate them and to reestablish them in breeding colonies in the wild. It costs nothing at all to award leases to companies that have a good safety record and to refuse leases to companies with bad records. It costs a little more to maintain good safety practices in drilling and transportation. It does cost more to transport oil in small, double-bottomed tankers with well-trained crews than to transport it in big ships flying flags of convenience, but the cost differential is very small compared to the value of the shipment.
In considering the economics of conservation, we have to weigh the costs of conservation against the value of the resource being exploited. Full-scale development of oil reserves on the Alaskan continental shelf would generate economic values on the order of ten billion dollars per year. Of this total, 0.1% would support a reasonably sized management program for the threatened resources. About 1% of the total, or 12¢ per barrel of oil, would not only support an ample management program but also permit management of many other coastal zone resources. Yet the experience of the last few years has shown that an increase in oil prices of 1% is barely noticed by consumers.
The point I am trying to make is that extracting oil carefully does not cost significantly more than extracting it carelessly. If we can solve the institutional problems—and I do not underestimate the difficulty of doing so—we are not talking about an irrational use of resources. Conservation is feasible; it is worthwhile; it is not expensive; and there is a public demand for it.
Practical conservation is an adaptive process. It is not at present a process that is firmly based in ecological theory. It is one in which we have to start by doing something, see whether it works, and then change our program in accordance with our early experience. I do not believe that we can wait for detailed knowledge of population sizes, or ecology, or demography, or trophic importance, or any other biological attribute of these birds before we start conservation and management. As scientists we do, of course, find it interesting and important to study these things. We should do so; we need to do so; but we should not use our ignorance of detail as an excuse for delaying action. If this seabird resource is worth conserving, we should start now.
The marine birds of the northern North Pacific Ocean, Bering Sea, and adjacent seas constitute one of the great neglected biological resources of the world.
This resource is impressive in terms of both total numbers (probably of the order of 100 million birds) and species diversity. A number of species are endemic to the area and hence of special interest.
The resource is international in that it includes major populations of migrants from Chile, New Zealand, Australia, Japan, the Soviet Union, and other countries. Several migrant species appear to use this area exclusively in their nonbreeding season and should be included in the list of endemics.
The general relation between the distribution and abundance of seabirds and other marine resources is beginning to be understood. However, comparatively little is known about the distribution of seabirds in winter, and there is a serious dearth of information about breeding success, survival, and demography.
Seabirds in the north Pacific Ocean and adjacent seas are concentrated over the continental shelf and in areas of high biological productivity. Hence they are especially vul[Pg 315]nerable to human exploitation.
Seabirds of the northern Pacific Ocean have already been damaged by human activities in the past and present. Experience in other areas shows that seabirds are extremely vulnerable to human activities and their populations are often very slow to recover.
The most important threats to the seabird resource are oil drilling and transportation and introduced predators, especially foxes. Other identifiable threats include mineral exploitation, fishing, ocean dumping of toxic chemicals, and human disturbance, including both hunting and tourism.
Experience in other parts of the world, especially in the North Atlantic, has shown that seabird populations can be protected and restored through modest programs of management and public education. The principal exception has been the failure to regulate discharges of oil at sea, which continue to cause major damage to seabird populations in many areas.
In the North Pacific and Bering Sea areas, the most urgent conservation needs are effective regulation of prospective oil exploitation, control of introduced predators, and public education. Regional management plans should be developed. Public access to bird colonies should be managed carefully to combine protection with public education.
Conservation programs for seabirds can be justified as a response to increasing public demand for rational management of natural resources. Conservation programs are inexpensive in relation to the economic values generated by oil and mineral development. They represent a rational allocation of economic resources.
The following priorities for further study are suggested:
• Study of productivity and demography in a few carefully selected species to provide basic life table data that will permit rapid identification of future changes.
• A base-line census of some carefully selected breeding colonies, including precise photographic surveys that can be used to measure future population changes.
• Surveys of the distribution of seabirds of the North Pacific and Bering Sea in winter, with special emphasis on areas close to shore where birds may be vulnerable to oil pollution.
• Special studies of endemic species.
• Studies of the way in which seabirds locate and use patchily distributed food resources.
The following conservation measures are suggested:
• Adoption of regulations governing exploitation and transportation of oil which would provide strong incentives for safe performance and severe penalties for safety violations.
• A conservation tax of a few cents per barrel of oil to cover the costs of managing the major seabird colonies and to establish a trust fund for restoring depleted populations.
• Equivalent measures for mining and other exploitative industries in the coastal zone with a prospective impact on marine resources.
• Prohibition of dumping of toxic chemicals in biologically productive waters.
• A program to eliminate introduced predators from the Aleutian Islands and from important bird colonies elsewhere.
• Promulgation of effective regulations to protect birds under the Migratory Bird Treaty with Japan.
• Negotiation of migratory bird treaties with other affected countries, including the Soviet Union, Australia, New Zealand, and Chile.
• Acquisition of major unprotected seabird colonies into the national wildlife refuge or other federal landholding systems.
• Formulation of regional and international management plans for localized species, especially endemic species of the Bering Sea.
• Regulation of public access to major seabird colonies.
Conservation of marine birds of northern North America—the symposium theme, by Nathaniel P. Reed
The marine environment of birds: an overview, by N. Philip Ashmole
Environmental hazards to birds from petroleum development in the Beaufort Sea, by Kees Vermeer [in lieu of a paper on the status of birds in the Beaufort Sea]
Birds of the marine habitat off northwestern North America, by Gerald A. Sanger and James G. King
The pelagic and nearshore birds of the Beaufort and Chukchi Seas, by George J. Divoky and George E. Watson
Dispersal and migratory movements, by George E. Watson, George M. Jonkel, and F. Graham Cooch
Summary of the session "Status of Marine Bird Populations," by David N. Nettleship
Breeding habitat of marine birds, by Gerald A. Bertrand
Exposure of marine birds to environmental pollutants, by Harry M. Ohlendorf, Robert W. Risebrough, and Kees Vermeer[83]
Summary of the session "The Biology and Ecology of Marine Birds in the North," by Hugh Boyd
Summary of the session "Conflicts Between the Conservation of Marine Birds and Uses of Other Resources," by Joseph P. Linduska
Programs and authorities of the United States Government related to marine bird conservation, by Omar Halvorson
Programs and authorities related to marine bird conservation: Canadian Government, by Hugh Boyd
Programs and authorities of the State of Alaska related to marine bird conservation, by Robert E. LeResche
Seabird programs of private organizations, by C. Eugene Knoder
Summary of the session "Programs and Authorities Related to Marine Bird Conservation," by William H. Drury
Conservation of marine birds in Antarctica, by William J. L. Sladen
Seabirds and their conservation in Western Europe, by W. R. P. Bourne
Summary of the session, "Conservation of Marine Birds in Other Lands," by Joseph J. Hickey
[83] This paper, because of its length, was published separately as Wildlife Research Report 9.
U.S. GOVERNMENT PRINTING OFFICE: 1979-681-016/29
P. 228 the sentence "The factors in Table are largely self-explanatory" was missing the table reference.
Silently corrected simple spelling, grammar, and typographical errors.
Retained anachronistic and non-standard spellings as printed.