
BRITISH MUSEUM (NATURAL HISTORY)
CROMWELL ROAD, LONDON, S.W.
MINERAL DEPARTMENT.
WITH A LIST OF THE METEORITES REPRESENTED IN THE COLLECTION.
BY
L. FLETCHER, M.A., F.R.S.,
KEEPER OF MINERALS IN THE BRITISH MUSEUM; FORMERLY FELLOW OF UNIVERSITY COLLEGE AND MILLARD LECTURER AT TRINITY COLLEGE, OXFORD.
TENTH EDITION.
[This Guide-book can be obtained only at the Museum; written applications should be addressed to "The Director, Natural History Museum, Cromwell Road, London, S. W."]
PRINTED BY ORDER OF THE TRUSTEES.
1908.
[All rights reserved.]
LONDON:
PRINTED BY WILLIAM CLOWES AND SONS, LIMITED,
DUKE STREET, STAMFORD STREET, S.E., AND GREAT WINDMILL STREET, W.
In the accompanying list, the topographical arrangement has been continued for those meteorites of which the circumstances of the fall are without satisfactory record. This mode of arrangement brings near together fragments which have been found in the same district at different times; in some cases they belong to the same meteoritic fall. As the dates of discovery of the masses and the dates of recognition of meteoric origin, upon which other lists of meteorites are based, have been stated very differently in the publications of the principal museums, a reference in each instance to the best available report, and a brief extract from it, are given.
Even as regards the dates of fall of the remaining meteorites there has been much discrepancy in the various lists: every case in which the date here given has been found to differ from that recorded in any other list has been verified by reference to the published reports of the fall.
For the convenience of collectors there has been added (page 107) an alphabetical list of those meteorites of which specimens have been first acquired since the issue of the last list (January 1, 1904).
L. Fletcher.
May 1, 1908.

| PAGE | ||
|---|---|---|
| Arrangement of the Collection | 7 | |
| History of the Collection | 8 | |
| An Introduction to the Study of Meteorites | 17 | |
| List of the Meteorites represented in the Collection on May 1, 1908:— | ||
| I. | Siderites or Meteoric Irons | 66 |
| II. | Siderolites | 91 |
| III. | Aerolites or Meteoric Stones | 95 |
| List of Recent Additions | 107 | |
| List of British Meteorites | 107 | |
| Appendix to the List of the Meteorites:— | ||
| A. | Native Iron (of terrestrial origin) | 108 |
| B. | Pseudo-meteorites | 109 |
| List of the Casts of Meteorites | 110 | |
| Index to the Collection | 111 | |
Plan of the Mineral Gallery
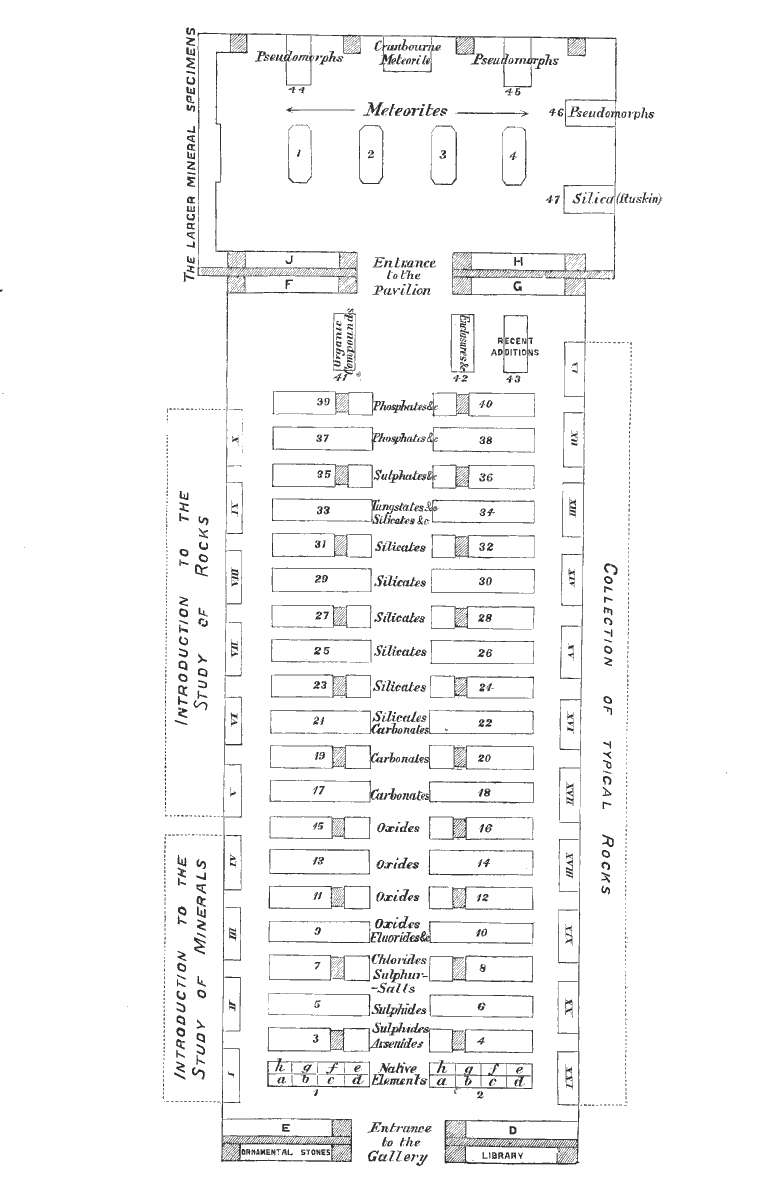
[pg 7]By ascending the large staircase opposite to the Grand Entrance and turning to the right, the visitor will reach a corridor leading to the Department of Minerals.
From the entrance of the Gallery the large mass of meteoric iron, weighing three and a half tons, found about 1854 at Cranbourne, near Melbourne, Australia, and presented to the Museum in 1862 by Mr. James Bruce, can be seen in the Pavilion at the opposite end of the Gallery.
The other meteorites will be found in the same room, the smaller specimens in the four central cases, and the larger on separate stands. The casts of meteorites are exhibited in the lower parts of the cases.
The specimens referred to in the 'Introduction to the Study of Meteorites' are in case 4, and are arranged, as far as is practicable, in the order of reference.
The remaining specimens are classified as:—
Siderites, consisting chiefly of metallic nickel-iron (panes 1a-2d):
Siderolites, consisting chiefly of metallic nickel-iron and stony matter, both in large proportions (panes 2e, 2f): and
Aerolites, consisting chiefly of stony matter (panes 2g-3o).
At the beginning of each class are placed those meteorites of which the fall has been observed.
The position of any meteorite in the cases may be found by reference to the Index (p. 111) and to the second column of the List of the Collection (p. 66).
[pg 8]Until nearly fifty years after the establishment of the British Museum, meteorite collections nowhere existed, for the reports of the fall of stones from the sky were then treated as absurd, and the exhibition of such stones in a public museum would have been a matter for ridicule; a few stones, which had escaped destruction, were scattered about Europe, and were in the possession of private individuals curious enough to preserve bodies concerning the fall of which upon our globe such reports had been given. Hence it happened that in 1807 not more than four meteoric stones were in the British Museum: three of them, Krakhut, Wold Cottage and Siena, had been presented in 1802-3 by Sir Joseph Banks; the fourth was a stone of the L'Aigle fall, presented in 1804 by Prof. Biot, the distinguished physicist. A fragment of the mass met with by the traveller Pallas had been presented by the Academy of Sciences of St. Petersburg as early as 1776; this, and the fragments of Otumpa and Senegal River, were long regarded by scientific men as specimens of "native iron," and of terrestrial origin.
In the year 1807, happily for the future development of the Mineral Collection, Mr. Charles Konig (formerly König) was appointed Assistant-keeper, and six years later was promoted to the Keepership of the then undivided Natural History Department; it thus came about that for thirty-eight years the senior officer of the Natural History Department of the Museum was one who had an intense enthusiasm for minerals and made them his own special study. It was in Mr. Konig's time that Parliament voted (1810) a special grant of nearly £14,000 for the purchase of the minerals which had belonged to the Rt. Hon. Charles Greville; with these passed into the possession of the Trustees fragments of seven meteorites, including Tabor, which had been acquired [pg 9] by Mr. Greville with the mineral cabinet of Baron Born. The increase of the Natural History Collections was such that in 1827 the Botanical, and in 1837 the Zoological, specimens were assigned to special Departments, after which Mr. Konig, as Keeper of "Minerals (including Fossils)," was left free to devote his attention to those parts of Natural History to which he was more particularly attached.
During Mr. Konig's Keepership, though numerous and excellent mineral specimens were acquired, no great effort was made to render the meteorite collection itself complete; at his death in 1851, 70 falls were represented by specimens. The following had been presented:—
Stannern: by the Imperial Museum of Vienna, in 1814.
Red River: by Prof. A. Bruce, in 1814.
Mooresfort: by Mr. J. G. Children, F.R.S., in 1817, and by Dr. Blake, in 1819.
Adare: by Dr. Blake, in 1819.
The large Otumpa iron, and a piece of the Imilac siderolite: by Sir Woodbine Parish, K.C.B., F.R.S., in 1826 and 1828 respectively.
Bitburg: by Mr. Henry Heuland, in 1831.
Krakhut: by Mr. Wm. Marsden, in 1834.
Cold Bokkeveld meteorite: by Sir John Herschel, Bart., F.R.S., Sir Thos. Maclear, F.R.S., and Mr. E. Charlesworth, in 1839 and 1845.
Zacatecas: by Mr. T. Parkinson, in 1840.
Akbarpur: by Captain P. T. Cautley, in 1843.
Braunau and Seeläsgen: by the Royal Society, in 1848.
After the death of Mr. Konig, Mr. G. R. Waterhouse, palæontologist, was appointed Keeper of the composite Department. It was natural that the palæontological side should then have its turn of special development, and in fact the palæontological collections, already important, increased from that time with great rapidity; the mineralogical side, however, had additions made to it, though not in the proportion allotted during the preceding years. During the Keepership of Mr. Waterhouse (1851-7), only specimens of two additional meteorites were added to the collection; one of them, Madoc, was presented in 1856 by Sir Wm. E. Logan, [pg 10] F.R.S.; also additional fragments of Imilac were presented by Mr. W. Bollaert in 1857.
In the year 1857, a further division of the Natural History Collections took place; the mineralogical and the palæontological specimens being assigned to special Departments, and the Minerals placed in the Keepership of Prof. Story-Maskelyne. Under him the Mineral Collection was rendered as complete as possible in all its branches; and it is owing entirely to the unflagging energy he displayed, both in the search for, and in the acquisition of the best obtainable specimens, that the Mineral Collection was brought to its present position of general excellence. Perhaps the greatest relative advance was made in the improvement of the Collection of Meteorites. Perceiving that only half of the falls represented at Vienna were represented in the British Museum, and that the difficulty of making a fairly complete collection of such bodies must increase enormously as time goes on, owing to the absorption of the specimens by public museums, Mr. Maskelyne immediately after his appointment tried to fill up the gaps. In the first place, the meteorite collections of Dr. A. Krantz, Mr. R. P. Greg, and Mr. R. Campbell, and many meteorites belonging to Mr. W. Nevill and Prof. C. U. Shepard, were acquired by purchase in 1861-2. During the interval (1857-63), the whole or parts of many meteorites were presented to the Museum:—
From Great Britain.—Perth: by Mr. W. Nevill.
From Russia.—Tula: by Dr. J. Auerbach of Moscow.
From India.—Bustee, Dhurmsala, Durala and Shalka: by the Secretary of State for India.
Assam, Butsura, Futtehpur, Khiragurh, Manegaum, Mhow, Moradabad, Segowlie and Umballa: by the Asiatic Society of Bengal.
Nellore and Parnallee: by Sir W. T. Denison, K.C.B.
Kusiali and Pegu: by Dr. Thos. Oldham, F.R.S.
Kaee: by Sir Thos. Maclear, F.R.S.
Dhurmsala: by Mr. G. Lennox Conyngham.
[pg 11]From Australia.—The large Cranbourne iron: by Mr. James Bruce.
From South America.—Vaca Muerta: by Mr. W. Taylour Thomson.
Imilac: by Mr. W. Bollaert.
An Atacama iron: by Mr. Lewis Joel.
From North America.—Tucson: by the Town Authorities of San Francisco.
During the same interval, exchanges were made with the museums of Paris, Vienna, Berlin, Copenhagen, Heidelberg, and Göttingen, through Professors Daubrée, Haidinger, Rose, Hoff, Bunsen, and Wöhler, respectively: and also with the following private collectors:—Dr. Abich of Dorpat, Dr. J. Auerbach of Moscow, Mr. R. P. Greg of Manchester, Prof. C. U. Shepard of New Haven, U.S.A., and Dr. Sismonda of Turin.
The result was that by the end of 1863 the number of meteoric falls represented in the collection was 204, and thus had been almost trebled during Mr. Maskelyne's first six years of office.
Meanwhile, although Mr. Maskelyne, with the help of a single assistant (Mr. Thomas Davies), was then rearranging the general collection of minerals according to a new system of classification, time was found for a scientific examination of the meteorites thus being acquired. At that time the Department was without a chemical laboratory, and not even a blowpipe could be used, owing to the necessity of guarding against a possible destruction of the Museum by fire. Hence recourse was had to the microscope, and as early as 1861, a microscope fitted with a revolving graduated stage and an eye-piece goniometer was constructed, under the Keeper's directions, for the examination of thin sections of meteorites with the aid of polarised light.
Working in this way, and with the simplest chemical tests, Mr. Maskelyne was the first to announce in 1862 the discovery in the Bustee meteorite of a mineral, unknown in terrestrial mineralogy, to which he gave the name of Oldhamite, and in 1863, the more than probable occurrence [pg 12] of Enstatite as an important meteoritic ingredient (Nellore). This method of determining the mineral constituents of a rock-section by means of the relation of the vibration-traces to known crystallographic lines, thus first and of necessity employed for the discrimination of the minerals in meteorites, is now in general use in the investigation, not only of meteoritic, but of terrestrial rocks. About the same time, from the Breitenbach meteorite were extracted crystals of Bronzite, which yielded the first crystallographic elements obtained for that mineral: the measurements were made and published by Dr. Viktor von Lang, then assistant in the Department (1862-4) and now Professor of Physics at Vienna.
The microscope was further applied to the mechanical separation of the different mineral ingredients of a meteorite: and by picking out in this toilsome manner the different mineral ingredients from the crumbled material of the Bustee aerolite, and from the residue of the Breitenbach siderolite left after the iron had been removed by mercuric chloride, the several silicates contained in these meteorites were isolated for future analysis. From the particles of colourless mineral thus obtained from the Breitenbach meteorite, one kind was selected in 1867, of which the crystals presented a zone of orthosymmetry containing two optic axes, and yielded two similar cleavages in a zone perpendicular to the former. This ingredient was afterwards (1869) announced to consist wholly of silica, a substance which, before the isolation of this mineral, was only known to occur as quartz, when in crystals, and these belong to the hexagonal system: to the new mineral Mr. Maskelyne later assigned the name of Asmanite. In 1868 was published by Vom Rath the discovery of a species of terrestrial silica, the crystals of which were regarded as belonging to the hexagonal system, though their angular elements were distinct from those of quartz: this mineral, named by him Tridymite, has since been found (1878) to present optical and other characters inconsistent with true hexagonal symmetry, and is probably identical in its specific characters with the meteoritic asmanite.
[pg 13]Further, another mineral occurring as minute gold-yellow octahedra in the Bustee meteorite was recognised as new to mineralogy, and termed Osbornite.
It was not till 1867, when a laboratory was fitted up outside the Museum precincts, that it became possible to make a complete chemical examination of these materials, which had been gradually prepared and carefully picked for analysis. In that year the late Dr. Walter Flight was appointed to assist in the laboratory-work of the Department, and afterwards gave valuable help in the chemical analysis of the above materials; the results were quite confirmatory of those already obtained by aid of the microscope and the simple tests.
Since the great increase made during the first six years of Prof. Maskelyne's Keepership, the Collection has continued to grow, though necessarily at a less rapid rate.
Of the specimens added after 1863, the following have been presented:—
1864-7: Manbhoom, Muddoor and Pokhra: by Dr. Thos. Oldham, F.R.S.
1864: Agra: by Mr. Wm. Nevill.
1864: Atacama (stone): by Mr. Alfred Lutschaunig.
1865-70: Jamkheir, Lodran, Shytal, Supuhee and Udipi: by the Secretary of State for India.
1865: Nerft: by Prof. Grewingk.
1865: Ski: by Prof. Kjerulf.
1867-70: Goalpara, Gopalpur, Khetri, Moti-ka-nagla, Pulsora and Sherghotty: by the Trustees of the Indian Museum. Calcutta.
1867-75: Knyahinya and Zsadány: by the Hungarian Academy of Sciences.
1869: Krähenberg: by Dr. Neumayer.
1871: Searsmont: by Dr. A. C. Hamlin.
1873: Fragments of thirteen meteorites already represented: by Mr. Benj. Bright.
1874: Bethany (Wild): by the Trustees of the South African Museum, Capetown.
1875: Amana: by Dr. G. Hinrichs.
1876: Shingle Springs: by Mr. E. N. Winslow.
[pg 14]1876: Rowton: by the Duke of Cleveland.
1877: Khairpur and Jhung: by Mr. A. Brandreth.
1877: Verkhne-Dnieprovsk: by Prof. Koulibini.
1878: Cronstad: by Mr. John Sanderson.
1878: Santa Catharina: by Prof. Daubrée.
1879: Imilac, Mount Hicks and Serrania de Varas: by Mr. George Hicks.
1881: Middlesbrough: by the Directors of the North Eastern Railway.
1882: Veramin: by the Shah of Persia.
1882: Vaca Muerta: by Mr. F. A. Eck.
1883: Ogi: by Naotaro Nabeshima, formerly Daimiô of Ogi, Japan.
1885: Ivanpah: by Mr. H. G. Hanks.
1885: Youndegin: by the Rev. Charles G. Nicolay.
1885 et seq.: Ambapur Nagla, Bishunpur, Bori, Chandpur, Dokáchi, Donga Kohrod, Esnandes, Gambat, Heidelberg, Kahangarai, Kodaikanal, Lalitpur, Nagaria, Nammianthal, Nawalpali, Pirthalla, Sindhri, Wessely and Wöhler's iron: by the Director of the Geological Survey of India.
1885: Lucky-Hill: by the Governors of the Jamaica Institute.
1886: Nenntmannsdorf: by Dr. H. B. Geinitz.
1886: Jenny's Creek: by Mr. John N. Tilden.
1887: Djati-Pengilon: by the Government of the Netherlands.
1887, 1906: Albuquerque: by Dr. Richard Pearce.
1889: Bhagur and Kalambi: by the Bombay Branch of the Royal Asiatic Society.
1890: Bendegó River: by the Director of the National Museum, Rio de Janeiro.
1891: Dundrum: by the Board of Trinity College, Dublin.
1891: Farmington: by Dr. G. F. Kunz.
1891-1903: Barratta and Thunda: by Prof. A. Liversidge, F.R.S.
1894: Makariwa: by Prof. G. H. F. Ulrich.
1894: Bherai: by the Nawab of Junagadh, India.
[pg 15]1895: Concepcion: by Mr. W. Taylor.
1896: Madrid: by Don Miguel Merino of Madrid.
1897: Cold Bokkeveld: by Mrs. Whitwell.
1899, 1906: Caperr: by the Director of the La Plata Museum.
1899: El Ranchito (Bacubirito): by Mr. O. H. Howarth.
1899: Kokstad: by the Trustees of the South African Museum.
1899: Zomba: by Sir A. Sharpe, C.B., K.C.M.G., Mr. J. F. Cunningham, and Mr. J. McClounie.
1901: Ness City: by Dr. H. A. Ward.
1903: Caratash: by His Highness Kiamil Pasha.
1904: Narraburra: by Mr. H. C. Russell, C.M.G., F.R.S.
1905: Fukutomi, Oshima, Tanakami and Yonõzu: by Dr. C. Ishikowa.
1905: Kota-Kota: by Mr. A. J. Swann.
1907: Kangra: by Prof. W. N. Hartley, F.R.S.
1908: Uwet: by the Governor of Southern Nigeria.
Since the same year (1863) meteoritic exchanges have been made with the museums of Belgrade, Berlin, Blömfontein, Breslau, Calcutta, Calne, Cambridge, Chicago (Field Columbian Museum), Christiania, Debreczin, Dresden, Fremantle, Göttingen, Helsingfors, Munich, Odessa, Paris, Pau, Rio de Janeiro, Rome, St. Petersburg (Institute of Mines), South Africa, Stockholm, Sydney, Transylvania, Troyes, Utrecht, Vienna, Washington, Wisconsin University, and Yale College; and also with the following:—Dr. Abich of Dorpat, Dr. J. Auerbach of Moscow, Mr. S. C. H. Bailey of Cortlandt-on-Hudson, U.S.A., Prof. Baumhauer of Haarlem, Mr. C. S. Bement of Philadelphia, U.S.A., Dr. Breithaupt of Freiberg, Dr. A. Brezina of Vienna, Mr. J. B. Gregory of London, Prof. C. T. Jackson of Boston, U.S.A., Mr. Henry Ludlam of London, Prof. W. Mallet of Virginia, U.S.A., Prof. Vom Rath of Bonn, Prof. C. U. Shepard of New Haven, U.S.A., His Excellency Julien de Siemachko of St. Petersburg, Prof. Lawrence Smith of Louisville, U.S.A., Mr. J. N. Tilden of New York, U.S.A., and Dr. Henry A. Ward of Chicago, U.S.A.
[pg 16]In this way, by the generosity and self-denial of donors, by the somewhat difficult method of exchange, and by purchase, it has been possible to get together the fine representative collection of meteorites now in the British Museum.

AN INTRODUCTION
TO THE
STUDY OF METEORITES.
Most of the specimens here referred to are in Case 4 in the Pavilion at the end of the Mineral Gallery.
1. Till the beginning of the nineteenth century, the fall of stones from the sky was an event, the actuality of which neither men of science nor people in general could be brought to credit. Yet such falls have been recorded from the earliest times, and the records have occasionally been received as authentic by a whole nation. In most cases, however, the witnesses of such an event have been treated with the disrespect usually shown to reporters of the extraordinary, and have been laughed at for their supposed delusions: this is less to be wondered at when we remember that the witnesses of the arrival of a stone from the sky have usually been few in number, unaccustomed to exact observation, frightened both by what they saw and by what they heard, and have had a common tendency towards exaggeration and superstition.
2. De Guignes in his Travels states that, according to old Chinese manuscripts, falls of stones have again and again been observed in China; the earliest mentioned is one which happened about 644 B.C.
A stone, famous through long ages,1 fell in Phrygia and [pg 18] was preserved there for many generations. About 204 B.C. it was demanded from King Attalus and taken with great ceremony to Rome. It is described as "a black stone, in the figure of a cone, circular below and ending in an apex above."
In his History of Rome, Livy tells of a shower of stones on the Alban Mount, about 652 B.C., which so impressed the Senate that a nine days' solemn festival was decreed; as the shower lasted for two days, it was doubtless the result of volcanic action; other instances of the "rain of stones" in Italy, mentioned by the same author, had possibly a similar origin.
Plutarch relates the fall of a stone in Thrace about 470 B.C., during the time of Pindar, and according to Pliny, the stone was still preserved in his day, 500 years afterwards. The latter records two other falls, one in Asia Minor, the other in Macedonia.
3. These falls from the sky, when credited at all, have been deemed prodigies or miracles, and the stones have been regarded as objects for reverence and worship. It has even been conjectured that the worship of such stones was the earliest form of idolatry. The Phrygian stone, mentioned above, was worshipped at Pessinus by the Phrygians and Phœnicians as Cybele, "the mother of the gods," and its transference to Rome followed the announcement by an oracle that possession of the stone would secure to the State a continual increase of prosperity. Similarly, the Diana of the Ephesians, "which fell down from Jupiter," and the image of Venus at Cyprus, appear to have been, not statues, but conical or pyramidal stones. A stone, of which the history goes back far beyond the seventh century, is still revered by the Moslems as one of their holiest relics, and is preserved at Mecca built into the northeastern corner of the Kaaba. The late Paul Partsch,2 for many years Keeper of Minerals in the Imperial Museum of Vienna, considered that the meteoric origin of the Kaaba stone was sufficiently proved by descriptions which had been submitted to him. A stone which fell in Japan in Pane 4c. [pg 19] the year 1741, and was presented to the British Museum in 1883, had long been made an annual offering in a temple of Ogi at one of the Japanese religious festivals. It may be added that a stone which lately fell in India3 was decked with flowers, daily anointed with ghee (clarified butter), and subjected to frequent ceremonial worship and coatings of sandal-wood powder. The stone was placed on a terrace constructed for it at the place where it struck the ground, and a subscription was made for the erection of a shrine.
4. The oldest undoubted sky-stone still preserved is that which was long suspended by a chain from the vault of the choir of the parish church of Ensisheim in Elsass, and is now kept in the Rathhaus of that town. The following is a translated extract from a document which was preserved in the church:—
"On the 16th of November, 1492, a singular miracle happened: for between 11 and 12 in the forenoon, with a loud crash of thunder and a prolonged noise heard afar off, there fell in the town of Ensisheim a stone weighing 260 pounds. It was seen by a child to strike the ground in a field near the canton called Gisgaud, where it made a hole more than five feet deep. It was taken to the church as being a miraculous object. The noise was heard so distinctly at Lucerne, Villing, and many other places, that in each of them it was thought that some houses had fallen. King Maximilian, who was then at Ensisheim, had the stone carried to the castle: after breaking off two pieces, one for the Duke Sigismund of Austria and the other for himself, he forbade further damage, and ordered the stone to be suspended in the parish church."
5. Three French Academicians, one of whom was the afterwards renowned chemist Lavoisier, presented to the Academy in 1772 a report on the analysis of a stone said to have been seen to fall at Lucé on September 13, 1768. As Pane 4c. [pg 20] the identity of lightning with the electric spark had been recently established by Franklin, they were in advance convinced that "thunder-stones" existed only in the imagination; and never dreaming of the existence of a "sky-stone" which had no relation to a "thunder-stone," they somewhat easily assured both themselves and the Academy that there was nothing unusual in the mineralogical characters of the Lucé specimen, their verdict being that the stone was an ordinary one which had been struck and altered by lightning.
6. In 1794 the German philosopher Chladni, famed for his researches into the laws of sound, brought together numerous accounts of the fall of bodies from the sky, and called the attention of the scientific world to the fact that several masses of iron, of which he specially considers two, had in all probability come from outer space to this planet.4
One of them is the mass still known as the Pallas or Krasnojarsk iron.5 This irregular mass, weighing about 1500 lbs., of which the greater part is in the Museum at St. Petersburg, was met with at Krasnojarsk by the traveller Pallas in the year 1772, and had been found in 1749 by a Cossack on the surface of the highest part of a lofty mountain between Krasnojarsk and Abakansk in Siberia, in the midst of a schistose district: it was regarded by the Tartars as a "holy thing fallen from heaven." The interior is composed of a ductile iron, which, though brittle at a high temperature, can be forged either cold or at a moderate heat; its large sponge-like pores are filled with an amber-coloured olivine; the texture is uniform, and the olivine equally distributed; a vitreous varnish had preserved it from rust. The fragment in the case, weighing about 7 lbs., was presented to the Trustees in 1776 by the Academy of Sciences of St. Petersburg.
A second specimen referred to is that which in 1783 Don Michael Rubin de Celis was sent by the Viceroy of Rio de la [pg 21] Plata to investigate;6 it had been found by Indians, searching for honey and wax, and trusting to rain for drink, projecting about a foot above the ground near a place called Otumpa, in the Gran Chaco Gualamba, South America, and was at first thought to be the outcrop of an iron vein. Don Rubin de Celis estimated the weight of this mass of malleable iron at thirty thousand pounds, and reported that for a hundred leagues around there were neither iron mines nor mountains nor even the smallest stones, and that owing to the absence of water, there was not a single fixed habitation in the country. There were several smaller masses at the locality; one of them, weighing 1400 lbs., is shown on a separate stand in the Pavilion: according to Sir Woodbine Parish, who presented it to the Museum in 1826, it had been removed to Buenos Ayres at the beginning of the struggle for Independence; it was a complimentary gift to Sir Woodbine on the occasion of his being sent by Canning to acknowledge the Independence of the State. Pane 4c.A slice of this iron is shown in case 4c.
7. Chladni argued that these masses could not have been formed in the wet way, for they had evidently been exposed to fire and slowly cooled: that the absence of scoriæ in the neighbourhood, the extremely hard and pitted crust, the ductility of the iron, and, in the case of the Siberian mass, the regular distribution of the pores and olivine, precluded the idea that they could have been formed where found, whether by man, electricity, or an accidental conflagration: he was driven to conclude that they had been formed elsewhere, and projected thence to the places where they were discovered; and as no volcanoes had been known to eject masses of iron, and as, moreover, no volcanoes are met with in those regions, he held that the specimens referred to must have actually fallen from the sky. Further, he sought to show that the flight of a heavy body through the sky is the direct cause of the luminous phenomenon known as a fire-ball.
8. About seven o'clock on the evening of June 16, 1794, as if to direct attention to Chladni's just published theory, there fell a shower of stones at Siena, in Tuscany.
[pg 22]The event is described in the following letter, dated Siena, July 12, 1794, from the Earl of Bristol to Sir William Hamilton, K.B., F.R.S., at that time British Envoy-Extraordinary and Plenipotentiary at the Court of Naples:—7
"In the midst of a most violent thunderstorm, about a dozen stones of various weights and dimensions fell at the feet of different persons, men, women and children. The stones are of a quality not found in any part of the Siennese territory; they fell about 18 hours after the enormous eruption of Mount Vesuvius: which circumstance leaves a choice of difficulties in the solution of this extraordinary phenomenon. Either these stones have been generated in this igneous mass of clouds which produced such unusual thunder, or, which is equally incredible, they were thrown from Vesuvius, at a distance of at least 250 miles: judge, then, of its parabola. The philosophers here incline to the first solution. I wish much, Sir, to know your sentiments. My first objection was to the fact itself, but of this there are so many eyewitnesses, it seems impossible to withstand their evidence."
9. Soon afterwards there fell a stone in England itself. About three o'clock in the afternoon of December 13, 1795, a labourer working near Wold Cottage, a few miles from Scarborough, in Yorkshire,8 was terrified to see a stone fall about ten yards from where he was standing. The stone, weighing 56 lbs., was found to have gone through 12 inches of soil and 6 inches of solid chalk rock. No thunder, lightning, or luminous meteor accompanied the fall; but in the adjacent villages there was heard an explosion likened by the inhabitants to the firing of guns at sea, while in two of them the sounds were so distinct of something singular passing through the air towards Wold Cottage, that five or six people went to see if anything extraordinary had happened to the house or grounds. No stone presenting Pane 4b. [pg 23] the same characters was known in the district. The stone is preserved in the Museum Collection.
10. It seemed to be now impossible for any one to doubt the fall of stones from the sky, but the reluctance of scientific men to grant an extra-terrestrial origin to them is shown by the theories referred to in the above letter to Sir William Hamilton, and is rendered even more evident by the theory proposed in 1796 by Edward King, who suggested that the stones had their origin in the condensation of a cloud of ashes, mixed with pyritical dust and numerous particles of iron, coming from some volcano. As the stones fell at Siena out of a cloud coming from the North, while Vesuvius is really to the South, he gravely suggested that in this case the cloud had been blown from the South past Siena, and had then before its condensation into stone been brought back by a change of wind. As to the fall of a stone near Wold Cottage, he was not prepared either to believe or disbelieve the witnesses until the matter had been more closely examined; but in case the statements should prove worthy of credit, he points out the possibility of the necessary dust-cloud having come from Mount Hecla in Iceland.
11. Later came a well-authenticated account of a more wonderful event still. At 8 o'clock on the evening of December 19, 1798, many stones fell at Krakhut, 14 miles from Benares, in India; the sky was perfectly serene, not a cloud had been seen since December 11, and none was seen for many days after. According to the observations of several Europeans, as well as natives, in different parts of the country, the fall of the stones was preceded by the appearance of a ball of fire, which lasted for only a few instants, and was followed by an explosion resembling thunder.
12. Fragments of the stones of Siena, Wold Cottage, and Krakhut, as also of a stone said to have fallen on July 3, 1753, at Tabor, in Bohemia, came into the hands of Edward Howard, and the comparative results of a chemical and mineralogical investigation (the latter by the Count de Bournon) of the stones from the above four places are [pg 24] given in a paper read before the Royal Society of London, on February 25, 1802. Howard concludes as follows:—
"The mineralogical descriptions of the Lucé stone by the French Academicians, of the Ensisheim stone by M. Barthold, and of stones from the above four places (Siena, Wold Cottage, Krakhut and Tabor) by the Count de Bournon, all exhibit a striking conformity of character common to each of them, and I doubt not but the similarity of component parts, especially of the malleable alloy, together with the near approach of the constituent proportions of the earth contained in each of the four stones, will establish very strong evidence in favour of the assertion that they have fallen on our globe. They have been found at places very remote from each other, and at periods also sufficiently distant. The mineralogists who have examined them agree that they have no resemblance to mineral substances properly so called, nor have they been described by mineralogical authors."
13. This paper aroused much interest in the scientific world, and, though Chladni's view that such stones come from outer space was still not generally accepted in France, it was there deemed more worthy of consideration after Poisson9 (following Laplace) had shown that a body shot from the moon in the direction of the earth, with an initial velocity of 7592 feet a second, would not fall back upon the moon, but would actually, after a journey of sixty-four hours, reach the earth, upon which, neglecting the resistance of the air, it would fall with a velocity of about 31,508 feet a second.
14. Whilst the minds of the scientific men of France were in this unsettled condition, there came a report that still another shower of stones had fallen, this time in their own Pane 4c. country, and within easy reach of Paris. To settle the matter finally, if possible, the physicist Biot, Member of the French Academy, was directed by the Minister of the Interior to inquire into the event upon the spot. After a careful [pg 25] examination of the stones and a comparison of the statements of the villagers, Biot10 was convinced that—
1. On Tuesday, April 26, 1803, about 1 P.M., there was a noise as of a violent explosion in the neighbourhood of L'Aigle, in the department of Orne, followed by a rolling sound which lasted for five or six minutes: the noise was heard for a distance of 75 miles round.
2. Some moments before the explosion at L'Aigle, a fire-ball in quick motion was seen from several of the adjoining towns, though not from L'Aigle itself.
3. There was absolutely no doubt that on the same day many stones fell in the neighbourhood of L'Aigle.
Biot estimated the number of the stones at two or three thousand; they fell within an ellipse of which the larger axis was 6·2 miles, and the smaller 2·5 miles; and this inequality might indicate not a single explosion but a series of them. With the exception of a few little clouds of ordinary character, the sky was quite clear.
The exhaustive report of Biot, and the completeness of his proofs, compelled the whole of the scientific world to recognise the fall of stones on the earth from outer space as an undoubted fact.
15. Since that date many falls have been observed, and the attendant phenomena have been carefully investigated. These observations teach us that meteorites, as they are now called, fall at all times of the day and night, and at all seasons of the year, while they favour no particular latitudes: also they are found to be quite independent of the weather, and in many cases have fallen when the sky has been perfectly clear; even where stones have fallen in what has been called a thunder-storm, we may reasonably suppose that in most cases the luminous phenomenon has been mistaken for a variety of lightning, and the loud noise for thunder.
16. From observations of the path and the time of flight of the luminous meteor, it is calculated that meteorites enter the earth's atmosphere with absolute velocities ranging [pg 26] from 10 to 45 miles a second: the velocity actually observed is that relative to a person at rest on the earth's surface; for the determination of the absolute velocity of the meteorite, the motion of the observer with the earth (about 18 miles a second) must be allowed for. Let us attempt to follow the course of a small compact body moving at such a rate. So long as the body is traversing "empty space," the only heat it receives is that sent direct from the sun and stars; in general, the meteorite will thus be probably very cold, and, owing to its small size and want of luminosity, it will be invisible to an observer on the earth's surface. After the meteorite enters the earth's atmosphere a very speedy change must take place. The resistance of the air. Assuming the law of resistance of the air for a planetary velocity to be the same as that deduced from experiments with artillery, the astronomer Schiaparelli11 has shown that if a ball of 8 inches diameter and 32-1/3 lbs. weight enter the atmosphere with a velocity of 44¾ miles a second, its velocity on arriving at a point where the barometric pressure is still only 1/760th of that at the earth's surface will have been already reduced to 3-1/6 miles a second. From this it is clear that the speed of the meteorite after the whole of the atmosphere has been traversed will be extremely small, and comparable with that of an ordinary falling body. From experiments made by Professor A. S. Herschel, it has been calculated that the velocity of the meteorite which fell at Middlesbrough, in Yorkshire, on March 14, 1881, was, on striking the ground, only 412 feet a second. From the depth of the hole (20 to 24 inches) made in stiff loam by the stone which fell at Hvittis, in Finland, on October 21, 1901, it has been estimated by Mr. Borgström that the meteorite had a velocity of 584 feet a second when it reached the earth. He further calculates that the stone would have acquired virtually the same velocity if it had been merely allowed to fall, from a position of rest, under the action of gravity, through an infinite atmosphere having the same density as at the earth's surface. In the case of the [pg 27] Hessle fall, several stones fell on the ice, which was only a few inches thick, and rebounded without either breaking the ice or being broken themselves.
17. Further, Schiaparelli pointed out that, in the case imagined by him, the energy already converted into heat would be sufficient to raise 198,400 pounds of water from freezing point to boiling point under the ordinary barometric pressure. The greater part of this heat is, no doubt, carried off by the air through which the meteorite passes; but still the wonder is, not that a meteorite is small on reaching the earth's surface, but that any of it is left to "tell the tale."
This sudden generation of heat will cause fusion, and even luminosity, of the outer material of the meteorite, and in some cases a combustion of some of its constituents: the products of the thermal and mechanical action sufficiently account for the cloud from which the meteorite is generally seen to emerge as a ball of fire, and also for the visible trail often left behind. The ball of fire has often an apparent diameter larger even than that of the moon, and is sometimes too bright for the eye to gaze upon.
18. Owing to the quick reduction of speed, the luminosity will be a feature of the higher, not the lower, part of the course. The Orgueil meteorite of May 14, 1864, was so high when luminous that, notwithstanding its almost easterly motion, it was seen over a space of country ranging from the Pyrenees to the north of Paris, a distance of more than 300 miles.
19. Next we may remark that the time of flight in the earth's atmosphere will be very short, and reckoned only by seconds. Even when the meteorite is wholly metallic, if we may judge from the time one end of a poker may be held in the hand whilst the other end is in the fire, the heat will not have had time to get far below the surface before the body Pane 4d. will have reached the ground.
[pg 28]As a matter of fact, meteorites are almost invariably found to be covered with a crust or varnish, such as would be caused by strong heating, and its thinness shows the slight depth to which the heat has had time to penetrate; in the case of the stones, the greater part of the suddenly heated superficial material must chip off and be left behind at all parts of the track of the meteor. The aspect of the crust varies according to the mineral constitution of the meteorites: it is generally black, and in most cases dull, as Pane 4d. in High Possil, Zsadány and Orgueil, but sometimes shiny, as in Stannern, or partly dull and partly shiny, as in Dyalpur; rarely, it is of a dark grey colour, as in Mezö-Madaras and some of the stones which fell in the neighbourhood of Mocs. In the case of the Pultusk meteorite of Panes 4efg. January 30, 1868, several thousands of stones, varying from the size of a man's head to that of a small nut, were picked up, each covered with a crust: fifty-six of the stones of this fall are shown in the case.
20. The crust is not of equal thickness at every point; for, the form of the meteorite being a result of oft-repeated fracture, the constantly changing surface must be very irregular, and its different parts must be heated to different temperatures and be exposed to different amounts of mechanical action. Sometimes, owing to the motion of the meteorite through the air, the crust is so marked as to indicate the position of the meteorite in regard to its line of motion at a certain part of its course; and this relation is rendered more clear in some cases by evidence that melted material has been driven to the back of the moving mass. The Nedagolla iron Pane 4h. and the Goalpara stone illustrate this peculiarity.
21. Further, the surface of a meteorite is generally covered with pittings, which have been compared in form to thumb-marks: stones from the Supuhee, Futtehpur, and Pane 4h. Knyahinya falls present good examples of this character. It is remarkable that pittings bearing a close resemblance to those of meteorites have been observed on the large partially burned grains of gunpowder, which have been Pane 4h. picked up near the muzzle after the firing of the 35-ton and 80-ton guns at Woolwich. The pitting of the gunpowder grains is attributed to unequal combustion, but that of meteorites seems to be due not so much to inequality of combustibility as to that of conductivity, fusibility and frangibility of the matter at the surface.
[pg 29]22. As picked up, complete and covered with crust, meteorites are not spherical, nor have they any definite shape: in fact, they are always irregular angular fragments, such as would be obtained on breaking up a rock presenting no regularity of structure.
In the case of the Butsura fall of May 12, 1861,12 fragments of the stone were picked up three or four miles apart, and, wonderful to say, it was possible to reconstruct Pane 4h. with much certainty the portion of the meteorite to which they once belonged: a model of the reconstructed portion is Pane 4a. shown in the case. Two of the fragments, in other respects fitting perfectly together, are even on the faces of the junction now coated with a black crust, showing that one disruption took place when the meteorite had a high velocity; two other fragments found some miles apart fitted perfectly, and were neither of them incrusted at the surface of fracture, thus indicating another disruption at a time when the velocity of the meteorite had been so far reduced that the material of the new faces was not blackened through the generation of heat. Sometimes, as in the case of the meteorite of Orgueil, the fragments reach the ground before the detonation is heard, proving that the fracture has taken place at a part of the course where the velocity of the meteorite was considerably greater than that of the sound-vibrations (1100 feet a second).
23. The sudden condensation of air in front of the meteorite, the consequent generation of heat and expansion of the outer shell, have been held to account not only for the break-up of the meteorite into fragments, but partly also for the crash like that of thunder which is a usual accompaniment of the fall. Others have referred this noise solely to the sudden rush of air into the space traversed by the meteorite in the early part of the course. It has, however, now been discovered that the mere flight of a projectile through the air with a velocity exceeding that of sound (1100 feet a second) is itself sufficient to cause a loud detonation; neither explosion, like that of a bomb-shell, [pg 30] nor simple fracture of the meteorite by reason of pressure or sudden heat, is a necessary preliminary to the production of the loud noise. It is found, in fact, that when a projectile is fired with high initial velocity, say 2350 feet a second, an observer near the path of the projectile begins to distinguish two detonations as soon as his distance from the cannon reaches 500 feet; the first of them, a sharp one, appears to come from that part of the projectile's path which is nearest to the observer, and travels with the velocity of the projectile; the later and duller one appears to come from the cannon itself, and travels with the velocity of sound. If the projectile is intercepted near the cannon, only a single detonation is heard by an observer in the same position as before, and it travels at the rate of 1100 feet a second. If the initial velocity of the projectile is less than that of sound, only a single detonation is heard, and it starts from the cannon.
The rolling sound, which follows the detonation of a meteorite, is due, as in the case of thunder, to echoes from the ground and the clouds.
The detonations due to the different members of a swarm of meteorites will combine to form a single detonation unless they are separated by perceptible intervals of time.
24. After the detonation, sounds are generally heard which have been variously likened to the flapping of the wings of wild geese, the bellowing of oxen, Turkish music, the roaring of a fire in a chimney, the noise of a carriage on the pavement, and the tearing of calico: these sounds are probably due to the whirling and oscillation of the fragments while traversing the air, with small velocity, near the observers, and correspond to the hiss or hum observed in the case of a projectile travelling with a velocity less than that of sound.
25. As to the kinds of elementary matter13 of which meteorites are composed, about one-third, and those the most common, of the elements at present recognised as [pg 31] constituents of the earth's crust have been met with: no new elementary body has been discovered. The most frequent or plentiful in their occurrence are:—
while, less frequently or in smaller quantities, are found:
26. In addition to the above, the existence of minute traces of several other elements has been announced; of these special mention may be made of gallium, gold, iridium, lead, platinum and silver.
27. Most of the above elements are present in the combined state; the iron occurring chiefly in combination with nickel, and the phosphorus almost always combined with both nickel and iron. Some of them are found also in their elementary condition: perhaps hydrogen and nitrogen; carbon, both as indistinctly crystallised diamond and as graphitic carbon, the latter being generally amorphous, but occasionally in cubic crystals (cliftonite); free phosphorus has been found in Saline Township; free sulphur has been observed in one of the carbonaceous meteorites, but may have been separated from the unstable sulphides since the entry into our atmosphere.
28. Of the constituents of meteorites, the following are by many mineralogists regarded as being at present unrepresented among the terrestrial minerals:—
Cliftonite, a cubic form of graphitic carbon,
Phosphorus,
Various alloys of nickel and iron,
[pg 32]Moissanite, silicide of carbon,
Cohenite, carbide of iron and nickel; corresponding to Cementite, carbide of iron, found in artificial iron,
Schreibersite, phosphide of iron and nickel,
Troilite, proto-sulphide of iron,
Oldhamite, sulphide of calcium,
Osbornite, oxy-sulphide of calcium and titanium or zirconium,
Daubréelite, sulphide of iron and chromium,
Lawrencite, protochloride of iron,
Asmanite, a species of silica,
Maskelynite, a singly refracting mineral with the composition of labradorite.
Weinbergerite, silicate intermediate in chemical composition between pyroxene and nepheline.
Of the above, Troilite is perhaps identical with some varieties of terrestrial pyrrhotite: Asmanite, the form of silica obtained in 1867 by Prof. Maskelyne from the Breitenbach meteorite, was announced by him in 1869 to be optically biaxal, and thus to belong to a crystalline system different from the hexagonal to which both tridymite, then just announced by Vom Rath, and quartz had been assigned. Later investigations of tridymite have shown that its optical characters and crystalline form are inconsistent with the hexagonal system of crystallisation, and it is not impossible that asmanite and tridymite may be specifically identical. It has been found that tridymite becomes optically uniaxal at a moderate temperature, and its general characters appear to be essentially identical with those of asmanite. According to one view, Maskelynite is the result of fusion of a plagioclastic felspar; according to another, it is an independent species chemically related to leucite.
29. Other compounds are present, corresponding to the following terrestrial minerals:—
Olivine and forsterite,
Enstatite and bronzite,
Diopside and augite,
Anorthite, labradorite and oligoclase,
Leucite,
[pg 33]Magnetite and chromite,
Pyrites,
Pyrrhotite,
Breunnerite.
Further, from one of the Lancé stones, chloride of sodium, and from the carbonaceous meteorites, sulphates of sodium, calcium and magnesium, have been extracted by means of water.
In addition to the above, there are several compounds or mixtures of which the nature has not yet been satisfactorily ascertained.
30. Quartz, the most common of terrestrial minerals, is absent from the stony meteorites; but in the undissolved residue of the Toluca iron microscopic crystals have been found, some of which have important characters identical with those of quartz, while others resemble zircon. As mentioned above, free silica is present in the Breitenbach meteorite as asmanite.
31. As to the conditions14 under which such compounds can have been formed, we may assert that they must have been very different from those which at present obtain near the earth's surface: in fact, it is impossible to imagine that phosphorus, the metallic nickel-iron and the unstable sulphides can either have been formed, or have remained unaltered, under circumstances in which water and atmospheric air have played any prominent part. Still, what little we do know of the inner part of our globe does not shut out the possibility of the existence of similar elementary and compound bodies at great depths below the surface. Daubrée,15 after experiment, inclines to the belief that the iron is due, in many cases at least, to reduction from an olivine rich in diferrous silicates, and this view perhaps acquires some additional probability from the fact that hydrogen and carbonic oxide are given off when meteoric iron is heated: the existence, however, of such siderolites as that of Krasnojarsk, which is rich both in metallic iron and in [pg 34] orthosilicate of iron and magnesium (olivine), and yet presents no traces of the intermediate metasilicate of iron and magnesium (bronzite), offers a weighty objection to the general application of this view.
32. Meteorites may be conveniently arranged in three classes, which pass more or less gradually into each other: the first includes all those which consist mainly of iron, and have, therefore, been called by Prof. Maskelyne aero-siderites (aer, air, and sideros, iron), or, more shortly, Siderites; the second is formed by those which are composed chiefly of iron and stone, both in large proportion, and are called aero-siderolites, or, shortly, Siderolites; while those of the third class, being almost wholly of stone, are called Aerolites (aer, air, and lithos, stone).
33. In the Siderites the iron generally varies from 80 to 95 per cent., and the nickel from 6 to 10 per cent.; in the Santa Catharina siderite (of which the meteoric origin is somewhat doubtful) 34, and in that of Oktibbeha County 60, per cent. of nickel have been found: the nickel is alloyed with the iron, and several of the alloys have been distinguished by special names. Owing to the presence of the nickel, meteoric iron is often so white on a fractured surface as to be mistaken for silver by its finder; it is also less liable to rust than ordinary iron is. Troilite is frequently present as plates, veins or large nodules, sometimes surrounded by graphite; schreibersite is almost always found, and occasionally also daubréelite.
Further, various chemists have proved that hydrogen, nitrogen, marsh gas, and the carbonic oxides are evolved when meteoric iron or stone is heated; in one case a trace of helium was detected. Probably the gases were not present in the occluded state, but resulted from the decomposition or interaction of non-gaseous constituents during the experiments.
34. The want of homogeneity and the structure of meteoric iron are beautifully shown by the figures generally called into existence when a polished surface is exposed to the action of acids or bromine; they are due to the inequality of the action on thick or thin plates of various [pg 35] constituents, the plates being composed chiefly of two nickel-iron materials termed kamacite and tænite. A third nickel-iron material, filling up the spaces formed by the intersection of these plates of kamacite and tænite, is termed plessite; it is probably not an independent substance but an intergrowth of the first two kinds.
In the Agram iron, investigated by Widmanstätten in 1808, the plates are parallel to the faces of the regular octahedron; such figures are well shown by the exhibited slice of the Toluca iron; different degrees of distinctness of such "Widmanstätten" figures are illustrated by specimens Pane 4l. of Seneca River, Zacatecas, Charcas, Burlington, Jewell Hill, Lagrange, Victoria West, Nelson County, and Seeläsgen. The large Otumpa specimen, mounted on a separate pedestal, furnishes a good example of the less distinct, and more or less damascene, appearance presented by the etched surface of some meteoric irons of octahedral structure.
The Braunau iron gives no "Widmanstätten" figures, but has cleavages parallel to the faces of a cube; on etching it yields linear furrows which were found (1848) by Neumann to have directions such as would result from twinning of the cube about an octahedral face; as illustrations of the "Neumann lines," etched specimens of Braunau and Salt Pane 4l. River are exhibited.
For meteoric irons of cubic structure the percentage of nickel is lower than 6 or 7; for those of octahedral structure it is higher than 6 or 7, and the plates of kamacite are thinner, and the structure therefore finer, the higher the percentage of that metal. A considerable number of meteoric irons, however, show no crystalline structure at all, and have percentages of nickel both below and above 7; it has been suggested that these masses have been metamorphosed, and that crystalline structure was once present, but has disappeared as a result of the meteorites having been heated, not merely superficially during their passage through the earth's atmosphere, but throughout their mass while travelling in outer space.
[pg 36]35. Though meteoric iron has been at some time, presumably, in a state of fusion, and its present structure is a result of the particular circumstances of the cooling of the liquid and afterwards solid material, attempts to produce such structures by the cooling of fused meteoric iron or artificial mixtures of nickel and iron have not yet been successful. It will be useful, therefore, to consider briefly some of the manifold changes which are found to take place during the passage of fused mixtures and of solutions to the solid state, and during the cooling of such solids to ordinary temperatures.
If a fused mixture of antimony and bismuth is allowed to cool, the solid which first separates is neither pure antimony nor pure bismuth, but a material which has a percentage composition depending on, though not identical with, that of the original mixture. The temperature for the beginning of the solidification is different for different proportions of the two metals, and is intermediate between 622° and 268°, the solidifying temperatures of antimony and bismuth, respectively; it approaches the latter more and more closely as the percentage of the bismuth is increased. The solid first separated is somewhat richer in antimony than the original mixture; the still fused part, therefore, is somewhat richer in bismuth than before, and does not begin to solidify till a lower temperature is reached; the temperature thus gradually falls, instead of remaining constant, during the solidification. In the cooling of such fused mixtures the changing composition of the part still fused has for effect a changing composition of the solid already separated; whence the slower the cooling of the fused material, the greater is the homogeneity of the final solid.
A fused mixture of silver and copper behaves in a different way. When the percentage weight of the silver is 72, and that of the copper, therefore, is 28, solidification begins, not at a temperature between 960° and 1083°, the solidifying temperatures of silver and copper, respectively, but at a temperature below both, namely, 770°. The solid which first separates has the same percentage composition as the original mixture; the part still fused has thus itself the same percentage composition as before, and continues to [pg 37] Cooling of fused mixtures and of solutions. solidify at the same temperature, and in the same way, until the solidification is complete. Such a mixture, having a definite composition and a definite temperature of solidification, was for a time regarded as a definite chemical compound with a complex chemical formula, but on microscopic examination the resultant solid is found to be heterogeneous; minute particles of the silver and copper are seen to lie side by side, the particles being granular or lamellar in form according to the circumstances of the cooling. If the percentage of silver is different from 72, whether it be higher or lower, the solidification begins at a higher temperature than 770°; whence the mixture containing 72 per cent. of silver has been conveniently termed eutectic (i.e. very fusible); the term was suggested by Prof. F. Guthrie,16 to whom our knowledge of the existence of such mixtures is due.
36. When the silver is in excess of 72 per cent., the excess of silver gradually collects together and solidifies at various parts of the cooling fused mass; the still fused portion thus gradually becomes poorer in that metal, and the temperature, instead of remaining constant, gradually falls during the separation of the solid. At length the percentage of silver in the fused portion falls to 72 per cent. and the temperature to 770°; the solid which now begins to form is no longer pure silver, but a material containing 72 per cent. of that metal; and it continues to have the same percentage composition as the surrounding liquid, and the temperature of solid and liquid to be 770°, until the solidification is complete. The final solid thus consists of blebs of silver scattered through a fine groundmass of eutectic mixture of silver and copper. Similarly, if the copper is in excess of 28 per cent., the final solid consists of blebs of copper scattered through a fine groundmass of eutectic mixture of silver and copper.
If the two metals are copper and antimony, instead of copper and silver, the results are more complicated; for the first two metals are capable of combining together to form a definite chemical compound represented by the formula Cu2Sb, and each of the metals forms a eutectic mixture with the latter. According to the percentage composition of the original mixture, the solid which first separates during cooling from fusion may be either copper or antimony or the compound Cu2Sb; the separation continuing, and the temperature falling, until the first eutectic proportion and its corresponding temperature are reached.
[pg 38]37. Analogous results are obtained during the cooling of solutions; for instance, during the cooling of a solution of sodium chloride (common salt) in water. A solution containing 23·5 per cent. of sodium chloride begins to solidify at -22° C.; the separating solid is not simple sodium chloride or simple ice, but has the same percentage composition as the original solution, and thus the temperature remains -22° until the whole material has become solid. On microscopic examination the solid is seen to be heterogeneous, and to consist of small particles of sodium chloride and ice lying side by side. If the percentage of sodium chloride is different from 23·5, whether higher or lower, solidification begins before the temperature has fallen to -22°. The characters of this particular solution are thus closely analogous to those of the eutectic mixtures described above. If the sodium chloride exceeds 23·5 per cent., the excess of sodium chloride begins to separate, and solidify, at various parts of the liquid, at a temperature higher than -22°; it continues to separate, and the temperature to fall, until the proportion of sodium chloride in the residual liquid is reduced to 23·5 per cent. and the temperature to -22°. Afterwards the separating solid has the same composition as the residual liquid (23·5 per cent. of sodium chloride), and the temperature remains constant, until the residual liquid has been wholly transformed into a solid fine-grained mixture of sodium chloride and ice. The final solid thus consists of large particles of sodium chloride dispersed through a fine groundmass consisting of eutectic mixture of sodium chloride and ice. Similarly, if the water is in excess of 76·5 per cent., the final solid consists of large particles of ice dispersed through a fine groundmass consisting of eutectic mixture of sodium chloride and ice.
The results of the cooling of a solution of ferric chloride are still more complicated; for this substance enters into [pg 39] chemical combination with water, and in no fewer than four different proportions. The solid which first separates from the cooling solution may thus, according to the percentage of ferric chloride, be either ferric chloride or water, or any one of the various compounds of the two; and to each pair of compounds nearest to each other in composition corresponds a different eutectic mixture and a different temperature for its formation.
38. Some solid bodies, during cooling, show changes analogous to those observed in solutions, and are therefore termed "solid solutions." For instance, if a hot physically homogeneous solid obtained from the fusion of iron with carbon is cooled, there may result a separation in the solid of particles of either iron or cementite, the latter being a chemical compound of iron and carbon represented by the formula Fe3C; the particular substance separated depending on the percentage composition of the original solid. This separation continues, and the temperature falls, until the residual physically homogeneous material contains 0·9 per cent. of carbon and the temperature is 690°; the temperature then remains constant, although the body is surrounded by a cooling medium, until this residual physically homogeneous material has been wholly transformed into a fine-grained mixture of iron and cementite, containing 0·9 per cent. of carbon. This particular kind of mixture has been termed eutectic, though the transformation has taken place, not by solidification from fusion, but in a body which was already solid. Prof. Rinne has proposed for such cases the substitution of the term eutropic, thus avoiding the suggestion of fusion. The eutectic mixture of iron (or ferrite) and cementite is known as pearlite.
39. Just as water may be cooled so quietly that it is still liquid at a temperature much below the normal freezing point, a mixture may be cooled in such a way as to pass much below the eutectic (or eutropic) point without the normal transformation taking place; it is then said to be overcooled. The equilibrium, however, is very unstable, and the transformation, once begun, takes place almost instantaneously throughout the whole mass.
[pg 40]40. A structure analogous to that shown by the Widmanstätten figures, though on a finer scale, has been observed by Prof. J. O. Arnold and Mr. A. McWilliam17 in cast steel containing 0·4 per cent. of carbon; the plates of iron (or ferrite) in the cast steel correspond to the plates of kamacite in meteorites. Further, it has been found that the plates in the cast steel disappear during the process of annealing; similarly, there are no Widmanstätten figures, and the structure of the material is granular, near the outer surface of an unweathered meteoric iron; presumably as a result of the high temperature to which the outer part of the mass has been raised during the passage of the meteorite through the earth's atmosphere.
41. At present it is generally imagined that kamacite and tænite are definite alloys, or perhaps solid solutions, of iron and nickel, the former being poor in nickel (6 or 7 per cent.) and the latter rich in that constituent (25 to 38 per cent.), that kamacite and tænite separate in succession from the molten mass or solid solution until the residual part is so rich in nickel that a eutectic (or eutropic) proportion is reached; the residual material then forms plessite, which, according to this view, is a eutectic (or eutropic) mixture of kamacite and tænite. But it is difficult to understand how the thin plates of tænite are deposited on the plates of kamacite, seeing that they contain more nickel than kamacite and plessite, and yet have an intermediate epoch of formation, prior to the epoch of formation of that tænite which is a constituent of the plessite; one suggestion is that the thin plates of tænite have been deposited on the plates of kamacite owing to the temperature having fallen well below the eutectic (or eutropic) point after the separation of the kamacite and before the eutectic transformation of the residual material has taken place. And Prof. Rinne18 himself is of opinion that the Widmanstätten structure has been wholly developed in meteoric iron after the solidification of the mass; further, as the relations of the kamacite, tænite and plessite to the enclosed troilite indicate that the troilite [pg 41] was solid before the octahedral structure was developed, and as that mineral, under normal circumstances, solidifies at about 950°, he infers that the structure was developed below that temperature. In the case of the Jewell (Duel) Hill meteorite it was discovered by Dr. Brezina that, notwithstanding the pronounced octahedral structure, plates of troilite are embedded, not in accidental positions nor between successive octahedral layers, but parallel to the faces of the corresponding cube; whence Prof. Rinne suggests that this iron, now of octahedral structure, and possibly all others of a similar character, had a cubic structure at the epoch when they entered upon the solid condition. But, as both Prof. Rinne and Dr. Brezina19 have pointed out, a fused mixture of nickel and iron, cooling undisturbedly in outer space, may have solidified at a temperature even below 950° and thus have been much overcooled.
42. In the course of a recent elaborate investigation of the changes of the magnetic permeability of the Sacramento meteoric iron with changing temperature, Mr. S. W. J. Smith20 has been led to infer that the magnetic behaviour can only be explained by imagining the meteorite to consist largely of plates of nickel-iron, containing about 7 per cent. of nickel (kamacite), separated from each other by thin plates of a nickel-iron constituent (tænite), containing about 27 per cent. of nickel and having different thermo-magnetic characters from those of kamacite; he suggests, however, that tænite is not a definite chemical compound, but is itself a eutectic (or eutropic) mixture, and consists of kamacite and a nickel-iron compound containing not less than 37 per cent. of nickel. And he points out that, while the tænite mechanically isolated from meteorites for analysis has approximately the lower percentage (27 per cent.), the tænite chemically isolated through the prolonged action of dilute acid (which would remove much of the admixed kamacite) has a higher percentage, which in several cases approximates to 40 per cent.
[pg 42]43. The Siderites actually observed to fall, or found soon after a luminous meteor had been seen, or a detonation heard, by people in the neighbourhood, reach only the small number of nine; they are, Agram, Charlotte, Braunau, Victoria West, Nedagolla, Rowton, Mazapil, Cabin Creek, and N'Goureyma. The remaining specimens in collections of Siderites are presumed to be of meteoric origin by reason of the peculiarity of their appearance and chemical composition, and of the characters of the material in which they have been found (Art. 7).
The large Cranbourne meteorite, mounted in a special case in the Pavilion, before rusting weighed 3½ tons. The two largest known were found in Western Greenland and Mexico, respectively, and are both of very irregular shape. The Greenland mass is 11 feet long, 7½ feet wide, and 6 feet thick, and its weight, which had been variously estimated at from 50 to 100 tons, has been determined to be 36½ tons; the mass had long been known to the Eskimos, and was inquired after by Captain John Ross in 1818; it was shown by a native to Lieutenant Peary in 1894, who afterwards transported it from Melville Bay to New York; it is now preserved in the American Museum of Natural History in that city. The Mexican mass is 13 feet long, 6 feet wide, and 5 feet thick, and has an estimated weight of 50 tons; it is the property of the Mexican Government, and is still lying at El Ranchito, near Bacubirito, Province of Sinaloa.
44. The difficulty of distinguishing an iron of terrestrial from one of meteoric origin was rendered very evident by the prolonged controversy as to the origin of the large masses of iron, containing one or two per cent. of nickel, and weighing 9,000, 20,000, and 50,000 lbs., respectively, found in 1870 by Baron N. A. E. Nordenskiöld on the beach at Ovifak, Disko Island, Western Greenland.
A careful examination of the rocks of the neighbourhood shows that the basalt contains nickeliferous iron disseminated through it, and that the large masses of iron, Pane 4m. at first thought to be meteorites, are very probably of terrestrial origin, and have been left exposed upon the seashore through the weathering of the rock which originally [pg 43] enclosed them. Some of the malleable metallic nodules extracted from the basalt were found to contain as much as 6·5 per cent. of nickel. In 1880 Professor K. J. V. Steenstrup21 found ferriferous basalt in situ in three different parts of the island. At Assuk (Asuk) the enclosed balls of iron reach a diameter of nearly three-quarters of an inch. Some assert that the basalt and the nickel-iron have been expelled together from great depths below the earth's surface, while others consider that the nickel-iron is due to the reduction of the iron-compounds in the basalt by the passage of the lava through the beds of lignite and other vegetable matter found in the vicinity.
45. With the Ovifak iron in the case are shown other specimens of iron which have been brought by various explorers from West Greenland, and were formerly thought to have had a meteoric origin. The discovery of ferriferous basalt, not only in situ in several places, but also deposited in a Greenlander's grave (1879) along with knives (similar to those given to Captain John Ross in 1818) and the usual stone tools, renders it clear that the Eskimos were not dependent solely on meteorites for their metallic iron, as had long been supposed.
Mr. Skey announced in 1885 the discovery of terrestrial nickel-iron in New Zealand. Grains of the alloy (Awaruite), containing as much as 67·6 per cent. of nickel, are found in the sand of the rivers flowing from a range of mountains composed of olivine-enstatite rocks, in places altered to serpentine: similar particles have been found in the serpentine itself. Similarly, in the sand of the stream Elvo, near Biella, in Piedmont, and of the river Fraser, British Columbia, grains of nickel-iron containing 75 or 76 per cent. of nickel have been found: and in the placer gravel of a stream in Josephine and Jackson Counties, Oregon, U.S.A., large quantities of waterworn pebbles, which enclose an alloy (Josephinite) of nickel and iron containing 72 per cent. of the former metal, have been met with. Professor Andrews many years ago established the presence of minute particles of metallic iron in some basalts; Dr. Sauer has lately found [pg 44] a single nodule of malleable iron of the size of a walnut in the basalt of Ascherhübel, in Saxony; Dr. Hornstein has described large nodules of (nickel-free) iron found in basalt in a quarry at Weimar, near Cassel; Dr. Beckenkamp has described nodules of metallic iron found in clay at Dettelbach, near Würzburg; and Dr. Johnston-Lavis has announced the find of an enclosure of metallic iron in a leucitic lava of Monte Somma; Dr. Hoffmann has noted the occurrence of minute spherules of brittle iron both in perthite and quartzite in Ontario; Dr. Hussak has recorded the discovery of metallic iron in an alluvium of Brazil, and Dr. Högbom has found it associated with topaz, quartz, felspar, and other minerals, in limonite from an unspecified place in South America; two minute grains of iron were found by Mr. Osaka in the débris of an agglomerate at Nishinotake, Japan.
46. The stony part of the siderolites and aerolites is almost entirely crystalline, and in most cases presents a peculiar "chondritic" or granular structure, the loosely coherent grains being composed of minerals similar to those which enclose them, and containing in most cases minute particles of iron and troilite disseminated through them: glass-inclusions are found to be present. The minerals mentioned above as occurring in meteorites are such as are very characteristic of the more basic terrestrial rocks, such as dunite, lherzolite and basalt, which have been expelled from considerable depths below the earth's surface.
47. Several attempts to classify aerolites according to their mineralogical constitution have been made, but it cannot be said that any of them is very satisfactory; seeing that even in the same stone there may be much difference in its parts, a perfect classification on such a basis is scarcely to be hoped for.
About eleven out of every twelve of the stony meteorites belong to a division to which Rose22 gave the name of chondritic (chondros, a grain): they present a very fine-grained but crystalline matrix or paste, consisting of olivine and enstatite or bronzite, with more or less nickel-iron, [pg 45] troilite, chromite, augite and anorthic felspar; through this paste are disseminated round chondrules of various sizes (up to that of a walnut) and with the same mineral composition as the matrix; in some cases the chondrules consist wholly or in great part of glass.23 In mineral composition chondritic aerolites approximate more or less to terrestrial lherzolites. Some meteorites consist almost solely of chondrules, others contain only few; in some cases the chondrules are easy separable from the surrounding material. Of the chondritic division Knyahinya, Pegu, Muddoor, Seres, Pane 4n. Judesegeri, Khiragurh, Utrecht and Nellore (pane 4p) afford good illustrations.
A few meteorites belonging to this division are remarkable as containing carbon in combination with hydrogen and oxygen. Of these the Alais and Cold Bokkeveld meteorites Pane 4n. are good examples: the former has a bituminous smell; it yields sulphates of magnesium, calcium, sodium and potassium, if steeped in water.
48. The remaining aerolites are not chondritic, and they contain little or no nickel-iron; of these we may specially mention for their mineral composition the following:—
Juvinas and Stannern, consisting essentially of anorthite and augite.
Petersburg, consisting of anorthite, augite and olivine, with a little chromite and nickel-iron: both Juvinas and Petersburg may be compared to terrestrial basalt.
Sherghotty, consisting chiefly of augite and maskelynite.
Angra dos Reis, consisting almost wholly of augite; olivine is present in small proportion.
Bustee, of diopside, enstatite and a little anorthic felspar, with some nickel-iron, oldhamite and osbornite.
Bishopville, of enstatite and anorthic felspar, with occasional augite, nickel-iron, troilite and chromite.
Roda, of olivine and bronzite.
Chassigny, consisting of olivine with enclosed chromite, and thus mineralogically similar to a terrestrial dunite.
49. The importance of the examination and classification [pg 46] of meteorites, with a view to a possible recognition of periodicity of fall of specimens presenting the same characters, need only be mentioned to be appreciated: such a determination is, however, rendered very difficult by the close similarity of structure and composition presented by the great majority of the aerolites of the large chondritic division.
50. Attention has been already directed to the fact that although many masses of meteoric iron, some of them like that of El Ranchito, near Bacubirito, in Mexico, weighing very many tons, have been found at various parts of the earth's surface, very few of them have been actually observed to fall: in the case of the stony meteorites just the opposite holds good, for they are never very large, and few are known which have not an authenticated date of fall. This may be due to the fact that a meteoric stone is less easily distinguished than is a meteoric iron from ordinary terrestrial bodies, and will thus in most cases remain unnoticed unless its fall has been actually observed; while, further, a quick decomposition and disintegration must set in on exposure to atmospheric influences. The smaller size of the meteoric stones may be due to the greater ease with which they break up on the sudden increase of temperature of their outer surface, consequent on their entry into the earth's atmosphere. The largest meteoric stone preserved in a Museum is one which fell as part of a shower at Knyahinya, Hungary, in 1866: it weighs 647 lbs. and is at Vienna. A larger stone (723 lbs.) fell at Tabory, Russia, in 1887, but was broken to pieces by the impact on the earth; fragments of a still larger single stone, weighing at least 1244 lbs., were found near together at Long Island, Kansas, U.S.A., but the fall was not observed.
51. If we now examine minutely the structure of the meteoric stones, it will be seen that almost all of them appear to be made up chiefly of irregular angular fragments, and that some of them bear a close resemblance to volcanic tuffs. In the large group of chondritic aerolites, chondrules or spherules, some of which can only be seen under the microscope while others reach the size of a [pg 47] walnut, are embedded in a matrix, apparently made up of minute splinters such as might result from the fracture of the chondrules themselves. In fact, until recently, it was thought by some24 that the chondrules owe their form, not to crystallisation, but to friction, and that the matrix was actually produced by the wearing down of the chondrules through collision with each other either as oscillating components of a comet or during repeated ejection from a volcanic vent of some small celestial body. Chondrules have been observed, however, presenting forms and crystalline surfaces incompatible with such a mode of formation, and others have been described which exhibit features resulting from mutual interference during their growth.
The crystallisation of the chondrules is independent of their form, and must have started, not at the centre, but at various places on their surfaces; Dr. Sorby25 argued that some at least of the chondrules must once have fallen as drops of fiery rain, and have assumed their shape in an atmosphere heated to nearly their own temperature. The chondritic structure is different from anything which has been observed in terrestrial rocks, and the chondrules are distinct in character from those observed in perlite and obsidian. After much study, Dr. Brezina26 lends his weighty support to the hypothesis that the structural features of meteorites are the result of a hurried crystallisation: and Prof. Wadsworth27 accepts the same interpretation.
52. Since the time of their consolidation some meteoric stones, as Tadjera, appear to have been heated throughout their mass to a high temperature: and in the case of Orvinio, Chantonnay, Juvinas, and Weston, fragments are cemented together with a material having the same composition as the fragments themselves, thus giving rise to a structure resembling that of a volcanic breccia. Others seem to have experienced a chemical change, for some of the chondrules in [pg 48] Knyahinya and in Mezö-Madaras, when examined with the microscope, are found to be surrounded by spherical and concentric aggregations of minute particles of nickel-iron, perhaps due to the reducing action of hydrogen at a high temperature. Others, as Château-Renard, Pultusk and Alessandria, present what in terrestrial rocks would probably be called faults: in some cases the fissures are seen to have been filled with a fused material after the chondrules have been broken and one side of the fissure has glided along the other. These peculiarities of structure suggest that the small body which reaches the earth is only a minute fragment of a much larger mass. It has been suggested that the chondritic structure is of metamorphic origin, and a mere result of enormous pressure on the stony material during the passage through the earth's atmosphere; according to still another view, the structure, though metamorphic, is of extra-terrestrial origin, and due to the quick cooling of a tuff-like stone which has been partially melted, for instance, by the heat from a neighbouring new star or by traversing the hot vapours on the limits of an old one.
53. The idea that meteorites arrive at our own atmosphere, not as fragments of rock, but as mere clouds of gas or dust, has been recently revived and again discarded. According to this hypothesis, the air, instead of dispersing the entering cloud, acts in the contrary way, and in a few seconds of time presses the particles together to form solid bodies. This idea is open to various objections, and in any case one can scarcely understand how large masses of iron, presenting a wonderful regularity of crystalline structure, can have been the result of so hurried a process: and if we once grant that the irons enter the atmosphere as solid bodies, it is difficult to believe that the same is not the case with the stones.
54. From the above it will be evident that the old hypotheses that meteorites are terrestrial stones which have been struck by lightning, or carried to the sky by a whirlwind, or are concretions in the atmosphere, or are due to the condensation of a dust-cloud coming from some volcano, or have been shot recently from terrestrial volcanoes, are inconsistent with [pg 49] later observation; it may be granted that the bodies reach our atmosphere from outer space. From what part or parts of space do they come? Their general similarity of structure and chemical composition, and more especially the presence of nickeliferous iron in almost every one, suggest that most, if not all of them, have had a common source, and that they are chips of a single celestial body.
55. Dr. Sorby suggested that they are probably ejected from the sun itself, though this is difficult to reconcile with the fact that some of them are easily combustible. Others, among whom we may mention Laplace, have suggested that they come from volcanoes of the moon which are now active; but the suggestion, although mathematically sound, has no physical basis, for, so far as one can discover, active volcanoes do not there exist: and Sir Robert Ball28 has virtually excluded the lunar volcanoes, which were active in times now long past, by pointing out that if a projectile from the moon once misses the earth, its chance of ever reaching the earth is too small to be worthy of mention. It has further been shown that, although the explosive force necessary to carry a projectile so far from one of the smaller planets that it will not return, is not very large, yet the initial velocity requisite to carry the body as far as the earth's orbit is so considerable, and the chance of hitting the earth so slight, that a more probable hypothesis is, to say the least, desirable. If these bodies have been shot from volcanoes of any planet, Sir Robert Ball is himself inclined, upon mechanical grounds alone, to believe that the projection was from our own in bygone ages; for as such projectiles, having once got away from the earth, would take up paths round the sun which would intersect the earth's orbit, every one of them would have a chance of some time or other meeting with the earth again at the point of intersection, and of appearing as a meteorite. The size and initial velocity requisite for the escape of a projectile through a lofty atmosphere would be enormous: even then the difficulty would still remain that meteorites generally differ, both in structure and material, from anything known to have been ejected from existing terrestrial [pg 50] volcanoes. To meet these difficulties, Sir Robert has speculatively suggested that the matter was expelled before the surface of the earth became solid, and at a time when there was as much activity in the terrestrial planet as there is now in the material of the sun itself.
Nor is it probable that they are portions of a lost satellite of the earth, or are due to a collision of two planets; for in each of these cases we should expect to have received some of the larger fragments which must at the same time have been produced.
Much light is thrown on the history of meteorites by the discovery of a relationship with shooting stars and comets.
56. The meteorite-yielding fireball, referred to in Art. 17, is not the only luminous meteor, apart from lightning, with which we are acquainted. On a clear dark night any one can see a star shoot now and then across the firmament: it is estimated that on the average as many as fourteen are visible to a single observer every hour. Are the shooting, or, as they are often called, falling stars products of our own atmosphere, or do they, like the meteorites, come from outer space? In 1794 Chladni, in the memoir already referred to, gave reasons for believing that a meteoritic fireball and a shooting star are only varieties of one phenomenon.
57. But long after the cosmic origin of meteorites had been generally acknowledged, the atmospheric origin of the shooting stars was still asserted, and it was not till the wondrous star-shower of November 12-13, 1833,29 that the cosmic origin of any of the shooting stars was finally established. During that night upwards of 200,000 shooting stars, according to a rough estimate, were seen from a single place; and the remarkable observation was made at various localities, widely distributed over North America, that the apparent paths of the shooting stars in the sky, when prolonged backwards, all passed through a point in the constellation Leo: this point of radiation appeared to rotate with the heavens during the eight hours for which the shower was visible.
[pg 51]Hence it was manifest that the star-shower was independent of the earth's rotation and must therefore have come from outer space; that the radiation of the paths was only apparent and due to perspective; and that, relatively to an observer, the flights of all the shooting stars were really parallel to the direction of the apparent radiant point.
On the same day of November in each of the three following years the shower was repeated though on a less grand scale, and the constancy of the radiant point was confirmed: similar small showers had been seen also in 1831 and 1832 before the radiation had been noticed. Though in the years immediately before and after 1831-6 no remarkable display of November meteors took place, it was remembered that a similar shower had been chronicled by Humboldt and by Ellicott, as observed by them on November 12, 1799; and a study of ancient documents revealed the fact that a grand star-shower had been recorded several times in October and November since A.D. 902, the date having gradually advanced, during that long space of time, from the middle of October to the middle of November.30 The only sufficient explanation of the observed facts is that a swarm of isolated small bodies, solid and non-luminous—meteorites in fact—is moving in an orbit round the sun, completing the circuit in 33¼ years; the orbit intersects that of the earth, and the earth meets the swarm at the place of intersection. The isolated bodies or meteorites become luminous, as already explained in Art. 17, after their entry into the earth's atmosphere. The swarm can be only a few hundred thousand miles thick, for the earth, travelling through space at the rate of 66,000 miles an hour, passes through the densest part in 2 or 3 hours, and through the whole in 10 to 15 hours: its length, however, must be enormous, amounting to hundreds of millions of miles; for, although the meteorites move with a velocity of twenty miles a second, the swarm takes 5 or 6 years to pass the place of intersection with the earth's orbit, thus causing star-showers, more or less dense, during that number of years.
Contrary to expectation, no large November star-shower [pg 52] occurred either in the year 1899 or in the years which have since elapsed.
Schiaparelli has shown that the unequal attraction of the sun for the individuals of a swarm of meteorites moving round it would scatter them along the orbit, and in the course of time produce a more or less complete ring; if this intersects the earth's orbit an annual star-shower must ensue.
58. A small annual star-shower occurs, in fact, on August 10-11,31 and has been observed since A.D. 830: it radiates from a point in the constellation Perseus. Schiaparelli calculated in 1866 the orbit and motion of the meteorites producing it, and was surprised to find that the numbers corresponded exactly with those calculated for one of the recently observed comets; in other words, a comet was moving in the path of the meteorites, and at exactly the same speed. At the same time Schiaparelli gave numbers defining the motions of the meteorites which would cause the periodic November star-showers.
59. Immediately afterwards, when the numbers calculated by Oppolzer for the orbit of the comet discovered by Tempel were published, it was seen that they were really identical with those already calculated by Schiaparelli for the orbit of the meteorites of the November star-shower, and that here again a comet and a swarm of meteorites were moving in exactly the same path at exactly the same rate.
Almost immediately afterwards it was shown that the radiant points of the small star-showers of April 20-21 and November 27-28 both correspond to the orbits of known comets.
It was evident that these could not be accidental coincidences, and that the comets and the attendant swarms of meteorites are closely related to each other.
[pg 53]60. An intimate connection between, if not complete identity of, meteorites, shooting stars and comets, had indeed long been suspected. Astronomers were convinced that comets, though occasionally of enormous size, are always of extremely small mass, since they pass by the earth and other planets without sensibly disturbing their motions; the comet of 1770 passed through the system of Jupiter's satellites without any perceptible action upon them: it has been calculated that the mass of a small comet may be about eight pounds. Again, the light of a comet, like that of a cloud or planet, was seen to be partially polarised: hence part, at least, must be reflected sunlight, for the plane of polarisation passes through the sun's place. Further, stars of very small magnitude have been seen not only through the tail, but even through the nucleus, of a comet without any apparent alteration of position by refraction: hence it was inferred that a comet is not a continuous mass, but consists of particles so far distant from each other that a ray of light may pass through the comet without meeting a single one of them. Such a constitution likewise accounts for the absence of phases of the reflected light: for although only half of each particle will be directly illuminated by the sun, the remaining half will receive light irregularly reflected from the particles more distant from the sun.
Among others, Chladni in 1817 had referred to the great similarity in the motions of comets and meteorites: Olmsted, in 1834, had calculated the orbit of a comet which would cause the November star-shower; his results were wrong owing to the assumption that the shower was annual: Cappocci, in 1842, gave reasons for believing that a meteorite is a small comet: Reichenbach, in 1858, in a most elaborate paper,32 sought to prove that a comet is a swarm of meteorites; that each chondrule of a meteorite had once been an individual of a cometary swarm, and owes its rounded shape to frequent collision with its fellows; that the rest of the stone consists of the broken splinters thus produced; and that the brecciated aspect of many meteorites is due to collisions in the denser part or nucleus of a comet. As already pointed out in Art. 51, later modes of investigation have led petrologists to reject this method of accounting for the rotundity of the chondrules.
[pg 54]61. In addition to the few radiant points which correspond to swarms moving in orbits identical with those of known comets, there are numerous radiant points which have not yet been recognised as related to existing comets, and may possibly be due to swarms produced by the dispersal of comets along their orbits; indeed, it has been inferred from observation of shooting stars that on the average there are no fewer than fifty distinct radiant points, and therefore showers, for any night of the year. But there are still others of which there is yet no satisfactory explanation. A cometary swarm is thin, and is passed through in a few hours; the stars are seen to radiate from the corresponding point of the sky for only that length of time: but there are other radiant points which have a duration of several months, and this is the case notwithstanding the constantly changing direction of the earth's motion in space.33 Since the position of the radiant point in the sky as seen by a terrestrial observer depends not only on the direction in which the swarm is moving, but also on the velocity and direction of motion of the observer through space, it is easily seen that a radiant point having a fixed position during some months corresponds to something quite distinct from a cometary swarm. It has been suggested by Mr. W. F. Denning (1899) that in some cases a long-continued radiant point may really be due, not to a single swarm, but to successive swarms not physically associated with each other. On the other hand, Professor H. H. Turner has shown that the average effect of the earth's attraction on a meteorite passing near it is to change only the position in our orbit at which we meet the meteorite (i.e. the time of year), not the relative-direction of motion or the relative speed; hence, a swarm of such meteorites must be spread out, in the course of ages, into a succession of rings, all of them equally inclined to the earth's orbit, but intersecting it at different places; the radiant point will then be of long duration. Professor A. S. Herschel34 made the suggestion that the radiant points of long duration may have resulted from the passage, in bygone epochs, of quickly moving streams of cosmical matter through a ring of small bodies circulating, as satellites, round the earth.
[pg 55]62. The rotation of the earth round its axis is such that the part furthest from the sun, for which it is therefore midnight, is moving in the same direction as the earth in its orbit; whence, at the part of the earth most forward in the orbit it is sunrise, and at the part most backward it is sunset. Thus, as Schiaparelli pointed out, the meteorites which enter the atmosphere in the first half of the night are more or less following the earth in its orbit, and have their velocity relative to the earth diminished by the earth's own motion of translation; they are thus less likely to produce shooting stars than those which enter the atmosphere in the second half of the night and are travelling more or less oppositely to the earth as it moves in its orbit, and have their relative velocity increased. Hence, if the directions of flight of meteorites were uniformly distributed in space, the number of shooting stars hourly visible at one place, a number which would be constant if the earth were at rest, would gradually vary during the night, reaching a maximum about 3 A.M.
Also, as the point in space towards which the earth is moving in its orbit varies in height above the horizon during the year, being highest in autumn and lowest in spring, the number of shooting stars hourly visible at one place will gradually vary from night to night, reaching a maximum in the former season and a minimum in the latter, if the directions of flight of the meteorites be themselves uniformly distributed in space.
63. The history of Biela's comet35 is of great interest as throwing light on the relationship of comets and swarms of meteorites. Though already observed in 1772 and in 1806, this comet was not recognised as periodic till it was seen by Biela in 1826, when its orbit was determined. On its returns in 1832 and 1845 it was found in its calculated positions, but in the latter year was seen to be double, a small comet being visible beside a larger one. Vast changes took place during the time the companions were visible. The smaller one grew both in size and brightness, each threw out a tail, the smaller threw out a second tail, afterwards the larger showed two nuclei and two tails, then the [pg 56] smaller became the brighter of the two companions; next three tails were shown by the primary, and three cometary fragments were visible round its nucleus. On the next return, in 1852, the two comets were farther apart, one being more than a million miles ahead of the other. The next favourable return was to be in 1866, and the orbit was by this time so well known that the positions of the two companions could be calculated beforehand with great precision; owing to the changes which had been visibly taking place, the arrival of the comets was looked forward to with great interest by astronomers. But neither in 1866, nor on the next occasion in 1872, were they to be seen in their calculated positions, and a careful examination of the whole sky failed to lead to their discovery.
The connexion between several comets and meteoritic swarms having in the meantime been established, it was now surmised that Biela's comet might have been scattered along part of its path, and that some evidence of the dispersal might perhaps be obtained on the next occasion, November 27, 1872, of the passage of the earth across the comet's orbit. In fact the star-shower of that date, with a radiant point corresponding to the orbit of Biela's comet, was observed to be much more dense than usual, the stars shooting across the sky at the rate of a thousand an hour for several hours.
64. Klinkerfues, a German astronomer, was struck with the idea that if this star-shower were really due to the passage of the earth through a moving swarm of meteorites, the latter might possibly be visible as it departed from our neighbourhood. The swarm having come from a radiant point in the northern sky, after passing the earth would need to be sought near the opposite point in the southern sky; he telegraphed, therefore, to the Madras observatory, asking Pogson, the astronomer, to search for the swarm in the direction opposite to the radiant point. The search was successful; on two mornings a small comet was distinctly seen, and on the second morning it showed a tail with an apparent length equal to one-fourth the apparent diameter of the moon. Bad weather came on, and the comet got away without being again seen. The two Madras observations [pg 57] agree with a motion in the orbit of Biela's comet, and show that the earth had passed excentrically through the small comet seen by Pogson. This small comet was probably a third fragment of Biela's, for it was 200 million miles behind the calculated position of the first two. From these two observations it is inferred that a swarm of meteorites, though only manifesting itself by a star-shower when passing through the earth's atmosphere, at some distance from us may be visible as a comet by reflected sunlight.
65. A dense star-shower36 recurred on the same day of the month (November 27) in 1885, the principal part being over in six hours. The hourly number visible at one place at the time of greatest density was estimated at 75,000. In the densest part of the stream, the average distance of the individuals from each other was about twenty miles.
During this star-shower a piece of iron weighing about 8 lbs. was seen to fall at Mazapil in Mexico:37 in external characters and chemical composition it is similar to the other meteoric irons: the simultaneity was probably accidental.
66. It may be asked why, if star-showers are caused by the entry of solid bodies into our atmosphere from without, there is only one authentic instance of material being actually seen to fall and being picked up during such a shower. As it is absolutely beyond question that star-showers do come from outer space, we can seek an explanation only in the size or speed of the entering individuals, or in the nature of their material. A sufficient reason is to be found in the small size of the individuals; for the meteorites which actually reach the ground rarely weigh more than a few pounds, and are often quite minute; a small diminution of the original individual would thus ensure its complete destruction before the planetary velocity was exhausted: that the individuals of a swarm are extremely minute follows from the fact that the total mass of the biggest swarm is small, while the number of the individuals seems almost infinite.
67. Between the small silent shooting star visible only with the telescope and the large detonating meteorite-yielding [pg 58] fireball there is every gradation; during the star-showers themselves many fireballs of great size and brilliancy are seen, while the smaller individuals appear in no way different from the solitary shooting star. The luminous meteors, large and small, are in the upper atmosphere, few higher than 100 miles, few lower than 30 miles from the earth's surface; they all have velocities of the same order of magnitude, comparable with that of the earth in its orbit; in each there must be a solid body, as is proved by the long path in the sky, for attendant gas or vapour would be immediately scattered or burnt; large and small present similar varieties of colour, and leave similar luminous trails; examination with the spectroscope teaches us that the light of the meteors is such as would result from the ignition of such meteorites as have actually reached the ground. The frequent absence of detonation may likewise be due in many cases to the small size, or small relative velocity, of the entering meteorite.
68. That part of the light of a comet is reflected sunlight is confirmed by examination with the spectroscope, in which instrument is seen a feeble continuous spectrum crossed by dark lines, identical with those afforded by the direct light of the sun. But a comet is also more or less self-luminous; for, in addition to the continuous spectrum, there are bright flutings and bright lines to which much attention has been given. The three ordinary bright flutings were found by Sir William Huggins in 1868 to be identical with the spectrum obtained when an electric spark is passed through olefiant gas, and they are now recognised as due to carbon. The carbon is presumed to be combined with hydrogen, sometimes also with nitrogen; in the case of comets approaching very near the sun, the lines of sodium, and others which have been supposed to be iron-lines, are seen.38
69. The discovery made by Schiaparelli proves, as already pointed out, that there is a relationship between comets and meteoritic swarms; Schiaparelli himself held the view that a comet and its attendant swarms are merely of identical [pg 59] origin. In 186939 Tait discussed, from a purely dynamical point of view, the question as to whether the swarm of meteorites attending a comet may not really be part of the comet itself; he showed that many cometary characters can be mechanically explained on the assumption that comets are really swarms of small meteorites, and pointed out that the self-luminosity may be produced by the heating of the individuals through collision with each other.
70. Flutings exactly identical with those seen in the spectrum of a comet were obtained by Professor A. W. Wright in 187540 on allowing the electric glow to pass through a heated tube, in which, after the introduction of fragments of the Iowa meteorite, the gaseous density had been reduced by an air-pump. The bright lines, too, in the spectrum of a comet, even when nearest to the sun, are found by Sir Norman Lockyer to be identical with those yielded when the electric glow is passed over ordinary meteorites at comparatively low temperatures; and further, the changes in these lines as the comet approaches and recedes from the sun are exactly those which take place on variation of the temperature of the meteorites enclosed in the glow-tubes.
71. From these facts it is inferred that a comet may be in every instance a swarm of isolated large or minute meteorites, at a not very high temperature, shining partly by reflected sunlight and partly by the electric glowing of the gases evolved owing to the action of the sun's heat on the meteorites: further, some of the heat may be due to the clashing together of the meteorites, the grouping of which becomes more and more condensed as the swarm approaches the sun.
The gases driven from the meteorites by the sun's heat would be quite sufficient in quantity to form the tail of the comet: as pointed out by Professor Wright, a meteorite like that which fell at Cold Bokkeveld would furnish 30 cubic miles of gas measured at the pressure of our own atmosphere, and in space itself this gas would expand to enormous dimensions owing to the small mass and attraction [pg 60] of the meteoritic swarm. We are still uncertain, however, as regards the actual physical condition of the matter composing the tail of a comet.
72. Clerk-Maxwell proved, as long ago as 1857, that the stability of the rings which revolve round the planet Saturn is inconsistent with their being formed of continuous solid or liquid matter; and has shown, by mechanical reasoning, that they must be revolving clouds of small separate bodies, like cannon-shot, each moving as a satellite and almost independent of the rest in its motion: determination of the motions of the inner and outer parts of the ring-system made with the help of the spectroscope supports this conclusion.
73. Reichenbach, in 1858, before the self-luminosity had been proved by means of the spectroscope, had imagined a nebula to be a cloud of isolated meteorites, illuminated by some neighbouring sun: Chladni, long before, had supposed a nebula to be a cloud of phosphorescent dust. But, in 1864, it was established by Sir William Huggins that the light is due, not to reflection or phosphorescence, but to incandescence, for the spectrum consists of bright lines such as are yielded by glowing gas. Tait,41 in 1871, suggested that the nebulæ may be clouds of mutually impinging meteorites, mingled with glowing gases developed by the impacts; he pointed out that the heat produced by the clashing of the individuals of such an immense group as a nebula evidently is would be quite adequate for the production of their light. Sir Norman Lockyer finds that the bright lines (generally accompanied by a certain amount of continuous spectrum) which have been observed in nebular spectra are consistent with this suggestion, and regards them as closely related to the low temperature lines obtained when a gentle electric glow is passed over meteorite-fragments in a tube containing gases given out by them, and of which the density has been reduced by the air-pump; further, he points out that the nebular spectrum is identical with that of the comets of 1866 and 1867 when distant from the sun. According to this suggestion, a nebula and a comet are of [pg 61] identical constitution, and a comet is merely a nebula which has become entangled in the solar system. On the other hand, Sir William Huggins has expressed (1891) the opinion that the spectrum of the bright-line nebulæ is certainly not such as we should expect to result from the collision of meteorites like those which have reached the earth, and that it is suggestive of a high temperature; he points out that the particles which have just been in collision may be at high temperatures and yet the average temperature of all the particles may be low.
74. The examination and classification of the spectra of the stars has likewise led to remarkable conclusions. Secchi, following Rutherfurd, found that the stars could be distributed into classes according to the characters of their spectra,42 and his classification has since, with little modification, been adopted by Vogel and Dunér, by whom several thousand star-spectra have now been systematically mapped. The first three classes are characterised by absorption, the fourth by radiation.
In the spectra of Class I the absorption is small and simple, the dark lines being broad and few; the stars themselves are white: in one division of this class, represented by Sirius and Vega, the principal lines are due to hydrogen; in another important division, represented by β, γ, δ, ε, ζ Orionis, lines of helium are very pronounced.
In Class II the dark lines are thinner and more numerous; the stars are bluish-white to reddish-yellow: to this class belong the Sun, Arcturus, Capella.
The absorption in Class III manifests itself predominantly as flutings, though there are also many thin lines: the stars are orange or red: in one division (a) of this class the darkest part and the sharpest edge of each fluting is towards the violet end of the spectrum, as in Betelgeux; in a smaller division (b) the darkest part of each fluting is towards the red end, as in star 152 Schjellerup; the fluting absorption of the latter division being due to carbon.
The remaining Class IV is an extremely small one: the spectra are characterised by bright lines: some of the lines [pg 62] are due to hydrogen, and others to substances not yet recognised in terrestrial chemistry.
75. Soon after the classification suggested by Secchi had been announced, it was surmised that the differences in the stars of the first three classes might be due, not so much to differences of matter, as to differences of temperature, and that a very hot star such as, from its brightness and distance, its small and simple absorption, and the development of the blue end of its spectrum, Vega is believed to be, would, on getting older and colder, pass from Class I to Class II, and thence to one or other of the divisions of Class III.
76. In 1866 a star of 9th or 10th magnitude burst into greater brilliancy and nearly reached the intensity of Vega; the spectrum showed the presence of brilliantly glowing hydrogen. Almost as suddenly the light went down again, and within a month returned to its original brightness. Ten years later, another new star of the 3rd or 4th magnitude appeared at a place in the sky where no star had been noticed before; its spectrum showed numerous bright lines; gradually, in the course of a year, it dwindled down to the 10th magnitude, then giving the telescopic appearance and the spectrum of a nebula. Several other new stars have since been observed, the most notable being Nova Persei, which appeared in 1901. In each case, as the star faded, its spectrum changed into that which is characteristic of the nebulæ.
The appearance of a new star has been generally attributed to the collision of two bodies in space; Sir Norman Lockyer43 has pointed out that the rapidity of the change in the brilliancy, so different from that of other stars, may be due to the smallness of the mass, and that such a star may be produced by the collision of two swarms of widely separated meteorites. He has shown that the changes in the spectrum as such a star varies in brightness are confirmatory of this view.
77. That the heat of our own sun was originated by the falling together of smaller bodies was, until lately, generally [pg 63] acknowledged;44 for the only other conceivable natural cause, known to exist from independent evidence, namely, chemical combination, was quite insufficient; the greatest amount of heat obtainable from the most advantageous chemical combination of any of the then known elements, having a total mass equal to that of the sun, would not cover the sun's expenditure for more than three thousand years, while there is no difficulty on the meteoritic explanation in providing a supply of heat sufficient to cover the loss by radiation during 20,000,000 years. But the discovery that compounds of radium maintain themselves at a higher temperature than that of surrounding bodies and are only inappreciably changed though continuously emitting an appreciable amount of heat, shows that the meteoritic hypothesis as to the cause of the sun's high temperature is not necessarily the true one: there may be an analogous heat-yielding material in the sun.
In any case the present loss of the sun's heat by radiation is probably not covered by the fall of bodies into the sun; for the requisite mass would, if from distant regions, visibly affect the motions of the planets by its attraction, and, even if circulating round the sun at no great distance from it, would seriously disturb the motions of some of the comets. Further, much heat will result from the shrinkage of the volume of the solar aggregate.
78. By study of the spectra, at various temperatures, of the elements and compounds found in those meteorites which have reached our earth and been preserved, Sir Norman Lockyer45 has been led to support the view that the stars are not at present all cooling down, but that some, on the contrary, are rising in temperature; he suggests that many of the stars, like the nebulæ, are constituted of separate meteorites in continual relative motion, and become hotter and hotter through contraction of the grouping, collision, and transformation of the energy of position and motion into heat. This increase of temperature must continue during [pg 64] successive ages, until the energy of position and motion of the separate meteorites is wholly transformed, the separate masses having then combined to form a single white hot body which will gradually cool down to the state in which our own moon now is. If a swarm of meteorites forming one nebula be subjected to the external action of another moving swarm of meteorites, intermediate stages resembling the conditions of Saturn and of the solar system may ensue.
According to this spectroscopic affirmation of the nebular theory, all the heavenly bodies are constituted of the same kinds of elementary matter, those in fact which are found in meteorites and our own earth, and the difference is solely due to temperature; and a nebula in its gradual passage to the lunar condition will show every phase of spectrum observed in the stars as now existent.
79. Finally, it may be asked whether or not meteorites bring us any tangible evidence of the existence of living beings outside our own world. To this we may briefly answer, that while an organic origin can scarcely be claimed for the graphite present in the meteoric irons, there are no less than six meteoric stones which contain, though in very minute quantity, carbon compounds of such a character that their presence in a terrestrial body would be regarded as doubtlessly an indirect result of animal or vegetable existence. On the other hand, the stony matter is such that in a terrestrial body an igneous origin would be assumed.
Professor Maskelyne has pointed out that these carbon compounds can be completely removed without a preliminary pulverisation of the stone, and thus seem to be contained merely in the pores; he suggested that they may have been absorbed by the stones in their passage through an atmosphere containing the compounds in a state of vapour. In any case, it is impossible to prove that there is a necessary relation between these compounds of carbon and the existence of living beings.
80. In 188046 descriptions were given of sponges, corals, [pg 65] crinoids and plants, found in several meteorites, chiefly in that of Knyahinya, but the memoir has been generally regarded as an elaborate jest. The chondrules with their excentrically radiating crystallisation are there classified and named as sponges, corals and crinoids, while the structure of meteoric iron, revealed by the Widmanstätten figures, is regarded as a result of plant life. There can be no hesitation in asserting that as yet no organised matter has been found in meteorites.
Footnotes
REPRESENTED IN THE COLLECTION ON MAY 1, 1908.
The references in the second column correspond with numbers and letters on the cases, and indicate the pane behind which the meteorite will be found.
Weights under one gram are not given. 1,000 grams are equivalent to 2·205 avdp. lbs.
I. SIDERITES
or Meteoric Irons
(consisting chiefly of nickeliferous iron, and enclosing schreibersite, troilite, graphite, &.).
A. Fall Recorded.
[Arranged chronologically.]
| No. | Pane. | Name of Meteorite and Place of Fall. | Date of Fall. | Weight in grams. | |
|---|---|---|---|---|---|
1 | 1c | Agram (Hraschina), Croatia, Austria. | May 26, | 1751. | 282 |
2 | 1c | Charlotte, Dickson County, Tennessee, U.S.A. | July 31, or
| 1835. | 77 |
3 | 1c,4l | Braunau (Hauptmannsdorf), Bohemia. | July 14, | 1847. | 554 |
4 | 1c,4l | Victoria West, Cape Colony, South Africa. | Fell in | 1862. | 153 |
5 | 1c,4h | Nedagolla, Mirangi, Vizagapatam, Madras, India. | Jan. 23, | 1870. | 4,280 |
6 | 1c | Rowton, near Wellington, Shropshire. | April 20, | 1876. | 3,109 |
7 | 1c | Mazapil, Zacatecas, Mexico. | Nov. 27, | 1885. | 14 |
8 | 1c | Cabin Creek, Johnson County, Arkansas, U.S.A. | March 27, | 1886. | 5 |
9 | 1c | N'Goureyma, Djenne, Massina, North-West Africa. | June 15, | 1900. | 871 |
B. Fall not Recorded.[pg 67]
[Arranged topographically.]
| No. | Pane. | Name of Meteorite and Place of Find. | Report of Find. | Weight in grams. |
|---|---|---|---|---|
10 | 1c | La Caille, near Grasse, Alpes Maritimes, France. For about two centuries it was in front of the church of La Caille and was used as a seat: its meteoric origin was recognised by Brard in 1828. | Acad. Sci. Bordeaux, 1829, p. 39. | 374 |
11 | 1c | São Julião de Moreira de Lima, Minho, Portugal. Known since 1883: described by Ben-Saude in 1888. | Comm. da commiss. d. trab. geol. de Portugal, 1888, vol. 2, p. 14. | 728 |
12 | 1a | Obernkirchen near Bückeburg, Schaumburg-Lippe, Germany. Found in a quarry on the Bückeberg 15 feet below the surface, and thrown aside: recognised as meteoric by Wicke and Wöhler, in 1863. | Pogg. Ann. 1863, vol. 120, p. 509. | 34,700 |
13 | 1d | Bitburg Rhenish Prussia. Dug up about 1807, taken to Trèves and put into a furnace: afterwards thrown away with the waste: later, fragments of having been recognised by Gibbs as meteoric, the mass was searched for by Nöggerath and re-discovered in 1824. | Schweigg. Journ. 1825, vol. 43, p. 1. | 1,349 |
14 | 1d,
| Seeläsgen Brandenburg, Prussia. Found in draining a field: several years afterwards, in 1847, it was met with by Hartig and recognised as meteoric. | Pogg. Ann. 1848, vol. 73, p. 329; 1849, vol. 74, p. 57. | 9,846 |
15 | 1d | Schwetz Prussia. Found in 1850 in making a road; it was about 4 feet below the surface: described by Rose in 1851. | Pogg. Ann. 1851, vol. 83, p. 594. | 1,062 |
16 | 1d | Nenntmannsdorf, Pirna, Saxony. Found in 1872 about 2 feet below the surface: reported by Geinitz in 1873. | Sitzungs-Ber. d. n. G. Isis in Dresden, 1873, p. 4. | 15 |
17 | 1d | Tabarz, near Gotha, Germany. Said to have been seen by a shepherd to fall on Oct. 18, 1854: described in 1855 by Eberhard, to whom the rust seemed incompatible with a recent fall. | Ann. Chem. Pharm. 1855, vol. 96, p. 286. | 9 |
[pg 68]18 | 1d | Elbogen, Bohemia. Preserved for centuries at the Rathhaus of Elbogen: its meteoric origin was recognised by Neumann in 1811. | Gilb. Ann. 1812, vol. 42, p. 197. | 94 |
19 | 1d | Bohumilitz, Prachin, Bohemia. Laid bare by heavy rain in 1829. | Verh. Ges. Mus. Böhm. April 3, 1830, p. 15. | 118 |
20 | 1d | Lénárto, Sáros, Hungary. Found in 1814: described by Tehel in 1815. | Gilb. Ann. 1815, vol. 49, p. 181. | 2,018 |
21 | 1d | Arva (Szlanicza), Hungary. Made known by Haidinger in 1844. | Pogg. Ann. 1844, vol. 61, p. 675. | 9,110 |
22 | 1d | Nagy-Vázsony, Veszprim, Hungary. Found in 1890: described by Brezina in 1896. | Ann. d. k. k. Naturh. Hofmus. Wien,1896, vol. 10, pp. 284, 356. | 69 |
23 | 1d | Tula (Netschaëvo), Russia. Found in 1846 in making a road: it was 2 feet below the surface: recognised as meteoric by Auerbach in 1857. | Wien. Akad. Ber., 1860 (1861), vol. 42, p. 507. | 1,076 |
24 | 1d | Sarepta, Saratov, Russia. Found in 1854: reported by Auerbach in the same year. | Bull. Soc. Nat. Moscow, 1854, p. 504. | 283 |
25 | 1d | Verkhne-Dnieprovsk, Ekaterinoslav, Russia. Found in 1876. | 24 | |
26 | 1d | Augustinovka, Ekaterinoslav, Russia. Known before 1893; fragment described by Meunier in that year. | Comptes Rendus, 1893, vol. 116, p. 1151. | 950 |
27 | 1d | Bischtübe, Nikolaev, Turgai, Russia. Found in 1888: described by Kislakovsky in 1890. | Bull. de la Soc. Imp. des Natur. de Moscou, 1890, vol. 4, p. 187. | 1,750 |
28 | 1d | Petropavlovsk (gold washings), Mrasa River, Tomsk, Asiatic Russia. Found about 32 feet from the surface: given to the Director of the Kolyvani Works in 1841 and described by Sokolovskji in the same year. | Erman's Archiv f. wiss. Kunde von Russland, 1841, vol. 1, p. 314. | 12 |
[pg 69]29 | 1d | Toubil River (Taiga), Petropavlovsk, Yeniseisk, Asiatic Russia. Found in 1891: described by Khlaponin in 1898. | Verhandl. russ.-kais. min. Ges., 1898, ser. 2, vol. 35, p. 233. | 490 |
30 | 1d | Ssyromolotovo Keshma, Yeniseisk, Asiatic Russia. Known since the year 1873: described: by Göbel in 1874. | Bull. Ac. Imp. des Sc. de St. Pétersb. 1874, vol. 19, p. 544. | 3 |
31 | 1e | Verkhne-Udinsk (Niro River), Transbaikal, Asiatic Russia. Found in 1854: noted by Buchner in 1865. | Pogg. Ann. 1865, vol. 124, p. 599. | 2,904 |
32 | 1e | Nochtuisk, Jakutsk, Asiatic Russia. Found in 1876. |
| 4 |
33 | 1b | Nejed (Wanee Banee Khaled), Central Arabia. Said to have been seen to fall in 1863; probably this is a mistake and the time of fall unknown: described by L. F. in 1887. | Mineralog. Magazine, 1887, vol. 7, p. 179. | 58,160 |
34 | 1e | Kodaikanal Palni Hills, Madura, Madras, India. Known since 1898: reported by Holland in 1900: described by Berwerth in 1906. | Proc. Asiatic Soc. of Bengal, 1900, January, p. 2. Tschermak's Min. u. Petrog. Mitth. 1906, vol. 25, p. 179. | 2,355 |
35 | 1e | Tanokami (-yama), Kurifuto-gōri, Ōmi, Japan. Found about 1885: described by Ōtsuki in 1900, and Jimbo in 1906. | Jour. Geol. Soc. Tōkyō, 1900, vol. 7, p. 85. Beiträge zur Mineralogie von Japan. Herausgegeben von T. Wada, 1906, No. 2, p. 42. | 178 |
36 | 1e | Uwet, Southern Nigeria, Africa. |
| 6,948 |
37 | 1e | Bethany, Great Namaqualand, South Africa. (a) Many large masses were reported by Alexander in 1838 to be lying N.E. of Bethany and near the Great Fish River. {None of the fragments given to Alexander seem to have been placed in Museum Collections.} L. F. | Jour. Roy. Geog. Soc. of London,1838, vol. 8, p. 24. |
|
(b) Bethany (Lion River). A large mass said to have been found near Lion River, Great Namaqualand, was described by Shepard in 1853. | Amer. Jour. Sc. 1853, ser. 2, vol. 15, p. 1. | 388 | ||
[pg 70](c) Bethany (Wild). A large mass which had long been known to the missionaries of Bethany was brought to CapeTown by Wild in 1860: described by Cohen in 1900. | Annals of the South African Mus. 1900, vol. 2, part 2, p. 21 | 1,434 | ||
(d) Bethany (Mukerop). Four large masses were met with in 1899 at Mukerop, Gibeon, Great Namaqualand: described by Brezina and Cohen in 1902. | Jahreshefte des Vereins für Vaterl. Naturk. Württ., 1902, vol. 58, p. 292. | 4,320 | ||
(e) Bethany (Springbok River). A fragment (9 grams) found with the label "Spring Bok River," among Dr. H. J. Burkart's minerals, after his death in 1874. {All the above masses may have been transported at some time or other from the place indicated by Alexander; their etched figures are similar.} L. F. | Mineralog. Magazine, 1904, vol. 14, p. 28. | 9 | ||
38 | 1e | Orange River District, South Africa. Sent from the Orange River District in 1855: described by Shepard in 1856. | Amer. Jour. Sc. 1856, ser. 2, vol. 21, p. 213. | 95 |
39 | 1e | Hex River Mountains Cape Colony, South Africa. Found in 1882: described by Brezina in 1896. | Ann. d.k.k. Naturh. Hofmus. Wien, 1896, vol. 10, pp. 291, 349. | 245 |
40 | 1e | Cape of Good Hope: between Sunday River and Bushman River (west of Great Fish River), Cape Colony, South Africa. Known long before 1793: mentioned in "Barrow's Travels into the Interior of South Africa," 1801, vol. i. p. 226: full particulars were given in 1805 by von Dankelmann. | Mag. für den neuesten Zustand der Naturkunde, von J. H. Voigt, 1805, vol. 10, p. 12. | 342 |
41 | 1e | Kokstad, Griqualand East, South Africa. | Ann. South African Mus. 1900, vol. 2, p. 9. | 243 |
42 | 1e | Prambanan Surakarta, Java. Known as early as 1797, and probably earlier: described by Baumhauer in 1866. | Arch. Néer. Haarlem, 1866, vol. 1, p. 465. | 8 |
43 | 1f | Thunda, Windorah, Diamantina District, Queensland, Australia. Described by Liversidge in 1886. | Jour. and Proc. Roy. Soc. of New South Wales, 1887, vol. 20, p. 73. | 396 |
[pg 71]44 | 1f | Mungindi, New South Wales, Australia. Found on the Queensland side of the border in 1897: mentioned by Card in 1897 and figured by Ward in 1898. | Rec. Geol. Surv. of New South Wales, 1897, vol. 5, p. 121. Amer. Jour. Sc. 1898, ser. 4, vol. 5, p. 138. | 368 |
45 | 1f | Boogaldi, Coonabarabran, New South Wales. Found in 1900: described by Baker in 1900 and by Liversidge in 1902. | Jour. and Proc. Roy. Soc. New South Wales, 1900, vol. 34, p. 81; and 1903, vol. 36, p. 341. | 179 |
46 | 1f | Cowra, Bathurst, New South Wales. Known since 1888: described by Card in 1897. | Records of the Geol. Survey of New South Wales, 1897, vol. 5, p. 51. | 192 |
47 | 1f | Narraburra, Temora, New South Wales. Found in 1855: described by Russell in 1890 and by Card in 1897. | Jour. and Proc. Roy. Soc. of New South Wales, 1890, vol. 24, p. 81. Rec. Geol. Surv. of New South Wales, 1897, vol. 5, p. 52. | 1918 |
48 | 1f | Nocoleche, Wanaaring, New South Wales. Known in 1895: described by Cooksey in 1897. | Records of the Australian Mus. 1897, vol. 3, p. 51. | 687 |
49 | 1f | Rhine Villa, Rhine Valley, South Australia. Described by Goyder in 1901. | Trans. of the Roy. Soc. of South Australia, 1901, vol. 25, p. 14. | 193 |
50 | Sep.
| Cranbourne, near Melbourne, Victoria, Australia. (a) Two large masses, found nearly four miles apart, have been known since 1854: described by Haidinger in 1861. | Wien. Akad. Ber. 1861, vol. 43, Abth. 2, p. 583. | 3,500,000 |
(b) A much smaller mass was found later at Beaconsfield, six miles from Cranbourne: described by Cohen in 1897. | Sitzungsber. k. pr. Ak. d. Wiss. zu Berlin, 1897, vol. 46, p. 1035. |
| ||
1f |
(c) {Fragments found in Abel's collection of minerals with the label "Yarra Yarra River—Date 1858" had probably been detached from one of the two masses of Cranbourne.} L. F. | 214 | ||
[pg 72]51 | 1e | Youndegin, 70 miles E. of York, Western Australia. Found in 1884: described by L. F. in 1887. | Mineralog. Magazine, 1887, vol. 7, p. 121. | 13,187 |
52 | 1f | Roebourne (200 miles south-east of), Western Australia. Found in 1892: described by Cooksey in 1897 and by Ward in 1898. | Records of the Australian Mus. 1897, vol. 3, p. 59. Amer. Jour. Sc. 1898, ser. 4, vol. 5, p. 135. | 1,502 |
53 | 1f | Mount Stirling, Western Australia. Known in 1892: described by Cooksey in 1897. | Records of the Australian Mus. 1897, vol. 3, p. 58. | 1,888 |
54 | 1f | Ballinoo, Murchison River, Western Australia. Found in 1892: described by Cooksey in 1897 and by Ward in 1898. | Records of the Australian Mus. 1897, vol. 3, p. 55. Amer. Jour. Sc. 1898, ser. 4, vol. 5, p. 136. | 3,160 |
55 | 1f | Mooranoppin, Western Australia. Found in or before 1893: described by Cooksey in 1897 and by Ward in 1898. | Records of the Australian Mus. 1897, vol. 3, p. 58. Amer. Jour. Sc. 1898, ser. 4, vol. 5, p. 140. | 261 |
56 | 4m | Melville Bay, 35 miles east of Cape York, West Greenland (Ross's iron). Two knives or lance-heads with bone handles given to Captain John Ross in 1818 by the Eskimos of Prince Regent's Bay: one of them was figured by Ross on page 102 of his work. According to the Eskimos, the iron had been obtained from a neighbouring mountain called Sowallick. | Voyage of Discovery, &., by Captain John Ross. London, 1819. |
|
The locality of the three large masses was shown by an Eskimo to Lieut. Peary in 1894: by him they were later transported to New York. | Northward over the Great Ice, by R. E. Peary. London, 1898, vol. 2, p. 556. |
| ||
57 | 1f | Madoc, Hastings County, Ontario, Canada. Found in 1854: described by Hunt in 1855. | Amer. Jour. Sc. 1855, ser. 2, vol. 19,p. 417. | 205 |
[pg 73]58 | 1f | Welland, Ontario, Canada. Ploughed up in 1888: described by Howell in 1890. | Proc. Rochester Ac. of Sc. 1890, vol. 1, p. 86. | 466 |
59 | 1f | Thurlow, Hastings County, Ontario, Canada. Found in 1888: described by Hoffmann in 1897. | Amer. Jour. Sc. 1897, ser. 4, vol. 4, p. 325. | 189 |
60 | 1f | Iron Creek, Battle River, North Saskatchewan, Canada. Removed about 1869: described by Coleman in 1886. | Proc. and Trans. Roy. Soc. of Canada, 1887, vol. 4, sec. 3, p. 97. | 79 |
61 | 1h | Lockport (Cambria), Niagara County, New York, U.S.A. Turned up by plough: described as meteoric by Silliman in 1845. | Amer. Jour. Sc. 1845, ser. 1, vol. 48, p. 388. | 5,329 |
62 | 4l | Seneca River, Cayuga County, New York, U.S.A. | Amer. Jour. Sc. 1852, ser. 2, vol. 14, p. 439. | 54 |
63 | 1g, | Burlington, Otsego County, New York, U.S.A. Turned up by plough some time previous to 1819, and described by Silliman in 1844. | Amer. Jour. Sc. 1844, ser. 1, vol. 46, p. 401. | 290 |
64 | 1g | Pittsburg (Miller's Run), Alleghany County, Pennsylvania, U.S.A. Described by Silliman in 1850: date of find unknown. | Proc. Amer. Assoc. Fourth Meeting, held Aug. 1850, vol. 4, p. 37. | 208 |
65 | 1g | Mount Joy, Adams County, Pennsylvania, U.S.A. Found in 1887: described by Howell in 1892. | Amer. Jour. Sc. 1892, ser. 3, vol. 44, p. 415. | 730 |
66 | 1g | Emmittsburg, Frederick County, Maryland, U.S.A. Found in 1854. | 6 | |
[pg 74]67 | 1g | Staunton, Augusta County, Virginia, U.S.A. Five masses have been found. Three masses, of which two at least were found in 1869, were described by Mallet in 1871. | Amer. Jour. Sc. 1871, ser. 3, vol. 2, p. 10. | 2,893 |
A fourth was found about 1858-9, thrown away, used in the construction of a stone fence, then as an anvil; was next built into a wall: in 1877 it was taken out, and its meteoric nature was recognised by Mallet. | Amer. Jour. Sc. 1878, ser. 3, vol. 15, p. 337. |
| ||
A fifth was described by Kunz in 1887. | Amer. Jour. Sc. 1887, ser. 3, vol. 33, p. 58. |
| ||
68 | 1g | Indian Valley Township, Floyd County, Virginia, U.S.A. Found in 1887: described by Kunz and Weinschenk in 1891. | Tschermak's Min. u. Petrog. Mitth. 1891, vol. 12, p. 182. | 82 |
69 | 1g | Greenbrier County (near the summit of the Alleghany Mountain, 3 miles north of White Sulphur Springs), West Virginia, U.S.A. Found about 1880: described by L. F. in 1887. | Mineralog. Magazine, 1887, vol. 7, p. 183. | 2,238 |
70 | 1g | Jenny's Creek, Wayne County,West Virginia, U.S.A. The first piece was found before the Spring of 1883 and lost sight of; two other pieces were found in 1883 and 1885 respectively: reported by Kunz in 1885. | Proc. Amer. Assoc. for the year 1885, vol. 34, p. 246. | 78 |
71 | 1h | Smith's Mountain, Rockingham County, N. Carolina, U.S.A. Reported by Genth in 1875 to have been found in 1866. | Rep. Geol. Surv. N. Carolina, by Kerr: Raleigh, 1875, vol. 1, app. C, p. 56. | 77 |
Reported by Smith in 1877 to have passed into the hands of Kerr about 1863. | Amer. Jour. Sc. 1877, ser. 3, vol. 13, p. 213. |
| ||
No mention of date of find by Genth when describing the meteorite in 1885. | Minerals and Mineral Localities of North Carolina, by Genth and Kerr: Raleigh, 1885, p. 15. |
| ||
[pg 75]72 | 1h | Deep Springs (farm), Rockingham County, N. Carolina, U.S.A. Known since about 1846: described by Venable in 1890. | Amer. Jour. Sc. 1890, ser. 3, vol. 40, p. 161. | 170 |
73 | 1h | Guilford County, N. Carolina, U.S.A. Date of find unknown: first described by Shepard as terrestrial in 1830, but in 1841 its meteoric origin was recognised by him. | Amer. Jour. Sc. 1830, ser. 1, vol. 17, p. 140; and 1841, vol. 40, p. 369. | 15 |
74 | 1h | Lick Creek, Davidson County, North Carolina, U.S.A. Found in 1879: described by Hidden in 1880. | Amer. Jour. Sc. 1880, ser. 3, vol. 20, p. 324. | 19 |
75 | 1h | Linnville Mountain, Burke County, N. Carolina, U.S.A. Found about 1882: described by Kunz in 1888. | Amer. Jour. Sc. 1888, ser. 3, vol. 36, p. 275. | 21 |
76 | 1h | Ellenboro', Rutherford County, N. Carolina, U.S.A. Found in 1880: described by Eakins in 1890. | Amer. Jour. Sc. 1890, ser. 3, vol. 39, p. 395. | 52 |
77 | 1h | Bridgewater, Burke County, N. Carolina, U.S.A. Found by a ploughman: described by Kunz in 1890. | Amer. Jour. Sc. 1890, ser. 3, vol. 40, p. 320. | 51 |
78a | 1h, | Jewell Hill, Walnut Mtns., Madison County, N. Carolina, U.S.A. (a) One was given to Smith in 1854, and described by him in 1860. | Amer. Jour. Sc. 1860, ser. 2, vol. 30, p. 240; and Orig. Res. in Min. and Chem. by Lawrence Smith, 1884, p. 409. | 130 |
78b | 1h | (b) A second was found in use in 1873, supporting a corner of a rail-fence: described as from Duel Hill by Burton in 1876. The etched figures are different for the two masses. | Amer. Jour. Sc. 1876, ser. 3, vol. 12, p. 439. The Minerals and Mineral Localities of North Carolina, by Genth and Kerr: Raleigh, 1885, p. 14. | 12 |
79 | 1h | Black Mountain, 15 m. E. of Asheville, Buncombe County, N. Carolina, U.S.A. Found about 1839, and described by Shepard in 1847. | Amer. Jour. Sc. 1847, ser. 2, vol. 4, p. 82. | 71 |
[pg 76]80 | 1h | Asheville (Baird's Plantation, 6 m. N. of), Buncombe County, N. Carolina, U.S.A. Found loose in the soil: described by Shepard in 1839. | Amer. Jour. Sc. 1839, ser. 1, vol. 36, p. 81; and 1847, ser. 2, vol. 4, p. 79. | 111 |
81 | 1h | Murphy, Cherokee County, N. Carolina, U.S.A. Found in 1899: described in the same year by Ward. | Amer. Jour. Sc. 1899, ser. 4, vol. 8, p. 225. | 1,521 |
82 | 1k | Chesterville, Chester County, S. Carolina, U.S.A. Ploughed up several years before 1849, when it was described by Shepard. | Amer. Jour. Sc. 1849, ser. 2, vol. 7, p. 449. | 2,197 |
83 | 1k | Laurens County, S. Carolina, U.S.A. Found in 1857: described by by Hidden in 1886. | Amer. Jour. Sc. 1886, ser. 3, vol. 31, p. 463. | 61 |
84 | 1k | Ruff's Mountain, Lexington County, S. Carolina, U.S.A. Date of find not stated: described by Shepard in 1850. | Amer. Jour. Sc. 1850, ser. 2, vol. 10, p. 128. | 499 |
85 | 1k | Lexington County, S. Carolina, U.S.A. Found in 1880: described by Shepard in 1881. | Amer. Jour. Sc. 1881, ser. 3, vol. 21, p. 117. | 271 |
86 | 1k | Union County, Georgia, U.S.A. Found in 1853: described by Shepard in 1854. | Amer. Jour. Sc. 1854, ser. 2, vol. 17, p. 328. | 55 |
87 | 1k | Whitfield County (Dalton), Georgia, U.S.A. First specimen found in 1877: particulars of find, and description, given by Hidden in 1881. | Amer. Jour. Sc. 1881, ser. 3, vol. 21, p. 286. | 288 |
A second specimen was found in 1879, and described by Shepard in 1883. | Amer. Jour. Sc. 1883, ser. 3, vol. 26, p. 337. |
| ||
88 | 1l | Losttown (2½ m. S.W. of), Cherokee County, Georgia, U.S.A. Ploughed up in 1868: described in the same year by Shepard. | Amer. Jour. Sc. 1868, ser. 2, vol. 46, p. 257. | 6 |
[pg 77]89 | 1l | Canton, Cherokee County, Georgia, U.S.A. Ploughed up in 1894: described by Howell in 1895. According to Brezina, Canton and Losttown probably belong to the same fall. | Amer. Jour. Sc. 1895, ser. 3, vol. 50, p. 252. | 330 |
90 | 1l | Holland's Store, Chattooga County, Georgia, U.S.A. Found in 1887: described by Kunz in the same year. | Amer. Jour. Sc. 1887, ser. 3, vol. 34, p. 471. | 204 |
91 | 1l | Forsyth County, Georgia (notN. Carolina), U.S.A. Found about 1892: described by Schweinitz in 1896 and Cohen in 1897; the former gives the State as "N. Carolina." | Amer. Jour. Sc. 1896, ser. 4, vol. 1, p. 208.
| 324 |
92 | 1l | Locust Grove, Henry County, Georgia, (? N. Carolina), U.S.A. Found in 1857: described by Cohen in 1897, who gives the State as "N. Carolina." | Sitzungsber. k. pr. Ak. d. Wiss. zu Berlin, 1897, p. 76. | 365 |
93 | 1l | Putnam County, Georgia, U.S.A. Found in 1839: described by Willet in 1854. | Amer. Jour. Sc. 1854, ser. 2, vol. 17, p. 331. | 112 |
94 | 1l | Chulafinnee, Cleberne County, Alabama, U.S.A. Ploughed up in 1873: described by Hidden in 1880. | Amer. Jour. Sc. 1880, ser. 3, vol. 19, p. 370. | 60 |
95 | 1l | Auburn, Lee (not Macon) County, Alabama, U.S.A. Ploughed up some years before 1869, when it was described by Shepard. | Amer. Jour. Sc. 1869, ser. 2, vol. 47, p. 230. | 37 |
96 | 1l | Summit, Blount County, Alabama, U.S.A. Known since 1890: described by Kunz in the same year. | Amer. Jour. Sc. 1890, ser. 3, vol. 40, p. 322. | 47 |
97 | 1h | Walker County, Alabama, U.S.A. Found in 1832: described by Troost in 1845. | Amer. Jour. Sc. 1845, ser. 1, vol. 49, p. 344. | 22,040 |
98 | 1l | Claiborne (Lime Creek), Clarke County, Alabama, U.S.A. Mentioned in 1834: described by Jackson in 1838. | Amer. Jour. Sc. 1838, ser. 1, vol. 34, p. 332. | 19 |
[pg 78]99 | 1l | Tombigbee River, Choctaw and Sumter Counties, Alabama, U.S.A. Various masses found about 1859 and afterwards: described by Foote in 1899. | Amer. Jour. Sc. 1899, ser. 4, vol. 8, p. 153. | 7,875 |
100 | 1l | Oktibbeha County, Mississippi, U.S.A. Found in an Indian tumulus: described by Taylor in 1857. | Amer. Jour. Sc. 1897, ser. 2, vol. 24, p. 293. | — |
101 | 1l | Cocke County (Cosby's Creek), Tennessee, U.S.A. Described in 1840 by Troost: date of find unknown. | Amer. Jour. Sc. 1840, ser. 1, vol. 38, p. 253. | 50,460 |
102 | 1l | Babb's Mill, Green County, Tennessee, U.S.A. Turned up by a plough: first mentioned in 1842: described by Troost in 1845. | Amer. Jour. Sc. 1845, ser. 1, vol. 49, p. 342. | 2,127 |
103 | 1l | Tazewell, Claiborne County, Tennessee, U.S.A. Turned up by a plough in 1853: described by Shepard in 1854. | Amer. Jour. Sc. 1854, ser. 2, vol. 17, p. 325. | 336 |
104 | 1l | Waldron Ridge, Claiborne County, Tennessee, U.S.A. Known since 1887: described by Kunz in the same year. | Amer. Jour. Sc. 1887, ser. 3, vol. 34, p. 475. | 70 |
105 | 1l | Cleveland, Bradley County, Tennessee, U.S.A. This mass was acquired in 1867 by Lea, and described by Genth in 1886. | Proc. Ac. Nat. Sc. Philad. 1886, p. 366. | 209 |
106 | 1l | Jackson County, Tennessee, U.S.A. Date of find unknown: described in 1846 by Troost. | Amer. Jour. Sc. 1846, ser. 2, vol. 2, p. 357. | 91 |
107 | 1m | Carthage, Smith County, Tennessee, U.S.A. Found about 1844: described in 1846 by Troost. | Amer. Jour. Sc. 1846, ser. 2, vol. 2, p. 356. | 24,610 |
108 | 1l | Caney Fork, De Kalb County, Tennessee, U.S.A. Turned up by a plough, near the mouth of the Caney Fork ("Caryfort"), date not mentioned: described by Troost in 1845. | Amer. Jour. Sc. 1845, ser. 1, vol. 49, p. 341. | 4 |
[pg 79]109 | 1l | Smithville, De Kalb County, Tennessee, U.S.A. Three masses were ploughed up in 1892-3: described by Huntington in 1894. | Proc. Amer. Ac. Arts & Sci. 1894: new series, vol. 21, p. 251. | 1,683 |
110 | 1l | Murfreesboro', Rutherford County, Tennessee, U.S.A. Found about 1847-8: described in 1848 by Troost. | Amer. Jour. Sc. 1848, ser. 2, vol. 5, p. 351. | 2,790 |
111 | 1l | Coopertown, Robertson County, Tennessee, U.S.A. Sent to Smith in 1860: described by him in 1861. | Amer. Jour. Sc. 1861, ser. 2, vol. 31, p. 266. | 179 |
112 | 1m | Kenton County (8 miles south of Independence), Kentucky, U.S.A. Found in 1889: described by Preston in 1892. | Amer. Jour. Sc. 1892, ser. 3, vol. 44, p. 163. | 2,520 |
113 | 1m, | Lagrange, Oldham County, Kentucky, U.S.A. Found in 1860: described by Smith in 1861. | Amer. Jour. Sc. 1861, ser. 2, vol. 31, p. 265. | 216 |
114 | 1m | Frankfort (8 miles S.W. of), Franklin County, Kentucky, U.S.A. Found in 1866: described (1870) by Smith. | Amer. Jour. Sc. 1870, ser. 2, vol. 49, p. 331. | 216 |
115 | 1m, | Salt River, about 20 miles below Louisville, Kentucky, U.S.A. Date of find not mentioned: described by Silliman in 1850. | Proc. Amer. Assoc. Fourth Meeting, held Aug. 1850, vol. 4, p. 36. | 524 |
116 | 1m, | Nelson County, Kentucky, U.S.A. Turned up by a plough in 1860: described by Smith in the same year. | Amer. Jour. Sc. 1860, ser. 2, vol. 30, p. 240. | 4,341 |
117 | 1m | Casey County, Kentucky, U.S.A. Mentioned in 1877 by Smith. | Amer. Jour. Sc. 1877, ser. 3, vol. 14, p. 246. | 45 |
118 | 1m | Scottsville, Allen County, Kentucky, U.S.A. Found in 1867: described by Whitfield in 1887. | Amer. Jour. Sc. 1887, ser. 3, vol. 33, p. 500. | 404 |
119 | 1m | Smithland, Livingston County, Kentucky, U.S.A. Found about 1839-40, and described in 1846 by Troost. | Amer. Jour. Sc. 1846, ser. 2, vol. 2, p. 357. | 2,545 |
[pg 80]120 | 1m | Marshall County, Kentucky, U.S.A. Described by Smith in 1860. | Amer. Jour. Sc. 1860, ser. 2, vol. 30, p. 240. | 80 |
121 | 1m | Wayne County (near Wooster), Ohio, U.S.A. Found about 1858: described by Smith in 1864. | Amer. Jour. Sc. 1864, ser. 2, vol. 38, p. 385. | 5 |
122 | 1m | Grand Rapids, Kent County, Michigan, U.S.A. Found in 1883 about 3 feet below the surface: reported by Eastman in 1884. | Amer. Jour. Sc. 1884, ser. 3, vol. 28, p. 299. | 1,135 |
123 | 1m | Reed City, Osceola County, Michigan, U.S.A. Found in 1895: described by Preston in 1903. | Proc. Rochester Ac. U.S.A. of Sc., 1903, vol. 4, p. 89. | 876 |
124 | 1m | Howard County (7 miles S.E. of Kokomo), Indiana, U.S.A. Found in 1862 or 1870 at a depth of 2 feet: described by Cox in 1872 and by Smith in 1874. | Amer. Jour. Sc. 1873, ser. 3, vol. 5, p. 155; and 1874, ser. 3, vol. 7, p. 391. | 45 |
125 | 1m | Plymouth, Marshall County, Indiana, U.S.A. Found in 1893 by a ploughman: described by Ward in 1895. | Amer. Jour. Sc. 1895, ser. 3, vol. 49, p. 53. | 445 |
126 | 1m | Independence County (about 7 miles east of Batesville), Arkansas, U.S.A. Found in 1884: described by Hidden in 1886. | School of Mines Quarterly, 1886, vol. 7, No. 2, Jan., p. 188. | 372 |
127 | 1m | South-East Missouri, U.S.A. Found in 1863 in the Museum of 1869, St. Louis, labelled "South-East, Missouri": reported by Shepard in 1869. | Amer. Jour. Sc. ser. 2, vol. 47 p. 233. | 102 |
128 | 1n | St. Genevieve County, Missouri, U.S.A. Found in 1888: described by Ward in 1901. | Proc. Rochester Ac. of Sci., 1901, vol. 4, p. 65. | 6,445 |
129 | 1p | Central Missouri, U.S.A. Found about 1850-60: described 1900, by Preston in 1900. | Amer. Jour. Sc. ser. 4, vol. 9, p. 285. | 988 |
[pg 81]130 | 1n | Butler, Bates County, Missouri, U.S.A. Turned up by a plough: long afterwards came to the knowledge of Broadhead, who mentioned it in 1875. | Amer. Jour. Sc. 1875, ser. 3, vol. 10, p. 401. | 389 |
131 | 1n | Billings, Christian County, Missouri, U.S.A. Found in 1903: described by Ward in 1905. | Amer. Jour. Sc. 1905, ser. 4, vol. 19, p. 240. | 633 |
132 | 1n | Arlington, Sibley County, Minnesota, U.S.A. Found in 1894: described by Winchell in 1896. | Amer. Geologist, 1896, vol. 18, p. 267. | 56 |
133 | 1n | Trenton, Washington County, Wisconsin, U.S.A. Turned up by a plough in 1858: described by Dörflinger in 1868. | Smithson. Rep. for 1869: p. 417. | 223 |
134 | 1n | Hammond Township, St. Croix County, Wisconsin, U.S.A. Ploughed up in 1884: described by Fisher in 1887. | Amer. Jour. Sc. 1887, ser. 3, vol. 34, p. 381. | 62 |
135 | 1n | Algoma, Kewaunee County, Wisconsin, U.S.A. Found in 1887: described by Hobbs in 1902 (1903). | Bull. Geol. Soc. America, 1903, vol. 14, p. 97. | 18 |
136 | 1n | Dakota, U.S.A. Described in 1863 by Jackson. | Amer. Jour. Sc. 1863, ser. 2, vol. 36, p. 259. | 224 |
137 | 1n | Jamestown (15 or 20 miles south-east of), Stutsman County, N. Dakota, U.S.A. Found in 1885: described by Huntington in 1890. | Proc. Amer. Ac. Arts & Sci. 1890, vol. 25 (new ser., vol. 17), p. 229. | 1,627 |
138 | 1n | Niagara, Grand Forks County, N. Dakota, U.S.A. Found in 1879: described by Preston in 1902. | Jour. of Geology, 1902, vol. 10, p. 518. | 17 |
139 | 1n | Nebraska (25 m. N.W. of Fort Pierre), Dakota, U.S.A. Brought away in 1857: described by Holmes in 1860. | Trans. of St. Louis Acad. of Sc. 1857-60, vol. 1, p. 711. | 2,016 |
140 | 1n | Crow Creek, Laramie County, Wyoming, U.S.A. Found in 1887: described by Kunz in 1888. | Amer. Jour. Sc. 1888, ser. 3, vol. 36, p. 276. | 583 |
[pg 82]141 | 1n | Illinois Gulch, Deer Lodge County, Montana, U.S.A. Found in 1899: described by Preston in 1900. | Amer. Jour. Sc. 1900, ser. 4, vol. 9, p. 201. | 637 |
142 | 1n | Tonganoxie, Leavenworth County, Kansas, U.S.A. Found in 1886: described by Bailey in 1891. | Amer. Jour. Sc. 1891, ser. 3, vol. 42, p. 385. | 260 |
143 | 1n | Russel Gulch, Gilpin County, Colorado, U.S.A. Found in 1863: described in 1866 by Smith. | Amer. Jour. Sc. 1866, ser. 2, vol. 42, p. 218. | 245 |
144 | 1n | Bear Creek, Denver, Colorado, U.S.A. Found in 1866: described by Shepard in the same year. | Amer. Jour. Sc. 1866, ser. 2, vol. 42, pp. 250, 286. | 52 |
145 | 1n | Franceville, El Paso County, Colorado, U.S.A. Found in 1890: described by Preston in 1902. | Proc. Rochester Ac. of Sci., 1902, vol. 4, p. 75. | 772 |
146 | 1n | Hayden Creek, Lemhi County, Idaho, U.S.A. Known in 1895: described by Hidden in 1900. | Amer. Jour. Sc. 1900, ser. 4, vol. 9, p. 367. | 79 |
147 | 1m | Willamette, Clackamas County, Oregon, U.S.A. Found in 1902: described by Ward in 1904 and by Hovey in 1906. | Proc. Rochester Ac. of Sci., 1904, vol. 4, p. 137.
| 976 |
148 | 1o | Canyon City, Trinity County, California, U.S.A. Found in 1875: described by Shepard in 1885 and by Ward in 1904. | Amer. Jour. Sc. 1885, ser. 8, vol. 29, p. 469; and 1904 ser. 4, vol. 17, p. 383. | 193 |
149 | 1o | Oroville, Butte County, California, U.S.A. Found in 1893. |
| 373 |
150 | 1o | Shingle Springs, El Dorado County, California, U.S.A. Found 1869-70: described by Silliman in 1873. | Amer. Jour. Sc. 1873, ser. 3, vol. 6, p. 18. | 84 |
[pg 83]151 | 1o | Ivanpah, San Bernardino County, California, U.S.A. Described by Shepard in 1880, shortly after its discovery. | Amer. Jour. Sc. 1880, ser. 3, vol. 19, p. 381 | 33 |
152 | 1o | Surprise Springs, Bagdad, San Bernardino County, S. California, U.S.A. Found in 1899: described by Cohen in 1901. | Mittheil. naturw. Verein für Neu-Vorpommern und Rügen, 1902, Jahrg. 33, p. 29. | 97 |
153 | Sep. | Cañon Diablo, Arizona, U.S.A. Found in 1891: described by Foote in the same year. | Amer. Jour. Sc. 1891, ser. 3, vol. 42, p. 413. | 83,369 |
154 | 1n | Weaver's Mountains, Wickenburg, Arizona, U.S.A. Found in 1898. |
| 155 |
155 | 1o | Tucson, Arizona, U.S.A. Two large masses, long preserved at Tucson, had been transported to that town from the Puerto de los Muchachos, a pass about 20 or 30 miles south of Tucson. Their existence has been known for centuries. One of them has been termed the Signet or Irwin-Ainsa iron, the other the Carleton iron. | Mineralog. Magazine, 1890, vol. 9, p. 16. | 161 282 |
156 | 1o | Costilla Peak, Cimarron Range, New Mexico, U.S.A. Found in 1881 by a sheep-herder: described by Hills in 1895. | Proc. Colorado Scient. Soc. 1895, vol. 5, p. 121. | 1,595 |
157 | 1o | Capitan Range, New Mexico, U.S.A. Found in 1893 by a sheep-herder: described by Howell in 1895. | Amer. Jour. Sc. 1895, ser. 3, vol. 50, p. 253. | 956 |
158a | 1o | Glorieta Mountain, 1 m. N.E. of Canoncito, Santa Fé County, New Mexico, U.S.A. Found in 1884: described by Kunz in 1885. | Amer. Jour. Sc. 1885, ser. 3, vol. 30, p. 235. | 1,528 |
158b | 1o | A specimen probably from this locality was sent in 1884 to Denver from Albuquerque, New Mexico, as silver bullion: described by Pearce and Eakins in 1884-5. | Proc. Colorado Scient. Soc. 1884, vol. 1, p. 110; 1885, vol. 2, pp. 14, 35. | 61 |
[pg 84]159 | 1o | Sacramento Mountains, Eddy County, New Mexico, U.S.A. Known in 1896: described by Foote in 1896 (1897). | Amer. Jour. Sc. 1897, ser. 4, vol. 3, p. 65. | 14,050 |
160 | 1o | Luis Lopez, Socorro County, New Mexico, U.S.A. Found in 1896: described by Preston in 1900. | Amer. Jour. Sc. 1900, ser. 4, vol. 9, p. 283. | 425 |
161 | 1o | Oscuro Mountain, Socorro County, New Mexico, U.S.A. Found in 1895: described by Hills in 1897. | Proc. Colorado Scient. Soc. 1897, vol. 6, p. 30. | 494 |
162 | 1o | Brazos River, Wichita County, Texas, U.S.A. Known to the Comanches for many years: removed in 1836: described by Shumard in 1860, and by Mallet in 1884. | Trans. of St. Louis Acad. of Sc. 1857-60, vol. 1, p. 622.
| 1,397 |
163 | 1o | Denton County, Texas, U.S.A. After discovery it remained with a blacksmith for several months; in 1859 it came into the possession of Shumard, by whom it was described in the following year. | Trans. of St. Louis Acad. of Sc. 1857-60, vol. 1, p. 623. | 122 |
164 | 1o | Red River (Cross Timbers), Johnson County, Texas, U.S.A. Mentioned in 1808 to Captain Glass, and reported by Gibbs in 1814. | Amer. Min. Jour. by Bruce: 1814, vol. 1, pp. 124, 218.
| 507 |
165 | 1n | Carlton, Hamilton County, Texas, U.S.A. Ploughed up in 1887-8: described by Howell in 1890. | Proc. Rochester Ac. of Sc., 1890, vol. 1, p. 87.
| 6,180 |
166 | 1o | Kendall County, San Antonio, Texas, U.S.A. Mentioned in 1887 by Brezina, and fully described later by Brezina and Cohen. | Ann. d. k. k. Naturhist. Hofmuseums, 1887, band II., Notizen, p. 115;
| 556 |
167 | 1o | Mart, McLennan County, Texas, U.S.A. Found in 1898: described by Merrill and Stokes in 1899 (1900). | Proc. Washington Acad. Sci. 1900, vol. 2, p. 51. | 430 |
[pg 85]168 | 1o | San Angelo, Tom Green County, Texas, U.S.A. Found in 1897: described by Preston in 1898. | Amer. Jour. Sc. 1898, ser. 4, vol. 5, p. 269. | 771 |
169 | 1o | Fort Duncan, Maverick County, Texas, U.S.A. Found in 1882: described by Hidden in 1886: similar to Coahuila; perhaps transported from the same district by way of Santa Rosa. | Mineralog. Magazine, 1890, vol. 9, p. 116. | 4,520 |
170a | 2c | Coahuila, Mexico. Since 1837 many masses have been brought to Santa Rosa, from a district of small area about 90 miles north-west of that town. An account of a visit by Hamilton was published by Shepard in 1866; he designated the iron by the name Bonanza: eight large masses were removed to the United States by Butcher in 1868. | Mineralog. Magazine, 1890, vol. 9, p. 107. | 246,924 |
170b | 2c | Sanchez Estate, Coahuila, Mexico. Found in 1853 by Couch in use as an anvil at Saltillo. It was said to have been brought to that town from the "Sancha Estate," but had probably been acquired still earlier at Santa Rosa, and been got at the north-west locality. | Mineralog. Magazine, 1890, vol. 9, p. 113. | 572 |
171 | 2c | Sierra Blanca, Huejuquilla or Jimenez, Chihuahua, Mexico. The occurrence at Sierra Blanca was recorded in 1784: the only specimen known—that from the Bergemann collection—is now thought to be of doubtful authenticity; in its etched figures it is like Toluca. | Mineralog. Magazine, 1890, vol. 9, p. 149. | 15 |
172 | 2c | Concepcion: (Huejuquilla or Jimenez, Chihuahua, Mexico). Masses of iron, some of them probably belonging to one fall, have been known for centuries to exist near Huejuquilla: the mass is said to have been transported to Concepcion from Sierra de las Adargas in 1780. | Mineralog. Magazine, 1890, vol. 9, p. 140. | 47 |
173 | 2c | Chupaderos, Chihuahua, Mexico. Mentioned to Bartlett in 1852. | Mineralog. Magazine, 1890, vol. 9, p. 148. | 1,087 |
[pg 86]174 | 2c | Casas Grandes (de Malintzin), Chihuahua, Mexico. Reported by Tarayre in 1867. | Mineralog. Magazine, 1890, vol. 9, p. 119. | 989 |
175 | 2c | Moctezuma, Sonora, Mexico. |
| 170 |
176 | 2c | Arispe, Sonora, Mexico. Found in 1898: described by Ward in 1902 and Wuensch in 1903. | Proc. Rochester Ac. Sci. 1902, vol. 4, p. 79.
| 1,910 |
177 | 2c | El Ranchito, Bacubirito, Sinaloa, Mexico. Found in 1871: described by Castillo in 1889. | Mineralog. Magazine, 1890, vol. 9, p. 151. | 1,085 |
178 | 1a | Rancho de la Pila, Labor de Guadalupe, Durango, Mexico. Ploughed up in 1882: described by Häpke in 1883. | Mineralog. Magazine, 1890, vol. 9, p. 153. | 44,220 |
179 | 2c | Cacaria, Durango, Mexico. Reported by Castillo in 1889: described by Cohen in 1900. | Mineralog. Magazine, 1890, vol. 9, p. 154.
| 310 |
180 | 2b | San Francisco del Mezquital, Durango, Mexico. Brought from Mexico by General Castelnau, and described in 1868 by Daubrée. The above is the old name for the capital of Mezquital. | Mineralog. Magazine, 1890, vol. 9, p. 154. | 7,095 |
181 | 2c | Bella Roca, Sierra de San Francisco, Santiago Papasquiaro, Durango, Mexico. Acquired by Ward in 1888: described by Whitfield in 1889. | Amer. Jour. Sci. 1889, ser. 3, vol. 37, p. 439. | 3,537 |
182 | 2c | Rodeo, Durango, Mexico. Found about 1852: described by Farrington in 1905. | Field Columbian Museum. Publication 101. Geol. series 1905, vol. 3, No. 1. | 409 |
183 | 2c,2p | Descubridora, Catorce, San Luis Potosi, Mexico. Found before 1780, and described by a committee in 1872. | Mineralog. Magazine, 1890, vol. 9, p. 157. | 4,474 |
[pg 87]184 | 4l | Charcas, San Luis Potosi, Mexico. Mentioned in 1804 by Sonneschmid; it was then at the corner of the church, and was said to have been brought from San José del Sitio, 12 leagues distant. In 1866 it was removed to Paris. | Mineralog. Magazine, 1890, vol. 9, p. 160. | 333 |
185 | 2c,4l | Zacatecas, Mexico. Mentioned in 1792; it was said to have been found long before near the Quebradilla Mine. | Mineralog. Magazine, 1890, vol. 9, p. 162. | 3,848 |
186 | 1a | Toluca, Mexico. Before 1776 it was known that masses of iron occurred in the neighbourhood of Xiquipilco, Valley of Toluca. | Mineralog. Magazine, 1890, vol. 9, p. 164. | 120,089 |
187 | 2c | Cuernavaca, Morelos, Mexico. Mentioned by Castillo in 1889. | Mineralog. Magazine, 1890, vol. 9, p. 168. | 1,024 |
188 | 2c | Yanhuitlan, Misteca alta, Oaxaca, Mexico. Mentioned by Del Rio in 1804. | Mineralog. Magazine, 1890, vol. 9, p. 171. | 316 |
189 | 2c | Apoala, Oaxaca, Mexico. Found in 1889: mentioned by Cohen in 1900. | Cohen, Meteoritenkunde, 1905, Heft III., p. 384. | 283 |
190 | 2d | Rosario, Honduras, Central America. Found in 1897. |
| 126 |
191 | Lucky Hill, St. Elizabeth, Jamaica. Found in 1885 about 2 feet elow the surface. |
| Rusted. | |
192 | 2d | Santa Rosa (Tocavita), near Tunja, Boyaca River, Colombia, S. America. (a) In 1824 Rivero and Boussingault made known a large mass of iron in use as an anvil at Santa Rosa. | Ann. Chim. Phys. 1824, vol. 25, p. 438. | 996 |
In 1874 the mass was placed on a pillar in the market-place of Santa Rosa (de Viterbo); in 1906 the town was visited by Ward, who then obtained a large piece of the mass. (b) With other small pieces it had been found on a neighbouring hill, called Tocavita, in 1810: Rivero and Boussingault collected several specimens themselves. The large mass and the other small pieces have different characters. | Amer. Jour. Sc. 1907, ser. 4, vol. 23, p. 1. | 105 | ||
[pg 88]193 | 2d | Rasgata, Colombia, S. America. Other masses of iron were seen by Rivero and Boussingault at Rasgata, and were said to have been found there. | Ann. Chim. Phys. 1824, vol. 25, p. 442. | 58 |
194 | 2b | El Inca mass, from Pampa de Tamarugal, Iquique, Chili. Found in 1903: of "octahedral" structure, described by Rinne and Boeke in 1907. | Neues Jahrb. f. Min. Festband, 1907, p. 227. | 6,235 |
A fragment, having "cubic" structure, from a large mass lying at a place similarly defined had been described by Rose in 1873. | Festsch. zur Feier d. hundertjähr. Bestehens d. Gesellsch. Naturf. Freunde zu Berlin, 1873, p. 33. |
| ||
195 | 2d | Tarapaca, Chili, S. America. Known since 1894. |
| 14 |
196 | 2d | La Primitiva, Desert of Tarapaca, Chili, S. America. Known in 1888: mentioned by Howell in 1890. | Proc. Rochester Ac. Sci. 1890, vol. 1, p. 100. | 78 |
197 | 2a | Mount Hicks, Mantos Blancos, about 40 miles from Antofagasta, Atacama, Chili. Found about 1876, and described by L. F. in 1889. | Mineralog. Magazine, 1889, vol. 8, p. 257. | 9,015 |
198 | 2d | Serrania de Varas, Atacama, Chili. Found about 1875, and described by L. F. in 1889. | Mineralog. Magazine, 1889, vol. 8, p. 258. | 1468 |
199 | 2d | San Cristobal, Antofagasta, Atacama, Chili. Known since 1896: described by Cohen in 1898. | Sitzungsb. d. k. preuss. Ak. d. Wissens. zu Berlin, 1898, I. p. 607. | 145 |
200 | 2d | Cachiyuyal, Atacama, Chili. Found in 1874: described by Domeyko in 1875. | Mineralog. Magazine, 1889, vol. 8, p. 259. | 28 |
201 | 2d | Ilimaë, Atacama, Chili. Known since 1870: described by Tschermak in 1872. | Mineralog. Magazine, 1889, vol. 8, p. 260. | 39 |
202 | 2d | Merceditas, 10 or 12 leagues east of Chañaral, Atacama, Chili. Known since 1884: described by Howell in 1890. | Proc. Rochester Ac. of Sc. 1890, vol. 1, p. 99. | 1,917 |
[pg 89]203 | 2d | Pan de Azucar, Atacama, Chili. Found about 67 miles from the port of Pan de Azucar in 1887. |
| 19,280 |
204 | 2d | Juncal, Atacama, Chili. Found in 1866 between Rio Juncal and the Salinas de Pedernal: had possibly been transported to that place: described by Daubrée in 1868. | Mineralog. Magazine, 1889, vol. 8, p. 261. | 72 |
205 | 2d | Puquios, Copiapo, Atacama, Chili. Found about 1885: described by Howell in 1890. | Proc. Rochester Ac. of Sc. 1890, vol. 1, p. 89. | 176 |
206 | 2d | The Joel Iron, Atacama, Chili. Found in 1858 in an unspecified part of the desert: described by L. F. in 1889. | Mineralog. Magazine, 1889, vol. 8, p. 263. | 1,144 |
207 | 2d | Sierra de la Ternera, Atacama, Chili. Described by Kunz and Weinschenk in 1891. | Tschermak's Min. u. Petrog. Mitth. 1891, vol. 12, p. 184. | 5 |
208 | 2d | Barranca Blanca, between Copiapo and Catamarca, South America. Found in 1855, and described by L. F. in 1889. | Mineralog. Magazine, 1889, vol. 8, p. 262. | 11,910 |
209 | 2d | Chili. Owing to an interchange of labels, the specimen was described in 1868 by Daubrée as having been found in an unspecified locality in Chili. According to Domeyko it was supposed to have been found in the Cordillera de la Dehesa, near Santiago. | Mineralog. Magazine, 1889, vol. 8, p. 256. | 2 |
210 | 2d | Angelas (Oficina), Chili. |
| 5,545 |
211 | Sep. | Otumpa, Gran Chaco Gualamba, Argentine Republic. The occurrence of metallic iron at this locality having been reported, Don Rubin de Celis was sent in 1783 to investigate the matter: his report was published in 1788. | Phil. Trans. 1788, vol. 78, pp. 37, 183.
| 634,000 |
212 | 2d | Bendegó River, Bahia, Brazil. Found in 1784: described by Mornay in 1816. | Phil. Trans. 1816, vol. 106, p. 270. | 3,119 |
[pg 90]213 | 2d | Santa Catharina (Morro do Rocio), Rio San Francisco do Sul, Brazil. Discovered in 1875: described by Lunay in 1877: it is regarded by some mineralogists as probably of terrestrial origin. | Comptes Rendus, 1877, vol. 85, p. 84. | 6,455 |
214 | 2d | Caperr, Rio Senguerr, Patagonia. Known before 1869: described by L. F. in 1899. | Mineralog. Magazine, 1900, vol. 12, p. 167. | 313 |
215 | 2d | Locality unknown (from Prof. Wöhler's Collection). Described by Wöhler in 1852. | Ann. Chem. Pharm. 1852, vol. 81, p. 253. | 30 |
216 | 2d | Locality unknown (from Smithsonian Museum Collection). Described by Shepard in 1881. | Amer. Jour. Sc. 1881, ser. 3, vol. 22, p. 119. | 5 |
217 | 2d | Locality unknown (from United States National Museum Collection). Slice of a complete meteorite which was found in a collection of minerals formed by the late Col. J. J. Abert: described by Riggs in 1887. | Amer. Jour. Sc. 1887, ser. 3, vol. 34, p. 59. | 47 |
[pg 91] II. SIDEROLITES (consisting chiefly of nickeliferous iron and silicates, both in large proportion).
A. Fall Recorded.
[Arranged chronologically.]
| No. | Pane. | Name of Meteorite and Place of Fall. | Date of Fall. | Weight in grams. |
|---|---|---|---|---|
218 | 2e | Taney County, Missouri, U.S.A. A fragment, sent from Taney County, Missouri, about 1857-8, was described by Shepard in 1860. Amer. Jour. Sc. 1860, ser. 2, vol. 30, p. 205. A fragment of a meteorite was given to Cox by Judge Green of Crawford County: no mention of place or date of find. Sec. Rep. Geol. Reconn. Arkansas, 1860, p. 408. Green's fragment was described under the name of Newton County (Arkansas) by Smith in 1865. Amer. Jour. Sc. 1865, ser. 2, vol. 40, p. 213. A large mass was obtained by Kunz and reported by him in 1887 to have really fallen in Taney County, Missouri, about thirty years before, and to have been afterwards taken to Newton County, Arkansas. Amer. Jour. Sc. 1887, ser. 3, vol. 34, p. 467. | Fell about 1857‑8. | 2,454 |
219 | 2e | Lodran (Lodhran), Mooltan, Punjab, India. | Oct. 1, 1868. | 59 |
220 | 2e | Estherville, Emmet County, Iowa, U.S.A. | May 10, 1879. | 116,618 |
221 | 2e | Veramin, Teheran, Persia. | May, 1880. | 238 |
222 | 2e | Marjalahti, Viborgs Län, Finland. | June 1, 1902. | 2,990 |
A. Fall Not Recorded.[pg 92]
[Arranged topographically.]
| No. | Pane. | Name of Meteorite and Place of Find. | Report of Find. | Weight in grams. |
|---|---|---|---|---|
223 | 2e | Finmarken, Norway. Found in 1902: described by Cohen in 1903. | Mittheil. naturw. Verein für Neu-Vorpommern und Rügen, Jahrg. 35, 1903, p. 1. | 1,306 |
224 | 2e | Hainholz, Minden, Westphalia. Found in 1856: described by Wöhler in 1857. | Pogg. Ann. 1857, vol. 100, p. 342. | 484 |
225a | 2e | Steinbach, Erzgebirge, Saxony. Reported as "native iron" by J. G. Lehmann in 1751. | Kurze Einleitung in einige Theile der Bergwerks-Wissenschaft, 1751, p. 79. | 130 |
225b | 2e | Rittersgrün, Erzgebirge, Saxony. Found in (1833 or) 1847: reported by Breithaupt in 1861. | Zeitsch. deutsch. geol. Gesell. 1861, vol. 13, p. 148. | 694 |
According to Weisbach it was really found in 1833. | Der Eisenmeteorit von Rittersgrün im sächsischen Erzgebirge: von A. W.: Freiberg, 1876. | |||
225c | 2e | Breitenbach, Erzgebirge, Bohemia. Found in 1861: described by Maskelyne in 1871. | Phil. Trans. 1871, vol. 161, p. 359. | 6,230 |
Steinbach, Rittersgrün, and Breitenbach are within five English miles of each other, on the border of Saxony and Bohemia; the siderolites probably fell at the same time. Breithaupt suggests that this was the fall reported to have taken place at Whitsuntide in the year 1164: Buchner (p. 124) suggests a fall which took place between 1540 and 1550. | Berg-und hütt. Zeitung, 1862, Jahrg. 21, p. 321. |
| ||
226 | 2e | Brahin, Minsk, Russia. Found in 1809, 1810 or 1820. | Bull. des. Sc. par la Soc. philom., Paris, 1823, p. 86. Partsch's Die Meteoriten zu Wien. 1843, p. 90. Erman's Archiv. f. wiss. Kunde von Russland, 1846, vol. 5, p. 183. | 22 |
[pg 93]227 | 2e,4c | The Pallas iron Found in 1749 between the Ubei and Sisim rivers, Yeniseisk, Asiatic Russia, and transported to Krasnojarsk: reported by Pallas in 1776. | Reise d. versch. Prov. d. russ. Reichs: von P. S. Pallas. St. Petersburg, 1776. Part iii. p. 411. | 3,365 |
228 | 2e | Pavlodar, Semipalatinsk, Asiatic Russia. Found in 1885. | 58 | |
229 | 2e | Senegal River, West Africa. "Native Iron" was found by Compagnon in 1716 to be in very common use in many parts of the kingdoms of Bambuk and Siratik. | Allgemeine Historie der Reisen zu Wasser und Lande: von J. J. Schwabe. Leipzig, 1748, vol. 2, Book 5, Ch. 13, p. 510. | 396 |
230 | 2e | Mount Dyrring, Bridgman, Singleton District, New South Wales. Found in 1902: described by Card in 1903. | Records of the Geol. Survey of N. S. Wales, 1903, vol. 7, p. 218. | 248 |
231 | 2e | Powder Mill Creek, Cumberland County, Tennessee, U.S.A. Found in 1887: described in the same year by Whitfield and Kunz. | Amer. Jour. Sc. 1887, ser. 3, vol. 34, pp. 387, 476. | 1,167 |
232 | 2e | Eagle Station, Carroll County, Kentucky, U.S.A. Found in 1880, and described by Kunz in 1887. | Amer. Jour. Sc. 1887, ser. 3, vol. 33, p. 228. | 708 |
233 | 2e | Brenham Township, Kiowa County, Kansas, U.S.A. Found about 1886: described by Kunz in 1890. | Amer. Jour. Sc. 1890, ser. 3, vol. 40, p. 312. | 2,008 |
234 | 2e | Admire, Lyon County, Kansas, U.S.A. Found about 1892: described by Merrill in 1902. | Proc. U.S. Nat. Mus. 1902, vol. 24, p. 907. | 1,076 |
235 | Sep. | Imilac, Atacama, Chili. Known in 1822: probably the specimen found at Campo de Pucará in 1879 had been carried at some time or other from Imilac. | Mineralog. Magazine, 1889, vol. 8, p. 243. | 212,136 |
236 | 2e | Ilimaes, 12 leagues south of Taltal, Atacama, Chili. Found about 1874-5: described by Ward in 1906. | Proc. Roch. Acad. of Science, 1906, vol. 4, p. 225. | 266 |
[pg 94]237 | 2f | Vaca Muerta, Atacama, Chili. Mentioned in 1861, and described in 1864 by Domeyko as found at Sierra de Chaco. Specimens probably got from the same place are known by various names (Mejillones, Jarquera or Janacera Pass, &.). | Mineralog. Magazine, 1889, vol. 8, p. 234. | 7,285 |
238 | 2f | Llano del Inca, 35 leagues S.E. of Taltal, Atacama, Chili. | Proc. Rochester Ac. of Sci. 1890, vol. 1, p. 93. | 376 |
239 | 2f | Doña Inez, Atacama, Chili. The meteorites of Llano del Inca and Doña Inez were found in these localities in 1888, and were described by Howell in 1890: "polished sections of the two meteorites are in many cases not distinguishable," and Howell is inclined to think that they belong to a single fall. (Some of the polished faces are not to be distinguished from those of Vaca Muerta.) L. F. | Ibid. | 1,015 |
240 | 2f | Copiapo, Chili. Numerous masses of this type have been brought to Copiapo since 1863: some of them, owing to an interchange of labels, have been supposed to come from the Sierra de la Dehesa (Deesa), near Santiago. | Mineralog. Magazine 1889, vol. 8, p. 255. | 769 |
III. AEROLITES[pg 95]
or Meteoric Stones
(consisting generally of one or more silicates, and interspersed particles of nickeliferous iron, troilite, &.).
A. Fall Recorded.
[Arranged chronologically.]
| No. | Pane. | Name of Meteorite and Place of Fall. | Date of Fall. | Weight in grams. | ||
|---|---|---|---|---|---|---|
241 | 4c | Ensisheim, Elsass, Germany. | Nov. | 16, | 1492 | 458 .0 |
242 | 2g | Schellin, near Stargard, Pomerania, Prussia. | April | 11, | 1715 | —.0 |
243 | 2g | Plescowitz, near Reichstadt, Bohemia. | June | 22, | 1723 | 25 .0 |
244 | 4c | Ogi (Haruta), Hizen, Kiusiu, Japan. | June | 8, | 1741 | 4,175 .0 |
245 | 4c | Tabor (Krawin, Plan, Strkow), Bohemia. | July | 3, | 1753 | 151 .0 |
246 | 2g | Luponnas, Ain, France. | Sept. | 7, | 1753 | 7 .0 |
247 | 2g | Albareto, Modena, Italy. | July | 1766 | 52 .0 | |
248 | 4c | Lucé, (Maine), Sarthe, France. | Sept. | 13, | 1768 | 5 .0 |
249 | 2g | Mauerkirchen, Upper Austria. | Nov. | 20, | 1768 | 302 .0 |
250 | 2g | Sena, Sigena, Aragon, Spain. | Nov. | 17, | 1773 | 0·7 |
251 | 2g | Eichstädt, Wittmess, Bavaria. | Feb. | 19, | 1785 | 47 .0 |
252 | 2g | Kharkov (Jigalowka, Bobrik), Russia. | Oct. 12 (not 13), | 1787 | 437 .0 | |
253 | 2g | Barbotan, Landes, France. | July | 24, | 1790 | 782 .0 |
254 | 4c | Siena, Cosona, Italy. | June | 16, | 1794 | 123 .0 |
255 | 4b | Wold Cottage, Thwing, Yorkshire. | Dec. | 13, | 1795 | 20,682.0 |
256 | 2g | Bjelaja Zerkov, Kiev, Russia. | Jan. 15 or 16 | 1796 | 9 .0 | |
257 | 2g | Salles, near Villefranche, Rhône, France. | March 8 or 12, | 1798 | 165 .0 | |
258 | 2g,4c | Krakhut, Benares, India. | Dec. | 19, | 1798 | 510 .0 |
259 | 2h,4c | L'Aigle, Orne, France. | April | 26, | 1803 | 2,201 .0 |
260 | 2h | Apt (Saurette), Vaucluse, France. | Oct. | 8, | 1803 | 37 .0 |
261 | 2h | Mässing (St. Nicholas), Bavaria. | Dec. | 13, | 1803 | —.0 |
262 | 2h | Darmstadt, Hesse, Germany. | Fell before | 1804 | 1·6 | |
263 | 4d | High Possil, near Glasgow, Scotland. | April | 5, | 1804 | 91 .0 |
264 | 2h | Hacienda de Bocas, San Luis Potosi, Mexico. | Nov. | 24, | 1804 | —.0 |
265 | 2h | Doroninsk, Irkutsk, Asiatic Russia. | April | 6, | 1805 | 9 .0 |
266 | 2h | Asco, Corsica. | Nov. | 1805 | —.0 | |
267 | 4n | Alais, Gard, France. | March | 15, | 1806 | 13 .0 |
268 | 2h | Timochin, Juchnov, Smolensk, Russia. | March | 25, | 1807 | 139 .0 |
269 | 2h,4o | Weston, Fairfield County, Connecticut, U.S.A. | Dec. | 14, | 1807 | 1,034 .0 |
270 | 2h | Borgo San Donino, Cusignano, Parma, Italy. | April | 19, | 1808 | 9 .0 |
[pg 96]271 | 2h | Stannern: Iglau, Moravia, Austria. (a) Stannern, (b) Langenpiernitz. | May | 22, | 1808 |
|
272 | 2h | Lissa, Bunzlau, Bohemia. | Sept. | 3, | 1808 | 169 .0 |
273 | 2h | Moradabad, North-West Provinces, India. | Fell in | 1808 | 17 .0 | |
274 | 2h | Kikino, Viasma, Smolensk, Russia. | Fell in | 1809 | 28 .0 | |
275 | 2h | Mooresfort, County Tipperary, Ireland. | Aug. | 1810 | 243 .0 | |
276 | 2h | Charsonville: Meung, Loiret, France (a) Charsonville, (b) Bois de Fontaine, (c) Fragment of a stone labelled Chartres. | Nov. | 23, | 1810 |
|
277 | 2h | Kuleschovka, Poltava, Russia. | March | 12, | 1811 | 58 .0 |
278 | 2h | Berlanguillas, near Burgos, Spain | July | 8, | 1811 | 26 .0 |
279 | 2k | Toulouse Haute Garonne, France. | April | 10, | 1812 | 31 .0 |
280 | 2k | Erxleben Magdeburg, Prussia. | April | 15, | 1812 | 31 .0 |
281 | 2k | Chantonnay, Vendée, France. | Aug. | 5, | 1812 | 1,352 .0 |
282 | 2k | Adare, County Limerick, Ireland. | Sept. | 10, | 1813 | 161 .0 |
283 | 2k | Luotolaks, Viborg, Finland. | Dec. | 13, | 1813 | 20 .0 |
284 | 2k | Gurram Konda, between Punganur and Kadapa, Madras, India. | Fell in | 1814 | 9 .0 | |
285 | 2k | Bachmut (Alexejevka), Ekaterinoslav, Russia. | Feb. | 15, | 1814 | 41 .0 |
286 | 2k | Agen, Lot-et-Garonne, France. | Sept. | 5, | 1814 | 40 .0 |
287 | 2k | Chail, Allahabad, North-West Provinces, India. | Nov. | 5, | 1814 | —.0 |
288 | 2k | Durala, N.W. of Kurnal, Punjab, India. | Feb. | 18, | 1815 | 12,000.0 |
289 | 4o | Chassigny, Haute Marne, France. | Oct. | 3, | 1815 | 40 .0 |
290 | 2k | Zaborzika, Czartorya, Volhynia, Russia. | April 11 (not 10), | 1818 | 16 .0 | |
291 | 4n | Seres, Macedonia, Turkey. | June | 1818 | 399 .0 | |
292 | 2k | Slobodka, Juchnov, Smolensk, Russia. | Aug. | 10, | 1818 | 27 .0 |
293 | 2l | Jonzac, Charente Inférieure, France. | June | 13, | 1819 | 9 .0 |
294 | 2l | Pohlitz, near Gera, Reuss, Germany. | Oct. | 13, | 1819 | 87 .0 |
295 | 2l | Lixna (Lasdany), Dünaburg, Vitebsk, Russia. | July | 12, | 1820 | 58 .0 |
296 | 4o | Juvinas, near Libonnez, Ardèche, France. | June | 15, | 1821 | 940 .0 |
297 | 2l | Angers, Maine-et-Loire, France. | June | 3, | 1822 | 22 .0 |
298 | 2l | Agra (Kadonah), India. | Aug. | 7, | 1822 | 38 .0 |
299 | 2l | Epinal (La Baffe), Vosges, France. | Sept. | 13, | 1822 | 1·6 |
300 | 2l,4h | Futtehpur (Fatehpur): N. West Provinces India (a) Futtehpur (b) Bithur. | Nov. | 30, | 1822 |
|
301 | 2l | Umballa (40 miles S.W. of), Punjab, India. | Fell in | 1822-3 | 20 .0 | |
[pg 97]302 | 2l | Nobleborough, Lincoln County, Maine, U.S.A. | Aug. | 7, | 1823 | — .0 |
303 | 2l | Renazzo, Cento, Ferrara, Italy. | Jan. | 15, | 1824 | 15 .0 |
304 | 2l | Zebrak (Praskoles), near Horzowitz, Bohemia. | Oct. | 14, | 1824 | 83 .0 |
305 | 2l | Nanjemoy, Charles County, Maryland, U.S.A. | Feb. | 10, | 1825 | 325 .0 |
306 | 2l | Honolulu, Hawaii, Sandwich Islands. | Sept. | 27, | 1825 | 81 .0 |
307 | 2m | Pavlograd (Mordvinovka), Ekaterinoslav, Russi | May | 19, | 1826 | 161 .0 |
308 | 2m | Mhow, Azamgarh District, North-West Provinces, India. | Feb. | 16, | 1827 | 163 .0 |
309 | 2m | Drake Creek, Nashville, Tennessee, U.S.A. | May | 9, | 1827 | 19 .0 |
310 | 2m | Bialystock (Jasly), Grodno, Russia. | Oct. | 5, | 1827 | 4 .0 |
311 | 2m | Richmond, Henrico County, Virginia, U.S.A. | June | 4, | 1828 | 169 .0 |
312 | 2m | Forsyth, Georgia, U.S.A. | May | 8, | 1829 | 72 .0 |
313 | 2m | Deal, near Long Branch, New Jersey, U.S.A. | Aug. | 14, | 1829 | —.0 |
314 | 2m | Krasnoi-Ugol, Rjäsan, Russia. | Sept. | 9, | 1829 | 5 .0 |
315 | 2m | Launton, Bicester, Oxfordshire. | Feb. | 15, | 1830 | 1,023 .0 |
316 | 2m | Perth (North Inch of), Scotland. | May | 17, | 1830 | 1·5 |
317 | 2m | Vouillé, near Poitiers, Vienne, France. | May | 13, | 1831 | 61 .0 |
318 | 2m | Wessely (Znorow), Hradisch, Moravia, Austria. | Sept. | 9, | 1831 | 3 .0 |
319 | 2m | Blansko, Brünn, Moravia, Austria. | Nov. | 25, | 1833 | —.0 |
320 | 2m | Okniny, Kremenetz, Volhynia, Russia. | Jan. | 8, | 1834 | 7 .0 |
321 | 2m | Charwallas (Chaharwala), near Hissar, Delhi, India. | June | 12, | 1834 | 37 .0 |
322 | 2m | Mascombes, Corrèze, France. | Jan. | 31, | 1835 | 5 .0 |
323 | 2m | Aldsworth, near Cirencester, Gloucestershire. | Aug. | 4, | 1835 | 520 .0 |
324 | 2m | Aubres, Nyons, Drôme, France. | Sept. | 14, | 1836 | 487 .0 |
325 | 2m | Macao, Rio Grande do Norte, Brazil. | Nov. | 11, | 1836 | 6 .0 |
326 | 2m | Yonōzu, Nishikambara, Echigo, Japan. | July | 14, | 1837 | 34 .0 |
327 | 2m | Nagy-Diwina, near Budetin, Trentschin, Hungary. | July | 24, | 1837 | 3 .0 |
328 | 2m | Esnandes, Charente Inférieure, France. | Aug. | 1837 | 2 .0 | |
329 | 2n | Kaee, Sandee District, Onde, India. | Jan. | 29, | 1838 | 209 .0 |
330 | 2n | Akbarpur, Saharanpur, North-West Provinces, India. | April | 18, | 1838 | 1,569 .0 |
331 | 2n | Chandakapur, Berar, India. | June | 6, | 1838 | 760 .0 |
332 | 2n | Montlivault, Loir-et-Cher, France. | July | 22, | 1838 | 11 .0 |
333 | 2n,4n | Cold Bokkeveld, Cape Colony. | Oct. | 13, | 1838 | 1,079 .0 |
334 | 2n | Little Piney (Pine Bluff), Pulaski County, Missouri, U.S.A. | Feb. | 13, | 1839 | 104 .0 |
335 | 2n | Karakol, Ajagus, Kirghiz Steppes, Russia. | May | 9, | 1840 | 24 .0 |
[pg 98]336 | 2n | Uden (Staartje), North Brabant, Netherlands. | June | 12, | 1840 | — .0 |
337 | 2n | Cereseto, near Ottiglio, Alessandria, Piedmont, Italy. | July | 17, | 1840 | 124 .0 |
338 | 2n | Grüneberg, Heinrichsau, Prussian Silesia. | March | 22, | 1841 | 30 .0 |
339 | 2n | Château-Renard, Triguères, Loiret, France. | June | 12, | 1841 | 3,250 .0 |
340 | 2n | Milena, Warasdin, Croatia, Austria. | April | 26, | 1842 | 147 .0 |
341 | 4o | Aumières, Lozère, France. | June | 3, | 1842 | 43 .0 |
342 | 2n | Bishopville, Sumter County, S. Carolina, U.S.A. | March | 25, | 1843 | 509 .0 |
343 | 2m,4n | Utrecht (Blaauw-Kapel), Netherlands. | June | 2, | 1843 | 186 .0 |
344 | 2n | Manegaum (Manegaon), near Eidulabad, border of Khandeish, India | June | 29, | 1843 | 11 .0 |
345 | 2n | Klein-Wenden, near Nordhausen, Erfurt, Prussia. | Sept. | 16, | 1843 | 5 .0 |
346 | 2n | Cerro Cosina, near Dolores Hidalgo, San Miguel, Guanaxuato, Mexico | Jan. | 1844 | 42 .0 | |
347 | 2n | Killeter, County Tyrone, Ireland. | April | 29, | 1844 | 101 .0 |
348 | 2n | Favars, Aveyron, France. | Oct. | 21, | 1844 | 6 .0 |
349 | 2n | Le Teilleul (La Vivionnère), Manche, France. | July | 14, | 1845 | 2 .0 |
350 | 2n | Monte Milone (now called Pollenza), Macerata, Italy. | May | 8, | 1846 | 8 .0 |
351 | 2n | Cape Girardeau, Missouri, U.S.A. | Aug. | 14, | 1846 | 78 .0 |
352 | 2n | Schönenberg, Mindelthal, Schwaben, Bavaria. | Dec. | 25, | 1846 | 42 .0 |
353 | 2o | Linn County (Hartford), Iowa, U.S.A. | Feb. | 25, | 1847 | 942 .0 |
354 | 2o | Castine, Hancock County, Maine, U.S.A. | May | 20, | 1848 | 2 .0 |
355 | 2o | Marmande (Montignac), Aveyron, France. | July | 4, | 1848 | 4 .0 |
356 | 2o | Ski, Amt Akershuus, Norway. | Dec. | 27, | 1848 | 5 .0 |
357 | 2o | Cabarras County (Monroe), N. Carolina, U.S.A. | Oct. | 31, | 1849 | 385 .0 |
358 | 2o | Kesen(-mura), Kesen-gōri, Rikuzen, Japan. | June | 12, | 1850 | 1,280 .0 |
359 | 2o | Shalka, Bancoorah, Bengal, India. | Nov. | 30, | 1850 | 1,132 .0 |
360 | 2o | Gütersloh, Westphalia, Prussia. | April | 17, | 1851 | 109 .0 |
361 | 2o | Quinçay, Vienne, France. | Summer, | 1851 | 10 .0 | |
362 | 2o | Nulles, Catalonia, Spain. | Nov. | 5, | 1851 | 27 .0 |
363 | 4p | Nellore (Yatur), Madras. | Jan. | 23, | 1852 | 10,400.0 |
364 | 2o,4d | Mezö-Madaras, Transylvania. | Sept. | 4, | 1852 | 733 .0 |
365 | 2o | Borkut, Marmoros, Hungary. | Oct. | 13, | 1852 | 40 .0 |
366 | 4o | Bustee (Basti), between Goruckpur and Fyzabad, India. | Dec. | 2, | 1852 | 1,398 .0 |
367 | 2o | Girgenti, Sicily. | Feb. | 10, | 1853 | 233 .0 |
368 | 2o | Segowlie, Bengal, India. | March | 6, | 1853 | 1,205 .0 |
369 | 2o | Duruma, Wanikaland, E. Africa. | Fell in |
| 1853 | —.0 |
370 | 2o | Linum, Brandenburg, Prussia. | Sept. | 5, | 1854 | 2 .0 |
[pg 99]371 | 3c | Oesel (Gesinde Kaande, near Piddul), Baltic Sea. | May | 11, | 1855 | 15 .0 |
372 | 3c | Gnarrenburg (Bremervörde), Hanover. | May | 13, | 1855 | 808 .0 |
373 | 3c | St. Denis-Westrem, near Ghent, Belgium. | June | 7, | 1855 | 1·3 |
374 | 4o | Petersburg, Lincoln County, Tennessee, U.S.A. | Aug. | 5, | 1855 | 52 .0 |
375 | 3c | Trenzano, Brescia, Italy. | Nov. | 12, | 1856 | 157 .0 |
376 | 3c,3a | Parnallee, Madras, India. | Feb. | 28, | 1857 | 60,941.0 |
377 | 3c | Heredia, San José, Costa Rica. | April | 1, | 1857 | 53 .0 |
378 | 3c | Stavropol, north side of the Caucasus, Russia. | April | 5, | 1857 | 22 .0 |
379 | 3c | Kaba, Debreczin, Hungary. | April | 15, | 1857 | 104 .0 |
380 | 3c | Les Ormes, near Joigny, Yonne, France. | Oct. | 1, | 1857 | 12 .0 |
381 | 3c | Ohaba (Veresegyhaza), near Karlsburg, Transylvania. | Oct. | 11, | 1857 | 39 .0 |
382 | 4n | Pegu (Quenggouk), British Burmah. | Dec. | 27, | 1857 | 654 .0 |
383 | 3c | Kakowa, Temeser Banat, Hungary. | May | 19, | 1858 | 160 .0 |
384 | 3c | Ausson: Haute Garonne, France. (a) Ausson, (b) Clarac, | Dec. | 9, | 1858 |
|
385 | 3c | Molina, Murcia, Spain. | Dec. | 24, | 1858 | 6 .0 |
386 | 3d | Harrison County, Indiana, U.S.A. | March | 28, | 1859 | 38 .0 |
387 | 3d | Pampanga (Mexico), Philippine Islands. | April | 4, | 1859 | 1·8 |
388 | 3d | Beuste, near Pau, Basses-Pyrénées, France. | May | 1859 | 40 .0 | |
389 | 3d | Bethlehem, near Albany, New York, U.S.A. | Aug. | 11, | 1859 | —.0 |
390 | 3d | Alessandria (San Giuliano Vecchio), Piedmont, Italy. | Feb. | 2, | 1860 | 35 .0 |
391 | 4n | Khiragurh, S.E. of Bhurtpur, India. | March | 28, | 1860 | 353 .0 |
392 | 3d,3b | New Concord, Muskingum County, Ohio, U.S.A. | May | 1, | 1860 | 19,724.0 |
393 | 3d | Kusiali, Kumaon, India. | June | 16, | 1860 | 4 .0 |
394 | 3c | Dhurmsala (Dharmsala), Kangra, Punjab, India. | July | 14, | 1860 | 12,410.0 |
395 | 4h | Butsura (Batsura): Bengal, India. (Qutahar Bazaar) (Chireya) (Piprassi) (Bulloah) | May | 12, | 1861 |
|
396 | 3d | Canellas, near Barcelona, Spain. | May | 14, | 1861 | 1·5 |
397 | 3d | Grosnaja, (Mikenskoi), Banks of the Terek, Caucasus, Russia. | June | 28, | 1861 | 167 .0 |
398 | 3d | Klein-Menow, Alt-Strelitz, Mecklenburg, Germany. | Oct. | 7, | 1862 | 1,132 .0 |
399 | 3d | Pulsora, N.E. of Rutlam, Indore, Central India. | March | 16, | 1863 | 48 .0 |
400 | 3d | Buschhof (Scheikahr Stattan), Courland, Russia. | June | 2, | 1863 | 98 .0 |
401 | 3d | Pillistfer (Aukoma), Livland, Russia. | Aug. | 8, | 1863 | 157 .0 |
[pg 100]402 | 3d | Shytal 40 miles north of Dacca, India. | Aug. | 11, | 1863 | 462 .0 |
403 | 3d | Tourinnes-la-Grosse, Tirlemont, Belgium. | Dec. | 7, | 1863 | 203 .0 |
404 | 3d | Manbhoom, Bengal, India. | Dec. | 22, | 1863 | 123 .0 |
405 | 3d | Nerft, Courland, Russia. | April | 12, | 1864 | 69 .0 |
406 | 3d | Orgueil near Montauban, Tarn-et-Garonne, France. | May | 14, | 1864 | 612 .0 |
407 | 3d | Dolgovoli, Volhynia, Russia. | June | 26, | 1864 | 3 .0 |
408 |
4h | Supuhee: Goruckpur District India. (a) Mouza Khoorna, Sidowra, (b) Bubuowly Indigo Factory, Supuhee, | Jan. | 19, | 1865 |
|
409 | 3e | Vernon County, Wisconsin, U.S.A. | March | 26, | 1865 | 52 .0 |
410 | 3e | Gopalpur, Jessore, India. | May | 23, | 1865 | 147 .0 |
411 | 3e | Dundrum, Tipperary, Ireland. | Aug. | 12, | 1865 | 245 .0 |
412 | 3e | Aumale, (Senhadja), Constantine, Algeria. | Aug. | 25, | 1865 | 34 .0 |
413 | 4k,4o | Sherghotty (Umjhiawar), near Gya, Behar, India. | Aug. | 25, | 1865 | 117 .0 |
414 | 4n | Muddoor, Mysore, India. | Sept. | 21, | 1865 | 407 .0 |
415 | 3e | Udipi (Yedabettu), South Canara, India. | April | 1866 | 3,320 .0 | |
416 | 3e | Pokhra, near Bustee, Goruckpur, India. | May | 27, | 1866 | 46 .0 |
417 | 3e | St. Mesmin, Aube, France. | May | 30, | 1866 | 110 .0 |
418 | 3d,4d, 4h,4n | Knyahinya, near Nagy-Berezna, Hungary. | June | 9, | 1866 | 11,325.0 |
419 | 3e | Jamkheir, Ahmednuggur, Bombay. | Oct. | 5, | 1866 | 16 .0 |
420 | 3e | Cangas de Onis (Elgueras), Asturias, Spain. | Dec. | 6, | 1866 | 97 .0 |
421 | 3e | Khetri (Saonlod, Sankhoo, Phulee, &c.), Rajpootana, India. | Jan. | 19, | 1867 | 13 .0 |
422 | 4o | Tadjera near Guidjel, Setif, Algeria. | June | 9, | 1867 | 39 .0 |
423 | 3e,4e-g | Pultusk (Siedlce, Gostkóv, &c.), Poland. | Jan. | 30, | 1868 | 18,029.0 |
3e | Lerici Lerici, Spezia, Italy. | Jan. | 30, | 1868 | 8 .0 | |
424 | 3e,4d | Daniel's Kuil, riqualand, South Africa. | March | 20, | 1868 | 446 .0 |
425 | 3e | Slavetic, Agram, Croatia, Austria. | May | 22, | 1868 | 20 .0 |
426 | 3e | Ornans, Doubs, France. | July | 11, | 1868 | 1,019 .0 |
427 | 3e | Sauguis, St. Étienne, Basses-Pyrénées, France. | Sept. | 7, | 1868 | 16 .0 |
428 | 3e | Danville, Morgan County, Alabama, U.S.A. | Nov. | 27, | 1868 | 27 .0 |
429 | 3e | Frankfort (4 miles S. of), Franklin County, Alabama, U.S.A. | Dec. | 5, | 1868 | 32 .0 |
430 | 3e | Moti-ka-nagla, Ghoordha, Bhurtpur, India. | Dec. | 22, | 1868 | 407 .0 |
431 | 4o | Angra dos Reis, Rio de Janeiro, Brazil. | Jan. | 1869 | 6 .0 | |
432 | 3e,4d | Hessle, near Upsala, Sweden. | Jan. | 1, | 1869 | 909 .0 |
433 | 3e | Krähenberg, Zweibrücken, Rhenish Bavaria. | May | 5, | 1869 | 10 .0 |
434 | 3e | Cléguérec (Kernouvé), Morbihan, France. | May | 22, | 1869 | 9,231 .0 |
[pg 101]435 | 3e | Tjabé, Tennasilm | Sept. | 19, | 1869 | 134 .0 |
436 | 3e | Stewart County (12 miles S.W. of Lumpkin), Georgia, U.S.A. | Oct. | 6, | 1869 | 17 .0 |
437 | 3f | Ibbenbühren, Westphalia, Prussia. | June | 17, | 1870 | 3 .0 |
438 | 3f | Cabeza de Mayo, Murcia, Spain. | Aug. | 18, | 1870 | 3 .0 |
439 | 4o | Roda (4 miles from), Huesca, Spain. | Spring | 1871 | 7 .0 | |
440 | 3f | Searsmont, Waldo County, Maine, U.S.A. | May | 21, | 1871 | 51 .0 |
441 | 3f | Laborel, Drôme, France. | June | 14, | 1871 | 291 .0 |
442 | 3f | Bandong, Java. | Dec. | 10, | 1871 | 14 .0 |
443 | 4d | Dyalpur, Sultanpur, Oude, India. | May | 8, | 1872 | 269 .0 |
444 | 3f | Tennasilm (Sikkensaare), Esthonia, Russia. | June | 28, | 1872 | 15 .0 |
445 | 3f | Lancé: Authon and Lancé, Vendôme, Loir-et-Cher, France. | July | 23, | 1872 | 332 .0 |
446 | 4o | Orvinio, near Rome, Italy. | Aug. | 31, | 1872 | 63 .0 |
447 | 3f | Jhung (Jhang), Punjab, India. | June | 1873 | 1,770 .0 | |
448 | 3f | Khairpur, 35 miles east of Bhawalpur, India. | Sept. | 23, | 1873 | 3,286 .0 |
449 | 3f | Santa Barbara, Rio Grande do Sul, Brazil. | Sept. | 26, | 1873 | 1·7 |
450 | 3f | Aleppo, Syria. | Fell about | 1873 | 77 .0 | |
451 | 3f | Sevrukovo, near Belgorod, Kursk, Russia. | May | 11, | 1874 | 20 .0 |
452 | 3f | Nash County (near Castalia), N. Carolina, U.S.A. | May | 14, | 1874 | 29 .0 |
453 | 3f | Virba, Vidin, Turkey. | May | 20, | 1874 | 38 .0 |
454 | 3f | Kerilis, Mael Pestivien, Côtes-du-Nord, France. | Nov. | 26, | 1874 | 74 .0 |
455 | 3f | Amana (Colony) [Homestead, West Liberty], Iowa County, Iowa, U.S.A. | Feb. | 12, | 1875 | 3,800 .0 |
456 | 3f | Sitathali (Nurrah), S.E. of Raepur, Central Provinces, India. | March | 4, | 1875 | 600 .0 |
457 | 4d | Zsadány, Temeser Banat, Hungary. | March | 31, | 1875 | 25 .0 |
458 | 3f | Nagaria, Fathabad, Agra, India. | April | 24, | 1875 | 13 .0 |
459 | 3f | Mornans, Bourdeaux, Drôme, France. | Sept. | 1875 | 973 .0 | |
460 | 4n | Judesegeri, Kadaba Taluk, Mysore, India. | Feb. | 16, | 1876 | 114 .0 |
461 | 3g | Vavilovka, Kherson, Russia. | June | 19, | 1876 | 10 .0 |
462 | 3g | Ställdalen, Nya Kopparberg, Orebro, Sweden. | June | 28, | 1876 | 1,575 .0 |
463 | 3g | Rochester, Fulton County, Indiana, U.S.A. | Dec. | 21, | 1876 | 8 .0 |
464 | 3g | Warrenton, Warren County, Missouri, U.S.A. | Jan. | 3, | 1877 | 82 .0 |
465 | 3g | Cynthiana (9 miles from), Harrison County, Kentucky, U.S.A. | Jan. | 23, | 1877 | 154 .0 |
466 | 3g | Hungen, Hesse, Germany. | May | 17, | 1877 | 5 .0 |
467 | 3g | Jodzie Ponevej, Kovno, Russia. | June | 17, | 1877 | 1·6 |
468 | 3g | Soko-Banja (Sarbanovac), N.E. of Alexinatz, Servia. | Oct. | 13, | 1877 | 1,995 .0 |
[pg 102]469 | 3g | Cronstad, Orange River Colony, S. Africa. | Nov. | 19, | 1877 | 1,228 .0 |
470 | 3g | Bhagur (Dhulia), India. | Nov. | 27, | 1877 | 6 .0 |
471 | 3h | Tieschitz, Prerau, Moravia. | July | 15, | 1878 | 17 .0 |
472 | 3h | Mern, Præsto, Denmark. | Aug. | 29, | 1878 | 39 .0 |
473 | 3h | Dandapur, Goruckpur, India. | Sept. | 5, | 1878 | 2,370 .0 |
474 | 3h | Rakovka, Tula, Russia. | Nov. | 20, | 1878 | 372 .0 |
475 | 2h | La Bécasse, Dun le Poëlier, Indre, France. | Jan. | 31, | 1879 | 19 .0 |
476 | 3h | Itapicuru-mirim, Maranhão, Brazil. | March | 1879 | 6 .0 | |
477 | 3h | Gnadenfrei, Prussian Silesia. | May | 17, | 1879 | 54 .0 |
478 | 3h | Nagaya, Entre Rios, Argentine Republic. | July | 1, | 1879 | 31 .0 |
479 | 3h | Tomatlan (Gargantillo), Jalisco, Mexico. | Sept. | 17, | 1879 | 135 .0 |
480 | 3h | Kalambi (Kalumbi), Bombay, India. | Nov. | 4, | 1879 | 28 .0 |
481 | 3h | Takenouchi (-mura), Yabu-gōri, Tajima, Japan. | Feb. | 18, | 1880 | 2 .0 |
482 | 3h | Middlesbrough (Pennyman's Siding), Yorkshire. | March | 14, | 1881 | 22 .0 |
483 | 3h | Pacula, Jacala, Hidalgo, Mexico. | June | 18, | 1881 | 28 .0 |
484 | 3h | Gross-Liebenthal, 12 miles S.S.W. of Odessa, Russia. | Nov. | 19, | 1881 | 62 .0 |
485 | 3h, | Mocs, Kolos, Transylvania. | Feb. | 3, | 1882 | 14,677.0 |
486 | 3k | Fukutomi (-mura), Kijima-gōri, Hizen, Japan. | March | 19, | 1882 | 230 .0 |
487 | 3k | Pavlovka, Balachev, Saratov, Russia. | Aug. | 2, | 1882 | 78 .0 |
488 | 3k | Pirgunje, Dinagepur, India. | Aug. | 29, | 1882 | 732 .0 |
489 | 3k | Saint Caprais-de-Quinsac, Gironde, France. | Jan. | 28, | 1883 | 9 .0 |
490 | 3k | Alfianello, Brescia, Italy. | Feb. | 16, | 1883 | 2,515 .0 |
491 | 3k | Ngawi, Madioen, Java. | Oct. | 3, | 1883 | 51 .0 |
492 | 3l | Pirthalla, Hissar District, Punjab, India. | Feb. | 9, | 1884 | 427 .0 |
493 | 3l | Djati-Pengilon, Alastoeva, Java. | March | 19, | 1884 | 469 .0 |
494 | 3l | Tysnes (Midt-Vaage), Hardanger Fiord, Norway. | May | 20, | 1884 | 895 .0 |
495 | 3l | Chandpur, 5 miles N.W. of Mainpuri, North-West Provinces, India. | April | 6, | 1885 | 490 .0 |
496 | 3l | Nammianthal, South Arcot, Madras, India. | Jan. | 27, | 1886 | 1,615 .0 |
497 | 3l | Assisi, Perugia, Italy. | May | 24, | 1886 | 153 .0 |
498 | 3l | Alatyr (Novo-Urei), Karamzinka, Petrovka, Nijni Novgorod, Russia. | Sept. | 4, | 1886 | 22 .0 |
499 | 3p | Oshima(-mura) [Yenshigahara, Oynchimura], Kitaisa-gōri, Satsuma, Kiusiu, Japan. | Oct. | 26, | 1886 | 31,354.0 |
500 | 3l | Bielokrynitschie, Zaslavl, Volhynia, Russia. | Jan. | 1, | 1887 | 53 .0 |
501 | 3l | Lalitpur North-West Provinces, India. | April | 7, | 1887 | 82 .0 |
502 | 3l | Tabory, Ochansk, Perm, Russia. | Aug. | 30, | 1887 | 1,012 .0 |
[pg 103]503 | 3l | Lundsgård, Ljungby, Sweden. | April | 3, | 1889 | 214 .0 |
504 | 3l | Migheja,Olviopol, Elizabetgrad, Kherson, South Russia. | June | 21, | 1889 | 234 .0 |
505 | 3l | Ergheo, Brava, Somaliland. | July | 1889 | 926 .0 | |
506 | 3l | Jelica, Servia. | Dec. | 1, | 1889 | 1,879 .0 |
507 | 3m | Collescipoli (Antifona), Terni, Italy. | Feb. | 3, | 1890 | 421 .0 |
508 | 3m | Baldohn, Misshof, Courland, Russia. | April | 10, | 1890 | 134 .0 |
509 | 3m | Winnebago County (Forest City), Iowa, U.S.A. | May | 2, | 1890 | 2,556 .0 |
510 | 3m | Kahangarai, Tirupatúr, Salem, Madras, India. | June | 4, | 1890 | 122 .0 |
511 | 3m | Nawapali, Sambalpur District, Central Provinces, India. | June | 6, | 1890 | 21 .0 |
512 | 3m | Farmington, Washington County, Kansas, U.S.A. | June | 25, | 1890 | 802 .0 |
513 | 3m | Indarch, Elissavetpol, Transcaucasia. | April | 7, | 1891 | 393 .0 |
514 | 3m | Cross Roads, Wilson County, N. Carolina, U.S.A. | May | 24, | 1892 | 11 .0 |
515 | 3m | Guareña, Badajoz, Spain. | July | 20, | 1892 | 69 .0 |
516 | 3m | Bath, S. Dakota, U.S.A. | Aug. | 29, | 1892 | 2,119 .0 |
517 | 3m | Pricetown, Highland County, Ohio, U.S.A. | Feb. | 13, | 1894 | 10 .0 |
518 | 3m | Bherai, Junagadh, Kathiawar, Bombay. | April | 28, | 1894 | 17 .0 |
519 | 3m | Beaver Creek, West Kootenai District, British Columbia. | May | 26, | 1894 | 685 .0 |
520 | 3m | Zabrodje, Wilna, Russia. | Sept. | 22, | 1894 | 3 .0 |
521 | 3m | Fisher, Polk County, Minnesota, U.S.A. | April | 9, | 1894 | 603 .0 |
522 | 3m | Bori,Badnúr, Betul District, Central Provinces, India. | May | 9, | 1894 | 1,270 .0 |
523 | 3m | Savtschenskoje, Kherson, Russia. | July | 27, | 1894 | 62 .0 |
524 | 3m | Bishunpur (and Parjabatpur), Mirzapur District, North-West Provinces, India. | April | 26, | 1895 | 392 .0 |
525 | 3m | Nagy-Borové, Liptau, Hungary. | May | 9, | 1895 | 53 .0 |
526 | 3m | Ambapur Nagla, Sikandra Rao Tahsil, Aligarh District, North-West Provinces, India. | May | 27, | 1895 | 1,075 .0 |
527 | 3m | Madrid, Spain. | Feb. | 10, | 1896 | 18 .0 |
528 | 3m | Ottawa, Franklin County, Kansas, U.S.A. | April | 9, | 1896 | 90 .0 |
529 | 3n | Lesves, Namur, Belgium. | April | 13, | 1896 | 56 .0 |
530 | 3n | Kangra North Eastern Punjab, India. | Before Aug. | 1897 | 395 .0 | |
531 | 3n | Meuselbach, Thuringia, Germany. | May | 19, | 1897 | 19 .0 |
532 | 3n | Lançon, Bouches-du-Rhône, France. | June | 20, | 1897 | 199 .0 |
533 | 3n | Zavid, District Zwornik, Bosnia. | Aug. | 1, | 1897 | 267 .0 |
534 | 3n | Higashikoen, Fukuoka, Chikuzen, Japan. | Aug. | 11, | 1897 | 32 .0 |
535 | 3n | Gambat, Khairpur State, Sind, India. | Sept. | 15, | 1897 | 1,752 .0 |
536 | 3n | Saline Township, Sheridan County, Kansas, U.S.A. | Nov. | 15, | 1898(?) | 172.0 |
537 | 3n | Zomba, British Central Africa. | Jan. | 25, | 1899 | 2,413 .0 |
538 | 3e | Bjurböle, Borgå, Finland. | March | 12, | 1899 | 153 .0 |
[pg 104]539 | 2e | Allegan, Michigan, U.S.A. | July | 10, | 1899 | 763 .0 |
540 | 2e | Donga Kohrod, Bilatpur, India. | Sept. | 23, | 1899 | 39 .0 |
541 | 2e | Sindhri, Thar and Parkar District, Bombay, India. | June | 10, | 1901 | 1,199 .0 |
542 | 2e | Andover, Oxford County, Maine, U.S.A. | Aug. | 5, | 1901 | 20 .0 |
543 | 2e | Hvittis, Åbo Län, Finland. | Oct. | 21, | 1901 | 159 .0 |
544 | 2e | Palézieux, Lausanne, Switzerland. | Nov. | 30, | 1901 | 29 .0 |
545 | 2e | Mount Browne, Evelyn County, New South Wales. | July | 17, | 1902 | 148 .0 |
546 | 2e | Caratash, Smyrna, Asia Minor. | Aug. | 22, | 1902 | 8 .0 |
547 | 2e | Crumlin, County Antrim, Ireland. | Sept. | 13, | 1902 | 3,860 .0 |
548 | 2e | Bath Furnace, Bath County, Kentucky, U.S.A. | Nov. | 15, | 1902 | 1,013 .0 |
549 | 2e | Uberaba, Minas Geraes, Brazil. | June | 29, | 1903 | 52 .0 |
550 | 2e | Dokáchi, Dacca District, Bengal, India. | Oct. | 22, | 1903 | 622 .0 |
551 | 2e | Shelburne, Grey County, Ontario, Canada. | Aug. | 13, | 1904 | 1,791.0 |
B. Fall not Recorded.
[Arranged topographically.]
| No. | Pane. | Name of Meteorite and Place of Find. | Report of Find. | Weight in grams. |
|---|---|---|---|---|
552 | 3o | Mainz, Hesse, Germany. Described in 1857 by Seelheim: it had been turned up by a plough some years before. | Jahrb. d. Ver. für Naturk. im Nassau, 1857, p. 405. | 33 .0 |
553 | 3o | Oczeretna, Lipovitz, Kiev, Russia. Found in the summer of 1871. | 109 .0 | |
554 | 3o | Assam, India. Found in 1846 in the refuse of the "Coal and Iron Committee's" collections, probably obtained from Assam. | Proc. Asiatic Soc. Bengal, June, 1846, pp. xlvi, lxxvi. | 539 .0 |
555 | 4h | Goalpara, Assam, India. Found among some specimens obtained from the neighbourhood of Goalpara: described by Haidinger in 1869. | Wien. Akad. Ber. 1869, vol. 59, part 2, p. 665. | 1,187 .0 |
556 | 3o | Kota-Kota, Marimba District, British Central Africa. |
| 333 .0 |
557 | 3o | Warbreccan, Windorah, Diamantina District, Queensland. |
| 61,223.0 |
[pg 105]558 | 3o | Barratta, Deniliquin, New South Wales. One person thought he saw it fall in the month of May, about 1860: another reports that he saw the mass lying on the ground in 1845. | Trans. Roy. Soc. of New South Wales, 1872, vol. 6, p. 97. | 2,724.0 |
Two other masses were described by Liversidge in 1902. | Jour. and Proc. Roy. Soc. New South Wales, 1902, vol. 36, p. 350. | |||
559 | 3o | Gilgoin, New South Wales: described by Russell in 1889. | Jour. & Proc. Roy. Soc. New South Wales, 1889, vol. 23, p. 47. | 1,975.0 |
A second mass, found later, was described by Liversidge in 1902. | Jour. & Proc. Roy. Soc. New South Wales, 1902, vol. 36, p. 354. | |||
560 | 3o | Makariwa, Invercargill, New Zealand. Found in clay, about 2½ ft. from the surface, in 1879: described by Ulrich and L. F. in 1893-4. | Proc. Roy. Soc., 1893, vol. 53, p. 54: Mineralog. Magazine, 1894, vol. 10, p. 287. | 62 .0 |
561 | 3o | Tomhannock Creek, County, New York, U.S.A. Found about the year 1863: described by Bailey in 1887: Brezina points out a close likeness of this stone, and also of "Yorktown," to those of Amana. | Rensselaer Amer. Jour. Sc. 1887, ser. 3, vol. 34, p. 60: Ann.d.k.k. Naturh. Hofmus. Wien, 1896, vol. 10, p. 251. | 21 .0 |
562 | 3o | Morristown, Hamblen County, Tennessee, U.S.A. Found in 1887: described by Eakins in 1893. | Amer. Jour. Sc. 1893, ser. 3; vol. 46, p. 283. | 560 .0 |
563 | 3o | Elm Creek, Admire, Lyon County, Kansas, U.S.A. Found in 1906: described by Howard in 1907. | Amer. Jour. Sci. 1907 ser. 4, vol. 23, p. 379. | 912 .0 |
564 | 3o | Waconda, Mitchell County, Kansas, U.S.A. Found in 1873 in the grass, upon the slope of a ravine: described by Shepard and by Patrick in 1876. | Amer. Jour. Sc. 1876, ser. 3, vol. 11, p. 473: Trans. Kansas Ac. Sc. 1876, vol. 5, p. 12. | 369 .0 |
565 | 3o | Prairie Dog Creek, Decatur County, Kansas, U.S.A. Reported and described by Weinschenk in 1895. | Tschermak's Min. und Petrog. Mitth. 1894-5, vol. 14, p. 471. | 529 .0 |
566 | 3o | Long Island, Phillips County, Kansas, U.S.A. Reported and described by Weinschenk in 1895. | Ibid. | 1,288 .0 |
[pg 106]567 | 3o | Oakley, Logan County, Kansas, U.S.A. Found in 1895: described by Preston in 1900. | Amer. Jour. Sc. 1900, ser. 4, vol. 9, p. 410. | 2,495 .0 |
568 | 3o | Kansada, Ness County, Kansas, U.S.A. Found in 1894. |
| 2,005 .0 |
569 | 3o | Ness City, Ness County, Kansas, U.S.A. Found in 1898: described by Ward in 1899. | Amer. Jour. Sc. 1899, ser. 4, vol. 7, p. 233. | 667 .0 |
570 | 3o | Utah, U.S.A. Found in 1869 on the open prairie between Salt Lake City and Echo, Utah: described by Dana and Penfield in 1886. | Amer. Jour. Sc. 1886, ser. 3, vol. 32, p. 226. | 4 .0 |
571 | 3o | McKinney, Collin County, Texas, U.S.A. |
| 290 .0 |
572 | 3o | Bluff, 3 miles S. W. of La Grange, Fayette County, Texas. Found about 1878, and described by Whitfield and Merrill in 1888. | Amer. Jour. Sc. 1888, ser. 3, vol. 36, p. 113. | 12,565.0 |
573 | 3o | Pipe Creek, Bandera County, Texas, U.S.A. Found in 1887: described by Ledoux in 1888-9. | Trans. of New York Ac. of Sc., 1888-9, vol. 8, p. 186. | 822 .0 |
574 | 4a | Estacado, Hale County, Texas, U.S.A. Found in 1902: described by Howard in 1906. | Amer. Jour. Sc. 1906, ser. 4, vol. 22, p. 55. | 17,103.0 |
575 | 3o | Cobija, Tocopilla, Antofagasta, Chili, S. America. Found in 1902: described by Ward in 1906. | Proc. Rochester Ac. Sci. 1906, vol. 4, p. 229. | 252 .0 |
576 | 3o | The Lutschaunig stone, Atacama, Chili. | Mineralog. Magazine 1889, vol. 8, p. 234. | 92 .0 |
577 | 3o | Carcote, Atacama, Chili, S. America. Known since 1888: described by Sandberger in 1889. | Neues Jahrb. f. Min., 1889, vol. 2, p. 173. | 2 .0 |
578 | 3o | Santiago, Chili. |
| 301 .0 |
579 | 3o | Minas Geraes (?), Brazil. Found without label among specimens which may have been brought from Minas Geraes: mentioned by Derby in 1888. | Revista do Observatorio, Rio de Janeiro, 1888. | 65.0 |
580 | 3o | Indio Rico, Buenos Ayres, Argentina. Described by Kyle in 1887. | Anales de la Sociedad Científica Argentina, 1887, vol. 24, p. 128. | 1·5 |
(Meteorites for the First Time Included in the List.)
Angelas | No. | 210 | Mern | No. | 472 |
Billings | No. | 131 | Narraburra | No. | 47 |
Boogaldi | No. | 47 | Rodeo | No. | 182 |
Canyon City | No. | 148 | Santiago | No. | 578 |
Cobija | No. | 575 | Shelburne | No. | 551 |
Dokáchi | No. | 550 | Tanokami | No. | 35 |
El Inca | No. | 194 | Uberaba | No. | 549 |
Elm Creek | No. | 563 | Uwet | No. | 36 |
Estacado | No. | 574 | Warbreccan | No. | 557 |
Ilimaes | No. | 236 | Weaver's Mountains | No. | 154 |
Kangra | No. | 530 | Willamette | No. | 147 |
Kota-Kota | No. | 556 | Yonōzu | No. | 326 |
Of the preceding meteorites the following have fallen within the British Isles:—
| Name | Date of Fall. | |||
|---|---|---|---|---|
1. In England— | Wold Cottage | December | 13, | 1795 |
Launton | February | 15, | 1830 | |
Aldsworth | August | 4, | 1835 | |
Rowton | April | 20, | 1876 | |
Middlesbrough | March | 14, | 1881 | |
2. In Scotland— | High Possil | April | 5, | 1804 |
Perth | May | 17, | 1830 | |
3. In Ireland— | Mooresfort | August, | 1810 | |
Adare | September | 10, | 1810 | |
Killeter | April | 29, | 1844 | |
Dundrum | August | 12, | 1865 | |
Crumlin | September | 13, | 1902 | |
One of them, Rowton, is a meteoric iron; the rest are meteoric stones.
NATIVE IRON (of terrestrial origin).
(Pane 4m.)
| Name of Iron and Place of Find. | Report of Find. |
|---|---|
Niakornak, Jakobshavn District, West Greenland. (Rink's iron). Part of a lump obtained (1848-50) by Dr. Rink from a Greenlander who lived at Niakornak: it had been found not far from his home, lying loose on a pebble-strewn plain near the coast. | Oversigt over det koniglike danske vidensk. selsk. forh. 1854, p. 1. |
Jakobshavn, West Greenland (The Pfaff-Öberg iron). Part of a lump given by Dr. Pfaff of Jakobshavn to Dr. Öberg in 1870: it was said to have been found in the neighbourhood (perhaps near Niakornak). | Geological Magazine, 1872, vol. 9, p. 520. |
Ovifak, Disko Island, West Greenland. Found by Baron N. A. E. Nordenskiöld in 1870. | Geological Magazine, 1872, vol. 9, p. 460. |
New Zealand (Jackson's Bay). Found in 1885, and described by Skey in the same year (Awaruite). | Trans. and Proc. of New Zealand Institute, 1885, vol. 18, p. 401. |
South America. Found in an old collection; described by Högbom in 1902. | Bull. of the Geol. Instit. of the Univ. of Upsala, 1902, vol. 5, p. 277. |
PSEUDO-METEORITES
which have been described as Meteorites.
(In Drawers.)
Aachen, Rhenish Prussia.
Braunfels, Coblenz.
Campbell County, Tennessee, U.S.A.
Canaan, Connecticut, U.S.A.
Collina di Brianza, Milan, Italy.
Concord, New Hampshire, U.S.A.
Gross-Kamsdorf, Saxony.
Haywood County, N. Carolina, U.S.A.
Heidelberg, Germany.
Hemalga, Desert of Tarapaca, S. America.
Hommoney Creek, Buncombe County, N. Carolina, U.S.A.
Igast, Livland, Russia.
Kamtschatka, Asiatic Russia.
Leadhills, Lanarkshire, Scotland.
Long Creek, Jefferson County, New York, U.S.A.
Magdeburg, Prussia.
Nauheim, Giessen, Germany.
New Haven, Connecticut, U.S.A.
Newstead, Roxburghshire, Scotland.
Nöbdenitz, Saxon Altenburg.
Richland, S. Carolina, U.S.A.
Rutherfordton, N. Carolina, U.S.A.
St. Augustine's Bay, Madagascar.
Scriba, Oswego County, New York, U.S.A.
South America.
Sterlitamak, Russia.
Voigtland,Saxony.
Waterloo, New York, U.S.A.
Meteorites are generally represented in collections by mere fragments of the original specimens, which often fail to give any idea of the original size and shape. Before division of a specimen a cast of it is sometimes prepared, and a representation of the size and shape is thus preserved.
Casts of most of the following meteorites are exhibited in the lower parts of the cases:—
The Trustees possess moulds of those meteorites in the preceding list of which the names are printed in italics, and casts may be obtained on payment of the necessary expenses. Applications should be made in writing to the formatori, D. Brucciani & Co., 254 Goswell Road, London, E.C.
TO THE METEORITES REPRESENTED IN THE COLLECTION ON MAY 1, 1908.
The names adopted for the meteorites are printed in thick type: the other names are synonyms.
The numbers correspond with those of the first column of the meteorite list.
Aachen, (pseudo-meteorite).
Abert iron (unknown locality), 217
Adare, 282
Admire, 234
Aeriotopos v. Bear Creek, 144
Agen, 286
Agra, 298
Agra v. Khiragurh, 391
Agram, 1
Aigle v. L'Aigle, 259
Ainsa iron v. Tucson, 155
Akbarpur, 330
Akershuus v. Ski, 356
Alais, 267
Alatyr, 498
Albareto, 247
Albuquerque v. Glorieta Mountain, 158b
Aldsworth, 323
Aleppo, 450
Alessandria, 390
Alexejevka v. Bachmut, 285
Alexinatz v. Soko-Banja, 468
Alfianello, 490
Algoma, 135
Allahabad v. Futtehpur, 300
Allegan, 539
Allen County v. Scottsville, 118
Amana, 455
Ambapur Nagla, 526
Andover, 542
Angelas, 210
Angers, 297
Angra dos Reis, 431
Antifona v. Collescipoli, 507
Apoala, 189
Apt, 260
Arispe, 176
Arlington, 132
Arva, 21
Asco, 266
Asheville, 80
Asheville v. Black Mountain, 79
Assam, 554
Assisi, 497
Aubres, 324
Auburn, 95
Augusta County v. Staunton, 67
Augustinovka, 26
Aukoma v. Pillistfer, 401
Aumale, 412
Aumières, 341
Ausson, 384
Authon v. Lancé, 445
Babb's Mill, 102
Bachmut, 285
Bacubirito v. El Ranchito, 177
Bahia v. Bendegó River, 212
Baird's Farm v. Asheville, 80
Baird's Plantation v. Asheville, 80
Baldohn, 508
Ballinoo, 54
Bambuk v. Senegal River, 229
Bancoorah v. Shalka, 359
Bandong, 442
Barbotan, 253
Barranca Blanca, 208
Barratta, 558
Basti v. Bustee, 366
[pg 112]
Bates County v. Butler, 130
Bath, 516
Bath Furnace, 548
Batsura v. Butsura, 395
Beaconsfield v. Cranbourne, 50b
Bear Creek, 144
Beaver Creek, 519
Bécasse v. La Bécasse, 475
Behar v. Sherghotty, 413
Belaja-Zerkov v. Bjelaja Zerkov, 256
Belgorod v. Sevrukovo, 451
Bella Roca, 181
Bendegó River, 212
Benares v. Krakhut, 258
Berar v. Chandakapur, 331
Beraun v. Zebrak, 304
Berlanguillas, 278
Bethany, 37
Bethlehem, 389
Beuste, 388
Bhagur, 470
Bherai, 518
Bhurtpur v. Moti-ka-nagla, 430
Bialystock, 310
Bielokrynitschie, 500
Billings, 131
Bischtübe, 27
Bishopville, 342
Bishunpur, 524
Bissempore v. Shalka, 359
Bitburg, 13
Bithur v. Futtehpur, 300
Bjelaja Zerkov, 256
Bjurböle, 538
Blaauw-Kapel v. Utrecht, 343
Black Mountain, 79
Blansko, 319
Bluff, 572
Bocas v. Hacienda de Bocas, 264
Bogota v. Rasgata, 193
Bohumilitz, 19
Bois de Fontaine v. Charsonville, 276
Bokkeveldt v. Cold Bokkeveld, 333
Bolson de Mapimi v. Coahuila, 170a
Bolson de Mapimi v. Sanchez Estate, 170b
Bonanza iron v. Coahuila, 170a
Boogaldi, 45
Borgo San Donino, 270
Bori, 522
Borkut, 365
Brahin, 226
Braunau, 3
Braunfels (pseudo-meteorite).
Brazos River, 162
Breitenbach, 225c
Bremervörde v. Gnarrenburg, 372
Brenham Township, 233
Bridgewater, 77
Bubuowly v. Supuhee, 408
Budetin v. Nagy-Diwina, 327
Bückeburg v. Obernkirchen, 12
Bueste v. Beuste, 388
Bugaldi v. Boogaldi, 45
Bunzlau v. Lissa, 272
Burlington, 63
Buschhof, 400
Bustee, 366
Butcher iron v. Coahuila, 170a
Butler, 130
Butsura, 395
Cabarras County, 357
Cabeza de Mayo, 438
Cabin Creek, 8
Cacaria, 179
Cachiyuyal, 200
Caille v. La Caille, 10
Callac v. Kerilis, 454
Cambria v. Lockport, 61
Campbell County, (pseudo-meteorite).
Campo del Cielo v. Otumpa, 211
Campo de Pucará v. Imilac, 235
Canaan (pseudo-meteorite).
Canara v. Udipi, 415
Canellas, 396
Caney Fork, 108
Cangas de Onis, 240
Cañon Diablo, 153
Canton, 89
Canyon City, 148
Cape Girardeau, 351
Cape of Good Hope, 40
Caperr, 214
Capitan Range, 157
Caracoles v. Imilac, 235
Caratash, 546
Carcoar v. Cowra, 46
Carcote, 577
Carleton iron v. Tucson, 155
Carlton, 165
Carroll County v. Eagle Station, 232
Carthage, 107
Caryfort v. Caney Fork, 108
Casale v. Cereseto, 337
Casas Grandes, 174
Casey County, 117
Castalia v. Nash County, 452
Castine, 354
Catorze v. Descubridora, 183
Central Missouri, 129
Cereseto, 337
Cerro Cosina, 346
[pg 113]
Chail, 287
Chandakapur, 331
Chandpur, 495
Chantonnay, 281
Charcas, 184
Charkow v. Kharkov, 252
Charleston v. Jenny's Creek, 70
Charlotte, 2
Charlottetown v. Cabarras County. 357
Charsonville, 276
Chartres v. Charsonville, 276
Charwallas, 321
Chassigny, 289
Château-Renard, 339
Cherokee Mills v. Canton, 89
Cherson v. Vavilovka, 461
Chesterville, 82
Chili, 209
Christian County v. Billings, 131
Chulafinnee, 94
Chupaderos, 173
Cirencester v. Aldsworth, 323
Claiborne, 98
Claiborne County v. Tazewell, 103
Clarac v. Ausson, 384
Clarke County v. Claiborne, 98
Claywater Stone v. Vernon County. 409
Cleberne County v. Chulafinnee, 94
Cléguérec, 434
Cleveland, 105
Coahuila, 170a
Cobija, 575
Cocke County, 101
Cold Bokkeveld, 333
Colfax v. Ellenboro', 76
Collescipoli, 507
Collina di Brianza (pseudo-meteorite).
Commune des Ormes v. Les Ormes. 380
Concepcion, 172
Concord (pseudo-meteorite)
Coneyfork v. Caney Fork, 108
Coopertown, 111
Copiapo, 240
Cosby's Creek v. Cocke County, 101
Cosona v. Siena, 254
Cossipore v. Manbhoom, 404
Costa Rica v. Heredia, 377
Costilla Peak, 156
Cowra, 46
Cranbourne, 50
Crawford County v. Taney County. 218
Cronstad, 469
Cross Roads, 514
Cross Timbers v. Red River, 164
Crow Creek, 140
Crumlin, 547
Cuernavaca, 187
Cusignano v. Borgo San Donino, 270
Cynthiana, 465
Czartorya v. Zaborzika, 290
Dacca v. Shytal, 402
Dakota, 136
Dalton v. Whitfield County, 87
Dandapur, 473
Daniel's Kuil, 424
Danville, 428
Darmstadt, 262
Davis Strait v. Melville Bay, 56
Deal, 313
Debreczin v. Kaba, 379
Decatur County v. Prairie Dog Creek. 565
Deep Springs, 72
Deesa v. Copiapo, 240
De Kalb County v. Caney Fork, 108
Denton County, 163
Denver v. Bear Creek, 144
Descubridora, 183
Dhulia v. Bhagur, 470
Dhurmsala, 394
Dickson County v. Charlotte, 2
Disko Island v. Ovifak (telluric).
Djati-Pengilon, 493
Dokáchi, 550
Dolgaja Wolja v. Dolgovoli, 407
Dolgovoli, 407
Doña Inez, 239
Donga Kohrod, 540
Dooralla v. Durala, 288
Doroninsk, 265
Drake Creek, 309
Duel Hill v. Jewell Hill, 78b
Dundrum, 411
Durala, 288
Duruma, 369
Dyalpur, 443
Eagle Station, 232
East Tennessee v. Cleveland, 105
Echigo v. Yonōzu, 325
Echo v. Utah, 570
Eichstädt, 251
Eifel v. Bitburg, 13
Elbogen, 18
Elgueras v. Cangas de Onis, 420
El Inca, 194
Ellenboro', 76
Elm Creek, 563
[pg 114]
Elmo v. Independence County, 126
El Ranchito, 177
Emmet County v. Estherville, 220
Emmittsburg, 66
Ensisheim, 241
Epinal, 299
Ergheo, 505
Erxleben, 280
Eschigo v. Yonōzu, 326
Esnandes, 328
Estacado, 574
Estherville, 220
Faha v. Adare, 282
Farmington, 512
Fatchpur v. Futtehpur, 300
Favars, 348
Fayette County v. Bluff, 572
Fekete v. Mezö-Madaras, 364
Finmarken, 223
Fisher, 521
Fish River v. Great Fish River, 37a
Floyd, County v. Indian Valley Township, 68
Fomatlan v. Tomatlan, 479
Forest City v. Winnebago County, 509
Forsyth, 312
Forsyth County, 91
Fort Duncan, 169
Fort Pierre v. Nebraska, 139
Franceville, 145
Frankfort (Alabama), 429
Frankfort (Kentucky), 114
Franklin County v. Frankfort, 114, 429
Fürstenburg v. Klein-Menow, 398
Fukutomi, 486
Fulton County v. Rochester, 463
Futtehpur, 300
Gambat, 535
Gargantillo v. Tomatlan, 479
Garz v. Schellin, 242
Gera v. Pohlitz, 294
Ghazeepore v. Mhow, 308
Ghent v. St. Denis-Westrem, 373
Ghoordha v. Moti-ka-nagla, 430
Gilgoin, 559
Girgenti, 367
Glorieta Mountain, 158a, 158b
Gnadenfrei, 477
Gnarrenburg, 372
Goalpara, 555
Gopalpur, 410
Gran Chaco v. Otumpa, 211
Grand Rapids, 122
Great Fish, River v. Bethany, 37a
Great Fish, River v. Cape of Good Hope, 40
Great Nama
ualand v. Bethany, 37
Greenbrier County, 69
Green County v. Babb's Mill, 102
Grenade v. Toulouse, 279
Griualand v. Daniel's Kuil, 424
Grosnaja, 397
Gross-Diwina v. Nagy-Diwina, 327
Gross-Kamsdorf, (pseudo-meteorite)
Gross-Liebenthal, 484
Grüneberg, 338
Guareña, 515
Guernsey County v. New Concord, 392
Gütersloh, 360
Guilford County, 73
Gurram Konda, 284
Hacienda de Bocas, 264
Hainholz, 224
Hamblen County v. Morristown, 562
Hamilton County v. Carlton, 165
Hammond Township, 134
Harrison County, 386
Hartford v. Linn County, 353
Hauptmannsdorf v. Braunau, 3
Hawaii v. Honolulu, 306
Hayden Creek, 146
Haywood County, (pseudo-meteorite).
Heidelberg (pseudo-meteorite).
Heinrichsau v. Grüneberg, 338
Hemalga (pseudo-meteorite).
Heredia, 377
Hessle, 432
Hex River Mountains, 39
Higashikoen, 534
High Possil, 263
Holland's Store, 90
Homestead v. Amana, 455
Hommoney Creek (pseudo-meteorite).
Honolulu, 306
Horzowitz v. Zebrak, 304
Howard County, 124
Hraschina v. Agram, 1
Huesca v. Roda, 439
Hungen, 466
Hvittis, 543
Ibbenbühren, 437
Igast (pseudo-meteorite).
[pg 115]
Iglau v. Stannern, 271
Iharaota v. Lalitpur, 501
Ihung v. Jhung, 447
Ilimaë, 201
Ilimaes, 236
Illinois Gulch, 141
Imilac, 235
Indarch, 513
Independence County, 126
Indian Valley Township, 68
Indio Rico, 580
Iowa v. Amana, 455
Iquique v. El Inca, 194
Iron Creek, 60
Irwin-Ainsa iron v. Tucson, 155
Itapicuru-Mirim, 476
Ivanpah, 151
Jackson County, 106
Jakobshavn (telluric).
Jamaica v. Lucky Hill, 191
Jamestown, 137
Jamkheir, 419
Janacera Pass v. Vaca Muerta, 237
Japan v. Ogi, 244
Jarquera v. Vaca Muerta, 237
Jasly v. Bialystock, 310
Jelica, 506
Jenny's Creek, 70
Jewell Hill, 78
Jharaota v. Lalitpur, 501
Jhung, 447
Jigalowka v. Kharkov, 252
Jodzie, 467
Joel iron, 206
Johanngeorgenstadt v. Steinbach, 225a
Jonzac, 293
Juchnow v. Timochin, 268
Judesegeri, 460
Juncal, 204
Juvinas, 296
Kaande v. Oesel, 371
Kaba, 379
Kadonah v. Agra, 298
Kaee, 329
Kahangarai, 510
Kakangarai v. Kahangarai, 510
Kakowa, 383
Kalambi, 480
Kamtschatka (pseudo-meteorite).
Kangra, 530
Kansada, 568
Karakol, 335
Karand v. Veramin, 221
Karlsburg v. Ohaba, 381
Kathiawar v. Bherai, 518
Kendall County, 166
Kenton County, 112
Kerilis, 454
Kernouvé v. Cléguérec, 434
Kesen, 358
Khairpur, 448
Kharkov, 252
Kheragur v. Khiragurh, 391
Khetri, 421
Khiragurh, 391
Kikino, 274
Kiowa County v. Brenham Township, 233
Killeter, 347
Klein-Menow, 398
Klein-Wenden, 345
Knasta v. Bialystock, 310
Knoxville v. Tazewell, 103
Knyahinya, 418
Kodaikanal, 34
Köstritz v. Pohlitz, 294
Kokomo v. Howard County, 124
Kokstad, 41
Kota-Kota, 556
Koursk v. Sevrukovo, 451
Krähenberg, 433
Krakhut, 258
Krasnoi-Ugol, 314
Krasnojarsk v. Pallas iron, 227
Krasnoslobodsk v. Alatyr, 498
Krawin v. Tabor, 245
Kuleschovka, 277
Kusiali, 393
La Baffe v. Epinal, 299
La Bécasse, 475
Laborel, 441
La Caille, 10
Lagrange, 113
L'Aigle, 259
Laissac v. Favars, 348
Lalitpur, 501
Lancé, 445
Lançon, 532
Langenpiernitz v. Stannern, 271
Langres v. Chassigny, 289
La Primitiva, 196
Lasdany v. Lixna, 295
Launton, 315
Laurens County, 83
La Vivionnère v. Le Teilleul, 349
Leadhills (pseudo-meteorite).
Lebedin v. Kharkov, 252
Lénárto, 20
Lerici, 423
Les Ormes, 380
Lesves, 529
Le Teilleul, 349
Lexington County, 85
[pg 116]
Lexington County v. Ruff's Mountain, 84
Libonnez v. Juvinas, 296
Liboschitz v. Plescowitz, 243
Lick Creek, 74
Lime Creek v. Claiborne, 98
Limerick v. Adare, 282
Linn County, 353
Linnville Mountain, 75
Linum, 370
Lion River v. Bethany, 37b
Liponnas v. Luponnas, 246
Lissa, 272
Little Piney, 334
Livingston County v. Smithland, 119
Lixna, 295
Ljungby v. Lundsgård, 503
Llano del Inca, 238
Lockport, 61
Locust Grove, 92
Lodran, 219
Long Creek (pseudo-meteorite).
Long Island, 566
Lontolax v. Luotolaks, 283
Losttown, 88
Louisiana v. Red River, 164
Louvain v. Tourinnes-la-Grosse, 403
Lucé, 248
Lucky Hill, 191
Luis Lopez, 160
Lumpkin v. Stewart County, 436
Lundsgård, 503
Luotolaks, 283
Luponnas, 246
Lutschaunig stone, 576
Macao, 325
Macayo v. Macao, 325
Macedonia v. Seres, 291
Macerata v. Monte Milone, 350
Macon County v. Auburn, 95
Madagascar v. St. Augustine's Bay (pseudo-meteorite).
Maddur taluk v. Muddoor, 414
Madioen v. Ngawi, 491
Madoc, 57
Madrid, 527
Mael Pestivien v. Kerilis, 454
Maêmê v. Oshima, 499
Mässing, 261
Magdalena v. Luis Lopez, 160
Magdeburg (pseudo-meteorite).
Magdeburg v. Erxleben, 280
Magura v. Arva, 21
Mainz, 552
Makariwa, 560
Mánbazar pargama v. Manbhoom, 404
Manbhoom, 404
Manegaum, 344
Mantos Blancos v. Mount Hicks, 197
Marimba District v. Kota-Kota, 556
Marion v. Linn County, 353
Marjalahti, 222
Marmande, 355
Marmoros v. Borkut, 365
Marshall County, 120
Mart, 167
Maryland v. Nanjemoy, 305
Mascombes, 322
Mau v. Mhow, 308
Mauerkirchen, 249
Mauléon v. Sauguis, 427
Mazapil, 7
McKinney, 571
Medwedewa v. Pallas iron, 227
Mejillones v. Vaca Muerta, 237
Melbourne v. Cranbourne, 50
Melville Bay, 56
Menow v. Klein-Menow, 398
Merceditas, 202
Mern, 472
Meuselbach, 531
Mexico v. Pampanga, 387
Mezö-Madaras, 364
Mhow, 308
Middlesbrough, 482
Midt-Vaage v. Tysnes, 494
Mighei v. Migheja, 504
Migheja, 504
Mikenskoi v. Grosnaja, 397
Miljana v. Milena, 340
Milena, 340
Milwaukee v. Trenton, 133
Minas Geraes, 579
Misshof v. Baldohn, 508
Missouri v. South-East Missouri, 127
Misteca v. Yanhuitlan, 188
Mocs, 485
Moctezuma, 175
Modena v. Albareto, 247
Molina, 385
Monroe v. Cabarras County, 357
Montauban v. Orgueil, 406
Monte Milone, 350
Montignac v. Marmande, 355
Montlivault, 332
Montréjean v. Ausson, 384
Mooltan v. Lodran, 219
Mooranoppin, 55
Mooresfort, 275
Moradabad, 273
Morbihan v. Cléguérec, 434
Mordvinovka v. Pavlograd, 307
Mornans, 459
[pg 117]
Morristown, 562
Morro do Rocio v. Santa Catharina. 213
Moteeka Nugla v. Moti-ka-nagla, 430
Moti-ka-nagla, 430
Mount Browne, 545
Mount Dyrring, 230
Mount Hicks, 197
Mount Joy, 65
Mount Stirling, 53
Mount Zomba v. Zomba, 537
Mouza Khoorna v. Supuhee, 408
Muddoor, 414
Mukerop v. Bethany, 37d
Mungindi, 44
Murcia v. Cabeza de Mayo, 438
Murcia v. Molina, 385
Murfreesboro', 110
Murphy, 81
Muskingum County v. New Concord. 392
Nagaria, 458
Nagaya, 478
Nagy-Borové, 525
Nagy-Diwina, 327
Nagy-Vázsony, 22
Nammianthal, 496
Nanjemoy, 305
Napoléonsville v. Cléguérec, 434
Narraburra, 47
Nash County, 452
Nashville v. Drake Creek, 309
Nauheim (pseudo-meteorite).
Nawapali, 511
Nebraska, 139
Nedagolla, 5
Nejed, 33
Nellore, 363
Nelson County, 116
Nenntmannsdorf, 16
Nerft, 405
Ness City, 569
Ness County v. Kansada, 568
Ness County v. Ness City, 569
Netschaëvo v. Tula, 23
Newberry v. Ruff's Mountain, 84
New Concord, 392
New Haven (pseudo-meteorite).
Newstead (pseudo-meteorite).
Newton County v. Taney County, 218
New Zealand (telluric).
Ngawi, 491
N'Goureyma, 9
Niagara, 138
Niakornak (telluric).
Nidigullam v. Nedagolla, 5
Nobleborough, 302
Nochtuisk, 32
Nocoleche, 48
Nöbdenitz (pseudo-meteorite).
North Inch of Perth v. Perth. 316
Novo-Urei v. Alatyr, 498
Nulles, 362
Nurrah v. Sitathali, 456
Oakley, 567
Oaxaca v. Yanhuitlan, 188
Obernkirchen, 12
Ocatitlan v. Toluca, 186
Oczeretna, 553
Oesel, 371
Oficina Angelas v. Angelas, 210
Ogi, 244
Ohaba, 381
Okniny, 320
Oktibbeha County, 100
Oldham County v. Lagrange, 113
Orange River, 38
Orgueil, 406
Orléans v. Charsonville, 276
Ormes v. Les Ormes, 380
Ornans, 426
Oroville, 149
Orvinio, 446
Oscuro Mountain, 161
Oshima, 499
Oswego County v. Scriba (pseudo-meteorite).
Otsego County v. Burlington, 63
Ottawa, 528
Ottiglio v. Cereseto, 337
Otumpa, 211
Oude v. Kaee, 329
Ovifak (telluric).
Oynchimura v. Oshima, 499
Pacula, 483
Palézieux, 544
Pallas iron, 227
Pampa de Tamarugal v. El Inca. 194
Pampanga, 387
Pan de Azucar, 203
Parma v. Borgo San Donino, 270
Parnallee, 376
Pavlodar, 228
Pavlograd, 307
Pavlovka, 487
Pegu, 382
Penkarring Rock v. Youndegin, 51
Pennyman's Siding v. Middlesbrough. 482
Perth, 316
[pg 118]
Petersburg, 374
Petropavlovsk, 28
Pfaff-Öberg v. Jakobshavn (telluric).
Philippine Islands v. Pampanga, 387
Phillips County v. Long Island, 566
Pillistfer, 401
Pine Bluff v. Little Piney, 334
Pipe Creek, 573
Pirgunje, 488
Pirthalla, 492
Pittsburg, 64
Plescowitz, 243
Ploschkowitz v. Plescowitz, 243
Plymouth, 125
Pohlitz, 294
Pokhra, 416
Politz v. Pohlitz, 294
Poltawa v. Kuleschovka, 277
Poltawa of Partsch v. Slobodka, 292
Powder Mill Creek, 231
Prachin v. Bohumilitz, 19
Prairie Dog Creek, 565
Prambanan, 42
Praskoles v. Zebrak, 304
Pricetown, 517
Pulaski v. Little Piney, 334
Pulsora, 399
Pultusk, 423
Puquios, 205
Pusinsko Selo v. Milena, 340
Putnam County, 93
Quenggouk v. Pegu, 382
Quinçay, 361
Raepur v. Sitathali, 456
Rakovka, 474
Ranchito v. El Ranchito, 177
Rancho de la Pila, 178
Rasgata, 193
Red River, 164
Reed City, 123
Reichstadt v. Plescowitz, 243
Renazzo, 303
Rhine Valley v. Rhine Villa, 49
Rhine Villa, 49
Richland (pseudo-meteorite).
Richmond, 311
Rink's iron v. Niakornak (telluric).
Rittersgrün, 225b
Robertson County v. Coopertown, 111
Rochester, 463
Rockwood v. Powder Mill Creek, 231
Roda, 439
Rodeo, 182
Roebourne, 52
Rokičky v. Brahin, 226
Roquefort v. Barbotan, 253
Rosario, 190
Ross's iron v. Melville Bay, 56
Rowton, 6
Roxburghshire, v. Newstead (pseudo-meteorite).
Ruff's Mountain, 84
Russel Gulch, 143
Rutherford County v. Murfreesboro', 110
Rutherfordton, (pseudo-meteorite).
Rutlam v. Pulsora, 399
Saboryzy v. Zaborzika, 290
Sacramento Mountains, 159
Saharanpur v. Akbarpur, 330
St. Augustine's Bay (pseudo-meteorite).
St. Caprais-de-Quinsac, 489
St. Denis-Westrem, 373
St. Genevieve County, 128
St. Julien v. Alessandria, 390
St. Mesmin, 417
St. Nicholas v. Mässing, 261
Saintonge v. Jonzac, 293
Saline Township, 536
Salles, 257
Saltillo v. Sanchez Estate, 170b
Salt Lake City v. Utah, 370
Salt River, 115
Sáluká v. Shalka, 359
San Angelo, 168
San Bernardino County v. Ivanpah. 151
Sanchez Estate, 170b
San Cristobal, 199
San Francisco del Mezquital, 180
San Francisco Pass v. Barranca Blanca, 208
San José v. Heredia, 377
San Pedro v. Imilac, 235
Santa Barbara, 449
Santa Catharina, 213
Santa Rosa, 192
Santa Rosa v. Coahuila, 170a
Santa Rosa v. Sanchez Estate, 170b
Santiago, 578
São Julião de Moreira, 11
Saonlod v. Khetri, 421
Sarbanovac v. Soko-Banja, 468
Sarepta, 24
Saskatchewan v. Iron Creek, 60
Sauguis, 427
Saurette v. Apt, 260
[pg 119]
Savtschenskoje, 523
Scheikahr Stattan v. Buschhof, 400
Schellin, 242
Schie v. Ski, 356
Schobergrund v. Gnadenfrei, 477
Schönenberg, 352
Schwetz, 15
Scottsville, 118
Scriba (pseudo-meteorite).
Searsmont, 440
Seeläsgen, 14
Segowlie, 368
Sena, 250
Seneca River (or Falls), 62
Senegal River, 229
Senhadja v. Aumale, 412
Seres, 291
Serrania de Varas, 198
Sevier County v. Cocke County, 101
Sevrukovo, 451
Shahpur v. Futtehpur, 300
Shaital v. Shytal, 402
Shalka, 359
Shelburne, 551
Sherghotty, 413
Shingle Springs, 150
Shytal, 402
Sidowra v. Supuhee, 408
Siena, 254
Sierra Blanca, 171
Sierra de Chaco v. Vaca Muerta, 237
Sierra de Deesa v. Copiapo, 240
Sierra de la Ternera, 207
Signet iron v. Tucson, 155
Sikkensaare v. Tennasilm, 444
Silver Crown v. Crow Creek, 140
Sindhri, 541
Siratik v. Senegal River, 229
Sitathali, 456
Ski, 356
Slavetic, 425
Slobodka, 292
Smithland, 119
Smith's Mountain, 71
Smithsonian iron (unknown locality). 216
Smithville, 109
Socrakarta v. Prambanan, 42
Soko-Banja, 468
South America (telluric).
South Arcot v. Nammianthal, 496
South Canara v. Udipi, 415
South-East Missouri, 127
Sowallick Mountain v. Melville Bay. 56
Springbok River v. Bethany, 37d
Ssyromolotovo, 30
Staartje v. Uden, 336
Ställdalen, 462
Stannern, 271
Staunton, 67
Stavropol, 378
Steinbach, 225a
Sterlitamak (pseudo-meteorite).
Stewart County, 436
Stinking Creek v. Campbell County (pseudo-meteorite).
Stutsman County v. Jamestown, 137
Summit, 96
Supuhee, 408
Surakarta v. Prambanan, 42
Surprise Springs, 152
Szadany v. Zsadány, 457
Szlanicza v. Arva, 21
Tabarz, 17
Tabor, 245
Tabory, 502
Tadjera, 422
Taiga v. Toubil River, 29
Takenouchi, 481
Tamarugal v. El Inca, 194
Taney County, 218
Tanokami, 35
Tarapaca, 195
Tarapaca Desert v. Hemalga (pseudo-meteorite).
Tazewell, 103
Teilleul v. Le Teilleul, 349
Tennasilm, 444
Terni v. Collescipoli, 507
Texas v. Red River, 164
Thunda, 43
Thurlow, 59
Tieschitz, 471
Timochin, 268
Tipperary v. Mooresfort, 275
Tjabé, 435
Tocavita v. Santa Rosa, 192b
Toluca, 186
Tomatlan, 479
Tombigbee River, 99
Tomhannock Creek, 561
Tonganoxie, 142
Toubil River, 29
Toulouse, 279
Tourinnes-la-Grosse, 403
Trenton, 133
Trenzano, 375
Triguères v. Château-Renard, 339
Tucson, 155
Tucuman v. Otumpa, 211
Tula, 23
Turuma v. Duruma, 369
Tysnes, 494
[pg 120]
Uberaba, 549
Uden, 336
Udipi, 415
Umballa, 301
Umjhiawar v. Sherghotty, 413
Union County, 86
Utah, 570
Utrecht, 343
Uwet, 36
Vaca Muerta, 237
Varas v. Serrania de Varas, 198
Vavilovka, 461
Venagas v. Descubridora, 183
Veramin, 221
Veresegyhaza v. Ohaba, 381
Verkhne-Dnieprovsk, 25
Verkhne-Udinsk, 31
Vernon County, 409
Victoria West, 4
Virba, 453
Voigtland (pseudo-meteorite).
Vouillé, 317
Waconda, 564
Waldron Ridge, 104
Walker County, 97
Warbreccan, 557
Warrenton, 464
Washington v. Farmington, 512
Waterloo (pseudo-meteorite).
Wayne County, 121
Weaver's Mountains, 154
Welland, 58
Werchne v. Verkhne
Wessely, 318
West Liberty v. Amana, 455
Weston, 269
Whitfield County, 87
Wichita County v. Brazos River, 162
Wild v. Bethany, 37c
Willamette, 147
Winnebago County, 509
Witim v. Verkhne-Udinsk, 31
Wittmess v. Eichstädt, 251
Wöhler's iron (unknown locality), 215
Wold Cottage, 255
Xiquipilco v. Toluca, 186
Yanhuitlan, 188
Yarra Yarra River v. Cranbourne, 50c
Yatur v. Nellore, 363
Yenshigahara v. Oshima, 499
Yodzé v. Jodzie, 467
Yonōzu, 326
Yorktown v. Tomhannock Creek, 561
Youndegin, 51
Zaborzika, 290
Zabrodje, 520
Zacatecas, 185
Zavid, 533
Zebrak, 304
Ziquipilco v. Toluca, 186
Znorow v. Wessely, 318
Zomba, 537
Zsadány, 457
THE END.
BRITISH MUSEUM (NATURAL HISTORY)
CROMWELL ROAD, LONDON, S.W.
GUIDE BOOKS.
A General Guide to the British Museum (Natural History). 58 Woodcuts, 2 Plans, and 2 Views of the Building. 8vo. 3d.
Zoological Department.
Guide to the Galleries of Mammals (other than Ungulates). 52 Woodcuts and 4 Plans. 8vo. 6d.
—— Great Game Animals (Ungulata). 53 Illustrations. 8vo. 1s.
—— Horse Family (Equidæ). 26 Illustrations. 8vo. 1s.
—— Domesticated Animals (other than Horses). 24 Illustrations. 8vo. 6d.
—— Gallery of Birds. 24 Plates and 7 Woodcuts. Royal 8vo. 2s. 6d.
—— —— Part I. General Series. Royal 8vo. 6d.
—— —— Part II. Nesting Series of British Birds. 4 Plates. Royal 8vo. 4d.
—— Gallery of Reptilia and Amphibia. 76 Illustrations. 8vo. 6d.
—— Gallery of Fishes. 96 Illustrations. 8vo. 1s.
—— Exhibited Series of Insects. 62 Illustrations. 8vo. 1s.
—— Shell and Star-fish Galleries. 125 Woodcuts and 1 Plan. 8vo. 6d.
—— Coral Gallery (Protozoa, Porifera or Sponges, Hydrozoa, and Anthozoa). 90 Illustrations and 1 Plan. 8vo. 1s.
[Guides to other sections are in preparation.]
Geological Department.
A Guide to the Fossil Mammals and Birds. 6 Plates and 88 Woodcuts. 8vo. 6d.
—— Fossil Reptiles and Fishes. 8 Plates and 116 Woodcuts. 8vo. 6d.
—— Fossil Invertebrate Animals. 7 Plates and 96 Text-figures. 8vo. 1s.
—— Elephants (Recent and Fossil). 31 Text-figures. 8vo. 6d.
Mineral Department.
A Guide to the Mineral Gallery. Plan. 8vo. 1d.
The Student's Index to the Collection of Minerals. Plan. 8vo. 2d.
An Introduction to the Study of Minerals, with a guide to the Mineral Gallery. 41 Woodcuts. Plan. 8vo. 6d.
—— Study of Rocks. Plan. 8vo. 6d.
—— Study of Meteorites, with a List of the Meteorites represented in the Collection. Plan. 8vo. 6d.
Botanical Department.
List of British Seed-plants and Ferns. 8vo. 4d.
Guide to Sowerby's Models of British Fungi. 93 Woodcuts. 8vo. 4d.
Guide to the British Mycetozoa. 45 Woodcuts. 8vo. 3d.
Special Guides.
No. 1. Guide to an Exhibition of Old Natural History Books. 8vo. 3d.
No. 2. Books and Portraits illustrating the History of Plant Classification. 4 Plates. 8vo. 4d.
No. 3. Memorials of Linnæus. 2 Plates. 8vo. 3d.
The Guide-Books can be obtained only at the Natural History Museum. Written communications respecting them should be addressed to The Director.
LONDON: PRINTED BY WILLIAM CLOWES AND SONS, LIMITED, DUKE STREET, STAMFORD STREET, S.E., AND GREAT WINDMILL STREET, W.
BRITISH MUSEUM
(NATURAL HISTORY).
DAYS AND HOURS OF ADMISSION.
The Exhibition Galleries are open to the Public, free, every week-day, in
January, from 10 A.M. till 4 P.M.
February, 1st to 14th, from 10 A.M. till 4.30 P.M.
February, 15th to end, from 10 A.M. till 5 P.M.
March, from 10 A.M. till 5.30 P.M.
April to August, from 10 A.M. till 6 P.M.
September, from 10 A.M. till 5.30 P.M.
October, from 10 A.M. till 5 P.M.
November and December, from 10 A.M. till 4 P.M.
Also, from May 1st to the middle of July, on Mondays and Saturdays only, till 8 P.M.,
and from the middle of July to the end of August, on Mondays and Saturdays only, till 7 P.M.
The Museum is also open on Sunday afternoons throughout the year.
The Museum is closed on Good Friday and Christmas Day.
By Order of the Trustees.
LONDON: PRINTED BY WILLIAM CLOWES AND SONS, LIMITED, DUKE STREET, STAMFORD STREET, S.E., AND GREAT WINDMILL STREET, W.
TRANSCRIBER'S NOTES:
| page | Original text | Replaced with |
| all | Ditto marks | Repeated the actual text |
| 66–106 | The various lists of meteorites were inconsistent in the use of a full stop at the end of the place of fall. | Standardised by placing a full stop at the end of every place of fall. |
| 95–104 | In one of the lists of meteorites the typesetter occasionally used large curly brackets (e.g No. 271, 276, 300, 384, 395, 408, 423, 445) to collect together multiple falls of meteorites and their constituent weights to a single entry and place of fall. | Omitted the curly brackets as each numbered entry is now spaced from each other, putting the place of fall following the adopted name for the meteorites. |
| 68 | 1750 | 1,750 |
| 69 | 6948 | 6,948 |
| 104 | 1791 | 1,791 |
| 112 | Batsúra | Batsura |
| 114 | Haywood County, pseudo-meteorite) | Haywood County, (pseudo-meteorite) |
| 116 | Montauban v Orgueil, 406 | Montauban v. Orgueil, 406 |
| 119 | Senhadja v Aumale, 412 | Senhadja v. Aumale, 412 |
| 120 | Werchne v. Verkhne | Werchne v. Verkhne |
| 121 | Shell and Star-fish Galleries. 125 Woodcuts and 1 Plan. 8vo, 6d. | Shell and Star-fish Galleries. 125 Woodcuts and 1 Plan. 8vo. 6d. |
| Back cover | October | October, |