
The Project Gutenberg EBook of Cooley's Cyclopædia of Practical Receipts
and Collateral Information in the Arts, Ma, by Arnold Cooley and Richard Tuson
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
Title: Cooley's Cyclopædia of Practical Receipts and Collateral Information in the Arts, Manufactures, Professions, and Trades..., Sixth Edition, Volume I
Author: Arnold Cooley
Richard Tuson
Release Date: May 19, 2012 [EBook #39733]
Language: English
Character set encoding: UTF-8
*** START OF THIS PROJECT GUTENBERG EBOOK COOLEY'S CYCLOPÆDIA ***
Produced by Chris Curnow, Leonora Dias de Lima, Henry
Gardiner and the Online Distributed Proofreading Team at
http://www.pgdp.net


v
Some one has said that “when a book reaches a fifth edition it scarcely requires a preface.” If such be true of a fifth, it is probably still truer of a sixth edition, and therefore this issue of ‘Cooley’s Cyclopædia’ might fairly be sent forth to the public without any prefatory remarks whatever. It is, however, desirable to point out that the present edition is larger than the last by about six hundred pages; that much greater space than hitherto is devoted to Hygiène (including sanitation, the composition and adulteration of foods) as well as to the Arts, Pharmacy, Manufacturing Chemistry, and other subjects of importance to those for whom the work is intended.
The articles on what is commonly termed ‘Household Medicine’ have been amplified and numerically increased.
Short accounts of the more common diseases, their causes, symptoms, and treatment, affecting the domesticated animals have been introduced. “Here, however, it may be useful to repeat the cautions given in other parts of this volume, as to the impropriety of unnecessarily meddling with the healing art or neglecting a prompt application” (where and when possible) “to a duly qualified practitioner in all cases demanding medical or surgical aid.” These remarks of Mr Cooley are as applicable to cases of Veterinary as to those of Human Medicine.
Numerous authors have necessarily been consulted; a list of them, and the titles of their works from which information has been derived, will be found at the end of the second volume. When extracts have been introduced verbatim the authority is quoted in the body of the book.
Many of my scientific confrères have rendered me valuable aid in preparing this edition; but I am particularly indebted to my accomplished and zealous friend Mr John Gardner for his hearty and constant co-operation; to Dr Lionel Beale for his kindness in revising the articles on “Urine,” “Urinary Diseases,” &c., as well as for thevi use of cuts from his celebrated works on these subjects; to my friend and former pupil Mr F. Woodland Toms for revising and rewriting the articles on “Sewage” and “Water;” and to my assistants Mr James Bayne and Mr Cuthbert Neison for correcting “proof.”
The laborious task of preparing a sixth edition of ‘Cooley’ having been accomplished, it is hoped that, due consideration being given to the magnitude of the work and to the great variety of the subjects treated, it will be found to be practically free from important errors, and that it will meet with, at least, the same gratifying reception as that accorded to its predecessors.
vii
The design of the present work is briefly, but not completely expressed in its title-page. Independently of a reliable and comprehensive collection of formulæ and processes in nearly all the industrial and useful arts, it contains a description of the leading properties and applications of the substances referred to, together with ample directions, hints, data, and allied information, calculated to facilitate the development of the practical value of the book in the shop, the laboratory, the factory, and the household. Notices of the substances embraced in the Materia Medica of our national pharmacopœias, in addition to the whole of their preparations, and numerous other animal and vegetable substances employed in medicine, as well as most of those used for food, clothing, and fuel, with their economic applications, have been included in the work. The synonymes and references are other additions which will prove invaluable to the reader. Lastly, there have been appended to all the principal articles referred to brief, but clear, directions for determining their purity and commercial value, and for detecting their presence and proportions in compounds.
The sources from which I have derived the vast mass of materials forming this volume are such as to render it deserving the utmost confidence. I have invariably resorted to the best and latest authorities, and have consulted almost innumerable volumes, both British and foreign, during its compilation. Secondary channels of information have been scarcely ever relied on when original authorities were within my reach. A large portion of the work has been derived from my personal experience and observations in the departments of applied chemistry and hygiene, and from the processes of various laboratories and manufactories, many of which I can the more confidently recommend from having either inspected or witnessed their employment on an extensive scale. The indiscriminate adoption of matter, without examination, has been uniformly avoided, and in no instance has any formula or process been admitted into this work, unless it rested on some well-known fact of science, had been sanctioned by usage, or come recommended by some respectable authority. The settlement of doubtful or disputed pointsviii has often occupied me a greater number of hours, and not unfrequently a greater number of days, than that of the lines of letter-press which convey the results to the public. In all cases precedence has been given to the standard formulæ of our national pharmacopœias, and to those processes which long experience, or well-conducted experiments, have shown to be the most successful, profitable, and trustworthy. In general, the sources of information have been indicated, for the purpose of enabling the reader to form a better estimation of their value. Whenever this is not the case, in reference to borrowed formulæ and data, the omission has arisen from the impossibility of determining to whom the merit is justly due.
I have endeavoured as much as possible, in the present work, to avoid confusion of the medical weights with those commonly used in trade and commerce—an attempt which, so far as I am aware, has not been successfully carried out in any other quarter. For this purpose I determined to entirely abandon the usual arbitrary signs or characters employed to represent the divisions of the apothecaries’ pound, and to distinguish the two weights from each other, by simply printing, in different type, the plain English names and abbreviations representing their several denominations. The medical signs for the imperial gallon and its subdivisions have also been abandoned for their common English names. It would have afforded me pleasure to have reduced all the quantities to one uniform standard, had it been practicable, or, in all cases, advisable.
Under the names of most of the leading diseases that could be profitably noticed in the present work, such explanations and directions have been given as accord with the prevailing opinions and practice of the faculty at the present day. These, when judiciously applied, will prove invaluable to emigrants, travellers, voyagers, and other parties beyond the reach of legitimate medical assistance; and, under opposite circumstances, will, in general, enable those who have the care of the sick the better to second and carry out the instructions and efforts of the physician for the benefit of their charge. Here, however, it may be useful to repeat the cautions given in other parts of this volume, as to the impropriety of unnecessarily meddling with the healing art, or neglecting a prompt application to a duly qualified practitioner, in all cases demanding either medical or surgical aid. It is an indubitable fact that the best efforts of the inexperienced and uninitiated in the mysteries of medical science must be always enormously behind those of parties whose whole lives and study have been devoted to the subject.
The nature of a condensed alphabetical arrangement not permittingix numerous articles to come under distinct heads, or to be referred to under all their synonymes, the casual reader may often be led to suppose that this book is most deficient where in reality it is the most copious. In general I have attempted, as much as possible, to bring together subjects of a closely allied character, and compounds which are analogous to each other, either in constitution or the mode of their preparation. Thus, most of the formulæ for Mixtures, Ointments, Pills, &c., follow in alphabetical order the general articles under these heads; whilst those for the Oxides, Salts, &c., follow the names of their respective bases. In like manner, a notice of a number of preparations will be found included in that of their principal ingredients. The names under which the leading substances appear are generally those which are most familiar to well-informed practical men, and which have commonly reference to either their acknowledged chemical constitution, or to some long-known and easily recognised quality. The following extract conveys an important lesson on this subject, with which I perfectly agree:—“We have been unwilling to make any unnecessary changes in the nomenclature of substances whose names are sanctioned by the usage of the present day; for these names have been, for the most part, rightly assigned by our predecessors, or confirmed by lapse of time. We are indeed aware that every improvement in the knowledge of things ought to be embodied in their names; but we must be careful, in selecting or forming these names, not to make those points appear certain and established which are as yet doubtful, for it is safer to be in the rear than advance of natural history.”[1]
I have exerted myself to the utmost to ensure the accuracy and completeness of this volume, but I feel conscious that, after all my efforts for this purpose, some errors have crept into it, that many subjects which deserve insertion in it have been omitted, and that many others have been either imperfectly or too briefly noticed. “Yet these failures, however frequent, may,” I trust, “admit of extenuation and apology. To have attempted much is always laudable, even where the enterprise is above the strength that undertakes it. To rest below his aim is incident to every one whose fancy is active, and whose views are comprehensive; nor is any man satisfied with himself because he has done much, but because he conceives little.” When I commenced this work I resolved to leave nothing within its legitimate limits unexamined or unelucidated; and I flattered myself with a prospect of the hours which I should thus “revel away” in a pursuit so congenial to my desires—“the treasures with which I expected every search into those neglected mines to reward my labour—and the triumph with which I should display my acquisitions to mankind.” But these were thex dreams of a poet, doomed at last to wake a “Cyclopædist”. The long task which I had undertaken soon exhibited its truly onerous character, and daily grew in urgency, until that which promised to be a pleasure had been transformed into an exhausting and continuous labour. At first, a sacrifice of the hours of leisure only seemed necessary to the undertaking—next, those assigned to professional and business avocations were demanded, and absorbed; but, ere long, one by one, the hours usually devoted to repose were sucked into the insatiable vortex, until the bright beams of the rising sun not unfrequently illumined the lamp-lit study or the gloomy laboratory, and surprised the author, no longer an enthusiast, at his still-enduring task. But long ere this I had learned that to carry out my original resolutions in all their completeness and entirety was impossible, and “that to pursue perfection was, like the first inhabitants of Arcadia, to chase the sun, which, when they had reached the hill where he had seemed to rest, was still at the same distance from them.”[2] All I can further say in reference to this point is simply to assure the reader that three of the elements usually deemed essential to give value to a technological work—viz. zeal, industry, and capital—have not been wanting in the production of the present one;—the first two depending on the author, and the other chiefly on the liberality and enterprise of the publisher.
As heretofore, I beg to solicit my readers to apprise me of any inaccuracies or omissions in this volume which may come beneath their notice. I shall also thankfully receive any hints or suggestions tending to the improvement of future editions of this work. Such communications, to be useful, must, however be written on only one side of the paper. Parties who may thus kindly afford me assistance will, in due course, have their services publicly acknowledged; and their names and addresses, unless when otherwise requested, will be published in full.
I have endeavoured to render the present volume as self-explanatory as possible, and, in general, have appended ample directions to the several formulæ and processes that seemed to me likely to cause embarrassment to those inexpert in chemical manipulation; but should any party find it otherwise, I shall be happy to reply, gratuitously, to any reasonable questions tending to elucidate the difficulty.
In conclusion, I may add that, having now for nearly a quarter of a century devoted my attention to the applications of chemistry in most of the useful arts and manufactures, both British and foreign, and in sanitation, I am in possession of many valuable processes and formulæ, hitherto wholly unknown, or but partially developed, with variousxi improved plans of factories, laboratories, ventilation, &c., which the limits of this work will not permit me to describe in its pages, but on which I should be happy to communicate with parties interested in the same. Persons desirous of establishing any new branch of manufacture, or of improving an existing one, or of determining the purity or value of articles of food, wines, liqueurs, medicines, &c., or of obtaining formulæ or processes which are not contained in this work, may, in like manner, have their wishes complied with, by enclosing to me samples, or the requisite information.
2
These, for the most part, consist of the first syllable, or the initial letter or letters of the words they stand for. As Prep., preparation; Pur., purity; Purif., purification; Obs., observations; Var., varieties, &c.—Ph., stands for pharmacopœia; B. P., for British Pharmacopœia; Ind. Ph., for Indian Pharmacopœia; Cod., for Codex.—L., E., D., P., U. S., &c., associated with the last two abbreviations, are the initial letters of the cities and countries which produced the respective works; as, London, Edinburgh, Dublin, Paris, United States, &c. When no dates are given, the last editions of the pharmacopœias are referred to.
lb., oz., dr., respectively represent the pound, ounce, and drachm (1⁄8 oz.), Avoirdupois weight. This is the only weight employed in the British and last Dublin Pharmacopœias.
lb., oz., dr., and gr., refer to the pound, ounce, drachm, and grain, Apothecaries’ or Troy weight.
The word ‘drop’ in all cases indicates a measured drop or minim.
The names of individuals which appear in this work are those to whom the immediately attached information or formula is usually attributed, or on whose recommendation or authority it has been selected.
3
A-, ab-, abs-. [L.] In composition, from, denoting distance, departure, separation, or opposition; as in aberration, abstraction, abnormal, &c.
A-, an-. [Gr.] In composition, no, not, without, denoting the absence or loss of some quality or thing; as in achromatic, anhydrous, amorphous, &c.
AB′ACA (kăh). A species of vegetable fibre, of several varieties, obtained in the Philippine Islands, and remarkable for its brilliancy, strength, and durability. The finer kinds are woven into muslins, and other delicate fabrics; the coarser are formed into mats, cordage, and sail-cloth. It has been recently employed in Paris for the manufacture of various articles of furniture and dress; including bonnets, tapestry, carpets, network, hammocks, &c. The fibre, and fabrics made of it, may be bleached and dyed in a similar manner to flax and linen.
ABATTOIR. A public slaughter-house for cattle, &c., usually erected within the walls or precincts of a continental town or city.
ABBREVIATION. One or more of the earlier letters of a word used to express the whole.
A.B., Bachelor of Arts.
A.D., In the year of our Lord.
A.I.C., Associate of the Institute of Chemistry.
A.I.C.E., Associate of the Institute of Civil Engineers.
A.M., Master of Arts.—Before noon.
A.R.A., Associate of the Royal Academy.
B.A., Bachelor of Arts.
Bart., Baronet.
B.C., Before Christ.
B.D., Bachelor of Divinity.
B.Sc., Bachelor of Science.
C.B., Companion of the Bath.
C.E., Civil Engineer.
C.S., Civil Service.
D.C.L., Doctor of Civil Laws.
D.D., Doctor of Divinity.
D.G., By the Grace of God.
Dr., Doctor.—Debtor.
D.Sc., Doctor of Science.
D.V., God willing.
Ed., Editor, or Edition.
e.g., for example.
F.C.P., Fellow of the College of Preceptors.
F.C.S., Fellow of the Chemical Society.
F.G.S., Fellow of the Geological Society.
F.I.C., Fellow of the Institute of Chemistry.
F.L.S., Fellow of the Linnean Society.
F.R.A.S., Fellow of the Royal Astronomical Society.
F.R.C.P., Fellow of the Royal College of Physicians.
F.R.C.S., Fellow of the Royal College of Surgeons.
F.R.G.S., Fellow of the Royal Geographical Society.
F.R.S., Fellow of the Royal Society.
F.R.S.E., Fellow of the Royal Society of Edinburgh.
H.M.S., Her Majesty’s Ship.
H.R.H., His (or Her) Royal Highness.
i.e., That is.
Inst., Instant (the present month).
I.H.S., Jesus the Saviour of Man.
K.B., Knight of the Bath.
K.C.B., Knight Commander of the Bath.
K.G., Knight of the Garter.
Knt., Knight.
K.St.P., Knight of St. Patrick.
K.T., Knight of the Thistle.
L.A.C., Licentiate of the Apothecaries’ Company.
Lat., Latitude.
L.D., Licentiate in Dentistry.
LL.D., Doctor of Laws.
L.M., Licentiate in Midwifery.
Loc. cit., The part referred to.
Lon. or Long., Longitude.
M.A., Master of Arts.
M.B., Bachelor of Medicine.4
M.C., Master of Surgery.—Master of the Ceremonies.
M.C.P., Member of the College of Preceptors.
M.D., Doctor of Medicine.
M.I.B.A., Member of the Institute of British Architects.
M.R.C.P., Member of the Royal College of Physicians.
M.R.C.S., Member of the Royal College of Surgeons.
M.R.C.V.S., Member of the Royal College of Veterinary Surgeons.
M.R.I., Member of the Royal Institution.
M.R.I.A., Member of the Royal Irish Academy.
MS., Manuscript.
MSS., Manuscripts.
Mus. Doc., Doctor of Music.
N.B., Mark well.
Nem. con., Without opposition.
O.H.M.S., On Her Majesty’s service.
Op. cit., The work quoted.
Per cent. (often expressed by the sign %), By the hundred.
Ph.D., Doctor of Philosophy.
P.M., Afternoon.
Prox., The next (month).
P.S., Postscript.
Q.C., Queen’s Counsel.
Qy. (?), Query, Question.
R.A., Royal Academician—Royal Artillery.
R.E., Royal Engineers.
R.H.A., Royal Horse Artillery.
R.M., Royal Marines.
R.N., Royal Navy.
Tr., Translator.
Ult., The last (month).
v. or vide, See.
W.S., Writer to the Signet.
&, ampersand, and.
&c., et cetera, And so on.
A. aa., ana (Greek), of each. Equally by weight or measure.
Abdom., abdomen, the abdomen, the belly.
Abs. febr., absente febre, fever being absent.
Ad 2 vic., ad secundum vicem, to the second time; or ad duas vices, for two times.
Ad gr. acid., ad gratam aciditatem, to an agreeable acidity.
Ad def. animi, ad defectionem animi, to fainting.
Ad del. an., ad deliquium animi, to fainting.
Ad libit., ad libitum, at pleasure.
Add., adde, or addantur, add, or let them be added; addendus, to be added.
Adjac., adjacens, adjacent.
Admov., admove, admoveatur, admoveantur, apply, let it be applied, let them be applied.
Ads. febre, adstante febre, while the fever is present.
Alter. hor., alternis horis, every other hour.
Alvo adstr., alvo adstrictâ, when the bowels are confined.
Aq. astr., aqua astricta, frozen water.
Aq. bull., aqua bulliens, boiling water.
Aq. com., aqua communis, common water.
Aq. fluv., aqua fluviatilis, river water.
Aq. mar., aqua marina, sea water.
Aq. niv., aqua nivalis, snow water.
Aq. pluv., aqua pluviatilis, or pluvialis, rain water.
Aq. ferv., aqua fervens, hot water.
Aq. font., aqua fontana, or aqua fontis, spring water.
Bis ind., bis in dies, twice a day.
Bib., bibe, drink.
BB., Bbds., Barbadensis, Barbadoes, as aloë Barbadensis.
B.M., balneum mariæ, or balneum maris, a warm-water bath.
B. P., or B. Ph., British Pharmacopœia.
But., butyrum, butter.
B.V., balneum vaporis, a vapour bath.
Cærul., cæruleus, blue.
Cap., capiat, let him (or her) take.
Calom., calomelas, calomel, subchloride of mercury.
C. C., cornu cervi, hartshorn; it may also signify cucurbitula cruenta, the cupping-glass with scarificator.
C.C.U., cornu cervi ustum, burnt hartshorn.
Cochleat., cochleatim, by spoonfuls.
Coch. ampl., cochleare amplum, a large (or table) spoonful; about half a fluid ounce.
Coch. infant., cochleare infantis, a child’s (or tea) spoonful.
Coch. magn., cochleare magnum, a large spoonful.
Coch. med., cochleare medium, } a middling or moderate spoonful; that is,
Coch. mod., cochleare modicum,} a dessert-spoonful—about two fluid drachms.
Coch. parv., cochleare parvum, a small (or tea) spoonful; it contains about one fluid drachm.
Col., cola, strain.
Col., colatus, strained.
Colet., coletur, colat., colatur, let it be strained; colaturæ, to the strained liquor.
Colent., colentur, let them be strained.
Color., coloretur, let it be coloured.
Comp., compositus, compounded.
Cong., congius, a gallon.
Cons., conserva, conserve; also (imperat. of conservo) keep.
Cont. rem., or med., continuentur remedia, or medicamenta, let the remedies, or the medicines, be continued.
Coq., coque, boil; coquantur, let them be boiled.
Coq. ad med. consumpt., coque or coquatur ad medietatis consumptionem, boil, or let it be boiled to the consumption of one half.
Coq. S. A., coque secundum artem, boil according to art.
Coq. in S. A., coque in sufficiente quantitate aquæ, boil in a sufficient quantity of water.5
Cort., cortex, bark.
C. v., cras vespere, to-morrow evening.
C. m. s., cras mane sumendus, to be taken to-morrow morning.
C. n., cras nocte, to-morrow night.
Crast., crastinus, for to-morrow.
Cuj., cujus, of which.
Cujusl., cujuslibet, of any.
Cyath. theæ, cyatho theæ, in a cup of tea.
Cyath., cyathus, vel, a wine-glass; from an ounce and half...
C. vinar., cyathus vinarius; to two ounces and half.
Deaur. pil., deaurentur pilulæ, let the pills be gilt.
Deb. spiss., debitur spissitudo, due consistence.
Dec., decanta, pour off.
Decub. hor., decubitûs horâ, at the hour of going to bed, or at bedtime.
De d. in d., de die in diem, from day to day.
Deglut., deglutiatur, let it be swallowed.
Dej. alv., dejectiones alvi, stools.
Det., detur, let it be given.
Dieb. alt., diebus alternis, every other day.
Dieb. tert., diebus tertiis, every third day.
Dil., dilue, dilutus, dilute (thin), diluted.
Diluc., diluculo, at break of day.
Dim., dimidius, one half.
D. in 2 plo., deter in duplo, let it be given in twice the quantity.
D. in p. æq., dividatur in partes æquales, let it be divided in equal parts.
D. P., directione propria, with a proper direction.
Donec alv. bis dej., donec alvus bis dejecerit, until the bowels have been twice opened.
Donec alv. sol. fuer., donec alvus soluta fuerit, until the bowels have been loosened.
Donec dol. neph. exulav., donec dolor nephriticus exulaverit, until the nephritic pain has been removed.
D., dosis, a dose.
Eburn., eburneus, made of ivory.
Ed., edulcorata, edulcorated.
Ejusd., ejusdem, of the same.
Elect., electuarium, an electuary.
Enem., enema, a clyster.
Exhib., exhibeatur, let it be administered.
Ext. sup. alut. moll., extende super alutam mollem, spread upon soft leather.
F., fac, make; fiat, fiant, let it be made, let them be made.
F. pil., fiant pilulæ, let pills be made.
Fasc., fasciculus, a bundle.
Feb. dur., febre durante, during the fever.
Fem. intern., femoribus internis, to the inside of the thighs.
F. venæs., fiat venæsectio, let venesection be performed.
F. H., fiat haustus, let a draught be made.
Fict., fictilis, earthen.
Fil., filtrum, a filter.
Fist. arm., fistula armata, a clyster-pipe and bladder fitted for use.
Fl., fluidus, fluid.
F. L. A., fiat lege artis, let it be made by the rules of art.
F. M., fiat mistura, let a mixture be made.
F. S. A., fiat secundum artem, let it be made according to art.
Gel. quav., gelatina quavis, in any jelly.
G. G. G., gummi guttæ gambæ, gamboge.
Gr., granum, a grain; grana, grains.
Gr. vj pond., grana sex pondere, six grains by weight.
Gtt., gutta, a drop; guttæ, drops.
Gtt. quibusd., guttis quibusdam, with some drops.
Guttat., guttatim, by drops.
Har. pil. sum. iij, harum pilularum sumantur tres, of these pills let three be taken.
H. D., or hor. decub., horâ decubitûs, at bedtime.
H. P., haustus purgans, purging draught.
H. S., horâ somni, at the hour of going to sleep.
Hor. un. spætio, horæ unius spatio, at the expiration of one hour.
Hor. interm., horis intermediis, in the intermediate hours.
Hor. 11mâ mat., horâ undecimâ matutinâ, at 11 o’clock in the morning.
Ind., indies, daily.
In pulm., in pulmento, in gruel.
Ind. Ph., Indian Pharmacopœia.
Inf., infunde, infuse.
Inj. enem., injiciatur enema, let a clyster be thrown up.
Jul., julepus, julapium, a julep.
Kal. ppt., kali præparatum, prepared kali (potassæ carbonas).
Lat. dol., lateri dolenti, to the affected side.
M., misce, mix; mensurâ, by measure; manipulus, a handful; minimum, a minim.
Mane pr., mane primo, early in the morning.
Man., manipulus, a handful.
Min., minimum, a minim, the 60th part of a drachm measure.
M. P., massa pilularum, a pill mass.
M.R., mistura, a mixture.
Mic. pan., mica panis, crumb of bread.
Mitt., mitte, send; mittantur, let them be sent.
Mitt. sang. ad ℥xij, mitte sanguinem ad ℥xij, take blood to twelve ounces.
Mod. præscr., modo præscripto, in the manner directed.
Mor. dict., more dicto, in the way ordered.
Mor. sol., more solito, in the usual way.
Ne tr. s. num., ne tradas sine nummo, do not deliver it without the money.
No., numero, in number.
N. M., nux moschata, a nutmeg.
O., octarius, a pint.
Ol. lini s. i., oleum lini sine ligné, cold-drawn linseed oil.
Omn. hor., omni horâ, every hour.
Omn. bid., omni biduo, every two days.6
Omn. bih., omni bihorio, every two hours.
O. M., or omn. man., omni mane, every morning.
O. N., or omn. noct., omni nocte, every night.
Omn. quadr. hor., omni quadrante horæ, every quarter of an hour.
O. O. O., oleum olivæ optimum, best olive oil.
Ov., ovum, an egg.
Oz., the ounce avoirdupois.
P. æ., part. æqual., partes æquales, equal parts.
P. d., per deliquium, by deliquescence.
Past., pastillus, a pastil, or ball of paste.
P., pondere, by weight.
Ph. D., Pharmacopœia Dubliniensis.
Ph. E., Pharmacopœia Edinensis.
Ph. L., Pharmacopœia Londinensis.
Ph. U. S., Pharmacopœia of the United States.
Part. vic., partitis vicibus, in divided doses.
Per. op. emet., peractâ operatione emetici, the operation of the emetic being over.
Pocul., poculum, a cup.
Pocill., pocillum, a small cup.
Post sing. sed. liq., post singulas sedes liquidas, after every loose stool.
Ppt., præparata, prepared.
P. r. n., pro re nata, occasionally.
P. rat. ætat., pro ratione ætatis, according to the age.
Pug., pugillus, a pinch, a gripe between the thumb and the two first fingers.
Pulv., pulvis, pulverizatus, a powder, pulverised.
Q. l., quantum lubet, } as much as you
Q. p., quantum placet,} please.
Q. s., quantum sufficiat, as much as may suffice.
Quor., quorum, of which.
Q. V., quantum vis, as much as you will.
Red. in pulv., redactus in pulverem, reduced to powder.
Redig. in pulv., redigatur in pulverem, let it be reduced into powder.
Reg. umbil., regio umbilici, the umbilical region.
Repet., repetatur, or repetantur, let it, or them, be repeated.
S. A., secundum artem, according to art.
Scat., scatula, a box.
S. N., secundum naturam, according to nature.
Semidr., semidrachma, half a drachm.
Semih., semihora, half an hour.
Sesunc., sesuncia, half an ounce.
Sesquih., sesquihora, an hour and a half.
Si n. val., si non valeat, if it does not answer.
Si op. sit, si opus sit, if it be necessary.
Si vir. perm., si vires permittant, if the strength allow it.
Signat., signatura, a label.
Sign. n. pr., signetur nomine proprio, let it be written upon, let it be signed with the proper name (not the trade name).
Sing., singulorum, of each.
S. S. S., stratum super stratum, layer upon layer.
Ss., semi, a half.
St., stet, let it stand; stent, let them stand.
Sub fin. coct., sub finem coctionis, towards the end of boiling, when the boiling is nearly finished.
Sum. tal., sumat talem, let the patient take one such as this.
Summ., summitates, the summits or tops.
Sum., sume, sumat, sumatur, sumantur, take, let him or her take, let it be taken, let them be taken.
S. V., spiritus vini, spirit of wine.
S. V. R., spiritus vini rectificatus, rectified spirit of wine.
S. V. T., spiritus vini tenuis, proof spirit.
Tabel., tabella, a lozenge.
Temp. dext., tempori dextro, to the right temple.
T. O., tinctura opii, tincture of opium.
T. O. C., tinctura opii camphorata, camphorated tincture of opium.
Tra., tinctura, tincture.
Ult. præscr., ultimo præscriptus, last prescribed.
U. S. Ph., United States’ Pharmacopœia.
V. O. S., vitello ovi solutus, dissolved in the yolk of an egg.
Vom. urg., vomitione urgente, the vomiting being troublesome.
V. S. B., venæsectio brachii, bleeding from the arm.
Zz., zingiber, ginger.
See Formula, Prescriptions, Symbols, &c.
ABDO′MEN. [Eng., Fr., L.] In anatomy, the belly, or lower belly; the great cavity of the body extending from the thorax, or chest, to the bottom of the pelvis. It contains the stomach, intestines, liver, spleen, kidneys, bladder, &c.; and in the female, the uterus, ovaria. &c.
AB′ERNE′THY MEDICINES. These originally consisted of a calomel pill, and subsequently of a mercurial or ‘blue’ pill, to be taken over-night, followed by an aromatised black draught in the morning. The quantity of either of the former, for an adult, was about 3 gr. to 31⁄2 gr., increased a little in bulk by the addition of some liquorice powder; that of the latter, from 1 to 11⁄2 fl. oz. As, however, when frequently taken, these pills sometimes occasioned salivation, which proved prejudicial to their sale, a little compound extract of colocynth (Ph. L., 1836) was introduced into their composition, by which this objection was obviated. Ultimately, their composition was settled at 3 gr. of mercurial pill, and 2 gr. of compound extract of colocynth; and these proportions are still followed as the best by those who prepare and sell them.7 Persons who object to black draught, will find a dose of castor oil, or of any other mild purgative medicine that may be more agreeable to them, equally efficacious.
The occasional use of these medicines seldom fails to prove highly beneficial to the plethoric, bilious, and dyspeptic. In ordinary cases of constipation, headache, &c., arising from deranged stomach or liver, wherein the administration of mercurials is not contra-indicated, they will be found of great service. It need scarcely be added that these medicines are named after Mr Abernethy, the celebrated surgeon, who is said to have frequently employed them in his practice.
ABERRA′TION. [Eng., Fr.] Syn. Aberra′tio, L. A wandering or deviation from the usual course, or from the normal condition. In optics, the deviation of the rays of light from the true focus, when inflected by a lens or speculum. This arises from a difference in the physical nature of the rays, from the figure of the lenses or specula, or from the nature of the materials of which the media traversed are composed. See Achromatism, Lens, &c.
Aberration of mind. Mental alienation or wandering; insanity. A term frequently applied, in familiar language, to a mild form of incipient insanity or dementia, which is more or less occasional or continued, trifling or severe, according to circumstances. The studious, nervous, slothful, and those who are engaged in sedentary occupations and spend much of their time in ill-ventilated apartments, or who indulge in irregular or vicious habits, as well as ‘fast livers,’ are the most liable to this affection. It also frequently arises from disordered physical health.
Treat., &c. Change of scene, out-door exercise, agreeable company, pleasing and continued mental occupation, and due attention to diet, clothing, ventilation, &c., with the judicious use of some mild aperient medicine and tepid bathing, will generally alleviate, and frequently effect a cure. For the prevention of its accession, or its recurrence, care should be taken to promote the general health, and also, where necessary, to elevate the spirits and to divert the mind.
ABLU′TION. [Eng., Fr.] Syn. Ablu′tio, L. In a general sense, washing, cleansing, or purification by water.
Ablution. In hygiène and the toilet, a washing of the whole body, or any part of it. The value of frequent and copious affusions of pure water to the surface of the body is well known. During life, the skin is continually subjected to abrasion, and the processes of reproduction and decay, by which the cuticle, its exterior portion, is being constantly thrown off as effete and useless matter, in the shape of very minute scales or dust. This, mingling with the oily and saline products of the skin, acquires sufficient adhesiveness to attach itself to the surface of the body and clothing, as well as to attract the waste particles of the dress, and the dust and soot floating in the atmosphere. In this way, if occasional ablutions be not had recourse to, the channels of perspiration will become choked, and the clothing itself rendered unwholesome and unfit for use. The consequence of the pores of the skin being obstructed is impeded transpiration, by which its functions, as a respiratory organ, are interfered with or suspended. This adhering pellicle of refuse matter also acts as an irritant, and forms a favorable medium for the absorption, and the transmission into the body, of effluvia, miasmata, poisonous gases, and the infectious and contagious matters of disease. “The greater part of (contagious) poisons are conveyed to us through the external surface of our bodies; and it is fully proved that poison, already communicated, has been by cleanliness removed, before it could actually produce any bad effects. I here allude, in particular, to frequent washing, bathing, rinsing the mouth, combing and brushing the hair, and often changing the linen, clothing, and bedding.” (Hufeland.) Such are the immediate effects of neglected ablution of the skin; the further consequences are of an equally serious character. The blood being deprived of one of its sources of oxygen, and one of the outlets for its carbon, the functions of nutrition become imperfect, and the animal temperature lessened. The matters which would be thrown out of the system in the form of perspiration are retained, and must be eliminated by other channels. The lungs, the kidneys, the liver, and the bowels, are each, in their turn, overtasked to perform the functions of another organ. The oppressed viscera suffer from exhaustion, and incipient disease soon follows. Their particular offices are languidly performed, the equilibrium of health is disturbed, and skin diseases, or consumption, diarrhœa, dropsy, liver-complaints, visceral obesity, or some other serious diseases of the vital organs, ensue. When it is added, that no dirty or imperfectly washed skin can long continue healthy, and ceasing to be healthy must also cease to be agreeable and beautiful, the argument in favour of the daily use of water of good quality to the whole surface of the body, when possible, will surely be complete. The inculcation of habits of personal cleanliness cannot be too forcibly emphasized. The fact, however, cannot be overlooked, that in order to introduce habits of cleanliness amongst the poorer classes, a plentiful supply of water, combined with cheap baths, are requisite. Every officer of health should inquire into the amount as well as the character of the water supply in the district over which he has supervision. The body should be washed all over every morning with either cold or lukewarm water and soap. This custom is more necessary for workmen employed in laborious and dirty occupations than for those who live sedentary lives; but all people8 perspire, and from every drop of perspiration the water evaporates, and leaves a fraction of solid matter on and around the pores that excrete the perspiration. If this solid matter be not washed off, it accumulates and may derange the health. Instances have occurred in which persons suffering from extensive bodily burns have died, not from the effect of the injury, but from the destruction of the pores or excreting vessels, with which the skin is covered. It is well, therefore, to bear in mind that a dirty skin does not always come from without, but also from within. Cold ablution, that has been so indiscriminately recommended, is not half so efficacious, nor so safe, as lukewarm. The German aurists ascribe the presence of the large amount of deafness in England to our habit of washing the head and ears each morning with cold water.
Ablution. In medicine, the washing the body, externally, as by bathing; or internally, by diluting drinks. In ancient medicine, according to Galen, internal ablution was accomplished by the use of profuse libations of milk-whey; an object now aimed at, by the hydropathists, by the copious administration of pure cold water. To neglect the daily ablution of an infant is to discard one of the greatest aids to its healthy development and physical wellbeing. That disregard of this precaution is a fertile source of most of the skin diseases that affect infants and children there seems little question about amongst medical men. Water at a temperature ranging from 80° to 90° F. should always be used. Mr Chevasse, in his ‘Counsel to a Mother,’ is emphatic in his advocacy of rain water. He also advises the employment of Castile soap, and of glycerine soap, should there be any excoriation of the skin. Of course the same remarks apply to children as to infants, with this difference, that the ablution is to be performed with water a few degrees colder; and both infants and children should be rubbed dry with a dry soft towel. There are doubtless many persons who deem themselves cleanly washed, if in addition to their hands and arms, neck and face, undergoing duly daily ablution, they wash their feet once a week. These individuals cannot reflect that, because of their less exposure to the depurating influence of the atmosphere, the feet require to be more frequently washed than either the hands or face. See Bathing, Baths, Hydropathy, &c.
ABNORM′AL. [Eng., Fr.] Syn. Abnor′mis, L. In medicine and the collateral sciences, contrary to, or without system or rule; irregular; deformed; unnatural. In a diseased or unhealthy state.
ABORTION IN COWS. Abortion is the expulsion of the contents of the pregnant womb before the full period of gestation is complete, and occurs much more frequently in cows than in any other of the lower animals. Abortion is often induced by shocks and injuries, feeding on ergotised grasses, but more commonly by causes which are less obvious. Thus, bad smells, pasturing on flooded meadows, rich and stimulating food, and even association with other cows while aborting, are among the exciting causes of this malady. The premonitory signs are an irritable excited state of the animal, a discharge from the vagina, looseness and fulness of the external organs of generation, and, occasionally, sudden enlargement of the udder. These symptoms may continue for several days, and, if noticed before straining or other signs of calving have appeared, the animal should be copiously bled and placed in a comfortable loose-box, kept as quiet as possible, moderately supplied with soft laxative food, and, if the bowels be costive, with a pound or two of treacle daily. Powerful purgatives are too irritant, and must, therefore, be studiously avoided. Two ounces of laudanum, with the same quantity of sweet spirits of nitre, should be given twice a day until all danger is over. To prevent the continuance and spread of the evil, place the cow by herself as soon as she aborts; remove and bury the fœtus beyond the reach of other cows; feed off the cow, if practicable, but if she be again bulled, it ought not to be for several weeks, and until the period of heat is passing off; remove all disagreeable smells, and see that the remainder of the herd are moderately fed and carefully watched, so that the earliest symptoms of abortion may be noticed.
ABRA′SION. [Eng., Fr.] Syn. Abra′sio, L. The rubbing or wearing down of surfaces by friction. In the arts, the reduction or figuration of materials by the use of an abrasive tool, or grinder, of which the effective portion is an exact counterpart of the form to be produced.
Abrasion. In numismatics, the ‘wear and tear,’ or waste of the substance of coins, in the pocket and circulation. It forms a large item in the expense of a metallic currency. The means employed to obviate, or to reduce it, consist in either alloying the metal to render it tougher and harder, or raising the borders so as to lessen the surface exposed to friction. In well-formed coin both methods are adopted.
Abrasion. In pathology and surgery—1. A superficial removal or injury of the skin by fretting or friction.
Treat., &c. When the injured surface is large, or exposed, it should be protected from dirt and further injury, by applying a piece of lint or soft linen rag, covered with spermaceti cerate, or some other simple ointment; over which a piece of strapping, or bandage of any sort, may be placed to keep it on. In many cases, a piece of common sticking-plaster will be found quite sufficient.
2. A very superficial ulceration or excoriation of the intestinal or other mucous membrane. Treat. Aperients of castor oil, demulcents,9 and a light nutritious diet. See Excoriations.
ABRUS PRECATORIUS. (Ind. Ph.) Indian Liquorice Plant. Habitat. Tropical portions of both hemispheres, Officinal part. The root (Abri Radix, Indian Liquorice). Occurs in pieces of various lengths, from 1⁄2 to 1 inch in diameter; pale brown externally, yellowish internally; inodorous, taste sweetish and mucilaginous, much resembling officinal liquorice root. Properties and uses. Similar to those of liquorice, for which it forms an excellent substitute. Preparation. Extract of Abrus (Extractum Abri). Prepared as Extractum Glycyrrhizæ.
ABSCESS. A formation of matter or pus, resulting from inflammation, either acute or chronic. The symptoms are pain, swelling, heat, and redness, a conical projection on the swelling, often with a white point at the apex. Abscess or suppuration may come on any part of the body. When the local inflammation does not yield to cold lotions, apply poultices; a pledget of lint dipped in cold water and kept moist by means of oil-silk; a slice of bread softened with boiling water or milk, or linseed meal, make the best poultices. Should the pain be severe add laudanum, and additionally rub it round the swelling. Or apply common white paint by laying it on gently with a brush, or else tincture of marigold or arnica in the same manner. Chronic abscesses in the glands in the neck are usually scrofulous, and should be opened. Abscesses in the breast should not be opened too early, or others are formed. Those in the gums may be cut early, not so if in the tonsils. After opening with a needle or lancet-point external abscesses, continue to poultice till the hardness disappears, then dress with spermaceti ointment spread on lint. When the abscess is of a dangerous nature, lose no time in consulting a medical practitioner.
Treatment for horses and cattle. Mr Finlay Dun prescribes fomentations, poultices, counter-irritants, the knife, cauterisation, carbolic-acid dressing, stimulating injections, and the administration of sulphites and chlorate of potash.
ABSINTHE. [Fr.] Absinthium, L.; Wormwood, E.; Wermuth, G. This article is met with in commerce in the form of the dried herb with the flowers of Artemisia Absinthium, having a whitish-grey appearance, a soft feel, an aromatic and unpleasant odour, and an extremely bitter and aromatic flavour. The plant is indigenous, and grows in thickets, in mountainous districts, and on waste ground. Its odour is due to its containing an essential oil; its bitterness is referable to absinthin, a crystallisable principle which may be extracted from the herb by water or spirit. The name absinthe is also given to an intoxicating liqueur which is extensively drunk on the Continent, and which unfortunately appears to be rapidly attracting consumers in this country. The remarks on this subject by Blyth in his admirable ‘Dictionary of Hygiène’ are so pregnant with important facts that they will be here produced verbatim et literatim. “An analysis recently made at the Conservatoire des Arts shows that absinthe now contains a large quantity of antimony, a poison which cannot fail to add largely to the irritant effects necessarily produced on the alimentary canal and liver by constant doses of a concentrated alcoholic liquid. And we have recently received the results of some experiments made by M. Magnan, of Paris. By means of successive distillations he has been able to isolate various products—(1) a blue oil; (2) a yellowish oil; (3) an oxygenated substance. There was besides a yellowish residue left in the glass. These various substances were tried on animals; ten grammes of the yellow sediment given to a small dog produced no effect; thirty centigrammes of the blue oil produced from eight to ten epileptiform attacks. The oxygenated product proved, however, the most powerful toxic agent. Fifteen centigrammes of it, injected into the veins of a large dog, caused the most violent epileptic attacks, which followed in rapid succession, and ended in death. There was an extraordinary rise of temperature, from 39° to 42° Centigrade, and the post mortem showed various apoplectic centres. Dr Decaisne regards the terrible evil of this almost universal absinthe-drinking as the greatest national calamity that has ever befallen France, and has made an eloquent appeal to the Government to strike at once a decisive blow at the trade in this liqueur. Originally the only important ingredient in its composition besides alcohol was the essential oil of absinthium or wormwood; and though this without doubt added something to the mischievous effects of the liqueur, it would be impossible to trace to it, or to the other comparatively trivial ingredients, the more serious of the special results which are now observed to occur to victims of absinthe, though the habitual drinking even in small doses of good absinthe is believed by Dr Decaisne, sooner or later, to produce disorders in the animal economy. Now various deleterious substances are added, the most important of these being antimony. As at present constituted, therefore, and especially when drunk in the disastrous excess now common in Paris, and taken, as it frequently is, on an empty stomach, absinthe forms a chronic poison of almost unequalled virulence, both as an irritant to the stomach and bowels, and also as a destroyer of the nervous system. The effect of absinthe is to produce a superabundant activity of the brain, a cerebral excitement, which at first is agreeable; intoxication comes on rapidly; the head swims, and the effect produced is nearly the same as that of poisoning by a narcotic, which certainly does not occur with an equal dose of brandy. With the absinthe-drinker,10 as with the opium-eater, the excitement the spirit produces diminishes daily in intensity. Each day he is obliged to augment the dose in order to bring himself up to the right pitch. The diseases brought on by the excessive drinking of ardent spirits are produced with greater rapidity by the use of absinthe.” The amount of absinthe consumed in London has during the last few years been enormously on the increase. See Liqueurs.
ABSINTHIN. C16H22O5. The bitter principle of wormwood (Artemisia absinthium). A hard crystalline solid, having an intensely bitter taste; slightly soluble in water, very soluble in alcohol, less so in ether. Its physiological effects resemble those of extract of wormwood. Dose. 1⁄2 gr. to 2 gr., or more; in dyspepsia; as a stomachic, to promote the appetite, &c.; as a substitute for quinine in intermittents; and in worms.
ABSINTH′IUM. [L.] See Absinthe.
ABSOLUTE. Syns. Absolutus, L.; Absolu, Fr.; Undebingt, G. In chemistry, pure, unmixed; as absolute alcohol, pure spirit of wine, i.e. free from water.
ABSORBED′ (-sorbd′). Syn. Chilled; Absorbé, Fr. In painting, a term among French connoisseurs, to represent that state of a picture in which the oil has sunk into the canvas or ground, leaving the colours ‘flat,’ and the touches indistinct. The remedy consists in rubbing the surface of the picture, previously well cleaned, with a soft sponge dipped in a little drying oil, and after some days varnishing it; when it should be kept in a warm room until perfectly dry.
ABSORB′ENT. Syn. Absorb′ens, L.; Absorbant, Fr.; Absorbirend, Ger. Imbibing; that imbibes or sucks up; variously applied in science and art. (See below.)
Absorbent Ground. In painting, a picture-ground prepared wholly or chiefly in distemper or water colour, in order that the redundant oil in the colours subsequently applied may be immediately ‘absorbed,’ by which expedition is permitted, and brilliancy imparted to them.
Absorbent Surfaces. In the arts, these are usually rendered non-absorbent, preliminary to their being bronzed, gilded, painted, or varnished, by giving them one, or more, coats of thin size, so as to destroy their porosity; care being taken to allow each coat to become thoroughly dry before the application of the next one; and also, finally, to remove any unabsorbed excess of size from the surface, by means of a sponge dipped in warm water. This applies to ALABASTER, PAPER, WOOD, PLASTER CASTS, &c.; and to WALLS and CEILINGS which are not exposed to the weather, and which there is not time to prepare with drying oil. See Bronzing, Maps, Varnishing, &c.
Absorption and consequent adherence in porous moulds, as those of plaster, are usually prevented by thoroughly saturating the pores of the mould with melted tallow, or a mixture of tallow and bees’ wax; or for delicate objects or the electrotype, with white wax. The ‘dry moulds’ are either heated before the application of these substances, or they are boiled in them; any portion that may finally remain unabsorbed, being carefully removed with cotton-wool or a soft rag. Another method is to wash the moulds over two or three times with drying oil, or to boil them in it; after which they must be exposed to the air for some days, to dry and harden. Before being used for plaster, composition, &c., the surface of these prepared moulds require to be slightly moistened with sweet oil.
Plaster moulds are generally prepared for sulphur, wax, and gutta percha casts, by simply placing them (upright) with the back immersed in a little water, contained in any shallow vessel, as a saucer or plate; and letting them remain there until moisture begins to appear on the surface. The materials to be cast, or moulded, should then be used at the lowest possible temperature, to prevent the formation of air-bubbles.
The adherence of wax or mixtures containing it, and of gutta percha, is best prevented by moistening the surface of the mould (whether of plaster, metal, or gutta percha), immediately before use, with soft soap reduced to the consistence of thin cream with water. See Casts, Moulds, Electrotype, &c.
ABSORB′ENTS. In anatomy and physiology, two distinct sets of small, delicate, transparent vessels, which imbibe or suck up fluid substances, and convey them to the blood. They are termed lacteals or lymphatics; the former take up the chyme from the alimentary canal, the latter pervade almost every part of the body in which they absorb lymph.
Absorbents. In botany and vegetable physiology, the origins of the different vessels constituting the vascular tissue, as they are found in the root, where they imbibe or suck up the nutritive fluids from the soil. See Plants and Vegetables.
Absorbents. In agriculture and chemistry, substances which possess the power of withdrawing moisture from the atmosphere; as soils, argillaceous earths, &c. Also (but less frequently) substances which neutralise acids; as chalk, lime, and magnesia. Absorbents differ from ‘deliquescent salts’; the latter attract moisture and dissolve in it; whilst the former merely suck it into their pores, as a sponge does water. See Absorption.
Absorbents. Syn. Absorben′tia, L. In medicine and pharmacy, substances which remove acidity from the stomach and bowels. Of these the principal are—magnesia, carbonate and bicarbonate of magnesia, prepared chalk, and the carbonates and bicarbonates of potash, soda, and ammonia. The first four are popularly called earthy absorbents; and the others, alkaline absorbents. See Antacids.
The following absorbent mixtures are taken from Dr Kirby’s valuable work, ‘Selected Remedies’:11
1. Infusion of rhubarb, 11⁄2 oz.; compound spirit of ammonia, 11⁄2 dr.; compound infusion of gentian to 6 oz. Two tablespoonfuls to be taken 3 times a day.
2. Bicarbonate of potash, 11⁄2 dr.; syrup, 2 drs.; compound spirit ammonia, 11⁄2 dr.; compound infusion of gentian to 6 oz. Two tablespoonfuls to be taken 3 times a day.
3. Bicarbonate of soda, 11⁄2 dr.; spirits of chloroform, 11⁄2 dr.; infusion of calumba to 6 oz. Two tablespoonfuls to be taken 3 times a day.
ABSORP′TION. [Eng., Fr.] Syn. Absorp′tio, L.; Einsaugung, Ger. The act or the power of absorbing, in various applications. (See below.)
Absorption. In agriculture, the power possessed by soils of absorbing moisture from the atmosphere. The more a soil is divided by labour and vegetation, the greater is its absorbent power, and, consequently, its fertility. Indeed, the latter chiefly depends on its capacity for imbibing moisture, and may be illustrated by reference to recent and disintegrated lava. (Leslie.) The finely divided state, most penetrable by the delicate fibres of plants, appears to derive its superior power of acting on atmospheric vapour from the augmentation of its surface and the multiplication of its points of contact. (Ure.) This method of increasing the fertility of a soil is well known to scientific farmers, and seldom neglected by them. (Loudon.) That soil must be regarded as the most fertile which possesses this power in the greatest degree. Garden-mould has the highest absorbent power of any mineral substance. (Leslie.)
Process of ascertaining the ABSORBENT POWER OF SOILS, and other substances. Thoroughly dry the article by the suitable application of a heat not exceeding 212° Fahr., continued for several hours, and transfer it, while still warm, into a clean dry phial furnished with a perfectly tight ground-glass stopper. When cold, quickly and cautiously introduce it, along with a delicate hygrometer, into a large wide-mouthed glass bottle, the atmosphere of which has been previously rendered as damp as possible, by suspending a piece of moistened rag or filtering paper in it. It must now be kept closed for some hours, when the hygrometer will indicate the degree of dryness of the enclosed air, and, consequently, the absorbent power of the substance examined.
Obs. Experiments of this nature are only relatively correct, and must be performed under exactly similar circumstances, to furnish reliable comparative results. The whole process, in each case, must be as similar as careful manipulation can possibly make them. With this reserve, they will be found invaluable to the agriculturist.
Absorption. In chemistry the passage of gases and vapours into liquid and solid substances. Thus, water absorbs the oxygen of the air, lime absorbs water, charcoal absorbs ammoniacal and other gases.
Absorption. In medicine and toxicology, see Medicines and Poisons.
Absorption. In perfumery, see Enfleurage.
Absorption. In physics, see Heat, Light, Refrigeration, &c.
Absorption. In physiology (animal and vegetable), the function of sucking, or taking up, of appropriate substances, by the ‘absorbent vessels.’ It is one of the chief vital functions, the primary object of which is to convey to the circulatory organs the proper supply of the materials necessary for the support and growth of the body; and subsequently, to remove and convey to these organs its effete and useless portions, in order to their ultimate elimination from the system.
Absorption. In surgery, the natural process by which tumours and their contents, morbid growths, and, sometimes, even healthy glands, &c., are gradually taken up and disappear, by the action of the ‘absorbents.’
Absorption (of Surfaces, Moulds, &c). See Absorbent Surfaces.
ABSTERG′ENTS. Syn. Abstergen′tia, L. In medicine and pharmacy, substances which cleanse or clear away foulness from the surface of the body or sores; as soap, lotions, &c. See Detergent, which has a nearly similar meaning, and is in more general use.
AC′ARI (-rī). [L.; prim. Gr.] Syn. Acar′idans; Acar′ides (dēz); Acarid′iæ. (-e-ē). In entomology, a division of arachnidans, including the mite and tick. All the species are either microscopic or extremely minute, and possess such tenacity of life as to resist for some time the action of boiling water, and to live with comparative impunity in alcohol. Leuwenhoek had one that lived eleven weeks glued on its back to the point of a needle, without food. The following are well known—ACARUS AUTUMNA′LIS, the harvest-bug or wheal-worm; A. DOMES′TICUS, the domestic tick; A. DYSENTE′RIÆ, the dysentery-tick; A. FARI′NÆ, the meal mite (fig. a); A. RI′′CINUS (rĭc-), the dog-tick; A. SAC′CHARI, the sugar-mite (fig. b); A. SI′′RO, the cheese-mite (fig. c); A. SCABIE′I, the itch-insect (fig. d).
The irritation of the skin, caused by these vermin, may be relieved by a lotion of equal parts of sal volatile and water; and they may be destroyed by tobacco water, or a lotion or ointment of stavesacre. See Itch, Mange, Parasites, Pediculi, Scab, &c.
Acarus Farinæ, or meal-mite (fig. a). This insect is found only in damaged flour, and is more frequently met with in the flour of the leguminosæ (beans, peas) than in that of the gramineæ (wheat, rye, oat).
Now and then a single acarus may occasionally be found in good flour, but even one should be regarded with suspicion, and the12 flour should afterwards be frequently examined to see if they are increasing.
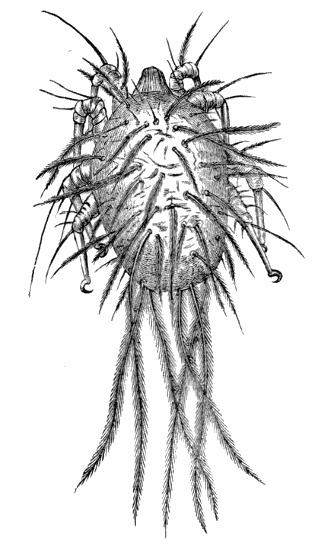 Fig. a. Mag. 250 diams.
Fig. a. Mag. 250 diams.
Acarus Sacchari, or sugar-mite (fig. b).
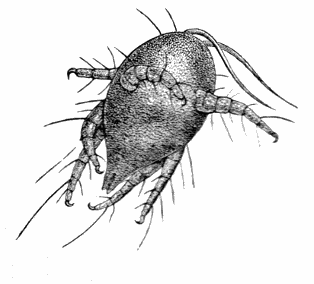 Fig. b. Mag. 260 diams.
Fig. b. Mag. 260 diams.
Most of the brown sugars of commerce are infested by this pest, which is of a size sufficiently large to be visible to the naked eye. The following method of proceeding will lead to its detection:
Dissolve 2 or 3 teaspoonfuls of sugar in a large wineglass of tepid water, and let the solution remain for an hour or so, at the expiration of which time the acari may be found, some on the surface of the liquid, some attaching themselves to the sides of the glass, and some at the bottom, mixed up with the copious and dark sediment, made up of fragments of cane, woody fibre, grit, dirt, and starch granules, which usually subside on dissolving even a small quantity of sugar in hot water. When first hatched this acarus is hardly visible.
Acari of all sizes—that is, in all stages of growth—may be met with in most samples of sugar.
Dr Hassall, in seventy-two samples of sugar which he examined, found sixty-nine containing them.
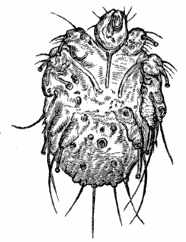 Fig. c.
Fig. c.
Acarus Siro, the cheese-mite (fig. c). The dry and powdery parts of decayed cheese, which by careful watching may very frequently be seen in movement, consist almost wholly of this insect and their eggs in different stages of development. The cheese-mite can hardly be seen without the aid of the microscope. They are very tenacious of life, even when kept without food. Mr Blyth says that under these circumstances “it is no uncommon sight to see them killing and devouring each other; and that cheese is rapidly destroyed by them; they crumble it into minute pieces, and emit a liquid substance which causes the decayed parts to spread speedily.” They may be destroyed by being exposed to a strong heat, or by putting the cheese for a short time in whisky.
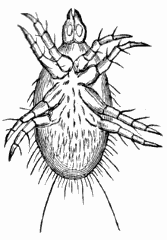 Fig. d.
Fig. d.
Acarus Scabiei, the itch-insect (fig. d). The parasitic character of the disease known as the itch was first demonstrated by Dr Bononio, who13 on turning out the contents of one of the little bladders that show themselves between the fingers of those affected with the complaint, and placing the fluid under the microscope, discovered a minute animal, very nimble in its movements, covered with short hairs, having a short head, a pair of strong mandibles or cutting-jaws, and eight legs, terminating in remarkable appendages, each provided with a sucker and setæ.
It has no eyes; but when disturbed it quickly draws in its head and feet, and then somewhat resembles the tortoise in appearance, its march being precisely the same. It usually lays sixteen eggs, which are carefully deposited in furrows under the skin, and ranged in pairs; these are hatched in about ten days.
“To find the itch-insect,” says Mr Jabez Hogg, “the operator must carefully examine the parts surrounding each pustule; he will then see a red line or spot communicating with it; this part, and not the pustule, must be probed with a fine-pointed instrument. The operator must not be disappointed by repeated failures.”
ACCIDENT′AL COLOURS. See Colours (Complementary).
ACCIDENTS. Black eye. Bathe the eye frequently with a soft piece of linen rag dipped in a lotion composed of one part of tincture of arnica and seven parts of water.
Burns and Scalds. Refer to Burns and Scalds.
Charcoal, combustion of, poisoning by. Refer to Carbonic Anhydride.
Choking, or suffocation from substances sticking in the throat. Refer to Choking.
Cut Finger. Refer to Cuts.
Precautions against Fires. Refer to Fires.
Precautions against Lightning. To take refuge under a tree during a thunderstorm accompanied by lightning is to expose oneself to a double danger—firstly, because by keeping the clothes dry these are prevented becoming the non-conductors they would be if damp; and secondly, because the tree, serving as a point of attraction for the lightning, conducts it to the ground, and in doing so frequently rends the trunks or branches, and kills any person or animal who happens to be close to, or in contact with, it at the time.
Never, therefore, if overtaken by a storm of thunder and lightning fly to the dangerous cover of a tree, pillar, hay-rick, wall, or hedge, but seek shelter in the nearest dwelling; or if this is not at hand, get to a part of the road or field where there is no object to attract the lightning, and there remain till the storm has expended itself. Also avoid particularly the proximity of iron gates, palisades, bronze statues, bell wires, iron railings, and such like. When in the house, do not sit or stand near the windows, doors, or walls, but place yourself in the middle of the room, unless there should be a lamp or chandelier hanging from the ceiling. Franklin recommends persons to keep away from the neighbourhood of fireplaces.
Treatment of persons struck by lightning. In case of any person being struck by lightning, immediately strip the body and throw bucketsful of cold water over it for ten or fifteen minutes; continued frictions and inhalations of the lungs must also be employed, and electricity should be tried if it be possible.
Accidents by Poison. The means to be adopted in cases where poison is taken, if the poison be known, are embodied in the antidotes, which will be found given in this volume under the respective poisons.
Under all circumstances, however, medical aid should be sought as expeditiously as possible, since many of the antidotes themselves being of a dangerous, if not poisonous, character, should only be administered under medical supervision. Pending the arrival of the doctor, no time should be lost in giving an emetic, consisting of a teaspoonful of flour of mustard in half a pint of warm water, supplemented by copious draughts of warm water, and tickling the throat with the finger if necessary.
Fish poisoning. It is a not unfrequent occurrence to find fish when eaten giving rise to a species of poisoning of a more or less violent form, such as a sense of weight at the stomach, accompanied with nausea, vertigo, headache, heat about the head and eyes, pains in the stomach, thirst, and often an eruption of the skin resembling nettle-rash. These symptoms may be sometimes due to the nature of the fish itself; sometimes to its being in a state unfit to be taken as food, as, for instance, when it is in a stale or decomposing condition; and occasionally to the peculiarity of constitution of those who partake of it, even if in a perfectly fresh condition. Whenever any of the symptoms above described follow from eating fish, an emetic of mustard and water (a teaspoonful of mustard in half a pint of water) should be administered. If subsequently a rash should appear, it would be well to take a dose of brisk purgative medicine, and, if necessary, a few doses of carbonate of soda 3 or 4 times during the day.
Poisonous Mushrooms. The same treatment should be followed as for fish. With some people the edible mushroom acts as a poison.
Sinks. See that these be securely trapped, and in the event of any unpleasant smell from them, pour down some disinfectant, such as chloride of lime, carbolic acid, or Condy’s fluid. The foul emanations from a sink ought to be regarded as of a most dangerous and pestilential nature.
Accidents to Children. Many, if not most, of the casualties to which children are exposed are given above, together with the best course to be pursued in the event of their being overtaken by any of them. There are, however, a few forms of disaster which seem more especially14 peculiar to children. Of these we may select—
Swallowing a piece of broken glass. In this case avoid giving purgatives, but give solid farinaceous food, so as to envelope the glass and enable it to pass through the bowels without causing injury by coming in contact with them.
Swallowing a coin. Give a dose or two of castor oil, and examine the stools until the coin is perceived.
A small coin sticking in the windpipe. Seize the child by the legs, letting his head hang downwards, then administer several brisk blows on the back with the palm of the hand, when very frequently the coin will be coughed out of the mouth and on to the floor. If this plan do not succeed, send immediately for medical aid.
ACCLI′MATE, or ACCLI′MATISE. In botany and zoology, to inure a plant or animal to a climate to which it is not indigenous. When so inured it is said to be ACCLIMATED. In medicine, to habituate the body to a foreign climate, so that it may not be peculiarly liable to its endemic diseases; or to become so habituated. Thus, a person who has resided several years at New Orleans without an attack of yellow fever, or having had an attack has satisfactorily recovered, is said to be ACCLI′MATISED.
ACCOM′PANIMENTS. In cookery and housekeeping, see Trimmings.
ACCUMULA′TION. [Eng., Fr.] Syn. Accumula′tio, L. In medicine, a term applied when the effects of the first dose of any substance still continue when the second is administered (accumulation of action); or when several doses of insoluble substances remain inactive in the system until their energy is developed by chemical influence (accumulation of doses). See Medicines, Poisons, &c.
ACEPH′ALANS. Syn. Aceph′ala, Cuv. In malacology, a class of aquatic mollusca, having no apparent head, but a mouth between the folds of their mantle. Several of them, as the oyster, cockle, mussel, scallop, &c., are consumed for food.
ACERB′ITY. Syn. Acerb′itas, L.; Acerbité, Fr.; Herbigkeit, Ger. In chemistry, &c., sourness, with bitterness and astringency, or harshness. See Cider, Fruit, Wine, &c.
ACERBO’S ANTI-RHEUMATIC AND ANTI-CATARRH OIL. For various horse diseases. Gum euphorbium, 10 parts; absolute alcohol, 10 parts; olive oil, 80 parts. Digest in a warm-water bath for 24 hours, then boil until all the spirit has evaporated, and, when cold, strain through cotton. (Hager.)
ACER′IDES. Plasters that do not contain wax.
ACES′CENT. Syns. Aces′cens, L.; Acescent, Aigrelet, Fr.; Säurlich, Ger. In chemistry, &c., growing sour; slightly tart or acid; having a tendency to sourness, or to run into the acetic fermentation, as wine, beer, malt-wort, &c. Hence, ACES′CENCE or ACES′CENCY (acescen′tia, L.; acescense, aigreur, Fr.; säurlichkeit, Ger.), the tendency to become slightly acid, or the quality of being so. See Acetification, Malt-liquors, Wine, Wort, &c.
ACETA′′RIOUS (-tāre′-e-ŭs). Used for salads (as plants); relating to salads (which see).
AC′ETATE (ăs′-). Syn. Ace′tas, L.; Acetate, Fr.; Essigsäure salze, Ger. In chemistry, a salt consisting of C2H3O2 (sometimes called the acid-radical of the acetates) with hydrogen, a metal, or a compound basic radical; e.g.,
| Hydrogen acetate (acetic acid) | HC2H3O2 |
| Potassium acetate | KC2H3O2 |
| Lead (plumbic) acetate | Pb(C2H3O2)2 |
| Ammonium acetate | NH4C2H3O2 |
Salts of acetic acid (HC2H3O2) with the alkaloids are likewise termed acetates; e.g.,
Morphia acetate . C17H19NO3 . C2H4O2
Prep. That of the commercial acetates, and of many others, is noticed under the respective metals. In general, they may all be formed by direct solution of the carbonate, hydrate or oxide of the metal whose acetate it is desired to form, in dilute acetic acid; or from a solution of an acetate and of another salt of the metal, by double decomposition. In either case, the resulting solution must be carefully evaporated by a gentle heat, and, where possible, crystallised.
Prop., &c. All the neutral acetates, except those of molybdenum and tungsten, are more or less soluble in water, several so much so as to be uncrystallizable; many dissolve in alcohol; they suffer decomposition at a dull red heat, and by distillation, at that temperature, yield acetone and water, or acetone and acetic acid, and leave a carbonaceous residuum; at a full red-heat, those of potassium, sodium, barium, strontium, calcium, and magnesium, are converted into carbonates, whilst the other metallic acetates leave behind the pure metal, or its oxide. The aqueous solutions of the alkaline acetates soon turn mouldy and suffer decomposition. No more of them should, therefore, be dissolved at once than is required for immediate use.
Char., tests, &c. The acetates are known—1. By evolving fumes of acetic acid, recognisable by its peculiar and characteristic odour, on the addition of strong sulphuric acid:—2. By evolving the vapour of acetic ether (known by its peculiar and agreeable odour) when heated with a mixture of about equal parts of concentrated sulphuric acid and alcohol.
AC′ETATED (ăs′-). In chemistry and pharmacy, combined or impregnated with acetic acid or vinegar.
ACE′TIC. Syn. Ace′ticus, L.; Acétique, Fr. Of or relating to vinegar; made with acetic acid, as perfumes, &c. (See below.)15
ACETIC ACID. HC2H3O2. Syn. Pyrolig′neous acid (pure); Acid of vinegar; Acidum ace′ticum, L.; Acide acetique, Fr.; Acido acetico, It.; Essigsäure, Ger.; Azynzuur, Dut.; Eisel, Sax. When free from water it crystallises on cooling, and is distinguished as—Acetic hydrate, Hy′drated acetic acid, Monohy′drated a. a., Gla′cial a. a., Monohydrated a. a., Ace′tum glacia′le, Acidum ace′ticum G., L., &c. the sour principle of vinegar.
Var. Commercial acetic acid is found under the form of the pure acid of the chemist and pharmaceutist (glacial and dilute), and of vinegar, of which there are several varieties, which are noticed under their respective heads.
Sources. Fermented liquors; the vinegars of commerce; alcoholic liquors; wood, from which it is obtained, as pyroligneous acid, by distillation; the commercial acetates of soda, potassa, lime, lead, copper, &c. The pure acetic acid of the chemist and of commerce is almost wholly obtained from the acetates, either by the action of a strong acid, which seizes on the base, setting the acid free; or, by dry distillation, in which the high degree of heat employed separates the acetic acid from the base in the form of vapour. It is also obtained by the oxidation of alcohol.
Prep. The following are the principal processes at present adopted to obtain pure acetic acid:—
1. From the Acetates in the moist way:—
a. From ACETATE OF SODA:—
1. Commercial acetate of soda (i.e., the ‘pure acetate’ of the pyroligneous acid works), in crystals, is put into the body of a stout copper still, and a deep cavity being made in the centre of the mass, about 35% of sulphuric acid of a sp. gr. of not less than 1·84 is poured in; the walls of the cavity are then thrown in upon the acid, and the whole briskly agitated, for a very short time, with a large wooden spatula; the head of the still is next luted on, and the distillation conducted at a gentle heat, the receiver being changed as soon as the distillate begins to acquire a slight empyreumatic odour. The product, when the process is well managed, is an almost colourless acid of the sp. gr. of fully 1·05, containing about 40% of glacial acid, or between 34% and 35% of anhydrous acid. Any trace of colour or empyreuma is removed by agitation with some well-washed and recently ignited vegetable charcoal, or with a very small quantity of recently ignited purified animal charcoal, and subsequently passing it through a prepared calico bag-filter; or by allowing it to stand, for about a fortnight, in barrels containing some beech-wood chips; after which it is ready for sale, either as the ordinary acetic acid or pure pyroligneous acid of commerce, or (on dilution, &c.) as vinegar.
2. The acid of sp. gr. 1·05 (obtained as above) is distilled with fused chloride of calcium, the distillate being run into a refrigerator; the crystals that form are drained at a temperature below 40° or 45° Fahr., and after removal to a warmer temperature, where they liquefy, and agitation with a little peroxide of lead, are submitted to a second distillation, as before; and this is repeated until the whole of the acid crystallises at 51° Fahr. The product is the glacial acetic acid of commerce.
Obs. The above are the processes usually adopted, on the large scale, in this country.
3. (M. Mollerat’s process—without distillation.) Pure commercial acetate of soda, in coarse powder, is placed in a hard glazed stoneware or glass pan or receiver set in a cool situation, and 35% or 36% of concentrated sulphuric acid, of the sp. gr. 1·843, added, in such a manner that the acid may flow under the powder, and little heat be generated by the operation; the whole is then allowed to remain in contact (covered) for some hours, when crystalline grains of sulphate of soda are found covering the bottom and sides of the vessel, and hydrated acetic acid, partly liquid and partly in crystals, the upper portion. The temperature being now slightly raised to a point just sufficient to cause the liquefaction of the crystals of acetic acid (i.e., to from 62° to 65° Fahr.), the fluid is poured off, and a very small quantity of pure acetate of lime added to it gradually, until it ceases to yield any trace of free sulphuric acid on evaporation. After sufficient repose it is carefully decanted for use. An excellent commercial strong acetic acid is thus obtained, without distillation, owing to the insolubility of sulphate of soda in acetic acid; and from which glacial acid may be procured by refrigeration. If, however, the process be badly managed, or the proportions of the ingredients be not carefully observed, the product will be contaminated with either a little sulphuric acid or saline matter. It is also important to the success of this process that it be performed in a cool apartment, and in well-cooled vessels. Perfectly pure acetic acid may easily be obtained by rectification from this acid. The above plan of superseding a troublesome distillation is one of the greatest improvements yet introduced into the manufacture of acetic acid.
4. (Liebig’s process.) Pure acetate of soda, thoroughly dried and finely powdered, 3 parts, is placed in a capacious retort, and pure concentrated sulphuric acid, 9·7 parts, poured over it through the tubulature. One eighth of the acetic acid passes over by the heat developed by the reaction of the ingredients. The heat of a sand bath is next applied and continued until the contents of the retort become quite liquid. The distillate, carefully rectified, yields two parts of pure acid, containing only 20 per cent. of water. On exposing the latter portion which comes over in16 a closed vessel to a temperature below 40° Fahr., crystals of hydrated acetic acid are deposited. The weaker, or liquid portion, being poured off, the crystals are again melted and re-crystallised by cooling. The crystals of the last operation, separated from the liquid, and carefully drained in a cool and closed vessel, are perfectly pure hydrated acetic acid.
Obs. The above is an excellent process for obtaining a chemically pure acid. The excess of sulphuric acid left from the process may be recovered by distillation; or the whole residuum may be employed in a second distillation with fresh acetate.
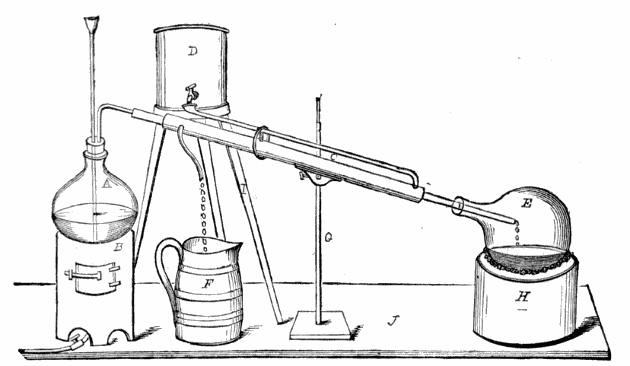 (C.) A Liebig’s Condenser. (The other reference
letters are self-explanatory.)
(C.) A Liebig’s Condenser. (The other reference
letters are self-explanatory.)
Although a retort is recommended by Liebig for the distillation, and is usually adopted, on the small scale, for the purpose, a flask closed by a cork perforated by two tubes, as exhibited by the engr., will be found more convenient and safe; as the product is then less likely to be contaminated by the ‘spirting’ of the ingredients over the brim of the vessel. The heat of a diffused gas-flame may also be often advantageously substituted for a sand bath.
b. From ACETATE OF POTASH:—
1. Acetate of potash (fused and powdered) is placed in a still, or other suitable vessel, and 50% of the strongest sulphuric acid (‘oil of vitriol’ of fully 1·84 sp. gr.) being added, the mixture is distilled to dryness, as before. The product is 50 to 51% of the weight of the acetate employed, with a sp. gr. of about 1·0735 to 1·074, containing about 66% of anhydrous acetic acid, or nearly 80% of ordinary glacial acid. By rectification from a little dried acetate of lead a perfectly pure acid of almost any strength may be obtained. The ingredients are nearly in equiv. proportions.
c. From ACETATE OF LEAD:—
1. (Ure.) Take of dried acetate of lead, 4 parts; strongest oil of vitriol, 1 part. Distil slowly to dryness. Nearly equal to the last.
2. (Liebig.) Acetate of lead, 3 parts; sulphuric acid, 8 parts; as before.
3. (Dollfuss’ Concentrated Acetic Acid.) Take of dried acetate of lead, 12 oz.; sulphuric acid, 6 oz.; distil 7 ounces.
d. From ACETATE OF LIME:—
1. (Christl.) Raw acetate or pyrolignate of lime (prepared by Völckel’s process), 100 parts, is mixed with hydrochloric acid (20° Baumé, or sp. gr. 1·1515), 120 parts; and after 12 hours, distilled in a copper vessel, with a gradually applied heat. The product is 100 parts or lbs. of acetic acid of 8° Baumé (sp. gr. 1·0556), containing about 47% of hydrated acid, only slightly coloured and empyreumatic, fit for various manufacturing purposes. The advantage of this process is the low price of hydrochloric acid, and the product not being contaminated with sulphuric or sulphurous acid.
Obs. It will be found that pyrolignate of lime generally contains 60% to 70% of neutral acetate; but should it contain either more or less, a proportionate quantity must be employed. When the proper proportions are used the distillate gives only a scarcely perceptible turbid cloud when tested with nitrate of silver. If the hydrochloric acid used has the sp. gr. 1·16, a less quantity being employed, the product will have the sp. gr. of 1·058 to 1·061, and will then contain from 48 to 51% of the monohydrate, or 41 or 42% of anhydrous acetic acid. The resin sometimes17 found floating on the mixed ingredients should be carefully removed, by skimming, before distillation.
As acid of the above strength is rarely required, and as the distillation is more easily conducted when the ingredients are less concentrated, a little water may be conveniently added either before or towards the end of the distillation. Hence the following proportions have been recommended:—
2. (Völckel.) Acetate of lime (as last), 100 parts; hydrochloric acid (sp. gr. 1·16), 90 to 95 parts; water, 25 parts; mix, and proceed as before. Prod. 96 to 98 parts of an excellent acid, well adapted to trading purposes, having a sp. gr. about 1·050, and containing nearly 40% of hydrated acetic acid. It has been correctly remarked, that the acetic acid produced with hydrochloric acid is always of better quality than that produced with sulphuric acid; being not only less coloured, but also entirely free from sulphurous acid. The distillation uniformly proceeds with ease and regularity, and the whole of the acetic acid passes over between 212° and 248° Fahr.; by which the danger of contamination with other products, resulting from a high degree of heat, is obviated.
3. An Acetic acid sufficiently strong and pure for many ordinary purposes may be obtained without distillation, by pouring strong sulphuric acid, 60 parts, diluted with water, 5 parts, on well-dried acetate of lime, 100 parts; digesting, with occasional agitation in a close vessel, decanting the clear liquid, and straining the remainder.
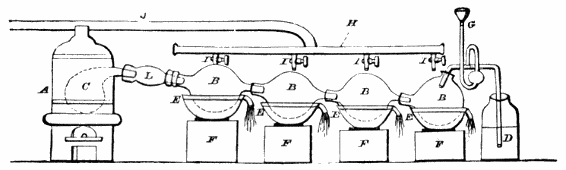 A, Furnace.
A, Furnace.II. From the Acetates by dry distillation with a sulphate:—
a. From ACETATE OF LEAD:—
1. Acetate of lead (dried), 5 parts; and sulphate of iron (gently calcined), 2 parts; are separately powdered; and after thorough mixture, carefully distilled, by the heat of a sand bath, into a well-cooled receiver. An economical process for a strong acid, under certain circumstances; but one now seldom adopted.
2. (Bardollier’s Strong Acetous acid.) Dried acetate of lead, 10 oz.; calcined green vitriol, 12 oz.; as the last.
b. From the ACETATES OF COPPER:—By substituting acetate or diacetate of copper, in equiv. proportions, or better with excess of the sulphate. Seldom used.
c. From ACETATE OF POTASH, as the last.
III. From the Acetates per se:—
a. From ACETATE OF COPPER:—Aromatic v.†; Spirit of verdigris†; Spiritus ven′eris†, L.; Esprit de venus, Fr.; Acidum ace′ticum, (Ph. L. 1787.) Process. Carefully dry crystallised verdigris (diacetate of copper) by a very gentle heat, and introduce it into a large stoneware retort (see engr.), the bottom of which has been previously coated with a mixture of clay and horse-dung, to render it more capable of standing the fire. Next place it in a suitable furnace, and connect it, by an adapter, with 3 or 4 double tubulated globes, the last of which must be furnished with a vertical tubulature, to which a double Welter’s safety-tube should be adapted; the other end being immersed in a basin half-filled with distilled vinegar or water, while the funnel portion communicates with the atmosphere. Then place each globe in a basin of water, kept cool by a stream constantly passing through it; and cover the upper portion with cloths kept continually wet with cold water. After 15 or 20 hours, fire may be applied, and must be so regulated that the drops follow each other with considerable rapidity from the end of the adapter, whilst the bubbles of air cause no inconvenience at the other end of the apparatus. If otherwise, the fire must be damped a little. The operation should be continued, and the fire gradually increased, until vapour ceases to come over, known by the globes gradually cooling, notwithstanding the heat of the furnace. The operation being concluded, the whole may be allowed to cool, and the acid collected preparatory to rectification. This may be effected in a similarly arranged apparatus, except that it must be wholly of glass; and the retort should not be much more than half-filled. The operation must now be very18 carefully conducted, and discontinued before barely the whole of the acid has distilled over; as the last portion is apt to injure the flavour and colour of the rest. The first portions which come over are very weak, and should be kept separate, until the sp. gr. reaches to about 1·372, when the receiver should be changed, and the product collected in separate portions, as noticed below.
Obs. Good diacetate of copper yields, by careful management, at a temperature of 400° to 560° Fahr., fully one half its weight of a greenish-coloured acid, of the sp. gr. of about 1·061, containing above 50% of hydrated acetic acid, or 43% of anhydrous acid. 20 lbs. of the ordinary acetate yields 93⁄4 lbs. of this rough acid, leaving a residuum of about 61⁄2 lbs. of metallic copper mixed with a little charcoal, in the retort; the remainder (nearly 2⁄10ths of the acid in the acetate) being decomposed by the heat, and lost. This 93⁄4 lbs. of crude acid yields by rectification, and dividing the products, 1⁄2 lb. of acid of the sp. gr. 1·023; 3 lbs. of the sp. gr. 1·042; and 6 lbs. of the sp. gr. 1·065; exclusive of a little acetone which comes over with it. In the first distillation, the strongest acid is found in the third receiver, and the weakest in the first. The acid obtained in this way is always accompanied with a little fragrant pyro-acetic spirit; which renders it preferable for aromatic vinegar and perfumery. It dissolves camphor, resins, and essential oils with facility. This is one of the oldest methods of obtaining glacial acetic acid, and the product is still preferred for some purposes. It is the RADICAL VINEGAR of the alchemists, and it is that which is preferred by the perfumers. Well-dried acetate of lead, or of iron, as well as several other acetates, may be substituted for acetate of copper in the above process; but are less economical and convenient. In all cases, great care must be taken to avoid over-firing, as thereby the quantity obtained is lessened, and the quality injured. The residuum of the distillation is pyrophoric and frequently inflames spontaneously, on exposure to the air. Due caution must be therefore observed regarding it.
IV. From Wood, by dry distillation. See Pyroligneous Acid. The preparation of the purified acid, by converting it into an acetate, and subsequent distillation with a strong acid, is noticed above.
V. From Alcohol. (Alcohol vinegar, German acetic acid.) In a bell-glass, or an oblong glass case, perforated shelves are arranged, a few inches apart, one above another, on which are placed a number of small flat dishes of porcelain, earthenware, or wood. These dishes are filled with spirit of wine or dilute alcohol; and over each is suspended a watch-glass or capsule containing a portion of platinum-black; the whole being arranged so that the platinum-black and the surface of the alcohol are not more than 11⁄2 to 2 inches apart. Strips of porous paper are next so hung in the case, that their bottom edges are immersed in the spirit, to promote evaporation; and lastly, the apparatus, loosely covered, is set in a light place at a temperature of from 70° to 90° Fahr.—the sunshine, when convenient. In a short time the temperature of the platinum rises, and the formation of acetic acid begins; and the condensed vapour trickles down the sides of the glass and collects at the bottom of the case, whence it is removed once or twice a day. (See engr.) The product of a case of twelve cubic feet content, with 7 or 8 oz. of platinum-powder, is capable of producing daily, if well managed, nearly 1·31 lb. of hydrated acetic acid from 1 lb. of absolute alcohol; 25 lbs. of platinum-powder and 300 lbs. of alcohol will, in like manner, furnish a daily supply of nearly 350 lbs. of pure acid, and of other strengths in proportion. Theoretically, the product should be 130 parts of the hydrated acid for every 100 parts of alcohol consumed; but this is never quite obtained in practice, owing to a small portion of the alcohol mixing with the newly formed acid, and escaping decomposition; and from another small portion of both the alcohol, and of the newly formed aldehyd, being carried off by the air that permeates the apparatus. The platinum-powder does not waste, and the most inferior spirit may generally be employed.
Rationale. In this process, the alcohol (as in other cases of acetification) is first converted into aldehyd; and this, as rapidly as formed, absorbs oxygen and passes into hydrated acetic acid. The simultaneous formation of aldehyd during the oxygenation of19 that already formed, may be detected by its odour.
Obs. During the mutual action of the platinum-black and the vapour of alcohol, the temperature increases, and continues to do so until all the oxygen contained in the air enclosed in the case is consumed, when the acetification stops. On opening the case for a short time, to admit of a fresh supply of air, the operation recommences, thus showing its dependence on the oxygen of the atmosphere. For this transmutation, 100 grains of alcohol require 71 grains (equal to 200 cubic inches) of oxygen, or about 1000 cubic inches of atmospheric air. To render the process continuous and rapid, a fresh supply of air must, therefore, be constantly provided. This may be effected by either having a loosely covered opening at the top of the case, and several much smaller ones near its lower part; or (and preferably) by means of two small glass tubes passing through the lid or cover, one of which terminates just below the point of insertion, whilst the other divides into branches which reach to within a short distance from the bottom, as shown in the engraving. In this way a very slow current of fresh air will always be kept up in the apparatus.
In practice, we find, that by loosely spreading the platinum-black on pieces of platinum-gauze, and supporting these on small tripods or bars of glass or porcelain (or even wood), the watch-glasses and their troublesome suspension may be dispensed with; as also may be the strip of porous paper, provided a temperature of not less than 90° Fahr. be maintained in the case or acetifier, which may easily be done by the application of artificial heat in the absence of sunshine. On the large scale, a case of wood with a glass roof, or even a well-seasoned cask or vat may be employed, in which case the temperature of the apparatus must be kept up either by means of steam-pipes or flues, or by the supply of warm air. On the small scale, a hand bell-glass placed on a dish, with a single watch-glass or piece of platinum-gauze, and a single capsule containing alcohol, may be used, provided the bell-glass be supported on three very small wedges, to admit of a supply of air. A modification of this is sometimes employed, in which the alcohol is supplied, in drops, to the platinum-black, by means of a long, tubular funnel passing through the mouth of the bell-glass, and having its lower extremity drawn to a very fine point, as shown in the engr. To ensure success, the platinum-black should be either fresh-prepared, or recently washed and very gently heated, before placing it in the acetifier. Spongy platinum, though ordered by many chemical compilers, does not answer well for this process.
By the above elegant and economical process, perfectly pure acetic acid of considerable strength may be produced from even impure alcohol; but it is impossible in this way to obtain a concentrated acid without a subsequent operation, because the action of platinum-black on absolute alcohol, or even on strong alcohol, is so violent that the platinum soon begins to glow, and inflammation ensues. Unfortunately the revenue laws of this country, until lately, stood in the way of the adoption of this beautiful process, unless duty-paid alcohol or methylated spirit be employed; but there is no statute that prevents an individual employing pure spirit, of any strength, on the small scale, for private consumption. In Germany, and in the United States of America, vinegar is manufactured on this plan, and from the low price of crude alcohol there, it will no doubt prove ultimately to be the cheapest source of both pure acetic acid and culinary vinegars.
VI. Miscellaneous Formulæ:—
1. An excellent acetic acid, of considerable strength, may be made by soaking fresh-burnt and perfectly dry charcoal in common vinegar, and then subjecting it to distillation. The water comes over first, and on increasing the heat, the acid follows. Vinegar-bottoms and waste vinegar may be used.
2. By exposing vinegar, or dilute acetic acid, to the air in very cold weather, or to freezing mixtures, the water separates in the form of ice, and the strong acetic acid may be obtained by draining it into suitable glass vessels, observing to do so at a temperature sufficiently low to keep the water solid. Said to answer well in cold climates.
3. Acetic acid containing 20% of water may be deprived of a good deal of its superfluous water by standing over dry sulphate of soda. (Liebig.) It may then be used either with or without distillation.
4. Acetic acid, of ordinary strength, may be concentrated to any degree, by rectification once, or oftener, from dry acetate of potash or soda, rejecting the first and last portions. The same acetate may be used repeatedly. The temperature need not exceed 400°, and must not rise above 570° Fahr.
Acetic acid. (B. P.) Syn. Acidum aceticum. Water mixed with 33% of hydrated acetic acid. Prepared by distilling acetate of soda with sulphuric acid. Colourless sour liquid. Sp. gr. 1·044.
Prop. Pure hydrated acetic acid is a thin, colourless liquid above 62 Fahr.; at 50° to 55° it crystallises in large, brilliant, colourless, transparent needles and plates, and even at 60° if a crystal of the acid be dropped in; at 40° it is a solid crystalline mass. Sp. gr.—liquid,20 1·063 (Mollerat) to 1·0635 (Mohr);[3]—crystallised, 1·135 at 55° Fahr. (Ure). Odour, intensely pungent when concentrated, but grateful, fragrant, and refreshing, when diffused; taste, intensely sour and acrid, becoming agreeable and refreshing, on sufficient dilution with water; volatile; inflammable, burning with a white flame; vapour of boiling acid highly combustible; dissolves camphor, resins, gum resins, volatile oils, gelatin, gliadin, coagulated albumen, and fibrin (as muscle or the crassamentum of the blood); it coagulates casein, but not liquid albumen (as the serum of the blood and white of egg): miscible with alcohol, ether, and water in all proportions; boils at 248° Fahr.;[4] and is decomposed at a red heat. Its salts are called ACETATES (which see).
Char., Tests, &c.—1. Free acetic acid reddens litmus paper, like the other acids; and may be readily recognised by its odour and volatility:—2. Sesquichloride of iron being added, and the acid then nearly saturated with ammonia, the fluid acquires a deep dark-red colour.
Estim. See Acetimetry. Organic mixtures that cannot be thus tested, or from which the acid cannot be obtained by simple distillation, may be neutralised, if acid, with carbonate of lime, boiled for a few minutes, cooled, filtered, the lime precipitated with dilute sulphuric acid, and the whole submitted to distillation, when the acid contents of the distillate may be estimated as above.
Pur. By heat, it escapes (entirely) in vapour; nothing is precipitated on the addition of either hydrosulphuric acid, nitrate of silver, or chloride of barium. Sometimes it is contaminated with sulphurous acid, which may be recognised by putting a fluid drachm of the acid, mixed with an ounce of distilled water and half a drachm of pure hydrochloric acid, also a few pieces of granulated zinc, into a flask. While effervescence continues suspend a slip of white blotting-paper, moistened with solution of sub-acetate of lead, in the upper part of the flask above the liquid, for about five minutes. The paper should not be discoloured, and thus indicate the absence of sulphurous acid.
Adult. The acetic acid of the shops is chiefly adulterated with water. Sulphurous acid and lead are accidental contaminations; that of the latter often reaches 2%, making the acid poisonous.
Phys. eff., &c. In its concentrated state it is a corrosive and an acrid poison. Taken internally, it acts by dissolving the animal tissues, and by thus destroying the organisation causes death, like the other acids. In the dilute form it acts as a stimulant, rubefacient, alterative, refrigerant, and escharotic.
Uses. Acetic acid is much employed by the chemist and pharmaceutist, in the manufacture of various preparations, and in analysis; by the perfumer, in the composition of several of his most refreshing and agreeable scents; and in medicine, as an antiseptic, stimulant, rubefacient, alterative, refrigerant, and escharotic. Acetic acid (B. P.) applied by means of a piece of rag tied to the end of a small stick, is a nearly certain cure for ring-worm and scaldhead—one or two applications generally effecting a cure, and the severe smarting it causes is only of short duration; as a caustic, it removes warts and corns; a piece of lint or blotting-paper wetted with it and applied to the skin (evaporation being prevented), forms a useful extemporaneous blister. It was once employed as a disinfectant; but is now only used as a fumigation, to disguise the unpleasant smell of the sickroom and crowded assemblies. It is a popular refreshing scent in faintings, asphyxia, and nervous headache; and is a valuable rubefacient, astringent, and local stimulant. It is also used as a rubefacient and caustic in veterinary practice.
In the arts, the commercial acid (pure pyroligneous acid) is used by the engraver to etch his plates; as an antiseptic in pickling and preserving animal and vegetable substances used as food, and anatomical preparations; in dyeing and calico printing, and in the manufacture of medicated vinegars and other pharmaceutical preparations.
In the dilute state, its properties and applications are similar to those of ordinary vinegar, and are noticed under that head.
Poisoning from acetic acid is rare. When concentrated, it is capable, by its corrosive and solvent action, of perforating the coats of the stomach and digestive canal; and it colours the mucus of these organs by the chemical action it exerts upon the blood. Vinegar in an excessive quantity acts in a similar way, but in a slighter degree. The treatment and antidotes are similar to those directed in cases of poisoning by the other acids. See Poisons.
Duty, Excise, &c. See Vinegar.
Gen. commentary. Acetic acid, on the large scale, is principally prepared from acetate of soda, which yields by a comparatively inexpensive, and not a difficult operation, an acid sufficiently strong and pure for commercial purposes, without the necessity of rectification. In this process shallow vessels of wood or of copper formed without rivets or solder (except silver solder) in those parts exposed to the action of the acid, are generally employed for the purpose of the distillation. A coil of drawn21 copper pipe, heated by steam having a pressure of 30 to 40 lbs. to the inch, traverses the bottom of the apparatus, to impart the necessary heat. The refrigerator consists of well-cooled earthenware, Berlin ware, or glass vessels; and the adopter pipe is also of the same materials. Another common form, which is even still more convenient, is a stout copper still, furnished with a cast-iron jacket to hold high-pressure steam, the usual refrigeratory being employed. In a few instances the space between the still and jacket is filled with sand, oil, tallow, or fusible metal; in which case the apparatus is set in brickwork, and heated by a naked fire. Stills of earthenware are also frequently employed; and even worms and condensers of silver, or silvered copper, are sometimes used, and with advantage. With a leaden worm the product is always contaminated with a little of that metal; the efforts of the manufacturer to the contrary, by the exclusion of air, and by rejecting the first and last portions of the distillate, only lessening and not preventing this evil. A lute (if any) composed of linseed meal and water, with or without a little powdered plaster of paris, may be employed; but flat bands and short tubes of well-seasoned vulcanised india rubber are infinitely more convenient and efficacious. The ingredients being placed in the still, and well but hastily stirred together with a wooden spatula, the head is luted on, and the distillation soon afterwards commenced. The chief care now should be to increase the heat gradually as the distillation proceeds; and when a steam-heat is not used, to carefully avoid over-firing, particularly towards the close of the operation. A little acetic ether is added by some manufacturers. In this way 4 lbs. of acid of the sp. gr. 1·050, is obtained for every 3 lbs. of acetate of soda employed. Should rectification be had recourse to, the addition of about 2 or 3% of bichromate of potash, peroxide of manganese, or red oxide of lead, will remove empyreuma, if present. The first of these substances is the most effective; the power of the others being in the order in which they are printed. In distilling the weaker acids and vinegars, it is found useful to add from 25 to 30% of chloride of sodium, which, by raising the boiling-point of the liquid, allows the acid the more freely to pass over (Stein); but this addition proves disadvantageous when any sulphuric acid is present, in which case sulphate of soda may be employed instead. If this addition be not made, the whole of the acid cannot be obtained without distillation to dryness, and the generation of empyreuma.
On the small scale, glass retorts are usually directed to be used, but glass alembics or flasks are more convenient and safe, as already noticed. In the preparation of the pure acid, care should be taken that the acetate of soda does not contain common salt, as the carbonate of soda prepared by calcination, and frequently used to form the acetate, is generally contaminated with it, and yields up its hydrochloric acid or chlorine during the process of distillation, thus vitiating the product. In all the methods given the product becomes more concentrated in proportion to the dryness of the acetate and the strength of the oil of vitriol or muriatic acid employed. By using the one dry, and the other concentrated, glacial acid may always be obtained by collecting separately the last two fifths that come over, and submitting this to refrigeration.
According to Melsens, pure GLACIAL ACETIC ACID is most advantageously obtained by distilling pure and dry acetate of potash with an excess of strong and moderately pure acetic acid, rejecting that which first passes over.
Acetate of soda may be safely dried at a temperature of 400° to 450°, provided care be taken to avoid ignition from contact with sparks. A less heat is, however, quite sufficient to drive off the whole of its water of crystallisation. It is known to be dry by its assuming the appearance of a smooth oily liquid whilst hot. If, whilst heated, it emits fumes, it is suffering decomposition. The same applies to the other commercial acetates. Crystallised acetate of soda loses about 2⁄5ths of its weight by thorough drying.
When acetate of soda and sulphuric acid are the ingredients employed in the production of acetic acid, sulphate of soda is formed, which, in the large way, the chemist returns to the manufacturer of acetate of soda (i. e. to the pyroligneous acid maker), who employs it in the decomposition of fresh acetate or pyrolignite of lime. In this way the same soda-salt is employed over and over again, acting merely as the vehicle for the separation of the crude acetic acid in the solid form, and its easy and cheap transportation from one point to another. This ingenious method of mutual assistance resulting from the application of chemical science to provide for the wants of everyday life, offers some explanation of the extraordinarily low price at which acetic acid may now be purchased.
The acetic acid of commerce (pure pyroligneous acid) is almost wholly obtained from the acetates of soda and lime. The principal supply of crude acetate (pyrolignite) of soda is from America, Norway, and Sweden; but much is also obtained from our home manufactories. See Acetification, Acetimetry, Fermentation, Pyroligneous Acid, Sodium, (Acetate of), Vinegar, &c.
More recently, acetic acid has been obtained by decomposing with hydrochloric acid the double salt of chloride of calcium and acetate of lime, mentioned by Fritzsche (‘Ann. de Poggend,’ xxviii, 123). For this purpose, solutions of acetate of lime and chloride of calcium are mixed and evaporated, the combined salts readily crystallising in large needles. These are freed from the mother-liquor and distilled with common muriatic acid.22
The acid furnished by this method requires redistillation, and is, moreover, contaminated with some of the fatty products always present in the crude pyrolignite.
Anhydrous Acetic Acid. Syn. Acetic Anhydride. Acetic acid deprived of the elements of water.| Acetic Acid. | Water. | Acetic Anhydride. |
| 2C2H4O2 | - H2O | = C4H6O3. |
Aromat′ic Acetic Acid. Syn. Aromatic vinegar; A. spirit of v.; Acidum Ace′ticum aromat′icum, L.—Prep. 1. (Ph. E. 1839.) Dried rosemary and origanum, of each 1 oz.; lavender flowers, 1⁄2 oz.; bruised cloves, 1⁄2 dr.; acetic acid (sp. gr. 1·068), 11⁄2 pint; macerate for 7 days, express, and filter. A fragrant and refreshing perfume. Omitted in Ph. E. 1841 and P. B. 1867.
2. (Ph. E. 1817.) As the last, but using distilled vinegar instead of the strong acid of the Pharmacopœia. Inferior.
3. (P. Cod. 1839) Camphor, 2 oz.; oil of lavender, 10 gr.; oil of cinnamon, 20 gr.; oil of cloves, 30 gr.; concentrated acetic acid, 1 pint. Very fragrant and refreshing.
4. (Ph. Bor. 1847; Cod. Med. Hamb. 1845.) Oil of cloves, 1 dr.; oils of lavender and citron, of each 2 scrup.; oils of bergamot and thyme, of each 1 scrup.; oil of cinnamon, 10 drops; strongest acetic acid, 1 oz.; mix. Limpid; yellow-brown; highly fragrant and refreshing. See Acetic Acid (Camphorated), and Vinegar (Aromatic).
Beaufoy’s Acetic Acid. A superior commercial acetic acid (i. e. purified pyroligneous acid), having a sp. gr. of about 1·044; or containing about 28% of real acetic acid, or 32 to 33% of the hydrated acid. Same strength, &c., as Acetic Acid P. B.
Cam′phorated Acetic Acid. Syn. Camphorated vinegar; Acidum ace′ticum camphora′tum, L.—Prep. 1. (Ph. E. 1841.) Camphor, 1⁄2 oz.; pulverise it by means of a few drops of spirit of wine, and then dissolve it in acetic acid (Ph. E.), 61⁄2 fl. oz.
2. (Ph. D. 1850.) Camphor, 1 oz.; rectified spirit, 1 fl. dr.; pulverise, and dissolve in strong acetic acid (acid. acet. fort. Ph. D.), 10 fl. oz.
Obs. This preparation is intended as a substitute for the aromatic acetic acid of the shops and previous pharmacopœias. It is also useful as an embrocation, in rheumatism and neuralgia; as an extemporaneous vesicant and counter-irritant; and as a fumigation in fevers, &c.
Dilute′ Acetic Acid. Syn. Acidum aceticum dilu′tum, L. Acetic acid, 1 pint; distilled water, 7 pints; mix, Sp. gr. 1·006. One fluid ounce corresponds to 16 grains of anhydrous acid (3·63 per cent.).
Glacial Acetic Acid. Syn. Acidum aceticum glaciale. Acetate of soda, 20 oz., is liquefied by a gentle heat, stirred till it becomes pulverulent, and then further heated until it fuses; it is at once removed from the fire, and, when cool, the mass is broken up, placed in a 3-pint stoppered retort connected with a Liebig’s condenser, and then treated with sulphuric acid, 8 fl. oz. When the distillation slackens heat is to be applied, and the process continued until 6 fl. oz. of acetic acid have passed over. If a little of the product strikes a blue colour when mixed with a solution of iodate of potassium containing mucilage of starch, the whole product must be agitated with perfectly dry black oxide of manganese, 1⁄4 oz., and redistilled. Sp. gr. 1·065; contains 85% of anhydrous acid.
ACETIC ANHYDRIDE. See Anhydrous Acetic Acid.
ACETICA. [L.] Medicated vinegars.
ACETIDUX, Dr DELFER’S. Made by Döllinger, of Berlin. For the radical and painless removal of warts, corns, hard skin, &c. A solution of 5 grms. of chromic acid in 15 grms. of water. (Schädler.)
ACETIFICATION. Syn. Acetifacio, L.; Acetification, Fr.; Essigmachen, Einsaüern, Ger. In chemistry, the act or process of converting into vinegar; also the state of undergoing such conversion.
Acetic acid is produced either by the partial dehydrogenation and subsequent oxidation of bodies containing its elements, or by their destructive distillation. The first is effected—by their exposure, in a finely divided state, to the action of air or atmospheric oxygen, as in the quick process of making vinegar; or—by submitting them, in combination with ferments, to contact with a free supply of atmospheric air, as in the old field process of making vinegar; or—by exposure to the direct action of chemical or mechanical oxidizing agents, as condensed air (platinum-black process), chromic and nitric acid, &c. In general, it is alcohol more or less dilute, particularly as it exists in fermented liquors, which is thus converted into acetic acid. In the second process (destructive distillation), wood is the substance usually employed, and heat is the agent which develops the acid.
The conversion of alcohol into acetic acid is not immediate and direct. The atmospheric oxygen first oxidises two atoms of its hydrogen, aldehyd and water being formed; and this aldehyd uniting with one atom of oxygen produces one molecule of ACETIC ACID. The changes are represented in the following equations:—
| Alcohol. | Oxygen. | Aldehyd. | Water. | |
| 1. | C2H6O | + O | = C2H4O | + H2O |
| Aldehyd. | Oxygen. | Acetic Acid. | |
| 2. | C2H4O | + O | = HC2H3O2 |
After the first formation of aldehyd, the two processes, unless artificially checked, go on simultaneously, as long as any undecomposed alcohol is present.
The conversion of alcohol into acetic acid, although greatly accelerated by the presence of nitrogenised organic matter (according to23 Mulder, of a fungus—the Mycoderma Vini or Vinegar Plant), is rather a case of eremacausis (slow combustion) than of fermentation. Acetification effects combination, as shown by the foregoing equations, whereas fermentation resolves complex bodies into simpler ones, e.g. sugar into alcohol and carbonic anhydride. Moreover, the presence of ferments is not essential to the change, since pure alcohol becomes acetified when exposed to the oxidising agents already named.
Another remarkable distinction between acetification and fermentation is, that the former requires the continued presence of atmospheric oxygen; whilst the vinous fermentation after being once established, proceeds perfectly without it.
During the oxidation of the alcohol of vegetable solutions, some of the other organic matters present also suffer change. A white gelatinous mass (mother of vinegar)[5] is commonly deposited; but this is a secondary result of the process, and not, as formerly supposed, one essential to it. In ordinary cases acetification occurs only at or near the surface of the liquid; which accounts for the length of time required for the operation under the old process of ‘fielding,’ and the shorter time in which it is accomplished by the improved process of Mr Ham. It proceeds favorably at temperatures ranging from 60° to 90° Fahr.; and most rapidly at 95° Fahr. (Liebig). In the ‘quick process’ of making vinegar a temperature of 90° to 92° is generally aimed at; but it often rises to 100°, or even to 105°, Fahr. As the temperature falls acetification proceeds more slowly, and at 46 to 50° Fahr. it ceases altogether (Liebig).
Aldehyd (see above) is an exceedingly volatile substance, and easily dissipated by a slight heat. It is, therefore, of the highest importance to duly regulate the temperature, as well as the supply of air, during acetification. In the ‘quick process’ of making vinegar the loss from this cause is always considerable, and often very great. This loss may be diminished by passing the heated air, as it escapes from the acetifier, through a porcelain or silvered copper worm or refrigerator, set in a chamber containing water of a temperature not higher than 40° to 45° Fahr.; the connection being made at the lower end of the worm, whilst the upper end is open to the air. On the small scale, as in the platinum-black process, the loss may be almost entirely prevented by causing the upper air tube to pass through a vessel containing ice or a freezing mixture; or by uniting it with the lower end of a Liebig’s condenser.
In liquors undergoing the vinous fermentation, a portion of the newly formed alcohol is invariably acetified whenever the temperature rises above 51° Fahr.; and at a higher temperature, this proceeds with a rapidity often highly injurious to the quality of the liquor. In this way there is frequently a useless loss of the alcohol, which is rendered more apparent by the incipient, and sometimes the actual, souring of the liquor.
ACETIM′ETRY. Syn. Acetom′etry; Acétométrie, Fr.; Acetime′tria, &c., L. The art or process of determining the quantity of pure acetic acid in vinegar, or in any other liquid. The plans generally adopted for this purpose are—
I. From the saturating power of the acid, as in the common methods of acidimetry:—
1. The molecular weight of commercially pure bicarbonate of potash, in crystals, being 100, whilst that of absolute acetic acid is 60, it is evident that every ten grains of the bicarbonate will exactly equal 6 grains of the acid. To apply this practically, we have only to exactly neutralise 100 gr. of the vinegar or solution under examination with the bicarbonate, observing the usual precautions; then, as 10 is to 6, so is the number of grains used, to the per-centage strength required. In this, as in other like cases, it is convenient to form a test-solution with the bicarbonate, by dissolving it in sufficient water to fill the 100 divisions of any simple form of ‘acidimeter,’ as a, b, or c; when the quantity of the solution, and, consequently, of the salt used, may be read off at once from the graduated portion of the tube. Still greater accuracy may be obtained by dissolving the bicarbonate in exactly 1000 gr. of distilled water contained in a ‘Schuster’s alkalimeter,’ previously very carefully weighed; in which case each grain of the test-solution will indicate 1⁄10th of a grain, or 0·1% of absolute acetic acid, whilst every 10 grains will be equal to 1 grain, or 1%.
The test-solution may also be prepared from bicarbonate of soda, or from the carbonates of soda or potash, care being taken that the quantity of the salt dissolved be in proportion to its molecular weight.
2. (Brande.) A small piece of white marble, clean and dry, is weighed, and then suspended by a silk thread in a weighed sample (say 100 or 1000 grs.) of the vinegar or acid under examination; the action being promoted by occasionally stirring the liquid with a glass rod, until the whole of the acid is saturated, as shown by no further action on the marble being observable on close inspection. The marble is then withdrawn, washed in distilled water, dried and weighed. The loss in weight24 which it has sustained will be nearly equal to the acetic acid present, or strictly, as 50 (marble) to 60 (absolute acetic acid). The only precautions required are, to avoid striking the piece of marble with the rod whilst stirring the solution, or causing loss of substance in it after its withdrawal; and to allow ample time for the action of the acid on it. If the sample consists of strong acid, it should be diluted with twice or thrice its weight of water before suspending the marble in it.
3. (Ure.) 100 grains of the sample under examination is slightly reddened with tincture of litmus, and ammonia of the sp. gr. 0·992 is added drop by drop (from an acetimeter holding 1000 water-gr. measure, divided into 100 divisions) until precise neutralisation is effected, indicated by the blue colour of the litmus being restored. The number of the divisions of the acetimeter used, multiplied by 60, and the first two right-hand figures of the product cut off as decimals, gives a number which represents the exact quantity of absolute acetic acid in the sample. In practice, it is found more convenient to keep the test-ammonia ready tinged with litmus.
The mode of estimating the per-centage of acetic acid in beers, when finding their original gravities, is a slight modification of the above. A test-solution of ammonia is prepared of such a strength that a given bulk of it will exactly neutralise one per cent. of absolute acetic acid in an equal bulk of beer, so that, if 100 fluid grains of the solution are sufficient to neutralise the acid in 1000 fluid grains of beer, such beer contains one tenth per cent. of acid. A solution of ammonia, diluted with distilled water until it has the sp. gr. ·9986 at 60°, is of the exact strength required.
An acetimeter holding 1000 grains, and graduated downwards to 100 equal divisions, is filled to 0 of the scale with the test-ammonia, which is then added, drop by drop, to 1000 measured grains of the beer, until neutralisation takes place. Every division of the acetimeter (corresponding to ten fluid grains), so emptied, indicates ·01 per cent. of acetic acid in the beer. The progress of the neutralisation is tested from time to time with a slip of reddened litmus paper, which should be suffered to become faintly blue before ceasing to add the ammonia. By this method the exact per-centage of absolute acetic acid in any sample may be accurately determined. The only precaution necessary is to be certain that the ‘test-ammonia’ has the required sp. gr. (·9986). Test-solutions may also be prepared with pure potash or pure soda.
II. From the specific gravity of the liquid after it has been neutralised with hydrate of lime:—
Common hydrate of lime (freshly slaked lime), in powder, is added gradually to the sample under examination, until it is saturated, when the sp. gr. of the resulting clear solution of acetate of lime is taken by Taylor’s ACETIMETER. This instrument is so adjusted and graduated as to float at the mark on the stem called ‘proof,’ in a solution containing 5% of absolute acetic acid (No. 24 vinegar). For vinegars stronger than proof small weights are provided, each of which indicates an additional 5 per cent. To ascertain the per-centage of real acid, 5% must therefore be added to the acetimeter number. Thus, without being loaded, the instrument, floating at the ‘proof mark,’ indicates a vinegar of 5%; with one weight, a vinegar of 10%; with two weights, 15%, and so on. According to this system of notation, each 5% is called a ‘vinegar.’ An acid of 10% is said to contain two vinegars; one of 15%, three vinegars, &c. It is also common to speak of the degrees of the acetimeter as proof or over-proof. Thus, No. 24 vinegar is said to be proof; one of 5 acetimeter degrees, 5 over-proof; one of 10 degrees, 10 over-proof, &c. For malt and wine vinegars, which contain gluten and mucilage, this method is not strictly accurate, as a portion of these substances escapes precipitation by the lime, and consequently alters the specific gravity. A small weight marked ‘M’ is generally supplied with the acetimeters for trying such vinegars.
III. From the specific gravity:—
The sp. gr. of the sample (carefully determined by any of the usual methods) is sought in one of the following Tables, when the corresponding per-centage content of acetic acid is at once seen.
This method furnishes reliable results only with pure, or nearly pure solutions which do not contain much above 50% of glacial acid, or which have a sp. gr. not higher than 1·062. It is also more to be depended on for weak solutions than strong ones. By carefully diluting a strong acid with an equal weight, or twice or thrice its weight of water, and allowing the mixture to again acquire its normal temperature, the sp. gr. may be taken as a guide in all cases in which great accuracy is not required. When such dilution is made it only becomes necessary to multiply the indication furnished in the Tables by 2, 3, or 4, as the case may be. As, however, authorities are not agreed as to the precise sp. gr. of the monohydrate or glacial acid, and of its solutions, extreme accuracy must not be expected by this method.
| sp. gr. | per cent. | ||
| 1·0085 | contains of anhydrous | or real acetic acid | 5 |
| 1·0170 | ” | ” | 10 |
| 1·0257 | ” | ” | 15 |
| 1·0320 | ” | ” | 20 |
| 1·0470 | ” | ” | 30 |
| 1·0580 | ” | ” | 40 |
25
| Absolute Acetic Acid, per cent. | Sp. Gr. | Absolute Acetic Acid, per cent. | Sp. Gr. | Absolute Acetic Acid, per cent. | Sp. Gr. | Absolute Acetic Acid, per cent. | Sp. Gr. |
| Pure acid or | |||||||
| 100 | 1·0630 | 74 | 1·0732 | 48 | 1·0582 | 22 | 1·0311 |
| 99 | 1·0648 | 73 | 1·0728 | 47 | 1·0568 | 21 | 1·0292 |
| 98 | 1·0663 | 72 | 1·0721 | 46 | 1·0557 | 20 | 1·0275 |
| 97 | 1·0677 | 71 | 1·0718 | 45 | 1·0553 | 19 | 1·0264 |
| 96 | 1·0685 | 70 | 1·0713 | 44 | 1·0544 | 18 | 1·0253 |
| 95 | 1·0696 | 69 | 1·0711 | 43 | 1·0535 | 17 | 1·0241 |
| 94 | 1·0704 | 68 | 1·0708 | 42 | 1·0525 | 16 | 1·0229 |
| 93 | 1·0708 | 67 | 1·0702 | 41 | 1·0518 | 15 | 1·0218 |
| 92 | 1·0715 | 66 | 1·0701 | 40 | 1·0513 | 14 | 1·0200 |
| 91 | 1·0721 | 65 | 1·0693 | 39 | 1·0502 | 13 | 1·0173 |
| 90 | 1·0726 | 64 | 1·0692 | 38 | 1·0492 | 12 | 1·0172 |
| 89 | 1·0729 | 63 | 1·0685 | 37 | 1·0482 | 11 | 1·0161 |
| 88 | 1·0730 | 62 | 1·0679 | 36 | 1·0473 | 10 | 1·0150 |
| 87 | 1·0731 | 61 | 1·0675 | 35 | 1·0460 | 09 | 1·0131 |
| 86 | 1·0732 | 60 | 1·0672 | 34 | 1·0449 | 08 | 1·0121 |
| 85 | 1·0733 | 59 | 1·0665 | 33 | 1·0439 | 07 | 1·0102 |
| 84 | 1·0734 | 58 | 1·0662 | 32 | 1·0425 | 06 | 1·0085 |
| 83 | 1·07343 | 57 | 1·0653 | 31 | 1·0413 | 05 | 1·0071 |
| 82 | 1·0735 | 56 | 1·0645 | 30 | 1·0402 | 04 | 1·0057 |
| 81 | 1·0738 | 55 | 1·0641 | 29 | 1·0392 | 03 | 1·0042 |
| 80 | 1·0743 | 54 | 1·0632 | 28 | 1·0380 | 02 | 1·0025 |
| 79 | 1·0742 | 53 | 1·0628 | 27 | 1·0364 | 01 | 1·0012 |
| 78 | 1·0740 | 52 | 1·0616 | 26 | 1·0352 | 00 | 1·0000 |
| 77 | 1·0739 | 51 | 1·0610 | 25 | 1·0341 | or Pure water. | |
| 76 | 1·0736 | 50 | 1·0602 | 24 | 1·0330 | ||
| 75 | 1·0731 | 49 | 1·0593 | 23 | 1·0320 | ||
Concluding remarks. Before applying the above processes, account should be taken of any mineral acid which may be present in the sample, such being not unfrequently added to vinegar to impart artificial strength; and in those depending on the sp. gr., gum, gluten, &c., must also be allowed for. The methods depending on the saturating power of the acid will be found appropriate to acetic acid of all strengths, when unadulterated with the mineral acid. The method based on the sp. gr. is also very convenient, and is sufficiently accurate for distilled vinegars and for pure acids of moderate strength.
It is found that the decimal fraction of the sp. gr. of pure or nearly pure vinegar is doubled by its conversion into acetate of lime. Thus, 1·0085 in vinegar becomes 1·0170 when converted into a solution of acetate of lime. In malt vinegar, however, 0·005 may be deducted from the sp. gr. for mucilage and gluten. The quantity of foreign matter present in vinegar may therefore be approximatively ascertained, by deducting the decimal of the sp. gr. of the solution of acetate of lime from double that of the decimal part of the sp. gr. of the vinegar. Thus:—the sp. gr. of a sample of vinegar being 1·014, and after saturation with hydrate of calcium 1·023, the sp. gr. of the pure vinegar would be 1·009, and that due to foreign matter ·005. For—
and—
The reason why proof-vinegar is called, in commerce, No. 24, is that 1 fl. oz. of it requires exactly 24 gr. of pure anhydrous carbonate of soda to neutralise it. Weaker vinegars are represented in the same ‘notation’ by the Nos. 22, 20, 18, &c., according to their respective strengths estimated by their saturating power.
ACETINE. An essence for the removal of corns. Concentrated vinegar (1·04 sp. gr.) slightly tinged with fuchsine, 15 grms. (Hager.)
ACETINE, HOCHSTETTER’S. Prepared by J. C. F. Witte, Berlin. A remedy for corns, warts, and hard skin. Diluted vinegar, coloured with blue carmine, 16 grms. (Schälder.)
ACETOLATS. [Fr.] Syn. Esprits acétiques. In French pharmacy, medicated vinegars obtained by distillation.
ACETOLES. [Fr.] In French pharmacy, medicated vinegars obtained by maceration.
ACETOUS FERMENTATION. See Acetification.
ACETUM. [L.] Vinegar.
ACETYL. Syn. Acetyle. A name originally given to a hypothetical body, having the formula C2H3, and regarded by Berzelius as the radical of 26the acetates and their congeners. The acetyl of Gerhardt (C2H3O) is, however, according to that chemist, the true radical of the acetates. Williamson, in order to remove the confusion of terms occasioned by the application of the same name to compounds of different composition, proposed the title of othyl for the radical C2H3O.
ACHAR. See Pickles.
ACEIILE′INE (-kĭl-). A peculiar bitter principle obtained from achillé a millefolium (Linn.), or yarrow.
A′CHOR, (-kŏr). [Gr.] See Scald-head.
ACHROMAT′IC (ăk-ro-). Syn. Achromatique, Fr. In optics, devoid of colour; bodies that transmit light without decomposition, and consequently, without the formation of coloured rings or fringes; applied to compound lenses, prisms, &c., and to instruments fitted with them.
ACRO′MATISM. Syn. Achromatisme, Fr. In optics, the state of being achromatic; the absence of coloured fringes in the images of objects seen through a lens or prism.
Light is not homogeneous, but decomposable by refraction, absorption, or reflection, into coloured rays of unequal refrangibility. A ray of white light, in passing through a glass prism, is entirely separated into the coloured rays forming the ‘prismatic spectrum,’ and when it passes through a lens, an analogous resolution into coloured rays still occurs, though not so readily observed, and that to an extent often incompatible with distinct vision. Now, if a convex lens be regarded as a number of prisms united by their bases round a common centre, and a concave lens, as a similar number of prisms with their apices in contact, the action of lenticular and prismatic glasses on light will be reduced to a common principle. A beam of light thrown on a simple converging lens not only suffers refraction at the spherical surface (SPHERICAL ABERRATION), but the different coloured rays of which it is composed, from the causes mentioned, being unequally bent or refracted, diverge from their original course (CHROMATIC ABERRATION), forming as many foci on the axis of the lens as there are colours, and fall separately, instead of together, on the eye or object which receives them. Hence arise the coloured fringes or halos that surround objects viewed through ordinary glasses, and which form the great impediments to the construction of perfect lenses. This effect, like the refractive power and focal distance, varies in degree in different diaphanous substances.
The correction of the chromatic aberration of lenses is commonly effected by combining two, or more, made of materials possessing different ‘dispersive’ powers. Thus, the spectrum formed by flint glass is longer than that formed by crown glass, for the same deviation. When the two are combined, so as to form a compound lens, the one tends to correct the ‘dispersion’ of the other. On this principle ACHROMATIC GLASSES are generally formed in this country. A convex lens of crown glass is combined with a weaker concave lens of flint glass, the latter counteracting the dispersion of the former, without materially interfering with its refractive power. The resulting combination is not absolutely achromatic, but is sufficiently so for all ordinary purposes. According to Dr Blair, a compound lens perfectly achromatic for the intermediate, as well as for the extreme rays, may be made by confining certain fluids, as hydrochloric acid, between two lenses of crown glass. In order to produce nearly perfect achromatism in the object-glasses of telescopes, microscopes, cameras, &c., a concave lens of flint glass is commonly placed between two convex lenses of crown or plate glass, the adjacent surfaces being cemented with the purest Canada balsam, to prevent the loss of light by reflection from so many surfaces.
Obs. The production of perfect achromatism in lenses is a subject not less fraught with difficulty than with practical importance to the astronomer, the mariner, the microscopist, and the photographer; and it has hence engaged the attention of the leading mathematicians and artists of Europe up to the present time. All the larger object-glasses lately manufactured are said to consist of only two lenses; the resulting achromatism proving sufficiently exact for all useful purposes. Those of recent production have come chiefly from the workshops of Dollond, of London, and the opticians of Bavaria and Switzerland. The achromatism of prisms depends upon the same principles, and it is effected in the same way as that of lenses.
ACIC′ULAR. Needle-shaped; slender or sharp pointed; spicular; in botany, applied to leaves, and in chemistry, to crystals. The last are also sometimes termed ACIC′ULÆ.
ACID, Syn. Acidum, L.; Acide, Fr.; Acido, Ital.; Säure, G. In familiar language, any substance possessing a sour taste. In chemistry, substances are said to be acid, or to have an acid reaction, when they are capable of turning blue litmus red. In chemistry, also, the term acid is applied to a very large class of compounds containing hydrogen (hydrogen salts), and in which one or more atoms of that element may be replaced by an equivalent quantity of a metal or other basic radical; e.g.—
1. The one atom of hydrogen in hydrochloric acid (HCl) may be replaced by sodium, producing the salt sodium chloride (NaCl).
2. The one atom of hydrogen in nitric acid (HNO3) may be replaced by silver, producing the salt silver nitrate (AgNO3).
3. One atom of hydrogen in acetic acid (HC2H3O2)[6] may be replaced by the basic radical ammonium (NH4), producing the salt ammonium acetate (NH4C2H3O2).
Acids which, like those mentioned in the foregoing examples, contain one atom of replaceable hydrogen are called monobasic; those which contain two such atoms (e.g. sulphuric acid, H2SO4; tartaric acid, H2C4H4O6),[7] dibasic; those which contain three such atoms (e.g. phosphoric acid, H3PO4; citric acid, H3C6H5O7),[7] tribasic; and so on with acids of higher basicity. Acids of greater basicity than unity are frequently termed polybasic.
Besides containing replaceable or basic hydrogen, acids are further characterised by the property of combining with alkaloids to form salts; e.g.—
| Sulphuric Acid. | Quinia. | |
| H2SO4 | + 2C24H24N2O2 | = |
| Quinia Sulphate. |
| (C20H24N2O2)2 . H2SO4 |
| Acetic Acid. | Morphia. | |
| HC2H3O2 | + C17H19NO3 | = |
| Morphia Acetate. |
| C17H19NO3 . HC2H3O2 |
Dibasic Acids. See Acid.
Fatty Acids. Acids separable from fats or oils; e.g. stearic acid, oleic acid, butyric acid, &c.
Inorganic Acids. Same as Mineral Acids (which see).
Mineral Acids. Acids chiefly or wholly derived from the mineral kingdom. In medicine, sulphuric, hydrochloric, and nitric acids, are commonly so called.
Monobasic Acids. See Acid.
Organic Acids. Acids formed by, or derived from organic substances; e.g. acetic acid, tartaric acid, uric acid, &c.
Polybasic Acids. See Acid.
Pyro-acids. Acids resulting from the decomposition by heat of other acids, e.g. gallic acid, when heated, yields pyro-gallic acid.
Tribasic Acids. See Acid.
ACIDIFICA′TION. [Eng., Fr.] Syn. Acidifica′tio, L. In chemistry, the act, process, or state of acidifying, or of making, becoming, or impregnated with acid.
ACIDIM′ETER. Syn. Acidom′eter; Acidime′trum, &c., L.; Acidimètre, Fr. An instrument or apparatus employed in acidimetry.
The ordinary acidimeters of the chemist are small tubes, constructed to hold exactly 1000 grains of distilled water, at 60° Fahr., within the limits of their scale, which is accurately graduated into 100 divisions. They are used to contain the alkaline solutions (TEST-LIQUORS, NORMAL or STANDARD SOLUTIONS) employed in the following processes.
Beaumé’s Acidimeter, and others of the same class, are HYDROMETERS, and are described under that ‘head.’
ACIDIM′ETRY. Syn. Acidom′etry; Acidime′tria, &c., L.; Acidimétrie, Fr. The estimation of the strength or quantity of acid, in a free state, contained in any liquid. It is the reverse of ‘alkalimetry.’ Acidimetrical assays are understood to refer to the relative strengths of the same acids (i. e., the quantity of real acid of the same kind contained in the solutions examined), and not to the comparative strengths of acids of different composition or names.
Acidimetrical processes. These are founded chiefly on the capacity of the acids to saturate the bases; and, in some of the liquid acids, on the specific gravity.
a. Volumetrically:—
1. The sample of the acid to be examined (100 gr., or any convenient aliquot part thereof) is placed in a suitable glass vessel, and if it be one of the stronger acids, diluted with six or eight times its weight of water, or if solid (as oxalic, or citric acid), dissolved in a like quantity. This liquid is then exactly neutralised with an alkali.
This point is usually determined, by the addition of a small quantity of litmus solution, which turns just blue when the solution is neutralised, but when a carbonate is used for the alkaline solution, the acid must be boiled a short time after each addition to expel the carbonic acid. The quantity of the alkaline solution consumed for this purpose represents an equivalent quantity of acid, and thus gives us the acid content of the sample under examination. The common practice is to dissolve one equivalent of the alkaline test in grains or grammes in water, and to make up the solution to exactly 1000 parts by measure (i. e., 1000 ‘water-grains’ or grammes), so as to accurately fill the 100 divisions of an acidimeter; when the quantity, in grains or grammes, of the sample tested, bears the same proportion to the equivalent number of the acid under examination, that the number of acidimeter divisions of the test-liquor consumed bear to the per-centage of acid sought. Thus:—suppose 50 gr. of a sample of sulphuric acid take 25 acidimeter divisions (300 parts or water-grains measure) of the test-liquid to neutralise it, what is its content of real acid?
The equivalent of sulphuric acid is 49 (half its atomic weight); so, by the rule of proportion,
50 : 49 :: 25 : 241⁄2
It therefore contains 241⁄2 parts of real sulphuric acid, in 50.
If the 1000 parts or grain-measures, instead of the number of the acidimeter divisions, be taken for the calculation, it will, of course, be necessary to point off the first right-hand figure of the result as a decimal. Thus; repeating the above example—
50 : 49 :: 250 : 24·5
Or, since the equivalent of the test-liquid is 100, it will bear the same proportion to the equiv. of the acid examined as the number of the acidimeter divisions of the test-liquid28 consumed in neutralising 100 gr., do to the per-centage sought. Thus:—50 gr. of hydrochloric acid take 45 acidimeter divisions to effect neutralisation, what is its real strength?—The equiv. of hydrochloric acid is 36·5: therefore—
100 : 36·5 :: 45 : 16·425%
and, since only 50 gr. (instead of 100 gr.) were examined—
Some operators prefer employing 100 gr. instead of the equivalent weights of the given tests in making their test-solutions, in which case each gr. or 1000th part represents 1⁄10th, and each acidimeter degree 1 gr. of the alkali or carbonate employed; when a similar proportion will obtain to that first above given.
In technical analysis it is more convenient if the number of acidimeter divisions of the ‘test-liquid’ consumed express the per-centage strength of the acid, without further calculation. For this purpose the number of grains of the acid taken for the assay should correspond to the equivalent number of such acid (see Table I, below); or to some convenient aliquot part of it, as the 1⁄2, 1⁄4, 1⁄5, or 1⁄10th; the per-centage answer, in the last case, being doubled, quadrupled, &c., according to the aliquot part taken. The reason of this is obvious.
For the test-solutions, ammonia, and the dry and crystallised carbonates and bicarbonates of potash and soda, are used, and are made by dissolving in water their constituents except ammonia, of which 1000 grains, or one litre, of solution of specific gravity 0·992 contains exactly one equivalent.
53 grains (or grammes) of pure anhydrous carbonate of soda, prepared by gradually heating to redness the crystallised salt, constitute one equivalent (half the atomic weight), and 69 grains (or grammes) of pure dry carbonate of potash. Of the crystallised salt 143 grains of carbonate of soda will be required, and 84 grains (grammes) of the crystallised bicarbonate of soda, and 100 of the crystallised bicarbonate of potash. Occasionally solutions containing in one thousand parts, 50 of pure carbonate of lime or chalk, or 28 of pure caustic lime, are used.
Besides these, a process known as Kiefer’s is practised, and an ammoniacal solution of oxide of copper is employed as the ‘test-liquor,’ and the ‘point of neutralisation’ is known by the turbidity observed as soon as the free acid present is completely saturated.
The normal solution or test-liquor is prepared by adding to an aqueous solution of sulphate of copper, pure ammonia water, until the precipitate, which at first forms, is just redissolved, carefully avoiding excess. Or better, by adding a rather strong solution of sulphate of copper, to a quantity of a rather strong solution of ammonia containing exactly 17 gr., or one equiv. of pure ammonia, as long as the precipitate which forms is redissolved on agitation; the resulting liquid being afterwards diluted with pure distilled water, until it accurately measures 1000 water-grains, or fills 100 divisions of an acidimeter, at 60° Fahr. In either case, the strength of the resulting ‘test-solution’ must be carefully determined by means of standard sulphuric acid, and adjusted, if necessary.
This method answers well with all the stronger acids (excepting oxalic acid), even when dilute; and it has the advantage of not being affected by the presence of a neutral metallic salt with an acid reaction, as sulphate of copper, or of zinc.
Besides this process a solution of lime in sugar may be used, as proposed by M. Peligot, and made as follows:—
Pure caustic lime is carefully slaked by sprinkling with water, and 50 grains (or grammes), made up by water to a milky solution, and 100 grains of pure sugar candy dissolved in 1000 grains of water, are added, and the whole well shaken. It is allowed to settle in a closed bottle, and the clear solution poured off and diluted, until 1000 grains neutralise exactly 100 grains of pure hydrochloric acid of sp. gr. 1·1812. Of course it only answers with acids whose calcium salts are readily soluble in water.
b. Gravermetrically:—
The test-liquors or standard solutions of the above methods are made up so as to weigh exactly 1000 grains, instead of to ‘measure’ 100 acidimeter divisions. Every grain of the test-liquor thus represents 1⁄10th gr. of alkali; and every 10 gr., 1 gr. of alkali; or respectively, 1⁄10th per cent. and 1 per cent. The vessel used for containing the solutions is carefully weighed whilst empty, and 1000 gr. being placed in the opposite scale, the test-solution, containing exactly one equivalent of base, is poured in, and the whole made up with distilled water (if necessary) so as to restore the balance to an equilibrium. After the process of neutralisation, the acidimeter, with its contents, is again placed in the scales; its previous weight still remaining there. The number of grains required to restore the equilibrium of the balance (i.e., the loss of weight), gives the exact weight of the test-liquor consumed. In all other respects the process is the same as in the ‘volumetrical method’ already described.
Another method for estimating the strength of the sample of acid is by weighing the amount of carbonic acid expelled during saturation. (Method of Fresenius and Will.) This depends on the weight of gaseous carbonic acid which a given weight of the acid-sample under examination is capable of expelling from pure bicarbonate of soda (or of potash), which is estimated by the loss of weight in the acidimeter, or apparatus, after the gas, rendered perfectly dry by passing through sulphuric acid, has escaped into the air.29
| 17 | gr. | of pure ammonia.[8] | are exactly neutralised by | 51 Acetic acid (anhydrous). |
| 31 | ” | anhydrous soda.[9] | 60 Acetic acid (crystallised or glacial). | |
| 40 | ” | hydrate of soda.[9] | 99 Arsenious acid (dry). | |
| 53 | ” | dry carbonate of soda.[10] | 35 Boracic acid (anhydrous). | |
| 143 | ” | crystallised carbonate of soda.[11] | 62 Boracic acid (crystallised). | |
| 84 | ” | crystallised bicarbonate of soda. | 22 Carbonic acid (dry). | |
| 47 | ” | anhydrous potassa.[9] | 67 Citric acid (crystallised). | |
| 56 | ” | hydrate of potassa.[9] | 85 Gallic acid (dried at 212°). | |
| 69 | ” | dry carbonate of potassa.[10] | 94 Gallic acid (crystallised). | |
| 100 | ” | crystallised bicarbonate of potassa. | 1271⁄2 Hydriodic acid (dry or gaseous). | |
| 50 | ” | pure chalk or pure marble. | 27 Hydrocyanic acid (anhydrous). | |
| 28 | ” | pure caustic lime. | 361⁄2 Hydrochloric acid (dry or gaseous). | |
| 37 | ” | hydrate of lime (fresh). | 1091⁄2 Hydrochloric acid (liquid, sp. gr. 1·162). | |
| 44 | ” | dry carbonic acid (when the bicarbonate of potassa or soda is used for testing in the process of Fresenius and Will). | 1661⁄2 Iodic acid. | |
| 22 | ” | dry carbonic acid (when a dry carbonate is used). | 54 Nitric acid (anhydrous). | |
| 671⁄2 Nitric acid (liquid, sesquihydrated, sp. gr. 1·5033 to 1·504). | ||||
| 72 Nitric acid (liquid, binhydrated, sp. gr. 1·486). | ||||
| 90 Nitric acid (liquid, sp. gr 1·42). | ||||
| 36 Oxalic acid (anhydrous). | ||||
| 63 Oxalic acid (crystallised). | ||||
| 72 Phosphoric acid (anhydrous). | ||||
| 81 Phosphoric acid (glacial). | ||||
| 50 Succinic acid (dry or anhydrous crystals). | ||||
| 59 Succinic acid (ordinary crystals). | ||||
| 40 Sulphuric acid (anhydrous). | ||||
| 49 Sulphuric acid (liquid, monohydrated, sp. gr. 1·8485). | ||||
| 75 Tartaric acid (crystallised). | ||||
| 12 Tannic acid (carefully dried). | ||||
Oper. A determined amount of the acid under examination is accurately weighed into the flask A (see engr.); and if it be a concentrated acid, or a solid, it is mixed with or dissolved in 6 or 8 times its weight of water. The little glass tube (e) is then nearly filled to the brim with pure bicarbonate of soda, in powder, and a fine silken thread is tied round the neck of the tube, by means of which it can be lowered down into the flask (A), so as to remain perpendicularly suspended when the cork is placed in the latter; the cord being held between the cork and the mouth of the flask. The flask (B) is next about half filled with oil of vitriol, and the tubes being arranged in their places, as represented in the engr.; and time having been allowed for the mixture of acid and water to cool completely, after the increase of heat caused by mixing, the whole apparatus is very accurately weighed. The cork in the flask (A) is then slightly loosened, so as to allow the little tube containing the bicarbonate of soda to fall into the acid, and is again instantly fixed AIR-TIGHT in its place. The evolution of carbonic acid now commences, and continues until the acid in the flask (A) is neutralised. When this takes place, which is easily seen by no bubbles being emitted on shaking the apparatus, the flask (A) is put into hot water (120° to 130°30 Fahr.), and kept there, with occasional agitation, until the renewed evolution of gas has completely ceased. The little wax stopper is then taken off the tube (a), the apparatus taken out of the hot water, wiped dry, and suction applied, by means of a perforated cork, or a small india-rubber tube, and the mouth, to the end of the tube (d), until the sucked air no longer tastes of carbonic acid. The whole is then allowed to become quite cold, when it is replaced in the balance (the other scale still containing the original weights), and weights added to restore the equilibrium.
The loss of weight represents the exact quantity of dry carbonic anhydride, or anhydrous carbonic acid gas, that has been expelled from the bicarbonate of soda, by the action of the acid in the sample examined.
The quantity of real acid it contained is then deduced by the following calculation:—One equivalent of gaseous carbonic anhydride, or anhydrous carbonic acid (= 44) bears the same proportion to one equivalent of the acid in question, as the amount of the carbonic anhydride expelled does to the amount of the acid sought. Thus, suppose a dilute sulphuric acid expels 3 gr. of carbonic anhydride, the arrangement is—
44 : 49 :: 3 : 3·349
Consequently the sample operated on contained 3·5 (nearly) grains of true sulphuric acid.
Instead of the above calculation, we may multiply the weights of the respective acids required to expel 1 gr. of carbonic acid (as exhibited in the following table) by the number of gr. of dry carbonic acid evolved during the above operation. The product represents the per-centage strength, when 100 gr. of the acid have been examined. When only 50, 25, 20, or 10 gr. have been tested, this product must, of course, be doubled, quadrupled, &c., as the case may be.
| Multipliers. | |
| Acetic acid (anhydrous) | 1·159 |
| Acetic acid (hydrated or glacial) | 1·364 |
| Citric acid (crystallised) | 1·523 |
| Hydrochloric acid (dry or gaseous) | ·829 |
| Hydrochloric acid (sp. gr. 1·16) | 2·478 |
| Nitric acid (anhydrous) | 1·227 |
| Nitric acid (sp. gr. 1·5) | 1·523 |
| Nitric acid (sp. gr. 1·42) | 2·045 |
| Oxalic acid (crystallised) | 1·432 |
| Sulphuric acid (anhydrous) | ·909 |
| Sulphuric acid (sp. gr. 1·8485) | 1·114 |
| Tartaric acid (anhydrous) | 1·500 |
| Tartaric acid (crystallised) | 1·705 |
Even this easy calculation may be avoided, in technical analysis, by simply taking for the assay such a weight of the respective acids as is capable of disengaging exactly 10 gr. of dry carbonic acid from the bicarbonate. In this case, the loss of weight in grains, from the operation, multiplied by 10, at once indicates the exact per-centage strength sought. The proper weight of any acid to be taken to give per-centage results is found by simply dividing ten times the equiv. of that acid by 44. For, taking sulphuric acid as an example,
as— 44: 49 :: 10 : 11·1318
or 11·13 nearly.
On this principle are obtained the weights to be taken, as given in—
| Grains. | |
| Acetic acid (anhydrous) | 11·59 |
| Acetic acid (hydrated or glacial) | 13·64 |
| Citric acid (crystallised) | 15·23 |
| Hydrochloric acid (dry or gaseous) | 8·29 |
| Hydrochloric acid (sp. gr. 1·16) | 24·78 |
| Nitric acid (anhydrous) | 12·27 |
| Nitric acid (sp. gr. 1·5) | 15·23 |
| Nitric acid (sp. gr. 1·42) | 20·45 |
| Oxalic acid (crystallised) | 14·32 |
| Sulphuric acid (anhydrous) | 9·09 |
| Sulphuric acid (sp. gr. 1·845) | 11·14 |
| Tartaric acid (anhydrous) | 15·00 |
| Tartaric acid (crystallised) | 17·05 |
2. A convenient modification of the preceding method of acidimetry consists in using the common apparatus figured in the margin and employing fused chloride of calcium to dry the evolved carbonic acid gas, instead of concentrated sulphuric acid. The mode of conducting the process and obtaining the results is precisely the same as in that last explained, and need not, therefore, be repeated. In this case, however, suction must be applied to31 the small tube (g), instead of (d) in the accompanying engraving.
Obs. These methods, though apparently complicated, are not difficult to perform, when once well understood. The application of heat after the completion of the operation is indispensable, as, if it were neglected, from 0·3 to 0·4 of a gr. of carbonic acid would be retained in the liquid. The bicarbonate of soda must be pure, and perfectly free from any neutral carbonate or sesquicarbonate of soda. To ensure this, the bicarbonate of commerce is reduced to a uniform powder, put into a glass jar, and covered with its own weight of cold distilled or rain water, and allowed to stand for twenty-four hours, with frequent stirring. It is then placed upon a funnel, the tube of which is stopped with loose cotton, so as to allow the lye to drain off. It is next washed several times with small quantities of cold distilled or rain water, and after being dried by pressure between some sheets of blotting-paper, without the aid of heat, is kept for use in a well-closed glass bottle. Before use, it may be tested to ascertain its purity. If pure, it neither reddens turmeric paper, nor gives a brick-red precipitate with a solution of bichloride of mercury. Pure bicarbonate of potassa may be used instead of bicarbonate of soda; but in either case it is always proper to use an excess, so as to leave some undecomposed carbonate after the operation has ended. The presence of a little sodium chloride or sulphate in the bicarbonate will not interfere in the least, but the absence of every trace of neutral carbonate is a sine quâ non.
The two above methods of estimating the amount of acid are only superior to the generally used methods first described, when the presence of colouring matter interferes with the reaction of the litmus used to show the point of neutralisation.
Observations. When great accuracy is required in conducting the neutralisation of the solution in estimating volumetrically with litmus as an indicator, it is proper to prepare and keep standard solutions of sulphuric acid and oxalic acid, with which occasionally to try the alkaline test-liquor. The only difficulty in the process is to avoid over-saturation of the acid-sample. Great care must be taken not to exceed the precise point of neutralisation of the acid. After adding each portion of the test-liquor, the solution should be well stirred up, and as soon as the effervescence becomes languid the greatest caution must be observed in adding more. The proper point is arrived at when the liquor ceases to redden litmus, and does not alter the colour of turmeric paper; if it turns the latter brown, too much of the test-liquid has been added, and the operation becomes useless. Towards the end of the experiment, when great precision is required, a gentle heat may be applied, in order to expel the free carbonic acid in the liquor; but otherwise this is unnecessary. The peculiar soapy odour gradually acquired by the liquor as it nears saturation will materially assist the operator when testing vinegars, and some of the other vegetable acids. A good method is to tint either the acid-sample or the test-liquid with a few drops of litmus, as noticed under Acetimetry; when the reddish shade will gradually deepen into ‘purple,’ or the purple into ‘red,’ as the point of saturation is approached; and the blue colour will be perfectly restored as soon as this point is reached. Dr Ure recommends keeping the ammonia-test ready tinged with litmus, and the same applies to other test-liquors.
In commerce, the strength of acids is frequently reckoned with reference to a standard, termed 100 acidimetric degrees. This is taken from the circumstance that 91 gr. of commercial oil of vitriol, of a sp. gr. of 1·845, exactly saturate 100 gr. of dried carbonate of soda. An acid requiring only 35, 50, or any other number of grains of the carbonate to saturate it, is in like manner termed of so many degrees strong; the number of grains representing in each case an equal number of degrees. This method originated with the French chemists, and though only conventional, and principally confined to commercial purposes, is especially adapted to practical men but little conversant with chemistry, yet very ready in retaining or calculating anything on the centesimal scale, from its similarity to monetary language and reckoning.
ACID′ITY. Syn. Acid′itas, L.; Acidité, Fr.; Säure, Ger. In chemistry, the state of being acid. In physiology, &c., the impression given to the organs of taste by tart or acid substances. Sourness. See Fermentation, Malt-liquors, Wines, &c.
Gas′tric Acidity. Acidity of the stomach; a common and well-known symptom of weak or disordered digestion.
Treat., &c. Small doses of absorbents or antacids, three or four times daily, to which some tonic bitter, as calumba, cascarilla, chamomile, gentian, or orange-peel, may be added. Stomachic stimulants, as capsicum, ginger, mustard, or wine, &c., taken with, or after, meals, are also useful. The diet should be light and nutritious; and acescent vegetables,32 over-ripe fruit, and weak new beer or other liquors avoided as much as possible. The bowels should be kept regular, but not open, by the occasional use of mild aperients, as rhubarb, aloes, castor oil, senna, or mercurial pill, or compounds containing them. Excessive looseness or diarrhœa may be checked by a few doses of carbonate of soda, chalk-mixture, or astringents.
In INFANCY this affection is usually accompanied by restlessness, continual crying, drawing up of the legs forcibly towards the body, hiccups, vomiting, diarrhœa, sour eructations, griping pains, green stools, and debility; often followed, when the irritation is considerable, by convulsions. The treatment consists in relieving the bowels of all offending matter by a few doses of rhubarb-and-magnesia. The looseness or diarrhœa may be checked by a few small doses of carbonate of soda or chalk mixture; or better, in an infant which is fed by lime-water (1 or 2 fl. oz.) mixed with as much milk. Two or three drops of caraway, cinnamon, dill, or peppermint water, on sugar (not with the food) will tend to promote the expulsion, and prevent the undue generation of gases. The flatulence usually disappears with the acidity. The occasional administration of 1 to 3 gr. of quicksilver-with-chalk (‘grey powder’), will frequently remove the complaint, and prevent its recurrence, when all other means fail. The diet of both nurse and infant should be carefully regulated.
See Antacids, Dyspepsia, &c.
Treatment for Horses. Alkalies, their carbonates and bicarbonates; alterative doses of aloes with alkalies; chalk, carbonate of magnesia; mineral acids; bismuth, arsenic, nux vomica, or strychnia.
ACIDS, EFFECTS OF, ON VEGETATION. This subject has been ably investigated of recent years by Dr Angus Smith and Mr Rothwell, and the practical importance of their labours is shown by the circumstance that an Act of Parliament passed in 1875 renders it penal for the proprietors of alkali works to condense not less than 95 per cent. of the hydrochloric acid evolved in the process of manufacturing ‘soda,’ also to allow air, smoke, or chimney gases to escape into the atmosphere containing more than one fifth of a grain of hydrochloric acid per cubic foot. Every owner of an alkali work is likewise required to ‘use the best practical means of preventing the discharge into the atmosphere of all other noxious gases arising from such work, or of rendering such gases harmless when discharged.’
The injurious effects of acids on vegetation are indicated chiefly by the shrivelled-up appearance which the leaves of herbage, trees, &c., exhibit in the vicinity of chemical works in which the condensation of noxious gases (hydrochloric acid, sulphurous acid, sulphuric acid, sulphuretted hydrogen, nitric acid, and oxides of nitrogen and chlorine) is not effectually carried out. According to Mr Rothwell, ‘in fields exposed to acid vapours handfuls of dead grass may be pulled up in the spring, smelling strongly of the vapour, and that trees, under similar influences, become bark-bound.’
The following is a list of trees arranged in the order of their susceptibility. (Rothwell.)
Forest Trees. Larch, spruce fir, Scotch fir, black Italian poplar, Lombardy poplar, ash, oak, elm, birch, alder, sycamore.
Fruit Trees. Damson, greengage, Halewood plum, Jacob plum, pears, apples, cherries.
Shrubs, Evergreens, and Wild Plants. British laurels, Portugal laurels, Aucuba Japonica, Barberry evergreen, hazel, guelder rose, sloe thorn, hawthorn, raspberries, gooseberries, blackberries, gorse, hollies.
Farm Crops. Potatoes, mangel, white clover and rhubarb, red clover, trefoil, rye-grass, wheat, oats, barley, common turnips, swedes.
Second list of Plants affected by Noxious Vapours, mixing the classes according to the effects produced on each.
I. Fern—only in the summer.
Scotch firs, spruce, and larches—a little in winter.
Clover (white and red), trefoil, rye-grass, poplars, hawthorn, potatoes—receive damage in winter to roots.
II. Wheat receives some damage in winter.
Oats in May, when in the grass state, soon receive damage.
Barley, mangel, common turnips, rhubarb.
III. Laurels (British and Portugal), aucubas, yews, holly, gorse—receive damage in winter, but more in summer.
Old grass meadows and pastures receive much damage in winter.
IV. Ashes, oaks, hazels, horse-chestnuts, walnuts, Spanish chestnuts, sloe thorn.
V. Swedish turnip and cabbages, damson, other fruit trees, beech, elm, birch, alder, sycamores.
ACIDULÆ. [L. pl.] In medicine, mineral waters rich in carbonic acid.
ACIDULATED. Syn. Acidulatus, L.; Acidulé, Fr. Blended or flavoured with an acid; made slightly sour. See Kali (Acidulated), Drops, Lozenges, &c. In chemistry, the addition of an acid to a neutral or alkaline liquid until it reddens blue litmus paper.
ACIDUM. [L.] An acid.
ACNE. [Syn. Pimpled Face.] There are two forms of this affection. 1st. In young persons of both sexes; generally in phlegmatic habits. The disease shows itself by hard pimples, with a small black spot on the apex, unaccompanied with redness or inflammation at first, but after a while they become red and inflamed, and sometimes suppurate, with a greasy look of the skin between them. In this form of acne the black spots should be picked out with a needle or a small pair of33 tweezers. A long piece of thick matter, like a worm, is extracted; but is no worm. Afterwards wash the face with water in which a small piece of Quillar bark has been steeped, or with bitter almond emulsion, or borax, one drachm, water 4 oz. When there is no inflammation, use Eau de Cologne, or a few drops of oil of rosemary dissolved in spirit of wine, taking a small dose of magnesia in the morning, or milk of sulphur daily. When the pimples are very sluggish the cautious application of tincture of iodine, or of ointment of nitrate of mercury, will be found serviceable.
2nd. Arises from intemperance. In this case a gradual change of habits is essential. The use of soap should be avoided, and recourse had to warm fomentations of slippery elm, or thin oat gruel. The following should be applied to the pimples:—Cold cream, 1 oz., Goulard’s extract 20 drops, mixed together; or lemon juice diluted, or solution of borax in water. The internal administration of the mineral acids combined with bitter tonics, or small doses of iodide of potassium, will be found effectual.
Treatment. Fomentations, poultices, chloride of zinc solution externally; sulphur and alteratives internally.
ACOLOGY. Syn. In medicine, the doctrine of, or a discourse on, remedies or the materia medica.
ACONITE. (-nite). Syn. Acon′itum, L.; Aconit, Fr.: Akonitum, Eisenhut, Sturmhut, Ger. Monkshood; wolfsbane. In botany, a genus of exogenous plants. Nat. ord., Ranunculaceæ; Sex. syst., Polyandria Trigynia. They are characterised by showy purple or yellow helmet-shaped flowers growing in panicles, deeply cut leaves, and perennial (usually) tap-shaped or tapering roots. The whole plant is highly poisonous, the roots being more poisonous than the leaves. In medicine and materia medica, the plant Aconitum Napellus (which see).
Symptoms. Numbness and tingling in the mouth and throat, which are parched; followed by giddiness, dimness of sight, and (sometimes) delirium, but seldom complete coma; there is numbness and tingling of the limbs, a loss of power in the legs, (in some cases) frothing at the mouth, severe abdominal pains, nausea, vomiting, and diarrhœa; tremors or twitchings of the voluntary muscles, (sometimes) convulsions (in animals, but not in man); sharp cries; pupil (generally) dilated, very rarely contracted; pulse fitful and sinking; skin cold and livid; difficulty of breathing; general prostration; loss of sensation or feeling, insensibility, general trembling, fainting, and sudden death. The eyes are often glaring; and, in some cases, the patient is completely paralysed, yet retains consciousness to the last. The case generally proves fatal in from 1 to 8 hours. If it last beyond this period there is hope of recovery. (Fleming.)
Antidotes. Ammonia, or brandy, with artificial respiration if necessary: cold affusion and friction, with warm towels to the back and limbs. See Alkaloids.
ACONITE LEAVES (B. Ph.). Syn. Aconiti folia, L. The fresh leaves and flowering tops of aconitum napellus, Linn., gathered when about one third of the flowers are expanded, from plants cultivated in Britain.
Char. Leaves smooth, palmate, divided into five deeply cut wedge-shaped segments; excizing slowly, when chewed, a sensation of tingling. Flowers numerous, irregular, deep blue, in dense racemes.
Prep. Extractum aconiti.
ACONITE ROOT. (B. Ph.). Syn. Aconiti radix, L. The dried root of aconitum napellus. Imported from Germany, or cultivated in Britain, and collected in the winter or early spring before the leaves have appeared.
Prep. Aconitia, the active principle; Linimentum Aconiti, 1 ounce to 1 fluid ounce; Tinctura Aconiti, 541⁄2 grains to 1 fluid ounce.
Char. Usually from one to three inches long, not thicker than the finger at the crown, tapering, blackish-brown, internally whitish. A minute portion, cautiously chewed, causes prolonged tingling and numbness.
ACONITI FOLIA. See Aconite Leaves.
ACONITI RADIX. See Aconite Root.
ACONITIA. C30H47O7N. (B. P.) Syn. Aconitia, L. An alkaloid obtained from aconite.
Take of
| Aconite root, in coarse powder, 14 pounds. | |
| Rectified spirit | of each a sufficiency. |
| Distilled water | |
| Solution or ammonia | |
| Pure ether | |
| Diluted sulphuric acid |
Pour upon the aconite root three gallons of the spirit, mix them well, and heat until ebullition commences; then cool and macerate for four days. Transfer the whole to a displacement apparatus, and percolate, adding more spirit, when requisite, until the root is exhausted. Distil off the greater part of the spirit from the tincture, and evaporate the remainder over a water bath until the whole of the alcohol has been dissipated. Mix the residual extract thoroughly with twice its weight of boiling distilled water, and when it has cooled to the temperature of the atmosphere, filter through paper. To the filtered liquid add solution of ammonia in slight excess, and heat them gently over a water bath. Separate the precipitate on a filter, and dry it. Reduce this to coarse powder, and macerate it in successive portions of the pure ether with frequent agitation. Decant the several products, mix and distil off the ether until the extract is dry. Dissolve the dry extract in warm distilled water acidulated with the sulphuric acid; and, when the solution is cold, precipitate it by the cautious addition of solution of ammonia diluted with four times its bulk of distilled water. Wash the precipitate on a filter with a small quantity of cold distilled34 water, and dry it by slight pressure between folds of filtering paper.
Characters and Tests. A white, usually amorphous, solid, soluble in 150 parts of cold, and 50 of hot water, and much more soluble in alcohol and in ether; strongly alkaline to reddened litmus, neutralising acids, and precipitated from them by the caustic alkalies, but not by carbonate of ammonia or the bicarbonates of soda or potash. It melts with heat, and burns with a smoky flame, leaving no residue when burned with free access of air. When rubbed on the skin it causes a tingling sensation, followed by prolonged numbness. It is a very active poison.
ACONITIA, CRYSTALLISED. C27H40NO10. Exhaust the root of wild aconite, carefully picked and powdered, with very strong alcohol, to which 1 per cent. of tartaric acid has been added. Distil at a gentle heat, and sheltered from the air, to recover the alcohol. Treat the extract with water to separate all the fatty and resinous matters. The solution which contains the aconite in the state of acid tartrate is first shaken with ether to remove colouring matters, and then the alkaloid is set free by the addition of alkaline bicarbonate, until the cessation of effervescence. A fresh treatment with ether of this alkaline solution removes the alkaloid, which crystallizes upon the concentration of the ethereal liquid, with an addition of petroleum spirit. The crystals are colourless tables, rhombic or hexagonal, according to the modifications produced principally in the acute angles. Crystallized aconitia is soluble in alcohol, ether, benzine, and chloroform; insoluble in petroleum oils and glycerine.
Aconitia Nitrate, Crystallised. Crystallised aconitine q. s.; nitric acid, sp. gr. 1·442, q. s. Saturate the nitric acid with the aconitine and evaporate. Voluminous crystals are easily obtained (from ‘Formulæ for New Medicaments adopted by the Paris Pharmaceutical Society’).—‘Pharm. Journal.’ Owing to the decomposition which this alkaloid undergoes in the animal organism, as well as to its liability to decompose during the process of evaporation, and exposure to the air, it often becomes extremely difficult, if not impossible, to obtain it in a separate state in conducting a post-mortem examination. The physiological effects seem to furnish the most prominent and characteristic evidence of its presence in such cases, or at any rate these may serve as a valuable guide to the toxicologist.
Uncrystallised aconitia is sometimes contaminated with delphinia, as well as with aconella, another constituent of aconite root. For the dissection of these see Alkaloids. One fiftieth of a grain of aconitia is stated to have killed a dog.
Antidotes. See Aconite.
ACONITIC ACID. (Identical with Pyrocitric Acid.) An acid extracted by Peschier from aconitum napellus, and by Bracconnot from equisetum fluviatile. It exists in these plants chiefly in the form of aconitate of calcium.
Properties. A white, colourless, semi-crystalline mass.
ACONITINA. See Aconitia.
ACONITINE. See Aconitia.
ACONI′TUM. [L.] Aconite. The pharmacopœial name of aconitum napellus(see below).
Aconitum Ferox. (Ind. P.) Habitat. Temperate and sub-Alpine Himalaya, at 10,000 to 14,000 feet elevation, from Gurhwal to Sikkim.
Officinal part. The dried root (Aconiti ferocis Radix), in common with those of other Himalayan species, viz., aconitum napellus, a. palmatum, and a. luridum, constitutes the drug well known in the bazaars of Upper India under the Hindostani name of Bish or Bikh.
It occurs in the form of tuberous roots of a more or less conical form, from two to three inches in length, and from half an inch to one inch in thickness at their upper end. They have usually a shrunken appearance, and are covered with a dark shrivelled bark; fracture shining and resinous; sometimes waxy, varying in colour from pale to deep brown. Some specimens are white and spongy; and these, it is asserted, are superior in activity to the more compact kinds. Inodorous; taste at first slightly bitter, leaving a peculiar sense of numbness on the tongue and fauces. Active principle, aconitia.
Medical Properties and Uses. Similar to those of aconitum napellus of Europe. Preparations. This root may be advantageously used for the manufacture of aconitia, the proportion of this alkaloid being much larger than in the European drug; and also for the preparation of Linimentum Aconiti. From its greater activity, however, it is unsuited for the preparation of this tincture, which is intended for external use.
Aconitum Hetorophyllum. (Ind. P.) Habitat. Western temperate Himalaya, at 8000 to 13,000 feet elevation; from Indus to Kumaon. Officinal part. The dried root (Aconiti heterophylli Radix). Ovoid tuberous roots, tapering downwards to a point, from one to one and a half inches or more in length, and from three eighths to half an inch in thickness. The surface, which is covered with a thin greyish epidermis, is slightly wrinkled longitudinally, and marked here and there with root scars. It is inodorous, and of a bitter taste, devoid of acridity. Does not contain aconitia. It may be readily distinguished from other roots sold in the bazaars under the same vernacular name (Atis) by its characteristic bitterness. Properties. Tonic and antiperiodic. It may be administered internally with safety, as it contains no poisonous principle. Therapeutic uses. In convalescence35 after debilitating diseases, and in intermittent and other paroxysmal fevers, it has been found an efficient remedy. Doses. Tonic, 5 to 10 grains thrice daily; antiperiodic, 20 to 30 grains of the powdered root every three or four hours, irrespective of the presence of pyrexia.
Aconitum Napell′us. [Linn.] Syn. Aconi′tum, Ph. L., E., & D.; Aconitnapèl, chaperon de moine, Fr.; Eisenhut, Blauersturmhut, Ger. Early blue wolfsbane, or deadly aconite. Hab. Various parts of Europe; grows wild in England, flowering in June and July. The fresh and dried leaves (ACONITI FO′′LIUM), Ph. L. & E. The root (ACONITI ra′dix), Ph. L. & D. This is the species of aconite ordered in the pharmacopœias, and commonly used in medicine. When chewed it imparts a sensation of acrimony, followed by a pungent heat of the lips, gums, palate, and fauces, which is succeeded by a general tremor and chilliness. The juice applied to a wound or the unsound skin affects the whole nervous system. Even by remaining long in the hand, or on the bosom, it produces unpleasant symptoms. Fatal cases of poisoning, by eating the root in mistake for horseradish, have been common of late years. The two roots may be, however, easily distinguished from one another; when scraped aconite emits an earthy, and horseradish its well-known pungent odour. Moreover, the shape of the roots is very different. In the accompanying figure a represents aconite root, and b horseradish root.
The leaves should be gathered as soon as the flowers appear. The root should be taken up in autumn. When the whole plant is employed, it should be gathered as soon as the flowers begin to open. The strength (richness in aconitia) varies considerably with the time of the year. 1 oz. of the fresh root contains 1⁄4 to 3⁄4 gr. of aconitia; 1 lb. of the dried English root contains from 12 to 36 gr. (Herapath). The leaves possess the greatest activity just before flowering; the root, after it. The root is at all times fully six times as strong as the leaves or herb. The wild plant contains much more aconitia than that which is cultivated. The herb, and all its preparations, lose their efficacy if long kept. The powder, more particularly, cannot be relied on. Mr Holmes says it is difficult to find in a commercial sample of aconite root one root in a dozen, which upon fracture appears sound and in good condition.
Properties, Antidotes, &c. See Aconite.
Tests, &c. See Aconite.
Uses, &c. In small doses aconite is narcotic, powerfully diaphoretic, and sometimes diuretic; in larger ones, the symptoms are similar to those produced by aconitia. It acts as a powerful sedative on the heart’s action, and destroys sensibility without disturbing the mental faculties. It has been given in chronic rheumatism, gout, paralysis, scirrhus, scrofula, cancers, venereal nodes, epilepsy, amaurosis, intermittents, &c.; but its exhibition requires the greatest possible caution. As a topical benumber it has been used with great advantage in painful affections depending on increased sensibility of the nerves. Externally it “is most valuable for the cure of neuralgic and rheumatic pains. In neuralgia, no remedy, I believe, will be found equal to it. One application of the tincture produces some amelioration; and after a few times’ use, it frequently happens that the patient is cured. In some cases, the benefit appears almost magical. In others, however, it entirely fails to give permanent relief.” “I do not think that in any (case) it proves injurious.” “When it succeeds, it gives more or less relief at the first application. When the disease depends on inflammation, aconite will be found, I think, an unavailing remedy.” “In rheumatic pains, unaccompanied with local swelling or redness, aconite is frequently of very great service.” (Pereira, iii, 691.) Dose, of the powder, 1 to 2 gr., gradually increased to 6 or 8. Dr Stocrk was the first who gave wolfsbane internally, about the year 1762. It has since been successfully employed in Germany in cases of chronic rheumatism, gout, &c., some of which were of long standing and had resisted every other remedy. In England it has been less extensively used.
Aconitum Panicula′tum. Panicled wolfsbane; a species formerly ordered in the Ph. L.; and, with a. napellus, also in the Ph. U. S. It is less active than the officinal species.
A′CORN. Syn. Glans. quer′cus, L. The seed or fruit of the oak. In the early ages of the world, acorns probably formed one of the principal articles of the food of man. (Ovid, Met., i, 106; Virgil, Georg., i, 8; &c.) In modern times, during periods of scarcity, they have been consumed as food on the Continent. Besides starch, they contain a peculiar species of sugar, which crystallises in prisms, and is unfermentable; they also contain tannic and gallic acids. Mannite and dulcose are the substances which it most nearly resembles. (M. Dessaignes.) During the autumn, acorns are said to be sometimes poisonous to cattle36 and sheep. Supposed cases of so-called acorn poisoning are best treated by withdrawing the supply of acorns, or removing the animals from the pastures on which the acorns fall, and by the administration of aperients, alkalies, and stimulants.
AC′ORUS CAL′AMUS. See Sweet Flag.
ACOTYLE′DONS (-ko-te-lē′-). Syn. Acotyle′dones (dŏn-ēz; L., prim. Gr.), Jussieu; Acotylédons, Fr.; Ohne samenlappen, Ger. In botany, plants whose seeds are not furnished with distinct cotyledons or seed-lobes. Acotyledonous plants form one of the two great divisions of the vegetable kingdom, according to the natural system. They are remarkable by increasing chiefly in length, by additions to their end; and not by addition to the outside, as in Exogens; nor to the inside, as in Endogens. They are also termed Asex′ual and Flowerless Plants, and answer to the Cryptogamia of the Linnean system. See Acrogens, Cellulares, Thallogens, &c.
ACOUS′TICS (-kow′-). The science of audition and sound; that branch of physics which treats of their cause, nature, and phenomena. The doctrine of the production and transmission of sound is termed Diacous′tics; that of reflected sound Catacous′tics.
Acoustics. In medicine, remedies employed to relieve deafness. See Deafness and Drops, Acoustic.
ACQUETTA. [It., Little Water.] Syn. Aqua Toffana; A. Toffania; Acquetta di napoli della Toffana, It. A celebrated poison, prepared by an Italian woman named Toffano, or Tophana, and in great request in Rome about the middle of the 17th century. The composition of this poison has been a matter of frequent controversy. Pope Alexander VII, in his proclamation, described it as “aquafortis distilled into arsenic.” This would produce a concentrated solution of arsenic acid. The Emperor Charles VI, who was governor of Naples during Toffano’s trial, declared to his physician, Garelli, that it was arsenic (arsenious acid) dissolved in aqua cymbalariá. According to Gerarde this cymbalarià was an aquatic species of pennywort, highly poisonous. The only objection to the latter statement is the smallness of the dose, regard being had to the comparative insolubility of arsenious acid; but if the woman Toffano prepared two poisons, as is probable from history—one, a single dose of which was fatal, and another, of which the dose required repetition, and which was more gradual in its activity—the discrepancy will be at once removed.
AC′RID. Syn. Ac′er, Ac′ris, L.; Acre (âcre), Fr.; Beissend, Scharf, Ger. In chemistry and medicine, sharp, pungent, acrimonious. Acrid substances are such as excite a sensation of pungency and heat when tasted, and which irritate and inflame the skin; as mustard, turpentine, cantharides, &c.
ACRIDITY. Syn. AcretÉ, Fr.; Acritudo, L. The quality of being acrid.
AC′RIMONY. Syn. Acrimo′nia, L.; Acrimonié, Acreté, Fr.; Scharfe, Ger. In medicine and chemistry, the quality or property of inflaming, irritating, corroding, dissolving, or destroying other bodies.
ACROGENS. Syn. Acrogenæ, L.; Acrogènes, Fr. In botany, acotyledonous or cryptogamic plants, in which stems and leaves, or an organisation approaching leaves, are distinguishable; which have stomates or breathing spores on their surface, are propagated by spores, and increase by the growth of the stem at the point only. Ferns and club-mosses are examples of this class of plants.
ACROLEIN. Syn. Acrylic Alcohol. This substance occurs amongst the products of decomposition when glycerine or any of its compounds is subjected to ordinary distillation. It derives its name from its violently irritant effect upon the mucous membranes of the eyes and respiratory organs. It is best prepared by the process of Redtenbacher (see ‘Leibig’s Ann.,’ xlvii, 114), by distilling in a capacious retort, a mixture of glycerine with phosphoric anhydride, or with hydric-potassic sulphate (the acid sulphate or bisulphate of potash); the vapours must be condensed in a properly cooled receiver, which is luted on to the retort and provided with a tube opening into a chimney having a good draught. The distilled liquid separates into two layers, the upper one consisting of acrolein, and the lower one of an aqueous solution of the same substance mixed with a quantity of acrylic acid. This distillate, after digestion with finely powdered litharge, with the object of neutralising the acid, must be rectified by the heat of a water bath: the acrolein so obtained must be submitted to a second rectification from calcic chloride. All these operations must be conducted in vessels filled with carbonic anhydride (carbonic acid) because acrolein becomes rapidly oxidized when exposed to the air.
Acrolein is a clear colourless liquid, lighter than water, boiling at about 125° F. It has great refracting power and a burning taste; when pure it is neutral to test paper.
AC′ROSPIRE (-spire). Syn. Acrospi′ra, L.; Plumule, Fr.; Blattkeim, Ger. The shoot or sprout of a seed, when it begins to grow; the part of a germinating seed termed the plume, or plumula.
When the growth of a seed begins to be developed, the germ, from which the stem originates, shoots forth under the form of a delicate curved fibre, which, gradually bursting its covering, makes its appearance at the end of the seed. The fibrils of the radicle first sprout forth from the tip of the grain; a white elevation appears, that soon divides into three or more radicles, which rapidly grow larger, and are succeeded by the plumula, which peeps forth at the same point, in the form of a pale green leaflet, which, twisting thence beneath the husk to the other end of37 the seed, ultimately bursts its prison-house, and becomes a perfect leaf. See Germination and Malting.
ACTINIC RAYS. See Actinism.
ACTINISM. Syn. Actinic Rays; Chemical Rays. A term given to a supposed principle accompanying the heat and light of the sunbeam. Actinic rays chiefly exist beyond the violet extremity of the solar spectrum, and are characterised by the power of exciting chemical change, e.g., the decomposition of certain silver salts (in photography); the combination of a mixture of chlorine and hydrogen, &c. The so-called vital functions of animals and plants are also greatly influenced by the actinic or chemical rays.
ACTINOGRAPH. An instrument for registering the intensity of the chemical influence (actinism) of the sun’s rays.
ACT, TOWNS IMPROVEMENT CLAUSES, 1847 (10 & 11 Vict., c. 34), The following provisions of this Act are incorporated in the Public Health Act, 1875, and refer exclusively to urban districts:—
1. With respect to naming the streets and numbering the houses.
2. With respect to improving the line of the streets and removing the obstructions.
3. With respect to ruinous or dangerous buildings.
4. With respect to precautions during the construction and repair of sewers, streets, and houses.
5. With respect to the regulation of slaughter houses.
Notices for alterations under the 69th, 70th, and 71st sections, directions under the 73rd section, and orders under the 74th section of the said Towns Improvement Clauses Act, may, at the option of the urban authority, be served on owners instead of occupiers, or on owners as well as occupiers, and the cost of works done under any of these sections may, when notices have been so served on owners, be recovered from owners instead of occupiers; and when such cost is recovered from occupiers, so much thereof may be deducted from the rent of the premises where the work is done as is allowed in the case of private rates under the Act.
AC′TUAL. Real, effectual, absolute; as opposed to that which is merely virtual or potential. In surgery, a red-hot iron, or any other heated body, used as a cautery, is termed the ACTUAL CAUTERY; whilst a caustic or escharotic so employed is called the POTENTIAL CAUTERY.
ACTUAL CAUTERY. See Actual.
ACUTE′. Syn. Acut′us, L.; Aigu, Fr.; Heftig, Hitzig, Spitzig, Ger. Sharp, pointed, sensitive. Applied to the senses, as acute hearing, eyesight, &c. In pathology, diseases exhibiting violent symptoms, and whose course is short, are said to be acute diseases.
ADAPTER. In chemistry, a tube placed between two vessels (commonly a retort and receiver) for the purpose of uniting them or increasing the distance between them, so as to facilitate the condensation of vapour in distillation. (See figure.)
ADDER’S TONGUE. Syn. Common adder’s tongue; Ophioglos′sum vulga′tum, Linn. A perennial plant, of the natural order Filices (DC.), growing wild in England. It is found in our woods and pastures, and flowers in May and June. It was once used to form a celebrated traumatic or vulnerary ointment and is still highly esteemed among rustic herbalists.
ADEPS. Syn. Lard. See Adeps præparatus, Fat, and Lard.
ADEPS BENZOATUS. Syn. Benzoated Lard.
ADEPS PRÆPARATUS. Syn. Axunge; Prepared Lard.
ADHE′SION (-hē-zhün). Syn. Adhæ′sio, L.; Adhesion, Fr.; Anhängung, Arxlebung, Ger. The act or state of sticking or being united.
Adhesion. In physics, the force with which bodies remain attached to each other when brought into contact; e.g., ink adheres to paper, paint adheres to wood, &c. It differs from ‘cohesion’ in representing the force with which different bodies cling together; whereas cohesion is the force which unites the particles of a homogeneous body with each other, e.g., particles of iron cohere and form a mass of iron; particles of water cohere and form a mass of water, &c.
Adhesion. In pathology, the morbid union, from inflammation, of parts normally contiguous but not adherent.
Adhesion. In surgery, the reunion of divided parts, by the adhesive inflammation; as when incised wounds heal by what is termed the ‘first intention.’
ADHE′SIVE. Syn. Adhæsi′vus, L.; Adhésif, Fr.; Adhäsive, Verwachsend, Ger. In pharmacy, &c., having the quality or property of sticking or adhering. Hence adhe′siveness.
AD′IPOCERE (-sēre). Syn. Grave-wax‡; Adipoce′′ra, L.; Adipocire, Fr.; Fetewachs, Ger. A substance resembling a mixture of fat and wax, resulting from the decomposition of the flesh of animals in moist situations, or under water. It is chiefly margarate of ammonium. Lavoisier proposed to produce this substance artificially, for the purposes of the arts. Attempts have since been made to convert the dead bodies of cattle (carrion) into adipocere, for the purposes of the candle-maker and the soap-boiler, but without success. Besides, dead animal matter38 can be worked up more profitably than in making artificial adipocere.
Hatchettine or rock-fat is sometimes called ‘adipocere’; and bog-butter is a substance nearly similar to it.
AD′JECTIVE. Syn. Adjecti′vus, L.; Adjectif, Fr. In dyeing, depending on another, or on something else; applied to those colours which require a base or mordant to render them permanent. See Dyeing.
AD′JUVANT. [Eng., Fr.] Syn. Ad′juvans, L.; Aidant, &c., Fr. Assistant; helping. (As a substantive—) In prescriptions, see Prescribing (Art of).
ADULTERATION. Strictly speaking, this term ought only to be applied to the practice of adding substances to articles of commerce, food or drink, for the purposes of deception or gain, but a wider interpretation is frequently placed on the word than the definition given by magistrates and analysts, these latter often regarding accidental impurity, or even, in some instances, actual substitution as acts of adulteration.
The following definition of an adulterated substance has been adopted by the Society of Public Analysts—
A substance shall be deemed to be adulterated—
A. In the ease of food or drink:
1. If it contain any ingredient which may render such article injurious to the health of a consumer.
2. If it contain any substance that sensibly increases its weight, bulk, or strength, or gives it a fictitious value, unless the amount of such substance present be due to circumstances necessarily appertaining to its collection or manufacture, or be necessary for its preservation, or unless the presence thereof be acknowledged at the time of sale.
3. If any important constituent has been wholly or in part abstracted or omitted, unless acknowledgment of such abstraction or omission be made at the time of sale.
4. If it be an imitation of or sold under the name of another article.
B. In the case of drugs:
1. If when retailed for medical purposes under a name recognised in the ‘British Pharmacopœia’ it be not equal in strength and purity to the standard laid down in that work.
2. If when sold under a name not recognised in the ‘British Pharmacopœia’ it differs materially from the standard laid down in approved works on materia medica, or the professed standard under which it is sold.
Limits. The following shall be deemed limits for the respective articles referred to:
Milk shall contain not less than 9·0 per cent., by weight, of milk solids, not fat, and not less than 2·5 per cent. of butter fat.
Skim Milk shall contain not less than 9·0 per cent. by weight, of milk solids not butter fat.
Butter shall contain not less than 80 per cent. of butter fat.
Tea shall not contain more than 8·0 per cent. of mineral matter, calculated on the tea dried at 100° C., of which at least 3·0 per cent. shall be soluble in water, and the tea as sold shall yield at least 30 per cent. of extract.
Cocoa shall contain at least 20 per cent. of cocoa fat.
Vinegar shall contain not less than 3 per cent. of acetic acid.
The practice of fraudulent adulteration has been indulged in for centuries. In every civilised state there have been enactments against it. The Romans had their inspectors of meat and corn. In England an Act to prohibit adulteration was passed as early as 1267, and penalties against it were in force in 1581, 1604, 1836, 1851. In 1822, Accum published a work having the sensational title of ‘Death in the Pot,’ and in 1855 appeared Dr Hassall’s book, ‘Food and its Adulterations.’ The information conveyed in these works, added to the revelations of the ‘Lancet’ Sanitary Commission, and the contributions to scientific literature on the subject of food by Letheby, Pavy, Parkes, Blyth, and others, together with the published evidence given before the House of Commons Commission appointed to carry out an inquiry into the subject, roused public attention to such a degree as to lead to the passing by the legislature of the Adulteration Acts.
The sophistications may be divided into several distinct classes:
1. To give weight or volume, such as water added to butter, plaster of paris to flour, &c.; red earths to annatto, sand to tea-leaves, &c.; water to milk, &c.; all these, therefore, are substitutions of worthless or very cheap articles which take the place of the real.
2. To give a colour which either makes the article more pleasing to the eye, or else disguises an inferior one, e.g., Prussian blue, black lead, &c., to green teas; annatto to cheese, &c.; arsenite of copper to sweetmeats, &c.
3. Substitutions of a cheaper form of the article, or the same substance from which the strength has been extracted put in the place of the real, e.g., tea mixed with spent leaves, &c.
4. A very small class where the adulteration is really added with no fraudulent intent, but to enhance the quality of the goods sold—alum to bread in small quantities.
The following, according to Blyth (‘Dic. of Hygiène’), is a list of articles most commonly adulterated, with the names of the substances used in their sophistication:—
Aconitia with other alkaloids, e.g., delphinia, aconella, &c.
Ale, common salt, Cocculus indicus, grains of paradise, quassia, and other bitters, sulphate of iron, alum, &c.
Allspice, mustard husks.39
Anchovies, other fish, and colouring matters, e.g., Armenian bole, Venetian red, &c.
Annatto, all sorts of starch, soap, red ferruginous earths, carbonate and sulphate of lime, salts, &c.
Arrowroot, various other fecula, such as sago, tapioca, potato, and others.
Balsam of Copaiba, turpentine and fixed oils.
Beef (Potted), Armenian bole.
Bismuth, carbonate of lead, sometimes arsenic (this latter is an impurity not intentional).
Bloaters (Potted), Armenian bole.
Brandy, water, burnt sugar, &c.
Bread, potatoes (mashed), alum, inferior flour, &c., &c.
Butter, water, salt, colouring matter, lard, tallow, and other fats.
Cajuput Oil, copper, camphor dissolved in oil of rosemary, and coloured with copper as a substitute.
Calamine, coloured sulphate of baryta.
Calomel, sulphate of baryta, chalk, white precipitate, white lead, pipe-clay, &c., &c.
Calumba, tinged bryony root, root of Frasera Walteri, and others.
Camboge, starch, &c.
Camphor, a substitution of Borneo camphor has been made.
Cantharides, golden beetle, artificially coloured glass, &c.
Carbonate of Lead, sulphate of baryta, sulphate of lead, chalk, &c., &c.
Carmine (Cochineal), sulphate of baryta, bone black, &c.
Cassia (Senna), leaves of Solenostemma argel, and other foreign leaves.
Castor Oil, other oils, often small quantities of croton oil.
Cayenne, ground rice, vermilion, Venetian red, turmeric.
Champagne, gooseberry and other wines as substitutes, different colouring matters, &c.
Cheese, annatto, bole (Armenian), and other colouring matters.
Chicory, colouring matters, such as ferruginous earths, and burnt sugar, Venetian red, &c., and different flours, such as wheat, rye, beans, &c., and sometimes sawdust.
Cider, lead (as an impurity, not intentional).
Cigars, substitutions of hay and other rubbish, inferior tobacco, leaves sometimes darkened by some brown vegetable dye.
Cinnamon, cassia, clove stalks, and different flowers.
Claret, brandy, and substitution of inferior wines.
Cloves, clove stalks.
Cocoa and Chocolate, cheaper kinds of arrow-root, such as Tous les mois and East Indian, animal matter, corn, sago, tapioca, &c.
Coffee, chicory, roasted wheat, rye flowers, and colouring matters, such as burnt sugar, &c.
Cod-liver Oil, other oils mixed with it.
Colocynth (Compound Extract of), the extract is not unfrequently made with the pulp and seeds.
Confectionery, injurious colouring matters, such as arsenite of copper, chromate of lead, &c.
Confection, Aromatic (Aromatic Chalk Powder), expensive ingredients omitted, turmeric substituted for saffron, &c., &c.
Copal, gum dammar, resin, &c.
Curry-powder, red lead, ground rice, salt.
Cusparia Bark, the bark of Strychnos Nux Vomica is said to have been substituted.
Custard and Egg Powder, turmeric, chrome yellow, and different flours.
Elaterium, starch, flour, chalk, &c.
Epsom Salts, chloride magnesium, chalk, &c.
Ether, alcohol.
Flour, other and inferior flours, as the flour from rice, bean, Indian corn, potato, &c., sulphate of lime, alum.
Gelatine, salt and sugar.
Gin, water, sugar, capsicum, flavouring matters of different kinds, turpentine, alum, tartar.
Ginger, turmeric, and husks of mustard, flour from wheat, sago, &c.
Guaiacum Resin, other resins.
Honey, flour, cane sugar, &c.
Hops, Cocculus indicus, grains of paradise, &c., &c.
Iodide of Potassium, water, carbonate of potash, chlorides of soda and potash, iodate of potash, iodine, &c.
Iodine, water, plumbago, charcoal, black oxide of manganese, &c.
Ipecaquanha, other roots, extraneous woody fibre; when in powder, chalk, flour, &c., have been added.
Isinglass, gelatine.
Jalap, raspings of guaiacum, false jalap root, &c.
Lard, carbonate of soda, salt, potato, flour, and lime.
Lemon Juice, a mixture of sugar and water, acidulated with sulphuric acid, has been substituted.
Liquorice, rice, chalk, gelatine, and different flours.
Magnesia, Magnesia Sulphate, lime, carbonate of magnesia.
Magnesia, Carbonate, lime, sulphate, &c., &c.
Marmalade, apple, or turnip pulp.
Mercury, lead, tin, zinc, bismuth, &c.
Mercury Green Iodide of, red iodide of
Mercury Red Oxide of, brick-dust, red lead, &c.
Mercury Ammoniated (WHITE PRECIPITATE), chalk, carbonate of lead, plaster of Paris, &c., 40&c.
Milk, water.
Mustard, turmeric, wheat flour.
Myrrh, gum bdellium, and other gum resins.
Oatmeal, barley flour, rubble.
Opium, stones, sand, clay, vegetable extracts, sugar, treacle, water, &c.
Pareira Root, different roots substituted.
Pepper, linseed meal, different flours, mustard husks, &c.
Pickles, salts of copper, acetate of copper.
Porter and Stout, sugar, treacle, water and salt.
Potash, carbonate, sulphate, and chloride of potash, lime, iron, and alumina.
Potash, Acetate of, sulphates, and chlorides of potash.
Potash, Carbonate of, sulphates, and chlorides of potash.
Potash, Bicarbonate of, carbonate of potash.
Potash, Citrate of, sulphates of potash.
Potash, Chlorate of, chloride of potassium.
Potash, Tartrate of, tartrate of lime.
Potash, Nitrate of, sulphate or chloride of potassium.
Preserves, salts of copper.
Quinine, sulphate of lime, chalk, magnesia, cane-sugar, sulphate of cinchonine, &c.
Rhubarb, turmeric, and inferior varieties substituted for Turkey.
Rum, water, cayenne, burnt sugar.
Sago, potato flour.
Sauce, treacle, salt, cochineal, Armenian bole, and other colouring matters.
Scammony, chalk, starch, guaiacum, jalap, dextrin, &c.
Senega, guiseng, gillenia.
Senna, leaves of cynanchum argel.
Sherry, sulphates of potash, soda, brandy, burnt sugar, &c.
Snuff, carbonate of ammonia, glass, sand, colouring matter, &c.
Soda, Bicarbonate, carbonate and sulphate of soda.
Soda, Carbonate, sulphate of soda.
Soda, Phosphate of, phosphate of lime.
Spices, colouring materials, substitutions, and different flours.
Squills (Powdered), wheat flour.
Sugar (Moist), sand, flour, &c.
Sulphur, sulphurous acid (as an impurity).
Sulphuric Acid, lead, water, arsenic, hydrochloric acid, &c.
Tapioca, mixing inferior starches with the pure tapioca.
Tea, sand, iron filings, exhausted tea leaves, foreign leaves; and in green teas, black lead, Prussian blue, China clay.
Tobacco, inferior tobacco, water.
Turmeric, yellow ochre, carbonate of soda, or potash.
Uva Ursi (Bearberry Leaves), leaves of red whortleberry, and others.
Vinegar, sulphuric acid, and metallic impurities.
Wines, water, jerupiga, bitartrate of potash, substitution of inferior wines, brandy, spirits, and various other matters.
Zinc, Oxide of, chalk, carbonate of magnesia.
“The Sale of Food and Drugs Act” has now supplemented several Acts which were passed during the present century for the prevention of adulteration. An Act prohibiting the mixture of injurious ingredients with intoxicating liquors remains unrepealed, as do also one or two statutes relating to trade frauds as for example the Adulteration of Seeds Act, 1809. These latter have not been incorporated in “the Sale of Food and Drugs” Act.
Æ (ē). [L.] For words sometimes written with this initial diphthong, and not found below, look under E.
ÆGI′RINON (-jī′-). [Gr.] See Ointment.
ÆGYPTI′ACUM† (-jĭp-tī′-). [Lat.] Syn. Unguen′tum Ægyptiacum, L. Oxymel or liniment of verdigris. The name originated with Hippocrates, who is said to have learned its composition in Egypt.
ÆOL′IPILE (-pĭle). A hollow ball of metal, having a slender neck with a very small orifice, contrived to exhibit the conversion of water into steam by the action of heat, and to account for the natural production of winds. It was known to the ancients, is mentioned by Vitruvius, and was studied by Descartes and others. It has been used in surgery to produce eschars, in the same cases as moxas; the effect of the steam being limited by means of a piece of perforated pasteboard. When filled with alcohol, and the jet of vapour inflamed, it is sometimes employed as a blowpipe. M. Soyer used an apparatus of this kind to supply the heat in his portable furnace. The liquid, however, which he employed was camphine.
A′ER, (ā′-ĕr). [L. prim. Gr.] Air.
A′ERATED (ā′-ĕr-rāte-ĕd). In chemistry, &c., impregnated with carbonic acid. See Alkali, Lemonade, Waters, Mineral.
AE′′RIAL (ā-ēre′-e-ăl). Belonging to the air or atmosphere; produced by, consisting of, depending on, or partaking of the nature of the air.
AERIFICA′TION (ā-ĕr-e-). Syn. Aërifica′tio, L.; Aérification, Gazéification, Fr. In chemistry, the conversion of a body into gas.
A′ERIFORM (ā′-ĕr-). Syn. Aëriform′is, L.; Aériforme, Gazéiforme, Fr. Luftformig, &c., Ger. In chemistry, air-like, gaseous.
AEROL′OGY. Syn. Aërolo′gia, L.; Aérologie, Fr., Ger. In physics, a discourse or treatise of the air. In physiology and hygiène, the doctrine of the air, more especially with regard to its salubrity and action on organised beings.
AEROM′ETER. Syn. Aërome′trum, L.; Aéromètre, Fr. An instrument used in aërometry.
AEROM′ETRY. Syn. Aërome′tria, L.; Aérométrie, Fr.; Luftmesskunst, &c., Ger.41 In chemistry and physics, the art of measuring gases, and of determining their densities.
AERONAUT′ICS. Syn. Aéronautique, Fr. The art of sailing in, or of navigating the air. See Balloons.
AEROPHO′BIA. [L.] Syn. Aérophobie, Fr. In pathology, a dread of air (wind); a common symptom in hydrophobia, and occasionally present in hysteria and phrenitis.
AEROSTAT′ICS. Syn. Aérostat′ica, L.; Aérostatique, Fr. That branch of pneumatics which treats of air, and other elastic fluids, in a state of rest.
AEROSTA′TION. [Eng., Fr.] Syn. Aërosta′tio, L. The art of weighing the air; aërial suspension and navigation. See Balloons.
ÆRU′GO (ē-). [L.] The rust of brass, bronze, or copper; verdigris.
ÆSCULIN. C21H24O13. A crystalline fluorescent substance existing in the bark of the horse-chestnut (æsculus hippocastanum) and of other trees of the genera Æsculus and Paria. In the above-named sources Æsculin is associated with another fluorescent body called Pariin.
Æ′′THER. See Ether.
ÆITHE′′REA (-thēré-). [L. pl.] Ethers.
ÆSTHET′ICS (ēz-). Syn. Æsthet′ica, L. Medicines or agents which affect sensation. See Anæsthetics and Hyperæsthetics.
ÆTHIOPS. See Ethiops.
AFFEC′TION. [Eng., Fr.] Syn. Affec′tio, L. In pathology, a term nearly synonymous with disease.
AFFINITY. Syn. Chemical affinity; Affinitas, L.; Affinité, Fr.; Verwandtschaft, Ger. If oil and water be shaken together they produce no change upon one another, as is proved by their separating into two layers with their properties unaltered, when the mixture is allowed to remain at rest for a short time. Such bodies are said, in chemical language, to have no affinity for one another. If iodine and metallic mercury be rubbed together in a mortar they will unite in definite proportions by weight, and form a combination possessing properties totally distinct from those of its constituents. Thus, iodine is a greyish, metallic-looking solid, convertible into a violet vapour by heat, perceptibly soluble in water, and capable of producing a blue compound with starch. Mercury is a metallic, silvery-looking liquid. The product of their union (biniodide of mercury) is a scarlet powder, destitute of metallic lustre, convertible into vapour by heat, without the production of violet fumes, insoluble in water, and incapable of developing a blue colour with starch. Again, the greenish-yellow and intensely poisonous gas, chlorine, unites in definite proportions by weight with the soft, wax-like, and highly poisonous metal sodium to produce the white crystalline solid chloride of sodium (common salt), a compound which, except in very large quantities, is not only not poisonous, but actually beneficial to health.
Such combinations are called chemical compounds, and the force which binds their constituents together is distinguished from all other attractive forces by the term affinity or chemical affinity. Bodies united by affinity are also said to have united chemically.
Affinity is in most cases exerted between different substances, in which respect it resembles adhesion; but bodies united by adhesion, e.g. ink to paper, paint to wood, &c., unlike those united by affinity, suffer no change of properties.
Affinity is exerted at immeasurable distances, therefore substances to be submitted to its influence must be brought into (apparently) actual contact. This condition is frequently fulfilled by the vaporisation, fusion, or solution of one or more of the bodies to be submitted to its action.
In many instances substances which have no affinity for one another at ordinary temperatures manifest this power when heated.
Whenever chemical union takes place, heat is invariably evolved; conversely, the decomposition of a chemical compound is always accompanied by an apparent loss of heat or reduction of temperature.
Finally, the most striking phenomena characteristic of, and accompanying, chemical affinity are, development of heat, change of properties, and union in definite or constant proportions by weight.
AFFUSION. In chemistry, the washing of a precipitate, &c., for the purpose of removing soluble matters. In medicine, affusion is of three kinds:—
1. Lotions, which consist in washing a part of the body with a sponge or rag soaked in a liquid.
2. Aspersions, which consist in throwing a liquid drop by drop, like rain, upon the body.
3. Shower baths, which consist in allowing a number of small streams of water to fall from a height upon the surface of the body. If the water fall from a considerable height, affusion is then termed douche by the French.
AFT′ER-DAMP. Syn. Choke-damp. Carbonic acid gas resulting from explosion of air and fire-damp (light carbonetted hydrogen) in coal mines.
AFT′ER-PAINS. Those following childbirth. The only remedy is patience; they may, however, be frequently alleviated by small doses of morphia or liquor opii sedativus. Heated cloths and warm fomentations are sometimes useful, particularly if assisted by moderate but sufficient pressure on the abdomen, by means of a broad bandage. They seldom follow with severity the first birth.
Treatment for Animals. Remove clots from parts, raise the hind-quarters. Give clysters of linseed tea, lukewarm, and laudanum or42 belladonna extract. Syringe out parts with Condy’s fluid considerably diluted. Give internally belladonna, opium, or chloroform. Draw away milk.
AFT′ER-WASH (wŏsh). In the art of the distiller, the liquor in the still after the spirit has been drawn over.
AG′ARIC. [Eng., Fr.] Syn. Agar′icum, Agar′icus, L.; Blätterschwamm, Pilz, Schwamm, Ger. In botany, a genus of fungi, of numerous species, embracing the mushrooms and champignons. Of these plants, some are edible; others poisonous. The term is also commonly applied to the boletus found on oaks (TOUCHWOOD), and on larches (MALE AGARIC). See Mushrooms.
Fly-agaric. Syn. Fly mush′room; Agar′icus musca′′ria, Linn.; Amani′ta m. One of the most narcotic and poisonous of our fungi, producing, in small doses, intoxication and a pleasing species of delirium; for which purpose it is commonly employed in Kamschatka. (Hooker.) It possesses the singular property of imparting an intoxicating quality to the urine, which continues for a long time after taking it. This secretion is, therefore, commonly saved by the natives during a scarcity of the fungus. “Thus, with a few amanitæ, a party of drunkards may keep up their debauch for a week;” and the intoxication so produced is capable of “being propagated through five or six individuals.” (Langsdorff.) Water in which it has been boiled is poisonous; but the boiled fungus itself is inert. The liquid from it is used as a fly-poison; whence the name mushroom is derived. It may be known by its rich orange-red colour in autumn.
AG′ATE (-āte, -ĕt‡). [Eng., Fr.] Syn. Acha′tes (-kā′-tēz), L. A semi-pellucid uncrystallised species of quartz, remarkable for its hardness, variety of colour, and susceptibility of receiving a high polish. It is an aggregate of various siliceous minerals, of which chalcedony appears generally to be the base. Carnelian, jasper, amethyst, and other similar minerals, often enter into its composition. The colours are often delicately arranged in stripes, bands, or clouds. Those which take an angular form, as the Scotch pebble, are called FORTIFICATION AGATES. It is the least valuable of the precious stones, and is chiefly made into rings, seals, beads, burnishers, &c., on account of its hardness. Its powder is used for cleansing and polishing iron, brass, &c., and to sharpen edge-tools.
AGEING LIQUOR. Dissolve 3 lbs. of chlorate of potash in 4 galls. of boiling water. Add 20 lbs. of powdered white arsenic to 20 lbs. of solution of caustic soda at 60° Tw., and boil until the arsenic is completely dissolved. Add the latter solution to the former, with stirring, until the mixture stands at 28° Tw.
AG′NAIL. See Whitlow.
AGRYPNOT′ICS (-grĭp-). Syn. Anthypnot′ics (-hĭp-); Agrypnot′ica, Anthypnot′ica, L. In medicine and pharmacology, agents or substances which prevent sleep; as tea, coffee, digitalis, vinegar, &c.
A′GUE (-gŭ). Ague may be defined as febrile phenomena occurring in paroxysms, and observing a certain regular succession, characterised by chill, abnormal heat, and unnatural cutaneous discharge, which prove to be a temporary crisis and usher in a remission. These phenomena are developed in an uninterrupted series or succession more or less regular, which pass into each other by insensible stages. Ague is paludal fever, and has always been observed to prevail in marshy moist districts, and in low, swampy humid countries, in which seasons of considerable heat occur.
The neighbourhood of marshes, or of a district which has been at some recent time under water; the banks of extensive lakes, and the shores of rivers and seas where the water flows sluggishly, and in some places stagnates; shallow rivers; extensive level tracts of forest land, where moisture is always present; and the surface of the land constantly covered with excavation from the ground,—these are the terrestrial physical conditions, in which marsh and littoral fevers are almost universally to be found, although it must be admitted that there are some marshy districts in which the disease does not show itself.
In these latter localities the effects of the miasmatic poison, show themselves in cholera or typhus. No precise knowledge of the nature and source of this subtle poison which, in default of a better name we call malaria, has yet been acquired; indeed it has yet to be proved that malaria has a distinct existence. Science has as yet been unable to discover the presence of any poisonous principle in the air of ague on other regions.
Ague may exist without any alteration of structure being set up; but in the milder forms of this fever a greater number of organs and tissues are morbidly altered than perhaps in any other form of disease. The parts so affected are the liver, spleen, lungs, heart, brain, and the serous and mucous membranes of the body generally. Within certain limits, the specific action of the malarial poison may be said to be in the inverse ratio of the intensity of the fever which attends its action. The affections of the liver and spleen also vary greatly according to the locality in which the patient is attacked; for instance, whilst in some parts of India the spleen is the organ principally involved, in other districts of the same continent it is the liver. In England, under proper medical treatment, the patient usually recovers without any manifest derangement either of structure or impairment of function of any organ or tissue. The liver may, however, become affected if the patient suffering from the disease has been neglected for any length of time.43
Notwithstanding the opinions of Finke and Professor Colin, there appears to be considerable ground for the supposition that ague may be caused by drinking marsh and surface water. In an interesting paper on the ‘Indian Annals’ for 1856, Mr Bettington, of the Madras Civil Service, says:—“It is notorious that the water produces fever and affections of the spleen.” In confirmation of this assertion, he brings forward what seems to be some remarkably strong evidence. He cites cases of villages placed under the same conditions as to marsh-air in some of which fevers were prevalent, whilst in others they were absent; and he found on inquiry that whilst the latter villages were supplied with pure water, the inhabitants of the former had to drink marsh or mullah water, full of vegetable débris. In one village there were two sources of supply—a spring and a tank, the first fed by surface, and the other by marsh water. Those only who partook of the tank water were attacked by fever. Again, in Tulliwaree the fever was so universal that scarcely any inhabitant escaped it. In this village Mr Bettington caused a well to be dug, and the result was that the fever disappeared. Similar cases have occurred in this country. Twenty years ago Mr Blower, of Bedford, directed the attention of medical men to a case that occurred in a village, in which ague had nearly disappeared when a well was dug; and to another instance which occurred in the village of Houghton. In this parish almost the only family which escaped ague was that of a farmer; the members of this family partook of well water; whilst those who did not escape the disease drank ditch water.
In the ‘Indian Annals’ for 1867 is a paper by Dr Moore, confirming the opinion that ague may be produced by the causes already stated, and M. Commaille (‘Rec. de Mêm. de Med. Mil.,’ Nov., 1868) states that in Marseilles, paroxysmal fevers, formerly unknown, have made their appearance, since the water supply to that city has been drawn from the Marseilles Canal.
In his report for 1870 Dr Townsend, the Sanitary Commissioner for the central provinces of India, states that the natives of India hold an opinion that the use of river and tank water during rainy seasons (when the water always contains an increased quantity of vegetable matter) will almost always cause ague. Boudin (‘Traité de Géographie et de Statistiques Médicale,’ 1857, t. i, p. 142), records an extraordinary case. Eight hundred soldiers, in good health, embarked in three vessels to pass from Bona, in Algiers, to Marseilles, in the year 1834. They all arrived at Marseilles the same day. In two vessels there were 680 men, without a single sick one amongst them. In the third vessel, the Argo, there had been 120 soldiers; 13 died during the short passage, and of the 107 survivors no less than 98 were disembarked suffering from all forms of paludal fevers. We may presume that the diagnosis was correct, since Boudin himself examined the men. When the vessels started the crew of the Argo had not a single sick man aboard. The crew and soldiers of all the boats were exposed to the same atmospheric conditions. The influence of air must, therefore, be excluded. There is no mention of food, but it has never been suggested that food has ever been concerned in the production of malarious fever. It was a very different matter, however, with the water supply. In two of the vessels the water was good, whilst the Argo had been supplied with marsh water, which was offensive to the smell, as well as unpalatable. This latter was supplied to the soldiers, whilst the crew drank uncontaminated water. Amongst those who deny that marsh water is the cause of ague must be quoted Professor Colin. The professor, who is regarded as an authority on intermittent fever, in his work De l’Ingestion des Eaux Marécageuse comme cause de la Dysenterie et des Fièvres intermittentes,’ instances numerous cases in Algiers and Italy in which impure marsh water gives rise to indigestion, diarrhœa, and dysentery, but in no case to intermittent fever; and he states that in all his observations he has never met with an instance of ague having such an origin. Without contesting the case of the Argo, he views it with considerable suspicion, and doubts whether Boudin is correct in his details. Finke also states that, in Hungary and Holland, marsh-water is daily drank without causing any ill-effects. The inhalation of the fumes of oxide of zinc appears to produce in workers of this metal a variety of ague termed by Shackrah “brass ague,” and by Dr Greenhow, “brass-founder’s ague.” The symptoms of the malady are tightness and oppression of the chest; with indefinite nervous sensations, followed by shivering, an indistinct hot stage, and profuse perspiration. These attacks, however, are not periodical.
It is open to doubt whether the malarious poison exists in the form of a gas, for the observations of microscopists go to show the extreme minuteness of the germs of disease, which are probably not more than 1⁄70000th of an inch in size, and it is regarded as probable that the real cause of ague is the entry into the circulation of some low forms of spores of fungi, or of some minute animalcules. Ague is always to be met with in places where fungi grow, and is always associated with what Pettenkofer calls “the ground air”—that is, the air contained in the interstices of the soil, no inconsiderable volume of which is drawn into every house which has a fire on the floor which rests on the earth. That animalcules (?) may exist in the blood is evidenced by the discovery of Dr Lewis, who found hair-like worms in the circulation; and whilst considering this point, we must bear in mind that the44 remedial agents employed to check ague, quinine, arsenic, &c., are drugs capable of destroying animal life, and it is not impossible that they may exercise a beneficial effect in destroying the spores or animalcules to which the disease may be due.
The best means to be employed to combat malarial fevers in any district are thorough and efficient drainage (and it must be remembered that drainage purifies both the ground-air and the ground water) and a supply of wholesome water free from decomposing vegetable matter.
That the adoption of the above means cannot fail to succeed is incontestably proved by the fact, that during the last 200 years, ague in England has diminished to a wonderful extent, in short, as good drainage and a pure water supply have prevailed, there has been a proportionate diminution of paludal poisoning.
During the protectorate of Cromwell great mortality prevailed in London, from the ravages of ague; at that time London was as swampy as the fens of Lincolnshire. See Fever (Intermittent).
Ague-cake. The popular name of a tumour felt under the false ribs on the left side, formed by enlargement and induration of the spleen, following protracted ague; also, sometimes, of indurations of the liver following ague.
Ague-drop. See Drops.
Ague-salt (sŏlt). Disulphate of quinine.
Ague-tree. Sassafras.
Ague-weed. The herb thorough-wort (‘Eupato′′rium perfolia′tum,’ Linn.).
AIG′REMORE (ĕg′r-mor). [Fr.] Pulverised charcoal in the state it is used to make gunpowder.
 Attelettes from Soyer.
Attelettes from Soyer.
AIGUILLETTE (Attelette). [Fr.] In cookery, a term applied to several small dishes, from the articles of which they consist being mounted on silver needles, or skewers, with ornamental handles or tops. (See engr.) They form one of the varieties of the ‘hors-d’œuvres’ of Soyer; and are commonly served on a napkin. The skewers should be about four inches long, and of the thickness of an ordinary packing needle. The person eating what is served on them takes the head of the skewer between the thumb and fingers of the left hand, and picks it off with his fork. Those noticed by Soyer are—
Aiguillettes à l’Éperlan (smelts);
Aiguillettes aux Huitres (oysters);
Aiguillettes de Filets de Sole (soles);
Aiguillettes de Homard (lobsters);
Aiguillettes de Langue de Bœuf (ox-tongue);
Aiguillettes de Ris de Veau (sweetbread of veal);
Aiguillettes de Volaille à la Jolie Fille (fowl);—
all of which are prepared in a nearly similar manner, merely varying the sauces, &c., to suit the article and palate. See Attelettes, Hors-d’œuvres, &c.
AHORNZUCKER (genuine American maple sugar). For coughs, hoarseness, and all affections of the throat and chest caused by cold. The raw maple sugar as imported. (Hager.)
AILANTHUS. The inner bark of the ailanthus glandulosa, a common tree growing in northern China, said by Dr Dudgeon to have proved very successful in dysentery.
The ailanthus glandulosa is also well known throughout the United States. Professor Hétet, of Toulon, tried the effect of the powdered bark, leaves, and various preparations of the bark or drugs, with the result of their administration being attended with purgative effect—and the discharge of worms.
The powdered bark has been given in small cases of tape-worm in the human subject, with marked success. The dose of the powder found sufficient for the expulsion of the tapeworm was from seven or eight to thirty grains.
AIL′MENT. Pain, indisposition; disease. Its use is generally restricted to the non-acute, and milder forms of disease.
AIR. [Eng., Fr.] Syn. Aer, L. (from αηρ Gr.); Luft, Ger.; Atmospheric Air; The Atmosphere. This name was formerly given to any aëriform body; thus, by the old chemists ammoniacal gas was called alkaline air; oxygen,—dephlogisticated, vital, or empyreal air; carbonic anhydride (carbonic acid), fixed air; hydrogen, inflammable air; heavy carbonetted hydrogen, olefiant gas, heavy inflammable air; nitrogen,—mephitic, phlogisticated, or nitrous air. At the present time the term air is usually restricted to the gaseous envelope surrounding the solid and liquid parts of our globe.
Air, Atmospheric (or simply, The Air). The air chiefly consists of a mechanical mixture of four volumes of nitrogen and one volume of oxygen, or more accurately—
| By volume. | By weight. | |
| Nitrogen | 79·1 | 76·8 |
| Oxygen | 20·9 | 23·2 |
| ——— | ——— | |
| 100 | 100[12] |
We may premise our description of the functions of the constituents of the atmosphere by the following quotation from Mr Blyth’s ‘Dictionary of Hygiène and Public Health’:—“One of the most important properties of air is its power of penetration and its universality. Air is, indeed, present everywhere; there is scarcely a solid, however compact it may appear to be, which does not contain pores, and these pores filled with air. The soil contains no small quantity; indeed, if it were not so the numberless insects, worms, &c., which burrow in its interstices would cease to exist. The most compact mortar and walls are penetrated with it, and water of natural origin contains a large quantity of air in solution. The atmosphere is supposed to extend to a very great height, from 200 to 300 miles; it used to be considered only five (forty-five) miles high, but observations on shooting stars, &c., show that this opinion is erroneous. Owing to the force of gravity, the air is much denser near the earth, and gets more attenuated layer by layer as you ascend. If, then, the atmosphere were possessed of colour, it would be very dark just round the globe, and the tint would gradually fade into space. The air is by no means wholly gaseous; it contains, indeed, an immense amount of life, and small particles derived from the whole creation. In the air may be found animalcules, spores, seeds, pollen cells of all kind, vibriones, elements of contagion, eggs of insects, &c., and a few fungi, besides formless dust, sandy, and other particles of local origin; for example, no one can ride in a railway carriage without being accompanied with dust, a great portion of which is attracted by a magnet, and is, indeed, minute particles of iron derived from the rails. The purest air has some dust in it. There probably never fell a beam of light from the sun since the world was made which did not show, were there eyes to see it, myriads of motes; these, however, generally speaking, are quite innocuous to man—some, indeed, may possibly be beneficial. Another most important property of air is its mobility; on the calmest day and in the quietest room there are constant currents of air which rapidly dilute any noxious odours of gases.”
The chief functions of the oxygen are to maintain respiration and support combustion, while the office of the nitrogen is to dilute the oxygen and control its energy.
Besides nitrogen and oxygen, aqueous vapour, carbonic anhydride, ammonia, and nitric acid are met with in the atmosphere, the last especially during and shortly after thunder storms.
Although, doubtless owing to local conditions, trifling variations may occur in the proportion of oxygen present in the atmosphere, this variation is so trifling that the difference of the amount in air from places separated by very long distances will be found in the second decimal place only; thus, whilst a portion of air taken during a balloon ascent by Mr Green gave on analysis 20·88 per cent. by vol., Dr Frankland found in air collected by himself on the summit of Mont Blanc 20·96 per cent. by vol. A still nearer approximation in uniformity in the amount of oxygen present in atmospheric air is exhibited in the following table, which gives the results of 95 analyses by Regnault on air obtained from nine different localities:—
| 100 | from Paris gave in 100 parts, by vol. of oxygen | 20·913 to | 20·999 |
| 9 | from Lyons and around gave in 100 parts, by vol. of oxygen | 20·918 to | 20·966 |
| 30 | from Berlin gave in 100 parts, by vol. of oxygen | 20·908 to | 20·998 |
| 10 | from Madrid gave in 100 parts, by vol. of oxygen | 20·916 to | 20·982 |
| 23 | from Geneva and Switzerland gave in 100 parts, by vol. of oxygen | 20·909 to | 20·993 |
| 15 | from Toulon and Mediterranean gave in 100 parts, by vol. of oxygen | 20·912 to | 20·982 |
| 5 | from Atlantic Ocean gave in 100 parts, by vol. of oxygen | 20·918 to | 20·965 |
| 1 | from Ecuador gave in 100 parts, by vol. of oxygen | 20·960 | |
| 2 | from Pichincha gave in 100 parts, by vol. of oxygen | 20·949 to | 20·981 |
| ——— | ——— | ||
| Mean of all foregoing | 20·949 | 20·988 | |
| Mean of the Paris specimens | 20·96 |
Vapour of water is essential to the respiration of animals and plants, in order that the organs concerned in this operation may be kept in a soft and moist condition.
Carbonic anhydride is evolved during combustion, putrefaction, and fermentation; it is also a product of the respiration of animals, and highly poisonous to them, even when diluted with large proportions of air. This gas is, however, greedily absorbed by plants, which decompose it; they assimilate the carbon and return the oxygen to the atmosphere, ready to be again consumed in supporting the life of the animal world.
Dr Angus Smith has defined a very pure air to be one that contains with 20·99 per cent. of oxygen 0·30 of carbonic acid (anhydride).
This latter varies in amount in the atmosphere of cities, as will be seen upon inspection of the subjoined table, extracted from Dr Smith’s work ‘Air and Rain’:46—
| Per cent. | |
| Air of Madrid, outside the walls, mean of 12 analyses, by Luna | ·045 |
| Mean of 12 analyses, within the walls of Madrid, by Luna | ·051 |
| Mean of 14 analyses, by Angus Smith, in Manchester suburbs | ·369 |
| In Manchester streets | ·403 |
| Usual weather | ·0403 |
| During fogs | ·0679 |
De Saussure’s analyses show that there is more carbonic acid on the mountains than in the plains, as might be inferred from the comparative absence of vegetation in elevated positions. Dr Pietra Santa states that the air of hills or mountains, at the height of 2300 feet, is lighter than common air, contains a smaller proportion of oxygen, and is impregnated with a largely increased amount of aqueous vapour. It also contains a large quantity of ozone. He considers such a climate peculiarly soothing to persons suffering from chest diseases.
Dr Angus Smith’s analysis of the air from the mountainous districts of Scotland confirms the above statement of Dr Pietra Santa’s. The heaths and mountains of that country are remarkably healthy localities, and the air from them gave on analysis 20·94 per cent. by vol. of oxygen, and only ·033 of carbonic acid.
Ammonia is derived from the putrefaction of animal and vegetable substances. It is from atmospheric ammoniacal compounds that plants obtain much of the nitrogen which is essential to the formation of many parts of their structure.
Nitric acid, like ammonia, is absorbed, and its nitrogen assimilated, by plants.
In addition to the gases and vapours already enumerated, as well as others which exist in minute quantity, or which are of only occasional occurrence, Pasteur and other investigators have discovered in the air living germs which are capable of exciting putrefaction and fermentation, and which are competent, in some instances, to engender disease when they are injected into the blood of animals. In fact, the spread of infectious diseases, e.g., smallpox, typhus fever, cattle plague, &c., is attributed to the presence in the atmosphere of the germs of such maladies. These germs are believed to be living beings, which develope and multiply at the expense of the tissues of the larger animals into whose systems they have found entrance.
Air, Vitiated. As has been stated in the previous article, the air consists chiefly of two gases, oxygen and nitrogen. In all open places it has a similar composition, as might be concluded from the constant mingling which takes place by the agency of currents continually in movement, although sometimes to an inconsiderable extent only. Dr Angus Smith regards air as very pure when it contains not less than 20·99 per cent. by volume of oxygen, and 0·030 of carbonic anhydride (acid). According as the proportion of the former gas diminishes and that of the latter increases beyond certain limits in the air by which we are surrounded, it becomes more or less deteriorated and unfit to be breathed, particularly as the increased amount of carbonic acid is, in crowded dwellings, assembly rooms, theatres, and confined inhabited spaces, associated with deleterious and putrescent exhalations from the person.
| Per-centage | |
| by volume. | |
| Chancery Court, closed doors, 7 feet from the ground, March 3 | ·193 |
| Same, 3 feet from ground | ·203 |
| Chancery Court, doors wide open, 4 feet from ground, 11·40, March 5 | ·0507 |
| Same, 12·40 p.m., 5 feet from ground | ·045 |
| Strand Theatre, gallery, 10 p.m. | ·101 |
| Surrey Theatre, boxes, March 7, 10·30 p.m. | ·218 |
| Olympic, 11·30 p.m. | ·0817 |
| Same, 11·55 p.m. | ·1014 |
| Victoria Theatre, boxes, March 24, 10 p.m. | ·126 |
| Haymarket Theatre, dress circle, March 18, 11·30 p.m. | ·0757 |
| Queen’s Ward, St. Thomas’s Hospital, 3·25 p.m. | ·052 |
| Edward’s Ward, St. Thomas’s Hospital, 3·30 p.m. | ·052 |
| Victoria Theatre, boxes, April 4. | ·076 |
| Effingham, 10·30 p.m., April 9, Whitechapel | ·126 |
| Pavilion, 10·11 p.m., April 9, Whitechapel | ·152 |
| City of London Theatre, pit, 11·15 p.m., April 16 | ·252 |
| Standard Theatre, pit, 11 p.m., April 16 | ·320 |
Dr Angus Smith states that out of 339 specimens of air obtained from various mines he found 35 normal or nearly so, 81 decidedly impure, and 212 exceedingly bad; he also adds that owing to the frequent firing of charges of gunpowder within the mines, and from other causes, the atmosphere is further contaminated with sulphuretted hydrogen, sulphate, carbonate, sulphide, sulphocyanide of potassium, and nitrate of potassium, carbon, sulphur, carbonate of ammonia, organic matter, sand, and sulphurous and arsenious acids.
The air of large cities, which are the seats of manufacturing industry, is always more or less charged with the exhalations given off by chemical and other works. The sulphuric-acid works contribute sulphuric, sulphurous, nitrous, and arsenious acids; copper works, in which pyrites is employed, give off large quantities47 of sulphurous acid, mixed with arsenic and a little copper; manure works, in many cases, send out compounds of fluorine, besides sulphuric acid; glass works, sulphuric and hydrochloric acids; and alkali works, hydrochloric acid (although in small quantities), which very frequently contains arsenic. Of ammonia, Angus Smith remarks: “It is one measure of the ‘sewage’ of the air; it is the result of decomposition. It is not, in these small quantities, hurtful, so far as we know. The ammonia is in no case free, but combined probably with hydrosulphuric, hydrochloric, and sulphuric acid in towns. In country places it is, at all events partly, united to carbonic acid.
| Date. | Place. | Time of Day. | Carbonic Acid, per cent. | Oxygen, per cent. | |
| 1869. Nov. | 12. | Tunnel between Gower Street and King’s Cross Stations; specimen taken at the open window, first-class carriage. | 10 a.m. | ·150 | 20·60 |
| ” | 12. | Tunnel between Gower Street and King’s Cross Stations; specimen taken at the open window, first-class carriage. | 7·30 p.m. | ·078 | 20·79 |
| ” | 12. | Tunnel Praed Street; specimen taken at the open window, first-class carriage. | 10·30 a.m. | ... | 20·71 |
| ” | 15. | Specimen taken during journey between Gower Street and King’s Cross, first-class carriage, window open. | 10·15 a.m. | ·338 | 20·66 |
| ” | 15. | Same | 3 p.m. | ·155 | 20·70 |
| ” | 15. | Same | 11 p.m. | ·150 | 20·74 |
| Average | ·1452 | 20·70 |
| Name of Mine, and depth from surface, in fathoms. | Description of place, where taken and time when taken. | Thermometer, Fahr. | Number of Men working in it. | Oxygen, per cent. | Carbonic Acid, per cent. |
| Hurst | End, 300 ft. beyond a rise, 9 ft. high, 7 ft. wide. | ... | 2 | ... | 1·99 |
| Old Gang | End of level | ... | 2 | 20·58 | ·48 |
| ” | End of level | ... | 2 | ... | ·28 |
| ” | (a) Rise 7 ft. high, 132 ft. from current. | ... | 2 | 20·25 | ·39 |
| Grassington | (b) End of cross cut, 480 ft. from rise. | ... | 2 | 20·94 | ·06 |
| ” | End, 480 ft. from rise. | ... | 2 | 19·53 | 1·59 |
| ” | Rise 60 ft. high in shale. | ... | 2 | 19·52 | 1·72 |
| ” | End, 60 ft. from rise. | ... | 2 | 20·47 | 1·06 |
| ” | (c)End, 840 ft. from rise. | ... | 2 | 20·08 | ·94 |
The following table, showing the amount of ammonia present in rain collected at the different places named, is from Dr Smith’s work, ‘Air and Rain.’
| Comparative. | Ammonia. |
| That of Valentia (Ireland) taken as 1 or 100. | |
| Ireland, Valentia | ·1 |
| Scotland, sea-coast, country places, west | 2·69 |
| Scotland, inland, country places, west | 2·96 |
| Scotland, sea-coast, country places, average | 4·10 |
| Scotland, sea-coast, country places, east | 5·51 |
| England, inland, country places, east | 5·94 |
| England, sea-coast, country places, west | 10·55 |
| German specimens | 10·61 |
| London, 1869 | 19·17 |
| Scotland, towns (Glasgow not included) | 21·22 |
| St. Helen’s | 25·33 |
| Runcorn | 25·72 |
| England, towns | 28·67 |
| Liverpool | 29·89 |
| Manchester, 1869 | 35·33 |
| Manchester, 1869 and 1870, average | 35·94 |
| Manchester, 1870 | 36·54 |
| Glasgow | 50·55 |
The effects resulting from breathing an impure atmosphere are necessarily dependent upon the extent of the pollution and other conditions. When the contamination is moderate the first effect is headache, accompanied with lassitude, and a general paleness of the face and skin, owing to a diminution of the red corpuscles of the blood or to their imperfect aëration; the pulse becomes lowered, and at the same time the breathing is accelerated. When in addition to breathing such air from day to day is superadded the misfortune of an insufficiency of food, scrofula and consumption very often follow. Dr Guy has demonstrated the great mortality that is caused by consumption in those trades in which workmen pursue their calling in hot, close, gas-lit rooms, in comparison with those who pass most of their time in the open air. The amount of air required by each person in a room is no less than 2100 feet per hour; when the ventilation does not supply this amount of fresh air, the apartment smells stuffy, the furniture becomes coated with a film of organic matter, unless constantly cleaned, and the carbonic acid becomes increased beyond its normal quantity.
Dr Parkes has shown that bronchitis and consumption are more frequently than not contracted by those who live in an atmosphere of foul air. In the years 1834 to 1847 the proportion of deaths in the ill-ventilated prison of Leopoldstadt in Vienna was 86 per 1000, out of which number 51·4 per 1000 was due to phthisis or consumption; while in the well-ventilated House of Correction in the same city the deaths were 14 per 1000, of which 7·9 were from phthisis; hence 43·5 cases per 1000 of the deaths were clearly traceable to foul air and nothing else.
Mr Noel Hartley, in his valuable little manual, ‘Water, Air, and Disinfectants,’ says: “During the outbreak of cattle plague in 1866, in sheds containing twenty to thirty cows—which the owners kept closed to such an extent that all chinks in the doors and windows were stuffed with straw and matting, under an ignorant belief that thus the plague could be kept out—very frequently the entire stock died in two or three days after the first appearance of disease; while in other cases where animals were housed in a well-cleaned and tidily-kept shed, with a plentiful supply of fresh air, not only did some of them escape the disease altogether, but the deaths were reduced to one third of the number of beasts attacked.”
The large supply of fresh air necessary in hospitals for contagious diseases is fully recognised by medical men, and more especially so in America. Wounds carefully protected from contact with impure air do not suppurate, and organic fluids do not putrefy. On the other hand, in a bad atmosphere sores become unhealthy, and are difficult to heal, erysipelas and hospital gangrene frequently set in, while the best prevention and the best means of cure for such afflictions is the greatest possible exposure to fresh air.
Vitiated air, as a consequence of over-crowding, aids the spread of measles, scarlet fever, and the much to be dreaded smallpox; it brings on ophthalmia, a troublesome inflammation of the eyes, and is not unfrequently the cause of the ricketty and scrofulous condition of children. Although exposure to cold does cause such affections as bronchitis, pneumonia, cold in the head, sore throat, and other affections of the respiratory organs, it is more frequently the case that they are the result of a sudden change of temperature, such as experienced in coming out of a crowded assembly in a close, badly-ventilated building, than by actually cold weather. This is decidedly and strikingly shown by the fact which Dr de Chaumont has quoted, that the British Army when in the Crimea, when lodged in tents during extremely rigorous weather, experienced a wonderful condition of health, such a thing as a cold being an unknown complaint; but when some of the men were placed in huts which were much warmer, and into which there was a smaller circulation of fresh air, the sick rate increased, and coughs and colds began to put in an appearance. Persons who during summer and winter sleep with their windows more or less open cannot endure a night spent in the chamber with the chimney closed and the window shut. A less refreshing sleep occupies the night, and a somewhat feverish sensation is felt next morning.
If in cold weather the window be opened only one inch at the top, the difference in the air in the bedroom is something quite beyond comprehension to those who have not paid attention to these things. See Ventilation.
Air, Analysis of. Priestley’s discovery of oxygen gas in 1774 prepared the way for the knowledge of the real composition of air, which was discovered about the same time by Scheele and Lavoisier. Scheele’s method of operating was by exposing some atmospheric air to a solution of sulphide of potassium. Lavoisier effected the same object by the combustion of iron wire and phosphorus, and subsequently by heating mercury on a flask filled with air for some time, just below its boiling point.
These, however, were but elementary methods, which, however creditable to the ingenuity of the great founders of modern chemistry, not only failed in accuracy, but49 took no account of the presence and amount of two most important constituents in the atmosphere, viz. carbonic anhydride (acid) and ammonia.
Determination of Aqueous Vapour. To effect this an aspirator must be used (see Aspirator). This instrument is easily made, and is not expensive. The accompanying figure will illustrate the arrangement generally adopted: a is an aspirator made of galvanised iron or sheet zinc. It holds from 50 to 200 litres (from 11 to 44 gallons). By this means a known volume of air is drawn through the tubes marked b, c, d, e, which may be filled with pumice-stone moistened with strong sulphuric acid; but if the carbonic acid is to be estimated as well, b and c are filled with moist hydrate of lime (potash used to be employed, but hydrate of lime is to be preferred, as the potash absorbs oxygen), and d and e as above. Each of the tubes is accurately weighed previously to connecting them with the apparatus.
It is imperative to have each of the tubes connected by perfectly air-tight joints. The gain of weight in d and e gives the water in b and c the carbonic acid.
Determination of Carbonic Acid. A better and perhaps more exact means of determining the carbonic acid is that invented by Pettenkofer. It may be briefly described as follows:—Baryta water of definite strength is prepared and accurately standardised by a standard solution of oxalic acid. A portion of this baryta water is then made to act upon a definite quantity of air. It will absorb the whole of the carbonic acid in that air.
The alkalinity of the liquid will in consequence be diminished; it will take less of the oxalic-acid solution than before, which shows so much less caustic baryta, and from which the carbonic acid absorbed may be easily calculated.
The actual Analysis. Two kinds of baryta water may be used, the one containing 7 grammes to the litre, the other three times that strength; 1 c. c. of the stronger = 3 m. grms. of carbonic acid; 1 c. c. of the weaker = 1 m. grm. The baryta water is best kept in the bottle represented below.
The bottle (a) contains the baryta water. It has an accurately-fitting double-perforated stoppered caoutchouc. The left-hand tube is connected with the tube (b) containing pumice-stone moistened with potash, while the right-hand one is a syphon. When required for use the stop-cock (f) is opened, and suction applied by a glass tube to F. The syphon is thus filled and the stop-cock closed. If a pipette is required to be filled its nozzle is inserted at F, the stop-cock compressed, and the fluid immediately rises into the pipette.
The air entering the bottle as the fluid decreases in a is, of course, thoroughly deprived of its carbonic acid by the tubes at b.
The first thing to be done is to standardise the baryta solution by a solution of oxalic acid, containing 2·8636 grammes of crystallised oxalic acid to the litre.
Thirty c. c. of baryta solution are run into a small flask, and the oxalic acid run in from a Mohr’s burette with float, the vanishing-point of the alkaline reaction being ascertained by delicate turmeric paper. As soon as a drop placed on turmeric paper does not give a brown ring the end is attained.
The actual analysis is performed by filling a bottle of known capacity, with the aid of a pair of bellows, with the air to be analysed, then distributing over its sides 45 c. c. of the baryta water it is left for half an hour. The turbid water is poured into a cylinder, closely secured, and allowed to deposit; then take out 30 c. c. by a pipette of the clear fluid, run in the solution of oxalic acid, multiply the volume used by 1·5, and deduct the produce from the c. c. of oxalic acid used for 45 c. c. of the fresh baryta water. A different method has been suggested by Dr Angus Smith, viz. to measure the carbonic anhydride by the turbidities of the baryta water; this is, in fact, a colorimetric test. For rough approximative results Dr Smith’s process will be found a very useful and convenient one. It depends upon the fact that the amount of carbonic acid in a given quantity of air will not produce a precipitate in a given quantity of lime or baryta water unless the carbonic acid is in excess. The following is one of his tables:—Columns 1 and 250 give the rates of carbonic acid in the quantity of air which will produce no precipitate in half an ounce of lime water. Column 3 is the same as column 2; but 14·16 c. c. (half an ounce) is added to give the corresponding size of the bottle, and column 4 gives the size of the bottle in ounces.
| Carbonic Acid in the Air, per cent. | Volume of Air in cubic centimètres. | Size of bottle in cubic centimètres. | Size of bottle in ounces Avoirdupois. |
| ·03 | 185 | 199 | 7·06 |
| ·04 | 139 | 154 | 5·42 |
| ·05 | 111 | 125 | 4·44 |
| ·06 | 93 | 107 | 3·78 |
| ·07 | 79 | 93 | 3·31 |
| ·08 | 70 | 84 | 2·96 |
| ·09 | 62 | 76 | 2·69 |
| ·10 | 56 | 70 | 2·46 |
| ·11 | 51 | 65 | 2·29 |
| ·12 | 46 | 60 | 2·14 |
| ·13 | 43 | 57 | 2·01 |
| ·14 | 40 | 54 | 1·90 |
| ·15 | 37 | 51 | 1·81 |
| ·20 | 28 | 42 | 1·48 |
| ·25 | 22 | 36 | 1·29 |
| ·30 | 19 | 33 | 1·16 |
| ·40 | 14 | 28 | 1·04 |
| ·50 | 11 | 25 | ·89 |
| ·60 | 9 | 23 | ·89 |
| ·70 | 8 | 22 | ·78 |
| ·80 | 6 | 20 | ·72 |
| 1·00 | 5·5 | 19·7 | ·70 |
Mr Wanklyn’s process for the determination of carbonic acid in the atmosphere is as follows:—A solution of carbonate of soda is first made as follows: 4·47 grammes of gently-ignited carbonate of soda are dissolved in one litre of water, giving a solution of such a strength that 1 c. c. contains exactly 1 c. c. of carbonic acid (= 1·97 milligrammes of CO2); a large quantity of baryta water (strength about 0·1 per cent.) is prepared.
If now 100 c. c. of clear baryta water be treated with 1 c. c. of carbonate of soda, just described, a certain degree of turbidity is produced.
If 2 c. c. of the solution be taken another degree of turbidity is produced, and so on. If, then, a bottle capable of holding 2000 c. c. of air, together with 100 c. c. of baryta water, be filled with the sample of air to be tested, there will be a certain depth of turbidity produced by shaking it up. Having got the air to expend itself on 100 c. c. of baryta water the degree is to be found by comparison with another 100 c. c. of baryta water, in which a like turbidity has been induced by means of the standard solution of carbonate.
Every c. c. of soda solution counts for a c. c. of carbonic acid in two litres of air. A consumption of 1 c. c. will correspond to ·05 volumes of carbonic acid per cent. Good air should accordingly not take more than 1 c. c. of soda solution, air which takes already 2 c. c. being already bad.
In order practically to carry out this method of estimating carbonic acid the following apparatus is required:—Several bottles capable of holding 2·210 c. c., and well stoppered (failing bottles of exactly the right capacity Winchester quart bottles will answer); a small pair of bellows; several colourless glass cylinders marked at 100 c. c. capacity—the Nesslerising cylinders will answer for this purpose—a graduated pipette or burette to deliver tenths of a c. c. of solution, the standard solution of carbonate of soda, and the baryta water, which may be of moderate strength.
The testing is managed thus: Winchester quart bottles having been made clean are rinsed with distilled water, and allowed to drain a little. They are then closed with their stoppers, and are ready for use. The operator having provided himself with two or three of these bottles and a small pair of bellows enters the room the air of which is to be tested. The stopper is then removed from one of the bottles, and some air of the room blown through with the bellows, and then the stopper is replaced, and the bottle carried away to be tested.
The testing is done by pouring into the bottle 100 c. c. of clear baryta water, shaking up for two or three minutes, and then pouring out into a cylinder of colourless glass, and observing the depth of the turbidity in various lights and against various backgrounds. The turbidity is to be exactly imitated by means of the standard solution of carbonate of soda. In order to imitate the turbidity produced by a Winchester quart full of good air only 1 c. c. of this solution of carbonate of soda is required.
If 2 c. c. or more than 2 are required, the air is bad and the ventilation is defective.
In place of the first c. c. of solution of carbonate of soda the carbonic acid naturally present in a Winchester quart of good average air may be used, and a little practice and intelligence will suggest the necessary precautions.
Estimation of the Oxygen.—To determine this Angus Smith has recourse to the endiometer. Five or six of Bunsen’s endiometers were used at once and the mixed gases were exploded by means of a powerful battery and a Ruhumkorff’s coil. In his ‘Inorganic Chemistry,’ Miller thus explains the principle upon which the action of the endiometer is based: “By means of the endiometer various gaseous mixtures may be analysed with great exactness. Many different forms of this instrument are in use. One of the most convenient is Hoffmann’s. It consists of a stout syphon tube. (See next figure.) Into the sides of the51 tube, near the sealed end, two platinum wires (a, b) are fixed for the purpose of transmitting an electric spark through the cavity of the tube. The sealed limb is accurately graduated to tenths of a c. c. or other suitable divisions. Suppose it be desired to ascertain the proportion of oxygen in atmospheric air. The instrument is first filled with mercury, after which a small quantity of air is introduced; the bulk of the air is accurately measured, taking care that the liquid metal stands at the same level in both tubes, which is easily effected by adding mercury, or by drawing off the mercury if needed, through the caoutchouc tube, which is fixed upon the small inlet tube just above the bend, and which is closed by means of a screw tap (c).
A quantity of pure hydrogen, about equal in bulk to the air, is next introduced, and the bulk of the mixture is then accurately measured. The open extremity of the tube is now closed with a cork, below which a column of atmospheric air is safely included. This portion of air acts as a spring, which gradually checks the explosive force, when the combination is effected by passing a spark across the tube by means of the platinum wires. The mixture is then exploded by the electric spark. The remaining gas now occupies a smaller volume, owing to the condensation of the steam which has been formed. Mercury is, therefore, again poured in the open limb until it stands at the same level in both tubes, and the volume of the gas is measured a third time. One third of the reduction of the bulk experienced by the gas will represent the entire volume of oxygen which the mixture contained. Liebig’s method is as follows. It is based upon the fact that an alkaline solution of pyrogallic acid absorbs oxygen:
1. A strong measuring tube holding 30 c. c., and divided into one fifth or one tenth c. c., is filled to two thirds with the air intended for analysis. The remaining part of the tube is filled with mercury, and the tube is inverted over that fluid in a tall cylinder widened at the top.
2. The volume of air confined is measured—a quantity of solution of potash of 1·4 sp. grf. (1 part of dry hydrate of potash to 2 parts of water), amounting from 1⁄40th to 1⁄50th of the volume of the air, is then introduced into the measuring tube by means of a pipette with the point bent upwards (see drawing), and spread over the entire inner surface of the tube by shaking the latter. When no further diminution of volume takes place the decrease is read off. The carbonic acid is thus removed.
3. A solution of pyrogallic acid containing 1 gramme of the acid in 5 or 6 c. c. of water is introduced into the same measuring tube by means of another pipette similar to the above. The mixed fluid (the pyrogallic acid and the solution of potash) is spread over the inner surface of the tube by shaking the latter, and when no further diminution of volume is observed the residuary nitrogen is measured.
4. The solution of pyrogallic acid mixing with the solution of potash of course dilutes it, causing thus an error from the diminution of its tension; but this error is so trifling that it has no appreciable influence upon the results. It may, moreover, be readily corrected by introducing into the tube, after the absorption of the oxygen, a small piece of hydrate of potash, corresponding to the amount of water in the solution of the pyrogallic acid.
There is another slight error on account of a portion of the fluid adhering to the inner surface of the tube, so that the volume of the gas is never read off with absolute accuracy.
In conducting these endiometric experiments the necessary corrections for temperature and barometric pressure must, of course, be made.
Estimation of the Nitrogen. The amount of this gas is usually determined by deducting the aqueous vapours, oxygen and carbonic acid, from the volume of air examined.
Determination of Ammonia and Organic Matter. These are best determined by drawing a known volume of air through absolutely pure water. To obtain this latter it is best to redistil distilled water, to reject the first portions, then to add an alkaline solution of permanganate of potash, and to discard any portions of the distillate which give the52 slightest reaction with the Nessler test. The water through which the air is drawn must be kept cool, and afterwards submitted to the proper tests, which will be found under Ammonia and Water Analysis. Mr Blyth says, “Solid bodies such as vibrionic germs, dust, fungi, &c., may be obtained by using an aspirator, and drawing the air either through a drop of glycerine or water. Organic matter may also be obtained by suspending glass vessels filled with ice water, over or in the places to be investigated, and submitted to the microscope. High powers, such as immersion lenses, are requisite for the investigation of germs,” &c.
Of these germs Dr Angus Smith says:—“They may probably be divided into many kinds—the useful and the deleterious, those which promote health and those which bring disease. The idea of any of them bringing health is not founded on anything positive, but we can scarcely imagine these numberless forms to be all useless. The idea that they bring disease is, I think, one well confirmed.” See a paper by the same author “On the Air and Rain of Manchester.” ‘Memoirs of the Literary and Scientific Society of Manchester,’ vol. x. See Air, Vitiated.
AIR-GAS. Air deprived of its carbonic acid and moisture, and then impregnated with the vapours of very volatile fluid hydrocarbons, such as benzine and benzoline, can be used as an illuminating agent. It is requisite, however, to use burners with wide openings, and to apply a low pressure, because if the current be too rapid the flame becomes too much cooled, and is readily extinguished. Apparatus for preparing air-gas have been devised and constructed by Marcus, Mille, Methei, and others.

AIR-PUMP. An instrument designed for the removal of air from closed vessels. The simplest form of air-pump is the exhausting syringe, which consists of a cylinder fitted with a stop-cock, and having a valve at the bottom opening inwards. Another valve opening outwards is attached to a piston working inside the cylinder, and by screwing the instrument on to a vessel, and alternately elevating and depressing the piston, all except a very small quantity of residual and comparatively inelastic air is pumped out of the vessel (Figs. a and b). The accompanying figures show relative positions of the valve during (a) the elevation, and (b) the depression of the piston. In the usual and more convenient form of air-pump, a brass tube passes from the bottom of the syringe and terminates in the centre of a disk of brass or glass ground accurately; the vessel from which the air is to be exhausted has its edge very accurately ground, and is mounted upon the plate as shown in the subjoined figure.
Air-pump, Bunsen’s Water. (See figure on page 53.)
This consists of a wide glass tube, a, into which another tube, b, b′, b′′, passes air-tight. c is an india-rubber tube connecting a with the water supply, d is a clamp to stop the flow of water through c. e is another clamp to regulate the flow, f is a reservoir to prevent any water which may accidentally come over from getting into j. g is a plug to let out any water from f. h is a screw for connecting a air-tight to a piece of tubing, which should pass 32 feet, if possible, below the level of a. i is a piece of strong india-rubber tubing to connect the pump with the vessel to be exhausted. The water rushes in at c and down h, carrying bubbles of air with it till the exhaustion is complete. The figure illustrates a common application of this pump to the rapid filtration of liquids which ordinarily pass through paper with difficulty. a is represented as being about half full of water. k is a funnel fixed air-tight in the india-rubber stopper of the bell-jar j. l is a small cone of platinum foil to prevent the paper filter which fits into it from being broken. m is a plate of ground glass, n is a beaker to receive the filtrate.53
 Bunsen’s water-air-pump.
Bunsen’s water-air-pump.
Air-pump, Sprengel’s. This apparatus depends on the principle of converting the space to be exhausted into a torricellian vacuum.
In the subjoined figure, c, d is a glass tube longer than a barometer, open at both ends, and connected by means of india-rubber tubing with a funnel, A, filled with mercury and supported by a stand. Mercury is allowed to fall in this tube at a rate regulated by a clamp at C; the lower end of the tube, c, d, fits in the flask B, which has a spout at the side a little higher than the lower end of c, d; the upper part has a branch at x to which a receiver R can be tightly fixed. When the clamp at C is opened, the first portions of mercury which run out close the tube and prevent air from entering below. As the mercury is allowed to run down the exhaustion begins, and the whole length of the tube from x to d is fitted with cylinders of air and mercury, having a downward motion. Air and mercury escape through the spout of the bulb B, which is above the basin H, where the mercury is collected. It is poured back from time to time into the funnel A, to be repassed through the tube until the exhaustion is complete.
 Sprengel’s air-pump.
Sprengel’s air-pump.
AIRY’S (Dr.) NATURE’S MEDICAL TREATMENT is the title of a pamphlet which recommends four secret remedies against 166 diseases:
a. The Pain Expeller, a mixture of about 35 parts of tincture of capsicum, 20 parts of diluted spirit, and 20 parts of spirit of ammonia.
b. Sarsaparillian, a fluid extract of sarsaparilla and China root, containing 1 per cent. of iodide of potassium.
c. Pills composed of powdered iron, jalap resin, jalap powder, and marsh mallow powder, made into a mass with some bitter extract. Each pill weighs 0·1 gramme.
d. Calming Pastilles are thick, hard tablets, composed of sugar, with oil of anise, and coloured with liquorice juice. (Hager.)
AKUSTICON (an ear essence). A proved remedy for every kind of ear disease, by Pserhofer. This may be imitated by dissolving in common glycerine one fifth of its weight of fir tar, filtering, and adding a few drops of cajeput oil dissolved in spirit (Hager.)
AL-. [Ar.] An inseparable article equivalent to the English the. It is found in many chemical and other words derived from the Arabic; as alchemy, alcohol, alembic, almanac, &c.
AL′ABASTER. Syn. Albâtre, Fr.; Alabas′ter, Alabastri′tes, Alabas′trum, L. A54 soft, white species of calcareous and of gypseous stone, used by sculptors. There are several varieties, all of which may be ranged under two heads:—
1. Calca′′reous alabaster; Orient′al, a.; Calc-sin′ter. A sub-variety of carbonate of calcium, formed by the deposition of calcareous particles in the caverns of limestone rocks. It has a foliated, fibrous, or granular structure, and a pure, soft, rich, semi-translucent whiteness, generally agreeably variegated with undulating zones or stripes of various shades of yellow, red, or brown. This variety is that most esteemed by sculptors, and for the manufacture of alabaster ornaments. The ancients used it for ointment and perfume boxes. At the baths of San Filippo (Tuscany), the process of its formation may be examined by the observer. The natural spring of boiling water holds carbonate of lime in solution by means of sulphuretted hydrogen, which, escaping into the air, leaves the lime as a precipitate, which is gradually deposited in a concrete form. (M. Alex. Brogniart.)
2. Gyp′seous or common alabaster; Gypsum. A natural hydrated sulphate of calcium, containing a little carbonate of calcium. That from the quarries of the Paris basin contains about 12% of the latter substance. When calcined or roasted, and powdered, it forms the substance known under the name of Plaster of Paris. The more compact, fine-grained specimens of this variety are, like the preceding one, sculptured into almost numberless articles of ornament and utility, such as vases, clock-stands, statuettes, &c. The inferior kinds only are manufactured into the ‘plaster of Paris’ of the shops. The best specimens are obtained from the lower beds of the gypsum quarries, and are white, and granular, not unlike Carrara marble. It takes a high polish; but from its softness and liability to become discoloured, articles formed of it require more careful treatment than even those of ‘calcareous alabaster.’
Alabaster is wrought, turned, and fashioned, in a nearly similar manner to the softer varieties of marble. The tools resemble those employed for the like operations in ivory and brass. Machinery is now often applied to this purpose.
Alabaster is polished, first with pumice-stone, and then with a paste or pap made of whiting, soap, and milk or water; and lastly, with dry flannel. A better method, however, is to rub it first with dried shave-grass (equisetum), and afterwards with finely powdered and sifted slaked lime formed into a paste with water. The surface is then ‘finished off’ by friction with finely powdered talc or French chalk, until a satiny lustre is produced, or with putty powder, in a similar way to marble.
Alabaster is engraved with tools resembling those employed for other soft minerals. It is etched by covering every part of the surface, except that to be acted on, with a solution of white wax in oil of turpentine (1 to 4), thickened with a little finely powdered white lead, and subsequent immersion in water acidulated with acetic acid or hydrochloric acid, for the calcareous variety; and in spring water, for 20 to 50 hours (according to the effect desired), for the gypseous variety. The varnish is washed off with oil of turpentine, and the etched parts carefully brushed over with finely powdered gypsum.
Alabaster is joined and repaired by means of white of egg, or rice glue, thickened with finely powdered quicklime; or by a paste of newly baked and finely powdered gypsum, mixed up with the least possible quantity of water.
Calcareous alabaster is usually cleaned with a brush and warm soap-and-water, or with tepid water to which a few grains of carbonate of soda or of ammonia have been added; followed in either case by rinsing in clean water. If much discoloured, thoroughly cover the article with a paste of freshly slaked lime and water, and let it remain twenty-four hours; then wash off the paste with soap and water, rubbing hard the stains.
Delicate objects in gypseous alabaster can only be safely cleaned with benzol, or with pure oil of turpentine. If necessary, the surface must be repolished. Grease spots may be removed from either variety with a little benzol or oil of turpentine.
Alabaster is occasionally stained or coloured, and, for the calcareous variety, in a similar way to marble, except that heat is not employed; and for the gypseous variety, in the manner noticed under Plaster OF Paris. The gypseous variety is also bronzed and hardened in a similar way to that adopted for casts in the latter substance.
Obs. Gypseous alabaster is dissolved by water; and the beauty of both varieties is almost irrecoverably destroyed by grease, coloured oils, varnishes, smoke, &c. It is, therefore, unfitted for garden ornaments, or other objects exposed to the rain or weather, unless it be painted or bronzed; and is even then very perishable. Contact with acids, alkalies, and ammoniacal and sulphurous fumes, also injure, and, if prolonged, destroy it. Even an uncorked phial of smelling-salts placed on a mantel-piece beside an alabaster vase will soon destroy its beauty. Thus, all delicate objects in alabaster should be protected by a glass shade.
Alabaster, Orient′al (Factitious). Figures, basso relievos, &c., of considerable hardness and beauty, may be formed by imitating the process adopted at the baths of San Filippo, before referred to.
Proc., &c. Moulds of sulphur are placed either vertically or obliquely in an open tub or cistern, having a freely perforated bottom. Surmounting the whole are two or more pieces of wood in the form of a cross or star. The sulphurous calcareous water, falling on this55 cross, is scattered into spray or streamlets, and losing the gaseous portion which holds the lime in solution, deposits it in the form of oriental alabaster on the surface of the moulds. In from 1 to 4 months, according to the nature of the article, a sufficiently thick deposit is obtained. The object is then removed from the mould, and trimmed and polished. It is found that the more vertical the position of the mould, the finer is the grain of the resulting deposit. The water of the Spring of San Filippo may be exactly and easily imitated by the chemist; and the whole process offers a new and valuable ornamental art for the amusement and profit of the ingenious and enterprising.
Alabaster, Shand’s Chinese. Carbonate of lime. (Chandler.)
Alabaster Tablets, John Swine’s Chinese. Carbonate of lime. (Chandler.)
ALAMODE′ (ăl-ăh-mōdé). [Fr., à la mode.] According to the prevailing mode or fashion. In cookery, applied to several dishes, but more particularly to one of beef (alamode beef), commonly shortened by the lower class of Londoners into “alamode.” See Beef, Stewing, &c.
ALAN′TINE. [Eng., Fr., Ger.] Syn. Alanti′na, L. A substance identical with inulin, found in the roots of garden angelica (‘angelica archangelica,’ Linn.).
ALBA′TA. [L., Eng.] A name given to several alloys resembling silver. See Alloys, German Silver, &c.
ALBION (Parisian). “Will preserve the skin white and free from wrinkles.” An aromatic water with chloride of lead and calomel suspended in it. (Landerer.)
ALBOLITH. A cement powder prepared by W. Riemann, Breslau. Made with calcined magnesia (obtained from magnesite) and chloride of magnesium. It is recommended for painting walls, stairs, and wooden articles. (Hager.)
ALBU′MEN. [Eng., L.] Syn. Albumin; Albumine, Fr.; Eiweiss, Eiweistoff, Ger. Literally, the white of egg; a peculiar nitrogenous substance which enters largely into the composition of animal bodies. It abounds in the blood, muscles, bones, coagulable lymph, vitreous and crystalline humour of the eye, fluid of dropsy, &c. The white of egg consists of nearly pure albumen dissolved in water.
A substance identical with albumen is found in many vegetables. It enters largely into the composition of all the emulsive seeds. According to Seguin, it exists in considerable quantity in all those vegetables and fruits that afford a vinous liquor without the addition of yeast.
Prep. The white of egg and the serum of blood, when strained through muslin, furnish albumen, in solution, in a sufficiently pure state for all the ordinary purposes of the arts. Pure solid albumen may be prepared as follows:—
1. Agitate strained white of egg with 10 or 12 times its bulk of alcohol, collect the precipitated flocculi on a muslin filter, and suffer it to dry at a temperature not exceeding 120° Fahr.
2. Add a little water to white of egg, mix, filter, exactly neutralise with acetic acid, and then largely dilute with pure cold water; the precipitate which falls may be collected on a filter and washed. Strained serum of blood may be used instead of white of egg, in both the above forms.
Comp., &c. The following is the composition of albumen according to Lieberkühn:—
| Carbon | 53·3 |
| Hydrogen | 7·1 |
| Nitrogen | 15·7 |
| Oxygen | 22·1 |
| Sulphur | 1·8 |
| —— | |
| 100·0 |
Chatin found iodine in the white of egg; it also contains chloride, sulphate, phosphate, and carbonate of sodium, phosphate of calcium, and traces of potassium in it; but, unlike the sulphur, none of these substances form a constituent part of pure albumen, though probably always present in white of egg.
Prop. Pure solid albumen (unaltered by heat) is nearly colourless, inodorous, and tasteless; scarcely soluble in water, but readily so in water, containing an exceedingly small quantity of caustic soda or potash, and in a strong solution of nitrate of potassium. When dried by a gentle heat it shrinks into a translucent horny mass; and when exposed to a sufficient temperature, yields the usual ammoniacal odour and products of animal matter. Its solution (as white of egg) is solidified or coagulated by a heat of from 145° to 165° Fahr., forming a white, opaque mass; when very dilute, on boiling (only) it separates in fine light flocks. When thus coagulated, it is insoluble in water at a less temperature than 302° Fahr. (Wöhler and Vögel), unless alkalised. Ordinary solutions of albumen give precipitates with sulphuric, hydrochloric, nitric, and metaphosphoric acids, with tannin and astringent solutions, and with most of the metallic salts; but are not affected by either acetic acid or tribasic (common) phosphoric acid. Alcohol, in quantity, also precipitates albumen. Strong oil of vitriol turns it black in the cold, but on applying a gentle heat, a gorgeous, red-coloured liquid is produced. Strong hydrochloric acid gives a deep violet-blue solution. White of egg or serum exposed in a thin stratum to the air, dries up into a pale, yellow, gum-like substance, and in this state may be kept for any length of time, retaining its property of redissolving when immersed in slightly warm water.
Tests.—1. Both heat and alcohol (or strong spirit) coagulate it:—2. A solution of perchloride56 of mercury dropped into a fluid containing albumen occasions a white precipitate:—3. Subacetate of lead acts in the same way. Either of the last two will render turbid a solution containing only the 1-2000th part of fresh white of egg, or the 1-10,000th part of dry albumen:—4. Tannin and tincture of galls give yellow, pitchy precipitates:—5. If dry caustic potash or soda be triturated with either liquid or solid albumen, ammoniacal fumes are evolved, and the mixture on calcination yields ferrocyanide of potassium:—6. Its coagulability by heat, and its incoagulability by acetic acid, distinguish it from casein.
Uses, &c., Independently of its value as an alimentary substance, albumen is largely employed in photography as a glaze or varnish, for fixing colours in calico printing, as a cement, &c., and more particularly as a clarifier for wines, syrups, vegetable solutions, and other liquids. Its efficacy for the last purpose depends on its entangling the impurities in its meshes during coagulation, and either rising to the surface with them as a ‘scum,’ or sinking with them as a precipitate. In France it is prepared on an extensive scale, at the abattoirs, by being spread in thin layers to dry; the source of supply being of course the stream of the blood of the slaughtered animals. When the liquid operated on does not spontaneously coagulate albumen, it is necessary to apply heat to it. In cases of poisoning by the mineral acids, corrosive sublimate, nitrate of silver, sulphate of copper, bichloride of tin, or sugar of lead, the white of egg (or indeed the yolk as well) is one of the best antidotes that can be administered.
Albumen, Flake. Syn. Albumen in powder, Solid a., Soluble a., Planter’s a. Prep. Expose strained white of egg or serum of bullock’s blood, in a thin stratum, to a current of dry air, until it concretes into a solid transparent substance, resembling horn. In this state it may be kept any length of time, or it may be further dried until brittle, and then reduced to coarse powder.
Use. It is extensively employed as a ‘clarifier’ in the sugar plantations of the West Indies, and elsewhere. It is prepared for use by soaking and stirring it with cold water until it is dissolved, when it is whisked to a froth in the usual way, and agitated with the liquid to be clarified.
Albumen, Iodised. 1. To the white of every egg employed add 71⁄2 grains of iodide of potassium dissolved in an equal weight of distilled water. Beat the mixture to a froth, let it stand until insoluble matters have settled, pour the clear portion into a wide-mouthed bottle, and keep in a cool place. 2. Dissolve 50 grains of iodide of potassium and 10 grains of bromide of ammonium in 21⁄2 oz. of distilled water, and add 120 minims of strong liquor ammoniæ. Add this solution to 10 oz. of albumen, let the mixture stand to settle, and filter. This preparation is said to keep good for a long time.
Albumen, Solution of (B. P.). Take of white of one egg; distilled water, four fluid ounces. Mix by trituration in a mortar, and filter through clean tow, first moistened with distilled water. This solution must be recently prepared.
Albumen, Vegetable. This substance, long considered to be a distinct proximate principle peculiar to the vegetable kingdom, has been shown, by recent researches, to be identical with animal albumen. It is particularly abundant in carrots, turnips, cabbages, green stems of peas, and oleaginous seeds.
ALBU′MEN. In botany, the solid, fleshy, or horny substance found in many seeds, between the integuments and the embryo. It is the part that furnishes the flour of the ‘cereals,’ the flesh of the ‘cocoa-nut,’ and the great mass of the seeds of coffee and other vegetables. However poisonous the plants which produce it may be, this substance is never deleterious.
ALBUMENISED PAPER. A French paper highly glazed, having a fine surface, and made by Rive; a German paper having a more uniform texture, and made by Saxe; also a paper by Towgood, are recommended for the preparation of albumenised paper. Positive paper may be albumenised as follows:—Add 15 grains of finely pulverised common salt to the white of every egg used, and whisk until the mixture is entirely converted into a white froth. Allow this froth to stand in a glazed earthenware pan which must be rather larger than the sheets of paper to be albumenised, for about twelve hours. At the end of this pour the clear portion of the liquid into a flat porcelain tray. Mark the inferior side of the paper, slightly damp it, lift it by its ends, and float it carefully on the prepared albumen, keeping its inferior and dry side uppermost. Then raise the paper at each end, and if any air bubbles are seen remove them with a card or brush and replace the paper in the bath. Remove the paper from the bath and suspend it at the corners by clips. Albumenised paper should be kept dry by enclosing it in tin or zinc cases.
ALBUMENOIDS. A term applied to albumen, fibrin, casein, and similar bodies.
ALBU′MENOUS. Syn. Albumino′sus, L.; Albuminé, Abumineux, Fr.; Eiweisstoffhaltig, Ger. Formed of, containing, or having the properties of albumen.
Albuminous Plants. In botany, all plants whose seeds contain albumen in a separate state; as in the cereals, palms, &c.
Albuminous Principles or Substances. Albumen, casein, fibrin, gluten, &c.
ALBURN′UM. [L.] Syn. Alburn*; Sapwood. In botany, the white and softer parts of the wood of exogenous plants, lying between the inner bark and the heartwood. It consists of empty or nearly empty tubes or57 cells, which gradually acquire solidity by the deposition of resins, tannin, and other products of vegetation, and in time becomes wood. It is through the alburnum that the ascending sap chiefly flows.
ALCARAZ′ZA. [Sp.] A species of porous earthenware, or a vessel formed of it, made in Spain from a light, sandy marl, and but slightly fired. Their value as ‘coolers’ arises from the copious evaporation of the water, which gradually transudes. A similar ware and articles are made in France, under the name of HYGROCERA′MEN; and in England, under the names of POROUS WARE, WATER COOLERS, WINE COOLERS, BUTTER COOLERS, &c. The following are forms said to be used in our potteries:—
Prep. 1. Take of sandy marl, 2 parts; brine, q. s.; make a dough, and then knead in of common salt, in fine powder, 1 part. Bake the pieces slowly, and lightly.
2. Good clay, 2 parts; fine siliceous sand, 3 parts; brine, q. s.; common salt, 1 to 2 parts; as before.
3. Powdered clay, 2 parts; powdered charcoal, 3 parts (by weight); water q. s. to form a stiff dough. The kilning must be so arranged that the heat is applied gradually, and the vessels exposed to a current of hot air; and it must be continued until all the charcoal is burnt out, carefully avoiding over-firing.
AL′CHEMY (-kĭm-). Syn. Al′chymy (-kĭm-); Hermetic Art*; Alchem′ia, Alchym′ia, L.; Alchimie, Fr.; Alchemie, Ger.; Alchimia, It. The romantic forerunner of the modern science of chemistry. An imaginative art or science, having for its objects the discovery of a substance (PHILOSOPHER’S STONE) capable of transmuting the baser metals into gold—a panacea, or universal remedy (ELIXER VITÆ), by which disease and death were to be avoided by its possessor—an alkahest, or universal solvent—a universal ferment; and other like absurdities. A mixed metal formerly used for utensils was also called by this name.
AL′COHOL. C2H6O. [Eng., L.; B. P.] Syn. Al′kohol, Eng., L.; Alcoöl, Alcohol, Fr.; Alkohol, Höchst Rectifieirter Wein-geist., Ger.; Alcoöle, It. A term commonly applied to one kind of spirit—that obtained by the distillation of any fermented saccharine liquid, and forming the characteristic principle of wines, beers, spirits, and other intoxicating liquors.
Etym. Kohol, a Hebrew-Syriac word, is the name given to a preparation of powdered antimony used by Oriental ladies to paint their eyebrows. In course of time this term was applied to other fine powders, and ultimately to highly rectified spirits.
Hist., &c. Although the art of distillation was probably known at a comparatively early age of the world, the preparation of pure rectified spirit is a discovery of modern times. It was not until the 13th century that Raymond Lully first showed the way to concentrate spirit by means of carbonate of potash; after which date pure concentrated spirit gradually rose into note as an article of trade and commerce in Europe. In the 16th century its distillation was in common practice in these countries. (Burns.) By means of chloride of calcium, Dr Black obtained alcohol of sp. gr. 0·800 (about A.D. 1760); and Richter afterwards procured it of a sp. gr. so low as 0·796 at 60° Fahr. (Crell’s ‘Annals,’ 1796.) Lavoisier first demonstrated the composition of alcohol (about 1780). Its analysis was subsequently perfected by M. Saussure, jun., and confirmed by MM. Dumas and Boullay, and Gay-Lussac; and by many others since.
Nat. Hist. Alcohol is peculiar to the organic kingdom, being exclusively produced, in the natural way, by the process of fermentation.
Sources, &c. Dilute alcohol may be procured, by the ordinary process of distillation, from all fermented liquors. When drawn from wine (as in France), it constitutes BRANDY; when from the refuse juice of the sugar-cane, it is called RUM; when from malt, grain, or molasses (as in England), it is called MALT, RAW-GRAIN or MOLASSES SPIRIT; and when from rice or palm-wine, ARRACK. Brandy, rum, Hollands, and whisky, contain only about half their volume of alcohol; and gin much less. When distilled from any of these spirituous liquors, the alcohol contains, besides water, variable quantities of essential oils, ethers, and other flavouring matters, which, by one or more redistillations with charcoal or lime, it for the most part loses, and then becomes commercial spirit of wine. By a further rectification from chloride of calcium, lime, carbonate of potash, or any other substance having a strong affinity for water, the water is retained, and a strong spirit passes over containing not more than 10 per cent. of water. By repeating the process, and using the proper precautions, it may be obtained almost entirely free from water, and is then called absolute or anhydrous alcohol.
Preparation I. Of Absolute Alcohol:—
a. Alcohol (highly rectified spirit), of 85% (sp. gr. ·835 to ·822), is mixed, in a tubulated retort, with about half its weight of fresh-burnt quick-lime, in coarse powder; and the whole, after securely stopping the neck with a cork, and agitation, is allowed to repose for several days. The alcohol is then carefully distilled off, drop by drop, by the heat of a water bath, until the weight of the distillate nearly equals that of the ‘anhydrous alcohol’ in the spirit operated on. The sp. gr. of the product should be ·795 or ·796; but by carefully repeating the process with the distillate and a fresh quantity of lime, and prolonging the last digestion with the latter for several weeks, absolute alcohol of the sp. gr. ·79381 at 60° Fahr. may be easily obtained.
b. (Drinkwater; Fownes.) The strongest58 rectified spirit of wine is digested in a stoppered bottle for several days, with about half its weight of anhydrous carbonate of potash, in powder, frequent agitation being had recourse to; the alcohol, after repose, is then decanted, and treated with sufficient fresh-burnt quick-lime to absorb the whole of the spirit. After 48 hours’ digestion, the spirit, when distilled, will have the sp. gr. ·793 at 60° Fahr.
c. (Liebig; Ure.) Alcohol of about 90% is saturated with fused chloride of calcium, in powder, and after repose for a few hours in a stoppered bottle, is submitted to distillation as before. The product should nearly equal the quantity of dry alcohol in the sample. Ure recommends equal weights of the spirit and chloride to be taken; and the process to be stopped as soon as about half the volume of the spirit employed has passed over, or the distillate acquires a higher sp. gr. than ·791 at 68°, or ·796 at 60° Fahr.
d. (B. P. 1867.) Take of rectified spirit, 1 pint; carbonate of potash, 11⁄2 ounce; slaked lime, 10 ounces. Put the carbonate of potash and spirit into a stoppered bottle and allow them to remain in contact for two days, frequently shaking the bottle. Expose the slaked lime to a red heat in a covered crucible for half an hour, then remove it from the fire, and, when it has cooled, immediately put the lime into a flask or retort, and add to it the spirit from which the denser aqueous solution of carbonate of potash, which will have formed a distinct stratum at the bottom of the bottle, has been carefully and completely separated. Attach a condenser to the apparatus, and allow it to remain without any external application of heat for twenty-four hours; then applying a gentle heat, let the spirit distil until that which has passed over shall measure 11⁄2 fluid ounce; reject this, and continue the distillation into a fresh receiver until nothing more passes at a temperature of 200° Fahr.
e. (Poggendorff.) Saturate alcohol with caustic potash, then add half its volume of water, and distil at a low temperature.
II. Of Hydrated or Commercial Alcohol:—
a. (Alcohol, Ph. L. 1836.) Take of rectified spirit (sp. gr. 0·838), 1 gal.; chloride of calcium, 1 lb.; proceed as above, and distil 7 pints and 5 fl. oz. Sp. gr. of product 0·815. It contains about 7% of water, by weight, and 5% by volume.
b. (Alcohol, Ph. D. 1826.) Rectified spirit, 1 gal.; pearl-ashes (dried and still hot), 31⁄2 lbs.; mix, digest in a covered vessel, with frequent agitation, for seven days; then decant the clear portion, and add to it of chloride of calcium, 1 lb.; agitate to effect solution, and distil off the spirit until the mixture in the retort begins to thicken. Sp. gr. of product 0·810. It contains about 5% of water, by weight.
c. (Without distillation.) Rectified spirit is agitated, in a closed vessel, with anhydrous carbonate of potash (prepared by heating the salt to redness, and still slightly warm), until the powder sinks to the bottom undissolved; the carbonate is then added in considerable excess, and the agitation repeated at short intervals for some hours or even days; lastly, after sufficient repose, the clear upper portion is decanted.—Obs. If a clean spirit, and pure carbonate of potash (or at least one perfectly free from caustic potash or any other impurity soluble in strong spirit), be used, an alcohol sufficiently pure and free from water for many common purposes may be thus obtained; otherwise the product contains a little potassa, &c., which can only be removed by distillation. For some purposes, however, this would not be objectionable. Sp. gr. about ·812.
A, A bottle with two necks, the upper furnished with a ground-glass stopper.
B, Loop of cord to hang the apparatus up by.
C, Bladder, containing spirit, filled by means of the bottle A.
D, Neck of bladder accurately secured to the under neck of the bottle A.
III. (Soëmmering.—Varnish-maker’s alcohol.) The bladder of an ox or calf, thoroughly cleansed from fat, and washed and dried, is nearly filled with rectified spirit, and then securely fastened and suspended in any dry situation, at a temperature of about 122° Fahr. In from six to twelve hours, when the heat is properly maintained, the spirit is generally sufficiently concentrated, and in a little time longer is rendered nearly free from water (anhydrous), or of the strength of 96 to 98%.—Obs. The same bladder will serve for more than one hundred operations. If not kept very nearly full, a portion of the spirit escapes through the empty part. To prevent this accident, a bottle with a double neck, of the shape represented in the engr., may be employed; by which means the bladder may be kept constantly full during the process. After the first or second time of using, the bladder gives alcohol sufficiently pure for all ordinary purposes. Before hanging the apparatus up, it is better to enclose it in a coarse potato-netting, to prevent any accident arising from the strain on the neck of the bladder. Soëmmering recommends both the inside and outside of the dry bladder to be smeared over59 2 or 3 times with a strong solution of isinglass; but this is not necessary to the success of the process.
IV. Rectified Spirit. (B. P. 1867.) Spiritus Rectificatus. Alcohol with 16 per cent. of water; obtained by the distilling of fermented saccharine fluids. Sp. gr. 0·838.
V. Proof Spirit. (B. P. 1867.) Spiritus Tenuior. Take of rectified spirit, 5 pints; distilled water, 3 pints. Mix. Sp. gr. of product 0·920.
Prop. of Alcohol. Light, transparent, colourless; highly volatile and inflammable, burning with a pale blue and smokeless flame; very mobile; odour, agreeable; taste, strong and pungent; miscible in all proportions with water, with the evolution of heat, and temporary expansion, but ultimate condensation of the mixture, some hours elapsing before the union is complete, and the normal temperature restored. The mixture has a higher sp. gr. than the mean of its constituents; and this is greatest when 54 vols. of alcohol are mixed with 49·77 vols. of water, the resulting compound measuring only 100 volumes. It absorbs water from moist air; dissolves resins, essential oils, camphor, bitumen, soaps, sugar, carbonic and boracic acid, iodine and the iodides, lime, ammonia, soda, potash, the alkaloids, wax and spermaceti (when boiling), all the deliquescent salts (except carbonate of potassa), and various other substances. It curdles milk, coagulates albumen, and (in quantity) separates both starch and gum from their mucilages. It boils, in the air, at 173° Fahr., when in the anhydrous state. When diluted with water its boiling point rises in proportion to the amount of water added. It boils, in vacuo, at 56° Fahr. Every volume of boiling alcohol yields 488·3 vols. of vapour at 212° Fahr. Its sp. gr. is 0·793811 at 60° Fahr., that of its vapour being 1·6133. It has never been frozen; when cooled to -166° Fahr., it acquired the consistence of castor oil, but did not solidify. It contracts by cold; between -15° and +99° Fahr., this occurs with great regularity, at the rate of ·00047 part of its volume for every degree of the thermometer. Its evaporation, like that of ether, produces intense cold. The products of its combustion are carbonic anhydride and water. It acts as a powerful antiseptic on organic substances immersed in it, and is in consequence extensively employed in the preservation of anatomical preparations. With the acids it forms ethers.
Phys. eff. Alcohol is a narcotico-acrid poison. In small doses it occasions excitement and intoxication; in larger ones, delirium, somnolency, coma, apoplexy, and death. It acts as a violent nervous stimulant, and, by abstracting water from the soft tissues of the stomach and primæ viæ, destroys their organisation. It is alike poisonous to all animals;—2 drs. will kill a dog. All strong spirits act in the same way, the effect being proportionate to the state of concentration and the quantity taken. On plants it acts as a rapid and fatal poison.
Ant., &c. Copious internal use of tepid water, with cold affusions to the head and spine, and injection of cold water into the ears. In the absence of vomiting, a strong emetic should be given, or the stomach-pump used. Ammonia may be used as a stimulant, and, added to water just in sufficient quantity to flavour it, is one of the best antidotes. The head should be kept elevated, and bleeding had recourse to, if cerebral congestion threatens.
Tests in cases of death. 1. The odour of the contents of the stomach and ejected matters, and their ready inflammability. 2. The spirit may be separated by digestion with water, filtration, the addition of carbonate of potash, and distillation.
Comp., &c. Its per-centage composition is—
| Dumas and Boullay. | Brande and Ure. | Ure. sp. gr. 0·812. | |
| Carbon | 52·37 | 52·18 | 47·85 |
| Hydrogen | 13·01 | 13·04 | 12·24 |
| Oxygen | 34·61 | 34·78 | 39·91 |
| ———— | ———— | ———— | |
| 99·99 | 100·00 | 100·00 |
This nearly represents 2 equivalents of carbon, 3 eq. of hydrogen, and 1 of oxygen. The atom of alcohol is now regarded as a multiple of these numbers, and formed by the breaking up of one atom of grape sugar (C13H28O11) into 4 eq. of alcohol, 8 eq. of carbonic acid, and 4 eq. of water. It was formerly regarded as a compound of 1 eq. of olefiant gas, and 1 eq. of water; but it is now generally viewed as HYDRATE OF THE OXIDE OF ETHYLE (C2H5.HO), or a compound of ethylene and water (C2H4.H2O). Grape sugar alone yields alcohol; cane sugar, before it undergoes the vinous fermentation, being first converted into this substance by contact with the ferment.
Purity. The presence of water is shown by the specific gravity (see Alcoholometry); the absence of other foreign matter by the following tests:—
1. Its colour and transparency is not affected by the addition of a little colourless oil of vitriol (Liebig), or by a solution of nitrate of silver, and subsequent exposure for some time to solar light (Vögel), unless either essential oil or organic matter be present, when it assumes a reddish tinge. 2. It should be neutral to test-papers, colourless, leave no residue on evaporation, and be miscible, in all proportions, with water and with ether. 3. Its boiling point should never be less than 170° Fahr.; a lower temperature suggests the presence of wood spirits, or acetone, or one of the ethers. To detect wood spirit (wood naphtha) see Nessler’s Test. For the reverse of this adulteration—the evasion of the duty by the introduction of spirit, under the disguise of naphtha, turpentine, &c.—see those articles.60
4. The presence of water in alcohol may be detected, not only by the sp. gr., but also by white anhydrous sulphate of copper burning blue when dropped into it. 5. Potassium placed on alcohol does not take fire, unless a considerable per-centage of water be present.
Tests, &c. 1. It may generally be recognised by its volatility, inflammability, odour, taste, miscibility with water, power of dissolving camphor and resins, and other qualities already described. 2. If a few fibres of asbestos be ‘moistened’ with a saturated solution of bichromate of potash in oil of vitriol, and exposed to the smallest possible portion of hot alcohol vapour, it is almost instantly turned green, owing to the formation of oxide of chromium. In practice, the asbestos may be inserted in the neck of a retort, or even of a bulbed glass-tube containing a few drops of the suspected solution, when the effect occurs as soon as distillation commences. Ether and pyroxylic spirit produce a nearly similar result; but the ‘first’ of these is distinguished from alcohol by its not being miscible with water in all proportions; and the ‘other’ by Nessler’s Test; whilst both may be readily distinguished by their peculiar and characteristic odour. 3. Dissolve 3 pts. crystallised carbonate of soda in 10 pts. water. To this solution add 1 pt. of liquid to be tested, and heat to about 160° Fahr. Lastly, add iodine in small pieces, till it has entirely dissolved, and the liquid has become colourless. If alcohol be present, iodoform will make its appearance on cooling, and sink to the bottom in the form of a yellow powder. As a similar result is obtained with wood spirit, this must be proved to be absent before applying this test.
The only reliable method of proving that a sample is ethylic alcohol is the production of ether, by acting on the suspected liquid with sulphuric acid. See Ether.
Uses. In the arts, alcohol is used by the varnish-maker to dissolve resins; by the perfumer, to extract the odour of plants, and dissolve essential oils, soaps, and other similar substances; by the pharmaceutist, to prepare tinctures and other valuable medicinals; by the instrument-maker, to fill the bulbs of thermometers required to measure extreme degrees of cold; by the photographer, in the preparation of collodion; by the chemist, in analysis, and in the manufacture of numerous preparations; by the anatomist and naturalist, as an antiseptic; and by the physician, for various purposes and applications as a remedy. It is also frequently burnt in lamps, and in parts of the world where it is inexpensive, it is employed in the manufacture of vinegar. Its uses, when dilute, as in the ‘spirituous liquors’ of commerce, are well known. In medicine, it is employed both concentrated (‘alcohol,’ ‘rectified spirit’) and dilute (‘proof spirit,’ ‘brandy,’ ‘gin,’ &c.), as a caustic, irritant, stimulant, tonic, &c. It has also been used in a multitude of other cases, and has been applied to an almost infinite variety of other purposes.
Gen. commentary. The selection of any one of the processes given above for the preparation of alcohol must greatly depend on the convenience or position of the operator. Chloride of calcium, and quick-lime, from their powerful affinity for water, and easy application, are the hygrometric substances most generally employed; but the processes involving the use of the other substances and methods already noticed, have all of them advantages under particular circumstances. Gay-Lussac has recommended the use of caustic baryta instead of lime; and others have employed dry alumina, as an absorbent of the water prior to distillation. Common proof spirit may be concentrated until its sp. gr. falls to about 0·825, by simple distillation in a water bath; at which sp. gr. it contains only about 11% of water, by weight, and is then nearly as volatile as pure alcohol.
A convenient apparatus for the preparation61 of alcohol, on the small scale, is that figured in the engr., and which will be self-explanatory to every one competent to use it. The tank (i) should be supplied with ice-cold water; and the receiver (g) should be covered with cloths kept continually wet with water of the same temperature. The capsule or basin (c) is a water bath heated by the little gas furnace (d). On the large scale, for commercial alcohol, a copper still, fitted with a glass refrigeratory and receiver, is commonly employed.
By surrounding the capital of a still, or other like apparatus, by a water bath kept at the proper temperature, the alcoholic richness or content of the product may be regulated to the greatest nicety, for any desired strength.
The different statements of chemical authors as to the boiling point, specific gravity, &c., of alcohol, already noticed, may be referred to their having either experimented with samples which have not been absolutely anhydrous, or to their not having made the proper corrections for temperature, and for the different materials of which their vessels and instruments were composed—some probably having been made of glass, and others of brass or some other metal. In some instances the differences are more apparent than real, as in the Tables by Tralles and Lowitz; in the former of which water, at its lowest sp. gr., is taken as the standard. Until recently, the only known source of alcohol was the fermentation of saccharine solutions. Its production by synthesis, though often attempted, is, however, erroneously said to have always failed. It had long been employed as an occasional source of bicarburetted hydrogen (olefiant gas) at a high temperature; but M. Berthelot succeeded in reproducing it, from bicarburetted hydrogen, by agitating the latter, in a closed vessel, with sulphuric acid and metallic mercury (‘Journ. de Chimie Med.,’ 1855, p. 175); and Henry Flennel, nearly thirty years before M. Berthelot’s discovery, found that pure olefiant gas is absorbed by agitation with concentrated sulphuric acid, with the formation of sulphovinic acid, and that by subsequent dilution with water, and distillation, alcohol passes over into the receiver.
ALCOHOLATE. Syn. Alcohate; Alcoholas, L. A salt in which alcohol appears to replace the water of crystallisation, as is the case with certain chlorides, nitrates, &c. Some of them may be formed by simple solution and crystallisation of the salt in alcohol. (Graham.) They are all very unstable, being readily decomposed by water.
ALCOHOLIC. Syn. Alcoholicus, L.; Alcoholique, &c., Fr.; Alkoholisch, Ger. Pertaining to, containing, of the nature of, or made with, alcohol.
ALCOHOLICA. [L.] Syn. Alcoöliques, Fr.; Weingeist-verbindungen, Ger. In pharmacy, liquids containing, or preparations made with, alcohol, as a characteristic ingredient.
ALCOHOLISATION. [Eng., Fr.] Syn. Alcoholisatio, L.; Alcoölisation, &c., Fr.; Alkoholiserung, Ger. In chem. and pharm., the development of the characteristic properties of alcohol in a liquid, or the use of it either as an addition or a menstruum; also the act or process of obtaining alcohol from spirit by rectification.
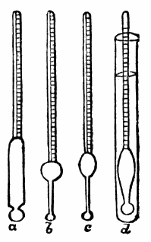
ALCOHOLOM′ETER (-lŏm′-). Syn. Alcohol′meter (hŏl′-; -hŏm′-‡); Alcoholométrum, L.; Alcoölomètre, Alcoömètre, Alcoholmètre, &c., Fr. An instrument or apparatus used in alcoholometry. Alcoholometers are simply ‘hydrometers’ adapted to the densities of alcohol, either concentrated or dilute. Some of these, as Baumé’s, Carter’s, &c., merely indicate the number of degrees corresponding to the state of concentration of the liquid. Others, of a like construction, as those of Richter (a), Tralles (b), and Gay-Lussac (c), have their stems so graduated as at once to indicate the proportion per cent. of alcohol present, either by weight, or by volume, at some standard temperature. (See engr.) A third class, as those of the Abbé Brossard-Vidal, Field, &c. are essentially thermometers, with scales which indicate the boiling points of spirits of different strengths, instead of the common thermometric degrees; whilst to a fourth class belong the alcoholometer of M. Silbermann, which is based upon the known rate of expansion of alcoholic liquors by heat, expressed in alcoholometric degrees; and that of M. Geissler, which depends on the measurement of the tension of the vapour of the liquid, as indicated by the height to which it raises a small column of mercury. In Syke’s hydrometer, used by officers of the Revenue, the scale of the instrument is enormously extended by the use of movable weights, with each of which it becomes, in fact, a separate instrument, adapted to a certain range of specific gravities.
A very convenient alcoholometer for ordinary purposes (d) has been lately produced by some of the instrument makers. It is of the usual form, but its stem on one side exhibits the per-centage richness of the sample in alcohol by volume; and on the other, the per-centage by weight. Thus, both results may be obtained at one trial. This instrument is sometimes called Richter’s alcoholometer, in England. A further improvement, still more recently introduced, is a similar ‘double-scale’ instrument, showing the degrees of Sykes on one side, and carrying a small spirit-thermometer in the bulb, to which62 a scale is fixed ranging from 35° to 82° Fahr.
ALCOHOLOM′ETRY. Syn. Alcohol′metry (-hŏl′-; -hŏm′-‡); Spirit testing‡; Alcoholme′tria, L.; Alcoölométrie, Alcoömétrie, &c., Fr. In chemistry, the art or process of ascertaining the richness of spirits in alcohol. In commerce, the determination of the quantity of spirit of a certain strength, taken as a standard, present in any given sample of spirituous or fermented liquors. In England, this standard is called “proof spirit.”
Hist., &c. The great importance of being able accurately to determine the strength of spirits in the United Kingdom, on account of the high duties levied on them, has induced the Government authorities, at various times, to investigate the subject. In 1790, the matter was referred to Sir C. Blagden, then Secretary to the Royal Society, who instituted an extensive series of experiments to determine the real specific gravities of different mixtures of alcohol and water. The results of his labours and researches were put forward, with ‘Gilpin’s Tables,’ in 1794, but no practical measures appear to have been taken in consequence. In 1832 a committee of the Royal Society, at the request of the Lords of the Treasury, examined into the accuracy of the Tables, and the construction and application of the instrument (Syke’s hydrometer) now used by the Revenue officers, on which they reported favorably, and declared that they were sufficiently perfect for all practical and scientific purposes. The errors introduced into calculations of the strength of spirits by these tables were found to be quite unimportant in practice, and did not, in any one instance, amount to unity in the fourth place of decimals. This method adapts the specific gravity as the test of the strength of spirits, and is founded on the fact that alcohol is considerably lighter than water, and that (with proper corrections for condensation and temperature) the sp. gr. regularly increases, or decreases, according to the relative proportions in which the two are mixed.
Several other methods of alcoholometry have been proposed, founded upon—the variations in temperature of the vapour of alcohol of different strengths—the heat involved by its admixture with water—its dilatation by heat—the tension of its vapour—the insolubility of carbonate of potash in alcohol—its volatility, boiling point, &c. &c., the more important and useful of which are noticed further on. The method adopted by the Boards of Inland Revenue and Customs is, however, the one which is almost exclusively employed in trade and commerce in Great Britain, not only on account of its simplicity and correctness, but for the purpose of the results exactly coinciding with the results obtained by the Revenue officers.
1. Methods based on the specific gravity, or per-centage strength, by VOLUME:—
a. With Sykes’ Hydrometer. Revenue system. The engraving below represents Sykes’ hydrometer, as made by Mr Bate, under the directions of the Commissioners of Inland Revenue and Customs. It consists of a spherical ball or float, with an upper and lower stem, and is made of brass, which (in the more expensive instruments) is usually coated with gold, to prevent corrosion from damp, and the acidity so generally present in spirituous liquors. The upper stem (A) is about four inches long, and is divided into ten parts, each of which contains five subdivisions. There are nine movable weights of the form b, of different sizes, numbered respectively 10, 20, 30, &c., to 90, each of which represents so many of the principal divisions of the stem, as its number indicates. In use, one of these weights is slipped on to the lower stems; and thus, by means of them, the instrument acquires a range of above 500 divisions, or degrees, extending from the Revenue ‘standard alcohol’ (sp. gr. ·825) to water. It is so formed as to give the sp. gr. with almost perfect accuracy, at 62° Fahr. When loaded with the weight 60 it sinks in proof spirit to the line marked (P) on the narrow edge of the stem at 51° Fahr.; and, by further placing the square weight or cap (also supplied with the instr.) on the top of the upper stem, it floats exactly at the same point in distilled water. This weight or cap is found to weigh 43·66 grs., which is practically 1-12th of the total observed weight of the instrument, and its poise 60, and hence shows the difference between the gravity of proof spirit and water, as explained hereafter. The whole is fitted up in a neat mahogany case, accompanied with a thermometer, and a book of tables containing corrections for temperature, &c.—Process. A glass tube of the form of fig. B is filled to about the mark (a) with the sample for examination; the thermometer is then placed in the liquor, and stirred about for two or three63 minutes (observing not to breathe upon the glass, nor hold it in the hand), and the temperature noted. The hydrometer is next immersed in a similar manner, and gently pressed down in the liquor to the 0 on the stem with the finger; it having been previously loaded with any one of the nine weights that will cause it to float with the surface of the spirit at some point on the graduated part of the scale. The indication at the point cut by the surface of the liquor, as seen from below, added to the number of the weight with which the float is loaded, gives a number which must be sought in the hook of Tables, which is always sold with the instrument. In this book, at the page headed “Temperature as observed by the Thermometer,” and against the part of the column appropriated to the given indication (weight), will be found the strength per cent., expressed in degrees over or under proof, by VOLUME, in whole numbers or decimal parts. In reading off the indication, to ensure accuracy, it is necessary to allow for the convexity of the liquor at the part where it immediately rests against the stem.
Obs. In an instrument requiring so much care and skill in its manufacture the purchaser should be careful to procure a perfect one. A very slight blow, friction from continual wiping with a rough cloth, and other apparently trivial causes, tend to injure so delicate an instrument. The shape of the weights occasionally vary; some being intended to be attached to the hydrometer at the bottom of the spindle, and others to rest on its top. The first plan is, perhaps, the best, as it tends to make the instrument float with greater steadiness in the liquor; but, at the same time, it renders its adjustment by the maker a matter of greater difficulty.
In employing this instrument, the Revenue officers are instructed to take the nearest degree above the surface of the mercury, when it stands between any two degrees of the thermometer; and the division on the scale of the hydrometer next below the surface of the liquid, when it cuts the stem between any two lines; thus giving the difference in favour of the trader in both cases.
By means of the Table at page 64 the hydrometer indication, or the degrees over or under proof, of the Revenue system, may be converted into ‘real specific gravities,’ by mere inspection; and the corresponding ‘per-centage richness’ in alcohol of any sample may be found, either by WEIGHT or VOLUME.
The specific gravities in this table are such as, on being referred to Gilpin’s Tables, will give the expressions of proof strength answering to the whole indications of the Revenue hydrometer. Intermediate values at fifths of indications may be had by taking proportional differences between the nearest tabular numbers. Thus, to find the specific gravity that should stand opposite to Indication 70·6, we first obtain the difference between the densities standing in a line with Indications 70 and 71 respectively, and then say, as 1 : 0·6 :: ·00192. 00·115, and ·94135 + ·00115 = ·94250, the specific gravity required.
b. With GLASS ALCOHOLOMETERS. That of Tralles, and most others of a like description (as made in England), gave the per-centage strength, by VOLUME, with tolerable accuracy, at the standard temperature of 60° Fahr. Gay-Lussac’s ALCOÖMETRE, which closely resembles that of Tralles, is adjusted for the temperature of 59° Fahr. (15° Cent.). All of these, to give at once accurate results, must, of course, be employed at the ‘normal temperature’ of the instrument. As, however, in practice, the experiment cannot be conveniently performed at any ‘fixed’ temperature but only at that of the atmosphere, it is obvious that certain corrections are constantly required in order to obtain results of any value. Perfect accuracy requires that table for every variation of the thermometer, founded on actual experiments, should accompany each instrument; as, without them, tedious and difficult calculations are necessary, which, in the hurry of the cellar and laboratory, or by persons inexpert at figures, are not easily performed. A series of such Tables were prepared by Gay-Lussac, and, with his instrument, are those which are almost exclusively used in France. For rough purposes, in the absence of Tables or nicer calculations, it may be useful to know that, for commercial spirits, at ordinary temperatures, a variation of—
| 5° | Fahr. | is equal to (about) | 1·00% | of Alcohol; or (about) | 1·794% | of Proof spirit. |
| 1° | ” | ” | 0·20% | ” | 0·359% | ” |
| 5° | Cent. | ” | 1·80% | ” | 3·229% | ” |
| 1° | ” | ” | 0·36% | ” | 0·646% | ” |
| 5° | Fahr. | is equal to (about) | 0·80% | of Alcohol; or (about) | 1·62% | ” |
| 1° | ” | ” | ·16% | ” | ·32% | ” |
| 5° | Cent. | ” | 1·43% | ” | 2·9% | ” |
| 1° | ” | ” | ·28% | ” | ·58% | ” |
64
| Sykes’ Hydrometer Indication. | Strength per cent. | Specific Gravity. | Per Cents. of Absolute Alcohol. | |
| By Measure. | By Weight. | |||
| O.P. | ||||
| 0 | 67·0 | ·81520 | 95·28 | 92·78 |
| 1 | 66·1 | ·81715 | 94·78 | 92·08 |
| 2 | 65·3 | ·81889 | 94·31 | 91·42 |
| 3 | 64·5 | ·82061 | 93·84 | 90·78 |
| 4 | 63·6 | ·82251 | 93·33 | 90·07 |
| 5 | 62·7 | ·82441 | 92·80 | 89·36 |
| 6 | 61·8 | ·82622 | 92·29 | 88·67 |
| 7 | 60·9 | ·82800 | 91·77 | 87·99 |
| 8 | 60·0 | ·82978 | 91·25 | 87·30 |
| 9 | 59·1 | ·83151 | 90·74 | 86·63 |
| 10 | 58·2 | ·83323 | 90·23 | 85·96 |
| 11 | 57·3 | ·83494 | 89·72 | 85·30 |
| 12 | 56·4 | ·83661 | 89·21 | 84·65 |
| 13 | 55·5 | ·83827 | 88·70 | 84·00 |
| 14 | 54·6 | ·83993 | 88·17 | 83·33 |
| 15 | 53·7 | ·84153 | 87·67 | 82·70 |
| 16 | 52·7 | ·84331 | 87·10 | 81·99 |
| 17 | 51·7 | ·84509 | 86·51 | 81·26 |
| 18 | 50·7 | ·84680 | 85·95 | 80·58 |
| 19 | 49·7 | ·84851 | 85·39 | 79·89 |
| 20 | 48·7 | ·85022 | 84·81 | 79·19 |
| 21 | 47·6 | ·85205 | 84·19 | 78·44 |
| 22 | 46·6 | ·85372 | 83·61 | 77·74 |
| 23 | 45·6 | ·85537 | 83·04 | 77·07 |
| 24 | 44·6 | ·85700 | 82·47 | 76·39 |
| 25 | 43·5 | ·85878 | 81·85 | 75·66 |
| 26 | 42·4 | ·86055 | 81·21 | 74·92 |
| 27 | 41·3 | ·86229 | 80·59 | 74·19 |
| 28 | 40·2 | ·86402 | 79·97 | 73·47 |
| 29 | 39·1 | ·86574 | 79·34 | 72·75 |
| 30 | 38·0 | ·86745 | 78·71 | 72·03 |
| 31 | 36·9 | ·86915 | 78·08 | 71·32 |
| 32 | 35·7 | ·87099 | 77·40 | 70·54 |
| 33 | 34·5 | ·87282 | 76·71 | 69·77 |
| 34 | 33·4 | ·87450 | 76·08 | 69·06 |
| 35 | 32·2 | ·87627 | 75·41 | 68·32 |
| 36 | 31·0 | ·87809 | 74·72 | 67·55 |
| 37 | 29·8 | ·87988 | 74·03 | 66·79 |
| 38 | 28·5 | ·88179 | 73·29 | 65·98 |
| 39 | 27·3 | ·88355 | 72·60 | 65·23 |
| 40 | 26·0 | ·88544 | 71·86 | 64·43 |
| 41 | 24·8 | ·88716 | 71·17 | 63·68 |
| 42 | 23·5 | ·88901 | 70·43 | 62·89 |
| 43 | 22·2 | ·89086 | 69·69 | 62·10 |
| 44 | 20·9 | ·89268 | 68·95 | 61·32 |
| 45 | 19·6 | ·89451 | 68·21 | 60·53 |
| 46 | 18·3 | ·89629 | 67·47 | 59·76 |
| 47 | 16·9 | ·89822 | 66·67 | 58·92 |
| 48 | 15·6 | ·89997 | 65·93 | 58·15 |
| 49 | 14·2 | ·90182 | 65·14 | 57·34 |
| 50 | 12·8 | ·90367 | 64·34 | 56·52 |
| 51 | 11·4 | ·90551 | 63·54 | 55·70 |
| 52 | 10·0 | ·90732 | 62·74 | 54·89 |
| 53 | 8·6 | ·90913 | 61·94 | 54·09 |
| 54 | 7·1 | ·91107 | 61·09 | 53·23 |
| 55 | 5·6 | ·91299 | 60·24 | 52·38 |
| 56 | 4·2 | ·91479 | 59·43 | 51·57 |
| 57 | 2·7 | ·91666 | 58·58 | 50·73 |
| 58 | 1·3 | ·91839 | 57·78 | 49·94 |
| U.P. | ||||
| 59 | 0·3 | ·92037 | 56·86 | 49·04 |
| 60 | 1·9 | ·92228 | 55·96 | 48·17 |
| 61 | 3·4 | ·92408 | 55·10 | 47·33 |
| 62 | 5·0 | ·92597 | 54·19 | 46·46 |
| 63 | 6·7 | ·92798 | 53·22 | 45·53 |
| 64 | 8·3 | ·92984 | 52·30 | 44·65 |
| 65 | 10·0 | ·93176 | 51·36 | 43·76 |
| 66 | 11·7 | ·93367 | 50·39 | 42·84 |
| 67 | 13·5 | ·93586 | 49·34 | 41·86 |
| 68 | 15·3 | ·93758 | 48·31 | 40·90 |
| 69 | 17·1 | ·93949 | 47·29 | 39·96 |
| 70 | 18·9 | ·94135 | 46·29 | 39·04 |
| 71 | 20·8 | ·94327 | 45·20 | 38·04 |
| 72 | 22·7 | ·94518 | 44·09 | 37·03 |
| 73 | 24·7 | ·94709 | 42·96 | 36·01 |
| 74 | 26·7 | ·94899 | 41·82 | 34·98 |
| 75 | 28·8 | ·95092 | 40·63 | 33·92 |
| 76 | 31·0 | ·95288 | 39·40 | 32·82 |
| 77 | 33·2 | ·95484 | 38·10 | 31·68 |
| 78 | 35·6 | ·95677 | 36·76 | 30·50 |
| 79 | 38·1 | ·95877 | 35·32 | 29·24 |
| 80 | 40·6 | ·96068 | 33·90 | 28·01 |
| 81 | 43·3 | ·96259 | 32·41 | 26·73 |
| 82 | 46·1 | ·96457 | 30·77 | 25·32 |
| 83 | 49·1 | ·96651 | 29·08 | 23·88 |
| 84 | 52·2 | ·96846 | 27·31 | 22·38 |
| 85 | 55·5 | ·97049 | 25·39 | 20·77 |
| 86 | 59·0 | ·97254 | 23·41 | 19·11 |
| 87 | 62·5 | ·97458 | 21·39 | 17·42 |
| 88 | 66·0 | ·97660 | 19·41 | 15·78 |
| 89 | 69·4 | ·97857 | 17·46 | 14·16 |
| 90 | 72·8 | ·98057 | 15·51 | 12·56 |
| 91 | 76·1 | ·98261 | 13·58 | 10·97 |
| 92 | 79·2 | ·98452 | 11·85 | 9·56 |
| 93 | 82·3 | ·98657 | 10·04 | 8·08 |
| 94 | 85·2 | ·98866 | 8·28 | 6·65 |
| 95 | 88·0 | ·99047 | 6·83 | 5·48 |
| 96 | 90·7 | ·99251 | 5·25 | 4·20 |
| 97 | 93·3 | ·99448 | 3·80 | 3·03 |
| 98 | 95·9 | ·99658 | 2·31 | 1·84 |
| 99 | 98·2 | ·99851 | ·997 | ·793 |
| 100 | ... | 1·00000 | ... | ... |
This Table {above} has been copied, by permission, from Loftus’s ‘Inland Revenue Officer’s Manual,’ and its correctness verified by W. H. Johnston, Esq., Surveying General Examiner.65
| Water taken as 1000. | |||||||
| Specific gravity. | Correction for each degree. | Specific gravity. | Correction for each degree. | ||||
| 810 | to | 820 | ± ·475 | 910 | to | 920 | ± ·434 |
| 820 | ” | 830 | ± ·473 | 920 | ” | 930 | ± ·424 |
| 830 | ” | 840 | ± ·472 | 930 | ” | 940 | ± ·406 |
| 840 | ” | 850 | ± ·471 | 940 | ” | 950 | ± ·381 |
| 850 | ” | 860 | ± ·471 | 950 | ” | 960 | ± ·340 |
| 860 | ” | 870 | ± ·466 | 960 | ” | 970 | ± ·269 |
| 870 | ” | 880 | ± ·460 | 970 | ” | 980 | ± ·165 |
| 880 | ” | 890 | ± ·456 | 980 | ” | 990 | ± ·090 |
| 890 | ” | 900 | ± ·450 | 990 | ” | 1000 | ± ·084 |
| 900 | ” | 910 | ± ·442 | ||||
Thus, by making the proper ADDITION to the apparent strength per cent., when the observed temperature is BELOW the normal temperature of the instrument, or a corresponding SUBTRACTION, when it is ABOVE it, the strength of the sample may be determined sufficiently near for all practical purposes.
The following Table, taken from Loftus’s ‘Inland Revenue Officer’s Manual,’ will be found of great value in making these corrections, and has the merit of being easily applied.
An example will show how this Table is to be used.
Example.—If a quantity of spirit is of the sp. gr. 894 at 73°, what will be its sp. gr. at 60°?
Here the sp. gr. being between 890 and 900, we must add ·450 for each degree of temperature between 73° and 60°. The sp. gr. at 60° would, therefore, be 894 + (·450 × 13) = 899·85. When the temperature is below 60°, the correction for each degree must be subtracted. When, however, very accurate results are desired, and the necessary Tables are not accessible, the sample for trial must be brought to the normal temperature of the instrument, in the manner explained under Hydrometry.
c. From the SPECIFIC GRAVITY. The temperature having been taken by a thermometer, and the specific gravity ascertained by any of the usual methods, but preferably by means of an accurate glass hydrometer, it merely becomes necessary to refer to Table I, where, against the number expressing the specific gravity, the alcoholic content per cent., by volume, of the sample examined, will be found for 60° Fahr., subject to the corrections just referred to, when the temperature is either above or below this point.
If the precise specific gravity sought cannot be found in the Table, the difference between it and the next greater specific gravity must be taken for the numerator of a fraction, having for its denominator the difference between the greater and the next less specific gravity in the table. This fraction, added to the per-centage of alcohol in the fourth column of the table, opposite the greater sp. gr., will give the true per-centage sought. Thus, the sp. gr. ·96051 is not in the table, and the next greater number is ·96068; the former must, therefore, be deducted from the latter, and the difference (17) put as the numerator of the fraction, having for its denominator 191, the difference between ·96068 and ·95877. The fraction (17⁄191) ·089, so found, added to the per-centage strength opposite ·96068 in the third column, gives 33·989 as the true per-centage of alcohol in the given sample.
The per-centage by volume may be converted into per-centage by weight, by multiplying the former by ·793811, the sp. gr. of absolute alcohol, and dividing the product by the sp. gr. of the sample. The quotient is the number of pounds of alcohol in 100 pounds of the given spirit. Thus:—Suppose 1000 grains by measure of alcohol to weigh 950·92 grains, and to contain (see Table I) 40·63 per cent. by volume of absolute alcohol, what per cent. by weight does the sample contain?
·793811 × 40·63 = 32·25254093, and this product divided by ·95092 = 33·917, the true per-centage by weight of absolute alcohol in the sample.
2. Method based on the specific gravity, or per-centage strength by WEIGHT:—
The specific gravity is ascertained and the Table used in precisely the same manner as in the “method by volume,” already described.
The per-centage by weight may be converted into per-centage by volume, by multiplying the former by the sp. gr. of the sample, and dividing the product by the sp. gr. of absolute alcohol. This is merely the reverse of the operation described above.
Obs. The preceding methods of alcoholometry, as well as all others depending on the sp. gr. refer to UNSWEETENED SPIRITS only; and are inapplicable to those holding sugar in solution, or any other organic matter capable of altering the sp. gr. For sweetened spirits, fermented worts, wine, beer, &c., one or other of the following processes must be adopted:—
3. Other methods, adapted to either SWEETENED or UNSWEETENED SPIRITS, Tinctures, Fermented Liquors, &c.—
a. By DISTILLATION as originally proposed by M. Gay-Lussac. 300 parts of the liquor under examination (measured in a graduated glass tube) are placed in a retort or small still, and a quantity exactly equal to one third (i.e., 100 parts), carefully drawn over; a graduated glass tube[13] being used as a receiver, and66 the operation stopped as soon as the distillate reaches the hundredth degree. The ‘alcoholic strength’ of the distilled liquor is then ascertained by any of the usual methods, and the result divided by three, when the per-centage of alcohol in the original liquor is at once obtained. If, from want of attention, more than 100 parts should be distilled over, the number which expresses the relation of the volume of the distilled product to the original bulk of the liquor tested, must be employed as the divisor. Thus, if 106 parts of liquor have distilled over (instead of 100), containing 33% of alcohol, the 300 must be divided by 106, which gives 2·83, and the 33% by this 2·83, which gives 11·66%, the true proportion of alcohol in the original liquor. The strength at ‘proof’ may be calculated from this in the usual way.
To ensure accurate results, the acidity (if any) of the liquor must be neutralised with carbonate of sodium, prior to distillation. It is also advisable to add 8% or 10% of common salt to the liquor in the retort or still; this, by raising the boiling point, causes the whole of the spirit to pass over into the receiver before the distillate has reached the required measure. This applies more particularly to weak liquors. With those of greater strength (as the stronger wines), it is better to distil over 150 parts, and divide the result by 2 instead of 3. To liquors stronger than 25% by volume of alcohol, or above 52% to 54% under proof, add about an equal volume of water to the liquor in the still, and draw over a quantity equal to that of the sample tested; when the alcoholic strength of the distillate gives, without calculation, the true strength sought. To liquors stronger than 48% to 50% (14 to 12 u. p.), add thrice their bulk of water, and do not stop the process until the volume of the distillate is double that of the sample tested, when the per-centage obtained must also be doubled. In each case a proportionate quantity of salt is employed.
Revenue Method. The following is the method adopted in the Inland Revenue and Customs Laboratories for the estimation of the per-centage of alcohol in wines, liqueurs, &c. A measure flask is filled up to a mark on its neck, with the wine, which is then carefully transferred to a distilling flask or retort, the traces of wine remaining in the former vessel being rinsed out with small quantities of distilled water, and the rinsings added to the wine in the latter vessel. About two thirds of the contents of the retort are then distilled over into the clean measure flask, and made up to the original bulk with distilled water, at the same temperature as the sample was previous to distillation. The strength is then taken by Sykes’ hydrometer, and this (if u. p.) deducted from 100, gives the per-centage of proof spirit in the wine. Thus:—
b. From the Temperature of the Vapour, as originally proposed by Gröning. The bulb of a thermometer is thrust through a cork into the head of the still, or other vessel employed, and the temperature of the vapour in which it is immersed being noted, is sought in the following table:—
| Temperature of the Vapour. Fahr. | Alcoholic content of the Distillate per cent. | Alcoholic content of the Boiling Liquid per cent. | Temperature of the Vapour. Fahr. | Alcoholic content of the Distillate per cent. | Alcoholic content of the Boiling Liquid per cent. |
| 170·0 | 93 | 92 | 189·8 | 71 | 20 |
| 171·8 | 92 | 90 | 192·0 | 68 | 18 |
| 172·0 | 91 | 85 | 194·0 | 66 | 15 |
| 172·8 | 901⁄2 | 80 | 196·4 | 61 | 12 |
| 174·0 | 90 | 75 | 198·6 | 55 | 10 |
| 174·6 | 89 | 70 | 201·0 | 50 | 7 |
| 176·0 | 87 | 65 | 203·0 | 42 | 5 |
| 178·3 | 85 | 50 | 205·4 | 36 | 3 |
| 180·8 | 82 | 40 | 207·7 | 28 | 2 |
| 183·0 | 80 | 35 | 210·0 | 13 | 1 |
| 185·0 | 78 | 30 | 212·0 | 0 | 0 |
This method is admirably adapted to the purposes of the distiller and rectifier, as it furnishes a ready means of approximately determining the strength of the spirit passing over, at every part of the process of distillation, as well as that of the wash left in the still.
c. From the BOILING POINT, as originally proposed by M. l’Abbé Brossard-Vidal. This method is founded on the fact, that the boiling points of mixtures of alcohol and water, unlike water alone, are scarcely disturbed by the addition of saline, saccharine, or extractive matter within certain limits. It hence offers a ready means of determining the proportion of alcohol present in spirits, wines, fermented67 liquors, &c., with sufficient accuracy for all ordinary purposes. In applying it, a thermometer, with a large bulb and a narrow bore, and a movable scale graduated from 180° to 212° Fahr., is usually employed. Before using it as an alcoholometer, it is set, with its bulb immersed, in a small metallic boiler (brass or copper) containing distilled water, which is then raised to the boiling-point, and the 212° of the scale accurately adjusted on a level with the surface of the mercury, should it vary from that point. This is necessary on account of variations of atmospheric pressure causing corresponding variations of the boiling-points of liquids. It is then ready for several hours’ operations, and, generally, for an entire business day, without further adjustment. The little boiler is next filled with the liquor to be examined, and the lamp again lighted. The temperature as shown by the scale of the instrument at the commencement of full ebullition being ascertained, may be sought in one of the following Tables, against which the alcoholic content of the liquor will be found (nearly).
| Boiling point. Fahr. | Alcohol per cent. by volume. | Boiling point. Fahr. | Alcohol per cent. by volume. |
| 205·34 | 5 | 179·96 | 55 |
| 199·22 | 10 | 179·42 | 60 |
| 195·8 | 15 | 178·7 | 65 |
| 192·38 | 20 | 177·62 | 70 |
| 189·50 | 25 | 176·54 | 75 |
| 187·16 | 30 | 175·46 | 80 |
| 185· | 35 | 174·92 | 85 |
| 183·38 | 40 | 174·2 | 90 |
| 182·12 | 45 | 173·14 | 95 |
| 181·58 | 50 | 172· | 100 |
| Boiling points. Fahr. | Per-centage strength. | Corresponding Sp. Gr. | |
| 178·5 | Proof. | ·9200 | |
| 179·75 | 10· | U.P. | ·9321 |
| 180·4 | 20· | ” | ·9420 |
| 182·1 | 30· | ” | ·9516 |
| 183·4 | 40· | ” | ·9600 |
| 185·6 | 50· | ” | ·9665 |
| 189· | 60· | ” | ·9729 |
| 191·8 | 70· | ” | ·9786 |
| 196·4 | 80· | ” | ·9850 |
| 202· | 90· | ” | ·9920 |
Obs. This method does not answer well with spiritous liquor above ‘proof,’ owing to the variations of their boiling point being so slight as not to be easily observed with accuracy; but with liquors under ‘proof,’ and particularly with wines, beer, and other fermented liquors, due care being observed, it gives results closely approximating to those obtained by distillation, and sufficiently accurate for all ordinary purposes. In testing strong alcoholic solutions it is, therefore, proper to dilute them with twice their bulk of water; and commercial spirits, with an equal bulk of water; the results obtained being doubled or tripled as the case may be.
d. From the EXPANSION of the LIQUID when heated: Silbermann’s DILATATOMETER. The expansion of alcohol between 0° and 212° Fahr. is triple that of water; and between 77° and 122° Fahr. it is much greater. Between -14° and -98° Fahr. the rate of expansion is about the ·00047th part in volume for every degree of Fahrenheit’s scale. The measurement of this expansion has been proposed as a new and ready method of alcoholometry, adapted to nearly all spirituous and fermented liquors. Silbermann’s instrument, which is based on it (see engr.), simply consists of a flat brass or ivory plate (A), on which are fixed a mercurial thermometer (D) graduated from 22° to 50° Cent. (= 77° to 122° Fahr.); and the DILATATOMETER (B), which is a glass pipette open at both ends. A valve of cork, or vulcanised india rubber, closes the tapering end (c); this valve is attached to a movable rod (C) which is fastened to the supporting-plate, and connected with a spring68 (f) and a handle (g) bearing a four-threaded screw, by which the lower orifice of the pipette can be opened or closed at will. In use, the pipette is filled with the liquor under examination, to a little above the zero point (0) on the scale. This is effected by suction, by means of a little piston of leather (i), which fits tightly in the long and wider limb of the pipette; the valve (d) being previously opened by turning the knob (h). The proper quantity of liquor being introduced, and the lower end closed, the piston is moved up and down two or three times, for the purpose of drawing the air-bubbles and absorbed air out of the liquid, the presence of which would vitiate the results of the trial. To allow the piston to be withdrawn without any shock, or the danger of dividing the column abruptly, the rod attached to it is made hollow throughout. In using it the operator applies the ball of his forefinger to the top of the piston-rod (E), in order to create a vacuum as he raises it; and then withdraws it, to readmit the air when he thrusts it down or removes it from the tube. The excess of liquid (if any) in the pipette is then run off until its upper surface is exactly level with the zero (0) of the scale, at 25° C., to which it is raised by immersion in a water bath of that temperature, as observed by the thermometer; which is done by very cautiously turning the rod which depresses the valve. The whole apparatus is now again immersed in the water bath; and, held by the upper portion of the plate, kept in gentle motion with the hand, until the temperature rises to exactly 50° C., when the coefficient of expansion is obtained, and hence also the proportion of alcohol—the scale of the instrument being so graduated, from actual experiments previously made upon mixtures of known composition, as to give, at once, the per-centage of alcohol by VOLUME (nearly).[14]
e. From the TENSION of the VAPOUR:—Geissler’s ALCOHOLOMETER. This method, for which we are indebted to M. Geissler, of Bonn, depends on the measurement of the tension or elastic force of the vapour of the liquid, as indicated by the height to which it raises a small column of mercury. The spirit, wine, or other liquor, of which it is desired to ascertain the strength, is put into the little flask (a), which, when completely filled, is screwed on to the curved glass-tube which contains the mercurial column (which is inverted for the purpose), and is closed by the stop-cock (b). The instrument (see engr.) is then placed erect, and the flask and lower part of the tube immersed in a water bath, as in the previous method. The number, on the graduated scale of the instrument corresponding to the height of the mercury, at the boiling point of the liquor under examination, gives the per-centage of alcohol by VOLUME (nearly).
This method furnishes approximative results with great facility and expedition; and, with proper care, these do not vary more than 1⁄3 to 1⁄2 of 1%, from those obtained by distillation. We find, that by having the diameter of the part of the tube at which the surface of the mercury is acted on by the vapour a little larger than that of the longer limb, and by previously abstracting the air from the sample, as in Silbermann’s method, or even by agitation and exposure in an open vessel, the two may be made to correspond almost exactly.
f. From the DIFFERENCE between the sp. gr. BEFORE and AFTER ebullitiom:—Taberié’s method and ŒNOMETER. The sp. gr. of the sample is first accurately determined by any of the usual methods. It is next carefully evaporated, in an open vessel, to one half its volume. The residuum, when cold, is made up with pure water to exactly its original measure at its original temperature, and the sp. gr. again ascertained. The difference between the two being due to the spirit originally present, furnishes the means of calculating a new sp. gr., from which the per-centage richness of the sample may be obtained by mere inspection of the Tables. The observed sp. gr. is the true one, whenever the liquor, after ebullition and restoration to its original volume, has the same sp. gr. as water (i. e., 1·000), at 60° Fahr. Taberié employs a peculiar instrument, which he calls an œnometer; but its use is not essential to his method of alcoholometry. The results are, of course, only approximative, though sufficient for all ordinary purposes. Prof. Mulder, however, says that he prefers it to any of the previous methods; and that the results, with care, are almost as accurate as those obtained by distillation.
g. By means of CARBONATE OF POTASH:—
g. a. (Brande’s Method.) The liquor for trial is poured into a long, narrow glass tube (graduated centesimally), until the vessel is half-filled, and, after the solution of about 12% or 15% of a strong solution of subacetate of lead, or a little finely powdered litharge, is agitated until the colour is entirely, or nearly removed. Anhydrous carbonate of potash, in powder, is next added, until it sinks undissolved, even after prolonged agitation of the liquid. The whole is now allowed to repose for a short time, when the alcohol is seen floating on the top of the aqueous portion of the liquid in a well-marked stratum. Its quantity, read off by means of the graduations of the tube, and doubled, gives the per-centage richness of the sample in alcohol, by volume.
This process answers well with cordials, wines, and the stronger ales; but with very weak liquors it is not to be relied on. The69 whole operation may be performed in two to five minutes, and (with these exceptions) furnishes very reliable approximative results. In most cases the decolouring part of the process may be omitted. The alcohol thus separated has a sp. gr. of from ·8061 to ·8118, and contains 3% or 4% of water; but for ordinary purposes it may be regarded as pure alcohol.
4. Alcoholometry of MINUTE QUANTITIES of liquid. When only a few drops, or a quantity too small for the application of the preceding methods, can be obtained, an organic analysis may be had recourse to, and the quantity of absolute alcohol calculated from that of the resulting carbonic anhydride and water; care being previously taken to free the sample from other volatile bodies, if it contains any of them.
Gen. commentary. The duties on spirits in England are charged on the number of proof gallons they contain, which is ascertained by gauging or weighing the spirit, and then trying its strength by Sykes’ hydrometer. The per-centage of proof spirit multiplied by the number of gallons gives the net amount of proof spirit to be charged.
‘Proof strength’ is an arbitrary standard, adopted for the purpose of facilitating calculations, for which it is well suited; although pure alcohol would, for this purpose, be more simple. As defined by Act of Parliament, 58 Geo. III, c. 28, “proof spirit” is such “as shall, at the temperature of 51° of Fahrenheit’s thermometer, weigh exactly twelve thirteenth parts of an equal measure of distilled water.”
Taking, therefore, water at 51° Fahr. as unity, the sp. gr. of “proof spirit” at 51° Fahr. is 12⁄13 of 1·000 or ·92308. When such spirit is raised to the temperature of 60° Fahr., its density is ·91984.
Spirit at “proof” contains very nearly equal weights of absolute alcohol and water; the exact proportions according to recent experiments are:—
| By Weight. | By Volume. | Sp. gr. at 60° Fahr. | |
| Bulk before admixture. | Bulk after admixture and condensation. | ||
| Alcohol. Water. | Alcohol. Water. | ||
| 100·00 + 103·08 | 100·00 + 81·80 | 175·23 | ·91984 |
| 49·24 + 50·76 | 57·06 + 46·68 | 100·00 | |
The standard alcohol of the Revenue authorities, and that on which Gilpin’s Tables are founded, is a spirit of the sp. gr. ·825 at 60° Fahr., which is said to contain, by weight, 89% of pure alcohol of ·796; and 92·6% of alcohol, by volume, which corresponds to about 62·5 o. p.
It is of great importance to the spirit dealer to be able to estimate correctly the number of ‘proof gallons’ in any quantity of his commodities, or in the whole or any portion of his stock, as disagreeable errors frequently result from ignorance on this point. Calculations of this kind are extremely simple. Thus, when we find, by the hydrometer, that a given sample of spirit is 10 per cent. over-proof, it means, that 100 gallons of such spirit contain as much alcohol as 110 gallons of proof spirit.
In over-proof spirit, the per-centage o. p. always represents the quantity of water which the given spirit requires to reduce it to proof. By adding this per-centage over-proof to 100, we obtain a number which, multiplied by any number of gallons, and divided by 100, gives the exact number of proof gallons which is contained in any quantity of the spirit referred to. Thus:—A puncheon of rum gauged at 91 galls., and shown by the hydrometer to be 21 o. p., contains—
| 21 o. p. of sample added to 100 | 121 |
| No. of gallons of rum | 91 |
| ——— | |
| 11011 |
In like manner when a spirit is said to be 11 u. p., or under-proof, it means that 100 gal. of such spirit contains 11 gal. of water, and 89 gal. of ‘proof spirit.’ By deducting the per-centage under-proof from 100, we not only obtain the number of proof gal. contained in 100 gal. of such spirit, but, as in the last case, a factor which multiplied by any number of gal., and divided by 100, gives the exact number of ‘proof gallons’ contained in any quantity of the given strength. Thus:—An ullage brandy piece containing 45 gal. of spirit at 10 u. p., would have the proof value of—
| Per cent. u. p. of sample 10, subtracted from 100 | 90 |
| No. of gall | 45 |
| ——— | |
| 4050 |
Or exactly 401⁄2 gallons.
The strength of absolute alcohol (sp. gr. ·7938) is estimated at 751⁄4% over-proof. It therefore contains 1751⁄4% of ‘proof spirit,’70 whilst proof spirit (sp. gr. ·91984) contains 57·06% of ‘absolute alcohol,’ both being by measure or volume. Thus—
And—
From which we derive the ‘constant multipliers’ 1·7525 (or roughly 13⁄4), and ·5706, applicable to any number of volumes or gallons. For—
and—
To ascertain what quantity of a spirit at any given strength is equiv. to or contains 100 lbs. of absolute alcohol, we have only to divide the constant number 2207·7 by the proof value per cent. of such spirit.[15] Thus—for a spirit 12 u. p.—this would be
and—
That is, 251⁄10 gal. of such spirit would contain 100 lbs. of absolute alcohol.
By removing the decimal point one place to the right, we have the equiv. measure of 1000 lbs. By removing it one, two, or three places to the left, we have it respectively for 10 lbs., 1 lb., and 1⁄10 lb.; from which the equiv. for all other weights may be easily obtained.
By reversing the above operation, the measure of alcohol corresponding to any given weight of spirit, at any strength, may also be easily found.
The weight of 1 gal. of absolute alcohol being 7·938 lbs.; that of 1 gal. of proof spirit, 9·2 lbs,; and that of the ‘alcohol’ in 1 gal. of proof spirit, 4·53 lbs.; the weight of any number of gallons or volumes of either, and their equivalents, may be easily found. Thus:—
| gallons of alc. × 7·938 | = | lbs. weight of alc. |
| gallons pf. sp. × 9·2 | = | lbs. w. of pf. spt. |
and—
| gallons | of alc. × 16·121 | = | lbs. weight of pf. spt. |
| ” | pf. spt. × 4·53 | = | content in lbs. weight of alc. |
In these cases a knowledge of the first four rules of decimal fractions is necessary, or, at least, advantageous; as the Excise officers carry their calculations to two figures of decimals, or 1⁄100ths. Their plan is to reject the third decimal figure when less than 5; but to carry 1 to the next figure on the left hand, when it exceeds 5. Thus, 5·432 is set down as only 5·43; but 5·437 is written 5·44. In the delicate chemical processes of the laboratory, even greater accuracy is observed.
Formerly, spirit was said to be 1 to 3, 1 to 4, &c., over-proof, by which it was meant that 1 gal. of water added to 3 or 4 gals. of such spirit would reduce it to ‘proof.’ On the other hand, 1 in 5, or 1 in 8, under-proof, meant that the 5 or 8 gals., as the case might be, contained 1 gal. of water, and the remainder represented the quantity of ‘proof spirit.’ This method of calculation has now long given way to the ‘centigrade system,’ which not only admits of greater accuracy, but is quite as simple. It should be adopted by every spirit-dealer in England, from being that which is employed by the Revenue officers, whose ‘surveys’ it is absolutely necessary that the trader should understand, in order that his own estimation of his stock and his business calculations should correspond with theirs.
Several other methods of alcoholometry, besides those already noticed, have been adopted at various times, but the majority of them possess so little accuracy as to be quite inapplicable to the purposes of trade, and of the laboratory. Thus, the strength was at one time estimated by what was called the ‘proof.’ A little of the spirit was poured upon a small quantity of gunpowder, contained in a spoon or saucer, so as just to moisten it, and was then inflamed. If at the end of the combustion the gunpowder took fire, the spirit was held to be ‘above proof,’ if it only languidly fizzed away, or slowly burnt, the spirit was said to be ‘proof,’ but if the gunpowder failed to ignite, the spirit was esteemed ‘below proof.’ Hence arose the terms ‘proof’ and ‘proof spirit,’ which have since been adopted by Act of Parliament. Another method was that of dropping oil into the spirit; if the oil floated, the spirit was considered to be ‘under proof,’ if it sunk, it was rated as ‘proof’ or ‘over-proof.’ The ‘gunpowder test’ is quite fallacious; for, if a certain quantity of a spirit is capable of firing the gunpowder, a little excess of a spirit 20% or 25% stronger will often fail to do so, so much water being formed as to prevent the ignition. The ‘Preuve d’Holland’ test, of the French, or the ‘BEAD,’ is still frequently employed by persons unacquainted with the use of the hydrometer. It consists in shaking the spirit in a phial, and observing the size, number, and duration of the bubbles or beads, as they are called. The larger and more numerous these are, and the more rapidly they break and disappear, the stronger the spirit is presumed to be. This method is unreliable, as the presence of sugar or acid, even in minute quantities, will sometimes give to a weak sample the appearance of one many degrees stronger. Lovi’s beads are also often employed to ascertain approximately71 the strength of spirit, when a hydrometer is not at hand.
The insufficiency of most of the methods of alcoholometry here referred to, throws us back on the Revenue System (Sykes’ hydrometer), or on the specific gravity for unsweetened spirits. For sweetened spirits, as cordials, wines, beers, &c., there are none of the tests which give such accurate results as the distillation test, previously described as the Revenue Method.
The spirituous liquors of commerce being sold by measure, and not by weight, the methods of alcoholometry which give the results, per cent., by volume, are those we have chiefly explained. In the laboratory, the method by weight is that most generally employed in delicate processes and in analyses. By weight, the per-centage of alcohol remains the same for all temperatures, for the same sample; whilst by volume, the per-centage varies with the temperature of the liquid. This variation explains the cause of many of the sudden apparent decreases and increases, which occur in large stocks of spirits. Persons purchasing spirits during very warm weather, and paying for them according to their apparent quantity and strength, lose considerably by selling the same spirit when the weather becomes colder, without being conscious of such loss from the hydrometer. The reason of this is obvious, for, whilst the relative proportions of the alcohol to the water continue the same, the sp. gr. and the volume alter with the temperature; the latter being increased by warmth, and decreased by cold, in exact opposition to the former. Accuracy requires, in all cases, that a spirituous liquor should be tested for its strength at the temperature at which it was measured; and measured at the same temperature at which its strength was determined.
A consideration of these facts has led some of the great houses to introduce the system of weighing their spirits, instead of measuring them, the weight of an imperial gallon at 60° Fahr. being taken as the standard gallon. This is the method adopted by the Inland Revenue, at all distilleries, for assessing the duty, and will be readily understood by the following example:—
| Cwts. | qrs. | lbs. | ||
| Gross weight of full cask | = | 13 | 2 | 27 |
| Tare | = | 2 | 2 | 5 |
| —— | —— | —— | ||
| Net weight of spirit | = | 11 | 0 | 22 |
or 1254 lbs. Let us suppose the hydrometer indication to be 43·0, the weight per imperial gallon would be 8·903 lbs. (see Table VI), and 1254 ÷ 8·903 = 140 gallons.
A = Indication on Sykes’ Hydrometer. B = Weight per Gallon. A B A B A B A B A B 0 8·145 8 8·509 6 8·878 4 9·264 2 9·667 2 8·157 21 8·512 8 8·881 6 9·267 4 9·671 4 8·161 2 8·516 42 8·885 8 9·271 6 9·674 6 8·164 4 8·519 2 8·889 63 9·275 8 9·678 8 8·168 6 8·523 4 8·892 2 9·279 84 9·682 1 8·171 8 8·526 6 8·896 4 9·283 2 9·686 2 8·174 22 8·530 8 8·899 6 9·286 4 9·690 4 8·178 2 8·533 43 8·903 8 9·290 6 9·694 6 8·181 4 8·537 2 8·907 64 9·294 8 9·698 8 8·185 6 8·540 4 8·911 2 9·298 85 9·702 2 8·188 8 8·544 6 8·914 4 9·302 2 9·706 2 8·191 23 8·547 8 8·918 6 9·305 4 9·710 4 8·195 2 8·551 44 8·922 8 9·309 6 9·714 6 8·198 4 8·554 2 8·926 65 9·313 8 9·718 8 8·202 6 8·558 4 8·929 2 9·317 86 9·722 3 8·205 8 8·561 6 8·933 4 9·321 2 9·726 2 8·208 24 8·565 8 8·936 6 9·324 4 9·730 4 8·212 2 8·568 45 8·940 8 9·328 6 9·733 6 8·215 4 8·572 2 8·944 66 9·332 8 9·737 8 8·219 6 8·575 4 8·947 2 9·336 87 9·741 4 8·222 8 8·579 6 8·951 4 9·340 2 9·745 2 8·225 25 8·582 8 8·954 6 9·344 4 9·749 4 8·229 2 8·586 46 8·958 8 9·348 6 9·753 6 8·232 4 8·589 2 8·962 67 9·352 8 9·757 8 8·236 6 8·593 4 8·965 2 9·356 88 9·761 5 8·239 8 8·596 6 8·969 4 9·360 2 9·765 2 8·242 26 8·600 8 8·972 6 9·363 4 9·769 4 8·245 2 8·603 47 8·976 8 9·367 6 9·773 6 8·249 4 8·607 2 8·980 68 9·371 8 9·777 8 8·252 6 8·610 4 8·984 2 9·375 89 9·781 6 8·255 8 8·614 6 8·987 4 9·379 2 9·785 2 8·258 27 8·617 8 8·991 6 9·382 4 9·789 4 8·262 2 8·620 48 8·995 8 9·386 6 9·792 6 8·265 4 8·624 2 8·999 69 9·390 8 9·796 8 8·269 6 8·628 4 9·002 2 9·394 90 9·800 7 8·272 8 8·631 6 9·006 4 9·398 2 9·804 2 8·275 28 8·635 8 9·009 6 9·401 4 9·808 4 8·279 2 8·639 49 9·013 8 9·405 6 9·812 6 8·282 4 8·642 2 9·017 70 9·409 8 9·816 8 8·286 6 8·646 4 9·021 2 9·413 91 9·820 8 8·289 8 8·649 6 9·024 4 9·417 2 9·824 2 8·292 29 8·653 8 9·028 6 9·420 4 9·828 4 8·296 2 8·656 50 9·032 8 9·424 6 9·832 6 8·299 4 8·660 2 9·036 71 9·428 8 9·836 8 8·303 6 8·663 4 9·039 2 9·432 92 9·840 9 8·306 8 8·667 6 9·043 4 9·436 2 9·844 2 8·309 30 8·670 8 9·046 6 9·440 4 9·848 4 8·313 2 8·674 51 9·050 8 9·444 6 9·852 6 8·316 4 8·677 2 9·054 72 9·448 8 9·856 8 8·320 6 8·681 4 9·058 2 9·452 93 9·860 10 8·323 8 8·684 6 9·061 4 9·456 2 9·864 2 8·326 31 8·688 8 9·065 6 9·459 4 9·868 4 8·330 2 8·692 52 9·069 8 9·463 6 9·872 6 8·333 4 8·695 2 9·073 73 9·467 8 9·876 8 8·337 6 8·699 4 9·076 2 9·471 94 9·880 11 8·340 8 8·702 6 9·080 4 9·475 2 9·884 2 8·343 32 8·706 8 9·083 6 9·479 4 9·888 4 8·347 2 8·709 53 9·087 8 9·483 6 9·892 6 8·350 4 8·713 2 9·091 74 9·487 8 9·896 8 8·354 6 8·716 4 9·095 2 9·491 95 9·900 12 8·357 8 8·720 6 9·098 4 9·495 2 9·904 2 8·361 33 8·723 8 9·102 6 9·498 4 9·908 4 8·364 2 8·727 54 9·106 8 9·502 6 9·913 6 8·368 4 8·730 2 9·110 75 9·506 8 9·917 8 8·371 6 8·734 4 9·114 2 9·510 96 9·921 13 8·375 8 8·737 6 9·117 4 9·514 2 9·925 2 8·378 34 8·741 8 9·121 6 9·517 4 9·929 4 8·382 2 8·745 55 9·125 8 9·521 6 9·934 6 8·385 4 8·748 2 9·129 76 9·525 8 9·938 8 8·389 6 8·752 4 9·132 2 9·529 97 9·942 14 8·392 8 8·755 6 9·136 4 9·533 2 9·946 2 8·395 35 8·759 8 9·139 6 9·537 4 9·950 4 8·399 2 8·763 56 9·143 8 9·541 6 9·955 6 8·402 4 8·766 2 9·147 77 9·545 8 9·959 8 8·406 6 8·770 4 9·151 2 9·549 98 9·963 15 8·409 8 8·773 6 9·154 4 9·553 2 9·967 2 8·412 36 8·777 8 9·158 6 9·557 4 9·972 4 8·416 2 8·781 57 9·162 8 9·561 6 9·976 6 8·419 4 8·784 2 9·166 78 9·565 8 9·981 8 8·423 6 8·788 4 9·170 2 9·569 99 9·985 16 8·426 8 8·791 6 9·173 4 9·573 2 9·989 2 8·429 37 8·795 8 9·177 6 9·576 4 9·994 4 8·433 2 8·799 58 9·181 8 9·580 6 9·998 6 8·436 4 8·802 2 9·185 79 9·584 8 10·003 8 8·440 6 8·806 4 9·189 2 9·588 100 10·007 17 8·443 8 8·809 6 9·192 4 9·592 2 8·446 38 8·813 8 9·196 6 9·596 4 8·450 2 8·817 59 9·200 8 9·600 6 8·453 4 8·820 2 9·204 80 9·604 8 8·457 6 8·824 4 9·207 2 9·608 18 8·460 8 8·827 6 9·211 4 9·612 2 8·464 39 8·831 8 9·214 6 9·615 4 8·467 2 8·835 60 9·218 8 9·619 6 8·471 4 8·838 2 9·222 81 9·623 8 8·474 6 8·842 4 9·226 2 9·627 19 8·478 8 8·845 6 9·229 4 9·631 2 8·481 40 8·849 8 9·233 6 9·635 4 8·485 2 8·853 61 9·237 8 9·639 6 8·488 4 8·856 2 9·241 82 9·643 8 8·492 6 8·860 4 9·245 2 9·647 20 8·495 8 8·863 6 9·248 4 9·651 2 8·498 41 8·867 8 9·252 6 9·655 4 8·502 2 8·871 62 9·256 8 9·659 6 8·505 4 8·874 2 9·260 83 9·663
⁂ For further information in connection with Alchoholometry see Alcohol, Beer, Brewing, Distillation, Ebullioscope, Hydrometer, Hydrometry, Liqueurs, Malt-Liquors, Organic Substances, Saccharine, Specific Gravity, Spirit, Sugar, Syrups, Tinctures, Wine, Wort, &c. 73&c.
ALCOHOL; EFFECTS OF ALCOHOLISM. Without entering into the controversy as to whether the moderate consumption of alcohol, or its total disuse, is the more conducive to personal health and comfort—whether, as Dr Anstie and others have asserted it acts, when prudently taken, as a food—or whether, as other medical authorities contend, even its moderate use is a disturbing factor in the human economy—there need be no qualification of the assertion, that when the drinking of spirituous liquids of any kind is indulged in to excess, the habit, if persisted in, must sooner or later terminate in impaired health, serious disease, and premature death.
A powerful array of facts could be brought in support of this statement. For instance, in Nelson’s statistics we find it mentioned that—
| A temperate person’s chance of living is— | An intemperate person’s chance of living is— | ||||||||
| At | 20 | = | 44·2 | years. | At | 20 | = | 15·6 | years. |
| ” | 30 | = | 36·5 | ” | ” | 30 | = | 13·8 | ” |
| ” | 40 | = | 28·8 | ” | ” | 40 | = | 11·6 | ” |
| ” | 50 | = | 21·25 | ” | ” | 50 | = | 10·8 | ” |
| ” | 60 | = | 14·285 | ” | ” | 60 | = | 8·9 | ” |
The average duration of life after the commencement of habits of intemperance is—
| Among | mechanics, working and labouring men | 18 | years. |
| ” | traders, dealers, and merchants | 17 | ” |
| ” | professional men and gentlemen | 15 | ” |
| ” | females | 14 | ” |
Again, Dr Dickinson, writing “on the morbid effects of alcohol in persons who trade in liquor,” gave the results of an examination of 149 traders in liquor, as compared with 149 persons of various trades. The general results were diseases of the liver much more common in those who dealt in alcoholic drinks. In the lungs tubercle affected sixty-one persons of the alcoholic, forty-four of the non-alcoholic.
Tubercle in the brain, liver, kidneys, spleen, bowels, mesenteric glands, and peritoneum were twice as common in the alcoholic as in the non-alcoholic. The verdict, therefore, is unavoidable that alcohol (in excess) engenders tubercle in the brain, inflammations, atrophy, hæmorrhages; in the heart and vessels atheroma, hypertrophy, and other affections, were all more common in the alcoholic than in the non-alcoholic series. The evidence in kidney disease did not appear so conclusive, but some forms of kidney disease appear to be increased. The author sums up thus:—“Alcohol causes fatty infiltration and fibroid encroachment; it engenders tubercle, encourages suppuration, and retards healing; it produces untimely atheroma, invites hæmorrhage, and anticipates age. The most constant fatty change, replacement by oil of the material of epithelial cells and muscular fibres, though probably nearly universal, is most noticeable in the liver, the heart, and the kidney.”
Alcohol also seems to be the cause of special diseases, besides those more common and generally known ones, delirium tremens, alcoholism, &c. Of these we may mention one recorded by M. Galezowski, a peculiar affection of the eyes, which the doctor found very74 prevalent during the siege of Paris in 1870-1. In the five months of the siege fifty patients were affected by it, whilst during the twelve months preceding the siege only nineteen were to be found. Dr Galowski ascribed the malady to the habit of taking alcoholic drinks in the morning fasting. A peculiar kind of palsy has also been referred to alcoholic poisoning.
The following table, compiled by Dr Joseph Williams, lends support to the fact that an indulgence in alcohol is either the cause of insanity, or that it tends to its increase:
| Total admission. | Proportion caused by intemperance. | |
| Charenton | 855 | 134 |
| Bicêtre and Salpêtrière | 2012 | 414 |
| Bordeaux | 156 | 20 |
| Turin, 1830-31 | 158 | 17 |
| Turin, 1831-36 | 390 | 76 |
| Gard | 209 | 4 |
| United States | 551 | 146 |
| Palermo | 189 | 9 |
| Caen | 60 | 16 |
| Dundee | 14 | 4 |
| M. Parchappe | 167 | 46 |
| M. Batten | 288 | 54 |
| ——— | ——— | |
| 5019 | 940 |
Commenting on these figures, Mr Walter Blyth remarks, “There may be another explanation of the fact that many mad people have been great drinkers. A large proportion of those subject to insanity are driven by their morbid minds to drink; so that it may be that insanity causes drink, and not drink causes insanity.”
Many medical writers who are no advocates for the total abandonment of alcohol limit its consumption, in healthy people, to one or two fluid ounces a day, in the form of wine, beer, or spirits and water; two fluid ounces is, we believe, the quantity apportioned daily to every able-bodied seaman in the Royal Navy. Any slight habitual departure from this standard—even when the evidences of excess are not perceptible to others—all authority, historical, pathological, and physiological (unless it be given as a medicine), shows to be injurious. The researches of Anstie, Parkes, and Count Wollowicz, appear to prove that any quantity of alcohol exceeding an ounce and a half taken by an adult showed itself in the urine, a circumstance which these writers look upon as tending to show that the system has taken more alcohol than can be used in the body itself. In slight doses the action of alcohol is to produce a sedative effect upon the nerves, to redden slightly the lining membrane of the stomach, and to stimulate the secretion of the gastric juice.
Thus, in small doses alcohol may, and doubtless does, promote appetite. In excess, however, all these effects are turned to evil, and then ensue an inflammatory condition of the stomach, compression of the gland ducts from thickening of the tissue around them, excessive mucous secretion, and great loss of appetite. When carried into the circulation it greatly increases the force of the heart’s action, and at the same time paralyses, as it were, the restraining nervous supply to the arteries and small vessels, so that they can no longer oppose themselves to the blood-current, but dilate. This action in a small degree, occurring in persons of a weak and languid circulation, is no doubt beneficial; on the other hand, when in excess, it is most dangerous, and is a cause of the greater part of the diseases of the heart and great vessels.
“There appears to be a slight fall of temperature with moderate doses of alcohol, a very decided fall with excessive doses; the muscular and nervous systems are transitorily stimulated, and may do more work when small doses are given in cases of fatigue, but in other cases there is a marked torpor of the nervous and a want of co-ordination of the muscular system.”—Blyth.
Notwithstanding the researches of Percy, Strauch, Masing, Lallemand, Duroy, Parkes, Dupré, Anstie, Thudichum, and others, there is still a considerable divergence of opinion as to how alcohol is eliminated from the body. By some of the authorities just named it is affirmed to be eliminated as aldehyd, by others as carbonic acid; as to the latter, the experiments of Dr E. Smith show that the carbonic acid is decreased when brandy and gin are drunk, and increased by rum.
The only probable supposition, which facts support, tends to show that the alcohol is turned into acetic acid in the body, some of which unites with potash and other bases, and some is destroyed. All are pretty well agreed that in the form of spirits alcohol as a food is valueless, but that in the form of beer and wine it is possessed of a slight dietetic power, naturally varying with the amount and nature of the different substances held in solution in these beverages.
The imports of spirits into this country, in the seven years from 1850 to 1857, amounted to 70,740,980 gallons; whilst the imports in the seven years following, viz. from 1857 to 1864, were 78,016,071 gallons, showing an increase of 7,305,091 gallons. The population has, however, increased in the time, and a deduction on that account, as well as correction on one or two other heads, are required; still, that there is an increase is indisputable.
As respects France, a considerable increase in the consumption of spirits has taken place of late years, as the following table by M. Husson will illustrate:75
| Litres. | Litres. | ||||||
| From | 1825 | to | 1830 | 8·96 | yearly. | ·024 | daily. |
| ” | 1831 | ” | 1835 | 8·74 | ” | ·023 | ” |
| ” | 1836 | ” | 1840 | 10·15 | ” | ·026 | ” |
| ” | 1841 | ” | 1845 | 11·14 | ” | ·031 | ” |
| ” | 1846 | ” | 1850 | 11·03 | ” | ·030 | ” |
| ” | 1851 | ” | 1854 | 14·25 | ” | ·039 | ” |
In the United States, during the period from 1807 to 1828, the average was 27 litres for every inhabitant, which is even greater than the highest of the two sets of figures just quoted.
The demoralisation of the French army during the late Franco-Prussian war has been also unanimously ascribed to the excessive consumption of spirituous liquids.
The following results of an inquiry instituted in 1870 by the Massachusetts Board of Health into the comparative sobriety of different nations are gathered from an able paper which appeared in the ‘Medical Times and Gazette’ of April 15th, 1872, by Dr Druitt, in which he dissects and summarises the results in question. Dr Druitt writes:
“Highest in the scale of temperance come the Turks and Arabs; next the Iberians, Levantines, Greeks, and Latin races; lower down the Japanese, Scandinavians, Belgians, and the Irish Celt; lowest of all the so-called Anglo-Saxon of either continent.”
Professor Levi contributes to our knowledge on this subject by giving the following statistics:—In 1860 the committals for drunkenness in England and Wales were 88,000, and in 1870 134,000, an increase of 50 per cent.
In Manchester the increase from 1860 to 1870 was 375 per cent., or computed according to the increase of population 35·3 per cent. In London drunkenness is in the proportion of 5·43 per 1000, in Leeds 7·40, in Manchester 31·13, and in Liverpool 42·82. It must, however, be remembered that these figures are based on mere committals, which greatly depend on the activity of the police, and the noisy or quiet character of the drunkard.
We quote the following from Dr Blyth’s work on ‘Hygiene,’ without, however, attempting either to endorse or controvert what he says on the subject.
“Whether is Alcohol necessary or not. All experience, both at home and abroad, shows by facts that cannot be disputed that a person can do quite as hard work without alcohol as with it; and probably as the limits between moderation and excess are easily passed, and as the generality of mankind, even without intending it, err on the latter side, the result is that a comparison between total abstainers and even temperate men generally terminates in favour of the former. It would appear that total abstainers live longer, are better citizens, and can do more work than the rest of mankind. The figures of the “United Kingdom Temperance and General Provident Institution” go far to prove the above. This insurance society is divided into two sections. One section consists of abstainers, the other of persons selected as not known to be intemperate. The claims for five years anticipated in the temperance section were £100,446, but the actual claims were only £72,676. In the general section of the anticipated claims were £196,352; the actual claims no less than £330,297. In war the march of 2000 miles in his War of Independence by Cornwallis and his troops (1783), the Maroon war of Jamaica, the 400 miles’ march of an English army across the Desert from Komer, on the Red Sea, a march of 1000 miles in the Kaffir war, experiences at sieges, in action, in hot, temperate, and cold climates, where abstinence was either forced through circumstances or followed, shows to every unprejudiced mind that soldiers endure more fatigue, are healthier, and fight better, without stimulants than with them; and this fact is endorsed by every commander of the present day.
The excess and abuse of spirits, as before remarked, lost the French their military prestige in the Franco-German war. In very hot and very cold climates the Indian observers and the Arctic explorers all unite in condemning its (that is, the use of alcohol) use in the slightest excess, or even in moderate doses. It does not warm the body in cold climates, and the reaction that follows the exciting of the circulation is followed by a dangerous depression; whilst in hot it combines with the climate, and quickly produces disease.”
ALCOHOLIC DRINKS, EFFECTS OF. In addition to the serious injury to health caused by an excessive or imprudent indulgence in spirituous stimulants (see previous article), even a moderate and not injudicious use of them may often be attended with very disagreeable consequences—a more or less mild or modified form of poisoning, in fact—if the beverages themselves are, as very frequently happens, contaminated, either accidentally or intentionally, with certain objectionable ingredients. These ingredients are described under the articles Beer, Wines, and the various SPIRITS, such as Gin, Brandy, Absinthe, &c. Of spirit drinking it may be observed, that this dangerous practice is intensified by what is to be feared is the too prevalent custom of taking them undiluted, or “neat,” as it is termed. There is no doubt that they constitute the very worst form of alcoholic drinks, and shorten the lives of those who indulge in them to excess more summarily than any other intoxicating potion. The greatest and most ineradicable drunkards are almost always found to be spirit drinkers.
Liebig remarked that less bread was consumed in families where beer was drunk, and there seems to be little doubt that the different species of beer, including porter and ale, when76 pure and free from adulteration, act, although in a small degree, as food. Probably there are some who will agree with, whilst others will dissent from, Benjamin Franklin, who said “there was more sustenance in a penny loaf than in a gallon of beer.” The starchy extractive matters of the beer no doubt perform the same function in the animal economy that sugar does. It is well known that those who drink freely of beer mostly become corpulent, as witness the portly forms of draymen. The hop contained in the beer has doubtless tonic and stomachic qualities. We can speak with less certainty about the free acids contained in malt fluids. It is very certain that some people cannot drink a glass of beer without experiencing rheumatic pains in the joints, which effect is generally ascribed to the acidity of the beer; but which is really supposed to be due to the decreased elimination of urea and pulmonary carbonic acid from the system caused by the alcohol of the beer.
The heavy low-priced beers occasion drunkenness of a peculiarly violent and savage kind, a fact which strongly favours the inference that this form of intoxication is due to some toxic agent, used as an adulterant. Of wines, the clarets and subacid wines are undoubtedly antiscorbutic in properties, and light wines as beverages are preferable to the stronger. Port, sherry, beer, stout, and ale are almost universally condemned in cases where there is a tendency to gout. The light clarets and Rhine wines are far more desirable beverages when this is the case, and the German wines are said to be valuable drinks in many lithic affections. It seems probable that the ethers and the vegetable salts, together with the sugar contained in wines, perform the most important part in the human economy.
It has been proposed to introduce the red subacid wines as drinks for our sailors, because of their antiscorbutic qualities. Some of the alcoholic drinks prepared in India frequently cause temporary madness.
ALCOHOLISM. Alcohol; EFFECTS OF ALCOHOLISM.
AL′COHOLS. In chemistry, a term applied to compounds possessing a composition, formulæ, and chemical properties similar to those of ordinary alcohol. They form a series presenting an unmistakable symmetry, and differ from one another by well-marked gradations, as shown below:—
| Methyl-alcohol (wood spirit). | CH4O |
| Ethyl-alcohol (ordinary alcohol) | C2H6O |
| Amyl-alcohol (füsel-oil) | C5H12O |
| Capryl-alcohol | C8H18O |
| Cetyl-alcohol | C16H34O |
| &c., &c. |
Alcohols. In commerce, pure spirits of a greater strength than about 58 o. p. (sp. gr. 8335), or containing more than about 85% by WEIGHT, or 90% by VOLUME, of pure alcohol, are commonly so called.
Alcohols. In perfumery, rectified spirit of wine, or commercial alcohol, holding essential oils or other odorous matters in solution.
Alcohols. In Fr. pharmacy, alcoholic tinctures and essences.
ALCOOLATIFS (alcoölatifs). [F.] Syn. ALCOHOLATI′VA, L. In Fr. pharmacy, alcoholic solutions of liniments, embrocations, &c., whether made by distillation, maceration, or solution.
ALCOOLATS (alcoölats). [Fr.] In Fr. pharmacy, spirits; applied by Béral, Henry and Guibourt, and others, to medicated distilled spirits.
ALCOOLATURES (alcoölatures). [Fr.] Syn. Alcoholatu′′ra, L. In. Fr. pharmacy, alcoholic tinctures, elixirs, &c. M. Béral confines the term to vegetable juices preserved by alcohol.
ALCOOLES (alcooölés). [Fr.] Tinctures; the ‘teintures alcoholiques’ of the Fr. Codex.
ALCOOLIQUES (alcoöliques). [Fr.] Syn. Alcohol′ica, L. In Fr. pharmacy, alcoholic or spirituous solutions. (Béral.)
AL′CORNINE (-nĭn). [Eng., Fr.] Syn. Alcor′nocine (-sĭn); Alcor′neum, Alcorni′na, L. A crystallisable substance, apparently intermediate between fat and wax, discovered by Biltz, in alcornoco bark.
ALCORNO′CO. Syn. A.-bark; Alcornoque, Fr.; Alkornoc, A.-rind, Ger. The bark of an unknown tree of South America. It is astringent and bitter, and has been highly extolled as a specific in phthisis; but appears to possess little medicinal virtue. The bark of the young branches of the cork tree (quercus suber), used in tanning, is also sometimes called alcornoco-bark; but possesses none of the characters of the former article.
AL′DECAY. The galls on the leaves of myrobalanus chebula (Gaertn.), a forest-tree of Bengal. Equal to the best oak-galls.
AL′DEHYD (-hīd). [al-(cohol)-dehyd (rogenatus).] C2H4O. Syn. Hydrated oxide of acetyle; Hydrate of othyle*; Hydroxide of O.* Literally, dehydrogenated alcohol. In chemistry, a peculiar ethereal liquid, first obtained in a pure form by Liebig, from alcohol. It is produced under various circumstances, particularly during the destructive distillation of certain organic matters, and in several processes of oxidation. The following are the most convenient methods of preparing it:—
Prep. 1. (Liebig.) Sulphuric acid, 3 parts; is diluted with water, 2 parts; and as soon as the mixture has cooled, alcohol of 80%, 2 parts, is added; and, subsequently, peroxide of manganese (in fine powder), 3 parts. The whole, after agitation, is then distilled at a very gentle heat, from a spacious retort into a receiver surrounded with ice, the connection between the two being perfectly air-tight. The process is continued until frothing commences, or the distillate becomes acid which generally occurs when about one third (3 parts) has passed over. The distillate is next agitated in a retort, with about its own weight of fused chloride of calcium, in powder; after which about one half only is drawn over at a very77 gentle heat (85° to 90° Fahr.), by means of a water bath. This rectification is repeated in a precisely similar way. The last distillate is ANHYDROUS ALDEHYD only slightly contaminated with foreign matters.
2. (Liebig.) Aldehyd-ammonia, 2 parts, is dissolved in an equal weight of distilled water; and, after being placed in a retort, sulphuric acid, 2 or 3 parts, previously diluted with rather more than its own weight of distilled water, and allowed to cool, is added. The whole is now distilled, by means of a water bath, into a receiver surrounded with ice, or (preferably) a freezing-mixture, the temperature of the bath at first being very low, and the operation being stopped as soon, or rather before the water begins to boil. The distillate is then placed in a retort connected with a well-cooled receiver, as before; and after all the joints are made perfectly tight, powdered fused chloride of calcium, in weight equal to that of the liquid in the retort, is added through the tubulature. The heat produced by the hydration of the chloride causes the distillation to commence, after which it is carried on, by means of a water bath, at a temperature ranging from 80° to 82° Fahr. This rectification being very carefully repeated, the last distillate is PURE ANHYDROUS ALDEHYD.
Prop., &c. Limpid, colourless, ethereal, neutral, inflammable; mixes in all proportions with alcohol, ether, and water; odour peculiar, penetrating, and, when strong, exceedingly suffocating, the vapour, in quantity, producing spasmodic contraction of the thorax; boils at 72° Fahr. (70°—Ure, 5th ed.); sp. gr. ·790 at 60°, and ·800 at 32° Fahr.; sp. gr. of vapour, 1·532; by exposure to air it is gradually converted into acetic acid, and speedily so under the influence of platinum-black; heated with caustic potash, a brown substance resembling resin (ALDEHYD-RESIN) is formed; gently heated with protoxide of silver, or its solutions, metallic silver is deposited on the inner surface of the vessel, in a uniform and brilliant film, whilst ALDEHYDATE OF SILVER remains in solution; heated with hydrocyanic acid it yields ALANINE. By age, even in close vessels, it passes into one or more isomeric compounds (ELALDEHYDE; METALDEHYDE), with change of properties. Aldehyde for experiments should, therefore, be always recently prepared; and it must be kept in a well-stopped bottle, in a very cold place, and preferably in ice.
Obs. Aldehyd is important for its assumed position in the acetyl-series, and the part which it plays in the process of acetification, &c. The word is now also commonly employed, by chemists, as a generic term for any organic substance which, by assimilating two atoms of hydrogen, yields, or would yield, a compound having the composition or properties of an alcohol; or which, by taking up one atom of oxygen, yields an acid. Many of the essential oils (as those of almonds, cinnamon, and cumin) are composed principally of bodies which may thus be called aldehyds. One of the most valuable properties of these substances, is their strong tendency to combine with the bisulphites of ammonium, potassium, and sodium; and by which they may be separated from complex mixtures.
AL′DEHYD-AMMO′NIA (-hĭd-). An ammonia-compound of aldehyd, discovered by Döbereiner and Liebig.
Prep. (Liebig.) Aldehyd (of process No. 1, above) is mixed with an equal volume of ether,[16] in a flask surrounded with ice, or (what is better) a freezing-mixture; and is then saturated with dry gaseous ammonia. The crystals which soon form, after being washed with ether, and dried by means of bibulous paper and a short exposure to the air, are pure aldehyd ammonia.
Prop., &c. It smells like a mixture of turpentine and ammonia; melts at 165° to 170°; volatilises, unchanged, at 212° Fahr.; decomposed by exposure to the air; very soluble in water; soluble in alcohol, and more or less so in most other menstrua, except ether; acids decompose it. With sulphuretted hydrogen it forms thialdine.—Use. Chiefly to make pure aldehyd (which see).
AL′DER (awl′-). Syn. Al′der-tree; Al′nus (ăl-), L.; A. glutino′sa (Gaertn.); Betu′la alnus, Linn.; Aune, Aulne, Fr.; Erle, Ger. A well-known English tree, chiefly growing in moist grounds near rivers. Its wood is used for hurdles, for various articles of turnery and furniture, and when converted into charcoal, for making gunpowder; it possesses considerable durability under water; but is otherwise of little value. Bark and leaves very astringent, and reputed vulnerary; decoction used as a gargle in sore throat, and, in double the dose of cinchona, as a febrifuge in agues; bark and sap used in dyeing and tanning. The following belong to different nat. orders and genera to the preceding:—
Alder, Black. Syn. Win′ter-berry; Pri′nos verticilla′tus, Linn. A tree growing in the United States of America. Bark febrifuge, tonic, and astringent; berries tonic and emetic. (Bigelow.) It has been much recommended in dropsies, diarrhœa, intermittents, &c. Dose (of the dried bark), 1⁄2 to 1 dr., 3 or 4 times a day.
Alder-tree, Black. Syn. Berry-bearing alder-tree; Rham′nus fran′gula, Linn. A large shrub found in the woods and thickets of England, &c. Wood, BLACK DOG′WOOD; bark, bitter, emetic, purgative; used to dye yellow; root-bark, a drastic purgative; berries, purgative, emetic; unripe berries yield SAP-GREEN; charcoal of the wood esteemed the best for gunpowder.
ALE. Syn. Barley Wine*; Aile, Fr.; Weiss-bier, Ger.; Ael, Eale, Sax.; Cerevis′ia alba, C. lupula′ta, A′la*, Al′la*, L.78 Pale-coloured beer, prepared from lightly dried malt, by the ordinary process of brewing. The ale of the modern brewer is manufactured in several varieties, which are determined by the wants of the consumer, and the particular market for which it is intended. Thus, the finer kinds of Burton, East India, Bavarian, and other like ales, having undergone a thorough fermentation, contain only a small quantity of undecomposed sugar and gum, varying from 1 to 5 per cent. Some of these are highly ‘hopped,’ or ‘bittered,’ the further to promote their preservation during transit and change of temperature. Mild or sweet ales, on the contrary, are less attenuated by lengthened fermentation, and abound in saccharine and gummy matter. They are, therefore, more nutritious, though less intoxicating, than those previously referred to.
In brewing the finer kinds of ale, pale malt and the best East Kent hops of the current season’s growth, are always employed; and when it is desired to produce a liquor possessing little colour, very great attention is paid to their selection. With the same object, the boiling is conducted with more than the usual precautions, and the fermentation is carried on at a somewhat lower temperature than that commonly allowed for other varieties of beer. For ordinary ale, intended for immediate use, the malt may be all pale; but, if the liquor be brewed for keeping, and in warm weather, when a slight colour is not objectionable, one fifth, or even one fourth of ‘amber malt’ may be advantageously employed. From 41⁄2 lbs. to 6 lbs. of hops is the quantity commonly used to the quarter of malt, for ‘ordinary ales,’ and 7 lbs. to 10 lbs. for ‘keeping ales.’ The proportions, however, must greatly depend on the intended quality and description of the brewing, and the period that will be allowed for its maturation.
The stronger varieties of ale usually contain from 6 to 8% of ‘absolute alcohol,’ ordinary strong ale, 41⁄2 to 6%; mild ale, 3 to 4%; and table ale, 1% to 11⁄2%; (each by volume); together with some undecomposed saccharine, gummy, and extractive matter, the bitter and narcotic principles of the hop, some acetic acid formed by the oxidation of the alcohol, and very small and variable quantities of mineral and saline matter. For the adulterants of ale, see Porter. See Beer, Brewing, Fermentation, Malt-liquors, &c.
Ale, Dev′onshire White. A liquor once generally drunk, and still in demand, in the neighbourhood of Kingsbridge and Modbury, Devon.
Prep. Ordinary ale-wort (preferably pale) sufficient to produce 1 barrel, is slowly boiled with about 3 handfuls of hops, and 12 to 14 lbs. of crushed groats, until the whole of the soluble matter of the latter is extracted. The resulting liquor, after being run through a coarse strainer, and become lukewarm, is fermented with 2 or 3 pints of yeast; and, as soon as the fermentation is at its height, is either closely bunged up for ‘draught,’ or is at once put into strong stoneware bottles, which are then well corked and wired.
Obs. White ale is said to be very feeding, though apt to prove laxative to those unaccustomed to its use. It is drunk in a state of effervescence or lively fermentation; the glass or cup containing it being kept in constant motion, when removed from the mouth, until the whole is consumed, in order that the thicker portion may not subside to the bottom.
Ales, Med′icated. Syn. Bryt′oles; Brutolés, Fr.; Cerevis′iæ Medica′tæ, L. In pharmacy, ale prepared by macerating medicinal substances in it, either at the ordinary temperature of the atmosphere, or when heated; infusions and decoctions, in which ale or beer is employed as the menstruum. The old dispensatories enumerate several medicated ales; such as CEREVISIA OXYDOR′CICA, for the eyes; C. ANTI-ARTHRIT′ICA, for the gout; C. CEPHAL′ICA, for the head; C. EPILEP′TICA, against epilepsy; &c. Preparations of this kind are now seldom ordered by the faculty, and their use is chiefly confined to the practice of empirics, and to domestic medicine. Bark, rue, savine, antiscorbutic plants, aromatic bitters, and stomachics, are the substances most commonly administered in this way. Ale in which wormwood, gentian, orange-peel, and the like, have been steeped, taken warm early in the morning, is much esteemed as a restorative tonic by drunkards and dyspeptics. See Beer, Purl, &c.
ALE′BERRY. A beverage made by boiling ale with spice, sugar, and bread-sops; the last commonly toasted. A domestic remedy for a cold.
ALE′GILL (g hard). Ale or beer flavoured or medicated by infusing the leaves of ground ivy in it; pectoral, stomachic, and nervine.
ALE′WIFE. The clupea serrata, an American species of herring. Its proper name is a′loof, although the established pronunciation and common orthography is ale-wife.
ALEM′BIC. Syn. Moors′head†; Alem′bicus, L.; Alambic, Fr.; Destillirkolben, Ger. An old form of distillatory vessel usually made of glass or earthenware, but sometimes of metal. The body (a) which holds the liquid for distillation is called the CU′CURBIT; the upper part (b) the HEAD or CAP′ITOL; (c) is the RECEIVER. It is still employed in the79 laboratory, in the distillation of articles that are apt to spurt over into the neck of the common retort, and thus vitiate the product.
ALEUROM′ETER. Syn. Aleuromètre, Fr. An instrument for determining the quantity and quality of gluten in wheat-flour, invented by M. Boland. It essentially consists of a hollow copper cylinder, about 6 inches long, and 3⁄4 of an inch internal diameter. This tube has two principal parts; the one, about 2 inches long, is closed at the lower end, forming a kind of cup, into which the gluten is placed; it screws into the remainder of the cylinder. The cup being charged with a sample of gluten, and the upper part of the cylinder being screwed on, it is exposed in an oven, or (preferably) in an oil bath, to a temperature of 350 to 380° Fahr.[17] From the length of the tube the gluten occupies in swelling, as measured by a graduated scale, its quality is determined. The ‘crude gluten’ of good wheat-flour augments to four or five times its original volume, when thus treated; but that from bad flour does not swell, becomes viscid and semi-fluid, and generally gives off a disagreeable odour; whilst that of good flour merely suggests the smell of hot and highly baked bread.
AL′GA. (-gă). [L.] Sea-weed. A common name of grass-wrack (‘zostera marina’—Linn.), though not one of the algæ.
AL′GÆ. (ăl′-jē). [L. pl.] Syn. Al′gals; Algæ (DC.), Al′gales (Lindl.), L.; Algues, Varech, Fr.; Alge, Meergrass, Seegrass, Ger. Sea-weeds. In botany, an order of Thallogens living in water or very moist places, nourished throughout their whole surface by the medium in which they live, having no distinct axis of vegetation, and propagated by zoöspores, coloured spores, or tetraspores. Linnæus defines them—“plants, the roots, leaves, and stems of which are all in one.” The algæ consist either of simple vesicles lying in mucus, or of articulated filaments, or of lobed fronds formed of uniform cellular tissue. Those that vegetate in salt water are popularly called SEA-WEEDS (fu′ci, L.) and LA′VER (ulvæ, L.); those found in fresh water CONFER′VÆ. One of their divisions (the Zoöspermeæ) comprehends the lowest known forms of vegetable life, being merely adhering cells, emitting, at maturity, seeds or sporules having a distinct animal motion. In Oscillatorias, the whole plant twists and writhes spontaneously; and Zymenas actually copulate like animals. Some of the Algæ possess great beauty. In the lower grades the colour is green; in the higher, red or purple.
Prop., Uses, &c. None of the Algæ are poisonous. Several are nutritious, emollient, and demulcent, from containing mucilage (carrageenin), starch, sugar (mannite), and a little albumen; and are hence used as esculents. The ash from the dried weed varies in different varieties from 9% to fully 25%; and contains variable quantities of potassa, soda, lime, magnesia, iron, manganese, and silica, with sulphuric acid, phosphoric acid, chlorine, and a little iodine and bromine. (Schweitzer; Forchhammer; Gödechens.) Sea-weeds, their charcoal, and their ashes, have been long regarded as alterative and resolvent; and anti-phthisic virtues have been attributed to them by Laennec and others. They were formerly much given in scrofulous affections and glandular enlargements; but their use is now almost superseded by that of iodine and its preparations. Dr Stenhouse has proposed some of the algæ as furnishing an economical source of mannite. The sea algæ are used for manure; their ashes form KELP.
The following table, showing the results of several analyses of different kinds of algæ, and illustrating the very large amount of nitrogen contained in them, is from Mr Walter Blyth’s excellent dictionary of ‘Hygiene and Public Health.’
| Kinds of Algæ. | Water. | Dry matter. | Per cent. Nitrogen in dry matter. | Protein contained in dry matter. |
| Chondrus crispus, bleached, from Bewlay Evans. | 17·92 | 82·08 | 1·534 | 9·587 |
| Chondrus crispus, unbleached, Ballycastle. | 21·47 | 78·53 | 2·142 | 13·387 |
| Gigastina mamillosa, Ballycastle. | 21·55 | 78·45 | 2·198 | 13·737 |
| Chondrus crispus, bleached, second experiment. | 19·79 | 80·21 | 1·485 | 9·281 |
| Chondrus crispus, unbleached second experiment. | 19·96 | 80·04 | 2·510 | 15·687 |
| Laminaria digitata, or dulse tangle. | 21·38 | 78·62 | 1·588 | 9·925 |
| Rhodomenia palmata. | 16·56 | 83·44 | 3·465 | 21·656 |
| Porphyra laciniata. | 17·41 | 82·59 | 4·650 | 29·062 |
| Iridæa edulis. | 19·61 | 80·39 | 3·088 | 19·300 |
| Alaria esculenta. | 17·91 | 80·09 | 2·424 | 15·150 |
80
From the above, we learn the important fact that the sea-weeds found on our coasts are amongst the most nutritious of vegetable substances, and that they, when dry, are even richer in nitrogenous matter than either oatmeal or Indian corn in the same state. The following are the chief varieties of algæ which are used as food by the dwellers on our coasts as well as on the continent:—Porphyra laciniata and VULGARIS, called laver in England, stoke in Ireland, and slouk in Scotland. Chondrus crispus, called carrageen or Irish moss, and also pearl-moss, and sea-moss. Laminaria digitata, known as the sea-girdle in England, tangle in Scotland, and red-ware in the Orkneys; and Laminaria saccharina, Alaria esculenta, or bladder-lock, called also henware, and honey-ware by the Scotch. Ulva latissima or Green Laver—Rhodomenia palmata or dulse of Scotland. Under the name of “marine sauce” the Laver was esteemed a luxury in London, where it may now occasionally be met with in the shops of provision merchants. The employment of the Chondrus crispus or Carrageen in the form of an aliment for consumptive and weakly persons, would seem from the analysis of it given above to be fully justified. In preparing the algæ for food, they must be soaked in water to remove the saline matter, and where they are possessed of a bitter flavour this may be removed by adding a little carbonate of soda to the water. They should then be stewed in water or milk till they are tender. The best flavourings are pepper and vinegar. See Jelly.
ALGARO′BA. Syn. Ca′′rob-tree, St. John’s Bread; Cerato′nia Sil′iqua, Linn. A leguminous tree of southern Europe, Palestine, and part of Africa. Pods (ALGAROBA BEANS), used for food, and to improve the voice; they contain a sweetish, nutritious powder, and are supposed to have been the ‘locusts’ on which St. John fed in the wilderness; their decoction has been used as a pectoral in asthma and coughs.
Algaroba or Algarovil′la. The astringent pods of prosopis pallida, p. siliquastrum, and Inga Marthæ (South American trees), bruised and more or less agglutinated by the extractive exudation of the seed and husks. They are used in tanning, for which purpose they have been strongly recommended; indeed that of Chili, and of Santa Martha (New Carthagena), is said to possess “four times the power of good oak bark” (Ure); and in dyeing are only inferior to oak-galls.
ALGONTINE. A mouth and tooth wash. An aqueous solution of nitrate of potassium, aromatised with oil of peppermint, tincture of myrrh, and tincture of cinnamon.
ALGOPHON (Bernhard, Salzburg). For pains in decayed teeth. A solution of ethereal oil of mustard (2 grms.) in spirit of cochlearia (30 grms.), coloured green by saffron and litmus. (Wittstein.)
AL′IMENT. [Eng., Fr.] Syn. Alimen′tum, L.; Nahrung, Speise, Ger. Food; nutriment; anything which nourishes or supports life.
ALIMENT′ARY Syn. Alimenta′′rius, L.; Alimentaire, Fr.; zur Nahrung gehörig, Ger. Pertaining to food or aliment; nutrimental; nourishing.
Alimentary Canal′. Syn. Alimentary duct; Cana′lis Alimenta′′rius, L. In anatomy, the cavity in the bodies of animals into which the food is taken for the purpose of being digested; the whole passage or conduit extending from the mouth to the anus. In some of the lower animals this is a simple cavity, with only one opening; when the same aperture which admits the food also gives egress to the excrementitious matter. In others it is a true canal, with both a mouth and an outlet. Another step, and we find this canal is divided into a stomach and intestines. In the higher grades, a mouth, pharynx, and œsophagus precede the stomach. Birds have one or two sacculi or crops added to the œsophagus. The stomach of the ruminants consists of four sacs or parts, each of which may be regarded as a separate stomach; that of the bottle-nose whale contains no less than seven of such sacs. The part below the stomach, forming the intestines, is also variously subdivided, complicated, and connected. In man, these subdivisions are termed—DUODENUM, JEJU′NUM, IL′EUM, CÆ′CUM, CO′LON, and REC′TUM; the lower end or orifice of the last being called the A′NUS. The existence of an alimentary canal is said to be the only true characteristic of an animal. Plants have no common receptacle for their food, nor canal for carrying away effete matter; but every animal, however low in the scale of being, possesses an internal cavity which serves it as a stomach.
Alimentary Sub′stances. Syn. Aliments; Mate′′ria alimenta′′ria, L. Substances employed as food.
ALIMENTA′TION. [Eng., Fr.] Syn. Alimenta′tio, L.; Nahrhaftigkeit, Ger. The act, process, power, or state of nourishing, or being nourished.
AL′IZARI. [Tur., ali-zari.] The commercial name of madder in the Levant.
ALIZARIN. C10H6O3 . 2H2O. Syn. Lazaric Acid. A red colouring matter obtained from madder.
Prep. 1. Exhaust madder with boiling water, and precipitate the decoction by sulphuric acid. Wash the precipitate, and, while yet moist, boil it with a concentrated solution of hydrate of aluminum in hydrochloric acid, and mix the solution with hydrochloric acid; red flakes of impure alizarin deposit. Dissolve this precipitate in alcohol or in dilute ammonia, and treat the solution with hydrate of aluminum. Boil the aluminum compound thus formed with carbonate of sodium, and, after freeing it from resinous impurities by digestion with ether, decompose it with hot hydrochloric acid. Wash the alizarin thus separated, dry it81 by simple exposure to air, and purify it by repeated crystallisation out of alcohol.
2. Sublime on a paper an alcoholic extract of madder. This method yields the purest alizarin.
Props. Red prisms; sublimes at 419° F.; odourless, tasteless, and neutral to test-paper; sparingly soluble in water, even at the boiling temperature; soluble in alcohol and ether; not decomposed by hydrochloric acid; dissolved, without decomposition, by strong sulphuric acid; soluble in solutions of the alkalies and their carbonates; acids precipitate alizarin from its alkaline solutions in orange-coloured flakes; alumina decolorises an alcoholic solution of alizarin, forming a red lake.
ALIZARIN, ARTIFICIAL. C14H8O4. This colour was first obtained by Graebe and Liebermann in 1869 from anthrachinon, an oxidation product of anthracen, this latter being a substance which is formed during the destructive distillation of coal-tar. These chemists converted anthracen into antichinon by means of nitric acid.
The crude anthracen is previously purified by treatment with benzoline (petroleum spirit), aided by heat, and by being subjected to the action of the centrifugal machine to fusion, and to sublimation.
According to the original method of preparing alizarin, the anthrachinon was first converted into a dibromide of anthrachinon by treatment with bromine, and this bromated compound, by further treatment either with caustic potash or soda at a temperature of 180° to 200° C., converted into alizarin-potassium (or alizarin-sodium if caustic soda has been used), from which the alizarin is set free by means of hydrochloric acid.
Alizarin is now procured from anthrachinon by treatment at a temperature of 260° C., with concentrated sulphuric acid of 1·84 sp. gr., the anthrachinon being converted into a sulpho-acid; this acid is next neutralised with carbonate of lime, the fluid decanted from the deposited sulphate of lime, and carbonate of potash added to it, with the object of throwing down all the lime. The clear liquid is then evaporated to dryness, the resulting saline mass is converted into alizarin-potassium by heating it with caustic potash. From the alizarin-potassium thus obtained the alizarin is set free by the aid of hydrochloric acid.
In another method the preparation of anthrachinon is avoided, and anthracen employed directly, by first converting it, by means of sulphuric acid and heat, into anthracen sulphonic-acid. After having been diluted with water, the solution of this acid is treated with oxidising agents (peroxides of manganese, lead, chromic acid, nitric acid), and the acid fluid is afterwards neutralised with carbonate of lime. When peroxide of manganese has been used, the manganese is also precipitated as oxide. The oxidised sulpho-acid having been previously converted into a potassium salt, the latter being heated with caustic potash, alizarin is obtained. The details of these two processes will be found set forth in the terms of the patent taken out by Messrs Caro, Graebe and Liebermann, further on.
The following method of preparing alizarin from anthracene paranaphthalene and their homologues is by Girard. The material used is that which distils between 290° and 360°; it is purified by distillation and pressure, the portion which passes over, between 300° and 305°, being collected separately. This mixture is treated with potassium chlorate and hydrochloric acid, whereby it is converted into tetra-chlorinated products. These are oxidised either by nitric acid in the water bath, or by a metallic oxide (red or brown oxide of lead), and sulphuric or acetic acid. In the first place a mixture of dichloranthraquinine and chloride of chloroxyanthranyl are obtained. These substances are treated in presence of a metallic oxide (oxide of zinc, oxide of copper, or litharge), with an alcoholic solution of sodium acetate. The metallic oxide removes the last atom of chlorine from the sodium chloroxyanthranilate, and converts it, like the dichloranthraquinine, into alizarin. The purification is effected by means of benzine, petroleum, &c., which dissolve out the foreign matters, and by successive precipitation from the alkaline solutions by mineral acids. The foreign matters may also be separated by means of a little alum, when it is necessary to work with neutral potash or soda salts.
Another method for the preparation of alizarin has been patented by Dale and Schorlemmer. It is as follows: 1 part of anthracen is boiled with 4 to 10 parts of strong sulphuric acid, then diluted with water, and the solution neutralised with carbonate of calcium, barium, potassium, or sodium. The resulting sulphates having been removed by nitration or crystallisation, the solution is heated to between 180° and 260° with caustic potash or soda, to which a quantity of potassium nitrate or chlorate has been added, about equal in weight to the anthracen, as long as a blue-violet colour is thereby produced. From this product the alizarin is separated in the usual way by precipitation with an acid. Several other patents have been taken out for the preparation of artificial alizarin.
The specification of Messrs Caro, Graebe, and Liebermann, and dated June 25th, 1869, was the first which was taken out in England. We quote it here because it enters more fully into detail than any of the others.
“Our invention is carried into effect by means of either of the two processes which we will proceed to describe.
“In the one process we proceed as follows—We take about one part by weight of anthraquinone and about three parts by weight of sulphuric acid of about specific gravity of82 1·488, and introduce the same into a retort, which may be made of glass, or porcelain, or of any other material not easily acted upon by sulphuric acid, and the contents are then to be heated up to about 260° Centigrade, and the temperature is maintained until the mixture is found no longer to contain any appreciable quantity of unaltered anthraquinone. The completion of this operation may be ascertained or tested by withdrawing a small portion of the product from time to time, and continuing the operation at the high temperature until such product upon being diluted with water is found to form a substantially perfect solution, thereby indicating that the anthraquinone has become either entirely or in greater part converted into the desired product. The products thus obtained are then allowed to cool, and are diluted with water; carbonate of lime is then added in order to neutralise and remove the excess of sulphuric acid contained in the solution; the mixture is then filtered, and to the filtrate carbonate of potash, or carbonate of soda, by preference in solution, is to be added until carbonate of lime is no longer precipitated; the mixture is then filtered, and the clear solution is evaporated to dryness, by which means the potash or soda salts of the sulpho-acids of anthraquinone are obtained, and which are to be treated in the following manner:—We take about one part by weight of this product, and from two to three parts by weight of solid caustic, soda, or potash; water may be added or not, but by preference we add as much water as is necessary to dissolve the alkali after admixture; we heat the whole in a suitable vessel, and the heating operation is continued at a temperature of from about 180° to 260° Centigrade, for about one hour, or until a portion of the mixture is found upon withdrawing and testing it to give a solution in water, which being acidulated with an acid, for example, sulphuric acid, will give a copious precipitate of the colouring matters. The heating operation having been found to have been continued for a sufficient time, the resulting products are then dissolved in water, and we either filter or decant the solution of the same, from which we precipitate the colouring matters or artificial alizarin, by means of a mineral or organic acid, such, for example, as sulphuric or acetic acid. The precipitated colouring matters thus obtained are collected in a filter or otherwise, and after having been washed may be employed for the purpose of dyeing and printing, either in the same way as preparations of madder are now used or otherwise.
“In carrying out our other process we proceed as follows:—We take about one part by weight of anthracene and about four parts by weight of sulphuric acid of specific gravity of about 1·848, and the mixture being contained in a suitable vessel, is heated to a temperature of about 100° Centigrade, and which temperature is to be maintained for the space of about three hours; the temperature is then to be raised to about 150° Centigrade, which temperature is to be maintained for about one hour, or until a small portion of the product when submitted to the two subsequent processes hereinafter described is found to produce the desired colouring matters; we then allow the result obtained by this operation to cool, and dilute it with water, by preference in the proportion of about three times its weight. To the solution thus obtained we add for every part of anthracene by weight which had been employed in the previous operations, from about two to three parts by weight of peroxide of manganese, preferring to employ an excess, and we boil the whole strongly for some time, and in order fully to ensure the desired degree of oxidation the mixture may be subsequently concentrated, and by preference be evaporated to dryness, and the heat be continued until a small portion of the oxidised product, when submitted to the subsequent processes hereinafter described will produce the desired colouring matters. We then neutralise and remove the sulphuric acid contained in this mixture, and at the same time precipitate any oxides of manganese that may be held in solution, by adding an excess of caustic lime, which we use by preference in the form of milk of lime, and we add the same until the mixture has an alkaline reaction. We then filter, and add to the filtrate carbonate of potash or soda, until there is no further precipitation of carbonate of lime. The solution is then filtered and evaporated to dryness, and we thus obtain the potash or soda salts of what we call the sulpho-acids of anthraquinone.
“In effecting the conversion of the oxidised products thus obtained into colouring matters, or into what we call artificial alizarin, we proceed as follows:—We take one part by weight of this product, and from two to three parts by weight of solid caustic soda or potash, and water may be added or not, but by preference we add as much water as may be necessary to dissolve the alkali. After admixture we heat the whole in a suitable vessel, and continue the heating operation at a temperature of about 180° to about 260° Centigrade for about one hour, or until a portion of the mixture is found to give a solution in water, which upon acidulation with an acid, for example, sulphuric acid, is found to give a copious precipitate of the colouring matters. The heating operation having been found to have been continued for a sufficient time, we then dissolve the product in water, and either filter or decant the solution of the same, from which we precipitate the colouring matters or artificial alizarin by means of a mineral or organic acid, such, for example, as sulphuric or acetic acid. The precipitated colouring83 matters thus obtained are collected on a filter or otherwise, and after having been washed may be employed for the purpose of dyeing and printing, either in the same way as preparations of madder are now used or otherwise.
“Instead of acting upon anthracen by means of sulphuric acid of the density before mentioned, fuming sulphuric acid may be employed, but we prefer to use the ordinary kind before described.
“In order to effect the process of oxidation, before referred to, other oxidising agents may be used in the place of the oxide of manganese, before mentioned, such, for example, as perioxide of lead, or chromic, nitric, or other acids capable of effecting the desired oxidation may be employed.”
Mr W. H. Perkin’s patent is similar in principle to that of Messrs Caro, Graebe, and Liebermann, and is dated only one day later.
The following is an outline of a patent taken out in France in May, 1869, by MM. Brœnner and Gutzkon, for the manufacture of artificial alizarin. One part of anthracen is heated with two parts of nitric acid, sp. gr. 1·3 to 1·5. The anthraquinone thus produced is washed and dissolved at a moderate heat in sulphuric acid. Mercuric nitrate is now added, which converts the anthraquinone into alizarin, The mass thus formed is dissolved in an excess of alkali, which precipitates the oxide of mercury, and retains the colouring matters in solution. The alkaline liquor is decanted and neutralised with sulphuric acid, and the precipitate thus formed is washed and collected. If not quite pure the treatment with alkali must be repeated. (The complete specification of this patent is published in the ‘Moniteur Scientifique,’ vol. xi, p. 865.)
In England a large quantity of artificial alizarin is manufactured by the process of Mr Perkin, and is used as a substitute for madder and madder extract, in Turkey red dyeing and topical styles. The largest makers of artificial alizarin on the continent are Messrs Gessert Frères, of Ebelfort, Messrs Maister, Lucius and Co., of Hæchst, near Frankfort, and the Badische Anilin und Soda Fabric, Mannheim.
The following recipes for printing with artificial alizarin are extracted from Mr Crookes’ ‘Practical Handbook of Dyeing and Calico Printing’:
The above diluted with 2 or 3 parts of thickening.
For double printing, when deep red is printed on first, the goods must be steamed one hour before the second printing takes place. After the second printing the goods are again steamed for one hour, and aged for twenty-four hours; they are then passed through one of the following baths, at from 120 to 140 F., remaining in the bath not longer than 1 to 11⁄2 minute:—
The goods are then washed, and cleaned as follows:—
Take, for 10 pieces of fifty yards each,—
Boil well and stir till cold; then add—
The printed goods are steamed for an hour or two, and then aged from twenty-four to thirty-six hours. They are then padded in the chalk and arseniate of soda bath; after which they are washed and soaped in a single soap-bath without tin crystals; and, if needful, cleaned in a weak solution of bleaching powder.
Boil well together, and stir till cold.84
Boil well together, and stir till cold.
The mordants in the above recipes are prepared as fellows:
Stir 30 lbs. of hydrate of alumina into six quarts of acetic acid, warm, filter, and reduce to the specific gravity required.
The hydrate of alumina is prepared by dissolving 72 lbs. of alum in 100 gals. of water, and 62 lbs. soda in 100 gals. of water. The two solutions are mixed, this precipitate is washed eight times by decantation, collected on a filter and pressed. It must be dissolved on the filter before it gets dry.
Dissolve and filter off the liquid from the precipitate, and dilute to proper standard.
The reds are turned more yellow by nitrate than by acetate of alumina, and when the former is used more acetate of lime is taken in addition.
A solution of acetate of lime at 25° Tw. contains 25 per cent. of acetate of lime; generally 1⁄10th of the weight of alizarin paste is required; but with a fresh quantity of alizarin it is safer to ascertain, on a small scale, the amount needed.
To obtain a yellower shade, for every quart of mixed colour, 1 oz. bark liquor, at 30° Tw., may be added.
Old spoiled red colours may be advantageously used for browns by adding per quart, 3⁄4 oz. to 1 oz. red prussiate, dissolved in water.
ALKALI. Syn. Alkali, Fr.; Langensalz, Ger. This word has been used in various senses, but is now usually applied to four substances only, viz. the hydrates of potassium, sodium, lithium, and ammonium (the latter being supposed to exist in the aqueous solution of ammonia). In a more general sense it is applied to the hydrates of barium, strontium, and calcium, which, for the sake of distinction, are called the alkaline earths. The following properties are characteristic of the alkalies:—(1) They are soluble in water, the alkalies proper more so than the alkaline earths. (2) They change the hue of many vegetable colouring matters; thus, they turn reddened litmus blue, yellow turmeric brown, and syrup of violets and infusion of red cabbage green. (3) They neutralise the strongest acids. (4) They precipitate most of the heavy metals from solutions of their salts as hydrates or oxides. (5) They saponify the fixed oils and fats. (6) They exert a caustic or corrosive action on animal and vegetable substances.
ALKALI ACTS. The principal alkali Act is the 26 and 27 Vict., c. 24, amended by 37 and 38 Vict., c. 43, the amended Act having come into operation in 1875.
Every alkali work must be carried on so as to ensure the condensation of not less than 95% of muriatic acid evolved therein; and it must be so condensed that in each cubic foot of air, smoke, or chimney gases, escaping from the works into the atmosphere, there is not contained more than one fifth part of a grain of muriatic acid. Penalty for first conviction, £50; for second and other offences, £100, or less (26 and 27 Vict., c. 124, s. 4; 37 and 38 Vict., c. 43, s. 4).
The owner of every alkali work is also bound “to use the best practicable means of preventing the discharge into the atmosphere of all other noxious gases arising from such work; or of rendering such gases harmless when discharged.”
The noxious gases are defined to be sulphuric acid, sulphurous acid (except that arising from the combustion of coals), nitric acid, or other noxious oxides of nitrogen, sulphuretted hydrogen and chlorine (37 and 38 Vict., c. 43, ss. 5 and 8).
The owner is liable for any offence against the Alkali Acts, unless he prove that the offence was committed by some agent, servant, or workman, and without his knowledge, in which case the agent, &c., is liable (26 and 27 Vict., c. 124, s. 5).
Every alkali work must be registered; penalty for neglect £5 per day (ibid., s. 6).
Powers are given to owners to make special rules for the guidance of their workmen (ibid. s. 13).
ALKALIM′ETRY. Syn. Alkalime′tria, L.; Alcalimétrie, Fr. In chemistry, the estimation of the strength of the commercial alkalies; the art or process of determining the quantity or proportion of pure caustic alkali, or of its carbonate, in any given sample or simple solution. It is the reverse of ‘acidimetry,’ and it should be understood that it does not apply to alkalies occurring under any other form or condition than those just mentioned. Alkalimetric assays are now also frequently and conveniently extended to the estimation of the alkaline earths and their carbonates, as hereafter noticed.
Alkalimetrical processes. These, like those85 of ‘acidimetry,’ are for the most part founded on—the capacity of the bases to saturate acids—the estimation of the quantity of dry carbonic acid liberated from a given weight of an alkaline carbonate under the influence of a stronger acid; and, in the case of the pure alkalies, the sp. gr. of their solutions. From any one of these results the exact amount of alkali, or of alkaline carbonate, present in a sample, is easily found or calculated. These processes are, indeed, precisely similar to those described under Acidimetry; but here the unknown quantity sought is the alkali, instead of the acid.
Assay. The SAMPLE is drawn from as near the centre of the cask containing the alkali as possible, and at once placed in a wide-mouthed bottle, which is then closely corked up and numbered. Before proceeding to the assay, the contents of the bottle are thrown on a piece of dry paper, the lumps crushed small, and the whole reduced to coarse powder as rapidly as possible. The number of grains required for the trial are then at once weighed, placed in a phial or small glass tube, and agitated with about 1⁄2 oz. of hot water. After a short time allowed for repose, the clear liquid is poured off into a beaker-glass or other vessel in which the trial is to be made. This process is repeated with a second and a third quantity of water, or until nothing soluble remains, shown by the last washings not affecting the colour of turmeric paper. The greatest care must here be taken not to waste the smallest portion of the liquid, which would render the results inaccurate.
To the solution in the beaker-glass a little solution of litmus is added, unless the acid is tinted with it when it is unnecessary. The solution is now heated until near its boiling point, and a piece of white paper or porcelain put behind it, to better show up the changes of colour. The alkaline solution is now treated with the standard test-acid, which is poured carefully from an alkalimeter or Mohr’s burette, until the solution, after turning a purple red, suddenly assumes a pink colour. Neutralisation being thus effected, the operator allows the sides of the alkalimeter or burette to drain, and then either ‘reads off’ the number of divisions which have been consumed, or (if using the test-acid by weight) determines the quantity by again weighing the alkalimeter. The common practice is to allow two drops (= 1⁄5th of an alkalimetrical division by VOLUME, or 2 gr. by WEIGHT) for over-saturation, which is, therefore, deducted from the ‘observed quantity’ of the test-liquor employed.
In testing solutions of the PURE or CAUSTIC ALKALIES, the colour, on neutralisation, suddenly changes from blue to pink or red, without any intermediate vinous or purple colour being produced.
The quantity of test-acid used gives the absolute or per-centage composition of the sample examined, according to the constitution of the test-acid used.
Standard Acids. The various test-acids in use as described below, each being used by different operators as they think best.
The most convenient test-acid, or normal solution, both for commercial and chemical assays, is perhaps dilute sulphuric acid, which, when intended to be used VOLUMETRICALLY, has the sp. gr. 1·032 at 60° Fahr., and contains in 100 alkalimetrical divisions 1000 water-grains measure, or 1 litre, exactly 49 gr. (or grammes) of sulphuric acid; and when intended to be used GRAVIMETRICALLY, or by weight, has the sp. gr. 1·033, and contains in 1000 gr. (or grammes) weight exactly 49 gr. (grammes) of sulphuric acid; and, in both cases, consequently corresponds to 1 equiv. of every other base. These dilute acids are easily prepared by mixing 1 part of the concentrated acid with 11 or 12 parts of distilled water; the precise quantity depending on the strength of the acid employed, and must be so arranged that 1000 grains shall exactly neutralise 1000 grains of water containing 53 grains of pure anhydrous sodium carbonate.
This acid (as well as all those hereafter mentioned) may be kept faintly tinged with litmus, which is often more convenient than tinging the alkaline solution at the time of making the assay.
It will at once be seen that every alkalimeter division of the first of the above acids, and every 10 gr. of the second, represent the 1⁄100th part, or 1% of alkali whenever the equivalent weight[18] of the latter is taken for the assay. Every 1-10th part of an alkalimeter-division (or every drop), and every grain weight (when a Schüster’s alkalimeter is employed) then respectively represents the 1⁄10 of 1%; and the result sought is obtained without the necessity of any calculation.
This is obvious—for if the equivalent of a pure alkali or of its carbonate (i. e. one of 100%) requires an equiv. (100 alkalimeter-divisions, or 1000 gr.) of test-acid to saturate it, an alkali or alkaline carbonate of 75%, 50%, or 25%, will respectively require only 75, 50, or 25 divisions, or 750, 500, or 250 gr.; and so of other strengths in proportion. The only precaution necessary is always to take the standard weight for the assay answering to the equiv. of the denomination of the per-centage result sought. Thus, in testing a carbonate of potash, we may either wish to determine its per-centage richness in ‘dry carbonate,’ or in ‘pure potassa,’ the latter being usually the case. To obtain the first, we must take 69 gr. for the assay; and to obtain the second, 47 gr. With CAUSTIC ALKALIES, or mixtures containing them, the weight, in grains, taken for the assay, must always correspond to the equiv. of the pure base. See Table II, at the end of this article.
In commercial assays, when 100 gr. (or some86 aliquot part thereof) are taken for trial, the per-centage result is obtained from the number of alkalimeter-divisions, or the number of grains, of the test-acid consumed, by the common Rule of Proportion. Thus:—A crude sample of potash having taken 90 alkalimeter-divisions of test-acid to neutralise it, would contain—
100 : 47 :: 90 : 42·30%
or nearly 421⁄3 per cent. of pure potassa. If only 50, 25, or 20 gr. are tested, the result must, of course, be double, quadruple, &c., as the case may be. Or the third term of the proportion may be multiplied by the denominator of the fraction representing the aliquot part. This, in the case of 50 gr. (repeating the above example), would be—
10 : 47 :: 45 × 2 : 42·30%
as before; but even these easy calculations may be simplified, as is shown below.
One of the advantages, and not the least, attending the use of test-acids corresponding to equivalents, is, that by means of the simple Rule of Three, the per-centage quantity of alkali may be found whether 100 or any other number of grains have been submitted to trial. For—The weight of the sample tested (in grains) bears the same relation to the equivalent weight of the alkali under examination, that the number of alkalimeter-divisions or of the grains of test-acid consumed do to the per-centage of alkali sought. Thus, with a sample of 33 gr. of pearlash taking 35 alkalimeter-divisions or 350 grains (every 10 gr. being = 1%) of test-acid for neutralisation, this would be—
33 : 47 :: 35 : 49·85%
or nearly 50 per cent. of pure potassa. By substituting the equiv. of the dry carbonate of potash (69), for that of pure potassa used above, the quantity of that article corresponding to the same weight of the pure alkali may be at once found. Repeating the last example this will be—
33 : 69 :: 35 : 73·18%
or nearly 731⁄4 per cent. The same applies to all the alkaline bases and their carbonates.
For commercial purposes, there is used, amongst others, an empirical solution, as a test-acid for potassa, soda, and ammonia, to save the necessity of calculation.
This is dilute sulphuric acid having a sp. gr. of about 1·071; 100 alkalimeter-divisions (1000 water-grains measure) exactly saturate 100 gr. of pure potassa, or 113 gr. of anhydrous carbonate of soda. The number of measures consumed, read off by mere inspection from the scale of the alkalimeter, gives the exact per-centage of alkali in the sample examined, for POTASH; and by multiplying it by ·66, that for SODA also. By employing ·362 as the multiplier, it gives the like result for AMMONIA. In fact, occasionally, in order to save the necessity of any calculation, two ‘test-acids’ are frequently employed—the one for potash and the other for soda.
These are made by diluting sulphuric acid to a sp. gr. of near 1·071 and 1·086 respectively; 1000 grains, by measure, of the first neutralising exactly 100 grains of pure potassa, or 113 of pure anhydrous soda carbonate, and the latter neutralising exactly 100 grains of pure soda, or 171 gr. of pure anhydrous sodium carbonate.
There is another system of preparing standard acids by means of a Faraday’s alkalimeter. A strong acid is prepared by diluting sulphuric acid to a sp. gr. of 1·1268 at 60°, and 455·7 grains exactly neutralise 100 of anhydrous carbonate of soda.
The glass tube here referred to, and known as Faraday’s ALKALIMETER, is graduated centesimally, in the usual manner; but opposite the numbers 22·1, 48·62, 54·43, and 65, are cut the words ‘soda,’ ‘potassa,’ ‘carbonate of soda,’ and ‘carbonate of potassa,’ to indicate the quantity of the test-acid to be employed for each of these substances. (See engr.) It is used by pouring the test-liquor into it until it reaches the line marked against the alkali, or carbonate, under examination, the remaining divisions being filled up with pure water, and the whole well mixed by placing the thumb on the orifice of the tube and shaking it well. The measure of the resulting dilute acid must then be very carefully observed, and more water added, if required, to bring it up to the zero (0) or 1000 gr. on the scale; careful agitation being again employed as before. The test-acid thus prepared is then added, with the usual precautions, to the sample until exact neutralisation is effected. The quantity consumed for this purpose, read off from the graduated scale, expresses the exact per-centage of the pure ALKALI, or of its CARBONATE, as the case may be, contained in the sample examined, provided 100 gr. have been taken for the assay.
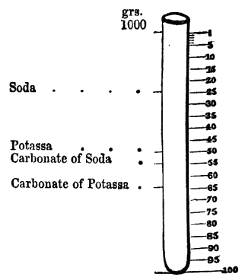
Another method sometimes used is that of M. Mohr, and practised as follows:—The alkaline solution, slightly coloured blue with litmus, is strongly super-saturated with a standard acid (sulphuric or oxalic) of known strength, supplied from an alkalimeter in the usual manner; the last traces of carbonic anhydride being removed by boiling, shaking, blowing into the flask, and, finally, sucking87 out the air. A standard solution of caustic soda (of a strength exactly corresponding to that of the test-acid already used) is now cautiously added, drop by drop, until the colour, rendered yellowish-red by the acid, just appears of a light blue. The difference between the quantity of the solution of the test-alkali and of the test-acid consumed, expresses the exact quantity of acid neutralised by the alkali, and hence also its strength.
Besides the above methods, the alkaline carbonates are analysed, by the loss of carbonic anhydride (carbonic acid) they suffer, by being decomposed by a strong acid. The best method in use is that of MM. Fresenius and Will, and depends on the same principle, and is performed in a similar manner and in a similar apparatus to that described under Acidimetry; the only difference being that here the uses of the small tube (e) is dispensed with, and that the alkali is tested under the form of carbonate, instead of bicarbonate.
Oper. The smaller flask (B) is about half filled with concentrated sulphuric acid, and the sample of alkali, in solution (under the form of carbonate), being placed in the larger flask (A), water is added until it is about one third full. The tubes are then fitted into the apparatus quite air-tight; the end of the tube (b) is fastened with a piece of wax, and the whole is very carefully weighed. The apparatus is now removed from the scales, and a perforated cork, or a small piece of india-rubber tube, being temporarily applied to the end of the tube (h), a few bubbles of air are sucked out of the flask (B) by means of the lips; the consequence of which is, that on removing the mouth the acid in (B) ascends to a certain height in the tube (c). If in a short time this little column of liquid maintains its height in the tube, it is a proof that the apparatus is perfectly air-tight, and as it should be. Suction is now again cautiously applied to the tube (h) and a little of the acid in (B) made to flow over into the flask (A), the quantity being proportionate to the vacuum produced by suction, and capable of being regulated at will. No sooner does the acid come into contact with the carbonate in the flask (A) than the evolution of carbonic acid commences, and this, from the construction of the apparatus, having to pass through the concentrated sulphuric acid, is rendered quite dry before it can escape by the tube (d) into the atmosphere. Whenever the effervescence flags, a little more acid is sucked over, until the whole of the carbonate is decomposed; after which an additional quantity is made to pass into (A), so as to raise the temperature considerably, for the purpose of expelling all the gas absorbed by the fluid during the operation. As soon as this is effected, the wax is removed from the aperture (b), and suction applied to (h), until all the carbonic acid in the apparatus is replaced by atmospheric air. The whole is now allowed to cool, and (together with the piece of wax removed) is again accurately weighed. The loss of weight gives the exact amount of dry carbonic anhydride, or anhydrous carbonic acid, which was contained in the specimen, from which the weight of PURE ALKALI is readily estimated, as every 22 gr. of dry carbonic acid gas evolved represents exactly 31 gr. of pure SODA, 47 gr. of pure POTASSA, &c. &c.; these numbers being the equivalents of the respective substances from which the per-centage strength may be found by the rule of proportion, as before explained.
Thus, in the case of a 100-gr. sample of carbonate of soda which has lost 151⁄4 gr. of carbonic acid, by the assay, this would be—
22 : 31 :: 151⁄4 : 21·48%
or nearly 211⁄2 per cent. of pure soda. If 53, the equiv. of anhydrous carbonate of soda, be taken, instead of 31 (the eq. of pure soda), the answer would have been, in the terms of that substance, 36·748%, or nearly 363⁄4 per cent. When an aliquot part of 100 gr. has been taken for the assay, either the result, or the third term of the proportion, must, of course, he multiplied by the denominator and divided by the numerator of the fraction representing such aliquot part.
By multiplying the weight of carbonic anhydride lost, by the numbers opposite the names of the respective alkalies and their carbonates in the second column of the following Table the equivalent per-centage value of the carbonates examined may be obtained in terms corresponding to the various denominations named therein, when 100 gr., or any aliquot part of 100 gr., have been tested; the result, in the latter case, being, of course, multiplied as before.
By taking certain standard weights for the assay, the quantity of carbonic acid evolved may be made to furnish the per-centage strength or value of the specimen in the terms of either the pure or carbonated alkalies, whether in their anhydrous or hydrated state. The numbers in the second column of the following Table represent the quantity in grains and decimal parts of each of the substances named in the first column, equivalent to one grain of carbonic anhydride. These numbers, as already mentioned, may be employed as factors for converting any numbers representing grains of that acid into the equivalents of these substances, true to 4 places of decimals; and further, they furnish us with the data for determining the exact number of grains which must be tested, so that the loss of weight in carbonic anhydride shall at once give us the per-centage richness of the sample in the terms of the denomination for which it is taken. The numbers in the third column of the Table, formed by simply moving the decimal point of the numbers in the second column one figure further to the right, indicate the weights to be taken for the assay, so that the loss of weight, reckoned in tenths of a grain, exactly represents the per-centage88 strength in the terms sought. The weights corresponding to the numbers in the fifth column give the same results, provided the loss of weight is reckoned in quarter-grains; those in the sixth column effect the same when the loss of weight is reckoned in half-grains; whilst those in the last column require that the gas eliminated should be counted in grains, and are simply the numbers in the second column of the Table multiplied by 100, or reproduced by moving the decimal point two figures to the right.
| NAMES, &c. | Factors or Multipliers for converting the weight of carbonic acid expelled into real strengths. | Quantity (in grains) to be taken, so that the per-centage value of the sample tested shall be shown in the terms of any of the denominations given, by the weight of the evolved Carbonic Acid reckoned— | ||||
| In tenths of a grain. | ||||||
| Whole numbers and decimals. | Nearest common numbers. | in quarter-grains. | in half-grains. | in grains. | ||
| Ammonia (pure, gaseous) | ·77273 | 7·727 | 73⁄4 | 191⁄3 | 385⁄8 | 773⁄0 |
| Carbonate of ammonia (neutral, anhydrous) | 1·77273 | 17·727 | 173⁄4 | 445⁄16 | 885⁄8 | 1771⁄4 |
| Carbonate of ammonia (neutral, crystallised) | 1·9773 | 19·773 | 193⁄4 | 497⁄16 | 987⁄8 | 1973⁄4 |
| Sesquicarbonate of ammonia (translucent) | 2·6818 | 26·818 | 2613⁄16 | 671⁄10 | 1341⁄10 | 2681⁄5 |
| Bicarbonate of ammonia (crystallised) | 3·5909 | 35·909 | 359⁄10 | 8913⁄16 | 1795⁄8 | 3591⁄10 |
| Potassa (anhydrous) | 2·1364 | 21·364 | 211⁄2 | 531⁄2 | 107 | 2133⁄8 |
| Hydrate of potassa | 2·54546 | 25·455 | 255⁄11 | 635⁄8 | 1271⁄4 | 2541⁄2 |
| Carbonate of potassa (anhydrous) | 3·1364 | 31·364 | 313⁄8 | 781⁄2 | 157 | 3131⁄2 |
| Carbonate of potassa (granulated) | 3·7727 | 37·727 | 371⁄2 | 943⁄10 | 1885⁄8 | 3771⁄4 |
| Carbonate of potassa (crystallised) | 3·9545 | 39·545 | 395⁄8 | 99 | 198 | 3951⁄2 |
| Bicarbonate of potassa (crystallised) | 4·5454 | 45·454 | 451⁄2 | 1133⁄4 | 2271⁄2 | 4541⁄2 |
| Soda (anhydrous) | 1·4091 | 14·09 | 141⁄10 | 351⁄4 | 701⁄2 | 141 |
| Hydrate of soda | 1·8182 | 18·182 | 181⁄5 | 451⁄2 | 91 | 182 |
| Carbonate of soda (anhydrous) | 2·4091 | 24·091 | 241⁄10 | 601⁄4 | 1201⁄2 | 241 |
| Carbonate of soda (crystallised) | 6·5 | 65· | 65 | 1621⁄2 | 325 | 650 |
| Sesquicarbonate of soda (dry; theoretical) | 2·9091 | 29·091 | 291⁄10 | 721⁄2 | 145 | 290 |
| Sesquicarbonate of soda (Ph. L., 1836) | 3·7273 | 37·273 | 371⁄4 | 931⁄4 | 1861⁄2 | 373 |
| Sesquicarbonate of soda (average commercial) | 3·7954 | 37·954 | 38 | 947⁄8 | 1893⁄4 | 3791⁄2 |
| Bicarbonate of soda (crystallised) | 3·8182 | 38·182 | 381⁄5 | 951⁄2 | 191 | 382 |
| Lithia (pure, anhydrous) | ·6818 | 6·818 | 613⁄16 | 171⁄20 | 341⁄10 | 681⁄5 |
| Baryta (pure, caustic) | 3·4773 | 34·773 | 344⁄5 | 867⁄8 | 1737⁄8 | 3473⁄4 |
| Lime (pure, caustic) | 1·2727 | 12·727 | 123⁄4 | 313⁄4 | 635⁄8 | 1271⁄4 |
| Magnesia (pure, anhydrous) | ·90909 | 9·091 | 91⁄11 | 223⁄4 | 451⁄2 | 91 |
In this ingenious method of alkalimetry it is absolutely necessary that the whole of the alkali in the specimen tested should be in the state of neutral carbonate. If a sample of potash contains any caustic alkali (as the potashes and pearlash of commerce generally do), Fresenius and Will direct it, previously to being tested, to be triturated with its own weight of pure quartzose sand, and about one third of its weight of carbonate of ammonia; and the resulting mixture, placed in a small iron capsule, or a porcelain crucible, to be moistened with water, and exposed to a gentle heat until it becomes quite dry, and all the ammonia is expelled. If the sample contains any bicarbonate or sesquicarbonate, it must be heated to dull redness before being placed in the apparatus and tested. In the case of crude soda (particularly soda ash), the proportion of carbonate of ammonia should be equal to at least one half the quantity operated on. With both alkalies, if the sample contains sulphides, sulphites, or hyposulphites, the same method is to be followed, except that solution of ammonia,89 instead of water, is to be employed for moistening the powder. To remedy the error which would arise from the apparent amount of carbonic anhydride liberated during the assay, being swelled by the disengagement of ‘sulphuretted hydrogen’ or sulphurous acid from these substances, a small quantity of neutral (i. e. yellow) chromate of potash may be added to the alkaline solution in the flask (A); by which they will be converted into sulphates, sulphur, and water, which will remain in the apparatus, the carbonic acid only being evolved. “As most sorts of soda of commerce contain one or other of the substances (just) named, and as it is far more simple to add at once some chromate of potassa to the soda solution, than to test the latter for either of the three salts, it is always advisable to make it a rule, in the examination of SODA, to add some chromate of potassa.” (Fresenius.)
If the sodium or other carbonate under analysis contains much chloride, the addition of more sulphuric acid than necessary must be avoided, and the carbonic anhydride expelled by gently heating over a warm bath, and not by the addition of excess of acid.
To obviate the difficulties, and to give greater precision and delicacy to volumetrical assays, the instrument known as Mohr’s ALKALIMETER, or Mohr’s BURETTE, and which is figured in the margin, may be employed. By means of it the test-acid in the graduated tube (a) may be added to the alkaline solution in (f), in any quantity at a time, however minute, by merely pressing the handles of the clamp (d) with the thumb and finger. The terminal tube (e) has its lower orifice very small, and it is connected with the burette by means of a small piece of vulcanised india-rubber tube, on which the clamp (d) acts. (See engr.) The inner cylindrical part of the arm (b) is lined with cork, to prevent injury to the glass burette, and to hold it the more firmly.
Generally the alkali in the specimen examined may be in either the caustic or carbonated state, or it may consist of any mixture of caustic alkali, or carbonates; but it is absolutely necessary for accurate results, that it should be free from sulphides, sulphites, and hyposulphites, as sulphuric acid acts upon these substances as well as on carbonates. The presence of chlorides does not interfere with the accuracy of the assay, unless a higher degree of heat is employed than that necessary for the expulsion of the absorbed carbonic acid. The SODA-ASH of commerce generally contains all these substances besides common salt, sulphate of soda, and insoluble matter, which do not interfere. Rough samples of POT-ASHES and PEARL-ASH also generally contain some sulphides, though not a large quantity. Various plans have been proposed to avoid this source of error. The best is that of MM. Fresenius and Will, given above, in which the value of the carbonates is estimated by their yield of carbonic anhydride.
The difference between an assay of a sample of the unprepared alkali and of another which has been treated as above, indicates the quantity of impurities contained in them under the forms just referred to. The presence of these substances in the commercial alkalies may be detected by the following tests:—
Sulphides. The addition of sulphuric acid causes the evolution of an odour like that of rotten eggs. The sample in solution yields a black precipitate with acetate of lead. But the most delicate test is the splendid violet-blue colour with nitro-prusside of sodium.
Sulphites and Hyposulphites. A solution of the alkali, insufficient for saturation, being added to sulphuric acid tinged reddish yellow with bichromate of potash, occasions a greenish tinge (owing to the formation of oxide of chromium), when these are present. Hydrochloric acid added to a clear solution, after some time, causes a turbidity and odour of sulphurous anhydride.
Chlorides yield a copious curdy precipitate with nitrate of silver, soluble in ammonia, and reprecipitated by excess of nitric acid.
The amount of pure caustic alkali in a sample of alkali is best determined by Fresenius’s method, as follows:—The total amount of pure alkali, both caustic and carbonated, expressed in per-cents. of carbonate of soda or carbonate of potassa, is ascertained by any of the usual methods. The apparent quantity of alkali per cent. is then determined, without previous treatment of the sample with carbonate of90 ammonia, by the method of Will and Fresenius (p. 86). The difference between the results indicates the per-centage of dry caustic alkali present; or if the volumetric method be in use, it can be often fairly estimated by adding the first portions of the test-acid very gradually to the sample, carefully observing the effect. When the effervescence at length commences the weight or measure of the test-liquor expended shows the quantity of pure caustic alkali under treatment (nearly). The result depends upon the fact, that little or no carbonic-acid gas is expelled from the liquid on the addition of the test-acid, until the caustic portion is very nearly neutralised.
The quantity of WATER or MOISTURE, per cent., present in an alkaline carbonate, is indicated by the loss of weight which 100 gr. suffer on gentle ignition in a loosely-covered iron dish or platinum crucible. So also with samples containing caustic alkali, except that here the water of hydration (= 1 equiv. = 9) is not expelled from the ‘caustic’ portion, and must therefore be determined by calculation.
Other matters deserving the serious attention of the operator are—hitting the exact point of neutralisation, and—preparing the test-acids of the proper strength. The method of effecting the former correctly has been already referred to in this article, and is also fully noticed under Acetimetry and Acidimetry.
Test-acids may be very simply prepared by gradually diluting concentrated sulphuric acid with water until it is reduced to the proper strength; the dilution being made in a glass vessel containing a ‘hydrostatic bead’ exactly corresponding to the desired specific gravity of the dilute acid. When the proper point is reached, and the mixture has again acquired the normal temperature of 60° Fahr., the bead rises from the bottom of the vessel, and floats about indifferently in the middle of the liquid. The sp. gr. may then he carefully ascertained by means of an hydrometer or a specific gravity bottle; after which the strength must be accurately determined by means of a standard solution of either pure anhydrous carbonate of soda or pure caustic soda. An acid of any given strength or saturating power may also be prepared in the following manner:—49 parts of commercial sulphuric acid (oil of vitriol), sp. gr. 1·825, contain nearly 40 parts or 1 equiv. of anhydrous sulphuric acid; if we, therefore, wish to prepare a dilute acid containing in every 1000 grains weight, or measure, exactly 1 equiv. of hydrated sulphuric acid, we have only to make 49 gr. of such acid up to 100 gr. weight or measure with pure water. After it has recovered the proper temperature, its sp. gr., or rather its saturating power, must be carefully tried, and, if necessary, readjusted. As, however, it very often happens that the oil of vitriol employed is not so strong as that above referred to, it is better first to test its strength with pure anhydrous carbonate of soda, and to calculate the quantity required by the Rule of Proportion. Every 53 gr. of the dry carbonate are equal to 40 gr. of ‘dry sulphuric acid.’ Suppose we find the oil of vitriol to contain only 72% of hydrated acid, then—
100 : 40 :: 72 : 55·55
or, instead of only 40 gr., fully 551⁄4 gr. will be required, which are to be made up with water to 1000 gr., as before. Finally, the diluted acid must be very carefully re-tested, and if found correct, at once put into a well-stoppered bottle, and labelled, for use. Too much care cannot be taken to ensure the test-liquid, whether for alkalies or acids, being of the proper strength, of which the specific gravity alone is an insufficient proof. In practice, so small a quantity only of test-acid as that referred to above is, of course, seldom made; but as any larger quantities are mere multiples of the smaller one, the necessary proportions to be employed are easily calculated. The common plan is to prepare one or more gallons or quantities of 10 lbs. each, and to preserve the liquid in stoppered green glass ‘Winchester-quart bottles,’ so that it may be always ready for use.
Although, as may be inferred from the text, sulphuric acid is generally used as the standard acid, yet oxalic acid in pure crystals is recommended by M. Mohr, and answers admirably, and is prepared and used exactly in the same manner.91
| Grains. | are equivalent to | Grains. |
| 22 Carbonic anhydride (dry). | 17 Ammonia (pure or gaseous). | |
| 63 Oxalic acid (crystallised). | 431⁄2 Carbonate of ammonia (neutral, hydrated). | |
| 49 Sulphuric acid (liquid, monohydrated, sp. gr. 1·8485). | 59 Sesquicarbonate of ammonia (Ph. L.; translucent, hydrated). | |
| 75 Tartaric acid (crystallised). | 79 Bicarbonate of ammonia (crystallised). | |
| 1000 Dilute sulphuric acid (sp. gr. 1·033). | 47 Potassa (anhydrous). | |
| 56 Hydrate of potassa (pure caustic potassa). | ||
| Water—gr. measure. | 69 Carbonate of potassa (anhydrous). | |
| 1000 Dilute sulphuric acid (sp. gr. 1·032). | 83 Carbonate of potassa (granulated, commercial). | |
| 87 Carbonate of potassa (crystallised). | ||
| 100 Bicarbonate of potassa (crystallised). | ||
| 31 Soda (anhydrous). | ||
| 40 Hydrate of soda (pure caustic soda). | ||
| 53 Carbonate of soda (anhydrous). | ||
| 143 Carbonate of soda (crystallised). | ||
| 84 Bicarbonate of soda (crystallised). | ||
| 831⁄2 Sesquicarbonate of soda (average commercial). | ||
| 84 Bicarbonate of soda (crystals, or cryst. powder, free from moisture). | ||
| 15 Lithia. | ||
| 24 Hydrate of lithia. | ||
| 37 Carbonate of lithia. | ||
| 761⁄2 Baryta (pure, caustic). | ||
| 851⁄2 Hydrate of baryta. | ||
| 981⁄2 Carbonate of baryta. | ||
| 28 Lime (pure, caustic; i. e. quick-lime). | ||
| 37 Hydrate of lime (slaked lime). | ||
| 50 Carbonate of lime (chalk; marble). | ||
| 20 Magnesia (pure, calcined). | ||
| 42 Carbonate of magnesia (dry, neutral). | ||
| 481⁄2 Carbonate of magnesia (ordinary commercial). | ||
| 52 Stronia (pure, caustic). | ||
| 61 Hydrate of strontia. | ||
| 74 Carbonate of strontia. |
ALKALOID. Syn. Vegetable Alkali, Organic Base; Alkaloïdes (pl., -IDES, or -IDÆ), L.; Alcaloïde, Alcali organique, Fr. In chemistry, a name commonly given to any proximate principle of vegetable origin possessing alkaline or basic properties, however feeble. In its most extended sense the term embraces all organic bases, whether obtained from the animal or vegetable kingdom, or produced artificially. The alkaloids form a numerous and important class of bodies. They exist in nature nearly always in the form of salts, the acid being often, like themselves, peculiar to the plant, or class of plants, in which they are found; whilst the medicinal activity of the latter, in most cases, almost entirely depends on their presence.
Prep. The following general methods of procuring the alkaloids will be found applicable to such as full directions are not given for under their respective heads:—
1. (When the base is insoluble in water, non-volatile, and existing in the plant in an insoluble form.) The bruised plant is boiled or macerated in water acidulated with hydrochloric or acetic acid, and the liquor, after filtration, is neutralised with an alkali (ammonia, potassa, lime, or magnesia); the resulting precipitate is purified by re-solution in dilute acid, digestion with a little animal charcoal, and subsequent crystallisation, or re-precipitation with an alkali; or the first precipitate is purified by dissolving it once, or, if necessary, several times, in boiling alcohol, which yields the pure alkaloid either on cooling or by evaporation.
2. (When the base is insoluble in water, and non-volatile, but existing in the plant as a soluble salt.) The bruised or sliced plant is boiled or macerated in water, and the filtered liquor precipitated and otherwise treated as before.
3. (When the base is soluble in water, and non-volatile.) An infusion made with very dilute acid, hydrochloric or acetic, is concentrated by a gentle heat; and the residual liquor treated with potassa (or concentrated solution of ammonia) and ether conjointly; after repose, the ethereal solution is decanted and evaporated. For those alkaloids which are insoluble in ether (as morphia and cinchonia), the previous process may be adopted.
4. (When the base is both soluble in water and volatile.) The vegetable, in a bruised or divided state, or its extract, is alkalised with potassa and distilled; the distillate is neutralised92 with dilute oxalic or sulphuric acid, and carefully evaporated to dryness; the residuum is next digested in alcohol, and the resulting tincture agitated with potassa and ether, the former being in quantity just sufficient to seize on all the acid; lastly, the ethereal solution thus formed, on careful evaporation, leaves the alkaloid nearly pure. It may be further purified by cautious distillation.
As some of the alkaloids are soluble in excess of the alkaline precipitant, over-saturation should be carefully avoided; or the precipitant may be used under the form of carbonate or bicarbonate. When lime and magnesia are employed, they are boiled for a few minutes with the solution.
Props. Alcoholic or aqueous solutions of the alkaloids generally exhibit an alkaline reaction with vegetable colours. Like the alkalies, also, they combine with acids to form salts which, when dissolved in water, are capable of producing the ordinary phenomena of saline double decomposition. Their taste is usually intensely bitter.
The majority of the natural alkaloids contain carbon, hydrogen, nitrogen, and oxygen, and are, at ordinary temperatures, solid, and not volatile without decomposition. Some natural alkaloids contain carbon, hydrogen, and nitrogen only; these are, for the most part, liquid at ordinary temperatures, and can be distilled without decomposition. The greater number of the artificial alkalies are composed of carbon, hydrogen, and nitrogen; some, however, contain oxygen in addition. Alkaloids have also been obtained artificially, in which nitrogen is replaced by phosphorus, arsenic, antimony, or bismuth. Most of the alkaloids, as they are obtained in the free state, correspond in function to ammonia, NH3, rather than to the fixed alkalies; that is to say, they form salts by direct union with acids, without elimination of water or any other substance. In order to make them strictly comparable to the fixed alkalies, they require, like ammonia, the addition of water (H2O) to their formulæ; they may then be considered as hydrates of compound radicles analogous to ammonium.
Physiological action. The alkaloids generally possess great medicinal power; some of them act with terrific energy, and are the most violent poisons with which we are acquainted. Perfectly pure aconitia is about 200 times more poisonous than arsenic, and at least 50 times more poisonous than ordinary medicinal prussic acid. The greater number act on animals in the same way as the plants which produce them, provided they are given in proportionately small doses. Many of them, when judiciously administered, are most valuable medicines.
Pois., Ant., &c. Some of the alkaloids act as narcotic or stupefying poisons; others are classed with the narcotico-acrid poisons, or those which produce both narcotism and irritation of the parts they touch. The general symptoms produced by opium and its preparations may be taken as an example of the former; those from aconite and strychnia, of the latter. In large doses of the greater number, narcotism predominates; in smaller ones, irritation; they are rarely coexistent.—Treatm. No common antidote to the effects of this class of substances has yet been discovered. The only safe treatment, of at all general application, is to immediately clear the stomach by means of a strong and quick-acting emetic (as sulphate of zinc), or the stomach-pump, and to administer copious and continued draughts of astringent vegetable solutions (as of tannin, nut-galls, oak-bark, or what is always at hand—very strong tea or coffee). These may be followed by or combined with a smart purge of castor oil, as soon as the stomach is thoroughly cleared of the poison. M. Bouchardat strongly recommends a solution of iodine, 3 gr., and iodide of potassium, 6 gr., in pure water, 16 fl. oz., in cases of poisoning by OPIUM, ACONITE, COLCHICUM, DEADLY NIGHTSHADE, HEMLOCK, NUX VOMICA, &c., or by the alkaloids obtained from them—ACONITINE, ATROPIA, COLCHICINA, CONIA, MORPHIA, STRYCHNIA, &c., or their salts; but not where foxglove or digitalin has been taken. The stomach having been well emptied by an emetic, the solution is to be given by wine-glassfuls for some time; the vomiting being still encouraged during the early part of the administration of the antidote. In the case of narcotics (as opium, morphia, &c.), this is to be followed by the free use of a strong infusion of coffee. According to Dr Garrod, purified animal charcoal is an ‘excellent antidote’ to many of the alkaloids, including those above enumerated, when taken in poisonous doses; as it not merely absorbs them, but, for the most part, renders them inert. To be serviceable it should be recently prepared and fresh-burnt; and should be given in doses of about an ounce at a time, diffused in warm or tepid water, and frequently repeated. The vomiting which follows its use, owing to the warm water, proves advantageous; but after a sufficient time may be lessened by employing less water, or cooler or even cold water. Drowsiness, if present, may be combated by the subsequent use of strong coffee or tea, as before. We have seen this plan succeed in several cases.—Lesions. These, like the symptoms, vary. In some cases there are redness and inflammation of the stomach and intestines, and turgescence of the vessels of the lungs and brain; in others, these appearances are either slight or wholly wanting. Wherever there has been much cerebral disturbance, traces of congestion are usually discernible.
Detec., Tests, &c. The identification of the pure alkaloids is extremely simple; but their detection, when combined with organic and colouring matters, is a task of considerable difficulty. One or other of the following plans may be adopted for this purpose:93—
1. (Merck.) The matter under examination is digested, for several hours, with concentrated acetic acid, added in sufficient quantity to produce a strongly acid reaction; the fluid portion is then strained from the insoluble matter, and the latter being washed with water acidulated with acetic acid, the mixed liquors are gently evaporated to dryness in a water bath; the residuum of the evaporation is boiled first with rectified spirit, and next with rectified spirit acidulated with acetic acid; the mixed liquors are again evaporated, the residuum redissolved or diluted with distilled water, and carbonate of soda or potassa added to feebly alkaline reaction, and the whole, after evaporation to the consistence of a syrup, set aside to repose for 24 hours; it is now again diluted with water, filtered, and the insoluble portion washed with cold distilled water, and digested with concentrated acetic acid; this last solution is diluted with distilled water, and decoloured with pure blood-charcoal (if it be necessary); the fluid, either at once, or after cautious evaporation, may then be tested for the alkaloids, in the usual manner. The charcoal previously used should also be tested in the way described below. This method answers admirably with all the NON-VOLATILE ALKALOIDS, and may be applied to the stomach and viscera, and their contents, and to food, &c., in cases of poisoning.
2. (Stas.) The suspected matter, in a finely divided state, is digested, at 160° to 165° Fahr., with twice or thrice its weight of strong alcohol acidulated (according to the quantity) with 1⁄2 dr. to 2 or 3 dr., or more, of pure oxalic or tartaric acid. After a sufficient time, and when the whole has become quite cold, it is thrown on a filter, and the undissolved portion, after being squeezed dry, is washed with strong alcohol. The mixed and filtered alcoholic liquids are then evaporated at a temperature not exceeding 95° Fahr., and, if no insoluble matter separates, the evaporation is continued nearly to dryness;[19] but if fatty or other insoluble matter separates during the process of concentration, the concentrated fluid is passed through a moistened filter, and the filtrate evaporated nearly to dryness, as before. The residuum is next digested with absolute alcohol, in the cold, the insoluble portion, after filtration, washed with alcohol, and the mixed filtrates again evaporated in the air, or in vacuo. The acid residue is now dissolved in a little distilled water, and bicarbonate of soda added as long as effervescence ensues. To this mixture 4 or 5 times its volume of ether is added, and after lengthened agitation (the bottle or tube being held in a cold wet cloth), the whole is allowed to repose for a short time. A little of the supernatant ether is now removed to a small glass capsule or watch-glass, and allowed to evaporate spontaneously.[19] When this leaves oily streaks upon the glass, which gradually collect into a small drop, which emits, when gently heated, a disagreeable, pungent, and stifling odour, the presence of a LIQUID VOLATILE BASE or ALKALOID is inferred; whilst a solid residue or a turbid fluid with small solid particles floating in it, indicates a NON-VOLATILE SOLID BASE.[20] In either case the blue colour of reddened litmus is permanently restored by the residuum. If no residuum is left on the capsule, some solution of pure soda or potassa is added to the liquid, the whole well agitated for several minutes, and the ether (after repose) decanted; an operation which is repeated with fresh ether a second, third, and even a fourth time. The base, or bases (if any are present), will now be found in the mixed ethereal solution, which is, therefore, tested as before. The presence of an alkaloid being detected, the mixed ethereal solutions are allowed to evaporate spontaneously, care being taken, if a volatile alkaloid be present, to neutralise the liquid with an acid before the final evaporation. The last residuum is then tested for the particular alkaloid present, as before.[21]
This method, according to Stas, answers well for all the ALKALOIDS which are soluble in ether; including—ACONITIA, ANILINE, ATROPIA, BRUCIA, CODEIA, COLCHICINA, CONIA, DELPHIA, EMETINA, HYOSCYAMINE, MORPHIA (?), NICOTIA, PETININE, PICOLINE, SOLANINE, STRYCHNIA, VERATRIA, &c. By means of it Stas found nicotia in the heart-blood of a poisoned dog. With such alkaloids as are, however, only very sparingly soluble in ether (as morphia for instance), the result must, necessarily, be doubtful. To detect these, as well as all the alkaloids which are insoluble in ether, it is, therefore, necessary, as directed by Otto, to add to the alkaline fluid left by the decantation of the ether, sufficient solution of soda to dissolve the morphia, &c. (if any has separated), and after the expulsion of the last traces of the ether by a gentle heat, to add a concentrated solution of hydrochlorate of ammonia, and to allow the mixture to repose for some time in the open air. When MORPHIA is present, it separates under the form of small crystals.[22] Or the alkaline liquor may be diluted with distilled water, and treated with charcoal, and this with alcohol, in the manner noticed under method 4 (below).
4. (Graham and Hoffmann—slightly modified.) 2 or 3 oz. of purified animal charcoal are digested in about 1⁄2 gal. of the (neutral or only slightly acid) aqueous fluid under examination, with frequent agitation, for 10 to 12 hours, or longer. The liquid is then filtered, and the charcoal left on the filter is washed twice with cold distilled water. The charcoal94 is then boiled for 1⁄2 an hour with about 1⁄2 a pint of rectified spirit of 80 or 90%; the ebullition being conducted in a flask having a very long tube, open at both ends, fitted air-tight through the cork, to prevent loss of the alcohol by evaporation. The spirit, which now contains the alkaloid (if any was present in the original liquor), is next filtered whilst hot, and the filtrate is submitted to distillation until the whole of the alcohol is removed. A small quantity (commonly a few drops) of solutions of potassa is then added to the residual aqueous liquor, followed by 1 to 2 fl. oz. of pure ether, after which the whole is well agitated for several minutes, and allowed to repose for a short time. Lastly, the supernatant ether is decanted, and allowed to evaporate spontaneously, when the residuum (if any) left in the capsule may be tested by reagents, as before.
This method was devised for the detection of STRYCHNIA and NUX VOMICA in malt-liquors; but it is equally applicable to the detection of ANY ALKALOID which is soluble in ether. The CHARCOAL TEST may also be employed to detect alkaloids which are insoluble in ether; but then the base must be sought in the aqueous residuum obtained by the evaporation of the alcohol.[23]
The presence of the alkaloids and their salts, in clear solutions, may be thus determined:—
I. (Fresenius).—1. The solution is rendered very slightly alkaline with dilute solution of potassa or soda, added drop by drop:—
a. No precipitate is formed; total absence of the alkaloids. (See 4, below.)
b. A precipitate is formed:—solution of potassa or soda is added, drop by drop, until the liquid exhibits a strong alkaline reaction:—
α. The precipitate redissolves; absence of Brucia, Cinchonia, Narcotina, Quina, Strychnia, and Veratria; probable presence of Morphia.
β. Precipitate does not redissolve, or not completely; probable presence of one or more of the first six of the above-named alkaloids:—the fluid is filtered from the precipitate, mixed with either bicarbonate of soda or of potassa, gently boiled nearly to dryness, and treated with water. If it dissolves completely; absence of morphia; an insoluble residue indicates Morphia.
2. The precipitate 1. b. β. is washed with cold distilled water, dissolved in a slight excess of dilute sulphuric acid, neutralised with a saturated solution of bicarbonate of soda, and allowed to repose a few hours:[24]—
a. No precipitate; absence of Cinchonia, Narcotina, and Quina:—the solution is gently evaporated nearly to dryness, and treated with cold water:—if it dissolves completely, pass on to 4; if there is an insoluble residue, it may contain Brucia, Strychnia, or Veratria. (See 3.)
b. A precipitate:—the filtered fluid is treated as directed at 2 a.; the precipitate is washed with cold distilled water, dissolved in a little hydrochloric acid, ammonia is added in excess, and subsequently a sufficient quantity of ether, agitation being had recourse to:—
α. The precipitate formed by the ammonia redissolves completely in the ether, and the clear fluid separates into two layers; absence of Cinchonia; probable presence of Quina or Narcotina.
β. The precipitate produced by the ammonia does not redissolve in the ether, or not completely; probable presence of Cinchonia, and perhaps also of Quina or Narcotina. The filtered liquid may be tested for these alkaloids as at a.
3. The insoluble residuum after the evaporation of the solution 2. a., or of the filtrate 2. b., is now dried in a water bath, and digested with absolute alcohol:—
a. It dissolves completely; absence of strychnia; probable presence of Brucia, Quina (?), or Veratria:—the alcoholic solution is evaporated to dryness, and, if quina has been already detected, the residue is divided into two portions, one of which is tested for Brucia, the other for Veratria.
b. It does not dissolve, or not completely; probable presence of Strychnia, and perhaps also of Brucia and Veratria:—the filtered fluid is divided into two portions, and tested separately as at a.
4. The original liquid 1. a. may contain Salicine, a proximate vegetable principle closely allied to the alkaloids:—a portion is boiled with hydrochloric acid for some time; the formation of a precipitate shows the presence of Salicin. (See 2, below.)[25]
II. (Larocque and Thibierge.) Terchloride of gold is recommended, by these writers, as a more decisive test for the alkaloids than the ‘double chloride of gold and sodium’ commonly employed for this purpose. The following are the colours of the precipitates which it produces with the aqueous solution of their salts:—Brucia, milk-brown, passing into coffee-brown, and lastly chocolate-brown:—CINCHONIA, sulphur yellow:—MORPHIA, yellow, then bluish, and lastly violet; in this last state the gold is reduced, and the precipitate is insoluble in water, alcohol, the caustic alkalies, and sulphuric, nitric, and hydrochloric acid; it forms with aqua regia a solution95 which is precipitated by protosulphate of iron:—QUINA, buff-coloured:—STRYCHNIA, canary-yellow:—VERATRIA, pale greenish-yellow. All these precipitates, with the exception mentioned, are very soluble in alcohol, insoluble in ether, and only slightly soluble in water. Those with morphia and brucia are sufficiently marked to prevent these alkalies from being mistaken for each other; and those with brucia and strychnia are, in like manner, easily distinguishable.
III.—Mr Wanklyn discriminates the different alkaloids from the estimation of the ammonia they evolve. His process is as follows:—A small flask with a lateral tube, and connected with a Liebig’s condenser, is charged with about 25 c. c. of an alkaline solution of permanganate potash made by dissolving 200 grammes of caustic potash and 8 grammes of crystallised permanganate of potash in 1 litre of water. A minute quantity of the alkaloid carefully and accurately weighed is now introduced, and the mixture slowly distilled. The most satisfactory results are obtained by treating from 1 to 5 milligrammes of the alkaloid in this way, but quantities so small as 1⁄10th of a milligram will in skilled hands give accurate results. The ammonia is formed in the distillate by Nesslerising it, as described under Water analysis. For all practical purposes the poisonous alkaloids may be divided into four classes:
(a) Those which yield from 5 to 2 per cent. of ammonia.
(b) Those which yield from 2 to 3 per cent. of ammonia.
(c) Those which yield from 3 to 5 per cent. of ammonia.
(d) Those which yield a larger quantity than 5 per cent., e.g.
| I. | |
| NH3 per cent. | |
| Solanine yields half its nitrogen as Ammonia | 0·98 |
| II. | |
| Morphia yields half its nitrogen as Ammonia | 2·98 |
| Codeine, ditto, ditto | 2·87 |
| Papaverine, ditto, ditto | 2·50 |
| Veratria, ditto, ditto | 2·87 |
| III. | |
| Atropia yields all its nitrogen as Ammonia | 5·73 |
| Narcotine, ditto, ditto | 4·11 |
| Strychnia yields half its nitrogen as Ammonia | 5·09 |
| Brucine, ditto, ditto | 4·32 |
| Aconite, ditto, ditto | 3·50 |
| Coneine, ditto, ditto | 4·60 |
| IV. | |
| Nicotine yields half its nitrogen as Ammonia | 10·49 |
IV. Dr Guy, as well as others, have made researches, having for their object the determination of the exact temperature at which the poisonous alkaloids melt and sublime. A very minute speck of the substance is placed on a porcelain plate or copper disc, and a square or oval of microscope-covering glass is placed over it, supported by a thin ring of glass or any other convenient substance.
Heat is then applied to the plate or copper, and the temperature, as indicated by a thermometer at which the substance fuses or volatilises, is carefully noted.
| Fahr. | Cent. | ||||
| Cantharidine sublimes as a white vapour without change of form or colour. | 212° | 100° | |||
| Sublime. | Melt. | ||||
| Fahr. | Cent. | Fahr. | Cent. | ||
| Morphine | Sublime, melt and yield carbonaceous residue. | 330° | 165° | 340° | 171° |
| Strychnine | 345° | 174° | 430° | 224° | |
| Melt. | Sublime. | ||||
| Fahr. | Cent. | Fahr. | Cent. | ||
| Aconitine | Melt, change colour, sublime, and deposit carbon. | 140° | 60° | 400° | 204° |
| Atropine | 150° | 66° | 280° | 138° | |
| Veratrine | 200° | 93° | 360° | 182° | |
| Brucine | 240° | 116° | 400° | 204° | |
| Digitalin | 310° | 154° | 310° | 154° | |
| Picrotoxin | 320° | 160° | 320° | 160° | |
| Solanine | 420° | 215° | 420° | 216° | |
Selmi’s method of extracting poisonous alkaloids in forensic investigations. The alcoholic extract of the viscera, acidified and filtered, is evaporated at 65° C., the residue taken up with water, filtered to separate fatty matters, and decoloured by means of basic acetate of lead, leaving the solution in contact with the air for 24 hours. It is then filtered, the lead precipitated by means of sulphuretted hydrogen, and the solution after concentration repeatedly extracted with ether. The ethereal solution is then saturated with dry carbonic anhydride, which generally causes a precipitate of minute drops adhering to the sides of the vessel, and containing some of the alkaloids. The ethereal solution is then poured into a clean vessel, mixed with about half its volume of water, and a current of carbonic anhydride96 passed for about twenty minutes, which may cause the precipitation of other alkaloids not precipitated by dry carbonic anhydride. Usually the whole of the alkaloids present in the ether are thrown down by these means, but if not, the solution is dehydrated by agitation with Barium oxide, and then a solution of tartaric acid in ether added to the clear liquid, taking great care not to employ excess of acid. This throws down any alkaloid that may remain. In order to extract any alkaloids that may still remain in the viscera, they are mixed with Barium hydrate and a little water, and then agitated with purified amylic alcohol; the alkaloids may subsequently be extracted from the alcohol by agitation with very dilute sulphuric acid.
A knowledge of the different solubilities of the alkaloids will be found an important auxiliary in their analysis. The following is a summary of the relative solubility of the most important of them. The figures denote the number of parts of the liquid required for their solution:—
Absolute alcohol.—Strychnine insoluble; brucine soluble.
Amylic alcohol.—Solanine (1061); digitalin sparingly soluble; morphine (133); strychnine (122); veratrine, brucine, atropine, aconitine, and picrotoxin, freely soluble.
Benzol.—All the poisonous alkaloids, except solanine, are soluble in benzol.
Chloroform.—Solanine (50,000); morphine (6550); strychnine (8); the rest freely soluble.
Ether.—Solanine (9000); morphine (7725); strychnine (1400); aconitine (777); brucine (440); veratrine (108); atropine, picrotoxin,[26] and digitalin, very soluble.
Water (cold).—Strychnine (8333); veratrine (7860); morphine (4166); aconitine (1783); solanine (1750); brucine (900); atropine (414); picrotoxin (150); digitalin very soluble.
The principal Alkaloids and their Salts, in the state of powder, or with ‘conia’ and ‘nicotia,’ in the state of an oily looking liquid, may be thus distinguished:—
1. a. The powder is treated with nitric acid:—It is coloured red; probable presence of Brucia, Delphia, Morphia, or commercial Strychnia. If the reddened acid becomes violet on the addition of ‘protochloride of tin,’ it is Brucia; if it becomes black and carbonaceous, it is Delphia. If the powder is fusible without decomposition, and strongly decomposes iodic acid, it is Morphia; if it is not fusible without decomposition, and does not decompose iodic acid, it is Strychnia.
b. If instead of a red, the powder strikes a green colour with nitric acid, it is Solania; if it is insoluble in ‘ether,’ and not reddened by ‘nitric acid,’ it is Emetia; if soluble in ether, not reddened by ‘nitric acid,’ but melts and volatilises when heated, it is Atropia; if it is thus affected by ether or nitric acid, but does not volatilise, it is Veratria. (See 2, below.)
2. a. The powder, or (with ‘conia and nicotia’) concentrated liquor, is treated with a drop or two of concentrated sulphuric acid:—A red colour is produced; probable presence of Brucia, Nicotina, Salicine, or Veratria. If the reddened mixture has at first a roseate hue, turning deep red on the addition of nitric acid, it is Brucia; if the original substance moistened with solution of potassa evolves the odour of tobacco, it contains Nicotine; if the red colour produced by the acid is permanent and of an intense blood-hue, and the powder agglutinates into lumps like resin, it is Salicine; if the colour is at first yellowish, changing to blood-red, and ultimately to crimson and violet, it is Veratria.
b. If instead of the substance being ‘reddened’ by strong sulphuric acid, no particular action ensues in the cold, it contains either Conia or Strychnia; if a small fragment of bichromate of potassa being now dropped in, produces a rich violet colour, it is Strychnia; if the original matter on being heated, or treated with solution of potassa, evolves a penetrating, disagreeable odour, somewhat analogous to that from ‘hemlock,’ or to a mixture of those from tobacco and mice, it is Conia.
“Reactions with ceroso-ceric oxide. This oxide exhibits characteristic colours with several alkaloids, especially with STRYCHNINE. When strong sulphuric acid is poured upon strychnine, and then a small quantity of ceroso-ceric oxide added, a fine blue colour is produced, similar to that which strychnine exhibits with potassium bichromate, but much more permanent. The blue colour gradually changes to cherry-red, and then remains unaltered for several days. This reaction is capable of detecting one part of strychnine in a million parts of liquid. Brucine similarly treated acquires an orange-colour, gradually changing to yellow; MORPHINE, olive-brown, finally brown; NARCOTINE, brown cherry red, finally wine-red; CODEINE, olive-green, finally brown; QUININE, pale-yellow; CINCHONINE and THEINE remain colourless; VERATRINE becomes reddish-brown; ATROPINE, dingy yellowish-brown; SOLANINE, yellow at first, finally brownish; EMETINE, brown; COLCHICINE, first green, then dirty brown; ANILINE, after a long time, acquires a blue colour extending from the edges inwards; CONINE becomes light-yellow. Piperine colours the sulphuric acid blood-red, and is turned dark-brown, almost black by the cerium oxide” (Sonnenschein).
“Reactions with picric acid. This acid is a very good precipitant for alkaloids, affording a very delicate test for many of them, and may perhaps also serve for separating them one from another. The precipitation takes place97 even in solutions containing a large excess of sulphuric acid, and is sometimes complete. Precipitated are, BRUCINE, STRYCHNINE, VERATRINE, QUINIDINE, CINCHONINE, and most of the opium alkaloids; not precipitated, MORPHINE, ATROPINE (English), PSEUDO-MORPHINE, CAFFEINE, and all glucosides” (Hager).
The presence of one or more of the alkaloids being shown by any of the preceding methods, a portion of the original clear solution or powder, or of the precipitates or filtrates above referred to, must be treated with their characteristic tests, as given under the individual notices of these articles, so as to set at rest all doubt as to their identity. No single test must ever be relied on as a positive proof. The presence of Brucia, Morphia and Strychnia may be determined in substances which after being mixed with the salts of these alkaloids have undergone the acetous, vinous, or putrefactive fermentation, as shown by Orfila, MM. Larocque and Thibierge, and many other eminent chemists and toxicologists, and confirmed, in numerous cases, by our own experiments. Opium and morphia may thus be readily detected in beer, wine, soup, and milk. A paper by Professor Dragendorf in the ‘American Chemist’ for April, 1876, may be consulted with advantage.
Concluding Remarks. It is a singular fact that none of the organic bases found in plants have yet been formed artificially, although several analogous substances have been thus produced. Closely allied to the alkaloids there also exists an extensive series of neutral proximate principles, which differ from those substances chiefly in the absence of basic properties, and in most of them being destitute of nitrogen. They are usually bitter, and, like the alkaloids, generally represent the active properties of the plants in which they are found; whilst some of them possess considerable medicinal energy. Of this kind are asparagin, elaterin, gentianin, picrotoxin, salicin, &c. These two classes of bodies, though actually distinct, are frequently confounded. See Alkali, Organic Bases, Poisons, Proximate Principles, Vegetables, Nomenclature, &c.; also the individual alkaloids under their respective heads.
ALKALOIDS OF ACONITE. The nature of the active principle of aconite root does not appear to have been satisfactorily determined. Messrs Groves, Wright, and Williams contend that the Aconitum napellus yields an active crystalline alkaloid, which they distinguish as Aconitine, and to which they assign the formula C33H43NO12; they add that additionally the root contains more or less of another active alkaloid, which they term Pseudaconitine, and which is represented by the formula C36H49NO11; they also assert that the extract of the roots contains varying quantities of certain decomposition products resulting from the saponification of the above bases by the acids, which are produced by the breaking up of part of the aconitine. The name of these decomposition products is Aconine and Pseudaconine. Of Aconitum ferox they report that it yields a comparatively large quantity of Pseudaconitine and a small quantity of Aconitine. They further affirm that the so-called aconitine of commerce is a mixture of true aconitine and pseudaconitine with variable quantities of their alteration products, aconine and pseudaconine, and of certain amorphous unnamed alkaloids.
Messrs Paul and Kingzett contest the accuracy of these deductions, and dispute the correctness of the formula given to aconitine. Dr Paul doubts whether the alkaloid to which the active properties of the root are ascribed has ever yet been obtained in an isolated condition. He thinks it probable that the substance obtained from aconite root was to a great extent a salt of an acid, like aconitic acid. For further information the reader is referred to the ‘Pharmaceutical Year Book’ for 1873, 1874, 1875, 1876, and 1877.
AL′KANET. Syn. Anchu′sa, L.; Orcanette, Fr.; Orkanet, Ger.; Or′chanet*, Dyer’s al′kanet, D. bu′gloss*. The anchu′sa tincto′′ria (Willd.; lithosper′mum tincto′′rium—Linn.), a deciduous herbaceous plant, with a perennial, dark blood-red root. Hab. Asia Minor, Greece, Hungary, &c. It is also largely cultivated in the neighbourhood of Montpellier. The dried root (ALKANET ROOT; RADIX ANCHUSÆ, R. A. TINCTORIÆ) is chiefly imported from the Levant. It contains a beautiful blood-red colour, which it freely gives out to oils, fats, wax, spirits, essences, and similar substances, by simply infusing it in them, and is consequently much employed to colour these articles. Wax tinged with it, and applied on warm marble, stains it of a rich flesh-colour, which sinks deep into the stone, and possesses considerable durability. Its spirituous tincture also imparts a deep red to marble.
Prop., &c. The colouring matter of alkanet was regarded by Pelletier as a fatty acid (ANCHUSIC ACID); but it has since been shown to be a species of resin (ANCHUSINE, PSEUDO-ALKANNINE, P.-ALKANIUM). According to Dr John, good alkanet root contains 51⁄2 per cent. of this substance. Anchusine melts at 140° Fahr.; is scarcely soluble in water, to which it only imparts a dirty red colour, but is very soluble in alcohol, oils, and acetic acid. Alkalies turn it blue. It is found wholly in the root-bark. In selecting this article, the smaller roots should therefore be chosen, as they possess more bark than the larger ones, in proportion to their weight. Exposure to ammoniacal fumes, or even handling it much with the fingers, changes its red to a crimson or purplish hue.
Uses, &c. It is much employed by druggists and perfumers to colour oils, lip-salves, plasters, pomatums, &c.; by varnish-makers, to tinge their varnishes and lacquers; by statuaries98 to stain marble; by dairy-farmers, to colour cheese; by wine-merchants and bottlers (in the form of tincture), to stain beforehand the corks of their port-wine bottles, in order to imitate the effects of age, and as colouring and flavouring for factitious port wine; and by dyers, and others. A species of crimson rouge was formerly prepared from it (hence its name).
ALLANTO′IC ACID. See Allantoin.
ALLAN′TOIN. C8H6O6N4. Syn. Allanto′ic acid*, Amniot′ic a.† Am′nic a.†; Allantoï′na, L. A substance discovered by Vauquelin and Buniva in what they imagined to be the liquor amnii of the cow, and hence named by them amniotic acid. It was afterwards shown by Dzondi and Lassaigne to exist in the fluid of the allantoïs, and not of the amnios. It has since been produced artificially by Wöhler and Liebig.
Prep. 1. The allantoïc fluid of the fœtal calf is evaporated to 1-4th or 1-5th of its volume, and then set aside for some time. The crystals thus obtained are purified by re-solution, digestion with animal charcoal, and re-crystallisation.
2. (Wöhler and Liebig.) Uric acid, 1 part; is dissolved in water, 20 parts; and freshly precipitated and well-washed binoxide of lead is added to the solution until the colour ceases to change; the liquid is next filtered while hot, evaporated until a pellicle forms on the surface, and then set aside to crystallise; the crystals being purified as before.
Prop., &c. Small, but very brilliant prismatic, transparent, colourless crystals; tasteless; neutral; soluble in 160 parts of cold water, and in much less at 212°; nitric acid converts it into ALLANTURIC ACID; oil of vitriol resolves it into ammonia, carbonic acid, and carbonic oxide; hot concentrated solutions of the caustic alkalies change it into ammonia and oxalic acid.
ALLANTOX′ICUM. [L.] Syn. Allantox′icum, L. (prim., Gr.). The poison developed, during putrefaction, in sausages made of blood, liver, &c. “It often proves speedily fatal.” (Kraus.)
ALLGEMEINE FLUSSTINCTUR (Sulzberger, Salzungen). For the relief of a number of diseases, among which are cholera and sea-sickness. Aloes, 1 part; spirit of wine, 2 parts. (Spau.)
ALLIA′CEOUS (-sh′us). Syn. Allia′ceus, L.; Alliacé, Ailiacé, Fr.; Knoblauchartig, &c., Ger. Garlick-like; an epithet applied to substances having the odour or properties of garlic or onions.
Alliaceous Plants. Chives, garlic, leeks, onions, rocambole, shallots, &c.
ALLIGA′TION. Syn. Alliga′tio, L. In commercial arithmetic, a rule for ascertaining the price or value of mixtures, and for determining the proportions of the ingredients that must be taken to produce mixtures of any given price, value, or strength. The first is called ALLIGATION ME′DIAL; the second, ALLIGATION ALTERN′ATE. Its principles and applications are explained under Mixtures (Arithmetic of).
ALLOP′ATHY. Syn. Allopa′thia, L. (from ἁλλος, other, different, and παθος, affection or disease, Gr.); Allopathie, Fr. In medicine, the method of curing disease by the use of remedies which tend to produce a condition of the system, either differing from, opposed to, or incompatible with the condition believed to be essential to the disease it is sought to cure. It is commonly employed to distinguish the ordinary system of medical practice from homœopathy (which see). Hence (an) ALLOP′ATHIST, and the corresponding adjective ALLOPATH′IC (allopath′icus, L.).
ALLOT′ROPY. Syn. Allot′ropism; Allotro′pia, Allotropis′mus, L. Literally, a difference in character; another form of the same substance. In chemistry, a term invented, by Berzelius, to express the state or condition, or the change of character, assumed by certain substances at different temperatures, or under different treatment, whilst their nature and composition continue the same. It more particularly relates to colour, hardness, solubility, texture, &c. Boron, carbon, silicon, iron, sulphur, and phosphorus, afford striking examples of the changes here referred to.
ALLOX′ANTIN. C8H4N4O7.3H2O. A crystallisable substance, first obtained by Dr Prout from uric acid.
Prep. 1. Uric acid, 1 part; is boiled in water, 32 parts; dilute nitric acid being added until solution is complete; the resulting liquid is evaporated to 2⁄3rds its volume, and then set aside for 10 or 12 hours; the crystals, which are deposited, are purified by re-solution and crystallisation.
2. Sulphuretted hydrogen gas is passed, in a full stream, through a moderately strong aqueous solution of alloxan, in the cold. The alloxantin, which is deposited as a crystalline mass, is purified by draining, cautious washing with cold water, re-solution in boiling water, and re-crystallisation. The impure mother-liquor from which crystals of alloxan have separated, if diluted with water, may be used for this purpose.
Prop., &c. Crystals, small colourless, transparent, four-sided, oblique rhombic prisms; scarcely soluble in cold water; solution reddens litmus; with baryta water it gives a characteristic violet-coloured precipitate, which disappears on heating; and with nitrate of silver a black precipitate of that metal; the crystals are reddened by ammoniacal vapours.
ALLOY′. Syn. Alliage, Fr.; Legirung, Vermischung durch schmelzen, Ger. In coinage, a compound of the precious metals with another, or others, of less value; also the least valuable metal, or metals, in such compounds. In chemistry and metallurgy, combinations of the metals with each other usually obtained by fusion. When mercury is one of99 the component metals, the compound is termed an AMALGAM.
Prep., &c. No General rules can be given for this purpose. Alloys of metals differing greatly in fusibility, are commonly made by adding the more fusible one, either in the melted state, or in small portions at a time, to the other melted, or heated to the lowest possible temperature at which a perfect union will take place between them. The mixture is usually affected under a flux, or some material that will promote liquefaction, and prevent volatilisation and unnecessary exposure to the air. Thus, in melting lead and tin together, for solder, resin, or tallow is thrown upon the surface; in tinning copper, the surface is rubbed with sal ammoniac; and in combining some metals, powdered charcoal is used for the same purpose. Quicksilver combines with many metals in the cold, forming AMALGAMS.
Comp. The following Table exhibits the composition of the more important compounds of this class:—
| Names. | Combining metals. |
| Albata | See German Silver. |
| Amalgams | Mercury and other metals. |
| Bath-metal | Copper and zinc. |
| Bell-metal | Copper and tin. |
| Brass | Copper and zinc. |
| Britannia metal | Tin with antimony, copper, and bismuth. |
| Bronze | Tin and copper. |
| Bronze aluminium | Copper and aluminium. |
| Cannon-metal | Tin and copper. |
| Dutch gold | Copper and zinc. |
| Fusible metal | Bismuth, lead, and tin. |
| German silver | Copper, nickel, and zinc, with, sometimes, a little iron and tin. |
| Gold (standard) | Gold with copper. |
| Gold (old standard) | Gold with copper and silver. |
| Gun-metal | See Cannon-metal. |
| Mosaic gold | Copper and zinc. |
| Or-molu | Copper and zinc. |
| Pewter (common) | Tin and lead. |
| Pewter (best) | Tin with antimony, bismuth and copper. |
| Pot-metal, Cock-metal | Copper and lead, with, sometimes, a little zinc. |
| Queen’s metal | Tin with antimony, bismuth, and copper. |
| Shot-metal | Lead with a little arsenic. |
| Silver (standard) | Silver and copper. |
| Solder | Tin and lead. |
| Speculum-metal | Tin and copper, and arsenic. |
| Stereotype-metal | Lead, antimony, and bismuth. |
| Tombac, Red Tombac | Copper and zinc. |
| Tutania | See Britannia metal. |
| Type-metal | Lead and antimony. |
| White copper (Packfong; Whitetombac) | Copper and arsenic. |
Prop., &c. Alloys generally possess characteristics unshared by their component metals. Thus, copper and zinc form brass, which has a different density, hardness, and colour to either of its constituents. Whether the metals tend to unite in atomic proportions, or in any definite ratio, is still undetermined. The evidence afforded by the natural alloys of gold and silver, and by the phenomena accompanying the cooling of several alloys from the state of fusion, goes far to prove that such is the case. (Rudberg.) The subject is, however, one of considerable difficulty, as metals and metallic compounds are generally soluble in each other, and unite by a simple fusion and contact. That they do not combine indifferently with each other, but exercise a species of elective affinity not dissimilar to other bodies, is clearly shown by the homogeneity and superior quality of many alloys in which the constituent metals are in atomic proportions. The variation of the specific gravity and melting-points of alloys from the mean of those of their component metals, also affords strong evidence of a chemical change having taken place. Thus, alloys generally melt at lower temperatures than those required for their separate metals. They also usually possess more tenacity and hardness than the mean of their constituents.
Matthiessen found that when weights are suspend to spirals of hard-drawn wire made of copper, silver, gold, or platinum, they become nearly straightened when stretched by a moderate weight; but wires of equal dimensions100 composed of copper-tin (12% of tin), silver-platinum (36% of platinum), and gold-copper (84% of copper), scarcely undergo any permanent change in form when subjected to tension by the same weight.
The same chemist gives the following approximative results upon the tenacity of certain metals and wires hard drawn through the same gauge (No. 23):
Breaking strain for:
| lbs. | ||
| Copper | 25-30 | |
| Tin | under | 7 |
| Lead | ” | 7 |
| Tin-lead (20% lead) | about | 7 |
| Tin-copper (12% copper) | ” | 7 |
| Copper-tin (12% tin) | ” | 80-90 |
| Gold | 20-25 | |
| Gold-copper (8·4% copper) | 70-75 | |
| Silver | 45-50 | |
| Platinum | 45-50 | |
| Silver-platinum (30% platinum) | 75-80 |
On the other hand, their malleability, ductility, and power of resisting oxygen is generally diminished. The alloy formed of two brittle metals is always brittle; that of a brittle and a ductile metal, generally so; and even two ductile metals sometimes unite to form a brittle compound. The alloys formed of metals having different fusing-points are usually malleable whilst cold, and brittle whilst hot. The action of the air on alloys is generally less than on their simple metals, unless the former are heated. A mixture of 1 part of tin and 3 parts of lead is scarcely acted on at common temperatures; but at a red heat it readily takes fire, and continues to burn for some time like a piece of bad turf. In like manner, a mixture of tin and zinc, when strongly heated, decomposes both moist air and steam with almost fearful rapidity.
The specific gravity of alloys is never the arithmetical mean of that of their constituents, as commonly taught; and in many cases considerable condensation or expansion occurs. When there is a strong affinity between two metals, the density of their alloy is generally greater than the calculated mean; and vice versâ, as may be seen in the following Table:—
| Greater than the mean of their constituents:— | Less than the mean of their constituents:— | ||
| Copper and | bismuth, | Gold and | copper, |
| ” | palladium, | ” | iridium, |
| ” | tin, | ” | iron, |
| ” | zinc, | ” | lead, |
| Gold and | antimony, | ” | nickel, |
| ” | bismuth, | ” | silver, |
| ” | cobalt, | Iron and | antimony, |
| ” | tin, | ” | bismuth, |
| ” | zinc, | ” | lead, |
| Lead and | antimony, | Nickel and | arsenic, |
| Palladium and | bismuth, | Silver and | copper, |
| Platinum and | molybdenum, | Tin and | antimony, |
| Silver and | antimony, | ” | lead, |
| ” | bismuth, | ” | palladium, |
| ” | lead, | Zinc and | antimony. |
| ” | tin, | ||
| ” | zinc. | ||
“Every alloy,” says Dr Ure, “is, in reference to the arts and manufactures, a new metal, on account of its chemical and physical properties. A vast field here remains to be explored. Not above sixty alloys have been studied by chemists, out of many hundreds which may be made, and of these very few have yet been practically employed. Very slight modifications often constitute very valuable improvements upon metallic bodies.” See Analysis, Assaying, Brass, Bronze, Electrotype, German Silver, Gold, Metals, Specific Gravity, &c.
ALL′SPICE. See Pimento.
ALLU′′VIAL. (-l’ōōv′-yăl). Syn. Allu′′vious*; Allu′′vius, L.; d′Alluvion, Fr. In geology, applied to partial deposits of mud, sand, gravel, &c., left by rivers and floods upon land not permanently submerged beneath water; in agriculture, applied to soils so formed or deposited.
ALLU′′VIUM. [L., Eng.] Syn. Alluvion, Fr.; Anflössung, Anschwemmung, Ger. In geol. and agr., alluvial deposit or soil. See Soils, &c.
AL′LYL (-lĭl). C3H5. In chemistry, the radical of the essential oils containing sulphur, as those of assafœtida, garlic, horseradish, mustard, onions, &c., which are either sulphides or sulphocyanides of allyl. Its probable existence was first shown by Captain Reynolds, who succeeded in producing several of its derivatives. It has since been obtained, in a separate state, by the action of sodium upon iodide of allyl. It is an oily substance with a high boiling point.
Allyl, Sulphide of, (C3H5)2S; obtained (artificially) by acting on sulphocyanide of allyl with sulphide of potassium. See Oil of Garlick.
Allyl, Sulphocy′anide of, C3H5CNS; obtained by submitting iodide of allyl to the action of sulphocyanide of potassium; or by gently heating a mixed alcoholic solution of sulphide of allyl and bichloride of mercury, with sulphocyanide101 of potassium. See Oil of Mustard (Volatile).
AL′MOND (ah′-mŭnd). Syn. Amyg′dala (also -US, -UM*), L.; Amande, Fr.; Mandel, Ger., Dut., Dan., Swed. The ‘almond-tree’ (amyg′dalus commu′nis—Linn.; Ph. L., E., and D.; Amandier—Fr.), a tree of the nat. ord. Rosaceæ, indigenous to Persia, Syria, and the north of Africa; but also extensively cultivated in southern Europe. The almond-tree is about the size of the peach-tree, which it much resembles in appearance. It is incapable of ripening its fruit in this country, and is, therefore, only grown here for the sake of its beautiful vernal flowers. There are several varieties, of which the most important are the sweet and the bitter, so named from the flavour of the seed or kernel. These, for the most part, resemble each other in appearance. De Candolle (‘Prodromus,’ ii, 530) gives five varieties of this species:—A. AMA′′RA (bitter-almond); A. DUL′CIS (sweet-a.); A. FRAGILIS (tender-shelled a.); A. MACROCAR′PA (large-fruited a., pista′chio a., sultana a.); A. PERSICO′ÏDES (peach a.).
Almond, Per′sian. The peach.
AL′MONDS. Syn. Amyg′dalæ, L.; Amandes, Fr.; Mandeln, Ger. The seed or kernels of the almond-tree. They are met with in commerce both in the shell (AMYG′DALÆ CUM PUTAM′INE, -ĭn-e, L.), and shelled (AMYGDALÆ, L.). In the retail shops, most commonly in the latter form. Those rancid, broken, or worm-eaten should be rejected.
Almonds, Bitt′er. Syn. Amyg′dalæ ama′′ræ, L.; Amygdala amara, Ph. E.; Amandes Amères, Fr.; Bittere mandeln, Ger. A variety imported from Mogadore, chiefly characterised by possessing the bitter flavour, and when rubbed with water, the odour of peach-kernels. They are also smaller and thicker than the sweet almond.
Uses, &c. Bitter almonds are used to relieve the flavour of sweet almonds, to clear muddy water, and to flavour confectionery, liqueurs, &c. By pressure, they yield their bland oil (OIL OF ALMONDS; O′LEUM AMYG′DALÆ, L.); the resulting cake (BITTER-A. CAKE; PLACEN′TA A. AMARÆ, L.) is distilled for the volatile oil (ESSENTIAL OIL OF A.; O. A. A., L.), and is afterwards again pressed into cakes (A.-CAKE), and used to fatten pigs, and for other purposes. Bitter almonds are now seldom employed in medicines, although it is said that they have cured ‘intermittents’ when bark had failed (Bergius), and that their emulsion has been found useful in pulmonary and dyspeptic affections, hooping-cough, and asthma; and externally as a lotion in acne. (Thomson.) In large quantities they are poisonous, and even in the smallest quantities have been known to produce nettle-rash (urticaria) and other unpleasant symptoms. They have long been in repute as an antidote to intoxication. The ancient bacchanals chewed them at their orgies, to lessen the effects of wine, and to enable them to take it in larger quantities with impunity.
Almonds, Blanched′ (blăncht′-). Syn. Amyg′dalæ decortica′tæ, L. Almonds from which the husk or seed-coat has been removed. This is effected by soaking them for a short time in warm water, until the skin can be easily removed by pressure between the thumb and forefinger. They are then peeled, rinsed in cold water, drained, and dried. When intended for the table, the last is effected by wiping them with a soft towel; but when they are intended to be powdered, or kept, they are dried by a very gentle heat in a stove, or in the sun.
Almonds, Burnt′. Syn. Roasted almonds; Almond coffee. Used to colour and flavour liqueurs and confectionery; and formerly, as a substitute for coffee.
Almonds, Guia′na. (ghe-āh′-nă; g hard). Brazil-nuts.
Al′monds, In′dian. The fruit of terminalia catappa (Linn.). They are oleaginous, and nutritious; and are used as a substitute for almonds.
Almonds, Ja′va (jāh′-). The nuts or kernels of canarium commune (Linn.). They are eaten, made into bread, and pressed for their oil.
Almonds, Sweet′. Syn. Almonds; Amyg′dalæ, L.; A. dulces, Ph. D.; Amygdala, A. Jordan′ica, Ph. L.; A. Dulcis, Ph. E., & Ph. L. 1836; Amandes, Amandes douces, Fr.; Süsse mandeln, Ger. These are the well-known dessert or table fruit of the name, and are the kind always referred to when ‘almonds’ (simply) are spoken of or ordered.
Comm. var.—1. Jor′dan Almonds, which are the finest, and are imported from Malaga. Of these there are two kinds; the one, above an inch in length, flat, and with a clear brown cuticle, sweet, mucilaginous, and rather tough; the other, more plump, and pointed at one end, brittle, but equally sweet with the former.—2. Valen′tia a. (which come next in quality) are about 3⁄8ths of an inch broad, not quite an inch long, round at one end, and obtusely pointed at the other, flat, of a dingy brown colour, with a dusty cuticle.—3. Bar′bary and Ital′ian a., which resemble the latter, but are generally smaller and less flattened.—4. A variety, of medium quality, imported in baskets from Spain.
Uses, &c. Sweet almonds are nutritive, emollient, and demulcent; but frequently disagree with weak stomachs. The husk is apt to occasion indigestion and nausea. Owing to a peculiar idiosyncrasy of some habits, dyspepsia, diarrhœa, œdematous swelling of the face, and urticaria (nettle-rash), sometimes, though seldom, follow the use of unblanched almonds. Blanched almonds do not produce these inconveniences, and, therefore, should be preferred for the table. In medicine, almonds are employed chiefly under the form of emulsion, confection,102 &c., and to suspend oily substances in water. Their uses for dietetical purposes are well known. Preparations of them are also employed as cosmetics. The cake left after expressing the oil (ALMOND-CAKE) is used for washing the skin, which it is said to render beautifully soft and clear. See Almond Paste, &c.
AL′NIGHT† (awl′-). A cake of wax with a wick in the midst. The forerunner of, and a rude form of the modern dumpy night-lights called MORTARS.
AL′OE (ăl′-o). Syn. Al′oë (-o-ē), L., Fr. (or ALOÈS), Ger., Ital., Sp., Belg., Dan., Dut., Swed. The aloe-tree. In botany, a genus of plants of the nat. ord. Liliaceæ (DC). The species, of which there are several, are succulent plants or small trees with endogenous stems, and stiff, fleshy, hard, pointed leaves, abounding in a purgative principle (ALOES), which is obtained from them by either evaporating the expressed juice or the decoction. They are all natives of warm climates, and most of them are indigenous to southern Africa.
Hist.  ,
aehleem (aloe-trees), were known to the sacred
historians; and both the plant and the inspissated juice are described by
Dioscorides[28] and Pliny.[29]
,
aehleem (aloe-trees), were known to the sacred
historians; and both the plant and the inspissated juice are described by
Dioscorides[28] and Pliny.[29]
Uses, &c. In Africa, the leaves of the Guinea aloe are made into ropes, fishing-lines, bow-strings, stockings, hammocks, &c. The leaves of another species are used to catch and hold rain-water. The expressed juice and decoction are also used by the natives as a distaff. (Vide infrà.) Comparative trials, made in Paris, of the strength of cordage and cables formed of hemp, and of the aloe from Algiers, are said to have shown the great superiority of the latter. Fabroni obtained a fine violet colour from the recent juice of the aloe, which has been proposed as a dye for silk.[30]
American Aloe. The agave Americana (Linn.) is a plant unconnected with the preceding, and belonging to the nat. ord. Bromeliaceæ. It is found in all parts of tropical America, and is largely cultivated on the shores of the Mediterranean; and less frequently, as an exotic plant in this country. It grows to the height of about 20 feet, and takes many years to produce its gigantic and magnificent pyramid of flowers; shortly after which it perishes, exhausted, as it were, by its efforts in bestowing its rare beauty on the floral world. The vulgar belief is that it blossoms only once in a century; but, as stated by the late Mr Loudon, it flowers sooner or later according to the culture bestowed on it. Its sap yields a kind of honey (AGAVE HONEY), and by fermentation an intoxicating liquor (PULQUE); desiccated juice, mixed with wood ashes, is used as soap, and lathers either with sea or fresh water; leaf-fibre, used as hemp to make thread and twine.
AL′OE-RESIN. Syn. Resi′na Al′oës, L. The resinous matter deposited by a decoction of aloes as it cools.
Prep. (Ph. L. 1746.) Boil aloes, 1 part, in water, 8 parts, and allow the decoction, strained whilst hot, to repose until the next day; then wash the deposited RESIN, and dry it by a gentle heat. It is probably a mixture of aloine and oxidised extractive.
AL′OES (-ōze). Syn. Bitt′er Aloes‡; Al′oë (-o-ē), L.; Aloès, Suc d’Aloès, Fr.; Aloe, Glausinde Aloe, Ger.[31] The inspissated juice or extract of several species of aloe.
Comp., Prep., &c. Aloes is a complex resinous substance containing a body called aloin, which is its active or purgative principle. It is completely soluble in boiling water, and in alcohol or rectified spirit. The decoction deposits an impure resin or resinoid on cooling.
Phys. eff., Uses, &c. Aloes is a warm stimulating purgative, in doses of 3 to 10 gr.; whilst even 1 or 2 gr. seldom fail to produce one motion without pain or inconvenience. It is considered highly serviceable in hypochondriacal, hysterical, and dyspeptic affections, particularly in phlegmatic habits, and in cases arising from deficiency of bile. As an emmenagogue, and a vermifuge, few medicines are more valuable. It acts on the large intestines, and principally on the rectum; and, therefore, should be administered with caution, or only in small doses, where there is a tendency to prolapsus or piles, and in cases where uterine stimulants (as in pregnancy, &c.) would be improper. “It is remarkable with regard to it, that it operates almost to as good a purpose in a small as in a large dose; and one or two grains will produce one considerable dejection, and twenty grains will do no more, except it be that in the last dose (case) the operation will be attended with griping, &c. It is one of the best cures for habitual costiveness.” (Cullen.) Many of the effects complained of arise from its slow solubility in the primæ viæ, and may be obviated by administering it in a liquid form, or in a solid form combined with soap, which renders it freely soluble in the juices of the stomach.
Aloes is more frequently taken than, perhaps, any known purgative. It enters into the composition of a majority of the aperient medicines prescribed by the faculty, and forms the principal ingredient of nearly all the advertised purgative, antibilious, and universal pills of the nostrum-mongers. The fact of aloetic pills not acting until about 8 to 10 hours after being swallowed—so that if taken on retiring to rest at night they do not generally disturb the patient before the usual time of rising in the morning—has contributed more than anything else to make such remedies popular with parties whose habits or business avocations would be otherwise interfered with.
Aloes is also extensively used in veterinary103 practice. It is the most valuable and reliable purgative for the horse of the whole materia medica; but is less to be depended on for cattle, sheep, and hogs. Barbadoes aloes is the best for this purpose. Cape aloes are, however, often employed, when 1-4th more must be given.—Dose (of the former), for a HORSE, 4 to 8 dr.;[32]—CATTLE, 3 to 6 dr. (followed by a purging drench);—HOGS, 5 to 15 gr.;—SHEEP, 15 to 30 gr.;[33]—DOGS (small ones), 10 to 30 gr., (middle-sized) 20 to 44, or even 60 gr., (large) 3⁄4 to 1 dr., or even 2 dr.
Aloes is also used in dyeing; and as a colouring matter in stains, lacquers, and varnishes. Aloes, and several of its preparations, are likewise extensively employed to adulterate porter.
Var. These, arranged in the order of their reputed medicinal value, are—Socotrine, Hepatic, Barbadoes, Cape, &c.; and alphabetically, as given below:—
Aloes, Barba′does. Syn. Aloes in Gourds; Al′oë Barbaden′sis, L., Ph. L. & E. Imported from Barbadoes and Jamaica, usually in gourds; sometimes in boxes. The best is the inspissated juice of the cut leaf of aloë vulga′′ris; an inferior quality is prepared from the decoction.—Char., &c. Opaque, lustreless, of a liver colour, a little tending to black, with a bitter nauseous taste, and a very disagreeable odour, especially when breathed on; powder a dull olive-yellow. It is the ‘hepatic’ aloes of most continental writers, and said to be the Αλοη of Dioscorides. It is more active than the other varieties of aloes; but is also more apt to occasion hæmorrhoids, and to gripe, than any of them.
Aloes, Cab′alline (-līne.) Syn. Fœt′id aloes, Horse a.; Aloë caballi′na, A. Guinien′sis, L.; Aloès Caballin, Fr. From aloë In′dica (O’Shaughnessy); or from aloë spica′ta by long and careful boiling. (Lindley.) Used only by farriers. Scarcely known in English commerce.
Aloes, Cape. Syn. Aloë Capen′sis, A. lu′cida (Geiger), L. Imported from the Cape of Good Hope, and obtained from aloë spica′ta, and other Cape species. Odour stronger and even more disagreeable than that of Barbadoes aloes; colour deep greenish-brown; appearance shining and resinous; fracture generally glassy; powder a lively greenish-yellow; almost completely soluble in boiling water, decoction paler than that of other kinds. It is weaker than Barbadoes or even hepatic aloes, and is more apt to gripe, &c., than the latter. A finer kind, known as ‘Bethelsdorp aloes,’ imported from Algoa Bay, is more of a liver colour, and softer than the preceding, and hence often called Cape hepatic-aloes.
Aloes, Hepat′ic. Syn. Bombay’ Aloes*, East-India a.*, Liver-coloured Socotrine a.*; Aloë hepat′ica, Ph. L. & D.; A. In′dica, Ph. E. Imported from Bombay and Madras. It is usually said to be obtained from “uncertain species of aloes;” but it is almost certain that it is “the juice of the Socotrine aloes plant which has been solidified without the aid of artificial heat.”[34]—Char., &c. “Opaque, of a liver colour, bitter taste, and an unpleasant odour.” (Ph. L.) It is less odorous, darker coloured, and more opaque than Socotrine aloes; its powder has also a duller colour, and weak spirit leaves much undissolved matter. Its decoction on cooling frequently deposits a yellow powder. The finer and brighter varieties of hepatic aloes are commonly sold for ‘Socotrines,’ and their medicinal virtues are nearly similar. (See below.)
Aloes, In′dian (various);—1. Deep brown or black, very opaque, and less soluble than ordinary aloes. Scarcely known in commerce.—2. Several varieties ranging in character from ‘Cape aloes’ to ‘hepatics,’ and occasionally to ‘Barbadoes,’ obtained from several species.
Aloes, Mo′cha (-kăh). Syn. Aloë de Mochâ, L. Imported from Muscat. An inferior kind of Indian aloes. (Christison.) It is obtained from the same plant as produces genuine hepatic aloes. (Lindley.) It holds an intermediate position between ‘Cape’ and ‘hepatics,’ but contains much impurity; the latter often amounting to upwards of 25%. Some specimens are, however, of excellent quality. When melted and ‘doctored,’ it is sold for Barbadoes, hepatic, and even Socotrine aloes.
Aloes, Soc′otrine (-trĭn; sŭk′-‡). Syn. Soc′otorine aloes, Smyr′na a., Tur′key a.; Aloë Socotri′na, Ph. L.; Aloë, Ph. L. 1836; A. Socotri′na, Ph. E. “The juice of the cut leaf of uncertain species hardened by the air.” (Ph. L.) Genuine Socotrine aloes is generally supposed to be obtained from aloë spica′ta; but is referred by De Candolle to a distinct species, a. Socotri′na; and by Martius, also to a. purpuras′cens. Formerly this variety was brought from the Island of Socotra or Zocotora (hence the name), by way of Smyrna and Malta; but it is now chiefly obtained from Bombay and Madras.—Char., &c. Colour garnet red to golden red; smell peculiar and aromatic, not unlike a decaying russet apple, especially when fresh-broken, or breathed on, or warmed; taste permanently and intensely bitter; fracture conchoidal; softens in the hand, and becomes adhesive, yet retains considerable brittleness; powder bright golden-yellow colour; central portions of the lumps often soft, especially when first imported. “It is brittle, bitter, of a reddish-brown colour, and an aromatic odour. Light permeates thin recently broken laminæ.” (Ph. L.) “In thin pieces, translucent and garnet red;104 almost entirely soluble in spirit of the strength of sherry. Very rare.” (Ph. E.)
Socotrine aloes are always preferred for medicinal purposes, and are the only variety used in perfumery, varnishes, and other nice purposes in the arts.
Aloes, Strained. Syn. Melted Aloes; Aloë cola′ta, L. Proc. 1. The aloes are melted in a copper pan, by the heat of steam or a water bath, and are then pressed through a strong hair or wire sieve, and allowed to cool.
2. As above, but with the addition of about twice its weight of water; the decoction being strained and evaporated.
Obs. Mocha, Indian, and other common aloes, treated in this way and coloured, are frequently sold for melted or strained ‘Socotrines’ and ‘hepatics.’ The colouring matter usually employed is the precipitated carbonate of iron (sesquioxide), or Venetian red, in very fine powder, with, sometimes, a little annatto. This fraud is not readily detected by mere inspection, by those unaccustomed to these matters; and hence the impunity with which it is perpetrated.
The object in melting aloes is to deprive it of the foreign matters, as sand, leaves, pieces of wood, &c., which the commoner kinds generally contain in large quantities. The action of the heat drives off much of their nauseous smell, at the same time that it deepens their colour, and renders their appearance more translucent and resinous, to the disguise of their original nature. The operation, on the large scale, is usually carried on at night, in consequence of the horribly nauseous fumes evolved, which may be smelt at a great distance, and contaminate the clothes of those engaged in it for a long time afterwards.
AL′OES HEMP. A plant growing in Peru, the East and West Indies, and Mexico (A. Americana, A. vivapara, A. fœtida, &c.), where the leaf is cultivated for its fibre, which is generally of a yellowish-white colour, and used for rope-making.
AL′OES WOOD. Syn. Al′oe-wood; Eaglewood; Agal′lochum (-kŭm), Lig′num al′oës, L. agal′lochi, L. a. ve′′ri, L. aq′uilæ, L. aspal′athi, L.; Agalloche, Bois d’aloès, Fr.; Aloeholz, Ger.; Calam′bac, Calam′bouc, Ind.; Xylo-al′oës†. A name applied to the wood of alöex′ylon agal′lochum (Lam.), a leguminous tree of Cochin China; and, though apparently less correctly, to that of aquila′′ria agallochum and a. ova′ta (Lour.), trees of tropical Asia, belonging to a different nat. order. Both are highly fragrant and aromatic; used in fumigations and pastilles, and occasionally by cabinet makers and inlayers. The essential oil of the wood, dissolved in spirit, was regarded by Hoffmann as one of the best cordials and invigorants known. The same has also been said of a tincture of its resin.
The same name and synonyms are popularly applied to the resin of the above woods (ALOES-WOOD RESIN), of which there are two varieties:—the one, light and porous, and filled with a highly fragrant resinous substance; the other, denser and less resinous. It is an oily concretion in the centre of the tree, the result of disease, which gradually hardens, and, in time, kills it. It is highly fragrant, and is said to be nervine, cephalic, cardiac, and stimulant. The powder is regarded as tonic and astringent. Of all perfumes this is said to be the one most esteemed by oriental nations.
ALOE′TIC. Syn. Aloët′icus, L.; Aloétique, Fr. Of or belonging to aloes. In medicine, pharmacy, &c., applied to any preparation containing aloes as a characteristic ingredient; made or obtained from aloes. Substantively, an aloetic medicine.
AL′OIN (-o-ĭn). C17H18O7. [Eng., Fr.] Syn. Al′öin; Aloï′na, L. The Messrs T. & H. Smith, of Edinburgh, have applied this name to a crystalline substance, which they assert to be the pure cathartic principle of aloes. Their process is to evaporate to the consistence of a syrup, in vacuo, a solution obtained by exhausting a mixture of aloes and sand, with cold water, and then to set it aside for a few days. The resulting dark crystalline mass is purified by pressure between folds of bibulous paper, and repeated crystallisation from hot water. Barbadoes aloes are commonly used for the purpose; but soft or semi-liquid Socotrine aloes, or the unevaporated Socotrine-aloes juice, is probably its best source. Tilden gives the following process for the preparation of aloin:—The aloes crushed small is to be dissolved in nine or ten times its weight of boiling water acidified with sulphuric acid. After cooling and standing for a few hours, the clear liquid is decanted from the resin, and evaporated. The concentrated solution deposits a mass of yellow crystals, which can be purified by washing, pressure, and recrystallisation from hot spirit. After several recrystallisations the aloin is obtained in the form of beautiful yellow needles, which are pretty soluble in water and in alcohol, but soluble with difficulty in ether.—Dose, 1 to 2 gr.
ALOPE′CIA (-sh′ă). [L.] Syn. Al′opecy, Fox′-evil; Alopécie, Fr.; Fuchsraude, Ger. In pathology, baldness from disease, often extending to the beard and eyebrows; as distinguished from ‘calvities,’ or ordinary baldness arising from attenuation of the scalp or defective nutrition. See Baldness.
ALPAC′A. A species of Llama, popularly known as the Peruvian Sheep, an animal intermediate between the camel and sheep, having long silky hair, nearly as fine as that of the Cashmere goat. It was introduced to the British manufacturers in 1834, when only 5700 lbs. of it was imported; but it soon became an important article of commerce, the quantity imported having gradually risen to105 above 21⁄4 millions of lbs. in 1853; whilst the price has risen from about 9d. to 2s. 7d. the lb., in the same time. The name is also given to fabrics woven from the wool of this animal; and to others in fine wool, made in imitation of them. The gigantic factory, &c., erected at Saltaire, Yorkshire, in 1852, for this manufacture, covers about 12 acres of land. See Llama.
ALPENKRAUTER-BRUST-TEIG (Grablowitz, Gras). Pectoral cakes of Alpine herbs. Gum arabic, 100 parts; sugar, 200 parts; extract liquorice, 1 part; saffron, 1⁄8th part. Each box contains 48 lozenge-shaped yellowish cakes. Made into a mass with decoction of marsh mallow. (Hager.)
ALPENKRAUTER GESUNDHEIT’S LIQUEUR (Rudolph Bohl). Medicinal liqueur of Alpine herbs. A bottle containing 350 grammes of a liqueur which is an extract of star anise, cassia, frangula bark, centaury, chicory, gentian, and a little aloes. (Hager.)
ALPENKRAUTER-MAGENBITTER (Hauber). Stomachic bitters of Alpine herbs. A brown liqueur of bitter, spirituous, and slightly aromatic flavour, containing in 100 parts: oil of anise, 0·5; oil of cloves, 0·5; aloes, 1·5; alcohol, 40; water, 50. 157 grammes in each bottle. (Wittstein.)
ALPHA-ORSELL′IC ACID. See Orsellic Acid.
ALPINE ROSE SOAP, SWISS. A preservative against syphilitic infection (G. A. Sarpe, Zurich). A glass cylinder corked and sealed, about 2 inches long, and containing a hard brownish-grey mass weighing 12 grammes, prepared thus:—Ammonia, 1 part; sublimate, 3 parts; tannin, 2 parts; chloride of lime, 24 parts; Castile soap, 190 parts; oil of cloves, 1 part; spirit of wine, q. s. (Hager.)
AL′QUIFOU (-ke-fōō). Syn. Black lead-ore, Potter’s ore. A native sulphide of lead used by potters to give a green glaze to coarse wares.
ALSTONIA SCHOLARIS. (Ind. Ph.) Habitat. Common in forests throughout India.—Officinal part. The bark (Alstoniæ cortex). It occurs in thick, irregular, more or less contorted pieces, easily broken. It consists of a rough greyish epidermis, investing a buff or pale cinnamon-coloured bark; internally, still lighter in colour, and of a spongy texture, having a very bitter taste, but devoid of odour.—Properties. Astringent, tonic, anthelmintic, antiperiodic—Therapeutic uses. In chronic diarrhœa and the advanced stages of dysentery; also as a tonic in debility after fevers, and other exhausting diseases.—Dose. 3 to 5 grains, either alone or combined, in bowel affections, with small doses of ipecacuanha and extract of gentian.—Preparations. Tincture of Alstonia (Tinctura Alstoniæ). Take of alstonia bark, bruised, 21⁄2 ounces; proof spirit, 1 pint. Macerate for seven days in a closed vessel, with occasional agitation; filter, and add sufficient proof spirit to make 1 pint. Or prepare by percolation, as Tincture of Calumba.—Dose, 1 to 2 fluid drachms.
Alstonia, Infusion of. (Infusum Alstoniæ.) Take of alstonia bark, bruised, 1⁄2 an ounce; boiling water, 10 fluid ounces. Infuse in a covered vessel for an hour and strain.—Dose. From 1 to 2 fluid ounces twice or thrice daily. A good serviceable tonic.
AL′TERATIVE (awl′-tĕr-ă-tĭv). Syn. Al′terant*; Al′terans (ăl′-), L.; Altérant, Altératif, Fr. In medicine, having power to alter; applied to substances and agents which occasion a change in the habit or constitution, and thus re-establish the healthy functions of the body, or any part of it, without producing any sensible evacuation or other obvious effect.
ALTERATIVE EXTRACT, or GOLDEN MEDICAL DISCOVERY (Dr Pierce, Buffalo), for the cure of all severe, acute, chronic, or long-standing coughs, inflammations, hoarseness, scrofulous, and syphilitic diseases. A clear light-brown fluid, 220 grms., composed of 15 grms. purified honey, 1 grm. extract of lettuce, 2 grms. laudanum, 100 grms. of proof spirit tasting of fusel oil and wood spirit, and 105 grms. water. (Hager.)
AL′TERATIVES (-tĭvz). Syn. Alteran′tia, L.; Altératifs, &c., Fr. Alterative medicines or agents. The preparations of mercury and iodine, when properly administered, are the most useful members of this class; and are those which are now the most generally employed.
ALTHE′IN (ăl-thē′-ĭn). Syn. Althæ′ina, L. The name given by Braconnot to a substance identical with asparagin, which he discovered in the ‘marsh-mallow’ (althæ′a officina′lis, Linn.).
ALTHOFF WATER (aqua mirabilis), for torpid ulcers. Wine vinegar, 750 parts; sulphate of copper, 100 parts; potash, 25 parts; ammonia, 30 parts; salt of sorrel, 8 parts; French brandy, 375 parts. Digest for a few days in a glass vessel and distil to dryness from a glass retort. (Wittstein.)
AL′UDEL (-ū-). In chemistry, a pear-shaped glass or earthen pot open at both ends, formerly much used for connecting other vessels in the process of sublimation. A number of them joined together are still employed for the distillation of quicksilver, in Spain.
AL′UM K2SO4.Al2(SO4)3.24Aq. Syn. Pot′ash-alum, Sul′phate of aluminum and potassium, Common alum; Alu′men, A. potas′sicum, L.; Alun, Sulfate d’alumine et de potasse, Fr.; Alaun, Ger.; Alume, Ital.
The principal alum-works in England, until recently, were those of Lord Glasgow, at Hurlett and Campsie, near Glasgow, and those of Lords Dundas and Mulgrave, at Whitby, Yorkshire (est. 1600); but those of Mr Spence, at Manchester, and at Goole (Yorkshire), and of Mr Pochin, at Manchester, are now among the largest, if they be not actually the largest in106 the world. There are also extensive alum-works at and near Newcastle-on-Tyne; but none of importance, that we know of, in any other part of these realms.
Nat. hist. Alum is found native in some places (NATIVE ALUM), either effloresced on the surface of bituminous alum-schist (Göttwigg, Austria); or united with the soil in the neighbourhood of volcanoes (Solfatara, Naples); when it may be obtained by simple lixiviation and evaporation, a little potash being commonly added to convert the excess of sulphate of alumina present into alum. It is also found in certain mineral waters (East Indies).
Sources. The alum of commerce is usually obtained from schistose pyritic clays, commonly termed alum-ores, aluminous shale, a.-schist, &c.; and from alum-rock, a.-stone, or alunite. At La Tolfa, Civita Vecchia, where the best Roman-alum is produced, the source is stratified alum-stone. On the Continent, and in Great Britain, it is generally pyritaceous clays, volcanic aluminous ores, aluminous shale, or alum-slate. These minerals contain sulphide of iron, alumina, bitumen or carbon, and frequently a salt of potassium. Of late years large quantities of alum have been prepared on the banks of the Tyne from aluminous clay.
Prep. The manufacture of alum is technically said to be conducted according to the natural process when prepared from alum-schist or alum-ore; and according to the artificial process when made by acting on clay with sulphuric acid, and adding a potassium salt to the resulting lixivium. The manufacture of alum and of sulphate of alumina from such materials as contain only alumina, to which consequently sulphuric acid and alkaline salts have to be added, has come largely into practice in England. The materials employed are, in addition to clay, cryolite or Greenland spar, a fluoride of aluminum and soda; bauxite, a hydrate of alumina, of more or less purity; and slag. The following are the details of these processes:—
a. From ALUM-ORE, ALUMINOUS SCHIST, or SHALE, &c.:—
1. The mineral (alum-ore, a.-schist, &c.) is placed in heaps, and moistened from time to time with water, when it becomes gradually hot, and falls into a pulverulent state. This decomposition commonly occurs either wholly, or partially, on the floor of the mine. If the ore does not possess this property on mere exposure to air and moisture, it is broken into pieces and laid upon a bed of brushwood and small coal, to the depth of about four feet, when the pile is fired and fresh lumps of the alum-mineral thrown on, until the mass becomes of considerable height and size. The combustion, as soon as established, is conducted with a smothered fire, until the calcination is complete; care being taken to prevent fusion, or the disengagement of either sulphurous or sulphuric acid, from contact between the ignited stones and the carbonaceous fuel.[35] To promote these ends the pile, at the proper time, is ‘mantled’ (as the workmen call it) or covered with a layer of already calcined and exhausted ore, in order to protect it from high winds and heavy rains; as also to moderate the heat, and let it proceed gradually, so that the sulphur present may not be lost or wasted by volatilisation. The roasting is finally checked by a thicker ‘mantling,’ and the whole allowed to cool. By this time the pile has usually lost about one half its bulk, and become open and porous in the interior, so that the air can circulate freely through the mass; the latter, in dry weather, as the heap cools, being usually promoted by sprinkling a little water on it, which, by carrying down some of the saline matter, renders the interior still more open to the atmosphere. The whole, when cold, or nearly cold, is, if necessary, still further exposed to the action of air and moisture. The time required to calcine the heap properly, including that taken by the burned ore to cool, varies, according to its size and the state of the weather, from three to nine, or even twelve months. The residuum of the calcination is next placed in large stone or brick cisterns, and edulcorated with water, until all the soluble portion is dissolved out; the solution is then concentrated in another stone cistern, so made that the flame and heated air of its reverberatory furnace sweep the whole surface of the liquor. (See engr.) The evaporation is continued until it just barely reaches the point at which crystals are deposited on cooling; when it is run off into coolers. After the sulphate of iron, always present, has been deposited in crystals, the mother-liquor, containing the sulphate of aluminum, is run into other cisterns, and a saturated solution of chloride of potassium, or of sulphate of potassium, or (sometimes) impure sulphate or carbonate of ammonium, or a mixture of them,[36] is added until a cloud or milkiness ceases to be produced on addition of more.[37] It is107 next allowed to settle and get thoroughly cold, and the supernatant ‘mother-liquor’ being drawn off with a pump or syphon, the precipitate, which is alum in the form of minute crystals (technically termed ‘flour’), is well drained, and subsequently washed by stirring it up with a little very cold water, which is then drained off, and the operation repeated a second time with fresh water. A saturated solution of the pulverulent alum (‘flour’) is next formed in a leaden boiler, and the clear portion is run or pumped off, while boiling hot, into crystallising vessels, called roaching casks (see engr.), the staves of which are lined with lead, and nicely adjusted to each other. After the lapse of a week or ten days, the hoops and staves of these ‘casks’ are removed, when a thick crust of crystallised alum is found, which exactly corresponds in form and size to the interior of the cask. A few holes are then made in the sides of this mass, near the bottom, to allow the contained mother-liquor to drain off, after which the whole is broken up and packed in casks for sale. Sometimes the alum thus obtained, or the lower portion of it, is washed with a little very cold water, and, if discoloured, or small or slimy, is purified by a second crystallisation.
| Chloride of | potassium | 15·7 |
| Sulphate of | ” | 18·4 |
| ” | ammonium | 13·9 |
In practice, the exact quantity required may be found by a previous trial of a little of the aluminous liquor; but the indications mentioned in the text will always show the operator when a sufficient dose is added.

2. As ammonia-alum (Spence’s process; see below), but using a potash-salt as the precipitant, either wholly or in part, instead of ammonia; and, in the latter case, supplementing the deficiency of potash with ammonia, as there explained.
b. From ALUMINOUS CLAY and OIL OF VITRIOL:—
1. Clay, free or nearly free from carbonate of lime and oxide of iron, is chosen for this purpose. It is moderately calcined (in lumps) in a reverberatory furnace, until it becomes friable; great care being taken that the heat be not sufficient to indurate it, which would destroy its subsequent solubility. It is next reduced to powder, sifted, and mixed with about 45% of its weight of sulphuric acid (sp. gr. 1·45), the operation being conducted in a large stone or brick basin arched over with brickwork. Heat is then applied, the flame and hot air of a reverberatory furnace being made to sweep over the surface of the liquor. The heat and agitation are continued for 2 or 3 days, when the mass is raked out and set aside in a warm place for a few weeks (6 to 8), to allow the acid the more perfectly to combine with the clay. At the end of this time the newly-formed sulphate of alumina is washed out, the solution evaporated until of a sp. gr. of about 1·38 (1·24 for ‘ammonia-alum’), and the salt of potash added. The remaining operations resemble those above described. Good alum may be produced by this process at about two thirds the cost of rock or mine alum.
2. (Process of Mr Pochin.) Fine China clay is heated in a furnace, and mixed with a suitable proportion of sulphuric acid; the latter being considerably diluted with water, in order to moderate its action, which would otherwise be far too violent. The mixture is then passed into cisterns furnished with movable sides, where, in a few minutes, it heats violently and boils. The thick liquid gradually becomes thicker, until it is converted into a solid porous mass; the pores being produced by the bubbles of steam which are driven through it, owing to the heat resulting from the reaction of the ingredients on each other. This porous mass (ALUM-CAKE; CONCENTRATED ALUM) appears perfectly dry, although retaining a large amount of combined water. It also contains all the silica of the original clay, but in such a state of fine division, that the whole appears homogeneous; whilst it imparts a dryness to the touch which can scarcely be given to pure sulphate of alumina. From this substance a solution of pure sulphate of alumina is easily obtainable by lixiviation, and allowing the resulting solution to deposit its silica before using it, but for many purposes the presence of the finely divided silica is not objectionable. The sulphate of alumina solution so obtained is adapted to all the purposes in dyeing for which alum is now employed; the sulphate of potash or of ammonia in the latter being an unnecessary constituent, and one merely added to facilitate the purification and subsequent crystallisation of the salt. To obtain ALUM from the porous alum-cake, the proper proportion of acid having been used in its preparation, or subsequently added, it is only necessary to precipitate its concentrated solution with a strong solution of a salt of potash, or of ammonia, or a mixture of them, and to otherwise proceed as before.
Ratio. In the above process the sulphide of iron of the shale or schist is converted by atmospheric oxygen into sulphate of iron and sulphuric acid; the sulphuric acid decomposes the clay, setting silica free, and producing sulphate of aluminum. The sulphate of iron is108 mostly got rid of by concentrating the solution of the mixed sulphates, and the mother-liquors are converted into alum by the addition of the salt of potassium. When chloride of potassium is used, it yields chloride of iron and sulphate of potassium, the latter combining with the sulphate of aluminum, and the former remaining behind in the mother-liquor. See Alums (in Chemistry).
Comp. Potassium alum has the formula K2SO4.Al2(SO4)3.24Aq.
c. From Cryolite.
1. (Thomson’s method.) Decomposition of cryolite by ignition with carbonate of lime. From the ignited mass the aluminate of soda is obtained by lixiviation with water, and into the solution carbonic acid gas is passed, when there result precipitated hydrated gelatinous alumina and carbonate of soda, which remains in solution. If it be desired to obtain the alumina as an earthy compact precipitate, bicarbonate of soda is used instead of carbonic acid. While the clear liquor is boiled down for the purpose of obtaining carbonate of soda, the precipitated alumina is dissolved in dilute sulphuric acid; this solution is evaporated for the purpose of obtaining sulphate of alumina (the so-called concentrated alum), or the solution after having been treated with a potassa or an ammonia salt is converted into alum.
2. (Sauerwein’s method.) Decomposition of cryolite by caustic lime by the wet way. Very finely ground cryolite is boiled with water and lime, the purer the better, and as free from iron as possible, in a leaden pan. The result is the formation of a solution of aluminate of soda, and insoluble fluoride of calcium (lime). When the fluoride of calcium has deposited, the clear liquid is decanted, and the sediment washed, the first wash-water being added to the decanted liquor, and the second and third wash-waters being used instead of pure water at a subsequent operation. In order to separate the alumina from the solution of aluminate of soda, there is added to the liquid while being continuously stirred very finely pulverised cryolite in excess, the result of the decomposition being alumina and fluoride of sodium, (soda). When no more caustic soda can be detected in the liquid, it is left to stand for the purpose of becoming clear. The clarified solution of fluoride of sodium is then drawn off, and the alumina treated as above described. The solution of fluoride of sodium having been boiled with caustic lime yields a caustic soda solution, which having been decanted from the sediment of fluoride of calcium is evaporated to dryness. Recently the fluoride of calcium occurring as a by-product has been used in glass-making.
3. The decomposition of cryolite by sulphuric acid yields sulphate of soda convertible into carbonate by Leblanc’s process, and sulphate of alumina free from iron. This method of decomposing cryolite is, however, by no means to be recommended, as owing to the liberation of hydrofluoric acid, peculiarly constructed apparatus are required, whilst the sulphate of soda has to be converted into carbonate.
d. From Bauxite. This mineral, occurring in some parts of Southern France, in Calabria, near Belfast, and in other parts of Europe, consists essentially (viz. 60 per cent.) of hydrate of alumina, more or less pure. In order to prepare alums and sulphate of alumina from it, the mineral is first disintegrated by being ignited with carbonate of soda, or with a mixture of sulphate of soda and charcoal; in each case the lixiviation of the ignited mass yields aluminate of soda, from which, by the processes already described under “Cryolite,” alum, or sulphate of alumina, and soda are prepared.
e. From blast-furnace slag. Lürmann recommends the slag to be decomposed by means of hydrochloric (muriatic) acid. From the resulting solution of chloride of aluminum the alumina is precipitated by carbonate of lime, any dissolved silica being precipitated at the same time. The alumina is dissolved in sulphuric acid, leaving the silica.
Prop. Alum crystallises in regular octahedrons, often with truncated edges and angles; (see engr.); and sometimes in cubes, but only when there is a deficiency of acid in its composition, with the alkali in slight excess of the proper quantity. (Löwel.)[38] It is slightly efflorescent in dry air: soluble in 18 parts of cold water, and in rather less than its own weight of boiling water; tastes sweet, acidulous, and very astringent; is styptic; and reddens litmus. When heated it melts, loses its water of crystallisation, and becomes white and spongy (DRIED ALUM); a strong heat, short of whiteness, decomposes it, with the evolution of oxygen and a mixture of sulphuric and sulphurous anhydride; calcined with carbonaceous matter it suffers decomposition, and furnishes a pyrophoric residuum (Homberg’s pyro′phorus). Ignited with alkaline chlorides, hydrochloric acid is liberated; which also occurs when their concentrated solutions are boiled together. Ammonia precipitates pure hydrate of aluminum from potassium alum; but only a subsulphate from the simple sulphate of alumina. Sp. gr. 1·724; but, when containing ammonia, often so low as 1·710.
Tests, &c. It is easily recognised by its crystalline form, its taste, and by its complete solubility in water. Its aqueous solution gives a white gelatinous precipitate soluble in excess; a platinum wire moistened with the solution imparts a violet colour to the blowpipe flame;109 and chloride of barium gives a white precipitate insoluble in nitric acid.
Pur. When pure, its solution is not darkened by tincture of galls, sulphuretted hydrogen or ferrocyanide of potassium; neither does it give any precipitate with solution of nitrate of silver. Heated with caustic potassa, or quick-lime, it does not evolve fumes of ammonia.
Adult., &c. The principal impurity, and one which renders alum unfit for the use of the dyer, is iron. This may be readily detected by the blue precipitate it gives with ferrocyanide of potassium, or the black precipitate with sulphide of ammonium, which are very delicate tests.[39] Lime, another very injurious contamination, may be detected by precipitating the alumina and iron (if any) with ammonia, and then adding oxalate of ammonia to the boiled and filtered liquid. The liquid filtered from the last precipitate (oxalate of lime) may still contain magnesia, which may be detected by the white precipitate caused on the addition of an alkaline phosphate. Common alum frequently contains ammonia, from urine, or the crude sulphate of the gas-works, having been employed in its manufacture. Powdered alum is frequently adulterated with common salt, in which case it gives a white curdy precipitate with nitrate of silver, turning black by exposure to the light.
Phys. eff. &c. In small quantities alum acts as an astringent; in larger doses as an irritant. It acts chemically on the animal tissues and fluids, is absorbed, and has been discovered in the liver, spleen, and urine (Orfila), the last often becoming acid (Kraus). Externally, it is astringent. The almost general use of alum by the English bakers is one of the most fertile sources of dyspepsia and liver and bowel complaints in adults; and of debility and rickets in children. Bad teeth and their early decay is another consequence of the daily use of alum in our food. The bone matter (phosphate of lime) of bread, instead of being assimilated by the system, is either wholly, or in part, converted into a salt of alumina, which is useless and incapable of appropriation. When alum has been taken in poisonous doses an emetic should be given, followed by warm diluents and demulcents, containing a little carbonate of soda; and subsequently by a purgative.
Uses, &c. The applications of alum in the arts and manufactures are numerous and important. It is used to harden tallow and fats; to render wood and paper incombustible; to remove greasiness from printers’ blocks and rollers; to prepare a paper for whitening silver and silvering brass in the cold; to help the separation of the butter from milk; to purify turbid water; to dress skins; to fix and brighten the colours in dyeing; to make lake and pyrophorus, &c., &c. It is also extensively used for clarifying liquors, and for many other purposes connected with the arts and everyday life. In medicine, alum is used as a tonic and astringent, in doses of 5 to 20 gr.; as a gargle (1 dr. to 1⁄2 pint of water); and as a collyrium and injection (10 to 15 gr. to 6 oz. of water). In lead colic, 1⁄2 to 1 dr. of alum (dissolved in gum-water), every 3 or 4 hours, is said to be infallible. Powdered alum is frequently applied with the tips of the fingers, in cases of sore throat and ulcerations of the mouth, &c. A teaspoonful of it is said to be one of the very best emetics in croup. (Dr Meigs.) Alkalies, alkaline carbonates, lime, magnesia, acetate of lead, astringent vegetables, &c., are incompatible with it.
Gen. commentary. In addition to the particulars of its manufacture given above, we may add, that the plan of getting rid of the ferric salts there referred to has to some considerable extent been successfully replaced by that of precipitating the alum, instead of the sulphate of iron, by adding alkaline matter to the lixivium. The crystalline precipitate is purified by draining, re-solution, and re-crystallisation; whilst the sulphate of iron and Epsom-salts contained in the mother liquor are obtained by subsequent evaporation and crystallisation; after which a fresh crop of alum may be got from it, by the use of an alkaline precipitant, as before.
In estimating the strength of his solution the alum-maker takes as a standard a measure or sp. gr. bottle capable of holding exactly 80 pennyweights of distilled water. The excess of the weight of liquor, in pennyweights, over 80, or that of water, is called so many ‘pennyweights strong.’ Thus one of 90 pennyweights (90 dwt.) is said to be ‘10 dwt. strong,’ or simply, ‘one of 90 dwt.’ These numbers correspond to 21⁄2 degrees of Twaddle’s hydrometer, and may easily be found by dividing Twaddle’s degrees by 2·5 or 21⁄2; or by multiplying them by 4, and pointing off the right-hand figure of the product for a decimal. The result is in alum-makers’ pennyweights.
By a patent now expired (Weisman’s, 1839) the ferric salts are precipitated by the addition of a solution of ferrocyanide of potassium (prussiate of potash); after which the supernatant clear liquor, which is now a solution of nearly pure sulphate of alumina, is decanted, and evaporated for future operations, until it either forms, on cooling, a concrete mass, which is moulded into bricks or lumps, for the convenience of ‘packing,’ or until it is sufficiently concentrated to be converted into ALUM by the addition of a salt of potash or of ammonia in the usual manner. The product, in each case, is perfectly free from iron. By a like addition of the ferrocyanide to a solution of ordinary sulphate of aluminia or alum, the dyer may himself easily render them free from110 iron, or iron-alum; when, as mordants for even the most delicate colours, they are equal to the very best Roman alum.
Another process has been patented (Barlow & Gore, 1851) for the manufacture of alum from the ash or residue of the combustion of Boghead-coal, which, though hitherto regarded as almost valueless, actually contains about 30% of alumina. It has not, however, been found a convenient material for the purpose.
By the latest and most approved processes the least possible quantity of boiling water or liquor is employed for making the solutions, so that they may crystallise without evaporation, and thus economise fuel; and the mother-liquors of previous operations are constantly employed for this purpose, when possible. Nor is anything which is convertible to use, from the drainage of the heaps, to the liquor and slime of the roaching casks, allowed to be wasted.
By whatever process, or from whatever materials alum is obtained, it is absolutely necessary for the successful and economical conduct of its manufacture, that the precise composition of the mineral or minerals employed should be exactly known. This can only be determined by actual analysis, which should be extended to several parts of the same bed, and particularly to the upper and lower strata, which frequently differ in composition from each other, and thus require different treatment, or may be most advantageously employed in combinations with each other. The necessity of this will be seen by reference to the composition of the following minerals, of which the top contains a larger proportion of iron-pyrites than the bottom, and the two require to be mixed, to equally diffuse the sulphuric acid generated by the calcination, &c., to which they are subjected.
The following is the per-centage composition of certain alum shales:—
| Whitby, Yorkshire. (Richardson.) | ||
| Top rock. | Bottom rock. | |
| Sulphide of iron (pyrites) | 4·20 | 8·50 |
| Silica | 52·25 | 15·16 |
| Protoxide of iron | 8·49 | 6·11 |
| Alumina | 18·75 | 18·30 |
| Lime | 1·25 | 2·15 |
| Magnesia | ·91 | ·90 |
| Oxide of manganese | traces | traces |
| Sulphuric acid (SO3) | 1·37 | 2·50 |
| Potassa | ·13 | traces |
| Soda | ·20 | traces |
| Chlorine | traces | traces |
| Coal | 4·97 | 8·29 |
| Water | 2·88 | ·00 |
| Loss | 4·60 | (?) |
| ——— | ——— | |
| 100· | 100· | |
| Campsie, near Glasgow. (Ronalds.) | |||
| Top rock. | Top rock. | Bottom rock. | |
| Sulphide of iron (pyrites) | 40·52 | 38·48 | 9·63(?) |
| Silica | 15·40 | 15·41 | 20·47(?) |
| Protoxide of iron | ... | ... | 2·18 |
| Alumina | 11·35 | 11·64 | 18·91(?) |
| Lime | 1·40 | 2·22 | ·40 |
| Magnesia | ·50 | ·32 | 2·17 |
| Oxide of manganese | ·15 | ... | ·55 |
| Sulphuric acid | ... | ... | ·05 |
| Potassa | ·90 | ... | 1·26 |
| Soda | ... | ... | ·21 |
| Carbon or bituminous matter | 27·65(?) | 28·80 | (?) |
| Coal | ... | ... | 8·51 |
| Water | ... | ... | 8·54 |
| Loss | 2·13(?) | 3·13 | 1·59(?) |
| ——— | ——— | ——— | |
| 100· | 100· | 100· | |
Alum-rock, or alum-stone, is a species of impure alunite, and is not of very common occurrence. That of Tolfa, near Civita Vecchia, according to Klaproth, consists of—
| Silica | 56·5 |
| Alumina | 19· |
| Sulphuric acid (SO3) | 16·5 |
| Potassa | 4· |
| Water | 3· |
| Loss | 1· |
| ——— | |
| 100· |
which exhibits an excess of about 3% of sulphuric acid, and about 14% of alumina, more than are requisite to form alum with the 4% of potassa; proportions which, therefore, require to be supplemented with a potassium salt during the process of manufacture. The alum-stone of Mont d’Or contains, according to Cordier, 1·4% of oxide of iron.
The presence of lime in alum-ore is most prejudicial, owing to its affinity for sulphuric acid being greater than that of either alumina or iron. Ores containing it in any quantity are, therefore, unfitted for the manufacture of alum. Magnesia is also prejudicial; but in this case the sulphate of magnesia left in the mother-liquors is not wholly valueless, as it may be crystallised and sold as111 ‘Epsom-salt,’—a thing which is actually done in some English alum-works.
The potash-salt employed by the alum-makers is either the sulphate or the chloride—chiefly the latter; its sources being the waste liquor of soap-works, saltpetre refineries, and glass-houses. Wood-ashes, although rich in potash, do not answer well unless freed by lixiviation from the large amount of carbonate of lime which is always present in them.
The ammonia-salt used in making alum is generally the crude sulphate prepared from the ammoniacal liquor of gas-works, or that from the manufacture of sal-ammoniac by the destructive distillation of animal matter. Both these liquors may be used without previous conversion into sulphate of ammonia whenever there is an excess of sulphuric acid in the aluminous solution.
Soda-salts are seldom, if ever, used as precipitants in the manufacture of alum, on account of the easy solubility of the resulting SODA-ALUM—a property which unfits them for this purpose. See Alums, Ammonia, Dyeing, Mordants, Potash, Sulphuric Acid, &c. (also below).
Alum, Ammonia. (NH4)2SO4 . Al2(SO4)3 . 24 Aq. Syn. (Alumen; Alum; B. P.), Alu′men Ammonia′tum, L.; Alun d’ammoniaque, A. ammoniacal, Fr. This is an alum in which the sulphate of potassium is replaced by an equivalent of sulphate of ammonium. It is prepared by adding crude sulphate of ammonium to solution of sulphate of aluminum; or gas-liquor, putrid urine, &c., to the acid-sulphate.
Much of the common alum, especially that prepared on the Continent, contains both potassium and ammonium; and recently enormous works for its manufacture have been established in England. As an astringent, and as a source of alumina in dyeing, it resembles potash-alum (i. e. ordinary alum). It may, however, be readily distinguished from the latter by the fumes of ammonia which are evolved when it is moistened and triturated, or heated, with caustic potassa or quick-lime; and by the residuum of its exposure to a white heat being pure alumina. See Alum (antè).
Alum, Basic. A variety of alum found native at Tolfa. On calcination and subsequent lixiviation it yields ordinary alum. A like substance falls as a white powder, when newly precipitated alumina is boiled in a solution of alum.
Alum, Baumé’s. Alum-white. See White Pigments.
Alum, Dried; Alum, Burnt. Syn. Alu′men us′tum, A. exsicca′tum (B. P.); Alun Sec, Fr.; Gebrannter alaun, Ger.; Alume calcinato, Ital. Alum deprived of its water of crystallisation by heat.
Prep. Take of alum, 4 oz. Heat the alum in a porcelain dish or other suitable vessel, till it liquefies, then raise and continue the heat, not allowing it to exceed 400°, till aqueous vapour ceases to be disengaged, and the salt has lost 47 per cent. of its weight. Reduce the residue to powder, and preserve it in a well-stopped bottle.
Prop., &c. Similar to those of common alum, but it is rather more astringent, and is less soluble. When moistened, or placed in contact with water, it resumes its water of crystallisation with evolution of heat.—Dose, 10 to 20 gr.; in colic (especially painters’ colic), hæmoptysis, &c. It is chiefly used as an escharotic, to destroy ‘proud flesh,’ &c. It must be kept in a stoppered bottle.
Alum, Chrome. See Alums (in Chemistry).
Alum, I′ron (-ŭrn). Syn. Alu′men Fer′ricum, Sul′phas fer′ri et potas′sæ, Fer′ri perox′idi potassio-sul′phas, &c., L.
Comp. K2SO4 . Fe2(SO4)3.24Aq.
Prep. Take of peroxide of iron, 9 lbs.; sulphuric acid 14 lbs.; dissolve, dilute the mixture with water, q. s., and add of potassium sulphate, 10 lbs.; evaporate, and crystallise.
Prop., &c. Crystals, beautiful octahedrons of a pinkish or pale violet colour. It is strongly recommended, by Dr Tyler Smith, as a chalybeate tonic, and has been used by him, at St. Mary’s Hospital with marked success. It has also been used as a mordant, in dyeing black.—Dose, 1⁄2 gr. to 5 gr.
Alum, Ro′man. Syn. Red alum*, Roach A., Roche A., Rock A.*; Alu′men Roma′num, A. ru′brum, A. ru′peum, &c., L.; Alun Romain, A. de roche, Fr.; Alume di rocca, It. In small fragments, covered with a reddish powder (ALUMEN RUBRUM VE′′RUM); originally imported from Civita Vecchia, where it occurs native. It is much esteemed by dyers from being nearly free from iron-alum. That now sold for it in England is ordinary alum coloured with Venetian red, Armenian bole, or rose-pink (ALUMEN RUBRUM SPU′′RIUM). This is done by shaking the fragments in a sieve over a vessel of hot water, and then stirring them up with the colour, until the surface is uniformly tinged with it. In genuine roach-alum the colour not only covers the surface, but also partially pervades the substance of the crystals. The name was formerly also applied to a pure white variety of alum, prepared at Tolfa; but it is now, in English commerce, exclusively given to common alum artificially coloured.
Alum, Saccharated. Alum, 6 oz., white lead 6 drms., sulphate of zinc 3 drms., sugar 11⁄2 oz. Mix the ingredients reduced to powder into a paste, with vinegar and white of egg. Used in eye waters and cosmetic washes.
Alum, So′da. Syn. Sulphas aluminæ et sodæ, L. Comp. Na2SO4 . Al2(SO4)3 . 24Aq. An alum in which the potassium sulphate of common alum is replaced by a like salt of sodium. It does not occur in commerce. (Vide suprà et infrà.)112
ALUM-EARTH. Alumina.
ALUM MOR′DANTS. In dyeing, mordants having for their basis either common alum or the acetate or sulphate of aluminum. See Alums and Mordants.
AL′UM-ROOT. Syn. Amer′ican san′icle; Heu′chera (Ph. U. S.), L. The root of heuchera America′na (Linn.), a plant of North America. It is powerfully styptic and astringent; and is used chiefly as an external application in cancer.
ALUM-WHITE. See White Pigments.
AL′UMS. Syn. Alu′mina (pl. of alu′men), L. In chemistry, a term applied to a series or group of salts having potassium alum for their type, which they resemble in crystalline form and constitution.
It is found that the aluminum of common alum may be replaced by any other metal having a like nature, without affecting the leading characteristics of the salt; and further, that in the newly formed compound, as in potassium-alum, the second sulphate may also be replaced under the like conditions. All the alums crystallise in octahedrons or cubes, and they all contain the same number of molecules of water. The alums of commerce (or alums proper) all contain aluminum sulphate and an alkaline sulphate.
Prep. All the alums may be made by mixing together solutions of the respective sulphates in equivalent proportions, when crystals may be obtained by evaporation in the usual manner. The presence of sulphuric acid, in slight excess, assists their crystallisation.
AL′UMED (al′ŭmd). Mixed or impregnated with alum. In dyeing, mordanted with alum.
ALU′MEN (-l′ōō-). [L.] Alum; the pharmacopœial name of alum. (See above.)
ALUMINIUM. Syn. Aluminum (which see).
ALUMINOUS. In mineralogy, of, resembling, or containing aluminum. In chemistry, containing or obtained from alum.
ALUMINUM. [Eng., Fr., L.] Syn. Aluminium, Eng., Fr., L.; Alumium, Ger. A metallic radical or element very abundantly distributed, united with silica. Discovered by M. Wöhler, who succeeded in obtaining it as a grey metallic powder (A.D. 1827); and later (1845), under the form of globules exhibiting the leading characteristics of the metal. In 1854, M. Dumas announced to the ‘Academy of Sciences,’ that M. St. Clair Deville had procured pure aluminum from clay, and exhibited several specimens of considerable size and beauty. The result was a general impression that it might be easily obtained in any quantity, and ultimately at a reasonable price; expectations which have been only partly, though to a great extent fulfilled, owing to the expense and trouble of the process, notwithstanding recent improvements.
Prep. (M. Deville; A.D. 1854-59.)—A quantity of chloride of aluminum, varying from 200 to 300 grammes (say from 6 to 10 oz.), is introduced into a wide glass or porcelain tube, between two plugs of asbestos to retain it in position, and a current of hydrogen (thoroughly dried by passing first through concentrated sulphuric acid, and then through a tube containing fused chloride of calcium) passed over it; a gentle heat being at the same time applied to the part of the tube containing the chloride, to drive off any free hydrochloric acid which might have been formed by the action of the air upon it. A small porcelain boat, containing sodium, is now introduced at the other extremity of the glass tube, which is then again closed; and when the sodium is fused, the chloride is sufficiently heated to cause its vapour to come into free contact with it. A powerful reaction ensues, with the evolution of much heat, and this continues as long as any undecomposed sodium remains to act on the passing vapour. The mass in the boat, which is now a mixture of the double chloride of aluminum and sodium, in which small globules of the newly reduced metal are suspended, is allowed to cool in the hydrogen; after which it is treated with water, to remove the soluble double chloride. The residuum, consisting of small globules of aluminum, is, lastly, reduced to a solid button or mass, by fusion, at a strong heat, under a layer of the fused double chloride of aluminum and sodium.
On a large scale two cast-iron cylinders are employed, instead of the glass or porcelain tube just referred to; the anterior one of which contains the chloride of aluminum, and the posterior one a tray holding the sodium, of which 10 or 12 lbs. are commonly operated on at once. These cylinders are united by means of a smaller intermediate one, filled with clean scraps of iron, which serve to separate iron, free hydrochloric acid, and chloride of sulphur, from the vapour of the chloride of aluminum, as it passes through them. During the passage of the vapour of the chloride this smaller cylinder, or tube, is kept heated to from 400° to 600° Fahr.; but the two other cylinders are only very gently heated, since the chloride is volatilised at a comparatively low temperature, and the reaction between it and the fused sodium, when once commenced, usually generates sufficient heat for the completion of the process.
Occasionally a mixture of the double chloride of aluminum and sodium, 40 parts; chloride of sodium 20 parts; fluor spar, 20 parts; each separately dried, powdered, and then blended together; sodium, in small pieces, 71⁄2 to 8 parts, are used instead of the last.
It is likewise made from a mixture of cryolite and fused chloride of potassium, of each, in powder, 5 parts; sodium, 2 parts; a cast-iron crucible being employed; the resulting minute globules being collected and fused to a button under a layer of the double chloride of aluminum and sodium.113
Prop., &c. Aluminum, when quite pure, closely approaches silver in appearance, except in being rather less white and lustrous than that metal. Ordinary specimens, called pure, have a slight bluish tint or tin-white colour, with a perfect lustre, but far inferior to that of pure silver. Sp. gr. 2·56, which by hammering may be raised to 2·67. It is both ductile and malleable; fuses at a temperature between the melting-points of zinc and silver; is not affected by either damp or dry air, or by oxygen at ordinary temperatures, or by water whether cold or boiling; even steam, at a red heat, is only slowly decomposed by it. It is not acted on by nitric acid, however concentrated, unless boiling, and then very slowly; nor by dilute sulphuric acid, sulphuretted hydrogen, and the sulphides, or even the fused hydrates of the alkalies. It is, however, readily dissolved by hydrochloric acid, with the evolution of hydrogen, even in the cold; and by a concentrated mixture of nitric and sulphuric acid. It is feebly magnetic, conducts electricity about eight times better than iron, and is more electro-negative than zinc. Commercial specimens, owing to the presence of iron and silicon, and often zinc, usually slowly tarnish in damp air, and possess the other properties described above in a somewhat diminished degree.
In a finely divided state, particularly in the state of powder or minute scales in which it was originally obtained, when heated to redness, it catches fire and burns with great rapidity in the air, and in oxygen gas with intense brilliancy, the product in each case being alumina.
Aluminum unites with the other metals, forming ALLOYS, of which some promise to be of great value in the arts. An alloy of 100 parts of aluminum with 5 parts of silver may be worked like the pure metal, but is harder and susceptible of a finer polish, whilst its property of not being affected by sulphuretted hydrogen and acids remains unimpaired; even 3% of silver is said to be sufficient to impart to it the full brilliance and colour of pure silver. An alloy containing 10% of gold is softer and scarcely so malleable as the pure metal. With 8% of iron, or 10% of copper, it still remains tough and malleable; but a larger proportion of either of these metals renders it brittle.
The presence of 2 or 3% of zinc destroys its ductility and malleability, and also impairs its colour and lustre; whilst less than even 1⁄4% of bismuth renders it brittle in a high degree. Small quantities of aluminum added to other metals change their properties in a very remarkable manner. Thus, copper alloyed with 10%; of aluminum has the colour and brilliancy of gold, is harder than bronze, very malleable, and may be worked at high temperatures easier than the best varieties of iron; and with 20% is quite white, and closely resembles silver. With more than 12% of aluminum the alloy is harder, but brittle. The alloy formed of 100 parts of silver with 5 parts of aluminum is as hard as the silver of our coinage, whilst the other properties of the latter metal remain unaltered.
Uses. The valuable properties of aluminum adapt it to numerous applications in the arts and everyday life. Hitherto these have been very limited, owing to its comparatively high price; which, notwithstanding it has fallen considerably, is still sufficient to prevent its general or even extensive application. The ‘eagles’ of the French army have been made of it, as well as certain articles of jewelry, plate, &c., as brooches, bracelets, chains, spoons, and other ornamental and useful objects. Owing to its low sp. gr., it has been used as a suitable material for the minute decimal weights of chemists, for military helmets, trumpets, &c. A few cornet-à-pistons, for which its lightness and sonorousness admirably adapt it, have actually been made of it. Its power of resisting oxygen, sulphuretted hydrogen, moisture, &c., would render it invaluable as a coating to metals, particularly iron and lead, to protect them from rust or corrosion, did not its price intervene. As an internal coating for water-pipes, cisterns, &c., no other substance, except gold and platinum, is so well adapted. In chemistry, capsules, tubes, &c., either made of or coated with it, may be often advantageously substituted for those of platinum.
In addition to what has been said above, it may be observed that, in preparing aluminum, the chief care should be to avoid accidents or failure by the employment of too high a temperature, and to avoid the product being contaminated with other metals or with carbon. To ensure the purity of the metal is a matter of the greatest difficulty, owing to the facility with which foreign matters are taken up, during the process, from the materials of which the apparatus is composed; and from the substances from which it is prepared being seldom absolutely pure. Indeed, it is not too much to assert that chemically pure aluminum has not yet been obtained; and that even a very close approximation to it is of very rare occurrence. Whenever a copper boat is used to hold the sodium, the product is always contaminated with copper. Chloride of aluminum always contains some of the chlorides of iron and silicon, both of which are volatile, and probably takes up a further portion from the porcelain or earthenware used to form the apparatus. Sodium also is seldom uncontaminated with carbon or some compound of it; in which case, and likewise when it is not carefully freed from the naphtha in which it has been preserved, the product always contains carbon. The crucible, whether of porcelain or iron, in which the final fusion is made, also contributes to contaminate the metal. Hence the inferior whiteness and brilliancy of commercial specimens of aluminum; a metal which, in its absolutely pure state, may be114 reasonably inferred to be as superior in the above respects to silver as silver is to tin. Commercial aluminum contains from 88 to 94 per cent. only of pure aluminum, and from 1 to 4 per cent. of iron, 1⁄2 to 3 per cent. of silicon, and from 1 to 6 per cent. of copper.
Aluminum salts are generally colourless, soluble, and crystallise with difficulty, and are distinguished as follows:—
Tests.—1. Ammonia and the alkaline carbonates throw down a bulky white precipitate (hydrate of aluminum) from solutions of its salts, which is insoluble in excess of the precipitant.—2. Pure potassa and soda throw down white gelatinous precipitates, freely soluble in excess of the precipitant; from which the hydrate of aluminum is reprecipitated by chloride of ammonium, even in the cold:—3. Phosphate of ammonium gives a white precipitate—4. Iodide of potassium produces a white precipitate, passing into a permanent yellow:—5. Sulphuretted hydrogen gives no precipitate:—6. Sulphydrate of ammonium precipitates alumina from these solutions:—7. Bisulphate of potassium, added to concentrated solutions, gives a precipitate of octahedral crystals of alum:—8. At a red heat its salts part with some of their acid; at a white heat, most of it, if not all:—9. Aluminum compounds, ignited on charcoal before the blowpipe, and afterwards moistened with a solution of nitrate of cobalt and again strongly ignited, give an unfused mass, which, on cooling, appears blue by day, and violet by candlelight; a test, however, which is inapplicable to fusible compounds of aluminum, and such as are not free, or nearly free, from other oxides.
Aluminum, Acetate of. Syn. Acetate of Alumina. Prep. Pure hydrate of aluminum is digested, to saturation, in strong acetic acid, in the cold; and the resulting solution, after being filtered or decanted, is either evaporated by a very gentle heat to a gelatinous, semi-solid consistence (its usual form), or is preserved in the liquid state. By spontaneous evaporation it may be obtained in long, transparent crystals.
Red liquor. From alum, in powder, 4 parts; warm water, q. s. to dissolve; acetate of lead, in powder, 3 parts; the solution and mixture being effected by lengthened agitation in a tub or other wooden vessels, and the clear liquid, after repose for a sufficient time, decanted or drawn off from the sediment.
From alum, 2 parts; (dissolved in) warm water, q. s.; solution of pyrolignite of lime (20° Baumé), 3 parts; as before, but allowing a longer time for the subsidence of the precipitate, and taking more care in the decantation than when acetate of lead is employed.
By decomposing a solution of crude sulphate of alumina with neutral or monobasic acetate of lead.
Prop. Its characteristic property is the feeble affinity existing between its acid and base, which, when it is used as a mordant, is counterbalanced by that of the fibres of the cloth or yarn to which it is applied. In other respects it resembles the other simple salts of alumina.
Uses, &c. In dyeing and calico printing, as a mordant. In medicine, properly diluted, in chronic diarrhœa; and, mixed with syrup of poppies, in slight cases of hæmoptysis (spitting of blood). It has been employed by M. Gannal as an injection to preserve animal bodies, which it will do for years.—Dose, 1⁄2 to 1 dr. daily, in divided portions, taken in thin mucilage or syrup, or in barley-water; as an injection, 10 to 20 gr., to water, 4 to 6 fl. oz., in gonorrhœa, leucorrhœa, &c.
Aluminum, Chloride of. Al2Cl6. Syn. Sesquichlo′′ride of Aluminum; Alumin′ii Chlori′di, &c., L. Prep. A thick paste made of dry precipitated alumina, lampblack, and oil, is strongly heated in a covered crucible until all the organic matter is carbonised. The residuum is transferred to a porcelain tube fixed across a furnace, one end of which is connected with another tube containing dry chloride of calcium, and the other end with a small tubulated receiver. The porcelain tube is then heated to redness, whilst chlorine, dried by passing through the chloride-of-calcium tube, is transmitted through the apparatus. In one or two hours, or as soon as the tube is choked, the whole is allowed to cool, and the newly-formed SESQUICHLORIDE collected and preserved in mineral naphtha for use.
On the large scale:—Chlorine, dried as before, is passed over a mixture of pure clay, lamp-black, and coal-tar, contained in an iron retort, similar to that used in the manufacture of coal-gas (previously ignited by means of a suitable furnace), and connected with a cool chamber accurately lined with tiles of earthenware. The vapours of the SESQUICHLORIDE condense in this chamber, as a yellowish crystalline mass, which is collected and preserved as before.
Prop., &c. It is volatile at a dull red heat; excessively greedy of moisture; and very soluble, with decomposition, hydrochloric acid and alumina being formed. Once dissolved, it cannot be again recovered. Its chief use is in the preparation of aluminum.
Obs. Although alumina, like magnesia, is freely soluble in hydrochloric acid, the sesquichloride of aluminum contained in this solution cannot be obtained in the anhydrous state, or even the solid form, by its evaporation; the chloride suffering decomposition, with the formation of hydrochloric acid, which is volatilised, and alumina, which is left behind.
Aluminum, Ni′trate of. Al2(NO3)6. Syn. Nitrate of Alumina; Alu′minæ Ni′tras, L. Prep. Similar to that of the acetate and citrate. Its concentrated acid solution deposits rhombic crystals, containing 18 equiv. of water.115
Aluminum, Oxide of (Al2O3), and Hydrate of (Al2(HO)6). Syn. Alumina.
Prep. Aluminum is precipitated as a hydrate from solutions of aluminum salts on the addition of an alkali or alkaline carbonate; and this precipitate, after being thoroughly washed and dried, on ignition loses its water and becomes anhydrous. The following are the best formulæ for the purpose:—
Alum is dissolved in about 20 times its weight of distilled water, and the solution is dropped slowly into pure solution of ammonia, until the latter is nearly but not entirely saturated, when the whole is set aside for some time. The clear supernatant liquid is then decanted, and the precipitate is carefully and thoroughly washed three or four times with tepid distilled water; after which it is collected on a filter, again well washed with water, and, lastly, pressed and dried between bibulous paper, either without heat, or at a temperature not higher than 120° Fahr. The product is pure hydrate of ammonium, and is converted into anhydrous alumina by exposure to a white heat in a covered crucible. The residuum, after ignition, is pure ANHY′DROUS ALUMINA, or SESQUIOX′IDE OF ALUMIN′UM.
A solution of alum is slowly added to a solution of carbonate of ammonia, avoiding excess; and the resulting precipitate, after being washed and pressed, is dried at a heat of from 120° to 180° Fahr.
Prop., &c. A soft white powder. The hydrate is freely soluble in the acids and in solution of caustic potassa and soda (from which it is precipitable by sal ammoniac); when anhydrous (as after ignition), it is scarcely acted on by acids, and when perfectly indurated, or crystallised, it is wholly insoluble; but on ignition with alkalies, alkaline ALU′MINATES are formed, and the alumina is then readily dissolved by acids, forming salts, which are mostly colourless, non-volatile, and soluble; they have a very astringent and somewhat sweetish taste, redden litmus paper, and lose their acids by ignition. Its most remarkable, or rather useful property, is its strong affinity for the fibres of organic bodies, as cotton, flax, silk, wool, &c., which are capable of taking it from its salts; and also for organic colouring matters. Hence its great use in dyeing, and in bleaching liquids and the preparation of lakes. Hydrate of aluminum agitated or digested with liquids containing vegetable colouring matter, combines with the latter, and either entirely, or to a great extent, removes it from the solution.
Moist precipitated alumina, dried at a heat between 70° and 80°, contains above 58% of water; dried at 212° Fahr., about 32% of water.
Estim. Aluminum is weighed as oxide, after ignition. The solubility of the moist or recently precipitated hydrate in solution of ammonia enable us to separate it from the ALKALINE EARTHS which, when present, are thrown down with it.
Uses, &c. The moist hydrate is used in several processes in the arts. It is the base of cobalt-blue, the lake-pigments, &c. In medicine, it is employed as an antacid and astringent, in acidity of the stomach, cholera, diarrhœa, and dysentery; in which it is said to be superior to the other absorbent remedies. (Ficinus.) It has also been highly recommended in the vomiting and diarrhœa of infancy. (Durr; Neumann; Weese; &c.)—Dose. Children 3 to 10 gr.; adults, 5 or 6 to 20 or even 30 gr., three to six times daily, suspended in water, by mucilage or simple syrup.
Aluminum, Sil′icate of. Al2(SiO2)3. Syn. Sil′icate of Alumina. A substance which, in its hydrous form, is the chief and characteristic ingredient of common clay; and which also occurs, in combination, in several other important and abundant minerals.
Aluminum, Sul′phate of. Al2(SO4)3. Syn. Sesquisul′phate of Alumina, Neutral s. of a., Alu′minæ sul′phas, A. sesquisul′phas, L. Prep. 1. Saturate dilute sulphuric acid with hydrate of aluminum, gently evaporate, and crystallise.
2. (Crude, commercial.) By mixing clay and oil of vitriol, in the way described under Alum. The product is the ‘concentrated Alum’ of the dyers.
Prop. Its crystals are needles and thin pearly plates; soluble in 2 parts of water; taste astringent, and somewhat sweetish; reaction acid; a full red heat expels its acid, leaving a residuum of pure alumina; with the sulphates of potassium, sodium, and ammonium, it forms alum.
Uses, &c. In the arts, chiefly as a substitute for alum; the sulphate of potassium in the latter, being found to be an unnecessary and costly ingredient, only useful to purify the salt from iron, by forming a compound of easy crystallisation; an object that may be effected with greater certainty by cheaper methods. In medicine, as a wash for foul and ill-conditioned ulcers; and as an astringent and antiseptic injection. M. Gannal has successfully employed a solution of this salt to preserve animal bodies, by throwing it into the arteries. Even an enema of 1 quart of it, or an injection of a like quantity into the œsophagus, will suffice to preserve a body for several weeks. The mineral called Al′unite or Alu′minite, found near Newhaven (Sussex), is a native subsulphate or basic sulphate (DISUL′PHATE) of alumina.
Aluminum, Sulphide of. Al2S3. Syn. Sul′phide of Aluminium, &c. A substance best obtained by passing the vapour of bisulphide of carbon over pure alumina, at a bright red heat. It is instantly decomposed by water, with the evolution of sulphuretted hydrogen. See Aluminum (above).
Aluminum Tann′ate. Syn. Tannate of Alumina, Eng.; Alu′minæ tann′as, L. Prep. Take of pure hydrate of aluminum (dried at 90°116 Fahr.), 1 part; tannic acid (dried at 212°), 2 parts; triturate them together for some time, adding just sufficient water to bring them to the consistence of a syrup, and carefully evaporate to dryness at a heat not higher than 120° Fahr.; lastly, reduce the residuum to powder.
Uses, &c. A combination of certain constitution, which is said to have been found very useful in obstinate vomiting and diarrhœa, in dysentery, and particularly in hæmoptysis, hæmorrhage, &c.—Dose, 3 to 12 or 15 gr.
Aluminium Bronze. See Bronze Aluminium.
AL′VINE (-vĭn). Syn. Alvi′nus, L.: Alvin, Fr. Of or from the belly or intestines; relating to the intestinal secretions.
AMABELE. Consists of crushed millets. See Millet.
AM′ADOU (-ăh-dōō). Syn. German tinder, Touch′wood, Pyrotech′nic sponge, Spunk‡§, Surgeon’s Ag′aric, A. of the oak, &c.; Agar′icus quer′cûs, A. quer′nus, A. Chirurgo′′rum, Fun′gus quer′cûs, &c., L.; Amadou, Agaric Amadouvier, Fr.; Zunderschwamm. Ger. A soft, spongy, combustible substance, being the prepared flesh of bole′tus fomenta′′rius (Linn.), an indigenous species of fungus found on the oak, birch, and a few other trees (REAL AMADOU or OAK-AGARIC); for which b. ignia′′rius (Linn.), a like fungus, found on the willow, cherry, plum, and other trees, is frequently substituted.
Collec., Prep., &c. The outer bark of the fungus (collected in Aug. or Sept.) having been removed with a knife, the inner spongy substance is carefully separated from the woody portion lying below, and after being cut into slices, is well beaten with a mallet until sufficiently soft and pliable. Sometimes it is first boiled in water, in order to separate the epidermis and porous parts, and to free it from soluble matter; after which it is beaten as before. In this state it is used in surgery, &c. To complete its manufacture for TINDER, it is soaked once, or oftener, in a strong solution of saltpetre (RED AMADOU; BROWN A.); or in a thin paste made of gunpowder and water, which is thoroughly forced into the pores (BLACK A.); after which it is dried, and well rubbed to free it from loose matter. The first is the more cleanly; the last the more combustible.
Uses, &c. A light brown or reddish-brown substance. In surgery, pharmacy, &c., it is used to stop local bleeding, to spread plasters on, as a compress, and for other like purposes. When covered with resin-plaster it forms an excellent article for the protection of abraded surfaces. A small piece thus prepared, of a circular shape, having a round hole cut in the middle, the size of the apex of the corn, is one of the very best corn-plasters known; as from its great softness it at once protects the part from pressure, and removes the cause. As a material for shoe-socks it is superior to all other substances. The amadou for surgical purposes must not contain nitre.
AMAL′GAM. [Eng., Ger.] Syn. Amal′gama, L.; Amalgame, Fr. In chemistry and metallurgy, an alloy containing quicksilver; more particularly one in which that metal plays a conspicuous part. Medallists improperly apply this term to all soft alloys.
Mercury unites with many of the metals by mere contact; and with some of them, as gold, silver, tin, and lead, in certain proportions, without losing its fluidity. In a few cases, as with potassium, this union is attended with considerable violence, and with the production of light and heat.
Prep. Most of these compounds may be formed by agitating or rubbing the mercury with the other metal, or metals, in the state of filings or small fragments, either with or without heat; or with the easily fusible metals, by adding it to them in the melted state; care been taken, in both cases, that the heat be not sufficient to volatilise the mercury.
Prop., Uses, &c. Some amalgams are solid, and not unfrequently crystalline; others are fluid. Of the latter several crystallise after a time, becoming solid; being, probably, merely solutions of the solid amalgams in excess of mercury. The amalgams of gold, silver, tin, zinc, &c., are extensively employed in gilding, silvering and dentistry, and in other useful arts and manufactures.
Amalgam, Ammonium. An unstable compound produced when a globule of mercury is placed in a small cavity formed in a piece of sal ammoniac, and the negative pole of a powerful galvanic battery is brought into contact with the metal, and the positive pole, with the ammoniacal salt. In a few seconds the new compound (ammonium amalgam) of the consistence of butter is formed. On withdrawing the influence of the battery, the whole returns to its former condition. By putting an amalgam of sodium into the moistened cavity of the sal ammoniac, similar results are obtained. The phenomena attending the formation of this new substance have been urged as evidence of the existence of the theoretical basic radicle AMMONIUM.
Amalgam, Elec′trical. Prep. 1. Take zinc and grain-tin, of each, 1 oz.; melt them in an iron ladle, remove it from the fire, and add of mercury (hot), 3 oz.; stir the whole well together with an iron rod, pour it into a well-chalked wooden box, and agitate it violently until cold; or, instead of this, it may be briskly stirred until cold, and then powdered. It should be preserved in a corked glass bottle.
2. (La Baumé.) Zinc, 2 oz.; grain-tin, 1 oz.; bees’ wax, 1⁄2 oz.; melt, add of mercury, 6 oz., and otherwise proceed as before. Preferred by some to all other mixtures.117
3. Zinc, 2 oz.; mercury, 5 oz.
Use. To cover the cushions of electrical machines. A little of the powder is poured on a piece of paper, crushed smooth with a flat knife, and then spread thinly on the surface of the cushion or rubber, previously slightly smeared with tallow; or the powder may be rubbed down with a little tallow, prior to the application of it.
Amalgam, Gild′ing. Syn. Amalgam of gold.
Prep. Take of grain-gold, 1 part; mercury, 8 parts; put them into a small iron saucepan, or ladle, and apply a gentle heat, using a smooth piece of iron as a stirrer; when the solution or combination is complete, pour it out on a clean plate or smooth stone slab.
Use. To gild brass, copper, &c., in the common process of wash or fire-gilding. A less proportion of gold than the above is used when a thin and cheap gilding is required; as by increasing the quantity of the mercury the same weight of the precious metal may be extended over a much larger surface.
Amalgam, Sil′vering.—a. For METALS. Syn. Amalgam of silver. Prep., Uses, &c. As the last, but substituting silver for gold.
b. For GLASS. Prep. 1. Lead, tin, and bismuth, of each, 1 oz.; bees’ wax or resin 1⁄4 oz.; melt, skim off the dross, cool to the lowest point at which the mixture will remain liquid, and add of quicksilver 10 oz.; mix well with an iron rod.
2. Lead and tin, of each, 1 oz.; bismuth, 2 oz.; quicksilver, 4 oz.; as the last.
Uses, &c. For silvering the insides of hollow glass vessels, globes, convex mirrors, &c. The glass being thoroughly cleaned and dried, is carefully warmed, and the amalgam, rendered fluid by a gentle heat, is poured in, and the vessel turned round and round, so as to bring the metal into contact with every part which it is desired to cover. At a certain temperature it will be found to readily adhere to the glass. The excess is then poured out, and the vessel set aside to cool.
Amalgams, Tooth. See Dentistry and Tooth-cements.
Amalgam, Var′nisher’s. Prep. Melt grain-tin, 4 oz., with bismuth, 1 oz.; add quicksilver, 1 oz., and stir till cold; then grind it very fine with white-of-egg or with varnish, and apply the mixture to the figure or surface with a soft brush. It is used in several of the ornamental trades.
Amalgamating Salts. Boil a solution of pernitrate of mercury with excess of equal parts of powdered persulphate and perchloride of mercury, and decant the liquid portion of the result for use. Chiefly used for amalgamating the zinc plates of galvanic batteries, also as a substitute for mercury in gilding by the amalgam process.
AMAL′GAMATED. Syn. Amalgama′tus, L.; Amalgamé, Fr. Compounded or blended with quicksilver; formed into an amalgam.
AMALGAMA′TION. [Eng., Fr.] Syn. Amalgama′tio, L.; Verquicken, Ger. The act or process by which an amalgam is formed; hence loosely, the mixing or blending of different things. In the art of the refiner, the operation of separating gold and silver from their ores by means of mercury.
AM′ANDINE (-dēne). Prep. 1. (Transparent.)—a. Fine new white or pale honey, 4 oz.; white soft-soap (prepared from lard and potassa), 2 oz.; mix thoroughly in a marble mortar, adding 1 or 2 teaspoonfuls (if necessary) of solution of potassa, until a perfectly homogeneous paste or cream is produced; then rub in, by degrees, and very gradually, of oil of almonds, 7 lbs. (or q. s.), previously mixed with essential oil of almonds, 1 oz.; essence (oil) of bergamot, 3⁄4 oz.; oil of cloves, 1⁄2 oz.; and balsam of Peru, 3 dr. The product, which should have a rich, transparent, jelly-like appearance and behaviour, is, lastly, put into pots for use or sale.
b. (G. W. S. Piesse.) Simple syrup, 4 oz.; white soft-soap (see above), 1 oz.; oil of almonds, 7 lbs. (previously scented with—); essential oil of almonds and bergamot, of each 1 oz.; oil of cloves, 1⁄2 oz.; the whole being mixed, &c., as before. Both the above are of very fine quality. Glycerin, in the proportion of about 1⁄2 oz. to each lb. of the products, added with the soap, improves their softening quality.
2. (Opaque.)—a. From white potash-soap and gum-mucilage (thick), of each 3 oz.; new white honey, 6 oz.; and the yelks of 5 large eggs; well mixed together, and afterwards intimately blended first, with oil of almonds (scented as before, or at will), 2 lbs.; and afterwards, with thick pistachio-milk (made of the fresh-peeled nuts and rose-water), 5 fl. oz.
b. From almond-paste, honey, white potash-soap, and glycerin, of each. 1 oz.; yelk of 1 egg; oil of almonds, 1⁄2 pint (holding in solution—); essential oil of almonds, 1 dr.; balsam of Peru, 1⁄2 dr.
Uses, &c. To whiten and soften the skin, and to prevent it chapping. A small portion, about half the size of a filbert, with a few drops of warm water, produces a very white and rich lather, with which the hands and face are lightly rubbed, and the skin, in a short time, gently wiped with a small napkin, whilst the water on it is still milky.
The manufacture of AMANDINE is a matter of some difficulty and labour. The details essential to success are given under Emulsines. It is sometimes coloured, which is done by infusing or dissolving in the oil, before using it, a little—spinach-leaves, for GREEN; and palm-oil, or annatto, for YELLOW and ORANGE. A beautiful SCARLET or CRIMSON tinge may be given to it by a little liquid rouge or carmine (ammoniacal), added just before removing it from the mortar. See Emulsines, Olivine, Paste, 118&c.
AMANI′TA MUSCA′′RIA. The fly-agaric or fly-mushroom. See Agaric.
AMANITINE. Syn. Amanitina, L. The name given by Letellier to the poisonous principle of amani′ta muscaria, and some other species of fungi. It is brown, uncrystallisable, and soluble.
AMARA. [L.] In medicine and pharmacology, the bitter tonics.
AMARANTH. Syn. Amaranth′us, L.; Amarante, Fr. The flower love-lies-bleeding (amaranthus caudatus—Linn.). In poetry, an imaginary flower that never fades. (Milton.) In chromatics, a colour inclining to purple.
AMARYTH′RINE. A bitter principle found, in certain lichens, associated with erythrine (which see).
AMASI. This, the native name given by the natives of Central Africa to sour milk, which they prepare by adding to the new milk, a small quantity of milk previously allowed to become sour. The milk thus acidified is considered by them far more wholesome than new milk.
AMAUROSIS. Syn. Gutta serena, Suffusio nigra. A diminution or total loss of sight, arising from paralysis of the retina or optic nerve.
AM′BER. Syn. Elec′tron, Gr.; Elec′trum, Suc′cinum (Ph. D.), L.; Ambre, Succin, Fr.; Bernstein, Ger.; Lynx-stone†, La′pis lyn′cis†, L. A well-known yellowish, semi-transparent, fossil resin, of which trinkets and the mouth-pieces of pipes are commonly made.
Nat. hist., &c. Amber is found in detached pieces on the sea-coast, and is dug up in diluvial soils. That of commerce comes chiefly from the southern coasts of the Baltic, where it is cast ashore between Königsberg and Memel; and from Ducal Prussia, Saxony, Poland, Sicily, and Maryland (U.S.), where it is dug out of beds or mines. It has also been found on the shores of Norfolk, and small pieces are occasionally dug up in the gravel pits round London. It is probably an antediluvian resin; and when found on the coast, is supposed to be disengaged, by the action of the sea, from neighbouring beds of lignite or fossil coal. Much diversity of opinion for a long time prevailed amongst naturalists and chemists as to the origin of amber, some referring it to the vegetable, others to the mineral, and some even to the animal kingdom; its natural history and analysis affording something in favour of each. The vegetable origin of amber has, however, been recently shown by various facts, and is now generally admitted. According to Sir David Brewster, its optical properties are those of an indurated vegetable juice. (‘Ed. Phil. Journ.,’ ii.) Insects and fragments of vegetables are frequently found imbedded in it; and this in a manner which could only have occurred when the resin was a viscid fluid. Microscopical researches have led to the conclusion that it is the production of some species of pine, closely allied to the pinus balsamea. (‘Entom. Trans.,’ i & ii.)
Manuf. Amber is WORKED in a lathe, POLISHED with whiting and water or rottenstone-and-oil, and FINISHED OFF by friction with flannel. During the operation the pieces often become hot and electrical, and fly into fragments; to avoid which they are kept as cool as possible, and only worked for a short period at a time. The workmen are said to often suffer considerably from electrical excitement. Amber is JOINED and MENDED by smearing the surface of the pieces with linseed or boiled oil, and then strongly pressing them together, at the same time holding them over a charcoal fire, or heating them in any other convenient way in which they will not be exposed to injury. The commoner varieties are HARDENED and rendered CLEARER, either by boiling them in rape oil for about 24 hours, or by surrounding the pieces with clean sand in an iron pot, and exposing them to a gradually increasing heat for 30 or 40 hours. During this process small fragments are kept in the sand at the side of the pot, for the purpose of occasional examination, lest the heat be raised too high, or be too long continued.
Prop., &c. Hard; brittle; tasteless; glossy; generally translucent, but sometimes opaque, and occasionally, though rarely, transparent; colour generally yellow or orange, but sometimes yellowish-white; becomes negatively electric by friction; smells agreeably when rubbed or heated; fracture conchoidal and vitreous or resinous; soluble in the pure alkalies, and, without decomposition, in oil of vitriol, which then becomes purple; insoluble in the essential and fixed oils without long digestion and heat; soluble in chloroform; melts at about 550° Fahr.; burns with a yellow flame, emitting at the same time a peculiar fragrant odour, and leaving a light and shiny coal. By dry distillation it yields inflammable gases, a small quantity of water, a little acetic acid, a volatile oil (OIL OF AMBER; O′LEUM SUC′CINI, L.) at first pale, afterwards brown, thick, and empyreumatic, and an acid (SUCCIN′IC ACID; ACIDUM SUCCIN′ICUM, L.); with residual charcoal 12 to 13%. Sp. gr. 1·065 to 1·09, but usually about 1·070. It cannot be fused without undergoing more or less chemical change.
Ident. Amber may be known from mellite and copal, both of which articles are occasionally substituted for it, by the following characteristics:—1. Mellite is infusible by heat, and burns white:—2. A piece of COPAL, heated on the point of a knife, catches fire, and runs into drops, which flatten as they fall:—3. Amber burns with spitting and frothing, and when its liquefied particles drop, they rebound from the plane on which they fall (M. Haüy):—4. Neither mellite nor copal yields succinic acid by distillation; nor the agreeable119 odour of amber when burnt; nor do they become so readily electric by friction.
Uses. It is chiefly made into mouth-pieces for pipes, beads for necklaces, and other ornaments and trinkets. It is also used as the basis of several excellent varnishes. In medicine, it was formerly given in chronic coughs, hysteria, &c.—Dose (of the powder), 10 to 60 gr.
Remarks. The finer sorts of amber fetch very high prices. A piece 1 lb. in weight is said to be worth from 10£ to 15£. 5000 dollars a few years since were offered in Prussia for a piece weighing 13 lbs., and which, it was stated by the Armenian merchants, would fetch from 30,000 to 40,000 dollars in Constantinople. It is more valued in the East than in England; and chiefly on account of the Turks and other Orientals believing it to be incapable of transmitting infection. In the royal cabinet, Berlin, there is a piece weighing 18 lbs., supposed to be the largest ever found. The coarser kinds alone are employed in medicine, chemistry, &c.
Amber, Ac′id of* (ăs′-). Succinic acid.
Amber, Bal′sam of. Syn. Bal′samum suc′cini, L. The thick matter left in the retort after the rectification of oil of amber; and which it resembles in its properties.
Amber, Facti′′tious (-tĭsh′-). Syn. Suc′cinum facti′′tium, L. Mellite, copal, and anime, have each been substituted for amber, especially for small fragments of it. Recently an imitation has been produced by acting on gutta percha with sulphur, at a high temperature, which, either alone or in combination with copal, is said to have been extensively passed off for genuine amber.
Amber, Liq′uid†. See Liquid-ambar.
Amber, Oil of. See Oils.
Amber, Re′sin of. See Pyrétine.
Amber, Salt of. Succinic Acid.
Amber, Sol′uble. Prep. Fragments of amber are cautiously heated in an iron pot, and as soon as it becomes semi-liquid, an equal weight of pale boiled linseed-oil, previously made hot, is very gradually stirred in, and the whole thoroughly blended. Used as a cement for glass and earthenware, and thinned with oil of turpentine to make varnishes. It will keep any length of time if preserved from the air.
AMBER-CAM′PHOR. See Pyrétine (Crystalline).
AM′BER DRINK†. Amber-coloured malt liquor.
AM′BER-SEED. Musk-seed (which see).
AM′BER-TREE. The popular name of a species of anthospermum, an evergreen shrub, of which the leaves, when bruised, emit an agreeable odour.
AM′BERGRIS (-grĭs; grēse‡). Syn. Grey amber*; Ambragri′′sea (grĭzh′-e-ă), L.; Ambregris, Fr.; Ambra, Ambar, Ger. An odorous, solid substance, found floating on the sea in tropical climates, and in the cæcum of the cachalot or spermaceti whale (physeter macrocephalus). It has been supposed by some to be a morbid secretion of the liver or intestines, analogous to biliary calculi; but according to Mr Beale, it consists of the mere indurated fæces of the animal, perhaps (as suggested by Brande and Pereira) somewhat altered by disease. “Some of the semifluid fæces, dried with the proper precautions, had all the properties of ambergris.” (Beale.) It is occasionally found in masses weighing from 60 to 225 lbs.
Prop., &c. Solid, opaque, ash-coloured, streaked or variegated, fatty, inflammable; remarkably light; highly odorous,[40] particularly when warmed, cut, or handled—the odour being peculiar and not easily described or imitated, of a very diffusive and penetrating character, and perceptible in minute quantities; rugged on the surface; does not effervesce with acids; melts at 140° to 150° Fahr. into a yellowish resin-like mass; at 212° flies off as a white vapour; very soluble in alcohol, ether, and the volatile and fixed oils. It appears to be a non-saponifiable fat, analogous to cholesterine. Sp. gr. 0·780 to 0·926.[41]
Pur. From the high price of genuine ambergris it is very frequently, if not nearly always, adulterated. When quite pure and of the best quality, it is—1. Nearly wholly soluble in hot alcohol and ether, and yields about 85% of ambreine:—2. It almost wholly volatilises at a moderate heat, and when burnt leaves no notable quantity of ashes; a little of it exposed in a silver spoon melts without bubble or scum; and on the heated point of a knife it is rapidly and entirely dissipated:—3. It is easily punctured with a heated needle, and on withdrawing it, not only should the odour be immediately evolved, but the needle should come out clean, without anything adhering to it (Normandy):—4. The Chinese are said to try its genuineness by scraping it fine upon the top of boiling tea. “It should dissolve (melt) and diffuse itself generally.” Black or white is bad. The smooth and uniform is generally factitious.[42]
Uses, &c. It is highly prized for its odour, which is found greatly to improve and exalt that of other substances; hence its extensive use in perfumery. In medicine it was formerly given as an aphrodisiac, in doses of 3 to 10 gr. “A grain or two, when rubbed down with sugar, and added to a hogshead of claret, is very perceptible in the wine, and gives it a flavour, by some considered as an improvement.” (Brande.)
Ambergris Facti′′tious. An article of this kind, met with in the shops, is thus made:—Orris-powder, spermaceti, and gum-benzoin, of each, 1 lb.; asphaltum, 3 or 4 oz.; ambergris,120 6 oz.; grain-musk, 3 dr.; oil of cloves, 1 dr.; oil of rhodium, 1⁄2 dr.; liquor of ammonia, 1 fl. oz.; beaten to a smooth hard mass with mucilage, and made into lumps whilst soft. This fraud is readily detected.
AM′BREINE (-bre-ĭn). Syn. Ambrei′na, L.; Ambreine, Fr.; Ambarstoff, Ger. The fatty, odorous principle of ambergris.
Prep. Digest ambergris in hot alcohol (sp. gr. 0·827) until the latter will dissolve no more, then filter. The AMBREINE will be deposited as the solution cools, in an irregular crystalline mass, which may be purified by recrystallisation in alcohol.
Prop., &c. Melts at about 90°; volatilises at 212° to 220° Fahr.; nitric acid converts it into AMBREIC ACID. It closely resembles cholesterine.—Prod. 85%.
AMBRETTE′ (-brĕt′). [Fr.] Musk-seed.
AMBROSIA, RING’S VEGETABLE (Tubbs, Peterborg, U.S.). A liquid with a sediment, containing 1 per cent. of lead. (Chandler.)
AMEISEN BALSAM. Von Dr Livingstone (Ahnelt, Charlottenburg). Balsam of ants. Castor oil, 72 grms.; balsam of Peru, 2 grms.; bergamot, 5 drops. (Hager.)
AMERICAN PILLS (A. H. Boldt, Lexington). For full-blooded, corpulent persons, and for those of sedentary habits, for irregular menstruation, and against contagious diseases. Made of scammony, rhubarb, and soap. (Schädler.)
AMERICAN MEDICINES, Dr SAMPSON’S (New York). Two kinds of pills of coca:—No. 1. 85 pills composed of coca extract and coca powder, and each pill containing about 0·006 grm. of a morphia salt. No. 2. 50 pills, also of coca, and each containing 0·05 grm. of powdered iron. Both kinds are rolled in lycopodium. (Hager.)
AMERICAN PILLS FOR ASTHMA. Gilded pills made of gum ammoniacum.
AMERICAN SCHAMPOO-FLUID FOR PROMOTING THE GROWTH OF THE HAIR. Spirit of wine and rum, with some carbonate of ammonia and potash.
AMERICAN DROPS FOR TOOTHACHE (Majewsky, Warsaw) have been found of various composition. Some which profess to have taken a prize at the Vienna Exhibition were composed of French brandy, containing common salt, and coloured with cochineal. The first was a spirituous solution of an ethereal oil with some oil of cloves, coloured rather reddish; No. 2 was a similar solution with some oil of peppermint and tincture of rhatany; and No. 3 was merely a diluted solution of No. 2. (Hager.)
AMERICAN UNIVERSAL BLOOD-PURIFYING HERB TEA (Dr Kuhr), for women’s diseases, hysteria, nervous debility, epilepsy, stomachic complaints, asthma, hæmorrhoids, gout, rheumatism, worms, and much besides. White horehound, marsh mallow, liquorice wood, and sassafras, of each, 10 parts; anise, coriander and fennel, of each, 5 parts; red poppy petals, 4 parts; lavender flowers, 2 parts; senna, peppermint, millefoil flowers, and valerian root, of each, 1 part. (Kuhr and Selle.)
AM′ETHYST (-thĭst). Syn. Purple rock-crystal; Améthyste, Fr.; Amethys′tus, L. A beautiful sub-species of quartz or rock crystal, of a violet-blue colour of varying intensity, in great request for cutting into seals, brooches, and other like articles of ornament. It was known and prized in the earliest ages of antiquity. Among the ancients, cups and vases were made out of this mineral; and it was an opinion of the Greeks and Persians, that an amethyst bound on the navel would counteract the effects of wine, and that wine drank out of an amethystine vessel would not intoxicate. See Gems.
Amethyst. In chromation, dyeing, &c., a rich variety of deep violet colour. Hence, AMETHYST′INE (ĭn), &c.
Amethyst, Orient′al. A rich violet-blue variety of transparent, crystallised corundum.
AM′IANTH (-e-ănth). Syn. Amianth′us, Amian′tus, L.; Amiante, Fr. The whiter and more delicate varieties of asbestos, particularly those which possess a satiny lustre.
AM′IDIN (-e-dĭn). [Eng., Fr.] Syn. Am′ydine; Amidi′na, L. A substance noticed by Saussure in starch-paste, when long kept. According to Caventou, it is formed at once by the action of boiling water on starch. It forms the interior substance of the starch-grains, and its properties are intermediate between those of starch and gum. It is, indeed, the soluble part of starch, of which a perfect solution can only be obtained by prolonged ebullition in a large quantity of water.
AMID′OGEN. NH2. Literally, the generator of amides; in chemistry, the name given by Kane to an hypothetical body, composed of two atoms of hydrogen and one of nitrogen. It forms AMIDES by combining with other bodies.
Amidogen Ba′ses. In chemistry, ‘amines’ in which only one equiv. of hydrogen is replaced by an organic radical; and hence called PRIMARY MON′AMINES.
AMMONIA. NH3. Syn. Ammonia gas, Ammoniacal gas, Anhydrous ammonia, Terhydride of nitrogen; Ammoniaque, Fr.; Ammoniak, Ger. At the present day the ammonia of commerce is chiefly prepared from the ammoniacal liquor of the gas-works and the manufactories of ivory black, animal charcoal, &c. Lant or stale urine is also an important source of ammonia. In these places a large quantity of crude ammoniacal liquor is produced; to which either sulphuric or hydrochloric acid is added, by which it is converted into a salt, which may be obtained nearly pure by evaporation, and one or more crystallisations, and, in the case of the hydrochlorate and carbonate, subsequent sublimation. Other sources and processes have been sought out and occasionally adopted for the preparation121 of the principal salts of ammonia (its sulphate, carbonate, and hydrochlorate); some of which have been patented, but few of them have got into general use, or have been carried out on the large scale. For many years the manufacture of ammonia and its compounds has incessantly engaged the attention of European chemists.
Many unsuccessful attempts have been made to directly convert the nitrogen of the atmosphere into ammonia. Of these we may mention one which consisted in passing a mixture of nitrogen, carbonic oxide and steam over red-hot hydrate of lime, whereby ammonia and carbonic acid are formed. A plan for the indirect application of atmospheric nitrogen in the preparation of ammonia was suggested by Margueritte, in which it was proposed that cyanide of barium should be prepared, and its nitrogen converted into ammonia by the aid of a current of superheated steam at 600° C. According to the description of this process in a patent, not, however, in practice, native carbonate of baryta is calcined with about 30% of coal-tar, for the purpose of rendering the mass porous as well as more readily converted into caustic baryta at a lower temperature. The carbonaceous mass is, after cooling, placed in a retort, and kept at a temperature of 300° C., while air and aqueous vapour are forced in, the result being the formation of ammonia in considerable quantity, and carbonate of baryta, which is again used.
Ammonia is evolved from ball soda while cooling; during the formation of cyanogen and cyanide of potassium in blast furnaces; and the formation of sal-ammoniac in the process of iron smelting.
Ammonia, in a state of combination, is found, in variable quantities, among the saline product of volcanoes, in sea and rain water, in bituminous coal, in urine, in guano, and in the atmosphere, especially that of large towns. The minute stellated crystals sometimes found on dirty windows in London, and other populous cities, consist of sulphate of ammonia. It is also found in clayey and peaty soils, and in minute quantity in good air and water. (Brande; Fownes; Letheby.) In the free state it exists in the juices of some plants, and in the living blood of animals, and it is freely developed during the decomposition of azotised vegetable substances, and during the putrefaction of animal matter.
Prep. A mixture of fresh hydrate of lime with an equal weight of sal ammoniac (both dry and in fine powder) is introduced into a glass flask or retort, the beak of which communicates with one end of a U-shaped tube filled with small fragments of recently burnt quick-lime, and from which extends another glass tube, about 18 inches long, having its further end bent up ready to be placed under a gas-jar, on the shelf of a mercurial pneumatic trough. (See engr.) The joints being all made air-tight by collars of india rubber, heat is applied by means of a spirit-lamp, and as soon as the air contained in the apparatus is expelled, the gas is collected for use. It cannot be dried by means of chloride of calcium. Powdered quick-lime may be substituted for the hydrate in the above process; in which case the evolved gas is anhydrous, but a much greater heat is then required for its liberation.
Comp. Ammonia is a compound of 3 volumes of hydrogen, and 1 vol. of nitrogen, condensed into two volumes; and by weight of 82·35 parts of nitrogen, 17·65 parts of hydrogen, or, in other words, of one atomic weight of nitrogen and three of hydrogen, having the formula NH3.
Prop. Gaseous, colourless, invisible; highly pungent, acrid, irritating and alkaline; irrespirable, unless very largely diluted with air; extinguishes combustion; burns slowly in oxygen; sp. gr. 0·589; 100 cub. inches weigh 18·26 gr. Under a pressure of 6·5 atmospheres, at 50° Fahr., it forms a transparent, colourless liquid of the sp. gr. 0·731; at 60° Fahr. this liquid expanded into 1009 times its volume of ammoniacal gas; at -40° Fahr., and the ordinary atmospheric pressure, it forms a subtle colourless liquid, which at -103° Fahr. freezes into a white, translucent, crystalline substance. (Faraday.) It is highly basic; all its salts are either volatilised or decomposed at, or under, a red heat—those with a volatile acid sublime unchanged—those with a fixed acid lose their ammonia. It is decomposed into its elements by transmission through a red-hot tube; and when in contact with metallic oxides or spongy platinum, at the same temperature, the newly evolved hydrogen unites with the oxygen of the oxide or of the atmosphere, forming water. Water at 50° Fahr. absorbs 670 times its volume of this gas, and the solution has the sp. gr. 0·875. Its concentrated aqueous solution boils at 130°, and freezes at -40° Fahr.
Tests, &c. Ammonia is recognised by—1. Its pungent odour:—2. By turning vegetable blues green, and vegetable yellows brown; but which soon regain their previous colours, especially on the application of heat:—3. By producing dense white fumes when brought in contact with those of hydrochloric acid:—4. By the Nessler test (see Water, Quantitative and Qualitative Analysis of):—5. If a saturated solution of arsenious acid is mixed with a solution of nitrate of silver (strength 2%) a trace of ammonia causes the formation of try-argentic122 arsenite:—6. Böttger says a very delicate test for ammonia is afforded by an aqueous solution of carbolic acid. On adding to a liquid containing the smallest quantity of ammonia, or an ammoniacal salt, a few drops of this solution, and then a small quantity of a filtered solution of chloride of lime, the liquid becomes green, especially when warmed.
Phys. eff., &c. Inhaled, undiluted with air, it is an irritant poison, producing spasms of the glottis, convulsions, and death; even when diluted it acts as a powerful acrid, and local irritant; applied to the skin it causes vesication. The use of the pungent odour of common ‘smelling salts,’ in syncope, headache, &c., is well known. Largely diluted with air, it has been recently highly extolled in chronic hoarseness, asthma, &c.; and as an antidote to the fumes of bromine, chlorine, and hydrocyanic acid. (Smee.)
Ant., &c. The vapour of acetic acid or common vinegar, freely inhaled. It may be produced by sprinkling a little on a piece of hot iron, as a heated shovel. If bronchial inflammation follows, it must be treated by purgatives and a low diet; and, if severe, and the patient be plethoric or robust, by venesection or cupping.
Uses. Ammonia is employed in numerous processes in chemistry and the arts; but chiefly in the form of ‘liquor of ammonia,’ ‘spirits of hartshorn,’ &c., and in combination, under the form of salts. In its pure or gaseous state it possesses little practical interest.
Ammonia, Solution of. Syn. Solution of ammonia, Liquor ammoniæ, Ammonium hydrate, Ammonia, Eng.; Ammoniaque liquide, Dissolution d’ammoniaque, Esprit de sal ammoniac, Fr.; Atzender ammonium-liquor, Salmiak-geist, Ger.; Liquore di ammoniaco, Ital. Ammonia gas readily dissolves in water, one volume of water absorbing about 670 volumes of ammonia, much heat being liberated, and the solution increases greatly in volume.
This solution is regarded in two very different lights; firstly and most generally as simply a solution of gaseous ammonia, a view rendered most probable by its general physical and by many chemical reactions; by a few, however, it is looked upon as a solution of ammonium hydrate.
Prepared by distilling, in a tubular retort, equal parts of sal ammoniac, hydrated lime, or slaked lime and water, and passing the gas evolved through a set of Wolff’s bottles partially filled with water, as in the figure above.
A, Cylindrical Iron Retort.
B, Furnace for ditto.
C C C C, Stoneware Receivers.
D D D D, Connecting Pipes.
E F, Waste Pipe and Receiver.
G, Safety Tube.
Commercially this article is prepared on the large scale, from a mixture of about equal parts of fresh-slaked lime and sal-ammoniac or sulphate of ammonia, which is heated in an iron cylinder or retort connected with a set of ‘refrigerators,’ the latter consisting of a row123 of stoneware bottles with double necks, containing water, and kept very cold. The general arrangement of the apparatus used in this manufacture is exhibited above, and with the accompanying references, will be easily understood. The ‘condensers,’ when in use, are surrounded with cloths (not shown in the engr.) kept wet with very cold water, whilst constant current of cold air is commonly made to pass over them. The pipe (D) leading from the retort is also several feet long, and is advantageously passed through a wooden screen in order that the radiated heat of the retort and brickwork of the furnace may be intercepted as much as possible.
Two different methods of proceeding are adopted in this process. In the one the dry pulverulent ingredients are mixed together, and the resulting gas distilled over into the water placed in the receivers. In the other the lime is made into a ‘pap’ with water, and the ammonia-salt, in coarse powder, being added, the whole is rapidly blended together, before closing the retort, and applying heat. In either case a proportionate quantity of water is put into the condensers, and the operation is nearly similar; but the latter method requires the least heat, and so far as the receivers and refrigerators are concerned, is, perhaps, the one most easily managed. It is that which is always, and necessarily followed, when sulphate of ammonia is employed.
Prop., Uses, &c. Highly pungent, caustic, and alkaline; lighter than water, and presenting in a liquid form most of the characteristics of pure ammonia. When strongest has a sp. gr. of ·875, and contains about 39 per cent. of ammonia, but the usual strong ammonia of commerce has a sp. gr. of but ·88. The liquor ammonia fortior, B. P., has a sp. gr. of about ·893, and contains 32·5 per cent. of ammonia, while the liquor ammoniæ B. P. has a sp. gr. of about ·940, and contains about 10 per cent. of ammonia. As a medicine it is antacid, diaphoretic, rubefacient, stimulant, and counter-irritant; and is used in various affections in which these remedies are indicated. As a vesicant it is superior to cantharides, and as a caustic it is used with advantage in the bites of rabid animals, especially those of serpents and insects. Its vapour is a common nasal stimulant in faintings, epilepsy, &c. In its concentrated form it is a corrosive poison.—Dose, 5 to 25 drops, in cold water, or milk and water. It enters into the composition of several valuable external remedies, and is in constant employment in the chemical laboratory, both as a reagent and for the preparation of other compounds.
Ant., &c. When the fumes have been inhaled, the patient should be exposed to a current of fresh air; and when the liquid has been swallowed, vinegar or lemon-juice mixed with water may be administered; followed by an emetic, or, on its failure, by the stomach-pump.
Estim. The quantity of gaseous ammonia in pure water of ammonia is easily determined from the specific gravity of the liquid, or from its saturating power. When impure or mixed with other substances, a given weight of the sample is placed in a small retort, the end of which is made to dip into a vessel containing dilute hydrochloric acid. A strong solution of caustic potassa is then poured into the retort, and heat applied by means of a small spirit lamp. When all the ammonia is distilled over, the acid solution is evaporated to dryness, by the heat of a water bath, and the residuum (chloride of ammonium) weighed. Each grain of the chloride thus found represents ·31804 gr. of pure ammonia; 53·5 parts of the former being equivalent to 17 of the latter. If the article for examination be a solid substance (as a salt), it may be dissolved in water, or in dilute acid, before being put into the retort.
In accurate experiments in the laboratory, ammonia is usually WEIGHED either as chloride of ammonium (see above), or as ammonio-bichloride of platinum (NH4Cl, PtCl2); every gr. of the latter representing ·07614 gr. of pure ammonia. Sometimes, though rarely, the quantity of ammonia is determined from the volume of nitrogen eliminated from it, of which 14 gr. represent 17 gr. of ammonia.
Concluding remarks, Patents, &c. Whatever form or process may be adopted for the preparation of liquid ammonia, it is absolutely necessary to keep the receivers as cool as possible, by means of snow, ice, or a current of very cold water, for the purpose of promoting the absorption of the gas, and to prevent its loss. On the small scale, the glass receivers or bottles may be most conveniently surrounded with ice, or a freezing mixture, and two, or more of them, should be furnished with safety-tubes, to prevent accidents. On the large scale, a capacious oblong retort, usually of iron (but sometimes, though seldom, of lead), with a large opening or tubulature conveniently situated for inserting the ‘charges,’ and withdrawing the residuum of the distillation, is employed. The tubulature, or opening, is closed by means of a large and accurately ground iron stopper, or with a door secured by screws, as the case might be. The stopper is well greased before insertion, and is removed by means of a powerful lever. Should it become so firmly fixed that it cannot be displaced in the usual manner, a cloth moistened with cold water, and carefully wrapped round it, without touching the neck of the retort, will generally cause it to contract sufficiently to enable the operator to remove it with facility. Sometimes a large iron kettle, with a moveable and accurately fitting lid secured in its place like that of a ‘Papin’s digester,’ and having a large and long tubulature in its centre, is employed instead of a retort, over which it has the advantage of exposing a larger opening for the removal of the residuum of the process. In either case the distillatory124 vessel is imbedded in sand supported by fire-brick, and is not exposed directly to the heat of the furnace. Before commencing the distillation the joints are all well luted, to avoid leakage. An excellent plan is to pass the gas, as it leaves the retort, through a silver or pewter ‘worm’ or ‘refrigerator’ set in a tub supplied with a stream of very cold water; by which it will be sufficiently cooled before it reaches the ‘receivers’ to obviate the necessity of any further attention to them than keeping the cloths wrapped round them constantly moistened with cold water. The lower end of the ‘worm’ should be connected, by means of a balloon-shaped ‘adopter,’ with the ‘still,’ and the upper end with the first ‘receiver,’ the use of the balloon being to intercept any volatilised ammonia-salt that might be accidentally driven over by the heat being too high, or too suddenly raised.
The heat should be gradually applied, and very gradually raised, to prevent any of the sal ammoniac or sulphate being volatilised undecomposed; and even towards the end of the process it should not even approach redness.
The lime is best ‘slaked’ and ‘papped’ with about 4 parts of water; as a lower heat is then required to expel the gas, and it passes over more easily and fully than when less water is employed. This is absolutely necessary when the sulphate is the ammonia-salt used; as otherwise the residuum of ‘sulphate of lime’ would become so hard that it could not be easily removed from the retort.
The gas being wholly expelled from the retort, or other distillatory vessel, it is disconnected from the receivers, and (when sal ammoniac has been employed) the heat is raised sufficiently high to fuse the residual chloride of calcium, which is then at once baled or poured out. Glass retorts often suffer fracture at this point; but if they escape now, it generally happens that they are broken when heat is applied for a second operation. Hence, according to Prof. Muspratt, it is rare to find a retort, even when carefully handled, that will stand two operations.
When crude sulphate of ammonia is employed it is advisable to have only a little water in the first receiver, which is placed there merely to purify the gas which passes through it, and to retain any traces of volatile empyreumatic or oily matter which may be carried over with it.
Pure solution of ammonia is most easily obtained from ‘sal ammoniac,’ but crystallised sulphate of ammonia, often crude, is more commonly employed, on account of its lower price.
The preparation of pure solution of ammonia admits of no other improvements than such as merely affect the form of the apparatus employed to produce it; and hence, unlike the ammonia-salts of commerce, has been little meddled with by inventors and patentees. Among the plans having for their object the production of an ammoniacal solution, more or less concentrated, fitted for many of the purposes of the arts, and for the preparation of salts, but not for chemical and medical use, besides those of Reece, Spence, Crane and Jullien, &c., already noticed, may be mentioned—
1. That of Watson (Patent dated 1838) in which gas-liquor mixed with a proper quantity of fresh-slaked lime is distilled from a spacious retort or still into a receiver containing cold water, until much steam passes over with the gas, when the strong alkaline liquor forming the distillate, and called the first portion, is drawn off. The distillation is then continued, when a weaker and impurer solution is obtained, called the second portion. The first portion is then reintroduced into a retort or still with a small quantity of fresh lime, and the distillation repeated. The product the patentee calls the first portion of the second distillation. The latter is a strong ammoniacal liquor sufficient for all the purposes of scouring, cleaning, conversion into commercial ammonia-salts, &c. It may be further purified by a third distillation; the second portion of each operation being transferred again to the still with the next fresh charge of gas-liquor.
2. A modification of Coffey’s still,[43] patented by Mr W. E. Newton (1841), under the name of the ‘AMMONIA STILL,’ is now extensively and successfully employed in this manufacture. By its use ammonia may be obtained from ‘gas-liquor,’ ‘bone-spirit,’ or any other ammoniacal liquor or solution, and even from solutions of the salts of ammonia, of almost any density, and of considerable purity; and this by a process which is continuous and inexpensive. The body of the apparatus is formed of wood, the chambers are lined with lead, and the diaphragms are of perforated sheet iron. The management of the apparatus varies with the form in which it is desired to obtain the product. When the ammonia is required to leave the upper chamber of the rectifier in the form of gas, either pure or impure, the steam which ascends, and the current of ‘ammoniacal liquor’ which descends, are regulated in such relative proportions that the latter remains at or near the atmospheric temperature during its passage through some of the upper chambers, becoming successively hotter as it descends, until at length it enters into ebullition; in which state it passes through the lower chambers, either to make its escape, or to enter a cistern provided to receive it. If, on the contrary, the ammonia is required to leave the upper chamber in combination with the vapour of water, the supply of steam entering below must be in such proportion to that of the ammoniacal125 liquor supplied from above, that the latter may be at or near the boiling temperature in the upper part of the apparatus. Crude liquor and ammonia-salts, before being thus submitted to distillation, are, of course, first treated with a proper quantity of quick-lime—in the one case to remove most of the impurities, and in the other to set the ammonia free by seizing on its acid.[44]
The water or solution contained in the first bottle or the first receiver is found to be the strongest, provided it has been kept well cooled; and that in the others, of progressively decreasing strength. By mixing the contents of one bottle with another a solution of almost any strength may be made. It is also easy to prepare liquor of ammonia of any required strength, or to ascertain the strength of that in the receivers, by observing the expansion of the liquid. Water, when fully saturated with ammonia, expands from 3 volumes to 5 vols.; and in less, but corresponding proportion, according to the quantity absorbed. All that is necessary in practice is, that each receiver be furnished with a gauge-pipe by which the degree of expansion may be noted. On the small scale, graduated glass receivers may be used.
3. Mallet’s Apparatus. This, which is employed in many of the large gas works, is shown in vertical section in the accompanying woodcut. Steam is forced into large receptacles, which are filled with gas water, by which means the carbonate of ammonia is volatilised. When lime, as is sometimes the case, is added, ammonia gas is evolved, and this being conveyed into weak sulphuric acid, sulphate of ammonia is the result.
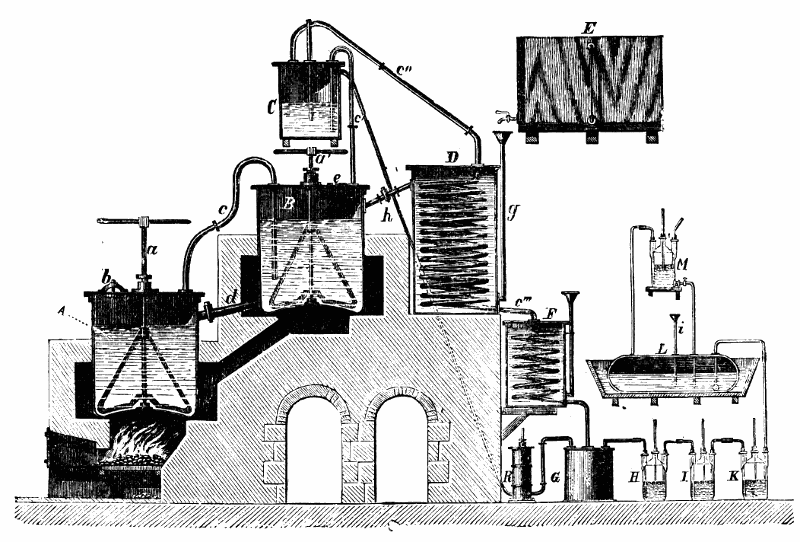
126
The apparatus consists of two cylindrical boiler-plate vessels, A and B. A is heated directly by the fire, and has a leaden tube, c, which dips into the liquid contained in B, this vessel being so placed as to catch the waste heat from the fire. b and e are man-holes; a and a′ are stirrers. By means of the tube d the fluid from B can be run off into A. Gas-water is poured into both vessels, and lime added; ammonia is liberated, whilst carbonate of lime and sulphide of calcium are formed, and these latter remain in the vessels after the volatilisation of the ammonia. The vessel D is also filled with ammoniacal water, and when the operation is in action this water, already warmed, is run by the aid of the tube h from D into B. E is a gas-water tank, from which D is filled by means of g. The ammonia set free in A is, with the steam, conveyed by the pipe c into B, thence through c′ into the wash-vessel C, and thence again through c′′ into the first condenser, D. The partially condensed vapour now passes into the condensing vessel F, the worm of which is surrounded by cold water. The dilute ammonia is collected in G, and forced by means of the pump (R) into C, from whence it is occasionally removed by means of a syphon into either A or B. The non-condensed ammoniacal gas is carried from G through a series of Wolfe’s bottles, the first bottle (H) containing olive oil, with the object of retaining any hydrocarbons that may be present in the gas; the bottle J contains caustic soda-ley, in order to purify the ammonia and retain impurities; the bottle K is half filled with distilled water. The ammoniacal gas having passed through K, is conveyed to the large wooden tank (lined with lead) L, filled with diluted sulphuric acid, if it is intended to prepare sulphate of ammonia, or with water, if solution of ammonia be required. The vessel L is placed in a tank of water; i is a small pipe for introducing acid, while the tube leading to M serves to carry off any unabsorbed ammonia, M being likewise filled with acid.
4. By means of Rose’s apparatus, the ammoniacal gas-liquor mixed with one third of slaked lime is heated in a boiler to a temperature of from 96° to 100°, the ammoniacal gas evolved being passed into hydrochloric acid, and thence through charcoal into vessels containing from 120 to 150 litres of water, which is converted into liquid ammonia of a sp. gr. 0·920.
5. In Lunge’s apparatus the gas-water is heated in a boiler, and the liberated ammoniacal gas passed into sulphuric acid.
Solution of ammonia is now seldom made by the druggist, or on the small scale, the large manufacturing chemists supplying it at a very low rate, and of very superior quality. In the shops it is kept of two or three strengths.
The estimation of the strength of ammonia solutions in commerce is known as ammonimetry, and depends upon their specific gravities. The per-centage richness of solutions of ammonia, or of its carbonates, may be most accurately determined, by ALKALIMETRY. For all the ordinary purposes of commerce, and of the laboratory, the strength of pure solutions of ammonia may, however, be inferred, with sufficient correctness, from their density; and to this the term AMMONIOMETRY is usually restricted.
The specific gravity of the sample being found either by the hydrometer[45] or specific gravity bottle, in the usual manner, its per-centage strength may be seen by inspection of the following Table and the Table on p. 127.
| Sp. Gr. by experiment. | Water of Ammonia of 900, per cent. | Pure Ammonia, per cent. | Water, per cent. |
| ·9000 | 100 | 26·500 | 73·500 |
| ·9045 | 95 | 25·175 | 74·825 |
| ·9090 | 90 | 23·850 | 76·150 |
| ·9133 | 85 | 22·525 | 77·475 |
| ·9177 | 80 | 21·200 | 78·800 |
| ·9227 | 75 | 19·875 | 80·125 |
| ·9275 | 70 | 18·550 | 81·450 |
| ·9320 | 65 | 17·225 | 82·775 |
| ·9363 | 60 | 15·900 | 84·100 |
| ·9410 | 55 | 14·575 | 85·425 |
| ·9455 | 50 | 13·250 | 86·750 |
| ·9510 | 45 | 11·925 | 88·075 |
| ·9564 | 40 | 10·600 | 89·400 |
| ·9614 | 35 | 9·275 | 90·725 |
| ·9662 | 30 | 7·950 | 92·050 |
| ·9716 | 25 | 6·625 | 93·375 |
| ·9768 | 20 | 5·300 | 94·700 |
| ·9828 | 15 | 3·975 | 96·025 |
| ·9887 | 10 | 2·650 | 97·350 |
| ·9945 | 5 | 1·325 | 98·675 |
⁂ Strengths corresponding to sp. gr. which are not in the above Tables may be found by the ‘method of differences’ explained under Alcoholometry.
⁂ The sp. gr. of any sample of liquid ammonia, expressed in three integers, deducted from ·998, and the remainder divided by 4, gives a number which represents the per-centage strength, nearly. (Ure.) This rule may be sometimes conveniently employed for rough calculations, in the absence of Tables.
Ammonia, Carbonates of. (B. P.) Syn. Ammoniæ carbonas. See Ammonium, Sesquicarbonate of.127
| Sp. Gr. of the Liquid Ammonia. | Pure Ammonia per cent., by Weight. | Sp. Gr. of the Liquid Ammonia. | Pure Ammonia per cent. by weight. | Sp. Gr. of the Liquid Ammonia. | Pure Ammonia per cent., by weight. |
| ·87500 | 34·694 | ·91750 | 21·837 | ·96000 | 10·119 |
| ·87625 | 34·298 | ·91875 | 21·477 | ·96125 | 9·790 |
| ·87750 | 33·903 | ·92000 | 21·118 | ·96250 | 9·462 |
| ·87875 | 33·509 | ·92125 | 20·760 | ·96375 | 9·135 |
| ·88000 | 33·117 | ·92250 | 20·403 | ·96500 | 8·808 |
| ·88125 | 32·725 | ·92375 | 20·046 | ·96625 | 8·483 |
| ·88250 | 32·335 | ·92500 | 19·691 | ·96750 | 8·158 |
| ·88375 | 31·946 | ·92625 | 19·337 | ·96875 | 7·834 |
| ·88500 | 31·558 | ·92750 | 18·983 | ·97000 | 7·511 |
| ·88625 | 31·172 | ·92875 | 18·631 | ·97125 | 7·189 |
| ·88750 | 30·785 | ·93000 | 18·280 | ·97250 | 6·867 |
| ·88875 | 30·400 | ·93125 | 17·929 | ·97375 | 6·547 |
| ·89000 | 30·016 | ·93250 | 17·579 | ·97500 | 6·227 |
| ·89125 | 29·633 | ·93375 | 17·231 | ·97625 | 5·908 |
| ·89250 | 29·252 | ·93500 | 16·883 | ·97750 | 5·590 |
| ·89375 | 28·871 | ·93625 | 16·536 | ·97875 | 5·273 |
| ·89500 | 28·492 | ·93750 | 16·190 | ·98000 | 4·956 |
| ·89625 | 28·133 | ·93875 | 15·846 | ·98125 | 4·641 |
| ·89750 | 27·736 | ·94000 | 15·502 | ·98250 | 4·326 |
| ·89875 | 27·359 | ·94125 | 15·158 | ·98375 | 4·011 |
| ·90000 | 26·984 | ·94250 | 14·816 | ·98500 | 3·698 |
| ·90125 | 26·610 | ·94375 | 14·475 | ·98625 | 3·386 |
| ·90250 | 26·237 | ·94500 | 14·135 | ·98750 | 3·074 |
| ·90375 | 25·865 | ·94625 | 13·795 | ·98875 | 2·763 |
| ·90500 | 25·493 | ·94750 | 13·456 | ·99000 | 2·453 |
| ·90625 | 25·123 | ·94875 | 13·119 | ·99125 | 2·144 |
| ·90750 | 24·754 | ·95000 | 12·782 | ·99250 | 1·835 |
| ·90875 | 24·386 | ·95125 | 12·446 | ·99375 | 1·527 |
| ·91000 | 24·019 | ·95250 | 12·111 | ·99500 | 1·220 |
| ·91125 | 23·653 | ·95375 | 11·777 | ·99625 | ·914 |
| ·91250 | 23·288 | ·95500 | 11·444 | ·99760 | ·609 |
| ·91375 | 22·924 | ·95625 | 11·111 | ·99875 | ·304 |
| ·91500 | 22·561 | ·95750 | 10·780 | 1·00000 | 0 or Water. |
| ·91625 | 22·198 | ·95875 | 10·449 |
⁂ The specific gravity of mixtures of pure solution of ammonia and pure water is precisely the mean of the specific gravities of their constituents. (Davy; Dalton; Christison.) In all solutions of ammonia, a quantity of anhydrous ammonia, weighing 2121⁄2 gr., displaces exactly 300 gr. of water, and reduces the sp. gr. of the liquid to the extent of ·00125. (Griffin.) The strongest solution of ammonia which it is possible to prepare at 62° Fahr. has the sp. gr. ·87500, and contains 34·694% of pure ammonia, by weight, or 21,251 gr. per gallon. (Griffin.)[46]
AMMONIUM. The name given to a group of atoms, which play the part of a compound basic, radical, or metallic element. This substance, whose formula is NH4 or (NH4)2, has never been isolated, although capable of forming most stable salts with the various acid radicals. Several attempts have been made, however, to obtain this compound radical, or group of elements, in a free state, and with more or less success, but on account of its great instability it invariably decomposes when set free into ammonia and hydrogen.
Ammonium salts are some of the most important chemical agents, and are usually recognised as follows, ammonia solution, however, usually acting in exactly the same manner as a solution of ammonium hydrate:—By imparting a deep blue tint to solutions of salts of copper. By exhalation of ammoniacal gas (recognised by its odour), when triturated or mixed and heated with caustic potassa, soda, or lime. Added to a solution of bichloride of platinum, they produce a heavy yellow, crystalline precipitate, consisting of minute octahedrons128 easily discernible under the microscope. With protonitrate of mercury, a black precipitate. With bichloride of mercury, a heavy, white precipitate. With a concentrated solution of tartaric acid, a crystalline, white precipitate, nearly similar to that given with salts of potassa. They are nearly all soluble in water, volatile, and crystallisable.
Except the carbonate, they are almost invariably estimated by conversion into ammonia, and estimation by volumetric analyses, as in alkalimetry. In the laboratory, however, for exact purposes, they are converted into the double chloride of ammonium and platinum.
Ammonium Salts:—
Ammonium, Acetate of. NH4C2H3O2. Syn. Ammo′′niæ ace′tas, L.; Acetate d’ammoniaque, Fr.; Essigsäures ammoniak, Ger. Prep. 1. Take of acetate of lime or of potassa and sal ammoniac, equal parts; mix and distil at a gentle heat. The oily liquid (BINACETATE OF AMMONIUM, HNH4(C2H3O2)2), in the receiver forms a radiated crystalline mass on cooling. Dry gaseous ammonia passed into this salt, melted by a gentle heat, transforms it into the solid and inodorous neutral acetate, NH4C2H3O2.
2. Strong acetic acid is saturated with ammonia or carbonate of ammonium, and the solution evaporated over sulphuric acid in vacuo; the resulting crystals, after being carefully drained, are dried by pressure between bibulous paper.
Prop., &c. Long, slender crystals, or a crystalline mass, freely soluble in both alcohol and water, and deliquescent in the air; taste sharp and cooling, and somewhat sweetish. Its solutions cannot be evaporated without loss of the ammonia; even the salt passes off in large quantities with the vapour of water. Its aqueous solution becomes alkaline on keeping, from decomposition of the acid. Distilled with anhydrous phosphoric acid, it is converted into ACETONITRILE. An aqueous solution of this salt was introduced into the Materia Medica by Boerhaave, and has since been extensively used as a diaphoretic and febrifuge, under the popular name of MINDERERUS SPIRIT, after Minderer or Mindererus, who extensively employed it and extolled its virtues. When pure, both the salt and its solutions are neutral to test-paper, and are wholly volatilised by heat. See Solutions.
Ammonium, Arseniate of. (NH4)3AsO4. Syn. Ammoniæ arse′nias, L. Prep. 1. (Neutral.) Saturate a warm concentrated solution of arsenic acid with carbonate of ammonium in slight excess; evaporate by a gentle heat, that crystals may form on cooling.
2. Ammonium, Binarseniate of. H(NH4)2AsO4. As above, but adding an additional equiv. of the acid, as soon as any excess of ammonia has been expelled by the heat employed to evaporate the solution.—Dose (of either). 1-24th to 1-12th gr.; in phthisis, certain skin diseases, &c. See Solutions (and below).
Ammonium, Arsenite of. NH4AsO2. Syn. Ammoniæ ar′senis, L. Prep. From a hot concentrated solution of arsenious acid, and sesquicarbonate of ammonium, as the last.—Used (chiefly) to make arsenite of iron. The properties and physiological effects of the above arsenical preparations are for the most part similar to those of arseniate and arsenate of potassa. They are all poisonous.
Ammonium, Benzoate of. Prep. 1. Dissolve benzoic acid in ammonia solution to saturation, then further add ammonia in slight excess, and crystallise by refrigeration, or in vacuo.
2. (Liquid; Solu′tio ammoniæ benzoa′tis, L.) As the last, but without evaporating the solution.
Prop., &c. Very soluble and very difficult to crystallise. If the solution is boiled for a short time and then abandoned to spontaneous evaporation, crystals of ACID BENZOATE OF AMMONIUM are deposited. It is used chiefly as a chemical test; but has been recently recommended in chronic bronchitis, old coughs, &c.; and to check the formation of chalk-stones and urinary calculi.—Dose, 10 to 15 gr.; (of the solution) 15 drops to 1 fl. dr., or more. See Benzoic Acid.
Ammonium, Bromide of. NH4Br. Syn. Ammo′′nii bromi′dum, A. bro′mis, L.; Hydrobromate d’ammoniaque, Bromure d’ammonium, Fr. A salt which is obtained from hydrobromic acid, bromide of iron, &c., by similar processes to those adopted for the iodide. The following process for the preparation of bromide of ammonium is from the formula for the new medicaments adopted by the Paris Pharmaceutical Society: “Add bromine very slowly to a solution of ammonia, with continual stirring, until the liquid remains faintly and persistently coloured by a slight excess of bromine.” It forms white prismatic crystals; and, in its general properties, resembles bromide of potassium. It is volatile, and easily decomposed.
Used as a nervine in hysterics; especially useful for sleeplessness where there is no organic disease; given in epilepsy when bromide of potassium fails.—Dose, 2 to 20 grains.
Ammonium, Carbonates of[47]—
Ammonium, Carbonate of. Syn. Neutral carbonate of ammonium. Equal parts of dry sal ammoniac and sodium carbonate are heated to form the neutral ammonium carbonate of commerce, which sublimes. Solid crystalline substance, with a strong ammoniacal odour, volatile and soluble.
Uses, &c. In the solid form it is not now used in medicine; but it is indirectly employed in several liquid preparations in which the sesquicarbonate is ordered. It is superior to any other preparation of ammonia for filling smelling bottles; as it is not only more pungent, but does not lose its pungency by keeping. It volatilises more quickly than the sesquicarbonate,129 and the residuum, unlike that of the latter salt, continues as odorous as ever. It is the basis of several of the most popular and esteemed advertised smelling salts of the shops. Spirit of hartshorn is an impure solution of this salt, originally obtained by distilling hartshorn or bones.
Ammonium, Sesquicarbonate of. Probably 2NH4HCO3 + NH4NH2CO3, i. e. a mixture or compound of bicarbonate of ammonium and carbamate of ammonium. Syn. (Carbonate of ammonia, Ammoniæ carbonas. B. P.). Carbonate d’ammoniaque, Fr.; Kohlensaures ammoniak, Ger. It is prepared on a very large scale commercially as follows:—Sal ammoniac or sulphate of ammonia, and chalk, equal parts, both dry and in powder, are mixed as before, and sublimed from a series of iron retorts or iron pots, into a well-cooled and capacious receiver lined with lead or earthenware; or, more generally, into such a receiver connected, by iron or lead pipes, with a second and similar one containing a stratum of water, to absorb the free ammonia evolved during the process.
The so-called “Volcanic Ammonia” is evolved during the manufacture of borax, from carbonate of soda and boracic acid. It is largely used in pharmacy.
Prop. The carbonate of ammonia, of commerce, usually occurs in the form of white, fibrous, translucent, or semi-translucent cakes, generally about two inches thick. It is less volatile and pungent than the neutral carbonate; soluble in 4 parts of water at 55° Fahr., 3·3 parts at 62°, 2·5 parts at 96°, and 2 parts at 120°; boiling water and alcohol decompose it, with the evolution of carbonic acid gas and ammonia; by age or exposure to air, the surface assumes an opaque white colour, from its carbonate flying off, and the remaining bicarbonate being less volatile. Unlike the carbonate, it can neither be resublimed nor digested or distilled with either alcohol or water, without suffering decomposition. Sp. gr. 0·966.
The exact composition of this salt varies, according to its method of preparation.
Uses, &c. It is commonly employed by bakers to give lightness to their fancy goods, and to make extemporaneous bread and pastry; by the chemist and pharmaceutist, for the preparation of other salts of ammonia, and in analysis, &c. In medicine it is used as a stimulant, antispasmodic, antacid, and diaphoretic, in acidity of the stomach, dyspeptic affections, gout, scrofula, hysteria, lowness of spirits, epilepsy, &c.; and in the convulsions attending dentition. It has been recently recommended, by Dr Barlow, in diabetes. It is also employed to make effervescing draughts; and externally as a counter-irritant and stimulant. Its use as a nasal stimulant in headaches, fainting, &c., is well known. In large doses it is emetic; in excessive doses poisonous. Its long-continued use, in quantity, is often productive of very serious consequences—slow fever, debility, emaciation, scurvy, loss of teeth, hæmorrhage, general cachexy, and even death. The antidote and restorative treatment are, the free use of lemon-juice, wine or malt-liquors, new milk, and antiscorbutic vegetables, with a generous diet, of which the red meats form a large proportion.—Dose. As a stimulant or diaphoretic, 5 to 15 gr., dissolved in cold water; as an emetic, 20 to 30 gr., in tepid water, repeated if necessary; as an effervescing saline draught, 15 to 30 gr. A few grains (8 or 10) dissolved in a tumbler of cold water is an excellent ‘refresher’ in lowness of spirits, or after fatigue; and is highly esteemed by drunkards; being, in each case, preferable to ‘spirit of sal volatile,’—Doses for Animals. Horse: 1 to 2 drachms. Cattle: 2 to 4 drachms. Sheep: 20 grains to 1 drachm. Pig: 20 grains to 1 drachm. Dog: 3 to 10 grains; in bolus, pill, or cold gruel.
Concluding remarks, Patents, &c. In extension of the above it may be added that, on the large scale, the distillation is usually carried on in cast-iron retorts, similar in size, shape, and character to those employed in the manufacture of coal-gas, and of which five, or more, are commonly set horizontally in the same furnace. (See engr.) Each retort has its mouth (a), through which the ‘charge’ is introduced, closed with a movable door, which is securely fastened in its place, in the manner shown in the engr.; and is furnished, at the upper part of its further end, with an iron pipe (c), to carry off the evolved vapours to the condenser or receiver. The latter consists of two large square wooden chambers (B, C), lined with lead, and either fitted with movable covers, secured by water-joints, or with doors in the side, to permit of the easy removal of the sublimed salt. The first receiver communicates with the second by means of a large lead tube (d) near its centre, and by another tube (d′), somewhat smaller, and nearer the bottom, but above the surface of the stratum of water in the second receiver, before alluded to. These chambers have also a lead pipe (e, e), stopped during the process with a plug or cock of lead, to allow of the liquid product of the distillation, &c., to be drawn off, or run into another receiver or cistern, at will. Both chambers are placed on strong wooden supports, or scaffolding, to bring them on a level with the retorts. When the impure sulphate or other ammonia-salt is used in the manufacture of the sesquicarbonate (which is generally the case), the resulting salt being impure and discoloured, is resublimed in iron pots (f, f, f), furnished with movable leaden heads, which are kept cool by a current of air passing over them; a little water being introduced into the subliming pots to render the product translucent. The heat is applied either by means of a flue passing from the retort-furnace (A, b), or by a water bath heated in the same manner; the latter being the preferable method, as the temperature130 should not be greater than about 200° Fahr., and need not exceed 150° to 155°. These pots are arranged in sets, as shown at D in the engraving.
The charge of a retort usually consists of about 70 to 72 lbs. of sulphate of ammonia or 57 to 58 lbs. of the hydrochlorate to 1 cwt. of chalk; or in these proportions. The product is about 40 lbs. of the crude salt, which, by careful resublimation, yields about 39 lbs. of marketable carbonate of ammonia.
Carbonate of ammonia, like the chloride and sulphate, is now scarcely ever prepared on the small scale, that of commerce being not only cheaper, but sufficiently pure for all the purposes of medicine and the arts.
Ammonium, Bicarbonate of. HNH4CO3. Prep. By digesting cold water on sesquicarbonate of ammonia in considerable excess, until the whole of the pungent neutral carbonate is dissolved out. If the salt is reduced to powder the operation is facilitated.
To powdered sesquicarbonate of ammonia add boiling water just sufficient to dissolve it, and immediately close the vessel; crystals form as the liquid cools, containing 21⁄2 equiv. of water.
Prop., &c. For the most part similar to the sesquicarbonate, except in having a taste and smell which is only faintly ammoniacal, and hence more palatable. Crystallises in oblique prisms, which, as usually obtained, contain about 23% of water. It requires 8 parts of cold water to dissolve it. It is distinguished from the previous carbonates by the almost entire absence of ammoniacal odour, and by its solution giving no immediate precipitate with chloride of barium, but by standing, or on the addition of a little liquor of ammonia, a white earthy precipitate, accompanied with the evolution of carbonic acid gas. A saturated solution of this salt, evaporated by a very gentle heat, or refrigerated, gives small prismatic crystals having neither smell nor taste.
Uses, &c. Similar to those of the other carbonates.—Dose, 6 or 7 to 20 or 25 gr.
Ammonium, Chloride of. NH4Cl. Syn. Muriate of ammonia, Sal ammoniac, Hydrochlorate of ammonia; Chlorohydrate d’ammoniaque, Sel ammoniac, &c., Fr.; Salmiak, Ger. A substance which, as already noticed, appears to have been originally obtained, by sublimation, from the soot of camels’ dung, in Egypt. In this country, at the present day, it is manufactured chiefly from the crude ammoniacal liquors obtained as secondary products in the manufacture of coal-gas and animal charcoal.
Prep. 1. From GAS-LIQUOR:—The crude ammoniacal liquor of the gas-works is, either at once, or after distillation,[48] neutralised with hydrochloric or sulphuric acid, the choice being given to the one which is the cheaper and more accessible at the place where the works are situated. When hydrochloric acid is employed, the SATURATION is usually effected by allowing the acid to flow from a large wooden vessel or tank lined with lead or gutta percha into a large underground reservoir or tank containing the ammoniacal liquor, and having an exit-tube passing into the chimney or shaft of the steam-engine, to carry off the sulphuretted hydrogen and other offensive gases liberated during the mixture. Sometimes the gas-liquor is accumulated in enormous covered wooden tuns, capable of holding from 10,000 to 20,000 gallons, or more; and the acid is added by raising the gutta-percha carboys containing it by means of cranes, and then thoroughly mixing it with the liquor by means of powerful ‘agitators,’ whilst the offensive fumes are either passed off as before, or made to traverse the fire of the steam-engine before entering the chimney-shaft. The quantity of acid employed to effect saturation must, of course, depend on the ammoniacal strength of the gas-liquor operated on. The usual proportions are 11⁄2 to 2 lbs. of the former, to each gal. of the latter; but in all cases sufficient should be added to impart a very faint acid reaction to the mixture. This last having been effected, the saline131 solution, now containing hydrochlorate of ammonia, is, after repose, ready to be pumped or run off into the evaporators.
The EVAPORATION of the crude saline solution is usually carried on in large square or rectangular cast-iron vats, of very moderate depth, and capable of holding from 1000 to 1500 gallons, or more. These are encased in brickwork, and are heated by a furnace, of which the flues pass in a sinuous course beneath the lining of brickwork on which the vats or pans rest. During the concentration of the liquid, the tar, &c., which separates and floats on the surface, and which thus seriously impedes evaporation, is, from time to time, removed by skimming. As soon as the sp. gr. reaches 1·25, any excess of acid in the solution is exactly neutralised with a little fresh ammoniacal liquor; by which any waste of acid is prevented, at the same time that any ferric salt present, and which would contaminate the ultimate product, is precipitated as sesquioxide. After settling for a short time, the hot liquor is ready to be transferred to the crystallisers.
The vessels employed in the CRYSTALLISATION are pans or tubs, usually circular and about 7 or 8 feet wide, by 21⁄2 to 3 feet deep; and are generally set on the ground, or are embedded either partially or wholly in it. The saline liquor being pumped or run into them at a little below the boiling temperature, crystallises as it cools; the only interference being occasional stirring or agitation, to prevent the formation of large crystals, which would be inconvenient in the subsequent part of the process. The time occupied in the crystallisation varies, according to the size of the ‘crystallisers,’ and the weather, from 3 or 4 to 8 or even 10 days. The ‘mother-liquor’ of the ‘crystallisers’ is pumped back into the evaporating pans for further concentration. The crude blackish salt (hydrochlorate) thus obtained is contaminated with tarry and oleaginous matter, free acid, water, &c.; from part of which it is freed by exposing it in a layer about 4 inches deep, on a cast-iron plate gently heated by a zigzag flue of a small furnace, until all the water is expelled; care being taken that the heat never rises high enough to volatilise the salt. This operation is generally performed under a dome, or the expanded throat of a large chimney. The salt will now have become of a greyish-white colour, and is ready for the next operation.
The crude dried salt of the last process is finally purified by sublimation. For this purpose cast-iron-pots lined with clay, and heated from below and by flues round their sides, are employed. (See engr.) The crude grey salt is beaten down into these pots until they are about 2-3rds filled, when the heads or capitols are fitted on, and heat applied. The latter are very heavy, being usually made of lead (sometimes of iron), and have the form of a dome, or a hemispherical cup, with a small tube or hole at the apex, in which a plug is loosely placed, to permit the escape of steam. These domes or heads are so made as to fit closely and firmly on the flat rim or flange of the ‘sublimers,’ and are retained in their places, during use, both by their weight, and by 2 or 3 clamps provided for the purpose. They are also furnished with 3 rings, set at equal distances, to allow of their being lifted off, or moved, by means of a pulley and chains. The due application and regulation of the heat is here of the utmost importance. If the temperature employed be too high, the sublimed salt will be contaminated with empyreumatic matter, while some of it will be carried beyond the dome and lost; and if it be extreme, the head may be altogether blown off, and the contents of the pan scattered about the building; whilst on the other hand, if the heat employed be too low, the resulting cake of sal ammonia will be soft, spongy, and either grey or yellowish. The proper temperature is said to be known by two or three drops of water readily boiling, and being dissipated in vapour, when placed on the head or cover of the sublimer; but it should not ‘spit’ or ‘dance about,’ or be raised by the heat out of contact with the metal. The usual practice is to keep the fires “briskly up until the sublimers and their surroundings attain a sufficient degree of heat; they are then slackened, and maintained at a mean temperature.” (Muspratt.) The sublimation occupies from 5 to 9 days; but it is customary to raise the heads once, or even twice a week, to ascertain the progress made; the fires having been purposely neglected or checked for some hours previously. The process is finally stopped132 before the whole of the crude salt in the pots is volatilised; since the heat required for that purpose would lead to the decomposition of the carbonaceous impurities, and cause them to emit volatile hydrocarbons, which would materially lessen the purity and beauty of the product. The unsublimed portion in the pots forms a conical mass, which is technically called the ‘yolk.’ This is shown in the second engr. (see below), in which the latest improvements in the form of the subliming apparatus are also exhibited.

The sublimation having been carried to a sufficient extent, the fires are allowed to die out. The domes, after cooling, are lifted off, and the attached hemispherical cakes or ‘bells’ of SAL AMMONIAC or HYDROCHLORATE OF AMMONIA at once removed. These vary from 2 to 5 inches in thickness, and from 45 or 50 lbs. to 1000 lbs., and upwards, in weight, according to the size of the sublimers in which they have been produced. They are generally nearly pure, except in the outer part which has been in contact with the metal. From the subliming-house they are taken to the store or packing-house, and after having been scraped, to remove the discoloured portion before alluded to, are either preserved entire, or are broken up into convenient pieces, which are then packed in casks or barrels, and in either state are ready for the market.
When sulphuric acid[49] is used to neutralise the ammoniacal liquor, the process is generally, for the most part, the same as when hydrochloric acid is employed; but here the brown salt obtained by the crystallisation, and subsequent desiccation, is crude SULPHATE OF AMMONIA, instead of the hydrochlorate. It is intimately mixed with about an equal weight of chloride of sodium (common salt) before being put into the sublimers.
In some cases, particularly where the ammoniacal liquor is rich in carbonate of ammonia, gypsum is employed as a source of sulphuric acid. (See below.)
Another method is to convert the solution of the crude sulphate into a solution of the hydrochlorate, during the process, by the addition of chloride of sodium. Both these last methods are described below.
2. From BONE-LIQUOR, &c.[50]—The ammoniacal liquor technically called ‘bone-liquor’ or ‘bone-spirit,’ and formerly known under the name of ‘spirit of hartshorn,’ is essentially a solution of carbonate of ammonia more or less contaminated with volatile empyreumatic oil. Its conversion into SAL AMMONIA may be easily effected by saturating it with hydrochloric acid, evaporating the resulting neutral solution in lead or iron boilers until a pellicle begins to form, then pumping or running off the hot liquors into the crystallisers, and, lastly, draining and drying the crystals. The salt thus obtained may be purified either by sublimation or by recrystallisation. The whole series of processes closely resemble those already described, except in being less troublesome, owing to the absence of the tarry and other foreign matters which impede and complicate them when gas-liquor is employed.
Another method adopted, particularly on the Continent, and one equally applicable to any crude ammoniacal liquor rich in free ammonia or its carbonates, is to employ sulphate of lime instead of sulphuric acid to neutralise the alkali. For this purpose the ammoniacal liquor is passed through a series of three or four covered wooden filters lined with lead, each containing a layer of crushed gypsum to the depth of 3 or 4 inches. These filters are usually set on ‘stages’ one above another, and each communicates with a cistern placed beneath it by means of a leaden pipe furnished with a stop-cock. This last is not opened untill the liquor has remained some little time in the filter; and a pump throws back once, or oftener, upon each filter, what has already passed through it, before it is allowed to run into the next lower one. The ‘liquor’ in each filter is not allowed to stand higher than from 2 to 3 inches above the surface of the gypsum; and the lowest or last filter is supplied with fresh gypsum at each separate charge of fresh liquor. A little water is lastly passed through the filters to wash out the portion of ammoniacal liquor absorbed or retained by the filtering media. In this way the gypsum of the filters is converted into carbonate of lime at the expense of the carbonate of ammonia in the solution; whilst the ammonia of the latter decomposes the gypsum, and becomes converted into sulphate of ammonia, which, with some free ammonia, is found in the filtrate. Sulphuric acid is next added to the filtered liquor to completely neutralise the free and carbonated alkali still existing in it; after which it is evaporated in a leaden boiler, with133 frequent skimming to remove floating oil, until of the sp. gr. 1·160. Chloride of sodium (common salt), in sufficient quantity to convert all the sulphate of ammonia in the liquid into hydrochlorate, by double decomposition, is now added, with constant stirring; after which the clear portion is either pumped or syphoned off into a somewhat deep reservoir or tank, where it is allowed to settle. The liquid after sufficient repose is pumped from the reservoir to the boilers, and evaporated, with frequent agitation, so long as the sulphate of soda now existing in it falls to the bottom in granular crystals. These crystals are, at intervals, scraped to the cooler portion of the pan or boiler, whence they are removed by copper rakes and shovels, into draining-hoppers, placed near the edges of the pan. The liquor in the boiler is now a strong solution of sal ammoniac, but still containing a little sulphate of soda, from which it has to be freed by crystallisation. With this object it is further concentrated, and then run or pumped into the crystallisers. In 30 or 40 hours, or longer, the mother-liquor is run or pumped off. The mass of newly-formed crystals is then drained, and slightly washed, first with a little weak solution of sal ammoniac, and next with a very little cold water; after which they are again well drained. The crude HYDROCHLORATE OF AMMONIA, thus obtained, is converted into the pure salts, by desiccation and sublimation, as before.
In France, where this method is very generally employed, the sublimation is commonly conducted in stoneware or earthenware balloons or bottles coated with loam, of about 18 to 20 inches in height in the body, and either surmounted with inverted ‘cups’ or ‘heads’ 10 or 12 inches high, or simply covered with a tile, when (in the latter case) the sublimate collects in the upper part or neck of the balloon, which is above the action of the fire. A number of these vessels are set on the dome of a furnace, which is perforated with holes or slits, to allow the heat to pass through; whilst their necks or heads are sheltered from the action of the fire by plates of iron or earthenware, having semi-circular indentations on their edges, so that when placed together they form a level surface, through which the necks of the sublimers protrude, and fit closely. The fire is nicely regulated, so as to cause the salts to condense in the upper and cooler part of the vessels, or in the heads, as the case may be; and great care is taken to occasionally clear the necks with a skewer, to prevent choking, and consequent bursting.
In Scotland, where a similar process is also commonly pursued, the sublimers, according to Dr Ure, are generally “cast-iron pots, lined with fire-proof tiles; the condensation being effected in globular heads of green glass, with which each of the iron pots are capped.”[51]
Ratio. Gas-liquor contains carbonate of ammonium (chiefly), with chloride, sulphate, hydrosulphate, cyanide, sulphocyanide, &c., of the same radical. On neutralisation with hydrochloric acid, or sulphuric acid, these are converted into chloride or sulphate of ammonium, according to the acid used. By sublimation with chloride of sodium, the sulphate of ammonium is converted, by double decomposition, into chloride of ammonium, which sublimes; and sulphate of sodium, which remains in the subliming pot. A similar change occurs when the solution of the sulphate, prior to crystallisation, is decomposed by the addition of chloride of sodium, or any other chloride. When the ‘gas-liquor’ is at once converted into chloride of ammonium by the addition of hydrochloric acid, the sublimation merely purifies the salt. Like changes occur when bone-spirit is employed.
Comp. Chemically considered, this salt consists of equal VOLUMES of gaseous ammonia and hydrochloric acid gas condensed into the solid form; or, by WEIGHT, according to the ammonia-theory, of—
| Atoms. | Equiv. wt. | Per cent. | |
| Ammonia (NH3) | 1 | 17· | 31·78 |
| Hydrochloric acid (HCl) | 1 | 36·5 | 68·22 |
| —— | ——— | ——— | |
| Hydrochlorate of Ammonia (NH3HCl) | 1 | 53·5 | 100· |
Or, according to the ‘ammonium-theory,’ of—
| Atoms. | Equiv. wt. | Per cent. | |
| Ammonium (NH4) | 1 | 18· | 33·65 |
| Chloride (Cl) | 1 | 35·5 | 66·35 |
| —— | ——— | ——— | |
| Chloride of Ammonium (NH4Cl) | 1 | 53·5 | 100· |
Prop. &c. The sal ammoniac of commerce is found under the form of large white hemispherical, cup-like cakes or masses (or in large fragments which are sections of them), possessing a tough, fibrous, semi-crystalline texture, and very difficult to powder. It is odourless, has a saline taste somewhat sharp or acrid, and sublimes without either fusion or decomposition. It slightly reddens litmus; dissolves in rather less than 3 parts of cold water, and in about 1 part of boiling water; is soluble in alcohol; and when crystallised from water, under favorable circumstances, forms distinct octahedra, or cubes, usually small and aggregated together in rays or feathery masses. By slowly evaporating its aqueous solution, it may be sometimes obtained in cakes an inch in thickness. It is anhydrous. Sp. gr. 1·450.
Pur. It should give a colourless solution with water; wholly sublime with heat; and neither chloride of barium, nor sulphuretted hydrogen, should affect its solution. A solution,134 to which a few drops of nitric acid have been added, should not yield a blue precipitate with ferrocyanide of potassium. It often contains sesquichloride of iron, and sometimes lead; both of which may be readily detected by the above tests. Its complete volatility may be easily determined by heating, in the flame of a candle, a small fragment held on the point of a knife.
Tests.—1. It is known to be a salt of ammonium by its cooling ammoniacal fumes when triturated with lime, or when moistened with caustic potassa or soda:—2. It is shown to be a chloride by its solution yielding, with nitrate of silver, a white curdy precipitate, insoluble in boiling nitric acid, soluble in ammonia.
Uses, &c. In the arts, chiefly in the coating and soldering of metals, and the preparation of alloys; in dyeing; and in the manufacture of ammonia-alum; also, in large quantities, to give a factitious pungency to snuff. In chemistry, as a reagent; and, owing to the cold produced during its solution, to form frigorific mixtures. In medicine it is chiefly used externally, as a stimulant and resolvent or discutient; and occasionally, internally, as a diuretic, stimulant, resolvent, alterative, tonic, &c., particularly in chronic inflammations of the mucous and serous membranes, in chronic glandular and visceral enlargements and indurations, and in amenorrhœa. In rather large doses, frequently repeated, it is said to prove often highly beneficial in chronic enlargement and induration of the prostate gland (M. René Vanoye); and also in other like cases.—Dose, 5 to 20 gr., 3 or 4 times daily, either in powder or solution, mixed with some demulcent; as a discutient or resolvent lotion, 1 to 11⁄2 oz., to 1⁄2 pint of water, either with or without 4 or 5 fl. oz. of spirits or strong vinegar (often serviceable in chilblains); as a weak lotion, or a collyrium or injection, 1 to 4 dr., to water, 1 pint. In very large doses it is poisonous; the treatment is emetics and mucilaginous or demulcent drinks.
Concluding remarks, Patents, &c. The methods already described are those by which commercial hydrochlorate of ammonia is usually if not almost entirely obtained; the various improvements or modifications, from time to time introduced, affecting chiefly the minor details, and the form or size of the apparatus and machinery employed, and not the general principles on which the processes are based. One of the most important of these has for its object the entire removal of the iron present in the crude salt, some of which, if it be not removed before sublimation, is volatilised and contaminates the ultimate product. To obviate this evil, Mr Brewer passes a few bubbles of chlorine through the hot concentrated solution of the salt, previous to its crystallisation; by which the protochloride of iron is converted into the perchloride, which, being acted on by the ammonia always present in the liquor, is precipitated as ferric hydrate, with the formation of a small additional quantity of sal ammoniac. The only precaution necessary is to avoid employing more chlorine gas than is necessary to peroxidise the iron; as beyond this a portion of the ammonia-salt itself is decomposed, with the evolution of nitrogen. The temperature of the liquor is kept up, after the action of the chlorine, until the whole of the brown flocculent oxide of iron has subsided, when it is at once decanted or filtered into the crystallisers.
Another modification which has been adopted in two or three places is to effect neutralisation of the crude ammoniacal liquor by distilling it, and passing the fumes in at the lower end of a hollow shaft or column filled with coke, down which the acid trickles; the resulting solution of sulphate or chloride of ammonium being received in proper cisterns, conveniently situated near the base of the column.
In Mr Spence’s method of obtaining ammonia-salts from gas-liquor or bone-spirit, a series of (usually four) cylindrical boilers, or reservoirs, so placed that the contents of each upper one may be drawn off into the one next below it are employed. Each boiler has an exit-pipe which carries the vapour generated in it to that next above it, whilst that of the highest boiler passes off to a trunk containing the acid necessary to form the salt. The top boiler is connected with the reservoir of gas-liquor (which is already mixed with milk of lime) by a charging pipe furnished with a stop-cock turned by a floating ball, so as to keep the surface of the liquor constantly at the same height. High-pressure steam enters the lower boiler, by which its ammonia is driven through the connecting pipe into the next boiler, and so on in succession, until it leaves the highest boiler in a concentrated state, and thus enters the acid-tank. When this last contains moderately strong hydrochloric or sulphuric acid, the resulting solution of CHLORIDE or SULPHATE OF AMMONIUM (as the case may be) is sufficiently concentrated to be at once run off into the crystallisers. As soon as the liquor in the lowest boiler is exhausted of its ammonia, its contents are drawn off, and replaced by that of the next boiler, which is followed by a like descent throughout the whole series.
Among improvements having for their object the substitution of cheap chlorides[52] for the more expensive commercial acids, may be mentioned those of—
1. Mr Laming (Patent dated 1843), who employs a strong solution of CHLORIDE OF CALCIUM for converting the ammonia of gas-liquor into the hydrochlorate.
2. Mr Hills (Patent dated 1846) employs CHLORIDE OF MAGNESIUM[53] in the same way; and by a subsequent patent proposes to convert the ammonia eliminated in the distillation of135 coal into the hydrochlorate, by mixing CHLORIDE OF MAGNESIUM with the coal in the retorts, or by introducing the chloride into a retort appropriated for the purpose. The heat dispels the chlorine of the chloride, in the form of hydrochloric acid, and this, uniting with the ammoniacal vapour, forms hydrochlorate of ammonia, which is retained in the liquor of the condenser. From this liquor the salt is obtained by evaporation, &c., in the usual way.
3. Mr Croll (Patent dated 1849) converts the crude ammoniacal vapours that issue with the gas from the common retorts into the hydrochlorate, and obtains a solution of it by passing the gas through a solution of crude CHLORIDE OF MANGANESE[54] (1 cwt. of the salt to about 40 galls. of water), contained in one of the ordinary vessels used for purifying coal-gas. The manganic solution absorbs the ammonia and its salts, converting them into the hydrochlorate, whilst a corresponding proportion of oxide of manganese is precipitated. As soon as the liquor in the purifier is fully saturated, it is drawn off, and replaced by a fresh quantity; whilst the saturated liquor containing the hydrochlorate, after subsidence, or filtration, is evaporated, &c., as before. Crude CHLORIDE OF IRON may be substituted for the chloride of manganese, in the above process: as may also SULPHATE OF MANGANESE, but then the product, of course, will be sulphate of ammonia, instead of the hydrochlorate.
4. Mr Laming (Patent dated 1850) also proposes the use of various salts and mixtures for retaining and condensing the ammoniacal vapour of coal-gas as it passes from the retorts through the purifiers. Of these the principal are CHLORIDE OF CALCIUM obtained by decomposing chloride of iron by hydrate of lime; CHLORIDE OF IRON, obtained by decomposing sulphate of iron with chloride of sodium; CHLORIDE OF MAGNESIUM; a mixture of SULPHATE OF LIME and SULPHATE OF IRON; or of moist precipitated oxide of iron with carbonate of lime, carbonate of magnesia, or magnesian limestone; or one containing sulphate of magnesia, or chloride of magnesium or calcium, or one or more of them, in combination with oxide of copper, either with or without lime or magnesia, or with both or either of them or their carbonates. These salts, or compounds, are mingled with sawdust, or some other porous substance not acted on by the gas, before being put into the purifiers; and after they become saturated with the vapour, the newly-formed hydrochlorate or sulphate (according to the salt or mixture employed) is washed out of the mass with water.
Besides the usual sources of SAL AMMONIAC (and the other ammonia-salts of commerce) it has been proposed to obtain it from guano, peat, shale, &c., as noticed under Sesquicarbonate of Ammonia (suprà); the substance employed to effect the neutralisation or decomposition of the ammoniacal liquor being, in this case either hydrochloric acid or a chloride.
In Young’s Patent (1841) for ‘obtaining AMMONIA and its SALTS,’ a mixture of 2 parts of guano, and 1 part of hydrate of lime, is distilled in a retort placed vertically, at a moderate heat, gradually increased until the bottom of the retort becomes red hot. The ammoniacal portion of the fumes evolved are absorbed by the cold water contained in a suitable condenser; whilst the other gases eliminated by the process pass off uncondensed. By subsequently passing carbonic acid gas into the liquor of the condenser, a solution of CARBONATE, BICARBONATE, or SESQUICARBONATE of AMMONIA is formed. By nearly filling the condenser with diluted hydrochloric or sulphuric acid, instead of with water, a solution of HYDROCHLORATE or of SULPHATE of AMMONIA is obtained.
Stale urine saturated with hydrochloric acid, or with sulphuric acid diluted with about twice its weight of water, yields SAL AMMONIAC, or SULPHATE OF AMMONIA (according to the acid used) on evaporation.
Hydrochlorate of ammonia is now wholly prepared on the large scale, and never by the dealer or retailer, by whom it is only occasionally refined or purified, in small quantities, for chemical and medical purposes. The sal ammoniac of commerce is found to be sufficiently pure for all its ordinary applications in the arts; but when wanted of greater purity, it is broken into pieces, and resublimed from an earthenware vessel into a large receiver of earthenware or glass. The product (REFINED SAL AMMONIAC, DOUBLE-REFINED S. A.; AMMONIÆ HYDROCHLO′′RAS PU′′RA, SAL AMMONI′ACUS DEPURA′TUS†, L.) is popularly known as FLOWERS OF SAL AMMONIAC (flo′res sa′lis ammoni′aci, L.), from being in a finely divided crystalline state.
The chemically pure chloride of ammonium may be prepared by bringing its gaseous constituents—ammonia and hydrochloric acid—into contact. During the combination much heat, and even light, is generated, and the anhydrous solid salt is precipitated in a minutely divided state, which, under the microscope, is seen to be crystalline. It may be also more easily and conveniently prepared by saturating pure and moderately dilute hydrochloric acid with ammonia or its carbonates, and evaporating the solution until a pellicle forms, when crystals of the chloride separate as the liquid cools. A similar but rather more violent reaction occurs when gaseous chlorine136 is brought in contact with gaseous ammonia, or is passed into a nearly saturated solution of ammonia or its carbonates; but in this case nitrogen is evolved at the expense of the ammonia; moreover, the process is attended with danger.
The manufacture of sal ammoniac is usually a distinct business, and is carried on to a very great extent in the neighbourhood of London. Indeed, the London makers now supply the chief portion of that used in England. A large quantity is now, however, made at Manchester and Liverpool. A small quantity is imported from Germany. That from Brunswick is in the form of sugar-loaves. An inferior quality is also imported, in chests, from the East Indies.
The red bands frequently seen in the sal ammoniac of commerce are said to arise from the workmen falling asleep, and allowing the fire to go down, and then suddenly raising the heat too high. (Muspratt.) They consist chiefly of ammonio-chloride of iron.
Ammonium, Citrate of. (NH4)2HC6H6O7. Syn. Diammonium citrate, Citrate of oxide of ammonia; Ammon′′niæ cit′ras, L.
Prep. A concentrated solution of pure citric acid, gently heated, is saturated with sesquicarbonate of ammonium, in fine powder (about 7 parts to 6), and slightly in excess; and the resulting liquid is crystallised by refrigeration in close vessels, or by evaporation in vacuo. If heat be employed in the evaporation of the solution, an acid citrate will be formed.
Uses, &c. Chiefly as a chemical test. An extemporaneous citrate, made with lemon-juice and drunk effervescing, is employed as a saline draught, and a mild aperient and diaphoretic, in fevers, &c.
Ammonium, Ferrocyanide of. (NH4)4 FeC6N6 . 3Aq. Syn. Ferrocyanate d’ammoniaque, Fr. Prep. 1. Saturate a solution of hydroferrocyanic acid with sesquicarbonate of ammonium, in slight excess; evaporate the solution at a heat below ebullition, and crystallise by refrigeration.
2. Digest ferrocyanide of lead or of iron in a solution of sesquicarbonate of ammonium, at a gentle heat, for some time; then filter, evaporate, and crystallise.
Prop., &c. It is isomorphous with ferrocyanide of potassium; it is easily crystallisable, very soluble in water, and is decomposed by ebullition.
Ammonium, Iodide of. NH4I. Syn. Hydriodate of ammonia; Ammo′′nii iodi′dum, L.; Hydriodate d’ammoniaque, Fr. Prep. An aqueous solution of hydriodic acid is neutralised with ammonia, or ammonium sesquicarbonate, in slight excess; and the resulting liquid is either carefully, but rapidly, evaporated to dryness over a water bath, or it is concentrated by the same means, and then caused to deposit crystals by refrigeration; in both cases care is taken to keep a slight excess of ammonia present during the evaporation. The crystals are dried by pressure between folds of bibulous paper; and the product, in either form, preserved in a stoppered bottle.
Pure iodine is triturated with a little distilled water, and solution of ammonium sulphydrate added, in small quantities at a time, with continued trituration, until the red colour of the iodine has entirely disappeared. The solution, after being gently boiled for a few seconds, to expel the sulphuretted hydrogen present, is filtered, slightly alkalised, with ammonia, and evaporated or crystallised, as before.
Prop., &c. Colourless; deliquescent; freely soluble in water, and in spirit; air and light turn it yellowish or brownish, with partial decomposition. It closely resembles iodide of potassium, than which it is more active, and thought to be better suited to irritable and relaxed habits.—Dose, 1 to 10 or 12 gr.
Ammonium, Lac′tate of. Syn. Ammo′′niæ lac′tas, L. An uncrystallisable salt prepared by saturating ammonia, or its carbonate, with lactic acid. It has been found useful in rickets, and in dyspepsia and worms, when occurring in debilitated habits. For this purpose it is best taken fresh-prepared, as a draught, flavoured with syrup of orange-peel, 3 or 4 times daily. See Lactate and Lactic Acid.
Ammonium, Nitrate of. NH4NO3. Syn. Ammo′′niæ ni′tras, L.; Nitrate d’ammoniaque, Fr. Prep. Saturate nitric acid (diluted with 3 or 4 times its weight of water) with sesquicarbonate of ammonium, evaporate by a gentle heat, and crystallise. When not required in a crystalline form, it is usually evaporated to dryness at about 212° Fahr.; and the heat being carefully raised to about 250°, the fused salt is poured out on a polished slab of iron or stone, and when solidified broken up and put into bottles.
Prop. When the evaporation of the solution is conducted at a heat under 100° Fahr., the salt is obtained in beautiful hexagonal prisms; when at 212°, in long silky fibres; when by rapid evaporation and fusion, it forms a white, compact, and usually foliated mass. It dissolves in about twice its weight of water; is slightly deliquescent; melts at 230°, and is decomposed into nitrous gas and water at 460° Fahr. It deflagrates, like nitre, on contact with heated combustible matter.
Uses, &c. Chiefly to prepare nitrous oxide or laughing gas (of which nearly 41⁄2 cubic feet may be procured from every lb. avoir.); and with water, to form freezing mixtures, for which purpose it may be used for any number of times by simply evaporating the solution to dryness, when the salt, obtained unaltered, is ready for another operation. Care, however, should be taken not to expose it to too great a heat, as at a certain temperature it deflagrates with violence. It is occasionally employed in the laboratory to promote the combustion of organic bodies during incineration; and sometimes, though seldom, in medicine, as a diuretic137 and diaphoretic. It is said to reduce the frequency of the pulse, and the animal heat, without affecting the head, chest, or stomach. (Wibmer.)—Dose, 10 to 30 gr.
Ammonium, Nitro-sulphate of. Syn. Ammo′′niæ nitro-sul′phas, L. Dissolve sulphite of ammonium, 1 part; in solution of ammonia, 5 parts; and pass nitric oxide gas through the solution; rapidly wash the crystals that form with solution of ammonia, dry in bibulous paper, without heat, and preserve them in a well-stopped bottle.—Dose, 10 to 20 gr.; in typhoid fevers, &c.
Ammonium, Oxalate of. (NH4)2C2O4. Syn. Ammo′′niæ ox′alis, L.; Oxalate d’ammoniaque, Fr. Neutralise a hot solution of oxalic acid with sesquicarbonate of ammonia; evaporate and crystallise.
Prop. It forms beautiful, colourless, long, rhombic prisms, which effloresce in the air; slightly soluble in cold water; freely soluble in hot water; heated in a retort, it yields ammonia, carbonate of ammonia, cyanogen, and carbonic acid, together with oxamide, which sublimes.
Uses, &c. In chemistry, chiefly as a test for calcium (with which it produces a white precipitate soluble in nitric acid), and to separate lime from magnesium, solutions of the salt of which it does not precipitate. A BINOX′ALATE may also be formed; but it possesses no practical interest.
Ammonium, Phosphate of. (NH4)3PO4. Syn. Ammo′′niæ phos′phas, L. Prep. Saturate a solution of phosphoric acid with sesquicarbonate of ammonium, in slight excess; gently evaporate and crystallise by refrigeration. Diuretic, discutient, and antilithic.—Dose, 3 to 10 gr., or 20 to 30 drops of a saturated solution, 3 or 4 times a day; in gout, rheumatism, and calculus, accompanied with the lithic-acid diathesis; also in rickets and certain forms of dyspepsia.
Ammonium Suc′cinate. Syn. Ammo′′niæ suc′cinas, L. Prep. 1. Succinic acid, 1 part; water, 4 parts; dissolve, neutralise with solution of ammonia, or of ammonium carbonate, in slight excess, and evaporate, and crystallise as directed under the ‘benzoate’ or ‘phosphate,’—Dose, 2 to 10 gr.
Ammonium, Sul′phate of. (NH4)2SO4. Syn. Sulphate of ox′ide of ammonia; Ammo′′niæ sul′phas, L,; Sulfate d’ammoniaque, Fr.; Schwefelsauer ammonium salz, Ger.; Glauber’s Secret salt†, G. SECRET SAL AMMONIAC†, Sal ammoni′acum secre′tum Glaube′′ri†, &c. Crude sulphate of ammonia exists in considerable quantity in the soot from pit-coal; and it is obtained, as a secondary product, from the ammoniacal liquor of gas-works and animal charcoal manufactories. These last are its chief sources. It is also found native, associated with sal ammoniac, in the neighbourhood of volcanoes, under the name of ‘mascagnine’ or ‘massagnine,’
Prep. 1. (Medicinal.) Saturate dilute sulphuric acid with sesquicarbonate of ammonia, in slight excess; filter, gently evaporate, and crystallise.
2. (Commercial.) From gas-liquor or bone-spirit, saturated with weak oil of vitriol, and, the clear portion of the liquid, after repose decanted, concentrated by rapid evaporation, and crystallised, in the manner noticed under Ammonium, Chloride of.
Prop. Crystals, long, flattened, six-sided prisms; soluble in 2 parts of cold, and 1 of boiling water; fuses, with loss of one atom of water, at about 280° Fahr.; and is volatilised, with entire decomposition, at about 535°. Even its solution, by long boiling, becomes acid from loss of ammonia. The anhydrous salt does not exist.
Uses, &c. Pure sulphate of ammonia is diuretic, aperient, resolvent, and stimulant.—Dose, 10 to 30 gr. It is now seldom employed in medicine. The crude sulphate is principally used in the preparation of sal ammoniac and sesquicarbonate of ammonia, and for manure. “A mixture of 10% of this sulphate with 20% of bone-dust, some gypsum, and farm-yard manure, forms a very fertilising compost, applicable to a great variety of soils” (Ure); and we may add—greatly superior to a very large portion of what is now so commonly vended under the name of ‘guano.’
Concluding remarks, Patents, &c. The manufacture of sulphate of ammonia, on the large scale, has been unavoidably explained in treating on the salts of that base already noticed. All that is necessary is to saturate with sulphuric acid the solution of ammonia, crude or otherwise, and obtained in any manner; and then to evaporate the solution until the salt crystallises out. At other times, however, instead of adding the acid to the ammoniacal liquor, the latter, either at once, or after treatment with lime, is submitted to distillation, and the evolved alkaline vapour is passed into the acid (previously somewhat diluted), contained in a large receiver or cistern, or a series of them; the salt being obtained from the resulting solution in the usual manner. By re-solution and a second crystallisation the sulphate is generally obtained sufficiently pure for all commercial purposes; but when the salt is intended for use as manure, or (unless very rough) for conversion into sal ammoniac, this need not be had recourse to.
Among modifications and improvements, not previously noticed, may be mentioned—
1. That of Dr Richardson (Patent dated Jan., 1850), who mixes SULPHATE OF MAGNESIA with the crude ammoniacal liquor, and thus forms a double sulphate of magnesia and ammonia, from which he obtains the SULPHATE OF AMMONIA by sublimation.
2. That of Michiel (Patent dated April, 1850), who prepares sulphate of ammonia by means of OXYSULPHATE OF LEAD obtained by roasting galena (sulphide of lead), by exposing it in a crushed state and thin layers for 2 or 3 hours,138 to the heat of a reverberatory furnace. The resulting mixture of sulphate and oxide of lead is reduced to the state of coarse powder, and well worked up with the ammoniacal liquor, when SULPHATE OF AMMONIA and sulphide and carbonate of lead are produced by the mutual reaction of the elements present. The first is removed by treatment with water; and the residuum serves for the manufacture of lead compounds, or may be reduced to the metallic state by fusion in the usual manner.
3. That of Mr Laming (Patent dated Aug., 1852), in which a stream of SULPHUROUS ACID GAS is transmitted through the liquor containing the ammonia, either in the free state or as carbonate, by which SULPHITE OF AMMONIA is formed. This salt he oxidises, and thus converts into the SULPHATE OF AMMONIA, by agitation and free exposure to the air.
Sulphate of ammonia, like the hydrochlorate, may also be obtained by saturating stale urine with the acid, and subsequent evaporation and crystallisation. See Ammonia; Ammonia, Carbonates of; Ammonium, Chloride of, and Manures, &c.
Ammonium, Sulphide of (neutral). (NH4)2S. Prep. Saturate strong solution of ammonia with pure sulphuretted hydrogen gas; then add a second portion of solution of ammonia, equal to that first used, and preserve it in a well-stoppered bottle.
Ammonium, Sulphydrate of. NH4HS. Syn. Sulphide of ammonium, Hydrosulphide of ammonium, Hydrosulphate of ammonia. Prep. By passing sulphuretted hydrogen gas, to saturation, through a mixture composed of strong solution of ammonia, 1 part, and distilled water, 4 parts.
Props. Prepared as above, it has a very fœtid odour. When pure it is wholly volatilised by heat, and does not disturb a solution of sulphate of magnesium. Mineral acids decompose it, with the evolution of sulphuretted hydrogen. By keeping, it decomposes and acquires a yellow colour. This yellow coloration does not, however, render it unfit for use as a reagent; but it must be borne in mind that it will now deposit sulphur when mixed with acids. In this state it proves valuable as a reagent to detect hydrocyanic acid, and as a solvent to separate metallic sulphides thrown down by sulphuretted hydrogen.
Uses, &c. It is principally employed by chemists as a reagent to precipitate metals, to separate metallic sulphides, &c.; and by the perfumers as a mordant in dyeing hair. In medicine it has been used by Cruickshank, Rollo, and others, to check the morbid appetite, and to increase the action of the stomach and general tone of the system in diabetes mellitus. It has also been used by Brauw, Gruithuisen, and others, in old pulmonary and vesical catarrhs. It is a powerful sedative, lessening the action of the circulatory system, causing nausea, vomiting, vertigo, drowsiness, &c.—Dose, 3 to 6 drops, three or four times daily, mixed with pure water, and instantly swallowed. In large doses it is poisonous.
Ant. Very dilute solution of chlorine, or of chloride of lime or soda, followed by a powerful emetic, or the stomach-pump. When the vapour has been respired, free exposure to fresh air, with the head a little elevated, and copious affusions of cold water, with moderate draughts of brandy-and-water, and the use of the smelling-bottle (ammoniacal) should be adopted. If need be, artificial respiration should be attempted, and the air around the patient should be slightly impregnated with the fumes of chlorine or chloride of lime.
Ammonium, Persulphide of. Syn. Boyle’s Fuming-liquor, Hoffman’s Vol′atile spirit of sulphur, &c.; Ammo′′niæ perhydrosul′phas, A. perhydrosulphure′tum, &c. Authorities differ as to the constitution of this liquid, which, since its introduction by Beguin in 1650, has passed under more ‘aliases’ than perhaps any other preparation. Its precise position amongst the ammonia-compounds is still undecided.
Prep. 1. (Beguin.) Sulphur, 1 lb; quick-lime, 1⁄2 lb; sal ammoniac, 4 oz.; mix and distil.
2. (Boyle.) Sulphur and sal ammoniac, of each, 5 oz.; quick-lime, 6 oz.; as last.
3. (Liebig.) Agitate the common hydrosulphate of ammonia with pure sulphur, until the latter ceases to be dissolved; and, after repose, decant the clear liquid.
Prop., &c. An orange-yellow, fuming, fœtid liquid, of an oily consistence, having the characteristics of the common sulphydrate in a remarkable degree. It may prove an excellent medicine. “Useful for wounds and ulcers.” (Beguin.) Diluted with three parts of spirit of wine, it formed the LIQUOR ANTIPODAG′RICUS of F. Hoffman; of which we are told that about 30 drops acted as a strong sudorific; and applied externally, mixed with camphor, “it relieved pain like a charm.” (Hoffman.) The sulphides of ammonium are now scarcely ever employed as remedies.
Ammonium, Sul′phite of. (NH4)2SO3.7Aq. Syn. Ammoniæ sulphis, L. Prepared by passing sulphurous acid gas into a solution of ammonia. It is crystallisable and very soluble in water.
Ammonium, Sulphocyanide of. NH4CNS. Prep. 1. Neutralise hydrosulphocyanic acid with ammonia, and gently evaporate the solution to dryness, by the heat of a water bath.
2. Digest hydrocyanic acid with yellow sulphydrate of ammonium, and, after a time, evaporate as before.
A deliquescent, white, saline mass, very soluble in water, but seldom employed out of the laboratory in a pure state. Of late it has been obtained in quantity as a crude product of the gas-liquors.
Ammonium, Tartrates of. Of these there are two:—
Ammonium, Neutral Tartrate of. (NH4)2C4H4O6. Syn. Ammo′′niæ tar′tras, L. Prep.139 Saturate a solution of crystallised tartaric acid, 150 grs.; with sesquicarbonate of ammonium, 118 grs.; and either evaporate the solution at a gentle heat, and crystallise; or evaporate to dryness, and powder the residuum.
Prop., &c. Prismatic crystals, or a crystalline mass; soluble and efflorescent. Its medicinal properties and doses resemble those of citrate of ammonium.
Ammonium, Bitartrate of. NH4HC4H4O6. Syn. Ammo′′niæ bitar′tras, L. Prep. To a strong solution of tartaric acid add another of sesquicarbonate of ammonium, or of tartrate of ammonium, as long as a precipitate falls; which must be collected and dried.
Prop., &c. A crystalline powder, only slightly soluble in water, closely resembling ordinary cream of tartar. It is diaphoretic, diuretic, and deobstruent, and is frequently, though improperly, sold for the preceding preparation.
Ammonium, Valerianate of. NH4C5H9O2. Syn. Ammo′′niæ valeria′nas, L. Prep. Saturate valerianic acid with strong solution of ammonia, and evaporate the resulting liquid to a syrupy consistence at a heat under 175° Fahr.; then add twice its volume of alcohol, and, after agitation, allow it to crystallise by spontaneous evaporation.—Dose, 2 to 8 or 10 gr.; in neuralgia, epilepsy, hypochondriasis, hysteria, low fevers of an intermittent kind, &c.; also in dyspepsia and debility complicated with these affections.
AMMONI′ACAL. [Eng., Fr.] Syn. Ammoniaca′lis, L. Pertaining to, or possessing the odour or properties of, ammonia. See Ammonia, &c.
AMMONI′ACUM. Syn. Gum ammoniacum, G. ammo′′niac†; Gomme ammoniaque, Fr.; Ammoniak, Ger. A gummy-resinous exudation from the stem of dorema ammoniacum, in tears and masses, of a pale cinnamon colour, brittle, and when broken has a white and shining surface. Collected in Persia and the Punjaub. (B. P.)
Gum ammoniacum has an unpleasant odour, especially when heated, and a nauseous and slightly bitter taste. It is a mild, stimulating expectorant and emmenagogue; and its effects on the system resemble those of assafœtida except in being weaker. Externally, it is resolvent.—Dose, 10 to 30 gr. in pills or emulsion.
Doses for Animals. Horse, 2 to 4 drachms. Cattle, 2 to 4 drachms. Sheep, 1⁄2 to 11⁄2 drachm. Pig, 1⁄2 to 11⁄2 drachm. Dog, 10 to 20 grains. Either by bolus or emulsion.
Ammoniacum, Strained′. Syn. Prepared ammoniacum; Ammoni′acum præpara′tum (Ph. L.), L. Prep. (Ph. L. 1851.) Boil ammoniacum in water just sufficient to cover it; strain the mixture through a hair sieve, and constantly stirring, evaporate in a water bath, until, on cooling, it becomes hard. The product, owing to a loss of volatile oil, is much weaker than the unprepared gum-resin. The process is only necessary with rough lump ammoniacum.
Ammo′′niated. Syn. Ammonia′tus, L. In pharmacy, perfumery, &c., applied to preparations containing ammonia.
AMMO′NIO-, Ammon′ico-. In chemistry, a common prefix to double salts containing ammonia; as ammonio-citrate, a.-chloride, or a.-tartrate of iron, &c. See the respective metals.
AMONTILLADO. [Sp.] See Sherry and Wine.
AMORPH′OUS (-morf′-us). Syn. Amorph′us, L.; Amorphe, Informe, Difforme, Fr.; Amorphisch, Misgebildet, Missgestaltet, Ger. Shapeless. In chemistry and mineralogy, applied to substances devoid of regular or crystalline form; as a lump of chalk, the majority of precipitates, &c. The corresponding substantives are AMORPH′ISM, AMORPH′OUSNESS* (amorphis′mus, L.; amorphisme, Fr.).
AMPHIB′IA (fĭb′-y′ă). [L. pl.; prim. Gr.] Syn. Amphib′ians (-yănz), Amphib′ials (-y′ălz). Animals that possess the faculty of living both in water and on land. In modern zoology it is restricted to those animals which possess both gills and lungs; as the batrach′ia or frog tribe. The term is also often applied, colloquially, to otters, seals, walruses, crocodiles, &c., none of which can breathe under water, although, from the languid nature of their circulation, they are able to remain a long time in it.
AMPHIB′IOUS (y′ŭs). Syn. Amphib′ius, L.; Amphibie, Fr.; Beydlebig, Ger. In botany and zoology, having the faculty of growing or living both on land and in water. See Amphibia.
AM′PHITYPE (-fe-). See Photography.
AMYGDALIN. C20H27NO11.3Aq. This substance exists in bitter almonds. It crystallises in pearly white plates, which are odourless and almost tasteless. It is nearly insoluble in hot and cold water and in cold alcohol, but soluble in boiling alcohol. To prepare amygdalin, boil well-pressed cake of bitter almonds twice in strong alcohol; strain through linen, and press the residue; remove any oil that may appear, heat the liquid again, and filter. In a few days part of the amygdalin crystallises out. Concentrate the residuary liquor to a sixth part, and add ether, which will throw down the amygdalin. Press it between blotting paper, wash it with ether, and set aside to crystallise.
AMYG′DALOID (-loyd). Syn. Amygdaloid′al; Amygdaloï′des (-dēz), L.; Amygdaloïde, Fr. Almond-shaped. In mineralogy, amygdaloid is ‘toadstone.’
AMYKOS (Galen, Upsala). A cosmetic and mouth-wash. Claims to be prepared according to an English patent. It is an aqueous extract of 420 grms. cloves, boiled in a gallon of water, in which 420 grms. of pure glycerine are dissolved, and to which 210 grms. of borax are added. (Hager.)140
AMYKOSASEPTIN is linen saturated with a hot solution of borax. (Nyström.)
AMYLA′CEOUS (ăm-e-lā′-sh′ŭs). Syn. Amyla′ceus, L.; Amylacé, Fr. Of or like starch; consisting of or abounding in starch; starchy. See Food, Nutrition, Starch, &c.
AM′YL (-ĭl). C5H11. The radical of the fusel-oil compounds (AMYL-SERIES).
Amyl, Acetate of. C5H11C2H3O2. Syn. Pear-oil. Prep. From fusel-oil, 1 part; acetate of potassa (dry), 2 parts; concentrated sulphuric acid, 1 part; distilled, with the usual precautions, from a glass retort into a cool receiver. The distillate is purified by washing it with very dilute solution of potassa, and redistilling it from fused chloride of calcium. A little litharge added to the liquid in the retort, before rectification, will remove any sulphurous odour, should it be present.
Prop., &c. Liquid, limpid, colourless; insoluble in water; soluble in alcohol; boils at 272° Fahr.; alcoholic solution of potassa converts it into an acetate of that base, with reproduction of fusel-oil.
Obs. The odour and flavour of this preparation are those of the Jargonelle pear. It is now extensively manufactured, and, after dilution with alcohol, is sold under the name of ESSENCE OF JARGONELLE PEAR, for flavouring liqueurs and confectionery.
Amyl, Vale′rianate of. C5H11C5H9O2. Syn. Apple-oil, A.-essence, &c. This compound is abundantly formed during the preparation of valerianic acid from potato oil, and is recognised by the offensive odour of rotten apples evolved during the process. By treating the crude product of the distillation with a weak solution of pure potassa, the valerianic acid is removed, and the volatile oil obtained nearly pure. Dissolved in rectified spirit it forms the ‘APPLE-ESSENCE’ now so much employed as a flavouring ingredient for confectionery and liqueurs. See Fruit Essences, Valerianic Acid, &c.
AMYL NITRITE. Syn. Amyl nitris, B. P. Produced by the action of nitric or nitrous acid on amylic alcohol.—Dose. By inhalation, the vapour of 2 to 5 minims. To be used with caution. It may be produced by passing a stream of nitrous acid gas through purified amylic alcohol at a temperature of 132° C.
For other methods of preparing it consult ‘Wood and Bache’s United States Dispensatory, 1877.’ Mr Umney (‘Pharm. Journal’) says that true nitrite of amyl should be made by passing nitrous acid into amylic alcohol which has been previously submitted to a fractional distillation, until the portion retained for use has a boiling point of 132° C. A nitrate so prepared, when deprived of any excess of acid it may contain by rectification over fused carbonate of potash, will have a boiling point of 98°-99° C.
AM′YLENE (-e-lēne). C5H10. [Eng., Fr.] Syn. Am′ilene*; Amyle′na, Amyle′num, L. A peculiar volatile, liquid hydrocarbon, discovered by Cahours.
Prep. From fusel-oil repeatedly distilled along with either anhydrous phosphoric acid, or a concentrated solution of chloride of zinc; the product being repeatedly rectified at a low temperature, until the boiling point sinks to 102° Fahr.
Prop., Uses, &c. An ethereal liquid, lighter than water, having an aromatic odour, slightly alliaceous. Sp. gr. of vapour, 2·68. Its vapour was several times successfully employed, by the late Dr Snow, as a substitute for ether and chloroform in producing anæsthesia, being, though less agreeable, also less pungent, and consequently easier to breathe, than either of them; but its use has since been given up owing to doubts as to its safety, two or three deaths having followed its inhalation.
ANADOLI (Kreller, Nuremburg). An oriental tooth-powder. Powdered soap, 42 parts; starch powder, 44 parts; levantine soapwort, 12 parts; oil of bergamot and lemon to flavour. (Wittstein.)
ANÆMIA. Deficiency of blood.
ANÆSTHE′SIA (ăn-ēz-the′-zh′ă; -sh′ă; -thēze′y′ăr). [L.; prim. Gr.] Syn. Anesthésie, Fr. In pathology, diminished or lost sense of feeling.
In surgery and obstetrics, the production of temporary anæsthesia, for the purpose of rendering operations painless, relieving the pangs of childbirth, &c., is effected by the use of—
ANÆSTHET′ICS. Syn. Anæsthet′ica, L.; Anesthétiques, Fr. In pharmacology and surgery, substances or agents which diminish or destroy sensibility, or which relieve pain. In its full extent this term includes both anodynes and narcotics; but it is now more generally confined to those substances which greatly diminish common sensibility, or entirely remove susceptibility to pain. Among the most useful, safe, and powerful of this class are chloroform, ether, nitrous oxide, and intense cold; besides several chlorinated compounds, such as the bichlorides of ethylen, methylen, and carbon.
More than 1500 years ago the Chinese are said to have used a preparation of hemp, or ma-yo, to annul the pain attendant upon cauterisation and other surgical operations. Mandragora (mandrake) was employed for a similar purpose by the Greeks and Romans; and we learn that as early as the thirteenth century the vapour from a sponge filled with tinctures of mandragora, opium, and other sedatives was used for a similar purpose.
Baptista Porta, in his work on natural magic printed in 1597, mentions a quintessence extracted from medicines by somniferous menstrua, of the nature of which he leaves us in ignorance. This quintessence was to be preserved in leaden vessels very perfectly closed, lest the aura should escape, for the medicine would vanish away. Furthermore, he adds, “when it is used, the cover being141 removed, it is applied to the nostrils of the sleeper, who draws in the most subtle power of the vapour by smelling, and so blocks up the fortress of the senses, that he is plunged into the most profound sleep, and cannot be roused without the greatest effort.” Dr Iron suggested that the volatile substance was sulphuric ether, which he says had been described more than fifty years before Porta wrote his book. In the year 1800 Sir Humphry Davy suggested the employment of nitrous oxide, or laughing gas, as it was then termed, for minor operations in surgery, and in 1828 Dr Hickman proposed carbonic acid as an anæsthetic. The vapour of sulphuric ether had been used in his practice by Dr Pearson as early as 1795, for the relief of spasmodic asthma. The fact that sulphuric ether was capable of producing insensibility was demonstrated by American physicians; viz. by Godwin in 1822, Mitchell in 1832, Jackson in 1833, and Wood and Bache in 1834; but the first practitioner to employ it to prevent the pain of an operation was Dr Morton, a Boston dentist, who successfully used it for this purpose in 1846. On the 19th of December of the same year Mr Liston, of University Hospital, London, and Mr Robinson, a dentist, operated upon patients who had been rendered insensible by means of the inhalation of the vapour of ether.
Throughout the year 1847 ether was employed as an anæsthetic both in England and France, but towards the end of that year the anæsthetic properties of chloroform were pointed out by Flourens. The first, however, to introduce this agent into surgical and obstetric practice was Dr I. T. Simpson, of Edinburgh. In 1849 a work on the inhalation of ether was published by Dr Snow, who afterwards introduced a new anæsthetic, viz. amylene, which was capable of producing effects similar to those of chloroform; but as two patients out of but a small number who inhaled the vapour of amylene died, this latter soon fell into discredit, and consequent disuse.
Except in dental practice, in which nitrous oxide gas is the anæsthetic invariably employed, chloroform is almost universally used in surgical operations, one advantage it possesses over ether being its much more rapid action, although this latter property must be regarded as one which constitutes the risk which, although very slight (when the exceedingly small per-centage of deaths resulting from its administration is taken into account), undoubtedly attends its inhalation.
Dr Sansom says of chloroform:—“The cause of its danger is its power of paralysing the cardiac and other motor sources of circulation. This property resides in large and sudden doses of its vapour.” He strongly recommends its dilution by air and alcohols. He further remarks that all anæsthetics modify the endosmotic condition of the blood discs, and contends that they affect the supply of arterial blood by altering the calibre of the channels which convey it. He advocates the substitution of one anæsthetic for another during the inhalation.
Methylene dichloride, introduced by Dr B. W. Richardson, is said to possess the disadvantage of causing considerable depression.
The mode of administering these agents is by causing the patient to inhale their vapour mixed with air.
Sometimes they are poured on to a sponge or a handkerchief, or piece of lint, either of which is then applied to the mouth and nostrils of the patient in such a manner that the air which passes into his lungs is saturated with the vapour. Except in extemporised cases, however, this method is pretty well abandoned, a proper apparatus having supplanted the sponge or handkerchief, &c. Part of the apparatus consists of a graduated bottle containing the anæsthetic, by means of which the operator is enabled to tell how much of this latter is being consumed, and thus to regulate the quantity inhaled.
The first effect that results from the administration of anæsthetics is a form of intoxication, caused by the action of the anæsthetic agent on the cerebral lobes, and as this action extends to the cerebellum, the patient becomes incapable of directing his movements—an effect like that caused by intoxication from alcohol.
In the next stage the spinal cord is attacked, unconsciousness supervenes, and all powers of motion and sensation are lost. The individual is now said to be in a state of anæsthesia; but the heart continues to beat, respiration is not impeded, and the other essential functions of the body go on as usual.
Should, however, the exhibition of the anæsthetic agent be incautiously continued too long, the bodily temperature falls, the movements of respiration and circulation become impaired, the heart ceases its action, and death finally ensues. The introduction of anæsthetics into surgical practice has been of great and invaluable service to the operator. The patient being motionless and free from pain, the surgeon is enabled to perform the operation at his ease, and consequently more efficiently; moreover, in the reduction of dislocations and of hernia, the muscles being flaccid, the obstacle produced by their contraction is removed. M. Velpeau endeavoured to produce local anæsthesia, or insensibility of the part of the body to be operated upon, by means of a freezing mixture composed of ice and salt; this method, however, was found impracticable, and was soon abandoned. Since then local anæsthesia as introduced by Dr Richardson, when had recourse to, is effected by means of a spray of ether directed on the part, the intense cold produced by the rapid evaporation of the ether entirely142 depriving the part of sensation. It is said that the pain resulting from the application of this method is a great barrier to its use.
Amongst anæsthetics, nitrous oxide gas occupies an important place, its use, as before stated, being almost wholly confined to operations in dental surgery.[55] As in the case of ether, the American practitioners were the first to employ nitrous oxide as an anæsthetic. Attention was directed to its anæsthetic properties in 1844 by Mr Horace Wells, an American dentist, but little interest seems to have been awakened by his application of it, since it was not until 1863 that Dr Cotton, of New York, drew attention to the subject by performing an operation on a patient under its influence.
In March, 1868, Dr Evans, residing in Paris, after a visit from Dr Cotton, directed the attention of medical men in England to the value of nitrous oxide as an anæsthetic in dental surgery, and shortly afterwards it was first employed to produce anæsthesia at the Dental Hospital. Nitrous oxide is obtained from nitrate of ammonia, and the particulars of its preparation may be found by referring to the article Nitrous oxide.
Immense quantities of the gas are used in dental operations. It has been computed that in 1870 Messrs Coxeter and Barth could not have prepared much less than 60,000 gallons in London alone. To fit it for transit it is reduced by compression. Fifteen gallons may thus be diminished in volume until it fills an iron bottle holding a quart. Five or six gallons of the gas are, on an average, required for each patient. In the preparation of nitrous oxide for surgical purposes Dr Evans advises it to be made at least 24 hours before it is used, and further recommends its being thoroughly washed. An apparatus for the preparation of the gas was devised by Mr Porter, a description of which will be found in the ‘Transactions of the Odontological Society of Great Britain’ for 1868, in which also mention is made of a face-piece for its administration, the invention of Mr Clover. By means of this latter instrument the desiderata that the nitrous oxide should be inhaled without admixture with atmospheric air, and contamination arising from the expired air given off by the patient, are accomplished, for it has been found that when excitement and talking attend the inhalation of the gas, these effects are due to the presence of the carbonic acid thrown off by the lungs.
When inhaled in the ordinary way, nitrous oxide gas induces exhilaration and narcotism, without asphyxia. When, however, the atmospheric air is carefully excluded, it produces, as we have just seen, anæsthesia without exhilaration. The time required to produce anæsthesia varies from 25 to 120 seconds, by from 10 to 60 inhalations. A patient has been subjected for 10 minutes to its action without experiencing any unpleasant symptoms or after effects. Mr Randle says it is perfectly safe in all short operations, and possibly in long ones also, provided there is due admission of air at proper intervals. It seems tolerably certain that nitrous oxide is largely absorbed by the blood-corpuscles, and it is probable that its presence in them may temporarily act to the exclusion of oxygen, and thus prevent for a time that combination of oxygen with hæmoglobin upon which the red colour of the corpuscles depends. Chemistry, however, has failed to show that nitrous oxide is decomposed in the blood, or that it exerts any of the chemical properties of oxygen on the constituent elements of the blood. Whenever the slightest anæsthetic effect is communicated to the nervous system, a simultaneous effect is produced upon the medulla oblongata, the spinal chord, as well as upon the cerebrum and cerebellum.
The whole available force in the body is undoubtedly due to oxidation. This oxidation is accomplished by means of the blood, and it is therefore evident that a continuous flow of oxygenated blood to the nerve centres is necessary as a source of power and of sensibility, as well as for the reintegration of nerve tissue. Any deficiency of oxygen in the blood is followed by a decreased arterialisation of the whole volume of the blood. Under these conditions the exhalation of carbonic acid is relatively less rapid than its formation, and life cannot continue if the blood in the arteries becomes thoroughly venous, as well in colour as in character. That nitrous oxide, when inhaled, changes the colour of the blood-corpuscles is evidenced by the livid appearance of the face and mucous surfaces; the latter, indeed, is a characteristic accompaniment of its administration, and the darkened colour of the blood may be observed as it flows from the severed vessels. This colour of the blood is probably in part due to uneliminated carbonic acid; but that nitrous oxide possesses in a high degree the property of darkening the blood-corpuscles may be easily demonstrated by directing a jet of the gas for a few seconds upon a little arterial blood in a test tube. Yet, from what has previously been advanced on this point, this latter result may more strictly be due to physical than to chemical causes. An interruption of the circulation in any part of the organism is soon followed by local insensibility in the tissues from which the blood supply may have been withdrawn; and it is beyond dispute that, during the anæsthetic state, the circulation of the blood through the capillary system becomes diminished in velocity. A tendency to stasis begins to appear, accompanied at the same time by a considerable reduction in the supply of arterial blood. These are facts that admit of experimental demonstration, as does also another fact, viz. that during the period of insensibility produced by the inhalation of nitrous oxide the143 brain itself is in a state of comparative anæmia. In short, it appears most probable that an arrest of the capillary circulation through the brain, to which several writers have attributed a potential influence as the cause of anæsthesia, is simply, so far as it may exist, a result of it.
The anæsthesia produced by the inhalation of nitrous oxide would, therefore, appear to be referable to an altered condition of the blood, whereby the molecular dynamic changes are interfered with, this interruption being probably due either to the retention of carbonic acid, or to the presence of nitrous oxide; or, as the result of both conditions, to the exclusion of oxygen.
For minor operations nitrous oxide possesses many advantages over other anæsthetics. The principal of these is its safety. In America, in 200,000 cases in which it had been administered, there was only one case of death. Furthermore its use is not contra-indicated in patients having any constitutional derangement, nor for women who are either pregnant or suckling.
Nitrogen, coal-gas, and carbonic acid have also been employed as anæsthetics.
The ‘British Medical Journal’ for June 13th, 1868, contains an account of some experiments performed by Dr Burdon Sanderson, at Middlesex Hospital, with nitrogen. It seems to have been longer in producing insensibility than nitrous oxide, but no lividity of countenance accompanied, nor sickness or headache followed, its administration.
ANALEP′TIC. Syn. Analep′ticus, L.; Analeptique, Fr. Restorative; that recruits the strength lost by sickness.
Analep′tics. Syn. Analep′tica, L.; Analeptiques, Fr. In pharmacology, &c., restorative medicines and agents.
ANAL′YSIS (-e-sĭs). [Eng. L., Gr.] Syn. Analyse, Fr.; Auslösung, Zerlegung, Ger. In a gen. sense, the resolution of anything, whether an object of the senses or of the intellect, into its elementary parts. In chemistry, the resolution or separation of a compound body into its constituent parts or elements, for the purpose of either determining their nature, or, when this is known, their relative proportions. It is divided into QUAL′ITATIVE ANALYSIS and QUAN′TITATIVE ANALYSIS; and these again into PROX′IMATE ANALYSIS and UL′TIMATE ANALYSIS. The first consists in finding the components of a compound, merely as respects their nature or names; the second, in finding not merely the component parts, but also the proportions of each of them; the third gives the results in the names of the proximate or immediate principles or compounds which, by their union, form the body under examination; whilst the fourth develops the chemical elements of which it is composed.[56] An analysis may also be made to determine whether a certain body is or is not contained in a compound (as lead in wine); or it may be undertaken to ascertain all the constituents present; the extent of an investigation being merely limited by the object in view.
For success in chemical analysis a thorough acquaintance with the various properties of bodies is required, as well as aptitude in applying this knowledge in discriminating them, and separating them from each other. Judgment and expertness in manipulation are, indeed, essential qualifications. The method pursued must likewise be such as to attain the object in view with unerring certainty, and in the most expeditious manner. “The mere knowledge of the reagents, and of the reactions of other bodies with them, will not suffice for the attainment of this end. This requires the additional knowledge of a systematic and progressive course of analysis, or, in other words, the knowledge of the order, and succession, in which solvents, together with general and special reagents, ought to be applied, both to effect the speedy and safe detection of every individual component of a compound or mixture, and to prove with certainty the absence of all other substances. If we do not possess this systematic knowledge, or if in the hope of attaining an object more rapidly, we adhere to no method in our investigations and experiments, analysing becomes (at least in the hands of a novice) mere guesswork, and the results obtained are no longer the fruits of scientific calculation, but mere matters of accident, which sometimes may prove lucky hits, and at others total failures.” (Fresenius.)
ANALYSIS, SPECTRUM. More than half a century ago Sir John Herschel employed the prism in the analysis of coloured flames, and in 1834 Fox Talbot, by means of the same instrument, distinguished the difference between the spectra given by strontium and lithium, notwithstanding the similarity of the two in colour. But it was reserved for Messrs Kirchkoff and Bunsen, as the inventors of the spectroscope, to devise the only efficient method of analysing flame, and, at the same time, to furnish chemists with a means whereby they may detect with unerring certainty the presence of any known element by observing the spectrum it gives when such element is submitted to a temperature sufficiently high for it to emit a luminous vapour. That certain chemical substances when heated in the flame of the spirit-lamp or the blow-pipe, or any other source of comparatively white light, imparted characteristic colours to the flame, was a fact that had long been known to chemists; for example, when a salt of sodium was so treated, an intense yellow colour was imparted to the flame. A salt of potassium produced under144 the same circumstances a violet, strontium, a crimson colour, &c. These results could only be produced when the substance under examination contained but one of the salts in question. If more than one were present, this method of qualitative analysis was comparatively, if not wholly, valueless, because the specific colour communicated to the flame by the presence of one element would be masked, and, consequently, destroyed by the colour developed by the vapour of another or other elements. For instance, so much more vivid is the yellow colour given to flame by sodium salts than the violet tint imparted by those of potassium, that a very small trace of sodium prevents the unaided eye from perceiving the violet, even when the potassium compound is present in large quantity.
Very different optical effects, however, follow if the rays from the various-coloured flames are made to pass through a prism. As is well known, if a ray of ordinary white light is made to traverse a prism, when it issues from the prism it has become decomposed or dissected into seven luminous rays of as many different colours, the coloured image thus produced being called a prismatic spectrum, or simply a spectrum.
This phenomenon is owing to the prism refracting or bending out of its course the beam of light sent through it, and to each coloured ray of which the beam is made up being differently refracted.
“If, however, instead of the white flame coloured flames are examined by means of a prism, the light being allowed to fall through a narrow slit upon the prism, it is at once seen that the light thus refracted differs essentially from white light, inasmuch as it consists of only a particular set of rays, each flame giving a spectrum containing a few bright bands. Thus, the spectrum of the yellow soda flame contains only one fine bright yellow line, whilst the purple potash flame exhibits a spectrum in which there are two bright lines, one lying at the extreme red, and the other at the extreme violet end. These peculiar lines are always produced by the same chemical element, and by no other known substance; and the position of these lines always remains unaltered. When the spectrum of a flame tinted by a mixture of sodium and potassium salts is examined, the yellow ray of sodium is found to be confined to its own position, whilst the potassium red and purple lines are as plainly seen as they would have been had no sodium been present.”[57]
Equally characteristic and well-defined spectra, the bands in which have each an invariable and fixed position in the spectrum, are also produced when the coloured flames arising from heating to the requisite point the remaining salts of the alkalies and alkaline earths are examined by the prism. On the opposite page the first spectrum shows some of the fixed dark lines that are always observed when a solar beam is examined by the spectroscope. These lines are compared with the position of some of the more important bright lines furnished by the spectra of the metals of the alkalies and alkaline earths, when their chlorides are heated upon a loop of platinum wire introduced into the flame of a Bunsen gas-burner. The characteristic bright lines given by each metal are denoted by the letters of the Greek alphabet, the earliest letter indicating the most strongly marked lines.
In the potassium spectrum the most characteristic bright lines are the red line K α, and violet line K β. In the case of sodium nearly the whole of the light is concentrated on the intense yellow double line Na α. In the lithium spectrum a crimson band, Li α, is the prominent line; Li β is seldom visible, but at the elevated temperature of the voltaic arc an additional blue line becomes very intense. In the spectrum of cæsium two lines in the blue, Cs α and Cs β, are strongly marked. In rubidium the lines Rb α and Rb β in the blue, and Rb γ in the red are almost equally specific. Thallium is recognised by the intense green line Il α. The spectra of the metals of the alkaline earths are equally definite, though more complicated.
By means of the spectroscope quantities so inconceivably minute as the 33,000th of a grain of chloride of rubidium, the 170,000th of a grain of chloride of cæsium, the 2,500,000th of a grain of sodium, and the 6,000,000th of a grain of lithium, have been detected, and have revealed themselves to the sight by their characteristic bands in the spectrum. Hence it is that in making use of this branch of analysis the chemist has been enabled to show the universality of many elements hitherto regarded as being very sparingly distributed throughout the globe.
Thus lithium, which until lately was supposed to be one of the rare elements, has been found as a constituent of tea, tobacco, milk, blood, and in almost all spring waters. Furthermore, the prodigiously sensitive reactions afforded by the spectroscope have not only revealed the presence of infinitesimal quantities of known elements, but have led to the discovery of new ones which had escaped detection by the older and less delicate processes of analysis. It was by means of spectrum analysis that the two alkali metals, cæsium and rubidium, were discovered by Bunsen and Kirchkoff in 1860 in a mineral water at Durkheim, and that Mr Crookes in 1861 discovered the metal thallium in the deposit found in the flue of a pyrites furnace; whilst still more recently Messrs Reich and Richter, in a spectrum examination of a zinc ore from Freiberg, discovered the metal indium.
The most brilliant spectra are given by those salts which are the most easily volatilised, such as the chlorides, iodides, and bromides146 of the different metals. But it is only the metals of the alkalies and alkaline earths that give spectra that are characteristic. When it is desired to obtain the spectra of the other metals, they may be raised to the requisite temperature by means of the electric spark, which in passing through the two points of the metal operated upon volatilises a minute quantity of it, and thus enables it to emit its particular light. The electric sparks are best obtained by means of Ruhmkorff’s coil. Thus each metal may be made to yield a spectrum which specially belongs to it, and to it alone. When the electric discharge is sent through a compound gas or vapour, owing to the intense temperature generated separation of its constituents must take place, since the spectra produced are those of the elementary components of the gas. The permanent gases give each their peculiar spectrum when they are strongly heated, by which they may be recognised; thus the spectrum of hydrogen is composed of three bands, one being bright red, one green, and the other blue. Nitrogen gives a very complicated spectrum.
The accompanying figure exhibits a very complete form of the spectroscope adapted to a single prism.
P represents a flint-glass prism supported on the cast-iron tripod F, and retained in its place by the spring c. At the end of the tube A nearest the prism is a lens, placed at the distance of its focus for parallel rays from a vertical slit at the other end of the tube. The width of the slit can be regulated by means of the screw e. One half of this slit is covered by a small rectangular prism designed to reflect the rays proceeding from the source of light D, down the axis of the tube, whilst the rays from the source of light E pass directly down the tube. By this arrangement the observer stationed at the end of the telescope B is able to compare the spectra of both lights, which are seen one above the other, and he can at once decide whether their lines coincide or differ. a and b are screws for adjusting the axis of the telescope so as to bring any part of the slit at e into the centre of the field of vision.
The telescope as well as the tube C is moveable in a horizontal plane around the axis of the tripod. The tube C contains a lens at the end next to the prism, and at the other end is a scale formed by transparent lines on an opaque ground; it is provided with a levelling screw, d. When the telescope has been properly adjusted to the examination of the spectrum, the tube C is moved until it is placed at such an angle with the telescope and the face of the prism, that when a light is transmitted through the scale the image of this scale is reflected into the telescope from the face of the prism nearest the observer. This image is rendered perfectly distinct by pushing in the tube which holds the scale nearer to the lens in C, or withdrawing it to a greater distance, as may be required. The reflected lines of the scale can then be employed for reading off the position of the dark or bright lines of the spectrum, as both will appear simultaneously overlapping each other in the field of the telescope.
By turning the tube C round upon the axis of the tripod any particular line of the scale can be brought to coincidence with any desired line of the spectrum. Stray light is excluded by covering the stand, the prism, and the ends of the tube adjoining it with a loose black cloth. The dispersive power upon the spectrum may be much increased by using several prisms instead of one. Kirchkoff used four prisms in his experiments upon the solar spectrum. Great care must be observed in placing the prisms; the refracting edge of each prism must be exactly vertical, and the position of minimum deviation for the rays to be observed must be obtained.
The preceding remarks have reference to the spectra produced when the vapours of certain elements are evolved in flame derived from artificial sources. When, however, solar light is examined by the spectroscope, results entirely the reverse follow.
If a beam of sunlight be sent through the slit of the spectroscope, the prismatic image is seen to be intersected by a number of fine black lines, varying in thickness and intensity, and invariably occupying the same relative position in the solar spectrum. These lines were first noticed so far back as 1815 by a German optician, Frauenhofer, after whom they were named Frauenhofer’s lines; but it was not until the invention of the spectroscope that the origin of these lines could be accounted for. By so arranging the instrument as to cause the spectrum from a solar beam, and that from a metallic element, to fall upon the field of the telescope, so that the solar spectrum shall be above the other, both being perfectly parallel; the bright bands or lines of the metal are all seen to be continued in the dark solar lines, for, as may be seen by consulting the plate of the different spectra, several lines are sometimes produced by one element alone. If, for instance, the sodium and solar spectra are thus compared, the bright yellow sodium line will be found to147 agree exactly not only in position, but also in intensity and breadth, with one of the dark solar ones. And the same thing occurs when the comparison is made with many of the other metals, the bright lines in the respective spectra furnished by them are each coincident with a particular dark line in the solar spectrum, and from every dark line in the latter a corresponding bright one can be found amongst the spectra of the metals. From what has just been stated, the inference seems irresistible that this coincidence between the dark solar lines and the bright lines of the metals cannot be accidental, but must be due to some intimate connection between them, and that this is the case can be proved beyond refutation by a simple experiment, in which the bright metallic lines can be changed into dark ones, corresponding in every particular with those of the solar spectrum. Thus the bright yellow soda lines coincident with Frauenhofer’s lines can be converted into dark ones by allowing the rays from a strong source of white light to pass through a flame coloured with sodium, and then making them fall upon the slit of the spectroscope. If we examine the spectrum obtained by this means, instead of seeing the usual bright double band upon a black ground, there will be presented to our sight a double dark line, corresponding exactly with the position and width of the sodium line, and instead of the black ground there will be a continuous spectrum of white light, as in the solar spectrum.
The explanation of this remarkable phenomenon is due to Kirchkoff, and is as follows:—When any substance is heated sufficiently to render it luminous, rays of a certain and definite degree of refrangibility are given out by it; whilst the same substance has also the power of absorbing rays of this identical refrangibility. In the above experiment, therefore, the yellow flame absorbed the same kind of light as it gave out, a corresponding decrease of intensity in its own particular position in the spectrum occurred, and a dark line showed itself in consequence.
In the same manner and under similar conditions the spectra of many other substances have been reversed.
Reasoning on these facts, Kirchkoff has been able to account for the presence in the solar spectrum of Frauenhofer’s dark lines. He supposes that in the luminous atmosphere surrounding the sun the vapours of various metals are present, each of which would give its characteristic system of bright lines; but behind this incandescent atmosphere containing metallic vapour is the still more intensely heated solid or liquid nucleus of the sun, which emits a brilliant continuous spectrum, containing rays of all degrees of refrangibility.
When the light of this intensely heated nucleus is transmitted through the incandescent photosphere of the sun, the bright lines which would be produced by the photosphere are reversed, and Frauenhofer’s dark lines are only the reversed bright lines which would be visible if the intensely heated nucleus were no longer there.
The correctness of this theory has been rigorously tested by Kirchkoff himself, who submitted the solar spectrum to a most minute and searching examination.
As a result of the knowledge thus obtained, the presence of certain metals in the sun’s atmosphere was an inevitable deduction. The metals hitherto detected in the solar photosphere are—iron, sodium, magnesium, calcium, chromium, nickel, barium, copper, zinc, strontium, cadmium, cobalt, manganese, aluminium, and titanium. Hydrogen also exists in large quantity as an incandescent gas, and gives rise to the red protuberances that may be observed during a total eclipse.
During the total eclipse of 1869, M. Janssen, a French astronomer, was enabled to obtain and figure the specimen of these red protuberances, which, taken exclusively from that source of light, gave not dark lines, but bright ones, corresponding in position with those of hydrogen, magnesium, and sodium.
The fixed stars, unlike the moon and planets, which shine only by reflected light, are not merely illuminated by self luminous bodies, and yield spectra, which show them to contain many elements known to us; their spectra are crossed by dark lines similar to, but not identical with those given by the sun’s light. The spectrum yielded by the star Aldebaran shows it to contain hydrogen, sodium, magnesium, calcium, iron, tellurium, antimony, bismuth, and mercury; in the spectrum of Sirius only sodium, magnesium, and hydrogen have been found; whilst in that of Orionis there is an absence of hydrogen. Most of the nebulæ and comets give spectra in which there are only bright lines. It is hence inferred that these celestial bodies are composed of masses of glowing gas, and, unlike the sun and stars, do not consist of a solid or liquid mass surrounded by a gaseous atmosphere. In the nebulæ hydrogen and nitrogen only have been found; and in comets, principally carbon.
ANANAS HEMP (Ananassa sativa, S. Brumelia ananas, as well as other species). This hemp comes from the West Indies and Central and South America, where the common ananas is cultivated. It is rather inferior to some varieties for spinning.
ANASTATIC PRINTING. See Printing and Zincography.
ANATHERIN BALSAM. The following formula is published by the Netherlands Society:—Tincture of myrrh, 160 grms.; tincture of catechu, 80 grms.; tincture of guaiacum, 40 grms.; tincture of rhatany, 40 grms.; tincture of cloves, 30 grms.; spirit of cochlearia, 20 grms.; oil of cassia, 20 drops; otto of roses, 1 drop; proof spirit, 630 grms.148
ANATHERIN BALSAM (J. G. Popp, Vienna). A mouth-wash. Red sandal wood, 20 parts; guaiacum wood, 10 parts; myrrh, 25 parts; cloves, 15 parts; cinnamon, 5 parts; oils of cloves and cinnamon, of each, 2⁄3 part; spirit, 90 per cent., 1450 parts; rose water, 725 parts. Digest and filter.
Dr Hager, who gives the above, says that on the expiration of the patent the following formula was published, but that a preparation made from that process had only a distant resemblance to the actual compound. Myrrh, 1 part; guaiacum wood, 4 parts; saltpetre, 1 part; to be macerated for a night with corn brandy, 120 parts; spirit of cochlearia, 180 parts. Then distil of this 240 parts, in which are to be digested for 14 days garden rue, cochlearia, rose leaves, black mustard, horseradish, pellitory root, cinchona bark, club-moss, sage-vetiver, and alkanet root, of each 1 part. Strain and filter, and to each 120 parts of the filtrate add 1 part of spirit of nitrous ether. (Hager.)
ANATOM′ICAL. Syn. Anatom′icus, L.; Anatomique, Fr.; Anatomisch, Ger. Belonging to anatomy or dissection.
Anatomical Prepara′tions. Objects of interest in both surgical and pathological anatomy, and specimens in natural history, preserved by subjecting them to antiseptic processes, to which is also frequently added injection with coloured fluids (which subsequently harden), amalgams, or fusible metal, in order to display more fully the minute vessels, or the microscopic anatomy of the several parts. See Fusible Alloy, Injections, Preparations, Putrefaction, Skeletons, Solutions, &c.
ANCH′OVY (-chō′-). Syn. Anchois, Fr.; Anchove, Anschove, Ger.; Acciughe, Anchiove. It.; Anchova, Port., Sp. The clu′pea encrasic′olus (Linn.), a small fish of the herring tribe, closely resembling the English sprat. It is common in the Mediterranean, and occurs in the greatest abundance and of the finest quality about the island of Gorgona, near Leghorn. It is taken in the night, during May, June, and July.
Anchovies are prepared for sale or exportation by salting or pickling them—the heads, intestines and pectoral fins having been first removed, but not the scales, and afterwards packing them, along with rock-salt, in the small kegs in which they are imported into this country. The small fish are valued more than the larger ones. For the table they are often fried to a pale amber colour, in oil or butter; having previously been scraped clean, soaked for an hour or two in water, wiped dry, opened (without dividing the fish), and had the back-bones removed. Before being put into the pan they are usually highly seasoned with cayenne; and after being again closed, are dipped into a rich light batter. They are also divided into fillets, and served as sandwiches, or in curried toasts. Anchovies are also extensively potted (POTTED ANCHOVIES), and made into butter (A.-BUTTER), and into sauce (A.-SAUCE), particularly the last.
The anchovy has a fine and peculiar flavour, and is eaten as a delicacy all over Europe. It was known to the Greeks and Romans, who prepared from it a kind of garum for the table. It is said to be aperitive, stimulant, and stomachic.
The high price of genuine Gorgona anchovies has led the fraudulent dealer to either substitute for them, or mix with them, fish of a less expensive kind. The most frequent SUBSTITUTIONS are Dutch, French, and Sicilian fish of allied species or varieties, sardines and even the common sprat. The genuine Gorgona fish is about the length of one’s finger; and may be known by its silvery appearance; by the greater thickness of its head, which is sharp-pointed, with the upper jaw considerably the longest, and the mouth deeply divided; the dusky brown colour of its back,[58] and the pink salmon colour of its flesh. When only 3 months old, its flesh is pale; when of 6 months, rather pink; when of 10 to 12 months (or in its prime), a beautiful deep pink colour; and when much older, darker, but less lively. The fin-rays, varying in number with the age of the fish, are—
| Yarrell. | Hassall.[59] | |
| Dorsal | 14, | 16 (?). |
| Pectoral | 15, | — |
| Ventral | 7, | — |
| Anal | 18, | 19 (?). |
| Caudal | 19, | 26 (?). |
These fins are delicate in structure and greenish-white; and the membranes connecting the rays almost transparent. “The length of the head, compared with the length of the body alone, is as 1 to 3; the depth of the body but 2-3rds of the length of the head, and compared to the length of the whole fish is as 1 to 7;” the tail is deeply forked, the gill covers are elongated, and the scales of the body large and deciduous.” “The breadth of the eye is 1-5th of the length of the whole head.”[60] Dutch fish may be generally known by being deprived of the scales, and the French fish by their larger size; and both by the paler or whiter colour of their flesh; and sardines and sprats by the flesh being white. The genuine fish may also be known by the pickle, after repose or filtration, being of a clear pinkish colour, without any red sediment; whilst that from spurious kinds is turbid and red only when agitated, and deposits a heavy red sediment (Armenian bole, Venetian red, or red ochre) on repose. See Butter, Potting, Powders, Sauces, &c.
Anchovies, Brit′ish. See Sprats.
ANCHU′SIC ACID (-kū′zĭk). See Anchusine.
ANCHU′SINE. (-kū′zĭn). [Eng., Fr.] Syn. Anchu′sic acid*, Pseu′do-alkann′ine*, Pseudo-alka′′nium*; Anchusi′na, L. The resinoid constituting the colouring matter of alkanet-root (which see).
ANCHYLO′SIS (ăngk-e-). [L.; prim. Gr.] Syn. Ankylo′sis, Ancylo′sis (ăn-se-), L.; Ankylose, Fr., Ger. In pathology, stiffness or immobility of a joint naturally moveable. Anchylosis is either true or complete, as when the extremities of the bones forming a joint are reunited and immovable; or false, or incomplete, where the affection depends upon a contraction of the tendons and ligaments surrounding the joints, which nevertheless admit of a small degree of motion. For the first there is no available remedy; for the second gentle and progressive flexion and extension of the part daily (carefully avoiding violence), friction with oleaginous and stimulating liniments, and the use of the hot bath, vapour bath, or hot-air or Turkish bath, and electricity, have been strongly recommended, and have frequently proved successful.
ANCYLO′SIS. See Anchylosis.
ANDITROPFEN (Kirchner and Menge Arolsen), for weak digestion. Senna, 20 parts; rhubarb, 3 parts; jalap, 6 parts; zedoary root, 2 parts; ginger, 2 parts; galangal, 3 parts; soda, bicarbonate, 5 parts; sugar, 15 parts; water, 300 parts; spirit, 65 parts. After digestion this is to be strained and mixed with an infusion of 30 parts of yarrow (with the flowers) in 300 parts of hot water. After standing some time filter. (Hager.)
ANDROGRAPHIS PANICULATA. (Ind. Ph.) Syn. Kariyát. Habitat. Commonly in shady places all over India.—Officinal part. The dried stalks and root (Andrographis Caules et Radix, Kariyat, Creyat). The stem, which is usually met with, with the root attached, occurs in pieces of about a foot or more in length, quadrangular, of a lightish-brown colour, and persistent bitter taste.—Properties. Bitter tonic and stomachic, very analogous to quassia in its action.—Therapeutic uses. In general debility, in convalescence after fevers, and in the advanced stages of dysentery.
Preparations:—
Compound Infusion of Kariyát (Infusum Andrographis compositum). Take of Kariyát, bruised, 1⁄2 an ounce; orange-peel and coriander fruit, bruised, of each, 60 grains; boiling water, 10 fluid ounces. Infuse in a covered vessel for an hour and strain.—Dose. From 11⁄2 to 2 fluid ounces, twice or thrice daily.
Compound Tincture of Kariyát (Tinctura Andrographis composita). Take of kariyát root, cut small, 6 ounces; myrrh and aloes, in coarse powder, of each 1 ounce; brandy, 2 pints. Macerate for seven days in a closed vessel, with occasional agitation; strain, press, filter, and add sufficient brandy to make two pints.—Dose. From 1 to 4 fluid drachms. Said to be tonic, stimulant, and gently aperient, and to prove valuable in several forms of dyspepsia, and in torpidity of the bowels.
ANDROPOGON (CYMBOPOGON) CITRATUM. Lemon Grass. (Ind. Ph.) Habitat. Commonly cultivated in gardens in India; also in Ceylon, upon a large scale, for the sake of its volatile oil.—Officinal part. The volatile oil (Oleum Andropogi Citrati, Lemon Grass Oil, Oil of Verbena), obtained by distillation from the fresh plant; of a pale sherry colour, transparent, extremely pungent taste, and a peculiar fragrant lemon-like odour.—Properties. Stimulant, carminative, antispasmodic, and diaphoretic; locally applied, rubefacient.—Therapeutic use. In flatulent and spasmodic affections of the bowels, and in gastric irritability. In cholera it proves serviceable by aiding the process of reaction. Externally, as an embrocation in chronic rheumatism, neuralgia, sprains, and other painful affections.
Dose. From 3 to 6 drops, on sugar or in emulsion. For external application it should be diluted with twice its bulk of soap liniment or any bland oil.
ANDROPOGON (CYMBOPOGON) NARDUS. Citronelle. (Ind. Ph.) Habitat. Madras Peninsula and Ceylon. The volatile oil of this plant has similar properties to A. citratum. and is used for the same purposes.
ANDROPOGON PACHNODES. (Ind. Ph.) The volatile oil of this plant possesses similar properties to that of A. citratum, and is used for the same purposes.
ANELEC′TRIC (ăn-e-). Non-electric; a non-electric.
ANEMOM′ETER (ăn-e-). Syn. Anemom′etrum, L.; Anémomètre, Fr.; Windmesser, Ger. An instrument or apparatus for measuring the force or velocity of the wind, or of a current of air. Various contrivances have been adopted for this purpose. The anemometer of Dr Lind being also applicable to the determination of the draught of a chimney, and the strength of air-current, in ventilation, may be usefully described here:—
Uses and Appl. The open end (a) is kept, by means of a vane, presented to the wind, which acting on the surface of the water, or other liquid in b, raises the level of the fluid in the arm (c). The difference of the level of the fluid in the two arms of the instrument is the measure of the force of the wind. To estimate the draught of a flue or chimney, the arm (c) is placed in the chimney, and the orifice (a) in the apartment.[61]
ANEMOM′ETRY. Syn. Anemome′tria, L.; Anémometrie, Fr.; Windmessen, Ger. In meteorology, physics, &c., the art or act of measuring the velocity or force of the wind, or of ascertaining its direction.
ANEM′ONE (ă-nĕm′-o-ne). Syn. Anem′ony; Anem′one, L., Gr.; Anémone, Fr. The wind-flower. In botany, a genus of beautiful flowering herbaceous plants, of the nat. ord. Ranunculaceæ. The double flowers of some of the species are among the most elegant ornaments of our gardens. Others are used in medicine. They are all acrid and stimulating.
Anem′ones, Sea. (-o-nēz). Syn. An′imal-flowers‡, Sea sun′flowers‡. Animals of the genus actin′ia, so called from the resemblance of their claws or tentacles, when expanded, to the petals of a flower. They are of various colours, are generally fixed by one end to rocks or stones in the sand, and are very voracious, being accused of occasionally swallowing a mussel or a crab as large as a hen’s egg for a meal. They belong to the highly organised polypes of Cuvier.
ANEMON′IC ACID. See Anemonine.
ANEMONIN. A crystalline substance found in the leaves of several species of anemone, viz. A. pulsatilla, A. pretensis, A. nemorosa. Water distilled from these leaves, after some weeks, deposits a colourless inodorous substance, which softens at 150° C, giving off water and acrid vapours. It is purified by repeated crystallisation from boiling alcohol. Anemonin is a poisonous body. It causes slight irritation when applied to the skin. By the action of alkalies anemonin is transferred into anemonic acid.
ANEM′OSCOPE (ăn′-e—Brande, Mayne). Syn. Anemosco′pium, L.; Anémoscope, Fr.; Anemoskop, Ger. An instrument to measure the force and velocity of the wind. See Anemometer.
AN′EROID (-royd)[62]. In physics, &c., not fluid, or not depending on water or a fluid for its action; applied to a certain form of barometer (which see)
ANEURISM. A tumour on an artery, produced by the rupture of the inner coat of the vessel, and the blood getting between it and the outer coat.
ANGEL′ICA (-jĕl′-). [L., Port., Sp.; Ph. E. & D.] Syn. Garden Angelica; Angélique, Fr.; Angelika, A.-wurzel, Angelkraut, Ger. The angelica archangel′ica of Linnæus, an aromatic herbaceous plant with a biennial, fleshy root, indigenous to the north of Europe, but frequently found wild in England, and largely cultivated in our gardens. Dried root (ANGELICA, Ph. E.), aperient, carminative, diaphoretic, and tonic; much esteemed by the Laplanders, both as food and medicine;—fruit or seed (Angelica, Ph. D.) resembles the root, but is weaker. The whole plant has been extolled as an aromatic tonic. As a masticatory, it leaves an agreeable glowing heat in the mouth. The aromatic properties of this plant depend on a peculiar volatile oil and resin.
Uses, &c. It has been recommended in diarrhœa, dyspepsia, debility, and some fevers; but is now seldom used in medicine. Dose, 30 gr. to 1 dr. The dried root and seeds are used by rectifiers to flavour gin and liqueurs; and the fresh root, tender stems, stalks, &c., are made by the confectioners into an aromatic candy. See Candying, Liqueurs, &c.
Angelica Atropurpu′′rea. [Linn.] Syn. Amer′ican Angelica; Angelica, Ph. U. S. Hab. North America. Resembles garden angelica, but placed by some botanists in a separate, though allied genus. It is a popular remedy for flatulent colic, indigestion, and cardialgia, in the United States; and is there regarded as tonic, cordial, and aphrodisiac.
ANGEL′IC ACID. HC5H7O2. A volatile substance, noticed by L. A. Buchner, jun., in angelica-root. It has a pungent sour smell, and a biting acid taste; is sometimes fluid and oleaginous, and sometimes crystallised in striated prisms.[63]
ANGO′LA Syn. Ango′la-wool, Ango′′ra-w., Ango′na-w., &c.; Poil de chevron d′Angora, Fr.; (Engoor′, Engour′, or Engu′ri) Tiftic, Tur. The wool of ‘ca′pra Angoren′sis’ or the Angora-goat, of which the shawls of Cashmere are made, and others in imitation of them. It is also used to make plush, light cloths for paletôts which are repellent of wet, &c.; and is extensively employed in France in the manufacture of lace more brilliant than that of Valenciennes and Chantilly, and at half the price. See Alpaca, Shawls, Wool, &c.
ANGOSTU′RA, Angustu′ra. (-tūre′-ă). See Cusparia.
Angostura, False. See Brucea, Cusparia and Strychnos.
ANGOSTU′′RINE, Angustu′rine (-ĭn). See Cusparin.
ANHYDRIDE. Most, if not all modern chemists, adopting Gerhardt’s practice of limiting the title of acid to a particular class of substances which contain hydrogen, now regard all true acids as salts of hydrogen. Formerly many bodies, such as silica or white arsenic, were looked upon as acids, though if we adopt the foregoing definition they are not really so until they have combined with water. Such bodies, because they contain no hydrogen, are now distinguished as anhydrides; the substances,151 for example, familiarly known as carbonic, sulphurous, and phosphoric acids, must, upon the above principle, be designated carbonic, sulphurous, and phosphoric anhydrides. We may also define an anhydride to be an oxide which forms an acid on treatment with water.
ANHY′DROUS (-drŭs; an′hydrous, as marked by Brande, is less usual). Syn. An′hydrus, L.; Anhydré, Fr.; Wasserfrei, Ger. Free from water; dry. In chemistry and mineralogy, a term frequently applied to substances, as acids, alcohol, gases, salts, minerals, &c., which do not contain either free or combined water. Gases may generally be rendered anhydrous by passing them through a tube containing fused chloride of calcium, or (e.g. AMMONIA and two or three others) quick-lime, in coarse powder; and some of them, by passing them through concentrated sulphuric acid. Salts may generally be dried by cautiously submitting them to the action of heat, or by exposure to a very dry atmosphere; and alcohol, and many other volatile fluids, by careful distillation from chloride of calcium, or some other highly hygrometric substance.
AN′IL. [Fr., Sp., L.] The indigof′era anil of botanists—one of the plants yielding ‘indigo’—a native of America, but now largely cultivated in the East Indies. See Indigo (and below).
AN′ILINE[64] (-een). [Eng., Fr.] C6H7N. Syn. Phenyl′amine; Anili′na, Anili′num, &c., L. A peculiar volatile organic base first noticed by Unverdorben in empyreumatic bone-oil, and afterwards obtained by Runge from coal-tar, and by Fritzsche, Zinin, A. W. Hofmann, and others, as a product of various reactions, processes, and decompositions, particularly those attending the destructive distillation of organic bodies.
Prep. Aniline is now almost invariably obtained, on the large scale, either directly or indirectly from coal-tar or indigo; and chiefly from the basic oil or naphtha, or the nitrobenzol, of which the former is the principal source. The following are the leading commercial and experimental processes:
1. From COAL-TAR or COAL-TAR NAPHTHA:—The basic oil or basic portion of coal-tar or coal-tar naphtha, forming the latter, denser, and least volatile products of the distillation or rectification of these substances, is strongly agitated, for some time, along with hydrochloric acid in slight excess, a glass globe, or, on the large scale, a suitable vessel of lead, or of enamelled iron, being employed for the purpose; the clear portion of the liquid (containing the hydrochlorates of the bases present) is then decanted and carefully evaporated over an open fire until acrid fumes begin to be disengaged, when it is again decanted or filtered; the clear liquor, or filtrate is next treated with potash or milk of lime in excess, by which the bases—chiefly aniline and chinoline—are liberated under the form of a brownish oil; the whole of the resulting mixture is now submitted to distillation, the portion which passes over at or about 360° Fahr., and which consists chiefly of crude aniline, being collected separately; the product is purified by rectification and recollection, once or oftener, at the same temperature, and, lastly, by fresh treatment with hydrochloric acid and careful distillation with excess of potash, or milk of lime, as before.
2. From NITROBENZOL:—a. (Zinin.) An alcoholic solution of nitrobenzol, after saturation with ammonia, is treated with sulphuretted hydrogen, until, after some hours, a precipitation of sulphur takes place; the brown liquid is then repeatedly saturated with fresh sulphuretted hydrogen, until no more sulphur separates, the reaction being aided by occasionally heating or distilling the mixture; an excess of acid is next added, and, after filtering the liquid, and the removal of the alcohol and unaltered nitrobenzol by ebullition or distillation, the residuum is lastly distilled with caustic potash, in excess. The ANILINE found in the receiver may be rendered quite pure by forming it into oxalate of aniline, repeatedly crystallising the salt from alcohol, and finally distilling it with excess of caustic potassa, as before.
The following is a cheaper and more convenient process; and probably the best, or one of the best, that has yet been invented for obtaining aniline:—
b. (M. Béchamps.) From nitrobenzol distilled along with basic protacetate of iron; or, what is better, by distilling a mixture of iron-filings, 2 parts, and acetic acid, 1 part, with about an equal volume of nitrobenzol, the reaction being assisted, whenever the effervescence flags, by the application of a gentle heat. The liquor found in the receiver consists of aniline and water, from which the first, forming the lower portion, is obtained, after sufficient repose in a separator; or more easily, by adding a very little ether, which by dissolving in the aniline, causes it to rise to the surface, when it is at once decanted. A very spacious glass or earthenware retort must be employed in the process, as the mass swells up violently; and it must be connected with the receiver, on the small scale, by means of a Liebig’s condenser, and, on the large scale, by an ordinary worm-pipe and tub, kept in good action by a sufficient flow of cold water.
The apparatus for carrying out Béchamp’s method was devised by Nicholson, and is exhibited in the subjoined plate.
“It consists essentially of a cast-iron cylinder (A) of 10 hectolitres (220 cubic gallons) capacity. A stout iron tube is fitted to this152 vessel, reaching nearly to the bottom of the cylinder. The upper part of this tube is connected with the machinery (G), while the surface of the tube is fitted with steel projections. The tube serves to admit steam, as well as acting as a stirring apparatus. Sometimes, instead of this tube, a solid iron axle is employed, and in this case there is a separate steampipe (D). Through the opening at K the materials for making aniline are put into the apparatus, while the volatile products are carried off through E. H serves for emptying and cleaning the apparatus. The S-shaped tube connected with the vessel B acts as a safety valve. When it is intended to work with this apparatus there is poured into it through K 10 parts of acetic acid at 8° B. (sp. gr. 1·060), previously diluted with six times its weight of water; next there are added 30 parts of iron filings, or cast-iron borings, and 125 parts of nitrobenzol, and immediately after the stirring apparatus is set in motion. The reaction ensues directly, and is attended by a considerable evolution of heat and vapours. Gradually more iron is added until the quantity amounts to 180 parts. The escaping vapours are condensed in F, and the liquid condensed in R is from time to time poured back into the cylinder A. The reduction is finished after a few hours.”

3. From INDIGO:—Powdered indigo is added to a boiling and highly concentrated solution of caustic potash, as long as it dissolves and hydrogen gas is liberated; the resulting brownish-red liquid is evaporated to dryness, and the residuum is submitted to destructive distillation in a retort, which, owing to the intumesence of the mass, should be strong and spacious. The ANILINE is found in the receiver under the form of a brownish oil mixed with ammoniacal liquor, and by separation from the latter, and subsequent rectification, is obtained nearly colourless. It may be further purified, as in the preceding processes.—Prod. 18 to 20% of the indigo employed.
4. By fusing, with proper precautions, a mixture of isatine and hydrate of potassium (both in powder); a retort connected with a well-cooled receiver, being employed as the apparatus. Said by Profs A. W. Hofmann and Muspratt to be “the most eligible process for isolating” aniline.[65]
5. From anthranilic acid mixed with powdered glass or sand, and rapidly heated in a retort.
6. By treating an alcoholic solution of benzine with a little zinc and hydrochloric acid.
7. By heating phenyl-alcohol with ammonia in sealed tubes.
In Zinin’s process the nitrobenzol is dissolved in alcohol, and the solution, after the addition of ammonia, is saturated with sulphuretted hydrogen. After standing some time the solution deposits a large quantity of sulphur, and the liquid yields aniline.
Many other reducing agents have been proposed for the conversion of nitrobenzol into aniline, such as arsenite of sodium, powdered zinc, &c., but on the large scale they have all been found inferior to the process of Béchamp. Kremer’s process consists in heating one part of nitrobenzol in a proper apparatus with five of water and two and a half of zinc dust. When the reaction is completed the aniline, amounting to about 65% of the weight of the benzol, is distilled off in a current of steam.
Prop., &c. A thin, oily, colourless liquid, with a faintly vinous odour, and a hot and aromatic taste; very volatile in the air; miscible in all proportions with alcohol and ether; very slightly soluble in water; neutral to ordinary test-paper, but exhibiting an alkaline reaction to dahlia-petal infusion and paper; dissolves camphor, sulphur, and phosphorus, and coagulates albumen; possesses a high refractive power; and precipitates the oxides of iron, zinc, and alumina, from solutions of their salts, and neutralises the acids, like ammonia. With the acids it forms numerous crystallisable compounds of great beauty, and which are easily formed, and are precisely analogous to the corresponding salts of ammonia. These, on exposure to the air, acquired a rose colour, in many cases gradually passing into brown. Its boiling-point is 359° to 360° Fahr.; sp. gr. 1·028.
Tests.—1. Chromic acid gives a deep greenish or bluish-black precipitate with aniline and its salts:—2. Hypochlorite of lime strikes an extremely beautiful violet colour, which is soon destroyed:—3. The addition of two or three drops of nitric acid to anhydrous aniline produces a fine blue colour, which, on the application of heat, passes into yellow, and a violent reaction ensues, sometimes followed by explosion:—4. With bichloride of platinum it yields a double salt (platino-chloride of aniline)153 analogous to the like salt of ammonia. These reactions distinguish it from all other substances.
Commercial aniline is a mixture consisting in great part of aniline, paratoluidine (solid), and orthotoluidine in variable proportions. In addition it contains small amounts of metatoluidine, nitrobenzol, odorine, &c., but for all practical purposes it may be regarded as a mixture of aniline and toluidine. These anilines are obtained from a portion of the light coal-tar naphtha boiling between certain temperatures, by treating it first with nitric acid to convert it into the nitro-compounds, and then reducing these with iron and acetic acid, as already described under Béchamp’s process. It is very plain that as the coal-tar naphtha contains variable proportions of benzol and toluidine, the resulting product must also vary in the quantities of aniline and toluidine it will contain. In order to distinguish between various samples of commercial aniline, Reimann submits them to fractional distillation and compares the results. He places 100 c. c. of the sample to be tested in a retort fitted with a thermometer and heated by means of an oil bath. The liquid as it distils is received in a narrow graduated cylinder, and the amount that passes over between every 5° C. (9° F.) is noted.
In order to obtain standards for comparison he first distilled a sample of light aniline, or kuphaniline, as he terms it, then one of heavy aniline or baraniline; afterwards mixtures of the two in varying proportions. In the accompanying table the results are given.
| Centigrade | K. | 100 | 90 | 85 | 80 | 75 | 60 | 50 | 25 | 0 |
| B. | 0 | 10 | 15 | 20 | 25 | 40 | 50 | 75 | 100 | |
| Below 180° | 81⁄2 | 7 | 21⁄2 | 51⁄2 | 7 | ... | 7 | 51⁄2 | ... | |
| 180°—185° | 54 | 50 | 291⁄2 | 22 | 51⁄2 | 7 | 41⁄2 | 21⁄2 | 2 | |
| 185°—190° | 34 | 34 | 561⁄2 | 551⁄2 | 551⁄2 | 37 | 71⁄2 | 41⁄2 | 11⁄2 | |
| 190°—195° | ... | 5 | 71⁄2 | 81⁄2 | 15 | 33 | 42 | 17 | 8 | |
| 195°—200° | ... | ... | ... | ... | 9 | ... | 19 | 36 | 18 | |
| 200°—205° | ... | ... | ... | ... | 41⁄2 | 16 | 10 | 16 | 39 | |
| 205°—210° | ... | ... | ... | ... | ... | ... | 31⁄2 | 8 | 19 | |
| 210°—215° | ... | ... | ... | ... | ... | ... | ... | 41⁄2 | 7 | |
| Residue | 31⁄2 | 4 | 4 | 81⁄2 | 31⁄2 | 7 | 61⁄2 | 5 | 51⁄2 |
To ascertain the quality of any sample it is only necessary to distil it in the manner already described, and compare the results with those in the above table.
(For further information consult Wagner’s ‘Chemical Technology,’ Calvert’s ‘Dyeing and Calico Printing,’ edited by Stenhouse and Groves; Crooke’s ‘Practical Handbook of Dyeing and Calico Printing,’ Ure’s Dictionary, edited by Hunt.)
Uses, &c. Chiefly in dyeing, for the production of colouring matter of various rich shades of purple and violet, some approaching pink, by the action of chromic acid; and of a splendid crimson, by the action of various oxidising agents. It forms the basis of the celebrated new dyes for silks lately patented by Mr W. H. Perkin, and others, and which are not only more delicate and gorgeous in tint, but also more permanent, than any produced by other substances.
Besides numerous salts, various substitution compounds of aniline have been formed, all of which possess vast scientific interest, and several are likely to prove of importance in the arts. See Dyeing, Indigo, Tar Colours, &c. (also below.)
Aniline, Chro′mates of. Prep. 1. (NEUTRAL CHROMATE.) From sulphate or oxalate of aniline and chromate of potash, by double decomposition.
2. (BICHRO′MATE:—Mr W. H. Perkin.) Sulphate of aniline and bichromate of potash, in equivalent quantities, are separately dissolved in water, and the solutions, after being mixed, are allowed to stand for several hours. The whole is then thrown upon a filter, and the black precipitate which forms is washed and dried. It is next digested in coal-tar naphtha (—? benzol), to extract a brown resinous substance; after which it is digested in alcohol, to dissolve out the colouring matter (BICHROMATE OF ANILINE), which is left behind on distilling off the spirit, as a coppery friable mass. Patented.
Aniline, Cy′anide of. Benzonitrile.
Aniline, Ox′alate of. (C6H7N)2C2O4. Obtained by saturating an alcoholic solution of oxalic acid with aniline; the salt separating as a crystalline mass. It is very soluble in hot water; much less so in cold water; only slightly soluble in alcohol; and insoluble in ether. It may be crystallised from hot water or boiling alcohol. Used chiefly to form other salts.
Aniline, Sul′phate of. (C6H7N)2SO4. Prepared by saturating aniline with dilute sulphuric acid, and gently evaporating the liquid until the salt separates. By re-solution in boiling alcohol, it crystallises out, as the liquor cools, under the form of very beautiful colourless plates, of a silvery lustre. It is freely soluble in water, and in hot alcohol; scarcely soluble in cold alcohol; and insoluble in ether.154 It is chiefly employed in the preparation of the new aniline dyes.
ANIMAL′CULE (-kūle). [Eng., Fr.; pl. animal′cules.] Syn. Animal′culum (pl., animal′cula[66]), L.; Thierchen, Ger. In zoology and physiology, a microscopic animal, or one so extremely small, that it is either invisible, or not distinctly discernible, without the aid of a lens or microscope; more especially one that is not perceptible to the naked eye. “A mite was anciently thought the limit of littleness; but there are animals 27,000,000 of times smaller than a mite.” A thousand millions of some of the animalcula found in common water are said to be collectively of less bulk than a single grain of sand; yet their numbers are so prodigious as sometimes to give the fluid they inhabit a pale red or yellow tinge. The milt of a single codfish is said to contain more of these minute animals than there are people in the whole earth. Animalcula were first scientifically observed by Leuwenhoek about the year 1677. Assisted by the microscope he unveiled, as it were, he created a new world for future naturalists and microscopists to explore.
“Take any drop of water,” says Professor Rymer Jones, “from our rivers, from our lakes, or from the vast ocean itself, and place it under the microscope; you will find therein countless living beings moving therein in all directions with considerable swiftness, apparently gifted with sagacity, for they readily elude each other in the active dance they keep up.... Increase the power of your glasses, and you will soon perceive inhabiting the same drop, other animals compared to which the former were elephantine in their dimensions, equally vivacious and equally gifted. Exhaust the art of the optician, strain your eyes to the utmost, until the aching sense refuses to perceive the little quivering movement that indicates the presence of life, and you will find that you have not exhausted nature in the descending scale.”
Amongst the most remarkable discoveries of modern science must be reckoned that of fossil animalcules in such abundance as to form the principal part of extensive strata. This discovery is due to Ehrenberg, who found the Polierschiefer (the polishing slate or tripoli) of Bilin to be almost entirely made up of the siliceous shields of a minute fossil animalcule, the length of one of which is about 1⁄288th of a line, so that about 23,000,000 of animalcules must have gone to form a cubic line, and 41,000,000,000 to form a cubic inch of the rock. Ehrenberg succeeded in discovering the formation of similar strata in deposits of mud at the bottom of lakes and marshes, the mud swarming with living animalcules, probably in their turn to be fossilised. The bergmehl, or mountain meal of Sweden and other parts of Europe, which is sometimes used as an article of food, is entirely composed of the remains of animalcules; not merely, however, of their siliceous shields, for it contains a considerable per-centage of dry animal matter. Some animalcules prefer waters impregnated with iron, and their death gives rise to an ochreous substance in which iron is a principal ingredient.
AN′IME (ăn′-ĭm-e). [Eng., L., Sp.] Syn. Gum-an′ime, A.-res′in; Animé, Fr.; Animeharz, Kourbarillharz, Ger.; Courbaril, Jutaiba, Nat. A pale brownish-yellow, transparent, brittle resin, which exudes from the hymenæa courbaril (Linn.) or locust-tree, the h. martiana, and other species of hymenæa growing in tropical America. It contains about ·2% of volatile oil, which gives it an agreeable odour; melts without decomposition; is (nearly) insoluble in alcohol and in caoutchoucine, but forms a gelatinous mass in a mixture of the two. (Ure.) It burns readily, emitting a very fragrant smell. Sp. gr. 1·054 to 1·057.
Uses, &c. As a fumigation in spasmodic asthma; in solution as an embrocation; and in powder as a substitute for gum guaiacum. In this country it is chiefly employed to make varnishes and pastilles (which see).
AN′ION (-y′ŭn—Br., We.; ă-nī′-ŭn—Smart). Literally, ‘upward going,’ in electro-chemistry, a substance which is evolved from the surface where the electrical current is supposed to enter the electrolyte; an electro-negative body, or one which passes to the positive pole, or anode, in electrolysis, as opposed to a CATION. See Anode, Ions, &c.
AN′ISATED. Syn. Anisa′tus, L.; Anisé, Fr. In pharmacy, the art of the liqueuriste, confectioner, &c., applied to articles or preparations impregnated or flavoured with aniseed.
AN′ISE (-ĭs). Syn. Ani′sum, Pimpinel′la a. (Linn.), A. officina′le, L.; Anis, Fr.; Anis, Gemeiner Anis, Ger. An annual plant of the nat. ord. Umbelliferæ (DC.). Hab., Egypt, Scio, and the Levant; but largely cultivated in Malta, Spain, Germany, and various other parts of Asia and Europe. “A considerable quantity is cultivated at Mitcham, in Surrey, chiefly for the use of the rectifiers of British spirits.” (Stephenson.) Fruit, aniseed. (See below.)
AN′ISEED. Syn. An′ise, An′ise-seed; Sem′ina ani′si, Fruc′tus a., L.; Anis, A. vrai, Graines d’anis, Semence d’anis, Fr.; Anis, Anisamen, Ger.; Anis, Sp.; Anice, It. The aromatic fruit or seed of the pimpinella anisum just noticed.
Prop., Uses, &c. Its aromatic properties depend on the presence of volatile oil. The seed and oil, and a spirit and a water prepared from them, are officinal in the pharmacopœias. Both the seed and its preparations are reputed155 stimulant, stomachic, carminative, pectoral, diuretic, and emmenagogue. They are commonly used to relieve flatulence and colicky pains, and to prevent the griping effects of certain cathartics; and they have long been popular remedies for coughs, colds, and other breath ailments. They are esteemed especially useful in warming the stomach and expelling wind, particularly during infancy and childhood; the distilled or flavoured water being usually employed. Nurses also take the latter to promote the secretion of milk, to which it at length imparts its peculiar odour and flavour. In veterinary practice the powdered seed is used as a carminative, pectoral, and corroborant. The essential oil is said to be poisonous to pigeons. (Vogel; Hillefield.) Aniseed is principally used to flavour liqueurs, sweetmeats, and confectionery.—Dose (of the powder), 10 gr. to 1 or 2 dr.; for a horse, 1⁄2 to 1 oz.; cattle, 3⁄4 to 2 oz.
Pur., &c. Powdered aniseed is nearly always adulterated, the adulterant being generally linseed meal. Sometimes, as for the horse, the latter is entirely substituted for it, a few drops of oil of aniseed being added to give it smell. The adulteration is not readily detected by the uninitiated, owing to the strong odour of aniseed; but readily by the microscope. The fruit of myrrhis odorata (sweet cicily), and of illicium anisatum (star-anise), also possess the odour and flavour of common aniseed; indeed, most of the essential oil now sold as ‘oil of aniseed’ is star-anise oil. See Liqueurs, Oils, Spirits, Waters, &c.
Anise, Star′. The fruit or seed of illi′′cium anisa′tum (Linn.), an evergreen tree growing in Japan and China. The odour and properties of both the seed and oil greatly resemble those of common anise. They are both employed by the liqueuriste. See Aniseed (above), &c.
ANISETTE′ (ăn-ĭz-ĕt′). [Fr.] Aniseed cordial. See Liqueurs.
ANISOCHILUS CARNOSUM. Nat. order Labiatæ. An Indian plant. It is stimulant, diaphoretic, and expectorant; is used in quinsy, and by the native doctors of Travancore in catarrhal affections. Dr Bidie, an Indian practitioner, characterises it as a mild stimulating expectorant, and as such particularly useful in the coughs of childhood. Its properties depend upon a volatile oil.
ANISOMELES MALABARICA. An Indian plant. Nat. order Labiatæ. Few plants are held in higher esteem, or more frequently employed in native practice in Southern India, than this. An infusion made of the leaves is very generally used in affections of the stomach and bowels, catarrhal complaints, and intermittent fevers.
Dr Wright says that in addition to its internal use in the case of fevers, patients are made to inhale the vapour of a hot infusion, so as to induce copious diaphoresis. An infusion of the leaves is reported to be powerfully diaphoretic, and to have been found very useful in the low continuous fevers of the natives. An oil obtained by distillation from the leaves is likewise stated to be an effectual external application in rheumatism.
ANI′SUM. Aniseed.
ANNEAL′ING. Syn. Nealing†§; Le recuit, Fr.; Das anlassen, Ger. The art of tempering by heat: appropriately, the process by which glass, porcelain, &c., are rendered less frangible, and metals which have become brittle by fusion, or long-continued hammering, again rendered tough and malleable.
Glass vessels, and other articles of glass, are annealed by being placed in an oven or apartment near the furnaces at which they are formed, called the ‘leer,’ where they are allowed to cool very slowly, the process being prolonged in proportion to their bulk.
Steel, iron, and other metals are annealed by heating them and allowing them to cool slowly on the hearth of the furnace, or in any other suitable place, unexposed to the cold. Steel is also annealed by being made red-hot, and in that state is placed in a heap of dry saw-dust till cold, when it will be found quite soft.
Cast-iron is rendered tough and malleable, without ‘puddling,’ by embedding it in ground charcoal or hæmatite, and thus protected, keeping it exposed at a high temperature for several hours, after which the whole is allowed to cool very slowly.
Prince Rupert’s drop may be mentioned as an example of unannealed glass, and common cast-iron of unannealed metals, to which heads the reader is referred.
ANNOT′TA. Syn. Anot′to, Annat′to, Annat′ta; Arnat′to, Arnot′to, &c.; Orlea′na, Ter′ra o.*, &c., L.; Roucol, Rocou, Roucou, Fr.; Orleans, Ger. A colouring matter forming the outer pellicle of the seeds of the bix′a orella′na (Linn.), an exogenous evergreen tree, common in Cayenne and some other parts of tropical America, and now extensively cultivated in both the E. and W. Indies. It is usually obtained by macerating the crushed seeds or seed-pods in water for several weeks, ultimately allowing the pulp to subside, which is then boiled in coppers to a stiff paste, and dried in the shade. Sometimes a little oil is added in making it up into cakes or lumps. A better method is that proposed by Leblond, in which the crushed seeds are simply exhausted by washing them in water (—? alkalised), from which the colouring matter is then precipitated by means of vinegar or lemon-juice; the precipitate being subsequently collected, and either boiled up in the ordinary manner, or drained in bags and dried, as is practised with indigo. Annotta so prepared is said to be four times as valuable as made by the old process.
Prop. Good annotta is of a brilliant red colour; brighter in the middle than on the outside; feels soft and smooth to the touch;156 has a good consistence, and a strongly characteristic but not a putrid smell. It is scarcely soluble in water; freely soluble in alcohol, ether, oils, and fats, to each of which it imparts a beautiful orange colour, and in alkaline solutions which darken it; acids precipitate it of an orange red hue; strong sulphuric acid turns it blue. Its most important property is the affinity of its colouring matter for the fibres of silk, wool, and cotton.
Pur. Annotta is very frequently adulterated; indeed, nearly always so. To what extent the sophistication of annotta is carried may be judged from the statement of Mr Blyth, who says that on examination of thirty-four samples of various kinds, as imported and obtained from English makers and as purchased from dealers, he found only two that were genuine. As annotta is often used to give colour to different articles of diet, it is important that it should be as pure as possible; otherwise injurious effects detrimental to health may be caused by partaking of any food to which it is added. Now, amongst the list of adulterants given below are three, at least, unmistakeable poisons, viz. red lead, orange chrome, and sulphate of copper. It is but right to state of the first of these substances (red lead) that Mr Blyth says it is extremely doubtful whether it is now employed to the extent it formerly was. He also ascribes its presence in annotta to the impure Venetian red which is used, the employment of this colour being a necessity because of the large quantities of flour and lime which are mixed with the annotta, which thereby becomes so reduced in colour that it is essential to have recourse to salt, alkalies, and the red earths to restore it to its original standard. The adulterants are generally meal, flour, or farina, and often chalk or gypsum, with some pearlash and oil, or even soap, to give it an unctuous character; turmeric, Venetian red, red ochre, orange chrome, or even red lead, to give it ‘colour,’ and common salt, and sometimes even sulphate of copper, to prevent decomposition—the last two being poisonous. Sometimes a little carbonate of ammonia is also added to it to improve the colour. When quite pure it contains about 28% of resinous colouring matter, and 20% of colouring extractive matter (Dr John), and should leave only a small quantity of insoluble residuum after digestion in alcohol, whilst the ash resulting from its incineration should not exceed 11⁄2 to 2%. The quantity, colour, &c., of the ash will give an easy clue to the inorganic adulterants, if any are present, which may be then followed up by a chemical examination. The presence of red lead may be detected by heating it on a piece of charcoal in the reducing flame of the blowpipe, by which a small bead of metallic lead will be obtained. If it contains chalk, ochre, gypsum, &c., the undissolved residuum of the washed ash gives the amount of the adulteration (nearly).
Microscopical Examination of Annotta.—When annotta is subjected to a microscopical examination the outer red portion will be found to present an almost homogeneous appearance, whilst the surface of the seed proper will be seen to consist of narrow or elongated cells or fibres disposed in a vertical direction, while the inner white portion will be seen to be made up of cells filled with starch corpuscles, well defined, of medium size, and resembling in the elongated and stellate hilum the starch granules of the pea and bean.
When the annotta is manufactured, and an unadulterated sample is examined, but little structure is met with. Portions of the outer cells may be seen; and in those samples which in the course of their preparation have not been subjected to the action of boiling water, a few starch granules may be observed.
Since annotta, when manufactured, presents so few evidences of structure, we are easily able, with the microscope at our command, to detect the presence of most foreign vegetable substances. These consist of turmeric powder, wheat, rye and barley starch, and sago flours. The salt and alkali present in the fraudulent annotta generally greatly alter the appearance of the turmeric. Most of the colouring matter of the cells is discharged, so that the starch corpuscles contained within them become visible. Loose starch granules of turmeric may also be frequently seen, and in a much enlarged condition, owing to the action of the alkali upon them.
The following process for conducting the assay of annotta is given by Mr Blyth:—
“In order to estimate the commercial value and detect adulteration in a sample, the quickest and best way is the following: Weigh accurately a gramme in a small platinum dish; dry in the water-bath for a couple of hours, then weigh; the loss is the water. Finely powder, and digest it for some hours in alcohol; then boil, filter and treat with successive portions of alcohol until all the colouring-matter is dissolved; filter, evaporate the filtrate down and weigh; the result is the resin. The insoluble portion will in a good commercial specimen consist of woody matter, extractive, gluten, &c. For the ash weigh another gramme in a platinum dish; dry for a short time over the water-bath; then powder and burn until it ceases to lose weight. It is prudent to fuse a little on charcoal with carbonate of soda before the blow-pipe before burning it in a platinum vessel, as there may be lead in the annotta. The ash should then be submitted to the various reagents in order to detect lime, alumina, &c. A correct determination of ash and resin is all that is required to definitely pronounce upon the purity or impurity of the samples.”
The following is the analysis of a fair commercial sample:—
The sample was in the form of a paste, colour deep red, odour peculiar, but not disagreeable.157
| Water | 24·2 |
| Resinous colouring matter | 28·8 |
| Ash | 22·5 |
| Starch and extractive matter | 24·5 |
| ——— | |
| 100·0 |
The following is an analysis of an adulterated specimen. The sample was in a hard cake of a brown colour, with the maker’s name stamped upon it, and marked “patent;” texture hard and leathery, odour disagreeable:
| Water | 13·4 |
| Resin | 11·0 |
| Ash, consisting of iron, chalk, salt, alumina, silica | 48·3 |
| Extractive matter | 27·3 |
| ——— | |
| 100·0 |
Thus, in the one the resin was 28%, the ash 22; in the other the resin was only 11%, the ash no less than 48%.
Uses, &c. To colour varnishes and lacquers; as a pigment for painting velvet and transparencies; as a colouring matter for cheese (1 oz. to 1 cwt. of curd), for which purpose it is not injurious, if pure; and as a dye-stuff for cotton, silk, and wool, particularly the second, to which it imparts a beautiful orange-yellow hue, the shade of which may be varied from ‘aurora’ to deep orange by using different proportions of pearlash with the water it is dissolved in, and by applying different mordants before putting it into the dye-bath, or different rinsing liquids afterwards. The hues thus imparted are, however, all more or less fugitive.
Annotta Cake. Syn. Flag annotta; Orlea′na in fo′liis, L. From Cayenne; bright yellow, firm and soft to the touch; in square cakes, weighing 2 or 3 lbs. each.
Annotta Egg. Syn. Lump annotta; Orlea′na in o′vulis, L. Generally inferior.
Annotta, Eng′lish. Syn. Trade a., Reduced’ a.; Orlea′na reduc′ta, L. A fraudulent mess commonly prepared from egg or flag annotta, gum tragacanth, flour, or farina, chalk, soap, train-oil, Venetian red, or bole, common salt, water, mixed by heat in a copper pan, and formed into rolls. Sold for genuine annotta, from which it is readily distinguished by its inferior quality and its partial solubility in alcohol.
Annotta, Liq′uid. See Solution of Annotta (below).
Annotta, Pu′′rified. See Orelline.
Annotta Roll. Syn. Orlea′na in rot′ulis, O. in bac′ulis, L. From the Brazils; hard, dry, brown outside, yellow within. When pure, this is the variety most esteemed, and the one preferred for colouring cheese.
Annotta, Solu′tion of. Syn. Essence of Annotta, Extract of a., Annotta-dye, &c.; Solu′tio orlea′næ, Extrac′tum o., &c., L. A strong aqueous solution of equal parts of annotta and pearlash, the whole being heated or boiled together until the ingredients are dissolved. Sold in bottles. See Annotta (above), Nankeen Dye, &c.
ANNUALS. Plants which bear flowers and fruit in the same year when raised from seed.
AN′O-. [Gr.] In composition, upwards, &c.; as in anocathar′tic (emetic).
AN′ODE. Literally, ‘upward way,’ in electro-chemistry, the ‘way in,’ or that by which the electric current is supposed to enter substances through which it passes, as opposed to the CATHODE, or that by which it goes out; the positive pole of a voltaic battery.
AN′ODYNE (-dīne). Syn. Ano′dynus (-dĭnŭs-), L.; Anodin, Fr.; Schmerzstillend, Ger. That allays pain; soothing; atalgic.
Anodynes. Syn. Ano′dyna (sing., ano′dy̆̆num), L.; Anodins, Remèdes a., Fr. In medicine and pharmacy, substances and agents which allay pain. Some (as the PAREGORICS) act by actually assuaging pain; others (HYPNOTICS) by inducing sleep; whilst a third class (NARCOTICS) give ease by stupefying the senses, or by lessening the susceptibility to pain. Among the principal anodynes are opium, morphia, henbane, camphor ether, chloroform, chloral hydrate, and other medicines of the like kind; to which must be added spirituous liquors, wines, and the stronger varieties of malt liquor. “The frequent use of anodynes begets the necessity of their continuance.” (W. Cooley.)
Anodyne, In′fantile (-īle). Syn. Ano′dynum infan′tile (-tĭl-e), L. Prep. Take of syrup of poppies, 1 oz.; aniseed-water, 3 oz.; French brandy, 3⁄4 oz. (or rectified spirit, 1⁄2 oz.); calcined magnesia, 1⁄4 oz.; mix. An excellent anodyne and antacid for infants.—Dose. A small teaspoonful as required.
ANODYN (Müller, Berlin.) Chiefly for rheumatic pains, toothache, &c. Oil of rosemary, 30 drops; oil of thyme, 10 drops; camphor, 5 grms.; spirit of ammonia, 12 grms.; spirit, 60 grms. (Hager.)
ANODYN′IA (-dĭn′-y′ă). Freedom from pain; anæsthesia.
AN′OREXY. Syn. Anorex′ia, L.; Anorexie, Fr., Ger. In pathology, want of, or morbidly diminished appetite, without loathing of food. It is usually symptomatic of other affections. See Appetite, Dyspepsia, &c.
ANOSMIN FOOT POWDER (Dr Oscar Bernar, Vienna). “An unfailing remedy for sweaty feet and bad odour of the feet.” Powdered alum, 21 parts; maize meal, 1 part. (Hager.)
ANOSMIN FOOT WATER (Koch), for a similar purpose. An aqueous solution of tartaric acid.
ANO ZABAGLIONE (-băl-y′ō′-nā). Prep. Put 2 eggs, 3 teaspoonfuls of sugar, and 2 small glassfuls of sherry or marsala, into a chocolate cup, placed in boiling water, or over the fire, and keep the mixture rapidly stirred until it begins to rise and thicken a little; then add158 1 or 2 teaspoonfuls of orange-flower water or rose water, and serve it up in wine-glasses. A pleasant Italian domestic remedy for a cold.
ANT (ănt). Syn. Emm′et, Pis′mire*‡ (pĭz′-); Formi′ca, L.; Fourmi, Fr.; Ameise, Ger.; Æmet, Sax. This well-known little insect belongs to the family formic′′idæ, and the order hymenop′tera. Like the bee, it is a social animal, lives in communities which may be compared to well-regulated republics, and is of three sexes—male, female, neuter. Those belonging to the last alone labour and take care of the ova and young. The red ant contains FORMIC ACID (acid of ants), and a peculiar RESINOUS OIL. Both of these may be obtained by maceration in rectified spirit. A tincture so prepared, and flavoured with aromatics, constitutes Hoffman’s Eau de Magnanimité, once greatly esteemed as an aphrodisiac. See Formica, Formic Acid, Formyle, &c.
ANTAC′ID (-tăs′-ĭd). Syn. Antac′idus, L.; Antacide, &c., Fr.; Säuretilgend, &c., Ger. An agent which neutralises acids or removes acidity. (See below.)
ANTAC′IDS (-tăs′-ĭdz). Syn. Antac′ida, L.; Antacides, &c., Fr. Antacid substances. In medicine, &c., substances which remove or prevent acidity of the stomach, and thus tend to relieve heartburn, dyspepsia, and diarrhœa.
The principal antacids are potassa, soda, ammonia, lime, and magnesia, with their carbonates and bicarbonates. Ammonia is one of the most powerful, and when the acidity is conjoined with nausea and faintness, or is accompanied with symptoms of nervous derangement or hysteria, is undoubtedly the best; when great irritability of the coats of the stomach exist, POTASH is to be preferred; when the acidity is accompanied with diarrhœa, carbonate of lime (prepared chalk), lime-water, or Carara-water; and when with costiveness, MAGNESIA. They may be advantageously combined with some simple aromatic, as ginger, cinnamon, or peppermint. Their preparation, doses, administration, &c., will be found under each in its alphabetical place; and formulæ containing them, under Draughts, Lozenges, Mixtures, &c.
ANTAL′GICS (-tăl′-). Syn. Antal′gica, L. Medicines which relieve pain; anodynes.
ANTAL′KALINES (ănt-ăl′-kă-lĭnz). Syn. Antalkali′na, L. Agents or medicines which correct alkalinity. All the acids except the carbonic are antalkaline.
AN′TE-. In composition, before, contrary, opposite; generally in the first sense. See Anti-.
ANTEPIDEMICUM UNIVERSALE (H. Müller, Copenhagen). “A valuable universal remedy for all sorts of contagious diseases in man or domestic animals.” A fluid like water, with a weak, almost imperceptible, odour of acetic ether. Is composed of spring water, in which perhaps two or three drops of pure carbolic acid are dissolved, and a few drops of acetic ether added to disguise it. (Hager.)
ANTHELMIN′TICS, Anthelmin′thics (-thĕl-). See Vermifuges and Worms.
AN′THIARINE (-ĭn). See Anthirine.
ANTHOK′YAN. Syn. Succ′us vi′olæ prepara′tus, L. The expressed juice of the sweet or purple violet (vi′ola odora′ta—Linn.), defecated, gently heated in glass or earthenware to 192° Fahr., then skimmed, cooled, and filtered; a little rectified spirit is next added, and the following day the whole is again filtered. It must be kept well corked, and in a cool situation.
Uses, &c. Chiefly to make syrup of violets, to colour and flavour liqueurs, and as a chemical test. The London druggists obtain it principally from Lincolnshire.
AN′THONY’S FIRE, Saint (-to-nĭz). See Erysipelas.
ANTHOSENZ (Dr Hess, Berlin). General tonic and anodyne balsam. Oil of cloves, 4 parts; oil of geranium, 2 parts; pine-apple essence, 1 part; spirit, 50 parts; coloured with alkanet root. (Hager.)
AN′THOTYPE. See Photography.
ANTHRACENE. C14H10. Anthracene is one of the last products passing over in the dry distillation of coal-tar. Dr Calvert says it is “found most abundantly in the ten or fifteen per cent. which comes over between the temperature at which soft pitch is produced and that at which hard pitch is formed.”
Coal-tar contains very variable quantities of anthracene, those tars procured from coals which are richest in naphtha yielding it most abundantly. The coals of South Staffordshire give the largest yield, whilst the Newcastle coals give very little. In consequence of the solubility of anthracene in the oily hydrocarbons which accompany it, owing to “slight elevation of temperature, its extraction can only be carried on advantageously in cold weather.”
Gessert prepares anthracene from coal-tar as follows: He places the last pasty portions (the ‘green grease’) of the coal-tar distillation (which must not be carried beyond the point at which white pitch is formed) first in a centrifugal machine, and then in a hydraulic press at 40°, or subjects the mass heated to 30°-40° directly to pressure in a filter press. The pressed mass consists of about 60% of anthracene; for further purification it is boiled with light tar-oil or petroleum naphtha, and finally heated till it melts. The residue contains 95% of anthracene.
The following method for the purification of crude anthracene contaminated with oily matters is by Schuller:—The crude anthracene is carefully heated to commencing ebullition in a capacious retort connected with a tubulated receiver of glass or earthenware, the lower aperture of which is closed with a fine wire sieve. A strong current of air is159 then blown into the retort with a pair of bellows, whereby the anthracene is driven over in a very short time nearly pure and dry, and condenses in the receiver as a faintly yellowish showy mass. By this method a quantity of anthracene, the purification of which by re-crystallisation or sublimation would take several days, may be purified in as many hours; moreover it is obtained in a pulverulent form, in which it is very readily acted on by oxidising agents. Anthraquinone prepared from crude anthracene may also be obtained by this method in the form of a light yellow powder, resembling flowers of sulphur.
Fritzsche obtained anthracene in crystals exhibiting a beautiful violet colour by exposing a solution of anthracene in coal-tar naphtha to sunshine, until the solution became colourless.
Pure anthracene assumes the form of fluorescent transparent crystals, consisting of four- or six-sided plates, which when seen by transmitted light are of a very pale blue colour, but of a pale violet by reflected light.
The process for obtaining pure anthracene is a very troublesome one. Mr Crookes says:—“A trustworthy method for determining the amount of pure anthracene either in commercial anthracene or in crude green grease is the following:—The melting-point of the sample in question is first determined. 5 to 10 grammes are sufficient for the operation. It is put between thick folds of blotting paper, and placed under a press, between plates which have been previously warmed. The anthracene remaining upon the paper after pressure is weighed. The residue after it has been boiled with a certain quantity of alcohol, filtered, washed with cold alcohol and dried, is weighed as pure anthracene. It is now advisable to determine the melting-point of the purified product, which will generally be 210° C.” Anthracene is only slightly soluble in alcohol, but rather more so in ether and bisulphide of carbon. It is more soluble in hot, but less so in cold benzene. Petroleum boiling between 160° and 195° F. dissolves less than benzene.
“Anthracene dissolves in concentrated sulphuric acid with a green colour, and forms conjugated monsulpho or bisulpho-anthracene acid, according to the temperature employed. Chlorine and bromine give rise to substitution products. Nitric acid acts on it with great violence, with formation of anthraquinone, nitro-anthraquinone, and other compounds according to the temperature and proportion of the substances taken. With picric acid anthracene forms a compound crystallising in very bright ruby-red needles, which by the aid of the microscope are seen to be prisms. To prepare it a saturated solution of picric acid in water at 80° F. is mixed with a saturated solution of anthracene in boiling alcohol; on cooling the compound is deposited in the crystalline state. It is rapidly decomposed by an excess of alcohol into picric acid and anthracene, the solution assuming a yellow tint. This reaction can be employed to distinguish anthracene from naphthalene and other hydrocarbons, naphthalin under similar circumstances forming a compound which crystallises in fine golden yellow needles, whilst chrysene gives rise to clusters of very small yellow needles.” (Calvert’s ‘Dyeing and Calico Printing,’ edited by Stenhouse and Groves). Another characteristic of anthracene, noticed by Fritzsche, is its deportment under the microscope with a solution of binitro-anthraquinone in benzene. In this reaction fine rhomboidal scales of a beautiful pink colour are formed, the purity and brilliancy of the colour depending on the purity of the anthracene.
In the ‘Bul. Soc. Chim.,’ vii, 274, several reactions by which anthracene is formed are described by Berthelot, as by the action of heat on other hydrocarbons, or by passing the vapours of ethylene, styrolene, and benzene through a porcelain tube heated to bright redness.
A great number of products are procured from anthracene, by far the most important of these being artificial alizarin.
See Alizarin, Artificial.
AN′THRACITE (sīte). [Eng., Fr.] Syn. Anthrac′olite, Glance′-coal, Stone′-coal‡, Mineral char′coal*; Anthraci′tes, L.; Glanzkohle, Ger. A species of coal found in the transition-rock formation, consisting chiefly of dense carbon. It has a conchoidal fracture, a semi-metallic lustre, and a sp. gr. usually varying from 1·4 to 1·6. It burns without either flame or smoke, emits an intense heat, and leaves scarcely any ash; but it is difficult to kindle, and requires a lively draught for its combustion. It is the common fuel in the United States of America, although, until recently, scarcely employed in Europe, and that chiefly in a few iron works and steam furnaces. Its adoption in this country would not merely at once remove the smoke nuisance, but would produce a vast annual saving to the community. By contracting the throat of the chimney a little, and avoiding the use of the poker, it may be burnt in a common grate. The Americans use a little charcoal as kindle, and seldom supply fresh coal to the fire oftener than once or twice a day.
The inferior varieties of anthracite are technically and provincially called culm; as is also the small and waste of the better kinds.
For the analysis, geology, calorific value, &c., of anthracite, see Coal, Culm, Evaporation, Fuel, Heat, &c.
De la Beche describes Anthracite as “a variety of coal containing a larger proportion of carbon, and less bituminous matter, than common coal.”
In the ‘Memoirs of the Geological Survey’ we read:—“We see the same series of coal beds becoming so altered in their horizontal160 range that a set of beds bituminous in one locality is observed gradually to change into anthracitic in another. Taking the coal measures of South Wales and Monmouthshire, we have a series of accumulations in which the coal-beds become not only more anthracitic toward the west, but also exhibit this change in a plane which may be considered as dipping south-south-east, at a moderate angle, the amount of which is not yet clearly ascertained, so that in the natural sections afforded, we have bituminous coals in the high grounds and anthracite coals beneath. This fact is readily observed either in the Neath or Swansea valleys, where we have bituminous coals on the south and anthracite on the north; and more bituminous coal-beds on the heights than beneath, some distance up these valleys, those of the Nedd and Tawe. Though the terms bituminous coal and anthracite, have been applied to marked differences, the changes are that there is no sudden modification to be seen. To some of the intermediate kinds the term “free burning” has been given, and thus three chief differences have been recognised.”
The term Culm is applied both to an inferior kind of anthracite only worked for lime-making and mixing with clay and to the small pieces of anthracite obtained in working the beds of true anthracite. It is also known under the names of Blind-coal, Glance-coal, and Kilkenny-coal.
There are three distinct trades in anthracite. The first one is that where the coal is sold just as it is brought from the pit. This is termed Through Culm, and is used for lime-burning. This coal is inferior in quality to that from which the large coal has been removed, and is sometimes called Bastard Stone-coal. The trade in the Neath district is exclusively of this kind. In Swansea and Llanelly it is partly of this kind and partly of the kind where the large coal is picked out and sold as stone-coal for the various purposes to which that coal is put, the small pieces being left for shipment to places where it is required for lime-burning, under the name of stone-coal culm. No “through culm” is shipped from Pembrokeshire. Four thousand tons almost in the condition of dust are annually shipped from Swansea, under the name of Lambskin, being sent to Cardiganshire, where it is used solely for mixing with clay. This mixture, which is known under the name of Fireballs, is used for household purposes. This mixture, made of the ordinary stone-coal culm, is also in very general use throughout parts of Pembrokeshire and Carmarthenshire.
Anthracite coal is found in this country at Bideford in Devonshire, at Walsall in Staffordshire, in the western divisions of the South Wales coalfield, in Ireland, and near Edinburgh. It is very abundant in America. In the ‘Transactions of the American Geologists’ it is stated by Professor Roger that in the great Apalachian coal-field, 720 miles in extent, with a chief breadth of 180 miles, the coal is bituminous towards the western limit, where it is level and unbroken, becoming anthracite towards the south-west, where it becomes disturbed. Anthracite coal is also found in the coal-measures of France, more particularly in the departments of Isère, the high Alps, Gard, Mayenne, and of Sarth. About 42,271,000 kilogrammes (of 22,046 avoirdupois pounds each) form the annual yield. Anthracite is also obtained in Belgium. “Anthracite is not an original variety of coal, but a modification of the same beds which remain bituminous in other parts of the region. Anthracite beds, therefore, are not separate deposits in another sea, nor coal-measures in161 another area, nor interpolations among bituminous coal; but the bituminous beds themselves altered into a natural coke, from which the volatile bituminous oils and gases have been driven off.”—Lesley on Coal.
| Locality. | Name of Coal. | Carbon. | Volatile matter. | Ashes. |
| Bituminous. | ||||
| Birtley Works, Newcastle-on-Tyne | 60·50 | 35·50 | 4·00 | |
| Alfreton, Derbyshire | 52·46 | 42·50 | 2·04 | |
| Anthracite. | ||||
| Neath Abbey | Pwlferon Vein, 5th bed | 91·08 | 8·00 | 0·92 |
| Swansea | Peacock Coal | 89·00 | 7·50 | 3·50 |
| Ystalyfera | Brass Vein | 92·46 | 6·04 | 1·50 |
| Cwm Neath | Nine-feet Vein | 93·12 | 5·22 | 1·50 |
| France | Anthracite, common | 79·15 | 7·35 | 13·25 |
| ” | Côte-d’Or | 82·60 | 8·60 | 8·80 |
| ” | Mais Saize | 83·80 | 7·50 | 9·50 |
| Pennsylvania | Beaver Meadow | 92·30 | 6·42 | 1·28 |
| ” | Shenoweth Vein | 94·10 | 1·40 | 4·50 |
| ” | Black Spring Gap | 80·57 | 7·15 | 3·28 |
| ” | Nealey’s Tunnel | 89·20 | 5·40 | 5·40 |
| Massachusetts | Mansfield Mine | 97·00 | 10·50 | 3·00 |
| Rhode Island | Portsmouth Mine | 85·84 | 10·50 | 3·66 |
| Westphalia | Shafberg, Alexander Seam | 82·02 | 8·69 | 9·29 |
Anthracite, the exclusive employment of which is for iron-making, steam engines, and for domestic uses in the United States, was some 60 years since regarded as incombustible refuse, and as such looked upon as rubbish and thrown away.
The foregoing analyses of bituminous and anthracite coals will sufficiently show the difference between the two.
| Specific Gravity. | Weight of a cubic yard in lbs. | ||
| South Wales— | Swansea | 1·263 | 2131 |
| Cyfarthfa | 1·337 | 2256 | |
| Ynscedwin | 1·354 | 2284 | |
| Average | 1·445 | 2278 | |
| Ireland— | Mean | 1·445 | 2376 |
| France— | Allier | 1·380 | 2207 |
| Tantal | 1·390 | 2283 | |
| Brassac | 1·430 | 2413 | |
| Belgium— | Mons | 1·307 | 2105 |
| Westphalia | 1·305 | 2278 | |
| Prussian Saxony | 1·466 | 2474 | |
| Saxony | 1·300 | 2193 | |
| Average of Europe | 2281 |
| Pennsylvania— | |||
| Lyken’s Valley | 1·327 | 2240 | |
| Lebanon Co., Grey Vein | 1·379 | 2327 | |
| Schuylkin Co., Lorberry Creek | 1·472 | 2484 | |
| Pottsville, Sharp Mount | 1·412 | 2382 | |
| Peach | 1·446 | 2440 | |
| Salem Vein | 1·574 | 2649 | |
| Tamaqua, North Vein | 1·600 | 2700 | |
| Maunch Chunk | 1·550 | 2615 | |
| Nesquehoning | 1·558 | 2646 | |
| Wilkesbarre, best | 1·472 | 2884 | |
| West Mahoney | 1·371 | 2313 | |
| Beaver Meadow | 1·600 | 2700 | |
| Girardville | 1·600 | 2700 | |
| Hazelton | 1·550 | 2615 | |
| Broad Mountain | 1·700 | 2869 | |
| Lackawanna | 1·609 | 2715 | |
| Massachusetts— | Mansfield | 1·710 | 2882 |
| Rhode Island— | Portsmouth | 1·810 | 3054 |
| Average in United States | 2601 | ||
The calorific value of anthracite coal is well shown by the following results from Dr Fyfe’s experiments, to compare Scotch and English bituminous coals with anthracite, in regard to their evaporative power, in a high-pressure boiler of a 4-horse engine having a grate with 8·15 square feet of surface; also in a waggon-shaped copper boiler, open to the air, surface 18 feet, grate 1·55:—
| Kind of Fuel employed. | A | B | C | D | E | F | G | H | Remarks. |
| Middlerig Scotch coal | 81·33 | 9 | 45° | 6·66 | 7·74 | 10·00 | 44·27 | ... | Pressure 17 lbs. per square in. |
| Scotch coal, different variety from preceding | 108 | 5 | 170° | 6·62 | 6·89 | 13·25 | 33·33 | ... | Ditto. |
| Anthracite | 47·94 | 81⁄2 | 45° | 8·73 | 10·10 | 5·88 | 75·09 | ... | Ditto. |
| Scotch coal, from near Edinburgh | 8·24 | 81⁄2 | 50° | 5·38 | 6·90 | 5·31 | 436·89 | 3·15 | Lower pressure, open copper boiler. |
| English bituminous coal | 6·07 | 8·4 | 50° | 7·84 | 9·07 | 3·91 | 503·08 | 3·06 | Ditto. |
Space will not admit of our entering fully into the question of the evaporative power of anthracite, but its advantages under certain conditions are fully established.
AN′THRACOKA′LI. [Eng., L.] Syn. Anthrakoka′li, Anthrak′ali; An′thracoka′li, Hamb. C. 1845. Prep. 1. (Polya.) Carbonate of potassa, 6 oz.; quick-lime, 31⁄2 oz.; water, 4 pints; proceed as directed for solution of potassa, then evaporate the clear liquid, in an iron capsule, to about 6 fl. oz., add of finely powdered mineral coal 5 oz., boil, with constant stirring, to dryness, and continue the stirring at a reduced heat, until the whole is converted into a homogeneous black powder, which must be at once placed in small, dry, and well-stoppered phials.
2. (Hamb. C. 1845; Ph. Baden, 1841.)162 Hydrate of potassa, 7 dr.; melt, add of cannel coal, 5 dr., and then proceed as before.
Prop. &c. A deliquescent black powder, with a caustic taste, and empyreumatic smell; 10 gr. with 1 fl. oz. of water, after filtration, forms a clear, dark brown solution, giving a precipitate with acids, without effervescence.—Dose, 1 to 3 gr., twice or thrice daily; and externally, made into a pomade or ointment (1⁄2 to 1 dr., to lard, 1 oz.); in skin diseases (particularly herpetic eruptions), scrofula, chronic rheumatism, &c. It has been highly extolled by Dr Gilbert, and by its inventor, Dr Polya; but apparently undeservedly.
Anthracokali Sulphuretted. Syn. Anthracokali sulphuretum, L. Prep. (Polya.) As formula 1 (above), but adding sulphur, 4 dr., immediately after stirring in the powdered coal.—Dose, use, &c., as the last. See Fuligokali.
ANTHRACOM′ETER. Syn. Antracom′etrum, L.; Anthracomètre, Fr.; Kohlensäuremesser, Ger. An apparatus used to determine the heating power or commercial value of coal, or other fuel; also an instrument for finding the proportion of carbonic acid in any gaseous mixture.
ANTHRAPURPURIN. C14H8O5.—A colouring matter obtained as a secondary product in the preparation of alizarin from anthracen. It may be prepared by dissolving the crude colouring matter in a dilute solution of carbonate of soda, and shaking up the resulting solution with freshly precipitated alumina, which combines with the alizarin, leaving the anthrapurpurin in solution. This is filtered off from the alizarin lake, heated to boiling, and acidified with hydrochloric acid. The colouring matter which is precipitated is thrown on to a filter, washed and dried.
Anthrapurpurin has about the same affinity for mordants as alizarin. It forms red with alumina, and purple and black with iron mordants. The reds are much purer and less blue in colour than those of the alizarin, whilst the purples are bluer and the blacks more intense. The anthrapurpurin colours resist soap and light quite as effectively as those produced with alizarin. When employed to dye Turkey-red, anthrapurpurin gives a very brilliant scarlet shade of colour, which is of remarkable durability.
ANTHYPNOTICS (-thĭp-). Syn. Antihypnot′ics (-hĭp-), &c. See Agripnotics.
AN′TI-. [Gr., αντι, against.] In composition, before, against, contrary to, corrective of, &c., more especially representing antagonism or opposition; whilst the Latin ante- is generally used in the sense of before, having reference to precedence either of place or time.
Anti- is a common prefix in English words derived from the Greek and Latin, especially those connected with pharmacology and medicine, the final i being either dropped or retained (but generally the first) before a, e, and h; as in antacid, antibilious, anti-emetic, anthelmintic, anti-corbutic, antiseptic, &c., whether used as adjectives or substantives. These compounds, which are very numerous, are in general self-explanatory.
AN′TIARINE (-ĭn; -ti′—Brande). [Eng., Fr.] Syn. An′thiarine, Eng., Fr.; Antiari′na, Anthiari′na, Antia′′ria, Upa′sia (-zh′ă), L. The active principle of the upas poison of Java. It is extracted from the partially inspissated juice (upas poison) of the upas tree by alcohol, and may be obtained under the form of small pearly crystalline scales by careful evaporation.—Prod. About 31⁄2% (Mulder).
Prop., &c. Soluble in 27 parts of boiling water; freely soluble in alcohol; scarcely so in ether; heat decomposes it. It is a frightful poison, to which no antidote is known. Even a minute quantity introduced into a wound rapidly brings on vomiting, convulsions, and death. “It renders the heart insensible to the stimulus of the blood.” (Sir B. Brodie.)
ANTI-ATTRI′′TION (-trĭsh′-) [Eng., Fr.] Syn. Antifriction Grease, Axle-grease, Friction compo′, Lu′bricating compound, &c. Prep. 1. Good plumbago (black lead), finely powdered and sifted, so as to be perfectly free from grit, is gradually added, through a sieve, to 5 times its weight of good lard contained in an iron pan and rendered semi-fluid, but not liquid, by a gentle heat; the mass being vigorously stirred with a strong wooden spatula, after each addition, until the mixture is complete, and the composition smooth and uniform. The heat is then gradually raised until the whole liquefies, when the vessel is removed from the fire to a cool situation, and the stirring, which should have been unremitted, continued until the mixture is quite cold. It is applied in the cold state, with a brush, about once a day, according to the velocity of the parts; and is said to be fully 3-4ths cheaper in use than oil, tallow, tar, or any of the ordinary compo’s. When intended for uses in which it will be exposed to warmth, and consequent waste by dripping, a part, or even the whole of the lard is replaced by hard strained grease or tallow, or a little bees’ wax is added during its manufacture.
2. Black lead, 1 part; tallow or grease, 4 parts; ground together until perfectly smooth, either with or without camphor, 3 to 5 lbs. per cwt. Expired patent.
3. Scotch soda, 60 lbs.; water, 30 galls.; dissolve in a capacious boiler, and palm oil and hard tallow, of each 11⁄4 cwt., and having withdrawn the heat, stir vigorously as before, until the mass is homogeneous and nearly solidified. In hot weather the proportion of tallow is increased, and that of the palm oil diminished; in winter, the reverse. Used for the axles of railway carriages and other coarse purposes. For express trains all tallow163 is usually employed, irrespective of the weather or season.
4. Melt, but avoid boiling, 16 lbs. tallow, and dissolve in it 21⁄4 lbs. of sugar of lead; then add 3 lbs. of black antimony. The mixture must be constantly stirred till cold. This composition is for cooling the necks of shafts, and may be of service where the shafts are not of the proper length, or the bearings are at fault.
5. Lard, 21⁄2 lbs.; camphor, 1 oz.; black lead, 1⁄2 lb. Rub the camphor in a mortar, into a paste with a small portion of the lard; then add the remainder of the lard and the black lead, and thoroughly mix.
6. (Railway Grease.)—For summer use, tallow, 1 cwt. 3 qrs.; palm oil, 1 cwt. 1 qr. For autumn or spring, tallow, 1 cwt. 2 qrs.; palm oil, 1 cwt. 2 qrs. For winter, tallow, 1 cwt. 1 qr.; palm oil, 1 cwt. 3 qrs. Melt the tallow in a boiler, then add to it the palm oil as soon as the mixture boils, and put out the fire. When the mixture, which should now be frequently stirred, has cooled down to blood heat (98° to 100° F.), it should be run through a sieve into a solution of from 56 to 60 lbs. of soda in about 3 galls. of water. Thoroughly mix by stirring.
7. Bean or rye flour, 1 cwt.; water, 6 cwt.; mix to a smooth paste, raise the heat until the mixture boils, and stir in first of milk of lime (of about the consistence of cream), 7 cwt.; resin-oil, 10 cwt.; and stir vigorously until cold. Inferior.
8. (Booth’s.)—a. From Scotch soda, 1⁄2 lb.; boiling water, 1 gall.; palm oil or tallow, or any mixture of them, 10 lbs.; as before, observing to continue the stirring until the mixture has cooled down to 60° or 70° Fahr.
b. Soda, 1⁄2 lb.; water and rape-oil, of each 1 gall.; tallow or palm-oil, 1⁄2 lb.; as last. Expired patent.
9. (Mankettrick’s.) From caoutchouc (dissolved in oil of turpentine), 4 lbs.; Scotch soda, 10 lbs.; glue, 1 lb.; (dissolved in) water, 10 galls.; oil, 10 galls.; thoroughly incorporated by assiduous stirring, adding the caoutchouc last.
10. (Liard, Fr.). Finest rape-oil, 1 gall.; caoutchouc (cut small), 3 oz.; dissolve with heat.
Uses, &c. To lessen friction in machinery, prevent the bearings rusting, &c. The simplest are perhaps the best. Of late years several different liquid hydrocarbons obtained from coal, and particularly paraffin oil, have been extensively employed in this way. See Friction, Lubrication, &c.
ANTIBIL′IOUS (-yŭs). Syn. Antibilio′sus, L.; Antibilieux, Fr. An epithet of medicines that are supposed to remove ailments depending on disordered action of the liver. Aperients, mercurials, and aloetic purgatives generally, belong to this class. See Abernethy Medicines, Bile, Pills, &c.
ANTICAR′DIUM. See Reviver (Black).
ANTI-CHOLERA ACID (H. Ludwig, Vienna; also an American preparation). “A proved cure and preventive of cholera.” Diluted sulphuric acid, 1 part; wine, 5 parts; water, 10 parts. (Hager, Buchner, and Wittstein.)
ANTI-CHOLERA WATER (Eau Anticholerique de Duboc, Paris), for lead colic and a preventive of cholera. Composed of water with some brandy and 1⁄2 per cent. of sulphuric acid. (Gmelin.)
AN′TICHLORE (-klōre). Among bleachers, any substance, agent, or means, by which the pernicious after-affects of chlorine are prevented. Washing with a weak solution of sulphite of soda (which converts any adhering ‘bleaching salt’ into sulphate, sulphide, or chloride) is commonly adopted for this purpose. Recently chloride of tin, used in the same way, has been recommended. A cheap sulphite of lime, prepared by agitating milk of lime with the fumes of burning sulphur, and draining and air-drying the product, has been lately patented in England and America, by Prof. Horsford, under the name of ‘Antichloride of lime,’ See Bleaching, &c.
AN′TIDOTE (-dōte). [Eng., Fr.] Syn. Antid′otum, Antid′otus, L.; Antidot, Gegengift, Ger. In medicine, toxicology, &c., a substance administered to counteract or lessen the effects of poison.
The principal poisons, with their antidotes, are noticed under their respective heads. Also see Poisons, Toxicology, &c.
ANTI-EPILEPTICUM (Wepler, Berlin), known as Wepler’s Krampfpulver. Magnesia alba, 5 parts; rad. dictamni, 15 parts; rad. zedoar, 12 parts; rad. artemis, 8 parts; soot, 1⁄2 part; ol. valerian, 1⁄2 part; ol. cajeputi, 1⁄4 part.
Dr Hager is the authority for the above, and he adds that formerly the same proprietor sold a remedy which consisted of a black powder made by carbonising hempen thread.
ANTIFER′MENT (pop. and more us., in this sense, an′tiferment′). [Eng., Fr.] Syn. Antifermen′tum, L. Any substance which prevents or arrests fermentation. Several nostrums are sold under this name in the cider-districts. The following are tried and useful formulæ:—
Prep. 1. Sulphite (not sulphate) of lime, in fine powder, 1 part; marble-dust, ground oyster-shells, or chalk, 7 parts; mix, and pack tight, so as to exclude the air.
2. Sulphite (not sulphate) of potassa, 1 part; new black-mustard seed (ground in a pepper-mill), 7 parts; mix, and pack so as to perfectly exclude air and moisture. Dose (of either), 1⁄2 oz. to 11⁄2 oz. per hhd.
3. Mustard-seed, 14 lbs.; cloves and capsicum, of each 11⁄4 lb.; mix, and grind them to powder in a pepper-mill. Dose, 1⁄4 to 1⁄2 lb. per hhd.
Uses, &c. The above formulæ are infinitely164 superior to those commonly met with in trade; and are quite harmless. A portion of any one of them added to cider, or perry, soon allays fermentation, when excessive, or when it has been renewed. The first formula is preferred when there is a tendency to acidity. The second and third may be advantageously used for wine and beer, as well as for cider. That of the third formula greatly improves the flavour and the apparent strength of the liquor, and also improves its keeping qualities. See Cellar-management, Fermentation, &c.
ANTI-FRIC′TION METAL. Prep. 1. From tin, 16 to 20 parts; antimony, 2 parts; lead, 1 part; fused together, and then blended with copper, 80 parts. Used where there is much friction or high velocity.
2. Zinc, 6 parts; tin, 1 part; copper, 20 parts. Used when the metal is exposed to violent shocks.
3. Lead, 1 part; tin, 2 parts; zinc, 4 parts; copper, 68 parts. Used when the metal is exposed to heat.
4. (Babbet’s.) Tin, 48 to 50 parts; antimony, 5 parts; copper, 1 part.
5. (Fenton’s.) Tin with some zinc, and a little copper.
6. (Ordinary.) Tin, or hard pewter, with or without a small portion of antimony or copper. Without the last it is apt to spread out under the weight of heavy machinery. Used for the bearings of locomotive engines, &c.
Obs. These alloys are usually supported by bearings of brass, into which it is poured after they have been tinned, and heated and put together with an exact model of the axle, or other working piece, plastic clay being previously applied, in the usual manner, as a lute or outer mould. Soft gun-metal is also excellent, and is much used for bearings. They all become less heated in working than the harder metals, and less grease or oil is consequently required when they are used. See Alloys, Friction, &c.
ANTIGUG′GLER. A small bent tube of glass or metal inserted into casks and carboys, to admit air over the liquor whilst it is being poured out or drawn off, so that the sediment may not be disturbed.
ANTIHECTICUM POTERII. Fuse together 4 parts of regulus of antimony, and 51⁄2 of fine tin; pour it on a metal plate, reduce it to powder, and deflagrate it in a red-hot crucible with 15 parts of nitre; keep it hot for some time, then wash it, and dry it with a gentle heat.—Dose, two to ten grains in hectic fevers.
ANTILITHIC. See Lithontryptics.
ANTIMO′′NIAL (-mōne′y-′ăl).[67] [Eng., Fr.] Syn. Antimonia′lis, L. Pertaining to, composed of, or containing antimony. In medicine and pharmacy, applied to preparations or remedies (Antimo′′nials; Antimonia′′lia, L.) in which antimony, or one of its compounds, is the leading or characteristic ingredient.
ANTIMO′NIATED. Syn. Antimonia′tus, L. Mixed or impregnated with antimony; antimonial.
ANTIMON′IC ACID. Syn. Acidum antimon′icum, L.; Acide antimonique, Fr.; Antimonsäure, Ger.
Prep. 1. Pure metallic antimony, in coarse powder, or small fragments, is digested in excess of concentrated nitric acid, until the oxidation and conversion is complete; the excess of nitric acid is then removed by evaporation nearly to dryness, and the residuum thrown into cold distilled water; after which the powder (ANTIMONIC ACID) is collected on a calico filter, washed with distilled water, and dried by a gentle heat. Pure.
2. Metallic antimony (in powder), 1 part; powdered nitre, 6 or 8 parts; are mixed and ignited or deflagrated in a silver crucible; the mass, when cold, is powdered; the excess of alkali washed out with hot water, and the residuum (ANTIMONIATE OF POTASSIUM) decomposed with hydrochloric acid; lastly, the precipitate (ANTIMONIC ACID) is washed and dried as before.
That obtained by the first process is dibasic, and has the formula H2Sb2O6, while that produced by the second process is tetrabasic, and has the formula H4Sb2O7; the former is called simply antimonic acid, the latter metantimonic acid.
Prop. Antimonic acid is a soft white powder, sparingly soluble in water, reddens litmus, and is dissolved, even in the cold, by strong hydrochloric acid and by potash. The hydrochloric solution, mixed with a small quantity
of water, yields, after a while, a precipitate of antimonic acid; but if diluted with a large quantity of water, it remains clear. Ammonia does not dissolve it in the cold. By heating with a large excess of caustic potash it is converted into metantimonic acid.
Metantimonic acid is more readily dissolved by acids than antimonic acid, and is dissolved by ammonia, after a while, even at ordinary temperatures. It is also perfectly soluble in a large quantity of water, and is precipitated therefrom by acids. It is very unstable, and easily changes into antimonic acid, even in water.
ANTIMONIC ANHYDRIDE (Sb2O5). Syn. Antimonic Oxide, Anhydrous Antimonic Acid, Pentoxide of Antimony. Antimonic or metantimonic acid, heated to a temperature below redness, loses water and yields the anhydride, Sb2O5. Antimonic anhydride is a yellowish-white powder, tasteless and insoluble in water and acids. Boiled with a solution of caustic potash, it is dissolved. If fused with carbonate of potassium, carbonic anhydride is expelled, and a salt is produced from which antimonic acid is precipitated by acids.
ANTIMONIOUS ACID. See Antimony, Tetroxide of.
AN′TIMONETTED. Syn. Antimo′′niuretted; Antimonia′tus, L. Combined with or containing antimony. See Hydrogen, 165&c.
AN′TIMONY (-te-mŭn-e). Syn. Metal′lic antimony*, Reg′ulus of a.†; Antimo′′nium, A. metal′licum, Stib′ium, Metal′lum antimo′′nii†, A. reg′ulus†, &c., L.; Antimoine, Fr.; Antimon, Spiessglanz, Spiessglas, Spiessglanzmetall, Ger.; Antimonio, It., Sp. The term formerly applied to the native sulphide or greyish-black semi-crystalline ore of antimony; but now solely appropriated to the pure metal.
Sources. Metallic antimony, in combination with silver and iron (NATIVE ANTIMONY), with sulphur (GREY SULPHIDE OF A.), or with nickel (NICKELIF′EROUS SULPHIDE OF A.) is found in Bohemia, Hungary, Germany, Sweden, France, England, Borneo, and America; and oxidised, combined with oxide of iron, &c. (ANTIMO′′NIAL, O′CHRE, RED ANTIMONY, WHITE A.[68]), forming ores, either small in quantity or of little value, in various parts of the world. Of these the only one in sufficient abundance for smelting is the common sulphide known as ‘grey antimony’ or ‘stibnite.’
a, b, Grate and fire-place.
c, Bridge.
d, Air-channel.
e, Concave space for ore, resting on a solid bed f, formed of sand and clay.
g, Door for introducing the ore, and abstracting residuary slag.
h, Pipe to convey away the liquid metal.
i, Chimney.
Prep. Native antimony is freed from impurities by fusion. The sulphide, after being melted from the gangue, is commonly oxidised by exposure on the concave hearth of a reverberatory furnace, and is then reduced to the metallic state by fusion in crucibles with coal-dust, crude tartar, or some other deoxidising agent. To free the product from iron, it is generally fused, or re-fused, with a little antimonic oxide; and when the ore contains arsenic, iron, or its oxide, and an alkaline carbonate or sulphate, are used in the same way. It is seldom prepared on the small scale. The following formulæ are in use, or are recommended:—
1. On the SMALL SCALE:—
a. From tersulphide of antimony, in coarse powder, 2 parts; iron filings, 1 part; fused together in a covered crucible, at a heat gradually raised to dull redness.
b. From the teroxide or the oxychloride of antimony, fused together, as before, with twice its weight of crude tartar.
c. (Ph. Castr. Ru. 1840.) Sulphide of antimony, 16 parts; cream of tartar, 6 parts; both in powder; throw the mixture, in small quantities at a time, into a vessel (an earthen crucible) heated to redness; when the reaction is over (having closely covered the vessel), fuse the mass, and after a quarter of an hour pour it out, and separate the metal from the slag.
d. From sulphide of antimony, 8 parts; crude tartar, 6 parts; nitre, 3 parts; as last.
e. (Wöhler.) Sulphide of antimony, 10 parts; nitre, 12 parts; dry carbonate of soda, 15 parts; deflagrate together; powder the resulting mass, and wash it thoroughly with boiling water; lastly, smelt the dried residuum with black flux. All the preceding are nearly pure; the impurity, if any, being traces of copper, lead, or iron.
f. (Berzelius.) From metallic antimony, in fine powder, 2 parts; teroxide of antimony, 1 part; fused together. The product will be pure provided the antimony employed is free from lead.
g. (Muspratt.) From antimony, 9 parts; peroxide of manganese, 1 part; fused together; the resulting metal being re-fused with 1-10th of its weight of carbonate of soda.
2. On the LARGE SCALE—commercial:—
a. See above (before 1 a.).
b. From sulphide of antimony, 100 parts; iron (in very small scraps), 40 parts; dry crude sulphate of soda, 10 parts; powdered charcoal, 21⁄2 parts; fused together.—Prod. 60 to 65 parts of antimony, besides the scoriæ or ash, which is also valuable.
c. (Berthier.) Sulphide of antimony, 100 parts; hammerschlag (rough oxide or iron from the shingling or rolling mills), 60 parts; crude carbonate or sulphate of soda, 45 to 50 parts; charcoal powder, 10 parts; as last.—Prod. 65 to 70 parts.
Prop., &c. Bluish-white, lustrous, with a lamellar texture, and a crystalline or semi-crystalline fracture, with fern-leaf markings on the surface, when pure (star antimony); extremely brittle (may be powdered); imparts brittleness to its alloys (even 1-1000th part added to gold renders it unfit for the purposes of coinage and the arts); melts at 809-810° Fahr., or just under redness; fumes, boils, and volatilises at a white heat, and, when suddenly exposed to the air, inflames with conversion into the teroxide, which is deposited in beautiful flowers or crystals; when perfectly pure and fused without contact with air or foreign matter, it bears an intense heat without subliming (Thénard); allowed to cool slowly from a state of perfect fusion, it crystallises in octahedrons or dodecahedrons; tarnishes, but does not rust by exposure to air or moisture at common temperatures; hot hydrochloric acid dissolves it, with the formation of TRICHLORIDE OF ANTIMONY; nitric acid, when concentrated, converts it into ANTIMONIC ACID; and when166 dilute, into TRIOXIDE OF ANTIMONY. Sp. gr. 6·7 to 6·8.[69]
Tests. Metallic antimony may be recognised by the above properties; its oxide, salts, &c., by the following reactions:—1. Sulphuretted hydrogen gives, with acid solutions, an orange-red precipitate, which is sparingly soluble in ammonia,[70] and insoluble in dilute acids; but readily soluble in pure potassa and alkaline sulphides, and in hot hydrochloric acid with the evolution of sulphuretted hydrogen gas:—2. Sulphydrate of ammonium gives an orange-red precipitate, readily soluble in excess of the precipitant, if this latter contains sulphur in excess; and the liquor containing the re-dissolved precipitate gives a yellow or orange-yellow precipitate on the addition of an acid:—3. Ammonia and potassa, and their carbonates, give (except in solutions of tartar emetic) a bulky white precipitate; that with ammonia and its carbonate being insoluble in excess of the precipitant; that with potassa, readily so; whilst that with carbonate of potassium is only soluble on the application of heat:—4. A rod of zinc throws down metallic antimony, as a black powder, from all its solutions not containing free nitric acid. If the experiment be made with a few drops of a solution of antimony containing a little free hydrochloric acid, and a small platinum dish or capsule be employed, the part covered by the liquid is soon stained brown or blackish, and the stain is irremovable by cold hydrochloric acid, but may be easily removed by warm nitric acid:—5. By ebullition of the acidulated liquid along with copper gauze, foil, or wire, as noticed under ‘Reinsch’s Test.’[71] The peculiar violet-grey of the deposit is characteristic, and may easily be distinguished from that given by arsenical solutions:—6. Mixed with dilute sulphuric acid and poured on some metallic zinc in a gas-generating flask, provided with a small bent tube (see engr.), it yields ANTIMONETTED HYDROGEN (Marsh’s test), recognised by burning with a bluish-green flame, and furnishing dense white fumes which adhere readily to any cold substance (as a porcelain plate) held over it; or, if the plate be depressed upon the flame, a deep black, and almost lustreless spot of metallic antimony; the fumes and spots in both cases being insoluble in water, and in dilute solution of chloride (crude hypochlorite) of soda. On heating the centre of the tube to redness with a spirit lamp, the bluish-green colour of the flame lessens in intensity, and a mirror of metallic antimony, of silvery lustre, forms inside the tube at the ignited part. On passing dry sulphuretted hydrogen through the tube, still heated by a spirit lamp, this mirror assumes a reddish-yellow colour, approaching black in its thicker parts; and by exposure to a feeble stream of hydrochloric acid gas, almost immediately, or in a few seconds, disappears, being carried off by the gas, which, if passed into a little distilled water, yields a solution of chloride of antimony, which may be further submitted to any of the usual tests.[71] If the substance be in the solid state, it must be reduced to powder and dissolved in water; or if insoluble in that menstruum, a solution must be obtained by digestion in either hot hydrochloric or nitrohydrochloric acid, before proceeding to examine it by this method.
a, Flask containing the suspected fluid, dilute sulphuric acid, and zinc.
b, Small tube, at the one end having an almost capillary orifice, where the gas is inflamed.
c, Spirit-lamp.
d, Support.
Estim. Antimony is generally WEIGHED under the form of tersulphide; but sometimes as antimonious anhydride, and—though more seldom—as pure metal:—
1. A solution being obtained as above, if necessary, it is strongly acidulated with tartaric acid, and the antimony thrown down as a sulphide by a stream of sulphuretted hydrogen. After warming the solution and allowing it to cool, the precipitate (TERSULPHIDE) is collected on a filter, dried, and weighed. A small portion digested in strong hydrochloric acid will completely dissolve if it be the pure sulphide; in which case the quantity of ANTIMONY sought will be equal to 711⁄2% (71·5%) of the weight of the sulphide found (very nearly).[72] Should only part of the precipitate be soluble, a known weight of it may be introduced into a flask, and a considerable quantity of fuming nitric acid added, drop by drop, and afterwards, a little hydrochloric acid, the mixture being digested, at a gentle heat, until the reaction is complete, and the whole of the sulphur is dissolved. The resulting solution diluted with water, strongly acidulated with tartaric acid, and solution of chloride of barium added as long as it disturbs the liquid, yields a precipitate, of which the weight, after it has been thoroughly washed, dried, and gently ignited, multiplied by 136, gives the quantity of SULPHUR in the sample; and which, deducted from the weight of the sulphide first found, gives the quantity of pure ANTIMONY, as before.
2. The quantity of PURE ANTIMONY in commercial samples may be determined by treating them (in powder) with nitric acid, which oxidises the antimony and leaves it in an insoluble state, whilst it dissolves the other metals. The resulting oxide is collected on a filter, washed, dried, ignited in an open porcelain crucible, and weighed—its weight multiplied by ·7898 gives the quantity of pure metal sought.
3. Dissolve a known weight of the sample in hydrochloric acid, immerse a blade of pure metallic tin in the solution, and keep the liquor acidulous, and in a state of gentle ebullition by the heat of a sand bath, when the whole of the ANTIMONY will be precipitated under the form of a black powder, and may be collected, washed, dried, and weighed. This is particularly adapted to alloys of antimony and tin. See Tests (above) and Pur. (below).
Pur. The antimony of commerce generally contains a little arsenic, with variable quantities of iron, lead, sulphur, and tin. These impurities may be thus detected:—
1. (Arsenic.) By fusing the sample, in powder, mixed with about an equal weight of tartrate or bitartrate of potassium, in a covered crucible, for 2 or 3 hours, and placing the resulting button, which is an alloy of antimony and potassium, in a ‘Marsh’s apparatus’ along with a little water, when the disengagement of hydrogen gas will commence, and may be tested in the usual manner. See Arsenic.
2. (Iron.) Dissolve the powdered sample in nitrohydrochloric acid, dilute the solution with a large quantity of cold water, filter, and pass a current of sulphuretted hydrogen through the filtrate as long as it produces a precipitate; again filter, boil the filtered liquor for a few minutes to drive off the sulphuretted hydrogen, and then test it with ferrocyanide of potassium, which will give a blue precipitate if iron be present; or supersaturate the last filtrate with ammonia, and then add hydrosulphydrate of ammonium, when, under like conditions, a black precipitate will be formed.
3. (Lead.) Digest the powdered sample in hot nitric acid, which will dissolve out the LEAD but leave the antimony behind. The whitish powdery residuum may be washed, dried, ignited, and weighed, as above; the clear decanted liquor may now be mixed with the first washings, evaporated to dryness, the residuum re-dissolved in water, and the solution submitted to reagents (see Lead). If lead is found to be present, a solution of sulphate of sodium may be added until it ceases to disturb the liquid, and the resulting precipitate (sulphate of lead) washed, dried, and gently ignited (alone) in a porcelain crucible; the weight of the ignited residuum furnishes a number which, multiplied by ·683, gives the weight of the LEAD sought.
4. (Sulphur.) The solution in nitrohydrochloric acid, when tested with either nitrate or chloride of barium, gives a white precipitate of sulphate of barium, insoluble in both water and acids, which when dried, ignited, and weighed, and the weight multiplied by ·136, gives the quantity of SULPHUR as before. In this case, as with the sulphides (see above), free sulphur maybe removed by digesting and washing the powdered sample in bisulphide of carbon, previous to its solution in the acid, by which the violence of the subsequent reaction will be lessened.
5. (Tin.) Two samples of equal weight are taken; the one is tested for ANTIMONY, as described above; the other is dissolved in a mixture of equal parts of hydrochloric and nitrohydrochloric acid, and a blade of zinc immersed in the solution (see above); the mixed precipitate of tin and antimony which forms is collected on a weighed filter, washed, dried, and weighed. The weight of antimony in the first sample subtracted from that now obtained, leaves a remainder which indicates the quantity of TIN in the original sample.
Phys. eff., &c. Nearly all the salts and preparations of antimony are emetic and cathartic, and in large doses poisonous—occasioning vomiting, profuse alvine dejections, acute colic, and inflammation of the stomach and bowels, often serious, though rarely resulting in death. Tartar emetic and BUTTER OF ANTIMONY are those from which accidents have principally occurred.—Ant., &c. Copious vomiting, if it has not already occurred, should be promoted, and the recently prepared hydrated sulphide of iron administered in considerable doses, followed or accompanied by mucilaginous drinks and diuretics. If much prostration follows, wine and stimulants may be had recourse to. In the absence of hydrated sulphide of iron, a solution of tannin, or decoction of galls; cinchona, or oak bark, or even powdered cinchona, mixed with tepid water, may be administered.
Uses. In the arts, antimony enters into the composition of several useful alloys, as TYPE-METAL, PEWTER, BRITANNIA-METAL, MUSIC-PLATE METAL, &c. It is added to the alloy for concave mirrors, to give them a finer texture; to bell metal, to render it more sonorous; and to various other metals to increase their hardness and fusibility; for the latter purpose it is employed in the casting of cannon balls.
Concluding Remarks. In ‘roasting’ or oxidising the native sulphide of antimony on the bed of the reverberatory furnace, as in the common method before referred to, care must be taken to regulate and gradually raise the heat, which, until towards the end of the process, need not be extreme, and then only should it approach dull redness. Without this precaution much of the undecomposed sulphide will be lost by volatilisation. During the whole time the ‘charge’ should also be well stirred with an iron spatula, to ensure the constant exposure of every part of it to the atmosphere. The process is complete when168 the whole mass assumes a greyish-white appearance. Earthen crucibles are commonly employed for the subsequent reduction, and after being charged and covered over with ground charcoal, are heated in a reverberatory furnace. The product is the crude metallic antimony of commerce. It is generally REFINED by smelting it with about 1-8th of its weight of the refined sulphide, and about 1-4th of its weight of carbonate or sulphate of soda; but if there be much iron present, more of the sulphide—even 1-4th—may be required; for unless there be sufficient sulphur to combine with the whole of the iron, the arsenic will not be oxidised, but remain as a contamination. When cold, the metal is carefully separated from the slag, and is frequently re-fused with a little fresh carbonate of soda (1 to 11⁄2 part); after which it is cast into pigs, lumps, or ingots. The crude metal, thus treated, commonly yields 94% of REFINED METAL of tolerable purity.
Should lead have been present in the sulphide or ore, it remains after a second, or even a third fusion, although proportionately reduced in quantity; and it can only be completely separated in the humid way. It is, therefore, always desirable to select an ore free from lead.
Antimony, Ash of. Syn. Antimony-ash, Calcined’ antimony*; Ci′nis antimo′′nii, Antimo′′nium calcina′tum*, L. Prepared by roasting the common grey sulphide of antimony on an iron plate set under a chimney, to carry off the fumes. The product is a mixture of teroxide of antimony, with some unburnt sulphide, and a little antimonious acid.
Prop., &c. Ash-grey; emetic in small doses. Used chiefly as a cheap substitute for teroxide of antimony by the manufacturers of tartar emetic; also to make metallic antimony.
Antimony, Butt′er of. See Antimony, Trichloride of.
Antimony, Calx of. Syn. Calx antimo′′nii, L. Sometimes applied to antimony-ash, but more commonly to crude, unwashed diaphoretic antimony.
Antimony, Calx of (Sul′phurated). Syn. Antimo′′nii calx sulphura′ta, L. Prep. (Hufeland.) Calcined oyster-shells, 10 parts; sulphur, 4 parts; crude antimony, 3 parts; powder, mix, and calcine in a luted crucible for an hour. Emetic, resolvent, and alterative.—Dose, 1 to 6 gr.; in gout, rheumatism, scrofula, &c.
Antimony, Ce′ruse of. Syn. Antimo′′nii cerus′sa, L. Prep. (Bate.) As diaphoretic antimony (over which it possesses no advantage), merely using the metal instead of the sulphide.
An old preparation made by igniting antimony in the sun’s rays, by means of a lens, was called ANTIMONII CERUSSA SOLA′′RIS.
Antimony, Chlo′′rides of (klōre′-īdz):—
1. Antimony, Trichloride of. SbCl2. Syn. Terchloride of antimony, Antimonious chloride, Chlo′′ride of antimony, Sesquichloride of a., Butter of a., Cau′stic antimony†, &c.; Antimo′′nii chlori′dum, A. TERCHLORI′DUM, A. bu′tyrum*, &c., L.; Chlorure d’antimoine, Beurre d’antimoine, &c., Fr.; Antimon-chlorid, Spiessglanz-butter, Ger. This is the substance of which common chloride, or butter of antimony, of the shops, is an impure concentrated solution containing free acid.
Prep. 1. Solid, anhydrous:—
a. Pure commercial tersulphide of antimony, in coarse powder, 1 part; concentrated hydrochloric acid, 5 parts; are mixed in a capacious stoneware or glass vessel set under a chimney with a quick draught, to convey away the fumes, the whole being constantly stirred, and, as the effervescence slackens, a gradually increasing gentle heat applied until solution is complete; the resulting liquid is put into a retort, and distilled, until each drop of the distillate, as it falls into the aqueous liquid which has previously passed over into the receiver, produces a copious white precipitate; the receiver is then changed, and the distillation continued, when pure TRICHLORIDE OF ANTIMONY passes over, and solidifies on cooling to a white and highly crystalline mass, which must be carefully excluded from the air.
b. From pure metallic antimony, 2 parts; bichloride of mercury, 5 parts; both in fine powder; mixed and distilled in a retort with a large neck, by a gentle sand-heat, into a suitable receiver. Chemically pure.
2. Liquid:—
a. (Liquor antimonii chloridi, B. P.) Syn. Solution of chloride of antimony.
Prep. Take of black antimony, 1 lb.; hydrochloric acid, 4 pints; place the black antimony in a porcelain vessel; pour upon it the hydrochloric acid, and, constantly stirring, apply to the mixture, beneath a flue with a good draught, a gentle heat, which must be gradually augmented as the evolution of gas begins to slacken, until the liquid boils. Maintain it at this temperature for fifteen minutes; then remove the vessel from the fire, and filter the liquid through calico into another vessel, returning what passes through first, that a perfectly clear solution may be obtained. Boil this down to the bulk of two pints, and preserve it in a stoppered bottle.
Characters and Tests. A heavy liquid, usually of a yellowish-red colour. A little of it dropped into water gives a white precipitate, and the filtered solution lets fall a copious deposit on the addition of nitrate of silver. If the white precipitate formed by water be treated with sulphuretted hydrogen it becomes orange-coloured. The specific gravity of the solution is 1·47. One fluid drachm of it mixed with a solution of a quarter of an ounce of tartaric acid in four fluid ounces of water, forms a clear solution, which, if treated with sulphuretted hydrogen, gives an orange precipitate,169 weighing, when washed and dried at 212°, at least 22 grains.
b. (Commercial.)—a. Take of ash or calx of antimony, 31⁄4 lbs.; common salt, 2 lbs.; oil of vitriol, 11⁄2 lb.; water, 1 lb.; proceed as before. Prod., 21⁄2 lbs.
c. From roasted sulphide or glass of antimony, 7 lbs.; salt, 28 lbs.; oil of vitriol, 21 lbs.; water, 14 lbs.; as before.
d. From crude sulphide of antimony (powdered), 25 lbs.; strongest commercial hydrochloric acid, 1 cwt.; nitric acid, 31⁄2 lbs.; as before; the product being coloured with a little pernitrate of iron, and made up to the sp. gr. 1·4. The quality is improved, and the process more easily conducted, if the crude antimony is roasted before dissolving it in the acid. The same applies to the other formulæ.
Prop., &c.—a. Solid. When pure, and nearly free from water, it somewhat resembles butter, melts with a gentle heat, and partially crystallises on cooling; is very deliquescent, and quickly passes into an oily liquid when exposed to damp air; very soluble in strong hydrochloric acid; water, according to its quantity, more or less decomposes it. When perfectly pure and anhydrous, it forms a white and highly crystalline mass, rapidly decomposed by air and moisture.—b. Solution. The sp. gr. of the solution of the shops varies from 1·25 to 1·4, in which state it is a transparent fuming yellow liquid (unless when artificially coloured), and extremely acid and caustic. Submitted to distillation, it at first parts with its water and excess of acid, after which the salt itself is volatilised. By changing the receiver as soon as the distillate concretes on cooling, or produces a copious white precipitate on falling into the liquid already passed over, the pure ANHYDROUS TRICHLORIDE may be readily obtained.
Phys. eff., Ant., Lesions, &c. See Antimony.
Uses. In medicine, only externally, and chiefly as a caustic or escharotic to the wounds caused by rabid and venomous animals, and to repress excessive granulations in ulcers. In pharmacy, as a source of both oxychloride and oxide of antimony. The residuum in the retort when corrosive sublimate is used, is sulphide of mercury, and was formerly called CINNABAR OF ANTIMONY.
2. Antimony, Pentachlo′′ride of. Sb2Cl5. Syn. Perchlo′′ride of antimony; Antimo′′nii pentachlori′dum, L. Prepared by passing a stream of chlorine gas over metallic antimony in fine powder, and gently heated. A mixture of TRICHLORIDE and PENTACHLORIDE OF ANTIMONY is found in the receiver, from which the latter may be separated by careful distillation. It is a colourless volatile liquid, forming a crystalline compound with a small quantity of water, but decomposed by a larger quantity.
Antimony, Cro′cus of. Syn. Saff′ron of antimony, Liv′er of a.; Cro′cus antimo′′nii C. metallo′′rum, He′par antimonii, L.; Crocus d’antimoine, Saffran d’a., Fr. Prep. 1. From black sulphide of antimony, and saltpetre, equal parts, deflagrated together by small portions at a time, and the fused mass (separated from the scoriæ) reduced to fine powder.
2. (Ant. crocus, Ph. L. 1788,) Sulphide of antimony, 1 lb.; nitre, 1 lb.; common salt, 1 oz.; as before.
Prop., &c. Its medicinal properties closely resemble those of diaphoretic antimony. It is a mixture of sulphate of potassium, antimoniate of potassium, teroxide of antimony, oxysulphide of antimony, sulphide of potassium, and undecomposed trisulphide of antimony, in variable and undetermined proportions. When repeatedly washed or boiled in water, and dried, it forms the WASHED SAFFRON OF ANTIMONY (C. A. LO′TUS, L.) of old pharmacy, and has then lost its sulphate of potassium, caustic potash, and sulphide of potassium. Formerly used to make tartar emetic. See Antimony, Liver of.
Antimony, Crude. Native sulphide of antimony melted from the gangue.
Antimony, Diaphoret′ic. Syn. Calx of antimony, Calcined’ a., Antimo′′niate of pot′ash, Stib′iated ka′li†, Diaphoretic min′eral†, &c.; Antimo′′nium diaphoret′icum, A. calcina′tum, Calx antimo′′nii, C. a. anglo′′rum†, POTAS′SÆ ANTIMO′′NIAS, Kali stib′icum†, &c., L. var.; Antimoine diaphorétique, Biantimoniate de potasse, Fr. An old preparation with numerous synonyms, of which the first two of the above are those which are now chiefly in use.
Prep. 1. Sulphide of antimony, 1 part; nitre, 3 parts; powder, mix, and deflagrate by spoonfuls in a red-hot crucible, then calcine for half an hour, and when cold powder the residuum.
2. Washed diaphoretic a., W. calx of a.; Antimonium diaphoreticum lo′tum, A. d. ablu′tum (Ph. Bor. 1847), A. calcina′tum (Ph. L. 1788); Antimoine diaphorétique lavé, &c., Fr.:—a. (Ph. L. 1788.) As the last, but the powder is subsequently deprived of soluble matter by repeated washings with water, after which it is collected and dried.
b. (Ph. Bor. 1847.) Metallic antimony, 1 part; nitre, 2 parts; as above, but drying the washed powder at a heat not exceeding 104° F.
Prop., &c. A white or greyish-white powder, without either smell or taste; gently diaphoretic and laxative; its activity greatly depending on the quantity of acid in the stomach.—Dose, 1 to 6 gr., or even 10 gr.; for horses, 1 to 3 or 4 dr. It was formerly in high repute; but is now almost superseded by the present pharmacopœial preparations.
Antimony, E′thiops of. Syn. Æ′thiops antimonia′lis, L. Prep. 1. From metallic mercury, 1 part; sulphide of antimony, 2 parts; triturated together until the globules of the former entirely disappear.—2. Sulphide of antimony,170 3 parts; black sulphide of mercury, 2 parts; triturated together for some time. An old remedy in certain skin diseases, still highly esteemed by some provincial practitioners.—Dose, 3 to 5 gr., gradually increased to 20 or 30 gr.
Antimony, Flow′ers of. Syn. Flo′′res antimo′′nii, L.; Fleurs d′antimoine, Fr. Prep. Throw powdered sulphide of antimony, by spoonfuls at a time, into an ignited tubulated retort with a short and very wide neck, until as many ‘flowers’ collect in the receiver as are required. An impure oxysulphide of antimony, with variable portions of trioxide, and undecomposed tersulphide. Emetic in doses of 1 to 3 grains.
Antimony, Flowers of (Ar′gentine). [-ĭn.] Syn. White ox′ide of antimony, Snow of a.†; Antimo′′nii flo′′res argenti′ni, A. nix†, L.; Fleurs argentine d’antimoine, Oxyde blanc d’antimoine, Fr. Prep. Melt metallic antimony in a vessel freely exposed to the air, and furnished with a cool place for the ‘flowers’ to rest on, and collect them as deposited; or, and what is better, heat the metal to a full red or white heat in a covered crucible, and then suddenly expose it to the air, when it will inflame, and the oxidised vapour condense as ‘flowers’ on any cool surface (as a partially inverted wide-mouthed flask) held at a little distance over it. The product is TRIOXIDE OF ANTIMONY in a crystalline form, and received the name of argentine flowers from its silvery whiteness and beauty.
Antimony, Flowers of (Helmont’s). Syn. Flo′′res antimo′′nii Helmon′tii. An old preparation formed by dissolving sulphide of antimony in aqua regia, expelling the free water and acid by heat, and subliming the residuum with an equal weight of sal ammoniac. Violently emetic, even in small doses, and unfit for internal use.
Antimony, Flowers of (Red). Syn. Flo′′res antimo′′nii ru′bri, L. From sulphide of antimony, and sal ammoniac, both in fine powder, mixed and sublimed together. Resembles the last.
Antimony, Ful′minating. See Fulminating Compounds.
Antimony, Glass of. Syn. Vit′rified antimony*, V. ox′ide of a.*, Grey o. of a.*; Antimo′′nii vit′rum, Antimo′′nium vitrifica′tum, A. vitrifac′tum (Ph. L. 1788), Ox′ydum antimonii vitrificatum, &c., L.; Verre d’antimoine, Oxysulfure d’antimoine silicaté, Fr. Prep. (Ph. L. 1788.) Roast sulphide of antimony in a shallow earthen vessel, over a moderate fire, stirring it constantly with an iron rod, until it turns whitish-grey and ceases to emit fumes at a red heat; put the residuum into a covered crucible which it shall only two thirds fill, and expose it to an intense heat (gradually raised), until it fuses, then pour it out on an iron plate. If calcined too much, a little more crude antimony may be added to make it run well.
Comp., Prop., &c. A mixture of sulphide and oxide of antimony contaminated with a little silica and iron. In fine powder it is emetic, in doses of 1 to 3 gr.; but owing to the uncertainty and violence of its operation, is now seldom employed. It has been used as a cheap source of the TEROXIDE by the manufacturers of tartar emetic.
Antimony, Glass of (Cera′′ted). Syn. Antimo′′nii vit′rum cera′tum, L. Prep. (Dr Young & Ph. L. 1746.) Glass of antimony, in very fine powder, 1 oz.; yellow wax, 1 dr.; melt together in an iron ladle, and keep it over a gentle fire free from flame (constantly stirring) for about half an hour, or until it acquires a snuff colour, then pour it out on a piece of white paper (or a plate), and when cold, powder it.—Dose, 2 to 10 gr., in dysentery, &c.
Antimony, Li′ver of. Syn. He′par antimo′′nii, L.; Hépar d’antimoine, Oxysulfure d’antimoine silicaté, Fr. Prep. From sulphide of antimony, 1 part; and dry carbonate of sodium or potassium, 2 parts; melted together, and heated until it acquires the proper colour, and then cooled and powdered.
Comp., Uses, &c. A mixture of trioxide of antimony, sulphide of potassium, carbonate of potassium, and undecomposed trisulphide of antimony. It is chiefly used by farriers, in doses of 1 to 2 dr., as an alterative purge for horses, in greasy heels, &c.; and sometimes by chemists, as a source of the crude oxide. Crocus of antimony, before noticed, sometimes passes under the name, and is sold for it.
Antimony, Ore of. Syn. Antimony-ore. Native sulphide of antimony.
Antimony, Oxide of. The B. P. name for Antimony, Trioxide of (which see).
Antimony, Oxides of. Antimony forms with oxygen three definite compounds, viz the—
| Trioxide or antimonious oxide | Sb2O3 |
| Tetroxide or antimonoso-antimonic oxide | Sb2O4 or Sb2O3 or Sb2O5 |
| Pentoxide or antimonic oxide | Sb2O5 |
Antimony, Trioxide of. Sb2O3. Syn. Teroxide of antimony, Antimonious oxide (B. P. Oxide of antimony, Eng.; Antimonii oxidum, L.). Prep. (B. P.) Take of solution of chloride of antimony, 16 fluid oz.; carbonate of soda, 6 oz.; water, 2 galls.; distilled water, a sufficiency. Pour the antimonial solution into the water, mix thoroughly, let the precipitate settle, remove the supernatant liquid by a siphon, add one gallon of distilled water, agitate well, let the precipitate subside, again withdraw the fluid, and repeat the processes of affusion of distilled water, agitation, and subsidence. Add now the carbonate of soda previously dissolved in two pints of distilled water, leave them in contact for half an hour, stirring frequently, collect the deposit on171 a calico filter, and wash with boiling distilled water until the washings cease to give a precipitate with a solution of nitrate of silver acidulated by nitric acid. Lastly, dry the product at a heat not exceeding 212°.
Char. and Tests. A greyish-white powder, fusible at a low red heat, insoluble in water, but readily dissolved by hydrochloric acid. The solution, dropped into distilled water, gives a white deposit, at once changed to orange by sulphuretted hydrogen. It dissolves entirely when boiled with an excess of the acid tartrate of potash.
Uses. Chiefly in making tartar emetic and some other salts of antimony; also in the preparation of pulvis antimonialis. Therapeutically, it is a diaphoretic and febrifuge.—Dose, 1 to 4 grains.
Antimony, Pentoxide of. See Antimonic Anhydride.
Antimony, Tetroxide of. Sb2O4 or Sb2O3.Sb2O5. Syn. Antimonoso-antimonic oxide, Antimonious acid. Found natural as Cervantite or Antimony ochre. Prepared by heating antimonic anhydride, by roasting the trioxide or trisulphide, or by the action of excess of nitric acid on finely powdered metallic antimony. Thus prepared, it is a white solid, unalterable by heat; slightly soluble in water, more so in hydrochloric acid.
Antimony, Oxychloride of. SbOCl. Syn. Powder of Algaroth. Thrown down as a white precipitate when trichloride of antimony is poured into water. Continued washing with water deprives it of nearly the whole of its chlorine, and converts it into the trioxide, a change which is more completely effected by aqueous solutions of the alkalies or their carbonates.
Antimony, Oxysulphide of. The compound Sb2O3.2Sb2S3 occurs native as red antimony. Antimony blende, Kermesome, Rothspiessglanzerz, Crocus of antimony, Glass of antimony, and similar preparations, are believed by some authorities to be crude oxysulphides of antimony. See Antimony, Sulphurated.
Antimony, Red. See Oxysulphide of Antimony, before noticed.
Antimony, Reg′ulus of. Syn. Reg′ulus antimo′′nii, L. Metallic antimony obtained by fusion. Alloys formed by fusing antimony with iron, tin, lead, or copper, and a little tartar, were respectively called MAR′TIAL REGULUS OF ANTIMONY (r. antimo′′nii martia′lis, L.), R. A. JOVIA′LIS (L.), R. A. SATURNI′NUS (L.), R. A. VEN′ERIS (L.), &c. (See below.)
Antimony, Ru′by of. Syn. Medic′inal (-dĭs′-) REG′ULUS OF ANTIMONY; Antimo′′nii rubi′nus, Reg′ulus medicina′lis, R. a. m., &c., L. From crude sulphide of antimony, 5 parts; fused with carbonate of potassa, 1 part; and the purified portion separated from the scoriæ. See Liver of Antimony.
Antimony, Saff′ron of. See Crocus of Antimony.
Antimony, Smelt′ed. Syn. Antimo′′nium purifica′tum, L. Crude antimony melted and poured into small conical moulds.—Uses, &c. Same as the ordinary tersulphide.
Antimony, Snow of. See Antimony, Flowers of.
Antimony, Sulphurated. B. P. Syn. Oxysulphuret, or Precipitated Sulphide of Antimony, Golden Sulphide of Antimony. Mix black antimony 10 oz. with solution of soda 41⁄2 pints, and boil for two hours, with frequent stirring, adding distilled water occasionally to maintain the same volume. Strain the liquor through calico, and before it cools add to it by degrees dilute sulphuric acid till the latter is in slight excess. Collect the precipitate on a calico filter, wash with distilled water till the washings no longer precipitate with chloride of barium, and dry at a temperature not exceeding 212° F.—Dose, 1 to 5 grains.
Antimony, Sulphantimonate. Syn. Schlippe’s Antimonial Salt. Mix eight parts of effloresced sulphate of soda, six of black antimony, and three of charcoal, and expose to a red-heat in a covered Hessian crucible till the fused mass ceases to throw up a scum. Boil the residue in a porcelain vessel with one part of sulphur and sufficient distilled water, and set the filtered liquor aside for crystallisation.
Antimony, Pentasulphide of (Sb2S5), is a yellowish-red powder, obtained (1) by passing hydrosulphuric acid gas through a mixture of pentachloride of antimony, water, and tartaric acid; or (2) through antimonic anhydride suspended in water. It is insoluble in water; hot hydrochloric acid decomposes it, producing trichloride of antimony, sulphur, and hydrosulphuric acid. With the more basic metallic sulphides it unites to form a class of salts called sulphantimonates.
Antimony, Trisulphide of. Sb2S3. Syn. Tersul′phide of antimony, Sul′phide of a., Sul′phuret of a., Black s. of a., Sesquisul′phuret of a., &c.; L′antimoine sulfure, Sulfure d’antimoine, &c., Fr.; Schwefel-spiessglanz, Anderthalb, &c., Ger. This is the grey or greyish-black substance commonly known as crude antimony, black antimony, or sulphide of antimony, in commerce, and from which the other compounds of antimony are chiefly obtained.
Nat. hist., Sources, &c. See Antimony.
The crude ore is freed from earthy impurities in the following manner:—The crushed ore is submitted to ‘eliquation’ in order to separate the SULPHIDE from the gangue or earthy matter with which it is contaminated; after which it is remelted and run into ‘loaves’ or large cakes, in which form it is sent to market. Formerly the operation was performed by introducing the ore into large pots or crucibles having a hole in the bottom, and which, after being closely covered, were set in a circle around a suitable furnace, by which172 they were heated. At the present time the process is commonly conducted in a ‘reverberatory furnace,’ similar to that figured in the engraving.
a, b, Grate and fire-place.
c, Bridge.
e, Concave space for ore formed by a solid bed (f) of clay and sand, and having a ‘hole’ near the bottom extending nearly horizontally through the wall of the furnace to ‘run off’ the fused sulphide.
g, Door for introducing ore, and removing residuum.
h, Chimney.
i, Damper, chain, and lever.
Native trisulphide of antimony treated in this way and ground to powder constitutes the BLACK ANTIMONY (ANTIMONIUM NIGRUM), B. P.
Antimony, Trisulphide of (artificially prepared). Saturate an aqueous solution of tartar emetic with hydrosulphuric acid; an orange precipitate will be thrown down. This precipitate, when collected on a filter, washed, and dried, is the pure trisulphide.
Prop., &c. (Native.) Anhydrous, inodorous, insipid, opaque, brittle, easily pulverisable, and of a dark leaden-grey or steel colour; it has a striated crystalline texture, and breaks with a rough spicular fracture; is insoluble in both water and alcohol; soluble, with decomposition, in hot strong acids and alkaline solutions; melts at a red heat, and is partly dissipated in white fumes, leaving an impure grey-coloured oxide mixed with some undecomposed tersulphide (ANTIMONY-ASH). Its powder is black, of peculiar richness, and stains the fingers. Sp. gr. 4·6 to 4·62. The pure precipitated (amorphous) tersulphide is of orange colour; is darkened by a gentle heat, with loss of water, and at a higher temperature passes from the amorphous to the crystalline condition, at the same time that it assumes the colour and appearance of the native sulphide. It dissolves in hot hydrochloric acid, evolving hydrosulphuric acid, and producing a solution of trichloride of antimony.
Pur. The crude commercial sulphide frequently contains lead, iron, copper, and arsenic, and sometimes manganese. Its goodness is commonly estimated by its compactness and weight, the largeness and distinctness of the striæ, and the volatility of its sulphide.
Uses, &c. Chiefly as a source of metallic antimony, and of the oxide in the preparation of other antimonials. Exhibited alone, it possesses little activity unless it meets with acid in the primæ viæ, when it occasionally acts with considerable violence both as an emetic and cathartic.—Dose, 10 to 30 gr., in powder; as an alterative and diaphoretic in rheumatism, gout, scrofula, and glandular affections, and in lepra, scabies, and some other skin diseases. It is a favourite alterative in veterinary medicine, particularly in skin diseases. Farriers and grooms frequently mix a little of it with the food of horses to improve their coat and promote their ‘condition,’—Dose. For a HORSE, 1 to 4 dr., in fine powder, often combined with nitre and sulphur; for CATTLE, 1⁄2 to 1 oz., or even 11⁄2 oz.; DOGS, 5 or 6 to 20 or 30 gr.; HOGS, 20 to 30 gr., twice or thrice daily. According to Dr Paris, it is one of the ingredients in Spilsbury’s Drops. It is also an ingredient in Tisane de Feltz.
Antimony, Tartarated. KSbOC4H4O6.Aq. Syn. Tartarized antimony, Tartar emetic, Emetic tartar, Potassio-tartrate of Antimony, Eng.; Antimonium tartaratum, B. P. Prep. Various methods have been devised for the preparation of this compound, but the following, which is taken from the ‘British Pharmacopœia,’ is to be preferred:—
Take of oxide of antimony 5 oz., acid tartrate of potash in fine powder 6 oz., distilled water, 2 pints. Mix the oxide of antimony and acid tartrate of potash with sufficient distilled water to form a paste, and set aside for 24 hours. Then add the remainder of the water, and boil for a quarter of an hour, stirring frequently. Filter, and set aside the clear filtrate to crystallise. Pour off the mother-liquor, evaporate to one third, and set aside, that more crystals may form. Dry the crystals on filtering paper at the temperature of the air.
Char. and Tests. In colourless transparent crystals exhibiting triangular facets, soluble in water, and less so in proof spirit. It decrepitates and blackens upon the application of heat. Its solution in water gives with hydrochloric acid a white precipitate, soluble in excess, and which is not formed if tartaric acid be previously added. Twenty grains dissolve without residue in a fluid ounce of distilled water at 60°, and the solution gives with sulphuretted hydrogen an orange precipitate which, when washed and dried at 212°, weighs 9·91 grains.
Phys. eff., Doses, &c. Externally tartar emetic acts as a powerful local irritant, causing a pustular eruption, which permanently marks the skin; for this purpose it is used in the form of solution, ointment, or plaster. Internally, in small doses (1⁄16 to 1⁄8, or even 1⁄6 gr.), it acts as a diaphoretic and expectorant; in somewhat larger doses (1⁄6 to 1⁄2 gr.) it excites nausea, and sometimes vomiting, occasioning depression and relaxation, especially of the muscular fibre; in larger doses (1 to 2 or 3 gr.) it acts as an emetic and sudorific (and often as a purge), depressing the nervous functions, and producing a feeling of feebleness,173 exhaustion, and relaxation, greater than that caused by other emetics; in certain doses (1⁄2 to 3, or even 4 gr.), it is used as a sedative and antiphlogistic, to reduce the force of the circulation, &c.; in excessive doses it acts as an irritant poison, and has in some instances caused death; and even small doses, frequently administered and long continued, have brought on a state of weakness, prostration, and distaste for food, which has led to a fatal termination. It is usually exhibited dissolved in distilled water, either with or without the addition of a little simple syrup. In acute rheumatism, inflammation of the lungs or pleura, chorea, hydrocephalus, and apoplexy, it is said to have been given in doses of 2 to 4, or even 6 gr., with advantage, by Laennec, Rasori, and others; but these extreme doses are not always safe, and cannot be commendable when smaller ones (1⁄4 to 1⁄2 gr., repeated every two hours) appear equally beneficial, and distress the patient less.[73] In doses of 1⁄2 gr. to 3⁄4 gr. each, combined with calomel, it is a powerful and excellent alterative in acute rheumatism and many skin diseases. Of all our sudorifics it is perhaps the most valuable, and the one most generally available. Triturated with 16 to 20 times its weight of sulphate of potassa, it forms an excellent substitute for antimonial powder and James’s powder, as a diaphoretic, in doses of 2 to 4 gr.
Whenever much gastric or intestinal irritation is present, tartar emetic should be avoided, or very cautiously administered, and then combined with an opiate, or some other sedative. It should also be given with caution to children; as, according to Messrs Goodlad and Noble, even in small doses it sometimes acts as a poison on them.
In veterinary medicine it is employed to promote diaphoresis and expectoration, and to reduce arterial action, particularly in fevers, and catarrhal affections, the dose for HORSES being 20 gr. to 1 dr., or even occasionally 11⁄2 dr., in gruel, thrice daily; also sometimes as a diuretic and vermifuge, in doses of 1 to 2 dr., combined with tin-filings, for 2 or 3 successive days, followed by a purge of aloes. The usual dose for CATTLE is 20 gr. to 1 dr.; SHEEP, 5 or 6 to 20 gr.; SWINE (chiefly as an emetic), 2 to 5 or 6 gr.; DOGS (chiefly as an emetic), 1 to 3 gr. It is sometimes, though seldom, used externally, as a counter-irritant, in chest affections, &c.; but its employment thus requires caution.
Pois., &c. That from large doses has been already noticed under ANTIMONY (which see). In poisoning the treatment is the entire disuse of all antimonials, followed by tonics, a light nutritious diet, the use of lemon-juice or ripe fruit, a little wine, warm baths, and mild restoratives generally.
Antimony, Tar′tarised. See Antimony, Tartarated.
Antimony, Vit′rified. See Antimony, Glass of.
ANTI-MIASMATICUM. A disinfecting powder, manufactured first in Berlin in 1866, and described as “prepared by steam.” Quicklime slaked with a solution of sulphate of iron and mixed with turf ashes, also probably containing some carbolic acid. Fluid anti-miasmaticum is a solution of sulphate of iron in impure acetic acid. (Hager.)
ANTIPHLOGIS′TIC (-flo-jĭs′-). Syn. Antiphlogis′ticus, L.; Antiphlogistique, Fr.; Antiphlogistisch, Ger. In medicine, the common epithet of remedies, agents, and treatment (ANTIPHLOGIS′TICS; ANTIPHLOGISTICA, L.), which lessen inflammatory action, or allay the excited state of the system which accompanies it. Of these the principal are bleeding, purging, a low diet, cooling beverages (as water and acidulous drinks), and sedatives generally.
ANTIPSILOTHRON, for preventing loss of hair (Hegewald, Berlin). A brownish-yellow, clear, pleasant-smelling liquid, which consists of a filtered extract of 2·5 grms. of nutgalls, with 50 grms. strong spirit and 30 grms. water; perfumed with several ethereal oils. The liquid is not made turbid by dilution with water. Sold in square bottles containing about 80 grms. The directions strongly recommend the supplementary use of a Swiss “vegetable oil,” which probably Switzerland has never seen. (Hager.)
ANTI-RHEUMATIC DROPS (Roll, Amsterdam). A turbid, dark-brown liquid, which consists of a solution of spirituous extract of aconite in a decoction of couch-grass root, and to which some tincture of opium with saffron and oil of valerian have been added.
ANTI-RHEUMATIC SALVE, Mrs HUNGERFORD’S (Wedecke, Berlin). Recommended for acute and chronic rheumatism, gout, and nervous pains. Camphor, 1 grm.; carbolic acid, 1 grm.; simple cerate, 12 grms. (Schädler.)
ANTISCORBU′TIC (-skor-bū′-). Syn. Antiscorbu′ticus, L.; Antiscorbutique, Fr.; Antiscorbutisch, Gut wider den scharbock, Ger. Good against scurvy. In medicine, an epithet of remedies, agents, &c. (ANTISCORBU′TICS; ANTISCORBU′TICA, L.), used in scurvy. Lemon-juice, ripe fruit, milk, the salts of potassa, green vegetables, potatoes, meal-bread, fresh meat, and raw or lightly boiled eggs, belong to this class.
ANTISEP′TIC. Syn. Antisep′ticus, L.; Antiseptique, Fr.; Antiseptisch, Fäulnisswidrig, Ger. An epithet of substances, agents, &c. (ANTISEP′TICS; ANTISEP′TICA, L.), that impede, arrest, or prevent putrefaction. The principal antiseptics in common use are culinary salt, saltpetre, spices, sugar, vinegar,174 carbolic acid, creasote, and alcohol; to which may be added intense cold, desiccation, and the exclusion of air. Among ANTISEPTIC MEDICINES, bark, dilute acids, quinine, wine, spirits, camphor, charcoal, and yeast, take the first rank. See Putrefaction, Solutions (Antiseptic), &c.
ANTISPASMOD′IC (-spăz-). Syn. Antispas′tic; Antispasmod′icus, L.; Antispasmodique, Fr.; Krampestillend, Ger. In medicine, an epithet of substances and agents (ANTISPASMOD′ICS; ANTISPASMOD′ICA, L.) which allay spasms and convulsions. It is frequently incorrectly applied to anodynes and narcotics, which soothe pain, but do not repress muscular spasm. Ammonia, assafœtida, bark, camphor, castor, chalybeates, chloral hydrate, chloroform, ether, Indian hemp and cannabine, musk, opium, saffron, and valerian, with many other similar substances, are regarded as antispasmodics.
ANTI-SPASMODIC SYRUP, for hooping-cough (Dessaga, Strasburg). A pleasant syrup, leaving a slightly sharp taste, containing a little carbonate of potash, and faintly coloured with rosaniline. (Hager.)
ANTISUDIN, a remedy for sweaty feet (Mandowski, Annaberg). Powdered alum. (Hager.)
ANTS (ănts). See Ant, Formic Acid, Gardening, Insects, &c.
AORT′A [L., Ger.] Syn. Aorte, Fr. In anatomy, the main trunk of the arterial system, arising immediately from the left ventricle of the heart, and giving origin to all the other arteries of the body, except the pulmonary artery and its ramifications, which permeate the air-vesicles of the lungs.
AP′ATITE (-tīte). In mineralogy, native tricalcium phosphate (phosphate of lime). It is found in Devonshire and Cornwall, and abundantly in Spain, whence it is imported for use as manure, and recently particularly for the manufacture of ARTIFICIAL GUANO. Its powder phosphoresces on burning coals. It differs from phosphorite in not containing fluorine.
Apatite (phosphate of lime of similar constitution to bone-earth, Ca3(PO4)2) is found in every fertile soil, and of which it is an essential ingredient.
APE′′RIENT (ă-pēre′-ĕ-ĕnt; -pĕr′-, as marked by Mayne and Smart, though etym. correct, is less usual). Syn. Aper′itive (-tĭv); Aper′iens, L.; Apéritif, Fr.; Abführend, ÖFFNEND, Ger. In medicine, opening, laxative, gently purgative; usually applied as an epithet to substances and agents (APE′RIENTS; Aperien′tia, Aperiti′va, L.) which, in moderate doses, and under ordinary circumstances, gently, but completely, open the bowels; and in this respect rank between the simple laxatives on the one hand, and the stronger purgatives and cathartics on the other. Among these may be named as examples—Aloes (when combined with soap or aromatics), Castile soap, castor oil, compound extract of colocynth (in small doses), compound rhubarb pill, confection of senna, cream of tartar, Epsom salts, Glauber’s salt, phosphate of soda (tasteless purging salt), pil. rufi, seidlitz powders, cold-water compress over the abdomen, &c. Several of these, in larger doses, become active purgatives or cathartics. See Purgatives, also Draughts, Mixtures, Pills, &c.
A′PIOL (-pe-ōle; or -ŏl). Prep. The soft alcoholic extract of parsley-seed is either digested or agitated for some time with ether; after sufficient repose in a cool place, the ethereal solution is decanted, and the ether removed by distillation; the residuum is purified by solution in rectified spirit, and agitation first with a little litharge, and next with animal charcoal; after which the spirit is removed by distillation from the filtered solution.
Prop., &c. A yellow, oily, non-volatile liquid, having a peculiar smell, and a highly disagreeable taste; soluble in alcohol, ether, and chloroform; insoluble in water; and coloured red by strong sulphuric acid. Sp. gr. 1·078. In small doses it excites the pulse and nervous system; and in larger ones it causes headache, giddiness, vertigo, &c. It is said to be powerfully febrifuge, and has been highly extolled by MM. Joret and Homalle as a substitute for quinine in intermittents.[74] It has also been found useful in intermittent neuralgias and the nocturnal sweats of phthisis. Dose, 5 to 15 drops, in capsules.
A′PIS. [L.] The bee. In entomology, a genus of hymenopterous insects of the family anthoph′ila or mellif′era, section apia′′riæ. (Latreille.) The mouth has two jaws, and a proboscis infolded in a double sheath; the wings are four; the two foremost covering the hinder ones when at rest. The sexes are three—prolific females or queens, unprolific females or workers commonly (termed neuters), and males or drones. The females and working bees have a sting. The honey or hive bee is distinguished from the other species of this genus by having the femora of the posterior pair of legs furnished with a smooth and concave plate on the outer side, and fringed with hair, forming a basket or pocket for the reception and conveyance of the pollen of plants; and also in being destitute of spines at the extremity. The Linnæan genus includes nearly 60 species. See Bee.
Apis Mellif′ica. [Linn.] The honey bee.
APLANAT′IC. In optics, applied as an epithet to lenses, of which the figure, as well as the materials of which they are composed, are such that, with a given index of refraction, the amount of aberration, both chromatic and spherical, is insignificant, or the least that can be possibly obtained. See Aberration, Achromatism, Lens, 175&c.
APLOTAXIS AURICULATA. Nat. ord., Compositæ. A plant growing in the North Western Himalayas. It was first shown by the late Dr Hugh Falconer to be the source of the Costus Arabicus of the ancients, which Dr Royle had previously identified with the Patchuck or Koot root met with in the Indian bazaars. Dr Irvine states that formerly, when opium was not produced in Rajwarra, this root was extensively smoked as a stimulant. He adds, that it is said to be a narcotic when thus used, and that formerly great quantities went to China for smoking purposes. It is chiefly used as a perfume, as for protection of bales of cloth against insects.
APO-. [Gr.] In composition, from; denoting derivation, separation, opposition, or departure. It is a common prefix in words from the Greek, and is etymologically the same as the latin ab-.
APOC′NYINE (-pŏs′-e-nĭn). Syn. Apocyni′na, L. A bitter, crystallisable substance, found in apŏ′′cynum cannabi′num (Linn.), or the Indian hemp of North America. See Alkaloid.
APOMORPHINE. Syn. Apomorphia. C17H17NO2. A remarkable base, obtained from morphia by Matthiessen and Wright. It is possessed of powerful emetic properties. Introduce into a strong glass tube, closed at one end, 1 part of pure morphia, and 20 parts of pure hydrochloric acid; these should not occupy more than one fifteenth of the tube. Seal the open end, and place the glass tube in another of cast iron, closed with a screw, and heat the whole in an oil-bath at a temperature between 140° and 150° C., during three hours. After cooling, the morphine has been converted into apomorphine, which can be purified as follows:
The tube is opened, and the liquid it contains diluted with water and neutralised by bicarbonate of soda; then an excess of this salt being added, the apomorphine is precipitated with any morphia that may remain. The liquid is decanted, and the precipitate is exhausted with ether or chloroform, which dissolves the apomorphine only. To the ethereal or chloroformic liquor are afterwards added a few drops of hydrochloric acid to saturate the base. Crystallised apomorphine then separates spontaneously, and is deposited on the sides of the vessel. These crystals are washed rapidly with cold water, and purified by crystallisation from boiling water. The apomorphine can be obtained by precipitating a concentrated solution of this hydrochlorate by bicarbonate of soda; the precipitate is white, but turns green rapidly in the air. It should be washed with a little cold water, and promptly dried to avoid this alteration.
AP′OPLEXY (-plĕks-e). Syn. Apoplex′ia, Apoplex′is, L. (from απο-πλησσω, I astound, or strike down, Gr.); Apoplexie, Fr.; Schlagfluss, Ger. A disease so named on account of the suddenness and violence of its attacks.
Symp. Sudden suspension or loss of the powers of sense and motion; the heart continuing to beat and the lungs to act, but generally with difficulty. During the fit the patient usually lies in a state resembling sleep, or the stupor induced by drunkenness. In some cases there is paralysis of one side of the body, and convulsions of the other. In the sanguineous or sthenic variety, or the one which is most common, the pulse is hard and full, the countenance flushed and bloated, and the breathing stertorous; in the serous or asthenic variety, the pulse is feeble, the skin cold, and the countenance pale. “The presence of convulsions is indicative of great danger.” (Dr Cheyne.) In both cases the patient is generally found lying on his back, in a state of complete insensibility, which defies every effort to arouse him; the eyelids almost cover the eyes, which are fixed and devoid of intelligence, whilst the pupils scarcely change their dimensions under the varying influence of light and darkness; the lips are usually purple or very dark; and both the lips and nostrils have generally a slight trembling movement communicated to them by the deep and laborious breathing of the patient.
Treat. In this disease, more than perhaps any other, medical aid should be immediately sought. In the mean time the patient should be placed in an easy posture, in a well-ventilated apartment, and in the sanguineous or sthenic variety, in as erect a position as possible; but in the asthenic variety, when the face is pale, with the head and shoulders only moderately elevated. The neckcloth should be removed, and the clothes loosened, and the head and neck laid bare. Crowding round the patient should be particularly avoided, and a free exposure to fresh air secured in every possible way. When medical aid cannot be immediately procured, blood should be freely taken (say 15 to 20 fl. oz., or more) from the arm, by any person competent to do so; unless the face be pale, and the pulse feeble, when cupping at the back of the neck, or leeches behind the ears, should be substituted for ordinary bleeding. Cold water should be dashed on the head, the legs placed in pretty warm water, and blisters or mustard poultices applied between the shoulders. In the mean time 8 or 10 gr. of calomel may be administered, and its action subsequently promoted by the use of saline purgatives and stimulating clysters. When there is a difficulty of swallowing, a couple of drops of croton oil may be applied to the tongue; or it may be poured on sugar, before placing it in the mouth. Indeed, this mode of relieving the bowels should be adopted in all extreme cases, as soon as possible. Emetics should be carefully avoided. The only exception to this rule is, when the stomach is distended by a heavy undigested meal; when an emetic is hazarded as the less of two evils.176 Nasal stimulants, as smelling salts or aromatic vinegar, should also be avoided. If the bleeding has not afforded some relief, it may be repeated in from 3 to 5 hours. When these means prove successful, the remainder of the treatment may consist in the administration of mild purgatives and diaphoretics, and the avoidance of stimulating food or drinks, and of other like exciting agents.
Prev., &c. The premonitory symptoms of apoplexy are giddiness, pain and swimming in the head, loss of memory, faltering in speech or using one word for another, diminished sensibility either of body or mind, or both, drowsiness, noises in the ears, specks floating before the eyes, nightmare, frightful dreams, laborious respiration, heavy yet unrefreshing sleep, an inclination to sigh without any moral cause, cramp in the legs at night when there is no irritation of the bowels to account for them, &c. &c. When any of these symptoms occur (especially in “free livers”) aperient medicines and a light diet should be at once had recourse to, and wine, beer, and spirits avoided as the most dangerous poisons. If the symptoms increase or continue, active purgation, a still lower diet, and even bleeding may be had recourse to. Pure air, early rising, regular habits, gentle muscular exercise, and loose, easy clothing, are powerful preventives of apoplexy. By attending to the admonitions of nature, and adopting the simple means which are within the reach of all, it is indisputable that many fatal cases of apoplexy might have been avoided, and a still larger number lessened in severity.
Robust, plethoric persons, with short thick necks, are universally accounted the most liable to apoplexy. In them the fit generally comes on without warning; and when once attacked with this malady they are especially liable to its recurrence. But it must be recollected that the possessor of no particular constitution or temperaments, to whatever class it may belong, enjoys immunity from the attacks of apoplexy—a disease more fatal among Englishmen than the natives of other countries.
Obs. A loss of consciousness exists alike in apoplexy, epilepsy, narcotism from opium and opiates, complete intoxication, and common fainting. These may be distinguished by observing that—in EPILEPSY there are almost always convulsions, and more or less rigidity of the limbs, with (generally) foaming at the mouth and gnashing or grinding of the teeth, and frequently, the utterance of noises often not unlike the barking of a dog; whilst stertor and laborious breathing, as a rule, are absent:—in the stupor produced by OPIUM, MORPHIA, &c., the face is pale, calm, and perspiring, and the respiration is tranquil and without stertor; whilst the patient can, in almost all cases, be temporarily aroused to consciousness and kept awake by being made to walk between two attendants; the odour of opium or laudanum is also frequently perceptible in the breath or ejected matter:—in the insensibility of INTOXICATION the pulse is usually feeble, and the patient may be temporarily roused by violent shouting in the ear, or by the application of nasal stimulants, particularly the common smelling-bottle (if strong); and the breath, and ejected matter (if any), smells of liquor:—in ordinary FAINTING the face and lips are pale, the breathing quiet, the pulse scarcely perceptible, the limbs mobile, and the fit lasts only a few minutes.
Treatment for Horses. Give in the first place a strong stimulant internally, and apply mustard embrocations to the belly and spine. Bleed, should the pulse be small and indistinct.—In the parturient apoplexy of cows. Bleed in the very earliest stage; give salts and croton; diluents; no solid food; let the body and legs be rubbed and clothed; use catheter; apply ice and refrigerants to head and neck; give frequent clysters of linseed gruel; remove milk every hour, and apply rubefacients to the spine.
APOSEP′EDIN (-dĭn). A substance found in putrid cheese, and supposed to be a product of the fermentation of caseine. Mulder and others have shown that it is merely impure leucine.
AP′OSTEME† (-tēme or -tĕm). Syn. Ap′ostem†; Aposte′ma†, L. An abscess or collection of purulent matter in any part of the body.
APPARA′TUS. [L., Eng.; class. pl., appara′tus; Eng. pl., appara′tuses—Webster.] Syn. Appareil, Fr.; Apparat, Geräthschaft, Ger. In technical language, the instruments, utensils, and mechanical arrangements, employed in any operation, experiment, or observation, or in any art or trade.
Apparatus. In anatomy and physiology, a catenation of organs all ministering to one general purpose or function; as the digestive apparatus, respiratory a., &c.
APP′ETITE. Syn. Appeti′tus, L.; Apétit, Fr.; Apetit, Begierde, Esslust, Ger. The natural desire of gratification, whether corporeal or mental. In physiology, the instinctive inclination to perform certain natural functions, as those of digestion and generation; but appr., the natural desire for food. In psychology and philosophy, the APPETITES (pl.) are affections of the mind directed to general objects, as fame, glory, or riches; these when subsequently turned to particular objects, constitute the PASSIONS, as envy, gratitude, revenge, or love. In its common and unqualified sense, the word appetite is confined to the desire for food; and in that sense chiefly concerns us here.
The sensations of hunger and thirst are seated in the stomach, and their recurrence at proper intervals is a necessary consequence of vital action, and is essential to the existence of the body in a state of vigour and health. Any alteration from their normal condition177 indicates diseased action of the stomach, or of the nervous system or circulation; or it may result from vicious habits. A healthy appetite for food is usually a most certain indication that nature requires a supply; but in the indulgence of this appetite certain regulations should be observed, and a boundary should be put to mere animal gratification. By slowly eating and thoroughly masticating the food, the stomach becomes gradually and equally distended, and the individual feels himself satisfied only after he has taken a quantity sufficient for the nourishment of his body; but, on the contrary, if the food be swallowed rapidly, and without proper mastication, it presses heavily and roughly against the sides of the stomach, and induces a sensation of fulness before a sufficient meal has been made. The consequences are, that hunger soon returns, and the party must either have recourse to food between the usual time of meals, or suffer the consequences of imperfect nutrition. Exercise and labour, within certain limits, promote the healthy functions of the stomach and bowels, through the action of the muscles of the abdomen increasing the peristaltic motion of these viscera. An inordinate appetite in persons leading a sedentary life is generally indicative of the food passing off imperfectly digested, or of the coats of the stomach being relaxed, or even diseased. More food is required in winter than in summer, in consequence of the greater radiation of the heat of the body; and hence the increased appetite which is usually an accompaniment of that season. In persons who lead a more sedentary life in winter than in summer, either no change of this kind occurs, or the reverse is the case; the want of exercise producing a diminution of appetite corresponding to the increase of it that would otherwise result from the seasonal change of atmospheric temperature, or even greater. Deviations of the appetite from the healthy standard, or the normal condition, constitutes DEFECTIVE or DISEASED APPETITE.
Deficiency or loss of appetite (AN′OREXY; ANOREX′IA, L.) generally arises from disordered stomach; but is also frequently symptomatic of other affections, particularly dyspepsia, biliousness, feverishness, and organic diseases of the lungs, stomach, and primæ viæ. It is a common consequence of sedentary life, and of extreme mental anxiety, excitement, or exhaustion. The treatment will necessarily vary with the cause. In simple spontaneous cases the appetite may generally be improved by outdoor exercise, and the occasional use of mild aperients, especially salines and aloetics. When the affection arises from the stomach being loaded with bile and crudities, an emetic in the evening, followed by a stomachic purgative the next morning, with an occasional aperient afterwards, will seldom fail to effect a cure. With heavy drinkers a gradual reduction of the quantity of the strong liquors usually consumed is generally followed by a restoration of the appetite and digestive powers. The change thus gradually effected in the course of 8 or 10 days is often almost magical. The excessive use of liquors—especially of spirits, wine, or beer, or even of warm weak ones, as tea, coffee, soup, &c.—is always prejudicial. Hence drunkards are particularly subject to defective appetite; and teetotallers and water-drinkers to a heartiness often almost approaching voracity. See Bile, Dyspepsia, &c.
Depraved appetite (PI′CA, L.), or a desire for unnatural food, as chalk, cinders, dirt, soap, tallow, &c., when an idiopathic affection or when depending on vicious tastes or habits (as is often the case in childhood), it may be treated by admixing very small doses of tartar emetic or ipecacuanha with the objectionable food or articles. When symptomatic of pregnancy, a plentiful and nutritious diet, including the red meats, with a little good malt liquor or wine, may be adopted with advantage. When symptomatic of chlorosis, to this diet may be added the use of chalybeate tonics, and sea or tepid bathing; when of dyspepsia, a light diet, bitter tonics, free exercise, fresh air, and cold bathing, will generally effect a cure.
Insatiable appetite (CANINE APPETITE, VORACITY; BULIM′IA, L.) is generally symptomatic of pregnancy, or worms, or diseases of the stomach or the viscera immediately connected with it; but sometimes exists as a separate disease, and is even said to be occasionally hereditary. When it occurs in childhood, worms may be suspected, and vermifuges administered. In adults, a common cause is imperfect digestion, arising from stomach complaints or gluttony, when the languor and gnawing pains of disease are mistaken for hunger. In this case the diet should be regulated and the bowels kept gently relaxed with mild aperients, and tonics (as bark and steel), or bitters (as orange-peel and gentian), may be administered. When pregnancy or vicious habits are the cause, the treatment indicated under DEPRAVED APPETITE may be adopted. When the affection is occasioned by acidity in the stomach, an emetic, followed by the moderate use of absorbents or antacids, will generally effect a cure. In those cases depending on a highly increased power of the stomach in effecting rapid and complete digestion, its contractile force and morbid activity may be often allayed by the copious use of salad oil, fat meat, &c., by the cautious use of opiates, or by the use, or freer use, of tobacco (either smoked or chewed, or both). A cathartic daily, with a dose of blue-pill, or mercurial powder, every second or third day, is also often advantageous. 25 or 30 drops of solution of potassa, in broth, twice or thrice daily, has also been recommended. See Bile, Dyspepsia, Worms, &c.
APP′LE (ăp′l). Syn. Ma′lum, Po′mum, L.; Pomme, Fr.; Apfel, Ger.; Appel, Dut.;178 Aple, Swed. This well-known fruit is the product of the cultivated varieties of pyrus malus (Linn.), or the crab-apple of our hedges; a tree of the nat. ord. Rosaceæ. The date of its amelioration from the wild state is probably very remote, as several kinds are noticed by Pliny in a manner that would lead to the inference of a high antiquity. Pippins, or ‘seedling improved apples,’ are said to have been introduced into this country from the South of Europe towards the end of the 16th century. Don enumerated 1400 varieties of the cultivated apple; there are now probably above 1650. Rennet apples (POMA RENETTIA) are those ordered in the P. Cod. to be used in pharmacy. In botany and composition, the term apple (POMUM) is used to designate any large, round, fleshy fruit, consisting of a ‘pericarp,’ enclosing a tough ‘capsule’ containing several seeds; as love-apple, pine-apple, &c.
The wood of the apple-tree is much used in turnery; that of the crab-tree is generally preferred by mill-wrights for the teeth of mortise-wheels.
The expressed juice of 1 cwt. of ripe apples, after the free acid has been saturated with chalk, yields from 11 to 13 lbs. of a very sweet, but uncrystallisable sugar.
Apples have been analysed by Fresenius, and were found to have the following composition:—
| Soluble Matter— | |
| Sugar | 7·58 |
| Free acid (reduced to equivalent in malic acid) | 1·04 |
| Albuminous substance | 0·22 |
| Pectous substances, &c. | 2·72 |
| Ash | 0·44 |
| Insoluble Matter— | |
| Seeds | 0·38 |
| Skins | 1·44 |
| Pectose | 1·14 |
| [Ash from insoluble matter included in weights given] | [0·13] |
| Water | 85·04 |
| ——— | |
| 100·00 |
Love′-apple‡. The tomato.
Mad′-apple‡. The larger Mecca or Bussorah gall. They are also called Dead-sea apples, a. of Sodom, &c. See Galls.
Acid of Apples. Malic acid.
A′PRICOT. Syn. A′pricock†; Armeni′acum ma′lum, Præco′tium, L.; Abricot, Fr.; Aprikose, Ger. The fruit of armeniaca vulgaris (Lamb.; prunus armeniaca, Linn.), a rosaceous tree indigenous in Armenia, Cachmere, &c., and now cultivated in every temperate region of the world. Under the name of præcox it was known in Italy in the time of Dioscorides; but it was not introduced into England until the reign of Henry VIII (A.D. 1540). Its cultivation has since been zealously attended to by our gardeners, and it is now one of the choicest and most esteemed of our wall-fruits, and is particularly valued for desserts. It is reputed to be nutritious, easy of digestion, laxative, and stomachic. The seeds are bitter and saponaceous.
Apricots are principally eaten as gathered; but are also dried, candied, and made into jam. In confectionery, the Brussels and Breda varieties are preferred to the larger and sweeter kinds. See Fruit, Preserves, &c.
Apricots, Briançon′. The fruit of armeniaca brigantiaca (Pers.). Acidulous; seeds or kernels, by expression, yield HUILE DE MARMOTE.
A′QUA (-kwă). [L.] Water.—Aqua destilla′ta or A. DISTILLA′TA, is distilled water; A. FLUVIA′LIS or A. EX FLU′MINE (-ĭn-e), river-water; A. FONTA′NA, spring-water; A. MARI′NA or A. MA′′RIS, sea-water; A. MINERA′LIS, mineral water; A. NIVA′LIS or A. EX NI′VE, snow-water; A. PLUVIA′LIS, A. PLU′′VIA, or A. IM′BRIUM, rain-water, soft water; A. PUTEA′NA or A. EX PU′TEO, well, pump, or hard water.
Aqua. In chemistry and pharmacy, this word was formerly applied to numerous preparations and articles now included under other heads. See Eau, Esprits, Hair-dyes, Liquors, Solutions, Waters, &c.
Aquafor′tis. [L.] Literally, ‘strong water,’ the name given by the alchemists to the acid obtained by distilling a mixture of nitre and sulphate of iron. The word is still commonly employed by mechanics and artists to designate the impure fuming nitric acid of commerce, and is thus also retained in trade. By these parties concentrated nitric acid is called ‘spirit of nitre.’ ‘Double aquafortis’ merely differs from the other in strength. See Nitric acid.
Aqua Amarella. A compound for hair-dyeing; is prepared with sugar of lead, common salt, and water.
Aqua Græ′ca, A. Orienta′lis. See Hair-dyes.
Aqua Mari′na. [L.] The beryl†.
Aqua Mirab′ilis†. [L.] Literally, ‘wonderful water,’ a cordial and carminative spirit distilled from aromatics, and formerly reputed to possess many virtues.
Aqua Re′gia. [L.] Nitrohydrochloric acid, originally so called, by the alchemists, from its power of dissolving gold.
Aqua Toffa′nia. [L.] See Acquetta.
Aqua Vi′tæ†. [L.] Literally, ‘water of life,’ a name familiarly applied to the leading native distilled spirit. Thus, it is whiskey in Scotland, usquebaugh in Ireland, geneva in Holland, and eau de vie or brandy in France. When the term is employed in England, French brandy is understood to be referred to. See Alcohol, &c.
Aqua Vitæ Aromatico-Amara. (F. Bolle, formerly J. B. Claude, Berlin). Galangal ginger, āā, 2 parts; orange berries, European centaury, gentian, cinnamon, angelica, āā, 1 part; alcohol, 30 parts; water, 26 parts. Digest and filter. (Hager.)179
AQUARIUM. A tank or vessel made of glass, containing either salt or fresh water, and in which either marine or fresh-water plants and animals are kept in a living state. In principle, the aquarium depends upon the interdependence of animal and vegetable life. The carbonic acid evolved by the animals is decomposed under the influence of solar light by the plants, and the oxygen necessary for the maintenance of the life of the animals is thus eliminated, whilst the carbonic acid essential to the existence of the plants is supplied by the animals. The aquarium, therefore, must be stocked both with plants and animals, and for the welfare of both, something like a proper proportion should exist between them. But even under these conditions the water should be frequently aërated, whether the aquarium contains fresh or salt-water. This may be done by simply blowing through a glass tube which reaches to near the bottom, or, still better, in the following manner:—Take a glass syringe which can be easily worked. Having filled it with water, hold it with the nozzle about two inches from the surface of the water in the aquarium, into which the contents are to be discharged quickly and with a sort of jerk. By this means a multitude of small bubbles are forced down into the fluid. This operation should be several times repeated. A simpler method is to take out a portion of the water from the aquarium and to pour it back again from a height. When, as not infrequently happens, the aquarium is provided with a fountain, this of course ensures a continual change of water; but even where this is the case the joint presence both of plants and animals is advantageous to the health of both. When sea-water cannot be procured for the marine aquarium a substitute for it may be made as follows:—Mix with 970,000 grains of rain-water 27,000 grains of chloride of sodium, 3600 of chloride of magnesium, 750 of chloride of potassium, 29 of bromide of magnesium, 2300 of sulphate of magnesia, 1400 of sulphate of lime, 35 of carbonate of lime, and 5 of iodide of sodium. These all being finely powdered and mixed first, are to be stirred into the water, from which a stream of air may be caused to pass from the bottom until the whole is dissolved. On no account is the water to be boiled, or even to be heated. Into this water, when clear, the rocks and seaweed may be introduced. As soon as the latter are in a flourishing state the animals may follow. Care must be taken not to have too many of these, and to remove immediately any dead ones. The loss that takes place from evaporation is to be made up by adding clear rain-water. The presence of a number of molluscous animals, such as the common periwinkle, is necessary for the consumption of the vegetable matter continually given off by the growing plants, and of the multitudinous spores, particularly of the confervæ, which would otherwise soon fill the water, rendering it greenish or brownish, and turbid. In a fresh-water aquarium the bottom should be covered with a layer of fine sand and shingle, and in this the weeds should be planted. The best for this purpose are valesneria spiralis, anacharis, and chara vulgaris. A few water-snails should also be put in; the best are planorbis, paludina, and amphibia glutinosa. One plant and two or three snails should be used for each gallon of water put into the aquarium.
AQUATINT′A. [L., Fr.] Syn. A′quatint, Eng.; Acquatinta, It. A species of etching on copper, producing an effect resembling a drawing in Indian ink.
A′QUEOUS (-kwe-). Syn. Aquose′*; A′queus, Aquo′sus, L.; Aqueux, Fr.; Wässerig, Wässerhaltig, Ger. Watery; made with, containing, or resembling water. In chemistry and pharmacy, applied to solutions, extracts, &c., prepared with water.
AR′ABESQUE (-bĕsk). [Fr.] In the Arabian manner; more particularly applied to a species of capricious, fantastic, and imaginative ornamentation, consisting of foliage, stalks, plants, &c., to the entire exclusion of the figures of animals. The designs of this class, now so much employed in cloth and leather binding, are produced by the pressure of hot plates or rollers having the pattern engraved on them. See Moresque.
AR′ABIN (-bĭn). C12H22O11. [Eng., Fr.] Syn. Soluble gum; Arabi′na, L. The pure soluble principle of gum acacia.
Prep. Dissolve white gum arabic in pure water, filter the solution, and add alcohol as long as it produces curdiness; collect the precipitate, and dry it by a gentle heat.
Prop. &c. Very soluble in water; basic acetate of lead, alcohol, and ether, precipitate it from its solutions. It is isomeric with crystallised cane sugar. It possesses no practical superiority over the best gum arabic, except its paler colour.
AR′ABLE (ăbl). Syn. Arab′ilis, L.; Arabile, Labourable, Fr.; Pflügbar, Ger. In agriculture, fit for or under tillage or aëration; ploughed.
Arable Land. In agriculture, land which is chiefly or wholly cultivated by the plough, as distinguished from grass-land, wood-land, common pasture, and waste. See Land, Soils, &c.
ARACHIS HYPOGÆA. Syn. Ground Nut Plant. Hab. Cultivated throughout the tropics of the Old and New World. Officinal part. The oil of the seeds (Oleum Arachis, Ground Nut Oil). Obtained by expression. Limpid, clear, light yellow, almost inodorous, or with a faint smell and bland taste. Sp. gr. 0·916.—Prop. and Uses. This oil affords a cheap and excellent substitute for olive oil for pharmaceutical and other purposes.
The following notice, by the Editor of this180 work, appeared in ‘The Veterinarian’ for October, 1876:—
“Having in the course of my analytical practice had occasion to examine some samples of Marseilles earth-nut cake, I take the opportunity of communicating the results obtained, in the hope of furnishing interesting information respecting a material which is chiefly employed in the sophistication of the more expensive feeding cakes, but which I think might in some instances be with advantage substituted for them.
“Arachis seeds constitute one of the varieties of food termed pulse, and the oil which exists in them to the extent of from 40 to 50 per cent., is rapidly being introduced in the making of soap in this and other countries. It is an article also of the Indian Pharmacopœia.
“By pressure the seeds yield all but about 7 per cent. of their oil, and the material which remains after the expression of the greater part of the oil is sent into commerce as earth-nut or ground-nut cake.
“Sometimes the husks of the seeds are first removed and only the kernels subjected to pressure for the sake of the oil; the cake so produced is called ‘decorticated earth-nut cake,’ at other times the entire seeds are subjected to this treatment, and then the resulting cake is known as ‘undecorticated earth-nut cake.’
“The following table shows the composition in 100 parts of both descriptions of cake, as well as that of linseed cake of first-rate quality; the last analysis being added for the sake of comparison:—
| Decorticated Earth-nut Cake. | Undecorticated Earth-nut Cake. | Linseed Cake. | |
| Moisture | 9·58 | 9·28 | 11·72 |
| Fat and heat producers | |||
| Oil | 7·40 | 6·99 | 12·00 |
| Starch digestible fibre, &c. | 27·63 | 23·66 | 25·29 |
| Flesh-formers (albumenoids) | 42·81[75] | 32·81[76] | 32·64 |
| Indigestible fibre | 7·87 | 23·80 | 11·79 |
| Ash | 4·71 | 3·45 | 6·47 |
| ——— | ——— | ——— | |
| 100·00 | 100·00 | 100·00 |
“From the foregoing analyses it will be seen that both descriptions of earth nut are exceedingly rich in flesh-formers, and that they contain a moderately large amount of oil. They also possess a sweet agreeable flavour, and are, I believe, very digestible. As these may, I am informed, be bought at from £6 to £8 per ton, it is evident that farmers would do well to give earth-nut cakes a trial in the feeding of their stock.
“Pure linseed cake does not contain starch, but in its stead mucilage. The feeding qualities of starch and mucilage are, however, very similar.”
ARAROBA. Syn. Araroba powder. Bahia powder. Goa powder. The pith or medulla of the stem and branches of a leguminous tree (a species of Centrolobium) growing in Brazil. It is in extensive use amongst the natives of India, who employ it in affections of the skin. It has been applied with success in shingles and ring-worm, in the form of ointment made as follows:—
| Araroba in powder | 20 grains. |
| Acetic acid | 10 drops. |
| Benzoated lard | 1 ounce. |
Dr Attfield found the powder to contain from 80 to 84 per cent. of chrysophanic acid, to which substance its remedial powers are doubtless due. It is now the chief source of this acid.
ARA′TION*. In agriculture, ploughing; culture by ploughing; tillage. Lands in a state of aration’ are those under tillage.
AR′BOR. [L.] A tree. The seventh family of vegetables in Linnæus’s system. In anatomy and chemistry, a term formerly applied to membranes and substances having some real or fancied resemblance to a tree or vegetation. An ar′boret is a little tree; an arborist, or ar′borātor†, is one who studies or cultivates trees.
ARBUTIN. C12H16O7. A substance obtained by Kawalier from the leaves of the red bearberry Arctostophylos uva ursi, and by Zwenger and Himmelmann from the leaves of a species of winter-green, Pyrola Umbellata. It is prepared by precipitating the aqueous decoction of the leaves of either of these plants, with basic acetate of lead, filtering, removing the excess of lead with sulphuretted hydrogen, and either treating the filtrate with animal charcoal and leaving it to crystallise or evaporating and digesting the residue with a mixture of eight parts of ether and one part of alcohol, which dissolves out the arbutin, and deposits it on evaporation in the crystalline state.
ARCA′NUM [L.] Syn. Arcane, Fr.; Geheimnis, Ger. A secret. In alchemy, a term applied to various preparations without any precise meaning. “Arcanum is a thing secret, incorporeal, and immortal, which can only be known to man by experience; for it is the virtue of each thing, which operates a thousand times more than the thing itself.” (Ruland) In ancient medicine and pharmacy; a nostrum. The word is still occasionally used in the plural181 (ARCA′NA, secrets, mysteries), in the titles of books; as, ‘Arcana of Chemistry,’ a book professing to contain a full exposition of the mysteries of that art.
Among the old chemists, ARCANUM AL′BUM was ‘pulvis Viennensis albus virgineus’ (see Powders); A. BEC′CHICUM, a sweetened aqueous solution of liver of sulphur; A. CORALLI′NUM, red oxide of mercury that had been digested in a solution of potash, washed with water, and then had spirit of wine burnt on it (once a favourite mercurial and escharotic); A. DUPLICA′TUM, sulphate of potash; A. D. CATHOL′ICUM, roots of colchicum and plantain (worn as an amulet against fevers and pestilential diseases); A. LUDEMAN′NI, oxide of zinc; A. TAR′TARI, acetate of potassa; A. VI′TÆ, elixir vitæ; &c.
ARCHE′US (-kē′-ŭs; ăr′*—Mayne). [L.] Syn. Archæ′us, L. A term invented by Paracelsus, and employed by the alchemists and older physicians, to imply the occult cause of phenomena, as well as the sub-causes or agents by which the effects were accomplished. Van Helmont and Stahl ascribe certain vital functions to the influence and superintendence of a ‘spiritus archæus’ or intelligent vital principle. According to others, the powers of ‘Archæus’ were indefinitely extended. He or it was an occult power of nature, the artificer of all things, physician-general to the universe, &c. &c., to the utmost bounds of absurdity and confusion.
From this word comes the adj. Arche′al or Archæ′al, hidden, operative.
ARCH′IL (artsh′-ĭl). Syn. Arch′el*, Or′chil; Archil′la, Orchil′la (ch as k), L.; Orseille, Fr., Ger.; Oricello, It. A violet-red, purple or blue colouring matter or dye-stuff, obtained from several species of lichens, but of the finest quality from roccella tinctoria (DC.), and next from r. fuciformis (DC.).
The archil of commerce is met with as a liquid paste, or as a thin liquid dye or stain of more or less intensity. The ordinary archil or orchil of the shops (ORCHIL-LIQUOR) is under the last form; and is known as either BLUE OR RED ARCHIL—distinctions which arise as follows:—
Prep. 1. Blue archil:—The bruised or coarsely ground lichen is steeped for some time in a mixture of stale urine, or bone-spirit, and lime or milk of lime, or in any similar ammoniacal solution, contained in covered wooden vessels in the cold; the process being repeated until all the colour is extracted.
2. Red or crimson archil:—The materials are the same as for the last variety, but rather less milk of lime is used, and the ‘steep’ is generally made in earthen jars placed in a room heated by steam, technically called a stove. The two kinds merely differ in the degree of their red or violet tint—the addition of a small quantity of lime or alkali to the one, or of an acid to the other, immediately bringing them both to the same shade of colour.
Prop. Archil has a disagreeable putrid ammoniacal odour. Its colouring matter is soluble in water, alcohol, urine, ammoniacal and alkaline lyes, and weak acid liquors; alkalies turn it blue, acids red; alum gives with it a brownish-red precipitate, and solution of tin a red one; the alcoholic solution gradually loses its colour when excluded from the air. Its colouring matter consists chiefly of orcein.
Pur. Archil is frequently adulterated with extract of logwood, or of Lima or Sapan-wood. It may be tested as follows:—1. A solution of 50 or 60 drops of pure archil in about 3 fl. oz. of water slightly acidulated with acetic acid, almost entirely loses its colour, or presents only a yellowish tinge, when heated to ebullition in a flask along with 50 drops of a fresh solution of protochloride of tin made with 1 part of the salt to 2 parts of water:—2. A drop of fluid extract of logwood treated in the same way, gives a distinct violet tint, which resists several hours’ boiling; but when only 3 or 4 per cent. of logwood is present, the boiled liquid has a permanent grey tint:—3. If the boiled liquid retains its red hue, extract of Sapan-wood is present:—4. The boiled liquor, when the archil is pure, re-acquires its colour by exposure to the air, and the addition of an alkali, particularly ammonia; whilst the colour produced by logwood is destroyed only by an alkaline solution of tin, and is restored by acids.
Uses, &c. It is employed to tinge the spirit used to fill the tubes of thermometers, and to stain paper, wood, &c. The aqueous solution stains MARBLE, in the cold, of a beautiful violet colour, of considerable permanence when not exposed to a vivid light. “Marble thus tinged preserves its colour unchanged at the end of two years.” (Dufay.) Its principal use is, however, in dyeing. By proper management it may be made to produce every shade of pink and crimson to blue and purple. Unfortunately, although the hues it imparts to silk and wool possess an exquisite bloom or lustre, they are far from permanent, and unless well managed, soon decay. It is hence generally employed in combination with other dye-stuffs, or as a finishing bath to impart a bloom to silk or woollens already dyed of permanent colours. In using it as a dye it is added to hot water in the required quantity, and the bath being raised to nearly the boiling-point, the materials are put in and passed through it, until the desired shade is produced. A mordant of alum and tartar is sometimes used, but does not add to the permanence of the colour. Solution of tin added to the bath increases the durability, but turns the colour more on the scarlet. (Hellot.) Milk of lime or salt of tartar is added to darken it; acids or solution of tin to redden it. A beautiful crimson-red is obtained by first passing the stuff through a mordant of tin and tartar, and182 then through a bath of archil mixed with a very little solution of tin. By the proper management of this dye, lilacs, violets, mallows, rosemary flower, soupes au vin, agates, and many other shades may be produced on silk or cloth, either alone or in conjunction with other dyes to modify it. 1⁄2 lb. of solid archil, or its equivalent in a liquid form, will dye 1 to 2 lb. of cloth. Herb-archil, it is asserted, will bear boiling, and gives a more durable tint than the other lichens, especially with solution of tin. (Hellot.) Recently Mr Lightfoot has patented a process for dyeing with archil with the aid of oil, after the manner followed for producing Turkey-red on cottons.
Archil, Facti′′tious:—1. From a mixture of onions (in a state of incipient putrefaction) with about 1-10th to 1-12th their weight of carbonate of potash and some ammonia, fermented together; and adding, after some days, 1-7th to 1-8th of the weight of the potash used in a salt of lead. The details of the process essential to success are, however, now unknown, the secret having died with a relative of the writer of this article.
2. Extract of logwood dissolved in juice of elderberries and putrid urine, with the addition of a little pearlash for the BLUE, and a very little oxalic acid or oil of vitriol for the RED variety. Used to stain wood.
Arch′il, Herb. Roccella tinctoria. See Archil (above), Lichens, and Mosses.
ARE (ăr; āre—Eng.). [Fr.] See Measures.
ARE′CA. [L.] In botany a genus of East Indian trees, of the nat. ord. Palmæ (DC.).
Areca Cate′chu. [L.; Linn.] Syn. Are′ca, A. In′dica, A. Faufel, Be′tel-nut tree. Hab. East Indies. Fruit (BETEL-NUT), astringent and narcotic; husk of fruit (PENANG or PINANG), sialagogue and stomachic; both are used as masticatories; wood and nut yield an inferior or bastard sort of catechu; charcoal of the nut highly esteemed as tooth-powder; also given in tape-worm in doses of 1⁄4 oz. and 1⁄2 oz.; said to be more efficacious in coarse than in fine powder.—Doses for Animals. HORSE, 4 to 6 drachms; CATTLE, 4 to 8 drachms; DOG, 30 grains to 2 drachms.
Areca Globulif′era. [L.] Properties similar to the last.
Areca Olera′cea. [L.; Willd.] Cabbage-palm.
ARENA′CEOUS (ăr-e-). Syn. Arena′ceus, L.; Arénacé, Sablonneux, Fr.; Sandig, Sandartig, Ger. In agriculture, mineralogy, &c., sandy; resembling sand; friable.
ARENA′′RIOUS (-nare′-). Syn. Arena′′rius, L.; Arénaire, Fr. Sandy, arenaceous. In agriculture and botany applied to soils (ARENARIOUS SOILS) in which sand is the prevailing and characteristic ingredient; also to plants that grow in sandy or arid soils.
ARENA′TION. Syn. Saburra′tion; Arena′tio, L.; Arénation, Fr.; Sandbad, Ger. In medicine sandbathing; a practice formerly prevalent, in dropsy, of applying hot sand, either by immersion or otherwise, to the feet, legs, or even the whole body.
ARENOSE′ (ăr-e-nōse’). Syn. Ar′enous*; Areno′sus, L.; Aréneux, Fr. Sandy; arenaceous (which see).
AREOM′ETER (ă-re- or ăr-re-; āre-e—Smart). Syn. Areom′etrum, L.; Aréomètre, Fr. Literally, a ‘measure of lightness’ or ‘rarity,’ originally applied to any instrument for determining the specific gravity of alcoholic and ethereal liquids; but since applied, like the word ‘hydrometer,’ to instruments adjusted to the densities of all liquids. In this country the term is principally confined to the aréomètres of Baumé, on account of their general use by Continental chemists. The relations and equivalents of Baumé’s scales, as now adopted in France, are shown in the first two of the following Tables:—
I.—Corresponding DEGREES of Baumé’s Areometers and REAL SPECIFIC GRAVITIES:—
| Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. |
| 10 | 1·0000 | 21 | 0·9300 | 32 | 0·8690 | 42 | 0·8202 | 52 | 0·7766 |
| 11 | 0·9932 | 22 | 0·9241 | 33 | 0·8639 | 43 | 0·8156 | 53 | 0·7725 |
| 12 | 0·9865 | 23 | 0·9183 | 34 | 0·8588 | 44 | 0·8111 | 54 | 0·7684 |
| 13 | 0·9799 | 24 | 0·9125 | 35 | 0·8538 | 45 | 0·8066 | 55 | 0·7643 |
| 14 | 0·9733 | 25 | 0·9068 | 36 | 0·8488 | 46 | 0·8022 | 56 | 0·7604 |
| 15 | 0·9669 | 26 | 0·9012 | 37 | 0·8439 | 47 | 0·7978 | 57 | 0·7556 |
| 16 | 0·9605 | 27 | 0·8957 | 38 | 0·8391 | 48 | 0·7935 | 58 | 0·7526 |
| 17 | 0·9542 | 28 | 0·8902 | 39 | 0·8343 | 49 | 0·7892 | 59 | 0·7487 |
| 18 | 0·9480 | 29 | 0·8848 | 40 | 0·8295 | 50 | 0·7849 | 60 | 0·7449 |
| 19 | 0·9420 | 30 | 0·8795 | 41 | 0·8249 | 51 | 0·7807 | 61 | 0·7411 |
| 20 | 0·9359 | 31 | 0·8742 |
| Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. |
| 0 | 1·0000 | 16 | 1·1176 | 32 | 1·2667 | 47 | 1·4476 | 62 | 1·6889 |
| 1 | 1·0066 | 17 | 1·1259 | 33 | 1·2773 | 48 | 1·4615 | 63 | 1·7079 |
| 2 | 1·0133 | 18 | 1·1343 | 34 | 1·2881 | 49 | 1·4758 | 64 | 1·7273 |
| 3 | 1·0201 | 19 | 1·1428 | 35 | 1·2992 | 50 | 1·4902 | 65 | 1·7471 |
| 4 | 1·0270 | 20 | 1·1515 | 36 | 1·3103 | 51 | 1·5051 | 66 | 1·7674 |
| 5 | 1·0340 | 21 | 1·1603 | 37 | 1·3217 | 52 | 1·5200 | 67 | 1·7882 |
| 6 | 1·0411 | 22 | 1·1692 | 38 | 1·3333 | 53 | 1·5353 | 68 | 1·8095 |
| 7 | 1·0483 | 23 | 1·1783 | 39 | 1·3451 | 54 | 1·5510 | 69 | 1·8313 |
| 8 | 1·0556 | 24 | 1·1875 | 40 | 1·3571 | 55 | 1·5671 | 70 | 1·8537 |
| 9 | 1·0630 | 25 | 1·1968 | 41 | 1·3694 | 56 | 1·5833 | 71 | 1·8765 |
| 10 | 1·0704 | 26 | 1·2063 | 42 | 1·3818 | 57 | 1·6000 | 72 | 1·9000 |
| 11 | 1·0780 | 27 | 1·2160 | 43 | 1·3945 | 58 | 1·6170 | 73 | 1·9241 |
| 12 | 1·0857 | 28 | 1·2258 | 44 | 1·4074 | 59 | 1·6344 | 74 | 1·9487 |
| 13 | 1·0935 | 29 | 1·2358 | 45 | 1·4206 | 60 | 1·6522 | 75 | 1·9740 |
| 14 | 1·1014 | 30 | 1·2459 | 46 | 1·4339 | 61 | 1·6705 | 76 | 2·0000 |
| 15 | 1·1095 | 31 | 1·2562 |
| Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. | Degrees Baumé. | Specific Gravity. |
| 0 | 1000 | 16 | 1125 | 32 | 1286 | 47 | 1485 | 62 | 1758 |
| 1 | 1007 | 17 | 1134 | 33 | 1298 | 48 | 1501 | 63 | 1779 |
| 2 | 1014 | 18 | 1143 | 34 | 1309 | 49 | 1516 | 64 | 1801 |
| 3 | 1022 | 19 | 1152 | 35 | 1321 | 50 | 1532 | 65 | 1823 |
| 4 | 1029 | 20 | 1161 | 36 | 1334 | 51 | 1549 | 66 | 1847 |
| 5 | 1036 | 21 | 1171 | 37 | 1346 | 52 | 1566 | 67 | 1872 |
| 6 | 1044 | 22 | 1180 | 38 | 1359 | 53 | 1583 | 68 | 1897 |
| 7 | 1052 | 23 | 1190 | 39 | 1372 | 54 | 1601 | 69 | 1921 |
| 8 | 1060 | 24 | 1199 | 40 | 1384 | 55 | 1618 | 70 | 1946 |
| 9 | 1067 | 25 | 1210 | 41 | 1398 | 56 | 1637 | 71 | 1974 |
| 10 | 1075 | 26 | 1221 | 42 | 1412 | 57 | 1656 | 72 | 2000 |
| 11 | 1083 | 27 | 1231 | 43 | 1426 | 58 | 1676 | 73 | 2031 |
| 12 | 1091 | 28 | 1242 | 44 | 1440 | 59 | 1695 | 74 | 2059 |
| 13 | 1100 | 29 | 1252 | 45 | 1454 | 60 | 1715 | 75 | 2087 |
| 14 | 1108 | 30 | 1261 | 46 | 1470 | 61 | 1738 | 76 | 2116 |
| 15 | 1116 | 31 | 1275 |
AREOM′ETRY. Syn. Areome′tria, L.; Aréométrie, Fr. The art or operation of ascertaining the specific gravity of liquids, and hence also their strength or commercial value; hydrometry. See Areometer (above), Hydrometry, Specific Gravity, &c.
ARE′CINA. C23H26N2O4. An alkaloid discovered by Pelletier and Comol, in white cinchona bark from Aréca. It is extracted from the bark by the same process as Quinine, viz., by boiling the bark with acidulated water, treating the liquor with lime, and digesting the lime-precipitate in alcohol. The solution filtered at the boiling heat yields a very dark-coloured liquid, which, after a time, deposits the greater part of the aricine in crystals. An additional quantity may be obtained from the mother-liquor by expelling the alcohol by distillation, treating the residue with a slight excess of hydrochloric acid, separating the greater part of the colouring matter by means of a saturated solution of common salts, then throwing down the aricine by ammonia, dissolving the precipitate in alcohol, decolourising with animal charcoal and crystallising.
ARGAMONE MEXICANA (nat. order Papaveraceæ). A tropical American plant, now a common weed growing in almost every part of India. A fixed oil is obtained from the seeds by expression, which has long been employed as an aperient in the West Indies. In half-drachm doses it is said to act as a gentle aperient, and at the same time it allays, apparently by its sedative qualities, the pain in colic. The smallness of the dose, and the mildness of its operation, commend it to the notice of the medical practitioner. Its efficiency is impaired by keeping, the freshly prepared oil proving more active and uniform in its action than that which has been long on184 hand. It is reported to exercise a well-marked and soothing influence when applied to herpetic eruptions and other forms of skin disease. By the natives of India the expressed yellow glutinous juice of the plant is held in high repute as a local application to indolent and foul ulcers.
ARGENT′INE (-ĭn). Syn. Argenti′nus, L.; Argentin, Fr.; Silberfarben, &c., Ger. Silver-like; pertaining to, resembling, or sounding like silver; argental.
Ar′gentine. (-tĭn). [Eng., Fr.] German silver*. In mineralogy, nacreous carbonate of lime, from its whiteness and silvery lustre.
ARGENT′UM. [L.] Silver. In old chemistry and pharmacy, ARGENTUM, FUGITI′′VUM†, A. MO′BILE† (-ĭl-e), was quicksilver; A. MOR′TUUM†, dead silver, grain-s; A. MUSI′VUM†, mosaic s., silver-bronze; A. NITRA′TUM†, lunar caustic; A. VI′VUM†, quicksilver; A. ZOÖTIN′ICUM†, cyanide of silver; &c.
AR′GIL† (jĭl). Syn. Argil′la, L.; Argile, Fr. Clay or potter’s earth.
ARGILLA′CEOUS (-jĭl-). Syn. Argilla′ceus, L.; Argilleux, Fr.; Thonig, Thonartig, Ger. Clayey; pertaining to, containing, or of the nature of clay or argil. In agriculture, an epithet of soils (ARGILLACEOUS SOILS) of which clay is the principal or characteristic ingredient.
Argil′lo-arena′ceous (-jĭl-). In agr., consisting chiefly of clay and sand.
Argillo-calca′′reous. In agr., consisting chiefly of clay and chalk.
AR′GOL. Syn. Argal*; Tar′tarus cru′dus, L.; Tartre brut, Fr.; Weinstein, Ger. Crude bitartrate of potash, as deposited by wine. That from red wine is RED ARGOL; that from white wine, WHITE ARGOL. See Tartar.
ARM′ATURE (-ă-tūre). Syn. Armatu′′ra, L. In magnetism, a piece of soft iron used to connect the poles of a horseshoe magnet, for the purpose of preventing loss of power.
AR′NICA. [L., Fr., Eng.] Syn. Arnique, Fr.; Arnika, Wolverlei, Ger. In botany, a genus of plants of the nat. ord. Compositæ (DC.). In the Ph. U. S., arnica montana (see below).
Arnica Monta′na. [L.; Linn.] Syn. Arnica, Moun′tain a., M. tobac′co, German leop′ard’s bane; Panace′a lapso′′rum*, L. Arnique, A. des montagnes, Tabac des Savoyards et des Vosges, Fr.; Arnika, Falkraut, &c., Ger. Hab. Meadows of the cooler parts of Europe, North America, and Siberia. It is now cultivated in our gardens. Flowers (ARNICA, Ph. U. S., Castr. Ruth., and Bor.) and leaves, diaphoretic, diuretic, stimulant, and narcotic; in large doses emetic and purgative; root discutient; whole herb diaphoretic, stimulant, and nervine.
Prop., &c. Arnica acts as an energetic stimulant on the cerebro-spinal system, and as an irritant on the stomach and bowels. It is much employed on the Continent, and is given in a great variety of diseases—amaurosis, chlorosis, convulsions, diarrhœa, dysentery, gout, paralysis, rheumatism, &c. It is much used in Germany, instead of bark, in intermittents, putrid fevers, and gangrene. In France it is commonly employed as an excito-tonic in paralysis. It has been greatly extolled, as a restorative, and in bruises and injuries from falls. The Savoyards and inhabitants of the Vosges both smoke and ‘snuff’ the leaves. In England it is little used except by homœopaths. It is said that no animal but the goat will eat this plant. (Thomson.) Its noxious properties chiefly depend on the presence of cytisine.—Dose. Flowers, 5 to 10 gr., in powder, with syrup or honey; root, 10 to 20 gr. It is most conveniently administered under the form of infusion or tincture. Severe abdominal pains and vertigo, and even tetanus and death, have followed excessive doses.
Obs. According to Dupuytren, the emetic action of infusion of arnica depends on minute particles of the down of the plant which remain suspended in it, and which may be removed by filtration. See Infusions, Tinctures, &c.
ARNATT′O, Arnott′o. See Annotta.
AR′NICINE (seen). This name has been applied to two substances—the one discovered by Pfaff; the other by Bastick:—
Arnicine (of Pfaff). The resinous matter extracted by alcohol from the roots and flowers of mountain arnica, and in which their acridity appears to reside.
Arnicine (of Bastick). Syn. Arnici′na, Arnici′′a (nīsh′-y′ă), L. Prep. 1. (Bastick.) From the flowers, by a similar process to that by which he obtains lobelina. 2. From the flowers (or root), as directed under Aricina.
Prop., &c. Bitter; acrid; crystallisable scarcely soluble in water; soluble in alcohol and ether; forms salts with the acids, the hydrochlorate and one or two others being crystallisable. Its physiological properties and dose have not as yet been accurately determined.
ARO′MA. [L.] Syn. Arome, Fr.; Arom, Geruchstoff, Ger. The characteristic odour of substances, particularly the peculiar quality of plants, and of substances derived from them, which constitutes their fragrance.
AROMA′TA. [L.] See Aromatic.
AROMAT′IC. Syn. Aromat′icus, L.; Aromatique, Fr.; Gewürzhaft, Ger. Fragrant; odoriferous; spicy; applied chiefly to plants and their products (Aromatics, A. plants; Aromat′a, AROMAT′ICA, L.; Aromatiques, ÉPICES, Fr.; Gewurz, Ger.) characterised by their spicy odour or aroma, and warm pungent flavour, and of which allspice, cinnamon, cloves, lavender, pepper, rosemary, sage, &c., are well-known examples. They are all stimulant, carminative, and antiseptic; and from remote antiquity have been regarded as prophylactic and disinfectant.185
Aromatic. In medicine, pharmacy, perfumery, &c., applied to substances, simple or compound, characterised by an agreeable odour or carminative properties, or both; as aromatic confection, a. pastilles, a. vinegar, a. bark (CORTEX AROMATICUS, white canella), &c.
AROMATIC SULPHUR-SOAP (Ed. Heger). For cleansing the teeth and mouth. A hard sulphur-coloured soap externally; on cutting, greyish-brown. Composed of soap with 10 per cent. of hyposulphite of soda, perfumed with a scent resembling oil of balm. (Hager.)
AROMATIQUE (Albin Müller, Brünn). Spirit (90 per cent.), 50 grms.; sugar, 45 grms.; extractive matter, 4 grms. (composed of cinnamon, cloves, galangal, zedoary, angelica, anise); water, 81 grms. Sold in wine-bottle-shaped bottles, and recommended for all derangements of the digestive organs. (Hager.)
ARQUEBUSADE′ (ar-ke-bŏŏ-zade′). [Fr.] Primarily, the shot of an arquebuse; but afterwards applied to an aromatic spirit (EAU D′ARQUEBUSADE, Fr.), originally employed as an application to gunshot (arquebuse) wounds.
AR′RACK (Syn. Rack) (arrack′—Brande). [Ind.] Syn. Arac, Arack, Rack‡§; Palm-spirit; Ar′ac′ca, Spir′itus Pal′mæ, S. SUC′CI P., S. ORY′ZÆ*, L.; Arack, Fr.; Arak, Ger. A spirituous liquor imported from the East Indies. The finer qualities are distilled from the fermented juice (toddy, palm-wine) of the cocoa-nut tree, palmyra tree, and other palms; and the other kinds, from the infusion of unhusked rice (rice-beer), fermented with cocoa-nut or palm-juice, either with or without the addition of coarse sugar or jaggery.
Prop., &c. It is colourless or nearly so, but like other spirit, when long kept in wood, gradually acquires a slight tinge, similar to that of old Hollands. The best kinds, when of sufficient age, are pleasant flavoured, and are probably as wholesome as the other spirits of commerce; but common arrack has a strong and somewhat nauseous flavour and odour, depending on the presence of volatile oil derived from the rice, and corresponding to that of corn-spirit. The inferior qualities are hence more heating and apt to disagree with the stomach than the other commercial spirits. In this country it is chiefly used to make punch. When sliced pine-apples are put into good arrack, and the spirit kept for some time, it mellows down and acquires a most delicious flavour, and is thought by many to be then unrivalled for making ‘nectarial punch’ or ‘rack-punch.’
Obs. Batavian arrack is most esteemed; then that of Madras; and next that of China. Others are regarded as inferior. The common par′iah arrack is generally narcotic, very intoxicating, and unwholesome; being commonly prepared from coarse jaggery, spoilt toddy, refuse rice, &c., and rendered more intoxicating by the addition of hemp-leaves, poppy-heads, juice of stramonium, and other deleterious substances.
Arrack, Facti′′tious. Syn. Mock ar′rack, Brit′ish a.; Vauxhall′nec′tar; &c. Prep. Good old Jamaica rum (uncoloured), rectified spirit (54 to 56 o. p.; clean flavoured), and water, of each 1 quart; flowers of benzoin, 1 dr.; sliced pine-apple, 1⁄4 oz. (or essence of pine-apple, 1⁄2 teaspoonful); digest, with occasional agitation, for a fortnight; then add of skimmed milk 1 wine-glassful; agitate well for 15 minutes, and in a few days decant the clear portion.
The crude Indian arrack, when subjected to distillation until it has a sp. gr. ·920, is employed in India, as proof spirit, in the preparation of official tinctures, and for other pharmaceutical purposes. A very useful stimulating application, known in India as toddy poultice, and intended as a substitute for yeast poultice, is prepared by adding freshly drawn toddy to rice flour, till it has the consistence of a soft poultice, and subjecting this to heat over a gentle fire, stirring constantly till fermentation commences.
The light brown cotton-like substance from the outside of the base of the fronds belonging to the Palmyra palm is employed by the Cyngalese doctors as a styptic for stopping the hæmorrhage of superficial wounds.
AR′ROW-ROOT. The common name of maran′ta arundina′cea (Linn.; m. Indi′ca—Tuss.); a plant of the nat. ord. Marantaceæ (Lindl.; Cannaceæ—Endl.). It was originally brought from the island of Dominica to Barbadoes, by Col. James Walker. It has since been extensively cultivated in the West Indies.
Tubers yield true ARROW-ROOT; when fresh and good they contain about 26% of starch, of which 23% may be obtained as arrow-root, and the rest by boiling.
Arrow-root. Syn. Maran′ta, Am′ylum Maran′tæ, Fæc′ula M., L.; Racine Fléchière, Pivot, Fr.; Pfeilwurz, P.-satzmehl, Ger. The starch or fecula obtained from the rhizoma or tubers of maran′ta arundina′cea (Linn.; see above), and which forms the true ‘arrow-root’ of commerce.
Prep. The fecula is extracted from the tubers when they are about 10 or 12 months old, by a process similar to that by which the farina is obtained from potatoes. In Bermuda the tubers, after being washed, are deprived of their paper-like scales and every discoloured and defective part by hand; they are then again washed and drained, and next subjected to the action of a wheel-rasp, the starch being washed from the comminuted tubers with rain-water; the milky liquid is passed through a hair sieve, or a coarse cloth, and allowed to deposit its fecula. This is then allowed to drain, after which it is again carefully washed with clean water, again drained, and, after being thoroughly dried in the air or sun, is at once packed for market. (Cogswell.) In St. Vincent (on the Hopewell Estate), a186 cylindrical crushing-mill, tinned-copper washing machines, and German-silver palettes and shovels are employed; whilst the drying is effected in extensive sheds, under white gauze, to exclude insects. In Jamaica the washed tubers are generally pulped in deep wooden mortars; machinery being seldom employed in any part of the process.
Prop., &c. A light, dull, dead-white, tasteless, inodorous powder or small pulverulent masses, feeling firm to the fingers, and crackling when pressed or rubbed; viewed by a pocket lens it appears to consist of glistening particles, which are shown by a microscope to be convex, irregular, ovoid or truncated granules, most of them, according to Mr Jackson, being ·0010 of an inch in length, and ·0008 of an inch in breadth; mixed with others varying from about double to only half that size. In its action with boiling water, and its general properties it resembles the other starches; than which, however, it is freer from any peculiar taste and flavour; and thus agrees better with the delicate stomachs of invalids and infants than the ordinary farinas.
 West Indian Arrowroot (Maranta Arundinacæa). Scale
1-1000th of an inch.
West Indian Arrowroot (Maranta Arundinacæa). Scale
1-1000th of an inch.
Comp. Similar to that of the other starches.
Pur. A large portion of the arrow-root of the shops consists either wholly or in part of the fecula or farina of potatoes or of inferior starches such as cacuma, or East Indian arrow-root, jatropha, or Brazilian arrow-root, canna, or tous les mois; or is more or less mixed with sago-meal or rice-meal: such materials can be readily detected by the microscope. Potato starch is known in commerce as ‘FARINA’ or ‘British arrow-root,’ or simply ‘arrow-root,’ whereas genuine arrow-root is always described as ‘Bermuda,’ ‘St. Vincent,’ ‘St. Kitts,’ or, at least, as ‘West Indian arrow-root.’ The substitution of the inferior farinas for genuine arrow-root is not only fraudulent on account of their inferior value, but is reprehensible in a hygienic point of view; as some of them are offensive to a delicate stomach, and exert of themselves, and still more when carelessly manufactured, a laxative action on the bowels; whereas the effect of true arrow-root is that of a slight and soothing tonic.
Uses, &c. As an agreeable, non-irritable article of diet for invalids and children, in the form of cakes, biscuits or puddings, or boiled with milk or water and flavoured with sugar, spices, lemon-juice, or wine, at pleasure. For young children a little caraway or cinnamon water is to be preferred. It is especially useful in irritation or debility of the stomach, bowels, or urinary organs, and in all cases in which a demulcent or emollient is indicated. It must not, however, be employed to the entire exclusion of other food, as, being destitute of the nitrogenous elements of nutrition, it is incapable alone of supporting life. Arrow-root jelly is prepared by first rubbing the powder up with a very small quantity of cold water, and then gradually adding the remainder boiling, stirring well all the time. Beef tea, veal broth, or milk may be used instead of water. Some persons boil it for a few minutes. This jelly, flavoured with a little genuine port wine and nutmeg, is almost a specific in cases of simple diarrhœa arising from habit or debility.
Obs. Arrow-root is imported in tins, barrels, and boxes, from all the West India Islands; and from Calcutta and Sierra Leone. The best quality was, until recently, solely obtained from Bermuda; but of late equally fine samples have been produced on the Hopewell Estate, St Vincent, and, according to Dr Ure, with the advantage of being prepared with the purest spring water, in profusion, instead of rain water.
In commerce, the word arrow-root is now often loosely used as a generic term to indicate any white, tasteless, and edible starch or fecula.
Arrow-root, Brazil′ian. Cassava-starch or tapioca-meal.
Arrow-root, East In′dian. Curcuma starch; from the tubers of the curcuma angustifolia or narrow-leaved turmeric. The maranta arundinacea is now also extensively cultivated in India under the name of maranta Indica, and the fecula therefrom extensively exported, which might, with equal propriety, be called East Indian arrow-root; but this is not the case in commerce, the whole passing as W. I. arrow-root irrespective of the place of its production.
Arrow-root, Eng′lish. Potato-starch.
Arrow-root, Portland. From the underground tubers of arum maculatum (Linn.) or wake-robin.
Arrow-root, Tahi′ti. Tacca starch or Otaheite187 salep; from the tubers of tacca oceanica.
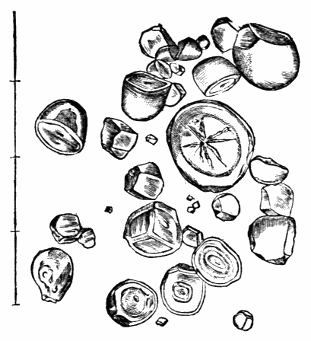 Rio, or Manihot Arrow Root. Scale 1-1000th of an inch.
Rio, or Manihot Arrow Root. Scale 1-1000th of an inch.
ARSE′′NIATE. Syn. Ar′senate; Arse′′nias, Ar′senas, L.; Arséniate, Fr.; Arse′′niksaure salze, Ger. A salt consisting of AsO4 and a metal or other basic radical; e.g., ammonio-magnesium arseniate, NH4Mg9AsO4.
AR′SENIC (-se-nĭk). As. Syn. Arsenium; Arsen′icum, Arse′nium, L.; Arsenik, A.-metall, Ger. Arsenico, Sp., It. The brittle, grey-coloured metal, or metalloid, which forms the base of the white arsenic and orpiment of commerce. Discovered by Geber in the eighth century, but first accurately described by Brandt (A.D. 1773). The poisonous properties of arsenious acid were not generally known for some centuries after its discovery. As a medicine it was first employed in intermittents in Hungary.
Sources. Arsenic is peculiar to the mineral kingdom. The metallic arsenic of commerce is obtained by roasting arsenical pyrites (MISPICKEL), in earthen tubes, or in tubular earthen retorts; the arsenic sublimes, and sulphuret of iron remains behind. On the small scale it is prepared by sublimation from a mixture of arsenious acid and charcoal or black flux. Combined with oxygen it frequently exists in mineral waters; and, in a larger quantity, in certain rivulets and streams.
Prep. A mixture of arsenious acid, 1 part; and black flux, 2 or 3 parts; is exposed to a low red heat in a Hessian crucible over which is luted a deep empty crucible, or an earthen tube, to receive the metal; the latter being kept as cool as possible. Charcoal or even oil may be substituted for black flux, and a retort of hard glass may be used, with the same result. Or the following method may be used:—White oxide of arsenic, of commerce, 2 dr.; is placed at the sealed end of a hard German-glass tube (1⁄2 × 18 inches), and covered with about 8 inches of dry and coarsely powdered charcoal; the portion of the tube containing the latter is then raised to a red heat, whilst a few ignited coals are placed beneath the oxide to effect its slow sublimation. The sublimed metal gradually attaches itself to the inside of the tube at its cool extremity. A small charcoal furnace similar to that used for organic analysis should be employed, and the process conducted under a flue to carry off any fumes that may escape. The open end of the tube should be loosely closed with a cork.
Prop. Very brittle, so much so that it may be easily powdered in a mortar; lustre highly metallic; colour steel-grey or bluish-white; texture crystalline; crystals rhombohedrons; sublimes, without fusion, at 356 to 360° Fahr., (and slowly at lower temperatures), in close vessels unaltered, but when exposed to the air with conversion into arsenious acid; at a higher temperature, in open vessels, it burns with a pale-blue flame. Its vapour or fumes have a characteristic alliaceous odour; it is slowly oxidised and dissolved by boiling water; but may be preserved unchanged in pure cold water; it rapidly tarnishes in the air, particularly when moist, a black film, consisting of metallic arsenic and arsenious acid forming on its surface; with chlorine, iodine, sulphur, and hydrogen, it unites to form definite compounds. With oxygen it forms acids, but no basic oxide. It combines with the metals in a similar manner to sulphur and phosphorus, the latter of which it resembles in many respects. These compounds are termed AR′SENIDES, formerly Arseniurets. Sp. gr. 5·7 to 5·9; sp. gr. of vapour, 1·0362.
Uses, &c. With copper it forms a white alloy (PACKFONG); and it is added to some other alloys to increase their whiteness, hardness, and fusibility. In medicine it is only used in combination. In the metallic state it is inert; but, from its great affinity for oxygen, it rapidly becomes oxidised and poisonous; and hence acts as a powerful poison when swallowed, or when rubbed on the skin. Its fumes are also highly poisonous. See Arsenious Acid (and below).
Arsenic, Tribro′mide of. AsBr3. Syn. Terbro′mide of arsenic, Sesquibro′mide of a.; Arsen′ici Bromi′dum, L. Prep. Add metallic arsenic, in powder, cautiously and in a very small quantity at a time, to pure bromine, contained in a vessel set in ice or a freezing mixture, until light ceases to be emitted; then cautiously distil into a well-cooled receiver.
Prop., &c. Solid below 68° Fahr.; above it, a yellowish fuming liquid, which boils at 428° Fahr.
Arsenic, Trichlo′′ride of. AsCl3. Syn. Chlo′′ride of a., Arsen′ici terchlori′dum, &c., L. Prep. 1. From a mixture of white arsenic, 1 part; and bichloride of mercury, 6 parts; both in powder, carefully distilled into a well-cooled receiver.188
2. Gently boil powdered white arsenic for some time in hydrochloric acid to which a little nitric acid has been added; then concentrate cautiously by evaporation, and distil as before. It is also produced, with the disengagement of heat and light, when powdered metallic arsenic is thrown into gaseous chlorine.
Prop., &c. A colourless, volatile, highly poisonous liquid, decomposed by water into arsenious acid and hydrochloric acid. It has been employed as a caustic in cancer and venereal warts; but its use requires the greatest caution.
Arsenic, Flu′oride of. AsF3. Syn. Arsenic trifluoride, Terflu′oride of arsenic. A fuming volatile liquid, prepared as the bromide.
Arsenic, Trii′odide of. AsI3. Syn. Teriodide of arsenic, Iodide of arsenic; Arsen′ici iodi′dum, A. teriodi′dum, L.; Arsenic iodure, &c., Fr. Prep. 1. From finely-pulverised metallic arsenic, 2 parts; iodide, 11 parts; mixed and gently heated in a bent glass tube, or a suitable retort, until combination is complete; the heat being then raised, and the sublimed iodide collected, and at once put into a well-stopped phial.
2. Arsenic, in fine powder, 1 part; iodine, 5 parts; triturate them together, place the mixture in a small flask or retort just large enough to contain it, and apply a gentle heat until liquefaction is complete, avoiding the formation of iodine vapour; when the odour of iodine is no longer perceptible, and the mass assumes a reddish-yellow colour and crystallises on the sides of the vessel, the operation is complete, without having recourse to sublimation. A very easy and excellent process.
Prop., &c. A deep orange-red, crystallisable solid; soluble in water, and highly volatile and poisonous. Its aqueous solution yields the iodine unchanged by rapid evaporation, but when slowly concentrated and set aside, white pearly plates are obtained, consisting of arsenious acid and the teriodide. As a medicine it combines the properties of both arsenious acid and iodine, but its use requires great caution. It has been successfully employed by Dr A. T. Thomson, Biett, and others, in obstinate skin diseases (lepra, impetigo, herpes, lupus, psoriasis, &c.), and in real or stimulated cancer.—Dose, 1⁄16 to 1⁄12 gr. (in pills or solution), gradually increased to 1⁄6 or even 1⁄3 gr. (A. T. Thomson.) Externally, 21⁄2 gr., to lard 1 oz.; of which 1 dr. may be used at a time. (Biett.)
Arsenic, Disulphide of. As2S2. Syn. Arsenic bisulphide, Bisul′phide of a., Red sul′phide of a., &c., Realgar; Réalgal, Arsenic rouge sulfure, Orpin rouge, &c., Fr.; Rothes schwefelarsenik, &c., Ger. This substance is found native at Solfaterra, near Naples, and in several other volcanic districts; but that of commerce is often prepared by distilling arsenical pyrites, or a mixture of sulphur and white arsenic, &c., in the proper proportions, as noticed under Reälgar and Red Pigments.
Prop., &c. A fusible, volatile substance; scarlet or ruby-red in mass, but orange-red in powder, by which it is distinguished from cinnabar; crystals, oblique rhombic prisms. Sp. gr. 3·3 to 3·6. Its chief use is as a pigment and in pyrotechny to make white fire. The factitious sulphide has not the rich colour of the native mineral, whilst it is much more poisonous. It is improved by re-sublimation.
Arsenic, Trisul′phide of. As2S3. Syn. Tersul′phide of arsenic, Yellow sul′phide of a., Sesquisul′phide of a., Or′piment; A. sesquisulphure′tum, Orpimen′tum, L.; Orpiment, Sulfure jaune d’arsenic, &c., Fr.; Auripigment, Operment, Rauschgelb, Ger. This sulphide, like the last, is found ready formed in nature; and is prepared artificially, by sublimation, from a mixture of arsenious acid and sulphur, as noticed under Orpiment and Yellow Pigments. It also falls as a precipitate when a stream of sulphuretted hydrogen gas is passed through an acid solution of arsenious acid or of an arsenite.
Prop., &c. Golden-yellow crystalline lumps, or a fine golden-yellow powder; crystals, right rhombic prisms; volatile; fusible; very soluble in pure alkalies, by which it is distinguished from sulphide of cadmium; and from trisulphide of antimony by being soluble in hydrochloric acid. The factitious sulphide (KING’S YELLOW) of the shops often contains 80 to 90% of white arsenic; and is, therefore, much more poisonous than the native trisulphide. Sp. gr. (native) 3·44 to 3·60.
Use, &c. As a dye, as a pigment, and as an ingredient in fireworks, and in some depilatories. Silk, woollen, or cotton goods soaked in a solution of pure orpiment in ammonia, and then suspended in a warm apartment or stove-room, rapidly lose their ammonia, and become permanently dyed of a superb yellow colour. The native sulphides (both red and yellow) are much less soluble, and hence less poisonous, than those prepared artificially. They also possess the richest colour; and are, therefore, preferred by artists and dyers. In former times, orpiment, like realgar, was employed in medicine. See Arsenic.
Arsenic, Pentasul′phide of. As2S5. Syn. Sulpharsen′ic acid, &c.; Arsen′ici pentasulphure′tum, &c., L. When a stream of sulphuretted hydrogen is transmitted for some time through a solution of arsenic acid, a precipitate of the PENTASULPHIDE is deposited after some hours’ repose. Its formation is accelerated by boiling the liquid.
Prop., &c. It greatly resembles the tersulphide in its appearance and general properties.189
Arsenic, White‡. See Arsenious anhydride.
Arsenic, Yell′ow. Trisulphide of arsenic.
ARSENIC ACID. H3AsO4. Syn. Acidum arsen′icum, L.; Acide arsénique, Fr.; Arsenicsäure, Ger.
Prep. 1. Arsenious acid, in fine powder, 2 parts; concentrated nitric acid, 6 parts; hydrochloric acid, 1 part; mix in a flask or tubulated retort, and digest, with heat, until solution is complete; after repose, decant the clear portion and evaporate, to the consistence of a thick syrup.
2. Dissolve arsenious acid in hot hydrochloric acid, and when the solution is cold add concentrated nitric acid, in small quantities at a time, until red vapours cease to be evolved, then proceed as before.
Prop. Thick syrup, occasionally forming clear transparent crystals, very deliquescent, readily soluble in water, and converted by heat into the anhydrous acid. Extremely poisonous.
Arseniates. Prep. Most of the metallic arseniates may be formed by adding a solution of a soluble salt of the metal to another of an alkaline arseniate, as long as a precipitate falls; which must be collected, washed, and dried. The alkaline arseniates may be prepared by adding the base or its carbonate to a solution of the acid, to alkaline reaction, and then evaporating and crystallising the liquid.
Prop., &c. The arseniates of the alkalies are soluble in water; those of the earths and metals insoluble, except in acids. They are isomorphous with the corresponding phosphates.
Tests. Nitrate of silver added to the solution of an arseniate gives a highly characteristic reddish-brown precipitate, which distinguishes it from arsenious acid. Nitrate of lead gives a white precipitate, and the salts of copper greenish-blue ones. Pure lump-sugar dissolved in an aqueous solution of this acid becomes, in a few hours, of a reddish colour, and afterwards of a magnificent purple. Heated with charcoal it evolves a garlic-like odour, and is reduced to the metallic state. The suspected liquid being treated with sulphurous acid and boiled for a short time, the arsenic acid loses oxygen and is converted into arsenious acid, which may be tested for as such. Sulphuretted hydrogen does not precipitate a solution of arsenic acid, or an acidified arseniate, until after the lapse of several hours; and alkaline and neutral solutions not at all.
ARSENIC ANHYDRIDE. As2O5. Syn. Anhydrous arsenic acid, Arsenic acid; Acidum arsenicum, L.; Acide arsénique, Fr.; Arseniksäure, Ger. Best prepared by igniting the arsenic acid, in a platinum crucible, at a low red heat, as long as water is given off.
Prop. White deliquescent substance, and violent poison, readily soluble in water to the acid.
ARSENIOUS ACID. See Arsenious anhydride.
ARSE′′NIOUS ANHYDRIDE. As2O3. Syn. Ar′senious acid, Ar′senic, White a.; Acide arsénieux, Arsenic blanc, Oxyde, Fr.; Arsenigsäure, Arsenichste s., Ger.; Arsenico bianco, It.; A. blanco, Sp. The arsenic, or white arsenic, of the shops.
Sources. The white arsenic of commerce is principally imported from Germany, where it is obtained in the process of roasting arseniuretted cobalt ores, in making zaffre. At Altenburgh it is procured from arsenical iron pyrites (mispickel); and at Reichenstein from native arsenide of iron. About 900 to 1000 tons are also annually collected at Cornwall, being principally a secondary product of the process of roasting grey copper ore and white mundic. The British arsenic works in that county are perhaps the finest in the world. The usual plan is to roast the powdered ore in muffle-furnaces; by which its arsenic is converted into arsenious anhydride, which escapes as vapour (smelting-house smoke), and passing into the condensing-chambers, is deposited in a pulverulent state, forming the flowers of arsenic, or rough white arsenic, of the smelters, (the giftmehl or poison-flour of the Germans). The crude article obtained in this way is purified by re-sublimation in suitable iron pots or other iron vessels, before it is fit for sale. It then forms a semi-transparent vitreous cake, which gradually becomes opaque, and of snowy whiteness, by exposure to the air, and at length acquires a more or less pulverulent state on the surface.
In Silesia the crude arsenious anhydride obtained from arsenical pyrites is refined by190 sublimation as follows:—For this purpose the cast-iron vessels (a) are employed. Upon these are placed iron rings or collars (b, c, d) and a hood (e), communicating by means of tubes with a series of chambers, of which the first only is shown in i. The flanges of the cast-iron collars and all other joints having been thoroughly luted, the fire is lighted and the heat so increased as to cause the semi-fusion of the arsenious anhydride, which, after cooling, exhibits a peculiarly porcelain-like appearance, at first being as transparent as glass.
Prop. Crystals (obtained by careful sublimation, or by cooling a boiling aqueous solution), usually transparent, regular octahedrons (fig. 1), but sometimes, though rarely, assume the form of tetrahedrons (see 2). When prepared on the large scale it forms large, glassy, colourless or yellowish-white, transparent or semi-transparent cakes or porcelain-like masses (vitreous arsenious anhydride, glacial a. a.), which soon becomes opaque on their exterior, and often friable and pulverulent; odourless; volatilises at 380° Fahr.; fumes odourless, unless carbonaceous organic matter be present, when they smell strongly like garlic; heated under pressure it liquefies and forms a transparent glass; taste faintly sweetish, with a slight acidity and astringency, not perceived until some minutes after being swallowed. The opaque variety is soluble in 80 parts of water at 59° Fahr., and 7·72 parts of boiling water; but on cooling to 60°, only about one third of this quantity continues in solution. The transparent variety is soluble in 103 parts of water at 59°, and 9·3 parts of boiling water. Both soluble in alcohol, syrups, oils, and spirits, and freely so in alkaline lyes and hydrochloric acid; organic matter generally impedes its solution; solutions redden litmus; heated with organic matter it is reduced to the metallic state. Sp. gr. 3·5 (lowest opaque var.) to 3·8 (highest transp. var.).
Arsenites. True arsenious acid (HAsO2) has never been obtained in a satisfactory condition, but its salts are readily obtained by dissolving arsenious anhydride in a solution of the base, or by double decomposition. They are generally white, nearly all insoluble, except those of the alkalies, and all soluble in acids.
Tests, Detec., &c. Owing to the importance of the subject, and for convenience and facility of reference, the leading tests for the arsenites and arsenious anhydride are noticed alphabetically below; to which a few general remarks on their application, under the various circumstances that occur to the chemist and toxicologist, are appended. When not otherwise stated, it is to be understood that they are to be applied to pure, or nearly pure and colourless solutions of arsenious acid or the arsenites. Ammonio-nitrate of silver gives a well-marked yellow precipitate of arsenite of silver in an aqueous or arsenious anhydride solution which is soluble in ammonia and in dilute nitric acid.
Crystallisation Test.—A very minute quantity of arsenious acid placed in a small tube (arsenic-tube), and heated in the flame of a spirit lamp, gives a crystalline sublimate, which collects on the cooler portion of the tube, and which, when examined by a pocket lens, is found to consist of sparkling octahedral crystals (see engr.)
 (Magnified.)
(Magnified.)
Ellis’s Test.—This is a modification of the ‘nascent hydrogen test,’ in which the suspected gas is passed through a tube containing slips of copper leaf or riband, or still better pure oxide of copper, gently heated; the end of the tube communicating with the atmosphere being drawn to a capillary size, at which the gas may be inflamed and tested, as in ‘Marsh’s Apparatus.’ (See engr.) If arsenic be abundant in the gas, the copper will be almost instantly covered over with a coating of metallic arsenic; and after continuing the heat for a few minutes it will present a beautiful silvery surface, and may then be submitted to further examination.

a, Flask containing the suspected fluid, dilute sulphuric acid, and zinc.
b, Funnel.
c, Tube containing the copper-leaf or c.-riband, and heated by the lamp d.
e, Support.
f, Capillary end of tube c, with the gas inflamed.
Lassaigne’s Test. (Adopted by the French Academy.) This consists in passing the gas generated in the suspected liquid, through a solution of nitrate of silver. (See engr.) When arsenic is present black flocculi of metallic silver are deposited, and arsenious acid remains in solution mixed with nitric acid and some arsenide of silver. The filtered191 liquor, treated with ammonia, will now give a characteristic yellow precipitate of arsenite of silver; or a little dilute hydrochloric acid may be cautiously added to precipitate any remaining nitrate of silver, and the liquid, after filtration, tested for arsenic either in a Marsh’s apparatus, or with any of the liquid tests; or it may be evaporated to dryness, when its arsenious acid will be converted into arsenic acid by the nitric acid present, and will then be found to give the usual brick-red precipitate of arseniate of silver with a solution of the nitrate of that metal. See Marsh’s Test.
a, Bottle containing dilute sulphuric acid, zinc, and suspected fluid.
b, Funnel for supplying the bottle with acid.
c, c, Supports.
d, Tube filled with asbestos.
e, Bent tube to convey the liberated gas.
f, Glass vessel containing a solution of nitrate of silver.
Marsh’s Test. Some of the suspected liquid is mixed with dilute sulphuric acid until strongly acid, and is then poured upon some pure granulated zinc, or clippings or other small pieces of zinc, previously placed in the apparatus; hydrogen gas is immediately evolved, and, if arsenic be present, unites with it, forming arseniuretted hydrogen gas, which escapes by the aperture b (see engr.), and may be recognised as follows:—
It possesses a garlic-like odour.
It burns with a bluish-white flame and emits a whitish smoke.
If a piece of window-glass, or a white porcelain plate or saucer, be held a short distance above the flame, a fine pulverulent film of arsenious acid is deposited on it. See (fig.) above.
If the cold plate be held in the flame, so as to slightly impede the combustion of the gas, a blackish-brown deposit of metallic arsenic is obtained, more or less deep, brilliant, and glistening. Both these deposits may be obtained simultaneously by holding nearly vertically over the flame a glass tube about 8 or 10 inches long and 3⁄8ths of an inch in diameter. See (fig.) above.
A solution of arsenious acid may be obtained by letting the flame play upon 3 or 4 drops of water placed on the under side of the piece of glass or china, to which the liquid tests may be then applied. Another plan is to apply drops of the liquid tests to the plate as above, and to let the flame play on them successively.
The true arsenical spot or film is of a blackish-brown colour, and generally of a very deep hair-brown, usually surrounded at the circumference, with a white film of arsenious acid; whilst that of antimony, which in some points is similar, is of a deep black colour, and but feebly lustrous, and, when viewed by transmitted light, appears smoky black; whereas an arsenical spot viewed in the same way appears brown. It is further distinguished from others by—Treated with concentrated nitric acid, it instantly disappears, leaving upon the surface of the liquid traces of the metal, which only dissolve on the application of heat. This solution, gently and carefully heated, leaves a white residuum, which, when cold, gives with a concentrated solution of nitrate of silver a dull-red precipitate of arseniate of silver.—The nitric solution treated with a few drops of sulphurous acid, and subsequently with sulphuretted hydrogen, gives a canary-yellow precipitate of trisulphide of arsenic, which readily redissolves, forming a colourless solution with ammonia.—The arsenical spot, when heated, is turned bright yellow by sulphuretted hydrogen, and is then readily dissolved, as before, by ammonia, and by its bicarbonate; whereas one of antimony is turned of a deep orange-red, or reddish-brown, by sulphuretted hydrogen, is not readily dissolved by ammonia, and is scarcely or not at all affected by bicarbonate of ammonia.—It is freely soluble in and removed by hypochlorite of soda; a reagent which does not affect antimonial spots. Heated by a flame of pure hydrogen an arsenical stain rapidly disappears. A mixed stain of antimony and arsenic does not disappear by the action of the last two reagents, and is shown to contain arsenic by the two first tests above. When hydrochloric acid is present zinc stains192 are sometimes formed, but they do not resemble those from arsenic. The flame which produces it is very pale blue or bluish-white; whereas antimoniuretted hydrogen burns with a pale green or greenish-yellow flame, and a white smoke, both of which are characteristic.
Obs. Marsh’s test is admirable for its simplicity, delicacy, and trustworthiness, as well as for the ease of its application. It is adapted to all liquids, whether colourless or coloured, which are not so glutinous as to inconveniently froth during the extrication of the hydrogen.[79] Various modifications of the original apparatus have been proposed to obviate this difficulty; among which the one chiefly deserving notice is figured in the margin. It consists of a bent tube having two large bulbs blown in it, and fitted with a stop-cock and jet in the usual manner. In this case the grains or fragments of zinc are put into the lower bulb (a). It is, however, worthy of remark, that, with ordinary care and skill, a simple wide-mouthed bottle, furnished with a tube and cock, will often be found to answer quite as well as more costly apparatus; as the fluid is less liable to froth than in a narrow tube. Even a common quinine-phial, or a 4-oz. or 6-oz. medicine phial, fitted with a piece of glass tube of very small bore, or even with a piece of a common tobacco-pipe, for a burner (see engr.), may be used when no more convenient instrument is at hand.
A film of oil placed on the surface of the liquid tends considerably to lessen the frothing.
Objec., precau., &c. Objections have been raised to this mode of testing, from the great frothing which often occurs with organic mixtures, and from antimony and imperfectly charred organic matter also forming crusts somewhat resembling, to the inexperienced eye, those produced by arsenic. But these objections are invalid, because there are easy means of purifying the liquid before testing it, and of discriminating between true arsenical spots or deposits and false ones. Another objection is, that both zinc and sulphuric acid sometimes contain arsenic; but to obviate this difficulty, we have only to use them when perfectly pure; and to test them by means of the apparatus before pouring the suspected liquid into it. Indeed, these objections apply with equal force to all those tests which depend on the production of nascent hydrogen. The precaution necessary to success, and to reliable results, is to set the apparatus with simple zinc, acid, and water, and after it has worked a short time to test the evolved gas for arsenic (as above); when, if no trace of that substance is detected, the suspected fluid, in which the organic matter (if necessary) has been destroyed by any one of the methods hereinafter pointed out, may be added, and the operation continued. Care should also be taken not to light the jet of gas before all the atmospheric air is expelled from the apparatus, as without this precaution an explosion may take place.
Modification of Marsh’s Test.—Davy. This process consists in the use of sodium amalgam instead of zinc and sulphuric acid, both of which are liable to be contaminated with arsenic. Sodium, on the other hand, has never been found to contain arsenic, and mercury only very rarely; but should it exist in that metal, it can be easily removed by digesting the mercury in dilute nitric acid, and afterwards well washing it with water.
One part by weight of sodium to 8 or 10 parts of mercury forms a very good amalgam. The mercury is placed in a test-tube, and the sodium gradually added in small portions; the metals readily combine, forming an alloy, liquid whilst hot, but hard and brittle when cold.
The author uses this amalgam by placing the suspected solution, or solid substance, along with a little water in a test-tube, then adding a small piece of amalgam about the size of a grain of wheat, and quickly covering it with a piece of white filtering paper or the lid of a porcelain crucible moistened with a dilute solution of silver nitrate slightly acidified with nitric acid. If arsenic is present, a dull black or deep brown stain on the paper or porcelain will be developed on the moistened part, owing to the silver being reduced to the metallic state by the arseniuretted hydrogen. The solution may be made by dissolving 20 gr. of nitrate of silver in an ounce of distilled water acidulated with 2 drops of strong nitric acid.
It is advisable to place between the moistened paper or lid and the tube a small disc of bibulous paper, to prevent any particles of the liquid producing minute black spots, and thus interfering with the results. 1⁄1000th part of a grain of arsenious acid in 1 c. c. of distilled193 water gives a very decided effect in a few moments, but much smaller quantities may be detected, e.g., the 1⁄100000th or even 1⁄1000000th part of a grain in 1 c. c.
This method is applicable not only to arsenic as arsenious acid, but also to other compounds of arsenic, soluble or insoluble in water, e.g., orpiment and realgar, the alkaline arsenates, and even the metal itself if in powder. Organic matter interferes but very little with this method. Antimony, as in Marsh’s process, will produce, with the sodium amalgam, results similar to those of arsenic; this, when brought into contact with the nitrate of silver, forms a black antimonide of that metal.
Fleitmann, however, pointed out that antimoniuretted hydrogen is not evolved from strongly alkaline solution, and, as in this case, the action of the sodium amalgam is to render the mixture quickly alkaline, only a very small quantity of antimony present will be evolved, and by previously rendering the mixture strongly alkaline the evolution of that gas may be almost entirely prevented.
It may be occasionally necessary to determine whether the stains on the paper moistened by the silver solution are due to arsenic or antimony. It is then best to digest the paper-stain in sulphide of ammonium, the metal present being converted into a sulphide, and dissolving in the excess of the alkaline salt, leaving the silver sulphide undissolved; the alkaline solution when evaporated will, in the case of arsenic, leave a bright yellow residue, almost insoluble in hydrochloric acid; whereas in the case of antimony an orange-coloured residue will remain soluble in that acid. Dr Russell observes that hydrogen alone is capable of reducing silver solution to the metallic state, but acknowledges that this action is exceedingly slow. Pellet, on the other hand, maintains that pure hydrogen when passed through solutions of soda and nitrate of silver has no action at the ordinary temperature; but he states that the silver salt which has been fused possesses an alkaline reaction in solution, and hydrogen thus produces a slight precipitate, which can be prevented by adding a drop or two of nitric acid.
Davy, however, found in his experiments only the faintest possible effect of the reducing action of pure hydrogen in solutions of caustic soda and nitrate of silver.
Finally, the author mentions that where paper is used with the silver solution we must not forget that the silver alone will after some time blacken the paper, especially if exposed to light; but this gradual change is very unlike the quick effect produced by arseniuretted or antimoniuretted hydrogen. (‘Chem. News,’ xxxiii, 58-63.)
Nascent Hydrogen Test. The apparatus used may be similar to that figured in the engr. The plan followed in the laboratory of Giessen is to heat the long tube through which the gas passes to redness in several parts, to produce distinct metallic mirrors; and then to remove the tube from the hydrogen apparatus and transmit a very feeble stream of dry sulphuretted hydrogen through it, the metallic mirrors being at the same time heated by means of a common spirit lamp from the outer towards the inner border or extremity. If arsenic alone is present, yellow trisulphide of arsenic is formed within the tube; if antimony alone is present, an orange-red or black trisulphide of antimony is produced; and if the mirror consists of both metals, the two sulphides appear side by side, the sulphide of arsenic, as the more volatile, lying invariably before the sulphide of antimony. If dry hydrochloric acid gas be now transmitted through the tube, without application of heat, no alteration will take place if sulphide of arsenic alone is present, even though the gas be transmitted through the tube for a considerable time. If sulphide of antimony alone is present, this will entirely disappear; and if both sulphides are present, the sulphide of antimony will immediately volatilise, whilst the yellow sulphide of arsenic will remain. If a small quantity of ammonia be now introduced into the tube, the sulphide of arsenic is dissolved, and may thus be readily distinguished from sulphur, which perhaps may have separated.
Reduction Test. A small quantity of the suspected sample, in the state of powder, is mixed with twice its weight, or more, of some reducing agent or flux, and the mixture is placed at the bottom of a very small glass tube, and heated in the flame of a spirit lamp for some time, when the arsenic gradually sublimes, and condenses in the cooler portion of the tube, under the form of a metallic crust, mirror, or ring. A common test-tube, if of very small diameter, may be employed; but those known as the reduction tubes of Liebig, Rose, or Berzelius are undoubtedly the most convenient and efficient. (See engr.)
Liebig’s method is by using a mixture of equal parts of dry carbonate of sodium and cyanide of potassium. The suspected substance, perfectly194 dry and in powder, being first introduced into a Berzelius’ tube, is then covered with 6 times the quantity of this mixture, and so that the whole will not more than half fill the bulb. A very gentle heat is next applied, to expel any adhering moisture from the powder and the tube, after which a strong heat is applied to the bulb, and continued for some time, to effect the entire reduction and sublimation of the arsenical compound.
a, The arsenical mixture.
b, Arsenical ring.
The best fluxes to use are ferrocyanide of potassium dried at 212° F., calcined bitartrate of potassium, cyanide of potassium, and powdered charcoal.
The metallic ring is proved to be arsenical by the properties and tests previously noticed. Should it be imperfectly formed, or masked by decomposed organic matter, the portion of the tube which contains it may be cut off with a file, next coarsely powdered, then reintroduced into another arsenic tube, and the exposure to heat repeated.
The characteristics most simple and well-marked are—
The volatility of the deposit when heated, shown by its escaping from the hotter portion of the tube and condensing on the cooler part higher up or further on.
Its conversion into minute octahedral crystals of arsenious anhydride, when repeatedly chased up and down the tube by the cautious application of the flame of a spirit lamp first to one part, and then to another. The character of these crystals with respect to volatility, lustre, transparency, and form, is so exceedingly well marked that a practised eye may safely identify them, though their weight should not exceed the 1⁄100th or even the 1⁄250th part of a grain. A pocket lens is here serviceable. The form of the crystals is very evident with a microscope of 4 powers. Oxide of antimony never forms octahedrons, but only prisms.
In employing this test, particular care must be taken to avoid soiling the sides of the tube in inserting the mixture, and that the substances operated on are perfectly dry; as unless this is attended to, the experiment does not succeed. The common plan is to introduce the mixture through a small paper funnel or tube extemporised for the purpose. The heat at first should be gentle, and merely sufficient to expel any adhering moisture from the mixture and the inner surface of the tube; after which (except where otherwise ordered) the upper portion of the mixture should be strongly heated, and then the bulb or bottom of the tube exposed to the full flame. After the operation is complete the bulb or lower portion of the tube is usually removed by a file, and the portion containing the deposit hermetically sealed, when it may be preserved, unaltered, for any length of time, ready to be produced as evidence if required.
This test is usually regarded as decisive; as we here actually obtain the arsenic in a solid form, recognisable by the most unequivocal characters.
Reinsch’s Test; Cupro-arsenical Test. The suspected solution is strongly acidulated with hydrochloric acid (1 to 6 or 8), and after being raised to ebullition in a porcelain or glass vessel, a piece of bright and clean metallic copper about 1⁄2 inch long and 1⁄4 inch wide in the form of gauze or foil, but preferably the first, is added, and the whole boiled together. The time required for the ebullition varies according to the strength of the solution; when weak it should be continued for at least a quarter of an hour. When the quantity of arsenic in the suspected liquid is very small, at least half an hour should elapse before the removal of the copper. In solutions containing a notable quantity of arsenic, a few seconds is often sufficient to obtain a coating; but which, for safety sake, may be extended to two or three minutes, or even longer. Liquids rich in organic matter also require longer boiling than those nearly free from it. The coated copper, which has now acquired a characteristic iron-grey colour, is then taken from the liquid, carefully washed in distilled water, in alcohol, and (if greasy) in ether, next dried on blotting-paper, and then either cut into small pieces, or rolled into a small coil or cylinder. It is then heated in a reduction-tube over a spirit lamp, when the metallic arsenic forming the coating is volatilised, and yields a sublimate of minute octahedral crystals of arsenious anhydride; or, if the tube be very small, or any reducing agent be added, a bright metallic ring. When the coating on the copper is sufficiently thick, it may be scraped off with a knife, and heated separately in an arsenic-tube.
This test is invaluable as affording a certain and ready means of abstracting arsenic from its solution, whether pure or mixed with organic matter. The contents of the stomach or other viscera may thus be at once examined, without any tedious preliminary operations. In this way Dr Christison discovered the presence of arsenic upwards of four months after interment; and we have ourselves found it two years and eight months after interment. The195 coated copper may be preserved unharmed for years. Dr Taylor found that the 1-8th of an inch in one of these deposits that had been kept in paper nearly fourteen years gave a well-marked ring of octahedral crystals when heated.
Sulphuretted Hydrogen Test; Sulphur Test. This produces a bright yellow precipitate of trisulphide of arsenic (orpiment) in solutions containing a free acid; but acts slowly and imperfectly on pure and neutral solutions, and does not disturb those that possess an alkaline reaction. The suspected liquid should therefore be slightly acidulated with hydrochloric or acetic acid before applying this test, unless it be already acid, when it is better first to neutralise it with an alkali, and then to add the acid. The transmission of the gas through the liquid (see engr.) should be continued for at least half an hour; when the end of the conducting tube, after being well rinsed in the liquid, is removed, and the glass, lightly covered with a piece of porous paper, set aside in a temperature of about 100° Fahr., until the odour of sulphuretted hydrogen is completely lost. The precipitate is now collected on a small filter, washed with pure water, and dried by a gentle heat. It is then placed in a watch-glass or small capsule, and redissolved in a little liquor of ammonia, which is then again expelled by heat; or it may be at once submitted to confirmatory tests. It is shown to contain arsenic by its ready and perfect solubility in ammonia, and in solutions of the fixed alkalies, their carbonates and bicarbonates, and in alkaline sulphides; by being nearly insoluble in hydrochloric acid, even when concentrated and boiling; and by yielding a metallic mirror when mixed with a flux and submitted to the reduction-test (which see).
Sulphuretted-hydrogen water and sulphydrate of ammonium act in a similar way to gaseous sulphuretted hydrogen; but much less effectively.
For accuracy, the sulphuretted hydrogen should be washed by passing it through a small bottle containing a little pure water, or dilute sulphuric acid, before allowing it to enter the arsenical liquor. The reduction of the newly precipitated sulphide is generally regarded as the most important part of the investigation, and requires great care and attention. An extremely elegant and sensitive method of effecting this is by heating the mixture in a stream of dry carbonic acid gas. This method has been followed by Drs Babo and Fresenius with the most satisfactory results, and is thus performed:—(A) is a capacious flask for the evolution of carbonic acid, half filled with rather large pieces of solid limestone or marble (not chalk). To one aperture of the doubly perforated cork, a funnel-tube (a) is adapted, which nearly reaches to the bottom of the vessel; to the other aperture a tube (b), by means of which the gas evolved is conducted into a flask of smaller size (B), in which it is washed and dried by concentrated sulphuric acid. The tube (c) conducts the carbonic acid into the reduction-tube (C), which is shortened in the engr., and must be made of difficultly fusible glass. When the apparatus is prepared, the sulphide of arsenic intended for reduction is rubbed in a small basin, previously heated in a water-bath, with about twelve parts of a well-dried mixture consisting of 3 parts of dry carbonate of sodium and 1 part of cyanide of potassium (prepared by Liebig’s method). The mixed powder is then placed on a small strip of card-paper beat into the shape of a gutter, which is next pushed into the reduction-tube up to the point (f), and the tube is turned half round. In this manner the mixture is deposited without soiling any other part of the tube; after which the strip of card-paper is cautiously withdrawn.196 The reduction-tube is then, by means of the cork (e), fixed in its place; a moderate stream of carbonic acid gas is evolved by pouring hydrochloric acid into the funnel-tube (a), and the mixture carefully dried, by very moderately heating the tube along its whole length, by means of a small spirit lamp. When the gas-stream has become so low that the bubbles pass through the sulphuric acid at intervals of about a second, the spot (k) is heated to redness by means of a spirit lamp. When this point is attained another strong spirit-flame is applied to the mixture, progressing from (d) to (f), until all the arsenic is reduced and volatilised (the first flame at the same time continuing in action at (k)).
The reduced arsenic recondenses at the spot (g), forming a mirror, whilst an exceedingly small portion escapes at the capillary orifice (h), and fills the air with its garlic-like odour. The second spirit lamp is at last slowly advanced towards the other lamp, or the spot (k), so as to drive towards (g) all the arsenic which has adhered to the walls of the wider part of the tube. Both lamps are then removed, the tube closed at the point (h) by fusion, and heat applied, progressing from the point (h) towards (g), to contract the mirror on that side also, which increases its beauty and distinctness. The tube is then cut off at (f), and hermetically closed and sealed. In this state it becomes a permanent evidence which may be referred to in any future proceedings. Neither sulphide of antimony nor any other compound of antimony yields a metallic mirror or ring when treated in this way. Less than 1⁄300 gr. of trisulphide of arsenic thus gives a very distinct and beautiful mirror; and even 1⁄500 gr. a clearly perceptible one.
Voltaic Test. The wires from the opposite poles of a voltaic battery are immersed or brought in contact with a little of the arsenious solution placed in a capsule or on a piece of window glass. If arsenic be present it is developed at the negative pole; and if this be formed of copper wire, it becomes whitened and assumes the appearance of polished steel or silver, in consequence of the formation of arsenide of copper.
Detection of Arsenic in Organic Mixtures. Of the tests those which act by producing coloured precipitates are only applicable, with any degree of certainty, to perfectly limpid and colourless liquors. Those depending on the extrication of arseniuretted hydrogen are partially free from this inconvenience; but even here, if the suspected liquid be more than slightly charged with organic matter, so much frothing ensues, as to render the process nearly unmanageable. In this respect Reinsch’s Test possesses advantages over all others, as it may be applied even to coloured liquids containing a considerable quantity of organic matter, without these being subjected to any preliminary process, and without danger of failure. In some cases also, as with liquids possessing only a slight degree of consistency or colour, the arsenic may be separated, after simple filtration and acidulation with hydrochloric acid, by a stream of sulphuretted hydrogen, in the usual manner. The reduction-test is only applicable to solid arsenious acid, or to compounds of arsenic obtained by means of other tests or processes. In toxicological examinations the poison is almost always to be sought for in mixtures loaded with organic matter, and under other conditions even more embarrassing. Soon after arsenic is swallowed it enters the circulation, contaminates the various tissues, localises itself in certain viscera, and is eliminated in the excretions. Hence it becomes necessary not only to examine the solids and liquids in which it is suspected the poison has been administered, the vomited matter, and the contents of the stomach and primæ viæ, but also, in fatal cases, the stomach itself, the liver, blood, muscles, and more especially the urine.[80] In such cases the stomach is the part first laid open, and a careful examination is made of its contents and coats in order to detect any undissolved particles of the poison, a pocket lens being employed, if necessary, in the search. If any particles, however minute, are found they are carefully collected and submitted to the reduction-test. If the reverse be the case, the stomach (cut into small pieces), together with its contents, is submitted to some further process, to obtain a solution suitable for the application of the usual tests. The liver, also some muscle, and any other portion of the body that may be selected, are likewise separately treated in the same manner. We have here both solid and liquid organic matter to operate on, and the problem for solution is the abstraction of their arsenic in the simplest and most certain manner, and in a form in which its presence may be demonstrated by tests. This subject has long engaged the attention of the most eminent chemists and toxicologists, and various plans have been proposed for the purpose, among which the following appear to be the most valuable and that usually adopted:—
(Reinsch.) Solids (as the stomach, liver, &c.) are cut into small fragments and boiled in a glass vessel with water acidulated with about 1-4th of its volume of hydrochloric acid, until the tissues or fragments are entirely broken down into flakes or grains, when the whole, after filtration, is again heated to the boiling-point, and tested as described under Reinsch’s test (see ANTIMONY). Liquids do not require this preparation.
Reinsch’s test is inapplicable when, as sometimes happens, the arsenic sought after may be in the state of one of the sulphides—either197 as orpiment or realgar—a not improbable contingency, when it is remembered that, although arsenious anhydride or white arsenic is the form most generally used for criminal or suicidal purposes, the yellow and the red varieties being largely employed in workshops where fireworks are manufactured, have not unfrequently been had recourse to. Again, when the examination of a corpse long buried and disinterred takes place, it must be borne in mind that the arsenious anhydride taken by the deceased has, by the decomposition of the body, become converted into sulphide. In these cases the hydrochloric acid necessary for the performance of Reinsch’s test fails to effect the solution of the sulphide.
Mr Blyth says: “It is found that the post-mortem change into orpiment is never quite complete, so that for the detection of arsenic in solid organic substances, such as the tissues of the body, the best general method is most decidedly to convert the arsenic, if present, into the volatile chloride; and according to Dr Taylor, there is always sufficient arsenic (if present at all) unchanged into sulphide to ensure success. The only necessary caution is that the substance be thoroughly dried, and that the reagents be pure. After drying it is placed in a retort with fuming hydrochloric acid, and slowly distilled by the heat of a sand-bath. The distillate contains chloride of arsenic (if arsenic was present), and may be submitted to further tests.”
Estim. This may be effected in various ways:—
1. Gravimetrically:—Arsenic is usually WEIGHED under the form of arsenate of lead, arsenate of sesquioxide of iron, tersulphide of arsenic, (metallic) arsenic, or (directly) as arsenious anhydride. The last three only, as the more simple and convenient, will be noticed here:—
As trisulphide:—The whole of the arsenic being precipitated by a stream of sulphuretted hydrogen, with the necessary precautions, in the manner already noticed, the precipitate, after being carefully collected, washed, and dried, is purified by redissolving it in pure ammonia water, and evaporating the resulting solution in a weighed watch glass or capsule by the heat of a water-bath. It is then dried at a temperature not above 212° Fahr., and finally weighed. Each grain of the tersulphide so found corresponds to ·80487 gr. of arsenious acid, or ·61 gr. of metallic arsenic.
As (metallic) Arsenic:—Obtained by one of the processes already given. Each gr. represents 1·32 gr. arsenious acid.
As Arsenious anhydride:—Obtained in a weighed capsule or tube, either by the crystallisation or sublimation test. The weight is the answer sought for arsenious anhydride. Each gr. of this is equiv. to ·75758 gr. of metallic arsenic.
Volumetrically. (Method of F. Mohr.) This depends on the fact that an aqueous solution of arsenious acid, or of an alkaline arsenite, when mixed with an excess of saturated solution of pure bicarbonate of soda and a little starch-paste, has its arsenious acid converted into arsenic acid by a solution of iodine. A standard solution of iodine is, therefore, an appropriate arsenim′eter for the above mixture. The solution of iodine is added until the blue starch-reaction just begins to appear, the arsenious solution having been previously exactly neutralised with pure carbonate of soda if acid, or with pure hydrochloric acid if alkaline. The results are accurate when no substance capable of oxidising or decomposing iodine is present in the liquid tested.
Phys. eff., &c. Arsenious anhydride or white arsenic is alike destructive to vegetable and animal life. Seeds soaked in any but a very weak solution of it lose their power of germination, and buds plunged in it become incapable of expanding into flowers. When applied to the leaves, roots, or stems, absorption takes place, and the plant soon perishes. On combustion it evolves the characteristic garlic-like odour of arsenic, and arsenic may be discovered in its substance by chemical tests. According to Jäger, Gilgenkrantz, and Pereira, a few of the lower order of the algæ are occasionally developed in solutions of arsenious acid. To all animals, from the infusoria up to man, arsenic proves deleterious, although in different degrees, the highest susceptibility of its effects existing in man on account of the superiority of his development. In all of them death is preceded by inordinate actions and increased evacuations, especially from the mucous surfaces. Difficult respiration, thirst, vomiting, and convulsions are the leading symptoms which gradually develope themselves as we approach the higher grades of the system. (Jäger.) In very small or therapeutical doses, properly administered, it is a valuable medicine, and acts as a tonic, alterative, and antispasmodic attenuant, and externally as an escharotic. In slightly increased medicinal doses, or long-continued small doses, nausea, vomiting, purging, griping, debility, emaciation, and all the effects of slow-poisoning, occur in succession—a gradual sinking of the powers of life, without any violent symptom; a nameless feeling of illness, failure of the strength, an aversion to food and drink, and to all the enjoyments of life. Redness of the conjunctiva and eyelids, headache and giddiness, spasms, eczematous eruptions, numbness and paralysis of the limbs, and ptyalism, are also frequent and well-marked symptoms of slow poisoning by arsenic. In an excessive or poisonous dose the symptoms are rapid and violent, usually indicating extreme gastro-intestinal inflammation and disorder of the cerebro-spinal system, and often occasioning death in from one to three days. The smallest fatal dose found recorded by Christison is 41⁄2 gr., taken in solution. The subject was a child 4 years old, and death occurred in six hours. 21⁄2 gr. destroyed198 a robust girl in 36 hours. (Letheby.) 2 gr., in solution, are suspected to have caused the death of a full-grown woman. 2 or 3 gr. may be a fatal dose. (Dr A. Taylor.) Notwithstanding these facts much larger quantities have been taken, under peculiar circumstances, with comparative impunity; and cases are not wanting in which even enormous quantities have produced very trifling effects.
The dose for animals is—Cattle, 5 to 10 grains. Horse, 5 to 10 grains. Sheep, 1 to 2 grains. Pig, 1⁄2 to 2 grains. Dog, 1⁄15th to 1⁄10th of a grain.
Under all circumstances arsenious anhydride is, undoubtedly, one of the most powerful of the mineral poisons; and in whatever form or way it is introduced into the system it exerts the same deleterious influence. In all cases, in sufficient doses, its action is to increase the secretions, diminish the contractility of the voluntary muscles, and to produce convulsions, prostration and death.
Arsenic is a non-accumulative, irritant poison, and exerts no decided chemical or corrosive action on the tissues. (Taylor.)
Pois., &c.—Symp. These sometimes begin to appear within half an hour after the poison has been taken, or even sooner; but much more generally, not until after the lapse of some hours. They usually commence with nausea and distress at the stomach, followed by thirst, often intense, and a sense of burning heat in the bowels; then come on constriction of the œsophagus, violent vomiting, severe colic pains, tenesmus, and excessive and painful purging, the stools being occasionally bloody; but pain, vomiting, &c., do not invariably occur. The pulse is generally quick, small, feeble, and irregular—sometimes scarcely perceptible, and the heart’s action is irregular and tumultuous. The tongue is dry and furred; the respiration difficult and panting; the urino-genital apparatus is often affected; there is pain and difficult micturition, and sometimes entire suppression of urine; faintings, coldness of the limbs, and cold sweats, with other signs of debility, intervene. Itching, and eczematous eruptions of the skin, trembling, painful cramps, and contractions of the extremities, and violent convulsions often follow; and after these, a greater or less prostration of strength, which induces a deceitful calm. At length the heart’s action abates, the skin becomes suffused with a cold clammy sweat, and the sufferer dies from exhaustion. The progress, succession, and precise character of the symptoms are modified by the idiosyncrasy of the individual, the quantity of the poison, and the manner in which it has been taken; and are seldom all present in the same person.
Treatm. If vomiting has commenced it should be promoted by tickling the throat, and administering a large quantity of gelatinous hydrated peroxide of iron, or other appropriate antidote, in divided doses, mixed with a large quantity of warm or tepid water, strongly sweetened with sugar. If vomiting has not commenced, which is rare, it must be excited by administering 15 to 20 gr. of sulphate of zinc, or ipecacuanha (or in the absence of these, a teaspoonful of flour of mustard) in a tumbler of tepid water, and tickling the throat as before. If these means fail in rapidly inducing copious vomiting, the dose must be repeated, or the stomach-pump had recourse to. Altogether as much as 16 to 18 oz. of the hydrated peroxide of iron may be administered. If the poison has been swallowed several hours previously, and hence may have passed the pylorus, a strong dose of castor oil or a purgative clyster may be administered, and, after its action, another clyster containing the antidote. As soon as the stomach and bowels are cleared, diuretics and sudorifics should be given in abundance. Lastly, any remaining irritation must be relieved by demulcent and soothing remedies; or if urgent, by slight general or local bleeding, which cannot be earlier practised without danger; and opium, camphor, and ether, followed by tonics, may be had recourse to, to recruit the system.
Lesions. Redness and inflammation of the whole primæ viæ; and sometimes of the mouth, fauces, and œsophagus, but more usually the contrary. Sometimes also, though seldom, there is no marked appearance of inflammation in the stomach and intestines. The stomach is usually highly injected, and frequently marked with extravasations; lungs gorged with blood; mucous lining of trachea reddened; heart generally flabby, and exhibiting deep red or blackish stains, and the right cavities more or less loaded with blood; the conjunctiva is sometimes very vascular; and redness, extravasation of blood, and effusion of serum is occasionally seen in the brain. The blood is frequently, though not invariably, fluid after death, and dark coloured. Under certain circumstances, the mucous membrane of the stomach and intestines is lined with a multitude of brilliant points or grains, which have been mistaken for arsenious anhydride; but which, according to Orfila, are composed of fat and albumen. Placed on burning coals, they decrepitate on drying, and produce a species of explosion or detonation. These grains are also met with in the stomach of persons who have not been poisoned. Digested in water, the liquid obtained from them does not show the presence of arsenic when submitted to reagents.
Ant. In the order of their assumed efficiency:—Moist peroxide of Iron.—See under the preparations of Iron (Arsenici Antidotum, G.). Hydrated or gelatinous sesquioxide or peroxide of iron (for an adult—a tablespoonful, in water, every 8 or 10 minutes until 12 or 16 oz., or more, have been taken). Hydrated sulphide of iron (as the last). Gelatinous hydrate of magnesia (as the last).199 Calcined magnesia (taken as the first). Salad or olive oil, or almond oil, and oil or fats generally (ad libitum), are all highly effective in lessening, if not destroying the action of arsenious anhydride.[81] Albumen (white of egg), or liquids containing it (in cold water, ad libitum). Milk, wheat-flour, oatmeal gruel (with water, ad libitum). Lime water, with milk (as the last). Chalk, with milk and water (as the last). Infusion or decoction of bark, or better, of nut-galls (as the last). Sugar or syrup (ad libitum). See Treatm. (above); also the above substances under their respective heads.
Uses, &c. Arsenious anhydride and its compounds are extensively employed in the arts and medicine. It is used by the dyer, it furnishes the artist with several of his most beautiful pigments, and the glass-maker and enameller with a flux or material to whiten and decolour their wares. In agriculture, it is used (in solution) as an anti-smut for seed-wheat; and as an anti-vermin lotion or dipping for sheep and cattle. In small (therapeutical) doses it is a valuable remedy in intermittent fevers, chronic skin diseases (especially lepra and psoriasis), and in several nervous affections (as neuralgia, epilepsy, chorea, tetanus, &c.). It is the active ingredient of the tasteless ague-drop; of Fowler’s and Pearson’s solutions; and in the Tanjore pills, long celebrated in India for the cure of the bite of the cobra di capello and other venomous serpents, as well as of hydrophobia. It has been given in syphilis, chronic rheumatism, typhus, and several other diseases, with more or less advantage. Cautiously administered in phthisis, it frequently restores the appetite and strength and greatly retards, and in some cases arrests, the progress of the disease. It has been recently used to relieve toothache arising from caries. Externally, it is employed in the form of powder, lotion, and ointment, for the cure of cancer. Plunkett’s ointment, Pâte arsénicale, Davidson’s Remedy for Cancer, and several other like preparations, owe their activity to arsenious anhydride. Water in which white arsenic has been steeped has become a favorite cosmetic wash with many ladies, since its assumed property of softening the skin was announced in a certain popular periodical. It is also the prime ingredient in the papier moure, a popular fly paper. Its use, whether internal or external, is, however, attended with considerable danger in unskilful hands, and should, therefore, never be adopted but under proper advice.—Dose, 1⁄20 to 1⁄8 gr., made into pills with crum of bread and lump sugar; or in solution, 3 to 5 or 6 drops, twice or thrice daily, gradually and cautiously increased to 12, or even 15 drops. As a rule, arsenical preparations should be taken soon after a meal, and by no means on an empty stomach. (Dr A. T. Thomson.) The dose should be suspended, or greatly reduced, as soon as the conjunctiva is affected (Hunt); or if dryness of the mouth or throat, or irritation of the stomach or bowels, ensues. Mr Maculloch found the pills more efficacious than the solution; they act differently, and cannot be substituted for one another.
Arsenic is a favorite tonic and alterative with farriers, who often administer it very carelessly to horses, to the serious injury of these animals. It is also a favorite with grooms, who have imbibed the notion that small doses of it contribute to improve the condition of the skin. The best-informed veterinarians, however, either wholly avoid it, or use it with very great caution.[82]—Dose (for a HORSE), 2 to 5 or 6 gr., twice or thrice daily; in farcy or glanders, 10 to 12 gr. In solution it is often employed as a wash or dipping to destroy vermin in cattle and sheep; but its use is not free from danger, particularly to the shepherds or dippers.
Gen. commentary. The necessary length of the preceding article, owing to the great importance of the subject in its relations to toxicology and medical jurisprudence, has left us little space for further remark here. In addition to what has been said on arsenical testing, it may be useful to caution the reader of the absolute necessity of only employing tests and reagents which are themselves absolutely pure; and in which the operator has, by personal examination, failed to detect the slightest trace of arsenic. Commercial sulphuric, nitric, and hydrochloric acids, potash, soda, nitre, iron, and zinc, frequently contain200 arsenic; from which, however, they may be freed by chemical processes; or they may be purchased in the pure state from respectable dealers in chemicals. But no assurance of the vender should be regarded as a proof of their purity. In all judicial investigations the absence of arsenic in the several tests and reagents, and the apparatus employed, must be demonstrated and sworn to. We may further add, that the results afforded by no single test can be depended on. In matters of such vast importance, the most ample confirmatory evidence must be sought.
Marsh’s, Reinsch’s, Lassaigne’s, the sulphur, and the Reduction Tests, and their modifications, are those now generally preferred by toxicological chemists; each of which, with its confirmatory tests, are amply sufficient for the indisputable identification of arsenic.
Modern toxicologists have abandoned most of the old processes for the detection of arsenic, and have adopted one of two, which have been found more expeditious as well as more certain. These are the tests of Marsh and Reinsch, preferably the latter.
Herapath’s Method is to obtain deposits by Reinsch’s Test on 4 or 5 pieces of No. 13 copper wire; each piece being about 21⁄2 inches long, and previously flattened and planished with a polished hammer for about one half its length. The deposit, with some of the adhering copper, scraped from one of these coated pieces, is sealed up hermetically in a tube for future production. The scrapings from three pieces of wire are separately submitted to the sublimation test in tubes bent in the form of an obtuse V capillary at one end, and about 3⁄10ths of an inch in diameter at the other; the capillary leg being about three times as long as the larger one. The scrapings are placed in the bent part of the tube; and the flame of a small spirit lamp is so applied as to slowly drive the sublimate into the narrower portion of the tube, which is held rather higher than the other. If the deposit so obtained be mercury, it condenses in white shining globules;—if lead or bismuth, it does not rise but melts into a yellowish glass, which adheres to the copper; if tellurium, it falls as a white amorphous powder; if antimony, it does not rise at that low temperature; but if it be arsenic, it sublimes as arsenious anhydride, which condenses as minute octahedral crystals, looking, with the microscope, like very transparent grains of sand. One of these tubes containing the sublimed arsenious anhydride is then sealed up, like the first one, for future production. The capillary part of another tube containing the sublimate is then cut off, and carefully boiled in a few drops (10 to 15) of distilled water; and, when cold, 3 or 4 drops of the resulting solution is poured on a plate of white porcelain, and to this, by means of a glass rod, one drop of solution of ammoniacal sulphate of copper is added. The mixture is then carefully conducted on to a piece of white filtering-paper set on the surface of a smooth, clean, and dry chalk-stone, by which the moisture is absorbed, and the smallest portion of Scheele’s green produced by the test rendered more conspicuous. The ammonio-nitrate of silver test is then applied, in a similar manner, to 3 or 4 drops of the remaining solution; after which the pieces of paper with the spots are dried, and sealed up in separate tubes, as before, observing to exclude the light from that containing the yellow precipitate of arsenite of silver. A stream of sulphuretted hydrogen is then passed through the remaining tube containing the arsenical sublimate, by which the latter is converted into the yellow tersulphide—this too is sealed up. Here are now five tests—the metal, the acid, arsenite of copper, arsenite of silver, and yellow tersulphide of arsenic.
It is now well known that certain soils contain arsenic, either as arsenite of lime or sulphide of arsenic; and which, under favorable circumstances, may permeate or be absorbed by a body, after interment. In judicial investigations following disinterment it is, therefore, necessary to examine portions of the cemetery-earth taken from the grave, as well as from parts more or less distant from it. For this purpose the earth should be thoroughly dried in a water-bath, drenched with pure and concentrated hydrochloric acid, and allowed to stand for twenty-four hours. The mixture is then distilled, and the distillate tested for arsenic by Reinsch’s or Marsh’s test. Should the product of one distillation yield no evidence of arsenic, it should be returned to the retort, if necessary, a second or even a third time, and the distillation repeated.
The practice of employing an alkaline solution of white arsenic as an anti-smut steep for wheat, has lately arrested the attention of chemists. M. Audouard states that he has detected traces of arsenic in the crops raised from seed-wheat thus treated. But that which appears to be likely to prove much more dangerous is the introduction of arsenic into crops by the employment of crude superphosphate of lime as manure—a substance often rich in this poison. Dr Edmund Davy positively states that arsenic, as it exists in artificial manures, is taken up by plants growing where those manures have been applied! He found cabbages and turnips taken from fields manured with superphosphate give unmistakeable evidence of being ‘arseniated.’ These facts have some important bearings; for though the quantity of arsenic which occurs in such manures is not large when compared with their other constituents, and the proportion of that substance which is thus added to the soil must be necessarily small, still plants during their growth, as in the case of the alkaline and earthy salts, take up a considerable quantity of this substance. Further, as arsenic is well known to accumulate in soils, though not an accumulative poison in201 the animal system, the effects after some time will probably be, that vegetables raised on those continuously so manured will ultimately be found to contain such a proportion of arsenic as will exercise an injurious effect on the health of man and animals. The statement of M. Audouard has been disputed by M. Girardin, because he failed to detect arsenic in corn under the circumstances; and it is also denied by Dr A. S. Taylor, and others; but our own experiments, very carefully performed, confirm the assertions of both Audouard and Davy. The ultimate consequences of pouring into the Thames such enormous quantities of disinfectants contaminated with arsenic, as has been done during the last three or four years, is another matter deserving consideration, and one which has been ably pointed out by Dr Letheby, in his reports as Officer of Health to the City of London.
Dr Lois has found arsenic, often in large quantities, in ordinary brass, and brass utensils; and we have ourselves repeatedly found arsenic in the Britannia-metal, German-silver, and other cheap white alloys at present in such general use.
The preceding facts are recommended to the careful attention of medical jurists.
By an Act of Parliament[83] it is provided—1. That every vender of arsenic shall, before the delivery of the same to the customer, enter in a book or books kept for the purpose, the date of sale, name, and residence of the purchaser, in full, his or her condition or occupation, the quantity so sold, and the purpose or purposes for which it is required, in a form set forth in the schedule to the Act; which form or schedule shall be signed by the vender, and by the said purchaser, unless he be unable to write, when such fact shall be recorded in the said schedule by the vender; and this schedule, when a witness is required to the sale, shall also bear his signature, together with his place of abode:—2. Arsenic is not to be sold to a stranger, unless in the presence of a witness acquainted with both vender and purchaser:—3. No person to sell arsenic unless it be previously mixed with at least 1 oz. of soot or 1⁄2 oz. of indigo to the pound; unless such admixture would be injurious to the object for which it is intended, when not less than 10 lbs. is to be sold at any one time:—4. Penalty for evading the Act, either as vender, purchaser, or witness, £20:—5. Act not to extend to arsenic used in compounding prescriptions nor to the wholesale trade:—6. The word ‘arsenic’ to include ‘arsenious anhydride,’ and the arsenites, arsenic acid and the arseniates, and all other colourless poisonous preparations of arsenic. See Arsenic, Arsenic Acid, Lotions, Pills, Sheep-dipping, Soaps, Solutions, Wheat-steeps, Iron, Potassa, Soda, and other Bases, &c. &c. (also below).
Self-detect′ing Arsenious Anhydride. Prep. (Dr Cattell.)—1. Ordinary white arsenic to which is added a small quantity of a mixture of dry calomel and quick-lime; or of dried sulphate of iron and powdered gall-nuts. The product is white, but immediately turns black when mixed with liquids:—2. As the last, but adding a mixture of thoroughly dried sulphate of iron and ferrocyanide of potassium. Strikes a blue:—3. As last, but using dried phosphate of sodium and dried sulphate of iron. Strikes a green. Proposed as a method of preventing arsenic being used as a poison.
ARSENICAL PIGMENTS, EFFECTS OF. The composition of those substances which are compounds of copper with arsenious, very frequently combined with acetic acid, will be found under Green Pigments, under their respective commercial names of Scheele’s Green, Mineral Green, Emerald Green, and Schweinfurt Green. The purity of tint and durability of these arsenical salts have, not unnaturally, caused them to be employed in many branches of industry, the products of which are everywhere around us, and as the colouring material of these, they are placed in conditions very favorable to their being taken into the stomach or lungs. This will be apparent when we name a few of the materials in which they are employed:—wafers, candles, wall-papers, window curtains, confectionery.
A curious illustration of the risks attending their use may be cited from the ‘Medical Times and Gazette’ of April, 1854, which states that some loaves found to contain arsenic were discovered on inquiry to have got the dangerous intruder from having been allowed to stand on shelves freshly painted a bright green colour. Arsenical-coloured wafers may be pronounced free from danger, so long as they are kept out of the reach of children; and although the arsenical vapours given off by burning a green wax taper would not be sufficient to induce toxic results, the fact of the extreme sensibility of some people to the action of this poison, when taken in by the lungs, renders the use of these tapers a very objectionable one, particularly if they are generally employed in a household. The burning of wax candles, coloured with arsenical green, is, of course, still more strongly to be condemned, because from its superior mass, when compared with the taper, the candle gives off a greater amount of the poisonous fumes. An arsenical taper weighing 17·69 grains was found upon analysis by Mr Bolas, late of Charing Cross Hospital, to contain 0·276 grains of arsenious acid. “A Christmas tree,” says Mr Blyth, “brilliantly illuminated with Christmas candles, may be taken as an extreme instance of the danger likely to arise from this source.” That the employment of arsenical green in the manufacture of sweetmeats was not abandoned in 1873 may be evidenced from a circumstance quoted by Mr Blyth in his interesting work on ‘Hygiène.’ “During the Christmas of 1873 a large cake in which was imbedded202 a green card labelled “for the bairnies,” was seized in a baker’s shop at Greenock. The card was coated with sugar, and on being submitted to analysis, was found to contain 7·04 grains of arsenious acid.
A curious case, illustrating the effect of arsenical wall-papers, is furnished by Dr Dalzell, of Malvern. He was attending a lady ill with scarlet fever, and during the attack her husband occupied a small bedroom. The first night he slept in it his slumbers were most unrefreshing and disturbed by horrible dreams, and on rising in the morning he felt languid and weak, had lost his appetite, and had a dull headache. Towards the evening these unpleasant symptoms had nearly vanished. On the second night (when he occupied the same dormitory) and on the day following the same disagreeable symptoms returned. He then changed his bedroom, and forthwith they troubled him no more. A servant, who next occupied the chamber, was affected as her master had been. Dr Dalzell suspecting the wall-paper as the cause, examined it, and found it to contain a large quantity of arsenic.
Some little time since Mr Bolas examined a sample of wall-paper containing 27·53 grains of arsenious acid in the square foot, and in this case the pigment was so loosely fixed that the slightest friction was sufficient to detach a portion and diffuse it through the air. Nor is this surprising when we consider how slightly the arsenical colour is attached to the surface of the paper, as well as how easily it may become liberated from it by the desiccation of the air of the room when heated by a fire. This may be exemplified by drawing the sleeve of a black or dark-coloured coat over an arsenical wall-paper, and observing the green deposit that is left on the garment.
After this we shall be prepared for the following statement: “Hamberg drew, by means of aspirators, the air of a room, the walls of which were papered with a very old green paper, through various tubes containing cotton wool and silver nitrate. On examination scarcely any solid particles could be discovered. The cotton-wool was fused with sodium nitrate and carbonate, and gave a little ferric oxide and a trace of arsenic, but the solution of nitrate of silver gave decided evidence of arsenic, as well as of sulphide of silver.” (‘Phar. Jour.’)
Not many years since Professor Fleck showed that the arsenious acid in the Schweinfurt green, when in contact with moist organic substances, and especially starch-sizing, forms arseniuretted hydrogen, which diffuses in the room, and which is no doubt the cause of some of the cases of arsenical poisoning from green papers. So that a contrary condition to a dry atmosphere, viz. a moist or damp one, may also lead to results nearly, if not quite as objectionable, when rooms are papered with arsenical papers. We have Mr Blyth’s word for the assertion, that the most dangerous of the arsenical papers, viz. those covered with a thick, unvarnished, loosely coherent layer of Seheele’s green are most frequently to be met with in our nurseries, where the beds are placed next the wall, and where the attrition of the bedclothes frequently removes portions of the poisonous colouring matter. The fine cupro-arsenical dust which thus becomes diffused through the room, now and then produces in children symptoms resembling those of violent catarrh. Some of the wall-papers of these nurseries have been found to yield 18 grains of arsenious acid in a square foot. It would appear that the use of arsenical pigments is by no means restricted to green wall-papers. Very recently an analytical chemist examined a great number of samples of wall-papers of different colours, and was surprised to find arsenic in most of them. Within the last year the writer examined the pigment which he could disengage without much difficulty from a very small piece of green muslin window curtain, and found it yield a large quantity of arsenic. In Paris alone there are more than 15,000 people who earn their living by making artificial flowers, a quarter at least of these workers being engaged in that branch of the manufacture in which Schweinfurt green is used. From the instances already adduced of the ill effects caused, although in a mild degree, by occasional and accidental exposure to arsenical pigments, we shall be prepared to learn that the danger and the damage to health is very much more intensified when, as in the case of these poor artisans, the workman is constantly handling the deadly material, and incessantly inhaling an atmosphere laden with its particles. Dr Vernois has published a most interesting description, which we subjoin, of the artificial flower-maker at work. He says:—“These greens are formed either from arsenite of copper alone, or mixed in variable proportions with acetate of copper (English green). Arsenical greens are employed to colour different herbs, to tint the fabric destined to prepare the leaves of artificial flowers, as they are painted directly on the leaves or petals of flowers worked on cloths of various textures. For these various purposes they buy the Schweinfurt or the English green (vert Anglaise), either in powder or in aqueous solution, and add to it, according to the effect desired, a certain quantity of Flanders glue, starch, gum, honey, or turpentine. Sometimes it is applied in the dry state, in order to sprinkle it over the things already coloured by the arsenical green. They frequently also, in order to modify the colour, mix with it a certain quantity of chromate of lead or picric acid.
The preparation of herbs is carried on as follows:—The workman plunges into a shallow vessel, containing a sufficiently liquid solution of Schweinfurt green, one or several stalks203 of natural plants, perfectly dried, and agitates them quickly, seizing them by their roots with a pair of forceps. This operation, which is termed ‘steeping,’ stains the fingers, the arms, the person, and the clothes of the workman, and the surrounding objects are covered with traces of this kind of paint. The plants thus prepared are hung on a line, and there allowed to dry for thirty-four or forty-eight hours. At the end of that time all the stalks are gathered and formed into bundles, which are used finally for bouquets. Often enough, to satisfy some freak of fashion, they are sprinkled with powdered arsenite of copper. This is the powdering. The bouquet-work constitutes one of the principal dangers; for the colouring matter not having been fixed by any mordant, detaches itself in the form of a fine dust, which penetrates the skin of the hands, and which the workman breathes constantly. This danger is still more increased when he handles bouquets covered with arsenical powder. At other times, however, in the manufacture of the plants, the Schweinfurt green is diluted with a sufficient quantity of turpentine. In this way the colour takes a smooth appearance, not altered by contact with water, and does not escape immediately in the form of powder by gentle handling; but when it is thoroughly dry it falls to the ground in little flakes, and may again rise in the air with ordinary dust. Thus the danger is modified, a little retarded, but always exists. There are then in this speciality of the florist the operations of steeping, drying, powdering, and arranging the flowers for bouquets, which in their details place the workman or the purchaser under the more or less direct, and more or less active influence of arsenical salt. This particular industry is exercised under conditions which render it still more injurious; for it is freely practised by a number of poor workpeople, by households living in one or two rooms, ill-ventilated, ill-lighted, and which they never sweep, and of which the floor like the furniture, and like the clothing of the workpeople, is continually impregnated by pigment and covered with arsenical dust. The preparers of the cloth destined for the manufacture of the artificial leaves by the aid of arsenical greens, comprehend the portion of the work most exposed to deleterious action. They use arsenite of copper alone, mixed principally with starch, and in rare instances associated with acetate of copper in variable proportions. Some use eublèe, a mixture of picric acid and of greenish indigo, in which they steep their stuffs. Other manufacturers use fabrics prepared with hot solutions by ordinary dyers. According to the hue which the Schweinfurt dyer wishes to obtain, the workman commences by giving the stuff a yellow shade, by plunging it into a solution of picric acid and pure alcohol. He squeezes it between his fingers, in order to completely impregnate it and dries it. It is this preliminary operation which stains the workman’s fingers yellow. Frequently the latter mixes picric acid by grinding it with the Schweinfurt green, and applies this paste immediately to the fabric. The paste is prepared by kneading the Schweinfurt green, already treated with water, with a solution of starch thick enough, yet sufficiently liquid, to be easily spread on the cloth. During this working up the paste the fingers, arms, and hands of the workman are covered with arsenical solution. This being ready, the workman lays out his stuff, distributes the paste over it, then beats it between his hands, in order to make the colouring matter thoroughly penetrate the cloth. The longer it is beaten the better is the quality of the article. During this operation the skin of the hands and arms is completely impregnated with the solution. Sometimes the cloth, having been touched here and there with arsenical paste, is attached to a hook in the wall, and twisted different ways—wrung as it were. In this way a very uniform colouring is obtained. This process is as bad to the workman as the former. Lastly, a process which is generally practised consists in placing the fabric, stained or not with picric acid, on a wooden table, and distributing on both sides the arsenical preparation with a brush, and then beating the stuff with a thick rubber. In this way the hands and arms of the workman are much less exposed to the paste than in the preceding processes. After the brushing and beating of the fabric comes the drying, to which operation attention must next be directed. Once impregnated with the green colour by whatever process, the pieces in squares of about 1 metre 50 cent. are hung on wooden frames, furnished with teeth, on which the borders of the cloth are transfixed. During this simple operation the workmen stain themselves much. When the stuffs are detached from the squares they are folded, and from every crease falls a fine dust, which may then be carried into the mucous membranes. The workmen then are liable to all the accidents of the manufacturers of flowers, especially in the operations of kneading the paste, or during the beating, brushing, drying, and folding of the cloths. From the hands of the fabricator the fabrics are very often immediately consigned to the manufacturers of artificial flowers, who press them, figure them (that is to say, make the nerves), arm them with a wire, and mount them with flowers. It may be at once understood how much all the manipulations I have just mentioned are liable to develop the arsenical dust. The paste has not been fixed on the stuffs by any mordant; the starch with which it is mixed has given it a very brittle consistence, and has predisposed it to be easily detached from the cloth.
The stamping is effected by putting a certain number of folded pieces one above the other, and submitting them to the pressure of a204 stamping instrument. Repeated blows of this instrument detach the paste in scales, and cover with dust the fingers and person of the workman. A series of small packets are taken from the stamping press, which contain, strongly pressed together, from twelve to twenty-four leaves. They are passed on to another workman, who is charged with the folding. This operation is performed by holding the little bundle of leaves between the thumb and index finger of the left hand.
The thumb of the right hand presses the edges quickly and sharply so as to separate the leaves one from another, as you separate the leaves of a book recently bound. During this process still more dust escapes. Then comes the figuring, which by reason of successive blows applied to each leaf covers the body of the operator with the same pulverulent material. Fixing a wire to the leaves at their lowest part by the aid of gum follows that operation.
Then the leaves are arranged together in dozens, and passed to the bouquet manufacturers, who mount them. From thence they go to the milliners, who adapt them to different articles of dress, and sell them to the public. Through all this series of transformations there are the same manipulations, the same production of dust, the same action on the skin and mucous membrane, only in a decreasing degree, from the first preparer to the milliner. There is, however, a process of preparing the cloth which diminishes notably the severity and frequency of the evils of the Schweinfurt green. It is that which immediately after the drying of the stuffs submits them at once to the “calendrage.” This operation causes the arsenical paste to penetrate mechanically into the fibres of the stuff, and gives it a smooth and glazed aspect, which only permits imperfectly the production of the arsenical dust. This process renders the successive workings of the cloth less injurious, but it would be an error to consider it as inoffensive. During the action of the press, and especially during the separating and the fixing of the flowers, a notable quantity of the toxic dust is still produced. However well prepared the fabrics may be, you have only to tear it, to detach the coating under the form of a palpable powder.
It is only necessary to add that the waxing of the leaves, after they have been separated and figured, and before putting them into bouquets, constitutes a protecting envelope against the effects of the powdered coating for workmen who then handle them, as well as for women who wear them; but this film of wax is only applied, comparatively speaking, to a small number of leaves, for it alters the green and vivacity of its colour.
In the preparing of the stuffs in the process of drying, Dr Vernois says:—A new condition and serious results appear. The multiplicity of sharp points fixed in the wooden squares inevitably pricks and scratches the skin of the workmen. An inoculation of the arsenical salt immediately takes place, as if it had been practised experimentally. The skin irritates and inflames, a vesicle first, then a large pustule covers the orifice of the prick, and undergoes all the stages of inflammation, which produces suppuration and often gangrene, below which a deep and painful ulceration is developed—all the more tedious to heal as the inoculation is renewed from day to day.
The action of picric acid mixed with the paste can only augment and aggravate the irritation of the wounds. If the ulcerations are numerous the workmen may absorb the arsenious acid and be liable to serious results. I have seen a certain number of workmen with glandular enlargements under the armpits, and the hands in such a state that they were obliged to come to the hospital, where they were only cured after one or several months of treatment. The aspect of the hand was then characteristic to the greenish-yellow tint of all the skin, and especially of the palmar aspect of the hands. To the greenish crust under the nails was nearly always added a yellow colour of the nails, produced by the repeated contact with picric acid.
When we add a generally diffused erythema, then a series of black points, or of inflamed pustules, and sometimes a whitlow, we shall have a faithful representation of the evils which most frequently present themselves in the preparers of stuffs, for artificial flowers tinted with Schweinfurt green.
Amongst the endeavours to counteract the evils entailed upon the workers in this branch of industry may be mentioned the attempt to substitute chrome for Schweinfurt green, as the less poisonous of the two substances, and the ingenious process of M. Bérard-Zenzilin, which consists in directly incorporating the arsenical colouring matter with a specially prepared collodion.
AR′SENIDE. Syn. Arsen′iuret; Arseniure′tum (-i-ū-), L.; Arséniure, Fr. A combination of arsenicum with a metal (including hydrogen), in definite proportion.
AR′SENITE (-nīte). Syn. Ar′senis, L.; Arsenite, Fr.; Arsenigsäure salz, Ger. A salt of arsenious acid.
ART. [Eng., Fr.] Syn. Ars (gen., ar′tis; pl., ar′tes), L.; τεχνη, (tech′ne), Gr.; Kunst, Ger. Primarily, strength, power, and hence also mental strength, skill; the application of knowledge or power to effect a desired purpose; the power or ability of doing something not taught by nature or instinct; practical skill guided by rules. Science is knowledge—Art, practical skill in applying this knowledge. Art is applied science; whilst SCIENCE is knowledge obtained by observation, experience,205 and ratiocination. This distinction is nowhere more fully seen than within the domain of chemistry, where knowledge, deduction, great power of generalisation, and great expertness are necessary elements of success. Art has filled the world with luxuries, conveniences, and comforts; and art—the ARTS—useful or fine—are the safest and surest civilisers of our race. See Science.
ARTESIAN WELL. A cylindrical perforation bored vertically down through one or more strata of the earth till it reaches a porous bed of gravel containing water, this fluid being placed under such incumbent pressure that it rises up the perforation either to the surface, or to a convenient height for the operation of a pump. When they rise to the surface these wells are called spouting or flowing. The name of these wells is taken from Artois, a province in the Departement du pays de Calais, where their use was revived. They have been in use for a long time in Italy and in the East. The accompanying drawing represents the manner in which rain may be supposed to distribute itself when it falls upon a portion of the surface of our globe. The figure represents a geological section, showing the succession of the different strata.
The figure is supposed to represent two beds, A, B, more porous, and consequently more absorbent than the rocks by which they are interstratified. The condensed dews and rains falling upon the distant hills pass rapidly by the outcrops of the strata to the lower levels, until the entire mass becomes thoroughly saturated with water. Supposing two such beds as are represented in the section to exist, fully charged with water, it is evident that if we bored down into them through the rocks as represented at C, D, the water would rise through those wells or borings, and spring out in the form of a jet to such a height above the surface as is due to the height of the hills from which the water has been obtained. The fountain derived from B would necessarily flow as much higher as that derived from the bed A, as is the height of B above A.
For particulars as to the modes of constructing artesian wells, the reader is referred to ‘Traité sur les puits Artesiens,’ by M Gamier, and to ‘Considérations Géologiques et physiques sur la théorie des puits forcés, ou fontaines Artésiennes,’ by M. le Vicomte Hericart de Thury, and to ‘Rudimentary Treatise on Well-digging, Boring, &c.,’ by J. G. Swindell, and also to Ure’s ‘Dictionary of Arts, Manufactures and Mines,’ edited by Mr Robert Hunt.
ARTHANI′TINE (-tĭn). [Eng., Fr.] Syn. Artaniti′ne; Arthaniti′na, L. A peculiar substance first obtained by M. Saladin, by the action of alcohol on the tuberous stems of the herb arthrani′ta, or sow-bread. It is acrid, colourless, and crystalline, and imparts its acridity to the plant.
AR′TICHOKE. Syn. Cin′ara, Cyn′ara; Scol′ymus, L.; Artichaut, Fr.; Artischocke, Ger. The cynara scoly̆̆mus (Linn.), a thistle-like perennial plant of the nat. ord. Compositæ (DC.). Hab. Southern Europe; but now extensively cultivated in our gardens, for its ‘bottom,’ or the sweet fleshy receptacle of its flowers, which is eaten as a pot herb. These are soaked in brisk boiling in water, stalk-ends uppermost, until tender; and take 1⁄2 to 1 hour according to their age. Sometimes they are preserved in brine (PICKLED ARTICHOKES); and also after depriving them of the ‘choke’ and spiny hairs and blanching them by immersion in boiling water, by drying in the sun (DRIED ARTICHOKES; CULS D’ARTICHAUT, Fr.), by which they retain their flavour for some time. Infusion of the flowers, used with rennet.
As an esculent the artichoke resembles asparagus in its general properties; but it is said to be more nutritious, and even more diuretic.
Artichokes, Jeru′salem. The helianthus tuberosus (Linn.), a perennial plant of the sun-flower family, and quite distinct from the preceding. Hab. The Brazils. The appellation “Jerusalem” is believed to be a corruption of the Italian word girasole—“a sunflower,” to which botanical family the plant belongs. It is cultivated in England for culinary purposes. Roots (tubers) resemble the artichoke in flavour; but are considered far from wholesome, being apt to produce flatulence and dyspepsia. They are diuretic, and impart the odour of turpentine to the urine. They are cooked by boiling (15 to 25 minutes, according to size), or frying; in the former case served with melted butter. They are also served mashed, like turnips. The flowers yield a volatile oil resembling that of turpentine.
| Nitrogenous matter | 3·1 |
| Sugar | 14·7 |
| Inulin | 1·9 |
| Pectic Acid | 0·9 |
| Pectin | 0·4 |
| Cellulose | 1·5 |
| Fatty matter | 0·2 |
| Mineral matter | 1·3 |
| Water | 76·0 |
| ——— | |
| 100·0 |
From the above it will be seen that this esculent contains no nitrogen.
ARTIFICIAL FOODS. See Farina.
ASARABAC′CA (ăs-ă-). Syn. As′arum, A. Europæ′um: (Linn.), Nar′dus monta′na*, &c., L.; Asaret, A. d’Europe, Cabaret, Azarum C., Nard sauvage, Oreille d’homme, &c., Fr.; Hazelwurtzel, Ger. The ασαρον of Dioscorides, a small round, hard, stemless, hardy herbaceous plant, bearing chocolate-coloured flowers; and of the nat. ord. Aristolochieæ (DC.). It grows freely in central France, and is found in woods and shady places in Lancashire, Westmoreland, and other parts of England. Hab. Europe, between 37° and 60° latitude.—Root & rhizome (AS′ARI RA′DIX) has a pepper-like odour and an acrid taste:—Leaves (A. FO′LIA) less odorous, though bitter-tasted, acrid, and aromatic; formerly officinal in the pharmacopœias:—Whole plant (ASARABACCA of the shops) nauseant, emetic, and purgative. Before the introduction of ipecacuanha it was the common emetic (6 to 9 of the green leaves in whey); but, owing to the violence of its action, it has long fallen into disuse. Its common name in France (CABARET, or public-house plant) is said to have arisen from its frequent employment to relieve the stomach of those who had drunk too hard. It is now almost solely used as a sternutatory or errhine, and is probably one of the best.
According to Gräger[84], asarabacca contains three volatile, oily principles, which may be obtained by distillation with water:—Volatile oil (o′leum as′ari):—As′arite, an odourless, tasteless, and crystalline solid; fusible and volatilisable, yielding white and very irritating fumes:—As′arum-cam′phor, differing chiefly from the last in being precipitated, by water, from its alcoholic solution in cubes or six-sided prisms, instead of delicate flexible needles. Also a brownish, bitter, crystallisable principle (AS′ARINE, AS′ARUM-BIT′TER), which is soluble in alcohol.
Uses, Dose, &c. Dried leaves, 20 to 30 gr., or root, 10 to 12 gr.; as a purge or emetic. As an errhine—leaves, 3 to 5 gr.; root, 1 to 3 gr.; in powder, snuffed up the nose every day, or every other day, at bedtime. It excites irritation and a copious watery discharge, more or less muculent, which frequently continues to flow for several days, and occasionally proves highly useful in certain affections of the brain, eyes, mouth, nose, ear, and throat, on the principle of counter-irritation. It has been found “particularly serviceable in cephalalgia (headache), obstinate headache, chronic ophthalmia (inflammation of the eyes), and some other lethargic affections.” (Dr A. T. Thomson.) In dimness of sight (especially that arising from fatigue or congestion), deafness, and slight paralytic affections of the mouth, tongue, lips, or eyelids, not of a serious organic character, and particularly in chronic earache, it also sometimes affords relief after other remedies have failed. It constitutes the basis of several Cephalic snuffs, Asarabacca-snuff, Baron McKinsey’s medicinal powder (or SNUFF), and several other like nostrums, which are much extolled by their venders, and sold at marvellously high prices. See Patent Medicines, Powders, Snuffs, &c. (also below).
AS′ARIN (-rĭn). C20H26O5. Syn. Asarone. A species of stearopten, discovered by Görtz, in asarabacca. It has an aromatic taste and an odour resembling camphor, and is said to be emetic. It is probably a mixture of asarum-camphor and some partially oxidised volatile oil. (See above.)
As′arine (of Gräger). Syn. Asari′na, L. The crystallisable bitter principle of asarabacca, noticed above. It is said to greatly resemble cytisine.
AS′ARITE (-rīte). See Asarabacca.
ASBES′TOS. Syn. Asbes′tus (ασβεστος, incombustible, unconsumable, Gr.), Amianth′us, La′pis A., &c., L.; Asbeste, Amiante, Fr.; Asbest, Steinflachs, Ger. In mineralogy, a soft, fibrous substance, composed of flexible or elastic filaments which, in their most highly developed form, greatly resemble those of flax or silk, and which bear exposure to a very considerable degree of heat without suffering decomposition. It has been proposed to clothe our firemen in dresses of asbestos; but without freedom of respiration could be insured in a heated and poisonous atmosphere, this envelope would be of little service. Gloves are sometimes made of it, for holding red-hot crucibles. It is also used as a filtering medium for corrosive liquids. A kind of felt made of asbestos is now used as a substitute for wire gauze to support beakers, retorts, &c., over lamps.
Var. Of these there are several; as Am′ianth or ELAS′TIC ASBESTOS, LIG′NIFORM A., MOUNTAIN-CORK, M.-LEATHER, M.-WOOD, &c.; varying from a grey, brown, or green colour, to pure white, and from extreme flexibility and softness, to rigidity and hardness, as indicated by the respective names.
ASCARIS LUMBRICOIDES. A parasite belonging to the genus entozoa, commonly known as the round worm, and found in the intestines of man, the horse, the ox, the pig, and some other of the lower animals. It is of a greyish-red colour and in size and general appearance like the common earthworm.
Children are very frequently infested by them. Their usual habitat is the small intestines.207 But they are occasionally found in the stomach, and have been known to transport themselves into the gall-ducts, frontal sinuses, nostrils, and mouth. The males are smaller than the females and much more rare. The females produce eggs in great numbers, but it is doubtful if the young are ever developed in the intestine in which the parent worm dwells.
It is probable that the ova gain access to the intestines of the animals of which they eventually become the pests from various outer sources. They are said to be very frequent in persons who partake much of raw leaves and roots. Dr Paterson, of Leith, noticed that families who drank certain water from a well supplied from a dirty pool, which contained various vermiform animalcules, were much infested with this particular species of intestinal worm; whilst others in the same street, who had recourse to a different water supply, entirely escaped. For medicinal treatment, see Worms.
ASCARIS MYSTAX. A parasitic round worm infesting the cat. It has been also occasionally found in man.
ASH. Syn. Frax′inus, L.; Frêne, Fr.; Esche, Ger. The popular name of several species of valuable hardy trees bearing apetalous flowers (except in the ‘flowering ash’), belonging to the nat. ord. Oleaceæ (DC.), and gen. Fraxinus; but appropriately the—
Ash. Syn. Comm′on ash; Frax′inus, F. excel′sior (Linn.), F. apet′ala (Lamb.), F. or′nus (Scop.), L.; Frêne, F. commun, Fr.; Gemeine esche, Ger. A large tree common to our woods and hedges; timber (ASH or ASH-WOOD) used by carpenters, cabinet-makers, and machinists, and much esteemed for its great toughness and elasticity; bark febrifuge, diuretic, resolvent, and tonic; has been successfully exhibited in agues; seeds acrid, bitter, and diuretic; leaves purgative, diuretic, and febrifuge; sometimes used instead of senna. In southern Europe it exudes an inferior kind of MANNA, and its medicinal properties are much greater than in our climate.—Dose. (Leaves) 1⁄4 oz. to 11⁄2 oz. (made into an infusion), as a purge; seeds, 1 dr., as a diuretic, &c.
Ash, Flow′ering. Syn. Man′na-ash; Frax′inus or′nus (Linn.), L. A small tree of southern Europe. Yields MANNA. The ‘round′-leaved flowering-ash’ (Cala′brian-ash; FRAX′INUS ROTUNDIFO′′LIA, Lamarck) is a smaller variety of the preceding, and a native of Calabria and the Levant. Said to yield the best MANNA. The ‘small′-leaved flowering-ash’ (FRAX′INUS PARVIFO′′LIA, Lam.) is another manna-yielding species, indigenous to Asia Minor.
ASH. Ashes (which see).
ASH-BALLS. The ashes of land-plants, especially ferns, damped and made into balls. Used as a substitute for soap in washing, and in cleaning paint.
ASH′ERY. [Amer.] A place where potash or pearlash is made or kept.
ASH′ES. (-ĭz). [Eng. pl.] Syn. Ash; Ci′nis, L.; Cendres (pl.), Fr.; Asche, Ger. The remains of anything burned. In antiquity, the remains of a body consumed on the funeral pyre; and hence, figuratively, the remains of the dead. The word, in English, has properly no singular; although ‘ash’ is very commonly heard; and is now almost exclusively used in composition, as in pearlash, potash, soda-ash, &c.
Ashes. In commerce, the residuum of the combustion of vegetable substances containing either carbonate of potassium (‘land-plants’), or carbonate of sodium (‘marine plants’), and from which the commercial alkalies are obtained. Their value depends upon their richness in ‘alkali,’ which is determined in the manner explained under ALKALIMETRY. The word is also commonly employed as a general term for the crude carbonates of potash of commerce (which see).
Ashes of Plants. See following page, on which will be found a table giving the chemical composition of the ashes of a few well-known plants used as food for men and animals. See also Manures, Plants, Vegetation, &c.
A careful determination of the ash of different substances is of great use to the analyst, by enabling him to detect adulteration; for instance, almost every plant on being burnt yields a very constant amount of ash, and not alone the quantity is constant, but the different proportions of the various components are also, within certain limits, tolerably unvarying. Many plants have the power of extracting from the soil certain elements; for instance, the ash of the tobacco contains lithium; tea, manganese; seaweed, iodine. It seems by no means improbable that by the examination of the ashes of plants by means of the spectroscope new elements may be discovered. Appended is a short list of the amount of ash, contained in a few important substances:—
| Total Ash. | |||
| Cayenne pepper, | from | 5 to 6 | per cent. |
| Chicory | ” | 5 | ” |
| Cocoa | ” | 3 to 4 | ” |
| Coffee | ” | 4 | ” |
| Flour | ” | ·7 to 1·5 | ” |
| Mustard | ” | 3 to 4·5 | ” |
| Pepper | ” | 4·3 to 5 | ” |
| Rice | ” | 5 | ” |
| Tea | ” | 5·6 | ” |
| Turmeric | ” | 5 to 6 | ” |
The ashes of plants are employed by the agriculturist according as the nature and proportion of the different salts they contain is suited to the soil and to the crops it is desired to raise. M. Soulange Bodin says that ashes hold the middle place between stable-dung and pasture manure. They act mechanically by dividing soils that are too compact, hygroscopically by absorbing moisture, and they appear to have an action similar to lime in accelerating the decomposition of the mould. They also probably exercise a stimulating209 effect on the soil. In the case of low-lying lands they are particularly suited for very damp clayey soils. In Picardy the ashes of turf are made use of; in England, the low countries and the north of France, coal ashes are employed.
| Peas. | Beans. | Red Clover. | Sainfoin. | Wheat Grain. | Straw. | Barley. | Oats. | Turnip Root. | |
| Potassa | 42·43 | 36·72 | 18·44 | 31·90 | 29·76 | 10·51 | 20·07 | 17·70 | 23·70 |
| Soda | 3·27 | 0·14 | 2·79 | ... | 5·26 | 1·03 | 4·56 | 3·84 | 14·75 |
| Lime | 5·73 | 12·06 | 35·02 | 24·30 | 2·88 | 5·91 | 1·48 | 3·54 | 11·82 |
| Magnesia | 5·92 | 6·00 | 11·91 | 5·03 | 11·06 | 1·25 | 7·45 | 7·33 | 3·28 |
| Sesquioxide of Iron | 0·44 | 0·65 | 0·98 | 0·61 | 0·23 | 0·07 | 0·51 | 0·49 | 0·47 |
| Sulphuric acid | 6·23 | 4·28 | 3·91 | 3·28 | 0·11 | 2·14 | 0·79 | 1·10 | 16·13 |
| Silica | 1·74 | 1·52 | 4·03 | 3·22 | 2·23 | 73·57 | 32·73 | 38·48 | 2·69 |
| Carbonic acid | 4·38 | 1·63 | 12·92 | 15·20 | 0·22 | ... | ... | ... | 10·47 |
| Phosphoric acid | 29·92 | 33·74 | 5·82 | 9·35 | 48·21 | 5·51 | 31·69 | 26·46 | 9·31 |
| Chloride of potassium | ... | ... | ... | 6·24 | ... | ... | ... | 0·92 | ... |
| Chloride of sodium | ... | 3·26 | 4·13 | 0·78 | ... | ... | ... | ... | 7·05 |
| —— | —— | —— | —— | —— | —— | —— | —— | —— | |
| Total amount | 99·96 | 100·00 | 99·95 | 99·96 | 99·96 | 99·99 | 99·98 | 99·96 | 99·93 |
| Per-centage of dry ash in dry substance | 2·60 | 2·90 | 7·87 | 6·37 | 2·05 | ... | 2·50 | 2·50 | 6·00 |
| Per-centage of ash in the fresh substance | 2·24 | 2·54 | 6·77 | 5·65 | 1·81 | ... | 2·25 | 2·27 | 0·75 |
| Turnip Leaves. | Beet Root. | Carrot Root. | Potatoes. | Lettuce Leaves and Stalks. | Olive-tree Wood. | Hops. | Hay. | Clupea Sprouts. | |
| [85] | [86] | [87] | [88] | [89] | [90] | ||||
| Potassa | 11·56 | 21·68 | 37·55 | 25·41 | 22·37 | 20·60 | 24·88 | 11·93 | 17·23 |
| Soda | 12·43 | 3·13 | 12·63 | ... | 18·50 | ... | ... | 1·07 | 1·19 |
| Lime | 28·49 | 1·90 | 9·76 | 2·34 | 10·43 | 63·02 | 21·59 | 14·76 | 23·57 |
| Magnesia | 2·62 | 1·79 | 3·78 | 4·17 | 5·68 | 2·31 | 4·69 | 5·30 | 3·01 |
| Sesquioxide of Iron | 3·02 | 0·52 | 6·74 | 0·50 | 2·82 | ... | 1·75 | 2·75 | 0·28 |
| Sulphuric acid | 10·36 | 3·14 | 6·34 | 4·71 | 3·85 | 3·09 | 7·27 | 0·20 | ... |
| Silica | 8·04 | 1·40 | 0·76 | 3·64 | 11·86 | 3·82 | 19·71 | 53·43 | ... |
| Carbonic acid | 6·18 | 15·23 | 15·15 | ... | ... | ... | 2·17 | ... | ... |
| Phosphoric acid | 4·85 | 1·65 | 8·37 | 10·38 | 9·38 | 4·77 | 14·47 | 6·34 | 43·52 |
| Chloride of potassium | ... | ... | ... | 12·40 | ... | 1·09 | ... | ... | ... |
| Chloride of sodium | 12·41 | 49·51 | 4·91 | Trace | 15·09 | ... | 3·42 | 2·27 | 11·19 |
| —— | —— | —— | —— | —— | —— | —— | —— | —— | |
| Total amount | 99·96 | 99·96 | 99·99 | 100·00 | 99·99 | 100·00 | 99·95 | 100·00 | 100·00 |
| Per-centage of dry ash in dry substance | 16·40 | 11·32 | 5·12 | 4·86 | ... | 0·58 | 5·95 | 6·97 | ... |
| Per-centage of ash in the fresh substance | 1·97 | 1·02 | 0·77 | ... | ... | ... | ... | 6·15 | ... |
Coal ashes, when mixed with excrement, besides disinfecting the latter, make an excellent manure.
ASPAR′AGIN (-ă-jĭn). C4H8N2O3. [Eng., Fr.] Syn. Althe′ine, Aspar′amide, Mal′amide*; Asparagi′na, Asparagi′num, L.; Agédoïle, Fr.; Spargelstoff, Ger. A peculiar azotised principle discovered by Vauquelin and Robiquet in asparagus, and since found in the potato, marsh-mallow, liquorice, climbing vetch, and several other plants. Many plants which do not naturally contain it may be made to yield it by growing them in dark damp cellars; whilst many which only normally contain it in very small quantities are found to yield much more when allowed to vegetate in the same manner.
Prep. 1. From ASPARAGUS-SPROUTS:—The expressed juice, after being heated to the boiling-point (to coagulate albumen) and carefully skimmed and filtered, is evaporated, at a gentle heat, to a syrupy consistence, and then abandoned to spontaneous evaporation in a warm dry atmosphere for several days; the resulting crystals being purified by cautious washing with very cold water or very strong alcohol, re-solution, and re-crystallisation.
The following are cheaper and more convenient processes.
2. From MARSHMALLOW-ROOT:—a. The root (chopped small, or grated) is macerated for several days in milk of lime, in the cold; the filtered liquid precipitated with carbonate of ammonium, and the clear solution evaporated in a water-bath, and otherwise treated as before.
b. From the expressed juice, 2 parts; milk of lime, 1 part; agitated well together; the liquid portion, after some hours, being decanted, filtered, and evaporated, &c., as before.
3. From the ETIOLATED SHOOTS OF VETCHES:—The expressed juice of the young shoots when from 2 or 3 to even 12 or 15 inches long, is gently simmered for 8 or 10 minutes, to coagulate the albumen; and, after straining or clarification, the clear liquid is gently evaporated to the consistence of a thin syrup, and set aside to crystallise, as before. The resulting brown crystals are purified by washing with very cold water, re-solution in boiling water, and re-crystallisation, as in No. 1; or, and what is better, the hot liquid, before evaporation to a syrup, is digested for a short time with a little pure animal charcoal in coarse powder, and then filtered, when large and beautifully white crystals are obtained by the first operation.[91] An excellent and very economical process.
Prop., &c. Crystals brilliant, transparent, colourless, right rhombic prisms; neutral to test-paper; non-basic; having a faint, cooling, and scarcely nauseous taste; scarcely soluble in cold water; freely soluble in hot water; insoluble in strong alcohol and ether; solution unaffected by alkaline sulphurets, oxalate of ammonia, acetate of lead, or infusion of galls; triturated with quick-lime, ammonia is evolved; heated to 212° Fahr. the crystals lose two equiv. or 12% of water; heated with water under pressure in a closed vessel, or boiled along with an acid or an alkali, or dissolved in a saccharine liquid and then submitted to fermentation it is converted into ammonium and aspartic acid; aqueous solutions of asparagin and aspartic acid treated with a current of nitrous acid evolve pure nitrogen, with the formation of malic acid which remains in solution. It was called asparamide under the impression that it is aspartite of ammonia minus 1 atom of water; and malamide, for similar theoretical reasons.
Uses. It is sedative and diuretic.—Dose, 1 to 6 gr.; in dropsies, heart-affections, &c.
ASPAR′AGUS. [L., Eng.] In botany, a genus of low, spiny plants, with scale-like leaves, many of which are shrubs and climbers, of the nat. ord. Asparageæ (DC.).; Liliaceæ (Lindl.). The following species, which is that best known in England, is, however, an exception to this description, as it is neither climbing nor spinose.
Asparagus Officina′lis. [Linn.; L.] Syn. Aspar′agus, Comm′on a., Gard′en a.; Spar′agus§, Spar′row-grass§, Sper′age†§; Asperge, Fr.; Spargel, Ger. A well-known perennial plant, and one of the oldest and most delicate of our culinary vegetables.—Young shoots, from the underground eyes (TURIO′NES ASPAR′AGI, L.), the asparagus of our tables; diuretic; communicate a peculiar fœtid odour to the urine, and, when eaten in excess, occasion bloody urine and accelerate fits of gout; formerly esteemed emmenagogue and aphrodisiac.—Root (RA′DIX ASPAR′AGI, L.), properties resemble those of the young shoots, but stronger; one of the five ‘greater aperient roots’ (RAD′ICES APERIEN′TES QUIN′′QUE MAJO′′RES, L.) of old pharmacy. The tops and roots, though no longer officinal in the British Pharmacopœias, are both occasionally employed as popular remedies in dropsy and stone—the first being eaten in the usual way at table; and the second made into an infusion or decoction (1⁄2 oz. to the pint), taken ad libitum.
As an article of food, asparagus, in moderation, is both wholesome and nutritious. It is cooked by simply boiling it rather quickly until tender, like the other soft green vegetables;210 and is either served up plain, or on toast with melted butter or sauce Hollandaise in a boat (Soyer; Rundell.) When very small and green, it is frequently dressed and served like green-peas, the tender portion of each shoot being cut into bits of equal size, and about 1-3rd of an inch long. (Miss Acton.)
Choice, &c. “The large grass is generally preferred; although the smaller has the fullest flavour for a dish.” (Soyer.) Unlike other plants, the asparagus officinalis has not produced a single well-marked permanent variety by cultivation.[92]
Asparagus Petræ′a. [L.] Syn. Rock′-aspar′agus; Corruda; Aspar′agus acutifo′′lia, L,; Corrude, Fr. Resembles the last in its general qualities; but is said to contain more asparagin.
ASPAR′AMIDE (-mĭd). See Asparagin.
ASPAR′TIC ACID. HC4H6NO4. Syn. Malam′ic acid; Acidum aspar′ticum, L.; Acide aspartique, Fr. An acid first obtained, by Plisson, from asparagin, by boiling it along with hydrate of lead or of magnesia. Its salts are called ASPAR′TATES (Eng., Fr.; ASPAR′TAS, L. sing.) See Asparagin.
AS′PEN (-pĕn). Syn. Asp*, Trem′bling pop′lar‡; Pop′ulus trem′ula (Linn.), L.; Tremble, Fr.; Aespe (äspe), &c. Ger. A large tree, of the nat. ord. Amentaceæ; (DC.), not uncommon in the moist woodlands of England, and found native on many of the Scottish mountains. It derives its name from the trembling motion of its leaves, which, owing to the peculiar flattening of the leafstalks, are agitated by the slightest impulse of the air. Bark and leaves contain POP′ULIN associated with SAL′ICIN. Both bark and leaves have been used with advantage in strangury and intermittents.
ASPHALT′ (-fălt′). Asphaltum.
ASPHALT′UM. [L., prim. Gr.] Syn. Asphalt′, Compact bitumen, Mineral pitch, Jew’s pitch, Foss′il bitu′men, Vit′reus b., &c.; Asphal′tus, Bitumen fos′sile (-e-le), B. Juda′icum, B. sol′idum, B. vit′reum, Mu′mia†, M. minera′lis*, &c., L.; Asphalte, Bitume massif, B. solide, Poix juive, &c., Fr.; Asphalt, Erdpech, Judenpech, &c., Ger. A black, hard, brittle, and glossy variety of bitumen found on the shores of the Dead Sea (hence called La′cus Asphalti′tes), on and near the shores of the Great Pitch Lake of Trinidad, and as a mineral product in various other parts of the world.
Prop., &c. Melts without decomposition, and, when pure, burns without residue. It is distinguished from other varieties of bitumen by its more difficult fusibility, and by its fracture being clean, conchoidal, and vitreous. Distilled by itself it yields about 36% of a peculiar bituminous oil (crude PETROLENE), together with combustible gases, traces of ammonia and water. To anhydrous alcohol it yields 5% of a yellow resin, soluble in rectified spirit and ether; by digesting the residuum in ether, a further 70% of a brownish-black resin is obtained, which is freely soluble in the volatile oils and in about 5 times its weight of mineral naphtha. The portion (25%) left undissolved by ether is very soluble in the oils of turpentine and petroleum. These three resinous principles dissolve altogether, when digested, in the oils of anise, rosemary, and turpentine, and in the fixed oils. (John.) According to others, asphaltum consists almost entirely of asphaltene. (Boussingault.) Paranaphthaline has been found in some varieties. (M. Laurent.) Average sp. gr. 1 to 1·68. By friction it affords negative electricity. It is soluble in oil of turpentine, benzole, mineral and coal-tar naphtha, the fixed oils, solutions of the caustic alkalies, and several other liquids, by the aid of heat.
Sources. That of commerce is chiefly obtained from the shores of the Dead Sea; but much of that of the shops is a spurious article of the most worthless character. A short time since some specimens of the purest and most beautiful description, from the Great Bitumen Lake of Trinidad, were given us by our respected and venerable friend, the late Earl of Dundonald, who stated that the supply of both liquid and indurated bitumens, of every grade of quality, was unlimited from that source; but that owing to injudicious importations of inferior kinds (those most easily shipped), a prejudice had been created against them in the London market. Our personal investigations have since confirmed the accuracy of these statements.
Uses. The finer varieties are chiefly used as a ‘glazing colour’ by artists, and in the manufacture of black varnishes and japans. The inferior kinds are applied to the same purposes as ordinary solid bitumen. The Egyptians used it in embalming under the name of MU′MIA; and the Babylonian builders are said to have employed it, as a cement, in lieu of mortar. It is, however, doubtful whether the hard semi-vitreous variety of bitumen, properly termed ‘asphaltum,’ was that which was thus employed; its present hardness being probably due to time. As a medicine it is stimulant; and it was formerly used as an ingredient in certain plasters and ointments. See Bitumen, Pitch, &c. A mixture of asphalt, chalk, sand, ground sandstone, &c., is used as a pavement for making water-tight tanks and covers, as a coating for gas and water pipes, and for various other similar purposes. Sometimes the pitchy residue obtained by distilling off the more volatile portions of gas tar is employed to replace the asphalt in the foregoing mixture; the product is called artificial or gas-tar asphalt.
Asphaltum, Facti′′tious (-tĭsh-′ŭs). Syn. Asphal′tum facti′′tium, L. That of the shops,211 when not an inferior kind of true asphaltum, is commonly made from the bottoms of Barbadoes tar, and other mineral bitumens, by heating them until quite hard. Sometimes a little Scio turpentine, balsam of copaiba, or even common resin, is added. Colour, hardness, &c., inferior to those of native asphaltum.
Asphaltum, Liq′uid. Syn. Prepared’ asphaltum; Asphal′tum liq′uidum, L. Prep. 1. Scio turpentine, 2 oz.; melt; add asphaltum (in powder), 1 oz.; mix, cool a little, and reduce with hot oil of turpentine.
2. (Wilson’s.) Asphaltum, 1⁄2 lb.; melt; add of hot balsam of copaiba, 1 lb.; and, when mixed, thin it with hot oil of turpentine. Both are used as ‘black japan’ or ‘varnish,’ and as a ‘glazing colour’ by artists.
ASPHYX′IA (-fĭk′-sh′ă; -fĭks′-e-ă‡). [L., Gr.] Syn. Asphyx′y‡ (-e), Eng.; Asphyxie, Fr.; Pulslosigkeit, Scheintod, Gr. Literally, absence of pulse; hence, a fainting fit; apparent lifelessness. Its use is now generally confined to a suspension of vitality from some cause interrupting respiration, but in which life is not actually extinct, and may, under favorable circumstances, be revived.
Asphyxia is commonly divided into four varieties by nosologists:—
1. Asphyxia algida:—Cause. Exposure to intense cold.—Symp. Countenance pale, livid, and shrivelled; limbs rigid.
2. Asphyxia elec′trica:—Cause. Stroke of lightning or electricity.—Symp. Countenance pale, limbs flexible, blood incoagulable.
3. Asphyxia mephit′ica:—Cause. Inhalation of irrespirable gases or fumes.—Symp. Countenance pallid, lips wan, &c.
4. Asphyxia suffocatio′nis:—Cause. Suffocation or strangulation, as from drowning, hanging, &c.—Symp. Countenance turgid and livid.
Treatm., &c. No general rules can be given exactly suitable to each variety. Whenever it is possible to procure medical aid, it should, of course, be immediately sought, as the delay of even a single minute may render it unavailing. In the treatment of suspended animation the principal object is to effect a restoration of the respiratory and circulatory functions; the former of which has been arrested by the external condition of the patient; the latter by the contact of morbidly carbonised blood with the capillary vessels of the lungs. The first thing to be attempted is the restoration of warmth by active friction with the warm hands, flannels, &c.; the second, the re-establishment of natural respiration by an available means, of which, perhaps, none is simpler or better than alternate pressure and its relaxation, applied to the thorax and abdomen, so as to induce expiration first, and inspiration immediately afterwards, by the natural action and elasticity of the ribs and diaphragm. Cold water may also be suddenly dashed on the face and general surface previously warmed by the frictions, in the hope of inducing a more decided inspiration. If these measures fail, artificial respiration should be promptly had recourse to. (Dr Marshall Hall.) The warm bath, and slight electrical shocks, or continued streaming electricity, may also be applied.
See Charcoal, Cold, Drowning, Hanging, Respiration (Artificial), Sewers-gas, Strangulation, Suffocation, &c.
ASPHYX′IATED. Syn. Asphyxia′tus, L.; Asphyxié, Fr.; Asphyktisch, Scheintodt, &c., Ger. Affected with or labouring under asphyxia. (See above.)
ASP′IC†. Spike lavender or French lavender; also the male lavender, spica nardi, or pseudo-nardus of old writers.
Aspic. In cookery, “savory jelly extracted from the succulence of meat.” (Soyer.)
Prep. (Miss Acton.) Calf’s feet, 2 in no.; veal, 4 lbs.; ham, 3 lb.; onions, 2 (large); carrots, 3; water, 1 gall.; boil 5 or 6 hours, or until reduced to less than one half, strain, and when cold, put the jelly into a stew-pan with the whites of 4 eggs well beaten, a large bunch of savoury herbs, 3 blades of mace (in shreds), a teaspoonful of white peppercorns, and salt, q. s.; keep it well stirred until pretty hot, then let it gently simmer for about 15 minutes, and, after settling, pass it through a jelly-bag till quite clear. After cooling a little, it is fit for use; or it may be allowed to cool and be at any time remelted. French cooks commonly flavour it with tarragon-vinegar, added after clarification.
Uses, &c. “Cold poultry, game, fish, plovers’ eggs, truffles, and various dressed vegetables, with many other things often elaborately prepared, and highly ornamental, are moulded, and served in it, especially at large déjeûners and similar repasts. It is also much used to decorate raised pies and hams, and for many other purposes.”[93]
ASPIRATOR. An apparatus for drawing a stream of air through a tube or other vessel. There are several forms of aspirator; that invented by Brunner is perhaps one of the most convenient. It consists of two equal cylindrical vessels placed one above the other, and communicating by tubes which can be opened or closed, so that when the water has run from the upper to the lower vessel, the apparatus turning for the purpose on a horizontal axis may be inverted so as to bring the empty vessel to the bottom and the full one to the top; the water may then again be made to flow without the trouble of refilling. See Air, Analysis of.
ASS (ăss). Syn. As′inus, L.; Ane (âne), Fr.; Esel, Ger. The e′quus as′inus (Linn.), a well-known animal found almost everywhere.
ASSAFŒTIDA. [L. and Eng.] Syn. Assafetida, Devil’s dung, Eng.; Assafœtida gummi, L.; Stinkasand, Stinkender asand, Teufels-dreck, Ger. A gum resin exuded212 from the excised root of narthex assafœtida (B. P.); from ferula assafœfida, and probably from ferula Persica. It yields its virtues to alcohol, and forms a clear tincture, which becomes milky on the addition of water. It is imported into Europe from Persia, viâ Bombay, in cases, mats, and casks.
Comp. Assafœtida contains from 4 to 5% of a peculiar volatile oil, and from 50 to 60% of resin of a whitish colour, turning rose-red and reddish-brown by exposure to the air, and giving a greenish solution with concentrated sulphuric acid. Brande resolved this resin into two others—one soluble in ether; the other insoluble in that menstruum.
Pur. The assafœtida of the shops is generally in masses of a whitish, reddish, or violet hue, formed principally of adhering tears or grains, possesses a peculiar fœtid, alliaceous odour, and forms an emulsion with water in all proportions. Hot sulphuric acid blackens it and forms a dark blood-red liquid, sulphurous fumes being evolved. This solution diluted with water, and then saturated with potassa, has a blue colour, which is most visible by reflected light. Digested first in alcohol, and afterwards in weak spirit-and-water, the residuum should not exceed 16%. Sp. gr. 1·325 to 1·330. It is frequently adulterated with inferior gums, and with chalk, clay, sand, &c. The purest and best is that which is clear, of a more or less pale-red colour, full of white tears, and very fœtid.
Prop., Uses, &c. Assafœtida is stimulant, antispasmodic, emmenagogue, expectorant, aphrodisiac, and anthelmintic, and is the most powerful of all the fœtid gum-resins. It is administered with advantage in several uterine diseases, hysteria, chorea, flatulent colic, hooping-cough, infantile convulsions, spasmodic asthma, and some other affections of a spasmodic and convulsive character.—Dose, 5 or 6 to 30 gr.; in pills, or preferably made into an emulsion; as an enema, 2 dr., with warm water, q. s.—Dose for Animals. Similar to Assafœtida. Some oriental nations esteem it highly as a condiment. The Brahmins use it against flatulence, and to correct the coldness of their vegetable food. In Persia the leaves of the plant are eaten as salad; and the root, after being roasted. In cookery it is now frequently employed as a substitute for garlic. “I am assured by an experienced gastronome that the finest relish which a beef-steak can possess may be communicated by” (slightly) “rubbing the gridiron on which the steak is to be cooked with assafœtida.”[94]
ASSAFŒTIDA, PREPARED. As Ammoniacum, prepared.
ASSAMAR. A substance described by Reichenbach, and found by him in the crust of bread. It possesses the faculty of retarding tissue metamorphosis.
ASSAY′ (-sā). Syn. Essai (anc., asaie), Fr. Prüfung, &c., Ger. Literally, a ‘trial’ or examination. In chemistry, the determination, by any chemical means, of the richness of a substance in its essential material or more valuable ingredient; more particularly applied to quantitative analyses of the commercial alkalies, bleaching-powder, oxide of manganese, ores, and other like articles that are employed on the large scale. In docimacy and metallurgy the determination of the quantity of metal in any ore, alloy, or other metallic compound, particularly in the ‘dry way,’ or by the process of cupellation; and more especially of the quantity of pure gold, or pure silver, contained in coin, bullion, and the commercial alloys and ores of these metals. The substance assayed*. See Assaying, &c.
ASSAY′ING. Syn. Assay′, Doc′imacy (dŏos′-) Docimas′tic art; Coupellation, Fr.; Abtreiben auf der capelle, Ger. The art of assay, or of determining the quantity of gold and silver in ores and alloys of these metals, in the ‘dry way,’ or by cupellation. It differs from chemical analysis in merely furnishing the quantity of the precious metal contained in the sample examined; instead of the nature and proportions of all, or any, of the ingredients in the compound, at the will of the operator.
Materials, Appar., &c. These are—furnace, muffle, cupels, charcoal, &c., all of which must be provided and properly arranged for use before an assay can be made:—
The FUR′NACE employed at the Royal Mint and at Goldsmiths’ Hall, London, is figured in section in the above fig., and has the following dimensions:—Total height, 21⁄2 feet; from the bottom to the grate, 6 inches; grate, muffle-plate, and bed of loam that covers it, 3 inches;213 space between the grate and the bottom of the funnel or chimney, 211⁄2 inches; funnel, 6 inches. A furnace of any other shape and size may be employed, provided it affords a sufficient heat, and allows of the easy introduction of the muffle.
The MUFF′LE (mŭf′l) is a vessel made of clay (see engr.), and furnished with an opening to admit of the introduction of the cupels, and the complete inspection of the process. It is placed on the muffle-plate (see above), by which it is introduced into the furnace.
The CU′PEL (kū′-pĕl) is a small, porous, shallow crucible, usually made of bone ashes or burnt horn. The powder (slightly moistened with water) is placed in a circular steel mould, and after being pressed down tight, is finished off with a rammer having a convex face of polished steel, which is forcibly struck with a mallet, until the mass becomes sufficiently hard and adherent. The newly formed cupel is then carefully removed and exposed in the air for a fortnight or three weeks to dry. Fig. 1 represents a cupel in section, and fig. 2 the tongs used for charging it. The best weight for cupels ranges between 180 and 200 gr. Those used at the Royal Mint are made of the calcined cores of ox-horns.
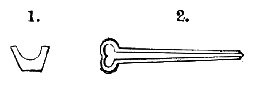
Proc. of Ass. The muffle, with the cupels properly arranged on the ‘muffle-plate,’ is placed in the furnace, and the charcoal added and lighted at the top by means of a few ignited pieces thrown on last. After the cupels have been exposed for about half an hour, and have become white-hot, the lead (see below) is put into them by means of the tongs. As soon as this becomes bright red and ‘circulating,’ as it is called, the specimen for assay, wrapped in a small piece of paper or lead-foil, is added. The fire is now kept up strongly until the metal enters the lead and circulates well, when the heat, slightly diminished, is so regulated that the assay appears convex and more glowing than the cupel itself, whilst the ‘undulations’ circulate in all directions, and the middle of the metal appears smooth, with a margin of litharge which is freely absorbed by the cupel. When the metal becomes bright and shining, or, in technical language, begins to ‘lighten,’ and prismatic hues suddenly flash across the globules, and undulate and cross each other, followed by the metal becoming very brilliant and clear, and at length fixed and solid (called the ‘brightening’), the separation is ended and the process complete. The cupels are then drawn to the mouth of the muffle, and allowed to cool slowly. When quite cold, the resulting ‘button,’ if of silver, is removed by the pliers or tongs from the cupel, and after being flattened on a small anvil of polished steel, with a polished steel hammer, to detach adhering oxide of lead, and cleaned with a small hard brush, is very accurately weighed. The weight is that of the pure silver; and the difference between the weight of the alloy before cupellation, and that of the button of pure metal, represents the proportion of alloy in the sample examined. (See below.) In the case of gold, the ‘button’ has to undergo the subsequent operations of quartation, parting, and annealing, before it is weighed, as described under that metal.
Assayer’s weights, &c. The materials used in assaying are accurately weighed in a balance of the most susceptible description; and the weights are given in terms of the ‘notation’ employed by assayers. The ‘fineness,’ ‘richness,’ or ‘degree of purity’ of gold is expressed in carats. Pure gold is spoken of as 24 carats fine; and any other sample containing in 24 parts only 12, 18, 22, &c., parts of pure gold, is said to be of as many carats fine. Every carat is nominally divided into 4 ‘assay-grains,’ each assay-grain into ‘quarters,’ and each quarter into ‘eighths’ (= 1⁄32 carat), giving 768 “reports” for gold. On this system fractional alloys are commonly spoken of as of so many ‘carats and thirty-seconds fine.’ The real quantity taken for assay, technically termed the ‘assay-pound,’ is, however, very small, generally either 12 gr. or 6 gr., which makes each assayer’s eighth-grain, or “report,” equal to either the 1⁄64 or 1⁄128 gr. Troy, as the case may be. The nominal assayer’s gold carat is 12 gr. The “journey-weight of gold” is 15 lbs. Troy (= 701 sovereigns = 1402 half-sovereigns).
The ‘fineness,’ ‘richness,’ or ‘purity’ of silver was formerly expressed in pennyweights; but is now generally reckoned in 1000ths, which admits of greater accuracy. Pure silver was said to be silver of 12 pennyweights.” If it contained 1, 2, or 3 parts of alloy, it was termed “silver of 11, 10, or 9 pennyweights,” as the case might be. Every assayer’s pennyweight was nominally divided into 24 gr., and hence gave 288 fine grains, or ‘reports,’ for silver. The fineness of specimens containing odd grains was given in pennyweights and fine grains. The ‘assay-pound’ for silver, on this system, may be 24 Troy gr., when 2 real grains are equal to 1 ‘fine pennyweight,’ and 1⁄12 real gr. equal to 1 ‘fine-grain.’ In the decimal method pure silver is = 1000. The usual weight of silver taken for the ‘assay-pound,’ when the fineness214 is reckoned in 1000ths, is 20 Troy gr., every real grain of which represents 50⁄1000th of fineness; and so on of smaller divisions. The mint “journeyweight of silver” is 60 lbs. Troy (=3920 shillings, or a like value in other denominations).
Ratio. Cupellation, which is the distinctive and most important operation in assaying gold and silver, is founded upon the feeble affinity which these metals have for oxygen, in comparison with copper, tin, and other cheaper metals; and on the tendency which these latter metals have to oxidise rapidly in contact with lead at a high temperature, and to sink with it into any porous earthen vessel, in a thin, glassy or vitriform state. The conditions essential to the success of the process, and which are found in the precious metals, are—that “the metal from which we wish to part the oxides must not be volatile;” and that “it should also melt and form a button at the heat of cupellation; for otherwise it would continue disseminated, attached to the portion of oxide spread over the cupel, and incapable of being collected.”[95]
Concluding Remarks. The art of assaying requires very great care, skill, and experience, for its due exercise; and from the costliness of the precious metals, and their general employment for coin, jewelry, plate, &c., is of the utmost importance both to individuals and governments. Such is the extreme delicacy of the operation of cupellation that, without the requisites alluded to, it is more likely to fail than to give reliable results. An assay is thought to be good when the ‘button’ or ‘bead’ separates readily from the cupel, has a round form, with a brilliant upper surface, and the lower one granular and of a dead metallic lustre. When the upper surface is ‘dead’ and ‘flat,’ too much heat has been employed; and in the case of silver, some of the metal may have been lost by fuming or absorption. When the bead adheres to the cupel, or is spongy, variegated, or has scales of litharge still adhering to it, either too little heat has been used, or the process has been stopped before the assay was complete. The remedy is re-exposure to heat in the cupel, adding a little powdered charcoal or a few small pieces of paper, and continuing the heat until the metal ‘brightens’ and ‘circulates’ freely. The lead employed must be absolutely pure, or that technically called ‘poor lead,’ and, for this purpose, is commonly prepared by the reduction of refined litharge mixed with some carbonaceous matter, by heat; but, according to the late T. H. Henry, “lead reduced from the litharge of commerce usually contains from 10 to 15 dwt. of silver per ton.” These remarks apply equally to gold and silver.
The process of assaying by the cupel, however skilfully conducted, gives much less accurate results, especially with silver, than the method of chemical analysis, often termed ‘humid’ or ‘volumetrical assay,’ whilst it is, in all cases, much more troublesome and expensive, and with compounds containing only small quantities of the precious metals, is not to be depended on. See Gold, Silver; also Carat, Cupellation, Parting, Liquation, Quartation, Refining, &c. (and below).[96]
Assay of the Touch. The fineness of JEWELRY, and of small quantities of GOLD which it is either impossible or inconvenient to assay according to the usual method, is generally determined by means of touch-needles and touch-stones. The former are made in sets, containing gold of different degrees of fineness, and differently alloyed with copper and silver. The latter are usually of black basalt; but pieces of good black pottery answer the purpose very well. The mode of using them is to mark the stone with the sample under examination, and to compare its appearance, hardness, colour, &c., with that produced by one or more of the needles. When the two are similar, the quality or ‘fineness’ is considered to be the same. The marks are then further examined by heating the ‘touch-stone’ to redness, and moistening the strokes with aquafortis, when the appearance resulting from oxidation, &c., differ according to the nature and quantity of the alloy. A nearly similar method is sometimes adopted with SILVER; but the characteristics are scarcely so distinct with the metal. (See above.)
Hu′mid Assaying, Humid Assay. Terms applied to the estimation of the quantity of gold and silver in ores and alloys in the moist way, more especially by the method known as volumetrical analysis. See Gold (Estim.), Silver (Estim.), Volumetrical Analysis, &c.
ASSIMILA′TION. [Eng. Fr.] Syn. Assimila′tio, L.; Aneigung, Verähnlichung, &c., Ger. In physiology, the conversion of food into nutriment, and finally into the substances which compose the bodies of animals and plants; the function of nutrition.
ASTHEN′IC. Syn. Asthen′icus, L.; Asthénique, Débile, Fr.; Schwach, Ger. Weak; debilitated. In pathology, an epithet of diseases (ASTHEN′IC DISEASES) accompanied by great and well-marked debility.
ASTHEN′OPY. Syn. Astheno′pia, L. In pathology, incapacity to keep the eyes fixed on near or small objects for any length of time without confusion of vision. The common causes are over-exertion of the eyes, particularly by artificial light, or by a very brilliant one, or during convalescence; congestion of the ocular vessels; debilitating discharges or indulgences; and general nervous debility, however produced. It “appears to consist in weakness of the apparatus by which the eye is adjusted for the vision of near objects;” and215 along with this “there is an irritable state of the retina, connected in some manner with a tendency to internal congestion of the eyes.”[97] The treatment may consist of rest to the eyes, and ablution of them in cold water, with such other efforts to restore their tone and the general health as are noticed under Amaurosis. The prospect of complete cure, when the cause is not removable, is unfavorable; but even when confirmed the disease is not likely to end in blindness. The use of convex spectacles of very low power will generally be found serviceable. See Eye, Spectacles, Vision, &c.
ASTH′MA (ăst′mă[98]). [Eng., Ger., L., Gr.] Syn. Asthme, Fr.; Engbrüstigkeit, Ger. In pathology, a well-known disease coming on by fits, and characterised by shortness and difficulty of breathing, accompanied by a wheezing sound, cough, stricture and tightness of the chest, with other like symptoms. These gradually increase until the patient can no longer remain in a recumbent position, being, as it were, threatened with immediate suffocation; and they generally terminate, after the lapse of a few hours, in copious expectoration. The attack usually commences towards evening, and the symptoms increase in urgency during the night—often occurring suddenly after the first sleep—until at length, on the approach of morning, a remission takes place, and, in all probability, the patient, worn out and exhausted, falls into a sound sleep. On awaking in the morning he still feels the ‘tightness’ at the chest, breathes with some difficulty, which is increased by moving, and cannot lie in bed unless his head and shoulders are greatly raised. After a repetition of the fits for some nights, they at length moderate, and after more considerable remissions, pass off at last, leaving the patient in his usual state of health for a time, or until fresh exciting causes produce a return of the disease. For an evening or two previous to the fit the patient generally feels drowsy, indolent, and low-spirited, and experiences a sensation of fulness about the stomach, with headache, general uneasiness, and indigestion—these are the premonitory symptoms.
Asthma is principally confined to the later periods of life, and appears in many cases to be hereditary. It is generally severest in the heat of summer, or in the foggy or damp or windy weather of winter. The fits vary in duration from two to several hours. Sometimes copious expectoration commences early, which has led to the division of asthma, by nosologists, into two kinds—dry, nervous, or spasmodic asthma (ASTHMA SIC′CUM, L.) and humid a. (A. HU′MIDUM, L.).
The exciting causes of asthma are exposure to sudden changes of temperature, particularly from heat to cold; unwholesome effluvia, hard drinking, heavy meals, indigestion, violent exercise, and cold, damp, foggy, and sometimes windy weather.
Treatm. A dry, warm, and airy situation as a residence should, if possible, be sought. The use of flannel next the skin, and tepid or warm bathing is also advantageous. The bowels should be kept regular by mild aperients, and the stomach preserved in order by the adoption of a light and wholesome diet; particularly avoiding excess in either eating or drinking. The severity of the paroxysm may be generally lessened by adopting the sitting posture, and inhaling the vapour of hot water or of an infusion of chamomile. Small doses of camphor, ether, and opium, frequently repeated, may also be tried. The inhalation of the vapour of a little tar liquefied by heat is said to often produce considerable relief. The fumes arising from the slow combustion of porous paper dipped in a solution of nitre, and dried, have also been recommended. “The fumes of a piece as big as one’s hand being placed on an earthenware plate, and ignited, presently become sensible throughout the room; and within a quarter of an hour their influence in many cases is rendered evident, in clearing the passages and gradually opening the air tubes.” “Of calming vapours that of chloroform is, however, the one likely, in respect of its soothing power, to supersede all others. Inspired in moderate quantity, far less than is requisite to produce general insensibility, it has been found of singular efficacy in allaying, at once, the spasmodic distress of an asthma-fit. But it is a remedy too potent and subtle to be entrusted to the discretion of the patient himself.” (Dr Watson); unless, indeed, he well understands its properties and nature, and has some friend near him to restrain his using it too freely—a thing he is, unfortunately, often tempted, by the urgency of the symptoms, to do. “Bleeding is an imprudent operation in every species of asthma” (Dr Bree); and has often proved highly injurious, especially in elderly persons. It is only in full plethoric habits, or when the paroxysms are very severe, and attended with signs of congestion of the lungs and brain, indicated by lividity of the countenance, stupor, extreme dyspnœa, &c., that blood should be taken; and then only by ‘cupping’ between the shoulders, or by leeches to the chest. Emetics and active purgatives must also be avoided during the paroxysm; at which time costiveness may be best removed by an aperient clyster containing assafœtida. At other times, emetics (of ipecacuanha) and diaphoretics, followed by mild purgatives, may be administered with advantage; indeed, an emetic, taken a few hours before an impending fit, will frequently prevent its accession. Dyspeptic symptoms must be treated in the usual manner. “Chalk and opium will astonish the asthmatic, by the excellence of their effects216 when the irritation proceeds from dyspepsia of the first passages only.” (Dr Bree.) The same authority also states that vinegar, separately administered, counteracts the flatulence and distension of the stomach.
Various other remedies have been recommended for asthma; among which are the smoking of tobacco and stramonium. In using the latter herb, the root and lower parts of the stem are chopped up and placed in the bowl of a common tobacco-pipe, and a few whiffs are occasionally taken. Drinking at the same time should be avoided. Lately lobelia inflata (Indian tobacco) has been highly extolled in asthma, in doses of—tincture, 20 or 30 drops, to 2 teaspoonfuls—powder, 5 to 15 or 20 gr.; taken at the commencement or shortly before the accession of the fit, and repeated after the interval of an hour, if nausea or expectoration does not intervene. Sir John Floyer is said to have been cured of an asthma of 60 years’ standing, at the age of 80, by the constant use of very strong coffee. Sir John Pringle adopted the same remedy with great success. He remarks, “One quality occurred to me which I have observed of that liquor (coffee), confirming what you have said of its sedative powers. It is the best abater of periodic asthma which I have seen. The coffee used ought to be the best Mocha, newly burnt, and made very strong immediately after grinding it. I commonly order an ounce for one dish, which is to be repeated with fresh coffee after the interval of a quarter or half an hour; and which I direct to be taken without milk or sugar.”[99]
Very recently cigars and cigarettes of datura tatula, Linn.—a peculiar species of stramonium—have been prepared by Messrs Savory and Moore; and are strongly recommended by Drs Watson, Latham, Fergusson, and many other physicians of eminence, as the very best remedy yet introduced for asthma.
A change of diet and habits, and particularly a change of residence, will often produce a marked improvement in asthmatic patients, and even effect a cure, when medicines have failed. The use of bark and bitters, or mild chalybeate tonics (when not contra-indicated), tends to improve the tone of the system, and may be adopted, in nearly all cases, with perfect safety. See Bath, AIR (Compressed), Cigars, Datura, &c.
Treatment for Horses. Ether and belladonna; chlorodyne; inhalation of chloroform; or amyl nitrite; subcutaneous injection of morphine or atropine; arsenic; and regular digestible diet.
Asthma, Grind′er’s. See Melanosis.
ASTHMA CURE. 1. (Dr Aubrée, Ferte Vidame, Eure et Loire, France.) Decoction of senega (10 parts of the root), 250 parts; iodide of potassium, 50 parts; extract of opium, 4 parts; simple syrup, 500 parts; weak spirit, 200 parts. Coloured with some cochineal tincture. (Hager.)
According to a later analysis by Schröppel, this remedy is thus composed:—Iodide of potassium, 9 parts; French lactucarium, 1 part; water, 288 parts; simple syrup, 48 parts; chloric ether, 11⁄2 part.
2. (Kubale, Klitschdorf, near Bunzlau.) This is a solution of iodide of potassium, bromide of potassium, and sugar in water, strongly coloured with a cochineal tincture containing alum. It is supplied in six bottles, numbered 1 to 6, No. 1 being the weakest, and No. 6 the strongest in the iodide and bromide. In No. 3, for example, we found:—Iodide of potassium, 5 grms.; sugar, 21⁄2 grms.; alum, 1⁄3 grm.; cochineal colouring matter, 1⁄2 grm.; water, 200 grms. (Hager.)
ASTHMA TEA (Dr Orleïn). Recommended for difficulty of breathing, dry coughs, loss of sleep, loss of appetite, &c. Liquorice, 8 parts; marshmallow root, 6 parts; Iceland moss, 5 parts; a sort of buckbean, 2 parts; horehound, 2 parts. (Schädler and Selle.)
ASTHMATIC PASTILLES (S. Kittel’s, now Daniel White & Co., New York). Set fire to the pastilles and inhale the smoke. An analysis found in 100 parts:—Nitrate of potash, 20·1 parts; impure resin of scammony, 3·5 parts; gum and sugar, 35· parts; charcoal, plant-stems, and leaves, 40·7 parts. (Dr Fleck.)
ASTRIN′GENT (-trĭnje′-). [Eng., Fr.] Syn. Astrin′gens, L.; Zusammenziehend, Ger. That straitens or causes wrinkling or constriction. In pharmacology, an epithet of substances or agents (ASTRIN′GENTS; ASTRINGEN′TIA, L.) which constrict animal fibre and coagulate albuminous fluids, and thereby obviate relaxation and check excessive secretion or discharges. In modern use, the word, both as an adj. and subst., is chiefly applied to internal remedies, those of a like character, employed externally, being usually termed ‘styptics,’ ‘desiccants,’ &c.
The principal astringents are—alcohol, alum, chalybeates (generally), sulphate of copper, sulphate and perchloride of iron, acetate and diacetate of lead, lime, bichloride of mercury, nitrate of silver, vegetable astringents (see below), acetate, carbonate, chloride, oxide, and sulphate of zinc, &c. See Desiccants, Styptics, Tonics, &c.
Astringents, Min′eral. See Astringent (above).
Astringents, Veg′etable. Of these the principal are—alkanet, bistort, catechu, the cinchona barks and their alkaloids, dragon’s blood, French or red rose, galls, kino, logwood, mastiche, oak-bark, red sanders wood, rhatany, tormentil, tannic acid, gallic acid, and areca nut. (See above.)
Astringent Prin′ciple. A term formerly restricted to tannin; but now commonly applied to the astringent matter of any vegetable.217
ATMOM′ETER. Syn. Atmidom′eter; Atmom′etrum, &c., L.; Atmomètre, &c., Fr. In chemistry and meteorology, an instrument for measuring the rate of evaporation from a humid surface. It is of very simple construction, and possesses some practical value. It consists of a long glass graduated tube divided into inches, having attached to the bottom a hollow ball made of porous earthenware, similar to that used in water bottles. When used, water is poured in at the top until it rises to the zero point of the scale. The outside of the porous ball being always covered with dew, the more rapidly the evaporation takes place, the more quickly will the water fall in the tube.
AT′MOSPHERE (-fēre). Syn. Atmosphe′′ra, L.; Atmosphère, Fr.; Atmosphäre, Dunstkreis, Ger. Primarily, a ‘vapour-sphere,’ appr., the assemblage of respirable gas and aëriform vapours which surround the earth; fig., any surrounding medium or influence.
Comp., Chem. prop., Pur., Uses, &c. See Air (Atmospheric).
Mechanical properties of the atmosphere:—
Colour:—The prevailing colour of the atmosphere is blue; at considerable elevations this blue tint is lost, and the sky appears deep black. The prevalence of blue is referred to the greater facility with which the blue and violet rays are reflected, whilst the glowing tints of morning and evening are conceived to arise from the red rays possessing greater momentum than the other rays of the spectrum.
Density:—The density of the atmosphere diminishes with the distance from the earth’s surface, and this is the duplicate ratio of the altitude. Thus, if at a given altitude the density of the air is only one half what it is at the level of the sea, at twice that elevation it possesses only one fourth that density. On this fact depends the application of the barometer to the determination of the elevation or depression of any point above or below the level of the sea, taken as a standard.
| Height above the level of the Sea in miles. | Volume of Air. | Height of the Barometer. |
| 0· | 1 | 30 |
| 2·705 | 2 | 15 |
| 5·41 | 4 | 7·5 |
| 8·115 | 8 | 3·75 |
| 10·82 | 16 | 1·875 |
| 13·525 | 32 | ·9375 |
| 16·23 | 64 | ·46875 |
Height, &c.:—If the density of the air were uniform throughout its whole extent, the height of the atmosphere, measured by a corresponding column of mercury, would be barely 51⁄4 miles. As, however, its density decreases with the distance from the earth’s surface, its real height must be considerably greater. Kepler found that the reflection and refraction of the sun’s rays by the atmosphere, producing twilight, ceases when that luminary descends 18 degrees below the horizon, whence it is calculated that the atmosphere cannot have a greater altitude than 45 miles. On the other hand, there is reason to believe that it cannot be much less than this sum. “With a good air-pump air may be rarefied 300 times; supposing this to be the utmost limit to which rarefaction can be carried, the atmosphere would still extend to an altitude of above 40 miles.” Whether, in a state of extreme tenuity in which its grosser properties are lost, it extends indefinitely into space, was formerly a subject of controversy. That its boundaries are limited, and that it belongs exclusively to our earth appears almost certain. “We are warranted in concluding that the atoms of air are not infinitely divisible, and consequently that the atmosphere has a limit; and the limit must be situated at that height above the earth where the gravitation of the atoms is just equal to the force of their repulsion.”[100] Under ordinary circumstances the mercury of the barometer falls about one inch for every 1000 feet of elevation.
Pressure:—The weight or pressure of the atmosphere is shown by the rise of water in the barrel of the common ‘lifting pump’ and the suspension of the mercurial column in the tube of the barometer. The last affords a ready means of determining the actual pressure of the air, the column of mercury, and the column of air by which it is suspended, resembling two weights in equilibrio, at the opposite extremities of the same balance. The mean height of the barometer at the level of the sea, in England, is 28·6 inches (= about 331⁄2 feet of water); and as a cubic inch of mercury weighs 3425·92 gr., or ·48956 lb., it follows that the weight of a column of mercury whose base is a square inch is 14·6 lbs. avoirdupois. The pressure of the atmosphere is not merely downwards, but is equally diffused in all directions, and exerts a most powerful effect in the economy of organic beings. On the surface of the body of an adult of ordinary size (say = 15 sq. feet, or 2160 inches), it amounts to the enormous weight of 31,536 lbs., which is not sensible, only because it is balanced by the force of the elastic fluids in the interior of the body. Were this equilibrium to be suddenly destroyed, the consequence would be, either that the body would be instantly torn to pieces with explosive violence, or that it would be crushed under the overwhelming weight that would suddenly fall upon it. Even the comparatively slight variations of atmospheric pressure which occur with changes of wind, weather, and218 season, exercise a perceptible effect on the functions of life.
| Lat. | Height (inches). | Lat. | Height (inches). | Lat. | Height (inches). |
| 0° | 29·930 | 40° | 30·019 | 541⁄2° | 29·926 |
| 10 | 29·975 | 45 | 30·000 | 60 | 29·803 |
| 20 | 30·064 | 49 | 29·978 | 64 | 29·606 |
| 30 | 30·108 | 511⁄2 | 29·551 | 67 | 29·673 |
Temperature:—The temperature of the atmosphere, independently of changes arising from variations of latitude and season, diminishes, like its density, with its elevation. In general, every 100 yards of ascent causes the temperature to fall 1° Fahr. See Air (Atmospheric), Epidemics, Ventilation, &c.
Atmosphere. In engineering and pneumatics, the pressure of a column of mercury at 0° Cent. or 32° Fahr., which is 76 centimètres or 29·9218 inches high, at the mean level of the sea in latitude 45°, taken as a standard of that exerted by other elastic fluids. In practice this is assumed to be 15 lbs. to the square inch, under a barometrical pressure of 30 inches. Thus, steam or air condensed so as to exert a pressure of 30 lbs. per sq. inch is said to be of two atmospheres; at 45 lbs., of three atmospheres, &c.
AT′OM (-ŭm). Atomic Weight, Atomic Theory. Syn. At′omus, L.; Atome, Fr.; Atom, Untheilbare THEILCHEN, Ger.
Atomic Weight. When the elements unite chemically, they invariably do so in the proportions by weight represented by the numbers attached to them in the following table, or in multiples of these proportions. Dalton accounted for this law by supposing that the constituent particles of matter are indivisible, and believed that, if it were possible to place such particles in the balance, their relative weights would be found to correspond with the numbers given in the table.[101] In other words, the term atom, which is derived from the Greek ατομος, indivisible, is applied in modern chemistry to the smallest quantity by weight of an element which is capable of existing in a chemical compound, hydrogen being taken as unity.
| Name. | Symbol. | Atomic weight. | Atomic volume. |
| ALUMINUM | Al | 27·5 | |
| Antimony | Sb | 122 | |
| Arsenic | As | 75 | 1⁄4 |
| Barium | Ba | 137 | |
| Bismuth | Bi | 208 | |
| Boron | B | 11 | |
| BROMINE | Br | 80 | 1 |
| Cadmium | Cd | 112 | 2 |
| Cæsium | Cs | 133 | |
| CALCIUM | Ca | 40 | |
| CARBON | C | 12 | |
| Cerium | Ce | 92 | |
| CHLORINE | Cl | 35·5 | 1 |
| Chromium | Cr | 52·5 | |
| Cobalt | Co | 58·8 | |
| COPPER | Cu | 63·5 | |
| Didymium | D | 96 | |
| FLUORINE | F | 19 | 1 |
| Glucinum | G | 14 | |
| Gold | Au | 196·7 | |
| HYDROGEN | H | 1 | 1 |
| Indium | In | 74 | |
| IODINE | I | 127 | 1 |
| Iridium | Ir | 198 | |
| IRON | Fe | 56 | |
| Lanthanum | L | 92 | |
| LEAD | Pb | 207 | |
| Lithium | Li | 7 | |
| Magnesium | Mg | 24 | |
| MANGANESE | Mn | 55 | |
| MERCURY | Hg | 200 | 2 |
| Molybdenum | Mo | 92 | |
| Nickel | Ni | 58·8 | |
| Niobium | Nb | 97·6 | |
| NITROGEN | N | 14 | 1 |
| Osmium | Os | 199 | |
| OXYGEN | O | 16 | 1 |
| Palladium | Pd | 106·5 | |
| PHOSPHORUS | P | 31 | 1⁄4 |
| Platinum | Pt | 197·4 | |
| POTASSIUM | K | 39 | |
| Rhodium | Rh | 104 | |
| Rubidium | Rb | 85·5 | |
| Ruthenium | Ru | 104 | |
| Selenium | Se | 79 | 1 |
| SILICON | Si | 28·5 | |
| SILVER | Ag | 108 | |
| SODIUM | Na | 23 | |
| Strontium | Sr | 87·5 | |
| SULPHUR | S | 32 | 1 |
| Tantalum | Ta | 137·5 | |
| Tellurium | Te | 128 | |
| Thallium | Tl | 204 | |
| Thorium | Th | 231·5 | |
| Tin | Sn | 118 | |
| Titanium | Ti | 50 | |
| Tungsten | W | 184 | |
| Uranium | U | 120 | |
| Vanadium | V | 51·2 | |
| Yttrium | Y | 68 | |
| ZINC | Zn | 65 | 2 |
| Zirconium | Zr | 90 |
219
Atomic Volume. The volume or space occupied by the atomic weights of gases at a temperature of 60° F., and under a pressure of 30 inches of the barometer, compared with that occupied by one part by weight of hydrogen under the same conditions.
In the same table the most important elements are distinguished by the largest type, those next in importance by medium type, and those of rare occurrence, or of which we know but little, by the smallest type.
ATOMIC WEIGHTS. See Atom.
ATON′IC. Syn. Aton′icus, L.; Atonique, Fr.; Atonisch, Schlaff, Ger. Weak; debilitated; deficient in tone or strength. In pathology, applied to diseases or conditions of the body (ATONIC DISEASES; ATONY) in which debility is the leading feature. In pharmacology, ATONICS are agents which relax or lower the tone of the system.
AT′ONY. Syn. Ato′nia, L.; Atonie, &c., Fr., Ger. In pathology, loss of tone, relaxation, morbid diminution of vital energy or power; commonly applied to debility of any kind.
AT′ROPHY (-fe). Syn. Atro′phia, L.; Atrophié, &c., Fr.; Atrophie, Ger. In pathology, wasting or emaciation, with loss of strength, and unaccompanied by fever or other sensible cause; defective nutrition; decline.
Classif., Causes, &c. It is either local, as in the case of a limb which is small, imperfectly developed, or withered; or general, affecting the whole body. Gen′eral atrophy appears to depend on deficient nutrition, arising from a want of due balance between the functions of assimilation and absorption, or from profuse evacuations draining off the materials necessary for the support of the body. In the former case only may it be regarded as an independent disease. Lo′cal atrophy commonly arises from some cause which lessens the normal circulation of blood in the part; or from a diminution of the nervous influence, as in paralysis. General atrophy is most frequent in infancy, childhood, and old age. In the first two it may be often traced to bad nursing, worms, or a scrofulous taint; and not unfrequently to continually inhaling impure or damp air. In adults, the causes are impaired digestion and imperfect action of the chyliferous organs, and sometimes diseased action of the liver. In many cases it results from the use of tobacco.
Treatm. This consists in a close attention to diet (which should be liberal and nutritious), exercise, clothing, ventilation, warmth, &c., with gentle stimulants, and chalybeate tonics where not contra-indicated; and, in the case of adults, the moderate use of pure generous wine or malt-liquor. Among special remedies, both in this disease and anæmia, may be mentioned pure sweet cod-liver oil, which seldom fails to arrest or greatly retard the progress of the disease, and in very many cases effect an entire cure. When this affection is symptomatic of any other disease, as worms, stomach or liver complaints, &c., the removal of the latter must of course be first attempted. See Anæmia, Chlorosis, Tabes, &c.
ATRO′′PIA (trōpe′y′ă). C34H23NO6. [L.; B. P.] Syn. At′ropine (-pin; sometimes atro′pĭne‡), Eng., Fr.; Atropi′na, Atro′′pium*, L. An alkaloid discovered by Brandes in at′ropa belladon′na or deadly nightshade.
Prep. 1. (B. P. Process.) Take of belladonna-root, recently dried, and in coarse powder, 2 lbs.; rectified spirit, 10 pints; slaked lime, 1 oz.; diluted sulphuric acid, carbonate of potash, of each a sufficiency; chloroform, 3 fl. oz.; purified animal charcoal, a sufficiency; distilled water, 10 fl. oz. Macerate the root in 4 pints of the spirit, for 24 hours, with frequent stirring. Transfer to a displacement apparatus, and exhaust the root with the remainder of the spirit by slow percolation. Add the lime to the tincture placed in a bottle, and shake them occasionally several times. Filter, add the diluted sulphuric acid in very feeble excess to the filtrate, and filter again. Distil off three fourths of the spirit, add to the residue the distilled water, evaporate at a gentle heat, but as rapidly as possible, until the liquor is reduced to one third of its volume and no longer smells of alcohol; then let it cool. Add very cautiously, with constant stirring, a solution of carbonate of potash so as nearly to neutralise the acid, care, however, being taken that an excess is not used. Set to rest for six hours, then filter, and add carbonate of potash in such quantity that the liquid shall acquire a decided alkaline reaction. Place in a bottle with the chloroform; mix well by frequently repeated brisk agitation, and pour the mixed liquids into a funnel furnished with a glass stop-cock. When the chloroform has subsided, draw it off by the stop-cock, and distil it on a water-bath from a retort connected with a condenser. Dissolve the residue in warm rectified spirit; digest the solution with a little animal charcoal: filter, evaporate, and cool until colourless crystals are obtained.
2. Expressed juice of belladonna is evaporated over a water-bath to the consistence of an extract, and then triturated in a marble or porcelain mortar with a strong solution of caustic potassa; the resulting mass is digested and well agitated for some time, at the temperature of 75° to 80° Fahr., with benzole, q. s.; and, after repose, the benzole-solution is carefully separated, and its volatile hydrocarbon is distilled off by the heat of a water-bath; the residuum in the retort is now exhausted with water acidulated with sulphuric acid, and the resulting ‘acid-solution,’ after filtration, precipitated with carbonate of soda; the precipitate is crude ATROPIA, which is collected220 on a filter, pressed between folds of bibulous paper, and dried; after which it is purified by one or more re-solutions, in alcohol, and crystallisations, which may or may not be modified in the manner noticed. The proportion of potassa should be about 1 dr. to every quart of the expressed juice. An excellent and economical process. The product is 0·3 to 4% of the weight of the plant from which the juice has been obtained.
3. (Mein and Liebig.) Belladonna-root (fresh-dried and coarsely powdered) is exhausted by alcohol (sp. gr. 0·822); slaked lime (1 part for every 24 of the dried root employed) is then added to the tincture, and the whole digested, with agitation, for 24 hours; sulphuric acid is next added, drop by drop, to slight excess, and, after filtration, rather more than one half the spirit is removed by distillation; a little water is now added to the residue, and the remainder of the alcohol evaporated as quickly as possible by a gentle heat; after again filtering, the liquid is reduced by further evaporation to the 1⁄12th part of the weight of the root employed, and a concentrated solution of potassa dropped into the cold liquid (to throw down a dark greyish-brown matter), carefully avoiding excess or rendering the liquid in the slightest degree alkaline; in a few hours the liquid is again filtered, and carbonate of potassa added as long as a precipitate (ATROPIA) falls; after a further interval of from 12 to 24 hours, this precipitate is collected and drained in a filter, and after pressure between folds of blotting-paper, dried by a very gentle heat. It is purified by making it into a paste with water, again squeezing it between the folds of blotting-paper, drying it, re-dissolving it in 5 times its weight of alcohol, decolouring it with pure animal charcoal, distilling off greater part of the alcohol, and evaporation and crystallisation by a very gentle heat; or only about one half the spirit is distilled off, and 3 or 4 times its volume of water gradually agitated with it, the resulting milky liquid being then heated to boiling, and allowed to cool very slowly, when nearly the whole of the ATROPIA crystallises out after a few hours. The same may be effected by at once agitating 6 or 8 volumes of water with the alcoholic solution, and setting aside the mixture for 12 to 24 hours, by which time the crystallisation will be completed. This process originated with Soubeiran, was improved by Mein, and subsequently, with slight modifications, adopted by Liebig. The product is about 0·3% of the weight of root operated on.
4. (Bouchardat and Cooper.) The filtered tincture is precipitated with iodine dissolved in an aqueous solution of iodide of potassium, the resulting ioduretted hydriodate of atropia, decomposed by zinc-and-water, the metallic oxide separated by means of carbonate of potassa, and the alkaloid thus obtained dissolved in alcohol and crystallised.
5. (Mr Luxton.) The dry leaves of belladonna are gently boiled for 2 hours in distilled water just sufficient to cover them, and the resulting decoction is strained through a coarse cloth into a large precipitating jar; this process is repeated with a second quantity of distilled water, and the two decoctions mixed; concentrated sulphuric acid is now added in the proportion of 2 dr. to every pound of leaves operated on, by which the vegetable albumen of the decoction is precipitated, and the liquid becomes clear and sherry-coloured; the clear liquor is now decanted or syphoned off, and, if necessary, filtered; the filtrate is now decomposed by either passing a stream of gaseous ammonia through it, or by suspending in it a lump of carbonate of ammonia. The effect is that the liquid turns black, and crystals of ATROPIA are slowly formed and deposited. At the expiration of a day or two, the supernatant mother-liquid is removed with a syphon, and the crystals thrown on a filter to drain and dry.[102] It may be purified by re-solution and crystallisation. 1 lb. of leaves yields 40 gr.; or at the rate of fully ·57%.
6. (Rabourdin.) To the crystallised juice of the plant (previously heated to coagulate its albumen, filtered, and allowed to cool), 1 quart, is added of caustic potassa 1 dr., and afterwards of chloroform 1 oz.; the whole is then agitated well, and after half an hour’s repose, the supernatant liquor is poured from the discoloured chloroform, which, after being washed with distilled water as long as it gives any colour to that liquid, is placed in a small retort, and the chloroform distilled off by the heat of a water-bath; the residuum is dissolved in a little water acidulated with sulphuric acid, and precipitated with carbonate of potassa, in slight excess; the precipitate is redissolved in alcohol, and the solution, by spontaneous evaporation, yields crystals of ATROPIA.
7. (Ure.) From the expressed juice of the fresh, or the watery extract of the dry plant, by treating it with caustic soda, in slight excess, and then agitating the mixture with 1½ times its volume of ether; the ATROPIA taken up by the ether is again deposited after repose for some time, and is then purified by repeating the treatment with fresh ether as often as necessary.
8. Freshly precipitated hydrate of magnesia is added to the coagulated and filtered expressed juice, and the mixture evaporated to dryness, as quickly as possible, in a water-bath; the residuum is pulverised and digested in strong alcohol, and the clear liquid allowed to evaporate spontaneously. The crystals may be purified by repeated re-solutions in alcohol.
Prop., Tests, &c. The crystals obtained from hot concentrated solutions, colourless, transparent, silky prisms; from solutions in dilute spirit, silky needles, like those of disulphate221 of quinine. It is colourless; has a bitter, acrid, and somewhat metallic taste; dissolves in 200 parts (300 parts—Thomson) of cold and 50 to 54 parts of boiling water, in 11⁄2 parts of cold alcohol, and in 25 parts of cold, and 6 parts of boiling ether; it has an alkaline reaction, fuses at about 194° Fahr., is slightly volatile at common temperatures, and freely rises in vapour at 212° Fahr.; at higher temperatures it volatilises with partial decomposition; with the acids it forms salts, of which several are crystallisable.
Tests.—1. Nitric acid forms with it a yellow solution:—2. With cold sulphuric acid it gives a colourless solution, which becomes red only when heated:—3. Aqueous solutions of atropia and its salts are—a, turned red by tincture of iodine—b, gives a citron-yellow precipitate with terchloride of gold—c, a flocculent whitish precipitate with tincture of galls, and—d, a yellowish-white one with bichloride of platinum:—4. Heated with caustic potassa or soda, it suffers decomposition, and ammonia is evolved:—5. A weak solution cautiously applied to the eyelid or conjunctiva, produces dilation of the pupil lasting for several hours.
Pur., &c. Alkaloid prepared from the root of atropa belladonna. Crystals; white, in the form of prisms; soluble in water and rectified spirit. It leaves no ash when burned with free access of air (B. P.).
Phys. eff. It is a very powerful narcotico-acrid poison.[103] Its effects are similar to those of belladonna, but considerably more powerful. “A very minute (imponderable) quantity applied to the eye is sufficient to dilate the pupil.” (Pereira.) The 1⁄12 to 1⁄10 gr. often causes very serious effects in the human subject. The 1⁄6th of a grain accelerates the pulse, affects the brain, causes dryness of the throat, difficulty of deglutition, dilation of the pupil, dimness of sight, giddiness, strangury, numbness of limbs, sense of formication in the arms, rigidity of thighs, depression of pulse, and sometimes feebleness or loss of voice. These symptoms continue for from 12 to 24 hours. In larger doses death ensues.
Ant., &c. These may be similar to those described under Belladonna and Alkaloid.
Uses. Chiefly as an external agent, as a substitute for belladonna, to cause dilation of the pupil; and as a local anæsthetic or anodyne, especially in facial neuralgia. Internally, it has been occasionally given in hooping-cough, chorea, and a few other nervous diseases.—Dose, 1⁄30 gr., gradually increased to 1⁄20, or, occasionally, even 1⁄15 gr. in solution, or made into a pill with liquorice powder and honey, or syrup, or used endermically; for a collyrium, 1 gr. to water 1 oz., a few drops only being applied to the eye at a time, the greatest caution in each case being observed. It is also employed to make the sulphate. In dispensing it a single drop of acetic acid, or dilute sulphuric acid, will be found to facilitate and ensure its perfect solution. See Belladonna and Belladonine.
Atropia, Sul′phate of. Syn. Atro′pia sul′phas, L. Prep. (B. P.) Take of atropia, 120 gr.; distilled water, 4 fl. dr.; diluted sulphuric acid, a sufficiency.
Mix the atropia with the water and add the acid gradually, stirring them together until the alkaloid is dissolved and the solution is neutral. Evaporate it to dryness at a temperature not exceeding 100°.
Characters and Tests.—A colourless powder, soluble in water, forming a solution which is neutral to test-paper, and when applied to the eye dilates the pupil as the solution of atropia does. It leaves no ash when burned with free access of air.
Intended for external application. It is a powerful poison.
Uses, &c. The same as those of the pure alkaloid.—Dose, 1⁄25 to 1⁄20 gr., either in solution or pills; 1 to 3 gr. to water 1 fl. oz., as a collyrium, of which a few drops seldom fail to produce full dilation of the pupil in about a quarter of an hour; 1 to 2 gr. to lard 1 dr. forms an excellent ointment in neuralgic affections.
Obs. Sulphate of atropia (which is intended for external use only) is rather difficult to crystallise, as it has a tendency to assume an amorphous or gum-like condition. It is more soluble than the pure alkaloid; and, like it, is a terrific poison.
ATROPIA, VALERIANATÈ. The Paris Codex directs this salt to be prepared as follows:—Dissolve valerianic acid in ether, and add atropia just sufficient to saturate the acid. Let the ether evaporate.
ATROP′IC ACID. Syn. Acidum Atrop′icum, L. The name given by Richter to a volatile crystallisable substance, possessing acid properties, found in atropa belladonna or deadly nightshade. In many respects it resembles benzoic acid, from which, however it is distinguished by not precipitating the salts of iron.
ATROPI′NA, At′ropine. See Atropia.
AT′TAR. See Otto and Volatile Oils.
ATTELETTES (-lĕts′). [Fr.] In cookery, small skewers, generally of silver, with ornamental heads. The term is also applied to small dishes (ENTRÉES, &c.) in which the articles are mounted on attelettes. Small fish, as smelts, are often served in this way. See Aiguillette.
ATTEN′UANT (-ū-ănt). Syn. Atten′uans, L.; Atténuant, Fr.; Verdünnend, Ger. That makes thin, or less dense or viscid; diluting. In medicine, applied to remedies (ATTEN′UANTS, SPANÆM′ICS) which are supposed to act by thinning, diluting, or impoverishing the blood.
ATTENUA′TION. Syn. Attenua′tio, L.; Atténuation, Fr.; Verdünnung, Ger. A thinning or diminishing; a reducing in consistence. In medicine, see the adj. (above);222 in brewing, the decrease of the density of worts during fermentation, arising from the gradual conversion of their ‘saccharine’ (sugar) into alcohol. See Brewing, Distillation, Worts, &c.
ATTRAC′TION. [Eng., Fr.] Syn. Attrac′tio, L.; Anziehung, Ger. The power that draws together matter and resists its separation. That force which attracts bodies towards the centre of the earth, and which keeps on its surface those that are movable, is called GRAVITY, or the attraction of gravitation. It is exerted at sensible, often at immense, distances, and determines the figure and motions of the planets and comets, and causes the descent of heavy bodies to the ground. This force it is which confers the property of weight upon matter.
That force which unites particles of the same kind of matter, so as to cause them to assume the condition of solid or liquid masses, e.g. particles of chalk to form a mass of chalk, particles of water to form a mass of water, is called COHESION, or the ATTRACTION OF COHESION. That force which binds together different substances without changing their properties, as when paint sticks to wood, ink to paper, &c., is called ADHESION, or the ATTRACTION OF ADHESION. Capillary attraction is a modification of adhesion, and is characterised by being exerted between liquids and the internal surfaces of tubes and pervious bodies. The absorption of water by a sponge, the ascent of oil in the wick of a lamp, are examples of this power. The CHEMICAL FORCE or AFFINITY differs from all other kinds of attraction in being exerted between definite and constant quantities (atoms) of matter, usually of dissimilar natures, and producing combinations possessing properties different from those of their components. (See Affinity.) This force, as well as cohesion and adhesion, is exerted at distances so small as to be immeasurable.
The terms ELEC′TRIC ATTRACTION and MAGNET′IC ATTRACTION are employed in physics to denote phenomena which we imperfectly understand, and which operate between bodies at sensible distances, and simulate those of the attraction of gravitation.
ATTRI′′TION (trĭsh′-ŭn). [Eng., Fr.] Syn. Attri′′tio, L.; Abreibung, Aufreibung, Ger. In mechanics, the wearing away of parts by friction. In medicine, a graze, abrasion, or solution of continuity of the cuticle, or the act which causes it. In surgery, the crushing or tearing away of any exterior portion of the body by violence. See Abrasion, Anti-attrition, Friction, &c.
AURANTIA′CEÆ (-she-ē). [Lat.; DC.] The orange tribe. In botany, an extensive and important natural order of exogenous trees and shrubs, found exclusively in the temperate and tropical parts of the Old World, and unknown in a wild state in America. The fruit is pulpy, succulent, sub-acid, and eatable, and separated into cells by membranous partitions, and is covered with a leathery aromatic skin or rind. Some of the genera embrace plants of great beauty and utility. A few of the Indian species are climbers. The genus CIT′RUS, which includes the orange, lemon, citron, lime, bergamot, and shaddock, is that best known in Europe.
AURAN′′TIIN (-she-ĭn). Syn. Hesperidin; Auran′tine* (-tĭn), Eng., Fr.; Aurantii′na, &c., L. The bitter principle of the peel of oranges and lemons.
Prep. The exterior or yellow peel of the Seville orange (carefully separated from the white matter, and air-dried) is steeped in hot water, and the filtered liquor gently evaporated to dryness.
Prop., &c. It possesses the bitter properties of the peel without any of its glutinosity or fragrance, and is said to agree better with delicate stomachs. It may be taken in water either with or without the addition of a little sugar or capillaire, or dissolved in wine.
AU′′RIC (aw′- or awr′-). Syn. Auri′cus, L. Of or relating to gold, or containing it, or formed from it.
AURIF′EROUS. Syn. Au′′rifer, Aurif′erus, L.; Aurifère, Fr.; Goldhaltig, Ger. In, mineralogy, that yields or contains gold; as auriferous sand, a. quartz, &c.
AURIPIGMEN′TUM†. [L.] Literally, paint of gold; appr., native orpiment. See Arsenic.
AURO-CHLO′′RIDES (klōre′-īdz). Compounds of terchloride of gold with chlorides of other bases. They may be prepared by mixing the terchloride of gold with the chloride of the base, in atomic proportions, and setting aside the solution to crystallise.
Prop., &c. Most of the auro-chlorides crystallise in prisms, dissolve in both alcohol and water, have an orange or yellow colour, and are decomposed at a red heat.
AURO-CY′ANIDES (ĭdz). In chemistry, compounds of cyanide of gold with cyanides of other bases. They may be formed in a similar manner to the auro-chlorides. Auro-cyanide of potassium is much used in electro-gilding.
AURORA BOREALIS. This luminous phenomenon, which is occasionally seen in our own country on clear frosty nights, and much more frequently and vividly by the dwellers in more northern latitudes, has been supposed to have an electrical origin, and to be occasioned by the passage of electricity through the rarefied strata of the upper regions of the atmosphere from the poles towards the equator. But physicists look upon this explanation as unsatisfactory, and inadequate to account for the effects produced. The hypothesis, however, seems to derive some support from the following fact:—
If one of Gassiot’s vacuum tubes be brought near to a powerful electrical machine, both while the machine is in motion and for some223 time after, flashes of light may be seen passing from the wire at one end of the tube to the other extremity, which flashes bear a great resemblance to the auroral rays. The great doubt, however, is whether the conditions necessary to the production of the aurora are similar to those prevailing during this experiment, a doubt not lessened by the difficulty of satisfactorily accounting for the rarefied state of the atmosphere which is assumed to exist.
The forms which the aurora assumes are very varied and of great beauty; there appears, however, to be some general similarity in its aspect at the same locality. Its appearance is briefly as follows:—A dingy aspect in the heavens in a northernly direction is usually the precursor of the aurora; and this gradually becomes darker in colour, and assumes the form of a circular segment surrounded by a luminous arch, and resting at each end on the horizon. This dark segment presents the appearance of a thick cloud, and is frequently seen as such in the fading twilight, before the auroral light manifests itself. The density of this segment must, however, be very inconsiderable, as stars may sometimes be seen shining brightly through it.
This dark segment is bounded by a luminous arch of a blueish-white colour, which varies in breadth from 1 to 6 diameters of the moon, having the lower edge sharply defined, and the upper edge only when the breadth of the arch is small. This arch may be considered to be a part of a luminous ring, elevated at a considerable distance above the earth’s surface and having its centre corresponding with some point near the north pole. The preceding description indicates the general features of the appearance of the aurora borealis; but several auroras have been described which presented striking peculiarities. Sometimes the phenomenon assumed the form of one or more curtains of light, depending from dingy clouds whose folds were agitated to and fro as if by the wind. Sometimes this curtain appeared to consist of separate ribbons of light, arranged side by side in groups of different lengths, and attaining their greatest brilliancy at the lower edges. In this country the aurora borealis seldom assumes the distinctness and brilliancy which characterise its appearance in northern latitudes, but the description thus given indicates the type to which such appearance of the meteor more or less approaches. During the winter that prevails in the northern hemispheres the inhabitants of the arctic zone are deprived for months together of the sun’s light, and their long dreary night is relieved by the light emanating from this beautiful meteor, which shines with great frequency and brilliancy in those regions.
A remarkable connection has been observed between the aurora and the earth’s magnetism, the magnetic needle showing great disturbance during a display of the aurora. The arches of the aurora most commonly traverse the sky at right angles to the magnetic meridian, though deviations from this direction are not rare. Sir J. Franklin found that the disturbance of the needle was not always proportionate to the agitation of the aurora, but was always greater when the quick motion and vivid light were observed to take place in a hazy atmosphere. The aurora is most frequent and vivid in high latitudes, towards either pole, but the meteor is not confined to these parts, as Dr Hooker states that one of the most brilliant displays he ever witnessed was under the tropical sky of India; and other observers have recorded instances of its appearance in the equatorial districts of the globe.
The attitude of the aurora varies considerably; there appears to be little doubt, however, that it frequently occurs at small elevations. Both Franklin and Parry record instances where it appeared below the level of the clouds, which they describe as having been hidden behind the masses of its light, and as reappearing when the meteor vanished. It would seem that there are two distinct kinds of aurora one dependent upon local causes, as in the cases last given, while in the other causes are probably cosmical, and the auroral effects are seen at very distant points of the earth’s surface.
AURORA POMADE. For promoting the action of the skin. Cocoa butter with orris.
AUTOG′ENOUS (tŏj′-). Syn. Autoge′′neal; Autog′enus (tŏj′). L. Self-generating or affecting; acting without the aid of foreign matter. In anatomy, &c., developed from distinct and independent centres; as parts or processes. Among metallists, it denotes a method of joining metals by fusing the parts in contact, by means of a flame of hydrogen, or of a mixture of hydrogen and common air, without the intervention of a fusible alloy or solder. Lead, and even ordinary hard solders, are, however, sometimes so employed, and the name, though improperly, retained.
AUTOMAT′IC. Syn. Automati′cus, Autom′atus, L.; Automatique, Fr.; Automatische, Ger. Self-acting or self-moving, or that seems to be so; mechanical; of or resembling an automaton. In physiology, involuntary, applied to functions which are performed without the operation of the will; as the movements in respiration, the contractions and dilations of the heart, the persistent contraction of the sphincters, &c. In mechanics, &c., moving and acting from concealed machinery; also, as applied to machinery, self-regulating and directing, within the limits prescribed by its author, though moved by external power. To the last class belongs the self-acting machinery of our flax and cotton mills, our engineering establishments, &c.; in which the elemental powers are made to animate, as it were, millions of complex organs, infusing into forms of wood, iron, and brass, an agency resembling that of intelligent beings.224 The manufactures in which such machinery is employed are termed the AUTOMATIC ARTS.
AUTOPSY. Literally, personal observation or examination; ocular view. The term, however, is now applied, rather loosely, to a post-mortem investigation. A post-mortem may be performed with the object of endeavouring to ascertain the cause of death in a medico-legal inquiry, or in the furtherance of the study of pathology. It is also a preliminary to embalmment, and is sometimes had recourse to as a means of saving the child when a woman dies in full pregnancy.
In France no post-mortem examination is permitted to take place until at least 24 hours after death, this delay being enforced as a safeguard against the possibility of the body operated upon being still alive. In England no post-mortem can be made without the consent of the friends of the deceased, unless by warrant from a coroner; although in many public institutions this consent is dispensed with. Whenever, however, a prisoner dies in gaol an inquest and post-mortem are held on the body.
An autopsy is to be discouraged in cases where a person has died from infectious disease; but should the law require it to be undertaken, disinfectants both during and after the operation should be liberally had recourse to.
AUTUMNAL FEVER. This term is chiefly employed by American medical writers to designate typhoid fever, because of its prevalence in the autumn.
AUXILION. A packet of small plasters for the painless and radical cure of corns. Each plaster is to be worn for about a week, and then the horny pustule is to be removed with a sharp knife. The plaster is a compound of 1 part of resin plaster and 2 parts of lead plaster, and is likely to promote the removal and solution of the thick skin of the corns. (Hager.)
AVA. Syn. Kava-kava. The native names of the root, a species of piper, the piper methysticum, cultivated in Tahiti, Hawaii, the Society and Tongan Islands, the natives of which make it into an intoxicating drink. It is said to have been used in France with excellent effect in gonorrhœa; and a tincture of it has been strongly recommended both for external and internal administration in gout. “For medicinal purposes it is used in the form of infusion,” a drachm of the scraped root being macerated in a quart of water for five minutes. Its action appears to vary with the amount taken; in small doses it is generally stated to act as a stimulant and tonic, but when taken in large doses it produces an intoxication which differs from that caused by alcohol, in being of a silent and drowsy nature accompanied by incoherent dreams” (‘Pharmaceutical Journal,’ August 19th, 1876, which consult for further information.’)
AVE′NA. [L.] The oat; oats.
AVE′NIN (-nĭn). Syn. Avena′ine* (ăv-e-) Aveni′na, &c., L.; Avénine, &c., Fr. A nitrogenous compound, analogous to, and probably identical with, casein, obtained from oats, and on which its nutritiveness chiefly depends.
Prep. The grain, reduced to the state of powder or meal, is washed on a sieve, and the milky liquid, after being allowed to deposit its starch, is heated to about 200° Fahr., to coagulate the albumen; when cold, acetic acid is added as long as a white powder falls, which is AVENIN; this is collected on a filter, drained, and dried by a gentle heat.
AVEN′TURIN, Avant′urin (-ū-rĭn; -vŏng-tōō—Knowles and Smart). [Eng. Fr.] A beautiful iridescent variety of rock crystal, minutely spangled throughout with yellow scales of mica (AVENTURIN, A. QUARTZ). A variety of felspar (A. FELSPAR) of somewhat similar appearance is found in the Continent and the Peninsula, of which the finer kinds are called A. ORIENTALE and PIERRE DE SOLEIL by the lapidaries. Both varieties are now imitated by the glass and porcelain manufacturers. See Glass, Glaze, Paste, &c.
A′VIARY (-ve-). Syn. Avia′′rium, L.; Volière, Fr.; Vogelhaus, Vogelhecke, Ger. A place for keeping birds; generally applied to an enclosed space or building in which birds are kept, or bred, on account of their rarity, plumage, or song; and not for food.
Situa., &c. For exotic birds, a place should be selected where the temperature can be maintained at a proper degree throughout the year, and which is well protected from the weather. This is commonly done by choosing a space attached to the summerhouse or hot-house. When the aviary is only intended for birds of climates similar to our own, any part of the open garden may be chosen, and a portion closed in, either with trellis-work or wire-work, or netting; care being taken to provide, in some easily accessible portion of it, full protection from vicissitudes of weather and season. Nor must cleanliness, and due ventilation and protection from foul air or noxious fumes, be left unattended to.
AVIGNON′ BERRIES (ăv-veen-yong). French berries.
AV′OIRDUPOIS′ (ăv-ĕr-du-pois′). The common weight of 16 oz. or 7000 gr. to the lb., used in these realms for all kinds of goods, except jewelry and the precious metals, and medicines in dispensing, or as ordered in the ‘British Pharmacopœia’ of 1867.
AX′IS. [L., Eng., Fr.] Syn. Axe, Fr.; Achse, Ger. Primarily, that on or around which anything acts or performs; an axle or axle-tree. In anatomy, that on or around which any organ or part rests, gravitates, or centres. In astronomy, the diameter on or about which a celestial body revolves. In botany, part or parts about which particular organs are arranged; an imaginary line passing from the base to the apex of a pericarp225 &c. In crystallography, imaginary lines passing through the central points of a crystal, and about which the molecules or particles of matter composing it may be conceived to be symmetrically built up. In geology, the centre of a mountain-group. In mechanics, the straight line, real or imaginary, about which any body oscillates or revolves. See Crystal, &c.
AX′LE, Ax′le-tree (ăks′l). Syn. Essieu, Fr.; Axe (am rade), &c., Ger. In mechanics, the pin, rod, or material line, on which a wheel, &c., turns. See Anti-attrition, Friction, &c.
AX′UNGE (-ŭnje). Syn. Axun′gia, L. Primarily, ‘wheel-grease,’ the lard or fat of an animal; restricted in pharmacy to hog’s lard.—Axungia cura′ta, A. PREPARA′TA, is prepared or washed hog’s lard (which see).
AYER’S PILLS. Sold in long wooden boxes, each containing 25 pills, covered with sugar and starch, and composed of pepper, colocynth, gamboge, and aloes. (Hager.)
AZADIRACHTA INDICA. (Ind. Ph.) Nim or Margosa Tree. (Ind. Ph.) Habitat. Common throughout India; often cultivated in gardens. Officinal parts.—1. The bark (Azadirachtæ cortex, Nim bark). It varies much in appearance, according to the size and age of the tree producing it. The bark from the trunk of a tree above three or four years of age is covered with a thick scaly epidermis, and varies in thickness from 1⁄4 to 1⁄2 inch. That from the smaller branches is smooth, of a dullish purple colour, marked by longitudinal lines of ash-coloured epidermis, from 1⁄8th to 1⁄12th of an inch apart. The inner layer of the bark, of a whitish colour in the fresh state, is powerfully bitter, far more so than the outer dark-coloured layer, which, however, possesses a greater amount of astringency. It contains a crystallisable principle (margosine) and an astringent principle (catechin).—2. The fresh leaves (Azadirachtæ folia, Nim leaves).—Properties. Bark astringent tonic and antiperiodic; leaves stimulant.—Therapeutic uses. In intermittent and other paroxysmal fevers, in general debility, and convalescence after febrile and other diseases, the bark has been employed with success. The leaves form a useful application to ulcers and skin diseases when a mild stimulant is required.—Dose. Of the powdered bark, a drachm three or four times a day.
Preparations. Decoction of Nim Bark (Decoctum Azadirachtæ). Take of the inner layer of nim bark, bruised, 2 oz.; water, a pint and a half. Boil for 15 minutes, and strain whilst hot.—Dose. As an antiperiodic, from 11⁄2 to 3 fl. oz., every second hour previous to an expected paroxysm. As a tonic, 1 or 2 fl. oz. twice or thrice daily. As this decoction soon decomposes in hot weather, it should be prepared fresh for use when required.
Tincture of Nim Bark (Tinctura Azadirachtæ). Take of the inner layer of nim bark, bruised, 21⁄2 oz.; proof spirit, 1 pint. Macerate for seven days in a closed vessel, with occasional agitation; strain, press, filter, and add sufficient proof spirit to make 1 pint. It may also be prepared by percolation in the same manner as Tincture of Calumba, q. v.—Dose. From 1⁄2 to 2 fl. dr. as a tonic.
Poltice of Nim Leaves (Cataplasma Azadirachtæ). Take of fresh nim leaves a sufficiency; bruise and moisten with tepid water. A good stimulant application to indolent and ill-conditioned ulcers. Should it cause pain and irritation, as it sometimes does, equal parts of rice-flour and linseed-meal may be added. The bitter oil of the seeds is held in high repute by the natives as an anthelmintic, and as an external application in rheumatism. It is also said to be an insecticide.
AZOERYTH′RYN (-rĭth′-rĭn). A substance obtained, by Kane, from archil. It is insoluble in alcohol, ether, and water; but is very soluble in alkaline lyes, to which it imparts a port-wine colour.
AZO′IC. Syn. Azöot′ic; Azo′icus, Azöot′icus, &c., L. Lifeless; wholly destitute of organic life. In geology, &c., applied to strata which do not contain organic remains.
AZOLIT′MIN (ăz-o-lĭt′-mĭn). A dark-red substance obtained, by Kane, from litmus, of which it forms a large portion of the colouring matter. It is insoluble in alcohol, and in water unless alkalised.
AZ′OTE* (ăz′ōte; a′-zōte). [Eng., Fr.] Syn. Azo′tum*, L.; Azot*, Ger. Nitrogen (because it is unfit for respiration, i.e. destroys life).
AZOT′IC. Syn. Azot′icum, L.; Azotique, Fr.; Azotisch, Ger. Of or like azote, or containing it or formed from it; irrespirable; destructive to life.—Azotic Acid† is nitric acid; A. GAS†, nitrogen.—Azo′tous acid† was nitrous acid.
AZ′OTISED (-tīzd). Syn. Nitrogenised, Containing azote or nitrogen; a common epithet of nitrogenous substances used as food.
AZ′URE (ăzh′-ūre; ā′zhure—Knowles, Smart, Walker). Syn. Cæru′leum, L.; Azur, Fr.; Hellblau, Himmelblau, Ger. In dyeing and painting, sky-blue; also the name of one or more pigments which possess this colour. See Blue Dyes, Blue Pigments, Smalts, Ultramarine, &c.
AZ′URE-STONE. Lapis lazuli.
AZ′URITE (-īte). In mineralogy, lazulite; blue malachite; sometimes, lapis lazuli (the name being, unfortunately, very loosely applied by different writers).
AZ′YMOUS† (-e-mŭs). Syn. Az′ymus, L. Unleavened; unfermented; as sea-biscuit. Unleavened bread was formerly termed AZ′YME† (-e-me) and AZ′YMUS† by theologists.
BAB′LAH. The rind or shell of the fruit of mimosa cineraria. According to Dr Ure, it contains a considerable quantity of gallic acid,226 some tannin, a red colouring principle, and an azotised substance, and is the article imported from the East Indies and Senegal under the name of NEB-NEB.—Used as a cheap dye-stuff for various shades of drab and grey.
BAC′CA (băk′-ă). [L.; pl., bac′cæ, băk′-sē.] A berry.
BACK. [D., bak, a bowl or cistern.] Syn. Bac. In brewing, a large, open, flat reservoir or cistern; commonly that in which wort is cooled. In distillation, the vessel into which the wort is pumped from the coolers, in order to be ‘worked’ with yeast. The LIQUOR-BACK in a brewery, distillery, or rectifying house is the water reservoir or cistern.
BACKS. In the leather trade, the thickest and stoutest portion of the hide, used for sole-leather.
BACON (bā′-kn). [W., baccun, prob. from Ger., bache, a wild sow; “old Fr., for dried flesh or pork”—Craig.] The flesh of swine salted and dried, and subsequently either smoked or not. The term is usually restricted to the sides and belly so prepared; the other parts of the animal having distinctive names. Sometimes, though rarely, the term is extended to the flesh of bears, and of other like animals, cured in a similar manner.
Qual., &c. When bacon has been properly prepared from young and well-fed animals, and is neither ‘stale’ nor ‘rusty,’ it forms a very wholesome and excellent article of food, especially adapted for a light or hasty meal, or as a relish for bread or vegetables. For persons with a weak stomach, and for invalids, great care should be taken to cook it without injuring its flavour, or rendering it indigestible. This is best effected by cutting it into slices of moderate thickness, and carefully broiling or toasting it; avoiding dressing it too hastily, too slowly, or too much. The common practice of cooking it in almost wafer-like slices, until it becomes brown and crisp, renders it not merely indigestible, but also a most fertile source of heartburn and dyspepsia. Fried bacon is remarkably strong, and is hence more likely to offend the stomach than when it is broiled, or preferably toasted before the fire; the last being, of all others, the best way of dressing it so as to preserve its delicacy and flavour. Gourmands, however, often esteem, as ‘une bonne bouche,’ bacon dressed in the flame arising from the dropping of its own fat.
Choice. Good bacon has a thin rind, and an agreeable odour, the fat has a firm consistence and a slightly reddish tinge; the lean is of a pleasing red colour, is tender, and adheres, whilst raw, strongly to the bone. When the fat is yellow, it is either ‘rusty’ or becoming so, and should be avoided. The streaky parts are not only those which are most esteemed, but are the most wholesome.
Bacon should be broiled or toasted in front of the fire. The rashers should be in thin slices, and the rind should be removed. The melted fat from the bacon should never be wasted. To partake of all broiled meats in perfection they should be served up as soon as they are taken off the gridiron.
BACTERIUM (Bacterion, a little rod). Since the publication of the researches of Professor Cohn, of Breslau, upon the nature of this organism, the idea previously entertained by Ehrenberg and others as to its animal origin has been long abandoned, and microscopists now very generally regard it as belonging to the vegetable kingdom. It is probably one of the lowest and most simple forms of vegetable or animal life, and consists of an envelope more or less enclosing protoplasm—the nitrogenous substance from which the cell nucleus is formed. Dr Lionel Beale very carefully crushed a very large bacterium while under observation by the microscope, and when the external membrane was ruptured the protoplasm was seen to escape, and to exhibit what Dr Beale regards as vital movement. In form, bacteria may be either globular, rod-shaped, egg-shaped, or filamentous. Cohn has described a variety presenting the appearance of beaded chains, or aggregations.
Bacteria vary considerably in size, some being as much as 1⁄3000th of an inch in length, whilst others are less than 1⁄10000th, and are only visible by the aid of a glass of very high power, such as the 1⁄50th of an inch objective. Dr Beale says, “The germs from which the little particles spring are far more minute and more difficult to identify. They appear as minute specks, the largest of them exhibiting a circular outline, and probably being spherical. The smallest are too minute to be discerned with the highest magnifying powers at our command. If a specimen of fluid in which these particles are rapidly growing and multiplying be carefully examined, many points will be observed to appear from time to time. After watching with great care for a considerable time a given spot I have assured myself that new particles actually come into existence; and that one does not, after intently watching for a time and concentrating the attention upon a certain space, merely see one coming into view one after another, as star after star. The material in which the minute germs of bacteria are imbedded, and which, at least in part, consists of formed material produced by the bacteria, is much softer than the matter of which the capsule of fungi consists. It is, perhaps, almost as soft as mucus. I believe that even the most minute bacterium germ is surrounded by a layer of such soft formed matter, in which very minute particles of bioplasm (protoplasm) divide and subdivide before they attain even the 1⁄100000th of an inch in diameter. When, therefore, bacteria in an early stage of development dry, it is not possible to identify them. When moistened, the dry mass swells up, and the bioplasm in the soft mucus-like matter grows, each particle producing a fresh investment of formed material, and then if the conditions are favorable,227 the germs either at once divide and subdivide for a time, or grow into perfect bacteria, which move freely and grow and multiply in this more advanced stage of development.”
Bacteria increase by bisection, and when the surrounding conditions are favorable their rate of production is marvellous. It has been computed that an individual bacterium will generate nearly 17,000,000 of its fellows within twenty-four hours. The very probable vegetable origin and nature of bacteria insisted upon by Professor Cohn not only appears to derive great support from his researches into the metamorphoses they undergo during development, &c., but also from their behaviour with certain chemical reagents. For instance, it was found that boiling them in solution of potash had no effect, and also when treated with sulphuric acid and iodine they deported themselves somewhat as cellulin does under like circumstances; although from their extreme minuteness any changes that take place in their tissue are very difficult to observe. Another remarkable analogy presented between bacteria and plants is the manner in which they both assimilate the elements of which they are built up; for they derive their nitrogen not from previously existing albuminous compounds, but from ammonia.
They may be made to develop themselves in any fluid if the fluid contains an organic substance in which carbon is present, a nitrogenous substance which need not be organic, and a phosphate. They appear to derive their carbon by the decomposition of almost any substance, containing this element except carbonic acid, and they will obtain their nitrogen from a nitrate, the nitrate becoming reduced to the state of a nitrite. A knowledge of these facts will of course indicate the method to be followed if we wish to obtain bacteria. All that we have to do is to prepare a liquid that fulfils the conditions just stated. Dr J. Burdon Sanderson gives the following formula for one:—Phosphate of potassium 1⁄2 per cent., sulphate of magnesium, 1⁄2 per cent., dissolve in water having a trace of phosphate of calcium in suspension, and then add a per cent. of tartrate of ammonium, and boil the mixture. If properly boiled the liquid will be free from bacteria; but the contact of almost any organic substance, for example, a drop of water, a pinch of hay, a morsel of meal, &c., will cause their appearance.
The tenacity of life exhibited by the bacteria is extremely great. Dr Beale says, “Extreme dryness does not destroy them, and they withstand a temperature far below the freezing point; and that under adverse circumstances they remain dormant, and are not destroyed by a degree of heat which is fatal probably to every other living organism.” Bastian says that the germs of bacteria are destroyed at a temperature of 160° F., but others are of opinion that under certain circumstances these germs are not killed at 212°, and that they may increase and multiply after having been exposed to this degree of heat. Professor Tyndall indeed has shown that in one experiment heating for a quarter of an hour at a temperature of 230° F. was insufficient to destroy them, whilst in another the five minutes’ exposure of an atmosphere containing them to the incandescence of the voltaic current failed to kill them.
Cohn relates that manufacturers of pots of preserved peas at Lubek have since 1858 been obliged to cook them in a solution of 28 per cent. of salt, at a temperature of 226° F., to prevent the putrefaction of their contents, as in warm years nearly half the pots were found to be spoiled. In experiments made in conjunction with Dr Hare, Cohn found that in infusions boiled for less than fifteen minutes organisms were, without exceptions, developed. Somewhat lower temperature proved fatal to the great majority of bacteria. Those that survived were all found to belong to the genus Bacillus, and among bacilli to the species Bacillus subtilis.
The experiments of Drs Ferrier and Burdon Sanderson would seem to show that bacteria do not nominally exist in the fluids and tissues of the body, but that their presence in the animal fluids may be traced to external surface contamination with ordinary water, the extent of their development being in proportion to the amount of the contamination. They contend that different varieties of water possess different degrees of what they term the ‘zymotic power.’ They examined the waters supplied by the several London water companies, and they found them to consist of varying degrees of bacterian impurity. They assert that all except freshly distilled water teems with invisible germs of bacteria. Writing of the universality of the presence of bacteria and bacterian germs, Dr Beale remarks:—“It would be difficult to say where bacterium germs do not exist. In air, in water, in the soil adhering to tiny particles of every kind, in every region of the earth, from the poles to the equator, they are found. In the substance of the tissues—nay, in the cells of almost all plants, and in the interstices of the tissues of many animals—bacteria germs exist. I know not what part of the body of man and the higher animals is entirely destitute of particles which under favorable circumstances develop into bacteria. Upon the skin and the surface of the mucous membranes they exist in profusion, and they abound in the mouth and in the follicles and glands.”
Dr Eberth, of Zurich, states that he has found on ordinary sweat small oval-shaped bacteria which are frequently united in strings of two or three, and endowed with rather active movements. The author thinks that they very likely conduce to produce certain chemical modifications of sweat.
Drs Ferrier and Sanderson appear to have satisfactorily proved that fungi are not developed228 from microzymes, and that their apparent association is one of juxtaposition only. They give the following reasons for adopting this conclusion:—(1) The quick appearance of torula cells in Pasteur’s solution whenever it is exposed to the air, and the rapid development and luxuriant fructification of the higher form (penicillium) show that so far as the chemical composition of the liquid is concerned, there exist in it all the conditions favorable to the process. (2) When precautions are taken to prevent contamination by impure surfaces or liquids, the development which ends in penicillium goes on from first to last without the appearance of microzymes. (3) Whenever it is possible to impregnate the test-liquid with microzymes, without at the same time introducing torula cells or germs, the development of the former begins and continues by itself without any transformation into the latter. Thus fungi are not developed, notwithstanding the presence of microzymes in the same liquid in which, microzymes being absent, but air having access, they appear with the greatest readiness. As we have already seen the germs of bacteria exist largely in air; the experiments of Hiller, of Berlin, would seem to negative the theory of Ferrier and Sanderson, as they tend to show that bacteria have little influence on putrefaction.
We are indebted to Dr Lionel Beale for these illustrations, which are taken from his very interesting work on ‘Disease Germs.’
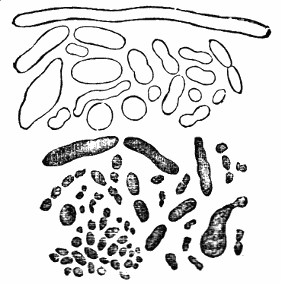
Hiller’s experiments tend to show that putrefaction is independent of the presence of bacteria, that bacteria can develop in liquids such as urine without producing its decomposition, and that the degree of their development and the rate of their multiplication depend upon the amount of assimilable material.
The following is the definition given to the word ‘microzyme’ (which occurs above) by its originator, Dr Sanderson: “I proposed the word ‘microzyme’ as a convenient general term for the first organic forms which present themselves in organic nitrogenous liquids when about to undergo spontaneous decomposition.”
From the experiments of Béchamp it appears that under some circumstances the mother of vinegar, a conglomeration of microzymes, can be transformed into bacteria, and under other circumstances into a cellular ferment which can excite normal alcoholic fermentation in cane sugar. Subsequent researches have shown that the converse of this is also true, and that the cellular ferment may be transformed into microzymes and bacteria.
A mixture of starch and yeast kept at a temperature of 24° to 35° soon liquefies, and the yeast undergoes remarkable changes. The cells swell, become transparent, and gradually disappear. Myriads of microzymes of great agility spring into existence, then vibrios appear, and as these increase the microzymes diminish. The vibrios in their turn are succeeded by myriads of bacteria, and finally the bacteria disappear, leaving nothing but microzymes, single or coupled together. During these changes a small quantity of gas is disengaged, no butyric acid is formed, and but little acetic or lactic acids.
As then the mother of vinegar when changed into bacteria becomes lactic or butyric ferment, and when transformed into cellular matter becomes alcoholic ferment, and as beer yeast becomes lactic or butyric ferment when reduced to microzymes, vibrios, or bacteria, it is evident that the property of being a ferment of any particular nature does not depend essentially upon the nature of the ferment, but upon its organisation or structure.
A contributor to the ‘Medical Times and Gazette’ of February 2nd, 1878, advances the opinion that many of the bacteria are only parts of a plant which has other forms and other modes of growth and propagation when not confined to the living organism or to fluids, and regards the bacterium as a transitional or provisional and not as a permanent form, but an abnormal phase of life thrust upon the plant by accident.
BACTERIA AS ORIGINATORS OF DISEASE.—The researches of many eminent microscopists and physiologists afford abundant evidence of the presence of bacteria in the blood of persons affected with various infectious diseases. For instance, Core and Feltz, of Strasbourg, found a linked bacterium in the blood of those attacked with septicæmia, typhoid, and puerperal fevers. The same investigators also discovered bacteria in the blood of patients suffering from scarlet fever; this blood when injected into the veins of rabbits set up a feverish disease that proved fatal.
Again, in the blood of man and the sheep attacked with smallpox, a bacterium of the globular or sphere-shaped variety was found by Keber, Hallier, and Zurn.
Bacteria have also been found in the blood during measles, and in the splenic apoplexy of sheep and cattle. They have likewise been identified in diphtheritical exudations both from the kidneys and womb, as well as in the blood during an attack of rheumatic fever, and229 they are undoubtedly present in the same fluid during many feverish disorders. Drs Lewis and Cunningham failed to discover them in the blood of cholera patients. Professors Cohn and Koch stand prominently forward as the advocates of the germ theory of disease by bacteria. Professor Cohn divides the bacteria into groups, genera, and species, and assigns to each species a different function.
For instance, he considers the ferment of contagion to be due to the presence of a variety of the sphere-shaped bacterium—one of his groups. He divides the whole group into three—the chromogen, zymogen, and pathogen, the bacteria of pigmentation, of fermentation, and of contagion, respectively. He says those organisms are exceedingly minute, darkish or coloured granules, so small as to be immeasurable. They frequently present the appearance of beaded chains or the form of aggregations. They are motionless and are occasionally found with the Bacterium termo in putrefying organic liquids.
Messrs Chauveau and Sanderson have discovered a bacterium in vaccine lymph which believers in the germ theory class among the pathogen bacteria, and which they have named the Micrococcus vaccinæ. Amongst the pathogen bacteria they also include the Micrococcus dipthericus and Micrococcus septicus, the former found in the epithelium of certain organs during certain forms of pyæmia, and the latter in the miliary eruption of typhus, pyæmia, and other diseases. The chromogen or pigmentary bacteria have occasionally been the means of working miracles. Several instances of bread exuding blood, under supernatural circumstances, are related by Rivola. Ehrenberg found this colour on some bread in the house of a patient who had died of cholera, and he ascertained the pigment to be due to the presence of the Monas prodigiosa, small round bodies which Professor Cohn classes with the micrococci, a variety of the sphere-shaped bacterium.
The recent investigations of Koch were directed to the cause of splenic fever, and Cohn on examining his specimens found that they were examples of Bacteria of the species called Bacillus anthracis, which seems to present little or no difference to the Bacillus subtilis of hay infusions. Koch found that bacilli increase with enormous rapidity in the blood, and in the fluid of tissues of living animals, by developing in length and dividing transversely. The animals employed were chiefly mice, and a small incision being made at the root of the tail, as minute a drop as possible of the fluid containing the bacilli was injected into the system. The spleen invariably became enormously swollen, and filled with a large number of crystalline-looking rods of varying size, never exhibiting movement or spore formation; they increased in numbers solely by division. The number of bacilli found in the blood varies in different animals; thus in the guinea-pig it was enormous, sometimes exceeding that of the blood-corpuscles; in the rabbit much smaller, so that sometimes several drops had to be examined before any were found, in the mouse often nil. In the blood of dead animals or other suitable fluids the bacilli grow to very long straight leptothorax-like filaments (within certain limits of temperature, and with the presence of air), while the formation of numerous spores goes on at the same time.
Kohl believes that it is to the presence of the spores that the occurrence of splenetic fever appears to be referable. When living, inoculation with them always produced the disease; but if killed, as by drying, or a high temperature, inoculation failed; it was necessary either that living spores should be present, or that the filaments should be capable of generating spores, in order that the disease should be propagated by inoculation.
Koch tried whether the poisonous bacilli spores could gain entrance through the digestive organs, but found that mice and rabbits could eat them with impunity. Koch draws attention to the similarity of splenic fever to typhus and cholera. He says it presents analogies to typhus in its dependence on soil-water, its preference for low grounds, its sporadic occurrence throughout the year, and its development into an epidemic in the late summer and autumn. Like cholera, again, he says, it is connected with soil-water, and it also agrees with cholera in the point which has been so well made out by Pettenkofer, that on board ship an interval of three or four weeks is sufficient to prevent its further development.
Hence Koch is disposed to hope that the contagium of typhus and cholera may still be discovered in the form of some Schizophyte or spheroidal bacterium, though practical observers have hitherto sought for them in vain.
Many pathologists, however, refuse to accept the accuracy of these deductions, and regard the presence of bacteria in the blood and tissues during disease as of no significance; whilst they deny that it is satisfactorily proved that they are the cause of disease.
Dr Lionel Beale says:—“Changes in the processes of digestion are soon followed by the multiplication of bacteria in every part of the alimentary canal, and within a few hours countless millions may be developed. They multiply in the secretions under certain circumstances, almost as soon as these are formed, and I have adduced evidence to show that bacteria germs exist even in healthy blood. In the very substance of some cells I have seen them, and in many cases in which little granules have been discerned in connection with bioplasts. There is reason to believe that some of them are really bacteria germs, passive as long as the higher life is maintained in its integrity, but ready to grow and multiply the instant a change favorable to them, and adverse to us, shall occur.”230
And again he remarks:—“Bacteria prey upon morbid structure, and upon the substances resulting from the death of bioplasm (protoplasm). We ought not, therefore, to be surprised at their existence in disease. They are found in great numbers amongst pus-corpuscles which have ceased to live, and they grow and multiply with great rapidity in fluids which contain disease germs, as soon as these begin to lose their specific powers and to undergo decomposition.” See Germs.
BAD′′GER (băj′-ĕr). Syn. Me′les, L.; Blaireau, Fr.; Dachs, Ger. The ur′sus me′les (Linn.), one of the plantigrade carnivora, a burrowing nocturnal animal, common in Europe, Asia, and North America. Since the extirpation of the bear, the badger is the sole representative of the ursine family in our indigenous zoology. Its habits are “nocturnal, inoffensive, and slothful; its food consists of roots, earth-nuts, fruits, the eggs of birds, insects, reptiles, and the smaller quadrupeds; its noxious qualities are consequently few and of slight moment, and by no means justify the exterminating war unintermittently waged against it.” (Brande.) Its “muscular strength is great, its bite proverbially powerful; and a dog must be trained and encouraged to enter willingly into combat” with it. (Id.)
Uses, &c. The flesh of the badger is prized as food; the skin used for pistol furniture; the hair made into brushes. The American badger is commonly called the GROUND-HOG. The Cape badger produces HYRACEUM (which see).
BAD′IANE (-e-ăhn). [Fr.] Syn. Bad′ian, B.-seed. Star-anise seed.
BADI′′GEON (bă-dĭzh′ōne; băd′-e-zhŭn‡, or bă-dĭj′ŭn‡—Smart). Among operatives and artists, any cement used to fill up holes and to cover defects in their work. Among statuaries, a mixture of plaster and free-stone is commonly used for this purpose; among joiners and carpenters, a mixture of sawdust and glue, or of whiting and glue; and among coopers, one of tallow and chalk. The name is also given to a stone-coloured mixture used for the fronts of houses, and said to be composed of wood-dust and lime, slaked together, stone powder, and a little ochre, umber, or sienna; the whole being mixed up with weak alum water to the consistence of paint, and laid on in dry weather.
BAEL. [Nat.] Syn. Indian bael, Bel*; Bael, B. in′dicus, Be′la, B. in′dica, L. The œg′le marmelos (Correa; cratæva m., Linn.) one of the Aurantiaceæ (DC.). Dried half-ripe fruit imported from the E. Indies, under the name of Indian bael. Astringent and refrigerant; highly extolled in chronic dysentery, diarrhœa, English cholera, and relaxations generally. It is also used in bilious fevers, hypochondriasis, melancholia, &c. Root-bark, stem-bark, and expressed juice of the leaves, particularly the first, also used in the same cases in India. Ripe fruit fragrant and delicious; used, in the E. I., as a warm cathartic, and regarded as a certain cure for habitual costiveness. Mucus of the seeds used by painters as size; also as a cement. Unripe fruit used to dye yellow. It is generally administered under the form of DECOCTION or EXTRACT (which see).
BAGASSE′ (-găs′). [Fr.] The dry refuse stalks of the sugar cane as they leave the crushing-mill.—Used as fuel in the colonial sugar-houses.
BAGG′ING. The cloth or materials of which bags or sacks are made. In agriculture, applied to a method of reaping corn by a chopping, instead of a drawing cut. See Rats, &c.
BAHIA POWDER. See Araroba.
BAHR’S NON-POISONOUS MEDICAL SNUFF. A snuff largely advertised in the Berlin journals, composed chiefly of powdered galls. (Hager.)
BAIN-MARIE. [Fr.] In old chemistry, a water bath; also, sometimes, a sand bath. In cookery, a shallow vessel containing heated water, in which saucepans, &c., are placed, when it is necessary either to make them hot, or to keep them so, without allowing them to boil. It is extremely useful in making sauces, warming soups and small dishes, and when dinners are delayed after they are ready to be served.
BA′′KING (bāke′-). Syn. Action de cuire au four, Fr. The process of cooking, or of heating, drying, and hardening any substance in an oven or kiln, or by the rays of the sun; the art or trade of a baker‡; also technically, a batch or ovenful, or the quantity baked at once (= FOURNÉE, Fr.).
In cookery, baking is, perhaps, of all others, the cheapest, most convenient, and best way of dressing dinners for small families, where a good domestic oven is at hand. Though the flavour of baked meat is generally considered barely equal to that of the same parts roasted, there are some joints and dishes to which it appears particularly suitable. Among these may be mentioned legs and loins of pork, legs and shoulders of mutton, fillets of veal, &c. A baked pig, if it has been occasionally basted, and the heat has not been too great, eats equal to a roast one. Geese and ducks treated in the same way are also excellent. A baked hare which has been basted with raw milk and butter also eats well; and so do various pieces of beef, especially the buttock. Cooks tell us that this last should be sprinkled with a little salt for a day or two before dressing it, and after being washed is preferably baked, along with about a pint of water, in a glazed earthen pan tied over with writing paper, ‘three or four times thick.’ A baked ham is said to be preferable to a boiled one; to be tenderer, fuller of gravy, and finer flavoured. It should be soaked in water for about an hour, wiped dry, and covered with a coarse thin paste or batter. Ordinary dishes require similar treatment in baking to that given them when roasted.231
For domestic use, where the kitchen-range does not include a really good oven, the portable articles known as a ‘Dutch-oven,’ and an ‘American oven,’ form an excellent substitute, admirably adapted for small joints, poultry, &c., all of which, when these utensils are skilfully employed, possess a delicacy and flavour fully equal to the same when roasted; whilst not more than one half the fire is required for the purpose. According to Miss Acton they also “answer excellently for delicate sweet puddings, and for cakes.” See Bread, Cakes, Roasting, &c.
Baking Pow′der. See Powders.
Baking Powder, American. For making light pastry. Tartaric acid and chalk. (Reichardt.)
Baking Powder, Borwick’s German, is an artificial fermentation powder, compounded with coarse maize-flour. (Gädike.)
Baking Powder, Goodall’s, is a compound of 2 parts of rice flour with 1 part of a mixture of tartaric acid and bicarbonate of soda. (K. Boschan.)
Baking Powder or Yeast Powder, Professor Horsford’s (Cambridge, U.S.). This is a powder supplied in two packets. The one contains an acid phosphate of lime and magnesia made up with a certain quantity of flour, and the other is bicarbonate of soda, with a little chloride of potassium.
BAL′ANCE. As in the process of what is termed gravimetric analysis the chemist has to determine the weights of the different substances employed as well as found, it will be self-evident that for his results to be trustworthy the balance he employs must be perfectly accurate and reliable.
The accompanying drawing, from Roscoe, represents a common form of chemical balance.
The apparatus consists of a perforated brass beam (AA), vibrating about its centre, at which is fixed a triangular knife-edge of agate (C); this rests upon a horizontal agate plane attached to the upright brass pillar. To each end of the beam light brass pans (BB) are hung, each pan being suspended by an agate plane, upon an agate knife-edge fixed on the end of the beam at DD. This arrangement is rendered necessary in order to reduce as much as possible the friction of the edges on their supports, which friction, if unchecked, would seriously impair the sensibility of the balance.
In order to prevent the agate edges being worn away by constantly rubbing on the agate planes, the beam and the ends (DD) are supported by the brass arm (EE), when the balance is not in use, so that the agate surfaces are not in contact. The beam and pans are released when required by turning the handle (F). The movement of the brass arm (EE) is accompanied by means of a rod descending through the upright brass pillar, and resting on a simple eccentric, by the turning of which by the handle (F) it may be gradually raised or lowered. The substance to be weighed (held by a tube, watch glass, &c.) is placed in one of the pans, and weights added one by one in the other, until the beam is in equilibrium: this is ascertained by the long pointer(G) oscillating to an equal distance on each side of the central mark or index, this latter being subdivided into equal spaces, so that the oscillations can be measured. A spirit level is also a necessary appendage to the instrument, since it enables the operator to place the beam on an exactly horizontal level.
The beam of the balance is generally graduated into decimals. This saves the trouble of placing a weight on the scale, since it enables the operator to weigh the milligramme and its fractions by suspending a centigramme rider or hook on or between the indicated points of a graduated line.
The balance is enclosed in a glass case, which serves not only to protect it from dust, but to allow of the weighing being carried on away from the outer air, in which the prevalence of draughts proves a source of considerable annoyance to the weigher. The front of the scale case generally consists of three parts, viz. a fixed centre piece and two lateral frames or doors, all of course of glass. It is of importance that the air inside the balance case should be perfectly dry, since a humid atmosphere would not only affect the weight of many hygroscopic substances when placed in the pans, but likewise be liable to attack the instrument itself. To guard against these casualties a small beaker containing oil of vitriol, or chloride of calcium, or freshly-burnt lime, should be kept in the232 case. A balance capable of weighing 70 or 80 grammes in each scale will be found to meet the needs of most chemists.
Fresenius says, “The ACCURACY of a balance depends upon the following conditions:
“a. The fulcrum must be placed above the centre of gravity of the beam.
“b. The suspension points of the scales must be on an exact level with the fulcrum.
“c. The beam must be sufficiently strong and inflexible to bear without bending the greatest weight that the construction of the balance admits of.
“d. The arms of the balance must be of equal length; i.e. the points of suspension must be equidistant from the fulcrum or point of support.
“The SENSIBILITY or DELICACY of a balance depends upon the following conditions:
“a. The friction of the edges upon their supports must be as slight as possible.
“b. The centre of gravity must be as near as possible to the fulcrum.
“c. The beam must be as light as possible.”
The following are the tests given by the same authority for the accuracy and sensibility of a balance:
“1. The balance is in the first place accurately adjusted, if necessary, either by the regulating screws, or by means of tinfoil, and a milligramme weight is then placed in one of the scales. A good and practically useful balance must turn distinctly with this weight; a delicate chemical balance should indicate the one tenth of a milligramme with perfect distinctness.
“2. Both scales are loaded with the maximum weight the construction of the balance will admit of; the balance is then accurately adjusted, and a milligramme added to the weight in one scale. This ought to cause the balance to turn to the same extent as in 1. In most balances, however, it shows somewhat less on the index.
“3. The balance is accurately adjusted should it be necessary to establish a perfect equilibrium between the scales by loading the one with a minute portion of tinfoil (this tinfoil must be left remaining upon the scale during the experiment); both scales are then equally loaded, say with about fifty grammes each, and if necessary the balance is again adjusted (by the addition of small weights, &c.). The load of the two scales is then interchanged, so as to transfer that of the right scale to the left, and vice versâ. A balance with perfectly equal arms must maintain its absolute equilibrium upon the interchange of the weights of the two scales.
“4. The balance is accurately adjusted; it is then arrested, subsequently set in motion, and again allowed to recover its equilibrium; the same process should be repeated several times. A good balance must invariably reassume its original equilibrium.
“A balance of which the end edges afford too much play to the hook resting upon them, so as to allow the latter slightly to alter its position, will show perceptible differences in different trials. This fault, however, is possible only with balances of defective construction.
“A balance to be perfectly useful for the purposes of quantitative analysis must stand the first, second, and last of these tests. A slight inequality of the arms is of no great consequence, since this may be readily and completely remedied by the manner of weighing.” See Weights.
Balance, Hydrostat′ic. See Specific Gravity.
Balance, Tor′sion. A delicate instrument, invented by Coulomb, for measuring the intensities of the electrical and magnetic forces.
BALD′NESS (bawld′-). Syn. Cal′vitas, Calvi′′ties (vĭsh′-e-ēz), L.; Calvitie, Chauveté, Fr.; Kahlheit, Kahlkopf, Kahlköpfigheit, Ger. Primarily, absence or loss of any natural covering; appr., destitution or loss of hair, more especially of that of the top and fore-part of the head. In botany, absence of beard or awn.
Grey hair and baldness dependent on old age are natural consequences of man’s infirmity, and must be regarded as evidence of failing vigour, rather than in the light of a disease. Premature loss of hair may be induced by various causes. It is common after severe fevers, and is frequently caused by external pressure, friction, or violence, and by such other local actions and conditions which, when long continued, interrupt the normal functions of the skin. Persons with a consumptive, scorbutic, scrofulous, or syphilitic taint, or of a general bad habit of body, frequently lose their hair early. In these cases it probably arises from debility or paralysis of the cutaneous vessels, and the consequent insufficient nutrition of the hair-bulbs. When it occurs in persons under the middle age, and apparently enjoying good health, it may be often traced to the pernicious practice of constantly wearing a hard non-ventilating hat, or to disordered stomach, habitual smoking or hard drinking, irregular habits, or late hours. Excessive anxiety or grief, and intense study and thoughtfulness, also tend to promote the early decay of the hair. The natural baldness of the aged, and frequently the premature baldness of earlier years, arises from the gradual attenuation of the scalp, which ultimately becomes too thin to afford room for the performance of the functions of the hair-producing organs, and too scantily supplied with blood for their due nutrition and support.[104]
Treatm. The baldness of senility and that arising from the permanent injury or destruction of the hair-bulbs, admit of no cure, notwithstanding the daily assurances of advertising impostors to the contrary. In other cases, when a disposition to baldness exists, shown by the hair falling off in large quantities, or ceasing to grow with its usual vigour and rapidity, the frequent but gentle use of the hair-brush, and of any bland stimulating oil, pomade, or wash, if adopted in time, will generally prove sufficient to arrest the progress of decay, and, very frequently, to restore the hair to its pristine condition. The head may be advantageously washed in cold water, at least once a day; or what is better, a shower bath may be taken on rising in the morning. Should this plan not succeed, the head, or the upper part of it, may be shaved, and a wig, or a scalp, adopted for a time. The effect of keeping the hair closely cropped or shaved is to make it grow thicker, stiffer, and stronger, and this often when all other means fail.
Among more active and less common remedies for baldness may be mentioned—mild streaming electricity, stimulant fomentations, cantharidised, ioduretted, phosphoretted, &c., oils and lotions, a night-cap that, without pressing injuriously on the head, lifts, as it were, the scalp into its natural position, &c., all of which are noticed elsewhere.
The celebrated John Wesley recommended rubbing the part morning and evening with a raw onion until it became red, and then applying a little honey. The vendors of Rowland’s ‘Macassar Oil’ recommend the head to be rubbed with a towel (or hair-brush), until somewhat red, each time before applying their nostrum; and the advice is certainly good, as independent of the stimulus thus given to the skin, and the increased flow of blood through the minute vessels of the scalp, it is rendered more absorbent and sensitive to the action of medicaments. At the same time the reader must be cautioned against placing any reliance on external applications, unless he assists their action by due attention to diet, exercise, ventilation, and such other matters as tend to promote the general health and vigour of the body.
The substances usually employed to medicate hair-cosmetics, the general management of the hair, and the formulæ for various applications to promote its growth, preservation, and beauty, are noticed in the articles Hair, Hair-cosmetics, Pomades, Oils, Washes, &c., to which the reader is referred.
BALEEN′ (-lēne′). [Fr. baleine.] The fisher’s name for whalebone.
BALL (bawl). [Eng., Ger., Swed.] Syn. Balle, Boule, Fr.; Bal, Bol, Dau.; Glob-u-lus, Pi′la, L. In commerce, veterinary medicine, perfumery, &c., applied to various substances made up into a globular, spheroidal, or even a cylindrical form, as ash-balls, horse-balls, soap-balls, &c.
BALLOON′ (-lōōn’). Syn. Ballon, Fr., Ger. Any hollow spherical body of which the sides are extremely thin or attenuated in comparison with its diameter or bulk. In aërostatics, a machine or apparatus for elevating and sustaining bodies in the air. In chemistry, a globular glass-receiver, with either one or two necks (= GROS RÉCIEIENT, Fr.; GROSSE R., Ger.). In pyrotechny, a hollow case or ball of pasteboard filled with fire-works or combustibles, which explodes in the air on being fired from a mortar.
Balloon. In aërostation, a bag or hollow pear-shaped vessel, made of varnished silk or other light material, and inflated with some gas or vapour lighter than the air, as hydrogen, carburetted hydrogen, heated air, &c., so as to rise and float in the atmosphere. When filled with gas it is called by way of distinction an AIR-BALLOON (aérostat, &c., Fr., lufball, luft-schiff, &c., Ger.); when with heated air, a FIRE-BALLOON or Montgolfier b. (ballon à feu, &c., Fr.).
In the early days of aërostation, and indeed for some years afterwards, balloons were inflated with hydrogen gas, obtained by the action of sulphuric acid and water on iron filings or small fragments of iron; but this method of filing them ultimately gave place to the cheaper and more convenient supply afforded by the gas-light companies. Of late years, the coal-gas furnished by the gas-works has been generally, if not solely, used for the inflation of balloons.
The principles of ballooning may be referred to the well-known difference in the specific gravity of bodies, and to the physical properties of the atmosphere. Pure hydrogen, weighed at the level of the sea, is about 16 times lighter than common air; but when prepared on the large scale, and containing water, and other impurities, it is only from 7 to 11 times lighter than the atmosphere. A globe of atmospheric air of 1 foot in diameter, under like circumstances, weighs 1⁄25 lb.; a similar globe of hydrogen (reckoning it only as 6 times lighter than common air, will, therefore, have an ascensional force of 1⁄30 lb.). Now, the weight of the body of air which a balloon displaces must exceed the gross weight of the balloon and all its appendages, in order for the latter to ascend in the atmosphere. The difference of the two weights expresses the ascensional force. The aërostatic power of balloons is proportional to their dimensions, in the ratio of the cubes of their diameters. Thus, it appears that a balloon of 60 feet diameter filled with common hydrogen will ascend with a weight of nearly 7000 lbs., besides the gas case; whilst one of only 1½ foot in diameter will barely float, owing to the less proportionate volume of gas to the weight of the case containing it. In round numbers the buoyancy of a balloon may be reckoned as equal to 1 oz.234 for every cubic foot of hydrogen it contains, less the weight of the case and appendages. The carburetted hydrogen supplied by the gas-works is much heavier than hydrogen gas, and consequently much less buoyant, for which due allowance must be made. That which possesses the least illuminating power is the lightest, and consequently the best adapted for aërostation.
The fabric of which the cases of air-balloons are made is strong thin silk, covered with an elastic varnish of drying oil or india rubber, or, what is better, a solution of india rubber in either chloroform or bisulphide of carbon; the netting is of strong light silk or flaxen cord; and the car of basket-work. Fire-balloons, on the small scale, are generally made of silver-paper, and are inflated with the fumes of burning spirit of wine, by means of a sponge dipped in that liquid, and suspended just within the mouth of the apparatus.
Owing to the increasing rarity of the atmosphere as we ascend from the earth’s surface, balloon cases are made very much larger than is required to contain the necessary quantity of gas, to allow for its expansion as it rises into a rarer medium. A cubical foot of gas measured at the level of the sea occupies a space of two feet at an elevation of 31⁄2 miles.
The following Table will prove useful to the amateur aëronaut or balloonist:—
| Diameters. | Surfaces. | Cubical contents. |
| 1 | 3·141 | ·523 |
| 2 | 12·567 | 4·188 |
| 3 | 28·274 | 14·137 |
| 4 | 50·265 | 33·51 |
| 5 | 78·54 | 65·45 |
| 10 | 314·159 | 523·6 |
| 15 | 706·9 | 1767·1 |
| 20 | 1256·6 | 4189· |
| 25 | 1963·5 | 8181· |
| 30 | 2827· | 14137· |
| 40 | 5026· | 33510· |
See Atmosphere, Gas, Hydrogen, Parachute, Varnish, &c.
BALLOON′ING‡. Syn. Balloon′ry†*. The act, art, or practice of ascending or travelling in balloons; aërostation. A BALLOON′IST‡ is an aëronaut (particularly an amateur or enthusiastic one).
BALLS. The application of this term in commerce, perfumery, veterinary medicine, &c., has been already noticed. (See Ball.) The following may be inserted here:—
Balls, Al′mond (ah′-mŭnd). Syn. Boules d’amande, Fr. Prep. 1. Spermaceti, 4 oz.; white wax (pure) 8 oz.; oil of almonds, 1 pint; melt them together in a glazed earthenware-vessel, by the heat of a water bath, and when the mixture has cooled a little, add essential oil of almonds, and expressed oil of mace, of each 2 dr.; stir assiduously until it begins to cool, and then pour it into the moulds, which may be ounce-gallipots with smooth bottoms (very slightly warmed), when it will form beautiful hemispherical cakes. Very fine.
2. Hard clarified suet, 11⁄4 lb.; white wax, 1⁄4 lb.; ess. oil of almonds, 11⁄2 dr.; oil of cloves (or of pimento), 1⁄2 dr.; as before.
Uses, &c. To soften the skin, and in winter to prevent chaps and chilblains. Sometimes these balls are coloured, which is done whilst the mixture is in the liquid state. A rich pink or red may be given by a little alkanet-root or dragon’s blood; a yellow, by palm oil or annotta; a blue, by a little finely powdered indigo; and a green, with spinage (steeped in the oil before use), or a few grains of verdigris. The most appropriate tint for them is a pale yellow or amber.
Balls, Barèges (-rāzhe’). Syn. Boules de Barèges, Fr. Prep. 1. Extract of soap-wort, 3 oz.; good glue or gelatin, 11⁄2 oz.; water, 4 oz.; dissolve with heat, and add of sulphide of calcium, 6 oz.; common salt, 1 oz. (both in powder); mix thoroughly, and form the mass into balls weighing 21⁄2 oz. each, adding a little powdered gum, if required, to thicken it, and using powdered starch to roll them in.
2. Gelatin, 8 oz.; sulphide of calcium, 12 oz.; common salt, 2 oz.; water, q. s.; after solution and admixture, add carbonate of soda and Castile soap, of each (in powder), 21⁄2 oz. One ball is added to the water of a bath for an adult, to be used as a substitute for that of Barèges.
Balls, Bitter. Prep. 1. Powdered gentian, 2 lbs.; extract of gentian, 1 lb.; grains of paradise (ground), 1⁄2 lb. syrup, q. s.; mix with heat, and divide into half-pound rolls. For ALE.
2. To the above add of Spanish-juice, 11⁄2 lb.; previously softened with a little boiling water For PORTER and STOUT. Both are used by fraudulent brewers; and by publicans in reducing their beer.
Balls, Black′ing. Prep. 1 (Bailey’s). Gum-tragacanth, 1 oz.; water, 4 oz.; dissolve, add of sugar candy, 4 oz.; and afterwards, ivory-black and Prussian blue (in very fine powder), of each 2 oz.; neat’s foot oil, 2 fl. oz.; thoroughly incorporate, and evaporate by a gentle heat, constantly stirring, until of a proper consistence, then pour it into oiled moulds.
2. Gum-arabic, moist sugar, and ivory-black, of each 1⁄2 lb.; lamp-black, 1⁄4 lb.; glue (melted with a little water), 2 oz.; water, 1 quart, or q. s.; neat’s foot oil, 1⁄4 pint; as before.—Used by the shoemakers, harness-makers, &c., to blacken and polish leather. See Balls, Heel.
Balls, Breech′es. See Balls, Scouring.
Balls, Bronze. See Balls, Copying.
Balls, Cam′phor. Syn. Cam′phor-cakes, Chap′-balls‡, Chil′blain b.‡, &c.; Glob′uli camphora′ti, Placen′tæ camphora′tæ, &c.,235 L. Prep. 1. Spermaceti and white wax, of each 2 oz.; almond or olive oil, 1⁄4 pint; melt together by a gentle heat, add of camphor (in small pieces), 1 oz.; when dissolved, stir until partly cold, and then pour it into moulds, as directed under ALMOND-CAKES (above).
2. Clarified suet, 1 lb.; spermaceti and white wax, of each, 3 oz.; camphor, 2 oz.; as before.
3. Spermaceti cerate (Ph. L.), 1 lb.; spermaceti, 2 oz.; camphor, 11⁄2 oz.; as before.
4. To either of the above add of balsam of Peru, 1⁄4 to 1⁄2 oz.; and, after solution, either strain the mixture through muslin, or allow it to settle, and decant the clear portion from the dregs.
Use, &c. A popular preventive of chapping and chilblains. A little is well rubbed into the skin, previously washed clean and wiped dry. Some persons add colour and scent; but they are generally sold without either. The only suitable colours are amber, pink, or yellow. The best perfumes are allspice, ambergris, cassia, cloves, mush, nutmeg, rondoletia, vanilla, and violets. See Balls, Almond (antè).
Balls, Clothes. See Balls, Scouring.
Balls, Contrayer′va. Syn. La′pis contrayer′væ, Glob′uli c., L. Compound contrayerva-powder made into balls with gum-water. An obsolete preparation, once in great repute as a stimulant, tonic, diaphoretic, and absorbent.
Balls, Cop′ying. These have a similar composition to ‘heel-balls’ (see below). For BLACK, the best colouring matter is lamp-black or plumbago with about half its weight of indigo; for a BRONZE-COLOUR, bronze-powder is substituted; and for a mellow BROWN, burnt terra di Sienna. These should be all in very fine powder. The semi-fluid mass is poured into small flat cylindrical moulds—paper pill-boxes answer the purpose well.—Used by artists and amateurs to copy inscriptions, monumental brasses, and other slightly raised or sunken patterns; the ball being rubbed over the paper previously laid flat on the design, and held securely in its place. They are sometimes rendered more permanent by damping the wrong side with a sponge dipped in water, strong spirit, or oil of turpentine; or by passing the wrong side over a hot iron held with the face upwards.
Balls, Cosmet′ic. See Savonettes, &c. (also above).
Balls, Cream. See Savonettes, Soap Balls, &c.
Balls, Dog. See Dogs.
Balls, Gas′coign’s. Syn. Glob′uli Gascoig′nii, L. Gascoign’s powder made up into small balls with thin mucilage. See Powders.
Balls, Heel. Prep. 1. (Ullathorne’s.) Bees’ wax, 1 lb.; suet, 4 oz.; melt together, and stir in of ivory-black (very finely powdered), 4 oz.; lamp-black (sifted), 3 oz.; gum arabic and sugar-candy, of each (in very fine powder) 2 oz.; and, when thoroughly mixed and partly cold, pour the composition into tin or leaden moulds.
2. To the last add of resin, 3 oz.; oil of turpentine, 2 oz.
3. Hard suet and bees’ wax, of each 4 oz.; powdered gum, sugar candy, and Venice turpentine, of each 1 oz.; ivory-black and lamp-black, of each 2 oz.; as before.
4. Suet and bees’ wax, of each 4 oz.; lamp-black and brown sugar, of each 8 oz.; common size, 5 oz.; melt together and stir until incorporated.
Uses, &c. Employed to black leather, and more especially by shoemakers for the edges of the soles; the ball being first rubbed on, and the part afterwards smoothed over with a burnisher or polished iron tool gently heated. Also used by artists to copy inscriptions, basso relievos, &c. To produce a good article, the gum, colouring matter, and sugar, must be in the state of extremely fine powder, and the mixture very carefully made; no lumps being left. Some persons dissolve the gum in a little water, and then stir the mixture over the fire until it acquires the proper consistence for moulding (as in No. 4, above); but the first is accounted the best method.
Balls, Horse. See Veterinary Medicine, &c.
Balls, Martial. Syn. Glob′uli martia′les, L. Prep. 1. Those of the P. Cod. consist of tartarised iron mixed with aromatics, and made up into small globular masses.
2. (Boules de Nancy.) Equal parts of iron filings and red tartar, in fine powder, made into balls with proof spirit or brandy. Both are used as chalybeate tonics, either in the form of pills or dissolved in hot water. Seldom employed in England.
Balls, Physic. (Vet. Med.) See Masses.
Balls, Poultry. See Poultry.
Balls, Scent. See Pastils (Toilet), Perfumery, Pomambra, Scents, &c.
Balls, Scouring. Syn. Breech′es balls, Clothes b., Carpet b., &c. Prep. 1. Curd soap (sliced), 1 lb.; water, 2 oz.; melt in a water bath, or a glue-pot, and when cooled a little, add ox-gall and oil of turpentine, of each, 51⁄2 oz.; mix well and roll or mould the mass into balls or cakes.
2. Fuller’s earth, 2 lbs.; curd-soap, 1 lb.; beat to a stiff paste with ox-gall, q. s.
3. Soft soap and fuller’s earth, equal parts, beat up with a little oil of turpentine, and either with or without a little essence of lemons.—Obs. The above are used to remove paint, grease, and dirt from cloth, carpets, &c. The spot, first moistened with hot water, is rubbed with the cake, and allowed to soak a few minutes, or to become nearly dry, when it is well rubbed with a little warm water and a brush or piece of woollen cloth, and afterwards rinsed in clean water, and finally rubbed dry and smoothed off with a piece of dry236 cloth or a dry brush. The last formula produces the composition so commonly vended about the streets of London in penny cakes.
4. Whiting and pipe-clay, equal parts; water, q. s. Used for soldiers’ belts, trousers, &c.
5. Pipe-clay, 2 lbs.; fuller’s earth, 1 lb.; whiting, 1⁄2 lb.; water, q. s.
6. Bath brick, 1 lb.; pipe-clay, 2 lbs.; soft soap, 1⁄4 lb.; ox-gall, 1⁄2 pint.
7. To the last add of pumice-stone, in very fine powder, 6 oz.—Obs. The last four are used for cloth and leather, especially for drab and light-coloured coats, trousers, leather breeches, belts, and gloves. Rose pink, yellow ochre, umber, Irish slate, or other like colouring matter, may be added to produce any desired tint. White pepper, cloves, &c., are also occasionally added to drive away moths and insects; and orris root, or essence of bergamot or of lemon-grass, as perfume.
Balls, Sweet. See Balls, Scent (antè).
Balls, Tan. The muddy sediment of tan-pits made into balls or lumps.—Used by the poor for summer fuel.
Balls, Wash. See Savonettes, Soap, &c.
BALM (bahm). Syn. Bal′samum, L.; Baume, Fr.; Balsam, Ger. Primarily, balsam (of which it is a contraction); formerly and still popularly applied to anything assumed to be soothing, healing, or genial in its action, particularly if also aromatic or fragrant; but chiefly to medicines and liqueurs, supposed to possess these properties. See Balsams, Liqueurs, Quack Medicines, &c.
Balm. Syn. Com′mon balm, Gar′den b.; Melis′sa, L.; Baume, Mélisse, Fr. The melis′sa officina′lis (Linn.), an aromatic perennial herb, a native of the south of Europe, but commonly cultivated in our gardens. It is reputed to be diaphoretic, diuretic, emmenagogue, exhilarating, nervine, and stomachic; and under the form of infusion (BALM-TEA) has long been a popular remedy in hypochondriacal, hysterical, and nervous affections, and in amenorrhœa and chlorosis. It is still sometimes ordered as a drink in fevers and in hypochondriasis.
Balm of a Thousand Flowers. Chandler says this is a thick yellow emulsion, free from injurious metallic ingredients.
Balm of White Lilies, for preserving and beautifying the skin (H. A. Hoadley, New York). This, also according to Chandler, is a red-coloured water containing a large quantity of chalk in suspension, but with no injurious metallic ingredient.
BAL′SAM (bawl′-săm; -sŭm‡§—Knowles, Walker). [Eng., Ger.] Syn. Bal′samum (băl′-), L.; Baume, Fr. Originally, any strong-scented oleo-resinous vegetable juice or exudation, of about the fluidity of treacle, and supposed to possess medicinal virtues. In modern chemistry and pharmacy, any vegetable production which is either semi-liquid, or which naturally becomes concrete, and which contains either benzoic acid, or cinnamic acid, combined with resin and aromatic essential oil. Several of the substances popularly termed balsams contain no benzoic acid, and are consequently now classed with the turpentines. This distinction, however, is far from being universally adopted, and a late high authority defines balsams to be “Exudations from plants, which are liquid or soft solid, and consist of a substance resembling a resin, either combined with benzoic acid, or with an essential oil, or both.” (Brande.)
The leading properties of the true natural balsams are—Insolubility in water, almost entire solubility in alcohol, and partial solubility in ether and in the volatile and fixed oils; the possession of a powerful, and generally, an agreeable odour, a hot, resinous or terebinthinate taste, and the usual stimulant and tonic properties of the milder turpentines. Distilled with water, ethereal oil and some acid pass over, and the residuum consists chiefly or entirely of acid-resin.
The TRUE BALSAMS, as those of benzoin Peru, styrax, and tolu, and the celebrated Chinese varnish-balsam, contain either benzoic or cinnamic acid. Among those falsely termed balsams, are copaiba, opobalsam, Japan lac-varnish, and some of the turpentines.
The following list includes most of the substances, natural and artificial, which pass, or have passed, under the name of balsams:[105]—
Balsam, Acous′tic. See Drops.
Balsam, Amer′ican†. Balsam of Peru.
Balsam, An′odyne. Syn. Sooth′ing balsam; Bal′samum ano′dynum (-dĭn-), B. tranquil′lans, L.; Baume anodin, B. tranquille, B. tranquillisant, &c., Fr. Prep. 1. (Bate’s.) See Patent Medicines.
2. (Guy’s.) A vulnerary balsam invented by Guy, of Caliac, once in great repute, but now obsolete. It consisted of aloes, amber, ammoniacum, balsam of Peru, bdellium, caranna, castor, galbanum, labdanum, myrrh, olibanum, storax, tacamahaca, and Venice turpentine, digested in alcohol.
3. (B. TRANQUILLANS, P. Cod.) Fresh leaves of belladonna, henbane, night-shade, tobacco, poppy, stramonium, of each two ounces; dried leaves of costermary, rosemary, rue, and sage, of each half an ounce; dried tops of wormwood, hyssop, sweet marjoram, peppermint, buckbean, and thyme, of each half an ounce; flowers of lavender and elder, of each half an ounce; olive oil, fifty ounces. Heat the green plants in the oil gently until all their water is dissipated; keep on the fire until the oil becomes of a green colour, and whilst still hot, mix in the other plants, carefully dried, and cut up. Digest for twelve hours on a water bath, strain, and filter.
4. (Baume tranquille de Chomel.) Henbane, hound’s tongue, and tobacco, of each237 1 lb.; white wine, 3 pints; boil down to a quart; press, strain, and add to the hot ‘strained liquor’ of olive oil, 1 quart, and again boil.
Balsam, Ap′oplexy. Syn. Bal′samum apoplec′ticum, B. ad apoplec′ticos (Ph. E. 1744), L. Prep. 1. Amber, civet, musk, Peruvian balsam, and some volatile oils, made into a balsam.
2. (Ph. E. 1744) Expressed oil of nutmeg, 1 oz.; liquefy by a gentle heat, and stir in of the oils of cloves, lavender, and rosemary, of each, 1⁄2 dr.; oil of amber, 10 drops; balsam of Peru, 1 dr. Both were formerly used to anoint the head and nostrils of apoplectic patients, and were believed to be of great efficacy.
Balsam, Asiat′ic†. Balm of Gilead.
Balsam, Bate’s. See Balsam, Anodyne.
Balsam, Berlin, for burns, cuts, bruises, and wounds of every kind, sores and ulcers, frost-bites, &c. Chloride of lime with impure glycerine.
Balsam Bilfinger, for rheumatism and gout. Black soap, 25 grms.; water, 40 grms.; spirit of wine, 10 grms.; camphorated spirit, 10 grms.; liquor ammoniæ caustic, 20 grms.; tinct. capsici, 5 grms. (Schädler.)
Balsam, Brazilian. Balsam of copaiba.
Balsam, Calaba′. Syn. Tacamaha′ca. A fragrant resinous substance produced by calophyl′lum cal′aba, or Santa Maria tree.
Balsam, Cam′phor. Syn. Cam′phorated balsam; Bal′samum camphora′tum, &c., L. Prep. 1. As camphor-liniment, Ph. L.
2. (B. Ace′ticum c., Sanchez’s Gout-b.:—Pelletier.) Curd-soap and camphor, of each 5 drs.; oil of thyme, 2 scru.; acetic ether, 5 oz.; digest together in a stoppered bottle until the solids are dissolved. Recommended as an efficacious anodyne liniment in certain forms of rheumatism and gout.
Balsam, Can′ada. See Turpentines.
Balsam, Cana′ry. A volatile oleaginous substance obtained by distillation from draco-ceph′alum Moldavi′cum.
Balsam, Carpa′thian. Riga Balsam.
Balsam, Cephal′ic. (Saxon.) Syn. Bal′samum cephali′cum Saxon′icum, L. A liquid preparation obtained from the essential oils of amber, lavender, marjoram, nutmeg, pennyroyal, rue, sage, &c., distilled together. Once in high repute; but long disused in England.
Balsam, Chil′blain. See Liniments.
Balsam, Chi′na Varnish. The aromatic varnish-like exudation of au′gia sinen′sis, used by the Chinese as a varnish or lacquer, for which purpose it is, perhaps, unequalled. It is highly fragrant, and abounds in benzoic acid.
Balsam, Command′er’s†. Compound tincture of benzoin.
Balsam, Copalm′. Liquid-ambar.
Balsam Egyp′tian. Balm of Mecca.
Balsam, Eye, Augsburg. Red oxide of mercury, ·75 grm.; extract of belladonna, ·5 grm.; tincture of opium, ·5 grm.; fatty substance, 7 grms. (Hager.)
Balsam, Eye (Müller, Berlin). Red oxide of mercury, 5 parts; opium, 3 parts; unsalted butter, 100 parts.
Balsam, Eye, (Müller’s Widow, Berlin). Red oxide of mercury, ·2 grm.; unsalted, unusually rancid, butter, 10 grms.
Balsam, Fe′male. Syn. Bal′samum embryo′num, A′qua e., L. An obsolete preparation made by digesting misletoe, civet, musk, and several other aromatics, in a mixture of wine and various medicated waters, and submitting the whole to distillation. Formerly taken both internally and externally, as a tonic for both fœtus and mother; and particularly to prevent abortion, &c.
Balsam, Fri′ar’s. Compound tincture of benzoin.
Balsam, Gen′oa. Locatelle’s balsam.
Balsam, Glyc′erin (glĭs′-). Syn. Bal′samum gly̆̆ceri′næ, L. Prep. To white wax and spermaceti, of each, 1 oz.; almond oil, 1⁄2 lb.; melted together, add of glycerin, 2 oz.; balsam of Peru, 1⁄2 oz.; and stir or agitate until nearly cold. 12 or 15 drops of otto of roses may be substituted for the balsam.—Used to soften and whiten the skin, and to prevent chaps and chilblains.
Balsam, God′bold’s Vegetable. See Patent Medicines.
Balsam, Goulard’s′. Syn. Bal′samum Goular′dii, B. satur′ni, L.; Baume de Goulard, Fr. Prep. (Van Mons.) Acetate of lead (in fine powder, and quite dry) is triturated, for some time, with hot oil of turpentine, in a heated mortar, or until no more will dissolve; after repose, and whilst still hot, the clear portion is decanted. Recommended as a useful application to foul and painful ulcers, and to scalds and burns.
Balsam, Green. Syn. Bal′samum vir′ide, &c., L.; Baume vert, Fr. Prep. 1. Linseed-oil, 6 lbs.; gum-elemi, 1 lb.; heat them together; add of powdered verdigris, 3 oz., or q. s. to impart a rich green colour, and, after repose, decant the clear portion.
2. Linseed oil strongly coloured with verdigris. Both were formerly much used by surgeons as detergents. ‘Green-oil’ or ‘oil of elder-leaves’ is now commonly sold for it.
A natural balsam, brought from Peru, and produced by chlorox′ylon verticilla′tum, is also popularly called GREEN BALSAM (of Peru).
Balsam, Guaiacum. (Ph. Lond. 1745.) Guaiac, 1 lb.; balsam of Peru, 3 dr.; rect. spirit, 2 pints.
Balsam, Gurgun′ (-gōōn’) Syn. Gurgi′na balsam, Wood-oil (of India). From dipterocar′pus tri′nervis, and other species, by applying a slow fire to a notch or wound made in the trunk. Has a mixed smell of copaiba and naphtha. Properties and dose similar to those of balsam of copaiba. Sp. gr. ·962 to ·964. See Copaiba and Wood-oil.238
Balsam, Hill’s, of Honey. See Patent Medicines.
Balsam, Hungarian. Syn. Bal′samum Hungar′icum, L. A terebinthinate exudation from the extremities of the branches of pi′nus pumil′io (Willd.) or mountain-pine. It is also obtained by pressure from the ‘cones’ of the same tree.
Balsam, Ioduretted. See Liniments.
Balsam, Japan Varnish. Syn. Japan lacq′uer. Exudes from incisions made in the trunk of melanorrhœ′a usitatis′sima, according to Wallich; or stagma′′ria, avernicif′lua according to Lindley. It constitutes the celebrated lac-varnish of the Japanese. It differs from that of China, and from the true balsams, in not containing benzoic acid. It is extremely acrid and irritant; and even its fumes affect the eyes and respiration.
Balsam†, Jews′. Balm of Gilead.
Balsam of Life, Professor Cook’s. Recommended especially for toothache and skin diseases. Borax, 20 parts; boiling water, 250 parts; camphor, 11⁄2 part. (Hager.)
Balsam, Locatelle’s′. Syn. Locatel′li’s balsam; Bal′samum Locatel′li, B. Lucatel′li, B. Ital′icum, B. Genofe′væ, &c., L. var. Prep. 1. (Original Formula.) Olive oil, 6 oz.; yellow wax, 4 oz.; sherry wine, 5 fl. oz.; red sanders (in very fine powder), 4 dr.; simmer them together until the moisture is nearly evaporated, then add of Strasburgh turpentine, 6 oz.; balsam of Peru, 2 dr.; strain through linen, and stir until nearly cold.
2. (Ph. E. 1744.) Olive oil, 24 fl. oz.; yellow wax, 1 lb.; melt, and add of Venice turpentine, 11⁄2 lb.; and, when cooled a little, further add, powdered dragon’s blood, 1 oz.; balsam of Peru, 2 oz.; and stir until cold.
3. (Ph. L. 1746.) Olive oil, 16 fl. oz.; Venice turpentine and yellow wax, of each 1⁄2 lb.; red sanders, 6 dr.
Uses, &c. A once highly esteemed pectoral, and still occasionally used, by the lower classes, in phthisis and chronic coughs (mixed with an equal weight of conserve of roses), and as a mild stimulating ointment.—Dose, 1⁄2 dr. or more.
Balsam, Mercu′′rial†. Ointment of nitrate of mercury.
Balsam, Metz’s. Syn. Bal′samum vir′ide Meten′sium, L.; Baume vert de Metz, Fr. Prep. (Guibourt.) Linseed oil and olive oil, of each 6 oz.; oil of laurel-berries, 1 oz.; common turpentine, 2 oz.; melt by a gentle heat, and add of verdigris 3 dr.; aloes, 2 dr.; sulphate of zinc, 11⁄2 dr. (all in powder); mix well, strain or pour the liquid into a bottle, and add oil of juniper, 4 dr.; oil of cloves, 1 dr. Used on the Continent as a common detergent dressing to wounds and ulcers.
Balsam, Mex′ican†. Balsam of Peru.
Balsam, Nat′ural†. That which exudes from plants, as opposed to those formed by art.
Balsam, Ner′vine. See Ointments.
Balsam, Odontal′gic. See Drops.
Balsam, Opodel′doc. See Opodeldoc (French).
Balsam, Pec′toral. Syn. Bal′samum pectora′le, L.; Baume pectoral, Fr. Prep. 1. Tincture of tolu and compound tincture of benzoin, of each, 2 oz.; rectified spirit, 4 oz.; mix. Dose, 1⁄2 to 1 teaspoonful, night and morning; in chronic coughs, hoarseness, &c.
2, 3. See Balsam of Honey, B. of Horehound, &c.
Balsam, Persian†. Friar’s Balsam.
Balsam, Peru′′vian. See Balsam of Peru.
Balsam, Poly′chrest. Syn. Elixir Polychreston. (E. 1745.) Guaiacum, 6 oz.; balsam of Peru, 1⁄2 oz.; rectified spirit, 32 oz. Digest in a sand bath for 4 days, and add oil of sassafras, 2 dr.
Balsam, Poser’s (E. Gross, Breslau), for chronic and local rheumatism. A yellow liquid, composed of:—Oil of rosemary, 4 grms.; camphor, 10 grms.; tincture of ants, 15 grms.; tincture of cantharides, 5 grms.; spirit of wine, 90 grms.; tincture of saffron, 10 drops. (Hager.)
Balsam, Potsdam (aromatic balsamic perfume). Liquid storax, 4 grms.; cloves, 2 grms.; oil of cloves, 3 grms.; oil of cassia, 1 grm.; oils of bergamot, lemon, and lavender, āā, 2 grms.; oil of curled mint, 1⁄4 grm.; rectified spirit, 200 grms.; macerate and filter. (Hager.)
Balsam, Riga. (rē′-). Syn. Carpa′thina balsam; Bal′samum carpath′icum, B. lib′ani, &c., L.; Baume de Carpathes, Fr. A pellucid white fluid obtained by careful distillation from the young shoots of pi′nus cem′bra (Linn.) or Siberian stone-pine. It much resembles oil of juniper; and, like that article, is powerfully diuretic. It is regarded as vulnerary, and is highly esteemed by some in sprains and bruises. The bottoms of oil of juniper are commonly sold for it in the shops. The spirit distilled from pine-tops (spiritus turionum pini) is also frequently, although incorrectly, called BIGA BALSAM.
Balsam, Sanchez’s Gout. See Balsam, Camphorated.
Balsam, Sooth′ing. See Balsam, Anodyne.
Balsam, St. Genevieve. Thick turpentine, 5 parts; olive oil, 30 parts; bees’ wax, 25 parts; spermaceti, 5 parts; camphor, 1 part; red sanders, 4 parts.
Balsam, St. John Long’s (liniment), used for application to the chest in cases of phthisis, is a thick emulsion composed of:—Turpentine, 25 parts; yolk of eggs, 50 parts; concentrated vinegar, 5 parts; rose water, 15 parts; and a few drops of essence of lemon.
Balsam, Stomach′ic (-măk′-). Syn. Bal′samum stomach′icum, L.; Baume stomachique, Fr. Prep. (Ph. Slesv.-Hols. 1831.) Oils of cloves, mace, wormwood, and peppermint, of each 1 dr.; balsam of Peru, 2 dr.; oil of nutmeg, 2 oz.; mix. 1 to 5 or 6 drops, on sugar, or dissolved in spirit.239
Balsam, Syr′ian. Balsam of Mecca.
Balsam, Thibaut’s. See Patent Medicines.
Balsam, Tooth′ache. See Drops, &c.
Balsam, Traumat′ic. Compound tincture of benzoin.
Balsam, Tur′key. Syn. Tur′key balm. The distilled oil of the dracocephalum moldavicum.
Balsam, Tur′lington’s. See Patent Medicines.
Balsam, Univer′sal. Syn. Bal′samum universa′le, L. Prep. (Ph. Slesv.-Hols. 1831.) Rape oil (recent), 11⁄2 lb.; yellow wax, 1⁄2 lb.; acetate of lead (in fine powder), 3 oz.; powdered camphor, 1⁄2 oz.; melted together; observing to triturate the acetate with a small portion of the oil before adding it to the mixture, and not to add the camphor until the heat is reduced a little.—Obs. This name has also been given to ‘compound cerate of lead,’ and even to ‘cerate of acetate of lead.’
Balsam, Vervain’s†. Compound tincture of benzoin.
Balsam, Wound. Several vulnerary preparations have been so called; but FRIAR’S BALSAM (comp. tinct. of benzoin) is that usually intended.
Balsam of Acou′chi. A yellowish aromatic liquid, of a terebinthinous nature and consistence, obtained from the wounded branches and shoots of the icica heterophylla (DC.). It is highly esteemed as vulnerary by the Caribs of Guiana. (Lindley.)
Balsam of Alpi′nus. Balm of Gilead; because Prosper Alpinus wrote a learned (?) treatise on it.
Balsam of Am′ber. Syn. Bal′samum succini, L.; Baume d’ambre, Fr. The article to which this term is usually applied has been already noticed. Oil of amber was also formerly so called; and the same name has been given to the following and other like preparations by their inventors:—
1. (Radius.) Oil of amber, 4 fl. oz.; oil of myrrh, 2 fl. oz; oil of turpentine, 1 fl. oz.; mix with a gentle heat.
2. (Bate.) See Balsam Anodyne. They are all stimulant and antispasmodic, and are used either internally or as a friction, like oil of amber.
Balsam of Arcæ′us. Syn. Bal′samum Arcæ′i, L.; Baume d’Arcæus, Fr. A digestive ointment formerly in great repute, and still much employed on the Continent. It is now superseded in England by the comp. elemi ointment of the Pharmacopœias. In the original formula, boiling water, 4 parts, were ordered to be stirred in.
Balsam of Can′ada. See Turpentines.
Balsam of Cloves. Syn. Aromat′ic balsam of cloves; Bal′samum caryophyl′li, L. Prep. (Bories.) Oil of cloves and oil of nutmeg, of each 1⁄2 dr.; spirit of juniper berries, 3 oz.; mix. Rubefacient and diuretic.—Used chiefly as a stimulating friction. Internally, 1⁄2 to 1 teaspoonful.
Balsam of Copai′ba. See Copaiba.
Balsam of Fern. Oil of male fern.
Balsam of Fiovaren′ti. Syn. Bal′samum Fiovaren′ti, L. Prep. (P. Cod.) Venice turpentine, 16 oz.; amber, elemi, galbanum, myrrh, styrax, and tacamahaca, of each 3 oz.; aloes, 1 oz.; bay-berries, 4 oz.; cinnamon, cloves, galangal, ginger, nutmegs, and zedoary, of each 11⁄2 oz.; dittany of Crete, 1 oz.; rectified spirit, 8 lbs.; macerate a week and distil off 7 lbs. The distilled spirit constitutes this notable preparation of, professedly, many virtues. It is reputed aromatic, diuretic, antispasmodic, and stimulant. One of its applications is as a collyrium—a drop or two being rubbed on the palm of the hands, which are then held to the eyes, so as to cover, without touching them—in chronic ophthalmia, conjunctivitis, &c.
Balsam of Gil′ead. See Balsam of Mecca.
Balsam of Gua′iacum (gwā-yă-). Syn. Bal′samum guai′aci, B. guaiaci′num, L. Prep. (Ph. L. 1745.) Gum-guaiacum, 1 lb.; balsam of Peru, 3 dr.; rectified spirit, 1 quart; digest 10 days and filter. Diaphoretic, arthrodynic, and anodyne.—Dose, 30 to 60 drops, in milk or water; in agues, rheumatism, &c. Externally, reputed also anti-suppurative.
Balsam of Honey. Syn. Pec′toral balsam, P. b. of honey; Bal′samum mel′lis, B. pectora′le, B. p. mellis, L.; Baume de miel, &c., Fr. Prep. 1. Balsam of tolu, 1 lb.; honey (finest), 21⁄2 lbs.; rectified spirit, 1 gall.; turmeric, 1 oz.; make a tincture.
2. To the last, before maceration, add of powdered opium, 2 oz.
3. (Hill’s.) See Patent Medicines. Uses, &c. A good pectoral in colds, tickling chronic coughs, hoarseness, &c., when unaccompanied with fever.—Dose. For an adult, 1⁄2 to 1 teaspoonful, twice or thrice a day; an occasional dose of some mild aperient being also taken. Tincture of balsam of tolu, or a mixture of the tinctures of tolu and benzoin, is frequently sold in the shops under the name of ‘balsam of honey.’ See Pectoral Balsam, &c.
Balsam of Hore′hound. Syn. Bal′samum marru′bii, L. Prep. 1. Extracts of horehound and liquorice, of each 2 oz.; hot water, 1⁄2 pint; dissolve, and when cold, add of paregoric, 3⁄4 pint; oxymel of squills, 6 oz.; tincture of benzoin, 2 oz.; honey, 10 oz.; and, after thorough admixture, strain through flannel.
2. (Ford’s.) See Patent Medicines.
Uses, &c. A popular pectoral.—Dose, &c., same as of BALSAM OF HONEY (above).
Balsam of Houmi′′ri. [Nat.] From humir′ia balsamif′era, or the houmiri-tree of Guiana.240 It resembles ‘balsam of umiri’ produced by another tree of the same genus. (See below.)
Balsam of Lead. See Balsam, Goulard’s.
Balsam of Life. Syn. Balm of life; Bal′samum vi′tæ, L.; Baume de vie, Elix′ir de vie, &c., Fr. Several compound medicines have been called by this name. Those of Gabius, Hoffman, and Turlington, are noticed under Patent Medicines (which see). The following are distinct preparations:—
1. Baume de vie externe:—Soap liniment, 2 parts; oil of turpentine, 1 part; mix. Stimulant and rubefacient. Used with friction.
2. Baume de vie purgatif; Elixir de vie:—a. (Briett.) Socotrine aloes and saffron, of each, 2 drs.; rhubarb, 6 drs.; liquorice-root, 1 oz.; proof spirit or brandy, 1⁄2 pint; digest a week, and filter.
b. (Original Swedish formula.) Aloes, 9 drs.; agaric, gentian, rhubarb, saffron, theriaca, and zedoary, of each 1 dr.; proof spirit or brandy, 1 quart. A mild stomachic purge.—Dose. 1 to 6 drs. Tincture of rhubarb-and-aloes (Ph. E.) is commonly substituted for it. See Elixirs.
Balsam of Liq′uorice. See Patent Medicines.
Balsam of Mec′ca. Syn. Balm of Gil′ead, B. of Mec′ca, Opobal′sam (-bawl′-), Jews’ balsam†, Oil of b.†, &c., Eng.; Bal′samum (băl′-) Gileaden′se, B. è Mec′ca, Opobal′samum (-băl′-) &c., L.; Baume de la Mecque, B. de Mecca*, B. de Judée, Opobalsamum, &c., Fr. Bal′samum Ægypti′acum†, B. Alpi′ni†, B. Antiquo′′rum Genui′num†, B. Asiat′icum†, B. Syri′acum†, O′leum Bal′sami†, &c., L. A fragrant oleo-resinous substance, obtained from balsamoden′dron gileaden′se (Kunth.; amy′ris gileaden′sis, Linn.; a. opobal′samum, Forsk), a middle-sized tree of the nat. ord. Terebinthacæ (DC.), growing in Arabia Felix, Asia Minor, and Egypt. It is the BALM of the Old Testament, and the βἁλσαμον of Theophrastus and Dioscorides. It is chemically classed with the turpentines.
Prop., &c. When fresh it is turbid and whitish, but becomes by degrees transparent, of a rich golden colour, and slightly thicker; and by exposure, eventually solid. It possesses a penetrating and delicate fragrance; tastes sharp, bitter, spicy, and somewhat astringent; is not entirely soluble in rectified spirit, but dissolves more or less completely in both the fixed and volatile oils, which then assume the fragrance of the balsam. A drop let fall on hot water spreads itself over the whole surface, like a film of oil, and again contracts on the water cooling. This, with its fragrance, is the common test of its genuineness in Turkey. The inferior qualities, or those of commerce, are generally opaque and thick, rapidly resinifying and turning of a dull yellow by age. When applied to the skin it causes redness and swelling. It was formerly regarded as possessing the most varied and exalted virtues, particularly as an antiseptic, stimulant, vulnerary, and nervine; and its fumes were supposed to prevent barrenness. It is still highly prized in the East as a cosmetic and perfume; and is said to be unequalled for giving a healthy glow to the complexion and promoting the growth of the hair. Its medicinal qualities are intermediate to those of the aromatic turpentines and balsam of tolu.—Dose. From 3 to 6, or even 10 or 12 drops.
Obs. According to Bruce, and others, the best balm of Gilead is a spontaneous exudation from the tree; a second quality is obtained by cutting the bark with an axe, and receiving the juice which exudes in a small earthen bottle. A large branch is said to produce not more than 3 or 4 drops a day; and even the most resinous trees seldom yield more than 60 drops daily. Hence its scarcity and costliness. Both varieties are held in such high estimation by the Turks and Egyptians, that none of them are exported as an article of commerce. That which is sent to England is obtained by boiling the leaves and young twigs of the balsam tree in water, and is rejected by the Orientals as worthless. Most of that sold in the shops of England is entirely spurious (see below).
The cosmetics recently so much advertised as ‘balm of Mecca’ do not contain even a trace of this article; nor do we believe that there is a single drop of the genuine balm to be purchased in London.
The following formulæ are current in the trade for Fac′′titious Balm of Mecca:—
1. Gum-benzoin (bright, coarsely powdered), 4 oz.; liquid styrax (finest), 3 oz.; balsam of tolu, 2 oz.; Canadian balsam, 11⁄2 pint; are mixed together in a flask, and exposed (closed) to the heat of a water bath, with frequent agitation, until the liquid is saturated; when cold, the clear portion is decanted, and a sufficient quantity of the oils of lemon, cassia, rosemary, nutmeg, and vanilla, added to give it a strong aromatic odour.
2. From gum-benzoin and balsam of Peru, of each 1 oz.; vanilla and nutmeg, of each (cut small) 1 dr.; Canadian balsam, 1⁄2 pint; digested as before, and some essential oils added to the decanted liquid.
Balsam of Nut′meg. Syn. Bal′samum myris′ticæ, B. nucis′tæ. L. Prep. (Ph. Bor. 1847.) Expressed oil of nutmeg (—? mace), 3 oz.; olive oil, 1 oz.; yellow wax, 1⁄2 oz.; melt them together by a gentle heat, pour the mixture into paper moulds, and, when cold, cut the mass up into cakes.
Balsam of Peru’ (rōō′). Syn. Peru′vian balsam; Bal′samum Peruvia′num (Ph. L., E., and D.), L.; Baume du Pérou, B. Peruvien, Fr.; Peruvianischer balsam, Ger. A balsam obtained from Myroxylon Pereiræ (Myrospermum of Sonsonate). It exudes from the trunk of the tree after it has been scorched241 and removed. From Salvador, in Central America. B. P.
Prop., &c. A chocolate-coloured or a reddish-brown liquid, of the consistence of treacle, possessing a bitterish, rather pungent taste, and an agreeable aromatic odour somewhat similar to that of a mixture of vanilla and benzoin. It is reputed stimulant, tonic, and expectorant, and has long been a popular remedy in chronic asthma, catarrh, and other pulmonary affections, debility, &c. It is now, however, principally used as an ingredient in pomades, hair-oils, lip-salves, and other cosmetics, in which it is only inferior to ‘balm of Mecca,’ and in compound perfumery. It is also used to scent lozenges, pastils, and chocolate and liqueurs; for these last, chiefly as a substitute for ‘vanilla’ when it is scarce and dear.—Dose, 10 or 12 to 30 gr. (even 1 dr. is sometimes given), either on sugar, or made into a bolus with liquorice powder, or into an emulsion with honey, mucilage, or yolk of egg.
Pur., Tests, &c.—1. The sp. gr. should not be lower than 1·15; nor higher than 1·16:—2. Ether dissolves it readily and completely:—3. Soluble in 5 parts of rectified spirit:—4. It should undergo no diminution in volume when agitated with water:—5. 100 gr., by its benzoic or cinnamic acid, should saturate not less than 71⁄2 gr. of pure crystallised carbonate of soda:—6. Sulphuric acid converts it into resin, artificial tannin, or charcoal, according to the quantity employed; if, on adding water, a brittle resin is not formed, some fixed oil (probably castor oil) is present:—7. Treated with nitric acid, some hydrocyanic acid is formed, benzoic acid sublimes, and the residual matter is artificial tannin:—8. The alkalies and their carbonates form with it a thickish semi-crystalline mass, which, on being treated with sulphuric acid, deposits a peculiar resinous matter, with crystals of benzoic and cinnamic acid:—9. If a few drops are distilled, and, when iodine is added to the distillate, an explosion results, it has been adulterated with ‘copaiba’:—10. The genuine balsam contains about 61⁄2% of benzoic (cinnamic) acid:—11. (Hager). If two or three cubic centimètres of balsam of Peru be shaken with five or six cubic centimètres of petroleum spirit, the mixture separates upon being allowed to stand into a black-brown layer, and a limpid and colourless or slightly yellowish layer, and is easily decanted. If the balsam be adulterated, this latter layer is turbid and coloured, while the viscous residue which separates is more fluid, which renders decantation more difficult. Sometimes the brown residue is pulverulent.
Obs. Balsam of Peru was formerly very generally adulterated, and often entirely factitious; but, owing to its present reduced price, this is now only confined to a few of the most unprincipled venders. The following formulæ for this purpose are still extant in the trade:—
Balsam of Peru, Facti′′tious:—From gum-benzoin (in coarse powder), 3 lbs.; dissolved in the least possible quantity of rectified spirit, and then mixed with balsam of tolu, 1 lb.; and liquid styrax, 2 oz.; subsequently adding of rectified spirit, q. s.
Balsam, Reduced Peruvian:—1. Balsam of Peru, 3 lbs.; balsam of tolu, 2 lbs.; rectified spirit, q. s. to reduce it to a proper consistence:—2. Balsam of Peru, 3 lbs.; gum-benzoin (dissolved in a little rectified spirit), 1 lb.; as before. It is occasionally met with largely adulterated with liquid styrax.
Balsam of Rackasi′ri. Syn. Balsam of Rakasi′ra; Bal′samum Rackasiri, B. Racazzi′ræ, B. Rhadasi′ri. A species of balsamic turpentine, said to be obtained from the bursera balsamifera (Pers.), an Indian tree of the natural order Terebinthaceæ. It has a slightly bitter taste, adheres to the teeth when chewed, and, when heated, smells like balsam of tolu. It has been extolled as possessing the virtues of copaiba in an exalted degree. The nostrum vended under the name of BALM OF RACKASIRI by certain quacks, simply consists of English gin, coloured, sweetened, and aromatised.
Balsam, Saturnine. (Bate.) Acetate of lead 40 oz.; oil of turpentine 12 oz. Digest for some days.
Balsam of Soap. Soap-liniment.
Balsam of Soap (Ethe′′real). Syn. Bal′samum sapo′nis æthe′′reum, L. Prep. (Cottereau.) Castile soap (powdered) and camphor, of each 1 dr.; oil of thyme, 10 drops; acetic ether, 1 oz.; dissolve in a close vessel with the aid of a gentle heat, and decant the clear portion. Used as an embrocation or liniment in gout, rheumatism, &c.
Balsam of St. John’s Wort. See Oils.
Balsam of Sto′′rax. Liquid-ambar or styrax.
Balsam of Sul′phur. See Oils.
Balsam of Sul′phur, Anisated. (Ph. Edin. 1722). Originally made by digesting one part of sulphur; three of turpentine; and four of oil of aniseed. A mixture of one part of oil of aniseed with three or four of balsam of sulphur is usually sold for it.
Balsam of Sul′phur with Turpentine. Digest one part of sulphur with three of oil of turpentine till dissolved. Similar compounds were formerly made with sulphur and Barbadoes tar, and with the empyreumatic oils of amber, benzoin, &c.
Balsam of Syri′acum. See Balsam of Mecca.
Balsam of Tolu′ (-l′ōō). Syn. Tolu’ bal′sam*; Bal′samum toluta′num (Ph. L., E., & D.), B. de To′lû, L.; Baume de Tolu, Fr.; Tolutanischer balsam, B. von Tolu, &c., Ger. Balsam flowing from the incised trunk of “myrosper′mum toluif′erum.” (B. P.) The tree which produces it is a native of the mountains of Tolu, Turbaco, &c., in South America.
Prop., Uses, &c. When first brought over it is soft and tenacious, but by age and careless242 keeping becomes hard, and even brittle, somewhat similar to resin. It is perfectly soluble in alcohol and in ether, and gives out its acid (benzoic or cinnamic) to water. Its odour is fragrant, though less powerful than that of either styrax or balsam of Peru; and it has a pleasant sweetish taste. It softens under the teeth, melts readily, and burns with an agreeable odour. As a medicine it is a stimulating expectorant, and, as such, is employed in chronic bronchial affections unaccompanied with inflammatory action. It has long been a popular pectoral. Syrup of Tolu is an agreeable and common adjunct to pectoral mixtures, and, with Tolu lozenges, is often serviceable in tickling coughs. It is also used by confectioners, perfumers, &c., and in fumigating pastils.—Dose, 5 to 20, or even 30 gr., dissolved in spirit, or made into an emulsion.
Pur. This is shown by its perfect solubility in rectified spirit, forming a transparent tincture, and by its odour. When adulterated it has a weaker smell, is only partially soluble in alcohol, and the tincture formed with that fluid is opaque. The presence of colophony (or lac), according to Ulex, may be detected by the balsam, instead of dissolving in sulphuric acid, swelling up, blackening, and disengaging sulphurous fumes.[106] Castor oil may be detected in the way noticed under Balsam of Peru.
Balsam of Tolu, a Factitious, was formerly met with in trade, made of equal parts of orange-lac and white sugar, reduced to a proper consistence with rectified spirit, and ‘brought up’ with some tincture of benzoin, and a few drops of the oils of cassia and nutmeg dissolved in a little essence of vanilla.
Balsam of Tur′pentine (-tīne). Syn. Bal′samum terebin′thinæ, L. A name formerly given to Strasburgh, Venice, and other like turpentines.
Balsam of Tur′pentine (Emollient). Syn. B. terebinthina′tum, L. Prep. Olive oil, 6 oz.; oil of turpentine, 2 oz.; yellow wax, 1 oz.; balsam of Peru, oil of nutmeg, and camphor, of each 2 dr. A stimulant emollient; in contusions, ulcerations, engorgements, nephritic pains, &c.
Balsam of Umi′′ri. [Nat.] By incision, from the humir′ia floribun′dum (Mart.), or the umiri-plant of Para. It is fragrant, limpid, of a palish-yellow colour, and in its medicinal properties is said to combine those of the balsams of copaiba and Peru.
BALSAM′IC (băl-). Syn. Balsam′icus, Balsa′meus, Balsam′inus, L.; Balsamique, Fr.; Balsamisch, Ger. Of the nature of balsam, or containing or resembling it; bland, soothing, healing; balmy.
BAMBOO′ (-bōō′). [Nat.] Syn. Bambu′sa, L.; Bambon, Fr.; Bambus, Bambusrohr, Indianischer rohr, Ger. The name of several species of the genus ‘bambusa,’ but appr. of b. arundina′cea or ‘common bamboo.’ See Bambusa.
Bamboo′-habit (-hăb-). A species of ‘life-preserver,’ or ‘float,’ used in China and the Indian Archipelago, consisting of four pieces of bamboo tied together so as to form a square.
BAMBU′SA. [Endl.] The bamboo. In botany, a genus of magnificent arborescent grasses, of the nat. order Gramineæ (DC.), having hollow jointed stems, of a hard woody texture, externally coated with siliceous matter, and sometimes secreting a similar siliceous substance (TABASHEER′) in their internal cavities. They are all of rapid growth, and vary in height from 6 to 150 feet.
There, is, perhaps, scarcely any other plant besides the palm which serves for so many purposes useful to man, as the various species of bamboo. Its grain is used for bread; the young shoots are eaten like asparagus, and are also pickled; the smaller stalks are made into walking canes, umbrella and parasol sticks, flutes, &c.; whilst its fibres are manufactured into cloth, and even paper. It is employed extensively in the construction of houses, bridges, masts for boats, domestic furniture, boxes, mats, baskets, utensils of various kinds, fences, water pipes and vessels, quicksilver bottles, &c., and for numerous other purposes connected with everyday life. In localities where ordinary surgical appliances are not at hand, splints, of any required length or size, can be made with very little delay, from the stems of the bamboo. The older and drier stems are to be preferred for this purpose. The genus is confined to the East and West Indies and tropical America. See Cane, Pickles, Tabasheer, &c.
BANA′NA (nā′- or -nah′-). [Nat.] The mu′sa sapien′tum (Linn.), a species of plantain; also its fruit. The Banana contains about 27 per cent. of solid matter, and has nearly the same nutritive value as rice. It is largely consumed in the tropics, where the common allowance for a labourer is 61⁄2 lbs. of the fresh fruit or 2 lbs. of the dry meal, with a quarter of a pound of salt meat or fish. It is sometimes fried in slices and often made into preserves.
| Nitrogenous matter | 4·820 |
| Sugar, pectose, organic acid, and traces of starch | 19·657 |
| Fatty matter | 0·632 |
| Cellulose | 0·200 |
| Saline matter | 0·791 |
| Water | 73·900 |
| ———— | |
| 100·000 |
See Plantain.
BANCOUL, NUTS OF. This nut is the seed of a tree belonging to the Euphorbiaciæ, of which two or three species occur in Ceylon, Cochin-China, New Caledonia, Bourbon, 243&c. It is composed of a hard and woody endocarp, and of an oily kernel, containing:—
| Water | 5·000 |
| Oil | 62·175 |
| Nitrogenous matter | 22·653 |
| Non-nitrogenous matter | 6·827 |
| Mineral matter | 3·345 |
| ———— | |
| 100·000 | |
| Nitrogen | 3·625% |
This cake, after expression of the oil, contains 9 per cent. of nitrogen, and 4 per cent. of phosphoric acid, and is consequently of high value as a manure. The expressed oil is purgative, but as a lamp-oil it is superior to colza. Unfortunately the kernel forms only 33 per cent. of the entire weight of the nut. Hence, before it can become an article of commerce, it must be decorticated at the place of its birth. (Corenwinder.)
BAND′AGE (āje). Syn. Deliga′tio, Fas′′cia, Liga′men, Ligatu′′ra, Vinctu′′ra, L.; Bandage, Bande, Fr.; Binde, Verband, Ger. In surgery, the fillet, roller, or cloths, used to support parts, to exert pressure on them, or to retain dressings in their proper places.
Bandages are usually formed of long narrow slips of calico or linen, and, occasionally, of flannel, which are generally made into a roll (ROLLER) of moderate size, so as to be the more conveniently handled and applied. They are either SIMPLE, as the cir′cular, the spīral, or the unīt′ing bandage; or COMPOUND, as the T-bandage, suspen′sory b., &c.
The application of bandages, as in the dressing of wounds, ulcers, &c., though of such frequent occurrence, is often very carelessly and badly done. The form and nature of the part, and the object in view, should always receive consideration; as should also the condition of the patient after their application—whether of repose, exercise, or labour. Ordinary ingenuity will supply the rest. The safest, simplest, and most effective means of fastening them is, in most cases, furnished by a common needle and thread or cotton.
Bandage, Mus′tard. A woollen roller soaked in a mixture of the best flour of mustard and warm water, of the consistence of fresh cream, the excess of moisture being expelled by gentle pressure.—Used to envelope the body, or a limb, by repeatedly folding it round the part; in the cold stages of cholera, and in other cases requiring an energetic stimulant. Other medicaments, particularly those of a stimulating and anodyne character, are sometimes applied in the same manner. See Embrocations, Lotions, Poultices, &c.
BANDAN′A. [Ind.] Syn. Bandan′na. A handkerchief, originally from the East Indies, having white spots on a red, blue, or other dark ground. In calico-printing, a ‘discharge style’ in imitation of the Indian bandanas. The fabric, many folds thick, is placed between leaden plates having the pattern cut out of them; hydraulic pressure is then applied, and a clear solution of chloride of lime forced through, followed by a stream of pure water.
BAND′OLINE (-lĭn; -lēne‡). See Fixature.
BANE. Poison; anything deleterious or destructive; a word often found joined to another, in the popular and vulgar names of plants and disease, to denote their character; as BANE′-BERRY, the herb Christopher; BANE′-WORT, deadly-nightshade; SHEEP’S BANE, the rot; &c.
BANG, Bangue (băng′). [Nat.] See Hemp, Indian.
BAN′IAN (băn′-yăn). The fi′cus In′dicus (Linn.), or Indian fig. The fruit and young branches yield one species of gum-lac; and both the juice and bark are used medicinally.
Among sailors, BANIAN′ DAYS are those on which butcher’s meat is not served up at dinner.
BANN′OCK (-ŭk). In Scotland and the northern counties of England, a flat round cake made of oat, rye, or barley meal, baked on an iron plate over the fire, or on the hot hearth.
BARBS. Syn. Lampas, Skew. This occurs in horses from two to four years old, and arises from a little inflammation of the ridges that pass along the palate, above and behind the incisor teeth, occasionally preventing the animal from eating and setting up slight fever. The best treatment is to scarify the enlarged ridges freely with a lancet or penknife, and to give for a time bran mashes, soaked grain, and other soft food.
BAR′BERRY. Syn. Pep′peridge-bush‡, Thorny box′-tree*; Ber′beris, B. vulga′′ris (Linn.), L.; Epine-vinette, Vinettier, Fr.; Berberitze, Ger. A perennial bush or shrub common in woods and hedges. Berries (BAR′BERRIES, PEP′PERIDGES), gratefully acid, cooling, and astringent; used in pastry, but require, according to their degree of ripeness, from one half their weight to an equal weight of sugar. Both bark and berries were formerly esteemed in jaundice, biliary flukes, &c. The crushed berries with water form a refreshing fever-drink. The root dyes a fugitive yellow. See Berberine, Jams, Preserves, &c.
BAREGE (barège, băr-rāzhe’). [Fr.] A light woollen fabric so named from having been first made in the valley of Barèges. Of late years Paris has become celebrated for its barèges; but these are generally woven with the ‘warp’ of silk, and the ‘woof’ of wool. In the common imitations of the shops, the ‘warp’ is generally of cotton.
BAREGINE (barégine). See Glairine.
BARIL′LA. [Eng., Ger., L., Sp.] Syn. So′dæ car′bonas vena′le, L.; Barig′′lia, Baril′lor, Sp., Lev.; Barille, Soude, Fr. The alkaline residuum of the combustion of salsola, salicornia, chenopodium, and other species of the order Chenopodiaceæ. These244 plants, which are cultivated on the sea-coast for the purpose, are cut down when ripe, dried, and burned in heaps, on iron bars laid across pits dug in the earth. The alkali and saline matter contained in them is thus fused, and flows into the cavity below, forming, when cold, a hard grey or bluish porous mass which is BARILLA.
Comp. Carbonate, sulphate, chloride, and sulphide of sodium, carbonate and sulphate of calcium, alumina, silica, oxide of iron, and imperfectly consumed carbonaceous matter, with a little iodine and bromine. The proportion of soda varies in different varieties:—
Alicant’ barilla; obtained chiefly from several species of salso′la and from chenopo′dium setig′erum (-tĭj′-), &c.; contains from 25% to 40% of carbonate of soda. (Guibourt.)
Cana′′ry b.; from salso′la ka′li. (Loudon.) French barillas:—
a. Narbonne’ b., salicor; from salicor′nia ann′ua or herba′cea; contains 14% to 15% of carbonate of soda.
b. B. of aiguemortes, blanquette; from mixed plants; contains 3% to 8% of carbonate of soda. (Guibourt.)
c. Nor′mandy b., N. soda; from fuci.
Sic′ily barilla (sĭs′-). Principally from salso′la sati′va; furnishes 55% of carbonate of soda. (Fée.)
Good barilla, on the average, contains about 20% of real or available alkali, chiefly under the form of carbonate, besides sulphates, muriates, &c.
Assay. See Alkalimetry.
Uses, &c. Barilla is chiefly used in the manufacture of soap and glass; but the gross quantity imported, though annually increasing, only reached 54,608 cwt. in 1856; whilst the exports of soda in the same year reached to about 1,500,000 cwt., and in 1859 to above 2,000,000 cwt. This enormous quantity was chiefly furnished by our home manufactories.
Barilla is chiefly imported from Spain, Sicily, Teneriffe, and the Levant; but since the introduction of Le Blanc’s process for obtaining soda from common salt, its importance and value has considerably lessened. See Kelp, Soda, &c.
BARIUM. Ba. A metallic radical or element, of which baryta is the chief oxide, and somewhat extensively distributed. First obtained in 1808 by Sir H. Davy. Prepared from baryta by strongly heating it in an iron tube, through which the vapour of potassium is conveyed; the reduced barium being subsequently extracted from the mixed residuum by quicksilver, which is afterwards driven off in a small green-glass retort, in a vapour of mineral naphtha.
Prop., &c. Greyish-white, approaching silver in colour and lustre; decomposes water, and gradually oxidises in the air, with the formation of the ordinary oxide (BARYTA). It is malleable, fusible under a red heat; burns in contact with air with a deep red light, and has the sp. gr. 4·70.
Salts. Barium forms numerous salts, which are all either colourless or white, except a few, whose acids are coloured, as the chromate, manganate, &c. Some of them are soluble in water; one or two only are soluble in alcohol, and that very sparingly; and (with the exception of the sulphate) they are all extremely poisonous. They may be prepared by saturating solutions of the acids with either baryta-water, or carbonate of barium; and some of them may be prepared by double decomposition.
The various soluble barium salts are known by the following reactions, and they are all (except the sulphate) soluble either in water or in dilute hydrochloric acid, except the nitrate and chloride, which are not soluble in aqueous solutions of their respective acids. Their solutions give an immediate heavy white precipitate with dilute sulphuric acid, and with solutions of the sulphates, which is insoluble in dilute acids and solutions of the alkalies and of the salts of ammonia, that with a solution of sulphate of lime being very sensitive, and characteristic:—Hydrofluosilicic acid gives a very characteristic colourless crystalline and quickly subsiding precipitate, only slightly soluble in hydrochloric acid and nitric acid; alcohol, in equal volume, being added, so hastens and completes the reaction, that the filtrate is unaffected by sulphuric acid:—Chromate of potassium gives a bright yellow precipitate in neutral solutions, soluble in hydrochloric acid and in nitric acid, but insoluble in acetic acid:—Caustic potassa or soda (when quite free from carbonate), and caustic ammonia, cause no precipitate, except in highly concentrated solutions:—Alkaline carbonates give a heavy white precipitate with baryta-water or a solution of baryta, and which is all but insoluble in water, and freely soluble in dilute hydrochloric acid:—Heated with proof spirit, or pyroxilic spirit, the barium salts give a greenish-yellow tinge to the flame:—The barium salts, and particularly the chloride, when exposed on a platinum wire to the inner flame of the blowpipe, colour the outer flame yellowish-green:—Insoluble sulphate of barium may be mixed with powdered charcoal, and exposed for a short time to a full red heat, when sulphide of barium will be formed, which is freely soluble in water, and which, after being neutralised with hydrochloric acid, or acetic acid, will yield a solution suitable to the application of the usual tests. The carbonate, and the salts of barium with the organic acids, are all convertible into pure baryta by exposure to a bright red heat.
Baryta is distinguished from lime and from magnesia by its great solubility in hot water, and by the entire insolubility of its sulphate; from strontia, by being precipitated by hydrofluosilicic acid, and by not giving a red colour to the flame of alcohol; from alumina, by its245 causticity and alkaline reaction, and by not being precipitated from its solution in water by ammonium sulphydrate.
Pois., &c. The sulphate, owing to its insolubility, is the only salt of barium which is not poisonous.—Symp. Nausea, vomiting, pains in the head, ringing in the ears, vertigo, and intermitting cramps and convulsions; the respiration is frequently suspended for several moments, and the pupil is generally dilated. The symptoms, however, often vary, and are not very distinctive.—Treatm., Ant., &c. Vomiting, followed by copious draughts of water soured with sulphuric acid, or sulphate of soda (Glauber-salt) or sulphate of magnesia (Epsom-salt), dissolved in a large quantity of water. When carbonate of barium has been swallowed, a mixture of one of the above sulphates and weak vinegar should be taken after the vomiting, in order that a soluble barium salt may be first formed, on which the alkaline sulphate will act more readily. Subsequent irritation may be soothed by opium or morphia, and antiphlogistics.
Barium, Ac′etate of. Ba(C2H3O2). Syn. Bary′tæ ace′tas, L. Prep. From dilute sulphuric acid, neutralised with carbonate of barium, and the solution evaporated and crystallised. Very soluble in water; insoluble, or nearly so, in rectified spirit.—Uses, Dose, &c. Same as the chloride. It is seldom employed.
Barium, Arse′′niate of. Ba3(PO4)2. Syn. Bary′tæ arse′′nias, L. Prep. A solution of chloride of barium is added to another of arseniate of potassium or sodium, and the precipitate collected, washed, and dried. By dissolving this salt in a solution of arsenic acid, and crystallising, BINARSE′′NIATE OF BARIUM is obtained. Has been recommended in certain skin diseases, and in phthisis complicated with scrofula.—Dose, 1⁄16 to 1⁄4 gr.
Barium, Ar′senite of. Ba(AsO2)2. Syn. Bary′tæ ar′senis, L. Very slightly soluble.—Use, &c. As the last.
Barium, Bromide of. BaBr2. Syn. Ba′′rii bromi′dum, L.; Bromure de baryum, &c., Fr. Prep. Boil a solution of protobromide of iron with moist carbonate of barium, in slight excess; filter, evaporate to dryness, and heat the residuum to redness. By careful evaporation of its aqueous solution it may be obtained in crystals. It is soluble in both alcohol and water, and its physiological properties resemble those of iodide of barium.
Barium, Carbonate of. BaCO3. Syn. Carbonate of Bary′ta; Bary′tæ car′bonas, L.; Carbonate de baryte, &c., Fr.; Kohlensaures, baryt, &c., Ger. A heavy white mass or powder, very nearly insoluble in water, and decomposed by nearly all the acids. It is found in the crude state abundantly in nature, but can only be obtained absolutely pure by adding an alkaline carbonate to a solution of chloride of barium, or by saturating the hydrate with carbonic anhydride, and in either case washing and drying the precipitate. Native carbonate of barium (witherite) is ordered in the pharmacopœias, and is sufficiently pure for making the barium salts, the only purpose to which it is therein applied.
Uses. In pharmacy, &c., chiefly to prepare barium salts. In chemistry, to separate certain metallic oxides when occurring together in solutions. In the arts, as a base for certain delicate colours, as an ingredient in plate-glass, in the manufacture of beet sugar, &c. It is not used in medicine. It is extremely poisonous.
Barium, Bisulphide. This substance may be obtained as a fine yellow-coloured product by shaking a solution of barium chloride with a mixture of ammonium sulphide and carbon disulphide. It is insoluble in alcohol, soluble in water, and rapidly dissolved by slightly acidulated water.
Barium, Chloride of. BaCl2.2Aq. Syn. Chloride of barium; Barii chloridum, L.; Chlorure de baryum, Chlorhydrate de baryte, &c., Fr.; Salzsäure schwererde, Chlorbarium, Ger. Neutralise a hot dilute solution of hydrochloric acid with carbonate of barium, evaporate down, and crystallise. Sulphide of barium can be substituted for the carbonate. If required chemically pure, gaseous hydrochloric acid is transmitted through a concentrated solution of common or impure chloride of barium, as long as a precipitate forms; the resulting crystalline powder, which is nearly the whole of the chloride of barium present, is collected on a filter, and, after draining, is washed repeatedly with small quantities of pure hydrochloric acid, until the washings, diluted with water, and precipitated with sulphuric acid, give a filtrate which, upon evaporation in a platinum capsule or a watch-glass, leaves no residue; the last traces of acid having been removed by a little alcohol applied in a like manner, the powder is at once dried, and then carefully preserved from the air.—Used in analysis.
Prop., &c. Crystals, flat, four-sided tables, colourless and transparent; sometimes double eight-sided pyramids; slightly efflorescent in dry warm air, but otherwise permanent; decrepitate when heated, and lose their water of crystallisation; fuse at a red heat; volatilise at a white heat; insoluble in hydrochloric acid and in alcohol, slightly soluble in rectified spirit, and very soluble in water; water at 60° dissolves 431⁄2% of the crystals, and nearly 37% of the dry salt; and when boiling 75% of the former, and about 66% of the latter; a saturated boiling solution (223° Fahr.) contains 100 parts of water, and 78 parts of the crystallised salt.
The crystals contain 2 atoms of water; and a formula of BaCl2 + 2Aq.
Uses, Phys. eff., &c. In chemistry, it is employed as a test for sulphuric acid and the soluble sulphates. In medicine, it has been employed, both internally and externally, as an alterative, resolvent, and deobstruent, in scrofula, glandular swellings, and enlargements,246 scirrhous cancer, skin diseases, &c.; and more particularly in the first with marked benefit. In large doses it is poisonous. According to Sir B. Brodie, its action on animals is analogous to that of arsenic. Locally, it acts as an irritant. A very weak solution, used as a lotion, often proves serviceable in herpetic eruptions, and as a collyrium in scrofulous ophthalmia. Dose, 1⁄2 gr. thrice a day, in water, gradually increased to 2 or 3 gr.
Barium, Chlorate of. Ba(ClO3)2. Syn. Chlorate of bary′ta; Bary′tæ chlo′′ras, L. Prep. From a solution of chloric acid neutralised with freshly precipitated carbonate of barium; the resulting solution, after filtration, being crystallised by evaporation.
By passing chlorine through strong milk of hydrate or of carbonate of barium, in the same way as in making chlorate of potassium.
Prop., &c. Soluble in 4 parts of cold water. Used in pyrotechny, and to make chloric acid.
Barium, Ferrocy′anide of. Ba2FeC6N6. Syn. Ba′′rii ferrocyani′dum, L. From pure ferrocyanide of iron digested in baryta water. By careful evaporation, efflorescent prismatic crystals may be obtained, soluble in 41⁄2 parts of water.
Barium, Fluoride of. BaF2. Syn. Ba′′rii fluori′dum, &c., L. A white powder, formed by digesting freshly precipitated carbonate of barium in hydrofluoric acid, in excess.
Barium, Hydrate of. Ba(HO)2. Syn. Hydrate of baryta; Barytæ hydras, L. Prep. By digesting caustic baryta, or barium oxide, with a little water, or igniting gently the crystallised hydrate. It can be obtained crystallised as follows:
1. From a concentrated solution of either nitrate or chloride of barium, precipitated with a rather strong solution of pure potassa, or of pure soda, perfectly free from carbonic acid.
2. A strong solution of sulphide of barium is boiled with successive portions of black oxide of copper, until it ceases to give a black precipitate with a salt of lead; the liquid, after filtration, yields crystals of the hydrate on cooling.
Prop., Uses, &c. Forms a bulky white powder, containing 101⁄2% of water of hydration, which it retains even after ignition. In this state it is soluble in 20 parts of cold water, and in 2 parts of boiling water. The hot saturated solution, as it cools, deposits abundantly columnar crystals (CRYS′TALLISED HYDRATE OF B.), which contain 511⁄2% of water, of which they lose, by drying and ignition, 883⁄4% (= 43⁄4% of their weight), being reduced to the state of the common or amorphous hydrate. Of all the bases it has the strongest affinity for both sulphuric and carbonic acid, and hence its solution (BARY′TA-WATER) and those of its neutral salts (nitrate or chloride) form our most sensitive tests for these substances. Sp. gr. 4·3 to 4·7. The crystallised hydrate is converted into the ordinary hydrate at a gentle heat, and this last fuses at a low red heat without losing its water of hydration, which it only slowly and with difficulty begins to part with at higher temperatures. In chemistry, its uses are, for the most part, similar to those of Barium, Oxide of.
Barium, Iodide of. Ba2I. Syn. Ba′′rii iodi′dum, L.; Iodure de baryum, &c., Fr.
Prep. 1. Dissolve sulphide of barium in water, and add iodine (gradually) in excess; after the reaction is complete, filter, and either evaporate to dryness, or crystallise.
2. Digest freshly precipitated carbonate of barium, in excess, in a hot solution of protiodide of iron; filter and evaporate to dryness; then re-dissolve and crystallise.
3. By saturating hydriodic acid with oxide or carbonate of barium.
Prop., &c. A white or greyish-white mass, or acicular crystals (according to the mode of its preparation); very soluble in water and in alcohol; and decomposed by exposure to the air. It has been highly recommended as an alterative, resolvent, and liquefacient, particularly in scrofula, glandular swellings, chronic inflammations, and the other affections in which chloride of barium and iodine are given.—Dose, 1⁄12th to 1⁄8th gr. (gradually and cautiously increased to 1 gr.), in distilled water, 2 or 3 times a day. Externally, as an ointment (3 or 4 gr., to lard, 1 oz.), as an application to scrofulous swellings. (Biett.) It possesses all the irritant, corrosive, and poisonous properties of the chloride, but in a much more violent degree.
Barium, Nitrate of. Ba(NO3)2. Syn. Nitrate of baryta; Bary′tæ ni′tras, L. Prep. As the acetate or chloride of barium, substituting pure nitric acid for acetic or hydrochloric acid.
Prop., &c. Transparent, colourless octahedrons, which are anhydrous, insoluble in alcohol, and require about 8 parts of cold water, and about 3 parts of boiling water, for solution.
Uses. In chemistry, to prepare baryta, and as a test for sulphuric acid and the soluble sulphates; and in pyrotechny, to give a green tinge to flame.
Barium, Oxalate of. BaC2O4. Syn. Ox′alate of baryta; Bary′tæ ox′alas, L. Prep. By precipitating a barium salt with oxalate of ammonium. Very nearly insoluble.
Barium, Oxide of. BaO. Syn. Baryta, Bary′tes, Cau′stic baryta*, Ox′ide of ba′′rium, Protox′ide of b., Heav′y earth; Baryte, Oxide de barium, Terre pesante†, &c., Fr.; Baryt, Baryterde, Schwererde, &c., Ger. One of the earths discovered by Scheele in 1774.
Sources. Sulphate and carbonate of barium are abundant minerals, forming the ‘vein-stone’ of many lead mines. It is from the247 latter that baryta and the barium salts are almost exclusively obtained.
Prep. 1. A mixture of carbonate of barium and charcoal (both in fine powder and moistened) is strongly ignited, for some time, in a porcelain, Hessian, or black-lead crucible, and then allowed to cool out of contact with the air, from which it must also be subsequently carefully preserved.
2. (Pure.) Crystallised nitrate of barium is calcined in a capacious covered porcelain or Hessian crucible, at a bright red heat, until red (nitrous) vapours are no longer disengaged, even on raising the temperature; and the residuum, as soon as the temperature has fallen sufficiently, but whilst still warm, is at once transferred to a bottle, as before.
3. M. Rosenthiel’s process is founded upon the decomposition of sulphide of barium dissolved in boiling water by oxide of zinc. Caustic baryta and sulphate of zinc are formed.
Prop. A greyish-white, spongy, earthy-looking mass, fusible only before the oxyhydrogen blowpipe; highly caustic, corrosive, and alkaline, and slaking, like quick-lime, on the addition of water, but with the evolution of more heat.
Barium, Peroxide of. BaO2. Syn. Deutox′ide of barium; Ba′′rii binox′ydum, &c. L.; Binoxide de baryum, &c., Fr. Prep. Pure baryta is heated to full redness in a porcelain tube, and a stream of pure dry oxygen passed over it as long as the gas is absorbed.
Baryta, 4 parts, is heated as above in a platinum crucible, and chlorate of potassium, 1 part, gradually added to it; the chloride of potassium formed along with the binoxide being afterwards washed away with cold water.
Prop., &c. Grey or greyish-white; with water it forms a hydrate, which is slightly soluble in water, and undecomposed by it in the cold. It is interesting chiefly in its relations with peroxide of hydrogen and the oxygenised acids of M. Thénard.
Barium, Phosphate of. Ba(PO4)2. Prep. In a similar manner to the oxalate, which it resembles in being an almost insoluble white powder.
Barium, Sulphate of. BaSO4. Syn. Sulphate of baryta, Heav′y spar, Bolo′′gnian s., Cawk (mi); Bary′tæ sul′phas (Ph. E. & D.), Spa′thium pondero′sum, &c., L.; Sulfate de baryte, Spath pesant, &c., Fr.; Schwefelsaures baryt, Schwerspath, &c., Ger. This salt is found native, often in beautiful tabular crystals, but more frequently in white or reddish-white masses. It is also occasionally prepared artificially, as a pigment and chemical, by decomposing a solution of chloride of barium with dilute sulphuric acid, or with a solution of sulphate of sodium; the resulting precipitate being collected, well washed, and dried.
Prop., &c. When pure, or free from iron, its powder is white. It is insoluble in water, and nearly insoluble in all other menstrua; before the blowpipe it decrepitates, fuses with great difficulty (by which it is distinguished from the sulphates of strontium and calcium), and ultimately melts into a hard, white enamel. Mixed with charcoal, and heated to redness in a covered crucible, it is reduced to sulphide of barium. It is readily decomposed by fusion with alkaline carbonates; also very slightly so by their cold solutions; but ultimately completely, though slowly, by their boiling solutions. Sp. gr. 4·3 to 4·75.
Uses. Chiefly as a pigment (PER′MANENT WHITE), and to adulterate white-lead; for which purposes the native sulphate is commonly well washed, first in very dilute sulphuric acid, and afterwards in pure water, to remove any iron which may contaminate it, and impair its whiteness. It is also used to form sulphide of barium; and, in pyrotechny, instead of the more expensive nitrate.
Barium, Sulphide of. BaS. Syn. Sul′phide of barium, Sul′phuret of baryta; Ba′′rii sulphure′tum, &c., L.; Sulfure de baryum, &c., Fr. Prep. Sulphate of barium, well dried and in fine powder, 3 parts; powdered charcoal or powdered coal, 1 part; the mixture is pressed tightly into an earthen crucible, and the cover being fitted on, it is exposed for 11⁄2 to 2 hours, to a bright red heat; after it has cooled, the black mass thus obtained is powdered, and boiled in water, and the resulting solution allowed to crystallise. Some authorities recommend forming the mixed powders into a stiff paste with oil, or oil of turpentine, before calcination; but this is not at all necessary.
Prop., Uses, &c. Crystals, thin and nearly colourless plates, containing combined water; very soluble in hot water, less so in cold water; and rapidly decomposed by exposure to the air. It is principally used to form the BARIUM SALTS, and in organic analysis. Care should be taken in its preparation to expose the solution to the air as little as possible. Sulphides of a higher grade may be formed by boiling this compound with sulphur; but they possess little practical interest.
Barium, Sulphite of. BaSO3. Syn. Sul′phite of baryta. Prep. By testing a soluble barium salt with sodium sulphite, and washing the precipitate. Insoluble.
Barium, Tartrate of. BaC4H4O6. Syn. Tar′trate of baryta. Prep. Like that of oxalate of barium. White powder. Slightly soluble.
BARK. [Eng., Dan.] Syn. Cor′tex, L.; Écorce, Fr.; Baumrinde, Rinde, Ger. The rind or exterior covering of vegetables, corresponding to the skin of animals. It consists of the—cu′ticle or epiderm′is—cellular substance, containing colouring matter, &c., and—li′ber, the inner or true bark. The last is formed of woody fibre in great quantity, intermixed with cellular tissue. At the commencement248 of the annual growth of a tree, the bark separates spontaneously from the wood, in order to make room for the new matter forming beneath. It thus increases by yearly layers, and gradually perishes on the outside, owing to distension, from the growth of the interior portion. Its physiological uses are numerous and important. It is the depository of many of the secretions of plants, and it acts as a living filter, separating secretions from each other, and allowing a part of them to pass off horizontally through the medullary processes on their way to the centre of the tree. But its principal offices appear to be to act as a protection to the tender wood, and as a channel for the sap in its descent from the leaves. “True bark only exists in exogens and gymnosperms; in endogens its place is supplied by cortical integuments, which cannot be separated from the adjacent wood, without violence.” (Lindley.)
According to Liebig, the characteristic ingredients found in bark are excrementitious—“substances evidently expelled by the living organism.” True wood yields only ·25% to 2% of ash; whilst the bark of some trees give 6, 10, to 15 times more; and these, like the organic constituents, differ materially in their composition and characters.
The uses of different species of bark in medicine and the arts are well known. Cinchona-bark is invaluable in fevers; OAK-BARK furnishes the tanner with one of the most important materials of his trade; and the tenacious fibres of other varieties are manufactured into cordage and textile fabrics.
Barks should be collected at that season in which they can be most easily separated from the wood, which, with a few exceptions, is late in the spring; because at this time the active principles deposited in their cells are most abundant. Oak-bark, collected in spring, contains four times as much astringent matter as that collected in winter.
Bark. (In medicine.) See Cinchona.
Bark. (In tanning.) See Oak.
Bark, Jes′uit’s. Cinchona-bark.
Bark, Salt of (Essential). See Extracts and Salts.
BAR′LEY. Syn. Hor′deum, L.; Orge, Fr.; Gerste, Ger., Anglo-S. A well-known grain, the produce of several species of the genus hordeum.
Var., Cult., &c. Those principally cultivated in England are—TWO′-ROWED, LONG′-EARED, or COMM′ON BARLEY (hor′deum dis′tichon, Linn.); SPRING′-BARLEY, SQUARE′-B., or BERE (h. vulga′′re, Linn.); and SIX′-ROWED BARLEY, WINTER B., Scotch BERE or BIGG (h. hexas′tichon, Linn.). Put′ney, SPRAT, or BATT′LEDORE B. (h. zeocriton, Linn.), is another species less frequently met with. Of each of the above there are several varieties. In Spain and Sicily, two crops of barley are obtained in a year; but, in countries so far north as Britain, it produces only one, and is a delicate species of grain. In England it is generally adopted as a succession crop on light lands, following turnips or green crops. (Loudon.) The ‘yield’ per acre varies from 28 to 64 bushels, and is usually from 28 to 40 bushels. The average weight per bushel is 50 to 51 lbs.; but the best Norfolk and Essex samples weigh 53 to 54 lbs. per bushel.
Comp. The leading constituents of barley are nearly similar to those of wheat, but it is scarcely so rich in nitrogenised matter. According to Einhof, the ripe SEEDS or GRAINS are composed of—
| Meal | 70·05 |
| Husk | 18·75 |
| Moisture | 11·20 |
| ——— | |
| 100· |
According to Johnston, average fine BARLEY-MEAL contains—
| Starch | 68· |
| Albumen, gluten, &c. | 14· |
| Fatty matter | 2· |
| Ash or saline matter | 2· |
| Water | 14· |
| ——— | |
| 100· |
According to Payen, dried barley possesses the following composition—
| Nitrogenous matter | 12·96 |
| Starch | 66·43 |
| Dextrin | 10·00 |
| Fatty matter | 2·76 |
| Cellulose | 4·75 |
| Mineral matter | 3·10 |
| ——— | |
| 100·00 |
According to Dr Ure, the sp. gr. of English barley is 1·25 to 1·33 (average, 1·235), and the weight of the husk is about 1-6th; that of BIGG, 1·227 to 1·265, and weight of husk, 2-9ths.
The analyses of the following varieties of barley, gave as the composition of the ashes of the grains:—
| Unknown | Chevalier Barley | From Moldavia | Chevalier Barley | |
| Potash | 21·14 | 20·77 | 37·55 | 7·70 |
| Soda | 4·56 | 1·06 | 0·36 | |
| Lime | 1·65 | 1·48 | 1·21 | 10·36 |
| Magnesia | 7·26 | 7·45 | 10·17 | 1·26 |
| Sesquioxide of iron | 2·13 | 0·51 | 1·02 | 1·46 |
| Sulphuric acid | 1·91 | 0·79 | 0·27 | 2·99 |
| Silica | 30·68 | 32·73 | 24·56 | 70·77 |
| Phosphoric acid | 28·53 | 31·69 | 38·64 | 1·99 |
| Chloride of Sodium | 1·10 | 1·47 | 1·10 |
249
In the ‘Journal of the Agricultural Society’ for 1873 is a report by Messrs Lawes and Gilbert of twenty years’ experiments with barley. The soil of a field at Rothampstead, in which the barley had been grown for twenty years, consisted of heavy loam, with a subsoil of clay resting on chalk, and was previous to the barley being planted almost exhausted by cropping. The produce was found to be greatest during the absence of drought and sudden alterations of temperature, the rather cool but uniform season of 1854 giving the heaviest crops. The yield from farm-yard manure and nitrate of soda was found in dry seasons to be rather larger than that from ammonia salts. Barley manured with phosphates was found to ripen one to two weeks earlier than when the phosphate was omitted.
The average produce per acre of a few of the principal plots is given below. The “ammonium salts” are stated to be a mixture of equal parts of sulphate and chloride; the “alkali salts” consist of the sulphates of potassium, sodium, and magnesium; the “cinerials” consist of alkali salts, plus superphosphate:
| Manures per Acre. | A | B | C | D | E | F |
| Unmanured. | 20 | 113⁄4 | 2454 | 86·6 | 52·3 | -23·6 |
| Mixed cinerials. | 271⁄2 | 143⁄8 | 3162 | 96·4 | 53·4 | -20·2 |
| Ammonium salts, 200 lbs. | 321⁄2 | 181⁄2 | 3919 | 89·2 | 52·1 | -9·7 |
| Ammonium salts, 200 lbs., and alkali salts. | 35 | 203⁄4 | 4317 | 86·3 | 52·8 | -5·3 |
| Ammonium salts, 200 lbs., and superphosphate. | 47 | 275⁄8 | 5760 | 86·8 | 53·5 | +2·7 |
| Ammonium salts, 200 lbs., and cinerials. | 461⁄4 | 281⁄2 | 5817 | 83·2 | 54·0 | -·3 |
| Rape cake (mean 1300 lbs.) | 451⁄4 | 267⁄8 | 5571 | 87·3 | 53·8 | |
| Farmyard manure, 14 tons. | 481⁄4 | 281⁄4 | 5933 | 88·5 | 54·3 | +14·8 |
The authors direct attention to the results obtained by using the cinerial manure alone, as illustrating the unsoundness of the old “mineral theory,” according to which plants were supposed to possess a sufficient source of nitrogen in the atmosphere. They found a greater crop yielded by barley than wheat, when no manures were employed, as well as when cinerials were employed, a fact which they attribute to barley being better able than wheat to supply itself with nitrogen, notwithstanding the deeper roots of the latter. They state that with both wheat and barley the produce is slowly falling off under these circumstances. With ammonium salts alone, and with nitrate of sodium alone, there is much less falling off than when no nitrogenous manure is used. The falling off was least with the nitrate. The nitrate gives a rather larger crop for the same amount of nitrogen supplied, and they found this to hold when both nitrate and ammonia are applied with cinerials. The addition of superphosphate to ammonium salts or sodium nitrate greatly increases the produce; the further addition of potassium, sodium, and magnesium salts they found almost without effect.
The inference was that the barley had obtained an ample supply of potash from the natural soil, but an insufficient supply of phosphoric acid.
When ammonium salts are used alone, and the quantity of ammonia does not exceed 50 lbs. per acre, 3·68 lbs. of ammonia will yield an average increase of 1 bushel of corn and 63 lbs. of straw—total, 115 lbs.; the extremes in 20 years were 2·25-18·05 lbs. When ammonium salts are applied with superphosphate, 2·21 lbs. of ammonia will produce the same result; the extremes were 1·47-5·36 lbs.
Silicate of sodium had been applied for eight years and a half to half the barley plots receiving ammonia; no increase has resulted where ammonia and superphosphate are employed; but on the other three plots an increase had taken place, which, in the case of the plot receiving only ammonia and alkali salts, is very considerable.
The authors think this irregular reaction seems to show that the silicate has not produced its effect by furnishing silica to the crop, but by some reaction upon the plant-food of the soil. The rape cake supplied much more nitrogen than the ammonium salts, and also some phosphates and potash. Rape cake alone gives a better return than either ammonium salts or sodium nitrate applied alone; but when the three manures are mixed with superphosphate, the results for equal amounts of nitrogen show the rape cake to be decidedly inferior. From the above experiments it is inferred that a supply of carbonaceous matter does not increase the crop of barley.
A farm-yard manure containing about 0·64 per cent. of nitrogen supplied far more plant food than any of the other manures. On an250 average of twenty years it was found that about 8 lbs. of ammonia in the form of dung would produce a bushel of barley, with its equivalent of straw.
In all cases which were comparable it was found that barley appropriates more of the nitrogenous manure than wheat, save with farmyard manure. A large amount of nitrogen applied by manure is not taken up by the crop. Experiments in the barley field proved that large residues from ammonium salts and sodium nitrate show a small but distinct effect upon succeeding crops, the influence extending over many years. From an examination of the drainage waters from lands dressed with the nitrates of ammonium and sodium, the authors conclude that ammonium salts, as well as sodium nitrate, will be more economically applied in the spring than in the winter. Manures containing organic nitrogen are clearly not so liable to loss from drainage.
Experiments were made on the growth of barley after turnips, and also in an ordinary four-course rotation. After growing turnips ten years consecutively with purely cinerial manures, and carting off the produce, the yield of barley was much smaller than in the experimental field, where barley was grown after barley. The turnips, though very small crops, had exhausted the soil of nitrogen to a greater extent than corn crops would have done. On one plot where rape cake had been applied to the turnips, the produce of barley was 81⁄4 bushels more than when none had been used. In the rotation experiments barley was grown after turnips (carted off), and was followed by beans and wheat. In one series all the crops were unmanured; in another the turnips received superphosphate; in a third the turnips received an abundant cinerial and nitrogenous manure.
The mean produce of the six crops of barley obtained in twenty-four years of rotation was as follows:
| Character of Rotation. | Dressed Corn. | Straw and Chaff. |
| bushels. | cwt. | |
| Unmanured continuously | 383⁄8 | 213⁄4 |
| Superphosphate for turnips only | 293⁄8 | 161⁄2 |
| Mixed manure for turnips only | 443⁄8 | 251⁄4 |
| Mean produce of unmanured barley in barley field during the same season | 211⁄2 | 121⁄8 |
The unmanured turnips were so very small in quantity, that the barley in the first series was practically grown after a fallow; this barley, however, was a much larger crop than that grown after turnips manured with superphosphate only, the available nitrogen of the soil in this case being exhausted by the turnips.
In the last series the residue of the abundant manure applied to the turnip crop suffices to produce a good crop of barley.
Qual., Uses, &c. Its employment and value as food, and in the manufacture of malt, are well known. It forms good wholesome bread well adapted for persons who live luxuriously; but which, for the abstemious and the delicate, is inferior to that made of wheat, as it is rather less nutritious, and less easy of digestion, and commonly proves laxative to those unaccustomed to its use. Barley-flour and barley-meal are also more perishable than wheat-flour; being very apt to acquire a hot nauseous taste, which even the heat of the oven does not remove. In a medical point of view, barley is regarded as the mildest and least irritating of the cereals. It has always been in high estimation as a demulcent and emollient. The decoction (BAR′LEY-WATER), made with pearl barley, is a common and useful drink in inflammatory diseases, particularly in those of the chest and urinary organs. Among the Ancients, decoctions of barley (κραθη) were the principal aliments and medicines employed in acute diseases.
Barley was extensively cultivated by the Romans and many other nations of antiquity, as well as by the ancient inhabitants of Gaul. The Greeks are said to have trained their athletes on it.
The best tests of the genuineness of barley are its colour, freedom from dust, grit, and insects. The microscope will lead to the detection of any cheaper grains if mixed with it. It is rarely adulterated, although it is said to be extensively used for the purpose of sophisticating wheat, annatto, and roll liquorice.
 Barley Starch.
Barley Starch.
Barley, Cau′stic. Sabadilla.
Barley, Pat′ent. Syn. Fari′na hor′dei, L. Pearl barley reduced to fine powder by grinding in a mill.
Barley, Pearl. Syn. Pearl′ed barley*; Hor′deum decorticatum (B. P.), L.; Orge perlé, Fr.; Perlengraupen, Ger. The seeds of hordeum distichon deprived of the husks.251 That of commerce is usually made by steaming spring-barley, to soften the skin, then drying it, and grinding it in a mill with the stones set wide apart, so as to round and polish the grains, and to separate the whole of the husk except that left in the furrow of the seed. Scotch pearl-barley and French barley resemble the last, but are smaller, being generally made from winter-barley or bigg. Faro de Orzo is another variety made from sprat-barley. See Barley (above).
Barley, Scotch. Syn. Hulled barley‡, Pot-b.‡; Hor′deum munda′tum, L.; Orge mondé, Fr.; Gerstengraupen, Graupen, Ger. The grains deprived of the husk by a mill, as noticed above, but less completely, and without rounding them.
BAR′LEY SUGAR. See Confectionery, and Sugar.
BARM. See Yeast.
BAROM′ETER (baros, weight; metron, measure). Syn. Weather-glass‡; Barom′etrum, L.; Baromètre, Fr.; Barometer, Wetterglas, Ger. An instrument for measuring the weight or pressure of the atmosphere. It was invented by Torricelli, of Florence, A.D. 1643.
The barometer is made of several forms, but the principle of its construction, with the exception of the aneroid barometer, is the same in each, and essentially consists of a column of fluid (usually mercury) supported in vacuo, in a glass tube, by the pressure of the atmosphere on its surface. The annexed figures exhibit the principal varieties at present known; several of which have been proposed with the view of improving the original instrument, either by increasing its range, or its portability. None, however, equal in simplicity, cheapness, and usefulness, the old forms proposed by Torricelli, and represented by the figs. 1 & 2. To avoid confusion, the graduated scales and cases of the instruments are not shown.
Of the many forms of mercurial barometer, that perhaps known as Fortin’s is the best. In this instrument the cistern and the lower portion of the tubes is shown in the annexed figure.252

“The cistern is made of boxwood, with a movable leather bottom b b, and a glass cylinder, b, is inserted into it above, all except the glass being encased in brass. In the bottom of the brass box a screw, C, works on the upper end of which the leather rests, so that by elevating or depressing this screw, the bottom of the cistern, and with it the cistern level of the mercury, can also be raised or depressed at pleasure. A small ivory pin, p, ending in a point is fixed to the upper frame of the cistern, and when an observation is made, the surface of the mercury is made to coincide with the point of the pin as the standard level from which the barometric column is to be measured. The tube of the barometer, the upper part of which is shown in the lower figure, is enclosed in one of brass, which has two directly opposite slits in it for showing the height of the column, and on the sides of these the graduation is marked. A brass collar, d, d, slides upon the tube with a vernier, v, v, marked on it for reading the height with the greatest exactness and in which two oblong holes are cut, a little wider than the slits in the brass tube. When a reading is taken the collar is so placed that the last streak of light is cut off by the two upper edges of the holes or until they form a tangent to the convex mercurial curve. By this means the observer is sure that his eye is on a level with the top of the column and that the reading is taken exactly for this point. Fortin’s barometer is generally arranged so as to be portable, in which case the screw, c, is sent in until the mercury fills the whole cistern, by which the air is kept from entering the tube during transport, the leather yielding sufficiently at the same time to allow for expansion for increase of temperature. It packs in a case which serves as a tripod when the instrument is mounted for use. On this tripod it is suspended about the middle, swinging upon two axles at right angles to each other, so that the cistern may act the part of a plummet, in keeping the tube vertical—the position essential to all measurements.”[107]
How to Manage a Barometer.—It is of the first importance to have the instrument hung perfectly perpendicular. This is best effected by means of a plummet line. It should be placed in a good light, but protected from direct sunlight and also from rain. If air should accidentally find its way into a common cistern barometer, it may be got rid of by first fixing the ivory piston, so as to prevent the escape of the mercury, then by means of the screw raising the mercurial column nearly to the top of the tube, then by slowly inverting the instrument and tapping the cistern gently, the air may then perhaps ascend to the cistern and thus escape. In transporting a barometer from place to place it is best to carry it by hand; and if packed it is almost needless to say that the float must be firmly fixed and the mercurial column raised by means of the screw, so as to prevent any escape of the metal.
Reading the Barometer.—The mercury in the cistern must first be brought by means of the screw to the ‘zero,’ and then the vernier must be screwed up so that its horizontal edge forms a tangent to the mercurial curve. The vernier is an instrument for reading off the graduated scale of the barometer correctly to 1⁄100th or 1⁄500th of an inch.
Buchan gives the following description of the vernier and of the method of using it: “It consists (see figures a and b) of a piece similar to the scale of the barometer along which it slides. It will be observed from figure a that ten divisions of the vernier are exactly equal to eleven divisions of the scale, that is, to eleven tenths of an inch. Hence each division of the vernier is equal to a tenth of an inch, together with a tenth of a tenth, or a hundredth, or to ten hundredths, and one hundredth, that is, to eleven hundredths of an inch. Similarly two divisions of the vernier are equal to twenty-two hundredths of an inch, which expressed as a decimal fraction is 0·22 inch, three divisions of the vernier is 0·33 inch, &c. Suppose the vernier set as previously described—that is, having the zero line of the vernier a tangent to the convex curve of the253 mercury in the column. If the vernier and scale occupy the relative positions as in figure a, then the height of the barometer is 30·00 inches, but if they stand as in figure b, we set about reading it in this way: (1) The zero of the vernier being between 29 and 30, the reading is more than 29 inches, but less than 30 inches, and we obtain the first figure 29 inches. (2) Counting the tenths of an inch from 29 upwards we find that the vernier indicates more than seven tenths and less than eight tenths, giving the second figure seven tenths or 0·7 inch. (3) Casting the eye down the scale to see the point at which a division of the scale and a division of the vernier lie in one and the same straight line, we observe this to take place at line 9 of the vernier; this gives this last figure nine hundredths or 0·09 inch, and placing all these figures in one line we find that the height of the barometer is 29·79 inches. This sort of vernier gives readings true to the hundredth of an inch. If the inch be divided into half tenths or twentieths, and twenty-five divisions of the vernier equal twenty-four divisions of the scale, it follows that the difference of these divisions is two thousandths of an inch.”

A still more divided vernier is always used with the best barometers, and though a little troublesome to read at first, yet if the method of reading the simpler one just described be understood, the difficulty will be easily overcome.
Uses, &c. The barometer is employed for ascertaining the amount of atmospherical refraction in astronomical calculations, for measuring altitudes, and in prognosticating the weather. For the last purpose, on land, it sometimes proves a false prophet; but at sea, its monitions are highly trustworthy. As a mere weather-glass, the indications, as read off from the scale of the instrument, are generally sufficiently accurate; but in all observations connected with meteorology, altitudes, astronomy, &c., certain corrections must be made; the height of the mercury being influenced both by the size of the tube and by the temperature of the air by which it is surrounded, as well as by variations in the weight or pressure of the atmosphere. (See below.)
Barometrical Corrections:—
1. As to CAPILLARITY:—This applies to all cistern-barometers formed of tubes of very small diameters, owing to the mercury assuming a convex surface in the tube. As the tube increases in diameter, so the depression of the mercury lessens. Hence, the “interior diameter” of a barometer “should, in every case, exceed one-fourth of an inch.” (Brande.) Syphon barometers that have each of their legs of equal size, require no correction, as the depression is equal at both ends.
| Diam. of Tube. | Depression. | ||
| ·10 | inch. | ·1403 | inch. + |
| ·15 | ” | ·0863 | ” |
| ·20 | ” | ·0581 | ” |
| ·25 | ” | ·0407 | ” |
| ·30 | ” | ·0292 | ” |
| ·35 | ” | ·0211 | ” |
| ·40 | ” | ·0153 | ” |
| ·45 | ” | ·0112 | ” |
| ·50 | ” | ·0083 | ” |
| ·60 | ” | ·0044 | ” |
| ·70 | ” | ·0023 | ” |
| ·80 | ” | ·0012 | ” |
2. As to TEMPERATURE:—These depend on the expansion of the mercury, and of the scale on which the divisions are marked. The rule for reducing an observed height to the corresponding height at the freezing-point, or 32° Fahr., the usual standard temperature, is—Subtract 1·10000th part of the observed height of the barometer for every degree of Fahr. above 32° at the time of the observation. Or—
Measurement of Heights by the Barometer.—When a barometer is at the foot of a mountain, the pressure it sustains is greater than that to which it is subjected at the top, by the weight of the column of air intervening between the top and the bottom.
The height can be obtained from the following table by calculating the number of feet which must have been ascended to cause the observed fall; and then making a correction for temperature by multiplying the number obtained from the table, which may be called A, by the following formula: t is the temperature of the lower and t′ of the upper station:—
1 × ((t + t′ - 64) / ·900) × A.
254
| To lower the | barometer | from | 31 | in. to | 30 | = | 857 | feet must | be ascended. |
| ” | ” | ” | 30 | ” | 29 | = | 886 | ” | ” |
| ” | ” | ” | 29 | ” | 28 | = | 918 | ” | ” |
| ” | ” | ” | 28 | ” | 27 | = | 951 | ” | ” |
| ” | ” | ” | 27 | ” | 26 | = | 986 | ” | ” |
| ” | ” | ” | 26 | ” | 25 | = | 1025 | ” | ” |
| ” | ” | ” | 25 | ” | 24 | = | 1068 | ” | ” |
| ” | ” | ” | 24 | ” | 23 | = | 1113 | ” | ” |
| ” | ” | ” | 23 | ” | 22 | = | 1161 | ” | ” |
| ” | ” | ” | 22 | ” | 21 | = | 1216 | ” | ” |
| ” | ” | ” | 21 | ” | 20 | = | 1276 | ” | ” |
| ” | ” | ” | 20 | ” | 19 | = | 1341 | ” | ” |
| ” | ” | ” | 19 | ” | 18 | = | 1413 | ” | ” |
A very complex formula is given by mathematicians for finding very nearly the true height of a mountain from barometrical and thermometrical observations made at its base and summit. The following rule by Mr Ellis will be found to give very nearly the same results:—Multiply the difference of the barometric readings by 52,400, and divide by the sum of the barometric readings. If the result be 1000, 2000, 3000, 4000, or 5000, add 0, 0·2, 6, 14, respectively. Subtract 21⁄3rd times the difference of the temperature of the mercury. Multiply the remainder by a number obtained by adding 836 to the sum of the temperatures of the air and dividing by 900. A correction must also be given for latitude, which can be done by the annexed table.
| Latitude. | Factor. | Latitude. | Factor. |
| 80 | 0·99751 | 35 | 1·00090 |
| 75 | 0·99770 | 30 | 1·00265 |
| 70 | 0·99797 | 25 | 1·00170 |
| 65 | 0·99830 | 20 | 1·00203 |
| 60 | 0·99868 | 15 | 1·00230 |
| 55 | 0·99910 | 10 | 1·00249 |
| 50 | 0·99954 | 5 | 1·00261 |
| 45 | 1·00000 | 0 | 1·00265 |
| 40 | 1·00046 |
Fortin’s and Gay-Lussac’s barometers are employed for measuring heights. The aneroid can be used for altitudes reaching to 5000 feet. A delicate instrument will register for as small an ascent as 4 feet.
The Barometer as a Weather-glass.—Generally speaking when the mercurial column in the barometer falls, ‘rain’ is indicated, and ‘fair weather’ when it rises. When it continues steady, a continuance of the weather at the time is regarded as the forecast; when low, the weather is generally broken or bad; and when high, it is fair and settled. A storm is usually preceded by a sudden fall in the mercurial column, the violence of the storm being in proportion to the suddenness of the fall. An unsteady barometer indicates an unsettled condition of weather, whilst a gradual change in it indicates the approach of some permanent condition of it. The state and direction of the wind has also to be taken into consideration when studying the changes of the barometer, and forms an important element in the calculations of the meteorologist, each different wind indicating variations of weather. The connection between changes of weather and the pressure of the atmosphere does not seem to have been satisfactorily established.
One of the reasons assigned for the mercurial column in the barometer being lower in wet than in fine weather is that so long as aqueous matter remains in the air in the form of elastic vapour, its tension assists in supporting the barometric column, but that when this aqueous vapour is precipitated in the form of rain, this tension is lost or removed, and the column therefore falls.
The correspondence between wet and fine weather and an elevation and depression of the barometer seems, however, equally, if not more, dependent on the nature of the winds than on the preceding cause. “In western Europe, the south and south-western winds, which are the rain-bringing winds, are warm winds. Now, a column of warm air to be of the same weight as one of cold air must necessarily be higher, but this cannot well be the case in the atmosphere, for no sooner does the warm column rise by its lightness above the surrounding level of the upper surface of the aërial ocean, than it flows over and becomes nearly of the same height as the cold air around it. The interchange taking place less interruptedly, and consequently less slowly, in the higher strata than in those near the ground, it is some time before the equilibrium, thus disturbed, is restored; and meanwhile the barometer keeps low under the pressure of a rarer atmospheric column. On the other hand, the northerly and easterly winds, being comparatively cold and dry, are accompanied by fair weather and a high barometer. It is thus to the warmth, and not to the moisture of these winds, that the pressure is to be ascribed.”[108]
Barometer, An′eroid. An instrument invented, or at least perfected, by M. Vidi, of Paris, in which the pressure of the atmosphere is measured without the employment of a fluid, as in the ordinary barometer.[109] Externally, it255 somewhat resembles in appearance a carriage clock or a ship’s chronometer; internally, it consists of a small air-tight cylindrical box, formed of thin corrugated copper plates, and partially exhausted of air, the sides of which yield to the pressure of the atmosphere; the effect being regulated by a spring, multiplied by a system of levers, and ultimately recorded by the index on a graduated dial. Compensation for changes of temperature are self-effected, with almost perfect accuracy, by the elastic force of the spring being so adjusted to that of the air in the cylinder, that the loss of force in the one and the increased expansive force of the other shall, independently of changes of atmospheric pressure, preserve the lever in equilibrio.

The indications of the aneroid barometer closely correspond to those of the mercurial barometer at ordinary ranges; the differences never exceeding ·01 of an inch. It is so extremely sensitive that an ascent or descent of only a few feet is distinctly indicated by it; whilst its portability adapts it for service in situations for which an ordinary barometer is unfitted. On the other hand, it is liable to move by jerks, and the elasticity of the spring, and consequently the zero-point of the scale, has been found to be sometimes affected by time and a rough journey. On this account it is necessary to compare it occasionally with some standard mercurial barometer, to determine its amount or rate of variation, if any.
Barometer, Phi′al. This amusing philosophic toy is made by cutting off the rim and part of the neck of a common glass-phial with a file. The phial is then nearly filled with water, either pure or tinged blue or red; and the finger being placed on its mouth, it is inverted, and suspended in a vertical position by means of a piece of twine or wire, when the finger is withdrawn. (See engr.) In dry weather the under surface of the water remains level with the neck of the bottle, or even concave; in damp weather, on the contrary, a drop appears at the mouth and continues enlarging until it falls, and is then followed by another in the same way.
Barometer, Fitzroy. This, which is a very cheap instrument, is made on the syphon principle, but the cistern is formed by the lower limit, which is blown into a bulb.
Barometer, Port′able. The most accurate are those of Gay-Lussac and Bunten, and after them the aneroid. They should be set on universal joints, and well balanced. The common instrument made with a box and leather cistern seldom continues long correct.
Barometer, Wheel. The common form of the instrument having a dial-face and hands.
[For further information in connection with the above subject the reader is referred to the ‘articles’ Aneroid, Atmosphere, Gas, Heights, Steam, Storm-glass, Vapour, Weather, &c.]
BAR′OSCOPE† (-skōpe). [Eng., Fr.] Syn. Barosco′pium, L. A barometer; sometimes applied to the wheel-barometer of Hooke.
BAR′RAS. The concrete resinous exudation from the bark of fir-trees. That from pi′nus marit′ima is called GALIPOT.
BARSE. [Provincial.] The common perch.
BAR′WOOD. A red dye-wood imported from Angola and other parts of Africa. It closely resembles cam-wood and sanders-wood in its colouring matter being of a resinous nature, and scarcely soluble in water. In dyeing this difficulty is obviated by taking advantage of the strong affinity existing between it and the proto-salts of tin and iron. Thus, by strongly impregnating the goods with protochloride of tin, either with or without the addition of sumach, according to the shade of red desired, and then putting them into a boiling bath containing the rasped wood, the colour is rapidly given out and taken up, until the whole of the tin in the fibres of the cloth is saturated, and the goods become of a rich bright hue. In like manner the dark red of bandana handkerchiefs is commonly given by a mordant of acetate of iron followed by a boiling bath of this dye-stuff. See Dyeing, Mordants, &c.
BASALT′ (bă-sŏlt′). [Eng., Ger.] Syn. Basal′tes (-săl′-tēz), L.; Basalte, Fr. In geology, &c., a species of trap-rock, essentially composed of the minerals felspar and augite. It is of a fine compact texture, of a dark-green, grey, or black colour, and usually occurs in regular columns, of which the Giants’ Causeway and the Island of Staffa furnish magnificent examples. It is fusible; and when rapidly cooled forms a dark brittle glass; but when slowly cooled retains its original beauty and hardness almost unimpaired. Messrs Chance, Brothers, of Birmingham, have availed themselves of this property to apply it to decorative and ornamental purposes. Their process is to melt the material[110] in a reverberatory256 furnace, and, when sufficiently fluid, to pour it into red-hot moulds of sand encased in iron boxes. The corresponding adj. is BASALT′IC (-sōlt′-; BASAL′TICUS, -săl′-, L.; BASALTIQUE, Fr.).
BASE. [Eng., Fr.] Syn. Ba′sis (pl., ba′ses), L., Gr.; Grund, Grundfläche, Ger. In chemistry it was formerly, and is now occasionally, applied to metallic oxides which possess the property of forming salts with acids. The alkaloids are also designated organic bases. In pharmacy, the characteristic or principal ingredient in any medicine or compound preparation; or that on which its qualities or efficacy depends.
BAS′IL (-băz′-). Syn. Sweet bas′il, Cit′ron b.; Basil′icum, L.; Basilic, Fr.; Basilikum, Ger. The oc′ymum (ŏs′-) basil′icum (Linn.), an annual aromatic herbaceous plant, of the nat. ord. Labiatæ (DC.). It is a native of India, but is largely cultivated in every part of Europe as a pot-herb. Leaves strong-scented; popularly reputed emmenagogue; much used to flavour salads, soups, &c., especially in French cookery. Mock-turtle soup derives its peculiar flavour from this herb; as also did the original Fetter-lane sausages, once so highly esteemed by cockney gourmands. In India it is commonly employed as an anodyne in childbirth.
Bas′il (băz′-). Syn. Bas′an; Basane, Fr. A sheep-skin, tanned; particularly one dressed on the grain side, for book-binding.
BASIL′ICON. See Cerates and Ointments.
BAS′KET (băs′-). Syn. Coph′inus (kŏf′-), L.; Panier, Corbeille, &c., Fr.; Korb, Ger. Baskets are generally STAINED or COLOURED with the simple liquid dyes used for straw or wood; and that, for variegated work, the twigs, after being carefully peeled, washed, and wiped dry or slightly air-dried, are stained before being woven. See Osiers, &c.
BASS‡. [Provin.] The linden-tree; also a hassock or mat made of its inner bark. See Bast.
Bassia butyracea. A tree growing in the sub-tropical Himalayas. The seeds yield by expression a concrete oil, known by the name of Fulwa Butter, which does not become rancid by keeping. It is held in high esteem in India as an external application in rheumatic and other painful maladies.
BAS′SORIN (-rĭn). Syn. Bassori′na, L. A substance first noticed, by Vauquelin, in Bas′sora-gum. See Gum, Insoluble, Tragacanthine, &c.
BAST (băst). Syn. Bass (which see). The inner bark of the linden tree or tiel tree; also matting, &c., made of it.
BAS′TARDS (-tărdz). Syn. Bas′tard Sug′ar (shŏŏg′-), Pieces, &c. In sugar-refining, impure or damaged sugar resulting from the heat and chemicals used in the process of manufacture, and which will not pay for purifying.
BA′′SYL (bāse′ĭl). In chemistry, any simple or compound body, acting as a basic radical.
BATATA [Convolvulus batatas, or Sweet Potato]. This is a native of the East Indies, but is now cultivated in all tropical and sub-tropical countries for the sake of its tubers, which are highly esteemed as an article of food. They are eaten either roasted or boiled, and are sweet, wholesome, and nutritious, although somewhat laxative.
In some parts of America the Batata, next to maize, forms the principal diet of the poorer classes. The plant was introduced into England by Sir Francis Drake and Sir John Hawkins; but they do not bear the cold of our winters, and if grown here are raised in hot-houses, where they may be obtained without difficulty varying from 1 lb. to 2 lbs. in weight. They thrive better in the south of Europe. The tubers contain about 32 per cent. of solid matter, 16 of which is starch, 10 sugar, 1·5 albumen, 1·1 gum, 0·3 fat, 2·9 mineral matter. The leaves are used as a boiled vegetable.
BATH (bahth). Syn. Bal′neum, L.; Bain, Fr.; Bad, Ger., Sax. A place for bathing; a vessel or receptacle, natural or artificial, containing or adapted to contain water, and used to bathe in. In architecture and hygiene, a building fitted up for and appropriated to bathing.
Constr., &c. Here one of the first subjects which must engage our attention is the selection of the material of which the bath is to be formed. For FIXED BATHS polished white marble has always been in favour, owing to its cleanliness and beauty. For this purpose, slabs of sufficient thickness and free from flaws or cracks should be chosen; and they should be securely and properly bedded in good water-tight cement, in a well-seasoned wooden case. The objections to marble, independent of its costliness, are, that it is apt to get yellow or discoloured, and to lose its polish, by frequent and careless use; and that the restoration of its surface to its original purity, is a matter of considerable expense and difficulty. It is also only fitted to contain water with, at the most, soap, weak alkalies or alkaline carbonates, aromatics, or neutral organic principles; and cannot be employed with water medicated, however slightly, with acids, sulphurets, iodine, chlorine, salines (others than those just named), or calorific substances. As a cheaper material thick slabs of Welsh slate are often substituted for marble; but even this substance is attacked by chemicals, though much more slowly. A lining of large Dutch tiles is sometimes used: but here the joints are very apt to leak. For baths adapted to all the requirements of health and disease, and which are at the same time durable and comparatively inexpensive, we must, therefore, seek further. Porcelain, glass, and hard glazed stone-ware have been proposed, and are even sometimes used for baths; but they possess the disadvantages of being fragile, and257 very liable to crack when filled with hot water in cold weather. Wedgewood-ware is very beautiful and durable; but is expensive, and baths formed of it can only be obtained on special order. Stourbridge-ware, as produced of late years, is the only product of the potter’s art that appears entirely to meet the case; but even this yields in durability to enamelled iron as a material for baths adapted to all liquids and temperatures, and to rough or careless usage. (See engr. 1.) The better qualities of PORT′ABLE BATHS (see engr. 2) are generally made of copper. Stout tinned or galvanised iron, and even stout block-tin thickly covered with waterproof paint or japan, are also employed; but though less expensive than copper, they have the disadvantage of being much less durable. All these substances are, however, readily acted on by chemicals. A durable and cheap portable bath, adapted to all purposes, must, therefore, like a fixed one, be made of one or other of the materials already noticed. For MED′ICATED BATHS large wooden troughs are frequently employed, particularly for acidulated, ioduretted, and sulphuretted baths.
 1.
1.
 2.
2.
The arrangements for supplying cold and hot water must necessarily greatly depend on circumstances, and the quantity required. For a single fixed bath, or even for two or three of them, the common circulating water-heater or boiler, placed in some apartment on a rather lower level than the bath, is, perhaps, the most convenient; but where this is not attainable the water may be run, by means of a pipe, from a boiler situated on a somewhat higher level. In either case a supply of cold water must also be at hand, and conveyed in a like manner, to enable the bath to be reduced to any required temperature. On the large scale, as in our public baths, where numerous baths are in constant use during the day, the hot water is best supplied from a large cistern somewhere above the level of the bath-rooms, and which is heated by a coil of pipe supplied with high-pressure steam from a boiler situated on a lower level, as the ground floor or basement. The hot and the cold water, conveyed by separate pipes of about 11⁄2 inch diameter, unite in a two-way cock close to the bath, so as to enter it together, by which only one aperture in the end of the bath is required for the purpose. The bath is emptied, and excess of water removed, by a grated aperture in the bottom, also stopped by a cock which, like the former, has handles or keys so placed as to be accessible to the attendant outside the bath-room, as well as to the bather, whilst the danger of overflowing is obviated by a two-inch waste-pipe, opening into the bath at about two inches from the top.
For heating portable baths, so many plans are in use, and have been suggested, and even patented, that the reader cannot possibly be at a loss for one to suit his particular case. A small grate for burning charcoal is the one most commonly adopted; but where attainable, a ring or cross of small inflamed gas-jets, is more cleanly and manageable.
When the bath consists of a wooden tub, or any other deep vessel, a simple and inexpensive apparatus brought out in America, under the title of the ‘Ital′ian bath′-warmer’ (see engr. 3), and made of thin sheet-iron, will occasionally be found useful.[111]
a, Bath-tub.
b, The larger arm of the warming-tube by which the charcoal is introduced, and by which the fumes fly off.
c, The smaller arm to admit air to support the combustion.
d, The fire grate, to support the burning charcoal.
This situation and the minor details connected with the comfort and convenience of the bath, must greatly depend on the character of the building, and the sum to be devoted to the purpose. When possible, the bath-room should always be on the same floor as the bedrooms, of easy access to them, and so situated and arranged, that a plentiful and constant supply of pure water can be ensured, and the waste water removed without trouble or inconvenience. The basement story should always be avoided; for, as observed by Dr Ure, there is a coldness and dampness belonging to it, in258 almost all weathers, which is neither agreeable nor salubrious.
The ranges of the temperature of water appropriate to the respective baths, according to the common nomenclature, are shown in the following Table:—
| Name. | Temperature. Fahr. | ||
| Cold bath | 33° | to | 75° |
| Temperate bath | 75 | ” | 82 |
| Tepid bath | 82 | ” | 90 |
| Warm bath | 90 | ” | 98 |
| Hot bath | 98 | ” | 112 |
Concluding Remarks. The importance, and indeed the absolute necessity of frequent personal ablution, has been already insisted on and explained. But however important and beneficial the use of water in this way may be, the effects arising from the immersion of the body in that liquid, as in the practice of bathing, are far more extensive and complete. What the one does usefully but not completely, the other accomplishes readily, satisfactorily, and perfectly. There is no absolute succedaneum for the entire bath. Its physiological effects are peculiar to itself, and of the utmost importance in pathology and hygiene. The practice of wearing flannel, the daily use of clean linen, the mere washing of the more exposed parts of the body, are but poor attempts at cleanliness, without the occasional, if not frequent, entire submersion of the body in water. Nor should the action of judicious bathing in the promotion of personal comfort and personal beauty be forgotten. Intellectual and moral vigour are also gradually, but materially, influenced and promoted by the beneficial action of bathing on the system; for mind and conscience being linked to matter in the ‘house we live in,’ become perturbed, or lethargic, in almost exact accordance with the fluctuations of our physical health. The neglect of bathing in this country is, to us, an absolute enigma. We are always talking about health, and continually professing to be seeking it; but the practical applications of the principles which we advocate, and the doctrines which we teach, are, unfortunately, the exceptions and not the rule.
Our recommendation of bathing applies chiefly to the warm bath and the tepid bath, which are alike adapted to the delicate and the robust, and to every condition of climate and season. Cold bathing, in this climate, is only suited to the most healthy and vigorous, and can only be safely practised during the warmer months of the year, and in a mass of water sufficient to permit of the heat of the body being maintained by swimming or other active exercise. The plunge and shower baths are partial exceptions to these remarks; whilst sea-bathing, for the reasons given elsewhere, comes under another category. This last, “on account of its stimulative and penetrating power, may be placed at the head of those means which regard the care of the skin; and it certainly supplies one of the first wants of the present generation, by opening the pores, and thereby re-invigorating the whole nervous system.” “Besides its great power in cases of disease, it may be employed by those who are perfectly well, as the means most agreeable to nature for strengthening the body and preserving the health.” Another important advantage which sea-bathing has over bathing in fresh water is, that persons seldom take cold from indulging in it.
For old people, or those of middle age, the cold bath is not to be recommended, or if taken, considerable caution is required in using it. By such persons, also, bathing in very hot weather, or in the sea, should likewise be prudently practised. For these, the warm or tepid sponge-bath will be found the much safer method.
It sometimes happens that, both with the old and young, the cold bath gives rise to headache, palpitation, shortness of breath, loss of appetite, or great languor. Whenever any of these effects are produced, the bath should be at once given up.
The best time for taking a cold hath or for swimming is in the morning, not too early, but when the sun is well up. Immersion is best practised after a light meal, but not immediately following one. After breakfast, from 10 a.m. to noon, are the preferable hours. Should the bather be unable to swim, when going into the sea or into a river, he should keep briskly moving all the time his body is immersed in the water. If in a room bodily friction must be substituted for exercise. A desirable glow may often be produced by rubbing the body with either a rough towel, a flesh-brush, or a pair of horsehair gloves.
The above remarks are meant to apply only to persons in average health.
Weakly and delicate persons, even without any disease about them, would always do well to consult their medical adviser before taking to cold bathing.
We may add, that for bathing to produce its best effects the water should be soft and pure, and good soap sparingly, but regularly, employed whenever the skin requires it. See Ablution and Wash-houses.
The medical and hygienic properties of baths are noticed below, under their respective names:—
Bath. In chemistry, &c., a vessel or apparatus containing some medium in which the vessel holding the substance to be heated is immersed, instead of being exposed to the direct action of the fire; by which means a limited and uniform temperature may be ensured.
The highest temperature that can be given to any substance contained in a vessel placed in another of boiling water, is about 205° or 206° Fahr.; but by adding 1⁄5th part of common259 salt to the bath, a heat of fully 212° may be obtained. Baths of fusible metal, saturated solutions of salt, sand, and (on the large scale) steam, are also used for the same purpose. A bath of oil may be safely heated to about 500° Fahr. without suffering decomposition, and will be found an exceedingly appropriate and convenient source of heat in many processes. The simplest and most convenient form of water bath is that afforded by raising water to the boiling point in a copper basin placed over a gas lamp, and supporting the vessel to be heated over the basin, by means of a circular hoop of copper resting on the top of the basin. By this means the lower surface of the dish or vessel to be heated is brought in contact with the steam. Copper basins, fitted with a series of concentric copper rings, so as to render the basin capable of supporting dishes of different sizes, are made for this purpose.
For drying many substances an air bath is required. The accompanying cut represents a convenient form of air bath. It consists of a cylindrical copper vessel (A), the cover of which is moveable, and has two apertures, the middle one (E) serving for the escape of vapour, and the lateral one (C) for the insertion of a thermometer. The vessel holding the substance to be heated rests on a ring within the box, supported on a tripod.
A larger air bath, by means of which several small vessels can be heated at once, is seen below.
Air-baths are sometimes surrounded with a jacket, and may be converted into water or oil baths, according as the jacket is filled with either of the fluids. For a Table of Boiling-points, see Ebullition.
An air-bath of constant temperature between 100° and 200° C. has been contrived by Sprengel. It consists of an ordinary hot-water oven made of sheet-lead autogenously soldered, and filled with dilute sulphuric acid boiling at the required temperature.
In order that the temperature may remain constant, the water which distils from the dilute sulphuric acid is condensed and allowed to flow back again into the bath by means of a worm of lead cooled by the atmosphere, or a long vertical metal or glass tube.
Bath. In medicine, the medium in which the body, or a part of it, is bathed or immersed, for some object beyond that of mere personal cleanliness or enjoyment; the composition, use, or temperature of the medium being generally indicated by some epithet, as in the instances below. When only the last is pointed out, pure water is, of course, intended to be used.
Baths are divided by medical writers into classes, and even minor subdivisions, in a manner which is more ingenious than useful. They are said to be SIMPLE when water or its vapour forms the bath; and COMPOUND when the water or vapour is medicated by the addition of other substances (COM′POUND BATHS; BAL′NEA COMPOS′ITA, L.). The latter class is also subdivided into THERAPEU′TIC BATHS (MED′ICATED BATHS; BAL′NEA MEDICA′TA, B. THERAPEU′TICA, L.); and NUTRIT′IVE BATHS (B. NUTRIEN′TIA, B. NUTRI′′TIA*, B. NUTRITO′′RIA*, L.). Thus, besides the ordinary water and vapour baths, the medical uses of which are hereafter noticed, we have WINE′-BATHS, MILK′-BATHS, SOUP′-BATHS, &c. (used to convey nourishment, or to sustain the body, as in occlusion of the œsophagus, certain diseases of the stomach, &c.); CHLO′′RINE BATHS, SUL′PHUROUS B., MERCU′′RIAL B., &c. (used in skin diseases, syphilis, &c.); AROMAT′IC and CHALYB′EATE BATHS (employed as tonics); and ACID BATHS (sometimes used to remove the effects of mercury).
On the Continent a variety of substances are employed to medicate baths, which are seldom or never so used in this country.
The quantity of any medicinal substance used to medicate a bath, for an adult, may be, in general, for each gallon of water employed, about the same as that which is used to form a half-pint lotion of medium or rather weak260 strength. Thus; taking the quantity of bichloride of mercury to form the lotion at 5 gr., and that of sulphurated potash at 1⁄2 dr., the quantity required for a bath of 30 to 40 galls. will be about 21⁄2 dr. of the first, and about 13⁄4 oz. of the second of these substances. Much, however, depends on the nature of the case, the length of the immersion, the periods of recurrence, and the intended number of repetitions. In the case of very active remedies it will be safest and best to begin with less than (say 1⁄4 to 1⁄3) the quantity thus indicated.
Medicated baths are, in nearly all cases, taken warm or fully tepid.
⁂ In the following baths the quantity of the ingredients ordered, when not otherwise indicated, is that proper for an ordinary full-sized bath for an adult; viz., from 40 to 60 galls. Those which do not contain volatile substances may be used more than once; and many of them several times by adding a small quantity of fresh ingredients to keep up their strength.
Bath, Acid (ăs′-). Syn. Bal′neum ac′idum (ăs′-), L. See Hydrochloric, Nitric, Nitro-hydrochloric, and Sulphuric Acid Baths (below). Enamelled, hard-glazed, or wooden vessels must be used with all of them.
Bath, Air. Syn. Bal′neum pneumat′icum, L.:—a. (Cold.) Simple exposure of the body, in a state of nudity, for a short time to the atmosphere. Tonic, anodyne, and sedative; in febrile excitement, nervous irritability, and restlessness accompanied by a quick or full pulse, &c. Safe and often very effective. It will frequently induce sleep when all other means fail.
b. (Hot:—Assa, A. suda′tio, L.) An apartment to which dry heated air is admitted. Sometimes the arrangement is such that the air is not inhaled. Stimulant; sudorific; more so than even the vapour bath; produces copious perspiration, being, indeed, the most powerful and certain diaphoretic known. It has been advantageously employed in cholera (for which its advocates state that it is almost a specific), congestive fevers, chronic rheumatism, contractions, stiff joints, paralysis, scaly skin-diseases, dropsical swellings, and most of the cases in which the vapour bath is usually employed. The temperatures are—as a sudorific, 85° to 105° Fahr.; as a stimulant, 100° to 130°. When not inhaled it may be often raised, with advantage, 15° to 25° higher. See Bath, Turkish.
c. (Compressed.) Recommended, by M. Tarberie, in aphonia, &c. It has recently been employed in asthma, phthisis, and some other like diseases, with extraordinary success, at Ben Rhydding.
d. (Rarefied.) Applied locally. Revulsive; resembles CUPPING, DRY (which see).
Bath, Al′kaline. Syn. Al′kalised bath; Bal′neum alkali′num, B. alkaliza′tum, L. Carbonate of potash (salt of tartar), 3⁄4 lb. In itch, prurigo and chronic skin diseases accompanied with dryness and irritation, acute gout, lithic gravel, scurvy, diarrhœa, &c. Scotch soda, 1 lb., is sometimes substituted for the ‘potash’; but is less effective, and is theoretically objectionable.
Bath, Al′um. Syn. Bal′neum alu′minis, L. Alum (in powder, or previously dissolved in hot water), 3⁄4 lb. to 11⁄2 lb., or even 2 lbs. In troublesome excoriations, extensive burns, obstinate vesicular eruptions, diarrhœa, &c.; also in obstinate piles and prolapsus ani. See Bath, Astringent.
Bath, Ammoni′acal. See Hydrochlorate of Ammonia Bath (below).
Bath, Animal. Syn. Bal′neum anima′le, L. The skin or any part of an animal just killed, wrapped round the body or a limb. Once much esteemed; now, happily, disused in this country.
Bath, Antimo′′nial. Syn. Bal′neum antimonia′le, L. Tartar-emetic, 1 to 2 oz. (Soubeiran.) In lumbago and certain skin diseases; also as a counter-irritant.
Bath, Antipso′′ric. Syn. Bal′neum antipso′′ricum, L. See Bath, Sulphuretted (also others).
Bath, Aromat′ic. Syn. Bal′neum aromat′icum, L. Balm, chamomile, lavender, mint, rosemary, sage, thyme, with any other like aromatic herbs (at will), of each a handful, mixed together and steeped in a (covered) pail of boiling or very hot water, for an hour, and then strained, with pressure, into the bath. Sometimes 2 or 3 oz. of sal-ammonia, a 1⁄4 lb. of alum, or 1 lb. of common salt, is also added. Occasionally used in cutaneous affections, chronic rheumatism, diarrhœa, dyspepsia, stiff-joints, &c.; also in debility arising from loss of blood, spermatorrhœa, suppressions, hysteria, hypochondriasis, &c.
The AROMATIC VAPOUR BATH is made by causing the vapour to pass through the herbs.
Baths, Aromatic Malt (J. Hoff, Berlin). Wittstein says these consist of coarsely crushed barley malt at six times its selling value.
Bath, Astrin′gent. Syn. Bal′neum astrin′gens, L. Prep. (Most.) Alum (2 to) 4 lbs.; dissolve in boiling water; and add, whey, 6 or 8 pailfuls, or q. s. In extensive burns, piles, prolapsus ani, &c. See Bath, Alum, Bath, Oakbark, &c.
Bath, Balsamic. Syn. Bal′neum balsam′inum, L. Bordeaux turpentine and tar, of each 2 lbs. (or of tar alone, 3 to 4 lbs.); hot water, 6 or 7 galls.; stir continuously until nearly cold, then add the clear portion to water q. s. to form a bath. In mumps, pruriginous diseases of the skin, eczema, impetigo, &c.
Bath, Barèges (Factitious). Syn. Bal′neum Baretginen′se (Factitium), L. Prep. 1. Crystallised sulphide of sodium, 31⁄2 oz.; chloride of sodium, 11⁄2 oz.; gelatine (dissolved), 4 oz.
2. (Trousseau & Reveil.) Dry sulphide of potassium, 4 oz.; water, 16 oz.; dissolve, and add the solution to the bath; then further add, of sulphuric or hydrochloric acid, 1⁄2 oz., previously261 diluted with water, 8 oz. In itch, moist skin diseases, chronic diarrhœa, chronic rheumatism, lead colic, &c. See Balls, Waters, &c.
Bath, Benzo′ic. Syn. Bal′neum benzo′icum, L. 1. Benzoin (in powder), 1⁄2 lb.; water (at 90°) q. s. In irritations, hysteria, hypochondriasis, &c. It is also reputed to be feebly aphrodisiac. 2. A common warm bath, with a little powdered benzoin laid on a heated plate near the bather, so that the fumes may be inhaled. Slightly soothing or anodyne; in chronic laryngitis, relaxed uvula, &c.
Bath, Bichlo′′ride of Mer′cury. See Bath, Mercurial.
Bath, Bran. Syn. Bal′neum fur′furis, L. Bran, 5 to 7 lbs.; boiling waters, 2 or 3 galls.; digested together for an hour, or boiled for 15 minutes; the strained liquid being added to the bath. Emollient; in dry and scaly skin disease, and to allay itching and surfacial irritation; also to promote suppuration, &c.
Bath, Bromine. The saline waters of Kreuznach contain bromides. The salts derived from the evaporation of these waters are imported into this country, and are employed in baths. Or the following substitute may be used:—Artificial sea-salt, 11 lbs.; bromide of potassium, 4 oz.; mix, and let the above be added to a bath containing sufficient water for immersion. The bromine bath is more especially used for tumours of every kind. It requires to be continued in for a long time. When the patient does not possess the conveniences for taking the bath, flannels dipped in a strong solution of the salt and wrung out may be applied wet to the abdomen for some hours daily.
Bath, Cam′phor. Syn. Bal′neum cam′phoræ, B. camphoratum, L. Camphor, 3 or 4 dr., coarsely powdered, and placed on a plate heated by boiling water, in the bathroom. Anodyne, anaphrodisiac, and diaphoretic; in spasmodic asthma, chronic cough, relaxation of the uvula, ardor urinæ, nervous irritability, &c.
Bath, Carbon′ic. Syn. Carbon′ic acid bath; Bal′neum carbon′icum, B. ac′idum carbonicum, L.
1. Carbonic acid gas applied, by means of a suitable apparatus, to prevent its being respired. Antiseptic, diaphoretic, and excitant to the vascular system; in amenorrhœa, chlorosis, hysteria, scrofula, cancerous and other ulcers (particularly foul ones), &c.
2. Water, at 50° Fahr., charged with the gas. Powerfully antiseptic and sedative; in foul ulcers, gangrene, &c.
Bath, Chlo′′ride of Ammo′′nium. Syn. Bal′neum ammo′′nii chlori′di, B. ammo′′niæ hydrochlora′tis, L. Sal-ammoniac, 2 to 3 lbs., or even 4 lbs. In chronic inflammations, glandular enlargements and indurations, chronic rheumatism and affections of the joints, leucorrhœa, chilblains, frost-bites, &c.
Bath, Chlo′′ride of Soda. Solution of chlorinated soda, 11⁄2 lb.; water, 30 galls.
Bath, Chlo′′rine. Syn. Bal′neum chlorin′ii, B. chlorina′tum, L. Tepid water to which a little chlorine has been added. Antiseptic, stimulant, and subsequently sedative and antiphlogistic; in itch, foul and gangrenous ulcers, chronic liver affections, &c. Chloride of lime is commonly substituted for chlorine.
2. (Magendie; Wallace.) Chlorine gas (obtained from salt, 11⁄2 oz.; oil of vitriol and water, of each 1 oz.; and black oxide of manganese, 1⁄2 oz. to 1 oz.) diluted with air, at a temperature of 104° to 150° Fahr., and applied, by means of a suitable apparatus, for 10 minutes to 1⁄2 an hour; every possible precaution being taken to prevent it being inhaled. In chronic liver affections, gradually and cautiously increasing the ingredients to three times the above quantity, and decreasing the dilution with air until the gas is used nearly pure.[112]
Bath, Cold. Syn. Balneum frig′idum (-frĭj′-), Frigida′′rium, L.; Bain froid, Fr. Water, fresh, saline, or mineral, at a temperature varying from 33° to about 75°; but usually understood to apply to water between 50° and 70° Fahr. When below 50° it is considered very cold. At a temperature ranging from 60° to about 75° it is commonly used by the healthy and vigorous as a luxury, and for cleanliness.
“The immediate effects of the cold bath are a sensation of cold (speedily followed by one of warmth), contraction of the cutaneous vessels, paleness of the skin, diminution of perspiration, and reduction of the volume of the body. Shivering, and, as the water rises to the chest, a kind of convulsive sobbing, are also experienced. Continued immersion renders the pulse small, and ultimately imperceptible, and the respiration difficult and irregular. A feeling of inactivity succeeds; the joints become rigid and inflexible; pain in the head, drowsiness, and cramps, come on; the temperature of the body falls rapidly; and faintness, followed by death, ensues.” “Its primary effects constitute the SHOCK—its secondary effects, the REACTION or GLOW.”[113] Hence it is that immersion of the body in water below about 65° Fahr. cannot be tolerated for any length of time without such a loss of animal heat as frequently to induce highly sedative and depressing effects, from which the constitution does not readily recover. Water at a temperature of below about 50° Fahr. can only be safely used as a plunge-bath. The sedative effects of sea and mineral waters is much262 than that of pure water, or of spring or river water.
The cold bath, medically considered, is tonic, stimulant, and restorative, when judiciously taken, and when not too long continued or too often repeated. When beneficial, the patient feels a pleasant glow on the surface of the body immediately following it. If a sensation of coldness or shivering ensues, it acts injuriously, and should not be repeated. The duration of the immersion may vary from 2 to 15 minutes, the precise time depending upon the temperature of the water and the feelings of the bather; the longer period being only proper in fine weather, and when accompanied by swimming or violent exercise.
As a remedial agent, the cold bath is principally recommended to increase the tone and vigour of the system; and is contra-indicated when there is a tendency to apoplexy, or to chronic affections, functional or organic, of the heart, lungs, or kidneys. It should never be taken when the person feels chilly, languid, or depressed; or if drowsiness and shivering follow it.
The temperature of the water of the rivers and the coasts of England ranges, in summer, from 55° to 70 or 72° Fahr.
Bath, Creosote. Creosote, 2 dr.; glycerin, 2 oz.; boiling water, one gall. To be added to 29 galls. of water.
Bath, Douche. See Bath, Shower, Douche, &c.
Bath, Dry. Syn. Bal′neum siccum, L. The immersion of the body in any dry material, as ashes, salt, sand, &c. Earth-bathing, as administered by the once notorious quack, Dr Graham, was of this kind. In the sudatorium or sweating room of the ancients the body was immersed in heated sand.
Bath, Elec′tric. Syn. Bal′neum elec′tricum, L. The patient, placed on an insulated stool, is put in contact, by means of a metallic wire, with the prime conductor of an electrical machine in action. The surface of the body is thus rendered electro-positive, and the surrounding air, by induction, electro-negative. It has been recommended in chronic rheumatism, scirrhous tumours, &c.
Bath, Electro-chemical (of Dr Caplin). This is founded on the supposition that all diseases arise from the presence of mineral, or other extraneous morbific matter, in some organ, or the whole organism, and which is capable of removal by electrolysis. The patient is placed in an appropriately arranged voltaic bath, and there “saturated with the electric fluid.” This “decomposes everything which is foreign to the organism, the vital parts being protected by the law of conservation belonging to every organic production.” These foreign substances are said to be thus carried out of the system by the electric current, and to be “fixed and plated on the copper in the same way, and according to the same law and principle (only reversed), as in the process of electro-plating.”[114]
Bath, Fec′ula. Syn. Bal′neum am′yli, B. fæc′ulæ, L. Potato-starch or wheat-starch, 1 to 4 lbs.; boiling water q. s. to dissolve. Resembles the BRAN-BATH.
Bath, Ferru′ginous. Syn. Chalyb′eate bath; Bal′neum ferrugin′eum, B. Chalybeatum, L. 1. Green sulphate of iron, 1 to 2 lbs. A well-tinned copper, wooden, or japanned bath may be used. In general debility when chalybeates are indicated, and the stomach will not bear iron; also in piles and prolapsus. The stains on the towels used to wipe the patient may be removed by at once soaking them in water acidulated with hydrochloric acid.
2. (Ioduretted.) See Bath of Iodide of Iron.
Bath, Foot. Syn. Pedilu′vium, L. Warm (or hot). Revulsive, counter-irritant; in colds, menstrual and hæmorrhoidal suppressions, rheumatism, stiffness of the ankles, tender feet, &c. A little common salt, flour of mustard, or sal-ammoniac, is often added to render it more stimulant, to prevent ‘taking cold,’ &c. See Feet, &c.
Bath, Gelat′inous. Syn. Bal′neum gelatino′sum, B. gelatin′ii, L. Gelatin or fine Salisbury glue, 3 or 4 lbs.; dissolved in boiling water, 2 galls., or q. s.; and added to a warm bath. At the ‘Hospital for Cutaneous Diseases’ 8 lbs. of patent size are used for a bath of 30 to 35 galls. Emollient; formerly, but erroneously, considered nutritive. Used in skin diseases; generally combined with sulphur. See Bath, Barèges.
Bath, Glyc′erine (glĭs′). Syn. Bal′neum Glycerin′ii, B. g. compos′itum, L. Glycerine, 2 lbs.; gum arabic (dissolved), 1 lb. Used as a soothing emollient, in itching, dryness, irritation, and hardness of the skin, &c. Where expense is an object, 3 or 4 lbs. of good honey, and 1 oz. of salt of tartar, form an excellent substitute for the glycerine.
Bath, Hem′lock. Syn. Bal′neum co′nii, L. 1. Dried hemlock-leaves (or herb), 4 to 6 handfuls; water, 1 gall.; infuse 2 hours, and strain. The part to be immersed in, or bathed with, the warm infusion, observing not to apply it if the skin is unsound; or it may be added to the water of a bath in the usual manner. In gout, cancer, chronic rheumatism, and certain skin diseases.
2. (Cut. Hosp.) Extract of hemlock, 2 oz.; starch, 1 lb.; boiling water, 1 gall.; dissolve. For a bath of about 30 galls. As the last.
Bath, Hip. Syn. Coxælu′vium, L. Usually warm; sometimes fully warm, or somewhat hot. In inflammatory, spasmodic, and chronic affections of the abdominal and pelvic viscera; in suppressed and painful menstruation, hæmorrhoids, strangury, prolapsus, ischuria, &c.; also as a substitute for a full bath, when this last is contra-indicated by some affection of the263 lungs, heart, brain, or great vessels. Like full baths, it may be often advantageously medicated. See Bidet.
Bath, Hot. Syn. Bal′neum cal′idum, Calda′′rium, L.; Bain chaud, Fr. Usual temperature, 98° to 106° Fahr.
The hot bath has a remarkably tranquillising effect upon the nervous system, producing a strong tendency to quietude and sleep. It also acts as a powerful antispasmodic, and by determining the blood to the surface of the body tends to relieve visceral inflammation and congestion. In chronic affections arising from the action of cold and damp and from exhausted energy, in stiff joints, rheumatism, neuralgia, diarrhœa, and numerous other affections, its effects are often rapid and remarkable. At high temperatures it strongly stimulates the arterial system, and arouses nervous energy and vital action, producing excessive excitement and turgescence, followed by copious perspiration, which has been often found successful in cholera, paralysis, &c. If the immersion be too long continued, or the bath be injudiciously employed, lassitude, debility, and somnolency ensue, and the good effect of the bath is more or less lost. In these cases violent throbbing and painful distension of the vessels of the head, with a distressing feeling of suffocation and anxiety, are premonitory symptoms of impending apoplexy, an accident which sometimes, though seldom, follows its improper use.
Bath, Hydrochlo′′rate of Ammonia. See Bath, Chloride of Ammonium.
Bath, Hydrochlo′′ric Acid. Syn. Muriat′ic acid bath; Bal′neum hydrochlo′′ricum, B. acidum h., B. muriat′icum, &c., L. Commercial hydrochloric acid, 1 to 3 lbs. (in chronic liver affections); or 3 to 6 fl. oz. (in prurigo and lichen).
Bath, Hydrosul′phuretted. Syn. Bal′neum hydrosulphure′tum, L.—1. A tepid sulphuretted bath, with the addition of hydrochloric acid, 2 or 3 fl. dr., immediately before immersion. In rheumatism, chronic skin diseases, hooping-cough, and certain forms of paralysis:—2. A tepid bath to which 3 to 6 fl. oz. of (liquid) hydrosulphate of ammonia is added immediately before use. Used as the last. It often acts almost as a specific in hooping-cough and certain breath ailments.
Bath, I′odine of I′ron. Syn. Bal′neum fer′ri iodi′di, L. Prep. (Pierquin.) Iodide of iron, 1⁄2 oz. to 2 oz. In amenorrhœa, leucorrhœa, chlorosis, scrofula, &c.; gradually increasing the quantity of the iodide until 4 oz., or more, is used for a bath.
Bath, I′odine. Syn. Bal′neum iodin′ii, L.:—1. Iodine, 3 to 5 dr.; dry siliceous sand, 2 oz.; triturated together until reduced to fine powder, and then agitated with the water of a tepid bath for 10 or 15 minutes. 2. (Cutan. Hosp.) Iodine, 4 dr.; liquor of potassa, 4 oz.; water, 2 pints; dissolve; for a bath of 30 galls. In skin diseases complicated with scrofula, glandular enlargements, amenorrhœa, &c.
Bath, Io′duretted. Syn. Io′durated bath, I′odised b., Compound iodine-b., &c.; Bal′neum iodure′tum, B. iodura′tum*, B. potas′′sii superiodi′di, &c., L. Lugol, the leading authority on this subject, employs this bath of the different strengths, &c., shown in the following tables:—
| Degree. | Iodine. | Iodide of Potassium. | Water for the bath. |
| dr. | dr. | gal. | |
| 1 | 2 to 21⁄2 | 4 to 5 | 50 |
| 2 | 2 to 3 | 4 to 6 | 60 |
| 3 | 3 to 31⁄2 | 6 to 7 | 75 |
| Age. | Iodine. | Iodide of Potassium. | Water. |
| gr. | gr. | gal. | |
| 4 to 7 | 30 to 36 | 60 to 72 | 9 |
| 7 to 11 | 48 to 72 | 96 to 144 | 18 |
| 11 to 14 | 72 to 96 | 144 to 192 | 31 |
⁂ The dry ingredients of the first Table are to be dissolved in a pint of water, and of the second, in 1⁄2 pint of water, before adding them to the bath.
In scrofulous affections and the other cases in which the external use of iodine or the iodides is indicated. Enamelled ware, stoneware, or wooden vessels must be employed.
Bath, Lime. Syn. Bal′neum cum cal′ce, L. Lime, 3 lbs.; slaked, and added to the bath. In gout, lithic diathesis, itch, &c. See Bath, Vapour.
Bath, Mercu′′rial. Syn. Antisyphilit′ic bath; Bal′neum mercuria′le, B. hydrar′gyri bichlori′di, B. antisyphilit′icum, &c., L.; Bain mercuriel, B. antisyphilitique, &c., Fr. Bichloride of mercury, in fine powder, 1 to 3 dr., hot water, 1 pint; agitate together until solution is complete, before adding them to the bath, the ‘water’ of which (contained in an enamelled or wooden vessel) must be soft (rain) and pure. At the ‘Cutan. Hosp.’ hydrochloric acid (= 1⁄3rd the weight of the chloride) is commonly added; and at the ‘Fr. hospitals,’ an equal, or rather264 more than an equal weight, of sal-ammoniac. These additions facilitate the solution of the chloride, and retard its decomposition by any slight impurity in the water forming the bath.
Uses, &c. In syphilitic affections, either with or without skin disease; in chronic rheumatism, swelled joints, and chronic skin diseases generally, where the use of mercury is indicated, and the remedy is rejected by the stomach; especially in these affections in women and children (for the last, proportionately reduced in strength and quantity). Also used in it, and to destroy pediculi on the body.
Bath, Met′al. See Bath (in Chemistry), Fusible Metal, &c.
Bath, Mud. Syn. Bal′neum lu′teum, B. lu′ti, L. Mud-bathing (ILLUTA′TION) was common among the ancients. The slime of rivers, and the mud on the sea-shore, were especially prized for this purpose. The Tartars and Egyptians still employ baths of this description in hypochondriasis, scrofula, and scurvy. At Franzenbad, in Germany, an acidulous species of black bog-earth found there, is beaten up with warm water to a semi-liquid consistence, and used as a bath. This is said to render the skin satin-like and soft; and to be useful in debility, and in paralytic affections of a gouty origin. In France, hot dung (DUNG BATH) is occasionally used in rheumatism; and in Poland, in syphilis. The husk of grapes and the refuse of olives, after undergoing a partial fermentation, have been successfully employed in France against acute rheumatism.[115]
Bath, Muriat′ic. See Bath, Hydrochloric Acid.
Bath, Mus′tard. Syn. Bal′neum sina′pis, L.:—1. Flour of mustard, 2 lbs.; warm water, 1 gall.; make a thin soup; in fifteen minutes pour it into a coarse linen bag or cloth, and press out the liquid, which is to be stirred up with the bath. In cholera, diarrhœa simulating cholera, &c.; also to cause reaction; the patient remaining in the bath until a somewhat painful sense of burning and irritation is experienced:—2. Flour of mustard, 3 to 8 oz.; as before. Used as a gentle stimulant to excite the skin, and promote its healthy action, &c.
Bath, Ni′tro-hydrochlo′′ric. Syn. Ac′id bath‡ (ăs′-), Nitro-muriat′ic b.*, N. a. b.*; Bal′neum nitro-hydrochlo′′ricum. B. ac′idi (ăs′-), B. a. nitro-hydrochlo′′rici, B. a. nitro-muriat′ici*, &c., L.:—1. Water slightly acidulated with the acid, so that its sourness to the taste is about that of common vinegar. According to Ainslie, 1 oz. of acid is sufficient for 1 gall. of water.[116] Other formulæ in use are—
2. (Cutan. Hosp.) Nitric acid, 11⁄2 lb.; hydrochloric acid, 1 lb.; for a bath of 60 to 70 galls.
3. (Soubeiran.) Nitro-hydrochloric acid, 4 to 16 fl. oz.; according to the case.
4. (Dr Scott.) Nitric acid, 2 fl. oz.; hydrochloric acid, 3 fl. oz.; water, 5 fl. oz.; mix. 11⁄2 to 2 fl. oz. to each gall. of water for a general bath; 3 fl. oz. to the gall. for a foot, knee, or sponge bath.
Uses, &c. In its weaker forms, in skin-diseases depending on disordered liver; in others, chiefly in liver complaints, and to relieve the pain on the passing of gall-stones. It must be contained in an enamelled or wooden vessel, and may be used as a hip, knee, or foot-bath; a knee-bath being the one generally adopted in England. Dr Scott, of Bombay, who first brought this bath into notice, once plunged the Duke of Wellington up to his chin in one, in India, and thus cured him of a severe hepatic affection. In its stronger form it causes tingling and pricking of the skin, and a peculiar taste in the mouth, and affects the gums and salivary glands, often producing plentiful ptyalism, without which, indeed, its advocates regard its action as incomplete. Time of application, 15 to 20 minutes daily, for a fortnight or three weeks; and afterwards, every second or third day.
Bath, Oak-Bark. Syn. Bal′neum quer′cûs, B. quer′ci, L. Oak-bark, 3 or 4 handfuls for a child; 10 to 15 for an adult; made into a decoction, and strained with pressure into the bath. In hæmorrhoids, prolapsus, leucorrhœa, hernia, diarrhœa, ill-conditioned and bleeding ulcerations, &c. Drs Elaesser, Eberle, and Fletcher have successfully employed it in the intermittents of infancy and childhood, tabes mesenterica or scrofula, &c. It has also proved useful in phthisis.
Bath, Oil. Syn. Bal′neum oleo′sum, L. Olive or other oil (hot), strongly aromatised with the oils of cassia, cloves, nutmegs, cedron, and juniper; and digested for a week on ambergris and vanilla, of each (bruised), about 10 gr. to the gallon. Used, in the East, to anoint the body, as a preservative against the plague and other contagious diseases; also as a full bath or hip-bath, the immersion being for 15 to 30 minutes.
Bath, Pneumat′ic. See Bath, Air.
Bath, Saline′ (Gelatinous). Syn. Bal′neum sali′no-gelatino′sum, L.; Bain de Plombières, Fr. Prep. Common salt and Flanders glue, of each 2 lbs.; water, 1 gall.; dissolve separately, and add the solutions to the bath. In scrofula, &c.
Bath, Salt. See Bath, Saline, Bath, Sea, &c.
Bath, Sand. Syn. Bal′neum are′næ, L.; Bain de sable, Fr. See Bath (in Chemistry), Bath, Dry, &c.
Bath, Sea. Syn. Bal′neum mari′num, L.; Bain marin, Fr. Immersion in the sea or in recent sea water (temperate, tepid, warm, or hot). Owing to the saline matter which it contains, it possesses stimulant, alterative, and resolvent properties, superadded to those of pure water at the corresponding temperature. When taken, in summer, on our coasts, the reaction and glow follow more speedily and265 certainly than after a common water bath; and it may be taken with greater safety, and for a longer period. It often proves very serviceable in diseases accompanied with debility, in phthisis, scrofula, glandular enlargement, &c. A warm or hot sea-water bath is one of the most restorative imaginable; often removing the effects of fatigue and exposure—exhaustion, stiff joints, cramps, rheumatism, &c.—like a charm. Unless under sanction of a medical man, boys and girls should never be allowed to bath in the sea after the end of September. See Bath (above), Waters, &c.
Bath, Sea. (Factitious). Syn. Bal′neum mari′num facti′′tium, L. Artificial sea-water, or rather a substitute for sea-water, for this purpose, is commonly prepared by adding about 3% of common salt to ordinary water.[117] The following are, however, more serviceable imitations:—
1. As above, with the addition of 1 dr. of iodide of potassium to every 3 or 4 galls. of water.
2. (Cutan. Hosp.). Common salt, 8 lbs.; sulphate of magnesia, 2 lbs.; chloride of calcium, 1 lb.; water, 50 to 60 galls.
3. Salt, a handful; water, a pailful; flour of mustard, 1 oz. For a foot-bath.
Bath, Show′er. Syn. Implu′′vium, Bal′neum pen′sile, &c., L.; Douche, Fr. Similar in its effects to the cold bath or plunge-bath; but without many of its advantages. It is less alarming to nervous persons, and less liable to produce cramp, than immersion in cold water; whilst the reaction or glow follows more speedily and certainly. It is considered the best and safest mode of cold bathing, and is often highly serviceable in nervous affections. A good plan is to allow the water to remain in the bedroom all night, by which any undue degree of coldness is removed. Tepid water may be commenced with; and at first, in extreme cases, the patient may stand in hot or warm water at the time of taking the bath. The reaction following its use is greatly promoted by friction of the surface with dry rough towels.
Bath, Soap. Syn. Bal′neum sapo′nis, L. White soap, 2 to 3 lbs.; water, 3 quarts; dissolve by heat, and add it to a warm bath. Detergent, lubricating, and discutient; in itch and other skin diseases, &c.
Bath, Spon′′ging (spŭnje′-). This title explains itself. In the sponging bath exercise and ablution are combined, and its employment by persons of sedentary habit is highly advantageous.
Bath, Sulphur. Syn. Bal′neum sul′phuris, L. 1. Flowers of sulphur, 1⁄2 to 1 lb.; water, a pailful; mix, agitate occasionally for 12 to 24 hours, and then add the whole to an ordinary bath. Useful in various mild, but obstinate, skin diseases. Its occasional employment, even in health, seldom fails to render the skin soft, smooth, and delicate. Soap may be used with it.
2. (Compound; B. s. compos′itum, L.)—a. (Cutan. Hosp.) Precipitated sulphur, 2 lbs.; hyposulphite of soda, 1⁄2 lb.; water, 1 gall.; dissolve, and add of sulphuric acid, 1 dr. One pint to every 30 galls. of water. In various skin diseases (see below).
b. See Bath, Sulphuretted.
Bath, Sul′phurous. Syn. Sul′phurous acid bath; Bal′neum sulphuro′sum, B. sul′phuris‡, L. From sulphur, 1⁄2 oz., sprinkled on a hot plate placed under or near the patient; the proper precautions being taken as directed under CHLORINE BATH. In itch, lepra, psoriasis, &c. Cleanly, but seldom used, chiefly on account of the number of baths required to prove serviceable. See Bath, Sulphuretted.
Bath, Sul′phuretted. Syn. Bal′neum sulphuret′um, B. sulphura′tum, B. sulphu′′reum, &c., L.; Bain sulfuré, &c., Fr. 1 Sulphurated potash, 1 oz.; for every 10 or 12 galls. of water employed. Sometimes sulphurated soda, or (in the Ger. hosp.) suphurated lime, is the sulphur-salt employed. 1⁄2 dr. of sulphuric acid is also occasionally added to the bath; but this increases its fœtor, without adding much, if anything, to its curative power; whilst, without care, the evolved gas may impede respiration.
2. (Gelatinous; Gelat′ino-sul′phurous b.; B. s. gelatino′sum, L.) Flanders glue, 11⁄2 to 2 lbs.; dissolved and added to a ‘sulphuretted bath.’ Recommended, by Dupuytren, as a substitute for the ‘Barèges bath.’
Obs. The sulphur or sulphuretted bath, under any of its forms, is a powerful remedy in almost every description of skin disease. Leprosy, the most obstinate of all, has been completely cured by it; the common itch requires only one or two applications to eradicate it entirely; all the scurfy and moist skin affections, local irritation, pimples, inflammatory patches, &c., speedily yield to its influence; scrofula, and, indeed, all those affections in which the warm or vapour bath is serviceable, also derive powerful assistance from the sulphur bath.
Bath, Tem′perate. Syn. Bal′neum tempera′tus*, L.
Bath, Tep′id. Syn. Bal′neum tep′idum, B. egel′idum, Tepida′′rium, L.; Bain tiède &c., Fr. Approaches the warm bath in its hygiènic and medical properties; and is, perhaps the one best adapted for the mere purposes266 of personal cleanliness. In the spacious public tepid baths of London, swimming may be safely indulged in even in cold weather.
Bath, Tum′ble. An obsolete form of the shower bath.
Bath, Turk′ish. Syn. Bal′neum tur′cicum, L. A hot vapour bath or sweating bath, with massing or shampooing, ending with a warm bath or warm ablutions and friction. The Egyptian, Persian, and Russian baths are essentially similar. In the Anglo-Turkish bath, recently introduced to this country, hot dry air wholly takes the place of vapour. See Bath, Air (antè).
Bath, Turpentine. Syn. Bal′neum terebinthina′tum, L. Prep. (Dr T. Smith.) Camphine (rectified oil of turpentine), 1⁄4 to 1⁄2 pint; Scotch soda, 2 lbs.; oil of rosemary, 1⁄2 dr.; for an adult. It calms the pulse, softens the skin, and renders the perspiration freer.
Bath, Va′pour. Syn. Dew′-bath*; Bal′neum va′poris, B. ro′′ris†, As′sa suda′tio, A. vapora′tio, Vapora′′rium*, L.; Bain de vapeurs, Fr. The vapour of hot water, either pure or medicated.
The simplest form of vapour bath is, perhaps, produced by placing some wet cloths, or sprinkling a little water on two or three heated bricks, laid under a chair on which the patient is seated; both the patient and whole apparatus being covered with a sheet or blanket, or, better still, a spacious waterproof cloak, to keep in the heated vapour. A large lump of quick-lime, set in a pan or an old iron pot and sprinkled with a little water, or else wrapped up in a thick coarse towel which has been previously soaked in water, may be substituted for the hot bricks; and often advantageously so. The slaking of the lime and the consequent evolution of vapour may be kept up or renewed, when necessary, by sprinkling on a little more water. This forms the “POOR MAN’S VAPOUR BATH” of the French. Dr Serres has suggested, as something apparently original, that a lump of quick-lime, wrapped in a wet cloth and covered with a dry one, be placed on each side of the patient;[118] and the whole being covered up allowed to remain until copious perspiration is established. It must, however, be recollected that by none of these minor contrivances can the temperature of the vapour, and its supply, be regulated, as in a perfect bath, even a portable one, such as is shown in the engraving.
The following are the temperatures, &c., of this bath:[119]—
| Temperature of Vapour, Fahr. | ||
| Breathed. | Not breathed. | |
| Tepid vapour bath | 90° to 100° | 96° to 106° |
| Warm vapour bath | 100 to 110 | 106 to 120 |
| Hot vapour bath | 110 to 130 | 120 to 160 |
Uses, &c. It is one of the most powerful diaphoretics known, and is almost specific in nearly all those cases wherein warm or hot bathing proves advantageous. It is one of the most certain agents existing in cases of chronic rheumatism, contracted muscles and tendons, stiffness of joints, indurations, dysentery, diarrhœa, suppressions, &c. Instances are numerous in which the lame have thrown aside their crutches and the bedridden have again mixed with the world after a few applications of this bath. It is no uncommon thing to hear a patient start and shriek with agony before entering the bath, and to receive his congratulations and thanks on his coming out. They often exclaim—“It is wonderful. I could not have believed it!”[120]
Bath, Warm. Syn. Bal′neum cal′idum, B. calid′ulum, B. Therma′le, Therm′a, &c. L., Bain thermal, B. chaud, &c., Fr. A bath at a temperature equal, or nearly equal, to that of the human body.
The sensations attendant upon immersion in a warm bath are most delicious. Its first effect is to increase the circulation of the blood, and to determine it to the skin. After a few minutes an agreeable and universal increase of heat is experienced; the face and head are generally soon bedewed with perspiration; a pleasing and prevailing calm, both mental and physical, follows; and after remaining in it some 12 or 15 minutes the effect is of the most refreshing and happy character.
The idea that the warm bath is relaxing is erroneous. It is only so where persons remain in it too long, or take it too frequently. Nor are those who indulge in it more liable to take cold than others. On the contrary, they are less liable, unless they wilfully expose themselves, insufficiently clad (particularly about the neck and chest), to draughts of cold air.[121]
As a remedial agent, the warm bath is adapted to general torpor of the system, liver and bowel complaints, hypochondriasis, hysterical affections, morbid suppressions, dryness of the skin, nearly all cutaneous and nervous diseases, chronic rheumatism, &c. As a tonic or stimulant after excessive fatigue, great mental excitement, or physical exertion, it is unequalled, and furnishes one of the most wholesome, and at the same time luxurious sources of refreshment we are acquainted with. “To those who are past the meridian of life, who have dry skins and begin to be emaciated, the warm bath for half an hour, twice a week, I believe to be eminently serviceable in retarding the advances of age.” (Darwin.) The healthy longevity of the late Duke of Wellington, after a period of exposure and trials equal to the entire life of many individuals, has been by some, and we think correctly, mainly attributed to the free and constant use of the warm bath. A warm bath frequently gives great relief to infants suffering from griping or flatulence. See Bath (antè), &c.
Bath, Wa′ter. Syn. Bal′neum a′quæ, B. Aquo′sum, B. ma′′riæ, B. ma′′ris, L.; Bainmarie, Fr. A water bath; in chemistry and cookery, applied to a bath of hot or boiling water. See Bath (in chemistry), Bainmarie, &c.
BATH′ING (bāthé-). See Bath.
BATH METAL. A species of brass having the following composition:—
1. Zinc, 3 parts; copper, 16 parts; melted together under charcoal.
2. Fine brass, 32 parts; spelter, 9 parts. See Brass and Alloys.
BATH PIPE. See Pipes.
BATH, VICHY (ARTIFICIAL). Bicarbonate of soda, 17 oz.; water, 60 galls.
BATHS and WASH′HOUSES. See Bath.
BATTER. Ingredients beaten together so as to form a semi-fluid mass. In cookery, a semi-fluid paste, which becomes hard in dressing, formed of flour, and milk or water, or a mixture of them, enriched and flavoured with eggs, butter, and (frequently) spices, currants, &c., at will. Used for frying vegetables, fillets, &c., and as a material for fritters and pancakes; also to form puddings, which are either baked alone, or under meat; and to cover various articles during the operation of cooking them. Miss Acton gives the following formulæ:—1. (For the Frying-pan.) Butter, 2 oz.; boiling water (nearly) 1⁄4 pint; mix, and stir in, gradually, of cold water, 3⁄4 pint; when quite smooth, mix it by degrees, very smoothly with fine dry flour, 3⁄4 lb.; adding (for fruit) a small pinch of salt (but more for meat or vegetables); just before use, stir in the whites of two eggs (or the white and yelk of one), and fry until light and crisp. In humble cookery the eggs may be omitted.
2. (For Puddings.) Eggs (yelk and white), about 4 in no.; flour, 1⁄2 lb.; milk, q. s.
Obs. When fruit, &c., are added, the batter must be made thicker than when none is used, to prevent it sinking. When sufficiently dressed it should cut smoothly and not stick to the knife. Eggs increase its firmness.
BATT′ERY. In frictional electricity, a series of Leyden jars so arranged as to admit of being charged and discharged together. See Electricity, &c.
Battery. In electro-chemistry, galvanism, &c., a pair, or series of pairs, of ‘excited’ metallic plates, so arranged as to act in unison, producing an electrical current by chemical decomposition.
BAUME (Baumé). See Areometer.
Baume Nerval. See Ointments.
BAUXITE. A ferruginous aluminic hydrate containing 55·4 per cent. of alumina and 44·5 of ferric oxide. It is met with in roundish masses in the crystalline limestone of Baux (hence its name) near Arles, in France. Bauxite is one of the sources of alum.
BAY. See Sweet Bay.
BAY ESSENCE. Bay Rum. This compound, which is largely employed as a perfume in America, and is one of the articles of the United States’ Pharmacopœia, is, when genuine, imported from the West Indies, where it is said to be prepared by distilling rum, with the leaves of the bayberry tree. More than three fourths, however, of the bay rum consumed is undoubtedly an imitation of the imported essence, and is a mechanical mixture of the volatile oil of the bayberry tree, rum, and spirit; sometimes with the addition of aromatic spices and various colouring matters. The volatile oil from which this last preparation is made is frequently adulterated to a large extent.
Mr Rother, an American chemist, states that in one sample alone he found about fifty per cent. of fixed oil. The imported rum is far superior in point of fragrance to the artificial. When mixed with water the genuine essence remains clear, whilst the imitation almost always becomes turbid or milky.
Mr Rother finds the following formula to yield a satisfactory product, and one much stronger in aroma than the imported perfume:
| Oil of bayberry tree and ml xx. | 1 fl. oz. |
| Jamaica rum | 1 pint o.m. |
| Strong alcohol | 4 pints o.m. |
| Water | 3 pints o.m. |
Mix the rum, alcohol, and water, then add the oil; mix, and filter.
Bay Rum. One of the highly valued American head-washes, pleasant in use, cooling and cleansing, and promoting the growth of the hair. It is prepared by distilling rum from the leaves of Myrica acris (called “Bayberry” in America).
BDEL′LIUM (dĕl′-yŭm). The commercial name of two gum-resins:268—
Bdellium, Af′rican. Syn. Bdellium, Africa′num, L. From the heudola′tia africa′na (Guillem.), a terebinthaceous tree, of Senegal.
Bdellium, In′dian. Syn. In′dian myrrh, False m.; Bdellium (of Scripture); Bdellium in′dicum, L. From am′y̆̆ris commiph′ora (Roxb.), or balsamoden′dron Roxbur′gii, a terebinthaceous tree of India.
Prop., &c. Once considered slightly deobstruent; sometimes used as a pectoral and emmenagogue, and, externally, as a stimulant and suppurative. It is now seldom met with in this country.
BDELLOM′ETER (dĕl-). Syn. Mechan′ical leech; Bdellom′etrum, L.; Bdellomètre, Fr. In surgery, a contrivance combining the principle of the cupping-glass, scarificator, and exhausting-syringe in one small instrument.
BEACH’S (Dr) Specific against Hemorrhoids and Stomach Complaints of all kinds. A tin box containing about 160 grammes of a fine sulphur-yellow powder, and imbedded in it a vial with 40 grammes of a brown clear fluid. The powder is a mixture of 7 parts of washed flowers of sulphur, 21⁄2 parts cream of tartar, 1⁄6 part of an inferior kind of rhubarb, finely powdered. The drops consist of a solution of brown sugar in strong spirit, with traces of various ethers. (Hager.)
BEAD (bēde). Syn. Glob′ulus, sphær′ula, &c., L.; Grain (de collier), &c., Fr.; Bethe, Perle, &c., Ger. A little ball or spheroid pierced for stringing; any very small globular body‡; a bubble (‡ or tech.). A number of the first mounted on a thread or ribbon form a ‘string of beads’ or ‘chaplet.’
Materials, Manufac., &c. Beads are often formed of coral, gems, jet, pearls, porcelain, rock-crystal, &c.; but much more frequently of white and coloured glass. The mode in which these last are produced is as follows:—Glass tubes, appropriately ornamented by colour, reticulation, &c., are drawn out in various sizes, and from 100 to 200 feet in length. These tubes are cut into two-feet lengths, and then, by means of a steel knife, divided into pieces having, as nearly as possible, the same length as diameter. The resulting small fragments or cylinders are next well stirred with a mixture of sand and wood ashes, in order to prevent the closure of the perforations and their adhering together during the subsequent part of the process. They are then placed in a revolving cylinder and gradually heated until they become sufficiently spherical. They are next sifted from the sand and ashes, sorted into sizes, first by means of sieves, and afterwards by hand, and are lastly either put up in weighed parcels or strung by women and children for the market.
The manufacture of coral, gems, jet, and minerals generally, into beads, belongs to the lapidary.
Uses. Chiefly to form necklaces, bracelets, and other articles of personal ornament; by milliners to decorate head-dresses, &c.; and for other like purposes. They are also employed among Catholic and Mohammedan nations for devotional purposes; and among savage tribes in lieu of money. They are still sometimes worn as amulets. See Bugle, Coral, Glass, Paste, Pearls, &c.
Beads, Jum′ble (bēdz). The dried seeds of a′brus precatōr′ius (Linn.) or Jamaica wild liquorice. Hard and indigestible; accounted cephalic and ophthalmic by the vulgar.
Beads, Lo′vi’s. Syn. Specif′ic-grav′ity beads. Small hollow spheres of glass carefully adjusted and numbered, in sets, intended to supersede the hydrometer in determining the density of fluids. They are used by dropping them into the liquid, in succession, until one is found that exhibits indifference as to buoyancy, and will float under the surface at any point at which it may be placed. The number on this ball indicates, in thousandths, the sp. gr. sought. They are particularly serviceable in the hurry of the commercial laboratory, and have the advantage of being applicable to very small quantities of liquid; but their use, of course, requires the same precautions, and the results obtained the same corrections for deviations from the normal temperature, as with other instruments. See Hydrometer, Specific Gravity, &c.
Bead. Syn. Bead′ing‡. In architecture, cabinet-work, &c., any small moulding or continued projection of which the vertical section is semicircular.
Bead (of Liquors). [Tech.] The small bright iridescent bubbles, possessing some slight degree of permanence, which form on the surface of alcoholic liquors of sufficient strength, when agitated. See Alcoholometry, Proof, &c. (also below).
BEAD′ING. In the liquor-trade, anything added to commercial spirits to cause them to carry a ‘bead’ and to hang in pearly drops about the sides of the glass or bottle when poured out or shaken. The popular notion being that spirit is strong in proportion as it ‘beads,’ the object is to impart this property to weak spirit, so that it may appear to the eye to be of the proper strength. Various formulæ are current among the ‘knowing ones’ of the trade, most of which are unscientific, and many of them absolutely ineffective. The following are those now usually employed:—
Prep. 1. Oil of sweet almonds and oil of vitriol, of each 1 oz.; rub them together in a glass, porcelain, or wedgewood-ware mortar or basin, adding, by degrees, of crushed lump-sugar, 1 oz.; continue the trituration until the mixture becomes pasty, then add, gradually, sufficient rectified spirit (strongest) to render the whole perfectly liquid; pour it into a quart bottle, and wash out the mortar twice, or oftener, with a little fresh spirit, until about 1 pint of rectified spirit has been employed, adding the washings each time to the bottle;269 lastly, cautiously shake the bottle (loosely corked) until admixture appears complete, and then set it aside in a cool place. For use, this compound (after agitation) is thrown into a two-gallon can or measure, which is then filled, from a tap, with the spirit to be ‘beaded,’ when the whole is thrown into the cask, and the measure washed out by refilling it and returning it two or three times; after which the contents of the cask are well ‘rummaged up,’ Gin is usually ‘fined’ a few hours afterwards; but it is better not to add the ‘finings’ for two or three days. Other spirits are allowed to become ‘fine’ by simple repose. According to Mr Hartley, and others, this quantity is “sufficient for 100 galls. of any spirit;” but it is more commonly used for a puncheon of 80 to 85 gallons.
2. Oil of vitriol, 2 to 3 oz.; rectified spirit, (strongest), 1 pint; cautiously agitate them together in a loosely corked quart bottle; in 2 or 3 hours add another pint of rectified spirit, and again agitate. It will be fit for use in a week; as before.
3. Sulphuric ether, 1⁄2 lb.; strongest rectified spirit, 1 quart; mix. May be used at once, as before; but if otherwise, should be kept, like the last, closely corked, and in a cool place.
4. Soapwort-root (saponaria officinalis), bruised or rasped small, 1 lb.; rectified spirit and water, of each 1⁄2 gall.; macerate in a corked jar, with occasional agitation, for 8 or 10 days, strain with pressure, and, after a few days’ repose, decant the clear portion. Used as before.
Obs. The above are not injurious when employed for ‘beading,’ since the quantity employed is much too small to injure the wholesomeness of the liquor. The fraud consists in their being used to disguise the presence of 10 to 12% of water, which is thus sold at the price of spirit. Beyond a certain degree of dilution they fail, however, to produce the intended effect, the bubbles becoming ‘soapy,’ and without the requisite permanence. The addition of a little powdered white sugar (1⁄2 oz. to 11⁄2 oz. per gall.) increases the efficacy of all of them. This may be dissolved in the water, if any is added at the time; but its effect on the hydrometer must be recollected. See Alcoholometry, Gin, Spirit (Management of), &c.
BEAK′ER (bēke′-). Syn. Beak′er-glass. In chemistry, a beaked cup or glass, more or less of the tumbler-pattern, used to collect precipitates and to heat liquids in.
BEAL* (bēle). Syn. Bouton, Pustule, Fr. A pimple or pustule; a small inflamed tumour.
BEAM (bēme). See Balance, Scales, &c.
BEAM′-TREE. Syn. White beam-tree. The ‘pyrus aria’ or wild pear. Wood, hard, compact, and tough; used for axle-trees, naves and cogs of wheels, &c.
BEAN (bēne). [Sax., Eng.] Syn. Fa′ba, L.; Fève, Fr.; Bohne, Ger. The general name of leguminous seeds, as also of the plants which produce them; appr., fa′ba vulgār′is (Mönch.[122] vĭcia faba, Linn.) or common GAR′DEN-BEAN, phase′olus multiflōr′us (Willd.) or SCARLET-RUNN′ER,[123] and ph. vulgaris (Sav.), French bean, KID′NEY-B., or HAR′ICOT (-ko),[124] with their varieties, all of which are annuals cultivated in our gardens—the first chiefly for its seeds—the others both for their green pods and ripe seed. The name is also often popularly applied, as an appellative, to the fruit or seeds of other plants which, in size and appearance, resemble common beans, as noticed below.
Those principally cultivated in our gardens are the small Lis′bon, Sand′wich, Span′ish, Tokay′, Wind′sor, and Maz′agan (from north Africa), with almost innumerable sub-varieties of each. The exquisite perfume of beans in blossom is referred to by the poet Thomson:—
Preparations including their fragrant principle are highly prized in modern perfumery.
Qual., &c. The pods eaten in the green state, properly dressed, are regarded as antiscorbutic and wholesome; but are apt to produce flatulence, unless combined with spices. In the dried or ripe state they are rather difficult of digestion, and very apt to distend the stomach and intestines with wind. This objection does not exist, to the same extent, to their use in the form of flour or meal. The amount of nutritious nitrogenous matter in beans rather exceeds that in wheat, and independently of a disposition to produce constipation in some habits, and being rather less easy of digestion, they must be considered nearly as wholesome as that cereal. The London millers and bakers use immense quantities of bean flour to adulterate their flour and bread.
This sophisticant may be detected by the appearance it presents under the microscope. The meshes of cellulin are very much larger than those of the fourth coat of wheat, with which it has been sometimes confounded, and the starch grains present a totally different appearance. They are oval or reniform, or with one end slightly larger; they have no well-defined hilum or rings, but many have a deep central longitudinal cleft running in the longer axis, and occupying two thirds or three fourths the length, but never reaching completely to the end; this cleft is sometimes a line, sometimes a chasm, and occasionally270 secondary clefts abut upon it at parts of its course; sometimes, instead of a cleft, there is an irregular-shaped depression. If a little liquor potassæ be added the cellulin is seen more clearly. If the flour be added to a little boiling water, the smell of bean becomes evident.
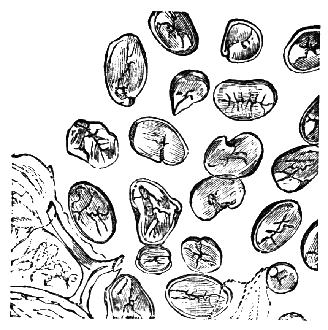
Green beans (pods or legumes) are cooked by simply throwing them into boiling water, and simmering them until quite tender, taking the precaution of removing the lid of the saucepan, a ‘pinch’ of salt of tartar, or a little common salt, being usually added to preserve their green colour. Young and small ones take from 12 to 18 minutes—large or older ones longer. The first are merely ‘topped and tailed’ with a knife before being dressed; the others require also the ‘side strings’ to be drawn off, and to be cut obliquely into pieces of a lozenge form, or else to be split lengthwise into strips, and then divided once across. Old ones never boil tender. Windsor beans, and other “shelled beans,” take 15 to 30 minutes according to age. These last are sometimes skinned after being dressed. All of them are commonly ‘served up,’ or eaten, with melted butter. Beans, although rich in nitrogenous, are deficient in carbonaceous constituents; hence it is curious to note how almost invariably they are when eaten combined with some substance rich in carbon. The Hindoo, for instance, mixes lentils with rice and ghee or a form of clarified butter. In Yucatan and throughout the whole of central Africa, where a black bean is extensively used as food, they are well boiled in water, and eaten with pepper, salt, and pork. In this country, beans and bacon always appear at table together, and have done so for centuries. See Leguminosæ, Pulse, &c. (also below).
Bean, Algaro′ba. See Algaroba.
Bean, Earth. American earth-nut.
Bean, French; Horse-bean; Kidney-bean; &c. See Bean (antè).
| Kidney beans. | Field beans. | |
| Water | 23·0 | 15·6 |
| Albumenoid bodies | 23·6 | 11·7 |
| Starch, sugar, gum, &c. | 44·7 | 58·3 |
| Oil and fat | 0·7 | 2· |
| Husk | 7·0 | 10·0 |
| Salts (ash) | 1·0 | 4·4 |
| ——— | ——— | |
| 100· | 100· |
Bean, St. Ignatius’s. The poisonous seed of the fruit of Igna′tia amār′a, Linn.; stry̆̆ch′nos Igna′tii, Berg.; a tree indigenous to the Philippine Islands.—Prop., Uses, &c. Similar to those of nux vomica. Contains Strychnine (which see).
BEAR (bare). Syn. Ur′sus, L.; Ours, Fr.; Bär, Ger.; Bera, Sax. In zoology, a Cuvierian genus of the ‘plantigrade carnivora,’ of several species, found both in the Old and New World. Those generally known under the name are omnivorous or frugivorous. The skin of the American black bear (ursus America′nus, Pallas) was formerly highly prized, and fetched an extravagant price. The brown bear (u. arc′tos, Linn.) supplies the Kamschatkans, and some other northern races, with many of the necessaries, and even the comforts of life. The fat or grease (BEAR’S GREASE; AD′EPS UR′SI, L.) of all the common species has long been highly esteemed for promoting the growth of the human hair; but apparently without sufficient reason. The mass of that sold under the name in England is simply hog’s lard or veal fat, or a mixture of them, variously scented and slightly coloured. The quantity annually consumed in Great Britain, and exported, is estimated at many tons; being a larger quantity than all the bears at present procurable in Europe would supply, if slaughtered and stripped of their fat.
BEAR′BERRY, Bear’s Bil′berry, &c. See Uva Ursi.
BEAR’S GREASE. See Bear (above), Hair Cosmetics, Marrow, Pomades, &c.
BEARD (bēerd). [Sax., Eng.] Syn. Bar′ba, L.; Barbe, Fr.; Bart, Ger., Dan.; Baard, Dut. The hair of the lips and chin; but appr., only the last—that on each lip being distinguished, in toilet-nomenclature, by a separate name. In popular botany and zoology, any beard-like appendage; the ‘awn’ of corn or grass; the ‘gills’ or breathing organs of oysters and other bivalves, &c.
Beard-cultivating Pomade, Royer’s (Royer & Co., Berlin). An ointment of 1 part pulv. cinchon. rub., and 11⁄2 parts of a hair pomade containing wax. (Hager.)
Beard-cultivating Tincture (Bergmann, Rochlitz). A spirituous extract of some271 agreeable bark, mixed with a little oil of rosemary and thyme. (Wittstein.)
Beard-cultivating Tincture, Royer’s (Royer, Berlin). 10 grammes kitchen salt, 150 grammes French brandy, fictitious and fuselly, and 2 grammes tincture of mace. (Schädler.)
Beard Tincture, American (Teinture americaine pour la barbe), for dyeing the beard black. Three fluids. No. 1, nitrate of silver solution; No. 2, tincture of galls; No. 3, sodium sulphide solution.
BEARD′ED. Syn. Barba′tus, L.; Barbu, Fr.; Bartig, Ger. In anatomy, botany, and zoology, having a beard, or a beard-like appendage; prickly, barbed, jagged; awned.
BEA′VER, (bē′-) Syn. Cas′tor, L.; Castor, Bièvre, Fr.; Biber, Ger. The fi′ber cas′tor (Linn.), an animal belonging to the rodentia of Cuvier, and remarkable for the great ingenuity which it exercises in the construction of its lodges or habitations. Hab. Europe and America. Those of the former are burrowers; those of the latter, builders. The fur has long been employed in the manufacture of the best quality of hats (BEAVER HATS). The fat was officinal in the Ph. L. 1618. Castor (CASTO′′REUM) is obtained from this animal.
BE′BEERINE (bēbe′-ĕr-in‡). C19H21NO3. [Eng., Fr.] Syn. Bi′birine (bē′-bĕr-ĭn); Bebeeri′na, Bibiri′na, &c., L. A peculiar alkaloid, discovered by Dr Rodie, in the bark and seeds of the beeberu, bibiri, or green-heart tree (nectan′dra Rodiæ′i, Schomb.), of British Guiana; and since minutely examined by Maclagan and Tilley, and by Von Planta.
Prep. 1. That of commerce, which generally contains some sipirine (—? altered bebeerine), and a little lime, is generally first obtained in the form of sulphate, by a process analogous to that employed in the preparation of sulphate of quinine; and from this salt it is precipitated by the addition of ammonia or an alkali.
2. (Pure.) By precipitating the sulphate with ammonia, washing the precipitate with very cold water, and triturating it, whilst still moist, with fresh hydrated oxide of lead; next drying the mixture by a gentle heat, exhausting the residuum with alcohol, distilling off the alcohol, and treating the last residuum with ether; the ethereal solution on evaporation leaves pure bebeerine, under the form of a white or yellowish-white, resinous-looking substance, which is pure white when powdered.
Prop., &c. Amorphous; uncrystallisable; non-volatile; bitter-tasted; inodorous; unalterable in the air; very slightly soluble in water; very soluble in alcohol; less so in ether; reaction alkaline; when quite pure, melts at 355° Fahr., and on cooling forms a vitreous or semi-vitreous mass (Winckler); at a higher temperature it suffers decomposition; ignited on platinum-foil, it burns without leaving any carbonaceous residue; neutralises acids forming uncrystallisable salts, most of which are soluble in water.—Prod. From the bark, 1·5 to 1·75%; dried seed, 2·5% (nearly).
Use, &c. Bebeeru-bark has been proposed and occasionally employed as a substitute for cinchona bark, and bebeerine for quinine, in the usual cases; but whether as a tonic, febrifuge, or antiperiodic, they appear less powerful and certain than these last.—Dose, 2 to 12 gr. or more. (See below.)
Sul′phates of Bebeerine. Of these there are two, both of which are obtained in a similar manner to the Ph. E. formula for sulphate of quinine, and merely differ in the amount of acid finally left in combination with the alkali:—
1. Sulphate. Syn. Neu′tral sulphate of bebeerine; Bibiri′næ sul′phas, &c., L. Easily soluble in water. Contains 86·4% of bebeerine, and 13·6% of sulphuric acid.
2. Subsul′phate. Syn. Bas′ic sulphate of bebeerine, Disul′phate of b.; Biberi′næ subsul′phas, &c., L. Soluble in alcohol; sparingly soluble in water unless acidulated. Contains 90·8% of bebeerine, and 9·2% of sulphuric acid. This is the sulphate of bebeerine of commerce, and the one usually employed in medicine. It is generally met with in thin brownish-yellow scales, which are formed in a similar manner to those of ammonio-citrate of iron.—Dose. As a tonic, 1 to 3 gr.; as a febrifuge or antiperiodic, 5 to 20 gr.; in similar cases to those in which disulphate of quinine is employed.
BECH′AMEL (bĕsh′-ă-mĕl‡[125]). Syn. Béchamel, Fr. In French cookery, a fine white sauce, essentially consisting of concentrated veal gravy or veal consommé and cream, with or without flavouring. See Sauces.
BE′CHIC* (-kĭk). Syn. Be′chous†; Be′chicus (bĕk′-‡), L.; Béchique, Fr.; Hustend, &c., Ger. In medicine, &c., of or for a cough; pectoral; also subst., applied to remedies (BE′CHICS; BE′CHICA, L.) used to relieve cough.
BED. [Eng., D., Sax.] Syn. Lit, Couche, Fr.; Bett, &c., Ger.; Cubi′le, Lec′tus, Lec′tulus, Graba′tus, &c., L. A couch; that in or on which we sleep; that on which anything is generated, deposited, or rests.
Bed curtains and valances are both unnecessary and objectionable as bed appendages, and as such should be discarded. Before making the bed in the morning the blankets and sheets should be stripped off and allowed to remain for an hour or two in a current of air, on the back of a chair or some other convenient support. If it does not rain, or is not too damp, they are best placed near the window. The night dress which has been slept in should be exposed in the same manner; and on going to bed it would be found a good plan, when removing the inner vest which has been worn272 during the day, to turn it inside out, and to hang it over the footboard of the bed. Under ordinary conditions the sheets should be changed every week. When it is remembered that on an average a third of a human being’s existence may be said to be passed in bed, the importance of his dormitory being kept scrupulously clean will be self evident. Every bedroom should therefore be well swept out each day, and the floor diligently scrubbed once a week. With the exception of a small strip beside the bed, the room should contain no carpet; a piece of New Zealand matting, being less able to retain dust, is preferable to carpeting. The door and windows of the bed-chamber should be kept more or less open during the day, so as to ensure a thorough draught of air through the room, and all slops and contents of chamber utensils should be immediately removed. No plants should be allowed in the bedroom.
There is no better form of mattress than one made of horsehair, both for children and adults. The pillows should also be made of the same material. Both pillows and mattress should be taken to pieces once a year, and their contents well ventilated by exposure to the air. When a child is ricketty, weak in the neck, inclined to stoop, or at all crooked, a pillow is best dispensed with. Cotton sheets have two advantages over linen ones—they are more absorbent, and feel less cold. In cases of sickness the comfortable construction of the patient’s bed, as well as the adoption of such means as shall ensure as much as possible its efficient ventilation, are matters of primary import. Hence because it permits of a more thorough circulation of air than any other kind, the horsehair mattress calls even more imperatively for adoption than in health. It may be placed upon the feather or wool bed, and should it be found too rough, or causing any discomfort, one or two blankets may be placed over it. The straw palliass should at the same time be removed. Both sheets and pillow cases should be frequently changed, more especially in fevers. If the patient perspire very profusely, fresh sheets and pillow cases should be supplied every twenty-four hours. If soiled by evacuation of any kind, it is most important that they should be changed at once, and so with the night dress. In all cases of eruptive and other fevers, and contagious diseases, all articles of wearing apparel (underclothing, as well as sheets, pillow cases, handkerchiefs, &c.) should when removed be placed in a vessel and covered with water.
In the articles on “air” and “vitiated air” the evil effects of ill-ventilated dormitories have been adverted to. Every bedroom should if possible contain an enclosed fireplace having free access to the chimney. Failing this a series of little holes about the size of a shilling should be bored in the lower part of the door, and the upper sash of the window should be opened to the extent of two or three inches.
[On the connection of BEDS and BEDDING with comfort and health, see Cotton, Damp, Feathers, Linen, Sleep, Ventilation, Vermin, &c.; also below.]
 1.
1.
 2. (Opened.)
2. (Opened.)
 3. (Closed.)
3. (Closed.)
Bed, Air. Beds, pillows, cushions, &c., when properly constructed and inflated with air, are clean, luxurious, and healthy substitutes for those in common use. For this purpose the air-proof part should be formed of separate cells or tubes, arranged in ridges (see engr.), or in any similar manner to admit of free ventilation; and in the case of beds, or of cushions for the sick, two or three folds of flannel, or blanket, or of any loose porous fabric, should be placed between them and the under sheet or the person of the sleeper or patient. Without this precaution, discomfort and restlessness, excessive warmth and perspiration and even bed-sores, are apt to follow their use by invalids, when badly constructed. To obviate these objections to articles of this class commonly sold, a new one has been produced under the name of the ‘Incom′parable bed’ (Aycbourn’s Patent), which is thoroughly applicable to all purposes—domestic, medical, naval, or military—and superior to any feather, flock, or spring bed, however good or carefully made up. This bed consists of an outer case made of ordinary bed-ticking divided internally into numerous separate cells, into each of which is placed a suitably constructed bag, which may be either wholly or partially filled with air or water; the latter either hot or cold. (See engr. 2, 3.) It is incapable of bursting, and is very agreeable to lie on. It retains its shape, saves the time, trouble, and wear and tear ordinarily273 bestowed or produced by servants in daily tossing about one of down or feathers, is easily washed and kept clean, allows all the ventilation essential to health, and is so portable that it may be easily packed in a carpet-bag. In almost an instant it may be converted into six, or more, separate life-preservers; and what is equally important it will stand any climate. Hitherto the use of air-beds and water-beds has been almost exclusively confined to the upper and wealthy classes, and to hospital practice; but the moderate prices[126] at which Aycbourn’s beds, cushions, &c., are sold, place these luxuries, and in many cases—absolute necessities—within the reach of the masses of the people.
Bed, Water. Water-beds, cushions, &c., are chiefly employed for patients labouring under bed-sores, paralysis, spinal affections, &c., or who are the subjects of active surgical treatment in which equable support for the body or a limb is absolutely necessary. Their construction and use are similar to those previously noticed, except that, instead of being inflated with air, they are filled with water, either warm or tepid. For the bedridden, and for long-continued use generally, they are much inferior to air-beds. See Air-bed (above).
Bed. Syn. Stra′tum, L.; Strate, &c., Fr.; Schicht, &c., Ger. In geology, a mineral layer, seam, or stratum, thick or thin.
Bed. In horticulture, a small plot of land, usually raised a little above the general surface, in which flowers, or other plants, are raised or grown.
BEDDING, PURIFICATION OF. To be efficiently disinfected, bedding must be taken to pieces and subjected to dry heat. This last condition can only be satisfactorily carried out in large ovens or disinfecting chambers. Any local authority may provide a proper place and necessary appliances for the disinfection of bedding. Any local authority may direct the detention of bedding, clothing, &c., which have been exposed to infection, and may give compensation for the same.
Any person giving, lending, selling, transmitting or exposing bedding, clothing, rags, &c., which have been exposed to infection, is liable to a penalty not exceeding £5.
Bedding or Litter. The following is from Col. Fitzwygram’s useful work, ‘Horses and Stables,’ “One great item in a horse’s comfort, and consequently in his aptitude to carry flesh, is a good bed. Every horse should be bedded down at mid-day. As regards economy of straw, it is essential not to give the horse a chance of eating it. With this view no fresh straw should be placed within his reach. The fresh straw should be brought in first, and put not merely at the bottom, but also in rear of the stall; then the old litter should be brought in and put at the top and in front. The horse will not readily eat at, and by the following morning the new straw will have become somewhat tainted, and may then be mixed and dried along with the rest. Again, great care should be taken in the morning to thoroughly shake up and cleanse the bedding from dung; and any parts which may have become rotten should be thrown out. Good straw rapidly deteriorates if these precautions are not taken. On the other hand careless servants often throw away along with the bad parts much good bedding which might be dried and used again. Bedding should be taken up, and turned over at least twice in each forenoon, so as to expose every part to the drying and purifying influence of the sun and air. It is, however, a mistake to expose it over-much to the action of a very hot sun, as it makes it too dry and brittle.” See Horse.
BED′EGUAR (-e-gahr). Syn. Bédéguar (or GAR), Fr. Sweet-briar sponge (which see).
BEE (bē). Syn. Hive-bee, Hon′ey-b. (hŭn′-), Domes′tic b.; A′pis, L.; Abeille, A. mellifique, &c., Fr.; Biene, Honigbiene, &c. Ger. The a′-pis mellif′ica (Linn.; Ph. L., E., & D.), one of the hymenop′tera best known and most useful to man. [Those desirous of studying the habits and economy of bees are referred to the works of Huber and Latreille; and for their management to Mr Cobbett’s little book on the subject.] See Apis, Hive, Honey, Wax, &c.
BEE′-BREAD. The pollen of flowers collected by bees as food for their young.
BEE′-GLUE, Syn. Prop′olis, L., Fr. The resinous matter with which bees cement the combs to the hives, and close up and repair the cells.
BEECH (bēche). Syn. Beech′-tree; Fa′gus, L; Hétre, H. commun, Fr.; Buche, Gemeine b., Ger. The fa′gus sylvat′ica (Linn.), a magnificent English forest-tree, of the nat. ord. Amentaceæ (DC.). Fruit (BEECH′-MAST, B.-NUTS), used to feed swine, and, sometimes, in obstinate headaches, and in gravel complaints; yields oil by expression; inner bark occasionally used in hectic fevers. Wood (BEECH, B.-WOOD), handsome and very hard, but brittle and perishable, and particularly liable to become worm-eaten; its durability is increased by steeping it, when fresh-hewn, for some time in water; chiefly used by cabinet-makers, coach-builders, millwrights, and turners; and, sometimes, by coopers; also burnt for charcoal.
BEEF (bēfe). Syn. Chaire de bœuf, Du bœuf, Fr.; Rindfleisch, &c., Ger.; Bu′bula, Ca′′ro bo′vis, &c., L. The flesh of bovine animals, generally; but ordinarily only that of the domestic ox, cow, or bull.
Qual. Good beef is highly wholesome and nutritious; and is well adapted to persons of good appetite, or that labour or take much exercise. For the delicate, especially those suffering from debility, partial anæmia, amenorrhœa, and similar ailments, it is, perhaps,274 superior to every other kind of animal food. If cooked so as to be left full of gravy, it sits lightly on the stomach, and its fat proves even more digestible than that of either veal or mutton.
It has been proved that under-done beef is one of the causes of tapeworm.
Choice. Ox-beef is known by having a fine smooth, open grain, a lively and agreeable red colour, and a tender texture, with the fat of a pleasing pale whitish-yellow or but slightly yellow, and the suet white and hard. When fine and well fed, the flesh is inter-grained or marbled with fat.—Cow-beef has a closer grain than ox-beef, and the lean is of a deeper red.—Bull-beef is closer still, the fat dark, hard, and skinny, the lean of a deep coarse red, and it has a strong smell and flavour.—Heifer beef resembles ox-beef, except in being smaller, often an advantage; but it lacks the rich flavour of the flesh of full-grown oxen.
Joints, Managem., &c. Beef is CURED, SALTED, and DRESSED, in all the ways common to the other meats; the only care necessary being in the selection of the appropriate joint or part. The ribs, sirloin, rump, and veiny piece are the proper joints for ROASTING or BAKING. The buttock or round, edge-bone, second round or mouse-buttock, brisket, flank, shoulder or leg-of-mutton piece, and the clod, those generally BOILED, STEWED, or SALTED. The choicest STEAKS are cut from the middle of the rump; the next best from the veiny piece, or from the chuck-rib. In summer, excellent ones may also be cut from the shoulder. In France, steaks cut from the sirloin (without bone) are preferred to all others, and are exceedingly delicate and tender. The neck may be either stewed or boiled, and is much used to make soup and gravy. In the country, the round, when fine, and well hung, is also often roasted or baked.
According to Miss Acton, “the finest part of the sirloin is the chump-end, which contains the larger portion of the fillet; of the ribs, the middle ones.”
Beef is in season during the whole year, but is finest—when it is most relished—during the winter months, when, owing to the temperature of the air, it may be ‘hung’ a long time, and thus increased in tenderness and flavour. See Ox, Baking, Boiling, Essences, Roasting, Salting, Tea, &c. (also below).
Beef, Alamode′. Syn. Bœuf à la mode, Fr. The true ‘beef à la mode’ is made as follows; and is not a mere kind of rich stew, such as is daily sold under the name in the ‘cook-shops’ of London:—
1. (M. Alexis Soyer.) Rump, sirloin, or rib of beef (about) 12 lbs.; lard it through with 10 or 12 long pieces of fat bacon; put it into an earthen pan with a calf’s foot, 4 onions, 2 carrots (sliced), a bunch of parsley, 2 bay leaves, 2 sprigs of thyme, 2 cloves, 1⁄2 teaspoonful of pepper, 1 do. of salt, 4 wine-glassfuls of sherry, 4 do. of water, and 1 lb. of streaky bacon (cut into small squares); place on the cover, make it air-tight round the edges with a little flour-paste, and expose it in a moderate oven for about 4 hours. Dish up with the vegetables and bacon placed tastefully round it, the gravy (skimmed) being poured over all. Or it may be eaten cold, in which case the pan should not be opened until the whole has thoroughly cooled.
2. (Mrs Rundell.) Rump of beef (or any part of the beef which will stew well), 3 or 4 lbs.; trim it, and cut off the fat; add several sorts (according to taste) of sweet herbs chopped very fine, a little shalot, and a great deal of spice (cayenne, white pepper, allspice, cloves, and mace; or mixed spices), and put them, with vinegar, into a saucer that has been rubbed with garlic; add fat bacon cut into long slips; lard the beef regularly on both sides, and rub it over with the herbs and spices; next flour it, and add a small piece of butter, and a pint of water; bake it in an oven until thoroughly ‘done,’ then strain the gravy, and serve it up with pickles on the top. Excellent either hot or cold.
Obs. Miss Acton—a high authority in these matters—tells us, that 7 or 8 lbs. of beef, thus treated, takes 4 to 5 hours to dress it properly; and that if a stew-pan be used, it should be as nearly the size of the meat as possible, the whole being allowed to simmer very gently, and the meat turned when half done. She also states that “veal dressed in this way is even better than beef;” but, of course, it takes less time in cooking.
Beef, Col′lared. Prep. 1. (Miss Acton.) The piece of beef is rubbed with a little coarse sugar, and set aside for two or three days; it is then slightly salted (about 1 oz. of salt, containing a little saltpetre, to each lb. of meat); and allowed to rest 8 to 10 days; the bones and tougher skin are next removed, and the under side is sprinkled thickly with parsley and other savoury herbs (shred small), after which it is very tightly rolled up, secured with a cloth, and bound as closely as possible with broad tape. A piece of 8 lbs. will require about 5 hours’ gentle boiling, and should be placed, in the same state, whilst still hot, under a heavy weight, or in a press, for a few hours. The ribs, or (better) the thinnest part of the flank, is generally selected. The last should be ‘hung’ in a damp place for a day or two before curing it.
2. (Mrs Rundell.) From stewed shin of beef and ox-tail, re-stewed with a glassful each of wine and ketchup, and some of the old broth, and then poured into moulds. Sweet herbs, sliced eggs, and pickles, may be added at will.
Beef, Dutch, Hung Beef. The round, rump, veiny-piece, or thick flank, cured, for 10 or 12 days, with dry salt to which a little saltpetre and some sugar and black pepper has been added; and afterwards ‘hung’ for use. It eats well if boiled tender with greens or carrots.275 If to be grated or shred, as Dutch, and eaten as a relish on bread and butter, then cut a lean bit, boil it till extremely tender, and while hot put it under a press. When cold, fold it in a sheet of paper, and hang it in a very dry place. It will then keep two or three months.
Beef, Pott′ed. See Potted Meats, &c.
Beef, Spiced (spīst’). Salted beef when spices (usually black pepper and allspice) have been added to the salt, &c., used in curing it. See Beef, Collared (above).
Beef Tea. An extract manufactured at Berlin, which contains the nutritive matter of the flesh in the highest state of potency. A pale blood-red(!) jelly, which will not keep long, and after a time passes into a state of odorous putrefaction. (A. Buchner.)
BEER (bēre). Syn. Bière, Fr.; Bier, D., Ger.; Birra, It.; Cerevi′′sia (-vĭzh′-’ă), Cervi′′sia (Pliny), C. Lupula′ta* (i.e. hopp′d or modern b.), Vi′num Anglic′anum*, V. hordea′ceum* (-sh′ŭm), Zy′thum* (or -THUS*; ζὑθος, Gr.), &c., L.; Bere, Beere, Sax.; Bir, W. An aqueous infusion of malted grain which, after being boiled with hops, has undergone the vinous fermentation; malt-liquor. The word BEER is now the common generic term for all fermented malt-liquors, and, indeed, for all other beverages prepared by a process of brewing. Whenever the term is used in a special sense, it is with a descriptive prefix, as, for example, spruce beer, ginger beer, &c.
Hist. Ale and wine are fabled to have been invented by Bacchus; the former, in Egypt, where the soil and climate would not permit of the cultivation of the grape. Herodotus ascribes the origin of the art of brewing to Isis, the wife of Osiris, and notices zythum ζὑθος, a beer obtained from barley. Malt-liquor was undoubtedly employed as a beverage in the fifth century before Christ; and, probably, very much earlier. Xenophon distinctly alludes to it in his famous retreat (B.C. 401). Aristotle speaks of ‘beer drunkenness,’ and Theophrastus calls it ‘barley-wine.’ The Romans learned the art of brewing from the Egyptians, and gave the liquor thus made the appropriate name of cerevisia (quasi Cererisia), from its being the product of corn, the gift of Ceres. The most celebrated beer of ancient times was the Pelus′ian pota′tion, so named after a town at the mouth of the Nile where beer was prepared in great perfection. The use of beer was likewise known to the ancient Gauls and Germans, and probably also to most other ancient nations inhabiting the temperate zone. Pliny says “Zythum is made in Egypt, ce′lia and ce′′ria in Spain, and many other sorts (of beer) in Gaul.” In our own country, ale was early known and valued as a beverage. The art of its preparation appears to have been obtained either from the Romans or the Saxons. According to Verstegan, “This excellent and healthsome liquor, beere, anciently called ale, as of the Danes it yet is, was of the Germans invented and brought into use.” Alehouses are mentioned in the laws of Ina, king of Wessex (A.D. 680). Alebooths were regulated by law, A.D. 728. By the beginning of the 13th century ale was drunk generally in England. By a statute of James III, of Scotland, it was made a capital offence to mix wine with beer (A.D. 1482). In 1492, a licence was granted to a brewer at Greenwich to export 50 tons of that “ale” called “beer” or “bere;” the distinction between the two apparently being, that the latter was flavoured with wormwood or other bitters; whereas ale was not. Ale was originally made from barley-malt and yeast alone, and those who put in anything else were held to sophisticate the liquor. Hops were introduced A.D. 1524; and to this date modern, or hopp′d beer, may be traced.
By statute of James I the “ale” called “bere” was taxed, and “one quart of the best thereof” ordered to be sold for a penny (A.D. 1610). Alehouses were first licensed in 1621, and during the reign of Charles II were, together with all malt-liquor, placed under the control of the Excise (A.D. 1660). By the Statutes 1 & 4 Will. IV (1834), previous enactments respecting malt liquors and their sale were reduced to their present form. Beer is now the common beverage in all European countries where the vine is not a subject of rustic husbandry.
Qual. Pure malt-liquor which has undergone sufficient fermentation is perhaps, when taken in moderation, one of the most wholesome beverages that can be drunk. Ale is the most nutritious variety, and, when moderately mature, is the one best adapted to the debilitated and delicate; but good porter, owing to being less rich in extractive and gummy matter, and from being slightly astringent from high-dried or scorched malt being used in its preparation, occasionally agrees better with bilious constitutions and the dyspeptic. Much, however, depends on acquired taste and habit. The most wholesome, and perhaps the least exceptionable beverages obtained from malt, are those known as East-India, Scotch, and Bavarian ales, when honestly prepared and not highly ‘bittered’ with the hop, as is, unfortunately, now so general. A late writer has described good beer as nutritious, from the sugar and mucilage which it contains; exhilarating, from its spirit; and strengthening and narcotic, from its hops. Pereira says, “Beer is a thirst-quenching, refreshing, intoxicating, and slightly nutritious beverage.” Its effects, when taken injudiciously, or in excess, for the most part resemble those of other intoxicating liquors—disease, misery, and crime; and these in direct proportion as it deviates from the true standard of purity and excellence.
All medical authorities agree in discountenancing the use of beer for infants and276 children. Water or milk with the child’s meals are the best beverages for them.
Var. The numerous varieties of malt liquor met with in commerce may be resolved into two great classes—ALE and PORTER. Ale of all kinds is brewed chiefly from pale malt, and is generally of a light amber colour. Pale ale is manufactured from the finest and lightest dried malt, and the choicest hops, the latter in excess. Mild ale differs from pale ale in being sweeter, stronger, and almost free from the flavour of the hop. Bitter ale or BITTER BEER has, as a rule, less body than pale ale, and is more highly hopped. Table beer is a weak liquor commonly containing three or four times the proportion of water usually present in ordinary beer or ale. Porter differs from ale chiefly in its being artificially coloured by the use of roasted malt, which also imparts to it a peculiar bitter flavour. In point of strength it stands about midway between light and strong ales, although frequently brewed of a strength very slightly above that of table beer. Stout, BROWN STOUT, &c., are simply richer or stronger descriptions of porter, and may be said to have nearly the same relation to the higher qualities of mild ale that porter holds with regard to pale ale or bitter beer. In London, PORTER is called BEER; and, indeed, in all parts of the kingdom, the prevailing beverage of this kind consumed by the masses, of whatever class, commonly goes by the name of beer.
The two great classes of malt liquor above referred to, are, independently of mere differences of strength, excellence, and commercial value, practically subdivided into an almost infinite number of varieties. Every county, every town, and almost every brewer, is distinguished by the production of a different-flavoured beer, readily perceived, and highly appreciated by their respective votaries. These differences may be traced to—variations in the quantity and quality of the materials employed in their manufacture—the temperature of the water used for mashing—the duration of the boiling—the temperature at which the fermentation is conducted, and the extent to which it is carried, together with numerous other circumstances, which, though usually of an accidental and uncertain character, are nevertheless sufficient to affect the flavour and quality of a brewing. Among these, those depending on the condition of the building, the locality, the apparatus, the water, the management, &c., are not the least important. In general, however, when the same quantity and quality of materials are employed, and the same time allowed for the maturation of the liquor, the chief causes of this diversity will be found to depend on the water used in the brewing, and the method followed in the preparation of the malt. Thus, Bavarian, Scotch, and Burton ales differ in style from other ales chiefly from being fermented at a lower temperature, and from the water employed in the brewing being that usually denominated ‘hard,’ whilst porter and stout differ from all these because they are brewed from a mixture of pale and roasted malt. It is from causes like these, though apparently trivial, that the many varieties of malt liquor met with, at the present day, originate.
The per-centage in English beers of malt extract (dextrin and sugar glucose) is least in bitter, and highest in the sweet ales. The hop extract (lupulin and hop resin) is in much smaller amount.
The alcohol varies considerably, as does also the free acidity.
The albuminous matter in most beers does not average more than 0·5 per cent.
The salts, which consist of alkaline chlorides and phosphates, and some earthy phosphates, average 0·1 to 0·2 per cent. Ammoniacal salts are found in small quantities. Caramel and assamar are found in the dark beers and porters. Carbonic acid is always more or less present. The average is 0·1 to 0·2 parts by weight per cent., or about 13⁄4 cubic inches per ounce. Volatile and essential oils are also present.
Parkes says, “Adopting means numbers; one pint (20 oz.) of beer will contain—
| Alcohol | 1 | ounce. | |
| Extractive (dextrin), sugar, &c. | 1·2 | ” | (534 grains). |
| Free acid | 25 | grains. | |
| Salts | 13 | ” |
The following beer analyses are given by Professor Wanklyn:
Bass’s bottled bitter ale contains in 100 cubic centimètres:
| 5·3 | grams of | alcohol. |
| 5·52 | ” | organic residue. |
| 0·36 | ” | ash. |
A sample of draught ale, costing 2d. per pint in London, contained in 100 cubic centimètres:
| 4·7 | grams of | alcohol. |
| 5·8 | ” | organic residue. |
| 0·32 | ” | ash. |
A sample of London porter in 100 cubic centimètres contained:
| 3·3 | grams of | alcohol. |
| 3·45 | ” | organic residue. |
| 0·30 | ” | ash. |
“A large number of analyses recently made show that in the various classes of malt liquor sold in London there is a variation in the amount of alcohol contents from 3·87 to 8·41 per cent. of absolute alcohol by weight, these two extremes corresponding to ·98 and 2·18 fluid ounces of absolute alcohol in the pint of beer. The amount of extract varies from 2·16 to 13·32 per cent. by weight, or from ·73 to 2·77 ounces per pint of beer, as will be seen from the accompanying table.277
| Kind of Malt Liquor. | Specific Gravity. | Per-centage of | Original Gravity of Wort. | Malt, per barrel. | Con. vols., per pint. | ||||
| Alcohol | Extract | Acetic Acid. | Alcohol, fl. oz. | Extract ounces. | Acid, grains. | ||||
| Burton ale (Allsopp’s) | 1040·38 | 8·25 | 13·32 | ·32 | 1121·63 | 4·50 | 2·16 | 2·77 | 29·12 |
| Bass’s barley wine | 1032·31 | 8·41 | 11·75 | ·23 | 1114·78 | 4·25 | 2·18 | 2·42 | 20·77 |
| Edinburgh ale | 1006·63 | 4·41 | 3·58 | ·19 | 1048·38 | 1·77 | 1·12 | ·72 | 16·73 |
| Guinness’s stout | 1015·51 | 6·81 | 6·17 | ·24 | 1078·06 | 2·88 | 1·74 | 1·25 | 21·32 |
| Truman, Hanbury, & Co.’s porter | 1013·16 | 4·02 | 5·12 | ·24 | 1051·33 | 1·90 | 1·03 | 1·01 | 21·27 |
| Whitbread’s porter | 1014·04 | 4·28 | 5·15 | ·18 | 1054·11 | 2·00 | 1·09 | 1·03 | 15·97 |
| Hoare’s porter | 1012·99 | 4·18 | 5·04 | ·18 | 1052·42 | 1·94 | 1·06 | 1·03 | 15·95 |
| Perry’s ale | 1006·48 | 3·87 | 3·65 | ·14 | 1045·82 | 1·69 | 0·98 | 0·73 | 7·97 |
“The relative proportions of alcohol and extract in beer will also have some influence on its fitness in a medical point of view for certain persons; and in some instances thin dry beer, that has had the fermentation carried so far as to reduce the amount of extract to a minimum, may be very preferable to beer containing a larger amount of extract. In this respect some samples of the Prestonpans’ beer are remarkable for the small proportion of extract they contain.
“In regard to the nutritive value of beer, over and above the stimulant and tonic actions due to the alcohol and to the bitter principle of the hop, it is worth notice that a pint of pale ale contains from 1⁄2 an ounce to an ounce of solid extract, while mild and old ale contain from 11⁄10 to 23⁄4 ounces in the pint.
“The amount of free acid in British beer appears to be uniformly larger than in the Viennese and Bavarian beer recently introduced here, and sometimes it is very much larger. This free acid is represented in the tables as acetic acid; but there is reason to believe that beer probably contains lactic acid or other fixed acids, together with a substance analogous to glucic acid, which, according to Graham, Hoffmann, and Redwood, appears to be produced in the fermentation of beer wort, as practised in this country.
“There appears to be great differences in the quality of beer sold by publicans at a given price. Thus, for instance, the variation in the pale ale sold at fourpence per pint is from 4·08 to 7·10 per cent. of alcohol, and from 3·22 to 7·53 per cent. of extract; in the mild ale sold at twopence per pint it is from 4·43 to 5·62 per cent. of alcohol, and from 5·01 to 5·56 per cent. of extract; and in old ale sold at fourpence per pint it is from 6·20 to 8·31 per cent. of alcohol, and from 4·56 to 6·2 per cent. of extract. These differences represent respectively 1·58, ·27, and ·49 bushels of malt per barrel of beer. From the great alcoholic strength of some kinds of old ale they partake more of the nature of wine than of beer, in the usual sense of this term. They are, in fact, quite equal in that respect to most of the cheaper wine imported from the Continent, while in flavour and general character old ale, such as that brewed at Burton-on-Trent and in Scotland, is far superior to any wine of the kind referred to, which can be sold here at a price even double that of the best old ale. This kind of ale, however, is but rarely sold by publicans.”—Dr Paul.
Materials, Manuf., &c. See Malt, Hops, Brewing, &c.
Purity. The leading characteristics of good beer are transparency, a fine colour, an agreeable semi-vinous flavour, and the property of remaining for several hours exposed in a glass or cup without becoming ‘flat’ or insipid. If the materials used were good, if the brewing was skilfully conducted, if the liquor has been carefully stowed in perfectly sweet casks or vessels, in a suitable cellar, for a sufficient time, and has not been tampered with, this will almost always be the case. Hence colour, transparency, and flavour, and the power of resisting exposure, are tests of the purity and quality of beer, which should not be lightly treated. There are none more simple and effectual; and, together with a good ‘palate,’ and a close observance of its effects on the head and on the stomach, will readily distinguish pure and wholesome beer from ‘doctored’ and inferior liquor. If, therefore, we find a sample of beer possessing the above qualities and in good condition, and on testing it for its alcohol and saccharine matter, find these substances in such quantities as fairly to represent the amount of malt which should have been used in the brewing of such a liquor, we may, in the absence of proof to the contrary, infer it to be pure; because the object for which adulteration is practised—the saving of malt and hops—did not exist in this case. To demonstrate the purity of beer requires an elaborate and troublesome analysis, which can only be performed by those accustomed to chemical operations. Good and pure beer should contain nothing but what exists in the malt, the hops, and the water, from which it is brewed, or which is produced from them278 in the processes of ‘mashing,’ ‘fermentation,’ and ‘maturation.’
Adulteration. Until the year 1862 nothing was allowed to enter into the composition of beer but malt and hops; and the Act 56 Geo. III, cap. 58, imposes a penalty of £200 on any “brewer, dealer, or retailer of beer,” who “shall receive, or have in his possession, or use, or mix with, or put into any worts or beer any molasses, honey, liquorice, vitriol, quassia, cocculus indicus, grains of paradise, Guinea-pepper, or opium, or any extract or preparation of these substances, for, or as a substitute for, malt or hops;” and a further penalty of £500 on any “druggist, or vender of, or dealer in drugs, or chemist, or other person whatever,” who shall “sell, send, or deliver to any licensed brewer,” &c., any of the above materials. However, by the Act 25 Vic., cap. 22, s. 20, so much of the above is repealed as relates to hops. This Act provides that—
“On and after the 16th Sept., 1862, so much of an Act passed in the 56th year of the reign of King George the Third, cap. 58, and of an Act passed in the 7th and 8th years of the reign of King George the Fourth, cap. 52, and of any other Act relating to the revenue of excise, as imposes any excise penalty upon any brewer of, or dealer in, or retailer of beer, for receiving into, or having in his possession, or using or mixing with any worts or beer, any article for, or as a substitute for hops, or as prohibits the sale of any such article to the said persons, shall be, and is hereby repealed: provided always, that nothing herein contained shall be construed to extend to repeal any such penalty or prohibition so far as regards any article which may be used as a substitute for malt, notwithstanding that it may be also a substitute for hops.”
Prior to this an Act (10 Vic., c. 5) had been passed allowing brewers to use sugar under certain restrictions.
As the law now stands, a brewer may use hops, quassia, wormwood, gentian, or any other simple bitter; but he is forbidden to use any substitute for malt, such as unmalted grain, sugar in a liquid state, molasses, or any substance which would give pungency or intoxicating properties to the beer, such as cocculus indicus, grains of paradise, tobacco, &c. It is a well-known and authenticated fact, that beer is commonly and sometimes dangerously adulterated. The cupidity of fraudulent brewers and publicans frequently induces them to introduce other ingredients than malt and bitters into their liquors, with a view of giving them a false appearance and strength. Thus, to give pungency—capsicum, grains of paradise, ginger, &c., have been added; to give intoxicating properties—opium, cocculus indicus, tobacco, &c.; as a substitute for malt—molasses, treacle, colouring, honey, &c.; to impart a false appearance of age—sulphuric acid, alum, green vitriol, glycerin, mustard, &c.; to remove acidity—pearlash, soda, chalk, &c.; and to impart a frothy head—alum, foots, table-salt, &c.
The publicans generally ‘reduce’ their strong beer with water (which they call ‘liquor’), and add treacle, together with a mixture of copperas, salt, and alum (termed ‘heading’), to make it bear a frothy head. The cheap beer sold in many of the low taverns of London is made by dividing the contents of two butts between three butts, filling them up with water, and adding a bladder of porter-extract (technically termed ‘P. E,’) to each. This ‘P. E,’ is a mixture of powdered cocculus, Spanish juice, caramel, capsicum, &c., boiled up with treacle and water to the consistence of a thin extract, and then put into bullocks’ bladders.
Exam., Tests, &c. The analysis of beer, both qualitative and quantitative, as already noticed, is a matter of considerable difficulty. We shall therefore defer its consideration until we come to the article Porter, as that description of beer, on account of its colour, is not only the one most difficult to examine, but also the one most frequently adulterated. See Ale, Brewing, Hops, Malt, Porter, &c.
Beer, Am′ber. Syn. Amber. A liquor, formerly much drank in London, brewed from a mixture of 3 parts of amber malt, and 1 part of pale malt, with about 6 lbs. of hops to the quarter. It was generally ‘tapped’ within a few days after it had done ‘working,’ and was chiefly used mixed with bitters, or made into ‘Purl,’
Beer, Wheat′en, Wheat′-malt Beer. See Mum.
⁂ Besides malt liquor, or BEER properly so called, a somewhat similar beverage, though of inferior quality, may be prepared from any vegetable substance rich in starch and sugar, as noticed in our article on Brewing. Certain summer beverages also pass under the name; but in both the cases referred to, the name of the characteristic ingredient, or that of the vegetable employed, is always conjoined; as in pea-shell beer, potato-beer, ginger-beer, &c. Examples of some of these are given below:—
Beer, Gin′ger. Syn. Cerevis′ia (-vĭzh′-) ZINGIB′ERIS, C. zingibera′ta*, C. cum zingib′ere* (-ĕr-e), L. Prep. 1. Lump-sugar, 1 lb.; good unbleached Jamaica ginger (well-bruised), 1 oz.; cream of tartar, 3⁄4 oz. (or tartaric acid, 1⁄2 oz.); 2 or 3 lemons (sliced); boiling water, 1 gall.; macerate, with frequent stirring, in a covered vessel, until barely lukewarm, then add of yeast, 11⁄2 or 2 oz. (about 2⁄3rds of a wine-glassful), and keep it in a moderately warm place, to excite a brisk fermentation; the next day rack or decant the liquor, and strain it through a jelly-bag or flannel; allow it to work for another day, or two, according to the weather; then skim it, again decant or strain, and put it into bottles, the corks of which should be ‘wired’ down.
2. Good white sugar, 18 to 24 lbs.; lemon-juice or lime-juice, 1 quart; finest Narbonne honey, 1 or 2 lbs.; bruised Jamaica ginger, 11⁄2279 lb.; pure soft water (that has been boiled, and then allowed to settle), q. s. Boil the ginger in 3 galls. of the water for half an hour; then add the sugar, the juice, and the honey, with sufficient water (see above) to make the whole measure 181⁄4 galls., and strain the mixture as before. When the liquor has become almost cold, add the white of 1 egg, and 1⁄2 fl. oz. of essence of lemon, and strongly agitate the cask or vessel for about half an hour. After standing 3 to 6 days, according to the state of the weather, bottle it, and place the bottles on their sides in a cellar, just as is done with wine or beer. It will be ready for use in about 3 weeks, and will keep good for several months. If wanted for immediate use, about 1⁄2 pint of yeast may be added, as in formula 1; but then it will not keep so well, or be quite so transparent and free from deposit. The lemon juice and essence of lemon may be replaced, at will, by cream of tartar (in powder) or tartaric acid, 4 oz.; and lemons (sliced) 11⁄2 to 2 doz.; added with the sugar, &c.; but the original formula is preferable.—Prod. 18 galls. = 24 doz. 1⁄2-pint bottles, or 30 doz. ordinary sized ones.
3. Extemporaneous:—a. Into each bottle put concentrated essence of ginger, 1 drop; simple syrup or capillaire, 1⁄2 oz. (or in lieu of them, syrup of ginger and simple syrup, of each a dessert-spoonful); and fill with aërated soda-water at the ‘bottling machine,’ in the usual way. Very superior.
b. Into each bottle put two or three lumps of sugar, fill them to the proper height with pure water, throw in (quickly) an effervescing ginger-beer powder, and instantly cork the bottle, and secure the cork with wire.
Use. As a cooling and refreshing drink in warm weather; and as a restorative after hard drink, fatigue, &c.
Obs. The products of all the above formulæ, if well managed, are excellent; those of No. 2, and 3a., of the very finest description, much stronger and superior to nine tenths of that sold for the best in the shops. They are often called, by way of distinction, Limo′′niated Ginger-beer, Imperial G.-b., &c. Cheaper articles are made by omitting some of the ingredients, and particularly a portion of the sugar. The ginger-beer vended at 1d. and 2d. a bottle, with that known as Ginger Pop, Impe′′rial Pop, &c., are generally made with moist sugar (1⁄2 to 3⁄4 lb. to the gall.), and merely flavoured with a little coarse ginger. No. 2, made with 2 lbs. of sugar to the gall. may be kept 2 years, if not bottled for six months, and well-stored; and with 3 lbs. to the gall., for 4 years, when it forms a splendid article (GINGER-CHAMPAGNE).
Beer, Pine. See, Beer Spruce.
Beer, Spruce. Syn. Cerevis′ia (-vĭzh′-) ABI′ETIS, C. abieti′na, C. abiet′ica*, L. Prep. 1. Sugar, 1 lb.; essence of spruce, 1⁄2 oz.; boiling water, 1 gall.; mix well, and when nearly cold, add of yeast 1⁄2 a wine-glassful; and the next day bottle like ginger-beer.
2. Essence of spruce, 1⁄2 pint; pimento and ginger (bruised), of each 5 oz.; hops, 1⁄2 lb.; water, 3 galls.; boil the whole for 10 minutes, then add of moist sugar, 12 lbs. (or good treacle, 14 lbs.); warm water, 11 galls.; mix well, and, when only lukewarm, further add of yeast, 1 pint; after the liquid has fermented for about 24 hours, bottle it.
Prop., Uses, &c. Diuretic and antiscorbutic. Regarded by some persons as an agreeable ‘summer-drink,’ and often found useful during long sea-voyages. When made with lump-sugar it is called WHITE SPRUCE-BEER; when with moist sugar or treacle, BROWN SPRUCE-BEER. An inferior sort is made by using less sugar, or more water. If made with 11⁄4 to 11⁄2 lb. of lump-sugar per gall., and without yeast, in a similar manner to that described under Ginger-beer (No. 2), it may be kept a twelvemonth or longer in a moderately cool place.
Beer, Sugar. Syn. Cerevis′ia (-vĭzh′-) sac′chari, L. From moist sugar (1 to 2 lbs. to the gall.) and a little hops; as treacle-beer.
Beer, Trea′cle (trē′kl-). Syn. Cerevis′ia fæ′cis sac′chari, &c., L. Prep. 1. From treacle or molasses, 3⁄4 to 2 lbs. per gall. (according to the desired strength); hops, 1⁄4 to 3⁄4 oz.; yeast, a table-spoonful; water, q. s.; treated as below.
2. Hops, 11⁄2 lb.; corianders, 1 oz.; capsicum-pods (cut small), 1⁄2 oz.; water, 8 galls.; boil for 10 or 15 minutes, and strain the ‘liquor’ through a coarse sieve into a barrel containing treacle, 28 lbs.; then throw back the hops, &c., into the copper, and reboil them, for 10 minutes, with a second 8 galls. of water, which must be strained into the barrel, as before; next ‘rummage’ the whole well with a stout stick, add of cold water 21 galls. (sufficient to make the whole measure 37 galls.), and, after again mixing, stir in 1⁄2 a pint of good fresh yeast; lastly, let it remain for 24 hours in a moderately warm place, after which it may be put into the cellar, and in two or three days ‘bottled,’ or ‘tapped’ on ‘draught.’ In a week it will be fit to drink. Very superior.—Prod. 1 barrel, or 36 gallons. For a stronger beer, 36 lbs., or even 1⁄2 cwt., of treacle, may be used. It will then keep good for a twelvemonth.
Obs. A wholesome drink; but one apt to prove laxative when taken in large quantities. See Brewing, Beer, Ginger, Powders, &c.
BEERS. (In pharmacy.) Syn. Cerevis′iæ (-vĭzh′-e-ē) medica′tæ, L. The general nature and preparation of these articles have been already noticed.[127] They are little employed in this country. The ingredients should be so proportioned that from 1⁄4 to 1⁄2 a pint may form the proper dose. The following are examples:—
Beer, Antiscorbu′tic. Syn. Cerevis′ia antiscorbu′tica, L.; Sapinette′, Fr. Prep. 1. (P. Cod. 1839) Scurvy-grass and buds of the280 spruce-fir, of each 1 oz.; horse-radish root, 2 oz. (all fresh, and bruised or sliced); new ale or beer, 31⁄2 pints (say, 1⁄2 gall.); macerate 4 days, press, and strain for use.
2. (Ph. Castr. Ruth. 1840.) Horse-radish (fresh), 4 lbs.; juniper berries, 3 lbs.; root of calamus aromaticus and buds of pinus abies, of each 1 lb.; ginger, 1 oz.; syrup (of brown sugar), 6 lbs.; beer, 120 lbs. (say, 12 galls.); macerate 4 days, or until it ferments, then decant, strain, and add of cream of tartar, 1⁄2 lb.; tincture of mustard (flour of mustard 2 oz., to proof spirit 12 oz.), 5 lbs. (say, 1⁄2 gall.). In scurvy, &c.
Beer, Cincho′na. Syn. A′gue-beer, Bark′-beer; Cerevis′ia cincho′næ, &c., L. Prep. 1. Bruised cinchona-bark, 1 oz.; proof spirit or brandy, 2 oz.; mix; the next day add of new beer, 1 quart, and in 3 days decant or filter.—Dose, 2 or 3 wine-glassfuls.
2. (Mutis.) Cinchona, 4 oz.; sugar, 2 lbs.; boiling water, 5 pints; when lukewarm, ferment with a little yeast, as for ginger-beer.—Dose, 1 or 2 wine-glassfuls.
3. (Ph. Ferrara.) Bruised Peruvian bark, 11⁄2 oz.; cinnamon, 2 dr.; nutmeg (rasped), 7 dr.; sugar, 25 oz.; yeast, 2 oz.; water, 5 pints; mix, ferment, decant, and strain, as before.—Dose, 3 or 4 wine-glassfuls. They are all administered during the intermission of ague.
Beer, Pipsissewa. Syn. Cervis′ia chimaph′ile, &c., L. Prep. (Dr J. Parrish.) Pipsissewa (chimaphila umbellata), 1⁄2 lb.; water, 1 gall.; boil, strain, add of sugar, 1 lb.; powdered ginger, 1⁄4 oz.; yeast, q. s.; and ferment, stain, and bottle, as for ginger-beer. In scrofulous affections; especially of the joints.—Dose. Half a tumblerful. It is a favorite remedy with some American practitioners.
Beer, Sarsaparil′la. Syn. Lis′bon di′et-beer, Span′ish Jarave; Cerevis′ia sar′zæ, C. sarsaparil′læ, Infu′sum s. para′tum fermentatio′ne, &c., L. Prep. 1. Compound extract of sarsaparilla, 11⁄2 oz.; hot water, 1 pint; dissolve, and when cold, add of good pale or East-India ale, 7 pints.
2. Sarsaparilla (sliced), 1 lb.; guaiacum-bark (bruised small), 1⁄4 lb.; guaiacum-wood (rasped), and liquorice root (sliced), of each 2 oz.; aniseed (bruised), 11⁄2 oz.; mezereon root-bark, 1 oz.; cloves (cut small), 1⁄4 oz.; moist sugar, 31⁄2 lbs.; hot water (not boiling), 9 quarts; mix in a clean stone jar, and keep it in a moderately warm room (shaking it twice or thrice daily) until active fermentation sets in, then let it repose for about a week, when it will be fit for use.
Obs. It is said to be superior to the other preparations of sarsaparilla as an alterative or purifier of the blood, particularly in old affections. That usually made has generally only one half the above quantity of sugar, for which treacle is often substituted; but in either case it will not keep well; whereas, with proper caution, the products of the above formulæ may be kept for one, or even two years. No yeast must be used.—Dose. A small tumblerful 3 or 4 times a day, or oftener.
Beer, Stomach′ic. Syn. Med′icated purl; Cerevis′ia stomach′ica, L. Prep. (Dr Quincy.) Centaury-tops and Roman wormwood, of each 4 handfuls; gentian root (bruised), 2 oz.; the yellow peels of 6 Seville oranges; Spanish angelica-root and Winter’s lard, of each (bruised) 1 oz.; new ale, or beer, 3 quarts (say, 1 gall.); digest for a few days, as before. One or two wine-glassfuls early in the morning, and an hour before a meal.
Beer, Sulphu′′ric Acid. Syn. Sulphuric lemonade; Cerevisia acidi sulphu′′rici, C. anticol′ica, L. Prep. 1. Treacle beer, or other weak mild beer or ale, to which a little concentrated sulphuric acid has been added, in the proportion of about 1 dr. to every 8 or 10 pints; the whole being well agitated together, and allowed a few hours to settle.
2. Treacle, 14 lbs.; bruised ginger, 1⁄2 lb.; coriander, 1⁄2 oz.; capsicum and cloves, of each 1⁄4 oz.; water, 121⁄2 galls.; yeast, 1 pint; proceed as for ginger-beer, and when the fermentation is nearly over, add of oil of vitriol, 11⁄2 oz. (diluted with 8 times its weight of water), and of bicarbonate of soda, 11⁄2 oz. (dissolved in a little water). It is fit to drink in 3 or 4 days.
Uses, &c. It is taken with great benefit by workers in lead, especially by those employed in white lead works; also in cases of lead colic, poisoning by lead or its salts, &c. A tumblerful twice or thrice daily. It is both harmless and wholesome.
Beer, Tar. Syn. Cerevis′ia pi′cis, C. p. liq′uidæ, L. Prep. (Duhamel.) Bran, 2 pints; tar, 1 pint; honey, 1⁄2 pint; water, 6 pints; mix, and gently simmer together for 3 hours; when lukewarm add of yeast, 1⁄2 pint; let it ferment for 36 hours, and strain. Pectoral, anti-asthmatic, anti-phthisic, &c.—Dose. One wine-glassful before each meal, in bronchial and chest diseases, and incipient consumption. See Beers (In Pharmacy; above).
BEES′WING. The second or pseudo-crust so much admired in port and a few other wines, and which forms in them only when kept for some time after the first or true crust has formed. It consists of minute, glittering, floating particles or lamellæ of tartar, purer, and freer from astringent matter, than that deposited in the first crust. See Crust, Wines, &c.
BEET (bēte). Syn. Be′ta, L.; Biet, D.; Bette, Fr.; Beete, Mangold, M.-kraut, Ger.; Bietola, It. The common name of plants of the genus ‘beta,’ and the nat. ord. Chenopodæ (DC.). There are said to be only two distinct species cultivated—beta vulgār′is and b. horten′sis—each of which occurs in several varieties; those of the first, and which we have chiefly to consider, producing a large fleshy root (BEET′-ROOT, MAN-GOLD-R.; RA′DIX BE′TÆ, L.; BETTERAVE, Fr.; ROTHE RÜBE,281 &c., Ger.), which is both sweet and succulent; those of the other, only succulent leaves. The varieties most useful, and now the most extensively cultivated in England, are of comparatively recent introduction; field-beet, the mangold-wurzel of the Germans, having been only brought under the notice of our agriculturists towards the end of the last century.
Beet, Field. See Beet, Hybrid (below).
Beet, Hy′brid. Syn. Common beet, Field′-b.; Be′ta Hy′brida, B. vulgar′′is, h.; L.; Bette commune, Betterave c., Racine d’abondance, R. de disette, &c., Fr.; Mangold, M.-wurzel, Mangel-w., &c., Ger. A variety of beta vulgaris (Linn.), and that usually cultivated by English farmers. Root red on the outside, white inside; chiefly grown as winter-food for cattle, being vastly superior to turnips. It has been used in Germany as a substitute for bread in times of scarcity. Leaves dressed and eaten like spinach.
Beet, Red. Syn. Cu′linary beet, Garden b., Beet′-radish, Beet′-rave, &c.; Be′ta ru′bra, B. vulg′′aris r., L.; Betterave, &c., Fr.; Rothe rübe, &c., Ger. Root tender, well-flavoured, and of a rich red colour throughout, and hence much used in salads, pickles, and cookery; also made into a conserve, jam, or confection. The kinds most esteemed for salads are the small red and the yellowish-red varieties of Castelnaudari.
Beet, Sea. Syn. Be′ta marit′ima, L. Said to be the best variety for dressing as spinach.
Beet, White. Syn. Be′ta al′ba, B. vulga′′ris a., B. ci′cla, L.; Bette blanche, Poirée, &c., Fr. A sub-variety of the red beet. Root white, and hence preferred for making sugar; that with a purple crown being the most esteemed.
Obs. The preceding varieties of beet resemble each other in their general properties. They are all antiscorbutic, detergent, emollient, and nutritious; and their roots contain about 8% of sugar, which, by proper treatment, may be obtained from them of excellent quality. The grated root is sometimes used to dress blisters and foul ulcers. When sliced, and dried in a malt-kiln, a very palatable beer may be brewed with it. The leaves of each variety are dressed and eaten like spinach. The roots, for the table, after being carefully washed, are dressed whole—neither scraped nor cut—and, according to their size and age, require from 1 to 4 hours’ simmering or baking. They are mostly served in slices, cold, intermingled with other winter salad vegetables. See Brewing, Cattle, Salads, Sugar, &c. (also antè).
BEET ROOT. See Beet.
BEE′TLE (bē′tl). Syn. Scar′ab†*, Scar′abee†* (-bē); Scärabæ′us, L.; Escarbot, Scarabeé, Fr.; Käfer, Ger.; Betel, Sax. In zoology, the common name of an extensive genus of insects (scarabæ′us, Linn.), of numerous species. It is also popularly applied to all coleopterous insects, or such as have hard or shelly wing-cases, especially to those of a dark or obscure colour. The common pests of our kitchens and basement floors which pass familiarly under the name of beetles, black beetles, or cockroaches, belong to the order orthoptera, and not to the coleoptera or beetle tribe, as the name implies. See Insects, &c.
Black Beetle; Domes′tic beetle. See Blatta, Cockroach, &c.
Blis′tering Beetle. See Cantharides.
BELL. Syn. Campa′na,[128] Campan′ula,[129] No′la*,[130] Tintinnab′ulum,[131] L.; Cloche, Clochette,[132] Grelot,[133] Fr.; Glocke, Schelle, &c., Ger.; Bell, Bella, Belle, Sax. A hollow vessel or body, usually of cast metal, with a wide cup-like mouth expanding outwards, so formed as to emit sound when suspended and struck with a hard substance. The word is also applied, either alone or in composition, to substances having the figure of a bell; as bells (of flowers), bell-animal, b.-flower, b.-glass, &c.
Form, Manuf., &c. Bells of “the common and well-known shape, with a thick lip or sound-bow, are the most effective known instruments for producing a loud and musical sound, such as you want when you erect a large public clock, or put up a peal of church-bells.” “After trying a number of experiments, at Messrs Warner’s, I am quite satisfied that there is nothing to be gained by deviating materially from the established proportions of the best old bells.”[134] This view is borne out by the researches of the Government commissioners[135] who visited the Paris Exhibition, who report, that among the ‘founders’ of France and Belgium, there are no traditions of the art, nor any discoveries or appliances of modern science, tending to the improvement of bells, or to provide efficient substitutes for them; nor is there any known improvement on the established mode and usual material (BRONZE or BELL-METAL) for casting them. Sir C. Barry, indeed, according to Mr Dennison, “seemed rather impressed with the merits of cast-steel bells;” but both Prof. Wheatstone and Mr Dennison differ from him in opinion. Undoubtedly some cast-steel bells, of small size, have been produced, capable of yielding sounds of extraordinary clearness and richness, but, in most cases, owing to the difficulty in giving the peculiar molecular condition to the metal essential to a high degree of sonorousness, their tones are comparatively harsh and282 disagreeable. Well-annealed glass offers a cheaper and better material than steel for large bells up to a certain size, whilst its tones are exquisite. As the depth of the tone of a bell depends chiefly upon the dimensions and weight of the sound-bow, it appears likely that by directing our experiments to the increase of these, and the diminishing of the thickness of the metal in the other parts, the quantity of metal required to produce large bells might be very greatly reduced. The sound of an Indian gong that may be easily held suspended by the hand is always rich and usually as loud and deep as a bell of ordinary construction which it would take several men to lift. The Chinese often use bells made of porcelain. Small hand-bells for the toilet and boudoir are often made of silver, and then yield tones which are remarkably soft, clear, and pleasing. The tongue, clapper, or hammer, of bronze bells should be of iron; and steel bells, of bronze. Glass and porcelain bells require the striking part of the tongue to be of box-wood, the proper weight being given by a ball of iron cast on the rod immediately above it, and a similar one screwed on the end of the rod immediately below it. In all cases the hammer-head, preferably globular, should strike the bell near the verge, and should be free from projections or asperities.
The casting, &c., of bells is essentially similar to that of other articles in bronze, of corresponding size, and particularly of cannon. See Bell-metal, Bronze, &c.
BELL′-METAL. Syn. Æs CAMPANA′′RUM, L.; Métal de cloche, Fr.; Glockengut, Glockenspeise, Ger. The alloy, usually bronze, of which bells, &c., are made.
The composition of bell-metal varies considerably, as may be seen below:—
1. (Standard.) Copper, 78 parts; tin, 22 parts;[136] fused together and cast in the manner described under Bronze. The most sonorous of all the alloys of copper and tin. It is easily fusible, and has a fine compact grain, and a vitreous-conchoidal and yellowish-red fracture. According to Klaproth, the finest-toned Indian gongs have this composition.
2. (Founder’s Standard.) Copper, 77 parts; tin, 21 parts; antimony, 2 parts.[137] Slightly paler and inferior to No. 1.
3. Copper, 80 parts; tin, 20 parts.[138] Very deep-toned and sonorous. Used in China and India for the larger gongs, tam-tams, &c.
4. Copper, 78 to 80 parts; tin, 22 to 20 parts. Usual composition of Chinese cymbals, tam-tams, &c.
5. Copper 75 (= 3) parts; tin, 25 (= 1) parts.[139] Somewhat brittle. In fracture, semi-vitreous and bluish-red. Used for church and other large bells.
6. Copper, 80 parts; tin, 101⁄4 parts; zinc, 51⁄2 parts; lead, 41⁄4 parts. English bell-metal, according to Thomson. Inferior to the last; the lead being apt to form isolated drops, to the injury of the uniformity of the alloy.
7. Copper, 68, parts; tin, 32 parts.[140] Brittle; fracture conchoidal and ash-grey. Best proportions for house-bells, hand-bells, &c.; for which, however, 2 of copper, and 1 of tin, is commonly substituted by the founders.
8. Copper, 72 parts; tin, 261⁄2 parts; iron, 11⁄2 part. Used by the Paris houses for the bells of small clocks or pendules.
9. Copper, 72 parts; tin, 26 parts; zinc, 2 parts. Used, like the last, for very small bells.
10. Copper, 70 parts; tin, 26 parts; zinc, 2 parts. Used for the bells of repeating watches.
11. Melt together copper, 100 parts; tin, 25 parts. After being cast into the required object, it should be made red hot, and then plunged immediately into cold water in order to impart to it the requisite degree of sonorousness. For cymbals and gongs.
12. Melt together copper, 80 parts; tin, 20 parts. When cold it has to be hammered out with frequent annealing.
13. Copper, 78 parts; tin, 22 parts. This is superior to the former, as it can be rolled out. For tom-toms and gongs.
14. Melt together copper, 72 parts; tin, 26 to 56 parts; iron, 1·44 part. Used in making the bells of pendules or ornamental Parisian clocks. For clock-bells.
Concluding remarks. Castings in bell-metal are all more or less brittle; and, when recent, have a colour varying from a dark ash-grey to greyish-white, which is darkest in the more cupreous varieties, in which it turns somewhat on the yellowish-red or bluish-red. The larger the proportion of copper in the alloy, the deeper and graver the tone of the bells formed of it. The addition of tin, iron, or zinc, causes them to give out their tones sharper. Bismuth and lead are also often added to modify the tone, which each metal affects differently. The addition of antimony and bismuth is frequently made by the founder to give a more crystalline grain to the alloy. All these additions are, however, prejudicial to the sonorousness of bells, and of very doubtful utility. Rapid refrigeration increases the sonorousness of all these alloys. Hence M. D’Arcet recommends the ‘pieces’ to be heated to a cherry-red after they are cast, and after having been suddenly plunged into cold water, to be submitted to well-regulated pressure by skilful hammering, until they assume their proper form; after which they are to be again heated and allowed to cool slowly in the air. This is the method adopted by the Chinese with their gongs, &c., a casing of sheet-iron283 being employed by them to support and protect the pieces during the exposure to heat. In a general way, however, bells are formed and completed by simple casting. This is necessarily the case with all very large bells. Where the quality of their tones is the chief object sought after, the greatest care should be taken to use commercially pure copper. The presence of a very little lead or any similar metal greatly lessens the sonorousness of this alloy; whilst that of silver increases it. This last metal has been detected in many old church bells remarkable for the richness of their tones—articles of silver plate having been cast into the crucibles of the founders, as votive offerings, by the pious Christians of former ages.
The specific gravity of a large bell is seldom uniform throughout its whole substance; nor can the sp. gr. from any given proportion of its constituent metals be exactly calculated owing to the many interfering circumstances. The nearer this uniformity is approached, or in other words, chemical combination is complete, the more durable and finer toned will be the bell.
In general it is found necessary to take about 1-10th more metal than the weight of the intended bell, or bells, in order to allow for waste and scorification during the operations of fusing and casting. See Bell, Bronze, Copper, &c.
BELLADON′NA (-dŏn′ă), [It., Sp., Port.; Eng., L., Ger.;[141] B. P.] Syn. Dead′ly night′shade, Dwale; Belledame, Belladonne, &c., Fr.; Tödtlicher Nachtschatten, Tollkersche, Tollkraut, Wolfskirsche, &c., Ger.; At′ropa letha′lis*, Sola′num furio′sum*, S. letha′le*, S. mania′cum*, S. melanocer′asus†, &c., L., Bot. var. Literally, fair lady; in materia medica, botany, &c., the usual name (adopted from the Ital.) of at′ropa belladonn′a (Linn.), an indigenous, poisonous, perennial, herbaceous plant, of the nat. ord. Solanæ (DC); Solanaceæ, Endl., (Lind.). It flowers in June and July, and its drooping, purple blossoms are common ornaments of our hedges and wastes where the soil is calcareous. It is supposed to be the ‘insane root’ of Shakespeare.[142]
The parts of this plant used in medicine and pharmacy are the “fresh leaves and branches to which they are attached; also the leaves separate from the branches, carefully dried, of atropa belladonna; gathered, when the fruit has begun to form, from wild or cultivated plants in Britain” (B. P.).
Prop., Uses, &c. Every part of this plant contains ATRO′PIA, and is consequently highly poisonous. Every part, except the berries, is fœtid when bruised, and of “a dark and lurid aspect, indicative of its deadly narcotic quality.”[143] Its berries, which are of a glossy violet-black, and of the size of a small cherry, are sweet-tasted, and not at all nauseous. Children and tired travellers and soldiers, allured by their beauty and the absence of disagreeable flavour, have frequently been induced to eat them; but in all cases poisoning, often fatal, has followed the indulgence.[144] Belladonna is, however, in qualified hands a safe and most valuable medicine. Its chief use is as an anodyne, antispasmodic, sedative, and discutient, and particularly to diminish sensibility and allay pain and nervous irritation in a variety of diseases—neuralgia, arthritic and migratory rheumatic pains, painful ulcers, cancer, spasmodic rigidity, strictures, and contractions (especially of the bladder and uterus), angina pectoris, iritis, epilepsy, chorea, hooping-cough, hysteria, mania, fevers, phthisis, asthma, &c.; also as a prophylactic of scarlet-fever,[145] hydrophobia, and salivation, as a resolvent in enlarged and indurated glands (particularly when painful), as an agent to produce dilation of the pupil during surgical examinations and operations, &c., &c. It is employed both internally and externally, and in various forms, as is noticed under its ‘preparations’ elsewhere. Dose. Of the powder, 1⁄2 to 1 gr. twice a day, gradually and cautiously increased until dryness of the throat or dilation of the pupil occurs, or the head is affected.
Pois., &c. Belladonna and its preparations are poisonous to all animals, but very much more so to the carnivora than to the herbivora. It also acts as a poison on vegetables.
Treatm. Ant., &c. These may be the same as those employed in poisoning by aconite, atropia, and opium. The stomach must be cleared as soon as possible, followed by active purgation. Unfortunately emetics have scarcely any action, and, therefore, must be given in large doses, assisted by tickling the fauces, &c. If copious vomiting does not rapidly follow, the stomach-pump may be had recourse to. When the poison has been removed from the stomach, copious and continued draughts of astringent vegetable solutions (weak decoction of galls or oak-bark, or strong coffee or green-tea), should be persisted in for some time; followed by like draughts of water soured with any mild vegetable acid (as vinegar, lemon-juice, citric or tartaric acid, &c.) Detec. The contents of the stomach or vomited matter may be searched for the berries, leaves, seed, or portions of the root; all of which are easily recognisable. The usual physiological and chemical tests of atropia may also be applied to these and to the organic liquids supposed284 to contain the poison. See Alkaloid, Atropia, Extracts, Ointments, Tinctures, Vegetable Juices, &c.
BELLADONNINE. Syn. Atropia, which see.
BELL′Y (-e). The abdomen (which see).
BELTS. In their connection with health and disease, see Bandage, Dress, Stays, &c.
BENEDICTINE’S HEALING-PLASTER (Hauber). 35 grammes of a dark brown plaster, prepared by digesting together 1 part litharge with 2 parts olive oil until they become blackish-brown, then adding 4 parts yellow wax, containing the heat for a short time, and then pouring out. (Wittstein.)
BENGAL′ (-gawl′). A thin fabric of silk and hair interwoven, originally from Bengal.
Ben′gal Light. A firework used as signals. See Fires (coloured).
Ben′gal Stripes. Cotton cloth, woven with coloured stripes, orig. from Bengal; gingham.
BEN′JAMIN†*. Benzoin.
BEN′ZENE. See Benzol.
BEN′ZINE (-zĭn). Benzol.
BEN′ZOATE (-zo-āte). [Eng., Fr.] Syn. Ben′zoäs, L. A salt in which one atom of benzoic acid is replaced by a metal or other basic radical. The benzoates may, in general, be easily prepared by either neutralising the acid with the base, or by double decomposition. Most of them are more or less soluble in water, and crystallisable. Those of the alkalies and ammonia are very soluble, and rather difficult to crystallise. See Benzoic Acid and the respective bases.
BEN′ZOENE*. See Tuluole.
BENZO′IC ACID (-zō′-ĭk). HC7H5O2 Syn. Flowers of benzoin′; Hy′drated ben′zoyl; Acidum benzo′icum (B. P.); Acide benzoïque, Fleurs de benjoin, &c., Fr.; Benzoesäure, &c., Ger. A substance which is commonly stated to be the characteristic constituent of the two balsams. Pure oil of bitter almonds suffers gradual conversion into this acid by exposure to the air.
Prep. The acid of commerce is principally obtained from gum-benzoin, either by sublimation (dry way), or by dissolving it out by means of an alkali, or an alkaline earth in the form of a salt (moist way); but chiefly by the first method.
1. By SUBLIMATION:—
a. Good benzoin, crushed small or in the state of coarse powder, is placed in a cylindrical iron pot with a flat bottom, and from 8 to 9 inches in diameter, so as to form a layer of from 1 to 2 inches deep. The open end of the pot is next covered with a sheet of soft and loose blotting-paper,[146] which is attached to the rim with paste. A cone, cap, or cylinder formed of strong thick paper (cartridge paper), open at its lower end, is then placed over the top of the pot, including the blotting-paper; and this is also attached with paste and string. The apparatus, thus prepared, is then placed on a sand bath,[147] and exposed for 4 to 6 hours to a gentle and uniform heat. It is next removed from the sand bath, and, when it has sufficiently cooled, inverted, and the string detached, when crystals of benzoic acid are found in the paper cone. If, owing to want of care in manipulating, the product is either coloured or empyreumatic, it must be enveloped in several folds of bibulous paper, then submitted to powerful pressure, and afterwards resublimed. The simple form of apparatus figured in the engraving answers well on the small scale, and is that recommended by Dr Mohr.
b. (Ph. D. 1850.) The subliming pot is ordered to be of sheet-iron. It is to be fitted into a circular hole in a sheet of pasteboard, and a collar of tow interposed between it and the flange, so as to produce a nearly air-tight junction. The paper receiver or cap is to be cylindrical, open at one end and about 18 inches high, with a diameter at least twice that of the pot; and it is to be secured in an inverted position on the pasteboard, and fastened to it by slips of paper and flour-paste. A couple of inches of the pot is to be passed through a corresponding hole in a plate of sheet-tin, which is to be kept from contact with the pasteboard by the interposition of a few corks; and a heat[148] only just sufficient to melt the benzoin is to be applied for at least six hours.
c. (Process adopted at Apothecaries’ Hall, London.) The best gum-benzoin is put into an iron pot, set in brickwork over a suitable small fire-place (or flue),[149] and communicating by a conical metal neck, with a wooden box (technically termed a ‘house’) lined with white blotting-paper, as a receiver for the flowers. A piece of fine muslin, or of bibulous paper, is interposed between the top of the subliming-pot and the receiver, to prevent the sublimate falling back into the former. The sublimation is conducted rather rapidly, and the acid condenses in beautiful white, soft, flexible crystals, which are at once ready for the market. When the process is conducted more slowly, the product is proportionately scaly.
Obs. Good samples of benzoin yield from 10 to 12%, or even 121⁄2%, of ‘flowers’ or ‘acid of the first sublimation.’ This, after being pressed in blotting-paper and again sublimed, gives 81⁄2 to 10% of nearly pure benzoic acid. The loss arising from a second sublimation is thus285 so great that the utmost care should be taken to avoid its necessity.
2. In the MOIST WAY:—
a. (Ph. D. 1826; Scheele’s Process.) Equal parts of benzoin and hydrate of lime, in fine powder, are intimately mixed together and boiled for about an hour, with 40 parts of water; the liquor, after filtration, is evaporated to 1⁄5th, and the lime saturated with hydrochloric acid; the benzoic acid crystallised out as the liquor cools, and is then either washed with very cold water, and dried by a gentle heat, or it is dried and sublimed in the manner already explained. The product of the sublimation is extremely white and pure.
Obs. An economical and productive process; but, to ensure success, a perfect mixture of the dry ingredients must be first made; as otherwise the benzoin runs into a solid mass in the boiling water, and the operation fails. Prod. “1 lb. of (gum) benjamin yields 1 oz. 6 dr. 2 scr. of flowers.” (Gray.)[150]
b. (Process of Stoltze.) The benzoin is dissolved in 3 times its weight of alcohol, the solution introduced into a retort, and a solution of carbonate of soda in weak spirit-and-water, is gradually added, until all the free acid present is neutralised; water, equal to about twice the weight of the benzoin employed, is next poured in, and the alcohol removed by distillation. The floating resin is now skimmed off the residual liquid and washed with a little water, and the washings added to the contents of the retort, which will deposit crystals of benzoate of soda on cooling, and more by subsequent evaporation. From this salt the benzoic acid is obtained by saturating the alkali with an acid (as the hydrochloric), and by subsequent sublimation of the crude precipitated crystals.
3. Other Methods:—
a. Ordinary hippuric acid is very gently boiled, for about 15 minutes, in nitric acid[151] (sp. gr. 1·42); water is then added, and the solution allowed to cool and crystallise. The crystals are collected on a filter, washed with a little very cold water, dried by pressure in bibulous paper, and lastly, purified by sublimation, as before.
b. From the urine of horses, cows, and other graminivorous animals, in a similar way to that by which hippuric acid is obtained, only allowing the urine to acquire a slight degree of putridity before evaporation, which last should be effected by a heat slightly under that of ebullition. The crude acid thus obtained is purified as previously directed.
Obs. Large quantities of benzoic acid are said to be obtained in this way on the Continent; but, owing to the process being clumsily conducted, it is generally of inferior quality, and hence unsaleable. It may, however, by skilful purification, be rendered quite equal to that obtained from gum benzoin.[152]
Prop. When obtained by sublimation benzoic acid forms soft, light, feathery, white, flexible crystals, which are transparent or semi-transparent, with more or less of a mother-of-pearl lustre; when by slowly cooling its aqueous solution, or by precipitation from a solution of a benzoate, it forms either thin plates or scales, or a dazzling white crystalline powder. It is inodorous when cold,[153] but acquires a faint balsamic odour when gently warmed; fuses at about 212° Fahr., and begins to sublime freely at a temperature a little above it, but does not boil until heated to about 460°; burns with a bright yellow flame; is very soluble in alcohol, dissolves in about 200 parts of cold water, and about 25 parts of boiling water; resists the action of ordinary nitric acid even when boiling; and forms salts (BEN′ZOATES) with the bases. Sp. gr. 0·667. Its vapour, which is very suffocating and irritating, has a density of 4·27. Added to fat and fatty substances it either prevents, or greatly retards, the accession of rancidity.
Test, &c. It may be recognised—1. By its physical properties (appearance, fusibility, volatility, odour, &c.) already enumerated:—2. By its ready solubility in solutions of the alkalies; and by being precipitated from these solutions, on the addition of one of the stronger acids, under the form of a dazzling white powder, which is only sparingly soluble in cold water:—3. By its neutral salts with the alkalies, or its neutral solution in an alkali, giving a bulky, flesh-coloured precipitate with perchloride of iron, which is insoluble in water:—4. By its solution not being precipitated by acetate of lead until after neutralisation with a fixed alkali, when the acetate produces a white, flocculent precipitate:—5. By a mixture of alcohol, ammonia, and solution of chloride of barium, neither disturbing a solution of the free acid, nor that of one of its salts with the alkalies.
It is chemically distinguished from cinnamic acid by not yielding essential oil of almonds when it is distilled with oxidising agents, as chromic acid or a mixture of bichromate of potassium and sulphuric acid; and from succinic acid, by its different deportment with sesquichloride of iron (Test 3, antè), and with a mixture of alcohol, ammonia, and solution of chloride of barium (T. 5, antè).
Estim.—1. By weighing it as benzoic acid, obtained either by precipitation, or by very careful sublimation in a glass apparatus:—2. By neutralising its alcoholic or aqueous solution, by the usual method of acidimetry:—3. By precipitating its neutral solution with acetate of lead, or with sesquichloride of iron, and weighing the carefully washed and dried286 precipitate either as benzoate of lead, or as ferric benzoate.
Pur., &c. White crystalline silky plates and needles, have an aromatic odour. Solubility in cold water, 1 in 300; in boiling water, 1 in 12; in spirit, 1 in 4. Also soluble in caustic alkalies and lime. Borax considerably increases its solubility in water; 1 of benzoic acid and 1 of borax are soluble in 100 of water. It sublimes without residue when heated. It is sometimes met with adulterated with hippuric acid, which may be easily detected by its altered form, by its diminishing solubility in cold water, and by its exhaling an odour of tonquin-beans, and afterwards of hydrocyanic acid, when sublimed. The presence of succinic acid may be readily detected by its greatly increased solubility in cold water; that of sugar, not only by its increased solubility, and partial volatility, but also by the odour of caramel being evolved on the application of sufficient heat, and the residuum being black and carbonaceous; that of camphor, by its peculiar odour when gently heated. Spermaceti, specially prepared for the purpose, is also an occasional adulterant, easily detected by its insolubility and other well-known properties. All these substances either destroy or lack the proper crystalline form of benzoic acid, which is one of the best proofs of its purity. They also greatly increase its sp. gr.
Uses, &c. Its chief use in medicine is as a stimulant and expectorant. It is an ingredient in the compound tincture of camphor (paregoric elixir) of the pharmacopœia.—Dose, 10 to 30 gr., dissolved in water by the aid of a little ammonia or potassa; in old coughs, &c.
BENZOIC AL′COHOL. A peculiar oily fluid, discovered by M. Cannizzaro, and obtained by the action of an alcoholic solution of potassa on pure oil of bitter almonds.
BENZOIN′, B. P. (-zoyn′; zō′-ĭn). Syn. Gum-benzoin*‡, Ben′jamin†*, Gum-b.†*; Benzöi′num, L., B. P.; Benjoin, Fr.; Benzöe, Ger. The balsamic resin exuded from incisions made in the stem of the styrax benzoin, a native of Sumatra, Java, Borneo, Laos, and Siam. Several varieties of benzoin are in the market; two only, however, are chiefly used in medicine, one in agglutinated masses, the other (from Siam), in tears, being the purer and having the stronger odour.
Prop., &c. Odour agreeable, and somewhat like that of vanilla, but more balsamic; fracture conchoidal; lustre greasy; sp. gr. 1·063 to 1·092. It fuses at a gentle heat and exhales white fumes, which, on condensation, are found to be benzoic acid contaminated with a little volatile oil. Alcohol dissolves the larger portion of it, ether much less, and the volatile and fixed oils only a little. It contains from 9% to 18, or (occasionally) nearly 20%, of benzoic acid, according to the quality. It burns with an agreeable odour. The resin and its alcoholic solution strike a bright red colour with oil of vitriol, and a green colour with chloride of iron.
Benzoin has occasionally been sold by fraudulent dealers after its benzoic acid has been removed by the wet method. When the gum has been thus treated it will not show the agglutinated tears, upon fracture, which commonly distinguishes it when intact.
Uses, &c. It is chiefly employed in perfumery, and as an ingredient in incense, fumigating pastilles, &c.; also in court-plaster, in certain cosmetics, and to scent the varnish used for snuff-boxes, walking-sticks, &c. As a medicine, its general effects resemble those of the other true balsams, and of benzoic acid.—Dose, 5 or 6 to 20, or even 30 gr., in powder, and usually in combination with some other remedy; chiefly in chronic pulmonary and bronchial affections, when occurring in torpid habits, and unaccompanied by inflammatory symptoms or gastric irritation. Also as a fumigation in the same diseases, hooping-cough, &c. Like benzoic acid, it is used to prevent rancidity in ointments, pomades, and other fatty preparations.
BENZOINUM. See Benzoin.
BEN′ZOL (-zōle). C6H6. [benz(oin)-oleum.] Syn. Ben′zene*, Ben′zĭne, Ben′zōle*, Hydrĭde of phe′ny̆̆l*, Phe′ne†, &c.; Benzo′leum, L.; Benzine, Fr.; Benzöl, Ger. A peculiar ethereal hydrocarbon discovered, by Faraday, among the products of the destructive distillation of whale oil and other organic substances (A.D. 1825); and subsequently shown, by Mitscherlich, to form the principal ingredient in the distillate procured by the action of heat on a mixture of benzoic acid and hydrate of lime. In 1849, Mr C. B. Mansfield[154] discovered its presence in coal-tar naphtha, from which the benzol of commerce is now chiefly, if not wholly, obtained.
Prep. 1. Pure:—a. A mixture of benzoic acid, 1 part; fresh-slaked lime, 3 parts; is submitted, in a coated glass or earthenware retort, to a heat slowly raised to redness; the oily portion of the resulting distillate is then separated from the water, and carefully rectified, with the proper precautions, at a temperature not exceeding 190° Fahr. The product is usually stated to be pure benzol; but to ensure this it may be submitted to one refrigeration and rectification, in the manner and at the temperature noticed below.
b. From good commercial benzol, agitated with 1-4th or 1-5th of its weight of concentrated sulphuric acid, and, after repose and decantation, rectified at a temperature under 195° Fahr.; the resulting distillate is exposed to a temperature below[155] 32° Fahr., and the mass of crystals that form are thrown on a funnel, kept at the same temperature, to drain, after which they are pressed between folds of287 bibulous paper,[156] and then allowed to liquefy by simple exposure, in a close vessel, to the ordinary temperature of the atmosphere. The product, after rectification at a temperature not exceeding 190,° is nearly pure benzol. It may be rendered absolutely pure by repeating the refrigeration a second and a third time, followed by a final rectification at 180-185° Fahr.
2. Commercial:—By submitting light coal-tar naphtha to distillation, either at once, or after it has been agitated with a little oil of vitriol, and decanted, care being taken that the temperature does not exceed 200° Fahr.
A drawing and description of the apparatus invented by Mansfield for the preparation of benzol from coal-tar naphtha is given below.
A is the still placed on a furnace R; C is filled with cold water. As soon as the oil in the still boils, the vapours are condensed in B, and flow back into A; this continues until the water in C has been heated to a certain temperature, when the vapours are condensed in the cooler D, the liquid flowing at n into the carboy S. As soon as the water in C begins to boil, all the substances contained in the coal-tar naphtha and volatile at 212° Fahr. are condensed and collected in S. A very pure benzol is obtained by this apparatus. By opening the tap m, the hydrocarbons which boil over 212° Fahr. can be rectified. The stopcock i is used for opening the still.
In the benzol works the apparatus shown below is employed.
A is the still, B the condenser, C a water-tank. At the commencement of the operation the water in C is heated by means of the steam-pipe D which communicates with the steam boiler. The tube G is attached to the still; i is a contrivance for filling, b for emptying it. The condensed water is carried off by means of H. By freezing benzol and pressing the solid substance obtained, it may be rendered quite pure.
Prop. Pure benzol is a clear, colourless, very mobile liquid, having a strong, characteristic, and rather agreeable ethereal odour. It is neutral to test-paper; exceedingly volatile at all temperatures; insoluble in water; miscible with alcohol and with ether; highly inflammable; burns with a brilliant flame, emitting clouds of smoke, which rapidly condense and fall as a shower of fine sooty, carbonaceous matter; boils at 176° Fahr.;[157] solidifies, at 32°, to288 a snowy white camphor-like mass, or when very slowly refrigerated, to beautiful transparent cruciform leaflets, which aggregate together into forms resembling fern-fronds; remelts at 40-1° Fahr.; and when solidifies burns, like camphor, without previous fusion. Sp. gr. ·850;[158] sp. gr. of vapour, 2·770.[159] It is unaffected by the ordinary hydrated acids, and has no action on the alkaline metals. Highly concentrated nitric acid readily dissolves it, and from this solution nitrobenzol is precipitated on the addition of water. Its vapour is dangerously inflammable, and, when mixed with the air, is highly explosive. Its solvent power extends over a numerous list of substances. Commercial benzol has a less agreeable odour, and not unfrequently a slight colour, with other modifications of the properties just enumerated, depending on the relative amount of impurities contained in it.
Pur.—1. It should be colourless, without action on either litmus or turmeric paper, and have the boiling-point, sp. gr.,[160] &c. already indicated:—2. A few drops thrown on a slip of glass or a piece of white paper should rapidly and entirely evaporate by simple exposure to the air without leaving a stain behind, or evolving any disagreeable or foreign odour:—3. Agitation with a little sulphuric acid should not discolour it:—4. It should not perceptibly lose weight or volume by agitation with a little cold water.
Detec.—1. From the physical and other properties already enumerated:—2. By converting it into aniline and then testing it accordingly. For this purpose a little of it is dissolved in concentrated nitric acid, and the nitrobenzol thus formed is precipitated by the addition of water. The fluid is then agitated with ether, to dissolve out the nitrobenzol, and the resulting ethereal solution is mixed with an equal bulk of alcohol and hydrochloric acid and a little granulated zinc at once added. Hydrogen is evolved, and by its action the nitro-compound is converted into aniline. The liquid is next alkalised with potassa in excess, and the alkaline fluid agitated with ether. The ethereal solution, on evaporation, leaves a residue (aniline), which, after the addition of a little water, may be tested with a few drops of solution of chloride of lime, when a characteristic purple colour will be developed, provided the original liquor was benzole, or contained it. In this way very minute traces of benzol may be detected.
Uses, &c. In its impure or commercial form, chiefly as a solvent for gutta percha and india rubber; but it leaves the first in a spongy, friable state, and the latter glutinous or sticky, unless heat is applied to it for some time; also as a solvent in the manufacture of varnishes, as a diluent in lieu of oil of turpentine, for oil-paints, as a material for the production of artificial light, &c., &c. In the pure or nearly pure form it is largely employed in the laboratory and in chemical analysis as a solvent of many resins,[161] mastic, wax, camphor, fat, the fixed and essential oils, sulphur, phosphorus, iodine, several of the alkaloids,[162] &c., &c. Under the name of BENZINE and BENZINE-COLLAS it has been recently extensively vended for the removal of spots of grease, paint, &c., from woven fabrics, which it does most readily and completely, without detriment to the material. As a source of artificial light it has been the subject of innumerable applications and patents. It may be burned in a ‘wickless’ lamp, provided a proper cap-burner be employed. Alcohol or pyroxilic spirit containing 1-3rd, or even 1-4th of it, burns with a rich white flame. Air driven through it becomes sufficiently inflammable to serve as illuminating gas; whilst ordinary coal-gas by merely passing over it yields a flame of greatly increased brilliancy; but in all these applications the greatest possible care is necessary to prevent accidents.[163] See Naphtha (Coal-tar).
Benzol, Nitrate of. See Nitro-Benzol.
BENZOLINE. A product of the fractional distillation of American rock oil. If used for burning purposes, care should always be taken to use a sponge lamp, so as to ensure the benzoline vapour (which is extremely inflammable) being well diluted with air when burnt.
BENZOYL. C7H5O. The radical of an extensive series of compounds, of which the hydride, C7H5OH (essential oil of bitter almonds), and benzoic acid, HC7H5O2 are the most important members.
Benzoyl, Hy′dride of. C7H5OH. Syn. Essence of bitter almonds, Essential oil of bitter almonds, Volatile oil of bitter almonds.289
Prep. 1. The crude oil of bitter almonds is agitated with a moderately dilute solution of protochloride of iron which has been previously mixed with fresh hydrate of lime in excess, and the whole, after having been placed in a retort connected with a suitable receiver, is subjected to distillation. The oil passes over mixed with water, from which it is easily separated after repose. By subjecting it to a second agitation and distillation with a fresh mixture of the protochloride and hydrate, and, after careful separation from the water which distils over with it, allowing it to remain for some hours in contact with fragments of fused chloride of calcium, to free it from all traces of adhering water, the product will be nearly chemically pure, provided the whole process has been conducted with as little access of air as possible.
2. (Liebig.) Agitate the crude oil of bitter almonds with mercuric oxide in slight excess, and, after a few days’ contact, rectify the oil from a little fresh oxide. The product is quite pure when the process is properly managed. The bicyanide of mercury thus formed may be either employed as such, or reconverted into oxide of mercury and hydrocyanic acid.
Prop., &c. A rather thin, colourless liquid, of great refractive power and characteristic and agreeable odour; soluble in 35 parts of water; miscible in all proportions with alcohol and ether; it boils at 356° Fahr.; on exposure to the air it rapidly absorbs oxygen, and becomes converted into a mass of crystallised benzoic acid; heated with solid hydrate of potassa hydrogen is evolved, and benzoate of potassium formed; with the alkaline bisulphites it forms beautiful crystalline compounds. Its flame, and that of its vapour, is bright but very smoky. Sp. gr. 1·043. It differs from the crude or common oil of bitter almonds chiefly in the absence of hydrocyanic acid, and consequently in not being poisonous. It has hence been proposed as a substitute for the crude oil as a flavouring ingredient in cookery, confectionery, liqueurs, &c.; but is unfitted for the purpose, owing to the rapid deterioration it suffers unless it be kept absolutely excluded from the air.
Formiate of Hydride of Benzoyle. See Formobenzoic Acid.
BER′BERINE (-een).[164] C20H17NO4. [Eng., Fr.] Syn. Bar′berĭne*, Ber′berite* (of Thomson); Berberi′na, L. A substance discovered by Buchner and Herberger in the root of the common barberry shrub (ber′beris vulga′ris, Linn.); and subsequently, by Bödecker, in calumba-root; and more recently by Mr Perrins, in the calumba-wood (menispermum fenestratum) of Ceylon, which contains a considerable quantity of it.
Prep. 1. A soft watery extract of the root, or of the wood, is digested in rectified spirit, with trituration, as long as anything is taken up; the resulting tincture, after repose, is filtered, and the alcohol gradually distilled off until the residuum has the consistence of a thin syrup. The crystals which form as the liquid cools are drained in a funnel, washed with a few drops of ice-cold water, pressed dry in bibulous paper, and then purified by solution and crystallisation, first in rectified spirit, and next in distilled water.
2. By digesting the root, or the wood (coarsely powdered) in rectified spirit, and then proceeding as before.
Prop. Berberine may be classed with the azotised colouring substances; or, from its composition and its possessing feeble basic properties, with the alkaloids. It crystallises in fine needles, or in stellated prisms, which are yellow, odourless, very bitter-tasted, neutral to test-paper, and contain 12 equiv. of water. At 212° Fahr. it acquires a red colour; but recovers its normal yellow on cooling. A much higher temperature decomposes it, yellow vapours being evolved. It is freely soluble in boiling water and in alcohol, from either of which solutions it may be readily obtained in crystals. It requires 500 parts of water at 60° to dissolve it, and very much more at lower temperatures. Its solutions are yellow; that in alcohol appears green by reflected light. The concentrated mineral acids destroy it. Its salts are more or less soluble.
Uses, &c. Chiefly in medicine, in similar cases to those in which the use of calumba-root is indicated. It has been highly recommended in dyspepsia and heartburn, in disturbed action of the liver, and, combined with iron (lactate, phosphate, or hyposulphite), in chlorosis, anæmia, &c. According to M. Altin, it is an effectual remedy for the mucal, colourless diarrhœa, and the derangement of the urinary secretions which commonly follow cholera.—Dose, 3 to 10 gr.; in larger doses it proves laxative. See Calumba, &c.
BERENIZON (Dr Charles Wortley). A preparation for promoting the growth of the hair. Balsam of Peru 3 grammes, castor oil 3 grammes, tinct. cinchona 4 grammes, spirit 85 grammes, rosewater 40 grammes. (Schädler.)
BERG′AMOT. Syn. Bergamo′ta, L.; Bergamote, Fr.; Bergamotte, Fr., Ger. The bergamot-lemon, or fruit of cit′rus ber′ga′mia; also sometimes, colloquially, the fragrant oil obtained from its rind. See Oils (Volatile).
BERGBALSAM—MOUNTAIN BALSAM (of G. Schmidt, Berlin). Recommended for hemorrhoids, want of appetite, headache, constipation, &c. Rhubarb 2 parts, cortex frangulæ 10 parts, milfoil flowers (Achillea millefolium) 1 part, tansy 1 part, crystallised soda 11⁄2 parts; be digested for some hours in warm water, the fluid expressed made up to 26 parts, 30 parts of sugar dissolved in it, and290 lastly mixed with 17 parts of rectified spirit. (Hager.)
BER′RY (bĕr′-re). Syn. Bac′ca (pl. bac′cæ, -sē), L.; Baie, Fr.; Beere, Ger. Any small succulent or pulpy fruit containing several naked seeds or granules. In botany, an indehiscent pericarp or seed-vessel, pulpy, many-celled, and many-seeded, the seeds being naked, and for a time connected by a slender membrane, from which they become detached at maturity, and then remain dispersed through the pulp. It is distinguished by its figure, &c., into several varieties.
The leading berries employed in domestic economy and the arts are noticed in their alphabetical places (which see).
BER′YL (bĕr-rĭl). Syn. Aquamarine′ (rēne); A′qua-mari′na, Beryl′lus, L.; Aigue-marine, Béril, Fr.; Beryll, &c., Ger.; Smaragd, It. A beautiful mineral, which, in its richer forms, is classed with the gems. It is usually of a green colour of various shades, passing into honey-yellow and sky-blue. It is allied in composition to the emerald; but occurs in much larger crystals than that gem, and owes its colour to oxide of iron instead of oxide of chromium. According to Gmelin its composition is—Silica, 68·7%; alumina, 17·6%; glucina, 13·4%; red oxide of iron, ·24%. Other (previous) authorities state that it contains fully 14% of glucina, 2% of lime, and 1% of oxide of iron.
The finest beryls come from Dauria on the frontiers of China, from Siberia, and from Brazil. Some of gigantic size have been found in the U.S., at Ackworth and Grantham, New Hampshire, and at Royalston, Mass. One of these measured 32 × 22 × 15 inches, and weighed 2900 lbs.; another, 12 × 24 × 45 inches, and weighed 1076 lbs.
Apatite or Saxony beryl, chrysolite or pierre d’asperge, coloured fluor-spar, and even natural crystals of phosphate of iron, are often worked up by the lapidaries and passed off as beryls, or false beryls, emeralds, topazes, &c. See Gems, Pastes, &c.
BERYL′LA*. See Glucinum, Oxide of.
BERYL′LIUM*. See Glucinium.
BETAINE. C5H11NO21. An alkaloid occurring in the juice of the mangold-wurzel. Scheibler prepares it as follows:—The expressed juice of the mangold-wurzel, strongly acidulated with hydrochloric acid, is mixed with a solution of sodium phosphotungstate;[165] the resulting precipitate containing albumen, colouring matter, woody fibre, and a small quantity of the base, is filtered as quickly as possible, and the filtrate, mixed with a fresh quantity of the precipitant, is left to itself for eight or ten days. It then gradually deposits on the bottom and sides of the vessel a crystalline precipitate, which is rinsed with a little water and treated with milk of lime, whereby insoluble calcium phosphotungstate is produced, while the betaine remains in solution. The filtered liquid freed from lime by carbonic acid, and evaporated, leaves impure betaine, which may be purified by recrystallisation from alcohol, with help of animal charcoal.
A hydrochlorate, a sulphate, an aurochloride, and a platinic chloride of betaine have been prepared.
BE′TEL (bē′tl). [Eng., Ger.] Syn. Be′tle, Be′tel-tree, B. pepper-tree; Bétel, Fr.; Wasserpfeffer, &c., Ger.; Pi′per be′tel (Linn.), Chavica betle (Miquel), L. A climbing plant of the nat. ord. Piperaceæ, common in India and the East. Its leaves, which somewhat resemble those of the citron, are bitter, stomachic, tonic, stimulant, and sialogogue.
Betel. A common masticatory in the East, where it is chewed in the same way as tobacco is by Europeans and Americans, but much more generally, being regarded by the Malays, Sumatrans, &c., as an absolute necessary of life. It is commonly formed by dividing areca-nuts[166] into four or six equal parts or slices, one of which is rolled up, with a little chunam,[167] in a sirih or leaf of the piper-betel,[168] and then constitutes a ‘quid’ ready for use.
Prop., &c. Betel, in those accustomed to its use, produces a species of pleasing excitement or intoxication, stimulates the action of the salivary glands, stomach, and kidneys, corrects acidity, diminishes cutaneous perspiration, restrains excessive discharges, increases the power of physical exertion and endurance, moderates the effects of climate, and appears to act as a general tonic on the system. It darkens the teeth, and tinges the saliva as well as the mouth and lips of a bright red colour. In those unhabituated to its use it causes giddiness, astringes and excoriates the mouth and fauces, and temporarily deadens the sense of taste. The Indians conceive that it preserves and fastens the teeth, cleanses and strengthens the gums, sweetens the breath, cools the mouth, assists respiration, and acts as a general aphrodisiac on both sexes. Peron states that he preserved his health during a long and very trying voyage by the habitual use of betel, whilst his companions, who did not use it, died mostly of dysentery.[169]
BE′TEL-NUT. Syn. Are′ca-nut; Nux are′cæ cat′echu, N.-be′tel, &c., L. The seed of the catechu-palm (are′ca, cat′echu, Linn.), divested of the husk or fibrous pericarp. The whole fruit (ARECA-NUT of commerce) is about the size of a small egg; the husked nut is of the size of a large nutmeg. The whole fruit291 is remarkable for its narcotic or intoxicating power. It has, however, been thought doubtful whether its intoxicating effect is not owing to the piper-leaf in which it is wrapped when eaten (chewed), rather than to any special property of its own. See Areca Catechu.
BETTNASSEN, Remedy for Incontinence of Urine (prepared by Dr Kirchhoffer, in Kappel by St. Galle). Thirty powders, each consisting of 2 grammes ferri carbonas, 4 grammes ergotæ pulv., ·03 grammes extract. sem. strychni. aquos. The prescription for the embrocation runs—Spirit serpylli 120 grammes, tinct. sem. strychni. 60 grammes, liq. ammon. 15 grammes. (Hager.)
BET′ULINE (-ū-lĭn; bē-tū). [Eng., Fr.] Syn. Betuli′na, L. A crystalline substance obtained from the bark of the white birch (be′tŭla al′ba, Linn.).
BE′ZOAR (-zōre). [Eng., L. indecl.; prim. Pers.[170]] Syn. Be′zoar-stone; Bezoär′dus, La′pis bezoär′dicus, &c., L.; Bézoar, Bézoard, Fr.; Bezoarstein, Ger. The name of preternatural concretions found in the stomach, intestines, &c., of certain animals, and formerly supposed to possess the most extraordinary antidotal power and medicinal virtues. So far, indeed, did this belief extend, that other substances regarded as antidotes were called BEZOAR′DICS†, or otherwise named after them; whilst the adj. BEZOAR′DIC† (bĕz-) and BEZOAR′TICAL† (bézoardique, Fr.; bezoar′dicus, L.), came to be synonymous with antidotal. Certain bezoars were once valued at even ten times their weight in gold. They were not only taken internally, but also worn as amulets. They have, however, long since fallen into disuse in this country.
Among the leading bezoars of old medicine are—
Bezoar, Ger′man. Syn. Be′zoär German′icum, B. capri′num, L. From the Alpine goat.
Bezoar, Hu′man. Syn. B. hom′inis, L. Falsely stated to be found occasionally in man.
Bezoar, Microcos′mic. Syn. B. microcos′micum, L. Human urinary calculi.
Bezoar, Mon′key. Syn. B. sim′æ, La′pis s., L. From certain species of ape or monkey, obtained by giving an emetic.
Bezoar Occiden′tal. Syn. West′ern b.; B. occidenta′le, L. Found in the fourth stomach of the chamois or wild goat of Peru, &c.; or, according to others, of a species of antelope.
Bezoar, Orien′tal. Syn. East′ern b.; B. orienta′le, Lapis b. orienta′lis, L. From the fourth stomach of ca′pra æga′grus, a species of goat inhabiting the mountains of Persia, &c.
Bezoar, Ox. Syn. B. bovi′num, L. From the ox, and other bovine animals.
Bezoar, Por′cupine. Syn. B. hys′tricis, B. hys′tricus, La′pis h., L. porci′nus, &c., L. Said to be found in the gall-bladder of the Indian porcupine. Chiefly from Malacca. Has an intensely bitter taste, which it imparts to water.
Bezoar, West′ern. See Occidental Bezoar (antè).
Of the preceding, those from the stomach of ruminants vary in size from that of a bean to that of a hen’s egg, and have a composition and appearance closely imitated by the following formula, the product of which is commonly sold for them:—
Bezoar, Facti′′tious. Prep. From pipe-clay, or clay and chalk, equal parts, made into a stiff paste with ox-gall; a little hair or wool being added, and the resulting mixture pressed by the hands into small masses of a flattened spheroidal or egg-like form. These give a yellow tint to paper rubbed with chalk, and a green one to quick-lime, which tests are used for genuine bezoars. Like the latter, they are antacid or absorbent, which is probably the only virtue they possess.
Amongst ‘chemical bezoars’ now obsolete even on the Continent were—
Bezoar, Ar′gentine†; B. luna′′re, L. Made by distilling butter of antimony with a solution of nitrate of silver. Once highly esteemed in epilepsy and head diseases.
Bezoar, Min′eral; B. minera′le, L. Powder of algaroth deflagrated with nitre in a red-hot crucible, and then well washed with water. Once used as a diaphoretic. Other similar preparations were B. JOVIA′LE (from tin), and B. MARTIALE (from iron).
Bezoar, Sat′urnine, B. of lead; B. satur′ni, L. Made by distilling a mixture of oxide of lead, butter of antimony, and nitric acid. Once highly esteemed in diseases of the spleen.
BHAURTA. In Indian cookery, a dish made of mashed potatoes and onions, strongly spiced with capsicum, and sometimes also with curry-powder, shaped in a mould, and then slightly baked.
BIBAS′IC. Syn. Bibas′icus, L.; Bibasique, Fr. In chemistry, having two bases, or two atoms of the base or basic radical in its composition. See Acid, Nomenclature, Salt, &c.
BIB′ERON (bĭb′-rōng). [Fr.] A sucking-bottle or ‘artificial mother.’ See Bottles.
BI′BIRINE (bē′-). See Bebeerine.
BIB′ULOUS (-ū-). Syn. Bib′ulus, L.; Spongieux, Fr. Absorptive; spongy.
BICAR′BONATE. A salt in which only half the hydrogen in (hypothetical) carbonic acid (H2CO3) is replaced by a metal, e.g. bicarbonate of sodium, NaHCO3.
BICE (bīse), Syn. Blue bice. See Blue Pigments.
Bice, Green. See Green Pigments.292
BICKEL′SCHER THEE, for constipation, flatulence, hemorrhoids, loss of appetite, stomach complaints, and similar diseases. Cassia lignea and anise, of each 3 parts; cumin and fennel seed, each 4 parts; senna leaves, 20 parts; to be bruised together. (Selle and Hager.)
BI′DERY (bē′-). Syn. Vi′dry. An alloy of which the chief seat of the manufacture is the city of Bider′, near Hyderabad, India. It was first brought under the notice of the British public at the International Exhibition of 1851, where many articles made of it were greatly admired for the elegance of their forms, and the gracefulness of their engraved and enchased patterns.
Prep. 1. Zinc, 31 parts; copper and lead, of each 2 parts; melted together, with the usual precautions, under a mixture of resin and beeswax, to prevent oxidation.[171]
2. (Dr Heyne.) Copper, 8 parts; lead, 2 parts; tin, 1 part; melted together, as before. For use, the resulting alloy is remelted, and to every 3 parts of it 16 parts of zinc are added.
Prop., &c. Colour between that of pewter and zinc; does not corrode by exposure to air or damp; yields little to the hammer, and can only be broken by extreme violence. It possesses a convenient degree of fusibility, above that of zinc and tin, but much lower than that of copper. For the turner it is usually cast in moulds of baked clay; but otherwise in moulds of iron or other hard metal. The beautiful black colour which the finished articles possess is imparted by dipping them into a solution of sal-ammoniac, saltpetre, sea-salt, and blue vitriol. See Brass, Bronze, Pewter, &c.
BIDET′ (bĭd-ĕt′; -ā′—Fr.). An article of bedroom furniture conveniently formed for laving the lower part of the body. Besides the value of its use as an instrument of personal cleanliness and health, it offers a ready means of medicating the parts, often highly serviceable in piles, prolapsus, affections of the scrotum and prostate gland, strangury, ischuria, suppressed or difficult menstruation, &c. See Ablution, Baths, &c.
BIELEFELDER TROPFEN—BIELEFIELDER DROPS (Bansi). A spirituous extract of wormwood, unripe oranges, rhubarb, cascarilla, cloves, and gentian. (Hager.)
BIEN′NIAL (bī-ĕn′-y′ăl). Syn. Bien′nis, L.; Biennal, Bisannuel, De deux ans, Fr.; Zweijährig, Ger. Occurring once in, or lasting, two years. In botany and gardening, applied to plants that do not produce flowers and seed until the second year or season of their growth, and which then die; subst., a biennial plant.
The existence of the biennials, like that of the annuals, may be prolonged by art; indeed, many of them, by carefully removing the flowers ere the seed-vessels begin to form, may be made to bloom a second season, and even for several seasons following, like perennials. See Annuals, Flowers, Plants, &c.
BIFF′IN. A baked apple, flattened by pressure.
Prep. The apples are placed in a cool oven 6 or 7 times in succession, and flattened each time by gentle pressure, gradually applied, as soon as they are soft enough to bear it; after which they are taken out, and as soon as cold put on clean dishes or glass plates. The sour or tart variety of apples is the best for baking. If the process be well managed, the appearance of the prepared fruit is very rich and the flavour delicious.
BIL′BERRY. The whortleberry.
Bilberry, Bear’s. Uva ursi.
BILE. Syn. Bi′lis,[172] Cho′le,[173] Fel,[174] L.; Bile, Fiel, Galle, Fr.; Galle, &c., Ger. A bitter fluid secreted by the liver, from venous blood; in part flowing from the intestines, and in part regurgitating into the gall-bladder. Its composition is of a very complex character; and its uses in the animal economy appear to be—to separate the chyle from the chyme, to promote the digestion and assimilation of oleaginous substances, and to assist in exciting the peristaltic action of the intestines. The fæces appear to owe their colour chiefly to the presence of bile; as, without it, they possess a dirty pipe-clay colour. Several of the substances which enter into its composition, or which are formed from those which do so, are noticed elsewhere, under their respective names. Its analysis, detection, and uses in the arts are given under Gall.
Bile (of Animals). See Gall.
BILE, Bil′iousness. Under these terms are popularly included all those slight affections of the stomach usually accompanied with derangement of the head and bowels, apparently arising from excess of bile. Persons subject to attacks of this description should be particularly careful to avoid excess in both eating and drinking, and should more especially shun those articles of food and those liquors which, from experience, they find are apt to disagree with them. A mutton chop, slightly under-dressed, is an excellent article for the breakfast, or the lunch, of bilious patients; and good beef or mutton, either broiled or roasted, so that the gravy be retained, is better for dinner than many dishes apparently more delicate. These, with fresh game and venison, form a good variety from which to choose a bill of fare. New beer and porter should be particularly avoided, as well as boiled meat, stews, soups, greasy or rich puddings, much butter or fat, and most articles of pastry, as they are very indigestible, and, by overtasking the powers of the stomach, very apt to derange293 it. Strong cheese,[175] salads (particularly cucumbers), over-ripe or unripe fruit, new bread and rolls, cabbages and green vegetables, and especially peas, beans, nuts, almonds, and the like, are also objectionable for parties with delicate stomachs or a bilious tendency. The bread eaten by such persons should be perfectly free from alum, and preferably prepared with meal retaining the whole of the bran in it; and should be two days, or at the least one day old. The quantity of animal food per day, except for the laborious, should be limited to from 6 or 8 to 12 oz.; and warm slops of all kinds, except moderately strong tea and coffee, should be taken as seldom as possible, and, in general, avoided altogether. Even cocoa and chocolate prove injurious to the delicate and bilious. Out-door exercise and plenty of fresh air are essential to the health of such persons. Those who indulge in them freely are never attacked with affections of this kind, unless it be after gluttonising or heavy drinking. Above all things heavy and late suppers should be abandoned; indeed, the better plan is to take nothing more than a hard biscuit, or dry crust, after tea.
In general, attacks of bile may be prevented by the exercise of moderate judgment and temperance in living; and in those hitherto subject to them by the occasional use of an aloetic, mercurial, or saline aperient; and they may be generally rapidly removed by an emetic, followed by a dose of castor oil, Epsom salts, or Seidlitz powder. A tumbler of pure cold water taken on retiring to rest, and another (or even two) on rising in the morning, will often remove both the tendency and the fit, when all the usual remedies have failed. See Abernethy Medicines, Antibilious, Dyspepsia, Stomach Affections, &c.
BILHARZIA HÆMATOBIA. A fluke-like parasite. It is bisexual. The body of the male is thread-shaped, round, white, and flattened anteriorly. The female is thin and delicate. This creature was discovered in the portal vein and bladder of man by Bilharz, of Cairo, after whom it was named. It is especially prevalent in those who dwell by the banks of the Nile, and is also very frequently met with amongst the inhabitants of the Cape of Good Hope. It is the cause of very serious disturbance in the human economy, and not infrequently of death.
The main symptoms of the disease this dangerous parasite sets up are those which point to derangement of the urinary organs; but its effects are not confined to these, since there seems little room to doubt that it is the chief cause of the dysentery so prevalent in Egypt, the eggs of the diatoma being found deposited within the intestinal vessels, or beneath the exudations of the swollen mucous membrane. Dr Harley has found the ova in the urine of persons affected with hæmaturia at the Cape of Good Hope. When death ensues from the presence of this parasite the post-mortem appearances are various. In the bowels, congestion, deposits upon the mucous membrane, and extensive ulcerations, degeneration and atrophy of the kidneys, dependent upon an infiltrated state of the ureters, and blocking of the portal vein, due to the presence of myriads of the parasites, are some of the most important pathological changes.
BIL′IARY AFFECTIONS (-yăr-e). See Bile (antè), Calculi, Jaundice, Liver, &c.
BI′LINE (-lĭn). Syn. Bili′na, L. This name has been loosely applied to two substances:—1. Bile, or pure bile, freed from the mucus of the gall-bladder, and gently evaporated to dryness. A gummy pale yellow mass, white when powdered:—2. Tauro-cholalic or choleic acid. See Gall, &c.
BIL′IOUS (-yŭs). Syn. Bilio′sus, L.; Bilieux, Fr.; Gallig, Gallicht, &c., Ger. Pertaining to, caused by, full of, or having excess of bile. See Bile, Biliousness.
BILIPH′EINE (-e-ĭn). Cholepyrrhine.
BILIV′ERDINE (-dĭn). A green colouring matter, identical with chlorophyll, found in bile, and in the green dejections of children.
BILL OF FARE. In cookery, domestic economy, &c., a list of things ready dressed or prepared for the table (CARTE, C. D’UN RESTAURANT, MENU, &c., Fr.); also a list of articles of food in season. For Tables of the latter, see Food.
BI′NARY. Syn. Bina′′rius, L.; Binaire, Fr. Consisting of two parts. In chemistry, compounded of two elements, or of two bodies performing the function of elements.
BINOC′ULAR (-ū-). Having two eyes. In optics, of or with two eyes, as binoc′ular vĭ′′sion; or formed with two eye-pieces or tubes, so as to be used with two eyes, as a b. mi′croscope, b. tel′escope, &c.
BIRCH. Syn. Be′tula, L.; Bouleau, Fr.; Birke, Ger. The common name of trees of the genus be′tula; appr., b. al′ba (Linn.), or white birch; also its wood. See Betuline, and below.
Birch, Black. Syn. Cher′ry b., Sweet b., Mount′ain mahog′any; Betulen′ta, L. A forest tree of N. America. Wood used for cabinet work; bark yields a volatile oil similar in odour and taste to that of gualtheria; juice obtained by tapping, saccharine, and yields BIRCH-SUGAR.
Birch, White. Syn. Birch, (or) Common b.; Be′tula, L. A tree found in the woods of England. Wood neither very hard nor durable; leaves formerly used in itch and dropsy; bark febrifuge, yields a pyroligneous oil by distillation. See Oils (and above).
BIRD[176] [Eng., Sax.] Syn. A′vis, L.; Oiseau,294 Fr.; Vogel, Ger. Any fowl or animal of the feathered kind. In fashionable and gourmandic cant, appr. a partridge. See Birds (below).
BIRD′LIME. Syn. Vis′cus, L.; Glu, Fr.; Vogelleim, Ger. Prep. The middle bark of the holly (gathered in June or July) is boiled for 6 to 8 hours in water, or until it becomes quite soft and tender; the water is then drained off, and it is placed in a heap, in a pit underground (commonly on layers of fern), and covered with stones. Here it is left to ferment for 2 or 3 weeks, and watered, if necessary, until it assumes a mucilaginous state. It is next pounded in a mortar until reduced to a uniform mass, which is then well kneaded with the hands in running water, until all the refuse matter is worked out. It is, lastly, placed in an earthen vessel, and covered with a little water; in which state it may be preserved from season to season. In about a week it is fit for use.
Prop. Greenish coloured: very gluey, stringy, and tenacious; when air-dried, brittle and pulverisable, but capable of gradually assuming its previous viscosity when moistened.
Uses. To cover twigs to catch birds, and other small animals. It is said to be discutient, but is now never employed in medicine.
Obs. Birdlime may also be made from mistletoe berries, the young shoots of the elder, the bark of the wayfaring-tree, and some other vegetables, by a similar process to that above described. Should any of it stick to the hands it may be removed by means of a little oil of turpentine.
A kind of factitious birdlime is made by boiling linseed oil either with, or without, a little yellow resin, until it forms a viscid, stringy paste when cold. This is chiefly used, spread on paper or cloth, to catch insects. See Fly-papers, &c.
BIRDS. Syn. A′ves, L. Birds, besides their value as food, play an important part in the economy of organic nature, and particularly in that of the vegetable kingdom. They are the best friends of the agriculturist and the gardener; and their presence, in numbers, appears essential to keep down the innumerable races of insects that prey upon our cereals, fruits, and culinary vegetables. M. Florent Prevost, who has for fifty years presided over the Natural History Museum of Paris, and who has, like the ancient Roman augurs, examined the entrails and stomach of fowls with scientific curiosity, avers, as the result of his long experience, that birds, of whatever sort, are an unmitigated blessing to the farmer, and that the detritus and organic particles found by inspection of them in whole hecatombs, which, by the assistance of the Royal Forest Rangers, he has sacrificed on the altar of utility, show an immense preponderance of insect corpuscula in their digestive organs, whilst the traces of cereal or other valuable products are infinitesimal in comparison. It is found that even sparrows, rooks, and owls—three of the feathered tribe the most persecuted by the farmer—are, in reality, the faithful and vigilant conservators of his fruits and crops. In one of the smaller states of Germany, where, owing to public rewards being given to their destroyers, the whole race of sparrows were exterminated, the crops failed to such an alarming extent that it became necessary to offer large premiums for the reintroduction of these useful birds from other parts. In some of the agricultural districts of France, where the destruction of small birds has been carried on with relentless activity for years, insects have so prodigiously multiplied as to attack everything green around them. Even the forest trees are, in many cases, denuded of leaves by them, and are rapidly perishing. Venomous species of caterpillars, previously scarcely known except to entomologists, have now become common; and cases of children losing their lives from attacks of them whilst birdnesting have been published in newspapers.[177] In our own country the extension of sparrow-clubs—associations disgraceful to the boasted intelligence of the nineteenth century—threatens similar results. Already the gardener finds his fruit-crops lessening year by year; and that many of them, particularly of the smaller and sweeter fruits, have become so precarious, that they now scarcely pay for cultivation. In our own neighbourhood, where small birds have for some years been destroyed by bushels at a time, it is almost impossible to raise a currant, gooseberry, cherry, or plum; whilst seedling flowers and culinary vegetables often entirely disappear on the first night after being planted, or are so completely deprived of the succulent portion of their leaves and stems, that the remaining skeleton of network in a few days withers and dies. But this is not all—the columns of our diurnals bring us continual reports of failing grain-crops in the neighbourhoods in which these bird-clubs have existed for any length of time, and that even on land previously remarkable for its fertility.[178] Did this loss fall only on the benighted beings who so wilfully cast back the blessings of an all-wise protecting Providence, it would be a just retribution; but, unfortunately, it affects the whole nation, and threatens, ere long, unless arrested by legislation, to prove a national calamity. The only apparent remedy for the evil, at present, is the diffusion of information tending to show that the farmer and the gardener, in destroying small birds, destroy their best friends.
[For further information respecting birds, see Aves, Bird (antè), Game, German Paste,295 Nests (Edible), Poultry, Putrefaction, Taxidermy, Trussing, &c.]
BIRKENBALSAM—BIRCH BALSAM (Dr Friedreich Lengiel). A cosmetic against wrinkles, small-pock marks, freckles, mole spots, red noses, acne, &c. 5 grammes water glass, 2 grammes potash, 1 gramme soap, 5 grammes gum arabic, 10 grammes glycerin, 400 grammes water. (Schädler.)
BIS′COTIN. [Fr.] A small biscuit. In cookery, &c., a species of confection made of eggs, flour, marmalade, and sugar, variously compounded and flavoured according to the taste of the operator.
BIS′CUIT (-kĭt). [Eng., Fr.] Syn. Buccella′tum, Pa′nis bis coc′tus, L.; Swieback, Ger.; Biscotto, It.; Bizcocho, Sp. Literally, ‘twice-baked,’ appr., a well-known variety of hard, dry, unleavened bread, made in thin flat pieces. Those prepared for seamen (SEA′-BISCUITS, CAP′TAIN’S B.[179]) are composed of flour and water only. When made of fine flour and a few caraway seeds are added, they are commonly called Ab′erne′thy biscuits. Fancy biscuits generally contain a little sugar and butter, to which almonds, caraways, mace, ginger, lemon, and other articles, technically called ‘flavourings,’ are frequently added.
Prep. On the small scale, biscuits are made by forming the flour and water into a dough by the common process of hand-kneading, occasionally assisted with a lever, as in making ordinary bread. The dough is then rolled into a sheet, and cut into pieces of the desired size and form. These, after being stamped, are exposed to the heat of a moderately quick oven, when a few minutes (12 to 18, according to their size) are sufficient to bake them.
On the large scale, the whole manual process, from preparing the dough to the point at which the newly-made biscuits are ready for baking is now generally performed by machinery. The articles so prepared are commonly known in trade as ‘MACHINE-MADE BISCUITS,’ and are not only much cheaper, but of fully equal quality to those ‘made by hand.’ In the bakehouses of her Majesty’s Victualling Yards at Deptford, Gosport, and Plymouth, the ingenious machinery invented by Mr T. T. Grant is employed. These establishments are said to be capable of producing annually above 8000 tons of sea-biscuits, at a saving of upwards of 12,000l. a year, from the cost that would have been incurred for the purpose on the old system. Under the latter it is stated that wages, and wear and tear of utensils, cost about 1s. 6d. per cwt. of biscuit; whilst under the new system the cost is only 5d.
The allowance of biscuit to each seaman in the royal navy is 1 lb. per day; or, on the average, six biscuits.
Biscuits Depuratifs (Olivier) are made with meal, milk, and sugar. Each biscuit contains 1 centigramme corrosive sublimate. (Foy.)
Biscuits, Fancy. The varieties of these are almost innumerable. In a printed list now before us we observe the names of upwards of one hundred different kinds. These are produced by varying the number and proportions, of the ingredients used in their composition, and the form and size in which they are turned out of hand. They are further modified by the relative heat of the oven, as well as the length of time they are allowed to remain in it. It would, therefore, be waste of space to give particular directions for the preparation of each. The proportion of butter and sugar, or either of them, may be from 1 oz. and upwards, to flour, 1 lb.; according to the degree of richness desired. In a few cases milk, or eggs, or both, are introduced. The ‘flavourings’ embrace a wide range of substances—bitter almonds, caraways, cassia, cinnamon, ginger, mace, nutmeg, lemon, orange-peel, orange-flower water, essence of peach kernels, vanilla, &c., &c.; many of which give their name to the biscuit.—Ar′row-root biscuits are usually made of equal parts of arrow-root and flour; MEAT′-BISCUITS, from about 1 part of lean meat (minced small and pulped) beaten to a dough with about 2 parts of flour, and a little seasoning, no water being added;[180] SODA BISCUITS, by adding 1 to 2 dr. of carbonate of soda to each lb. of flour. In most other cases, the mere inspection of the biscuit will convey to the experienced biscuit-baker and cook sufficient information to enable him to produce an exactly similar one, or at least a very close imitation. The richest kind of SPONGE-BISCUITS, as we are informed, are made as follows:—Add the whites and yelks of 12 eggs, previously well beaten, to 11⁄2 lb. of finely powdered sugar, and whisk it until it rises in bubbles, then add 1 lb. of the finest pastry-flour, and the grated rind of 2 lemons. Put it into ‘shapes,’ sift a little sugar over them, and bake them in buttered tin moulds, in a moderately quick oven, for nearly half an hour.[181]
Biscuits Purgatifs (Caroz). Each biscuit contains 2 decigrammes scammony. (Reveil.)
Biscuits Purgatifs (Sulot). Each biscuit contains 6 decigrammes scammony.
Biscuits Purgatifs et Vermifuges (Ferd. Gräf, Aschbach) contain 1⁄4 gramme resina scammonii in each.
Biscuits Purgatifs et Vermifuges au Calomel (Sulot). There are 3 decigrammes of calomel in each. (Reveil.)
Biscuits Vermifuges à la Santonine (Sulot.) Each biscuit contains 5 centigrammes of san tonin. (Reveil.)
Biscuits, dev′iled, in cookery, are captain’s biscuits (or any similar kind) buttered on both296 sides, peppered well, and then covered on one side with a slice of good cheese formed into a paste with made mustard; the whole being seasoned with a little cayenne pepper is, lastly, grilled. Chopped anchovies, or essence of anchovies, is a good addition.
BISMUTH. Bi. Bismuth, Etain de glace, Fr.; Bismuth, Wismuth, W.-metall, Ger. One of the metals.
Sources. Bismuth occurs in the mineral kingdom in the metallic state (NA′TIVE BISMUTH), and in combination with sulphur (BIS′MŬTHĬNE), and with oxygen (B. O′CHRE, &c.). That of commerce is mostly imported from Saxony, where it is chiefly obtained from native bismuth by the simple process of eliquation. The ore, sorted by hand from the gangue, and broken into pieces of about the size of nuts, is introduced into the ignited iron pipes of the furnace (see engr.) until these latter are filled to about one half their diameter and to three fourths of their length. From these the liquefied metal is allowed to flow into iron pans containing some coal-dust, and from these into a trough of water, in which it is granulated and cooled. It is subsequently remelted and cast into moulds so as to form ‘bars’ varying in weight from 25 to 56 lbs. each. In this state it usually contains a small admixture of arsenic, iron, lead, and sulphur; from the first of which it may be freed by exposure for some time, under charcoal, at a dull red heat. It is best obtained in a pure condition by heating to redness, in a covered crucible, a mixture of oxide, or subnitrate of bismuth, with half its weight of charcoal.
Prop. Colour greyish-white with a reddish tint; crystalline; very brittle (may be powdered); melts at about 480° Fahr., and does not re-solidify until cooled to 6 or 7° below this point; it volatilises at a strong heat, and, in close vessels, the fumes condense unchanged in crystalline laminæ; little acted on by the air, but when exposed to it at a high temperature burns with a faint blue flame, emitting yellow fumes which condense into a yellow pulverulent oxide; when slowly cooled, in large masses, it forms large cubic crystals or octahedrons of great beauty; nitric acid, somewhat dilute, dissolves it freely. It is highly diamagnetic. Sp. gr. 9·8 to 9·83, which, by careful hammering, may be increased to 9·8827. A bar of bismuth, when heated from 32° to 212°, expands exactly 1⁄710 in length.
Uses, &c. Bismuth enters into the composition of STEREOTYPE-METAL, SOLDER, PEWTER, FUSIBLE METALS, and several other alloys. Added to other metals it renders them more fusible. An alloy of tin, nickel, bismuth, and silver is said to hinder iron from rusting. A mixture of bismuth, lead, and tin is much employed for taking impressions from dies, forming moulds, and for other purposes.
Bismuth salts are usually insoluble, or decomposed by any quantity of water into free acid and a basic salt. They are nearly all colourless, and, except the chloride, more volatile. They are easily recognised by the following reactions:—
Their saturated or concentrated solutions giving a white precipitate on dilution with water:—Sulphuretted hydrogen blackens them, or gives a black precipitate:—The nitric solution is unaffected by the addition of sulphuric acid:—Chromate of potassium gives a yellow precipitate, which differs from that from lead, by being soluble in nitric acid, and insoluble in potassa.
Von Kobbell takes a mixture of potassium iodide and flowers of sulphur in equal proportions, and heats the whole on charcoal before the blowpipe; the production of bright scarlet, very volatile bismuth iodide ensues, even when only traces of bismuth are present.
For a method of volumetrically estimating bismuth, consult a paper by Mr Pattison Muir, in the ‘Journal of the Chemical Society,’ April, 1876.
Bismuth, Car′bonate. (Ph. B.) Mix nitric acid, four fluid ounces, with three fluid ounces of distilled water, and add in successive portions purified bismuth, in small pieces, two ounces. When effervescence has ceased apply for ten minutes a heat approaching ebullition; then decant the solution from any insoluble matter. Evaporate to two fluid ounces, and add this in small quantities at a time to a cold filtered solution of six ounces of carbonate of ammonia, in two pints of distilled water, constantly stirring. Collect precipitate on a calico filter; wash till washings pass tasteless. Remove water by slight pressure of the hands, and dry at a heat not exceeding 150°.—Dose, 5 to 20 grains.
Bismuth, Chlorides of:
Basic Chlo′′ride. Bi3Cl8. Syn. Subchlo′′ride of bismuth, Pearl′-powder; Bismu′thi subchlori′dum, L. Prep. A dilute solution of hydrochloric acid is dropped into another of bismuth (prepared by dissolving that metal in nitric acid); and the resulting297 precipitate, after being well washed in pure water, is dried in the shade.—Prop., Uses, &c. Similar to those of the subnitrate.
Chlo′′ride. BiCl3. Syn. Terchlo′′ride of bismuth. Prep. A mixture of corrosive sublimate, 2 parts; bismuth, 1 part; (both in powder) is exposed to heat until all the ‘mercury’ present is expelled, after which it is at once put into bottles. A greyish-white, granular substance.
Bismuth, Nitrates of:
Basic, Nitrate. BiONO3. Syn. Pearl-white, Bismuth subnitrate; Bismuthi subnitras, B. bismuthi nitras, L.; Blanc de Fard, B. d’Espagne*, &c., Fr.; Perlweiss, Schminkweiss, &c., Ger. Prep. Bismuth, 1 oz.; nitric acid, 11⁄2 fl. oz.; distilled water, 3 pints; mix 1 fl. oz. of the water with the acid, and dissolve the bismuth in the mixture; throw the solution into the remainder of the water, and, after repose, pour off the supernatant liquor, drain the powder that has subsided on a linen cloth, wash it with distilled water, and dry it with a gentle heat.
Prop. A pearly white, inodorous powder, insoluble in water, but freely soluble in nitric acid; long exposure to a strong light turns it greyish. When prepared from a neutral solution, it consists of very fine microscopic crystalline laminæ; but when prepared from acid solutions, with less water, the crystals are acicular, and more silky and lustrous. When moistened it exhibits an acid reaction with litmus paper.
Pois., &c. Like the other salts of bismuth, it causes vomiting, purging, giddiness, cramp, insensibility, &c. No certain antidote is known. The treatment may consist of an emetic, followed by the copious use of emollient drinks, as weak broth, barley water, milk and water, &c.; and subsequently, when necessary to prevent inflammation, by a low diet and aperients.
Uses, &c. In medicine, as a sedative, an astringent, or tonic, and an antispasmodic, in chronic affections of the stomach unaccompanied by organic disease of that organ, and apparently of a nervous character; particularly in gastrodynia, troublesome sickness and vomiting, pyrosis or waterbrash, and generally in gastro-intestinal affections attended with fluxes; also in intermittent fever, spasmodic asthma, &c.—Dose, 5 to 10, or even 20 gr.
Externally, made into an ointment with 4 parts of lard, it has long been employed in certain chronic skin diseases. Under the name of PEARL-WHITE it is commonly used by ladies as a cosmetic; but it is stated that it injures the skin, producing, after a time, paralysis of its minute vessels, rendering it yellow and leather-like—an effect which, unfortunately, it is usually attempted to conceal by its freer and more frequent application. In very large doses it is poisonous.
Both the basic nitrate and the basic chloride of bismuth pass under the names of PEARL-WHITE and PEARL-POWDER, owing to their extreme whiteness and beauty. That of the druggists, however, is usually the former; that of the perfumers usually the latter, but not unfrequently both.
Bismuth Powder, for beautifying the skin and removing freckles. (From North America.) Consists of calcium carbonate, with much clay, and is free from injurious metals. (Chandler.)
Bismuth, Purified. (Ph. Br.) Put bismuth, 10 ounces, and 1 ounce of powdered nitrate of potash, into a crucible, and heat them until both are fused. Continue the heat, constantly stirring, for fifteen minutes, or till the salt has solidified into a slag above the metal. Remove the salt, add nitrate of potash, 1 ounce, to bismuth in crucible, and repeat the process. Pour the fused bismuth into a suitable mould, and let it cool.
Nitrate. Bi(NO3)3. Syn. Neutral nitrate, Ternitrate. Purified bismuth (in small fragments), 2 oz.; nitric acid, 6 oz.; dissolve with heat, adding more acid, if necessary, to effect entire solution of the metal; to the resulting solution add half its volume of distilled water, filter through powdered glass, and evaporate until crystals form.
Use. Chiefly in chemistry, and as a source of the pure oxide and the subnitrate.
Bismuth Oxides:—
Bismuthous Oxide. Bi2O3. Syn. Teroxide of bismuth, Protoxide of bismuth. From either the neutral or the basic nitrate, by exposure, in a crucible, to gentle ignition. Pure. A straw-yellow powder, of rather difficult solubility.
Hy′drated:—By gradually dropping an acid solution of bismuthous nitrate into a concentrated solution of potassium hydrate perfectly free from carbonic acid, and washing and drying the resulting precipitate. Pure. A rich-looking white powder.
Prop., &c. Fuses at a high temperature, and then acts as a powerful flux on siliceous matter without itself imparting colour, a property of which the enameller and gilder has long availed himself. Like the basic nitrate, it has been used as an antispasmodic and as a cosmetic. Sp. gr. 8·211 to 8·355.
Bismuthic Oxide. Bi2O5. Syn. Bismuthic anhydride, Bismuthic acid. Suspend teroxide of bismuth in a strong solution of potassa, and pass chlorine through the mixture until decomposition is complete; treat the powder with dilute nitric acid (to remove any undecomposed teroxide), after which wash it in cold water, and dry it.
Prop., &c. A reddish powder, soluble in water. Its salts, of which little are known, are called BIS′MUTHATES. When heated it loses oxygen, and a bismuthate of bismuth is formed.
Bismuthous Sulphide. Bi2S3. This compound occurs native (BIS′MŬTHĬNE), and may be easily prepared artificially by either fusing its elements298 together, or by passing sulphuretted hydrogen through a solution of nitrate of bismuth.
Bismuthous Valerianate. Syn. Bismu′thi valeria′nas, L. Prep. An acid solution of nitrate of bismuth is decomposed with a solution of valerianate of soda in water containing a little free valerianic acid; the precipitate is carefully washed in distilled water, and dried in the shade. Recommended as superior to the subnitrate in some forms of gastrodynia, dyspepsia, intermittents, &c.—Dose, 2 to 6 gr., or more.
BIS′TRE (-ter). [Eng., Fr.] Syn. Bister, Ger. Prep. 1. The most compact, best coloured, and well-burnt portions of the soot of beechwood, or of peat,[184] are selected, reduced to powder and sifted through a very fine lawn sieve. It is then digested in clear warm water for several hours, with frequent stirring; after which it is allowed to settle, when the liquid portion is decanted from the sediment. This process is repeated a second, and even a third time. The paste is next poured into a tall narrow vessel, which is then filled with pure cold water, and well agitated. The grosser parts only are now allowed to subside,[185] and the supernatant liquor, containing the finer portion of the BISTRE in suspension, is poured off into another vessel, where it is left to deposit its contents. The deposit is next collected, and carefully dried and powdered; or it is only partially dried, and at once made into cakes with gum-water or isinglass-size, and then allowed to dry and harden for sale.
2. (Dr MacCulloch.) The tar-like liquid obtained from the dry distillation of wood is again carefully distilled until all volatile matter has passed over, and a brittle, pitch-like residuum is obtained, which is either brown or black according to the time and temperature employed; after which the heat is still further prolonged, but with increased care, until the brittle substance becomes pulverulent and carbonaceous. It is then ground and elutriated with pure cold water, as before.
Uses, &c. As a water colour to tint drawings, in the same way as Indian ink, to which it is esteemed superior when the subjects are intended to be afterwards tinted with other colours. It occupies the same place among water colours that brown-pink does in oil.
According to Dr MacCulloch, bistre from wood-tar, when carefully prepared, has great depth and beauty of colour, with all the fine properties of sepia; but that if the whole of the oils and acids have not been removed by the process, it is apt to collect in little flocks which interfere with its use.
BITES and STINGS. Syn. Mor′sūs (-SŪS, sing.) et Ic′tūs (-TŬS, s.), L. The treatment of the bites of non-venomous and non-rabid animals is the same as that of ordinary lacerated or punctured wounds, as the case may be; that of the bites and stings of venomous and rabid animals, serpents, insects, &c., often require, in addition, the use of special antidotes to destroy the virus or to prevent its absorption, or to neutralise its effects when absorbed and to promote its elimination from the system.
The bites and stings of ANTS, BEES, WASPS, HORNETS, and similar insects common to this climate may be treated by washing the part with spirit of hartshorn or dilute liquor of ammonia or eau de luce, or a weak solution of chloride of lime. Should considerable inflammation ensue, and the part become much swollen, a thing that rarely occurs, leeches may be applied, and a cooling purgative given. The stings of venomous reptiles may be similarly treated, excepting that the strength of the solutions of ammonia, chloride of lime, &c., should be stronger than in the former case, so as to produce some pain and smarting. In cases where the venom is of a very poisonous description, the wound should be first well washed with water of ammonia, and afterwards seared with lunar caustic in every part, including the interior and deep-seated portions. In extreme cases the surface of the wound, both internal and external, may be removed with the knife; or, in the case of a small joint, as a finger, the injured portion may be amputated. Prior to the use of the washes or caustic, dry-cupping or suction with the mouth may be had recourse to with great advantage. A ligature placed on the limb, above the wound, as soon as possible after the accident, will impede the absorption of the poison whilst the other treatment is in progress. A similar plan may be followed after the bite of a dog supposed to be mad. It has, indeed, been lately asserted by one of our most celebrated veterinarians that he and his colleagues have been repeatedly bitten by dogs that have afterwards been proved to be mad, but from having fearlessly applied caustic to the parts they have escaped uninjured.
The poison inserted by the stings and bites of several venomous reptiles is so rapidly absorbed, and of so fatal a description, as frequently to occasion death within a very short space of time, and before any remedy or antidote, under ordinary circumstances, can be applied. But even in these extreme cases it is probable that absorption, and consequently the rapidity of the action of the poison, might be considerably impeded or lessened by the immediate application of a ligature above the part, as before described, the patient accompanying the treatment by swallowing a large quantity of liquid, by which partial plethora would be produced, and the functions of the absorbents for a time nearly-suspended. A few minutes thus gained would permit of the application of appropriate antidotes, by which the poison might be neutralised before it would become necessary to remove the ligature, whilst the kidneys would be in full action. Unfortunately, these wounds are generally inflicted299 in parts of the world where precautionary measures are seldom thought of, and generally at times when people are least prepared to meet them, as well as so suddenly and unexpectedly as to stagger even those observers who may be in no absolute danger themselves. Such is the bite of the East Indian CO′BRA DI CAPEL′LO, against which two Asiatic (arsenical) pills are often prescribed by the Hindoos; but which are generally scarcely swallowed before the poison of the serpent has rendered the patient a stiffened corpse. Eau de luce, a favorite remedy in India, when liberally employed both internally and externally, is said to prove sometimes more successful. The bite of the PUFF-ADDER is of a similar, or even a more fatal description than that of the cobra. When the venom of any of these animals or of a rabid dog is once fully absorbed into the system, there appears to be no treatment that can save the patient. A bottle of Madeira wine or 1⁄2 a pint of brandy or rum diluted with twice its weight of water, drank in two doses about 3 or 4 minutes apart, is a popular remedy in India in such cases. Its effect is to impede absorption.
The secret antidote so long successfully employed by Mr Underwood, the ‘snake-king’ of Australia, for the bites of the WHIP-SNAKE and the DIAMOND-SNAKE, two of the most venomous of that region, is now positively asserted to be the common male fern (polypo′dium fi′lix mas, Linn.). Of the powdered root, or preferably, of the green leaves of this plant nearest the root, he prepares a sort of decoction, or broth, which he takes or administers liberally. A more convenient preparation would, perhaps, be a tincture prepared by digesting 1 oz. of the dried, or 3 oz. of the fresh leaves (bruised), in a pint of proof spirit or strong brandy or rum for a fortnight; as in this state it could be kept for any length of time, if well corked, without deterioration.
For Horses and Cattle. Mr Finlay Dun recommends ammonia solution; solution of caustic potash; carbolic acid; prussic acid and chloroform.
[See Hydrophobia, Poisons, Snake-bites, Stings, Venom, Wounds, &c.[186]]
BITT′ER. [Eng., Ger.] Syn. Ama′′rus, L.; Amer, Fr. Tasting like wormwood, quassia, or other similar vegetables; subst., a bitter plant, bark, or root (= AMA′′RUM, L.; see below).
Bitter App′le‡. Colocynth.
Bitter Cup. A cup or tumbler formed by the turner out of quassia wood. Liquor, by standing in it a short time, becomes bitter and stomachic. They are now common in the shops.
Bitter Earth*. Magnesia.
Bitter Herbs. See Bitters (infrà) and Species (Bitter).
Bitter Salt†, Bitter Pur′′ging-salt. Sulphate of magnesia.
Bitt′er-sweet. Woody nightshade.
Bitt′er-wort‡ (-wŭrt). Gentian.
BITT′ERN. The ‘mother-water’ or ‘bitter liquor’ of salt-works from which the chloride of sodium (sea-salt) has been separated by crystallisation.
Bittern. An intoxicating poisonous mixture sold by the brewers’ druggists, composed of 1 part each of extract of quassia and powdered sulphate of iron, with 2 parts of extract of cocculus indicus, 4 parts of Spanish liquorice, and about 8 parts of treacle; the liquorice being first boiled with a little water until dissolved, and the solution evaporated to a proper consistence before adding the other ingredients. Used by fraudulent brewers and publicans to impart a false bitter and apparent strength to their liquors.
BITT′ERS (-ĕrz). Syn. Ama′′ra, &c., L. Vegetable bitters are commonly regarded as tonic and stomachic, and to improve the appetite when taken occasionally and in moderation. The best time is early in the morning, or half an hour or an hour before a meal. An excessive, or a too prolonged use of them, tends to weaken the stomach, and to induce nervousness. They should not be taken for a longer period than about 8 or 10 days at a time, allowing a similar period to elapse before again having recourse to them.
Among the most useful and generally employed bitters are—calumba, cascarilla, chamomiles, gentian, hops, orange peel, quassia, and wormwood.
Bitters. In the liquor-trade, a compound prepared by steeping vegetable bitters, and some aromatics as flavouring, in weak spirit, for some 8 or 10 days; a little sugar or syrup being subsequently added to the strained or decanted tincture. In that of the taverns and gin-shops the menstruum is usually gin, or plain spirit reduced to a corresponding strength. Bran′dy-bitters and WINE′-BITTERS are prepared in a similar way with common British brandy, or some cheap white wine (Cape or raisin), as the case may be. Each maker has usually his own formulæ, which he modifies to suit the price and the palate of his customer.—This class of liquors has been justly charged with being the fertile cause of habitual intemperance, of disease, and even of death! Their occasional use as tonics or stomachics is also objectionable, owing to the trash, and even deleterious substances, which so frequently enter into their composition. See Liqueurs.
BITU′MEN. [Eng., L.] Syn. Bitume, Fr.; Erdpech, Erdtheer, &c., Ger. A term of a very comprehensive character, and, in general, very loosely applied, including a variety of inflammable mineral substances, consisting300 of varying proportions of hydrocarbons, having a strong smell and differing in consistence, all the varieties being found in the earth, of which asphaltum, naphtha, and petroleum may be mentioned as examples.
Asphalt is very extensively disseminated throughout Europe, Asia, and America. Considerable quantities are exported from the West Indian Islands, and from the Dead Sea, in Judæa; hence its commercial name, ‘Jewish bitumen,’ or ‘Jew’s pitch.’ The different kinds vary greatly in quality, according to the amount of earthy matter and other impurities contained in them; they may all, however, be reduced to a state of equal purity by boiling or macerating them in hot water, by which means the earthy and siliceous matters are more or less completely removed. These latter fall to the bottom of the vessel, and the bitumen rises to the surface, or forms clots on the sides of the boiler, when it is skimmed off, and thrown into a large cooler, where more water separates. At the Seyssel and Bechelbronn bitumen works the bitumen so obtained is thrown into large cauldrons and boiled for some time, by which means the volatile products and water accompanying it are driven off, and the remaining sand and impurities fall to the bottom of the cauldron, leaving the purified asphalt in the form of a thick fatty pitch, in which state it comes into the market or is applied to various purposes. In the following table we give the composition of a few bitumens:
| Carbon, per cent. | Hydrogen, per cent. | Oxygen, per cent. | Nitrogen per cent. | |
| Viscous bitumen of Bechelbronn | 88·0 | 12·0 | — | — |
| Virgin bitumen of Bechelbronn | 88·0 | 11·0 | — | 1·0 |
| Liquid bitumen from Hatten, Lower Rhine | 88·0 | 11·6 | — | 0·4 |
| Solid bitumen of Coxatambo, near Cuença, in Peru | 88·7 | 9·7 | 1·6 | |
| Bitumen of Bastennes. | Bitumen of Pont de Chateau, Auvergne. | Bitumen of Abruzzi. | Bitumen of Monastier, Haute Loire. | |||
| Crude. | Pure. | Crude. | Pure. | |||
| Oily matters, Bitumen | 20·0 | — | — | — | — | 7·0 |
| Carbon, Bitumen | 3·7 | 76·13 | 77·5 | 77·64 | 81·8 | 3·5 |
| Hydrogen | — | 9·41 | 9·6 | 7·86 | 8·4 | — |
| Nitrogen | — | 12·66 | 12·4 | 1·02 | 1·0 | — |
| Oxygen | — | 0·5 | 8·35 | 8·8 | — | |
| Water | — | — | — | — | — | 4·5 |
| Gas and vapour | — | — | — | — | — | 4·0 |
| Quartz sand and mica | 76·3 | — | — | — | — | 60·0 |
| Clay | — | — | — | — | Ferrug. 21·0 | |
| Ashes | — | 1·80 | — | 5·13 | — | — |
| ——— | ——— | ——— | ——— | ——— | ——— | |
| 100·0 | 100·0 | 100·0 | 100·0 | 100·0 | 100·0 | |
The solid bitumens are now extensively employed in the manufacture of bituminous mastic or cement and similar compositions, which are used for the lining of water-cisterns, and for various other hydraulic purposes; as also for roofs, floors, roads, pavements, &c. For the last purpose the native varieties of ‘asphaltic rock,’ consisting of a mixture of bitumen and calcareous earth, when tempered with a proper quantity of crushed granite, or calcareous sand or gravel, is found to be the most substantial and durable. The plan followed in laying down such pavements in Paris, where they have been the most extensively adopted, is—The ground having been made uniformly smooth, is edged, in the usual manner, with curb-stones rising about 4 inches above its level, and then covered, to the depth of 3 inches, with concrete (made with about 1-6th part of good hydraulic lime), which is well pressed upon its bed, the surface being subsequently smoothed over with a very thin coating of hydraulic mortar. On this, when perfectly dry,[187] the ‘bituminous mastic,’ rendered semi-fluid by being cautiously heated in a suitable iron cauldron,[188] is evenly spread over so as to form a layer three quarters of an inch, or for less solid work, half an inch thick. Some coarse sand is lastly sifted over and pressed down on the surface, when the work is complete; and in a few days the pavement becomes sufficiently compact and solid to be thrown open to foot passengers.
An important precaution to be observed in301 making asphalt pavements or roads is to boil the bitumen which is employed thoroughly, so as to expel the water and volatile oils, which if allowed to remain are found to render the mastic more liable to be affected by the extremes of heat and cold, as well as less able to stand the wear and tear of traffic.
Claridge’s Process. This consists in fusing the blocks of mastic in a suitable boiler, similar to that seen in fig. 1, and in adding a quantity of mineral tar, in the proportion of 1 lb. to every cwt. of the mastic. The tar is first fused in the boiler, 56 lbs. of the mastic are then introduced, and the whole repeatedly stirred so as to prevent the formation of a deposit. When the contents of the boiler are melted, the cauldron is covered over for a quarter of an hour, after which the remainder of the mastic is added, and its fusion proceeded with as before, the process being repeated until the boiler is full, allowing an interval of from ten to fifteen minutes between each operation.
When the mastic is sufficiently fluid it will drop freely from the stirrer, and jets of light smoke are observed to issue from it. If stiff mastic be required, the proportion of tar is lessened, and a quantity of coarse grit or river sand, to the amount of 20 or 30 lbs. to the cwt., is added.
 1.
1.
In laying the asphalt the greatest attention and care must be paid to the preparation of a solid and dry foundation.
This is usually accomplished by removing or ramming the loose earth, and placing upon the bed a layer of coarse sand mixed with powdered limestone, in the proportion of seven parts of the former to one of the latter, and the whole is pressed or beaten solid; upon this a second layer of finer materials is laid compacted and levelled; the bed thus prepared is allowed to dry before coating it with mastic.
Fig. 2 shows the manner in which ordinary asphalting is laid down. In this figure C is the bed of coarse concrete, B the second and finer layer of the same material, and A the superior layer of asphalt.
 2.
2.
The base or concrete must be perfectly dry when the mastic is poured on, or the work will be a failure, for the moisture will be converted into steam, which, issuing through the fluid mastic, will cause the formation of holes in the latter or blister it, and ultimately the surface will crack. To counteract in some measure the evil arising from the formation of steam, fine cinder dust is sifted over the bed of concrete previous to the application of the mastic.
When asphalting suspension bridges, a sheet of canvas is usually spread over the concrete.
In asphalting damp places, such as cellars and foundations, a brick invert is always laid in asphalt beneath the concrete. This is done by placing the bricks in rows, at the proper depth and slope, and pouring a coating of asphalt about a quarter of an inch thick upon them. Before the mastic solidifies, the bricks are separated a little by passing a knife between them, thus affording the mastic an opening by which to seal up more thoroughly the connection. The concrete is afterwards laid upon this bed, and the layer of mastic upon this in the usual way. The thickness of the layer of mastic varies according to the attrition to which it is to be subjected; but the usual depth is from a quarter to one and a quarter inch.
Artificial Asphalt. This is prepared from coal tar by distilling off the volatile oils which hold the tar in solution, the result being that a kind of fatty pitch is left, which must be boiled until a sample, when cooled, becomes nearly solid. The operation may be accomplished in the open air, but if this means of evaporation be adopted, the process is attended with a very unpleasant odour, and the volatile oils are dissipated. These volatile oils are used for the preparation of varnish, for lubricating machinery, and for the manufacture of a superior kind of lampblack. They have also been employed to increase the illuminating power of coal-gas, which purpose they accomplish by imparting their vapours to gas passed over them when they are placed302 in shallow vessels. Various forms of patent apparatus have been designed for this purpose.
When it is required to collect the oils, the coal tar is placed in a retort made of sheet iron, with a convex bottom, which is placed immediately over a fire. The products of the combustion after striking the bottom of the retort circulate round it, then proceed under a second boiler to heat the tar contained in it, and from which the retort is replenished when necessary. This vessel, when three quarters full, contains nearly 24 cwts. of tar; it should be perfectly embedded in masonry; the capital itself by which the volatile products escape should be surrounded with materials that are bad conductors of heat, such as ashes. But for this precaution the volatile oils would become condensed, and fall back into the evaporating vessel.
The volatile oils are collected by being made to pass through a tube cooled by a current of water, this tube running in a direction the reverse of that pursued by the vapours, and terminating in a closed vessel, which acts as the receptacle for the oils. A tube branching from the boiler conducts the uncondensed products outside the building in which the distillation is conducted.
When the tar has been boiled sufficiently long to give it the requisite consistence, it is removed by means of a pipe into a third hemispherical boiler of cast-iron. To prepare the bituminous mastic directly from this fatty pitch, the latter is kept in a state of fusion, and chalk in sufficient quantity is then added. If the chalk be previously heated, ground to a coarse powder, and sifted, the mixture is effected more rapidly and satisfactorily.
The asphalt becomes the more solid the greater the proportion of chalk added; on the other hand, it becomes less elastic and more brittle. The asphalt is moulded as follows:—A long table is covered with cast-iron plates, surrounded with a framework, which is subdivided into eight or ten equal compartments by means of rules of about six inches in height, introduced vertically into grooves formed at equal intervals in the long sides of the frame. The eight or ten moulds obtained by this means are coated internally with a paste composed of sixty parts of water and forty of chalk. This compound prevents the mastic adhering to the sides of the mould, and ensures its being easily detached.
Two barrels, or 9 cwts. of tar, lose by distillation one fourth of their weight, the loss consisting of 1 cwt. 3 qrs. 15 lbs. of volatile oils, and 1 qr. 13 lbs. of water.
Sometimes ground or fine sand enters into the composition of asphalt in proportions equal to the chalk; but in some cases only half as much sand as chalk is used.
In the manufacture of asphalt it is very important that the contents of the cauldron should be stirred during fusion, not only to prevent the tar adhering to the bottom, and so getting burnt, but to ensure the ingredients being brought into intimate combination, and a homogeneous and smooth compound being produced.
As soon as the whole is thoroughly incorporated, the proper consistence attained, and the vapours of the volatile oils and water come off in very minute quantities, the asphalt is run off into the moulds before described, and when sufficiently set may be removed, and is ready for use.
Dr G. H. Smith has patented a process for making artificial asphalt, waterproof concrete, &c., which promises to become of great value in the construction of sea walls, docks, and harbour works, &c. Dr Smith’s invention consists in filling up the interstices of any porous substance, such as brick, burned or unburned clay, soft stones, plaster of Paris, &c., with pitch or tar which has been boiled to such a consistence that the pores or cells of the material used are completely filled with solid matter when cold.
Other hydrocarbons, resins, or gums may be used instead of pitch or tar; but it is essential that the saturating substances, though naturally fluid or semifluid, can be so changed by boiling that they lose their fluidity when cold; or they must be, though hard under all ordinary temperatures of the atmosphere, capable of reduction by heat or otherwise to a fluid condition, so that they will penetrate the porous materials.
The asphalto-bitumen mine of the Val de Travers, in the Canton of Neufchâtel, is said to be the richest and most extensive in the world of its particular class. The calcareous bitumen which it yields contains 20% of nearly pure bitumen, and 80% of carbonate of lime; and it has a sp. gr. (2·115) approaching that of ordinary bricks.
The ‘Val de Travers Company,’ and the ‘Bastenne and Gaujac Company,’ are, it is said, those which have hitherto been the most successful in laying down asphalto-pavements. See Asphaltum, Petroleum, &c.
Bitumen, Elastic. Syn. Min′eral caou′tchouc (kōō′-chŏŏk), El′aterite. A rather rare species of bitumen, differing chiefly from the other solid varieties in being elastic.
Bitumen, Liq′uid. Petroleum.
BITU′MINOUS. Syn. Bitutmĭno′sus, L.; Bitumineux, Fr.; Erdpechig, Ger. Of bitumen, or resembling or containing it.
BIX′EINE (-e-ĭn). The red colouring-principle of annotta. It is obtained by treating bixine with liquid ammonia, with subsequent free contact of air.
Prop., &c. When pure, a rich deep-red powder, soluble in alcohol and in alkalies, and turned blue by sulphuric acid. It appears to be oxidised bixine.
BIXIN. The red resinous colouring matter of annatto. Bolley and Mylius prepare it by digesting the dried alcoholic extract of annatto with ether; repeatedly treating the303 least soluble portion (which contains the greater part of the colouring matter) with hot ether; dissolving the remainder in alcohol; precipitating the alcoholic solution with lead acetate; decomposing the washed precipitate with sulphuretted hydrogen; extracting the colouring matter therefrom by hot alcohol; and precipitating the alcoholic solution with water.
BIX′INE (-ĭn). The yellow colouring-principle of annotta.
Prep. A solution of annotta is precipitated with a solution of acetate of lead; the precipitate, after having been washed in cold water, is decomposed by sulphuretted hydrogen; the decanted liquor or filtrate yields crystals by cautious evaporation.
Prop., &c. Yellowish white, turning full yellow by exposure to air; soluble in water, and freely so in alcohol and in alkaline solutions; by oxidation it is converted into bixeine. For a correct knowledge of both of these substances we are indebted to M. Preisser.
BLACK. Syn. A′ter,[189] Ni′ger, L.; Noir, Fr.; Schwarz, Ger.; Blac, Blæc, Sax. In dyeing, &c., of the colour of lamp-soot, or of night; subst., a black colour.
Black Ash. The waste lye of the soapmakers is evaporated in large iron boilers, the salt separated as it falls down, and then heated in a reverberatory furnace, until it is partially decomposed and fused, when it is run into iron pots to cool. It is used in the manufacture of alum and common soap.
Black Col′ours (kŭl′-). See Black Pigments.
Black Draught. See Mixture, Senna (Compound).
Black Drop. See Drops, Patent Medicines, &c.
BLACK DYE. Syn. Teinte noire, Fr.; Schwarze farbe, Ger. The following are the processes and materials now commonly employed in dyeing black:—
a. For Cotton:—
1. The goods, previously dyed blue, are steeped for about 24 hours in a decoction of gall-nuts or sumach, then drained, rinsed in water, and passed through a bath of acetate of iron for a quarter of an hour; they are next again rinsed in water, and exposed for some time to the air; after which they are passed a second time through the bath, to which a little more iron-liquor is previously added. The whole process is repeated, if necessary, according to the intensity of the shade of black desired.
2. The goods are steeped in a mordant of acetate of iron, worked well, and then passed through a bath of madder and logwood for 2 hours. Less permanent than No. 1.
Obs. About 2 oz. of coarsely powdered galls, or 4 oz. of sumach, are required for every pound of cotton, in the process of galling. The first should be boiled in the water, in the proportion of about 1⁄2 gal. of water to every lb. of cotton. The sumach-bath is better made by mere infusion of that dye-stuff in very hot water.
3. (For 10 lbs. of cloth.) The goods are put into a boiling bath made of 3 lbs. of sumach, and allowed to steep, with occasional ‘working,’ until the liquor is perfectly cold; they are next passed through lime water, and, after having drained for a few minutes, immediately transferred to and worked for an hour in a warm solution of 2 lbs. of copperas; after free exposure to the air for about an hour they are again passed through lime water, and, after draining, ‘worked’ for an hour in a bath made with 3 lbs. of logwood, and 1 lb. of fustic; they are then ‘lifted,’ and 1⁄4 lb. of copperas being added, they are returned to the bath, ‘worked’ well for about 30 minutes, and finished. Good and deep.
Obs. Instead of copperas iron-liquor may be used, observing to take 11⁄2 pint of the latter (of the ordinary strength) for every lb. of the former ordered above.
b. For Flax and Linen:—
This, for the most part, closely resembles that employed for cotton; but, in some cases, a mordant of iron-liquor, or of copperas, followed by passing the goods through lime-water, and exposure to the air, precedes the dye-bath.
c. For Silk:—
Silk goods are dyed much in the same way as woollens, but the process is conducted with less heat:—
1. A bath of nut-galls is given for 12 to 36 hours, occasionally working the goods therein; they are next taken out, rinsed, and well aired, after which they are passed for a few minutes through a bath containing sulphate of iron, and are then again drained, rinsed, and aired. The steep in the nut-gall bath may be repeated, if necessary, followed, as before, by the iron-bath previously replenished with a little fresh copperas. The whole quantity of galls to be taken for 1 lb. of silk varies with their quantity from 1⁄2 to 3⁄4 lb., that of the copperas (for the first bath), from 3 to 4 oz.
2. (For 1 cwt. of silk.) Boil 22 lbs. of bruised Aleppo galls, for 2 hours, in 90 to 100 galls. of water, observing to add boiling water from time to time, to compensate for that lost by evaporation; to the clear bath add 32 lbs. of copperas, 7 lbs. of iron-filings, and 21 lbs. of gum; digest with agitation for 1 hour, and when the ingredients are dissolved, pass the silk (previously prepared [‘galled’] with 1⁄3rd of its weight of gall-nuts) through the bath for about an hour; then rinse and air it well; next leave it in the dye-bath for 6 to 12 hours; and this immersion or steep may be repeated, if necessary, at will. This is said to be the process commonly adopted for velvet at Genoa and Tours.304
3. (For 5 lbs. of silk.) Turn the goods for an hour through a mordant formed of 1 lb. of copperas and 2 oz. of nitrate of iron (dyer’s), with sufficient water; after rinsing in cold water and airing them, ‘work’ them for an hour in a decoction made of 5 lbs. of logwood and 1 lb. of fustic; then lift them from the bath, add 2 oz. of copperas, reimmerse, and ‘work’ them well for 10 or 15 minutes longer; lastly, rinse, air, and finish. A full deep black.
4. (For 5 lbs.) For the mordant use 1⁄2 lb. of copperas; rinse, and air; for the ‘dye-bath,’ a decoction of 4 lbs. of logwood to which 1⁄2 pint of stale urine has been added; after ‘lifting’ the goods add 2 oz. more of copperas to the bath, and work for 15 minutes, as before. A good black. By adding 2 oz. of dyer’s nitrate of iron to the mordant the same ingredients will give a deep black; and by substituting a little white soap for the urine, and omitting the addition of copperas to the logwood-bath, it will give a blue-black. The last may also be produced by first dyeing the goods deep blue as with ‘prussiate,’ and omitting the urine and soap, in which case one half only of the logwood will be required.
d. For Wool:—
To produce a good permanent black on wool or woollen goods, they must be first dyed of a deep blue in the indigo-vat, or, more cheaply, by the Prussian-blue process. When the goods are coarse or common, and price is an object, they are generally ‘rooted’ instead of being ‘blued.’ This consists in giving them a dun or brown colour with the husks of walnuts or the roots of the walnut-tree, or with other like cheap astringent substances.
1. (For 1 cwt. of wool.) Good logwood-chips, 20 lbs., and Aleppo-galls, 18 lbs.; are inclosed in a coarse bag, and boiled with water, q. s., for 5 or 6 hours; 1⁄3rd of this decoction is then transferred into another copper, with verdigris, 2 lbs., and a sufficient quantity of water having been added, the goods (previously dyed dark blue) are passed through the liquor for two hours, at a heat slightly below the boiling-point. The goods are next lifted and drained, another 1⁄3rd of the decoction of logwood and galls, with copperas, 9 lbs., added to the boiler, after which the fire is lowered, and as soon as the copperas is dissolved, the cloth is returned to the bath, and again well ‘worked’ for at least an hour. It is then taken out, thoroughly aired, and the remaining 1⁄3rd of the decoction added, with sumach, 20 lbs. The whole is then brought to a boil, and sulphate of iron, 2 lbs., together with a pailful of cold water, thrown in; after which the goods are put in a third time, and ‘worked’ for one hour; they are then taken out, rinsed, aired, and again passed through the bath for another hour. After being thoroughly rinsed, the goods are at once either ‘fulled,’ dried, and folded, or are further softened and beautified by passing them for 15 minutes through a hot weld-bath (not boiling), when they are rinsed, &c. (but not ‘fulled’), as before. A beautiful though expensive dye. With management the above quantities of the ingredients will dye 11⁄4 or even 11⁄2 cwt. of wool.
2. (For 1 cwt.) The cloth (previously dyed blue) is ‘galled’ with 5 lbs. of nut-galls, and then dyed in a bath made with 30 lbs. of logwood, to which about 5 lbs. of copperas has been added; after which it is rinsed, aired, and ‘fulled,’ as before. This is said, by Lewis, to be the usual proportions and plan adopted by the English dyers.
3. (For 1 cwt.) Make a bath, as before, with fustic, 2 lbs.; logwood, 5 lbs.; and sumach, 10 lbs.; work the (blued) cloth for 3 hours at the boiling heat, or near it; lift it out, add sulphate of iron, 101⁄2 lbs., and when dissolved, pass the cloth through it for 2 hours; rinse, air well, and again pass the goods through the bath for an hour; lastly, rinse until the water runs clear. Inferior to the last, but less expensive.
4. (For 1 cwt., without previous blueing or ‘rooting,’)—a. Work the goods at about 200° Fahr. for 1 hour, in a bath made with 6 to 7 lbs. of cam-wood; lift, add 61⁄2 lbs. of copperas, and again work the goods for an hour, after which withdraw the fire, and allow them to steep for 10 or 12 hours; next drain and rinse them, and work them in a second bath made with 60 lbs. of logwood for 11⁄2 hour; lift, add 3 lbs. of copperas, and again work for an hour; lastly, rinse, air, and finish:—b. The goods are first worked, for about two hours, in a bath of 3 lbs. of fustic, in which 5 lbs. of bichromate of potash and 4 lbs. of alum have been dissolved; after exposure to the air for about an hour and thorough rinsing they are worked for a second two hours in a bath made with 45 lbs. of logwood, 31⁄2 lbs. of barwood or cam-wood, and 3 lbs. of fustic; they are then lifted, and 3 lbs. of copperas having been added to the bath, are again immersed and worked for half an hour to an hour.
5. (For 10 lbs. of wool or w. cloth.) Work the goods for 1⁄2 an hour in a bath of 1⁄2 lb. of cam-wood; lift, add 7 or 8 oz. of copperas, and after working them for 20 minutes, withdraw the fire, and leave them in the liquor for 10 or 12 hours; next rinse them in cold water, drain, and then work them for an hour in a bath made with 5 lbs. of logwood, to which 1 pint of urine has been added; lift, add 4 oz. of copperas, work them for half an hour longer, and, lastly, wash and dry them.
6. (For 7 lbs.) Take of galls (bruised), 1⁄4 lb.; logwood chips, 11⁄2 lb.; for the bath; boil or work the goods for 2 hours, take them out, add of copperas, 1⁄4 lb.; and when it is dissolved, work the goods through the liquor for at least 2 hours, keeping the bath nearly boiling; again take them out, wash, and air; then add 1 oz. more of copperas to the bath, and pass the cloth through it for another hour; lastly, air, rinse, and finish.
7. (For 5 lbs.) For the first bath—bichromate305 of potash, 8 oz.; alum, 6 oz.; fustic, 4 oz.; for the second bath—logwood, 4 lbs.; barwood and fustic, of each 4 oz.; to which add, after the lift, copperas, 4 oz.; the process being conducted as in 4, b. This, as well as the two formulæ immediately preceding it, is particularly suited to articles of dress dyed in the small way, at home. When the articles are only re-dipped, as it is called, a proportionately smaller quantity of the ingredients may be taken.
Concluding Remarks. In dyeing black, particularly on wool, it is absolutely necessary to take the goods out of the dye-bath several times, and to expose them to the air. This is called “airing” them, and is done to allow the oxygen of the atmosphere to act upon the ingredients of the dye, and especially on the iron; as without this action of the air a good colour cannot be produced. The usual proportions employed by the dyers of England are 5 lbs. each of galls and copperas and 30 lbs. of logwood for every cwt. of cloth; but these weights are frequently increased for choice goods, just as they are always lessened for common ones. The other astringent substances used as substitutes for galls in dyeing black are taken in quantities proportionate to their respective strengths, that of good Aleppo gall-nuts being referred to as a standard.
The German wool-dyers usually commence their process with a mordant of Salzburg vitriol (3 parts) and argol (1 part); and after exposure of the goods in a cool place for 24 hours, work them in a bath of logwood (5 to 6 p.) and fustic (2 p.); after which the bath is restored by the addition of verdigris (1⁄4 p.) dissolved in vinegar and the goods again worked through it for about 1⁄2 an hour. This is for 20 parts weight of wool or cloth.
Black marinos are usually mordanted (hot) with about 1⁄10th of their weight of copperas, and then aired for 24 hours; after which they are dyed in a boiling bath made with about 1⁄2 their weight of logwood with the addition of about 2% of argol or tartar.
As black is the shade most commonly attempted by amateur dyers, it may be here necessary to call their attention to what is said on mixed fabrics in our article on DYEING; since an inattention to this point will inevitably cause the failure of their efforts.
According to Muspratt, a mixed fabric of silk and woollen may be dyed black by one process, as follows:—Work the goods an hour in a solution of 8 oz. each of tartar and copperas, and wash out; work for 15 minutes in a decoction of 4 lbs. of logwood; lift, add 1 oz. of bichromate of potash, work for 1⁄2 an hour, and dry. And a mixed fabric of cotton, silk, and woollen:—Steep for six hours in a bath made of 2 lbs. of sumach; then work for an hour in a solution of 6 oz. each of tartar, sulphate of copper, and copperas; wash, and work 1⁄2 an hour in a decoction of 4 lbs. of logwood; lift, add to the bath 1 oz. of copperas; work ten minutes, wash, and dry. If a very deep black be required, 1 lb. of bark is to be added with the logwood. See Dyeing, Mordants, &c.
BLACK JACK. This term is applied to burnt sugar, which is used to colour beverages, and more particularly for the adulteration of coffee. It is also known under the name of “coffee refined,” and as such is vended in tin canisters. It is moreover employed to give colour to vinegar, brandy, and rum. Butter, with which water has been largely incorporated, is also known as “Black Jack.” See Caramel.
BLACK LEAD (lĕd). See Plumbago.
BLACK PIG′MENTS. Syn. Pigmen′ta ni′gra, L. The principal black pigments of commerce are obtained by carbonising organic substances (particularly bones), by exposure to a dull red heat, in covered vessels out of contact with the air; or by collecting the soot formed during the combustion of unctuous, resinous, and bituminous matters. Artists and amateurs also prepare, on the small scale, a variety of blacks, many of which are not procurable at the colour-shops. This they effect either by the carbonisation of substances not usually employed for the purpose, or by simply reducing to powder certain mineral productions selected on account of the peculiar shades of colour which they respectively possess. Some of the last might, however, be more appropriately classed with browns. The following list embraces most of these articles:—
Black, An′imal. Bone-black.
Black, Aniline. See Tar Colours.
Black, Beech′. Carbonised beech-wood.
Black, Blue′. Vine-twigs dried and then carefully carbonised, in covered vessels, until of the proper shade. That of the ancients was made of wine-lees. Pit-coal, carefully burnt at a white heat, then quenched in water, dried and well-ground, forms a cheap, good, and durable blue-black, fit for most ordinary purposes. See Frankfort-black.
Black, Bone′. Syn. I′vory-black (of commerce); Car′bo os′sis, Os us′tum ni′grum, E′bur u. n. (vena′le), &c., L.; Noir d’os, &c., Fr.; Knochenschwartz, &c., Ger. Carbonised bones reduced to powder. That of commerce is usually the residuum of the distillation of bone-spirit. Inferior to true ivory-black; having a slight, but peculiar reddish tinge, from which the latter is quite free. Besides its use as a pigment, it is extensively employed in making blacking, as a material for the moulds of founders, as a clarifier and bleacher of liquids, &c. See Ivory-black and Charcoal, Animal.
Black, Cas′sel, Cologne′-Black. Ivory-black.
Black, Coal′. See Blue-black and Newcastle-black.
Black, Composi′′tion (zĭsh′-ŭn-). The selected portion of the residuum of the process of making prussiate of potash from blood and hoofs. Used both as a pigment and to decolour306 organic solutions, which it does better than bone-black.
Black, Cork′. Spanish-black.
Black, Flo′′rey, Florée d’Inde. The dried scum of the dyer’s wood-bath. A superior blue-black.
Black, Frank′fort, Noir de Francfort. From vine-twigs dried, carbonised to a full black, and then ground very fine. An excellent black pigment; also used by the copper-plate printers to make their ink. See Blue-black.
Black, Harts′horn. Resembles ivory-black, which is now usually sold for it. It was formerly prepared by carbonising the residuum of the distillation of spirit of hartshorn.
Black, I′vory. Syn. Car′bo eb′oris, E′bur us′tum ni′grum, L.; Noir d′ivoire, &c., Fr.; Elfenbeinschwartz, Kohle von elfenbein, Ger. From waste fragments and turnings of ivory, by careful exposure in covered crucibles, avoiding excess of heat or over-burning. The whole having been allowed to become quite cold, the crucibles are opened and their contents reduced to fine powder. For the first quality only the richest coloured portion of the charcoal is selected, and this, after being powdered, is ground with water on porphyry, washed on a filter with warm water, and then dried. A very rich and beautiful black. It is brighter than even peach-stone black, and is quite free from the reddish tinge of bone-black. With white-lead it forms a rich pearl-grey. See Bone-black.
Black, Jamai′ca (-mā′-). Sugar-black.
Black, Lamp′. Syn. Fuli′go lucer′næ, F. pi′nea, &c., L.; Noir de fumée, &c., Fr.; Kienruss, &c., Ger. Prep. 1. (On the small scale.) A conical funnel of tin-plate furnished with a small pipe to convey the fumes from the apartment, is suspended over a lamp fed with oil, tallow, coal-tar, or crude naphtha, the wick being large and so arranged as to burn with a full smoky flame. Large spongy, mushroom-like concretions of an exceedingly light, very black, carbonaceous matter, gradually form at the summit of the cone, and must be collected from time to time. The funnel should be united to the smoke-pipe by means of wire, and no solder should be used for the joints of either.
2. (Commercial.) On the large scale, lamp-black is now generally made by burning bone-oil (previously freed from its ammonia), or common coal-tar, and receiving the smoke in a suitable chamber. In the patented process of Messrs Martin and Grafton the coal-tar is violently agitated with lime-water until the two are well mixed, after which it is allowed to subside, and the lime-water having been drawn off, the tar is washed several times with hot water. After subsidence and decantation it is put into stills, and rectified. The crude naphtha in the receiver is then put into a long cast-iron tube furnished with numerous large burners, underneath which is a furnace to heat the pipe to nearly the boiling point. Over each burner is a sort of funnel which goes into a cast-iron pipe or main, which thus receives the smoke from all the burners. From this main the smoke is conveyed by large pipes to a succession of boxes or chambers, and thence into a series of large canvas bags arranged side by side, and connected together at top and bottom alternately. Fifty to eighty of these ‘bags’ are employed; the last one being left open to admit of the escape of the smoke, which has thus been made to traverse a space of about 400 yards. As soon as the bags contain any considerable quantity of black they are removed and emptied. The black deposited in the last bag is the finest and best, and it becomes progressively coarse as it approaches the furnace.
Obs. The state of minute division in which the carbon exists in good lamp-black is such as cannot be given to any other matter, not even by grinding it on porphyry, or by ‘elutriation’ or ‘washing over’ with water. On this account it goes a great way in every kind of painting. It may be rendered drier and less oily by gentle calcination in close vessels, when it is called burnt lamp-black, and may then be used as a water-colour; or its greasiness may be removed by the alkali-treatment noticed under Indian ink. It is the basis of Indian ink, printer’s ink, and most black paints.
Russian lamp-black is the soot produced by burning the chips of resinous deal. It is objectionable chiefly from being liable to take fire spontaneously when left for some time moistened with oil.
Black, Manganese′ (-nēze′-). Native binoxide of manganese. Durable and dries well.
Black, New′castle. From the richer-looking varieties of pit-coal by grinding, and elutriation. Brown black or, in thin layers, deep brown. It is, perhaps, “the most useful brown the artist can place on his palette; being remarkably clear, not so warm as Vandyke-brown, and serving as a shadow for blues, reds, and yellows, when glazed over them. It seems almost certain that Titian made large use of this material.” See Blue-black (antè).
Black, Opor′to. Carbonised wine-lees.
Black, Pa′ris, Noir de Paris. From turner’s bone-dust, treated as for ivory-black. Works well both in oil and water. It is commonly sold for real ivory-black, and for burnt lamp-black.
Black, Peach-stone. From the stones or kernels of peaches, cherries, and other similar fruits, treated as for ivory-black. A bright, rich black; works well with oil; with white-lead and oil it makes old grey.
Black, Pit′coal. Newcastle-black.
Black, Prus′′sian (prŭsh′-ăn). Composition-black.
Black, Rice′. Rice-charcoal. Inferior.
Black, Rus′′sian. See Lamp-black.
Black, Soot′ (sŏŏt′-). The soot of coal-fires, ground and sifted. Used in common paint,307 and to colour whitewash; with Venetian-red and oil, it makes chocolate-colour; also used to make grey mortar.
Black, Span′ish. From cork-cuttings carbonised, as bone-black. Resembles Frankfort-black, but works softer.
Black, Sug′ar (shŏŏg′-). Carbonised moist sugar. Has little body, but for washing drawings is equal in mellowness to Indian ink and bistre.
Black, Sun′derland. Newcastle-black.
Black, Tur′ner’s. Paris-black.
Black, Vine′-twig. Frankfort-black.
Black Wheat′ (hwēte′-) Carbonised wheat. It has a good colour, a full body, and dries hard and quickly with oil.
BLACK′BERRY. The popular name of ru′bus frutico′sus (Linn.) or the common ‘bramble.’ Fruit (BLACK′BERRIES; MÛRES DE RONCE, Fr.), antiscorbutic and wholesome, but in excess apt to sicken; twigs used in dyeing black; root astringent, formerly used in hooping-cough.
Blackberry, Amer′ican. The ru′bus villo′sus (Ait.). Root astringent and tonic; officinal in the Ph. U. S.
BLACK PUDDING. A pudding made of the blood of the pig, mixed with groats and fat. It contains about 11 per cent. of nitrogenous matter.
BLACK′ING. Syn. Cirage (des bottes), Noir (pour les souliers), Fr.; Schwärze, Schuhschwärze, Ger. An article too well known to require description.
Hist., &c. Blacking, and other polishes for leather, were undoubtedly in common use among the ancients; but the compound to which we now more particularly apply the name is of comparatively modern invention. The latter appears to have been first introduced into England from Paris, during the reign of Chas. II, but was not in common use among the masses of our population much before the middle of the 18th century.
The general and still increasing use of blacking as a polish for boots and shoes by all classes of the inhabitants of civilised countries, has given an extent and importance to its manufacture which a stranger to the subject would scarcely be led to suspect. The princely establishments of some of the firms who compound this sable article cannot fail to have arrested the attention of the passenger through the streets of this great metropolis; whilst the enormous fortune acquired by one of their late members, and, for the most part, bequeathed by him for purposes of charity and philanthropy, has invested both the donor and his craft with an interest and notoriety which they did not previously possess. At the present time the consumption of blacking is greater than at any former period; and of this, as of many other articles, England is the principal manufactory for the world, alike distinguished for the extent of her trade and the excellent quality of this product of her industry. In truth, England excels all other nations in the manufacture of common shoe-blacking; and perhaps in no other country is an equal attention paid to the cleanliness and appearance of the external clothing of the feet.
Prep. I. Liquid Blacking:—
1. Take of bone-black, 16 parts; treacle, 12 parts; oil of vitriol, 3 parts; sperm oil,[190] 2 parts; gum-arabic, 1 part; strong vinegar, or sour beer, 48 to 50 parts[191] (all by weight); place the bone-black in a capacious wooden, stone-ware, or enamelled iron vessel,[192] add the oil, and rub them well together; next gradually add the treacle, and actively and patiently grind or rub the mass, after each addition, until the oil is perfectly killed, and finally for some time afterwards, to ensure complete admixture; then cautiously dilute the vitriol with about three times its bulk of water, and add it, in separate portions, to the former mixture, observing to stir the whole together, as rapidly as possible, on each addition of the acid, and for some minutes after the whole is added, so as to render the mass thoroughly smooth and homogeneous; let it stand, covered over, for two or three days, or longer, stirring it, in the mean time, for 15 or 20 minutes daily; lastly, having dissolved the gum in the vinegar, add the solution gradually to the rest, and stir the whole together briskly for some time, and again daily for 3 or 4 days. It may be further diluted, at will, with a little more vinegar or beer, or with water; but unnecessary or excessive dilution should be avoided, as the richness and quality of the blacking become proportionately reduced. If all the ingredients (except the vitriol) be made hot before admixture, the shining quality of the product will be greatly improved, and the process may be shortened.[193]
2. Ivory-black, 16 parts; treacle, 8 parts; oil of vitriol, 4 parts; (diluted with) water, 2 parts; oil, 2 parts; gum-arabic, 1 part; soft water (for the final dilution, instead of vinegar), 64 parts; mixed, &c., as before. Excellent.
3. As the last; but taking only 6 parts of treacle, 1 part of oil, and omitting the gum-arabic. Good. A commoner article of liquid blacking does not sell.
4. (Bryant and James’s India-rubber Liquid Blacking. Patent dated 1836.) Take of india rubber (in small pieces), 18 oz.; hot308 rape oil, 9 lbs. (say 1 gall.); dissolve; to the solution add of ivory-black (in very fine powder), 60 lbs.; treacle, 45 lbs.; mix thoroughly; further add of gum-arabic, 1 lb., dissolved in vinegar (No. 24), 20 galls.; reduce the whole to a perfect state of smoothness and admixture by trituration in a paint-mill; throw the compound into a wooden vessel, and add, very gradually, of sulphuric acid, 12 lbs.; continue the stirring for 1⁄2 an hour, repeating it daily for 14 days; then add of gum-arabic (in fine powder), 3 lbs.;[194] again mix well, and repeat the stirring for 1⁄2 an hour daily for 14 days longer, when the liquid blacking will be ready for use or for bottling. The quality is very excellent; but this, probably, does not depend on the presence of the india rubber, but on the general correctness of the proportions, and the care and completeness with which they are mixed.
5. (Without Vitriol.) Take of ivory-black (in very fine powder), 2 lbs.; treacle, 11⁄2 lb.; sperm oil, 1⁄4 pint; mix, as before; then add of gum-arabic, 1 oz.; (dissolved in) strong vinegar, 1⁄2 pint; mix well; the next day further add of good vinegar, or strong sour beer, 3 to 4 pints (or q. s.); stir briskly for a 1⁄4 of an hour, and again once a day for a week. Excellent. A cheaper, but inferior article, may be made by the reductions and omissions noticed above.
6. From paste-blacking (see below), by reducing it with sufficient vinegar, sour beer, or water, to give it the liquid form.[195]
II. Paste Blacking:—
1. Qualities from good to super-excellent may be made from any of the preceding formulæ, by simply omitting the final dilution with the vinegar, sour beer, or water, therein ordered at the end of the process.
2. (Bryant and James’s India-rubber Paste Blacking.—Patent dated 1836.) Of india-rubber oil, ivory black, treacle, and gum-arabic, the same as for their liquid blacking (see I, 4, above), but dissolving the last in only 12 lbs. (say 5 quarts), instead of 20 galls. of vinegar; grinding to a smooth paste in a colour-mill, and then adding of oil of vitriol, 12 lbs. as before. The mass is to be stirred daily for a week, when it will be fit for use, or potting.[196] Excellent.
3. Ivory-black, 1 cwt.; treacle, 28 lbs.; rape oil (or other cheap oil), 1 gall.; mix, as before; then add of oil of vitriol, 21 lbs.; (diluted with) water, 2 galls.; mix them quickly and thoroughly by forcible stirring with a strong wooden spatula, and as soon as admixture is complete, but whilst still fuming, put the cover on the tub, and leave it till the next day, when (without further stirring) it will be fit for use or sale.[197] Good ordinary. Used for packets and tins.
4. As the last; but adding with the ivory-black, &c., 14 to 28 lbs. of coal-soot[198] (sifted), omitting one half of the oil, and diluting the vitriol with an extra gall. of water. Inferior. Chiefly used for 1d. and 1⁄2d. packets.[199]
5. (German Blacking). Ivory-black, 1 part; treacle, 1⁄2 part; sweet oil, 1⁄8 part; mix, as before; then stir in a mixture of hydrochloric acid, 1⁄8 part; oil of vitriol, 1⁄4 part (each separately diluted with twice its weight of water before mixing them). This forms the ordinary paste-blacking of Germany, according to Liebig.
6. (Without Vitriol.) As I, 5 (antè); but with the omission of the last 1⁄2 gall. of ‘vinegar.’
Concluding Remarks.—To produce a first-rate article of blacking it is absolutely necessary that the ingredients be of the best quality, and used in the proper proportions; and that the order of their admixture, and the general manipulations, be conducted under ordinary circumstances, in the manner described in the first of the above formulæ. The proportions of the treacle and the oil (the most expensive of the ingredients) should not be stinted; and, indeed, that of the latter may be safely increased in quantity, without materially affecting the polish, and with manifest advantage as far as the softness and durability of the leather to which it is applied is concerned. The manipulations required in the manufacture of both paste-blacking and liquid blacking are essentially the same; the difference between the two articles, when the same materials are used, depending entirely on the quantity of liquid added. Thus, as noticed before, by diluting paste-blacking with water, vinegar, or beer-bottoms, it may be converted into liquid blacking of a nearly similar quality; and, by using less fluid matter, the ingredients of liquid blacking will produce paste blacking. One thing must, however, be observed, and that is, that the ivory-black used for liquid blacking should be reduced to a much finer powder than for paste blacking; as, if this is not attended to, it is apt to settle at the bottom, and to be with difficulty again diffused through the liquid. Persons who object to the use of blacking containing oil of vitriol may employ formula I, 5, or II, 6 (above). The vitriol, however, greatly contributes to promote309 the shining properties of the blacking; and, in small quantities, or in the proper proportion, is not so injurious to the leather as some persons have represented; as it wholly unites itself to the lime of the bone-phosphate contained in the ivory-black, and is thus neutralised, insoluble sulphate of lime, and an acid phosphate or superphosphate, being formed. It is the latter that gives the acidity to a well-made sample of blacking, and not the sulphuric acid originally added to it. In this way the larger portion of the ivory-black is reduced to a state of extremely minute division, and with the other ingredients forms a strongly adhesive paste, which clings to the surface of the leather, and is susceptible of receiving a high polish by friction when in a scarcely dry state. This is the reason why lamp-black should never be employed for blacking to the exclusion of the necessary proportion of bone-black, as it has no earthy base to absorb or neutralise the acid, which, if left in a free state, would prove very hurtful to the leather. Oil of vitriol is now employed in the manufacture of all the more celebrated and expensive blackings; and that simply because no other substance is known so efficient, and so little injurious to the leather. In the common blackings of Germany, hydrochloric acid is often used to the entire exclusion of oil of vitriol; but blacking so prepared possesses several disadvantages from which that of England is free. In the best German blackings only a small portion of this acid is used, as may be seen by reference to formula II, 5 (above). The addition of white-of-egg, isinglass, and similar articles[200] to blacking, always proves injurious, as they tend to stiffen the leather and to make it crack, without at all improving its polishing properties. Even gum-arabic, in quantity, is on this account objectionable. Oil has an opposite tendency, and, as already stated, the quantity commonly used may be increased with advantage. Resin oil should be particularly avoided.
Dr Ure has recommended the use of a little copperas[201] in blacking; with the object, we presume, of striking a black with the tan in the leather; but except with new, or nearly new leather, this effect would not occur, whilst its presence, if not objectionable, would otherwise be useless.
The only improvement that has been introduced in the manufacture of blacking since the early days of the celebrated Day & Martin is, a few hours after the conclusion of the mixture of the ingredients (but before adding the vinegar, if any), to simmer the whole very gently, for about 8 or 10 minutes, observing to stir it assiduously all the time. The fire must then be withdrawn, and the pan covered over until it is quite cold, when half an hour’s lusty stirring will finish the process.[202] In this way a degree of maturity and brilliancy will be imparted to the product, which, without the application of heat, it would take months to acquire, if, indeed, it ever reached it.
As it is generally more convenient to measure than to weigh liquids, it may be useful to remind the reader that, in round numbers,
| 1 | gal. of | oil | weighs | 91⁄4 | lbs. |
| 1 | ” | sour beer | ” | 101⁄4 | ” |
| 1 | ” | vinegar | ” | 10 | ” |
| 1 | ” | water |
We may here further remark that the blackings of different houses vary considerably in some of their properties; as also do those of even the same maker by age. Some blackings dry off rapidly and give a very brilliant polish with very little labour; whilst others take a little longer to ‘dry off,’ and somewhat more labour to polish them. The former are best adapted to hasty use, and when a very brilliant surface is desired; the latter when depth of polish, without extreme brilliancy, satisfies the wearer. The first best meets the requirements of fashionable life; the last those of the middle classes and pedestrians exposed to dirt, mud, and the various vicissitudes of travelling and weather. To the one belong the ‘blackings’ of Everett, Day & Martin, &c.; to the other, those of Warren, Bryant & James, and most of the smaller manufacturers, with nearly all the paste-blacking of the more respectable shops. Time, however, equalises the qualities of these two classes. Blackings which are crude, moist, and oily lose these properties, and become drier and more brilliant by age. The practice of several of the first-class West-end boot and shoe makers is never to use a blacking which they have not had in their stock at least a twelvemonth.
Blacking, both liquid and paste, should be stored in a cool and moderately dry cellar; and when in use should be kept corked or otherwise excluded from the air. Exposure or desiccation destroys most of its best qualities.
The present annual value of the blacking consumed in the United Kingdom is estimated at 562,500l., or about 41⁄2d. per head for the whole population; while the collective yearly value of that exported is about 35,000l.
[See Balls, Blacking, Bone-black, Boots and Shoes, Leather, Sulphuric Acid, &c.; also below.]310
Blacking, Automat′ic. Syn. Self-shi′′ning blacking, Span′ish Japan, &c. Prep. 1. Gum-arabic, 4 oz.; treacle or coarse moist sugar, 11⁄2oz.; good black ink, 1⁄4 pint; strong vinegar, 2 oz.; rectified spirit of wine and sweet oil, of each 1 oz.; dissolve the gum in the ink, add the oil, and rub them in a mortar or shake them together for some time, until they are thoroughly united; then add the vinegar, and lastly the spirit.
2. Lamp-black, 3⁄4 oz.; indigo (in fine powder), 1 dr.; put them in a mortar, or basin, and rub them with sufficient mucilage (made by dissolving 4 oz. of gum in 1⁄4 pint of strong vinegar) to form a thin paste; add very gradually of sweet oil, 1 oz.; and triturate until their union is complete, adding toward the end the rest of the mucilage; then further add of treacle, 11⁄2 oz.; and afterwards, successively, of strong vinegar, 2 oz.; rectified spirit, 1 oz.; lastly, bottle for use.
3. Mix the whites of 2 eggs with a table-spoonful of spirit of wine, 2 large lumps of sugar (crushed), and sufficient finely powdered ivory-black to give the required colour and thickness, avoiding excess.
Obs. The above are chiefly used for dress boots and shoes. The first two are applied to the leather with the tip of the finger, or a sponge, and then allowed to dry out of the dust. The third is commonly laid on with a sponge or soft brush, and when almost dry or hard may have its polish heightened with a brush or soft rubber, after which it is left for a few hours to harden. It may also be used to revive the faded black leather seats and backs of old chairs. They all possess great brilliancy for a time; but are only adapted to clean, dry weather, or indoor use. They should all be applied to the leather as thinly as possible, as otherwise they soon crack off.
Blacking, Har′ness. Good glue or gelatine, 4 oz.; gum-arabic, 3 oz.; water, 3⁄4 pint; dissolve by heat; add of treacle, 6 oz.; ivory-black (in very fine powder), 5 oz.; and gently evaporate, with constant trituration, until of a proper consistence when cold; when nearly cold put it into bottles, and cork them down. For use, the bottle may be warmed a little to thin it, if necessary. Does not resist the wet.
2. Mutton suet, 2 oz.; bees-wax (pure), 6 oz.; melt, add of sugar candy (in fine powder), 6 oz.; soft soap, 2 oz.; lamp-black, 21⁄2 oz.; indigo (in fine powder), 1⁄2 oz.; when thoroughly incorporated, further add of oil of turpentine, 1⁄4 pint; and pour it into pots or tins.
3. Bees′-wax, 1 lb.; soft soap, 6 oz.; ivory-black, 1⁄4 lb.; Prussian blue, 1 oz.; (ground in) linseed oil, 2 oz.; oil of turpentine, 1⁄2 pint; to be mixed, &c., as before.
Obs. The above are used by laying a very little of them on the leather, evenly spreading it over the surface, and then polishing it by gentle friction with a brush, or a soft-rubber. The last two are waterproof. Numerous compositions of the class are vended by the saddlers and oilmen, but the mass of them are unchemical mixtures, badly prepared, and cause disappointment to those who use them. Such is not the case with the products of the above formulæ, if we may rely on the statements of those who have employed them for years. The last two are suitable for both harness and carriage leather. See Balls, Heel, &c.
BLADD′ER. Syn. Ves′ica, L.; Vessie, Fr.; Blase, Blatter, Ger. In anatomy, &c., a thin membranous sac or bag, in an animal, serving as a receptacle for some secreted fluid; appr., the urinary bladder. See Calculus, Inflammation, Rupture, &c.
Bladd′ers. (In commerce.) The better qualities of these articles are prepared by cutting off the fat and loose membranes attached to them, and washing them first in a weak solution of chloride of lime, and afterwards in clear water; they are then blown out and submitted to strong pressure by rolling them under the arm, by which they become considerably larger; they are next blown quite tight, dried, and tied up in dozens. Commoner qualities are merely emptied, the loose fat removed, and then blown out, and strung up to dry. Used chiefly by druggists and oilmen to tie over pots, bottles, and jars, and to contain pill-masses, hard extracts, and other similar substances; also in surgery, to cover wounds, sore heads, &c.—Obs. Bladders should never be purchased unless perfectly dry and air-tight; as, if the reverse be the case, they will neither keep nor prove useful, but will rapidly become rotten and evolve a most offensive odour. If purchased whilst damp, they should be at once hung up in a current of air, so as to dry as soon as possible.
BLAIN* (blāne). A boil; a sore; a pustule.
BLANC (blŏng). [Fr.] In cookery, a dish which, according to Mrs Rundell, is formed of grated bacon and suet, of each 1 lb.; butter, 1⁄2 lb.; 2 lemons; 3 or 4 carrots (cut into dice); 3 or 4 onions; and a little water; the whole being simmered for a short time, with or without the addition of a glass of sherry or marsala, before serving.
BLANCH′ING. Syn. Candica′tio, Dealba′tio, &c., L.; Blanchiment, &c., Fr.; Bleichen, &c., Ger. A whitening, or making white; a growing white. In some cases it means decortication. See Almonds, Bleaching, Decoloration, &c.
Blanching. In cookery, an operation intended to impart whiteness, plumpness, and softness, to joints of meats, poultry, tongues, palates, &c. It is usually performed by putting the articles into cold water, which is then gradually raised to the boiling point, when they are at once taken out, plunged into cold water, and left there until quite cold. They are subsequently removed and wiped dry, ready for being dressed.
Obs. The operation of blanching meat, although it renders it more sightly according to the notions of fashionable life, at the same311 time lessens its nutritive qualities, by abstracting a portion of the soluble saline matter which it contains, especially the phosphates, and thus deprives it of one of the principal features which distinguish fresh meat from salted meat. Animal food, before being dressed, may be washed or rinsed in cold water without injury, provided it be quickly done; but it cannot be soaked in water at any temperature much below the boiling-point without the surface, and the parts near it, being rendered less nutritious. Washing meat when first received from the butcher is, indeed, a necessary act of cleanliness; but soaking it for some time in water is unnecessary, and for the reasons stated should be avoided.
Strong acetic acid (concentrated vinegar) poured on or rubbed over hard lean meat gradually renders it soft and gelatinous. Ordinary household vinegar has the same effect, but in a less degree. Tough meat thus treated for a short time before dressing it becomes more tender and digestible, though somewhat less nutritious; whilst the taste and flavour of the vinegar is removed by the heat subsequently employed in dressing it.
BLANCMANGE′. (blo-mŏngzh′‡.) Syn. Blancmanger (blŏng-mŏng-zhā), Fr. Literally, white food; in cookery, a confected white jelly. It is commonly prepared by simmering 1 oz. of isinglass, 2 or 3 oz. of lump sugar, and a little flavouring,[203] in about a pint of milk, until the first is dissolved, when the whole is thrown into a jelly-bag, and the strained liquor is allowed to cool and solidify; it is next remelted by a gentle heat, and, when nearly cold, poured into moulds, which have been previously rubbed with a little salad oil and then wiped out again.
Obs. Good gelatine, or strong calves’ feet jelly, is often substituted for the isinglass. At other times the jelly is made with about 1⁄2 pint of water (instead of milk), when 1⁄2 pint of almond-milk, or of cream, is added to the remelted jelly. Sometimes ground rice or arrow-root is employed in lieu of isinglass, when the product is called RICE-BLANCMANGE, or West-Indian b., as the case may be. Transpa′′rent blancmange[204] is merely clarified isinglass-jelly, flavoured. See Cream (Stone), Isinglass, and Jelly.
BLANQUETTE′ (blang-ket’). [Fr.] In cookery, a species of white fricasee. It is also the name of a delicate species of white wine, and of a particular sort of pear.
BLAST′ING. In civil and military engineering, the disruption of rocks, &c., by the explosion of gunpowder, or other like material.
BLAST′ING POWDERS (Melville and Callow’s). Prep. 1. (Powder No. 1.) Chlorate of potassa, 2 parts; red sulphuret of arsenic, 1 part; to be separately carefully reduced to powder, and lightly mixed together, scrupulously avoiding the use of iron instruments, percussion, much friction, the slightest contact with acids, or exposure to heat.
2. (Powder No. 2.) Chlorate of potassa, 5 parts; red sulphuret of arsenic, 2 parts; ferrocyanide of potassium (prussiate of potash), 1 part; as No. 1.
3. (Powder No. 3.) Chlorate of potassa and ferrocyanide of potassium, equal parts.
Obs. These compounds are not permanently injured by either salt or fresh water, merely requiring to be dried to regain their explosive character. They possess fully eight times the force of ordinary powder. One of their advantages, especially to the underground miner, is the very trifling amount of smoke produced by their explosion. On the other hand, the extreme facility with which they explode by attrition, contact with a strong acid, and a slight elevation of temperature, render them unsuited to most of the purposes of ordinary gunpowder. On this account they should only be prepared in small quantities at a time, and with the utmost caution. Mr Callow, the inventor of them, lost several of his fingers, and was rendered a cripple for life, by an explosion of the kind referred to, which occurred only a few weeks after the sealing of his patent. A straw, or small strip of wood, only slightly wetted with oil of vitriol, and applied to a small heap of the powder, produces instantaneous explosion. Captain Wynand’s ‘Saxifragine’ is composed of nitrate of baryta, 76 parts; charcoal, 22 parts; and nitre, 2 parts. Schultze’s wood-gunpowder is composed of granulated wood treated with a mixture of nitric and sulphuric acid, afterwards impregnated with a solution of nitre. M. Bäudish has invented a method by which this wood-gunpowder may be compressed into a solid substance, exerting great power and free from danger by transport. Lithofracteur, a white blasting powder used in Belgium, is a substance similar to gun-cotton.
Messrs Nenmayer and Fehleisen’s haloxylin is composed of charcoal, nitre, and yellow prussiate of potash. See Gun-cotton, Gunpowder, Mining, &c.
BLATTA ORIENTALIS. The common cockroach, originally imported from the East, belongs to the family of orthopterous insects; and may be classed amongst the most offensive and objectionable of domestic pests. It is extremely voracious, not only devouring all kinds of provisions, but attacking and consequently destroying silk, flannel, and even cotton fabrics, in the absence of anything more eatable. The cockroach is nocturnal in its habits, and exceedingly active and swift of movement. Its flattened form enables it to insinuate itself easily into crevices, and so to escape detection. The American cockroach (Blatta Americana) is larger than the above. A still larger species (Blatta gigantea) is found in the West Indies where it is known by the name of the drummer.312 It is so called from the tapping noise it makes on wood, the sound so produced, when joined in by several of the creatures (as it usually is) being sufficient to destroy the slumbers of a household.
Cockroaches may be poisoned by means of wafers made of red lead, or caught by smearing a piece of wood with treacle, and floating it on a broad basin of water. When the fires and lights are extinguished they issue from their holes, and fall into the basin in their efforts to reach the bait. The chinks and holes from which they come should also be filled up with unslaked lime, and some lime should also be sprinkled about the ground.
Old Gerrard says they avoid any place in which the leaves of the mullein are strewn about.
The Blatta Orientalis, which was formerly supposed to possess remedial powers, and was hence employed in medicine by the more ancient therapeutists, has lately found advocates for his readmission into the animal materia medica. He is reported, when made into a tincture, to act as a diuretic, and to yield a crystalline body possessed of similar properties, but in a more concentrated form. Some of the American journals report that he may be given in the form of powder or infusion (from 15 to 30 gr.) 3 or 4 times a day, in dropsy, and to increase the secretion of urine as well as of perspiration.
BLEACH′ING, (blēche′-). Syn. Deälba′tio (-sh′o), Insola′tio,[205] &c., L.; Blanchiment, Blanchissage, Fr.; Bleichen, Ger. The process by which the colour of bodies, natural or acquired, is removed, and by which they are rendered white or colourless. It is more particularly applied to the decolorisation of textile filaments, and of cloths made of them.
Hist. Bleaching is a very ancient art, as passages referring to it in the earlier sacred and profane writers fully testify. It had probably reached a high degree of excellence among the inhabitants of the first Assyrian empire, and was certainly practised in Egypt long before the commencement of written history. We may fairly assume that fine white linen formed part of the “raiment,” which, together with “jewels of gold, and jewels of silver,” and “precious things,” Abraham sent as presents to the beautiful Rebekah and her family,[206] fully three centuries and a half before the Exodus. Subsequently, in Scripture, we have special mention of “fine linen, white and clean.” Herodotus, the earliest Greek historian, tells us, that the Babylonians wore “white cloaks;”[207] and in Athenæus we read of “shining fine linen,” as opposed to that which was “raw” or unbleached.[208] At this early period, and for many centuries afterwards, the operations of washing, fulling, and bleaching were not distinctly separated. The common system of washing followed by drying in the sun, adopted by the ancients, is a process which of itself, by frequent repetition, decolorises the raw materials of textile fabrics, and thus must inevitably have taught them the art of ‘natural bleaching’ of a character similar to that practised in Europe up to a comparatively very recent period. And this appears, according to the authority of ancient authors, to have been the case. Washing or steeping in alkaline and ammoniacal lyes, or in milk of lime, followed by exposure in the sun, formed the chief basis of their system; whilst woollens, then as now, were treated with soap and fuller’s earth, or with potter’s clay, marl, Cimolian earth, or other like mineral. Urine was highly esteemed among them; and we are told that in the time of the emperor Vespasian,[209] and undoubtedly long before it, cloths were sulphured. Indeed, according to Pliny, sulphuring was often had recourse to in ordinary washing, as well as in the bleaching process.[210]
Bleaching continued to be practised with no essential change of its principles until the discovery of chlorine, to which we shall presently refer. In the last century Holland obtained the best name for bleaching. The process passed then to Ireland and Scotland, and thence into England. It was even customary to send goods from this country to be bleached in Holland. The first attempt to vie with Holland was made, in Scotland, in 1749.
The first steps towards the modern or chemical system of bleaching were the investigations of Berthollet on chlorine, in 1784, but which were not communicated to the French Academy until the year 1787. The knowledge of the use of chlorine as a bleacher was soon afterwards brought to this country by the Duke of Gordon, and by Prof. Copeland of Aberdeen, and through them was practically applied by Messrs Milnes of that place. About the same time James Watt, a correspondent of Berthollet, successfully introduced its use in the neighbourhood of Glasgow, and then generously laid a statement of the results before the Manchester manufacturers. In enforcing the importance of the new substance and process on these gentlemen, he was ably followed and seconded by Dr Henry. In 1798, Mr Charles Tennant, of Glasgow, obtained a patent for a new bleaching liquor prepared by saturating lime water with chlorine; and another, in 1799, for dry chloride of lime, a substance which is still preferred as a bleacher to all other preparations of chlorine. The new or continuous process of bleaching, as it is called, and that which is at present in general use in all the chief bleach-works of Lancashire, was introduced by Mr David313 Bentley, of Pendleton, and patented by him in 1828.
Proc. Bleaching is commonly said to be natural when exposure to light, air, and moisture forms the leading part of the process; and to be chemical when chlorine, chloride of lime, sulphurous acid, or other like substances are employed. In some cases, as with linen, the two processes are combined. The subject requires to be noticed under separate heads, depending on the material operated on:—
I. Bleaching of Cotton:—Cotton is more easily bleached, and appears to suffer less from the process than most other textile substances. On the old plan it was first (1) thoroughly washed in warm water, to remove the weaver’s paste or dressing; then (2) ‘bucked’ or ‘bowked’ (boiled) in a weak alkaline lye, or in milk of lime, to remove colouring, fatty, and resinous matters, insoluble in simple water; and after being (3) again well washed, was (4) spread out upon the grass, or bleaching ground, and freely exposed to the joint action of light, air, and moisture (technically called ‘crofting’). The operation of ‘bucking’ in an alkaline lye, washing, and exposure was repeated as often as necessary, when the goods were (5) ‘soured’ or immersed in water acidulated with sulphuric acid, after which they (6) received a final thorough washing in clean water, and were (7) dried, finished, and folded for the market. From the length of the exposure upon the bleaching ground this method is apt to injure the texture of the cloth; and from the number of operations required is necessarily expensive and tedious. It is therefore now very generally superseded by the system of chemical bleaching briefly described below.
In the CHEMICAL SYSTEM of bleaching the goods are ‘washed’ and ‘bucked’ as on the old plan, then submitted to the action of a weak solution of chloride of lime, and afterwards passed through water soured with hydrochloric or sulphuric acid, when they have only to be thoroughly washed, and to be dried and finished, for the entire completion of the process.
The new or continuous process, before referred to,[211] is the method of chemical bleaching at present in the most general use; and, indeed, it has nearly superseded all other methods. In this system the pieces, previously tacked together endwise so as to form a chain, are drawn, by the motion of rollers, in any direction, and any number of times, through every solution to the action of which it is desired to expose them, and this entirely and completely under the control of the operator.
The following Table exhibits an outline of the several operations in the improved form of the continuous process as practised by Messrs McNaughten, Barton, and Thom, at Chorley, and in most other large bleach-works:—
1. Preliminary operations:—a. The ‘pieces’[212] are separately stamped with the printer’s name, a solution of silver, or sometimes coal-tar, being employed for the purpose.
b. They are tacked together endwise either by hand or a machine, so as to form one continuous piece of 300 to 350 yards in length, according to the weight of the cloth.
c. They are singed.[213]
d. They are crushed into a rope-like form by drawing them through a smooth aperture,[214] the surface of which is generally of glass or porcelain—the rope-form being given them to enable the water and other liquids to penetrate the goods more easily, and to allow them to be laid in loose coils in the kiers.
2. The pieces are bucked or boiled in milk of lime[215] for 12 to 14 hours,[216] followed by rinsing or cleansing in the washing-machine.
3. They are soured in water acidulated with hydrochloric acid,[217] and again washed; similar machines being employed for each.
4. They are bucked or boiled for 15 or 16 hours in a solution of resinate of soda,[218] and then washed as before.
5. They are chemicked by being laid in a wooden, stone, or slate cistern, when a solution of chloride of lime[219] is pumped over them, so as to run through the ‘goods’ into a vessel below, from which it is returned on them by continued pumping, so that the cloth lies in it for 1 or 2 hours; it is then washed.
6. They are bucked or boiled, for 4 or 5 hours, in a solution of 1 lb. of crystallised carbonate of soda, dissolved in 5 galls. of water, to every 35 lbs. of cloth; and washed.
7. They are again ‘chemicked,’ as before; and washed.
8. They are soured in very dilute hydrochloric acid;[220] and then left on ‘stillages’[221] for 5 or 6 hours.
9. They are, finally, thoroughly washed, well squeezed between rollers, dried over steam-heated tin-cylinders, starched or dressed, and finished.
This is the usual process for good calicos,314 Muslins, and other light goods, are handled rather more carefully; whilst for commoner ones the sixth and seventh operations are generally omitted. The whole usually occupies 5 days; but by using Mr Barlow’s high-pressure steam kiers, it may be performed in two days. Yarns, &c., may be bleached in a similar manner by first looping the skeins together.
Obs. According to the most reliable authorities, the strength of cotton-fibre is not impaired by its being boiled for two hours in milk of lime, under ordinary pressure, out of contact with the air; nor, according to the bleachers, even by sixteen hours boiling at the strength of 40 lbs. per 100 galls. It is said that lime is less injurious than ‘soda.’
Solution of caustic soda, sp. gr. 1·030, does not injure it, even by boiling under high pressure; but, in practice, soda-ash, or carbonate of soda, is used, and this only in the second bucking, and in the third, if there be one. The strength now never exceeds 25 lbs. of the crystals to the 100 galls., and is usually less.
Experiments have shown that immersion for 8 hours in a solution of chloride of lime containing 3 lbs. to the 100 galls., followed by souring in sulphuric acid of the sp. gr. 1·067, or for 18 hours in acid of 1·035, does not injure it.
By the improved method of previously treating the goods with lime or alkalies, little chloride of lime is required. Indeed, it is said that where 300 lbs. were formerly employed, 30 to 40 lbs. only are now used. At the same time it is right to mention, that though a solution at 1⁄2° Twaddle is usually regarded as the best and safest strength, yet in some bleach works, particularly for inferior and less tender goods, this is greatly increased, even up to 5°, the period of immersion being proportionately reduced, as it is not safe to expose the goods long to the action of such powerful solutions. With the higher strengths they are passed rapidly through the liquid with the calender, sufficient time only being allowed to soak them thoroughly; then immediately through the acid or souring, followed by washing as before.
In Scotland and Ireland the washing is generally performed by wash-stocks; whilst in Lancashire, dash-wheels, or washing machines with squeezers, are almost always used for the purpose.
Cotton loses about 1-20th of its weight by bleaching.
II. Bleaching of Linen:—Linen may be bleached in a similar way to ‘cotton,’ but the process is much more troublesome and tedious, owing to its greater affinity for the colouring matter existing in it in the raw state. Under the old system, several alternate buckings with pearlash or potash and lengthened exposure on the field, with one or two sourings, and a final scrubbing with a strong lather of soft soap, constituted the chief details of the process. In this way a high degree of whiteness, though not an absolutely pure or snow white, was ultimately produced. Grass-bleaching or crofting is still extensively used for linen; but it is more generally employed only for a limited time, and in combination with a modification of the system at present almost universally adopted for cotton goods; whilst, in some cases, crofting is omitted altogether, and the bleaching conducted wholly by the latter process. The following Tables exhibit the outlines of the new system as at present practised in Ireland and Scotland:—
a. For plain sheetings:—
1. They are bucked for 12 or 15 hours in a lye made with about 1 lb. of pearlash (or soda-ash) to every 56 lbs. of cloth, and washed.
2. Crofted for about 2 days.
3. Bucked in milk of lime.
4. Turned, and the bucking continued, some fresh lime and water being added; and washed.
5. Soured in dilute sulphuric acid at 2° Twaddle.
6. Bucked with soda-ash for about 10 hours, and washed.
7. Crofted, as before.
8. Bucked again with soda-ash, as before.
9. Crofted for about 3 days.
10. Examined, the white ones taken out, and the others again bucked and crofted.
11. Scalded or simmered in a lye of soda-ash of about only 2-3rds the former strength, and washed.
12. Chemicked, for 2 hours, at 1⁄2° Twaddle, washed, and scalded.
13. Again chemicked, as before.
14. Soured for 4 hours, as in No. 5; washed, and finished.
This occupies 13 to 15 days, according to the weather.
b. For shirtings, &c.:—As the preceding, but with somewhat weaker solutions.
c. For goods to be subsequently printed:—
1. Bucked in milk of lime for 10 or 12 hours.
2. Soured in dilute hydrochloric acid of 2° Tw., for 3 to 5 hours, and washed.
3. Bucked with resinate of soda for about 12 hours.
4. Goods turned, reboiled as before, and washed.
5. Chemicked at 1⁄2° Tw., for 4 hours.
6. Soured at 2° Tw., for 2 hours, and washed.
7. Bucked with soda-ash for about 10 hours, and washed.
8. Chemicked as in No. 5.
9. Soured, as at No. 6, for 3 hours; washed, and dried.[222]
Obs. The chief difficulty in bleaching linen arises from the fact that its colouring matter315 is insoluble in acid or alkaline solutions until it has been long acted upon by light, air, and moisture, as in the common process of grass-bleaching. Chlorine hastens the operation; but, unfortunately, it can only be employed towards the end of the process; as when earlier used, the colour of the raw cloth becomes set, and irremovable. To obviate this difficulty Mr F. M. Jennings, of Cork, has lately[223] introduced the joint use of an alkali and an alkaline hypochlorite (chloride) in the place of the ordinary chloride of lime. He prepares a bath of solution of soda at 5° Twaddle, which he raises by the addition of chloride of soda (or of potash) to 6 or 7°, and in this he steeps the cloth (after the first bucking and souring) for some hours, heat, or constant squeezing between rollers, being had recourse to, to facilitate the action. Souring and washing follow, when the goods are again put into the alkaline and chloride bath, as before; after which they are soured, and bucked again with soda. These last three operations are repeated until the cloth is almost white, when crofting for one half to one fourth the time required by the usual method renders it fit for the final bucking, and finishing. Indeed, it is said that if the process be very carefully managed it renders crofting unnecessary.
Raw linen loses about 1-3rd of its weight in bleaching.
III. Silk:—Silk is usually bleached by first steeping it, and then boiling it in solutions of white soap in water, after which it is subjected to repeated rinsings, a little indigo-blue, or archil, being added to the last water to give it a pearly appearance. When required to be very white (as for gloves, stockings, &c.), the goods are cautiously submitted, for 2 or 3 hours, to the action of the fumes of burning sulphur, and then finished by rinsing, as before.
Obs. Boiling or sulphuring is not required for the white silk of China. Raw silk loses from 4 to 5 oz. per lb. by bleaching.
IV. Wool:—In bleaching raw wool it is first deprived of the yolk or peculiar natural varnish with which it is covered. For this purpose it is steeped and stirred for about 20 minutes in rather warm water (135°—140° Fahr.), either with or without the addition of 1-4th part of stale urine; after which it is placed in baskets to drain, and soon afterwards thoroughly rinsed in a stream of water, when it is again allowed to drain, and it is hung up to dry. The further operations depend on circumstances, wool being sometimes whitened in the fleece, or in the yarn, but still more frequently and extensively not till woven. When it is intended to send it in the first two forms white to market, it is hung up or spread out, whilst still wet, and sulphured (see below); after which it is either at once rinsed for some time in cold water, or is previously treated with a very weak bath of soft soap.
In the case of woollen fabrics the operations of purifying or whitening the wool, beyond the removal of the yolk, are, for the most part, mixed up with the weaving and working of it. The pieces leave the hands of the weaver of a dingy grey colour, loaded with oil, dirt, and dressing. They then pass to the fulling-mill, where they are treated with fuller’s earth and soap, often preceded with ammonia or stale urine, after each of which they are well washed out or scoured with cold water, and are then ready for the dyer. When it is intended to obtain them very white, or to dye them of a very delicate shade, they are commonly sulphured; after which they are washed or milled in cold water for some hours, a little finely ground indigo being added towards the end, to increase their whiteness; an addition also made when the cloth is sufficiently white without the sulphuring process.
The usual mode of SULPHURING woollen goods is to hang them up on pegs or rails, or, in the case of fleece-wool, to spread it about, at the upper part of a close, lofty room or chamber, called a sulphur-stove. In each corner of this room is set a cast-iron pot containing sulphur, which, after the introduction of the goods, is set on fire, when the door at the lower part of the chamber is shut tight and clayed. This is commonly done over-night; and by the morning, the bleaching being finished, the goods are removed, washed, and azured.
Sulphuring, unless very skilfully managed, imparts a harsh feel to woollen goods, which is best removed by a very weak bath of soap-and-water (lukewarm); but the action of soap in part reproduces the previous yellowish-white tinge. Milling with cold, or lukewarm water, tinged with indigo, is the best substitute.
Obs. Raw wool loses from 35 to 45% of its weight by scouring, and 1 to 2% more in the subsequent operations of the bleacher; the loss being in direct proportion to the fineness of the staple.
⁂The above are the four principal applications of the art of bleaching; but, in technical language, the words bleaching, bleacher, bleachery, bleach-works, &c., when employed alone, are understood to have reference only to cotton and linen. This has arisen from the enormous extent of these manufactures, and from the process of bleaching them forming a business entirely distinct from that of weaving, dyeing, or printing them. The following, with the exception of the first, are of comparatively minor importance and interest:—
V. Materials for Paper:—Old rags for the manufacture of paper, and paper-pulp, are now almost universally bleached with chlorine or chloride of lime; the former being generally used in France, and the latter in England. The process usually consists in (1) boiling in an alkaline lye to remove grease and dirt,316 (2) washing, (3) pressing, (4) deviling or tearing up the pressed cake into fine shreds or pulp, (5) chemicking, with agitation, for about an hour, in a clear solution of chloride of lime,[224] followed by (6) washing, (7) souring with dilute hydrochloric acid at 1 or 2° Tw., or treatment with a solution of some antichlor, or both, and (8) a final washing and pressing. For the common kinds of paper, the operations included in No. 7 are omitted; but unless the whole of the lime-salt be removed from the pulp, the paper made of it is liable to turn brown and become rotten by age. In some cases rags are bleached before being divided and pulped. Cotton-waste is bleached in a similar way to rags.
In France, the chlorine, in a gaseous form, is passed from the generators into the bleach-cisterns containing the pulp, which in this case must be fitted with close covers.
VI. Printed Paper, as Books, Engravings, Maps, &c.—These when stained or discoloured may be whitened by (1) wetting them with pure clean water, (2) plunging them into a dilute solution of chloride of lime, (3) passing them through water soured with hydrochloric acid, and then (4) through pure water until every trace of acid be removed. This process may be further improved by further dipping them into a weak solution of some antichlor, and again washing them, before finally drying them. It is only rare and valuable original works or specimens of art that are worth this treatment, which, owing to the very nature of paper, requires considerable address to manage. In many cases a sufficient degree of renovation may be effected by simply exposing the articles, previously slightly moistened, to the fumes of burning sulphur, followed by passing them through a vessel of pure water.
VII. Straw, Straw-plait, and articles made of them, are, on the large scale, usually bleached by (1) a hot steep or boil in a weak solution of caustic soda, or a stronger one of soda-ash, followed (2) by washing and (3) by exposure to the fumes of burning sulphur. To effect the last, the goods are suspended in a close chamber connected with a small stove, in which brimstone is kept burning. On the small scale, a large chest or box is commonly employed. A piece of brick, or an old box-iron heater, heated to dull redness, is placed at the bottom of an iron crock or earthen pan, a few fragments of roll sulphur thrown on, the lid instantly closed, and the whole left for some hours. Care should be taken to avoid inhaling the fumes, which are very deleterious as well as disagreeable and annoying. Straw goods are now also frequently bleached by the use of a weak solution of chloride of lime, or of water strongly soured with oxalic acid or even oil of vitriol, followed by very careful rinsing in clean water; but here, as in the former case, the natural varnish, dirt, grease, &c., must be first removed by alkalies or soap, to enable the chlorine or acid to act on the fibres.
VIII. Wax. Wax is bleached by first melting it at a low temperature in a cauldron, from whence it is allowed to run out by a pipe at the bottom into a capacious vessel filled with cold water.
This vessel is fitted with a large wooden cylinder, which turns upon its axis; and the melted wax falls upon this cylinder. The surface of the cylinder being always wet, the wax does not adhere to it, but becomes solid, assuming the form of ribbons as it does so, and in this shape becoming distributed through water in the tub. The wax is then removed and placed upon large frames stretched upon linen cloth, which are supported about 18 inches above the ground, and erected in a situation exposed to the air, dew, and sun. The several ribbons thus placed on the frame should not exceed an inch and a half, and they ought to be so moved about from time to time as that each part may be equally exposed. If the weather be favorable the wax will become white in a few days. It is again remelted, formed into ribbons, and exposed as before. These operations are continued in until the wax is completely bleached, after which it is melted and run into moulds.
Concluding Remarks. The theory of bleaching, notwithstanding the giant strides of chemistry during the last 20 years, remains still unsettled; and hence the processes employed are still, for the most part, empirical. It appears probable that chlorine acts by uniting with the hydrogen of the water, or of other compounds present, or probably with that of both, and that it is the oxygen thus liberated, and whilst in the nascent state, that is the true operative agent. Hence bleaching by chlorine, or by the hypochlorites, may be regarded as an oxidation of the colouring matter; but whether the chlorine or the oxygen effects this oxidation is of little practical importance—the result being the same—the destruction of the compound, and the removal of the colour that depends on its existence. It is doubtful whether the bleaching power of sulphurous acid is due to it as an oxidising or a deoxidising agent; but the last is probably the case, with a like destruction of the compound constituting the colouring matter. It may, however, be supposed that sulphurous acid acts as an oxidiser, as it appears to do when it decomposes sulphuretted hydrogen; or it may act by simply altering the compound by inserting itself, a view receiving some support from the fact that wool whitened by317 sulphuring may be restored to nearly its previous colour by merely treating it with soap or alkalies.
The bleaching power of light depends on its actinic or chemical rays, which, like chlorine, appear to act as an oxidising agent.
Chlorates, chromates, chromic acid, manganates, &c., have been proposed as bleaching agents for textile filaments and fabrics, but without success or practical advantage. Immersion in water more or less strongly impregnated with sulphurous acid has, however, been successfully substituted for the common sulphuring process, particularly for silk.
To avoid the injury of the goods by sparks, and by drops of water highly saturated with sulphurous acid falling from the roof, Mr Thom has invented a method of passing them rapidly through, or keeping them in constant motion in the sulphuring chamber. His apparatus is constructed on the principle of the washing-machine, the fumes of burning sulphur being used instead of water.
M. Tessie du Motay has proposed a new method for bleaching. He takes about equal parts of permanganate of soda and sulphate of magnesia, and dissolves them in lukewarm water. The tissues, previously freed from grease, are to be plunged into this bath until they are covered with a brown coating. They are then to be placed in a bath of sulphuric acid at 4 per cent., and rinsed after the brown matter is removed. They may be finally passed through sulphurous acid. Mr Ramsay’s method consists in sprinkling with water equal parts of chloride of lime and sulphate of magnesia, when hydrochlorate of magnesia is formed. It may be remarked that none of the more modern methods of bleaching have been found, when reduced to practice, to be cheaper, better, or more advantageous to work than those sanctioned by long experience and use.
[Further information in connection with bleaching will be found under the heads Actinism, Blanching, Calico-printing, Charcoal, Chlorides (Bleaching), Chromates, Chromic Acid, Hypochlorites, Hypochlorous Acid, Light, Rinsing, Spots and Stains, Sulphuration, Washing, &c.; also under Bones, Engravings, Fat, Feathers, Horn, Ivory, Oil, Paper, Printed Books, Rags, Sponge, Straw-plait, Tallow, Wax, &c.[225]]
Bleaching Liq′uid. Solution of chloride of lime.
Bleaching Pow′der. Chloride of lime.
Bleaching Salts. The commercial hypochlorites.
BLEAR′-EYE (blēre′-ī). Syn. Lippitu′do, L.; Chassie, Lippitude, Fr. An exudation of a puriform matter from the margins of the eyelids, which are red, tumid, and painful; and frequently, during the night, glued together by the discharge.
Treatm. Mild astringent collyria, as those of sulphate of zinc or alum (6 or 8 gr. to 1 oz. of water). An ointment formed of 1 part of the ointment of nitrate of mercury (Ph. L.), diluted with 11 parts of sweet washed lard, may be advantageously applied nightly, by means of a camel-hair pencil, the smallest quantity possible only being used. Excess in eating and drinking should be avoided, and some aperient medicine taken.
BLEAK (blēke). Syn. Blay‡, Bley‡, (blā). The cypri′nus albur′nus (Linn.), a small river-fish, the scales of which are used in making artificial pearls (which see).
BLEB. A vesicle or blister. In some states of general derangement of health this arises spontaneously. It should be treated in the same way as scalds.
BLEED′ING (blēde′-). In the sense of a flow or loss of blood, see Hæmorrhage; in that of bloodletting, see Cupping, Leeching, Venesection, &c.
Bleeding Piles. Take every morning aperient doses of milk of sulphur, then a small teaspoonful of confection of black pepper every day. Wash externally with a sponge and cold water. Apply compound gall. ointment to the piles if external.
Bleeding from the Air Passages and Lungs. Let the patient at once go to bed, and keep perfectly quiet, avoiding movement of any kind as much as possible. Administer dilute acids in frequently repeated doses, with five drops of tincture of digitalis. The bowels should be kept open by means of Epsom salts in infusion of roses. Give iced drinks and let solid ice be sucked. Mustard plasters may be applied to the chest. A morphia lozenge may now and then be sucked gradually away, as well as a small piece of sal prunella. The cough must be allayed by the administration of small doses of morphia in gum water or barley water. All food should be taken cold.
The treatment that we have indicated in the last two forms of hæmorrhage is intended for the exclusive guidance of emigrants or of others so placed as to be unable to summon prompt medical aid. Wherever this can be obtained no time should be lost in at once seeking it.
Bleeding from the Nose. Apply cold water containing ice, if obtainable. It should be so applied to the nose as to cause a shock. A cold piece of metal, such as a key, placed on the naked back sometimes stops the hæmorrhage. If neither of the above means succeed inject with a syringe a solution of alum or sulphate of zinc (ten grains to the ounce), or snuff up the nostrils some gallic acid, powder of pomegranate, kino, or catechu, mixed with starch. A plug of lint may also be dipped in either of the above solutions, or rolled in the318 powders, and pushed up the nostrils, or some tincture of perchloride of iron, properly diluted and applied on a piece of lint, may be tried.
Bleeding from the Stomach. Syn. Hematemesis. In this case the blood is vomited usually in clots of a dark colour. It should be noticed whether it comes from the back of the nose or throat. The treatment consists in perfect repose in bed, and in the administration of dilute sulphuric acid in infusion of roses, with saline aperients. If these fail to give relief, tannin and krameria may be tried, and small doses of laudanum or five grains of alum may be given every four hours. If in pain, add to it 1⁄4 grain of acetate of morphia. All food and drinks should be taken cold, the latter iced. Pernitrate of iron in from 10 to 30 minim doses is a valuable remedy.
BLENDE (blĕnd). A name applied to several minerals; appr., zinc-blend, or native sulphuret of zinc—the black jack of miners.
BLIGHT (blīte). See Mildew, and Plants (Diseases of).
BLIND′NESS (blīnd′-). Syn. Ablep′sia, Cæ′citas, &c., L.; Aveuglement, Cécité, Fr.; Blindheit, Ger. Deprivation or want of sight.
Blindness may be congenital, or born with a person; or it may arise from accident, external violence, or disease. In the latter it may frequently be relieved by medical and surgical treatment. See Amaurosis, Cataract, Eyes, Ophthalmia, Vision, &c.
Blindness, Day. Syn. Night′-sight; Ny̆̆ctalo′pia, L. A disease of the eye in which vision is painfully acute or more or less extinct in a strong light, as that of day; but clear and pleasant in the dusk of evening and at night. Its chief causes are excessive exposure of the eyes to the direct influence of very strong or glaring light, or to heat, or both of them together; and is often one of the sequelæ of ophthalmia (which see).
Blindness, Night. Syn. Day′-sight; Hemeralo′pia, L. An affection of the eye, the reverse of the preceding, in which objects are clearly seen only in broad daylight. In the beginning of the complaint the patient continues to be able to see, though less clearly, for a short time after sunset, and even by moonlight, and perhaps distinctly by bright candle light; but after a short time this power is lost. It most frequently occurs in hot climates, and low latitudes at sea. Its chief causes are fatigue and exposure of the eyes to the glare of the tropical sun, probably coupled with gastric derangement. In some cases it is congenital, and is then generally incurable. The treatment consists in avoiding exciting causes, and endeavouring to restore the tone of the stomach, and the general health, by the usual methods. The eyes at the same time should be topically medicated by the frequent use of cold water, or mild astringent collyria. See Ophthalmia (Chronic).
BLIS′TER. Syn. Pap′ula, Pus′tula, L.; Pustule, Vessie, &c., Fr.; Blase, Blatter, Ger. A bladder or vesicle caused by the deposition of serous fluid between the cuticle and the derma or true skin, occasioned by the application of a vesicant, or by a burn, scald, or friction.
Blister. Syn. Vesicato′′rium, L.; Epispastique, Vesicatoire, Fr.; Blasen-pflaster, B.-stoff, Ger. A substance which vesicates or raises blisters; in pop. lang., a vesicating plaster or similar application.
The use of blisters is very ancient, and appears to date back long prior to the time of Hippocrates. Indeed, their value as cutaneous stimulants and counter-irritants appears to have been recognised by the medical faculty of all nations down to the present time. It is a principle sufficiently established with regard to the living system, that where a morbid action exists, it may often be removed by inducing an action of a different kind, as a state of excitement or irritation, in the same or a neighbouring part. In this way is explained the utility of blisters in local inflammation and spasmodic action, and it is this principle which regulates their application in pneumonia, gastritis, hepatitis, phrenitis, angina, rheumatism, colic, spasmodic affections of the stomach, &c.—diseases in which they are employed with the most marked advantage. A similar principle exists with respect to pain; exciting one pain often relieves another. Hence blisters frequently give relief in neuralgia, toothache, and other like painful affections. Lastly, blisters, by their operation, communicate a stimulus to the whole system, and raise the vigour of the circulation. Hence, in part, their utility in fevers of the typhoid kind, though in such cases they are used with still more advantage to obviate or remove local inflammation.
Blisters are commonly prepared with cantharides plaster, or with some other preparation of cantharides; and, in the former case, usually have their surface sprinkled over with powdered Spanish fly; whilst the blistering surface is surrounded with a margin spread with common adhesive plaster, for the purpose of causing them to adhere to the part to which they are applied. In order to prevent the action of the cantharides upon the mucous membrane of the bladder, or urinary organs, they are also often sprinkled with a little powdered camphor, or better still, are moistened with camphorated ether, which, on its evaporation, leaves a thin layer of camphor on the surface; but care must be taken that the layer be not too thick, as in that case the plaster would not take effect. With a like object, a piece of thin book-muslin or tissue-paper (silver-paper) is frequently placed between the blistering surface of the plaster and the skin; the efficacy of which may be still further heightened by first soaking the muslin or paper in olive or almond oil.
The usual time an ordinary blister of cantharides319 plaster is allowed to remain in contact with the skin is from 10 to 12 hours. It is then gently removed. The subsequent treatment depends on the object in view. When it is not wished to maintain a discharge from the blistered surface, the vesicle is cut with the point of a pair of scissors at its most depending part, to let out the fluid which it contains, followed by a dressing of spermaceti or other simple ointment; but when the case requires the blister to be kept open, or to be converted into a perpetual blister, as it is sometimes called, the whole of the detached cuticle is carefully removed with the scissors, and the part is dressed with either the ointment of cantharides or of savine, at first more or less diluted with lard or simple ointment, with an occasional dressing of resin cerate. According to Mr Crowther, the blistered surface is best kept clean by daily fomentation with warm water.
Of late years, to obviate the unpleasant effects occasionally arising from the common blister, various compounds having cantharides for their base, as well as fabrics spread with them, have been brought before the public. These are noticed hereafter. See Plaster, Vesicants, &c.
Blisters, Extempora′′neous. Among the best of these may be mentioned the following:—
1. A piece of lint dipped in the strongest vinegar of cantharides, and immediately after its application to the skin, covered over with a piece of strapping, or preferably a piece of sheet gutta percha or oiled silk, to prevent evaporation. Raises a blister in from 5 to 8 minutes.
2. Concentrated acetic acid, applied in the same way, has a similar effect.
3. (Dr Darcq.) Into a flat watch glass pour from 8 to 10 drops of highly concentrated liquor of ammonia; cover the liquid with a small piece of linen of rather less diameter than that of the glass, and at once apply this little apparatus to the previously shaved skin. The whole must be kept in its place by means of moderate pressure with the fingers, until a red ring, about 2 centimètres in breadth, is observed round the glass, when it is certain that vesication is effected. Sometimes scarcely 30 seconds are necessary for obtaining the result. The apparatus may then be removed, and the blistered part treated in the usual manner; the dressing being according to the object in view.
4. (Trousseau.) Bibulous paper slightly wetted with a little of the ethereal extract of cancharides, and instantly applied to the skin, the whole being covered with a piece of common adhesive plaster to prevent evaporation.
5. Boiling water applied by means of a suitably shaped tube, the adjacent parts being at the same time protected from injury. Instantaneous.
Blister, Horse. See Veterinary Medicines.
Blister*, Perpet′ual. See Blister (antè).
BLIS′TERING. Syn. Ves′icans, Vesicato′′rius, L.; Epispastique, Vésicant, Vésicatoire, Fr.; Blasenziehend, &c., Ger. In medicine, &c., that vesicates or raises blisters when applied to the skin.
Blistering Pa′per, Plas′ter, Tis′′sue (tĭsh-ū), &c. See Plasters, Vesicants, &c.
BLOAT′ER. See Blote.
BLONDE. [Fr.] Syn. Blond′-lace. Silk-lace. The name is now also applied to cotton-lace edged with silk. For the mode of cleaning it and getting it up, see Lace and Muslin.
BLOOD (blŭd). Syn. San′′guis, L.; Sang, Fr.; Blut, Ger. The general circulating fluid of animals, and that on which the nourishment and growth of their bodies depend, and from which all the secretions are formed. It is warm and red in vertebrated animals; and, for the most part, cold and white in the invertebrata. In man and all other mammals, and in birds—the two highest classes of the animal kingdom—the blood, though collectively forming but one circulating stream, varies considerably in appearance according to the part or vessels in which it is found. That contained in the left side of the heart, and in the arteries, possesses a very brilliant scarlet colour, and is called arte′′rial blood; whilst that found in the right side of the heart, and in the veins, has a darkish purple colour, and is called ve′nous blood. The two, however, differ little from each other in their chemical properties and composition; the most marked point of difference being that venous blood holds carbonic acid in solution, whilst oxygen predominates in the blood of the arteries. The fibrine of venous blood is also soluble in a solution of nitrate of potassa; whilst that of arterial blood is insoluble in that menstruum.
Comp. Blood consists of a transparent and nearly colourless fluid (plas′ma, se′′rum, sĕralbu′men), in which float about a countless multitude of microscopic round red bodies (blood-discs, blood-corpuscles), to which its colour is due, accompanied by a few colourless globules (white blood-corpuscles) of a somewhat larger size. The red corpuscles are found, on more minute examination, to consist of an envelope containing a solution of hæmatosin.
Prop. These are, for the most part, well known. It has an alkaline reaction, a saline and rather disagreeable sweetish taste, and when newly drawn evolves a peculiar odour or halitus, which almost immediately disappears. As it cools and on repose it coagulates, owing, according to some, to the spontaneous solidification of the fibrine.
The following table, based upon the observations of Schmidt and the analysis of Lehmann, is given by the latter, as representing the average quantitative relation of the principal constituents of normal blood. It will be noticed that the blood is here regarded as composed of two portions, one consisting solely of the red particles, and the other of the320 liquid, in which these red corpuscles are suspended, termed the liquor sanguinis, which consists of the serum holding fibre in solution:—
| 1000 parts blood-corpuscles contain— | |
| Water | 688·00 |
| Solid constituents | 312·00 |
| consisting of— | |
| Hæmatin (with iron) | 16·75 |
| Globulin and cell membrane | 282·22 |
| Fat | 2·31 |
| Extractive matters | 2·60 |
| Mineral substances (without iron) | 8·12 |
| Chlorine | 1·686 |
| Sulphuric anhydride (SO3) | 0·066 |
| Phosphoric anhydride (P2O5) | 1·134 |
| Potassium | 3·328 |
| Sodium | 1·052 |
| Oxygen | 0·667 |
| Calcium phosphate | 0·114 |
| Magnesium phosphate | 0·073 |
| 1000 parts of liquor sanguinis contain— | |
| Water | 902·90 |
| Solid constituents | 97·10 |
| consisting of— | |
| Fibrin | 4·05 |
| Albumen | 78·84 |
| Fat | 1·72 |
| Extractive matters | 3·94 |
| Mineral substances | 8·55 |
| Chlorine | 3·644 |
| Sulphuric anhydride (SO3) | 0·115 |
| Phosphoric anhydride (P2O5) | 0·191 |
| Potassium | 0·323 |
| Sodium | 3·341 |
| Oxygen | 0·403 |
| Calcium phosphate | 0·311 |
| Magnesium phosphate | 0·222 |
The ash of blood contains about 6·84 per cent. of ferric oxide. (Lehmann.)
The following table gives the results of the average composition of human blood in man and woman, according to the analyses of Becquerel and Rodie:
| Male. | Female. | ||||
| Specific gravity of defibrinated blood | 1·0600 | 1·0575 | |||
| Specific gravity of serum | 1·0280 | 1·0274 | |||
| Water | 779·00 | 791·00 | |||
| Fibrin | 2·20 | 2·20 | |||
| Fatty Matters | Serolin | 1·60: | 0·02 | 1·62: | 0·02 |
| Phosphorised fat | 0·49 | 0·46 | |||
| Cholesterin | 0·09 | 0·09 | |||
| Saponified fat | 1·00 | 1·05 | |||
| Albumen | 69·40 | 70·50 | |||
| Blood-corpuscles | 141·10 | 127·20 | |||
| Extractive matters | 6·80 | 7·40 | |||
| ——— | ——— | ||||
| 1000·10 | 1000·02 | ||||
| Salts | Sodium chloride | 3·10 | 3·90 | ||
| Other soluble salts | 2·50 | 2·90 | |||
| Earthy phosphates | 0·33 | 0·35 | |||
| Metallic iron | 0·57 | 0·54 | |||
| ——— | ——— | ||||
| 6·50 | 7·69 | ||||
The blood also contains, in solution, oxygen, nitrogen, carbonic acid, as well as a free alkaline carbonate, urea, and small traces of alcohol have also been detected in normal blood.
The following report of a commission composed of MM. Mialhe, Mayel, Lefort, and Cornil, appointed to devise the best method for the examination of blood stains, was published in 1873. The following translation of the report appeared in the ‘Chemical News’ of December 5th, 1873.
1st. When the stain is of recent date, or supposed to be so, the red corpuscles should be particularly examined, and every care taken to preserve them without change. The stains must not be washed with water, so that the hæmatin may not be altered. After insisting on the microscopic characters of the blood stains, isolated or compared with those of various animals, the commission enumerates with care the fluids which are destructive or preservative of blood-corpuscles. Among the first, water, and particularly hot water, acetic, gallic, hydrochloric, and sulphuric acids; and of alkalies, potash and soda, even in weak solution, and ether and chloroform, also many other reagents, so alter the blood-corpuscles as to cause them to entirely disappear. Alcohol, chromic and picric acids, and bichromate of potash, preserve the corpuscles, though they alter their form. The preservative fluids are those whose composition approaches nearest to serum, such as the iodised serum of Schultze, an excellent preparation made with amniotic fluid, to which are added a few drops of the tincture of iodine, so as to give it the colour of white wine; or, better, a fluid composed321 thus; white of egg, 30 grams; distilled water, 270 grams; and chloride of sodium, 40 grams; or even a fluid containing 0·5 per cent. of chloride of sodium, or 5 or 6 per cent. of sulphate of sodium. If the stains be wetted and softened by these fluids, and then examined, white and red corpuscles and fibroid particles will be observed.
2nd. In more difficult cases, when the microscope, owing to the alterations which time has effected in the hæmatin, can give but vague information, examination by the spectroscope and chemical analysis enables us to arrive at precise results. The use of these means being less known, and also more delicate, requires special study.
1. Spectrum analysis. Colouring matters have the power of absorbing certain coloured rays of white light—the same always for the same substance. This is the principle upon which spectroscopic examination is based. If into any analysing tube filled with water a few drops of solution of hæmoglobin be introduced, till it has the colour of peach-blossoms, the luminous rays of the spectrum passing through this fluid present two bands of absorption, in the lines D and E of Frauenhofer, in the yellow and the green. The same fact would be observed if a few drops of blood were substituted for hæmoglobin in the analysis.
In a case of doubt the hæmoglobin of the blood could be reduced by adding to this latter a reducing body. Destroyed hæmoglobin has a different spectrum from oxygenated hæmoglobin, a single absorption band as large as the two former bands united, and a little to the left of Frauenhofer’s line D.
2. In blood in a state of decomposition, or which has been treated with acids or caustic alkalies, hæmoglobin is changed into a new substance; hæmatin is formed, which, combined with hydrochloric acid, gives definite crystals.
In order to obtain them we must proceed thus:—A small fragment of dried blood is placed on a glass slide; it is dissolved in a drop of water, and a minute portion of sea-salt added. It is covered with a thin slide, and pure acetic acid is made to pass between the two slides, and it is heated over a spirit-lamp to boiling-point; acetic acid is again added, and it is heated afresh; and this is repeated till the crystals are obtained.
They are rhomboidal, of a dirty brown colour, quite characteristic, and require to be seen with a magnifying power of three hundred or four hundred diameters. With the smallest quantity of blood these two reactions can always be produced—the spectrum examination and the crystals of hydrochlorate of hæmatin; and they are so certain that the existence of one alone enables one to affirm the presence of blood.
3. The third process, though not so exact as the preceding, ought, nevertheless, never to be neglected. If to a very small quantity of blood dissolved in a little water be added a few drops of tincture of guaiacum and of binoxide of hydrogen, a persistent blue colour is immediately produced; but this very sensitive reaction can be obtained with other organic matter, such as nasal mucus, saliva, &c.; it therefore only gives a probability. We must proceed in the following manner:—A tincture of guaiacum is prepared with alcohol at 83 degrees, and guaiacum resin; a mixture of sulphuric ether and binoxide of hydrogen is also made, and enclosed in a stoppered bottle, and kept under water in the dark. This preparation is less liable to change than pure oxygenated waters. The object stained with blood, if it be white, is put into a little cup, then moistened with water to dissolve out the blood stain, and washed in distilled water; this water is then submitted to the action of these reagents.
If the thing stained be coloured, and the stain little or not at all visible, it must be moistened, and then pressed between two or three sheets of white blotting-paper, and tried first with the guaiacum. If the stain be of blood a reddish or brown spot will form on the paper.
One of the sheets should be treated with ammonia, and the stain will become crimson or green. A second sheet treated with tincture of guaiacum and ozonised ether will give a blue colour more or less intense, according to the quantity of the blood.
To recapitulate:—1. If the stains or scales of blood appear recent, the corpuscles may, after the necessary precautions, be examined under the microscope, and their presence, diameter, &c. observed, which will enable one to diagnose the origin of the blood, whether human or animal. 2. If the stains be old and the blood changed, the reaction with the tincture of guaiacum would make the presence of blood probable; but its actual presence cannot be affirmed without spectrum examination or the production of crystals of hydrochlorate of hæmatin; one of the two is sufficient. It is unnecessary to add that these reactions do not show whether the blood is human or animal.
Bullocks’ blood has of late years, more especially in France, come into use as a remedy for anæmia and pulmonary phthisis. A correspondent, writing from Paris to the ‘Medical Times and Gazette’ in 1872, says: “It is a curious sight to see the number of patients of both sexes and of all ranks and ages, who flock to the slaughter-house every morning to drink of the still fuming blood of the oxen slaughtered for the table. I was struck with the facility with which young ladies take to it, and I have heard many say that they prefer it to cod-liver oil.”
In a paper read in 1872 before the Academy of Sciences in Paris by M. Boussingault, detailing his researches into the composition of blood, the author expressed his surprise that bullock’s blood was not more generally used as a food, as it contains all the constituents of a322 perfect aliment. According to the above chemist, of all nutritive substances the blood of animals contains the largest amount of iron. In man, Boussingault found in 100 grammes of blood 51 milligrammes of iron; in that of the ox, 55 milligrammes; of the pig, 59 milligrammes; and in that of the frog, 42 milligrammes. But it was not only in red blood that iron was found, Boussingault detected it in white blood also; and he found the blood of snails to contain as much iron as that of the ox or calf.
A simple and ingenious method for the therapeutic administration of the serum of the blood of sheep and oxen has been lately devised by Dr Francis Vacher, the medical officer of Birkenhead. Dr Vacher takes the blood of these animals, allows it to stand until it clots, removes the clot, and dries it at a gentle heat in a hot-air chamber. By this means he obtains a nearly odourless and comparatively tasteless powder, which is ten times the strength of fresh serum. To this preparation he gives the name “serum sanguinis exsiccatum.” He believes that his dried serum will prove a valuable nutrient in consumption, scrofula, diabetes, and loss of flesh.
Uses, &c. That of bullocks is employed for the clarification of wines and syrups; also in the preparation of adhesive cements, as the vehicle in coarse paint for outdoor work, as a manure, as a bleaching powder, to make pure animal charcoal, and for several other purposes. The blood of sheep, pigs, and bullocks, mixed with flour or oatmeal, and seasoned, is eaten by the common people, but it is rather indigestible, and apt to induce disease. Gut-skins stuffed with this mixture form “black puddings.”
Bullock’s blood, dried by exposure in thin layers to a current of air, at a heat under 125°, and then reduced to powder, is exported in large quantities to the colonies, where it is used, as a ‘clarifier,’ in the sugar-works. Dried at a temperature ranging between 212° to 220°, then coarsely powdered, and the dusty portion sifted off, it is much used by fraudulent dealers to adulterate grain-musk. See Charcoal (Animal), Globulin, Hæmatosin, Plasma, Serum, Stains, Vision, &c.
Blood-purifying Tea, Gout and Rheumatic (Franz Wilhelm, Neunkirchen). Equal parts of senna leaves, sarsaparilla root, liquorice, rad. tritici, red sandalwood, bittersweet stalks, cut small and mixed. (Hager.)
Blood-purifying Tea (F. Köller, Graz). Senna leaves, 32 parts; guaiacum wood, 10 parts; juniper wood, restharrow root, rad. tritici, dandelion root, chicory root, of each 8 parts; alder bark, 3 parts; sassafras, 2 parts; star-anise, 5 parts, dirty and worm-eaten, roughly chopped, and mixed. (Hager.)
Blood, Spit′ting of. See Hæmoptysis.
Blood, Vom′iting of. See Stomach Diseases.
BLOOD′-ROOT. Syn. Red′-root, Puccoon′; Sanguina′′ria, L. The sanguinār′ia Canaden′sis (Linn.), a papaveraceous plant of North America; also its root (SANGUINA′′RIA, Ph. U. S.), which is the part used in medicine. Juice, blood-red, used in dyeing. In small doses (3 to 5 gr.) it is stimulant, diaphoretic, and expectorant; in large ones (10 to 20 gr.), narcotic, emetic, and purgative. The powder is sometimes used as an escharotic. See Sanguinarine.
BLOOD′STONE. A hard compact variety of hæmatite used to form burnishers. The name is also applied by lapidaries to the heliotrope.
BLOOM. In perfumery, &c., a name given to several calorific skin-cosmetics, of which the following are examples:—
Bloom of Almonds (ah′-mŭndz). Syn. Al′mond-bloom. Prop. Boil 1 oz. of ground Brazil-wood in 21⁄2 pints of soft water for 30 minutes, adding the juice of two lemons towards the end; strain, and add 3⁄4 oz. of isinglass, 1⁄4 oz. of powdered cochineal, 1 oz. of alum, and 1⁄2 oz. of borax; boil again for 4 or 5 minutes, and strain through muslin. Glass or earthenware vessels must be used, as metals injure its colour.
Bloom of Roses. Prep. 1. Dried red rose leaves, 11⁄2 oz.; boiling water, 1 pint; infuse in glass or earthenware for 2 hours, press out the liquor, and add the juice of 3 large lemons; the next day filter, or decant the clear portion. Both the above should be kept in a cool place, otherwise they soon spoil. A little spirit of wine (3 or 4 fl. oz. to the pint) is sometimes added to them to remove this objection. They are greatly inferior to the following:—
2. Carmine, 1⁄4 oz.; strong liquor of ammonia (not weaker than ·900), 1 oz.; put them into a stoppered bottle, set it in a cool place, and occasionally agitate it for two or three days, to effect a solution; then add of rose-water, 1 pint; and, after admixture, further add of esprit de rose, 1⁄2 fl. oz.; pure rectified spirit, 1 fl. oz.; again well agitate, and set the whole aside for a week; lastly, decant the clear portion from the dregs (if any), for use or sale. Very fine. A cheaper article is made by omitting a portion of the carmine, and the whole of the esprit and spirit; and a still inferior one by substituting 11⁄2 oz. of silver-grain cochineal (in powder) for the carmine, with digestion for a week in the ammonia previously diluted with one half of the water.
Bloom of Youth, or Liquid Pearl (G. W. Laird, New York). A colourless liquid holding in suspension 34 per cent. of zinc oxide entirely free from lead. (Chandler.)
BLOTE. To prepare or cure by drying and smoking; now only applied to fish.
BLO′TER. Syn. Bloat′er. A bloted fish; appr., a herring slightly salted, and only very slightly dried and smoked.
BLOW′PIPE (blō′-). Syn. Chalumeau, Fr.; Löthrohr, Ger. An instrument by means of which the flame of a candle or lamp, or a gas-jet, is directed upon any substance placed323 to receive it, which is thus subjected to an intense heat. The blowpipe is to the artist and the experimentalist what the wind-furnace is to the artisan; but it is proportionately more powerful, convenient, and economical.
Beginners are usually unable to maintain a continued stream of air from the jet of this instrument, although the doing so is really a very simple affair. The operation merely depends on a little artifice in using it, which is more difficult to describe than to acquire. The effect intended to be produced is a continual stream of air for many minutes, if necessary, without interruption, even for an instant. This is done by simply applying the tongue to the roof of the mouth, so as to interrupt the communication between the mouth and the passage of the nostrils; by which means the operator is at liberty to breathe through the nose, at the same time that by the muscles of the lips he forces a continued stream of air from the anterior part of the mouth through the blowpipe. When the mouth begins to be empty it is replenished by the lungs in an instant, while the tongue is withdrawn from the roof of the mouth, and replaced again in the same manner as in pronouncing the monosyllable tut. In this way the stream of air may be continued for a long time without fatigue, provided the flame be not urged too impetuously; and even should it be so urged no other inconvenience will be felt than that of slight fatigue of the muscles of the lips.
The hottest portion of the flame produced by the action of the blowpipe is at the tip of the outer white flame, which has also the property of rapidly burning or oxidising substances placed in it which are susceptible of such a change; and it is hence commonly called the OXIDISING FLAME. The interior blue flame is, for a like reason, called the DEOXIDISING or REDUCING FLAME, as it possesses the property of extracting oxygen from most bodies capable of being so affected.
Substances to be submitted to the action of the blowpipe-flame are placed on a support, which is either a piece of charcoal, or a wire or small spoon of platinum, gold, or silver, as the case may require. Sometimes a plate of cyanite is used. Pine-wood charcoal is preferred for this purpose; and the sides, not the ends of the fibres, are presented to the flame. When a very intense heat is required, the substance operated on should not exceed the size of half a peppercorn.
Several characteristic colour reactions may often be obtained in the examination of a substance for analysis, by fusing a small portion of it, with a bead of microcosmic salt, and exposing it for some time to the outer flame of the blowpipe. If the substance dissolve readily in the salt and rather copiously to a clear bead whilst hot, and is of a blue colour by candle light inclining to violet, it denotes COBALT. If it be green, upon cooling blue; in the reducing flame after cooling, red—COPPER. If green, particularly fine on cooling, unaltered in the reducing flame, CHROMIUM. If brownish red, on cooling light yellow or colourless; in this reducing flame, red whilst hot, yellow whilst cooling, then greenish—IRON. If reddish to brownish red, on cooling yellow to reddish yellow or colourless; in the reducing flame unaltered—NICKEL. If yellowish-brown, on cooling light yellow or colourless; in the reducing flame almost colourless, and blackish-grey on cooling—BISMUTH. If light yellowish to opal, when cold, rather dull; in the reducing flame whitish-grey—SILVER. If amethyst-red, especially on cooling; colourless in the reducing flame, not quite clear—MANGANESE. If the bead remains clear on cooling, ANTIMONY, ALUMINA, ZINC, CADMIUM, LEAD, LIME, and MAGNESIA are indicated, the latter five when added in somewhat large proportion to the microcosmic salt, give enamel white beads. The bead of oxide of LEAD saturated is yellowish. If the bead becomes enamel-white on cooling, even where only a small portion of the powder has been added to the microcosmic salt—BARYTA and STRONTIA are indicated.
If the substance dissolves in the microcosmic salt slowly and only in small quantity, the bead being colourless and remaining so after cooling, the undissolved portion looking semi-transparent, and if upon the addition of a little sesquioxide of iron it acquires the characteristic colour of an iron bead—this denotes SILICIC ACID.
For producing extreme degrees of heat the flame is blown with a jet of oxygen gas, the instrument being then called an OXYGEN BLOWPIPE; or a mixture of oxygen and hydrogen is burned, when it is called an OXY-HYDROGEN BLOWPIPE. The heat produced by the last is so great that no substance can stand exposure to it, even the most refractory native compounds being immediately fused. Gold is volatilised, and iron is rapidly consumed the instant it is placed in the flame.
 1. Hemming’s safety-jet for the oxy-hydrogen blowpipe.
1. Hemming’s safety-jet for the oxy-hydrogen blowpipe.The principal varieties of the blowpipe in324 general use are figured in the engravings above.
Beside the above there are several other varieties of the blowpipe occasionally employed; one in which the air is expelled by the pressure of a column of water, and hence called the HYDROSTATIC BLOWPIPE; another, in which the flame is blown with the vapour of boiling alcohol, is named the SPIRIT-BLOWPIPE.
Blowpipe, Herapath. For sealing and bending glass tubes and constructing glass apparatus of various forms, it is convenient to have the blowpipe mounted on a fixed support, and when a flame of considerable power is required, the blast must be supplied by bellows worked with the foot. A very convenient form of blowpipe for these purposes is that invented by Herapath, and represented in the following figure, a is a flexible tube attached to a stop-cock (b), which communicates with a tube (c d), bent at right angles at d, where a T-shaped tube (e f g) slips on by means of the piece f. The blow-pipe jet (h i) passes into the longer arm of the T-piece, and fits somewhat tightly; k l is a second piece of flexible tube, terminating in a mouthpiece, or connected with a blowing apparatus. On turning on the gas, it passes in the direction marked by the arrows, and is to be inflamed at e. On blowing with the mouth, or by means of a pair of bellows, into the tube k l, the ignited gas takes the form of a blow-pipe flame of great power, the nature of which is entirely under control by means of the stop-cock b, and also by regulating the quantity of air supplied through the tube (k l). The T-shaped piece is movable at f, so that the jet may be directed to any position. The apparatus may be mounted on a heavy foot, and connected with the gas-supply, by means of the flexible tube, so that it can be placed in any required position on the laboratory table; or it may be permanently fixed on a table specially devoted to the purpose, and having beneath it a pair of bellows worked by a treadle.
A simple and inexpensive apparatus for supplying a continuous blast of air for blowpipe or other purpose is figured below.
It consists essentially of a tin tube (to which is fixed a branch tube open to the air), through which water may be driven from a supply tap into a properly fitted bottle. Air becomes thus entangled with the water in its course through the tube, and carried with it into the bottle. The water is then got rid of by means of a syphon, and the air is conducted by an elastic tube to the blow-pipe.
BLUB′BER. Syn. Ad′eps balæna′′rum, L.; Graisse de baleine, Fr. The soft fat of whales, and of other large sea-animals, from which the oil (TRAIN′ OIL, WHALE′ OIL) is obtained by heat.
Blubber, Sea. The popular name of several species of marine animals of the genus medusa, having a body resembling a large mass of jelly. They are very plentiful in some parts of the coast of England, and are said to form a rich and cheap manure for pasture and arable land. They are used at the rate of about 1 ton to every 20 or 30 loads of mould, together with a chaldron of lime, per acre. In 3 or 4 months the land is usually found in prime condition. Pilchards, and other fish that swarm upon our coasts, and for which there is not a ready market, may be used in the same way, and are much richer, being, when properly managed, but little inferior to guano.
BLUE (bl′ōō). Syn. Cæru′leus, L.; Bleu, Fr.; Blau, Ger. Of the colour of the clear sky, or of any shade of it, whether lighter or darker; subst., a blue colour, blueness (COL′OR CÆRU′LEUS, L.); or a blue, colouring material or pigment (CÆRU′LEUM, L.).325
Blue Dye. Syn. Teinte bleue, Fr.; Blau farbe, Ger. The most permanent blue is that given by indigo, and particularly by what is called the ‘indigo-vat.’ A variety of shades, of great beauty, and considerable permanence, may also be given by the ‘Prussian-blue process.’ Cheaper blues are commonly dyed with logwood. Each of these is noticed at length under their respective heads. The following are also employed, and are well adapted for common goods, on the small scale and for domestic use.
1. Give the goods a mordant of alum, or of acetate of alumina (‘red liquor’), then rinse them well, and boil them in a bath of logwood, to which a small quantity of blue vitriol has been added; lastly, rinse and dry.
2. Boil the goods for a short time in a bath of logwood; then add to the liquor tartar and verdigris, in the proportion of 1 oz. of each to every lb. of logwood employed; and again boil for a short time.
3. Give the goods a mordant of tartar; lift, add a little chromate of potash; again work for 15 or 20 minutes, and rinse; next boil in a bath of logwood, adding towards the last a few grains more of the chromate; again boil, and finish. The whole quantity of chromate used should not exceed 1⁄4 oz. to each lb. of logwood taken for the bath. Very dark.
4. Bilberries, elder-berries, mulberries, privet-berries, and several other like vegetable substances, may be used to dye blue, as above, instead of logwood.
Obs. By increasing the proportion of alum or red-liquor the colour verges on purple; and by employing a little acetate of iron or green copperas, the darker shades of blue are produced. Verdigris, blue vitriol, and alkalies, turn it more on the blue; whilst a mordant of tin imparts a violet cast. If much more chromate be used than that ordered the result is a blue-black. See Dyeing, Indigo, Logwood, Mordants, Prussian blue, &c.
Blue Pig′ments. Syn. Cæru′lea, &c., L. The preparation of the principal blue pigments of commerce is described under their respective names. In the following list those for which directions are given are of a miscellaneous and less usual character.
Az′ure. Syn. Azure Blue. A name frequently given to smalts. That of the oil-painter is ultramarine; that of the ancients is noticed below. See Ultramarine, &c.
Blue, Barth’s. See Indigo, Sulphate of.
Blue, Berlin. Prussian blue.
Blue, Bice. Native blue carbonate of copper, prepared by grinding and elutriation. That of the shops is generally a factitious compound made from smalts.
Blue, Carmine. See Carmine and Indigo, Sulphate of.
Blue, Char′coal. Carbonised vine-stalks are triturated with an equal weight of salt of tartar or pearlash, the mixture put into a crucible, and heated over the fire until it ceases to swell, the mass being kept well stirred all the time; when cold, it is dissolved in water, and the excess of alkali saturated with dilute sulphuric acid. The liquid becomes blue, and a dark precipitate falls down, which turns of a brilliant blue colour when dried and cautiously heated.
Blue, Chi′na. Syn. Roy′al Smalts. The crude oxide of cobalt, or zaffre, is ground with an equal weight of potash, and about eight times its weight of felspar, the mixture submitted to fusion in a crucible, and when cold reduced to an impalpable powder. Used to paint pottery, and as a blue pigment.
Blue, Co′balt. Syn. Cobalt′ic Az′ure. This is commonly prepared by one or other of the following formulæ:—
1. Zaffre, 1 lb., is dissolved in nitric acid (diluted with an equal weight of water), 3⁄4 lb., by digestion for some hours; the solution is evaporated nearly to dryness, and the residuum redissolved in warm water; to this solution, after filtration, a solution of phosphate of soda is added as long as a precipitate forms; this last is collected on a filter, washed with cold water, and mixed, whilst still moist, with 8 times its weight of fresh-precipitated hydrate of alumina; the paste is then dried, and exposed to a cherry-red heat in a crucible, after which the mass is cooled and reduced to a very fine powder.
2. A solution of nitrate of cobalt is precipitated with ammonia-alum, and the precipitate washed, dried, and exposed to a cherry-red heat, as before. The products of the above formulæ are very beautiful and permanent. See Cobalto-ultramarine.
Egyp′tian Az′ure. Alexan′drian Frit, Azure of the Ancients. A mixture of carbonate of soda, 1 lb.; calcined flints, 11⁄2 lb.; copper filings, 1⁄4 lb. (all in fine powder); fused together in a crucible for 2 or 3 hours, and when cold, reduced to an impalpable powder. A beautiful and unchangeable sky-blue colour. Used in both oil and fresco painting; and as a substitute for smalts, of which, indeed, it is a variety.
In′digo (which see).
Blue, I′ron. Fer′ric blue. Ordinary phosphate of iron prepared by precipitating a solution of protosulphate of iron with another of phosphate of soda, the resulting powder being washed, and dried at a gentle heat. A lively sky-blue colour, but without much depth or body.
Blue, Lake. See Lakes and Indigo, Sulphate of.
Blue, Molybde′num. From sulphuret of molybdenum, dissolved in nitric acid, and some tin filings and a little muriatic acid added. After digestion for some time the clear liquid is poured off, and evaporated to dryness. The resulting powder is then mixed with moist hydrate of alumina (as in making cobalt blue), heated to a very dull red, and when it has326 again become cold, reduced to powder. Used both as a paint and an enamel-colour.
Blue, Moun′tain. Native carbonate of copper, mixed with more or less earthy matter, reduced to fine powder. That of the shops is often factitious.
Blue, Par′is. Prussian blue.
Blue, Pow′der. Smalts.
Blue, Prus′′sian (which see).
Blue, Queen’s. See Thumb-blue (below).
Blue, San′der’s. Ultramarine-ashes.
Blue, Sax′on. Saxon Az′ure. A compound of hydrate of alumina and Prussian blue, prepared as follows:—
1. To sulphate of iron, 1 oz.; and alum, 8 oz.; dissolved in water, 1 gall.; add, simultaneously, separate solutions of prussiate of potash and common pearlash, until they cease to produce a precipitate; after repose collect the deposit, wash it well with water, and dry it.
2. A solution of sulphate of iron is precipitated with another of prussiate of potash, and instantly mixed with the precipitate which has just been obtained by treating a solution of alum with a solution of pearlash; the mixed precipitates being finally treated as before.
Smalts (which see; also China-blue and Egyptian Azure, above).
Blue, Thénard’s. See Ultramarine (Cobaltic).
Blue, Thumb′. Cake′-blue, Crown′-blue, Fig′-blue, Knob′-blue, Mech′lenburg-blue, (mēk′-), Queen’s-blue, Stone-blue, &c. Names given to the lump-blue used in laundries, which vary according to the quality and the particular form given to it.
Prep. 1. A mixture of powdered starch with sufficient indigo (in impalpable powder) to give the necessary colour, made into a stiff dough with starch-paste, and then formed into lumps or cakes of the desired size and shape, and dried. This forms the ordinary ‘washerwoman’s blue’ of the shops.
2. As the last, but substituting cæruleo-sulphate of potassa or blue carmine[226] for the ‘powdered indigo’ ordered in the last formula. Very fine.
3. As No. 1, but substituting whiting for the powdered starch and weak size, or a decoction of Irish moss for the starch-paste. Inferior.
Uses, &c. Employed by laundresses to impart a faint blue tinge to linen, in order to increase its apparent whiteness. The common forms given to it are that of small balls of about 3⁄4 to 1 inch in diameter; the same, but rather larger, and pinched with the thumb and finger in three directions, so as to leave corresponding depressions (THUMB-BLUE); and cakes, which are cut out of the mass, previously rolled into a sheet, by a suitably shaped cutter.
Blue, Turnbull’s. Ferridcyanide of iron (which see; also Turnbull’s Blue).
Blue Verditer. See Verditer.
Ultramarine′ (-rēne′), U.-blue. See Ultramarine.
BLUSH′ING. Syn. Ru′bor, Rube′do, L. In physiology, &c., the red glow on the cheeks or face occasioned by confusion, bashfulness, surprise, or shame.
Blushing is caused by a sudden increase in the quantity and velocity of the blood in the capillaries, occasioning their turgescence; and, consequently, a heightening of the natural pale-reddish hue of the skin. It is referable to sudden mental emotions of an exciting character, such as surprise, confusion, consciousness of slight, injury, or indignity, and the like. Emotions of a depressing character frequently produce an opposite effect. This is termed pallor, and depends on the rush of blood from the skin and surface of the body upon the internal organs. The first, though often unpleasant, is never dangerous; the last always so. The cure of the habit of blushing consists in persisting efforts to maintain a sufficient degree of presence of mind and self-confidence to permit of reflection, or a calm view of the exciting circumstance, instead of sinking into a state of temporary mental imbecility and helpless confusion.
‘BLUTANDRANG UND LUFTROHREN-VERSCHLEIMUNG’ (remedy for congestion and obstruction of the air-vessels), manufactured and sold by the inventor, C. Tänzer, 18, Kesselstrasse, Berlin,’ is the title of a twelve-page pamphlet. For cold in the head, the apparatus, which consists of a small linen cushion to bind over the mouth, is moistened with 10 to 15 drops of the fluid. The fluid (150 grammes) is a mixture of spirit of wine and acetic ether, in which some arnica, milfoil, &c., have been macerated. (Hager.)
BLUTHENHARZ—FLOWER RESIN (Kwizda, Kornenburg). Against barrenness in domestic animals. A mixture of 9 parts powdered Bergundy pitch with 1 part pine pollen, 3⁄4 oz. (Hager.)
BOARDS, to make White. Boards may be rendered white and clean by scrubbing them, instead of with soap, with a mixture composed of one part of freshly slaked lime and three parts of white sand.
BOCKBIERESSENZ, for the artificial imitation of bockbier. A tincture of 1 part lupulin, 2 parts pyroligneous acid, and 8 parts spirit of wine. (Hager.)
BOG SPAVIN. In horses, a distension of the bursa or sheath of the true hock joint. Mr Finlay Dun prescribes rest; high-heeled shoe, fomentation, cold water, spring truss, counter-irritation, firing-iron; seton.
BOIL (boyl). Syn. Furun′culus, L.; Furoncle, Fr.; Beule, Eiterstock, Ger. In surgery, a well-known inflammatory tumour,327 of a superficial and more or less temporary character, which generally terminates by suppuration.
Boils (furun′culi) generally attack the healthy and robust during the period of youth and early manhood, and seldom trouble persons who have arrived at the middle age of life.
Treatm., &c. When boils begin to appear, and exhibit persistency by daily enlargement and increasing pain, suppuration should be promoted by warm poultices of bread and linseed-meal, to which a little fat or oil may be added, to prevent their getting hard. If poultices are inconvenient, warm and stimulating embrocations, or exposure to the vapour of hot water, or the application of stimulating plasters, may be adopted instead. When the tumour is sufficiently ‘ripe,’ the matter should be evacuated by gentle pressure, and the wound dressed with a little simple ointment spread on a piece of clean lint or linen. The diet may be full and liberal until the maturation of the tumour and the discharge of the matter, when it should be lessened, and the bowels kept gently open by saline purgatives, as Epsom-salt or cream of tartar. When there is a disposition in the constitution to the formation of boils, the bowels should be kept at all times regular, and tonics, as bark or steel, had recourse to, with the frequent use of sea-bathing when possible. An occasional dose of the Abernethy medicines (which see) also often prevents their recurrence. A course of sarsaparilla may be likewise taken with advantage. See Abscess, Tumours, &c.
Dr Sydney Ringer prescribes a 1⁄16th grain of sulphide of sodium, mixed with sugar of milk, three or four times a day on the tongue; but this should only be administered under medical supervision.
Treatment for Horses and Cattle.—Fomentations; poultices containing belladonna, cold water, carbolic acid dressing, counter-irritants, laxatives, sulphites, and chlorates.
BOIL′ERS. See Incrustation and Steam.
BOIL′ING. In cookery, the operation of dressing food in water at the point of ebullition, or one very closely approaching it. The practice of cooking animal food by boiling, although exceedingly simple, and often most convenient, is neither judicious nor economical when the broth or liquid in which it has been dressed is to be rejected as waste; as in this way the most nutritious portion of the flesh of animals, consisting of soluble saline and other matter required for the formation of bone, and the nutrition of the muscular tissues, &c., is to a great extent lost. This particularly applies to small pieces so dressed, and to those presenting a large surface to the action of the water in proportion to their weight. Large pieces of meat suffer less in proportion than smaller ones, for the same reason; but even with them the outside should be rejected, as it is both insipid and innutritious compared with the interior portion. To reduce the solvent and deteriorating action of the water to the lowest possible point, the articles to be boiled should not be put into the water until it is in a state of full ebullition, which should be maintained for 5 or 6 minutes afterwards, by which time the surface and the parts lying immediately beneath it will have become, to a certain degree, hardened, and will then act as a protective shield to the inner portion of the mass. The boiling being continued for 5 or 6 minutes cold water is added, until the temperature becomes about 150° F., and the cooking of the joint is carried on at this heat until the meat is done: meat loses nearly a fourth of its weight in boiling, salt meat, which is intended to be eaten cold, should be allowed to cool in the water in which it has been boiled. The practice of dressing meat by putting it into cold water, which is then gradually raised to the boiling-point, cannot be too much censured. A 1⁄4 of an hour per lb. for dressing young meat, poultry, and small pieces, and 20 minutes per lb. for old, tough, and larger ones are the usual times allowed by cooks for the purpose. See Bouilli, Food, &c.
BOIL′ING-POINT. See Ebullition.
BOIS DURCE (bwah dŭr-sā). [Fr.] The substance invented in France, and to which this name is given, is made from sawdust, which, under the influence of a high temperature and the enormous pressure of 600 tons, acquires a degree of hardness very much exceeding that of ordinary wood. It has a very fine grain, and is unaffected by atmospherical variations; but its principal merit is its adaptation to moulding, so that by the most economical processes forms and impressions are given to it which it would require, in any other way, considerable labour and workmanship.
BOLAS. Sweet light cakes which, according to Mrs Rundell, are prepared as follows:—Into flour, 2 lbs., pour of warm milk, 3⁄4 pint, a small teacupful of yeast, and 6 eggs; make a dough, add of butter 1 lb. (by degrees), and set it in a warm place to rise for an hour; then mix in of powdered sugar 1 lb.; and make the mass into cakes; put these into cups or tins previously well buttered, and ornament the top with candied orange or lemon peel; lastly bake them. See Cakes.
BOLDO (nat. ord. Monimiaceæ). A shrub growing in the Chilian Andes. The bark is used in tanning, and the wood makes a good charcoal. It is reported to be useful in affections of the liver and digestive organs. It has been employed as a tonic in cases where quinine is inadmissible. In large doses it provokes vomiting. The powder of the dried leaves is a sternutatory. See a paper by M. Claude Verne, translated into the ‘Pharm. Journ.,’ 3rd series, v, 405.
BOLE. Syn. Bo′lus, L.; Terre bolaire, &c., Fr. The name of several argillaceous328 minerals, varying in colour from white to yellow, red, and brown, which they owe chiefly to iron. See Ochres and Red and Brown Pigments.
BOLOG′′NA PHI′AL (-lawn′-yă). See Phials.
BO′LUS, [L., Eng.] Syn. Bol, Fr. Boluses, in pharmacy and medicine, are small, roundish masses of medicinal substances, which are taken in the same manner as pills, which they resemble, except in their larger size. Those persons who object to swallowing them in their common state may wrap them in soft paper, or introduce them into the emptied husks of raisins or grapes.
Boluses (bo′lī, L.) are prepared with the same ingredients, and in a similar manner to pills (which see).
Bolus, Guaiacum (Horne). Guaiacum resin 1⁄2 drachm, elder rob, enough to make into a bolus. Formerly given in quinsy.
Bolus for Ague. (The bolus ad quartanum of the French Hospital). Peruvian bark 1 ounce; carbonate of potash 1 drachm; tartarised antimony 15 grains; syrup, a sufficient quantity, one to be taken every four hours during the intermission.
Bolus, Vermifuge (Dr Campbell). Basilie powder one scruple, conserve of wormwood, a sufficient quantity to make into one bolus for an adult. (Foy.) Powdered pomegranate root 1 drachm, assafœtida half a drachm, croton oil 3 or 4 drops, syrup sufficient. Divide into 15 boluses; 5 daily for tapeworm. (French Hospital.) Wormseed 1 scruple, calomel 5 grains, camphor 15 grains, syrup sufficient. Make into 3 doses; one, two, or three in the day.
BON′-BON (bōng′-bōng). [Fr.] A sugarplum. See Confectionery and Sugarplums.
BONBONS VERMIFUGES OF GAROZ. A bonbon containing 15 centigrammes of scammony, and 2 centigrammes of santonin. (Reveil.)
BONE. Syn. Os, L., Fr.; Bein, Knochen, Ger.; Bán, Sax. The hard substance forming the interior skeleton of animals, or any single part of it.
| Human bones. | Ox bones. | |
| Animal matter soluble in boiling water | 32·17 | 33·30 |
| Vascular substance | 1·13 | |
| Phosphate of calcium, with a little fluoride of calcium | 53·04 | 57·35 |
| Carbonate of calcium | 11·30 | 3·85 |
| Phosphate of magnesium | 1·16 | 2·05 |
| Chloride of sodium and other salts | 1·20 | 3·45 |
| 100· | 100· |
The soluble animal matter is chiefly fat and gelatin.
Uses, &c. The bones of animals are employed for various purposes in the arts, manufactures, and domestic economy. Those of good meat form most excellent materials for making soups and gravies, as is well known to every cook. In France, soup is extensively made by subjecting bruised bones to a steam heat of 2 or 3 days’ continuance. In England the same is commonly effected in an iron Papin’s digester. When the earthy matter of a bone is dissolved out by digesting it in a large quantity of very dilute hydrochloric acid, a lump of gelatine is obtained, which, after being well washed with water, is equal to isinglass for all the purposes of making soups and jellies. The following is the process recommended by Proust for making the best of bones, in hospitals, gaols, and similar establishments:—
The bones, crushed small, are to be boiled for 15 minutes in a kettle of water, and the fat (which is fit for all common purposes) skimmed off as soon as cold. The bones are then to be ground, and boiled in 8 to 10 times their weight of water (of which that already used must form a part), until half of it is wasted, when a very nutritious jelly will be obtained. Iron vessels should alone be used in this process, as the jelly and soup act upon copper, brass, and the other common metals. The bones of fresh meat are the most productive; those of boiled meat come next, whilst those of roasted meat scarcely afford any jelly. As ‘boning’ meat before cooking is now a very general practice, a quantity of fresh bones may always be obtained.
Bones are, for the most part, WROUGHT, TURNED, BLEACHED, and DYED in a similar manner to ivory, but with less care, owing to their inexpensive and coarser character. Before being submitted to any of these operations they are, however, first submitted to long boiling, to deprive them of grease.
The bones of living animals may be dyed by mixing madder with their food. The bones of young pigeons may thus be tinged of a rose colour in 24 hours, and of a deep scarlet in 3 days; but the bones of adult animals take a fortnight to acquire even a rose colour. The bones nearest the heart become tinged the soonest. In the same way extract of logwood tinges the bones of young pigeons purple. See Bleaching, Dyeing, Ivory, &c.
In all manufacturing processes in which bones are operated upon, foul vapours, unless special precautions are observed, will be thrown off, to the great annoyance and discomfort of those living near the building where the operations are performed.
To avoid this the offensive vapours should always be carried by a flue made for the purpose into the furnace-fire, and there consumed. But this will not remedy another source of annoyance which arises from the disgusting stench caused by the putrefaction of the flesh adhering to the bones, which lie in heaps about the premises.329
The trade of a bone-boiler comes under the head of offensive trades (see ‘Public Health Acts,’ s. 112-114), and is under the control and regulation of an urban sanitary authority, which has also the power of preventing the bone-boiling being carried on within its district if it thinks proper.
BONE′-ASH. Impure triphosphate of calcium, obtained by calcining bones to whiteness, and reducing the ash to fine powder. Used to make pure phosphate of calcium, to form cupels, &c.; also sold for burnt hartshorn.
BONE′-DUST. Syn. Bone-manure. Bones (previously boiled for their grease) ground to different degrees of coarseness, in a mill. It is sown along with the seed in a drill. Wheat thus treated is said to yield 30 to 50 per cent, more weight in straw and grain than by the common methods. Turnip and other light soils it renders more than ordinarily productive. Bone manure is much used in the west of Yorkshire, Holderness, and Lincolnshire. The usual quantity per acre is 70 bushels, when used alone; but when mixed with ashes or other common manure, 30 bushels per acre is said to be enough. When coarse, and applied in the same manner as other manures, it has been found to remain upwards of seven years in the ground, the productiveness of which it has increased during the whole time.
BONE′-GLUE. See Gelatin.
BONE′-GREASE. From refuse bones, bruised, boiled in water, and the broth skimmed when cold. Prod. 1⁄8th to 1⁄4th of the weight of the dry bones. (Proust.) Used for making soap and candles. See Charcoal, Animal.
BONE′-PHOSPHATE. See Tribasic Phosphate of Lime.
BONE′-SHAVINGS. Syn. Bone′-dust (Turners’), Bone-turnings. This, by boiling with water, yields a beautiful jelly, which is nearly equal to that produced from hartshorn and ivory shavings, for which it is very frequently sold. Used to make jellies and blancmanges, to stiffen straw bonnets, &c.
BONE-SPAVIN. A bony enlargement on the antero-internal parts of the hock in horses. In recent cases it is best to apply cold applications, but in protracted and chronic cases, hot fomentations will be found best. In case of these failing, recourse should be had to blistering or firing, or if need be to a seton.
BOOK′BINDING (-bīnd-). Although a full description of the various operations of this well-known art, or handicraft, does not properly fall within the province of this work, a brief notice of them will probably, in many cases, prove useful to the amateur and the emigrant:—
The process of binding books is divided into several distinct operations, which, in large establishments, are usually performed by different persons; such a method being found to produce greater expedition, and better work, than when the whole is done by one person.
The sheets received from the hands of the printer are—
1. Folded, which is done correctly by observing the ‘marks’ or ‘signatures’ at the bottom of the pages. As the sheets are folded they are laid upon each other in proper order, and are ready to undergo—
2. The operation of beating. This is performed by either laying them upon a large stone and striking them with a heavy smoothed-faced hammer, or by passing them through a rolling-press. The former method is usually adopted in the small way, and the latter on the large scale.
3. The sheets are next fastened to bands, which is done by taking them up one by one, and sewing them to pieces of cord, stretched in a little frame screwed or fastened to the counter or table, called the sewing press. (See engr.) The number of bands used is generally 6 for a folio, 5 for a quarto, and so on proportionally, less than 4 being seldom employed even for small sizes. The ends of the cords being cut off to within about 2 inches of the back, the sheets are ready for—
4. Glueing. The back being knocked into shape with a hammer, and the sheets placed in the cutting-press, which is then slightly screwed up, melted glue is thinly and evenly applied. After a short time, to permit it to become sufficiently set and hard, the book is removed from the press, and the back properly adjusted with a hammer, when it is again put into the cutting-press, where it is screwed up very tight, and is then ready for—
5. Cutting. The instrument employed for this purpose is of a peculiar shape, and called a plough or plough-knife, which consists of a stout flat knife, double-edged at the ‘cutting point,’ firmly set in a kind of frame, in which it may be adjusted by screws.
6. Affixing the boards. The bands are now scraped out fine at the ends, and fastened to the pasteboard intended to form the covers, which is then properly adjusted, and further shaped, if necessary, with a large pair of shears. The edges now undergo the operation of—
7. Sprinkling, gilding, or other adornment. The first is performed with a stiff brush made of hog’s bristles, dipped in the colour; the brush being held in the one hand, and the hairs moved with the other, so as to scatter the colour in minute drops equally over the surface.
8. The external covering of leather, fancy cloth, or paper, is now applied, having been330 previously soaked in paste, to make it properly adhere. One or more of the blank leaves of the book are next pasted against the inside of the cover, to screen the ends that are turned over when the book is finished; or for choice work, is handed to a ‘finisher’ for—
9. Lettering, gilding, &c. Ordinary gold-leaf is applied by means of white of egg, the pattern being given by pressure with heated brass tools, having the design or letters on their surfaces. The whole is then glazed over with white of egg and polished.
10. Burnishing book edges. This is performed with a wolf’s or dog’s tooth, or a steel burnisher. Place the books in a screw press, with boards on each side of them, and other boards distributed between each volume. First rub the edges well with the tooth to give them a lustre. After sprinkling, or staining, or when the edges have become dry, burnish the front; then turning the press, burnish the edges at the top and bottom of the volume. Burnish the gilt edges in the same manner, after having applied the gold; but observe in gilding to put the gold first upon the front, and allow it to dry; and on no account commence the burnishing until the gold is quite dry.
The succession of the above operations sometimes slightly varies with the workmen, and with the nature of the binding. The examination of a bound book during their perusal will, however, render the whole quite familiar to the reader.
There are several varieties of binding, of which only the following deserve notice here:—
Boards. A book rather loosely done up, without cutting the edges, and covered with coloured paper or cloth, is said to be in ‘boards.’
Cloth, Cloth-binding. This is the style of binding in which the majority of works are now issued. It admits of great neatness and even beauty, is cheap, and when well executed is very durable.—The prepared cloth (hard-glazed or varnished calico), cut by a pattern to the proper size, is passed rapidly between the engraved cylinders of a rolling-press, by which the design is given to it. Paste is now applied to each piece of cloth, which is then placed over the volume previously prepared to receive it. In many cases the covers are prepared separately before being embossed, and are afterwards fastened in the finished state to the book by means of a piece of canvas or calico previously affixed to its back for the purpose, when all that is required is to paste the ends of it to the inside of the boards, with the last blank leaf over it. Books in cloth are seldom cut at the edges, unless they are otherwise highly finished.
Half-binding. Books forwarded in boards, and finished with leather backs and corners, are said to be ‘half-bound.’
Leather-binding. A book is only said to be ‘bound,’ or ‘fully-bound,’ when both its backs and sides are wholly covered with one piece of leather.—The leather is wetted by immersion in water, wrung or squeezed, stretched on a smooth board, cut to the proper size, pared thin on the edges, and covered with paste. It is then applied to the book (previously forwarded in boards, and cut), drawn tightly over it, turned down on the inside, rubbed smooth with a folding-stick, and otherwise adjusted; after which it is placed in some suitable situation, at a distance from a fire, to dry.
Rough calf requires to be damped on the grain side with a sponge and water before pasting and covering.
Russia-leather is well soaked in water for an hour, taken out, beaten, and rubbed; after which the paste is well worked into the flesh side before covering.
Morocco is first ‘grained’ by rubbing it on a board, with the grain side inside, and, after being pasted, left to soak for about a quarter of an hour; after which it is drawn on with a piece of woollen cloth, to preserve the grain.
Roan is either soaked in water, or left to soak when pasted.
School-binding. Originally applied to school-books strongly sewn and ‘done up’ in sheep-skin, which was either left of a plain brown, or sprinkled or marbled with copperas water. Similar works of a cheaper class are now often ‘done up’ in canvas, brown-holland, and even coarse and strong coloured glazed calico.
Concluding Remarks. Numerous patents for improvements in binding books, several of which possess very great merit and usefulness, have been obtained during the last 30 years. Among these, one known as ‘Hancock’s Patent Binding,’ from its extreme novelty, simplicity, durability, and inexpensiveness, deserves a passing notice here. By Mr Wm. Hancock’s method the sheets are folded in double leaves, and by being properly placed together and adjusted (by setting them vertically, with the edges forming the back of the book downwards, in a concave mould so formed that whilst giving shape it may leave the whole breadth, and nearly the whole length exposed), and firmly secured by a few turns of packthread, the book is subjected to the action of a press, and a strong and quick-drying solution of india rubber is smeared over the back with the finger, when the whole is left for 3 or 4 hours, or longer, to dry. The operation is repeated as often as necessary, after which fillets of cloth are cemented on with the same varnish, and the book is ready to have the boards attached. The sheets of books that cannot be folded in ‘double leaves’ may be strongly stitched through, separately, before adjusting them in the mould. In this way several of the usual operations of binding are dispensed with. We most willingly bear testimony to the strength and durability of this method, as well as to the331 great convenience it affords in allowing the books to open perfectly flat upon a table, or to be distorted in any possible manner, without injury to their backs. It is, undoubtedly, the best way of binding books for travellers. The Editor of the last edition of this work once had a large trunk of books, among which was a massive volume bound on Hancock’s plan. All the rest were nearly torn to pieces by a few months’ journey, but this one remained uninjured even after five years, during which time it accompanied him in his travels, extending, collectively, to upwards of 23,000 miles. See Gilding, Marbling, Sprinkles, Stains, &c.
BOONEKAMP OF MAAGBITTER. Dried orange berries, 100 grammes; bitter orange peel, 30 grammes; gentian root, 60 grammes; cascarilla bark, 30 grammes; turmeric, 15 grammes; cinnamon, 25 grammes; cloves, 15 grammes; rhubarb, 71⁄2 grammes; 90 per cent. spirit, 750 grammes: water, 1650 grammes; star-anise oil, 40 drops; sugar, 250 grammes; digested, expressed, and filtered. (Hager.)
BOOTS and SHOES. The cleaning of boots and shoes forms no unimportant part of the domestic duties of a large establishment; as on it being properly performed depend both their appearance and durability. A votary of St Crispin, in whom we place considerable reliance, assures us that to effect this object in the best style, all that is necessary is to employ very little blacking (merely enough to moisten the surface of the leather), and to brush it off whilst still damp. Never make the surface wet, nor allow the blacking to dry before applying the polishing brush. For this purpose a portion only of the boot or shoe should be attended to at a time. The dirt is, of course, to be carefully brushed off before applying the blacking. When it is desired to restore the shape of a boot or shoe, as well as to clean it, boot trees may be used. Of the brushes, we are told that there should be at least three—one (dirt brush) with bristles stiff, but not wiry nor scratchy, to remove mud and dirt; another (blacking-brush), with fine, flexible hair, and plenty of it, for applying the blacking; and a third (polishing-brush), covered with long, fine, springy, and slightly stiff hair, for giving the polish. The employment of inferior or worn-out brushes is said to be false economy, and proves particularly destructive to the lighter classes of leather.
The occasional use of a little oil or grease to the uppers of boots and shoes increases their softness and durability, as well as the ‘depth,’ but not the brilliancy of the polish, from common blacking. For this purpose some good tallow or ‘dubbing’ may be used; the absorption being aided by a very gentle heat. The soles or bottoms of new boots and shoes may be thoroughly saturated with similar substances, by which means their durability will be fully doubled. The common practice among the shoemakers is to moisten the surface of the leather with a wet sponge before applying the oil or grease; by which (they say) its pores are opened and its absorbent powers increased.
Varnish for Boots and Shoes.—1. Boil together in a pipkin one pint of linseed oil; 1⁄2 lb. of mutton suet, the same quantity of beeswax, and a small piece of resin; and when the mixture becomes milk-warm, apply it with a hair brush. After two applications the articles will become waterproof. Great caution must be exercised in melting the above ingredients, lest the mixture boils over, and so give rise to a conflagration.
2. Common tar may be made warm and brushed over the soles of boots or shoes. These latter are then put near the fire so that the tar may be absorbed. When the absorption has taken place, a second or third application may be given with advantage. This application is not suitable for the upper leathers.
3. India-rubber varnish will be found very useful for anointing the upper leather of boots and shoes; but the lower parts, which are exposed to the wear and tear caused by friction with the ground, are but little benefited by its application.
Patent-leather boots and shoes are best cleaned with a little sweet oil or milk (preferably the first), the dirt having been previously removed in the usual way.
India-rubber goloshes and overshoes may be cleaned with a sponge or brush, and water, care being taken not to wet the linings. The same applies to gutta percha. See Blacking, Leather, Waterproofing, &c.
The reasons why boots and shoes so commonly cause corns, and fatigue, and give pain in wear, are explained in our article on the Feet (which see).
Paramount in importance to the appearance of boots or shoes on the wearer is the desideratum, not only of having them so made as to ensure personal comfort in walking, but additionally to have them so constructed as to protect the feet from wet during damp and rainy weather. The evils arising from getting the feet damp cannot be overstated; amongst them are to be included—cold, cough, bronchitis, inflammation of the lungs, and rheumatism. In those inheriting a constitutional consumptive taint, a cold caught from wearing damp or leaky boots has very frequently been known to have precipitated the disease, that has ended in more or less speedy death. Hence arises not only the duty of changing damp boots or shoes as soon as ever the opportunity offers, but the wisdom of adopting the preventive precaution of wearing them of such stout construction as to be impervious to water during rainy weather. If the dangers arising from a neglect of this advice are visited with such serious consequences upon adults and grown persons, they affect infants and children with even far greater intensity, because of the much more tender and sensitive332 organisation of the latter. It therefore behoves every mother not only to see that her children are shod with good thick boots or shoes, but to take especial care that whenever these are damp they are removed at once.
Mr Chavasse, in his excellent work, ‘Counsel to a Mother,’ recommends “boots for walking out of doors and shoes for the house.” He adds, “that the constant wearing of boots in the house is weakening to the ankles, as weakening as tight lacing is to the waist; indeed it acts much in the same way, namely, by wasting away, by pressure, the ligaments of the ankles, as stays waste away the muscles of the waist.” In support of his argument he quotes Dr Humphrey, who says, “The notion is in both instances fortified by the fact that those persons who have been accustomed to the pressure either upon the ankle or upon the waist, feel a want of it when it is removed, and are uncomfortable without it. They forget, or are unconscious, that the feeling of the want has been engendered by the appliance, and that had they never resorted to the latter, they would never have experienced the former. The deduction to be drawn from Dr Hutchinson’s opinion is that no more fertile source of weak ankles exists than that of wearing laced boots during childhood. Boots with elastic sides, as exerting much more equal pressure, and allowing full scope for the ankles to play, are far preferable to tightly laced-up boots.
BOOT-POW′DER. French chalk reduced to powder by scraping or grating. Used to facilitate the ‘getting on’ of new or tight boots, a little of it being rubbed on the insides of the backs, heels, and insteps.
BOOT-TOP LIQ′UID. Syn. Boot′-top composi′′tion. There are numerous articles of this class extant, but, with few exceptions, they are most unchemical mixtures, not infrequently containing ingredients which are either unnecessary, or opposed to the action of the rest. The following are examples:—
Prep. a. White-top:—1. Oxalic acid and white vitriol, of each 1 oz.; water, 11⁄2 pint; dissolve. It is applied with a sponge, the leather having been previously washed with water; after a short time it is washed off with water, when the boot-tops are either dried in a current of air or by a gentle heat; they are lastly either polished with a brush, so as to appear like new leather, or they are left rough, as the case may require.
2. Sour milk, 1 quart; butter of antimony, cream of tartar, tartaric acid, and burnt alum, of each 2 oz.; mix.
3. Sour milk (skimmed), 3 pints; cream of tartar, 2 oz.; alum and oxalic acid, of each 1 oz.
4. Alum, cream of tartar, magnesia, and oxalic acid, of each 1 oz.; salt of sorrel and sugar lead, of each 1⁄4 oz.; water, 1 quart. The preceding are for white tops.
b. Brown-top:—Alum, annatto, and oxalic acid, of each 1 oz.; isinglass and sugar of lead, of each 1⁄2 oz.; salt of sorrel, 1⁄4 oz.; water, 1 quart; boil for 10 minutes.
c. Saffron, 15 grains; boiling water, 2 oz.; infuse and strain. Add tincture of rhubarb 11⁄2 oz.; concentrated infusion of rhubarb, to make up to 4 oz.
BORACIC ACID (-răs′-). H3BO3. Syn. Boric acid, Sedative salt†, S. s. of vit′riol†; Acidum boracicum (-răs′-), L.; Acide boracique, A. borique, Fr.; Boraxsäure, &c., Ger. The pure acid is obtained from common borax. That of commerce is extracted from the boracic acid lagoons of Tuscany.
Prep. 1. Borax, 1 part; boiling water, 4 parts; dissolve, and add sulphuric acid until the solution acquires a distinctly acid reaction, for which purpose about 1⁄2 the weight of the borax will be required. As the solution cools, crystals of BORACIC ACID will be deposited. These may be purified by placing them on a filter, and washing them with a little very cold water, followed by re-solution, in boiling water, and recrystallisation. Nearly pure.
2. As the last, but substituting hydrochloric acid for the sulphuric acid, there ordered. Very nearly pure.
3. By exposing the product of the first crystallisation of either of the preceding formulæ to heat in a platinum crucible, and redissolving and recrystallising the residuum. Chemically pure. Used in analysis.
Prop., &c. Odourless; bitter-tasted; dissolves in 25 times its weight of cold water, and in 3 times its weight of boiling water; very soluble in alcohol, which then burns with a bright green flame; reddens litmus; browns turmeric-paper (properties characteristic of this substance); when strongly heated it forms a brittle glass (VITRIFIED BORACIC ACID) on cooling. The crystallised acid contains 3 atoms, or 43·5% of water. Its salts are called BO′′RATES.
Uses. Boracic acid was once administered internally, in large doses, as an anodyne, antispasmodic, and sedative, but is now scarcely ever employed as a medicine. The crude acid is used in the manufacture of borax; the pure acid in the manufacture of certain chemicals.
Boracic acid is extensively used in Sweden and other countries for the preservation of milk. Meat which has been soaked in a solution of the acid for a few seconds, and milk to which a small quantity has been added, will keep much longer than they would otherwise do. In Sweden alone boracic acid to the amount of 75,000l. was consumed in one year. It is said to be a perfectly harmless antiseptic.
BORACIC ANHYDRIDE. See Boric Anhydride.
BORATE. [Eng., Fr.] Syn. Bo′′ras, L.; Boraxsäure salze, Ger. A salt in which the hydrogen of boracic acid is replaced by a basic radical. The borates may be formed by either digesting the hydrate of the base in a333 solution of the acid, with the assistance of heat, or from a solution of borax and a soluble salt of the base, by double decomposition. They are all decomposed by the stronger acids.
Tests. The borates may be tested by digesting them in a slight excess of oil of vitriol, evaporating the resulting solution to dryness, powdering the residuum, and dissolving it in alcohol; the resulting solution possesses the property of burning with a green flame if the sample examined was a borate, or contained a notable quantity of one. See Boracic Acid.
BORAX. [Eng., Fr.; Ger., L., B. P.] 2NaBO2.B2O3. Syn. Bibo′′rate of so′da, Bo′′rate of s*, Subbo′′rate of s.†, Gold solder†*, Refined′ tinc′al†*; So′dæ bibo′′ras, S. bo′′ras, L.; Chrysocolle, &c., Fr.; Boraxsaures natron, &c., Ger. Commercial biborate of soda. Borax is obtained either by purifying native borate of soda (TINC′AL, TINC′AR), or by saturating crude boracic acid with the alkali. It is never prepared on the small scale unless for chemical analysis.
Prop. Crystals, six-sided prisms, which contain 10 equiv. of water, and effloresce in dry air; soluble in 20 parts of cold, and in 6 parts of boiling water; solution has an alkaline reaction on test-paper; by heat it loses its water of crystallisation, and at a higher temperature fuses to a glass-like substance (see below).
Pur. This may be ascertained by determining the quantity of sulphuric acid required to neutralise a given weight of the sample under examination, as indicated by litmus paper. Common salt and alum are frequently mixed with borax to lower the value. The first may be detected by a solution in hot water giving a curdy-white precipitate with nitrate of silver, soluble in ammonia; the last, by water of ammonia, giving a bulky-white pulverulent precipitate. The former must be distinguished from the white pulverulent precipitate of borate of silver, which is thrown down from pure borax.
Uses, &c. Borax is extensively employed as a flux for metals, for soldering, and in medicine. Internally it is diuretic, sedative, emmenagogue, and refrigerant, in doses of 15 to 40 gr.; externally, made into a gargle for sore throat, and in powder as a detergent in aphthæ, and ulcerations of the month. Dissolved in rose-water, it is used as a cosmetic; and mixed with about 8 times its weight of lard, forms a useful ointment in piles and sore nipples.
The ‘Comptes Rendus’ (lxxx, 473) contains the results of some experiments made by M. Schnetzler, with the view of testing the antifermentative and anti-putrefactive properties of borax.
When the leaves of the Elodea Canadensis were plunged into a concentrated solution of borax, the living matter of the cell was killed, and the same result followed with the fresh leaves and spores of the Vaucheria clavata, the spores of the grape fungus (Oidium sacchari), and of yeast moulds, &c. Infusoria, rotifera, and entomostraca, placed in water containing borax, quickly ceased to move and then died. The larvæ of frogs placed in a solution of borax were killed in less than an hour after immersion. M. Schnetzler thinks the deduction to be drawn from these facts is that borax ought to act antagonistically to fermentation, if this latter be a chemical phenomenon accomplished under the influence of the life of the yeast. To test the correctness of this hypothesis experiments were undertaken with a view of determining the action of borax upon fermentable matters.
Ripe grapes and currants after being kept two years in a concentrated solution of borax, in a closed vessel, presented no trace of fermentation, although, however well preserved, they were not eatable. As a counter test grapes were placed in a well-closed vessel filled with ordinary water, when after a time, according to the temperature, fermentation took place, with evolution of carbonic acid. Thirty cubic centimètres of fresh milk were placed in a test tube with one gram of borax. The cream quickly formed a rather thick layer on the upper portion. Although the test tube was closed by a cork a mould was formed upon the cream, but the remainder of the liquid underwent no acid fermentation, and retained during several months the appearance of very clear creamed milk, and although afterwards under the influence of summer heat the liquid became perfectly limpid, and deposited the casein as a soft white matter, neither the deposit nor the liquid had an acid taste, and after three months they still had the odour of fresh milk. Fresh milk put into a well-closed tube without borax underwent fermentation in two or three days. A piece of sheep’s brain treated with powdered borax, after eight days, although it evolved sulphuretted hydrogen, gave no indications of putrefaction, and after retaining a soft consistence during some months, became hard and almost horny without any disagreeable smell.
A pound of beef was placed in a concentrated solution of borax, in a tin case not hermetically sealed.
The liquid into which the colouring matter of the blood, and some of the soluble nitrogenous substances of the meat had diffused, was three times removed during a year and a half, and the meat washed with cold water; but at the end of the above time it had not the least odour of putrefaction. It was of a yellowish colour, but soft and tender as fresh meat. Removed from the borax solution the meat remained in the same state in the air. Beef, veal and portions of sheeps’ brains were placed in a vessel which was filled with solution of borax and hermetically sealed. The liquid soon became clear red, and this colour remained during several months without alteration. The334 meat presented not the least disagreeable smell as long as excess of air was prevented. Meat placed in water in a flask hermetically sealed became rotten in a few days.
The peculiar odour of the meat preserved in borax in contact with air the author considers to be due to the decomposition of matters which result from the metamorphosis of substances that constitute the muscular and intermuscular fibre. Although probably the use of borax will not be applicable to the preservation of meat for culinary purposes, the author considers that it may be economically substituted for alcohol in the preservation of anatomical specimens. Moreover, its power of suspending life in the lower organisms would seem to indicate its probable utilisation in the treatment of wounds, &c.
In support of the above views as to the antiseptic properties of borax, M. Schnetzler refers to a letter from an English traveller in California, who there observed that in a soil containing borax the carcass of a horse had, for four months, remained without decomposition, the flesh continuing perfectly fresh, and the eye retaining its clearness and brightness. For most of the proposed applications of salicylic acid to the preservation of milk, and the products derived from it, it is affirmed that borax is equally efficacious, and has the advantage of being cheaper and more convenient.
Borax, Glass of. Borax dried at a gentle heat, and then melted by increasing the heat until it forms a vitreous mass on being cooled. Used in soldering, and as a flux, particularly in blowpipe experiments.
BO′′RIC ACID. See Boracic Acid.
BORIC ANHYDRIDE. B2O3. Syn. Anhydrous boracic acid, Boracic anhydride, Boric oxide. The only known oxide of boron. It can be produced by burning boron in oxygen, in the air, or in nitrous oxide, but is most easily and economically prepared by strongly heating boracic acid so as to deprive it of water. It is a brittle vitreous solid, not volatilised by heat except in the presence of water. Dissolves in water, forming boracic acid. Its alcoholic solution burns with a green flame, like that of boracic acid.
BO′RON. B. The base of boracic acid. It was discovered by Homberg in 1702; but, from attracting little notice, was soon forgotten. It was rediscovered, almost simultaneously, by Sir H. Davy and by Gay-Lussac and Thénard, in 1807-8.
Prep. Boron is prepared by a process similar to that employed to obtain silicium:—Potassium and perfectly dry boracic acid, or, preferably, boro-fluoride of potassium, intimately mixed together, are placed in a glass adopter-tube, and submitted to a low red heat. When cold, the loose cork that fastened its mouth is removed, and hot water poured in, in successive portions, until the whole matter is detached and all its soluble portion dissolved; the liquid is next allowed to settle, and the precipitate washed first with a solution of sal-ammoniac, and afterwards with alcohol; the residuum (boron) is lastly dried in a capsule, and put into a well-stoppered phial.
Prop., &c. A solid, tasteless, and inodorous powder, of a dark greyish-brown colour. With sulphur it unites at high temperatures, forming sulphurets (sulphides of boron); and when placed in chlorine gas it spontaneously inflames, and a gaseous chloride of boron is formed. The compounds of boron with basic radicals are termed BORIDES.
Obs. Among the most remarkable of the recent discoveries in chemistry are those of MM. Wohler and Deville, relative to silica and boron. Each of these substances is now proved to exist in three very different states, analogous to the three known states of carbon, namely, charcoal, graphite, and diamond. The last of these states is, of course, the most interesting. Crystallised boron possesses a hardness, brightness, and refractive power comparable to those of the diamond; it burns in chlorine, without residue, and with circumstances resembling those of the combustion of diamond in oxygen; it is not acted on by any of the acids, and appears to be the least alterable of all the simple bodies. Its powder is already used in the arts, instead of diamond-dust; and it seems not improbable that, when obtained by the chemist in crystals of a larger size, it may rival even the diamond as a gem.
Some late experiments by Wohler and Deville seem to have established the fact, that the so-called “graphitoidal” boron is really a boride of aluminium. Its formation on fusing aluminium with amorphous boron or boric oxide appears to take place more particularly when the heat applied is neither very strong nor long continued.
Boron, Terflu′oride of. See Fluoboric Acid.
BOTHRIOCEPHALUS CORDATUS. Leuckart was the first to describe this creature, which is a parasitic worm infesting the human intestines. It is, however, much more commonly met with in dogs than in man. The annexed engraving depicts—b, the head (back view), magnified five diameters; b′, upper part of body and head, magnified two diameters; a is a portion of the worm, natural size. See Bothriocephalus Latus.
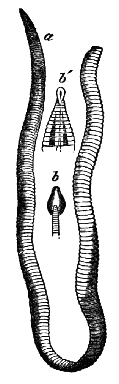
BOTHRIOCEPHALUS LATUS. A parasitic worm infesting the human intestines. Although classed with the tapeworms, it differs essentially from tænia. The head is of an elongated335 form, compressed with an anterior obtuse prominence into which the mouth opens.

The animal has the power of elongating and contracting the neck, so that it appears sometimes short, sometimes long. The joints or segments commence about three inches from the head; the anterior ones are nearly square, but the remainder are much elongated transversely. Each segment contains on its flat surface two orifices, the anterior connected with a male, the posterior with a female organ of generation. The parasite is of a brown colour, and from six to twenty feet in length.
Those who are affected by this worm never pass the single segments from the bowels, but void them in chains of many links. The ova are also frequently to be met with in the fæces; they are of an ovoid shape; the capsule is perfectly translucent, and the yolk is distinguishable. The yolk undergoes segmentation, and ultimately develops an embryo with six hooks at the anterior extremity, cased in a mantle studded with vibratory cilia, and the lid of the capsule then opens up; and the embryo escapes. If they do not obtain access to the intestines of an animal within a week, they lose their ciliated mantle and perish. Drinking-water is supposed to be the chief if not the only medium through which the parasite gains admissions to the intestines in man. It seems to be unknown in England, except when imported; but is common in Russia, Sweden, Norway, Lapland, Finland, Poland, and Switzerland.
BOTS. The larvæ of the gad-fly. The eggs are deposited by the female fly on the horse’s shoulder and on parts of the body within reach of the tongue, by which they are carried to the mouth and find their way to the stomach. They usually resist all attempts to expel them. The most promising treatment consists in rubbing down in hot water about 2 or 3 drms. each of aloes and assafœtida; and when the solution has cooled, adding to it 1 oz. each of turpentine and ether. Repeat this dose two or three times a week, omitting the aloes if necessary.
BOT′TLES (bŏt′lz). See Glass, Infancy, Lactation, Phials, &c.
BOTT′LING (bŏt′l-ĭng.) See Corks, Malt Liquors, Wine, &c.
BOUGIE (bōō′-zhē). [Fr.] Syn. Ce′reus, Cere′olus,[227] Cande′la probato′′ria*, L. In surgery, a long slender instrument, originally of wax,[228] introduced into the urethra, œsophagus, or rectum, in stricture and other diseases of those organs.
Prep. 1. (Prof. Pickel’s.) Amber (melted), 1 part; boiled oil, 3 parts; mix, cool a little, and further add of oil of turpentine, 1 part; spread the mixture, at 3 successive intervals, upon loose spun-silk cord or web; dry in a heat of 150° Fahr., and repeat the process until the instrument has acquired the proper size; lastly, polish it, first with pumice-stone, and afterwards with tripoli and oil. This is the original receipt of the once celebrated French professor Pickel, and is still generally used, slightly modified, on the Continent. At the present time, in Paris, a little caoutchouc, equal to about 1⁄20th of the weight of the oil employed, is generally added. For the best ELAS′TIC BOUGIES the process usually occupies from 6 to 8 weeks, to allow full time for the drying and hardening of the composition. When the bougie is required to be hollow, a piece of polished metallic wire is introduced into the axis of the silk; or tin-foil is rolled round the wire and the composition applied as before. When dry and hard the wire is withdrawn.
2. (Hunter’s.) Yellow wax, 2 parts; red lead, 3 parts; olive oil, 6 parts; slowly boiled together until combination takes place; strips of soft linen (rather wider at the one end than the other) are then dipped into the composition, rolled up firmly, and finished off on a polished slab.
3. (Piderit’s.) Olive oil, 1 part; wax, 6 parts; as before.
4. (Bell’s.) Lead-plaster, 11 parts; yellow wax, 4 parts; olive oil, 1 part.
5. (St. B. Hosp.) Wax, 12 parts; Chio turpentine, 4 parts; red sulphide of mercury, 1 part.
6. Caou′tchouc bougies:—In France, where ether is comparatively inexpensive, these are made by applying an ethereal solution of india rubber to the silk or foil prepared as before. In England, naphtha was, until recently, employed instead of ‘ether,’ but it furnishes a very inferior product. Now bisulphuret of carbon is generally used as the solvent. Sometimes strips of india rubber, previously boiled in water, or that have had their edges softened by moistening them with a little ether, or bisulphuret of carbon, are wound round the ‘wire or foil,’ and kept in their place by a piece of tape applied over them. They are afterwards carefully smoothed off and polished.
7. Gutta-per′cha bougies:—These are formed of gutta percha (previously softened by immersion in boiling water), by rolling it between plates of polished glass or marble. When skilfully prepared from the best (uncoloured) gutta percha, they are admirable instruments. A bougie of this description, of moderate size, and slightly oiled, or wetted with glycerin or gum-water, may be passed through the whole length of the urethra of a healthy person without causing the slightest pain. Gutta-percha catheters (hollow bougies) are still more flexible and easily introduced, and may remain in the urethra for a long time without causing irritation; an important advantage in such matters. The reader cannot, however, be too336 careful to avoid those made of coloured gutta percha, which, unfortunately, rapidly become very brittle by age. Those originally manufactured in this material were coloured black, and were constantly breaking whilst in use—a disaster from which several serious and even fatal cases ensued. There is no such danger to be dreaded from those made of the uncoloured material when of good quality.
BOULES DE NANCY. See Balls (Martial).
BOUILLI. [Fr.] A name frequently applied by cooks to dishes of boiled or stewed meat, as a refinement on its plain English synonymes. Thus, beef bouilli, beef in bouilli, &c., mean stewed or boiled beef, &c. As, however, the name is à la français, so must be the ‘accompaniments,’ which generally consist of herbs and vegetable seasoning in greater quantity and variety than is usually deemed essential for an humble dish of English boiled or stewed meat.
BOUILLON (bōōl′-yong). [Fr.] In cookery, broth, soup.
BOUQUET′ (bōō-kā′). [Fr.] A nosegay. In perfumery, highly scented spirits (esprits) adapted for the handkerchief are commonly called bouquets. The following are examples:—
Bouquet d’Amour. Prep. From esprits de rose, jasmin, violette, and cassie (flowers of acacia farnesiana), of each 2 parts; essences of musk and ambergris, of each 1 part; mix, and filter.
Bouquet de la Reine. Prep. 1. Essence of bergamot, 1 dr.; English oil of lavender, 25 drops; oil of cloves, aromatic vinegar, and essence of musk, of each 10 drops; alcohol, 1 fl. oz.; mix.
2. Oils of bergamot and lavender, of each 30 drops; neroli, 15 drops; oils of verbena and cloves, of each 5 drops; essences of musk, ambergris, and jasmin, of each 1⁄2 dr.; rectified spirit of wine (strongest, scentless), 2 fl. oz.; mix. A much-esteemed perfume.
BRA′GRAS. Tar, black resin, and the dregs of strained resin, melted together.
BRAIN (brāne). Syn. Brains‡; Cer′ebrum, L.; Cerveau, Fr.; Gehirn, Hirn, Ger. The soft whitish mass of nerve-matter contained in the skulls of animals, and, in man, supposed to be the seat of the soul and the mind.
Brains. (In cookery.) There appears to be scarcely anything which is at all eatable that the ingenuity and taste of the modern cook does not appropriate to his purposes, and clothe with delectability, or transform into something execrable. We observe that our chef de cuisine—no unimportant personage—has taxed every viscera and brought together every novelty and dainty to humour and excite the appetite. Animals which were guiltless of brains whilst living, are found by him to possess excellent ones when dead, from which he prepares a variety of miniature dishes which are truly novel and inviting. Let frugal housewives for the future carefully value their brains, and apply them to useful purposes in a double sense. When cleaned, washed, blanched, and flavoured with the necessary seasoning, they may be formed into a variety of hors-d’œuvres creditable to any table. Mrs Rundell tells us that “beat up with a little white pepper and salt, a sage-leaf or two (scalded and finely chopped), and the yelk of an egg, and fried, they make excellent cakes, fritters, &c.”
BRAMAELIXIR—GENUINE ASIATIC STOMACH BITTER (Ch. Rama Ayen, Hamburg). Cardamoms, cinnamon, cloves, of each 15 grammes; galangal, ginger, zedoary, pepper, of each 30 grammes; wormwood oil, 15 drops; 90 per cent. spirit, 830 grammes; water, 330 grammes; digest and filter. (Hager.)
BRAN. Syn. Fur′fur, L.; Bran, Son, Fr.; Kleie, Ger. The inner husk or proper coat of the cereal grains, sifted from the flour; appr., that of wheat. Comp. 100 parts of bran contain albuminoid bodies, 13·80; oil, 5·56; starch, fibre, &c., 61·67; ash, 6·11; water, 12·85.
Uses, &c. The bran of wheat, diffused through hot water, is largely employed by the calico-printers to remove the colouring matter from those parts of their goods which are not mordanted. A handful mixed with a pail of warm water forms an excellent emollient foot-bath. Infused in hot water (bran-tea), and sweetened, it forms a popular demulcent, much used in coughs and hoarseness, and which, taken in quantity, proves gently laxative. It also forms an excellent manure, and, from containing the ammonio-magnesian phosphate, is especially adapted as a ‘dressing’ for potatoes. It is frequently mixed with flour, and made into bread (bran-bread), which is eaten by the poorer orders for economy, and by the higher classes because it is recommended by the faculty as being more wholesome than white wheaten-bread.
Bran Mash. Put half a peck of bran or pollard into a bucket and pour on to it enough scalding water to wet it thoroughly; stir well with a stick or work with the hands; and let it stand, covered up, till new-milk warm. If a horse is not in work on Sunday, it is a good custom to give it on Saturday evening a bran mash in lieu of a feed of corn. Bran mash is cooling and slightly laxative. The bran should always be freshly ground. When intended to be nutritive, oats should be scalded with the bran.
BRANDISH’S ALKALINE (Liqueur de potasse des Anglais, Solutio Alkalina Anglica), used in England to add to meat and vegetables about to be cooked, to help in “drawing” tea and coffee, and as a medicine to neutralise acidity of the stomach and lubricate the digestive passages [die Verdanungswege schlüpfriger zu machen]. Preparation:—Crude carbonate of potash 3 parts, wood ashes 1 part, quicklime 1 part, warm water 40 parts. Add to the water the lime, carbonate, and ashes, digest one day, and filter. (Hager.)337
BRANDRETH’S PILLS, much used as a purging pill in North America, consist of gamboge, podophyllin, the inspissated juice of pokeberries, saffron adulterated with turmeric, powdered cloves, and peppermint oil. Gamboge is stated to be present in Brandreth’s pills on the authority of two American druggists and one dealer. The action of the pills does not, however, correspond with that ingredient, for in two persons five pills produced no loose stools. (Hager.)
BRAN′DY. Syn. Spir′itus Gal′licus, S. vi′ni Gal′lici (-sī; B. P.), A′qua vi′tæ†*, L.; Eau-de-vie, Fr.; Branntwein, Cognac, Ger.; Bran′dywine†. A well-known spirituous liquor obtained by the distillation of the wine of grapes. The name is also often, though improperly, given to the spirit distilled from other liquors, and particularly from the fermented juice of fruits; but in this case usually with some qualifying epithet.
When first distilled, brandy, like other spirituous liquors, is colourless (WHITE BRANDY), and continues so if kept in glass or stoneware; but if stored in new oak casks, as is usually the case, it gradually acquires a yellowish tint from the wood (PALE BRANDY). The deep colour that this spirit frequently possesses when it reaches the consumer is imparted to it by the addition of a little burnt sugar (caramel). Catechu, or terra japonica, in powder or solution, is also sometimes added to give a roughness to the spirit. The original intention was merely to imitate the appearance acquired by brandy from great age, when kept in wood; but in process of time the thing has come to be overdone. The natural colour which the spirit receives from the cask, however long it may be kept in it, never exceeds a light amber tint, about equal to that of pale Jamaica rum. Nothing, however, will now please the public taste but a spirit of lively and full ‘brandy-colour,’ as it is called. The consequence is that more colouring is commonly added than is compatible with a rich appearance or a very fine flavour.
The brandies most esteemed in England are imported from France, and are those of Cognac and Armagnac, the preference being generally given to the former. The brandies of Rochelle and Bordeaux come next in quality; while those obtained from Portugal, Spain, and Italy are very inferior.
The constituents of pure brandy are alcohol and water, together with small quantities of a volatile oil, acetic acid, acetic ether, œnanthic ether, colouring matter, and tannin. It is from the presence of the two ethers that the spirit derives its characteristic smell and flavour. The amount of absolute alcohol in brandy varies from 45 to 55 per cent. When first imported it is generally 1 or 2 over-proof, but its strength decreases by age, and by the time it is taken from the bonded store for sale, it is seldom stronger than 3 or 4 under-proof. Pure brandies of the best quality, even when new, seldom exceed proof, and are generally a little below it. The reason of this is that they are but slightly rectified, as redistillation tends to injure the ethereal oils, upon which the flavour of the brandy depends.
The quality and flavour of the brandy imported from France vary, and often considerably, from that which is drunk at the best tables on the Continent; this principally arises from it being prepared, or, as it is technically termed, ‘made up,’ for the London market; which means lowering it by the addition of plain spirit, colouring, &c. This is done to any extent desired by the English purchaser, and the quantity and prices of the substances so added are regularly set out in the invoice. The strength at which foreign brandy is sold in England varies from proof to 33 under-proof. In large quantities, and from bond, the strength, of course, depends much upon the age and quality of the spirit; a fine old brandy being, perhaps, 15 or 17 u. p., while one of the last year’s vintage, of a commoner quality, may be as strong as 2 u. p., or even 1 u. p. These matters are familiar to every experienced brandy dealer.
In France there are several varieties of brandy, which are known by names descriptive of their qualities, source, and strengths:—
“Eau-de-vie supérieure” is obtained from pale white wines by skilful distillation, and is remarkable for its rich and delicate flavour. It forms the finest variety of Cognac brandy, both ‘white’ and ‘pale,’ of the English drinker, being seldom artificially coloured. Its deepest tint, though long kept in wood, never exceeds a pale amber; and hence, even when thus coloured, it is frequently called ‘white brandy’ by the uninitiated.
“Eau-de-vie ordinaire,” or common brandy, is distilled from inferior or spoilt white or red wines; average sp. gr. about 0·9476 (from 22 to 27 u. p.). It forms the ‘ordinary brandy’ of the taverns and hotels; and, after being ‘made up’ with plain spirit to 1 or 2 u. p., a very large portion of that which is exported.
Of each of the above varieties there are numerous degrees of qualities, which are further increased in number by their admixture, and by the addition to them of plain spirit.
“Eau-de-vie de marc.” From the lees of sour, damaged, and inferior red wines, the marc or cake of grapes, &c., distilled by a quick fire, to drive over as much essential oil and flavouring matter as possible. Coarse flavoured and inferior. Used chiefly to mix with other brandy, or to flavour plain spirit.
“Eau-de-vie seconde.” The weak spirit that passes over, after the receiver has been changed. Very weak and inferior.
“Eau-de-vie à preuve d’Hollande.” Sp. gr. ·941 to ·942 (18 to 20 u. p.). The common strength at which brandy is retailed in France, and that at which it stands the ‘proof’ or ‘bead.’
“Eau-de-vie à preuve d’huile.” Sp. gr.338 ·9185 (about 23° Baumé, or 11⁄4 o. p.); pure, olive oil just sinks in it. It is the strongest brandy kept for retail sale in France.
“Eau-de-vie forte.” From common brandy distilled at a low temperature. It answers to our spirit of wine. Sp. gr. ·839 (38° Baumé, or 55° o. p.).
“Esprit de vin” is brandy or spirit, carefully rectified to ·861 (28° Baumé, or 42 o. p., and upwards).
Pur., &c. The method of determining the strength of brandy is explained under ‘Alcoholometry,’ Of the large quantity of this liquor consumed in England, we can assure the reader that a small fraction only escapes adulteration. Pure French brandy is indeed an article quite unattainable by the small consumer. The brandy of our shops and taverns is not only systematically ‘lowered’ a little (with spirit of wine or British brandy) by the wholesale dealer, but it undergoes a like process, but to a much greater extent, at the hands of the retailer. The only method to obtain perfectly pure brandy is either to take it direct from the bond store, or to buy it of some known respectable party, and to pay a price that offers no inducement to dishonesty. When this cannot be done, British brandy had better be at once purchased, by which money will be saved, and a more wholesome article obtained.
French brandy, as already noticed, is commonly ‘lowered’ with water, malt brandy, and spirit of wine, by which its original flavour is more or less weakened and injured. This species of adulteration is best detected by the palate. Another, and no very uncommon fraud practised by the retailers, is to reduce their brandy with a large quantity of water. As a natural consequence their liquor suffers so greatly in flavour, and its deficiency in alcohol becomes so apparent, that they soon see the necessity of either abandoning the nefarious practice, or resorting to others of a less harmless character to disguise it. The latter alternative is commonly adopted. An excess of burnt sugar is immediately introduced into the spirit, followed by sundry portions of cayenne pepper, grains of paradise, horse-radish, acetic ether, &c., to give it a pungency and ‘make-believe strength’ that “passes muster” with the petty consumer. This fraud may be detected by gently evaporating a little of the suspected liquor in a spoon or glass capsule, when the acrid matter, colouring, and sugar will be left behind, and may be readily detected by their flavour, sweetness, glutinosity, &c. A little perfectly pure brandy evaporated in a similar manner (on a watch-glass, for instance), merely leaves a trifling discoloration on the surface of the glass. Genuine French brandy always reddens blue litmus paper, from containing a little acetic acid; the old coloured varieties are also blackened by a solution of a persalt of iron. Another test for caramel (burnt sugar) is, let a small quantity of the brandy be well shaken with one sixth of its volume of white of egg, and the precipitate formed allowed to deposit, or be removed by filtration; the clear liquid ought to be colourless. Should caramel be present, however, it will retain its colour. Sometimes brandy is contaminated with a small quantity of lead or copper derived from the apparatus or utensils with which it has been prepared or measured. Sugar of lead has also sometimes been used by the ignorant dealer to clarify it. The presence of these highly deleterious substances may be detected in the following manner:—
1. Copper:—a. A small piece of clean polished iron or steel immersed in the suspected liquid for a short time (with agitation) becomes coated with a film of metallic copper, when that metal is present. To facilitate the precipitation of the metal, the sample under examination may be slightly acidulated with a few drops of pure acetic acid. Minute traces of copper may sometimes be detected on the surface of the iron with a lens, which would be passed over unnoticed by the naked eye.
b. (Böttger.) A little of the brandy is to be agitated with a few drops of pure olive oil. The latter will acquire a green colour if copper be present.
2. Lead:—a. Hydrosulphuric acid and sulphide of ammonium produce a black precipitate or discoloration in brandy containing lead.—b. A solution of sulphate of soda (Glauber-salts), or water soured with sulphuric acid, produces a heavy white precipitate, which turns black when moistened with sulphide of ammonium.
3. Methylated spirit is detected by rubbing a little of the suspected brandy on the hands, and then drawing a long breath with the hands over the mouth. The peculiar odour of the methylated spirit, if present, then becomes evident. This is a test, however, requiring practice and experience.
4. To determine the Alcoholic Strength.—Put 100 c. c. of the brandy into a small retort, or into a flask, with a lateral tube, and distil to dryness, or nearly so, condensing the distillate by means of a suitable receiver, and let the alcohol by means of the processes detailed under ALCOHOLOMETRY. The brandy may be roughly tested for fusel oil by burning a little of it in a dish, and depressing over the flame a saucer or other cold piece of porcelain. If a black stain is left, some of the lower alcohols are very probably present, and should be looked for by distilling half a pint of the spirit, and examining the later for heavier products. The vinic alcohol being the most volatile comes over first, the heavier fusel oil remaining until the later stages.
Concluding Remarks. In the ‘trade,’ the addition of water (‘liquor’) to spirit is technically called ‘reducing,’ whilst absolute adulteration is known under the questionable name ‘improving.’ Both of these operations have now been so long practised with impunity as339 to form the leading qualifications demanded in a cellarman.
The following formulæ for ‘reducing’ brandy are those of two large wholesale dealers, who consider themselves much more honest than their brethren in the same line:
1. Cognac brandy (10 u. p.), 20 galls.; British brandy (17 u. p.), 5 galls.; water, 41⁄2 galls. Strength of mixture, 25 u. p.
2. To 72 galls, of full-flavoured French brandy (5 u. p.) are added 10 galls, of spirit of wine (58 o. p.); 25 galls. of water, and 1 pint of good colouring. The whole is then well ‘rummaged up,’ and allowed to stand for two days, when it is fit for use. Strength of mixture, 22 u. p.
A liqueur, sold in London under the name of “brandy improver,” or “brandy essence,” consists of a thin sugar syrup, flavoured with acetic ether and essence of cayenne, and coloured with burnt sugar. It is said to heighten the true Cognac flavour, and restore lost alcoholic strength.
Brandy, British. Syn. Malt Brandy, &c. For a long time this liquor was distilled from spoiled wine and the dregs of wine, both British and foreign, mixed with beer-bottoms, spoiled raisins, and similar substances. Malt and molasses spirit were afterwards employed, as at the present day, for the purpose; but it was long considered as “an unpardonable and wicked misuse of these articles.” Modern experience, however, has proved that pure malt spirit is, in this country, the most convenient, if not the best kind, to form the basis of an imitation brandy.
Prep. 1. To 12 galls. of malt spirit (finest and flavourless) at proof, add, of water, 5 galls.; crude red tartar or wine-stone, 3⁄4 lb. (previously dissolved in 1 gall. of boiling water); acetic ether, 6 fl. oz.; French wine-vinegar, 2 quarts; French plums (bruised), 5 lbs.; sherry wine-bottoms, 1⁄2 gall.; mix in a sherry or French-brandy cask, and let them stand for about a month, frequently ‘rummaging up’ the liquor with a stick; next draw over 15 galls. of the mixture from a still furnished with an agitator. Put the ‘rectified spirit’ into a clean, fresh-emptied Cognac-brandy cask, and add of tincture of catechu, 1 pint; oak shavings, 1 lb.; and spirit colouring, 1⁄2 pint; agitate occasionally for a few days, and then let it repose for a week, when it will be fit for use. Prod., 15 galls, of BRANDY, 17 u. p. Age greatly improves it.
2. Malt spirit (as before), 99 galls.; red tartar (dissolved), 7 lbs.; acetic ether, 1⁄2 gall.; wine-vinegar, 5 galls.; bruised raisins or French plums, 14 lbs.; bitter-almond cake (bruised and steeped for twenty-four hours in twice its weight of water, which must be used with it), 1⁄4 lb.; water, q. s.; macerate as before, and draw over, with a quick fire, 120 galls. To the distilled spirit add a few lbs. of oak shavings; 2 lbs. of powdered catechu (made into a paste with hot water), and spirit-colouring q. s.; and ‘finish’ as in the last. Prod., 120 galls, of spirit, fully 17 u. p. Equal in quality to the last.
3. Clean spirit (17 u. p.), 100 galls; nitrous ether, 2 quarts; cassia buds (ground) 4 oz.; bitter-almond meal, 5 oz.; orris-root (sliced), 6 oz,; powdered cloves, 1 oz.; capsicum, 11⁄2 oz.; good vinegar, 3 galls.; brandy colouring, 3 pints; powdered catechu, 2 lbs.; full-flavoured Jamaica rum, 2 galls. Mix in an empty Cognac ‘piece,’ and macerate for a fortnight, with occasional stirring. Prod., 106 galls., at 21 or 22 u. p.
4. Malt spirit (17 u. p.), 100 galls.; catechu, 2 lbs.; tincture of vanilla, 1⁄2 pint; burnt sugar colouring, 1 quart; good rum, 3 galls.; acetic or nitrous ether, 2 quarts. Mix as the last.
5. Clean spirit (17 u. p.), 89 galls.; high-flavoured Cognac, 10 galls.; oil of cassia, 2 dr.; oil of bitter almonds, 3 dr.; powdered catechu, 1 lb.; cream of tartar (dissolved), 11⁄4 lbs.; Beaufoy’s concentrated acetic acid, 1⁄2 gall.; sugar colouring, 2 to 3 pints; good rum, 1 gall. When the above mixtures are distilled, the French brandy, colouring, and catechu, should be added to the distilled spirit.
6. To plain spirit (coloured), at 17 u. p., add a little tincture of catechu, and a sufficient quantity of eau-de-vie de marc, or of the oil distilled from wine-lees, to flavour it.
Obs. The oil referred to in the last formula is obtained by distillation from the lees of wine, either dried and made up into cakes, or in their wet state, mixed with about 7 or 8 times their weight of water. This oil should be kept dissolved in alcohol, as it is otherwise apt to lose its flavour. Brandy from any part of the world may be very closely imitated by distilling the oil from the lees of the wines produced in that particular district. Where black tea is cheap, as in the United States of America, it is very commonly employed to impart the roughness of brandy to the coloured spirit, and the subsequent addition of a little ‘flavouring’ greatly improves it. A really good article of cider-spirit thus treated forms a passable ‘mock brandy.’ In conclusion, we may remark that, as the strength and quality of ingredients frequently vary, and success depends greatly on skill in manipulation, much must be left to the experience, judgment, and discretion of the operator. In all cases he must recollect that a certain degree of ‘age’ is absolutely necessary to give a high character to any spirit. Indeed, to age in the one case, and its absence in the other, may be referred the reasons why French brandy and British brandy, apart from mere shades of flavour, so materially differ.
The production of a flavoured British spirit closely resembling French brandy is a subject well worthy of the attention of the ingenious chemist, rectifier, and cellarman, as a matter of profit; and of the amateur, as affording an interesting field for useful and amusing experiment.340
Brandy, Car′away. A species of cordial commonly prepared as follows:—1. Caraway-seeds (bruised), 4 oz.; lump sugar, 2 lbs.; British brandy, 1 gall.; macerate a fortnight, occasionally shaking the bottle.—2. Sugar, 1 lb.; caraways (bruised), 1 oz.; 3 bitter almonds (grated); spirit-colouring, 1 oz.; plain spirit or gin (22 u. p.), 1⁄2 gall.; as before. Some persons omit the colouring.
Brandy, Cher′ry. Prep. 1. Brandy and cherries (crushed), of each 1 gall.; let them lie together for 3 days, then express the liquor, and add 2 lbs. of lump sugar; in a week or two decant the clear portion for use.
2. To the last add 1 quart of raspberry juice, and 1⁄2 a pint of orange-flower water. Both the above are excellent.
3. Treacle, 1 cwt.; spirit (45 u. p.), 41 galls.; bitter almonds (bruised), 1 lb. (or more or less to taste); cloves, 1 oz.; cassia, 2 oz.; macerate a month, frequently stirring. This is the article now commonly vended in the shops and at stalls for cherry brandy.
4. German cherry juice 15 galls.; pure rect. spirits, 20 galls.; syrup, 5 galls.; oil of bitter almonds, 1 drachm.
Obs. Equal part of fully ripe Morello cherries and black cherries produce the richest cordial. Some persons prick each cherry separately with a needle instead of crushing them; in which case they retain them in the liquor, and serve up a few of them in each glass. The plan named in the first formula is, however, that usually adopted. On the small scale, the fruit is commonly bruised between the fingers. A portion only (if any) of the stones in the cherries should be crushed, to impart a nutty flavour. See Liqueurs.
Brandy, Ci′der. From cider and perry; also from the marc of apples and pears fermented. It is very largely manufactured in the United States of America and Canada, where it may be purchased for about 2s. 1d. a gallon. See British Brandy (above).
Brandy, Dant′zic. From rye, ground with the root of calamus aromaticus. It has a mixed flavour of orris and cinnamon.
Brandy, Guern′sey. Beet-root spirit flavoured.
Brandy, Lem′on. Prep. 1. Fresh lemons (sliced), 1 dozen; brandy, 1 gall.; macerate for a week, press out the liquor, and add of lump sugar, 1 lb.
2. Proof spirit, 7 galls.; essence of lemon, 3 dr.; sugar, 5 lbs.; tartaric acid, 1 oz.; (dissolved in) water, 2 galls.; turmeric powder or spirit-colouring, a dessert-spoonful; as before. Sometimes milk is added to the above, in the proportion of 1 quart (boiling hot) to every gallon.
Brandy, Malt. See British Brandy.
Brandy, Or′ange. As lemon brandy, but employing oranges.
Brandy, Pale. This article has been already referred to. (See p. 337.) That of the gin-shops and publicans is generally a spurious article, made by mixing together about equal parts of good brown French brandy, clean spirit of wine, and soft water, and allowing the whole to stand until the next day to ‘fine down.’ If the first is 9 u. p., and the second 58 o. p., the product will be 17 u. p. Any deficiency of strength is made up by adding a little more spirit of wine.
Brandy, Pat′ent. The article so much bepuffed under this name, by certain houses, is merely very clean malt-spirit mixed with about 1-7th of its bulk (or less) of strong-flavoured Cognac, and a little colouring.
Brandy, Peach. From peaches, by fermentation and distillation. Much used in the United States, where peaches are very plentiful, and consequently cheap. A cordial spirit under the same name is prepared as follows:—
1. From peaches, sliced and steeped in twice their weight of British brandy or malt-spirit, as in making cherry brandy.
2. Bitter almonds (bruised), 3 oz.; proof spirit (pale), 10 galls.; water, 3 galls.; sugar, 5 or 6 lbs.; orange-flower water, 1⁄2 a pint; macerate for 14 days. Add brandy-colouring, if required darker.
Brandy, Rais′in (rā′zn). See Spirit (Raisin).
Brandy, Rasp′berry (răz′-). From raspberries, as directed under Cherry brandy. Sometimes a little cinnamon and cloves are added. The only addition, however, that really improves the flavour or bouquet is a little orange-flower water, a very little essence of vanilla, or a single drop of essence of ambergris.
Brandy, White. See Brandy (p. 337) and Pale brandy (antè).
BRASS. Syn. Æs, Æ′′ris metal′lum, L.; Airain, Laiton, Cuivre jaune, Fr.; Erz, Messing, Ger.; Bræs, Sax. A well-known alloy of copper and zinc.
Prep. Brass is now generally manufactured by plunging copper, in slips, into zinc melted in the usual manner. The former metal rapidly combines with the fluid mass, and the addition is continued until an alloy somewhat difficult of fusion is formed, when the remainder of the copper is at once added. The brass thus obtained is broken into pieces, and remelted under charcoal, and a proper addition of either zinc or copper made, to bring it up to the colour and quality desired. It is next poured into moulds of granite. Before being submitted to the rolling-press for reduction to thin plates it undergoes the operation of annealing.
The proportions of the metals forming this alloy are varied according to the desired colour, and the purposes to which it is to be applied. The following formulæ are founded chiefly on analyses of standard brasses and yellow metals, made expressly for this work. Small fractions are omitted; the nearest whole numbers being generally taken:—
a. Fine Brass:—1. Copper, 2 parts; zinc,341 1 part; either combined, as explained above, or the two metals separately melted, suddenly poured together, and united by vigorous stirring.
2. Copper, 7 parts; zinc, 3 parts. Bright yellow; malleable.
3. Fine copper, 4 parts; zinc, 1 part. Deeper coloured than the last; an excellent and very useful alloy.
b. Malleable Brass:—1. Copper, 33 parts; zinc, 25 parts; as before.
2. Copper, 3 parts; zinc, 2 parts. These alloys are malleable whilst hot.
c. Red Brass. This name is commonly applied to all those alloys which do not contain more than 18 to 20% of zinc. In the deeper-coloured foreign varieties (RED TOM′BAC) the per-centage of copper occasionally amounts to 88, 90, or even 92%.
d. Yellow Brass. See Fine Brass (above).
e. Button-brass:—1. Copper, 8 parts; zinc, 5 parts. This is the ‘PLATIN’ of the Birmingham makers.
2. Yellow brass, 16 parts; zinc, 2 parts; tin, 1 part. Paler than the last.
3. Copper, 25 parts; zinc, 20 parts; lead, 3 parts; tin, 2 parts. Pale; used for common buttons.
f. For fine Castings:—1. As fine brass, according to the colour desired. (See above.)
2. Copper, 62 parts; zinc, 35 parts; lead, 2 parts; tin, 1 part.
3. Copper, 60 parts; zinc, 36 parts; tin, 4 parts. Both the last two are rather pale and brittle.
4. Copper, 90 parts; zinc, 7 parts; tin, 2 parts; lead, 1 part. Rich deep colour.
5. Copper, 91 parts; zinc, 5 parts; tin, 3 parts; lead, 1 part; as the last.
g. For Gilding:—1. As fine brass (above).
2. Copper, 64 parts; zinc, 32 parts; lead, 3 parts; tin, 1 part.
3. Copper, 82 parts; zinc, 18 parts; tin, 3 parts; lead, 1 part.
h. For Solder:—1. Fine brass, 12 parts; zinc, 6 parts; tin, 1 part; melted together.
2. Brass, 2 parts; zinc, 1 part; as before.
3. Brass, 3 parts; zinc, 1 part. Very strong. Used for soldering tubes and other like purposes requiring great strength. The above alloys form the ‘HARD SOLDER’ of the braziers. For certain purposes a little silver is added to them, when the compound receives the name of ‘SILVER-SOLDER,’
i. For Turning:—1. Fine brass, 98 parts; lead, 2 parts; melted together.
2. Copper, 61 parts; zinc, 36 parts; lead, 3 parts.
3. Copper, 65 parts; zinc, 33 parts; lead, 2 parts.
j. For Wire:—1. Copper, 72 parts; zinc, 28 parts; the resulting alloy being subsequently properly annealed.
2. Copper, 64 parts; zinc, 34 parts; as before.
3. To the last add of lead, 2 parts.
Anal. This may be briefly described as follows:—
a. 100 gr. of the alloy is digested in nitric acid. The insoluble portion is peroxide of tin, every 74 gr. of which, when washed and dried, contain 58 gr. of metallic tin.
b. Sulphuric acid is added to the nitric solution as long as a white precipitate falls; after a time the precipitate is collected on a filter, washed with a mixture of water and alcohol, and ignited in a porcelain crucible. Every 152 gr. of the residuum represents 104 gr. of metallic lead.
c. The liquid filtered from the precipitate of sulphate of lead is treated with a stream of sulphuretted hydrogen; the precipitate is collected on a filter, washed with water mixed with a little sulphuretted hydrogen, dried, and digested in pure nitric acid until the sulphur which separates acquires its natural full yellow colour; the resulting solution is next diluted with water, and reprecipitated with potassa, the whole being boiled until the precipitated oxide of copper becomes of a deep brown or black; it is then collected on a filter, washed, dried, ignited in a platinum crucible, and weighed therein immediately after it becomes cold. Every 40 gr. of oxide of copper thus obtained represents 32 gr. of pure copper.
d. The liquid poured from the precipitate of sulphide of copper is boiled for about a minute, when it is precipitated with a solution of carbonate of sodium; the whole is then boiled for a few minutes, and the precipitated oxide of zinc collected, washed, dried, and ignited. Every 40 gr. of this oxide contains 32 gr. of metallic zinc.
Concluding Remarks. In the adoption of his formula the operator should be entirely led by the object he has in view. The larger the proportion of copper, the deeper will be the colour, and the greater the density, and, within certain limits, the toughness of the alloy. Zinc lessens the specific gravity and colour. Tin gives it hardness and grain; whilst lead toughens it, and renders it fitter for turning. These facts are known to every experienced brass-founder. See Alloys, Copper, Mosaic Gold, Prince’s Metal, Tombac, &c.
BRASS BATH (FOR ELECTRO-PLATING). For steel, wrought and cast iron, and tin; using ordinary cyanide of potassium. Dissolve together in 14 pints of distilled or rain water:—Bisulphite of soda, 7 oz.; cyanide of potassium (containing 75 per cent. of real cyanide), 17 oz.; carbonate of soda, 34 oz.
To this solution add the following, made up to 31⁄4 pints of water:—Acetate of copper, 41⁄2 oz.; neutral protochloride of zinc, 31⁄2 oz.; the two liquors become colourless when mixed. Ammonia must not be used for brass electro-plating baths for iron, especially for solutions worked in the cold.342
BRASS BATH (FOR ELECTRO-PLATING). For zinc. Pure or rain water, 41⁄2 gallons; bisulphite of soda, 241⁄2 oz.; cyanide of potassium (containing 75 per cent. of cyanide), 35 oz. To this add the following solution:—Water, 9 pints; acetate of copper and protochloride of zinc, each 121⁄2 oz.; liquid ammonia, 14 oz.
The filtered bath is colourless, and gives, under the action of the battery, a brass deposit of a very fine shade, varying from red to green, by increasing the proportion of copper or that of zinc.
BRASS′-COLOUR. Syn. Brass-pigment, B.-bronze. Prep. 1. Grind copper filings, or the precipitated powder of copper, with a little red ochre. Red-coloured.
2. Gold-coloured brass, or Dutch leaf, reduced to a very fine powder. Yellow or gold coloured.
Obs. Before application these powders are mixed up with pale varnish, no more being worked up at once than is wanted for immediate use. They are also applied by dusting them over any surface previously covered with varnish to make them adhere.
BRASS-PASTE. Prep. 1. Soft soap, 2 oz.; rotten-stone, 4 oz.; beaten to a paste.
2. Rotten-stone made into a paste with sweet oil.
3. Rotten-stone, 4 oz.; oxalic acid (in fine powder), 1 oz.; sweet oil, 11⁄2 oz.; turpentine, q. s. to make a paste.
Obs. The above are used to clean brass-work, when neither varnished nor lacquered. The first and last are best applied with a little water; the second with a little spirit of turpentine or sweet oil. Both require friction with soft leather. See Brass-work, Pastes, &c.
BRASS PLATING. By simple dipping. A colour resembling brass is given to small articles of iron or steel by a long stirring in a suspended tub containing the following solution:—Water, 1 quart; sulphate of copper, and protochloride of tin crystallised, about 1-5th of an oz. each. The shades are modified by varying the proportions of the two salts.
BRASS-STAIN. Prep. 1. Sheet-brass (cut into small pieces) is exposed to a strong heat for 2 or 3 days, then powdered, and again further exposed in a like manner for several days; the whole is then reduced to fine powder, and exposed, a third time, to heat, testing it occasionally, to see if it be sufficiently burnt. When a little of it, fused with glass, makes the latter swell and froth up, the process is complete. It imparts to glass a green tint, passing into turquoise.
2. Equal parts of plate-brass and sulphur are stratified together in a crucible, and calcined, until they become friable; the whole is then reduced to powder, and exposed to heat as before. This imparts a calcedony red or yellow tinge to glass by fusion; the precise shade of colour being modified by the mode of using it.
Obs. The common practice in the glass-houses is to conduct the calcination by exposing the metal, placed on tiles, in the leer or annealing arch of the furnace; a plan both convenient and economical.
BRASS′-WORK. Articles of brass and copper, when not varnished or lacquered, may be cleaned and polished with sweet oil and tripoli, rotten-stone, or powdered bath-brick, applied with friction on flannel, and ‘finished off’ with leather; due care being taken to ensure the absence of anything gritty, which would scratch and disfigure the surface of the metal. A strong solution of oxalic acid in water gives brass a fine colour. Vitriol and spirits of salts make brass and copper very bright, but the polish thus obtained soon tarnishes, and the articles consequently require more frequent cleaning. A strong lye of roche alum and water also improves the appearance of brass. In all cases where acids or saline matter has been used, the metal should be at once well rinsed in clean water, and then wiped dry, and finally dry polished with soft leather.
Brass inlaid-work may be cleaned with tripoli and linseed oil, applied by a rubber of felt or leather; the whole being afterwards thoroughly rubbed off, and then finished with clean soft leather. The ornaments of a French clock, and similar articles, are said to be best cleaned with bread-crumb, carefully rubbed, so as not to injure the wood-work. Ormolu candlesticks, LAMPS, and BRANCHES, may be cleaned with soap and water. Lacquered and GILDED ARTICLES are spoiled by frequent rubbing, and by acids and alkaline leys.
1. A fine colour may be given to BRASS ORNAMENTS, when not gilt or lacquered, with a little sal-ammoniac, in fine powder, moistened with soft water. The articles must be afterwards rubbed dry with bran and whiting. Another plan is to wash the brass-work with a strong lye of roche alum (1 oz. to water 1 pint), and after rinsing it in clean water and drying it, to finish it off with fine tripoli. These processes give to brass the brilliancy of gold. See Brass-paste.
2. A gold varnish for giving a beautiful gilding to brass and bronze objects is prepared from 16 grams of shell-lac, 4 grams of dragon’s blood, 1 gram of turmeric-root, and 332 grams of rectified spirit of wine. The varnish is thinly stroked over the surface with a sponge, the metal being warmed over a small coal fire.
The surface at first appears dull, but soon after it appears as if most beautifully gilded. The ready-prepared spirituous varnish must be preserved in well-stoppered vessels.—Dingler’s Journal.
BRASS′ING. Syn. Brass-coating. 1. Copper-plates and copper-rods may be covered with a superficial coating of brass by simply exposing them, in a heated state, to the fumes given off by melted zinc at a high temperature.343 The coated plates and rods are rolled into thin sheets or drawn into wire. The spurious gold wire of Lyons is said to be made in this way.
2. Vessels of copper may be coated with brass, internally, by filling them with water strongly soured with hydrochloric acid, adding some amalgam of zinc and cream of tartar, and then boiling the whole for a short time. This plan may be usefully applied in certain cases to copper boilers in laboratories, and to other purposes.
3. By the electrotype (which see).
BRAUNETINCTUR—QUINSY OR BROWN TINCTURE (Netsch, Rauschau), an embrocation for the larynx, is a mixture of 3 parts oil of cloves and 1 part creosote. (Hager.) According to Leimbach 1 part creosote with 3 parts of a spirituous tincture of cochineal perfumed with oil of cloves.
BRAWN. A boar or its flesh. When young, the horny parts feel moderately tender. If the rind is hard, it is old. (Mrs Rundell.) Also in cookery, the flesh of the boar, or of swine, collared so as to squeeze out as much of the fat as possible, boiled, and pickled.
Brawn, Mock. Prep. (Mrs Rundell.) Take the head and belly-piece of a young porker, well saltpetred; split the head and boil it; take out the bones and cut it to pieces; then take 4 ox-feet, boiled tender, and cut them in thin pieces; lay them in the belly-piece with the head cut small; roll it up tight with sheet tin, and boil it 4 or 5 hours. When it comes out set it up on one end, put a trencher on it (within the tin), press it down with a heavy weight, and let it stand all night. The next morning take it out of the tin and bind it with a fillet, put it in cold salt-and-water, and it will be fit for use; it will keep a long time, if fresh salt-and-water are put into it about once every four days.
BRAXY. Inflammation of the bowels in sheep, chiefly affecting young sheep. It is most frequent during winter, and occurs in exposed wet localities. The symptoms are restlessness, thirst, tenderness along the spine or belly, and constipation.
Treatment. Bleed early, and give 3 oz. castor oil; 8 grains of calomel; 1⁄2 oz. of laudanum; 3 oz. treacle; beat up with two eggs, and mixed with about 6 oz. of warm water; let this be repeated in half doses every six hours.
This should be combined with clysters and hot fomentations to the belly. If, after two days, no benefit should be derived, give thrice daily, 5 grains of calomel, 20 grains of carbonate of ammonia, and 11⁄2 dram of laudanum, in gruel. The animal should be removed to a shed or sheltered place.
BRAZIL NUTS. The particular tree yielding these nuts (the Bartholetia excelsa) is a native of Brazil, whence the nuts are exported to the yearly annual amount of about 60,000 bushels.
When the kernels of the nuts are submitted to pressure they yield an oil in great repute for domestic purposes and for export, each pound of the nuts yielding nine ounces of the oil, valued at two shillings the pound. According to Martius, this oil consists of 74 per cent. of eldein, and 26 per cent. of stearin. The finely laminated inner bark of the trunks is also a valuable article of commerce, especially adapted for the caulking of ships and barges, and is worth about eighteen shillings the cwt.
The following analysis by Corenwinder gives the composition of the kernels taken from the nuts when in a fresh condition:
| Water | 8·00 | |
| Oil | 65·60 | |
| Nitrogenous matters | 15·31 | |
| Non-nitrogenous organic matters | 7·39 | |
| Phosphoric acid | 1·35 | 3·70 |
| Lime, potash, silica, &c. | 2·35 | |
| ——— | ||
| 100·00 | ||
BRAZIL′-WOOD (-zēle′-). Syn. Brazil‡; Lig′num brazilien′se, L.; Bois de Brésil, Fr. A dye-stuff furnished by several species of trees of the genus cæsalpin′ia, and much used in dyeing various shades of red. The usual practice is to boil it for some hours in hard spring-water, and to keep the resulting decoction for some time, or until it undergoes a species of fermentation; as it is thus found to yield more permanent and beautiful colours than when employed fresh. The following are examples of its application:—
a. For Cotton:—
1. The goods are first boiled in a bath of sumach, next worked through a weak mordant of solution of tin, and then run through the Brazil bath lukewarm. This gives a bright red.
2. The goods are alumed, rinsed, next mordanted with solution of tin, rinsed again, and then turned through the Brazil dye bath. This gives a rose colour.
b. For Linen:—This, for the most part, is similar to that adopted for cotton.
c. For Silk:—The goods, after being alumed in the same way as wool, but at a lower temperature, are rinsed, and passed through the Brazil-wood bath lukewarm.
d. For Wool:—The goods are first steeped or boiled in a weak mordant of alum and tartar, for 1 hour, and then allowed to lie in the cold liquor for 2 or 3 days, with frequent moving about; they are lastly boiled in the Brazil-wood bath for about 1⁄2 an hour.
Obs. The shades of colour given with Brazil-wood may be modified by varying the strength of the bath, the mordant, &c. The addition of a little alum turns it on the purple. A little alkali added to the bath, or passing the goods, after being dyed, through water holding a little alkali in solution, produces what is called false crimson. A deep344 crimson is obtained by adding a little logwood to the Brazil-wood bath. 1 lb. of Brazil-wood, 1⁄2 oz. of alum, and 2 oz. of tartar, are sufficient to dye from 20 to 28 lbs. of cotton, according to the depth of shade required. See Dyeing, Red Dyes, &c.
BRAZI′LIN. Syn. Breze′′lĭne, Sapan′ĭne. The colouring matter of Brazil-wood. It forms small orange-coloured needles, soluble in both water and alcohol. Alkalies turn it violet; acids, yellow. Bolly has shown it to be identical with the colouring matter of Sapan-wood.
BRA′′ZING. The operation of uniting pieces of copper, brass, iron, &c., by means of hard solder.
Proc. The edges, after being filed or scraped quite clean, are covered with a mixture of hard solder and powdered borax, made into a paste with water. The whole is then allowed to dry, and is afterwards exposed, in a clear fire, to a heat sufficient to melt the solder. See Autogenous, Soldering, Solders, &c.
BREAD (brĕd). Syn. Pa′nis, L.; Pain, Fr.; Brod, Ger.; Brood, Dut.; Bröd, Dan., Swed.; Breod, Sax. Loaves or cakes made from ground corn, and constituting the staple article of food of all civilised nations.
This important article of food is made of the flour of different cereal grains, but only those that contain gluten admit of conversion into light or spongy bread. In this respect wheat-flour is superior to all others. When this flour is made into a paste or dough with water, and the dough, previous to baking, is left for some time in a moderately warm place, a state of fermentation comes on, owing to the sugar of the flour gradually undergoing the process of conversion into alcohol, in every respect similar to that which takes place during the fermentation of wine and beer. In this process a large quantity of carbonic acid gas is liberated, and the toughness of the dough preventing its escape, the whole mass becomes puffed up and spongy, and a light porous paste is formed, the porosity of which is still further increased by the heat of the oven. The natural process of fermenting the dough just described is, however, tedious and uncertain, whilst the dough has a tendency to run into the acetous fermentation, and to acquire a sour and disagreeable taste, by which it is rendered less nutritious and less easy of digestion. This has led to the use of a ferment which produces a similar condition more speedily, and with greater certainty. Leaven or dough was originally employed for this purpose, and the bread so made was hence called LEAVENED BREAD. At the present time barm or yeast is almost universally used for this purpose. All that is essential to make a loaf of bread is to add a proper quantity of yeast to the dough, and to allow it to remain for a short time in a warm place, and as soon as it rises or becomes spongy, to subject it to the process of baking.
In preparing his dough, the modern baker takes a part of the water needed for the batch, and having rendered it tepid or lukewarm (80° to 90° Fahr.) by the addition of boiling water, dissolves his salt in it, and adds the yeast, together with a portion of the flour. With these he forms a thin dough, which he sets aside in a moderately warm place provided for the purpose, and technically called the ‘kneading trough,’ ‘prover,’ or ‘tryer,’ where it soon begins to ferment and swell up. This process is called ‘setting the sponge,’ and according to the proportion the water in it bears to the whole quantity that is to be used, it receives the name of ‘whole,’ ‘half,’ or ‘quarter sponge.’ Here the sponge heaves and swells, and ultimately the surface bursts and subsides, and if not checked swells again and again in a similar manner and would continue to do so until the whole of the ‘saccharine matter’ was destroyed, and the dough had become sour. The baker is careful, however, to stop it before it has communicated a sourness to the mass. After the first, or, at the furthest, after the second or third ‘dropping of the sponge,’ he adds the remaining quantity of flour, water, and salt, necessary to form the ‘batch,’ and then kneads the whole until it becomes sufficiently tough and elastic to bear the pressure of the hand without adhering to it. The ‘dough’ is now left to itself for a few hours, during which the fermentation still goes on. The inflated mass is then again kneaded, cut into pieces, weighed, and shaped into loaves. In an hour or two these unbaked loaves swell up to nearly double their former size, and are then placed in the oven and baked. During this operation they continue for a time to increase in size, in consequence of the dilation of the pent-up gas by the heat. At length the fermentation is checked, and the dough becomes too solid to admit of further alteration.
Such are the principles and practice of the art of baking. The operations are precisely the same on both the small and the large scale, and therefore need not be separately described.
The kneading of the dough by hand is not only a very laborious process, but it is unhealthy and additionally objectionable on account of its being uncleanly. Added to this, the uniform quality of the dough is not to be depended upon. Although it is impossible to perform by machinery any labour which absolutely requires the touch of the human hand, bread-kneading machines have been introduced wherever the making of only one and the same kind of bread is required. Amongst the numerous kinds of machines devised for bread-making, is Clayton’s. (See cut.)
The constituents of the dough are placed in the cylinder, A, mounted in the framework, b b, and provided with hollow axles, c and d,345 turning in their bearings at e. The interior of the cylinder is fitted with the framework, f, which may be made to revolve by the aid of the axles, g and h. The two halves of this framework are connected together by the diagonal knives i, i, which, when the machinery revolves, work up the dough; the trough or outer cylinder revolves in the opposite direction to the revolution of the framework. The crank, o, is connected with the axle of the trough or outer cylinder, the crank, p, with that of the inner framework; as the two cranks are turned in opposite directions, they impart opposite movements to trough and framework. The revolving of the machinery may be performed by one man by the aid of one crank, since the axle, h, of the crank, o, which is fitted to the inner frame by means of the hollow axle-tree, and revolves along with it, carries a conically shaped wheel, m, fitted to the wheel k, which, being connected with l, causes the trough also to revolve; when therefore the wheel m turns towards the right, the wheel t will revolve towards the left. Another kneading machine is that of Mr Stevens. It is employed at the Holborn Union, where more than 5000 lbs. of bread are made every week by one man and two boys.
Adult. The adulteration of both flour and bread is carried to a fearful extent, more especially in London. The baker’s flour is very often made of the worst kinds of damaged foreign wheat; and other cereal grains, and particularly beans, are mixed with them in grinding them into flour. In this capital no fewer than six distinct kinds of wheaten flour are brought into the market—fine flour, seconds, middlings, fine middlings, coarse middlings, and twenty-penny flour.
Among the principal substances which have been proved to have been used to adulterate wheat-flour and bread are the following:—
| ** | Alum. |
| * | Ammonia (Sesquicarbonate). |
| ** | Beans. |
| * | Bone dust. |
| * | Chalk. |
| Clay. | |
| Copper (Sulphate). | |
| Lime (Sulphate from the soda water makers). | |
| * | Magnesia (Carbonate). |
| * | Plaster of Paris. |
| * | Potash (Carbonate and bicarbonate). |
| ** | Potatoes. |
| ** | Rice. |
| ** | Soda (Carbonate and sesquicarbonate). |
| * | Starch (Potato). |
| ** | Water (in excess). |
| Zinc (Sulphate). |
Of these substances, those marked thus (*) are very frequently used; and those marked thus (**) almost universally so.
In the absence of chemical analysis the unalumed loaf may be roughly distinguished from the alumed one by the following characteristics: it is neither so white, so bulky, nor so symmetrical; it bites shorter, and it is free from the sour taste which accompanies the presence of alum. Again, unalumed bread a day or two old will be found to crumble with great readiness; alumed bread, however old, crumbles, on the contrary, with difficulty.
According to Mr Accum, the smallest quantity of alum that can be employed with effect to produce white, light, and porous bread, from the inferior kinds of flour commonly used by the bakers, is from 3 to 4 oz. to a sack of flour weighing 280 lbs. But Dr P. Markham states that the ordinary bread of the London baker is made of one sack or 5 bushels of flour; 8 oz. of alum; 4 lbs. of salt; 1⁄2 gall. of yeast; and about 3 galls. of water. Our own analyses, extending to many hundred samples of London bread, as well as those of other chemists, show that even this large quantity of alum is often very much exceeded by the bakers.
Alkaline substances, as the carbonates of ammonia, soda, and potash, are often employed to realise the important consideration of producing light and porous bread from spoiled, or, as it is technically called, sour flour. The first salt becomes temporarily converted into a gaseous state during the operation of baking, causing the dough to swell up in minute bubbles, which thus render it light and porous; the salt itself being at the same time, for the most part, volatilised. Alum is added, not only with a like intention, but also to enable the dough to carry more water. There are several instances of convictions on record of millers and bakers having used gypsum, chalk, and pipeclay in the manufacture of their goods. A gentleman lately writing from the346 North of England says that he found in one sample of flour which he recently examined upwards of 16% of gypsum; and in another, 12% of the same earth.
A few years since it was discovered that some of the bakers in France and Belgium added blue vitriol to their dough to make it take more water, in the same way as the English baker uses alum. 1 oz. of this sulphate was dissolved in a quart of water, and a wine-glassful of this solution added to the water necessary to make about 50 4-lb. loaves. This enormous crime was soon detected, and deservedly caused the ruin of its heartless perpetrators.
Exam. The following are the methods employed for the discovery of the principal sophisticants of bread, and as the chief of these, and the one most difficult of identification is alum, we have given prominence to the processes now generally adopted for the detection of this article:—
1. Alum:—a. (Robine and Parisot.) About 1⁄4 lb. of the suspected bread (somewhat stale or dry) is reduced to crumbs, macerated for 2 or 3 hours in cold water, and then squeezed through a clean piece of white linen. The liquid is next evaporated to dryness at a steam-heat, the residuum redissolved in a little hot water, and the solution filtered. Liquor of ammonia or a solution of sal-ammoniac, and a solution of chloride of barium added to the filtered liquid, give a white precipitate when ALUM is present.
When nearly the whole of the alum has suffered decomposition in the loaf, as is frequently the case, the following process is required:—
b. (M. Kuhlman.) 4 or 5 oz. of bread are reduced to ash, which is powdered and treated with nitric acid, the mixture evaporated to dryness, and about 1 oz. of hot water added. A little caustic potassa is added to the last solution (unfiltered), the whole boiled a few minutes, and passed through a filter. The filtrate is next tested with a solution of sal-ammoniac, and the whole again boiled for 2 or 3 minutes. If a precipitate forms it is alumina; every 50 gr. of which are equivalent to 332 gr. of crystallised alum.
c. The suspected sample is wetted with a weak solution of logwood, or, preferably, of cochineal. Pure bread is only slightly stained by this solution; bread containing alum strikes a lavender, lilac, or purple colour, according to the quantity of the adulterant present. If it acquires a pearl-grey or bluish tint, some alkali (potash, soda, or ammonia) is present.
d. (J. A. Wanklyn.) 100 grams of bread are incinerated in a platinum dish, capable of holding the whole quantity at once. The incineration is managed at a comparatively low temperature, and takes some four or five hours; the platinum dish being heated by means of a large Bunsen burner, abundantly supplied with air. It is well to continue the ignition until the bread-ash is nearly completely burnt, and it is advisable to weigh the dish containing the ash. The weight of the ash should not sensibly exceed 2 grams. The ash having been obtained is then moistened with 3 c. c. of pure strong hydrochloric acid, and then some 20 to 30 c. c. of distilled water is added, and the whole is boiled, filtered, and the precipitate washed several times with boiling water. In this manner a precipitate consisting of a silica, together with some unburnt carbon, is left on the filter, whilst the filtrate contains the phosphates. The precipitate, which, after being burnt, consists of silica, is weighed. The filtrate is mixed with 5 c. c. of ammonia (sp. gr. 0·880), whereby it is rendered powerfully alkaline and opaque, owing to the precipitation of the phosphates. It is finally mixed gradually with some 20 c. c. of strong acetic acid, and as the acid is being poured in, it is to be observed that the liquid is alkaline and opaque, until some 5 c. c. of the acid have been added; that when about 10 c. c. have been added the liquid is acid and much clearer, and that at least 10 c. c. of strong acetic acid are added after the establishment of a distinctly acid reaction. The liquid is then boiled and filtered, and the precipitates, consisting of phosphates of alumina and iron, well-washed with boiling water, ignited and weighed. The last step is the determination of the iron in the weighed precipitate, and this is accomplished either by reduction and titration with standard solution of permanganate in the well known manner, or else by a colour process, viz., by trituration with ferrocyanide of potassium. Having ascertained the amount of iron in the precipitate of mixed phosphates, it is only necessary to calculate it into phosphate of iron, and to subtract the weight of phosphate of iron from the total weight of the mixed phosphates, and the difference is the phosphate of alum yielded by 100 grams of the bread. The following results have been obtained by applying the above-described process to samples of bread presumed to be free from alum:—
| Bread-ash. | Silica. | Precipitate insoluble in acetic acid. | |
| Grams. | Grams. | Grams. | |
| A | 1·408 | ... | 0·010 |
| B | 1·378 | ... | 0·006 |
| C | 1·730 | 0·018 | 0·010 |
| D | 1·620 | 0·032 | 0·014 |
| E | ... | ... | 0·012 |
| (1)F | 1·383 | 0·030 | 0·012 |
| (2)F | 1·324 | 0·025 | 0·014 |
The precipitate insoluble in acetic acid contained in every instance a large proportion of iron, but in some cases at least did not wholly consist of phosphate of iron. On deducting the quantity of phosphate of iron from the total phosphates insoluble in acetic acid, there remains a residue of some five or six milligrams. It would therefore appear that unalumed347 bread is liable to contain a minute trace of alumina, which, expressed as phosphate of alumina (Al2O3PO5), equals five or six milligrams per 100 grains of bread, or 0·005 per cent. If the alum corresponding to this phosphate be calculated, it will be seen that 100 grams of unalumed bread may appear to contain 0·022 grams of alum; or expressed on the 4-lb. loaf, there may appear to be 6 grams of alum in it. This agrees very fairly with Dr Dupré’s observation.
e. (J. C. Thresh.) The author states that this process requires only a few hours, and quotes experiments, showing the accuracy of the results:—
Take 1250 gr. of bread (from middle of loaf) or flour, and char thoroughly in a platinum dish or on foil over a gas lamp. Powder the char and mix it with sufficient pure strong hydrochloric acid to make a thin cream. Boil gently for a few minutes, then add 100 c. c. of water, and continue the ebullition a few minutes longer. Dilute to 150 c. c., stir well, and filter off 120 c. c., which will contain the alumina from 1000 gr. of the bread or flour. To this filtrate add a slight excess of solution of ammonia, boil for a few seconds. Then let the precipitate subside, and decant the supernatant fluid. Add boiling water to the sediment, and again set aside to settle, and decant the clear fluid. Pass the fluids through a small filter to collect any particles of the precipitate which may have been suspended therein, and throw the filtrate away. Now add to the partially washed precipitate about a gram of pure caustic potash (or soda), warm, and pass the solution through the same filter employed for the previously decanted fluids. Wash the filter with hot water, to which a little KHO may be added, and proceed to precipitate the alumina in the filtrate by adding a few drops of dilute phosphoric acid and excess of pure acetic acid. Heat the solution and precipitate to the boiling point, and then wash the latter by decantation and filtration. Finally dry, ignite, and weigh. The weight of the resulting Al2PO4 in grams, multiplied by 400, will give the amounts of ammonia alum in grains present in one pound of the bread or flour.
f. (Mr Crookes.) The bread of which at least 500 grains should be taken is first to be incinerated on a platinum or porcelain dish, until all volatile organic matter has been expelled, and a black carbonaceous ash remains. The temperature must not be raised much beyond the point necessary to effect this. Powder the coal thus obtained and add about thirty drops of oil of vitriol, and heat until vapours begin to rise; when sufficiently cool, add water, and boil for ten minutes. Filter and evaporate the filtrate until the fumes of sulphuric acid begin to be evolved, when 10 gr. of metallic tin and an excess of nitric acid must be added, together with water, drop by drop, until action between the acid and metal commences. When all the tin is oxidised, add water, and filter. Evaporate the filtrate until fumes of sulphuric acid are again visible, when more water must be added, and the liquid again filtered if necessary. To the clear solution now add tartaric acid, then ammonia in excess, and sulphide of ammonium. Evaporate the liquid containing the precipitate suspended to it, in a dish, until all the smell of sulphide of ammonium has disappeared. Filter, evaporate to dryness, and ignite to get rid of the organic matter. Powder the black ash, boil it in moderately strong hydrochloric acid, filter, add a crystal of chlorate of potash, and boil for a minute. Now add chloride of ammonium and ammonia, and boil for five minutes. If at the end of that time any precipitate is observed, it will be alumina. From the filtered solution, if oxalate of ammonia be added, the lime will be precipitated; and if to the filtrate from this, ammonia and phosphate of soda be added, the magnesia will come down.
Dr Dupré is of opinion that no baker should be fined in whose bread the amount of alumina found corresponds with less than 10 grains of potash alum in the 2-lb. loaf, unless there is direct evidence of adulteration by alum independent of the result of analysis.
Mr Crookes says, “By treatment with a trace of alum, flour with a doubtful soundness is endowed with soundness. For this purpose a proportion of alum is required which does not exceed 20 grains to a 4-lb. loaf.
2. Copper:—a. Moisten the suspected bread with a few drops of a solution of ferrocyanide of potassium. It will assume a pinkish-brown colour if copper be present.
b. A little of the bread may be steeped in hot water, or, better still, in water soured with a little nitric acid, and the clear liquor squeezed or poured off, and tested with ferrocyanide of potassium, as before.
3. Magnesia:—Bread adulterated with magnesia, on digestion in hot water acidulated with sulphuric acid, furnishes a liquid which gives a white precipitate when tested with a solution of either carbonate of potassa or of carbonate of soda, especially on boiling.
4. Soda; Potassa:—Hot water after digestion on the ashes or charcoal turns turmeric paper brown. The liquid may be evaporated to dryness, redissolved in distilled water, slightly acidulated with hydrochloric acid, and tested with bichloride of platinum. If a yellow crystalline precipitate forms, either at once or after some hours, it is potash; otherwise the alkali present is soda.
5. Chalk, WHITING, BURNT BONES, plaster of Paris, and similar substances are easily detected by calcining a little of the flour or bread in a clean open vessel, when the amount of ash left will indicate the quantity of adulteration. The quantity of the ash left by genuine bread or flour is very trifling indeed, about 2%.348
Microscopic Characters of Bread. When bread is placed under the microscope, starch cells, broken up into angular masses, or greatly enlarged, and stringy masses of gluten are usually visible; besides these, when a microscope of high power is employed, bacteria of the rod-shaped variety may frequently be detected, the source of these being, probably, the yeast. Great caution and diligent observation are necessary to guard against the falling into the serious error of mistaking the many curious forms the broken-up wheat starch presents for adulterants. By practice and the constant examination of the characters of unadulterated bread, combined with a practical knowledge of the appearance different starch grains present, after being more or less changed in shape by cooking, the microscopist may identify rice-flour, bean-flour, and Indian millet. Barley flour and potatoes, however, are very difficult of detection. There is very little difference in the shape of the barley-starch granule and that of the wheat, and in the process of bread-making the potato granules are so changed as to confuse all their distinctive characters. Bone-dust and a few other mineral adulterations may be detected by the microscope.
Concluding Remarks. A number of processes are used by cooks and confectioners to make the different varieties of fancy bread, cakes, puddings, &c., which vary according to the peculiar characteristic it is desired to communicate to them; but none of these articles properly belong to the trade of the common baker. Thus, some kinds of cakes and pastes are made to eat ‘short,’ as it is called, or are rendered less tenacious, and a species of brittleness imparted to them by the addition of starch, rice-flour, or sugar. In pastry a similar effect and peculiar lightness is produced by butter or lard, whilst in some articles white of egg, gum water, isinglass, and other adhesive substances, are added to produce an exceedingly light and porous mass.
The chief varieties of bread at present in use in this country are known according to their shapes, as—Bricks, Coburg, Cottage, Batch, French rolls, and Rye bread. These vary in their quality, chiefly according to the flour of which they are formed, and their various flavours depend upon the heat of the oven in baking. The best WHITE BREAD is made from the purest wheat-flour; ordinary WHEATEN BREAD, of flour containing a little of the finest bran; SECONDS, from flour containing a still larger proportion of bran; and common HOUSEHOLD BREAD, from flour produced by grinding the whole substance of the grain, without any separation of the bran. The last variety is undoubtedly the most wholesome and nutritious, although that least frequently used. Symnel-bread, MANCHET or ROLL-BREAD, and French bread are varieties made of the purest flour, from the finest wheat, a little milk being usually added for rolls, and butter and eggs for choicer purposes. Several other minor kinds of bread are also made, varied by the addition of sundry trifles, as sugar, currants, and other palatable ingredients. The Scotch shortbread is made from a very thick dough, to which butter, sugar, orange-peel, and spices are added, according to the taste of the maker.
In the manufacture of white bread from damaged or inferior flour a large quantity of alum is employed by the fraudulent baker, as already noticed; but with the ‘best flour’ no alum is required. The utmost beauty, sponginess, and sweetness may be given to bread without the addition of one particle of alum, provided the best materials alone enter into its composition. As such materials are seldom employed by the bakers, the usual practice is to introduce 4 or 5 oz. of alum to every sack of flour, or about 1 oz. to each bushel; and very frequently fully double this quantity of alum is employed. But even this enormous quantity is often not the whole of the alum present in common bread; for the miller, in order to cheat the baker, puts in the ‘doctor,’ in the shape of 4 to 6 oz. of alum to the sack, whilst the baker, unconscious of this victimisation, subsequently uses a double dose of alum in order to cheat his customers.[229] The method of detecting this pernicious adulteration has been already explained. The proper quantity of salt is 4 lbs., and never more than 5 lbs., to the sack, or 1 lb. per bushel. One sack of the best flour, with 4 or 5 lbs. of salt, yields about 360 lbs. of good bread; and a sack of seconds, 345 to 350 lbs. of bread; each being moderately baked. If the loaves are well-baked or over-baked, the product will be from 345 to 350 lbs. only; but if they are slack-baked or under-baked, from 370 lbs. to 385 lbs. of crumbling bread may be obtained from 1 sack of good white flour.
The attention of chemists has, at various times, been directed in search of some method to rectify or lessen the effects of bad harvesting and improper storage on grain, so that a damaged or inferior article might be rendered serviceable, and available for human food. Prof. E. Davy recommends the addition of 1⁄4 oz. of carbonate of magnesia to about every 3 lbs. of sour, melted, heated, and similarly damaged flour. This substance materially improves the quality of the bread, “even when made from the worst new seconds flour;” whilst it is said to be perfectly harmless; and the bread so prepared, for temporary use, is certainly unobjectionable. What effects would arise from the daily consumption of such bread for several months has not been determined; but it is349 doubtful whether it would prove salutary. Indeed there are sufficient reasons for condemning the adoption of such bread in the general diet of a people for any very lengthened period.[230] Our own experiments in bread-making, extending over a long period of years, lead us to prefer carbonate or bicarbonate of soda for the purpose. Theoretically, the corresponding salts of potassa would be preferable. A mixture of equal parts of the bicarbonates of potassa and of soda will, perhaps, ultimately be found to be more useful than either substance used separately.
In times of scarcity and famine various substances, besides the flour of the cereals, have been made into bread, or have been mixed with it, in order to lessen the quantity of the former required by the people. For this purpose, almost every amylaceous vegetable at once plentiful and cheap has, in its turn, been eagerly appropriated. Acorns, beech-mast, the leguminous seeds, numerous starchy bulbous roots, and similar substances, have been employed, either in the form of meal, or made into an emulsion or jelly, which has been used instead of water to form the flour of bread-corn into a dough. At such times bran, the most nutritious and valuable portion of the grain, although usually rejected as worthless, has been retained in the flour, and has even been added to it in excess. Birkenmayer, a brewer of Constance, during a period of scarcity, succeeded in manufacturing bread from the farinaceous residue of beer (brewer’s grains). 10 lbs. of this substance, rubbed to a paste, with 1⁄2 lb. of yeast, 5 lbs. of ordinary meal, and a handful of salt, produces 14 lbs. of BLACK BREAD, which is said to be “both savoury and nourishing.” The nutritious quality of brewer’s grains is shown by their extensive employment at the present day as food for pigs and cattle, and particularly for milch cows. In like manner Iceland, Carragheen, and other mosses, have been made into bread, either alone, or mixed with flour or meal. They are used, in the first case, in the state of meal, in the same way as flour; in the second case, 7 lbs. of moss are directed to be boiled in 10 or 12 galls. of water, and the resulting glutinous liquid or jelly to be employed to make 70 lbs. of flour into dough, which is then fermented and baked in the usual way. It is said that flour thus produces fully double its weight of good household bread. A simpler plan is to mix 1 lb. of lichen meal with 3 or 4 lbs. of flour; the bitterness of the lichen having been first extracted by soaking it in cold water. Bread so prepared has of late been highly recommended for the delicate and dyspeptic. The modern baker is in the habit of mixing large quantities of potatoes with his bread, whenever he can purchase them at paying prices. Mealy potatoes are selected, and are carefully mashed or pulped, and the dry flour is worked into this pulp or dough, which is then mixed with the sponge in the usual manner. For inferior bread, equal weights of potato pulp and dry flour are often used. Bread so prepared eats ‘short,’ and is deficient in sponginess, and in that fine yellowish-white tint which forms one of the characteristics of pure wheaten bread, More recently, rice boiled with water to a jelly has got into very extensive use among the bakers. A ‘sponge’ is made with a portion of the jelly thickened with some flour, and the whole process is conducted in the ordinary manner, except that the fermentation is generally more slowly conducted and allowed to proceed for a longer period. Flour so treated yields fully 50% more bread than when merely mixed up with yeast and water. This constitutes the process of Messrs Morian, Martin, and Journet, of Paris, which was tested, a few years since, at Marylebone Workhouse. The experiment succeeded, but the only result to the public has been, that the common bakers have adopted the plan, and now very generally surcharge their bread with such an excess of water that, in many cases, it only possesses two thirds the amount of nourishment which it did before the publication of the system just referred to. Unfortunately, the cupidity of dishonest tradesmen appears to be continually impelling them to avail themselves of the exertions of philanthropists and the discoveries of science, in order to increase their profits, regardless alike of the quality of their commodities and the health of their customers. Bread containing an excess of water rapidly becomes sour and mouldy, and is apt to disorder the digestive functions of those who eat it.
From the experiments of Dr Colquhoun, it appears that the starch of flour is partially converted into sugar during the process of fermenting and baking the dough, and thus contributes to the sweetness of the bread. He proposes to add to the flour, arrow-root, the farina of potatoes, and similar amylaceous substances, made into a jelly with hot water, for this purpose. Dr Percival has recommended the addition of salep with the same intention. 1 oz. of salep, dissolved in 1 quart of water; 2 lbs. of flour; 80 grs. of salt; and 2 oz. of yeast, gave 3 lbs. 2 oz. of good bread. The same weight of materials, without the salep, gave only 23⁄4 lbs. If too much salep is added, it gives its peculiar flavour to the bread.
In reference to the above substitutions, and to the relative quantity of bread produced from any given weight of flour, the reader should remember that the mere increase of the weight or bulk of the product does not carry with it a corresponding increase of the nutritive elements contained in the flour. These remain the same in all cases; and just in proportion as the product, in bread, is greater, will be the decrease in the value of such bread as food. So also with potatoes, rice, and other farinaceous and pulpy substances used as substitutes350 for wheat-flour. Their poverty in nitrogenous matter, or flesh-formers, is so great, that the greatly increased quantity required as food to support the body, apart from mere inconvenience, more than compensates for their apparent low price. Thus, good wheaten bread, at 2d. per lb., is more than twice as cheap as potatoes at 1d.; for, assuming 2 lbs. of the first as a day’s food, 10 lbs. of the last will be required for the same purpose; and even this large quantity will scarcely effect the desired object. Liebig has demonstrated that, regard being had to the nutritive power of wheat, it is, under all ordinary circumstances, the cheapest article of food provided by nature for man.
We have not entered into particulars respecting oven management, because, on the large scale, it is thoroughly understood by every practical baker. For the instruction of the busy housewife, however, we may state that the oven should always be sufficiently heated before the bread is put into it, in order that the gas contained in the cells of the ‘sponge’ may be expanded as rapidly as possible by the heat, and the resulting light mass quickly rendered sufficiently solid to prevent its subsequent collapse. The heat should also be maintained at nearly the same temperature during the whole of the time the bread is submitted to its action. In general, with ordinary kitchen ovens properly heated, 30 minutes’ baking is sufficient for one-pound loaves and cakes; and 15 minutes in addition for every pound after the first for larger ones. Thus, a 1 lb. loaf requires 1⁄2 hour; a 2 lb. loaf 3⁄4 hour; and a 4 lb. loaf, 11⁄4 hour.
It is the common ambition of the English baker to give that peculiar tint to the crust of his bread in the process of baking which is so highly esteemed by connoisseurs, and so successfully produced by the Viennese and Parisians. It has been long known at Vienna that if the hearth of an oven be cleaned with a moistened wisp of straw, the crust of bread baked in it immediately afterwards presents a beautiful yellow tint. It was thence inferred that this peculiarity depends on the vapour, which being condensed on the roof of the oven, falls back on the bread. At Paris, in order to secure with certainty so desirable an appearance, the hearth of the oven is generally laid so as to form an inclined plane, with a rise of about 11 inches in 3 feet; and the arched roof is built lower at the end nearest the door, as compared with the further extremity. When the oven is charged the entrance is closed with a wet bundle of straw. By this arrangement the steam is driven down on the bread, and a golden-yellow crust is given to it, as if it had been previously covered with the yolk of an egg.
Pure wheaten bread is one of the most wholesome articles of food, and has been justly termed the ‘staff of life,’ and a certain proportion of it should be taken at every meal.
New and Stale Bread.—As has been just stated, bread which has been kept for 24 hours after baking is more digestible, and therefore preferable to that which has been newly baked. This latter exhibits a well-known elastic appearance, and possesses a certain degree of moisture which renders its taste more agreeable to most persons than bread which has been kept for a day or two, and has become firmer and drier in appearance, and which is commonly termed stale. It is very generally supposed that this change in properties in bread which has been kept for a few days, is owing to the loss of water.
This, however, is not the case. The crum of newly baked bread when cold contains about 45 per cent. of water, and that of stale bread contains almost exactly the same proportion.
The difference in properties between the two is due simply to difference in molecular arrangement. Boussingault found that a loaf which had been kept for six days, though it had become very stale, had not lost more than 1 per cent. of its weight when new. The same loaf was then placed in the oven for an hour, and at the end of that time it had acquired all the properties and appearance of new bread, although during the second baking it lost 31⁄2 per cent. of water. In another experiment a portion of bread was allowed to become stale when enclosed in a tight case, to prevent loss of water by evaporation; it was then heated, and was thus restored to the condition of new bread; these effects were produced alternately, many times in succession, upon the same piece of bread; a heat of about 131° F. was found to be sufficient to convert stale into new bread. Every person who has seen a thick slice of stale bread toasted may have satisfied himself that the crum has during this operation been converted into the same condition as that of new bread.
Fungi. When bread has been kept a few days and has become stale, certain species of fungi show themselves in it: these are the penicillium glaucum, which is the green mould of cheese; the fermentum cerivisiæ or yeast fungus; the oidium auriantiacum or orange-red mould; the puccinia graminis and others. Excess of salt added to the bread prevents the development of these fungi.
Diseases arising from the employment of unsound Flour and Bread.—The flour may be ergotised or grown, and fermenting from the presence of fungi. All the poisonous symptoms of ergot are induced from continuously partaking of bread made with ergotised flour. Dry gangrene is one of the most virulent forms of poisoning caused by partaking of ergotised bread. Severe intestinal derangement is an accompaniment of the milder forms of poisoning. Ergot is more frequently present in rye flour than in wheat. Fermenting bread is a fertile source of dyspepsia, whilst acid bread causes diarrhœa. This latter malady is also caused by the presence in bread of the oidium351 aurantiacum. Professors Varnell and Tuson state that mouldy oats, the mould being caused by a fungus (the aspergillus), have given rise to paralytic symptoms in horses, so that the presence of these fungi in oats used for making bread should always be regarded with considerable caution.
It has not been demonstrated that the acarus so common in flour has had any injurious effect when eaten. When well fermented and baked bread is very easy of digestion. It should never be eaten until it has stood at least 24 hours after being taken out of the oven. When newer, bread is apt to disagree with the stomach, frequently producing indigestion, biliousness, diarrhœa, dyspepsia, and other like ailments. Bread prepared from meal containing the whole of the bran is the most nutritious and digestible, and should alone be given to children and growing persons, and eaten by the dyspeptic and delicate. Young infants should never be fed upon bread. See Aleurometer, Alum, Flour, Wheat, &c.
Bread, Aërated. The best description of unfermented bread is that manufactured by the process of Dr Dauglish. The method of manufacture has this advantage:—During the whole of the operation neither the flour nor the dough comes into contact with the flesh of the workman. For a full description of the method of preparing this article, see Watts’ ‘Dic. of Chemistry.’ See Bread, Unfermented.
Bread, Amer′ican. From American barreled flour. “14 lbs. of American flour will make 211⁄2 lbs. of bread; whereas the best sort of English flour produces only 181⁄2 lbs. of bread.” (Mrs Rundell.) This arises from the superior quality of the wheat used in its production; and also from its being kiln-dried before grinding, by which much water is driven off.
Bread, Bee. The matter collected by bees to form the bottom of the hive. It resembles a mixture of resin and wax. Its fumes were formerly thought to be anti-asthmatic.
Bread, Bran. 1. From the whole meal, without sifting out any of the bran.
2. By adding about 3 oz. of bran to every lb. of ordinary flour.
Bread, Cassava, is made from the root of the manihot, by first expressing the juice, then grinding the residue into a coarse meal, and baking it in the form of cakes upon thin iron plates. When steeped in oil, and flavoured with cayenne, and slightly broiled upon a gridiron, it is not unpalatable.
Bread, Extemporaneous. See Bread, Unfermented.
Bread, French. Prep. 1. From fine flour, as the best white bread. For the better kinds, and for those intended for rolls and small fancy bread, the sponge and dough is commonly wetted with milk and water, and, occasionally, a very little butter is added. “When the rolls or small fancy loaves have lain in a quick oven about a quarter of an hour, turn them on the other side for about a quarter of an hour longer. Then take them out and chip them with a knife, which will make them look spongy, and of a fine yellow; whereas rasping takes off this fine colour, and renders their look less inviting.”
2. French Soup-bread. From fine flour, but employing fully double the usual quantity of salt. It is baked in thin loaves, so as to be nearly all crust, by which means it becomes more soluble in hot soup.
Bread, Hick’s Pat′ent. This is ordinary bread baked in an oven so arranged that the vapours arising during the process are condensed in a suitable receiver. The condensed liquor is a crude, weak spirit, produced during the fermentation of the dough, and possesses little commercial value; indeed, insufficient to pay for the expenses attending its collection. Besides which, the bread prepared under this patent was rejected by the vulgar, who flocked to the shops of the neighbouring bakers, who professed to sell their bread with “the gin in it.”
Bread, Household. This name is commonly given to bread made with flour from which only the coarser portion of the bran has been removed; and to bread prepared from a mixture of flour and potatoes. The following are examples:—
1. (Rev. Mr Haggett.) Remove the flake-bran from flour, 14 lbs.; boil the bran in 1 gall. of water until reduced to 7 pints; strain, cool, and knead in the flour, adding salt and yeast as for other bread. Very wholesome.
2. Flour, 7 lbs.; mealy potatoes (well mashed), 3 lbs.; as before. Objectionable for the reasons already given.
Bread, Leav′ened. (lĕv′-). Using leaven instead of yeast, and in the same way. About 1 lb. to each bushel of flour is usually sufficient. The more leaven used, the lighter the bread made with it will be; and the fresher and sweeter the leaven, the less sour will it taste. Leaven, except among the Jews and sailors, is now superseded by yeast.
Bread, London White. The common proportions of the London bakers are—Flour, 1 sack; common salt, 41⁄2 lbs.; alum, 5 oz.; yeast, 4 pints; warm water for the sponge (about), 3 galls. The process has been already noticed.
Bread, Paris White. The following has been handed to us as the plan commonly adopted by the Paris bakers for their best white bread:—On 80 lbs. of the dough (before the yeast was added) from yesterday’s baking, as much lukewarm water is poured as will be required to make 320 lbs. of flour into a rather thin dough; as soon as this has risen, 80 lbs. are taken out and reserved in a warm place as leaven for the next day’s baking; 1 lb. of dry yeast, dissolved in warm water, is then added to the remaining portion, and the whole lightly352 kneaded; as soon as it has sufficiently risen, it is made into loaves, and shortly afterwards baked; the loaves being placed in the oven without touching each other, so that they may become crusty all round.
Bread, Unfermented. Syn. Extemporaneous bread. Prep. 1. From Jones’s patent flour. Very wholesome and excellent; indeed, when skilfully made and baked, almost equal to French bread.
2. From Sewell’s patent flour. Slightly inferior to the last.
3. To each lb. of flour add, separately, 11⁄4 dr. of bicarbonate of soda, and 1 dr. of tartaric acid (both perfectly dry, and in very fine powder); rub them well together with the hands until thoroughly incorporated; then form the whole into a dough with water, as quickly as possible, and at once bake in a quick oven. About 8 or 9 oz. of water are required for every lb. of flour. Answers well when expertly managed.
4. Flour, 1 lb.; bicarbonate of soda, 1 dr.; mix; make a dough with water, q. s., to which 1 dr. of hydrochloric acid (commercial) has been added, and further proceed as before.
5. Whiting’s Patent bread:—This closely resembles the last. The proportions are—Flour, 7 lbs.; carbonate of soda and hydrochloric acid, of each 1 oz.; water, 23⁄4 pints. This method was suggested by Dr Henry in 1797, and was patented by Dr Whiting in 1836. If the proportions be not observed, or the mixture be not perfect, the quality of the bread suffers. The action of the acid on the soda forms common salt in the loaf.
6. Ammoni′acal bread:—Carbonate of ammonia, 3⁄4 to 1 oz.; cold water, q. s.; dissolve, add of flour, 7 lbs.; and make a dough, which must be formed into loaves and baked immediately, as before.—Obs. To ensure success the carbonate should be recent, and free from bicarbonate, the presence of which is known by its being white and powdery, and of inferior pungency. If any of the last salt be present, the bread will have a yellowish colour, and a slightly alkaline or urinous flavour. The process answers best for small loaves, cakes, and fancy bread. By employing pure carbonate of ammonia instead of the commercial sesquicarbonate, the process succeeds admirably, and the resulting bread is most wholesome. A late writer recommends the use of bicarbonate of ammonia, but evidently does so in ignorance, as in practice it is inapplicable, as the author verified by numerous carefully conducted experiments.
7. It has been at various times proposed to knead the dough with water highly charged with carbonic acid, on which Dr Ure observes that “the resulting bread will be somewhat spongy.” He states that he endeavoured to make bread in this way, but never could succeed in producing a light spongy loaf. The quantity of gas in the water is much too trifling for the purpose, and the greater part of it escapes in the process of making the dough, even though all the materials be well cooled, and the operation conducted in a cold place. The only way of obviating the difficulty is to conduct the kneading in a trough under considerable atmospheric pressure, and at a very low temperature, by means of machinery, as is done by Dr Dauglish, whose method is now protected by letters patent. This method is not, however, adapted either to domestic use or the small scale.[231]
Obs. Unfermented bread has been strongly recommended as being more wholesome, and generally better adapted to bilious and dyspeptic patients, than fermented bread. It must, however, be confessed, that the unfermented bread commonly met with has a slight ‘raw-grain’ taste, which is very disagreeable to some persons. But this taste is not necessarily present, being chiefly dependent on bad manipulation, the use of inferior flour, and insufficient baking. The process of fermentation doubtless modifies the condition of the starch and gluten of the dough, and renders them easier of digestion. This species of bread is sadly adulterated with a variety of indescribable messes. See Biscuits, Bread (antè), Flour, Gingerbread, &c.
Bread Fruit (Artocarpus incisa, nat. order Graminaceæ). The tree yielding the bread-fruit is a native of Central America, the South Sea Islands, and the Islands of the Indian Archipelago. It is principally composed of starch, sugar, and water, every 100 parts containing 80 of water. The fruit is gathered when the starch is in a mealy condition; it is then peeled, wrapped in leaves, and baked by placing it between hot stones. It then has the taste of sweetbread.
The natives of the countries where this fruit is found practise a method for preserving it, which consists in allowing the nitrogenous parts of the fruit to putrefy in water-tight pits. They thus obtain a mass resembling soft cheese in consistence, and this, when required to be eaten, is baked in the same manner as the fresh fruit.
BREAK′FAST (brĕk′-). Syn. Jentaculum L.; Déjeûner, Déjeûné, Fr.; Frühstück, Ger. The first meal of the day; or the food served at it.
The morning meal—the ‘early bit’ of the Germans—is perhaps the most important one of the day. According to Erasmus Wilson, it is usually “taken at eight or nine.” The proper time for the purpose must, however, depend upon that at which the party rises. About an hour, to an hour and a half, after leaving the bed, will generally be found the most appropriate time for breakfast, and appears to be the one pointed out by nature, and the most conducive to health. By that time the powers of the system have fully recovered from the inactivity of sleep, and the353 functions of the stomach and other viscera have again come into full play. The appetite is excited and seeks appeasing, and both instinct and reason direct us to the social board. If abstinence be now prolonged, the physical and mental energies, unsupported by the supply of food which indirectly gives them birth, gradually lessen, and incipient exhaustion ensues. The fluids of the stomach and the smaller intestines begin to act upon the coats of those viscera instead of on the food, and an unpleasant feeling of hunger or a loss of appetite comes on, with all its depressing consequences. When breakfast cannot be taken within a reasonable period after rising, the gap should be filled up by chewing a crust, a biscuit, or the like. A raw egg or two, sucked from the shell, or broken into a teacup and drank, will be found most valuable for this purpose. Raw milk, cheese, salted food, and other indigestible matter, should be particularly avoided at this early period of the day.
The articles of food to be chosen for the breakfast-table must depend entirely on the state of the health, the occupations, &c., of those assembled round it. Coffee appears to be, by common consent, the favourite beverage. For the delicate, the bilious, and the young, it should neither be taken too strong, nor very weak, and should be softened down with milk or cream, and well sweetened with sugar. Tea is more apt to affect the nerves and stomach than pure unchicoried coffee. Green tea, taken thus early in the day, often acts as an absolute poison, though a slow one. We have seen severe fits of vomiting and exhaustion follow its use.
The solid food for breakfast should be easy of digestion, and nutritious. Females, children, and persons leading a sedentary life, should confine themselves to a sufficient quantity of good meal-bread with only a moderate quantity of butter, to which an egg, or a small rasher of mild bacon, may be advantageously added. For very young children there is no better breakfast, where it agrees with them, than scalding-hot new-milk poured on sliced bread, with a slice or two of bread and butter to eat with it. Parties engaged in active occupations may extend their exploits somewhat farther, and add to this bill of fare a little ham or cold meat. When an undue time will elapse before the luncheon or dinner, and particularly during the colder season of the year, the broiled leg of a fowl, an under-dressed mutton chop, or a little tender beef-steak, will be found, by the parties last referred to, most useful; nay, in many cases, invaluable. But excess must be particularly avoided. The object is to take enough food to maintain the system in full energy and vigour, and particularly to avoid offending the stomach by overloading it; a misfortune easily effected at the breakfast table. Old commercial travellers—men wise in the mysteries of life and its enjoyments—are scrupulously careful to make a good, but not a heavy breakfast, before commencing the arduous duties of the day. See Déjeûner, Meals, &c.
Breakfast Pow′der. Syn. Rye′-coffee, Dillen′ius’s c., Hunt’s Econom′ical breakfast powder, &c. Rye, roasted along with a little fat, after the manner of coffee. It was once sold at 2s. 6d. the lb., and was formerly extensively used as a substitute for foreign coffee, of which it is one of the cheapest and best. Since the reduction of duty on coffee it has nearly fallen into disuse, unless it be by the grocers to adulterate that article.
BREAST (Sore). See Nipples.
Breast Pang. Syn. Angina pectoris. Symptoms.—A sudden pain occurring in the parts covered by the breast-bone and the throat, accompanied with a feeling of suffocation, and the apprehension of immediate death. The pain sometimes extends down the arms and through the back. Summon a medical man without a moment’s loss of time. Pending his speedy arrival give a drachm of ether with one third of a grain of acetate of morphia. Apply hot applications to the chest and stomach; likewise friction to the chest, back, and sides with spirits. If the relief be only partial, the dose of ether may be repeated after twenty minutes.
BREATH (Fetid). Scarcely anything is more disagreeable or disgusting than a stinking breath. Various means have been proposed to remove this annoyance, depending principally on the administration of aromatics, which by their odour smother it for a time; but these require continual repetition, and are liable to interfere with the functions of digestion. The real cause of stinking breath may generally be traced to a diseased stomach, or to decayed teeth. When the former is the case, mild aperients should be administered; and if these do not succeed, an emetic may be given, followed by an occasional dose of the Abernethy-medicines. When rotten teeth are the cause, they should be thoroughly cleansed, and then ‘stopped,’ or if this is impracticable, they should be removed. When this is impossible or inconvenient, the evil may usually be lessened by keeping them scrupulously clean. Dirty teeth also often cause the breath to smell; and hence the use of the tooth-brush should be a daily habit. Occasionally rinsing out the mouth with a little clean water to which a few drops of solution of chloride of lime, or of chloride of soda, has been added, is often an effective method. Mouth-washes of Condy’s fluid, and also of carbolic acid, both very greatly diluted, form useful remedies; as do also chlorate of potash and tannic acid in the form of mouth-washes. As a tooth-powder, fresh-burnt charcoal, and particularly areca-nut charcoal, is without comparison the best. Lozenges, such as the following, have been strongly recommended354 to sweeten and purify the breath:—Gum-catechu, 2 oz.; white sugar, 5 oz.; orris powder, 1 oz.; neroli, 5 or 6 drops; make them into a paste with mucilage, and divide the mass into very small lozenges. 20 or 30 drops of oil of cloves may be substituted for the orris and neroli, at will. One or two may be sucked at pleasure. When the breath of a child or infant, usually so sweet and fresh, smells unpleasantly, it indicates stomach derangement of some sort. Very frequently it is indicative of worms. See Cachou Aromatisé, Pastils, &c.
BREW′ING. The art of making beer.
The only ingredients allowed by law to enter into the composition of beer are malt, sugar, hops, or any substitute for hops, and water, together with a little yeast.
The apparatus and utensils required under the common system, in brewing beer, are—
1. A copper or boiler capable of holding fully two thirds of the quantity proposed to be brewed; with a gauge-stick to determine the number of gallons of fluid at any given depth therein; and a wooden cover to place over it before the boiling commences, or when not in use. A copper capable of holding not less than 140 galls. is a convenient size for brewing a quarter of malt, and is commonly known as a one-quarter copper.
2. A mash-tub or mash-tun capable of containing one third more than the copper.
3. One or more tuns or vessels, to ferment the beer in.
4. Three or four shallow coolers, to reduce the wort as rapidly as possible to a proper temperature for fermentation.
5. One or two copper or wooden bowls, for baling, &c.
6. A thermometer with a scale reaching from below 32° to a few degrees above the boiling-point of water (say to 225° or 230° Fahr.).
7. A saccharometer, for taking the density of worts and beer.
8. A suitable number of casks (clean and sweet), to contain the beer.
9. One or more large funnels or tunners.
10. Two or more clean pails.
11. A hand-pump of a size proportionate to the brewing.
12. A mill, for crushing the malt. Brewers, for sale, are restricted by law to the use of mills with plain metal rollers.
These articles will vary in value from £10, upwards to many hundreds, or even thousands, according to the extent of the brewing; but the whole of them necessary for a private family may be bought for less than the former amount, as the mill, pump, &c., may then be dispensed with, and the rest may be of the simplest and least expensive character possible. By proper care they will last for 30 or 40 years, and still continue in a useful state.
Preliminary proceedings:—
The malt is chosen according to the intended character of the brewing—pale, amber, roasted, or any mixture of them, as the occasion may require. It is bruised or crushed in a mill (malt-mill) before employing it in brewing, that it may be the more readily acted on by the water. It should not be ground too small, as it would then make the wort thick, and cause it to run with difficulty from the mash-tun. The crushed malt may advantageously lie for a few days in a cool situation, by which it will attract a considerable quantity of moisture from the air, and be the more easily exhausted by the water used in mashing. Pale malt may be used coarser than amber or brown malt. A bushel of good malt should measure 11⁄4 bushel when ground; and a quarter should yield between 91⁄2 and 10 bushels, the quantity slightly varying according to the degree of bruising it has undergone. On the large scale, malt is ground in crushing mills furnished with plain iron rollers; on the small scale, by wooden rollers or mills worked by hand. For private brewing, the malt is generally bought ready crushed or ground, for convenience sake.
The hops, after being taken from the ‘pockets’ or ‘bag,’ are crumbled with the hands ready to be thrown into the copper. For general purposes those grown in Kent, and of the present season, are preferred. For the finer sorts of ale, East Kent hops are commonly used; and when it is intended to keep the liquor for a long time, those known by the names of Country’s, Alton’s, or Farnham hops, are employed.
The quantity of hops required by a given measure of malt varies from 2 lbs. to 22 lbs. per quarter, according to the strength or gravity of the wort, the character of the beer intended to be brewed, and the climate which the beer may have to sustain. Export beer requires, as a rule, an exceptionally large amount of hops to enable it to bear without injury the heat of the country to which it is shipped. The following are the usual proportions:—
| Table beer | 2 | lbs. | 1 | qr. |
| Mild ale or porter | 4 | ” | 1 | ” |
| Brown stout | 5 | ” | 1 | ” |
| Scotch ale (best) | 5 | ” | 1 | ” |
| Strong ale (ordinary) | 51⁄2 | ” | 1 | ” |
| Strong ale (keeping) | 8 | ” | 1 | ” |
| Bitter ale | 10 to 14 | ” | 1 | ” |
| East India ale (export) | 12 to 22 | ” | 1 | ” |
When a strong, coarse hop is used, a less quantity suffices for the same strength brewed, but the flavour is always inferior.
The water, which should be clear, and free from all traces of decomposing animal and vegetable matter, must be provided in abundance. Of late years hard water has been preferred by many brewers, on the ground that beer brewed with it is self-fining, and hence requires no artificial clarification either in the vat or cask.
Hard water is also much to be preferred to355 soft in brewing stock beers; since by its rendering the albuminous matters contained in the mash insoluble, it prevents the fermentation to which these would otherwise give rise, and so assists in the preservation of the beer, and in keeping it free from acidity.
The German brewers, however, who do not brew beverages intended to be kept for any time, on the contrary, employ a soft water, by which means the albuminous substances contained in the malt are rendered soluble, and become diffused throughout the beer, and possibly add in some measure to its nutritive qualities. Hard waters are said to have the property, over soft ones, of enabling the beer to retain more saccharine matter, and hence to improve its flavour and to give it more body. The ales of Burton are pre-eminent for their excellent quality and keeping properties. In the neighbourhood of Burton there are extensive beds of new red sandstone and gypsum, by sinking wells into which the Burton brewers obtain the water from which they make their beers. From the subjoined analyses of Burton well waters it will be seen that this water is a very hard one, and contains, besides other salts, a very large quantity of sulphate of lime.
Analysis of the water used in Messrs Allsopp’s brewery (Dr Böttinger):—
| Amount of ingredients in the imperial gallon, represented in grains. | |
| Chloride of sodium | 10·12 |
| Sulphate of potash | 7·65 |
| Sulphate of lime | 18·96 |
| Sulphate of magnesia | 9·95 |
| Carbonate of lime | 15·51 |
| Carbonate of magnesia | 1·70 |
| Carbonate of iron | 0·60 |
| Silica | 0·79 |
| ——— | |
| 65·28 |
Analysis of water from a well at the brewery of Messrs Bass (Cooper):—
| Carbonate of lime | 9·93 |
| Sulphate of lime | 54·40 |
| Chloride of calcium | 13·38 |
| Sulphate of magnesia | 0·83 |
| ——— | |
| 78·54 |
The whole of the water used in the Burton breweries is obtained from wells, and not from the river Trent, as was at one time erroneously supposed. A factitious Burton water may, it is said, be obtained by adding sulphate of lime and salt to any soft water, in the proportions stated in the above analyses.
Dr C. Graham is of opinion that, although the properties of the Burton well waters are very greatly due to the large quantity of sulphate of lime contained in them, the chlorides of sodium and calcium are also important constituents.
The yeast must be fresh and good; and all the vessels and utensils perfectly sweet and clean. If the latter be neglected, even the most skilful brewing will prove a failure.
Process of brewing:—
1. Mashing:—The ground or bruised malt placed in the mash-tun is macerated for some time in hot water, and the infusion (wort) drawn off from a hole in the bottom, over which a strainer or false bottom is placed, to prevent the malt passing out along with the liquor. During the process of mashing a peculiar principle contained in the malt, called diastase, reacts upon the starch with which it is associated, and converts it into grape-sugar. The more completely this conversion is effected the richer will be the resulting wort in sugar or “saccharine,” and the stronger and more alcoholic the beer produced by its fermentation. It is, therefore, a desideratum with the brewer to mash at the temperature which most fully promotes this important object. The best temperature for this purpose ranges between 150° and 170° Fahr. When more than one mash is made, the first should be something lower than the first-named temperature; the second may be from 175° to 185°; and the third as high as 200° Fahr.
If the first mashing has been rightly conducted, the whole of the starch will be converted into sugar, and the action of the second and third mashings is merely to wash out any of the remaining saccharine matter still existing in the crushed grain.
In practice, as soon as the water in the copper acquires the temperature of 170°, 45 galls. are run into the mash-tun, and 1 quarter of crushed malt gradually added to it. The whole is now thoroughly mixed with the mash liquor, by means of oars, or machinery, the agitation (mashing) being continued for 30 or 40 minutes, when 36 galls. more water from the copper are added, and the whole again well agitated, as before. The mash-tun is now closely covered up, and the mash allowed to repose for about two hours, in order that the diastase may exert its saccharifying power upon the unconverted starch of the malt. At the end of this time the tap is set, and the wort run into the ‘underback.’ It generally amounts to about 50 galls. The second mash is then made with about 60 galls. of water, at 185° F., and the whole process repeated as before. After an hour the liquor is drawn off, and the malt drained ready for the third mash. This time only 35 galls. of water are added at 200° F., and the whole is seldom allowed to stand longer than half an hour. It is then run off, and the malt allowed to drain as dry as possible.
In some cases the worts of the first and second mashes only are used for strong beer; that of the third mashing being kept for table beer, or as liquor to mash a fresh quantity of malt.
Pale malt and mixtures of malt and raw grain should be mashed for a longer time, and356 at a somewhat lower temperature than brown or high-dried malt.
Instead of making second and third mashes as above described, it has long been the practice in Scotland, and is now becoming common in England, to sprinkle the surface of the grains in the mash-tun with water, at or about the temperature of 180° Fahr., by means of a simple revolving instrument termed a ‘sparger,’ and to let the liquor drain through the goods and run off by the tap with the last portions of the first wort. By this means the whole surface of the grain is continuously and regularly sprinkled with hot water.
When sugar is used it may be either mixed with the malt in the mash-tun, at the time of mashing, or put into the underback, just before setting the taps, and the hot wort run upon it. The proportions of malt and sugar vary according to the quality of the latter, but, on an average, from 170 lbs. to 200 lbs. of good raw sugar may be taken as the equivalent of a quarter of malt.
2. Boiling:—The wort is next transferred from the underback to the copper, and heated to the boiling-point as soon as possible, the object of this expedition being to prevent the formation of acid in the wort, by exposure to the air, before undergoing the changes which take place in the copper. As soon as the boiling of the wort commences the hops are added, and the boiling is continued for about 2 or 21⁄2 hours. A longer boiling is highly objectionable, owing to the extraction of a heavy, resinous bitter from the hop, and the danger of losing the volatile oil upon which the aroma depends. For mild beers the worts are seldom boiled so long; for strong keeping ales, sometimes a little longer. The boiling is known to be completed when the liquor ‘clears,’ as it is called, and albuminous flocks sink to the bottom of the copper.
The hops, strained from each wort, are returned into the copper with the following one.
The average loss by evaporation in the process of boiling varies from 1⁄6th to 1⁄7th of the original bulk of the wort. The gravity increases at the same time in about the ratio of 5 to 4; so that if the gravity be, at first, say 32 lbs. per barrel, it will at the end of the operation have risen to about 40 lbs.
3. Cooling:—The wort, under the common system, is ‘run off’ from the copper into the ‘hop-back,’ through a strainer which keeps back the hops. It is then pumped into large square shallow vessels called ‘coolers,’ where it is freely exposed to a current of air to reduce its temperature as quickly as possible, in order to avoid acidity or ‘souring.’ In 6 or 7 hours, or sooner, the temperature should fall to about 60° Fahr. In warm weather the depth of the liquor in the coolers should not exceed 3 or 4 inches; and in cold weather not more than 5 or 6 inches. As soon as the temperature has fallen to about 60° the liquor is ‘tunned’ and ‘yeasted.’
The loss by evaporation and condensation in the coolers varies from 13 to 18 galls. per quarter.
4. Fermentation:—The cooled wort is next run into the fermenting tuns or vessels (gyle-tuns). In small brewings these may be casks with one of their heads removed; but under any form they must not be more than 2⁄3rds filled. The yeast, previously mixed with a little wort, and kept until the whole has begun to ferment (technically termed ‘lobb’), is now added, and after agitation the vessel is covered up, and kept so, until the fermentation is well established. By this time the temperature has risen from 9° to 15°.
The quantity of yeast employed, and the temperature of the wort when it is added, differ in different breweries and for different kinds of beer. It seldom exceeds 2 lbs. per barrel unless the weather is unusually cold, or the yeast old or stale, when a larger proportion is required. The Scotch brewers generally take only 1 gall. of yeast to fully 4 hhds. of wort.
In England, the temperature at which the yeast is added varies from 55° to 65° Fahr. In Scotland, the common temperature is 51° to 52°. In cold weather the heat may be 5° or 6° higher than in mild and warm weather, and a little more yeast may also be advantageously employed. In cold weather ale is commonly tunned at 60°, porter at 64°, and weaker beers at 65° or 70° Fahr. In ‘warm weather’ strong beer should be 4° or 5°, and other beers 7° or 8° cooler than the ‘heats’ just mentioned. On the small scale, 1 to 11⁄4 pint of yeast may be used to every barrel of strong-beer wort, and 3⁄4 pint to every barrel of mild-beer wort.
The commencement of the fermentation is indicated by a line of small bubbles forming round the sides of the tun, and in a short time extending over the whole surface. A ‘crusty head’ soon forms, and then a ‘fine rocky head,’ followed by a ‘light frothy’ one. At length the head assumes a yeasty appearance, the colour becomes yellowish brown, and a vinous odour is developed. As soon as this last head begins to fall, the tun is skimmed every 2 or 3 hours, until no more yeast is formed. The object of this is, not only to check the violence of the fermentation, but also to remove a peculiar bitterness, with which the first portion of the yeast is impregnated. The beer is then put into casks, or ‘cleansed,’ as it is called. A minute attention to every stage of this process is necessary to secure a fine flavour and a brilliant beverage.
It may be regarded, as a rule, that the lower the temperature, and the slower, more regular, and less interrupted the process of fermentation, the better will be the quality of the brewing, and the less likely to change by age. A little more yeast is required in winter than in summer. When the fermentation becomes slack in the ‘gyle-tun,’ a little more ‘lobb’ is357 generally added, and the whole is well ‘roused up,’ On the contrary, if the temperature rises considerably, or the fermentation becomes too brisk, the wort is cooled a little and skimmed, or at once cleansed.
5. Cleansing:—This consists in running the beer from the gyle-tun into casks, or other vessels, set sloping, so that the yeast as it forms way work off the one side of the top, and fall into a vessel placed below to receive it. In small brewings the beer is often at once transferred from the gyle-tun to the ‘store-casks,’ which are sloped a little until the fermentation is over, when they are skimmed, filled, and bunged.
The process of cleansing is generally commenced as soon as the ‘saccharine’ in the fermenting wort falls to about 10 lbs. per barrel, a degree of attenuation which it usually reaches in about 48 hours. Some brewers add a little wheat-flour or bean-flour (about 1⁄4 lb. per barrel) to the beer in the gyle-tun, shortly before cleansing, to quicken the discharge of yeast; but it is not clearly ascertained whether such a plan is advantageous, or the contrary.
6. Storing:—As soon as the fermentation is concluded, which generally takes from 6 to 8 days, or longer, the clear liquor is pumped into the store-casks or vats, which are then closely bunged, and deposited in a cool cellar, if not already there, to mature. The preference, which at present exists in most parts of the United Kingdom, is for mild, freshly-brewed malt liquors; the good old or mature-vatted beer being now seldom met with. This, of course, is a source of increased profit to the brewer, as it enables him to turn over his capital more rapidly, and saves the risk and expense attendant on long storage.
7. Ripening:—After a period varying from one to twelve months or longer, according to the nature of the brewing, and the condition of the cellar, the liquor will have become fine, and sufficiently mature for use. During this period the casks, &c., should be occasionally examined to see that there is no leakage, and to open the vent-holes, should any oozings appear at the joints. As equable a temperature as possible should be maintained in the cellar, by ventilation, on the one hand, and the employment of artificial heat on the other, as circumstances and seasonal changes may render necessary.
8. Fining or Clarifying:—Beer which has been badly brewed or badly stored, or which from other causes may be thick or muddy, requires clarifying by artificial means. For a barrel about 1 to 11⁄2 pint of brewer’s finings (isinglass or fish-gelatin dissolved in sour beer) is put into a bucket, and some of the beer being gradually added, the whole is violently agitated with a whisk until a frothy head is formed. The mixture is then thrown into the cask of beer, and well ‘rummaged up,’ after which the bung is replaced, and the liquor allowed to repose for a week or ten days.
Sometimes the above method is found to fail with weak and bad-conditioned beer. When such is the case, the addition of a teaspoonful of sulphuric acid, or a table-spoonful of powdered catechu (previously dissolved in 1⁄2 a pint of boiling water), followed by agitation for a quarter of an hour, will generally cause the ‘finings’ to clarify the liquor; 2 or 3 oz. of tincture of catechu (mixed with a little water) may be used in the same way. A handful of hops, previously boiled for five minutes in a little of the beer, and then added to the barrel, and the whole allowed to stand for a few days, before proceeding to clarify it, will generally have a similar effect, and cause the ‘finings’ to act with certainty. It is the absence of the proper quantity of astringent matter in beer that usually renders them ineffective.
M. Brescius employs tannin for the clarification of beer. To 1000 litres of beer he adds 140 grains of tannin dissolved in 3⁄4 of a litre of water, which is thoroughly stirred up. After three or four days he adds one litre of isinglass or two of gelatin in the proportion of 1 kilo. to 100 litres. The complete clarification requires about eight days.
Gen. commentary. The preceding is a concise account of all the essential operations of the system of brewing at present practised in this country. On the large scale, extensive and costly apparatus and machinery are employed for the purpose. On the small scale, various modifications, of a minor character, or the several processes herein detailed, are frequently adopted according to the circumstances or ingenuity of the operator. The principles and practice of brewing beer are, however, essentially the same under all the conditions here referred to. In Scotland, only one mash is made, and that at a temperature of about 180° Fahr., with one third of the quantity of the water required for the brewing. The ‘mash-tun’ is then covered up for about half an hour, when the wort is drawn off, and the operation of ‘sparging’ begun. This operation is continued until the density of the mixed worts becomes adapted to produce the quality of the ale then under process of manufacture. The ‘gyle-tun’ (fermenting-tun) is set at from 50° to 60° Fahr., the fermentation being continued slowly for fifteen to twenty days; and the ale is not ‘cleansed’ before the degree of attenuation falls to about 1⁄2 lb. per day, and not more than one fourth of the original gravity of the wort remains. Scotch ale is justly celebrated for its superior quality. Its usual original gravity is from 34 to 45 lbs. per barrel.
In Bavaria, a country remarkable for the excellence of its beer, the wort is made to ferment at a low temperature, until all the substances which favour acetification have been rendered insoluble, and have separated from358 the liquor. The fermentation is conducted in wide, open, shallow vessels, which afford free and unlimited access to atmospheric oxygen; and this in a situation where the temperature does not exceed 45° to 60° Fahr. A separation of the nitrogenous constituents thus takes place simultaneously on the surface, and within the whole body of the liquid. The clearing of the fluid is the sign by which it is known that these matters have separated. The fermentation usually occupies three or four weeks, and is conducted during the cooler portion of the year only, and in a situation removed as much as possible from the influence of atmospherical changes of temperature. The sedimentary yeast (unterhefe), and not the surface yeast (oberhefe), of the Bavarian fermenting backs is employed.
The beers of England and France, as well as most of those of Germany, become gradually sour by contact with the air. This defect, as observed by Liebig, does not belong to the beers of Bavaria, which may be preserved, at pleasure, in half-full casks, as well as in full ones, without suffering any material alteration. This precious quality must be ascribed to the peculiar process employed for fermenting the wort, called by the German chemists ‘untergährung,’ or fermentation from below; and which “has solved one of the finest theoretical problems that had long taxed the ingenuity and patience of both the scientific and practical brewer.” (Liebig.)
The ‘Comptes Rendus,’ lxxvii, 1140-1148, contains a paper by M. Pasteur on the manufacture of an ‘unalterable beer.’ In this communication he states that the liability of beer to turn sour, ropy, &c., is due to the presence of special ferments derived from the air, and from the materials used. By boiling the infusion of malt and hops, cooling out of contact with air, and fermenting with pure yeast[232] in vessels to which only carbonic acid or pure air is admitted, a beer is produced of superior quality, which may be preserved without trouble for any time. Even a partial adoption of these precautions is attended with valuable results. In preparing pure yeast to start with, the author makes use of the fact that oxygen favours the growth of true yeast, but hinders the propagation of the other ferments. Pure yeast being obtained, the beer is afterwards fermented in an atmosphere nearly destitute of oxygen, as its quality is thereby improved. Pure yeast when kept in pure air undergoes no change, even at summer temperature. The mycoderma vini does not, as the author once thought, become changed into beer-yeast on submersion in a nutritive fluid; under these circumstances it acts as an alcoholic ferment, but does not propagate itself.
“In the ordinary fermentation of grape-juice and worts these liquids do not furnish a quantity of alcohol equivalent to the sugar which they contain; and this because a certain portion of the sugar serves for the oxidation of the gluten, and is not transformed like the rest. But wherever the liquor has arrived at the second period of transformation, the product in alcohol ought to be equivalent to the quantity of sugar present, as actually happens in all fermentations (sedimentary) which are not accompanied with a formation, but a disappearance of the yeast. According to Dr Ure, worts furnish, in the Bavarian breweries, from 10% to 20% more alcohol than they do by the ordinary process of fermentation (obergährung), or that excited by the use of ‘oberhefe’ or top-yeast.”
East-India Ale or Pale Ale, for exportation, is brewed from worts of a sp. gr. of from 1·063 to 1·070. For the best varieties, 15 to 16 lbs. of the finest East Kent hops are used to every quarter of pure malt. The pale ale or bitter beer of the publicans is commonly a very weak liquor (mere table beer), highly bittered with the hop, and too often with quassia, wormwood, and other still more objectionable substances. The process now adopted by the great brewers of pale ale at Burton-on-Trent combines all the most admirable points of both the Bavarian and Scotch systems of brewing.
Berlin White ale or Pale beer is brewed from wheat-malt mixed with about 1⁄6th part of barley-malt, the ‘wort’ being boiled with hops, 1⁄2 lb. to the bushel, and slightly fermented with ‘top-yeast,’ at a rather higher temperature.
The desire of evading the duty led to the discovery of its being only necessary to employ 1⁄3rd, or less, of the grain, in the form of malt; this portion being sufficient to convert into sugar, in the process of mashing, the starch of the unmalted grain forming the other part. This plan answers well when the wort is merely intended for the production of ‘grain spirit,’ but beer so made is insipid and inferior in quality to that brewed wholly of malt. Inferior kinds of beer have also been made from other ingredients than barley-malt, among which may be named the grain of the cheaper cereals, bran, potatoes, turnips, beet-root, carrots, parsnips, pea-shells, and other vegetable substances rich in starch and sugar, all of which will produce beer by being mashed with water in the common way, with about 9% or 10% of barley-malt.
One quarter of the best barley-malt yields, by skilful mashing, fully 84 lbs. of ‘saccharine,’ or soluble sweet extractive matter. This concentrated within the compass of one barrel (33 galls.) gives a sp. gr. of 1·234. In the process of mashing about 4⁄7ths of this quantity of saccharine (or 48 lbs.) is generally carried off in the first wort; 2⁄7ths (or 24 lbs.) in the second wort; and 1⁄7th (or 12 lbs.) in the third wort; the strengths of the worts being to each other respectively as 4, 2, 1. The average gravity obtained by the common brewers from malt of current quality ranges from 80359 to 81 lbs. Sugar may be used as a partial substitute for malt, with, in most cases, some degree of saving to the brewer, and without injury to the quality of the beer. The kind of sugar to be used will depend on the quality of the beer to be brewed, but it should be remembered that a bad sugar will not, any more than bad malt, yield a sound palatable beer. From 170 lbs. to 200 lbs. of good raw sugar may be taken as the average equivalent of a quarter of malt.
When the process of mashing has been properly conducted, the wort, after leaving the cooler, should not be turned blue by tincture of iodine, or by iodide of potassium mixed with a few drops of nitric acid. If it turns blue some of the starch has escaped conversion into sugar, and is dissolved in the liquor.
By multiplying the decimal part of the number representing the specific gravity of a wort by 360 (the weight in pounds of a barrel of pure water), we obtain the quantity of saccharine per barrel, corresponding to the given sp. gr.; and by dividing the joint weight of saccharine and water, per barrel, by 360, we obtain the specific gravity. Thus—
Suppose a sample of wort to have a specific gravity of 1·055, then—
Again, a barrel of wort weighs 379·8 lbs., that is, 360 lbs. for the weight of a barrel of water, and 19·8 lbs. for the weight of saccharine in the water, then—
It is usually stated in works on brewing that certain temperatures must be reached by each variety of beer, during the progress of the fermentation, in order for the liquor to acquire its characteristic flavour. Thus, it is stated that mild beer begins to acquire flavour when the heat of fermentation arrives at 75° Fahr., increases at 80°, and is highest at 90°, but sometimes even reaches 100°. Old ale is said to obtain its best flavour at a temperature not exceeding 75°; and porter at 70° Fahr. In order to reach these temperatures the worts are directed to be set at from 10° to 15° lower, the rise being due to the heat generated during the fermentation. That these statements refer principally to the old methods of brewing is shown by the fact that some of the brewers of Bavaria, Scotland, and Burton-on-Trent produce rich and high-flavoured liquors at temperatures vastly below those above enumerated. Still, however, the fact must not be concealed, that since the introduction of the new German system of brewing into England the general character of its beers, as they reach the consumer, are inferior in strength and flavour to those of a former period. We may now seek almost in vain for the fine vinous, high-flavoured, invigorating old beers vended in our early days by the common publicans and tavern-keepers, of whom the larger majority were their own brewers. Under the new system of chemical brewing, as worked by those huge monopolists, the ‘great brewers,’ the only object appears to be to obtain the largest quantity possible of saccharine out of the quarter of malt, and to convert this into the largest possible quantity of beer, with little regard to flavour or quality, but an excessive one for their own profits. In due course this liquor is forced on their helpless tenants the publicans, who, in their turn, ‘reduce’ and ‘doctor’ the liquor, until, by the time it reaches the consumer, its insipidity and low strength would have led even a brewer’s drayman of the last century to cast it into the kennel.
The best times for brewing are the spring and autumn; as at those periods of the year the temperature of the air is such as to permit of the easy cooling of worts sufficiently low, without having recourse to artificial refrigeration, or to the use of machinery for the purpose. Old ale cannot be conveniently brewed in summer.
Beers are classed by the brewers into—
The densities of the worts employed for different kinds of beer vary considerably, as will be seen by the following table:—
| Description. | Pounds per barrel. | Specific Gravity. |
| Burton ale, Class 1 | 40 to 43 | 1·111 to 1·120 |
| Burton ale, Class 2 | 35 to 40 | 1·097 to 1·111 |
| Burton ale, Class 3 | 28 to 33 | 1·077 to 1·092 |
| Ordinary ale | 25 to 27 | 1·070 to 1·075 |
| Common ale | 21 | 1·058 |
| Scotch ale, Class 1 | 40 to 44 | 1·111 to 1·122 |
| Scotch ale, Class 2 | 33 to 40 | 1·092 to 1·111 |
| Porter (ordinary) | 18 | 1·050 |
| Porter (good) | 18 to 21 | 1·050 to 1·058 |
| Porter (double) | {?} to 22 | 1·055 to 1·060 |
| Brown stout | 23 | 1·064 |
| Brown stout (best) | 26 | 1·072 |
| Table beer | 12 to 14 | 1·033 to 1·039 |
| Small beer (com.) | 6 | 1·017 |
Exportation of Beer:—When beer is exported from any part of the United Kingdom, either as merchandise or ships’ stores, the brewer or exporter of such beer is allowed a certain drawback of duty. The amount is proportional to the quantity of malt or sugar360 inferred to have been used in the brewing of the beer. Thus, if the original specific gravity of the worts from which the beer was brewed were not less than 1·040, a drawback is granted of 4s. 3d. per barrel. This is equivalent to a return of the duty on 11⁄2 bushels of malt, with an allowance of 3d. for licence duty, now charged in lieu of the abolished hop duty. For every additional 5 degrees of specific gravity, from 1040° to 1125° inclusive, a further sum of 5d. per barrel is allowed.
[For further information connected with the above subject the reader is referred to the separate articles—Ale, Beer, Dextrine, Diastase, Fermentation, Malt Liquors, Porter, Saccharometer, Specific Gravity, Wort, Yeast, &c.]
Brewing Uten′sils. The cleansing and preservation of brewing utensils, beer casks, &c., has frequently engaged the attention of practical men and brewers’ chemists. To preserve them sweet they should always be thoroughly cleaned before setting them aside. Contact with soap, or any greasy material, should be carefully avoided. A scrubbing-brush and scalding-hot water are generally sufficient to clean them. Great care should be taken to remove every particle of yeast or fur on the sides and bottom; and after being well drained they should be stowed away in some clean and cold situation, properly exposed to the fresh air. Should they become tainted or mouldy, a strong lye of pearl-ash, common salt, or quick-lime, may be spread over them, scalding hot, with a broom or scrubbing-brush. Washing them with oil of vitriol diluted with about 7 or 8 times its bulk of water, is another excellent and very effective method. Fresh-burnt charcoal has also been employed for the same purpose. In each case the vessels must be subsequently thoroughly washed out with clean water, as before. Steam, assisted by the action of a chain, has been successfully applied to clean casks in several breweries. Bisulphite of lime has, within the last few years, been highly recommended for sweetening and cleaning vats, casks, &c. It is also said to prevent beer from developing acidity. See Casks, Vats, Sporokton, &c.
BRICKS. Brick-making scarcely comes within the province of this work. In connection with hygiene, however, we may call the reader’s attention to the superior advantages of both hollow and waterproof bricks; the first, for ventilation and lightness; the last, for preserving the dryness and integrity of our homes under all the vicissitudes of climate, season, and weather, either on damp soils or dry ones. Workman’s “Patent Waterproof Bricks” received a strong commendatory notice from the Commissioners of the “Great International Exhibition” of 1851.
BRILLIANTINE. 1. Castor oil, 1 part; eau de Cologne, 4 parts. Mix. 2. Honey, 1 oz.; glycerin, 1⁄2 oz.; eau de Cologne, 1⁄2 oz.; spirit of wine, 2 oz. Mix.
BRINE (for Meat). Prep. 1. A nearly saturated solution of common salt, 1 lb.; and saltpetre, 1 oz.; in soft water.
2. To the last add of sugar or treacle, 1⁄2 lb. Bay-salt is recommended when the meat is to be kept for a very long period. Meat preserved in brine that has been used for curing several times is said to become poisonous. See Pickling, &c.
Brine, Red-Cabbage. Red-cabbage leaves steeped in a strong solution of common salt. Used as a test for acids and alkalies.
Brine, Vi′olet. From the petals of the blue violet, as the last. Used as a test for acids.
BRIOCHE PASTE (bre-ōsh′). In cookery, a species of paste, or crust, prepared of eggs and flour, fermented with yeast, to which a little salt, a large quantity of sugar, and about half as much butter as the weight of the flour used, are afterwards added, and well worked in. Used as an addition to soup, and as a casing for lobsters, patties, eggs, &c.
BRISK′NESS. The natural briskness and sparkling of fermented liquors depends on the gradual evolution of carbonic acid gas within the body of the fluid, by the process of fermentation. See Malt Liquors, Porter, Wines, &c.
BRIS′TLES (brĭs′lz). The stiff hair of swine, &c. They are commonly stiffened by immersion for a short time in alum-water; and are dyed by steeping them for a short time in any of the common dyes used for cotton or wool.
BRITAN′NIA METAL. Syn. Tutania. A superior species of pewter, used for teapots, spoons, &c.
Prep. 1. Plate-brass, bismuth, antimony, and tin, equal parts, melted together, and the resulting alloy added at discretion to melted tin, until it acquires the proper degree of colour and hardness.
2. To the first alloy, prepared as in No. 1, add one fifth of its weight of metallic arsenic, before mixing it with the melted tin.
3. Antimony, 1 part; brass, 4 parts; tin, 5 or 6 parts; melted together. See Queen’s Metal (Alloys), Pewter, &c.
4. Tin, 150 parts; copper, 3 parts; antimony, 10 parts.
5. Tin, 461⁄2 parts; copper, 1 part; antimony, 3 parts.
Britannia Metal for Casting. a. Tin, 100 parts; hardening (see below), 5 parts; antimony, 5 parts. b. Tin, 105 parts; copper, 2 parts; antimony, 12 parts.
Britannia Metal (Best) for Handles. Tin, 140 parts; copper, 2 parts; antimony, 5 parts.
Britannia Metal, Hardening for. Tin, 1 part; copper, 2 parts.
Britannia Metal (Best) for Lamps, Pillars, and Spouts. Tin, 75 parts; copper, 1 part; antimony, 33⁄4 parts.
Britannia Metal for Registers. Tin, 25 parts; antimony, 2 parts; hardening, 2 parts.361
Britannia Metal for Spinning. Tin, 25 parts; antimony, 1 part; hardening, 1 part.
Britannia Metal (Best) for Spoons. Tin, 20 parts; antimony, 2 parts; hardening, 1 part.
Britannia Metal for Spouts. Tin, 461⁄2 parts; copper, 1 part; antimony, 2 parts.
BRITANNIA SILVER. Under this name there is, or was, offered to the public at Vienna, and probably elsewhere, under the misleading recommendation that it is a perfect substitute for silver, a heterogeneous metallic composition, in the form of spoons, forks, candlesticks, cups, &c. The Britannia silver is sometimes, or always, light, silvered, Britannia metal (an alloy of 86 tin, 10 antimony, 3 zinc, 1 copper; or of 2 copper, 6 zinc, 21 antimony, 71 tin; or of 1·84 copper, 81·90 tin, 16·25 antimony, and 1 zinc). One firm announces that Britannia silver is silver-white throughout, a colour which can only be obtained in similar alloys by the addition of arsenic. Another firm sells candlesticks of inferior packfong as Britannia metal, and another actually sells tinned Bessemer steel-plate cups as guaranteed Britannia silver. (Ackerman.)
BRIT′ISH GUM. See Gum.
BRITISH WINES. See Wines.
BROC′COLI. [Eng., L., Ger.] Syn. Brocoli, Fr.; Broccolo, It. A well-known sub-variety of cauliflower. The qualities, and the mode of dressing broccoli, are similar to those of cabbages, noticed elsewhere. See Vegetables (Culinary), &c.
BROKEN KNEES (in Horses). The wound should first be thoroughly washed, and then sewn up, and fomented with tepid water. Afterwards cold-water dressings containing a little carbolic acid may be applied. Perfect rest is essential, and, where necessary, splints and slings must be had recourse to. After the wound has thoroughly healed blisters are recommended for restoring the hair.
BROKEN WIND (in Horses). Of the many remedies said to be useful in this malady few, if any, appear to exercise any permanent advantage. There is no reason, however, why a horse affected with broken wind should not be made serviceable if the precaution be taken to put him to moderately slow work, if the following precautions be followed. His food should be given him in small quantity and at frequent intervals. The oats should be bruised and the hay cut small, and both be slightly damped before he partakes of them. This dietary may be varied by small doses of carrots or turnips.
The amount of fluids should be restricted, and he should be fed and watered at least an hour before going to work. A mild physic ball should also be occasionally administered.
Dogs suffering from asthma should be subjected to the same treatment. To a full-sized dog ten drops each of ether and tincture of belladonna may be given every hour during an attack of spasm until the breathing becomes easier.
BRO′MA. Prep. 1. Pure cocoa, 1 lb.; sugar and sago-meal, of each 4 oz.; mix. British arrow-root (i. e. carefully prepared potato-starch) is often substituted for the sago.
2. As the last, but using fine wheat flour in lieu of sago-meal. Made into a beverage in a similar way to cocoa.
BRO′MAL. C2Br3HO. A colourless, oily liquid, obtained by the action of bromine on alcohol. Sp. gr. 3·34; boiling point above 212° F. Like chloral it yields a solid hydrate with water. Because of its powerful irritant properties it seems unlikely to prove useful, either as a hypnotic or as an anæsthetic.
BRO′MIDE (-mĭd). Syn. Bro′muret*, Hydrobro′mate*; Bromi′dum, Bromure′tum, Hydrobro′mas, L.; Bromide, Bromure. Fr. A chemical compound of bromine with another radical.
Prop., &c. The soluble bromides give white precipitates with nitrate of silver, acetate of lead, and protonitrate of mercury. That from the first of these is insoluble in dilute nitric acid and in ammonia water unless concentrated; and it has a slight yellowish tinge, changing to a violet on exposure to the light. A few drops of liquid chlorine poured upon a bromide, followed by agitation of the mixture with a little sulphuric ether, furnishes an ethereal solution of bromine. [For the other bromides see the respective bases.]
BRO′MINE (-mĭn), (bromos, a stink). Br. Syn. Brome*; Bro′mium, Bromin′ium, L.; Brôme, Fr. An elementary substance, discovered by M. Balard, of Montpellier, in 1826.
Prep. 1. A current of gaseous chlorine is passed through the uncrystallisable residuum of sea-water called bittern, which then assumes an orange tint, in consequence of bromine being set free from its combinations; sulphuric ether is then agitated with it, and the mixture is allowed to stand, in a close vessel, until the ethereal portion floats upon the surface. This is a solution of crude bromine, and for common purposes the ether may be at once evaporated by a very gentle heat. To render it pure, caustic potassa is added in excess to the ethereal solution, or the latter is agitated with a solution of potassa, by which means bromide and bromate of potassium are formed. The whole is evaporated to dryness, and submitted to a dull red heat. The residuum is next powdered and mixed with pure peroxide of manganese; the mixture having been placed in a retort, sulphuric acid (diluted with half its weight of water) is poured in. Red vapours immediately arise, and condense into drops of bromine, which are collected by plunging the neck of the retort nearly to the bottom of a small receiver362 containing a little very cold water. The bromine forms a stratum beneath the water, and may be collected and at once put into a stoppered bottle; or it may be further purified by distillation from dry chloride of calcium.
2. Leisler’s patent for a method of obtaining bromine consists in decomposing the lye containing the bromine salt by heating it with hydrochloric acid and bichromate of potash in a leaden still having an earthenware head. The volatilised bromine with the vapour of water is conducted into a receiver containing iron turnings, bromide of iron, which dissolves in the water contained in the receiver, being formed. The bromide of iron so produced is either converted into other metallic bromides by the usual processes, or the bromine is obtained in a separate state from the iodide by treatment with sulphuric acid and bichromate of potash.
3. Large quantities of bromine are extracted from the mother liquor of carnallite, a double chloride of magnesium of potassium occurring in enormous quantities in a bed of clay in the neighbourhood of Stassfurt, near Magdeburg. The mother liquid of the carnallite at 35° B. is first freed as much as possible from the chloride of calcium it contains, by means of refrigeration. It is next evaporated down until it acquires a density of 40° B. Frank says it cannot be concentrated to the above extent, because of a waste of bromine resulting from the formation of hydrobromic acid produced by the decomposition of the lye, owing to its being overheated at the bottom of the pan. Upon being cooled to 25° C. a quantity of chloride of magnesium crystallises out, whilst the remaining liquor contains from 0·3 to 0·5 of bromine as bromide of magnesium. The liquor is then put into a sandstone apparatus such as is used for the preparation of chlorine, and the requisite quantity of manganese and hydrochloric acid being added, steam is poured into the apparatus. After about a quarter of an hour the bromine is evolved in the form of vapour, which becomes condensed by being made to pass through a leaden worm cooled in water, and is finally collected as liquid bromine in Woolff’s bottles.
The crude bromine so obtained is purified by redistillation in glass retorts. It is stated that the sandstone apparatus can be charged six times in 24 hours. In order to free the bromine from the presence of any chloride it is shaken up with a solution of bromide of potassium.
The chlorine unites with the potassium, forming chloride of potassium and liberating an equivalent quantity of bromine in so doing. Dr Frank suggests the use of earthenware worms in preference to leaden ones, these latter being acted upon and corroded by liquid bromine. In Dr Frank’s bromine works at Stassfurt the distillation is conducted in cubic stoneware vessels, having a capacity of about three cubic metres. These vessels are surrounded with belts of iron, in case of the occurrence of fracture. It was found that few stones answered the purpose required of them, as by reason of their porous nature they permitted the chloride of manganese formed during the distillation to ooze through. To remedy this the stones had to be coated with tar, a process which entailed a very serious loss of bromine, from the formation of bromine compounds with the hydrocarbons of the tar, as well as a contamination of the bromine with the tar. Subsequently Dr Frank found in the neighbourhood of Porta Westphalia a stone which answered the purpose without requiring the previous objectionable and expensive preparation with tar.
It seems that the workmen discard the respirators which are provided for their use in the bromine works, and merely tie a cloth over the mouth and nose (sometimes neglecting this precaution) when decanting the bromine.
To lessen the evil effects of the vapours upon the health of the workmen under these circumstances, the building is rendered as airy as possible by being thoroughly ventilated throughout. No workmen afflicted with asthma or with any catarrhal affection are employed, whilst those engaged are strictly prohibited from taking spirituous liquids in any form, a custom which begets an irritability of the mucous membranes, which is found to be exceedingly dangerous; on the contrary, a generous diet, and one consisting of an abundant use of bacon and butter, was found very beneficial.
Bromine is sometimes contaminated with chlorine, iodine, and occasionally bromide of carbon. A small quantity of the bromine agitated with a solution of soda, in such proportion that the fluid is made very slightly alkaline, forms a colourless solution, which, if coloured by the further addition of a small quantity of the bromine, does not become blue on the subsequent addition of a cold solution of starch. This shows the absence of iodine. Chlorine may be detected by adding a small quantity of the suspected bromine to some warm solution of potash in a capsule, evaporating, drying the residue, and distilling with bichromate of potash and sulphuric acid. Bromide of carbon has a higher boiling-point than pure bromine.
Prop., &c. A dark, reddish-coloured, volatile liquid, having an odour intermediate between that of chlorine and iodine, but much more suffocating and offensive. It solidifies at about 19°, and boils at about 145° Fahr. It is slightly soluble in water, more so in alcohol, and abundantly so in ether. Its aqueous solution bleaches like chlorine, but less powerfully. With hydrogen it forms HYDROBRO′MIC ACID; and with the bases, compounds called BRO′MIDES. Its sp. gr. is 2·976; that of its vapour, 5·39.
Tests. It is readily recognised by its colour, odour, and volatility, and by the colour363 of its vapour; by its giving a yellowish-white precipitate with nitrate of silver, which is turned violet by the action of light; and by its solutions giving an orange or yellow colour to starch, and a red tinge to solution of chloride of gold.
Uses, &c. Bromine possesses very similar medicinal properties to iodine, and has been administered in goitre, scrofula, &c., in the form of an aqueous solution composed of 1 part of bromine to 40 of water, of which 5 or 6 drops is the dose; but it is more usually given under the form of bromide of potassium (which see). The compounds of bromine are also largely used in photography in the manufacture of certain coal-tar colours, and in scientific chemistry the solution has also been used as a lotion. Bromine is a good disinfectant. It is very poisonous; the antidotes, &c., resemble those for iodine. See Bromide, Solutions, &c.
BROMOCHLORALUM (Tilden & Co., New York), for the removal of bad smells, as a disinfectant, and antiseptic. A fluid, sp. gr. 1·43, containing 27·5 per cent. of solid matter. The latter consists of 18·5 per cent. of aluminium chloride, with chalk and a considerable quantity of alkaline salts. Free bromine is not present. (H. En{?}demann.)
BROMOFORM (CHBr3). A colourless liquid obtained by distilling bromide of calcium with alcohol. It has a sp. gr. of 2·90; and boils at 305·6° F., emitting a vapour having a density 8·632. It is somewhat similar in properties to chloroform, but much more irritating; hence it has been rarely employed medicinally.
BROMTHEE—BRAMBLE TEA (?)—is a mixture of 5 parts lime flowers cum bracteis, 5 parts senna leaves, 5 parts acacia flowers, 8 parts cort. frangulæ, and 2 parts sassafras chips. (Hager.)
BROHCHI′TIS (brŏng-kī′). [L.; prim. Gr.] In pathology, inflammation of the mucous lining of the bronchia or smaller ramifications of the windpipe. In its milder form it is popularly called a ‘cold on the chest.’
Symp. The usual symptoms are hoarseness, dry cough, and a slight degree of fever, followed by expectoration of mucus, at first thin, and afterwards thick and copious. In the severer forms there is more fever, cough, and oppression at the chest, &c.
Treatm. It generally yields to small and repeated doses of ipecacuanha and antimonial diaphoretics; a light diet and mild purgatives being at the same time adopted, but in every case it is safer to have recourse to medical aid.
Horses.—Finlay Dun prescribes the following:—Tincture of aconite, inhalation of the vapour of water, ether and belladonna, carbolic acid, sulphurous acid, mash diet, salines, chlorate of potash, the salts of ammonia, chloral hydrate, mustard externally, warm clothing, but cool air. Symptoms very similar to those of bronchitis are frequently caused in calves and young cattle by the presence in the bronchii of threadworms or filaria. The cause is generally removed by the administration of a dose or two of oil of turpentine, given at intervals of a day or two.
BRON′CHOCELE (brŏng′-ko-sēle). See Goitre.
BRONZE. [Eng., Fr., Ger.] Syn. Æs, L.; Bronzo, It. An alloy of tin and copper, remarkable for the exactness of the impressions which it takes by moulding and stamping, as well as for its great durability. It has hence been always extensively employed in the casting of buts, medals, statues, &c. In ancient times, when the manufacture of steel was ill-understood, cutting instruments were commonly made of it. It was also the general material of coins of small value; a use which, of late years, has been revived in several of the states of Europe, and still more recently in the coinage of these realms. Bell-metal, gun-metal, and speculum-metal are mere varieties of bronze.
Prep. On the small scale this alloy is prepared in crucibles; but for statues and larger works on reverberatory hearths. The fusion of the mixed metals is conducted as rapidly as possible under pounded charcoal, and the melted mass is frequently stirred together to produce a perfect mixture before casting.
The proportions of the materials so vary in different castings that it is almost impossible to say precisely what quantities are the best. The following are given as examples:—
a. For Edge-tools:—Copper, 100 parts; tin, 14 parts. When skilfully hardened and tempered this alloy is capable of receiving an edge nearly equal to that of steel.
b. For Gilding:—1. Copper, 82 parts; zinc, 18 parts; tin, 3 parts; lead, 2 parts.
2. From copper, 83 parts; zinc, 17 parts; tin, 2 parts; lead, 1 part.
c. For Medals:—1. Copper, 89 parts; tin, 8 parts; zinc, 3 parts. This alloy assumes a beautiful antique appearance by age, and takes a sharp impression by stamping.
2. (M. Chaudet.) Copper, 95 parts; tin, 4 or 5 parts. This is also excellent for any small castings.
d. For Mortars:—Copper, 93 parts; lead, 5 parts; tin, 2 parts.
e. For Statuary:—1. Copper, 88 parts; tin, 9 parts; zinc, 2 parts; lead, 1 part.
2. Copper, 821⁄2 parts; zinc, 101⁄2 parts; tin, 5 parts; lead, 2 parts. These are very nearly the proportions of the celebrated statue of Louis XV.
3. Copper, 90 parts; tin, 9 parts; lead, 1 part.
4. Copper, 91 parts; tin, 9 parts.
For a gold varnish for bronze objects refer to Brass.
Obs. Several analyses have been made of ancient cutting instruments, from which it appears that the proportion of tin varies from 4% to 15%; a fact which tends to prove that more depends upon the exact mode of tempering364 the alloy than on the relative proportions of the ingredients. Lead and zinc are inadmissible in bronze for this purpose. One or two per cent. of iron may, nevertheless, be added with advantage. The ancient bronze used for springs contained only 3% to 4% of tin. The edges and lips of bronze mortars must be carefully tempered by heating them to a cherry red, and then plunging them into cold water, as unless so treated they are very apt to be broken in use. See Bell-metal, Brass, Gun-metal, &c.
Bronze′-powder. Syn. Bronze. A name given to various powders having a rich metallic appearance, which they retain when applied on varnish, or when mixed with it, as in surface bronzing.
Prep. 1. Gold-coloured:—a. From Dutch-foil, reduced to an impalpable power by grinding. Cheap and looks well, and is very durable when varnished.
b. From gold-leaf, as the last.
c. Precipitated powder of gold.
d. From verdigris, 8 oz.; tutty powder, 4 oz.; borax and nitre, of each 2 oz.; bichloride of mercury, 1⁄4 oz.; grind them together, make the mixture into a paste with oil, and then fuse it; when cold, roll it into thin sheets or leaves, and grind it as in No. 1.
2. Iron-coloured:—Plumbago, in fine powder.
3. Red:—Sulphate of copper, 100 parts; carbonate of soda, 60 parts; mix, and apply heat until they unite into a mass; then cool, powder, and add of copper filings, 15 parts; again well mix, and keep the compound at a white heat for about twenty minutes; lastly, when cold, reduce the ‘residuum’ to an impalpable powder, wash it in pure water, and dry it.
4. Silver:—Bismuth and tin, of each 1 oz.; melt them together, and add of quicksilver, 1 to 11⁄2 oz.; when cold, powder it.
Obs. The above are used by painters, japanners, &c. See Bisulphide of Tin (Tin), Powders, &c.
BRONZ′ING. The process of giving a bronze-like, or an antique metal appearance, to the surface of copper, brass, and other metals. The following methods are recommended for this purpose:—
1. To the surface of the article, first thoroughly cleaned and polished, evenly apply with a brush the common crocus powder (‘jewellers’ rouge’), previously made into a smooth paste with water. When dry, place it in an iron ladle, or on a common fire-shovel, and expose it over a clear fire for about one minute; lastly, when sufficiently cold, polish it with a plate-brush. This gives a very rich appearance, similar to that on tea-urns; the shade depending on the duration and the degree of heat employed.
2. As the last, but substituting finely powdered plumbago for crocus powder. Equally beautiful, but deeper coloured and more permanent than that produced by No. 1.
3. As the preceding, but employing mixtures of plumbago and crocus in various proportions according to the shade desired.
4. A dilute solution of liver of sulphur (sulphurated potash), or of hydrosulphate of ammonia is applied with a camel-hair pencil to the metal previously slightly warmed; when dry, the surface is either left rough or brushed off. If liver of sulphur has been used, it will be better to wash it first in clean hot water; but without the slightest friction. This gives the appearance of very antique bronze.
5. Verdigris, 2 oz.; and sal-ammoniac, 1 oz.; are dissolved in vinegar, 1 pint; and the mixture is diluted with water until it tastes only slightly metallic, when it is boiled for a few minutes, and filtered for use. Copper medals, &c. (thoroughly clean) are steeped in the liquor at the boiling-point until the desired effect is produced. Care must be taken not to keep them in it too long. When taken out they are carefully washed in hot water, and dried. Effect as the last.
6. Verdigris and vermilion, of each 2 oz.; alum and sal-ammonia, of each 5 oz. (all in fine powder); vinegar, q. s. to form a thin paste. This is spread over the surface of the copper, which is then uniformly warmed by the fire, and afterwards well washed and dried. The tint may be deepened by repeating the process. The addition of a little blue vitriol inclines the colour to a chestnut-brown; and a little borax to a yellowish-brown. Used by the Chinese for copper tea-urns, &c.
7. Sal-ammonia, 1 oz.; cream of tartar, 3 oz.; common salt, 3 oz.; hot water, 1 pint; dissolve; then add of nitrate of copper, 2 oz., dissolved in 1⁄2 a pint of water; mix well, and with it repeatedly moisten the article (placed in a damp situation) by means of a soft brush. Produces a very antique appearance.
8. Salt of sorrel, 1 oz.; sal-ammoniac, 3 oz.; distilled vinegar, 1 quart; dissolve. As the last. Much used for bronze figures.
9. A very weak solution of bichloride of platinum, applied with a hair pencil or by immersion. Used for binding screws, holders, and other small articles of copper and brass.
10. Sulphate of iron and sulphate of copper, of each 1 oz.; water, 1 pint; dissolve; wash the surface of the articles with it; let them dry; then apply a solution of verdigris, 2 oz. dissolved in strong vinegar, 1⁄4 pint; when dry, polish them with a soft brush, and either some plumbago or colcothar. Used for tin castings.
11. The articles (properly cleaned) are either immersed in, or washed over, with a solution of sulphate of copper or of verdigris. In a short time they acquire a coating of pure metallic copper, and are then washed. This only answers with iron and steel goods. It is admirably suited for iron castings.
12. An antique appearance may be given to365 silver by either exposing it to the fumes of hydrosulphate of ammonia, or immersing it for a very short time in a solution of hydrosulphate of ammonia, or in dilute nitric acid.
Bronzing, Sur′face. A term commonly applied to the process of imparting a bronze-like or metallic appearance to the prominent portions of the surfaces of figures made of paper, wood, plaster of Paris, &c. It is effected by first giving them a coat of oil-varnish or size, and when this is nearly dried, applying, with a ‘dabber’ of cotton, or a camel-hair pencil, any of the ordinary metallic bronze-powders before referred to. Sometimes the powder is placed in a little bag of muslin, and dusted over the surface. The articles should be afterwards varnished.
Paper is bronzed by mixing the bronze-powders up with a little weak gum-water, and burnishing the surface when dry and hard.
Electrotypes, to Bronze. Green. Steep the medal or figure in a strong solution of common salt, or sugar, or sal-ammoniac, for a few days; wash in water, and allow to dry slowly; or suspend it over a vessel containing a small quantity of bleaching powder, and cover over. The length of time it is allowed to remain will determine the depth of colour.
Brown. Add four or five drops of nitric acid to a wine-glassful of water. The object is rubbed over with this gently, and allowed to dry, and when dry subjected to a gradual and equal heat; the surface will be darkened in proportion to the heat applied.
Black. Wash the surface over with a little dilute solution of hydrosulphate of ammonia, and dry at a gentle heat.
BROOM. The common name of the plant spar′tium scopa′′rium. A useful diuretic; of great service in dropsy. See Decoction.
Broom Ashes. From broom-stalks burnt. Formerly used as a diuretic in dropsy.
Broom, Salt of. Obtained by dissolving broom ashes in water, and filtering and evaporating the solution. It consists principally of carbonate of potassa. It was formerly used in dropsy, and as an antacid, &c.
BROSSÉ DE CORAIL. [Fr.] The root of lucerne (medicago sativa), cleaned, dried, and hammered at the end. Used as a tooth-brush.
BROTH. Syn. Jus (coctis carnibus), Jus′culum, L.; Bouillon, Jus, Fr.; Fleischbrühe, Ger. In cookery, the liquor in which flesh has been boiled. Broth is distinguished from soup by its inferior strength and quantity of seasoning, &c. It contains much of the nutriment of the meat. We extract the following from Dr Letheby’s work ‘On Food’:—
“A nutritious broth, containing the albumen of the meat or chicken, may be obtained by infusing a third of a pound of minced meat or chicken in 14 oz. of cold water, to which a few drops (4 or 5) of muriatic acid and a little salt (from 10 to 18 grains) have been added. After digesting for an hour or so, it should be strained through a sieve, and the residue washed with five ounces of water, and pressed, The mixed liquids thus obtained will furnish about a pint of cold extract of meat, containing the whole of the soluble constituents of the meat (albumen, creatin, creatinin, &c.), and it may be drunk cold, or slightly warmed, the temperature not being raised above 100° F., for fear of coagulating the albumen.”
Broth, Scotch. This, which is in very general use amongst the middle and working classes of Scotland, is made as follows:—Put into a pot three quarts of cold water along with a cupful of Scotch barley, and let it boil; add two pounds of neck of mutton. Allow it to stew gently for an hour, skimming occasionally. Then add turnips cut in squares, and onions sliced, and carrots and turnips uncut. The half of a small cabbage chopped in moderately fine pieces may be put in instead of all these vegetables; and leeks may be used instead of onions. Stew the whole for an hour longer. The broth is now ready. Season with salt and serve in a tureen. The meat is served in a separate dish, with the uncut pieces of turnip and carrot and a little of the broth as gravy. Any meat may be employed in the same way. Broths and soups contain the greater part of the saline matter of the meat, the crystalline principles, viz. creatin and creatinin, some of the albumen and fat, and an amount of gelatin, dependent upon the duration of the boiling process. They also contain nearly all the odorous matters of the meat. Cold water extracts from one sixth to one fourth of the solid ingredients of meat. The presence of a large quantity of highly nitrogenous crystalline principles in broths and soups accounts for their restorative powers. These, which are the creatin and creatinin, bear a close resemblance to the thein of tea and coffee, and the theobrominæ of cocoa, in their physiological effects.
Broth is contra-indicated for children at the breast, as it not unfrequently induces sickness, disorders the bowels, and induces fever. The same applies to beef tea. When, however, broth and beef tea are used as clysters in such quantities that can be retained, they act most beneficially. See Boiling, Soup, &c.
BROWN DYE. Every shade of brown may be produced, almost at will, by mixtures of reds and yellows with blues and blacks; or directly by simple dyes. The following are examples:—
a. For Cotton:—
1. Give the goods a mixed mordant of acetate of alumina and acetate of iron, followed by a bath of madder or of madder and fustic. Excess of acetate of alumina turns it on the AMARANTH TINT; the acetate of iron darkens it.
2. First ‘gall’ the goods, then turn them for a short time through the black bath; next give them a mordant of sulphate of copper,366 then pass them through a decoction of fustic, afterwards through a bath of madder, and again through the solution of sulphate of copper; drain, dry, rinse well, and finish with a boil in soap and water. This gives a CHESTNUT-BROWN.
3. First give the goods a mordant of alum, then a bath of madder, and next a bath of fustic to which a little green copperas has been added. This gives a CINNAMON-BROWN.
b. For Linen:—This varies little from that commonly employed for cotton.
c. For Silk:—
1. One of the above mordants is followed by a bath made by mixing equal parts of the decoctions of logwood, fustic, and Brazil-wood. The shade may be varied by altering the proportions of the decoctions; Brazil-wood reddening, logwood darkening, and fustic yellowing, the tint.
2. Annotta, 4 oz.; and pearlash, 1 lb.; are dissolved in boiling water, q. s.; the silk is passed through it for two hours, then taken out, and squeezed dry; it is next passed through a mordant of alum, and then through a bath of Brazil-wood, followed by another of logwood to which a little green copperas has been added.
d. For Wool:—
1. Boil the cloth in a mixed mordant of alum, common salt, and water, then dye it in a bath of logwood to which a little green copperas has been added. 2 oz. of alum, and 1 oz. of salt, are required for every lb. of wool.
2. Boil the goods in a mordant of alum and sulphate of iron, then pass them through a bath of madder. The more copperas the darker will be the dye. Good proportions are 2 parts of alum and 3 of copperas.
3. Give a mordant of alum and tartar, then pass the goods through a madder bath; next run them through a bath of galls and sumach or logwood to which a little acetate or sulphate of iron has been added.
4. Mordant the cloth as last, dye in a madder bath, remove the cloth, add a little acetate or sulphate of iron, and again pass it through the bath as long as necessary.
5. Give the cloth a light blue ground with indigo, and then a mordant of alum; rinse, and lastly run it through a bath of madder.
6. A mordant of alum and tartar, followed by, first a bath of madder, and afterwards a bath of weld or fustic to which a little iron-liquor has been previously added. In this way every shade, from MORDORÉ and CINNAMON to DARK CHESTNUT, may be produced.
7. Boil fustic-chips, 1 lb., for 2 hours; pass the cloth through the bath for 1 hour; take it out and drain; add of green copperas, 11⁄4 oz.; good madder, 4 oz.; boil for a short time, and again pass the cloth through the bath, until it acquires the proper tint. Bronze-browns, and every similar shade, may be thus given by varying the proportions.
e. The following are called SUB′STANTIVE or DIRECT BROWNS:—
1. Decoction of oak-bark. It dyes wool of a fast brown of various shades, according to the quantity employed. A mordant of alum brightens it.
2. Infusion or decoction of walnut-peels. Dyes wool and silk a brown, which is brightened by alum.
3. Horse-chestnut-peels. A mordant of chloride of tin turns it on the BRONZE; and sugar of lead, on the REDDISH-BROWN.
4. Catechu or Terra Japonica. For cottons. Blue vitriol turns it on the BRONZE, and green copperas darkens it, when applied as mordants. Acetate of alumina as a mordant brightens it. The French colour, CARMELITE, is given with 1 lb. of catechu, 4 oz. of verdigris, and 5 oz. of sal-ammoniac.
5. Sulphate or chloride of manganese. Dissolved in water with a little tartaric acid, it gives the bronze tint called SOLITAIRE. The stuff, after being passed through the solution, is turned through a weak lye of potash, and afterwards through another of chloride of lime, to heighten and fix it.
6. Prussiate of copper. This gives a fine BRONZE or YELLOWISH-BROWN to silk. A mordant of blue vitriol is commonly first given, followed by a bath of prussiate of potash.
BROWN PIG′MENTS. The principal and most useful of these are—umber, terra di Sienna (both burnt and raw), Spanish brown, and some of the ochres. Brown, of almost any shade, may be made by the admixture of blacks with reds and yellows, or with greens, in different proportions. See Bistre, Black, Newcastle,[233] Ochres, Sepia, &c.
Brown, Span′ish. See Ochres.
BROWN PINK. See Yellow Pigments.
BROWN′ING. In cookery, a fluid preparation used to colour and flavour gravies, soups, &c.
Prep. 1. Sugar, 4 oz.; and butter, 1 oz.; are melted in a frying-pan or ladle with about a tablespoonful of water, and the heat is continued until the whole has turned of a deep brown; the heat is then lowered a little, and some port wine (about 1 pint) is gradually poured in; the pan is now removed from the fire, and the mixture well stirred until the roasted sugar is entirely dissolved; it is then put into a bottle, and 1⁄2 oz. each of bruised pimento and black pepper, 5 or 6 shalots (cut small), a little mace and finely grated lemon peel, and 1⁄4 pint of mushroom catsup, added. The bottle is shaken daily for a week, and the clear liquid, after 5 or 6 days’ repose, decanted into another bottle. Rich flavoured, but expensive.
2. As the last, but using strong beer, or water, instead of wine. A glassful of spirit may be added after bottling it.
3. Sugar-colouring, 1 pint; salt, 1⁄4 lb.;367 mushroom-catsup, 1⁄2 pint; spice, q. s. Excellent for all ordinary purposes.
4. Lump sugar (powdered), 21⁄2 lbs.; salad oil, 1⁄2 lb.; heat as before; then add, of port wine, 1 quart; Cape wine, 3 quarts; shalots, 6 oz.; mixed spice, 4 oz.; black pepper, 3 oz.; mace, 1 oz; salt, 1 lb.; lemon juice, 1 pint; catsup, 1 quart; mix well.
5. Good spirit-colouring or sugar-colouring and mushroom catsup, of each 1 gall.; Jamaica pepper, black pepper, and shalots, of each 4 oz; cloves, cassia, and mace, bruised, of each 3⁄4 oz.; boil in a covered vessel for 5 minutes; digest for 14 days, and strain.
6. Colouring, 3 pints; mushroom catsup, 1 pint; common salt, 3⁄4 lb.; Chili vinegar (strongest), 1⁄2 pint; spice, q. s. Half a pint of British brandy or rum may be added.
Obs. The above are excellent additions to gravies, soups, &c.; and of themselves form most admirable sauces for fish, meat, and game.
Browning (for Gun-barrels). Prep. The following are current formulæ:—
1. Blue vitriol, 4 oz.; tincture of chloride of iron, 2 oz.; water, 1 quart; dissolve, and add aquafortis and sweet spirit of nitre, of each 1 oz.
2. Blue vitriol and sweet spirit of nitre, of each 1 oz.; aquafortis, 1⁄2 oz.; water, 1 pint; as last.
3. Butter of antimony and sweet oil, equal parts; well shaken together. To be applied to the iron previously warmed.
Obs. The above fluids are rubbed on the barrel (previously well polished and cleaned off with whiting to remove the oil), and allowed to remain on for some hours, or until the next day, when they are rubbed off with a stiff brush. The process may be repeated, if necessary. The barrel is next washed in water in which a little pearlash or soda has been dissolved, and afterwards well rinsed in clean water; it is then polished, either with the burnisher, or with a brush and beeswax. Sometimes a coat of tough shell-lac varnish is applied.
BRUCEA (-sh′ă). False cusparia (which see).
BRUCHBALSAM—RUPTURE BALSAM (Dr Tänzer).—No. 1. Compound rosemary cerate, nutmeg cerate, red Johannis oil, yellow wax, of each 1 part; fat, 5 parts. No. 2. Mixture of nutmeg cerate, 50 parts; tallow, butter, of each 10 parts, melted and mixed with 25 parts strongest liquor potassæ. No. 3. Compound rosemary cerate, oil of bayberries, of each 2 parts; nutmeg cerate, 4 parts; red Johannis oil, 6 parts; yellow wax, 3 parts; tincture of myrrh and tincture of aloes, of each 1⁄2 part; tr. opii, 1⁄4 part, melted and heated until the spirit has evaporated. (Hager.)
BRUCHPFLASTER—RUPTURE PLASTER (Krüsi Altherr). A spread plaster, the mass consisting of 5 parts Bergundy pitch and 2 parts turpentine. (Walz and Hager.)
Bruchpflaster—Rupture Plaster (Caspar Menet). Machine-made paper covered with thin gauze, and thinly spread with a mass of 9 parts wax, 3 parts turpentine, and 1 part elemi. (Hager.)
BRUCHSALBE—RUPTURE CERATE (Gottlieb Sturzenegger, Herisau, Canton Appenzell). A mixture of 50 parts fat and 1 part oil of bayberries. (Hager.)
BRUCHE, ruptures cured without medicine, operation, or pain, by Lavedan, chemist. A pelotte containing in it zinc and copper plate on which a solution of the “poudre electrochimique” (common salt) is dropped. (Hager.)
BRU′CIA. C23H26N2O4. Aq. [Eng., Fr.] Syn. Bru′cine; Bru′cina, L. An alkaloid discovered by Pelletier and Caventou, in the bark of bru′cia antidysenter′ica, and afterwards associated with strychnia, in nux vomica.
Prep. Ground nux vomica, or the bark of brucia antidysenterica, is boiled in dilute sulphuric acid, and the resulting decoction mixed with hydrate of lime (in excess); the crude precipitate thus obtained is boiled in alcohol (sp. gr. ·850), and the tincture filtered whilst hot. A mixture of crude strychnia and brucia is deposited as the fluid cools, and the remainder is obtained by evaporation. This is powdered and digested in cold alcohol, which dissolves out the brucia; the solution furnishes crystals on spontaneous evaporation. It may be further purified by recrystallisation from alcohol.
Prop. Soluble in 850 parts of cold, and about 500 parts of hot water; freely soluble in alcohol; added to the dilute acids until they are neutralised, it forms crystallisable salts, easily obtained by evaporation.
Tests. It is distinguished from strychnia, which in many respects it resembles, by its ready solubility in both dilute and absolute alcohol, and its insolubility in ether. With nitric acid it strikes a fine red colour, which is removed by sulphuretted hydrogen and sulphurous acid. Iodic acid, chloric acid, and chlorine, also turn it red.
Professor Sonnenschein has succeeded in converting brucia into strychnia. He says—“Brucia C23H26N2O4 and Strychnia C21H22N2O2 differ apparently considerably in their composition; but the former may easily be converted into the latter. Referring to the formulæ it will be seen that strychnia is produced by combining brucia with 4O, and eliminating 2H2O1 and 2CO2. This is effected as follows:—Brucia is moderately heated with 4 to 5 times its weight of diluted nitric acid, when a red colouration will be produced and gases evolved, which cause in a mixture of barium chloride and ammonia a white precipitate of carbonate of barium.
The red solution is concentrated in a water-bath, super-saturated with potassa, and agitated with ether, which, on spontaneous368 evaporation, leaves a reddish mass, containing a red colouring matter, a yellowish resin, and an alkaloid which is obtained pure by dissolving in an acid and crystallising. This base has the intensity, bitter taste, and other properties of strychnia, gives the characteristic reactions with potassium chromate, cerium oxide, and sulphuric acid, and yields with chlorine the sparingly soluble compound. The muriate crystallises in fine silky needles, from which 9·20 per cent. of chlorine was obtained.
The conversion of brucia into strychnia is not only highly interesting, but it is likewise of great importance in forensic analysis, proving again that in such cases the employment of oxidising agents is admissible only with great caution. A student who had received for analysis a mixture containing, among other substances, brucia and nitrate of lead, employed the process of Stas and Otto for the separation of the alkaloids, and found strychnia instead of brucia which had been oxidised by the liberated nitric acid.
“If strychnia is heated with a strong base like potassa, soda, or baryta, for some time, in a sealed glass tube placed in a water-bath, a body is obtained which no longer shows the reactions of strychnia, but resembles brucia in its reactions. The experiments on this decomposition, which is likewise of importance in forensic analysis, are not yet concluded.”
BRUISE (brōōze). Syn. Contu′sio, Contu′sum, L.; Contusion, Meurtrissure, Fr.; Brausche, Quetschung, &c., Ger. A contusion; but in popular language applied chiefly to cases in which there is an extravasation of blood owing to the rupture of the minute vessels, with consequent discoloration or tumefaction of the part.
Treatment.—In common cases, sufficiently serious, bruises may be rubbed with a little opodeldoc or soap-liniment; or, if the inflammation be considerable, they may be bathed with a little weak goulard water, or with vinegar and water. In more severe cases leeches may be applied. See Contusion.
Treatment for Animals.—The same as for man.
BRUNS′WICK BLACK. See Varnishes.
BRUNS′WICK GREEN. See Green Pigments.
BRUSHES. Brushes may be best washed in a moderately cold weak solution of borax. They should afterwards be rinsed in cold water and dried.
BRUSTBONBONS—PECTORAL BONBONS (Fr., Stollwerck, Cologne). Carageen, 3 parts; Iceland moss, 2 parts; red poppy petals, 11⁄2 parts; coltsfoot, 1 part; liquorice, 2 parts; marshmallow root, 2 parts; daisy (Bellis perennis), 11⁄2 parts; Souchong tea, 1 part; boiled with 24 parts of water till reduced to half, and the fluid afterwards mixed with refined sugar.
BRUSTGELEE—PECTORAL JELLY (Daubitz, Berlin). A yellowish-brown nearly clear jelly, with a sweet, weak anise, followed by a somewhat bitter taste, made of gelatin, 12 grammes; sugar, 60 grammes; and a herbal infusion, 120 grammes; the latter made from anise, star-anise, Iceland moss, &c.
BRUSTPULVER—PECTORAL POWDER (Beliol, Paris). For chronic pains in the chest. A mixture of 75 parts milk-sugar, 20 parts gum arabic, 5 parts Rochelle salt. (Mayer).
BRUSTSAFT PRAPARIRTER—PREPARED PECTORAL JUICE (Rudolph Büttner, Berlin). For coughs, hoarseness, tightness of the chest, &c. An ordinary pectoral tea made of an infusion of red poppy petals, which is boiled to a syrup with sugar (Hager).
BRUSTSYRUP WEISER MAYERSCHER—WHITE PECTORAL SYRUP (G. A. W. Mayer, Breslau). 4 parts powdered radish extracted with 5 parts water (according to others rose-water), the liquor expressed and filtered. 6 parts of the clear liquor digested with 10 parts of sugar to make a syrup. (Hager.) Frequently nothing but a simple solution of sugar.
Brustsyrup—Pectoral Syrup (Dr Moth). A mixture of syrup of marshmallow, 1000 parts; extract of horehound, 30 parts; oxymel of squills, 50 parts; aq. amygd. amar., 25 parts; aqua. foenic, 100 parts; spirit of ether, 10 parts.
BRUSTWARZEN—MITTEL ZUR HEILUNG WUNDER. Miraculous remedy for healing sore nipples. (From Paris.) A dirty brownish-yellow, somewhat turbid liquid, smelling of vinegar, and with a taste both sour and sweet. A solution of 11⁄4 parts litharge in 100 parts vinegar. (Wittstein.)
Brustwarzen—Mittel Gegen Wunde. Sore nipple preventive. (From Paris.) Acetic acid, 1 part; sugar of lead, 3 parts; camphor, 5 parts; water, 100 parts. (Terreil.)
BRUSTWARZENBALSAM, RIGAER—RIGA’S NIPPLE BALSAM. A mixture of the yolk of one egg with 10 to 12 grammes balsam of Peru.
BRY′ONIN (-nĭn). A peculiar bitter principle extracted from the root of white bryony (bryonia dioica, Jacq.). It is obtained from the dry extract of the expressed juice, by solution in alcohol, filtration, and cautious evaporation.
Prop., &c. A yellowish-white mass. It is a drastic purgative; and, in large doses, poisonous. It enters into the composition of several quack medicines.
BUBBLE-AND-SQUEAK. In cookery, a species of olla podrida variously prepared, as the materials and fancy of the maker dictate.
Prep. (Rundell.) Take slices of cold meat, fry them quickly until brown, and put them into a dish to keep them hot. Then clean the pan from the fat; put in it greens and carrots (previously boiled and chopped small); add a369 little butter, pepper, and salt; make them very hot, and put them round the beef with a little gravy. Cold boiled pork is a better material for bubble-and-squeak than beef. In either case the slices should be very thin and lightly fried.
BUB′BLE FEVER‡. See Pemphigus.
BU′CHU (-kū). The plant dios′ma crena′ta (which see).
BUCK′BEAN or BOG′BEAN. The menyanthes trifoliata. See Infusions.
BUCKINGHAM’S DYE for the whiskers; manufactured by R. E. Hall & Co., Nashua, N.H. This whisker dye is an ammoniacal solution of nitrate of silver, and consists of 1⁄2 gramme nitrate of silver, 21⁄2 grammes solution of ammonia, and 40 grammes distilled water. (Dr Schacht).
BUCK′THORN. Syn. Rham′nus, L. The rham′nus cathar′ticus (Linn.). Berries (BAC′CÆ RHAM′NI, L.), cathartic; juice of the berries (SUC′CUS R., L.) is officinal in the B. P. See Rhamnine, Syrups, &c.
BUCK′WHEAT. See Wheat.
BUG. Syn. Ci′mex, L.; Punaisé, Fr.; Wanse, Ger. A name popularly and very loosely applied to a vast number of insects that infest houses and plants; in zoology, hemipterous insects of the genus ‘cimex,’ of which there are many hundred species; appr., the bed-bug.
Bug. Syn. Bed′-bug, House′-b., Wall-b., Wall′-louse*, &c.; Ci′mex domes′ticus, C. lectula′′rius (Linn.), L.; Punaise, Fr.; Bettwanze, Häuswanse, Ger. An insect too well known in all the larger towns of Europe and America, and in the huts of squalid poverty everywhere, to require a description here. It is almost the only species of the bug kind that has undeveloped wings. Its introduction to England is believed to have occurred soon after the great Fire of London (A.D. 1666). Human blood appears to be its favourite food; but it will also eat grain, seed, flour, dried paste, size, soft deal, beech, osier, &c. Cedar, mahogany, and the odorous and harder woods are usually avoided by this insect. Aromatics, perfumes, and strong odours generally are unfavorable to its propagation.
Exterm., &c. Various means have been adopted to prevent the accession, and to destroy or drive away, these enemies of “tired nature’s sweet restorer, balmy sleep.” Among the most certain of these is thorough cleanliness and ventilation. The furniture brokers put articles infested with these insects into a room with doors and windows fitting quite close, and subject them to the fumes of burning sulphur or chlorine gas. In the small way poisonous washes are commonly resorted to. For this purpose nothing is more effective than chloride of lime or chloride of zinc; the latter being preferable to the other on account of its being comparatively scentless.
The following mixtures are in common use, or have been recommended for this purpose:—
1. Corrosive sublimate (in powder) and hydrochloric acid, of each 1 oz.; hot water, 3⁄4 pint; agitate them together until the first is completely dissolved. It is applied with a paint-brush, observing to rub it well into the cracks and joints. This is the common ‘bug-wash’ of the shops. It is a deadly poison!
2. As the last, but substituting 2 oz. of sal-ammoniac for the hydrochloric acid.
3. Oil of turpentine, 1 pint; camphor, 2 oz.; dissolve. Very cleanly and effective.
4. Tobacco-water, made by steeping 2 oz. of good shag in 1 pint of warm water for a few hours.
5. Crude pyroligneous acid.
6. Coal-tar naphtha. This, as well as No. 3 (above), should never be used by candle-light, as it is excessively inflammable. When the smell of the common naphtha is objectionable, benzol or benzine may be used instead. The celebrated nostrum vended under the name of ‘Insecticide’ is said to be nothing but benzol.
7. Sulphurated potash (in powder), 6 oz.; soft soap, 1⁄2 lb.; oil of turpentine, 1⁄4 pint or q. s. to make a species of soft ointment. The odour of the last three (Nos. 5, 6, 7) is rather persistent and disagreeable; but they are very effective.
8. Strong mercurial ointment, soft soap, and oil of turpentine, equal parts, triturated together. Rather greasy and dirty.
9. Scotch or Welsh snuff, mixed with twice its weight of soft soap.
10. Sulphur, or squills, in impalpable powder, blown into the cracks or joints, or scattered in a fine cloud, by means of a hollow ball or balloon of vulcanised india rubber filled with it and furnished with a small wooden jet or mouth-piece, or in any other convenient manner. Very cleanly and effective. Dumont’s ‘Patent Vermin Killer,’ as well as the whole host of imitations of it, is of this kind.
Obs. Out of the above list there is ample room for selection. The common practice is to take the bedstead or other piece of furniture to pieces before applying them.
These pests exist only in dirty houses. A careful housewife or servant will soon completely destroy them. The surest method of destruction is to catch them individually when they attack the person in bed. When their bite is felt, instantly rise and light a candle and capture them. This may be troublesome, but if there be not a great number a few nights will finish them. When there is a large number, and they have gained a lodgment in the timbers, take the bed in pieces, and fill in all the apertures and joints with a mixture of soft soap and Scotch snuff. A piece of wicker-work, called a BUG-TRAP, placed at the head of the bed, forms a receptacle for them, and then they may be daily caught till no more are left. Oil-painting a wall is a sure means of excluding and destroying them. It has been asserted that these insects are so fond of narrow-leaved dittany or370 pepperwort (lepidium ruderale), that if a bunch of it be suspended near their haunts they will settle in it, and may be thus easily captured. It is said to be commonly used as a bug-trap in some of our rural districts. Water, poured boiling from the spout of a kettle into the cracks and joints, is a cleanly and certain remedy, which we have often seen employed; so also is a jet of steam; they are both destructive to all insects, and will be found particularly so to beetles.
The proper time for attacking these pests is early in March, or shortly before they are revived from their dormant state by the warm weather. See Insects.
Bug, Harvest. See Acari.
BU′GLE (bu′gl). An elongated cylindrical glass bead. See Bead.
BUILDING STONES. Amongst the calcareous and magnesian stones used for building many of the fine-grained and porous varieties are liable to split into flakes after a few years’ exposure to the atmosphere, owing to the absorption by the stone of water, which, becoming frozen during severe weather, fractures the stone by its expansion. Brard invented a simple means of ascertaining whether a building stone is liable to this defect, which consists in taking a smoothly-cut block of the stone, one or two inches square, and placing it in a cold saturated solution of sodic sulphate. The temperature of the solution is gradually raised to the boiling point; it is allowed to boil for half an hour, and then the stone is left to cool in the liquid. When cold it is suspended over a dish, and once a day for a week or a fortnight plunged for a few moments into a cold saturated solution of sodic sulphate, and it is then again freely suspended in the air. The sulphate crystallises in the pores of the stone and splits off fragments of it. A similar experiment is made upon an equal-sized mass of stone which is known to be free from this defect. By the comparative weight of these fragments in the two cases the tendency of the stone to the defect in question may be estimated.
A stone that is placed in a building in a position similar to that in which it is found in the quarry, that is, with its seams lying horizontally, is found to resist the weather much more successfully than one that has not been so placed.
BUN. A well-known kind of light, sweet cake.
Prep. 1. Bath-buns:—As 6, but adding a little candied lemon and orange peel, and putting a little grated peel and a few caraway comfits on the top of each.
2. Cross-buns:—Flour, 21⁄2 lbs.; sifted sugar 1⁄2 lb.; coriander seeds, cassia, and mace, of each (powdered) a sufficiency; make a paste with butter, 1⁄2 lb.; (dissolved in) hot milk, 1⁄2 pint; work with three table-spoonfuls of yeast; set it before the fire for an hour to rise, then make it into buns, and set them before the fire on a tin for half an hour; lastly, brush them over with warm milk, and bake them to a nice brown in a moderate oven.
3. Madeira-buns:—Butter, 8 oz.; 2 eggs; flour, 1 lb.; powdered sugar, 6 oz.; half a nutmeg (grated); powdered ginger and caraway seeds, of each 1⁄2 teaspoonful; work well together, then add as much milk as required, and ferment; lastly, bake on tins in a quick oven.
4. Plain buns:—Flour, 2 lbs.; butter, 1⁄4 lb.; sugar, 6 oz.; a little salt, caraway and ginger; make a paste with yeast, 4 spoonfuls, and warm milk, q. s.; as before.
5. Penny-buns:—To the last add of currants, well washed, 1⁄2 lb.; and water, stained by steeping a little saffron in it, q. s., to give a light yellow tinge to them.
6. Rich buns:—Fine flour, 3 lbs; sugar, 1 lb.; butter, 2 lbs. (melted and beat with) rose water, 4 oz.; currants, 1 lb.; yeast, 1⁄4 pint; as before.
Obs. The great secret in producing good buns is the use of sweet yeast and the best currants only, and thoroughly washing these last in a sieve or colander, to remove grit, before adding them to the dough.
BUNION (-yŭn). A species of corn or swelling on the ball of the great toe, resulting from pressure, and irritation by friction. The treatment recommended for corns applies also to bunions; but in consequence of the greater extension of the disease, the cure is more tedious. A bunion may often be effectually stopped and removed by poulticing it, and, at the proper time, carefully opening it with a lancet. See Corns.
BURETTE. A graduated glass vessel employed in volumetric analysis for measuring liquids.
1. The first burette was invented by Gay-Lussac, a drawing of whose instrument is given below.
It rarely, if ever, has a capacity greater than 50 cubic centimetres, and consists of a narrow tube fused on to a wider one. The larger tube is about 33 centimetres long, the graduated portion occupying about 25 centimetres, and its internal diameter measures 15 millimetres; the narrow tube has a diameter of 4 millimetres, which in the upper bent end decreases to 2 millimetres. When used the instrument should be held in the left hand, the bottom part being allowed to lean a little against the chest. The operation is aided by giving the instrument from time to time a slight turn in the direction of its longitudinal axis, thereby placing the curve of the stout alternately in a more vertical, alternately in a more horizontal position. The volume must not be read off before the surface of the liquid has attained a constant height.
2. Geissler’s burette. This instrument differs from Gay-Lussac’s in having the narrow tube inside, instead of outside the wider one. It is found very convenient in use, and is less liable to fracture than Gay-Lussac’s.371
3. Mohr’s burette, which can be more easily and readily managed than either of the two preceding ones, is described and figured under Alkalimetry.
 Gay-Lussac’s Burette and Geissler’s Burette.
Gay-Lussac’s Burette and Geissler’s Burette.
In volumetric analysis the method of taking the readings of the burette is an operation of great importance, requiring considerable method and practice.
The first proceeding is to bring the eye to a level with the fluid, and to adopt a fixed and unalterable standard of what is to be considered the surface.
If you hold a burette partly filled with water between the eye and a strongly illumined wall, the surface of the fluid presents the appearance shown in fig. a. If you hold close behind the tube a sheet of white paper with a strong light falling on it, the surface of the fluid will present an appearance similar to that shown in fig. b.
In both cases you have read off at the lower border of the dark zone, this being the most distinctly marked line. Its distinctness may be heightened by adopting Mohr’s contrivance which consists in pasting on a sheet of very white cardboard a broad strip of black paper, and when reading off holding this close behind the burette in a position to place the border line between white and black from 2 to 3 millimetres below the lower border of the dark zone as shown in figure c.
 a. and b.
a. and b.
 c.
c.
Great care must be taken to hold the paper invariably in the same position, since if it be held lower down, the lower border of the black zone will move higher up.
To test the correctness of the graduation of a burette proceed as follows:—Fill the instrument up to the highest division with water at 60·8° F., then let the cubic centimetres of the liquid flow out into an accurately weighed flask, and determine the weight of these ten cubic centimetres in the usual way; then let another quantity of ten cubic centimetres flow out, and weigh again, and repeat the operation till the contents of the burette are exhausted. If the instrument is correctly graduated, every372 ten cubic centimetres of water at 60·8° F. must weigh 9·990 grammes.
BURG′LARIES. The common precautions of locks and bolts, alarum-bells and fire-arms, are frequently found useless in preserving houses from burglars; but a light in the upper part of the house, or a small dog on the ground-floor, with the means of running into a place of safety from its enemies, has been seldom known to fail. A combination of the two would undoubtedly be doubly effective. The bark of the dog and the fear of detection by the approach of the light would deter the majority of rogues of common pluck and feeling. A dog out of doors, and consequently accessible, however large and fierce, is easily pacified or silenced by men of the class referred to.
BURLS, REMOVAL OF, from Cloth and Wool. Introduce the wool or the woollen goods into 100 litres of sulphuric acid at 6° B., in which 500 grams of alum and 250 grams of salt have been dissolved. Work in this bath for one or two hours, drain in the centrifugal, and hang up at 100° to 120°. Wash for an hour and a half in clear water, treat for two hours with fuller’s earth, soda and lime, and wash again for two hours. Sulphuric acid is adapted only for whites and indigo blues. For coloured goods solutions of chloride of tin, and chloride of manganese at 6° B., are recommended.
BURNS[234] and Scalds.[235] Treatm. When the injury is superficial and slight, a little creosote may be applied to the part. If a scald, the vesicle should be first pierced with a needle, or what is better, snipped with a pair of scissors, and the water which it contains should be then gently squeezed out. When creosote is not procurable, a liniment formed of equal parts of soft soap, basilicon ointment, oil of turpentine, and water, may be used instead. When the part feels very hot and painful, a poultice may be applied, on the surface of which a few drops of creosote, or of the liniment, should be spread with a knife. This treatment will generally succeed in allaying the pain. It may be followed by a dressing of zinc ointment, or any other like simple emollient or unctuous preparation. Creosote, contrary to what is commonly asserted, produces scarcely any smarting or pain; whilst it rapidly removes the burning sensation, and the charred surface soon assumes a dry scabby appearance, which, by dressing with simple ointment, soon comes off and leaves the part beneath in a sound and healthy state. If a poultice be applied it is best to keep it on until the next day. Plunging the part into very cold water immediately after the receipt of an injury of this kind will frequently prevent any further remedy being required. Flexible collodion painted over a burn forms a good protective envelope. In all cases cooling laxatives should be administered; and the diet should be rather low until the inflammatory symptoms subside.
Treatment for Animals. Carbolic dressing, exclusion of air, cotton wool, linseed oil and lime water.
BURNING-GLASS. See Lens.
BUTEA FRONDOSA, Roxb. (Ind. Ph.) Syn. Bengal Kino Tree. Habitat. Common all over India.—Officinal part. The inspissated juice obtained from the stem by incision (Buteæ Gummi, Kino Bengalensis, Bengal kino). It occurs in the form of irregular shining fragments, seldom as large as a pea; more or less mixed with adherent pieces of greyish bark; of an intense ruby colour and astringent taste; soluble, but not freely so, in water and in alcohol. Its astringency is due to the presence of tannic and gallic acids.—Prop. & Uses. Similar to those of kino, for which it has been found an efficient substitute.—Prep. Same as those of kino.
BUTTER. [Eng., Ger.] Syn. Buty′rum, L.; Beurre, Fr.; Buter, Butera, Sax. The fatty matter obtained from cream by churning it.
Manuf. The process of making butter by the common operation of churning is extremely simple, and is well known. The chief objects to attend to are maintaining a proper temperature, and a certain degree of exposure to the air. Extreme cleanliness must also be observed; the churn and other utensils being frequently scalded out with water. When the butter is ‘come,’ it should be put into a fresh-scalded pan, or tub, which has been standing in cold water, cold water poured on it, and after it has acquired some hardness, it should be well beaten with a flat board until not the least taste of the butter-milk remains, and the water, which must be often changed, becomes quite colourless and tasteless. A little salt may then be worked into it; after which it may be weighed and made into ‘forms,’ which should then be thrown into cold water contained in an earthen pan provided with a cover. In this way nice and cool butter may be obtained in the hottest weather.
At Dumbarton the newly separated butter is put into a clean vessel, and a corn sickle is drawn several times crosswise through it, to extract any hairs that may adhere to it. This operation is performed in very cold spring water, and is followed by thoroughly washing it therein. 10 oz. of salt are now added to every stone-weight of the butter, and well mixed in.
In Devonshire the milk is generally scalded in copper pans over a charcoal or wood fire, and the cream collected as soon as it rises, or, and more frequently, when the whole has got cold. It is then churned in the usual way. On the small scale the butter is commonly obtained from this cream by patiently working it with the hand in a shallow pan or tub. Without care the cream is apt to absorb some of the373 fumes from the charcoal, which impart a peculiar taste to the butter. This is the reason why some of the Devonshire butter has a slight smoky flavour. It may be removed by thorough washing in cold water. Of late years, in the large dairy-farms of Devonshire, covered flues, with openings to receive the bottoms of the pans, have superseded open fires, by which the danger of contamination from the fumes is removed.
Choice. Fresh butter has a pleasant odour and is of an equal colour throughout its substance. If it smells sour, the butter-milk has not been well washed out; and if it is streaked or veiny, it is probably mixed with stale butter or lard. A good way to try butter is to thrust a knife into it, which should not smell rancid and unpleasant when withdrawn. Rancid and stale butter, when eaten in quantity, is capable of producing dangerous symptoms.
Pur. The cheaper kinds of butter are frequently adulterated with common wheat-flour, oatmeal, pea-flour, lard, and is sometimes mixed with suet and turnips, as well as with a large quantity of salt and water. The trick is concocted between the Irish factors and the London dealers. The higher priced article is seldom mixed with anything beyond an excess of salt and water, notwithstanding the assertions of alarmists to the contrary. The presence of lard may be detected by the flavour and paleness of the colour. A little of the sample adulterated with the other substances named, if melted in a glass tube or phial, will separate into strata, which are very marked when cold.
Quantitative Analysis of Butter.—1. The following process for the analysis of butter, by Mr A. H. Allen, is extracted from the ‘Chemical News’ (xxxii, 77):—The Society of Public Analysts has adopted 80·00 per cent. as the lowest limit of fat contained in a genuine butter.
The amount of water is best ascertained by heating 5 grams of the butter in a small weighed beaker to a temperature of about 110° or 120° C. for an hour or so. Some chemists merely heat the butter on a water bath. According to the author’s experience, perfect drying is next to impossible at that temperature.
The dried butter is next treated in the beaker with anhydrous ether, or commercial benzoline. The former liquid is expensive and inconveniently volatile, while it must be used in a perfectly anhydrous condition (to avoid solution of the salt), and except when boiling has but a limited solvent power for butter, especially when adulterated. Benzoline dissolves fat more readily than ether; it does not volatilise so rapidly at ordinary temperatures, it is always anhydrous and has the advantage of low price. The “benzoline” employed by the author is made by redistilling the commercial article from a retort immersed in a bath of boiling water. About one third of the original bulk usually comes over readily at 100° C., and has a gravity of 0·689.
On warming the beaker containing the benzoline the dry butter readily dissolves. The liquid is poured on a small dry filter and washed with warm benzoline, the filtrate being collected in a small wide beaker. If the filter had been previously weighed, its increase of weight, after careful drying, will of course give the quantity of curd and salt in the 5 grams of butter taken. Except in cases in which extreme accuracy is desired, it is preferable to scrape the residue off the filter and weigh it separately.
The error (owing to imperfect removal) only amounts to one or two tenths per cent. of the butter taken. As the salt is accurately estimated afterwards, the loss falls on the curd. The salt may be determined by careful ignition of the filter and residue, the incombustible matter consisting almost wholly of common salt, while the curd is ascertained by loss of weight. This method is not to be recommended; for without great care some of the salt will be volatilised and lost, the error causing the amount of curd to appear excessive.
Ignition also renders any further examination of the curd an impossibility. A far preferable plan is to return the weighed curd and salt to the filter, and to wash them with cold water. The filtrate is made up to 100 c. c., and the salt is estimated in a half of it by titrating with decinormal nitrate of silver. The remaining portion of the solution can be employed for the estimation of sugar, if desired. This is effected by inverse titration with Fehling’s copper solution, in the same way as grape sugar. The estimation of sugar may sometimes be of interest, as a means of ascertaining whether the aqueous portion of the butter consisted of mere water or of serum of milk. In other words, the estimation of the sugar may furnish a means of ascertaining whether an excess of water in the butter is due to insufficient removal of the butter-milk, or to subsequent incorporation of water. Every 0·001 gram of milk sugar represents about 0·022 of average milk serum.
The residue insoluble in cold water usually consists almost wholly of casein. If, however, the butter has been adulterated with mashed potatoes, flour, or other starchy matters—said to be occasionally employed—they will be found here. The presence of starch in the residue will, of course, be readily indicated by treating it with hot water, and testing the cooled liquid with solution of iodine. By pressing out a small portion of the butter between two slips of glass, so as to obtain a thin film, and observing it under the microscope (or by observing the caseous residue after treatment with cold water), the nature of the starch may be ascertained.
The solution of the fatty matter in benzoline is evaporated at 100° C. till it no longer374 decreases in weight. The average proportion of fatty matter in butter is about 85 per cent. If less than 80 per cent. the butter must be considered adulterated. It is evident that a careful estimation of the per-centage of fatty matter would often render separate estimations of the water, curd, and salt unnecessary; for unless the sum of the three latter constituents exceeded 20 per cent. the butter could not be considered as adulterated, unless by an admixture of other fats.
An easy and rapid method of estimating the fat in the undried butter is therefore a great desideratum; but unfortunately no satisfactory method is at present known. The indirect estimation of the fat, by subtracting the sum of the per-centages of water, curd, and salt from 100·00, ought to agree with the direct estimation of fat within 0·5 per cent., and the variation is often much less.
2. Dr Dupré adopts the following method: About 5 grams of the dry filtered butter fat are weighed in a small strong flask; 25 c. c. of a normal alcoholic soda solution are added; the flask is closed by means of a well-fitting cauotchouc stopper, firmly secured by a piece of canvas and string, and heated in a water-bath for about an hour. When cool the flask is opened, the contents—which are semi-solid—carefully liquefied by heat, and washed into a flask with hot water. This flask is now heated for some time on a water-bath to expel the alcohol, some more hot water is added, and 25 c. c. of diluted sulphuric acid somewhat stronger than the alkali used, are run in. The contents are allowed to cool, and the acid aqueous solution below the cake of fatty acids is passed through a filter. The fatty acids in the flask are washed by hot water in the manner recommended by Dr Muter, i.e., each time allowed to cool; all the washings are passed through a filter.
The author uses no cambric, but passes everything through paper. With care scarcely any of the fatty acid will find its way into the filter. After the washing with water is completed and the flask drained, he washes any fatty acid that may be on the filter into the flask by means of a mixture of alcohol and ether on a water bath, and finally dries the fatty acids in the flask at a temperature of 105° C. The drying can be done readily if the melted fat is now and then shaken briskly, so as to subdivide the water as much as possible. In this way the acids when once in the flask are not taken out until their weight has been taken, thus reducing the risk of loss to a minimum. Meanwhile the acidity of the aqueous filtrate and washings is estimated by decinormal soda solution. Subtracting from the amount required to the proportion necessary to neutralise the excess of acid added in decomposing the soap, the rest represents the soluble fatty acids contained in the butter taken, and on the assumption of its being butyric acid, we can, of course, calculate the amount of this acid present. When once the equivalent of the soluble acids present in butter is fairly determined, this, of course, will have to be substituted for that of butyric acid. The results thus obtained are very accurate, and the process is very simple in execution.
The author has satisfied himself by repeated experiments that the alkalinity of the alcoholic soda solution by itself is not altered by the process. The author places no reliance on the specific gravity test, as he finds that mutton dripping, and other fats likely to be used as adulterants of butter, may acquire a specific gravity above ·911 by being strongly and repeatedly heated. He thinks, however, that any sample of butter below ·911 may safely be pronounced adulterated.
In a subsequent note Dr Dupré states that he has effected the saponification, decomposition of the soap, and the washing and drying of the fatty acids at ordinary temperature, thus still further reducing the risk of breaking up the higher into lower acids. The saponification is readily effected by using a sufficiency of alcoholic soda. Between four and five grams of the dry butter fat were shaken up for several minutes with 100 c. c. of normal alcoholic soda. The butter soon dissolves, but after a time the solution gelatinises to a clear transparent mass. (The temperature of the laboratory at the time of these experiments ranged between 22° and 50°.) This jelly is now allowed to stand over night, during which time the smell of butyric ether, very strong at first, entirely disappears. In one of the experiments the alcohol was allowed to evaporate spontaneously before the acid was added; in the other (made with a different sample of butter) the soap was dissolved in about half a litre of water, and at once decomposed by the addition of hydrochloric acid.
The fatty acids which separated in white curdy masses were thoroughly washed on a filter with cold water, about four litres, dried in vacuo over oil of vitriol, and weighed. The results of experiment show that butter fat yields the same proportion of insoluble fatty acids when saponified with or without the aid of heat.
3. Mr Gatehouse. Rapid Method of Detecting the Adulteration of Butter with other Fats. The following comparative method is based upon the insolubility of potassium stearate in alkaline solutions when the stearate has been produced at high temperatures:
Before applying the test it is essential to remove all curd, butter-milk, and salt, by washing with hot water or dissolving in ether. Twenty grains of the butter are placed in a large test-tube one third full with water boiled thoroughly and allowed to stand till the fat separates. The fat is either dissolved in ether, and after evaporation saponified, or the lower layer of the liquid is drawn off by a pipette as follows:—A thin glass tube is drawn out to375 a fairly fine point and bent at the top to an obtuse angle. Whilst the butter is still liquid this nozzle is inserted into the bottom of the test-tube, placing the finger over the upper end to prevent any liquid from getting in till it reaches the bottom. When fairly cold the liquid may be withdrawn by a pipette attached to the tube. This process can be repeated till the washings are free from chlorides.
The saponification is effected by heating the purified butter with 1⁄3-1⁄2 of its own weight of pure solid potassium hydrate (purified by alcohol) to a temperature above 420° F.; applying the heat gently at first, and when the frothing ceases, heating it more strongly, till no further apparent action occurs. The ultimate temperature during saponification must be kept above 400° for some minutes, otherwise the sterate formed will be soluble instead of insoluble in the alkaline solution.
If the butter is pure, the colour of the residue will be at the utmost light yellow, but should the butter be adulterated to any extent, it may be almost black. Too much reliance must not, however, be placed on the colour.
After allowing the tube and its contents to cool, the mass is boiled with successive portions of distilled water till 6 oz. (or 200 c. c.) altogether have been used. If the butter is pure, a portion of this solution poured into a test-tube will present only a faint opalescence; if, on the other hand, the butter is impure, a decided opacity will be perceived, the degree depending upon the amount of adulteration.
The amount of adulteration in any sample is determined by first obtaining pure butter and adding to separate portions of it known per-centages of lard, &c. Each of these can be saponified as stated above; they are then corked up in tubes of equal diameter and labelled with the per-centage of lard they contain. On comparing them it will be seen that 2 per cent. of lard can be clearly indicated.
When a butter is analysed all that is wanted is to saponify, make up to the correct strength, and after cooling pour into a test-tube and compare with the specimen tubes.
4. Dr Redwood. The Determination of the Melting Points of Butter and other Fats. The apparatus in the form best suited for general use consists of a basin, two small beakers, and a thermometer. The author uses an enamelled iron basin about six inches in diameter, and three and a half inches deep. In this is placed a beaker four and a half inches deep and three inches in diameter, and within this beaker is placed another much smaller one, supported by its projecting rim on a disk of tinplate or copper, the outer edge of which rests on the mouth of the larger beaker. Some mercury is put in the smaller beaker to the depth of about an inch, and cold water into the larger beaker, so that its surface shall be half an inch or an inch higher than that of the mercury.
A small drop of the fat which has been previously melted and heated to several degrees above its melting-point, but has been allowed to cool again to near its setting point, is put on the surface of the cold mercury. This is best done by means of a thin glass rod about one eighth of an inch in diameter, the end of which has been rounded off in the blow-pipe flame.
It is important that the drop should be very small, and its temperature when placed on the mercury not much above its melting point, for if it be too hot it will spread over the surface of the mercury, which is not desirable.
If the rounded end of the rod be slightly dipped into the melted fat, and then brought to the surface of the mercury, a small hemispherical particle will attach itself there and speedily congeal, becoming more or less opaque in doing so. The weight of one of these hemispherical masses, which should not be more than the eighth of an inch in diameter, will be from 1⁄50 to 1⁄10 of a grain. Having placed the drop of fat upon the mercury, the bulb of a thermometer, with sufficiently minute graduations, is introduced into the mercury and hot water poured into the basin. The heat is thus communicated to the contents of the small beaker slowly through the water in the larger beaker, and the rise of temperature in the mercury may be easily regulated, and should take place at the rate of about one degree per minute.
The mercury, by virtue of its comparatively good conducting power, acquires a uniform temperature throughout, which is indicated by the thermometer, and at the same time communicated to the fat. The fat when the temperature approaches its melting point becomes partially transparent, and if the stem or elongated bulb of the thermometer be now brought up against it, the moment fusion takes place the liquid fat will run into the channel formed by the repulsion of the mercury and the outside of the thermometer tube. This process presents the following advantages:—
1. The heat-conducting power of the mercury, on which the fat is placed, ensures the equalisation of the temperature as indicated by the thermometer, and at the same time communicated to the fat.
2. The direct contact of the fat with the mercury, without the intervention of a bad conducting medium, such as glass, ensures a more immediate and correct indication of the temperature at which liquefaction takes place than would otherwise occur.
3. The minuteness of the quantity of fat operated upon reduces to a minimum the time occupied in its melting, and thus facilitates the determination with exactness of its melting point.
4. The time occupied in preparing small tubes and charging them with the fat is saved, and several experiments in succession may be easily and rapidly made with the same apparatus. The author observed that in butter as376 well as other fats, such as tallows, there were at least two melting points, dependent upon the way in which the fat had been previously subjected to the action of heat, and that they may differ in butter to the extent of 3° or 4° F.; the low melting point being that of the fat after it has been heated to several degrees above its first melting point, and the higher melting point being that of fat which has been previously melted to the lowest possible temperature, and then immediately allowed to congeal.
5. Professor Wanklyn carefully weighs one gram of butter, and heats it in a platinum dish of the size shown in the accompanying figure, from four to six hours or even more—in short, until it ceases to lose weight. The loss of weight is the water, which should be calculated and expressed in per-centages.
Fat. The dried butter is now to be heated with ether (the ether should be made to boil by floating the dish in hot water). Several successive portions should be taken, the whole passed through a filter, the filter well washed with ether, and the filtrate evaporated to dryness and weighed.
Caseine and Ash. The residue from which the fat and water have been extracted is now to be taken, carefully weighed, then burned down to a low red heat; the residue remaining is the ash, the loss the caseine.
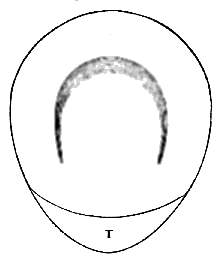 Butter-analysing dish.
Butter-analysing dish.
The amount of ash, practically speaking, is the salt, but if there be any doubt as to its composition, the chlorine maybe estimated by a volumetric solution of nitrate of silver, and further examined.
The following table shows the composition of a few genuine and other butters, examined according to the same, or at least to a similar process to the one described:—
| Fat. | Ash, principally Salt. | Water. | Caseine. | Quality. | ||
| Fresh Devonshire butter. | 82·7 | 1·1 | 16·2 | 16·2 | Good. | Wanklyn. |
| Normandy butter. | 82·1 | 1·8 | 16·1 | 16·1 | ” | ” |
| Jersey butter. | 78·491 | 8·528 | 10·445 | 2·536 | ” | Angell and Hehner. |
| Normandy butter. | 82·643 | 2·915 | 9·305 | 5·137 | ” | |
| Butter from Ventnor. | 86·280 | 6·600 | 3·831 | 3·289 | Found to be adulterated with foreign fat. | ” |
| Butter from London. | 87·50 | 1·559 | 23·981 | 6·880 | Adulterated with water. | ” |
| ” | 47·119 | 2·689 | 42·358 | 7·834 | Adulterated with water, and contains an excess of curd. | ” |
6. A Method of Detecting Meat Fats in Butter. Mr Horsley, writing to the ‘Chemical News,’ September, 1874, says:—“My starting point is, that fresh butter is permanently soluble in methylated ether, sp. gr. 0·730 at the temperature of 65° Fahr. But with the view of seeing if any other substance it may contain could be precipitated from it, I took, say, 20 or 25 grains of fresh butter, placed it in a small test-tube, and poured over it one drachm of methylated ether, and on corking the tube it readily dissolved after a few minutes’ agitation. I then added 30 drops of methylated alcohol, 63° o. p., and agitated again, but nothing was precipitated. I, therefore, made another experiment with 15 grains of butter and 10 grains of prepared mutton fat, dissolved them in 1 drachm of ether first, and added 30 drops of alcohol, when in less than half an hour the fat was precipitated in a room heated to 68° Fahr. Next, in order to see the effects upon mixtures of known fats,377 such as lard, beef, mutton, and tallow fats properly melted together in proportions of 60 grains of butter and 40 of fat, and stirring till cold, I found that each of them could, by a similar procedure, be precipitated in a few minutes. In one case, that of mutton, I filtered off the ethereal liquid, and collected the residue, and obtained as much as 30 per cent. of what had been used; so that there is no longer any doubt about easily detecting fatty adulterations in butter. Lastly, I would observe that crystallisation of butter, out of the ethereal solution at a lower temperature than 65°, must not be mistaken for the fats precipitated by alcohol alluded to, as the butter, besides being so much lighter, occupies the upper layer, and is different in character and easily remelted by the application of the warm hand for a minute or so.
“Further experiments have proved that half an hour suffices to effect the full precipitation of fats from the ethereal solutions by the addition of 20 drops or so of alcohol to the drachm of ether, containing not more than 25 grains of the adulterated butter; after which the tube should be agitated and its contents projected on to a small double filter, washed with a little alcohol, and the residue whilst moist scraped off, and transferred to a watch-glass to dry. In this way loss by melting and absorption into the paper is obviated.
“The following were the proportions of fats I recovered, viz.—
| Lard | 60 | per cent. |
| Mutton fat | 75 | ” |
| Beef fat | 95 | ” |
The precipitated mutton fat is powdery, and white as snow. Lard and beef are more adherent and greasy; for that reason mutton makes the firmest compound.”
7. On the Cooling of Fats. At a meeting of the University of Edinburgh Chemical Society, held on March 13th, 1878, a paper on the above subject was read by Mr Treharne, M.B.C.M., wherein the author states:—“If equal bulks of the fats of mutton, beef, pork, and butter, and palm oil be heated to 100° C. in small flasks fitted with a thermometer through the cork, and then allowed to cool by radiation under the same conditions for each, temperature is found to fall regularly to a certain point (which is different for each of the facts above named) and then to rise to a certain turning point. These turning points are approximately as follows:—
| For | Mutton | fat | 40° | C. |
| ” | Beef | ” | 28·5° | C. |
| ” | Pork | ” | 26·5° | ” |
| ” | Butter | ” | 23·5° | ” |
| ” | Palm oil | 21·0° | ” |
The extent of the rise in temperature is different in each fat, being greatest in that of mutton, and least in that of butter and palm oil. The extent of the rise is also greater within certain limits the greater the quantity of fat employed; but as a rule the turning point is pretty constant for the same fat. There is also a little difference in the turning points and the extent of rise according to the part of the carcase from which the fat has been taken. If temperature and time be taken as co-ordinates, and the rate of cooling be represented by curves, these latter will be characteristic of the respective fats. A mixture of equal parts of mutton and butter fats does not give a curve intermediate between those of its two components; but is such as to indicate that less heat is given out on cooling (to 20° C say) than in the case of butter, which, compared with mutton fat, gives off very little heat.”
For further information on the subject of ‘Butter’ the reader is referred to a Report by Mr Bell—the principal of the Chemical Laboratory at Somerset House—to the Board of Inland Revenue, included in a return made to the House of Commons in 1876.
Preservation. 1. Melt the butter in a stoneware or a well-glazed earthen pan set in a water bath at a heat not exceeding 180° Fahr., and keep it heated, skimming it from time to time until it becomes quite transparent; then pour off the clear portion into another vessel, and cool it as quickly as possible by placing the vessel in very cold water or ice. This is the method employed by the Tartars who supply the Constantinople market. In this state it may be preserved perfectly fresh for 6 or 9 months, if kept in a close vessel and a cool place. This is the plan so strongly recommended by M. Thénard. Mr Eaton states that butter melted by the Tartarian method, and then salted by ours, will keep good and fine-tasted for two years.
2. Saltpetre and white sugar, of each 1 oz.; best Spanish great-salt (or Cheshire large-grained salt), 2 oz.; all in very fine powder; mix thoroughly, and add 1 oz. of this mixture to every lb. of butter, and thoroughly incorporate them together. The butter thus prepared is then to be tightly pressed into clean glazed earthenware vessels (or well-seasoned casks), so as to leave no vacuities. This plan is recommended by Dr Anderson, who declares that “butter so prepared will keep in a cool place for years; and will bear a voyage to the East Indies, if packed (stowed) so as not to melt.” It does not taste well before it has stood for three or four weeks, after which it acquires a rich marrow-like flavour, which no other butter ever possesses. A good method to preserve the butter from the air is to fill the pots to within an inch of the top, then to lay on it some coarse-grained salt to the depth of a 1⁄4 to 1⁄2 an inch, and lastly to cover each pot with a slate, plate, or other flat article. The salt by long keeping runs to brine, which forms an air-tight layer on the top of the butter, and may at any time be very easily removed by turning the pot on one side.378
3. Fresh butter, 21 lbs.; salt, 1 lb.; saltpetre, 1 oz. These are the common proportions for the best salt butter of the shops.
4. Fresh butter, 18 lbs.; salt, 1 lb.; saltpetre, 11⁄2 oz.; honey or fine brown sugar, 2 oz. Superior to No. 3.
Concl. Remarks. It may be useful to know that rancid butter may be restored, or, in all cases, greatly improved, by melting it in a water bath with some fresh-burnt and coarsely powdered animal charcoal (which has been thoroughly freed from dust by sifting), and straining it through clean flannel. A better and less troublesome method is to well wash the butter first with some good new milk, and next with cold spring water. Butyric acid, on the presence of which rancidity depends, is freely soluble in fresh milk.
The turnip-flavour arising from the cows being fed on turnips or cabbages is said to be removed by one or other of the following methods:—1. When the milk is strained into the pans put to every 6 galls. 1 gal. of boiling water.—2. Dissolve 1 oz. of nitre in a pint of spring water, and put a 1⁄4 pint of the solution to every 15 galls. of milk.—3. Keep back a 1⁄4 pint of the sour cream when you churn, and put it into a well-scalded pot, into which you are to gather the next cream; stir that well, and do so with every fresh addition. Each of these methods come on good authority, but we are bound to say that our own experience does not confirm their constant success. We have found that the addition of a handful of salt to the water used to wash the butter is as good a plan as any.
Butter, Ancho′vy. From anchovies (boned and beaten to a paste), 1 part; butter, 2 parts; spice, q. s.
Butter-colouring (from Paris). A mixture of 40 per cent. of chrome yellow with some fat coloured with annatto. (Flückiger and Weil.)
Butter, Clar′ified. Fresh butter melted in a water bath, allowed to settle, and the clear portion poured into an earthenware basin or pot, set in cold water, so as to cool it as quickly as possible, without allowing it to crystallise. It keeps a long time without becoming rank. See Butter, No. 1 (antè).
Butter, Hon′ey. Fine Narbonne honey, 2 to 4 oz.; mixed with good butter, 1 lb. Used as a delicacy for children, and by the sick and aged.
Butter, Lem′on. See Butter, Orange.
Butter, Melt′ed. This well-known sauce may be prepared of excellent quality as follows:—Beat up about 1 oz. of fine flour with 4 oz. of butter, in the cold, until they are evenly and thoroughly mixed, then add 4 or 5 table-spoonfuls of hot milk, put the whole into a small saucepan, and continue shaking it, all in one direction, until it simmers very gently; lastly, remove it from the fire, and pour it into the butter-boats for use. These last should be filled with hot water, and then emptied and wiped dry, before putting the melted butter into them. See Sauces.
Butter, Or′ange. Prep. 1. From 6 eggs, 2 oz. of powdered sugar, and 4 oz. of butter, well beaten together with a little orange-flower water. Sometimes 1 or 2 oz. of blanched almonds, or of almond-paste, is added.
2. Butter, 1 lb.; syrup of orange peel, 4 oz. Both are eaten as a delicacy. Lemon butter, is made in a similar manner.
Butter of An′timony*‡. Trichloride of antimony.
Butter of Caca′o. See Co′coa, and Co′coa-nut Oil.
Butter of Nut′megs. Collected from the surface of the water in the still, after the distillation of the essential oil of nutmegs.
Butter of Ro′ses. Obtained by distilling damask roses. It separates slowly from the water in the receiver. It has little smell, and is hence used to dilute the odour of musk, ambergris, and civet.
Butter of Wax. Prepared by distilling bees′-wax. A factitious kind is also made.
Butter of Zinc*‡. Chloride of zinc.
Butter Powder (from the Adler-Apotheke Emmerich on the Rhine). Bicarbonate of soda. (Dr U. Kreusler.)
Butter Powder (Lemmel, Schleswig-Holstein). An impure bicarbonate of soda, coloured with turmeric. (Hirschberg.)
Butter Powder, Schuhrer’s (Emil Schührer, Mutzschen, Saxony). This, it is claimed, will considerably increase the yield of butter, shorten the process of churning, and yield a product which will be firm even in the height of summer, well-flavoured, of a handsome colour, and of excellent commercial value. It consists of a tolerably pure commercial bicarbonate of soda, with 1⁄2 per cent. of powdered turmeric. (Dr Peters.)
Butter Powder, Tomlinson’s (Tomlinson & Co., Lincoln, England). Ordinary bicarbonate of soda, coloured with 3⁄4 per cent. of annatto. (Dr Karmrodt.)
Butter-preservative Paste (from Spaa). Consists of common salt, 52 parts; nitre, 23 parts; syrup, 5 parts. (Wittstein.)
BUTTERINE. A substance known under this name, and intended as a substitute for butter, is imported into this country from New York.
Of butterine Dr Campbell Brown remarks:—“In general appearance, taste, and consistence, it is very similar to ordinary butter; but notwithstanding that its solidifying point is lower than that of some butters, it retains much of the peculiar crumbly texture and fracture of dripping.
“It softens at 78° F., and melts at 86°. When heated and slowly cooled it obscures the thermometer at 62° and solidifies at 60°. It contains379—
| Water | 11·25 | to | 8·5 |
| Salt | 1·03 | ” | 5·5 |
| Curd | 0·57 | ” | 0·6 |
| Fat | 87·15 | ” | 85·4 |
| ——— | ——— | ||
| 100·00 | 100·00 |
“The fat consists of oleine, palmitine, margarine, a trace of stearine, and about 5 or 6 per cent. of butter. When dissolved in about four times its weight of ether, and allowed to evaporate spontaneously, it does not deposit any fat until more than half of the ether has passed off, and if the temperature is not below 60° the deposit is not solid.
“The first deposit when dried fuses at 108°; the second deposit fuses at 88°, and solidifies at 64°.
“Under the microscope butterine does not appear to consist of acicular crystals of fat, but of irregular masses, containing a few butter globules, particles of curd, and crystals of salt. With polarised light the irregular crystalline structure is beautifully seen, and is clearly distinguishable from butter which has been melted and recongealed. When old and rancid it acquires the odour and taste of dripping, but it keeps longer undecomposed than butter. When fresh it is a wholesome substitute for real butter. No one can reasonably take exception to its sale.
“Butterine may be detected by the following characters:—
“1. Its crumbly fracture.
“2. Its loss of colour when kept melted for a short time at 212°.
“3. The behaviour of its ethereal solution.
“4. Its action on polarised light.”
The ‘American Chemist’ for 1876 contains an interesting paper by Mr Henry Mott on the manufacture of artificial butter, which is too lengthy for insertion here.
BUTTER-MILK. The liquid that remains after the butter is separated from the cream.
Qual., &c. Butter-milk left from the churning of sweet cream is not only very delicious, but exceedingly wholesome and nutritious. It is eaten with fruit, puddings, and cakes, and is said to possess the property of allaying the nervous irritability induced by excessive tea-drinking. It is an admirable beverage in rickets, diabetes, and many stomach affections. An American physician has recently asserted that it induces longevity. See Milk.
BUTTONS. See Brass, Gilding, &c.
BU′TYRATE. [Eng., Fr.] Syn. Bu′tyras, L. A salt in which the hydrogen of butyric acid is replaced by a basic radical.
Butyrate of Barium. Prep. Saponify butter with a boiling solution of caustic alkali, and decompose the resulting soap by adding a solution of tartaric acid; filter and distil; neutralise the distillate with hydrate of barium, and evaporate; the first crystals that form are caprate of barium; the next, caproate of barium; and the last, butyrate of barium. This salt is very soluble in water, and hence is easily separated from the others.—Use. Chiefly for making butyric acid.
BUTYRIC (-tĭr′-). Syn. Butyr′icus, L.; Butyrique, Fr. Of or from butter.
BUTYRIC ACID. HC4H7O2. Syn. Acidum Buty̆̆r′icum, L.; Acide butyrique, Fr.; Buttersäure, Ger. An oily acid, first obtained by Chevreul from butter.
Prep. From butyrate of barium or magnesium, by adding sulphuric acid in quantity not quite sufficient to decompose the whole of the salt; the clear liquid, filtered and distilled, yields butyric acid, from which the water may be removed by digestion with chloride of calcium.
Prop. A thin colourless liquid, of pungent rancid odour, and sour taste, miscible with water and alcohol. It boils and distils unchanged at 327° Fahr. Sp. gr. ·963. See Ethers.
BU′TYRIN (-in). [Eng., Fr.] An oily substance existing in butter, and of which it forms the characteristic portion. It was discovered by Chevreul.
Prep. Keep clarified butter in a porcelain vessel, at a heat of 66°, for some days; carefully collect the oily portion which separates, mix it with an equal weight of alcohol of the sp. gr. ·796, and agitate it frequently for 24 hours; after repose pour off the clear portion, and evaporate it; treat the oily residuum with a little carbonate of magnesium, to remove free acid, and wash off the butyrate of magnesium, thus formed, with water; lastly, heat the remaining fatty matter in alcohol, filter, and evaporate, by a gentle heat; the residuum is butyrin.
BUXINE (-ĭn). A substance detected by M. Faure in bux′us semper′virens, or the common box-tree.
CABBAGE. Syn. Bras′sica, L.; Chou, Fr.; Kohl, Ger. This common esculent, and all its numerous varieties, are merely cultivated specimens of the wild sea-cabbage of our coasts (bras′sica olera′cea, Linn.), one of an extensive and valuable genus of plants belonging to the nat. ord. Cruciferæ. After the potato, the cabbage is doubtless more extensively used by the masses of the people than any other fresh vegetable. When young, and properly dressed, it forms an agreeable and wholesome addition to animal food, the grossness of which, it is said, it tends to correct. It should be eaten only when fresh gathered and fresh cooked; and the unconsumed portion, as well as the water in which it has been boiled, should be at once thrown away. Persons troubled with a weak digestion, or who have a tendency to flatulence, diarrhœa, or worms, would do well to avoid them. Their use is particularly serviceable in scurvy, and in numerous skin diseases.
It has been asserted that cabbages, cauliflowers, broccoli, celery, and several other380 culinary vegetables, may be preserved in a fresh state for some time, by cutting them so that they may have about two inches of stem left below the leaves, scooping out the pith as far down as a small knife will reach, and then suspending them perpendicularly by means of a cord, in an inverted position, in some cool situation, and daily filling up the bottom part of the stem with clean cold water. In this way it is stated that a supply of green vegetables may be readily obtained during a severe winter, and on ship-board. Other methods, including those usually adopted with the same object, are noticed under Vegetables (Culinary).
Cabbages, broccoli, &c., are dressed by simply throwing them into boiling water, and simmering them until tender. A few minutes is sufficient for this purpose. A pinch of salt of tartar, or of carbonate of soda, is commonly added to the water, to preserve the green colour of the vegetables.
CACHOU AROMATISE (kăshoo ărŏmătēzā). [Fr.] A mouth-lozenge intended to sweeten and perfume the breath. Preparations of this description are much used by smokers and bacchanals. The form under which they are generally prepared for sale is that of 11⁄2 to 2 gr. pills, neatly silvered. Originally they were composed chiefly of catechu and sugar, flavoured and perfumed with the stronger aromatics; but at the present day the catechu, from which they derive their name, is not unfrequently omitted. Their preparation is described elsewhere. See Breath, Lozenges, Pastils, &c.
CAD′MIUM. Cd. [Eng., L.] Syn. Klapro′thium. A metal discovered by Stromeyer and Hermann, in the ores of zinc.
Prep. 1. (Stromeyer.) The cadmo-zincic ore is dissolved in an excess of dilute sulphuric or hydrochloric acid by heat; a stream of sulphuretted hydrogen is passed through the solution, the resulting precipitate (sulphide of cadmium) dissolved in nitric acid, and the solution evaporated to dryness; the residuum is dissolved in water, the solution precipitated with carbonate of ammonium in excess, and the precipitate (carbonate of cadmium) collected, mixed with charcoal, and heated to redness in a crucible apparatus so arranged as to condense the fumes; the cadmium sublimes.
2. (Wollaston.) A solution of the ore obtained as above is placed in a platinum capsule, and a piece of metallic zinc is plunged into it. In a short time the cadmium is precipitated, and attaches itself to the sides of the capsule, when it is collected, washed, and dried.
3. (Herapath.) When zinc is obtained by distilling its ores, per descensum, the first portion of the metallic fumes evolved burn with a brownish flame, and deposit oxide of cadmium, which is subsequently reduced by distillation with charcoal. Thousands of pounds of cadmium are yearly wasted at the zinc works which might be easily collected in a similar manner.
Prop., &c. Resembles tin in most of its physical properties, being white, soft, and malleable. Sp. gr. 8·61. Stromeyer gives its melting point as 442° Fahr., but Dr Wood, an American chemist, states that the metal requires for its fusion nearly the same heat as lead, and gives it as about 600° Fahr. It volatilises at a somewhat higher temperature, giving off orange-coloured, suffocating fumes, which, when inhaled too freely, leave a disagreeable, sweetish, styptic sensation upon the lips, and a persistent brassy taste in the mouth, with constriction of the throat, heaviness in the head, and nausea. The alloys of cadmium are said to be brittle by almost all who have treated of them, but Wood found that many were extremely tenacious, as, for instance, the combination of 2 parts of silver and 1 part of cadmium, which is perfectly malleable and very strong. The amalgam of equal parts of cadmium and mercury is also highly malleable. Like bismuth, cadmium has the property of promoting fusibility in certain alloys; thus, a remarkable fusible metal may be formed by melting together cadmium 1 to 2 parts, lead 2 parts, and tin 4 parts.
Tests. Its ores and salts are recognised as follows:—1. Mixed with carbonate of sodium, and exposed on a charcoal support to the reducing flame of the blowpipe, the charcoal becomes almost instantly covered with a reddish-yellow incrustation of oxide of cadmium, commonly forming a circle or zone.—2. Caustic soda and potassa give a white precipitate (hydrated oxide) in solutions containing cadmium, insoluble in excess of the precipitant.—3. Ammonia gives a similar white precipitate, freely soluble in excess.—4. The alkaline carbonates give white precipitates (carbonate of cadmium), insoluble in excess.—5. Sulphuretted hydrogen, and sulphydrate of ammonium, give a bright yellow precipitate (sulphide of cadmium), which is insoluble in dilute acid, alkalies, sulphides, and cyanide of potassium, but readily soluble in both hydrochloric acid and nitric acid, especially with heat.—6. The salts of cadmium are readily distinguished from those of arsenic, by the precipitated sulphide being insoluble in ammonia, and soluble in hydrochloric acid, and being capable of sustaining a white heat without subliming.
Cadmium, Car′bonate of. CdCO3. Syn. Cad′mii car′bonas, L. From a solution of sulphate or chloride of cadmium, and an alkaline carbonate; the precipitate being collected, washed, and dried by a gentle heat. A white powder.
Cadmium, Chlo′′ride of. CdCl2. Syn. Hydrochlo′′rate of cadmium, Mu′′riate of cadmium; Cad′mii chlori′dum, Cad′mii hydrochlo′′ras, L. Prep. 1. (Pure.) By dissolving carbonate or oxide of cadmium in hydrochloric acid, and crystallising by gentle381 evaporation. Prismatic crystals; very soluble in water.
2. (Turner.) By exposing the product of the last process to heat. Amorphous.
3. From crude cadmium or its oxide, and hydrochloric acid, as last.
Cadmium, I′odide of. CdI2. Syn. Hydri′odate of cadmium; Cad′′mii iodi′dum, C. hydrio′das, L.
Prep. (Crookes.) Cadmium in filings 1 part, pure iodine 2 parts, are to be placed together in a capacious flask, with alcohol sufficient to cover them. Action commences at once, attended with considerable evolution of heat; when it ceases, heat the mixture till it is colourless; then filter from a few grains of cadmium which will remain undissolved, evaporate and crystallise.
Uses. In photography this salt has lately been employed with great success for iodizing collodion. Being very stable, it is not decomposed, and the collodion iodized with it preserves its sensitiveness undiminished during many months. (See Collodion.) In medicine it is used occasionally as a substitute for iodide of lead.
Cadmium, Ox′ide of. CdO. Syn. Protox′ide of cadmium; Cad′mii oxy′dum, L. Prep. 1. (Hydrated.) From sulphate or chloride of cadmium, and a solution of caustic alkali; observing to well wash and dry the precipitate. A white powder, freely soluble in acids.
2. (Anhydrous.) By igniting the hydrated oxide, or the carbonate or nitrate of cadmium. That from the first two has a pale brown colour; that from the nitrate has a dark brown tint and a semi-crystalline appearance. The former has been proposed to be used as a pigment.
Cadmium, Sul′phate of. CdSO4. Syn. Cad′mii sul′phas, Cad′mium sulphu′ricum, Klapro′thrium sulphu′ricum, L. Prep. 1. From carbonate or oxide of cadmium and dilute sulphuric acid, as the chloride.
2. (Cottereau.) Oxide of cadmium, 1 oz.; sulphuric acid, q. s.; dissolve, evaporate, and crystallise.
3. (Pereira.) Sulphuric acid, 61⁄2 parts; water, 15 parts; mix; add cadmium, 7 parts; dissolve, evaporate to dryness, redissolve in water, filter, and evaporate by a gentle heat, so that crystals may form.
Prop., &c. Efflorescent, rectangular, prismatic crystals; very soluble in water; tastes astringent. It is about 4 times as strong as sulphate of zinc, and is used in similar cases. Dose, 3 to 10 gr. Externally (1⁄2 to 3 or 4 gr. to water, 1 oz.); in specks of the eye, opacity of the cornea, chronic ophthalmia, &c. As an ointment, 10 to 12 gr. to lard, 1 oz.
Cadmium, Sul′phide of. CdS. Syn. Cadmium yellow. This occurs native as Greenock′ite. It may be prepared artificially, either by fusing its elements together, or by passing a stream of sulphuretted hydrogen through a solution of the chloride, nitrate, or sulphate. When prepared artificially, it is of a bright yellow or orange colour, and is of great value to the artist. It has been used in making fireworks. See Fires, Coloured.
Cadmium, Yellow. See Cadmium, Sulphide of (above), and Yellow Pigments.
CÆSALPINA (GUILANDINA) BONDUCELLA. (Ind. Ph.) Habitat. Tropical portions of both hemispheres.—Officinal part. The seeds (Bonducellæ semina, Bonduc seeds); of a somewhat irregular sub-spherical or ovoid form, usually from 5⁄8 to 6⁄8 of an inch in diameter, smooth, hard, and lead-coloured, and contain an amylaceous white nucleus, having a bitter taste. They contain a fixed oil, resin, and a bitter principle.—Properties. Tonic and antiperiodic.—Therapeutic uses. In intermittent fevers; also in debility, and other cases requiring tonics.—Dose, 10 to 15 grains twice daily.
Compound Powder of Bonduc (Pulvis Bonducellæ compositus). Take of bonduc seeds, deprived of their shells and powdered, 1 oz.; black pepper, powdered, 1 oz. Mix thoroughly, and keep in a well-stoppered bottle.—Dose, 15 to 30 gr., three times a day.
CÆSIUM. [Eng., L.] Cæ. A metal belonging to the alkaline group, discovered by Bunsen in the mineral water of Durckheim by means of SPECTRUM ANALYSIS (which see), and so named by him from cæsius, greyish-blue, the colour of its characteristic ray.
CAFFE′IC ACID. Syn. Chloroge′nic acid. A white powder, discovered by Runge in coffee, in which it exists in combination with potassium (caffeiate of potassium), and caffeine, and is then very soluble in alcohol. Pfaff states that the aroma of coffee is dependent on the volatilisation, or, rather, the decomposition of this acid.
CAFFE′INE. C8H10N4O2. Syn. Caffe′ina, Théine, Guaranine. A peculiar nitrogenised principle, discovered by Robiquet in coffee. It is, moreover, the essential principle of tea, of Paraguay tea, and of Guarana, infusions of which are used as beverages in different parts of the world. The proportion of caffeine to the pound was found by Liebig to be as stated below in the six descriptions of coffee named:—
| Martinique | 32 | grains. |
| Alexandrian | 22 | ” |
| Java | 22 | ” |
| Mocha | 20 | ” |
| Cayenne | 19 | ” |
| St Dominique | 16 | ” |
In Hyson tea it exists in the proportion of from 2·5 to 3·4 per cent.; and in gunpowder tea from 2·2 to 4·1. In Paraguay tea, or maté as it is called in Brazil, and in Guarana, it exists in the proportion of ·13 per cent.
Prep. 1. Coarsely powdered raw or unroasted coffee is boiled in water, and subacetate of lead added to the filtered decoction to throw down the extractive and colouring matter;382 the excess of lead is next precipitated with sulphuretted hydrogen, and the liquid filtered and evaporated by a gentle heat; the residuum is dissolved in boiling water, the solution agitated with freshly burnt animal charcoal, filtered, evaporated, and crystallised. By redissolving the product in hot alcohol, it may be obtained in white, shining, silky filaments, as the solution cools.
2. (H. J. Versman.) Quick-lime, 2 lbs.; water, q. s. to form a hydrate; raw coffee (bruised), 10 lbs.; mix, put it into a displacement apparatus, and cause alcohol of 80% to percolate through the mixture, until the fluid obtained no longer contains caffeine; the mass in the percolator is then roughly ground to powder, mixed with a fresh quantity of quick-lime, and the process of percolation repeated with fresh alcohol, as before. The spirit is next distilled from the mixed tinctures in a retort, and the residuum washed with a little warm water to remove the oil; the evaporation is then gently conducted until a crystalline mass is obtained, which is further freed from adhering oil by pressure between folds of blotting paper. It is purified by redissolving it in boiling water or hot alcohol, &c., as before.
3. (A. Vogel.) An extract of powdered coffee is made with commercial benzol; this being distilled off, leaves an oil and caffeine behind; the oil is then removed by a little ether or by hot water, from which latter liquid the alkaloid crystallises on cooling.
4. From a hot infusion of tea-leaves by treatment with subacetate of lead and sulphuretted hydrogen, as in process 1 (above).
5. (F. V. Greene.) Powdered guarana is intimately mixed with three times its weight of finely divided litharge, and the mixture boiled in distilled water, the ebullition being continued until, on allowing the temperature to fall below the boiling point, the insoluble portion is found to subside rapidly, leaving the supernatant liquid clear, bright, and without colour. The quantity of distilled water required will be found to be about a pint for every fifteen grams of the guarana used in the experiment, and as the boiling has to be continued for several hours before the desired and all essential separation mentioned above takes place, water must be added from time to time to supply the place of that lost by evaporation. When cool, the clear liquid is decanted upon a filter, and when it has passed through, which it will be found to do with facility, the precipitate is to be transferred to the filter, and washed with boiling water, the washing to be continued as long as yellowish precipitates are produced with either phosphomolybdic acid solution, auric, or platinic chloride. A stream of sulphuretted hydrogen gas is now passed through the filtrate to remove the small quantity of lead that has been dissolved, and the sulphide thus formed separated by filtration. The solution is evaporated on a water bath to expel the excess of sulphuretted hydrogen, filtered to remove a trace of sulphur, finally evaporated to the crystallising point, and the caffeine which crystallises out in cooling removed from the mother liquor and pressed between folds of bibulous paper. After being thus treated the crystals will be found to be perfectly white. On diluting the mother liquor with distilled water, filtering, and evaporating, a second crop of crystals are obtained, which are also perfectly white, after being pressed as above. The crystals are now dissolved in boiling dilute alcohol, filtered, and the solution set aside to crystallise by spontaneous evaporation. The resulting crystals of caffeine are perfectly pure and colourless.
6. (O. Caillol and P. Cazeneuve.) The following is a process for the rapid preparation of caffeine:—Black tea is thoroughly softened with four times its weight of hot water; a quantity of calcium hydrate equal to that of tea used is then added, and the whole evaporated on a water-bath to perfect dryness. The dry residue is exhausted with chloroform in a displacement apparatus, and the chloroform recovered from the percolate by distillation. The residue left in the retort is a mixture of caffeine and a resinous substance containing chlorophyll. On treating it with hot water, filtering and evaporating the filtrate on a water bath, the caffeine is obtained in perfectly white crystals.
Prop., &c. Soluble in 100 parts of cold water; freely soluble in hot water and in water acidulated with an acid; slightly soluble in cold alcohol; it fuses at 352° Fahr., tastes slightly bitter, and possesses feeble basic properties. With the sulphuric and hydrochloric acids it forms crystallisable compounds. The salts of caffeine may be made by dissolving it to saturation in the dilute acid, and evaporating the solution by a very gentle heat. It forms splendid double salts with bichloride of platinum and terchloride of gold.
Uses. Caffeine has been recommended in those pains that affect only one side of the head (hemicrania); in doses of 1 to 3 gr. Its physiological action is very trifling, notwithstanding all that has been said to the contrary. Mr Cooley took 20 gr. daily of pure caffeine, for above a month, without experiencing any other effect than a very slight elevation of spirits after each dose, similar to that produced by a small quantity of spirits of sal volatile. It has been used lately with doubtful success as an antidote to the poisonous effects of opium. See Coffee, Tea, &c.
CAFFE′ONE. A brown, aromatic oil, formed during the roasting of coffee.
CAJ′EPUT OIL. See Oils (Volatile).
CAKES. A species of fancy bread or trifle familiar to every one.
Before proceeding to the actual operation of cake-making, the various materials which are to enter into their composition undergo a certain383 amount of preparation. For this purpose every article is got ready about an hour previously to its being wanted, and is placed before the fire, or upon a stove, that it may become gently heated. Without these precautions it is impossible to produce good cakes. The flour is thoroughly dried, and warmed. The currants are nicely washed in a hair sieve, wiped dry in a cloth, and then set before the fire. Before use they are dusted over with a little flour. The sugar is rubbed to a fine powder, and passed through a sieve. The eggs are well beaten in a basin, and strained. The butter is melted by being placed in a basin set in hot water, and is afterwards well beaten up with a little warm milk. The lemon peel is cut very thin, and beaten in a mortar to a paste or powder, with lump sugar; or for common purposes, it is grated. The caraways, ginger, and other flavouring ingredients are preferred in the form of fine powder, or are made into an essence, by digesting them in spirit of wine; the first is the most common method. The milk and water is made lukewarm. When all these things are ready and have stood a sufficient time, they are put into a pan, one after another, in the proper order, and well beaten together, by which the lightness of the cakes is considerably increased.
In plum cakes, as well as in some other varieties, a little yeast may be added after the butter, and the mass allowed to rise a little, and then again well kneaded, by which not only less butter and eggs may be used, but the products will be both lighter and more wholesome. Good stale bread, well soaked in hot milk or water, and then beaten to a paste, and passed through a fine sieve, forms an excellent thing to mix up the ingredients with, and produces a very light and nutritious cake. Cakes “wetted up” with milk are richer, but do not keep so well as those without it; they get stale sooner, and then in that state are far from agreeable to the palate. A kind of flour prepared from maize or Indian corn has been recently introduced to the notice of cooks, but it is better adapted for puddings than for cakes. See Corn-flour.
Cakes are preferably baked on flat tins or in little “tin shapes,” which should be first well buttered.
Cakes should be kept for store in tin canisters; wooden boxes, unless very well seasoned, are apt to give them an unpleasant taste. Brown-paper linings and wrappers should be avoided for the same reason. See Biscuits, Bread, Bun, Icing, Stains, &c.
Cakes, Al′mond. Prep. 1. From sweet almonds (blanched and beaten to a smooth paste), flour and powdered sugar, of each 1⁄2 lb.; 7 eggs, and the outside peel of 4 lemons (shredded small). The almonds, sugar, lemon peel, and eggs, are beaten together, until as white as sponge paste; the flour next worked in, and the paste put into buttered moulds, and baked in a slack oven, with 8 or 10 thicknesses of white paper under them and one or two over them.
2. Almonds, 1 lb.; sugar, 1⁄2 lb.; rose water or orange-flower water, 1⁄4 pint; flour, 3⁄4 lb.; 3 eggs; as above. Some persons ice these cakes.
Cakes, Ban′bury. Prep. From butter and dough fermented for white bread, of each 1 lb., as in making puff paste, then rolled out very thin, and cut into oval or triangular pieces, or other shapes. On these are placed a mixture of currants and moist sugar, equal parts, wetted with a little wine or brandy, and the paste being closed up, they are placed on a tin with the closed side downwards, and baked. A little powdered sugar, flavoured with candied peel (grated), or essence of lemon, is sifted over them as soon as they come out of the oven. In the common cakes of the shops the brandy is omitted, and lard is used for butter, but less of it.
Cakes, Bath. Prep. From butter, 1⁄2 lb., flour, 1 lb., 5 eggs, and a cupful of yeast; when risen, add powdered sugar, 4 oz., and caraways, 1 oz. Bake them on tins.
Cakes, Cheese. Prep. 1. Curdle some warm new milk with rennet, drain the curd in a linen bag, and add 1⁄4 of its weight, each, of sugar and butter, 6 eggs, some grated nutmeg, and a little orange flower or rose water.
2. (Almond Cheese Cakes.) To the above add as much blanched almonds, beaten to a smooth paste, as there is butter, and an equal weight of macaroni.
3. (Lemon Cheese Cakes.) To the first form add lemon peel (grated fine), or essence of lemon, q. s.
Cakes, Di′et. Syn. Diet bread. Prep. 1. Dissolve sugar, 1 lb., in milk, 1⁄2 pint; add 6 eggs, and whisk the mixture to a full froth, then cautiously stir in flour, 1 lb., beat it for 3⁄4 hour, and immediately bake it in a quick oven. It may be baked whole or divided into small cakes.
2. From fine flour and powdered sugar, equal parts; 6 eggs; and the juice and rind (grated) of 1 lemon.
Cakes, Drop. Prep. Eggs, 1 dozen; rosewater, 1 table-spoonful; powdered sugar, 1⁄2 lb.; fine flour, 1⁄2 lb.; and caraways, 1⁄2 oz. Drop it on wafer paper, and bake as before.
Cakes, Gin′ger. Prep. Sugar, 1 lb.; powdered ginger, 4 oz.; flour, 2 lbs.; water, 1 pint; butter, 1⁄2 lb.; candied orange peel, 8 caps (grated).
Cakes, Lem′on. Prep. Flour and sugar, of each 1 lb.; eggs, 1 dozen; grated peel and juice of 4 lemons; whisk the eggs to a bright froth; then gradually add the rest.
Cakes, Marl′borough. Prep. Beat 8 eggs and 1 lb. of pounded sugar 3⁄4 hour; then add fine flour, 1 lb.; and caraway seeds, 2 oz.
Cakes, Plain. Prep. 1. From flour, 4 lbs.; currants, 2 lbs.; butter, 1⁄2 lb.; caraway seeds, 1⁄4 oz.; candied lemon peel (grated), 1 oz.;384 yeast, 1⁄4 pint; milk, q. s. Let it rise well before baking.
2. Baker’s dough, 2 lbs.; currants, 1 lb.; butter, 1⁄4 lb.; 3 eggs; milk (hot), 1⁄4 pint.
3. (Rundell.) Baker’s dough, 4 lbs.; butter and moist sugar, of each 1⁄4 lb.; caraway seeds, a small handful. Well work it together, pull it into pieces the size of a golden pippin, and work it together again. This must be done three times, or it will be in lumps, and heavy when baked.
4. (Rich) Equal weights of flour, butter, sultana raisins, eggs, currants, and brown sugar, mixed up with milk, and seasoned with candied peel, nutmeg, &c., and baked in a quick oven. This resembles “pound cake.”
Cakes, Plum. Prep. 1. (Good.) From butter, 1⁄2 lb.; dry flour, 3 lbs.; Lisbon sugar, 8 oz.; plums and currants, of each 3⁄4 lb.; and some pimento, finely powdered; to be “wetted up” with 3 spoonfuls of yeast, and a Winchester pint of new milk (warmed); bake on a floured tin half an hour.
2. (Excellent.) From fresh butter, sifted sugar, flour, and currants, of each 1 lb.; 18 eggs; powdered spices, 2 oz. (viz. cloves, mace, cinnamon, nutmeg, and allspice); sliced almonds, 4 oz.; raisins (stoned and chopped), 1⁄2 lb.; and a large glass of brandy; bake in a hot oven. When sufficiently baked let the oven cool, and afterwards put in the cake and allow it to remain for several hours to dry. (Rundell.)
3. (Rich.) Take fresh butter and sugar, of each 1 lb.; flour, 11⁄2 lb.; currants, 2 lbs.; a glass of brandy; sweetmeats and peels, 1 lb.; sweet almonds, 2 oz.; 10 eggs; allspice and cinnamon, of each 1⁄4 oz.; bake in a tin hoop in a hot oven for 3 hours, and put 12 sheets of paper under it to keep it from burning. (Mackenzie.)
Cakes, Port′ugal. Prep. From flour, powdered sugar, and fresh butter, of each 1 lb.; 10 eggs; currants, 1⁄2 lb.; and a little white wine; bake in small tins only half filled.
Cake, Potato. A pound of cold potatoes, a quarter of a pound of flour or oatmeal, half a gill of warm milk (with a quarter of an ounce of yeast dissolved in it), a little salt and butter. Mash the potatoes, add the other ingredients, roll out the paste an inch and a half or two inches thick, place it in a greased tin, and bake it.
Cakes, Pound. Prep. 1. As plum cake; but using 1 lb. each of all the ingredients except the spices.
2. Using equal parts of sugar, flour, currants, and sultana raisins, and half that quantity each of butter, brandy, and candied peel, with spices as required.
Cakes, Queen. Prep. From about 1 lb. each of dried flour, sifted sugar, washed currants, and butter, with 8 eggs; the whole beaten for an hour, made into a batter, and baked in little tins, teacups, or saucers, only half filled. A little fine sugar is frequently sifted over them. Nutmeg, mace, and cinnamon are also sometimes added.
Cakes, Rat′ifia. Prep. Beat 1⁄2 lb. of sweet and 1 oz. of bitter almonds, in fine orange, rose, or ratifia water; mix in 1⁄2 lb. of pounded sugar; add the whites of 4 eggs (well beaten); set it over a moderate fire in a preserving-pan; stir it one way until it is pretty hot, and when a little cool form it into small rolls, and cut it into thin cakes; shake some flour lightly on them, give each a light tap, put them on sugar papers, sift a little sugar on them, and put them into a very slack oven.
Cakes, Rout. Prep. From flour, 2 lbs.; butter, sugar, and currants, of each 1 lb.; 3 eggs; 1⁄2 pint of milk; 2 glasses of white wine; and 1 glass of brandy; drop them on a tin plate, and bake them.
Cakes, Savoy. Prep. From flour and sifted sugar, of each 1 lb.; 10 eggs; and the rind of a lemon (grated); form a batter by degrees, put it into moulds, and bake in a slack oven.
Cake, Seed. Prep. 1. (Plain.) From flour, 1⁄4 peck; sugar, 1⁄2 lb.; allspice, 1⁄4 oz.; melted butter, 1⁄2 lb.; a little ginger; milk, 1⁄2 pint; yeast, 1⁄4 pint; add seeds or currants; and bake an hour and a half.
2. (Good.) To the preceding add of butter and sugar, of each 1⁄2 lb., and wet it up with milk previously mixed with 6 eggs.
3. (Rich.) Take of flour, 11⁄2 lb.; butter and sugar, of each 1⁄2 lb.; 8 eggs; 2 oz. of caraway seeds, 1 grated nutmeg, and its weight in cinnamon. Bake 2 hours in a quick oven.
4. (Scotch.) Nine eggs; sugar and butter, of each 1⁄2 lb.; mix well together, then add a little cinnamon, nutmeg, and cloves; 1⁄4 oz. of caraway seeds; 1⁄2 lb. of candied citron; 1⁄4 lb. of candied orange peel; 1⁄2 lb. of blanched almonds (pounded fine); flour, 3 lbs.; and brandy, 1⁄4 pint.
Cakes, Shrews′bury. Prep. From flour, 3 lbs.; sugar, 1 lb.; a little cinnamon and nutmeg; 3 eggs; a little rose water; and melted butter enough to make it into a dough.
Cakes, So′da. Prep. 1. From flour, 1 lb.; bicarbonate of soda, 1⁄4 oz.; sugar and butter, of each 1⁄2 lb.; make a paste with milk, and add candied orange, lemon, or citron peel, or the fresh peels grated, q. s. to flavour.
2. To flour, 1 lb.; sugar and butter, of each 2 oz.; candied peel, 1⁄2 oz.; sesquicarbonate of soda, 3 dr.; milk, q. s.
Obs. An equal weight of carbonate of magnesia, used instead of the soda, also makes good cakes. Both are suitable to delicate stomachs, especially in dyspepsia, with acidity.
Cakes, Sponge. Prep. From 8 eggs; lump sugar, 3⁄4 lb.; flour, 1⁄2 lb.; water, 1⁄4 pint; the yellow peel of a lemon; mix as follows:—Put the lemon peel into the water; when about to make the cake, put the sugar into a saucepan, pour the water and peel on it, and let it stand by the fire to get hot. Break the eggs into a deep earthen vessel that has been made quite hot; remove from the heat, whisk for a few385 minutes; make the sugar and water boil up, and pour it very gradually boiling-hot over the eggs; continue to whisk them briskly until they become thick and white; add the flour (quite warm), stir it lightly in, put the paste into tins lined with white paper, and bake them immediately in a moderately hot oven.
Cakes, Tea. Syn. Benton Cakes. Prep. From flour, 1 lb.; butter, 4 oz.; and milk, q. s.; bake on a hot hearth or slow oven plate.
2. To the last add 2 table-spoonfuls of yeast.
Cakes, Tip′sy. Prep. Small sponge cakes steeped in brandy, and then covered with grated almonds and candied peel; or almonds (cut into spikes) are stuck in them. They are commonly piled on a dish, surrounded with a custard, and covered with preserves drained as dry as possible.
Cakes, Wigg. Prep. From 1⁄2 pint of warm milk; 3⁄4 lb. of fine flour; and 2 or 3 spoonfuls of light yeast. Afterwards work in 4 oz. each of sugar and butter; make it into cakes, or wiggs, with as little flour as possible, add a few caraway seeds, and bake them quickly.
Cakes. (In medicine.) Cakes have been used as a form of administering medicinal substances to children, but have not been extensively employed in this country for the purpose, unless by quacks and in domestic practice. In preparing them the active ingredients are added in such proportions to the common materials of a sweet cake that one or two, as the case may be, are sufficient for a dose. See Gingerbread, Worm-cakes, &c.
CALA′BAR BEAN. Syn. Physostigmatis Faba. The seed of Physostigma venenosum. The plant is a native of Western Africa, where the bean is used as an ordeal poison. The bean itself is about the size of a large horse-bean, with a very firm, hard, brittle, shining coat of a brownish-red, pale chocolate, or ash-grey colour. It has an irregular kidney shape, with flat surfaces and a rounded border, which is for the most part boldly curved, and there marked with a broad furrow, with the central raised raphe in the centre, and ending at one extremity in the microphyle. The kernel consists of two cotyledons. It yields its properties to alcohol, and imperfectly to water. Calabar bean has been used in cases of strychnia poisoning and tetanus, as well as in epilepsy and St. Vitus’s dance. The dose of the powdered bean, according to Royle, is one to four grains. Locally applied it produces contraction of the pupil.
Until the researches of Harnack and Witkowsky the Calabar bean was supposed to owe its activity, when internally administered, to the presence of a powerful alkaloid called esernia or physostigma. These chemists, however, have lately succeeded in discovering in the bean, in addition to eserina, another very potent alkaloid, to which they have given the name calabaria or calabarine.
Calabarine appears to exert a physiological action antagonistic to that of eserine, and since the commercial preparations of the drug consist, according to the above chemists, of mixtures of the two alkaloids in varying proportions, the discordant effects frequently observed to follow the administration of any of the various preparations of the bean, admit of ready explanation. Wherever eserine predominated it appeared to suppress the effects of calabarine; on the other hand, if this latter preponderated, the paralysing effect on the spinal cord otherwise exercised by eserine would fail to be produced.
The necessity of having preparations of calabar free from calabarine, in cases where the drug is administered for tetanus, will be apparent when it is stated that calabarine itself induces the disease.
We quote the following from ‘New Remedies’ for June, 1877:—
“The well-known manufacturing chemist, E. Merk, in Darmstadt, has heretofore prepared and sold a substance which was supposed to be the only active principle of calabar, and which he called calabarine, but which was really eserine or physostigmine. He now accepts and confirms the results of Harnack’s and Witkowsky’s researches, and has put both of the active principles upon the market labelled with their correct name, viz. ‘Physostigmin’ (or eserine, being the same substance which he formerly sold as calabarine), and ‘Calabarin,’ distinguished by the addition of Harnack’s name (Harnack’s ‘Calabarine’). The attention of physicians and pharmacists is particularly directed to the change of appellations.”
Calabar bean is a powerful poison. The antidotes are:—Diffusible stimulants; the hypodermic injection of the 1⁄50th of a grain of sulphate of atropia, to be repeated if necessary at the end of two hours; and artificial respiration. See Eserine.
CAL′AMINE. See Zinc (Carbonate of).
CALCINA′TION. The operation of burning or roasting any solid body to expel its more volatile parts, as the conversion of chalk into lime by the expulsion of carbonic anhydride. The roasting of the ores in the first stage of the Welsh process of copper smelting and in the Silesian mode of extracting zinc is technically termed CALCINATION.
The method of conducting the process of calcination depends on the nature of the body operated on. Many substances, for delicate experiments, are calcined over a spirit lamp in a platinum spoon or crucible; others, in iron vessels or earthen crucibles, placed in a common furnace. When the action of the air proves injurious, as in the manufacture of charcoal, the process is performed in close vessels or chambers. In some cases the fuel is mixed with the articles, and they are both burnt together, as in the manufacture of lime, the roasting of ores, &c. The process of drying386 salts, or driving off their water of crystallisation by heat, is also frequently called CALCINATION; thus we have calcined copperas, alum, &c.
CAL′′CINER. A reverberatory furnace used for the calcination of metallic ores, particularly those of COPPER and ZINC (which see).
CAL′CIUM. [Eng., L.] Ca. The metal of which LIME is an oxide. Though it is a chemical curiosity when isolated, it is one of the most abundant substances in nature, forming a very large portion of the crust of the earth. It occurs in combination with fluorine as fluor-spar; with oxygen and carbonic acid as chalk, limestone, and marble; and with oxygen and sulphuric acid as gypsum. The metal was first obtained from lime by Sir H. Davy in 1808; but little was known of its properties until Dr Matthiessen formed it by the electrolytic decomposition of the chloride of calcium.
Prep. 1. By the action of a powerful voltaic current upon a paste of pure lime in contact with mercury, as in the original method of preparing barium.
2. By the electrolysis of chloride of calcium in a state of fusion.
3. (Caron.) Fused chloride of calcium in powder, 300 parts; distilled zinc, finely granulated, 400 parts; sodium, in small pieces, 100 parts; the whole placed in a crucible and heated to redness in an ordinary furnace. The action is very feeble at first, but after some time zinc flames arise. The heat must now be moderated to prevent the volatilisation of the zinc, but at the same time it must be maintained as high as possible. When the crucible has remained in this state for about a quarter of an hour it may be withdrawn. On cooling, a metallic button will be found at the bottom. This alloy of zinc and calcium, which generally contains from 10 to 15% of the latter metal, must be placed in a coke crucible and heated until the whole of the zinc is driven off. The alloy should be in pieces as large as possible. When proper precautions have been observed a button of CALCIUM is obtained, only contaminated with the foreign metals contained in the zinc.
Prop., &c. The metal belongs to the group which includes BARIUM, STRONTIUM, and MAGNESIUM; it is of a light yellow colour; is rather harder than lead, and very malleable. It melts at a red heat. It tarnishes in a day or two, even in dry air, and in contact with moist air it breaks up like ordinary lime. Its sp. gr. is 1·55.
Tests. Salts of calcium in solution produce a white precipitate with carbonate of ammonium; it becomes far less voluminous on heating the solution, and dissolves very readily in hydrochloric acid. Sulphuric acid, when added to concentrated solutions, gives an immediate white precipitate; if the solution is not concentrated, the precipitate may separate gradually, in minute crystals; and if it is very dilute, no precipitation will take place, because sulphate of lime is soluble in about 500 times its weight of water. With neutral solutions, even when very dilute, oxalate of ammonium gives a copious white precipitate, soluble in most dilute acids.
Calcium, Acetate of. Add prepared chalk to acetic (or purified pyroligneous) acid till fully saturated; filter and evaporate, that crystals may form. Diuretic. Dose, 10 to 20 grains.
Calcium, Acid Phosphate of. Syn. Superphosphate of lime, Soluble Acid Phosphate. CaH4,2PO4. This may be procured by treating bone-earth with two thirds of its weight of oil of vitriol, as in the preliminary stage of the extraction of phosphorus. It is extensively used as a manure for turnips.
Calcium, Bibasic Phosphate. Ca2H2P2O8 + 3H2O. Dissolve 608 grams of crystallised calcium chloride in 1000 grams of distilled water, and add gradually to this solution 1000 grams of sodium phosphate, dissolved in 10,000 grams of water. Allow the precipitate to deposit, and wash it five or six times with 10 litres of water each time; drain the precipitate on a moistened cloth. As soon as its consistence permits, detach from it irregular pieces, and place them to dry in the open air upon filtering paper; the spontaneous desiccation is sufficiently rapid.
From ‘Formulæ for New Medicaments adopted by the Paris Pharmaceutical Society.’
Calcium, Bro′mide of. CaBr2. Syn. Cal′cii bromi′dum, L. Prep. (Magendie.) To a solution of bromide of iron add hydrate of calcium in slight excess; filter, evaporate to dryness, redissolve in water, and again filter, and evaporate.
Calcium, Carbonate of. See Chalk.
Calcium, Chlo′′ride of. CaCl2. Syn. Cal′cii chlori′dum (B. P.). Prep. Hydrochloric acid and water, of each 10 fl. oz.; chalk, 5 oz.; evaporate the solution until the salt becomes solid, and dry the residue at about 400° F.
It is obtained in solution as a residuum in making several preparations of ammonia, as the liquor and carbonate, and in making carbonic acid by the action of hydrochloric acid on marble. The residuum is concentrated and set aside to crystallise, or evaporated to dryness.
Prop., Uses, &c. This salt crystallises in colourless, striated, hexagonal prisms, terminated by very acute points. It is very soluble in alcohol and water, the latter even at 32° dissolving more than its own weight, and at 60° three or four times its weight of this salt. When heated, the crystals undergo watery fusion. When dissolved in water, they produce great cold; and hence are frequently employed as an ingredient in FREEZING MIXTURES. These crystals contain nearly half their weight of water. They are very deliquescent, passing readily into the liquid state, and forming what used to be called oleum calcis, or oil of lime. The anhydrous chloride387 is hard and friable; slightly translucent; totally and readily soluble in water, and, like the crystallised salt, very deliquescent. In the laboratory chloride of calcium, either fused or merely dried, is continually used for drying gases and for absorbing the water from ethereal and oily liquids in organic analysis. The unfused is now generally preferred for this purpose, as it is more porous than the fused. The salt is also used in the rectification of alcohol, and to form a bath for heating stoneware stills and other apparatus liable to be cracked on the sand bath. As a chemical reagent it is employed chiefly in detecting certain organic acids. As a medicine it has been given in some scrofulous and glandular diseases. Dose, 10 to 20 gr. See Solutions.
Calcium, Flu′oride of. CaF2. Syn. Hydroflu′orate of lime. This occurs native as the mineral called fluor-spar. It is found in beautiful crystals in the lead mines of Alston Moor and Derbyshire, and in the concretionary crystalline masses known as Blue John or Derbyshire spar at Castleton. It may be prepared by the action of hydrofluoric acid upon lime, as directed under Barium, Fluoride of.
Calcium Hypophosphite. CaP2H4O4. Mix milk of lime (1 in 5) in porcelain capsule placed in a sand bath, with half its weight of phosphorus in small pieces, and heat it to ebullition, operating in the open air or under a chimney with a good draught. Spontaneously inflammable phosphuretted hydrogen is given off, the vapour of which should be avoided. Add from time to time a little warm water, to replace that which has evaporated. Discontinue the heat when the phosphorus has disappeared—that is, when inflammable bubbles cease to be produced. If the phosphorus remain in excess, add more milk of lime, and continue the heat until the complete disappearance of the metalloid. Allow the liquor to cool and then filter; then saturate it with a current of carbonic acid gas to eliminate any excess of lime remaining uncombined. Filter again, and concentrate the liquor in a water bath to dryness, keeping the temperature below 100° C., to avoid detonations. Preserve the salt from the air in well-closed bottles.
From ‘Formulæ for New Medicaments adopted by the Paris Pharmaceutical Society.’
Calcium, I′odide of. CaI2. Syn. Hydri′odate of lime; Cal′cii iodi′dum, Calcis hydrio′das, L. Prep. 1. (Magendie.) From a solution of protiodide of iron and hydrate of calcium, as directed under iodide of barium.
2. Dissolve lime or carbonate of lime in hydriodic acid.
Prop., Uses, &c. It is a deliquescent salt, easily soluble in water, and has a bitterish taste. It has been used in scrofulous affections, internally, in doses ranging from 1⁄8 to 2 gr., thrice daily, and externally in ointments containing 2 dr. or less to the oz.
Calcium, Lactophosphate. This product ought not to be employed except in the state of solution in water or in syrup. In the pasty or solid state its solubility varies, and it is always an indefinite compound.
Solution. Bibasic phosphate of lime, 17 grams; concentrated lactic acid, as little as possible; distilled water, 964 grams. Suspend the phosphate carefully in the distilled water, add the lactic acid, allow solution to go on for some minutes, and filter.
From ‘Formulæ for New Medicaments adopted by the Paris Pharmaceutical Society.’
Calcium, Oxide of. See Lime.
Calcium, Phosphate of. Syn. Calcis phosphas (Ph. B.). Digest bone-ash, 4 oz., in hydrochloric acid, 6 fl. oz., diluted with a pint of water, until it is dissolved.
Filter the solution, if necessary; add water, 1 pint, and afterwards solution of ammonia (Ph. B.), 12 fl. oz., or a sufficient quantity, until the mixture acquires an alkaline reaction, and having collected the precipitate on a calico filter, wash it with boiling distilled water as long as the liquid which passes through occasions a precipitate when dropped into solution of nitrate of silver acidulated with nitric acid. Dry the washed product at a temperature not exceeding 212° F.
Calcium, Phos′phide of. Syn. Phosphu′ret of lime; Cal′cii phosphure′tum, C. phosphi′dum, L. Prep. By passing the vapour of phosphorus over lime (in small fragments) heated to redness in a porcelain tube. A brownish substance, supposed to be a mere mechanical mixture of phosphide and phosphate of calcium. Thrown into water, it suffers instant decomposition, and phosphuretted hydrogen gas escapes.
Calcium, Sulphides of. Calcium forms with sulphur at least three different compounds:—
1. Calcium, Protosul′phide of. CaS. Prep.—a. From sulphate of lime, exposed at a high temperature to a stream of hydrogen gas.—b. From dried gypsum, 25 parts; lampblack or finely powdered charcoal, 4 parts; calcined together at a strong heat in a covered crucible.
2. Calcium, Bisulphide of. CaS2. Prep. From sulphur and quick-lime, equal parts; water, q. s.; slake the lime, add the sulphur, and boil until a solution is obtained, which on cooling deposits crystals.
3. Calcium, Pentasulphide of. CaS5. Prep. As the last, but increasing the quantity of sulphur, and continuing the boiling for a longer period. Little is known about it.
4. Calcium, Sulphate of. See Gypsum.
5. Calcium, Commercial Sulphuret of. Syn. Commercial sulphide of calcium. Prep.—a. As 1, b (above).
b. Sulphur, 1 part; hydrate of lime, 3 parts; water, 21⁄2 pints; boil it until it solidifies on cooling, then pour it out on a cold marble slab, and when solid break it into pieces and preserve it in a well-corked bottle.
c. (Guibourt.) Quick-lime, 7 parts; sulphur,388 4 parts; mix, and heat the compound for about 2 hours in a covered crucible.
d. (Cottereau.) Quick-lime, 2 parts; sulphur, 1 part; water, 5 parts; as 4, b (above).
Obs. The precise composition of the last three preparations is uncertain. They are acrid, caustic, stimulant, and diaphoretic. Dose, 1 to 3 gr. Sulphide of calcium has been used as a depilatory by applying it made into a paste with water, and washing it off in about 1⁄4 of an hour. Made into an embrocation, it has been strongly recommended in gout, scabies, &c. Its solution yields pure sulphur on the addition of hydrochloric acid.
CALCULA′TIONS (Useful). 1. To find the Value of a Dozen Articles. Take the price in pence as shillings, and if there are any farthings in the price, add threepence for each. Thus 2s. 8d., or 32 pence per yard, is £1 12s. per dozen.
2. To find the Value of One Hundred Articles. For every farthing take as many pence and twice as many shillings. Thus, 11⁄4d. each is—5d., and 10s. = 10s. 5d. per hundred.
3. To find the Value of a Pound at any price per Ounce. Take the price in farthings as shillings, and divide by three. Thus, 51⁄4d. per ounce is 21 farthings; taken as shillings, 21 ÷ 3 = 7s. per pound.
4. To find the Value of an Ounce at any price per Pound. Take the shillings as farthings, and multiply by three. Thus, at 6s.—6 × 3 = 18 farthings, or 41⁄2d. per ounce.
Obs. By reversing Nos. 1 and 2, the price of a single article or pound may be found from the price per dozen or hundred. For several other calculations, useful in domestic economy, chemistry, &c., see Brewing, Decimals, Equivalents, Measures, Per-centage, Weights.
CAL′CULUS. Syn. Stone. In medicine, a hard concretion formed within the animal body by the deposition of matters which usually remain in solution. The concretions most commonly found are those formed in the kidneys or bladder, and termed urinary calculi, and those formed in the gall-bladder or biliary ducts, which are called biliary calculi. Urinary calculi are in most cases composed of substances which are constituents of healthy urine, such as uric acid, urate of ammonia, and the phosphates of lime and magnesia; they are, however, sometimes composed of substances which are met with in unhealthy urine, such as oxalate of lime, cystine, &c.
Biliary calculi, or gall-stones, usually contain from 50 to 80 per cent. of cholesterin, a crystallisable fatty body, constituting a never failing ingredient in healthy bile, the rest of the concretion being made up of biliary resin and colouring matter, with a small quantity of inorganic salts.
Calculus or stone in the bladder, which is a prevalent disease in Norfolk, both among men and sheep, has been attributed to the use of the hard water of the district.
Both of these give rise to very painful symptoms, and may even threaten life. See Cholesterin.
CALEFACIENTS. Applications that excite warmth.
CAL′ENDAR. Syn. Calenda′rium, L.; Calen′drier, Fr. A table of all the days of the year, arranged in the order of days and weeks, to which are generally added certain astronomical indications and dates of great civil and religious events. The most remarkable calendars are the Hebrew calendar, the calendar of the Greeks, the Roman, or Julian calendar, the Gregorian calendar (now adopted by all Christian peoples except the Greeks and Russians), and the French Republican calendar, which, having remained in force about thirteen years, was abolished by Napoleon I on the 1st of January, 1806.
Calendar, Perpet′ual. A table which furnishes the general indications necessary to construct a calendar for any year, and to resolve, without error, many difficulties connected with the verification of dates.
CAL′ENDERING. The process of finishing by pressure the surface of linen or cotton goods. It is usually performed by passing the fabric between cylinders pressed together with great force. It is necessary that one of the cylinders, at least, shall be of a material combining considerable hardness with a slight degree of elasticity; for this purpose the paper cylinder is used. It is made by forcibly compressing a number of circular discs of thick pasteboard, each with a square hole in the centre, upon an iron axis, so as to form a solid cylinder, which is turned perfectly smooth and true in a lathe. The paper cylinder usually works against a hollow roller of copper or iron, heated by steam or metallic heaters. Before the final rolling in the calendering machine the fabric is lightly smoothed by passing over warm cylinders. Cotton goods are starched, and a fictitious appearance of stoutness is sometimes given to them by employing starch thickened with plaster of Paris, porcelain clay, or a mixture of these. Watering is a beautiful effect, produced by means of a hot cylinder with a pattern raised upon it. Glazing is produced by combined rubbing and pressure, the rollers being made to move with unequal velocities, so that one side of the fabric is rubbed as well as pressed by the roller whose surface moves with the greater speed. A copper cylinder is preferred for glazing, and is made so hot that if the machine stops it burns the goods. The old method of glazing consisted in burnishing the surface of the fabric with a polished flint.
CAL′ICO. See Cotton.
Cal′ico Printing. The art of producing figured patterns upon calico by means of dyes and mordants topically applied by wooden blocks, copper plates, or engraved cylinders. The goods are either directly printed in colour, or receive their patterns by being run through389 a colouring matter or mordant, when the dye is only produced upon that portion of the ground previously prepared for it. Of late this system of dyeing has been extended to silk and woollens.
The mordants are thickened with some glutinous substance, as flour, starch, or gum, to render them adhesive and to prevent their spreading.
The following are the principal styles of calico-printing, each requiring a different method of manipulation:—
In the madder, fast colour, or chintz style, the mordants are applied to the white cloth, and the colours are brought out in the dye bath. This is the method commonly followed for “permanent prints.”
In the padding or plaquage style, the whole cloth is passed through a bath of some particular mordant, and different mordants are afterwards printed on it before submitting it to the dye bath. By this means the colour of the ground and pattern is varied. Like the last, it is much used for gown pieces, &c.
In the reserve or resist-paste style, white or coloured figures are produced by covering those parts with a composition which resists the general dye afterwards applied to form the ground of the pattern. In this style the dye bath is indigo, or some other substantive colour.
The discharge, or rongeant style, is the reverse of the preceding; it exhibits bright figures on a dark ground, which are produced by printing with acidulous or discharge mordants after the cloth has been passed through the colouring bath.
Steam-colour printing consists in printing the calico with a mixture of dye extracts and mordants, and afterwards exposing it to the action of steam.
Spirit-colour printing is a method by which brilliant colours are produced by a mixture of dye extracts and solution of tin, called by the dyers “spirits of tin.”
Pigment printing consists in applying such colours as ultramarine, magenta, or aniline purple, to the cloth, and fixing them by such agents as casein, albumen, or solution of india rubber. This style of printing has been developed to a great extent since the introduction of the splendid mauves and purples obtained from aniline.
For further information on this subject the reader is referred to Ure’s ‘Dictionary of Arts, Manufactures, and Mines,’ Calvert’s ‘Dyeing and Calico Printing,’ edited by Stenhouse and Groves; Wagner’s ‘Clinical Technology,’ and Crooke’s ‘Practical Handbook of Dyeing and Printing,’ where he will find the several processes of calico printing fully treated on, and most ably and accurately described. To enter largely into the subject in this work might amuse the reader, but would be of no practical value; as calico printing is an art only practised on the large scale, and by men who obtain their whole knowledge of it in the laboratories and printing rooms of the factories.
CAL′OMEL. See Mercury (Chlorides of).
CALOTRO′PIS PROCE′RA., CALOTRO′PIS GIGAN′TEA., (Ind. Ph.). Syn. Mudar.—Habitat. One or other of these species, everywhere in India.—Officinal part. The root-bark, dried (calotropis cortex). Small flat or arched pieces, brownish externally, yellow-greyish internally, peculiar smell, and mucilaginous, nauseous, acrid taste. Its activity appears to reside in a peculiar extractive matter named mudarine.—Properties. Alterative tonic; diaphoretic, and, in large doses, emetic.—Therapeutic uses. In leprosy, constitutional syphilis, mercurial cachexia, syphilitic and idiopathic ulcerations, in dysentery, diarrhœa, and chronic rheumatism, it has been used with alleged benefit.
Powder of Mudar. (Pulvis Calotropis.) Take of the roots of mudar, collected in the months of April and May from sandy soils, a sufficiency; carefully remove, by washing, all particles of sand and dirt, and dry in the open air, without exposure to the sun, until the milky juice contained in it becomes so far inspissated that it ceases to flow on incisions being made in it. The bark is then to be carefully removed, dried, and reduced to powder. Preserve in well-corked bottles.—Dose. As an alterative tonic, 3 grains, gradually increased to 10 grains or more, thrice daily. As an emetic, from 1⁄2 to 1 drachm.
CAL′OTYPE. See Photography.
CALUM′BA. Syn. Calumbæ Radix, B. P. Calum′ba-root; Kalumb, Hind. The root of a plant of Eastern Africa, extensively used in medicine as a stomachic and mild tonic. Dose, 10 to 20 grains, three or four times a day. The botanical name of this plant is Jateorhiza palmata, or Cocculus palmatus. See Calumbine (below); also Infusions and Tinctures.
CALUM′BA WOOD. This wood, which is used as a tonic by the Cingalese, is not the produce of the true calumba plant, but of Menispermum fenestratum. It contains the alkaloid BERBERINE (which see).
CALUM′BINE. Syn. Calom′bine, Calum′bina. A bitter substance discovered by Wittstock in calumba root.
Prep. 1. Digest calumba root (in coarse powder) in water acidulated with acetic acid; express, filter, boil to one half, again filter, add carbonate of calcium, in slight excess, and evaporate to dryness in a water bath; reduce the residuum to powder, and digest it in boiling alcohol; the latter will deposit crystals of CALUMBINE on cooling.
2. (Wittstock.) Evaporate tincture of calumba root (made with rectified spirit) to dryness; dissolve the residuum in water, and agitate the solution with an equal bulk of ether; after repose for a short time, decant390 the ethereal portion, distil off most of the ether, and set the liquid aside to crystallise.
Prop., &c. Impure calumbine occurs as a yellow-brown mass; when pure, it forms rhombic prismatic crystals or delicate white needles; it is only slightly soluble in alcohol, ether, and water; 40 parts of boiling rectified spirit take up only 1 part of calumbine. Its best solvent is acetic acid; it is also soluble in acidulated and alkalised water. Neither nut-galls nor metallic salts affect its solution. Concentrated sulphuric acid dissolves it, and assumes first a yellow, and then a red colour. Its properties indicate that weak vinegar or sour wine would be the best menstruum for extracting the medicinal virtues of calumba root. Dose, 1 to 3 gr. twice a day as a tonic and stomachic, in dyspepsia, debilitated stomach, bilious vomiting, &c.; and in the later periods of dysentery and diarrhœa.
CALX. This term was formerly applied to the residuum of the combustion of any substance; or to any substance which had been exposed to a strong heat. See Calcination, Lime, &c.
CAMBOGE′. See Gamboge.
CAM′ERA LU′CIDA. [L. and Eng.] When a ray of light (r) falls upon a quadrangular glass prism (a), it is bent by two reflections (at c and d), and thrown upwards where it may be received by the eye, to which it will appear described on the table or sheet of paper (f) placed to receive it. The point of a pencil used to trace any object on the paper can also be seen, and by its means the picture can be easily copied. When the prism is mounted on a stand, and a thin brass plate with a small hole through it for the eyepiece adjusted thereto, it forms the CAMERA LUCIDA of the opticians. The image may be magnified or lessened by placing a lens so as either to intercept the rays before they strike the prism, or before they reach the eye. An ingenious person will readily be able to set up this instrument, than which a more useful one cannot exist.
CAM′ERA OBSCU′′RA. [L. and Eng.] An optical instrument for producing upon a screen the image of a field of view more or less extensive. It was invented by Baptista Porta in the 16th century. The principles and construction of the camera obscura may be thus described:—A convex lens (B) is placed in a hole admitting the light into a darkened box or chamber (A), which, falling on a white ground (D), produces an inverted picture of every object within its range. The image thus formed may be restored to its natural position, by allowing the rays of light to pass through two lenses instead of one, or by receiving the rays on a mirror placed at an angle of 45°, when the image will be thrown on the floor in its original position. The picture may be viewed through an oblong aperture cut in the box, or the experiment may be performed in a darkened room, by placing the lens in a hole in the shutter, and allowing the image to fall on the wall, or on a sheet of white paper stretched to receive it.
In the simplest form, when intended for taking views or portraits, the image is thrown upon a mirror placed at an angle of 45°, and resting on the bottom of the box, by which means it is thrown upwards against a plate of glass, also placed at a similar angle. On this is laid a piece of semi-transparent tracing paper, on which the object is distinctly seen painted, and may be traced out with a pencil. When the camera is used in photography, slides are provided to retain the sensitive paper in the proper position in the box or dark chamber to receive the image, and the whole apparatus is adjusted with screws, and slides of the most delicate description. Achromatic glasses are also employed. See Photography.
CAM′PHINE. The name given by the trade to rectified oil of turpentine when sold for burning in lamps, in order that purchasers may not be aware of the inflammable character of the liquid. Since the introduction of the hydro-carbon oils from coal, shale, and petroleum, camphine has been little used for burning. To rectify the turpentine, it is passed in vapour through a solution of caustic potash, soda, or lime; or through sulphuric acid.
CAM′PHOR. C10H16O. Syn. Cam′phire, Lau′rel Cam′phor; Campho′ra, B. P. A crystalline substance found in many plants; though only obtained in large quantities from two, namely, Camphora officinarum and Dryobalanops aromatica. The first, commonly known as the laurel camphor tree of China and Japan, yields the camphor of commerce; the latter, the Sumatra or Borneo camphor, and the peculiar fluid known as liquid camphor.
It is found that several of the essential oils, by carefully distilling off about one third their volume, yield a species of camphor. By collecting this, and redistilling the remainder of391 the oil 2 or 3 times, a farther quantity of camphor may be obtained. Oil of rosemary, treated in this way, yields about 10% of camphor; oil of sweet marjoram the same; oil of sage yields 13%; oil of lavender, 25%. By keeping the oils loosely corked, and in a cool place, they produce a larger portion of this camphor. Aniseed camphor is the congealable portion of oil of aniseed, separated from the liquid oil, which it resembles in odour and flavour.
Camphor, Am′ber. See Pyretine (Crystallised).
Camphor, Com′mercial (Crude). The produce of the laurel camphor tree, brought to Europe chiefly from China and the island of Formosa, in the form of greyish grains, aggregated into crumbling cakes.—Prep. The Chinese and Japanese extract the camphor by cutting the wood into small pieces, and boiling it with water in iron vessels, which are covered with large earthen capitals or domes, lined with rice straw. As the water boils, the camphor is volatilised along with the steam, and condenses on the straw.
Cam′phor, Commercial (Refined). Syn. White camphor; Campho′ra, B. P. Prep. 100 parts of crude camphor are mixed with 2 parts each of quick-lime and animal charcoal, both in powder, and the mixture is placed in a thin, globular, glass vessel, sunk in a sand bath. The heat is then cautiously applied, and the vessel gradually and carefully raised out of the sand as the sublimation goes on. When the process is complete, the subliming vessel is removed and allowed to cool.
Obs. The whole process of refining camphor requires great care and experience to ensure its success. If conducted too slowly, or at a heat under 375° Fahr., the product is found to be flaky, and consequently unsaleable, without remelting or subliming. An improvement on the common method is simply to sublime the above mixture in any convenient vessel furnished with a large and well-cooled receiver, and to remelt the product in close vessels under pressure, and to cool the liquid mass as rapidly as possible.
Prop., &c. A white, semi-crystalline solid, very volatile at common temperatures; freely soluble in alcohol, ether, bisulphuret of carbon, benzol, oils, and acetic acid, and sufficiently so in water (about 11⁄4 gr. to 1 oz.), to impart its characteristic smell and taste; 100 parts of alcohol (sp. gr. ·806) dissolve 120 parts of camphor; concentrated acetic acid dissolves twice its weight of camphor; average sp. gr. ·990. It fuses at 347°, boils at 400° Fahr., and when set fire to, burns with a bright flame. It evaporates slowly at ordinary temperatures, and crystallises on the inside of bottles. While floating on water it undergoes a curious rotatory movement.
Uses, &c. Camphor is sedative, narcotic, anodyne, diaphoretic, and anaphrodisiac. Dose, 2 to 10 gr. in the form of pill or bolus, or made into an emulsion with yolk of egg, mucilage, or almonds. In overdoses it is poisonous. The best antidote is opium or wine, preceded by an emetic. It is also used externally in ointments, liniments, and embrocations.
Camphor is frequently put into wardrobes and clothes-trunks, to keep away insects; it is used to make the white stars and fire of the pyrotechnist; and by the varnish-maker to increase the solubility of copal and other gums. Mixed with six times its weight of clay, and distilled, it suffers decomposition, and yields a yellow, aromatic, volatile oil, smelling strongly of thyme and rosemary, which is much used by the wholesale druggists and perfumers to adulterate some of the more costly essential oils, and by the fancy soap-makers to scent their soaps.
Camphor may be beaten in a mortar for some time, without being reduced to powder, but if it be first broken with the pestle, and then sprinkled with a few drops of rectified spirit of wine, it may be readily pulverised. By adding water to an alcoholic or ethereal solution of camphor, this drug is precipitated under the form of an impalpable powder of exquisite whiteness.
Tests. Pure camphor is entirely soluble in rectified spirit, oils, and strong acetic acid; a fragment placed on a heated spoon or in a warm situation will wholly disappear, and the evolved fumes will be highly fragrant (camphoraceous), and be free from an acid or terebinthinate odour. In an alcoholic solution of natural camphor ammonia gives but a slight precipitate, which is dissolved on shaking the mixture; a similar solution of artificial camphor under the like treatment gives a flocculent precipitate, which remains undissolved. See Camphor, Factitious (below).
Camphor, Facti′′tious. Syn. Hydrochlorate of tur′pentine, Hydrochlorate of camphene, Artificial camphor. Prepared by passing dry hydrochloric acid gas into pure oil of turpentine, cooled by a freezing mixture or pounded ice. After a time a white, crystalline mass is formed, which must be drained, and dried by pressure between folds of bibulous paper. It may be purified by solution in alcohol.
Prop., &c. It has a camphoraceous taste and odour; burns with a greenish, sooty flame, and when blown out evolves a terebinthinate odour; heated a little above the boiling-point of water, slight fumes of hydrochloric acid gas are perceptible.
Camphor, Hydrochlo′′rate of. Syn. Mu′′riate of camphor; Campho′ræ hydrochlo′′ras, L. By passing hydrochloric acid gas over camphor, in small fragments, until it ceases to be absorbed.
Camphor, Liq′uid. Syn. Camphor oil; O′leum campho′ræ, L. A pale yellowish, limpid fluid, which exudes from Dryobalanops aromatica, a tree growing in Sumatra and Borneo, when deep incisions are made in the392 trunk. It is supposed that the crystalline Sumatra camphor (see below) is deposited from this fluid. The liquid camphor has somewhat the odour of CAJEPUT OIL, and might, no doubt, be beneficially employed for the same purpose. It is sometimes imported into Europe.
Camphor, Monobromated. C10H15O1Br. Coarsely powdered camphor is introduced into a flask of about ten times the capacity of the amount it is intended to prepare. A fine stream of bromine is then allowed to fall upon the powder with continual agitation; the addition of bromine ceases when the camphor is liquefied. A large long abductor tube is then fitted to the flask, and the other end plunged into an alkaline solution, which will absorb the vapour that would otherwise incommode the operator. The flask is placed in a water bath that is raised to ebullition, when the reaction soon commences. This is at first rather active, there being an abundant evolution of hydrobromic gas, and some vapour of bromine and undecomposed camphor. The liquid, which is at first dark brown in colour, acquires an amber colour and the evolution of gas suddenly slackens. The operation should be carried out at a temperature between 80° and 90° C. The amber-coloured liquid that remains in the flask solidifies upon cooling, and appears then as a slightly citrine-coloured friable mass. It is purified by treating it several times with boiling 90° to 95° alcohol, filtering the liquor, and leaving it to crystallise. The crystals are to be dried in the air upon unsized paper.
Dr Bourneville advises monobromated camphor to be administered either in the form of pills, made up with conserve of roses, or of a mixture rubbed up with mucilage of gum arabic and syrup. He gives it in doses varying from twelve to thirty centigrams daily. Where it cannot be taken by the mouth he injects the following solution subcutaneously:—Monobromated camphor 3 gr., alcohol 35 gr., glycerin 22 gr.
Camphor, Nitrate of. Syn. Camphor oil; O′leum campho′ræ facti′′tium, L. Prepared by dissolving camphor in nitric acid, in the cold.
Camphor, Sul′phite of. From camphor and sulphurous acid gas, as hydrochlorate of camphor.
Camphor, Suma′tra. Syn. Bor′neo camphor, Hard c., Dragon’s brain perfume. Obtained from Dryobalanops aromatica, being found in natural fissures or crevices of the wood. It resembles ordinary camphor in most properties, but its odour is not of so diffusible a nature. This kind is not seen in European commerce.
CAMPHOR CAKES. See Balls (Camphor).
CAMPHOR′IC ACID. H2C10H14O4. Syn. Acidum camphor′icum, L. Prep. From camphor, 1 part; and nitric acid (sp. gr. 1·33), 4 parts; distilled together in a glass retort, with a gradually increasing heat, until vapours cease to be evolved; the camphor that has volatilised is then added to that in the retort, along with 4 or 5 parts more of nitric acid, and the process repeated again and again, until 20 parts of acid have been consumed, when crude camphoric acid crystallises out of the remaining liquor on cooling. The crystals are purified by washing with cold distilled water, solution in boiling water, and evaporating the solution until a pellicle forms; crystals of pure camphoric acid are formed as the liquid cools.
Prop., &c. Small, colourless, lamellar or acicular crystals; acid; bitter; fusible at 158° Fahr.; sparingly soluble in water; soluble in alcohol; alcoholic solution not precipitated by water, which distinguishes camphoric acid from benzoic acid. Its salts are called CAMPHORATES. The soluble camphorates may be made by digesting the carbonate or hydrate of the metal in a hot solution of the acid, and the insoluble camphorates by double decomposition. By distillation, camphoric acid yields a colourless, crystalline, neutral substance, which has been improperly called anhydrous camphoric acid.
CAM′WOOD. This dye-stuff resembles Brazil wood in its properties, and is used in a similar manner.
CAN′ADA BALSAM. Syn. Bal′samum Canaden′se, Terebinth′ina Canaden′sis, L. A thick, viscid oleo-resin obtained from the Abies balsamea (Lindley), a tree of common growth in Canada and the State of Maine. It is much employed as a medium for mounting microscopic objects. When pure it is perfectly transparent, has an agreeable odour (not terebinthinate), and is wholly soluble in rectified oil of turpentine, with which it forms a beautiful glassy and colourless varnish, much used for preparing a semi-transparent copying paper.
A mixture of 3 parts of Canada balsam and one of wax, if added to pile masses, is said to have the effect of binding together the component parts of the mass, and of keeping the piles made from it soft and in good shape.
Canada Balsam, Facti′′tious. Syn. Balsamum Canadense Facti′′tium, L. Prep. 1. Yellow resin, 3 lbs.; oil of turpentine, 1 gall.; dissolve, and add essence of lemon, 2 dr.; oil of rosemary, 11⁄4 dr.
2. To the last add of nut oil, 1 pint. Both are sold in the shops for Canada balsam.
CAN′DIES. See Candying.
CAN′DLES. Candle-making, once a rude and noisome trade, has, since the researches of Chevreul and Branconnot into the nature of the fats, developed into one of the most important branches of scientific industry, the progressive improvements in which, accompanied by a corresponding cheapening and immensely increased efficiency in one of our chief means of artificial illumination, have added greatly to the comfort and enjoyment of393 every civilised community. Candles are either dipped, moulded, or rolled. The cheaper sorts of tallow candles are formed by the first process, and wax candles by the last; all the other kinds are moulded. The moulds are tubes of pewter, well polished on the inside, eight or more being fitted into a frame, the upper part of which forms a trough to receive the melted candle material. When in the moulds the candles are inverted; in other words, the bottom of each mould corresponds to the top of the candle. The wick passes through a small hole at the lower extremity of the tube, and is held in the axis by a little bar placed across the top. At the factories of Price’s Patent Candle Company the frames of moulds are ranged close together in long benches, and are filled with hot candle material from cars running along little railways above them. When quite cold the candles are withdrawn. The plan of pulling them out one by one with the aid of a bodkin has been superseded at the factories above mentioned, by the ingenious device of blowing them out with compressed air.
The wicks of ordinary tallow candles are made of the rovings of Turkey skein-cotton, 4 or more of which, according to the intended thickness of the wick, are wound on a reel, from which they are again run off, and cut into the proper lengths. Of late years the wicks of the best candles have been made in such a way that they do not require snuffing. This object is effected by causing the wick to bend over, and its end to fall outside the flame, where it is exposed to the oxygen of the air. This bending over is variously brought about.—1. By twisting the wick with one strand shorter than the rest, which, being slightly stretched during the moulding of the candle, contracts again and bends the wick when the fat melts. 2. By plaiting the cotton into a flat wick, which naturally takes the required curve. Such a wick is generally dipped in a solution of borax, which preserves it from being acted upon by the flame except at its extreme point at the edge of the flame. A very fine wire is sometimes included in the plaited wick. 3. In Palmer’s patent two-wicked candles, which were formerly much used in lamps, the wicks are saturated with subnitrate of bismuth ground up with oil; they are then twisted tightly round a wire, which is withdrawn after the candle is moulded. In burning, the ends gradually untwist and stand out of the flame on either side. Other devices are said to be employed.
The wicks of candles should be free from knots and inequalities, as well as from adhering particles of cotton, the presence of all of which are the cause of the “guttering” one frequently sees in a burning candle. The finer the thread of which the wick is composed the more complete will be the combustion of the melted fatty material. Unless the above precautions are attended to, in selecting the wick, it will not be so entirely consumed as it ought to be.
Candles, Com′posite. Mould candles formed of a mixture of the hard fatty acid obtained from palm oil and the stearine of cocoa-nut oil. They were introduced in 1840. Other compositions are occasionally used, such as a mixture of spermaceti and hard white tallow, to which a little bleached resin is added.
Candles, Med′icated. These have been proposed as a convenient means of diffusing the active principles of certain volatile substances through the atmosphere, and for complete and partial fumigations. They are seldom employed in England.
Candles, Mercu′′rial. From the red sulphide or the grey oxide of mercury mixed with wax, and a wick of cotton inserted therein. Recommended by Mr Colles for partial mercurial fumigation. They are burnt under a glass funnel with a curved neck, the upper orifice of which is directed to the diseased part.
Candles, Par′affin. From the beautiful translucent substance paraffin (which see). These candles surpass all others in elegance, and are entirely free from odour and greasiness. The light produced by 98 lbs. of paraffin candles is equal to that of 120 lbs. of spermaceti, or 138 lbs. of wax, or 144 lbs. of stearic, or 155 lbs. of the best composite candles (Letheby). They are sometimes delicately tinted with red, mauve, violet, crimson, and rose colour. Aniline colours will not dissolve in paraffin. Stearic acid, however, is a solvent for them, and accordingly when the candles are tinted with the coal-tar colours these are previously dissolved in the stearic acid, always mixed with the paraffin. This insolubility of the aniline colours in paraffin has been suggested as a test for the purity of this hydrocarbon, and of its freedom from stearic or other fatty acids. For colouring paraffin candles black the paraffin is heated nearly to the boiling point with anacardium shells or nuts, which dissolve readily in the heated paraffin. The Belmontine Candles of Price’s Patent Candle Company are formed of the paraffin of Rangoon tar.
Previous to the paraffin being made into candles, it is necessary that it should be purified and bleached. Many processes for effecting these ends have been devised. In the works of Price’s Candle Company the method known as “Hodge’s” is had recourse to. This consists in first freeing the crude paraffin from the coarser impurities, melting it, casting it into cakes, and allowing it to cool sufficiently slowly, so as to form well-defined crystals. The cakes are then placed upon a bed of some porous and absorbent material, and subjected to a temperature not sufficient to melt the paraffin, but only the liquid hydrocarbons and other more easily fused bodies, the latter running off from between the crystals of the paraffin, and being absorbed by the porous substance upon which the394 paraffin rests. This process is repeated until the removal of the liquid hydrocarbons from the solid paraffin has been satisfactorily accomplished. If it be requisite to subject the paraffin to further purification, the following method is frequently adopted. The paraffin, previously melted by steam, is placed in a tank, with from 5 to 10 per cent. of strong sulphuric acid, and the mixture agitated for some hours by means of air (the time depending on the quality of the paraffin), the sulphurous acid fumes resulting from the reaction being carried off by a suitable contrivance. After the agitation is completed, the paraffin, after being allowed to stand for some time, is decanted into a suitable vessel containing animal charcoal, with which it is digested for some hours. Upon the subsidence of the charcoal the paraffin is drawn off if at all turbid, and is passed through a funnel heated by means of a steam jacket.
Another method, the invention of Messrs Fordred, Lambe, & Sterry, for the decolorisation of the paraffin employed in candle manufacture, consists in digesting the paraffin at a temperature of 230° F. with about 12% of powdered fuller’s earth. Of late this process has supplanted the charcoal one; and it may be employed, no matter by what means the previous purification of the paraffin has been carried out. The paraffin and fuller’s earth are to be well agitated together, and when the latter has fallen down the clear paraffin is decanted from it. The inventors affirm that their process answers quite as well if marl clay, or any other similarly constituted and equally abundant natural substance be substituted for fuller’s earth; and that no matter which of these bodies is employed, they may be re-used, and any adhering paraffin be removed by washing with agitation, or by other suitable contrivances.
Messrs Smith & Field’s patent for the removal of the colouring matters of the paraffin consists in the employment of silicite of magnesium. The patentees state that the successful issue of the operation depends not only upon the careful preparation of the salt used, but upon its being dried at a temperature of as exactly as possible 212° F. The careful preparation before insisted on of the magnesium salt, which is procured by the double decomposition of magnesium, sulphate, and sodium silicate, includes its thorough washing from adhering sodium sulphate previous to its desiccation. If this precaution be neglected, the porosity of the silicate will be impaired, and its bleaching effect more or less interfered with; and further, the patentees state that if the washed silicate be heated to redness, its decolourising power will also be lost.
It appears that the paraffin employed in making the candles consists of a mixture of paraffins having different melting points. The following are the melting points of some of the chief varieties of paraffin:—
| Paraffin | from | Boghead coal | at | 45° to 52° C. |
| ” | ” | Brown coal | ” | 56° C. |
| ” | ” | Peat | ” | 46·7° C. |
| ” | ” | Rangoon oil or tar | ” | 61° C. |
| ” | ” | Ozokerit | ” | 65·5° C. |
Paraffin candles contain from 5{?} to 15 per cent. of stearin, this addition being made for the purpose of diluting the paraffin as well as for raising the melting point of the paraffin where this is low. The stearin, moreover, serves to preserve the rigidity of the candle in the candlestick, and to prevent its bending out of the upright position. Paraffin candles are always moulded, but previous to this being done the moulds must be heated to a temperature above the melting point of the paraffin; this may vary from 60°, 70°, and 87° C., according to the paraffin employed. The moulds having been filled with the melted paraffin are, after one or two moments only, plunged into cold water, when the candle immediately becomes solid. Unless this were done the candle would be spoilt, owing to the crystallisation of the paraffin. A thin wick is required for paraffin candles.
Candles, Spermace′ti. From spermaceti (which see). These are very delicate in appearance, but rather expensive. They burn well, but as the melting point of spermaceti is low, 120° Fahr., they will not bear carrying about in the hand without guttering. They are generally adulterated with stearic acid or hard white tallow.
In candle-making “spermaceti is usually mixed with 3 per cent. of wax or paraffin to destroy its highly crystalline structure; it is moulded in the usual way with plaited wicks that require no snuffing. Occasionally the spermaceti candles are cast without any admixture of wax, the moulds being raised to a higher temperature just as with stearic acid. Some manufacturers, in order to make the spermaceti appear like wax, use gamboge to give the desired tint; such candles are known as transparent wax.”[236] Spermaceti candles are largely consumed in India.
Candles, Stear′ic. Under this head we may place the various sorts of candles moulded from the hard fatty acids of both animal and vegetable origin. The principal sources whence British manufacturers derive their acids are tallow, palm oil, and cocoa-nut oil. The processes employed for separating them are generally described under Stearic Acid. Candles formed of the fatty acids can now be prepared so as to imitate and almost rival those of wax and spermaceti; and they are quite as cheap as the nearly obsolete mould candles formed of common tallow. They are extremely hard; they do not grease the hands, and they burn away brightly and steadily, without giving off any offensive odour. Uncoloured, they are snowy white, but a yellow tint is frequently given them by gamboge.395
Candles, Tal′low. From ordinary tallow or from tallow which has been freed from much of its oleic acid by pressure. These have so unpleasant an odour and are so apt to gutter, that they will probably ultimately disappear from use. They are, however, sold at so low a price, that among the lower classes they must long retain their hold. For dip candles the wicks are immersed in melted tallow, and after rubbing with the hands are placed straight and allowed to harden, after which they are arranged upon the “broaches” ready for dipping. For mould candles the last operation is omitted. Great care is taken to select a cotton that yields the least possible quantity of ash after burning.
In the process of “dipping,” the “dipping cistern” being filled with tallow of a proper temperature from the boiler, one of the broaches covered with wicks is placed upon the end of the “dipping beam,” and pressed down gently into the melted fat; it is then withdrawn, the bottoms of the candles just touched against a board placed on one side of the cistern for the purpose, and the frame removed to the rack. This operation is repeated until the candles acquire a sufficient size, when they are finally cooled, sorted, weighed, and strung in pounds for sale.
The mould candles once in common use were made of the finer kinds of tallow only; a mixture of 3 parts of sheep, with 1 part of ox suet, being preferred. See Wax.
Candles, Wax. These are most frequently made by pouring melted white wax on to the wicks, which are hung upon frames and covered with metal tags at the ends to protect the cotton from the wax in those parts. The frames are made to turn round, and melted white wax is poured first down one wick, and then the next, and so on. When the wicks have been subjected to this operation once and have become sufficiently cooled, they have a second, and then a third coat given them, until they are of the required thickness. The candles are next rolled into proper shape on a marble slab or wooden board. The conical top is moulded by properly-shaped tubes, and the bottoms are cut off and trimmed. Wax candles are now seldom moulded, but if so the same processes are followed as for stearic and paraffin candles. The large altar candles, which frequently weigh from thirty to forty pounds, are made by hand.
Wax Tapers. These, which are of various degrees of thickness, are not made of pure wax, but of wax (usually vegetable wax) and tallow, the latter being added to give them flexibility. When they are required to be coloured, resin and turpentine are added to the tallow. For further particulars, consult Wagner’s ‘Chemical Technology,’ “Candle-making.”
CANDLE NUTS. The kernels of the alearites triloba, the candleberry tree, a plant growing in most tropical countries. The nuts when dried, and stuck upon a reed, are used by the natives of the Polynesian Islands as a substitute for candles. They contain a large amount of pure palatable oil, which is sometimes used by artists as a drying oil. After the expression of this oil the cake has been used as a food for cattle; also as a manure.
The following is the composition of the nuts:—
| Shells. | |
| Water | 3·71 |
| Organic matter | 89·90 |
| Mineral matter | 6·39 |
| Kernels. | |
| Water | 5·27 |
| Fat | 62·97 |
| Cellulose | 28·99 |
| Mineral matter | 2·79 |
| Ash of Kernel. | |
| Lime | 18·69 |
| Magnesia | 6·01 |
| Potash | 11·33 |
| Phosphoric acid | 29·30 |
CAN′DLESTICKS. Metallic, earthenware, and porcelain candlesticks, snuffers, and snuffer-stands, are recommended to be cleaned by pouring boiling hot water on them (previously placed in an earthen pan), and, after wiping them quite dry with a cloth, to clean them with a piece of wash leather; those made of silver, or of plated copper, may be finally polished with a little plate powder; those of white metal, with a little whiting or fine chalk, and those of brass, with a little rotten-stone or one of the polishing pastes. For articles of this kind, made of bronze and papier maché, the water should be used only hot enough to melt the tallow, and they should be only gently dabbed or rubbed off with a very soft cloth or leather. The common practice of placing candlesticks before the fire to melt off the grease is injudicious, as the solder or japan about them is almost certain to be injured. Hence the common annoyance of damaged or “crippled” candlesticks in houses where there are careless servants.
CAN′DYING. When the object is simply to form a confection or sweetmeat, imbued with the aroma, flavour, or medicinal property of any substance, candies are generally prepared by simply boiling lump sugar with a sufficient quantity of the infusion, decoction, tincture, expressed juice, or sometimes even the powder of the particular article, until a portion taken out and cooled becomes quite solid, when it is either poured out on a marble slab, or into tin, marble, or paper moulds, dusted with powdered lump sugar.
When the object is to preserve the form and character of the vegetable in the candy, the substance is boiled in water until soft, and then suspended in concentrated syrup (in the cold), until they become transparent; after which they are either dried in a current of warm air, or in a stove, at a heat not exceeding 120° Fahr. The syrup must be kept396 fully saturated with sugar by reboiling it once or twice during the process.
Another method occasionally employed by confectioners for almonds and the like is to put the substances into a syrup boiled until it forms a small thread between the opening fingers, and to stir the whole until it is nearly set. See Sugar Boiling.
The following are the principal candied articles kept at the shops:—
Candied Al′monds. From blanched almonds, roasted and halved.
Candied Angel′ica. Prep. 1. From the root. Boil the fresh roots (after slicing them and removing the pith) in water, to deprive them of part of their bitterness and aroma; then drain them and put them into syrup boiled to a full candy height, and boiling hot; let them remain until nearly cold, when they may be taken out and carefully dried.
2. From the stems. From the tender stems, stalks, and midribs of the leaves, as last. Used as a sweetmeat and dessert. It is said to be cordial, stomachic, tonic, and aphrodisiac.
Candied A′pricots. From the fruit, scarcely ripe, either whole or cut into quarters, immersed in the syrup (hot), without any further preparation.
Candied Cit′rons. From the peels.
Candied Erin′go. From the roots, slit and washed.
Candied Gin′ger. From the roots of green ginger.
Candied Hore′hound. From a strong decoction or infusion of the root, and lump sugar, 1 pint to 8 or 10 lbs. may be used. Boil the mixture to a candy height, and pour it whilst warm into moulds or small paper cases well dusted with finely powdered lump sugar; or pour it on a dusted slab and cut it into squares.
Candied Lem′on Peel. As Candied Citron.
Candied Or′ange Flow′ers. From the flowers deprived of their cups, stamina, and pistils (2 oz. to each lb. of sugar), as Candied Almonds, but poured out on a slab.
Candied Or′ange Peel. From the peel of the Seville orange, or common orange, as Candied Citron.
Candied Su′gar. See Sugar Boiling. The following are articles of a more special character.
Candy, Car′away. 1. From caraway seeds (in fine powder), 1⁄2 oz.; sugar, 1 lb.
2. Oil of Caraway, 1 dr.; sugar, 1 lb.
Candy, Diges′tive. Syn. Live-long candy. Prep. 1. Rhubarb and bicarbonate of soda, of each 1 dr.; ginger, 1⁄2 dr.; cinnamon, 20 gr. (all in fine powder); heavy magnesia, 1 oz.; powdered sugar, 2 oz.; mucilage of tragacanth, q. s. to form a lozenge mass; to be divided into small squares of 18 or 20 gr. each.
2. As the last, but adding finely powdered caraways, 1 dr.; oil of caraway, 15 drops; and sugar, 1 oz. Both are used as heartburn and digestive lozenges.
Candy, Gin′ger. Prep. 1. From ginger (in coarse powder), 3 oz.; boiling water, 11⁄4 pint; macerate in a warm place for 2 hours, strain, add lump and moist sugar, of each 5 lbs., and boil to a candy.
2. Ginger (in very fine powder), 1 oz.; powdered sugar, 2 lbs.; syrup, q. s. to make a paste. Stomachic and carminative.
For various sweetmeats which might come under the head of Candy, see Confections, Drops.
CANKER. This disease consists in a depraved condition of that part of the sensitive foot of the horse which secretes the horny frog and sole. It mostly occurs in coarsely-bred animals, and is the result of filth, damp, and bad ventilation. The treatment consists in first removing all loose horn, and allowing all pent-up matter to escape; the exuberant granulations must be carefully cut away, and the parts then washed with a tepid lotion of sulphate or chloride of zinc; after drying the surface dust it with oxide of zinc; apply tow dipped in a mixture of tar and lime, and “keep it in firm contact with the parts by means of a leather sole or strips of hoop iron underneath a shoe lightly tacked on. Dress in this manner daily, keeping up the dry pressure for a week.” (Finlay Dun.)
CAN′NON METAL. See Gun Metal.
CANTHAR′IDES. Syn. Spanish flies, Blistering f., Lyt′tæ; Canthar′is, B. P. The Cantharis vesicatoria of Latreille, commonly known as the Spanish fly, is an insect of the order Coleoptera; it abounds in the south of France, Spain, and Italy; and has spread into Germany and the south of Russia. When alive it exudes a strong fetid and penetrating odour.
Pur., &c. These insects should be preserved in well-closed bottles or tin canisters. The addition of a few drops of oil of cloves, or of strong acetic acid, or even of a few cloves in substance, will preserve them unchanged for a length of time in closed vessels. The best proof of their goodness is the smell. The powder is constantly adulterated. The plan of the wholesale druggists is to sort out the most worthless flies for powdering, and to compensate for their deficiency of vesicating power by adding 1 lb. of euphorbium to every 12 or 13 lbs. of flies. When a superior article is required, liquorice powder is added (4 or 5 lbs. to every 14 lbs.), along with about 1 lb. of euphorbium, and sufficient blue black or charcoal to turn the yellow of the liquorice to a greenish colour. The best mode of detecting this adulteration is by the microscope. It should be borne in mind that only those flies which have attained their full growth possess blistering properties. The immature or undersized insects are destitute of epigastric power.
Ant. An emetic of sulphate of zinc, followed by the stomach-pump, if necessary. The397 vomiting may be promoted by copiously drinking warm bland diluents, such as broth, linseed tea, milk, &c. Friction on the spine, with volatile liniment and laudanum, and the subsequent administration of draughts containing musk, opium, and camphorated emulsion, have been strongly recommended.
Tests. By the microscope very minute particles may be discovered in the stomach and intestines, on a post-mortem examination. Orfila thus found particles of cantharides in a body that had been interred nine months.
Uses, &c. Spanish flies are used externally to raise blisters, and internally as a stimulant and diuretic, generally in the form of tincture. In excess they produce strangury, bloody urine, satyriasis, delirium, convulsions, and death. See Tinctures, Vesicants, &c.
CANTHARI′DIN. C5H12O2. Isomeric with picrotoxin. This substance is found in, and is the vesicating principle of, the Spanish fly, Chinese blistering fly, and other coleopterous insects. Prep. Pulverised cantharides are allowed to remain in contact for 24 hours with twice their weight of chloroform, in a displacement apparatus. The chloroform is then drained off, and finally displaced by alcohol, and the solution is left to evaporate. The cantharidin crystallises out, saturated with green oil. In order to purify the cantharidin it is laid on bibulous paper, which absorbs the greater part of the oil, and then crystallised out of a mixture of alcohol and chloroform. (Procter.)
Prop. Prismatic crystals, melts at 200° C., volatilises in white fumes, which strongly irritate the eyes, nose, and throat, and condenses in rectangular prisms. Cantharidin is insoluble in water, but soluble in alcohol, ether, chloroform, acetic acid, and in the fixed and volatile oils. Its solution in any of the liquids above mentioned possesses vesicating properties, which, however, is not exhibited by solid cantharidin.
CAOUT′CHOUC. Syn. India rubber, Elastic gum. India rubber is the concrete juice of the Ficus elastica, Siphonia elastica, the Urceola elastica, and many other tropical plants. The fresh milky juice is spread over moulds of unbaked clay, and is then exposed to the heat and smoke of a fire, or torches, to dry it, whence it derives its dark colour. Successive coats of juice are laid on, and the operation of drying repeated until the bottles acquire sufficient thickness. When it has become thoroughly hard and dry, the clay is beaten out. In this form it is commonly imported.
Prop., &c. The general properties of india rubber, as well as its numerous applications, are well known. The fresh juice has a cream-like appearance and consistence, is coagulated by heat, and is miscible with water, alcohol, and wood naphtha; sp. gr. 1·012 to 1·041; it yields from 18% to 45% of solid caoutchouc, either by heat or evaporation. By excluding it from the air it may be preserved unchanged for a considerable period.
Solid caoutchouc has a sp. gr. ranging between ·919 and ·941; it melts at 248° Fahr. into a viscid mass, which does not again harden on cooling; it is unaltered by chlorine, hydrochloric acid, sulphurous acid, fluosilicic acid, ammonia, caustic alkaline lyes (even when boiling), and most similar substances; nitric acid and sulphuric acid act on it only by long contact when concentrated. Some specimens of caoutchouc are harder than gutta percha itself, and equally inelastic, whilst others never perfectly solidify, but remain in a condition resembling that of birdlime or printers’ varnish.
The best solvents of caoutchouc are rectified sulphuric ether (which has been washed with water to remove alcohol and acidity), chloroform, bisulphide of carbon, a mixture of bisulphide of carbon and absolute alcohol (94 of the first to 6 or 7 of the last), and caoutchoucin. All these liquids dissolve india rubber rapidly in the cold, and leave it unaltered on evaporation. The first two are, however, too expensive to be generally employed. The others have a disagreeable odour, but are much cheaper than the rest, and possess the advantage of leaving the film of caoutchouc in a firmer and stronger condition than other solvents. Pyrogenous oil of turpentine is another cheap and good solvent. Benzol, rectified mineral or coal-tar naphtha, crude petroleum, and oil of turpentine dissolve india rubber by long digestion and trituration (with heat), otherwise they merely form with it a glutinous jelly that dries very slowly and imperfectly, leaving it much reduced in hardness and elasticity. The fats and fixed oils also readily dissolve caoutchouc (with heat), forming permanently glutinous solutions or pastes; so also do most of the volatile oils, but the solutions with the majority of them dry with difficulty.
One of the most remarkable properties of india rubber is the great amount of heat which is disengaged during its condensation by pressure or in the exercise of its elasticity. During the process of kneading the raw caoutchouc in the “masticators,” the cold water thrown in to reduce the temperature soon becomes boiling hot. When no water is added, a temperature so high is often reached as to occasion the melting of the rubber. This is particularly the case during the process of “dry kneading” with quick-lime. A tube 21⁄4 inches in diameter, impactly secured, was subjected to a force of 200 tons. The result was a compression amounting to 1-10th; great heat was evolved, and the excessive elasticity of the substance caused a fly-wheel weighing five tons to recoil with alarming violence. Mr Brockedon states that he succeeded in raising the temperature of an ounce of water 2° in about fifteen minutes by collecting the heat evolved by the extension of a small thread of caoutchouc. He refers this effect to the398 change in specific gravity, and contends that the heat thus produced is not due to friction, because the same amount of friction is occasioned in the contraction as in the extension of the substance, and the result of this contraction is to reduce the caoutchouc thus acted upon to its original temperature.
The edges and surfaces of india rubber are readily and perfectly joined by mere contact and intense pressure. On the small scale the edges may be moistened with ether, naphtha, oil of turpentine, or some other solvent, or by long boiling in water, and immediately pressed tight together and held in contact for some time.
Elastic tubes are readily formed of india rubber by cutting it into uniform slips of proper thickness and winding them round rods of polished glass or metal, so that the edges are in close contact or “overlapping.” A piece of tape is then wound round outside it, and the whole boiled in water for 2 or 3 hours, after which time the edges will be found to be sufficiently adherent. A better plan is to immerse the “rubber” in a mixture formed of bisulphide of carbon, 95 parts, and rectified spirit, 5 parts, until it swells into a pasty mass, which may then be moulded into any desired form or passed through the die of a tubing machine. For chemical purposes, brewing, &c., vulcanised india-rubber tubing has now taken the place formerly occupied by the unprepared material.
The once celebrated “Mackintoshes” are made by spreading two or more coats of a paste made of caoutchouc and rectified coal-tar naphtha over the surface of the stuff or cloth, and, when it has become partially dry, pressing two such surfaces evenly together by passing the goods between a pair of cylinders or rollers. The articles are then placed in a stove room for the composition to harden, and to remove the odour of the naphtha. Of late years vulcanised or mineralised rubber (coloured) has been used for this purpose, and being spread on the outside of the stuff instead of the inside forms an ornamental and thoroughly waterproof material.
India-rubber thread is prepared by stretching it (previously cut into coarse filaments) to 5 or 6 times its length in boiling water or hot air, in which state it is allowed to cool slowly. This process is repeated again and again until it reaches 16,000 or 17,000 times its original length, when it is glazed by agitating it with powdered sulphur or French chalk. This thread is readily joined or “pieced,” as it is called, by paring the ends obliquely with a pair of scissors or a knife, and then pressing the clean ends strongly together with the fingers. When the coarse filaments from the cutting machine are simply stretched with the moistened thumb and finger in the act of “reeling” to about 8 or 9 times their length, they are said to be “inelasticated,” and are ready to be made into elastic braces, elastic web, and other like elastic tissues and fabrics in the braiding machine.
Caoutchouc, Vul′canised. Syn. Vulcanised india rubber, Mineralised i. r., Sulphuretted i. r. The discovery of the singular action of sulphur and the mineral sulphides on caoutchouc was made by Mr Charles Goodyear, of New York, in 1842, at which date the manufacture of vulcanised india rubber may be said to have commenced. In 1843 Mr Thomas Hancock patented a process for vulcanised india rubber in these countries, founded on that of Mr Goodyear. A sheet of caoutchouc immersed in melted sulphur absorbs a portion of it, and at the same time undergoes important changes in many of its leading characteristics. So prepared, it is no longer affected by changes of temperature; it is neither hardened by cold nor softened by any heat insufficient to destroy it. It loses its solubility in the solvents of ordinary caoutchouc, whilst its elasticity is greatly augmented, and has become permanent.
The same effect is produced when sulphur is kneaded into caoutchouc in a masticator, or by means of powerful rollers, as well as when common solvents (naphtha, spirit of turpentine, &c.) are charged with a sufficient amount of sulphur in solution to become a compound solvent of the rubber. In these cases articles may be made of any required form before heating them for the change of condition technically termed “vulcanisation.” It is necessary, however, for this purpose that the form should be carefully maintained both before and during the exposure to the heat.
“A vulcanised solid sphere of 21⁄2 inches in diameter, when forced between two rollers 1⁄4 inch apart, was found to maintain its form uninjured. In fact, it is the exclusive property of vulcanised caoutchouc to be able to retain any form impressed upon it, and to return to that form on the removal of any disturbing force which has been brought to act upon it.” (Brockedon.)
Caoutchouc combines with from 12% to 15% of sulphur; the quantity of sulphur added to the naphtha paste should not, therefore, exceed 10% or 12% of its weight.
The temperatures for vulcanisation by the common method range from 320° to 330°; and the period required is one hour or more, according to the temperature. A much lower temperature is, however, sufficient if the duration of the exposure is much extended or the compound mass is softened with any of the common solvents of india rubber.
The process of sulphuring, or mineralisation, is differently conducted in different manufactories. Under Mr Burke’s patent, oxysulphide or amorphous sulphide of antimony (formed by decomposing a solution of crude antimony in a lye of potash or soda with hydrochloric acid) is employed. This powder he combines with either india rubber or gutta percha, or mixtures of them, by kneading in a “masticator”399 for 2 or 3 hours, and after strong compression in a mould whilst still warm, he exposes the mass to a steam heat ranging from 250° to 280° Fahr. The block, so prepared, is afterwards cut into sheets, &c. The advantages possessed by the product are that it possesses no unpleasant odour, nor does the sulphur effloresce on its surface, as in ordinary vulcanised india rubber.
Under Mr Christopher Nickel’s patent (1849) 1 part of sulphur is kneaded with 6 parts of caoutchouc, and then pressed into moulds, as before. He also vulcanises rubber by exposing it in a cylinder heated in a steam jacket to the fumes of sulphur or to sulphuretted gases, given off from a retort connected with the apparatus. The rubber thus prepared he next subjects to hydraulic pressure in moulds, at a temperature ranging between 220° and 250° Fahr.
Small articles or sheets of india rubber may be extemporaneously vulcanised at common temperatures by simple immersion, for a minute or two, in a mixture of bisulphide of carbon, 971⁄2 parts, and protochloride of sulphur, 21⁄2 parts; after which they must be well washed first in weak alkaline lye, and next in pure water. Mr Parkes employs 100 instead of 971⁄2 parts of the bisulphide. This method is termed “cold sulphuring.”
An excellent method of vulcanisation, recommended by Mr Parkes, particularly applicable to small articles, consists in immersing them for about 3 hours in a close vessel containing a solution of polysulphide of potassium at 25° Baumé (sp. gr. 1·197), and of the temperature of 240° Fahr. It is afterwards washed in an alkaline lye, then in pure water, and dried.
Among the many applications of vulcanised india rubber those connected with its elasticity and its enormous contractile power when extended are particularly striking. Under Mr E. Smith’s patent, “torsion springs” for roller blinds, door springs, clock springs, carriage springs, &c., are made of it. Mr Hodges, in another patent, has availed himself of the same property as a new mechanical power. Short lengths of caoutchouc, which he terms “vulcanised power purchases,” are successively drawn down from or lifted to a fixed bearing, and attached to any weight which it is required to raise; when a sufficient number of these power purchases are fixed to the weight, their combined elastic force lifts it from the ground. Thus, 10 purchases of the elastic strength each of 50 lbs. raise 500 lbs. Each purchase is 6 inches long, and contains about 11⁄2 oz. of vulcanised caoutchouc. These 10 purchases, if stretched to the limit of their elasticity (not of their cohesive strength), will lift a weight exceeding 650 lbs.
The same principle has been applied to relieve and equalise the strain on ships’ cables, especially where several boats are towing one vessel; and as a projectile force. A number of power purchases, attached to the barrel of a gun constructed to project harpoons, will exert a power, if suddenly relieved, proportioned to their aggregate forces. By similar contrivances balls may be projected 200 yards or more, and a charge of No. 4 shot can be thrown 120 yards. A bow, in which the string alone is elastic (the reverse of the usual form), has been contrived which throws a 30-inch arrow 170 yards.
The last great improvement in the manufacture of caoutchouc is the discovery that by continuing the process of vulcanisation for a longer time at an increased heat and under pressure, a hard black substance is obtained, which can be turned in a lathe like ebony. This substance has already been applied to an extraordinary number of uses. See Vulcanite.
An exceedingly useful combination of cork and india rubber has lately been introduced. See Kamptulicon.
Caoutchouc, Facti′′tious. See Oil, Consolidated.
CAOUT′CHOUCIN. An extremely light fluid obtained by distilling india rubber.
Prep. (Barnard’s patent process.) A highly volatile fluid, discovered by Mr Barnard. India rubber or caoutchouc, as imported, cut into small lumps, containing about 2 cubic inches each, is thrown into a cast-iron still, connected with a well-cooled worm-tub (any flat vessel with a large evaporating surface will do, the entire top of which can be removed for the purpose of cleaning it out); and heat is applied in the usual way, until the thermometer ranges to about 600° Fahr., when nothing is left in the still but dirt and charcoal. The dark coloured fetid oil which has distilled over is next rectified along with 1⁄3rd its weight of water, once or oftener; and at each rectification becomes brighter and paler, until at about sp. gr. ·680 it is colourless, and slightly volatile. The product is then shaken up with nitro-hydrochloric acid, or chlorine, in the proportion of a 1⁄4 of a pint of the acid to 1 gallon of the liquid. To enable the dirt to be the more easily removed from the bottom of the still, common solder, to the depth of about 1⁄2 an inch, is thrown in.—Prod. 80%.
Prop., &c. Mixed with alcohol, caoutchoucin dissolves gums and resins, especially copal and india rubber, at the common temperature of the atmosphere, and it speedily evaporates, leaving them again in the solid state. It mixes with the oils in all proportions. It has been used in the manufacture of varnishes, and for liquefying oil paints, instead of turpentine. It is very volatile, and requires to be kept in close vessels. According to the researches of Himly, Gregory, and Bouchardat, the caoutchoucin of Barnard consists of several liquids, some of which have the composition of olefiant gas, and others that of oil of turpentine.400
CA′′PERS. The flower buds of various species of Capparis, particularly C. spinosa, caper tree, preserved in vinegar. They are chiefly imported from Spain, Italy, and the south of France, where the caper tree is largely cultivated for the purpose. The flower-buds are picked daily, and thrown into a cask of strong pickling vinegar, until it becomes full, when it is sold to the dealers by the collector. The former sort them into different sizes by means of copper sieves, in a similar way to that adopted for lead shot and gunpowder. In this way they are divided into nonpareilles, capuchins, capotes, seconds, and thirds, of which the former, or smallest, are regarded as the best; but much depends upon the quality of the vinegar.
The bright green colour of capers, so much valued by the ignorant, arises chiefly from the presence of copper derived from the sieves used in sorting them. In many cases, copper coin, as sous and halfpence, are added for the purpose. Thus the eye is gratified at the sacrifice of the stomach, and an insidious poison introduced into the system, simply to give an unnatural appearance to a condiment which tastes better without it. See Copper.
CAPILLAIRE′. [Fr.] Simple syrup, or a concentrated solution of sugar in water, flavoured with orange-flower water, or some other similar aromatic. The name was originally given to a mucilaginous syrup, prepared by adding to an infusion of maiden-hair (Adiantum capillus Veneris) some sugar and orange-flower water.
CAP′NOMOR. See Kapnomor.
CAP′RIC ACID. HC10H19O2. Syn. Ru′tic acid; Acidum cap′ricum, L. An acid discovered by Chevreul, and obtained by decomposing caprate of barium with dilute sulphuric acid, or primarily by the saponification of butter or cocoa-nut oil, when it appears combined with butyric, caproic and caprylic acids. It is also procured by acting upon oleic acid or oil of rue with nitric acid.
Obs. When butter is saponified with caustic potassa or soda, and the resulting soap decomposed by adding an acid, in excess, and distilling the mixture, the four acids above named pass over into the receiver, in combination with water. The mixed acids may be separated by saturating them collectively with baryta, and by taking advantage of the unequal solubility of the newly formed barium salts. The less soluble portion (equal to about 1⁄20th of the dry mass) contains capric and caprylic acid; the larger and more soluble portion, butyric and caproic acid. On the same plan the two groups are resolved into their separate acids. These acids are deprived of their uncombined water by means of chloride of calcium. It is advisable to employ the term rutic acid, as the older term is easily confounded with caproic and caprylic.
Prop. Capric or rutic acid crystallises in fine needles, which fuse at 86° Fahr., giving out an odour resembling that of a goat. It is sparingly soluble in boiling water.
Prep. (Miller.) Castor oil is saponified by means of potassa or soda, and afterwards an excess of the hydrated alkali is added, amounting to one half the oil used. The mass is heated in a retort, and an oily liquid covered with water distils over. This oily liquid, which is the octylic alcohol, is rectified several times with potassa until the residue is no longer coloured brown.—Prop. A colourless liquid, of powerful aromatic odour; insoluble in water, but dissolving readily in acetic acid, ether, and alcohol. Its boiling point is 356° Fr., its sp. gr, ·823. The caprylate of ethyl, erroneously termed caprylic ether, is a colourless liquid, with an agreeable odour of pine-apples.
CAPSAICIN. Until the researches of Mr Thresh proved to the contrary the active principle of the capsicum fruit, or cayenne pepper, and the one to which it was thought it owed its acrid and pungent properties, was believed to be an alkaloid, and was named capsicine in consequence. Mr Thresh succeeded in obtaining an alkaloid from the capsicum, but this was entirely wanting in acridity and pungency. Its discoverer states that capsaicin occurs only in the pericarp of the fruit. The details of the process by which it may be obtained are given in the ‘Year Book of Pharmacy’ for 1876-77, from which it will be seen that the substance may also be procured by preparing a strong tincture of capsicum, and submitting it to dialysis. Capsaicin when cautiously heated to 138° F., melts to a transparent oily fluid, and if then allowed to cool rapidly, it becomes solid, assuming a crystalline condition in doing so. It volatilises at 240° F., without suffering decomposition. Strong nitric acid acts violently on it, decomposing and dissolving it. The crystals dissolve very readily in ether, amylic, alcohol, acetic ether, benzine, and fixed oils, and still more readily in alcohol, and in rectified and proof spirit. In turpentine and carbon disulphide it dissolves much more slowly. It is not affected by boiling for some considerable time in dilute sulphuric acid, and the acid liquor shows no signs of glucose.
A specimen of capsaicin which Mr Thresh believes to have been in a pure condition was sent to Dr Flückiger’s laboratory for analysis, and Dr Buri, by whom the combustion was made, reports that it gave the following composition:—C19H14O2, a result which Mr Thresh found to agree very fairly with some capsaicin derived from a specimen fruit obtained from a different source from that sent to Dr Flückiger. Administered internally in doses of the 1⁄25th of a gram, capsaicin gave rise to violent griping and purging; and when a lotion consisting of one part diluted with forty of glycerin and spirit was placed on the arm, it soon gave rise to such pain, and caused so much inflammation, that the lint which401 was wetted with the solution had to be removed very shortly after being applied.
CAP′SICUM. [L. and Eng.] Syn. Chil′i, Red pepper. A genus of plants belonging to the natural order Solanaceæ, species of which yield the fruits which are used to form Cayenne pepper and Chili vinegar. The officinal capsicum of B. P. is the fruit of the species C. fastigiatum. See Pepper, Tinctures, Vinegars.
CAP′SULES. This term is now commonly applied to small egg-shaped or spherical vessels, in which medicines are placed, for the purpose of covering their nauseous taste at the time of swallowing them. They are commonly made of gelatin, mixtures of sugar and gelatin, or animal membrane.
Capsules, Gel′atin. Prep. 1. By dipping the bulbous extremity of an oiled metallic rod into a strong solution of gelatin. When the rod is withdrawn, it is rotated, in order to diffuse the fluid jelly equally over its surface. As soon as the gelatinous film has partially hardened, it is removed from the mould and placed on pins, furnished with suitable heads, and fixed on a cork table. When sufficiently dry, the capsules are placed upright in little cells, made in the table to receive them, and the liquid with which they are to be filled is then introduced by means of a small glass tube. They are next closed by dropping some of the melted gelatin on the orifice of each. Six parts of gelatin, and one part sugar, are now the common proportions.
2. (Simonin.) Oval balls of wax, of the requisite size, are prepared by pouring wax, into a wooden mould, consisting of two parts, and arranged for the reception of a row of these balls. These are afterwards stuck on iron needles, affixed to rods of convenient size, in rows. The balls are now uniformly coated all at once by dipping in the usual manner, then removed from the needles, and are next placed with the needle holes downwards, on a gently heated plate, when the wax flows out, and a round capsule is left behind.
Cap′sules, Gel′atin and Su′gar. Prep. (Giraud.) Gelatin, 6 parts; solution of gum and simple syrup, of each 1 part; water, 5 parts; melt in a water bath, remove the scum, and proceed as before.
Capsules, Glut′en. These, which form the subject of a French patent, are said to be formed of the gluten of wheat flour, a substance which is insoluble, although softened, by water. We have placed these capsules for twenty-four hours in warm water, and found them, at the expiration of that time, still unbroken, the enclosed medicine being completely enveloped. The mode of preparation is kept secret.
Capsules, Mem′branous. Syn. Organ′ic capsules. From gut-skin moistened and stretched over an oiled bulb of glass or metal, and filled in the common way. These have been patented, but they do not appear to be an improvement on the common capsule of gelatin.
Obs. The common capsules usually hold about 10 or 12 gr. of balsam of copaiba. Those of the shops in nine cases out of ten, are filled with adulterated copaiba, and at least 4-5ths of them are filled with train oil or linseed oil, to which a few drops only of the balsam are added.
Balsam of copaiba (capivi) and oil of cubebs, or a mixture of them, castor oil and cod-liver oil, are the substances most usually administered in this way. Baccæ copaiferæ factitiæ are officinal in the Ph. Castr. Ruth. Ratier has proposed to grease them and administer them per anum. Ricord has strongly recommended capsules of copaiba, coated with extract of rhatany, as much superior to the common ones of copaiba alone, in the treatment of gleet and gonorrhœa. They may be easily prepared by either of the following methods:
1. By immersing, for an instant, the common capsule in a mixture of extract of rhatany (newly prepared from the root), 3 parts; syrup of moist sugar, 1 part; mucilage of gum Arabic, 1 part; melted together in a water bath.
2. By forming the bodies of the capsules with the above mixture or composition, instead of with gelatin, and then following the same manipulations as for the manufacture of the common gelatin capsules.
These capsules are said to sit well upon the stomach, the tone of which they contribute to improve, and to act with greater certainty than those made of copaiba and gelatin alone.
CAR′AMEL. A dark-brown substance obtained by heating sugar. It is formed during the roasting of all materials containing sugar, such as coffee and malt. It is much used for colouring soups, wines, spirits, and other liquids.
Caramel, Crude. Syn. Spirit colouring, Burnt sugar. Prep. From cane sugar, by heating it to from 410° to 428° Fahr., as long as aqueous vapour is formed; dissolving the product in water, and concentrating the solution by evaporation.
Caramel, Pure. Prep. 1. (Graham.) Crude caramel, obtained as above, is placed on a parchment-paper dialyser. The undecomposed sugar and certain intermediate compounds diffuse out with considerable facility, and what ultimately remains on the dialyser possesses five times the colouring power of the original crude caramel, weight for weight. See Dialysis.
2. (Peligot.) Add strong alcohol to a filtered aqueous solution of crude caramel until it ceases to produce a precipitate; collect the precipitate, which is caramel, on a filter, wash with alcohol, and dry. Graham recommends that the product should be dissolved and precipitated four or five times, or till the mass402 thrown down, from being plastic at first, becomes pulverulent.
3. (J. J. Pohl.) Cane sugar is heated in a spacious metallic vessel by means of an oil bath to 410° or 419° Fahr. as long as aqueous vapours escape, the mass being occasionally stirred with a spatula. The mass is then finely powdered and digested with alcohol for two or three hours; the digestion is repeated until the fluid no longer tastes bitter.
Prop. A solution containing 10% of purified caramel is gummy, and forms a tremulous jelly on standing. Evaporated in vacuo, it dries up into a black shining mass soluble in water; but if the solution be evaporated to dryness by the heat of a water bath, the whole matter is rendered insoluble in hot or cold water. A very small proportion of caramel suffices to give a rich sepia tint to water.
CAR′AT. A weight of 4 grains used in weighing diamonds, which are spoken of as of so many carats weight. Among assayers, a carat is a weight of 12 grains; but more commonly a proportional weight or term, representing the number of parts of pure gold in 24 parts of the alloy; pure gold being spoken of as of 24 carats fine. It is commonly the 24th part of the “assay pound,” and is nominally subdivided into 4 assay grains, and these again into quarters. See Assaying.
CAR′AWAY. Syn. Caraway seed; Se′mena carui, L.; Carui, B. P. The fruit of the Carum Carui (Linn.), an umbelliferous plant, common in England and other parts of Europe. These fruits, commonly called “seeds,” form an agreeable and useful aromatic and carminative, and are especially esteemed in the flatulent colic of children. They are also largely employed as an adjuvant or corrective in various officinal preparations; and as a flavoring ingredient in cakes, biscuits, cordials, confectionery, &c. See Essences.
CARBAZOT′IC ACID. See Picric Acid.
CARBOL′IC ACID. H.C6H5O. Syn. Phenylic acid, Phenic acid, Phenol, Phenylic alcohol, Hydrate of phenyle, Hydrated oxide of phenyle. A powerful antiseptic substance obtained from coal-tar oil.
Prep. Crude, heavy coal oil is agitated with milk of lime, allowed to stand, and the aqueous portion separated from the undissolved oil and decomposed by hydrochloric acid. The oily liquid obtained is purified by distillation.
1. Crude coal oil is distilled in a retort furnished with a thermometer, and the portion which passes over when the heat ranges between 300° and 400° Fahr., is collected apart, and mixed with a hot saturated solution of caustic potassa; after standing for some time, a semi crystalline pasty mass forms, from which the supernatant liquid is decanted; the pasty mass is now agitated with a small quantity of water until dissolved; the solution thus formed separates into two portions, the denser of which contains carbolate of potassa; this being separated by decantation, is decomposed by hydrochloric acid. The solution of carbolic acid which rises to the surface is digested with chloride of calcium, to remove water, and purified by distillation; the distillate, by refrigeration, furnishes crystals of the acid, which must be drained, dried, and preserved from the air.
2. From salicylic acid. Mix intimately together equal weights of salicylic acid and powdered glass; introduce the mixture into a good German retort, and heat on a sand bath, gradually raising the heat till it becomes red hot at the bottom. The vapour is condensed in any convenient receiver. If the materials are perfectly dry, it solidifies to a mass of crystals as soon as it condenses, but if there be a trace of water present it remains liquid. The slower it distils over the lighter will be the colour, while if a high temperature be employed it comes over nearly black. It may be rendered colourless and anhydrous by rectification over quick-lime.
Of late years the manufacture of carbolic acid has increased to a great extent, and is generally found in a pale yellow clear solution, instead of as a dark hazy liquid. The pure anhydrous acid is in long, colourless, prismatic crystals, often, however, on keeping turning a beautiful pink, rose, or crimson, and which rapidly deliquesce in moist air, becoming converted into a colourless refractive liquid, having a faint odour of roses and tar. At 95° F. they become an oily liquid, having an odour and taste like creosote. Sp. gr. 1·065, boiling point 370° F. Exposed to the air the crystals absorb moisture and liquefy. The acid is slightly soluble in water, but freely soluble in glycerin, alcohol, and ether. Carbolic acid is poisonous, and is a powerful antiseptic.
Tests.—About a grain of hypochlorite of calcium, added to a little aqueous solution of carbolic acid, placed in a test-tube, produces after agitation, the addition of a few drops of ammonia, and the application of a gentle heat, a bright blue colour with a tinge of green. One drachm of the acid if pure completely dissolves on being shaken with half a pint of warm water.
Uses. The extraordinary antiseptic properties of carbolic acid have long been known, but its extended use has been delayed, owing to the difficulty experienced in obtaining it in considerable quantities. It is now, however, principally owing to the labours of the late Dr F. Crace Calvert, produced on a large scale, and this chemist has proposed its application to many valuable purposes. As a medical agent it seems to have all the useful properties of creosote in an exalted degree, with some peculiar actions of its own, and is being applied with marked success in the Manchester Royal Infirmary and similar institutions, in cases of chronic diarrhœa, obstinate vomiting (even after creosote has failed), and as a disinfecting wash for ill-conditioned ulcers and gangrenous sores. It has been said to have been used with403 marked success internally as a remedy for hooping-cough. It has also been applied successfully in cases of foot-rot, a disease which annually carries off large numbers of sheep. It has been employed for the preservation of gelatin solutions and preparations of size made with starch, flour, and similar materials, and of skins and other animal substances. It appears to act strongly as an antiferment, and Dr Calvert states that it is one of the most powerful preventives of putrefaction with which he is acquainted. Commercial creosote is frequently nothing more than hydrated carbolic acid.
Professor Lister, of Edinburgh, adopting the germ theory of putrefaction, and regarding the putrid discharge from wounds as the result of the presence of atmospheric organisms which find a suitable nidus in the decomposing animal tissue exposed by the wound, seeks to exclude the access of these germs by the use of antiseptics, particularly of carbolic acid, the destructive action of which on living organisms is well known. He applies to the wounds dressings of gauze previously prepared with carbolic acid, additionally using as a lotion the acid, well diluted with water; whilst during the dressing of the wounds and the performance of surgical operations carbolic acid is diffused in the form of spray into the surrounding atmosphere with the object of destroying the germs floating in it.
Antidotes.—Calcined magnesia, or bicarbonate of soda, in milk after short intervals. In the absence of these, chalk, soap and water, or the plaster from the ceiling. Olive oil additionally. More than fifty per cent. of the carbolic acid manufactured is used for the purpose of preparing the following pigments and dye materials:—
1. Picric acid. 2. Phenyl brown. 3. Grenat soluble. 4. Coralline. 5. Azuline. These will be found described under Tar colours.
CAR′BON. C. Syn. Carbo′nium, Car′bo, L.; Charbon, Fr.; Kohlenstoff, Ger. An elementary or simple non-metallic solid body, very widely diffused through nature. Its purest and rarest form is that of the diamond. Nearly pure, it occurs very abundantly in the forms of graphite and anthracite. In combination with oxygen, as carbonic acid, it exists in the atmosphere and in the waters of most springs, also in limestone, marble, chalk, and dolomite. Combined with hydrogen, it enters largely into coal, peat, and lignite. It is an essential constituent of organic matter, and hence it has been termed the “organic element.” Charcoal, lamp-black, and coke, are more or less pure forms of carbon. By strongly igniting lamp-black in a covered crucible the element is obtained sufficiently pure for most chemical purposes.
It is best obtained purest by burning a jet of pure olefiant gas in an atmosphere of pure chloride, collecting the amorphous carbon deposited, and igniting in vacuo at a red heat.
Forms several chlorides, sulphides, &c., of which the following are the chief:—
Carbon, Protochloride of. Obtained from the sesquichloride by subliming it repeatedly through a tube filled with fragments of glass heated to redness. A transparent colourless liquid, with aromatic odour.
Carbon, Sesquichloride of. C2Cl6. Obtained by exposing Dutch liquid with chlorine, in a glass vessel, to the direct rays of the sun, taking care to renew the chlorine as long as it is absorbed. The liquid is ultimately converted into the sesquichloride of carbon, which is a white crystalline, volatile substance.
Carbon, Tetrachloride of. Syn. Bichloride of Carbon. It may be obtained by passing chlorine (desiccated by being made to pass through a tube wetted with strong sulphuric acid), through a bottle containing bisulphide of carbon, and afterwards through a porcelain tube, wrapped in sheet copper, and filled with fragments of broken porcelain, maintained at a red heat, by a charcoal or gas furnace, and condensing the product in a bottle surrounded by ice. A mixture of tetrachloride of carbon and chloride of sulphur is thus obtained. By shaking this mixture with solution of potash, the chloride of sulphur is decomposed and dissolved, whilst the tetrachloride of carbon separates, and falls to the bottom. The upper layer having been poured off, the tetrachloride may be purified by distillation.
Tetrachloride of carbon is a colourless liquid, having a sp. gr. 1·6, and boiling at 172° F. It is insoluble in water, but dissolves in alcohol and ether. Its vapour, diluted with air, is employed as an anæsthetic.
Carbon, Oxychloride of. COCl2. Syn. Chlo′rocarbon′ic acid, Phosgene gas, Chloride of carbonyl. Equal measures of carbonic oxide and chlorine are exposed to the direct rays of the sun; they combine, and become condensed to half their volume. It is a colourless, suffocating gas, which is immediately decomposed by water into carbonic and hydrochloric acids.
Carbon, Sulphide of. CS2. Syn. Bisulphide of carbon, Carbon disulphide, Sulphuret of carbon. Bisulphide of iron (iron pyrites), 5 parts, and fresh dry charcoal, 1 part, are heated together in a stoneware retort, furnished with a glass tube, having the end bent, and passing nearly to the bottom of a bottle or receiver filled with pounded ice. The bisulphide of carbon collects at the bottom of the receiver, and is then purified from adhering moisture and sulphur by distilling it, at a low temperature, from fused chloride of calcium.
By passing the vapour of sulphur over fragments of charcoal, heated to bright redness in a porcelain tube, and collecting the product as before.404
Sulphide of carbon is best manufactured by means of Peroncele’s apparatus figured in the accompanying drawing.
A is a fire-clay gas retort supported on the fire-clay block B; E and E are openings, one being that of a porcelain tube firmly cemented into the cover of A, serving for the introduction of sulphur; the other opening is for the introduction of pieces of coke, with which before the operation commences the retort is filled. The vapours of the sulphide of carbon pass through the tubes H and I into the vessel J, wherein part of the sulphide is condensed, and flows through K into the flask L, filled with water, thence through M into O, finally being run off by the tap N. Any vapours not condensed in J pass through P P into the worm T, the condensed sulphide being collected in S. The crude sulphide is rectified by redistillation over zinc or perchloride of mercury by means of a steam or water bath. If the perchloride is employed it should remain in contact with the crude sulphide for at least 24 hours before redistillation.
Prop., Uses, &c. A colourless, pungent, fetid liquid, having the sp. gr. 1·27. It is exceedingly volatile, boiling at 118·5° Fahr., and has never been frozen. It is highly inflammable, burning with a pale-blue flame, and giving off sulphurous and carbonic-acid gases. It freely dissolves sulphur and phosphorus, and by spontaneous evaporation deposits the first in beautiful crystals. The solution of phosphorus is much used in electrotyping objects, which are coated with a conducting film by its means. Its refractive power is remarkably high, and on this account it is employed to fill hollow lenses for spectroscopes and other optical instruments. It produces intense cold by its evaporation. A spirit thermometer, having its bulb covered with cotton, if dipped into this fluid and suspended in the air, rapidly sinks from 60° to 0°, and if put into the receiver of an air-pump it will fall to -81° Fahr. A mixture of sulphide of carbon and solid carbonic anhydride forms almost the most powerful frigorific agent known. Sulphide of carbon is now prepared on the large scale, and extensively employed as a solvent.
It is thus used for extracting from the cake of fruits and seeds the oil remaining in them after they have been submitted to pressure. The sulphide is subsequently separated from the oil by distillation. In Algiers it is used for obtaining the essential oils contained in the rose, jessamine, and lavender. It is also employed for dissolving the fat from bones, and from the crude wool. Furthermore, it is an excellent solvent for caoutchouc, as well as for the ordinary resins.
Its vapour is employed by agriculturists to kill the larvæ infesting grain. Latterly, it has been employed as a disinfectant.
a. Carbon Bisulphide as an Antiseptic. By P. Zöller (‘Deut. Chem. Ges. Berl.,’ ix, 1080-1084). The author has continued his experiments on this subject with the object of determining (1) the minimum quantity of bisulphide required, and (2) whether articles of food preserved by means of it are fit for human consumption.
As regards the first point, he found that meat of all kinds, and even entire animals, in quantities up to 20 kilograms, kept perfectly well for several weeks in vessels of sheet zinc, into which 5 grams of carbon bisulphide had been introduced, the meat being either simply hung on hooks or wrapped in cloths and laid on perforated shelves in the vessels. Probably a smaller quantity of the bisulphide would suffice. Meat also kept well for 62 days in a vessel in which carbon bisulphide was liberated by introducing potassium xanthate and dilute sulphuric acid. Freshly baked bread, vegetables, and fruits of all kinds (asparagus, radishes, young beans, cucumbers, strawberries, raspberries, currants, cherries, peaches, apricots, lemons, &c.), and juices of fruits kept perfectly well in glass vessels, into which carbon bisulphide has been introduced,405 in the proportion of 5-10 drops for each litre of capacity.
Bread, vegetables, and fruit thus preserved are fit to eat after simple exposure to the air, and cannot be distinguished by taste or other qualities (except a slight loss of colour in some fruits) from fresh bread, &c. Meat retains even after exposure to air the disagreeable odour of carbon bisulphide. But besides this odour, which disappears on boiling or roasting, the meat has a slight smell of the volatile fatty acids and the taste of game. To most people, however, this taste is not unpleasant. The presence of fatty acids is to be attributed to decomposition taking place in the interior of the meat, and not preventable by the carbon bisulphide, the function of which is merely to kill germs present in the air or on the surface of substances submitted to its influence.
b. By Hugo Schiff (‘Deut. Chem. Ges. Ber.,’ ix, 828). Cocoons of silkworms which had been killed by exposure to the vapours of carbon disulphide underwent no change during six months’ keeping in flasks in the laboratory. The bodies of some pigs which had been used for physiological experiments were put into a stoppered vessel with a few c. c. of carbon disulphide in 1869, and have been perfectly preserved without decomposition. The same result was obtained with a lizard 35-45 centimetres long, which had been suffocated accidentally in 1869, and was bottled whole. In this case a small quantity of liquid collected at the bottom of the vessel, and the green hue of the skin became a dirty greyish green, but not the slightest putrefaction occurred. Similar results were obtained with the intestines of poultry immersed in water in 1872 with a little carbon disulphide, in a bottle with a greased stopper; with a lump of beef weighing 200 grams; and with the body of a finch killed with paraconine. The beef yielded a normal flesh fluid, and was eaten by a dog without hesitation even after several months.
Purification.—1. It is stated that the odour of sulphide of carbon can be readily removed by allowing it to stand over mercury or corrosive sublimate for some time, and then redistilling.
2. The following method by Kern is stated by him to be the best for purifying sulphide of carbon:—The impure product is well mixed in a tall glass vessel with some lead nitrate, and with a small quantity of metallic lead. When the salt turns dark the liquid is poured into another vessel with a fresh quantity of the lead salt; and so on until the salt remains nearly white while mixed with the liquor. The sulphide of carbon is then placed in a retort, and distilled over into a well-cooled receiver.
3. M. Yvon proposes a process which consists in adding copper turnings to the sulphide; no slaking is necessary. The sulphide soon becomes nearly colourless, and loses its usually unpleasant odour. Miller says reduced copper produces the same result.
Carbon sulphide is employed therapeutically in doses of 2 drops, gradually increased to 5, as a sudorific in rheumatism. It is also dropped (40 to 50 drops) on the part, to promote the reduction of strangulated hernia. Externally, it is employed in liniments for rheumatic pains.
CAR′BONATE, a salt in which the hydrogen of (hypothetical) carbonic acid (H2CO3) is replaced by a metal or other basic radical.
Prep., &c. The processes by which the commercial carbonates and many others are prepared are described under the respective bases. Most of the earthy carbonates are found abundantly in nature. In general the salts of this class may be formed by adding an alkaline carbonate to a salt of the metal in solution by double decomposition.
Prop. The carbonates of the alkalies are soluble in water; those of the other bases are for the most part insoluble, except the water is highly charged with carbonic acid. From most of them carbonic anhydride or anhydrous carbonic acid can be easily expelled by heat.
Tests. The carbonates are easily distinguished by the following reactions:—They dissolve with effervescence in hydrochloric acid and in most other acids; in some cases a gentle heat is required to promote the disengagement of the gas.—The gas evolved in the last, passed into lime water and baryta water, occasions white precipitates, which redissolve in acids with effervescence, and after the solution has been boiled are not reprecipitated by liquor of ammonia.—Chloride of calcium and chloride of barium give white precipitates in solutions of the neutral alkaline carbonates, but in solutions of the alkaline bicarbonates only after ebullition; and the precipitates are readily soluble with effervescence in acetic acid.
Estim. The quantity of the metal in an alkaline or earthy carbonate may be easily determined by the ordinary volumetric methods of alkalimetry (which see), and the quantity of carbonic acid, by the method of Fresenius and Will (see Alkalimetry). The apparatus figured on next page, or preferably that shown in the article on Alkalimetry, may be used instead of the more complicated contrivance of the German chemists.
A weighed sample of the carbonate to be examined is placed in the flask a along with a little water, and the small tube, b, filled with either sulphuric or hydrochloric acid, is carefully introduced. The cork, with its chloride of calcium tube, d, is then fitted to the flask, and the whole apparatus very accurately weighed.
On inclining the apparatus the acid escapes over the side of the small tube, and mixing with the liquor in the flask, expels the carbonic406 acid of the carbonate, which is then dried by passing over the chloride of calcium. After effervescence has ceased heat should be applied to the bottom of the flask, until it be filled with steam, to expel the carbonic gas it contains. The loss of weight gives the weight of the carbonic acid gas that was contained in the sample. The quantity of carbonic acid in the carbonates of the metals that do not contain water may be determined by heating them to redness in a platina crucible.
a, Flask containing the sample of carbonate for examination, stopped by a closely fitting cork, through which passes the bent tube c.
b, A small tube, sufficiently long to maintain a slanting position without falling, filled with sulphuric or hydrochloric acid.
c, A bent tube, connecting the flask with d.
d, Horizontal tube, filled with small fragments of fused or dried chloride of calcium, with a fine orifice at the extremity e.
CARBONIC ACID. H2CO3. True carbonic acid has not yet been obtained in any satisfactory condition, although the solution of carbonic anhydride (often called carbonic acid), or anhydrous carbonic acid, is generally regarded as such. It forms with bases an important series of salts, called the carbonates, by double decomposition.
CARBONIC ANHYDRIDE. CO2. Syn. Carbonic acid, Carbon dioxide, Fixed air, Choke damp; Acide carbonique, Fr.; Kohlen säure, Ger. A compound formed by the chemical union of carbon and oxygen.
Hist. Van Helmont recognised carbonic acid as a peculiar gas. Dr Black, in 1757, proved that it was a constituent of limestone, and gave it the name of fixed air; he also showed that the causticity of alkalies depended on its absence. Bergmann first described it as an acid, applying to it the term aërial acid. Lavoisier, in 1776, established its true nature, and gave it the name it now bears. Faraday, in 1823, by pressure at an extremely low temperature, reduced carbonic acid to a liquid, and a few years later Thiloria and Brunel obtained it in the solid form.
Nat. Hist. Carbonic acid is a constituent of the atmosphere, its presence being essential to the existence of vegetable life on the globe. It issues from the earth in many situations, as the Grotto del Cane in Italy, the Valley of Poison in Java, and near the Lake of Laach in Germany. It gives to many mineral springs their sparkling brilliancy, and is held in solution by all natural waters. Combined with the bases, lime and magnesia especially, it exists in large quantities in the crust of the earth. It is the chief product of combustion, and one of the products of fermentation. It is always being exhaled by animals in the process of respiration, and in smaller quantities by plants at night or in the shade. It forms the terrible “choke-damp” or “after-damp” of the coal mines. It is the gas disengaged during the effervescence of soda water and other aërated drinks, and the cause of the freshness of newly-drawn beer.
Prep. Hydrochloric acid, 1 part, diluted with water, 4 or 5 parts, is poured upon fragments of white marble, previously placed in a suitable generating apparatus.[237]
Carbonic acid is rapidly evolved, and may be collected, with some loss, over water in the pneumatic trough. If required dry, the gas must be passed over fragments of fused chloride of calcium, placed in a large tube, or through a small quantity of concentrated sulphuric acid, and collected by displacement or over mercury.
From oil of vitriol, 1 part; water, 6 parts; and chalk or whiting, 11⁄4 part; mixed in a suitable vessel, applying agitation.
Prop. Under ordinary conditions carbonic acid is a colourless, non-inflammable, irrespirable gas, possessing a slightly pungent odour, and an acidulous taste. Water absorbs its own volume of this gas, and by pressure may be made to take up enormous quantities, forming carbonated or aërated water. Its sp. gr. is 1·520; hence it may be poured from one vessel to another like water. By a pressure of thirty atmospheres at 32° Fahr. it is liquefied, the pressure required decreasing as the temperature gets lower. At -94° Fahr. it solidifies into a vitreous transparent mass.
Carbonic acid, even when greatly diluted with air, cannot be inhaled without insensibility following. An atmosphere containing more than its natural quantity of gas (1 part in 2500 parts by measure) acts upon the system as a narcotic poison; hence the danger of over-crowded rooms. It is a non-supporter of combustion, at once extinguishing a lighted candle, gas-jet, or even a piece of burning phosphorus, when these are placed in a jar of the gas.
Tests. It feebly reddens litmus paper, extinguishes the flame of a burning taper, and forms a white precipitate in aqueous solutions of lime and baryta, which is soluble in acetic acid. By the last test a very small quantity of this gas may be easily detected in the atmosphere of rooms, &c. A lighted candle is generally used to test an atmosphere suspected to contain carbonic acid: but it is found that407 air that will support combustion will contain sufficient of this gas to cause insensibility.
Ant., &c. The patient should be immediately removed into the open air, and placed on his back with the head slightly raised. Cold water should be dashed over the body, hot water or mustard poultices applied to the feet, and ammonia (carefully) to the nostrils. Brandy-and-water and other stimulants may be administered. Continued friction on the surface of the body is also very useful. If the patient has ceased to breathe artificial respiration should be attempted. This may be done by gently pressing down the ribs, and forcing up the diaphragm, and then suddenly withdrawing the pressure. The inhalation of air, mixed with very little chlorine gas, has also been recommended. Wells, cellars, or other underground apartments, containing carbonic acid in poisonous quantities, may be freed from this gas by pumping it out in the same way as water, observing to allow the suction hose to fully reach the floor or bottom of the place. Fresh slaked lime or milk of lime, copiously thrown in, will have a like effect, by absorbing the gas. Free ventilation, whenever it can be established, is, however, not only the cheapest, but the most efficient remedy. See Asphyxia.
CARBON′IC OXIDE. CO. Syn. Protoxide of carbon, Carbon monoxide, Ga′seous oxide of carbon; Oxy′dum carbon′icum, L. A gaseous compound of carbon and oxygen, containing less oxygen than is contained in carbonic acid.
Prep. 1. From carbonic acid gas passed over fragments of charcoal, heated to redness in a tube of porcelain or iron.
2. From crystallised oxalic acid, gently heated with 5 or 6 times its weight of strong sulphuric acid in a glass retort.
3. From ferrocyanide of potassium in fine powder, and 8 or 10 times its weight of concentrated sulphuric acid, heated together in a glass retort.
Obs. All the processes except the last give a mixture of carbonic acid and oxide. It is therefore necessary to pass the gas through a caustic alkaline solution or milk of lime to deprive it of carbonic acid. It may then be passed over dried chloride of calcium, to deprive it of moisture. It may be collected either over mercury or water, as the latter absorbs very little of this gas.
Prop. Carbonic oxide is colourless, inodorous, neutral, inflammable, and irrespirable. It is extremely poisonous, 1% mixed with air being sufficient to cause dangerous drowsiness. The deaths produced by the combustion of charcoal in close rooms are now attributed to this gas. The antidotes, &c., are the same as for poisoning from inhaling carbonic acid.
CAR′BUNCLE. A larger sized and dangerous form of boil, attended by extensive sloughing. The treatment consists in lancing, poulticing, and the adoption of a generous diet, with wine and stimulants. The safer plan, however, is to seek the advice of a medical man.
CAR′BURETTED HY′DROGEN. See Hydrogen.
CARD′AMOM. Syn. Card′amum; Cardamo′mum, B. P. The seed or fruit of the Elettaria Cardamomum forms the officinal cardamom. It is warm, pungent, carminative, and stomachic, and is largely used as a condiment in the East, and in Europe as an adjuvant in other medicines. Several kinds of cardamoms used medicinally and as spices are produced by the genus Amomum, belonging to the natural order Zingiberaceæ, the Ginger family.
CARD′BOARD. Cardboard, or sized pasteboard, is made of two to fifteen sheets of sized paper, pressed and stained. There are varieties of cardboard known as Bristol-board, London-board, the former being largely used for water-colour drawings, mounting-board, ornamental board, &c.
CAR′MINATIVES. Medicines that allay flatulency and spasmodic pains. Among the principal carminatives are ANISEED, CARAWAY SEED, CARDAMOMS, CASSIA, CINNAMON, GINGER, PEPPERMINT, and the PEPPERS. To these may be added ARDENT SPIRITS, and most of the AROMATIC ESSENCES and TINCTURES. See Mixtures, Patent Medicines, &c.
CAR′MINE. Syn. Carmine red, Vegetable scarlet; Carmi′num, L. A beautiful red pigment prepared from the cochineal insect.
Prep. The preparation of carmine is little understood, but success in its manufacture depends less on any mystery connected with the process than on the employment of the purest water and the best materials, and the exercise of moderate care, dexterity, and patience. The following forms will produce carmine of the richest hues down to ordinary and common, according to the skill possessed by the manipulator.
1. (Madame Cenette’s process.) Cochineal (in powder), 2 lbs., is boiled in pure river water, 15 galls., for 2 hours, when refined saltpetre (bruised), 3 oz., is added to the decoction, and the whole boiled for 3 or 4 minutes longer; salt of sorrel, 4 oz., is next added, and the boiling again renewed for 10 or 12 minutes; the heat is now removed, and the liquid allowed to settle for about 4 hours, after which time it is decanted with a syphon into shallow plate-like vessels, and set aside for three weeks. At the end of this time the film of mould which has formed on the surface is dexterously and carefully removed, without breaking it or disturbing the liquid beneath it. The remaining fluid is next very carefully removed with a syphon, and the adhering moisture, as far as possible, drained off, or sucked up with a pipette. The residuum, which is the carmine,408 is dried in the shade, and possesses extraordinary lustre and beauty.
2. (Alxon or Langlois’ process.) Powdered cochineal, 1 lb., is boiled in river water, 4 galls., for 10 minutes, when carbonate of soda, 3⁄4 oz., dissolved in water, 1 pint, is added, and the whole again boiled for 1⁄2 hour longer; when the decoction is cold, alum (in fine powder), 3⁄4 oz., is thrown in, and the liquid agitated rapidly until it is entirely dissolved; after 20 minutes’ repose it is decanted into another vessel, and clarified by heating it with the whites of 2 eggs; the perfectly clear liquid is then allowed to repose for 40 minutes or longer, when it is decanted, and the carmine which it has deposited is collected, drained on a filter, and dried on shallow plates covered with silver paper. The product by either of the above processes varies from 91⁄2 to 10% on the weight of the cochineal employed in them.
3. (China or Spirit process.) Cochineal, 1 lb., is boiled for 15 minutes, in water, 3 galls., powdered alum, 1 dr., is next added, and the whole again boiled for 5 or 6 minutes; when the liquid has become cold, the clear portion is decanted, and again heated, the solution of tin (spirits of tin) cautiously dropped in until all the carmine is precipitated; it is collected, drained, and dried, as before. Prod. 11⁄2 oz.
3. (French process.) From cochineal (in powder), 1 lb., boiled for 15 minutes, in water, 3 galls.; cream of tartar (in powder), 1 oz., is then added, the boiling further continued for 10 minutes, and powdered alum 11⁄2 oz., thrown in; after another 2 minutes’ boil the heat is withdrawn, and in 5 or 6 minutes more the clear portion is decanted into porcelain vessels, which are set aside until the carmine falls down.
4. (German process.) Powdered cochineal, 1 lb., water, 4 galls.; boil 15 minutes, add powdered alum, 1 oz.; boil 3 minutes longer, remove the heat, allow the liquor to settle for 5 minutes, pour off the clear portion into porcelain or earthenware vessels, and set them aside for 3 or 4 days. The carmine is found deposited on the bottom of the vessel, and must be now carefully drained and dried, as before. The decanted liquor yields more carmine by standing in fresh vessels. Product. About 11⁄2 oz.; besides 1⁄2 oz., or more, of an inferior quality obtained as a second deposit.
5. (English process.) From cochineal, 1 lb., and carbonate of potash, 1⁄2 oz., boiled in water, 7 galls., for 15 minutes; the vessel is then removed from the fire, and powdered alum, 1 oz., added; the liquor is then well agitated and allowed to settle for about 15 minutes longer; the clear liquid is next decanted into a clean copper, and isinglass, 1⁄2 oz., dissolved in water, 1 pint (and strained), added; as soon as a coagulum forms upon the surface, the heat is removed, and the liquid is strongly agitated with a bone or silver spatula, after which it is allowed to repose for 20 or 30 minutes. The deposited carmine must be drained and dried, as before.
Obs. The best black cochineal is generally used for the preparation of carmine. For ordinary qualities spirits of tin (bichloride) is added to the decoction as a precipitant, and the liquid being put into suitable vessels (wash-hand basins answer very well), a deposit of carmine slowly takes place. Neither exposure to solar light nor artificial heat is advisable during the drying, but the latter must nevertheless be effected with all possible expedition. Hence the finer shades of carmine can only be successfully made during certain states of weather; as in very hot weather the liquid rapidly sours or ferments, and the deposit is more or less dissolved; whilst in dull, damp weather it is difficult to dry the precipitate sufficiently, which is then apt to become mouldy, and to lose colour. The researches of Pelletier and Caventou tend to show that the solution of tin used as a precipitant should be at the maximum of oxidation or chlorination, to produce the richest shades of carmine. That first deposited is, in all cases, the most beautiful, and the quality gradually deteriorates as the process proceeds. 6 or 7 dr. only of carmine of the very finest quality can hence be obtained from 1 lb. of cochineal.
Prop., &c. Pure carmine is a very light, lustrous, scarlet powder, entirely soluble in ammonia, a test by which its purity is readily determined. Mr Warren De la Rue says the pure colouring principle of cochineal is carminic acid. By digesting ammonia on carmine until all the colour is taken up, filtering and adding acetic acid and alcohol, till the whole is precipitated; and lastly, carefully washing the precipitate with spirit of wine, at proof, and drying in the shade, carmine of the richest and most lustrous hue may be obtained even from samples of inferior quality.
Uses, &c. As a pigment in velvet and miniature painting, and for tinting artificial flowers, and as rouge for the complexion. The powdered cochineal (carmine grounds), from which the coloured liquor (liquid rouge, carmine liquor) has been decanted, is used by the paper stainers, and both are used in the preparation of carminated lake.
Carmine, Blue. See Indigo.
Carmine, Li′quid. Syn. Fluid carmine, Liquid rouge, Carmine ink. Prep. 1. A solution of carmine in ammonia water, or spirits of hartshorn. Very rich and beautiful.
2. The residual liquor of the process of making carmine. Inferior. The first is used in velvet and miniature painting, and for tinting artificial flowers; the second for common purposes, as a stain or wash.
Carmine, Pur′ple. See Murexide.
CARMIN′IC ACID. C14H14O8. Prep. (W. De la Rue.) The powdered insect, after treatment with ether to remove the fat, is digested in water. The decoction of cochineal is precipitated by adding a solution of acetate of409 lead, and the impure carminate of lead thus formed, after being washed with water, is suspended in water, and decomposed by a stream of sulphuretted hydrogen; the whole process is repeated with the decanted solution so obtained; the second solution is then evaporated to dryness (in vacuo over sulphuric acid), dissolved in absolute alcohol, digested on some washed crude carminate of lead (to separate a little phosphoric acid), and, lastly, mixed with ether (to precipitate some nitrogenised matter); the residuum obtained by careful evaporation (in vacuo) is pure carminic acid.
Prop., &c. A purple-brown mass, yielding a rich-red powder; it is freely soluble in water and alcohol; slightly soluble in ether; and without decomposition in oil of vitriol; it is feebly acid; its salts are termed carminates, only two or three of which have been examined. According to Mr De la Rue, this acid constitutes the pure colouring matter of cochineal.
CARNAUBA ROOT. The root of the corypha cerifera, a wax-bearing palm, growing on the shores of the Rio Francisco, in Brazil. Dr C. Symes (see ‘Pharmaceutical Journal,’ 3rd series, v, 661) says:—Two bales of this root have been imported into Liverpool, with the following remarks in Portuguese:—“This root is recognised by the professor as an excellent purifying agent, and has been successfully applied in the cure of various diseases arising from impurity of the blood. We are indeed astonished that it is not more widely known, as its therapeutic qualities, which are worthy of full credence, rival those of sarsaparilla. The carnauba root likewise has a diuretic power, and possesses unusual efficacy, in the cure of acute and chronic blennorrhœas. It is, furthermore, very cooling, and displays a vigorous action in purifying the blood.” Mr Cleaver, who submitted the root to analysis, found it to contain very minute quantities of an alkaloid, an acrid resinous body, a red colouring matter, a variety of tannic acid, and a small portion of volatile oil.
CAROBA. The leaves of a tree belonging to the family Bignoniaceæ, employed in Brazil as a diaphoretic, diuretic, and alterative tonic. Dr Alt states that he has used them extensively, and with much success, in old-standing cases of syphilitic eruptions, and after a course of mercurial treatment. They are usually administered either in the form of powder or decoction.
CAROT′INE. C18H24O. A crystalline, copper-red substance, obtained from the root of the Daucus carota (sativa) or garden carrot. It is tasteless; odourless; neutral; fusible; inflammable; insoluble in ether and water; slightly soluble in alcohol; and very soluble in the mixed and volatile oils.
CAR′PETS. Consideration of cleanliness and economy demand a few words on carpets and hearth-rugs. We are assured by an experienced person that before proceeding to sweep a carpet, a few handfuls of waste tea-leaves should be sprinkled over it (say some five or six minutes before). A stiff hair broom or hair brush only should be employed unless the carpet be very dirty, when a whisk or carpet-broom may be used first, followed by another made of hair, to take off the loose dust. The frequent use of a stiff “carpet-broom” (those made of cane or birch are here alluded to) soon wears off the beauty of the best carpet. An ordinary clothes-brush, or a clean one, resembling the dirt brush used for shoes, is best adapted for superior carpets. When carpets are very dirty they should be cleaned by shaking and beating. “If you must have a carpet, take it up two or three times a year, instead of once. A dirty carpet literally infects the room: if you consider the enormous quantity of organic matter from the feet of people coming in, which must saturate it this is by no means surprising.” (Miss Nightingale.) In laying down carpets it is very advisable, at first, to cover the floor beneath them with large sheets of thick paper, so as to prevent dust from rising between the boards. Old drugget, sacking, matting, or any similar substance, will effect the same purpose, and will, moreover, materially increase the durability of the carpet, by preserving it from the contact of the hard floor.
Brussels Carpets may be cleaned with ox-gall (1 pint to a pailful of water), and a scrubbing-brush, and floor-cloth; afterwards rinsing them in fresh water applied in the same way. They should be previously perfectly freed from dust by beating, and should be nailed down before commencing the above operations. Great care should be taken to rub them as dry as possible with a clean dry floor-cloth. A small portion only should be done at a time, and a dry windy day selected for the purpose. A carpet treated in this manner will be greatly refreshed in colour, particularly the greens.
Kidderminster Carpets will scarcely bear the above treatment without becoming so soft as to get speedily dirty again. This may in some measure be prevented by brushing them over with a hot weak solution of size in water, to which a little alum has been added. Curd soap, dissolved in hot water, may be used instead of ox-gall, but it is more likely to injure the colours if produced by false dyes. When there are spots of grease on the carpeting they may be covered with curd soap, dissolved in boiling water, and rubbed with a brush until the stains are removed, when they must be cleaned with warm water as before. The addition of a little gall to the soap renders it more efficacious. Some persons employ a mixture of soap, fuller’s earth, and turpentine, for the same purpose. Benzol rapidly removes the grease stains, and may be advantageously substituted for preparations of soap.
CAR′RAGEEN. Syn. I′′rish moss; Chondrus,410 L. The Chondrus crispus of botanists, a well-known alga or seaweed. It contains a large proportion of a peculiar jelly, called carrageen′in or pect′in. This may be purified by agitation with dilute alcohol and filtration. The jelly forms an agreeable article of diet. It is used to a limited extent for thickening colours in calico printing. In medicine, carrageen is used in the form of a jelly and decoction as a demulcent, and is often prescribed in pulmonary complaints. See Fixature, Algæ, Paste, Syrup.
CAR′ROT. Syn. Caro′ta, L. The seed is carminative and diuretic; the expressed juice of the root is anthelmintic. Scraped raw carrot is sometimes employed as a stimulant application to sore nipples; the boiled root as a poultice to sores and tumours. As an article of food, unless young and well dressed, carrots are rather indigestible. Carrots can be kept for many months if the tops are cut out, and they are then placed in damp sand.
| Water | 87·30 |
| Albumenoids | 0·66 |
| Cellular tissue, gum, and non-nitrogenous substance | 2·56 |
| Sugar | 5·54 |
| Fibre | 3·20 |
| Mineral matters | ·74 |
| ——— | |
| 100·00 |
CAR′THAMIN. C14H16O7. Syn. Pure rouge, Saf′flower carmine, Safflower lake. The red colouring matter of Carthamus tinctorius or safflower, formerly much used as a dye, particularly in the form of pink saucers for dyeing stockings.
Prep. 1. Safflower, exhausted by washing it with water (or with water acidulated with acetic acid), is dried, coarsely pulverised, and the powder digested in a weak solution of carbonate of sodium; pieces of clean white cotton or calico are then immersed in the solution, and acetic acid gradually added in slight excess; the cotton is next washed, dried, and digested in a fresh quantity of dilute solution of carbonate of sodium, and agitation employed until the whole of the colour is again dissolved; the new solution is filtered and slightly super-saturated with citric acid (or acetic acid); the carthamin, which falls down in rich carmine-red flocks, is lastly washed with cold distilled water, and dried.
2. Washed safflower (dried and powdered), any quantity; aqueous solution of carbonate of sodium (containing 15% of carbonate), q. s. to form a thick paste; after some hours press out the red liquor, nearly neutralise it with acetic acid, put in cotton as before, and add acetic acid in slight excess; the next day remove the cotton and wash it in water holding in solution 5% of carbonate of sodium, until the colour is dissolved out, after which precipitate with citric acid, as before.
Prop., &c. An amorphous, brilliant, greenish powder; nearly insoluble in water, soluble in alcohol, forming a gorgeous purple solution, and in weak alkaline lyes giving an equally beautiful red one.
CAR′THAMUS. Syn. Saf′flower. In botany, a genus of composite plants, the most important species of which is Carthamus tinctorius, the safflower. The florets of this yield a beautiful pink dye (see above), and are sometimes used to adulterate hay saffron. The “cake saffron” of the shops consists entirely of safflower and mucilage. The fruits, commonly called “seeds,” yield by expression the useful oil known in India as Koosum oil.
CARUM (PTYCHOTIS) AJOWAN. Ind. Ph. Syn. Ajwain or Omum plant. Habitat. Tropical Africa? Much cultivated in India.— Officinal part. The fruit (Fructus Ptychotis, Ajwain fruit). Occurs in the form of minute umbelliferous fruits, which, examined with a lens, are seen to be covered with prominent tubercles, extremely aromatic, evolving, when rubbed, a strong odour resembling that of common thyme. Taste somewhat bitter, and very pungent. Its virtues reside in a volatile oil.—Properties. Valuable stimulant, carminative, and antispasmodic.—Therapeutic uses. In flatulence, flatulent colic, atonic dyspepsia, and diarrhœa, it is a remedy of much value.
Oil of Ajwain, or Omum (Oleum Ptychotis). The oil obtained by distillation from the fruit. Recently prepared, colourless, but soon acquires a yellowish tinge. It has the odour of the fruit, and an acrid burning taste. Sp. gr. about 0·88.—Dose, 1 to 3 drops on sugar or in emulsion.
Ajwain, or Omum Water (Aqua Ptychotis). Take of ajwain fruit, bruised, 20 oz.; water, 2 galls. Distil a gallon.—Dose, 1 to 2 fluid ounces. A valuable carminative; also useful in disguising the taste of disagreeable drugs, especially castor oil, and obviating their tendency to cause nausea and griping.
CARYOPH′YLLIN. C10H16O. Syn. Clove camphor, Clove resin. A crystalline substance, isomeric with ordinary camphor, which deposits from oil of cloves in needles.
CARYOPH′YLLUS. See Clove.
CASCARIL′LA. Syn. Cascarillæ cortex (B. P.), L. The bark of Croton eleutheria or the seaside balsam, a tree growing in the Bahamas and Jamaica. It is an aromatic bitter, stomachic, and tonic—Dose, 10 gr. to 30 gr., in the form of powder, infusion, or tincture; in diarrhœa, dysentery, dyspepsia, low fevers, intermittents, &c.
CASCARIL′LINE. Syn. Cascaril′lina. Prep. (Duval.) Cascarilla is exhausted with cold water by percolation, precipitated with acetate of lead, and the filtrate treated with sulphuretted hydrogen; the filtered liquid, after agitation with animal charcoal and filtration, is gently evaporated to dryness. The powder is redissolved in boiling alcohol and crystallised by very slow or by spontaneous411 evaporation. It has a bitter taste and acid reaction; its aqueous solution is unaffected by the ferric salts and tincture of galls.—Dose, 1 to 3 gr.; in dyspepsia, &c.
CASE-HARD′ENING. Syn. Steel sur′facing. The operation of giving a surface of steel to iron goods. Tools, fire-irons, fenders, keys, &c., are usually case-hardened.
Process. 1. The goods (finished in every respect but polishing) are put into an iron box, and covered with animal or vegetable charcoal, and “cemented” at a red heat for a period varying with the size and description of the articles operated on: these, when taken out, are hardened by plunging into water, or oil, if they are of a delicate nature.
2. (Moxon.) Cow’s horn or hoof is baked or thoroughly dried and pulverised; to this is added an equal quantity of bay salt, and the whole is made into a paste with stale chamber-lye, or white wine vinegar; the iron is covered with this mixture, and bedded in it, in loam, or inclosed in an iron box. In this form it is laid on the hearth of the forge to dry and harden, then it is put into the fire, and blown till the lump has a blood-red heat (no higher). It is hardened as before.
3. Coat the goods with a paste made of a concentrated solution of prussiate of potash and loam; then expose them to a strong red heat, and when it has fallen to a dull red, plunge the whole into cold water.
4. The goods, previously polished and finished, are heated to a bright-red, and rubbed or sprinkled over with prussiate of potash. As soon as the prussiate appears to be decomposed and dissipated the articles are plunged into cold water.
Obs. The process of case-hardening has been well conducted when the surface of the metal proves sufficiently hard to resist a file. The last two plans are a great improvement upon the common method. By the topical application of prussiate of potash (ferrocyanide of potassium) any part of a piece of iron may be case-hardened without interfering with the rest.
Case-hardening Powders. Syn. Case-hardening compositions. 1. Prussiate of potash, dried and powdered.
2. Prussiate of potash, 3 parts; sal-ammoniac, 1 part; mix.
3. Sal-ammoniac and bone-dust, of each 2 parts; prussiate of potash, 1 part. (See above.)
CA′SEIN. Syn. Ca′seum, Ca′sein, Lactalbu′men, Albumen of milk. The nitrogenous principle of milk. Cheese made from skimmed milk and well pressed is nearly pure casein. (Liebig.)
Prep. 1. The curd obtained by adding dilute sulphuric acid to milk is well washed and dissolved in carbonate of soda. It is allowed to stand for 24 hours, to let the oil rise to the surface, and when this is properly skimmed off, the casein is precipitated by an acid. The process is repeated a second time, and the coagulum digested with alcohol and ether, and dried. With all these precautions the casein still contains some saline matter which cannot be removed.
2. Milk is coagulated by hydrochloric acid, and the curd then well washed with dilute acid, and finally with pure water. The curd so prepared is dissolved by digestion at 110° Fahr., with a large quantity of water; the solution, after filtration, is coagulated with carbonate of ammonia; the coagulum is washed with water, ether, and alcohol, and finally dried.
Prop., &c. Coagulated casein is readily dissolved by the alkalies and alkaline carbonates. The most remarkable property of casein is its coagulation by certain animal membranes, as in the process of cheese-making with rennet. See Lactarin.
CASKS. The care and management of casks is an important affair in a large establishment. It is found that they last longest when stored either in a dry situation, or in one uniformly very moist. Continual variations from the one to the other speedily rot them. As soon as casks are emptied they should be bunged down quite air-tight, with as much care as if they were full, by which means they will be preserved both sweet and sound. Should any of the hoops become loose they should be immediately driven up tight, which will at once prevent the liability of their being lost or misplaced, as well as the casks fouling or becoming musty from the admission of air. For this purpose those out of use should be occasionally hauled over and examined.
Numerous plans are adopted for CLEANING and PURIFYING CASKS, among which are the following:—
1. Wash them well out with oil of vitriol, diluted with an equal weight of water.
2. Wash them first with a little chloride of lime and warm water, and then with water soured with oil of vitriol.
3. Match them with sulphur, or with sulphur mixed with a little saltpetre.
4. Unhead them and whitewash them with fresh milk of lime, made pretty strong. This plan is commonly followed for brewers’ vats.
5. Remove the heads, and char the insides of the staves by the aid of a fire of shavings kindled within them.
6. A simpler, safer, and more effectual method of charring them than the last is to wash the dry casks out with strong oil of vitriol (sp. gr. 1·854). This not only purifies the surfaces of the staves, but penetrates into all the cracks, some of which might escape the action of the fire.
7. Steam has lately been applied to the insides of casks with great advantage. High-pressure steam is driven in at the bung-hole, at the same time that the cask is violently agitated (a heavy chain having been previously412 put into it), until all the dirt and bad smell is removed.
8. A lye of pearlash or soda, mixed with milk of lime, as well as strong hot brine, and other similar liquors, have been adopted by some persons, and are highly spoken of.
9. The coopers boil the staves for gin casks in a strong lye of alum before placing them together, to prevent their colouring the spirit, but washing with oil of vitriol is a better plan.
10. Some persons fill musty casks with water and add 3 or 4 lbs. of coarsely powdered fresh burnt charcoal, and agitate well for a few days.
11. Wash with bisulphite of lime.
Obs. In all the above processes the greatest care must be taken to scald or soak and well rinse out the casks after the treatment described. See Brewing Utensils, Sporokton, Matches, &c.
CAS′SAREEP. The expressed juice of the sweet cassava, concentrated by heat and flavoured with aromatics. It is used in the West Indies as a condiment. (See below.)
CAS′SAVA. A poisonous shrub cultivated in the West Indies and in many parts of South America for the sake of the starchy matter contained in its roots. It belongs to the natural order Euphorbiaceæ, and is known to botanists under the names Manihot utilissima (Pohl), Janipha manihot (Humboldt), and Jatropha manihot (Linn.), the former being that now generally adopted. The name “bitter cassava” is commonly given to it in the West Indies, to distinguish it from another species of the same genus, Manihot aipi (Pohl), which, from having no poisonous properties, is named the “sweet cassava.” The roots of both species yield the starch, but those of the poisonous plant are the richer.
The roots, after being well washed and scraped, are rasped or grated, and the pulp thus formed is subjected to strong pressure, to expel the poisonous juice which it contains. The compressed pulp is next thoroughly dried over the fire, being constantly stirred the whole time, by which any remaining portion of the noxious juice is either volatilised or decomposed. It now forms CASSAVA MEAL. When it is further prepared by grinding, it forms FINE CASSAVA MEAL or CASSAVA FLOUR. When the compressed pulp is baked on a hot plate, it forms CASSAVA BREAD or CASSAVA CAKES, the flavour of which greatly resembles that of Scotch oat-cakes. See Tapioca.
CAS′SIA. In botany, a genus of the natural order Leguminosæ, including several important medicinal plants. The “purging cassia,” Cassia fistula (Linn.), produces pods containing a soft, blackish pulp. (See below, also Senna.)
Cassia Pulp. Syn. Cassia præpara′ta, Cassiæ pulpa (B. P.), L. Prep. The cassia (pods or fruit), broken lengthwise, are macerated in sufficient distilled water to cover them for six hours, constantly stirring; and the purified pulp strained through a hair sieve, and evaporated to the consistence of a confection in a water bath.—Dose. As a mild laxative, 1 to 2 dr.; as a purgative, 3⁄4 oz. to 11⁄2 oz.
CAS′SOLETTES (Scented). See Pastilles and Perfumery.
CAS′TOR. Syn. Casto′′reum, L. (B. P.) “The follicles of the prepuce of the Castor fiber or beaver, filled with a peculiar secretion.” (Ph. L.) “A peculiar secretion from the præputial follicles.” (Ph. E. and D.) It is often sophisticated; a fraud readily detected by the “absence of the membranous partition in the interior of the bags, as well as by the altered smell and taste.” (Ure.) Russian castor, which is very rare, may be distinguished by a tincture of 1-16th part in alcohol, being of the colour of deep sherry, while that with American castor is of the colour of London porter. (Pereira.)—Dose, 1 to 2 dr. or more, in powder or made into pills; in nervous and spasmodic affections, especially in hysteria, epilepsy, and other like diseases of females.
CASTOR CAKE. The crushed and closely-pressed seeds of the Ricinus communis, after the expression of the oil, are said to be sometimes employed as a cattle food, and have the following composition:—
| Moisture | 9·95 |
| Organic matter | 81·07 |
| Phosphate of lime and magnesia | 4·49 |
| Alkaline salts | 1·80 |
| Sand | 2·69 |
| ——— | |
| 100·00 |
This cake, even when mixed with large quantities of linseed cake, &c., is intensely poisonous. A pupil of the Editor states, however, that in India castor cake, after exposure to the sun, is commonly and safely used as a food for cattle.
CAS′TOR OIL. See Oils.
CAS′TORIN. Syn. Castorin′a, Castoreum camphor. When castor is cut into small pieces and boiled in about 6 times its weight of alcohol, crystalline substance (castorin) is deposited by the filtered tincture in cooling. By re-solution in alcohol it may be obtained under the form of colourless, prismatic, acicular crystals.
Obs. Genuine Russian castor, although the most expensive, must be employed in the above process, as scarcely any castorin can be obtained from the American variety.
Prop., &c. Castorin has the odour of castor, and a coppery taste; it is inflammable, and is soluble both in ether and hot alcohol.
CASTS. In preparing casts and moulds with gelatin, wax, fusible metal, and similar substances, it is important to use them at the lowest temperature compatible with fluidity; as when only a few degrees hotter the water which adheres to the things from which the413 casts are taken is converted into vapour, and produces bubbles. Fusible metal may be allowed to cool in a teacup until just ready to set at the edges, and then poured into the moulds. In this way beautiful casts from moulds of wood, or of other similar substances, may be procured. When taking impressions from gems, seals, &c., the fused alloy should be placed on paper or pasteboard, and stirred about till it becomes pasty, from incipient cooling, at which moment the gem, die, or seal should be suddenly stamped on it, and a very sharp impression will then be obtained.
CATALEP′SY. Syn. Trance; Catalep′sis, Catalep′sia, L. A disease in which the organs of sense and motion cease to exercise their functions, and the heart and lungs feebly perform their offices, and in a scarcely perceptible manner. The paroxysm generally comes on without previous warning, and its duration varies from a few minutes to several days, and if medical reports are to be credited, sometimes for a much longer period. Dr Cullen seriously affirms that this disease is always counterfeited.
Treat. Ammoniacal stimulants applied to the nostrils, and spirituous liquors injected into the stomach, with general friction of the body, and free access to pure air are the best remedies. Electricity and galvanism should also be had recourse to when the necessary apparatus is at hand.
CAT′APLASMS. See Poultices.
CAT′ARACT. An opaque condition of the lens of the eye. It is a common cause of blindness. It can only be cured by a surgical operation.
CATARRH′. Syn. Catarrh′us, L. The “cold in the head,” or “cold on the chest,” of domestic medicine. Influenza is a severer form of this complaint, and has been called epidemic catarrh.
The common symptoms of catarrh are a copious discharge from the eyes and nose, a hoarseness, and generally a cough, more or less severe. The exciting causes are sudden changes of temperature and exposure to currents of cold air while the body is heated; hence the frequency of colds in hot and changeable weather.
Treat. A light diet should be adopted, and animal food and fermented and spirituous liquors should be particularly avoided. Some mild aperient should be administered; and when the symptoms are severe, or fever or headache is present, small diaphoretic doses of antimonials, accompanied by copious draughts of diluents, as barley water, weak tea, or gruel should be taken. This treatment, except in very bad cases, will generally effect a cure.
In HORSES catarrh is caused by sudden changes of temperature, draughts, and faulty ventilation. Let the animal have plenty of cool fresh air, the body being kept warm by means of horse-cloths and bandages. If necessary, give a mild physic-ball, or a clyster; keep it on a soft, laxative diet, and give it an ounce of nitre daily. Should there be sore throat or troublesome cough apply a mild blister of cantharides or mustard.
The following will be found a serviceable mixture:—Mendererus spirit, 11⁄2 oz.; sweet spirit of nitre, 2 drachms; syrup of sugar, 1⁄2 oz.; camphor mixture, enough to make a 6-oz. mixture. An adult may take two table-spoonfuls of this mixture every 3 or 4 hours. Should the cold in the head be severe and accompanied with cough, it has been recommended to inhale the vapour of pure washed ether by drawing it alternately into the nostrils from a wide-mouthed bottle holding about an ounce, and clutching it in the warm hand until about a fourth of the ounce has been volatilised. This repeated two, three, or four times in 48 hours is said to effect a cure within that time. Persons liable to colds are advised to use the cold bath.
Dr Ferrier’s Remedy for a Cold in the Head.—Hydrochlorate of morphia, 2 gr.; powdered gum Arabic, 2 drachms; subnitrate of bismuth, 6 drachms. Mix. Let a very small quantity be sniffed up the nose every five minutes for 20 or 30 minutes.
Another remedy: Carbolic acid, 10 drops; tincture iodine; chloroform, of each 71⁄2 grams. Place a few drops in a test-tube, and heat cautiously over a spirit-lamp, and when it boils remove, and inhale by the nose. Repeat after a few minutes. Two inhalations are said to be sufficient to cure a cold in the head. (‘Year-book of Pharmacy.’)
CAT′ECHIN. Syn. Catechu′ic acid, Resinous tan′nin. When cubical gambir or catechu, in powder, is treated with cold water, a portion remains undissolved. This is catechin. By repeated solutions in alcohol it may be obtained under the form of white, silky, acicular crystals.
Prop., &c. Catechin strikes a green colour with the salts of iron, but does not precipitate gelatin. When dissolved in caustic potassa, and the solution exposed to the air, it absorbs oxygen, and japonic acid is formed. If, instead of caustic potassa, carbonate of potassa is employed, it is converted into rutic acid.
CAT′ECHU. Syn. Cas′′hew, Cutch, Gam′bir; Cat′echu (Ph. L. E. & D.), Ter′ra Japon′ica, L.; Cachou, Fr. “The extract from the wood of Acacia Catechu, or from the leaf of Uncaria Gambir.” (Pale catechu, Catechu Pallidum, B. P.) Also of the “kernels of areca catechu; probably, too, from other plants.” (Ph. E.) The term is now applied to several extracts similar in appearance and properties to that of Acacia Catechu.
There are several varieties of catechu known in commerce, of which the principal are—
Catechu, Bombay. Firm, brittle, dark brown, of a uniform texture, and a glossy,414 semi-resinous, and uneven fracture, Sp. gr. 1·39. Richness in tannin, 52%.
Catechu, Bengal. Rusty brown colour externally; porous, and more friable than the preceding. Sp. gr. 1·28. Richness in tannin, 49·5%.
Catechu, Malabar. Resembles the last in appearance, but is more brittle and gritty. Sp. gr. 1·40. Richness in tannin, 45·5%.
Of the above varieties the first is the one generally employed in medicine, and which commonly passes by the name of catechu. The second popularly passes under the name of terra Japonica (Japan earth), from the old belief that it was of mineral origin.
Catechu, Pale, is prepared at Singapore and in the Eastern Archipelago. It generally occurs in cubical reddish-brown pieces, porous, bitter, and astringent in taste. Entirely soluble in boiling water; the solution, when cold, is not rendered blue by iodine. Of 100 parts, only 60 are dissolved by cold water, and the solution is bright. Thirty parts of isinglass precipitate the whole of the astringent matter.—Test. Sp. gr. 1·39. “The pale catechu being already in the Edin., the B. P. 1864 retained it with the black; but the black is the one adopted by all other pharmacopœias, and is preferred in the arts and manufactures; it is well known to be far superior to the pale in astringency, and is always to be had of good quality; it is therefore a matter of surprise and regret that it has been rejected from the ‘British Pharmacopœia.’” (Squire.)
Estim. It is often of importance to the tanner and dyer to determine the richness of this article in tannic acid or tannin. The following are two simple methods:—
1. Exhaust a weighed sample (in powder) with ether, and evaporate by the heat of a hot-water bath. The product, which is the tannin, must then be accurately weighed.
2. Dissolve the sample (in powder) in hot water, let it cool out of contact with the air, filter, and add a solution of gelatin as long as a precipitate falls. The precipitate, after being washed and dried at a steam heat, contains 40% of tannin.
Uses, &c. Catechu is extensively employed in medicine, both internally and externally, as an astringent. It is used to flavour British brandy, and by the tanners as a substitute for oak bark. With it the dyer produces, inexpensively, many of his most pleasing browns. Alum mordants are mostly employed in dyeing with catechu. “The salts of copper with sal-ammoniac cause it to give a BRONZE COLOUR, which is very fast; the protochloride of tin, a BROWNISH YELLOW; the perchloride of tin, with the addition of nitrate of copper, a DEEP-BRONZE HUE; acetate of alumina, alone, a REDDISH BROWN, and with nitrate of copper, a REDDISH-OLIVE GREY; nitrate of iron, a DARK-BROWN GREY. For dyeing a GOLDEN COFFEE-BROWN, it has entirely superseded madder; 1 lb. of it being equivalent to 6 lbs. of this root.” (Ure.)—Dose, 10 gr. to 30 gr. in solution, in water, or made into a bolus, or sucked as a lozenge.
CAT′GUT. The prepared and twisted intestines of animals. Prep. The guts, taken whilst warm from the animal, are thoroughly cleaned, freed from adherent fat, and well rinsed in pure water. They are next soaked for about 2 days in water, after which they are laid on a table and scraped with a copper plate, having a semicircular notch, beginning the operation at the smaller end. In this way the mucous and peritoneal membranes are removed. The guts are then put into fresh water, and soaked until the next day, when they are again scraped, the larger ends cut off, and after well washing, again steeped for a night in fresh water, and then for 2 or 3 hours in a weak lye of pearlash or potash (2 oz. to the gall.) They are lastly washed in clean water, and passed through a polished hole in a piece of brass to smooth and equalise their surface; after which they are twisted, and sorted, according to the purposes for which they are intended. For many purposes the prepared gut is dyed or sulphured, and rubbed with olive oil. It improves by age. Red or black ink, or any of the simple dyes or stains, are used to colour it.
Uses, &c. Catgut is employed in several of the arts. The strings of harps, violins, &c., are formed of this material. Whipcord is made from catgut, which is sewed together while soft with the filandre or scrapings, after which it is put into a frame and twisted. Bowstrings for hatmakers are made out of the largest intestines, 4 to 12 of which are twisted together, until the cord is extended to 15 to 25 feet in length. It is then rubbed perfectly smooth and free from knots, half dried, sulphured twice, again stretched and sulphured, and lastly dried in a state of tension. Clock-makers’ cords are made of the smallest intestines in a similar manner.
The best fine catgut is made at Venice or Rome, from the intestines of thin, sinewy sheep. That made in England is formed from the fat sheep killed for the shamble, and is, hence, inferior. Coarse catgut, for turning lathes, &c., is made from the intestines of horses, cut into 4 or 5 strips, by forcing a ball furnished with projecting knives placed cross-wise along them. These strips are next twisted, dried, and rubbed smooth with fish skin. Gutta percha and vulcanised india rubber are now applied to many of the purposes formerly exclusively occupied by catgut.
CATHAR′TICS. See Purgatives.
CATHAR′TIN. The purgative principle of senna, first noticed by Lassaigne and Fenuelle. A strong aqueous infusion of senna leaves is evaporated to the consistence of a syrup, out of contact with the air; this fluid extract is then digested in alcohol or rectified spirit, and415 the tincture, after filtration, is evaporated to dryness by a gentle heat.
Prop., &c. A reddish-coloured, uncrystallisable mass; having a peculiar odour and a bitter, nauseous taste; freely soluble in both water and alcohol, and strongly cathartic. Two or three grs. cause nausea, griping, and purging. It has been proposed to employ it, combined with aromatics, as a cathartic.
CATH′ETERS. Small tubes introduced into the bladder for the purpose of drawing off its contents. They may be regarded as hollow bougies.
Prep. 1. A piece of smooth catgut, or steel wire, bent to the proper shape, is coated with melted wax. When cold it is dipped repeatedly into an ethereal solution of india rubber, until a sufficient thickness is obtained, after which it is dried by a gentle heat, and then boiled in water to melt out the wax, and to allow the catgut to be withdrawn. A solution of india rubber in bisulphide of carbon is now generally employed instead of an ethereal solution.
2. From slips of india rubber, as directed under Bougies.
3. A smooth tissue of silk is woven over a bent wire, and then coated with a surface of india rubber, or elastic varnish, and finished off as before. See Bougies.
CAUDLE. Gruel enriched by various additions.
Prep. 1. Thick oatmeal gruel mixed with about one half its weight of good mild ale (made hot), and as much sugar, and mace, nutmeg, or ginger, as will make it agreeable.
2. To the last add an egg, well beaten.
3. Sugar, 3 or 4 lumps; hot water, a table-spoonful; dissolve; add 1 egg; beat well together; further add a glass of wine and a little nutmeg or ginger; mix well, and stir the mixture into good gruel (hot), 3⁄4 pint.
Uses, &c. A nourishing and restorative mixture during convalescence, much used among certain classes after accouchement. It is an excellent domestic remedy for colds, &c., unaccompanied with fever; for which purpose it should be taken on retiring to rest at night, preceded by a dose of castor oil during the day.
CAULIFLOWER. Like the cabbage, the cauliflower forms a very nutritious article of diet; rich in albumenoids and phosphates. The ash, as will be seen from the subjoined analysis, contains a large amount of mineral matter:—
| Potash | 34·39 |
| Soda | 14·79 |
| Magnesia | 2·38 |
| Lime | 2·96 |
| Phosphoric acid | 25·84 |
| Sulphuric acid | 11·16 |
| Silica | 1·92 |
| Phosphate of iron | 3·67 |
| Chloride of sodium | 2·78 |
CAUS′TIC. Syn. Caus′ticum, Escharot′i-cum, L. A substance that corrodes or destroys the texture of organised bodies. This action is popularly termed “burning.”
The principal caustics are nitrate of silver, caustic potassa, a mixture of caustic potassa and quick-lime, sulphate of copper, red oxide of mercury, verdigris, tincture of sesquichloride of iron, chloride of zinc, chloride of antimony, nitric acid, acetic acid, and carbolic acid.
Use. Caustics are employed to remove excrescences, morbid growths, granulations, &c., as corns, warts, and proud flesh; and to open issues, abscesses, &c. The first, second, and fourth are applied by gently rubbing them on the part previously moistened with water; the third is commonly made into a paste, with rectified spirit or glycerin, before application; red oxide of mercury and verdigris (in the form of powder) are often sprinkled over foul and indolent ulcers; whilst the acids and other liquid caustics are applied with a feather, camel-hair pencil, or glass rod. The same applies to the liquid preparations below. In all cases care should be taken to confine the application to the affected part.
Caustic, Ammoni′acal. See Ointments, and Caustic, Gondret’s.
Caustic, Antimo′′nial. Syn. Causticum antimonia′le, L. Chloride of antimony.
Caustic, Arsen′ical. Syn. Causticum arsenica′le, C. arsenio′sum, C. a. compos′itum, L. Prep. 1. See Caustic, Plunket’s.
2. (Cutan. Hosp.) Calomel, 21⁄2 oz.; red sulphide of mercury, 1 dr.; arsenious acid, 1 dr. to 2 dr.
3. (Van Mons.) Arsenious acid, 6 dr.; dragon’s blood, 2 dr.; animal charcoal, 11⁄2 dr.; cinnabar, 3 oz.
4. (Ratier.) Arsenious acid, 1 part; kino, 8 parts; cinnabar, 16 parts. The ingredients of the last three must be separately reduced to fine powder, and then carefully mixed. They are favourite applications on the Continent, in cases of cancer, cancerous sores, obstinate lepra, &c. They are either dusted over the part, or are made into a paste with mucilage or the saliva, and applied like an ointment on a piece of rag or lint; due caution being observed, and the effects watched. The last is much used in the French hospitals.
Caustic, Canquoin’s. See Zinc Caustic.
Caustic, Canthar′ides. Syn. Causticum Canthar′idis, L. Prep. 1. Powdered cantharides made into a paste with concentrated acetic acid.
2. (Cutan. Hosp.) Tannin, 1 oz.; cantharides (powdered), 2 oz.; strong acetic acid, 8 oz.; digest a week, and strain. Blisters.
Caustic, Common. See Potassa (Hydrate of), and Caustic of Potassa with Lime.
Caustic, Duville’s. Prep. 1. Aloes, 5 oz.; proof spirit, 10 oz.; oil of vitriol, 6 oz.; mix.
2. Aloes (in powder), 21⁄2 oz.; rum, 1⁄4 pint; mix, and the next day add, oil of vitriol, 1 oz.416 A favourite caustic in veterinary practice; especially in foot-rot.
Caustic, Filho’s. Prep. From caustic potassa, 2 parts; quick-lime (in powder), 1 part; melt together in a ladle, mix well, and pour it into small leaden tubes, the size of a large swan-quill. When cold, coat each piece with melted beeswax, to exclude the air. Used as a strong caustic in veterinary practice. It is applied like nitrate of silver.
Caustic, Golden. Syn. Caustic of chloride of gold; Causticum aur′eum, C. aur′′ii chlor′idi, L. Prep. 1. (Recamier.) Terchloride of gold, 6 gr.; nitro-hydrochloric acid, 1 oz.; dissolve.
2. (Legrand.) As the last, but using nitric acid. Both are recommended as caustics in syphilitic, scrofulous, and scorbutic ulcers, cancerous growths, &c.; applied by means of a dossil of lint.
Caustic, Gondret’s. Syn. Gondret’s ammoni′acal caustic; Pommade de Gondret, Fr.; Causticum ammoniaca′le, L. Prep. 1. See Ointment, Ammoniacal.
2. (Original formula.) Almond oil, 2 dr.; suet, 4 dr.; lard, 6 dr.; melt together in a wide-mouthed bottle, cool a little, add solution of ammonia, 12 dr.; and agitate until cold. A powerful rubefacient and counter-irritant; used to produce an immediate revulsion. If covered with a compress, it raises a blister in 4 or 5 minutes.
Caustic, I′odine. Syn. Causticum iodin′ii, L. Prep. (Lugol.) Iodine and iodide of potassium, of each 1 part; water 2 parts; dissolve. Used in similar cases to iodine paint, and to scrofulous growths and ulcers.
Caustic, Lu′′nar. Syn. La′pis inferna′lis, L. Prep. 1. Nitrate of silver fused and formed into sticks by pouring it into moulds.
2. (E. R. Squibb.) Nitrate of silver fused with a small quantity of chloride of iron, and formed into sticks or points. The chloride of iron gives toughness to the caustic.
Caustic, Mercu′′rial. Syn. Caustic of nitrate of mercury; Causticum ac′idi hydrar′′gyri nitra′tis, C. h. deutronitratis, L. From mercury, 1 part; commercial nitric acid, 2 parts; dissolve.
2. (Cutan. Hosp.) Mercury, 1 part; nitric acid (sp. gr. 1·5), 2 parts.
3. (P. C.) As No. 1, but evaporating the solution to 3⁄4ths its weight. These liquids are applied with a pencil or lint, in scrofulous and syphilitic ulcers and eruptions, and in lupus, psoriasis, lepra, and other obstinate skin diseases; but their use requires great care.
4. (With arsenic.—Cutan. Hosp.) Mercury, 1⁄2 oz.; nitric acid, 1⁄2 oz.; arsenious acid, 1⁄2 dr.; as before.
Caustic, Ni′tric. Syn. Solid′ified nitric acid; Causticum ni′tricum, L. Prep. (Dr Rivallie.) Concentrated nitric acid is gradually dropped on a piece of lint, placed in a saucer or glass; as soon as the lint is gelatinised, it is pressed into a suitable shape with a glass rod, and applied to the part; it must be removed in 15 minutes. In cancerous tumours, fungoid growths, &c.
Caustic, O′′piated. Syn. Causticum opia′tum, L. Prep. 1. Common caustic (potassa with lime), 4 dr.; powdered opium, 1 dr.; soft soap, q. s. to make a paste. Applied to fungous ulcers.
Caustic, Plunket’s. Upright crowfoot and lesser spear-wort, of each 1 oz.; sulphur, 5 scrup.; white arsenic (in very fine powder), 1 dr.; beat to a smooth paste, form it into balls, and dry them in the sun. In cancer; a portion of one of the balls is reduced to powder, which is mixed up with yelk of eggs, and applied on a piece of bladder.
Caustic of Potassa with Lime. Syn. Vienna paste. Rub together equal parts of hydrate of potash and quick-lime, and keep the powder in a well-stoppered bottle.
Caustic, Poten′tial. Fused caustic potassa.
Caustic, Recamier’s. See Caustic, Golden.
Caustic, Sulphu′′ric. Syn. Causticum sulphu′′ricum, C. ac′idi sulphu′′rici, L. Prep. 1. Plaster of Paris made into a paste with oil of vitriol.
2. Saffron, lint, or unsized paper, soaked in oil of vitriol, and triturated to a plastic mass.
Caustic, Zinc. Syn. Caustic of chloride of zinc, Dr Canquoin’s cancer caustic; Causticum zinc′i, C. z. chlorid′i, L. Prep. 1. (Dr Canquoin.)—a. From chloride of zinc, 1 dr.; flour, 2 dr.; made into a stiff paste with water, q. s.
b. From chloride of zinc, 1 dr.; flour, 3 dr.; water, q. s.; as the last.
c. From chloride of zinc, 1 dr.; flour, 4 dr.; water, q. s.; as before.
d. From chloride of zinc, 2 dr.; chloride of antimony, 1 dr.; flour, 5 dr.; as before.
Powdered opium may be mixed with either of the preceding to mitigate the pain.
2. (Alex. Ure.) As above, but substituting plaster of Paris for the flour there ordered.
Uses, &c. As a caustic in cancer, lupus, skin-marks (nævi), &c. It is formed into small cakes or wafers not exceeding 1 or 2 lines in thickness, one of which is applied to the part, and allowed to remain on from 6 to 12 hours, when it is removed, and the part covered with a poultice. It produces an eschar, often exceeding a quarter of an inch in depth. The chlorides must be in the form of powder, and well mixed with the flour previously to adding the water. The last (No. 1, d) is recommended in nodulated cancerous tumours.
CAUS′TICS (Ve′terinary). In veterinary practice, any of the substances enumerated in the forgoing list may be employed; but nitric acid, sulphuric acid, carbolic acid, chloride of zinc, and nitrate of silver, are those most commonly used. See Veterinary Medicines.
CAV′IARE. Syn. Cav′iar, Cav′iale. The salted roe of several species of sturgeon. It is much esteemed by the Russians, as well as by some other nations of northern Europe,417 and is occasionally eaten as a delicacy in this country. It is, however, very oily, indigestible, and unwholesome.
CAYENNE′. See Capsicum, Peppers.
CEDAR-WOOD (Oil of). See Oils.
Cedar-Wood (Tincture of). See Tinctures.
CE′DRAT. See Liqueurs.
CE′DRENE and CE′DROLA. The oil of cedar-wood, by careful distillation, is separable into two substances—a solid crystalline compound (cedrola), and a volatile liquid hydrocarbon (cedrene). The first may be converted into the other by distillation with phosphoric anhydride.
CELLULARES. In botany, a name given to cryptogams, or flowerless plants, upon the supposition that they consist entirely of cells.
CEL′LULOSE. See Lignin.
CEMENT′. Syn. Cement′tum, L. Any substance which, when applied to the surfaces of other bodies, causes them to adhere together when placed in contact. Those referred to below are amongst the most useful preparations of this class. The term cement is also applied by builders and architects to several species of mortars and like compositions employed either to unite stones and bricks into masses, or as a protective covering against the weather or water, or to make statues, cornices, and similar ornamental articles.
In general the thinner the stratum of interposed cement, the stronger is the junction of the surfaces operated on. This caution is necessary, as in their anxiety to unite broken articles persons generally defeat themselves by spreading the cement too thickly on the edges of the fracture; whereas the least possible quantity should be used, so as to bring the edges as close as possible together.
Cement, Al′abaster. 1. Prom plaster of Paris (in fine powder), made into a cream with water, and at once applied.
2. Yellow resin, 2 parts; melt and stir in plaster of Paris, 1 part.
3. Yellow resin, beeswax, and plaster of Paris, equal parts.
4. Resin, 8 parts; wax, 1 part; melt and stir in plaster of Paris, 4 parts, or q. s.
5. Sulphur or shell-lac, melted with sufficient plaster of Paris or colouring matter to give the desired shade. Used to join or mend pieces in alabaster, white marble, Derbyshire spar, porphyry, and other like substances; and to fill up cracks, supply chips out of corners, &c. The last four are applied hot, the surfaces to be united having been previously warmed. See Cement, Waterglass.
Cement, Architect′ural. 1. From paper (reduced to a smooth paste by boiling it in water), sifted whiting, and good size, equal parts, boiled to a proper consistence.
2. Paper paste, size, and plaster of Paris, equal parts; as before.
Obs. This is a species of papier-maché. It is used to make architectural ornaments, busts, statues, columns, &c. It is very light, and receives a good polish, but will not stand the weather unless it is well varnished or painted.
Cement, Arme′nian. Syn. Diamond cement, Persian c., Turkish c., Jewellers’ c. The jewellers of Turkey, who are mostly Armenians, have a singular method of ornamenting watch-cases, &c., with diamonds and other precious stones, by simply gluing or cementing them on. The stone is set in silver or gold, and the lower part of the metal made flat, or to correspond with the part to which it is to be fixed; it is then gently warmed, and the glue is applied, which is so very strong that the parts thus cemented never separate. This glue will strongly unite pieces of glass and china, and even polished steel, and may be applied to a variety of useful purposes.
Prep. 1. (Original Armenian formula; Eton.) Dissolve five or six bits of gum mastic, each the size of a large pea, in as much rectified spirit of wine as will suffice to render it liquid; and, in another vessel, dissolve as much isinglass, previously a little softened in water (though none of the water must be used), in French brandy or good rum, as will make a two-ounce phial of very strong glue, adding two small bits of gum galbanum or ammoniacum, which must be rubbed or ground till they are dissolved. Then mix the whole with a sufficient heat. Keep the glue in a phial closely stopped, and when it is to be used set the phial in boiling water.
2. (Keller’s Armenian Cement.) Soak isinglass, 1⁄2 oz., in water, 4 oz., for 24 hours; evaporate in a water bath to 2 oz.; add rectified spirit, 2 oz., and strain through linen; mix this, whilst warm, with a solution formed by dissolving gum mastic (best), 1⁄4 oz., in rectified spirit, 2 oz.; add of powdered gum ammoniac 1 dr., and triturate together until perfectly incorporated, avoiding loss of the spirit by evaporation as much as possible.
3. (Ure’s Diamond Cement.) Isinglass, 1 oz.; distilled water, 6 oz.; boil to 3 oz., and add rectified spirit, 11⁄2 oz.; boil for a minute or two, strain, and add, while hot, first a milky emulsion of ammoniac, 1⁄2 oz., and then tincture of mastic, 5 dr.
4. Isinglass soaked in water and dissolved in spirit, 2 oz. (thick); dissolve in this 10 gr. of very pale gum ammoniac (in tears), by rubbing them together; then add 6 large tears of gum mastic, dissolved in the least possible quantity of rectified spirit.
5. Isinglass dissolved in proof spirit (as above), 3 oz.; bottoms of mastic varnish (thick, but clear), 11⁄2 oz.; mix well.
Obs. When carefully made, this cement resists moisture and dries colourless. As usually met with, it is not only of very bad quality, but sold at exorbitant prices. “Some persons have sold a composition under the name of Armenian cement in England; but this composition is badly made; it is much too418 thin, and the quantity of mastic is much too small.” (Eton.) Methylated spirit may be used instead of the pure spirit in the above preparations. Mastic and mastic varnish are also used by jewellers as cements.
Cement, Beale’s. Chalk, 60 parts; lime and salt, of each 20 parts; Barnsey sand, 10 parts; iron filings or dust, and blue or red clay, of each 5 parts; grind together and calcine. Patented as a fire-proof cement.
Cement, Boil′er. Prep. Dried clay in powder, 6 lbs.; iron filings, 1 lb. Make into a paste with boiled linseed oil. Used to stop cracks and leaks in iron boilers, stoves, &c. See Cement, Iron, Steam-boiler c.
Cement, Bot′any Bay. Yellow gum (Botany Bay gum) and brickdust, equal parts, melted together. Used to cement coarse earthenware, &c.
Cement, Bot′tle. Prep. 1. Resin, 1 lb.; tallow or suet, 1⁄4 lb.; melt together, and stir in the colouring matter.
2. Resin, 5 lbs.; beeswax, 1 lb.; colouring, q. s.; as last.
3. (Red.) To each pound of the above add whiting (dry), 3 oz., and light red (burnt ochre), 4 oz.; or red bole, q. s. (all in fine powder).
4. (Black.)—a. To each pound of No. 1, or No. 2, add ivory black (bone black), q. s.
b. From black pitch, 6 lbs.; ivory black and whiting of each, 1 lb.; melted together. Used in the same way as common sealing-wax for bottle corks, cask bungs, &c. See Cement, Maissiat’s.
Cement, Brim′stone. Melted brimstone, either alone, or mixed with resin and brickdust. Cheap and useful.
Cement, Bru′yere’s. Clay, 3 parts; slaked lime, 1 part; mix and expose them to a full red heat for 3 hours, then grind to powder. Recommended as an hydraulic cement.
Cement, Build′ing. Syn. Artificial puzzolene′. From a mixture of clay or loam, broken pottery, flints, or siliceous sand, or broken bottle glass, and wood ashes, exposed to a considerable heat in a furnace, until it becomes partially vitrified; it is then ground to fine powder, sifted, and mixed with one third its weight of quick-lime, also in fine powder, after which it must be packed (tight) in casks to preserve it from the air and moisture. For use it is mixed up with water and applied like Roman cement.
Cement, Cap. Prep. 1. Resin, 5 lbs.; beeswax and dried Venetian red, of each 1 lb.; melted together.
2. (C. G. Williams.) Equal weights of red lead and white lead. Used for chemical and electrical purposes. For cementing glass tubes, necks of balloons, &c., into metal mountings. No. 2 is preferable to white lead alone, and may be depended on for temperature up to 212°.
Cement, Cheese. From grated cheese, 2 parts; quick-lime (in fine powder) 1 part; white of egg, q. s.; beat to a paste. Used for earthenware, &c.
Cement, Chem′ical. Syn. Soft cement. Prep. From yellow wax, 4 parts; common turpentine, 2 parts; Venetian red (well dried), 1 part; melted together. Used as a temporary stopping or lute for the ends or joints of tubes, which are not exposed to much heat; as in alkalimetry, &c. See Cement, Electrical.
Cement, Chi′nese. Syn. Shell-lac cement, Liquid glue. Prep. 1. Finest pale orange shell-lac (broken small), 4 oz.; rectified spirit (strongest), 3 oz.; digested together in a corked bottle in a warm place until dissolved. Very strong and useful; almost odourless. It should have about the consistence of treacle.
2. As before, but using rectified wood naphtha as the solvent. Inferior to the last, but excellent for many purposes.
3. (Without spirit.) Prep. Borax, 1 oz.; water, 3⁄4 pint; shell-lac, 3 oz.; boil in a covered vessel until dissolved, then evaporate to a proper consistence. Cheap and useful, but dries slowly.
4. Macerate for several hours, 6 parts of glue, in small pieces, in 16 parts of water; then add 1 part of hydrochloric acid, and 11⁄2 part of sulphate of zinc; and let the mixture be kept for 10 or 12 hours at a temperature of 68° or 70° C.
Uses, &c. Employed to mend glass, china, fancy work, jewelry, &c., for which it is only inferior to Armenian cement. The first formula produces a cement so strong that pieces of wood may be joined together, cut slopingly across the grain, and will afterwards resist every attempt to break them at the same place. In many of the islands of the Indian Ocean, in Japan, China, and the East Indies, a similar cement is used to join pieces of wood for bows, lances, &c. The fluid is thinly smeared over each face of the joint, a piece of very thin gauze interposed, and the whole pressed tightly together and maintained so until the next day. Joints so made will even bear the continual flexure of a bow without separating. It is admirably adapted for fishing rods. The product of the second formula is commonly sold as LIQUID GLUE. That of the last is much used by the druggists and oilmen, instead of gum, for fixing paper labels to tin, and to glass when exposed to damp.
Cement, Cop′persmiths’. Syn. Blood cement. From bullock’s blood thickened with finely powdered quick-lime. Used to secure the edges and rivets of copper boilers, to mend leaks from joints, &c. It must be used as soon as mixed, as it rapidly gets hard. It is cheap and durable, and is suited for many other purposes.
Cement, Curd. Prep. 1. The curd of skimmed milk (obtained by the addition of vinegar or rennet) is beaten to a paste with quick-lime, in fine powder.
2. Add vinegar, 1⁄2 pint, to skimmed milk, 1⁄2419 pint; mix the curd with the whites of 5 eggs; well beaten and powdered quick-lime, q. s. to form a paste. Used for mending glass and earthenware; they resist water and a moderate degree of heat.
3. Rub from two to four parts of the curd, with a cold solution of borax, till a thick liquid is obtained, that becomes clear on standing. This is an excellent cement for artificial meerschaums, and may be used to give consistency to silk goods, or to coat artificial flowers, and court plaster, to the latter of which it imparts more adhesiveness and firmness.
Cement, Cut′lers’. Prep. 1. Black resin, 4 lbs.; beeswax, 1 lb.; melt, and add finely powdered and well-dried brickdust, 1 lb.; mix well.
2. Equal weights of resin and brickdust, melted together.
Use. To fix knives and forks in their handles. It is put into the hollow of the handle, and the metal, previously made hot enough to melt the composition, pressed into its place whilst warm, and the whole kept upright and still until quite cold.
Cement, Di′amond. See Cement, Armenian.
Cement, Egg. White of egg thickened with finely powdered quick-lime. Used to mend earthenware, glass, china, marble, alabaster, spar ornaments, &c. It does not resist long exposure to moisture unless it has been exposed to heat.
Cement, Elas′tic. Prep. 1. Caoutchouc (in small pieces), 1 part; chloroform, 3 parts; dissolve.
2. (Lenher.) Caoutchouc, 5 parts; chloroform, 3 parts; dissolve, and add gum mastic (powdered), 1 part. Elastic and transparent.
3. Gutta percha, 3 parts; caoutchouc, 1 part (both cut small); bisulphide of carbon, 8 parts; mix in a close vessel and dissolve by the heat of a water bath. This is to be gently warmed before it is applied.
4. Gutta percha, 1 lb.; caoutchouc, 4 oz.; pitch, 2 oz.; shell-lac, 1 oz.; linseed oil, 2 oz.; melted together. This must be melted before being applied.
Obs. The cements 1 and 2 are elastic and transparent, and are applicable to many uses. The others, 3 and 4, are used for uniting leather, cloth, &c.
Cement, Elec′trical. Syn. Chemical cement. From black resin, 7 lbs.; red ochre, 1 lb.; plaster of Paris, 1⁄2 lb. (both well dried and still warm); melted together, and the heat and agitation continued until all frothing ceases, and the liquid runs smooth; the vessel is then withdrawn from the fire, and the mixture stirred until cooled sufficiently. Used to cement the plates in galvanic troughs, join chemical vessels, &c. See Cement, Cap, Cement, Singer’s, &c.
Cement, Engineers′′. Prep. 1. Ground white lead, mixed with as much red lead as will make it of the consistence of putty.
2. Equal weights of red lead and white lead, mixed with boiled linseed oil, to a proper consistence. Used by engineers and others to make metallic joints. A washer of hemp, yarn, or canvas, smeared with the cement, is placed in the joint, which is then “brought home,” or screwed up tight. It dries as hard as stone. It also answers well for joining broken stones, however large. Cisterns built of squares stones, put together, while dry, with this cement, will never leak or come to repair.
Cement, Extempora′′neous. 1. Shell-lac, melted, and run into small sticks the size of a quill. Used to join glass, earthenware, &c. The edges are heated sufficiently hot to melt the cement, which is then thinly smeared over them, and the joint made while they are still hot. This is the cement so commonly vended in the streets of London, and which used to surprise us in our boyhood days.
2. Tears of gum mastic, used in the same way. Commonly employed by jewellers and others.
Cement, Fire′proof. Prep. From fine river sand, 20 parts; litharge, 2 parts; quick-lime, 1 part; linseed oil, q. s. to form a thin paste. Applied to walls, it soon acquires a stony hardness. It is also used to mend broken pieces of stone, stone steps, &c. See Cement, Beale’s, &c.
Cement, Flour. Syn. Paste, Flour paste. This useful and well-known article is made by mixing about a tablespoonful of wheat flour with cold water, (say) 1⁄2 pint, adding the latter gradually, and thoroughly stirring in each portion before pouring in more; the vessel is then placed over the fire, and the whole assiduously stirred until it boils, great care being taken to prevent caking on the bottom, or burning. Some persons add about 1⁄3 of a teaspoonful of powdered alum to the water, which is said to strengthen the product; the shoemakers add a little quantity of powdered resin to the flour, with the same intention. The addition of a little brown sugar and a few grains of corrosive sublimate will prevent it turning mouldy, and is said to preserve it for years. When too hard or dry, it may be softened by beating it up with a little hot water.
Cement, French. Mucilage of gum Arabic, thickened with starch powder or farina; a little lemon-juice is sometimes added. Used by naturalists in mounting specimens; by artificial-flower makers; and by confectioners, to stick paper, wafer papers, ornaments, &c., on their fancy cakes. Plain mucilage is often used in the same way.
Cement, Gad’s. Syn. Gad’s hydraulic cement. From clay (well dried and powdered), 3 parts; oxide of iron, 1 part; mixed together, and made into a stiff paste with boiled oil. Used for work required to harden under water.
Cement, Glass. Syn. Glass flux. Prep.420 Red lead, 3 parts; fine white sand, 2 parts; crystallised boracic acid, 3 parts; mixed and fused; it is levigated, and applied with thin mucilage of tragacanth. Used for mending broken china, &c. The repaired article must be gently heated, so as partially to fuse the cement.
Cement, Gibbs’. Mr Gibbs patented in 1850 various processes for making admirable building and architectural cements, equal in hardness and duration, and superior in colour, to the best Roman and Portland cements at present in use. His materials are obtained from “the vast beds of (natural) argillaceous marls and marly limestones, or marl stones, which contain the due admixture of lime, silica, and alumina, from which hydraulic cements and artificial stones may be manufactured.” These materials he finds in “the chalk formation, the Wealden formation, the Purbeck beds, the lias formation, the mountain limestone, and the lowest strata of the coal-measures.” After duly choosing his materials according to the particular object in view, he prepares them “by burning in kilns, and grinding in mills, in the way cement is now manufactured.” Marls and limestones are to be “first dried in kilns or ovens, at a heat fit for baking, until all moisture be driven off, and that then the calcination be prolonged as much as possible; the heat being kept as low as is only just sufficient to effect complete calcination—this being indispensable, to avoid the commencement of vitrification, which would destroy the adhesive properties of the cement.”
Cement, Glue. Prep. 1. From glue, 1 lb. melted with the least possible quantity of water, and then mixed with black resin, 1 lb., and red ochre, 4 oz.
2. Glue, melted as above, and mixed with about 1⁄4th of its weight each of boiled oil and red ochre.
3. (Ure.) Melted glue (of the consistence used by carpenters), 8 parts; linseed oil, boiled to varnish with litharge, 4 parts; incorporate thoroughly together.
4. Glue (melted as last), 4 parts; Venice turpentine, 1 part.
Obs. The first three dry in about 48 hours, and are very useful to render the joints of wooden casks, cisterns, &c., watertight; also to fix stones in frames. The last serves to cement glass, wood, and even metal to each other. A good cement for fixing wood to glass may be made by dissolving isinglass in acetic acid, in such quantities that it becomes solid when cold. When applied let it be heated. They all resist moisture well.
Cement, Grind′ers’. Prep. 1. From pitch, 5 parts; wood ashes and hard tallow, of each 1 part; melted together.
2. Black resin, 4 lbs.; beeswax, 1 lb. melt, and add of whiting (previously heated red hot, and still warm), 1 lb.
3. Shell-lac, melted and applied to the pieces slightly heated. Used to fix pieces of glass, &c., whilst grinding. The last is used for lenses and fine work.
Cement, Hamelin’s. Syn. Hamelin’s mastic. From siliceous sand, 60 parts; Bath or Portland stone (in fine powder), 40 parts; lime-marl, 20 parts; litharge, 8 parts; ground together. For use it is mixed up with linseed oil and used like mortar. When this cement is applied to the purpose of covering buildings intended to resemble stone, the surface of the building is first washed with linseed oil.
Cement, Hensler’s. Litharge, 3 parts; quick-lime, 2 parts; white bole, 1 part (all in fine powder); linseed-oil varnish, q. s. to make a paste. Used for china, glass, &c. It is very tenacious, but long in drying.
Cement, Hœnle’s. Shell-lac, 2 parts; Venice turpentine, 1 part; fused together, and formed into sticks. It is used like extemporaneous cement for glass and earthenware.
Cement, Hydraulic. Hydraulic mortars or cements are those which set or become hard under water. Common lime does not possess this property; but limestone containing from 8% to 25% of alumina, magnesia, and silica, yield a lime on burning, which does not slake when moistened with water, but forms a mortar with it, which hardens in a few days when covered with water, although it does not acquire much solidity in the air. Puzzolana, septaria, and argillaceous or siliceous earths, burnt, either with or without the addition of common limestone, and then ground to powder, form excellent hydraulic cements. The reniform limestone, commonly called “cement stone,” which is found distributed in single nodules or lenticular cakes, in beds of clay, is the substance most commonly used in this country for the manufacture of the cements in question.
“A very good hydraulic mortar is made by slaking lime with water containing about 2 per cent. of gypsum, and adding a little sand to the product. The presence of the gypsum tends to delay the slaking of the lime, and also to harden the substance formed after the slaking.
“If water containing a little lime in solution be added to burnt gypsum, a very hard compact mass is obtained. This substance is much used as an imitation marble, as by polishing it with pumice stone, colouring it, and again polishing with oil, it may be made to resemble natural marble very closely. Hardened gypsum treated with stearic acid, or paraffin, and polished, is used as a substitute for meerschaum, which it much resembles.”[238] See Gad’s, Hamelin’s, and Parker’s Cements, &c.
Cement, Iron. This cement, which is much used for closing the joints of iron pipes and similar purposes, is formed of the borings or turnings of cast iron, which should be clean and free from rust, mixed with a small quantity of sal-ammoniac and flowers of sulphur.421 For use, it is stirred up with just enough water to thoroughly moisten it, and it is rammed or caulked into the joints with a blunt caulking chisel and hammer, after which the joint is screwed up by its bolts as tightly as possible. If the turnings and borings are very coarse they are broken by pounding in an iron mortar, and the dust sifted off before use. The following are good proportions:
1. Sal-ammoniac (in powder), 2 oz.; flowers of sulphur, 1 oz.; iron borings, 5 lbs.; water, q. s. to mix.
2. Sal-ammoniac, 2 oz.; sulphur, 1 oz.; iron borings, 12 lbs.; water, q. s. to mix.
3. Sal-ammoniac, 2 oz.; iron borings, 7 or 8 lbs.; water, q. s. to mix.
4. Iron borings, 4 lbs.; good pipeclay, 2 lbs.; powdered potsherds, 1 lb.; make them into a paste with salt and water.
Remarks. The first of these forms is that generally employed for common purposes, but formerly much more sulphur and sal-ammoniac were used. We are told by one of the leading engineers in London that the strongest cement is made without sulphur and with only 1 or 2 parts of sal-ammoniac to 100 of iron borings (see the third form); but that when the work is required to dry rapidly, as for the steam joints of machinery wanted in haste, the quantity of sal-ammoniac is increased a little, and a very small quantity of sulphur is added. This addition makes it set quicker, but reduces its strength. As the power of the cement depends on the oxidation and consequent expansion of the mass, it is evident that the less foreign matter introduced the better. No more of this cement should be made at a time than can be used at once, because it soon spoils. I have seen it become quite hot by standing even a few hours, when it contained sulphur; and I have been informed by workmen that when much sulphur is used, and it has been left together in quantity all night, combustion has taken place. The last form produces a cement that gets very hard when allowed to dry slowly, and is excellent for mending cracks in iron boilers, tanks, &c.
Cement, Japanese. Syn. Rice glue. From rice flour, mixed with a little cold water, and boiling water gradually poured in until it acquires a proper consistence; when it is boiled for 1 or 2 minutes in a clean saucepan or earthen pipkin. It is beautifully white, and almost transparent, for which reason it is well adapted for fancy paper work, which requires a strong and colourless cement. It is superior to French cement. (See antè.)
Cement, Keene’s Marble. Baked gypsum or plaster of Paris, steeped in a saturated solution of alum, and then recalcined, and reduced to powder. For use it is mixed up with water, as ordinary plaster of Paris.
Obs. This cement has been most extensively applied as a stucco. It is susceptible of a high polish, and when coloured produces beautiful imitations of mosaic and other inlaid marbles, scagliola, &c. It is not adapted to hydraulic purposes, or for exposure to the weather, but it is admirable for internal decorations, and from its extreme hardness is very durable. It may be coloured or tinted of any shade, by diffusing mineral colours (levigated, if in powder) through the water used to mix up the cement with. A pleasing tint is given to this cement by adding a little solution of green copperas to the alum liquor.
Cement, Laboratory. Syn. Chemical mastic. From equal parts of pitch, resin, and plaster of Paris (thoroughly dried), mixed together. Used for the masonry of chlorine chambers, vitriol works, &c.; and as a lining for casks intended to hold chloride of lime.
Cement, Letter-fixing. Prep. Copal varnish, 15 parts; drying oil, 5 parts; turpentine, 3 parts; oil of turpentine, 2 parts; liquefied glue (made with the least possible quantity of water), 5 parts; melt together in a water bath, and add fresh slaked lime (perfectly dry, and in very fine powder), 10 parts. Used to attach metal letters to plate glass in shop windows, &c.
Cement, Mahogany. Prep. 1. Melt beeswax, 4 oz.; then add Indian red, 1 oz., and enough yellow ochre to produce the required tint.
2. Shell-lac, melted and coloured as above. Very hard. Both are used to fill up holes and cracks in mahogany furniture by the cabinet makers. Red putty is also used for the same purpose.
Cement, Maissiat’s. India rubber is melted either with or without about 15% of either beeswax or tallow; quick-lime (in fine powder) is gradually added; and the heat continued until change of odour shows that combination has taken place, and until a proper consistence is obtained. Used as a waterproof and air-tight covering for corks, bungs, &c.
Cement, Marine. See Glue, Marine, and Cement, Elastic.
Cement, Martin’s. This is manufactured in the same way as Keene’s, only carbonate of soda or carbonate of potash is used as well as alum, and the burning is carried on at a higher temperature.
Cement, Opticians’. Prep. 1. Shell-lac softened with rectified spirit or wood naphtha. For fine work.
2. Beeswax, 1 oz.; resin, 15 oz.; melt and add whiting (previously made red hot, and still warm), 4 oz.
3. Resin, 1 lb.; melt and add plaster of Paris (dry), 4 oz. The above are used to fix glasses, stones, &c., while polishing and cutting them. The last is a very strong cement for rough purposes.
Cement, Oxychlo′′ride of Zinc. (Sorel.) Prep. In solution of chloride of zinc, marking from 50° to 60° of Baumé’s hydrometer (i.e. sp. gr. 1·490 to 1·652), dissolve 3% of borax or sal-ammoniac; then add oxide of zinc which has422 been heated to redness, until the mass is of a proper consistence.
Obs. This cement becomes as hard as marble. It may be cast in moulds like plaster of Paris, or used in mosaic work, &c.
Cement, Parabol′ic. Syn. Universal cement. Prep. Curdle skim milk with rennet or vinegar, press out the whey, and dry the curd by a very gentle heat, but as quickly as possible. When it has become quite dry grind it in a coffee or pepper mill, and next triturate it in a mortar until reduced to a very fine powder. Mix this powder with 1⁄10th of its weight of new dry quick-lime, also in very fine powder, and to every ounce of the mixture add 5 or 6 gr. of powdered camphor; triturate the whole well together, and keep it in wide-mouth 1-oz. phials, well corked. Used to join glass, earthenware, &c. It is made into a paste with a little water, as wanted, and applied immediately.
Cement, Parian. Is prepared as Keene’s, substituting a solution of borax (1 part of borax to 9 of water) for a solution of alum.
Cement, Park′er’s. This cement is made of the nodules of indurated and slightly ferruginous marl, called by mineralogists “septaria,” and also of some other species of argillaceous limestone. These are burnt in conical kilns, with pit coal, in a similar way to other limestone, care being taken to avoid the use of too much heat, as if the pieces undergo the slightest degree of fusion, even on the surface, they will be unfit to form the cement. After being properly roasted the calx is reduced to a very fine powder by grinding, and immediately packed in barrels, to keep it from the air and moisture.
Uses, &c. This cement is tempered with water, and applied at once, as it soon hardens, and will not bear being again softened down with water. For foundations and cornices exposed to the weather it is usually mixed with an equal quantity of clean angular sand; for use as a common mortar, with about twice as much sand; for coating walls exposed to cold and wet, the common proportions are 3 of sand to 2 of cement, and for walls exposed to extreme dryness or heat, about 21⁄2 or 3 of sand to 1 of cement; for facing cistern work, water frontages, &c., nothing but cement and water should be employed. Under the name of compo’ or Roman cement it is much employed for facing houses, water cisterns, setting the foundations of large edifices, &c.
Cement, Pew’s. Quick-lime, 1 part; baked clay, 2 parts (both in powder); mix and calcine; then add gypsum (fresh baked and in fine powder), 1 part, to powdered baked clay, 2 parts; mix well, add the former mixture, and incorporate them well together. Used to cover buildings. It is applied like mortar, and is very hard and durable. See Cement, Gibbs,’ &c.
Cement, Plumb′ers’. Black resin melted with about an equal weight of brick-dust. Some times a little pitch or tallow is added.
Cement, Port′land. From clay and chalk, or argillaceous river-mud and chalk or limestone, calcined together, and then ground to powder. See Cement, Parker’s.
Cement, Ro′man. Genuine Roman cement consists of puzzolene (a ferruginous clay from Pozzuoli, calcined by the fires of Vesuvius), lime, and sand. The only preparation which the puzzolene undergoes is that of pounding and sifting. It is generally mixed up with water, like most other cements, but occasionally with bullock’s blood and oil, to give the composition more tenacity. That used in this country is now generally prepared from the septaria of either Harwich or Sheppy, or of the lias formation, or from the cement stone found in the upper division of the lias formation, or in the shale beds of the Kimmeridge clay. It is also prepared from several artificial mixtures of ferruginous clay and lime, calcined together. It must be kept in close vessels, and mixed with water when used. See Cement, Parker’s and Gibbs’.
Cement, Seal Engra′′vers’. Resembles plumbers’ cement. Used to fix the pieces of metal while cutting, and also to secure seals and tools in their handles. It grows harder and improves every time it is melted.
Cement, Sin′ger’s. Prep. 1. Melt together resin, 5 lbs., and beeswax, 1 lb., and stir in finely-powdered red ochre (highly dried and still warm), 1 lb., and plaster of Paris, 4 oz.; continuing the heat a little above 212° Fahr., and stirring constantly till all frothing ceases.
2. Resin, 6 lbs.; dried red-ochre, 1 lb.; calcined plaster of Paris, 1⁄2 lb.; linseed oil, 1⁄4 lb. Used to cement the plates in voltaic troughs, to join chemical vessels, &c. No. 2 is specially applicable to troughs. See Cement, Electrical.
Cement, Steam-boiler. Prep. Litharge, in fine powder, 2 parts; very fine sand and quick-lime (that has been allowed to slake spontaneously in a damp place), of each 1 part; mix and keep it from the air. Used to mend the cracks in boilers and ovens, and to secure steam joints. It is made into a paste with boiled oil before application.
Cement, Steam-pipe. Prep. Good linseed oil varnish is ground with equal weights of white lead, oxide of manganese, and pipeclay.
Cement, Stucco. This is a compound of powdered gypsum or strong gelatin. It is used for coating walls, and also for ornamenting ceilings. It takes a high polish, and coloured designs can be painted on it. When employed on walls a coarser kind is first laid on, which is followed by a coating made of choicer specimens of gypsum, or glue, or isinglass. When this latter and outer coat becomes dry it is polished with pumice, tripoli, and linen. The colour is incorporated with the outer coatings of the stucco by mixing the metallic pigments with it, and then423 applying it to the wall, after which a very thin coating of gypsum and isinglass, or sometimes of oil, is given to it, and when the whole is partially dried the tint is brought out by polishing, as before stated. Generally the finest effect is obtained by oil.
Cement, Transpar′ent. See Cement, Elastic.
Cement, Turn′ers’. Prep. Beeswax, 1 oz.; resin, 1⁄2 oz.; pitch, 1⁄2 oz.; melt, and stir in fine brickdust, q. s.
Cement, Univers′al. See Cement, Parabolic.
Cement, Var′ley’s. Syn. Varley’s mastic. Black resin, 16 parts; beeswax, 1 part; melt, add whiting (sifted, dried by a dull-red heat, and allowed to cool), 16 parts; and stir until nearly cold.
Cement, Water. Prep. 1. From good grey clay, 4 parts; black oxide of manganese, 6 parts; limestone (reduced to powder by sprinkling it with water), 90 parts; mix, calcine, and powder.
2. Mix white iron ore (manganese iron ore), 15 parts, with lime, 85 parts; calcine and powder as above. Both this and the preceding must be mixed up with a little sand for use. A piece thrown into water rapidly hardens.
3. Fine clean sand, 1 cwt.; quick-lime, in powder, 28 lbs.; bone ashes, 14 lbs. The above are beat up with water for use. See Cement, Hydraulic, &c.
Cement, Waterglass. For glass, earthenware, porcelain, and all kinds of stoneware, these cements are excellent. A cement for glass and marble is prepared by rubbing together one part of fine pulverised glass, and two parts of pulverised fluorspar, and then adding enough waterglass solution to give it the consistency necessary in a cement.
Waterglass mixed with hydraulic cement to a thick dough makes a good cement for the edges and joints of stone and marble slabs. It is well to mix but little at a time, as it hardens very quickly. (‘Journal of Applied Chemistry.’)
Cement, Wa′terproof. Several compounds of this class have been already noticed. The celebrated “waterproof cement of Dihl” consists of porcelain clay or pipeclay, dried by a gentle heat, and powdered, mixed up to the consistence of a paste with boiled linseed oil, and, sometimes, a little oil of turpentine. It is coloured by adding a little red or yellow ochre, or any similar pigment. It is used to cover the fronts of buildings, roofs of verandahs, &c.
Concluding Remarks. For mending broken CHINA, EARTHENWARE, GLASS, and WOOD, the preparations generally used are the cements described above as Armenian, Botany Bay, Cheese, Chinese, Curd, Egg, Extemporaneous, Glass, Glue, Hensler’s, Hœnle’s, Mahogany, and Parabolic. For SPAR, MARBLE, and similar materials, the Alabaster cement is specially adapted; the Egg and Parabolic cements will, however, answer the same purpose. For CLOTH, LEATHER, PAPER, CARD, and LIGHT FANCY WORK, the most suitable cements are the Elastic, Chinese, Flour, French, and Japanese. The cements adapted for CHEMICAL and ELECTRICAL APPARATUS, and for SEALING BOTTLES, are also termed Bottle, Brimstone, Cap, Chemical, Electrical, Laboratory, Maissiat’s, and Varley’s. The BUILDING and HYDRAULIC CEMENTS are described under the heads Architectural, Beale’s, Bruyere’s, Fireproof, Gad’s, Gibbs’, Hamelin’s, Hydraulic, Keene’s, Oxychloride, Parker’s, Pew’s, Portland, Roman, Water, and Waterproof. The cements used for METAL-WORK, &c., in different trades, are noticed under the heads Coppersmiths’, Cutlers’, Engineers’, Grinders’, Iron, Letter-fixing, Opticians’, Plumbers’, Seal-engravers’, Steam-boiler, Steam-pipe, and Turner’s. See Glue, Lute, Mortar, Tooth-cement, &c.
CEMENTA′TION. The process of imbedding a substance in, or covering it with, some powder or composition capable of acting on it when heated, and in this state exposing it to a red heat. Iron is converted into steel, and glass into Réaumur’s porcelain, by cementation.
CEN′TAURIN. Syn. Centaurin′a. The bitter extractive matter of Erythæa centaurium, or common centaury. Combined with hydrochloric acid, it has been highly recommended as a febrifuge.
CER′ASIN. Syn. Prun′ine. The insoluble portion of cherry-tree gum. It is identical with bassorin. Dr John applies the term to all those gums which, like tragacanth, swell, but do not dissolve in water. See Bassorin.
CE′RATE. Syn. Cera′tum, L. A thick species of ointment containing wax. Cerates are intermediate in consistence between ointments and plasters; but are less frequently employed than either of those preparations. The medicinal ingredients which enter into the cerates are very numerous; indeed, almost every kind of medicine capable of exercising a topical effect may be prescribed in this form.
It is a general custom with the druggists to use a less quantity of wax for their cerates than that which is necessary to give them a proper consistence, and in many cases it is omitted altogether, and its place supplied by hard suet, or stearine, and frequently by common resin. Lard is also very generally substituted for olive oil. Indeed, in no class of pharmaceutical preparations are the instructions of practitioners and the colleges more commonly disregarded. The operation of melting the ingredients should be performed in a water bath or steam bath, and the liquid mass should be assiduously stirred until cold.
All the medicated cerates may be prepared by adding the active ingredients, in the form of fine powder, soft extract, solution, &c., as the case may be, to either simple cerate or424 spermaceti cerate, in the proportions indicated under the head of “Doses” appended to every article of importance noticed in this work. The mixture, which must be complete, may be effected by working the articles together on a marble or glass slab or tile, or, still better, by trituration in a clean wedgwood mortar. In some cases the simple cerate is melted by a gentle heat, and the whole stirred or triturated until nearly solid; in others, digestion with heat is employed.
Cerate. Syn. Sim′ple cerate, Simple dres′sing; Ceratum (Ph. L.), C. sim′plex (Ph. L. 1824). Prep. (Ph. L.) Yellow wax, 20 oz.; melt by a gentle heat; add olive oil, 1 pint; and stir until it begins to solidify.
Used as a simple emollient dressing. The corresponding preparations of the other colleges will be found noticed under Ointments. The ceratum simplex of the Ph. E. is SPERMACETI CERATE.
Cerate, Ac′etate of Lead. Syn. Ce′rate of sugar of lead; Cera′tum plum′bi aceta′tis (Ph. L.), L. Prep. (Ph. L.) White wax, 5 oz.; olive oil, 18 fl. oz.; melt together; add, acetate of lead (in fine powder), 5 dr., previously triturated with olive oil, 2 fl. oz., and stir till they unite (begin to solidify). Used as a cooling dressing to burns, excoriations, and inflamed sores.
Cerate, Ammoni′acal. Syn. Cera′tum ammoniaca′le, L. Prep. (Rechoux.) Simple cerate, 1 oz.; carbonate of ammonia, 1 dr.; mix. As a counter-irritant in croup, &c.
Cerate, Arsen′ical. Syn. Cer′atum arsen′ici, C. a′cidi arsenio′si, L. Prep. 1. (Ph. U. S.) Arsenious acid (in very fine powder), 20 gr.; simple cerate, 1 oz.
2. (Sir A. Cooper.) Arsenious acid and sublimed sulphur, of each 1 dr.; spermaceti cerate, 1 oz. The above ingredients must be very carefully triturated together. The first is used as a dressing to cancerous sores; the second is applied on lint as a caustic in like cases.
Cerate, Belladonn′a. Syn. Cerate of deadly nightshade; Cera′tum belladonn′æ, L. Prep. 1. (W. Cooley.) Extract of belladonna, 3 dr.; simple cerate, 1 oz.; olive oil, 1 dr.; triturate together in a warm mortar, until nearly cold. Used in frictions to indolent tumours.
2. (Compound; C. b. compos′itum, L.) Prep. (W. Cooley.) Belladonna cerate, 1 oz.; iodide of gold, 12 gr.; carefully triturated together. Used as a friction to scrofulous and syphilitic tumours, and to remove syphilitic and rheumatic pains. A most active and excellent preparation.
Cerate, Brown. See Plasters.
Cerate, Caca′o. Syn. Caca′o pommade. Prep. Butter of cacao, white wax, and oil of almonds, equal parts, melted together and strained. Used as a cosmetic for chapped hands and lips, &c.
Cerate, Cal′amine. Syn. Turner’s cerate, Healing salve; Ceratum calami′næ (Ph. L. & E.), C. la′pis calamina′ris (Ph. L. 1788), L. Prep. 1. (Ph. L.) Yellow wax, 71⁄2 oz.; olive oil, 1 pint; melt together, remove the vessel from the fire, and when they first begin to thicken, add prepared calamine, 71⁄2 oz., and stir constantly until they cool.
2. (P. E.) Prepared calamine, 1 part; simple cerate (Ph. E.), 5 parts; mix.
3. (Ph. D.) See Ointment.
4. (Commercial.) Hard suet, 5 lbs.; lard, 3 lbs.; melt and sift in, gradually, calamine, 4 lbs.; agitate well for a few minutes, or until the whole is perfectly mixed, and after one minute’s repose pour it off into another vessel, the coarse sediment that has fallen to the bottom being carefully avoided; lastly, stir assiduously, until it is nearly cold. This forms the Turner’s cerate of the wholesale druggists. In many cases nothing but lard and calamine are used.
Uses, &c. When honestly prepared with genuine calamine, it is a most valuable desiccant and astringent application to excoriations, ulcers, burns, scalds, sore nipples, &c. It has long been held in popular esteem as a drying and healing dressing for sores.
Cerate, Cal′amine with Mercury. Syn. Cera′tum calami′næ cum hydrar′gyro, L. Prep. (Ph. Chirur.) Calamine cerate, 1 lb.; red oxide of mercury, 1 oz.; mix. Used as a stimulant application to foul and indolent ulcers, psorophthalmia, &c.
Cerate, Cal′omel. Syn. Cera′tum calomela′nos, C. hydrar′gyri chlor′idi, L. Prep. 1. Calomel, 1 dr.; spermaceti cerate, 7 dr. In herpes, and some other skin diseases.
2. (Compound; C. c. compos′itum, L.) Calomel, 2 dr.; calamine cerate, 1 oz.; olive oil, 1 dr.
Cerate, Cam′phor. Syn. Cera′tum camphora′tum, C. camphor′æ, L.; Pommade du frère cosme, Fr. Prep. Olive oil, 1 lb.; white wax, 1⁄2 lb.; camphor, 3 dr. As an application to chaps, chilblains, abrasions, excoriations, and slight wounds. See Balls, Camphor.
Cerate, Canthar′ides. Syn. Blistering cerate; Cera′tum lytt′æ, C. canthar′idis, L. Prep. 1. (Ph. L.) Cantharides (in very fine powder), 1 oz.; spermaceti cerate, 6 oz.; mix.
2. (Parrish.) Cantharides, 12 parts; lard, 10 parts; yellow wax and resin, of each 7 parts; incorporated by fusion. Irritant; used to keep blisters open, and to stimulate issues, and indolent ulcers and tumours.
Cerate, Chalk. Syn. Cera′tum cre′tæ, L. Prep. 1. Chalk (thoroughly dried, and in fine powder), 2 dr.; simple cerate, 6 dr.; almond oil, 3 dr. Used in piles and foul ulcers.
2. (Acetated.) See Cerate, Kirkland’s Neutral.
3. (Compound; Cera′tum cre′tæ compos′itum, L.)—a. To simple chalk cerate, 1425 oz., add powdered catechu, 1⁄2 dr. In piles, and foul and indolent ulcers.
b. (U.S. Hospital.) Lead plaster and olive oil, of each 8 oz.; white wax, 3 oz.; melt together; add solution of subacetate of lead, 6 oz.; thoroughly incorporate, and then further add, chalk (in fine powder), 5 oz. Cooling and astringent. Useful in inflamed sores, excoriations, piles, &c.
Cerate, Cher′ry-laur′el. Syn. Cera′tum lauro-cerasi, C. calmans, L. Prep. (Roux.) Simple cerate, 1 oz.; cherry-laurel water, 1⁄2 oz. As an application to burns.
Cerate, Cincho′na. Syn. Bark cerate; Cera′tum cincho′næ:, L. Prep. 1. Extract of bark, 2 dr.; simple cerate, 1 oz.
2. (Van Mons.) Simple cerate, 8 oz.; camphor, 11⁄2 dr.; melt together by a gentle heat, then add gradually, decoction of Peruvian bark (concentrated), 1 oz., and triturate until cold. Used as a dressing for ill-conditioned ulcers.
Cerate, Cin′nabar. Syn. Cera′tum ru′brum, C. cinnaba′ris, C. hydrar′gyri sulphure′ti ru′bri, L. Prep. 1. Camphor, 20 gr.; vermilion, 60 gr.; simple cerate, 1 oz. This is Alibert’s “Antiherpetic pommade.”
2. (Ph. Chirur.) Yellow wax and lard, of each 1⁄2 lb.; yellow resin, 1⁄2 oz.; red sulphide of mercury, 1 dr. Used as a common dressing.
Cerate, Cit′rine. See Cerate, Resin, Nitrate of Mercury c.
Cerate, Copai′ba. Syn. Cera′tum copai′bæ, L. Prep. 1. Spermaceti cerate, 3 oz.; melt by a gentle heat, then add, balsam of copaiba, 1 oz.
2. (Dr Houlton.) White wax, 1 oz.; balsam of copaiba, 2 oz.; mix, as last. Both the above have been recommended as topical applications to wounds and ulcers of the rectum, vagina, and urethra; especially in those of a fistulous character; and in piles, &c.
Cerate, Cop′per. Syn. Cupria′ted cerate; Cera′tum cu′pri; C. c. ammonia′ti, L. Prep. (Swediaur.) Simple cerate, 8 parts; melt, and add solution of ammoniuret of copper, 1 part. As a stimulant dressing for indolent ulcers; and in psorophthalmia, &c.
Cerate, Cosmet′ic. Syn. Cold cream, Cerate of Ga′len; Cera′tum cosmet′icum, C. Galeni, Cremor frigida, L.; Pommade en crême, Fr. Prep. 1. Oil of sweet almonds, 1 lb.; white wax and spermaceti, of each 2 oz.; melt, pour the mixture into a marble or wedgwood mortar, which has been heated by standing for some time in boiling water; add, gradually, rose water, 10 fl. oz., assiduously stirring until an emulsion is formed; then further add, oil of bergamot, 1⁄2 oz.; oil of lavender, 1 dr.; and continue the stirring or trituration until the whole has become cold.
2. To the last add otto of roses, 1 dr.; oil of rosemary, 15 drops.
3. Oil of almonds, 5 oz.; spermaceti, 5 dr.; white wax, 4 dr.; rose water, 31⁄2 oz.; balm of Mecca (genuine), 8 drops.
4. As the last, with essence of vanilla, 15 drops; essence of ambergris, 10 drops.
5. (P. C.) White wax, 1 part; oil of almonds, 4 parts; rose water, 3 parts; as before.
6. (Van Mons.) White wax and butter of cacao, of each 1 part; oil of almonds and rose water, of each 4 parts.
Obs. The above are used as agreeable and cooling emollients for irritable surfaces, excoriations, sore nipples, &c. See Cold cream and Ointments.
Cerate, Cro′ton. Syn. Cera′tum croto′nis, L. Prep. (Caventou.) Lard, 5 parts; wax, 1 part; melt, and when nearly cold add croton oil, 2 parts. Used as a counter-irritant; but is apt to affect the bowels.
Cerate, Goulard’s. See Cerate, Lead.
Cerate, Hemlock. Syn. Cera′tum co′nii, L. Prep. (St. B. Hosp.) Spermaceti, 2 oz.; white wax, 3 oz.; melt, and add of hemlock ointment, 12 oz. Used for inveterate cancerous, scrofulous, and other sores.
Cerate, Hon′ey. Syn. Cera′tum mel′is, L. Prep. 1. Simple cerate, 3 parts; honey, 1 part; oil of lemon-grass, 6 drops. Used as cold cream.
2. (Ph. Chirur.) Olive oil, 1⁄2 lb.; wax and lead plaster (or galbanum plaster), of each 4 oz.; melt, and add honey, 1⁄2 lb. As a cooling emollient dressing.
Cerate of Honey with Turpentine. (Paracelsus). Common turpentine, 1 lb.; the yolk of 20 eggs; honey, 1 lb.; beat together the honey and yolk, and add the turpentine, softened by heat.
Cerate, Is′sue. Syn. Cera′tum ad fonticulos, L. As issue plaster, but adding a little almond oil.
Cerate, Kirk′land’s. Syn. Kirkland’s neutral cerate; Cera′tum neutra′le, C. cre′tæ aceta′tis, L. Prep. 1. Lead plaster, 8 oz.; olive oil, 4 oz. melt, sift in chalk, 4 oz.; mix well, then add gradually Goulard’s extract, 1⁄2 oz.; distilled vinegar, 4 oz.; and stir until cold.
2. (Paris.) Lead plaster, 8 oz.; olive oil and chalk, of each 4 oz.; sugar of lead, 3 dr., (dissolved in) distilled vinegar, 4 fl. oz. As a cooling dressing to irritable ulcers and excoriated parts.
Cerate, Lead (Compound). Syn. Gou′lard’s cerate; Cera′tum plum′bi compos′itum (Ph. L.), L. Prep. (Ph. L.) Olive oil, 16 fl. oz.; yellow wax, 8 oz.; melt, remove the vessel from the fire, and when they begin to thicken, add gradually solution of subacetate of lead (slightly warmed), 6 fl. oz.; and stir constantly until the whole is nearly cold; then add camphor, 1 dr., dissolved in olive oil, 4 fl. oz. (by heat), and stir until the cerate is quite cold. Used in similar cases to Kirkland’s cerate (which see). See also Acetate of Lead cerate.
Cerate, Mar′shall’s. Prep. 1. Palm oil and426 calomel, of each 2 oz.; acetate of lead, 1 oz.; ointment of nitrate of mercury, 4 oz.; triturated together in a wedgwood mortar.
2. (Paris.) Palm oil, 5 oz.; calomel, 1 oz.; acetate of lead, 1⁄2 oz.; citrine ointment, 2 oz.; as the last. Applied to the eyes, &c.
Cerate, mercu′′rial. Syn. Cera′tum mercuria′le, C. hydrar′′gyri, L. Prep. 1. (Guibourt.) Strong mercurial ointment and simple cerate, equal parts.
2. (Ph. L. 1746.) Strong mercurial ointment and yellow wax, of each 6 oz.; lard, 3 oz. Both are used as dressings to venereal ulcers.
3. (Compound; Cera′tum mercuria′le compos′itum, C. hydrar′′gyri, L.) Prep. (Ph. L.) Mercurial ointment (strong) and soap cerate, of each 6 oz.; camphor (in powder), 11⁄2 oz.; triturate together. Alterative and discutient; used to disperse indolent tumours and swellings, and as a resolvent in enlarged joints, &c.
Cerate, Meto′′pium. Syn. Cera′tum meto′′pii, L. Prep. (Dr Barham.) Hog-gum (from Rhus Metopium), and lard, of each 4 oz.; white wax and root of Sweet Aristolochia (powdered), of each 2 oz.; yellow resin, 1 oz.; in stiff joints and rheumatic pains.
Cerate, Mez′ereon. Syn. Cera′tum mez′erei, L. Prep. 1. Extract of mezereon, 1 part; (dissolved in) alcohol, 5 parts; add beeswax, 5 parts; olive oil, 11 parts; melt together, and continue the heat until all the alcohol is evaporated.
2. Green oil of mezereon, 1 part; simple cerate, 20 parts; melt together. Both are used to keep up the discharge from blistered surfaces, and as a stimulant application to indolent sores.
Cerate, Neu′tral. See Cerate, Kirkland’s.
Cerate, Ni′trate of Mer′cury. Syn. Cit′rine cerate; Cera′tum hydrar′′gyri nitra′tis, L. Prep. (St. B. Hosp.) Citrine ointment and simple cerate, equal parts. See Ointments.
Cerate, O′pium. Syn. Laud′anum cerate, An′odyne c.; Cera′tum o′′pii, C. opia′tum, C. anody′num, L. Prep. 1. Tincture of opium and olive oil, of each 2 dr.; simple cerate, 1 oz.; digest with heat until all the spirit and water is evaporated, constantly stirring the mixture all the time.
2. (Gilbert.) Wine of opium, 1 dr.; simple cerate, 1 oz.
3. (Lagneau.) Opium (in fine powder), 1⁄2 dr.; yolk of 1 egg; mix, then triturate it with simple cerate, 1 oz.
Uses. The above are applied to painful swellings, piles, and ulcers, and in chronic ophthalmia, &c.
Cerate, Phosphora′ted. Syn. Cera′tum phospho′ri, C. phosphora′tum, L. Prep. 1. Phosphorus, 6 gr.; simple cerate, 3 oz.; heat together in a phial placed in a water bath, with frequent agitation for 2 hours; and after repose for 10 minutes, pour off the clear portion, and stir it well until cold.
2. (Foy.) Phosphorated ether, 5 parts; simple cerate, 24 parts.—Uses. Both of the above have been recommended as frictions in obstinate cutaneous affections, and in rheumatism of the joints.
Cerate, Pitch. Syn. Ceratum pi′cis Bergundi′cæ, L. Prep. (Beral.) White wax, 3 parts; suet, 4 parts; Bergundy pitch, 6 parts; melted together. A mild stimulant and detergent dressing. See Ointments.
Cerate, Quin′ine. Syn. Cera′tum quiniæ, L. Prep. 1. Sulphate of quinine, 5 or 6 gr.; simple cerate, 1 dr. Applied to the denuded dermis (endermically).
2. Sulphate of quinine and olive oil, of each 1 dr.; simple cerate, 6 dr. As a friction. Both are used in intermittents.
Cerate, Res′in. Syn. Basil′icon, B. cerate, B. ointment, Yellow b., Cit′rine cerate; Cera′tum citri′num (Ph. L. 1788), C. resi′næ fla′væ (Ph. L. 1745), C. resi′næ (Ph. L. 1809 and since), L. Prep. 1. (Ph. L.) Yellow resin and beeswax, of each 15 oz.; melt, add olive oil, 1 pint; strain through a cloth, and stir the mixture until cold.
Obs. The above is the formula of the London College, but the basilicon of the shops is seldom, if ever, made in this manner. The following forms are those commonly used in trade, but the products are much inferior to that made according to the directions in the Pharmacopœia.
2. (Commercial.)—a. Yellow resin, 10 lbs.; beeswax, 2 lbs.; linseed oil, 7 lbs.; melt together, and stir until cold.
b. As the last, but using nut oil instead of linseed oil.
c. Nut oil, 1 gall.; beeswax, 5 lbs.; yellow resin, 14 lbs.
d. Lard (common) and linseed oil, of each 3 lbs.; yellow resin, 9 lbs.; as before.
Uses, &c. This cerate is a mild stimulant, detergent, and digestive application; and as such is employed to dress foul and indolent ulcers, blistered surfaces, burns, &c. For the corresponding preparations of the other colleges, see Ointments.
3. (Compound; Desh′ler’s cerate; Cera′tum resi′næ compos′itum, L.) Prep. (Ph. U. S.) Resin, suet, and beeswax, of each 1 lb.; turpentine, 1⁄2 lb.; flax-seed oil (linseed oil), 1⁄2 pint; as above. Rather more stimulating than resin cerate, but used for the same purposes.
Cerate, Rose. Syn. Lip salve; Cera′tum rosa′tum, L. Prep. (P. C.) Oil of almonds, 16 parts; white wax, 8 parts; alkanet root, 1 part; digest, with a gentle heat, until sufficiently coloured, then strain, and for every ounce of the cerate, add otto of roses, 2 drops. See Lip Salve.
Cerate, Sav′ine. Syn. Cera′tum sabi′næ (Ph. E.; and Ph. L. 1836), L. Prep. 1. (Ph. E.) Beeswax, 1 part; lard, 4 parts;427 fresh savin (leaves bruised), 2 parts; boil together until the leaves become crisp, then strain, with pressure, through a linen cloth.
2. (Ph. L. 1836.) Lard, 2 lbs.; savin leaves, 1 lb.; beeswax, 1⁄2 lb.; as last.
3. (Ph. L. 1851.) In the B. P. this preparation is included among the OINTMENTS (which see); in trade, however, the old name (Ph. L. 1836) is still generally retained.
Obs. The preparation of this cerate requires caution, as the active principle of the savin, being volatile, is injured by long boiling and a high temperature. The leaves are usually boiled until they are crisp, but as this takes some time, the essential oil, and consequently the odour, is nearly all dissipated. A better plan is to express the juice from the leaves, and to add it to the wax and oil melted together, and just beginning to cool. As usually met with in the shops, this cerate has a lively green colour, and the odour of the fresh plant; but neither of these is derived from the leaves in the common process of making it. The first is caused by the addition of powdered verdigris, and the last by adding a little of the essential oil of savin to the compound when nearly cold. The preparations of the British Colleges have only a very pale green colour, and even that rapidly changes by exposure to the air. A uniform green colour may therefore be regarded as a proof of adulteration; as the unadulterated compound, however, skilfully prepared, is of a dingy green colour, of little intensity; and after it has been made a short time, it fades on the surface, and the under portion becomes streaky and mottled. A greater quantity of colour is obtained from the leaves by long digestion in the fat and wax in earthen vessels, at a moderate heat, than by hasty boiling. In this way a lively green is sometimes produced, but it rapidly changes in the manner just mentioned.
The following forms are those commonly adopted by the wholesale druggists for the manufacture of this cerate:—
4. Lard and suet, of each 6 lbs.; yellow wax, 2 lbs.; melt them together in an earthen vessel; add 2 oz. of distilled verdigris (previously rubbed down smooth in a mortar with an equal weight of sweet oil); strain, whilst hot, into a large earthen pot, and when the whole has cooled a little, add of oil of savin, 1 oz., and stir until cold.
5. Savin leaves, 4 lbs.; yellow wax, 2 lbs.; lard, 8 lbs.; boil until the leaves become crisp; then strain, and add, of green ointment (lively coloured), 5 lbs.; when cooled a little, further add, of oil of savin, 3 dr., and stir briskly until cold. Prod., 131⁄2 lbs.
Uses, &c. Savin cerate and ointment are chiefly employed to keep up the discharge from blisters (perpetual blisters), for which purpose it is preferable to preparations of cantharides. The practice of colouring this cerate with verdigris, which is general in trade, cannot be too severely censured, as its therapeutic action is thereby altered. The copper may be detected by burning down a little in a platinum or Hessian crucible, washing out the ashes with a little dilute nitric acid, placing the liquor in a glass tube, and applying the usual tests. See Copper and Ointments.
Cerate, Sim′ple. Syn. Cera′tum sim′plex, L. Prep. 1. (Ph. E.) Spermaceti, 1 part; white wax, 3 parts; olive oil, 6 parts; melt by a gentle heat, and stir until cold. This preparation is similar to Simple Ointment (Unguentum Simplex), B. P. (which see).
Cerate of Snails. White wax, 3 parts; spermaceti, 3 parts; oil of almonds, 32 parts; mucilage of snails, 24 parts; otto of rose, sufficient to scent it.
Cerate, Soap. Syn. Compound soap cerate; Cera′tum sapo′nis (Ph. L. 1836), C. saponis compos′itum (Ph. L. 1851), L. Prep. 1. (Ph. L.) Boil litharge, 15 oz., in distilled vinegar, 1 gall., until dissolved, stirring continually; then add of Castile soap, 10 oz.; again boil until all the moisture is evaporated; then add, gradually, beeswax, 121⁄2 oz., and olive oil, 1 pint, previously melted together, and stir until nearly cold. Similar to Soap cerate plaster (Emplastrum Cerati Saponis), B. P. (which see).
2. (Wholesale.) Distilled vinegar, 6 galls.; litharge, 5 lbs.; soap, 33⁄4 lbs.; yellow wax, 41⁄2 lbs.; olive oil, 6 pints. Mix as above. Good nut or poppy oil may be used instead of olive oil.
Obs. Unless the instructions contained in the above formulæ are followed in every particular, the process is apt to miscarry. When this is the case, the cerate, on cooling, separates into two portions, and is commonly full of hard, gritty particles. To prevent this, care should be taken to use soap of the best quality. This mishap cannot be got over by long boiling and stirring, as is generally supposed. The only remedy is the addition of a little more soap, previously melted with some water, and again evaporating to a proper consistence. A small quantity of solution of potassa has a similar effect.
The colour and consistence of soap cerate chiefly depends on the length of time it is kept heated after the addition of the oil and wax. As evaporation proceeds, so the colour and consistence increase. Its usual colour is that of a lively, pale chocolate-brown, but occasionally it is much paler. This arises from its containing an undue quantity of moisture. When it has been kept heated for a period beyond that usually adopted, it attains greater hardness, and is then frequently called hard soap cerate (Cera′tum saponis durum); but by over-boiling it is apt to become gritty.
Uses, &c. Soap cerate is resolvent, cooling, and desiccative, and is chiefly employed as a cooling dressing for scrofulous swellings, &c. It may be spread on linen and applied like a plaster. It is sometimes used as a support for428 fractured limbs, and forms an excellent dressing for soft corns.
Cerate, Spermace′ti. Syn. White cerate, White lip salve, Simple c.; Ceratum sim′plex (Ph. E.), C. album (Ph. L. 1745), C. sperma′tis ce′ti (Ph. L. 1788), C. ceta′cei (Ph. L. 1809, and since), L. Prep. 1. (Ph. L.) Spermaceti, 2 oz.; white wax, 8 oz.; melt by a gentle heat; add, olive oil (warm), 1 pint, and stir with a spatula until they cool.
2. (Ph. E.) See Cerate, Simple.
3. (Ph. D.) The corresponding preparation of the Ph. D. is classed under Ointments, and contains lard.
4. (Commercial.) On the large scale lard or suet is substituted for oil, by which means less wax is required. The following is a good form where a cheap article is wanted, and is that commonly adopted in the wholesale trade:—
Clarified mutton suet, 51⁄2 lbs.; white wax and spermaceti, of each 3⁄4 lb.; as above.
Obs. The materials should be melted by a very gentle heat (that of a water bath is best) in a clean stoneware vessel, and as soon as perfect liquefaction takes place, the heat should be withdrawn, and the fluid cerate strained into a clean vessel, and stirred with a clean wooden spatula until it solidifies. To facilitate the cooling, the vessel may be placed in cold water or in a current of cold air. In this way the product is rendered both whiter and finer than when the liquid mass is allowed to cool by itself. By adding a little flowers of benzoin with the oil, or a little nitric ether when the cerate is about half cold, this, as well as other like preparations, will keep for years without becoming rancid or suffering any material change of condition.
Uses, &c. Emollient and cooling. It is commonly employed as a soft, cooling dressing, as a lip salve, as an application to chaps, chilblains, &c.
Cerate, Sul′phur. Syn. Cera′tum sulphu′ris, C. sulphura′tum, L. Prep. (P. C.) Washed sulphur, 2 parts; cerate of Galen, 7 parts; almond oil, 1 part; mix. In itch, &c.
Cerate, Sul′phide of Mer′cury. Prep. (Swediaur.) Yellow resin, 1⁄2 oz.; yellow wax and lard, of each 1⁄2 lb.; vermilion, 20 gr. As a dressing to unhealthy ulcers. See Cerate, Cinnabar.
Cerate, Tobac′co. Prep. Beeswax, 3 oz.; yellow resin, 1 oz.; olive oil, 6 oz.; tobacco juice, 4 oz.; mix and evaporate to dryness, and when nearly cold, add bergamot, 2 dr. Used to destroy pediculi, &c.
Cerate, Touch. Syn. Cera′tum pro tec′tu, L.; Cerat pour le toucher, Fr. Prep. (Soubeiran.) Spermaceti and yellow wax, of each 1 part; olive oil, 16 parts; melt, add caustic soda, 1 part, and stir until cold. Used in hospitals for practising the touching in accouchements.
Cerate, Turner’s. See Cerate, Calamine.
Cerate, Ver′digris. Syn. Cera′tum æru′ginis, C. cu′pri diaceta′tis, L. Prep. 1. Resin cerate, 19 parts; verdigris (in fine powder), 1 part.
2. (For. Ph.) Wax and resin, of each 6 parts; Venice turpentine, 5 parts; linseed oil, 2 parts; verdigris, 1 part. Used as a mild escharotic and stimulant to fungous ulcers, warts, corns, &c.
Cerate, White. See Cerate, Spermaceti.
Cerate, Zinc. Syn. Cera′tum zinc′i, C. z. oxy′di, L. Prep. 1. Oxide of zinc, 20 gr., spermaceti cerate, 1 oz. Used in sore nipples, excoriations, &c.; and in chronic ophthalmia.
2. (Compound; Cera′tum zinci compositum, L.)—a. To the last add calomel, 10 gr. Used as the last, and in scrofulous ophthalmia.
b. (Mid. Hosp.) Zinc ointment and compound lead ointment, equal parts. Cooling, astringent; in excoriations, and as a dressing for ulcers.
c. (Hufeland.) Oxide of zinc and lycopodium, of each 15 gr.; simple cerate, 1⁄2 oz. In sore nipples, ulcerations of the breast, tetters, &c. It acts best when diluted with half its weight of spermaceti cerate.
d. (U. S. Ph.) Precipitated carbonate zinc, 2 oz.; simple cerate, 10 oz. A substitute for calamine cerate.
CEREB′RIC ACID. A peculiar acid compound, first noticed by M. Frémy, obtained along with oleo-phosphoric acid when the brain and nerves are treated with hot alcohol. It is solid, white, crystalline; freely soluble in boiling alcohol, and forms a solid gelatinous mass with hot water; fusible with decomposition, exhaling a peculiar odour, and leaving much charcoal behind. It has been found also in the yolk of eggs, in seminal fluid, and in pus. With the alkalies it forms insoluble salts termed cerebrates.
CEREB′ROLEIN. When oleo-phosphoric acid is boiled in water, it is resolved into a fluid neutral oil and phosphoric acid, which dissolves. The former is cerebrolein.
CE′RIN, HC27H53O2. (Brodie.) Syn. Cerotic acid. When pure beeswax (bleached) is digested in boiling alcohol for some time, a solution of myricin and cerin is formed. The former is deposited as the liquid cools, and the latter may be obtained by evaporating the decanted portion. Cerin is a white, crystallisable substance, soluble in 16 parts of boiling alcohol; it fuses at 144° Fahr.; and is readily saponified with caustic alkaline lyes. It greatly resembles white wax, of which, indeed, it forms from 70%; to 80%.
CERISIN. A substance obtained from ozokerit or fossil wax, very similar in appearance and properties to white wax, for which it has been proposed as a substitute in pharmaceutical preparations. At present it is chiefly used in the manufacture of candles. Cerisin appears to be one of the paraffins. It differs, however, from ordinary paraffin in not being unctuous to the touch, in being non-translucent and firmer in texture, and in429 having a higher fusing point. It seems to be intermediate between ordinary paraffin and wax.
CE′RIUM. Ce. A metal discovered in 1803 by Hisinger and Berzelius in the mineral named cerite.
Cerium Oxalate. (Ph. B.) It may be obtained as a precipitate by adding a solution of oxalate of ammonia to a soluble salt of cerium.—Dose, 1 to 2 gr. Given in the vomiting of pregnancy.
CE′ROMEL. Prep. (Van Mons.) Beeswax, 1 oz.; honey, 4 oz.; melt together and stir until cold. An excellent application to irritable ulcers, abraded surfaces, sore nipples, &c.
CERO′TIC ACID. See Cerin.
CESSPOOLS. It may be well to point out that the local authorities of any district in which a cesspool is situated are required by the Public Health Act—1. To see that it is so constructed and kept as to prevent its becoming either a nuisance or detrimental to health. 2. That an examination of any cesspool can be made by the sanitary inspector, or by any officer appointed by the local authority, after notice of entry has been served upon those who are the occupiers of the premises on which it is situated. 3. The local authority may itself undertake the cleansing of a cesspool, or it may enact bye-laws imposing this duty on the occupiers of the premises. 4. If the local authority, after having undertaken the cleansing of a cesspool, fail to do its duty, it becomes liable, after notice from an occupier, for the payment to the said occupier of a penalty not exceeding five shillings a day during default. 5. Any person in an urban district who allows the contents of a cesspool to overflow, or to soak therefrom, incurs a penalty of forty shillings for each offence, and a further charge of five shillings a day for the continuance of the offence after notice. 6. Information of any nuisance under the said Act in the district of any local authority may be given to such local authority by any person aggrieved thereby, or by any two inhabitant householders of such district, or by any officer of such authority, or by the relieving officer, or by any constable or officer of the police force of such district.
It does not come within our province to enter into details as to the best method of building a cesspool.
We may, however, state, that owing to the defective and leaky construction of a cesspool, it very frequently becomes a serious source of dangerous contamination to the wells in the neighbourhood, as well as a ready means of contagion, when it contains the excreta of fever patients. The outbreak of typhoid fever at the west end of London in 1874, the origin of which was traced to the milk supply, was owing to the vessels in which the milk was collected in the country having been washed out with water taken from a well near a cesspool, into which ran the contents of a privy belonging to a house, some of the inmates of which were labouring under typhoid fever.
For a cesspool not to be injurious to health it should be water-tight and ventilated by a shaft; it should never be allowed to overflow; and should be sunk at as great a distance from houses or dwellings as possible.
CE′TIN. C32H64O2. Chevreul applied this name to pure spermaceti. Prep. Dissolve spermaceti in boiling alcohol, and collect the crystals that are deposited as the solution cools. Bright pearly crystals, melting at 120°, and subliming at 670° Fahr. See Spermaceti.
CETRAR′IC ACID. H2C18H14O8. Syn. Cetrar′in. The bitter principle of Iceland moss (Cetraria Islandica). It exists, in the free state, in the cortical portion of the thallus.
Prep. 1. Iceland moss (bruised), 1 part; rectified spirit, 6 parts; boil in a covered vessel for half an hour; express the liquor whilst hot, filter, and distil off the spirit; redissolve the residuum in boiling alcohol, decant the clear, and let the solution cool slowly; lastly, collect the crystals and preserve them out of contact with air.
2. (Herberger.) Iceland moss (in coarse powder), 1 lb.; alcohol (·883), 4 lbs.; boil as before, cool until vapours cease to rise, express the tincture, add hydrochloric acid, 3 dr., (dissolved in) water, 2 oz.; let it rest for a night in a closed matrass; then decant, throw the deposit on a filter, press it in bibulous paper, and whilst still moist wash it with both alcohol and ether; lastly, purify it by digestion in boiling alcohol, as before.
Prop., &c. Pure cetraric acid occurs under the form of minute, shining, acicular crystals; it is intensely bitter, non-volatile, scarcely soluble in water, ether, and cold alcohol; soluble in alkaline solutions forming soluble salts, which give a red colour with the persalts of iron, and a yellow one with acetate of lead. The compounds are called cetrarates.—Dose, 2 to 4 gr. every three hours, as a febrifuge; 1 to 3 gr. thrice daily, as a tonic.
CHA′BERT’S OIL. Syn. Chabert’s empyreumat′ic oil; O′leum empyreumat′icum Chaberti, O. contra tæniam Chaberti, L. Prep. (Ph. Bor. 1847.) From empyreumatic oil of hartshorn, 1 part; oil of turpentine, 3 parts; mix and distil over three fourths only in a glass retort, and keep it in well-stopped bottles. In tapeworm.—Dose, 2 teaspoonfuls in water, night and morning, until 4 to 6 or even 7 oz. have been taken; a cathartic being also administered from time to time.
CHAFING. See Excoriations.
CHAIRS. The black leather work of chairs, settees, &c., may be restored by first well washing off the dirt with a little warm soap and water, and afterwards with clean water. The brown and faded portions may now be430 retained by means of a little black ink, or preferably, black reviver, and when this has got thoroughly dry, they may be touched over with white of egg, stained and mixed with a little sugar-candy. When the surface is nearly dry, it should be polished off with a clean brush.
CHALK. Syn. Soft carbonate of lime, or Carbonate of calcium, Earthy c. of l.; Cre′ta, L. Chalk is largely used in the arts and manufactures, and in medicine. The natural varieties are remarkable for the fossils which they contain. The COLOURED CHALKS which are used as pigments and for crayons generally contain both clay and magnesia, as well as oxide of iron, and are minerals quite distinct from WHITE CHALK, or CHALK properly so called. The latter is an AMORPHOUS CARBONATE OF LIME. Exposed for some time to a red heat, it is converted into QUICK-LIME; ground in mills and elutriated, it forms WHITING; the same process performed more carefully and on a smaller scale produces the PREPARED CHALK used in medicine. When prepared artificially (by precipitation), it is the PRECIPITATED CHALK of modern pharmacy. (See below.)
Chalk, Black. A variety of drawing slate.
Chalk, Brown. A familiar name for umber.
Chalk, Cam′phorated. Syn. Cretaceous tooth powder, Cam′phorated t. p.; Cre′ta cam′phorata, C. cum campho′ra, L. Prep. 1. Camphor, 1 oz.; add a few drops of spirit of wine, reduce it to a very fine powder, and mix it (perfectly) with precipitated chalk, 7 oz.; lastly, pass it through a clean, fine sieve, and keep it in a corked bottle. These proportions make the strongest “CAMPHORATED TOOTH POWDER” of the shops.
2. Camphor, 1 oz.; precipitated chalk, 15 oz.; as before. These are the best and safest proportions, and those now generally adopted by the West-end perfumers.
3. As either of the above, but using prepared chalk in lieu of precipitated chalk. Less white and velvety, but cleans the teeth better than the softer article.
Uses, &c. Camphorated chalk is much esteemed as a dentifrice; especially by smokers, and those troubled with foul teeth, or offensive breath. It may be scented with a few drops (3 or 4 to each oz.) of otto of roses, oil of cloves, or neroli, or of the essences of ambergris, musk, or vanilla; but care must be taken not to overdo it. When the teeth are much furred or discoloured, it may be mixed with about one seventh of its weight of finely powdered pumice stone (sifted through lawn), which will render it more effective. A little carmine, rouge, light red (burnt ochre), red coral, or rose pink, is also sometimes added to give it a tinge approaching that of the gums. The quantity of camphor (1 to 3 or 4) commonly ordered in certain books is absurdly large, and would render the compound not only unpleasant in use, but actually detrimental to the teeth. See Dentifrices.
Chalk, French. Soap stone or steatite, a soft magnesian mineral, possessing the property of writing on glass. It is used by tailors for marking cloths. Its powder (obtained by scraping) is very soft, velvety, and absorbent of grease. It forms the boot powder of the boot- and shoe-makers.
Chalk Mixture. Syn. Mistura Cretæ, L. Prepared chalk, 1 part; gum arabic (in powder), 1 part; syrup, 2 parts; cinnamon water, 30 parts; mix by trituration.—Dose, 1 to 2 oz., with astringent tinctures and opium. Care should be taken to use the prepared chalk as directed; the precipitated chalk has a crystalline character, and is said to occasion irritation of the bowels. (Squire.)
Chalk, Precip′itated. Syn. Precipitated car′bonate of lime; Cre′ta præcipita′ta, Cal′cis carb′onas præcipita′tum, L. Prep. 1. By adding to a solution of chloride of calcium, any quantity, another of carbonate of soda (both cold), and well washing the precipitate with pure water, and drying it out of the dust.
2. (Ph. D.) Solution of chloride of calcium (Ph. D.), 5 parts; carbonate of soda, 3 parts; (dissolved in) water, 4 parts.
3. (B. P.) Dissolve chloride of calcium, 5 oz.; and carbonate of soda, 13 oz.; each in two pints of boiling distilled water; mix the two solutions, and allow the precipitate to subside. Collect this on a calico filter, wash it with boiling distilled water, until the washing cease to give a precipitate with nitrate of silver, and try the product at the temperature of 212° F.
Uses, &c. It is chiefly employed for making aromatic confection, cretaceous powder, and chalk mixture. That of the shops is seldom pure, the refuse of the soda-water makers (sulphate of lime) being commonly sold for it. When pure it is wholly soluble, with effervescence, in dilute hydrochloric acid. (See below.)
Chalk, Prepa′′red. Syn. Cre′ta (Ph. E. & Ph. L. 1836), Cre′ta prepara′ta (Ph. L. 1851), Cre′ta al′ba (Ph. D.), L. Prep. 1. (Ph. D. 1836.) Rub chalk, 1 lb., with sufficient water, add gradually, until reduced to a smooth cream; then stir this into a large quantity of water, and, after a short interval, to allow the coarser particles to subside, pour off the supernatant water (still turbid) into another vessel, and allow the suspended powder to settle; lastly, collect the chalk so prepared and dry it. In the same way shells are prepared, after being first freed from impurities and washed with boiling water.
2. (Commercial; Whi′ting.) On the large scale the chalk is ground in mills, and the elutriation and deposit made in large reservoirs. It is now seldom prepared by the druggist.
Pur. Almost entirely soluble in dilute hydrochloric acid, provided it contains no sulphate431 of lime or silica, giving off small bubbles of carbonic acid gas.
Test. The salt formed by dissolving the chalk in hydrochloric acid, if rendered neutral by evaporation to dryness and redissolved in water, gives only a very scanty precipitate on the addition of a saccharated solution of lime, indicating absence of phosphate. (B. P.)
Uses, &c. In medicine, as an absorbent, antacid, and desiccant; in acidity, heartburn, dyspepsia, and other like stomach affections, and in diarrhœa, depending on acidity or irritation; in the latter, generally combined with aromatics, astringents, or opium. It forms a valuable dusting powder in excoriations, ulcers, &c., especially in those of children.—Dose, 10 gr. to a spoonful, in a little water or milk, or made into a mixture with mucilage or syrup.
Chalk, Red. A natural clay containing about 18% of protoxide and carbonate of iron.
CHALYB′EATES. Syn. Chalybea′ta, Ferrugin′ea, L. The medicinal qualities of the preparations of iron are noticed under the name of that metal. Those most frequently employed in medicine are—IRON FILINGS; Quevenne’s iron; the BLACK OXIDE, MAGNETIC OXIDE, and SESQUIOXIDE OF IRON; the AMMONIO-CHLORIDE and SESQUICHLORIDE; the CARBONATE and SACCHARINE CARBONATE; the CITRATE and AMMONIO-CITRATE; the IODIDE, LACTATE, and SULPHATE; the TARTRATE, AMMONIO-TARTRATE, and POTASSIO-TARTRATE OF IRON; and the CHALYBEATE MINERAL WATERS. For the doses, &c., see the respective articles.
CHAM′OMILE. Syn. Anthe′mis, L. The flowers of the Anthemis nobilis (Anthemidis Flores, B. P.). They are bitter, stomachic, and tonic; in dyspepsia, loss of appetite, intermittents, &c. They are an effectual remedy for nightmare; and, according to Dr Schall, the only certain remedy for that complaint.—Dose, 10 gr. to 1⁄2 dr., or more, in powder or made into a tea. Fomentations are also made with it. See Extracts, Oils, Pills, &c.
CHAMPAGNE′. See Wines.
CHAPS. These are too well known to require description. Chapped hands are common amongst persons with a languid circulation, who are continually “dabbling” in water during cold weather. Chapped lips generally occur in persons with pallid, bluish, moist lips, who are much exposed to the wind in dry cold weather; especially in those who are continually moving from heated apartments to the external air. The application of a little COLD CREAM, POMATUM, SPERMACETI OINTMENT, LARD, or any similar article, will generally prevent chaps on the lips, and chaps and chilblains on the hands. Persons employed in oil and tallow works, or about oil, and who have consequently their hands continually in contact with greasy matter, never suffer from these things. A little oil or unguent of any kind, well rubbed on the hands on going to rest (removing the superfluous portion with a cloth), will not only preserve them from cold, but tend to render them both soft and white. See Chilblain.
CHAR (Potted). The flesh of the Salmo Alpinus (Linn.), or trout of the Alps, common in the lakes of Lapland, preserved by the common process of potting.
CHAR′BON-ROUX [Fr.]. See Charcoal, Wood (below).
CHAR′COAL. Charcoal is made by charring organic substances, such as wood, bone, blood, &c., and is, in other words, the fixed residuum of vegetable or animal matter exposed to a high temperature out of contact with atmospheric air.
There are several different varieties of charcoal, the chief of which, however, are wood and animal charcoal.
Charcoal, Animal. Syn. Animal black, Bone black, Ivory black, Carbo animalis. The charcoal obtained by igniting bone in close vessels, but often applied likewise to any charcoal obtained from animal matter.
Commercial. Bones (deprived of their grease by boiling) are broken to pieces, and put into small cast-iron pots, varying from 3⁄8 to 1⁄2 an inch in thickness. Two of these being filled, are dexterously placed with their mouths together and then luted with loam. A number of these vessels are then placed side by side and piled on each other, in an oven resembling a potter’s kiln, to the number of 100 or 150, or even more. The fire is next kindled, and the heat kept up strongly for 10 or 12 hours, according to circumstances, until the process is completed. The whole is then allowed to cool before opening the pots.
A more economical method is by distillation, as under:—
Bones (previously boiled for their grease) are introduced into retorts similar to those used in gas works, and heat being applied, the volatile products are conveyed away by iron pipes to cisterns where the condensable portion is collected. As soon as the process of distillation is finished, the solid residuum in the retorts, while still red hot, is removed through their lower ends into wrought-iron canisters, which are instantly closed by air-tight covers and luted over. These are then raised to the ground by a crane, and set aside to cool.
The bones, having been carbonised, are ground in a mill, and the resulting coarse powder, sorted by sieves into two kinds, one, granular, somewhat resembling gunpowder, for decolorising liquids, and the other, quite fine, to be used as a pigment. The first is sold under the name of animal charcoal; the second as bone or ivory black. The latter and other fine varieties of animal charcoal are fully described under the head of Black pigments.
Uses, &c. This crude animal charcoal possesses432 the valuable property of taking lime and other saline matter from syrups and other aqueous solutions, especially organic ones, at the same time that it decolours them. Its power as a decoloriser may be tested by adding it to a solution of brown sugar or of molasses, or to water containing 1⁄1000 part of indigo dissolved in sulphuric acid. The test should be made in a small glass tube. By well washing and carefully reburning it, this charcoal may be used any number of times. As a decoloriser and deodoriser, animal charcoal is vastly superior to vegetable charcoal.
Dr Stenhouse has invented a charcoal respirator to cover over the mouth and nostrils of a person going into an infected atmosphere. Charcoal is also used with excellent effect to prevent the escape of noxious vapours and offensive effluvia from the ventilating openings of sewers. The charcoal condenses and oxidises the escaping sewer gas in its pores. Dr Garrod has proposed animal charcoal as a general antidote in cases of poisoning.
Prepared Animal Charcoal. Hydrochloric acid, 1 lb.; water, 1 pint; mix, add bone black, 7 lbs.; make a paste; in 2 or 3 days stir in boiling water, 1 quart; and the next day wash it with fresh water until the washings cease to affect litmus paper or a solution of carbonate of sodium; then collect it in a cloth, and drain, press, and dry it; lastly, heat it to redness, as before. Used to decolour syrups, &c.; and occasionally by the distillers and rectifiers.
The most powerful charcoal is prepared by calcining blood, and well washing the residue, and which is the method of the last ‘London Pharmacopœia.’ The B. P. directs it to be made by burning bones in a closed vessel.
Concluding Remarks. Animal charcoal, however prepared, if intended to be used as a deodoriser or decoloriser, should be kept thoroughly excluded from the air, as by exposure it loses all its valuable properties, and becomes absolutely inert. Freshly burnt charcoal is therefore to be employed whenever it can be obtained.
Charcoal, Wood. Syn. Veg′etable charcoal; Car′bo lig′ni, L. The residue obtained after heating wood without access of air to about 572° Fahr. It is extremely porous, and retains the structure of the wood from which it is derived. It consists essentially of carbon and of the fixed or inorganic matter which exists in wood; but if carbonisation be imperfectly effected, it may contain a sensible amount of hydrogen.
Charcoal burning is effected in the open air in piles or stacks provided with a yielding cover, in pits, in closed chambers of brick or stone, and in iron retorts heated externally like common gas retorts. The latter method is only practised by the manufacturers of pyroligneous acid and gunpowder.
Charcoal for Fuel, &c. The method of pile burning is that which is most extensively practised. Pieces of wood of equal length are piled concentrically round a sort of chimney formed by driving 3 stakes in the ground; those nearest the centre are almost vertical, and the surrounding pieces have a slight but gradually increasing inclination; a second row, and in the case of very large piles even a third, may be stacked in a similar manner one above the other. The pile is covered with turf and soil, and kindled by filling the space within the 3 central stakes with easily inflammable wood, which is ignited. The character of the smoke which issues from vents made in the piles indicates exactly the degrees of carbonisation in different parts. When the charcoal is drawn from the pile it is extinguished by cold water, or if that is not at hand, by charcoal dust or dry soil. In some parts of Sweden the wood is charred in large rectangular stacks, and in China the method of charring in pits is practised.
Charcoal for Gun′powder; Cyl′inder Charcoal. The charcoal employed in the manufacture of gunpowder is burnt in close iron cylinders, and has hence received the name of cylinder charcoal. For this and other nice purposes it is essential that the last portion of the tar and vinegar should be suffered to escape, and the reabsorption of the crude vapours prevented by cutting off the communication between the cylinders and the condensing apparatus; as without this precaution, on the fire being withdrawn, a retrograde movement of the product takes place, and the charcoal is much reduced in quality. Alder and willow are the woods chiefly used for making charcoal at Waltham Abbey. The Dutch white willow, and after that the Huntingdon willow, are said to yield the best charcoal for gunpowder. The charcoal from the cylinders of the pyroligneous acid (wood vinegar) works is also called cylinder charcoal, and is that chiefly used for chemical purposes; but it is inferior to that prepared for gunpowder.
Charcoal for Scientific Purposes. The box-wood charcoal, employed in voltaic electricity, is prepared by putting prismatic pieces of box-wood, about 1 inch long by 1⁄2 inch thick, into a crucible, which is then filled with clean, dry sand, covered up, and exposed to a red heat for about an hour.
Uses, &c. These are numerous and varied. Charcoal is extensively employed as a fuel; and in metallurgy for tempering metals, making steel, &c.; reduced to powder, it is used to surround vessels and bodies required to retain their heat for some time; a coating of charcoal, formed on piles and stakes of wood by charring them, promotes their preservation. Fresh burnt charcoal, in coarse powder, restores tainted meat and putrid water, discolours vegetable solutions, deodorises fetid substances, and withdraws lime from syrups filtered through it. Exposed on trays it is used as a disinfectant and deodoriser in433 the wards of hospitals and in dissecting rooms, also as a material for water filters.
In medicine, charcoal is principally used as a deodoriser and disinfectant, either in the form of powder or made into a poultice. It has been given internally in dyspepsia, diarrhœa, dysentery, heartburn, agues, constipation, sickness of pregnancy, and various other diseases, with advantage. As a prophylactic of cholera and fevers it is invaluable and superior to all other substances. It forms the best tooth powder known, as it both whitens the teeth and deodorises the breath.—Dose, 10 gr. to a teaspoonful, or more ad libitum. An ointment made with lard and charcoal has been successfully employed in some skin diseases. In all cases, to be useful, the charcoal must be both fresh-burnt and fresh-powdered, and carefully preserved, out of contact with the air, until about to be administered. Fresh carbonised bread forms an excellent charcoal, both for a prophylactic and a tooth powder.
Charcoal varies in its qualities according to the substance from which it is prepared: that of the soft woods (willow or alder) is best for crayons and gunpowder; that of the hard woods for fuel, and for blowpipe supports. That made by a low red heat, not exceeding cherry red, and which has a dull surface, is the most valuable. If the heat be carried much beyond this point, the charcoal acquires a brilliant surface, and deteriorates in quality. Chestnut charcoal is preferred by smiths for forging, as it not only burns slowly, but deadens as soon as the blast ceases. Areca-nut charcoal is preferred as a dentifrice; but the willow charcoal or box-wood charcoal is usually substituted for it by shopkeepers.
Ant., &c. Poisoning or suffocation, resulting from respiring the fumes of burning charcoal, has been already alluded to, and the treatment briefly pointed out. See Carbonic anhydride.
CHAR′GES (for Cattle). See Veterinary Medicine.
CHAR′RING (Surface). The operation by which the surface of wood is carbonised, to prevent its decay from exposure to air and moisture. Stakes and piles are generally thus treated before they are driven into the ground. Casks are charred on the inside by coopers when they are intended to hold water. In both these cases the fire is commonly applied directly to the wood. A new method has, however, been lately employed with apparent success. This consists in washing the wood with the strongest oil of vitriol. In this way not only the outer surface, but the surface of all the cracks and holes, get carbonised, which is not the case when heat is employed. It succeeds admirably with musty casks and vats.
CHATHAM LIGHT. A flash light used for military signals. It is produced by blowing a mixture of pulverised rosin and magnesium dust through the flame of a spirit lamp.
CHEESE. Syn. Ca′seum, Ca′seus, L. The curd of milk compressed into a solid mass. That of commerce is usually salted and dried, and in some varieties it is also coloured and flavoured.
The process of cheese-making is one which is eminently interesting and scientific, and which, in every gradation, depends on principles which chemistry has developed and illustrated. When a vegetable or mineral acid is added to milk, and heat applied, a coagulum is formed, which, when separated from the liquid portion, constitutes cheese. Neutral salts, earthy and metallic salts, sugar, and gum Arabic, as well as some other substances, also produce the same effect; but that which answers the purpose best, and which is almost exclusively used by dairy farmers, is rennet, or the mucous membrane of the last stomach of the calf. Alkalies dissolve this curd at a boiling heat, and acids again precipitate it. The solubility of casein in milk is occasioned by the presence of the phosphates and other salts of the alkalies. In fresh milk these substances may be readily detected by the property it possesses of restoring the colour of reddened litmus paper. The addition of an acid neutralises the alkali, and so precipitates the curd in an insoluble state. The philosophy of cheese-making is thus expounded by Liebig:—
“The acid indispensable to the coagulation of milk is not added to the milk in the preparation of cheese, but it is formed in the milk at the expense of the milk-sugar present. A small quantity of water is left in contact with a small quantity of a calf’s stomach for a few hours, or for a night; the water absorbs so minute a portion of the mucous membrane as to be scarcely ponderable; this is mixed with milk; its state of transformation is communicated (and this is a most important circumstance), not to the cheese, but to the milk-sugar, the elements of which transpose themselves into lactic acid, which neutralises the alkalies, and thus causes the separation of the cheese. By means of litmus paper the process may be followed and observed through all its stages; the alkaline reaction of the milk ceases as soon as the coagulation begins. If the cheese is not immediately separated from the whey, the formation of lactic acid continues, the fluid turns acid, and the cheese itself passes into a state of decomposition.
“When cheese-curd is kept in a cool place a series of transformations takes place, in consequence of which it assumes entirely new properties; it gradually becomes semi-transparent, and more or less soft, throughout the whole mass; it exhibits a feebly acid reaction, and develops the characteristic caseous odour. Fresh cheese is very sparingly soluble in water, but after having been left to itself for two or three years it becomes (especially if434 all the fat be previously removed) almost completely soluble in cold water, forming with it a solution which, like milk, is coagulated by the addition of the acetic or any mineral acid. The cheese, which whilst fresh is insoluble, returns during the maturation, or ripening, as it is called, to a state similar to that in which it originally existed in the milk. In those English, Dutch, and Swiss cheeses which are nearly inodorous, and in the superior kinds of French cheese, the caseine of the milk is present in its unaltered state.
“The odour and flavour of the cheese is owing to the decomposition of the butter; the non-volatile acids, the margaric and oleic acids, and the volatile butyric acid, capric and caproic acids are liberated in consequence of the decomposition of glycerin. Butyric acid imparts to cheese its characteristic caseous odour, and the differences in its pungency or aromatic flavour depend upon the proportion of free butyric, capric, and caproic acids present.” In the cheese of certain dairies and districts, valerianic acid has been detected along with the other acids just referred to. Messrs Jljenko and Laskowski found this acid in the cheese of Limbourg, and M. Bolard in that of Roquefort.
“The transition of the insoluble into soluble casein depends upon the decomposition of the phosphate of lime by the margaric acid of the butter; margarate of lime is formed, whilst the phosphoric acid combines with the casein, forming a compound soluble in water.
“The bad smell of inferior kinds of cheese, especially those called meagre or poor cheeses, is caused by certain fetid products containing sulphur, and which are formed by the decomposition or putrefaction of the casein. The alteration which the butter undergoes (that is, in becoming rancid), or which occurs in the milk-sugar still present, being transmitted to the casein, changes both the composition of the latter substance and its nutritive qualities.
“The principal conditions for the preparation of the superior kinds of cheese (other obvious circumstances being of course duly regarded) are a careful removal of the whey, which holds the milk-sugar in solution, and a low temperature during the maturation or ripening of the cheese.”
Cheese differs vastly in quality and flavour, according to the method employed in its manufacture and the richness of the milk of which it is made. Much depends upon the quantity of cream it contains, and consequently, when a superior quality of cheese is desired, cream is frequently added to the curd. This plan is adopted in the manufacture of Stilton cheese and others of a like description. The addition of a pound or two of butter to the curd for a middling size cheese also vastly improves the quality of the product. To ensure the richness of the milk, not only should the cows be properly fed, but certain breeds chosen. Those of Alderney, Cheddar, Cheshire, Gloucestershire, Guernsey, and North Wiltshire deserve a preference in this respect.
The materials employed in making cheese are milk and rennet. Rennet is used either fresh or salted and dried; generally in the latter state. The milk may be of any kind, according to the quality of the cheese required. Cows’ milk is that generally employed; but occasionally ewes’ milk is used; and sometimes, though more rarely, that from goats.
In preparing his cheese, the dairy farmer puts the greater portion of the milk into a large tub, to which he adds the remainder, sufficiently heated to raise the temperature to that of new milk. The whole is then whisked together, the rennet or rennet liquor added, and the tub covered over. It is now allowed to stand until completely “turned,” when the curd is gently struck down several times with the skimming-dish, after which it is allowed to subside. The vat covered with cheese-cloth is next placed on a “horse” or “ladder” over the tub, and filled with curd by means of the skimmer, care being taken to allow as little as possible of the oily particles or butter to run back with the whey. The curd is pressed down with the hands, and more added as it sinks. This process is repeated until the curd rises to about two inches above the edge. The newly formed cheese, thus partially separated from the whey, is now placed in a clean tub, and a proper quantity of salt added, as well as of annotta, when that colouring is used, after which a board is placed over and under it, and pressure applied for about 2 or 3 hours. The cheese is next turned out and surrounded by a fresh cheese-cloth, and then again submitted to pressure in the cheese press for 8 or 10 hours, after which it is commonly removed from the press, salted all over, and again pressed for 15 to 20 hours. The quality of the cheese especially depends on this part of the process, as if any of the whey is left in the cheese it rapidly becomes bad-flavoured. Before placing it in the press the last time the common practice is to pare the edges smooth and sightly. It now only remains to wash the outside of the cheese in warm whey or water, to wipe it dry, and to colour it with annotta or reddle, as is usually done.
The storing of the newly-made cheese is the next point that engages the attention of the maker and wholesale dealer. The same principles which influence the maturation or ripening of fermented liquors also operate here. In England, a cool cellar, neither damp nor dry, and which is uninfluenced by change of weather or season, is commonly regarded as the best for the purpose. If possible, the temperature should on no account be permitted to exceed 50° or 52° Fahr. at any portion of the year. An average of about 45° is preferable when it can be procured. A place exposed to sudden changes of temperature is as unfit for435 storing cheese as it is for storing beer. “The quality of Rochefort cheese, which is prepared from sheep’s milk, and is very excellent, depends exclusively upon the places where the cheeses are kept after pressing and during maturation. Those are cellars, communicating with mountain grottoes and caverns, which are kept constantly cool, at about 41° to 42° Fahr., by currents of air from clefts in the mountains. The value of these cellars as storehouses varies with their property of maintaining an equable and low temperature. Giron mentions that a certain cellar, the construction of which had cost only 480l. (12,000 francs), was sold for 8600l. (215,000 francs), being found to maintain a suitable temperature, a convincing proof of the importance attached to temperature in the preparation of these superior cheeses.” (Liebig.)
It will thus be seen that very slight differences in the materials, in the preparation, or in storing of the cheese, materially influence the quality and flavour of this article. The richness of the milk—the addition to or subtraction of cream from the milk—the separation of the curd from the whey with or without compression—the salting of the curd—the collection of the curd, either whole or broken, before pressing—the addition of colouring matter, as annotta or saffron, or of flavouring—the place and method of storing—and the length of time allowed for maturation, all tend to alter the taste and odour of the cheese in some or other particular, and that in a way readily perceptible to the palate of the connoisseur. No other alimentary substance appears to be so seriously affected by slight variations in the quality of the materials from which it is made, or by such apparently trifling differences in the methods of preparing it.
Var. The varieties of cheese met with in commerce are very numerous, and differ greatly from each other in richness, colour, and flavour. These are commonly distinguished by names indicative of the places in which they have been manufactured, or of the quality of the materials from which they have been prepared. Thus, we have Dutch, Gloucester, Stilton, skimmed-milk, raw-milk, cream, and other cheeses; names which explain themselves. The following are the principal varieties met with in Europe:—
Cheese, Brickbat. From its form; made in Wiltshire of new milk and cream.
Cheese, Cheddar. A fine, spongy kind of cheese, the eyes or vesicles of which contain a rich oil; made up into round, thick cheeses, of considerable size (150 to 200 lbs.).
Cheese, Cheshire. From new milk, without skimming, the morning’s milk being mixed with that of the preceding evening, previously warmed, so that the whole may be brought to the heat of new milk. To this the rennet is added, in less quantity than is commonly used for other kinds of cheese. On this point much of the flavour and mildness of the cheese is said to depend. A piece of dried rennet, of the size of half-a-crown, put into a pint of water over night, and allowed to stand until the next morning, is sufficient for 18 or 20 gallons of milk. In large, round, thick cheeses (100 to 200 lbs. each). They are generally solid, homogeneous, and dry, and friable rather than viscid.
Cheese, Cottenham. A rich kind of cheese, in flavour and consistence not unlike Stilton, from which, however, it differs in shape, being flatter and broader than the latter.
Cheese, Cream. From the “strippings” (the last of the milk drawn from the cow at each milking), from a mixture of milk and cream, or from raw cream only, according to the quality desired. It is usually made in small oblong, square, or rounded cakes, a general pressure only (that of a 2 or 4 lb. weight) being applied to press out the whey. After twelve hours it is placed upon a board or wooden trencher, and turned every day until dry. It ripens in about three weeks. A little salt is generally added, and frequently a little powdered lump sugar.
Cheese, Derbyshire. A small, white, rich variety, very similar to Dunlop cheese.
Cheese, Dunlop. Rich, white, and buttery; in round forms, weighing from 30 lbs. to 60 lbs.
Cheese, Dutch. (Holland.) Of a globular form. 5 to 14 lbs. each. Those from Edam are very highly salted; those from Gouda less so.
Cheese, Gloucester. Single Glo′ster; from milk deprived of part of its cream; Double Glo′ster, from milk retaining the whole of the cream. Mild tasted, semi-buttery consistence, without being friable; in large, round, flattish forms.
Cheese, Green or Sage. From milk mixed with the juice or an infusion or decoction of sage leaves, to which marygold flowers and parsley are frequently added.
Cheese, Gruyère. A fine description of cheese made in Switzerland, and largely consumed on the Continent. It is firm and dry, and exhibits numerous cells of considerable magnitude. Its flavour is peculiar, and is not generally liked by English people.
Cheese, Lincoln. From new milk and cream; in pieces about 2 inches thick; soft, and will not keep over 2 or 3 months.
Cheese, Neufchâtel. A much-esteemed variety of Swiss cheese; made of cream, and weighs about 5 or 6 oz.
Cheese, Norfolk. Dyed yellow with annotta or saffron; good, but not superior; in cheeses of 30 lbs. to 50 lbs.
Cheese, Parmesan. (Parma, &c.) From the curd of skimmed milk, hardened by a gentle heat. The rennet is added at about 120°, and an hour afterwards the curdling milk is set on a slow fire until heated to about436 150° Fahr.; during which the curd separates in small lumps. A few pinches of saffron are then thrown in. About a fortnight after making the outer crust is cut off, and the new surface varnished with linseed oil, and one side coloured red.
Cheese, Roquefort. From ewes’ milk; the best prepared in France. It greatly resembles Stilton, but is scarcely of equal richness or quality, and possesses a peculiar pungency and flavour.
Cheese, Slipcoat or Soft. A very rich white cheese, somewhat resembling butter; for present use only.
Cheese, Stilton. The richest and finest cheese made in England. From raw milk to which cream taken from other milk is added; in cheeses generally twice as high as they are broad. Like wine, this cheese is vastly improved by age, and is therefore seldom eaten before it is 2 years old. A spurious appearance of age is sometimes given to it by placing it in a warm, damp cellar, or by surrounding it with masses of fermenting straw or dung.
Cheese, Suffolk. From skimmed milk; in round, flat forms, from 24 lbs. to 30 lbs. each. Very hard and horny.
Cheese, Swiss. The principal cheeses made in Switzerland are the Gruyère, the Neufchâtel, and the Schabzieger or green cheese. The latter is flavoured with melilot.
Cheese, Westphalian. In small balls or rolls of about 1 lb. each. It derives its peculiar flavour from the curd being allowed to become partially putrid before being pressed. In small balls or rolls of about 1 lb. each.
Cheese, Wiltshire. Resembles poor Cheshire or Glo′ster. The outside is generally painted with a mixture of reddle or red-ochre or whey.
Cheese, York. From cream: it will not keep.
Qual., &c. Cheese has been objected to as an article of diet, but without sufficient reason, since it is, when of good quality, eminently nutritious, wholesome, and digestible. Like all other food, cheese digests more readily when well masticated, and the neglect of this precaution is one reason why it frequently disagrees with delicate stomachs. It is rendered more agreeable to many palates by toasting it, but becomes less digestible by that operation. The basis of cheese is casein or coagulated curd, a protein substance; it therefore cannot fail to prove nutritious, provided it is properly digested. Cheese-curd, carefully freed from water and milk by expression, and the addition of salt, is a mixture of casein and butter. It contains all the phosphate of lime and part of the phosphate of soda of the milk. (Liebig.) When taken as a condiment, especially when rich and old, it powerfully promotes the secretion of the saliva and gastric juice, and thereby aids the stomach in performing its proper functions. Rotten cheese is very unwholesome.
We give below the composition of some of the principal varieties of cheese:—
| Cheddar. | Double Gloucester. | Skim. | |
| Water | 36·64 | 35·61 | 43·64 |
| Casein | 23·38 | 21·76 | 45·64 |
| Fatty matter | 35·44 | 38·16 | 5·76 |
| Mineral matter | 4·54 | 4·47 | 4·96 |
| ——— | ——— | ——— | |
| 100·00 | 100·00 | 100·00 | |
| Stilton. | Cotherstone. | ||
| Water | 32·18 | 38·28 | |
| Butter | 37·36 | 30·89 | |
| Casein | 24·31 | 23·93 | |
| Milk, sugar, and extractive matters | 2·22 | 3·70 | |
| Mineral matter | 3·93 | 3·20 | |
| ——— | ——— | ||
| 100·00 | 100·00 | ||
| Gruyère. | Ordinary Dutch. | ||
| Water | 40·00 | 36·10 | |
| Casein | 31·50 | 29·40 | |
| Fatty matter | 24·00 | 27·50 | |
| Salts | 3·00 | ·90 | |
| Non-nitrogenous organic matter and loss | 1·50 | 6·10 | |
| ——— | ——— | ||
| 100·00 | 100·00 |
Concluding Remarks.—It is surprising that cheese is not more frequently made an article of domestic manufacture, especially by housewives resident in the country. The operations of cheese-making are all exceedingly simple, and not laborious, and will, in most cases, amply repay the outlay for the milk. Besides, cheese is not unfrequently coloured with stains and pigments which are injurious, and even poisonous, the risk of taking which is not encountered when it is made at home. Several persons have nearly lost their lives from eating cheese coloured with annotta, for instance. This substance, though harmless in itself, is frequently adulterated with red lead, so that the cheesemonger may very innocently introduce a poison, when he only intends to improve the colour of his goods.
When a whole cheese is cut, and the consumption small, it is generally found to become unpleasantly dry, and to lose flavour before it is consumed. This is best prevented by cutting a sufficient quantity for a few days’ consumption from the cheese, and keeping the remainder in a cool place, rather damp than dry, spreading a thin film of butter over the fresh surface, and covering it with a cloth or pan to keep off the dirt. This removes the objection existing in small families against purchasing a whole cheese at a time. The common practice of buying small quantities of cheese should be avoided, as not only a higher price is paid for any given quality, but there is little likelihood of obtaining exactly the same flavour twice running. Should cheese become too dry to be agreeable, it may be used437 for stewing, or for making grated cheese or Welsh rare-bits.
Cheese, Ap′ple. The pomace or ground apples from the cider press.
Cheese, Dam′son. Prep. From damsons boiled with a little water, the pulp passed through a sieve, and then boiled with about one fourth the weight of sugar, until the mixture solidifies on cooling; it is next poured into small tin moulds previously dusted out with sugar. Cherry cheese, gooseberry cheese, plum cheese, &c., are prepared in the same way, using the respective kinds of fruit. They are all very agreeable candies or confections.
Cheese, Facti′tious Roque′fort. Prep. (Roulle.) The gluten of wheat is kneaded with a little salt, and a small portion of a solution of starch, and made up into cheeses. It is said that this mixture soon acquires the taste, smell, and unctuosity of cheese, and when kept a certain time is not to be distinguished from the celebrated Roquefort cheese, of which it possesses all the peculiar pungency. By slightly varying the process other kinds of cheese may be imitated.
Cheese, Legumin. The Chinese prepare an actual cheese from peas, called “tao-foo,” which they sell in the streets of Canton. The paste from steeped ground peas is boiled, which causes the starch to dissolve with the casein; after straining the liquid, it is coagulated by a solution of gypsum; this coagulum is worked up like sour milk, salted, and pressed into moulds.
Cheese, Toasted. This much relished article is seldom well prepared. The following has been recommended as an excellent receipt:—Cut the cheese into slices of moderate thickness, and put them into a tinned copper saucepan, with a little butter and cream; simmer very gently until they are quite dissolved, then remove the saucepan from the fire, allow the whole to cool a little, add some yolk of egg, well beaten, add spice, make the compound into a “shape,” and brown it before the fire. See Fondue.
CHELSEA PENSIONER. Prep. From gum guaiacum, 1⁄4 oz.; rhubarb, 1⁄2 oz.; cream of tartar, 2 oz.; flowers of sulphur, 4 oz.; nutmegs, 2 in number (all in powder); honey, 11⁄2 lb., or q. s.; made into an electuary by beating them together in a mortar.—Dose, 1 to 2 table-spoonfuls, night and morning, in gout and chronic rheumatism. The name is said to have been given to it from the circumstance of a Chelsea pensioner having cured Lord Amherst with it.
CHEL′TENHAM SALTS. See Salts.
CHEM′IQUE or CHEM′IC BLUE. See Indigo.
CHEROOT. A species of cigar imported from Manilla, in the Philippine Islands, distinguished by extreme simplicity of construction as well as delicacy of flavour. The cigars now so commonly sold as cheroots in England are, for the most part, made of inferior tobacco, and are often much adulterated articles.
CHER′RIES are the fruit of different species of the genus Cerasus. They are regarded as wholesome, cooling, nutritive, laxative, and antiscorbutic. Brandy flavoured with this fruit or its juice is known as cherry-brandy. Morello cherries preserved in brandy are called brandy cherries. See Brandy, Fruit, &c.
CHER′RY LAUR′EL. Syn. Lau′rel. The Cerasus Lauro-Cerasus, a shrub common in every garden in England, and often confounded with the true laurel or Sweet Bay, which does not possess any of its deleterious properties. Leaves, occasionally used instead of bay leaves in cookery. The distilled oil and distilled water are both poisonous. See Oil, Water.
CHESTNUT. Both the horse-chestnut and the edible variety have been employed for the adulteration not only of coffee, but of chicory.
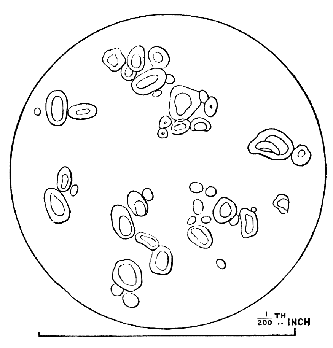 Microscopic view of the chestnut.
Microscopic view of the chestnut.
CHI′CA. The red colouring matter deposited by a decoction of the leaves of Bignonia Chica in cooling. Used by the American Indians to stain their skin. It is soluble in alcohol, ether, oil, fat, and alkaline lyes, and slightly so in boiling water.
Chi′ca. See Maize beer.
CHIC′ORY. Syn. Wild suc′cory; Cichor′ium Inty′bus (Linn.), L. A plant belonging to the natural order Compositæ. It is indigenous to this and many other countries of Europe, and is extensively cultivated for the sake of its roots, which are sliced, roasted, and ground, to form the chicory of the shops. Nearly 100 millions of pounds are annually consumed in Europe. Much of the chicory used in Britain is of home growth; but still more is imported in a raw state from Holland and other parts of the Continent. A blue dye has been prepared from the leaves of this plant.438
The FRESH ROOT OF CHICORY (ra′dix chico′rii re′cens) is reputed to be alterative, attenuant, diuretic, febrifuge, hepatic, resolvent, and tonic; and in large doses aperient. It is now seldom used in medicine, although it appears to possess similar qualities and equal activity to those of dandelion. “An infusion of the root, mixed with syrup, becomes thick; forming the GOMME SACCHO-CHICORICE of Lacarterie.” (Fee.)
| Moisture | 77·0 |
| Gummy matter (like pectin) | 7·5 |
| Glucose, or grape sugar | 1·1 |
| Bitter extractive | 4·0 |
| Fatty matter | 0·6 |
| Cellulose, inulin, and woody matter | 9·0 |
| Ash | 0·8 |
| ——— | |
| 100·0 |
The ROASTED ROOT is prepared by cutting the full-grown root into slices, and exposing it to heat in iron cylinders, along with about 11⁄2% or 2% of lard, in a similar way to that adopted for coffee. When ground to powder in a mill, it constitutes the CHICORY of the grocers (CHICORY COFFEE, SUCCORY C.; RADIX CHICO′RII TORREFAC′TA, R. C. T. CONTRI′TA); so generally employed both as a substitute for coffee and as an adulterant of that article. The addition of 1 part of good, fresh roasted chicory to 10 or 12 parts of coffee forms a mixture which yields a beverage of a fuller flavour, and of a deeper colour than that furnished by an equal quantity of pure or unmixed coffee. In this way a less quantity of coffee may be used, but it should be remembered that the article substituted for it does not possess in any degree the peculiar exciting, soothing, and hunger-staying properties of that valuable product. The use, however, of a larger proportion of chicory than that just named imparts to the beverage an insipid flavour, intermediate between that of treacle and liquorice; whilst the continual use of roasted chicory, or highly chicorised coffee, seldom fails to weaken the powers of digestion and derange the bowels. “There can be no doubt that roasted chicory must, when taken largely, have a tendency to excite diarrhœa.” (Pereira.)
Pur., &c. The ground chicory of the shops, like ground coffee, is almost universally adulterated. Pigments are added to it to colour it, and various vegetable substances to lessen its value. The following articles have been reported to have been detected in roasted chicory, or to have been known to be used to adulterate it:—Venetian red, reddle, and red clay; roasted acorns, beans, carrots, damaged dog-biscuits, damaged bread, damaged wheat, horse-chestnuts, mangel wurzel, parsnips, peas, rye, and sugar; coffee flights (coffee husks), coffina (roasted lupins), Hambro’ powder (roasted peas coloured with reddle), and the marc of coffee; exhausted bark (from the tan yards), logwood dust, mahogany dust, &c. It has also been asserted that the scorched livers of bullocks, horses, and dogs have been applied to the same purpose; but of this there is not sufficient evidence. The only way to avoid being thus cheated or poisoned is to buy the chicory whole, and to grind it at home.
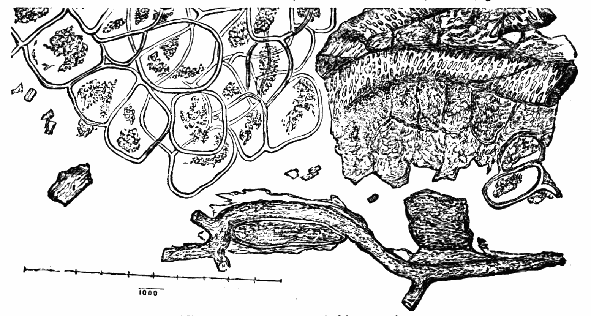 Microscopic appearance of chicory root.
Microscopic appearance of chicory root.
Roasted chicory is highly absorbent of moisture, and should, therefore, be always kept in close vessels (bottles or canisters), the same as coffee. If the lumps become tough or soft, or the powder cakes together, it is unfit for use; but in some cases it may be recovered, by exposing it on a plate in an oven until it again becomes perfectly dry or brittle.
Tests.—1. Powdered chicory thrown on water turns it reddish-brown and rapidly sinks, leaving light impurities either floating or diffused through the liquid.—2. The cold decoction tested with tincture, or solution of iodine, gives a brown colour; if it turns purple, blue, or black, it indicates the presence of roasted peas, beans, rye, or some other439 like substance, containing starch.—3. Persulphate or perchloride of iron, added in the same way, should not materially affect the liquid; if it turns it bluish or blackish, it indicates the presence of roasted acorns, oak-bark tan, or some other substance containing tannin.—4. Water acidulated with vinegar, digested on the powder, should not be blackened, or even materially darkened, by tincture of galls or a solution of red prussiate of potash; the contrary shows the presence of ferruginous colouring matter.—5. The dry powder, when incinerated, should not leave more than 41⁄2 to 5% of ash, which should be of a greyish or fawn colour; the contrary indicates the presence of reddle, red clay, ochre, or the like.—6. To the above may be added attention to the odour, colour, and appearance, both to the naked eye and under the microscope; by the latter, adulteration may be easily detected. See Coffee.
CHIL′BLAIN. Syn. Per′nio, L. An inflammatory swelling, of a purple or lead colour, produced by the action of cold. Chilblains (PERNIO′NES) exclusively attack the extremities of the body, and are generally confined to the fingers, toes, and heels. The common symptoms are itching and irritation, more or less intense, often accompanied with shooting pains, and tenderness, and tumefaction of the parts. Children, especially those of a scrofulous habit, and elderly persons, are generally the most liable to chilblains. The common cause of chilblains is holding the hands or feet to the fire after exposure to cold. The sudden change of temperature partially destroys the vitality of the minute surfacial vessels, and thus prevents the proper flow of blood through the part. The best preventives of chilblains are woollen socks or stockings, good waterproof shoes, woollen gloves, exercise, and friction. These act by promoting the circulation of the blood in the extremities, and protecting them from vicissitudes of temperature. When chilblains have once formed, the best treatment is friction with stimulants, as spirits of wine and camphor, oil of turpentine, opodeldoc, dilute spirits, camphorated oil, hartshorn and oil, &c. Linnæus recommends bathing the part with dilute muriatic acid, just strong enough to faintly prick the skin. When the inflamed parts have ulcerated, they are commonly called KIBES. In this state they should be dressed with a little resin cerate or elemi ointment. If fungous granulations appear, they must be removed by touching them with nitrate of silver or blue vitriol. See Chaps, &c.
Remedies for Chilblains.—The following have been strongly recommended by different parties, and may all prove useful in their turns, as circumstances and convenience may suggest:—
1. Sulphate of copper, 1 oz.; rosemary water, 1 pint; dissolve.
2. Sal-ammoniac, 1 oz.; vinegar, 1⁄2 pint.
3. Sal-ammoniac, 1 oz.; rum, 1⁄2 pint; camphor, 1 dr. The affected part is wetted with the above night and morning, and when dry is touched with a little simple ointment, cold cream, or pomatum.
4. Soap liniment, 2 oz.; tincture of cantharides, 1 oz.; oil of cajeput, 2 dr.
5. Oil of turpentine, 2 oz.; camphor, 3 dr.; oil of cajeput, 1 dr. The application of the last two is accompanied by gentle friction.
6. (Dr Graves’ Preventive.) Sulphate of copper, 20 gr.; water, 1 oz. As the first three.
7. (Lejeune’s Balsam.) See Chilblain Balsam.
8. (Linnæus’ remedy.) Hydrochloric acid, 1 oz.; water, 11 oz. As No. 3.
9. (Morton’s Liniment.) Calomel and camphor, of each 1 dr.; spermaceti ointment, 4 dr.; oil of turpentine and cocoa-nut oil, of each 2 dr. As No. 5.
10. (Wahler’s Ointment.) Black oxide of iron, bole, and oil of turpentine, of each 1 dr.; resin cerate, 1 oz. For broken chilblains. We have found a mixture of equal parts of calamine cerate and resin cerate answer quite as well. See Cerate, Ointment.
11. (Russian remedy.) The rind of perfectly ripe cucumbers dried, with the soft parts attached. For use they are placed with the inner side, previously soaked in warm water, over the soft parts. (Med. Zeitung.)
12. (Rhein.) Dissolve 1 oz. of tannin in a pint of water, and 74 grains of iodine in 13⁄4 oz. of spirit of wine; the solutions are then mixed, and enough water added to make the whole up to 21⁄2 pints. In applying it, which is best done at bedtime, the mixture is gently warmed over a slow fire; the affected part is dipped into it while still cold, and retained in it till the liquid, on being stirred, feels uncomfortably hot. The vessel is then withdrawn from the fire, and the affected part dried over it. The vessel must be of earthenware or porcelain, and care must be taken not to use too much iodine, especially when abrasions are present.
13. (L′Union Médicale.) Oxide of zinc, 2 parts; tannic acid, 1 part; glycerin, 10 parts; balsam of Peru, 8 parts; camphor, 4 parts.
14. (Swediaur’s Paste.) Bitter almonds, 8 oz.; honey, 6 oz.; powdered camphor and flour of mustard, of each 1⁄2 oz.; burnt alum and olibanum, of each 1⁄2 oz.; yolks of 3 eggs; beat to a paste. To be applied night and morning.
15. (Vance’s Cream.) Ointment of nitrate of mercury, 1 oz.; camphor, 1 dr.; oil of turpentine, 2 dr.; oil of olives, 4 dr.; mix well together. To be applied by gentle friction 2 or 3 times daily.
Obs. All the preceding preparations are intended for chilblains before they break. The liniments of ammonia, camphor, opium, soap,440 and turpentine, as well as the compound camphor liniment of the British Pharmacopœia, are also excellent preparations for the same purposes.
CHIL′DREN (Care of). See Infancy.
Children (Diseases of). See the respective heads, and Diseases.
CHIL′LIES. See Capsicum, Peppers.
CHIM′NEYS were not introduced into England until the reign of Queen Elizabeth, and for a considerable period the principles of their construction were ill-understood. When the air inside and outside a chimney is at the same temperature, an equilibrium exists; there is no draught in the chimney, because the downward tendency of that within is counteracted by the upward pressure of that without. Let a fire be kindled in the grate; hot air is evolved, the chimney is heated, the air it contains suffers expansion, and a portion is expelled. The chimney now contains a smaller weight of air than it did before; the external and internal columns no longer equibalance each other, the warmer and lighter air is forced upwards from below, and its place is occupied by cold, and consequently heavier air. If the fire continues to burn, and the chimney retains its temperature, the second portion of air is disposed of like the first, and the ascending current continues, so long as the sides of the chimney are hotter than the surrounding air. Should the reverse happen to be the case, as sometimes occurs from sudden atmospheric changes, the column of air within the chimney rapidly contracts in volume, the deficiency is filled up from without, the column of air becomes heavier than one of a corresponding height on the outside of it, or in the apartment, and, obeying the common laws of gravitation, it falls out of the throat of the chimney or fire-place just as a heavy body sinks in water, and has its place occupied by air from above. In this way a descending current, of more or less intensity and duration, is established, and, if there is a fire in the grate, the chimney “smokes,” or, if the grate is empty, perhaps the smoke from neighbouring chimneys finds its way into our apartments. By the judicious arrangement of the fire-place, and the throat and flue of a chimney, an upward current may be constantly ensured so long as there is a fire in the grate, or the air of the apartment is warmer than the external atmosphere.
Count Rumford was the first who scientifically investigated the construction of chimneys. He showed that more heat is obtained from the fire by reflection when the coverings are placed in an oblique position. He also directed that the fire itself should be kept as near to the hearth as possible, and that the throat of the chimney should be constructed much narrower than was then the practice, in order to prevent the escape of so much heated air as happened with wide throats. By contracting the open part of the fire-place immediately over the fire, as by lessening the width of the hobs, or by bringing the throat of the chimney closer to the fire, and by contracting the throat of the chimney itself, within certain limits, any desired amount of draught may be obtained. When the space above the fuel is too small, the throat too near the burning fuel, or the throat itself too contracted, the draught of a common chimney is often too strong, and much fuel and heat is wasted. When the reverse is the case, the draught is commonly too languid, the fire draws badly, a portion of the smoke escapes into the room, and all the usual annoyances of a smoky chimney are suffered. By a proper attention to these conditions a common fire-place may be adapted for the combustion of bituminous or easy burning coal, or of anthracite, and varieties of coal that require a considerable draught. It may even be converted into a wind furnace; whilst the inconvenience of smoky chimneys may be always avoided, and, when existing, easily cured. This is presuming, however, that a sufficient supply of air exists in front of the fire-place (i.e. in the apartment), not only for the combustion of the fuel, but also for the upward current of the chimney. Many chimneys smoke simply from the apartment being so ill-ventilated that the supply here alluded to is not provided. It may be further stated, as a rule, that the greater the length of a chimney the stronger will be the draught. Hence, the chimneys of the upper rooms of a house often smoke, whilst the fires in the rooms beneath them burn pleasantly and vigorously. Such cases are commonly relieved by a chimney-pot or cowl, of which numerous varieties are now before the public. The more crooked or tortuous the course of a chimney the less likely is it to be affected by eddies and gusts of wind from neighbouring buildings or hills. See Fire, Grate, Smoke Prevention, Stove.
CHI′NA. In the purchase of china, glass, and earthenware, care should be taken to select those patterns which in case of breakage can be the most readily matched. Peculiar or rare patterns should be avoided, for if any such be broken, it will generally be found very difficult and expensive, and frequently impossible, to replace them.
China, glass, and earthenware, when very dirty, are best cleaned with finely powdered fuller’s earth and warm water, followed by rinsing in clean water. A little clean soft soap may be added to the water instead of fuller’s earth. See Packing, Porcelain.
CHIN′OIDINE. See Quinoidine.
CHINOLINE BLUE. See Cyanine.
CHINTZ (to Wash). Boil 2 lbs. of rice in two gallons of water till soft; and pour the mixture into a tub; let it stand until it attains a warmth generally used for coloured linens; then put the chintz in it, and wash it with the rice instead of soap, until all the441 dirt has disappeared. Next boil another 2 lb. of rice, as above, in another two gallons of water, but strain the rice from the water, and mix it in warm water. Wash the chintz in this till quite clean, and afterwards rinse it in the water the rice was boiled in. This will answer the same end as starch, as no wet will affect it, as it will be stiff while it is worn. If a gown, it must be taken to pieces; and when dried, it must be hung as smooth as possible, after which it must be dry-rubbed with a smooth stone, but no iron must be used.
CHIRETTA. Chirata. The entire plant (Ophelia chirata) is employed in medicine. Northern India. The plant is pulled up by the root when the flowers begin to decay, and the capsules are formed. The dried plant, sometimes with, but more commonly without, the root, is the form in which the chiretta is generally met with in commerce. The whole plant is intensely bitter, but is without odour. In its physiological action it bears a great resemblance to gentian. Instead of a constipating, it appears to possess a slightly relaxing effect. It is an excellent stomachic and carminative, and is said to diminish the tendency to acidity, and to be of great service in the dyspepsia accompanying gout. No vegetable alkaloid has been obtained from it. If given in powder, the dose of chiretta is twenty grains. It is, however, more generally given in the form of an infusion or tincture (which see).
CHI′TIN. This name has been given to the hard, insoluble matter forming the shells and elytra of insects. It is obtained by boiling the elytra of the cockchafer with water, alcohol, ether, acetic acid, and alkalies.
CHIT′TICK’S REMEDY. Dr Chittick’s remedy for stone consisted of a fixed alkali, administered in veal broth. (Paris.)
CHLORAL. C2HCl3O. A peculiar liquid first obtained by Liebig, by the action of chlorine on alcohol. The name was intended to express its origin from chlorine and alcohol.
Prep. (Liebig.) Anhydrous alcohol is placed in a tubulated retort, and dry chlorine gas passed through it, at first in the cold, but afterwards with the application of a gentle heat, until the chlorine passes unchanged through the liquor on raising it to the boiling temperature; on cooling, the whole forms a crystalline mass of what was at one time thought to be chloral hydrate, but which subsequent researches have shown to be chloral alcoholate; this is melted by a gentle heat, and agitated with three times its volume of oil of vitriol; on increasing the heat a little, an oily stratum of impure chloral rises to the surface. It is purified by boiling it for some time (to drive off free hydrochloric acid and alcohol), next distilling it with an equal volume of oil of vitriol; and lastly, rectifying it from some powdered quick-lime, the process being stopped as soon as the surface of the lime becomes dry.
Prop., &c. Chloral is an oily liquid, possessing an ethereal smell; it is soluble in alcohol, ether, and water; with a small quantity of the latter it rapidly changes into a semi-solid crystalline mass (chloral hydrate), which is soluble in a larger quantity of water; boils at 201°; sp. gr. 1·502. It is decomposed by the caustic earths and alkalies. By age it is converted into a white, solid, translucent substance (insoluble chloral), which is reconverted by heat and by sulphuric acid into ordinary chloral.
Obs. In operating as above the chlorine is most conveniently introduced by a tube inserted into the tubulature of the retort, and a long tube, bent upwards, should be connected with the beak to convey away the hydrochloric acid gas extricated, and to allow the volatilised alcohol and chloral to condense, and flow back into the retort.
Chloral, Camphorated. Hydrate of chloral and camphor, equal parts. Rub them together in a warm mortar until they liquefy. It forms clear mixtures with oil of turpentine and chloroform, but not with solution of ammonia. It is a counter-irritant, and applied externally it has been found to give relief in rheumatic pains and neuralgia. It should be painted on the affected part with a camel’s-hair brush.
Chloral, Hydrate. C2HCl3O. Aq. Syn. Hydrate of chloral. Prep. “Pass dry chlorine gas, for several days, through absolute alcohol, sp. gr. 0·795, until it becomes a thick viscid liquid of sp. gr. 1·57. At the beginning of the operation the alcohol is well cooled to prevent inflammation and explosion, but towards the end of the operation the alcohol is heated nearly to the boiling point. The resulting liquid, which after a day or two solidifies to a mass of crude chloral hydrate, is agitated with four times its bulk of concentrated sulphuric acid, and the anhydrous chloral which floats on the surface is separated and purified by fractional distillation. The purified anhydrous chloral is placed in a still, mixed with 11 per cent. of water, and distilled over chalk to remove any hydrochloric acid that may be present; the resulting solid distillate is then fused and poured out into shallow vessels to cast into cakes.” (Squire.) The purest chloral hydrate is said to be that which has been crystallised two or three times out of pure bisulphide of carbon.—Prop. White opaque solid, having a pungent odour resembling that of a ripe melon. Soluble in water, glycerin, and alcohol. Gradually volatilises in the air, and may be distilled without decomposition. From 100 gr. dissolved in 1⁄2 fl. oz. of water, well shaken with 1 fl. oz. of solution of potash (B. P.), and allowed to stand for several hours, at least 46 grain-measures of chloroform should separate.
Chloral hydrate may be obtained in crystals442 by mixing the cake with about half its bulk of chloroform, and putting aside in a cool place. When the crystallisation is complete (which is generally in about 8 or 10 days) the crystals are freed from the mother liquor by a centrifugal machine, and afterwards dried at a gentle heat. The mother liquor may be utilised for future crystallisations.
Uses. An excellent sedative, antispasmodic, hypnotic, anodyne. It has done good service in hypochondriacal and other nervous affections, as well as in the insomnia of the insane, and of dipsomaniacs; also in asthma, hooping-cough, and scarlet fever. It has also the reputation of being an efficient preventive of sea-sickness, especially on short voyages, such as crossing the channel, which can be accomplished during the sleep occasioned by the agent.—Dose, from 10 to 60 gr.
It was introduced into medical practice by Dr Liebreich, of Berlin. Immense quantities are imported into this country from Germany.
CHLORALUM. An impure aqueous solution of chloride of aluminum, sp. gr. 1·15, 1 fl. oz. of the liquid contains 75 grains of anhydrous chloride. Introduced by Professor Gamgee as an antiseptic and disinfectant, for which purposes it is recommended to dilute the article as sold with four times its bulk of water.
CHLO′′RATE. Syn. Hyperoxymu′′riate†, Chlo′′ras, L. A compound in which the hydrogen of chloric acid, HClO3, is replaced by a metal or other basic radical, e.g. KClO3, chlorate of potassium. Chlorates may be prepared by dissolving the hydrate or oxide in chloric acid, and crystallising. The alkaline chlorates, however, are made by passing chlorine into solutions of the hydrate or carbonate of potassium or sodium, boiling the resulting liquid, and separating the chlorate from the chloride, which is also formed by crystallisation. They are very similar to the nitrates, both in their general properties and composition. They are all decomposed at a red heat, metallic chlorides being formed and oxygen gas given off. Like the nitrates, they deflagrate with inflammable substances, but with greater facility and violence. A mixture of this kind will detonate with a slight blow or friction. All the chlorates are soluble in water.
Char., Tests, &c. The chlorates are known by their deflagrating when placed on red-hot charcoal. By evolving a yellow gas when treated with concentrated sulphuric acid, in the cold, which gas also communicates to the liquid a red or yellow tinge. By evolving oxygen gas when heated alone in a test-tube. This test is not characteristic, unless carried a stage further, by dissolving the residual chloride out of the tube, and adding to the filtered solution a few drops of nitrate of silver; then the formation of a white precipitate, insoluble in nitric acid, will show that the salt treated was a chlorate, and not a nitrate. Pure chlorates give no precipitate with nitrate of silver.
CHLORHY′DRIC ACID. See Hydrochloric Acid.
CHLO′′RIC ACID. HClO3. Syn. Hyperoxymuriat′ic acid; Acidum chlo′′ricum, L. An acid discovered by Chenevix, but first obtained in a separate form by Gay-Lussac.
CHLO′′RIDE (-ĭd). Syn. Chlo′′ruret†; Chlori′dum, L. A chemical compound of chlorine with a metal or other basic radical, e.g. NaCl, chloride of sodium; C2H5Cl, chloride of ethyl.
Prep. The majority of the metallic chlorides may be made by simply dissolving the metal or its carbonate, oxide, or hydrate, in hydrochloric acid (previously diluted with about twice its weight of water), and evaporating and crystallising the solution in the usual manner. Zinc, cadmium, iron, nickel, cobalt, and tin, dissolve readily in hydrochloric acid; copper only in strong boiling acid; silver, mercury, and gold, not at all. The insoluble chlorides, as those of silver and mercury, may be readily prepared by precipitating any of their corresponding soluble salts with hydrochloric acid, or a soluble chloride, such as common salt. Anhydrous chlorides are generally prepared by the direct action of chlorine on the bases.
Char., Tests, &c. Most of the metallic chlorides are soluble in water. Many fuse when heated, and volatilise unchanged, but others are completely or partially decomposed at a red heat. All, with the exception of those of the alkali and earth metals, are decomposed at a red heat in a current of hydrogen. They are recognised by the following reactions:—1. Heated with a little peroxide of manganese and sulphuric acid, chlorine is evolved, and easily detected by its colour, smell, and bleaching properties:—2. The soluble chlorides may be readily detected by their solutions, slightly acidulated with nitric acid, giving with a solution of nitrate of silver a white, curdy precipitate (chloride of silver), insoluble in nitric acid, freely soluble in liquor of ammonia, and blackened by the light:—3. The insoluble chlorides may be tested by digesting them in a little liquor of potassa, when a solution of chloride of potassium will be formed, which may be treated as just directed (2); or the chloride may be dissolved in nitric acid, and tested with nitrate of silver as before.
CHLORIM′ETRY. See Chlorometry.
CHLORINA′TED LIME. See Lime.
CHLORINA′TED SO′DA. See Sodium.
CHLO′′RINE. Syn. Chlorin′ium, L.; Chlore, Fr.; Chlor, Ger. An elementary substance discovered by Scheele in 1774, and at first supposed to be a compound body. In 1809 MM. Gay-Lussac and Thénard suggested the probability of it being a simple substance; but it was reserved for Sir H. Davy, shortly afterwards,443 to demonstrate the truth of the suggestion of these foreign chemists.
Nat. Hist. It exists in nature chiefly in the form of chloride of sodium, which constitutes rock salt when deposited in inland beds, sea salt when dissolved in masses of water. The sea also contains chlorides of potassium, calcium, and magnesium. It is a constituent of several well-known minerals. It has been met with in the air of volcanic districts, combined with hydrogen, as hydrochloric acid.
Prep. Strong hydrochloric acid is poured on half of its weight of finely-powdered peroxide of manganese, previously placed in a glass flask or retort; chlorine gas is immediately evolved, even in the cold, but much more rapidly on the application of a gentle heat, and is collected in clean, dry bottles by displacement. The tube conducting the gas is so arranged as to reach to the bottom of the bottle, and the chlorine, being heavier than the air, displaces the latter without mixing with it. The bottle is known to be full by the gas, easily perceived by its green colour, overflowing the top of the vessel. The bottle is then closed up with an accurately fitting stopper, previously greased, and an empty one put in its place, which is subsequently treated in like manner. To free the gas entirely from hydrochloric acid it is passed through a wash bottle containing a small quantity of water; and to render it quite dry it is passed over fused chloride of calcium. When the presence of moisture is no object chlorine may be collected over warm water, or, what is better, a saturated solution of common salt, in the pneumatic trough. The mercurial trough cannot be employed, as the chlorine rapidly acts upon the metal, and becomes absorbed.
Commercial.—From oil of vitriol and water, of each 7 parts, cautiously mixed, and allowed to cool; chloride of sodium (common salt), 4 parts, mixed intimately with peroxide of manganese, 3 parts. The dilute acid is placed in a retort or other generating vessel, and the powder added. The gas comes off slowly at first, but the application of a gentle heat causes it to rush forth in large quantities. Of late years, owing to the general demand for bleaching agents, numerous new methods and suggestions for obtaining chlorine have been patented, of which the following are the most important.
1. Elliott. By this method the reconversion of the chloride of manganese to peroxide was attempted as follows:—The manganese residues left in the still are first heated to dryness. They are then roasted in a current of steam, the result being the formation of hydrochloric acid (which is condensed), and a residue consisting of a mixture of protoxide and peroxide of manganese.
2. Gatty. In this process the manganese residues, after evaporation to a suitable consistence, are mixed with nitrate of soda, and the nitrate of manganese and chloride of sodium formed, when dried, are strongly heated in an iron retort, the fumes of nitric acid which come off being employed in the manufacture of sulphuric acid. The residue in the retort, consisting of peroxide of manganese, being lixiviated, yields the peroxide in a pure state:
| Mn(NO3)2 + 2(NaCl) + O2 = |
| MnO2 + 2NaCl + 2(NO3). |
3. Hoffman. This process consisted in the regeneration of the manganese by means of soda waste. In this process the chloride of manganese is, by the addition of the yellow ley obtained from the lixiviation of soda waste, converted into sulphide of manganese. The precipitate so obtained consists of
| Sulphide of manganese | 55·00 |
| Sulphur | 40·00 |
| Protoxide of manganese | 5 |
| ——— | |
| 100·00 |
This is dried and then calcined, the sulphurous acid evolved being conducted into the sulphuric acid chamber.
The residue, which has the following composition—
| Sulphate of manganese | 44·50 |
| Peroxide of manganese | 18·90 |
| Protoxide of manganese | 36·60 |
| ——— | |
| 100·00 |
—is next mixed with nitrate of soda and heated to 300° C., yielding sulphate of soda and nitrate of manganese, the latter, however, being at once decomposed into peroxide of manganese, and nitrogen peroxide, thus:—
| a. | MnSO4 + 2NaNO3 | = | Mn(NO3)2 + Na2SO4. |
| b. | Mn(NO3)2 | = | MnO2 + 2NO2. |
After the mass has cooled, the sulphate of soda is washed out, the residue yielding, according to the inventor, a material equal to native peroxide of manganese.
4. Schlösing. Manganese is acted upon with a mixture of hydrochloric and nitric acids, the degree of concentration of the acids being so regulated by the addition of water that the mixture yields only chlorine, whilst protonitrate of manganese is formed; this salt being calcined yields peroxide of manganese and nitric acid. The nitric acid aids the oxygen of the air in decomposing the hydrochloric acid.
The following equation will explain the successive stages of the reaction:—
| a. | 2HCl + 2HNO3 + MnO2 | = | Cl2 + Mn(NO3) + 2H2O. |
| b. | Mn(NO3)2 | = | MnO2 + 2NO2. |
| c. | 2NO2 + H2O + O | = | 2HNO3. |
5. Vogel. By decomposing chloride of copper by heat. The chloride in the crystalline444 state is mixed with half its weight of sand and heated in earthenware retorts to 200° to 300° C., yielding chlorine gas, while the remaining protochloride of copper is reconverted into perchloride by the action of hydrochloric acid.
According to Laurens the reaction is as follows:—
| a. | 2CuCl2 | = | Cl2 + 2Cu22Cl2. |
| b. | Cu2Cl2 + 2HCl + O | = | H2O + 2CuCl2. |
6. MacDougal, Rawson, and Shanks. This is effected by decomposing chromate of lime by means of hydrochloric acid, the result being the formation of chloride of chromium, chloride of calcium, and the evolution of free chlorine; thus
| 2CaCrO4 + 16 HCl | = | Cr2Cl6 + 2CaCl2 + 8H2O + Cl6 |
158 parts of chromic acid yield 106 parts of chlorine. The chloride of chromium is again precipitated with carbonate of lime, and by ignition converted into chromate of lime. Only three eighths of the chlorine contained in the hydrochloric acid is given up, whilst manganese yields one half.
7. Hargreaves proposes to evaporate a solution of protochloride of iron to dryness, and then to heat the dried substance in a current of air, at a temperature of about the melting point of zinc, by which means perchloride of iron and free chlorine would be obtained. Thielbierge’s suggestion consists in passing air over protochloride of iron, and so giving rise to peroxide of iron and chlorine. This, like Hargreaves’ proposition, possesses the disadvantage of furnishing the chlorine very largely diluted with air and nitrogen.
8. Jessie de Mothney. This chemist has proposed a continuous process which is as follows:—Peroxide of manganese either alone or mixed with lime is put into a retort, which, when heated to redness, has a current of hydrochloric acid gas passed into it. A disengagement of chlorine and steam takes place, and there remains in the retort a mixture of undecomposed peroxide of manganese with chlorides of manganese and calcium. The retort containing the undecomposed peroxide and chlorides being still kept at a red-heat, air or oxygen is passed over them, the result being that the manganic chloride is decomposed at once.
The chlorine liberated by this last operation is conveyed into vats containing a mixture of lime and manganous oxide, which substances have been previously formed by the decomposition of the manganous chloride by lime, the soluble chloride of calcium having been run off. Sesquioxide of manganese and hypochlorite of calcium are formed in the vats, and these two, reacting on each other, give rise to peroxide of manganese and chloride of calcium. With fresh hydrochloric acid this latter product yields more chlorine for use in the chambers. Magnesia may be substituted for lime.
9. Dunlop. This process, which may be regarded as the first practical method for utilising the whole of the exhausted manganese residues, and rendering them capable of reapplication in the production of chlorine, was devised by Mr Dunlop in 1855, since which time it has been in use in the alkali works of the Messrs Tennant, at St. Rollox, Glasgow. The operation consists in precipitating the chloride of manganese in the still liquor by carbonate of calcium; the resulting manganese carbonate being decomposed by heat. The liquors are previously mixed with a little milk of lime, which frees them from ferric oxide, alumina, and silica.
Being allowed to stand until these and all other insoluble matters are precipitated, the clear solution containing the chloride of manganese is mixed with finely divided chalk, when the following reaction ensues:—
| MnCl2 + CaCO3 | = | MnCO3 + CaCl2. |
The resulting milky liquid is then run into large iron boilers, through each of which passes horizontally an iron shaft furnished with a number of projecting arms. This shaft having been put into revolution so as to keep the contents of the boilers agitated, steam is admitted into them under a pressure of from two to four atmospheres, and by the combined effects of heat and pressure the decomposition of the manganese chloride by the calcium carbonate is accomplished in about four hours. The manganese carbonate is then allowed to subside, the calcium chloride solution is drawn off, the precipitate carefully washed, and thrown up in heaps on an inclined surface to drain. When partially dried the carbonate of manganese is placed in small low wagons, made of sheet-iron, supported on rollers, and slowly drawn through a furnace by means of a chain. The furnace holds forty-eight of these little wagons. The furnace is 50 feet long, 12 feet wide, and 10 feet high. “A fire-brick flue runs down the centre of the bottom of the furnace, and is connected at the far end with two return metal pipes, which lie on each side of the flue. A uniform heat at about 660° F. is maintained in the furnace, in which four lines of rails are laid for the small wagons to run along. The half-dried substance loses all its moisture and part of its carbonic acid as the wagons pass along the first line of rails, and as they return down the second line a further escape of carbonic acid ensues, and eventually the expulsion of all the acid, and the peroxidation of the manganese is completed during the passage of the wagons on the third and fourth lines.” The operation lasts about forty-eight hours, the substance gradually changing in colour from white to brown, and lastly to black.
The ends of the furnace are closed by loose hanging doors, so as to ensure the entrance of a sufficient supply of air. The fire-place is situated below the floor of the furnace, and requires very careful watching. The resulting445 product is a mixture of oxides of manganese, and contains about 72 per cent. of peroxide of manganese.
9a. A. Dunlop. Another process designed by Mr Dunlop, and also in use at Messrs Tennant’s, is as follows:—Nitrate of sodium (Chilian nitre) and chloride of sodium are decomposed by being heated in cast-iron cylinders with sulphuric acid. The gaseous products are made to pass through leaden Woulff’s bottles containing sulphuric acid, which absorbs the nitric peroxide formed, and allows the passage of the chlorine into the chambers. The reaction may be represented by the following equation:—
| NaCl + NaNO3 + H2SO4 | = | Cl + NO2 + Na2SO4 + H2O. |
The sulphuric acid charged with nitric peroxide is used in the manufacture of chamber acid.
10. Weldon. a. The process by which the greater part of the chlorine employed in the manufacture of bleaching compounds is now obtained and which has hitherto proved the most practical is that of Mr Walter Weldon. We have the authority of Mr Kingzett, in his work on ‘The Alkali Trade,’ for the statement, that out of 90,000 tons of bleaching powder made in Great Britain in 1874, 50,000 were procured by Weldon’s method; and turning to the Continent we find the process largely adopted in Germany, France, and Belgium. The utilisation and regeneration of the residual product left in the still after the evolution of the chlorine which it will be presently seen is accomplished by the above process, is not the only advantage accruing from it, since it has also been the means of removing an extensive source of contamination of many of our streams and rivers, into which the then useless chloride of manganese was thrown previous to Mr Weldon’s invention. It is true that the only waste product formed in the course of the operations, viz., chloride of calcium, is got rid of by being run into the nearest waters, but it is stated by the Rivers Pollution Commission, beyond making these harder, no other objectionable effect is caused by it.
Mr Weldon’s process is based upon the fact, that if protoxide of manganese be suspended in a solution of chloride of calcium, and an excess of lime be added, the protoxide will become readily converted into peroxide if air be forced into the liquor. It had long been known that it was possible to convert into a peroxide the protoxide of manganese obtained by treating the residual still liquors with an equivalent of lime, but all attempts to reduce this knowledge to practical account had proved unsuccessful until Mr Weldon attempted it.
Mr Weldon made the important discovery, that whilst protoxide of manganese is by itself, when treated in the wet way with air, only capable of being converted into peroxide, at the greatest, to the extent of one half; the addition to the protoxide so treated of a certain quantity of lime converted the whole of it into peroxide in less than a twentieth the time required to peroxidise half the protoxide if lime were absent. It will be seen that it is the employment of an excess of lime which constitutes the success of Mr Weldon’s process, which is as follows:—
The residual liquors remaining in the still after the chlorine has been evolved by the action of hydrochloric acid on peroxide of manganese, and in which chloride of manganese is by far the predominating constituent, are run into a receptacle termed the neutralising well, which is usually six feet in depth by twenty in diameter. In this well the free hydrochloric acid of the still-liquor is neutralised by the addition of limestone or chalk, which at the same time serves to decompose the soluble ferric and aluminic chlorides present in the liquid, and to precipitate them as insoluble oxides. During this process the contents of the well are kept in a state of brisk agitation by means of a suitable stirrer. After this treatment the now neutral liquor consists of chlorides of manganese and calcium in solution, of a small quantity of suspended ferric and aluminic oxides and chalk. It additionally contains also in suspension a by no means small quantity of sulphate of lime, derived from the sulphuric acid always present in varying amount in the commercial hydrochloric acid used.
From the neutralising well the liquor is pumped to a height of some forty feet into tanks, called the chloride of manganese settlers, in which after from two to four hours it deposits the solid matters suspended in it, the supernatant clear liquor assuming a pale rose-coloured appearance.
The next operation is to draw off by means of syphons, which can be lowered or raised in the settlers to any desired level, this clear liquid, containing the chlorides of manganese and calcium, into a vessel called the oxidizer; this latter being an iron cylinder, of from eight to twelve feet in diameter, and from twenty-two to thirty-five feet deep. Two pipes go down nearly to the bottom of the oxidizer; the larger one being used for conveying a blast of air from a blowing engine, and the smaller for the injection of steam. The introduction of steam is only had recourse to in case the liquor when drawn into the oxidizer should not have the requisite temperature, viz. 130° or 140° Fahr. Immediately above the oxidizer a reservoir containing milk of lime is placed. A great deal depends upon the careful preparation of the milk of lime, since on the degree of fineness in which the lime is added to the manganese chloride in solution depends the rapidity with which it acts in the oxidizer. The milk of lime is kept constantly agitated, to ensure its being of uniform consistency, and should contain446 from 15 lbs. to 20 lbs. of hydrate of lime in every cubic foot of the mixture.
A charge of the clear liquor having been drawn into the oxidizer, and raised if necessary to the requisite temperature, the blowing in of air is begun, whilst at the same time the milk of lime is run into the oxidizer as rapidly as possible, the flow of milk of lime being only discontinued when a sample of the filtrate drawn off, by means of a tap placed near the bottom of the oxidizer, ceases to give the manganese reaction when mixed with a solution of bleaching powder. This reaction consists in the production of a purple colour caused by the formation of permanganate of calcium. More milk of lime is then added, when the contents of the oxidizer are found to consist of a thin white mud, composed of a solution of calcium chloride, holding in suspension a mixture of protoxide of manganese and lime. The injection of air being continued the white mud rapidly becomes darker in colour, and soon changes into a thin black mud composed of solution of calcium chloride, holding in suspension certain compounds of peroxide of manganese partly combined with protoxide of manganese, but chiefly with lime, which compounds Mr Weldon terms “manganites.” Mr Weldon suggests that the manganites so formed may be regarded as salts in which the basic radical is calcium or manganese, and the acid radical MnO3; and may be represented by the formulæ CaMnO3, and MnMnO3; and possibly also CaMnO2 (MnO2)2. “The quantity of lime which has to be put into the oxidizer before the filtrate from a sample of its contents ceases to yield the manganese reaction varies very considerably. Recently precipitated protoxide of manganese dissolves very appreciably in neutral solution of chloride of calcium, its solution therein comporting itself with reagents exactly like solutions of manganese salts. It dissolves also in solution of oxychloride of calcium, that is to say, in solution of chloride of calcium containing dissolved lime; its solution in oxychloride of calcium not giving the ordinary manganese reactions.”
“Hence even if all portions of the lime added to the chloride of manganese in the oxidizer were capable of acting on chloride of manganese equally readily, manganese could not cease to be so in solution as to be detectible by ordinary reagents, until more than an equivalent of lime had been added—that is to say, until enough had been added not only to decompose all the chloride of manganese, but also to form a certain quantity of oxychlorate of calcium. It is never the case, however, that all portions of the lime used are capable of acting on the chloride of manganese with equal readiness. The lime used always contains a larger or smaller proportion of particles coarser than the rest, which coarser portions cannot of course act so rapidly as the finer ones; and as the decomposition of the chloride of manganese requires to be completed as quickly as possible, those portions of the lime which will not act upon it instantly are scarcely allowed time to act upon it at all.
“These coarser portions of the lime thus contribute very little to the decomposition of the manganese, though they afterwards dissolve completely in the hot solution of chloride of calcium, and then play their full part in the reactions which take place during the subsequent blowing. The proportion of lime which thus does not act on the chloride of manganese varies with the source of the lime, and with the manner in which it is prepared, so that the quantity of lime which has to be added to a charge of chloride of manganese liquor in the oxidizer, before the filtrate from a sample of the resulting mixture ceases to become coloured on the addition of solution of bleaching powder, varies from about 1·1 to 1·45 equivalent.
“The further quantity of lime which is added after that point has been reached is now usually so much as to raise the total quantity to about 1·5 to 1·6 equivalents, being from one half to six tenths in excess of the quantity which actually takes part in the decomposition of the chloride of manganese.”[239]
As previously stated, Mr Weldon found that if only so much lime is employed as is necessary to precipitate the manganese, not more than half the protoxide of manganese will be converted into the peroxide, and that even this result will be accomplished very tardily. And, as has been already mentioned, a greater and more rapid yield of protoxide can only be obtained by using a larger proportion of lime. Any excess, however, of lime over that absolutely required for the peroxidation of the protoxide of manganese must be sedulously avoided, since a superabundance of lime leads to the formation of compounds that are not readily peroxidised. Should such compounds be formed, it is necessary to destroy them, and this may be done by the addition of a fresh quantity of chloride of manganese. The objectionable compounds in question are lime and protoxide of manganese, which are known in the process under the name of “bases;” and the reason why it is desirable to prevent as much as possible their formation will become evident when it is remembered that they cannot furnish chlorine when treated with hydrochloric acid, but that they merely dissolve in this latter.
The injection of air into the oxidizer, which constitutes the blowing operation, varies from two to four hours.
The quantity required to be blown in is chiefly dependent upon the depth of the oxidizer, and upon the amount of protoxide of manganese contained in a given volume of the charge.447
The greater the depth of this latter the more rapidly does the peroxidation take place; and the greater the number of molecules of protoxide in a given volume of the charge, the larger is the total surface presented to the action of injected air, and consequently the greater is the proportion of the oxygen absorbed.
“In one instance 175,000 cubic feet of air were blown in during five hours, and of the oxygen contained in this, 14·8 per cent. (equal to rather more than 4 cwt.) was absorbed in the production of 22 cwt. of peroxide of manganese.”[240]
The expenditure of mechanical power in forcing the air into the oxidizer averages between seven and eight horse-power for every 100 lbs. of peroxide of manganese obtained. In theory, to produce the quantity of chlorine contained in a ton of bleaching powder containing 37 per cent. of chlorine, 1020 lbs. of peroxide of manganese would be required; but it is found in practice that instead of this quantity of peroxide giving the above result, 1100 lbs. are needed.
“The consumption of lime averages 14 cwt. per ton of bleach. By this process 1 ton of bleach is made, using 2832 lbs. of hydrochloric acid, generated by the decomposition of 47·5 cwt. of salt, viz. a quantity which theoretically yields 3334 lbs. of hydrochloric acid. There is therefore a loss of acid of 15 per cent. The loss of manganese varies from 4 to 10 per cent. The whole of the lime is lost, and two thirds of the total chlorine (in combination with calcium) contained in the acid used.[241]
When sufficient air has been blown into the oxidizer, the contents which consist of a solution of chloride of calcium, holding in suspension peroxide and protoxide of manganese and lime, are run into one of a range of settling tanks placed below the level of the oxidizer. These tanks are known as mud settlers. In these the manganese mud is left to deposit until about half its volume has become clear. It generally requires 3 or 4 hours to deposit. The clear part, which consists of chloride of calcium, being then decanted by means of a swivel-pipe, is usually thrown away. The mud remaining in the settlers, which contains in a cubic foot from 4 lbs. to 5 lbs. of peroxide of manganese, is now in a fit condition to be placed in the still, where it is to be exposed to the action of the hydrochloric acid. The stills, which are made of slabs of hard siliceous sandstone or of Yorkshire flagstones, and are usually in the shape of an octagonal prism, are about 8 feet square, and 10 feet in depth. Mr Kingzett says “the new Weldon stills are polygonal in shape, about 12 feet across, and 7 feet to 8 feet deep.” Contrary to the course formerly followed, when native manganese was used in the Weldon process, the still is charged with hydrochloric acid first, and the manganese mud is run in upon the acid in a small, steady stream, the flow of which can be regulated by a stopcock. Steam being carefully admitted into the still at the same time, the mud dissolves very rapidly in the acid, and the chlorine is evolved in an even current, the force and flow of which is dependent upon and can be very accurately regulated by the admission of the mud.
The time occupied before the reaction between the acid and the manganese is completed varies in different works from two to six hours. At the end of this time the contents of the still are run off into the well placed below it, and are afterwards submitted to the various operations already described, which we have seen to accomplish the regeneration of the residue and effect its reconversion into peroxide of manganese. The process is a continuous one, and theoretically the original quantity of manganese should be capable of being used over and over again for an unlimited number of operations. In practice, however, there is always found to be a loss of a small per-centage of manganese, arising from some of the chloride of manganese being carried down by the sulphate of lime and the ferric and aluminic oxides in the settlers, and not being thoroughly recovered when the deposit is washed; for, though an exhaustive washing of the precipitated matters could be easily managed, the bulk of the wash waters would render the recovery of the chloride of manganese from them a non-paying affair. This loss of chloride varies according to the statements of different manufacturers from 2 to 10 per cent.
It is stated that not only is the chlorine yielded by Mr Weldon’s process of very pure quality, and the bleaching powder manufactured from it very high of strength and excellence; but that over from 20 to 25 per cent. more bleach is obtained from a given quantity of hydrochloric acid, when artificial peroxide of manganese is used instead of the native. This advantage is chiefly owing to the artificial manganese (of the manganese mud) from its physical condition being much more accessible than the native form of manganese to the action of the hydrochloric acid, and from its dissolving in the acid so much more readily and thoroughly, and neutralising as much as from 95 to 99 per cent. of it, a much larger amount than the native ores are capable of neutralising.
Again, the bleaching powder produced by the above process stands not only very high in point of strength, but varies very little in the amount of chlorine it contains, as may be gathered from the following table, which shows the average strength for thirteen consecutive weeks of the bleaching powder made at six large and different manufactories:448—
| Factory. | ||||||
| Week. | I. | II. | III. | IV. | V. | VI. |
| 1st | 36·9 | 37·8 | 36·7 | 36·3 | 36·6 | 36·6 |
| 2nd | 36·1 | 36·8 | 36·7 | 36·2 | 35·1 | 37·4 |
| 3rd | 36·5 | 38·0 | 36·6 | 36·1 | 37·2 | 37·9 |
| 4th | 35·9 | 37·1 | 35·9 | 36·2 | 36·8 | 37·1 |
| 5th | 35·8 | 36·9 | 36·0 | 36·3 | 36·5 | 37·8 |
| 6th | 36·0 | 37·0 | 36·2 | 35·9 | 36·5 | 37·2 |
| 7th | 36·5 | 36·0 | 36·3 | 36·6 | 35·8 | 36·8 |
| 8th | 36·3 | 36·8 | 36·3 | 36·3 | 35·0 | 37·1 |
| 9th | 36·4 | 36·3 | 36·8 | 35·7 | 35·2 | 37·0 |
| 10th | 36·5 | 36·7 | 36·3 | 36·0 | 36·2 | 37·6 |
| 11th | 36·8 | 36·8 | 36·2 | 35·9 | 36·0 | 37·2 |
| 12th | 36·8 | 35·9 | 35·6 | 35·6 | 36·1 | 37·2 |
| 13th | 36·7 | 35·2 | 35·9 | 36·1 | 36·9 | 37·5 |
Spite of the expensive plant required to work Mr Weldon’s process, it is said to possess very decided advantages over the old methods as far as regards cost of production.
In connection with Mr Weldon’s process may be mentioned Mr Valentin’s modification of it, for which a provisional patent was taken out by this latter gentleman. Instead of adding more lime, after the neutralised still liquors have been precipitated by an equivalent of lime, as is done in the above process, Mr Valentin adds a solution of potassium ferricyanide, and air being blown in, the peroxidation of the manganese is effected much more quickly than in Mr Weldon’s process. It was also calculated that, by Mr Valentin’s method, bleaching powder could be produced at a cost of about ten shillings per ton less than when made by Mr Weldon’s.
For the successful working of Mr Valentin’s process it is necessary that the ferricyanide should be recovered, not only because of its cost, but also because its presence gives rise to the production of cyanogen compounds, which would enter the chamber with the chlorine. Hitherto no economical plan for the recovery of the salt has been devised, and consequently Mr Valentin’s proposed modification of Mr Weldon’s process has failed to be adopted.
b. A second process for obtaining chlorine, called “the magnesia process,” has been devised by Mr Weldon. In the previous method, or “lime process,” two thirds of the chlorine contained in the hydrochloric acid, as we have seen, is lost, passing away in the form of waste chloride of calcium.
In the “magnesia process” all the chlorine is utilised, the acid employed being made to yield the whole of its gas in the free state. The regeneration of the manganese peroxide being likewise accomplished, and the process being a continuous one, theoretically no loss of material should take place.
Beyond the employment of liquor pumps, no machinery is requisite for carrying out the operation, which, being very simple in its details, requires the employment of little skilled and, consequently, expensive labour. Further, the inventor claims for it the production of bleaching powder at a less cost per ton than by any other process. The “magnesia method” is worked as follows:—
The spent liquors of the still, consisting of chloride of manganese and free hydrochloric acid, are neutralised with magnesite, or, as it is sometimes called, Greek stone—a very pure native form of carbonate of magnesia. Sometimes the magnesite is calcined, and the magnesia thus obtained used instead.
The neutralisation may be effected either in the still itself, or in a well made of cast iron. The liquid is next pumped into the settlers, in which it deposits its ferric and aluminic oxides and sulphate of lime. The clear liquor containing the chlorides of manganese and magnesia is then run into an iron evaporating pan, where it is concentrated by boiling until it reaches a temperature of between 300° and 320° F. At this point the magnesium chloride begins to be decomposed by the water, and hydrochloric acid is given off. When it has reached the above degree of concentration, it is conveyed into a muffle furnace. This furnace is divided into two compartments, separated by an iron door, which can be opened or shut by means of a pulley placed outside. The desiccation of the mass which is accompanied with the evolution of a little chlorine and a large amount of hydrochloric acid, having been completed in one of the divisions of the furnace, it is broken up by constant stirring into thin cakes and raked into the second division, where it is gently heated with access of air; when the operation is complete the residue which left the first compartment as a mixture of manganese and magnesium chloride becomes converted into manganate of magnesia (MgMnO3), its chlorine having been driven off partly in the free state and partly as hydrochloric acid. “As long as water is present in the furnace hydrochloric acid is evolved, and as the main evaporation takes place in the first division of the furnace, it is chiefly hydrochloric acid that is there generated. In the second division it is chiefly chlorine which is evolved, but it is, of course, mixed with some hydrochloric acid. It is, indeed, doubtful whether much manganese chloride is decomposed by the water so long as there remains any chloride of magnesium, as this body is far more readily decomposable.”[242]
It is stated that all the manganese is not converted into peroxide in the furnace, but that a certain portion of it is left as protoxide; which, with the magnesia, constitutes the useless “bases.” The completion of the process is known when portions of the cake drawn from time to time from the furnace cease to indicate any increase in the quantity of peroxide of manganese.449
The finely-divided black powder—manganate of magnesium—thus obtained, after leaving the furnace and when sufficiently cold, is ready for the stills—where, in contact with hydrochloric acid, it is again employed in the generation of chlorine.
The chlorine leaves the furnace mixed with much hydrochloric acid, nitrogen, and air. The gaseous mixture is drawn by a chimney draught through the coke towers, as in the making of salt-cake. By this contrivance the hydrochloric acid is recovered, yielding a solution strong enough to react upon fresh manganese in the still. The diluted chlorine may be made to ascend leaden towers, where it comes in contact with a shower of milk of lime, which absorbs the gas and forms ordinary bleaching liquid, whilst sometimes it is employed in the production of potassium chlorate.
We have seen that the chlorine yielded by the “magnesia process” is partly in the concentrated, and partly in the dilute condition. The ratio of strong chlorine generated in the still to that of weak chlorine produced in the furnace may be anything between one to one, and one to about four, at pleasure.
“When working so as to obtain strong chlorine and weak chlorine in about equal proportions, the quantity of the liquor to be boiled down per ton of total bleaching powder made was about 105 cubic feet. As the proportion of the weak chlorine increased, the quantity of liquor to be boiled down diminished until, when the proportion of the weak chlorine to that of the strong became as four to one, the quantity of liquor to be boiled down per ton of total bleaching powder made was only about 40 cubic feet.”[243]
[243] ‘Chemistry, Theoretical, Practical, and Analytical,’—Mackenzie & Co.
11. (Deacon.) As we have already seen, Vogel proposed to obtain chlorine by the decomposition by heat of cupric chloride, and to reconvert the resulting cuprous chloride into the cupric salt by treatment with hydrochloric acid.
Chlorine may be produced by passing a mixture of gaseous hydrochloric acid and air over heated bricks or other porous substances, a reaction which Oxland unsuccessfully attempted to turn to account for the production of chlorine for manufacturing purposes. The cause of failure appears to have been the great heat necessary to effect the decomposition of the acid atmospheric oxygen.
In the late Mr Deacon’s process both Vogel’s and Oxland’s methods are combined. He discovered that to be able to generate chlorine and water from gaseous hydrochloric acid and air, a very much lower temperature than that employed by Oxland was necessary, and he found that this diminished temperature could be attained, if the gas and air to be decomposed were passed over porous bricks saturated with a solution of sulphate of copper, and heated to a temperature of 700° to 750° F.
Beyond this point he found the heat ought not to be carried; for at 800° the cupric chloride formed begins to volatilise, and to condense in the cooler parts of the apparatus (presently to be described), thereby interfering with the draught through it, and delaying the working, since its removal becomes necessary. It was found that below 400° the reaction does not take place. Experience has demonstrated that the best temperature to effect this decomposition is 625° F.
The hydrochloric acid obtained either from a soda furnace or evolved from an aqueous solution is immediately mixed with a quantity of air containing an excess of oxygen over that required for liberating all the chlorine from the evolved hydrochloric acid, and passed through heated U-shaped tubes of cast-iron, from which the gaseous mixtures obtain the necessary temperature. The original plant was so contrived that the heated gases were conveyed from the U-shaped tubes into a series of nine towers made of iron or other suitable material. Entering by a pipe at the bottom of the first tower, and passing on to the second, the gases came into contact with a series of ordinary agricultural drain pipes of small bore arranged with vertical spaces, these pipes being saturated with a solution of sulphate of copper and sulphate of soda, it being subsequently found that this latter addition increased the efficacy of the copper sulphate, as well as its power to resist decomposition. From the first two towers of the series the mixed gases traversed the remaining ones, where they encountered small pieces of common brick, fire brick, or burnt clay also impregnated with the copper and soda sulphates, after reacting upon which they passed out of the apparatus, called the ‘decomposer,’
In the more recently made decomposers we believe the nine towers were abolished, and one chamber substituted for them, the drain-pipes being at the same time abandoned for pieces of brick and clay marbles.
A decomposer upon this latter principle is said to have been in use for several months at a factory in Berlin, and to have worked perfectly satisfactorily. After leaving the decomposer, the gaseous mixture, which now consists of chlorine, water, nitrogen, unconsumed oxygen, and undecomposed hydrochloric acid, after being cooled, is passed through water, by which means it is deprived of its hydrochloric acid.
It is next made to ascend a tower, where, meeting with a stream of sulphuric acid running over coke, it is deprived of its water. The chlorine (diluted with nitrogen and oxygen) is now ready for the lime chamber.
One great objection urged against the adoption of the above process, viz., that in consequence of the large volume of the evolved gases enormously large chambers for450 the preparation of the bleaching would be necessitated, seems to have been met by passing the gas through a series of chambers, in which the first contains nearly finished bleaching powder; the second, lime in a less saturated condition; and so on, until the last chamber contains merely slaked lime.
The following table, exhibiting the amount of chlorine contained in different batches of bleaching powder made by Deacon’s process, is extracted from ‘Chemistry, Theoretical, Practical, and Analytical,’ published by Mackenzie:—
| Strength. | Strength. | ||||
| July | 14 | 36·0 | July | 22 | 34·3 |
| ” | 15 | 34·8 | ” | ” | 36·5 |
| ” | ” | 36·1 | ” | 24 | 36·8 |
| ” | 17 | 36·4 | ” | ” | 37·5 |
| ” | ” | 36·0 | ” | 25 | 36·1 |
| ” | 18 | 37·2 | ” | ” | 36·7 |
| ” | ” | 37·9 | ” | ” | 36·8 |
| ” | 19 | 37·2 | ” | 26 | 36·2 |
| ” | ” | 37·0 | ” | ” | 36·9 |
| ” | 20 | 37·9 | ” | 27 | 36·9 |
| ” | ” | 36·7 | ” | ” | 35·5 |
| ” | 21 | 36·0 | ” | 28 | 37·2 |
| ” | ” | 35·3 | ” | ” | 37·0 |
| ” | ” | 37·7 | ” | ” | 36·75 |
Writing on this process in his late work, ‘The Alkali Trade,’ Mr Kingzett says:—“The process bearing Mr Deacon’s name was first brought before the public at the British Association Meeting in 1870.
“It excited at that time much attention, and indeed for some period it was doubtful whether it would not rival or even displace the Weldon process. Further experience, however, discovered difficulties in the practical working of this beautiful method, which exercise a deteriorating influence on its value, and lessen its applicability. Although several plants have been erected in connection with this mode of manufacturing chlorine, most of them have been since abandoned, and at the present time most of the chlorine is manufactured according to the process of Mr Weldon.”
Dr Jurisch, in a communication to ‘Dingler’s Polytechnic Journal,’ 1876, remarks that when Deacon’s process was first taken up within a short time by more than twelve English and two German establishments, the view was generally entertained that the balls of clay steeped in solution of copper would ensure an uninterrupted production of chlorine gas for a year or two, if not longer. Before many months had elapsed complaints were heard of the action of the balls. He, therefore, undertook to determine what can be the cause of these balls declining so rapidly in their efficacy. His conclusion is, that the true cause of this speedy decrease in the decomposition is due to sulphuric acid, which passes through the interstices of the clay-balls mixed with the other gases. This injurious action, according to Hasenclever and Sartori, is probably to be explained by the following reaction:—The vapour of sulphuric acid in contact with sulphate of alumina at a dull red-heat, as is found in the balls, is resolved into sulphurous acid, watery vapour, and oxygen; the sulphurous acid thus formed is reoxidised at the expense of the free chlorine, is again decomposed, and thus keeps up a destructive circulation in the apparatus, which reduces or totally checks the chlorine.[244]
[244] Extracted from the ‘Chemical News.’
Prices of Bleaching Powder (Clapham).
| In | 1805 | £120 | 0 | 0 | per ton. |
| ” | 1810 | 84 | 0 | 0 | ” |
| ” | 1815 | 80 | 0 | 0 | ” |
| ” | 1820 | 47 | 0 | 0 | ” |
| ” | 1825 | 27 | 0 | 0 | ” |
| ” | 1830 | 23 | 0 | 0 | ” |
| ” | 1832 | 21 | 0 | 0 | ” |
| ” | 1835 | 23 | 0 | 0 | ” |
| ” | 1840 | 21 | 0 | 0 | ” |
| ” | 1846 | 18 | 0 | 0 | ” |
| ” | 1850 | 13 | 15 | 0 | ” |
| ” | 1855 | 10 | 15 | 0 | ” |
| ” | 1857 | 13 | 10 | 0 | ” |
| ” | 1860 | 11 | 0 | 0 | ” |
| ” | 1868 | 10 | 12 | 0 | ” |
Prop., Uses, &c. Chlorine is a gas possessing a yellowish-green colour, and a pungent, suffocating odour. It is one of the heaviest substances that are gaseous at ordinary temperatures, being nearly 21⁄2 heavier than atmospheric air; sp. gr. 1·47. It is soluble to a considerable extent in water, that liquid at 60° Fahr. absorbing about twice its volume. It is non-inflammable, but its union with some of the elements is attended with the phenomena of combustion; thus, phosphorus, copper leaf, powdered antimony and arsenic, and several other substances thrown into chlorine immediately inflame. Under a pressure of 4 atmospheres it is condensed into a yellow, limpid liquid. Moist chlorine gas cooled to 32° Fahr. condenses into yellow crystals, containing 351⁄2 parts of chlorine and 90 parts of water. The most remarkable property of chlorine is its power of destroying almost all vegetable and animal colours, and the putrid odour of decomposing organic matter; hence its value as a bleaching agent, and as a disinfectant and fumigator. When first proposed as a bleaching agent by Berthollet, it was used much the same way as sulphur is now in bleaching woollen goods; afterwards a solution of the gas in water was employed, but the final improvement was Tennant’s patent of combining the gas with lime to form “chloride of lime.” With the bases chlorine forms an important series of compounds called chlorides.
451
Tests. Free chlorine is readily distinguished from other gases by its colour, suffocating odour, and bleaching properties. The aqueous solution dissolves gold leaf, and with nitrate of silver gives a white, curdy precipitate.
CHLORINE STILLS. The accompanying figure represents a section of one of the earlier forms of still used in the preparation of chlorine.
 Fig. 1.
Fig. 1.
These stills were sometimes made of strong sheet lead, the lower part of which was enclosed in a jacket of cast iron, into which steam was forced, by which means the contents of the still were heated. The steam was injected from an ordinary boiler through the pipe H, and the materials, after the decomposition had been completed, were drawn off by the pipe G. The four openings, C, D, E, F, were secured by water lutes, capable of bearing a pressure greater than that required in the chamber where the saturation took place. In some cases the lower half of the still was made of cast iron, and fitted into a groove made in the upper part, the two sections being united by means of a strong cement. In the latter case the heating of the still was effected by a naked fire applied to the bottom. Into the orifice C the said materials employed were introduced, whilst the acid was poured through the opening F. The gas evolved passed off through the pipe E to the purifier and chamber, where it was absorbed by the lime, and converted into bleaching powder, and the shaft of the agitator passed up through D.
The use of the leaden stills survived for a longer time in France than in this country. In some parts of Germany large glass globes with long necks were employed, in which the chlorine was generated from a mixture of hydrochloric acid and manganese. But these were only applicable in cases where comparatively small quantities of bleaching powder were to be manufactured. When the chlorine is obtained from a mixture of manganese, common salt, and sulphuric acid, the apparatus, being required to withstand a greater heat, is made entirely of metal.
 Fig. 2.
Fig. 2.
In fig. 2. a a represents a shallow iron pan, fitted with the tube b, for the purpose of emptying the contents of the leaden cylinder d d. This iron vessel serves as the lower part of the cylinder d d, the top of which is provided with an opening for a funnel syphon tube, for the introduction of the acid, and another opening, f, for the manganese. The entire apparatus stands on a flue leading from the furnace.
 Fig. 3.
Fig. 3.
452
The foregoing drawing represents a vessel for the manufacture of chlorine on a large scale, and is extensively used in Germany.
It consists of a cylindrical vessel of sandstone, the lower half of which, A, is carved out of a single block; the upper half, B, also of one piece, fits into the lower by means of a grooved joint, the two parts being united by means of a cement made of clay and boiled linseed oil. About six inches from the bottom the cylinder widens by 2 inches, and the rim thus formed carries a perforated bottom, C, upon which the manganese is deposited in large lumps. The tube D, likewise of stone, passes beneath the perforated bottom, and is at the other end joined to the steam-tube E. The steam must therefore, when introduced, enter the cylinder through the perforations of the false bottom. The top of the cylinder is closed by a lead cover, K, which is fastened down by means of iron clamps; this lid has an aperture, G, and the tubes E, F, H, pass through it; tube E serves, as already stated, for the introduction of the steam; tube F is for the delivery of chlorine; the bent tube, H, which ends in a funnel, for the introduction of the hydrochloric acid; and the opening G for throwing the lumps of manganese into the cylinder. The solution of manganese chloride, resulting from the action of the hydrochloric acid upon the manganese, is removed through I, which is kept closed by a wooden stopper whilst the reaction proceeds.
See also, under Chlorine, the description of Weldon’s stills, and of Deacon’s apparatus.
CHLORITE. A salt in which the hydrogen of chlorous acid, HClO2, is replaced by a metal or other basic radical. See Chlorous Acid.
CHLOROCHROMIC ACID. CrOCl. Syn. Chlorochromic anhydride. Prep. Bichromate of potassium, 3 parts; common salt, 31⁄2 parts; are intimately mixed together, put into a glass retort, and oil of vitriol, 9 parts, added; heat is next applied and maintained as long as dense, red vapours are given off. The product in the receiver is a heavy, deep-red liquor, greatly resembling bromine in appearance. Water resolves it into hydrochloric and chromic anhydride.
CHLORODYNE. See Patent Medicines.
Chlorodyne (Dr Browne’s). Acid muriat. conc., 5 parts; ether, chloroform, tinct. cannab. Ind., tinct. capsici, of each 10 parts; morphia, prussic acid, of each 2 parts; oil of peppermint, 1 part; syrup, 50 parts; tinct. hyoscyami, tinct. aconiti, of each 3 parts.
Chlorodyne, English. A filtered mixture of 5 grammes tinct. aromat., 4 grammes tinct. opii simp., 1 gramme morph. mur., 10 grammes aq. amygd. amar., 80 grammes syrup of liquorice, 1 gramme extract of liquorice, 40 grammes 90 per cent. spirit of wine, 5 drops oil of peppermint, 10 drops ether, 30 drops chloroform.
CHLOROFORM. CHCl3. Syn. Terchloride of formyle, Formyl-chloride; Chloroformyl, Trichloromethane, Chloroformum, L. A remarkable fluid discovered by Liebig in 1830, and independently by Soubeiran in 1832, and carefully examined in 1834 by Dumas. In 1842 its action upon animals was investigated by Dr M. Glover, and in 1847 it was introduced to the medical profession as an anæsthetic agent by Dr Simpson of Edinburgh.
It was first obtained by the action of caustic alkali upon chloral, but it is more easily prepared by distilling alcohol or wood spirit with chloride of lime. It may also be procured from wood spirit, acetone, oil of turpentine, and several essential oils, as well as from amylic alcohol, acetic acid, tartaric acid, and phenol; when these different bodies are severally subjected to the action of chloride of lime. When chlorine is made to act on marsh gas, or when chloral is treated with an alkali, chloroform is also produced.
Prep. 1. Chloride of lime (in powder), 4 lbs.; water, 12 lbs.; mix in a capacious retort or still, add of rectified spirit, 12 fl. oz., and cautiously distil as long as a dense liquid, which sinks in the water it passes over with, is produced; separate this from the water, agitate it with a little sulphuric acid, and, lastly, rectify it from carbonate of barium.
2. Chloride of lime, 4 lbs.; water, 10 pints; rectified spirit, 1⁄2 pint; proceed as last, using a spacious retort that the mixture will only 1-3rd fill, and the heat of a sand bath. When ebullition commences remove the fire as quickly as possible, lest the retort be broken by the suddenly increased heat, and let the solution distil into a receiver as long as there is nothing which subsides, the heat being restored if it be at all needed. Add to the distilled liquid four times as much water, and shake the whole well together; next cautiously separate the heavier part as soon as it has subsided, and to this add chloride of calcium, broken into fragments, 1 dr.; and shake occasionally during an hour; finally, let the fluid again distil from a glass retort into a glass receiver.
3. Hydrate of lime, 1 part, is suspended in cold water, 24 parts, and chlorine passed through the mixture until nearly the whole of the lime is dissolved; hydrate of lime, q. s. just to restore the alkaline reaction of the liquid, is then added; and, afterwards, rectified spirit of wine or wood spirit, 1 part, is mixed in; the whole, after repose for 24 hours in a covered vessel, is cautiously distilled as before.
4. (B. P.) Take of chlorinated lime 10 lbs.; rectified spirit, 30 fluid ounces; slaked lime, a sufficient quantity; water, 3 gallons; sulphuric acid, a sufficient quantity; chloride of calcium in small fragments 2 oz.; distilled water, 10 fluid ounces. Place the water and the spirit in a capacious still, and raise the mixture to a temperature of 100° F. Add the chlorinated lime, and 5 lbs. of the slaked lime, mixing thoroughly. Connect the still with a453 condensing worm, encompassed by cold water, and terminating in a narrow-necked receiver; and apply heat so as to cause distillation, taking care to withdraw the fire the moment that the process is well established. When the distilled product measures 50 fluid ounces the receiver is to be withdrawn. Pour its contents into a gallon bottle, half filled with water; mix well by shaking, and set it at rest for a few minutes, when the mixture will separate into two strata of different densities. Let the lower stratum, which contains crude chloroform, be washed by agitating it in a bottle with 3 fluid ounces of the distilled water. Allow the chloroform to subside, withdraw the water, and repeat the washing with the rest of the distilled water, in successive quantities of 3 oz. at a time. Agitate the washed chloroform for five minutes in a bottle with equal volume of sulphuric acid, allow the mixture to settle, and transfer the upper stratum of liquid to a flask, containing the chloride of calcium, mixed with 1⁄2 oz. of slaked lime, which should be perfectly dry. Mix well by agitation. After the lapse of an hour connect the flask with a Liebig condenser, and distil over the pure chloroform by means of a water bath. Preserve the product in a cool place in a bottle furnished with an accurately ground stopper. The lighter liquid which floats on the crude chloroform after its agitation with water, and the washings with distilled water, should be preserved and employed in a subsequent operation. Sp. gr. 1·456.
Prop., &c. Liquid; transparent; colourless; odour fragrant, ethereal, and apple-like; taste ethereal, sweetish, but slightly acrid; soluble in 2000 parts of water; mixes in all proportions with alcohol and ether; dissolves (readily) bromine, camphor, caoutchouc, gutta percha, iodine, oils, resins, wax, and several other like substances; boils at 141·8° Fahr.; kindles with difficulty; burns, when strongly heated, with a greenish flame; and communicates a dull, smoky-yellow colour to the flame of alcohol. Sp. gr. 1·48 (1·497, Miller); density of vapour 4·2. The vapour has the remarkable property of rendering a person breathing it temporarily insensible to pain.
Chloroform is frequently adulterated with alcohol and ether; and owing to careless manipulation, is also sometimes contaminated with other substances, as chloral, hydrochloric acid, free chlorine, aldehyde and certain chlorinated oils. These latter compounds are not only the most objectionable and prejudicial of the impurities found in chloroform, but if present in it to any appreciable extent, they render its anæsthetic administration not only inefficient, but frequently absolutely dangerous. These deleterious chlorinated oily compounds may be removed by agitation with strong sulphuric acid, or by distillation from it. Chloroform made from wood spirit is said to be more impure than that from alcohol. When pure it is free from colour, and of a pleasant odour. It is not perfectly soluble in water; and does not turn the colour of litmus red. Rubbed on the skin it quickly evaporates, scarcely leaving any odour. Dropped into water, it falls to the bottom and remains bright and limpid; but if it contain alcohol the surface of the drop becomes opaline. If the same experiment be made with diluted sulphuric acid, sp. gr. 1·44, the drop of pure chloroform will fall to the bottom; but that which contains spirit, if not shaken, will float or remain suspended in the acid solution. When contaminated with heavy hydrocarbon oils, a drop evaporated from the palm of the hand leaves behind a strong smell. Hydrochloric acid and free chlorine are detected by the ordinary tests.
Mr Shuttleworth, writing to the ‘Canadian Pharmaceutical Journal,’ says:—“In regard to the restoration of chloroform which has become spoiled, I would recommend that the chloroform be well agitated with a dilute solution of hyposulphite of soda.
“It should then be separated by means of a glass funnel from the supernatant liquid, and again washed; this time with simple water. After being separated the chloroform should be passed through filtering paper to free it from traces of moisture, when it will be found much improved and comparatively sweet, good enough in any case for external use.
“There are, of course, certain other impurities of chloroform which the hyposulphite will not remove. These are of a more stable character, and as they possess a higher boiling point than chloroform, may be separated by distillation, or by treatment with sulphuric acid in the usual manner.”
Uses, Action, &c. Chloroform is anodyne, antispasmodic, sedative, stimulant, and anæsthetic. In small doses (5 to 12 or 15 drops, in water, mixed with a little syrup or mucilage) it is employed in spasmodic disorders, and as a stimulant and diaphoretic. It is now chiefly used as an anæsthetic to produce insensibility to pain during surgical operations. The dose for inhalation is 1 fl. dr., which is repeated, in a few minutes, if no effect is produced, until 3 fl. dr. have been thus exhibited; the effects being carefully watched, and the source of the chloroform vapour removed as soon as a sufficient degree of anæsthesia is produced, or any unpleasant symptoms develop themselves.
Chloroform in large doses depresses the heart’s action, and causes profound coma, and death. It is therefore dangerous in all cases complicated with diseases of the heart or brain, or any visceral affections of a congestive character.
The treatment of asphyxia from chloroform is—the horizontal position, cold affusion to the head and spine, artificial respiration, and, if possible, either the application of electricity, or the inhalation of protoxide of nitrogen or oxygen gas, largely diluted with atmospheric air.
454
Concluding Remarks. The preparation of chloroform is not unattended with danger, and frequently miscarries in careless or inexperienced hands. This arises chiefly from the violent reaction which immediately follows the application of the heat. The common plan is attended with danger of explosion, or of the liquid in the still being forced over into the receiver, owing to the extraordinary rapidity with which the vapours are eliminated, and the ingredients, in consequence, swell up. A method which is successfully adopted on the large scale is to employ a very broad and shallow capsule-shaped still, having a flat rim round it, with a head or capital furnished with a corresponding rim at its lower part. In use, a flat, endless band of vulcanised india rubber is placed between the two rims, which are then held air-tight together by means of small, iron clamps. The application of heat is also delayed for some time after the admixture of the spirit with the other ingredients, and the process is interrupted as soon as the first violence of the reaction has subsided, by which time the whole product of chloroform will have passed over into the receiver. If the distillation is continued beyond this point, the remaining product is water. On the small scale, a very capacious, flat-bottomed retort or cucurbit should be employed. A similar condenser may be used to that noticed under ether.
CHLOROFORMIC ANODYNE (George Harley) is said to be an alcoholic tincture of opium with prussic acid and chloroform.
CHLOROHYPONITRIC GAS (NOCl) and CHLORONITROUS GAS (N2O2Cl4 are two peculiar compounds, formed when nitric acid and hydrochloric acid are mixed.
CHLOROMETER. Syn. Chlorim′eter. An instrument or apparatus employed in chlorometry. The chlorometers in common use are graduated measures and tubes precisely similar to those used in ACIDIMETRY, ALKALIMETRY, &c.
CHLOROMETRY. Syn. Chlorim′etry. The estimation of the available chlorine in the bleaching powder of commerce, which is valued and sold in this country by its per-centage of that element. The plans generally adopted are applicable to the so-called chlorides of soda and potassa, as well as to the ordinary bleaching powder, chloride of lime. Most of them depend on the oxidising effect of water when undergoing decomposition through the action of chlorine.
Dalton’s Process. The test solution is prepared as follows:—Pure protosulphate of iron (previously dried by strong pressure between the folds of cloth or bibulous paper), 78 gr., are dissolved in distilled water, 2 oz., and a few drops of hydrochloric or sulphuric acid added. This quantity of protosulphate requires for complete peroxidation just the quantity of oxygen liberated by 10 gr. of chlorine; in other words, the solution exhibits the indirect effect produced by exactly 10 grains of the bleaching element.
Exactly 50 gr. of the sample of chloride of lime to be examined are next weighed, and well mixed in a glass or wedgwood mortar with tepid water, 2 oz.; and the mixture poured into a graduated tube or chlorometer. The tube is next filled up to 0, or zero, with the washings of the mortar, and the whole well mixed, by placing the thumb over the orifice and shaking it. The solution of chloride of lime, thus formed, is next gradually and cautiously added to the solution of sulphate of iron, previously noticed, until the latter is completely peroxidised, which may be known when it ceases to be affected by a solution of red prussiate of potash. When a drop of the latter test, placed upon a white plate, ceases to give a blue colour on being touched with the point of a glass stirrer or rod dipped in the liquor under examination, enough of the solution of the chloride has been added. The number of measures thus consumed must now be carefully read off from the graduated scale of the chlorometer, from which the richness of the sample may be estimated as follows:—As 100 of the chlorometer divisions contain exactly 50 gr. of the chloride under examination, each measure will contain only 1⁄2 gr., and, consequently, the number of measures consumed will represent half that number of grains of the chloride examined; and the weight of the chloride thus used will have contained 10 gr. of chlorine—the constant quantity of that substance required to peroxide the test solution of sulphate of iron. Thus:—If 80 measures of the liquor in the chlorometer have been consumed, this quantity will represent 40 gr. of chloride of lime, and 10 gr. of chlorine. By dividing 1000 by this number, the per-centage of chlorine will be obtained. In the present instance this would be—
Crum’s Process. Equal weights of water and hydrochloric acid are mixed together, and cast-iron borings digested in the diluted acid until saturation is complete; a large excess of iron being purposely employed, and the liquid kept at the heat of boiling water for some time. One measure of the solution, marking 40° on Twaddell’s scale (sp. gr. 1·200), is then mixed with an equal quantity of acetic acid (sp. gr. 1·048). This forms the test-liquid. When mixed with 6 or 8 parts of water it is quite colourless, but chloride of lime occasions the production of peracetate of iron, which gives it a red colour.
The above proof-solution is next poured into 12 two-oz. vials, of exactly equal diameters, to the amount of 1⁄9th of their capacity; these are filled up with bleaching liquid of various strengths; the first at 1⁄12th of a degree of Twaddell, the second 2⁄12ths, and so on up to455 12⁄12ths of 1°. They are then well corked up, and, after agitation, arranged side by side on a tray, furnished with holes to receive them. (See engr.) To ascertain the strength of an unknown sample of bleaching liquor, the proof-solution of iron is put into a phial, exactly similar to the 12 previously used, and in precisely the same proportion (1⁄9th). The phial is then filled up with the bleaching liquor, well shaken, and placed beside that one of the 12 already prepared which it most resembles in colour. The number on that phial expresses the strength of the sample under examination, in twelfths of a degree of Twaddell’s hydrometer.
Obs. The preceding method is admirably suited for weak solutions, such as are employed for bleaching textile fabrics, and is well adapted (from its simplicity) to the purposes of practical men. Indeed, it is quite astonishing to see with what ease and accuracy it is applied by unlettered operatives. This gives it great practical value. It has been for some time in extensive use in the bleaching houses of Scotland.
Table exhibiting the quantity of Bleaching Liquid, at 6° on Twaddell’s scale (sp. gr. 1·030), required to be added to a weaker liquor, to raise it to the given strengths. Adapted from Mr Crum’s table by Mr Cooley.
| Strength of Sample in 1⁄12°. | Required Strength. | Proportions required. | |
| Given Sample. | Liquor at 6°. | ||
| Parts. | Part. | ||
| Water. | 8⁄12° | 8 | 1 |
| 1 | ” | 91⁄4 | 1 |
| 2 | ” | 11 | 1 |
| 3 | ” | 131⁄2 | 1 |
| 4 | ” | 17 | 1 |
| 5 | ” | 23 | 1 |
| 6 | ” | 35 | 1 |
| 7 | ” | 71 | 1 |
| Water. | 6⁄12° | 11 | 1 |
| 1 | ” | 131⁄2 | 1 |
| 2 | ” | 17 | 1 |
| 3 | ” | 23 | 1 |
| 4 | ” | 35 | 1 |
| 5 | ” | 71 | 1 |
| Water. | 4⁄12° | 17 | 1 |
| 1 | ” | 23 | 1 |
| 2 | ” | 35 | 1 |
| 3 | ” | 71 | 1 |
| Water. | 3⁄12° | 23 | 1 |
| 1 | ” | 35 | 1 |
| 2 | ” | 71 | 1 |
According to Mr Crum, the range of strength within which cotton is “safe” is very limited. A solution at 1° of Twaddell’s scale (sp. gr. 1·005) is not more than safe, while one at 1⁄2° is scarcely sufficiently strong for the first operation on stout cloth, unless it is packed more loosely than usual.
Gay-Lussac’s Indigo Process. One part of the best indigo is dissolved in 9 parts of strong sulphuric acid by the aid of a gentle heat; this solution is then mixed with distilled water, in such proportion that 1 volume of chlorine gas shall exactly decolour 10 volumes of this solution. Each measure so decoloured is called a degree, and each degree is divided into fifths. 5 gr. of the best chloride of lime, dissolved in 500 gr. measures of water, possess the above power, and indicate 10° or proof; or in other words, will decolour 10 times its volume of the indigo solution.
Obs. This method of chlorometry is objectionable, and liable to error, from the indigo solution altering by keeping. When, however, the proper precautions are used, it may be safely trusted for weak bleaching liquors.
Arsenious Acid Process. This depends on the conversion, by oxidation, of arsenious acid into arsenic acid, in the presence of chlorine and water.
To prepare the test-liquor, pure arsenious acid, 100 gr., are dissolved in about 4 fl. oz. of pure hydrochloric acid (free from sulphurous acid), and the solution diluted with water until, on being poured into a graduated 10,000 grains-measure-glass, it occupies the volume of 7000 grains measure marked on the scale. Each 1000 grains measure of this liquid now contains 14·29 gr. of arsenious acid; corresponding to 10 gr. of chlorine, or 1⁄10th gr. of chlorine for every division or degree of the scale of the chlorometer.
100 gr. of the chloride of lime to be examined are next dissolved in water as before, and poured into a tube graduated up to 2000 grains measure. The whole is now well shaken, in order to obtain a uniformly turbid solution, and half of it (1000-grains-measure) transferred to a graduated chlorometer, which is, therefore, thus filled up to 0°, or the zero of the scale, and contains exactly 50 gr. of the chloride of lime under examination; whilst each degree or division of the scale contains only 1⁄2 gr.
1000 grains measure of the arsenious acid test-liquor are now poured into a glass beaker, and a few drops of solution of sulphate of indigo added in order to impart a faint but distinct blue colour to it; the glass is then shaken so as to give a circular movement to the liquid, and whilst it is whirling round, the chloride-of-lime solution from the chlorometer is gradually and cautiously added, until the blue tinge given to the arsenious acid test-liquor is destroyed; care being taken to stir456 the mixture well during the whole process, and to stop as soon as the decolorisation is completed.
Let us suppose now that, in order to destroy the blue colour of the 1000 grains measure of the arsenious acid test-liquor, 90 divisions or degrees of the chloride-of-lime solution have been employed. These 90 divisions, therefore, contained the 10 gr. of chlorine required to destroy the colour of the test-solution; and since each division represents 1⁄2 gr. of chloride of lime, 45 gr. of chloride of lime (10 gr. of chlorine) were present in the 90 divisions so employed, from which the per-centage strength may be ascertained. For—
45 : 10 : : 100 : 22·22
The chloride of lime examined, therefore, contained 221⁄4 per cent, (nearly) of chlorine.
Obs. This method is extremely simple and trustworthy when properly employed; but to ensure accuracy, certain precautions must be adopted. Instead of pouring the test-liquor into the solution of the sample (as in alkalimetry), the solution of the sample must be poured into the test-liquor.
Vogel found that in a normal solution of arsenious acid that had been prepared for using in the above process, half the quantity of the arsenious acid became oxidised to arsenic acid in the course of about a year. He therefore recommends that the standard solution, if kept for some time, should be tested by a magnesium salt. The formation of a precipitate would show the solution had undergone such a change, as to render it unfit for volumetric estimations.
Penot’s Process. This is a modification of the previous process. For the arsenious acid solution arsenite of soda is substituted, and for the indigo solution a colourless iodised paper, which is turned blue by the smallest quantity of free acid. The paper is prepared in the following manner:—1 gram of iodine, 7 grams of carbonate of soda, 3 grams of starch, and a quarter of a litre of water are mixed. When the solution becomes colourless it is diluted to half a litre; in this fluid, white paper is soaked. The arsenical fluid is prepared by dissolving 4·44 grams of arsenious acid, and 13 grams of crystallised carbonate of soda in 1 litre of water. This solution is added by means of a burette to the solution of chloride of lime intended to be tested (10 grams of the sample to 1 litre) the completion of the reaction being known by the paper remaining uncoloured.
Lunge says that the same piece of moist iodine test paper may be made use of repeatedly, since the spots produced by testing usually disappear after about twenty-four hours if exposed to the air. The paper must, however, be kept away from dust.
Wagner’s Process. This method is based upon the fact that a solution of chloride of lime separates the iodine from a weak (1 to 10) and slightly acidified iodide-of-potassium solution, the iodine being quantitatively estimated by means of hyposulphite of soda:—
| Iodine 21, | yield | Iodine of sodium, 2NaI, |
| Hyposulphite of | Tetrathionate of sodium, Na2S4O6, | |
| soda, 2Na1SO3 | Water, 5H2O. | |
| +5H2O, |
The test is performed as follows:—100 c. c. =1 gram of bleaching-powder solution, obtained by dissolving 10 grams of chloride of lime in 1 litre of water, are mixed with 25 c. c. of solution of iodide of potassium acidified with dilute hydrochloric acid. The resulting clear, deep brown-coloured solution is treated with hyposulphite of soda solution until quite colourless. The hyposulphite of soda solution is composed of 24·8 grams of that salt to 1 litre of water; 1 c. c. of this solution neutralises 0·0127 grams of iodine, and 0·00355 grams of chlorine.
Otto’s Process. This method is based upon the following data. Two molecular weights of protosulphate of iron when brought into contact with chlorine, in presence of water, and free sulphuric acid, give one molecule of persulphate of iron, and two molecules of hydrochloric acid, the process consuming one molecule of chlorine. Two molecules of crystallised sulphate of iron = 556, correspond to 71·0 of chlorine, or in other terms 0·7839 grams of the crystallised sulphate correspond to 0·1 gram of chlorine.
Bunsen’s Process. This consists in adding iodide of potassium to the bleaching-powder liquor, acidulating the mixture with hydrochloric acid, and running the solution of arsenite of soda into it till only a yellow tint shows itself. A little starch paste is now added, and the arsenite solution cautiously introduced drop by drop, till the blue colour just disappears. The solutions must all be standardised. To preserve the starch paste Mohr advises the addition to it of a little chloride of zinc.
Mr Davies uses glycerin as a solvent for the arsenious acid. He prepares a standard solution as follows:—13·95 grains of arsenious acid in 40 c. c. of glycerin and fitted up to 1 litre. Every c. c. corresponds to 0·1 grain of chlorine. Indigo sulphate solution is used as an indicator, and the bleaching liquor is run into the glycerin solution until the blue colour of the latter is changed to a brownish yellow.
Dr Ure’s as follows:—Liquor of ammonia, of a known strength, tinged with litmus, is added to a solution of a given weight of the chloride under examination, until the whole of the chlorine is neutralised, which is known by the colour being destroyed. From the quantity of ammonia consumed the strength of the sample is estimated.
The value of bleaching powder is estimated in England, America, and Germany by degrees corresponding to the per-centage of available chlorine contained in a sample of457 chloride of lime by weight; but in France the degrees denote the number of litres of chlorine gas at 0° c. and 760° Mm. Bar., which 1 kilo of bleaching powder can evolve. In the following table the chlorometrical degrees of France and England are contrasted:—
| French. | English. |
| 63 | 20·02 |
| 65 | 20·65 |
| 70 | 22·24 |
| 75 | 23·83 |
| 80 | 25·42 |
| 85 | 27·01 |
| 90 | 28·60 |
| 100 | 31·80 |
| 105 | 33·36 |
| 110 | 34·95 |
| 115 | 36·54 |
| 120 | 38·13 |
| 125 | 39·72 |
| 126 | 40·04 |
The per-centage may be calculated by multiplying the French degrees by the coefficient 0·318.
CHLOROCARBONIC ACID. (COCl2). Syn. Phosgene Gas, Carbonic oxydichloride. This compound may be produced by the direct combination of equal volumes of carbonic oxide and chlorine gases under the influence of sunlight (whence its name of “phosgene gas”), when the mixture gradually becomes colourless, and contracts to half its original volume. Chlorocarbonic acid has a peculiar pungent smell, and fumes strongly when exposed to moist air, the moisture of which it decomposes, producing at the same time hydrochloric and carbonic acids.
It is sometimes employed in chemical research for the removal of hydrogen from organic compounds, and the substitution of carbonic oxide, or its elements for the hydrogen.
CHLOROPHYLL. The green colouring matter contained in the leaves, stalks, unripe fruit, and juices of most plants.
CHLORO′SIS. Syn. Green sickness. A disease which principally affects young unmarried females.
Symp. Languor, listlessness, fatigue after the least exercise, palpitation of the heart, flatulency, indigestion, acidity of stomach and bowels, constipation (generally), appetite for unnatural food, general debility, &c. As the disease advances, the skin at first pale, assumes a peculiar greenish tint, the respiration becomes affected, the feet and legs swell, and various organic affections of the viscera ensue. During the early stages of this disease the catamenia are usually pale and scanty, and return at irregular intervals, and as it progresses they disappear altogether.
Treat. This should be tonic and restorative. That recommended under Anæmia may be adopted with advantage. See also Appetite, Atrophy.
Chlorosis, Electuary for—Female Electuary. A greenish-black thick syrup, consisting of sugar, bayberries, carbonate of iron, iron filings, and water. (Buchner.)
Chlorosis Powder—Female Powder—consists of a mixture of anise, sugar, and 14 per cent. of iron filings. (Wittstein.)
Chlorosis Powder—Female Powder, according to Schott and Strauss, is a mixture of violet root, gum Arabic, and a tasteless green powder with 33 per cent. of steel filings. According to Hager, it is composed of 2 parts ferri pulvis, with 3 parts powdered sweet-flag root.
Chlorosis Powder—Female Powders. Steel filings, starch powder, and knot grass, of each 1 part, Florentine orris root, 4 parts.
Chlorosis Powder—Female Powders. A mixture of 1 part steel filings and 2 parts of a vegetable powder composed of gum Arabic, Florentine orris, knot grass, &c. (Egb. Hoyer.)
Chlorosis Water (Dr Ewich) contains in 10,000 parts 11 of sodium carbonate, 9 of sodium chloride, 11⁄2 sodium sulphate, 7 calcium carbonate, and 1·2 iron carbonate with an excess of carbonic acid. (Hager.)
CHLOROUS ACID. HClO2. Syn. Acidum chloro′sum, L. Prep. From chlorate of potassium, 4 parts; arsenious anhydride, 3 parts; nitric acid, 12 parts; (diluted with) water, 4 parts; heated together in a glass flask, furnished with a bent tube, and placed in a water bath. It must be collected in the same way as chlorine, or passed into water, when it forms a solution of chlorous acid.
Prop., &c. Chlorous acid is a greenish-yellow gas, non-condensable by a freezing mixture of salt and ice, but liquefiable by extreme cold. The aqueous solution undergoes gradual decomposition, yielding chloric acid and chlorine. Chlorous acid possesses powerful oxidising and bleaching properties; with the bases it forms salts called CHLORITES. These are all soluble in water, and bleach like the acid. They may be recognised by the evolution of chlorous acid gas when acted on by an acid. The use of the arsenious acid is to deoxidise the nitric acid employed in the process. Tartaric acid, or other deodorising agent, may be substituted for it.
CHOC′OLATE. Syn. Chocola′ta, L.; Chocollati, Mexican; Chocolat, Fr. A beverage or paste made from the roasted seeds of the Theobroma Cacao, or Cocoa. Strictly speaking, the term “chocolate” is applicable to all genuine preparations of cocoa, but it is now generally used to distinguish those which contain sugar, and, commonly, flavouring substances. Of late years great attention has been paid to the manufacture of chocolate in England; our principal makers now import the finest descriptions of cocoa, and produce varieties of the manufactured article which are scarcely inferior to those of their French458 rivals. The different kinds of cocoa, and the processes of roasting, sweating, &c., are described under Cocoa, to which article we refer the reader also for particulars respecting the chemistry of chocolate.
Prep. The cocoa nibs[245] are ground in a mill consisting of stone or metal rollers, which are usually heated either by charcoal fires or by steam, so as to soften or melt the natural fat.[246] The warm, smooth paste which passes from the mill is then placed in a mixing mill, and incorporated with refined sugar, and usually vanilla or other flavouring substance. The trituration is continued until the whole paste is converted into an entirely homogeneous mass, which is finally shaped, by means of suitable moulds, into various forms, as blocks, loaves, tablets, lozenges, &c.
[245] The bruised, roasted seeds, freed from husk and membrane.
[246] Cacao- or cocoa-butter.
Obs. Chocolate, prepared as above, without the addition of aromatics, is known in the trade as PLAIN CHOCOLATE. The Spaniards flavour it with vanilla, cloves, and cinnamon, and frequently scent it with musk and ambergris. With these additions it is termed Spanish chocolate. In general, they add too large a quantity of the last four articles. The Parisians, on the contrary, use little flavouring, and that principally vanilla. They employ the best kinds of cocoa, and add a considerable quantity of refined sugar. So prepared, it is called French chocolate.
Proportions. 1. French Chocolate:—The proportions used for the best description are said to be—2 beans of vanilla, and 1 lb. of the best refined sugar, to every 3 lbs. of the choicest cacao nuts.
2. Spanish Chocolate:—The following forms are said to be commonly adopted:—
a. Caracas cocoa, 11 lbs.; sugar (white), 3 lbs.; vanilla, 1 oz.; cinnamon (cassia), 1⁄4 oz.; cloves, 1⁄2 dr.
b. Caracas cocoa, 10 lbs.; sweet almonds, 1 lb.; sugar, 3 lbs.; vanilla, 11⁄4 oz.
c. Caracas cocoa, 8 lbs.; island cocoa, 2 lbs.; white sugar, 10 lbs.; aromatics, as above.
d. Island cocoa, 7 lbs.; farina, q. s. to absorb the oil. Inferior.
3. Vanilla Chocolate. Syn. Chocolat à la vanille, Fr. A variety of French or Spanish chocolate highly flavoured with vanilla. The following proportions have been recommended:—
a. Caracas cocoa, 7 lbs.; Mexican vanilla, 1 oz.; cinnamon, 1⁄2 oz.; cloves, 3 in no.
b. Best chocolate paste, 21 lbs.; vanilla, 4 oz.; cinnamon, 2 oz.; cloves, 1⁄2 dr.; musk, 10 gr.
Obs. The vanilla used in making chocolate is reduced to powder by rubbing it with a little sugar before adding it to the paste.
Pur., &c. The chocolate commonly sold in England is prepared from the cake left after the expression of the oil, and this is frequently mixed with the roasted seeds of ground peas, and maize or potato flour, to which a sufficient quantity of inferior brown sugar, or treacle and mutton suet, is added to make it adhere together. Inferior sweet almonds are also employed in the same way.
Since the above paragraph was written there has been a vast improvement in English chocolates, though the cheaper sorts of certain makers are still much adulterated. Genuine chocolate should dissolve in the mouth without grittiness, and should leave a peculiar sensation of freshness; after boiling it with water the emulsion should not form a jelly when cold, for if it does starch or flour is present. The presence of animal fat may generally be detected by a cheesy or rancid flavour. See Cocoa.
Qual., &c. Chocolate is nutritive and wholesome if taken in moderation, but is sometimes apt to disagree with weak stomachs, especially those that are easily affected by oily substances or vegetable food. When this is the case, by adopting the simple plan recommended under Butter, chocolate may generally be taken with impunity, even by the dyspeptic. The quantity of aromatics mixed with the richer varieties of chocolate improve the flavour, but render them more stimulant and prone to produce nervous symptoms and head complaints.
Chocolate is taken in the solid form, or made into a beverage; or, combined with sugar, is made into various articles of confectionery.
Chocolate for the Table is prepared by slicing or scraping very finely the required quantity into a jug, and adding to it a small quantity of boiling water. This is worked into a thin, smooth paste, and the jug immediately filled up with boiling milk-and-water. A froth is produced by the same means that eggs are beaten up. The operation of “milling,” performed by rapidly twirling a notched cylinder of wood in the emulsion, raises the froth very quickly. Sugar may be put in with the scraped chocolate, or added afterwards at pleasure.
Chocolate should never be made for the table before it is wanted, because beating it again injures the flavour, destroys the froth, and separates the body of the chocolate, the oil of the nut being observed, after a few minutes’ boiling, or even standing long by the fire, to rise to the top. This is one of the principal reasons why chocolate offends the stomach.
Preparations of chocolate, intended either as nutritious articles of food for convalescents, or as vehicles for medicine, are common among the pharmacopœial and magistral formulæ of the Continent. The following are a few examples:—
Chocolate, Aromat′ic. Prep. (Weiglebt.) Cocoa beans and sugar, of each 16 oz.; cinnamon, 1⁄2 oz.; cloves, 2 dr.; cardamoms and vanilla, of each 1 dr.
459
Chocolate, Car′rageen. See Chocolate, White (Nos. 1 and 2).
Chocolate, Chalyb′eate. Syn. Ferrugin′eous chocolate; Chocola′ta chalybea′ta, C. Mar′tis, L. Prep. 1. (Trousseau.) Spanish chocolate, 16 oz.; carbonate of iron, 1⁄2 oz.; mix, and divide into 1-oz. cakes. One at a time; in anæmia, amenorrhœa, chlorosis, &c.
2. (Pierquin.) Iodide of iron, 2 dr.; chocolate, 16 oz. For 1⁄2-oz. cakes; as above, and in scrofulous and glandular affections.
Chocolate, Guarana′. Syn. Paullin′ia chocolate; Chocola′ta paulin′iæ, C. guaran′æ, L. Prep. From guarana and white sugar, of each 1 oz., triturated together, and afterwards thoroughly mixed with good plain chocolate, 18 oz. Recommended as a restorative in debility, chlorosis, and other diseases of debility, especially those of a nervous character.
Chocolate, Ice′land Moss. Syn. Chocola′ta cetrar′iæ island′icæ, C. lichen′is, L. Prep. 1. (P. C.) Simple chocolate (P. C.), 32 parts; sugar, 29 parts; dried jelly of Iceland moss, 11 parts; mix.
2. (Cadet.) Chocolate, 4 lbs.; sugar, 2 lbs.; Iceland moss (freed from its bitter, and powdered), 11⁄2 lb.; tragacanth and cinnamon, of each 4 oz.; water, q. s.; to be beaten in a warm mortar, or ground with a muller on a warm slab to a paste. Recommended in pulmonary affections, general debility, weakness of stomach, &c. See Cocoa (Iceland Moss).
Chocolate, Pur′gative. Syn. Chocola′ta pur′gans, C. cathar′tica, L. Prep. 1. Jalap, 1 oz.; chocolate, 9 oz.; mix, and divide into 1-dr. cakes.—Dose, 1 to 2 cakes, as a purge.
2. Jalap, 2 oz.; calomel and sugar, of each 1 oz.; triturate together, then add chocolate, 20 oz.; for 1-dr. cakes.
3. Scammony, 2 dr.; chocolate, 3 oz.; for 1 dozen cakes. The last two are given in worms.—Dose (for an adult), 1 cake, taken fasting.
Chocolate, Sal′ep. Syn. Sal′oop chocolate; Chocola′ta cum sal′ep, L. Prep. 1. (P. C.) Chocolate, 16 oz.; powdered salep, 1⁄2 oz.
2. (Cadet.) Cacao paste and sugar, of each 1 lb.; powdered salep, 1 oz. Arrowroot chocolate and tapioca chocolate are made in the same manner. (See below.)
Chocolate, Sim′ple. Syn. Hygien′ic c., Homœopath′ic c.; Chocola′ta, C. sim′plex, C. salu′tis, L.; Chocolat de santé, Fr. Prep. (P. C.) Caracas and Maragnan cocoa, of each 96 lbs.; sugar, 160 lbs.; cinnamon, 1 oz. (to 2 oz.); triturated together in the usual manner, and formed into cakes or powder.
Chocolate, Vanil′la. Syn. Chocola′ta cum vanil′la, L. Prep. 1. (P. C.) Chocolate (plain,—P. C.), 16 oz.; vanilla, 1⁄2 dr.
2. (Cotterau.) Cocoa paste, 6 lbs.; sugar, 10 lbs.; vanilla, 11 dr.
See forms previously given.
Chocolate, Ver′mifuge. Syn. Chocola′ta vermifu′ga, L. See Chocolate, Purgative (Nos. 2 and 3, above).
Chocolate, White. Syn. White cocoa, Car′rageen c.; Chocola′ta cum chon′dro, Pas′ta caca′o cum chon′dro, P. c. c. lichen′e carraghen′o, L. Prep. 1. As Iceland moss chocolate, but employing carrageen moss.
2. (Ph. Dan.) Roasted and decorticated cocoa seeds (reduced to a subtile mass in a warm iron mortar) and powdered white sugar, of each 2 lbs.; powdered carrageen (debitterised), 3 oz.
3. (Cottereau.) Sugar, 6 lbs.; rice flour, 13⁄4 lb.; potato starch and butter of cocoa, of each 1⁄2 lb.; gum Arabic 1⁄4 lb. (dissolved); tincture of vanilla, 1⁄2 fl. oz.; boiling water, q. s.; triturate to a stiff paste. The above are highly nutritious, and are recommended as articles of diet for convalescents and debilitated persons.
CHOKE-DAMP. Syn. Aft′er-damp. The term applied by miners to carbonic anhydride (carbonic acid) and other irrespirable gases and vapours evolved in mines. See Carbonic Acid, Fire Damp, Ventilation, &c.
CHOKING. Threatened choking may occur either in the gullet or swallow—or in the windpipe. If in the gullet press down the tongue with the handle of a spoon, and pass the fingers down without any hesitation, when the substance may generally be dislodged or pulled up. When it is small, and has got out of reach, it may mostly be removed by filling the mouth with liquid and swallowing it at a gulp, or by swallowing a large piece of bread. Foreign bodies thus swallowed generally pass harmlessly through the bowels.
If the choking occur in the windpipe or trachea, it is usually dislodged by the paroxysm of coughing which accompanies the act. Should it fail to be so, and a sense of suffocation ensues, accompanied with blueness of countenance and difficulty of breathing, place the patient, and follow the directions given in the article “Suspended animation,” while a medical man is immediately sent for.
Treatment for Horses or Cattle.—Remove any foreign body by hand, as directed above, or have recourse to the probang. It may perhaps be necessary to call in a veterinary surgeon, in case the above methods fail, to extract the obstruction by cutting into the gullet.
CHOLAGOGUES. Medicines which promote a flow of bile.
CHOLALIC ACID. C24H40O5. Syn. Chol′ic acid. A non-nitrogenous acid existing in bile. It is best prepared by boiling the resinous mass precipitated by ether from an alcoholic solution of ox bile with a dilute solution of potassa, for 24 to 36 hours, till the amorphous potassa salt that has separated begins to crystallise. The dark-coloured soft mass is then removed from the alkaline liquid, dissolved in water, and hydrochloric acid460 added. A little ether will cause the deposition of the CHOLALIC ACID from this solution in crystals. With sulphuric acid and solution of sugar it strikes a purple-violet colour; this constitutes Pettenkofer’s test for bile.
CHOLE′IC ACID. Syn. Tauro-cholalic acid. A peculiar conjugated compound of cholalic acid with a substance called taurine, which contains both nitrogen and sulphur. In combination with soda, choleic acid constitutes a principal ingredient in bile.
CHOL′ERA. This word, which, from its derivation, can be only applied correctly to a bilious affection of the stomach and bowels, has been of late years very loosely extended to a malignant disease, the most marked characteristic of which is a total suspension of the functions of the biliary organs.
Cholera, En′′glish. Syn. Com′mon cholera, Bil′ious c.; Chol′era mor′bus, L. A disease characterised by bilious vomiting and purging, accompanied by more or less pain and debility. Diarrhœa is the most common precursor of the disease, and ought to be attended to without delay, particularly if the weather be warm. Cholera most frequently occurs towards the end of the summer and early in the autumn, when the increased heat of the sun stimulates the liver to an inordinate secretion of bile, by which the whole system becomes overloaded with it. Among secondary and accidental causes are sudden changes of temperature, checked perspiration, and the use of indigestible food, and food and beverages in a state of incipient decomposition. It is usually accompanied by fever, thirst, and severe colic, and sometimes by cold sweats, extreme debility, feeble pulse, &c., under which the patient sinks in 24 hours.
Treat. In most cases this complaint is not dangerous, and yields to proper treatment in a few days. As soon after the commencement of the attack as possible, some mild aperient should be administered. Opiates may be employed, both topically and by the mouth. Jeremie’s solution is stated to be very efficacious in the diarrhœa which so generally precedes cholera. A teaspoonful or two of laudanum, rubbed over the region of the stomach and bowels, is a simple application which will generally allay the pain. 10 to 20 drops of laudanum, mixed with a table-spoonful of good brandy, or a few grains of cayenne pepper, may also be taken every hour if the pain is severe. Should the stomach reject the medicine, or the vomiting be apparently increased by drinking warm diluents, a few spoonfuls of ice-cold water, or of a mixture of lemon-juice and water, may be taken instead, until the sickness abates. Dr Copeland recommends spirit of turpentine in violent attacks, both internally and as an external application in the form of warm epithems. When the violence of the symptoms has abated, tonics and bitters (as calumba, gentian, orange-peel, &c.) may be advantageously had recourse to. Calumba, in the form of a weak infusion, conjoined, if necessary, with aromatics, is, perhaps, the most valuable agent we possess for the after-treatment of the disease. See Diarrhœa.
Cholera, Malig′nant. Syn. Asiat′ic cholera, Epidem′ic c, Blue c, Pestilen′tial c, Spasmod′ic c.; Chol′era Asiat′ica, C. asphyx′ia, C. malig′na, L. This fearful disease first became known in this country in the autumn of 1831. The attack usually begins with sickness and purging; this discharge, however, is not bilious, as in ordinary cholera, but a thin, colourless fluid, like rice-water; at the same time there is great prostration of strength, and cold, clammy sweats. In a short time dreadful cramps assail the extremities and afterwards the abdomen; the body becomes bent, the limbs twisted, the countenance cadaverous, the pulse almost imperceptible, and the eyes sunken; the patient sinks into a state of apathy, and unless a favorable change speedily takes place, soon expires from exhaustion. When there is a reaction the pulse gradually returns, the natural warmth of the body is restored, and the spasms and difficulty of breathing give way. Frequently, however, the reaction is accompanied by fever closely resembling typhus, and which often terminates fatally in from four to eight days. The symptoms of epidemic cholera are not always of this terrible character.
Treat. In giving a few of the many remedies that have been recommended for this terrible disease, we may preface the list, by urgently counselling the sufferer to lose no time in sending for a medical man, in case of being attacked by this appalling malady.
1. (American Remedy.) Equal parts of maple sugar and powdered fresh-burnt charcoal, made into a stiff paste with lard, and divided into pieces the size of a filbert.—Dose. One, occasionally, swallowed whole.
2. (Austrian Specific.) The proportions of the ingredients in the following formulæ are founded on Mr Herapath’s analysis of this celebrated preparation, and are given in the nearest available whole numbers:—
a. Sulphuric acid (sp. gr. 1·845), 20 gr.; nitric acid (sp. gr. 1·500), 12 gr,; sugar and gum, of each 15 gr.; distilled or pure soft water, q. s. to make the whole weigh exactly 1 oz.
b. Sulphuric acid, 3 dr.; nitric acid, 2 dr.; simple syrup, 6 dr.; water, q. s. to make the whole weigh exactly 10 oz. A single drop of essential oil of lemon may be added.
Doses, &c. One table-spoonful is ordered to be taken in water, on the first appearance of premonitory symptoms, followed by the free use of very cold water. In half an hour a second dose is to be taken. This (as asserted) is generally sufficient to arrest the progress of the disease. A table-spoonful is then to be added to a pint of cold water, and drunk ad libitum. In more obstinate cases it is said that461 4 or 5 doses are generally required to effect a cure. When collapse sets in, double doses are ordered to be given, and to be repeated after every attack of vomiting until the sickness and cramp abate. After the vomiting abates the doses are still to be repeated until 5 or 6 doses are retained by the stomach. Should quiet sleep or drowsiness come on, it is not to be interfered with. The free use of cold water or soured water is to be allowed until perspiration sets in and the warmth of the body returns. According to the report, the use of warm liquors, wines, spirits, &c., must be carefully avoided as so much poison.
Obs. A bottle of the above remedy was handed to the late Mr Wm. Herapath by the superintendent of the Birmingham police, who had received it from the head of the Austrian police, as being in general use in Austria, under the sanction of the medical department of the government, and being found to act almost as a specific in cholera. In 1831-2 it was first tried on some criminals with perfect success, and soon afterwards with similar results on thousands of the general public. In 1849 the Austrian government ordered its use in the public establishments of the empire, since which not a single case of failure had occurred in which it had been fairly tried.
3. (Mr Buxton’s Remedy.) From dilute sulphuric acid (spirit of vitriol), 25 drops; water, 1 fl. oz. For a draught; as the last.
4. (College of Physicians and Board of Health; for Premonitory Diarrhœa.) Chalk mixture, 1 oz.; aromatic confection, 10 to 15 gr.; tincture of opium, 5 to 15 drops; to be repeated every 3 or 4 hours, or oftener, if required, until the looseness is arrested.
5. (Dr Graves’s Astringent Pills.) Acetate of lead, 20 gr.; opium, 1 gr.; conserve of roses, q. s.; for 12 pills.—Dose. One every 1⁄2 hour or hour, at first; then one every two hours.
6. (Homœopathic Preventive.) Camphor, 1 dr.; rectified spirit, 6 dr.; dissolve, and preserve it in a well-corked bottle.—Dose. 2 drops on a lump of sugar, sucked as a lozenge two or three times a day.
7. (Homœopathic Remedy.) As the last, repeating the dose every 10 or 15 minutes, followed by draughts of ice-cold water, until the symptoms abate.
8. (Mr Hope’s Remedy.) (a.) Red nitrous acid, 2 dr.; peppermint water or camphor julep, 1 oz.; tincture of opium, 40 drops; mix.—Dose. One to two teaspoonfuls in a cupful of thin gruel every 3 or 4 hours.
b. Spirit of wine, 1 oz.; spirit lavender, 1⁄4 oz.; oil of orizinanum, 1⁄4 oz.; compound tincture benzoin, 1⁄2 oz.; spirits camphor, 1⁄4 oz.—Dose, 20 drops on moist sugar. To be rubbed outwardly also.
9. (Liverpool Preventive Powders.) Bicarbonate of soda, 20 gr.; ginger, 10 gr.; for a dose. One to be taken in a glass of water after breakfast and supper daily.
These powders are said to have been used with good effect among the workmen in the mining and manufacturing districts during a former visitation of cholera.
10. (Police Remedy; Mr B. Child’s Remedy.) Rectified sulphuric ether and tincture of opium, of each 30 drops; for a dose for an adult; especially during the earlier stages.
11. (Mr Ross’s Astringent Pills.) Each pill contains 1 gr. of nitrate of silver, made up with crum of bread, q. s.—Dose. One pill, to be repeated after the interval of half an hour or an hour, should the symptoms continue unabated.
12. (Russian Remedy.) Sumbul, in the form of tincture, concentrated essence, in decoction, in cold infusion, and in powder in the form of pill.—Doses. Tincture, from 20 to 60 drops; essence, from 5 to 10 or 20 drops; in a little camphor julep or plain water. The physicians of Moscow and St. Petersburg ascribe to the virtues of this drug the saving of thousands of lives during the last epidemic. See Sumbul.
13. (Dr Stevens’ Saline Powders.) Bicarbonate of soda, 1⁄2 dr.; common salt, 20 gr.; chlorate of potassa, 7 gr.; for a dose.
14. (Sir M. Tierney’s Remedy.) Cajeput oil, in doses of 20 to 30 drops, every two or three hours. The oil excites the nervous system and equalises the circulation. The late Sir M. Tierney and others prescribed it frequently, it is said, with considerable success.
15. (Common Remedies of the Shops.) These generally consist of chalk mixture, with a little laudanum, and some aromatic or carminative, as cassia, cinnamon, cardamoms, nutmeg, or peppermint. In a few, some astringent, as tincture of catechu, or extract of logwood, is added.
16. (Dr Beaven’s Preventative and Remedy.) The Preventative.—Sulphite of magnesia, 2 dr.; sulphurous acid, 2 oz.; water, 2 oz.; tincture of capsicum, 1⁄2 oz. Mix and dissolve, a teaspoonful night and morning.
The Remedy.—Sulphite of magnesia, 2 dr., sulphurous acid, 2 oz.; water, 2 oz.; tincture of capsicum, 1⁄2 oz.; sulphate of morphia, 2 gr. Mix and dissolve; a teaspoonful every half hour until relieved.
CHOLERA MEDICINE. The expressed juice of dandelion and milfoil mixed with brandy spirit. (Dr Horn).
CHOLES′TERIN. C26CH44O.H2O. This substance is found in the bile, brain, nerves, blood, &c., and forms the principal ingredient of biliary calculi (gall-stones).
Note.—The remedies containing astringents are the most efficacious.
CHOL′IC ACID. Syn. Glyco-cholal′ic acid. A peculiar acid, existing as cholate of sodium, and associated with choleic acid in the bile. It is a conjugate compound of cholalic acid with a nitrogenised substance called glycocin.
CHON′DRIN. Gelatin obtained from cartilage. It differs from ordinary gelatin in462 being precipitable by acetic acid, alum, and acetate of lead.
CHOREA. [Syn. St. Vitus’s Dance.] A spasmodic disease affecting children and young persons, especially girls, between eight and sixteen years of age. It is caused by a debilitated condition of the nervous system, as well as by brain disease, scrofula, imprudent diet and worms.
The treatment recommended is the regulation of the bowels by mild purgatives. If the disease can be traced to worms, these should be removed by the proper remedies. If worms are not the cause, recourse should be had to the cold, or shower-bath. The hot hip-bath will be found serviceable in some cases. Where there is paleness of the skin any of the iron preparations will prove of great use, the bowels being kept regular. The best preparations of iron are either the tincture of the perchloride, or nitrate, or the citrate of iron and quinine. Some practitioners recommend arsenic—five drops of the solution (for an adult) twice a day after meals; others valerianate of zinc.
Treatment for the Horse and other Animals.—Similar to the above.
CHRISTOFIA is a stomachic brandy or wine made of 1500 parts white wine, 20 parts cinnamon, 10 parts cloves, 60 parts bitter almonds, digested several days; 300 parts of sugar and 500 of spirit are then added, and the whole filtered. (Hager).
CHROMACOME. For dyeing the hair black. This is said to be prepared from harmless vegetable materials, but really consists of pyrogallic acid and nitrate of silver.
Chromacome. This is a French preparation which “contains nothing injurious to health.” This hair dye consists of two fluids. The first, “Le chrômacome, teinture supérieure de William W. A. T., No. 1, Bonn,” weighing about 45 grammes, is tincture of galls. The other, No. 2, is a solution of acetate of iron with a little nitrate of silver. When grey hair is moistened first with No. 1, then with No. 2, it becomes blackish-brown or black.
CHRO′MATE. Syn. Chro′mas, L. A salt in which the hydrogen of (hypothetical) chromic acid, HCrO4, is replaced by a metal or other basic radical.
Chromates:—
Prep. The insoluble chromates, as those of barium, zinc, lead, mercury, silver, &c., may be made by mixing a soluble salt of those bases with neutral chromate of potassium. The first three are yellow; the fourth brick-red; and the fifth reddish-brown, or ruby red when crystallised. The soluble chromates may all be made by direct solution of the base in the acid, or by double decomposition. The chromates of commerce are prepared from either chrome ore or chromate of potassium.
Prop., Uses &c. The chromates are characterised by their yellow or red colour, the latter predominating when the acid is in excess; and except those with the alkaline bases, they are, for the most part, insoluble in water. Both the chromate and the bichromate of potassium are extensively used in dyeing and calico-printing. The former is employed in conjunction with sulphuric acid in the laboratory as an oxidising agent and in the manufactory for bleaching sperm oil. The bichromate of ammonium and potassium are used in photography.
They are readily recognised by the following tests:—
On boiling a chromate in hydrochloric acid mixed with alcohol, chromic acid is first set free, and then decomposed, forming a green solution of chloride of chromium. Sulphuretted hydrogen and sulphurous acid effect similar changes. With acetate of lead the chromates give a yellow precipitate; with nitrate of silver, a reddish-brown; with nitrate of mercury, a red one.
CHROME ALUM. See Alums.
CHROME GREEN. See Green Pigments.
CHROME IRON. See Iron.
CHROME RED. See Red Pigments.
CHROME YELLOW. See Lead, Chromate of.
CHROMIC ACID. See Chromic Anhydride.
CHRO′MIUM. Cr. A metal discovered in native chromate of lead by Vauquelin in 1797. It is found in the state of oxide, combined with oxide of iron, in some abundance, in the Shetland Islands, and elsewhere; as chromate of lead it constitutes a very beautiful material.
Prepared in an impure condition as a white, very infusible, hard metal, by igniting the oxide with charcoal, at a white heat, in a lime crucible.
Chromous Chloride. CrCl2. Syn. Protochloride. Prep. Ignite the chromic chloride in a current of dry hydrogen. A white, foliated mass, soluble in water (evolving much heat), and yielding a blue solution, which absorbs atmospheric oxygen with astonishing rapidity, acquiring a deep-green colour, and passing into the state of oxychloride of chromium. It is the most powerful reducing or deoxidising agent known.
Chromic Chloride. Cr2Cl6. Syn. Sesquichloride. Prep. Pass dry chlorine over a mixture of sesquioxide of chromium and charcoal, heated to redness, in a porcelain tube. The chloride collects as a sublimate, of a peach or violet colour, in the cool part of the tube.
Dissolve chromic oxide in hydrochloric acid and evaporate to dryness; the residue is chromic chloride. It forms a dark green mass, containing water, which is evolved by igniting at a temperature of 400°, turning a purplish red.
Chromium Oxides:—
Chromous Oxide. CrO. Syn. Protoxide of chromium. This oxide has not yet been obtained in a satisfactory manner, but the hydrate is prepared by the addition of potassium463 hydrate solution to a solution of chromous chloride or sulphate. A brownish-red powder, speedily passing to a deep foxy-red, with disengagement of hydrogen, and forming pale blue-coloured salts with the acids, which absorb oxygen with avidity, whilst the metal passes into a higher state of oxidation.
Chromic Oxide. CrO3. Syn. Sesquioxide. Prepared by igniting potassium bichromate at a red heat and well washing the residue, and as hydrate by cautiously adding equal parts of hydrochloric acid and alcohol or sugar to a boiling solution of chromate of potassa in water, in small portions at a time, until the red tint disappears, and the liquid assumes a green colour; pure ammonia, in excess, is next added, and the precipitate which subsides is collected and washed with water.
Prop., &c. The anhydrous oxide is a rich crystalline, green powder, insoluble in both water and acids; fused with borax and glass, it imparts a beautiful green colour.
The hydrate is soluble in the acids and in alkaline lyes; with the first it forms salts which have a green or purple colour. These compounds may be made by direct solution of the hydrate in the dilute acids. Chromic sulphate combines with the sulphates of potassium and ammonium, giving rise to salts (CHROME ALUMS) which crystallise in magnificent octahedrons of a deep claret colour. The finest crystals are obtained by spontaneous evaporation.
These salts of chromium are the most important, the chromous salts being seldom met with, and are best recognised by the following reactions:—Caustic alkalies precipitate the hydrate, easily soluble in excess of the precipitant. Ammonia the same, but the precipitate is nearly insoluble. The carbonates of potassium, sodium, and ammonium throw down a green precipitate of carbonate and hydrate, slightly soluble in a large excess. Sulphuretted hydrogen causes no change.—Sulphydrate of ammonium precipitates the hydrate of a bluish-green colour.
Chromic Anhydride. CrO3. Syn. Chromic acid, Anhydrous chromic acid, Chromic trioxide. Prep. By conducting gaseous fluoride of chromium into a silver or platinum vessel, the sides of which are just moistened with water, and the aperture covered with a piece of moist paper, the anhydride will be deposited under the form of red, acicular crystals, which will nearly fill the vessel. When the process is skilfully conducted, the product is of exquisite beauty and chemically pure. The fluoride referred to above is obtained from fluor spar, 3 parts; chromate of lead, 4 parts; fuming (or the strongest) sulphuric acid, 5 parts; mixed cautiously in a silver or leaden retort. A red-coloured gas is evolved, which acts rapidly on glass, forming fluosilicic acid gas, and upon water forming hydrofluoric acid and chromic anhydride. The moisture of the atmosphere is sufficient to effect the decomposition last referred to; the former substance escaping as gas, and the latter being deposited in small crystals.
It is also prepared nearly pure by adding a cold saturated solution of potassium bichromate to once and a half its bulk of pure strong sulphuric acid. As the liquor cools, the anhydrous chromic acid is deposited under the form of brilliant crimson-red prisms; the mother-liquor is then poured off, and the crystals, placed between two tiles of glass or porcelain, are submitted to strong pressure for some time, under a bell-glass or jar, when the anhydride will be found sufficiently dry. It may be deprived of a little adhering moisture by placing it over sulphuric acid for a short time in vacuo.
Commercially, it is prepared by one of the two following processes:—
To a saturated solution of chromate of potassium, 100 parts, add oil of vitriol (sp. gr. 1·845), 49 parts; and let the whole cool. This is the common process. The product contains sulphate of potassium, but this does not much interfere with its value as a bleaching agent.
From chromate of barium, decomposed by concentrated nitric acid. The anhydrous chromic acid is separated from the nitrate of barium by decantation, or, which is still better, by filtration through glass or asbestos. It is then evaporated to dryness, when the nitric acid is volatilised, and pure chromic anhydride left behind. The volatilised nitric acid may be condensed, and again used for the same purpose. The only precautions necessary to ensure the purity of the anhydrous chromic acid prepared by this plan are—to use a sufficient quantity of nitric acid and to take care that the nitric acid is sufficiently concentrated and pure.
Prop., &c. Forms ruby-red anhydrous prisms, very soluble in water, with formation of true chromic acid, and extensively manufactured for the purpose of oxidising and bleaching substances.
CHROME IRON-STONE. Syn. Chrome Iron-ore. FeO.Cr2O3. This, which is the principal ore of chromium, corresponds in composition to brown oxide of chromium and to the magnetic oxide of iron; part of the iron, however, is generally displaced by the isomorphous metal magnesium, and part of the chromium by aluminium.
Chrome iron-stone is often met with in the form of octohedral crystals. Acids fail to dissolve it, and it cannot be fused in the furnace, but when heated it absorbs oxygen from the air. This oxidation may be effected very readily if the chrome ore reduced to very fine powder be mixed with a carbonate of one of the metals of the alkalies or alkaline earths, a chromate of the base being formed.
CHRYSENE. C18H12. A hydrocarbon found by Laurent in crude anthracene. It occurs in bright yellow, glistening scales. It464 may be obtained colourless by heating the yellow crystals with hydriodic acid and amorphous phosphorus to 240°. It cannot be sublimed without decomposition. Chrysene is very slightly soluble in cold alcohol, ether, benzene, and glacial acetic acid. In carbon disulphide it dissolves somewhat more readily. Its melting point is from 248° to 250°, and it boils at a temperature beyond that which can be registered by the mercurial thermometer.
CHRYSOPHANIC ACID. See Rhein.
CHYLE. Syn. Lymph. This is the name given to the nutritious milky fluid generated during digestion, and absorbed from the intestines by a set of vessels called the lacteals, which carry it to the thoracic duct, whence it is immediately conveyed into the circulation.
CHYME. The pulpy mass formed by the food in its first great change, in the process of digestion.
CIDER. Syn. Cyder; Pomaceum, L. Cider is the fermented juice of the apple, and is a very ancient beverage. Pliny calls cider and perry the “wine of apples and pears.”
The attention of the cider farmer should be first directed to the culture of the apple tree. The situation most appropriate for an orchard is one on rising ground, rather dry than moist, and unexposed to sea air or high winds. The soil should be strong, but not too heavy, and should be rich in the alkaline and earthy bases, especially the phosphates. The selection of the proper varieties of the apple for grafting is also a point on which particular care should be taken. It is found that the juices of different kinds of apples vary in the quantity of saccharine matter which they contain, as well as in other particulars that influence the quality and flavour of the cider prepared from them. As a general rule, those varieties should be chosen that yield a juice rich in sugar, and contain no undue amount of acid, and which, after the period of active fermentation is past, furnishes a liquor which clarifies itself and keeps well. This quality of the juice may generally be determined from its specific gravity. The heaviest and clearest is the best, other points being equal. The specific gravity of the juice of the different varieties of apple varies from 1·060 to 1·100.
Cider apples are classed under three heads—bitter, sweet, and sour. The first are the best; their juice has the greatest specific gravity, is the richest in sugar, ferments the most freely, clarifies spontaneously the quickest, and keeps the best after fermentation. They contain a minute quantity of extractive matter which is not present in other apples. The juice of sweet apples ferments tumultuously, clears with difficulty, and the resulting cider does not keep so well as that produced from the first variety. The juice of sour apples contains less sugar and more acid than the other two, and consequently not only produces the weakest, but the worst cider; it, however, “fines” well, although it “stores” badly. Sour and “rough-tasted” apples are usually preferred by farmers for making cider. This preference, which is very decided in the West of England, may be readily accounted for. The sour and rough-tasted apples contain less sugar and more malic acid than some of the other varieties, and the presence of this acid impedes the conversion of the alcohol of the cider into vinegar; a change which their rude mode of operating renders otherwise inevitable. But cider made with such apples never equals in quality that prepared at a low temperature, from fruit abounding in sugar, provided equal skill is exercised in the manufacture as in the process of converting malt-worts into beer.
The process of making cider varies in different places, but in every case essentially consists of the collection of the fruit, the expression and fermentation of the juice, and the storing and management of the fermented liquor.
The collection of the fruit should not be commenced before it has become sufficiently mature, and should be performed with greater care than is commonly bestowed upon it. The apples, after being gathered, are usually left for 14 or 15 days in a barn or loft to mellow, during which time a considerable portion of the mucilage is decomposed, and alcohol and carbonic acid developed. If this “ripening” is allowed to go too far, loss arises, notwithstanding the vulgar prejudice in its favour. The spoiled apples are then separated from the sound ones, as they not only impart a bad flavour to the cider, but impede its spontaneous clarification.
The expression of the juice is the next step in the process of cider-making. The apples are crushed or ground in mills consisting of two fluted cylinders of hard wood or cast-iron, working against each other. The common practice is next to sprinkle the pulp with 1⁄6th to 1⁄4th of its weight of spring or river water, and then to allow it to remain in tubs or wooden cisterns for 12 or 14 hours, during which time incipient fermentation commences, and the breaking up of the cells of the membrane takes place, by which the subsequent separation of the juice is facilitated. This plan, though general among cider manufacturers, is prejudicial to the quality of the future liquor; as not only is a portion of the newly formed alcohol lost, but the skins and pips often impart to it a disagreeable flavour. By employing more efficient crushing machinery this system of vatting is rendered quite unnecessary. A machine furnished with a revolving circular rasp, similar to that used in making potato starch, is admirably adapted to this purpose.
The pulp of the crushed or ground apples is now placed on a kind of wicker frame, or in hair-cloth or coarse canvas bags, and after being allowed to drain into suitable tubs or receivers, is subjected to powerful pressure, gradually applied, in the cider press. The465 liquor which runs off first is the best, and is usually kept separately; whilst that which follows, especially the portion obtained by much pressure, tastes of the pips and skins.
The expressed juice or “must,” obtained as above, is next put into clean casks with large bung-holes, and freely exposed to the air and the shade, where they are placed on “stillions,” with flat tubs under them to catch the waste. They are now constantly attended to and kept quite full, in order that the yeast, as it forms, may froth over and be carried off from the surface of the liquor. After 2 or 3 days for weak cider, and 8 or 10 days for strong cider, or as soon as the sediment has subsided, the liquor is “racked off” into clean casks, which have been (according to the common practice) previously sulphured with a cooper’s match. The casks containing the “racked cider” are then stored in a cellar, shaded barn, or other cool place, where a low and regular temperature can be ensured, and are left to mature or ripen. By the following spring the cider is commonly fit for use, and may be “re-racked” for sale.
The marc, or pressed pulp, is generally again sprinkled with 1⁄3 or 1⁄2 its weight of water, and re-pressed. The resulting liquor, when fermented, forms a weak kind of cider (cider moil, water moil), which is reserved for domestic use in the same way as table-beer. The refuse-pulp (apple-marc, pomace, pommage, apple cheese) is used as food for pigs and store cattle, and is very acceptable to them.
The storing and management of cider are matters of vast importance to the cider farmer, the factor, the wholesale dealer, and the bottler. The principles by which these should be directed are precisely similar to those which are explained under the heads Brewing, Fermentation, and Malt Liquors; and which, indeed, refer, with slight modifications, to all fermented liquors.
Preparatory to bottling cider it should be examined, to see whether it is clear and sparkling. If not so, it should be clarified in a similar way to beer, and left for a fortnight. The night before it is intended to put it into bottles the bung should be taken out of the cask, and left so until the next day, and the filled bottles should not be corked down until the day after; as, if this is done at once, many of the bottles will burst by keeping. The best corks should alone be used. Champagne bottles are the variety generally chosen for cider. It is usual to wire down the corks, and to cover them with tinfoil, after the manner of champagne. A few bottles at a time may be kept in a warm place to ripen. When the cider is wanted for immediate use, or for consumption during the cooler portion of the year, a small piece of lump sugar may be put into each bottle before corking it; or, what is the same thing in effect, the bottles may be corked within 2 or 3 hours after being filled. In summer, and for long keeping, this practice is, however, inadmissible. The bottled stock should be stored in a cool cellar, when the quality will be greatly improved by age. Cider for bottling should be of good quality, sound and piquant, and at least a twelvemonth old. When out of condition it is unfit for bottling.
Qual., &c. Cider, when of good quality, and in good condition, is doubtless a very wholesome liquor. Cider consumers, living in the cider districts, appear to enjoy almost an immunity from cholera, and often from other diseases which are common in other parts of the kingdom. At the same time, however, it is right to mention, that the dry colic or belly-ache (colica pictonum) is far from uncommon in these districts, but is wholly confined to those who drink early, hard, or inferior cider, made from harsh, unripe fruit. We believe that, in most cases, it may be referred to the acid of the common cider having acted on the lead, pewter, or copper of the articles or utensils with which it has come in contact, and of which it has dissolved a very minute portion. The best cider contains from 8% to 10% of absolute alcohol; ordinary cider from 4% to 6%.
Concluding Remarks. Much of the excellence of cider depends upon the temperature at which the fermentation is conducted; a point utterly overlooked by the manufacturers of this liquor. Instead of the apple-juice, as soon as it is expressed from the fruit, being placed in a cool situation, where the temperature should not exceed 50° or 52° Fahr., it is frequently left exposed to the full heat of autumn. In this way much of the alcohol formed by the decomposition of the sugar is converted into vinegar by the absorption of atmospheric oxygen, and thus the liquor acquires that peculiar and unwholesome acidity known in the cider districts by the name of “roughness.” When, on the contrary, the fermentation is conducted at a low temperature, nearly the whole of the sugar is converted into alcohol, and this remains in the liquor, instead of undergoing the process of acetification. The acetous fermentation, by which alcohol is converted into vinegar, proceeds most rapidly at a temperature of about 90° Fahr., and at lower temperatures the action becomes gradually slower, until at 46° to 50° Fahr. no such change takes place. (Liebig.) It is therefore evident that if the saccharine juice of apples, or any other fruit, is made to undergo the vinous fermentation in a cool situation, less of the spirit resulting from the transformation of the sugar will be converted into acetic acid, and, consequently, more will be retained in an unaltered state in the liquor, to improve its quality, and by its conservative and chemical action to preserve it from future change. This is the principal cause, other circumstances being alike, of the difference in the quality of the cider made by466 persons living in the same district. The one has probably a cooler barn and cellar than the other to store his liquor in, and is more careful to keep the pulp and juice cool during the early part of the process. In Devonshire the pressing and fermentation are conducted in situations where the temperature varies little from that of the external air, and fluctuates with all its changes; the result is that Devonshire cider, of the best class, will rarely keep more than 4 or 5 years, and seldom improves after the second or third year; whilst the cider of Herefordshire and Worcestershire, where these operations are more carefully attended to, will keep for 20 or 30 years.
When the pressing the apples for the juice is deferred until late in the season, it sometimes happens that the fermentation is sluggish. Though the juice has been set on the old system, in November or December, the working hardly commences until March. At this time the cider is sweet; it now rapidly becomes pungent and vinous, and is soon ready to be racked for use. If the fermentation still continues, it is again racked into a clean cask that has been sulphured; or two or three cans of the cider are put into a cask, and a brimstone-match burned in it. The cask is then agitated, after which it is nearly filled with the cider. By this process the fermentation is checked, and the cider in a short time becomes fine. Great care must be taken that the sulphuring be not overdone, as it is apt to impart a slightly unpleasant flavour to the liquor. If, on the first operation, the fermentation is not checked, the process of ‘racking’ is repeated, until the liquor becomes clear, and is continued from time to time, till the cider is in a quiet state and fit for drinking.
A common practice in Devonshire is to add a stuff called ‘stum,’ sold by the wine-coopers, or an article called ‘antiferment,’ sold by the druggists, for the purpose of checking the fermentation, but a much better plan is that described above.
To improve the flavour of weak cider, or to render ordinary cider more vinous, various plans are followed by the cellarmen and bottlers. An excellent one is to add to each hogshead 11⁄2 gall. of good brandy or rum, with 2 oz. of powdered catechu (dissolved in water), 10 lbs. of good moist sugar or honey, 1⁄2 oz. each of bitter almonds and cloves, and 4 oz. of mustard seed (all in powder). These must be well ‘rummaged’ into the liquor, and the whole occasionally stirred up for a fortnight, after which it must be allowed to repose for 3 or 4 months, when it will usually be found perfectly ‘bright,’ and no bad substitute for foreign wine. Should this not be the case, the liquor must be ‘fined’ with a pint of isinglass finings, or a dozen eggs, and allowed to rest for a fortnight. If the cider is preferred pale, the catechu must be omitted, and instead of isinglass, a quart of skimmed milk is to be used as ‘finings.’ When desired of a pinkish tint, 1 oz. of cochineal (in powder) may be added instead of the catechu.
About 13 cwt. of November apples commonly yield one hogshead of cider. In Devonshire about 6 sacks or 24 bushels are the common quantity for the hogshead of 63 galls.
The best cider made at the present day is that of Normandy, Herefordshire, and New Jersey (U.S.), and next that of Devonshire and Somersetshire. See Antiferment, Fermentation, &c.
Cider, Champagne. This name is given in the United States of America to a fine, pale variety of cider, much used for bottling, which has a great resemblance to inferior champagne. The best variety comes from New Jersey. The name is also applied in this country in a similar manner. The following is a good form for a ‘made’ cider of this class:—
Prep. Good pale vinous cider, 1 hhd.; proof spirit (pale), 3 galls.; honey or sugar, 14 lbs.; mix well, and let them remain together in a temperate situation for 1 month; then add orange-flower water, 3 pints; and in a few days fine it down with skimmed milk, 1⁄2 gall. A similar article, bottled in champagne bottles, silvered, and labelled, is often sold to the ignorant for champagne.
Cider, Made. An article under this name is made in Devonshire, chiefly for the supply of the London market, it having been found that the ordinary cider will not stand a voyage to the metropolis without some preparation. The finest quality of ‘made’ cider is simply ordinary cider racked into clean and well-sulphured casks; but the mass of that which is sent to London is mixed with water, treacle, and alum. The cider sold in London under the name of Devonshire cider would be rejected even by the farmers’ servants in that county.
Cider, Raisin. This is made in a similar way to raisin wine, but without employing sugar, and with only 2 lbs. of raisins to the gall., or even more, of water. It is usually fit for bottling in 10 days, and in a week longer is ready for use.
CIDER SPIRIT. See Brandy.
CIGAR. Syn. Segar; Cigarre, Fr.; Cigarro, Span. A small roll of tobacco-leaf used for smoking. The leaf is stalked or stripped of its midrib, and damped before it passes into the hands of the cigar-roller. The envelope or skin is cut from a smooth, unbroken leaf, and is quickly rolled round sufficient tobacco to form the inside. To secure the loose end of the envelope a small quantity of paste, coloured brown with chicory, is generally used. Only those who have had great practice can make cigars of a good shape. A full account of the manufacture of cigars does not come within the scope of this work. Although cigars of British make cannot compete in point of flavour with those manufactured in tobacco-growing countries, they467 have obtained a high degree of favour from the excellent manner in which they are made, and from their comparative cheapness. For information respecting the adulteration of cigars, and the influence of their use upon health, see Tobacco.
CIGARS. (In pharmacy.) Syn. Med′icated cigars, M. cigarettes′. The administration of medicinal agents in the form of cigars is of recent introduction, and as yet in only very limited use. The medicinal substance, if of a suitable description, as the leaves of plants, is made up into small rolls, like cheroots, and then smoked in the usual manner. In some cases, common cigars, or paper cigars (cigarettes), are medicated by moistening them in a preparation of the article to be administered. When the narcotic property of the tobacco would prove injurious, it is first exhausted by soaking and washing it in water.
Cigars, Aromatic. Syn. Aromatic cigarettes; Cigarettæ aromat′icæ, L.; Cigarettes aromatiques, Fr. Aromatic spices, lavender flowers, &c., made into cigarettes. Smoked for their odour; and in tooth-ache, face-ache, &c. See Cigars, Scented.
Cigars, Arsenical. Syn. Cigarr′æ arsenicales, L. Prep. Dissolve arseniate of soda, 1 part, in water, 30 parts; dip white, unsized paper into the solution, and form it into small rolls, 3 or 4 inches long. Used in pulmonary consumption; 4 or 5 whiffs as many times a day.
Cigars, Balsamic. Syn. Balsamic cigarettes; Cigarræ balsamicæ, Cigarettæ b., L. Thick, unsized paper is soaked in a solution of saltpetre and dried; after which it is brushed over first with tincture of cascarilla, and when again nearly dry, with compound tincture of benzoin; in about half an hour it is cut into pieces (11⁄2 × 4 inches), and rolled into cigarettes. Used in hoarseness, loss of voice, asthma, &c.
Cigars, Belladonna. Syn. Belladonna cigarettes; Cigarettæ belladonnæ, L. Prep. 1. Belladonna leaves made into cigarettes of 1 dr. each.
2. (Compound—C. b. compos′itum.) From belladonna leaves, 4 parts; moistened with tincture of opium (Ph. L.), 1 part; dried and made into 1 dr. cigarettes, as before.
Used as an anodyne and antispasmodic, in troublesome coughs, hooping-cough, toothache, sore throat, tic douloureux, &c.
Cigars, Camphor (Raspail, Paris). A remedy for various chest diseases, such as catarrh, hoarseness, loss of voice, coughs, spasms, hooping-cough, phthisis; also, if the saliva be swallowed, for heartburn, pains in the stomach, and gastritis. They consist either of a straw or quill filled with broken camphor, or of a bone or horn mouthpiece, furnished at the outer end with a little capsule for the camphor. (Wittstein.)
Cigars, Cam′phor. Syn. Camphor cigarettes; Cigarett′æ campho′ræ, L.; Cigarettes de camphre, Fr. Prep. 1. Bibulous paper, moistened with 2 or 3 drops of essence of camphor, and rolled into cigarettes. For use they are loosely placed in a tubular cigar-holder.
2. (Raspail.) These are made by loosely filling a quill or large straw with small fragments of camphor, closing the open end with a little cotton wool or bibulous paper, and piercing the closed end with a pin, to allow the passage of air.
Obs. Both the above are used unlighted by drawing the air through them into the mouth, which then becomes very slightly charged with the vapour of camphor. In cold weather the vaporisation is promoted by holding the cigarette for a few minutes in the warm hand. The homœopathists regard them as prophylactic of cholera, and the common people hold them to possess the same virtue in reference to contagious diseases generally, but especially typhus and scarlet fever. They should not be employed oftener than 3 or 4 times a day.
Cigars, Hen′bane. Syn. Cigarr′æ hyoscy′ami, L. From henbane leaves, as directed under Belladonna Cigars.
Cigars, Indian Hemp. The plant is made into cigarettes, which are used in asthma. They must be used with caution.
Cigars, Mercu′′rial. Syn. Cigarr′æ mercuria′les, L. Prep. (Paul Bernard.) Ordinary cigars are deprived of their narcotic properties by soaking them in water, and are then wetted with a weak solution of corrosive sublimate, to which a little opium is generally added. The proportion may be, of corrosive sublimate, 1 gr.; rectified spirit, 20 drops; dissolve; add laudanum, 15 drops; with this solution 6 cigars are to be equally moistened to within about 11⁄2 inch of the mouth end, and then set aside to dry.
Used by persons afflicted with syphilitic affections of the throat and palate, as a convenient method of mercurial fumigation. For those accustomed to the use of tobacco, mild cigars, undeprived of their nicotine, may be employed for the purpose.
Cigars, Scent′ed. Syn. Perfu′′med cigars; Cigarr′æ aromat′icæ, L. Prep. 1. By moistening ordinary cigars with a strong tincture of cascarilla, to which a little gum benzoin and storax may be added. Some persons add a small quantity of camphor, or of oil of cloves or cassia.
2. By soaking the tobacco, of which the cigars are to be made, or the cigars themselves, for a short time in a very strong infusion of cascarilla, and then allowing them to dry by a very gentle heat.
3. By simply inserting very small shreds of cascarilla bark between the leaves of the cigar or in small slits made for the purpose.
Obs. The above yield a very agreeable odour when smoked; but are said to intoxicate468 quicker than unprepared cigars of equal strength and quality. They lose much of their fragrance by age.
Cigars, Stramo′′nium. Syn. Datu′ra cigars; Cigarræ stramo′′nii, L. From the leaves of Datura stramonium, or preferably those of the eastern species, Datura tatula. See Asthma, Datura.
CINCHONA BARKS. Syn. Cinchonæ cortex; Peruvian bark; Jesuit’s bark. The native names are quinquino and quina, quina. Of the nearly forty different known species of cinchona trees, the barks of about a third are employed, some either directly in medicine, but by far the larger number as sources of quinine and the other cinchona alkaloids. The original habitat of the genus Cinchona is the Andes, where it is found at a height of between 3000 and 12,000 feet above the sea, growing mostly in patches, distributed amongst the palms, plantains, and other tropical trees that form the vast forests, for the most part clothing the eastern slopes of the Cordilleras, and extending from 10° north to about 19° south latitude. In this district there is always an abundance of moisture and a mean temperature of about 62°. In 1853 the Dutch government introduced the cinchona into Java, and in 1861 the East Indian government, following their example, introduced it into British India, where it is now acclimatised, large plantations of it growing on the Neilgherries and in the valleys of the Himalayas. The cinchona is now also successfully cultivated in Ceylon and Jamaica.
The method followed in the collection of the bark by the Peruvians is a very wasteful and destructive one, and consists either in stripping the bark from the trees when they have attained a sufficient age, or in felling the tree a little above the roots. If the latter method be adopted, the roots give out a growth of suckers, which yield a good bark. The bark is never removed during the rainy season.
Previous to being stripped off, the bark is sometimes cleaned with a brush, and then peeled off in pieces varying from 15 to 18 inches long, and from 4 or 5 in width. The thinnest pieces, which are derived from the branches or the trunks of small trees, are dried in the sun, and thus acquire the well-known quill-like form. The larger trunks yield the flat specimens, which are submitted to a kind of pressure as they are being dried. The inferior specimens being rejected, the dried barks (mostly of the same kind) are sewed in canvas, and thus conveyed to the nearest depôt, from whence, previous to being shipped, they are enclosed in another envelope of fresh hide, the package being then known under the name of a seron.
Structure of Cinchona Barks. A few general observations on the structure of the bark of cinchona will be appropriate here. The epidermis is only found on the youngest bark, before it has attained sufficient age for medicinal use; it is then replaced by the corky layer. In most species this cracks, and is easily separable, but in some it is firmly attached to the internal layers. These are composed of the middle layer of the bark or mesophlæum, formed of parenchyma, and the innermost layer endophlæum, or liber. The middle layer disappears in some barks, which are thus wholly composed of liber. This is a means of distinguishing them. The liber is traversed by medullary rays, which project into the mesophlæum. It is, therefore, composed of woody fibres (prosenchyma) and soft parenchyma.
The arrangement of the woody fibres, their colour, size, and shape, give a special character to the cinchona barks.
As compared with other barks, the fibres of the liber are shorter and more loosely arranged, being for the most part separate or united into very short bundles. The fibres, therefore, are easily isolated; they are spindle-shaped, sub-quadrangular, rarely exceeding 1-10th of an inch in length, usually straight, and are very brittle, the cavity of the cell of which each is composed being reduced by secondary deposits to a fine canaliculus. This short and loose fibrous structure is not found in other barks.
In some cinchona bark a system of lactiferous vessels is found between the liber and mesophlæum.[247]
[247] Royle.
The parenchyma of the barks abounds in starch and oxalate of lime, or else contains a soft brown deposit.
The ‘British Pharmacopœia’ divides the cinchona barks into the three classes of—
1. Yellow Cinchona Bark. Syn. Cinchonæ flavæ cortex. The Cinchona Calisaya of Weddell.
2. Pale Cinchona Bark. Syn. Cinchonæ pallidæ cortex. The bark of Cinchona officinalis; var. Condaminea of Hooker. This bark is also known under the name of Crown-bark, from its having formerly been used by the royal family of Spain.
3. Red Cinchona Bark. Syn. Cinchonæ rubræ cortex. The Cinchona succirubra of Pavon.
The therapeutic properties of the cinchona barks are due to the following alkaloids:—
Quinia, or quinine, having the composition C20H24N2O2.
Quinidia, or quinidine, having the composition C20H24N2O2.
Cinchonia, or cinchonine, having the composition C20H24N2O.
Cinchonidia, or cinchonidine, having the composition C20H24N2O.
Quinamina, or quinamine, having the composition C20H24N2O2.
Besides the above, an alkaloid, which has been named Paracina, has been obtained from the bark of the Cinchona succirubra; whilst in those barks which contain only small portions of the more active constituents above named there have been found two alkaloids, named respectively Aricia and Cusconia, which have lately been accurately investigated by Hesse, who has determined their chemical constitution (Liebig’s ‘Annalen und Berichte der Chemische Gesselschaft in Berlin’).
469
The following Prospectus of the principal Species of Cinchona is from Flückiger and Hanbury’s ‘Pharmacographia,’
| Species (excluding Sub-species and Varieties) according to Weddell. | Where figured. | Native Country. | Where cultivated. | Product - Cinchona Barks. |
| I. Stirps Cinchonæ officinalis— | ||||
| 1. Cinchona officinalis, Hook. | ‘Bot. Mag.,’ 5364 | Ecuador Loxe, Peru | India, Ceylon, Java | Loxa, or Crown Bark, Pale Bark. |
| 2. Cinchona macrocalyx, Pav. | Howard, ‘N. Q.’ | Peru | — | Ashy Crown Bark. The sub-speciesC. Palton affords an important sort called Palton Bark, much used in the manufacture of quinine. |
| 3. Cinchona lucumæfolia, Pav. | Howard, ‘N. Q.’ | Ecuador, Peru | — | Carthagena Bark, confounded with Palton Bark, but is not so good. |
| 4. Cinchona lanciolata, R. and P. | Howard, ‘N. Q.’ | Peru | — | Columbian Bark. Imported in immense quantities for manufacture of quinine. The soft Columbian Bark is produced by Howard’s var. oblonga. |
| 5. Cinchona lancifolia, Mutis. | Karst., tab. 11, 12 | New Granada | India | Ditto. |
| 6. Cinchona amygdalifolia, Wedd. | Wedd., tab. 6 | Peru, Bolivia | — | A poor bark, not now imported. |
| II. Stirps Cinchonæ Rugosæ— | ||||
| 7. Cinchona Pityrensis, Wedd. | Karst., tab. 22 (G). Triane | New Grenada, Popayan | India | Pitayo Bark. Very valuable; used by makers of quinine. It is the chief source of quinidine. |
| 8. Cinchona rugosa, Pav. | Howard, ‘N. Q.’ | Peru | — | Bark unknown, probably valueless. |
| 9. Cinchona Mutisii, Lamb. | Howard, ‘N. Q.’ | Ecuador | — | Bark, not in commerce, contains only aricine. |
| 10. Cinchona hirsuta, R. and P. | Wedd., tab. 21 | Peru | — | |
| 11. Cinchona Carabayensis, Wedd. | Wedd., tab. 19 | Peru, Bolivia | — | Bark, not collected. |
| 12. Cinchona panudiana, How. | Howard, ‘N. Q.’ | Peru | India, Java | A poor bark, yet of handsome appearance; propagation of tree discontinued. |
| 13. Cinchona asperfolia, Wedd. | Wedd., tab. 20 | Bolivia | — | Bark not collected. |
| 14. Cinchona umbelluliferæ, Pav. | Howard, ‘N. Q.’ | Peru | — | Bark not known as a distinct sort. |
| 15. Cinchona glandulifera, R. and P. | Howard, ‘N. Q.’ | Peru | — | Bark not known as a distinct sort. |
| 16. Cinchona Humboldtiana, Lamb. | Howard, ‘N. Q.’ | Peru | — | False Loxa Bark, Jaen Bark. A very bad bark. |
| III. Stirps Cinchonæ Micranthæ— | ||||
| 17. Cinchona Australis, Wedd. | Wedd., tab. 8 | South Bolivia | — | An inferior bark, mixed with Calisaya. |
| 18. Cinchona scrobiculata, H. and B. | Wedd., tab. 8 | Peru | — | Bark formerly known as Red Cusco Bark or Santa Anna Bark. |
| 19. Cinchona Peruviana, How. | Howard, ‘N. Q.’ | Peru | India | |
| 20. Cinchona nitida, R. and P. | Howard, ‘N. Q.’ | Peru | India | Grey Bark, Huanuco, or Lima Bark. |
| 21. Cinchona micrantha, R. and P. | Howard, ‘N. Q.’ | Peru | India | Chiefly consumed on the Continent. |
| IV. Stirps Cinchonæ Calisayæ— | ||||
| 22. Cinchona Calisaya, Wedd. | Wedd., tab. 9 | Peru, Bolivia | India, Ceylon, Java, Jamaica, Mexico | Calisaya Bark, Bolivian Bark, Yellow Bark. The tree exists under many varieties; bark also very variable. |
| 23. Cinchona | elliptica, Wedd., tab. 9 | Peru, Carabaya | — | Carabaya Bark. Bark scarcely now imported. C. cuneura, Miq. (flower and fruit unknown), may perhaps be this species. |
| V. Stirps Cinchonæ ovatæ— | ||||
| 24. Cinchona purpurea, R. and P. | Howard, ‘N. Q.’ | Peru, Huamalies | — | Huamalies Bark, not now imported. |
| 25. Cinchona rufinervis, Wedd. | Howard, ‘N. Q.’ | Peru, Bolivia | — | Bark a kind of light Calisaya. |
| 26. Cinchona succirubra, Pav. | — | Ecuador | India, Ceylon, Java, Jamaica | Red Bark; largely cultivated in British India. |
| 27. Cinchona ovata, R. and P. | Howard, ‘N. Q.’ | Peru, Bolivia | India (?), Java (?). | Inferior brown and grey barks. |
| 28. Cinchona cordifolia, Mutis. | Karst., tab. 8 | New Granada, Peru | — | Columbian Bark (in part). Tree exists under many varieties; bark of some used in manufacture of quinine. |
| 29. Cinchona Tucujensis, Karst. | Karst., tab. 9 | Venezuela | — | Maracaibo Bark. |
| 30. Cinchona pubescens, Vahl. | Wedd., tab. 16 | Ecuador, Peru, Bolivia | — | Areca Bark (Cusco Bark from var. Pelletieriana). Some of the varieties contain aricine. C. caloptera, Miq., is probably a variety of the species. |
| 31. Cinchona purpurascens, Wedd. | Wedd., tab. 18 | Bolivia | — | Bark unknown in commerce. |
470
The cinchona barks vary greatly in the amount of alkaloids they contain and in their proportion to each other, these being dependent upon the species or varieties, and many other circumstances. Of the alkaloids, quinia and cinchonia were till lately the most abundant, but since the introduction of cinchona cultivation into India, cinchonidia has been found in very large quantity. Royle says:—“Good Calisaya bark usually contains from 5 to 6 per cent. of quinia,” but actually South American calisaya containing such an amount of quinia is rare in the market. Some barks, however, derived from cinchonas cultivated in India, such as C. Calisaya, var. Ledgeriana, and some varieties of C. officinalis, yield even a still higher per-centage of quinine.
The South American crown, or loxa bark, is very variable, and contains chiefly cinchonia.
Red bark also varies considerably, yielding from 3 to 10 per cent. of alkaloids, of which quinia forms only a small fraction, whilst generally cinchonidia is predominant. The development of the alkaloids is greatly influenced by cultivation, but particularly by the “renewing process,” which, applied to the C. succirubra, trebles the amount of quinine in the bark.
In addition to the alkaloids already mentioned, the cinchona barks contain the following acid principles:—Kinic acid, Cincho-tannic acid, and Quinovic or Chinovic acid. The quinovic acid is accompanied by an amorphous bitter substance, named Chinovin or Quinovia, which is present in much greater proportion than the acid, of which generally there are only traces. A description of these bodies will be found by referring to them under their respective names. Cinchona-red is another amorphous substance which is the body to which the red hue of the cinchona barks is due. It is produced when cincho-tannic acid is boiled with dilute sulphuric acid, sugar being formed at the same time.
When fused with potash, proto-catechinic acid is formed. Cinchona red dissolves sparingly in alcohol, freely in alkaline solutions, but neither in water nor ether. Thick red bark contains it to the amount of more than 10 per cent.
Cinchona red is the product of the oxidation of cincho-tannic acid, and is contained largely in South American red bark, because this is the product of old trees; but sparingly in Indian red bark, because this is always collected from trees not more than fourteen years old.
Medicinal Properties of the Cinchona Barks. The therapeutic effects of the cinchona barks are doubtless due to the alkaloids they contain; but spite of their variability of composition in this respect, which has been shown to be very great, they are very extensively employed in medical practice in the forms of powder, decoction, tincture, and extract.
Dr de Vrij, the eminent quinologist, is of opinion that the therapeutic effects of bark are chiefly due in part to the alkaloids, and in part to the cincho-tannic acid they contain; and as red Indian bark is rich in both these constituents, he considers it the best suited for medical practice. See Quinetum.
Garrod says:—“Given in small doses, bark causes an increase of appetite, especially in weak patients, and at the same time improves the condition of the muscular system; hence the improvement of the blood and general health. It may, therefore, be well designated a tonic.
Its power in bracing up the system is also seen in the check given to the colliquative sweating occurring in extreme debility. The pulse is not quickened by the use even of large doses of quinine, although it is frequently made stronger, nor does bark itself, in the majority of cases, increase the heart’s action.
Bark also produces a peculiar influence upon the nervous system, which is exhibited in the extraordinary power it possesses of arresting the progress of certain diseases characterised by a periodical recurrence of their symptoms, as ague, the different forms of neuralgia, and certain inflammatory affections; how this effect is produced is at present unknown. Bark acts likewise as an astringent, and this property, combined with the tonic and antiperiodic powers, is often of much therapeutic value.”
For the method of estimating the alkaloids in cinchona bark, see Quinometry, Quinine, Quinidine, Quinoidine, Quinicone, Quinamine, Cinchonine; also the different pharmaceutical preparations of Cinchona bark.
CINCHONIDINE. Syn. Cinchonidia. C20H24N20. This cinchona alkaloid is isomeric with cinchonine. It occurs in large, shining striated, rhombic prisms, which are anhydrous. It dissolves in ·76 parts of ether and 20 of spirit of wine. The solutions are fluorescent, but do not answer to the chlorine and ammonia tests.
“The great powers and activity of this alkaloid have only of late been appreciated. As a protoplasm-poison, and probably in every other physiological action, it comes next to quinine and quinidine, and decidedly above cinchonine.”[248]
[248] Dr C. D. Phillips.
If it is chemically pure, cinchonidine belongs to the non-fluorescent alkaloids.
471
CINCHONINE. Syn. Cinchonia. C20H24N2O. This alkaloid abounds most in the paler varieties of the cinchona barks. It occurs in clear, colourless, four-sided prisms, which are soluble in 30 parts of water, and in about 400 parts of ether and 120 of spirits of wine. With acids it forms soluble salts, which do not fluoresce in solution, and are turned lightish brown-yellow by the chlorine and ammonia tests. Of its salts, the hydriodate is readily soluble in water, and still more so in alcohol, whether dilute or strong. Cinchonine may be prepared from its sulphate or disulphate in the same way as quinine.
Cinchonine, Sulphate of. Syn. Cinchoniæ sulphas. (Ph. U. S.) Take of the mother-water remaining after the crystallisation of sulphate of quinia in the process for preparing that salt a convenient quantity, solution of soda, alcohol, diluted sulphuric acid, animal charcoal in fine powder, each a sufficient quantity. To the mother-water add gradually with constant stirring solution of soda, until the liquid becomes alkaline. Collect on a filter the precipitate formed, wash it with water, and dry it. Then wash it with successive small portions of alcohol to remove other alkaloids which may be present, mix the residue with 8 times its weight of water, and having heated the mixture, add gradually diluted sulphuric acid until it is neutralised and becomes clear. Then boil the liquid with animal charcoal, filter it while hot, and set it aside to crystallise. Lastly, drain the crystals and dry them on bibulous paper. By evaporating the mother-liquid more crystals may be obtained.
CINCHO-TANNIC ACID. This acid is precipitated from a decoction of bark by acetate of lead, after the decoction has been freed from cinchona red by means of magnesia.
If the cincho-tannate of lead thus formed be decomposed by sulphuretted hydrogen, and the solution carefully evaporated in vacuo, the acid may be obtained as an amorphous, hygroscopic substance, readily soluble in water. A ferric salt added to a solution of this acid imparts a greenish colour to it.
Cincho-tannic acid is very soluble in water, but not in acids. Therefore a concentrated watery infusion (1 to 4) of Indian bark gives a precipitate upon the addition of strong hydrochloric acid. By this means a rough estimation may be formed of the amount of cincho-tannic acid in a sample of bark.
CINCHOVATINE. The substance known under this name does not exist as an alkaloid, sui generis. It is nothing more than quinidine, or cinchonidine, or a mixture of both.
CINNABAR. Syn. Native Vermillion. This compound, which is one of the most abundant of the ores of mercury, is a product of considerable importance in the arts, and some portions of it are sometimes sufficiently pure in colour to be used after mere levigation. Generally, however, the factitious kind is employed. See Vermilion.
CINNAMEIN. C16H14O2. Syn. Oil of balsam of Peru. A volatile oil existing in balsam of Peru.
CINNAMIC ACID. HC9H7O2. A colourless, transparent, crystalline substance, obtained from oil of cinnamon, liquid storax, balsam of Peru, and balsam of tolu. It is freely dissolved by alcohol, but nearly insoluble in water. At 248° Fahr. it fuses, and at 560° Fahr. it sublimes unchanged. Distilled with dichromate of potassium and sulphuric acid it is converted into benzoic acid. Its salts are called cinnamates.
CINNAMON. Syn. Cinnamon Bark; Cinnamomi cortex (B. P.), L. The inner bark of shoots from the truncated stock of the Cinnamomum Zeylanicum, imported from Ceylon, and distinguished in commerce as Ceylon cinnamon. The best is obtained from branches about three years old.
Used in medicine as a carminative and astringent, chiefly as an adjuvant to other medicines, e.g. with chalk, in diarrhœa.—Dose, 10 to 20 grains.
Obs. Owing to the high price of this drug it has become a general practice to substitute the bark of cassia (Cassia; Cortex cinnamomi cassia) for it, which so closely resembles it in flavour that the uninitiated regard them as the same. Cassia, however, is not only thicker and coarser than cinnamon, but its fracture is short and resinous, and its flavour is more biting and hot, whilst it lacks the peculiar sweetish taste of cinnamon. The thickness of cinnamon seldom exceeds that of good drawing paper.
CISTERNS. See Tanks.
CITRATE. A salt in which the hydrogen of citric acid is replaced by a metal or other basic radical.
CIT′RIC ACID. H3C6H5O7,H2O. Syn. Acid of lemons, Concrete a. of l.; Ac′idum limo′nis, Acidum cit′ricum (B. P.), L.; Acide citrique, Fr.; Citronensaüre, Ger. An acid peculiar to the vegetable kingdom. It is obtained in large quantity from the juice of lemons and other fruits of the genus Citrus; it is also found in gooseberries, currants, cranberries, whortleberries, cherries, &c.; and Dr Wright has lately found it in great abundance in unripe mulberries, in conjunction with malic acid.
When currants or gooseberries are employed as a source of citric acid, they are first subjected to pressure, and the juice so obtained from them is then fermented. The fermented liquor is next submitted to distillation, and the alcohol collected.
The residue in the retort containing the citric acid is saturated with chalk, and the resulting citrate of lime is decomposed by means of sulphuric acid.
100 lbs. of the fruit are said to yield 10 lbs. of spirit and 1 lb. of acid.
Prep. The citric acid manufacture consists in separating it from the mucilage, sugar, and472 other foreign matter with which it is combined in the juice of lemons and limes.
1. (Ph. L. 1836,—Scheele’s process.) Take of lemon juice 4 pints; prepared chalk, 41⁄2 oz.; diluted sulphuric acid, 271⁄2 fl. oz.; distilled water, 2 pints. Add the chalk by degrees to the lemon juice, made hot, and mix well; set by, that the powder may subside, and afterwards pour off the supernatant liquor. Wash the precipitated citrate of lime frequently with warm water; then pour upon it the diluted sulphuric acid, mixed with the distilled water, and boil the whole for 15 minutes in glass, stoneware, or lead; press the mixture strongly through a linen cloth, and filter it. Evaporate the filtered liquor with a gentle heat, and set it aside, that crystals may form. To obtain the crystals pure, dissolve them in water a second and a third time; filter each solution, evaporate, and set it apart to crystallise.
2. (Ph. L. 1851.) Merely placed in the materia medica.
3. (Ph. E. 1841.) Similar to that of Ph. L. 1836, except that the washed citrate of lime is ordered to be squeezed in a powerful press, and that the filtered solution of citric acid is ordered to be tested with nitrate of baryta, and if the precipitate is not nearly all soluble in nitric acid, to add a little citrate of lime to the whole liquor, till it stands this test.
4. (Ph. D. 1826.) Same as that of Ph. L. 1836.
5. (Ph. D. 1851.) Included in the materia medica.
6. (P. B. 1867.) Differs from the process of the Ph. L. 1836 in some unimportant detail only.
7. (Dr Price.) The crude juice is saturated with ammonia, potassa, or soda (carbonates), or with the ammoniacal product distilled from gas-liquor; chalk, 150 parts, or hydrate of lime, 90 parts, are then added for every 192 parts of citric acid contained in the liquor, and the whole stirred well together; heat is next applied, and the ammonia distilled into another quantity of lemon juice; the citrate of lime thus obtained is then decomposed with dilute sulphuric acid, and the whole process conducted as before. When potassa or soda is used the distillation is omitted, and the expressed liquor, after filtration, used to decompose fresh lemon juice.
8. (Ordinary manufacturing process.) To crude lemon or lime juice, mixed with water, is added ground chalk; the precipitate is washed to free it from the impurities dissolved in the water, and afterwards decomposed by sulphuric acid. If the citric acid is not sufficiently white, it is decolorised by digestion with animal black.
9. (Kuhlman.) This chemist proposes saturating the hot lemon juice as far as possible with very finely divided barium carbonate, and afterwards completing the neutralisation with barium hydrate or sulphide. The precipitated barium citrate is then to be washed, and decomposed with the requisite quantity of sulphuric acid. The advantage of barium over lime as a precipitant is the more ready crystallisability of the citric acid from the solution thus obtained. Sulphate of baryta is absolutely insoluble in solution of citric acid, whilst sulphate of lime is not; and the presence of the latter impedes the crystallisation of the acid.
Obs. If the lemon or lime juice be allowed to ferment a short time, the mucilage and other impurities will, to a certain extent, separate and subside. See Concluding Remarks.
Prop., Uses, &c. Citric acid forms rhomboidal prisms, which are clear, colourless, odourless, sour, and deliquescent in a moist atmosphere. It is an agreeable acid, at once cooling and antiseptic. It is much used in medicine as a substitute for lemon juice, and to form effervescing draughts, citrates, &c.
17 gr. citric acid, in crystals, or 1⁄2 fl. oz. of lemon juice, are equivalent to—| 25 | gr. | bicarbonate of potash; |
| 20 | ” | carbonate of potash; |
| 15 | ” | carbonate of ammonia; |
| 20 | ” | bicarbonate of soda; |
| 35 | ” | carbonate of soda. |
The bicarbonate of potassa is that generally preferred for making saline draughts with citric acid; and when flavoured with a little tincture of orange peel and simple syrup, or syrup of orange peel alone, it forms a most delicious effervescing beverage. Citric acid in pure crystals or in lime juice is much used by the calico-printer, being the best known ‘resistant’ for iron and alumina mordants.
Pur. Citric acid is frequently met with adulterated with tartaric acid; the fraud is easily detected by dissolving the acid in a little cold water, and adding to the solution a small quantity of acetate of potash. If tartaric acid be present, a white, crystalline precipitate of cream of tartar will be produced on agitation. When pure it is devoid of colour, is entirely, or almost entirely, decomposed by heat. It is soluble in water and in spirit, and what is thrown down from its watery solution by acetate of lead is dissolved by nitric acid. No salt of potassium precipitates anything with citric acid except the tartrate. When a few drops of a solution of citric acid are added to lime water, a clear liquid results, which, when heated, deposits a white powder, soluble in acids without effervescence. By the action of nitric acid citric acid is converted into oxalic acid.
When the crystals of citric acid are very deliquescent, the presence of free sulphuric acid may be suspected. This latter may be detected with facility by dissolving the citric acid in a little water, strongly acidifying the solution with hydrochloric acid, and adding473 chloride of barium, when, if sulphuric acid be present, an insoluble precipitate of sulphate of barium will fall down after a short time. Oxalic acid is sometimes present in citric acid, the cause of its presence being explained further on. To test for it proceed as follows:
Dissolve a small quantity of the citric acid in water, and add to the solution an excess of ammonia; acidify with acetic acid, filter, and test the filtrate with calcium sulphate.
Estim. See Acidimetry and Lime juice.
Tests. See above.
Concluding Remarks. The preparation of citric acid has now become an important branch of chemical manufacture, from the large consumption of this article in various operations in the arts. In conducting the different steps of the process some little expertness and care are, however, necessary to ensure success. The chalk employed, which should be dry, and in fine powder, is added to the juice from a weighed sample, until the latter is perfectly neutralised, and the quantity consumed is exactly noted. The precipitated citrate of lime is next thoroughly washed with water, and the sulphuric acid, diluted with 6 or 8 times its weight of water, whilst still warm, is poured upon it, and thoroughly mixed with it. The agitation is occasionally renewed for 8 or 10 hours or longer, when the solution of citric acid is poured off, and the residuum of sulphate of lime thoroughly washed with warm water, the washings being added to the liquid acid. This last is then poured off from the impurities that may have been deposited, and evaporated in a leaden boiler, over the naked fire, or by high-pressure steam, until it acquires the gravity of 1·13, when the process is continued, at a lower temperature, until a syrupy aspect is assumed, and a pellicle appears on the surface of the liquor. Without great care at this part of the process the whole batch may be carbonised and spoiled. At this point the concentrated solution is emptied into warm and clean crystallising vessels, set in a dry apartment, where the thermometer does not fall below temperate. At the end of 4 days the crystals are found ready for removal from the pans. They are thoroughly drained, redissolved in as little water as possible, and after being allowed to stand for a few hours to deposit impurities, again evaporated and crystallised.
The acid of the second crystallisation is usually sufficiently pure for the market; when this is not the case a third, or even a fourth, crystallisation must be had recourse to. The mother-liquors from the several pans are now collected together, and a second or third crop of crystals obtained from them, by evaporation as before.
A frequent cause of difficulty in obtaining crystals from the solutions is the employment of too little sulphuric acid to decompose the whole of the citrate of lime; the consequence of which is that a little of that salt is taken up by the free citric acid, and materially obstructs the crystallisation. Forty parts of dry sulphuric acid are required to decompose 50 parts of chalk. Commercial sulphuric acid (oil of vitriol) is usually of the sp. gr. of 1·845, and it therefore requires 49 lbs. of this acid for every 50 lbs. of chalk employed in the process. In practice it is found that a very slight excess of sulphuric acid is preferable to a preponderance of undecomposed citrate of lime.
The first crop of crystals is called ‘brown citric acid,’ and is chiefly sold to the calico-printers. Sometimes a little nitric acid is added to the solution of the coloured crystals, for the purpose of bleaching them, but in this way a minute quantity of oxalic acid is formed. A more general plan is to bleach the citrate of lime by covering it with a weak solution of chloride of lime, exposing it in shallow vessels to the sun’s rays, and rewashing it before decomposing it with sulphuric acid. A safer plan is to dissolve the crude citric acid, digest with animal charcoal, and again concentrate the solution to the crystallising point.
When the aqueous solution of citric acid obtained, as already described, is concentrated by boiling in an open evaporating pan, the acid is not only liable to suffer partial decomposition by its long exposure to the air, but it not unfrequently acquires a brown colour from the carbonisation those portions of the liquid undergo which are in contact with the bottom of the pan, which being heated by high-pressure steam frequently reaches a temperature exceeding 200° F. This latter result is brought about in consequence of the slight movement in the dense acid liquor in the pan. To remedy the loss and inconvenience arising from the employment of the open evaporating pan, some years back Mr Pontifex devised an apparatus which effects the evaporation of acid liquor in vacuo (and therefore out of contact with air), and at a temperature never exceeding 130 F°. Moreover, in Mr Pontifex’s boiler the time necessary for the concentration of the citric-acid liquor is diminished to about an eighth, and as the strong ebullition keeps the liquid in constant motion its charring is entirely prevented.
Mr Row says that lemon juice may be purified to a great extent by diluting it with water until it contains about 12 oz. of acid to the gallon, and then filtering from the flocculent precipitate of mucilage thus thrown down. The citrate of lime obtained from juice so treated is comparatively pure.
Good lemon juice yields about 61⁄2% of crystallised lemon acid; 2 galls. yield fully 1 lb. of crystals. See Lemon juice, Lime juice. &c.
CIT′RON. The fruit of the citron tree (Citrus medica) is acidulous, antiseptic, and antiscorbutic; it excites the appetite, and stops vomiting; and, like lemon juice, has been greatly extolled in chronic rheumatism,474 gout, and scurvy. Mixed with cordials, it is used as an antidote to the manchineel poison.
Citron, Oil of. See Oil.
Citron Peel. This is prepared in the same way as candied orange and lemon peel, which it for the most part resembles.
Citron. Syn. Lem′on colour. The term applied to a pale and delicate shade of yellow. See Yellow Dyes, &c.
CIT′RONELLE. See Liqueurs and Oils (Lemon-grass).
CITRUS. A genus of plants belonging to the natural order Aurantiaceæ, the species of which yields useful fruits. From Citrus Aurantium, and its varieties, all the various descriptions of sweet oranges are obtained. The species C. Bigaradia or vulgaris yields the bitter or Seville orange; C. Limonum and its varieties, yield the lemons; C. Limetta is the source of the lime; C. medica of the citron; C. Decumana of the shaddock; C. paradisi of the forbidden fruit; C. Pampelmos of the Pampelmoose; and C. japonica of the kumquat.
Citrus Bergamia. (Ind. Ph.) Syn. The Lime tree. Habitat. Commonly cultivated in India and other tropical countries.—Officinal part. The fruit (lime) closely resembles the lemon, but is smaller, with a smoother, thinner rind, and of somewhat less fragrant odour. Its juice (lime juice) has the same pungent acid taste, and contains the same ingredients as lemon juice, though in somewhat different proportions, that of the citric acid being larger and that of the mucilage less in quantity. Much of the article imported into England under the name of lemon juice is obtained from the lime.—Properties and Uses. Very similar to those of the lemon, the juice being equally refrigerant and antiscorbutic; indeed, it is preferred by many tropical practitioners.
The fresh juice of the lime is procurable in almost every portion of the tropics, and is considered more effectual than preserved lemon juice.
Lime juice may be advantageously employed in the manufacture of citric acid, the proportion of this acid being larger than in lemon juice.
CIV′ET. Syn. Civet′ta Zybeth′um, L. A perfume obtained from the civet cat (Viverra civella, Linn.), a fierce, carnivorous quadruped, somewhat resembling a fox, found in China and the East and West Indies. The civet is secreted in a sort of pouch between the anus and the sexual organs. “Several of these animals have been brought into Holland, and afford a considerable branch of commerce, especially at Amsterdam. The civet is squeezed out in summer every other day, in winter twice a week; the quantity procured at once is from 2 scruples to 1 drachm or more.”
Civet is frequently adulterated with spermaceti and butter, and a similar substance to civet, but of a darker colour, obtained from the polecat. When pure it has an odour intermediate between that of musk and ambergris, but less refined; a pale-yellow colour; an acrid taste; and the consistence of honey. It is used in perfumery.
CLAIRET. See Liqueur.
CLAR′ET RAGS. Syn. Tournesol en drapeau, Fr.; Bezet′ta cœru′lea, L. 1. Pieces of clean linen coloured with Auvergne—or ground archil.
2. Pieces of linen dipped into the juice of mulberries, blood-red grapes, lees of red wine, &c. Used to colour jellies, confectionery, the rind of cheeses, &c.
CLARIFICATION. The act of clearing or making bright; commonly applied to the process of ‘clearing’ or ‘fining’ the liquids by chemical means, instead of by filtration. The substances used for this purpose are popularly known as ‘clarifiers’ or ‘finings.’
The substances employed in the clarification of liquids operate by either mechanically embracing the feculous matter, and subsiding with it to the bottom of the vessel, or by inducing such a change in its nature or bulk that it subsides by its own density, in each case leaving the liquor transparent. Albumen, gelatin, the acids, certain salts, blood, lime, plaster of Paris, alum, heat, alcohol, &c., serve in many cases for this purpose. The first is used, under the form of white of egg, for the clarification of syrups, as it combines with the liquid when cold, but on the application of heat rapidly coagulates and rises to the surface, carrying the impurities with it, forming a scum which is easily removed with a skimmer. It is also much used for fining wines and liqueurs, particularly the red wines and more limpid cordials. Gelatin, under the form of isinglass, dissolved in water or weak vinegar, is used to fine white wines, beer, cider, and similar liquors that contain a sufficient quantity of either spirit or astringency (tannin), to induce its precipitation. Sulphuric acid is frequently added to weak liquors for a similar purpose, either alone or after the addition of white of egg or gelatin, both of which it rapidly throws down in an insoluble form. A pernicious practice exists among some unprincipled manufacturers of using certain salts of lead and potash to clear their liquors; especially those that are expected to sparkle in the glass, as ‘cordial gin,’ &c. For this purpose a little sugar of lead, dissolved in water, is first mixed up with the fluid, and afterwards a little more than half its weight of sulphate of potassa, also dissolved in water, is added, and the liquor is again ‘roused’ up. By standing, the sulphate of lead, formed by this mixture, subsides, and leaves the liquor clear. Bullock’s blood is used in the same way as isinglass or white of eggs, for fining red wines, beer, and porter. Lime, alum, alcohol, acids, and heat, act by curdling or coagulating the feculencies, and thus, by increasing475 their density, induce their subsidence. Plaster of Paris acts, partly like the above, and partly like albumen, or gelatin, by developing and forcing down the suspended matter. Sand is often sifted over liquors (especially cordials and syrups), for the simple purpose of acting by its gravity, but appears to be quite useless, as it sinks too rapidly. The juices of plants are clarified by heat, which coagulates the albumen they contain. Marl or clay is frequently used to clear cider and perry. A strip of isinglass is generally employed to clarify coffee. See Wine, Brewing, Cordials, Coffee, Finings, Infusion, &c.
CLAY. Clay is formed from the disintegration of felspathic rocks, by the combined action of air and water. Its plasticity, when moist, and its capability of being made hard by heat, are properties which render it available for many useful purposes. The purest kind of clay is kaolin, or China clay, which consists almost entirely of silicate of aluminum. It is found in China; but a precisely similar substance is obtained from deposits in Cornwall and some parts of France. Pipe-clay, a white clay nearly free from iron, is found in large quantity in the island of Purbeck. Potter’s clay is found in many parts of Britain; that of Devonshire and Dorsetshire is much valued. Brick clay contains varying proportions of iron; hence the different colours of the bricks used in different countries. See Aluminum, Fuller’s earth, Ochre, &c.
CLEAN′ING. In domestic economy the best way to clean a house is to keep it clean by a daily attention to small things, and not allow it to get into such a state of dirtiness and disorder as to require great and periodical cleanings. Some mistresses, and also some servants, seem to have an idea that a house should undergo regular cleanings, or great washing and scrubbing matches, once every three or six months, on which occasions the house is turned almost inside out, and made most uncomfortable. All this is bad economy, and indicates general slovenliness of habits. (Chambers.) For hints upon cleaning, see Carpets, Clothes, &c.
CLEAN′LINESS. See Ablution, Bathing, and Sickness.
CLIPPING (Horses). Some horses should be worked in autumn in cloths, or with their coats on, as, on account of the extra sweating thus caused, they will be in better condition for the hunting season. Such horses should be clipped or shaved. The horse’s coat should be fully set before it is clipped. Those horses which sweat much in autumn should be singed. Singeing cannot be begun too early. The fresh growth must be removed every week. Singeing may be best accomplished by means of gas.
CLOTHES. Economy and cleanliness require due attention to be paid to every article of clothing, but more especially to those which are the most exposed to dirt and the weather. The following remarks, having reference chiefly to woollen articles, may prove useful to the reader:—If very dusty, hang them on a horse or line, and gently beat them with a cane; then lay them on a clean board or table and well brush them, first with a stiff brush, to remove the spots of mud and the coarsest of the dirt, and next with a softer one, to remove the dust and to lay the nap properly. If clothes are wet and spotted with dirt, dry them before brushing them, and then rub out spots with the hands. The hard brush should be used as little as possible, and then with a light hand, as it will, if roughly and constantly employed, soon render the cloth threadbare. Spots of tallow-grease on the clothes may be taken off with the nail, or, if that cannot be done, have a hot iron with some thick brown paper, lay the paper on the part where the grease is, then put the iron upon the spot; if the grease comes through the paper put on another piece, till it ceases to soil it. Moths may be prevented attacking clothes by putting a few cloves or allspice into the box or closet with them. See Balls, Clothes, and Scouring, &c.
CLO′′THING. In our changeable climate great care should be taken to clothe the body effectually; for when the skin is chilled the blood is determined in increased and injurious quantity to the internal organs, causing colds and inflammations. The ordinary materials for clothing are cotton, linen, woollen, and silk. Cotton is generally employed for undergarments, for which its softness and warmth render it well adapted. Linen is not nearly so warm, but it keeps its colour better; it is more expensive, and although it wears much longer, it is not so economical as cotton. Woollen garments are, in cold and variable climates, almost essential to comfort; the warmth obtained by wearing flannel next the body is very beneficial, and the slight stimulating effect arising from its roughness tends to keep the skin in healthy action.
The practice of dressing infants in long clothes is a very objectionable one, for besides being injurious to health it cramps the action of the legs, the feet, and the toes, and by so doing prevents their proper and healthy development.
An infant should be so clothed as to combine sufficient warmth with perfect freedom of the limbs; hence his garments should be loose instead of tight, more particularly round his waist.
In the selection of winter clothes for children, if for in-door wear, choice should be made of a dark woollen frock, and of stockings in preference to socks. The stockings should be of merino, and made to draw above the knees and fastened to the dress with a loop and tape instead of garters, which are very objectionable.
A child’s out-of-door attire in winter should additionally comprise a warm and properly-lined coat, made of cloth or some woollen476 fabric. It should button close to the chin and cover his neck. Mr Chavasse says, for this latter purpose a woollen neckerchief or scarf is preferable to furs. It is very important that the child’s feet and legs should be kept warm, but not too warm. In infancy and childhood—in summer as well as winter—the wearing of flannel next the skin is more necessary, and beneficial even, than when practised by adults.
CLOVE. Syn. Caryoph′yllum (B. P.), L. The flower-buds of the Ciryophyllus aromaticus (Linn.), or clove tree collected before they open, dried, and smoked. Cloves are aromatic, stimulant, carminative, and stomachic; and, according to some, possess febrifuge properties. They are chiefly used as an adjuvant in compound medicines. A few cloves kept in a closet or box prevent moths or mould attacking furs, woollens, &c.
It is a common practice to adulterate this spice in the same manner as cinchona bark. Cloves from which the oil has been distilled are dried and rubbed between the hands, previously moistened with a little sweet oil, to brighten their colour, after which they are mixed up with fresh spice for sale.
Cloves, Mother of. The unripe fruit of the clove tree; they are frequently imported preserved (preserved mother of cloves), and are reputed stomachic and antispasmodic.
Cloves, Oil of. Syn. O′leum caryoph′ylli (B. P.), L. This possesses similar virtues to the unexpanded flower-buds, and is esteemed as a remedy for the tooth-ache. Used to flavour liqueurs and confectionery. Sp. gr. 1·055-1·060.
M. Jacquemin recommends the following as a very delicate test for the presence of carbolic acid when used as an adulterant for oil of cloves. One drop of the suspected oil is mixed with a small trace of solution of aniline by means of a glass rod, and then shaken with 5 or 6 c. c. of distilled water. By the addition of a few drops of sodium hypochlorite to the mixture the characteristic blue coloration due to carbolic acid will be developed in a few minutes, whereas with the pure oil nothing but the purplish-violet colour of aniline will be perceived. Stirring or shaking must be avoided after the addition of the hypochlorite.
CLYS′TERS. See Enema.
COAL. The varieties of this valuable substance may be conveniently described under the three heads Anthracite, Lignite, and Pit-coal (which see). See also Fuel.
COAL-TAR. Coal-tar, one of the products of the destructive distillation of the coal employed in the manufacture of gas, is a very complex substance, consisting of various hydrocarbons, acids, and bases, together with certain resinoid and empyreumatic substances. The principal hydrocarbons yielded by coal-tar on distillation are: benzol, toluol, propyl, naphthalin, and anthracin; of these the first three are fluids, and the last two solids; the most important acids are: carbolic, cresylic, phlorylic, and nosolic; the chief bases are: aniline, chinoline, and lepidine. The quantity as well as the quality of the tar obtained from the distillation of coals varies considerably with the kind of coal used, as well as with the temperature at which the distillation is carried on, the yield of tar being smaller at very high temperatures than when lower ones are employed. Coal-tar, from its antiseptic properties (due chiefly to the carbolic acid it contains), is painted on wood to preserve the latter from decay when exposed to wind and weather. Mixed with coal-dust, saw-dust, and peat-dust, it forms a useful artificial fuel, and when incorporated with pebbles makes an excellent artificial asphalt for pavements. The chief value of coal-tar, however, consists in its being the source of those brilliant dye-stuffs, the coal-tar colours. These, together with the naphtha obtained from its distillation, have converted coal-tar from a worthless and unwelcome waste product of gas manufacture—for the removal of which from their premises the gas makers were formerly only too glad to pay—into a very considerable and important branch of profit and revenue.
The different constituents of the tar are separated from each other by distillation, the various products so obtained being further purified by various processes.
See Tar Colours, Naphtha, Benzol, Anthracene, &c.
CO′BALT. Co. Syn. Reg′ulus of cobalt; Cobalt′tum, L. A metal discovered by Brandt, in 1733. It generally occurs in the same ore as nickel, and the separation of the two metals is a task requiring great patience and expertness. Speiss cobalt and cobalt glance are the ores from which the metal is commonly extracted.
Prep. 1. Dissolve oxide of cobalt in hydrochloric acid, and pass sulphuretted hydrogen gas through the solution, until all the arsenic is thrown down; filter, and boil with a little nitric acid, then add carbonate of potassium, in excess, and digest the precipitate in a solution of oxalic acid, to remove any oxide of iron; wash and dry the residuum (oxalate of cobalt), and expose it to great heat, in a covered crucible lined with charcoal; the product is pure metallic cobalt.
2. Mix equal parts of oxide of cobalt or roasted Cornish cobalt ore, and soft soap, and expose them to a violent heat in a covered crucible.
3. Pass hydrogen gas over oxide of cobalt strongly heated in a porcelain tube.
Prop., Use, &c. Cobalt is a white, brittle metal; unchanged in the air; feebly acted on by dilute hydrochloric and sulphuric acids; has a high melting-point, and is strongly magnetic; sp. gr. 8·5. It is seldom employed in the metallic state, from the great difficulty of reducing its ores, but its oxide (black oxide) is477 largely employed in the arts. It forms salts with the acids, which are interesting from the remarkable changes of colour which they exhibit. See Ink, Smalts, Zaffre, and below.
Char., Tests. Solutions of the salts of cobalt are known as follows:—1. Ammonia gives a blue precipitate, slightly soluble in excess, giving a brownish-red colour.—2. Potassa gives a blue precipitate, turning to violet and red when the solution is heated.—3. Carbonate of ammonium and carbonate of sodium give pink precipitates; that from the former is soluble in excess.—4. Cyanide of potassium gives a yellowish-brown precipitate, soluble in excess; and the clear solution, after being boiled, is unaffected when mixed with hydrochloric acid.—5. Sulphuretted hydrogen produces no change in acid solutions.—6. Sulphydrate of ammonium gives a black precipitate in neutral solutions.—7. Melted with borax, before the blowpipe, it gives a bead of a magnificent blue colour, almost verging on black, if much is present. Phosphate of sodium and ammonium give a similar bead; but the colour is less intense.
Cobalt, Ac′etate of. Co(C2H3O2)2. Prep. From the carbonate or protoxide and acetic acid. It forms a sympathetic ink which turns blue when heated.
Cobalt, Arseniate of. Co32AsO4, 8H2O. A hydrated native tricobaltous arseniate of cobalt, known as “cobalt bloom.”
Cobalt, Car′bonate of. CoCO3. Prep. By adding an alkaline carbonate to a solution of the nitrate or sulphate. A pale peach-coloured powder, soluble in acids. It contains some hydrate.
Cobalt, Chlo′′ride of. CoCl2. Syn. Hydrochlo′′rate of c., Mu′′riate of c. Prep. By dissolving the carbonate or protoxide in hydrochloric acid; the solution deposits deep rose-red crystals on standing, which contain water. By evaporating the solution by heat, anhydrous blue crystals of the chloride are obtained. Both of them yield a deep rose-red solution with water, which is turned green by a little acid. This solution forms a well-known sympathetic ink, the traces of which become blue when heated. If the solution contains either chloride of iron or chloride of nickel, the traces become green. (Klaproth.) The addition of a little nitrate of copper to the above solution forms a sympathetic ink, which by heat gives a very rich greenish-yellow colour. (Ure.) The addition of a very little common salt makes the traces disappear with greater rapidity, on the withdrawal of the heat. In each case, when the paper is laid aside, moisture is absorbed, and the writing once more disappears. If, however, much heat has been used the traces become permanent.
Cobalt, Ni′trate of. Co(NO3)2. Prep. As the last, substituting nitric for hydrochloric acid; it forms deliquescent crystals.
Cobalt, Ox′alate of. CoC2O4. Prep. As the acetate, from oxalic acid and the carbonate or protoxide; or by double decomposition.
Cobalt, Ox′ides of. Of these there are two, the protoxide and the sesquioxide; besides an acid compound of cobalt and oxygen, to which the name cobaltic acid has been given.
1. Cobalt, Protox′ide of. CoO. Syn. Oxide of cobalt, Grey o. of c., Black o. of c., Cobalt black. Prep. 1. By precipitating a solution of sulphate of chloride of cobalt with carbonate of sodium, and washing, drying, and igniting the powder which subsides.
2. By boiling powdered bright-white cobalt ore (from Cornwall) in dilute nitric acid, and adding a solution of carbonate of potassium, very gradually, until the clear liquor, after the impurities have settled, becomes of a rose colour; and then as long as a precipitate falls; wash and dry it as before.
Prop., &c. A grey powder, turning black on exposure to the air; strongly basic; and forming salts with the acids, having a fine red tint. It is remarkable for the magnificent blue colour it communicates to glass, and by this character its presence may be readily detected before the blowpipe; the substance to be examined being fused with borax on a loop of platinum wire. Used to make blue colours for painters, stains and glazes for enamellers, glass-melters, potters, &c. In medicine it has occasionally been given as a remedy for rheumatism.
2. Cobalt, Sesquiox′ide of. Co2O3. Syn. Perox′ide of Cobalt. A black, insoluble, neutral powder, obtained by mixing solutions of cobalt and of chloride of lime; or, by heating the protoxide to redness in an open vessel.
Cobalt, Phos′phate of. Co3(PO4)2. Prep. As the acetate, substituting phosphoric for acetic acid. An insoluble purple powder, which, when heated along with eight times its weight of gelatinous alumina, produces a blue pigment (COBALT BLUE, COBALT ULTRAMARINE), almost equal in beauty to ultramarine. (See below.)
Cobalt, Sul′phate of. CoSO4. By boiling sulphuric acid on the metal, or by dissolving the oxide in the acid. It forms reddish crystals, soluble in 24 parts of water.
Cobalto-Ultramarine. A fine blue pigment, prepared by mixing freshly precipitated alumina, 8 parts, with phosphate or arseniate of cobalt, 1 part; drying the mixture, and then slowly heating it to redness. By daylight the colour is pure blue, but by artificial light it is violet. See Blue pigments.
COCA. Erythroxylon Coca. This plant is grown largely in Peru and Bolivia. The Bolivian coca is said to be much superior to the Peruvian. The best kind is believed to come from the province of Yungas, and the most inferior description from Peru. The consumption of Coca in Peru, Bolivia, and in some of the provinces of the Argentine Confederation478 is enormous. In small doses it is supposed to act as a stimulant and to aid digestion; in large ones it is said to possess dangerous narcotic properties. The mountaineers in South America state they are enabled to reach high elevations without difficulty of respiration, and to stave off the feeling of hunger by chewing the leaves during their ascents. “Good quality coca should have its leaves unbroken, of a medium size, bright green in colour, and of an odour somewhat combining that of hay and chocolate. The taste is bitter, and when masticated, coca is said to yield easily to the teeth. Infused in hot water, it has a beautiful green colour, which, however, is much darker from inferior leaves. An infinite number of varieties are recognised between the best and the lowest quality, which has a disagreeable smell and a colour resembling roasted coffee. The leaves are also bent and broken, scarcely a whole leaf being found amongst them.”[249] The statements as to the effects of coca are conflicting, as will be seen from what follows:—Sir R. Christison, writing to the ‘British Medical Journal,’ April 29th, 1876, states he was hardly sensible of the fatigue of two mountain descents made from Ben Vorlich after chewing coca leaves. That, as a consequence of his doing so, hunger and thirst were suspended for a long time, but that eventually appetite and digestion were unaffected. He made trial during the first descent of 60 grains, and of the second, undertaken eight days after, of 90 grains of coca.
[249] ‘Pharmaceutical Journal.’
Mr Dowdeswell, in a communication to the ‘Lancet,’ May 6th, 1876, says that, contrary to the experience of Sir R. Christison, he found no decided effects produced after consuming nearly a pound of the leaves, which were taken in all forms and at all hours for nearly a month. They failed to produce the slightest excitement, not giving rise even to the feeling of buoyancy and exhilaration which is experienced from mountain air or a draught of spring water.
In the ‘Canadian Pharmaceutical Journal’ for August, 1877, there is a paper by Mr Shuttleworth, wherein results the opposite to those of the last-named gentlemen are recorded. Mr Shuttleworth states that the members of a club established at Toronto for the purpose of playing at La Crosse, a very violent and fatiguing pastime, were almost unanimous in ascribing their invariable success over their numerous adversaries to the use of coca leaves during their contests; their opponents not employing the plant. The antagonists of the club were men of stronger build and physique as well as more accustomed to out-of-door pursuits, and were besides trained players.
The same writer says that in South America care is taken to procure the leaves in as fresh a state as possible, and that many writers have ascribed the want of effect to old leaves.
The ‘British Medical Journal’ of March 10th, 1877, contains a communication from Dr T. McBean, who states that he has found the administration of coca leaves useful in typhoid fever, as well as in other febrile diseases.
CO′COA (kō′-ko). Syn. Caca′o. An alimentary substance formed of the roasted seeds of the Theobroma Cacao, a tree belonging to the natural order Byttneriaceæ. This definition is equally applicable to chocolate, but we commonly class the preparations containing sugar and flavouring substances under that head, and the unsweetened and cheap preparations under COCOA. The cocoa-seed or berry must not be confounded with the cocoa-nut, which is the fruit of a palm (Cocus nucifera). The cocoa tree is a native of Mexico, and is now more or less extensively grown throughout Central America, Brazil, Peru, Venezuela, Caraccas, Ecuador, Grenada, Demerara, Essequibo, Guayaquil, and Surinam; with some of the West India Islands, foremost among which stands Trinidad. It has also been introduced with more or less success into Africa, the Mauritius, Madagascar, Bourbon, the East Indies, Australia, and the Philippine Islands. The following is a list of the principal kinds of cocoa, in the order of their commercial value:—Caraccas, Surinam, Trinidad, Grenada, Jamaica, Dominica, Guayaquil, Venezuela, Bahia, Brazil, St. Lucia. It seems probable that some of the highest kinds of cocoa do not find their way into this country, but are consumed by the inhabitants of Spain.
Prep. The pods containing the seeds are gathered when ripe, and after having lain for a day and a night are opened, and the seeds, which are taken out by hand, are submitted to what is termed the sweating process. They are first placed on a sloping floor or in baskets, so that the chief part of the pulp in which they are enveloped may drain off, and are then shut up in a close box, and left for 24 to 48 hours, according to the season and weather, after which they are turned out in the sun to dry. Upon a nice performance of the sweating process, which may be likened to malting, the value of the cocoa greatly depends. When quite dry, the seeds are packed in barrels or bags, and are ready for shipment. The process of roasting is effected in a metal cylinder, with holes at each end, through which the vapour generated is allowed to escape. When the aroma is sufficiently developed the seeds are cooled, and then passed to a ‘kibling mill,’ which removes the husks and skins from the ‘nibs’ (see below).
Prop., Constituents, &c. Cocoa, when unadulterated, forms a wholesome and highly nutritious beverage. Its active principle is theobromine, an alkaloid greatly resembling caffeine, the active principle of coffee and tea. A peculiar concrete oil, called cocoa-butter, or, more correctly, butter of cacao, is another important constituent, forming more than half the weight of the seed. The presence of479 about 20% of albumen gives to cocoa its nutritive character.
| Per cent. | |
| Fat (cocoa butter) | 50·00 |
| Albumen, fibrine, and gluten | 18·00 |
| Starch | 10·00 |
| Gum | 8·00 |
| Colouring matter | 2·60 |
| Water | 6·00 |
| Theobromine | 1·50 |
| Ash | 3·60 |
| Loss | 0·30 |
| 100·00 |
Dr Letheby calculated that a pint of cocoa made with 1 oz. of ground nibs would contain the following proportions of nutritious matters:—
| Nitrogenous matters | 96·2 | grains |
| Patty matter | 218·8 | ” |
| Gum, sugar, and extractive | 65·6 | ” |
| Mineral matters | 17·5 | ” |
| ——— | ||
| Total extracted | 398·1 |
Adult. Much of the cheap stuff sold as genuine cocoa is shamefully adulterated. Out of 68 samples of cocoa and chocolate examined by the ‘Lancet’ commission, 39 contained coloured earthy substances, as reddle, Venetian red, umber, &c. To some, chalk or plaster of Paris had been added, for the purpose of increasing the weight. Many of the samples consisted of sugar and starch, with only sufficient cocoa to impart a flavour. Cocoas containing a moderate amount of arrow-root or other starch must not be considered adulterated articles, for it is impossible to render cocoa soluble, or rather emulsive, without the addition of some diffusible substance.
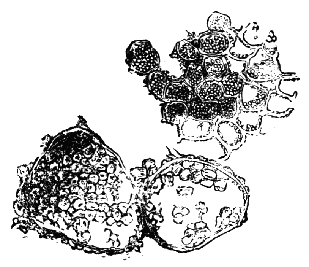
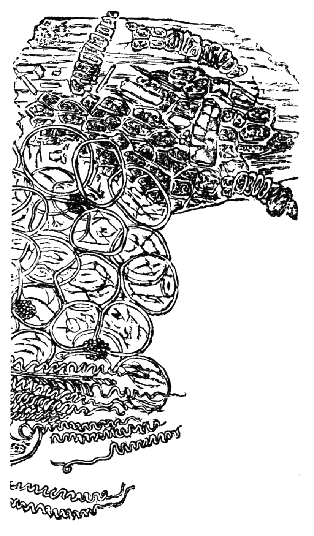
By an examination of the ash the presence of any mineral adulterant may be detected. Mr Blyth says the amounts of ash in genuine cocoa should never exceed 5 per cent. The seed of the cocoa consists of husk and seed proper. Under the microscope the husk exhibits on its surface a number of tubular480 fibres, filled with granular matter and minute corpuscles. It consists of three membranes; the first being a single layer of elongated cells; the second (forming the chief portion of the husk) of angular cells, enclosing mucilage, and also containing a few spiral vessels and woody fibres. The third membrane is very thin and delicate, and is made up of small angular cells containing minute globules of fat. The seed is composed of minute cells containing starch. The starch corpuscles are very small, with a trace of inulin. (See cuts on previous page.)
Cocoa, Flake. This is formed by grinding the nibs in a mill, consisting of two cones, working one inside the other. Pure flake cocoa is not a diluted or amalgamated article; in other words, it contains no sugar, and but a trace of starch.
Cocoa Nibs. The bruised, roasted seeds, freed from husk and membrane. They ought to be of a dull-red or greyish colour, but are frequently given a bright-red colour by a coating of Venetian red.
Cocoa, Sol′uble. From cocoa nibs and substances which are readily soluble or diffusible in water, ground together. Sugar and sago or arrow-root are the diluents used by respectable makers, but all kinds of starches, coloured with Venetian red, are used for the trashy articles which are sold to the poor. No form of cocoa is really soluble, but by the addition of easily diffusible substances an article is produced which is capable of forming an emulsion with boiling water. The following are the principal varieties of the so-called soluble cocoa:—
1. Cocoa, Granulated. From cocoa nibs and sufficient sugar and arrow-root to keep the fatty particles from forming a pasty mass. As it is impossible to granulate the nibs without the admixture of some other substance, those makers who declare that their granulated cocoas are perfectly pure do not act honestly towards their customers.
2. Cocoa, Homœopathic. A kind of soluble cocoa prepared with arrow-root, but without sugar.
3. Cocoa, Iceland-moss. From cocoa and Iceland moss, freed from its bitter principle, cetrarine. This form of cocoa was introduced by Messrs Dunn and Hewett, and is said to form a very valuable article of diet for invalids.
4. Cocoa, Maravilla. This is stated to be “the perfection of prepared cocoa.” It consists of cocoa, sugar, and sago flour, the last two being in great excess.
5. Cocoa, Caraccas. This is similar to the last, being a mixture of cocoa, sugar, and sago flour. The cocoa used in its manufacture is said to be imported from the Caraccas, on the north coast of South America, and to possess a peculiarly delicious flavour.
The amount of flour or starch in these so-called soluble cocoas frequently exceeds 40 per cent., and the amount of sugar 20 per cent. They have been not inaptly called “soups.”
Within the past year or two a new variety of soluble cocoa has been brought into the market. It is sold under various names, thus, ‘Theobromine, or Concentrated Cocoa,’ ‘Cocoa Essence,’ ‘Cocoatina,’ &c. We have examined many of these varieties, and find them to consist of pure cocoa deprived of about two thirds of its fat. It appears very suitable for people of weak digestion.
Obs. No warm drink that we take approaches cocoa in its nutritive character, because, while performing to a certain extent the exhilarating work of coffee or tea, it presents to the stomach a very considerable quantity of nitrogenous and carbonaceous matter; this advantage is partly due to the fact that cocoa is taken in the form of an emulsion, instead of an infusion or decoction.
Cocoa for the table is readily prepared from the soluble varieties by simply pouring boiling water upon the powder. From cocoa nibs, or flaked cocoa, the beverage is prepared by first pouring boiling water upon them, and then allowing the mass to simmer from 4 to 6 hours. The cocoa must on no account be allowed to boil, for in that case a coagulum will be formed, which cannot be dissolved in water.
COCOA-NUT OIL. A species of vegetable butter obtained from the common cocoa nut—the fruit of Cocos nucifera, the cocoa palm. It is separated from the dried kernel by hydraulic pressure. It contains olein, and a solid fat often used as a candle material. Large plantations of the cocoa palm, connected with Price’s candle company, exist in Ceylon. Cocoa-nut oil is often confounded with cocoa- or cacao-butter, which is the produce of a very different plant, namely, Theobroma cacao. See Cocinic acid, Cocoa, Stearic acid, &c.
COC′CULUS IND′ICUS. Syn. Indian berries, Indian cockles, Levant nut, Louse grains; Bac′ca Orienta′lis, Cocoulus piscator′ius, &c., L. The fruit of the Anamirta paniculata, a shrub which abounds on the sandy shores of Malabar, and several other islands in the Indian Ocean. The kernels should fill at least two thirds of the fruit.
It is a dark, tough, hard, wrinkled berry, about the size of a cherry, and possesses an intensely bitter taste. The berry consists of two parts, the husk and the kernel, the former being hard and difficult to bruise, and the latter soft and containing a large proportion of fatty matter.
Uses, &c. Cocculus indicus is poisonous to all animals, and to most vegetables. It is never employed internally in medicine, but an ointment, formed by mixing the powder with lard, has been used to destroy pediculi and in porrigo. Its active principle is picrotoxin, a peculiar needle-shaped, crystalline substance, possessing all the poisonous properties of the berry in an exalted degree, and of481 which it contains about 2 per cent. Its effects on the system are, to produce giddiness, convulsions, and insensibility, frequently ending in death. A small portion of the cocculus indicus imported is used by poachers, and a still smaller quantity to destroy vermin, the remaining, and by far the greater part, being employed to adulterate beer and even wine. “In our own analytical experience we have seldom found this substance in beer purchased from a respectable house. We have detected it, however, in beers purchased in the lowest localities in London and elsewhere, but have every reason to suspect that the adulterants had been added by the publican himself, in the form of an extract known in the trade by the name of ‘B. E,’ or black extract.” (Harkness.)
Chemists and druggists are liable to severe penalties if they are found supplying cocculus indicus, or any extract of the same, to brewers or publicans. See Beer, Porter, &c.
COCH′INEAL. Syn. Coc′cus (B. P.), L. Grana fina, Span. The Coccus Cacti (Linn.), an insect found upon the cactuses of Mexico. It is of great value as a dye stuff. The female insects, when matured, are brushed off the plants and dried by artificial heat. The entire insect is used. There are two varieties known in commerce—silver cochineal, which has a purplish-grey or silver-grey colour; and black cochineal, which is smaller, and of a reddish or purplish-black colour. The former is that commonly met with.
Adult. Genuine cochineal has the sp. gr. 1·25. It is commonly increased in weight by slightly moistening it with gum-water, and then rouncing it in a bag, first with sulphate of baryta, and then with finely powdered bone-black. In this way its sp. gr. is raised to 1·35, in consequence of being loaded by about 12% of useless foreign matter.
Herr Durwell, a German chemist, states that he found a sample of cochineal adulterated with sulphate of zinc. He thinks the sophistication was probably effected by immersing the cochineal in sulphate of zinc, and then in an alkali, whereby the white pulverulent aspect of the genuine article was imparted, and the weight increased.
The following is a method which has been given for estimating the value of samples of cochineal:—Grind the samples to be tested to a fine powder, weigh out 2 or 21⁄2 grammes, and boil this amount in a capacious narrow-necked flask, with 750 c. c. of water for 1 hour; filter immediately through dry paper filters, and allow it to cool. To test it 50 c. c. are measured in a flask of that capacity, and poured into another flask of about 200 c. c., and the measuring vessel rinsed with a definite quantity of water, say 10 to 15 c. c. A weak solution of permanganate is then run in from a burette with a glass cock, the flask being shaken after the addition of every 10 c. c. So much permanganate solution is then added that the cochineal extract shall be charged from its original colour to a pink of the faintest shade—almost yellow, in fact, but never reaching a full yellow. This pink shade should be persistent, that is, it should not turn yellow after standing fifteen minutes; and after a little practice it will be found very easy to obtain the tinge, which shows that the colouring matter is almost but not quite destroyed.
When a number of samples are to be compared, arrange an equal number of 200 c. c. flasks and test-tubes on the table, a tube standing in its rack in front of each flask. Then the same number of c. c. of the permanganate solution (which should be, at least, so weak that bulk for bulk of this and the cochineal solution will be required) is run into each flask, taking care to use too little to completely destroy the colouring matter in all.
The flasks are well shaken and allowed to stand for ten minutes. Part of the contents of each is then poured into the corresponding test-tube, and a glance of the tubes as they stand side by side will show which is the least affected by the bleaching liquid. This sample having been selected to serve as a standard, the contents of the test-tube are returned to the flask, and more permanganate solution is cautiously added, until a very faint pink tinge, which a fraction of a c. c. will turn to a full yellow, is obtained. The number of c. c. used having been noted, a fresh trial is made, in which the c. c. required, minus one, are used, the flask agitated, and the last c. c. or part of it, as the whole may not be necessary added.
If the two results agree, the next sample is treated in the same way, and so until all are tested.
A final trial may be made by measuring 50 c. c. of each solution into its flask, running in the permanganate in the ascertained amount into each as quickly as possible, letting the flasks stand ten minutes, and then making a comparison of all in the test-tubes. If the shades are not exactly alike, a pretty good guess can generally be made of the fractions of c. c. required, which should be added, the contents of the tubes being joined to that in the flasks, and a second or third comparison thus made.
This is a rather long description of what is in practice a very simple and good process, the three principal points to be borne in mind being—
1st. To use a weak solution of permanganate.
2nd. To have a very faint pink colour as a standard of comparison.
3rd. To let the liquids remain after agitation together ten or fifteen minutes before comparing them.
Uses, &c. Cochineal is principally used to prepare lake and carmine, and in dyeing. Its colouring principle is freely soluble in water. It imparts every variety of scarlet and crimson to textile fabrics previously prepared with482 alum, tin, and other mordants. It is also used to colour liqueurs, tinctures, and confectionery. It has been recommended as an antispasmodic and anodyne, in hooping-cough and neuralgia.—Dose, 10 to 60 gr., in powder, confection, or tincture. See Carmine and Carminic acid.
COCIN′IC ACID. Syn. Cocostear′ic acid. A crystalline, fatty acid, obtained by the saponification of COCOA-NUT OIL. See Stearic acid.
COCK-METAL. Syn. Pot Metal. Copper, 20 lbs.; lead, 8 lbs.; litharge, 1 oz.; antimony, 3 oz. Another variety consists of copper, lead, and sometimes a little zinc.
COCKROACH. See Blatta.
COD. Syn. Ga′dus Mor′rhua (Ph. L.), Mor′rhua vulgaris (Linn.), Asel′lus (Pliny), L. A fish common in the seas of the northern hemisphere, from about 40° to 75° of latitude. The flesh forms a most wholesome and excellent article of food. The best fish are very thick about the neck; and, when fresh, are marked by the redness of the gills, freshness of the eyes, and the whiteness and firmness of the flesh. The fish so largely imported from Newfoundland (Newfoundland fish) are cod beheaded, split open, gutted, and salted. They are caught by millions on the ‘Grand Bank.’ Cod-sounds are pickled in brine and also made into isinglass. The spawn is made into CAVIARE, and the liver is both pressed and boiled for its oil (see below).
Cod is generally cooked by boiling it, but is sometimes baked, or cut into slices and broiled or fried. Cod’s head and shoulders with oyster sauce is a favorite dish. Shrimp and anchovy sauce are also good additions.
COD-LIV′ER OIL. Syn. Mor′rhuæ o′leum, B. P.; O′leum jecor′is Asel′li, L.; Huile de Morue, Fr. The oil obtained from the liver of the common cod (oleum e jecore comparatum).
Prep. 1. The livers, being removed from the fish, are piled on layers of fir-twigs placed in tubs perforated at the bottom, and are allowed to remain for a considerable time exposed to the sun and air. As the livers putrefy, the oil runs out and flows through the holes in the tubs into vessels placed to receive it.
2. The partially decomposed livers, cut into pieces, are heated in iron pots without water, and the oil is poured off and set aside to deposit impurities.
3. (Savory.) The livers taken from the fresh fish are carefully washed. The large veins are then divided through their whole length, and any blood in them is carefully rinsed away. The livers are now cut into pieces, again washed and drained, and afterwards placed with a small quantity of water in vessels gently heated by steam. As the heat increases, the oil separates and rises to the surface, from which it is skimmed off; and after well cooling, to allow the deposit of some of the margarin, it is repeatedly filtered through flannel bags and finally through paper. This process gives a fine, clear, straw-coloured oil, having but a slight smell and taste.
4. (Donovan.) The perfectly fresh livers are placed in a metallic vessel and heated with constant stirring to 180° Fahr., by which treatment they break down into a uniform pulpy, liquid mass. This mass is immediately transferred to calico bags, whence the oil drains out; after filtration, while still warm, this oil is sufficiently pure for use.
Obs. Three kinds of cod-liver oil are usually distinguished—the pale yellow, pale brown, and dark brown. The latter is the most impure; its odour and taste are extremely disagreeable. The most conflicting opinions have been expressed by medical men as to the relative value of the light brown and yellow varieties. Ozonised cod-liver oil is said to be prepared by passing oxygen into the oil, and then exposing it to sunlight. Dr Letheby applied the most delicate tests to this much-vaunted remedy, but was not able to detect the slightest trace of ozone.
Prop. and Uses. Cod-liver oil has acquired much reputation for its remedial powers in pulmonary consumption, scrofulous and other glandular affections, chronic gout and rheumatism, certain skin diseases, and several other ailments. It is generally supposed that the iodine and bromine, which are present in minute quantities in this fish, are the substances to which it owes its efficacy. “Dr De Jongh refers its virtues to the presence of both iodine and the elements of the bile. Our own researches lead us to infer that one of its most active constituents is free phosphorus. Good cod-liver oil contains fully ·02 of this substance, as well as about ·09 of phosphoric acid. Now, the marked action of minute doses of phosphorus on the nervous, vascular, and secreting organs, is well known to every experienced surgeon. The difficulty, however, of bringing it into a form adapted for administration has hitherto prevented phosphorus being extensively employed as a therapeutic agent. This obstacle is removed by the employment of cod-liver oil. Nature has here provided a simple remedy, which the ingenuity of man has failed to produce artificially. This opinion is borne out by the facts, that cod-liver oil cures those forms of scrofula and other diseases which do not yield to iodine, and that those varieties of the oil are the most active which contain the most free phosphorus. We, therefore, think it reasonable to conclude, that the efficacy of cod-liver oil depends on the joint action of the minute quantities of iodine, phosphorus, and the elements of the bile which it contains, and not on any one separately; and that no substance, at present known, can be used as a substitute for it.” (Cooley.)—Dose, 1 to 8 dr., in water, syrup, or orange juice; or made into an emulsion with 1 fl. oz. of peppermint water.
483
M. Duquesnel states that cod-liver oil flavoured with essence of eucalyptol, in the proportion of one part of the essence to a thousand, has neither the taste nor the odour of cod-liver oil. It is taken with facility, only leaving at the back of the mouth and on the tongue the taste of the essence. M. Duquesnel adds that the offensive eructations arising from cod-liver oil are completely corrected.
Cod-liver Oil Jelly. Take of cod-liver oil, 85 parts; isinglass, 3 parts; sugar, 8 parts; water, 4 parts. It forms a semi-transparent jelly of a yellowish-green colour, having a strong odour, but less strong taste of the oil. The advantages of this preparation are—its easy administration, complete retention, and assimilation by the weakest stomach. A teaspoonful is said to be equal to a tablespoonful of the ordinary oil. A lemon flavour may be imparted to it with advantage if desired.
Cod-liver Oil and Lacto-Phosphate of Lime. (Shinn.) Cod-liver oil, 1 pint; oil of bitter almonds, peppermint, and winter green, of each 10 drops; powder of gum Arabic 4 oz.; sugar, 6 oz.; solution of lacto-phosphate of lime (60 gr. to 1 fl. oz.), 61⁄2 fl. oz.; lime water, 61⁄2 fl. oz. Mix the gum and sugar in a capacious mortar, and make a smooth mucilage with the lime water and 3 oz. of the solution of lacto-phosphate of lime. Add the volatile oils to the cod-liver oil, and gradually triturate them with the mucilage, until a perfect emulsion is formed. Finally, add the rest of the solution of the lacto-phosphate of lime, and mix thoroughly. The solution of lacto-phosphate of lime is made by saturating a solution of lactic acid with freshly precipitated phosphate of lime.
Cod-liver Oil with Iodide of Iron. Triturate iodide of iron, with cod-liver oil, 4 gr. to the ounce, until dissolved. Horsley’s patent is as follows:—Dissolve 22 scruples of iodine in a gallon of cod-liver oil, at a temperature of 140° Fahr., in a water-bath. Add to the solution 8 scruples of iron (reduced by hydrogen), and heat to 180° Fahr., until the combination is complete.—Dose, 1 dr. to 1⁄2 oz.
Cod-liver Oil, Phosphorated. (Lancet.) Pure unoxidised phosphorus, 2 gr.; almond oil, 2 oz. Put into a bottle, stoppered, and immerse the same in a water-bath; apply heat until the temperature of the oil is about 180° Fahr., as directed by the B. P., in the preparation of oleum phosphoratum; shake up occasionally, and again put the bottle into the water if necessary, until a perfect solution is obtained; then add about 10 oz. of cod-liver oil, and again immerse in the water-bath; finally, make up the measure with cod-liver oil to 25 oz. One drachm so prepared will contain over the 1⁄100th of a grain of pure phosphorus.
Cod-liver Oil, Emulsion of. Cod-liver oil, 8 fl. oz.; tragacanth, 1 dr.; powdered white sugar, 4 dr.; oil of gaultheria, 9 drops; oil sassafras, 1 drop; oil bitter almonds, 10 drops, water, 8 oz. Dissolve the tragacanth and sugar in water, and strain. Add to this first the essential oils, and then incorporate the cod-liver oil.
Cod-liver Oil and Hypophosphites, Emulsion of. (Canadian ‘Pharmaceutical Journal’). Powder of gum tragacanth, 1⁄2 oz.; glycerin, 3 oz.; water, 9 oz. Rub the tragacanth with the glycerin, and add the water gradually. To this mucilage add the following solution:—Hypophosphite of lime, 41⁄2 dr.; hypophosphite of soda, 21⁄4 dr.; hypophosphite of potash, 21⁄4 dr.; sugar, 3⁄4 lb.; boiling water, 12 oz. Make the admixture gradually with brisk trituration. To this medicated mucilage add the following:—Otto of almonds, bitter, 10 drops; otto of cinnamon, 5 drops; otto of canella, 5 drops; alcohol, 6 oz. The whole will now form a semi-transparent mucilaginous liquid of about 37 fl. oz. in bulk. To this add gradually an equal measure of cod-liver oil, and mix thoroughly. In practice it is advisable to work on small quantities, say half a pint of each in a No. 8 mortar. If care is taken the product will be very satisfactory.
CODE′IA. C18H21O3. Aq. Syn. Code′ine. An alkaloid discovered by Robiquet associated with morphia.
Prep. Dissolve commercial hydrochlorate of morphia in water, and precipitate the morphia with ammonia. Codeia is left in solution, and is obtained in octahedral crystals by spontaneous evaporation. It may be further purified by solution in ether. By the addition of a little water to the ethereal solution and spontaneous evaporation it may be obtained quite pure and in a crystalline state.
Obs. The morphia may be recovered by digesting the precipitate in weak solution of potassa.
Prop., &c. Freely soluble in alcohol and ether; soluble in 80 parts of cold and 17 parts of boiling water. Its solution in the latter, by slow evaporation, yields large, transparent octahedra. With the acids it forms crystallisable salts. These possess the singular property of producing a general and violent itching of the surface of the body when administered internally. The same symptoms frequently follow the exhibition of opium and hydrochlorate of morphia, and are referred to the presence of codeia. The commercial muriate of morphia frequently contains 3% to 4% of codeia.
Tests. It is distinguished from morphia by not becoming blue on the addition of perchloride of iron, nor turning red with nitric acid; and by not being precipitated by ammonia, when dissolved in hydrochloric acid and mixed with a large quantity of water. Unlike morphia, it is insoluble in weak solution of potash, and is soluble in ether. The salts of codeia are known by tincture of galls throwing down a copious precipitate from their solutions; this does not occur with the salts of484 morphia. It is distinguished from meconia by its aqueous solution showing an alkaline reaction with test-paper.
COFFEE. The seeds or berries of the Coffea arabica (Linn.) or coffee plant; a shrub of the natural order Cinchonaceæ, sub-order Coffeæ, indigenous in the low mountainous districts of Arabia Felix, and largely cultivated in various other parts of the world. About 40 millions of pounds of coffee are annually consumed in this country, and the consumption for the whole world has been estimated at about 600 millions of pounds. The seeds are roasted and ground, and used in the form of a decoction or infusion. The term coffee is applied to the prepared beverage as well as to the seeds. The valuable properties of coffee are mainly due to the presence of the alkaloid CAFFEIA or CAFFEINE.
Payen gives the following as the composition of the coffee-berry:—
| Water | 12·000 |
| Woody tissue | 34·000 |
| Fixed fatty matters | 10 to 13·000 |
| Gum, sugar, and vegetable acids | 15·500 |
| Nitrogenous matter allied to legumin (vegetable casein) | 13·000 |
| Free caffein | 0·800 |
| Compound of caffein with potash | 3.5 to 5·000 |
| Solid fatty essence | 0·002 |
| Aromatic essential oil | 0·001 |
| Saline matters | 6·697 |
| ———— | |
| 100·000 |
Prep., &c. The finest kind of coffee is that called mocha, from Aden, but that in common use is principally supplied from the British plantations in the West Indies. The selection being made, the berries are carefully roasted in revolving cylinders by a gradually applied heat, until the aroma is well developed and the toughness destroyed. Too much heat is avoided, as the volatile and aromatic properties of the coffee, and, consequently, the flavour, are thereby injured; whilst, on the other hand, if the berries are roasted too little, they produce a beverage with a raw, green taste, very liable to induce sickness and vomiting. When properly roasted, coffee has a lively chocolate-brown colour, and should not have lost more than 18% of its weight by the process. If the loss exceeds 20%, the flavour suffers in proportion. The roasted coffee should be placed in a very dry situation, and excluded from the air as soon as possible. It loses flavour by keeping, and also powerfully absorbs moisture from the atmosphere by reason of its hygrometric power.
Qual., &c. Coffee promotes digestion, and exhilarates the spirits, and when strong, generally occasions watchfulness, but in some phlegmatic constitutions induces sleep. Drunk in moderation, especially if combined with sugar and milk, it is perhaps the most wholesome beverage known. The various qualities that have been ascribed to it by some persons such as dispelling or causing flatulency, removing dizziness of the head, attenuating the blood, causing biliousness, &c., appear to be wholly imaginary. In a medical point of view it has been regarded as a cerebral stimulant and anti-soporific, and as a corrector of opium. As a medicine it should be strong, and is best taken only lukewarm.
Adult., &c. The principal substances used for the purposes of adulteration are caramel, roasted chicory, roasted locust beans, roasted corn, &c. Chicory being now charged with the same amount of duty as coffee, is not considered in a revenue point of view an adulteration; nevertheless, when we contrast coffee with chicory, we at once see the vast superiority of the former over the latter, thus:—
Coffee is the fruit of a tree, whilst chicory is the root of an herbaceous plant, and it is well known that more virtues exist in fruits and seeds than in roots.
Coffee contains three active principles, viz. an essential oil, caffeia, and tannic acid, and these exercise a powerful influence on the system, retarding the waste of the tissues of the body, exciting the brain to increased activity, and exhilarating without intoxicating. Chicory contains none of these constituents.
Coffee exerts on the system highly beneficial physiological effects; chicory possesses medicinal properties, which are not desirable in an article of food.
Chicory, therefore, is very objectionable, and when a dealer sells a mixture of coffee and chicory for pure coffee, as is almost invariably the case, he is guilty of selling an adulterated article, and ought to be punished accordingly.
The adulteration with caramel or chicory may readily be detected as follows:—
1. A spoonful of pure coffee placed gently on the surface of a glass of cold water will float for some time, and scarcely colour the liquid; if it contains caramel or chicory, it will rapidly absorb the water, and, sinking to the bottom of the glass, communicate a reddish-brown tint as it falls. Another method of applying this test is by expertly shaking a spoonful of the suspected coffee with a wine-glassful of cold water, and then placing the glass upon the table. If it is pure, it will rise to the surface, and scarcely colour the liquid; but if caramel or chicory is present, it will sink to the bottom, and the water will be tinged of a deep red as before.
2. The brown colour of decoction or infusion of roasted coffee becomes greenish when treated with a per-salt of iron; and a brownish-green, flocculent precipitate is formed. The colour of chicory is only deepened, but not otherwise altered, and no precipitate is formed, under the same treatment. A mixture of chicory and coffee retains a brownish-yellow485 colour after the precipitate has subsided, and the liquid appears brownish yellow by refracted light. The addition of a little weak ammonia water aids the subsidence of the precipitate.
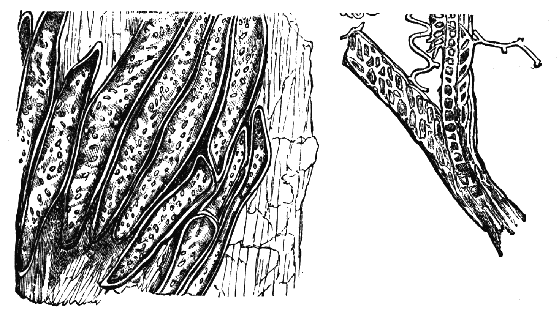
3. Under the microscope (see Chicory) the presence of chicory may be readily detected by the size, form, and ready separation of the cells of the cellular tissue, and by the presence and abundance of the pitted tissue or dotted ducts, which are absent from coffee, and by the size of the spiral vessels, which are very small in coffee. The most characteristic structure, however, and that by which chicory can be easily identified, is the lactiferous tissue. Roasted corn, and other amylaceous substances, may also be detected, in the same way, by the peculiar size and character of their starch grains.
Under the microscope the berry is seen to consist of a hard, tough tissue, that resists even long soaking. The testa covering the berry is made of lengthened cells with oblique markings resting on a thin membrane, almost structureless. These oblique markings of the cells are so characteristic as to render the cells distinguishable from every other tissue. The substance of the berry consists of angular cells, each one of which contains minute drops of oil. This oil is in some measure driven off during the process of roasting, which, however, leaves the structure unimpaired where it is not charred.
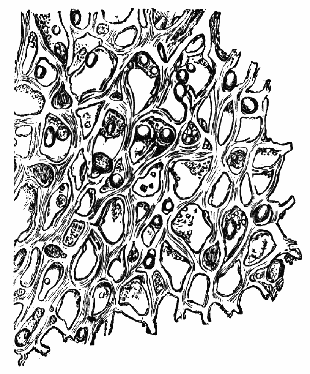
Roasted corn, beans, &c., may be detected by the cold decoction striking a blue colour with tincture of iodine. Pure coffee is merely deepened a little in colour by this substance.
4. (A. H. Allen.) The amount of ash in genuine coffee does not exceed 4·5 per cent.; chicory yields 5 per cent. The silica in coffee ash never exceeds 1 per cent., while in chicory it varies from 10-36 per cent. The average soluble ash in coffee is 3·24, while in chicory it is 1·74 per cent. By determining the soluble ash S, the per-centage of pure coffee C may be calculated thus:—
The density of coffee-infusion is determined by heating the powder with 10 times its weight of cold water, raising the liquid to the boiling point, filtering, and taking the gravity at 15·5° C. Taking the density of pure coffee-infusion at 1008·6, and that of chicory at 1020·6, the per-centage of pure coffee C in the sample may be calculated from the equation
where D represents the density of the infused sample. The relative tinctorial power of an infusion of a sample of coffee is determined by boiling a given weight with 20 c. c. of water for a few minutes, filtering, and again boiling the residue until thoroughly exhausted. An equal weight of a standard mixture of equal weights of pure coffee and chicory is treated in a precisely similar manner. The standard solution is486 made up to 200 c. c., that of the sample to 100 c. c.; 10 c. c. of the latter are put into a narrow burette, and some of the standard into a test tube of exactly equal bore. If the tints are exactly the same, the sample consisted of pure coffee; if chicory is present, water must be added to the sample until the tints are the same. Each c. c. of water represents 5 per cent. of chicory. The presence of leguminous seeds or cereals may be detected by boiling the sample with animal charcoal and water, filtering, and testing for starch in the cold liquid with iodine. Neither coffee nor chicory contains starch.
Obs. A few years ago the attention of the scientific world was drawn to the value of roasted coffee leaves, as furnishing materials for a beverage unexcelled in excellence by the coffee berry itself. It appears that the leaves, prepared for use, may be purchased for 11⁄2d. per lb., or packed ready for export at 2d. per lb. “That this preparation contains a considerable amount of the nutritious principles of coffee is evident from the analysis; but as the leaves can only be collected in a good state at the expense of the coffee-bush, it is doubtful whether the coffees produced by the berries be not, after all, the cheapest, as it certainly is the best.” (Jury Report, Exhibition, 1851.) Coffee for the table is best prepared with the aid of a French cafetière, or coffee biggin, furnished with a percolator or strainer, which will permit a moderately rapid filtration. To produce this beverage in perfection, it is necessary to employ the best materials in its preparation—fresh roasted and fresh ground. “At least 1 oz. of coffee should be used to make 3 common-sized coffee-cupfuls, with 1 teaspoonful of freshly roasted and ground chicory. If desired strong, the quantity of both should be doubled.” (Cooley.) Many habitual coffee drinkers cannot tolerate the use of chicory, which is a doubtful improver of coffee. The prevailing fault of the coffee made in England is its want of strength and flavour. The coffee-pot should be heated previously to putting in the coffee, which may be done by means of a little boiling water. The common practice of boiling coffee is quite unnecessary, for all its flavour and aroma is readily extracted by boiling hot water. Indeed, all the “useful and agreeable matter in coffee is very soluble, it comes off with the first waters of infusion, and needs no boiling.” (Ure.) Should prejudice, however, induce the housewife or cook to boil her coffee, it should be only just simmered for a minute, as long or violent boiling injures it considerably.
When coffee is prepared in a common pot, the latter being first made hot, the boiling water should be poured over the powder, and not, as is commonly the plan, put in first. It should then be kept stirred for 4 or 5 minutes, when a cup should be poured out and returned again, and this operation repeated 3 or 4 times, after which, if allowed to repose for a few minutes, it will generally become fine of itself. In all cases, where a percolator is not used, the liquor should be well stirred up several times before finally covering it up to settle for use.
Amongst the various descriptions of coffee pots in use we may mention those of French make, consisting of two cylindrical vessels, the upper having a metal strainer, on which the ground coffee is placed, and through which the clear infusion runs into the lower one; Loysell’s—an apparatus making very good coffee, and as one of the latest, an ingenious and inexpensive coffee pot, known as the “Kaffee Kanne,” devised by Mr Ash, of Oxford Street. Ash’s “Kaffee Kanne” consists of an ordinary biggin, surrounded by a jacket containing boiling water. The coffee is made by percolation in the inner vessel, and being kept at the point of ebullition by the surrounding boiling water, yields a beverage of excellent flavour and aroma.
Coffee is sometimes clarified by adding a shred of isinglass, a small piece of clean eel- or sole-skin, or a spoonful of white of egg. An excellent plan, common in France, is to place the vessel containing the made coffee upon the hearth, and to sprinkle over its surface half a cupful of cold water, which from its greater gravity descends, and carries the ‘foulness’ with it. Another plan sometimes adopted is to wrap a cloth, previously dipped into cold water, round the coffee-pot. This method is commonly practised by the Arabians in the neighbourhood of Yemen and Moka, and rapidly clarifies the liquor, unless a very large quantity of chicory is present. It should be recollected that the use of isinglass, white of egg, and all like artificial finings, remove much of the astringency and vivacity of the liquor.
The French, who are remarkable for the superior quality of their coffee, generally allow an ounce to each large coffee-cupful of water, and they use the coffee both newly ground and freshly roasted. A shred of saffron, or a little vanilla, is frequently added, whilst the percolating coffee-pot is generally employed. When the Parisian uses a common coffee-pot he generally divides the water into 2 parts. The first portion he pours on boiling hot, and allows it to infuse for 4 or 5 minutes; he then pours this off as clear as possible, and boils the grounds for 2 or 3 minutes with the remaining half of the water. As soon as this has deposited the sediment it is decanted, and mixed with the infusion. The object of this process is to obtain the whole of the strength as well as the flavour. The infusion is thought to contain the latter, and the decoction the former; a plausible, but erroneous idea, since both of them were carried off by the first water.
A much better method, and one we can recommend from experience, is to divide the coffee into 2 parts. Boil the first portion in the coffee-pot for 4 or 5 minutes, then add the other portion, and allow it to infuse slowly487 for about 10 minutes, the coffee-pot lid being kept well closed. This gives a coffee possessing a flavour which even the French cannot excel.
Coffee, Essence of. A highly concentrated infusion of coffee, prepared by percolation with boiling water, gently and quickly evaporated to about 1⁄3rd or 1⁄4th of its bulk, and mixed with a thick aqueous extract of chicory and syrup of burnt sugar, so as to give the whole the consistence of treacle. The proportions of the dry ingredients should be—coffee, 4 parts; chicory, 2 parts; burnt sugar (caramel) 1 part. It should be kept in well-corked bottles in a cool place. This preparation is very convenient for making extemporaneous coffee; but the beverage so made, though superior to much of that sold at coffee-houses, is inferior in flavour, aroma, and piquancy, to that we are accustomed to drink at home. Much of the so-called ‘Essence of Coffee’ is simply treacle and burnt sugar, flavoured with coffee.
Coffee, Searle’s Patent. This is prepared by mixing condensed milk with a very concentrated essence of coffee and evaporating at a low temperature (in vacuo, if possible), until the mixture acquires the consistence of a syrup (coffee syrup), paste (coffee paste), or candy (coffee candy). The last may be powdered (coffee powder, dry essence of coffee).
Coffee, Substitutes for. These are numerous, but are now seldom employed, owing to the cheapness of the genuine article, and the stringency of the revenue laws. Among the principal are the following:—
1. Coffee, Acorn. From acorns deprived of their shells, husked, dried, and roasted.
2. Coffee, Bean. Horse-beans roasted along with a little honey or sugar.
3. Coffee, Beet-root. From the yellow beet-root, sliced, dried in a kiln or oven, and ground with a little coffee.
4. Coffee, Dandelion. From dandelion roots, sliced, dried, roasted, and ground with a little caramel.
5. Coffee, German. Syn. Succory c., Chicory c. From chicory or succory. Used both for foreign coffee, and to adulterate it.
Obs. All the above are roasted, before grinding them, with a little fat or lard. Those which are larger than coffee-berries are cut into small slices before being roasted. They possess none of the exhilarating properties or medicinal virtues of foreign coffee.
COINS. See Medals and Electrotype.
COKE. Charred or carbonised coal. The principle of its manufacture is similar to that of charcoal. There are three varieties of coke:—
1. Kiln-made coke; STIFLED COKE. Made by burning pit-coal in a pile, kiln, or stove. It has a dull-black colour, and produces an intense heat when used as fuel. By condensing the bituminous vapours which are given off during the process, about 3% of tar may be obtained from common coal, and from some strong coal, by careful treatment fully 10% of its weight. The screenings of dust coal, separated from the better kinds of bituminous coal, is the sort commonly used for making coke in ovens.
2. Gas coke; DISTILLED COKE. The cinder left in the gas retorts. Grey; produces a weak heat, insufficient to smelt iron.
3. Shale Coke; MINERAL CARBON. From bituminous shale, burnt in covered iron pots, in a similar way to that adopted for making bone-black; or in piles. Black and friable. Used to clarify liquids, but is vastly inferior to bone-black, and does not abstract the lime from syrups. See Fuel, Pit-coal, &c.
COLCHICIN′A. Syn. Col′chicine. Colchicia. A peculiar principle discovered by Gieger and Hesse in the seeds of the Colchicum autumnale or common meadow saffron. It also exists in the corms or bulbs.
Prep. Macerate the bruised seeds in boiling alcohol, add magnesia, to throw down the alkaloid, digest the precipitate in boiling alcohol, and filter. By cautious evaporation colchicine will be deposited, and maybe purified by re-solution and crystallisation in alcohol.
Prop., &c. Odourless; bitter; soluble in water and alcohol; form salts with the acids. It is very poisonous. 1⁄10th of a grain, dissolved in spirit, killed a cat in 12 hours. It differs from veratria in being soluble in water and crystalline, and in the non-production of sneezing when cautiously applied to the nose. Strong oil of vitriol turns this alkaloid of a yellowish-brown; nitric acid turns it of a deep violet, passing into indigo-blue, green and yellow. It is not used in medicine.
COL′CHICUM. Syn. Mea′dow saf′fron; Colchicum autumnale (Linn.), L. The recent and dried corms or bulbs (colchici cormus), as well as the seeds (colchici semina), are official in the British Pharmacopœia. The corms are ordered to be dug up in the month of July, or before the autumnal bud has projected. The dry coatings having been torn off, cut the corms transversely in thin slices, and dry, at first with a gentle heat, but afterwards slowly increased to 150° Fahr.
Dose (of the corms), 2 to 8 or 9 gr.; (of the seeds), 2 to 7 gr., made into a pill or bolus with syrup or conserve; chiefly, as a specific in gout, to alleviate or check the paroxysm. This drug forms the base of almost all the advertised gout nostrums. It is, however, an active poison, and its administration requires care. “After all that has been said respecting colchicum in gout, and admitting that it rarely fails to allay pain and check a paroxysm, I would record my opinion that he who would wish to arrive at a good old age should eschew it as an ordinary remedy, and consider that he is drawing on his constitution for a temporary relief, with a certainty of becoming prematurely bankrupt in his vital energies.” (Collier.)
Antidotes. An emetic consisting of one488 scruple of sulphate of zinc dissolved in water, followed by a brisk dose of castor oil, then stimulants, and also charcoal.
COL′COTHAR. See Oxides of Iron.
COLD. Syn. Fri′gus, L. The privation of heat. The term is also applied to the sensation and effects which this privation produces.
When the body of an animal is immersed in an atmosphere at a temperature below the healthy standard, a sensation of coldness is experienced, produced by the passage of the caloric or heat of the body into the colder medium. If this extraction of caloric exceeds the quantity produced by the vital system, the temperature of the body decreases, until it sinks below the point at which the functions of life can be performed. This declination of the heat of the body is gradual; the extreme sensation of coldness changes into a disinclination for voluntary motion; next comes on drowsiness, followed by numbness and insensibility. At this point if the sufferer is not rescued, and remedial measures had recourse to, death inevitably and rapidly ensues.
The prevention of the effects of cold consists in the use of ample food and clothing proportioned to the inclemency of the weather, the exposure to be endured, and the habits of the wearer. The circulation of the blood should be promoted by active exercise, and any disposition to sleep shaken off by increased bodily exertion. The principal endeavour should be to keep the extremities and chest warm, as, if this can be accomplished, no danger need be feared.
In cases of asphyxia produced by intense cold, the patient should be laid in a room remote from the fire, and bathed with cold salt-and-water, or water to which some brandy or vinegar has been added; after which the body should be wiped dry, and friction assiduously applied by the hands of the attendants (warmed); as many operating at once as can conveniently do so. Gentle stimulants should be administered by the mouth, and the bowels excited by some mild, stimulating clyster. The lungs should also be inflated, and an effort made to re-establish the respiration. As soon as symptoms of returning animation are evinced, and the breathing and circulation restored, the patient should be laid in a bed between blankets, and a little wine-and-water administered, and perspiration promoted by heaping an ample quantity of clothing on the bed. Should the patient have suffered from hunger as well as cold, the appetite may be appeased by the administration of a limited quantity of light food, taking especial care to avoid excess, or anything indigestible or exciting, &c. See Asphyxia, Bronchitis, Catarrh, &c.
COLD CREAM. A snow-white, bland ointment, about the consistence of good lard, and an admirable substitute for that excipient where expense is no object, especially for applications about the face. It is commonly sold as a lip-salve and as a healing application to abraded and chapped surfaces generally. The ordinary receipts are given under the head of Cosmetic Cerate (which see). The following produces a superior article.
Prep. (Dr L. Turnbull.) From white wax, 1 oz.; oil of almonds, 4 oz.; rose-water, 2 oz.; borax, 1⁄2 dr.; oil of roses, 5 drops. Melt, and dissolve the wax in the oil of almonds by a gentle heat; dissolve the borax in the rose-water, which is then to be warmed a little and added to the heated oil; lastly, add the oil of roses, stirred.
COL′IC. Syn. Col′ica, L. The belly-ache or gripes. The name is popularly given to all severe griping abdominal pains, without reference to the cause. There are several varieties of this disease, as noticed below.
Colic, Accident′al. Produced by improper food, and poisons. The treatment may be similar to that recommended for bilious or flatulent colic.
Colic, Bil′ious. In this variety the pain is intermittent and transient, accompanied by constipation, nausea, and vomiting. The fæces, if any, are bilious, dark-coloured, and offensive. The common remedies are, a full dose of blue pill, calomel, colocynth, or aloes, followed by a sufficient quantity of Epsom salts or Glauber’s salts. Warm fomentations are also serviceable.
Colic, Flat′ulent. Marked by constipation, and the irregular distension of the bowels by gas, accompanied by a rumbling noise, &c. It is commonly produced by the use of indigestible vegetables and slops. The remedies are, a full dose of tincture of rhubarb combined with a few drops of essence of peppermint. If this does not afford relief, an Abernethy pill may be taken, washed down with a glass of any cordial water, as peppermint, cinnamon, or caraway. When the pain is extreme, warm fomentations to the belly, or a carminative clyster, will generally give relief. The Editor has found castor oil and Collis Browne’s chlorodyne of great benefit in this complaint.
Colic, Paint′er’s. Syn. Plumb′er’s colic, Devonshire c., Lead c.; Col′ica picto′num, L. The dry belly-ache. It is marked by obstinate costiveness, acrid bilious vomitings, violent pains about the region of the navel, convulsive spasms in the intestines, and a tendency to paralysis in the extremities. It is most prevalent in the cider counties, and amongst persons exposed to the fumes of lead. The remedies are the same as for the spasmodic variety. Should these fail, after the bowels have been thoroughly evacuated, small doses of camphor and opium may be administered, and sulphuric beer or sweetened water very slightly acidulated with sulphuric acid, had recourse to as a beverage. Mr Benson, the managing director of the British White-lead Works at Birmingham, says:—“Although during several weeks after the addition of the sulphuric acid to the treacle beer, drank at the works, little advantage seemed to be derived,489 yet the cases of lead colic became gradually less frequent, and since October of that year, or during a period of fifteen months, not a single case of lead colic has occurred amongst the people.” (‘Lancet.’) See Beer, Sulphuric Acid, and Sulphuric Acid.
Colic, Spasmod′ic. Marked by a fluctuating pain about the navel, which goes away and returns by starts, often leaving the patient for some time. The belly is usually soft, and the intestines may often be felt in lumps, which move about under the hand, or are wholly absent for a time. It is unaccompanied by flatulency. The remedies are warm fomentations, warm clysters, and carminatives, accompanied by small doses of camphor and opium.
Colic, Stercora′ceous. Marked by severe griping pains and constipation of the bowels. The remedies are powerful cathartics, as full doses of calomel, aloes, colocynth, jalap, &c., followed by purgative salts, as sulphate of magnesia, or sulphate of soda.
Colic in Horses. First give the horse a sharp trot, and apply friction over the belly, and follow this up with a drench of warm gruel to which has been added a glass or two of whisky or gin. Should these fail to give relief, let 4 dr. of aloes be rubbed down in a pint of hot water, and when the mixture becomes cool enough, add from thirty to sixty drops of strong solution of ammonia. Where the spasms and distress continue severe, and with little intermission, the physic may be followed in an hour with 2 dr. of tincture of aconite, given with an ounce of spirit of chloroform, in a little water, and repeated every hour; soap and water clysters should be administered every half hour, and friction and hot fomentations applied to the abdomen.
COLLO′DION. Syn. Collo′dium, L., B. P. A viscid fluid formed by dissolving pyroxylin (Schönbein’s gun-cotton) in a mixture of ether and alcohol. In surgery it is used in its natural state, and combined with certain elastic and medicinal substances. In photography it is used in combination with agents that render it sensitive to the action of light.
Collodion. Syn. Plain collodion. The following are the best methods of preparing plain collodion for surgical purposes:—
Prep. 1. (Ph. U. S.) Nitrate of potassa, in powder, 10 oz.; sulphuric acid, 81⁄2 fl. oz.; triturate together in a wedgwood mortar until uniformly mixed; then add of fine carded cotton (free from impurities), 1⁄2 oz.; and by means of the pestle or a glass rod, saturate it thoroughly with the liquor for a period of about 3 or 4 minutes; next transfer the cotton to a vessel containing water, and wash it in successive portions of pure water, with agitation and pressure, until the washings cease to affect litmus paper or a solution of chloride of barium; it is then to be spread out and dried by a very gentle heat, and dissolved by agitation in a stoppered bottle with rectified sulphuric ether, 1 quart, to which rectified spirit (alcohol), 1 fl. oz., has been previously added.
2. (Mialhe.) Nitrate of potassa, 40 parts; concentrated sulphuric acid, 60 parts; carded cotton, 2 parts; proceed as last until the dry cotton is obtained, then take of the prepared cotton, 8 parts; rectified sulphuric ether, 125 parts; mix in a well-stoppered bottle, and agitate it for some minutes; then add gradually, rectified alcohol, 1 part; and continue to shake until the whole of the liquid acquires a syrupy consistency. It may be now passed through a cloth; but a better way to prevent loss is to let it repose for a few days, and then decant the clear portion.
3. (Lauras.) This process only differs from No. 2 in the following particulars:—The cotton is immersed for 12 minutes, then rinsed 2 or 3 times in cold water, and afterwards immersed in a solution of carbonate of potassa, 4 parts, and water, 200 parts. Lastly, it is plunged again into simple water, and dried at a temperature of 77° to 86° Fahr.
4. (B. P.) Pyroxylin, 1 part; rectified spirit, 12 parts; ether, 36 parts; mix the ether and spirit, and add the pyroxylin. Keep in a well-corked bottle.
5. (Parrish.) Thoroughly saturate clean carded cotton, 1⁄2 oz., with fuming nitric acid and sulphuric acid, of each 4 fl. oz., previously mixed and allowed to become cool; macerate for 12 hours; wash the cotton in a large quantity of water; then free it from the water by successive washings in alcohol, and dissolve in ether, 3 pints.
Obs. For success in the manufacture of collodion it is absolutely necessary to avoid the presence of water. The ordinary commercial oil of vitriol, sp. gr. 1·84, may be used. Professor Procter, of Philadelphia, gives preference to the process with the mixed acids (No. 5), and directs that the cotton should be allowed to macerate for four days. In drying the cotton great care should be taken to prevent an explosion.
Uses, &c. In surgery plain collodion is employed as a dressing for wounds, and as a protection to abraded surfaces. On drying, it unites the former closely, and preserves the latter from the action of the air. It is impervious to water, and being transparent, it admits of the progress of the wound being inspected when necessary. Such is its adhesive power, that a piece of cloth cemented with it to the dry palm of the hand will support a weight of 25 to 30 lbs. The parts to which it is applied should be freed from moisture. See Collodions, Coloured, Elastic, Medicated and Vesicating (below).
Collodion, Blis′tering. See Vesicating Collodion.
Collodion, Col′oured. Syn. Collodium tinctum, L. Prep. (Cutan. Hosp.) Collodion, 2 oz.; palm oil, 1 dr.; alkanet root, q. s. to colour (say 15 gr.); digest and decant the clear. Colour bears a greater resemblance to490 the skin than that of common collodion, whilst it is more flexible; but it is weaker than the latter.
Collodion, Elas′tic. Prep. 1. (Lauras.) Heat together Venice turpentine, 2 parts; castor oil, 2 parts; and white wax, 2 parts; add sulphuric ether, 6 parts; and mix all with the product of No. 3 (above), that is, to the collodion formed with 8 parts of prepared cotton, 125 ether, and 8 alcohol.
2. (C. S. Rand.) Dissolve prepared cotton (No. 5, above), 2 dr., in sulphuric ether, 5 fl. oz.; then add, Venice turpentine, 2 dr., and complete the solution by slight agitation.
Obs. The collodion made by either of the above processes, when applied to the skin, forms a transparent pellicle, more pliable and more difficult to remove than that of ordinary collodion.
Collodion, Flexible. Collodion Flexile. (B. P.) Mix collodion (B. P.), 6 fl. oz., with Canada balsam, 120 gr.; and castor oil, 1 fl. dr., and keep in a well-corked bottle.
Collodion, Hemostatic. Collodion, 10 parts; carbolic acid, 1 part; tannic acid, 1⁄2 part; benzoic acid, 1⁄2 part; all by weight. To be applied with a pencil brush.
Collodion, Iodised. This may be made at one operation; it should be kept two days before being used, but is less reliable if kept for any length of time than the sensitised collodion described below. It is made as follows:—Place 16 grains of gun-cotton in a bottle, add 18 grains of iodide of cadmium in powder, 6 grains of bromide of cadmium in powder, and 11⁄2 oz. of spirits of wine (sp. gr. 0·805). Shake the bottle until the iodide and bromide are dissolved, then add 3 oz. of ether, sp. gr. 0·720, and shake until the cotton is dissolved. After settling for twenty-four hours, decant the clear portion into small well-stoppered bottles.
Collodion, Med′icated. It has been proposed to medicate collodion in several ways, but the practice has not found much favour with the medical profession. The following preparations have been described:—
Collodion, Aconite. From aconite root, by a similar formula to that of Belladonna c. (below).
Collodion, Belladonna. Prep. Macerate select belladonna leaves, powdered, 8 oz., in ether, 12 fl. oz., with alcohol (95%), 4 fl. oz., for six hours. Pack in a percolator, and pour en alcohol till a pint of tincture is obtained; in this dissolve pyroxylin (gun-cotton), 1 dr., and Canada balsam, 1⁄2 oz. Used as a substitute for BELLADONNA PLASTER.
Collodion, Cantharidin. See Collodion, Vesicating.
Collodion, Iodine. Prep. Dissolve iodine and Canada balsam, of each 1⁄2 oz., in collodion, 1 pint. Used as a substitute for IODINE OINTMENT.
Collodion, Morphia. (L′Union Medicale.) Dissolve 1 part of hydrochlorate of morphia in 30 parts of flexible collodion, and apply with a camel-hair brush.
Collodion, Photograph′ic. 1. There are so many methods adopted for preparing photographic collodion, that a large volume might be filled with notices of them. We have retained Mr Hardwich’s forms, which were formerly much esteemed by practical photographers, and appended to them modern formulæ which are now, we believe, in much greater demand, and for which we are indebted to Mr Ernest Spon’s valuable book ‘Workshop Receipts.’
2. Pyroxylin, and iodide of cadmium, or ammonium, of each 15 gr.; ether, 31⁄2 oz.; alcohol, 11⁄2 oz. Place the two first in a dry bottle, then pour on the spirits of wine, shake the mixture well, then add the ether, shake again and let it stand for 12 hours. Decant the clear portion into a wide-mouthed bottle, keep well stoppered, and in the dark. Avoid shaking the bottle when about to use the collodion, and never quite use all the bottle contains, as the sediment which will accumulate at the bottom would spoil the picture. The preparation of a sensitive collodion, whether positive or negative, includes three distinct operations, namely, the formation of the pyroxylin or gun-cotton, the conversion of this into plain collodion, and the final process of iodising the collodion.
Collodion, Plain. Mix in a bottle gun-cotton, 450 gr.; ether, 25 oz.; spirits of wine, 7 oz. Shake these well together, and leave to settle several days. Keep well corked.
Collodion, Pos′itive. (Hardwich.) To form the PYROXYLIN:—Take sulphuric acid, sp. gr. 1·845, at 60° Fahr., 12 fl. oz.; nitric acid, sp. gr. 1·45, at 60°, 12 fl. oz.; water, 31⁄2 fl. oz.; mix, and allow the temperature to fall to 140°; then immerse cotton, 300 grains. (If the cotton is found to gelatinise or dissolve in the acid mixture, the quantity of water is too great, and may be reduced to 3 fl. oz.) The cotton should be well pulled out in pieces, weighing about 30 grains each; and should be left in the acid for about 8 minutes, the vessel being covered over. It is taken out with a glass spatula, squeezed to remove acid, washed for at least 24 hours by a stream of water, then squeezed in a cloth, and pulled out to dry. To form the PLAIN COLLODION:—Shake up the dry pyroxylin, 48 grains, with alcohol, sp. gr. ·805, 11⁄2 fl. oz., and then add ether, sp. gr. ·725, 41⁄2 fl. oz. The solution should be allowed to rest for a week or ten days, when the clear fluid should be decanted from the sediment. To prepare the IODISING SOLUTION:—Take of iodide of ammonium, 11⁄2 dr.; iodide of cadmium, 11⁄2 dr.; bromide of ammonium, 40 grains; powder, and dissolve in alcohol, sp. gr. ·805 to ·816, 10 fl. oz. The collodion is iodised by adding the solution to it in the proportion of 1 part solution to 3 parts collodion. The iodised collodion should be kept for at least six weeks before using. If required491 for immediate use, add a few drops of an alcoholic solution of iodine, formed by dissolving 5 grains of iodine in 1 fl. oz. of alcohol.
Obs. Mr Hardwich recommends that the cotton, before being converted into pyroxylin, should be cleansed by boiling for two hours in a solution of caustic potassa (2 oz. to the gallon), and by being afterwards repeatedly washed and dried. The purest nitric acid, sp. gr. 1·45, should be employed, but the ordinary commercial sulphuric acid (oil of vitriol) is sufficiently pure for use. To purify the ETHER and to get rid of a certain ozonised principle which would decompose the iodising solution, Mr Hardwich recommends the following process:—Take the best washed ether of commerce and agitate it thoroughly with a small portion of dilute sulphuric acid, and then introduce it into a retort, and distil over one third. The alcohol used is of the strength of that sold for absolute alcohol; it should be pure.
Collodion, Neg′ative. (Hardwich.) To form the PYROXYLIN:—Take of sulphuric acid, sp. gr. 1·845, at 60°, 18 fl. oz.; nitric acid, sp. gr. 1·475, at 60°, 6 fl. oz.; water, 51⁄4 fl. oz.; cotton, 300 grains. Mix, and allow the temperature to fall to 150° Fahr. The weight of the pyroxylin ought to be 375 grains. To form the PLAIN COLLODION:—Take alcohol, sp. gr. ·806, 1⁄2 gallon; ether, sp. gr. ·725, 1 gall.; pyroxylin, 1900 grains. Saturate the pyroxylin with the alcohol, then pour in half a gallon of the ether, agitate for 3 or 4 minutes, and repeat the process in adding the remainder. Decant the clear liquid from the sediment after a week or ten days’ rest. The following forms for IODISING SOLUTIONS are recommended:—a. (Potassium Iodiser.) Iodide of potassium, 135 grains; alcohol, sp. gr. ·816, 10 fl. oz. Powder and dissolve in the alcohol, previously heated to 140°.—b. (Cadmium Iodiser.) Iodide of cadmium, 170 grains; alcohol, sp. gr. ·816, 10 fl. oz. Dissolve in the cold, and filter.—c. (Bromo-iodiser.) Bromide of ammonium, 40 grains; iodide of ammonium, 90 grains; iodide of cadmium, 90 grains; alcohol, sp. gr. ·816, 10 fl. oz. Pulverise and dissolve in the cold. To sensitise the collodion, add to three parts one part of either a, b, or c.
Obs. Most of the practical directions given under the head of POSITIVE COLLODION apply equally to NEGATIVE COLLODION. Nothing but patient and intelligent practice will ever lead to success in preparing collodion for photographic purposes. Although formulæ of undoubted excellence may be used, it continually happens that the results are entirely nugatory from some trifling cause. See Photography.
Collodion, Sensitised. Add to 1 oz. of the plain collodion 6 drams of spirits of wine; 13⁄4 oz. of ether; and 3 drams of iodide and bromide solution (see below). Shake the bottle well; the mixture is then ready, but is improved by being kept four or five hours before using. In hot weather a little more alcohol and less ether; in very cold weather more ether and less alcohol must be used. As sensitised collodion does not keep well, it is better not to mix the plain collodion and the iodide and bromide solution until shortly before required for use.
Iodide and Bromide Solution. Iodide of cadmium 154 grains; bromide of cadmium 54 grains; spirits of wine, 31⁄2 ounces. Pound the iodide and bromide very fine in a mortar, adding the spirit gradually; when the iodide and bromide are dissolved, pass the solution through a filter paper into a bottle. Must be kept in a closely-stoppered bottle.
Collodion, Styptic. Syn. Styptic colloid. (Dr Richardson.) To a saturated solution of tannic acid in alcohol and ether, in equal parts, add as much pyroxylin as the liquid will dissolve.
Collodion, Ves′icating. Syn. Blis′tering collodion, Canthar′idin c.; Collo′dium ves′icans, L. Prep. 1. (Tichborne.) Coarsely powdered cantharides, 6 oz., are placed loosely in a displacement apparatus (provided with a tap to regulate the flow), and treated with ether from methylated spirit, 13 fl. oz., and glacial acetic acid, 2 fl. oz., previously mixed together. After the fluid has passed through, it will be found that the débris has retained by absorption 7 fl. oz., which must be displaced by the gradual addition of methylated spirits of wine, 7 fl. oz. If properly managed, there is not the least danger of the admixture of the spirits with the percolated menstruum, as the animal substance of the flies swells considerably under the prolonged influence of the spirits of wine, so that the same bulk will be insufficient to quite displace the ether. The ethereal solution should be made to measure exactly 15 fl. oz. with a little spirit, and may then be converted into a collodion by the addition of pyroxylin, 1⁄2 oz.
Obs. The glacial acid plays a double part in this preparation. It dissolves the cantharidin, and at the same time gives to the collodion film the essential property of porosity. Ordinary collodion is useless as an excipient, for it produces a tough and contractile film, which really screens the skin from the action of the greater part of the blistering material.
2. (Ilisch.) Cantharidin, 15 gr.; pyroxylin, 20 gr.; rectified ether, 11⁄2 oz.; acetic ether, 1⁄2 oz.; dissolve.
3. (Œttinger.) Ether of cantharides and collodion, equal parts.
Use. Vesicating collodion is used as an irritant. No. 1 was introduced in 1862, and has many advantages over the other two. Mr Tichborne thus described the most effectual method of using it in the ‘Pharm. Journ,’:—“The part upon which the blister is to be raised should be painted with the vesicant492 to the desired extent, bearing in mind that the blister produced always extends to about one tenth of an inch beyond the margin of the space covered. Care should be taken to give a coating of considerable thickness, and to ensure this result the brush should be passed over and over again, until about 1⁄2 dr. has been used to the square inch, or less when operating upon a tender epidermis. It is desirable to place over the intended blister a piece of oil silk, or, what is still better, a piece of sheet gutta percha, somewhat larger than the surface painted, as this will stop the exhalations of the skin, and so render it moist and permeable. In ten minutes, or a quarter of an hour if the cuticle is hard, the collodion should be wiped off with a little cotton-wool moistened with ether, when the blister will almost instantly rise.”
COL′LOID. See Dialysis.
COLLYR′IUM. [L.] In medicine and pharmacy, a topical remedy for diseases of the eye. Formerly the term collyrium was applied to any medicament employed to restrain defluxions.
Collyr′ium, Dry. Syn. Eye powder; Collyr′ium sic′cum, L. Prep. 1. (Dupuytren.) White sugar, 1 dr.; red oxide of mercury, 10 gr.; oxide of zinc, 20 gr.; mix.
2. (Lagneau.) Sugar candy, 2 parts; nitrate of potassa, 1 part.
3. (Falconer.) Chloride of barium, 1 gr.; sugar candy, 1 dr.
4. (Radius.) Calomel and white sugar, of each 1⁄2 dr.; opium, 10 gr.
5. (Recamier.) Oxide of zinc and sugar candy, equal parts.
6. (Velpeau.) Trisnitrate of bismuth and sugar candy, equal parts.
7. (Wiseman.) Acetate of soda, 10 gr.; powdered opium, 1 gr.; sugar candy, 1⁄2 dr.
Obs. It is absolutely necessary that the ingredients in the above preparations should be reduced to an impalpable powder by careful trituration in a wedgwood mortar. For use, a small pinch is placed in a quill or straw, and blown into the eye previously opened with the fingers. On the whole, they may be regarded as unnecessary preparations, and are unsafe, except in skilful hands.
Collyrium, Liq′uid. See Waters (Eye).
Collyrium, Unct′uous. See Ointments (Eye).
COL′OCYNTH (sĭnth). Syn. Colocynth pulp., Colocynthidis pulpa, B. P. Bit′ter ap′ple, Bitter gourd, Bitter cu′cumber, Peeled colocynth; Coloquint′ida, Colocynth′is (B. P.), L. The decorticated fruit or pulp of the Citrellus Colocynthis (Schrad.—Ph. L.), or Cucumis Colocynthis (Linn.—Ph. E. & D.). It is an acrid, drastic purge and hydragogue, and cannot be given alone with safety; but, in combination with other substances, it forms some of our most useful cathartic medicines.
COLOCYNTH′IN. Syn. Colocynth′ium, L. The bitter, purgative principle of colocynth.
COL′OPHENE. Formed by distilling oil of turpentine with concentrated sulphuric acid. A colourless, viscid, oily liquid; with a high boiling-point; and exhibiting a bluish tint by reflected light.
COL′OPHONY. See Resin.
COLORADO BEETLE. Syn. Doryphera decemlineata. The Colorado potato beetle belongs to the family Chrysomelidæ, and is a native of the eastern slopes of the Rocky Mountains. It measures nearly half an inch in length, and has a tawny or yellowish cream-coloured body, darkly spotted; with wing cases which are marked with ten black longitudinal stripes. It has been gradually migrating eastward toward the more cultivated lands of the Northern states, until it has reached the Atlantic coast. It is now found over all the central and northern parts of the United States east of the Rocky Mountains, as well as throughout Canada, on the potato crops of all of which regions it has committed incalculable ravages. The leaves and stalks are the parts of the potato plant principally attacked; the depredators being, for the most part, the larvæ, of which three broods are said to be produced annually.
In America, we believe, the only means of destroying these insects as well as their eggs and larvæ consists in the application to the plant of the highly poisonous and dangerous pigment, Scheele’s green, a hydrated arsenite of copper. M. Girard recommends in preference to the arsenical salt a liberal use of sulpho-carbonate of potash.
COLOUR BLINDNESS. Syn. Dal′tonism. A curious defect of vision, from which the eye is incapable of distinguishing colours. It is of three kinds:—1. An inability to distinguish any colour properly so called, the person being only able to distinguish white and black, light and shade. 2. An inability to distinguish between the primary colours, red, blue, and yellow, or between these and the secondary or tertiary hues, such as green, purple, orange, and brown. 3. An inability to distinguish nicer shades and hues, as greys and neutral tints. The first form is rare; the second and third are common. Dr George Wilson found that of 1154 persons examined by him in Edinburgh, 65, or 1 in 177, were colour blind; of these, 21 confounded red with green, 19 brown with green, and 25 blue with green.
COL′OURING. Syn. Brandy colouring, Brewer’s c., Spirit c. Car′amel; Essen′tia bi′na, L. Prep. Brown sugar is melted in an iron vessel over the fire until it grows black and bitter, stirring it well all the time, after which water is added, and it is boiled to a syrup. In the making of brandy colouring white sugar is more frequently used.
Obs. Some persons use lime-water to dissolve the burnt sugar. Care must be taken not to overburn it, as a greater quantity is493 thereby rendered insoluble. The heat should not exceed 430°, nor be less than about 400° Fahr. The process, for nice experiments, is best conducted in a bath of melted tin, to which a little bismuth has been added to reduce its melting-point to about 435°; a little powdered resin or charcoal or a little oil being put upon the surface of the metal, to prevent the oxidisement of the alloy. See Caramel.
COL′OURS. White light from the sun is of a compound nature, and may be decomposed into rays of different colours. Newton distinguished seven PRIMITIVE COLOURS, namely, violet, indigo, blue, green, yellow, orange, and red. Sir D. Brewster is disposed to think that four of these colours are really compound, and that three, namely, blue, yellow, and red, alone deserve the name of primitive. The colours of natural objects are supposed to result from the power possessed by their surfaces of absorbing some of the coloured rays of light, while they reflect or transmit, as the case may be, the remainder of the rays. Thus, an object appears red because it absorbs or causes to disappear the yellow and blue rays composing the white light by which it is illuminated. Black and white are not colours, strictly speaking.
A body is said to be black when it absorbs or quenches a large proportion of all the rays of white light falling upon it. A body is said to be white when it receives the white light, and reflects all the rays with moderate strength. Grey may be regarded as a luminous black or dark white. The names given to colours are far from being satisfactory, for although many thousand shades may be distinguished by a practised eye, it is a question whether there are fifty names which would convey the same idea of shade to any ten colourists in the world. The names taken from natural coloured objects, as indigo, violet, orange, lilac, amber, emerald, &c., are the least objectionable. M. Chevreul has devised an ingenious system of naming and classifying colours. He employs only 6 fundamental names, which are those of the three elementary colours, red, yellow, and blue; and of the three secondary colours, orange, green, and violet. By the direct union of the elementary and secondary colours, 6 tertiary colours are formed. He arranges the twelve colours in a circle, like the spokes of a wheel, commencing with the red, and going to the right, thus:—Red, red-orange, orange, yellow-orange, yellow, yellow-green, green, blue-green, blue, blue-violet, violet, red-violet. The chromatic circle is completed by placing 5 shades between the red and red-orange, 5 between the red-orange and orange; and so on between each of the other couples. This chromatic circle of 72 colours is not imaginary, but actually exists, composed of dyed wools. The shades are distinguished by numbers; thus there are red, 1 red, 2 red, 3 red, 4 red, and 5 red, &c. Each of the 72 shades has, moreover, 20 different degrees of depth, from the lightest that can be discerned from pure white to the most intense depth, approaching to brown and black. These degrees of depth are called tones or tints. The addition of these tones to the chromatic circle brings up the number of tints to 1440. To indicate any one of these tints we have merely to write the number of the shade, and after it the number of the tone, as, for example, 3 blue-violet, 13 tone. By mixing each of the 1440 tints with grey or black, so as to darken it in different degrees, a total of 14,440 colours may be defined. This part of the system is generally regarded as unnecessary. Mr O′Neill, in his valuable ‘Dictionary of Calico Printing and Dyeing’ (to which work we refer the reader for a full account of Chevreul’s classification), gives a long list of colours and coloured bodies, which are pretty well defined in common language with the names of the colours, according to this ingenious system. We select from this list the following examples:—
For notices of Dyes, Pigments, &c., refer to the principal colours.
Colours, Cake. Syn. Artists’ Colours. These are made by grinding by means of a glass muller and a slab, the respective pigments previously reduced to powder, into a smooth paste with equal parts of isinglass size, and thin gum water. The paste is then compressed into squares as tightly as possible, and dried with a very gentle heat. Old crumbling cake colours should be powdered very finely in a biscuit-ware mortar, sifted through fine muslin, and ground up as above, the gum water being omitted. The powders rubbed up with honey to the consistence of cream constitute moist colours.
Colours, Complement′ary. Syn. Accident′al Colours. Colours are said to be complementary to each other which, by blending together, produce the perception of whiteness. According to Mayer, all colours are produced by the admixture of red, yellow, and blue light, in certain proportions; and by intercepting either one or more of these coloured rays in a beam of light, those which meet the eye will consist of the remaining coloured rays of the spectrum. Thus, by intercepting the red rays in a beam of white light, the remaining yellow and blue rays will produce a494 green colour; by intercepting the blue rays, the remaining yellow and red will give an orange; and so on of other cases; so that red and green, blue and orange, are COMPLEMENTARY COLOURS. If we look for some time, with one eye, on a bright-coloured object, as a wafer, placed on a piece of paper, and subsequently turn the same eye to another part of the paper, a similarly shaped spot or mark will be seen, but the colour will vary, though it will be always the same under like circumstances. Thus, if the original spot or wafer be of a red colour, the imaginary one will be green; if black, it will be white; the imaginary colour being always complementary of that first gazed upon. The colour so perceived is often called an ACCIDENTAL COLOUR, to distinguish it from the real colour. It is a general maxim in design that “colours look brightest when near their complementary colours.”
Colours, Drug′gists’ Show. See Show Bottles.
Colours, Flame. See Fires (Coloured).
COLTS′FOOT. This popular herb is the Tussilago farfara of Linnæus. It is a demulcent bitter, and is slightly stomachic and tonic. It is much esteemed by the lower classes in coughs, shortness of breath, and other affections of the chest. The leaves form the basis of most of the British herb tobaccos, and have been recommended to be smoked in asthma and difficulty of breathing.—Dose. One or two wine-glassfuls of the tea or decoction (1 oz. to the pint) ad libitum.
COLUM′BIC ACID. See Tantalic Acid.
COLUM′BIUM. See Tantalum.
COMA. A deep, heavy sleep, from which the patient cannot be aroused. See Apoplexy.
COMACHROME FOR DYEING THE HAIR BLACK. Nitrate of silver solution, with pyrogallic acid. (Reveil).
COMBINA′TION. In chemistry, the union of dissimilar substances. The great general laws which regulate all chemical combinations admit of being laid down in a manner at once simple and concise. The laws of COMBINATION BY WEIGHT are as follows:
“1. All chemical compounds are definite in their nature, the ratio of their elements being constant.
“2. When any body is capable of uniting with a second in several proportions, these proportions bear a simple relation to each other.
“3. If a body, A, unite with other bodies, B, C, D, the quantities of B, C, D, which unite with A, represent the relations in which they unite among themselves, in the event of union taking place.
“4. The combining quantity of a compound is the sum of the combining quantities of its components.” (Fownes.)
There is a remarkable relation between the specific gravity of a body in the gaseous state and its chemical equivalent or combining proportion—a relation of such a kind that quantities by weight of the various gases, expressed by their equivalents, or, in other words, quantities by weight which combine occupy, under similar circumstances of pressure and temperature, either equal volumes or volumes bearing a simple proportion to each other. This relation accounts for the law of COMBINATION BY VOLUME discovered by Gay-Lussac, and thus expressed:—
When gases combine, chemical union invariably takes place, either between equal volumes or between volumes which bear a simple relation to each other.
Gerhardt assumes that equal volumes of the elementary gases and vapours, when compared under similar conditions of pressure and temperature, contain the same number of atoms. Consult the chemical works of Fownes, Roscoe, Watts, &c. See Affinity, Atomic Theory, Equivalents, &c.
COMPOUND CHINESE TABLET OF ALABASTER (John Irvine). A cosmetic powder for the skin. It consists of chalk, free from injurious metals. (Chandler).
Compound Chinese Tablet of Alabaster (Shand). Identical in use and composition with the last-mentioned powder.
COMPOUND SUGAR-COATED MAY-APPLE PILLS (Dr Scott). Recommended as “antibilious, cathartic, chemical family pills.” Sugar-coated pills, consisting of bitter extract, powdered podophyllum root, rhubarb, jalap, and pepper. (Hager).
COMPRESSES DESINFECTANTES DE LE PERDRIEL. Charcoal powder incorporated with paper.
CONCENTRATED CASTOR OIL in Capsules of Gelatin (Taylor). 24 gelatin capsules filled with castor oil, containing ·5 per cent. of croton oil. (Hager).
CONCENTRA′TION. The volatilisation of part of a liquid in order to increase the strength of the remainder. The operation can only be performed on solutions of substances of greater fixity than the menstrua in which they are dissolved. Many of the liquid acids, solutions of the alkalies, &c., are concentrated by distilling off their water.
In pharmacy, the term CONCENTRATED is commonly applied to any liquid preparation possessing more than the usual strength. Thus, we have concentrated infusions, decoctions, liquors, solutions, tinctures, and essences, most of which are made of 8 times the common strength. This is generally effected by using 8 times the usual quantity of the ingredients, with a given portion of the menstruum, and operating by digestion and percolation; the latter being generally adopted when the articles are bulky. When the menstruum is water, a little spirit is added, to make the product keep. See Decoction, Infusion, &c.
CON′CRETE. A compact mass or cement,495 composed of pebbles, lime, and sand, employed in the foundations of buildings. The best proportions have been said to be—60 parts of coarse pebbles, 25 of rough sand, and 15 of lime; but Semple recommends 80 parts of pebbles, 40 parts of river sand, and only 10 parts of lime. The pebbles for concrete should not exceed about 1⁄2 lb. each in weight.
CON′DIMENTS. Substances taken with the food, to season or improve its flavour, or to render it more wholesome or digestible. The principal condiments are COMMON SALT, VINEGAR, LEMON-JUICE, SPICES, AROMATIC HERBS, OIL, BUTTER, SUGAR, HONEY, and SAUCES. Most of these, in moderation, promote the appetite and digestion, but their excessive use tends to vitiate the gastric juice, and injure the stomach.
CONDY’S FLUID (from England). A weak solution of permanganate of soda. (Wittstein.)
CONFEC′TION. Syn. Confectio, L. Anything prepared with sugar; a sweetmeat, or candy. In medicine the name is commonly applied to substances, usually pulverulent, mixed up to the consistence of a soft electuary, with powdered sugar, syrup, or honey. In the ‘London Pharmacopœia’ (1836 and 1851) both CONSERVES and ELECTUARIES are included under this head, though there appears to be some little distinction between them.
In the preparation of confections all the dry ingredients should be reduced to very fine powder, and passed through a sieve, not coarser than 80 holes to the inch; and the pulps and syrups used to mix them up should be perfectly homogeneous, and of a proper consistence. The mixture should be intimate and complete, in order that the characteristic constituents may be equally distributed throughout the mass. The consistence of the newly made confection should be sufficiently solid to prevent a separation of the ingredients, and yet soft enough to allow of it being easily swallowed without previous mastication.
Confections should be preserved in stone jars covered with writing paper, and placed in a cool and not too dry a situation. Without this precaution they are apt to mould on the top. If at any time the mass ferments and swells up, the fermentative process may be arrested by placing the jar in a bath of boiling water, for an hour or two, or until the whole becomes pretty hot; when it should be removed from the heat, and stirred occasionally until cold. Should the sugar crystallise out of the confection, or ‘candy,’ as it is called, the same method may be followed. Or, the mass may be well rubbed in a mortar until the hard lumps of sugar are broken down and a uniform consistence again produced. On the large scale it may be passed through the mill.
As remedial agents, the officinal confections possess little value, and are chiefly used as vehicles for the administration of more active medicines. See Conserves and Electuaries.
Confection of Acorns. Syn. Confec′tio sem′inum quer′cus, L. Prep. (Bories.) Powdered acorns, 3 oz.; red coral and catechu, of each 11⁄2 oz.; confection of dog-rose, 10 oz.; syrup of red roses, q. s. to make a confection.—Dose, 1 dr., every 4 hours; in chronic diarrhœa, &c.
Confection of Almonds. Syn. Almond paste, Conserve of almonds; Confec′tio amyg′dalæ (Ph. L.), Conser′va amygdala′rum (Ph. E.), Confec′tio amygdala′′rum (Ph. D. 1826), L. Prep. (Ph. L.) Sweet almonds, 8 oz.; white sugar, 4 oz.; powdered gum Arabic, 1 oz.; macerate the almonds in cold water, then remove the skins, and beat them with the other ingredients until reduced to a smooth confection. The Ph. E. form is similar. See Powders, Compound Powder of Almond.
Uses, &c. To prepare EMULSION of MILK OF ALMONDS. A little of this paste or powder, triturated with a sufficient portion of water and strained through a piece of calico, forms emulsion of almonds. “This confection will keep longer sound if the almonds, first decorticated (blanched), dried, and rubbed into the finest powder, be mixed with the acacia and sugar, separately powdered, and the mixed ingredients be kept in a well-stoppered bottle.” (Ph. L.) The same effect may be arrived at by simply well drying the blanched almonds before mixing them with the gum and sugar. The addition of even a small quantity of water or syrup causes the confection “to become soon mouldy, or rancid, or both.” (Brande.)
Confection of Al′um. Syn. Confectio alu′minis, L. Prep. 1. (St. B. H.) Alum (in fine powder), 1 dr.; conserve of roses, 6 dr.
2. (Foy.) Alum, 1 dr.; conserve of roses, 1 oz.—Dose, 1 dr., 2 or 3 times a day; in lead colic, and as an astringent in diarrhœa and other affections.
Confection, Aromat′ic. Syn. Aromatic elec′tuary; Confec′tio aromat′ica (Ph. L. & D.), Electua′′rium aromat′icum (Ph. E.), L. Prep. 1. (Ph. L.) Nutmegs, cinnamon, and hay saffron, of each 2 oz.; cloves, 1 oz.; cardamoms, 1⁄2 oz.; prepared chalk, 16 oz.; white sugar, 2 lbs.; reduce the whole to a very fine powder, and keep it in a closed vessel. When wanted for use, mix it with water to the consistence of a confection.
2. (Ph. E.) Aromatic powder (Ph. E.), 1 part; syrup of orange peel, 2 parts; mix.
3. (Ph. D.) Aromatic powder and simple syrup, of each 5 oz.; clarified honey, 2 oz.; powdered saffron, 1⁄2 oz.; mix, and add, oil of cloves, 30 drops.
4. (Commercial.)—a. Hay-saffron, cassia, and turmeric, of each 4 oz.; cardamoms, 1 oz.; starch, 8 oz.; precipitated chalk, 2 lbs.;496 white sugar, 4 lbs.; oil of nutmeg, 2 dr.; oil of cloves, 3 dr.; reduce the dry ingredients to fine powder, and pass it through a sieve (80 holes); then add the oils, and after well mixing them in, pass the whole through a coarse sieve (about 40 holes to the inch), to ensure perfect admixture.
b. Hay-saffron, 4 oz.; turmeric, 3 oz.; powdered starch, 8 oz.; precipitated chalk, 2 lbs.; white sugar, 4 lbs; oil of cloves and cassia, of each 3 dr.; oil of nutmeg, 2 dr.; essence of cardamoms, 1 oz.; boil the saffron and turmeric in 1 gallon of water, placed in a bright copper pan, for 10 minutes, then, without straining, add the chalk, starch, and sugar; mix well, and continue stirring until the mixture becomes quite stiff, then break it up, dry it thoroughly by the heat of a steam or water bath; next reduce it to fine powder, which must be passed through a fine sieve, as before; the oils and tincture are now to be added, and after being well mixed, and passed through a coarse sieve, it should be placed in a jar or bottle, and bunged up close. Very bright coloured.
Obs. In the wholesale trade this article is kept under two forms—one, in powder, as ordered by the College, and commonly called for distinction sake PULV′IS CONFECTIO′NIS AROMAT′ICÆ; the other, mixed up ready for use. In preparing the latter, it is a common plan to make a strong infusion or decoction of the saffron, and to use it to mix up the other ingredients, adding the aromatics last. (See 4, b.) When the price of precipitated chalk is an objection to its use, prepared chalk may be used instead. There is much anxiety evinced by the wholesale druggists to prepare this confection of a rich colour, without an undue expenditure of saffron, which is generally economised on account of its costliness. This confection is cordial, stimulant, antacid, and carminative.—Dose, 10 to 60 gr., either as a bolus or stirred up with a glass of water; in diarrhœa, acidity of stomach, heartburn, and any like affection, if accompanied by looseness of the bowels. In diarrhœa, English cholera, and flatulent colic, 1⁄4 gr. of powdered opium may be added to each dose. See Powders, Powder of Chalk, Compound.
Confection of Bark. Syn. Confec′tio cincho′næ, L. Prep. 1. Yellow bark and white sugar, of each 1 oz.; capsicum, 1 dr.; simple syrup, 4 oz.
2. (St. B. Hosp.) Yellow bark, 6 dr.; ginger, 1⁄2 dr.; treacle, 31⁄2 oz.—Dose, 1 to 6 dr., where the use of bark is indicated.
Confection of Cas′sia. Syn. Confec′tio cas′siæ (Ph. L.), L. Prep. (Ph. L.) Prepared cassia, 1⁄2 lb.; manna, 2 oz.; prepared tamarinds, 1 oz.; syrup of roses, 8 fl. oz.; mix with heat, and evaporate to a proper consistence.—Dose, 2 dr. to 6 dr.; or more, as a laxative.
Confection of Cat′echu. Syn. Confec′tio cat′echu compos′ita (Ph. D.), L. Prep. (Ph. D.) Compound powder of catechu, 5 oz.; simple syrup, 5 fl. oz.—Dose, 10 gr. to 20 gr.; as an astringent, in diarrhœa, &c.; either alone or combined with chalk.
Confection of Copai′ba. Syn. Confec′tio copai′bæ, L. Prep. 1. (Berton.) Copaiba and powdered cubebs, of each 2 oz.; alum, 1 oz.; opium, 5 gr.; mix well.
2. (Swediaur.) Turpentine, 1 oz.; copaiba, 1⁄2 oz.; mix; add mucilage of gum Arabic, 1 oz.; triturate to an emulsion, and further add, conserve of roses, 4 oz.
3. (Traill.) Copaiba, 2 oz.; oatmeal, q. s. to form an electuary; then add, conserve of roses, 1 oz.
4. (Voght.) Copaiba and powdered cubebs, of each 41⁄2 dr.; yolk of 1 egg; conserve of roses, 1⁄2 oz. All the above are excellent medicines in gonorrhœa.—Dose, 1 to 3 dr., three or four times a day, made into boluses, and covered with the fresh emptied skin of a prune before being swallowed; in gonorrhœa, gleet, &c.
Confection of Cream of Tar′tar. Syn. Confection of bitar′trate of potas′sa; Confec′tio potas′sæ bitartra′tis, L. Prep. 1. Cream of tartar and powdered sugar, of each 1 oz.; simple syrup, 2 oz.; 1 nutmeg, grated.—Dose, 2 dr. to 6 dr.
2. (St. B. Hosp.) Bitartrate of potassa and simple syrup, of each 3 oz.; ginger, 1 dr.—Dose, 11⁄2 dr. to 5 dr. Both are laxatives well adapted for women and children.
Confection of Hem′lock. Syn. Confec′tio co′nii, L. Prep. (Marshall Hall.) Fresh hemlock leaves beaten up with an equal weight of sugar.—Dose, 10 to 20 gr. as a bolus, 2 or 3 times daily, where the use of hemlock is indicated. The confection of other narcotic plants may be made in the same way.
Confection of Hips. Syn. Con′serve of hips, Confection of dog-rose, Conserve of d.-r.; Confec′tio ro′sæ; cani′næ (Ph. L.), Conser′va ros′æ fruc′tûs (Ph. E.), L. Prep. 1. (B. P.) Hips, 1 part; refined sugar, 2 parts; beat the hips in a stone mortar, rub the pulp through a sieve, add the sugar, and mix thoroughly.—Dose, 60 grains or more.
2. (Ph. L.) Fruit of the dog-rose, without the seeds (carpels), 1 lb.; pound it to a pulp, add, gradually, powdered white sugar, 20 oz.; and beat them together until thoroughly incorporated.
3. (Ph. E.) Pulp of hips, 1 part; white sugar, 3 parts; as No. 1.
4. (Wholesale.) Pulped hips, 2 cwt.; fine white sugar, 3 cwt.; incorporate them without applying heat.
Obs. Both this and the confection of red roses have a brighter colour, if made without heat, or touching metallic vessels. On the small scale it is generally made by beating the ingredients together in a marble mortar, but in large quantities by grinding in a mill. Great care must be taken to remove the seeds497 (carpels) with the hair surrounding them, before pulping the fruit, as they are apt, like the hairs of cowhage, when swallowed, to produce vomiting, itching about the anus, &c. This conserve is slightly laxative, and is principally used for forming pills. It is very apt to candy by keeping.
Confection of Ipecacuan′ha. Syn. Confec′tio ipecacuan′hæ, L. Prep. (Bories.) Ipecacuanha, 12 gr.; sulphur, 20 gr.; orris root, 1 dr.; syrup of mallows and manna, of each 2 oz.—Dose. A teaspoonful, 2 or 3 times daily; in hooping-cough, dyspepsia, &c.
Confection of Iron, Subcarbonate. (St. B. Hosp.) Subcarbonate (peroxide of iron), 1⁄2 oz.; treacle, a sufficient quantity.—Dose, 1⁄2 dr.
Confection of Jal′ap. Syn. Confec′tio jal′apæ, C. j. compos′ita, L. Prep. (St. B. Hosp.) Jalap, 4 dr.; ginger, 1 dr.; bitartrate of potassa, 3 oz.; treacle, 5 oz.—Dose, 1 to 3 dr. as a purgative.
Confection of Kermes. (L. P. 1745.) Strained juice of kermes, 3 lbs.; rose water, 6 fl. oz.; white sugar, 1 lb.; oil cinnamon, 10 gr.
Confection of Mer′cury. Syn. Confec′tio hyrar′gyri, C. mercuria′lis, L. Prep. 1. Stronger mercurial ointment (Ph. L.), 1 part; conserve of roses, 3 parts.
2. (Dr D. Davis.) Mercury and manna, equal parts; treacle, q. s.; triturate until the globules of mercury disappear.
Dose, &c. The same as those of mercurial pill.
Confection of Ni′tre. Syn. Confec′tio potas′sæ nitra′tis, L. Prep. 1. Nitre, 1 part; confection of roses, 6 parts; oil of juniper, a few drops.
2. (St. B. Hosp.) As the last, without the juniper. Both are used in gonorrhœa.
Confection of O′pium. Syn. Confec′tio o′pii (B. P.), Electua′′rium o′pii (Ph. E.), L. Prep. 1. (B. P.) Compound powder of opium, 192 gr.; syrup, 1 oz.
2. (Ph. L.) Powdered opium, 6 dr.; long pepper, 1 oz.; ginger, 2 oz.; caraways, 3 oz.; tragacanth, 2 dr.; reduce to fine powder, and keep it in a closed vessel; for use, add to it by degrees hot syrup, 16 fl. oz. (i.e. 31⁄2 dr. of the powder to each fl. oz. of syrup). It contains 1 gr. of opium in every 36 gr.
3. (Ph. E.) Aromatic powder, 6 oz.; senega, 3 oz.; opium, diffused in a little sherry, 1⁄2 oz.; syrup of ginger, 1 lb. Contains 1 gr. of opium in every 43 gr.
Uses, &c. This confection is intended as a substitute for the once celebrated Mithridate, philonium, and theriaca of the old Pharmacopœias. It is stimulant, anodyne, and narcotic.—Dose, 5 to 30 gr.; in flatulent colic and diarrhœa unaccompanied by fever.
Confection of Or′ange Flowers. Syn. Confec′tio flor′um auran′tii, L. Prep. 1. Orange flowers, 1 part; white sugar, 2 parts; beat together to a confection.
2. (Tadei.) Orange flowers, 1 part; simple syrup, 3 parts; evaporate to a proper consistence. Both are used as agreeable adjuncts or vehicles for other medicines. The first is the best article.
Confection of Or′ange Peel. Syn. Confection of orange, Conserve of orange peel; Confec′tio auran′tii (Ph. L.), Conser′va auran′′tii (Ph. E.), Conser′va aurantio′′rum (Ph. L. 1824), L. Prep. (Ph. L. and E.) External rind of the fresh orange, separated by rasping, 1 lb.; beat it in a stone mortar with a wooden pestle to a pulp, then add white sugar, 3 lbs.; and beat them together until incorporated.
Uses, &c. This confection is an agreeable tonic and stomachic; it is much used as an adjunct to bitter and purgative powders, and as a vehicle for the sesquioxide of iron.
Confection of Pep′per. Syn. Confection of black pepper, Conserve of b. p.; Ward’s paste; Confec′tio piperis (B. P.), C. p. ni′gri (Ph. D. & Ph. L. 1836), Electua′′rium pip′eris (Ph. E.), L. Prep. 1. (B. P.) Black pepper, in fine powder, 2 parts; caraway, in fine powder, 3 parts; clarified honey, 15 parts; triturate.—Dose, 60 to 120 gr.
2. (Ph. L.) Black pepper and elecampane, of each 1 lb.; fennel, 3 lbs.; white sugar, 2 lbs.; reduce to a very fine powder, and keep it in a covered vessel; for use, add it, gradually, to honey, 2 lbs.; and beat the whole to a paste (i. e., 2 oz. of honey to each 7 oz. of powder).
3. (Ph. E.) As the last, but using liquorice powder instead of elecampane, and at once making a confection.
4. (Ph. D.) Black pepper and liquorice root, of each 1⁄2 oz.; refined sugar, 1 oz.; oil of fennel, 1⁄2 fl. oz.; honey, 2 oz.; mix.—Dose, of each of the above, 1 to 3 dr., two or three times daily, for 3 or 4 months; in piles, fistula, &c., unaccompanied with inflammatory symptoms. Or, it may be used as a suppository. It is intended as a substitute for the once celebrated nostrum, ‘Ward’s Paste for the Piles.’
Confection of Pep′permint. Syn. Confectio men′thæ piperi′tæ, L. Green peppermint, 4 oz.; white sugar, 12 oz. Anti-emetic and anti-flatulent; in colic, diarrhœa, &c.; in the form of a bolus, or made into a mixture.
Confection of Res′in. Syn. Confectio resin′æ, L. Prep. (Dr Watson.) Powdered resin, 1 oz.; balsam of copaiba, 1⁄2 oz.; honey, 5 oz.—Dose, 1 to 3 dr.; in piles and gleet. It is best combined with a little confection of orange peel, which effectually covers the taste of the copaiba.
Confection of Ro′ses. Syn. Confection of red roses; Confec′tio ro′sæ (Ph. L. & D.), Conser′va ro′sæ (Ph. E.), Confectio ro′sæ Gal′licæ (B. P.), Conserva r. G. (Ph. L. 1824), L. Prep. 1. (B. P.) Fresh red-rose petals, 1 lb.; white sugar, 3 lbs.; mix as confection of hips.
498
2. (Ph. E.) Fresh petals, 1 part; sugar, 2 parts.
3. (Ph. D.) a. Fresh petals, 3 oz.; sugar, 8 oz. Or—
b. Dried petals, 1 oz.; water, 2 fl. oz.; macerate for 2 hours; then add refined sugar, 8 oz.; and beat to a mass as before.
Obs. It is astringent and tonic, but is principally used as an elegant vehicle for more active medicines. It keeps well, and does not candy like confection of hips.—Dose, 1 to 2 drs., eaten off a spoon, either alone or combined with chalk; in slight cases of diarrhœa, vomiting in pregnancy, &c. See Conserve.
Confection of Rue. Syn. Conpectio ru′tæ (Ph. L.), L. Prep. (Ph. L.) Fresh rue (bruised), caraways, and laurel berries, of each 11⁄2 oz.; prepared sagapenum, 1⁄2 oz.; black pepper, 2 dr.; honey, 16 oz.; water, q. s.; rub the dry ingredients to a flue powder, then add, gradually, the sagapenum, previously dissolved in the water and honey over a slow fire, and mix well. In the Ph. L. 1836 dried rue was ordered. Carminative and antispasmodic. In flatulent colic, and in the convulsions of children, when there is no inflammation.—Dose, 15 to 60 gr.; either by the mouth, or made into an enema with gruel.
Confection of Scam′mony. Syn. Confec′tio scammo′nii (B. P.), Electua′′rum scammo′′nii (Ph. D.). Prep. (B. P.) Scammony, in fine powder, 24 parts; ginger, in fine powder, 12 parts; oil of caraway, 1 part; oil of cloves, 1⁄2 part; syrup, 24 parts; clarified honey, 12 parts; rub the powders with the syrup and the honey into a uniform mass, then add the oils and mix.—Dose, 10 gr. to 30 gr.; as a warm cathartic, and in worms, &c.
Confection of Scurvy Grass. (P. Codex.) Fresh leaves of scurvy grass, 1 oz.; sugar, 3 oz. Beat to a pulp and pass through a hair sieve.
Confection of Sen′na. Syn. Len′itive elec′tuary, Elec′tuary of Senna; Confec′tio sen′næ (Ph. L. & D.), Electua′′rium sen′næ (Ph. E.), L. Prep. 1. Senna, 8 oz.; corianders, 4 oz.; rub them together, and by a sieve separate 10 oz. of the mixed powder; also boil figs, 1 lb., and fresh liquorice, bruised, 3 oz., in water, 3 pints, until reduced to one half; press, strain, and evaporate the strained liquor in a water bath to 24 fl. oz.; then add sugar, 21⁄2 lbs.; dissolve, and further add, prepared tamarinds, cassia, and prunes, of each 1⁄2 lb.; remove from the heat, and when the whole has considerably cooled, add the sifted powder, by degrees, and stir until the whole is thoroughly incorporated.
2. (Ph. E.) Senna, 8 oz.; corianders, 4 oz.; liquorice root, 3 oz.; figs and pulp of prunes, of each, 1 lb.; white sugar, 21⁄2 lbs.; water, 31⁄4 pints.
3. (Ph. D.) Senna leaves, in fine powder, 2 oz.; corianders (in fine powder), 1 oz.; oil of caraway, 1⁄2 dr.; mix, and add them to pulp of prunes, 5 oz.; pulp of tamarinds, 2 oz.; brown sugar, 8 oz.; water 2 fl. oz.; previously brought to a smooth paste by the heat of a water bath.
4. (Ph. B.) Boil figs, 12 oz., and prunes, 6 oz., gently in distilled water, 24 oz., in a covered vessel for hours, then, having added more distilled water to make up the quantity to 24 fluid ounces, add tamarinds, 9 oz., and cassia pulp, 9 oz.; macerate for two hours, and press the pulp through a hair sieve, rejecting the seeds, &c. Dissolve refined sugar, 30 oz., and extract of liquorice, 3⁄4 oz., in the mixture with a gentle heat; and while it is still warm, add to it gradually senna in fine powder, 7 oz., and coriander in fine powder, 3 oz., and stir diligently until all the ingredients are thoroughly combined. The resulting confection should weigh 75 oz.
Uses, &c. Confection of senna is a gentle and pleasant purgative, and well adapted for persons suffering from piles, and as a laxative during pregnancy. The dose is 1 dr. to 1⁄2 oz., taken at bedtime or early in the morning.
Obs. There is no one pharmacopœial preparation which it is more difficult to obtain of good quality than confection of senna. The absolute cost of an article prepared according to the directions of the Colleges is greater than the price at which many wholesale houses are vending the drug. Dr Paris very truly remarks, that “the directions of the Pharmacopœia are very rarely followed.” Considerable quantities are manufactured, into which unsound and spoilt apples enter as a principal ingredient; whilst the substitution of jalap for the whole, or a portion of the senna, is a very common practice. We have seen the following forms employed in the trade.
5. Powdered senna’ pulp of tamarinds, cassia, and prunes, of each 11⁄2 lb.; powdered corianders, 3⁄4 lb.; Spanish juice, 1⁄2 lb.; simple syrup, 12 lbs.
6. As the above, but omitting the cassia pulp, and adding 2 lbs. more tamarind pulp. Both these articles are labelled “P. L.” and sent out as genuine, and that when no competition as to price exists. The cheaper article is made as follows:—
7. Common prunes and tamarinds, of each 16 lbs.; treacle, 3⁄4 cwt.; species (a compound of senna dust and small senna, mixed with 3 lbs. of coriander seeds, and strengthened with jalap; all ground to a fine powder), 181⁄4 lbs. To this is frequently added, of rotten or inferior apples, 1⁄4 cwt., which are pulped with the prunes and tamarinds. This article is commonly labelled “Conf. Sennæ Ver.” by its manufacturer.
Confection of Sponge. Syn. Elec′tuary of burnt sponge; Confec′tio spongii, C. s. us′tæ, L. Prep. 1. Burnt sponge, 3 parts; confection of orange peel and hips, of each 1 part; simple syrup, q. s.
2. (St. B. Hosp.) Burnt sponge, made into a confection with syrup of orange peel. The499 first form produces the most agreeable confection.—Dose, of either, 1⁄2 dr. to 2 dr., twice or thrice daily; in scrofula, &c.
Confection of Steel. Prep. 1. Confec′tio fer′ri sesquiox′idi, L.—a. From confection of orange and sesquioxide of iron (Ph. L.), of each 2 oz.; white sugar, 3 oz.; syrup, 11⁄2 oz.; mix.—Dose, 1 dr. to 3 dr.
b. (St. B. Hosp.) Sesquioxide of iron, 1 oz.; treacle, q. s.—Dose, 1⁄2 dr. to 1 dr. Both are given in the usual cases wherein iron is indicated; especially in anæmia, chlorosis, and amenorrhœa.
2. (Confec′tio Fer′ri Tartariza′ti.—St B. Hosp.) Cream of tartar, 11⁄2 oz.; tartrate of iron, 2 dr.; ginger, 1 dr.; treacle, 21⁄2 oz., or q. s.—Dose, 1 dr. to 2 dr., 2 or 3 times daily.
Confection of Sul′phur. Syn. Brimstone and treacle; Confec′tio sulphu′ris, L. Prep. 1. Sublimed sulphur, 2 oz.; treacle, 4 oz.—Dose. A spoonful night and morning for a week or longer, as an alterative or purifier of the blood; in skin diseases, &c.
2. (St. B. Hosp.) Precipitated sulphur, 1 oz.; cream of tartar, 2 dr.; honey or treacle, 2 oz. As the last.
3. (B. P.) Sublimed sulphur, 4 oz.; cream of tartar, 1 oz.; syrup of orange peel, 4 fl. oz.—Dose, 1 to 2 dr.; as a laxative, in piles, gonorrhœa, &c.
Confection of Tin. Syn. Confec′tio stan′ni, L. Prep. (Hosp. Form.) Powdered tin, 1 oz.; confection of roses, 2 oz.; mix.—Dose, 2 to 4 dr., every morning; in worms.
Confection of Turpentine. Syn. Confec′tio terebinth′inæ, L. Prep. (B. P.) Oil of turpentine, 1 fl. oz.; liquorice powder, 1 oz.; triturate together, then add clarified honey, 2 oz.—Dose and use, as the last.
Confection of Worm-seed. Syn. Confec′tio cin′æ, C. s. cinæ. L. Prep. 1. (Ph. Slesvico-Holsat. 1831, and Ph. Suec. 1845.) Worm-seed, 2 oz.; heat it in a pan over a gentle fire, add white sugar, boiled to a low candy height, 4 oz.; and stir together until they become dry; then pick out those seeds which are covered with sugar, and repeat the process with the others.
2. Powdered worm-seed and syrup of orange peel, equal parts.—Dose, 1 to 2 dr., night and morning, followed by a brisk purge; in worms.
CONFEC′TIONERY. See Candies, Drops, Lozenges, Sugar, &c.
CONGELA′TION. The conversion of a substance from the fluid to the solid state by the abstraction of heat. See Ice and Refrigeration.
CONGESTION. “A common condition of disease in an undue flow of blood into any part, or accumulation within it. The vessels seem to lose the power of emptying themselves, which they possess in health. Congestion, although an effect of both visitation and inflammation, may exist irrespective of either. Two forms of it are distinguished, active and passive. The first is when some excitement causes the blood to pass more rapidly into a part than its vessels can transmit out of it; the second when from some inherent debility the vessels cannot get rid of the fluid ordinarily thrown into them. Congestion of organs disturb their functions, and through them the general health.”
CONGLU′TINUM (Bracy Clarke’s). Sulphate of zinc (white vitriol), 4 oz.; dissolved in water, 1 pint. Used as an astringent lotion in veterinary practice, and much diluted with water (a dessert-spoonful to 1⁄4 pint or more of water), as a collyrium in chronic inflammation of the eyes.
CO′NIA. C8H15O. Syn. Co′nine, Con′icine. An alkaloid, discovered by Gieseke in hemlock. It exists in every part of the plant, but is present in the largest quantity in the seed.
Prep. (Gieger.) The seeds of hemlock, or their alcoholic extracts, is distilled with water and potassa. The conia passes over into the receiver, and floats on the top of the water, which also contains a little conine in solution. It is purified in the way directed for the volatile bases. (See Alkaloid.) When the alcoholic extract is employed, about half its weight of potassa should be used.
Prop., &c. Pure conia is an oily-looking liquid, smelling intensely of hemlock, or rather of a combination of the odours of tobacco and mice; volatile at common temperatures; reddens turmeric; boils at about 340° Fahr., but readily distils over with water at 212°; sp. gr. ·89; with the acids it forms salts, some of which are crystallisable. Six lbs. of fresh and 9 lbs. of dried seeds yielded 1 oz. of conia. (Gieger.) Forty lbs. of the ripe but green seeds yielded 21⁄2 oz. of hydrated conia. (Christison.)
Conia is remarkably poisonous. 1 drop, placed in the eye of a rabbit, killed it in 9 minutes; 5 drops, poured into the throat of a dog, killed it in less than a minute. It has been employed in some convulsive and spasmodic diseases, but is now seldom used medicinally. “The patient cries, the contortions, and the rigidity of the limbs, which have always preceded death (caused by conia), leave no doubt as to the cruel pains which this kind of poisoning brings on.” (Boutron-Chalard and Henry.) The treatment may be that recommended under Aconite and Hemlock.
CONSERVATEUR FUR HAARBLEINDE. A preventive of hair diseases (Edm. Bühligen, Leipzig). Consists of 10 grammes tinct. arnica, 5 grammes glycerine, 10 grammes spirit, and 60 grammes water. (Schädler.)
CON′SERVE. Syn. Conser′va, L. Recent vegetable matter, as flowers, herbs, roots, fruit, and seed, beaten with powdered sugar to the consistence of a stiff paste, so as to preserve them as nearly as possible in their natural500 freshness. Conserves are made both by the confectioner and the druggist; by the first as SWEETMEATS; by the other chiefly as vehicles for more active medicines. The London College of Physicians now includes both conserves and electuaries under the general head of CONFECTIONS. The term appears, however, in some cases, scarcely appropriate. The word confection has a more general application, and implies any sweetmeat or composition in which sugar is the principal ingredient. See Confection and Electuary.
Conserve of Ac′etate of Potas′sa. Syn. Conser′va potas′sæ aceta′tis, L. Prep. (Bories.) Acetate of potassa, 1⁄2 oz.; sulphate of soda, 1 dr.; juices of scurvy grass, fumitory, and dandelion, of each 2 oz. (reduced to one half by gentle evaporation?; sugar, q. s. to make a conserve. A teaspoonful 2 or 3 times daily, as a diuretic aperient; in obstruction of the bowels, &c.
Conserve of Al′monds. See Confections.
Conserve of Angel′ica. Syn. Conser′va angel′icæ, L. Prep. (Giordano.) Fresh angelica root, 2 parts; water, 16 parts; macerate for a few hours, clarify the liquor, add sugar, 3 parts; cook the root in the syrup, and preserve it in this state (confection), or dry it (to a candy). Used as an agreeable tonic, stomachic, and carminative.
Conserve, Antiscorbu′tic. Syn. Conser′va antiscorbu′tica, L. Prep. (Selle.) Horse-radish, water-cress, and water-trefoil, orange-juice, and radish-juice, equal parts; powdered white sugar, q. s. to make a conserve. In scurvy, &c.
Conserve of A′′rum. Syn. Conser′va a′′ri, C. a. macula′ti, L. Prep. From fresh arum tubers (cuckow-pint or wake-robin), 1⁄2 lb.; sugar, 21⁄2 lbs. As a diuretic and attenuant in dropsy, or as an expectorant in chronic coughs.—Dose, 1⁄2 teaspoonful, gradually increased.
Conserve of Broom. Syn. Conser′va scopa′′rii, L. Prep. (Van Mons.) Broom flowers, 1 part; sugar, 2 parts.—Dose, 1⁄2 to 2 teaspoonfuls, 2 or 3 times a day; in dropsy, gout, rheumatism, &c.
Conserve of Hips. See Confection.
Conserve of Lavender. Syn. Conser′va lavendu′læ, L. Lavender flowers, 1 part; powdered lump sugar, 3 parts; beaten together to a smooth paste. Used to sweeten the breath. In a similar way conserves are made from various other leaves and flowers; but mostly with only twice their weight of sugar, when they are not very odorous or active.
Conserve of Lem′on Peel. Syn. Conserva limo′nis, C. l. cort′icis, L. As Confection of Orange Peel.
Conserve of Mal′lows. Syn. Conser′va mal′væ, L. From the flowers, as Conserve of Lavender.
Conserve of Or′ange Peel. See Confection.
Conserve of Pep′permint. See Confection.
Conserve of Rose′mary. Syn. Conser′va rosmari′ni, L. As Conserve of Lavender.
Conserve of Roses. 1. See Confection.
2. (Acidula′ted Conserve of Roses Conser′va ro′sæ ac′ida, L.) Prep. (Hosp. F.) Confection of roses and powdered gum, of each 1 oz.; sulphuric acid, 1 dr. to 11⁄2 dr.; (diluted with) water, 2 dr. An excellent substitute for tamarinds.
Conserve of Sav′in. Syn. Conser′va sabi′næ, L. Prep. (Ph. Han.) Fresh savin, 1 part; sugar, 2 parts. As an emmenagogue, in amenorrhœa, &c. Three parts of sugar make a better conserve.
Conserve of Scurvy Grass. Syn. Conser′va cochlea′′riæ, C. c. horten′sis, L. Prep. (Ph. Aust. 1836.) Fresh scurvy grass, 1 lb.; sugar, 3 lbs. Stimulant and antiscorbutic.
Conserve of Sea Worm′wood. Syn. Conser′va absinth′ii mariti′mi, L. Prep. (Ph. L. 1788.) From sea wormwood, as the last. As a stomachic bitter and vermifuge; in dyspepsia, &c.
Conserve of Sloes. Syn. Conser′va pru′ni sylves′tri, L. Prep. (Ph. L. 1788.) From the pulp of the fruit, 1 part; sugar, 3 parts. Astringent. Useful in simple diarrhœa, &c.; either alone or combined with chalk.
Conserve of Squills. Syn. Conser′va scil′læ, L. Prep. (Ph. L. 1788.) Fresh squills, 1 oz.; sugar, 5 oz. Diuretic, attenuant, and expectorant; in dropsy, chronic coughs, &c.—Dose, 10 to 20 gr.
Conserve of Tam′arinds. Syn. Conser′va tamarind′orum, L. Prep. (P. Cod.) Tamarind pulp, 2 oz.; white sugar, 3 oz.; evaporate by the heat of a water bath to the consistence of honey.
Conserve of Vi′olets. Syn. Conser′va vio′læ, C. v. odora′tæ, L. Prep. (Soubeiran.) Flowers, 1 part; sugar, 3 parts; beat to a paste. Demulcent and laxative; used as a purge for infants, and by ladies to perfume the breath.
Conserve of Wa′ter-cress. Syn. Conser′va nastur′′tii, L. Prep. (Ph. Græca, 1837.) From fresh water-cresses, as the last. In scurvy; taken ad libitum.
Conserve of Worm′wood. See Conserve of Sea Wormwood.
CONSTIPA′TION. Syn. Constipa′tio, Obstipa′tio, L. Surgeons distinguish between costiveness and constipation. The first applies to that condition of the body in which the bowels act tardily, and in which the fæces are abnormally and inconveniently indurated; the last implies the absence of the proper alvine evacuations. The one rapidly undermines the health; the other destroys life in a period varying from a few days to three or four weeks. In popular language, however, the words are frequently used synonymously. The use of bread containing alum, and water containing much lime (very hard water), and especially the want of sufficient exercise, are common causes of constipation.
501
Treatment. When the affection is merely accidental or occasional, a dose of some aperient or cathartic is the only treatment necessary; but when it is habitual it calls for further attention. Great benefit may generally be secured by adopting a diet free from astringents, and consisting of a large portion of green vegetables and ripe fruit; particularly avoiding the use of over-cooked, salted, or dried animal food. Brown bread may be eaten, as it acts as a gentle laxative, from the bran it contains. The occasional use of aperient and emollient enemata may be had recourse to; but their habitual administration, as well as that of purgative medicines generally, by the mouth, is not to be recommended. The bowels, accustomed to the continual use of stimulants, act but languidly or scarcely at all without their application. In females, especially of the higher classes, the want of proper exercise is commonly the chief cause of this affection. With such persons a short walk, two or three times daily, will often do wonders, particularly if a little ripe fruit, a few raisins or tamarinds, or, still better, 2 or 3 drum figs, be occasionally eaten. In some cases of obstinate constipation a cold-water dressing, placed over the pit of the stomach or the abdomen, will cause the bowels to act in the course of an hour or two. When the inactivity of the bowels arises from a deficiency of bile (one of the most common causes), no remedy is more natural, or more effective, than inspissated ox-gall. In cases complicated with nervous, hypochondriacal, or hysterical affections, in chlorosis, dyspepsia, depraved appetite, and numerous other ailments, this remedy frequently succeeds, after the most active articles of the materia medica have been tried in vain.
In the treatment of the constipation of infants, castor-oil (1⁄2 teaspoonful occasionally), or manna 1⁄4 to 1⁄2 oz., sucked at will, may be given. The introduction (very gently) of a little slip of writing paper, parsley stalk, or suet, is a method sometimes adopted successfully by nurses. Friction on the stomach and bowels with the warm hand, or a piece of soft flannel, should also be employed. See Gall, Purgative, &c.
Treatment for Animals. Mr Finlay Dun prescribes laxative clysters, aloes, or oils. Calomel for horses; croton and gamboge for cattle. Salts, calomel and jalap, castor oil, linseed oil, and emetics for carnivora. Oil of turpentine by mouth or rectum; clysters of tobacco, nux vomica, electricity.
Treatment for Horses. When the animal is constipated administer 4 dr. of aloes and 1 dr. of calomel, rubbed down with gruel; inject soap and water every hour, taking care to let the horse have walking exercise, and to apply friction to the belly. If, after twelve hours, no effect is produced, let the aloes and calomel be repeated, with the addition of three or four drops of croton oil and a wine-glassful of spirit of nitre, ether, gin, or whisky.
CONSTITUTION BALLS, Vegetable (A. H. Bôldt). Two parallelopiped hard brown balls, each of which weighs 58 grammes, and is made by melting together 2 parts of aloes and 1 part coarsely powdered gentian. (Hager).
CONSUMP′TION. See Phthisis.
CONTA′GION. By ‘contagion’ is usually meant the communication of disease by means either of actual contact or through a medium, such as the air. By some a contagious disease is regarded as one arising from direct contact only, in contradistinction to an infectious one, which is believed to act at a distance. See Disinfectant.
CONTU′SION. A hurt, or injury to the flesh, such as might be caused by a blunt instrument or by a fall, without breach or apparent wound. For treatment, see Bruise.
CONVALESCENCE. Convalescence may be described as the period between the cessation of an attack of serious illness and the restoration, if not to a perfect, to an accustomed state of health. Convalescent patients should particularly guard against excess in eating or drinking, or unnecessary and imprudent exposure to cold or damp weather, during this interval, as well as against premature exertion of the limbs or voice; such and all of which are acts of imprudence that may give rise to a return of the disease. In order to avoid this latter risk, as well as to aid in complete recovery, repose both of body and mind are generally needed, more particularly in the earlier stages of convalescence.
It should be borne in mind that convalescents from many infectious diseases, such as measles, scarlet fever, smallpox, typhus, &c., are much more likely to propagate these diseases than when they are labouring under them in the acute form. During the period of their recovery the skin and other organs are throwing off the poison in large quantities, and thus exposing those in contact with, or in the near neighbourhood of the convalescent, to the great and imminent risk of contagion. Even if not contagious himself, the convalescent’s clothes, if they be the same as those worn by him during his illness, may also convey the disease.
CONVULSIONS. Spasmodic contractions of the muscles producing motions of the limbs, generally accompanied with unconsciousness. Convulsions occur at all periods of life, but in adults they are only symptoms of other diseases. In children they are very common. They are of frequent occurrence in teething; and a swollen and inflamed state of the gums is said to excite them. Dr Gardner, in his very useful work, ‘Household Medicine,’ says they may be brought on by “improper food, e.g. the milk of a nurse suffering from some violent emotion. At the siege of Berlin nearly all the suckling children died of convulsions.” They may also be induced by feverish attacks, hooping-cough, strong purgatives, or suppressed eruptions. In the case502 of a dangerous attack of convulsion no time should be lost in sending for a medical practitioner. Pending his arrival, the patient should be placed as promptly as possible in a hot-water bath. A better plan is to loosen all the dress, to place the child across the arms, and sway it up and down gently, and to allow cool air to play on the face and chest; give an enema of soap and water, and apply mustard plasters for a few seconds only to the pit of the stomach. If these fail to give relief, apply leeches (number according to the age) to the temples, and cold to the head. Lance the gums if inflamed. When the fit is over keep the head cool. If there have been white stools, give a grain or two of calomel, and repeat it every three or four hours for three or four times until the stools become green or dark. Keep the bowels open by castor oil, and let the patient be put on a milk diet. The latter part of the above treatment is inserted for the benefit of the emigrant or other individual having no means of obtaining proper medical aid.
COPAHINE. Copaiba balsam made into a mass with wax and powdered cubebs, divided into hard egg-shaped pills weighing 5 decigrammes each and sugar coated.
COPAHINE MEGE DE JOZEAU. A fixed quantity of copaiba balsam is mixed with concentrated nitric acid, and constantly stirred as long as effervescence continues. The oxidised balsam is then washed, first with warm then with cold water, till the washings cease to have an acid reaction. From one part of this balsamum copaivæ acido nitrico correctum with 1⁄10 part powdered cubebs, 1⁄10 part bicarbonate of soda, 1⁄16 part calcined magnesia, with some mucilage, a mass is prepared and divided into oval pills, which are afterwards coated with sugar, mixed with gum and carmine.
COPAI′BA. Syn. Copai′va, Copaiva balsam, Capiv′i, Balsam of capiv′i; Copai′ba (Ph. L. E. & D.), L.; Baume de Copahu, Fr.; Cobaiva balsam, Ger. “The oleo-resin, of a brown colour, obtained by incision from the trunk of Copaifera multijuga.” (B. P.) Most of the balsam of commerce is obtained from Para and Maranhao. It is packed in casks containing from 1 to 11⁄2 cwt. each, or in large bottles, or in cylindrical tin boxes.
Prop., Purific., &c. Copaiba, though usually called a ‘balsam,’ is not correctly so named, as it contains no benzoic or cinnamic acid. It is correctly described in the B. P. as an ‘oleo-resin.’ Considerable variation exists in the colour, odour, consistence, and transparency, as well as in the proportion of oil and resin yielded by different samples, scarcely any two of which exactly agree. The sp. gr. varies from ·950 to ·996. Brazilian copaiba is thin, clear, and pale; whilst the West Indian variety is thick, golden yellow, less transparent, and has a less agreeable and somewhat terebinthinate smell. Some varieties are opaque, and continue so unless filtered. This is often a most troublesome operation. The opacity generally arises from the presence of water, which it retains with great tenacity. The following is the plan we have found to answer on the large scale:—Place the casks upon their ends in a warm situation, and leave them so for 10 days or a fortnight, or longer, if convenient. They may then be tapped a little above the bottom, when the contents of some of them will generally be found quite transparent, and may be drawn off and vatted, care being taken to avoid shaking up the bottom. The copaiva that remains foul must be filtered through one or more long Canton flannel bags, sunk in the bottom of a tin cistern, placed over a suitable receiver, in a similar way to that adopted for oils; a few pounds of coarsely powdered charcoal being mixed up with the first 5 or 6 gallons thrown in. This will rapidly fill up the pores of the bag, and make the balsam soon flow clear and pale. The “bottoms” of the casks, containing the water and impurities, may be poured into a large can or jar, and allowed to settle for a few days, when the copaiba may be poured off the top and filtered. A sudden change of temperature will frequently turn a transparent sample of this article opaque or milky; it is not, therefore, deemed fit to send out by the wholesale trade, unless it stands this test. To ascertain this point a common practice is to fill a small bottle with the copaiba, and to leave it out of doors all night in an exposed situation.
Pur., Tests, &c. This substance is frequently adulterated; indeed, fully one half that sold for copaiba does not contain 10% of the genuine balsam. This is particularly the case with that sold in capsules, at low prices, in the shops. Pure balsam of copaiba may be recognised by the following characters:—
1. (Ph. E.) It is transparent; free of turpentine odour when heated; soluble in 2 parts of alcohol; and dissolves one fourth of its weight of carbonate of magnesia with the aid of a gentle heat, and continues translucent.
2. (Chevallier.) A drop of the balsam, placed on a piece of unsized paper, and heated until all the essential oil is expelled, forms a semi-transparent, well-defined spot; but if the balsam has been adulterated with a fatty oil, it is surrounded by an oily areola.
3. (Planche.) 21⁄2 parts of balsam shaken with 1 part of solution of ammonia, sp. gr. ·965, forms a mixture which becomes clear and transparent in a few moments, and may be heated to 212° Fahr. without becoming opaque.
4. (Vigne.) Boiled with 50 times its weight of water for 1 hour, it should lose at least half its weight.
5. (Adder.) By agitating the suspected sample with a lye of caustic soda, and setting the mixture aside to repose, the balsam after a time rises to the surface, and the fatty503 oil present (if any) forms a soapy, thick mass below.
6. (‘Journ. de Pharm.,’ 1842.) Pure copaiba may be adulterated with 50 per cent. of a fat oil (nut, almond, or castor oil), without it ceasing to give a clear solution with 2 parts of alcohol; but it combines badly with magnesia and ammonia. Excess of alcohol, however, separates the oil in all cases. It was formerly considered that the best test for detecting the fat oils was pure alcohol, to which some caustic potash had been added.
7. (Dr Hager.) Copaiba which is adulterated with Gurgun balsam is not quite clear, and frequently exhibits prisms of gurginic acid under the microscope. The author states that the adulteration may be easily detected by mixing the suspected sample with four volumes of petroleum ether; the mixture at once becomes turbid, and gradually deposits a sediment, which, after half an hour’s settling, occupies the same volume as the copaiba operated upon. A mixture of pure copaiba with petroleum ether is clear at first, and either remains clear upon standing or it deposits after several hours a very slight sediment, which merely covers the bottom of the test tube like a thin film. Benzol may be used in place of petroleum ether.
8. (Muter.) Three to four grams of the sample are weighed into a clean, dry flask, and saponified on the water bath with 50 c. c. of alcohol, and a lump of caustic soda weighing not less than 5 grams. When all is dissolved water is added, and the whole washed into a half-pint basin, so as to nearly fill it, and evaporated to 100 c. c. over a low gas flame. Dilute sulphuric acid is then added till the whole just becomes permanently turbid, and then solution of caustic soda is dropped in till it just clears again. By this means a solution is obtained with the least possible excess of alkali, and with a good amount of sodium sulphate. The whole is now to be evaporated to perfect dryness on the water bath, stirring towards the end, so that the sulphate may mix with the soaps, and produce an easy pulverulent residue. The residue is moved from the basin into a small, wide-mouthed, stoppered bottle, treated with 70 c. c. of ether-alcohol, and well shaken up. As soon as it is fairly settled the fluid is filtered off through a quick filter, and this is repeated with two successive quantities of 70 c. c., making 210 c. c. in all of the solvent used. The residue in the bottle and in the filter now consists of sodium oleate and sulphate if the balsam be impure, and of the latter only if pure, with a little trace of the insoluble resin soap already referred to. The contents of the bottle and filter are then dissolved in warm water, and after heating until all smell of ether is gone the whole is boiled freely acidulated with hydrochloric acid, and set to cool.
If, when cold, nothing but a few specks of brown resin should rise to the surface, the balsam is pure; but if an oily layer be formed it is adulterated, and the smell of the separated oleic acid will at once determine whether it is actually castor oil or not.
In the case of the presence of oil, 2 grams of pure and dry white wax are added, and the whole heated till the wax melts with the oleic acid. On cooling, a solid cake is formed, which is detached from the side of the beaker, and the fluid below passed through a filter. The cake is once more melted in boiling water, cooled, detached, dried by gentle pressure between blotting paper, dried in a water-oven in a weighed platinum dish, and then weighed, and the weight of the wax used deducted. The beaker, filter, rod, &c., used are, if at all dirty, dried, extracted with ether, and the residue left, after evaporation, weighed and added to the total.
The calculation is then performed as follows:—
(1.) To the weight in grams found add ·20 for loss of oleic acid in solvent, and then say as 95 : 100 :: total oleic acid.
(2.) Calculate the per-centage from the quantity taken, and from this deduct 6 per cent. for possible altered resin in the balsam. The error, owing to the correction, of course, increases with the amount of oil present; but it is stated to be always an error in the direction of under-estimation, which is the great point for public analysts. When working on 3 to 4 grains with an admixture of not over 25 per cent. the errors due to loss of oleic acid and insoluble resin soap are said to so nearly balance each other, that any correction is unnecessary, and the actual amount of oleic acid found may be taken as correct within a per cent.
9. (B. P.) According to the British Pharmacopœia, copaiba should be soluble in an equal bulk of benzol.
10. (The evaporation test.) Mr Siebold says: “This is an excellent and exceedingly simple test, but is clumsily applied by many. Instead of boiling the balsam with water for many hours, a small quantity (about 1 to 1·5 gram) of the sample should be carefully heated in a watch-glass until all the oil is driven off, which is the case as soon as the residue has assumed a rich brown colour. A few minutes suffice for the experiment.
“If the remaining resin is perfectly brittle and pulverisable there is no fatty matter present, for 1 per cent. of oil would diminish the brittleness of the resin, so that it cannot be reduced to a fine powder. One per cent. of oil is thus readily detected, and with larger quantities of the adulterant (3 to 5 per cent.) the resin feels quite sticky.
“On heating the resin castor oil and linseed oil may be distinguished by the odour. By mixing the adulterated balsam with ten, twenty, forty, and fifty volumes of pure maranham balsam respectively, and testing each dilution in this manner, it is easy to find in504 which the oil has been reduced to below 1 per cent., and thus to ascertain whether the adulterant amounted to more than 10, 20, 30, 40, or 50 per cent., and this, I think, would be sufficiently near the mark for the purpose of public analysts.”
Uses, &c. Balsam of copaiba is considered detersive vulnerary, diuretic, and astringent; and appears to possess a sort of specific power over diseases of the mucous membranes of the urino-genital organs. It is hence a favourite remedy in gonorrhœa, as soon as the first inflammatory symptoms have subsided, antiphlogistic and soothing measures being previously adopted. Dose, 20 to 60 drops on sugar, floating on water, or made into an emulsion with yolk of egg or gum arabic, 3 or 4 times daily, if the stomach will bear it. The addition of a few drops of sweet spirits of nitre and laudanum have been recommended, to allay the nausea. By adding 1 dr. of oil of orange (ol. aurantii) to each oz. of the balsam, its flavour becomes far from disagreeable, and it sits well upon the stomach. Copaiba is also given in capsules and pills. See Capsules, Emulsion, Oil, Pills, &c.
Obs. Numerous preparations of this article are sold under such names as ‘soluble copaiba,’ ‘specific solution,’ ‘salt of copaiba,’ &c.; none of these appear to possess equal activity and certainty of operation to the natural balsam. As the whole virtue of copaiba as a medicine depends on the essential oil it contains, the value of any of these preparations may be estimated by the quantity of that article which is found in them. In the case of the first two articles above named the quantity is very small indeed, and in the last it is wholly deficient.
The following forms are current in the trade for the reduction (adulteration) of balsam of capivi:—
1. Balsam of copaiba, 4 lbs.; castor oil, 3 lbs.; mix well.
2. Balsam, 7 lbs.; castor oil, 4 lbs; yellow resin, 2 lbs.
3. Equal parts of balsam of copaiba and Canada balsam.
4. To the last add Venice turpentine, 1 lb.
5. Balsams of Canada and copaiba and nut or castor oil, equal parts.
6. Copaiba, 7 lbs.; nut oil, 3 lbs.; yellow resin, 2 lbs.; Canada balsam, 1 lb. Used to fill the cheap capsules; and to sell in the lower parts of London and in the manufacturing districts. See also Copaiba, Factitious (below).
Copaiba, Facti′′tious. Syn. Cobai′ba facti′′tia, Bal′samum copai′bæ facti′′tium, L. Prep. 1. Castor oil (warm), 7 quarts; copaiba bottoms, 1 quart; mix, and filter through flannel.
2. Castor, oil, 1 gal.; yellow resin, 3 lbs.; Canada balsam, 2 lbs.; oil of juniper, 2 oz.; oil of savin, 1 oz.; essences of orange and lemon, of each 1⁄2 oz.; powdered benzoin, 1 oz.; melt the resin with the castor oil and benzoin, and when nearly cold add the essences.
3. Canada balsam, 9 lbs.; castor oil, 7 lbs.; yellow resin, 1 lb.; Venice turpentine, 2 lbs.; oils of rosemary, juniper, and savin, of each 1 dr.; essential oil of almonds, 20 drops.
4. Canada balsam, 3 lb.; Venice turpentine, 1 lb.; oils of fennel, juniper, and savin, of each q. s.
Used chiefly to fill capsules. It is readily distinguished from balsam of copaiba by the proper tests. (See above.) Train oil or nut oil is frequently substituted for the castor oil.
Copaiba and Ka′li. Syn. Copaiba cum Potassâ, L. Prep. Carbonate of potassa and water, of each, equal parts; dissolve, and add gradually, transparent balsam of copaiba, until the fluid, at first milky, turns quite clear. Resembles miscible copaiba (see below).
Copaiba, Miscible. Prep. From balsam of copaiba (pure and transparent), mixed with half its volume of solution of potassa made of double the strength ordered in the B. P.
Obs. As different samples of copaiba often require slightly different quantities of the solution of potassa, it is best to mix the two gradually and cautiously together. Should the mixture be opaque, a little more of one or other of the ingredients, as the case may be, will render it clear. No heat must be used. This article is miscible with water, with which it forms a kind of milk; and from containing all the volatile oil of the copaiba, is a very valuable preparation. Its activity is considered equal to that of the balsam itself, and it is given in similar doses.
Copaiba, Sol′uble. Syn. Copai′ba solubil′is, L. Prep. 1. Heat miscible copaiba in an earthen, glass, or bright-tinned copper vessel, to nearly the boiling-point, pour it while still hot in a separator, cover it up, and allow it to cool very slowly. After a few days, draw off the clear portion from a cock or hole placed at or near the bottom of the vessel, observing to reject the first few drops which pass through, and to stop the stream before any of the floating oil (oleum copaibæ) reaches the orifice. A very little concentrated liquor of potassa, added before applying the heat, renders it more soluble. Thick, transparent, soluble in pure water, and resembles the natural balsam in appearance.
2. Balsam of copaiba and solution of potassa (B. P.), equal parts, by volume; mix, boil for a few minutes, and then proceed as before. Thinner than the last.
Prop. Less powerful than miscible copaiba, but it sits better on the stomach, and is about four times as strong as specific solution of copaiba. See Solution.
Copaiba, Res′in of. Syn. Copai′bæ resi′na, L. The residuum of the process of distilling the oil of copaiba from the balsam. It consists principally of copaibic acid. It has been recommended505 for gonorrhœa, but is nearly inert, even in 1⁄2 oz. or 3⁄4 oz. doses. See Oil.
Copaiba, Salt of. Syn. Sal copai′bæ, L. There are two preparations sold under this name; the one, crude copaibic acid; the other, copaibate of an alkali. Neither of them possesses the valuable properties of copaiba, which reside almost entirely in its essential oil, “We have taken the ‘sal copaibæ,’ and have watched its action on others, but have not been able to perceive any good effects to result from its administration.” (Cooley.)
COPAI′BIC ACID. Syn. Capiv′ic acid. Yellow resin of copaiba. An amber-coloured, brittle, semi-crystalline, resinous substance, obtained from resin of copaiba, soluble in alcohol, rectified spirit, ether, and oils, reddens litmus paper, and forms salts with the bases, called copaibates.
CO′PAL. Syn. Copal′, Gum copal. A resinous substance, which exudes spontaneously from various trees belonging to the genera Hymenæa, Guibourtia, and Trachylobium. The varieties commonly met with in commerce are East Indian copal, or anine, which is the produce of Hymenæa Courbaril, and West Indian copal, obtained from numerous species.
Prop. When of good quality it is too hard to be scratched by the nail, has a conchoidal fracture, and a sp. gr. ranging from 1·059 to 1·072. Unlike other resins, it is dissolved with difficulty by alcohol and essential oils; and this property, combined with its extreme hardness, renders it very valuable for making varnishes. See Varnish.
COP′PER. Cu. Syn. Cu′′prum, L.; Cuivre, Fr.; Kupfer, Ger.
Sources. Metallic copper (native copper) is found in many parts of the globe, diffused in isolated particles in the form of thin laminæ, in loose grains intermixed with quartz (copper sand, copper barilla), in dendritic pieces, and in solid blocks, occasionally of many tons weight. The richest deposits of native copper are those of Lake Superior, in North America. More frequently and more abundantly it occurs as an ore, e.g. red oxide, black oxide, green carbonate of copper or mal′achite, blue carbonate of copper, vitreous sulphide of copper, purple copper, copper pyrites, or yellow copper ore, with sulphur, antimony, or arsenic, and other metals (true grey copper ore or fah′lerz), as an impure hydrated silicate (chrys′ocolla), and as an impure hydrated oxychloride (atac′amite). The most abundant and important ore is copper pyrites. It is principally obtained from the mines of Cornwall, Devonshire, and Cuba. The carbonates of copper are now largely imported from Australia; the metal produced by smelting them is generally of the best quality.
Prep. We will not attempt to give a minute description of the various complex processes by which the reduction of copper from its ores is effected, but will merely give an outline of the common or Welsh process. This process includes six distinct operations, as follows:—1. The ore (copper and iron pyrites), containing from 8 to 10% of copper, is roasted in a reverberatory furnace, called a ‘calciner,’ by which much of the sulphide of iron is converted into oxide. 2. The calcined ore is melted with ‘metal slag’ (a product of a subsequent operation—No. 3), in a melting furnace called the ‘ore furnace.’ The products are a regulus, termed ‘coarse metal,’ containing about 35% of copper, and ‘ore-furnace slag,’ which is thrown away. Much of the iron, and the whole of the so-called earthy matter of the ore, are thus separated as slag. 3. The coarse metal, having been granulated by causing it to flow from the furnace into water, is calcined with free access of air in a calciner, and a considerable amount of sulphur is expelled. 4. The calcined granulated, coarse metal is melted with the addition of matters rich in oxides of copper, namely, ‘roaster’ and ‘refinery slags’ (from the two remaining operations, Nos. 5 and 6, respectively), and native carbonates of copper, or ores containing oxide of copper. The products are a regulus, termed ‘metal,’ which contains about 75% of copper, and metal slag (see No. 2). The metal should be in the state of ‘white metal,’ compact and brittle, with a feeble metallic lustre and a dark, bluish-grey colour. It is tapped off into sand moulds. 5. The pigs of regulus obtained by the last operation are roasted in a furnace through which air passes. The temperature is so regulated that the regulus may be melted in from 6 to 8 hours. The slag is skimmed off, and after a time the heat is lowered, to allow the regulus to solidify. It is again melted and tapped into sand moulds, the product being called ‘blister copper.’ 6. This, the last operation, is termed ‘refining.’ From 6 to 8 tons of blister copper, in pigs, are melted in a furnace, and kept exposed for about 15 hours to the oxidising influence of the air. The slag is skimmed off through the end opening. When the oxidation has been sufficiently prolonged, anthracite or free-burning coal, as pure as possible, is thrown upon the surface of the metal, and after a short time the thick end of a long birch or oak pole is plunged into the molten mass. This part of the operation is termed ‘poling.’ The wood in contact with the copper is rapidly decomposed; much gas is evolved, which causes the metal to be splashed about, and every part of it to be exposed to the reducing action of the coal. When the refiner finds the metal to be at the state of ‘tough pitch,’ the pole is taken out, and the coal pushed back from the end opening, through which the copper is then ladled out as quickly as possible, and cast into suitable moulds. For full details of this and other processes, the reader is referred to Dr Percy’s work on ‘Metallurgy,’ and Ure’s ‘Dictionary of Arts, Manufactures, and Mines.’
In the laboratory copper is commonly employed under the following forms:506—
1. Bean-shot copper. Produced by simply lading the melted copper from the refining furnace into hot water. In small lumps like peas and beans; hence its name. Used to make alloys, solutions, &c.
2. Electrotype copper. A very pure form, obtained by decomposing sulphate of copper in an electrotype apparatus. It does not contain lead, whereas most varieties of commercial copper do contain that metal.
3. Feather-shot copper, granulated C. Produced by lading the refined copper from the furnace into cold water. In small pieces, with a feathered edge. Used to make calamine, brass, solution of copper, &c.
4. Copper in plates or foil. Those of commerce (best, annealed) are generally employed.
5. Copper in powder.—a. A solution of sulphate of copper is heated to the boiling-point, and precipitated with distilled zinc; the precipitated copper is then separated from the adherent zinc by dilute sulphuric acid, washed with water, and dried by exposure to a moderate temperature.
6. Copper prepared by the hydrometallurgical method.—One of the oldest processes of this kind, is that known as the ‘cementation’ method, and consists in precipitating copper from a solution of the sulphate of the metal, by means of metallic iron. In some mines solutions of the sulphate are met with occurring naturally, in others they are prepared artificially by treating poor ores containing oxide of copper, with sulphurous acid or diluted sulphuric acid, and sometimes by roasting copper pyrites and afterwards washing them with water to extract the resulting sulphate. The copper obtained by any of the above processes is called ‘cementation copper.’ In the Isle of Anglesea the cementation liquid containing the dissolved sulphate of copper, is first run into large vessels where the suspended matters are allowed to subside; from these it is conveyed to tanks containing old scrap-iron, which serves as the precipitating agent. The scrap-iron is occasionally stirred up so as to renew the metallic surface presented to the solution. The muddy liquor which contains metallic copper as a spongy mass, besides impurities, is run into vessels where it deposits the copper, which after the removal of the supernatant fluid, is removed and dried in a furnace.
7. Wet Process. (Henderson’s process.) The ores (Spanish and Portuguese pyrites) treated by this method vary very slightly in composition, rarely containing much more than 3 per cent. of copper, nearly 50 per cent. of sulphur, from 43 to 44 per cent. of iron, with small quantities of lead, arsenic, zinc, lime, &c. The ores are first employed by the vitriol manufacturers, as a source of sulphuric acid. In the process of burning they lose about 30 per cent. of their sulphur. The copper is extracted from the residue by subjecting this latter to the following processes, which are thus described in the ‘Encyclopædia Brittanica.’
I. Grinding. The burnt ore, as received from the acid burners, is first mixed with about 15 per cent. of common salt, and ground to a fine powder by passing it between a pair of heavy cast-iron rolls. As the amount of sulphur left in the burnt ore is apt to vary, it is necessary to ascertain its proportion in each parcel of burnt pyrites. When the sulphur falls short of the proportion necessary for effecting the decomposition which follows, a sufficient quantity of ‘green’ or unburned pyrites is added to produce a proper balance. If, on the other hand, the sulphur has been sufficiently extracted, dead roasted ore is added.
II. Calcination. This operation is accomplished in several kinds of furnaces, that used by the Tharsis Sulphur and Copper Company, being a large muffle or close furnace. By others a patent furnace with a revolving hearth and mechanical stirring arrangement has been adopted with good results; and some use open reverbatory furnaces heated by gas from Siemens’s generators. During the roasting the mixture is frequently stirred, and in the case of hard-worked furnaces, turned with long rabbles, and the completion of the operation is ascertained by test assays. When the copper has been brought into a soluble condition, the charge is raked out of the furnace and permitted to cool under a screen at its mouth. By the calcination the sulphur in the compound is first oxidised, sulphate of sodium is formed, and at the same time the chlorine from the sodium chloride unites with the copper to form cupric chloride. A small proportion of cuprous chloride is also formed, and special precautions have to be taken to prevent the extensive formation of this compound which is dissolved only with difficulty. The hydrochloric acid and other gaseous products evolved during the calcination are condensed as ‘tower liquor’ in ordinary condensing towers, and the product is used in the subsequent process of lixiviation.
III. Lixiviation. The calcined ore is conveyed to tightly caulked wooden tanks, in which it receives repeated washings with hot water, tower liquor, and dilute hydrochloric acid till all the soluble copper is thereby extracted. The product of the latter washings is pumped or drawn up by a modification of Gilford’s injector, to serve as a first liquor for subsequent charges of the lixiviating tanks, and no solution under a definite strength is permitted to pass on to the next stage in the process. The insoluble residue in the tanks consist of “purple ore,” an almost pure ferric oxide, largely used in “settling” blast furnaces, and for smelting purposes; besides which it is available as jewellers’ rouge.
IV. Precipitation. The precipitation of metallic copper from the solution of its chloride is accomplished in large tanks by means507 of metallic iron in the same way that cementation copper is obtained from solutions of the sulphate. The solution is run into the tanks in which there are miscellaneous heaps of old malleable iron; the chlorine combined with the copper unites with the iron, and metallic copper in the state or fine division is thrown down. The completion of the precipitation is ascertained by dipping a bright steel knife into the solution in the tank, and when no deposit of copper covers the steel, the liquor is run off and a new charge conveyed into the tank. The tanks are drained periodically for removing the precipitate, which is first roughly separated from the small pieces of iron, after which it is more thoroughly freed from iron, &c., by washing in water in a rocking sieve apparatus. The precipitate so obtained should contain 80 per cent. of metallic copper, which is either smelted directly for blister copper, or may be fused with the white metal of the ordinary smelting process, and subsequently roasted. It has been found possible to extract in this process with profit the small proportions of lead, silver and gold, which Spanish pyrites is known to contain. Two processes are in operation for this purpose—one devised by Mr P. Claudet, and the other by Mr W. Henderson, the original patentee of the wet process. The liquors from the first three washings contain practically, all these metals, and they alone are treated. Mr Claudet precipitates them from the solution by means of iodide of potassium. Mr Henderson dilutes his solution from 20° to 25° Twaddell, and adds a very weak solution of lead salt, such as the acetate by which he obtains a cream-coloured precipitate containing 5 or 6 per cent. of silver, and 3 oz. of gold to each ton of the precipitate. The importance of the wet process may be estimated from the fact, that although it originated only in 1860, already 14,000 tons of copper, are annually produced by it in Great Britain alone, out of an annual production for the whole world estimated at from 126,000 to 130,000 tons.
Prop., &c. Copper has a brilliant yellowish-red colour, a nauseous, styptic taste, and emits a disagreeable odour when rubbed; is very malleable and ductile; unchanged in dry air; in damp air it soon becomes covered with a greenish rust (carbonate of copper); slightly soluble in dilute sulphuric and hydrochloric acid; freely soluble in boiling oil of vitriol (sulphurous anhydride being evolved); dilute nitric acid dissolves it readily with copious evolution of nitric oxide; heated to redness in the air, it rapidly becomes covered with a black scale (oxide); it fuses at a full red heat; its crystals are either octahedra or dodecahedra; sp. gr. 8·8 to 8·96; it forms numerous compounds (alloys and salts) with other bodies, all of which are more or less poisonous; its salts are either blue or green, and most of them (when neutral) are soluble in water.
Tests. Metallic copper may be recognised by the above properties; its oxides, salts, &c., by the following characters and reactions:—The solutions of copper possess a blue or green colour, which they retain even when considerably diluted with water:—With caustic potassa they give a light-blue, bulky precipitate, turning blackish-brown or black on boiling the liquid:—Ammonia and carbonate of ammonium produce a bluish-white precipitate, soluble in excess, yielding a rich deep-blue solution:—The carbonates of potassium give a light precipitate, insoluble in excess:—Ferrocyanide of potassium gives a reddish-brown precipitate:—Sulphuretted hydrogen and sulphydrate of ammonium give blackish-brown or black ones:—A polished rod of iron, on immersion in an acidulated solution, quickly becomes coated with metallic copper.
Estim., &c. Copper is generally WEIGHED under the form of black oxide, but sometimes as pure metal:—By throwing it down from its solution by pure potassa, after which it must be carefully collected, washed, dried, ignited in a platinum crucible, and weighed therein as soon as it is cold. Every 5 parts of the ignited precipitate (oxide) represents 4 parts of copper (nearly); or, more accurately, every 39·7 parts are equal to 31·7 of pure metallic copper:—By immersing a piece of polished steel in the solution, and weighing the resulting precipitate of the copper (see above). Less delicate than the preceding.
Copper can be separated from the other metals by means of the following processes:—
From lead. By adding sulphuric acid to the nitric solution, and evaporating to dryness, when water digested on the residuum will dissolve out the sulphate of copper, but leave the sulphate of lead behind. From this solution the oxide of the copper may be thrown down as before.
From tin. By digestion with hot nitric acid, which dissolves out the tin.
From zinc. By sulphuretted hydrogen, which throws down the sulphide of copper from an acid solution.
From silver. By digesting it in the state of filings or powder in a solution of chloride of zinc, which dissolves the first, but leaves the last unchanged.
Copper may be separated, in a state of great purity, from ANTIMONY, ARSENIC, BISMUTH, LEAD, IRON, TIN, ZINC, &c., as it exists in bell-metal, brass, bronze, gun-metal, mosaic gold, and other commercial alloys, by fusing it in a crucible for about half an hour, along with copper scales (black oxide) and ground bottle-glass, or other like flux. The pure metal is found at the bottom of the crucible, whilst the impurities are either volatilised or dissolved in the flux. The proportions for refining commercial copper are, metal, 10 parts; copper scales and bottle-glass, of each 1 part. The Society of Arts conceived this process to be so508 valuable, that they presented one of their gold medals to its inventor, Mr Lewis Thompson.
Uses, &c. The ordinary uses of copper are well known. In medicine, 3 or 4 gr. of the filings or powder were formerly given in rheumatism, and to prevent hydrophobia. Some of its salts are still used as astringents, emetics, and caustics. Its alloys are of great value. With zinc it forms BRASS; with tin, BRONZE, BELL-METAL, Gun-metal, and SPECULUM-METAL. White copper is formed by the addition of metallic arsenic, and German silver is a mixture of nickel, zinc, and copper.
Ant. Copper in the metallic state is almost inert, but all its compounds are poisonous. The antidotes are—the white of egg, milk, or flour, mixed with water. The hydrated sulphides of iron, iron filings, and ferro-cyanide of potassium have also been strongly recommended, and are exhibited in the same way. Sugar is likewise highly spoken of as an antidote. In all cases a strong emetic should be first given.
Obs. Culinary and pharmaceutical vessels are very commonly made of copper, but too much caution cannot be exercised in their employment. Acid syrups, vegetable juices, aqueous extracts, soups, stews, &c., prepared in copper saucepans, or boilers, receive a metallic contamination proportional to the length of time they are exposed to the action of the metal. Such vessels are frequently tinned, for the purpose of protecting the copper from contact with their contents, but this film of tin is necessarily very thin, and soon becomes imperfect by constant use. When copper vessels are allowed to remain wet or dirty, or, more especially, greasy, a poisonous green rust forms upon the surface, somewhat similar to verdigris. If articles are prepared in them in this state, serious consequences may ensue. Cases of poisoning from this cause are frequently met with, and instances of vomiting following the use of such articles are almost of daily occurrence, without the reason being suspected. We have occasionally seen confections and extracts, prepared in copper pans, deposit a coating of that metal upon the knives used to stir them. The ashes of the inspissated juices of fresh vegetables, and especially the pulps of fruit, prepared in vessels of this metal, have exhibited the presence of copper on the application of chemical tests. Ketchup is frequently rendered poisonous in this way. The most wholesome material for culinary utensils is thin sheet iron, or tinned iron plate (TIN), which is very durable if kept clean and dry when not in use. Copper vessels of every kind should be cleaned out, immediately before use, even though they may not appear to require it, and on no account should they be employed for any fluids that are the least acidulous, or that may have to remain long in them.
The following enamel is recommended in Dingler’s Polytechnic Journal for coating the inside of the copper vessels, used for cooking fruit or vegetables:—12 parts of white fluor-spar, 12 parts of unburnt gypsum, and 1 part of borax, are finely powdered, intimately mixed, and fused in a crucible. The fused mass is then poured out, and after cooling, is rubbed up to a paste. The copper vessel is then coated inside with this preparation, which is applied by means of a brush, and the vessel is placed in a moderately warm place, so that the coating may dry uniformly, when it is subjected to a gradually increasing heat, till at length the preparation fuses. On cooling, the vessel is found to be protected internally by a white opaque enamel, adhering very firmly to the copper, not chipping off by ordinary knocking and rubbing, and impervious to vegetable acids.
Copper may be cleaned by applying a small portion of the following paste, and rubbing it dry by a flannel or leather:—1 oz. oxalic acid, 6 oz. rotten stone, 1⁄2 oz. gum arabic, all in powder, 1 oz. of sweet oil, and sufficient water to make a paste.
Copper, Neu′tral Acetate of. Cu(C2H3O2)2. Syn. Nor′mal cupric acetate, Acetate of copper, Crys′tallised ver′digris. Prep. Dissolve common verdigris or cupric hydrate in hot acetic acid, so as to form a highly concentrated solution; filter and place in a cool situation to crystallise.
Prop. Beautiful dark, bluish-green prisms, which dissolve in 14 parts of cold and 5 parts of boiling water.
Copper, Ba′sic Acetates of. Syn. Ba′sic cu′pric acetates, Sub-ac′etates of copper. Common verdigris is a mixture of several basic acetates which have a green or blue colour. One of these (SESQUIBASIC ACETATE) is obtained by digesting powdered verdigris in tepid water, filtering, and leaving the soluble part to spontaneous evaporation. It may also be obtained in a state of purity by adding liquor of ammonia in small portions to a boiling concentrated solution of the neutral acetate till the precipitate is just redissolved, and leaving the solution to cool. It forms a blue, crystalline mass, but little soluble in cold water. The green, insoluble residue of the verdigris, after treatment with tepid water, contains another acetate (TRIBASIC ACETATE); this may be formed by digesting neutral acetate of copper with the hydrated oxide. A third salt (DIBASIC ACETATE, BLUE VERDIGRIS) is prepared on a large scale in France by exposing copper to the air in contact with fermenting wine-lees.
Copper, Ammo′′nio-sul′phate of. Syn. Sulphate of cuprammonium. Cu′pro-sulphate of ammo′′nia; Cu′pri ammo′′nio-sulphas, L.; Cuivre ammoniacal, Fr.; Kupfer salmiak, Ger. Prep. Sulphate of copper, 1 oz.; sesquicarbonate of ammonium, 11⁄2 oz.; rub together until carbonic acid ceases to be evolved, then wrap it in bibulous paper, and dry it in the air.
Pur. Pulverulent; dark blue; at an intense heat it is changed into oxide of copper, at first sesquicarbonate of ammonia, and, afterwards,509 sulphate of ammonia, being thrown off. It is soluble in water to a splendid purple-blue solution, from which the salt is precipitated by alcohol in blue crystals. This solution has the peculiar property of dissolving CELLULOSE (cotton, paper, &c.). The cellulose may be precipitated from the solution in colourless flakes by the addition of acids.
Uses., &c. It is occasionally employed in pyrotechny. In medicine, it has been given in chorea, epilepsy, hysteria, &c., but is now principally used as an injection, as a wash for foul ulcers, used as a collyrium, in opacity of the cornea.—Dose, 1⁄4 gr., gradually increased to 5 gr., twice a day. Great care must be taken in drying, as it is apt not only to lose a large portion of its weight, but to become of an inferior colour. Both the ingredients should be separately reduced to powder before mixing them.
Copper, Ar′senite of. Cu(AsO2)2. See Green Pigments (Scheele’s Green).
Copper, Carbonate of. CuCO3. Syn. Diba′sic carbonate of copper, Dicarbonate of c.; Cupri carbonas, L. Prep. Add carbonate of soda in excess to a solution of sulphate of copper, and warm the mixture till the pale-blue, flocculent precipitate becomes sandy and assumes a green tint. Used as a pigment. See Green Pigments and Verditer.
Obs. As prepared above, the carbonate contains 2 equivalents of water. The beautiful green mineral, MAL′ACHITE, has a similar composition, but contains only 1 equiv. of water. Another carbonate (TRIBASIC C., BLUE C.), occurs as a natural ore in large, transparent crystals, of the most intense blue; it has not yet been artificially imitated.
Cuprous Chloride. CuCl. Syn. Dichloride of copper, Subchloride of copper. Prep. By exposing the neutral chloride of copper to the action of heat.
Prop. White; fusible; slightly soluble in water; and decomposed by exposure to the air.
Copper, Chloride of. CuCl2. Syn. Neutral chloride of copper. Prep. From copper scales or black oxide of copper dissolved in hydrochloric acid, and the solution evaporated and crystallised.
Prop., &c. Green, acicular crystals; deliquescent; soluble in alcohol, the flame of which it colours green. When gently heated it loses water, and assumes the form of a yellowish-brown powder (ANHYDROUS CUPRIC CHLORIDE, or CHLORIDE OF COPPER); at a high temperature it loses half its chlorine, and becomes converted into cuprous chloride.
Cupric Iodide. CuI2. Syn. Iodide of copper, Dini′odide of copper; Cu′pri iodi′dum. L. Prep. By adding iodide of potassium to a solution of sulphate of copper, and washing out with alcohol the free iodine from the precipitate formed. A greenish-white precipitate.
(Commercial.) To a solution of sulphate of copper, 1 part, and protosulphate of iron, 3 parts, add a solution of iodide of potassium, and wash and dry the precipitate. This is the preparation commonly known in trade by the name of ‘iodide of copper.’
Cupric Nitrate. Cu(NO3)2. Syn. Nitrate of copper; Cu′pri ni′tras, L. Prep. By dissolving the copper in dilute nitric acid to saturation; evaporating to dryness; redissolving in distilled water; filtering, evaporating, and allowing to crystallise; or from black oxide of copper and nitric acid in the same manner.
Prop., Uses, &c. Deep-blue prismatic crystals, very soluble in water and deliquescent, soluble in alcohol. Generally used in medicine externally, in injections, or as a caustic, but sometimes given internally, dissolved in mucilaginous liquids.—Dose, 1⁄8 to 1⁄4 gr.
Cuprous Oxide. Cu2O. Syn. Red oxide of copper, Dinox′ide, Suboxide; Cupri subox′ydum, L. Prep. Add grape sugar to a solution of sulphate or acetate of copper, then further add caustic potassa in excess; the blue solution heated to ebullition deposits the suboxide, which must then be collected, washed, and dried.
A solution of cane sugar, 27 parts, in water, 60 parts, is poured over hydrated oxide of copper (weighed in the compressed and still moist state), 9 parts; a solution of caustic potassa, 18 parts, in water, 60 parts, is then added, and the whole mass well agitated together at the ordinary temperature, and strained through linen. If the dark-blue filtrate is next heated (continually stirring), over a water bath, anhydrous cuprous oxide is disengaged, and the liquor becomes nearly colourless.
Prop., Uses, &c. A superb red powder, with a metallic lustre. It often occurs in beautiful transparent, ruby-red crystals, associated with other ores of copper, and can be obtained in this state by artificial means. It is used as a pigment and a bronze, and as a stain for glass and enamels, to which it gives a rich red colour. By heat it is converted into the black oxide. With ammonia it forms a colourless solution, which rapidly becomes blue from the action of the air.
Cupric Oxide. CuO. Syn. Ox′ide of copper, Black oxide, Protoxide; Cu′pri protox′ydum. Prep. By heating the nitrate or carbonate of copper to redness. When it ceases to lose weight the conversion is completed, and the oxide appears as a heavy, black powder.
By heating in the air the hydrated oxide thrown down from solutions of copper by pure potassa.
By adding caustic potassa, in excess, to a solution of a cupric salt, and heating the whole to a boiling-point; the precipitate is then collected, washed, and dried. A heavy, dark-brown powder.
Uses, &c. Protoxide of copper is unchanged by heat unless combustible matter is present, when it readily parts with its oxygen; hence its general use in ORGANIC ANALYSIS as a510 source of that element. It communicates a beautiful green colour to glass and enamels. With the acids it produces the ordinary salts of copper.
Cupric Sulphate. CuSO4.5Aq. Syn. Sulphate of copper, Blue cop′peras, B. vit′riol; Cu′pri sul′phas, L.; Sulfate de cuivre, Fr.; Kupfer vitriol, Ger.; Neela tootia, Hind. Prep. (Commercial.) The sulphate of copper of commerce is obtained by the oxidation of native sulphide of copper (COPPER PYRITES); by the joint action of air, heat, and moisture, the copper is converted into an oxide, and the sulphur into sulphuric acid. The resulting salt is washed out, and the solution evaporated and crystallised. The water found in and issuing from copper mines often furnishes such a solution ready to the hands of the manufacturers. A large quantity of sulphate of copper is also obtained as a secondary product in the refining of silver, and is occasionally prepared by dissolving in sulphuric acid an oxychloride of copper, made for the purpose by exposing sheets of copper to the joint action of air and hydrochloric acid.
(Pure.) By the direct solution of the metal, or preferably, of its oxide or carbonate in sulphuric acid, or by purifying the commercial salt by recrystallisation, &c.
Prop., Uses, &c. Fine blue crystals, slightly efflorescent, having an intensely styptic and metallic taste. By heat the blue salt loses its water of crystallisation, and becomes a white, anhydrous powder. It dissolves in 4 parts of water at 60° Fahr., and in 2 parts at 212°; is insoluble in alcohol and ether; and is decomposed at an intense heat into protoxide of copper, sulphurous acid, and oxygen. It has been used to prevent the dry rot in timber and in dyeing. It is largely employed as a source of metallic copper in the ELECTROTYPE. Grain is steeped in a weak solution of it by the farmer, to prevent the ‘smut,’ As a medicine, it is employed chiefly as a styptic (in solution) and caustic (in substance) to destroy ‘proud flesh,’ and, less frequently, as an astringent or tonic (from 1⁄4 gr. to 2 gr.), and an emetic (3 or 4 gr. to 10 or 12 gr). It is exceedingly poisonous.
COP′PERAS. This is a generic name for the CRUDE METALLIC SULPHATES. When used without a qualifying adjective, it generally means sulphate of iron.
Copperas, Blue. Crude sulphate of copper. See Copper (above).
Copperas, Calcined′. From green copperas, heated in an unglazed earthen pot until it becomes white and dry. Used as an astringent and ‘drier,’ and in making ink and dyeing.
Copperas, Green. Syn. Copperas. Crude sulphate of iron. See Iron.
Copperas, White. Crude sulphate of zinc. See Zinc.
COP′PERING. Iron may be covered with a thin film of copper by merely immersing it (previously scoured clean) in an acidulated solution of sulphate of copper, after which it must be rinsed in clean water. This film soon rubs off, but still it lasts long enough to deceive the travelling tinker’s customers, who imagine that their copper kettles are properly repaired. Metals may be conveniently coated with compact copper to any desired thickness by means of voltaic electricity. See Electrotype.
COP′ROLITE. Syn. Dung′stone, Fossil manure. This mineral is the petrified dung of carnivorous reptiles. (Buckland.) Coprolites are found in all the secondary and tertiary strata. They contain a considerable proportion of phosphate of lime, for which reason they are largely employed in the manufacture of artificial manures. They form the bases of Lawes’ SUPERPHOSPHATE OF COPROLITE MANURE. The nodules, after being washed, are ground to powder in a mill, and mixed with an equal weight of oil of vitriol.
COPTIS TEETA. (Ind. Ph.) Syn. Coptis, or Mishmi tita. Hab. Mishmel mountains, east of Assam. Officinal part. The dried root (Coptidis Radix), imported into Bengal from Assam in small rattan baskets, each containing from 1 to 2 ounces of the drug. This consists of pieces of a woody rhizome, of the thickness of a small goose-quill and from 1 to 2 inches in length, often contracted at one extremity into a short woody stem; the surface is usually rough, irregular, more or less annulated, and marked with the remains of rootlets in the shape of short spiny point. Externally, yellowish-brown; internally, much brighter, frequently of a golden-yellow colour, exhibiting on fracture a radiated structure. Taste, persistently bitter, and when chewed tinges the saliva yellow. Contains neither tannic nor gallic acid, but abounds with a yellow, bitter principle, soluble in water and alcohol.—Prop. Pure bitter tonic.—Therapeutic uses. In debility, convalescence after fevers, and other debilitating diseases, atonic dyspepsia, and in mild forms of intermittent fevers.—Dose, 10 to 15 gr. of the powdered root, thrice daily.
Tincture of Coptis (Tinctura Coptidis). Take of coptis root, in coarse powder, 21⁄2 oz.; proof spirits, 2 pints. Macerate for 7 days in a closed vessel, with occasional agitation; strain, press, filter, and add sufficient proof spirit to make 1 pint.—Dose. 1⁄2 to 2 fl. oz.
Infusion of Coptis (Infusum Coptidis). Take of coptis root, in coarse powder, 5 dr.; boiling water, 1 pint. Infuse in a covered vessel for 2 hours, and strain.—Dose, 1 to 2 fl. oz., thrice daily.
COR′AL. Syn. Coral′lium, L. The comprehensive term for all calcareous or stony structures secreted by the marine asteroid polypes, or zoophytes. The RED CORAL of commerce, which is so largely employed for beads, earrings, and other ornaments, may be described as the internal skeleton of Corallium rubrum.
511
Coral, Red (Facti′′tious). Syn. Coral′lium ru′brum facti′′tium, L. Prepared chalk, coloured with a little sesquioxide of iron or rose pink, and passed through a sieve. Sold by the druggists for powdered coral.
Coral, Prepared’ Red. Syn. Coral′lium ru′brum prepara′tum. Levigated coral was formerly used in medicine as an antacid or absorbent, and is still occasionally employed as a dentifrice. It consists almost entirely of carbonate of lime, coloured with red oxide of iron, and possesses no advantage over good chalk. It is prepared in a similar manner as chalk.
CORAL, to Bleach. Immerse the coral in a mixture composed of one part of hydrochloric acid, and thirty parts of water; and keep it in this liquid until it becomes quite white. It should then be taken out, washed well in cold water, and allowed to dry.
COPPER, CYANIDE (CuCy2). This salt is much used in electro-coppering. It may be obtained by adding to a solution of a copper salt, a solution of ferrocyanide of potassium; when a precipitate is obtained, which dried, is of a brown colour, and is cyanide of copper.
CORALLINE. See Tar Colours.
CORD′IALS. Syn. Cardi′aca, L. Warm, stimulating, restorative medicines, that tend to raise the spirits and promote the circulation. The principal cordial medicines are noticed under the heads Tincture and Syrup. See also Patent Medicines.
Cordials. Aromatised and sweetened spirits used as beverages. See Liqueur.
CORIAN′DER. Syn. (Coriander fruit, Coriandri fructus, (B. P.); Corianders, C. seed; Coriandrum (Ph. L. E. & D.), L. “The ripe fruit of the Coriandrum sativum, dried.” (B. P.) Coriander is chiefly used by confectioners and distillers as a flavouring ingredient. In the East it is much employed as a condiment, being an ingredient in CURRY POWDER. It is aromatic, carminative, and stimulant; and more effectually covers the taste of senna than any other substance.—Dose, 20 to 60 gr.; chiefly used as a corrective or adjuvant in compound medicines.
CORK. The outer bark of the Quercus Suber or cork oak, a tree common in southern France, Italy, and Spain. The bark obtained from the younger branches of the same tree is employed for tanning. See Alcornoco.
Cork. A stopple or plug for a bottle or jar cut from the above substance. The common practice of employing inferior corks for the purpose of stopping the mouths of bottles is often productive of considerable loss, from the air being only partially excluded, and the contents suffering in consequence. Many a large bin of valuable wine has become, from this cause, in less than a year, little better than sour ‘Cape.’ Chemical preparations often suffer from a similar cause. The best corks are those called ‘velvet corks,’ and of these the finest qualities are imported from France. No pains should be spared to obtain sound and soft cork for connecting the combustion- and drying-tubes used in organic analysis.
Ruschhaupt gives the following process for preparing corks for corking bottles containing alcoholic or caustic liquids:—Paraffin is fused in a suitable vessel, the dry corks are added, and immersed in the paraffin by means of a perforated coon or disk. The air is now easily expelled from the pores of the corks, which after about five minutes, are removed and cooled; they may now be cut and bored like wax, are easily driven into the necks of bottles, and readily removed, retain their smoothness and are gas-tight throughout.
Several attempts have been made to introduce cork-cutting by machinery, but they have hitherto failed to supersede hand labour.
Cork-bo′′rer. A thin brass tube, filed to a cutting edge, used for piercing holes through corks. Several tubes of different sizes, which fit into each other, are generally sold together. This simple and convenient instrument was introduced into the laboratory by Dr Mohr.
CORN. Syn. Cla′vus, L. A horny induration of the skin, with a central nucleus, very sensitive at the base. The common cause of corns is continued pressure over the projection of the bones, from tight or stiff boots or shoes. They are of two kinds, hard and soft. The first grow on the exposed portions of the joints; the last, between the toes.
Preven. This consists in keeping the feet clean, by frequent ablution with warm water, and in the use of easy, soft boots and shoes. Without the latter precaution, corns will generally return, even after they appear to have been perfectly removed.
Treatment. After soaking the feet in warm water for a few minutes, pare the corns as close as possible with a sharp knife, taking care not to make them bleed. They may now be touched over with a little lunar caustic, or nitric acid, or a little concentrated acetic acid or aromatic vinegar. The last two do not stain the skin. The first is used by merely rubbing it on the corns, previously slightly moistened with water; the others, by moistening the corns with them, by means of a small strip of wood, or, preferably, a rod of glass; due care being taken not to allow the liquid to touch the neighbouring parts. This treatment, adopted every 3 or 4 days for 10 days or a fortnight, accompanied by the use of soft, loose shoes, will generally effect a cure. It has been recommended to remove large corns by ligatures of silk, applied as close to their base as possible, and tightened daily until they drop off; but this plan is tedious, and often inconvenient, and is not always successful. Another mode of extirpation is, the application of a small blister, which will frequently raise them with the skin out of their512 beds. In this case the exposed surface must be dressed with a little simple ointment. Soft corns may be removed by applying ivy leaf, previously soaked in strong vinegar changing the piece every morning; or by placing a dressing of soap cerate, spread on a bit of lint or old rag, between the toes. One of the simplest and best remedies for hard corns, and which has received the sanction of high medical authority, is to wear upon the toe or part affected a small, circular piece of soft leather, or, still better, a piece of amadou, spread with diachylon, or some other emollient plaster, and having a hole cut in the centre, corresponding to the size of the corn. (Sir B. Brodie.) By this means the pressure of the boot or shoe is equalised and the apex of the corn protected from injury. The following are among the most useful of the POPULAR REMEDIES FOR CORNS:—
Corns, Caus′tic for. Prep. From tincture of iodine and chloride of antimony, of each, 1 dr.; iodide of iron, 3 grs.; mix. It is applied with a camel-hair brush, after paring the corn. 2 to 4 applications are said to effect a cure.
Obs. Most of the remedies noticed below really act as caustics.
Corns, Lo′tion for. Prep. 1. A solution of sal-ammoniac, 1 part; in proof spirit, 4 parts.
2. A concentrated aqueous solution of sulphate of copper. To be applied night and morning.
Corn Plasters. Prep. 1. From white diachylon, 3 parts; yellow resin, 2 parts; verdigris, 1 part; melted together, and spread on leather.
2. From galbanum plaster, 1 oz.; verdigris, 1 dr.; as the last.
3. From resin plaster, 2 oz.; black pitch, 1 oz.; verdigris and sal-ammoniac, of each 1⁄2 dr.
4. To the last add powdered opium, 1 dr. Recommended to allay pain, &c.
5. (W. Cooley.) A piece of spread adhesive plaster is placed upon a table, and a piece of card paper having a round hole cut in it the size of the central portion of the corn is laid upon it; the exposed part is then softened by holding a piece of heated iron for a second or two near it; the card paper is then instantly removed, and nitrate of silver, in fine powder is sprinkled over the part which has been warmed. As soon as the whole is cold, the loose powder is shaken off, and the plaster is ready for use. Very cleanly and convenient. Two or three applications seldom fail to effect a cure.
6. (Mechanical Corn Plasters.) From common adhesive plaster spread on buckskin, amadou, or vulcanised india rubber, cut into pieces, and a circular hole corresponding to the size of the corn punched in each.
Corn Sol′vent. Prep. 1. Carbonate of potassa or pearlash, contained in an open jar or bottle, set in a damp place, until it deliquesces into an oil-like liquid (oil of tartar). Applied by means of a feather, or a small piece of rag dipped in it is bound on the corn.
2. Hydrate of potassa, 1 dr.; rectified spirit 1 oz.; dissolve. As No. 1.
3. Carbonate of potassa, with smalts, ochre, or bole, q. s. to give it the required colour. It must be kept dry, in a well-corked bottle. A pinch is placed on the corn, and confined by means of adhesive plaster or rag.
4. Carbonate of soda, 1 oz., finely powdered and mixed with lard, 1⁄2 oz. Applied on linen rag every night.
5. (Sir H. Davy’s.) Carbonate of potassa, 2 parts; salt of sorrel, 1 part; each in fine powder; mix, and place a small quantity on the corn for four or five successive nights, binding it on with a rag.
Obs. Care must be taken, in all cases, to pare the corn moderately close before applying the remedy; but in no case should any of the above be applied to a raw surface.
Corns, Pomade’ for. Prep. 1. Powdered verdigris, 1 dr.; savine ointment, 7 dr.
2. Dried carbonate of soda, 3 dr.; lard, 5 dr.; verdigris or smalts, q. s. to give a slight tinge of green or blue. Applied on a piece of rag.
Treatment for Horses.—“Pare out carefully the seat of corn, removing all reddened and diseased horn; reduce the crust of the quarter slightly, where it is unduly strong, but leave the bars and frog untouched. They must be religiously preserved, especially in weak feet, to afford a wide bearing for the bar shoe that should afterwards be used. To soften the parts, apply, in bad cases, a poultice for a day or two, and a few drops of nitric acid, when the horn is dry and scurfy; keep the hoof soft with soft soap and lard, or any emollient dressing, and pare out the corn every fortnight. In horses subject to corns, shoe and pare out frequently; and along with leather pads, use a bar shoe made with a wide heel on the inside quarter, and nailed only on the outside, or with one nail toward the inside toe.”[250]
[250] Finlay Dun.
CORRO′SIVE SUBLIMATE. See Mercury.
CORUN′DUM. See Emery.
CORYZA. Cold in the head. See Catarrh.
COSMET′ICS. Syn. Cosmet′ica, L.; Cosmetiques, Fr. External applications employed for the purpose of preserving or restoring personal beauty. The term is generally understood to refer to substances applied to the cuticle, to improve the colour and clearness of the complexion; but some writers have included under this head every topical application used with the like intention. Hence cosmetics may be divided into—CUTANEOUS COSMETICS, or those applied to the skin; HAIR COSMETICS, or such as are employed to promote the growth and beauty of the hair; and TEETH COSMETICS, or such as are used to cleanse and beautify the teeth. See Baldness, Cosmetique, Dentifrices, Depilatory, Hair-Dye, Pomade, Tooth Powder, 513&c.
COSMETIC VINEGAR (Acetum cosmeticum) is a mixture of tinct. benz., 60 parts; bals. Peruv., 10 parts; eau de Cologne and bals. vitæ Hoffm. ph. bor. āā 150 parts; aceti puri, 300 parts; allowed to precipitate and filtered clear.
COSMETICUM (Dr Henry’s):—For scalp diseases and an application for the hair. Spirit, 180 parts; oil of lemon, 3 parts; oil of bergamot, oil of rosemary, and oil of lavender, of each 1 part. (Hager.)
Cosmeticum (Siemerling) for skin affections, freckles, &c. Sweet almonds, 30 grammes; bitter almonds, 15 grammes; blanched and emulsified with 330 grammes of water; the emulsion strained and mixed with 25 grammes tinct. benzoin and 15 grammes lemon juice. (Wittstein.)
COSMETIQUE. [Fr.] Hard pomatum, formed into a cake or stick for the toilet. It is sometimes coloured black or brown, the pigments being added in the state of an impalpable powder.
1. (Black—Cosmetique Noir.) From good lard, 5 parts; wax, 2 parts; (or, hard pomatum, 7 parts;) melt, stir in levigated ivory black, 2 parts; and pour it into moulds of tinfoil; which are afterwards to be placed in paper sheaths.
2. (Brown—Cosmetique Brun.) As the last, but using levigated umber for ‘plain brown,’ and levigated terra di Sienna for ‘auburn’ and ‘chestnut.’
3. (White, or Plain—Cosmetique Blanc.) The same, without colouring matter.
Obs. They are generally scented with musk, ambergris, or cassia.
Use. The above are used to colour moustaches, eyebrows, whiskers, &c., as well as to keep the hair in its place. The labels on the packets before us have—“pour fixer et lisser les cheveux.” The application must be renewed daily, as the cosmetique is gradually removed by friction, and perfectly so by soap-and-water.
COSMOLINE. Syn. Cosmolin. Under the names of Cosmoline and Vaseline some fatty substances melting at 32° to 85° or even 95° C. have lately appeared in commerce. They are very variable mixtures of solid paraffin with paraffin oil, neutral oil, lubricating oil, &c., and are the residues left after the distillation of petroleum slightly purified by means of charcoal. (Miller.)
Cosmoline has been examined by Mr Naylor, who states his belief that it consists of a mixture of paraffins. Comparing Mr Naylor’s results with those obtained by Mr Moss, in an analysis made of a body imported from America, and called “Vaseline,” there seems little reason to doubt that if this latter and “Cosmoline” are not the same substance, they differ from each other only in a very minute degree, this difference not improbably being due to the varying temperature employed in producing them. Cosmoline was found to have the composition:—
| Hydrocarbons (paraffins?) | 98·59 |
| Moisture | 0·69 |
| Ash | 0·04 |
| ——— | |
| 99·32 |
It melts at 40°C., and has a sp. gr. of 0·866 at 45°C. The composition of Vaseline is as follows:—
| Hydrocarbons (paraffins?) | 97·54 |
| Moisture | 0·50 |
| Ash | 0·05 |
| ——— | |
| 98·09 |
It melts at 37° C., and has a sp. gr. of 0·840 at 55° C.
Both bodies are pale yellow in colour, translucent, slightly fluorescent, and semi-solid, and both are alike insoluble in water, slightly soluble in alcohol, and freely so in ether, whilst they are unaffected by hydrochloric acid and solution of potash. The processes by which it is believed cosmoline and vaseline are obtained, consist in separating the various volatile hydrocarbons from crude petroleum by distillation, the residuum is then brought into contact with superheated steam, and finally purified by filtration through animal charcoal. Vaseline has been also named “petroleum jelly.” Professor Otto, of New York, says that vaseline is very extensively used throughout the United States, as a substitute for lard in the preparation of ointments, a purpose for which the freedom from smell, the negative properties and unalterable qualities when exposed to the air, of both substances, seem highly to commend their superiority to lard for this purpose. They have also been employed very successfully for lubricating surgical instruments, and we believe are, when properly scented, used largely as the basis of hair pomades, whilst their suitability for the preparation of suppositories and pessaries has been urged.
This has been demonstrated by the much greater length of time during which certain ointments made by them remain fresh and undecomposed when compared with those in which lard was used.
The ‘American Journal of Pharmacy’ for March, 1877, gives the following formula as a substitute for cold cream, by E. J. Davidson:—Cosmoline, 24 oz.; white wax, spermaceti, of each 12 oz.; glycerin, 3 fl. oz.; oil of geranium, 1 fl. dr.
COSMOS POMADE (J. Pohlmann, Vienna), 11⁄2 parts white wax, 3 parts spermaceti, 2 parts castor oil, 8 parts almond oil, 2 parts glycerine, 9 parts extract of mignonette, 1⁄2 part eau de Cologne. (Hager.)
COTARN′INE. A crystallisable substance obtained from the mother-liquors of opianic acid. It is basic, very soluble, and bitter. Hydrochlorate of cotarnine is soluble and crystalline.
514
COTO BARK. A bark said to be imported from the interior of Bolivia, and thought by Dr Wittstein to belong to a lauraceous or a terebinthinaceous plant. In one specimen examined by Jobst was found a yellowish-white crystalline substance with the biting taste of the bark, which Jobst believes to be its active principle, and to which he gives the name Cotoin. Another sample, however, analysed by Jobst in conjunction with Hesse, failed to yield any cotoin, but gave instead a crystalline mass which consisted principally of three crystalline bodies, to which these chemists purpose applying the names paracotoin, oxyleucotin, and leucotin. Dr Gietel reports that he made trial of the bark therapeutically with some patients in the general hospital of Munich, and the results he obtained were such that he regards it as a specific against diarrhœa in all its varieties. Sometimes he administered it in the form of powder, and at others in that of tincture, the latter being made in the proportions of one part of bark to ten of spirit. He gave of the powder 1⁄2 grain four to six times a day, and of the tincture 10 minims every two hours. Herr Burkhart, similarly making trial of the cotoin and paracotoin instead, was equally successful as far as regarded its anti-diarrhœic action, paracotoin, however, exercising a slighter effect than the cotoin. Herr Burkhart administered paracotoin either in powder 1⁄10th of a gram, with 1⁄6th of a gram of sugar every three hours, or 1⁄2 a gram rubbed up as an emulsion.
COT′TON. Syn. Gossypium, L. The cotton of which textile fabrics are made consists of hairs covering the seeds of certain plants belonging to the natural order Malvaceæ, or the Mallow family. Our commercial cotton appears to be derived from four distinct species, viz.—
Gossypium arboreum. The tree cotton, an Indian species. Unlike the other cotton plants, it has the dimensions of a small tree. The cotton-hairs are remarkably soft and silky, and are woven by the natives into very fine muslin, used for turbans by the privileged classes only.
Gossypium Barbadense. The ‘Barbadoes’ or ‘Bourbon cotton plant.’ This is the species which yields all our best cotton. In the small American islands which fringe the coast from Charlestown to Savannah, this plant has produced the celebrated ‘sea-island cotton,’ which is unrivalled for the length of its ‘staple,’ its strength, and silkiness.
Gossypium herbaceum. The common cotton plant of India. It produces the Surat cotton of commerce.
Gossypium Peruvianum or acuminatum. A species supposed to be indigenous to America. It furnishes the South American varieties of cotton, as Pernambuco, Peruvian, Maranham, and Brazilian.
Identif. See Linen.
Dyeing. The fibres of cotton have nearly the same affinity for mordants and the colouring matter of dyed stuffs as linen, and may be treated in the same manner. See Dyeing, Linen, &c.
Cotton Cake. The cake remaining after the expression of the oil from the seeds of the cotton plant (Gossypium) is used as a cattle food. The decorticated is preferred to the undecorticated variety, as the latter is said to occasionally set up dangerous internal irritation amongst the animals partaking of it.
Composition of cotton-cake (decorticated).
| Moisture | 9·18 |
| Oil | 16·05 |
| Albuminous compounds | 41·25 |
| Non-nitrogenous principles | 16·45 |
| Phosphates and insoluble earthy matters | 8·15 |
| Woody fibre | 8·92 |
| ——— | |
| 100·00 |
COTTON, GUN-. See Pyroxylin.
COUGH. Syn. Tus′sis, L. The sudden and violent expulsion of air from the lungs. It is generally symptomatic of other affections, but is sometimes idiopathic, or a primary disease. Many cases of cough depend upon the extension of catarrh to the trachea and bronchiæ, which thus become loaded with mucus or phlegm, which they endeavour to throw off by the convulsive effort called coughing. In some cases it is caused by a vitiation and inspissation of the secretions, arising from the imperfect action of the absorbents; this is the common cause of the dry cough of old people. Idiopathic cough is not considered dangerous in itself, or while running its regular course, but it is often productive of most serious consequences, by superinducing the inflammation of some organ, or laying the foundation of phthisis.
Cough is sometimes attended by copious expectoration, and at other times exists without any; it has hence been distinguished into moist or mucous cough, and dry cough.
Treatment. That of common catarrhal cough consists in allaying the irritation as much as possible, by demulcents and expectorants, as mucilaginous drinks and lozenges, which act upon the glottis, and sympathetically upon the trachea and bronchiæ. Among the first may be mentioned almond milk, barley water, refined Spanish juice, gum Arabic, and a mixture of the last two made into lozenges; among the second, the most innocent and convenient is ipecacuanha, in the shape of lozenges, 2 or 3 of which maybe sucked whenever the cough is troublesome. A light diet should be adopted, the bowels kept slightly relaxed by the use of gentle aperients, and a mild and equable temperature sought as much as possible. When this plan does not succeed, recourse may be had to an emetic, followed by small doses of Dover’s powders, and extract or tincture of henbane or squill pill. When a cough is troublesome at night and unattended with515 fever, a small dose of laudanum, or tincture of henbane, taken on going to rest, will generally procure sleep. In the treatment of dry cough the more stimulating expectorants are useful, as garlic, ammoniacum, styrax, and benzoin, combined with narcotics and sedatives, as henbane, hemlock, and opium. A diaphoretic opiate is also very useful, especially in the cough of old people. See Draught, Emulsion, Mixture, Pills, &c.
COU′MARIN (kōō). Syn. Cu′marin. The odorous principle of the fruit or bean of Dipteryxodorata (tonquin bean). It exists in several other plants, as Melilotus officinalis, Asperula odorata, and Anthoxanthum odoratum.
Prep. From the sliced tonquin beans, by macerating in hot alcohol; straining through cloth, and distilling off the greater part of the spirit. The syrupy residue deposits, on standing, crystals of Coumarin, which must be purified from fat oil by pressure, and then crystallised from hot water.
Prop. Slender, brilliant, colourless needles; fusible at 122° Fahr., and distilling at a higher temperature without decomposition. It has a fragrant odour and burning taste; it is very slightly soluble in cold water, more freely in hot water, and also in alcohol.
COUNTER-IR′RITANTS. In medicine and pharmacy, substances applied to the surface of the body to establish a secondary morbid action, with the view of relieving one already existing. In painful and spasmodic affections, as neuralgia, spasms, and cramp; in rheumatism, lumbago, swelled and painful joints; in headache, sore throat, sprains, languid glandular tumours, and many other cases, this class of medicine often proves extremely valuable. The counter-irritants which are best known are blisters, mustard poultices, hartshorn-and-oil, and liniment of ammonia.
COURT PLAS′TER. See Plaster.
COW DUNG. This substance was formerly employed in large quantities by the calico printers. Recently a mixture of sulphate, carbonate, and phosphate of lime and soda, with British gum or bran, has been successfully tested as a substitute for it, and has the advantage of cleanliness and economy.
COW′HAGE. Syn. Cow′itch; Mucun′a (Ph. L. E. & D.), L. “The hairs of the fruit Mucuna pruriens” (Ph. L.). “The hairs from the pods” (Ph. E.). “The hairy down” (Ph. D.). It occasions violent itching when it comes in contact with the skin, which can only be allayed by a solution of green vitriol, or by oil. It is frequently administered as a vermifuge, made into a confection, by scraping the hair off a pod into treacle, syrup, or honey, for a morning dose, which is repeated for 3 or 4 successive days, followed by a brisk purge. It acts more effectually if its administration has been preceded by a gentle emetic.
COW-POX. [Variola Vaccina.] A disease affecting the udder in cows. The treatment consists in fomenting the udder and applying poultices of spent hops, giving laxative and saline medicines, and in drawing off the milk with a teat-syphon.
COWS. See Dairy.
CRAB. See Shell-fish.
CRACKNELS. Small, brittle cakes or biscuits, made by first boiling and then baking paste. Prep. To flour, 1 pint, add a little grated nutmeg, the yolks of 2 eggs, 2 or 3 spoonfuls of rose-water, and cold water, q. s. to make a paste; then roll in butter, 1⁄2 lb., and make it into shapes. In one hour put them into a kettle of boiling water, and boil them until they swim, then throw them into cold water; take them out; and when dry, bake them on tins. Those of the shops contain less butter, and the rose-water is omitted.
CRACK′NUTS. Thin and sweet cakes or wafers. Prep. 1. Flour, 1 lb.; sugar, 3⁄4 lb.; melted butter, 1⁄2 lb.; 6 or 7 eggs, well beaten; make a paste with a glassful of raisin wine and a little water; add caraways, roll it out as thin as paper, cut it into shapes with a tumbler, wash the pieces with the white of egg, and dust them over with powdered sugar.
2. As the last, but using 1⁄2 lb. more flour.
CRAMP. See Spasms.
CRAPE is cleaned by rinsing it in ox-gall and water, to remove the dirt; afterwards in pure water, to remove the gall; and lastly, in a little gum-water, to stiffen and crisp it. It is then clapped between the hands until dry.
CRAY-FISH. See Shell-fish.
CRAY′ONS. Colouring substances made up into small cylinders or any other convenient form for use in writing or drawing.
Crayons, Draw′ing. Prep. 1. Spermaceti, 3 oz.; boiling water, 1 pint; agitate together till they form a species of emulsion; add bone ash, 1 lb. (or more, previously reduced to an impalpable powder), and colouring matter, q. s. to give the proper tint; reduce the whole to a perfectly homogeneous paste, and form it into crayons.
2. Pipeclay and the finest prepared chalk, equal parts; or pipeclay alone, q. s.; colouring, a sufficient quantity; make them into a paste with pale mild ale.
3. White curd or Castile soap, cut into thin shavings, 1 oz.; boiling water, 1 pint; dissolve, and when cold, add gradually as much rectified spirit of wine as will render the liquid barely transparent. With this fluid make equal parts of the finest elutriated clay and chalk into a stiff paste, adding colouring matter, q. s., as before. For common qualities, the spirit of wine may be omitted, but the mass will then dry more slowly.
4. Curd soap, 11⁄2 oz.; gum Arabic, 1⁄2 oz.; boiling water, 11⁄4 pint; dissolve, and use it as the last. General Lomet uses a similar mixture to work up the softest varieties of hematite, with which he thus forms superior red crayon.
516
5. (Process of the Brothers Joel, of Paris.) Shell-lac, 3 parts; spirit of wine, 4 parts; oil of turpentine, 2 parts; dissolve, add pure clay, 6 parts; colouring matter, q. s.; form the mass into crayons, and dry them by a stove heat.
6. Pale shell-lac, 5 parts; wood naphtha, 12 parts; dissolve, and with this fluid mix up the colouring powder, previously stirred up with an equal weight of fine pale-blue clay; dry by a stove heat, as before. When this process is well managed, it produces crayons equal to those of the best Parisian houses.
Obs. The composition may be formed into crayons by simply rolling it on a slab; but to ensure their solidity the manufacturers generally employ a metallic cylinder of 2 or 3 inches in diameter, with one end open and the other firmly secured to a perforated plate, having holes of the same size as the intended crayons. The crayon composition, in the state of a stiff paste or dough, is introduced into the open end, and is forced down and through the holes, by means of a small plug or piston, that exactly fits the inside of the cylinder, and which is driven by the equable motion of a small screw. The pieces that pass through the holes are then cut into lengths and dried.
The substances employed as colouring matters for crayons are very numerous, and their choice offers a wide field for the skill and fancy of the artist. The pigment having been selected, it may be reduced to any shade or tint by admixture with other pigments, and by ‘dilution’ with a proper quantity of elutriated or prepared chalk. As, however, crayon colours do not admit of being mixed together at the time of using them, like liquid colours, it is usual to make 3 to 6 different shades of each colour, so as to enable the artist at once to produce any effect he chooses.
Crayons, Black. From prepared black-lead, ivory-black, lamp-black, &c. Black chalk and charcoal are frequently made into crayons by simply sawing them into suitably sized pieces. They may then be put into a pipkin of melted wax, and allowed to macerate for an hour; after which they should be taken out, drained, and laid on a piece of blotting paper to dry. Drawings made with these crayons are very permanent, and if warmed slightly on the wrong side, the lines will adhere, and become almost as durable as ink.
Crayons, Blue. From indigo, smalts, Prussian blue, verditer, &c.
Crayons, Brown. From umber (raw and burnt), terra di Sienna (raw and burnt), Cullen’s earth, brown ochre, &c.; and some peculiar shades, from a mixture of black, carmine, and either of the above colours.
Crayons, Green. From a mixture of king’s yellow, or yellow ochre, with blues.
Crayons, Purple. From any of the more brilliant blues, mixed with carmine, lake, or vermilion.
Crayons, Red. From carmine, carminated lakes, vermilion, hematite, and any of the earthy or mineral colours commonly used as pigments. Crayons of red chalk may be prepared in the manner pointed out for crayons of black chalk.
Crayons, White. From pure clay and chalk.
Crayons, Yellow. From king’s yellow, Naples yellow, orpiment, yellow ochre, &c.
Crayons, Lithograph′ic. Prep. 1. Tallow-soap, 7 parts; white wax, 6 parts; melt by a gentle heat, and add lamp-black, 1 part; keep it melted with constant stirring, for 20 or 30 minutes, then let it cool a little, and cast it into moulds.
2. White wax, 4 parts; shell-lac and hard tallow-soap, of each 2 parts; lamp-black, 1 part; as last.
3. Spermaceti, white wax, and hard tallow-soap, of each equal parts; lamp-black, q. s. to colour.
Obs. Some makers melt the soap, wax, and lamp-black in an iron ladle, over a brisk fire, and allow the mixture to blaze for a few seconds before adding the shell-lac, which is no sooner thoroughly incorporated than the heat is increased until the mass again kindles, when it is at once removed from the fire and stirred until it is cool enough to be poured into the moulds. This method leads to trouble and loss, without any corresponding advantage. These crayons are used to draw designs upon lithographic stones.
Crayons for Writing on Glass. Prep. 1. From French chalk, cut into suitable pieces. Marks made with these crayons, when obscured or rubbed out, may be several times revived by simply breathing on the glass.
2. (Brunquelle.) Spermaceti, 4 parts, tallow, 3 parts, wax, 2 parts, are melted together in a cup; and red lead, 6 parts, and carbonate of potassa (in fine powder), 1 part, stirred in; the mass is kept melted and stirred for about half an hour longer, then poured into glass moulds (tubes) of the thickness of a common pencil, and cooled as rapidly as possible. The mass may be screwed up and down in the tube, and cut to a point with a knife. A crayon is thus obtained which will readily write upon clean, dry glass.
CREAM. Syn. Crem′or, C. lac′tis, Flos lac′tis, L. The oleaginous portion of milk, which collects in a thin stratum upon the surface, when that fluid is left undisturbed for some time. By violent agitation, as in the process of churning, the fatty globules unite together, forming butter; whilst the liquid portion, consisting of caseum, serum, and a little butter, constituting the residuum, is called butter-milk. This separation is effected the most readily when the cream has become partially sour and coagulated by being kept a few days, a change which occurs in consequence of the conversion of some of the sugar of the serum into lactic acid, which precipitates the caseous matter contained in the small portion of the milk with which the517 cream is mixed. On these simple facts chiefly depend the successful manufacture of butter. The cream intended for churning should therefore be kept until it turns slightly sour, and assumes the condition above referred to, as then the butter will readily ‘come.’ If churned while quite sweet the operation will be tedious, and will frequently fail. When this happens the dairy maids declare the milk is ‘charmed’ or ‘bewitched,’ and reluctantly proceed with the operation. The addition of a little rennet or vinegar is the proper remedy in this case, and will cause the almost immediate separation of the butter.
When cream is suspended in a linen bag, and allowed to drain, it gradually becomes drier and harder, by the separation of the liquid portion, and then forms what is known by the name of cream cheese. By the application of slight pressure the separation of the whey is more completely effected, and the product is not only better, but will keep longer.
Qual. Cream, in a dietetic point of view, may be regarded in the same light as butter, as it is converted into butter in the process of digestion. On this account much cream should never be taken at once by persons of delicate stomachs. In eating cream with fruit persons are hardly aware of the large quantity they consume, until they find it disagree with the stomach, when the condiment is blamed for the indiscretion of those who take it.
Mr Wanklyn gives the following as the composition of six different samples of cream:—
| 1. | 2. | 3. | 4. | 5. | 6. | |
| Water | 72·20 | 71·2 | 66·36 | 60·17 | 53·62 | 50·00 |
| Fat | 19·00 | 14·1 | 18·87 | 33·02 | 38·17 | 43·90 |
| Milk, Sugar, Casein, and Ash. | 8·80 | 14·7 | 14·77 | 6·81 | 8·21 | 6·10 |
A quart of good cream generally yields from 13 oz. to 15 oz. of commercial butter.
Mr Blyth says: “The analysis of cream is conducted on exactly the same principle as that of milk; but the cream must be weighed, not measured; and smaller quantities may be evaporated to dryness in order to estimate the water, if the ratio of water to the solids not fat is such that adulteration may be suspected; for this ratio, although occasionally disturbed by some of the casein rising with the fat, is practically the same as in milk.” Mineral adulterations, such as carbonate of magnesia, will be detected, if present, in the ash. See Milk, Butter, &c.
Cream, Al′mond. Prep. From sweet almonds, 2 oz.; bitter almonds, 4 in no.; blanched and beaten in a mortar to a smooth paste, adding a teaspoonful of water to prevent oiling; and afterwards a pint of cream, and enough powdered lump sugar to sweeten; the whole is then whisked to a froth, the glasses filled with the liquor, and some of the froth placed on the top of each. Some persons add the juice of a lemon.
Cream, Bran′dy. Prep. To the last add the yolks of 6 eggs; heat it gently over the fire until it thickens, keeping it well stirred, then farther add two or three glassfuls of brandy, and pour it into small cups or shallow glasses.
Cream, Burnt. Prep. Cream, 1 quart; cassia, a small stick; peel of half a lemon; boil for 5 minutes, cool a little, take out the spice, and add the yolks of 9 eggs, and sugar, q. s. to sweeten; stir until cold, put it into a dish, strew pounded sugar over it, and bake it until brown.
Cream, Choc′olate. Prep. Chocolate, scraped fine, 1 oz.; thick cream, 1 quart; sugar (best), 6 oz.; heat it nearly to boiling, then remove it from the fire, and mix it well; when cold, add the whites of 8 or 10 eggs; whisk rapidly, and take up the froth on a sieve; serve the cream in glasses, and pile up the froth on the top of them.
Cream, Cof′fee. Prep. 1. As the last, omitting the chocolate, and using a pint of the strongest made coffee.
2. Add a teacupful of very clear, concentrated, made coffee to 1 pint each of clarified calf’s feet jelly and good cream; sweeten with lump sugar, give it one boil up, and pour it into shapes or glasses when nearly cold.
Cream, Cold. See Cosmetic, Cerate and Granulated Cream (below).
Cream, Costorph′in. After a village near Edinburgh, where it is commonly made. Prep. The milk of 3 or 4 consecutive days, together with the cream, are allowed to remain until sour and coagulated; the whey is then drawn off, and fresh cream added. It is eaten with sugar and fruit, especially with strawberries and raspberries.
Cream, Dev′onshire. Prep. 1. (Devonshire raw cream.) From sour cream mixed with an equal quantity of fresh cream, and sweetened with sugar. Eaten with fruit.
2. (Devonshire scalded cream, D. clouted c.) The milk of yesterday is set in a polished, shallow, brass pan, over a clear fire free from smoke, and gradually heated until very hot, care being taken not to let it boil; when the undulations on the surface look thick, and form a ring round the top of the fluid, the size of the bottom of the pan, it is removed from the fire and allowed to cool; the next day it is skimmed off for sale. Used with either tea or coffee, and excellent with both; it is also eaten with sugar and fruit, and is made into butter. See Cream (above).
Cream, D’Illotte’s. Syn. Crystallised cream, Vegetable c. The ingenious manufacturer whose anagrammatic powers have converted his patronym of Elliott into one less familiar to vulgar English ears, prepares this really elegant hair cosmetic as follows:—Oil of almonds, 3 oz., and spermaceti, 1⁄2 oz., are melted together; and bergamot, neroli, and verbena, of each 5 drops, and huile au jasmin, 10 drops, are then stirred in, and the518 mixture is at once poured into small, wide-mouthed bottles, to crystallise. If preferred harder, 1⁄2 dr. more spermaceti may be used, but the precise quantity to produce the best crystalline appearance depends greatly on the season of the year, more being required in winter than in summer.
Cream, Facti′′tious. Syn. Mock cream. Prep. 1. Beat 3 eggs, with 2 oz. of sugar, and a small piece of butter, until the combination is complete; then add warm milk, 1 pint; put the vessel into another containing hot water, and stir it one way until it acquires the consistence of cream.
2. Arrowroot, 1 spoonful; wet it with a little cold milk, then add, gradually, boiling milk, 1⁄4 pint; mix well, and further add, of fresh butter, 1 oz.; sugar, 11⁄2 oz.; cold milk, 3⁄4 pint; and continue stirring until the whole is quite cold.
Cream, Ice. See Ice.
Cream, Fruit. Prep. From pulped or preserved fruit, 1 lb.; cream, or good raw milk, 1 quart; sugar q. s.; boil for 1 minute; cool, and add a glassful of brandy. A froth is raised on these creams with a chocolate mill. It is taken off and placed on a hair sieve, and some of it, after the glasses are filled with the cream, placed on the top of each. The expressed juice of raspberries, of currants, and several other kinds of fruit, also make delicious creams. In winter, raspberry jelly, jam, or syrup may be used. A glass of good brandy improves these creams.
Cream, Fur′niture. See Polish.
Cream, Gran′ulated. Syn. Granulated cold cream. Prep. (Owen.) Almond oil, 6 oz., white wax and spermaceti, of each 2 oz., are melted together, and a little otto of roses added; the liquor is then poured into a large Wedgwood-ware or marble mortar, previously warmed, and containing 11⁄2 to 2 pints of warm water; brisk agitation with the pestle is then had recourse to, until the oleaginous portion is well divided, when the whole is suddenly thrown into a vessel containing a gallon or two of clean cold water; lastly, the granulated cream is thrown on a muslin filter; and as much water as possible is shaken (gently) out of it; after which it is put up for use.
Cream, Lem′on. Prep. From cream, 1 pint; yolks of 3 eggs; powdered sugar, 6 oz.; the yellow rind of 1 lemon (grated), with the juice; mix, apply a gentle heat, and stir until cold. If desired white, the whites of the eggs should be used instead of the yolks.
Cream, Or′ange. Similar to lemon cream, but using oranges.
Cream, Pis′′tachio. From the kernels of pistachio nuts, as almond cream.
Cream, Rasp′berry. See Cream, Fruit.
Cream, Sat′urnine. Syn. Crem′or plum′bi Aceta′tis, L. Prep. (Dr Kirkland.) Cream, 1 oz.; solution of diacetate of lead, 1 dr.; mix. Cooling, sedative, and astringent; a useful application in certain cases to irritable ulcers, sore nipples, &c. It is poisonous.
Cream, Scotch Sour. Prep. (Gray.) Skimmed milk is put over night into a wooden tub, with a spigot at the bottom, and this tub is put into another filled with hot water; in the morning the small tub is taken out and the thin part of the milk (‘wigg’) drawn off until the thick, sour cream begins to come. This process requires practice as to the heat of the water; when it succeeds, skimmed milk yields nearly one half of this cream, which is eaten with sugar as a delicacy; it is only distinguishable from cream by its taste, and sells for double the price of fresh milk.
Cream, Stone. Syn. Cream blancmange. Prep. From isinglass, 1⁄2 oz., dissolved in boiling water, a teacupful, adding cream, 1 pint, and sugar, 4 oz.; stirred until nearly cold, and then poured over fruit or preserves, placed on the bottom of glass dishes.
Cream, Tarax′acum. Syn. Crem′or tarax′aci, L. Prep. (Dr Collier.) From washed dandelion roots (sliced), sprinkled with spirit of juniper, and then pressed for their juice.—Dose. A table-spoonful twice or thrice daily, as a stomachic and tonic, in dyspepsia, &c.
Cream, Vanil′la. Prep. 1. Boil a stick of vanilla (grated), and isinglass, 1⁄2 oz., in milk, 1 pint, until the latter is dissolved; strain, add sugar, 6 oz., and cream, 1 pint; stir till nearly cold, then pour it into moulds like blancmange.
2. Cream and strong isinglass jelly, of each 1 pint; sugar, 6 oz.; essence of vanilla, 1⁄4 oz.; mix as before.
Cream, Vel′vet. Prep. As the last, but, instead of vanilla, flavour with the rind and juice of a lemon, and about a teacupful of white wine.
Cream, Whipped′. Prep. From the whites of 12 eggs; cream, 1 quart; pale sherry, 1⁄2 pint; essence of musk and ambergris, of each, 10 drops; essences of lemon and orange peel, of each, 3 or 4 drops; whisk to a froth, remove the latter on to a sieve, fill the glasses with the cream, and then pile the froth on the top of them.
CRE′ASOTE. See Kreasote.
CRE′ATINE. See Kreatine.
CREAT′ININE. See Kreatinine.
CRÉME. [Fr.] Syn. Cream. This name is applied to several compound spirits and cordial liquors, especially by the French liqueuristes, who pride themselves on the superior quality and cream-like smoothness of their manufactures. Like the cordials of the English, they are mostly dilute spirit, aromatised, and sweetened. See Liqueurs.
CREME DE BEAUTÉ. A cosmetic consisting of an emulsion of bitter and sweet almonds.
CREN′IC ACID. A brown substance discovered by Berzelius in certain mineral waters. It is a modification of HUMUS, and is produced by the decay of vegetable matter.
519
CRESYLIC ACID. C7H8O. Syn. Cresol, Kresylic acid, Kresol. One of the homologues of carbolic acid, found in coal tar. Cresylic, like carbolic acid, is a useful disinfectant.
CRIB-BITING.—The use of deal or any unseasoned wood for the manger may induce this habit in horses. To remedy it the stable fittings should be of iron. As the habit very frequently arises from acidity of stomach in horses, the administration of chalk or other antacids has been recommended.
CRICK′ETS. These insects may be destroyed by putting Scotch snuff into their holes, or by placing some pieces of beetle wafers for them to eat.
CRINUM ASIATICUM. (Ind. Ph.) Habitat. Low humid localities in Bengal, the Concans, and other parts of India; also cultivated in gardens; Ceylon, the Moluccas, and Cochin China.—Officinal part. The fresh root (Crini Radix); bulbous, with a terminal stoloniferous fusiform portion issuing from the crown of the bulb; emits an unpleasant narcotic odour; readily dried in a stove, and reducible to powder after desiccation.—Properties. Emetic; in small doses nauseant and diaphoretic.—Therapeutic uses. Analogous to those of squill.
Juice of Crinum (Succus Crini; Infusum Crini, Beng. Ph.). Take of the fresh root of crinum, 1⁄2 an ounce; cold water, 2 ounces. Bruise the root in a stone mortar, gradually adding the water. Strain, with pressure, through calico.—Dose. From 2 to 4 fluid drachms, every twenty minutes, until the desired effect is produced.
Syrup of Crinum (Syrupus Crini). Take of the fresh root of crinum, sliced, 8 ounces; boiling water, 1 pint; refined sugar, 1 pound. Macerate the root in the water for two hours, bruise in a mortar, press through calico, add the sugar, and dissolve with the aid of gentle heat.—Dose. About 2 fluid drachms, repeated as required. Used as a nauseant and emetic for children.
CROTON CHLORAL. Syn. Butyl chloral. A colourless oleaginous liquid, having an odour somewhat like that of ordinary chloral; insoluble in water. Croton chloral may be prepared by the process of Krämer and Pinner, who were the first to obtain it. A current of chlorine gas is passed into aldehyd during twenty-four hours. At the commencement of the operation the action is very energetic; so much so that it is necessary to surround the vessel containing the aldehyd with a refrigerating mixture, and it is only towards the end that the temperature is raised to 100° C. Large quantities of hydrochloric acid are generated during all the time the chlorine is acting on the aldehyd. The resulting product is submitted to fractional distillation, and the liquid passing over between 163° and 165° C. is croton chloral. Croton chloral is the hydride of trichlorcrotonyl (C4H2Cl3OH), or the aldehyd of crotonic acid (C4H5OOH) in the radical of which three atoms of hydrogen have been replaced by three atoms of chlorine. Like ordinary chloral, croton chloral combines with water to form a crystallised hydrate which is the substance used in medicine. Croton chloral hydrate occurs in white nacreous spangles. It is very slightly soluble in cold water, more so in warm, and extremely soluble in alcohol. A convenient solvent for it is glycerin, in which it dissolves much more easily than in water. The dose of the hydrate as a hypnotic is from 8 to 15 grains, for neuralgia 5 grains are given three times a day. Dr Liebreich, who first introduced croton chloral to the notice of the medical profession, says he has failed to discover that it exercises any hurtful effects on the stomach and other organs. On the contrary, Dr Worms asserts that he finds it not so generally tolerated as ordinary chloral, and Gay affirms that it is more uncertain in its narcotic effects.
CRO′TON OIL. Syn. Oleum croto′nis (B. P.), O. tiglii (Ph. L. & D.), L. The “oil expressed from the seeds of Croton tiglium” or purging croton. This oil is a drastic purgative, and a powerful local irritant and rubefacient. Rubbed on the skin, it produces a pustular eruption, and frequently purges. In this way (diluted with thrice its weight of olive oil) it is occasionally used as a counter-irritant.—Dose (as a purge), 1 to 2 drops; in obstinate constipation, lead colic, &c.
The residuum from which the oil has been expressed is sometimes used in veterinary practice under the name of croton cake, or croton farina; but as the amount of oil it contains varies greatly, it is irregular and uncertain in its effects.
CROUP. Syn. Cynan′che laryn′′gea, C. suffoca′tiva, C. trachea′lis, L. An inflammatory disease affecting the larynx and trachea.
Symp. A permanently laborious and suffocative breathing, accompanied by wheezing, cough, a peculiar shrillness of the voice, and more or less expectoration of purulent matter, which continually threatens suffocation. There are two varieties, acute croup and chronic croup. The latter is very rare.
Treat. Bleeding by leeches or cupping, over the region of the trachea, should be immediately had recourse to, when the symptoms are urgent; or violent local irritants, as pieces of lint dipped in strong acetic acid, or blisters, may be applied to the same part. In weakly subjects of irritable constitution bleeding should be avoided. Dr Larroque recommends repeated vomiting in the croup of children; and M. Marotte and M. Boudet have adopted this plan with great success. The treatment consists in making the patient attacked with croup vomit a great number of times within the day, so as to detach the pseudo-membrane from the larynx nearly as520 fast as it is formed. For this purpose M. Marotte employs one or other of the following formulæ:—
1. Tartar emetic, 11⁄2 gr.; syrup of ipecacuanha, 1 oz.; water, 2 oz.
2. Impure emetine, 3 gr.; syrup of ipecacuanha and water, of each 11⁄2 oz.
These draughts are administered by spoonfuls every ten minutes, until there has been a sufficient number of vomitings. In this manner he says he has been always able to make the patient expectorate a certain quantity of false membrane. This treatment is accompanied by the use of small doses of calomel, leeches to the throat, and blisters to the nape of the neck; but it is the opinion of M. Marotte that the vomitings alone effect the cure. Out of 25 cases that occurred at the Hôpital des Enfans[Enfants], the only authenticated case of cure among all these was effected by emetics. (M. Boudet.)
The croup is a very dangerous disease, and medical aid should be immediately sought wherever it can be procured. It is principally confined to infancy, or to children under 9 years of age; but occasionally attacks adults. One of our early friends, a young medical practitioner of great promise, died of it prematurely, after only about 20 hours’ illness.
CROWDIE. Mix the liquor in which a leg of mutton has been boiled with half a pint of oatmeal, and two onions cut very fine; and add pepper and salt. Make the oatmeal into a paste with a little of the liquor over the fire, stir in the remainder of the ingredients, and let them boil gently for twenty minutes. This forms a very nutritious and cheap dish.
CROWING, IN CHILDREN. Syn. Childcrowing. Spurious croup. Spasmodic croup. This very formidable disorder almost always occurs during teething. It comes on in paroxysms. In the intervals between the spasms the respiration is quite natural; but during the attack there is great difficulty of breathing accompanied with a crowing noise, and with violent struggling on the part of the little sufferer. Convulsions and faintness also sometimes occur. In his ‘Advice to a Mother’ Mr Chavasse prescribes the following treatment:—
“The first thing, of course, to be done is to send immediately for a medical man. Have a plentiful supply of cold and hot water always at hand, ready for use at a moment’s notice. The instant the paroxysm is on the child, plentifully and perseveringly dash cold water upon his head and face. Put his feet and legs in hot salt-mustard-and-water, and if necessary place the child up to his neck in a hot bath, still dashing water upon his face and head. If he does not quickly come round, sharply smack the back and buttocks. As soon as a medical man arrives, he will lose no time in thoroughly lancing the gums, and in applying appropriate remedies. During the intervals, great care and attention must be paid to the diet. If the child be breathing a smoky, close atmosphere, he should be immediately removed to a pure one. Indeed in this disease there is no remedy equal to a change of air—to a dry bracing neighbourhood. Even if it be winter, change of air is the best remedy, either to the coast or to a healthy farmhouse. In a case of this kind where it is not practicable to send a child from home, then let him be sent out of doors during the greater part of every day; let him, in point of fact, almost live in the open air. I am quite sure from an extensive experience that, in this disease, fresh air, and plenty of it, is the best and principal remedy.”
CRU′CIBLE. Syn. Melting Pot; Crucibulum, L.; Creuset, Fr. A vessel used by metallurgists and chemists for holding substances whilst they are exposed to a high temperature. The crucibles commonly used for fusing metals are formed of clay, or a mixture of plumbago and clay. For certain purposes, crucibles of platinum, gold, silver, iron, porcelain, and lime, are employed.
Crucibles, Earth′en. Syn. Clay crucibles. From fire-clay, mixed with silica, coke, burnt clay, or other infusible matter.
Manuf. The materials, having been ground and kneaded, are generally moulded by hand upon a wooden block of the shape of the cavity of the crucible. Another method of shaping a crucible consists in ramming the ingredients into a suitable mould, formed of steel or gun-metal. (See engr.)
a a. External steel mould.
b b, Clay or composition for forming the crucible.
c, Internal steel mould.
d d, Wooden stand.
e, Cord or chain to withdraw the internal mould or plug.
Small crucibles are sometimes formed by pouring ‘slip,’ that is, clay mixed with sufficient water to give it the consistence of cream, into porous moulds, made of a species of stucco. A series of these moulds are placed upon a table and filled with the semifluid composition. By the time the whole (say 50 or 60) are filled, the ‘slip’ may be poured out of the one first filled, leaving only a very small quantity behind to give the requisite thickness to the bottom. The second and third may then be treated in the same way, until the whole number have been attended to. In each mould a perfect crucible is formed, by the abstraction of the water of that portion of the ‘slip’ in immediate contact with the stucco, and the crucible is either thicker or thinner in proportion to the time this absorbent action has been allowed to go on. 70 or521 80 crucibles may thus be easily made in less than 15 minutes. The moulds and their contents are next placed in a stove or slow oven. In a short time, from the contraction of the clay in drying, the crucibles may be removed, and the moulds, as soon as they have become dry, may be again filled; by care they will last for years.
Earthen crucibles are used both in the burnt and unburnt state. Small crucibles are generally kiln-burnt before they are used, but the large Stourbridge clay ‘casting-pots,’ which are extensively employed in brass foundries, are never previously burnt.
The following kinds of earthen crucibles are much used in the arts:—
Crucibles, Cornish. From Teignmouth clay, 1 part; Poole clay, 1 part; sand from St. Agnes’s Beacon, Cornwall, 2 parts. When smaller and less refractory crucibles are needed, the same mixture is employed, with the addition of an eighth part of China clay, or Kaolinite from St. Austell. These crucibles are generally made round, and of two sizes, of which one fits into the other; the larger being 3 inches in diameter at the top, and 31⁄2 inches high outside measure. They are coarse in grain, and of a greyish-white colour, spotted with dark specks. They are always kiln-burnt. Of all crucibles, none are more generally useful for metallurgical experiments.
Crucibles, Hessian. From a mixture of equal weights of Almerode clay and sand. They are generally triangular in shape, so that the melted metal may be conveniently poured out from each corner. They are usually sold in ‘nests’ of six crucibles, fitting one in another. In the character of their body, and in composition and qualities, they closely resemble the Cornish.
Crucibles, London. From a very refractory clay. They have a reddish-brown colour, and are close in grain. They are exceedingly useful in assaying, as they resist the action of fused oxide of lead much better than most clay crucibles. Being very liable to crack, they require to be used with care.
White Fluxing-pots. From a peculiar kind of foreign clay. They are manufactured by the Patent Plumbago Crucible Company, and are much esteemed by metallurgists, being well moulded and very refractory. They have a smooth surface, and withstand the action of fluxes satisfactorily.
Crucibles, Stourbridge-clay. From Stourbridge clay, 4 parts; burnt clay, obtained by pounding and grinding old glass pots, 2 parts; pipe-clay and coke-powder, of each 1 part.
Anstey’s Patent. From Stourbridge clay, 2 parts; hard gas-coke (previously ground and sifted through a sieve of 1⁄8th-inch mesh), 1 part.
Obs. These crucibles of Stourbridge clay are made large enough to hold forty pounds or more of melted brass. They are only dried, and not baked. For use they are warmed, placed on the furnace, bottom upwards, the burning coke gradually heaped round them, and the firing continued until they acquire a fully red heat. They are then quickly taken out of the furnace, and put in again with the mouth upwards. If placed in the furnace with the mouth upwards at first, they are sure to crack. After they have been once used and allowed to become cold they are worthless.
Crucibles, Plat′inum. These are indispensable instruments in the laboratory of the analytical chemist. They are chiefly employed in the ignition of precipitates, and in the fusion of silicates with carbonated alkalies to render them soluble, a preliminary step to their analysis. The most ordinary form of the platinum crucible is that of a cup with a flat bottom. They are always provided with lids, which are sometimes so constructed that they may be used, when separated from the crucibles, as capsules for ignitions and evaporations. Platinum crucibles are not acted on by carbonated alkalies at a high temperature, but they are liable to be seriously damaged by the caustic alkalies. Precipitates of the more reducible metals must never be ignited in these crucibles, as the reduction of the metals would infallibly destroy the vessels.
Crucibles, Gold, are exceedingly useful for many operations, on account of the way which they stand caustic and carbonated alkalies, and nitric acid, which destroy platinum or silver crucibles respectively. Their drawbacks are their great expense and ready fusibility.
Crucibles, Silver. These are much used for fusions of alkalies, being much less acted on than platinum crucibles, and also for water analyses, from their cheapness and light weight. They are easily destroyed, however, by acids.
Crucibles, Plumba′go. Syn. Graphite c., Blacklead c., Blue pots. From graphite, ground and sifted, mixed with sufficient refractory clay to render it plastic. They are shaped by hand on an ordinary potter’s wheel, or by moulds of metal like that figured above under the head of Crucibles, Earthen.
Prop., &c. Good blacklead crucibles, even when of the largest size, support the greatest and most sudden alternations of temperature without cracking, and may be used after repeated heating and cooling. Their surface, within as well as without, may be made very smooth, so that particles of melted metal will not hang about the sides. They are now almost universally used for melting the precious metals.
Crucibles, Por′celain. These beautiful vessels are now made in Germany and France of all shapes and sizes. They are formed of the most exquisitely white, thin, and hard porcelain, which does not crack when heated, and which is but little acted on by the most energetic chemical reagents. For some operations they supersede platinum crucibles, particularly in the ignition of the precipitates522 of the more reducible metals. They do not retain colouring matter, and are not porous. Their covers are excellently adapted for delicate cases of testing, the whiteness of the porcelain showing the changes of colour in a single drop of liquid most distinctly.
Crucibles, Iron. Used chiefly for preparing common reagents, as sulphide of iron, calcic chloride, &c., and also for preparing pure caustic potassa from the nitrate.
CRUMP′ET. A sort of muffin or tea-cake, very light and spongy. Prep. From flour, 2 lbs., made into a dough with warm milk-and-water, adding a little salt, 3 eggs (well beaten), and 3 teaspoonfuls of yeast, mixed to the consistence of thick batter; after standing before the fire for a short time, to rise, it is poured into buttered tins, and baked slowly to a fine yellow. For the table, crumpets are toasted lightly on both sides, buttered, piled on a hot dish, and cut into halves.
CRUST. The paste with which pies, tarts, &c., are made, or covered.
1. (Fine.) From flour, 1 lb.; sugar, 1⁄4 lb.; melted butter, 1⁄2 lb.; 3 eggs; milk, q. s. Requires little baking.
2. (Raised crust, for meat pies, &c.) As the last, but using 6 oz. of lard for the butter, and 2 instead of 3 eggs.
3. (Short.) From flour, 1 lb.; butter and sugar, of each 2 oz.; eggs, 2 in no.; made into a stiff paste.
Obs. The quality is improved if the whole or a portion of the butter is employed in the way directed under Puff paste. For further information hereon, consult the cookery books of Acton, Beeton, Rundell, and Soyer.
CRY′OLITE (3NaF1AlF3). A native double fluoride of aluminium and sodium, found in large quantities in Greenland, employed in the manufacture of alum, and also as a source of metallic aluminium.
CRYOPH′ORUS. See Refrigeration.
CRYS′TAL. A solid body, having a regular geometrical form. The plane surfaces by which a crystal is bounded are termed faces; these intersect in straight lines or edges; and these again meet in points, and form angles. The axis of a crystal is an imaginary line passing through its centre, and terminating either in the middle of two faces or of two edges, or in two angles; and axes terminating in similar parts of a crystal are named similar axes. When the axes of a crystal are properly chosen, and placed in a right position, the various faces are observed to group themselves in a regular and beautiful manner around these axes, and to be all so related to them as to compose a connected series, produced according to definite laws. The multitudinous forms of crystals have been distributed by mineralogists and chemists into six primary classes or systems, distinguishable from one another by the relative positions and lengths of the three axes about which the planes or faces are arranged; while the different figures of any particular system are distinguishable by the arrangement of the planes in respect to the axes. Thus, the cube or hexahedron, the rhombic dodecahedron, and the octahedron all belong to the regular system, which is characterised by 3 equal axes cutting one another at right angles. But in the cube each plane cuts 1 axis, and is parallel to 2 axes; in the dodecahedron each plane cuts 2 axes, and is parallel to a third; while in the octahedron each plane cuts the 3 axes. The names and definitions of the six crystalline systems are given below:—
| 1. Regular system. | The 3 axes equal and rectangular. |
| 2. Square prismatic s. 2 equal axes. | The 3 axes unequal, and rectangular. |
| 3. Right prismatic s. All unequal. | |
| 4. Rhombohedral s. | The 3 axes equal, but not rectangular. |
| 5. Oblique prismatic s. 1 axis rectangular to 2. | The 3 axes not equal, and not rectangular. |
| 6. Doubly o.p.s. None rectangular. |
CRYSTALLISATION. The act or process by which crystals are formed. The frequent reference to this subject in the pages of this work, and the constant employment of the process of crystallisation in the manufacture of salts, &c., in the laboratory, seem to point out the necessity of a few explanatory remarks thereon under this head. When fluid substances are suffered to pass with adequate slowness to the solid state, or when solutions of solids are slowly concentrated by evaporation, or the solvent powers of the menstruum, gradually lessened by cooling, the ultimate particles of matter frequently so arrange themselves as to form regular geometrical bodies, familiarly known by the name of crystals. This wonderful property, which is possessed by a great variety of substances in the mineral kingdom, and by nearly all saline bodies, is resorted to for many useful and important purposes in the chemical arts. It is by means of crystallisation that the majority of salts are obtained in a state of purity; for in the act of passing into the crystalline state, the foreign substances with which they are united are left behind in the mother-liquor.
Salts are crystallised, either by allowing their hot and saturated solutions to cool slowly, or by simply evaporating the menstrua as long as crystals form. In the first case the liquid is commonly evaporated until a pellicle appears on the surface, when the vessel is set aside in some sheltered situation until cold, at which time the crystals are collected, and the process repeated for fresh crystals. In the second case the crystals are usually removed from the liquid as soon as they are deposited. The first method is adopted for those salts that are considerably523 more soluble in hot than in cold water, as carbonate of soda, Epsom salts, &c.; the last method, for those that possess nearly equal solubility in both cases, and also for many salts which are not required in handsome crystals; thus common salt and chromate of potash are crystallised in this way. Many of the alkaloids, and their salts, are obtained in crystals, by allowing their solutions (generally alcoholic or ethereal) to evaporate spontaneously. By repeating the processes of solution and crystallisation two or three times with the same body, the crystals obtained by the last operation will usually be found to be quite pure.
Many solids may be readily obtained in a crystalline state by melting them and allowing them to cool very slowly. Thus, iodide of sulphur is crystallised by melting it in a flask placed in a salt-water bath, and allowing it to remain in the water until the whole becomes cold. Sulphur and many metals are crystallised by pouring them, in a state of fusion, into a hot vessel having a plug in the bottom, which is withdrawn as soon as the surface becomes cool, when the liquid portion runs out, and leaves the under surface in the form of a mass of agglomerated crystals. Perfectly pure wax, stearine, and spermaceti have a very pleasing appearance when treated in this way.
CRYS′TALLOID. See Dialysis.
CU′BEBIN. A peculiar substance obtained from cubebs.
Prep. From cubebs (from which the oil has been expelled by distillation), by digestion in alcohol, evaporating the resulting tincture to one fourth, filtering, and then evaporating the remaining fluid almost to dryness. The residuum is left in a cold place until it assumes a semi-crystalline appearance, when it is thrown on a filter, and the fluid portion (the ‘cubebine’ of M. Cassola) allowed to drain off. In 24 hours the substance left on the filter is dissolved in 4 times its weight of boiling alcohol (sp. gr. ·90), the solution allowed to deposit its undissolved resin (still maintaining it near the boiling temperature), after which the clear portion is decanted. The crystals deposited as the liquid cools are cubebin. It is purified by redissolving it in boiling concentrated alcohol, and the addition of a little boiling water and animal charcoal, when long, white needles will be deposited if the solution is allowed to cool very slowly.
Prop., &c. It is insoluble in water, and nearly so in cold alcohol, but very soluble in boiling alcohol. It strikes a fine crimson colour with sulphuric acid, which remains unaltered for some hours; a property which distinguishes it from piperin. Its physiological action has been but little studied. According to Dr Görres, this for the most part resembles that of cubebs.
CU′BEBS. Syn. Cubeb pepper; Cubeba (B. P. & U. S.), Cubebæ (B. P.), L. The immature and stalked fruit of Piper cubeba or Cubeba officinalis. Cubebs are stimulant, stomachic, and aromatic, like the other peppers; they are also diuretic, and appear to possess a specific influence over the urino-genital organs.—Dose, 10 to 20 gr., in affections of the bladder and prostate gland, and in gleet and leucorrhœa; 1 to 3 dr., in the early and inflammatory stages of gonorrhœa, in piles, &c. They may be taken in water, milk, or bitter ale.
CU′CUMBER. The fruit of the Cucumis sativus (Linn.). Used as a salad vegetable. It is somewhat indigestible, but when properly dressed, with plenty of oil, it may be eaten without the slightest fear of evil consequences. The practice of pouring off the natural juice extracted from the cucumber by salt cannot be too strongly condemned. See Elaterium.
CUD′BEAR. Syn. Persio. A dye-stuff obtained from Lecanora tartarea and other lichens, by a process nearly similar to that used in making ARCHIL. The lichen is watered with stale urine or other ammoniacal liquor, and suffered to ferment for 3 or 4 weeks, after which the whole is poured into a flat vessel, and exposed to the air until the urinous smell has disappeared, and it has assumed a violet colour. It is then ground to powder. Its use is confined to a few cases of silk dyeing, where it is employed to yield shades of ruby and maroon; upon wool it gives deep-red shades. The colours produced by it are very fugitive. Like archil, there are two varieties of this dye-stuff—BLUE CUDBEAR and RED CUDBEAR. See Archil.
CULM. In mineralogy, a slaty kind of ANTHRACITE, occurring in Wales and North Devon. The term is also applied to any impure, shaly kind of coal.
CU′MARIN. See Coumarin.
CU′MIN. Syn. Cymini semina, Cyminum, L. The fruit (seed) of Cuminum cyminum. It is carminative and aromatic, like the caraway and anise. See Plaster.
CU′MINOL. A colourless, transparent oil, of powerful odour. It exists with CYMOL in OIL of CUMIN. See Cymol.
CU′PELLATION. The process of assaying gold and silver and their alloys by means of the CUPEL. See Assaying.
CUP′PING. This method of topical bleeding is performed as follows:—
The skin being softened by means of a sponge and warm water, and the hair and other extraneous substances being previously removed, one of the small bell-like glasses (CUPPING-GLASSES; CUCURBITU′LÆ), having the air contained in it rarefied by being passed over the flame of a spirit-lamp, is immediately applied to the part. From the formation of a partial vacuum beneath the cup, the pressure of the air on the surrounding surface causes that portion immediately under the cup to swell, and the vessels to become turgid. When this has taken place the cup is removed, and several incisions are instantly made by means524 of a scarificator, an instrument containing numerous lancets, which, by means of a spring, make a number of incisions at the same moment; the depth of these incisions being regulated by means of a screw which protrudes or withdraws the lancets, according to the vascularity of the part, or the quantity of blood to be abstracted. The cupping glass is now again applied. When a sufficient quantity of blood has been collected in the cup, it is removed by gently introducing the nail of one of the fingers under the upper edge, by which means, air being allowed to enter, the cup becomes detached. The part being washed with warm water to remove any clots of blood, another cup is applied as before, and the operation continued until a sufficient quantity of blood is withdrawn. Sometimes, especially when applied to the scalp, the cups fill so rapidly with blood as to become detached almost immediately on being applied. This method of local bleeding is frequently called ‘CUPPING WITH SCARIFICATIONS,’
When cupping-glasses are applied without the use of the lancet or scarificator, the operation is called ‘DRY CUPPING,’ and is much used to cause a speedy irritation of the skin and reaction, for the relief of oppressive breathing, local pains, &c. To obtain the full benefit from this operation, the cups should be suffered to remain upon the part until they cause an exudation of a small quantity of serum, or a considerable amount of irritation of the part. Dry cupping has been found extremely beneficial in poisoned wounds; as it acts not only by abstracting the poison, but also, by the pressure the glasses exercise on and around the part, in preventing the absorption of it.
Obs. For the operation of cupping, a basin of hot water, sponges, and clean, soft towels, should be provided. In clumsy hands, cupping is occasionally a severe and painful operation; but this is not the case with the skilful operator. A good cupper does not exhaust much of the air in the cup before applying it, but simply passes its mouth rapidly over the flame of the lamp. When it is held over the flame even for a few seconds, the compression of the edge of the cup upon the skin is so great, that it checks the flow of the blood to the scarified part. A good cupper also removes the cup without spilling the contents, and completes the whole operation quickly and neatly. There are, however, few persons, who are not professional cuppers, who are sufficiently expert to exhaust the air in the cup by means of the common lamp; although it is by far the best. A good plan is to rarify the air in the cup by means of a small cone of paper, dipped in spirits of wine, or strong brandy; this is ignited and thrown in the cup, which is instantly to be applied to the proper spot. Where cupping-glasses and the scarificator are not to be had, wine-glasses, or any very small tumblers, may be substituted for the first; and small incisions by means of a thumb lancet will answer the purpose of the other.
The cicatrices of the scarification leave permanent marks on the skin; on which account, when blood is to be drawn from the head or neck, the glasses should be applied behind the ears, and a portion of hair removed in such a manner that the part may be covered by what remains.
A most convenient cupping apparatus is manufactured by Mr Bigg, the eminent surgical instrument maker of Leicester Square, consisting of cups and an exhausting syringe, so arranged that the use of the spirit-lamp is rendered unnecessary, and the operation of cupping may be performed nearly as expertly by an inexperienced nurse as by the most accomplished professional operator. It is invaluable in places remote from town.
CURAR′INE. Syn. Curaria. The vegeto-alkaline base of curara, urari, woorara, woorali, or wourali, the arrow-poison of Central America.
In physiological effects curarine is antagonistic to strychnia, a fact which has led to its being proposed as an antidote for the latter poison. Curarine is also said to have been employed in Germany in the treatment of hydrophobia with such success that the patient to whom it was administered recovered. It is a most potent poison, and should not be allowed to come into contact with the fingers.
CURB. In horses. An enlargement at the back of a horse’s hock caused by injuring a ligament in this region. See Sprain.
CURCU′MIN. The yellow colouring matter of turmeric, obtained by digesting the alcoholic extract of the powder in ether, and evaporating the clear ethereal solution to dryness. A brownish-yellow mass, yielding a bright-yellow powder. It is scarcely soluble in water, but very soluble in both alcohol and ether. Boracic and hydrochloric acids redden it; alkalies turn it reddish brown.
CURD. Coagulated casein. See Cheese.
CUR′RANTS. The currants of our garden are varieties of the Ribes rubrum and Ribes nigrum. (Linn.) The first includes RED CURRANTS and WHITE CURRANTS; the fruit of both of which are gently acidulous, cooling, and wholesome. The juice makes excellent wine. The fruit of the last (BLACK CURRANTS, QUINSY-BERRIES) is aperitive, and has been used in calculous affections; the juice is made into wine, jellies, jams, lozenges, &c. The young leaves are used as a substitute for tea; one or two buds, or half a small leaf, impart to black tea the flavour and fragrance of green. The currants of the grocers (Zante currants) are a small variety of dried grapes. The word “currant” is a corruption of Corinth, whence the fruit originally came.
CUR′RY. Syn. Currie. A noted dish in Indian cookery, much esteemed throughout the East. Curries are simply stews, of which rice usually forms a characteristic ingredient,525 highly flavoured with fried onions and curry powder, to which sliced apples and lemon juice are sometimes added. They are made from every variety of fish, meat, poultry, game, &c., according to the fancy of the parties.
To make a Dish of Curry.—Cut an onion into slices and fry it with an apple, finely chopped, in two ounces of dripping; then add slices of cold meat; mix a dessert-spoonful of curry powder and one of flour in half a pint of water; pour it over the meat, and shake the whole over the fire till it boils.
Cur′ry Powder. Prep. (Kitchener.) From coriander-seed, 1⁄4 lb.; turmeric, 1⁄4 lb.; cinnamon-seed, 2 oz.; cayenne, 1⁄2 oz.; mustard, 1 oz.; ground ginger, 1 oz.; allspice, 1⁄2 oz.; fenugreek-seed, 2 oz.; all dried thoroughly, pounded in a mortar, rubbed through a sieve, and mixed together.
The famous Ceylon curry powder is said by Dr Balfour to have the following rather indefinite composition:—A piece of green ginger, two fragments of garlic, a few coriander and cumin seeds, six small onions, one dry Chili, eight peppercorns, a small piece of turmeric, half a dessert-spoonful of butter, half a cocoa-nut, and half a lime. For it to be in perfection the powder should be made the day on which it is cooked.
Obs. The above must be regarded as merely a substitute for Indian curry powder, which contains many ingredients not to be obtained in England. It should be kept in a bottle closely corked or stoppered. The curry powder sold at the present time consists of coriander-seed, turmeric, cayenne, fenugreek-seed, and a large proportion of sago-flour.
CUS′CONINE. See Aricine.
CUSPA′′RIA. Syn. Cusparia bark (B. P.), Angostu′′ra b.; Cor′tex angostu′′ræ, C. cuspa′′riæ, Cusparia (Ph. L. and E.), L. “The bark of Galipea cusparia” (Ph. L.), or Galipea officinalis (Ph. E.). A valuable drug, imported directly or indirectly from South America.—Dose, 10 gr. to 30 gr., as a tonic, stomachic, and febrifuge, in similar cases to those in which Cascarilla, Calumba, and Cinchona, are commonly given.
| Characters. | False Angostura. | True Angostura. |
| Form | Thick, rugous, rolled upon itself. Edges cut perpendicularly. | Flat or rolled up, little wrinkled, edges bevelled. |
| Colour | Brown, or greenish-yellow, presenting protuberances or excrescences, produced by the great development of the corky layer, which has a still more yellow colour. | Greyish-yellow. |
| Taste | Very bitter. | Bitter. |
| Reaction with Nitric Acid. | Red colour when dropped upon the bark. | Yellow colour. |
Angostura or cusparia bark has fallen into comparative disuse, in consequence of nux vomica or false angostura bark having formerly, in several instances, been mistaken for it, and administered with fatal results. The leading characteristics of these two barks have been pointed out by M. Gibourt. (See previous table.)
CUSPAR′IN. Syn. Angostu′′rin, Angostu′′ra. The bitter principle of Cusparia-bark. It is neutral; crystallises in tetrahedrons; is easily fusible; soluble in rectified spirits, in acids, and in alkaline solutions. It is precipitated of a whitish colour by tincture of galls.
CUS′TARD. A composition of milk, or cream, and eggs, sweetened with sugar, and variously flavoured. Custards may be cooked either in the oven or stew-pan.
Prep. 1. (Soyer.) Milk (boiling), 1 pint; sugar, 2 oz.; thin yellow peel of half a lemon; mix, and set it aside for a short time; then take eggs, 4 in no., beat them well in a basin; add, gradually, the milk (not too hot), pass the mixture through a colander or sieve, and fill the custard cups with it; these are then to be placed over the fire in a stew-pan, containing about one inch of hot water, and left there for 12 minutes, or till sufficiently set. The above is for PLAIN CUSTARDS; but it forms a good basis to receive any of the usual flavouring ingredients, as fresh or stewed fruit, peels, essences, orange-flower water, brandy, or other spirits, &c.
2. (Rundell.) As the last, but using cream instead of milk, or equal parts of the two, with 2 additional eggs. Very rich; like the last, any suitable flavouring matter may be added to it.
3. (Almond Custards,—Rundell.) As either of the above, adding blanched sweet almonds, 4 oz.; bitter do., 6 in no.; beaten to a smooth paste.
4. (Baked Custards,—Rundell.) From cream, 1 pint, with 4 eggs; flavoured with mace, nutmeg, and cinnamon, and add a little white wine, rose-water, and sugar; bake in cups.
5. (Coffee Custards,—Soyer.) Hot milk and strong-made coffee, of each 1⁄2 pint; sugar, 2 oz.; dissolve, and add it, gradually, to 4 eggs (well beaten), and proceed as in No. 1. Chocolate custards and cocoa custards are made in the same way.
6. (Cold Custard, for invalids,—Dewees.) 1 egg; sugar, a tablespoonful; beat well together; and add, gradually, constantly stirring, cold water, 1⁄2 pint; rose water, 2 teaspoonfuls; and a little grated nutmeg. An agreeable and nutritious demulcent. A wine-glassful every 2 or 3 hours, or ad libitum.
7. (Lemon Custards,—Rundell.)—a. As No. 1 (nearly), using a little more lemon peel.526 In the same way orange custards are made, but using orange peel.
b. From candied lemon peel and lump sugar, of each 2 oz., beaten in a mortar quite fine, and added to either No. 1 or No. 2. Orange and citron custards may be made in the same manner. A little orange-flower water, or marsala, or sherry, may be also added at pleasure. They are best baked.
8. (Orange Custards.) As above, No. 7, a and b.
9. (Rice Custards,—Rundell.) Boil 1⁄2 a cupful of the best ground rice in a pint of milk until dissolved, then mix it with a quart of cream; flavour with nutmeg, mace, and a little brandy, and put it into a cup or a dish.
CUTCH. See Catechu.
CUTTLE-FISH. The bone or skeleton of the Sepia officinalis of Linnæus, or common cuttle-fish (CUTTLE-FISH BONE; OS SE′PIÆ), is used by the law-stationers to erase ink-marks from paper and parchment, an application familiar to most schoolboys of the present generation. Reduced to powder (PUL′VIS SE′PIÆ), it forms a valuable dentifrice and polishing powder, and is used for forming the moulds for small silver castings.

The Sepias, which inhabit the seas of all quarters of the globe, like the other cephalapoda, are carnivorous. They are able to exercise considerable locomotive powers, by means of their tentaculæ or arms which surround the mouth, and which are usually provided with numerous suckers. Head downward, they walk on these arms at the bottom of the ocean. The sepias are also fleet swimmers; effecting their progress through the water either by making the expansion of their skin perform the same office as fins; or by the forcible projection of water from the cavity of their mouths, the reaction accompanying which operation drives them rapidly through the water in a different direction. They are provided sometimes with eight, and sometimes with ten tentaculæ, and have naked bodies. The black fluid which the animal is capable of ejecting from its ink-sac, when pursued by its enemies, was formerly employed in the manufacture of the pigment called from its source “sepia.”
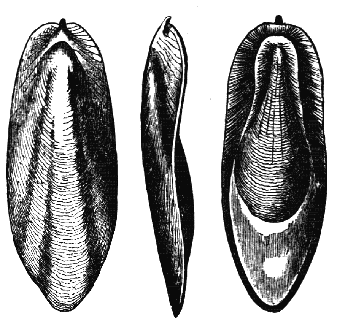
CUTS. These are incised wounds of greater or less extent, and must be treated accordingly. The divided parts should be drawn close together, and held so with small pieces of strapping or adhesive plaster stretched across the wound. If the part is covered with blood, it should be first wiped with a damp sponge. When the wound is large and it is much exposed, a good method is to sew the parts up. The application of a little creasote or a spirituous solution of creasote will generally stop local bleeding, provided it is applied to the clean extremities of the wounded vessels. A good way is to place a piece of lint, moistened with creasote, on the wound, previously wiped clean, or to pour a drop or two of that liquid on it. An excellent method is to cover the part with a film of collodion. Friar’s balsam, quick-drying copal varnish, tincture of galls,527 copperas water, black ink, &c., are popular remedies applied in the same way. A bit of the fur plucked from a black beaver hat is an excellent remedy to stop the bleeding from a cut produced by the razor in shaving. A cobweb is said to possess the same property.
CY′ANATE. Syn. Cy′anas, L. A salt in which the hydrogen of cyanic acid is replaced by a metal or other basic radical.
CYAN′IC ACID. HCNO. Syn. Acidum cyan′icum, L. Prep. 1. Cyanuric acid, deprived of its water of crystallisation, is distilled in a retort, and the product collected in a well-cooled receiver.
2. (Liebig.) A current of sulphuretted hydrogen gas is passed through water in which cyanate of silver is diffused, the process being suspended before all the cyanate of silver is decomposed.
Prop., &c. Cyanic acid is a limpid, colourless liquid; it reddens litmus; is sour to the taste; possesses a modified sulphurous odour, similar to that which is always perceived when any of its salts are decomposed by an acid; it forms salt with the bases called CYANATES; when in contact with water it suffers decomposition in a few hours, and is converted into bicarbonate of ammonia; it cannot be preserved for any time, as shortly after its preparation it spontaneously passes into a white, opaque, solid mass, to which the name CYAMELIDE has been given. By distillation this new substance is reconverted into cyanic acid.
CY′ANIDE. Syn. Cyan′uret; Cyan′idum, Cyanure′tum, L. The compound formed by the union of cyanogen with a metal or other radical. See Cyanogen, Hydrocyanic Acid, and the respective bases.
Cyanide, Al′kaline. Syn. Crude cyanide of potassium and sodium. Prep. (R. Wagner.) Dry ferrocyanide of potassium, 4 parts, dry carbonate of soda, 1 part, are melted together in an iron crucible at a red heat, and continually stirred until the iron rod comes out covered with a white crust, when the heat is withdrawn, and after a few moments’ repose the supernatant liquid portion is poured out on a clean iron slab. This crude mixed cyanide is quite as useful as the more expensive one of Liebig, and is equally fit for technical applications, as electrotyping, gilding, silvering, &c. See Potassium, Cyanide of.
CY′ANINE. A base discovered by Mr G. Williams in CHINOLINE BLUE. See below.
Cyanine, I′odide of. Syn. Chin′oline blue. The action of iodide of amyl upon chinoline gives rise to iodide of amylchinoline. Addition of excess of soda to an aqueous solution of this iodide produces a black resinous precipitate, which dissolves in alcohol with a magnificent blue colour. This precipitate is the IODIDE OF CYANINE, or CHINOLINE BLUE. Many attempts have been made to use it in dyeing; they have, however, failed on account of the instability of the colour.
CYAN′OGEN. CN or Cy. A highly important compound radical or quasi element, discovered by M. Gay Lussac in 1815.
Best obtained by carefully igniting dry cyanide of mercury in a small retort, and collecting the gas over mercury.
Prop., &c. A colourless gas, possessing a pungent and peculiar odour, resembling that of peach-kernels or prussic acid; under a pressure of about 4 atmospheres, at a temperature of 45°, it assumes the liquid form (Faraday), and this fluid again becomes gaseous on withdrawal of the pressure; water absorbs nearly 5 times its bulk of cyanogen at 60° Fahr., and alcohol about 23 times its volume; with hydrogen it forms hydrocyanic acid, and with the metals a most interesting and important class of bodies called cyanides or cyanurets; when kindled, it burns with a beautiful purple flame, carbonic acid and nitrogen being evolved. Sp gr. 1·806. See Hydrocyanic acid, &c.
Forms a bromide and iodide when the cyanide of mercury is distilled with bromine or iodine, and which are colourless, volatile, highly poisonous solids; and two isomeric chlorides, one a very volatile liquid, prepared by passing chlorine over moist cyanide of mercury, and the other in white volatile needles, prepared by exposing aqueous hydrocyanic acid to chlorine in sunshine.
CYANU′′RIC ACID. H3C3N3O3. Syn. Pyro-u′′ric acid†. A peculiar acid, discovered by Scheele. It is a product of the decomposition of the soluble cyanates by dilute acids, or of urea by heat, &c.
CY′DER. See Cider.
CY′DONINE. The peculiar gum of quince seed. It resembles bassorin in most of its properties.
CY′MIDINE. An oily base, homologous with aniline, obtained by the action of iron filings and acetic acid on nitro-cymol.
CY′MOL. A peculiar hydrocarbon found in oil of cumin, in admixture with cuminol. The two bodies are separable in a great measure by distillation, cymol being the most volatile portion of the oil.
CYNAPINE. An alkaloid obtained from Æthusa cynapium, or fool’s parsley. It possesses no practical interest.
CYSTICERCI. These parasites are embryo tænia or tapeworm, infesting the bodies of men and different animals. One variety of the cysticerci has its habitat in the organisms of men, pigs, oxen, horses, camels, sheep, and roe-deer; another in the muscles and internal organs of cattle; a third is found in cattle, sheep, horses, the reindeer, squirrels, certain kinds of monkeys, and occasionally in man; whilst a fourth—the Cystercus cellulosæ—is more especially met with in measly pork. Professor Gamgee believes that probably 5 per cent. of the pigs in Ireland are affected with this last cystercus.
The following figure represents a piece of528 measly pork infested with cysticerci. Professor Leuckart seems to have shown pretty conclusively that man may become infested with a certain form of tapeworm by partaking of imperfectly cooked veal or beef, infested with the second variety of the parasite.

CYST′INE. C3NH7SO2. Syn. Cyst′ic oxide. Obtained from cystic oxide calculi (in powder) by digestion in solution of ammonia. By spontaneous evaporation the ammoniacal solution deposits small, colourless crystals of cystic oxide. It forms a saline compound with hydrochloric acid, and is decomposed by the strong alkalies.
CY′TISINE. A purgative bitter principle, extracted from the Cytisus Laburnum (Linn.), or common laburnum, and some other plants.
DAGUERRE′OTYPE. See Photography.
DA′′HLIA DYE (dāle′-y′ă). The shade of colour which is commonly termed ‘dahlia’ is a reddish lilac. It is produced by combining a blue or purple with red when a compound colour is used. Upon wool and silk it can be obtained directly by means of archil or cudbear, either alone or ‘blued’ by a small quantity of sulphate of indigo. Upon cotton indifferent shades of dahlia are obtained by macerating in sumac liquor, working in tin solution, and dyeing in logwood mixed with some red wood.
DA′′HLINE. A species of fecula obtained from the tubers of the dahlia. It is identical with inuline. It is not employed in the arts.
DAIR′Y. The place where milk is kept, and cheese and butter made. The best situation for a dairy is on the north side of the dwelling-house, in order that it may be sheltered from the sun during the heat of the day. Ample means should be provided to ensure ventilation, and at the same time to exclude flies and other insects. The temperature should be preserved, as much as possible, in an equable state, ranging from 45° to 55° Fahr. To lessen the influence of external variations of temperature, the walls should be double, or of considerable thickness, and the windows provided with shutters or doors. In summer the beat may be lessened by sprinkling water on the floor, which will produce considerable cold by its evaporation. Dairies built of mud or ‘cob’ are preferred in the West of England; and this preference arises from the uniform temperature they maintain, on account of the great thickness of the walls, and their being very bad conductors of heat. In large dairy-farms, where butter and cheese are made, the dairy is generally a separate building, and divided into 3 or 4 apartments; one of which is called the ‘milk-room,’ a second the ‘churning-room,’ a third the ‘cheese-room,’ containing the cheese-press, &c.; and a fourth the ‘drying-room,’ where the cheeses are placed to dry and harden. To these may be added a scullery, furnished with boiler, water, &c., for scalding and cleaning the dairy utensils.
Cleanliness is very essential in all the operations of the dairy, and in none more so than in the milking of the cows. The hands and arms of the milkmaid should be kept scrupulously clean, and should be well washed with soap and water after touching the udder of a sick cow, as without this precaution the sores may be conveyed to the healthy ones. The milk-cans should be scalded out daily, and, as well as all the other dairy utensils, should be kept clean and dry. Before placing the milk on the shelves of the dairy it should be strained through a hair sieve or a searce covered with clean cheese-cloth, as by this precaution any stray hairs that may have fallen into the milk-pail will be taken out.
The average produce of a milch cow, supplied with good pasturage, is about 3 gallons daily from Lady-day to Michaelmas, and from that time to February about 1 gallon daily. Cows of good breed will be profitable milkers, to 14 or 15 years of age, if well fed. See Butter, Cheese, Cream, &c.
D’ALBESPYRE’S BLISTERING TISSUE. Lard and ship’s pitch, of each 1 part; resina flav. and yellow wax, of each, 4 parts; finely powdered cantharides, 6 parts; melted together, and spread over taffety.
DAMAS′CUS BLADES. See Steel.
DAMENPULVER—Ladies’ Powder (J. Pohlmann, Vienna). A face powder composed of 14 parts white lead, 7 of talc, 1 of magnesia, coloured with carmine and perfumed with volatile oil.
DAMMAR. A resin employed in mounting many microscopic objects; such as teeth, hair, hard bone, and most tissues which have been previously hardened by treatment with alcohol529 and chromic acid. Dammar is prepared for use as follows:—
a. Gum dammar, 1⁄2 oz.; oil turpentine, 1 oz.; dissolve and filter.
b. Gum mastic, 1⁄2 oz.; chloroform, 2 oz.; dissolve and filter. Add solution a to solution b. (Dr Klein.)
If allowed to become thick by drying, dammar may be used as luting.
DAMP, under any form, should be avoided. A humid atmosphere or situation is one of the commonest causes of agues, asthmas, rheumatism, and numerous other diseases.
Damp Linen is very injurious, and should be especially avoided. In travelling, when it is expected that the bed has not been properly aired, a good plan is to sleep between the blankets. To ascertain this point the bed may be warmed, and a cold, dry, glass tumbler immediately afterwards introduced between the sheets, in an inverted position. After it has remained a few seconds, it should be examined, when, if it is found dry, and undimmed by steam, it may be fairly presumed that the bed is well aired; but if the reverse should be the case, it should be avoided. When it is impossible to prevent the use of damp linen, as articles of dress, the best way to obviate any ill effects is to keep constantly in motion and avoid remaining near the fire, or in a warm apartment, or in a draught of cold air until sufficient time has elapsed to allow of the escape of the moisture. The effect of evaporation is the reduction of the temperature of the body; hence the depressing action of damp linen.
Damp Walls. Ivy planted against the sound wall of a house is said to exclude dampness. If a wall is already damp, ivy planted against it will, when grown up, cause it to become dry, provided the brickwork is sound and the dampness does not arise from moisture attracted upwards from the foundation. Sometimes, when ivy is propagated from flowering branches, it will not adhere to a wall at all; the way to get over this difficulty is to cut it back to near the surface of the ground. The young wood which will then form will take hold and cling immediately to almost anything.
The following is said to be a good application for damp walls:—Dissolve 3⁄4 lb. of mottled soap in 1 gallon of water. This composition is to be laid over the brickwork steadily and carefully with a large thick brush, but not in such a manner as to form a froth or lather on the surface. It must be allowed 24 hours to dry on the walls. Now mix 1⁄2 lb. alum with 4 gallons of water; let it stand 24 hours, and then apply it over the coating of soap. The operation must be performed in dry weather.
DAM′SON. A species of small black plum, much used in the preparation of tarts, &c. Damsons are rather apt to disagree with delicate stomachs, and also to affect the bowels. See Plum.
DAN′CING. The practice of dancing as an amusement or exercise must be almost as old as the human race itself. Yet, notwithstanding its antiquity and prevalence amongst all nations, both barbarous and refined, the propriety and advantages of its cultivation are of a very questionable character. In a hygienic point of view it can claim no preference, as an exercise, over the more simple ones of walking and running; whilst, from the associations it frequently induces, and the heated and confined atmosphere in which its votaries commonly assemble to indulge in it, it becomes the fertile parent of immorality and consumption. A celebrated cyclopædist has, perhaps harshly, but truthfully, defined dancing to be “a silly amusement for the idle and thoughtless.”
DANDELI′ON. Syn. Piss-a-bed; Tarax′acum (Ph. L. E. & D.), L. A common British plant of the natural order Compositæ. It is known among botanists by the names Taraxacum officinale, T. dens leonis, and Leontodon Taraxacum (Linn.). Its root (Taraxaci Radix, B. P.) is employed in medicine, being diuretic and tonic. It is roasted and used as coffee, and when mixed with an equal weight of foreign coffee constitutes the article once so much puffed under the name of ‘dandelion coffee.’ A similar mixture prepared with chocolate forms the ‘dandelion chocolate’ of the shops. The blanched leaves are used in salads, and the inspissated juice, extract, and decoction are employed in medicine, and are considered as detergent, aperitive, and deobstruent. Ground roasted dandelion root cannot now be sold under the name of ‘dandelion coffee’ or mixed with coffee unless it has previously paid the chicory duty. See Decoction, Extract, &c.
DANDRIFF. This is a scaly disease affecting the head, and giving rise to the formation of the small troublesome particles known as scurf. A serviceable application is two drachms of borax dissolved in a pint of camphor water; the head to be washed with this lotion once or twice a week, or much benefit may also be derived by washing the head with tepid water, agitated with a piece of quillar bark until a strong lather is produced; or with water containing salt of tartar, in the proportion of two drachms of the salt to a pint of tepid water. As a general rule, the use of soap is to be discountenanced.
DAN′IELL’S BATTERY. See Voltaic Electricity.
DAPH′NIN. A peculiar bitter principle, discovered by Vauquelin in the Daphne mezereum or mezereon. It is procured by separating the resin from the alcoholic tincture of the bark by evaporation; afterwards, diluting the residue with water, filtering, and adding acetate of lead. A yellow substance falls down, which, when decomposed by sulphuretted hydrogen, yields daphnin, under the form of small, colourless, transparent, radiated needles.530 It is bitter; volatile; sparingly soluble in cold water; freely soluble in hot water, and in alcohol and ether. It possesses basic properties. See Mezereon.
DARNEL. The powder of the seed of the Lolium temulentum, a poisonous grass, is not unfrequently found mixed with the flour of wheat, oats, and other cereals, and when these latter, under these circumstances, are partaken of as food, they give rise to more or less alarming symptoms of poisoning, which are thus enumerated by Dr Pareira:—Headache, giddiness, languor, ringing in the ears, confusion of sight, dilated pupil, delirium, heaviness, somnolency, trembling convulsions, paralysis, and great gastro-intestinal irritation. One of the most specific signs of poisoning by darnel seeds is said by Seeger to be the trembling of the whole body. Dr Taylor mentions a case in which thirty persons who had partaken of bread containing darnel seeds were violently attacked with the above symptoms; and another case is on record of sixty persons in a prison at Cologne being similarly attacked from the same cause. Hassall states that the flour of the darnel seed presents the following appearance under the microscope:—“The starch corpuscles are polygonal, and resemble in this respect those of rice. They are, however, much smaller, and frequently united into compound grains of various sizes, the larger grains consisting of some fifty or sixty starch corpuscles.” The structure of the testa is very different from that of either rice or oat, or indeed any of the other cereal grains. It is formed of three coats or membranes. The cells of the outer coat form but a single layer, and, contrary to the arrangement which exists in the oat, their long axes are disposed transversely, in which respect they resemble rice. The fibres of the husk of rice and the cells of the testa of Lolium are, however, very distinct in other respects. In the former the cells are long and narrow, forming fibres, while in the latter they are but two or three times as broad.
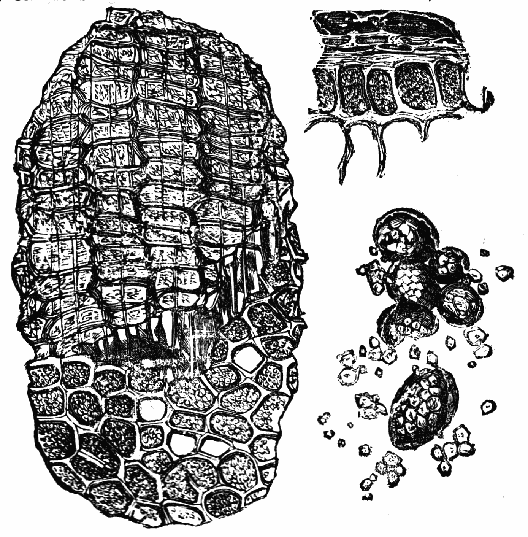
The cells of the second coat, which are ranged in two layers, follow a vertical disposition, an arrangement which is contrary to that which obtains in all the other cereal grains with the exception of rice. The cells of the third coat form but a single layer, and resemble those of other grains.
DATU′RA. Syn. Thorn-apple; Stramo′nium (Ph. L. E. & D.), L. A genus of plants belonging to the nightshade order, or ‘Atropaceæ,’ The species Datura Stramonium is an important medicinal plant, the leaves and seeds being officinal in B. P. It is anodyne and sedative, but not hypnotic, though it will often induce sleep by relieving pain. It affects the constitution in much the same way as belladonna.—Dose, 1 to 4 or 5 gr., in asthma, gout, headache, neuralgic and rheumatic pains, &c. In spasmodic asthma smoking the leaf often gives instantaneous relief, but must be exhibited with care, as the whole plant is intensely poisonous. No antidote is known.531 Another species, namely, Datura tatula, is now preferred for cigars or cigarettes. Cigars are made from Datura Stramonium more frequently than from Datura tatula. They are recommended for asthma. See Asthma, Cigars (Stramonium), Daturia (below).
DATU′RIA. Syn. Datu′rine, Daturin′a, Hyoscy′amine. An organic alkali, discovered by Geiger and Hesse in Datura Stramonium or thorn apple. It occurs also in Hyoscyamus niger or henbane. It is best obtained from the seeds. Daturia dissolves in 280 parts of cold and 72 parts of boiling water; it is very soluble in alcohol, less so in ether. It tastes bitter, dilates the pupil strongly, and is very poisonous. It may be sublimed unaltered, and may be obtained in prismatic crystals by the addition of water to its alcoholic solution. With the acids it forms salts, which are mostly crystallisable.
DAVIDS-THEE—David’s Tea (B. Fragner, Prague). Recommended as a domestic remedy for chronic catarrh of the lungs and air passages, and especially for tuberculosis. A mixture of equal parts of great centaury, hyssop, chervil (Scandix odorata), white horehound, milfoil, Iceland moss, and carduus benedictus.
Davids-Thee, Echter Karolinenthaler—Genuine Karolin’s Dale David’s Tea (Kràl). Recommended for the same diseases as the preceding. A mixture of white horehound, milfoil, Iceland moss, great centaury, and ground ivy. According to a communication from a Bohemian apothecary the original prescription reads thus:—Herba cerefolii (Scandicis, chervil), hb. centaurii minoris (lesser centaury), hb. marrub. (horehound), flor. millefol. (milfoil flowers), lichen. Isl., of each 6 parts; hb. hyssopi, 3 parts; hb. cardui benedicti, 2 parts. (A. Selle.)
DEAD, DISPOSAL OF. As every dead body during the process of the decomposition it undergoes gives rise to products that are not only in the highest degree offensive, but in an especial sense dangerous to the health and lives of a community, the disposal of the dead in a manner best calculated to ensure, with the removal of the corpse from amongst the survivors, the least injurious consequences of its subsequent decay, becomes a problem of supreme importance to the sanitarian.
Probably amongst the nations of antiquity the Romans, with their eminently practical minds, and as we may infer from their other enlightened sanitary arrangements, were the only ancient people who were not guided either by superstitious or religious ideas in the disposal of their dead, at one time burned, but afterwards buried them. The Greeks practised cremation, the Egyptians embalmment (previously disembowelling the body), under the belief that after the lapse of many thousands of years the soul would return to its earthly mansion. The Hebrews sometimes burned their dead, and sometimes interred them. Amongst the ancient Hindoos the body was got rid of sometimes by cremation, sometimes by being cast into the Ganges, or other sacred river, and sometimes by exposure, until eaten by vultures. The Icthyophagi, or fish-eaters, appear to be the only people of antiquity who disposed of their dead by throwing them into the sea.
Amongst modern civilised nations interment is the method almost universally adopted for disposing of the dead.
Embalmment has been of late years occasionally practised in America, and was frequently adopted during the late civil war in many cases, since it afforded the means of sending the bodies of the slain to surviving relatives at long distances. Sir Henry Thompson’s advocacy of cremation was the means of causing the establishment in England a few years back of a society for its introduction; but neither here nor in Germany, where it has occasionally been employed, has the practice being adopted, save in a very few instances, most of which seem to have been merely experimental. In Calcutta cremation is still practised to some extent by the native population; the process being very effectively carried out by a French company, which has been established for some years. We may mention here an important modification of the ordinary form of interment proposed by Mr Sydney Hadden. Mr Hadden’s proposal is to dispense with the coffin, and to place the corpse instead in a large wickerwork receptacle filled with flowers, then inter it.
Of the three modes of disposal of the dead, viz. by burial, by burning, and by consignment to the sea, the first, as we have already said, is the almost invariably prevalent custom amongst civilised communities. That in a sanitary point of view it is less to be commended than either of the other methods is, we think, not difficult of demonstration. When a body is burned the resulting gaseous products of combustion, which most probably consist mainly of carbonic acid, carbonic oxide, and nitrogen, diffuse harmlessly into the atmosphere, and there remains behind only the calcined bones which formed the skeleton. An experiment by Sir Henry Thompson has shown that cremation may be performed without giving rise to odour of any kind at a comparatively small expense, and within a very moderate space of time. He burned a body weighing 227 lbs. in fifty-five minutes by placing it in a cylindrical metallic vessel seven feet long and six feet in diameter, previously heated to incandescence. The evolved gases were made to pass over a large surface of strongly heated fire-bricks, which formed part of the furnace in which the metallic vessel was placed. The furnace and its fittings were designed by Dr Siemens. The remaining ashes weighed about 5 lbs. In his pamphlet ‘On Cremation,’ Sir Henry Thompson532 has proposed that the custom, if adopted, should be carried out in the following manner: “When death occurs, and the necessary certificate has been given, the body is placed in a light wood shell, then in a suitable outside receptacle preparatory to removal for religious rites or otherwise. After a proper time has elapsed it is conveyed to the spot where cremation is to be performed. There nothing need be seen by the last attendant or attendants than the placing of a shell within a small compartment, and the closing of the door upon it. It slides down into the heated chamber, and is left there an hour until the necessary changes have taken place. The ashes are then placed at the disposal of the attendants.” Sir Henry suggests that, previous to the burning of any corpse, proper officers appointed for the purpose should examine into and certify as to the cause of death, and if satisfied that it has resulted from natural events, that they should give the certificate he alludes to.
Sir Henry Thompson proposes that the functions of the officers appointed for this purpose should be the same as those of the medicins verificateurs, who are medical inspectors appointed by the municipality of Paris and the other large cities, whose duty consists in visiting each house where a death occurs, in assuring themselves that the person is really dead, and that there are no suspicious circumstances attending the demise.
In Paris alone there are more than eighty of such medical functionaries.
Burial by casting the corpse into the depths of the sea possesses the great advantage over ordinary interment of removing it from near the habitation of man, whilst the sea water, by its antiseptic properties, would be as little favorable to the dissemination of noxious putrescent compounds as cremation is. On the contrary, if the dead are disposed of by the ordinary method of burial, the objectionable effects arising from their decomposition in the earth are, under the most favorable conditions, only partially overcome; and the reason is obvious, since whilst deep-sea burial prevents animal decay altogether, and burning destroys the body, which, if not got rid of, would become putrid; burial in the earth permits its slow and lengthened decomposition to go on unchecked, and to thus very frequently become a source of contamination and danger to health.[251]
[251] “After death the buried body returns to its elements and gradually, and often by the means of other forms of life which prey on it, a large amount of it forms carbonic acid, ammonia, sulphuretted and carburetted hydrogen, nitrous and nitric acids, and various more complex gaseous products, many of which are very fetid, but which, however, are eventually all oxidised into the simpler combination. The non-volatile substances, the salts, become constituents of the soil, pass into plants, or are carried away into the water percolating through the ground. The hardest parts, the bones, remain in some soils for many centuries, and even for long periods retain a portion of their animal constituents.”—Parkes.
The atmosphere in the vicinity of graveyards and cemeteries is notoriously unhealthy, whilst water taken from wells situated near them is often so impure as to be wholly unfit for drinking. Several instances are on record in which the disturbance of an old graveyard has frequently been the means of disseminating disease. But although the disposal of the dead by means either of cremation or by consignment of the body to the deep caverns of the ocean are methods, in a hygienic point of view, immeasurably superior to earth-burial, there are, we think, certain obstacles to their adoption, even to a limited extent, by civilised communities, at any rate, for many years to come.
“Both cremation and deep-sea burial are open to the objection, that should the proposed officers appointed to inquire into the circumstances attending death have been mistaken in their verdict, as for instance in overlooking, or not suspecting a case of secret poisoning, not only would the murderer escape detection, but a sense of possible immunity from punishment might act as an encouragement to others who were equally guilty minded. The proposal that the stomach should be preserved, and kept for a certain time, and, in case of suspicion justifying it, examination, would in many instances fail to lead to detection, since, if certain alkaloids had been employed, they would have to be searched for, not in the stomach, but in the tissues of the dead body. Again, an obstacle to the universal adoption of deep-sea burial would be, in the case of vast continents, the difficulty of transmission from their interior, of the corpse, to the shore. But even if these objections against cremation and sea-burial could be overcome (and possibly they may be eventually), there would still remain the invincible repugnance of the survivors to what sentiment and feeling will persist in regarding as cruel indignity and irreverence toward the dead.
“Yet the eventual disposal of our frames is the same in all cases; and it is probably a matter merely of custom which makes us think that there is a want of affection, or of care, if the bodies of the dead are not suffered to repose in the earth that bore them.
“In reality, neither affection nor religion can be outraged by any manner of disposal of the dead which is done with proper solemnity and respect to the earthly dwelling-place of our friends. The question should be placed entirely on sanitary grounds, and we shall then judge it rightly.
“What is the use of preserving for a few more years the remains which will be an object of indifference to future generations? The next logical step would be to enshrine these remains in some way so as to ensure their preservation, and we should return to the vast burial mounds in Egypt.
“At present the question is not an urgent one, but if peace continue, and if the population of Europe increase, it will become so in another century or two.
533
“Already in this country we have seen, in our own time, a great change; the objectionable practice of interment under and round churches in towns has been given up, and the population is buried at a distance from their habitations. For the present that measure will probably suffice, but in a few years the question will again inevitably present itself.”[252]
[252] Parkes.
Since however, for the reasons above specified, earth-burial seems to be the only means of disposing of the dead likely to prevail for many years to come, the question arises as to how its attendant evils can be as much as possible minimised. The following suggestions, that may assist to effect this, are offered:—As quickly as possible after death the body should be covered with sawdust, to which carbolic acid has been added, a precaution which not only prevents the escape of fetid gases, but also of putrescent fluids from a badly jointed coffin. Charcoal, although an excellent disinfectant, from its colour, could not out of consideration for the feelings of the relatives or friends, be used until the coffin with its contents had been screwed down.
It is always desirable (save for some special reason) that the corpse should be interred within three or four days of the demise. If a body has to be kept above ground for some time, Mr Herbert Barker recommends a thin layer of sawdust and sulphate of zinc to be placed over it, in the proportion of two parts of the former to one of the latter. The coffin should be made of a material impervious to the air, and of such strength as to withstand the pressure of the overlying earth.
Mr Wynter Blyth, in his ‘Dictionary of Hygiène,’ recommends a coffin described by Mr Baker in his evidence before the sanitary commission. “The body being first of all placed in a common shell, this shell is placed in the coffin; the interval between the two is filled with common pitch, and then the outside coffin is coated over with pitch; so that it is as perfectly air-tight as a leaden shell. If desired a glass can be placed so as to leave the face exposed to the view of a jury when necessary with regard to the interment.
“The advantages of deep over shallow burying are obvious. The greater the mass of superincumbent earth into which the gaseous products of decomposition diffuse, the better the chance of their absorption and removal by the soil, and the less risk of their consequent escape into the contiguous atmosphere, as well as of the danger of contamination to the water of wells, &c. The depth of the grave varies in different countries. In Austria it is 6 ft. 2 in.; in Hesse Darmstadt, from 5 ft. 7 in. to 6 ft. 6 in.; Munich, 6 ft. 7 in.; Stuttgard, 6 ft. 6 in.; Russia from 6 ft. 10 in. In our country the practice is generally to make the depth about 6 feet, but then coffins may be placed one on the other, so that, as an actual fact, they often very closely approach the surface. The regulations followed at Stuttgard are much to be commended. In the cemeteries there the space allotted for each grave is an oblong piece of land 10 feet in length and 5 feet broad. In France the graves vary in depth from 11⁄4 metres (4·921 feet) to 2 metres (6·561 feet). They are 8 decimètres (2·264 feet) in breadth, and distant the one from the other from 3 to 4 decimètres (11·911 inches to 1·132 feet.)” (Blyth.)
To render a cemetery therefore as little prejudicial as possible to a community, not alone should deep burial be enforced, but only one body should be permitted to be deposited in a grave, at least, till after the lapse of some years. Some sanitary authorities recommend the use of quicklime or charcoal, advising them to be thrown into the grave previous to its being closed. Of the two, charcoal is the preferable disinfectant, although it does not entirely prevent putrefaction, nor the evolution of bad-smelling gases. No more efficient means of absorbing organic matters, and carbonic acid given off by the decaying corpse in the earth, can be devised than that of rapidly growing trees and shrubs in abundance around the graves.
For the funereal cypress and yew, which are slowly growing trees, why should a needless sentiment prevent the substitution of the much more sanitary and less sombre-looking eucalyptus in countries where this plant will flourish?
Old burial-grounds which have become offensive may be best disinfected by covering the ground with fresh earth to the depth of several inches, and by planting it with trees and sowing it with grass seed. Twenty-six city graveyards covering a superficial area of about 48,000 square yards, and in which, according to moderate calculation, there were not less than 48,000 tons of human remains, were successfully dealt with in this manner.
In the case of church vaults they should be first opened, a quantity of quicklime thrown into them, and thus freely exposed to the external air. The coffins should then be rearranged crossways like bricks in a building, and filled in with dry earth or masons’ rubbish, mixed with about 5 or 10 per cent. of vegetable charcoal. The vaults should next be ventilated by means of an upcast and downcast shaft of the size of a rain-water pipe, and the whole should then be closed in. In 1860, two hundred and fifty vaults in seventy-one city churches were thus disinfected.[253] These vaults contained the coffins and remains of at least 11,000 dead bodies, which, previous to the adoption of the above measures, were very offensive.[253] When bodies are removed from the vaults to other places, Dr Letheby recommends them to be in closed coffins and in cases containing an abundant supply of carbonate of lime powder.
[253] Letheby.
The disposal of the dead frequently becomes534 a matter of considerable difficulty in time of war or during a siege. Under these circumstances cremation may be found the most desirable method of getting rid of them. If the bodies are buried they should always be at as great a distance as possible from one another, and as deep as they can be. If procurable, charcoal should be thrown over them; if it cannot be obtained sawdust and sulphate of zinc, or carbolic acid, may be employed. Quicklime is also commonly used, but it is less useful.
At Metz, in 1870, the following plan was adopted:—A pit of about 17 feet in depth was filled with dead disposed of as follows:—A row of bodies was laid side by side; above this a second row was placed, with the heads laid against the feet of the first row; the third row were placed across, and the fourth row in the same way, but with the heads to the feet of the former; the fifth row was placed as No. 1, and so on.
Between each layer of bodies about an inch of lime, in powder, was placed. From 90 to 100 bodies were thus arranged on a length of 61⁄2 feet, and reached to about 6 feet to the surface; the pit was then filled up with earth, and though 8400 bodies were put in that pit there were no perceptible emanations at any time.
Around Metz the graves of men, horses, and cattle were disinfected with lime, charcoal, and sulphate of iron. Immense exertions were made to clean and disinfect the camps and battle fields, and in the month of May, 1871, from 1200 to 1600 labourers were employed by the Germans. Wherever practicable the ground was sown with oats or barley or grass. The hillocks formed by the graves were planted with trees.
In many cases at Metz bodies were dug up by the Germans, when there was any fear of water-courses being contaminated, or if houses were near. On account of the danger to the workmen, graves containing more than six bodies were left untouched, and the work was always done under the immediate superintendence of a physician. The earth was removed carefully, but not far enough to uncover the corpse; then one end of the corpse was uncovered, and as soon as the uniform or parts of the body were seen, chloride of lime and sawdust, or charcoal and carbolic acid, put in; the whole earth round the body was thus treated, and the body at length laid bare, lifted and carried away. The second body was then treated in the same way.
Near Sedan, where there were many bodies very superficially buried, burning was had recourse to. Straw mixed with pitch was put into the graves, and was lighted; one ton of pitch sufficed for from 15 to 20 bodies. Opinions as to this practice were divided, and it is not certain how many graves were thus dealt with. It seems probable that only the surface of the body was burnt, and when many bodies were together in the grave, some were not touched at all. On the whole the experiment appears to have been unsuccessful.
The Belgian experience at Sedan was in favour of employing chloride of lime, nitric acid, sulphate of iron, and chlorine gas. Carbolic acid did not answer so well. The sulphate of zinc and charcoal, which Barker found so useful, was not tried.[254]
[254] Parkes.
Various statutes have been framed for the burial of the dead and for the management and selection of the burial-grounds. In the carrying out of these enactments the local authorities have only an indirect voice, exception being made in the case of a local government district in which the vestry determines to appoint a burial board. The vestry then has power to constitute the local board so appointed the burial board of such district or parish, and to rule that the expenses of such burial board shall be met by a rate levied on such parish, after the manner of a general district rate.
Vict. 21 and 22, c. 90, s. 49, enacts that if such parish has been declared a ward for the election of members of the local board, such members are to form the burial board for the parish.
By Vict. 24 and 25, c. 61, s. 21, it is enacted that a sanitary authority may provide a proper place for the reception of dead bodies, as well as for those which are to be subjected to a post-mortem examination.
A sanitary authority is also empowered to make arrangements for interment. Any urban sanitary authority has the power of regulating these matters by by-laws. When once constituted a burial board, a sanitary authority has to see to the carrying out of the Burial Acts, to repair the fences of disused burial grounds, and generally to keep in proper order and regulate all burial grounds within its jurisdiction.
The law enacts that the proper sanitary authority shall close any burial ground which is detrimental to the health of those living in its neighbourhood, or of persons frequenting any church; and throws upon such sanitary body the duty of providing a proper place of interment elsewhere.
It may be well to know that by common law it is incumbent upon any person under whose roof a death has taken place to provide the corpse with interment. Such person may neither cast the body forth, nor carry it uncovered to the grave, but he must give it decent burial. This obligation is imposed upon public bodies as well as on private persons.
Upon presentation of a certificate signed by a properly qualified medical practitioner, a justice of the peace may order, under certain circumstances, the removal of the dead body to a mortuary.
Interment within the walls, or underneath the pavement or floor of any church, or other place of public worship, built in any urban535 district, has since August 31st, 1848, been interdicted under a penalty of £50.
DEAF′NESS. An imperfection or absence of the sense of hearing. When deafness is present in infancy and childhood, it is accompanied with dumbness, or imperfect articulation, in consequence of the impossibility of conveying a knowledge of the sounds necessary for the exercise of the imitative faculty of speech. Deafness frequently arises from some imperfection or obstruction of the passage leading to the membrane of the tympanum or drum of the ear. In some cases this passage is totally closed by a membrane, or some malformation of the tube, which may frequently be removed by a surgical operation. Even instances of partial obliteration of this passage have occurred, which have been successfully treated. The researches of Mr Yearsley have established the fact, that enlarged tonsils are a very common cause of deafness; and when such is the case, their excision will generally effect a cure. To this form of the affection Mr Yearsley applies the term ‘throat deafness.’ Another cause of deafness is the presence of foreign bodies in the aural passages or the accumulation of hardened wax. In these cases the best treatment is to inject warm water into the ear by means of a proper syringe. When deafness arises from imperfection of the tympanum or drum of the ear, the effects of the application of the artificial membrana tympani invented by Mr Yearsley (moistened cotton wool) are generally immediate and truly wonderful. By its aid persons previously so deaf as to be incapable of bearing their share in conversation have been enabled to hear an ordinary whisper. Insects may be destroyed by pouring a spoonful of warm olive oil, or camphorated oil, into the ear over night, retaining it there until the next morning by means of a piece of cotton wool, when it may be washed out with a little mild soap and warm water. When there is a deficiency of the natural secretion of wax, or a dryness of the aural passage, mild oleaginous stimulants may be employed. For this purpose a little olive oil, almond oil, to which a few drops of oil of turpentine, oil of juniper, or camphor liniment, have been added, may be used with advantage. A piece of cotton wool moistened with glycerin is an excellent application in such cases. When deafness is accompanied with continued acute pain, or a discharge of purulent matter, inflammation of the tympanum, or some other portion of the internal ear, probably exists, and medical advice should be sought as soon as possible. The deafness that frequently accompanies a violent cold is generally caused by obstructions in the Eustachian tubes, and goes off as soon as the secretions return to a healthy state. In some forms of deafness blisters behind the ears are useful. A clove of garlic wrapped in cotton or gauze, or a few drops of the juice introduced into the ear, is extremely efficacious in nervous deafness. When imperfect hearing depends upon obtundity of the auditory nerve, or an extensive obliteration or malformation of the internal ear, it scarcely admits of cure.
Deafness, Taylor’s Remedy for. Prep. From oil of almonds, 1 lb.; garlic, bruised, 2 oz.; alkanet root, 1⁄2 oz.; digest for a week, and strain. A little is poured into the ear in deafness.
DEATH. In cases of sudden death interment should be deferred till signs of putrefaction begin to appear, especially when no gradation of disease has preceded, as in cases of apoplexy, hysterics, external injuries, drowning, suffocation, &c. No sooner has breathing apparently ceased, and the visage assumed a ghastly or a death-like hue, than the patient, after his eyes are closed, is too often hurried into a coffin, and the body, scarcely yet cold, is precipitated into the grave. So extremely fallacious are the signs of death that the semblance has been frequently mistaken for the reality. By prompt means and judicious treatment, many persons, when in such a condition, have been happily restored to their families and friends. The effects of sound upon animal life is astonishing. The beat of a drum, for instance, has had a very beneficial effect upon persons in a state of suspended animation. At one time a scream, extorted by grief, proved the means of resuscitating a person who was supposed to be dead, and who had exhibited the usual recent marks of the extinction of life. In cases of catalepsy or trance, having the semblance of death, the action of the lungs and heart continues, though in a nearly imperceptible degree. By placing a cold mirror or piece of highly polished metal immediately over the mouth of the patient, symptoms of moisture will appear upon the surface if the most feeble respiration takes place.
DEBIL′ITY. Syn. Debil′itas, L. Weakness; languor; feebleness. When this arises from a diseased action of the stomach, the occasional use of mild aperients, followed by bitters and tonics, may be had recourse to. When from a general laxity of the solids, and there are no symptoms of fever, nor a tendency of blood to the head, a course of chalybeates generally proves advantageous. See Anæmia, Atrophy, &c.
DECANTA′TION. The operation of pouring or drawing off the clear portion of a liquid from the impurities or grosser matter that has subsided. It is commonly performed, either by gently inclining the vessel, or by the use of a syphon or pump. In the laboratory it is much resorted to in the purification of precipitates, or other similar operations, where repeated edulcoration or washing is required, for which purpose it is preferable to filtration, from being less troublesome and more economical. In these cases, after a sufficient time having been allowed for the subsidence of the precipitate or powder, or for the clearing of536 the supernatant fluid, the latter is decanted, and its place supplied by a fresh portion of water, which, after sufficient agitation, is similarly treated, and the whole operation repeated as often as necessary.
DECANT′ERS. There is often much difficulty experienced in cleaning decanters, especially after port wine has stood in them for some time. The best way is to wash them out with a little pearlash and warm water, adding a spoonful or two of fresh-slaked lime, if necessary. To facilitate the action of the fluid against the sides of the glass, a few small cinders or pieces of raw potato may be used. A spoonful of strong oil of vitriol will also rapidly remove any kind of dirt from glass bottles. Decanters which have become furred by holding hard water may be cleaned with a spoonful of hydrochloric acid (‘spirits of salt’), diluted with 3 or 4 times its weight of water. See Stoppers.
DECARBONISA′TION. This operation is performed on cast iron, to convert it into soft iron. The articles to be decarbonised are packed in finely powdered hæmatite, or native oxide of iron, to which iron filings are often added, and exposed for some time to a strong red heat, by which the excess of carbon is abstracted or burnt out. The process somewhat resembles annealing or cementation.
DECAY′. See Eremacausis.
DEC′IMALS. Syn. Decimal fractions. Fractions which have for their denominator 10, or some power of ten; as 100, 1000, &c.; the number of ciphers in the denominator being always equal to the number of figures in the numerator. Thus, ·2, ·25, ·125, respectively represent 2⁄10, 25⁄100, 125⁄1000. The denominator of decimals is never written, the dot placed before the first figure of the numerator expressing its value. Ciphers placed on the right hand of a decimal fraction do not alter its value; for ·5, ·50, ·500, are each equal to 1⁄2; but ciphers placed on the left hand of a decimal diminish its value in a tenfold proportion; thus, ·3, ·03, ·003, respectively answer to the common fractions 3⁄10, 3⁄100, and 3⁄1000. Every figure on the left-hand side of the dot or decimal sign is a whole number.
Addition and SUBTRACTION OF DECIMALS are performed in the same manner as with common numbers, care being taken to place the numbers under each other according to their several values; as, tens under tens, hundreds under hundreds, &c.
Multiplication of decimals is performed in precisely the same manner as with whole numbers, merely pointing off as many figures in the product as there are decimals in the multiplier and multiplicand put together.
Division of decimals. As the last, but pointing off as many figures in the quotient as the decimal places in the dividend exceed those of the divisor. If there are not figures enough in the quotient, the deficiency must be supplied by prefixing left-hand ciphers. Ciphers are also added to the right hand of the dividend, or to a remainder, where there are more figures in the divisor than in the dividend, by which the quotient may be carried on to any extent.
A vulgar fraction is reduced to a decimal by dividing the numerator (increased sufficiently with ciphers) by the denominator. Thus, 1⁄2 = ·5, 1⁄8 = ·125, &c.
The value of a decimal, of any denomination, is found by multiplying it by the number of parts in the next less denomination, and cutting off as many places to the right hand as there are decimals, and so on until the terms are exhausted. Thus, ·634 oz. is =
or, 5 dr. 41⁄3 gr. (nearly).
The constant use of decimals in the laboratory, in the surveys of the Excise, and in numerous chemical calculations, induces us to press the subject on the attention of operatives and others of neglected education. An attentive perusal of the above, and a few hours’ application, will make the matter familiar to them.
DECOC′TION. Syn. Decoct′um, L. An aqueous solution of the active principles of any substance obtained by boiling; also the process of preparing such solutions.
The effect of decoction in water differs greatly from that of infusion. At the temperature of 212° Fahr., the essential oils and aromatic principles of vegetables are dissipated or decomposed; while by infusion in hot water, in covered vessels, they remain for the most part uninjured. The solvent powers of boiling water are, however, much greater than those of hot water; and many vegetable principles scarcely acted on by the one are freely soluble in the other. This is the case with many of the alkaloids, on which the medicinal virtues of several vegetables depend. On the other hand, the solutions of many substances, though more readily made by boiling, are speedily weakened or rendered inert by ebullition, in consequence of the active principles being either volatilised along with the steam, or oxidised or decomposed by exposure to the atmosphere. This is particularly the case with substances abounding in extractive or astringent matter. When the medicinal properties of vegetables are volatile, or are injured by a strong heat, infusion should be had recourse to, in preference to boiling; but when a solution of the fixed constituents is alone sought, decoction is preferable.
The substances employed for making decoctions should be well bruised, or reduced to a537 very coarse powder, or, if fresh and soft, they should be sliced small. In the former case, any very fine powder or adhering dust should be removed with a sieve, as its presence tends to make the product thick and disagreeable, and also more troublesome to strain. The vessel in which the ebullition is conducted should be furnished with an accurately fitting cover, the better to exclude the air; and the application of the heat should be so conducted that the fluid may be kept simmering, or only gently boiling, as violent boiling is not only quite unnecessary, but absolutely injurious to the quality of the product. In every case the liquor should be strained whilst hot, but not boiling, and the best method of doing this is to employ a fine hair sieve, or a coarse flannel bag. In general it is found that, as decoctions cool, a sediment is formed, in consequence of the boiling water dissolving a larger portion of vegetable matter than it can retain in solution when cold. This deposit for the most part consists of the active principles of the solution, and, unless when otherwise ordered, should be mingled with the clear liquid by agitation, when the decoction enters into extemporaneous compositions, or when the dose is taken.
The length of time occupied by the ebullition is another point demanding some attention. Long boiling is in no case necessary, and should be avoided, especially in decoctions prepared from aromatic vegetables, or those abounding in extractive. The Colleges, in such cases, direct the ingredients “to be boiled for a short time,” or “for ten minutes;” or they limit the period of the ebullition by stating the quantity that must be volatilised, as—“boil to a pint, and strain.” The last method is generally employed for those substances that do not suffer by lengthened boiling.
In preparing compound decoctions those ingredients should be boiled first which least readily give up their active principles to the menstruum, and those which most readily part with them should be added afterwards. In many cases it is proper simply to infuse the more aromatic substances in the hot decoction of the other ingredients, by which means their volatile principles will be better preserved.
Distilled water, or perfectly clean rain water, should alone be used for decoctions, extracts, and infusions. Spring and river water, from containing lime, have much less solvent matter.
The aqueous solutions of organic matter, from the nature of their constituents, rapidly ferment or putrefy, at the ordinary temperature of the atmosphere. Neither decoctions nor infusions are fit to be used in dispensing, unless made the same day. They should, consequently, be only prepared in small quantities at a time, and any unconsumed portion should be rejected, as it would be imprudent for the dispenser to risk his own reputation, and the welfare of the patient, by employing an article of dubious quality.
It has of late years become a general practice for the wholesale houses to vend preparations under the name of ‘Concentrated Decoctions,’ which, with the exception of the compound decoction of aloes, are stated to be of 8 times the pharmacopœial strength; so that one drachm of these liquids added to seven drachms of water forms extemporaneous decoctions, professedly resembling those of the pharmacopœia. The decoction of aloes is made of only four times the usual strength, as the nature of its composition would not permit of further concentration. Such preparations are, however, very imperfect substitutes for the freshly made decoctions. The extreme difficulty of forming concentrated solutions of vegetable matter with bulky ingredients too often leads to the omission of a portion of the materials, or to the practice of concentrating the liquid by long evaporation. In the first case the strength is, of course, less than it should be; and in the second, the quality is injured, and perhaps the preparation is rendered nearly inert by the lengthened exposure to heat, and the consequent volatilisation or decomposition of its active constituents. The common practice of adding a considerable portion of spirit to these preparations, which is absolutely necessary to preserve them, is also objectionable, as, in many of the cases in which decoctions are prescribed, this article, even in small quantities, exerts a prejudicial action. Some concentrated decoctions have been recently offered for sale which do not contain alcohol, being preserved by the addition of sulphurous acid, or sulphite of lime.
Decoction of Alconorque. Syn. Decoctum alconorco. American alconorque bark, 1⁄2 oz.; water, 16 oz,; boil to 8 oz., and strain.—Dose, 1 oz. two or three times a day, in phthisis.
Decoction of Alder. Syn. Decoctum alni. Bark of common alder, 1 oz.; water, 20 oz.; boil to 16 oz.
Decoction of Alder, Black. Syn. Decoctum Rhamni Frangulæ. Black alder bark, dried, 1 oz.; water, 11⁄2 pint; boil to 1 pint, and strain.
Decoction of Al′oes. Syn. Compound d. of a., Balsam of life; Baume de vie, Fr.; Decoctum al′oës (Ph. E.), D. a. compos′itum (B. P. and Ph. D.), L. Prep. 1. (B. P.) Extract of liquorice, 1 oz.; extract of socotrine aloes, 2 dr.; powdered myrrh and saffron, of each, 11⁄2 dr.; carbonate of potassa, 1 dr.; tincture of cardamoms, 8 oz.; water, a sufficiency. Coarsely powder the extract of aloes and myrrh, and put them, together with the carbonate of potash and extract of liquorice, into a covered vessel, with a pint of distilled water; boil gently for five minutes, then add the saffron; let the vessel with contents cool, then add the tincture of cardamoms, and, covering the vessels closely, allow the ingredients538 to macerate two hours, finally strain through flannel, pouring as much distilled water over the contents of the strainer as will make the product measure 30 oz.
2. (Ph. E.) Aloes, myrrh, and saffron, of each 1 dr.; extract of liquorice, 1⁄2 oz.; carbonate of potassa, 40 gr.; water, 16 fl. oz.; boil to 12 fl. oz.; strain, and add of compound tincture of cardamoms 4 fl. oz.
3. (Ph. D.) As No. 1 (nearly), but using hepatic aloes.
A warm cathartic.—Dose, 1⁄2 to 11⁄2 oz.; in habitual costiveness, dyspepsia, jaundice, &c.
Obs. By boiling the saffron as ordered by the Dublin and Edinburgh Colleges, nearly the whole of its fragrance is dissipated. A better plan is to macerate it in the tincture for a few days, previously to adding the latter to the decoction of the other ingredients. After the tincture has been strained off from the saffron, the latter may be washed with a little water, to remove any adhering colour and odour, and this may be added to the decoction. The addition of the tincture produces a deposit of mucilaginous and feculent matter, which has been dissolved out of the liquorice, for which reason some houses omit the latter altogether, and supply its place with an equal quantity of sugar or treacle, and a little colouring. By this method the liquid, after being once obtained clear, will continue so for any length of time.
4. (Wholesale.) Solazzi juice, 11⁄2 lb.; kali (carbonate of potassa), 4 oz.; hepatic aloes, 51⁄2 oz.; myrrh (small), 5 oz.; water, 41⁄2 galls.; boil to 3 galls., strain through flannel, cool, and add, of compound tincture of cardamoms, 10 pints; previously digested for 10 days on saffron, 21⁄2 oz.; mix well, and add essential oil of nutmeg, 15 drops; oils of cassia and caraway, of each 10 drops; and oils of cloves and pimento, of each 5 drops; in a week decant the clear portion from the sediment, and preserve it in a cool place.
5. (Concentrated; D. a. concentra′tum, L.)—a. Lump sugar, 8 oz.; colouring, 1⁄4 pint; carbonate of potash, 2 oz.; aloes, 31⁄2 oz.; myrrh and saffron, of each 21⁄2 oz.; compound tincture of cardamoms, 1⁄2 a gall.; water, 3 pints; boil the first five in the water, until reduced to nearly one half; cool, and add the tincture, previously digested for a week, on the saffron; and proceed as above. 14 oz. of extract of liquorice may be used instead of the sugar and colouring.
b. Aloes, myrrh, liquorice, and potassa (all in powder), and saffron as last; compound tincture of cardamoms, 53⁄4 pints; digest a fortnight, and filter. In this way a very odorous and beautiful preparation is produced, which has been much admired. The above are said to possess four times the strength of the College preparation.
Decoction, Anticol′ic. Syn. Anticolic ap′ozem, Degland’s colic mixture; APOZ′EMA ANTICOL′ICUM, L. Prep. Senna leaves, 2 oz.; boiling water, 1 pint; simmer gently to 16 fl. oz.; press out the liquor, add sulphate of soda, 1 oz.; syrup of buckthorn, 2 oz.; and strain through flannel. Used by glassfuls in lead colic, or after poisoning by lead.
Decoction, Antidar′trous. Decoction of Bitter Sweet (see below).
Decoction of Apocynum. Syn. Decoctum apocyni. Root of Apocynum cannabinum, 1 oz.; juniper berries, 1 oz.; water, 3 pints. Boil to 2 pints. A wine-glassful frequently. In dropsy.
Decoction of Ar′nica. Syn. Decoctum arnicæ, L. Prep. 1. (Swediaur.) Flowers of Arnica montana, 1 oz.; water, 3 pints; boil to a quart; filter, and add of syrup of ginger, 3 oz.—Dose, 1 to 2 fl. oz. every two or three hours; in aphonia, paralysis of the voluntary muscles, rheumatism, &c.; and as a substitute for bark in putrid fever, agues, &c.
2. (Ph. Cast. Aust., 1841.) Arnica root, 2 dr.; water, 9 oz.; boil to 6 oz., and strain.—Dose, 1 oz.; as the last.
Decoction of Asparagus. Syn. Decoctum asparagi. Roots of asparagus, 1 oz.; water, 2 pints; boil for 10 or 15 minutes; diuretic.
Decoction, Astrin′gent. Syn. Decoctum astrin′gens, L. Prep. (Swediaur.) Oak-bark, pomegranate peel, and tormentil root, of each 2 dr; water and milk, of each 1 lb.; boil 12 minutes, add of cinnamon, 2 dr.; boil 2 or 3 minutes longer, and strain.—Dose. A wine-glassful.
Decoction of Avens Root. Syn. Decoctum Gei. (Dr A. T. Thompson.) Avens root, 1 oz.; water, 1 pint; boil for 15 minutes, and strain.
Decoction of Baobab Tree. Syn. Decoctum Adansoniæ. Bark of the baobab tree, 6 dr.; water, 11⁄2 pint; boil to a pint, and strain. Used as a substitute for decoction of bark.
Decoction of Bark. Syn. Decoction of cincho′na; Decoctum cincho′næ, L. Prep. 1. Ph. L.:—a. (D. of yellow b.; D. cinchonæ, B. P.) Yellow cinchona or calisaya bark (bruised), 11⁄4 oz.; distilled water, 1 pint; boil for 10 minutes in a lightly covered vessel; when cold, strain and pour on the marc sufficient water to make up 1 pint.
b. (D. of pale b.; D. c. pallidæ, Ph. L.) From pale cinchona or loxa bark, as above (a.)
c. (D. of red b.; D. c. rubræ, Ph. L.) From red bark, as above (a).
2. (Ph. E.) Brown, grey, yellow, or red cinchona (bruised), 1 oz.; water, 24 fl. oz.; boil for 10 minutes; when cold filter the liquor, and evaporate it to 16 fl. oz.
3. (Ph. D.) From pale or loxa bark, similar to the ‘Decoctum cinchonæ pallidæ’ of Ph. L. (1. b. above).
Dose, &c. 1 to 2 fl. oz., 3 or 4 times daily, as a tonic, stomachic, and febrifuge, when the stomach will not bear the administration of bark in powder; in fevers, dyspepsia, convalescence, &c. The plan recommended by the539 Edinburgh College of filtering the decoction when cold is absurd. According to Soubeiran, 146 gr. of the deposit thus removed contained 86 gr. soluble in alcohol, and rich in the cinchona alkaloids. This liquid should, therefore, be well shaken before pouring it out for use, instead of being filtered. The addition of a few drops of either sulphuric or hydrochloric acid to the water greatly increases its solvent power, and also, consequently, the medicinal value of this preparation. (See below.)
Decoction of Bark (Acid′ulated). Syn. Decoctum cinchonæ acidula′tum, L. Prep. 1. To the water for any one of the above, add dilute sulphuric acid, 11⁄2 fl. dr.; boil 10 minutes, and strain whilst hot.
2. (Sir J. Wylie.) Cinchona bark, 1 oz.; water, 16 fl. oz.; diluted sulphuric acid, 1 dr.; as last.
Decoction of Bark (Facti′′tious). Syn. Decoctum cinchonæ factitium, L. Prep. (Ph. Bor.) Willow bark and horse-chestnut bark, of each 1⁄2 oz.; calamus root and cloves, of each 1⁄4 oz.; water, 16 fl. oz.; boil to one half. Used as a substitute for decoction of cinchona bark, but is vastly inferior.
Decoction of Bark and Ser′pentary. Syn. Decoctum cinchonæ cum serpenta′ria, L. Prep. (Sir J. Pringle.) Peruvian bark, 3 dr.; water, 1 pint; boil to one half, and infuse in the hot decoction, serpentaria root, 3 dr. As a diaphoretic stimulant, and tonic, in fevers, and as a gargle in sore throat.
Decoction of Bar′ley. Syn. Barley-water; Decoctum hor′dei (B. P.), L. Prep. 1. (B. P.) Pearl barley, 1 oz. (washed clean); boil for 20 minutes in 15 oz. of water, and strain.
2. (Ph. D.) Similar to above. (See Obs. below.)
Decoction of Barley (Compound.) Syn. Pec′toral decoction, Fe′ver drink; Decoctum pectora′le, Ptisan′a commu′nis, Dec. hor′dei compos′itum (Ph. L.), Mistu′ra hor′dei (Ph. E.), L. Prep. 1. (Ph. L.) Decoction of barley (simple), 1 quart; figs (sliced) and raisins (stoned), of each 21⁄2 oz.; fresh liquorice (sliced), 5 dr.; water, 1 pint; boil to a quart, and strain.
2. (Ph. E.) Pearl barley, 21⁄2 oz.; water, 41⁄2 pints; boil to 3 pints; add figs and raisins, of each 21⁄2 oz.; liquorice root, 5 dr.; water, 1 pint; and boil to 2 pints, as before.
Obs. The above are used as demulcents in fevers, phthisis, strangury, &c., taken ad libitum. They are slightly laxative, and when this would be an objection to their use, a few drops of laudanum may be added. Mixed with an equal quantity of decoction of bark, barley-water forms an excellent gargle in cynanche maligna (ulcerated sore throat), and, with a like quantity of milk and a little sugar, a good substitute for the breast in dry nursing infants. It is, also, often acidulated with lemon juice or sulphuric acid, and sweetened (Decoctum hordei acidulatum). Gum Arabic, 4 dr., and nitre, 1 dr., to each pint, is a common addition in gonorrhœa. Cream of tartar, 1 dr., is occasionally added to render it more aperient.
Decoction of Bistort. Syn. Decoctum bistortæ. Bistort root, 2 oz.; water, 11⁄2 pints; boil 15 or 20 minutes, and strain.—Dose, 1 oz. to 2 oz.; astringent.
Decoction, Bit′ter. Syn. Decoctum ama′rum, L. Prep. 1. Dried tops of lesser centaury and wormwood, and leaves of germander, of each 3 dr.; water, 11⁄4 pint; boil to a pint.
2. Gentian root, 1⁄2 oz.; water, 11⁄2 pint; boil 10 minutes, take out the root, slice it, and add it again to the decoction with dried orange peel, 1⁄4 oz.; boil to 1 pint, and strain.
Decoction of Bitter Sweet. Syn. Antidar′trous ap′ozem; Apozema dulcama′′ræ, L. Prep. (Trousseau and Reveille.) Dulcamara, 1 dr.; water, 16 oz.; boil to 9 oz., and strain. To be taken in three doses during the day. Every other day the quantity is to be increased until 12 dr. or even 2 oz. are taken daily, “so that the patient may begin to feel dryness of the throat, and some disorder of vision and digestion;” and “continue at this quantity for several weeks in succession.” In obstinate skin diseases. See Decoction of Dulcamara.
Decoction of Blue Cardinal Flowers. Syn. Decoctum lobel′iæ, D. l. syphilit′icæ, L. Prep. 1. (P. Cod.) Root of Lobelia syphilitica, 1 handful; water, 12 lb.; boil to 7 lb., and strain.
2. (Swediaur.) Dried root, 5 oz.; water, 12 lb.; as last. Alterative, purgative, and diuretic.
Obs. This decoction was strongly recommended by Swediaur in certain complaints. He gave half a pint at first, twice daily, and afterwards 4 times a day, unless it acted too strongly on the bowels, when the frequency of the dose was diminished, or it was discontinued for 3 or 4 days, and then had recourse to again, until the cure was effected.
Decoction of Bran. Syn. Decoctum furfuris, L. Prep. 1. From bran, 1⁄4 lb.; water, 11⁄4 pint; boil to a pint. In diabetes; and sweetened with sugar, as a demulcent and laxative in cough and sore throat.
2. Bran, 1 quart; water, 11⁄2 gall.; boil 5 minutes, and add cold water, q. s. to bring it to the proper temperature. As an emollient foot-bath.
Decoction of Broom. Syn. Decoctum spar′tii cacumin′ium; D. scopa′′rii (Ph. D.), L. Prep. (Ph. D.) Broom-tops (dried), 1⁄2 oz.; water, 1⁄2 pint; boil 10 minutes, and strain.
2. (Ph. B.) Broom-tops (dried), 1 oz.; distilled water, 1 pint; boil for 10 minutes, and strain. (See below.)
Decoction of Broom (Compound). Syn. Decoctum spar′tii cacuminium c., D. scopa′′rii (Ph. E.), D. s. compos′itum (Ph. L.), L. Prep. 1. (Ph. L.) Tops of broom (recent and dried), juniper berries (bruised), dandelion540 root (bruised), of each 1⁄2 oz.; distilled water, 11⁄2 pint; boil to a pint, and strain.
2. (Ph. E.) Tops of broom and juniper, of each 1⁄2 oz.; cream of tartar, 21⁄2 dr.; water, 11⁄2 pint; boil to a pint, as last. The above are diuretic and laxative.—Dose, 1⁄2 to 1 wine-glassful, 3 or 4 times a day; in dropsy, especially of the belly (ASCITES).
Decoction of Buckbean. Syn. Decoctum menyanthis. Buckbean, 1 oz.; water, 11⁄2 pint; boil to a pint.
Decoction of Burdock. Syn. Decoctum arc′tii, D. barda′næ, L. Prep. 1. Bardana root, 6 oz.; water, 5 pints; boil to 3 pints, and strain.
2. (Wood.) Dried root, 2 oz.; water, 3 pints; boil to 2 pints, and strain. As an alterative; a pint or more daily, in all those cases in which sarsaparilla is recommended.
Decoction of Cabbage-tree Bark. Decoctum Geoffroyæ (Ph. E. 1817), D. g. inermis (Ph. D. 1826). Prep. (Ph. D.) Bark of the cabbage tree (bruised), 1 oz.; water, 1 quart; boil to a pint, and strain. Cathartic, narcotic, and anthelmintic.—Dose, 2 to 4 table-spoonfuls for an adult; 1 to 2 teaspoonfuls for a child, followed by demulcents and castor oil; in worms, &c.
Decoction of Calumba (Compound). Syn. Decoctum calum′bæ compos′itum, L. Prep. (Ph. U. S. 1831.) Calumba and quassia, of each 2 dr.; orange peel, 1 dr.; rhubarb, 20 gr.; carbonate of potassa, 30 gr.; water, 20 fl. oz.; boil to 16 fl. oz., strain, and, when cold, add of compound tincture of lavender, 1⁄2 fl. oz. Bitter, tonic, and stomachic.—Dose, 1 to 2 table-spoonfuls 3 or 4 times daily.
Decoction of Centaury. Syn. Decoctum cimicifuge, F. H. Lesser centaury, 2 oz.; water, 2 pints; boil for a few minutes, and strain.
Decoction of Cey′lon Moss. Syn. Decoctum fu′′ci amyla′cei, D. ploca′′riæ candi′dæ, L. Prep. From Ceylon moss, 2 dr.; water, milk, or whey, 1 pint; boil to 16 fl. oz., and strain. It may be sweetened and flavoured. In irritation of the mucous membranes and in phthisis.
Decoction of Cham′omile. Syn. Decoctum anthe′midis, D. chamæmeli, L. From chamomiles, 1 oz.; boiling water, 1 pint; digest for 10 minutes, simmer gently for 2 or 3 minutes longer, and strain with pressure. (See below.)
Decoction of Chamomile (Compound). Syn. Decoctum chamæmeli compositum, L. Prep. (Ph. D. 1826.) Chamomile flowers (dried), 1⁄2 oz.; fennel seed, 2 dr.; water, 16 oz.; boil a short time, and strain. Both the above are bitter, stomachic and tonic; the last is vermifuge. They are chiefly used as fomentations and clysters.
Decoction of Cherry Laurel Bark. Syn. Decoctum lauro-cerasi corticis. (Dr Kastner.) Cherry laurel bark, 2 oz.; water, 2 pints; boil, and strain.
Decoction of Chiret′ta. Syn. Decoctum Chiraytæ, L. Prep. From chiretta or chyrata, 5 dr.; water, 1 pint; boil 8 or 10 minutes and strain.—Dose, 1⁄2 to 1 wine-glassful 2 or 3 times daily, as a stomachic tonic; in flatulency and acidity, especially in the dyspepsia of gouty persons.
Decoction of Cincho′na. See Decoction of Bark.
Decoction of Coffee. Syn. Decoctum caffei. Boil 10 dr. of raw coffee berries in 8 oz. of water to 5 oz. To be given in 3 doses during the intermissions of intermittent fever.
Decoction of Col′ocynth. Syn. Decoctum colocynth′idis, L. Prep. (Ph. Bat.) Colocynth pulp, 1 dr.; water, 8 oz.; boil 10 minutes, and when quite cold, add of syrup of orange peel, 1 oz.; sulphuric ether, 1 dr.—Dose, 2 to 6 dr., 2 or 3 times a day; in dropsy, &c.
Decoction of Colts′foot. Syn. Decoctum tussilag′inis, L. Prep. (Pereira.) Fresh leaves of coltsfoot, 2 oz. (or flowers, 1 oz.); water, 2 pints; boil to a pint and strain. A popular remedy in chronic coughs and chest diseases. It is emollient and demulcent.—Dose. Half a teacupful, ad libitum. (See below.)
Decoction of Coltsfoot (Compound). Syn. Decoctum tussilaginis compositum, L. Prep. (Taddei.) Coltsfoot flowers, 6 oz.; figs, raisins, and jujubes, of each 2 oz.; water, 12 pints; boil down to 4 pints; add liquorice root, 2 oz.; again boil and strain. As the last.
Decoction, Com′mon. See Decoction of Mallows.
Decoction of Cor′sican Moss. Syn. Decoctum helminth′ocorti, L. Prep. From the moss, 5 dr.; water, 11⁄2 pint; boil to a pint.—Dose. A wine-glassful, three times a day; as a vermifuge. In 1822, Mr Farr brought it forward as a remedy for cancer.
Decoction of Cot′ton Root. Syn. Decoctum gossyp′ii, L. Prep. (Dr Bouchelle.) Inner part of the root of the cotton plant, 4 oz.; water, 1 quart; boil to a pint.—Dose. A wine-glassful, occasionally, as an emmenagogue; or, every 30 or 40 minutes, to produce uterine contractions, for which purpose it is said to be as effectual as ergot of rye.
Decoction of Dandeli′on. Syn. Decoctum tarax′aci (B. P.), L. Prep. 1. (B. P.) Fresh dandelion root (bruised), 1 oz.; water, 11⁄2 pint; boil to a pint, and strain.
2. (Ph. E.) Herb and root (fresh), 7 oz.; water, 1 quart; boil to a pint. Aperient, stomachic, and tonic.—Dose, 1 to 2 fl. oz., or more, 2 or 3 times daily.
Decoction, Diaphoret′ic. Syn. Decoctum diaphoreticum, L. Decoction of bark, 1 pint; liquor of acetate of ammonia, 4 oz.; aromatic confection, 1 oz.—Dose, 2 or 3 table-spoonfuls every 3 hours.
Decoction of Dog-grass. Syn. Decoctum gramin′is, L.; Ptisane chiendent, Fr. Prep. From dog-grass root (Triticum repens), 1 oz.; liquorice root, 1⁄2 oz.; water, 1 quart; boil 20541 minutes, and strain. Aperient and pectoral; by cupfuls, ad libitum. (See below.)
Decoction of Dog-grass (Ioduret′ted). Syn. Decoctum graminis iodure′tum, L. Prep. (Magendie.) Decoction of dog-grass, 32 fl. oz.; syrup of peppermint, 2 oz.; iodide of potassium, 1⁄2 dr.; mix. By cupfuls, ad libitum.
Decoction of Dog-wood. Syn. Decoctum cor′nus floridæ, L. Prep. (Ph. U. S.) Dog-wood bark (bruised), 1 oz.; water, 1 pint; boil 10 minutes, and strain whilst hot. Tonic and astringent; recommended as a substitute for bark.—Dose. A wine-glassful.
Decoction of Dulcama′ra. Syn. Decoction of bitter sweet, D. of woody nightshade; Decoctum dulcama′′ræ (Ph. L. E. & D.), L. Prep. 1. (Ph. L.) Woody nightshade or bitter sweet (the new shoots), 10 dr.; water, 11⁄2 pint; boil to a pint, and strain.
2. (Ph. E.) Dulcamara (chopped small), 1 oz.; water, 24 fl. oz.; boil to a pint, and strain.
3. (Ph. D.) Twigs of woody nightshade, 1 oz.; water, 1 pint; boil 10 minutes in a covered vessel, and strain. It should measure about 16 fl. oz. Alterative, diaphoretic, and diuretic.—Dose. A wine-glassful, or more, 2 or 3 times a day; in chronic coughs and chronic skin diseases, and in most of those cases wherein sarsaparilla proves useful. See Decoction of bitter sweet, also below.
Decoction of Dulcama′ra (Compound). Syn. Decoctum dulcama′′ræ composi′tum, L. Prep. 1. (Augustin.) Dulcamara (bitter sweet), 4 dr.; burdock root, liquorice root, sassafras chips, and guaiacum wood, of each, 2 dr.; water, 2 lbs.; boil to 16 fl. oz., and strain.—Dose, 1 to 2 wine-glassfuls 2 or 3 times a day.
2. (Foy.) As the last, but using dulcamara, 2 oz.—Dose, 1⁄2 to 1 wine-glassful; in similar cases to those in which the simple decoction is given, especially in chronic rheumatism and venereal affections.
Decoction of El′der Bark. Syn. Decoctum sambu′′ci, D. s. corticis. L. Prep. 1. (Sydenham.) Inner bark of elder, 1 oz.; water and milk, of each 1 pint; boil to one half, and strain.
2. (Collier.) Bark, 1 oz.; water, 16 fl. oz.; boil to 1⁄2 pint, and strain.
3. (Pereira.) Bark, 1 oz.; water, 1 quart; boil to one half.—Dose. One wine-glassful 2 or 3 times a day; as an aperient and resolvent in various chronic disorders, in dropsy, and in certain cutaneous affections; or, 2 wine-glassfuls, as before, as a hydragogue cathartic in dropsies.
Decoction of Elecam′pane. Syn. Decoctum helen′′ii, D. inu′læ, L. Prep. (Ph. U. S.) Elecampane root, 1⁄2 oz.; water, 1 pint; boil a few minutes and strain. Tonic and expectorant, and, in some cases, diuretic and diaphoretic.—Dose. A wine-glassful every hour or two. (See below.)
Decoction of Elecampane (Compound). Syn. Decoctum heleni compositum, D. inulæ c., L. Prep. (Rotier.) Elecampane, 1 oz.; hyssop and ground ivy, of each 2 dr.; water, 1 pint; boil 15 minutes, strain, and add of honey, 2 oz.—Dose, 1 to 3 table-spoonfuls; as the last.
Decoction of Elm Bark. Syn. Decoctum ul′mi (B. P.), L. Prep. Elm bark (cut in small pieces), 1 oz.; distilled water, 16 oz.; boil to 8 oz., and strain.—Dose, 2 to 4 oz., three or four times a day, as a cheap substitute for sarsaparilla in scaly skin diseases. (See below.)
Decoction of Elm Bark (Compound). Syn. Decoctum ulmi Compositum, L. Prep. (Jeffrey.) Simple decoction of elm bark, 8 pints; liquorice root, sassafras and guaiacum chips, of each 1 oz.; mezereon root, 3 dr.; boil for one hour, and strain. More active than the last.
Decoction of Er′got. Syn. Decoctum ergot′æ, D. seca′lis cornuti, L. Prep. (Pereira.) Ergot of rye (bruised), 1 dr.; water, 6 fl. oz.; boil 10 minutes, and strain.—Dose. One third at intervals of half an hour, until the whole is taken; as a parturifacient.
Decoction of Fern Root. Syn. Decoctum filicis; D. radicis f., L. Prep. (Dr Wood.) Dried fern-root, 1 oz.; water, 1 pint; boil to 16 fl. oz., and strain. By wine-glassfuls, fasting, until it excites slight nausea; as a vermifuge, more particularly for tapeworm.
Decoction of Figs. Syn. Decoctum fici, L. Prep. (Cadet.) Figs (chopped), 1 oz.; water, 1 pint; boil, and strain. Demulcent and pectoral; taken ad libitum. (See below.)
Decoction of Figs (Compound). Syn. Decoctum fici compositum, L. Prep. (Foy.) Figs and raisins (chopped), of each 2 oz.; liquorice root, 1⁄2 oz.; boiling water, 1 quart; boil 15 minutes, and strain. As the last.
Decoction for Ene′mas. Syn. Decoctum pro enema′te, L. Barley-water, or thin gruel, is commonly used under this name. See Decoction of Mallows, &c.
Decoction for Fomenta′tions. Syn. Decoctum pro fomento, L. Prep. (Ph. L. 1788.) Dried leaves of southern wood, tops of sea wormwood, and chamomile flowers, of each 1 oz.; laurel or bay leaves (dried), 1⁄2 oz.; water, 1 pint, boil a few minutes, and strain.
Decoction of Galls. Syn. Decoctum gal′læ, (Ph. L.) Prep. From galls (bruised), 21⁄2 oz.; water, 1 quart; boiled to one half, and strained. As an astringent, fomentation, enema, or injection, in prolapsus ani, piles, and leucorrhœa.
Decoction of Guaiac′um. Syn. Decoctum guaiac′i (Ph. E.), D. g. compositum (Ph. D. 1826), L. Prep. 1. (Ph. E.) Guaiacum shavings, 3 oz.; raisins (chopped), 2 oz.; water, 8 pints; simmer down to 5 pints, adding towards the end, sassafras (rasped or sliced), and liquorice root (bruised), of each 1 oz.
2. (Ph. D.) Guaiacum wood, 3 oz.; sassafras, 10 dr.; liquorice root, 21⁄2 oz.; water, 10 pints, as the last; to strain 5 pints.
542
Obs. The above form the once celebrated ‘Decoction of the Woods,’—Dose. A teacupful 3 or 4 times daily, or oftener, in chronic rheumatism, cutaneous diseases, after a course of mercury, &c. Although its virtues are of a very dubious kind, there is no doubt that it frequently does good, especially when persevered in with a sudorific regimen.
Decoction of Hairy Horehound. Syn. Decoctum ballotæ lanatæ, L. Prep. (Rehmann.) Siberian or woolly horehound (Ballota), 11⁄2 oz.; water, 1 quart; boil to one half.—Dose. A tumblerful, or more, twice a day; in rheumatic, gouty, and dropsical affections, especially the latter. See Decoction of Horehound.
Decoction of Harts′horn. See Mixtures.
Decoction of Hel′lebore. 1. (Decoction of Black hellebore; Decoctum hellebori nigri, L.) Prep. 1. (A. T. Thomson.) Black hellebore root, 2 dr.; water, 1 pint; boil 15 minutes.—Dose, 1 fl. oz., every 4 hours; in dropsy, worms, chronic skin diseases, &c., occurring in non-irritable habits.
2. (Decoction of white hellebore; Decoctum veratri, Ph. L. & D.) Prep. (Ph. L. 1836.) White hellebore (bruised), 10 dr.; water, 1 quart; boil to a pint, and when cold, add of rectified spirit, 3 fl. oz. Used as a lotion, in itch, lepra, psoriasis, scald-head, &c.; and to destroy pediculi. In most cases it should be diluted with water, and should never be applied to the unsound skin.
Decoction of Holly Leaves. Syn. Decoctum ilicis. (Foy.) Holly leaves, 1⁄2 oz.; water, 16 oz.; boil to 12 oz. For three doses.
Decoction of Horehound. Syn. Compound decoction of horehound; Decoctum marubii compositum, L. Prep. (Dr R. E. Griffith.) Dried horehound (Marrubium vulgare), 1 oz.; liquorice root and flax seed (bruised), of each 1⁄2 oz.; boiling water, 11⁄2 pint; macerate for 3 or 4 hours (boil a minute), and strain. An excellent demulcent and pectoral.—Dose, 1 to 2 fl. oz., as required, in coughs, &c.
Decoction of Horse-chest′nut Bark. Syn. Decoctum hippocastanei, L. Prep. (Dr Wood.) Horse-chestnut bark (coarsely powdered), 10 dr.; water, 1 pint; boil 10 minutes, and strain. Used for decoction of cinchona bark. A little liquorice root is frequently added. (See below.)
Decoction of Horse-chest′nut Bark (Compound). Syn. Decoctum hippocastanei compositum, L. Prep. 1. (Phœbus.) Horse-chestnut bark, 11⁄2 oz.; water, 18 fl. oz.; boil to one half, strain, and when quite cold, add of sulphuric ether, 1 to 2 dr.; syrup of orange peel, 1 oz. To be used during the intermission of an ague in wine-glassfuls at a time.
2. (Spielman.) Horse-chestnut bark and willow bark, of each 1⁄2 oz.; calamus aromaticus and root of water avens, of each 2 dr.; water, 16 fl. oz.; boil to one half. As the last.
Decoction of Iceland Moss. Syn. Decoction of liverwort; Decoctum cetrariæ (Ph. L.); D. lichenis Islandici (Ph. D.); D. lichenis (Ph. L. 1824.) Prep. 1. (Ph. L.) Liverwort (Iceland moss), 5 dr.; wecm 11⁄2; pint; boil to a pint, and strain.
2. (Ph. D.) Iceland moss, 1 oz.; water, 11⁄2 pint; boil for 10 minutes in a covered vessel, and strain. Nutritious, demulcent, pectoral, and tonic.—Dose, 1 to 4 fl. oz., every 3 or 4 hours; in chronic affections of the chest and stomach, especially pulmonary consumption, old coughs, dyspepsia, chronic diarrhœa, and dysentery. It may be flavoured and sweetened; milk is frequently added to it. The bitter matter may be removed by steeping the moss for some time in pretty warm water, or in cold water, to which a very little carbonate of potash has been added. Without this is done, it is intensely bitter and nauseous.
Decoction of Indian Ba′el. Syn. Decoction of Ægle marmelos; Decoctum bael, L. From the dried unripe fruit of Ægle marmelos (Indian bael), 2 oz.; water, 1 pint; boil to one third, and strain.—Dose, 2 fl. oz. two or three times a day; in dysentery, diarrhœa, and English cholera.
Decoction of Indian Pink. Syn. Decoctum spigeliæ, L. Prep. Indian pink root, 5 dr; water, 1 pint; boil 5 minutes; add senna, 4 dr.; digest 15 minutes, strain and add of manna, 1 oz.—Dose. A small teacupful, 3 times a day, for an adult; 1⁄2 oz. to 1 oz., or less, for children; as an anthelmintic purge.
Decoction of Indian Sarsaparil′la. Syn. Decoctum hemedes′mi, L. Prep. (Pereira.) Root of Indian sarsaparilla (Hemedesmus Indicus), 2 oz.; water, 11⁄2 pint; boil to a pint. Diuretic, alterative, and tonic.—Dose. By wine-glassfuls, as decoction of sarsaparilla.
Decoction of I′′rish Moss. Syn. Decoctum chon′dri. Prep. (Pereira.) Carrageen or Irish moss, 1 oz.; macerate in lukewarm water for 10 minutes, take it out and drain it, and then boil it in water (or milk), 3 pints, for 15 minutes, and strain through linen.
Obs. If twice the above weight of moss is employed, a mucilage (mucilago chondri) is produced, which may be flavoured with lemon juice, spices, &c., and forms a most nutritious article of spoon diet. It is taken in the same cases as decoction of Iceland moss; and is frequently employed in cookery, as a substitute for animal jelly, in the preparation of blancmanges, soups, &c.
Decoction of I′′singlass. See Lisbon Diet Drink.
Decoction of Jamaica Dogwood. Syn. Decoctum Cornus Floridæ. (U. S. Ph.) Bark of Jamaica dogwood, 1 oz.; water, 16 oz. o.m.; boil 10 minutes and strain, and make up to 1 pint o.m. As a substitute for cinchona, but is more astringent.
Decoction of Jujubes. Syn. Decoctum Jujubarum. Boil 2 oz. of jujubes (stoned),543 for an hour, in a sufficient quantity of water to produce 2 pints of decoction.
Decoction of Ju′niper Berries (Compound). Syn. Decoctum juniperi compositum, L. Prep. (St. B. Hosp.) Juniper berries, 2 oz.; cream of tartar, 3 dr.; water, 4 pints; boil to a quart, strain, and add compound spirit of juniper, 2 fl. oz. Diuretic.—Dose, 2 or 3 wine-glassfuls, 3 times a day, warm.
Decoction of Linseed (Compound). Syn. Decoctum li′ni compositum (Ph. D.), L. Prep. (Ph. D.) Linseed, 1 oz.; liquorice root (bruised), 1⁄2 oz.; water, 11⁄2 pint; boil for 10 minutes in a covered vessel, and strain whilst hot. Emollient and demulcent.—Dose. A wine-glassful ad libitum; in gonorrhœa, dysentery, pulmonary affections, &c. It may be flavoured with lemon peel, and sweetened. See Infusions.
Decoction of Liquorice. Syn. Decoctum glycyrrhizæ, L. Prep. (Ph. D. 1826.) Liquorice root (sliced), 11⁄2 oz.; water, 16 fl. oz.; boil 10 minutes and strain. A mild demulcent; it is taken either alone, by wine-glassfuls, or is used as a vehicle for more active remedies.
Decoction, Lisbon. See Lisbon Diet Drink.
Decoction of Liv′erwort. See Decoction of Iceland moss.
Decoction of Log′wood. Syn. Decoctum hæmatoxyli (Ph. L. E. & D.), L. Prep. 1. (Ph. L.) Logwood chips, 10 dr.; water, 11⁄2 pint; boil to a pint, and strain.
2. (Ph. E.) Logwood, 1 oz.; water, 1 pint; boil to 10 fl. oz., adding towards the last, cinnamon (in powder), 1 dr.
3. (Ph. D.) Logwood, 1 oz.; water, 1⁄2 pint. Astringent and tonic.—Dose, 1 table-spoonful to a wine-glassful; in diarrhœa, as required.
4. (Ph. B.) Logwood in chips, 1 oz.; cinnamon in coarse powder, 60 gr.; distilled water, 1 pint. Boil for 10 minutes, and strain to make up 1 pint.
Decoction of Mad′der. Syn. Decoctum rubiæ, D. r. tinctoriæ, L. Prep. 1. (Dewees.) Powdered madder, 1 oz.; boiling water, 1 pint; simmer for 15 minutes, and add of cloves (bruised), 1 dr.; when cold, strain.—Dose. A wine-glassful, 2 or 3 times daily; in amenorrhœa, chlorosis, &c.; or every 3 hours, a short time previous to the expected menstrual discharge.
2. (W. Cooley.) To the last add ammonio-citrate of iron, 3 dr.
3. (St. Marie.) Powdered madder, 1⁄2 oz.; hops, 1 dr.; English walnut leaves, 3 dr.; water, 1 quart; boil to 11⁄2 pint, strain, and when cold, add of tincture of tartrate of iron, 1 dr.—Dose, 2 fl. oz., night and morning; in scrofula, &c.
Decoction of Mal′lows. Syn. Common decoction; Decoctum com′mune, D. pro enema′te (Ph. L. 1787), D. malvæ compositum (Ph. L. 1836), L. Prep. (Ph. L. 1836.) Common mallows (dried), 1 oz.; chamomile flowers (dried), 1⁄2 oz.; water, 1 pint; boil 15 minutes, and strain. Used chiefly for fomentations and enemas.
Decoction of Malt. Syn. Decoctum bi′næ, D. bynes, D. malti, L. Prep. (Swediaur.) Ground malt, 3 oz.; water, 1 quart; boil to a pint, and strain. An oz. of syrup of lemons, or of saffron, may be added to the cold decoction; or, a little liquorice root, with the malt. Demulcent and laxative. A cupful ad libitum. Infusion of malt (sweet wort) is a more convenient and elegant preparation.
Decoction of Marshmal′low. Syn. Decoctum althæ′æ (Ph. D. 1826 and Ph. E. 1813), L. Prep. (Ph. D. 1826.) Dried root and herb of marshmallow, 4 oz.; raisins (stoned), 2 oz.; water, 7 pints (wine measure); boil down to 5 pints, strain, allow it to deposit the sediment and decant the clear liquid. Demulcent.—Dose. A cupful ad libitum, in coughs, colds, calculous affections, and other diseases of the urinary organs. See Mixtures.
Decoction of Matico. Syn. Decoctum matico′nis, L. Prep. (Dr Jeffreys.) Matico leaves, 1 oz.; water, 1 pint; boil 12 minutes, and strain. Astringent.—Dose, 1 fl. oz., 2 or 3 times a day; in hæmorrhagic and other discharges.
Decoction, Mercu′′rial. Syn. Decoctum hydrargyri, D. mercuriale, L. Prep. 1. Quicksilver, 4 oz.; water, 1 pint; boil in a glass or earthen vessel for an hour, adding water to replace that lost by evaporation.—Dose. A teacupful.
2. Mercurial pill, 1 oz.; water, 1 quart; boil to a pint.—Dose. A wine-glassful. Both were formerly taken for worms and the itch.
3. Corrosive sublimate, 1 gr.; (dissolved in) spirit of wine, 30 drops; extract of sarsaparilla, 3 dr.; decoction of sarsaparilla, 8 fl. oz.; mix.—Dose. One large table-spoonful, 3 times a day; in syphilis and obstinate skin diseases.
Decoction of Mezere′on. Syn. Decoctum mezerei (Ph. E. and Ph. D. 1826), L. Prep. (Ph. E.) Root-bark of mezereon, 2 dr.; liquorice root, 4 dr.; water, 1 quart; simmer to 11⁄2 pint, and strain. Stimulant and sudorific.—Dose. A wine-glassful, or more, three or four times a day; in chronic rheumatism, scrofula, secondary syphilis, lepra, and some other cutaneous affections. Much boiling injures the virtues of mezereon. (See below.)
Decoction of Mezere′on (Compound). Syn. Decoctum mezerei compositum, L. Prep. (Van Mons.) Mezereon, 2 dr.; bitter sweet, 4 dr.; burdock, 2 oz.; water, 2 quarts; boil to 3 pints, add of liquorice root, 2 dr., and strain. As the last, and in obstinate diseases of the skin.
Decoction of Mugwort. Syn. Decoctum artemisiæ vulgaris544. Mugwort root, 1 oz.; water, 24 oz. Boil for half an hour. In epilepsy.
Decoction of Myrrh. Syn. Decoctum MYRRHÆ. (Ph. D.) Myrrh, 2 dr.; water, 81⁄2 oz.; triturate the myrrh with the water gradually added; then boil for 10 minutes in a covered vessel, and strain.
Decoction, Narcotic. Syn. Decoctum anodynum, D. narcoticum, L. Prep. (Hosp. Form.) Common nightshade (dried), 1 oz.; poppy heads, 3 in no.; water, 1 pint; boil 10 minutes, and strain. As an anodyne fomentation, used warm.
Decoction of Ni′tre. Syn. Decoctum nitrosum, D. nitratum, D. potassæ nitratis, L. Prep. 1. Nitre, 1⁄2 oz.; white sugar, 2 oz.; cochineal, 20 gr.; water, 11⁄2 pint; boil a few minutes, and strain.
2. (Hosp. Form.) Barley-water, 1 pint; nitre, 5 dr.; dissolve. Diuretic, diaphoretic, and refrigerant. A wine-glassful, frequently; in gonorrhœa, sore throat, acute rheumatism, scurvy, &c.
Decoction of Oak Bark. Syn. Decoctum quercûs (Ph. L. E. & D.), L. Prep. 1. (Ph. L. & E.) Oak bark (bruised), 10 dr.; water, 1 quart; boil down to a pint, and strain.
2. (Ph. D.) Oak bark, 11⁄2 oz.; water, 11⁄2 pint; boil 10 minutes, and strain. Astringent. Used as a gargle in ulcerated sore throat, relaxation of the uvula, &c., and as a wash, and as an injection in piles, leucorrhœa, hæmorrhages, prolapsus ani, &c.
3. (Ph. B.) Oak bark bruised, 11⁄2 oz.; distilled water, 1 pint; boil for 10 minutes, and strain.
Decoction of Oats. Syn. Water gruel; Decoctum avenæ, L. Prep. 1. (Cullen.) Oatmeal, 1 oz.; water, 3 quarts; boil to a quart, strain, and when cold, decant the clear liquid from the sediment.
2. (A. T. Thomson.) Washed groats, 4 oz.; water, 4 pints; boil to a quart. Nutritious and demulcent. Taken ad libitum, to promote the action of purgatives, and as an enema, either alone, or as a vehicle for more active substances. It is too thin for food. See Gruel.
Decoction of Parei′ra. Syn. Decoctum pareiræ (Ph. L.), L. Prep. 1. (Ph. L.) Pareira brava root (sliced), 10 dr.; water, 11⁄2 pint; boil to a pint, and strain.
2. (Sir B. Brodie.) Pareira, 4 dr.; water, 3 pints; boil to a pint, as last. The above are given in gonorrhœa, leucorrhœa, and chronic inflammation of the bladder.—Dose. Of the first, 1⁄2 to 1 wine-glassful, 3 or 4 times a day; of the second, about twice that quantity, or more. It is commonly combined with some tincture of hyoscyamus; and when the triple phosphates are present in the urine, dilute hydrochloric or nitric acid may be added. See Pareira.
Decoction, Pec′toral. See Decoction of Barley.
Decoction of Pel′litory. Decoctum pyre′′thri, L. Prep. (Guy’s Hosp.) Pellitory root, 1 oz.; water, 11⁄2 pint; boil to a pint, and strain. Used as a gastric stimulant, and as a gargle in the relaxation of the uvula.
Decoction of Pome′granate. Syn. Decoctum grana′ti (Ph. L.), L. Prep. (Ph. L.) Pomegranate rind (fruit-bark), 2 oz.; distilled water, 11⁄2 pint; boil to a pint, and strain. Astringent. Used as a gargle and injection, in sore throat, leucorrhœa, &c.; and internally, in diarrhœa, dysentery, &c.—Dose, 1 fl. oz., or more.
Decoction of Pomegranate Root. Syn. Decoctum granati radicis (Ph. L.), L. Prep. 1. (Ph. L.) Root-bark of pomegranate (sliced), 2 oz.; water, 1 quart; boil to a pint, and strain.
2. (Collier.) Bark of the root, 2 oz.; water, 1 pint; boil to one half. This is the common form used in India.
Dose, &c. A wine-glassful, half-hourly, until the whole is taken, a light diet and a dose of castor oil having been taken the day previously. In tapeworm, Dr Collier recommends the whole of the last preparation to be given at 2 doses, at the interval of 2 hours. It purges, and in 5 or 6 hours frequently expels the worm; if this does not take place, it should be persevered in. “Look for the head of the tænia (tapeworm); for if that is not expelled, you have done nothing.” (Collier.) Oil of turpentine and kousso are now more frequently given in tænia in this country.
Decoction of Poppies. Syn. Decoction of poppy-heads, Fomentation of p.-h.; Decoctum papaveris (Ph. L. E. & D.), L. Prep. 1. (Ph. L.) Poppy-heads (bruised), 4 oz.; water, 2 quarts; boil for 15 minutes, and strain.
2. (Ph. E. & D.) As the last, but using only 3 pints of water. Used as an emollient fomentation, in painful swellings, excoriations, &c. The addition of a 1⁄4 pint of vinegar is said to promote its efficacy.
3. (Ph. B.) Poppy-heads bruised, 2 oz.; distilled water, 11⁄2 pint. Boil for 10 minutes and strain. The product should measure a pint.
Decoction of Quas′sia. Syn. Decoctum quassiæ, L. Prep. From quassia chips (small). 1 dr.; water, 11⁄4 pint; boil to a pint, and add syrup of orange peel, 2 oz.—Dose. A wine-glassful, occasionally, as a stomachic tonic. See Infusions.
Decoction of Quince. Syn. Decoction of quince seed, Mucilage of q. s.; Decoctum cydonii (Ph. L.), L. Prep. From quince seeds, 2 dr.; water, 1 pint; boil for 10 minutes, and strain. Used as an emollient and sheathing application to abraded or wounded surfaces, as cracked lip, nipples, &c.; and to the skin in erysipelas, to painful hæmorrhoidal tumours, and the like. Prepared with a little less water, it is used by the hairdresser as ‘bandoline’ or ‘fixateur.’
Decoction of Red Gum. Syn. Decoctum Gummi Rubri (Mr Squire.) Red gum, 1 oz.; water, 2 pints; boil 10 minutes, and strain.
Decoction of Rice. Syn. Rice water545, Rice drink; Decoctum ory′zæ, L. Prep. Rice, 2 oz.; water, 1 quart; boil to one half, and strain. Demulcent. A good drink in fevers, coughs, &c., either alone or sweetened and flavoured with a little lemon peel.
Decoction of Sarsaparil′la. Syn. Decoctum sar′zæ (Ph. L. & E.), D. sarsaparillæ (Ph. D.), L. Prep. 1. (Ph. L.) Sarsaparilla (sliced), 5 oz.; water, 2 quarts; boil to a quart, and strain.
2. (Ph. E.) Sarsaparilla, 5 oz.; boiling distilled water, 4 pints; macerate for 2 hours, in a vessel lightly covered, and placed in a warm situation; then take out the root, bruise it, return it again to the liquor, boil down to a quart, and strain.
3. (Ph. D.) Sarsaparilla, 2 oz.; boiling water, 11⁄2 pint; digest an hour, boil 10 minutes, cool, and strain.
4. (Ph. B.) Digest 21⁄2 oz. of Jamaica sarsaparilla cut transversely in 11⁄2 pint of boiling water for an hour, boil for 10 minutes, cool, and strain. Make up to one pint.
Obs. The medicinal virtues of sarsaparilla root reside wholly in the bark, or cortical portion; it is therefore quite unnecessary to bruise it, as directed in the Ph. E. By those houses which do largely in decoction of sarsaparilla, the root is seldom split or cut; the bundles in which it is made up being simply untied and spread open, to allow of the free exposure of every part to the solvent action of the water. By this plan the whole of the soluble portion of the bark is extracted, whilst the feculent matter that pervades the wood is only partially dissolved out. According to Soubeiran, a mere infusion is preferable. The dose is a teacupful to half a pint, 3 or 4 times a day.
An extemporaneous decoction of sarsaparilla is made by dissolving 3⁄4 oz. of the simplest extract in 1 pint of hot water. See Sarsaparilla, and below.
Decoction of Sarsaparilla (Concentrated). Syn. Decoctum sarzæ concentratum, L. Prep. 1. (Wholesale.) Sarsaparilla (Jamaica) 101⁄2 lbs., is placed in a large and well-cleaned copper boiler, and enough boiling water added to cover it; it is then left to macerate, without boiling, for 3 or 4 hours, after which it is boiled for about an hour, and the clear liquor drawn off into another clean copper pan; the root (after it has well drained) is then washed or ‘sparged’[255] with boiling water, until the latter runs off scarcely coloured; the washings are added to the decoction, and the whole evaporated as quickly as possible to 61⁄2 pints; it is then set to cool, and rectified spirit of wine, 11⁄2 pint, further added; after agitation, the whole is set aside in a well-corked bottle, in a cool place, for a week. In a few days it is usually found as clear and brilliant as brandy, with very little sediment, and will keep for any length of time uninjured. Some manufacturers, instead of washing the root, give it a second and third water, boiling it each time and evaporating the mixed liquors.
[255] For an explanation of the operation of ‘sparging’ see page 356.
2. (Extemporaneous.) Extract of sarsaparilla, 61⁄2 oz.; water, 12 fl. oz.; dissolve, add rectified spirit, 21⁄2 fl. oz., and water, q. s. to make the whole exactly measure a pint.
Obs. 1 drachm of this decoction, mixed with 7 drachms of water, forms a similar preparation to the Decoctum Sarzæ of the Ph. L., and is now very frequently substituted for it in dispensing. See Sarsaparilla, Extracts, and below.
Decoction of Sarsaparilla (Compound). Syn. Decoctum sarzæ compositum (Ph. L. & E.), D. sarsaparillæ c. (Ph. D.), L. Prep. 1. (Ph. L.) Decoction of sarsaparilla (boiling), 4 pints; sassafras chips, guaiacum wood (rasped), and fresh liquorice root (bruised), of each 10 dr.; mezereon (root-bark), 3 dr.; boil for 15 minutes, and strain.
2. (Ph. E.) As the last, but using 4 dr. of mezereon.
3. (Ph. D.) Sarsaparilla (sliced), 2 oz., sassafras, guaiacum turnings, and liquorice root (bruised), of each 2 dr.; mezereon root-bark, 1 dr.; boiling water, 11⁄2 pint; digest for an hour, then boil for 10 minutes, cool, and strain.
4. (Extemporaneous.) Compound extract of sarsaparilla, 71⁄2 dr.; boiling water, 1 pint; dissolve.
5. (Ph. B.) Jamaica sarsaparilla, cut transversely, 21⁄2 oz.; sassafras, guaiacum turnings, bruised liquorice root, of each 1⁄4 oz., mezereon root bark 60 grains; digest them with 11⁄2 pint of boiling water in a covered vessel for an hour, then boil for 10 minutes, cool, and strain. Make up to 1 pint.
Obs. This decoction is an imitation of the once justly celebrated ‘Lisbon Diet Drink.’ It is an alterative and diaphoretic.—Dose. A teacupful, or more, 3 or 4 times a day, either along with, or after, a mercurial course; and in syphilis, scurvy, scrofula, chronic rheumatism, lepra, psoriasis, and several other skin diseases, and especially in cachexia, or general bad habit of body. During its use the skin should be kept warm. See Sarsaparilla and below.
Decoction of Sarsaparilla (Concentrated Compound). Syn. Decoctum sarzæ compositum concentratum, D. sarsaparillæ c. c., L. There is a very considerable trade done in this article, in consequence of compound decoction of sarsaparilla being taken in large doses, both alone and in combination with other remedies, and the pharmacopœial preparation spoiling if kept longer than about 12 hours, in warm weather. Like the concentrated simple decoction, it is said to be of 8 times the usual strength, so that when mixed with 7 times its weight of water, it forms a similar preparation to the Decoctum Sarzæ Compositum,—Ph. L., for which it is very generally substituted in dispensing.
546
Prep. 1. (Wholesale.) Sarsaparilla (red Jamaica), 96 lbs.; mezereon root (not root-bark), 9 lbs.; liquorice root (bruised), 16 lbs. The mezereon and liquorice are first laid (loosely) on the bottom of a clean copper pan, and the bundles of sarsaparilla (untied and loosened) packed over them, in horizontal layers, alternately at right angles with each other. Three or four boards, with as many iron 1⁄2-cwt. weights, are next placed on the top of the whole. Water is now run in, to about ten inches higher than the ingredients, and heat is applied until ebullition commences. The materials are now allowed to macerate, without boiling, for 3 or 4 hours, after which the liquor is gently boiled for about an hour, care being taken to add fresh water from time to time, so as to keep the whole well covered. The decoction is next run off, and set evaporating as quickly as possible. The ingredients are then washed with successive portions of boiling water, by allowing it to descend from a species of shower-bath, after the manner of ‘sparging,’ described under Brewing.[256] This is repeated until the water runs off nearly colourless, the smallest quantity being employed that will effect the object in view. The whole of the liquid is now evaporated without delay, until reduced to 81⁄4 galls., when, after cooling, 2 dr. of essential oil of sassafras, dissolved in 2 galls. of rectified spirit of wine, are added, and afterwards 1 pint of essence of guaiacum. The liquid is then placed in a suitably sized barrel, set upon its head, and fitted with a small cock (not placed too near the bottom), and allowed to repose for a week, by which time it becomes clear and brilliant, and fit for sale. This is the form adopted by the large metropolitan drug-houses most celebrated for this preparation. The product that may be drawn off fit for sale is something over 10 galls. The residuum, forming the ‘bottoms,’ consists chiefly of fecula. The latter is well stirred up with 3 or 4 galls. of cold water, and allowed to settle. The clear decanted ‘washings’ are used as water or liquor in making the next batch of decoction.
[256] See page 356.
2. (Extemporaneous.) Compound extract of sarsaparilla, 71⁄2 oz.; boiling water, 12 fl. oz.; dissolve, then add of rectified spirit of wine, 21⁄2 fl. oz.; mix well, and further add of water, q. s. to make the whole measure a pint.
Obs. To conduct this process successfully, several large copper pans are required; one of which (to boil the ingredients in) must be capable of containing from 140 to 150 gallons at the least, and the others must be sufficiently large to receive the liquors as they are drawn off. Those for the evaporation should be very shallow, in order that it may proceed rapidly; and the whole should be heated by steam. An excellent plan is to employ large wooden vats, and to apply the heat by means of pipes laid along the bottom, and supplied with high-pressure steam. This method is less expensive than the use of double steam pans, as above. When essence of guaiacum is not used, 24 lbs. of guaiacum shavings, from which the dust has been sifted, are boiled with the other ingredients, instead. Those desirous of using the proportions of the ingredients ordered by the Colleges may do so by taking eight times the given quantities, and proceeding as above. The following are special preparations:—
Feltz’s Decoction of Sarsaparilla. Syn. Ap′ozem of Feltz; Decoctum sarzæ cum ichthyocol′la, L.; Ptisane de Feltz, Fr. Prep. From sarsaparilla (sliced), 3 oz.; isinglass and crude antimony (in powder), of each 1⁄2 oz.; water 5 pints; boil to one half, and strain. Used in skin diseases.
Jauperand’s Decoction of Sarsaparilla. Syn. Decoctum sarzæ cum radice Chinâ, L.; Ptisane de Jauperand, Fr. Prep. (Bories.) Sarsaparilla and China root, of each 2 oz.; senna and sassafras chips, of each 1⁄2 oz.; carbonate of potassa, 1 dr.; water, 2 galls.; simmer, gently, for several hours, and strain 12 pints; when cold, decant the clear.—Dose, 2 fl. oz., two or three times daily; in scrofula, &c.
Vinache’s Decoction of Sarsaparilla. Syn. Decoctum sarzæ cum sennâ, L.; Ptisane de Vinache, Fr. Prep. (Foy.) Sarsaparilla, China wood, and guaiacum wood, of each 11⁄2 oz.; crude antimony (tied in a rag), 2 oz.; water, 6 pints; macerate for 12 hours (7 in hot weather), boil to one half, add sassafras chips and senna, of each 1⁄2 oz., infuse 1 hour longer, and strain; when cold, decant the clear. Recommended in scrofula, secondary syphilis, and various cutaneous affections.
Zittmann’s Decoction of Sarsaparilla. Syn. Decoctum Zittmanni, L.; Ptisane de Zittmann, Fr. Prep. 1. (Stronger decoction; D. Z. Forte, Ph. Bor. 1847.) Sarsaparilla, 12 oz.; water, 72 lbs. (say 53⁄4 galls.); digest 24 hours, then add (suspended in a bag), white sugar and alum, of each 6 dr.; calomel, 4 dr.; cinnabar, 1 dr.; boil to 24 lbs., adding towards the end of the process, senna, 3 oz.; liquorice root, 11⁄2 oz.; aniseed and fennel seed, of each 1⁄2 oz.; finally strain, with pressure and after some time decant the clear portion. The formula in the ‘Ph. Suec.’ 1845 is similar; that in the ‘Hamburg Codex’ directs only 24 lbs. of water to be used, and the whole to be reduced to 16 lbs.
2. (Weaker decoction; D. Z. Tenue.—Ph. Bor. 1847.) Add to the residuum (waste) of the last preparation sarsaparilla, 6 oz.; water, 72 lbs. (say, 53⁄4 galls.); boil to 24 lbs.; adding towards the end of the process, lemon peel, cinnamon bark, liquorice root, and cardamoms (all bruised), of each 3 dr.; press, strain, &c., as before. In the ‘Ph. Suec.’ 1845 double the above weights of lemon peel and liquorice root are ordered, and in the ‘Hamburg547 Codex’ (1845) 24 lbs. of water only are ordered, and the whole is to be boiled down to 16 lbs.
Obs. Both the above are used in Germany and on the Continent generally, in the same cases as those in which compound decoction of sarsaparilla is administered in England. They may be drunk almost ad libitum. A trace of mercury may be detected in the stronger decoction, when properly prepared. See Sarsaparilla.
Decoction of Sen′ega Root. Syn. Decoction of American snake root, D. of rattlesnake root; Decoctum polygalæ, D. senegæ (Ph. L.), L. Prep. (Ph. L.) Senega or seneka root, 10 dr.; water, 1 quart; boil to a pint, and strain.—Dose, 1⁄2 to 2 wine-glassfuls three or four times daily; in humoral asthma, chronic cough, dropsy, &c. It is stimulant, expectorant, and diuretic, and, in large doses, emetic and cathartic. It is frequently conjoined with ammonia. It is the antidote employed by the Senega Indians against the bite of the rattlesnake. (Dr Tennant.)
Decoction of Simaru′ba Bark. Syn. Decoctum simaru′bæ, L. Prep. (Dr Wright.) Simaruba bark, 2 dr.; water, 24 fl. oz.; boil to one half, and strain. Tonic.—Dose, 1 to 2 fl. oz.; in chronic dysentery and diarrhœa.
Decoction of Squills (Compound). Syn. Decoctum scillæ compositum, L. Prep. (Ph. U. S. 1841.) Squills, 3 dr.; juniper berries, 4 oz.; snake root, 3 oz.; water, 4 lbs.; boil to one half, strain, and add of sweet spirits of nitre, 4 fl. oz. In chronic coughs and other chest affections, unaccompanied with active inflammatory symptoms.—Dose, 1 to 3 fl. oz., twice or thrice daily.
Decoction of Starch. Syn. Decoctum am′yli (Ph. L.), Mucila′go am′yli (Ph. E. & D.), L. Prep. (Ph. L. & E.) Starch, 1⁄2 oz.; add, gradually, water, 1 pint, and boil for a short time. The Dublin preparation is nearly twice as strong. Used as an enema in dysentery, diarrhœa, and excoriations of the rectum.
Decoction, Sudorif′ic. Syn. Decoctum sudorif′icum, L. The old name of the compound decoctions of sarsaparilla and guaiacum.
Decoction of Su′et. Syn. Artificial goat’s milk; Decoctum se′vi, L. Prep. Suet, 1 oz.; tie it loosely in a piece of muslin and simmer it in cow’s milk, 11⁄4 pint; adding towards the last, white sugar, 1⁄2 oz. In scrofulous emaciation and phthisis; taken ad libitum.
Decoction of Tam′arinds. Syn. Decoctum tamarind′orum, L. Prep. Tamarinds, 11⁄2 oz.; water, 1 pint; boil for 5 minutes, and strain. A pleasant drink in fevers, asthma, chronic coughs, &c.
Decoction of Tamarinds and Sen′na. Syn. Dec. tamarindorum cum sennâ (Ph. E. 1744), L. Prep. Tamarinds, 6 dr.; cream of tartar, 2 dr.; water, 11⁄2; pint; boil in a glazed earthen vessel until reduced to 16 oz.; then infuse therein for 12 hours, senna, 4 dr.; strain, and add of syrup of violets, 1 oz. A gentle aperient.—Dose. A wine-glassful, or more.
Decoction of Tar. Syn. Tar water; Decoctum pi′cis liq′uidæ, L. Prep. Tar, 1 oz.; water, 11⁄2; pint; boil to 1 pint.—Dose. A pint or more daily; in chronic catarrh; and as a wash in chronic skin diseases, especially those of the head, in children.
Decoction, Ton′ic. Syn. Strengthening decoction; Decoctum roborans, L. Prep. 1. Peruvian bark (bruised), 1⁄2 oz.; Virginian snake root, 2 dr.; water, 1 pint; boil to one half, strain whilst hot, and add, spirit of cinnamon, 11⁄2 fl. oz.; diluted sulphuric acid, 11⁄2 dr.—Dose, 2 oz. two or three times a day.
2. Decoction of bark, 5 oz.; tincture of bark, 6 dr.; aromatic confection, 1⁄2 dr.; salvolatile, 1 dr.—Dose, 1 or 2 table-spoonfuls night and morning; especially in diarrhœa.
Decoction of Tor′mentil. Syn. Decoctum tormentil′læ (Ph. L.), L. Prep. (Ph. L.) Tormentil root (bruised), 2 oz.; water, 11⁄2 pint; boil to a pint, and strain. Astringent.—Dose, 1 to 2 fl. oz., in chronic diarrhœa, &c.
Decoction of Tur′meric. Syn. Decoctum curcu′mæ, L. Prep. From turmeric root (in powder), 11⁄2 oz.; water, 1 pint; boil for 5 minutes, and strain. A mild aromatic stimulant and stomachic.—Dose. A wine-glassful ad libitum. It is principally used as a test for alkalies, which turn it brown. Unsized paper dipped into it and dried forms the turmeric test-paper of the chemist.
Decoction of Verbe′na. Syn. Decoctum verbe′næ, L. From verbena (vervain), 2 oz.; water, 11⁄2 pint; boil to 1 pint, and strain.
Obs. The Verbena officinalis was formerly highly recommended by Etmuller, Hartman, De Haën, Morley, and others, in scrofula, cephalalgia, &c., but afterwards fell into neglect. More recently, a decoction of the plant has been highly extolled by Boshanov and others as an anti-febrile.
Decoction, Vul′nerary. Syn. Decoctum vulnera′′rium, L. Prep. From ground ivy and broad-leaved plantain, of each 1⁄2 oz.; water, 3 pints; boil to 1 quart, strain, and add sugar, 1 oz. A popular pectoral and tonic, especially in old coughs; also to heal wounds.—Dose, 1⁄2 a teacupful or more twice a day.
Decoction of Wal′nut Bark. Syn. Decoctum jugland′is, L. Prep. (Ph. Gen.) Green bark of walnuts, 1 oz.; water, 1 pint; boil for 15 minutes, and strain. As an anti-syphilitic. Before the general introduction of sarsaparilla it was much esteemed in most cases in which that drug is now taken.—Dose, &c. The same as those of comp. dec. of sarsaparilla. Pearson says that “when the putamen (green rind) of the walnut has been omitted, either intentionally or by accident (from Decoctum Lusitanicum),548 the same good effects have not followed its use as when it contained this ingredient.
Decoction of Walnut Leaves. Syn. Decoctum jugland′is folio′′rum, L. Prep. (Negrier.) Walnut leaves, 1 handful; water, 1 quart; boil 15 minutes, and strain. Detersive, diaphoretic, and alterative.—Dose, &c. As the last, especially in chronic rheumatism, secondary syphilis, &c.
Decoction of Wa′terdock. Syn. Decoctum rumi′cis, D. r. aquat′ici, L. Prep. (A. T. Thomson.) Root of common waterdock (Rumex obtusifolius), 1 oz.; water, 1 pint; boil for 10 minutes, and strain.
Obs. This decoction is astringent, and was once much celebrated as a remedy for scurvy and some other cutaneous affections. “It is the only remedy which proves efficacious in that disease when the ulcers are healed, and the patient is attacked with asthma.” (Linnaeus, on the Scurvy of the Laplanders.)
Decoction, White (Sydenham’s). Syn. Hartshorn drink; Mis′tura cor′nu usti. Prep. Prepared burnt hartshorn, 2 oz.; gum Arabic, 1 oz.; water, 3 pints; boil to 1 quart, and strain. Mucilaginous; demulcent. Taken ad libitum.
Decoction of Whor′tleberry. Syn. Decoction of bear-berry, D. of uva-ursi; Decoctum uvæ ursi (Ph. L. & D.), L. Prep. 1. (Ph. L.) Whortleberry leaves, 1 oz.; water, 11⁄2 pint; boil to a pint, and strain.
2. (Ph. D.) Uva-ursi (the leaves), 1⁄2 oz.; water, 1⁄2 pint; boil 10 minutes, and strain.
Dose, &c. 1 to 3 fl. oz. two or three times daily; in phthisis and purulent affections of the urinary organs, unaccompanied with active inflammation; especially in chronic affections of the bladder.
Decoction of Wil′low Bark. Syn. Decoctum salicis, D. s. corticis, L. Prep. 1. (Wilkinson.) Willow bark (Salix latifolia), bruised, 11⁄2 oz.; macerate in water, 2 lbs., for 6 hours, then boil for 15 minutes, and strain. Tonic, astringent, and febrifuge.—Dose. A wine-glassful.
2. (Nieman.) Willow bark (Salix alba), 11⁄2 oz.; water, 3⁄4 pint; boil to one half.—Dose, 1 to 2 fl. oz. Both are used as substitutes for decoction of cinchona bark.
Decoction of Win′ter-green. Syn. Decoction of pyrola, D. of umbellated winter-green, D. of pipsissewa; Decoctum chimaphilæ (Ph. L.), D. pyrolæ (Ph. D.), L. Prep. 1. (Ph. L.) Chimaphila (dried herb), 1 oz.; water, 11⁄2 pint; boil to a pint, and strain.
2. (Ph. D.) Winter-green (dried leaves), 1⁄2 oz.; water, 1⁄2 pint; boil 10 minutes in a covered vessel, and strain. Tonic, stomachic, alterative, and diuretic.—Dose, 1 to 2 fl. oz.; in dropsies, scrofula, debility, loss of appetite, &c.; and in those affections of the urinary organs in which uva-ursi is commonly given.
Decoction of Worm′seed. Syn. Decoctum santonici, L. Prep. 1. Wormseed, bruised, 2 oz.; water, 1 pint; boil down to 16 fl. oz., and strain.
2. (Dr R. E. Griffith.) Fresh leaves of wormseed(Chenopodium anthelminticum),—Linn.), 1 oz.; water, 1 pint; orange peel, 2 dr.; boil (10 minutes), and strain. The above are bitter, stomachic, and vermifuge.—Dose. A wine-glassful twice a day; in worms. It is also used as an injection against ascarides.
Decoction of Yar′row. Syn. Decoctum millefolii, L. Prep. From milfoil or yarrow tops, 11⁄2 oz.; water, 11⁄4 pint; boil to a pint, and strain. Astringent, tonic, and vulnerary.—Dose. A wine-glassful thrice daily; in dropsies, &c. It is also used as a fomentation to bruises, &c.
Decoction of Black Snake Root. Syn. Decoctum Cimicifuge. Black snake root, 1 oz.; water, 16 oz.; boil for 10 minutes.—Dose, 1 oz. to 2 oz. in rheumatism and dropsy.
Decoction of Stavesacre. Syn. Decoctum Staphisagriæ. Stavesacre seed, 1 oz.; water, 2 pints; boil for a few minutes, and strain. For external use.
Decoction of Snails. Syn. Decoctum Limatum (Mars mouchon). Flesh of vine or garden snails (cleansed from shell and intestines), 5 oz.; water, 2 pints; simmer gently for 2 hours, adding towards the end, maiden hair 2 oz., and strain.
Decoction of Soapwort. Syn. Decoctum Saponariæ (Swediaur). Soapwort, 2 oz.; water, 4 lbs.; boil to 2 lbs., and strain.
Decoction of Wood-Soot. Syn. Decoctum Fuliginis (Dr Neligan). Wood-soot, 4 oz.; water, 11⁄2 pint.
DECOLORA′TION. The blanching or removal of the natural colour of any substance. Syrups and many animal, vegetable, and saline solutions are decoloured or whitened by agitation with animal charcoal, and subsequent subsidence or filtration. Many fluids rapidly lose their natural colour by exposure to light, especially to the direct rays of the sun. In this way castor, nut, poppy, and several other oils are whitened. Fish oils are partially deodorised and decoloured by filtration through animal charcoal. Cottons and linens are still commonly bleached by the joint action of light, air, and moisture. The peculiar way in which light produces this effect has never been satisfactorily explained. The decoloration of textile fabrics and solid bodies generally is called bleaching. See Blanching, Bleaching, Oils, Tallow, Syrups, Sugar, &c.
DECOMPOSI′′TION (-zĭsh′un). In chemistry, the resolution of compounds into their elements, or the alteration of their chemical constitution in such a manner that new products are formed.
DEFECA′TION. The separation of a liquid from its lees, dregs, or impurities by subsidence and decantation. It is commonly employed for the purification of saline solutions549 and glutinous or unctuous liquids on the large scale in preference to filtration; than which it is both more expeditious and expensive. See Clarification, Decantation, Filtration, &c.
DEFLAGRA′TION. The sudden combustion of any substance for the purpose of producing some change in its composition, by the joint action of heat and oxygen. The process is commonly performed by projecting into a red-hot crucible, in small portions at a time, a mixture of nitrate of potash, and the body to be oxidised.
DELIQUES′CENCE. Spontaneous liquefaction by absorption of the moisture of the atmosphere. Deliquescent salts are those which by exposure gradually assume the liquid state. They should all be kept in well-closed bottles or jars.
DELIR′IUM TRE′MENS. [L.] The madness of drunkards; a disease of the brain resulting from the excessive and protracted use of intoxicating liquors, particularly of ardent spirits. The early symptoms are extreme irritability and fretfulness, with unusual mobility of the body. Sleeplessness and unpleasant dreams soon follow. At length frightful dreams and visions harass the patient. He sees remarkable sights, hears extraordinary sounds, and labours under all the strange delusions of insane persons, which, however vague and unfounded, operate on him with all the force of realities till he becomes furiously mad. The fit almost always comes on after hard drinking; and the hands are usually, but not always, tremulous. A similar affection is occasionally produced by the abuse of opium, excessive mental anxiety, night watching, or depletion. According to Dr Armstrong, even respiring the fumes of ardent spirits will, under some circumstances, produce this disease. Persons who have undergone surgical operations under the influence of chloroform are more liable to attacks of this kind than other persons.
The treatment of delirium tremens consists mainly in the judicious use of opium, laudanum, or morphia, in rather large doses, frequently repeated. Thirty to sixty drops of laudanum may be given every hour or two during the fit, its effects being carefully watched. The object is to produce quiet sleep, from which the patient usually wakes free from the worst symptoms of the disease. Diaphoretics and mild aperients may also be given, and a light, nutritious diet adopted throughout. Depletion, especially bleeding, should be particularly avoided. Alcoholic stimulants and wine, in certain cases, have proved useful. Under this treatment, the patient, unless of a very bad habit of body, or much debilitated by previous excesses, usually recovers. He is, however, very liable to relapses and subsequent attacks, which are best prevented by judicious moral management.
The judicious administration of chloral hydrate, in doses of from thirty to sixty grains as well as of bromide of potassium in twenty-grain doses, either alone or combined with the chloral, has lately been had recourse to with the happiest results, for the production of sleep in cases of delirium tremens or in the insomnia of dipsomaniacs.
The repetition of the dose of chloral requires to be regulated with very great caution; and it is only in the case of emigrants and others unable to obtain medical aid that we would recommend it to be given, and then only should opium have failed to produce the desired effect. Not more than sixty grains of the chloral should be administered during the twenty-four hours. The internal administration of tincture of capsicum in moderately large doses, in the intervals of the opiates or chloral hydrate, has lately been tried in the treatment of this disease, it is said, with success.
DELPHIN′IC ACID. Syn. Phoce′nic acid. A fatty acid, obtained by saponifying the oil of the delphinus or porpoise. According to recent experiments, it is identical with valeric acid.
DELPHIN′INE. Syn. Del′phine, Del′phia, Delphin′ia. An alkaloid discovered by Lassaigne and Feneulle in Delphinium staphysagria or stavesacre.
Prep. 1. The husked seeds (in powder) are boiled in a little water, and pressed in a cloth; a little pure magnesia is then added to the filtered decoction, the whole is boiled for a few minutes, and refiltered; the residuum, after being well washed, is digested in boiling alcohol, which dissolves out the alkaloid, and gives it up again by gentle evaporation and cooling.
2. The bruised, but unshelled, seeds are digested in dilute sulphuric acid, the filtered liquor precipitated with carbonate of potassa, and the precipitate digested in alcohol as before.
3. (Parrish.) An alcoholic extract of the seeds is treated with dilute sulphuric acid, precipitated with an alkali, again dissolved in dilute sulphuric acid; the colouring matter precipitated by a few drops of nitric acid, the alkaloid by potassa. The alkaloid is then dissolved in absolute alcohol, and the solution thus formed is evaporated; one pound yields about one drachm.
Prop., &c. A light-yellowish or white, odourless powder; extremely acrid and bitter; scarcely soluble in water; dissolves in ether, and readily in alcohol; and has an alkaline reaction. Its alcoholic solution produces a burning and tingling sensation when rubbed on the skin, and a similar sensation is produced in various parts of the body when it is taken in doses of a few grains. It has been exhibited in neuralgia and rheumatism by Dr Turnbull.—Dose, 1⁄12 gr. every three hours, made into a pill with 1 gr. each of the extracts of henbane and liquorice. It is also550 used externally under the form of ointment and lotion.
DELPHINUM—A Boot Varnish. Shell-lac, 7·5 grammes dissolved in alcohol, 15 grammes, mixed with 20 drops fish oil, and ·1 gramme lampblack. (Geisse.)
DEMUL′CENTS. In medicine, substances which are calculated to soften and lubricate the parts to which they are applied. Though having the same signification as the word EMOLLIENTS, it is desirable to restrict the latter term to such as are intended for external application, and to include under the above head only such as are intended for internal exhibition. The principal demulcents are gum Arabic, gum tragacanth, liquorice, honey, arrow-root, pearl barley, isinglass, gelatin, milk, almonds, spermaceti, almond and olive oils, and most other mucilaginous, amylaceous, saccharine, and oily substances. For use, these are made into MUCILAGES, DECOCTIONS, EMULSIONS, or MILKS, with water, and form suitable beverages in dysentery, diarrhœa, catarrh, diseases of the urinary organs, and all other diseases where diluents are useful. See Emollients.
DENGUE. This disease is most commonly met with in the East and West Indies, and occasionally as an epidemic in America. In England it rarely shows itself in an epidemic character. The symptoms of dengue appear to combine those of rheumatism and scarlet fever. On the third or fourth day an eruption shows itself, accompanied with pains in the limbs, glandular swellings, and languor. The course of the disease is varied by frequent remissions. It does not come within our design to indicate the treatment, which appears to be the same as that pursued in scarlet fever.
DENS′ITY. Comparative masses of equal weights, or the quantity of matter contained in a given space. It is commonly used synonymously with SPECIFIC GRAVITY, which, however, refers to comparative weights of equal bulks. Thus, quicksilver is said to have a density greater than that of copper, and alcohol one less than that of oil of vitriol.
DENTI′FRICES. Syn. Dentifricia, L. Substances applied to the teeth, to cleanse and beautify them. The most useful form of dentifrices is that of powder (TOOTH POWDER); but liquids (TOOTH WASHES), and electuaries (TOOTH ELECTUARIES, TOOTH PASTES), are also employed. The solid ingredients used in dentifrices should not be so hard or gritty as to injure the enamel of the teeth; nor so soft or adhesive as to adhere to the gums, after rinsing the mouth out with water. Pumice-stone (in fine powder) is one of those substances that acts entirely by mechanical attrition, and is hence an objectionable ingredient in tooth powder intended for daily use. It is, however, very generally present in the various advertised dentifrices, which are remarkable for their rapid action in whitening the teeth. Bath brick is another substance of a similar nature to pumice, and, like that article, should be only occasionally employed. Cuttle-fish bone, coral, and prepared chalk, are also commonly used for the same purpose, but the last is rather too soft and absorbent to form the sole ingredient of a tooth powder. Charcoal, which is so very generally employed as a dentifrice, acts partly mechanically and partly by its chemical property of destroying foul smells and arresting putrefaction. For this purpose it should be newly burnt, and kept in well-closed vessels, until used, as by exposure to the air it rapidly loses its antiseptic powers. Powdered rhatany, cinchona bark, and catechu, are used as astringents, and are very useful in foulness or sponginess of the gums. Myrrh and mastic are employed on account of their odour, and their presumed preservative action and power of fixing loose teeth. Insoluble powders have been objected to on account of their being apt to accumulate between the folds of the gums and in the cracks of the teeth, and thus impart a disagreeable appearance to the mouth. To remedy this defect, a reddish or flesh-coloured tinge is commonly given to them with a little rose pink, red coral, or similar colouring substance, when any small portion that remains unwashed off is rendered less conspicuous. Some persons employ soluble substances as tooth powders, which are free from the above objection. Thus, sulphate of potash and cream of tartar are used for this purpose, because of the grittiness of their powders and their slight solubility in water. Phosphate of soda and common salt are also frequently employed as dentifrices, and possess the advantage of being readily removed from the mouth by means of a little water. Among those substances that chemically decolour and remove unpleasant odours, the only ones employed as dentifrices are charcoal and the chlorides of lime and soda. The first has been already noticed; the others may be used by brushing the teeth with water to which a very little of their solutions has been added. A very weak solution of chloride of lime is commonly employed by smokers to remove the odour and colour imparted by tobacco to the teeth. Electuaries, made of honey and astringent substances, are frequently employed in diseases of the gums. The juice of the common strawberry has been recommended as an elegant natural dentifrice, as it readily dissolves the tartarous incrustations on the teeth, and imparts an agreeable odour to the breath. See Paste and Powder (Tooth), also Washes (Mouth).
DENT′INE. The tissue of which the teeth are composed.
DENTISTRY. The art or practice of a dentist. Directions for the extraction of teeth, as well as elaborate details for stopping them, and for the manufacture of artificial ones, are branches of the dentist’s art, which, as they necessitate the exercise of considerable skill and long practice, do not call for notice in a551 work like the present. We shall confine ourselves, therefore, to that section of dentistry which concerns itself with stoppings for the cavities of decayed teeth, and for the preparation of which we give the following formulæ:—
1. (Soubeiran’s.) Powdered mastic and sandarach, of each 4 dr.; dragon’s blood, 2 dr.; opium, 15 gr.; mix with sufficient rectified spirit to form a stiff paste. A solution of mastic, or of mastic and sandarach, in half the quantity of alcohol, is also used, applied with a little cotton or lint.
2. Sandarach, 12 parts; mastic, 6 parts; amber, in powder, 1 part; ether, 6 parts. Applied with cotton. Or simply a paste of powdered mastic and ether. Or a saturated ethereal solution of mastic, applied with cotton.
3. Taveare’s cement is made with mastic and burnt alum. Bernoth directs 20 parts of powdered mastic to be digested with 40 of ether, and enough powdered alum added to form a stiff paste.
4. Gutta percha, softened by heat, is recommended. Dr Rollfs advises melting a piece of caoutchouc at the end of a wire, and introducing it while warm.
5. (Gauger’s Cement.) Put into a quart bottle 2 oz. of mastic and 3 oz. of absolute alcohol; apply a gentle heat by a water-bath. When dissolved, add 9 oz. of dry balsam of tolu, and again heat gently. A piece of cotton dipped in this viscid solution becomes hard when introduced into the tooth, previously cleansed and dried as above.
6. (Mr Robinson’s.) After washing out the mouth with warm water containing a few grains of bicarbonate of soda, and cleaning the cavity as above directed, he drops into it a drop of collodion, to which a little morphia has been added, fills the cavity with asbestos and saturates with collodion, placing over all a pledget of blotting paper.
7. (Ostermaier’s Cement.) Mix 12 parts of dry phosphoric acid with 13 of pure and pulverised quicklime. It becomes moist in mixing, in which state it is introduced into the cavity of the tooth, where it quickly becomes hard. [In some hands this has failed, from what cause we are not aware.] The acid should be prepared as directed under Acid, Phosphoric.
8. (Silica.) This name has been given to a mixture of Paris plaster, levigated porcelain, iron filings, and dregs of tincture of mastic, ground together.
9. (Wirih’s Cement.) It is said to consist of a viscid alcoholic solution of resins, with powdered asbestos.
10. (Metallic Cement.) Amalgams for the teeth are made with gold or silver, and quicksilver, the excess of the latter being squeezed out, and the stiff amalgam used warm. Inferior kinds are made with quicksilver and tin, or zinc. A popular nostrum of this kind is said to consist of 40 gr. of quicksilver and 20 of fine zinc filings, mixed at the time of using. Mr Evans states that pure tin, with a small portion of cadmium, and sufficient quicksilver, forms the most lasting and least objectionable amalgam. The following is the formula:—Melt 2 parts of tin with 1 of cadmium, run it into ingots, and reduce it to filings. Form these into a fluid amalgam with mercury, and squeeze out the excess of mercury through leather. Work up the solid residue in the hand, and press it into the tooth. Or, melt some beeswax in a pipkin over the fire, throw in 5 parts of cadmium, and, when melted, add 7 or 8 parts of tin in small pieces; pour the melted metals into an iron or wooden box, and shake them till cold, so as to obtain the alloy in a powder. This is mixed with 21⁄2 or 3 times its weight of quicksilver in the palm of the hand, and used as above.
Another cement consists of about 73 parts of silver, 21 of tin, and 6 of zinc, amalgamated with quicksilver. An amalgam of copper is said to be sometimes used. But this class of stoppings is altogether disapproved of by other authorities. Pure leaf-gold seems the least objectionable.
11. (Marmoratum.) Finely levigated glass, mixed with tin amalgam.
12. (Poudre Metallique.) The article sold under this name in Paris appears to be an amalgam of silver, mercury, and ammonium, with an excess of mercury, which is pressed out before using it.
13. (Fusible Metal.) Melt together 8 parts of bismuth, 5 of lead, 3 of tin, and 11⁄2 or 1·6 of quicksilver, with as little heat as possible. (Chaudet.)
14. (Non-expensive Metallic Tooth-stopping.) Take 1 part of sulphate of mercury, 1 part of copper in fine powder; rub them well together with a little warm water; when the amalgam is formed wash well, and remove the surplus of mercury by pressing it through chamois.—Pharm. Journ.
Expensive Metallic Tooth-stopping and much preferable. Take pure gold, pure gelatin, 1 part of each; pure silver, 2 parts; melt, and when refrigerated, reduce to a powder by means of a file; wash well and dry. In the moment of using it add sufficient mercury to form a plastic paste.—Pharm. Journ.
Paste for Destroying the Sensibility of the Dental Pulp previous to Stopping. Arsenious acid, 30 gr.; sulphate of morphia, 20 gr.; creasote, q. s. [Unsafe; it is only inserted by way of warning against what may prove an unsuspected cause of mischief.]
Pivots for Artificial Teeth. An alloy of platinum and silver.
Springs for Artificial Teeth. Equal parts of copper, silver, and palladium. (Chaudet.)
For Cachou Aromatisé, and other compounds552 for sweetening the breath, see Perfumery.
DENTI′TION. See Teething.
DEOB′STRUENT. In medicine, a substance which removes obstructions, and opens the natural passages of the fluids of the body, as the pores, lacteals, and glands. Iodine, mercury, sarsaparilla, and aperients, are deobstruents.
DEO′DORISER. Any substance having the power of absorbing or destroying fetid effluvia. Chlorine, chloride of lime, chloride of zinc, nitrate of lead, sulphate of iron, and freshly-burnt charcoal, are the most effective and convenient deodorisers. Peat charcoal has been highly recommended for deodorising manure, &c., on the large scale. When it is mixed with these substances their fetor is immediately destroyed, and a compost produced, which may be substituted for guano for agricultural purposes. ‘Biedermann’s Centralblatt für Agricultur Chemie’ for June, 1877, contains the results of some experiments undertaken by A. Eckstein on the comparative deodorising values of certain substances. Herr Eckstein found that 1 kilo of copperas dissolved in water destroyed the stench of sulphuretted hydrogen in a privy used daily by at least 100 persons. The action ceased after twelve hours. A solution of aqueous sulphate of copper produced a similar result. When 1 kilo of solid copperas was employed the action lasted for two days. The same result was obtained by using 1 kilo of a mixture compound of copperas, sulphate of copper, and carbonate of lime. Liquid sulphurous acid was found to act very rapidly, rendering the atmosphere difficult to breathe for an hour; its action ceased after twenty-three hours. Crude carbolic acid, which was used to the extent of 30 grams, gave so unpleasant a smell for two days as to render the result impossible to be arrived at. One kilo of copperas enclosed in a bag of parchment paper only began to act after two hours, and kept the place odourless for two days. One kilo of good chloride of lime, placed in a similar bag, did not lose its effect for nine days. With 60 grams of permanganate of soda the action commenced immediately, but the effect was over in twenty-four hours; when enclosed in parchment paper it was efficacious for two days. In Herr Eckstein’s opinion the most powerful deodoriser known is chloride of lime along with sulphuric acid. Powdered gypsum is a good absorber of ammonia, and for this purpose may be sprinkled over the floors of stables, manure heaps, &c. See Disinfectant.
DEOX′IDATION. See Reduction.
DEPIL′ATORY. A cosmetic employed to remove superfluous hairs from the human skin. Depilatories act either mechanically (MECHANICAL DEPILATORIES), or chemically (CHEMICAL DEPILATORIES). To the first class belong adhesive plasters, that, on their removal from the skin, bring away the hair with them. The second class includes all those substances which destroy the hair by their chemical action.
Lime or orpiment, and generally both of them, have formed the leading ingredients in depilatories, both in ancient and modern times. The first acts by its well-known causticity, and also, when an alkali is present, by reducing that also, either wholly or in part, to the caustic state. The action of the orpiment is of a less certain character, and its use is even dangerous when applied to a highly sensitive or an abraded surface. The addition of starch is to render the paste more adhesive and manageable.
In using the following preparations, those which are in the state of powder are mixed up with a little warm water to the consistence of a paste, and applied to the part. Sometimes soap lye is used for this purpose, and some persons spread the pulpy mass on a piece of paper, and apply it like a plaster. In 12 or 15 minutes, and sooner, if much smarting ensues, the whole should be washed off with warm water, and a little cold cream, lip-salve, or spermaceti cerate, applied to the part. The application of the liquid preparations is generally accompanied with gentle friction, care being taken to prevent them extending to the adjacent parts. All the following effect the object satisfactorily, with proper management; but some are much more effective than others. A small wooden or bone knife is the best for mixing them with. They must all be kept in well-stoppered bottles, and no liquid must be added to them until shortly before their application; and then no more should be mixed than is required for immediate use.
Depilatory, Arsen′ical. Orpiment (sulphide of arsenic) forms the principal ingredient in many fashionable depilatories, but its use is not free from danger. The following are well-known preparations:
1. (Colley’s D.) From nitre and sulphur, of each 1 part; orpiment, 3 parts; quicklime, 8 parts; soap lees, 32 parts; boil to the consistence of cream. Very caustic.
2. (Delcroix’s d.; ‘poudre subtile,’) Orpiment, 1 oz.; quicklime, 10 oz.; starch, 14 oz.
3. (Oriental d.; Oriental rusma.)—a. Quicklime, 3 oz.; orpiment, 1⁄2 dr.; strong alkaline lye, 1 lb.; boil together in a clean iron vessel until a feather dipped into the liquor loses its flue.
b. From pearlash, 2 oz.; orpiment, 3 dr.; liquor of potassa, 1⁄2 pint; boil together as before. One of the most caustic and consequently the most certain of depilatory preparations; but, with the rest of its class, open to the objections of containing orpiment. (See No. 7.)
4. (Paste d.; ‘Pâte épilatoire,’) To No. 1 add of orris root, 3 parts.
5. (Plenck’s d.; ‘Pasta epilatoria,’) Orpiment, 1 part; quicklime and starch, of each 12 parts.
553
6. (Soap d.; ‘Savon épilatoire,’) Turkish depilatory and soft soap, equal parts. Must not be mixed until about to be applied. (See No. 7.)
7. (Turkish d.; Turkish rusma.) Orpiment, 1 part; quicklime, 9 parts. For use, it is mixed up with soap lees, and a little powdered starch.
Depilatory, Boettger’s. Powdered sulphydrate of sodium, one part; washed chalk, three parts; made into a thick paste with a little water. Let a layer about the thickness of the back of a knife be spread upon the hairy surface. After two or three minutes the stoutest hairs are transformed into a soft mass which may be removed by water. A more prolonged action would attack the skin.
Depilatory, Boudet’s. Prep. Sulphide of sodium (crystallised), 3 parts; quicklime (in fine powder), 10 parts; starch, 10 parts; mix. To be mixed with water, and applied to the skin, and scraped off in 2 or 3 minutes with a wooden knife. Very effective and safe.
Depilatory, Cazenave’s. Syn. Mahon’s d.; Pommade épilatoire de Cazenave, Fr. Prep. Quicklime, 1 part; carbonate of soda, 2 parts; lard, 8 parts; mix. Applied as an ointment.
Depilatory, Chi′nese. Prep. 1. Quicklime, 8 oz.; pearlash (dry) and liver of sulphur, of each 1 oz.; all reduced to a fine powder; mixed, and kept in a close bottle.
2. (Roseate d.) As No. 1., but coloured with a little rose pink or light red.
These preparations are applied in the same manner as Boudet’s Depilatory.
Depilatory, Colley’s. See Depilatory, Arsenical.
Depilatory, Hydrosulphate of Lime. Prep. (Beasley.) Mix quicklime and water to a thick cream, and pass into the mixture 25 or 30 times its volume of sulphuretted hydrogen gas. When the gas ceases to be absorbed, stop the process. The pulpy mass is spread on paper, and applied for 12 or 15 minutes. It is very effective, but has a most disgusting smell. Spolasco’s depilatory is a very similar preparation (see below).
Depilatory, Mechan′ical. Syn. Depilatory plastee. Prep. From pitch and resin, equal parts, melted together and spread on leather. Applied as a plaster.
Depilatory, Rayer’s. Prep. Quicklime, 2 oz.; salt of tartar, 4 oz.; charcoal, 1⁄4 oz. Less active than Chinese Depilatory.
Depilatory, Redwood’s. Prep. A strong solution of sulphide of barium, made into a paste with powdered starch, and applied immediately. Mr Redwood says this is “the best and safest depilatory.”
Depilatory, Ro′seate. See Depilatory, Chinese.
Depilatory, Spolasco’s. Prep. Freshly prepared sulphide of calcium and quicklime, equal parts. Almost equal to Redwood’s (above).
DEPOSI′TION (of Metals). See Electro-type.
DERBY CONDITION POWDERS (J. Tobias Simpson, New York). Celebrated as a safe, infallible, and speedy remedy for glanders, coughs, colds, over feeding, worms, mouth disease, and loss of horns or hair, in horses and other valuable domestic animals. Tartar emetic, 2 grammes; black antimony, 20 grammes; sulphur, 10 grammes; nitre, 10 grammes; fenugreek, 40 grammes; juniper berries, 20 grammes. (Schädler.)
DER′BYSHIRE NECK. See Goitre.
DERMASOT (Apotheker Bertschinger, Baden, Switzerland). For profuse perspiration of the feet. Consists of acetate of alumina, 7·5 grammes; distilled water, 120 grammes; butyric ether, 2 drops; rosanilin to colour it slightly. (Weber.)
DESBRIERRE’S CHOCOLATE A LA MAGNESIE. 44 grammes of chocolate paste and 15 grammes of calcined magnesia, made into two tablets. (Reveil.)
DESIC′CANTS. Syn. Desiccan′tia, L. In pharmacology, substances that check secretion and dry sores of abraded surfaces, without acting as styptics, or constringing the fibres of the parts to which they are applied. See Astringents.
DESICCA′TION. Syn. Exsicca′tion. The evaporation or drying off of the aqueous portion of solid bodies. Plants and chemical preparations are deprived of their humidity by exposure to the sun, a current of dry air, an atmosphere rendered artificially dry by sulphuric acid, or by the direct application of heat by means of a water bath, a sand bath, or a common fire. Planks and timber are now seasoned, on the large scale, in this way, by which a condition may be produced, in 2 or 3 days, which on the old system is barely attainable in as many years. “Endeavours were made to enforce the importance and value of the desiccation of woods to the builder, cabinet maker, architect, and civil engineer, so long back as 1843, but without success. Since that period certain persons have availed themselves, commercially, of our ideas and experiments on the subject, without any acknowledgment, either verbal or pecuniary.” (Cooley.)
DESTEM′PER. Syn. Distemper. Colours ground up with size, gum, or white of egg, and water, as in scene painting. The art of executing work in distemper is called ‘distemper painting.’
DETER′GENT. An agent having the power of removing offensive matter from the skin. The name is now generally restricted to applications that tend to cleanse foul wounds and ulcers.
Detergent, Collier’s. Prep. From liquor of potassa, 2 fl. dr.; rose water, 51⁄2 fl. oz.; spirit of rosemary, 1⁄2 fl. oz.; mix. One of the best applications known to free the head from scurf, when the hair is strong and healthy.554 The head should be afterwards sponged with clean, soft water.
DETONA′TION. See Fulminating Compounds.
DEUTOX′IDE. See Oxides.
DEUTSCHE SIEGESTROPFEN—German Triumphal Drops (Schmidt). 480 grammes of a brown fluid with an agreeably sweet spirituous and aromatic taste, containing in a hundred parts five parts of the portion soluble in weak spirit of cloves and orange peel, 29 parts sugar, 36 parts alcohol, and 30 parts water. (Wittstein.)
DEW-POINT. The temperature at which dew begins to form, as observed by a thermometer. It varies with the humidity of the atmosphere.
DEX′TRIN. C6H10O5. Syn. Starch gum, Dextrina, Dextrinum, British gum. A soluble substance resembling gum, formed by the action of dilute acids at the boiling temperature, and by infusion of malt, at about 160° Fahr., on starch. It is also formed when potato starch and some of the other farinas are exposed to a heat of about 400°. See Diastase and Gum (British).
DEX′TRO-RACE′MIC ACID. See Racemic acid.
DIABE′TES. See Urine.
Diabetes (Saccharine). The symptoms observed in this generally fatal ailment are the passing of an excessive quantity of pale, straw-coloured urine, of high specific gravity, containing more or less grape sugar; great thirst and hunger, obstinate dyspepsia, constipation, an unpleasant odour from the feet, or perspiration of the arm-pits, and bodily debility, and emaciation. All these symptoms vary in intensity according to the course and duration of the disease, which is frequently accompanied with hectic fever, cough, and sometimes carbuncles, and generally ends in consumption or some organic disease. The flow of urine sometimes reaches as much as eight gallons in 24 hours; the average quantity, however, is about two gallons. The specific gravity of the urine varies between 1030 and 1070. The quantity of sugar excreted in the twenty-four hours differs greatly, ranging from half a pound to three pounds.
In the treatment of diabetes, great attention should be paid to diet, which should consist principally of digestible, broiled, or roasted meat, gluten and bran bread (these latter being substituted for ordinary bread, which with sugar must be especially avoided), liquids in moderate quantity, of which the most preferable are weak beef tea or mutton broth. If the thirst is extreme, it is best assuaged by drinking water acidulated with phosphoric acid. Spirituous liquids as well as saline aperients should be eschewed. Claret is, however, a suitable beverage.
Small doses of laudanum, given three or four times a day, have been found of great service.
Dr Watson recommends also the administration of creosote. The bowels must be regulated by means of mild aperients. Warm baths are also of use, as they augment the secretion of the skin. The disease may be kept under by administering from twenty to forty minims of tincture of perchloride of iron, 3 times a day. The above treatment is inserted for the guidance only of emigrants and others unable to obtain professional aid; wherever this can be obtained, no time should be lost in seeking it. This is the more important, since the earlier the patient has recourse to the proper remedies, the greater are the chances of recovery.
Horses. The disease occurs, although rarely, in horses. It is not known either in cattle or dogs. The treatment consists in depriving the animal for some weeks of food containing starch, or other matters capable of forming sugar. He must be fed on meat soup and cooked animal diet, to which he quickly becomes reconciled. The strength must be kept up by means of tonic. To counteract the intense thirst, Mr Finlay Dun recommends the following to be given three times a day in water:—A drachm of iodide of potassium, a scruple of iodine, and four drachms of carbonate of soda.
DIACH′YLON. See Plasters.
DIALY′′SER. In practical chemistry, an instrument for separating ‘crystalloids’ from ‘colloids,’ introduced by the late Prof. Graham. In its most convenient form it consists of a hoop of gutta percha, over which a circular piece of parchment paper is stretched. The paper is applied to the hoop while wet, and is kept stretched by a second hoop, by an elastic band, or by a few turns of string. The instrument, when complete, resembles an ordinary tambourine. It is distinguished as the ‘HOOP DIALYSER,’ The fluid to be ‘dialysed’ is poured into the hoop upon the surface of the parchment paper, to a small depth only, such as half an inch, and the dialyser is then floated upon water in a large glass basin. Another form of dialyser, termed the ‘BULB DIALYSER,’ consists of a small glass bell-jar, the mouth of which is covered by a piece of parchment paper. This is suspended or otherwise supported in a large vessel of water in such a manner that the parchment paper septum just dips below the surface. See Dialysis (below), Parchment paper.
DIAL′YSIS. In practical chemistry, the method of separating substances by ‘diffusion’ through a septum of gelatinous matter. When a solution having a sp. gr. greater is introduced into a cylindrical glass vessel, and then water very cautiously poured upon it, in such a manner that the two layers of liquid remain unmoved, the substance dissolved in the lower liquid will gradually pass into the supernatant water, though the vessel may have been left undisturbed, and the temperature remain unchanged. The gradual passage of a dissolved substance from its original solution into pure555 water taking place, notwithstanding the higher sp. gr. of the substance which opposes this passage, is called the ‘diffusion of liquids.’ From the investigation of the phenomena of this diffusion, the late Prof. Graham derived the remarkable results upon which the method under notice is based. Different substances, when in solution of the same concentration, and under other similar circumstances, diffuse with very unequal velocity. “The range in the degree of diffusive mobility,” says Prof. Graham, “exhibited by different substances, appears to be as wide as the scale of vapour-tensions. Thus, hydrate of potassa may be said to possess double the velocity of diffusion of sulphate of potassa, and sulphate of potassa again double the velocity of sugar, alcohol, and sulphate of magnesia. But the substances named belong, as regards diffusion, to the more volatile class. The comparatively fixed class, as regards diffusion, is represented by a different order of chemical substances (marked out by the absence of the power to crystallise), which are slow in the extreme. Among the latter are hydrated silicic acid, hydrated alumina, and other metallic peroxides of the aluminous class, when they exist in the soluble form; with starch, dextrine, and the gums, caramel, tannin, albumen, gelatin, vegetable and animal extractive matters. Low diffusibility is not the only property which the bodies last enumerated possess in common. They are distinguished by the gelatinous character of their hydrates. Although often largely soluble in water, they are held in solution by a most feeble force. They appear singularly inert in the capacity of acids and bases, and in all the ordinary chemical relations. But, on the other hand, their peculiar physical aggregation, with the chemical indifference referred to, appears to be required in substances that can intervene in the organic processes of life. The plastic elements of the body are found in this class. As gelatin appears to be its type, it is proposed to designate substances of this class as ‘COL′LOIDS,’ and to speak of their peculiar form as the ‘colloidal condition of matter.’ Opposed to the colloidal is the ‘crystalline condition.’ Substances affecting the latter form will be classed as ‘CRYSTAL′LOIDS,’ The distinction is, no doubt, one of intimate molecular constitution.”[257] A certain property of colloidal substances comes into play most opportunely in assisting diffusive preparations. The jelly of starch, that of animal mucus, of pectin, of vegetable gelose, and other solid colloidal hydrates, all of which, strictly speaking, are insoluble in cold water, are themselves permeable when in mass, as water is, by the more highly diffusive class of substances. But such jellies greatly resist the passage of the less diffusible substances, and cut off entirely other colloid substances like themselves that may be in solution. A mere film of the jelly has the separating effect. Now, parchment-paper, when wetted, acts just like a layer of animal mucus or other hydrated colloid, by permitting the passage of crystalloids, but not of colloids; consequently this substance may be used for dialytic septa (see Dialyser, above). The following experiments recorded by Graham will give some idea of the results which may be obtained by dialysis:—
[257] ‘Philosoph. Trans.’ for 1861.
1. Half a litre of urine was placed in a hoop dialyser, which was then floated on a considerable quantity of pure water. Dialysed for 24 hours, the urine gave its crystalloidal constituents to the external water. The latter, evaporated by a water bath, yielded a white saline mass. From this mass urea was extracted by alcohol in so pure a condition as to appear in crystalline tufts upon the evaporation of the alcohol.
2. By pouring silicate of soda into diluted hydrochloric acid (the acid being maintained in large excess), a solution of silica is obtained. But in addition to hydrochloric acid, such a solution contains chloride of sodium, a salt which causes the silica to gelatinise when the solution is heated, and otherwise modifies its properties. Now, such a solution placed for 24 hours in a dialyser of parchment paper was found to lose 5% of its silicic acid (silica) and 86% of its hydrochloric acid. After 4 days on the dialyser, the liquid ceased to be disturbed by nitrate of silver. All the chlorides were gone, with no further loss of silica. What remained was a pure solution of silicic acid, which could be boiled in a flask, and considerably concentrated, without change.
3. Half a litre of dark-coloured porter, with ·05 gramme of arsenious acid added (1⁄10000th part of arsenious acid), was placed on a hoop dialyser, 8 inches in diameter, and the whole floated in an earthenware basin containing 2 or 3 litres of water. After 24 hours the latter fluid had acquired a slight tinge of yellow. It yielded, when concentrated and precipitated by sulphuretted hydrogen, upwards of one half of the original arsenious acid in a fit state for examination.
DIAMANTKITT—Diamond Cement. 50 parts graphite, 15 parts litharge, 10 parts milk of lime, 5 parts slaked lime, intimately mixed with enough linseed oil to make a firm mass. (Hager.)
DIAMANTTROPFEN—Diamond Drops (Dr Allinhead). A combination of the juices of mysterious herbs of tropical climes, which has the power to make all men transparent.
DI′AMOND. The diamond is pure carbon, and differs from the carbon of charcoal and lampblack simply in being limpid, colourless, and highly refractive of light, properties which are generally referred to its crystalline form. The weight, and, consequently, the value of diamonds, is estimated in carats, one of which is equal to 4 grains; and the price of one diamond, compared to that of another of equal colour, transparency, purity, form, &c., is as the squares of the respective weights. The556 average price of ROUGH DIAMONDS that are worth working is about £2 for the first carat; that of a CUT DIAMOND is equal to that of a rough diamond of double weight, exclusive of the price of workmanship. “To estimate the value of a wrought diamond, ascertain its weight in carats, double that weight, and multiply the square of this product by £2.” (Ure.) Thus, a cut diamond of—
| 1 | carat is | worth | £8 |
| 2 | carats | ” | £32 |
| 3 | ” | ” | £72 |
| 4 | ” | ” | £128 |
| &c., &c. See Carbon, Gems. | |||
Di′amond Dust. Genuine diamond dust is the powder produced by the abrasion of diamonds against each other in the process of cutting and polishing them. It possesses the valuable property of polishing the gems, and giving “the finest edge to every kind of cutlery.” The discovery of the latter fact, a few years since, led certain dishonest persons to extensively advertise spurious preparations, consisting chiefly of emery powder or powdered quartz, under the name of diamond dust. The factitious articles acquired a very short and bad notoriety. Instead of sharpening cutting instruments, they infallibly destroyed their edge, and were particularly unfortunate in converting razors into saws.
DIAPEN′TE. Syn. Pulvis diapente. Prep. 1. (Ph. E. 1744) Bay-berries, birth-wort, gentian, ivory dust, and myrrh, equal parts. An excellent warm tonic, especially useful in the debility and rickets of children. The substance sold under this name in the shops is an inferior mixture, used principally as a tonic in veterinary practice. The following are the forms commonly adopted in its preparation:—
2. Turmeric, 4 lbs.; laurel berries and mustard, of each 3 lbs.; gentian, 2 lbs. (all in fine powder); mix.
3. Bay-berries, gentian, mustard, and turmeric, equal parts.
4. Gentian, 6 lbs.; bay-berries, 1 lb. This is the formula generally used by the farriers. Sometimes mustard, 1 lb., is added.
DIAPHORET′ICS. Syn. Sudorif′ics; Diaphoretica, Sudorifica, L. Medicines which promote or increase the perspiration. Those that produce this effect in a very marked degree are more particularly called ‘sudorifics.’ The principal diaphoretics are:—warm diluents, as barley-water, gruel, tea, &c.; salts of the alkalies, as the citrates of potassa and soda, acetate of potassa, acetate and carbonate of ammonia, sal-ammoniac, nitre, &c.; preparations of antimony, as antimonial powder, tartar emetic, &c; also alcohol, camphor, Dover’s powder, ipecacuanha, opium, wine, &c.
The use of diaphoretics is indicated in nearly all diseases accompanied by fever and a dry skin, and particularly in febrile and pectoral affections.
DI′APHRAGM (frăm). A partition through or across; a dividing substance. In anatomy, the term is applied to the midriff, a muscle separating the chest or thorax from the abdomen or lower belly. In astronomy and optics the term is applied to a circular ring placed in a telescope or other instrument to cut off the marginal portions of a beam of light. In electricity the name is commonly used to denote the porous partition, cell, or vessel, that separates the fluid containing the positive plate from the fluid which surrounds the negative plate, in a constant voltaic battery. Thin partitions of sycamore, or other porous wood, are occasionally used, but cells made of thin biscuit ware are the most convenient and durable diaphragms. Plaster of Paris, animal membrane, coarse and tightly wove canvas, &c., are used also for the purpose. Plaster cells are also formed by surrounding an oiled cylinder of wood with a hoop of paper, and pouring plaster of Paris, mixed up with water, into the space between the two. See Electrotype.
DIARRHŒ′A. A purging or looseness of the bowels. The causes of diarrhœa are various, but among the most common are the presence of irritating matter, worms, or acidity in the stomach or bowels; and exposure to cold (especially cold to the feet) or sudden changes of climate or temperature.
Treatment. In general, it will be proper to administer a mild aperient, for which purpose rhubarb or castor oil is usually preferred. The dose of the first may be from 20 to 30 grains in sugar, or made into a bolus; that of the second, from 1⁄4 oz. to 1⁄2 oz., with a little mint or peppermint water. After the due operation of this medicine, opium, astringents, and absorbents, may be taken with advantage, but not in excessive doses, as is commonly the practice. The first and second are indicated when great irritability exists, and the third, in cases of diarrhœa arising from the presence of acidity. Chalk mixture, to which a few drops of laudanum have been added, or the compound powder of chalk and opium, are excellent medicines, and will generally quiet the bowels. A small piece of catechu or hard extract of logwood, sucked in the same way as a lozenge, is a pleasant method of taking either of these powerful astringents, and will generally cure cases of simple diarrhœa arising from excessive peristaltic motion, or want of tonicity of the muscular coats of the intestines.
In bilious diarrhœa, characterised by the bright yellowish-brown colour of the dejections, a dose of blue pill or calomel, assisted by mild diluents and demulcents, and warmth, generally proves efficacious. Small doses of opium are also useful in some cases.
In catarrhal diarrhœa, chylous diarrhœa, and the like varieties, characterised by the dejections being nearly colourless, and consisting chiefly of water and mucus; or white and milky, showing the entire absence of bile; or, being entirely liquid, limpid, and serous (in557 some cases resembling the washings of flesh), opinions are divided as to the treatment. The majority of the best authorities regard purging as injurious in these varieties, and rely chiefly on warm baths and warm fomentations, with the internal administration of mild salines and diaphoretics, followed by astringents, tonics, and occasional doses of opiates. Choleraic diarrhœa demands a nearly similar treatment.
The diet in every variety of diarrhœa should be light and non-irritating. Glutinous broths, beef tea, and arrow-root, are among the best articles which can be taken. To these may be added a little dry toast. Arrow-root (genuine), either with or without a spoonful of port wine or brandy (preferably the former), will of itself cure all ordinary cases of diarrhœa, if accompanied with repose and a recumbent posture.
Among external remedies, warm and stimulating fomentations, liniments, &c., to the epigastrium and abdomen, will be found useful adjuncts to other treatment. A spoonful or two of laudanum, used as a friction, will generally allay pain, and in many cases settle the bowels when all other remedies have been tried in vain.
Treatment for Animals. If for the horse, give at the commencement of the attack from 2 to 4 dr. of aloes, mixed with 1 oz. of bicarbonate of soda, and the same quantity of ginger in powder; administer clysters occasionally. Cattle may be treated by having administered to them 3⁄4 lb. of Epsom or common salt, or a pint of linseed oil. Whichever of the two is employed, it must be combined with 2 oz. each of bicarbonate of soda and ginger, and 1⁄2 lb. of treacle; 1 oz. of laudanum should be added to the above drenches whenever there is much pain and straining, whether in the horse or cow. Should laxatives fail, aromatics and astringents are called for, and 1 oz. each of tincture of catechu, ginger, and gentian, given in a pint of warm ale, may be tried several times a day for a horse. For cows a double dose is required. Sheep need only half the dose.
DI′ASTASE. A peculiar azotised substance contained in malt, which effects the conversion of starch, first into dextrin, and then into grape sugar.
Prep. A cold infusion of malt is heated to 158° Fahr. (to coagulate in albumen); it is then allowed to cool, and alcohol is added to the filtered liquor, when diastase is precipitated, under the form of a tasteless white powder, which is freely soluble in water.
Prop., &c. Diastase seems to resemble vegetable albumen, but very little is known respecting it, as it has never been got in a state of purity. One part of diastase is capable of converting 2000 parts of starch into grape sugar. Malted barley is said to contain 1⁄500th part of this substance; yet this small portion is quite sufficient to convert the starch of the malt into sugar during the operation of mashing, in the manufacture of beer. See Brewing, Dextrin, &c.
DICTA′MIA. A nutritious, dietetic article. Prep. (Beasley.) Sugar, 7 oz.; potato arrow-root, 4 oz.; flour of brent barley (Triticum monococcum), 3 oz.; Trinidad and Granada chocolate, of each 1 oz.; vanilla, 15 gr.; triturate together.
Dictamia. A strengthening and restorative preparation. Arrowroot, 6 parts; meal of triticum monococcum, 6 parts; chocolate, 4 parts; vanilla, 1⁄4 part (Richter). Sugar, 217 parts; bran extract, 92 parts; starch, 125 parts; Caracas and Maragnan cocoa, 30 parts; vanilla, 1 part. (Chevallier.)
DIDYM′IUM. Di. A rare metal, found associated with cerium and lanthanium in the Swedish mineral cerite. See Cerium.
DI′ET. Food or victuals. In medicine food regulated by certain rules, or prescribed for the cure or prevention of disease. The dietetic part of medicine is no inconsiderable branch, and deserves a much greater share of regard than it commonly meets with. A great variety of diseases might be removed by the observance of a proper diet and regimen, without the assistance of medicine, were it not for the impatience of the sufferers. On all occasions it may come in as a proper assistant to the cure, which sometimes cannot be performed without a due observance of the non-naturals.
Writers on dietetics (DIETETICA, L.) have taken much trouble to divide and classify the numerous articles of food suitable to the various conditions of the body in health and disease; but little practical advantage has resulted from their labours. Low diet, middle diet, full diet, milk diet, farinaceous diet, fruit diet, and vegetable diet, are terms which, under most circumstances, are sufficiently simple to be almost self-explanatory.
DIGES′TION. In chemistry and pharmacy the operation of exposing bodies to a gentle and continuous heat. The best digesters are thin glass flasks and beakers, and the most convenient source of heat is the sand bath. Digestion is often performed to soften and otherwise modify bodies that are to be distilled. In physiology the term is applied to the conversion of food into chyme, or the process of dissolving food in the alimentary canal, and preparing it for circulation and nourishment. In surgery digestion signifies a method of treating ulcers, wounds, &c. See Digestives (below).
| Foods. | How cooked. | Mean time of chymification in stomach. |
| h. m. | ||
| Rice | Boiled | 1 |
| Eggs, whipped | Raw | 1 30 |
| Trout, salmon, fresh | Boiled | 1 30 |
| Venison, steak | Broiled | 1 35 |
| Sago | Boiled | 1 45 |
| Milk | Boiled | 2 |
| Eggs, fresh | Raw | 2 |
| Milk | Raw | 2 15 |
| Turkey | Boiled | 2 25 |
| Gelatin | Boiled | 2 30 |
| Goose, wild | Roasted | 2 30 |
| Pig, sucking | Roasted | 2 30 |
| Lamb, fresh | Broiled | 2 30 |
| Beans, pod | Boiled | 2 30 |
| Potatoes, Irish | Baked | 2 30 |
| Chicken | Fricassed | 2 45 |
| Oysters, fresh | Raw | 2 55 |
| Eggs, fresh | Soft-boiled | 3 |
| Beef, lean, rare | Roasted | 3 |
| Mutton, fresh | Boiled | 3 |
| Bread, corn | Baked | 3 15 |
| Butter | Melted | 3 30 |
| Cheese, old, strong | Raw | 3 30 |
| Potatoes, Irish | Boiled | 3 30 |
| Beef | Fried | 4 |
| Veal, fresh | Broiled | 4 |
| Fowls, domestic | Roasted | 4 |
| Ducks, Domestic | Roasted | 4 |
| Veal, fresh | Fried | 4 30 |
| Pork, fat, and lean | Roasted | 5 15 |
| Cabbage | Boiled | 4 30 |
The results recorded in the above table, giving the respective time required for the digestion of different foods, were obtained by Dr Beaumont, through his being enabled to watch the process of digestion actually going on in the stomach of a man, who had received a wound in that organ, by which part of it was laid bare, and could thus be seen into.
The above data were controlled by a series of independent experiments, which consisted in digesting different foods in a solution of gastric juice, and heating the mixture to 100°. The relative results of both sets of experiments were found to agree pretty closely; and they have since, on the whole, being confirmed by the researches of other physiologists.
DIGES′TIVES. In surgery substances which, when applied to wounds or tumours, induce or promote suppuration. All stimulating applications are of this class. Heat is a most powerful digestive agent. The action of digestives is opposed to that of DISCUTIENTS, which repel or resolve tumours and indurations.
DIGITA′LIN. Syn. Digita′lia. A vegetable principle discovered by M. Royer in Digitalis purpurea, or purple foxglove.
Prep. 1. (Majendie.) Foxglove leaves (powdered), 1 lb., are digested in ether, first in the cold, and then heated under pressure; when the whole has again become cold, the liquor is filtered (rapidly), and the ether is distilled off in a water bath; the residuum is dissolved in water, the filtered solution treated with hydrated oxide of lead, the whole gently evaporated to dryness, and the dry residuum again digested in hot ether; from this solution the alkali is obtained, by evaporation and repeated resolutions, in a crystalline form.
2. (Homolle and Henry.) Foxglove leaves (carefully dried and powdered), 21⁄2 lbs., are digested in rectified spirit, and the tincture expressed in a tincture press; the spirit is then distilled off, and the residual extract treated with distilled water, 1⁄2 pint, acidulated with about 2 fl. dr. of acetic acid, a gentle heat being employed; some animal charcoal is then added, and the whole filtered; the filtrate is then diluted with water, and partly neutralised with ammonia; a fresh-made, strong decoction of galls is next added; a copious precipitation of tannate of digitalin ensues; the precipitate is washed with water, and mixed with a little alcohol, after which it is triturated with litharge (in fine powder) and exposed to a gentle heat; the whole is now digested in alcohol, the tincture treated with animal charcoal, and evaporated; the dry residuum is, lastly, treated with cold sulphuric ether, which takes up some foreign matter, and leaves the digitalin. 2 lbs. 8 oz. of the dried leaves yield 140 to 150 gr. of the digitalin.
Prop., &c. White, inodorous, porous masses, or small scales; it crystallises with difficulty, is intensely bitter, and excites violent sneezing when smelled to; dissolves freely in alcohol; scarcely soluble in cold ether; and takes 2000 parts of water for its solution; it is neither basic nor alkaline; concentrated colourless hydrochloric acid dissolves it, forming a characteristic solution which passes from yellow to a fine green. (Homolle.) It is one of the most powerful of known poisons, being fully 100 times stronger than the powdered leaves of the dried plant. It is used in the same cases.—Dose, 1⁄60 to 1⁄30 gr.; either made into pills or dissolved in alcohol and formed into a mixture. Owing to the difficulty and uncertainty connected with dispensing such small quantities, it is now seldom employed in this country.
Digitalin, Crystallised. Digitalis leaves from the Vosges, in rather fine powder, 1000 grams; neutral lead acetate, 250 grams; distilled water, 1000 grams. The digitalis should be collected in its second year just when the first flowers appear. With respect to the lead acetate, it is very important that it should not have an alkaline reaction; a slight acidity would be preferable. The lead salt is dissolved in the cold water, the powder added and thoroughly mixed, the whole passed through a sieve and left in contact twenty-four hours, taking care to mix it from time to time. The mixture is then packed sufficiently in a displacement apparatus, and exhausted with 50° alcohol, until it no longer yields any bitterness. About six parts of liquor are thus obtained, and this is neutralised exactly with sodium bicarbonate dissolved to saturation in cold water; about 25 to 30 grams will be required. When effervescence ceases, the alcohol is distilled, and the liquor remaining is evaporated559 in a water bath down to 2000 grams; it is then left to cool and diluted with its weight of water. Two or three days afterwards the clear liquor is decanted off, by means of a syphon, and the precipitate drained upon a linen filter. When freed from the extractive liquor the precipitate weighs about 100 grams. It is suspended in 1000 grams of 80° alcohol, and the whole passed through a metal sieve or a fine cloth; the turbid liquor obtained is heated to ebullition, and to it is added a solution containing 10 grams of neutral lead acetate; the heating is continued a few moments, and the liquor is then left to cool and filtered. The deposit in the filter is washed with alcohol to remove any liquor it may retain, and then pressed. To this liquor is added 50 grams of finely powdered vegetable charcoal that has been washed with acid and afterwards with water until quite neutral, and it is then distilled, the residue being heated for some time in a water bath, it being very important that all the alcohol should be driven off. A little water is added to replace what may evaporate; the residue is allowed to cool, then drained upon the cloth that was used to separate the precipitate, and the carbonaceous mass is washed with a little water to remove the last portion of the coloured liquor. The carbonaceous residue is then dried completely in a stove at a temperature not exceeding 100° C., and exhausted by displacement with pure chloroform until it passes colourless. This liquid is distilled to dryness, and a few grams of 95° alcohol are placed in the retort, and evaporated to drive off the last traces of chloroform.
The residue is crude digitalin with viscous and oily matter. It is dissolved with heat in 100 grams of 90° alcohol, and 1 gram of neutral lead acetate dissolved in a little water added, together with 10 grams of animal charcoal in fine granules without powder that has been treated with hydrochloric acid, and washed until the washings are no longer acid. After boiling for ten minutes it is allowed to cool and settle, and then filtered in a glass cylinder furnished with a tight cotton plug, through which it passes quickly and clear. The black deposit is added last, and exhausted of all bitterness with alcohol. After distillation the digitalin remains as a grumous crystalline mass, now only contaminated with the coloured oil. A little aqueous liquor that occurs with it is separated and the weight of the impure digitalin is taken in the previously tarred vessel. The digitalin is then dissolved with heat in exactly sufficient 90° alcohol for its solution (from 6 to 12 grams). Any alcohol evaporated is replaced, and then to the cooled solution is added, sulphuric ether rectified at 65°, to half the weight of the alcohol employed; after mixing, distilled water equal to the weight of the alcohol and ether combined is added, and the flask is closed and shaken. Two layers are formed: the upper is coloured, and consists of the ether which has taken up the fat oil; the lower is colourless, and contains the digitalin, which being now free quickly crystallises. The flask is placed in a cool place. Two days afterwards the whole is thrown into a small cylinder furnished with a moderately tight cotton plug; the mother liquor runs off, and then the coloured layer, and the small quantity that remains adherent to the crystals, is removed by a little ether. Thus obtained, this first crystallisation of digitalin is slightly coloured; it is sufficiently pure, however, for its weight to be taken in an analysis; one tenth being deducted for the digitalin it still contains. To obtain it perfectly white, two purifications are necessary, but first a treatment with chloroform is indispensable to separate the remainder of the digitalin which injures its purity.
The digitalin, well dried and reduced to a fine powder, is dissolved in 20 parts of chloroform, and the solution is filtered in a cylinder through a tight cotton plug. The liquor passes limpid; it is distilled to dryness, and a little alcohol is poured into the retort, and evaporated to remove the last traces of chloroform.
The digitalin is dissolved in 30 grams of 90° alcohol, 5 grams of washed granular animal black added, and the whole boiled for 10 minutes; the liquor is filtered and the charcoal exhausted as before indicated; and, lastly, it is distilled, the digitalin in dry crystals is found on the sides of the vessel, but it is still slightly coloured. To obtain it perfectly white it is dissolved with heat in exactly sufficient 90° alcohol (about 6 to 8 grams); to the solution is added ether equal to half the weight of alcohol employed, and double the quantity of distilled water, and the flask is closed and agitated; the crystallisation commences quickly. The ether does not separate. It is exposed to the coolness of the night, and by the next day nearly the whole of the digitalin is deposited in small groups of white needles, that which retains colouring matter remaining in the mother liquor. The whole is thrown into a cylinder and the crystals washed with ether as before described. 1000 grams of Vosges digitalis of good quality yields about 1 gram of crystallised digitalin. Digitalin occurs under the form of very white light crystals, consisting of short slender needles, grouped around the same axis. It is very bitter and scarcely soluble in water. 90° alcohol dissolves it well, anhydrous alcohol dissolves it less freely. Pure ether dissolves only traces. Chloroform is its best solvent. Brought into contact with a small quantity of hydrochloric acid, digitalin is coloured emerald green, and this reaction is favoured by a very slight heat. From ‘Formulæ for New Medicaments, adopted by the Paris Pharmaceutical Society.’
DILL. Syn. Anethum (Ph. L. & E.), L. The fruit (seed) of Anethum graveolens, or garden dill, Anethi fructus, B. P. Dill is an aromatic560 stimulant and carminative. The Cossacks employ it as a condiment; and in this country it is frequently employed to heighten the relish of soups and pickles, especially cucumbers. Dill water is a favorite remedy of nurses to promote the secretion of milk, and to relieve the flatulence and griping of infants.—Dose, of the powder, 10 gr. to 1⁄2 dr., or more. Oil of dill (OLEUM ANETHI) and dill water (AQUA ANETHI) are officinal in the pharmacopœias.
DIL′UENTS. Syn. Diluentia, D. Aqueous liquors; so named because they increase the fluid portion of the blood. Tea, barley-water, water-gruel, and similar articles, are the most common diluents, after pure water. The copious use of diluents is recommended in all acute inflammatory diseases not of a congestive character, and to promote the action of diuretics and sudorifics.
DINNER PILLS. See Pills.
DIOS′MA. Syn. Bookoo, Buku; Folia barosmæ, F. diosmæ, L.; Buchu (Ph. L.), Bucku (Ph. E.), Diosma (Ph. L. 1836). “The leaves of Barosma serratifolia, B. crenulata, and B. crenata.” (Ph. L.) These species were all included by De Candolle in the genus Diosma. Buchu is principally employed in chronic affections of the urino-genital organs, especially that of the mucous membrane of the bladder, attended with copious discharge of mucus.—Dose, 20 gr. to 1⁄2 dr. of the powder, taken in wine; or made into an infusion or decoction.
The officinal buchu leaves are “glabrous, glandular; either linear-lanceolate with small serrations, or ovato-oblong, obtuse, crenated, ovate or obovate, serrated.” (Ph. L.) Their odour somewhat resembles that of rue, and their taste is warm and mint-like.
DIOS′MINE. A bitter extractive matter, obtained by Brande from buchu leaves. It is very soluble in water, but not in alcohol and ether.
DIPHTHERIA. A contagious disease affecting the throat and adjoining parts. A false membrane forms on the mucous lining of the several parts of the throat. This alarming malady generally commences with a little soreness of throat attended with fever; sometimes, however, the fever may not come on for some days after the sore throat has shown itself. An ash-coloured spot makes its appearance on one or both tonsils. This spreads to other parts, extending in doing so, over the soft palate and uvula, inclosing the latter in a sheath. Sometimes a thin red line surrounds the opaque membrane thus formed. As the disease proceeds this opaque and false membrane tends to enlarge itself, and may spread down the gullet into the stomach, or, what is more dangerous still, it may involve the mucous membrane of the larynx, and thence extend along the windpipe into the bronchial tubes. When this is the case the disease is accompanied with cough, and the peculiar noise of croup; harsh, noisy breathing. There also frequently runs from the nostrils a thin acrid secretion, smelling very offensively, and often tainting the whole atmosphere of the room.
By the inexperienced diphtheria is almost always mistaken for ulcerated sore throat.
As in croup, part of the exudation or false membrane is often coughed up; sometimes it peels off from the tonsils. Some pathologists think that minute particles of this membrane, loosely adhering to drinking vessels, linen, sheets, the night-dress, &c., of the patient, may be the means of communicating the disease; by others, however, this surmise is not accepted. The absence of certainty on this point does not remove the stringent necessity of thoroughly cleansing and disinfecting everything with which the secretions of the patient come into contact.
The foregoing has been written with the object of enabling the reader to detect the only symptoms by which this dangerous disease manifests itself, in order that he may seek medical assistance with which to combat the complaint as promptly as possible.
After stating thus much, it is needless to say that we only recommend the adoption of the following treatment, in the extreme case of emigrants and others unable to obtain the services of a medical practitioner. A saturated solution of borax in the form of a gargle should be first used, and used without stint. Should this fail to arrest the formation of the membrane, then a strong solution of alum should be employed instead; or alum in powder should be applied to the throat every half hour or hour. When children are to be submitted to this treatment, the alum instead of being used as a gargle may be mixed with honey, and in this condition laid on the parts with a feather; or the powdered alum may be blown on the affected parts with a tube. Tincture of perchloride of iron, diluted hydrochloric acid, and chlorate of potash are also said to have been used successfully as topical applications. The diet should consist of rich nourishing food, such as strong beef tea and mutton broth, aided by port wine. The internal remedies embrace quinine or bark, tincture of perchloride of iron, pernitrate of iron, chlorate of potash, and small, but repeated doses of the mineral acids. Dr Gardner, in his useful work ‘Household Medicine,’ says, “a definite plan said to have been successful is to employ as a gargle a solution of chlorinated soda half an ounce, syrup half an ounce, water six ounces, mixed. At the same time give internally four drops of the solution every two hours, for two days, then add quinine. We may add that if this latter prescription be used the diet before indicated should be adopted.”
DISCU′TIENTS. In surgery, substances or agents which disperse or resolve tumours, &c. See Digestives.
DISH-COVERS. As these are made of561 various materials they must be cleaned and polished with the substances best adapted for each. All kinds of dish-covers directly they come from table should be washed free from grease and wiped dry.
Plated and silver dish-covers should be polished with plate powder; that free from mercury should be preferred. When dish-covers (as is usually the case) are made of block tin, they should be polished, by first rubbing them with sweet oil, and then dusting over the oil finely powdered whiting; finishing off the polishing with soft rags. All the best covers are provided with movable handles, which must be removed during the process of cleaning.
DISINFECT′ANT. An agent which absorbs, neutralises, or destroys, putrescent effluvia, miasmata, or specific contagia, and thus removes the causes of infection. The principal disinfectants are chlorine, iodine, bromine, the so-called chlorides of lime and soda, chloride of zinc, ozone, carbolic acid, the alkaline manganates and permanganates, peat charcoal, the fumes of nitric and nitrous acid, sulphurous acids, heat, and ventilation. The last two are the most efficient and easily applied. The clothing, bedding, &c., of patients labouring under contagious diseases may be effectually (?) disinfected by exposure to a temperature a little higher than that of boiling water, for about an hour. Neither the texture nor colour of textile fabrics is injured by a heat of even 250° Fahr. (Dr Henry.) See Disinfecting Chambers.
It is a practice at some of the workhouses to bake the clothes of the paupers who have the itch, or who are infested with vermin. The soaking and boiling of clothes in the absence of being able to bake them is by no means a bad method for disinfecting them. The process is rendered the more effective by adding to the water in which they are immersed or boiled 1 part of strong solution of chloride of lime to 20 or 30 parts of water; or carbolic acid in the proportion of 1 part of the pure acid to 100 parts of water. In the German army, if the clothes cannot be baked they are soaked for 24 hours in water containing 1 part of sulphate of zinc to 120 of water, or 1 part of chloride of zinc to 240 of water, after which they are washed in soap and water and dried.
Quicklime rapidly absorbs carbonic acid, sulphuretted hydrogen, and several other noxious gases, and is therefore commonly used as a wash for the walls of buildings. Acetic acid, camphor, fragrant pastilles, cascarilla, brown paper, and other similar substances, are frequently burnt or volatilised by heat, for the purpose of disguising unpleasant odours, but are of little value as disinfectants. The sulphates of iron and zinc have the property of rapidly destroying noxious effluvia. A quantity of either of these sulphates thrown into a cesspool, for instance, will in a few hours render the matter therein quite scentless. Of gaseous disinfectants, “sulphurous acid gas (obtained by burning sulphur) is preferable, on theoretical grounds, to chlorine. No agent checks so effectually the first development of animal and vegetable life. All animal odours and emanations are immediately and most effectually destroyed by it.” (Graham.) See Antiseptic, Deodoriser, Fumigation, Infection, Ozone, Carbolic acid, Salicylic acid, Bacteria as originators of disease, Lime, Chloride, Charcoal, also the Disinfecting Compounds given below.
Dr Wynter Blyth divides disinfectants into two great classes:—Gaseous, and solid or liquid.
1. Substances which, like the halogens, appear to form substitution compounds, e.g.
2. Substances which probably combine chemically, and thus destroy contagion:
3. Oxidising substances, such as—
4. The volatile oils, &c. Feeble disinfectants, supposed, however, to oxidise—
1. The chlorides of different metals, earths, or bases:
2. All soluble sulphates, especially sulphates of iron and aluminium.
3. All soluble sulphites.
4. Some acetates, as acetate of iron.
5. Some nitrates, such as the nitrates of potash and soda.
6. Certain agents which appear to arrest putrefaction or condense certain gases, &c., without either destruction or oxidation:
7. Preservative liquids and solutions. Many of these act by coagulating the albumen of organised bodies:
8. Destructive agents. Not true disinfectants, they act not by disinfection, but by destruction:
9. Agents which act in many ways, partly by condensing gases, partly by absorbing moisture, and partly by a peculiar action on organic matter analogous to tannin:
The table on the next page is a summary by the late Dr Letheby of some experiments made by Drs Dougall and Calvert, with the view of determining the relative powers possessed by certain substances of arresting putrefaction, as measured by their action in preventing the germination of animalcules and fungi, and the development of vaccine lymph.[258]
[258] ‘On the Relative Power of various Substances in Preventing the Germination of Animalculæ,’ by John Dougall, 1871. Calvert, ‘Proceedings of the Royal Society,’ vol. xx, p. 185.
Disinfecting Compounds. 1. (Sir Wm. Burnett’s Disinfecting Liquid.) A concentrated solution of chloride of zinc. See Zinc.
2. (Collins’ Disinfecting Powder.) A mixture of dry chloride of lime, 2 parts, and burnt alum, 1 part. Used either dry or moistened with water. See Lime.
3. (Condy’s Disinfecting Fluids.) Solutions of the alkaline manganates and permanganates. Although this is an excellent and rapid deodoriser, and makes a most serviceable dressing for fetid sores, it must be borne in mind that it is in no sense an aërial disinfectant, its action being limited to the solid or liquid matters only with which it is brought into immediate contact. It exercises no corrosive action, but it is open to the objection that it leaves a brown stain upon linen. See Manganese.
4. (Ellerman’s Deodorising Fluid.) This is said to consist chiefly of the perchlorides and chlorides of iron and manganese.
“In a report addressed to the Metropolitan Board of Works in 1859, Drs Hoffmann and Frankland stated that the perchloride of iron was the cheapest and most efficient deodoriser that could be applied to sewage.” (Beasley.)
5. (Labarraque’s Disinfecting Solution; Liquor sodæ chlorinatæ, Ph. L. & D.) A solution of chlorinated soda, or, as it is commonly called, ‘chloride of soda.’ M. Labarraque made known this valuable disinfectant in 1822, and obtained the prize of the French ‘Society for Encouraging National Industry’ for its introduction.
6. (Ledoyen’s Disinfecting Fluid.) A solution of nitrate of lead, 1 part, in about 8 parts of water; or, of litharge, 131⁄2 oz., in nitric acid (sp. gr. 1·38), 12 oz., previously diluted with water, 6 pints. Sp. gr. 1·40.
7. (Siret’s Disinfecting Compounds.)—a. A mixture of sulphate of lime, 53 lbs., sulphate of iron, 40 lbs., sulphate of zinc, 7 lbs., and peat charcoal, 2 lbs., made into balls.
b. Sulphate of iron, 20 parts; sulphate of zinc, 10 parts; tan or waste oak-bark (in powder), 4 parts; tar and oil, of each 1 part; as before. Used for deodorising cesspools, &c.
8. (Bisulphide of Carbon.) This generates, when burnt, sulphurous acid, and is, therefore, a very valuable disinfectant. Its highly inflammable nature, however, renders the adoption of certain precautions necessary in its use. A method of employing it in the form of fumigation will be found under the article “Fumigation.”
9. Dry salicylic acid volatilised from a hot plate purifies the air, and perfectly disinfects the walls of a closed room. (Von Heyden.)
10. “Sanitas” is the name given by Mr Kingzett, its discoverer, to a new liquid antiseptic and disinfectant, containing peroxide of hydrogen and camphoric acid, and obtained by the atmospheric oxidation of turpentine. Sanitas is said by its inventor to possess the great advantages of being non-poisonous, and to exercise no injurious effects either on clothing or furniture. It is stated that its antiseptic power is distributed between the peroxide of hydrogen and camphoric acid, the peroxide of hydrogen being able to evolve large quantities of oxygen, which in this state is nascent, and of a powerful and oxidising character.
11. Cooper’s Universal Disinfecting Powder. According to Professor Wanklyn this powder contains 70 per cent. of mixed chloride of sodium and chloride of calcium, and about 6 per cent. of anhydrous sulphate of zinc (equal to about 12 per cent. of hydrated sulphate), a little insoluble matter, and 15 per cent. of moisture.
12. Dr Bond’s Cupralum and Ferralum. The first of these disinfectants is stated to be a mixture of the sulphates of copper and aluminium, with potassic dichromate and turpentine. Its inventor claims for it that it possesses great power of coagulating albumen and high value both as an antiseptic and deodorant. Ferralum is a mixture of ferrous and aluminic sulphates, turpentine, and carbolic acid. Its chief use is for flushing sewers and in deodorising cesspools, urinals, &c.
13. Bayard’s Disinfectant. A mixture of sulphate of iron, clay, lime, and coal tar.
563
| Substances used. | EXPERIMENTS MADE BY DR JOHN DOUGALL, OF GLASGOW. | EXPERIMENTS BY DR CRACE CALVERT. | |||||||||||||
| F | A | K | B | P | C | ||||||||||
| G | H | I | J | D | E | Q | R | S | T | ||||||
| L | M | N | O | ||||||||||||
| Acids. | |||||||||||||||
| Mineral. | |||||||||||||||
| Sulphurous | Acid. | 250 | 50 | 50 | 117 | Death. | 24 | 4 P. | 8 | Over 100 | Killed. | 11 | Over 40 | 21 | Over 40 |
| Nitric | ” | 400 | 400 | 200 | 333 | ” | 18 | 4 P. | 15 | 5 T. | ” | 10 | 50 | 10 | 23 |
| Hydrochloric | ” | 500 | 400 | 100 | 333 | ” | 28 | 4 P. | 9 | Over 100 | ” | — | — | — | — |
| Sulphuric | ” | 800 | 500 | 100 | 467 | ” | Over 100 | Over 100 | 30 | 10 T. | — | 9 | — | 9 | 11 |
| Chromic | ” | 4000 | 1400 | 1200 | 2200 | — | 78 | 38 P. | Over 100 | Over 100 | — | — | — | — | — |
| Organic. | |||||||||||||||
| Carbolic | Neutral. | 300 | 300 | 200 | 267 | None. | 12 | 50 T. | 38 | 36 P. | None. | Over 40 | Over 40 | Over 40 | Over 40 |
| Cresylic | ” | — | — | — | — | ” | — | — | — | — | ” | ” | ” | ” | ” |
| Acetic | Acid. | 350 | 25 | 10 | 125 | — | — | — | — | — | Killed. | 30 | — | 9 | 50 |
| Picric | ” | 350 | 350 | 350 | 350 | Death. | 44 | 11 P. | Over 100 | 44 P. | — | 17 | Over 40 | 19 | Over 40 |
| Benzoic | ” | 700 | 700 | 200 | 533 | ” | Over 100 | Over 100 | ” | Over 100 | — | — | — | — | — |
| Alkalies. | |||||||||||||||
| Lime | Alk. | — | — | — | — | — | — | — | — | — | — | 13 | 19 | Over 40 | Over 40 |
| Potash | ” | 300 | 50 | 10 | 120 | Death. | — | — | — | — | — | 16 | — | — | — |
| Soda | ” | — | — | — | — | — | — | — | — | — | — | 23 | 31 | 18 | 29 |
| Ammonia | ” | — | — | — | — | — | — | — | — | — | — | 24 | 50 | 20 | Over 40 |
| Haloids. | |||||||||||||||
| Iodine tincture | Neutral. | 400 | 400 | 50 | 283 | Death. | 1 | 80 T. | 15 | Over 100 | — | — | — | — | — |
| Chlorine gas | Acid. | — | — | — | — | — | — | — | — | — | Killed. | 7 | 21 | 21 | — |
| Chloride lime | Alk. | 200 | 200 | 25 | 142 | Death. | 27 | 27 T. | 40 | Over 100 | ” | 7 | 18 | 16 | — |
| Chloride zinc | Acid. | 300 | 300 | 300 | 300 | ” | 4 | Over 100 | 18 | ” | — | Over 40 | Over 40 | 50 | Over 40 |
| Chloride aluminum | ” | 2000 | 500 | 300 | 933 | — | 19 | 4 P. | Over 100 | 8 P. | — | 10 | ” | 21 | 50 |
| Sulphates, &c. | |||||||||||||||
| Bisulphite lime | Acid. | 100 | 50 | 25 | 58 | Death. | 4 | 92 T. | 9 | Over 100 | — | 11 | 21 | 14 | Over 40 |
| Sulphate zinc | ” | 300 | 300 | 200 | 267 | ” | 30 | 4 P. | 90 | 70 P. | — | — | — | — | — |
| Sulphate iron | ” | 500 | 500 | 100 | 367 | ? | 14 | 5 T. | 35 | 40 T. | — | 7 | Over 40 | 15 | — |
| Common alum | ” | 800 | 500 | 100 | 467 | — | 14 | 3 P. | 38 | 15 T. | — | — | — | — | — |
| Sulphate copper | ” | 1000 | 1000 | 800 | 933 | Death. | 86 | 20 P. | Over 100 | Over 100 | — | — | — | — | — |
| Permanganate potash | Neutral. | 500 | 200 | 125 | 275 | None. | — | — | — | — | — | 9 | 50 | 22 | Over 40 |
| Alcohol | ” | 350 | 50 | 20 | 140 | Death. | 4 | 4 T. | 10 | Over 100 | — | — | — | — | — |
| Camphor | ” | 300 | 150 | 50 | 167 | None. | — | — | — | — | None. | — | — | — | — |
| Turpentine | ” | — | — | — | — | — | — | — | — | — | — | 14 | Over 40 | 42 | Over 40 |
Note.—In the first set of Dr John Dougall’s experiments 3 drachms of a solution of the strength mentioned were treated with 1 drachm of a filtered infusion of hay, or with half a drachm of urine or half a drachm of the mixture of beef juice and egg-albumen. In the second set of experiments equal parts of a putrid solution of beef juice and egg-albumen, full of living animalcules, and of the solution of the various substances of the strength known to be preventive of life (as in third column), were mixed together, and the results immediately noted. In the third set of experiments 31⁄2 drachms of distilled water, containing 1 in 500 of the substances named, were treated with half a drachm of filtered beef juice, or half a drachm of a solution consisting of 1 part white of egg to 4 parts water. In the last set of experiments, separate minims of vaccine lymph were exposed to the several vapours for 24 hours, and the dried spot in each case was moistened with glycerin and water, and sealed in a capillary tube until an opportunity for vaccination occurred, when the whole of the diluted lymph was used in one insertion so as to ensure its full effect.
In Dr Crace Calvert’s experiments, 0·026 of a gramme of the substance was added to 26 grammes (1 to 1000) of a solution of albumen containing 1 part white of egg to 4 parts pure distilled water.
The Animalcules observed were Monads (microphymes), Vibrios, and their cell segments (microerphymes), Bacteria (microzymes), Amœba, &c.; and the Fungi were Torula, Mycelium, Penicilium, &c., indicated in Table by letters T and P. Putrefaction was always characterised by a putrid odour, an alkaline reaction, and the presence of animalcules; whereas Mouldiness and Fermentation were distinguished by a mouldy or musty odour, an acid reaction, and the presence of Fungi.
564
14. Larmande’s Antimephetic Liquor. A solution of the sulphates of zinc and copper.
15. Thymol. From experiments made with this substance it appears to be a very powerful and valuable antiseptic, and likely, because of its non-poisonous and non-irritant qualities, to supplant carbolic acid in various branches of surgical practice, in which this latter agent has hitherto been employed; such, for example, as a dressing for wounds, ulcers, and as a topical application for certain skin eruptions, &c. Its difficult solubility and price (spite of its much greater antiseptic power), however, for the present at any rate, preclude it from being made available as an ordinary common disinfectant, as this term is generally understood. See Thymol.
16. Silicate of Soda. It is stated that this salt has considerable anti-putrefactive powers.
17. Aluminised Charcoal. This is recommended by Dr Stenhouse as a cheap and very efficient decolorising agent. It is made by dissolving in water 54 parts of the sulphate of alumina of commerce in water, and mix it with 921⁄2 parts of finely powdered wood charcoal. When the charcoal is saturated it is evaporated to dryness, and heated to redness in covered Hessian crucibles till the water and acid are dissipated. The charcoal contains 71⁄2 per cent. of anhydrous alumina.
The natural disinfectants are air and water.
Air, when in violent motion, as is the case during a hurricane, has in many instances been known to arrest the course of certain epidemics; whilst in the form of ordinary ventilation, although inadequate alone to destroy the causes (whatever they may be) of contagion or infection, it is nevertheless found to supplement, to a considerable extent, the application of artificial and specific disinfectants. Hence the paramount necessity of perfect ventilation in all apartments in which the sick are placed, and hence also the measures taken in all hospitals to ensure by this means an increasing supply of fresh air to the wards in which the patients are lying.
The diminution in the amount of sickness prevailing in an army caused by the removal of the soldiers from barracks and placing them in sheds or under canvas is another illustration, tending to show the disinfectant properties possessed by an atmosphere in a state of circulation, when, of course, other hygienic precautions are not neglected.
In Hammond’s ‘Hygiène’ for 1863 the author, who was surgeon-general in the United States army, says that he only met with one instance of hospital gangrene in a wooden pavilion hospital, and not a single one in a tent; and the same result is recorded by Kraus, of the Austrian army in 1859, who says he never discovered that gangrene originated in a tent; that, on the contrary, cases of gangrene at once began to improve when those suffering from the disease were sent from hospital wards into tents. In his work on ‘Practical Hygiène’ Dr Parkes advises all cases of typhus occurring in barracks, whenever practicable, to be sent to tents or wooden huts having badly-jointed walls.
The great solvent power of water, superadded to its being able to hold matters in suspension, renders it a most important disinfectant, and thus enables it in the form of rain to remove from the atmosphere many noxious and pestilential bodies that would doubtless, if allowed to increase, become a source of disease. The air-current which constitutes the ventilation of the House of Commons, before entering the Commons’ chamber, is made to pass over a fine spray of water, by which means it has any dust or other organisms washed out of it. The beneficial effect of rain also in flushing drains and canals, and sweetening the superincumbent air, and of washing out of it many solid as well as gaseous objectionable impurities, is well known. The year 1860 was one of the wettest on record, as it was also one of the healthiest. Dr W. Budd recommends that when a room is to be disinfected, a short time before the process is commenced a tub of boiling water should be placed in the apartment, so that the steam may become condensed on the walls, and diffused throughout the air, as he believes there is a greater chance of ensuring the destruction of the disease germs by the aërial disinfectants than if these latter were allowed to act on the germs in the dry state.
We have already enforced in these pages the importance of the habit of personal cleanliness as being one of the greatest aids to the preservation of health; and although the unstinted use of soap and water will alone fail to effect the removal of any infectious or contagious maladies, their use will be found important auxiliaries in assisting recovery. But personal ablution is not the sine quâ non. The frequent cleansing of our dwellings, streets,[259] alleys (more particularly culs-de-sac), lanes, and the sheds and habitations of animals, by soap and water, or water alone, as well as the removal of all decaying or refuse materials from our midst, is of equal importance, and must not be disregarded, if we desire to make our sanitary surroundings such as they ought to be.
[259] In streets where there is much traffic the air above has been found to contain large quantities of dust composed, amongst other matters, of the remains of horse droppings; hence the great importance of assiduously watering and cleansing the thoroughfares of all large cities and towns. A plan for laying the dust of streets has been suggested by Mr Cooper, and consists in watering them with waste chlorides of calcium and magnesium. Carbolic acid has been employed for the same purpose by many urban authorities for some years past.
We extract the following from Dr Parkes’ valuable and standard work—‘Practical Hygiene,’
565
“Disinfection of Various Diseases.
“Exanthemata, Scarlet Fever, and Rothëln. The points to attack are the skin and throat. The skin should be rubbed from the very commencement of the rash until complete desquamation, with camphorated oil, or oil with a little weak carbolic acid. The throat should be washed with Condy’s fluid, or weak solution of sulphurous acid.
“Clothes to be baked, or to be placed at once in boiling water, as directed further back. The clothes should not be washed at a common laundry. Chlorine or euchlorine should be diffused in the air, the saucer being put some little distance above the head of the patient. Carbolic acid and ether or carbolic-acid spray may be used instead.
“Smallpox.—In this, as in all cases, there can be no use in employing aërial disinfectants, unless they are constantly in the air, so as to act on any particle of poison which may pass into the atmosphere.
“The skin and the discharges from the mouth, nose, and eyes are to be attacked. There is much greater difficulty with the skin, as inunction cannot be so well performed. By smearing with oil and a little carbolic acid and glycerin, or, in difficult cases, applying carbolised glycerin to the papules and commencing pustules, might be tried. The permanganate and sulphurious acid solutions should be used for the mouth, nose, and eyes. The clothing should always be baked before washing, if it can be done.
“The particles which pass into the air are enclosed in small dry pieces of pus and epithelial scales; and Bakewell, who has lately examined them, expresses great doubts whether any air purifier would touch them. Still it must be proper to use euchlorine or carbolic acid. Iodine has been recommended by Richardson and Hoffmann.
“Measles.—Oily applications to the skin and air purifiers, and chlorides of zinc and aluminium in the vessels receiving the expectoration, appear to be the proper measures.
“Typhus (Exanthematicus).—Two measures seem sufficient to prevent the spread of typhus, viz. most complete ventilation and immediate disinfection and cleansing of clothes. But there is also more evidence of use from air purifiers than in the exanthemata. The nitrous acid fumes were tried very largely towards the close of last century and the beginning of this in the hulks and prisons where Spanish, French, and Russian prisoners of war were confined. At that time so rapidly did the disease spread in the confined spaces where so many men were kept, that the efficacy even of ventilation was doubted, though there can be no question that the amount of ventilation which was necessary was very much underrated. Both at Windsor and Sheerness the circumstances were most difficult. At the latter place (in 1785), in the hulk, 200 men, 150 of whom had typhus, were closely crowded together; 10 attendants and 24 men of the crew were attacked; 3 medical officers had died when the experiments commenced. After the fumigations one attendant only was attacked, and it appeared as if the disease in those already suffering became milder. In 1797 it was again tried with success, and many reports were made on the subject by army and naval surgeons. It was subsequently largely employed on the Continent, and everywhere seems to have been useful.
“These facts lead to the inference that the evolutions of nitrous acid should be practised in typhus-fever wards, proper precautions being taken to diffuse it equally through the room, and in a highly dilute form.
“Hydrochloric acid was employed for the same purpose by Guyton de Morveau in 1773, but it is doubtless much inferior to nitrous acid. Chlorine has also been employed, and apparently with good results.
“In typhus it would seem probable that the contagia pass off entirely by the skin, at least the effect of ventilation, and the way in which the agent coheres to the body linen seems to show this.
“The agent is not also enclosed in quantities of dry discharges and epidemics, as in the exanthemata, and is therefore less persistent and more easily destroyed than in those cases. Hence possibly the greater benefit of fumigations, and the reason of the arrest by ventilation. The clothes should be baked, steeped, and washed, as in the exanthemata.
“Bubo Plague. The measures would probably be the same as for typhus.
“Enteric (typhoid fever). The bowels’ discharges are believed to be the chief, if not the sole agents in spreading the disease; the effluvia from them escape into the air, and will adhere to walls and retain power for some time, or the discharges themselves may get into drinking-water. Every discharge should be at once mixed with a powerful chemical agent; of these, chloride and sulphate of zinc have been chiefly used, but sulphate of copper (which Dougall found so useful in stopping the growth of animalculæ), chloride of aluminium, or strong solution of ferrous sulphate (1 ounce to a pint of water), or carbolic acid. After complete mixing the stools must be thrown into sewers in towns; but this should never be done without previous complete disinfection. In country places they should be deeply buried at a place far removed from any water supply; they should never be thrown on to manure heaps or on to middens, nor into earth closets, if it can be possibly avoided. As the bedclothes and beds are so constantly soiled with the discharges, they should be baked, or, if this cannot be done, boiled immediately after removal with sulphate or chloride of zinc. It would be less necessary to employ air purifiers in this case than in others.
“Cholera. There can be little doubt that566 the discharges are here also the active media of the conveyance of the disease, and their complete disinfection is a matter of the highest importance. It is, however, so difficult to do this with the immense discharges of cholera, especially when there are many patients, that the evidence of the use of the plan in the last European epidemic is very disappointing.
“The ferrous sulphate (green vitriol), which has been strongly recommended by Pettenkofer as an addition to the cholera evacuations, was fully tried in 1866 at Frankfurt, Halle, Leipzig, in Germany, and at Pill, near Bristol, and in those cases without any good result. In other places, as at Baden, the benefit was doubtful. It seemed to answer better with Dr Budd and Mr Davies at Bristol; but other substances were also used, viz. chlorine gas in the rooms, and chloride of lime and Condy’s fluid for the linen. On the whole it seems to have been a failure. Ferric sulphate, with or without potassium permanganate, has been recommended by Kühne instead of ferrous sulphate, but I am not aware of any evidence on the point. Carbolic acid was largely used in England in 1866, and appeared in some cases to be of use, as at Pill, near Bristol, and, perhaps, at Southampton. It failed at Erfurt, but, as it is believed the wells were contaminated by soakage, this is perhaps no certain case. Chloride of lime and lime were used at Stettin without any good result, and, on the whole, it may be said that the so-called disinfection of the discharges of cholera does not seem to have been attended with very marked results. At the same time it cannot be for a moment contended that the plan has had a fair trial, and we can easily believe that unless there is a full understanding on the part of both medical men and the public of what is to be accomplished by this system, and a conscientious carrying out of the plan to its minutest details, no safe opinions of its efficacy or otherwise can be arrived at. It would be desirable to try the effect of chromic acid or bichromate of potash.
“With regard to air purifiers little evidence exists. Chlorine gas diffused in the air was tried very largely in Austria and Hungary in 1832, but without any good results. Nitrous-acid gas was used in Malta in 1865, but apparently did not have any decided influences, although Ramon da Luna has asserted that it has a decided preservative effect, and that no one was attacked in Madrid who used fumigations of nitrous acid. But negative evidence of this kind is always doubtful. Charcoal in bulk appears to have no effect. Dr Sutherland saw a ship’s crew severely attacked, although the ship was loaded with charcoal.
“Carbolic-acid vapour diffused in the atmosphere was largely used in 1866 in England; the liquid was sprinkled about with water, and sawdust moistened with it was laid on the floors and under the patients. The effect in preventing the spread of the disease was very uncertain.
“Yellow Fever. In this case the discharges, especially from the stomach, probably spread the disease, and disinfectants must be mixed with them.
“Fumigations of nitrous acid were employed by Ramon da Luna, and it is asserted that no agent was so effectual in arresting the spread of the disease.
“Dysentery. It is well known that dysentery, and especially the putrid dysentery, may spread through an hospital from the practice of the same close stool or latrines being used. As long ago as 1807, fumigations of chlorine were used by Mojon to destroy the emanations from the stools, and with the best effects. The chlorine was diffused in the air, and the stools were not disinfected; but this ought to be done as in enteric fever, and especially in the sloughing form. It is probable that carbolic acid in large quantity would be efficacious.
“With respect to Erysipelas, Diphtheria, Syphilis, Gonorrhœa, Glanders, and Farcy, local applications are evidently required, and carbolic acid in various degrees of strength, and the metallic salts, are evidently the best measures.
“Cattle Plague. The experiments made by Mr Crookes on the disinfectant treatment of cattle plague with carbolic acid vapour have an important bearing on human disease. Although the observations fall short of demonstration there are grounds for thinking that when the air was kept constantly filled with carbolic acid vapour, the disease did not spread.
“So also euchlorine was employed in Lancashire by Professor Stone of Manchester, with apparent benefit. Dr Moffat employed ozone (developed by exposing phosphorus to the air), and he believes with benefit. As such experiments are very much more easily carried out on the diseases of animals than on those of men, it is much to be wished that the precise effect of the so-called disinfectants should be tested by continuing the experiments commenced by Mr Crookes, not only in cattle plague in the countries where it prevails, but in epizootic diseases generally.
“It may be said, in conclusion, that although positive evidence is so deficient, yet, taking into consideration the decidedly great and known effect of many so-called disinfectants, and air-purifiers on organic matters, and the fact that the infectious organic agencies are certainly easily destroyed in most cases (since free ventilation renders many of them inert, and few of them retain their power very long), it is highly probable that the specific poisons of the so-called zymotic diseases are destroyed by some of these chemical methods, and at any rate the careful and constant use of chemical agents for the destruction of the specific poisons in the excreta and discharges from the body, and when they pass into the air, is not567 only warranted, but should be considered comparative.
“Purification of rooms after infectious diseases. In addition to thorough cleansing of all woodwork with soft soap and water, to which a little carbolic acid has been added (1 pint of the common liquid to 3 or 4 gallons of water), and to removal and washing of all fabrics which can be removed, the brushing of the walls, the room should be fumigated for 3 hours with either the fumes of sulphurous or nitrous acids. Both of these are believed to be superior to chlorine, especially in smallpox. All doors and windows, and the chimney being closed, and curtains taken down, the sulphur is ignited as directed in our article Fumigation.
“In white-washed rooms the walls should be scraped, and then washed with hot lime to which carbolic acid is added.
“Mortuaries and dead-houses are best purified with nitrous acid.”
These directions may be supplemented by the following:—The towels, sheets, articles of clothing, &c., should be boiled in water, or plunged in boiling water containing one to two handfuls of soda to the gallon, before being taken from the room, after which treatment they should be steeped in water containing 4 fluid ounces of carbolic acid to a gallon of water.
Fabrics soiled by the discharges, &c., such as rags, bandages, and dressings, if of little value should be immediately consigned to the flames; but if this be not convenient, they may be treated with carbolic acid and water, in the same manner as directed for towels, sheets, &c.
As soon as any infectious disease sets in, the room of the patient should be at once stripped of curtains, carpet, bed-curtains, valances, and all unnecessary garments, whether in a wardrobe or drawers, as well as of all superfluous furniture, especially chairs stuffed with wool or covered with fabric of any kind.
Disinfections of the apartment by fumigations must be postponed until it is vacated; as before such a time thorough disinfection is impossible.
Infected bedding, &c., should be removed in the boxes made for the purpose, and subjected to the heating process. In most towns provision is made by the Board of Guardians, and under the directions of the medical officer of health, for the disinfection process to be efficiently carried out. See Disinfecting Chambers.
The disinfection of articles of food is accomplished by thorough cooking, boiling in the case of milk, boiling and filtration in the case of water, and complete roasting, stewing, and frying of meat.
The experiments of Mr Crookes (to which reference has been made in the extract taken from Dr Parkes’ ‘Practical Hygiène’) with carbolic acid during the cattle plague possess great practical interest both for the chemist and physiologist.
Of the use of carbolic acid as a disinfectant Mr Crookes, in the Appendix to the Report of the Cattle Plague Commissioners, writes as follows:
“According to the principles laid down, the air must be treated, and where there is no disease there is only a secondary use in treating anything besides the air. Several cowhouses have been treated with carbolic acid with very excellent results. The mode has been, first, to remove from the floor the mass of manure, which too often adheres to it; secondly, to sprinkle the floor with strong carbolic or cresylic acid; next, to wash the walls, beams, and rafters, and all that is visible in the cowhouse, with lime, in which is put some carbolic acid, 1 to 50 of the water used, or with strong carbolic acid alone. Next, to make a solution containing 1 of carbolic or cresylic acid to 100 of water, or, perhaps still better, 60 of water, and to water the yard and fold until the whole place smells strongly of the acid. Only a few farms have been treated in this way, so far as I know, but in each it has been successful. It may be well to give the cattle a little of the weak solution of carbolic acid, but this has not been so fully tried as the external use. The washing of the mouth and of the entire animal with the weak solution may be attended with good results, especially in the early stage of disease; but I know nothing of cure, and speak only hopefully of prevention.
“The animals seem to have an instinct for disinfection, and lick substances touched with this acid. They must not be allowed to drink it, as when strong it blisters the skin, and especially the mouth and tongue.”
Mr Crookes also tried the effect of the acid by injecting it into the veins of the animals, and thus details the results of his experiments:—“It appeared evident that if harm were to follow the injection of carbolic acid, the mischievous effect would be immediate; but that if the fluid could pass through the heart without exerting its paralysing action on that organ, and could get into the circulation, no present ill effects need be anticipated. I therefore determined to push these experiments as far as possible, increasing the quantity of carbolic acid, until it produced a fatal result.
The next operation was on cow No. 11, in which 3 oz. of solution (containing 521⁄2 gr. of pure carbolic acid) were very slowly injected; no bad effect followed. Increasing the dose, cow No. 12 had injected into her vein 41⁄2 oz. of solution (equal to 783⁄4 gr. of carbolic acid); this also was followed by no immediate ill effect. Cow No. 13 was then treated with 6 oz. of solution (containing 105 gr. of pure carbolic acid), in two portions of 3 oz. each, five minutes’ interval elapsing between each injection. The first 3 oz. produced a slight trembling,568 but not so severe as in the case of cow No. 10, as she seemed better in a few minutes. The second dose of 3 oz. was injected. This proved too much, or was pumped in too hurriedly, for almost before I had finished the animal trembled violently, its eyes projected, its breathing became laborious, it fell down and expired. The result could scarcely be attributed to the accidental injection of air into the vein, for the distress began with the injection of the first syringeful, and was only increased by the second; nor is it likely that this accident would happen twice consecutively. I was particularly careful on this point, and the construction of the instrument rendered such an occurrence scarcely possible with ordinary precaution. It is probable that the injection was performed too rapidly, or that the vital powers were lower than usual. In the case of the remaining animal, No. 14, I decided to inject as large a dose as it would bear, stopping the operation at the first sign of trembling, and delivering the liquid very gradually. The first syringeful caused no bad symptoms, and I had just finished injecting the second dose when trembling commenced. It was rather violent for a short time, but soon went off, and in five minutes the animal appeared as well as before.
This cow, therefore, bore without inconvenience the injection of 6 oz. of a 4 per cent. solution, containing 105 gr. of pure carbolic acid. Careful observations with the thermometer were taken before each operation. There were no more diseased beasts on the farm, or I should have carried my experiments still further. On visiting the farm the next day I was told that all the animals seemed better, and on testing them with the thermometer that statement was confirmed. I gave directions that each animal was to be drenched with half a wine-glassful (1 oz.) of carbolic acid in a quart of warm water every morning, but in other respects they might be treated as Mr Tomlinson, a skilful cow doctor, should direct.
“Business now calling me to London, I was unable to watch the further progress of these cases.
“This is to be regretted, as a series of daily thermometric observations would have been of great value in suggesting further experiments. I had, however, frequent accounts sent me. Cow No. 14 continued to improve slowly until convalescent; she is now quite well. Nos. 10, 11, and 12 remained apparently in the same state for four days; they then changed for the worse, and died. It is not improbable that had I been able to inject a further quantity of carbolic acid during the four days in which they were thus hovering between recovery and relapse, it would have turned the scale, and some of them at all events would be now alive and well.
The following table gives the thermometric observations;—
| No. | Grains of Carbolic Acid Injected. | Temperature before Injection | Second Day. | Third Day. | |
| F. | F. | ||||
| 10 | 261⁄2 | 105·4 | 103·8 | Better. | Died on 6th day. |
| 11 | 521⁄2 | 103·8 | 102·8 | ” | Died on 6th day. |
| 12 | 783⁄4 | 104·8 | 104·4 | ” | Died on 6th day. |
| 14 | 105 | 103·7 | 103·1 | ” | Recovered. |
If future experiments prove that injection of carbolic acid or other antiseptic will do good, it is an operation very easily performed. I have injected five animals, and taken thermometric observations within an hour. Sulphite or bisulphite of soda apparently occasions some pain, as the animals struggle very much; with carbolic acid I found them tolerably quiet. I have calculated the proportion which the carbolic acid bore to the whole quantity of blood in these operations. Taking the whole amount of blood in the animal at 150 lbs., there were injected into—
| No. | 10, | 1 part of | carb. acid, in | 40,000 | of blood. |
| ” | 11, | ” | ” | 20,000 | ” |
| ” | 12, | ” | ” | 13,300 | ” |
| ” | 14, | ” | ” | 10,000 | ” |
It is worth mentioning incidentally, that in the case of cow No. 14 (which recovered) the proportion of carbolic acid injected into the blood would have been enough to keep from decomposition the whole quantity of that liquid for a considerable time. In Nos. 10, 11, and 12 the proportion of carbolic acid would probably not have been sufficient for that purpose. I am informed by Dr Calvert that cresylic acid has much less coagulating power on albumen than carbolic acid, and my own experiments entirely confirm this statement.”
We have described under “Charcoal” the disinfecting properties of that substance. These properties have been turned to excellent account by Dr Stenhouse, who has invented a charcoal respirator, which, causing the wearer to breathe air drawn through a layer of that substance, and by thus depriving the air so inspired of any noxious gases or exhalations, if present, becomes, if worn in an infected atmosphere, a great safeguard against disease. Dr Letheby was accustomed to use a charcoal respirator when analysing dead bodies and other putrid matters of suspected poisoning, and by so doing never experienced any ill effects, nor was he conscious569 of the offensive odour which but for its adoption he must have encountered.
Professor Tyndall has suggested for the same purpose a respirator of cotton wool, by means of which the air, being filtered before it enters the lungs, becomes deprived of minute particles of various substances suspended in it, as well as of the germs, which so many pathologists believe to be always present during the prevalence of epidemic maladies, and the cause, when inhaled, of the maladies themselves.
DISINFECTING CHAMBERS. The sanitary authorities of most large cities have made provision for the purification of mattresses, linen, wearing apparel, &c., by means of disinfecting chambers or ovens, in which receptacles the infected articles are subjected for a certain time to hot air. The simplest form of apparatus for this purpose, and one that could be used on an emergency, provided the articles to be disinfected were not too bulky, is a baker’s oven. The drying closet of a good laundry would be so far unsafe, because it would occasionally fail to give a heat sufficient for the destruction of the noxious principles.
The disinfecting chambers employed in Liverpool are arched ovens of solid brickwork, having a depth of 7 feet from front to back, a width of 5 feet from side to side, and a height of 61⁄2 feet from the floor to the crown of the arch. The doors are made of wrought iron, tightly fitting into cast-iron framework. The floors are made of double iron gratings, having alternate openings, so arranged as to admit at pleasure hot air into the chamber. At the top of the arch there is an opening fitted with an iron valve, by which the air of the chamber escapes into an exhausting shaft which is connected with the chimney. The heating is accomplished by means of a cast-iron cockle, the smoke from which escapes by two cast-iron smoke flues, which, after forming a coil for the purpose of affording as great a heating surface as possible, pass along the hot-air passage under the chamber, into a chimney situated at the opposite end.
The cold air is drawn into a brick flue placed underneath the floor of the stokehole into a cavity on each side of the cockle, and thence into a space underneath the chamber, whence it becomes heated by the radiation from the surface of the two cast iron flues. From this cavity or passage it is conveyed at will through the gratings as already described. At the entrance of the cold air flue there is a damper, by which the temperature of the air may be regulated. A heat equal to 280° F. has been registered in this chamber, and as high as 380° in a drying closet over the cockle. Dr French, the medical officer of health for Liverpool, says “that, if necessary, a temperature reaching 500° F. can be attained in these chambers; but this temperature is of course never employed. Experience has proved that from 220° to 250° F. is the most suitable. Instances have been known where fabrics, after being exposed for some length of time to a temperature above 212° F., have sustained injury from being scorched.
In some of the chambers, carbolic acid powder is sprinkled on the floor.
We have taken the liberty of transcribing the following description and plates illustrative of the disinfecting stove used in the Royal Victoria Yard, Deptford, from that very useful publication, ‘Chemistry, Theoretical, Practical, and Analytical,’ published by Mr W. Mackenzie.
“This stove consists of a brick chamber with a slightly arched roof, and an iron movable floor in two pieces. The chamber is 7 feet deep, 6 feet 9 inches wide, and 5 feet 81⁄2 inches high in the centre of the arch. It is heated by a flue below the iron floor passing round 3 sides of the chamber and up a chimney. There is an opening in the upper part of the chamber in its centre, which passes along in the roof to the side, from thence down in the wall entering beneath the fire; this carries away any of the foul air of the clothes from the chamber through the fire and up the flue. This proceeding takes place after the clothes have been in the chamber say an hour and a half in the following manner:—The damper in the foul air shaft is withdrawn, and the furnace door is shut; any draught that gets to the fire comes to the chamber. Over the opening into the furnace is a square opening, fitted with a glass, inside of which is a fixed thermometer. When this shows a temperature of 200° F., the interior of the chamber is at 250° F., the highest point at which it is allowed to be. In the interior of the chamber at the sides there are little movable cranes, three rows of three supporting rods of iron on which wooden trays rest, and on which the clothes are placed when the iron cart is not used. The cranes move fore and aft to be out of the way when the cart is used. The cart is of iron on wheels, and runs into the chamber on tramways to keep it in position; in the interior of the cart are three iron trays for laying the clothes on. The lowest tray is always the hottest, so that it is prudent to use the cart, the iron bottom of which prevents burning. The iron ends of the cart are removed when it is placed in the chamber; so is the handle. It is usual to keep the clothes at the temperature of 250° F. for two hours.
There is a trap door 8 inches square about 14 inches above the upper edge of the furnace, and on a level with the iron floor of the chamber, for disinfectants. Carbolic acid and sulphur are used; the former is placed on a flat plate, the latter is sprinkled over the floor. These are used as the last, and after that has been the clothes are fit to be used without danger to any one.
Elevation plan (fig. 5) shows the front of570 the chamber with the doors closed; the openings (Nos. 1, 2, and 3) are for inserting the long thermometer, which is pushed into the clothing to be disinfected; they correspond with the three trays. The thermometer can be withdrawn and examined without allowing much cold air to enter; plugs fit into these three openings when not used for the thermometer.
Section.—The chamber is shown about the centre of its depth; the foul-air shaft (B) passes along the roof down the side wall, and beneath the fire (C); the opening where the fixed thermometer is placed is marked with dotted lines. The damper for the foul-air shaft (E) is represented as shut, and the damper for the chimney (F) is also shut.
571
The ground plan shows the flue beneath the iron plates, which form the floor of the chamber, the dotted lines showing the foul-air flue (B), as it passes beneath the fire. In the flue (C) there are openings at D, D, D, for the purpose of cleaning it.
Another form of disinfecting chamber is that invented by Dr Esse, of Berlin, and employed in that city. The apparatus consists of two iron cylinders, one fitted within the other, with a space between, into which steam under pressure is introduced. The outer cylinder is surrounded with wood and the top with felt, to prevent the escape of heat. The articles to be disinfected are put in at the top of the inner cylinder, the inside of which soon becomes heated up by the surrounding steam. A pulley is used to lift the lid of the inner cylinder, around which the clothes are hung on pegs, not being allowed to touch the side of the cylinder. At the top of the inside cylinder is a brass box pierced with holes at the bottom, which dips a little way down, through which the air from the interior can rise. In this box the bulb of a thermometer being placed, the temperature of the inner chamber can be registered.
When the steam condenses in the space between the cylinders it is carried off by means of a valve, which is lifted when the water reaches a certain point in the condenser. In an hour’s time the temperature of the interior cylinder can be raised to 235° Fahr.
For heating mattresses another apparatus has been devised by Dr Esse. It consists of an iron case with a spiral steam pipe in the centre, the steam inside the pipe being compressed to two atmospheres.
Dr Ransome has invented, for the use of the Nottingham hospital, a gas stove in the form of an iron box, well packed with a non-conducting material, which surrounds the outside. A channel leads to the interior of the box, and inside this channel gas is kept burning in such a manner by a modification of Kemp’s regulator, that the temperature of the box shall range day and night between 235° and 255° Fahr.
An apparatus put to great use by the Holborn District Board during the epidemic of smallpox in 1871 was one made by Fraser’s patent. Mr Fraser’s disinfecting chamber consists of an oven or receptacle made of brick, with doors in front. Situated on the lower portion of this chamber is a covered furnace connected with flues, by means of which the interior space is heated to the desired temperature. By a particular arrangement the air laden with the noxious vapours given off by the tainted clothing is conveyed into the furnace, and so consumed. Belonging to the apparatus is a covered truck or cart, fitted with doors and dampers, and provided inside with racks and shelves for holding the materials to be purified, which are thus brought from the infected dwelling and placed, truck and all, inside the chamber. The infected materials, as well as the truck containing them, are then heated to the necessary point, disinfection being assisted by sulphurous acid gas, or some other material adapted for the purpose. When the process is finished the carriage with its contents is drawn back to the house from which they were originally taken, and the purified articles are restored to the owners. It will be seen that by this arrangement the vehicle is disinfected as well as the clothes it contains.
DISLOCA′TION. Syn. Luxation; Dislocatio, L. The forcible displacement of a bone from its socket, either by violence or disease. The latter happens when the textures forming the joint have been destroyed by some independent organic affection. “A considerable share of anatomical knowledge is required to detect the nature of these accidents;572 and it is much to be lamented that students neglect to inform themselves sufficiently on the subject.” (Sir A. Cooper.) In common cases the bones may be frequently replaced by forcibly extending the limb. This should be done as early as possible, and before inflammation sets in. The latter should be combated by aperients, local bleeding, refrigerant lotions, &c. Dislocations frequently exist without the fact being suspected, the swelling and inflammation being referred to other causes.
DISPLACE′MENT. See Percolation.
DISTEM′PER. A disease among dogs, usually characterised by a running from the nose and eyes, and a short dry cough; followed by wasting of the flesh, and loss of strength and spirits. At length the brain suffers, and fits, paralysis of the extremities, or convulsions come on. Laxatives and emetics are the best remedies. If there is much diarrhœa, astringents may be afterwards given. The violence of the fits may be mitigated by the administration of antispasmodics, and by the warm bath. The distemper is a contagious disease, and is generally fatal to weakly and very young dogs. Fits in the advanced stages of the disease are seldom followed by recovery. Impatience of light, red eyes, obstinate diarrhœa, spasmodic twitchings, a yellow colour of the skin, and a pustular eruption, are also bad symptoms.
Distemper Powders (Blane’s). The basis of these is said to be ‘aurum musivum,’ or bisulphide of tin. That of another advertised nostrum is a mixture of mercury and chalk, with a little rhubarb and ipecacuanha.
DISTILLA′TION. The evaporation and subsequent condensation of the vapour of fluids, by means of a still and refrigerator, or other similar apparatus. Dry distillation is a term applied to the distillation of substances per se, or without the addition of water or other volatile fluid. Destructive distillation is the distillation of substances at temperatures sufficiently high to decompose them, by which their elements are separated, or evolved in new combinations. Fractional distillation is the separation of substances having different boiling-points, by distilling the mixture with a gradually increasing heat, and collecting the products which come over at different temperatures in separate receivers. See Hydrocarbon, Still, &c.
Distillation. The art of the distiller; the manufacture of spirituous liquors as practised on the large scale.
The process of distillation, as carried on in the distilleries of Great Britain, may be divided into four general operations, viz.—1. The mashing, or formation of a saccharine infusion from certain vegetable matters, as malt, barley, oats, rye, &c. 2. The cooling of this wort or liquor. 3. The fermentation, or process by which the sugar of the cooled wort is converted into alcohol. 4. The separation of the spirit so formed by means of a still and refrigerator. By the first operation the materials for the formation of the alcohol are obtained; by the second, they are brought to a temperature most favorable to the transformation that takes place in the third, after which it only remains to free the product of the last operation from the foreign matter with which it is associated; this is done in the fourth, which, correctly speaking, constitutes the only part of the process which can be called distillation.
The general principles of the first three of the preceding operations are noticed in the articles Brewing, Fermentation, &c. It will there be seen that the amylaceous or starchy matter of the grain is first ‘saccharified,’ and afterwards converted into alcohol, and that certain precautions are necessary to render the process successful and economical. In many of the distilleries of Great Britain molasses and analogous saccharine substances are employed, in which case the vegetable principle (sugar) essential to the formation of alcohol is already present, and merely requires simple solution in water of a proper temperature, to be ready to be subjected to immediate fermentation. In general, however, the sources of spirit in England are the various kinds of grain; barley, rye, maize, and rice are those commonly employed. These are ground and mixed with bruised malt, in various proportions, and are mashed in a similar manner to malted grain. The fermentation is carried on until the density of the liquor ceases to lessen or ‘attenuate,’ which is determined by an instrument called a saccharometer. When this point is arrived at, the ‘wash’ is submitted to distillation, to prevent the access of the acetous fermentation, which would lessen its alcoholic value.
During the process of distilling off the spirit of the fermented ‘wash’ or ‘wort’ a hydrometer is employed to ascertain the ‘strength’ of the liquor that passes over. As soon as this has fallen to a certain point, the operation is stopped, and the ‘spent wash’ removed. The spirits obtained by the first distillation are generally called ‘low wines,’ and have a specific gravity of about ·975. By rectification or ‘doubling,’ a crude milky spirit, abounding in oil, at first comes over, followed by clear spirit, which is received in a separate vessel. The process is continued until the alcoholic content of the distilled liquor has considerably diminished, when the remaining weak spirit that distils over, called ‘faints,’ is caught separately, and mixed with the low wines preparatory to another distillation. The strongest spirit passes over first, and the condensed liquor gradually becomes weaker, until it ceases to contain alcohol. By receiving in separate vessels any given portion of the product, spirit of any required strength, within certain limits, may be obtained. The same object is more conveniently effected by surrounding the top573 of the capital of the still with a water bath, of a temperature corresponding to that of alcoholic vapour of the strength it is desired to obtain. Thus, if we keep the temperature of the water at about 198° Fahr., we shall obtain proof spirit; if at 192°, a spirit 20 o. p.; and so on for other strengths.
It is found from experience, and is readily accounted for by theory, that the lower the temperature at which the distillation is conducted, the stronger will be the product, and the less quantity of oil or other volatile matter will come over along with it. To promote this, it has been proposed to carry on the process in vacuo, but on the large scale this has never been adopted. The distillation of the wash is usually performed in a separate set of stills to those employed for the rectification of the low wines. For very strong and tasteless spirit, a third and even a fourth rectification is employed, conjointly with other methods, to abstract the water and to remove any foreign matter that vitiates its odour or flavour. A portion of soap is generally put into the still with the wash, to prevent excessive frothing.
We have said that the processes of mashing, &c., in the distillery are similar to those adopted in brewing beer. We may add that, as richness in alcohol, and not flavour, is the object aimed at in the distiller’s wash, not only is a large quantity of unmalted grain employed, but the process of boiling the wort with hops is omitted altogether. The wort is commonly ‘set’ at 70° Fahr., and the fermentation and attenuation of the liquor pushed as far as possible by large and repeated doses of the best ‘top-yeast’ of the porter brewers.
It often happens that raw spirit prepared from damaged grain is contaminated with a highly acrid and volatile fatty substance, which is powerfully intoxicating and irritating to the eyes and nostrils, and possesses an odour very similar to that of an alcoholic solution of cyanogen. This may be got rid of by dilution with water and skilful rectification, when most of it passes over with the first and last ‘runnings,’ the intermediate portion being less loaded with it. Another plan is to filter the spirit successively through 6 or 7 separate vessels containing pine or willow charcoal before rectifying it. In some distilleries the contaminated spirit is well agitated with a considerable quantity of olive oil, and after repose decanted, diluted with water, and rectified as before. The ordinary corn oil or fusel oil of raw spirit is generally, for the most part, intercepted by a self-regulating bath arranged between the still-head and the refrigeratory.
The quantity of spirit obtained from various substances, and even from pure sugar, depends upon the skill with which the several operations are conducted. By theory, pure sugar should yield 51% of alcohol; but in practice 11·925 galls. of proof spirit is the largest quantity which has yet been obtained from 112 lbs. of sugar. By the revenue authorities this weight of sugar is estimated to afford 111⁄2 galls. of proof spirit. The average product is, perhaps, about 1 gall. of spirit of this strength for every 10 lbs. of sugar. According to Harmstädt, 100 lbs. of starch yield 35 lbs. of alcohol, or 7·8 galls. of proof spirit; and 100 lbs. of the following grains produce the accompanying quantities by weight of spirit of sp. gr. ·9427, or containing 45% of pure alcohol:—wheat, 40 to 45%; rye, 36 to 42%; barley, 40%; oats, 36%; buckwheat, 40%; maize, 40%; the mean being 3·47 galls. of proof spirit. It is found that a bushel of good malt yields 2 galls. of proof spirit, and that the largest quantity of proof spirit obtained from raw grain, mashed with 1⁄5 or 1⁄6 of malt, does not exceed 22 galls. per quarter.
The distiller is allowed to produce worts from any substance, and at any specific gravity, provided such gravity can be correctly ascertained by the saccharometer approved of by the Board of Inland Revenue. He is not, however, allowed to mash and distil at the same time. See Alcohol, Brandy, Fermentation, Fusel Oil, Gin, Still, &c.
DISTOMATA. A genus of fluke-like parasites infesting men and the higher vertebrate animals. The egg is about the 1⁄280th of an inch long and 1⁄270th inch wide.
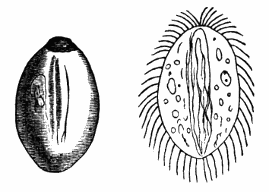
The embryo is frequently met with in sewage water, from which, if it be removed and placed in pure or distilled water, it soon dies. The embryo which does not become a distoma gives rise to a progeny (gradually formed from germ-cells within it) consisting sometimes of one, but much more often of a number of bodies of various forms and structures, each of which possesses powers of movement and locomotion. But the creatures of this second development are not distomata; neither are the offspring to which they in their turn give rise. Like their immediate progenitors, this offspring produce in their interior germ-cells which develop into minute worms having tails, and displaying great vivacity when placed in water. These latter alone exhibit the characters of true distomata. “These cercaria now either become enclosed, like a chrysalis in a pupa state, or they penetrate into the bodies of soft animals, become encysted and parasitic. It appears probable that the distomata enter the human intestinal canal as cercaria, and then pass into the biliary passages.”[260]
[260] Blyth.
574
A case is on record of two distomata having been extracted from the foot of a woman, into which it has been surmised they gained an entrance as cercaria whilst the woman was bathing.
It is thought that shell-fish, as well as uncooked fish when eaten, may be the means of introducing these parasites into the human system. The embryos of the Distomata hepaticum swim about and live in water, which may probably, when drunk, be the means of conveying them into the bodies of men and sheep. The ailments and symptoms to which these pests give rise depend upon the particular organ or portion of the body in which they establish themselves.
In man they are a frequent cause of hæmaturia and dysentery. In sheep they occasion fearful mortality, giving rise to the disease known as ‘the rot,’ and killing thousands of flocks annually.
“The number of species affecting men are usually enumerated as nine—viz. Fasciola hepatica, Distoma crassum, D. lanceolatum, D. ophthalmobium, D. heterophyes, Bilharzia hæmatopia, Tetrastoma renale, Hexathrydium renarum, and H. pinguicola.”[261]
[261] Blyth.
DISTORTIONS. In treating of this subject we shall confine ourselves to those distortions which are preventable—or rather, we may say, of two out of the three which will be discussed, which are voluntary.
One very common form of bodily distortion is crooked or curved spine. It is mostly met with in young girls of from ten to sixteen years of age; and first shows itself either in the elevation of one shoulder above the other, or in a growing out of one of the shoulder blades, or of one side of the bosom beyond the other. The elevated shoulder is generally the right one. At the same time the right side of the chest is unnaturally high, and rounded; whilst the opposite or left shoulder and chest are on the contrary depressed and concave. Very frequently these conditions are accompanied by a projection of the left hip, and a curving inwards of the loins on the right side. With persons so afflicted the spine presents an appearance that has not inaptly been compared to a long italic f.
Spinal curvature arises from a weakened state of the muscles, ligaments, and bones of the backbone. It is most frequently met with in those whose occupation compels them to stand the greater part of the day; as well as in persons who pass many hours at the desk or at needlework. Spinal curvature is also common in young fragile girls acting as nursemaids, and as such unduly subjected to carrying heavy infants on one side. Amongst the children of the poor, those of tender years are much too frequently put to this objectionable form of drudgery. Any one’s recollections of a walk through a poor neighbourhood will enable them to call to mind many instances they must have seen of smaller babes being nursed by larger ones. Those subjected to too long standing, no doubt because the posture affords them relief, unconsciously contract the habit of frequently standing on the right leg—of standing at ease on it, as it is called—and at the same time of bending the left knee a little; and since this position causes the right shoulder to be raised, and the left side of the pelvis to be thrown out of its place, it will be evident from what has been already said that, if persisted in, it will end in distorting the spine in the manner above indicated.
The same results will also follow in those other cases, such for instance as in too long an application at the desk or at the needle, as well as the carrying for an undue length of time a heavy child in the arms; these all being occupations in which one side of the body is subjected to an undue and unequal strain over the other.
“Why one-sided postures should cause distortion must be evident, when it is considered that the intervertebral substance is compressible to such an extent that an adult man of middle stature loses about an inch of his height after having been in the erect posture during the day, and does not regain it till after some hours of rest. Since the united thickness of the intervertebral substance in an adult man is about 3·875 inches, we see that they lose nearly one fourth by compression, which they do not recover till after some hours of rest. But if the weight of the body falls unequally on the spine day after day, it must be evident that they will become compressed on one side more than the other; and that if their elasticity be impaired, and the muscles and ligaments be weak, and the bones soft, as they are in young persons who have not a sufficiency of fresh air, wholesome food and active exercise, this lateral distortion will become permanent.[262]
[262] Dr Druitt.
Another cause tending to distortion of the575 spine is the foolish habit of using corsets, a practice which contributes to weaken the dorsal muscles. When the shoulders are continually supported by a corset, the dorsal muscles upon which the support ought to fall have their functions usurped by the corset, and hence fail to receive their proper development, and consequently lose their power; the result being an inability on the part of the body to support itself without the corset, and a sinking and bending of the spine when it is removed. In boys, who never wear corsets, spinal curvature is rarely met with. In girls, who do, it is constantly to be found. To guard against spinal distortions, bad and awkward positions of the body should, wherever possible, be prohibited. Amongst the prejudicial postures indulged in by the young, we have already mentioned the habit of standing on one leg and of carrying heavy loads on one side of the body.
To these may be added the habit of lying crooked in bed, and that of young girls spending a long time in a constrained position in dressing their own hair. Every one-sided motion may lead to distortion if it be frequently repeated, and the tendency once existing, the evil grows day by day. The use of corsets should be strenuously discountenanced. The early detection of spinal distortion is a matter of considerable importance. Hence the advisability of mothers, nurses, governesses, and other guardians of children or young girls, frequently examining the bodies of their charges to note if they present any of the peculiarities we have indicated at the commencement of this article. Should any of these develop themselves, aid should immediately be sought of a skilful medical practitioner.
Dr Lewis Sayre, in his work ‘Spinal Disease and Spinal Curvature’ says:—“The great object in the treatment of Pott’s disease is to maintain rest of the affected part by such means as will not debar the patient from the benefits of fresh air, sunlight, and change of scene. The patient should not be permitted to assume the upright position before he has been fitted with some artificial support capable of removing all pressure from the bodies of the diseased vertebræ. This object may be obtained by straightening the spinal column in such a manner that the weight of the body is borne by the transverse processes and not by the bodies of the vertebræ.” Acting on these principles, Dr Sayre partially envelopes the patient in a jacket of plaster of Paris, surrounding the body from the pelvis to the axillæ.
Although Dr Sayre’s work is almost entirely devoted to a much more serious affection of spinal curvature than that treated of here—viz. posterior angular curvature, in which actual disease of the bones of the vertebræ is concerned—his treatment is no less applicable to the milder form of distortion to which our remarks have been directed. Dr Sayre himself states that 300 cases have been treated by his method with very signal success, and very many eminent surgeons bear testimony to the soundness of the principles concerned in it. For the details of its application consult the author’s work before alluded to.
Serious as are the effects very frequently arising from spinal curvature, amongst which may be included lameness, lung disease, and inability to perform the functions of maternity; still worse results in addition to the two last of these ensue in the case of a persistence in another form of distortion, which is none the less dangerous because it is voluntary. The distortion to which we refer is that caused by the practice of tightlacing.
Foremost among the conditions absolutely essential for the preservation of health and bodily well-being, is the due performance of the function of the lungs, heart, liver, kidneys, stomach and other important organs. The object of the ribs within which most of these organs are more or less wholly contained is to protect these latter from external pressure, and therefore injury; as well as to allow them unimpeded and unrestricted action. To ensure this freedom of movement for the parts and organs within the ribs, it will be evident that every possible obstacle tending in any degree to compress them, or circumscribe their limits should be especially avoided.
Instead of the avoidance of such dangers, however, what course do the silly votaries and dupes of that most senseless and remorseless of all tyrants—Fashion—pursue? One the very reverse; and which is opposed, not only to personal comfort and common sense, but, since it mars nature’s outlines, to symmetry and our proper canons of the grace of the female figure. By means of corsets, tight stays, and other implements of torture the ribs are pressed inwards to such an extent that all the conditions we have insisted on as essential to health are imperilled, and eventually become overthrown. Now, this mischievous and unnatural pressure exerted on the stomach pushes that organ out of its proper position, and in doing so forces the diaphragm also out of its place; a disturbance which so curtails the space in which the movements of the lungs and the heart are performed, that if the pernicious custom be persevered in these latter organs become seriously and incurably diseased. The liver also shares in the damage inflicted, and frequently becomes incapable of discharging its office. The very much larger number of young women than of young men who die of consumption is undoubtedly referable to the fact that a large proportion of the majority are the victims of tightlacing. Nor is it difficult to understand why this should be, since we know that if the lungs are prevented exercising their full powers of expansion, unnaturally diminished function will set up disease in them, which, if there be a predisposition, will probably be consumption. This cause also, by preventing the blood becoming properly oxygenated, gives576 rise to a large class of disorders due to impurity of the vital fluid. Organic disease of the heart is by no means an uncommon contingency if tightlacing be persevered in; for that organ is not allowed room to beat, nor the blood to circulate. One effect of this is seen in frequent fainting fits.
Again, tightlacing not infrequently stops the growth and arrests the development of a young girl’s mammæ, thus seriously incapacitating her from suckling her babe when she becomes a mother. It also indirectly has a very prejudicial effect upon health by preventing its votaries from taking sufficient walking exercise; free bodily movement with accompanying expansion of the lungs becomes impossible with those encased in a vice of unyielding armour, such as constitutes pestilent stays and corsets. Amongst the minor evils wrought by the baleful custom, we may mention indigestion (for the pressure of the stays weakens the stomach, and sets up this troublesome complaint), with its accompaniments of flatulence, heartburn, pain in the chest, &c. Constipation is also another of its attendant ills; so also are bad breath and a red nose.
“I recollect Dr A. Todd Thomson, in his excellent lectures, relating a case he had attended where a young lady appeared to be dying from the evil effects of tightlacing. He cut open her stays and she gradually came to herself. If the worthy doctor had not quickly done what he did, she would soon have been a corpse! Dr Thomson has kindly favoured me with the following interesting particulars of the case for publication:[263]—‘Some years since I was requested to hasten to a house not far from my own to see a lady who had fallen from her chair in a fit whilst eating her dinner. On being ushered to the drawing room of the house where the circumstance had taken place, I saw a lady lying upon a sofa, apparently dead, and several ladies hanging over the couch in great distress. I found little appearance of life except that the temperature of the body was natural; the pulse had ceased to beat, and no respiratory action could be detected. On laying my hand over the region of the heart, I felt that the stays were extremely tightly laced; and conceiving that the suspension of animation arose from that cause, I requested a penknife to be given me, with which I instantly ripped down the stays and gown. In an instant the chest dilated, on the binding matter giving way, which was almost like splitting an overbraced drum; and in a few seconds respiration recommenced and animation returned. In this case the waist was drawn in to a degree that gave a complete hour-glass appearance to the figure, and prevented the descent of the diaphragm, whilst the blood could not circulate, or be renewed in the lungs from the general obstruction of many of the cells and smaller tubes. The quantity of residual air also in the lungs was too small; and this was still diminished by the warmth of some soup, which the lady was eating when she fell from the chair, dilating the gas in her stomach, and consequently pressing that enlarged organ upwards on the lungs. Had I not lived close by, the time necessary to get medical aid from a greater distance might have rendered it unavailable.’ The above narrative by Dr Thomson is valuable not only as illustrating the dangers arising from tightlacing, but also as emphasizing the rationale of its action as stated by ourselves. In the present article we have explained why it is the use of corsets is to be deprecated. We hope we have succeeded in showing how imperatively the abandonment of stays is called for.
[263] Dr Chavasse, ‘Counsels to a Mother.’
Another variety of distortion is that brought about by wearing tight boots and shoes, or boots and shoes constructed upon false principles; for, a boot or shoe may be productive of considerable inconvenience to the wearer, as well as the cause of a certain amount of twisting out of place of the bones of the foot, without necessarily being too small. Amongst the consequences arising from the adoption of tightly fitting or badly constructed boots or shoes may be mentioned the following:—Considerable bodily discomfort, and pain in walking; corns and bunions; growing in of the nails; chronic enlargement of the base of the great toe; caries or ulceration of the bones of the feet; and flat feet. That these are not altogether minor evils may be inferred when it is stated that, in order to obtain relief from the effects of a bunion, partial amputation of the foot has been sometimes found necessary; that the first attacks of gout mostly seize the joint of the ball of the great toe when that joint has become weakened by displacement following the use of faulty boots and shoes; and that a flat foot interferes with the proper performance of walking.
The above figure (No. 1) represents the skeleton of the foot with the bones which form it in their natural position, and in which they are admirably adapted for executing the various movements required of them.
577
It will be seen to consist of twenty-six bones, fourteen of which constitute the toes; the remaining twelve bones enter into the formation of what are termed the tarsus and metatarsus.
The five long bones (a) are the metatarsal bones. The toes form joints with the fore part of these metatarsal bones. The remaining seven are the tarsal bones; b, which is one of these, is named the astragalus, and being gripped on each side by a continuation from the bones of the leg called the malleolus, thus forms the ankle-joint.
 Fig. 2.
Fig. 2.
Fig. 2 gives a representation of the inner aspect and side view of the foot. It will be seen that it is an arch resting in front on the anterior heads of the five metatarsal bones, a, but chiefly on that of the great toe, and on the calcaneum or heel (b) behind.
The astragalus, c, forms the key-stone of the arch. This arch, which supports the superincumbent weight of the body, retains its curved form by means of strong ligaments or bands, which unite the bones which compose it into a compact but withal flexible mass. The arch, owing to the pressure thrown upon it from above, becomes flattened when the foot is resting on the ground; but when this pressure is removed and the foot hangs free, the curvature of the arch increases. In front of the metatarsal bones are placed the toes, which are connected with the metatarsal bones by joints. The great toe has one joint; each of the smaller ones has two.
 Fig. 3.
Fig. 3.
Fig. 3 depicts the skeleton of a foot with the bones thrown out of their natural position, the contortion being the result of wearing tightly fitting or unscientifically constructed boots or shoes. The following extracts from Dr Hermann Meyer will best illustrate how nature’s simple mechanical arrangements must be thwarted when coverings for the feet are permitted to be constructed which can give rise to distortions such as those represented in Figure 3. Dr Meyer says “the great toe plays by far the most important part in walking; because when the foot is raised from the ground with the intention of throwing it forwards, we first raise the heel, then rest for a second on the great toe, and in lifting this from the ground the point of it receives a pressure which impels the body forwards. Thus, in raising the foot the whole of the sole is gradually, as it were, ‘unrolled’ up to the point of the great toe, which again receives an impetus by contact with the ground.
 Fig. 4.
Fig. 4.
 Fig. 5.
Fig. 5.
The great toe ought, therefore, to have such a position as will admit of its being unrolled in the manner described; that is to say, it must so lie that the line of its axis, when, carried backwards, will emerge at the centre of the heel; and this is its position in the healthy foot. The sole of an almost sound foot is given in Fig. 4, and the true position of the great toe is indicated by the dotted line. This relation is still better brought out in Fig. 5, which represents the well-preserved foot of a child about two years old. The line drawn through both figures is that in which the foot unrolls itself from the ground. The smaller toes, however, are by no means without their uses. In standing they rest on the ground and give lateral support to the foot;578 while in walking they are bent in a peculiar manner, so that they are firmly pressed against the ground; and here too they support the foot laterally. The first joint is strongly bent upwards, while the second is hollow above. This peculiar curvature enables the toe in a measure to lay hold of the ground as with bird’s claws.”
Dr Meyer then proceeds to show how the application of these principles is entirely disregarded in the manufacture of our boots and shoes, and to demonstrate that their neglect gives rise to the objectionable consequences we have before alluded to. As boots and shoes are at present constructed, the foot is made to adapt itself to the sole, not the sole to the foot. This pernicious system must be abandoned if we wish to preserve our feet, as well as our personal comfort.
“A sole,” says Dr Meyer, “is of the proper construction when a line (see Fig. 6, c d) drawn at half the breadth of the great toe distant from, and parallel to, the inner margin of that toe shall, when carried backwards, pass through the centre of the heel. In the usual form of a sole this line passes out of the inner margin of the heel (see Fig. 7). If, then, the preservation of the primary straight line is, as has been already shown, the principal point in the formation of a proper sole, it follows that, if it be thought desirable to have pointed shoes, the pointing must be effected from the outer side, as indicated in the annexed Fig. 8. In a pair of shoes made on these principles, placed side by side with the heels in contact, the inner margins of the front part of the foot are also brought close together” (Fig. 9).
Dr Meyer’s pamphlet contains the following strictures on ‘High heels’ to boots and shoes: “It is usual, in all shoes of even moderate strength, to make the heel a little higher by means of what is called the heel-piece. These heel-pieces are generally of some little use, especially in dirty weather, and we cannot wholly deny their right to existence. But at the same time they ought to be as low as possible, and heels an inch thick, as is at present very commonly the case, have very serious disadvantages indeed.
“The weight of the body is by this means thrown in a disproportionate ratio on the toes, the joints of which are consequently overstrained. Moreover, with a high heel the sole is so oblique in its direction that the foot must be constantly gliding forwards and forcibly pressing the toes into the point of the shoe. The toes, therefore, even when the shoe is sufficiently long, are subjected to the same injuries and disfigurations as if it were too short, and the effects are doubly hurtful when the form of the sole is also incorrect. High heels, especially if they are also very small; are peculiarly liable to wear obliquely, and so the shoe gets trodden on one side; they must, therefore, be peculiarly favorable to origin of flat-foot.
 Fig. 6. and Fig. 7.
Fig. 6. and Fig. 7.
 Fig. 8. and Fig. 9.
Fig. 8. and Fig. 9.
High and small heels are therefore quite unsuitable. The heel-piece ought to be as low and broad as possible.”
Further and more explicit knowledge on this subject may be obtained from Dr Meyer’s579 excellent little pamphlet entitled ‘Procrustes ante portas,’ very ably translated into English by Mr J. T. Craig, L.R.C.E., under the title of ‘Why the Shoe Pinches.’
DIURE′SIS. See Urine.
DIURET′ICS. Syn. Diuretica. Medicines which promote the secretion of urine. The principal diuretics are—aqueous fluids, which act by increasing the watery portion of the blood, and—substances which promote the action of the kidneys. Most of the first produce copious diuresis if the skin is kept cool. Among the last are acetate, bitartrate, and nitrate of potassa; oils of juniper, turpentine, cajeput, and copaiba; dilute spirit, and sweet spirits of nitre; decoction of common broom, &c.
DIVIDIVI. An astringent substance imported from Jamaica. It contains above 5% of tannin; whilst gall-nuts contain less than 3·5%, and the best oak-bark only 1·35%. Hence its value in tanning.
DOBEREINER’S LAMP. A portable apparatus for obtaining instantaneous light by the action of a jet of hydrogen on a small piece of spongy platinum.
DOCHMIUS DUODENALIS. An intestinal parasitic worm. Its length is from 1⁄3 to 1⁄2 an inch and its breadth about 1⁄60th of an inch. It is furnished with hooklets. It is found in the duodenum, the ileum, and the jejunum of man, and Greisinger seems to have pretty conclusively established that it is the cause of the disease so prevalent in Egypt, and known as the Egyptian chlorosis. Anemia, dysentery, and hemorrhoids and liver diseases are also frequently caused by it amongst the natives of Arabia, Brazil, and Northern Italy. In India it is also stated to give rise to some very alarming maladies. Leuchart affirms that it obtains an entrance into the system through drinking impure water.
DOC′IMACY or DOCIMAS′TIC ART. See Assaying.
DOG. The effect of medicines on dogs is much the same as on man; but there are some striking exceptions to this rule. Thus, whilst the dog can take a dose of aloes six or eight times as large as that given to man, the administration of half as much calomel or oil of turpentine would be productive of serious injury to the animal. The idea usually entertained, therefore, that medicines may be given to dogs in doses equalling those taken by man requires considerable modification. Dogs have a short and straight alimentary canal, in consequence of which purgative medicines act more quickly upon them than they do on other veterinary subjects. The facility with which dogs can be made to vomit is also another peculiarity possessed by them. Vomiting may be produced by their swallowing nauseous or unpalatable matters, as well as from their eating various sorts of grass. A good plan to prevent dogs vomiting their medicines is to keep the head well raised for an hour after the administration; and this may be easily accomplished by attaching a chain or cord to the collar, and fixing it at the requisite height, to any object. The kidneys are acted upon with much more difficulty than with the horse, whilst the skin seems nearly, if not altogether incapable of being affected. We give below a list of medicines for dogs; premising that the doses required vary considerably according to the strength, size, and age of the dog, all of which should always be duly taken into account. The doses prescribed in the following formulæ are for moderately large dogs:—
Physic Balls and other Purgative Medicines:—
1. Barbadoes aloes, 8 oz., antimonial powder, 1 oz., ginger, 1 oz., palm oil, 5 oz.; beat together into a mass.—Dose. From 1⁄2 dr. to 2 dr. every 4 or 6 hours, till the bowels are relieved. (Youatt.)
2. The same, with the addition of 1 oz. of calomel. He directs from 45 gr. to 2 dr. for a dose. (Clater.)
3. Aloes, 1⁄2 dr. to 2 dr. made into a ball with syrup of ginger.
4. Aloes, 1⁄2 dr. to 11⁄2 dr., calomel, 2 to 5 gr., syrup to form a ball; in inflammation of the bowels, and in worms. (Blaine.)
5. Cape aloes, 1⁄2 dr. to 1 dr., calomel, 2 to 3 gr., oil of caraway, 6 drops, syrup to form a ball. (M′Ewen.)
6. Calomel, 12 gr., aloes, 3 dr., opium, 1 gr., syrup q. s. to form a mass, for 4, 6, or 8 balls; one every 4 or 5 hours till the bowels are relieved. (Blaine.)
7. Croton oil, 1 drop, Castile soap, 20 gr., conserve to form a ball.
8. Castor oil, 3 parts, syrup of buckthorn, 2 parts, syrup of poppies, 1 part.—Dose. From 1 to 2 tablespoonfuls.—Mr Youatt’s purge. [Mr Clark says syrup of buckthorn for dogs should be made with treacle, and the spices omitted.]
9. Epsom salts, from 1 to 4 dr., wrapped in tissue paper, dividing the doses into convenient-sized packets.
10. In costiveness with inflammation: 1⁄2 oz. to 2 oz. castor oil. (Mr Spooner.)
Alterative Balls and Powders:—
1. Sulphur, 21⁄2 lb.; nitre, 1⁄2 lb.; Æthiops mineral, 4 oz.; linseed meal, 1⁄2 lb.; palm oil, 1 lb., or as much as may be required; beat together, and keep in a jar for use.—Dose, from 2 scruples to 11⁄2 or 2 dr. (Clater.)
2. Ethiops mineral, 20 to 40 gr.; cream of tartar, 20 to 40 gr.; nitre, 5 to 10 gr.; night and morning, made into a ball with butter. (Spooner.)
3. Tonic Alterative. Mercurial pill, 1 dr.; aloes 2 dr.; myrrh, benzoin, balsam of Peru,580 of each 11⁄2 dr.; to be divided into 10, 15, or 20 pills: one every evening, for the yellows, after aloes and calomel. (Blaine.)
4. Alterative Powder. Æthiops mineral, 2 to 5 gr.; cream of tartar, 4 to 10 gr., tartarised iron, 1 to 3 gr., once a day. (Clater.)
5. To give a fine skin. Give a table-spoonful of tar made up with oatmeal. (Mayer.)
Astringent Balls, &c.:—
1. Catechu, 11⁄2 dr.; sulphate of quinine, 20 gr.; opium, 5 gr.; ginger, 1 dr.; conserve of roses, q. s. to form a mass, to be divided into 8, 6, or 4 balls. (Blaine.)
2. Prepared chalk, 2 oz.; powdered gum Arabic, 1⁄2 oz.; powdered catechu, 1⁄2 oz.; powdered oak bark, 1⁄2 oz.; powdered ginger, 1⁄4 oz.; opium, 15 gr.; palm oil, 1 oz.; beat well together.—Dose, 1⁄2 dr. to 2 dr., morning, noon, and night, in the advanced stage of distemper. (Clater.)
3. Opium, 5 gr.; catechu, 2 dr.; gum Arabic, 2 dr.; ginger, 1⁄2 dr.; syrup of poppies, q. s.; divide into 12, 9, or 6 balls: in diarrhœa. (Blaine.)
4. Myrrh, 1 dr.; ipecacuanha, 1 scruple; opium, 3 gr.; chalk, 2 dr.; carbonate of iron, 1 dr.; as No. 3. (Blaine.)
5. In obstinate cases: Alum, 1 dr.; chalk, 2 dr.; opium, 6 gr.; resin, 3 dr.; into 4, 6, or 8 balls.
6. In diarrhœa, after 1 to 4 dr. of Epsom salts; prepared chalk, 1 to 3 scruples; catechu, 5 to 10 gr.; opium, 1⁄4 to 2 gr.; twice a day. (Spooner.)
Cough Balls in Asthma, &c.:—
1. After a few emetics. Calomel, 3 gr.; foxglove, 3 gr.; cream of tartar, 1 dr.; antimonial powder, 12 gr.; honey to form 6 boluses. One twice a day. (Blaine.)
2. Digitalis, 20 gr.; antimonial powder, 40 gr.; nitre, 2 dr.; sulphur, 3 dr.; palm oil, 3 dr., or q. s. Divide into 10, 15, or 20 balls, according to the size of the dog, morning and night, interposing an emetic every third or fourth day. (Clater.)
3. In old cases. P. squill, 1⁄2 gr. to 1 gr.; gum ammoniac, 5 gr.; balsam of Peru, 8 gr.; benzoic acid, 1 gr.; balsam of sulphur to form a ball.
4. Extract of hemlock, 1⁄2 dr.; extract of henbane, 10 gr.; p. digitalis, 20 gr.; conserve of roses to form a mass. Divide into 8, 10, or 6 balls. One night and morning. (Blaine.)
Distemper Medicines:—
1. Turbeth mineral, 1 to 3 gr.; assafœtida, 1⁄2 dr.; aloes, 20 gr.; soap, 10 gr.; syrup of poppies to form a ball. To be preceded by an emetic, and given every third day.
2. After an emetic, give a physic ball; and afterwards the following, two or three times a day:—Antimonial powder, 2, 3, or 4 gr.; nitre, 5, 10, or 15 gr.; ipecacuanha, 2, 3, or 4 gr.; form a ball. If the disease proceed to the debilitating stage, give the Tonic Ball No. 2; in the putrid or malignant stage give the Astringent Ball No. 1. (Blaine.)
3. After the Emetic Powder No. 1 (which should be repeated every 3rd or 4th day) give the Cough Ball No. 2, from 1⁄2 dr. to 2 dr. in weight. And if the dog lose flesh, give equal parts of the cough ball and the Tonic Ball (No. 1). In the more advanced stages give the tonic alone; or the astringent ball if diarrhœa comes on. (Clater.)
4. Give a third of a paper of James’s powder mixed with butter, and afterwards warm broth or milk. In 2 hours, another third; and if this neither vomit nor purge, give the other third at the end of 4 hours. (Daniel.)
5. Blaine’s distemper powders, which are sold in packets, with directions for use.
6. Camphor, 3 to 5 gr.; charcoal, 10 gr.; opium, 1 gr.; aromatic confection, q. s. to form a ball.—In the malignant stage, with diarrhœa.
7. Antimomal powder, 2 to 4 gr.; nitre, 5 to 10 gr.; digitalis, 1⁄4 to 2 gr. Afterwards the Tonic Pills No. 4. (Spooner.)
Poudre Kusique: a French nostrum. Mix 45 gr. of nitre, 45 of sulphur, and 1 charcoal. Divide into 3 doses. Give 1 for 2 successive mornings, and the third on the 4th morning, mixed with lard or butter, or in milk. For a large dog a second packet (of 3 powders) may be required. (Habert.)
Another French nostrum. Hemel’s Powder is of a similar kind.
8. A strong solution of salt, to the amount of 1⁄2 pint daily.
9. Powdered tin, sulphur, gunpowder, of each 1 oz.; lard sufficient to form a mass. The size of a nutmeg to be given twice or thrice a week.
10. Physic Ball No. 11.
11. 1⁄4 oz. to 1 fl. oz. of cod-liver oil twice a day, according to size.
12. Emetics, gentle laxatives, milk diet, and from 5 to 15 gr. of chlorate of potash twice a day. (Finlay Dun.)
Worm Medicines:—
1. Carbonate of iron, 1⁄2 oz.; Æthiops mineral, 1 dr.; gentian, 1 oz.; ginger, 1⁄2 oz.; levigated glass, 1 oz.; palm oil, 9 dr.; beat well together.—Dose, from 3⁄4 to 2 dr. (Clater.)
2. As much very finely-powdered glass as will lie on a sixpence, mixed with butter (Blaine). Mr Youatt says from 1⁄2 dr. to 1 dr.; powdered glass, with a little ginger, made into a ball with lard.
3. Aloes, sulphur, prepared hartshorn, and juice of wormwood, made into a mass; the size of a hazel nut to be given three times a week, fasting, wrapped in butter. (Daniel.)
4. Tin filings, or pewter filings, 1⁄2 dr. to 1 dr., with butter or lard.
5. Jalap, 10 to 15 gr.; calomel, 2 to 3 gr. mixed with butter; no cold liquid should be allowed. (White.)
6. Cowhage, 1⁄2 dr.; iron filings, 4 dr.; conserve q. s. to form a mass, to be divided into581 4, 6, or 8 balls; one every night and morning; and afterwards the purgative No. 4. (Blaine.)
7. Epsom salts, 1 oz.; common salt, 1 dr.; give a small or large teaspoonful daily.
8. Give green walnut leaves boiled in milk. (Mayer.)
9. From 1⁄2 dr. to 2 dr., according to size. Betel nut in coarse powder, made into a ball.
10. For Tapeworm.—Oil of turpentine, 1⁄2 dr., mixed with yolk of egg; for very large dogs, 2 scruples. Some writers prescribe larger doses (1 to 2 dr.), but these sometimes prove fatal. (Blaine.) 2 to 6 dr. of cusso according to size.
11. For Tapeworm.—Oil of turpentine and olive oil, of each 1⁄2 oz.; mix, and give carefully; 3 or 4 hours after give 1 oz. castor oil. But see No. 9. (White.)
12. For Stomach Worms.—Give the emetic powder (see further back) and afterwards a physic ball.
13. Threadworms.—These are destroyed by an aloetic clyster.
Ointments and Lotions for the Mange:—
N.B.—An alterative ball should be given daily and a physic ball occasionally. Bleeding is sometimes prescribed.
For Scabby Mange.—Sulphur, 4 oz.; sal ammoniac, 1⁄2 oz.; aloes, 1 dr.; Venice turpentine, 1⁄2 oz.; lard, 6 oz.; mix. After four applications, wash well with soap and water. (Blaine.)
2. Horse turpentine and palm oil, each 1⁄2 lb.; train oil, 1⁄2 pint. Melt together, and while cooling, stir in 3 lbs. of flowers of sulphur. (Clater.)
3. Aloes, 2 dr.; hellebore, 1⁄2 oz.; sulphur, 4 oz.; lard or train oil, 6 oz. (McEwen.)
4. Sulphate of zinc, 1 dr.; snuff, 1⁄2 oz.; white hellebore, 1⁄2 oz.; sulphur, 4 oz.; aloes, 1⁄4 oz.; soft soap, 6 oz. (Blaine.)
5. Charcoal powder, 2 oz.; sulphur, 4 oz.; salt of tartar, 1 dr.; Venice turpentine, 1⁄2 oz.; lard, 6 oz.
6. For Red Mange.—Add 1 oz. of strong mercurial ointment to 6 oz. of either of the above.
7. Charcoal, 1 oz.; chalk, 1 oz.; sugar of lead, 1 dr.; white precipitate, 2 dr.; sulphur, 2 oz.; lard, 5 oz. (Blaine.)
8. Wash for Red Mange.—Corrosive sublimate, 20 gr.; spirit of wine, 2 dr.; dissolve and add milk of sulphur, 1⁄2 oz.; lime-water, 1⁄2 pint. Apply by means of a sponge. (Clater.)
9. For Ulcerated Mange.—Ointment of nitrated quicksilver, 2 dr.; sugar of lead, 20 gr.; flowers of sulphur, 1⁄2 oz.; lard, 1 oz.; mix. (Blaine.)
Fleas:—
1. Rub the skin with the powdered resin and bran.
2. Let the dog sleep on deal shavings.
3. Scotch snuff steeped in gin. (Meyer.) (This requires caution.)
4. Oil of aniseed. (Finlay Dun.)
5. Persian insect powder.
DOG-BALLS (A. H. Bôldt, Genf). Hard pills, weighing 15 grammes, of irregular shape and unequal size, composed of aloes with 1⁄3 of gentian, and covered with a brown powder containing liquorice root. (Hager.)
DOORS. Much annoyance is sometimes experienced from the creaking of doors. This may be prevented by rubbing a little soap or a mixture of tallow and black-lead on the hinges; or by applying to them with a feather a little sweet oil once or twice a year. The trifling trouble and expense (a penny or two a twelvemonth) will be amply repaid by their noiselessness and greater durability. To prevent the noise of doors slamming, a small piece of vulcanised india rubber, cork, or leather may be placed so as to receive the shock.
DOSE. In medicine the quantity taken or prescribed at one time. The doses of medicaments vary with the sex, age, temperament, constitutional strength, habituation, and idiosyncrasies of individuals. Different circumstances, especially of climate, exercise an important influence on the activity of medicines. Thus, the inhabitants of England and the northern countries of Europe bear much larger doses in their own climates than when they remove to warmer latitudes. Warmth, indeed, appears to promote the action of most medicaments, whilst cold acts in a contrary way. Nor does the same rule apply to all medicines. Calomel, for instance, is generally borne better by children than by adults; while opium affects them more powerfully, and requires the dose to be diminished considerably below that indicated by mere calculation or analogy with other medicines.
Prescribers ought not to forget that the action of medicines is not simply proportioned to the amount, but that each remedy has a dose below which it either produces no effect or one contrary to that which we desire it to produce. Dr Paris remarks, “that powerful doses are disposed to produce local rather than general effects;” and Dr Barlow gives it as his opinion that “practitioners often err, especially in the treatment of chronic maladies, from requiring an obvious effect from each dose administered.” Adult women are said to require only three fourths the full dose for men. The following rules and tables have been framed chiefly with reference to age; but, as Dr R. E. Griffith correctly observes, “no scheme can be devised, founded on age alone, to which there are not many exceptions.”
For children under 12 years, the doses of most medicines must be diminished in the proportion of the age to the age increased by 12. Thus, at 2 years, the dose will be 1-7th of that for an adult.
582
For an adult, suppose the dose to be 1, or 1 drachm (60 grains).
| Under | 1 | year | will require | 1⁄12 or 5 grains. |
| ” | 2 | years | ” | 1⁄8 or 8 grains. |
| ” | 3 | ” | ” | 1⁄6 or 10 grains. |
| ” | 4 | ” | ” | 1⁄4 or 15 grains. |
| ” | 7 | ” | ” | 1⁄3 or 1 scruple. |
| ” | 14 | ” | ” | 1⁄2 or 1⁄2 drachm. |
| ” | 20 | ” | ” | 2⁄3 or 2 scruples. |
| ” | 21 | to 60, the full dose, or 1 or 1 drachm. | ||
| Above this age an inverse gradation must be observed. | ||||
| Age— | Years | 80 | 65 | 50 | 25-40 20 | 16 | 12 | 8 | 5 | 2 |
| Doses | 5⁄8 | 3⁄4 | 7⁄8 | 1 | 7⁄8 | 3⁄4 | 5⁄8 1⁄2 | 3⁄8 | 1⁄4 | |
| ” | Months | 12 | 6 | 2 | 1 | |||||
| Doses | 1⁄5 | 1⁄8 | 1⁄15 | 1⁄24 |
DOUCHE. [Fr.] Syn. Douche bath. A species of bath much employed by hydropathists, both for the relief of local affections, and to give a healthy stimulus to the whole system. The douche consists of a single jet of cold water, varying in size from the thickness of a quill to that of a man’s arm; it is projected with great force, either from above, below, or on one side, upon a particular part of the body. See Bath (Shower).
DOUGLAS′ DISINFECTING POWDER. A mixture of sulphite of calcium, chalk, and carbolic acid, or of sulphite and carbonate of lime.
DOVER’S POWDERS. See Powder.
DRAB DYE. 1. (For cotton.) For 40 lbs. Boil 6 lbs. of fustic; scald 21⁄2 lbs. of Lima wood and 2 lbs. of sumach. Decant into a wooden vessel, capable of containing 100 gallons; reduce with cold water to handling heat; enter, 6 turns; wring out; sadden with 8 ounces of copperas; 4 turns; wring out again, and give 4 ounces of bluestone.
2. (For silk.) For 100 yards. Boil 4 lbs. of fustic and 6 ounces of logwood, 21⁄2 ounces of cudbear, 11⁄4 ounce of copperas. Cool to 200° Fahr.; enter, winch 20 minutes; air out; repeat; then take a little of the liquor out of the boiler, dissolve the copperas, reduce it to handling heat with water, and give one or two shots through it, as the pattern requires; one water out of the saddening; then give a warm but weak sour to clear the colour, wash in two waters, and dry.
3. (For Wool.) Dark drab. For 50 lbs. 7 lbs. of fustic, 8 ounces of madder, 4 ounces of cudbear, 2 lbs. of alum, 8 ounces of tartar. Enter between the cold and 160° Fahr.; after heating up boil from 10 to 30 minutes; wash in two waters. All dark shades of this may be slightly prepared with chrome; wash in two waters.
4. (For wool.) Light drab. For 56 lbs. 4 lbs. of fustic, 13⁄4 lb. of alum, 4 ounces of madder, 4 ounces of tartar, 31⁄2 ounces of cudbear. Work as for dark drab.
DRACONINE. Syn. Dra′cine, Red resin of dragon’s blood. A peculiar vegetable principle discovered by M. Melandre in dragon’s blood.
Prep. Dragon’s blood is dissolved in alcohol, the solution filtered, concentrated, and precipitated with cold water; the red, spongy precipitate is well washed, neutralised with dilute sulphuric acid, again liberated by means of an alkali, and well washed with water.
Prop., &c. Draconine has a fine red colour; is tasteless, inodorous, and flexible; it fuses at 131° Fahr. The smallest quantity of carbonate of lime in filtering paper may be detected by sulphate of draconine, the yellow colour instantly turning red.
“DRAGEES AU LACTATE DE FER.” (Gélis & Conté.) 100 grammes of lactate of iron made into 2,000 very small pills with powder and mucilage of marshmallow, and coated with eleosaccharate of anise. (Reveil.)
DRAGEES DE COPAHU DE FORTIN. 30 grammes balsam of copaiba made into 72 dragées, with 1·2 grammes calcined magnesia, and coated first with gum arabic and then with sugar. (Reveil.)
DRAGEES DE CUBEBE AU COPAHU. Cubebines (Labelonye). 2 parts balsam of copaiba, 2 parts extract of cubebs, 1 part yolk of egg, with sufficient liquorice powder to make a mass, which is divided into oblong pills, each weighing 7 decigrammes. These are dried and coated with white or raw sugar. (Hager.)
DRAGEES DE POUGUES. (Garnier.) Chloride of calcium, 50 parts; chloride of magnesium, 50 parts; chloride of iron, 10 parts; dissolved in water and precipitated with sodium carbonate. The precipitate is washed, pressed, and mixed with 100 parts bicarbonate of soda. Of this mixture 25 parts are made into a mass with 475 parts of a paste of sugar, peppermint, oil, and mucilage. The mass is then divided into dragées weighing 5 decigrammes, which are coated with gum and sugar. (Reveil.)
DRAGON’S BLOOD. Syn. Sanguis draconis, L. A rich red-coloured resin, obtained from various species of the genus Calamus. Its colour, in the lump, is a dark brownish-red; in powder, bright red. It is friable, breaks with a shining fracture, and has a sp. gr. not higher than 1·196 or 1·197. When pure, it readily dissolves in alcohol, ether, and oils, yielding rich red, transparent solutions. Adulterated and factitious dragon’s blood is only partly soluble, and lacks the rich colour of the genuine article. Dragon’s blood is chiefly used to tinge varnishes and lacquers.
Dragon’s Blood, Factitious. Prep. 1. Shell-lac, 4 lbs.; melt, remove from the fire, and add, Canada balsam, 6 oz., and gum benzoin, 2 oz.;583 mix well, stir in red sanders wood, 11⁄2 lb., and Venetian red, 3⁄4 lb. (both in fine powder); and form the mass into sticks.
2. As the last, omitting the red Venetian.
DRAINS. The salubrity of a dwelling-house is largely dependent upon the sound condition, the unimpeded outlet from, and the proper construction and position of, its drains, supplemented by like conditions in the various house-pipes which run from the sinks and closets into them.
The sense in which we shall use the term “drain” is that defined by the Public Health Act of 1875:—“‘Drain’ means any drain of, and used for the drainage of one building only, or premises within the same curtilage, and made merely for the purpose of communicating therefrom with a cesspool or other like receptacle for drainage, or with a sewer into which the drainage of two or more buildings or premises occupied by different persons is conveyed.”
There can be no doubt that the almost universal system pursued with regard to house drainage is radically irrational and dangerous. As the drains lead into the main sewer or cesspool it is most important that the house-pipes which communicate with the drains should be so connected with them and arranged, as to remove all risk of the foul air of the sewers passing into them through the drains, and thus (should the pipes be imperfect) escaping into and defiling the atmosphere of the house—a very possible contingency for two reasons, first, because the traps connecting the pipes with the drains may be defective, and, second, because the aspiratory power of the warm house is constantly tending to draw air (the sewer air) through the water in the water trap. Instead of making the connection, as is now so universally done, between the sink and closet and other pipes and the drain, by means of a water trap, underneath the basement, this junction should be effected outside the house. The drains should, therefore, always terminate outside, and not under the house; and wherever practicable all house-pipes should be carried outside, and not inside, or between the walls of any dwelling, the objections to which have been already stated. In cases, however, in which this arrangement is impossible of execution, and a pipe can only be carried through the house from the front to the rear, it is far better to take it above the basement than underneath.
The interposition of the water in the trap at the point of union between the house-pipe and the drain is not alone sufficient to effect the necessary amount of disconnection between them. There must be thorough ventilation and communication with the outer air at the point of junction, otherwise there is danger that the emanations from the sewer may find their way into the house, as in the former case.
A great number of methods have been devised for disconnecting the house-pipes and the outside drains, the simplest of which consists in placing the trap just outside the house, in opening the drain on the side of the trap the furthest from the house, and in carrying up from it a four-inch pipe to as great a height as is convenient. By this means any noxious gas that might escape from the drain is diverted from the house, and ascends into and diffuses into the superincumbent atmosphere.
In the arrangement of the house-pipes it is desirable not to carry the pipes which convey away the sink water and the waste water into the closet-soil pipes, but, wherever it can be managed, to let them discharge over a grating into the drain trap. Where we have to deal with soil or water-closet pipes, or with a pipe formed by the junction of these with the waste-water pipe (if such an arrangement is unavoidable), it is most important that there should be complete disconnection between the pipe and the drain by means of one of the many ventilating contrivances so well known to sanitary engineers.
The best material for the manufacture of drain pipes is hard, well-burnt, smooth, and glazed earthenware; bricks and porous earthenware are particularly ill adapted for the purpose; so also are iron pipes, unless they are thoroughly cemented inside.
In the laying of drain pipes care should be taken to place them on concrete, in loose soils, and on well-worked puddled clay, in the case of clay soils. When they are laid in very loose soils it is sometimes necessary, besides employing concrete, to additionally use even piling for the depth of a foot. Leakage and consequent soakage of the soil are sure to take place sooner or later if the drain pipes are not laid on a good foundation, as they are when the drains are badly and carelessly joined.
Messrs Brooke, of Huddersfield, have invented a combined drain and subsoil pipe, the latter, on which the drain pipe rests, being perforated, carries off the subsoil water. This contrivance is adapted for wet soils.
When junction pipes are required for uniting the drain pipes those known as “oblique junctions” only should be used. The junctions known as “square junctions” should be avoided, as they are always sure to become blocked up.
With respect to the fall of drain pipes Dr Parkes says, “one in forty-eight is frequently given, or three quarters of an inch in every584 yard; a fall of one in sixty-five in drains of six inches diameter, and one in eighty-seven in drains of eight inches diameter, will give a velocity of 220 feet per minute.
In order that drain pipes may be properly cleaned it is desirable to have them so made that they can be opened at intervals by means of lids or caps. The following cuts represent a few of the many kinds of pipes adapted for this purpose.
In addition to this method of cleansing them, drain pipes should be regularly flushed out at least once a month. House pipes are usually cleaned out by means of a flexible bamboo, or by jointed rods fitted with screws and rollers, which serve to loosen sediment. A frequent examination of all house pipes and traps should be made, and every joint and bend of the former well looked to. Unfortunately, however, they are so frequently covered in, that this is impossible.
Where it can be done all skirting boards and covers under which the pipes and traps are concealed should be removed. When, however, this cannot be managed the following plan of examination into their condition may be followed:—Pour water down the pipe, and observe if there be any smell; if there be, the pipe is full of foul air, and requires ventilation; or else the trap is defective, and the bad smell is due to sewer gas. Or, instead of pouring down water, a lighted candle or a piece of smouldering brown paper may be held over the entrance of the pipe, or the grating over a trap, when the air will be driven back. If the condition of the pipe be tested by throwing water down it, it should be noticed whether the water runs away at once or whether it is checked in its progress. This is all that, under the circumstances, can be done inside the house; but though an examination of the pipe is precluded inside, it may be possible to remove the earth on the outside, and so to get down to and open the drain with which the pipe communicates. Under these circumstances water mixed with lime should be poured down the house pipe; if the milky-looking water is long in making its appearance, and runs only in driblets, the drain requires flushing; if the milky-looking water is much coloured and mixed with dirt, then the pipes and trap are foul, or there is a sinking or depression in some part of the drain where the water is lodging.
Afterwards a pailful of lime and water should be poured down the pipe, which should be afterwards flushed by pouring water down it until the water flows off nearly clear.
Referring to the construction and position of the pipes which carry off the waste water, soil, &c., from our houses into the drains, Dr Parkes writes—“Builders are always anxious to conceal tubes, and thus carry them inside the walls, or in the case of hollow walls, between the two. The consequence is that any escape of air must be into the house. I have known a case in which the leakage of a closet pipe carried down in a hollow wall constantly contaminated the air of a house. It would be infinitely better to run the pipes at once through the wall to the outside. Few persons have any idea of the carelessness of plumbers’ work—of the bad junctions, and of the rapidity with which pipes get out of order and decay. When a leaden pipe carrying water is led into a water-closet discharge pipe, it is frequently simply puttied in, and very soon the dried putty breaks away, and there is a complete leakage of gas into the house. Even if well joined the lead pipe will, it is said, contract and expand, and thus openings are at last formed. Dr Fergus, of Glasgow, has directed particular attention to this in the case of lead closet pipes, which become easily perforated, and which have only a limited duration of wear.” See Traps, Sewers.
DRAUGHT. Syn. Haustus, L. A single dose of liquid medicine, usually dispensed in one-and-a-half-ounce or two-ounce phials. Draughts are almost exclusively extemporaneous compounds, and differ from ‘mixtures’ only in containing one dose; whereas mixtures contain several. The latter have now very generally superseded draughts among all but the higher classes, when the dose is to be frequently repeated. Draughts possess the advantages of extreme convenience, and, from only one phial being opened at a time, of preserving the preparation better than when it is exposed to the air by the frequent removal of the cork. They are usually taken from a wine-glass, which they about 2⁄3rds fill.
In the preparation of draughts the same precautions are observed as are pointed out under585 Mixture; regard being had to the increased volume of the dose. The ingredients of a six-ounce mixture, for example, containing (say) 12 doses, may be equally distributed among a dozen draught phials, after which each may be filled up with distilled water, or any other simple vehicle. In most cases a little syrup may be advantageously added. In many instances no addition will be required, the doses of each form of preparation being the same.
The following are useful formulæ, which will serve as examples for others of the class. The number might be easily multiplied, and, indeed, might be extended so as to include 3⁄4ths of the whole materia medica; but such a plan would lead to useless repetitions, and occupy much space. See Mixture, Prescribing, &c.
Draught, Abernethy’s. See Abernethy Medicines and Mixture.
Draught, Ace′tate of Ammo′′nia. Syn. Haustus ammoniæ acetatis, L. Prep. 1. (St. B. Hosp.) Solution of acetate of ammonia, 4 fl. dr.; water to make 11⁄2 fl. oz.
2. (Dr Paris.) Camphor mixture, 11⁄2 fl. oz.; liquor of acetate of ammonia, 4 fl. dr.; antimonial wine, 20 drops; mix. As a refrigerant and diaphoretic in febrile affections; taken late in the evening.
Draught, Ac′etate of Potas′sa. Syn. Haustus potassæ acetatis, L. Prep. (Mid. Hosp.) Acetate of potassa, 30 gr.; bicarbonate of potassa, 20 gr.; peppermint water, 1 fl. oz. Diuretic, antacid, and laxative.
Draught, Ammoni′acal. Syn. Haustus ammoniacalis, H. ammoniæ, L. Prep. (Brande.) Liquor of ammonia, 20 to 30 drops; compound tincture of cardamoms and tincture of gentian, of each 1⁄2 fl. dr.; campho-mixture, 11⁄2 fl. oz. An aromatic absorbent and stomachic; in heartburn, acidity, low spirits, &c.
Draught, An′odyne. Syn. Haustus anodynus, L. Prep. 1. Tincture of opium, 15 drops; pimento water and syrup of poppies, of each 2 dr.; water, 1 fl. oz.
2. (Copland.) Nitre, 6 gr.; laudanum, 12 drops; compound spirit of ether, 1 fl. dr.; syrup of poppies, 2 fl. dr.; camphor mixture, 9 fl. dr.
3. (Ellis.) Tincture of opium, 15 to 25 drops; syrup of poppies, 2 fl. dr.; spirit of cinnamon, 1 fl. dr.; distilled water, 11⁄2 fl. oz.
4. As the above, but substituting a like quantity of solution of either acetate or hydrochlorate of morphia in lieu of the laudanum. All the above are given as soothing draughts to allay pain and produce sleep, especially the last thing at night. No. 4 is to be preferred if there are febrile symptoms present.
Draught, Antac′id. Syn. Haustus antacidus, L. Prep. 1. Bicarbonate of soda, 20 gr.; tincture of calumba, 3 fl. dr.; tincture of hops, 1 fl. dr.; syrup of orange peel, 2 fl. dr.; water, 6 fl. dr. To improve the appetite in heartburn and dyspepsia; taken 1 hour before a meal.
2. Liquor of ammonia, 16 drops; syrup of saffron, 2 fl. dr.; infusion of gentian, 3 fl. dr.; water, 7 fl. dr. As the last, taken occasionally, especially in debility, low spirits, &c.
3. (Collier.) Compound tincture of cardamoms, 1 fl. dr.; solution of bicarbonate of magnesia (fluid magnesia), 9 fl. dr.; simple syrup, 2 fl. dr. Twice a day; in dyspepsia, heartburn, &c., especially in gouty patients.
4. (A. T. Thomson.) Magnesia, 1 dr.; peppermint water, 11⁄2 fl. oz.; tincture of orange peel, 1 fl. dr. In dyspepsia, &c., with acidity or diarrhœa.
5. As No. 1, but using bicarbonate of potassa for bicarbonate of soda. In acidity, diarrhœa, &c., accompanied by great irritability of the coats of the stomach.
6. Prepared chalk, 30 gr.; spirit of nutmeg and tincture of opium, of each 12 to 20 drops; syrup of saffron, 3 dr.; cinnamon water, 1 fl. oz. In acidity, with extreme looseness of the bowels.
Draught, Anti-arthrit′ic. Syn. Haustus anti-arthriticus, L. Prep. 1. Tincture of colchicum seeds (L.), 1⁄2 fl. dr.; syrup of orange peel, 21⁄2 fl. dr.; water, 1 fl. oz. In gout; taken over-night, followed by another in the morning.
2. (Brande.) Wine of colchicum, 1⁄2 fl. dr.; carbonate of magnesia, 15 gr.; cinnamon water, 1⁄2 fl. oz.; water, 1 fl. oz. As the last.
3. (Sir C. Scudamore.) Magnesia, 18 gr.; Epsom salts, 11⁄2 dr.; vinegar of colchicum, 1 fl. dr.; simple syrup, 1 fl. dr.; cinnamon water, 9 fl. dr. As the last.
4. (Sir H. Halford’s Gout Preventive.) Compound infusion of gentian, 11⁄2 fl. oz.; tincture of rhubarb, 1 fl. dr.; bicarbonate of potassa, 15 gr.
Draught, Anti-asthmat′ic. Syn. Haustus anti-asthmaticus, L. Prep. Vinegar of squills, 1⁄2 fl. dr.; ipecacuanha wine, 15 drops; cinnamon water, 11⁄2 fl. oz. Expectorant. One to be taken three times daily during the attack.
Draught, Anti-emet′ic. Syn. Haustus anti-emeticus, L. Prep. 1. Juice of 1 lemon; liquor opii sedativus, 10 drops (or laudanum, 15 drops); ether, 20 drops; simple syrup, 2 dr.; water, q. s.
2. (Haustus anti-emeticus Riverii,—P. C.) Bicarbonate of potassa, 30 gr.; lemon juice, 4 dr.; syrup of lemon, 1 oz.; water, 3 oz.; mix quickly, and tie down the cork. To check nausea and vomiting. This is best given effervescing.
Draught, Anti-hyster′ic. Syn. Haustus anti-hystericus, L. Prep. Cyanide of potassium, 1 gr.; lettuce water (distilled), 2 fl. oz.; syrup of orange flowers, 11⁄2 oz.; water,586 51⁄2 fl. oz.; for 6 draughts. One to be taken when the fit is expected, and a second in half an hour. Should the fit come on, the dose may be repeated at intervals of about 15 minutes until 3 or 4 have been altogether administered. The symptoms, however intense, are generally either at once arrested, or greatly alleviated by this treatment.
Draught, Antilith′ic. Syn. Haustus anti-lithicus, L. Prep. 1. (Venables.) Borax, 8 gr.; bicarbonate of soda, 10 gr.; aerated water, 8 fl. oz. For a draught; in red gravel.
2. (Dr Paris.) Carbonate of soda, 12 gr.; tincture of calumba, 1 fl. dr.; infusion of quassia, 1 fl. oz.; water, 3 fl. dr. In dyspepsia and gravel, attended with the lithic-acid diathesis.
Draught, Anti-neural′gic. Syn. Haustus anti-neuralgicus, H. narcotinæ, L. Prep. (Jeston.) Narcotine, 2 gr.; diluted sulphuric acid, 20 drops; infusion of roses, 11⁄2 fl. oz. One every 2 hours in the intermissions of neuralgia.
Draught, Antisep′tic. Syn. Haustus antisepticus, L. Prep. (Dr Collier.) Decoction of yellow bark, 1 fl. oz.; tincture of opium, 5 drops; spirit of pimento and water, of each 2 fl. dr. In putrid fevers, gangrene, &c.
Draught, Antispasmod′ic. Syn. Haustus antispasmodicus, L. Prep. 1. (Dr Collier.) Tincture of castor, 1 fl. dr.; sulphuric ether, 10 drops; peppermint water, 11 fl. dr.; mix. In hysteria, and that species of irregular muscular action dependent on debility.
2. (Dr Gregory.) Fetid spirit of ammonia, 1⁄2 to 1 fl. dr.; camphor mixture, 10 fl. dr.; syrup of saffron, 1 fl. dr. In cases complicated with low spirits, debility, &c.
3. (A. T. Thomson.) Musk mixture, 14 fl. dr.; liquor of ammonia, 16 drops; tincture of castor, 1 fl. dr.; syrup of poppies, 1⁄2 fl. dr.; mix. Three or four times daily, in hysteria and convulsive affections, after the bowels have been well cleared by some aperient.
4. (A. T. Thomson.) Oil of aniseed, 10 drops; magnesia, 20 gr.; tincture of senna, 2 fl. dr.; peppermint water, 10 fl. dr.; mix. In flatulence and spasms of the stomach.
Draught, Ape′′rient. Syn. Haustus aperiens, L. Prep. 1. (Paris.) Infusion of senna, 1 fl. oz.; tincture of senna, tincture of jalap, and syrup of senna, of each 1 fl. dr.; tartrate of potassa, 1 dr.; mix.
2. (Ryan.) Epsom salts, 4 dr.; tincture of senna, 11⁄2 fl. dr.; syrup of ginger, 1 fl. dr.; spirit of sal-volatile, 20 drops; infusion of senna, 11⁄2 fl. oz.
3. (Thomson.) Tartrate of potassa, 3 dr.; tincture of senna and syrup of saffron, of each 1 dr.; infusion of senna, 11⁄2 oz. The above are good aperients, and in their composition and action resemble the ordinary “black draught.”
4. (Effervescing A. D.)—a. (Dr Barker.) Bisulphate of potassa, 73 grs.; carbonate of soda, 72 gr.; water, q. s.; dissolve the two in separate glasses, mix the solutions, and drink whilst effervescing, in the same way as soda water.
b. (W. Cooley.) Bicarbonate of soda, 1 dr.; potassio-tartrate of soda, 2 drs.; dissolve in about 1-3rd of a glassful of cold water; and pour it on another like quantity of water, holding in solution tartaric acid, 40 grs., and syrup of orange peel, 11⁄2 fl. dr.; and drink it instantly.
c. (Paris.) Potassio-tartrate of soda, 2 dr.; bicarbonate of soda, 40 grs.; dissolve, and add lemon juice, 1 or 2 tablespoonfuls.
d. (Young.) Cream of tartar, 3 dr.; carbonate of soda, 21⁄2 dr.; throw them into a soda-water bottle three parts filled with cold water, cork immediately, and wire down the cork. The last three are examples of factitious effervescing Seidlitz water, and are good saline aperients. The method of taking them may be varied by mixing the dry ingredients (in fine powder) on a piece of paper, and throwing the mixture suddenly into a tumbler 2-3rds filled with water, and drinking the liquid whilst effervescing. See Cathartic D. (below.)
Draught, Ap′petite. See Draught, Dinner.
Draught, Aromat′ic. Syn. Aromatic antacid, draught; Haustus aromaticus, L. Prep. 1. Aromatic confection, 1 dr.; spirit of sal-volatile, 1⁄2 dr.; syrup of saffron, 2 drs.; pimento water, 9 fl. dr. Excellent in dyspepsia, with acidity, and in diarrhœa, preceded by an aperient.
2. (H. arom. cum rheo.—St. B. Hosp.) Aromatic confection, 1 dr.; infusion of rhubarb and cinnamon water, of each, 6 fl. dr. In diarrhœa and dyspepsia, especially when there is acidity and deficiency of bile.
Draught, Astrin′′gent. Syn. Haustus astringens, L. Prep. 1. Tannin, 3 gr.; rectified spirit, 1 fl. dr.; simple syrup, 2 fl. dr.; water, 6 fl. dr.
2. (Dr Paris.) Chalk mixture, 11⁄2 fl. oz.; tincture of catechu, 1 fl. dr.; laudanum, 15 drops.
3. (Thomson.) Extract of logwood, 12 gr.; tincture of catechu, 1 fl. dr.; cinnamon water, 15 fl. dr. The above are excellent remedies in diarrhœa (preceded by a purgative), and in dysentery, &c. One may be taken after each motion.
Draught, Black. See Mixture.
Draught, Cam′phor. Syn. Haustus camphoræ, L. Prep. (Guy’s Hosp.) Camphor, 6 gr.; rectified spirit, q. s. to powder; white sugar, 1 dr.; mucilage, 3 dr.; water, 11⁄2 fl. oz. Anodyne and diaphoretic, &c.
Draught, Cas′tor Oil. Syn. Haustus olei ricini, L. Prep. (Guy’s Hosp.) Castor oil, 4 dr.; yelk of egg, q. s. (2 in no.); simple syrup, 1 fl. dr.; cassia or cinnamon water, 1 fl. oz. Aperient.
Draught, Cathar′tic. Syn. Haustus catharticus,587 L. The following are given as additions to those under Aperient d., and other heads:—Prep. 1. (Dr Thomson.) Tartrate of potassa, 5 dr.; tincture of senna, 1 fl. dr.; infusion of senna, 141⁄2 fl. dr.; syrup of saffron, 1⁄2 fl. dr.; mix. In acute diseases, taken early in the morning.
2. (Thomson.) Epsom salts and manna, of each 2 dr.; infusion of roses, 14 fl. dr.; dilute sulphuric acid, 10 drops. In inflammatory affections, and to check vomiting in low fevers.
3. (Thomson.) Carbonate of magnesia, 1 dr.; powdered rhubarb, 20 gr.; peppermint water, 12 fl. dr. In dyspepsia, attended with costiveness and acidity, taken an hour before dinner.
4. (Thomson.) Castor oil, 5 fl. dr.; powdered gum, 20 gr.; rose water, 1 fl. oz.; compound tincture of lavender, 8 drops; syrup of poppies, 1 fl. dr. In colic and calculus. The above differ from aperient draughts simply in their greater strength.
Draught, Chalk. Syn. Haustus cretæ, L. Prep. 1. Powdered gum, chalk, and simple syrup, of each 1 dr.; aromatic water (as that of caraway, cinnamon, nutmeg, pimento, or peppermint), 11⁄2 fl. oz.
2. Chalybeated c. d.; Haustus cretæ et ferri, L.—Paris.) Chalk mixture, 7 fl. dr.; compound mixture of iron, 3 fl. dr.; sesquicarbonate of ammonia, 5 or 6 gr. In diarrhœa, particularly in that arising from debility and anæmia.
3. (C. d. with rhubarb; Haustus cretæ cum rheo, L.)—a. Chalk mixture (see above), 11⁄2 fl. oz.; powdered rhubarb, 12 gr.
b. (Lond. Hosp.) Powder of chalk with opium, 12 gr.; rhubarb, 15 gr.; syrup of saffron and compound tincture of cardamoms, of each 1 fl. dr.; caraway water, 10 fl. dr. In heartburn, dyspepsia, and certain forms of diarrhœa.
Draught, Chlo′′rine. Syn. Haustus chlorinii, L. Prep. (Copland.) Chlorine water, 1⁄2 fl. dr.; water, 11⁄2 fl. oz.; mix, and add of syrup of poppies, 1⁄2 fl. dr. One every 6 hours; in the worst form of typhus fever, and other putrid diseases, &c.
Draught, Cit′rate of Ammo′′nia. Syn. Haustus ammoniæ citratis, H. a. sesquicarbonatis effervescens, L. Prep. (Guy’s Hosp.) Sesquicarbonate of ammonia, 20 gr.; water, 1 fl. oz.; dissolve, and add of lemon juice, 1⁄2 fl. oz. An agreeable, cooling, saline draught in febrile cases.
Draught, Cit′rate of Potas′sa. Syn. Haustus potassæ citratis, L. Prep. From carbonate of potassa, 24 gr. (or bicarbonate, 29 gr.); water, 1 fl. oz.; dissolve, and add of lemon juice, 5 fl. dr. As the last. 20 gr. of citric acid may be used instead of the lemon juice.
Draught, Col′chicum. See Draught Anti-arthritic.
Draught, Copai′ba. Syn. Haustus copaibæ, L. Prep. (St. B. Hosp.) Balsam of copaiba, 1⁄2 fl. dr.; mucilage (thick), 4 fl. dr.; pimento water, 3 fl. dr.; water, 5 fl. dr. In gonorrhœa, &c.
Draught, Cough. See Mixture.
Draught, Diaphoret′ic. Syn. Haustus diaphoreticus, L. Prep. 1. (Collier.) Infusion of serpentary, 11⁄2 fl. oz.; tincture of serpentary, 1 fl. dr. Tonic and diaphoretic.
2. (Thomson.) Sesquicarbonate of potassa, 20 gr.; fresh lemon juice, 4 fl. dr.; tartrate of antimony, 1⁄6 gr.; water, 11 fl. dr.; syrup of poppies, 1 fl. dr. Antifebrile and diaphoretic.
3. (Thomson.) Liquor of acetate of ammonia, 6 fl. dr.; camphor mixture, 10 fl. dr.; nitrate of potassa, 10 gr.; syrup of tolu, 1⁄2 fl. oz. Anodyne and diaphoretic. All the above are used in inflammatory affections.
Draught, Din′ner. Syn. Appetite draught; Haustus dictus ante cibum. Prep. 1. Tinctures of cascarilla, hops, and rhubarb, of each 1 fl. dr.; spirit of sal-volatile, 1⁄2 fl. dr.; tincture of capsicum, 20 drops; syrup of orange peel, 2 dr.; water, 11⁄2 fl. oz.
2. Compound tincture of gentian, 1⁄2 fl. oz.; sal-volatile, 1⁄2 a teaspoonful; cinnamon water, 1 fl. oz.; compound tincture of cardamoms, 1 teaspoonful. Either of the above to be taken an hour before a meal.
Draught, Diuret′ic. Syn. Haustus diureticus, L. Prep. 1. (Collier.) Tincture of jalap, 2 fl. dr.; vinegar of squills, 1 fl. dr.; peppermint water, 10 fl. dr.; mix.
2. (Copland.) Acetate of potassa, 1⁄2 dr.; infusion of quassia and cinnamon water, of each 6 fl. dr.; vinegar of squills and sweet spirits of nitre, of each 1⁄2 fl. dr.
3. (Thomson.) Nitre, 8 gr.; tincture of digitalis, 16 drops; infusion of roses, 13 fl. dr.; syrup of roses, 1 fl. dr.
4. (Turner.) Nitre and powdered gum, of each 15 gr.; almond mixture, 11⁄2 fl. oz. The above are used as diuretics in dropsy; the last, also in scurvy, and in the incontinence of urine of children.
Draught, Donovan’s. Syn. Draught of hydriodate of arsenic and mercury; Haustus hydriodatis arsenici et hydrargyri, L. Prep. (Donovan.) Liquor of hydriodate of arsenic and mercury (Donovan’s), 2 fl. dr.; distilled water, 31⁄2 fl. oz.; syrup of ginger, 1⁄2 fl. oz.; mix for 4 draughts. One, night and morning; in lepra, lupus, psoriasis, and some other obstinate cutaneous affections. It must not be allowed to touch anything metallic.
Draught, Efferves′cing. Prep. (Lond. Hosp.) Sesquicarbonate of soda, 30 gr.; water, or peppermint water, 11⁄2 fl. oz.; syrup of orange peel, 2 fl. dr.; tincture of calumba, 1⁄2 fl. dr.; tartaric or citric acid, 25 gr.; add the acid last, and drink whilst effervescing. Stomachic, tonic, and anti-emetic; in acidity, dyspepsia, &c. (See antè.)
Draught, Emet′ic. Syn. Haustus emeticus, L. Prep. 1. Sulphate of zinc, 15 gr. to588 30 gr.; water, 9 fl. dr.; dissolve. In cases of poisoning, and at the commencement of an attack of ague.
2. (Copland.) Ipecacuanha, 30 gr.; sesquicarbonate of ammonia, 20 gr.; tincture of capsicum, 30 drops; oil of chamomile, 10 drops; mint water, 2 fl. oz. As a stimulant emetic in cases of poisoning by laudanum or other narcotics.
3. (Guy’s Hosp.) Antimonial wine, 2 fl. dr.; ipecacuanha wine, 6 fl. dr.; water, 4 fl. dr. For unloading the stomach in ordinary cases.
4. (Mid. Hosp.) Tartar emetic, 1 gr.; ipecacuanha, 20 gr.; syrup, 2 fl. dr.; water, 10 fl. dr. As the last.
5. (Dr Pickford.) Sulphate of zinc, 20 gr.; sulphate of magnesia, 4 dr.; water, 13⁄4 oz. When it is also desired to act rapidly on the bowels.
6. (Rodier.) Sulphate of copper, 10 gr.; water, 2 fl. oz. In poisoning by laudanum.
7. (Sprague.) Ipecacuanha, 30 gr.; sesquicarbonate of ammonia, 20 gr.; tincture of capsicum, 1 fl. dr.; peppermint water, 3 fl. oz. In poisoning by narcotics.
8. (A. T. Thomson.) Ipecacuanha, 20 gr,; ipecacuanha wine, 2 fl. dr.; water, 10 fl. dr. For unloading the stomach, in ordinary cases.
9. (Trousseau.) Ipecacuanha, 8 gr.; syrup of ipecacuanha, 1 fl. oz.; water, q. s. for 4 draughts. One every 10 minutes, until vomiting occurs.
Draught, E′ther. Syn. Haustus æthereus, L. Prep. (Neligan.) Sulphuric ether, 1 fl. dr.; spermaceti, 3 gr.; rub together (expertly), and add of peppermint water, 10 fl. dr. An excellent stimulant and antispasmodic, febrile symptoms being absent.
Draught, Expec′torant. Syn. Haustus expectorans, L. Prep. 1. (Collier.) Mixtures of ammoniacum and almonds, of each 6 fl. dr.; tincture of squills, 12 drops. In hoarseness, chronic coughs, &c.
Draught, Hen′bane. Syn. Haustus hyoscyami, L. Prep. 1. Tincture of henbane, 30 to 60 drops; syrup of saffron, 1 fl. dr.; water, 10 fl. dr. Anodyne and soporific. Used to allay nervous excitement, and induce sleep, when laudanum is inadmissible.
2. (Henbane and Squills d.; Haustus hyoscyami cum scillâ, L.—Dr Bree.) Extract of henbane, 3 gr.; tincture of squills, 10 drops; dilute nitric acid, 6 drops; water, 11⁄2 fl. oz. Anodyne and expectorant; in asthmas, chronic coughs, &c.
Draught, Hydrocyan′ic. Syn. Haustus hydrocyanicus, L. Prep. 1. (Donovan.) Cyanide of potassium, 1 gr.; syrup of lemons, 1⁄2 fl. oz.; distilled water, 71⁄2 fl. oz. For 8 draughts. One for a dose.
2. (Dr S. Dickson.) Medicinal Hydrocyanic acid (L.), 15 drops; liquor of ammonia, 20 drops; syrup of orange flowers (or simple syrup), 3 fl. dr.; water, 81⁄2 fl. oz.; mix, and divide into 6 draughts. One—2 or 3 times a day; in gastrodynia, and all those nameless nervous and hysterical affections arising from excessive irritability, mental anxiety, &c. In a case that came under our notice, in which life was an absolute burden to the patient, relief was afforded by the first draught, and 4 or 5 effected a comparative cure, although almost every other remedy had been tried in vain.
Draught, Laennec’s. Syn. Laennec’s contra-stimulant draught; Haustus contrastimulans, L. Prep. From tartar emetic, 2 grs.; syrup of poppies, 2 fl. drs.; orange-flower water, 11⁄2 fl. oz. Every two hours in pneumonia, &c.
Draught, Lax′ative. Syn. Haustus laxans, L. Prep. 1. See Draughts, Aperient.
2. (Dr Copeland.) Infusion of senna and compound infusion of gentian, of each 6 fl. dr.; sulphate of potassa, 20 to 30 gr.; extract of taraxacum, 30 to 40 gr.; compound tincture of cardamoms, 11⁄2 fl. dr. Aperient, stomachic, and alterative.
Draught, Mor′phia. Syn. Haustus morphiæ, L. Prep. (Brera.) Morphia, 1⁄4 gr.; syrup of poppies, 1 fl. dr.; water, 11 fl. dr. Two or three drops of acetic acid may be advantageously added. At bed-time, as a soporific.
Draught, Narcot′ic. Syn. Haustus narcoticus, H. opiatus, L. Prep. 1. (St. B. Hosp.) Laudanum, 12 to 20 drops; syrup of red poppies, 1 fl. dr.; pimento water, 3 fl. dr; water, 1 fl. oz. To induce sleep in slight cases, when fever is absent.
2. (A. T. Thomson.) Camphor mixture, 11⁄2 fl. oz.; laudanum, 35 drops; sulphuric ether and syrup of saffron, of each 1 fl. dr. In intermittent headache.
3. (Thomson.) Carbonate of ammonia, 15 gr.; fresh lemon juice, 1⁄2 fl. oz.; water, 1 fl. oz.; spirit of nutmeg, 1 fl. dr.; syrup of orange peel, 1⁄2 fl. dr.; tincture of hemlock, 10 drops. In diseases of increased irritability.
4. (Thomson.) Carbonate of potassa, 20 gr.; fresh lemon juice, 1⁄2 fl. oz.; peppermint water, 1 fl. oz.; laudanum, 25 drops; syrup of tolu, 1⁄2 fl. dr. To procure sleep in the majority of diseases. (See above.)
Draught, Nux Vom′ica. Syn. Haustus nucis vomicæ, L. Prep. (Dr Joy.) Nux vomica (in fine powder), 3 gr.; powdered gum, 2 dr; compound tincture of cardamoms, 1 fl. dr.; cinnamon water, 10 fl. dr. Diuretic, narcotic, stimulant, and tonic; in paralysis, impotence, debility, &c., unaccompanied by inflammation of the nervous centres. See Strychnine.
Draught, Refri′′gerant. Syn. Haustus refrigerans, L. Prep. 1. Carbonate of potassa, 20 grs.; syrup of orange peel, 1 fl. dr.; spirit of nutmeg, 1⁄2 fl. dr.; water, 11⁄2 fl. oz.
2. (Thomson.) Nitre, 12 gr.; almond mixture, 11⁄2 fl. oz.; syrup of tolu, 1 fl. oz.
3. (Collier.) Carbonate of potassa, 20 gr.; antimonial wine, 20 drops; syrup of orange peel, 1 fl. dr.; tincture of orange peel, 1⁄2 fl. dr.;589 water, 11⁄2 fl. oz.; mix, and add a large table-spoonful of lemon juice. In inflammatory diseases, &c.
Draught, Saline′. See Draught, Effervescing, &c.
Draught, Stomach′ic. See Draught, Dinner, &c.
Draught, Ton′ic. Syn. Strengthening draught; Haustus tonicus, L. Prep. 1. (Collier.) Disulphate of quinine, 2 gr.; tincture of orange peel, 1 fl. dr.; diluted sulphuric acid, 5 drops; laudanum, 10 drops; infusion of cascarilla, 11⁄2 fl, oz. In pyrosis, &c., 1 hour before dinner.
2. (A. T. Thomson.) Infusion of yellow bark, 11⁄2 fl. oz.; compound tincture of cinchona, 1 fl. dr.; powdered cinchona, 40 gr.; syrup of orange peel, 1 fl. dr. In intermittents and acute rheumatisms.
3. (Thomson.) Infusion of cascarilla, 11⁄2 fl. oz.; tincture of cascarilla and ginger, of each 1 fl. dr. In dyspepsia, arising from intemperance.
4. (Walton.) Infusion of cascarilla, 9 fl. dr.; tinctures of rhubarb and ginger, of each 1 fl. dr.; syrup of saffron, 1⁄2 fl. dr.; ammonio-citrate of iron, 6 gr.; tincture of capsicum, 5 drops. In anæmia, and debility accompanied by paleness and relaxation.
Draught, Ver′mifuge. Syn. Haustus vermifugus, H. anthelminthicus, L. Prep. (M. Levacher.) Castor oil, 4 dr.; oil of turpentine, 2 dr.; mint water, 2 fl. oz.; syrup, 1 fl. oz.; powdered gum, 2 dr.; for an emulsion. In tapeworm.
DRAW′INGS. Chalk and pencil drawings may be fixed so as not to suffer from slight abrasion, by washing them with skimmed milk, or with water holding in solution a little isinglass or gum. When the first is used, great care must be taken to deprive it of the whole of the cream, as the latter substance would cause the drawing to look streaky. An easy way of applying these fluids is to pour them into a shallow vessel, and to lay the drawing flat upon the surface of the liquid; after which it should be gently removed and placed on white blotting paper, in an inclined position, to drain and dry.
DRENCHES. Syn. Drinks. In veterinary practice, these terms are applied to liquid medicines or mixtures which are administered to horses and neat cattle, and chiefly to the latter. A drench for a HORSE should not be less than half a pint, nor more than a quart; about a pint is, perhaps, the best quantity; that for a COW or OX should measure about a quart, and not more than about 5 half pints. See Veterinary Medicine.
DRES′SING. In the industrial arts, a preparation of gum, starch, size, &c., employed in stiffening or “finishing off” textile fabrics and paper. In surgery, the term is appropriated to any application to a wound or sore, made by means of lint, linen, or leather. Simple dressing is simple cerate or spermaceti cerate. Among cooks, the stuffing of fowls, pork, veal, &c., is commonly called ‘dressing.’
DRIERS. Driers are substances employed to facilitate the drying of paints. The driers most commonly employed are sugar of lead, litharge, and white copperas. Either of these when well ground, and mixed in small proportion with paints, very materially hastens their drying. Indeed, some colours will not dry without them. Red lead is also well adapted for a drying agent, and in cases where its colour does not preclude it, is much used. The best drier is sugar of lead. Its cost, however, is somewhat higher than that of the other driers. It is important to bear in mind that in the finishing coats of delicate colours driers are not generally had recourse to, as they have a slight tendency to injure the colour. A drying property may be imparted to linseed oil by boiling it with drying substances; it then becomes a very useful vehicle for some purposes. See Oils, Drying.
DRIFFIELD OILS.—For the prevention of gangrene and for healing incised and other wounds, bruises, sprains, swellings, and external inflammations. A dusky brownish-green clear oil, consisting of olive oil, digested with wormwood, savin, and arnica, and afterwards perfumed with a mixture of oils of rosemary, thyme, and juniper, 1 pint (474 grammes). (Hager.)
DRINKS (Summer). See Beer, Ginger, Lemonade, Sherbet, &c.
DRINK, CORDIAL (Dr Cherwy). A herbal lemonade to heal all chronic and scrofulous diseases. It contains 115 grammes water, 15 grammes spirit, 2 grammes potassium iodide, 5 grammes bitter almond water, 10 grammes sugar, and 3 grammes burnt sugar. (Hager.)
DRIPPING TO CLARIFY. Put the dripping into a stewpan over the fire, and let it boil, and as it does so, skim it carefully. When it boils pour it into a basin, in which you have previously put a little cold water. It must stand till cold. It is then to be taken out of the water. The dripping will now be in the form of a cake, at the bottom of which will be found adhering little pieces of meat, skin, &c. These must be scraped off, and the dripping will have been purified. Another method is to mix boiling water with the dripping, to stir well, let it get cold, and then to take it out and scrape it as above.
DROP. See Measures.
DROPS, CHOLERA—CHOLERATROPFEN (A. Bastler, Vienna). Oils of anise, cajeput, and juniper berries, of each 20 parts; spirit of ether, 60 parts; tincture of cinnamon, 120 parts; Haller’s acid elixir, 5 parts.—Dose, 30 to 50 drops. (Wittstein.)
DROPS (Confectionery). These are confections of which the principal basis is sugar. They differ from lozenges chiefly in the ingredients being combined by the aid of heat. Occasionally they are medicated.
590
Prep. Double refined sugar is reduced to powder, and passed through a hair sieve (not too fine), and afterwards through a gauze sieve, to take out the fine dust, which would destroy the beauty of the drop. It is then put into a clean pan, and moistened with any favorite aromatic, as rose or orange-flower water, added slowly, stirring it with a paddle all the time, from which the sugar will fall as soon as it is moist enough, without sticking. The colouring (if any) is next added, in the liquid state, or in very fine powder. A small, polished copper, or tinned-copper pan, furnished with a lip, is now one half or three parts filled with the paste, and placed over the fire, or over the hole of a stove, or preferably on a sand bath, and the mixture stirred with a little bone or glass spatula until it becomes liquid. As soon as it almost boils, it is taken from the fire, and if it is too moist, a little more powdered sugar is added, and the whole stirred, until it is of such a consistence as to run without too much extension. A tin plate, very clean and smooth, and very slightly oiled, being now ready, the pan is taken in the left hand, and a bit of bright iron, copper, or silver wire, about 4 inches long, in the right. The melted sugar is next allowed to fall regularly on the tin plate, the wire being used to remove the drop from the lip of the pan. In two or three hours afterwards the drops are taken off with the blade of a knife, and are at once put into bottles or tins. On the large scale, ‘confectionery drops’ are moulded by a machine consisting essentially of two metal rollers covered with hollows. A sheet of the warm and soft composition, on being passed between the rollers, is at once converted into a batch of symmetrical drops, the upper and lower halves being moulded by the corresponding hollows of the upper and lower rollers. See Candying, Confection, Essence, Stains (Confectioner’s), Sugar Plums.
The following are a few of the principal confectionery drops kept in the shops:—
Drops, Acid′ulated. Syn. Acid Drops. Prep. Tartaric acid, 1⁄2 oz., dissolved in a very little water, is added to each lb. of sugar, as above; with essence of lemon, orange, or jargonelle pear, to flavour, as desired.
Drops, Chocolate. Prep. Chocolate, 1 oz., is reduced to fine powder by scraping, and added to powdered white sugar, 1 lb.; when the mixture is made into drops, as above, care being taken to avoid heating it a second time.
Drops, Cof′fee. Prep. A clarified, concentrated infusion of coffee, 1 oz., is used for each lb. of sugar.
Drops, Fruit. These are prepared according to the general description. (See above.) The flavouring essences (volatile oils or essences of lemon, orange, citron, raspberry, jargonelle pear, &c.) not being added until the sugar is melted, to avoid, as much as possible, loss by evaporation. The colouring matter may be any of the transparent ‘stains’ usually employed for cakes, jellies, and confectionery. In this way are made the majority of the first-class fruit drops and bon-bons of the sugar-bakers. In some cases the plan is varied by adding the clarified concentrated juice, or jelly of the fruit to the sugar. One variety of raspberry and currant (red and black) drops are made in this way.
Drops, Ginger. Prep. From essence or tincture of ginger, as above. An inferior kind is made in the way described under Candy, Ginger.
Drops, Jargonelle′. Fruit drops flavoured with essence of jargonelle pear (SOLUTION OF ACETATE of AMYLE).
Drops, Lem′on. Acidulated drops flavoured with essence of lemon. They are usually stained with an infusion of turmeric. (See above.)
Drops, Pep′permint. From the whitest refined sugar, flavoured with English oil of peppermint or its spirituous solution (essence of peppermint), or with peppermint water.
Drops, Rasp′berry. See Drops, Fruit (above.)
Drops (Med′icated). Syn. Guttæ, L. This term is commonly applied to compound medicines that are only taken in small doses. At the present time they are almost exclusively confined to empirical and domestic medicine. The plan of directing liquids to be measured by dropping is objectionable, because the drops of different fluids vary in size, and are also further influenced by the size of the bottle and the shape of its neck, as well as the quantity of liquid it is poured from. See Essence, and below.
Drops, Acoust′ic. Syn. Acoustic balsam; Guttæ acousticæ, Balsamum acousticum, L. Prep. 1. Oil of almonds, 1 oz.; laudanum and oil of turpentine, of each 1 dr.; mix. For hardened wax, and to allay pain.
2. Tinctures of benzoin, castor, and opium, of each, 1 fl. oz.; essential oil of assafœtida, 5 drops. As the last, and in deafness arising from debility of the organism.
3. (Baumé’s.) Tinctures of ambergris, assafœtida, castor, and opium, of each, 1 oz.; terebinthinated balsam of sulphur and oil of rue, of each, 15 drops. In atonic deafness.
4. (Bouchardat.) Compound spirit of balm, 21⁄2 dr.; oil of almonds, 5 dr.; ox-gall, 10 dr.; cresote, 10 or 20 drops. In cases complicated with hardened wax, fetid discharges, &c.
5. (Dr Hugh Smith.) Ox-gall, 3 dr.; balsam of Peru, 1 dr. In fetid ulcerations of the ear. One or two drops of the above are poured into the ear; or a piece of cotton wool moistened therewith is introduced instead. The last is the safest plan.
6. Glycerin, either alone or diluted with water. In deficiency of the natural secretions of the ear; used in sufficient quantity to moisten the first passages. See Deafness, Glycerin.
591
Drops, A′gue. Prep. White arsenic, 1⁄2 gr.; hot water, 1 oz.; dissolve.—Dose, 1⁄2 to 1 teaspoonful, twice a day. See Solution (Arsenite of Potassa).
Drops, An′odyne. Syn. Guttæ anodynæ, L. The solutions of acetate and hydrochlorate of morphia are commonly vended in the shops under this name.
Drops, Ant′acid. Syn. Guttæ antacidæ, L. Prep. (U. C. Hosp.) Liquor of potassa, 3 fl. oz,; powdered myrrh, 1 oz.; triturate together until thoroughly incorporated, add of liquor of ammonia, 1 fl. oz., mix well, place the mixture in a stoppered bottle, and the next day decant the clear portion. Antacid, tonic, and stomachic.—Dose, 10 to 20 drops, or more, in water.
Drops, Antihyster′ic. Syn. Guttæ antihystericæ, L. Prep. Cyanide of potassium, 2 gr.; rectified spirit, 5 fl. dr.; syrup of orange flowers, 3 fl. dr.—Dose, 10 drops to 1⁄2 teaspoonful, when the attack is expected, and repeated occasionally as required; in hysterical affections, gastrodynia, &c.
Drops, Antiscorbu′tic. Syn. Guttæ antiscorbuticæ, L. Prep. 1. Expressed juice of water-cress, 2 fl. oz.; salt of tartar, 1 oz.; agitate together occasionally for a few hours, and in 2 or 3 days decant.—Dose, 12 or 15 drops, to a teaspoonful, twice a day, in a cupful of new milk.
2. Citrate of potassa, 4 dr.; ammonio-citrate of iron, 2 dr.; water, 10 fl. dr.—Dose. As the last, in water.
3. (Green’s Antiscorbutic Drops.) Merely a disguised solution of corrosive sublimate. Most of the other ‘antiscorbutic’ and ‘anti-venereal drops’ advertised by quacks have a like composition.
Drops, Antiscrof′ulous. Syn. Guttæ antiscrofulosæ, L. Prep. 1. Iodine, 10 gr.; iodide of potassium, 1 dr.; water, 1 fl. oz.
2. (Augustin.) Chlorides of iron and barium, of each, 1⁄2 dr.; distilled water, 1 fl. oz.—Dose, 10 to 30 drops, 2 or 3 times a day.
Drops, Antispasmod′ic. Syn. Guttæ antispasmodicæ, L. Prep. Tinctures of castor, valerian, and assafœtida, of each, 2 dr.; tincture of capsicum and balsam of Peru, of each, 1 dr.; camphor, 20 gr.; acetate of morphia, 3 gr.—Dose, 10 to 20 drops, as required.
Drops, Bateman’s. See Drops, Pectoral.
Drops, Battley’s. See Liquor Opii Sedativus.
Drops, Bitter. Syn. Guttæ amaræ, L.; Gouttes amères, Fr. Prep. From nux vomica (rasped), 1 lb.; liquor of potassa, 1⁄2 fl. oz.; bistre, 1 dr.; compound spirit of wormwood, 32 fl. oz.; digest 10 days, express the tincture, and filter. A most unscientific preparation; said to be tonic and stomachic.—Dose, 1 to 8 drops in water or any bitter infusion. In large doses it is poisonous.
Drop, Black. Syn. Armstrong’s black drop, Lancaster’s b. d., Quaker’s b. d., Toustall’s b. d., Braithwaite’s genuine b. d.; Gutta nigra, L. This celebrated preparation was originally prepared nearly a century and a half ago by Edward Toustall, a medical practitioner in the county of Durham, and one of the Society of Friends. The formula passing into the possession of a relative of his (John Walton, of Shildon), was found among his brother’s papers, and, by the permission of Thomas Richardson, of Bishop’s Wearmouth, one of his executors, was handed to Dr Armstrong, who subsequently published it in his work on typhus fever.
Prep. 1. (Original formula.) Opium (sliced), 1⁄2 lb.; good verjuice, 3 pints; nutmegs, 11⁄2 oz.; saffron, 1⁄2 oz.; boil them to a proper thickness; then add, of sugar, 1⁄4 lb., and yeast, 2 teaspoonfuls. Set the whole in a warm place, near the fire, for 6 or 8 weeks, then place it in the open air until it becomes of the consistence of a syrup; lastly, decant, filter, and bottle it up, adding a little sugar to each bottle. To yield two pints of strained liquor.
2. (Acetum Opii, L.—U. S.) Opium, 8 oz.; nutmeg, 11⁄2 oz. (both in coarse powder); saffron, 1⁄2 oz.; distilled vinegar, 24 fl. oz.; digest on a sand bath with a gentle heat for 48 hours, and strain; digest the residuum with an equal quantity of distilled vinegar for 24 hours; then put the whole into a percolator, and return the filtered liquid as it passes until it runs clear; afterwards pour on the material, fresh distilled vinegar until 48 fl. oz. of filtered liquor shall be obtained; in this dissolve sugar, 12 oz., and gently evaporate the whole to 52 fl. oz.
3. (Wholesale.) Opium, 10 oz., and distilled vinegar, 1 quart, are digested together for about a fortnight, and after sufficient repose the clear portion is decanted. This is the form commonly adopted by the wholesale trade in England.—Dose, 5 to 10 drops. It is usually considered to be of fully 4 times the strength of laudanum.
Drops, Carmin′ative. Syn. Guttæ carminativæ, L. Prep. (Radius.) Oil of mace, 1 dr.; nitric ether, 3 dr.—Dose, 6 to 10 drops on sugar; in flatulent colic, &c.
Drops, Cham′omile. See Essence.
Drops, Dalby’s. See Patent Medicines (Dalby’s Carminative).
Drops, Durande’s. Syn. Guttæ ætheris terebinthinatæ, L. Prep. (M. Durande.) Rectified sulphuric acid, 3 parts; oil of turpentine, 1 part.—Dose, 20 to 30 drops, or more; in the passing of gall-stones.
Drops, Dutch. Syn. Haerlem drops, Turpentine drops; Balsamum terebinthinæ, L. The genuine or imported ‘Dutch Drops’ is the residuum of the rectification of oil of turpentine. It is also prepared by distilling resin, and collecting the product in different portions. At first a white, then a yellow, and lastly a red oil, comes over. The last is the balsam. The article commonly sold under the592 name in this country is prepared by one or other of the following formulæ:—
1. Oil of turpentine, tincture of guaiacum, and sweet spirit of nitre, of each 1 oz.; oils of amber and cloves, of each 15 drops.
2. Balsam of sulphur, 1 part; oil of turpentine, 5 parts. This last is the form most generally employed. They are all regarded by those who use them as detergent, diuretic, stimulant, and vulnerary.
Drops, Female. Syn. Emmenagogue drops; Guttæ emmenagogæ, L. Prep. (Brande.) Compound tincture of aloes and tincture of valerian, of each, 2 fl. oz.; tincture of sesquichloride of iron, 1 fl. oz.—Dose. A teaspoonful in water or chamomile tea; in obstructed menstruation, &c.
Drops, Fit. Syn. Soot drops; Tinctura fuliginis, Guttæ f., L. Prep. From wood-soot, 2 oz.; sal-ammoniac, 1 oz.; salt of tartar, 1⁄2 lb.; soft water, 4 lbs.; digest a week and filter. Reputed antispasmodic, and also useful in scurvy and certain skin diseases.—Dose. A teaspoonful or more, occasionally, in water.
Drops, Golden. Syn. De la Motte’s g. d.; Bestucheff’s nervous tincture; Guttæ aureæ, L.; Elixir d’or, Gouttes d’or du Général Lamotte, Fr. Prep. 1. (Original.) Chloride of iron (obtained by distilling iron pyrites with twice its weight of corrosive sublimate), 3 oz.; alcohol, 7 oz,; expose the mixture in a closely stoppered bottle to the rays of the sun until it becomes decoloured.
2. Chloride of iron, 1 part; alcohol and ether, of each, 3 parts. These drops have the remarkable property of losing their yellow colour in the sun, and recovering it in the shade. They are taken in gout, hypochondriasis, and nervous complaints, in doses of from 10 to 60 drops.
Drops, Hooping-Cough. Syn. Guttæ anti-pertussicæ, L. Prep. 1. (Dr Graves.) Tincture of assafœtida and compound tincture of camphor, of each 1⁄2 fl. oz.; compound tincture of bark, 5 fl. oz.—Dose. A teaspoonful, 2 or 3 times a day.
2. (Potestates Succini.) Oil of amber, 1 oz.; carbonate (not sesquicarb.) of ammonia, 1⁄2 oz.; strongest rectified spirit (alcohol), 1⁄2 pint; digest 3 or 4 days, and decant the clear portion.—Dose, 10 drops to 1 dr., applied as a friction.
Drops, Infantile. Several anodyne, carminative, and absorbent preparations, which pass by this name, will be found under Mixtures, &c.
Drops, Jes′uits’. Syn. Elixir antivenereum, L. Prep. 1. Gum guaiacum, 7 oz.; balsam of Peru, 4 dr.; root of sarsaparilla, 5 oz.; rectified spirit of wine, 1 quart; digest for 14 days.
2. (Quincy.) Copaiba, 1 oz.; gum guaiacum, 2 dr.; oil of sassafras, 1 dr.; salt of tartar, 1⁄2 dr.; rectified spirit, 5 fl. oz.; digest a week.
3. (Walker’s.) Copaiba, 6 oz.; gum guaiacum, 1 oz.; chio turpentine and salt of tartar, of each, 1⁄2 oz.; cochineal, 1 dr.; rectified spirit, 1 quart; digest a week. See Comp. Tincture of Benzoin.
Drops, Kœchlin’s. Prep. (Augustin.) Solution of ammonio-chloride of copper and mercury, 1 fl. dr.; water, 10 fl. dr. In obstinate venereal affections, scrofula, &c.—Dose. A teaspoonful after each meal.
Drops, Lav′ender. Syn. Red drops; Guttæ lavendulæ, L. The same as compound tincture of lavender.
Drops, Life. Syn. Salmon’s drops of life; Guttæ vitæ, L. Prep. Tincture of castor, 8 fl. oz.; antimonial wine and water, of each 1 lb.; opium, 3 oz.; saffron, 1⁄2 oz.; cochineal, camphor, and nutmegs, of each 2 dr.; digest for 10 days and filter. Anodyne and diaphoretic.—Dose, 20 to 60 drops.
Drops, Mercu′′rial. Syn. Guttæ hydrargyri bichloridi, L. Prep. 1. Bichloride of mercury, 2 gr.; hydrochloric acid, 3 drops; rectified spirit and distilled water, of each, 1⁄2 fl. oz.—Dose, 12 to 20 drops.
2. Bichloride of mercury, 2 gr.; sal-ammoniac, 3 gr.; compound decoction of sarsaparilla, 2 fl. oz.—Dose. A teaspoonful.
3. (Sir A. Cooper.) Corrosive sublimate, 1 gr.; dilute hydrochloric acid, 1⁄2 dr.; dissolve, and add tincture of bark, 2 fl. oz.—Dose. As the last. They are all taken 2 or 3 times daily, as alteratives in scrofula, syphilis, cancer, &c. It should not be measured in a metal spoon.
Drops, Norris’s. An aqueous solution of tartar emetic, mixed with spirit of wine, and coloured.
Drops, Odontal′gic. Syn. Tooth-ache drops; Guttæ odontalgicæ, L. Prep. 1. (Dr Blake.) Alum (in fine powder), 1 dr.; sweet spirit of nitre, 7 fl. dr.; agitate together occasionally for an hour.
2. (Dr Copland.) Powdered opium and camphor, of each 10 gr.; oils of cloves and cajeput, of each 1 dr.; highly rectified spirit and sulphuric ether, of each 1⁄2 fl. oz.
3. (Cottereau.) A saturated ethereal solution of camphor, to which a few drops of liquor of ammonia is added.
4. (Dr R. E. Griffith.) Wine of opium, Hoffman’s anodyne, and oil of peppermint, equal parts. Used as a friction on the cheek or gum, as well as applied to the teeth.
5. (Perry’s.) A concentrated ethereal tincture of camphor and pellitory.
6. (Righini.) Creosote, 6 dr.; rectified spirit, 4 dr.; tincture of cochineal, 2 dr.; oil of peppermint, 1⁄2 dr.
7. Camphor, 2 dr.; rectified spirit, 1 oz.
Obs. The above are applied to the tooth with a camel-hair pencil, or a little wad of lint or cotton wool is moistened with them, and placed in or against the tooth.
Drops, Pectoral. Syn. Bateman’s p. d.; Guttæ pectorales, L. Prep. 1. Paregoric, 10 fl. oz.; tincture of castor, 4 fl. oz.; laudanum,593 1 fl. oz.; tincture of saffron or of cochineal, 1⁄2 fl. oz.; oil of aniseed, 15 drops.
2. Castor, 1 oz.; oil of aniseed, 1 dr.; camphor, 5 dr.; cochineal, 11⁄2 dr.; opium, 3⁄4 oz.; treacle, 1 lb.; proof spirit, 1 gal.; digest for a week.
3. (Phil. Coll. of Pharm.) Camphor, catechu, powdered opium, and red sanders wood, of each 2 oz.; oil of aniseed, 4 fl. dr.; proof spirit, 4 old wine-gallons; digest 10 days, and filter.—Dose. A teaspoonful, or more, in coughs, colds, hoarseness, &c., assisted by an aperient.
Drops, Rheumat′ic. Syn. Guttæ rheumaticæ, L. Prep. 1. Iodide of potassium, 1 dr.; tincture of guaiacum, 2 fl. oz.; dissolve.—Dose, 20 to 30 drops. In both chronic and occasional rheumatism, assisted with the copious use of lemon juice.
2. (Lampadius.) Bisulphuret of carbon and ether, of each 2 fl. dr.—Dose, 6 to 12 drops, on sugar, or in milk.
3. (Wutzer.) Bisulphuret of carbon, 1 fl. dr.; alcohol, 2 fl. dr.—Dose. As No. 2. The last two are sudorific, alterative, resolvent, and emmenagogue, and, besides rheumatism, have been used with advantage in amenorrhœa, in some cutaneous affections, in glandular swellings, &c.
Drops, Rousseau’s. See Wine of opium (by Fermentation.)
Drops, Sed′ative. Syn. Guttæ sedativæ, L. The solutions of acetate and hydrochlorate of morphia, black drop, Rousseau’s drop, and Battley’s liquor opii sedativus, are frequently sold under this name by the druggists. The anti-hysteric drops (antè) is also an excellent sedative.
Drops, Spilbury’s. Prep. 1. (Dr Paris.) From bichloride of mercury, gentian root, and dried orange peel, of each 2 dr.; precipitated sulphuret of antimony and red sanders wood, of each, 1 dr.; proof spirit, 16 fl. oz.; digest ten days and strain.
2. Levigated crocus metallorum (‘crocus of antimony’), 6 dr.; corrosive sublimate, 45 gr.; red sanders, 1⁄2 dr.; gentian root and dried orange peel, of each 2 dr.; brandy (or equal parts of rect. sp. and water), 16 fl. oz.; digest as before.—Dose, 5 to 30 drops; as an antiscorbutic, &c.
Drops, Steel. See Tincture of Sesquichloride of Iron.
Drops, Ton′ic. Prep. (Collier.) Elixir of vitriol, 2 fl. dr.; tincture of calumba, 6 fl. dr. A teaspoonful three times daily, in a wine-glassful of cold water.
Drops, Torrington’s. See Tincture of Benzoin (Comp.).
Drops, Van Swieten’s. An aromatised solution of corrosive sublimate.
Drop, Ward’s White. Prep. From quicksilver, 4 oz.; nitric acid, 16 fl. oz.; dissolve, add sesquicarbonate of ammonia, 7 oz.; evaporate and crystallise; then dissolve the resulting salt by the heat of a sand bath, in 4 times its weight of rose-water. Very poisonous.—Dose, 5 to 15 drops, as an antiscorbutic, antivenereal, &c.
Drops, Worm. Syn. Guttæ anthelminticæ, G. vermifugæ, L. Prep. 1. Creosote, 1 dr.; oil of turpentine, 7 fl. dr.—Dose. A teaspoonful, 3 or 4 times a day.
2. (Peschier.) Oil of male-fern, 3 fl. dr.; rectified oil of turpentine, 5 fl. dr. As the last; in tapeworm.
3. (Schwartz’.) Barbadoes tar, 1 fl. oz.; tincture of assafœtida, 11⁄2 fl. oz.—Dose, 30 to 40 drops, three times a day; in tapeworm.
DROPS (Scouring). Prep. 1. Oil of turpentine and oil of lemons, equal parts. Both of the ingredients should have been recently distilled or rectified.
2. Oil of lemon bottoms, 13⁄4 lb.; oil of turpentine, 1 quart; mix well, and distil by the heat of a sand bath, until 3 pints have come over, or as long as the distillate is clear, pale, and sweet. Used to remove paint, grease, &c., from cloth.
DROPSY. Syn. Hydrops, L. An unnatural collection of aqueous fluid in any part of the body. Dropsy has received different names, according to the part of the body affected by the disease. When it occurs in the cellular membrane it is called ANASARCA; when in the cavity of the abdomen, Ascites; in the cavity of the cranium, HYDROCEPHALUS; in the scrotum, HYDROCELE; in the uterus, HYDROMETRA; and in the chest, HYDROTHORAX. Dropsy is mostly a symptom of extreme debility and a broken-down constitution, and frequently follows lengthened attacks of exhausting chronic diseases.
The treatment of dropsy, perhaps, more than any other disease, depends upon the circumstances with which it is connected, and, more especially, upon those which have caused it. The acute inflammatory forms of dropsy generally require depletion. In most other cases, tonics may be advantageously administered. To promote the absorption of the accumulated fluids, diuretics are commonly resorted to. Confirmed dropsy (especially HYDROCEPHALUS and HYDROTHORAX), occurring in patients either much debilitated by previous disease or of a bad habit of body, is seldom curable.
DROWNING. The cause of death from submersion in water is the entire seclusion of air from the lungs, by which the aëration of the venous blood is prevented. In consequence of this deprivation of air, venous blood circulates through the arterial system, whilst the pulmonary vein ceases to convey oxygenated blood to the heart. Under ordinary circumstances, in the course of 4 or 5 minutes after the access of air has been cut off, life becomes extinct. Many cases have, nevertheless, occurred of persons being submerged for 15 or 20 minutes, and even longer, and where perfect insensibility has existed, in which recovery has taken place.
Prev. The specific gravity of the human594 body is less than that of water, so long as the lungs are partially filled with air; and this difference is sufficient to keep the body floating with the mouth and nostrils free for respiration, provided the face is turned upwards by throwing the head back on the shoulders, by which the weight of the head is sustained by the water. When a person throws himself into the water, the body rises rapidly to the surface and assumes nearly the erect position, the upper part of the head, down to a little below the eyes, remaining above the surface of the water. This arises from the greater density of the legs and thighs compared to that of the chest, which acts as a species of float or buoy to the rest of the body. In this situation the head may be thrown back, so that the face may form the exposed portion, as before mentioned, when respiration may be carried on without inconvenience in still water, and regularly, but sufficiently, so as to sustain life for some time, even in a rough sea. The adoption of this simple precaution would have saved thousands of valuable lives.
Another point which should be remembered by every person in danger of drowning is, that there is always a considerable amount of residual air in the lungs, in a nearly deoxidised state, and that if this air is expelled by two or three forced inspirations, and a deep inspiration is then taken, a larger quantity of vital air will be introduced into the lungs, and the blood will continue aërated for a proportionally longer time; and consequently, a longer period will elapse before another inspiration will be required. If we prepare ourselves by taking two or three forced inspirations, and then take a full inspiration, we may remain for 11⁄2 or 2 minutes before a second attempt at respiration need be made. This is the plan adopted by the pearl fishers, and other divers who are remarkable for remaining beneath the surface of the water for some time. A person in danger of shipwreck, or expecting immediate submersion, in any other situation, should have recourse to this expedient, which would prevent the dreadful effects of attempting respiration whilst under water.
Treat. The first object is the restoration of the animal heat. For this purpose, the wet clothes should be removed, and the body, after being well dried, surrounded with warm air. In the absence of a warm-air bath, the body may be laid between well-heated blankets, and bottles of hot water applied to the feet and armpits. Gentle friction with warm flannel or the hands should also be assiduously employed. Meanwhile attempts should be made to excite respiration artificially; and when the apparatus is at hand, slight shocks of electricity should be kept up at the same time. On the appearance of returning life, such as sighing or convulsive twitching, a vein may be opened. The throat may be tickled with the finger or a feather, to excite vomiting, and a teaspoonful of warm water administered. If the power of swallowing exists, a table-spoonful of warm wine or brandy and water may be given. Even if no symptom of returning animation appear, these means of recovery should be persisted in for three or four hours.
In the treatment of this species of asphyxia, nasal stimulants, as ammonia, aromatic vinegar, &c., should be avoided, as well as the injection of tobacco smoke, both of which have been found highly prejudicial. The practice of holding the body with the head downwards, which is sometimes adopted by the vulgar and ignorant, under the idea of allowing the water to run out by the mouth, is still more dangerous and absurd. The supposition that water is inhaled by drowning persons instead of air is perfectly fallacious. The peculiar mechanism of the glottis, or upper portion of the windpipe, is such as to prevent, by the spasmodic closure of the epiglottis, the entrance of more than a very trifling and accidental quantity of water, which is altogether too insignificant to produce any very injurious effects. See Asphyxia.
DRUGS. Substances used in medicine, sold by druggists, and compounded by apothecaries and physicians. Our continental neighbours, wiser than ourselves, not merely require that persons engaged in selling and dispensing drugs and pharmaceutical preparations shall be fully qualified by previous education and training for the task, but also that the various articles they sell and use shall be commercially pure and of the proper quality. In the United States of America this subject has also engaged the attention of the government and legislature. Under the Act of the 26th June, 1848, inspectors were appointed to examine the quality of imported articles of this class before allowing them to pass the Customs for home use. An abridged copy of the order addressed to the “collectors and other officers under this act” is appended, and will be useful to the reader, as assisting to establish a standard by which the value of the substances named therein may be estimated.
[264] Of whatever denomination.
[265] Root in powder.
[266] Only Turkey, East Indian, and Russian, admissible.
DRUM′MOND LIGHT. See Light (Artificial).
DRUNK′ENNESS. See Abstinence, Intemperance, &c.
DRY′ERS (Painter’s). Prep. 1. Litharge (best) ground to a paste with drying-oil. For dark colours.
2. From white copperas and drying oil; as the last.
3. From sugar of lead and drying oil. The last two are for pale colours.
4. From white copperas and sugar of lead, of each 1 lb.; pure white lead, 2 lbs. For ‘whites,’ and opaque light colours, greys, &c.
Dryers are employed, as the name implies, to increase the drying and hardening properties of oil paints. A little is beat up with them at the time of mixing them with the oil and turpentine for use.
DRY′ING. See Desiccation, &c.
DRY′ING-OIL. See Oils.
DRY-ROT. A peculiar disease that attacks wood, and renders it brittle and rotten. It is generally caused by dampness and the subsequent development of the spores of fungi, particularly those of Merulius lacrymans and vastator and Polyporus destructor. The dry-rot principally attacks ‘ill-seasoned’ timber, and more particularly that of ships and badly ventilated buildings.
Prev. Various means have been proposed to prevent the attacks of dry-rot and to arrest its progress when it has commenced, among which the process called ‘Kyanizing’ (Kyan’s patent) is that most generally known and most extensively adopted. It consists in immersing the timber in a bath of corrosive sublimate. The process termed ‘Paynizing’ (Payne’s patent) consists in first filling the pores with a solution of chloride of calcium, under pressure, and next forcing in a solution of sulphate of iron, by which an insoluble sulphate of lime is formed in the body of the wood, which is thus rendered nearly as hard as stone. Wood so prepared is now largely employed in our public works and railways. Sir W. Burnett’s process (patented in 1836) consists in impregnating the timber with a solution of chloride of zinc. Mr J. Bethell’s process (patented in 1838) consists in thoroughly impregnating the wood with oil of tar containing creasote and a crude solution of acetate of iron, commonly called ‘pyrolignite of iron.’ The impregnation is effected in a strong cylindrical vessel, connected with a powerful air-pump, so that in the first instance a vacuum being formed, and subsequently a pressure of several atmospheres applied, the liquid may as much as possible be forced into all the pores of the wood. The above processes for ‘seasoning’ preserve the timber not only from dry-rot, but from the influence of the weather and the attacks of insects and worms.
“The construction of air-drains or passages around wood-work to be preserved is, where the method is applicable, a great aid to the preservation of wood. Dry-rot is both prevented in new buildings and cured in old ones by filling up the spaces between the floor-joists with ‘tank-waste’ from alkali works. This can also be applied to the ends of beams resting in walls.”—Chemical News.
DUB′BING. Prep. 1. By boiling the waste cuttings of sheep-skins in crude cod oil. 2. Black resin, 2 lb.; tallow, 1 lb.; crude cod oil or train oil, 1 gall.; boil to a proper consistence. Used by the curriers to dress leather, and by shoemakers and others to soften leather, and to render boots and shoes waterproof.
DUBOISIA MYOPOROIDES. (Nat. order, Solanacæ.) A small tree growing in Australia, New Caledonia, and New Guinea. The leaves have been used in Brisbane and Sydney as a substitute for atropine, and extract of belladonna; to both of which Mr Tweedy believes them to be superior in prompt and energetic action. Mr Tweedy further states that, in every case in which he has used duboisia to produce dilatation of the pupil of the eye, its action has been beneficial, and he is induced to conclude, more advantageous than that of atropine. According to Dr Ringer, duboisia, besides causing dilatation of the pupil, quickens the pulse, parches the tongue, stops the secretions of the skin, and induces headache and drowsiness. He also reports that it is antagonistic in its action to muscarine, and produces tetanus after the lapse of some hours or days.
For an account of the botanical properties of the plant, the reader is referred to a paper by Mr E. M. Holmes in the ‘Pharmaceutical Journal’ for March 9th, 1878; and to the ‘Lancet’ of March 2nd, 1878, for some experiments on its physiological effects by Messrs Ringer and Tweedie. The Duboisia myoporoides596 was introduced into medical practice by Dr Bancroft, of Brisbane.
Since the above has been written, Mr Gerrard has obtained a powerful alkaloid from an extract of the leaves of the Duboisias, very similar in chemical properties to aconite, and possessed of the same physiological qualities as the extract.
DUCK. See Poultry.
DUCTIL′ITY is the property of being drawn out in length without breaking. See Metals.
DULCAMA′RA. See Nightshade (Woody).
DUMB′NESS. Syn. Aphonia, L. As speech is an acquired and imitative faculty, persons who are either born deaf or become so in early infancy are also, necessarily, dumb. The first step in treating dumbness must therefore be directed to the removal of the deafness on which the imperfection rests. The exertions of modern philanthropists have, however, been so far successful in such cases as to enable the deaf-mute to converse with those around him by signs. Those interested in the subject may consult an admirable treatise on ‘Deaf-dumbness,’ by M. E. Hubert-Valleroux, of which an excellent translation appeared in the ‘Medical Circular,’ vol. ii, for 1853. See Deafness.
DUMPLINGS, Norfolk. Mix half a pound of flour with half a teaspoonful of baking powder and a pinch of salt; make into a little dough with cold water; fall into small balls, put them into boiling water immediately, and boil for twenty minutes.
DUNGER—MANURE (Boutin, Paris). A bluish-green fluid, containing about 190 grammes of solid matter per litre. The residue consists of sulphates of copper, iron, magnesia, and soda, sal ammoniac, nitrates of potash and soda, common salt, and none or a mere trace of phosphoric acid. The blue deposit which separates on standing is ultramarine. (Keller, Karmrodt, and Nessler.)
DUNG′ING. Syn. Cleansing. One of the principal processes in the arts of calico printing and dyeing, its object being to free the cloth from loose matters, which would interfere with the dyeing. After the thickened mordants have been applied to the fabric and properly fixed, it is necessary to remove the now useless thickening matter, together with the excess of mordant, which has not come into actual contact with the cloth. Formerly a bath formed of cow-dung, diffused through hot water (130° to 212° Fahr.) was always used to wash away these loose matters; but now various manufactured substances are successfully employed for the purpose. The best dung substitutes are the arsenite and arseniate of soda, the silicate of soda, and phosphate of lime. Experience proves that, in the case of these substitutes, a final rinse in cow-dung before dyeing is advantageous. A process very similar to ‘dunging’ is employed after dyeing, to clear and give purity to the undyed parts. This subsequent process is distinguished by the term ‘clearing.’ Cow-dung has been used in ‘clearing’ operations, but its employment is not to be recommended. Bran scalded and mixed with water is employed for certain goods, but bleaching powder is the most generally used ‘clearing agent.’
DUST, ATMOSPHERIC. When a ray of sunlight is admitted into a dark room, or an electric beam is transmitted through a glass tube, myriads of little motes are revealed, which move and dance about in all directions.
In ordinary daylight these minute particles are invisible. Nevertheless, they are always more or less present in the atmosphere wherever (except under special conditions) this permeates, and they constitute that more or less attenuated, impalpable, generally dry, or dessicated form of matter which we denominate dust.
As with every inspiration we take into our bodies more or less of this suspended material, the study of the composition and characters of the different substances which compose it is one possessed of paramount interest, both for the pathologist and sanitarian.
Amongst solid, inorganic matters found in the open air are silica, peroxide of iron, silicate of alumina, carbonate and phosphate of lime, sand, carbon, chloride of sodium, and metallic iron. These, of course, are of telluric origin, and are carried into the atmosphere by strong currents and winds, which latter have the power of transporting dust to great distances, e.g. red sand from the interior of Africa has been found in the sails of ships 600 or 800 miles distant from the African coast, whilst particles of carbon, sand, and dried mud, ejected to great heights from volcanoes into the air, have been transported over still greater distances.
Some doubt appears to prevail as to whether all dust storms originate on the earth, it having been conjectured that some of the solid matters found in the atmosphere may be of meteoric origin, and may have entered it from the realms of space. The chloride of sodium (which the chemist knows is so omnipresent that he cannot heat an ordinary platinum wire in a Bunsen burner without indications of its presence) is derived from the spray of the sea, lifted and diffused into the air by the wind; the iron dust from the rails over which railway trains are constantly passing; the silica, amongst other sources, from the traffic over macadamised thoroughfares.
The organised and organic substances contained in the external air are very numerous. The animal kingdom is the source of the wings of moths, butterflies, and other insects, spiders’ legs and webs, hair, wool, epithelium, and eggs, many of these bodies being mere débris.
The vegetable kingdom contributes spores, pollen, cells, cotton fibre, and the germs of vibriones and monads. Besides these are many living creatures, brought by the agency of monsoons and cyclones from extensive597 deserts. Showers of sand derived from these wastes occur in different parts of Europe. Ehrenberg submitted the sand obtained during seventy of these showers to microscopic examination, and found, in addition to sand and oxide of iron, numerous organic forms, amongst which 194 Polygastrica, 145 Phylolithariæ, besides Polythalmia, &c. Silvestre found four species of diatoms and living infusoria in the sand obtained from a dust shower in Sicily in 1872. But, besides the presence of these organisms in the external air, which may be regarded exceptional, it contains, under ordinary conditions, numerous living creatures, some brought into it from the earth by the force of winds, others growing in it. More than 200 forms—rhizopods, tardigrades, and Anguilulæ—have been found in it by Ehrenberg. So tenacious of life are these latter that, even if dried, they will retain their vitality for months, and even years.
Of the organisms found in the air the following are the most important:—1. Extremely small, round, and oval cells, which, that they may be rightly examined by the microscope, require a power of 600 or 1000 diameters. They are found sometimes growing together and sometimes cleaving, when they present an appearance like the figure 8. Sulphuretted hydrogen in the air is said to stimulate their growth and carbolic acid to check it. Although existing in the open air, they are by far more abundant in the atmosphere of dirty prisons. They are also met with in the sweat of the prisoners inhabiting these localities. Observers believe they increase rapidly by cleavage. No ill effects have been traced to them.
“To the same class, perhaps, of these round and oval cells the bacteria and monads, which have been described as gathered from the air, must be assigned; the development of these cells into vibriones and rod-like bacteria, though asserted, has not yet been definitely proved, and, indeed, Burden-Sanderson’s observations rather throw doubt on the statement that true bacteria exist in the air.
“2. Spores of fungi are not infrequent in the open air; they occur most commonly in the summer (July and August); they are not in this country more frequent with one wind than another; the largest number found by Maddox in ten hours was 250 spores; on some days not a spore can be found. Maddox leaves undetermined the kind of fungus which the spores developed under cultivation; the spores were pale or olive coloured and oval, probably from some form of smut. Angus Smith found in water through which the air of Manchester was drawn innumerable spores. Mr Dancer has calculated that in a single drop of the water 250,000 fungoid spores as well as mycelium were present; but as the water was not examined for some time there may have been growth. Mycelium of fungus seems uncommon in the air, but is sometimes found.
The cells of the Protococcus pluvialis are not uncommon, neither, perhaps, are those of other algæ. On the whole the experiments of Maddox show that in his locality (near Southampton) it is incorrect to speak of the air being loaded with fungoid spores; they can be found, but are not very numerous.”[267]
[267] Parkes.
Amongst other suspended matters are minute fragments of dried horse droppings, derived from the original substance, reduced to powder by the traffic, and carried by aerial currents into the atmosphere. In the ‘Chemical News’ for October, 1871, Professor Tichborne gives the results of some analyses of the street dust of Dublin. Some dust taken from the top of a pillar 134 feet high contained 29·7 per cent. of organic matter, whilst that collected from the street consisted of as much as 45·2 per cent. This organic matter was principally composed of comminuted stable manure; it was capable of acting as a ferment, and was possessed of deoxidising powers sufficient to reduce nitrate to nitrite of potash.
This evidence of the presence of suspended known matters in the air has led some pathologists to conjecture that certain formless substances found in it, undeterminable by the microscope, may in reality be disease germs, which, being transported through long distances by the wind, may also be the means of spreading certain maladies from one locality to another. In this manner cholera has been supposed to have been propagated from India, the particles of the dried excreta of cholera patients being supposed to be the carriers of the formidable disease; this hypothesis of its origin, however, is not yet, at any rate, universally accepted. “In the case of smallpox and scarlet fever the distance to which the ‘contagions’ spread by means of the air is certainly inconsiderable.”[268]
[268] Ibid.
Hitherto we have spoken only of the nature of the dust occurring in the external air. The composition of that met with in confined spaces is, of course, largely influenced by surrounding conditions and circumstances; for instance, in indifferently ventilated apartments, in addition to the substances already enumerated, the dust of the confined air has been found to contain small particles of food, bits of the hair of human beings, domestic animals, and feathers of birds, as well as of coals, cinders, charred wood, linen, cotton, and wool fibres, varieties of epithelium, and certain round cells resembling nuclei.
In the apartments of the sick it is additionally charged with a very large quantity of organic matter.
The spores of the Tricophyton and Acorion have been discovered in and seem peculiar to skin hospitals. In that taken upon two occasions from the ward of St. Louis (the Skin Hospital of Paris), and submitted to examination, one specimen was found additionally to598 contain 36 per cent. and in the other 46 per cent. of organic matter.
“The scaly and round epithelia found in most rooms are in large quantity in hospital wards, and probably in cases where there is much expectoration and exposure of pus or puriform fluids to the air the quantity would be still larger.”[269]
[269] Parkes.
The investigation of the air of a cholera ward in 1849 by Britain and Swayne, at Clifton, revealed the presence of bodies resembling fungi; minute scales of variolous matter have been found by Bakewell in smallpox wards, and cells of pus and epithelium in the sheds and stables of animals affected with cattle disease and pleuro-pneumonia. Dr Watson detected in the air of a ward for consumptive patients at Netley, together with pus cells, bodies bearing a great resemblance to the cells met with in tuberculous matter, these latter not being discoverable in the open air or in the rooms of non-consumptive persons; whilst Rainy, examining the air of the cholera ward at St. Thomas’s Hospital, found bacteria in it, besides fungi. The presence of these bodies was, however, detected in the open air.
The atmosphere of mines, workshops, manufactories, and rooms in which handicraft of any kind is carried on, is more or less laden with small particles of substances employed in the arts, manufactures, and various industries. The nature of these floating substances, as well as a list of the diseases, together with the amount of mortality they produce, will be found under the article “Trades, certain, their effects on Health.”
Dr Wynter Blyth gives the following instructions for collecting atmospheric dust for examination:—
“The most simple way to obtain the emanations from a sick room for microscopical observation is to suspend a common water bottle from the ceiling filled with iced water. The moisture of the air condenses, and brings with it organic matters; or the moisture may be gathered which adheres to panes of glass in cold weather; or a bottle may be taken containing some distilled water, absolutely free from impurities of any kind, and filled several times with the air of the place. The water may then be submitted to microscopical and chemical examination.
“Metallic dust, such as iron, may be attracted by a magnet. The most usual and successful way is, however, by aspiration, either by an aspirator made for the purpose [see Aspirator], or by means of an ordinary cask, by which a considerable volume of air is drawn through a small quantity of distilled water, glycerin, or other liquid. The indirect way for the organic matter, &c., mentioned above, viz., analysis of the rain water, and the obvious way of collecting the dust, by carefully sweeping it off shelves, &c., may be also enumerated.
“Examination of dust. The dust obtained by any or all of these methods should now be examined microscopically and chemically. Low powers should be used at first, and then (if looking for germs) the highest that can be obtained. If the dust is in any quantity it can be submitted to chemical examination, but a knowledge of what class it belongs to—animal, mineral, or vegetable—is sufficient for most purposes.”[270]
[270] ‘Dictionary of Hygiene.’
DUST-BIN. A dust-bin on any premises may become a nuisance and a peril to health if certain precautions are not observed with respect to it.
It should have a tolerably tight-fitting cover, and one that is waterproof also, if, especially as it ought to be, the dust-bin is situated in the open air. The bottom should never be the bare earth, but one that is properly bricked or tiled. It should be lime-washed occasionally, in summer time the most frequently. Only dry refuse, such as ashes and the sweepings of rooms, &c., should be thrown into it.
On no account should fragments of vegetable or animal nature be put in, such as fishbones, potato parings, cabbage stalks, dirty or discarded pieces of apparel, or bits of rags or dusters. These should be at once burnt on the kitchen fire; the best kind of stove for consuming these is that known as the kilnhouse. Meat bones should be got disposed of as soon as possible, as they frequently give rise to unpleasant and offensive odours. Finally, the dust-bin should not be too large. If too capacious, it acts as a guile for servants not to have it cleaned out as often as it should be, the frequent removal of its contents being a most essential condition toward the preservation of health.
DUSTING. This very important branch of household labour is sometimes very inefficiently performed. Very frequently the dust of an apartment is not removed, but merely disturbed or driven from one place to settle down on another.
It should always as much as possible be got rid of by means of a duster or a brush and dust-pan.
As the dust should adhere to the former, this should from time to time be taken out into the open air and shaken. During the time a room is being dusted the furniture should be collected in as small a space as possible, and enveloped in the dusting-sheet. The dusting-sheet on its removal should be carefully folded together, taken into the air and shaken. The furniture may then be dusted and returned to the proper places.
A duster should never be rubbed over furniture standing close to a wall, or a dirty mark on the wall-paper will be the result. The same caution applies to mantel-pieces, where599 the paper may soon be spoilt by the act of dusting, unless contact with the duster be avoided.
DUTCH DROPS. The dark-coloured residue left by the dry distillation of turpentine. (Hager.)
DUTCH GOLD. See Alloy.
DUTCH LIQ′UID. See Olefiant Gas.
DYE′ING. The act of tinging or colouring absorbent materials by impregnating them with solutions of colouring matters or dye-stuffs. The colouring matters which impart their tints without the intervention of other substances are called ‘substantive colours’; while those which require such aid are called ‘adjective colours.’ The bodies employed to fix and develop the latter class are called ‘mordants.’ The exact way in which dye-stuffs act upon fibrous materials has not yet been investigated as fully as it deserves; the generally received opinion is that the fibre has a chemical affinity for the colouring matter in the case of substantive dyes, and likewise for the mordant, which, in its turn, has an affinity for the colouring matter of adjective dyes. Another opinion is that the fibres have pores, which, when expanded by heat or chemical agents, admit particles of colouring matter. However this may be, it is certain that different materials ‘take’ dyes in different proportions; thus, silk and wool take the coal-tar dyes in the most perfect manner, but cotton requires the intervention of a most powerful mineral or animal mordant. Wool takes the colouring matters of most dye-stuff so well that the deepest tints can readily be produced. Silk and COTTON are dyed with greater difficulty, whilst LINEN shows still less disposition to take dyes. The operations which take place in dyeing are ‘mordanting,’ ‘ageing,’ ‘dunging,’ ‘dyeing,’ and ‘clearing.’ The first of these operations is noticed under Mordant. After the fabric has been mordanted, it is generally hung up in a room through which a current of steam and air is passing, by means of which the union between the fibre and the mordant is quickened very considerably. This exposure to moist air is the step in the process to which the term ‘ageing’ is applied. The operations of ‘dunging’ and ‘clearing’ are noticed above (see Dunging). The ‘dyeing’ proper, which follows the ‘dunging,’ is effected by running the fabric through the solution of the dye-stuff, the colour being modified more or less by the nature of the mordant used. Under the names of the different colours the means used to dye such colours are minutely described. See Black Dye, Blue Dye, &c.
The following particulars respecting the production of the more common colours may prove interesting to the reader, who merely requires some general information on the subject:—
Black is usually produced by logwood or galls with an iron mordant. Common black silks are dyed with logwood and fustic, iron being used as a mordant. The best silks are dyed black on a blue ground. Woollen goods are first dyed blue with indigo, and afterwards with sumac, logwood, and green or blue copperas. Cotton and linen goods are dyed black in a very similar manner.
Blue is commonly produced from indigo, either in the form of sulphate or in aqueous solution. Prussian blue, with a persalt of iron or tin as a mordant, gives a very splendid dark blue. Of late several blues of novel shades have been produced from coal-tar.
Red is obtained in various shades by using cochineal, safflower, lac-dye, madder, or logwood, with a tin mordant.
Purple. Until the last few years the dyer was dependent for his purples on orchil or cudbear, but he has now at his disposal the magnificent series of aniline, or coal-tar, colours, ranging from the most delicate violet, or ‘mauve,’ to the full crimson-purple, known as ‘magenta.’ See Purple Dye.
Yellow. The most important yellow dyes are made from quercitron, fustic, turmeric, arnotto, and French and Persian berries. For further information, see Bleaching, Calico-printing, &c.
DYER’S SPIRITS. See Tin Mordants.
DYES. See Dyeing, and the names of the principal colours.
DYE-STUFFS. The colouring materials used in dyeing are so called. The more important of them are noticed under the respective names.
DYNAMITE. Nobel’s dynamite consists of a mixture of 75 parts of nitroglycerin incorporated with 25 parts of an infusorial earth known as ‘kieselghur,’ found at Luneburgh, and consisting of the fossil shells of infusoria. Kieselghur is almost pure silica. Dynamite is in regular use on the Continent for mining operations, and its manufacture and transport appear to be subject only to reasonable precautions. If ignited in the open air, or even when loosely packed, it burns quietly away, with the evolution of a small quantity of nitrous acid. Although the first cost of dynamite is four times that of gunpowder, it is said to be really only half as expensive, since it possesses eight times the explosive power of the latter; added to which the labour of boring blast-holes is avoided. It also possesses the advantage of not being impaired in efficiency by damp.
When required for use the dynamite is rammed into a thick paper cartridge, into which a fusee is passed, by means of which it is ignited. Although dynamite when once made may be comparatively harmless until exploded at will; that great risk is incurred in its manufacture may be inferred from the fact that, upon two occasions the manufactory on the Continent in which it is prepared has been twice entirely destroyed. On the occasion of the last accident it was impossible to600 learn the cause of the disaster, since every one in the building was blown to atoms.
Diralin is said to be a mixture of nitroglycerin with sawdust or wood-pulp as used in paper-mills, the two latter substances having been previously treated with nitric and sulphuric acids.
DYNAMOM. (Dr Momma Düsseldorf.) A galvano-electric curative apparatus. A small capsule of horn, containing a disc secured to a pedicel. On the disc a number of sharp needles are fixed. By gently moving the apparatus, and afterwards withdrawing it, artificial pores are produced in the skin by punctures which are not very painful. These are then to be rubbed with a certain oil, probably containing cantharides. (Wittstein.)
DYS′ENTERY. Syn. Bloody flux; Dysenteria, L. A disease arising from inflammation of the mucous membrane of the large intestines, and characterised by stools consisting chiefly of blood and mucus, or other morbid matter, accompanied with griping of the bowels, and followed by tenesmus. There is generally more or less fever, and the natural fæces are either retained or discharged in small, hard balls (scybala). The common causes of this disease are marsh miasma, improper diet, excessive exhaustion, and fatigue, and, above all, exposure to the cold and damp air of night after a hot day.
Treat. The common dysentery of this country generally gives way to gentle aperients (castor oil or salts-and-manna), to cleanse the bowels, followed by mild opiates or morphia, to allay irritation. The chronic symptoms, which frequently hang about for some time, are best combated by mild tonics and vegetable bitters (bark, calumba, cascarilla). Occasionally, chalybeates (ammonia-citrate of iron, lactate of iron, wine of iron, saccharine carbonate of iron) will be found useful during convalescence. Throughout, the diet should be light and nutritious.
The contagious dysentery, of camps and hot climates, is a severe and often fatal disease, in which the preceding symptoms are complicated with remittent or typhoid fever. Its treatment is tedious and difficult, and depends chiefly on judiciously meeting the several symptoms as they develop themselves. Aperients, diaphoretics, and nauseants, followed by tonics, are the remedies generally relied on. The febrile symptoms must be treated according to their inflammatory or putrid tendency. This variety of the disease frequently gives rise to organic diseases of the abdominal viscera, dropsy, &c. It is regarded by some as contagious, but without sufficient reason.
DYSMENNORHŒ′A. See Menstruation.
DYSPEP′SIA. [L.] Syn. Dyspep′sy, Indigestion. This complaint pervades every rank of society, and is, perhaps, of all others, the most general. Few indeed are there who wholly escape it, in one or other of its forms. The common symptoms of dyspepsia are—want of appetite, sudden and transient distensions of the stomach, frequent eructations, heartburn, stomachic pains, occasional vomiting, and, frequently, costiveness or diarrhœa. Sometimes the head is affected, and dimness of sight, double vision, muscæ volitantes, and slight vertigo, are experienced, along with a multitude of other symptoms, depending on a derangement of the functions of the nervous system.
The causes of dyspepsia are numerous. In the higher ranks of society it is a common consequence of over-indulgence in the luxuries of the table, of late hours, or of the want of proper exercise, both of body and mind. In the studious, and those who lead a sedentary life, it is usually caused by excessive mental exertion or anxiety, or by the fatigues of business, and the want of sufficient bodily exertion and of pure air. In the lower orders of society it generally results from inebriety, or a deficiency of proper food and clothing, bad ventilation, &c.; and is not unfrequently occasioned by the physical powers being over-taxed, especially soon after meals.
The treatment of dyspepsia depends less on medicine than on the adoption of regular habits of life. Moderation in eating, drinking, and the indulgence of the passions; early rising, due exercise, and retiring to rest at an early hour, will do much to restore the tone both of the stomach and nerves. Excessive study and mental exertion should be avoided, and recourse should frequently be had to society and amusements of a lively and interesting character. If the bowels are confined, mild aperients should be taken, and if diarrhœa is present, antacids and absorbents may be had recourse to with advantage. The stomach may be strengthened by the use of mild bitters, tonics, and stimulants, and sea bathing, or the shower or tepid bath, may be taken, when convenient, to strengthen the nervous system. When dyspepsia is a secondary or symptomatic disease, the cause should be sought out, and the treatment varied accordingly. Among the aperient medicines most suitable to dyspepsia may be mentioned—Epsom salts, phosphate of soda, and Seidlitz powders, each of which should be taken largely diluted with water. An occasional dose of the ‘Abernethy Medicines’ (which see) has also been recommended. Among antacids, are the bicarbonates and carbonates of potassa and soda, either of which may be taken in doses of half a teaspoonful dissolved in water; or, if the spirits are depressed, one or two teaspoonfuls of spirit of sal volatile will be more appropriate; and in cases accompanied by diarrhœa, a little prepared chalk. As bitters, the compound infusion of orange peel, or of gentian, are excellent. As tonics, small doses of bark, or of sulphate of quinine, to which chalybeates may be added, if there is pallor of countenance, or a low pulse, with no disposition to fever or headache.
601
When dyspepsia is complicated with hysteria, hypochondriasis, or chlorosis, the treatment noticed under those heads may be conjoined to that above recommended. When it depends on constipation, or a deficiency of bile, the mildest and most effective of all remedies will be found supplied in inspissated ox-gall. “In all cases of incipient constipation, ox-gall is a remedy of undoubted efficacy; and even in protracted cases, when hope has almost fled—but where evidences of strangulation are not unequivocally manifested—it should never be omitted by the practitioner. In habitual or chronic constipation, accompanied by indigestion, clay-coloured stools, and a feeling of oppression after food has been taken, it acts with almost specific certainty. When, however, the liver begins to assume its healthy action, its employment should be discontinued, and it will then produce all the symptoms of regurgitation of bile into the stomach. This state will be readily recognised as a favorable omen of returning power.” (Dr Allnatt.)
DYSPNŒ′A. Difficulty of breathing. It is generally symptomatic of some other affections. When it occurs in persons of a nervous or irritable habit of body, perfect quiet, a semi-recumbent posture, fresh air, and some small doses of ether, ammonia, or opium, will generally effect a cure. Those of a full habit require aperients and depletion. To prevent attacks of the kind, excess in eating and drinking, and the use of stimulants, should be avoided.
DYSU′′RIA. [L.] Syn. Dys′ury. Difficult urination. It is generally symptomatic of disease of the kidneys, bladder, or urethra. The treatment depends on the exciting cause.
EAR (Inflamma′tion of). Syn. Otitis, L. This affection, when it attacks the internal part of the ear, is generally accompanied with confusion of sound, deafness, and more or less fever. It is most frequent among children, and is commonly produced by exposure to draughts of cold air, and, occasionally, by foreign matters, as cherry-stones, insects, &c., having got into the external ear. In such cases, the removal of the offensive matter, and due attention to warmth and cleanliness, with a dose of laxative medicine, will be all the treatment required. The pain may generally be relieved by throwing warm water into the ear by means of a syringe, and fomenting the surrounding parts with decoction of poppy-heads and chamomile flowers. Should this treatment not succeed, a drop or two of laudanum, with one drop of oil of cloves and a little oil of almonds, may be dropped in the ear, and a piece of cotton wool introduced afterwards. Cases of acute inflammation of the internal ear are occasionally met with in adults, which assume a very serious character, and demand the most careful treatment. See Deafness.
Earache. Pain in the ear may arise from various causes, amongst which, in the absence of organic disease, cold, and that peculiar derangement of health popularly called ‘nervousness,’ are the most common. In the one case, the proper remedy is warmth; in the other, the attention should be directed to the restoration of the body to the healthy standard.
Earache, Simple Cure for. Take a common, tobacco-pipe, place a wad of cotton in the bowl, drop upon it 8 or 10 drops of chloroform, and cover with another wad of cotton; place the stem to the affected ear, then blow into the bowl, and in many cases the pain will cease almost immediately.—Amer. Journ.
EARTHS. In chemistry, a group of metallic oxides. The principal earths are baryta strontia, lime, magnesia, alumina, berylla or glucina, yttria, zirconia, and thoria. The first four are termed ALKALINE EARTHS; the remainder, together with the oxides of the very rare metals erbium, terbium, norium, cerium, lanthanum, and didymium, constitute the EARTHS PROPER.
The term earth was very loosely applied by the older chemical and pharmaceutical writers, and the practice is still common among the vulgar at the present day. Thus, ABSORBENT EARTH (chalk); ALUMINOUS E., ARGILLACEOUS E. (alumina); BOLAR E. (bole); BONE-E. (phosphate of lime); FULLER’S E. (an absorbent clay); HEAVY E. (baryta); Japan e., or terra Japonica (catechu); SEALED E. (bole), &c., are names even now frequently encountered both in trade and in books.
EARTHEN-WARE AND GLASS, to prevent the Cracking of. When quite new, all vessels of glass and earthenware should be laid to soak in cold water, and after some hours, this water, covering the vessels, should be gradually heated to the boiling point. It is a good plan to place a little hay on the top of the water.
Glass and earthenware vessels thus treated are far less liable to crack when subjected to the heat of boiling water than it would otherwise be.
EARTH-NUT. See Arachis Hypogœa.
EAU. (Fr.) Water. This word, like its English synonym, is applied to numerous substances, differing in their composition, sensible properties, and uses, of which the following are a few useful examples:—Eau douce, fresh or river water; Eau de mer, sea or salt water; Eau de fontaine, Eau de source, spring water; Eau de puits, well or pump water; Eau de rivière, river water; Eau distillée, distilled water; Eau de rose, rose water; Eau de vie, brandy; Eau de Cologne, Cologne water; Eau d’Hongrie, Hungary water; Eau bénite, holy water; Eau forte, aquafortis; Eau de savon, soapsuds; Eau de senteur, scented water, &c.
Eau Athenienne. (Hte. Bourgeois, Paris.) Pour nettoyer la tête et enlever les pellicules—for cleaning the head and removing scurf. An alcoholic solution of potash-soap,602 with some solution of potash and aromatic oil. (Dr P. Goppelsröder.)
Eau Berger for Dyeing the Hair. Two fluids for consecutive application. No. 1 is a solution of 1·3 grammes sulphate of copper, ·25 grammes nitrate of nickel, 30 grammes distilled water, 4 grammes ammonia. No. 2 is a solution of calcium sulphide, made by passing sulphuretted hydrogen into milk of lime until it ceases to be absorbed, and then filtering from the excess of lime. (W. Engelhardt.)
Eau Capillaire Progressive, pour rétablir la coleur naturelle des cheveux et de la barbe. Formule rationelle, succès garanti. Progressive hair-wash for restoring the natural colour of the hair and beard. Formula rational, success guaranteed (Dr R. Brimmeyer, chimie-pharmacien, Echternach, Luxembourg). (Schädler.)
Eau d’Afrique, for dyeing the Hair Black. Three fluids to be consecutively applied. No. 1 is a solution of 3 parts nitrate of silver in 100 parts water. No. 2 is a solution of 8 parts sodium sulphide in 100 parts water. No. 3 is a solution of nitrate of silver like No. 1, but perfumed. (Reveil.)
Eau d’Atirona. An elegant fluid cosmetic soap, by the use of which all imperfections of the skin will be easily and painlessly removed. It consists of 25 grammes of a spirituous tincture of cinnamon and cloves, 4 grammes soda soap, and a drop of peppermint oil. (Wittstein.)
Eau de Bahama. A black dye for the hair. It is a solution of sugar of lead perfumed with oil of anise, and containing flowers of sulphur in suspension. (Reveil.)
Eau de Beauté, Eau de Paris sans pareille, or Eau de Princesses (August Renard, Paris); with a German title, “Rhümhehst bekanntes cosmetisches Wasser genannt Prinzessen-Wasser.” The well-known and renowned cosmetic called Princesses’ Water.’ To experience the brilliant effects of this marvellous fluid we need only, after washing, habitually pass a small sponge moistened with the fluid gently over the skin, and allow it to dry without rubbing. By so doing our complexion will remain white, smooth, clear, and soft, even to extreme old age. Those, however, who are troubled with freckles, heat-spots, or any other eruption should use the water several times a day as directed. They need suffer no longer from any defect of the skin. Princesses’ Water when shaken is a milk-white fluid contained in an oval bottle with a long neck, which holds 125 grammes. On standing it deposits a white precipitate. It is made from 2·5 grammes calomel, ·45 grammes corrosive sublimate (so altered by the added perfume that the usual tests do not reveal it), and 122 grammes orange-flower water.
Eau de Botot. A mouth wash. Tincture of cedar wood, 500 grammes; tincture of myrrh and tincture of rhatany, of each 125 grammes; peppermint oil, 5 drops. (Winkler.)
Eau de Capille (Kamprath & Schwartze). A hair dye. A mixture of 16 grammes glycerine, 8 grammes hyposulphite of soda, 1 gramme sugar of lead (or an equivalent quantity of Liq. Plumbi subacet.), about 2 grammes precipitated sulphur and 130 grammes water, perfumed with a small quantity of eau de Cologne. (Hager.)
Eau de Charbon, Dr Chattam’s (A. Ahnelt, Charlottenburg, the African traveller). A prophylactic and specific against syphilis. 150 grammes of a slightly red fluid, consisting of a watery solution of carbolic acid coloured with aniline and perfumed with 1 drop peppermint oil and 8 drops chloroform dissolved in 20 grammes spirit. (Hager.)
Eau de Cythère. A hair dye. A solution of 4 parts chloride of lead and 8 parts crystallised hyposulphite of soda in 88 parts distilled water. (Hager.)
Eau de Docteur Sachs. For promoting the growth of the hair, preventing its turning grey, for protecting the scalp from all injurious influences, and for preserving it in a state of purity and health. A solution of castor oil in spirit containing picrotoxin. (Dr C. Schacht.)
Eau de Fée—Fairy Water. A natural hair wash (Lattke, Chemiker, Kiel). Recommended as a preparation consisting solely of harmless vegetables. It consists mainly of a strong solution of nitrate of lead. (Himly.)
Eau des Fées—Fairy Water. A hair wash. A solution of 11⁄4 parts lead sulphite in about 3 parts sodium hyposulphite, 73⁄4 parts glycerin, and 88 parts water. According to the directions for use, more than three bottles of 120 grammes of the Fairy Water should not be used before the hair has been treated with Eau de Poppée, and, to raise it to the highest possible degree of beauty, with Huile régénératrice d’Hygie. (Hager.)
Eau de Hebe. For freckles. To be applied with a small sponge in the evening and washed off in the morning. Lemons, cut small, digested in a closed flask with distilled vinegar, lavender vinegar, oil of lemon, and rosemary, and filtered.
Eau de Java Anticholerique is a solution of camphor and carbolic acid in spirit. (Casselmann.)
Eau de la Floride. A colourless fluid with a greenish-yellow deposit consisting of sugar of lead, 50 parts; flowers of sulphur, 20 parts; distilled water, 1000 parts. (F. Eymael.)
Eau de Lechelle may be replaced by a filtered mixture of 200 parts aqua aromatica, 300 parts aqua dest., 10 parts acid. carbol., 10 parts ol. thymi, 20 parts acid. tannic.
Eau de lys de Lohse (Lohse formerly—before the French war—Lohsé, Berlin). A cosmetic consisting of 2 grammes zinc oxide,603 2 grammes prepared talc, 4 grammes glycerin, and 200 grammes rose water. (Schädler.)
Eau de Mont Blanc. A hair dye. A solution of nitrate of silver.
Eau de Naples. Neapolitan washing solution. A mixture of 12 parts borax, 100 parts distilled water, 50 parts rose water, 1 part camphor, 4 parts tinct. benzoin. (W. Hildwein.)
Eau de Quinine—Glycerin Hair Wash, with Extract of Peruvian Bark (A. Heinrich, Leipzig). For removing scurf and strengthening the hair. 2 grammes balsam of Peru, 6 grammes castor oil, 60 grammes rum, 35 grammes water, 5 grammes tincture of red cinchona. (Hager.)
Eau de Vienne. A hair dye from Paris. Two fluids, one of which is a solution of nitrate of silver in ammoniacal water, and the other a solution of pyrogallic acid.
Eau Dentifrice de Mallard. Star anise, common anise, cinnamon, cloves, of each, 8 parts; guaicum wood, 10 parts; brown cinchona, 6 parts; rose leaves, 5 parts; nutmegs, 2 parts, are placed in a displacement apparatus and percolated with 3 parts cochineal; 12-15 parts water, 1000 parts sp. vini; sp. gr. ·860. The tincture is displaced with water and 1000 parts are mixed with 7 parts of a mixture of peppermint oil, spirit of scurvy grass, and tinct. of benzoin, allowed to stand and filtered.
Eau Dentifrice des Cordillères. An Indian recipe. 360 parts strong spirits, 330 parts water, 21⁄2 parts extract of red or yellow cinchona, 1 part oil of cinnamon, 2 parts oil of cloves, 3 parts oil of anise, 5 parts oil of peppermint. (Hager.)
Eau Ecarlate—Scarlet Water. (Bürdel). For renovating red linen and woollen fabrics. Oxalium, 25 parts; soda, 16 parts; potash, 5 parts; water, coloured with cochineal and slightly perfumed, 1000 parts. (Sauerwein.)
Eau Lajeune. A hair dye. An elegant pasteboard box, in which are three bottles of fluid and two bone-handled tooth-brushes. No. 1 contains a clear fluid consisting of pyrogallic acid 1·5 gramme, ·3 gramme colouring matter of alkanet, 17·5 grammes spirit of wine, 27 grammes water. No. 2 is filled with a thick brown fluid, which from decomposition has produced a deposit sometimes brown, sometimes grey. This partly-decomposed fluid was originally a mixture of silver nitrate, 3·5 grammes; ammonia, 4·5 grammes; gum arabic or some similar mucilage, 2·5 grammes; distilled water, 23 grammes. No. 3, labelled “Fixateur,” contains 7·5 grammes fluid, consisting of ·5 gramme sodium sulphide, 7 grammes distilled water. The directions for use, translated into various languages, say—Dissolve 10 grammes subcarbonate of soda in half a litre of warm or cold rain water, and with this wash the grease from the hair. Afterwards rinse it in clear water, and dry it thoroughly with a cloth. Pour one part of fluid No. 1 into a saucer, and with brush No. 1 apply it to the roots of the hair. Allow it two or three minutes to dry, then rub the hair with an old linen cloth to remove the superfluous moisture. Next repeat the operation, using fluid and brush No. 2, and without waiting wash the hair with warm or cold soapy water. This hair dye is quite harmless, and leaves no marks on the skin behind it. To use it for the Beard.—The process is the same as that for the hair, except that instead of the soda solution, ordinary soap is to be used to cleanse the beard from grease. It often happens that when the user of the dye has not taken ordinary care in cleansing the hair, the latter becomes of a false and unnatural tint. In this case the Fixateur should be used. A small sponge should be moistened with this and passed over the hair, which will make the colour natural and glossy. The Fixateur as well as the sponge must only be used in this way. It may be employed two days after the first operation without it being necessary to dye the hair anew. (Hager.)
Eau Medicinales are either simply watery solutions (HYDROLÉS, HYDROLATURES, SOLUTIONS PAR L′EAU), or distilled water (EAUX DISTILLÉES); or they are vinous or alcoholic tinctures or solutions of essential oils, aromatics, or more active drugs. See Cordials, Hair Dyes, Perfumery, Spirits, Tinctures, Waters, &c.
Eau Tonique de Chalmin is a perfumed solution of tannin.
Eau Tonique Parachûte des Cheveux. To prevent the falling off of the hair. Macerate some pieces of violet root for some days in 120 grammes rose water, filter, and add to the fluid 2 decigrammes sulphate of iron, 3 drops vinegar, 1·3 gramme each of tincture of benzoin and balsam of Peru, 7·5 grammes Provence oil, and 10 drops oil of bergamot. (Dr Casselmann.)
Eau Virginale (Chable). Lead acetate, zinc sulphate, of each 1 part; distilled water, 28 parts; eau de Cologne, 12 parts. Dissolve and mix; allow to stand for a month and filter. A spoonful mixed with a glass of water to be used as a vaginal injection. (Reveil.)
Eaux, in perfumery, are solutions of the fragrant essential oils in spirit, as eau de Cologne, eau de bouquet, &c.; or they are distilled waters, largely charged with the odorous principles of plants, as eau de rose, eau de fleurs d’oranges, &c.
Eaux, of the liqueuriste, are aromatised spirits or cordials.
EB′LANINE. The yellowish-red, crystallisable, solid substance, which is left behind in the retort, when wood spirit is rectified from quicklime. It is insoluble in water, and sublimes without fusion at 273° Fahr.
EBONITE. The only difference between this and vulcanite, consists in the colouring materials used. See Caoutchouc.
604
EB′ONY. The wood of the Diospyrus Melanoxylon, an East Indian tree, of the natural order Ebenaceæ. Two other species of the same genus, namely, Diospyrus Ebenus and D. Ebenaster, yields respectively Mauritius ebony and the BASTARD EBONY of Ceylon. Pale-coloured woods are stained in imitation of ebony (FACTITIOUS EBONY), by washing them with or steeping them in a strong decoction of logwood or of galls, and, when dry, washing them over with a solution of sulphate or acetate of iron. They are then rinsed in clean water, and the process is repeated, if required. The wood is lastly polished or varnished.
EBRI′ETY. See Intoxication.
EBULLI′′TION. The state of boiling, or the agitation of a liquid arising from its rapid conversion into vapour by heat. Ebullition occurs in different liquids at very different temperatures, such temperatures being called their ‘boiling-points.’ Under the same circumstances the boiling-points are constant, and by observing them the chemist is often able to distinguish liquids which much resemble each other. The boiling-point of the same liquid may, however, vary considerably under different circumstances. The causes which induce variation are increased or diminished atmospheric pressure, the greater or less depth of the liquid, and the character of the containing vessel. Thus boiling water is colder by some degrees when the barometer is low, in bad weather, or at the top of a hill, than when the barometer is higher, in fine weather, or at the bottom of a valley or mine. There is a very simple and beautiful experiment, illustrative of the effect of diminished pressure in lowering the boiling-point of a liquid. A little water is made to boil for a few minutes in a flask or retort placed over a lamp, until the air has been expelled, and the steam issues freely from the neck. A tightly fitting cork is then inserted, and the lamp at the same moment withdrawn. When the ebullition ceases, it may be renewed at pleasure for a considerable time by the affusion of cold water, which, by condensing the vapour within, occasions a partial vacuum. Liquids in general boil from 60° to 140° lower than their ordinary boiling-points when heated in vacuo.
The following table furnishes very exact information respecting the effect of increasing pressure upon the boiling-point of water:—
Boiling water contained in a deep vessel is hotter than that in a shallow one, on account of the greater resistance in the one case than the other to the escape of the steam. It is also found that fluids boil at a lower temperature and more quietly in vessels with rough and spicular surfaces, than in those with smooth or polished ones. The boiling-point of water, as marked on the scale of the thermometer, is 212° Fahr., but in glass vessels, under common circumstances, it varies from 212·254° to 215·6°; whilst in perfectly pure and smooth glass vessels water may be heated to 221° Fahr. without boiling. That the elevation of the boiling-point in this case is due to the nature of the surface, may be at once demonstrated by throwing into water, about to boil in a glass matrass, a little iron filings or coarsely powdered glass, when ebullition will commence with almost explosive violence, at the same time that the temperature of the fluid will sink about 2° Fahr.
| Boiling-point ° Fahr. | Barometer Inches. | Boiling-point ° Fahr. | Barometer Inches. |
| 184 | 16·676 | 200 | 23·454 |
| 185 | 17·047 | 201 | 23·937 |
| 186 | 17·421 | 202 | 24·441 |
| 187 | 17·803 | 203 | 25·014 |
| 188 | 18·196 | 204 | 25·468 |
| 189 | 18·593 | 205 | 25·992 |
| 190 | 18·992 | 206 | 26·529 |
| 191 | 19·407 | 207 | 27·068 |
| 192 | 19·822 | 208 | 27·614 |
| 193 | 20·254 | 209 | 28·183 |
| 194 | 20·687 | 210 | 28·744 |
| 195 | 21·124 | 211 | 29·331 |
| 196 | 21·576 | 212 | 29·922 |
| 197 | 22·030 | 213 | 30·516 |
| 198 | 22·498 | 214 | 31·120 |
| 199 | 22·965 | 215 | 31·730 |
The boiling-point of water contained in ordinary vessels may be raised considerably above 212° Fahr., by the addition of saline matter, as will be seen in the following table, extracted from Mr C. G. Williams’s excellent ‘Handbook of Chemical Manipulation,’—
| Name of Salt. | Boiling-point. | |
| Chloride of calcium | 355° | Fahr. |
| Acetate of soda | 256 | ” |
| Nitrate of soda | 246 | ” |
| Sal-ammoniac | 236 | ” |
| Common salt | 224 | ” |
| Cream of tartar | 214 | ” |
The above solutions are suitable for chemical baths. With the exception of the first, they furnish in their boiling-points temperatures, as nearly as can be obtained, 10° above each other. They were chosen by Mr Williams because, in ‘fractionating’ volatile substances, it is usual to separate the distilled products by differences of temperature equal to 10° Fahr. In long operations it is found inconvenient to employ a saturated saline solution for a bath (by which the highest temperature would be obtained), as the constant evaporation of the water induces the crystallisation of605 the salt. It is hence usual to keep it considerably below that point.
The following table, compiled chiefly from the pages of Dr Miller’s ‘Elements of Chemistry,’ gives the boiling-points of several interesting substances.
| Name of Substance. | Boiling-point. | |
| Liquid carbonic acid | -108° | Fahr. |
| Liquid sulphurous acid | + 17·6 | ” |
| Chloric ether | 51·9 | ” |
| Aldehyd | 69·4 | ” |
| Ether | 94·8 | ” |
| Bisulphide of carbon | 118·5 | ” |
| Bromide | 145·4 | ” |
| Wood spirit | 149·9 | ” |
| Alcohol (sp. gr. ·815) | 173·1 | ” |
| Benzol | 176·8 | ” |
| Dutch liquid | 184·7 | ” |
| Acetic acid | 243·1 | ” |
| Sulphur (melted) | 609· | ” |
| Mercury | 662· | ” |
EBUL′LIOSCOPE. Syn. Ebullition alcoholometer, Thermo-alcoholometer. “This instrument is essentially a thermometer, and its application to alcoholometry is based upon the fact that the boiling-point of a spiritous liquid is scarcely altered by the presence, within certain limits, of the substances which may be dissolved in it, and which, by increasing its specific gravity, render the ordinary alcoholometers or hydrometers useless for the purpose of indicating its alcoholic richness. The ebullioscope was invented by the Abbé Brossard-Vidal, of Toulon, and in its original form consisted of a spirit-lamp surmounted by a small boiler, into which a large cylindrical glass bulb was plunged, having an upright stem of such calibre that the quicksilver contained in them, by its expansion and ascent when heated, raised before it a little glass float in the stem, which was connected by a thread with a similar glass bead, hanging in the air. This thread passed round a pulley, which, turning with the motion of the beads, caused an index to move along a graduated circular scale, which represented on its face the per-centage of absolute alcohol in spirituous liquors of different boiling-points. This form of the apparatus being found inconvenient and liable to get disarranged, various improvements were made in it by MM. Conaty, Lerebour, and others. The modification of the instrument now in use, and known as Field’s Patent Alcoholometer, was made by the late Dr Ure, and can scarcely be improved on. It consists of a thermometer having a very minute bore and a large bulb, similar to that employed to determine the height of mountains from the boiling-point of water, but instead of thermometric degrees being marked upon the scale the per-centage under proof is placed on the left-hand side of the stem, and the per-centage content of proof spirit on the right-hand side. These commence at 178·5° Fahr., the temperature at which ‘proof spirit’ boils, and which here forms the bottom of the scale. The succeeding number are based upon the boiling-points of mixtures of alcohol and water. The little boiler being charged, and about a teaspoonful of salt (35 gr.) being added, to prevent loss of alcohol by evaporation, the thermometer is set in its place, and the spirit-lamp lighted. When the mercury begins to rise out of the bulb of the thermometer, the ‘damper-plate’ is pushed in a little way, to moderate the heat. The eye is now kept steadily on the instrument, and as soon as the liquor boils freely, and the mercury becomes stationary in the stem, the indication is carefully noted, and the damper-plate pushed home to extinguish the flame.
“The ebullioscope is adjusted to the mean boiling-point of water under an atmospheric pressure of 29·5 inches. When the pressure is either higher or lower, both water and alcohol boil at a somewhat different temperature, to meet which a barometrical equation is attached to the thermometer by means of a small subsidiary scale. It is therefore necessary, prior to commencing the operation of testing any liquor, to charge the little boiler with pure water only, and to fix the thermometer in its place. When the water boils freely, the mercury becomes stationary in the stem, exactly opposite the true barometrical indication at the time. Should this be against the line 29·5, no correction will be required; but should it stand at any other line, above or below, then the various boiling-points will bear reference to that boiling-point only. In the latter case, the boiling-point of the water on the barometrical indicator must be set against the boiling-point of the liquid on the scale, when opposite the line—29·5 will be found the true strength. Thus:—the barometer being at 30 inches, and the indication or boiling-point being 72 u. p., 30 on the indicator must be placed against 72 u. p. on the thermometer, when against the line of 29·5 will be seen 69·6 u. p., the real strength of the sample tested.
“When a spirit is stronger than the ‘excise proof,’ its boiling-point varies too little with its alterations of strength to render the ebullioscope of much practical value. To make it applicable to the stronger spirits, it is therefore necessary to dilute them with exactly their own bulk of pure water before testing them, and then to double the resulting indication, as suggested by Dr Ure. Our own plan is always to do this when the spirit is stronger than 20 u. p.
“By means of the ebullioscope the alcoholic content of beer, wines, and spirits, of every variety and class, may be readily determined with sufficient accuracy for all practical purposes; and by methods which we shall hereafter point out, the amount of saccharine606 extractive, or sugar, in cordialised spirit, malt liquors and wines, may also be ascertained.
“The ebullioscope (Field’s Alcoholometer) employed by us in numerous and extensive investigations connected with public hygiène, was made by Mr Long, of Little Tower Street, and is an instrument which should be in the hands of every wine and spirit merchant and licensed victualler, as well as every private gentleman who feels interested in the quality of the liquors in his cellar. The instrument is accompanied by a useful little pamphlet of directions and tables, which has been very accurately got up, as we understand, by the late Dr Ure, expressly for Mr Long.” (A. J. Cooley.)
ECHINOCOCCUS HOMINIS. This creature, which is the larva of the Tinæa Echinococcus, is a very common parasite infesting man, and has been found in the human lungs, heart, kidneys, liver, spleen, ovaries, breasts, membrane of the throat, and the bones. The disease to which it gives rise is of a very long and painful nature, frequently terminating fatally, and one in which no remedies have hitherto been found of any avail. The part of the human economy most frequently attacked by the ravages of the Echinococcus is the liver, in the substance of which it gives rise to the formation of a hydatid tumour. This tumour is composed of a thick-walled cyst or bag, within which is another of a much more delicate texture. “This latter membrane is the mother-sac of the Echinococcus embryo” (Huxley), and corresponds with the germinal membrane of Professor Goodsir. It is studded with innumerable transparent cells, varying as extremes of measurement from 1⁄10000th to 1⁄3000th of an inch. It is the seat of development of innumerable Echinococci, and to this membrane, in a fresh hydatid tumour, they are found connected by a delicate membrane, either singly, or more commonly in clusters, the number of individuals on the cluster varying from 10 to 100 or more, as shown in the annexed woodcut.”[271]
[271] Aitken.
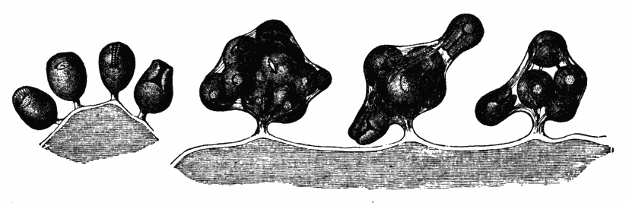
The size of the embryo varies from 1⁄18th to 1⁄20th of a line to 1⁄10th to 1⁄18th, according as it is elongated or contracted. Fig. 2 represents two Echinococci. In the one the head is drawn within the vehicle, and in the other it is extruded.
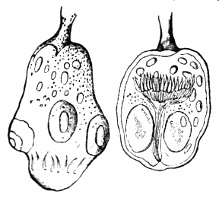 Fig. 2.
Fig. 2.
 Fig. 3.
Fig. 3.
Fig. 3 represents a transverse view of an echinococcus; S S are suctorial discs; the hooklets may be seen encircling a membranous disk.
 Fig. 4.
Fig. 4.
In Fig. 4 we have a representation of the circlet of these hooklets, B, which are thirty-four in number; C gives various views of separate hooklets; b is the base; c the central607 extremity; e the hooklets viewed upon their concave or inferior border. The dotted lines connecting f, g, k, represent the outer surface of the neck, and runs through the fixed point of the three hooks which move upon the central fixed process, as on the pivot.
The inhabitants of Iceland are said to suffer severely from the effects of the Echinococcus Humanus; it has been computed that a sixth of the population of the island are attacked by it.
ECLECTIC REMEDIES. These are medicines chiefly employed by a sect of American practitioners, self-styled “Eclectics.” The medicinal properties appended to each of these preparations are those ascribed to them by the Eclectics themselves.
Apocynin. From the roots of Apocynum and Rosœmifolium. Given in jaundice, hepatic torpor, and constipation.—Dose, 1⁄2 to 2 gr.
Asclepedin. From Asclepias tuberosa. Expectorant, diaphoretic, and tonic.—Dose, 1 to 5 grains three times a day.
Baptistin. From Wild Indigo. Given in liver affections.—Dose, 1⁄4 to 1⁄2 grain.
Barosmin. From Buchu. Diuretic, alterative, antispasmodic.—Dose, 2 to 4 grains.
Caulophyllin. From Caulophyllum thalictroides. Tonic and alterative, acts on the uterus.—Dose, 1⁄4 to 1 grain three times a day; as a parturient, 2 to 4 grains.
Cerasein. From the Cerasus virginiana. Given as a substitute for quinine when this latter is inadmissible.—Dose, 5 to 10 gr.
Cimicifugin. Syn. Macrotin. From Black Snakeroot. Tonic, alterative, nervine, antiperiodic, and in chorea.—Dose, 1 to 6 gr.
Cornine. From Cornus Florida (Dogwood). Antiperiodic.—Dose, 10 grains.
Eupatorine. From Eupatoreum purpureum. Diuretic.—Dose, 3 to 5 grains.
Euphorbin. From Euphorbia corollata. Emetic, cathartic, expectorant, and vermifuge.—Dose, 1 grain or less.
Gelsemin. From Gelsemium sempervirens. Given in pneumonia, hysteria, and dysmenorrhœa.—Dose, 1⁄4 to 2 grains. This must not be confounded with the powerful alkaloid, Gelsimia.
Geranin, or Geraniin. From Geranium maculatum. Astringent.—Dose, 1 to 5 gr.
Hamamelin. From Hamamelis Virginica (Witch hazel). Astringent.—Dose, 5 grains.
Hydrastin. From Hydrastis Canadensis. Tonic.—Dose, 3 to 5 grains. This must not be confounded with the alkaloid Hydrasta.
Iridin. From the Blue flag. Alterative, sialagogue, anthelmintic.—Dose, 1⁄2 to 5 gr.
Inglandin. From Butter-nut. Given in chronic hepatic disorders and constipation.—Dose, 2 to 4 gr.
Leptandrin. From Leptandra Virginica. Given in liver affections, chronic dysentery, diarrhœa, and typhus.—Dose, 2 to 4 gr.
Lycopin. From Lycopus Virginicus. Given in hæmorrhage, diabetes, and dysentery.—Dose, 2 to 3 grains.
Myricin. From Myrica cerifera. Stimulant, astringent, and antispasmodic.—Dose, 2 to 10 grains.
Populin. From Populus tremuloides. Tonic and febrifuge.—Dose, 4 to 8 grains.
Prunin. From Wild Cherry Bark. Stimulant, tonic, and expectorant.—Dose, 1 to 2 gr.
Rumin. From Rumex crispus. Action like rhubarb.—Dose, 3 grains.
Sanguinarin. From Sanguinaria Canadensis. Hepatic and alterative.—Dose, 1⁄2 to 2 grains.
Stillingin. From Stylingia sylvatica. Given in bronchitis and laryngitis. Used externally as a stimulant. Internally, 1 drop with mucilage.
EDELENZIANWURZELSAFT—Noble Gentian-root Juice—Enzian Extract—Extract of Gentian. A water-clear, colourless Schnapps, which contains much fusel oil and has had some of the spirit removed, distilled from gentian plant. (Hager.)
EDIBLE EARTHS. There seems little reason to doubt that the inhabitants of many countries, especially during famine and in times of scarcity, use certain kinds of earth as food. In Spain, a particular kind of earth known as bucaro is eaten; the Russian peasant partakes of his rock-flour; the Thuringian of his rock-butter; the Swede, of his bergmehl or mountain meal; the native of Java of an earth known as teneampa; the Hindoo, of the so-called Patna earth; and the Persian of a species of soil known as Gheli Giveh.
Mr Molvar has analysed an earth, eaten by the poorer classes of the Neograd district in Hungary, and finds it has the following composition:—
| Carbonic acid | 40·357 |
| Lime | 51·488 |
| Magnesia | 0·110 |
| Volatile matter | 5·545 |
| Ferrous oxide | 0·158 |
| Alumina | 2·272 |
As the volatile matter seemed to be the probable means of nourishment, it was subjected to a special examination, and was found to contain, besides empyreumatic substances, 0·067 water, and 0·010 nitrogen.
Dr Schmidt, a German chemist, gives the following as the composition of 100 parts of the air-dried powder from the coast of the White Sea:—
| Water given off at 100° C | 0·260 |
| Given off at a low red heat | 0·835 |
| Alumina | 40·797 |
| Ferric oxide | 0·310 |
| Magnesia | 0·618 |
| Lime | traces |
| Soda | 1·829 |
| Potassa | 9·845 |
| Silicic acid, trace of fluorine, and loss | 45·506 |
608
This earth is eaten by the Laplanders, who mix it with the flour of which they make their bread.
The Persian edible earth called Gheli Giveh contains:—
| Magnesic carbonate | 66·963 |
| Calcic carbonate | 23·634 |
| Sodium chloride | 3·542 |
| Sodic sulphate | 0·293 |
| Sodic carbonate | 0·598 |
| Magnesic hydrate | 1·311 |
| Ferric oxide | 0·092 |
| Alumina | 0·227 |
| Silicic acid | 0·765 |
| Water combined at 120° | 1·153 |
| Hydroscopic moisture | 1·422 |
The ‘Chemical News’ (xxxvi, 202) contains the analysis by Mr Paterson Muir of a clay from Mackenzie County, South Island, New Zealand, which is largely eaten by sheep. It consists of:—
| Silica | 61·25 |
| Alumina | 17·97 |
| Ferric oxide | 5·72 |
| Lime | 1·91 |
| Magnesia | 0·87 |
| Sodium chloride | 3·69 |
| Potassium chloride | trace |
| Water | 7·31 |
| Organic matter | 1·77 |
| ——— | |
| 100·49 |
See Animalculæ.
EDULCORA′TION. The affusion of water on any substance for the purpose of removing the portion soluble in that fluid. Edulcoration is usually performed by agitating or triturating the article with water, and removing the latter, after subsidence, by decantation or filtration. It is the method commonly adopted to purify precipitates and other powders which are insoluble in water. The washing-bottle is a most useful instrument for the edulcoration of precipitates. In its simplest form, it is a bottle fitted with two bent glass tubes, one drawn to a fine point and reaching to the bottom of the bottle, the other only entering the cork a few inches. By blowing down the latter tube, the water is forced out of the former in a fine stream. See Wash-bottle.
EEL. Syn. Anguilla, L. A family of fishes belonging to the ‘apodal’ section of the malacopterygii. At least three species of eels are found in this country—the ‘sharp-nosed,’ the ‘broad-nosed,’ and the ‘snig.’ The first, which is common in streams and lakes, attains the greatest size—sometimes 25 lbs. or even 30 lbs. The ‘snig’ is considered superior to other kinds for the table. As articles of food, eels are said to be laxative and far from wholesome. The fat (EEL FAT; ADEPS ANGUILLÆ, OLEUM A.) is among the simples of the Ph. L. 1618, and was formerly considered ‘good against stripes,’ and is even now used by the vulgar as a friction for stiff joints. For the table, eels are generally dressed by stewing, frying, baking, or potting, which is done in the usual way, the fish being cut into pieces 2 or 3 inches long, and melted butter, onions, sweet herbs, and anchovy sauce, added at will. The CONGER EEL is a distinct and gigantic species of the same family. Its flesh is coarse and oily, but is much esteemed by the inhabitants of the southern coast of Devon, on which it abounds.
Letheby states the following to be the composition of the Eel:
| Nitrogenous matter | 9·9 |
| Fat | 13·8 |
| Saline matter | 1·3 |
| Water | 75· |
| ——— | |
| 100·0 |
Payen’s analysis differs from the above, in giving a larger proportion of nitrogenous matter, and a still greater quantity of fat.
The native inhabitants of New Zealand and kindred races suffer largely from scrofula, the prevalence of which disease amongst them has been attributed to their partaking so largely of eel as a common article of diet.
EFFERVES′CENCE. The rapid escape of gas in small bubbles from a liquid. See Draught, Powder, &c.
EFFLORES′CENCE. The spontaneous conversion of a crystalline solid into a dry pulverulent form. Crystals which in a dry atmosphere lose their water of crystallisation, and become crusted over with a mealy powder, are said to be EFFLORESCENT.
EGG. Syn. Ovum, L. A body produced in the females of birds and certain other animals, containing an ′embryo’ of the same species, or body, from which a similar animal may ultimately be produced. The eggs of the common domestic fowl are nutritious and easily digestible; and when lightly cooked by boiling and eaten with a little salt, are admirably adapted as an aliment for the sick and delicate. When boiled hard or fried, they are rendered less digestible, and possess no advantage in this respect over butcher’s meat. A new-laid egg, beaten up in a cup of tea, coffee, or chocolate is an excellent ingredient in the breakfast of a person with a poor appetite, and is very supporting. A glass of wine, beer, or porter, similarly treated, along with a biscuit, has been recommended as a light and nutritious luncheon or supper, well suited to the debilitated and dyspeptic. Raw eggs may be advantageously substituted for cod-liver oil in all the cases in which this last is ordered, occurring in persons with delicate or irritable stomachs. The addition of fresh salad oil vastly increases their medicinal virtues. A fresh egg is said to contain about the same amount of nourishment as 11⁄2 oz. of fresh meat and 1 oz. of wheaten bread, but in a more digestible form.
609
| Composition of the contents of the egg. | |
| Water | 74·02 |
| Albumen | 14·08 |
| Oil and fat | 10·25 |
| Mineral matter | 1·65 |
| ——— | |
| 100·00 |
| Composition of the white of egg. (Thompson.) | |
| Nitrogenous matter | 20·40 |
| Fatty matter | |
| Saline matter | 1·60 |
| Water | 78·00 |
| ——— | |
| 100·00 |
| Composition of the yolk. (Thompson.) | |
| Nitrogenous matter | 16·00 |
| Fatty matter | 30·70 |
| Saline matter | 1·30 |
| Water | 52·00 |
| ——— | |
| 100·00 |
Egg, White of (ALBUMEN OVI), is officinal in the B. P. Yelk of egg (vitellus ovi) is an ingredient in the BRANDY MIXTURE (MISTURA SPIRITUS VINI GALLICI) of the London College. It is also a popular application to chaps, cracked nipples, abrasions, &c., and is largely used to render oleaginous substances miscible with water, in the preparation of emulsions.
The average weight of the new-laid egg of a hen is about 21⁄2 oz., and its sp. gr. is 1·080 to 1·090; the white generally weighs about 11⁄2 oz.; the yolk, a little under 3⁄4 oz.; and the shell and skin, 1⁄4 oz. Dr Prout found that an egg, on being kept for 2 years in a dry situation, lost 5443⁄10 gr., from the evaporation of a portion of its water through the shell. By boiling in water an egg loses from 2% to 3% of its weight.
Choice. The larger end of a new-laid egg feels cold when placed against the tongue. New-laid eggs appear semi-transparent when placed between the eye and a strong light, and have a small and perceptible division of the skin from the shell, which is filled with air. This mode of examination among the trade is called ‘candling.’ When they shake they are stale. The eggs of turkeys and pea-hens are much esteemed for some purposes; those of ducks and geese are coarse and inferior.
Sound eggs will sink if put into a solution, consisting of 1 oz. of salt in 10 oz. of water; in the same solution indifferent ones will float, whilst bad or worthless ones will swim even in pure water.
Pres. Eggs may be preserved for any length of time by excluding them from the air. One of the cleanest and easiest methods of doing this is to pack them with the small end downwards, in clean dry salt, in barrels or tubs, and to place them in a cool and dry situation. We have eaten eggs thus preserved that were more than a twelvemonth old, and that had been for some months on shipboard in a tropical climate, and which yet retained all the peculiar sweetness of new-laid eggs. With a like intention, eggs are placed in vessels containing milk of lime or strong brine, or are rubbed over with butter, lard, or gum water, all of which act by excluding the air. Eggs for keeping should never be laid on their sides, and when kept in the air should be occasionally turned to prevent the yolk attaching itself to the side instead of floating in the albumen. Some persons place the eggs in a netting or on a sieve or colander, and immerse them for an instant in a caldron of boiling water before packing them away. The practice of packing eggs in damp straw, or anything else that can convey a flavour should be carefully avoided. The shells of eggs are porous, and readily admit the passage of gaseous substances, especially of fetid odours. It is from inattention to this point that a large number of the eggs imported from the coast of France have a less delicate flavour than those of our poultry yards. Damp chopped straw, as well as most other organic substances exposed to warmth and moisture, readily ferment or putrefy; and during fermentation a considerable increase of temperature takes place, as any one may readily perceive by examining the common hotbeds in our gardens, which are merely masses of organic matter in a state of decomposition. Eggs, as long as they retain the embryo of the future chick in a vital state, possess in themselves a certain degree of warmth, which tends materially to promote the decomposition of the substances they are packed in, particularly in the presence of moisture.
A correspondent of the ‘Chemical News’ says: “Eggs may be kept fresh for a whole year by subjecting them to the following process. The fresh eggs are carefully placed in a mixture of five kilogrammes of alum, dissolved in five litres of water, heated to from 45° to 50° C., and left in that liquid for from thirty to forty minutes; the eggs are next drained, and in the meantime the solution of alum is heated to boiling-point. The eggs are again immersed in the liquid and kept therein from ten to fifteen seconds; after having been drained and cooled, they are packed in either dry bran, sawdust, cork-dust, sifted ashes, or in cotton-wool.”
M. Durand, of Blois, proposes to preserve eggs by coating them with silicate of soda.
To Preserve Eggs fresh for many weeks.—As the eggs are taken from the nest, brush each one separately with a thin solution of gum Arabic, being careful to leave no portion of the shell uncovered by it. The half of each egg must first be done, and left to become dry before the remainder is touched, that the gum may not be rubbed off any part by its coming in contact while wet with the hand as it is held to be varnished, or610 with the table when it is laid down to harden.—Eliza Acton.
Eggs to boil in the Shell.—Eggs brought from a cold place and suddenly plunged into boiling water are very frequently liable to crack, and thus to allow of the partial escape of their contents. In winter it will be found a good plan to hold them for an instant over the steam of the saucepan before they are placed in it, which they should be, very gently. By boiling for three minutes, the whites will become in a partially solid state. Exactly five minutes will harden the whites and leave the yolks liquid. Eight or ten minutes will render them hard. Eggs should always be boiled in water sufficient to entirely cover them. They should be boiled 15 minutes for salad-dressings.
Eggs, to Poach.—Take for this purpose a wide and delicately clean pan about half filled with the clearest spring water; throw in a small saltspoonful of salt, and place it over a fire quite free from smoke. Break some new-laid eggs into separate cups, and do this with care, that the yolks may not be injured. When the water boils, draw back the pan, glide the eggs gently into it, and let them stand till the whites appear almost set, which will be in about a minute; then without shaking them move the pan over the fire, and just simmer them from two minutes and a half to three minutes. Lift them out separately with a slice, trim quickly off the ragged edges, and serve them upon dressed spinach or upon minced veal, turkey, or chicken; or dish them for an invalid upon delicately toasted bread, sliced thick and free from crust; it is an improvement to have the bread buttered, but it is less wholesome.
Comparative time of poaching eggs: swan’s eggs, 5 to 6 minutes (in basin, 10 minutes); turkey’s eggs, 4 minutes; hen’s eggs, 3 to 31⁄2 minutes; Guinea fowl’s, 2 to 3 minutes; bantam’s, 2 minutes.
Obs. All eggs may be poached without boiling if kept just at simmering point, but one boil quite at last will assist to detach them from the stewpan, from which they should always be very carefully lifted on what is called a fish or egg slice. There are pans made on purpose for poaching and frying them in good form; but they do not, we believe, answer particularly well. If broken into cups slightly rubbed with butter and simmered in them, their roundness of shape will be well preserved.—Eliza Acton.
Egg, Elas′tic. Take a good and sound egg, place it in strong vinegar, and allow it to remain for 12 hours; it will then become quite soft and elastic. In this state it can be squeezed into a tolerably wide-mouthed bottle; when in, it must be covered with water having a little soda dissolved in it. In a few hours the egg will be restored to nearly its original solidity; after which the liquid may be poured off and the bottle dried, the whole being kept as a curiosity to puzzle one’s friends for an explanation how the egg got there. (‘Parlour Pastime.’)
Egg Flip. Prep. 1. Beer, 1 pint; eggs, 3 in no.; sugar, 2 oz.; nutmeg and ginger, q. s. to flavour; the eggs are broken into one half of the beer, the sugar added, and the whole beaten well together; the mixture is then placed in a clean warmer, and heated over the fire to nearly the boiling-point, and stirred one way all the time, care being taken not to let it either boil or curdle; the other portion of the beer and the spices are then added, and the whole mixed well together.
2. As above, but adding a glass of spirit. Some persons also add a little lemon peel.
Eggs, Packing, for Shipboard. The following plan is now adopted by many firms shipping eggs:—“In the bottom of the box may be placed bran, cut hay, and sawdust. Tear up old newspapers to about 8 or 10 inches square. The paper should be about medium—that is, not too stiff nor too soft. Place one of these pieces of paper on the hand, and on this an egg, on one end; close the lower hand so as to bring the paper up all round the egg; with the other hand crumple the loose corners and edges of the paper down over the other end of the egg; lay another piece of paper on the hand, on which place the same egg, but the other end up; bring up the new paper and crumple down as before. This gives a good cushion to both ends, and a fair one over the centre. Repeat this till you have six thicknesses of paper, reversing the egg each time, and always keeping it on the end. This gives you a ball about 3 to 31⁄2 in. thick by 31⁄2 to 4 in. long. Care should be taken not to press the paper too closely to the egg while covering. Place on one end in the box or basket; place alongside and press them together close enough to prevent their becoming loose in the box, fillings at the ends and on top with crumpled paper.”—J. P.
Egg, Glaire of. Prep. Separate the whites from the yolks, and whisk them to a froth, let them stand 24 hours, and strain them through muslin. Used as a glaze or varnish by bookbinders and others.
Egg, Liquid. Prep. (Jayne.) From lime, 1 bushel (slaked with water); common salt, 2 or 3 lbs.; cream of tartar, 1⁄2 lb.; water, q. s. to form a mixture strong enough to float an egg. Used to preserve eggs, which it is stated it will do for two years, by simply keeping them in it. Simple milk of lime answers quite as well.
Egg Wine. As egg flip, but using equal parts of white wine and water, instead of beer.
ELA′IDINE. A fatty compound of elaïdic acid and glycerin, formed by the action of nitrous acid or nitrate of mercury on olive oil. It is neutral; melts at 90° Fahr.; and is very soluble in ether, scarcely so in alcohol. It is one of the components of CITRINE OINTMENT.611 By saponification it is resolved into its two constituents.
ELA′IN. See Olein.
ELAIOM′ETER. Syn. Oleom′eter. An instrument for ascertaining the specific gravity of oils. See Hydrometer and Oil.
ELAL′DEHYD. A peculiar crystalline substance which forms in ALDEHYD when kept for some weeks at a temperature of 32° Fahr. It melts into a colourless liquid at about 38°, in which state it is miscible with water, alcohol, and ether. It is isomeric with aldehyd, but its vapour has about three times the density of that substance, whilst it neither combines with ammonia nor comports itself with potassa and solution of silver like aldehyd.
ELAOP′TENE. See Oil (Volatile).
ELAT′ERIN. Syn. Momordicine. The active principle of ELATERIUM. It was discovered by Dr Clutterbuck in 1819, but first obtained in a state of purity in 1830 by the late Mr Hennel.
Elaterin. Syn. Elaterium. (Dr Morries.) Obtained by evaporating tincture of elaterium (made with rectified spirit) to the consistence of thin oil, and throwing it in boiling distilled water. When cold, the crystalline precipitate is collected, and dried with a gentle heat.—Dose, to commence with 1-16th of a grain.
Prep. 1. (Dr Morries.) Elaterium is digested in hot alcohol, the resulting tincture filtered, evaporated to the consistence of thin oil, and then thrown into boiling distilled water. When the whole is cold, the precipitate is collected and purified by redissolving it in alcohol and precipitation by water, as before.
2. (Hennel.) The alcoholic extract of elaterium is digested in ether, and the residuum dissolved in hot alcohol; crystals form as the solution cools.
3. An alcoholic tincture is evaporated to the consistence of a syrup, and thrown into a mixture of equal parts of liquor of potassa and water at a boiling temperature. Almost pure elaterin separates as the liquid cools.
Obs. Elaterin forms delicate, white, silky crystals, having a bitter taste; it is fusible at about 365° Fahr.; tastes bitter; odourless; neutral; insoluble in water; and dissolves readily in hot alcohol. Its medicinal action is similar to that of elaterium, differing only in its greater activity.—Dose, 1⁄16 gr. to 1⁄20 gr.
ELATER′IUM. Syn. Squirting cucumber. In pharmacy, ‘the fresh unripe fruit’ of the wild cucumber, ‘Ecbalium officinarum—Richard,’ Ph. L. (Momordica Elaterium, Linn.). According to present usage, the word is more generally applied to the feculence deposited from the juice of the wild cucumber. It is thus applied in Ph. B. E. & D. See below.)
Elaterium. B. P. Syn. Extract of elaterium, E. of squirting cucumber; Extractum elaterii (Ph. L.), Elaterium (Ph. E. & D.,) L. The feculence of the juice of the above fruit.
Prep. 1. (Ph. L.) Slice wild cucumber before it is quite ripe in the long direction, and strain the juice, very gently expressed, through a fine hair sieve; then set it aside for some hours, until the thicker part has subsided. The thinner supernatant fluid being rejected, dry the thicker portion with a gentle heat. The processes of the other colleges are essentially the same.[272]
[272] At the Mitcham Gardens, elaterium is manufactured in much the same way, only that considerable force is used in the expression of the juice, and the product therefore less potent, though more in quantity. The manufacture usually commences about the second week in September. (Dr Royle).
2. (Dr Clutterbuck.) The cucumbers (fully ripe) are cut longitudinally, and sprinkling with cold water, and the juice allowed to strain through a fine sieve into an earthenware vessel. The seeds and surrounding pulp are next placed on the sieve, with the split fruit, and washed repeatedly with cold water. The washings being received in the same vessel with the juice, the whole is allowed to repose for a few hours, when the clear portion is decanted and the sediment spread thinly on fine linen, and dried by exposure to the air and a gentle heat avoiding the sunshine or a bright light. Quality very fine. Forty fruits, by this process, yield only 6 gr. of elaterium.
3. (Apothecaries’ Hall.) The fruit, slit into halves, is placed in hempen or horsehair bags, and submitted to slight pressure in a tincture press. The juice, as it runs off, passes through a fine hair sieve into a cylindrical glass jug or jar, where it is allowed to remain for two hours, when the clear supernatant liquor is poured off, and the thick portion containing the sediment is poured on a paper filter, supported on linen, and allowed to drain, after which it is dried by a gentle heat in a stove. The product has a green colour, and constitutes the finest elaterium of commerce. A darker and inferior article is obtained from the liquor, poured from the first sediment by placing it in shallow pans, and allowing it again to deposit.
Prop., &c. Elaterium is sold in thin cakes, and when pure has a pale-gray or greenish-gray colour, floats on water, is easily pulverised by pressure, and forms with rectified spirits a rich, green-coloured tincture. Elaterium obtained as a second deposit (ELATERIUM NIGRUM), is dark and inferior. Alcohol dissolves from 50% to 60% of good elaterium. “When exhausted by rectified spirit, the solution, concentrated, and poured into hot dilute solution of potassa, deposits, on cooling, minute silky, colourless crystals (of ELATERIN), weighing from 1⁄7th to 1⁄4th of the elaterium operated on.” (Ph. E.)
Obs. To procure a fine sample of elaterium it is necessary to remove it as soon as it is612 deposited, as a heavy mucilage falls down soon afterwards, which materially injures its quality and appearance. English elaterium is the best. The foreign is uniformly adulterated with chalk or starch, and coloured with sap green.
Dose, 1⁄16 gr. to 1⁄2 gr., formed into a pill with extract of gentian and liquorice powder; as a hydragogue and cathartic in dropsies, twice a day, repeated every other day for a week or ten days. Its use must be avoided when there is much debility or any inflammatory symptoms. Larger doses than 1⁄2 gr. of pure elaterium are poisonous. The antidotes are emetics, followed by demulcents, opium, and stimulants.
EL′DER. Syn. Sambucus (Ph. L. & E.), L. A large shrub or small tree belonging to the natural order Caprifoliacæ. It is indigenous in Europe, and has long been valued for its medicinal properties. “The recent flowers of the Sambucus nigra” (Ph. L.) or common elder are regarded as diaphoretic and pectoral, and a distilled water (ELDER-FLOWER WATER; AQUA SAMBUCI) is made of them. The inner bark of the same tree is purgative and emetic, and is used in dropsy; the leaves are purgative; the juice of the fresh berries is made into wine (ELDER WINE), and is largely used to make FACTITIOUS PORT WINE, and to adulterate the real wine. See Waters (Distilled).
ELECAMPANE′. Syn. Inula (Ph. L.), L. “The root of Inula Helenium” (Ph. L.). A plant of the nat. order Compositæ. Tonic, diaphoretic, and expectorant.—Dose, 20 gr. to 1 dr., or more, either in the form of powder or decoction; in catarrh, dyspepsia, &c. It is now seldom used.
ELECTRANODYN. For the cure of neuralgia, headache, migrain, faceache, and apoplectic attacks. As a necklace for children for toothache, as a preventive of quinsy, &c. A tissue paper converted into a nitrogenous material (pyroxylin or düppelpapier) by immersion in a mixture of sulphuric and nitric acid, and containing besides an insignificant proportion of wax and resin. (Hager.)
ELEC′TRIC. Syn. Electrical. Exhibiting the effects of ELECTRICITY when ‘excited’ by friction; pertaining to, derived from, or produced by electricity.
Electric. Syn. Insulator, Non-conductor. A substance which may under ordinary circumstances be readily made to evince electrical properties by friction. Electrics do not transmit, or conduct, electricity; whilst, on the other hand, ANELECTRICS are good transmitters or conductors of electrical action. The most perfect electrics are shell-lac, sulphur, amber, jet, resinous bodies, gums, gun-cotton, glass, silk, diamond, agate, and tourmaline; dry fur, hair, wood, feathers, and paper; turpentine and various oils; dry atmospheric air and other gases, steam of high elasticity, and ice at 0° Fahr. The most perfect anelectrics or conductors are the metals, charcoal, and saline fluids.
Electric Eel. The Gynotus electricus, a fish having the power or giving violent electric ‘shocks’; which power it exerts for killing or stunning its prey. It is an inhabitant of the fresh-water lakes and rivers of the warmer regions of America, Africa, and Asia.
Electrical Machine. An instrument for the excitation and collection of electricity. The term is only applied to contrivances in which friction is the immediate cause of the electrical disturbance; those which act through chemical force, magnetism, or heat, being known by various distinctive names, as ‘voltaic battery,’ ‘electro-magnetic machine,’ ‘induction-coil,’ ‘thermo-electric pile,’ &c.
The electrical machines in common use are composed of a hollow glass cylinder, or circular plate of glass, turning on an axis, and rubbing against two or more leather rubbers covered with silk, the electricity being collected by sharp points fixed in a metal rod standing on a glass pillar. A description of these instruments, however, would be out of place in the present work, which does not aim at giving information that may be easily obtained from other sources.
Cylinder machines are seldom made of greater size than 13 inches by 9, and are about as powerful as an 18-inch plate machine. The latter are commonly made up to 3 and 4 feet diameter, and will, with a suitable condenser, give 15 inch sparks in air.
ELECTRI′′CITY (-trĭs′-ĭt-e). The name given primarily to one of the great forces of nature, and secondarily to that department of physical science which embraces all that is known respecting this particular force. Many theories respecting the nature of electricity have been advanced for the purpose of explaining electrical phenomena. The theory of Dr Franklin supposed the existence of a single homogeneous, imponderable fluid, of extreme tenuity and elasticity, in a state of equable distribution throughout the material world. This fluid is assumed to be repulsive of its own particles, but attractive of all other matter. When distributed in bodies, in quantities proportionate to their capacities or attraction for it, such bodies are said to be in their ‘natural state.’ When we increase or diminish the natural quantity of electricity in any substance, excitation is the result, and the substance, if ‘overcharged,’ is said to be electrified ‘positively,’ or if ‘undercharged,’ ‘negatively.’ These theories, and all others based upon the assumption that electricity is a form of matter, have been found to be inadequate for the elucidation of electrical phenomena.
At the present day, however, two kinds of electric forces are recognised, and distinguished as negative and positive, but they are both assumed to be analogous in principle, and very generally assumed to be simply due to different analogous motions of matter. For a full exposition, however, the reader must refer to some of the especial works on the subject.
613
ELECTRICITY, Iron reduced by. Gelatin capsules of the size of a 2-grain pill, filled with powdered blacksmith scales (black oxide of iron). (Hager.)
ELECTRO-CHEM′ISTRY. That branch of chemistry which treats of the agency of electricity in effecting chemical changes.
ELECTRO-ETCH′ING. See Etching.
ELECTROL′YSIS. (-trŏl′-e-sĭs). Electro-chemical decomposition. The voltaic current has the power of loosening and separating the constituents of certain compound bodies when these are interposed in the circuit. The substances which are thus susceptible of decomposition are termed electrolytes. They are all binary compounds, containing single equivalents of their components, which are held together by very powerful affinities. The amount of electrical power required to effect decomposition varies greatly with different electrolytes: solution of iodide of potassium, melted chloride of lead, hydrochloric acid, water mixed with a little sulphuric acid, and pure water, demand very different degrees of decomposing force, the resistance increasing from the first-mentioned substance to the last, which latter it has been denied can be decomposed. One of the indispensable conditions of electrolysis is fluidity. When a liquid is electrolysed its components are discharged solely at the limiting surfaces, where, according to the usual figurative mode of speech, the current enters and leaves the liquid, all the intermediate portions appearing quiescent. The terms ‘anode’ and ‘cathode’ have been proposed respectively for the surfaces which are supposed to receive and let out the current of positive electricity. The anode is therefore directly against or opposite the positive pole of the battery, or, according to the improved nomenclature, the positive electrode; and the cathode against the negative pole, or electrode. The bodies which are set free by the action of the current are termed ions; those which go to the anode and appear at the positive electrode being distinguished by the term anions, and those which go to the cathode and appear at the negative electrode by the term cathions. This nomenclature has, however, been but partially adopted, and is making but slow way, if any, many preferring the old terms of electro-positive for anions, and electro-negative for cathions.
The relative decomposing effects produced by the same current in different electrolytes are exactly expressed by the chemical equivalents of the electrolytes. Thus, if a current be made to traverse acidulated water, iodide of potassium, and chloride of lead, these three electrolytes will suffer decomposition at the same time, but by no means to the same extent; for the current which decomposes but 9 parts of water will separate into their elements 166 parts of iodide of potassium and 139 parts of chloride of lead. The electrolysis of metallic salts is now carried out on a large scale in the beautiful arts, which we notice under the general head of Electrotype.
ELECTROMOTIVE ESSENCE (Romershausen). An embrocation for restoring the suspended functions of the skin by stimulating the flow of vital electricity and the functions of the nerves. A solution of oils of turpentine and rosemary in the ninth dilution of alcohol previously coloured red with some vegetable dye. (Reithner.)
ELECTRO-PLA′′TING and GILDING. See Electrotype.
ELECTROPH′ORUS. A simple instrument for exciting electricity, generally used in the chemical laboratory for charging small Leyden jars when gases have to be exploded by the electric spark. To construct it, a plate of tinned iron is made into a circle of about 12 inches diameter; a raised border is then turned up for about half an inch, and the extreme edge is turned outwards over a wire to avoid a sharp border. A mixture of equal parts by weight of shell-lac, Venice turpentine, and resin, is made by gently heating them together with stirring until well fused and thoroughly incorporated. This composition is poured into the plate, to quite fill it, and kept melted until all bubbles have disappeared. Another portion of the instrument, serving the same purpose as the conductor of an electric machine, is a circle of wood, rather smaller than the resinous plate, rounded at the edge, and neatly covered with tin-foil. An insulating handle, formed of a piece of stout glass rod, is cemented into the centre of this wooden disc. Before using the instrument it must be carefully dried and slightly warmed. The resinous surface is excited by beating it obliquely with a folded piece of warm flannel. When this has been done for about a minute, the warm dry cover of the instrument is to be placed upon the resinous plate, and touched with the finger. If the cover is then raised a few inches, and the knuckle approached, a powerful spark of positive electricity will pass; and if the cover be again replaced, touched, and raised, a second spark will pass. This may be repeated many times without again exciting the resinous plate. By receiving the sparks with the knobs of a Leyden jar, a charge strong enough to give a powerful shock, or explode a gaseous mixture, may be rapidly obtained. Other forms have been given to the instrument, but the essential part of every one is a plate of some resinous substance.
ELECTROTYPE. Syn. Electro-met′allurgy, Galvan′o-plas′tic. The art of working in metals by the aid of electricity. Strictly speaking, the term electrotype is only applicable to one branch of ‘electro-metallurgy’—that which relates to the production of copies of engraved plates, medals, coins, and other works—but it is now commonly employed in the sense indicated by our definition. According to this extended signification of the term, the614 art of electrotype includes ELECTRO-PLATING, and ELECTRO-GILDING.
General Principles.—If a current from a voltaic battery be passed, by means of platinum electrodes, through water to which some sulphuric acid has been added, electrolysis, takes place, hydrogen appearing at the cathode, and oxygen at the anode. If into the acid liquid some crystals of sulphate of copper be now thrown, electrolysis will still go on, but only one of the elements of the water, namely oxygen, will be evolved; for the hydrogen, on being released, will take the place of the copper in the solution, and the copper thus liberated will be deposited on the platinum plate or wire which constitutes the negative electrode. This experiment may be continued until all the copper is extracted from the solution. Let this experiment be repeated with a copper plate for the positive electrode, and it will be found that neither of the gases will be evolved. The hydrogen, as before, will take the place of the copper in the solution; the oxygen, instead of escaping at the anode, will combine with the copper of the electrode and the sulphuric acid to form sulphate of copper. The chemical forces called into action by the current are so beautifully balanced, that in the last experiment the quantity of copper supplied by the positive electrode exactly equals the quantity withdrawn from the solution and deposited upon the negative electrode. The whole art of electrotype consists in applying the metals thus released from their solutions to artistic or useful purposes. To obtain compact and brilliant deposits, many precautions have to be observed. The solutions must be kept saturated, or nearly so; the mould to be copied, or object to be coated, must not be too small, or out of proportion to the size of the zinc plate of the battery; in fine, the power employed must be carefully regulated according to the work to be done. In all arrangements the moulds or objects which receive the deposits act as negative electrodes, and are consequently in connection with the zinc of the battery or generating cell.
Electrotype Processes. Although reguline deposits of many metals can be obtained through the agency of voltaic electricity, we shall only treat of those of copper, silver, gold, and platinum. When copper is deposited, the object is generally to produce a substantial copy of a medal, an engraved plate, or other work of art; but when solutions containing the precious metals are electrolysed, the deposits are nearly always used for covering the surface of inferior metals. We shall notice the operations connected with the deposition of copper, and those relating to electro-plating under separate heads.
1. Deposition of copper:
The moulds or models intended to receive the deposited metals may be formed of various materials. For medals and similar small works, moulds of fusible metal, white wax, stearine, stearic acid, and gutta percha, are commonly used. The first are formed by dropping or pressing the medals to be copied upon the melted metal, taking care that the former are quite cold, and that the surface of the metal is bright or free from oxide. To make a mould in gutta percha, the material must be softened in warm water, and then pressed upon the medal by means of a strong screw press. With the other materials the manipulation is very easy. A ribbon of cardboard or thick paper is placed round the medal, so as to form a rim; the material, which has been melted in an earthen vessel, is then poured on, and allowed to remain until quite cold and hard, when it is cautiously removed. For large works, moulds of plaster of Paris are usually employed; these require to be saturated with wax or tallow, by standing them in a shallow vessel containing these substances in a melted state. For copying seals and small coins, impressions in ordinary sealing-wax may be used as electrotype moulds. Non-metallic moulds must be coated with some substance which has the property of conducting electricity before they can be used as negative electrodes. The substance commonly employed is plumbago or black-lead. It must be in the condition of an impalpable powder. It is rubbed briskly over the surface of the mould (wax, stearine, plaster, &c.) by means of a strong fine camel-hair brush, till the whole presents the well-known black-lead polish. The adhesion of the plumbago may be often promoted by breathing slightly on the mould. To cause it to adhere to sealing wax impressions, the wax may be slightly moistened with spirits of wine, or exposed to the vapour of ether. Delicate moulds and objects, which cannot well be black-leaded, may be covered with a conducting film of silver, by first dipping them in bisulphuret of carbon holding about 1⁄20th part of phosphorus in solution, and then, after a few seconds, immersing them in a weak solution of nitrate of silver, and allowing them to dry in the light. Metallic moulds require no preparation.
The voltaic apparatus used may now be described. The single-cell arrangement, used for small works, is formed on the principle of Daniell’s Constant Battery. It consists of a vessel of glass, earthenware, or wood, containing a smaller cell of thin biscuit ware, or other porous material; a rod or plate of amalgamated zinc, placed within the porous cell, and a wire connecting the zinc with the mould to be copied; the latter being placed in the outer vessel. The annexed figure represents a convenient form of the single-cell:—
The battery arrangement has many advantages over that described above, and should always be employed when large objects are to be electrotyped, or when a number of small moulds are to be operated upon. In this arrangement the copper solution is electrolysed615 in a separate vessel, termed the decomposition cell, and the current generated by one or more cells of a Daniell’s or Smee’s battery. This arrangement is shown in the following engraving:—
a. An oval vessel of salt-glazed earthenware or wood nearly filled with a saturated solution of sulphate of copper.
b. A porous diaphragm, containing the cylinder or plate of zinc (c), and filled with dilute sulphuric acid.
d. A small bar of brass or copper fastened to the vessel by the binding screws (e, e), and supporting the plate of zinc (c), by the hook of copper wire (f), and the mould (g), by the hook (h).
i. A small shelf or partition to support crystals of sulphate of copper, to keep up the strength of the solution.
a. A constant battery cell.
b. Decomposition cell, a cubical vessel made of wood or earthenware, and filled with a mixture of 1 part of dilute sulphuric acid (1 acid + 9 water), and 2 parts of saturated solution of sulphate of copper by measure.
c, c, c. Moulds suspended to the brass rod (f), and connected with the zinc or positive element of the battery (a), by means of the screw (g.)
d, d. Pieces of sheet copper suspended on the brass rod (h), and connected with the zinc end of the battery, by means of the screw (i), employed to keep up the strength of the cupreous solution in the decomposition cell.
To connect the moulds with the zinc or positive element, stout copper wires or strips of thin sheet copper are employed. In the case of a non-metallic mould, the wire must lead directly to the plumbagoed surface, or, what amounts to the same thing, the plumbago must be extended to the point of attachment. The connecting wires, and the backs and edges of metallic moulds, must be covered with sealing-wax varnish, or other non-conducting substance, to prevent them receiving the deposit. Before a mould is placed in the copper solution it is advisable that everything should be arranged, so that the immersion may occasion immediate voltaic action. If the connection between the zinc and the mould is not effected until after the immersion, the solution may act chemically on the surface of the mould, and cause the deposit to appear dark and dirty. When a mould has remained in the solution long enough to receive a complete coating of copper, it may be lifted out with impunity for examination. If everything is going on well, the deposited metal will present a brilliant, light, copper-coloured surface. When sufficiently thick, the deposit is removed with care, washed and placed to dry. Electrotype medals may be polished with wash-leather and the plate brush, or bronzed. Various natural objects such as insects, fruits, &c.; small works of art, such as busts and statuettes; chemical vessels, particularly glass flasks and retorts; and numerous classes of articles, may be rendered less fragile by coating them with copper by the electrotype process.
II. Deposition of the precious metals—
The solutions generally employed as electrolytes from which silver and gold are respectively separated, are those of the argento-cyanide and the auro-cyanide of potassium. These compounds are what chemists call double salts; for instance, cyanide of potassium is simply a compound of potassium and cyanogen; but argento-cyanide of potassium is cyanide of silver united with cyanide of potassium. When a solution of this double salt is electrolysed silver appears at one electrode and cyanogen at the other, while a proportionate amount of the simple cyanide of potassium is formed in the solution. But if the positive electrode is of silver, the cyanogen combines with it, and forms cyanide of silver, which unites with the liberated cyanide of potassium, and so keeps up the strength of the solution.
As in the deposition of copper, the apparatus used for plating or gilding may be the single cell or the decomposition cell and battery. The necessity of economising solutions of silver and gold has, however, led to certain modifications in the apparatus. The single-cell arrangement consists, as before (see above), of an outer vessel of glass or earthenware, containing a cell of porous biscuit ware; but the object to be silvered or gilded is placed, with the cyanide solution in the latter, while the zinc is placed in the outer vessel, with the dilute sulphuric acid.[273] The zinc is usually employed in the form of a cylinder, completely surrounding the porous cell. In the battery arrangement the decomposition cell may be of porcelain or glass; the silver or gold employed to keep up the strength of the solution may be in plates, wires, or ingots. For plating small objects, a single cell of a Daniell’s battery will afford ample decomposing power; gilding may be better accomplished by using three such616 cells. The battery arrangement is much more convenient, effective, and economical than the single-cell arrangement.
[273] The strength of the acid water acting upon the zinc must be regulated according to the work to be done. If the action between the acid and the zinc be too energetic, the electricity developed will be more than sufficient to release pure metal, and hydrogen will be evolved, which will interfere with the deposition.
On a large scale, electro-plating is carried out in oblong vats, occasionally holding from 200 to 250 gallons of solution. Silver plates connected with a powerful voltaic or magneto-electric battery, are placed at intervals in the vats; they form the positive electrodes, and correspond in extent of surface with the articles to be coated, and face them on both sides. The articles (tea-pots, cruet-frames, forks, spoons, &c.) act as the negative electrodes, and are suspended by copper wires from brass rods laid lengthways over the vats, and connected with the battery. The articles plated are usually formed of nickel silver or German silver, which is chosen on account of its silvery whiteness, a quality of great importance when portions of the coating of noble metal have been worn away by use.
To prepare the articles for plating, they are first boiled in a solution of potassa, to free them from grease; they are then quickly dipped in red nitrous acid, to remove any oxide that may have formed on the surface, and after this well washed in water, to remove every trace of acid. They are then suspended from copper wires, and dipped into a solution of mercury in cyanide of potassium, or some other mercurial solution, and afterwards washed in water, as before. The amalgamation of the surface effected by the last operation promotes the adhesion of the film of silver. The articles having been weighed, are now immersed in the silvering solution, and left until a sufficient amount of silver has been deposited on them. Their condition at any time may be ascertained by weighing a test-object removed from the solution. In some electro-plating establishments the silvering solution is kept constantly stirred by simple mechanical arrangements; in others, continual motion is given to the suspended articles. On being removed from the vats the plated articles are well brushed with brushes of fine brass wire attached to a lathe, and cleaned with fine Calais sand; they are afterwards polished on revolving brushes with rottenstone, then by hand with soft leather and rouge, and, lastly, with the naked female hand. A lasting polish is given to some articles by burnishing with a burnisher formed of highly polished hardened steel, bloodstone, agate, or flint. The process of electro-gilding on the large scale is nearly the same as that of electro-plating or silvering, but, of course, plates of gold are suspended in the solution instead of silver plates.
Various solutions for silvering, plating, and platinising, have been recommended. We give below those generally employed.
1. Solvent solution. Cyanide of potassium, 2 oz; distilled water or rain water, 1 pint; dissolve. Other proportions may be employed. Used as a general solvent for salts of silver, gold, and platinum.
2. Silver solution. Oxide of silver?[274] (not dried), 1 oz.; the solvent solution (No. 1), 1 pint. Used for the single-cell apparatus, its strength being maintained as the deposition proceeds by a fresh supply of oxide from time to time.
[274] Precipitated from pure solution of nitrate of silver by excess of lime water. It should be well washed, and preserved in bottles with distilled water.
Cyanide of silver dissolved in solvent solution (No. 1). This is the solution generally employed for plating with a separate decomposition cell.
3. Gold solution. Add to a pint of No. 1 oxide of gold, 1⁄4 oz. Used in the same manner as the second silver solution.
Cyanide of gold dissolved in solution of cyanide of potassium (No. 1). Used as last.
4. Platinum solution. The double chloride of platinum and potassium, dissolved in solution of caustic potassa. Other solutions have been proposed, but this appears to be decomposed with the greatest ease.
The above sketch of the electrotype art is necessarily very imperfect. For minute details respecting manipulation, the reader is referred to the excellent treatises on the subject that have been written; more particularly to Ernest Spon’s valuable work, entitled ‘Workshop Receipts.’
ELEC′TUARY. Syn. Electuarum, L. Electuaries (ELECTUARIA) are formed of light powders, generally vegetable, mixed up with honey, syrup, or sugar, to the consistence of a stiff paste. In the present Pharmacopœia they are included under the title Confection, but this arrangement is manifestly improper, as the words are not synonymous. In Conserves and Confections the addition of the saccharine matter is in much larger proportion, and is designed to preserve the vegetable matter; in Electuaries, the syrup is designed merely to communicate the required form. (Dr Murray.)
The preparation of electuaries is similar to that of confections and conserves, and the same precautions must be observed to reduce the dry ingredients to very fine powder before adding them to the syrup or other substances used to give them form. Care must also be taken to diffuse the ingredients equally through every portion of the mass, by patient and laborious stirring. The neglect of this point has often led to disagreeable consequences, from some portion of the electuary being nearly inert, while another portion has possessed increased activity. See Confection, Conserve, Linctus, &c.
Electuary of Ac′etate of Potassa. See Conserve.
Electuary of Al′um. Syn. Electuarium aluminis, L. Prep. 1. (Phœbus.) Alum, 1 dr.; extract of logwood, 4 dr.; balsam of Peru, 6 drops; water of sage, q. s. Astringent and antiseptic; in diarrhœa, sponginess of the gums, 617&c.
2. (St. Marie.) Alum, 1 dr.; catechu and extract of bark, of each 2 dr.; conserve of roses, 6 dr.; simple syrup, q. s.—Dose. A teaspoonful, every 4 hours; in chronic diarrhœa, leucorrhœa, hæmorrhages, &c. See Confection.
Electuary, An′odyne. Syn. Electuarium anodynum, L. Prep. See Confection of Opium.
Electuary, Anti′monial. Syn. Electuarium antimonii, Fr. Prep. Electuary of senna, 1 oz.; guaiacum resin, æthiops mineral, prepared sulphuret of antimony, each 1⁄2 oz.; syrup, q. s.—Dose, 1 dr. to 2 dr. twice a day.
Electuary, Anti-rheumatic. Syn. Electuarium antirheumaticum; Chelsea pensioner. Prep. Guaiacum resin, 1 dr.; rhubarb, 2 dr.; bitartrate of potash, 1 oz.; sulphur, 2 oz.; one nutmeg; mix the powders with 1 lb. of honey. Take two spoonfuls night and morning.
Electuary, Ar′abic. Syn. Electuarium sarzæ compositum, E. Arabicum, L.; Electuaire Arabique, Fr. Prep. From sarsaparilla, 5 oz.; senna and China root, of each, 3 oz.; dried walnut peel, 1 oz. (all in fine powder); honey, q. s.—Dose, 1 to 4 dr. See Traitement Arabique.
Electuary, Aromat′ic. Syn. Electuarium Aromaticum (Ph. E.). This preparation differs from the aromatic confection of the other British colleges, in not containing chalk. It is aromatic and stomachic, but not antacid or absorbent. Confection.
Electuary, Bath. Syn. Electuarium anti-cachecticum, E. Martiale, E. ferri compositum, L. Prep. From blacksmiths’ clinkers, reduced to an impalpable powder, and made into an electuary with honey or treacle, q. s.; afterwards adding powdered ginger and carbonate of magnesia, of each, 1 oz., to every lb. of the mixture.—Dose. A teaspoonful night and morning every day, for 3 or 4 days, and again, after an equal interval, as long as thought necessary; as a chalybeate tonic, and in worms.
Electuary of Bitar′trate of Potas′sa. Syn. Electuarium potassæ tartratis, L. Prep. (Monro.) Cream of Tartar, 1 oz,; powdered ginger and conserve of roses, of each 1 dr.; syrup of orange peel, q. s.—Dose, 1 to 3 dr.; as a hydragogue purge. It is also a useful laxative in common cases. See Confection of Cream of Tartar.
Electuary, Black. Syn. Trousseau’s electuary, Trousseau’s black tonic; Electuarium nigrum, E. ferri tannatis, L. Prep. From sesquichloride of iron, 4 dr.; tannin, 1 dr.; confection of roses, 2 oz.; syrup of orange peel, 1 oz. Tonic and astringent.—Dose, 5 to 30 gr.
Electuary of Black Pep′per. See Confection of Pepper.
Electuary of Burnt Sponge. Syn. Electuarium spongiæ ustæ, L. Prep. (Hulse.) Burnt sponge, 10 gr.; rhubarb, 4 gr.; conserve of roses, q. s. For a dose, to be taken night and morning; in scrofula, glandular swellings, &c. See Confection of Sponge.
Electuary of Cas′sia. Syn. Electuarium cassiæ (Ph. D. 1826.), E. c. Fistulæ (Ph. E.), L. Prep. (Ph. D. 1826.) Fresh cassia pulp and syrup of orange, of each, 1⁄2 lb.; manna, 2 oz.; tamarind pulp, 1 oz.; mix, and evaporate to a proper consistence.—Dose, 2 dr. to 1 oz.; as a gentle laxative for children, or as a vehicle for other cathartics. That of the shops is commonly made with equal parts of tamarind and cassia pulps, mixed with 1⁄8th of manna, and flavoured with a few drops of tincture of orange peel, without any evaporation. See Confection.
Electuary of Cat′echu. Syn. Electuarium catechu, Confectio c., C. japonica, L. Prep. (Ph. E.) Powdered catechu and kino, of each, 4 oz.; cinnamon and nutmegs, of each, 1 oz.; opium (dissolved in a little sherry), 11⁄2 dr.; syrup of red roses (evaporated to the consistence of honey), 11⁄2 pint. See Confection, and below.
Electuary of Cat′echu (Compound). Syn. Electuarium catechu compositum (Ph. D.). See Confections. Both the above are astringent, aromatic, and anodyne.—Dose, 15 gr. to 1 dr., or more; in diarrhœa, dysentery, &c.
Electuary, Cathar′tic. Syn. Electuarium catharticum, L. Prep. 1. Confection of senna, 11⁄2 oz.; flowers of sulphur, 1⁄2 oz.; syrup of roses or of orange peel, q. s.—Dose. A teaspoonful, 3 or 4 times a day, in piles; or, 2 to 3 teaspoonfuls, as a gentle laxative for females, and in skin diseases, gonorrhœa, &c. A mild and excellent medicine. It may be safely given in larger doses.
2. (Brera.) Aloes, 8 gr.; cream of tartar, 2 dr.; honey, q. s. For a dose. In amenorrhœa, attributed to abdominal engorgement.
Electuary, Cephal′ic. Syn. Electuarium cephalicum, E. valerianæ compositum, L. Prep. (Hosp. F.) Valerian root and mistletoe of the oak, of each 1 oz.; honey, 11⁄2 oz.; tincture of henbane, q. s. to make an electuary. In nervous and rheumatic headache, &c.; assisted by an aperient.
Electuary of Char′coal. Syn. Electuarium carbonis, E. carbonii, Confectio c., L. Prep. 1. (Hosp. F.) Confection of senna, 2 oz.; fresh burnt charcoal, 1⁄2 oz.; carbonate of soda, 1⁄4 oz.; syrup of orange peel, q. s.
2. (Radius.) Electuary of senna, 2 oz.; powdered charcoal and carbonate of soda, of each, 1 dr. Both the above are given in obstinate constipation.—Dose, 1 to 3 teaspoonfuls twice a day. See Electuary for the Teeth.
Electuary for Chol′era. Syn. Electuarium anti-cholericum, L. The preparations that come under this name are numerous, including aromatic confection, and several like absorbent or astringent preparations. This618 name has been given to the American remedy for cholera, noticed at page
Electuary of Cincho′na Bark. Syn. Electuary of bark; Electuarium cinchonæ, L. Prep. 1. From yellow bark and simple syrup, of each 1 oz.; conserve of red roses and confection of orange peel, of each 1⁄2 oz. Tonic and febrifuge.—Dose, 1 to 4 dr.; in debility, agues, &c.
2. (Radius.) Peruvian bark, 1 oz.; syrup of orange peel, q. s. As the last.—Dose, a teaspoonful or more, 3 or 4 times daily. (See below.)
Electuary of Cinchona (Compound). Syn. Electuarium cinchonæ compositum, L. Prep. 1. (Acidulated,—Copland.) Yellow bark, 1 oz.; confection of roses, 1⁄2 oz.; diluted sulphuric acid, 1 dr.; syrup of ginger, 11⁄2 oz.
2. (Astringent,—Saunders.) Powdered Peruvian bark, orange peel, and conserves of roses and hips, of each 6 dr.; crabs’ eyes (or prepared chalk), 2 dr.; syrup of catechu, q. s.—Dose. A teaspoonful, 2 or 3 times daily; in chronic diarrhœa, &c.
3. (With Catechu,—Pierquin.) Peruvian bark, 1 oz.; catechu and balsam of tolu, of each 1 dr.; syrup of comfrey (Symphytum officinale,—Linn.), q. s.—Dose. As the last; in spitting of blood, hæmorrhages, &c.
4. (With Cloves,—Dewees.) Peruvian bark, 2 oz.; cloves, 1 dr. (better, 4 dr.); simple syrup, q. s. A piece the size of a walnut, every hour or two, during the intermission of an ague.
5. (With Iron,—Cadet.) Peruvian bark, 6 dr.; oxide of iron and confection of opium, of each 2 dr.; syrup of cinnamon, q. s.—Dose. A teaspoonful, or more, twice a day; in dropsy of the belly, after the evacuation of the fluid, and as a tonic in debility, accompanied by nervous excitement, &c., in the absence of fever.
6. (Quarin’s.) Red bark, 1 oz.; ammoniated iron, 1 dr.; made into an electuary with equal parts of oxymel of squills and syrup of the ‘five roots’ (diuretic). Tonic, febrifuge, and pectoral.
7. (With Sal-Ammoniac,—P. Cod.) Gray bark, 21⁄4 oz.; hydrochlorate of ammonia, 1 dr.; honey and syrup of wormwood, of each 2 oz. In intermittents occurring in scrofulous subjects.
8. (With Soda,—P. Cod.) Powdered cinchona, 1 oz.; carbonate of soda, 2 dr.; thin mucilage, q. s. to mix. Tonic, febrifuge, and stomachic.—Dose, 2 dr., 2 or 3 times a day; in agues, complicated with acidity and dyspepsia.
9. (With Sulphur,—Cadet.) Peruvian bark, 1 dr.; sulphur, crabs’ eyes (chalk), and spermaceti, of each 2 dr.; extract of opium, 4 dr.; conserve of roses, 4 dr.; syrup of milfoil, q. s. Highly praised in debility from phthisis.—Dose. A teaspoonful, 2 or 3 times a day, assisted with the liberal use of raw or lightly boiled eggs and cod-liver oil.
10. (With Tin,—Cadet.) Peruvian bark 1 oz.; tin filings and valerian root, of each 1⁄2 oz.; syrup of saffron, q. s. In epilepsy, worms, &c.—Dose. A teaspoonful, morning and evening. See Confection of Bark.
Electuary of Copai′ba. Syn. Electuarium copaibæ, L. Prep. 1. Copaiba and powdered cubebs, equal parts; conserves of roses and orange peel, of each (in equal quantities), q. s.
2. (Caspar.) Blanched almonds, 6 dr.; powdered marsh-mallow root, 1 dr.; catechu, 1⁄2 dr.; balsam of copaiba, 3 dr.
3. (Ricord.) Confection of almonds, 1 oz.; copaiba, 1⁄2 oz.; hard extract of rhatany, 3 dr.; syrup of orange peel, q. s. All the above are excellent in gonorrhœa, gleets, &c. The last two agree better with the stomach than most other like preparations.—Dose, 1 teaspoonful, or more (rapidly increased to 2 or 3 dr.), 3 or 4 times daily. See Confection.
Electuary of Cow′hage. Syn. Electuarium dolichos, E. mucunæ, L. Prep. 1. Dip the pods of dolichos in treacle, allow them to drain a moment, and then scrape off the hairs for use.
2. (Chamberlain.) As the last, nearly.
3. (Correa.) Cowhage (the hairs or setæ), 40 gr.; syrup, 1⁄2 oz.
4. (Ellis.) Cowhage (hairs), 1 dr.; honey, q. s.
5. (Guy’s Hosp.) Cowhage (hairs), any quantity, made into an electuary with treacle, q. s. In worms.—Dose. For a child, a teaspoonful; for an adult, a table-spoonful; in the morning, fasting, and at night, for 3 or 4 days; followed by a dose of castor oil, to which a teaspoonful of turpentine may be advantageously added. See Cowhage.
Electuary of Cu′bebs. Syn. Electuarium cubebæ, L. Prep. 1. See Electuary of Copaiba.
2. (Beral.) Cubebs and copaiba, of each 2 oz.; powdered alum, 1 oz.; extract of opium, 5 or 6 gr.; mix.
3. (Bouchardat.) Cubebs, 1⁄2 oz.; copaiba, 1 oz.; sweet spirit of nitre, 1⁄2 fl. dr.; oil of peppermint, 8 or 10 drops; powdered sugar, q. s.
4. (Radius.) Cubebs, 1⁄2 oz.; honey, 1 oz. In gonorrhœa, mucous discharges from the vagina, bladder, &c.—Dose, 1 teaspoonful, afterwards increased to 2 or 3 teaspoonfuls, twice or thrice daily. See Confection of Copaiba, Electuary of C., &c.
Electuary, Demul′cent. Syn. Electuarium demulcens, L. Prep. From spermaceti, syrup of poppies, and syrup of tolu, of each 2 dr.; powdered gum tragacanth, 1 dr.; confection of roses, 6 dr.; nitre, 1⁄2 dr.—Dose. A piece the size of a small filbert, frequently; as a pectoral and demulcent in coughs, hoarseness, &c.
Electuary, Deob′struent. Syn. Electuarium deobstruens, L. Prep. (Copland.) Confection of senna, 11⁄2 oz.; cream of tartar, 1619 oz.; sulphur and syrup of ginger, of each 6 dr.; borax, 3 dr.; syrup of poppies, 2 dr.—Dose. A teaspoonful, or more, nightly; in the obstinate constipation of females, painful and suppressed menstruation, &c.
Electuary for Dys′entery. Syn. Electuarium anti-dysentericum (Ph. E. 1744), L. Electuary of catechu, mixed with half its weight of Locatel’s balsam.
Electuary, Emmen′agogue. Syn. Electuarium emmenagogicum, L. Prep. From myrrh, 1 dr.; ammoniated iron, 1 scrup.; syrup of ginger, q. s. to mix.—Dose, 1⁄2 dr. to 1 dr., night and morning; in deficient or suppressed menstruation.
Electuary for Epilep′sy. Syn. Electuarium anti-epilepticum, L. Prep. 1. See Electuary of Cinchona (Comp.), No. 10.
2. (Dr Mead.) Powdered cinchona, 1 oz.; valerian and tin (both in powder), of each, 1⁄2 oz.; syrup, q. s. to mix.—Dose. A teaspoonful, night and morning.
Electuary, Feb′rifuge. See Electuary of Cinchona, &c.
Electuary, Compound Guaiacum. Syn. Electuarium guaiaci compositum. (Mid. H.) Prep. Guaiacum resin, 2 dr.; rhubarb, 1 dr.; sulphur, 2 dr.; nitre, 2 dr.; syrup of poppies, q. s.; mix.—Dose, 1⁄2 dr. to 1 dr.
Electuary of In′digo. Syn. Electuarium indigi, E. pigmenti indici, L. Prep. (Phœbus.) Powdered indigo, 4 dr.; aromatic powder, 1⁄2 dr.; syrup, 1 fl. oz. or q. s. In spasmodic diseases, especially in epilepsy, chorea, and hysteria, and the convulsions of children. It has also been used with advantage in that species of impotence in which strychnia is useful. The above quantity is to be all taken, in divided doses, during the day. To be of permanent advantage, it should be continued for several weeks.
Electuary of Ipecacuan′ha. See Confection.
Electuary of Jal′ap. See Confection.
Electuary of Kermes. Marmelade de zanetti; Electuarium kermetis, E. k. mineralis, L. Prep. From manna, 4 oz.; pulp of cassia and oil of almonds, of each 2 oz.; butter of cacao, 1⁄2 oz.: Kermes mineral, 10 gr.; syrup of marsh-mallow, 3 fl. oz.; syrup of orange flower, q. s. A diaphoretic laxative.—Dose, 1 to 4 teaspoonfuls, or more.
Electuary of Lau′rel Ber′ries. See Confection of Rue.
Electuary, Len′itive. See Confection of Senna.
Electuary, Mahomed’s. Prep. 1. From grocer’s currants, 2 oz.; powdered senna, 1⁄2 oz.; powdered ginger, 1 dr.; finely powdered capsicum and cloves, of each 20 gr.; croton oil, 3 drops; conserve of roses and syrup of saffron, of each in equal parts, q. s. to mix.
2. (Bateman.) Currants, 1 oz.; senna, 1⁄2 oz.; ginger, 1⁄2 dr.; syrup of roses, q. s.; croton oil, 1 drop.—Dose, 1 or 2 teaspoonfuls, early in the morning; in dyspepsia and habitual constipation. The first formulary produces a most useful medicine, particularly for free-livers.
Electuary of Male Fern. Syn. Electuarium felicis maris, L. Prep. 1. Powder of male fern, 3 dr.; conserve of roses, 1 oz.
2. (Radius.) Ethereal extract of male fern, 1⁄2 dr.; honey of roses, 1 oz. The half of either to be taken at night, and the remainder the next morning. In worms.
Electuary, Mustard. Syn. Electuarium sinapis. (Guy’s H.) Prep. Mustard seed, lightly bruised, 1 oz.; sulphur, 2 dr.; syrup of orange peel, 1 fl. oz.—Dose, 1 dr., 3 or 4 times a day.
Electuary of Ni′tre. Syn. Electuarium potassæ nitratis, L. Prep. (Hosp. F.) Nitre, 3 dr.; confection of roses, 2 oz.—Dose. A piece of the size of a filbert, where the use of nitre is indicated. See Confection.
Electuary, Olibanum. Syn. Electuarium olibani. [Fr.] Prep. Olibanum, 1⁄2 oz.; balsam of copaiva, 1⁄2 oz.; conserve of hips, 1 oz.; syrup, q. s.—Dose, 2 dr. twice a day.
Electuary of O′pium. See Confection of Opium.
Electuary, Pec′toral. Syn. Electuarium pectorale, L. Prep. 1. (Ph. E. 1744.) From conserve of roses, 2 oz.; compound powder of tragacanth, 4 dr.; flowers of benzoin, 1 dr.; syrup of tolu, q. s.—Dose. A little, ad libitum.
2. Oxymel of squills, syrup of marsh-mallows, mucilage of gum Arabic, and syrup of tolu, of each 1⁄2 oz.; powdered lump sugar, 2 oz. As the last.
Electuary of Pep′per. See Confection, and above.
Electuary for Piles. Syn. Electuarium hæmorrhoidale, L. Prep. 1. See Confection and Electuary of Pepper.
2. (Dr Copland.) Cream of tartar, 1 oz.; precipitated sulphur (pure), 3 dr.; confection of senna, 2 oz.; syrup of orange peel or ginger, q. s. to mix.
3. (Dr Graves.) Confection of senna and sulphur, of each 1 oz.; balsam of copaiba and cream of tartar, of each 1⁄2 oz.; jalap and ginger, of each 1 dr.; syrup of orange peel, q. s.
4. (Hosp. F.) Confection of senna, 2 oz.; black pepper and precipitated sulphur, of each 1⁄2 oz.; oil of cubebs, 1 dr.; syrup, q. s. The last three are useful laxatives in piles, and by their preventing the accumulation and hardening of the fæces, often remove the affection.—Dose. A teaspoonful, three or four times a day. From the difficulty experienced in procuring pure precipitated sulphur, washed sublimed sulphur may be advantageously substituted.
Electuary of Pomegran′ate Syn. Electuarium granati, L. Prep. 1. From the root-bark, 1 dr.; assafœtida, 1⁄2 dr.; croton oil, 6 drops; conserve of roses, 1 oz.—Dose. A teaspoonful, night and morning.
620
2. (Radius.) Extract of the root-bark, 6 dr.; lemon juice, 2 fl. dr.; linden water, 3 fl. dr.; gum tragacanth, q. s. to make an electuary. One half to be taken at once; the remainder in an hour. Both are given in tape-worm.
Electuary of Prunes. Syn. Electuarium Prunonum. (Zwelfer.) Prep. Pulp of prunes boiled to a due consistence, 2 lbs.; pure sugar, 1 lb.
Electuary of Resin. See Confection of Resin.
Electuary of Rhubarb. Syn. Electuarium rhei, L. Prep. (Saunders.) Powdered rhubarb, 11⁄2 dr.; sulphate of potassa, 1 dr.; cream of tartar, 4 dr.; pulp of tamarinds, 2 oz.—Dose. A teaspoonful, as a mild stomachic laxative.
Electuary, Compound Saffron. Syn. Electuarium Croci Compositum. Confection d’Hyacinthe, [F.] Prep. Prepared Armenian bole, 8 oz.; levigated crab’s eyes, 8 oz.; cinnamon, 3 oz.; yellow sandal, red sandal, myrrh, of each 1 oz.; dittany of Crete, 1 oz.; all in fine powder. On the other hand, dissolve 11⁄2 lb. of honey in 3 lbs. of syrup of pinks, over a gentle fire, and strain, and when nearly cold stir into it 1 oz. of saffron in powder. Let stand twelve hours, and then stir in carefully the powders first mentioned.
Electuary of Scam′mony. See Confection.
Electuary for Scur′vy. See Conserve (Antiscorbutic).
Electuary of Sen′na. See Confection of Senna.
Electuary of Squills. Syn. Electuarium scillæ, L. Prep. 1. Oxymel of squills, 2 fl. oz.; cream of tartar and powdered sugar, of each 11⁄2 oz.—Dose, 1 to 2 teaspoonfuls, as a laxative and expectorant; in old coughs, &c.
2. (Radius.) Squills, nitre, gum ammoniacum, and tartrate (bitartrate) of potassa, of each 2 dr.; sal-ammoniac, 20 gr.; syrup of cinnamon, q. s.—Dose, 2 dr.; three times a day; in dropsies. See Conserve of Squills.
Electuary of Steel. Syn. Electuarium ferri, E. chalybeatum, L. Prep. 1. (Dr Collier.) Potassio-tartrate of iron, 1⁄2 oz.; confection of roses, 1 oz.; syrup q. s. to mix.
2. (Collier.) Precipitated sesquioxide of iron, 1 oz.; honey, 2 oz.; ginger syrup, 1⁄2 fl. oz. Both the above are tonic and emmenagogue.—Dose. One teaspoonful, thrice a day. See Confection.
Electuary, Stim′ulant. Syn. Electuarium stimulans, L. Prep. From gum ammoniacum (strained), 1 oz.; vinegar of squills, 1⁄2 oz.; mixed with a gentle heat, and spread on leather. Applied to the chest or pit of the stomach, as a mild counter-irritant and antispasmodic; and as a discutient to tumid glands and indolent tumours. It is wrongly called an electuary.
Electuary, Stomach′ic. Syn. Dinner electuary; Electuarium stomachicum, Confectio stomachica, L. Prep. 1. Rhubarb, ginger, and extract of chamomile, of each 1 dr.; confection of orange peel, 4 dr.; syrup q. s.
2. Rhubarb and gentian, of each, 11⁄2 dr.; extract of hops and powdered capsicum, of each 1⁄2 dr.; oil of chamomile, 12 drops; confection of hips and syrup of orange peel, of each 1⁄2 oz.
3. Green peppermint, lump sugar, and confection of orange peel, equal parts.—Dose. A teaspoonful, an hour before a meal. They are all excellent stomachics, and are useful to improve the appetite, and in dyspepsia.
Electuary of Sul′phur. See Confection of sulphur, and below.
Electuary of Sulphur (Compound). Syn. Electuarium sulphuris compositum, L. Prep. 1. Sulphur, 3⁄4 oz.; cream of tartar, 1 oz.; confections of senna and black pepper, of each 2 oz.; syrup of ginger, 1 fl. oz. An excellent medicine in piles.—Dose. A teaspoonful, twice a day.
2. (With Borax.) Flowers of sulphur, 1 oz.; cream of tartar, 11⁄2 oz.; borax, 1⁄2 oz.; confection of senna, 21⁄2 oz.; syrup of orange peel q. s. to mix.—Dose. 1 to 3 teaspoonfuls, in diseases of the uterine organs and lower bowels. See Confection.
Electuary for the Teeth. Syn. Electuarium dentifricum, L. See Tooth Paste, Dentifrice, &c.
Electuary of Tin. See Confection of tin, and below.
Electuary of Tin (Compound). Syn. Electuarium stanni compositum, L. Prep. 1. Powdered tin, 1 oz.; confection of oil of turpentine, 2 oz.
2. (Dr Cheston.) Tin filings, 4 dr.; carbonate of iron (sesquioxide), 1 dr.; conserve of wormwood, 3 dr.
3. (Foy.) Powder of tin, 1 oz.; extract of wormwood and powdered jalap, of each, 1 dr.; compound syrup of chicory, q. s. In worms.-Dose. A tablespoonful, or more, for 2 or 3 successive mornings, fasting; followed by a purge.
Electuary of Tur′pentine. Syn. Electuarium terebinthinæ, L. Prep. 1. (St. B. Hosp.) Common turpentine, 1 oz.; honey, 2 oz.—Dose, 1 to 2 teaspoonfuls; in complaints of the urinary organs, worms, &c.
2. (Radius.) Turpentine, soap, and rhubarb, of each, 1 dr.; syrup of wormwood, q. s.—Dose. Three teaspoonfuls a day; in dropsy, worms, &c.
3. (E. Olei Terebinthinæ,—Copland.) As confection of turpentine,—Ph. D. See Confection.
Electuary, Ver′mifuge. Syn. Electuarium anthelminticum, E. vermifugum, L. Prep. 1. (Bresmer.) Worm-seed and tansy-seed, of each 4 dr.; powdered valerian root, 2 dr.; jalap and sulphate of potassa, of each 11⁄2 to 1⁄2 dr.; oxymel of quills, q. s. to mix.—Dose. A teaspoonful, or more; repeated night and morning, followed by a brisk purge.
2. (Rosenstein.) Worm-seed, 10 gr.; sulphate621 of iron, 4 gr.; jalap and honey, of each 20 gr. For two doses, as the last. 2 or 3 dr. of confection of senna are often substituted for the jalap and honey.
3. (Foy.) Aloes, 1⁄2 oz.; common salt, 3 dr.; flour, 2 oz.; honey q. s. to form a stiff paste. Used as a suppository in ascarides.
4. Flowers of sulphur, 4 oz.; powdered jalap, 1 oz,; powdered bark, 1 oz.; syrup of buckthorn q. s.—Dose. Two or three teaspoonfuls, every morning early. See Confection and Electuary of Tin, Turpentine, Worm-seed, &c.
Electuary for Worms. See Electuary Vermifuge (above).
EL′EMENTS. Syn. Elementary bodies, Simple b.; Elementa, L. In chemistry, those substances or bodies which have hitherto resisted every attempt which has been made to decompose them, or to resolve them into simpler forms of matter. Earth, air, fire, and water, were regarded by the ancients as simple bodies, of which all others are composed, and they still constitute the ‘four elements’ of the vulgar. The imaginary principles or elements of the alchemists were termed salt, sulphur, and mercury. About sixty-four different kinds of matter are at present recognised as elementary bodies. They are substances having the most diverse characters. The great majority exist in the solid state; bromide and mercury are liquid; while oxygen, hydrogen, nitrogen, and chlorine, are gaseous. About four fifths of the elements are metallic, as instanced by gold, silver, copper, iron, &c.; the remainder are non-metallic, as instanced by carbon, sulphur, phosphorus, &c. A list of the known elements is given under the head of Atomic Weights (which see).
EL′EMI. Syn. Gum elemi; Elemi (B. P.). “A terebinthinate concretion, from an uncertain plant.” (Ph. L.) Mexican elemi is known to be the produce of a species of the genus Elaphrium. Manilla elemi is probably the product of Canarium commune.
Prop., &c. The elemi of commerce is of a pale-yellow colour, brittle without, but soft and tough within; it has a warm bitter taste, and a fragrant aromatic smell, partaking of fennel and juniper. It is only partially transparent even in thin plates, is very fusible, and has a density a little greater than that of water. It contains 121⁄2 per cent. of volatile oil (oil of elemi). It is used to give toughness to lacquers and varnishes, and in medicine in the preparation of ELEMI OINTMENT.
Pur. The elemi of the shops is often adulterated, but more frequently a factitious kind is sold for the genuine gum. This fraud may be detected by exposing the suspected article to heat, along with a little water, when the factitious fragrance of the spurious article evaporates, and the coarse terebinthinate smell of the resin used to adulterate it, or which is sold for it, becomes readily distinguishable.
Elemi, Facti′′tious. Prep. 1. Yellow resin, 8 lbs.; melt, add Canada balsam, 2 lbs; withdraw the vessel from the heat, and further add of oil of juniper, 2 dr.; oil of sweet fennel, 1 dr.; oil of nutmeg, 1⁄2 dr.
2. Yellow resin, 7 lb.; Canada balsam, 1 lb.; juniper oil bottoms, 4 dr.; oil of mace, 3 dr.; mix as before.
EL′EMIN. The crystalline resin of gum elemi.
ELIX′IR. In pharmacy, a name formerly applied to various compound tinctures, and to preparations supposed to contain the quintessence of other substances. (It is still applied to several popular remedies.) The elixirs of the alchemists were solutions employed in their fruitless attempts to transmute the baser metals into gold.
Elixir, Ac′id. Syn. Elixir acidum, L. Prep. 1. (Dippell’s) Sulphuric acid, 1 part, dropped gradually into rectified spirit of wine, 5 parts; placed in a large flask, and afterwards coloured by digestion on animal kermes and saffron, of each 1 part.
2. (Haller’s,—Ph. Sax. 1837.) From sulphuric acid and rectified spirit, of each 1 part; as before.
3. (Vogler’s.) From sulphuric acid and nitrous ether, equal parts, as above. Astringent and antiseptic.—Dose. A few drops, in water.
Elixir of Al′oes. Syn. Compound tincture of aloes; Elixir aloës, L. See Tincture.
Elixir of Aloes (Compound). Syn. Elixir of aloës compositum, L. Prep. (Dr Copland.) Acetate of potassa, inspissated ox-gall, socrotine aloes, and myrrh, of each 2 dr.; hay saffron, 1 dr.; brandy (or proof spirit), 21⁄2 fl. oz.; digest a week, and strain. Stomachic and laxative.—Dose. A teaspoonful, or more; in dyspepsia, constipation, &c.
Elixir, Anti-asthmat′ic. Syn. Elixir anti-asthmaticum, L. Prep. 1. Oil of aniseed, camphor, and balsam of tolu, of each 1 oz.; cochineal, 1 dr.; proof spirit, 1 gal.; digest a week, and filter.
2. As the last, adding powdered opium, 11⁄4 oz.—Dose. A teaspoonful to allay irritation, assisted by an occasional dose of aperient medicine; in asthma, chronic coughs, &c.
3. (Boerhaave’s.) Aniseed, asarabacca, elecampane, liquorice root, orris root, and sweet flag (calamus), of each equal parts; made into a tincture, with brandy.—Dose, 20 to 40 drops.
Elixir Antigoutteux de Villette is a tincture of 100 parts brown cinchona bark, 50 parts poppy petals, 25 parts sassafras, 50 parts guaiacum in 4,000 parts rum, mixed with 2,500 parts syrup of sarsaparilla. (Hager.)
Elixir, Anti-scrof′ulous. Syn. Elixir anti-scrofulosum, L. Prep. 1. (P. Cod.) The ammoniated tincture of gentian. See Tincture.
2. (Desforges.) Guaiacum, 5 oz.; cinchona bark and pellitory of each 3 oz.; cloves,622 5 dr.; orange peel and benzoin, of each 2 dr.; hay saffron, 1⁄2 dr.; rectified spirit and brandy, of each 1⁄2 pint; digest a week, and filter. Used as an application to scorbutic gums.
Elixir, Bitter. Syn. Elixir amarum (Ph. Germ.) Prep. Extract of buckbean, extract of orange peel, of each 2 parts; peppermint water, alcohol (68 per cent.), of each 16 parts; spirit of ether (made of 3 parts of alcohol and 1 part of ether), 1 part. Dissolve and mix.
Elixir, Boerhaave’s Asthmat′ic. See Elixir Antiasthmatic (above).
Elixir, Boerhaave’s Vis′ceral. Syn. Elixir Boerhaavii, E. B. viscerale, L. Prep. (Ph. Han.) Aloes, myrrh, and saffron, of each 1 oz.; tartrate of potassa, 2 oz.; alcohol (strongest rectified spirit), 14 oz.; water, 1 oz.; macerate 3 days, and filter. This preparation “has been highly praised in visceral obstruction.” (Dr Griffith.)—Dose, 1 to 3 teaspoonfuls.
Elixir of Celery (Dr Wilkinson’s.) For increasing, preserving, and producing virility. Juniper berries, angelica root, lovage root, of each 1 part; spirit, 12 parts; orange-flower water, rose water, of each 4 parts; spring water, sufficient. Distil 20 parts, and mix the distillate with 12 parts clarified honey. (Hager.)
Elixir, Claude’s. Syn. Elixir Clauderi, L. 1. (Pideret.) Salt of tartar, sal-ammoniac, strained aloes, and myrrh, of each 1 oz.; elder-flower water, 11⁄4 pint, digest, with agitation, for 24 hours, and filter.
2. (Parrish.) Carbonate of potassa, 1 oz.; aloes, guaiacum, myrrh, saffron, and rhubarb (contused), of each 2 dr.; water, 18 fl. oz. Macerate a few days, and decant.—Dose, 1 to 2 teaspoonfuls; in amenorrhœa, constipation, scurvy, visceral obstructions, &c.
Elixir, Cough. Syn. Elixir anti-catarrhale, L. Prep. 1. See Anti-asthmatic Elixir.
2. (Hufeland.) Extracts of blessed thistle and dulcamara, of each 1 dr.; cherry-laurel water, 1 fl. dr.; fennel-water, 1 fl. oz.—Dose, 1 to 2 teaspoonfuls, 3 or 4 times a day. It is a most useful remedy in coughs occurring in nervous, hysterical, or irritable patients. See Elixir of Ipecacuanha, Elixir Lettsom’s, &c. (below).
Elixir, Daffy’s. Syn. Elixir salutis, E. sennæ compositum, Tinctura sennæ composita, L. This is an aromatised and sweetened tincture of senna, to which other cathartics are generally added. Nearly every drug-house has its own formula for this article. The following are those employed in the London trade:—
Prep. 1. East India senna, 11⁄2 lb.; jalap, 5 oz.; coriander seed and aniseed, of each 1⁄2 lb.; rhubarb, 1⁄4 lb.; red sanders wood, 2 oz.; salt of tartar, 2 oz.; treacle, 7 lbs.; rectified spirit of wine, 21⁄2 galls.; water, 31⁄4 galls. All the solids are well bruised, and macerated in the mixed fluids for 14 days, when the whole is pressed, and strained through a flannel bag. It is too glutinous to run through filtering paper.
2. Senna, rhubarb, and aniseed, of each 2 lbs.; jalap and caraways, of each 1 lb.; red sanders wood, 1⁄2 lb.; brown sugar, 7 lbs.; proof spirit, 10 galls.; as the last.
3. Senna, 56 lbs.; aniseed, 7 lbs.; rhubarb (East India), 14 lbs.; coriander seed, 6 lbs.; caraway seed and red sanders wood, of each 5 lbs.; cassia bark and jalap, of each 3 lbs.; proof spirit, 100 galls.; digest for 14 days, press, strain, and add molasses, 84 lbs.; mix well, and either clarify or strain through flannel.
4. For proof spirit in the last two formulæ, equal parts of spirit of wine and water are employed by the smaller houses.
5. (Redwood.) Senna, 1⁄2 lb.; aniseed, caraways, and jalap, of each 1 oz. 2 dr.; juniper berries, 21⁄2 oz.; proof spirit, 6 pints; macerate for 14 days, then add of treacle, 101⁄2 oz.; water, 1 lb. 5 oz.; mix and strain.
6. (Dicey’s.) Senna, 1 lb.; guaiacum shavings, elecampane root (dried), aniseed, caraway seed, coriander seed, and liquorice root, of each 1⁄2 lb.; stoned raisins, 2 lbs.; proof spirit or brandy, 9 quarts; macerate for 10 days.
7. (Swinton’s.) Senna, 1 lb.; jalap, 3 lbs.; coriander seed, caraway seed, liquorice root, and elecampane root, of each 4 oz.; moist sugar, 2 lbs.; rectified spirit of wine and water, of each 1 gal.; as the last.
Obs. Daffy’s elixir is a favorite purge with drunkards, and is a common and very popular remedy in flatulent colic, dyspepsia, diarrhœa, &c.—Dose, 1 to 4 table-spoonfuls, or more.
Elixir de Pepsin Digestif. (Grimault & Co.) For loss of appetite and disordered digestion. Contains pepsine, in quantities not at all proportionate to the price of the article.
Elixir Deslauriers Toni-Febrifuge au Quinquina et Caffé. A tonic febrifuge. Yellow cinchona (Köningschina), 20 grammes; brown cinchona, 8 grammes; powdered coffee beans, slightly roasted, 16 grammes; wine, 250 grammes; sugar, 15 grammes; citric acid, 2·5 grammes. Boil once after standing some time in a warm place, and filter. Add to the filtered liquid 85 grammes sugar and 15 grammes spirit.
Elixir de St. Hubert pour les Chasseurs is a solution of 2 parts carbolic acid in 50 parts spirit. (Casselmann.)
Elixir, Devil’s. Syn. Elixir capsici compositum, L. Prep. From pods of capsicum, and cloves (bruised), of each 1 oz.; ginger and saffron, of each 3 oz.; cantharides, 5 dr.; proof spirit, 7 lbs.; digest for 10 days.—Dose, 1⁄2 dr. to 3 dr., in mixtures. It is stimulating, anti-choleraic, and aphrodisiac.
Elixir of Garlic. Syn. Elixir Allii, L. Prep. From garlic roots (bruised), 80 in no.; rectified spirit, 1 pint; digest, distil to dryness,623 and repeat the process with the same spirit from fresh roots, a second and a third time; lastly, add camphor, 2 dr. Diaphoretic and pectoral.—Dose. A teaspoonful, twice a day; in asthma, old coughs, diarrhœa from debility, &c.
Elixir, Garus’s. Syn. Elixir Gari, L.; Elixir de Garus, Fr. Prep. 1. Myrrh, 1 oz.; aloes and saffron, of each 1⁄2 oz.; cinnamon, cloves, and nutmeg, of each 1 dr.; proof spirit, 1 quart; digest a week, add water, 5 fl. oz., and distil over 1 quart; to the distillate (alcoolat de Garus) add of syrup of maidenhair, 2 lbs.; orange-flower water, 11⁄2 fl. oz.
2. (Foy.) Compound tincture of saffron, 8 pints; syrup of maidenhair, 10 pints; mix; add caramel, q. s. to colour, dissolved in orange-flower water, 1⁄2 pint.
3. (P. Cod.) Aloes and saffron, of each 1 oz.; myrrh, cinnamon, and cloves, of each 1⁄2 oz.; nutmeg, 1⁄2 dr.; proof spirit, 12 pints; orange-flower water, 16 fl. oz.; macerate 2 days, distil 6 pints, and add to the distillate (alcoolat de Garus), of syrup of capillaire, 71⁄2 pints; and colour with saffron q. s.
4. (Soubeiran.) Socotrine aloes and saffron, of each 1 oz.; myrrh, canella alba, citron, and nutmegs, of each 1⁄2 oz.; spirit (sp. gr. ·923), 20 lbs.; orange-flower water, 16 fl. oz.; macerate as last, distil 10 lbs., and add to the distillate (alcoolat de Garus), of syrup of capillaire, 121⁄2 lbs.; orange-flower water, 8 fl. oz.; with saffron q. s. to colour.
5. (Thierry.) Aloes, myrrh, and saffron, of each 2 dr.; nutmeg, 4 dr.; canella alba and cloves, of each 1 oz.; spirit (·864), 13 lbs.; draw over 12 lbs. of ‘alcoolat,’ add to the residue of the distillation rose water, 10 lbs.; distil 6 lbs., and add as much of this aromatic water to the alcoolat as will raise its sp. gr. to ·890. Then to every 11 lbs. of the above mixed liquor add of simple syrup, 15 lbs.; tincture of vanilla and orange peel, of each 21⁄2 fl. oz.; fresh milk (skimmed), 1 lb.; and tincture of saffron q. s. to colour; digest with agitation for two days, and filter. Used as a stomachic, carminative, and stimulant, in doses of a wine-glassful. That prepared without distillation forms an excellent stomachic purge. With the exception of that from the 2nd formula, the products may be regarded as agreeable cordial liquors rather than medicines. It is much employed on the Continent.
Elixir of Gold. Syn. Elixir aurii, L.; Elixir d’or, Fr. Prep. 1. De la Motte’s Golden Drops.
2. Terchloride of gold, 20 gr.; rectified spirit, 6 fl. dr.; ether, 3 fl. dr.; dissolve.—Dose, 5 to 15 drops, taken in distilled water; in gout, scrofula, nervous diseases, cancer, indurated glands, secondary syphilis, &c. This last preparation is often confounded with the gouttes d’or du Général de la Motte; but the two are evidently distinct articles. See Drops.
Elixir, Haller’s. See Elixir acid (above).
Elixir, Hoffman’s Visceral. Syn. Elixir Hoffmanni, E. H. viscerale, L. Prep. 1. As Elixir of Orange-peel,—Ph. Bor. 1847.
2. Thin outside peel of orange (dried), myrrh, and centuary, of each 2 dr.; extracts of carduus benedictus, cascarilla, and gentian, of each 1 dr.; white wine (sherry), 1 quart. Aromatic and stomachic.—Dose. A dessert-spoonful, or more.
Elixir for Impotence in Males (Dr Ludwig Tiedemann). Prepared from directions given in the Puntsaou from genuine ginseng root. 135 grammes of a dark brown aromatic vinous liquid, prepared by digesting orange berries in wine. The embrocation is an equal quantity of a pleasantly-smelling liquid consisting of spirit with tincture of storax and a small admixture of volatile oils. (Hager.)
Elixir of Ipecac′uanha. Syn. Elixir ipecacuanhæ, L. Prep. (Cadet.) Powdered ipecacuanha and balsam of tolu, of each 4 dr.; flowers of benzoin, opium, and saffron, of each 2 dr.; oil of aniseed, 1 dr.; camphor, 40 gr.; alcohol (rectified spirit), 11⁄2 pint; digest a week and filter.—Dose, 1 to 2 dr., as a stimulant, diaphoretic, expectorant, and stomachic; in chronic coughs, asthmas, and old colds, and in certain forms of deficient appetite, dyspepsia, diarrhœa, &c.
Elixir of Jal′ap. Syn. Elixir jalapæ compositum, L. Prep. From jalap, 4 oz.; scammony, 4 dr.; gamboge, 2 dr.; proof spirit, 1 quart.—Dose, 1⁄2 dr. to 3 dr., as a purgative; especially in worms.
Elixir Karoly pour les Fourrures. A solution of camphor and carbolic acid in strong spirit, mixed with a clear brown acrid tincture, perhaps tinct. pyrethri rosei. (Casselmann.)
Elixir, Lettsom’s. Prep. (Augustin.) Oil of aniseed, 1 dr.; camphor, 11⁄2 dr.; benzoic acid, opium, and saffron, of each 2 dr.; ipecacuanha and balsam of tolu, of each 4 dr.; rectified spirit, 2 lbs.; digest 10 days and filter.—Dose, 5 to 15 drops, for a child; 1⁄2 to 1 teaspoonful, for an adult; in ordinary coughs, hooping-cough, &c.
Elixir of Life, Bitter (Jacob Wolff). For strengthening the constitution. A brandy prepared from 1 gramme aloes, 10 grammes cinnamon, 2·5 grammes sweet flag, 5 grammes angelica root, ·6 grammes cake saffron, 10 grammes caramel, 215 grammes glycerin, 180 grammes spirit, 350 grammes water. (Hager.)
Elixir, Live-long. Syn. Elixir of long life; E. longæ vitæ, L. Prep. 1. See Tincture of Rhubarb and Aloes.
2. (Elixir vitæ Matthioli.) A mixture of several aromatics and stimulants, made with rectified spirit.
Elixir of Myrrh. Syn. Elixir myrrhæ, L. See Tincture of Savine (Comp.),—Ph. L. 1788.
624
Elixir d’Or. See Elixir of Gold.
Elixir of Or′ange Peel. Syn. Elixir aurantiorum compositum, L. Prep. 1. (Ph. Bor. 1847.) Orange peel, 6 oz.; cinnamon, 2 oz.; carbonate of potassa, 1 oz.; Madeira wine, 4 lbs.; macerate 6 days, express the tincture, and add of extracts of buckbean, cascarilla, gentian, and wormwood, of each, 1 oz.; dissolve, and after repose for subsidence, decant and filter. An excellent aromatic bitter and stomachic.
2. (Moscati.) Orange peel, 1 oz.; cascarilla, 1⁄2 oz.; waters of citron peel and wormwood, and rectified spirit, of each 1⁄2 pint; digest a week. Resembles the last.—Dose (of either). A table-spoonful to a wine-glassful.
Elixir, Parego′ric. Syn. Elixir paregoricum, L. See Tincture of Camphor (Comp.).
Elixir, Paregoric (Scotch). Syn. Elixir paregoricum Scoticum, L. See Tincture of Opium (Ammoniated).
Elixir, Pec′toral. Syn. Elixir pectorale, L. (Ph. E. 1745.) Balsam of tolu, 2 oz.; gum benzoin, 11⁄2 oz.; saffron, 1⁄2 oz.; rectified spirit, 32 fl. oz.; digest in a gentle heat for 4 days and filter.—Dose, 1⁄2 to 1 teaspoonful. (See above.)
Elixir, Pol′ychrest. Syn. Elixir polychreston, L. Prep. (Ph. E. 1745.) Guaiacum (gum), 6 oz.; balsam of Peru, 1⁄2 oz.; rectified spirit, 23 fl. oz.; digest as last, strain, and add oil of sassafras, 2 fl. dr. Pectoral and anti-rheumatic.—Dose, 10 to 60 drops, or more.
Elixir, Paracelsus’s. See Elixir Proprietatis (below).
Elixir Proprieta′tis. [L.] Syn. Paracelsus’s elixir of propriety; Elixir de propriété de Paracelse, Fr. An old preparation, nearly corresponding to the compound tincture of aloes of modern pharmacy, and which is now sold for it. Prep. 1. (Soubeiran.) Tincture of myrrh, 4 oz.; tinctures of aloes and saffron, of each 3 oz. (‘Trait. Pharm.’ 1847.)
2. (Elixir Proprietatis cum Acido.)—a. The last, slightly acidulated with oil of vitriol, and filtered.
b.—Ph. Bor. 1847.—Aloes and myrrh, of each 2 oz.; saffron, 1 oz.; spirit (sp. gr. ·900), 2 lbs.; dilute sulphuric acid (1 to 5), 2 oz.; macerate 4 days, and filter.
3. (Elixir Proprietatis Tartarizatum; E. P. Alkalizatum.) From elixir proprietatis, alkalised with salt of tartar, and filtered. The last two are old preparations, now seldom inquired for in this country, except in places remote from London.
Elixir, Radcliffe’s. Prep. 1. From socotrine aloes, 6 dr.; rhubarb, 1 dr.; cinnamon (cassia), cochineal, and zedoary root, of each 1⁄2 dr.; syrup of buckthorn, 2 fl. oz.; brandy, 11⁄4 pint; digest 10 days and strain.
2. As the last, but substituting proof spirit, 1 pint, and water, 1⁄4 pint, for the brandy. Aromatic, stomachic, and aperient.—Dose, 1 to 4 dr.; in similar cases to those in which ‘Daffy’s Elixir’ is taken.
Elixir of Ro′ses. Syn. Elixir Rosæ, L. Prep. 1. Eau de rose, 2 fl. oz.; spirits of horseradish and scurvy grass, of each 1 fl. oz.; otto of roses, 3 drops; camphor and cochineal (both in powder), 12 gr.; powdered sugar-candy, 1⁄2 oz.; digest, with frequent agitation, for a week, and after repose decant the clear, and strain through a piece of muslin. Used as an elegant application in scurvy of the gums, and also to perfume the breath.
2. (Beasley.) Cinnamon, 3 oz.; ginger, 2 oz.; cloves, 1 dr.; essence of peppermint, 1 oz.; oil of orange peel, 1 dr.; otto of roses, 15 (? 25) drops; rectified spirit, 21⁄2 pints; digest 15 days and filter. Used as a tooth cosmetic.
Elixir Sa′crum. Tincture of aloes and rhubarb.
Elixir Salu′tis. Syn. Elixir of health. The compound tincture of senna of old pharmacy. See Elixir, Daffy’s.
Elixir of Scam′mony. Syn. Elixir scammonii, L. Prep. (Guibourt.) Scammony (pure), 2 dr.; proof spirit, 8 fl. oz.; mix in a suitable vessel, apply heat, set the spirit on fire, and add of sugar, 4 oz.; when the whole is dissolved (melted down), extinguish the flame, and further add of syrup of violets, 2 fl. oz.; mix well, and after sufficient repose decant the clear portion from the dregs. The product should be 12 oz., containing 12 gr. of scammony per oz.—Dose, 1 to 2 dessert-spoonfuls in milk or aromatised water; or made into an emulsion with aromatics; in worms, &c.
Elixir, Squire’s. Prep. 1. (Original Formula.) Aurum musivum, 3 oz.; opium, 2 oz.; camphor, 1 oz.; cochineal, 1⁄2 oz.; sweet fennel, 1⁄4 oz.; tincture of serpentary, 1 pint (old meas.); spirit of aniseed, 1 gal. (old meas.); sugar, 1 lb.; dissolved in water, 1 pint (old meas.); digest 10 days and filter.
2. Powdered opium, 2 oz.; ginger, red sanders wood, and camphor, of each 1 oz.; oil of aniseed, 1⁄2 oz.; oil of sweet fennel, 1⁄2 dr.; tincture of serpentary, 1 pint; proof spirit, 5 pints; water, 1 quart; as last. Stimulant, anodyne, diaphoretic, and pectoral.—Dose, 1 to 2 teaspoonfuls; in chest affections, nervous headaches, &c., in the absence of inflammatory symptoms.
Elixir, Stomach′ic. Compound tincture of gentian was formerly so called.
Elixir, Stoughton’s. Prep. 1. Raisins (stoned and bruised), 1 lb.; gentian root, 3⁄4 lb.; dried orange peel, 6 oz.; serpentary, 1⁄4 lb.; calamus aromaticus, 11⁄2 oz.; cardamoms, 1⁄2 oz.; sugar colouring, 1⁄4 pint; brandy or proof spirit, 2 galls.; digest a week and strain.
2. Tincture of gentian (compound), and brandy or proof spirit, of each 1 quart; tincture of serpentary and syrup of saffron, of each 1 pint; tinctures of aloes and rhubarb, of each 1⁄4 pint; bitter almonds (bruised), 8 in no.; digest as before.
625
3. (Foy.) Aloes and cascarilla, of each 1 dr.; rhubarb, 4 dr.; gentian, germander, dried orange peel, and wormwood, of each 6 dr.; rectified spirit, 32 fl. oz.; as before. Stimulant, tonic, and stomachic.—Dose, 20 drops to a teaspoonful.
Elixir, Ton′ic. Syn. Elixir roborans. See Tincture of Crown Bark (Comp.,—Ph. Bor. 1847).
Elixir Tonique Antiglaireux de Guillé. A stomachic tonic for diarrhœa. Calumba root, 90 parts; orris root, 60 parts; gentian root, 8 parts; jalap root, 1500 parts; aloes, 13 parts; saffron, 60 parts; sulphate of quinine, 16 parts; tartar emetic, 2 parts; nitre, 16 parts; yellow sandal, 30 parts; syrup prepared from barley sugar, rectified spirit, and water, of each 11,000 parts. Macerate the drugs in spirit for 24 hours, and dissolve the salts in the water. Filter the liquids, mix and leave for 24 hours, then add the syrup, stand and filter next day. (Reveil and Hager.)
Elixir, Tooth. Syn. Elixir dentifricum, L. Prep. 1. (Lefandinière’s.) Guaiacum raspings and cloves, of each 1 oz.; pellitory of Spain and nutmeg, of each 2 dr.; oil of rosemary, 20 drops; bergamotte, 10 or 12 drops; brandy, 1 quart; macerate a fortnight, and filter.
2. Cinnamon, cloves, and nutmeg, of each, 1 dr.; vanilla, 1⁄2 dr.; camphor, 10 gr.; tincture of pellitory, 2 fl. oz.; brandy or proof spirit, 1⁄2 pint; digest as before. See Antiscrofulous and Rose Elixirs (above).
Elixir Valerianatis Ammonici (Goddard). Valerianic acid, 3 grammes dissolved in 40 grammes distilled water and neutralised with ammonium carbonate. Add this to 35 grammes spirit, 50 grammes syrup, 1 drop bitter almond oil, 2 drops oil of orange peel, 30 grammes diluted bitter almond water, 12 grammes tincture of red sandal, 3 grammes tincture of orange peel, 2 grammes burnt sugar, and filter.
Elixir, Vis′ceral. Syn. Elixir viscerale, L. See Elixirs, Boerhaave’s and Hoffman’s (above).
Elixir of Vit′riol. 1. The old name for aromatic Sulphuric Acid (which see).
2. (Mynsicht’s.) See Tincture (Acid Aromatic).
3. (Scourer’s.) Dilute sulphuric acid 1 to 5). Used to scour metals.
4. (Sweet E. of V.; E. Vitrioli Dulci, L.) The old name for aromatic Spirit of Ether (which see).
5. (Virgani’s). Prep. From spirit of sulphuric ether, 2 lb.; aromatic tincture, 3 lb.
Elixir, Woroneje. Capsicum, 1 oz.; nitre, 1⁄2 oz.; sal-ammoniac, 2 dr.; nitro-hydrochloric acid, 2 fl. dr.; vinegar, 11⁄2 pint; native white or rose naphtha, or petroleum, 11⁄2 fl. dr.; olive oil, 1 fl. oz.; oil of peppermint (Mitcham), 15 fl. oz.; strongest rectified spirit, 6 quarts; digest 12 days, with constant agitation, and filter.—Dose, 2 teaspoonfuls every 15 minutes; in cholera, diarrhœa, &c.
ELLAG′IC ACID. HC7H2O4.Aq. When an aqueous infusion of nut-galls is left for some time exposed to the atmosphere, the tannic acid gradually disappears, and is replaced by gallic acid, and an insoluble grey powder, to which the term ellagic acid was applied by Chevreul. It is soluble in alkalies, forming salts, and is precipitated by acids.
ELM. Syn. Ulmus, L. A genus of tree forming the type of the natural order Ulmaceæ. The interior bark of the Ulmus campestris, or common small-leaved elm (Ulmi cortex), is officinal in B. P. This substance is demulcent, diaphoretic, and diuretic, and slightly febrifuge, astringent, and tonic. It has been employed in agues, and as a substitute for sarsaparilla in cutaneous eruptions, but is now little used. The leaves of the elm-tree are reported to be vulnerary. See Decoction and Ulmin.
ELUTRIA′TION. Cleansing by washing. The term is commonly applied to the operation of washing insoluble powders with water, to separate them from foreign matter, or the coarser portion. It is usually performed by grinding or triturating the mass with a little water until reduced to a very fine powder, and this paste is suddenly diffused through a large quantity of water, contained in a deep vessel, from which, after the subsidence of the grosser portion, the liquid is poured into another vessel, and allowed to deposit the fine powder it still holds in suspension. When this has taken place, the clear supernatant liquor is decanted, and the sediment drained and dried. The coarse sediment deposited in the first vessel is now submitted to a fresh grinding and diffusion through water, and the entire operation is repeated until the whole of the pulverisable portion is washed over. The proper length of time for the liquid to remain in the first vessel depends solely on the density of the powder and the degree of fineness required in the product; heavy powders subsiding almost immediately, while light ones often take several minutes to deposit the coarser portion. Sometimes three or more vessels are employed, and the muddy liquor, after remaining a short time in the first, is poured into the next one, and this, in a short time longer, into the third, and so on, until the last vessel is filled, by which means powders of different degrees of fineness are obtained, that deposited in the last vessel being in the minutest state of division. The elutriated paste or moist powder is then drained, and dried. On the small scale the trituration is performed with a stone and muller, or in a mortar; on the large scale, in a mill, driven by either horse or steam power. Antimony chalk, bistre, and other pigments, as well as various other substances insoluble in or unacted on by water, are commonly obtained in the state of an impalpable powder by elutriation,626 or ‘washing over,’ as it is called by amateurs and operatives.
ELYDOR′IC PAINTING. A method of painting invented by M. Vincent, of Montpetit, in which the pigments are mixed up with an emulsion of oil and water. It is said to add the fresh appearance of water colours, and the finish of miniature painting, to the mellowness of oil colours.
EMBALM′ING. Syn. Mummification. The preservation of the dead bodies of animals. See Putrefaction.
EMBOS′SING. The formation of ornamental figures in relief on cloth, leather, paper, and wood, has now been brought to such perfection as to place this species of decoration within the reach of almost every class of society. Embossed cloth and PAPER are now employed by the bookbinder to cover even the low-priced volumes that pass through his hands; whilst the EMBOSSED LEATHER that encloses the album or ornaments our furniture frequently bears the richest patterns of the arabesque or moresque. Cloth and paper are usually embossed by machinery; leather and wood more frequently by hand labour.
EMBROCA′TION. Syn. Embrocatio, L. A fluid medicine for external and local use. Embrocations do not differ, materially, from liniments and lotions, and are applied in the same manner. (See those preparations, and below.)
Embrocation, Guestonian. Syn. Embrocatio terebinthinæ cum acido L. Prep. (Dr Paris.) Oil of turpentine and olive oil, of each 11⁄2 oz.; dilute sulphuric acid, 3 fl. dr.; agitate together until mixed. Used in rheumatism, &c.
Embrocation, Lynch’s. Olive oil (coloured with alkanet root), 5 fl. oz.; oils of amber, rosemary, and turpentine, of each 1 dr. In bruises, rheumatism, &c.
Embrocation, Roche’s. Prep. 1. (Dr Paris.) Olive oil, mixed with half its weight of the oil of cloves and amber.
2. Olive oil, 2 oz.; oil of amber, 1 oz.; oils of cloves and lemons, of each 1 dr. For hooping-cough.
Embrocation, Ward’s. See Essence.
Embrocation of Cantharides. Syn. Embrocatio Cantharides. (Dr Struve, in hooping-cough.) Prep. Tartarized antimony, 1 scruple; water, 2 oz.; tincture of cantharides, 1⁄2 oz. To be rubbed over the region of the stomach, covering the part afterwards with flannel.
Embrocation of Delphinia. Syn. Embrocatio Delphiniæ. (Dr Turnbull.) Prep. Delphinia, 1 scruple to 1 dr.; rectified spirit, 2 oz.
Embrocation of Quinine. Syn. Embrocatio Quiniæ. (Dr Gustamacchia.) Prep. Sulphate of quinine, 8 to 12 gr.; rectified spirit, 1 oz.
Embrocation of Veratria. Syn. Embrocatio Veratriæ. (Dr Turnbull.) Prep. Veratria, 1 scruple to 1 dr.; rectified spirit, 2 oz.
EMBROID′ERY. Gold and silver fancy work of this description may be cleaned with a little spirit of wine, either alone or diluted with an equal weight of water. Gin is frequently used for the same purpose. The common practice of using alkaline or acid liquors is very injurious, and frequently destroys the beauty of the articles instead of cleaning them.
EM′ERALD. Syn. Smaragdus; Emeraude [Fr.] This beautiful deep-green gem ranks next to the diamond in value. The finest are brought from Peru, but fair varieties are found in Bavaria, Siberia, and India. A fine emerald weighing 4 or 5 gr. is worth as many pounds; one of 10 gr., about £2 per gr.; one of 15 gr., £3 to £4 per gr.; and so on in proportion to the increase in size. One of 24 gr., if of pure water, is worth about £100. According to the analysis of Vauquelin, the purest specimens consists of 65 parts silica, 14 alumina, 13 glucina, 2·56 lime, and 3·50 oxide of chromium, to which last the gem owes its rich green colour. See Beryl, Gems, Pastes, &c.
Emerald Green. See Green Pigments.
EMERY is an impure, amorphous, compact, and opaque variety of corundum, and consists of alumina, with a small per-centage of silica and peroxide of iron. It occurs in Spain, the isles of Greece, and other localities, and derives its name from Cape Emeri, in the island of Naxos. Its hardness is so great, that it scratches and wears down nearly all minerals except the diamond; hence the use of its powder for cutting and polishing glass and various other hard substances. For commercial purposes, the lumps of emery, as taken from the mine, are broken into pieces about the size of a hen’s egg, which are then crushed under stampers, similar to those used for pounding metallic ores. The coarse powder is then sifted through sieves covered with wire-cloth of different degrees of fineness, by which it is sorted into different sizes. In this state it forms the emery of the shops, or flour emery. For delicate purposes, it is afterwards prepared by elutriation.
Emery Cakes are formed by melting emery flour with a little beeswax, and after thorough admixture, forming it into solid lumps of suitable sizes. Used to dress the edges of buff and glaze wheels.
Emery Cloth is prepared by brushing the surface of thin cotton cloth over with liquid glue, and sifting the emery powder over the surface while still warm.
Emery Paper is made in the same way as emery cloth. Both are used either with or without oil, in the same way as glass paper.
Emery Sticks are made of pieces of wood in the same way, and are used for the same purposes, as emery paper.
Emery Stones are formed of emery, of the627 requisite coarseness, mixed with about half its weight of good Stourbridge loam, and water q. s. to make a stiff paste, which is forced into metallic moulds by a powerful press. The pieces, when thoroughly dry, are exposed in a muffle for a short time to a temperature just under a full white heat. In this way ‘discs’ and ‘laps’ are generally made. For ‘wheels,’ only 1⁄4th of loam is used. Another method, applicable for ‘cutting stones’ generally, is to press the flour emery, previously moistened with water, into moulds, with strong pressure, as before, without any other addition, and then to fire it at nearly a full white heat.
EMETIA. Syn. Emetin, Emetina. A feebly basic or alkaloidal body, existing in and forming the active principle of ipecacuanha.
Prep. 1. (Medicinal—Emetic Extract.)—a. Ipecacuanha (in coarse powder) is digested first in ether, and then in rectified spirit for 3 or 4 days; the alcoholic tincture is next expressed and evaporated (distilled) to dryness; the residuum is dissolved in distilled water, and the solution precipitated with acetate of lead; the precipitate is then diffused through distilled water, in a tall glass vessel, and sulphuretted hydrogen is passed through it, to throw down the lead; after which the liquor is decanted, filtered, evaporated to the consistence of a thick syrup, and spread in a thin layer on warm plates of glass, and allowed to dry in a current of warm air, or by a gentle heat in a stove. The maceration in ether is frequently omitted.
b. Ipecacuanha, 1 part; rectified spirit (·835), 7 parts; make a tincture, distil off the spirit, dissolve in cold distilled water, 5 parts; filter the solution, and evaporate, &c., as before. Inferior to the last.
c. (P. Cod.) As the last, nearly.
Obs. Medicinal or impure emetia is brownish, red, deliquescent, and emetic in doses of 1⁄4 to 1⁄2 gr.
2. (Pure.)—a. Ipecacuanha (in coarse powder), 1 part, is digested for 24 hours in distilled water, 10 parts; together with calcined magnesia, added in slight excess; the deposit is then thrown on a filter, washed carefully with very cold water, and dried; it is next dissolved in rectified spirit and neutralised with dilute sulphuric acid; the filtered solution is decoloured with animal charcoal, again filtered, and again precipitated by digestion with magnesia; the last deposit forms a colourless solution with rectified spirit, which, by gentle evaporation, gives up its emetia as a yellowish white pulverulent mass, which may be rendered perfectly white by redissolving it in alcohol, &c., as before. The process is rendered easier by first digesting the powdered ipecacuanha in ether.
b. (P. Cod.) Alcoholic extract of ipecacuanha, 1 part; water, 10 parts; dissolve, filter; add calcined magnesia, 1 part; evaporate to dryness, wash the product on a filter with very cold water, 5 parts; dry it again, and dissolve it in boiling alcohol; evaporate the filtered tincture to dryness, redissolve the residuum in a little water, acidulate (slightly) with dilute sulphuric acid, decolour with animal charcoal, filter, precipitate with liquor of ammonia, and dry the precipitate by a gentle heat.
c. (Ph. Suec. 1845.) Powdered ipecacuanha, 1 part; water, acidulated with sulphuric acid, 6 parts; digest, filter; add lime, 1 part, and evaporate to dryness over a water bath; the residuum is then exhausted with boiling rectified spirit, and otherwise treated as in the last formula.
Prop., &c. Pure emetia is white, pulverulent, inodorous, and bitter; fusible at 122° Fahr.; very soluble in alcohol and boiling water, but only slightly so in ether, oils, and cold water. It restores the blue colour of reddened litmus, and partially neutralises the acids, forming scarcely crystallisable salts. It is reddened by nitric acid, and this red colour is deepened by ammonia. Tincture of iodine produces a reddish precipitate in an alcoholic solution of emetia. With tincture of galls this solution behaves like morphia; but, unlike the last substance, the salts of iron produce no change of colour in it. These reactions, combined with its emetic properties, are sufficient for its identification.—Dose. White and pure emetia is emetic in doses of 1⁄20 to 1⁄16 gr. The large doses ordered in certain pharmaceutical compilations, evidently in error of the difference between the strengths of the pure and the impure or medicinal emetia, have, in several cases which have been reported on, produced very serious consequences.
The ‘Journal de Pharmacie et de Chemie,’ for September, 1875, contains a new process for the extraction of emetia, by M. A. Glenard. This process is based upon the combined use of lime and ether. It consists in treating with ether a suitably prepared powder, or an extract of ipecacuanha and lime, or the precipitate formed upon adding an excess of lime to a solution obtained by treating ipecacuanha in the cold with water acidulated by sulphuric acid. Either of these mixtures, or the precipitate, when treated with ether, will yield all the alkaloid it contains.
The alkaloid may be obtained from the ethereal solution by distilling it to dryness, and treating the residue with acidulated water, or by at once shaking the solution with acidulated water. A more or less acid aqueous liquid is thus obtained, which upon the addition of ammonia, yields the emetine almost colourless, and much more pure than that produced by the process ordinarily employed.
Preparation of Crystallised Hydrochlorate and Pure Emetine.—When water, acidulated with hydrochloric acid, is employed to remove the emetine from the ether, an acid solution is obtained, which, when sufficiently concentrated by evaporation, forms a nearly colourless, solid, crystalline mass. This mass is formed of extremely delicate needles, formed628 in bundles that radiate around a central point, and form small spheres with an embossed surface, resembling mulberries in appearance. Upon pressing these crystals in a cloth the more or less coloured mother liquid runs off, and the crystals redissolved in water give a colourless solution, from which a fresh crystallisation of perfectly pure hydrochlorate of emetine can readily be obtained.
The production of this crystallised hydrochlorate of emetine is worthy of notice, since it does not accord with what has been stated by different authors, who have all considered emetine to be incapable of forming crystallisable salts. It is especially interesting in that it furnishes a convenient and certain method of obtaining perfectly pure emetine, for which it is only necessary to precipitate a solution of the hydrochlorate with an alkali. But it is important to observe that ammonia does not precipitate all the emetine of the hydrochlorate, and that the precipitate is less in proportion as the salt is more acid.
It might appear from this that emetine is soluble in hydrochlorate of ammonia. But the author finds that it is the result of a decomposing action exercised by the emetine upon the hydrochlorate of ammonia, as is shown by the following two experiments. If a little dry powdered emetine be placed in a glass containing a solution of hydrochlorate of ammonia, it may be observed to agglomerate and become transformed into a soft resinoid mass, at the same time the disengagement of ammonia may be recognised, and the resinoid mass gradually undergoes a kind of metamorphosis, and becomes white and crystalline. Again, if emetine in powder be suspended in water, and solution of hydrochlorate of ammonia be gradually added, the emetine is dissolved, and upon evaporation of the solution crystals of double hydrochlorate of emetine and ammonia is obtained.
The author believes the decomposition of hydrochlorate of ammonia by an organic alkali to have been hitherto unobserved. It does not appear, however, that emetine is alone in this action, as the author has observed that quinine, under similar conditions, behaves in the same manner.
Zinoffsky (‘Jour. de Pharm. d’Anvers,’ xxix, 490) gives the following process for the quantitative determination of emetia:—Treat fifteen grams of powdered ipecacuanha with alcohol of 85 per cent., acidified with a few drops of sulphuric acid, so as to form a volume of 150 cubic centimètres. Filter, and after expelling the alcohol from 100 cubic centimètres of the liquid by distillation, add to the residue a titrated solution of iodo-hydrargyrate of potassium until a filtered portion ceases to be affected by this reagent. The number of cubic centimètres of iodo-hydrargyrate multiplied by 0·0189 (0·0001 of the equivalent of emetine) gives the quantity of emetine contained in ten grains of the root.
A normal solution of iodo-hydrargyrate is obtained by mixing aqueous solutions of 13·546 grams of bichloride of mercury, and 49·8 of iodide of potassium, adding water to make one litre. One cubic centimètre of this solution precipitates 0·0001, or 0·00005 of an equivalent of alkaloid.
Wine of ipecacuanha can be titrated by the same process.
Composition of Emetine and its Hydrochlorate.—These substances dried at 110° C., gave upon analysis results corresponding with following centesimal composition:
| Emetine. | Hydrochlorate of Emetine. | |
| Carbon | 72·25 | 63·00 |
| Hydrogen | 8·61 | 8·15 |
| Nitrogen | 5·36 | 4·75 |
| Oxygen | 13·78 | 11·64 |
| Chlorine | 12·46 |
From these figures the author has constructed the following formulæ:
Emetine: C30H22NO4. Hydrochlorate of Emetine: C30H22NO4HCl.
Preparation and Composition of Emetine (J. Lefort and F. Wurtz, ‘Comptes Rendus,’ lxxxiv, 1299). When ipecacuanha is dissolved in water, and a concentrated solution of potassium nitrate added, a thick mass is produced, consisting of emetine nitrate. It is washed with water, dissolved in alcohol, and the solution poured into milk of lime. The mixture is evaporated to dryness, and digested with ether, which dissolves out the emetine, leaving it as a yellowish mass on evaporation. On dissolving this mass in sulphuric acid, and pouring the solution into dilute ammonia, the alkaloid is obtained as a white precipitate, which is dissolved in alcohol, from which it separates in minute radiate groups of needles. By analysis it gave numbers leading to the formula C28H40N2O3.[275]
[275] Note by the translator (‘Journal of Chem. Soc,’): “This is printed C28N2H40O5 in the formula for emetine nitrate, and as no data are given, it is impossible to tell which are correct.”
Pure emetine nitrate was prepared, and was found to have the formula C28N2H40O5NOH; this in conjunction with Glenard’s results, shows that emetine does not form basic salts.
EMET′ICS. Syn. Vomits, Anacathartics; Anacathartica, Emetica, Vomitoria, L. Medicines which induce vomiting. The principal emetics are ipecacuanha and tartarised antimony, and their preparations; and the sulphates of zinc and copper. Of these the first is commonly employed either in substance or infused in wine (ipecacuanha wine), when it is merely wished to evacuate the contents of the stomach, when that organ is in a disordered state or overloaded with food; and is the one most adapted in ordinary cases for children and females. Tartar emetic (tartarated antimony) (dissolved in water) and antimonial629 wine, either alone or combined with ipecacuanha, are preferable at the commencement of fevers and other inflammatory disorders, in consequence of the nausea, relaxation, and depression of the muscular power and circulation which commonly follow their use. When poison has been taken, sulphate of zinc is generally preferred as an emetic, on account of the promptness and completeness of its action, and its effects ceasing as soon as it is ejected from the stomach. Sulphate of copper is employed in the same cases as sulphate of zinc, but its action is more violent and disagreeable, whilst its intense metallic taste is a great objection to its use. 25 to 30 gr. of either of the above sulphates are dissolved in 3 or 4 fl. oz. of warm water, and a fourth of the solution is given every ten minutes, until copious vomiting ensues. In the absence of other substances, when an immediate emetic is required, a teaspoonful of flour of mustard (an article always at hand), stirred up with half a pint of warm water, and drank at a draught, will generally act easily and effectively, and relieve the stomach before other remedies can be obtained and applied.
The operation of emetics is powerfully promoted by drinking copiously of diluents, especially of warm or tepid water. The latter, in fact, is itself an emetic, when taken in quantity. Its use will also prevent that dreadful straining and retching which makes emetics so much dreaded by the nervous and delicate.
The timely administration of an emetic at the commencement of fevers and other inflammatory affections will frequently cause copious diaphoresis, and produce a cure, or at least greatly mitigate the severity of the symptoms. Dropsies have also been cured by vomiting; and swelled testicle, bubo, and other glandular swellings, have occasionally been dispersed by the action of emetics. Visceral obstructions, in both sexes, have also yielded to the same treatment. Small and repeated doses of emetics are frequently administered, with advantage, to produce nausea, in many diseases of the lungs and stomach. Certain chronic and obstinate diseases, as rheumatism and asthma, are sometimes relieved by emetics, when every other line of treatment has failed.
Emetics should be avoided in plethoric habits, in hernia, pregnancy, and whenever visceral inflammation is suspected. They should also be given with great caution to young children and females, and to the nervous and delicate. In such cases, wine or powder of ipecacuanha should alone be employed.
Emetic Cups. Syn. Antimonial cups; Pocula emetica, Calices vomitorii, L. Small cups made of metallic antimony. Wine left in them for 10 or 12 hours becomes emetic.
Emetic Tartar. See Antimony, Tartarated.
EM′ETINE. See Emetia.
EMMEN′AGOGUES. Syn. Emmenagoga, L. Medicines which are considered to have the power of promoting the menstrual discharge when either retained or suspended. There are, probably, few remedies which exert this specific power on the uterus, the majority of repeated emmenagogues acting rather by their influence on the system generally, or on parts contiguous to the uterus, than in the uterus itself. Among the substances usually arranged under this class are—aloes, black hellebore, birthwort, borax, cubebs, ergot, gamboge, gin, iodide of potassium, iodine, madder, mercurials, the peppers, rue, savine, stimulants (generally), stimulating diuretics, stinking goosefoot, stinking orache, wine, &c.
Of the above, ergot and madder are the only articles which exercise a direct power on the uterus, and that rather in increasing its expulsive energy than in promoting the menstrual function, though they are advantageously employed for the latter purpose. Several of the other substances named are drastic purgatives, or possess cerebro-spinal properties, or local powers of irritation, by which they increase the pelvic circulation, or produce excitement in the neighbouring parts, in many cases of a dangerous and irreparable character. Hence many writers on pharmacology deny the existence of emmenagogues.
To ensure the successful administration of this class of medicines, the system must be previously prepared for their use by invigorating it, if there is either relaxation or debility; and an opposite course should be pursued when there is an undue degree of arterial action. In the majority of cases, the restoration of the discharge is rather attributable to a proper regulation of the system than to any specific power in the medicine administered.
EMOLL′IENTS. Syn. Emollientia, L. In pharmacy and therapeutics, demulcents of an oleaginous, saponaceous, or emulsive character, applied to surfaces (generally external), to soften and relax the fibres. See Demulcents.
EMUL′SIN. Syn. Synaptase. An azotised substance, forming a large proportion of the white pulp of both bitter and sweet almonds. It is yellowish-white, soluble in cold water, and coagulated by heat and alcohol. Its most remarkable property is its action on amygdalin by which the volatile oil of almonds and hydrocyanic acid, with other products are formed. It has never been obtained in a state of purity.
EMULSINES. See Emulsion.
EMUL′SION. Syn. Emulsio, L. A milky fluid, formed by the mechanical admixture of oil and water, by means of some other substance that possesses the power of combining with both. The emulsions of the Pharmacopœia are in the ‘British Pharmacopœia’ included in the class Misturæ (which see).
630
In the preparation of emulsions, the oily or resinous ingredients are usually suspended by means of mucilage of gum arabic, almonds, or yolk of egg. 1 dr. of the first, made with equal parts of gum and water; 1 oz. of the second (usually 26 in number); and one in no. of the last, will form 2 dr. of any oil into an emulsion with about 1 oz. of water, gradually added. In some cases, instead of the above substances, a little liquor of potassa is employed, by which a saponaceous emulsion is formed. In all cases the mucilage or other viscid substance should be put into the mortar before anything else. The oil or resinous matter may then be very gradually rubbed in, taking care not to add it more quickly than it can be subdued by the pestle; and if, during this part of the manipulation, the mixture should begin to assume a breaking or curdling appearance at the edges, a few drops of water must be immediately incorporated with it, before adding the remainder of the oil. From the want of this precaution, it is common for an emulsion suddenly to lose its tenacious consistence in the mortar, and it is then in vain to endeavour to restore it. After the oil is thoroughly incorporated, some care is requisite to avoid separating it again by too hasty an effusion of the water or other fluid of the mixture. If any alcoholic or acid liquid is to be added, it must be at the very end of the process. Indeed, the addition of an acid liquid, even a slightly acescent syrup, will often entirely destroy an emulsion. Mixtures of copaiba are frequently spoiled by the addition of spirit of nitric ether; a misfortune which might be avoided by first diluting it with one or two parts of water.
An excellent method of preparing emulsions of resins and gum-resins, is to put the article into a marble or wedgwood mortar, and to pour over it about 4 times its weight of rectified spirit, which is then to be ignited, and the mixture triturated until an equal consistence is obtained. The liquid is then to be added gradually, and the whole patiently triturated or shaken until cold. Yolk of egg or mucilage may be added to the fluid resin or gum-resin, if desired, as in the common method, but an excellent emulsion may be made without them.
The presence of soluble salts in an emulsion is apt to occasion the separation of the oleaginous portion. Spirit produces the same effect in those which are made with yolk or mucilage; and acids in those made with an alkali. The addition of these substances to emulsions should be therefore avoided as much as possible. Emulsions of wax, spermaceti, oil of turpentine, and balsam of copaiba, are the most readily and completely formed with yolk of egg. Volatile oils are more readily made into emulsions if mixed with an equal volume of some simple fixed oil, before proceeding to operate on them. Scammony is generally formed into an emulsion with milk; and resin of jalap, with almonds and water.
In a paper read before the American Pharmaceutical Association by Mr Gregory, the author recommends the use of powdered gum instead of mucilage in the preparation of emulsions. He thinks that three drachms of acacia in fine powder are necessary to emulsify one ounce of any of the volatile oils, and that a little less (about two drachms) will answer for the fixed oils and balsams, and that to this quantity of gum four drachms and a half of water must be added (no more and no less), and that either the water or the oil may be added first to the gum, but it is quickest to add the oil the first; and well triturate before adding the water. Less gum can be made to yield a good result by a careful operator, but, as a general practical working rule, it may be said that three drachms are necessary for one ounce of oil.
The following formulæ, for certain emulsions, are merely given here for examples. Various others will be found under Mixture, Lotion, Wash, &c.
Emulsion of Al′monds. Syn. Milk of almonds, Almond mixture; Emulsio amygdalæ, Mistura a., L. Prep. 1. Blanched almonds, 1 oz.; beat them to a smooth paste, add, gradually, water, 1⁄2 pint; and when the whole is thoroughly incorporated, strain through a piece of gauze.
2. As the last, adding sugar, 1 oz.; or syrup (either simple or flavoured), 11⁄2 fl. oz. See Emulsion of Oil of Almonds (below).
Emulsion of Assafœt′ida. Syn. Emulsio Assafœtidæ, Mistura a., L. Prep. (Duclow.) Assafœtida, 1 oz.; powdered gum, 2 oz.; oil of almonds, 31⁄2 fl. oz.; water, 6 fl. oz. Antispasmodic.—Dose, 1 to 2 table-spoonfuls; in hysterical affections, &c.
Emulsion of Cam′phor. Syn. Emulsio camphoræ, E. camphorata, Mistura camphoræ (Ph. E.), L. Prep. 1. (Ph. Castr. Ruth. 1840.) Camphor, 1⁄2 dr.; triturate with milk, 1⁄2 fl. oz., gradually added; then further add of water, 71⁄2 fl. oz.
2. (Ph. E.) Camphor, 20 gr.; lump sugar, 1⁄2 oz.; triturate together, and add of blanched almonds, 1⁄2 oz.; again triturate, then gradually add of water, 1 pint. Stimulant, antispasmodic, and diaphoretic.—Dose, 1 fl. oz. to 2 fl. oz.
Emulsion of Cas′tor Oil. Syn. Emulsio olei ricini, Mistura o. r., L. Prep. 1. Castor oil, 1 oz.; thick mucilage, 11⁄2 oz.; syrup of orange peel, 1 fl. oz.; water, 6 fl. oz.
2. As the last, but using milk instead of water.—Dose. One third; as an aperient for females who object to taking the unprepared oil.
Emulsion of Copai′ba. Syn. Emulsion of capivi; Emulsio copaibæ, Mistura c., L. Prep. 1. Balsam of copaiba and syrup of orange peel, of each 2 oz.; yolks of 5 eggs; milk, 14 fl. oz.
631
2. (Beral.) Copaiba and mucilage, of each 2 oz.; water, 12 fl. oz.—Dose, 1⁄2 oz. to 1 oz., 2 or 3 times a day; where the use of copaiba is indicated.
Emulsion of Cubebs. Syn. Emulsio cubebæ. (Dublanc.) Prep. Essence of Cubebs, 4 oz.; mucilage, 4 oz. Mix them.
Emulsion of Gum. Syn. Emulsio acaciæ, Mistura acaciæ (Ph. E.), L. Prep. From sweet almonds (blanched), 10 dr.; white sugar, 5 dr.; mucilage, 3 fl. oz.; water, 1 quart. Demulcent. In coughs, &c., ad libitum.
Emulsion of Indian Hemp. Syn. Emulsio Cannabis Indicæ. (Mr Bromfield.) Prep. Rub 1 scruple of extract of Indian hemp in warm water with 1 fl. dr. of olive oil; then add gradually, still triturating the mixture, 4 dr. of mucilage of acacia and 71⁄2 oz. of distilled water.
Emulsion of Oil of Almonds. Syn. Emulsio olei amygdalæ, L. Prep. From oil of almonds, 3 dr.; thick mucilage and simple syrup, of each, 5 dr.; rose water, 1 fl. oz.; distilled water, 3 to 4 fl. oz. An elegant and efficient substitute for almond milk. See Emulsion of Almonds (above).
Emulsion, Pancreatic. See Pancreatin.
Emulsion of Peru′vian Balsam. Syn. Emulsio balsamica, E. balsami Peruviani, L. Prep. 1. As emulsion of copaiba.
2. (Hosp. F.) Balsam of Peru, 1⁄2 oz.; oil of almonds, 6 dr.; powdered gum, 1 oz.; triturate together, and add, gradually, rose water, 4 fl. oz.—Dose, 1 or 2 table-spoonfuls; in old asthmas, chronic coughs, winter coughs, &c.
Emulsion of Poppies. Syn. Emulsio papaveus. Prep. Poppy seeds, 2 drachms; water, 8 oz. Make into an emulsion and strain.
Emulsion of Raw Meat. (Yvon.) Raw meat 250 grammes; sweet almonds, 75 grammes; bitter almonds, 5 grammes; white sugar, 80 grammes. After blanching the almonds beat them up with the rest of the ingredients in a marble mortar until a rose-coloured uniform paste is obtained. This may be easily made into an emulsion with water, and will not unmix for 24 hours. It can be made still more nourishing by the addition of the yolks of two eggs, and by the substitution of milk for water. This emulsion is frequently prescribed by continental physicians.
Emulsion of Resin of Jalap. Syn. Emulsio purgans cum resinæ jalapæ. (Par. Pharm.) Prep. Resin of jalap, 8 gr.; white sugar, 1 oz.; orange-flower water, 2 dr.; water, 4 oz. Triturate the resin with a little of the sugar, add gradually half the yolk of an egg, triturate for a long time, then add gradually the rest of the sugar and the water.
Emulsion of Scam′mony. Syn. Emulsio scammonii, Mistura s. (Ph. E.), L. Prep. 1. (Ph. E.) Resin of scammony, 7 gr.; new milk, 3 fl. oz. For a dose.
2. (Planche.) Aleppo scammony, 7 gr.; sugar, 2 dr.; new milk, 3 fl. oz.; cherry-laurel water, 5 drops. For a dose. Purgative; in torpor of the intestines, dropsy, worms, &c. The formula of the Paris Codex is similar.
Emulsion of Spermace′ti. Syn. Emulsio cetacei, Mistura c., L. Prep. As emulsion of wax. Demulcent.
Emulsion of Tur′pentine. Syn. Emulsio terebinthinæ, Mistura t., L. Prep. 1. Chio turpentine, 2 dr.; white sugar, 1 oz.; yolk of 1 egg; milk of almonds, 4 fl. oz. In gleets.—Dose, 2 table-spoonfuls, 3 or 4 times a day.
2. (Clossius.) Venice turpentine, 11⁄2 dr.; yolk of 1 egg; peppermint water, 41⁄2 fl. oz. (See below.)
Emulsion of Oil of Turpentine. Syn. Emulsio olei terebinthinæ, Mistura o. t., L. Prep. (Carmichael.) Rectified oil of turpentine, 1 fl. oz.; yolk of 2 eggs; emulsion of almonds, 4 fl. oz.; syrup of orange peel, 2 fl. oz.; spirit of lavender, 4 fl. dr.; oil of cinnamon, 5 or 6 drops.—Dose, 1 fl. oz., twice or thrice a day; in nephritic pains, and that variety of ophthalmia termed iriditis. (See above.)
Emulsion of Wax. Syn. Emulsio ceræ, E. ceræ albæ, Mistura c., Lac c., L. Prep. (Guibourt.) White wax, 1 oz.; powdered gum, 11⁄2 dr.; water, 24 fl. oz.; simple syrup, 4 fl. oz.; put the syrup and gum into a warm mortar, add the wax, and triturate with a warm pestle until united; then add the water (warm) gradually, and continue the agitation till the whole is quite cold. Demulcent. Ad libitum.
ENAM′EL. A species of vitreous varnish, coloured with metallic oxides, applied in a thin stratum to brightly polished metallic surfaces (copper or gold), on which it is fused by the flame of a lamp urged by the blowpipe, or by the heat of a small furnace.
The basis of all enamels is a highly transparent and fusible gloss, called ‘frit,’ ‘flux,’ or ‘paste,’ which readily receives a colour on the addition of metallic oxides. It may be made by one or other of the following formulæ:
Prep. 1. Red lead, 16 parts; calcined borax, 3 parts; powdered flint glass, 12 parts; powdered flints, 4 parts; fuse in a Hessian crucible for 12 hours, then pour it out into water, and reduce it to a powder in a biscuit-ware mortar.
2. Tin, 3 parts; lead, 10 parts; mix, calcine in an iron pot at a dull cherry-red heat, and scrape off the oxide as it forms, observing to obtain it quite free from undecomposed metal; then reduce it to fine powder by grinding and elutriation. In this state it is known among enamellers as ‘flux’ or ‘calcine.’ 4 parts of this ‘calcine’ are next mixed with an equal weight of pure sand or powdered flints, and 1 part of sea salt, or other alkaline matter; the mixture is then partially fused in a Hessian632 crucible, by which it undergoes semi-vitrification.
3. (Chaptal.) Lead and tin, equal parts; calcine as above, and take off the mixed oxides or ‘calcine’ and ground flints, of each, 1 part; pure carbonate of potash, 2 parts; and proceed as before.
4. (Wynn.) Flint glass, 3 oz.; red lead, 1 oz.; as last.
5. (Wynn.) Red lead, 18 parts; borax (not calcined), 11 parts; flint glass, 16 parts; as last.
6. (Wynn.) Powdered flints, 10 parts; nitre and white arsenic, of each, 1 part; as last.
Obs. The precise qualities of the products of the above processes depend greatly upon the duration and degree of heat employed. By increasing the quantity of sand, glass, or flux, the enamel is rendered more fusible, and the opacity and whiteness is increased by the addition of oxide of tin. The use of borax should be avoided, or it should be used sparingly, as it is apt to make the enamel effloresce and lose colour.
Enamel, Black. Prep. 1. Calcined iron (protoxide), 12 parts; oxide of cobalt, 1 part; mix, add an equal weight of white flux, and fuse as before.
2. (Clouet.) Pure clay, 3 parts; protoxide of iron, 1 part. A fine black.
3. Peroxide of manganese, 3 parts; zaffre, 1 part; mix, and add it, as required, to white flux.
Enamel, Blue. Prep. 1. White ‘frit’ or ‘flux,’ coloured with oxide of cobalt.
2. Sand, red lead, and nitre, of each 10 parts; flint glass or ground flints, 20 parts; oxide of cobalt, 1 part, more or less; depending on the desired depth of colour.
Enamel, Brown. Prep. 1. Manganese, 5 parts; red lead, 16 parts; flint powder, 8 parts; as before.
2. (Wynn.) Manganese, 9 parts; red lead, 34 parts; flint powder, 16 parts.
3. Red lead and calcined iron, of each, 1 part; antimony, litharge, and sand, of each, 2 parts. To be added in any required proportion to white ‘frit,’ according to the colour desired. A little oxide of cobalt or zaffre is frequently added to alter the shade.
Enamel, Green. Prep. 1. ‘Flux’ or ‘frit,’ 2 lbs.; black oxide of copper, 1 oz.; as before.
2. As the last, but adding red oxide of iron, 1⁄2 dr. Less decisive.
3. Copper dust and litharge, of each 2 oz.; nitre, 1 oz.; sand, 4 oz.; ‘flux’ or ‘frit,’ q. s.
4. From transparent ‘frit,’ any quantity; oxide of chromium, q. s. to colour. Colour superb; it will stand a great heat; in common hands, however, it frequently turns on the dead-leaf tinge.
5. Transparent ‘flux,’ 5 oz.; black oxide of copper, 20 to 40 gr.; oxide of chromium, 2 gr. Resembles the emerald.
6. From blue and yellow enamel mixed in the required proportions.
Enamel, Ol′ive. Prep. Blue enamel, 2 parts; black and yellow enamel, of each, 1 part. See Enamel Brown.
Enamel, Or′ange. Prep. 1. Red lead, 12 parts; red sulphate of iron and oxide of antimony, of each, 1 part; flint powder, 3 parts; calcine together, powder, and melt with ‘flux,’ 50 parts.
2. (Wynn.) Red lead, 12 parts; oxide of antimony, 4 parts; flint powder, 3 parts; red sulphate of iron, 1 part; calcine, then add ‘flux,’ 5 parts, to every 2 parts of this mixture.
Enamel, Pur′ple. Prep. 1. ‘Flux’ or ‘frit,’ coloured with oxide of gold, purple precipitate of cassius, or peroxide of manganese.
2. Sulphur, nitre, green vitriol, antimony, and oxide of tin, of each, 1 lb.; red lead, 60 lb.; mix, fuse, cool, powder, and add rose copper (red oxide), 19 oz.; zaffre, 1 oz.; crocus martis, 11⁄2 oz.; borax, 3 oz.; and of a compound formed of gold, silver, and mercury, 1 lb.; fuse, stirring the melted mass with a copper rod all the time, then place it in crucibles, and submit them to the action of a reverberatory furnace for 24 hours. This is said to be the purple enamel used in the mosaic pictures in St. Peter’s at Rome.
Enamel, Red. Prep. 1. ‘Paste’ or ‘flux,’ coloured with the red oxide or protoxide of copper. Should the colour pass into the green or brown, from the partial peroxidation of the copper, from the heat being raised too high, the red colour may be restored by the addition of any carbonaceous matter, as tallow, or charcoal.
2. By tinging the glass or ‘flux’ with the oxide or salts of gold, or with the purple precipitate of cassius. These substances produce shades of red, inclining to crimson or purple of the most exquisite hue. The enamel often comes from the fire quite colourless, and afterwards receives its rich hue at the lamp.
3. (Wynn.) Sulphate of iron (calcined dark), 1 part; a mixture of 6 parts of ‘flux’ (No. 5), and 1 of colcothar, 3 parts. Dark red.
4. (Wynn.) Red sulphate of iron, 2 parts; ‘flux’ (No. 1), 6 parts; white lead, 3 parts. Light red.
Enamel, Rose-col′oured. Prep. Purple enamel (or its elements), 3 parts; ‘flux,’ 90 parts; mix, and add silver leaf or oxide of silver, 1 part, or less.
Enamel, Transpa′′rent. The ‘frit’ or ‘flux’ described above.
Enamel, Vi′olet. Prop. 1. Purple enamel, 2 parts; red enamel (No. 2), 3 parts; ‘frit,’ 6 parts.
2. Saline or alkaline ‘frit’ or ‘flux,’ any quantity; peroxide of manganese, q. s. to colour. As the tint depends on the metal being at the maximum of oxidation, contact with oily or carbonaceous substances should be particularly avoided.
Enamel, White. Prep. 1. ‘Calcine’ (from 2633 parts of tin and 1 part of lead), 1 part; fine crystal glass or ‘frit,’ 2 parts; manganese, a few grains; powder, mix, melt, and pour the fused mass into clean water; again powder, and fuse, and repeat the whole process 3 or 4 times, avoiding contamination with smoke, dirt, or oxide of iron. A fine dead white.
2. Washed diaphoretic antimony, 1 part; fine glass (free from lead), 3 parts; mix, and proceed as before. Very fine.
3. Lead, 30 parts; tin, 33 parts; calcine as before, then fuse 50 parts of this ‘calcine’ with an equal weight of flints, in powder, and 100 parts of salt of tartar. A fine dead white enamel.
Obs. For white enamel, the articles must be perfectly free from foreign admixture, as this would impart a colour. When well managed, either of the above forms will produce a paste that will rival the OPAL.
Enamel, Yellow. Superior yellow enamels are less easily produced than those of most other colours; they require very little flux, and that mostly of a metallic nature. The following come highly recommended by experienced artists:—
Prep. 1. From ‘frit’ or ‘flux,’ fused with oxide of lead, and a little red oxide of iron.
2. Lead, tin, ashes, litharge, antimony, and sand, of each 1 oz.; nitre, 4 oz.; mix, fuse, and powder; and add the product to ‘flux’ or ‘frit,’ q. s.
3. White oxide of antimony, alum, and sal-ammoniac, of each 1 part; pure carbonate of lead, 1 to 3 parts, or q. s. (all in powder); mix, and expose them to a heat sufficiently high to decompose the sal-ammoniac. Used as the last. Very bright coloured.
4. (Wynn.) Red lead, 8 oz.; oxide of antimony, and tin, calcined together, of each 1 oz; mix, and add of ‘flux’ (No. 5), 15 oz.; mix well and fuse.
5. Pure oxide of silver added to the metallic ‘fluxes.’ The salts of silver are also used, but are more difficult to manage. If a thin film of oxide of silver be spread over the surface of the enamel to be coloured, exposed to a moderate heat, then withdrawn, and the film of reduced silver on the surface removed, the part under will be found tinged of a fine yellow. (Clouet.)
Enamelling of Cast-Iron. Wagner in his ‘Chemical Technology’ gives the following account of this process:—The surface of the cast-iron to be enamelled is first carefully cleaned by scouring with sand and dilute sulphuric acid, next a somewhat thickish magma, made of pulverised quartz, borax, feldspar, kaolin and water is brushed over the clean metallic surface as evenly as possible, and immediately after a finely powdered mixture of feldspar, soda, borax, and oxide of tin, is dusted over, after which the enamel is burnt in by the heat of a muffle. In France an enamel is applied which consists of 130 parts of flint glass, 201⁄2 parts of carbonate of soda, and 12 parts of boric acid fused together, and afterwards ground to a fine powder.
It would appear, however, from the statements contained in a paper read by Mr Tatlock, F.R.S.E., F.C.S., that the enamel used for iron vessels is frequently of a less harmless kind than that described by Wagner. Mr Tatlock states that in some instances the milk-white porcelainous enamel, with which cast-iron cooking vessels are now so commonly prepared, has a composition such as to render it highly objectionable, on account of the facility with which it is acted upon by acid, fruits, common salt, and other ordinary dietetic substances, by which means lead, and even arsenic, are dissolved out in large quantity during cooking processes.
Mr Tatlock gives the analysis of three samples of enamel from the interior of three cast-iron pots obtained from different manufacturers. These iron vessels were all employed for cooking:—
| No. 1. per cent. | No. 2. per cent. | No 3. per cent. | |
| Silica | 61·00 | 42·40 | 42·00 |
| Alumina | 8·00 | 2·88 | 6·06 |
| Oxide of iron | 1·10 | 2·04 | 4·04 |
| Lime | 3·02 | 0·16 | 0·78 |
| Magnesia | 0·28 | 0·10 | 0·21 |
| Oxide of lead | absent | 25·89 | 18·48 |
| Potash | 5·61 | 7·99 | 6·46 |
| Soda | 20·67 | 14·67 | 19·25 |
| Phosphoric acid | trace | trace | trace |
| Arsenious acid | 0·02 | 0·42 | 1·02 |
| Carbonic acid | 0·30 | absent | absent |
| Borax | absent | 3·45 | 1·70 |
| ——— | ——— | ——— | |
| 100·00 | 100·00 | 100·00 | |
| ——— | ——— | ——— | |
| Total bases | 38·58 | 53·73 | 55·28 |
The author showed that it was not so much on account of the presence of large proportions of lead and arsenic that the enamels are so dangerous, but because they are so highly basic in character, that they are acted upon with facility by feebly acid solutions, the lead and arsenic being thereby easily dissolved out.
He demonstrated that the ratio of the bases to the silica in No. 1 was 1 to 1·58; in the No. 2, as 1 to 0·79; and in the No. 3, as 1 to 0·76. A one per cent. solution of citric acid boiled in the No. 1 did not affect it in the slightest, while in the case of the No. 3, the glassy surface of the enamel was at once roughened and destroyed, and lead dissolved out to such an extent as to give immediately a dense black precipitate with sulphuretted hydrogen. He thought that no enamel was fit to be used unless it were totally unaffected by boiling with a one per cent. solution of citric acid, which was a very moderate test, and gave it as his opinion that either the use of such poisonous ingredients as lead and arsenic in large quantity should be entirely abandoned, or that the composition otherwise of the enamel should634 be of such a character as to ensure that none of the poisonous substances could be dissolved out, in the circumstances under which the enamelled vessels are used.
ENCAU′STIC. See Painting (Encaustic).
ENDEMIC. Indigenous. Peculiar to a district. Those are called endemic diseases, which are produced by causes more or less local. The word is often confounded with epidemic.
ENE′MA. Syn. Clyster; En′ema (pl. Enem′ata), L. A medicine, usually liquid (sometimes gaseous), thrown into the rectum or lower bowels.
Clysters usually consist of some weak glutinous or mucilaginous fluid, to which the active ingredients are added; or a decoction or infusion is made of the medicaments, which is then used, either alone, or after the addition of a little gum, starch, or sugar. The proper vehicle for astringent vegetable matter, metallic salts, and the mineral acids, is pure water. Oleaginous and resinous substances are made into emulsions before being employed for enemas. In all cases the fluid is administered warm. The quantity of fluid forming a clyster, for an adult, may vary from 1⁄2 to 3⁄4 pint; that for an infant within a month old, should be about 1 fl. oz.; for a child of one year, about 21⁄2 fl. oz.; from one to seven years, from 3 or 4 fl. oz.; and from seven to twelve or fourteen, 6 or 7 fl. oz.; after that age to puberty, 1⁄2 pint may be employed.
The quantity or dose of the active ingredients in a clyster should be 4 or 5 times as great as that of the same medicines when taken by the mouth; as it is generally regarded that the susceptibility of the rectum is only 1⁄5th that of the stomach, and that to exert a like absorbent action it occupies 5 times as long as the latter viscus. The dose, and the interval between its repetition, should, therefore, be proportionately increased. Narcotics, as opium, tobacco, &c., should, however, be given in only twice or thrice the quantity that would be exhibited in the usual manner.
Enemata are usually administered by means of a syringe, bladder, or elastic bag, furnished with a rectum tube; but many ingenious and elegant pieces of mechanism, adapted for self-administration, are made by the instrument makers. Great care should be taken to avoid injuring the coats of the rectum by the use of a rough or improperly shaped pipe, or one that is too long. The extremity of the pipe or tube should also be perfectly smooth and well rounded (rather spherical than pointed), and in using it no force should be employed. A neglect of this point often produces very serious consequences, especially in young children.
Tobacco smoke may be administered by means of a double pair of bellows, supplied with air from a small funnel under which the herb is burning,—and gaseous matter, by connecting the rectum tube with a small gasometer, exerting a trifling pressure on the confined gas.
The number of substances employed in the preparation of enemata is very great. The following are some of them, arranged according to their effects:—
1. (Anodyne and Narcotic.) Opium, henbane, &c., are employed to allay spasms of the bowels, stomach, uterus, bladder, &c.
2. (Aperient or Cathartic.) Aloes, colocynth, senna, various purging salts, gruel, decoction of marshmallows, decoction of linseed, warm water, &c., are commonly employed to promote the peristaltic action of the bowels, or to destroy worms.
3. (Demulcent and Emollient.) Decoction of starch, gum, isinglass, glue, &c., either alone or combined with opium, are used to protect the coats of the intestines, and to allay irritation; and also to restrain diarrhœa, especially when combined with astringents, as logwood, catechu, or oak bark.
4. (Nutrient.) Animal jelly, soups, broths, milks, &c., are frequently used as injections to convey nourishment to the body.
5. (Sedative.) Tobacco infusion or smoke, and tartar emetic (in solution), are employed to relax the powers of the body, to remove spasms, depress the circulation, and to produce syncope.
Enemata or clysters are now very frequently employed in our large towns, especially among the higher classes; but a great prejudice exists among many persons against their use, arising from a fastidious and mistaken delicacy. The introduction of improved apparatus of late years, by which the administration of these remedies is attended with less difficulty and exposure than formerly, has removed much of the repugnance which previously existed.
Clysters are invaluable when it is necessary to evacuate the bowels as speedily as possible, and when the stomach will not bear the administration of a purgative by the mouth, as well as in cases requiring a direct medication of the lower bowels, as in dysentery, colic, &c. As a mere laxative, an injection of tepid water, milk-and-water, or water gruel, will generally be found sufficient. By the addition of 1 or 2 table-spoonfuls of common salt, Epsom salts, salad oil, or molasses, to this laxative enema, it will form an excellent purgative one, which will, in most cases, induce a full discharge. In all cases, the patient should be directed to retain the injection for as long a time as possible, and not to attempt to empty his bowels immediately after the reception of the medicine. “In irritation of the bladder, rectum, or uterus, an anodyne injection or enema often affords much relief. In diseases of the lower bowels, clysters are also of almost indispensable utility, as also in the dislodgment of ascarides seated in the rectum; nor are they less beneficial in those cases of sudden sinking of the powers of life where deglutition is impossible, and yet a prompt stimulating impression is requisite to635 save the patient; under such circumstances, clysters of some of the diffusible stimuli have proved of the greatest benefit.”
The injection of large quantities of liquid matter into the bowels, as well as the constant use of clysters (even of warm water only), is deemed by the highest medical authorities to be injurious, and occasionally dangerous. The practice should not, therefore, be allowed to grow into a habit. The bowels continually accustomed to a stimulant cease to act without one. The same remarks apply to aperients taken by the mouth.
The following formulæ embrace the whole of the enemas (ENEMATA) of the ‘British Pharmacopœia,’ as well as a few others in common use:—
Enema of Albu′men. Syn. Enema albuminis, L. Prep. (Ricord) Infusion of linseed, 12 oz.; whites of 2 or 3 eggs; mix. In chronic diarrhœa, and as a nutritient clyster in debility from stomach diseases. The reason for rejecting the yolks of the eggs is not very obvious, as the preparation is much more effective with them.
Enema of Al′oes. Syn. Enema aloës (B. P.), L. Prep. From aloes, 2 scrup.; carbonate of potassa, 15 gr.; mucilage of starch, 1⁄2 pint. In ascarides, atonic amenorrhœa, &c. It should not be employed when irritability of the rectum, bladder, or genitals, exists; nor in piles, or when there is a tendency to prolapsus ani or prolapsus uteri.
Enema, An′odyne. See Enema of Opium.
Enema, Antispasmod′ic. Syn. Enema antispasmodicum, L. Prep. From tincture of assafœtida, 3 fl. dr.; laudanum, 30 to 60 drops; water gruel or barley water, 1⁄2 pint. In spasmodic affections of the bowels. (See below.)
Enema of Assafœt′ida. Syn. Fetid clyster, Antispasmodic c.; Enema assafœtida (B. P.), E. fœtidum (Ph. E. & D.), L. Prep. 1. (B. P.) Assafœtida, 30 gr.; water, 4 oz.; rub together until mixed.
2. (Ph. E.) To cathartic enema (Ph. E.), add of tincture of assafœtida, 2 fl. dr.
3. (Ph. D.) Warm water, 12 fl. oz.; tincture of assafœtida, 2 fl. dr.
4. (St. B. Hosp.) Assafœtida, 2 dr.; yolk of an egg; barley water, 7 fl. oz. Stimulant, antispasmodic, and carminative. An excellent remedy in hysteria, flatulent colic, hooping-cough, infantile convulsions, worms in the lower bowels, &c. See Enema Hooping-cough.
Enema, Astrin′′gent. Syn. Enema astringens, L. Prep. 1. Tincture of catechu, 1 fl. oz.; barley water, 9 fl. oz.
2. Extract of rhatany, 2 dr.; syrup, or made starch, 2 oz.; water, 7 fl. oz.
3. Decoction of galls, oak-bark, pomegranate, or other like astringent substance, 3 or 4 fl. oz.; water or barley water, 6 or 7 fl. oz.
4. (Hosp. F.) Electuary of catechu, 2 dr.; water and lime water, of each 41⁄2 fl. oz. In diarrhœa, &c., arising from a relaxed condition of the coats of the lower bowels; and in fissures of the anus, &c.
Enema of Bark. Syn. Enema Cinchonæ. Decoction of bark is used.
Enema of Belladonna. Syn. Enema Belladonnæ (Ratier.) Prep. Belladonna, 10 gr.; water, 6 oz.; infuse.
Enema of Cam′phor. Syn. Enema camphoræ, L. Prep. 1. Camphor liniment, 4 fl. dr.; yolks of 2 eggs; water gruel, 7 fl. oz.
2. Camphor, 1 dr.; rectified spirit, 2 dr.; triturate till dissolved, then add, gradually, of simple syrup, 1 oz.; when thoroughly incorporated, further add of thin gruel, 7 fl. oz. Anodyne, antispasmodic, and diuretic. In difficult or obstructed micturition.
Enema of Cas′tor Oil. Syn. Enema olei ricini, L. Prep. 1. (Hosp. F.) Castor oil and mucilage, of each, 1 oz.; gruel, 1⁄2 pint.
2. Castor oil, 1 oz.; liquor potassa, 2 fl. dr.; triturate, and add of honey, 1 oz.; when mixed, further add of hot gruel, 1⁄2 pint; and agitate until cool enough to be administered.
Enema, Cathar′tic. Syn. Purgative clyster; Enema catharticum (B. P., Ph. E. & D.), E. laxativum, E. purgativum, L. These have been already alluded to. By increasing the quantity of the active ingredients, a mild laxative or aperient clyster is converted into an active purgative or cathartic one.
Prep. 1. (Ph. E.) Senna, 1⁄2 oz.; boiling water, 16 fl. oz.; infuse an hour, then add of Epsom salts, 1⁄2 oz.; sugar, 1 oz.; when dissolved, further add of olive oil, 1 oz.; and mix them by agitation.
2. (Ph. D.) Epsom salts, 1 oz.; olive oil, 1 fl. oz.; mucilage of barley. 16 fl. oz. Same as enema of sulphate of magnesia, B. P., except that in the latter mucilage of starch is substituted for mucilage of barley.
3. (Ph. D. 1826.) Manna, 1 oz.; compound decoction of chamomile, 1⁄2 pint; dissolve, and add, of olive oil, 1 oz.; Epsom salts, 1⁄2 oz.
4. Compound decoction of mallows, 1⁄2 pint; Epsom salts, 3⁄4 oz.; sweet oil, 2 fl. oz.; mix, as above.
Obs. The above are employed in all ordinary cases where the use of an immediate cathartic is indicated.
Enema of Cevidina. Syn. Enema Cevidinæ (Soubeiran.) Cevadilla, 2 dr.; water, 10 oz.; boil to 7 oz.; strain and add milk, 8 oz. To destroy ascarides.
Enema of Chlo′′ride of Lime. Syn. Enema chloridi calcis, E. antiputrescens636, L. Prep. 1. Chloride of lime, 10 gr.; tepid water, 1 fl. oz.; triturate, then add of barley water, or plain tepid water, 7 fl. oz.
2. (Pereira.) Chloride of lime, 10 to 15 gr., added to a common enema. As a deodoriser, when the alvine evacuations are unusually fetid.
Enema of Chloride of Soda. Syn. Enema Sodæ Chlorinatæ. Prep. Labarraque’s solution, 24 drops; decoction of mallows, 16 oz.
Enema of Chloride of Sodium. Syn. Enema Sodii Chloridi. Prep. Common salt, 1 oz.; barley water, 1⁄2 pint; olive oil, 1 oz.
Enema for Col′ic. Syn. Enema anticolicum, L. Prep. From oil of cajeput or peppermint, 15 drops; dissolved in sweet spirit of nitre, 60 drops; laudanum, 35 drops; infusion of chamomile, 1⁄2 pint.
Enema of Col′ocynth. Syn. Enema colocynthidis (Ph. L.), L. Prep. 1. (Ph. L.) Extract of colocynth, 1⁄2 dr.; soft soap, 1 oz.; triturate, and add of water, 1 pint.
2. (Ph. L. 1836.) As the last, but using compound extract of colocynth.
3. (Guy’s Hosp.) Colocynth pulp, 1 dr.; water, 3⁄4 pint; boil so as to strain 1⁄2 pint; and add of common salt, 1⁄2 oz.; syrup of buckthorn, 1 fl. oz. An efficient enema in colic and obstinate constipation, in the absence of spasms and inflammatory symptoms.
Enema, Com′mon. Syn. Enema commune, L. Gruel or barley water, either with or without the addition of a little common salt or oil, are generally so called. The first are simply laxative; the latter, purgative. Decoction of mallows, linseed tea, or water gruel, are also commonly used as the vehicle.
Prep. 1. (St. Bar. Hosp.) Barley water, 1 pint; common salt, 1 oz.; dissolve.
2. (Guy’s Hosp.) Water gruel, 10 to 15 fl. oz.; common salt, 1 oz.
3. (U. C. Hosp.) Water gruel, 8 to 12 fl. oz.; salt, 1 oz.; linseed oil, 2 fl. oz.
Enema of Copai′ba. Syn. Enema copaibæ, L. Prep. 1. From balsam of copaiba, 2 dr.; liquor opii sedativus, 15 drops; yolk of egg, q. s.; barley water, 71⁄2 fl. oz.
2. (Collier.) To the last add, of extract of opium, 1 gr.; oil of turpentine, 4 fl. dr.
3. (Velpeau.) Copaiba, 2 dr.; laudanum, 20 drops; yolk of 1 egg; water gruel, 8 fl. oz. In ascarides, gonorrhœa, and some affections of the lower bowels and bladder, when the stomach rejects the balsam.
Enema of Creosote. Syn. Enema Creosoti. (Dr Wilmot.) Creasote, 1 dr.; decoction of starch, 12 oz. In epidemic dysentery.
Enema of Croton Oil. Syn. Enema Olei Crotonis. (Sundelin.) Prep. Croton oil, 2 to 4 drops; linseed oil, 2 oz.; gruel, 4 oz.
Enema of Cubebs. Syn. Enema Cubebæ. (Velpeau.) Prep. Decoction of mallow, 10 oz.; powdered cubebs, 6 dr.
Enema, Domes′tic. Syn. Enema domesticum, L. This name has been applied to an enema of warm water, either with or without the addition of a little sugar, honey, or milk. The effect is laxative.
Enema, Emoll′ient. Syn. Enema emolliens, E. demulcens, L. Prep. From decoction of linseed, barley, or starch, 1 pint; linseed or olive oil, 1 oz. Soothing and laxative; in excoriations of the lower bowels. 20 to 40 drops of laudanum may be added when there is much pain or looseness.
Enema of Ergot. Syn. Enema Ergotæ. (Boudin.) Prep. Infuse 1 dr. of ergot in 8 oz. of hot water and strain.
Enema, Feb′rifuge. Syn. Enema febrifugum, L. Prep. 1. (Collier.) Water gruel, 12 fl. oz.; sugar, 1 fl. oz. In low fevers.
2. (Brande.) Vinegar, 2 fl. oz.; infusion of chamomile, 5 or 6 fl. oz. In typhus.
Enema, Fe′tid. See Enema of Assafœtida.
Enema of Galls and Opium. Syn. Enema Gallæ et Opii. (Dr Ryan.) Prep. Decoction of galls, 8 oz.; tincture of opium, 1⁄2 dr.
Enema for Hoo′ping-cough. Syn. Enema pertussiculaire, L. Prep. 1. See Enema Assafœtida.
2. (M. Reiken). Assafœtida, 8 gr.; yolk of 1 egg; water, 1⁄2 pint.
Obs. This quantity is sufficient for 10 or 12 clysters for children under 1 year; 5 or 6 for those under 3 years; and 2 or 3 for those under 7. Two clysters are prescribed daily in hooping-cough. According to M. Reiken, this is more successful in removing hooping-cough than any other remedy. To ensure success, it should not be administered until the feverish symptoms have passed. M. Reiken sometimes uses an ointment of assafœtida as well as the clyster.
Enema of Ipecacuanha. Syn. Enema Ipecacuanhæ. (U. C. Hosp.) Ipecacuanha root (bruised), 1 dr.; boiling water, 8 oz. Macerate for an hour and strain.
Enema, Lax′ative. See Enemas (Cathartic, Common, &c.).
Enema of Lead. Syn. Enema plumbi, L. Prep. (Dr Newbold.) Acetate of lead, 6 gr.; tepid distilled water, 6 fl. oz. In strangulated hernia; repeated in two or three hours.
Enema of Morphia. Syn. Enema Morphiæ. (Beera.) Prep. Morphia, 1 gr.; oil of almonds, 1 oz. Triturate and add infusion or decoction of linseed, q. s.
Enema, Nitrate of Silver. Syn. Enema Argenti Nitratis. (Boudin.) Prep. Nitrate of silver, 1 to 3 gr.; distilled water, 5 oz.
Enema, Nu′trient. Syn. Feeding clyster; Enema nutriens, L. Prep. 1. Strong beef tea, 12 fl. oz.; thickened a little with arrow-root or hartshorn shavings.
2. (M. Nasse). Strong meat soup, 3⁄4 pint; dilute hydrochloric acid, 1⁄2 fl. dr.
3. Yolks of 2 eggs; brown sugar and salad oil, of each 1 oz.; mutton broth, 12 fl. oz. To nourish the body, when aliments cannot be taken or retained by the stomach.
Enema, Oi′′ly. See Enema (Emollient).
Enema of O′pium. Syn. Enema opiatum, E. opii (B. P. and Ph. L.), E. opii vel ANODYNUM (Ph. E.), L. Prep. 1. (B. P.) Mucilage of starch, 2 fl. oz.; tincture of opium, 1⁄2 dr.
2. (Ph. E.) Starch, 1⁄2 dr.; water (boiling), 2 fl. oz.; mix, and when cool enough add of tincture of opium, 1⁄2 to 1 fl. dr.
637
3. (Ph. D. 1826.) Laudanum, 1 dr.; warm water, 6 fl. oz.
Obs. The above are the orders of the colleges, but in practice the quantity of laudanum is frequently doubled; this should, however, be done with great care. Opium clysters are used in dysentery, colic, cholera, and various painful affections of the intestines, bladder, &c. The bowels should be emptied before their administration, and in inflammatory complaints they should not be used for the first 48 hours. Clysters containing opium, even in small quantities, are dangerous remedies for young children; yet there are cases in which they sometimes succeed when every other remedy has failed. This is particularly so in the low chronic diarrhœa of infancy and early childhood. A case of this kind occurred in the family of the writer. The family medical attendant, as well as the physician he consulted, abandoned the little sufferer to apparently inevitable death, as beyond the reach of further assistance. A small opium clyster was given, and the child recovered.
Enema of Ox-gall. Syn. Enema fellis, E. f. bovis, L. Prep. (Dr Allnatt.) Fresh ox-gall, 2 fl. oz.; water gruel, 8 fl. oz.
2. (Dr Clay.) Ox-gall, 2 fl. oz.; water, 4 or 5 fl. oz. To soften indurated fæces, and in costiveness arising from deficiency of bile.
Enema of Percyanide of Iron. Syn. Enema Ferri Percyanidi. Prep. Triturate 5 gr. of Prussian blue, with 2 oz. of water; to be used daily, increasing the dose if necessary. An American remedy for ascarides.
Enema of Pop′pies. Syn. Enema papaveris, L. Prep. 1. Decoction of Poppies.
2. Poppy-heads (with the seeds), 5 dr.; water, 3⁄4 pint; boil to 12 fl. oz., and strain. Anodyne; as a substitute for opium clyster.
Enema, Pur′gative. See Enema Cathartic.
Enema of Quinine. Syn. Enema Quiniæ. Sulphate of quinine, 5 to 15 gr.; decoction of starch, 6 oz.
Enema of Rue. Syn. Enema Rutæ. Prep. Confection of rue, 20 to 60 gr; thin gruel, 6 oz. to 8 oz.
Enema, Sim′ple. Barley water, rice water, thin-made starch, and decoction of mallows, are frequently so called, from being used either for simple laxative enemas, or as the vehicle for more active substances.
Enema of Soap. Syn. Enema saponis, L. Prep. (St. B. Hosp.) Soft soap, 6 dr.; hot water, 1 pint; dissolve. To soften indurated fæces, &c.; and as a detergent in certain ulcerations of the rectum.
Enema of Soot. Syn. Enema Fuliginis. Wood soot, 4 oz.; water, 11⁄2 pints; boil to a pint.
Enema of Starch. Syn. Enema amyli, L. See Enema Simple (above).
Enema, Stim′ulant. Syn. Enema Stimulans, L. The ordinary cathartic clysters are often so called. The following belong to a different class:—
Prep. 1. Tincture of capsicum, 1 fl. oz.; barley water or thin arrow-root, 1⁄2 pint; mix. In cholera, especially the cold stages.
2. To the last add, of ether, 2 fl. dr.; laudanum, 30 drops.
3. Decoction of poppies, 1⁄2 pint; tincture of capsicum, 3 fl. dr.; oil of nutmeg, 10 drops. In diarrhœa.
Enema of Tobac′co. Syn. Enema tabaci (Ph. L. E. & D.), Infusum tabaci (Ph. D. 1826), L. Prep. 1. (B. P.) Tobacco leaf, 20 gr.; boiling water, 8 oz.; infuse half an hour, and strain.
2. (Ph. E.) Tobacco, 15 to 30 gr.; boiling water, 8 fl. oz.; as last.
3. (Ph. D.) Tobacco, 1 scrup.; boiling water, 8 fl. oz.
4. (Ph. L. 1836.) Tobacco, 1 dr.; boiling water, 1 pint.
Obs. Tobacco clyster is used in strangulated hernia, obstinate constipation, retention of urine, &c. It is violently depressing and relaxing; producing fainting, and even death, when improperly or injudiciously administered. “It is not to be forgotten that 2 dr., 1 dr., and even 1⁄2 dr., of tobacco, infused in water, have proved fatal.” “The cautious practitioner, therefore, will not use more than 15 or 20 gr.” (Pereira.) Three parts of Virginia tobacco are equal to seven parts of any other kind. (Davy.)
Enema of Tur′pentine. Syn. Turpentine clyster; Enema terebinthinæ (Ph. L.), E. olei t., L. Prep. 1. (B. P.) Oil of turpentine, 1 oz.; mucilage of starch, 15 oz.
2. (Ph. L.) Oil of turpentine, 1 fl. oz.; yolk of 1 egg; triturate together, then add of decoction of barley, 19 fl. oz.
3. (Ph. E.) As the last, but using simple water instead of barley water.
4. (Ph. D.) Oil of turpentine, 1 fl. oz.; mucilage of barley, 16 fl. oz.
5. (Dr Neligan.) Oil of turpentine, 1⁄2 fl. oz.; syrup of garlic, 1 fl. oz.; barley water, 6 or 7 fl. oz. In ascarides, and as an antispasmodic and purgative in colic, obstinate constipation, calculus, peritonitis, tympanitis (DRUM-BELLY), &c.
Enema, Ver′mifuge. Syn. Enema anthelminticum, E. vermifugum, L. Prep. 1. Castor oil, 1 oz.; mucilage, 3⁄4 oz.; decoction of the root of male fern, 7 fl. oz. In worms, especially tape-worm.
2. (Collier.) Oil of turpentine, 1 fl. oz.; olive oil (warm), 1⁄2 pint. In ascarides.
3. (Dr Darwall.) Tincture of sesquichloride of iron, 1 dr.; water, 7 or 8 fl. oz. In ascarides, especially when occurring in childhood; the quantity used being proportionately lessened. See Enemas of Aloes, Assafœtida, Turpentine, &c.
Enema of Vinegar. Syn. Enema acetici. (Brande.) Prep. Vinegar, 2 oz.; infusion of chamomile, 4 oz. In typhus fever.
638
Enema of Wine. Syn. Enema vinosum, L. Prep. From sherry wine and hot water, of each 7 fl. oz. In suspended animation. Sometimes a wine-glassful of brandy is added.
ENERGY, relative values of Food as sources of. Chemists and physiologists, although they agree that muscular power is derived from the action of the oxygen supplied during respiration upon the digested portions of the food, differ in their conclusions as to whether the nitrogenous or non-nitrogenous principles of the food, form the chief source of this power or not. The opinion of Liebig, Playfair, Ranke, and others, that the oxidation and metamorphosis of the nitrogenous tissue is the fountain of muscular force has of late years been contested, and on the opposite view adduced, viz. that it is principally to the oxidation of the carbonaceous or non-nitrogenous constituents of the food, that animal dynamic power is due.
This latter view has received support from the experiments of Frankland, Lawes, and Gilbert (from their observations on the feeding of cattle), Edward Smith, Meyer, Pettenkofer, Voit, Wislicenus, Fick, Parkes, and others.
The data upon which it is based are those derived from the observation of the amount of heat generated by the combustion of a definite quantity of food out of the body; which, it is affirmed with certain deductions, represents the quantity of heat evolved by the oxidation of the same food within the body; and as heat is the equivalent of muscular force or energy, that aliment which, in burning, gives off the most heat, must, it is supposed, necessarily be the richest in the production of animal motive power. Of course these conditions will, amongst others, be very considerably modified by the extent to which the processes of the animal economy, such as digestion, assimilation, &c., can liberate the elements of the food so as to become available as sources of this energy.
Were these processes perfect, all the carbon of the carbonaceous, as well as that of the nitrogenous constituents of the diet, after deducting the carbon which passes off as urea (one part of dry nitrogenous matter yielding about a third of its weight of urea) would be utilised and converted into heat-producing power. But even under these circumstances a considerable portion of this thermotic power would be expended in sustaining the internal movements of the body, such as respiration and the heart’s action, which it has been computed are daily maintained by a force capable of raising 600,000 pounds a foot high.
No wonder if, with such varying factors introduced into the problem, physiologists and physicists should differ so widely in their calculations; and that, whilst one inquirer believes that food practically yields only about half the force which, according to theory, it actually contains; another estimates it at only one fifth.
The following table by Dr Frankland shows the amount of force which different foods yield when burned. The results agree very closely with those theoretically given by Playfair and others.
Energy developed by one gramme, or one ounce of the following substances, when oxidised in the body.
| Name of Substance | Per cent. of Water | 1 gramme will equal kil.-metres of energy. | 1 ounce will equal foot-tons of energy, or in other words, would raise the under-given number of tons, 1 foot high.[276] |
| Beef (lean) | 70·5 | 604 | 55·0 |
| Veal (lean) | 70·9 | 496 | 45·3 |
| Ham (lean, boiled) | 54·4 | 711 | 64·9 |
| Bread crumb | 44·0 | 910 | 83·0 |
| Flour | ... | 1627 | 148·5 |
| Ground rice | ... | 1591 | 145·3 |
| Oatmeal | ... | 1665 | 152·0 |
| Pea meal | ... | 1598 | 146·0 |
| Potatoes | 73·0 | 422 | 38·5 |
| Carrots | 86·0 | 220 | 20·0 |
| Cabbage | 88·5 | 178 | 16·2 |
| Butter | ... | 3077 | 280·9 |
| Egg (white of) | 86·3 | 244 | 22·3 |
| Egg (yolk) | 47·0 | 1400 | 127·0 |
| Cheshire cheese | 24·0 | 1846 | 168·5 |
| Arrowroot | ... | 1656 | 151·3 |
| Milk | 87·0 | 266 | 24·3 |
| Sugar (lump) | ... | 1418 | 129·5 |
| Ale (Bass’ bottled) | 88·4 | 328 | 30·0 |
| Porter (Guinness’ stout) | 88·4 | 455 | 41·5 |
[276] The amount of work done is generally estimated in this country as so many lbs. or tons lifted 1 foot. In France it is expressed as so many kilogrammes lifted 1 metre,—and called ‘the kilogrammemetre,’ as above.
“A table of this kind,” says Dr Parkes, “is useful in showing what can be obtained from our food, but it must not be supposed that the value of food is in exact relation to the energy which it can furnish. In order that the force shall be obtained, the food must not only be digested and taken into the body properly prepared, but its energy must be developed in the place and in the manner proper for nutrition. The mere expression of potential energy cannot fix dietetic value, which may be dependent on conditions in the body unknown to us. For example, it is quite certain, from observation, that gelatin cannot take the place of albumen, though its potential energy is little inferior, and it is easily oxidised in the body. But, owing to some circumstances yet unknown, gelatin is chiefly destroyed in the blood and gland-cells, and its energy, therefore, has a different direction from that of albumen. So also of the639 potential energy, it is quite possible that all is not usefully employed. The tables of energy give broad indications, and can be used in a general statement of the value of a diet; but at present they do not throw light upon the intricacies of nutrition.”
ENFLURAGE. See Pommade.
ENGRA′′VING. The art of producing designs or figures on metal, wood, &c., by incision or corrosion, usually for the purpose of being subsequently printed on paper, calico, or other materials. The mechanical operations of the engraver do not come within the province of this work. Several of the materials which he employs in his trade will, however, be found noticed under their respective heads.
There is this important difference between engraving on metal plates and wood-engraving: in the former all the lines and dots that are to print black are hollowed out with a graving-tool, or ‘bitten in’ by acid; in the latter all the parts that are to appear white in the impression are cut away, and the lines which produce the imprint are left on the face of the block.
Casts of wood-blocks, or ‘stereos.’ are often used instead of the original blocks when a great number of impressions is required. To produce them stucco moulds are prepared, and from these the casts in type metal are taken. The casts are usually about 1⁄8 in. thick, and have to be screwed upon wooden blocks to bring them to the height of the types which are printed with them. As soon as one cast is worn out another may be taken, and the original block is thus preserved in the state in which it left the engraver’s hands.
For the reproduction of engraved metallic plates the ELECTROTYPE PROCESS is commonly employed. Woodcuts are also copied, though less frequently, by this process. The mode by which the postage-stamp plates are multiplied is as follows:—240 ‘queen’s heads’ or stamps (a pound’s worth) are engraved on one steel plate. This plate is then hardened, and an impression of it taken on a softened steel roller. This roller, in its turn, is also hardened, and softened steel plates being passed under it, an impression precisely like that of the original plate is produced on each of them. These plates are then hardened and employed for printing the penny postage stamps for sale. They last a long time; and when they are worn out they are destroyed, and their place is supplied by fresh ones, which are produced by the cylinder before referred to, which continues ready to supply any number that may be required. Bank-note plates are reproduced in the same manner. See Electrotype, Etching, &c.
Engravings, to Clean. Place the engraving on a smooth board, and cover it thinly with finely powdered and very clean common salt. Next squeeze lemon-juice upon the salt so as to dissolve a considerable portion of it. Now elevate one end of the board so that it may form an angle of about forty-five or fifty degrees. Next pour on the engraving boiling water from a tea-kettle until all the salt and lemon-juice are washed off. The engraving will then be found to be perfectly clean and free from stains. Care must be taken to dry it on the board or on some smooth surface very gradually. It will acquire a yellow tint if dried by the sun or before a fire.
Engravings, to Mount. Strain thin calico on a frame, then carefully paste on it the engraving, so as to be free from creases; afterwards, and when dry, give the engraving two coats of thin size (made by putting a piece of glue the size of a small nut into a small cupful of hot water); finally, when this dries, varnish the engraving with a varnish known as ‘white hard.’
ENTERI′TIS. See Inflammation of the Bowels.
ENTOZO′A. Parasitic animals which infest the bodies of other animals. See Worms.
ENTRY, Powers of. The Public Health Act thus defines the power of any local authority to enter into premises whereon a nuisance is supposed to exist; and the conditions under which this power is to be exercised.
“The local authority or their officer shall be admitted to any premises for the purpose of examining as to the existence of a nuisance thereon, or of enforcing the provisions of any Act in force within the district requiring fireplaces and furnaces to consume their own smoke at any time between the hours of nine in the forenoon and six in the afternoon, or in the case of a nuisance arising in respect of any business, then at any hour when such business is in progress or is usually carried on.
“Where under the Public Health Act a nuisance has been ascertained to exist, or an order of abatement or of prohibition has been made, the local authority or their officer shall be admitted from time to time into the premises between the hours aforesaid until the nuisance is abated or the works ordered to be done are completed, as the case may be.
“Where an order of abatement or prohibition has not been complied with or has been infringed, the local authority or their officer shall be admitted from time to time at all reasonable hours or at all hours during which business is in progress or is usually carried on into the premises where the nuisance exists, in order to abate or remove the same.
“If admission to premises for any of the purposes of this section is refused, any justice on complaint thereof on oath by any officer of the local authority (made after reasonable notice in writing of the intention to make the same has been given to the person having custody of the premises) may, by order under his hand, require the person having custody of the premises to admit the local authority or their officer into the premises during the hours aforesaid; and if no person having640 custody of the premises can be found, the justice shall, on oath made before him of that fact, by order under his hand, authorise the local authority or their officer to enter such premises during the hours aforesaid.
“Any order made by a justice for admission of the local authority or their officer on premises shall continue in force until the nuisance has been abated, or the work for which the entry was necessary has been done.
“Any person refusing to obey a justices’ order for admission of the local authority or their officers is liable to a penalty not exceeding five pounds. Power of entry at reasonable times is given to the medical officers of health and inspector of nuisances to inspect food, &c. Penalty for obstruction, five pounds and under.”
ENURE′SIS. See Urine.
EPHESTIA ELETELLA—The Chocolate Moth. The larvæ of this moth frequently cause serious damage to cocoa, flour, or biscuits when these are stored. Professor Huxley proposes to guard against the ravages of the insect by the adoption of the following precautions—
1. Have no cocoa stored in any place in which biscuits are manufactured.
2. Lead up all biscuit puncheons as soon as they are full of the freshly-baked biscuit.
3. Coat puncheons with tar after they are leaded up, or at least work lime-wash well into the joints and crevices.
4. Line the bread-rooms of the ships with tin, so that if the Ephestia has got into a puncheon it may not infest the rest of the ship.
5. If other means fail, expose the woodwork of puncheons to a heat of 200° F. for two hours, or they might be destroyed by driving into the puncheon a stream of carbonic oxide, and afterwards exposing it well to the air. Weevils in biscuit have frequently been exterminated by this method, and there appears to be no reason why this treatment should not be equally efficacious for getting rid of the larvæ of the Ephestia Eletella.
EPHIAL′TES. See Nightmare.
EPIDEM′IC. Common to many people. In pathology, an epidemic disease (EPIDEMIC; EPIDEMY) is one which seizes a number of people at the same time and in the same place, but which is not dependent on any local cause, but on some extraordinary condition of the air. When a disease is peculiar to a people or nation, and appears to depend on local causes, it is said to be ‘ENDEMIC’ or ‘ENCHORIAL,’ Thus, Asiatic cholera may be taken as an example of the first, and the agues of low countries, and the goitre of the Alps, as examples of the other.
Epidemics may be divided into indigenous and exotic. Amongst the former may be included scarlet fever, measles, hooping-cough, influenza, typhoid; whilst the latter embrace such as are imported, viz. Asiatic cholera, plague, &c.
No year passes without the prevalence of an epidemic of one kind or the other in this country.
The following enactments for the prevention of epidemic diseases are now in force:—
“Whenever any part of England appears to be threatened with, or is affected by, any formidable epidemic, endemic, or infectious disease, the Local Government Board may make, and from time to time alter and revoke, regulations for all or any of the following purposes, viz.:—
“(1) For the speedy interment of the dead; and—
“(2) For house-to-house visitation; and—
“(3) For the provision of medical aid and accommodation, for the promotion of cleansing, ventilation, and disinfection, and for guarding against the spread of the disease;—
“and may by order declare all or any of the regulations so made to be in force within the whole or any part or parts of the district of any local authority, and to apply to any vessels as well as arms or parts of the sea within the jurisdiction of the Lord High Admiral of the United Kingdom, or the Commissioners for executing the office of the Lord High Admiral for the time being, for the period in such order mentioned, and may by any subsequent order abridge or extend such period.” (P. H., s. 134.)
“All such regulations, &c., made by the Local Government Board are to be published in the ‘London Gazette,’ and such publication is to be held as conclusive evidence.” (P. H., s. 135.)
“The local authority of any district within which, or part of which, regulations so issued by the Local Government Board are declared to be in force, shall superintend and see to the execution thereof, and shall appoint and pay such medical or other officers or persons, and do and provide all such acts, matters, and things as may be necessary for mitigating any such disease, or for superintending or aiding in the execution of such regulations, or for executing the same, as the case may require. Moreover, the local authority may from time to time direct any prosecution or legal proceedings for or in respect of the wilful violation or neglect of any such regulation.” (P. H., s. 136.)
“The local authority and their officers shall have power of entry on any premises or vessel for the purpose of executing or superintending the execution of any regulations so issued by the Local Government Board as aforesaid.” (P. H., s. 137.)
“Whenever, in compliance with any regulation so issued by the Local Government Board as aforesaid, any poor-law medical officer performs any medical service on board any vessel, he shall be entitled to charge extra for such service, at the general rate of his allowance641 for services for the union or place for which he is appointed, and such charges shall be payable by the captain of such vessel on behalf of the owners thereof, together with any reasonable expenses for the treatment of the sick.
“Where such services are rendered by any medical practitioner who is not a poor-law medical officer, he shall be entitled to charges for any service rendered on board, with extra remuneration on account of distance, at the same rate as those which he is in the habit of receiving from private patients of the class of those attended and treated on shipboard, to be paid as aforesaid. In case of dispute in respect of such charges, such dispute may, where the charges do not exceed twenty pounds, be determined by a court of summary jurisdiction; and such court shall determine summarily the amount which is reasonable, according to the accustomed rate of charge within the place where the dispute arises for attendance on patients of the like class as those in respect of whom the charge is made.” (P. H., s. 138.)
“The Local Government Board may, if they think fit, by order authorise or require any two or more local authorities to act together for the purposes of the provisions of this Act relating to prevention of epidemic diseases, and may prescribe the mode of such joint action, and of defraying the costs thereof.” (P. H., s. 139.)
“Any person who—
“(1.) Wilfully violates any regulation so issued by the Local Government Board, as aforesaid; or,
“(2.) Wilfully obstructs any person acting under the authority or in the execution of any such regulation shall be liable to a penalty not exceeding five pounds.” (P. H., s. 140.)
EPIGAS′TRIC. In anatomy, pertaining to the EPIGAS′TRIUM, or the part of the abdomen over the stomach.
EPILEP′SY. Syn. Falling sickness; Epilepsia, Morbus caducus, L. The popular name of this disease arises from the patient, when attacked by it, suddenly falling to the ground. The other leading symptoms consist of convulsions, stupor, and, generally, frothing at the mouth. It comes on by fits, which after a time go off, leaving a certain amount of lassitude and drowsiness behind. Sometimes certain peculiar symptoms precede the attack. Among these, a sensation of coldness or of a current of cold air from the extremities of the body towards the head (AURA EPILEPTICA), palpitation, flatulency, stupor, and an indescribable cloud or depression, are the most common. The occurrence of these symptoms are not, however, uniform, even in the same patient; but it generally happens that the party falls down suddenly, and without the slightest warning.
Epilepsy is often symptomatic of other affections, as excessive irritation of the primæ viæ from worms, indigestible or noxious food, or poison; or it depends on local injuries, particularly those of the head, accompanied with lodgments of water on the brain, tumours, pressure, &c. Violent affections of the nervous system, sudden frights or fits of passion, violent mental emotions, the sudden suppression of old evacuations, and, in childhood, difficult teething, are also common causes of sympathetic epilepsy. Occasionally it arises from mobility of the sensorium, induced by plethora, or by excessive debility. In such cases the treatment must be energetically directed to the removal of the exciting cause.
When epilepsy occurs as an idiopathic or primary affection, or when it cannot be referred to any apparent cause, more especially when the attack commences about the age of puberty, and the fits are frequent, it is generally hereditary, and there is great danger of its terminating either in apoplexy, or lunacy, or imbecility.
The treatment of idiopathic epilepsy is principally directed to the improvement of the general health, and the diminution of nervous irritability by sedatives and tonics. Among the first, camphor, ether, henbane, hemlock, musk, oil of cajeput, opium, and morphia, and, more recently, hydrocyanic acid, have been principally relied on. Among the second, bark, cascarilla, quinine, strychnia, valerian, the sulphate of iron, zinc, and copper, arsenious acid, and nitrate of silver, have each their zealous advocates. The objection against the last preparation is the danger of its disfiguring the patient, by tinging the skin of a permanent dull, leaden hue. In cases accompanied with plethora, a low diet, daily out-of-door exercise, and the frequent use of aperients, with occasional blistering, cupping, and other depletive measures, are indicated; whilst in those marked by inanition and debility, an entirely opposite course must be adopted. When the disease is complicated with syphilis, a mild course of mercury may be given; and when with scrofula, iodine, iodide of potassium, or cod-liver oil, assisted by sea-bathing, will be proper.
Among other methods of treatment may be mentioned the administration of an active emetic or purgative, twice weekly, in the morning, when the stomach is empty. The first has now few supporters; but the second is said to be often productive of great benefit.
During a fit of epilepsy the only thing that can be done for the patient is to prevent the sufferer injuring himself, and to loosen every part of his dress that presses on his head, neck, or chest. When premonitory symptoms occur, a brisk emetic, a large dose of laudanum and ether, a cold plunge or shower bath (when not contra-indicated), or anything else which gives a sudden shock to the system or raises its tone, frequently prevents the accession of the fit.
Epilepsy more commonly attacks children than adults, and boys than girls. “Its returns642 are frequently periodical, and its paroxysms commence more frequently in the night than in the day, being somewhat connected with sleep. It is sometimes counterfeited by street impostors in order to excite the charity of the passers-by.
For Animals. All animals are subject to attacks of epilepsy, more particularly dogs and pigs. The animal seized with the fit loses the senses of sight and hearing, and falling down exhibits the same symptoms as those which accompany the disease in human beings. Cattle, although they bellow greatly during an attack, rarely die from it; but it not infrequently suffocates dogs, and is in them a not unusual cause of sudden death. The fit, which lasts from ten to fifty minutes, when it passes off, leaves the animal dull, and is apt to return. Epileptic fits are a frequent accompaniment of distemper in dogs. They are often induced in cattle by tough and indigestible food, and in these as well as in dogs, by intestinal worms. Hot weather and excitement, especially in dogs, are a frequent cause of an epileptic fit. By energetic treatment after the first attack the further course of the malady may often be arrested. The best treatment is to give, when the fit is over, a brisk purge, with an ounce of oil of turpentine in horses or cattle, and twenty to forty drops in dogs. If the disease is caused by worms give the medicines ordered in such cases.
EPISPAS′TICS. See Blister and Vesicant.
EP′ITHEM. Syn. Epithema, L. Any external liquid medicine for local application; as an embrocation or lotion. Some writers confine the term to those preparations which are intended to be applied by means of a cloth dipped into them. See Liniment, Lotion, &c.
Epithem, Astrin′′gent. Syn. Epithema astringens, L. Prep. 1. Powdered ice, 7 dr.; powdered catechu, 1 dr.; mix.
2. (Brera.) Powdered bole and rhatany, of each 1 oz.; vinegar of roses, q. s. to form a paste. Both are applied to the nostrils and forehead to stop bleeding at the nose.
Epithem, Gly′′cerin. Syn. Epithema glycerinæ, L. Prep. (Mr Startin.) Glycerin, 1 oz.; rose water and lime water, of each 3 or 4 fl. oz.; powdered gum tragacanth, q. s. to form a thin mucilage. In scalds, burns, and excoriations.
Epithem, Vermifuge. Syn. Epithema vermifugum, L. (Hoffmann). Wormwood and centaury, beaten up with aloes and colocynth, and applied over the belly.
Epithem, Vesica′ting. Syn. Epithema vesicatorium, L. Prep. 1. (Alibert.) Rye or barley meal, made into a paste with vinegar, and 30 to 40 gr. or more of powdered Spanish flies sprinkled over the surface.
2. (Ph. L. 1746.) Spanish flies (in fine powder) and wheat flour, equal parts, made into paste with vinegar, q. s. As a blister.
Epithem, Vol′atile. Syn. Epithema volatile, E. ammoniæ, L. Prep. (Ph. L. 1764.) Common turpentine and water of ammonia, equal parts. An excellent counter-irritant; either with or without the addition of a little olive oil.
EPIZOOTIC DISEASES. These are diseases which attack different species of domestic animals in the same manner that epidemics do man. These maladies ravage large tracts of country, frequently causing great mortality amongst the various animals inhabiting the localities visited by them; different animals being assailed by different forms of epizootic disease.
For instance, there is the rinderpest, or plague peculiar to cattle, the typhoid or gastric fever which prevailed so largely amongst horses in this country in 1854, and 1861 and 1862; the smallpox of sheep; the diphtheria affecting oxen, sheep, goats, and pigs; the influenza of horses, and the charbon of pigs. Dogs, cats, tame and wild birds, fish,[277] silkworms and bees, each suffers from a special variety of epizootic disease.
[277] Great mortality has prevailed amongst the salmon during the present year, owing to the attacks of a peculiar white fungus, called the Saprolegnia ferox, a parasite that multiplies so rapidly as speedily to envelope any fish it attacks.
Epizootic diseases are met with in most European countries. They are very common in Russia, where they commit great devastation amongst the horned cattle, 400,000 of which are said to die annually from their ravages. Of epizootics, Mr Finlay Dun says:—“They extend at the same time over large tracts of country, attack in a similar manner great numbers of animals, tend to assume a typhoid form, and withstand badly all depletive treatment. They depend upon some general causes as yet unknown, but which it has usually been thought sufficient to term “atmospheric;” but are always most common and fatal amongst animals breathing impure air, densely crowded, badly fed, or exposed to cold winds; and are generally prevented or robbed of their virulence by guarding against such debilitating causes, and maintaining a high standard of health.”
EQUISE′TIC ACID. In chemistry, a substance identical with ACONITIC ACID (which see).
EQUIV′ALENT. (Equivalency.) In modern chemistry, the equivalent of a body is that weight of it which will exactly replace in a compound 1 atom of hydrogen or 1 atom of either of the other monivalent elements (see Table, below).
Monivalent elements are those which replace one atom of hydrogen in chemical combinations in the ratios of their atomic weights.
One atom of a divalent, trivalent, tetrivalent, pentivalent, and hexivalent element replaces respectively, or is equivalent to, two, three, four, five, or six atoms of hydrogen or of any other monivalent element. (For further information643 on this subject consult the works on chemistry by Fownes, Miller, Kay-Shuttleworth, &c.)
Table of the Elements, arranged according to their Equivalency.
| Monivalent. | Divalent. | Trivalent. | Tetrivalent. | Pentivalent. | Hexivalent. |
| Hydrogen | Oxygen | Boron | Carbon | Nitrogen | Sulphur |
| Fluorine | Barium | Gold | Silicon | Phosphorus | Selenium |
| Chlorine | Strontium | Tin | Vanadium | Tellurium | |
| Bromine | Calcium | Titanium | Arsenic | Tungsten | |
| Iodine | Magnesium | Thorium | Antimony | Molybdenum | |
| Cæsium | Zinc | Niobium | Bismuth | Osmium | |
| Rubidium | Didymium | Tantalum | Iridium | ||
| Potassium | Lanthanium | Zinconium | Ruthenium | ||
| Sodium | Yttrium | Aluminium | Rhodium | ||
| Lithium | Glucinum | Platinum | Chromium | ||
| Thallium | Cadmium | Palladium | Manganese | ||
| Silver | Mercury | Lead | Iron | ||
| Copper | Cobalt | ||||
| Nickel | |||||
| Uranium | |||||
| Cerium |
ERB′IUM. According to Prof. Mosander, the substance usually called yttria is a mixture of the oxides of three metals—yttrium, erbium, and terbium, which differ in the character of their salts, and in some other important particulars. The first is a powerful base; the others, very weak ones. The latter are separated with extreme difficulty, and possess no practical importance.
ERDMAN’S FLOAT. This useful little instrument, invented as its name implies, by Erdman, is used to ensure accuracy in the readings of Mohr’s burette.
It is in the form of an elongated glass bulb, loaded with a globule of mercury at the bottom, the same as a hydrometer, and with a glass hook at the top, by means of which it can be placed in or removed from the liquid in the burette at pleasure. The float has a circular mark scratched by a diamond, running round the middle, which, when the instrument is placed in the fluid in the burette, should correspond with the graduation or degree on the burette at which the fluid stands. The actual height of the fluid in the burette is of no consequence, since, if the operation be commenced with the line on the float opposite the 0 gradation on the burette, the same proportional division is always maintained. It is most essential that, when the fluid is being drawn off, the float should accompany it in its descent without wavering, and that the circular mark upon it should always be parallel to the graduations of the burette. Another condition is, that when the float has been pressed down in the fluid of the closed burette it should slowly rise again. A correspondent in Liebig’s ‘Annalen der Chemie und Pharmacie’ for April, 1875, states that Erdman’s floats generally become lined internally with a green or yellow layer, from the oxidation of the mercury, and are thus rendered opaque and consequently useless. He proposes to place the mercury in a distinct cell, hermetically sealed from the upper part of the float which carries the circular mark. He has had floats of this construction in use for years.[278] See Burette.
[278] ‘Chemical News.’
EREMACAU′SIS. Slow burning; decay. This expression was applied by Liebig to the peculiar decomposition which moist organic matter undergoes, when freely exposed to the air, by the oxygen of which it is gradually burned or destroyed, without any sensible elevation of temperature. See Putrefaction.
ER′GOT. Syn. Ergot of rye, Spurred rye, Horned r., Cockspur r., Obstetrical r. Ergota (B. P.), L. The diseased seeds of Secale cereale (Linn.), or common rye.
Ergot of rye deteriorates greatly by age, being subject to the attacks of a description of acarus resembling the cheese mite, but much smaller, which destroys the whole of the internal portion of the grain, leaving nothing but the shell, and a considerable quantity of excrementitious matter. To prevent this the ergot should be well dried, and then placed in bottles or tin canisters, and closely preserved from the air. The addition of a few cloves, or drops of the oil of cloves, or strong acetic644 acid, or a little camphor, or camphorated spirit of wine, will preserve this substance for years in close vessels. M. Martin proposes to steep the dry ergot in strong mucilage, and then to dry it on a sheet of white iron. This operation he repeats once or oftener, and finally preserves the prepared and thoroughly dried ergot in a well-corked glass flask. (‘Jour. de Chimie Méd.’) The wholesale druggists generally keep it in well-covered tin canisters or tin boxes.
H. Ducros (‘Zeitschr. des Oesterr. Apoth. Ver.,’ 1876-8), on the strength of many years’ experience, recommends powdered wood-charcoal for the preservation of ergot of rye.
The ergot is placed in a wide-mouth stoppered bottle, and covered with a thick layer of the powdered charcoal. Whenever it is required for use some of the ergot is transferred to a piece of paper, and freed from the adhering charcoal by blowing and rubbing. What is not required is returned to the bottle.
N. B. Gionovié (‘Zeitschr. des Oesterr. Apoth. Ver.,’ 1876, 126) states he has used the following process with the best success. A small quantity of ether is dropped on the ergot contained in a bottle, and the latter closed with a well-fitting stopper. The addition of ether is repeated every time the bottle is opened.
Ergot of rye is much used to restrain uterine hæmorrhage, and to accelerate the contraction of the uterus in protracted labour. It is also much used as an emmenagogue.—Dose. To facilitate labour, 20 to 30 gr., either in powder or made into an infusion; repeated at intervals of 20 or 30 minutes until 3 or 4 scruples have been taken. In other cases (leucorrhœa, hæmorrhages, &c.) the dose is 5 to 12 gr., three times daily, for a period not longer than a week or ten days at a time.
M. Tancret states that he has succeeded in obtaining an alkaloid from ergot of rye, which he names ergotinine. The isolation of ergotinine is said to be attended with great difficulty, owing to its great tendency to undergo spontaneous changes, a short contact with the air being sufficient to decompose it; a circumstance which may perhaps help to explain the rapid change that the powder ergot experiences. Professor Dragendorff, however, refuses to admit that ergotinine is the active principle of ergot, or that it is a distinct chemical substance. He ascribes the therapeutic power of the drug mainly to sclerotic acid, which body, after various unsuccessful attempts, he has obtained from ergot, with certain other determinate compounds, by the following process:—“Very finely powdered ergot is exhausted with distilled water, the solution concentrated in vacuo, and the residuary liquid mixed with an equal volume of 95 per cent. alcohol. This causes the precipitation of a peculiarly shiny substance (scleromucin), together with a portion of the salts, and the greater part of the suspended fatty matter. The mixture having been allowed to stand on ice for twenty-four to forty-eight hours, it is filtered, and the filtrate mixed with a further quantity of 95 per cent. alcohol, sufficient to precipitate all the sclerotic acid in combination with the bases (chiefly as calcium sclerotate). The separation of the precipitate is promoted as before, by placing the mixture on ice for some days. This causes the deposited mass, which has a brownish colour, to adhere firmly to the walls of the vessel, so as to permit the supernatant liquid to be easily poured off. The precipitate is kneaded with alcohol of 80 per cent., and immediately thereafter dissolved in a sufficient quantity of 40 per cent. alcohol, when the remainder of the scleromucin and another large portion of the foreign salts are left behind. The filtered liquid is now mixed with absolute alcohol, whereby sclerotic acid is precipitated in conjunction with certain bases and other substances. The impure product, when carefully dried over sulphuric acid, was found on analysis to contain 8·5 per cent. of potassium, about 0·36 per cent. of calcium, 4·3 per cent. of sodium, 2·74 per cent. of phosphoric acid, or altogether 12·9 per cent. of ash.
“The greater part of these admixtures may be removed, and the sclerotic acid obtained free, by adding before the final precipitation with absolute alcohol a considerable quantity of hydrochloric acid (for every 100 c. c. of solution 5-6 gm. of the acid, sp. gr. 1·100), allowing to stand at ordinary temperature for a few hours, and then proceeding to precipitate. In this manner the amount of ash may be brought down to 3 per cent., and by repeated solution, addition of acid, and precipitation, it may further be reduced to less than 2 per cent. or 3 per cent. A more complete purification is difficult and hazardous, because every addition of hydrochloric acid causes the decomposition of a small quantity of sclerotic acid, while at the same time a portion of the latter is lost by remaining in solution.
“The resulting product, although not chemically pure, is nevertheless physiologically pure, as it always produces constant and identical results, no matter from what sample of (good) ergot it was obtained.
“Good ergot contains about 4 to 4·5 per cent. of the acid, although samples are met with which contain scarcely 1·5 to 2 per cent.”[279]
[279] From ‘New Remedies.’
Frogs are stated to have been thrown into a state of palsy by the hypodermic injection of 0·02 to 0·04 gram of sclerotic acid.
See Decoction, Extract, Infusion, Oil, Tincture, &c.
ER′GOTIN. Syn. Ergotina, L. Prep. 1. (Bonjean’s.) Powdered ergot is exhausted with cold water, by displacement, and the resulting solution is heated in a water bath to about 200° Fahr., and filtered; the filtered liquor is then evaporated to the consistence of645 a syrup, and when cold, is treated with rectified spirit, in considerable excess, to precipitate its gummy matter; after repose, the clear portion is decanted, by the heat of a water bath, to the consistence of a soft extract. Prod. 15%. According to M. Bonjean, this preparation possesses all the ‘hæmostatic’ without any of the ‘poisonous’ qualities of ergot. It has a reddish-brown colour, a bitter taste, and an odour somewhat resembling that of roasted meat. Its aqueous solution is red, limpid, and transparent.—Dose, 4 to 10 gr., either made into a pill or dissolved in water.
2. (Wigger’s.) Powdered ergot is first digested in ether, to remove the fatty matter, and then in boiling alcohol; the alcoholic tincture is evaporated, and the resulting extract treated with water; the undissolved portion, dissolved in hot alcohol and filtered, yields pure ergotine by gentle evaporation.—Prod. 11⁄4%. It has a brownish-red colour; is resinous, acrid, bitter, insoluble in water and ether, soluble in alcohol, and poisonous. It evolves a peculiar odour when warmed. Its therapeutical action has not been determined. See Extract.
ER′RHINES. Syn. Errhina, L. Substances applied to the pituitary membrane of the nose, for the purpose of producing an increased discharge of nasal mucus. When they are given to excite sneezing, they are called STERNUTATORIES or PTARMICS. Asarabacca, euphorbium, several of the labiatæ (herbæ vel flores), sal-ammoniac, powdered sugar, subsulphate of mercury, tobacco, and white hellebore, are the principal substances of this class.
Errhines act as local irritants, and are occasionally employed in chronic affections of the eyes, face, ears, and brain; as in amaurosis, ophthalmia, deafness, weak sight, headache, &c.
Errhine, Al′um. Syn. Errhinum aluminis, L. Prep. (Radius.) Alum and Armenian bole, of each, 1 dr.; kino, 1⁄2 dr.; red oxide of iron, 2 dr. (all in powder); mix and triturate. In bleeding at the nose. A little is snuffed up the nostrils.
Errhine, Hæmostat′ic. Syn. Errhinum hæmostaticus, L. Prep. From powdered catechu, 1 dr.; opium, 5 gr.; sugar, 2 dr. As the last.
ERUPTIONS (of the Skin). For brevity and convenience, these cutaneous affections may be divided into 5 classes:—
Eruptions, Animal′cular. These are due to the presence of minute parasites (ACARI), which burrow and breed in the scarf-skin, and occasion much local irritation. See Itch.
Eruptions, Pap′ular. Syn. Dry pimples. In these the surface is raised into little elevations or pimples, which sometimes show themselves on the surface, and at others are only appreciable by the touch. They are usually accompanied with a greater or less degree of cutaneous irritation and troublesome itching, in attempting to relieve which they are frequently converted into disagreeable and painful sores and excoriations, which are often difficult to heal.
Treat. In simple cases, where there is not much disarrangement of the general health, these eruptions commonly yield to the occasional use of mild saline aperients, and warm or tepid bathing, or frequent ablution with warm soap and water. Sea-bathing is also a powerful remedy. Stimulants of all kinds should be avoided, and ripe fruit and vegetables should form a prominent part of the diet. Lemonade, made by squeezing a lemon into a tumbler of water, and sweetening the mixture with a little sugar, is one of the best beverages on these occasions. To relieve the itching, brisk friction with a soft flesh-brush may be had recourse to, followed by the use of a lotion formed by adding the juice of a lemon or a wine-glassful of distilled vinegar, to 3⁄4 of a pint or a pint of water, either with or without the addition of a table-spoonful of glycerin. Occasional single pimples, depending on local causes, generally require no particular treatment. See Lichen, Prurigo, Red Gum, and Toothrash.
Eruptions, Pus′tular. Syn. Mattery pimples. These are distinguished by the pimples (pustules) containing an opaque yellow fluid or matter (pus, lymph). “They are generally developed on a ground of inflamed skin; and the degree of this inflammation of the skin is the basis of their division into two groups, termed technically ‘IMPETIGO’ and ‘ECTHYMA,’ The former presents the slighter degree of inflammation, and, sometimes, there is scarcely any redness of the skin; the latter is always accompanied by considerable inflammation and redness.” “The little bubbles attain their full size in the course of two or three days, and either dry up without breaking, or more frequently burst and then dry, forming a hard crust, which offers considerable variety of colour, being sometimes yellowish, sometimes brownish, and sometimes almost black.” The latter form is popularly known as ‘crusted tetter.’ In ecthyma the pustules “are generally of the size of a split pea, and surrounded at their base by a broad halo of redness. They are usually separate, not clustered like impetigo, scattered over various parts of the body, and followed either by a hard black crust or by a sore.”
Treat. The inflammation and pain may be generally alleviated by the application of a lotion formed of rectified spirit of wine, 1 part; and water, 5 or 6 parts; to which a table-spoonful of distilled vinegar is often added. The crusts or scabs, when they become hard or troublesome, may be removed by a warm fomentation or an emollient poultice; a little simple cerate being afterwards applied to allay irritation. When the constitution is full and inflammatory (as it usually is in impetigo), a depletive treatment may be adopted,646 when it is low and debilitated (as it usually is in ecthyma), tonics and a more liberal diet, with the free use of lemon juice diluted with water, as a beverage, should be had recourse to. Sea-bathing is also highly useful. See Tetters.
Eruptions, Sca′ly. Syn. Dry tetter. This is a form of inflammatory condition of the true skin (DERMA), which commonly makes its appearance as a small dull red, salmon-red, or liver-coloured spot, slightly raised above the level of the surrounding skin, constituting a broad, flat, pimple-like prominence, about the size of a split pea. Upon the surface of this prominence the scarf-skin becomes slightly roughened, and after a little while a very distinct but circular scale is produced, which increases in thickness by the addition of fresh layers, and after assuming various colours in different varieties of the disease, ultimately separates and falls off, either leaving a permanently bare surface, or being followed by crops of other like scales, which also fall off, and are replaced in rapid succession. This class of eruptions is more obstinate than any of the other varieties, and often defies medical skill. Each particular form generally requires special treatment. In all, however, endeavours should be made to restore the general health of the body in the manner which existing circumstances may indicate. The red meats, ripe fruit, and antiscorbutic vegetables should form a large portion of the diet; and sea-bathing, or shower, sulphuretted, or ioduretted baths, should be taken daily, if possible. Dry friction with a flesh-brush, and daily exercise to perspiration, are also highly recommended. See Leprosy, Psoriasis, Tetters, &c.
Eruptions, Vesic′ular. Syn. Watery pimples. These consist of little vesicles or bladders, filled with a small quantity of a transparent and colourless liquid. They result from a similar action to that which produces ordinary blisters. Inflammation is excited in the sensitive skin by an inward or outward cause, and the inflamed vessels pour out the watery part of the blood, and so raise the scarf-skin from off the sensitive layer, in the form of a small dome, which in some situations is conical, in others a segment of a sphere. They present great variety in point of number and size; some are so minute as to be scarcely discernible without close inspection, whilst others increase to the magnitude of a hen’s egg. They are numerous in the inverse ratio of their size; the smaller ones being very abundant, and the larger ones scanty and few.
Treat. This consists chiefly in the due attention to the general principles of health—cleanliness, exercise, food, and raiment, as already pointed out, assisted by such special remedies as the particular case or circumstances may demand. Antiphlogistics or tonics must be had recourse to, according to the condition of the system, and local irritation allayed by the usual means. Simple cases frequently yield to a dose or two of some saline aperient and a change of diet. See Acne, Erysipelas, Pemphigus, Rupia, Tetters, and Skin.
ERVALEN′TA. The meal of lentil (Ervum lens,—Linn.), variously doctored with other substances. In some cases the article sold under the name does not contain a particle of lentil meal.
Prep. 1. (Paris Ervalenta.) Indian-corn meal (fine), and bean flour, of each 14 lbs.; salt and sugar, of each 1 lb.; mix, and pass the compound through a sieve.
2. (Warton’s.) Lentil powder, 1 part; durra or Turkey millet flour (Sorghum vulgare), 2 parts. Some persons assert that it contains a large quantity of the flour of Indian corn. See Revalenta and Lentils.
ERYN′′GO. Syn. Eryngium, L. The root of the Eryngium campestre, a plant common in middle and southern Europe. It is sweet, aromatic, and tonic, and formerly enjoyed much repute in gonorrhœa, suppressed menstruation, and visceral obstructions generally, especially those of the gall-bladder, liver, and uterus. Candied eryngo (ERYNGIUM CONDITUM, ERYNGII RADIX CONDITA), according to Lindley, “has the credit of being a decided aphrodisiac,” and has a considerable sale. Eryngium aquaticum (bitter snake-weed) and E. maritimum (sea eryngo, sea holly) furnish the eryngo of the Ph. U. S. See Candying.
ERYSIP′ELAS. Syn. St. Anthony’s fire, The rose. A peculiar form of inflammation, which chiefly attacks the skin, and is generally accompanied or followed by an eruption of a very red colour, sometimes vesicular, and by tumefaction. It commonly attacks the head and face, and is at its height from the third to the sixth day, but the duration and progress of the symptoms are variable. From the eighth to the twelfth day the eruption usually scabs or scales off. Sometimes suppuration occurs, especially of the eyelids and scalp, and during the latter stages of the disease there is, in general, a tendency to debility. In many cases erysipelas is attended by typhoid symptoms, and is then a dangerous and often fatal disease.
Treat. Aperients and diaphoretics, assisted by a cooling diet. When the inflammatory symptoms run high, blistering and cupping are frequently had recourse to. Local irritation may be subdued by milk-and-water, or cooling or evaporating lotions, or by sprinkling starch, hair-powder, or arrow-root, on the part. The tendency to debility in the latter stages should be combated with bark, quinine, or other like tonics. When shiverings, sickness, and delirium, attend the height of the disorder, wine, bark, ammonia, and other stimulants, are usually prescribed, and depletion must be avoided. The same treatment is also adopted in the gangrenous forms of the disease, to which doses of opium and calomel are also commonly added. When suppuration and647 sloughing of the cellular membrane have taken place, it is usual to make incisions to give exit to the discharge, and relieve the tension of the limb. These may be about 11⁄2 inch in length, and from 2 to 4 inches apart, and should be made in the direction of the long dimensions of the limb. Mr Higginbottom, of Nottingham, applies (freely) lunar caustic to the inflamed skin, and also to the healthy skin, to the extent of an inch or more beyond it. The result, in many cases, is a complete change of action in the part, and a resolution of the disease. Iodine paint is often successfully used in the same way.
Wherever practicable medical assistance should be called in on the first appearance of this dangerous disease.
Erysipelas is generally symptomatic of a debilitated or bad constitution. It is also a common sequel of surgical operations in crowded and ill-ventilated hospitals, where it often appears to be contagious. In these cases cleanliness, ventilation, and change of air, are the only remedies. We need scarcely add, that this disease should never be tampered with, but the best medical advice sought, whenever it can be procured.
For animals. The bowels should be kept gently open, by small doses of medicine, and laxative glysters. If there be any feverish symptoms, saline diuretics should be administered; and as a lowering treatment is objectionable, tonics and stimulants should be had recourse to at an early stage of the disease. The affected parts should be kept constantly moist with a lotion composed of one part of Goulard’s extract to thirty parts of distilled or freshly boiled water. Nutritious food, fresh air and general comfort must not be neglected. Cold applications are hurtful. Horses are seldom attacked by erysipelas.
ERYTHORE′TIN. Syn. Red resin of rhubarb. A yellow or reddish-yellow substance, forming one of the three resins found by Schlösberger and Dœpping in rhubarb. It is very soluble in alcohol; less so in ether; with ammonia and potassa it forms soluble compounds of a rich purple colour. See Rhubarb.
ERYTH′RIC ACID. Prep. The lichen Roccella tinctoria (Canary or herb-archil) is boiled with milk of lime, and the filtered solution precipitated with hydrochloric acid; the dried precipitate is dissolved in warm alcohol, and filtered; as the solution cools, crystals of erythric acid are deposited.
Prep., &c. Feebly acid; colourless; inodorous; scarcely soluble in water; soluble in alcohol and ether; chloride of lime turns its solutions of a blood-red colour.
ERYTH′RINE, Amarythrine, Erythriline, Pseudo-erythrine, and Telerythrine. Substances obtained by Kane and Heeren from Roccella tinctoria, Parmelia roccella, Leconara Tartarea, &c. They are of little practical importance.
ESCHAROT′ICS. Syn. Escharotica, L. Substances that destroy the texture of living organic bodies, with the production of an ‘eschar’ or ‘scab.’ Escharotics have been divided into two classes—mechanical and chemical. Among the former are actual cauteries, as a heated iron, moxas, &c.; among the latter are all those substances commonly known as caustics. Some writers have subdivided chemical escharotics into ERODING ESCHAROTICS, as blue vitriol, red precipitate, burnt alum, &c.; and into CAUSTIC ESCHAROTICS, as lunar caustic, pure potassa, strong sulphuric acid, nitric acid, &c.; but these distinctions possess little practical value. “In cauterising with a heated iron, this should be at a white heat, as, at this temperature, it occasions less pain to the patient, from its causing an immediate death of the parts to which it is applied.” “The surrounding surface should be protected by some non-conductor of heat, but not by wet paper or cloth, as the sudden extrication of steam will produce a blistered surface around the burn, and will much increase the pain.” (Dr R. E. Griffith.) See Caustic, Solution, &c.
ES′CULENTS. Substances used for food. The more important esculents are noticed under their respective heads.
ESCU′LIC ACID. A peculiar acid found by M. Bussy in the bark of the horse-chestnut. It is but little known, and has not been applied to any use.
ESERINE. Powder of Calabar bean, 100 parts; tartaric acid, 1 part; potassium bicarbonate in powder, q. s.; alcohol (90°) q. s.; rectified and washed ether q. s. Exhaust the bean mixed with tartaric acid by several digestions in alcohol at the heat of a water-bath, alcohol equal to about three times the weight of the powder being used for each maceration. Distil the combined liquors and filter; heat the residue in a water-bath exposed to the air until it contains no more alcohol. After cooling suspend the extract in a small quantity of distilled water, and filter through paper to separate the insoluble resin.
Agitate the filtrate with rectified and washed ether, until the ether is no longer sensibly coloured; two or three treatments are usually sufficient. Treat the aqueous liquor which contains the eserine in the state of acid tartrate, with a slight excess of potassium bicarbonate, until the reaction is alkaline. Shake this liquor several times with ether, which removes the liberated eserine, and deposits it upon evaporation. The product is purified by fresh crystallisations from ether.
Pure eserine is colourless or slightly rose coloured; it crystallises in thin laminæ having a rhomboid form. Most frequently it occurs in commerce under the form of yellowish spangles, or amorphous masses more or less coloured by the action of the air.
It is slightly soluble in water, but dissolves freely in alcohol, ether, and chloroform. When a one per cent. solution of it is treated with648 potash or soda it rapidly acquires a characteristic rose colour. Heated in a flask with a water-bath in contact with excess of ammonia, it gives upon evaporation of the liquor in the open air, a magnificent blue colour, very soluble in the water. This solution treated with acids, produces a very fine dichroic liquor, violet and transparent by transmission, and carmine red and turbid by refraction. Eserine has the property of contracting energetically the pupil of the eye.
A kilogram of Calabar beans yields on the average one gram of eserine (from ‘Formulæ for new Medicaments, adopted by the Paris Pharmaceutical Society.’) See Calabar Bean.
Eserine, Neutral Hydrobromate of. This body is prepared with colourless hydrobromic acid in the same manner as the sulphate. The solution evaporated to a syrupy consistence, crystallises in the course of a few days in fibrous masses, rarely colourless and non-deliquescent.
The neutral hydrobromate of eserine is employed like the sulphate and in the same doses, although it contains a little less eserine. (From ‘Formulæ for New Medicaments,’ adopted by the Paris Pharmaceutical Society.) See Calabar Bean.
Eserine, Neutral Sulphate of. This salt is obtained by saturating directly and exactly a known quantity of eserine with dilute sulphuric acid (1 in 10); or better still, by shaking a solution of the eserine with a titrated solution of sulphuric acid so as not to exceed the point of saturation. The filtered solution of sulphate of eserine is evaporated rapidly to dryness by the aid of a gentle heat.
Sulphate of eserine can be crystallised in long prismatic needles, combined in radiating groups, but it is very difficult. It is preferable to preserve it in the amorphous state, and in well-stoppered bottles, as it is very deliquescent.
Sulphate of eserine is employed like eserine, internally under the form of granules containing up to one milligram. It is employed also for the eyes as a solution, containing two to five centigrams of the salt to ten grams of distilled water.
Solutions containing eserine, pure or combined, alter rapidly in contact with the air, becoming red; they should only be prepared in small quantities as required. (From ‘Formulæ for New Medicaments,’ adopted by the Paris Pharmaceutical Society.) See Calabar Bean.
ESPRIT. [Fr.] Spirit. This term is commonly applied to alcoholic solutions of the essential oils, and to various odorous and aromatic essences sold by the perfumers and druggists as articles of the toilet. See Essence, Spirit, &c.
ES′SENCE. Syn. Essentia, L. The active and characteristic portion of a substance, or that on which its most remarkable properties depend. The term has been very loosely applied to various preparations presumed to contain these essential principles or qualities, disencumbered of grosser matter. Modern systematic writers generally restrict its application to the volatile oils obtained from vegetable substances by distillation, or to a strong solution of them in alcohol. In pharmacy and perfumery, the word ‘essence’ is applied to concentrated preparations that differ vastly from each other. Thus, concentrated effusions, decoctions, liquors, solutions, and tinctures, are frequently called ‘essences’ by those who vend them; but the term ‘fluid extracts’ would be more appropriate, if those already mentioned are not deemed sufficiently showy and attractive. We shall here confine ourselves to a brief notice of the principal compound essences, or those that undergo some preparation beyond being merely extracted from vegetables by distillation along with water. The latter will be considered under the article Oil.
The concentrated preparations of the pharmaceutist, termed ‘essences,’ are mostly prepared by digesting the active ingredient or ingredients in rectified spirit of wine, either with or without the addition of a certain portion of water; or they are extemporaneously formed by dissolving a portion of the essential oil of such substances in the spirit. In this way are made the essences of lavender, musk, ginger, &c. When it is desired only to obtain the aromatic and volatile portion of the ingredients, the latter are usually digested in the spirit for a few days, and then submitted to distillation, when the alcohol comes over loaded with aromatic essential oil, or other volatile matter. In this way are prepared most of the fragrant essences of the perfumer and druggist, when simple solution of the essential oils in alcohol is not resorted to. In many cases the active principles of the ingredients are partly volatile and partly fixed, or at least do not readily volatilise at the temperature at which alcohol distils over. This is the case, for instance, with the active portion of ergot and Jamaica ginger. In such cases digestion alone should be adopted. When the principles of organic substances, of which it is desired to obtain a concentrated solution, are resinous or oily, or little soluble in weak spirit (which is mostly the case), the strongest rectified spirit of wine should alone be employed. In the preparation of essences without distillation, the method by percolation or displacement is preferable to that of simple maceration and expression, when the nature of the ingredients and other circumstances render it applicable, as it is not only more economical, but a more concentrated solution may thereby be obtained. At the same time, however, the reader should remember, that this mode of operating requires much greater experience and skill to ensure success than the former method. This clumsiness of manipulation is the common cause of the failures which are so frequently met with in the preparation of these articles.
649
The ingredients for the preparation of essences must undergo the same operations of bruising, powdering, or slicing, as directed under ‘Tincture,’ previous to digestion in the spirit, or other menstruum; and the length of time they should be allowed to infuse, when this method alone is adopted, should not be less than ten days; but this time may be advantageously extended to a fortnight, or even longer. During the whole of this period frequent agitation should be employed, and when the ingredients are so bulky as to absorb the whole of the fluid, the vessel which contains them should be securely fastened by a bung or stopper covered with bladder, and inverted every alternate day. By this means every portion of the ingredients will be equally exposed to the action of the menstruum. In all such cases the method of displacement, or percolation, is preferable. For the essences used as perfumes and for flavouring, not only must the spirit be perfectly tasteless and scentless, but it must be also quite devoid of colour.
The following formulæ embrace most of the essences met with in the shops. Those not found among them may be readily prepared by applying the general directions given above, or by employing the formula given for the preparation of the essence of some similar substance, merely varying the characteristic ingredient. Thus, were it desired to form an essence of ambergris or of myrrh, and no formulæ could be found for these preparations, the tyro would consider in what menstruum the active principles of these substances were most soluble. This, he would immediately see by reference to their properties, is rectified spirit of wine. He would next have to decide on the proper strength of his essence. In this he must be guided, either by the strength of the like preparations of other makers, or by his own judgment of what would be useful, novel, or convenient. Suppose he decided that his essence should represent 1-10th of its weight of the solid ingredient. He would then take 2 oz. of ambergris or myrrh, and 20 oz. of rectified spirit, which he would digest together for 10 days or a fortnight in the manner described above. Had the required preparation been an essence of senna (for example), he would probably recollect, or might easily ascertain by reference, that the active properties of senna are soluble in both water and weak spirit. Then, to make an essence 4 times as strong as the tincture of the pharmacopœia, 7 oz. of senna, and 1 pint of proof spirit, should be employed, with due digestion, as before.[280] The same applies to other preparations. See Concentration, Decoction, Infusion, Liquor, Spirit, Tincture, &c.
[280] See directions given under Tincture.
Essence of Ac′onite. Syn. Essentia aconiti, L. Prep. From aconite (herb, dried, and powdered), 8 oz.; rectified spirit, 16 oz.; macerate for 4 days at a temperature of 68° Fahr., press, and strain; the marc or residuum is again macerated with (a little) spirit, and pressed as before, so that the weight of the mixed tinctures may amount to double that of the herb.—Dose, 3 to 6 drops. See Tincture.
Essence of All′spice. Syn. Essence of pimento; Essentia pimentæ, L. Prep. From essential oil of pimento or allspice, 1 fl. oz.; strongest rectified spirit of wine, 1 pint; agitate until perfectly united, and the next day decant the clear portion, if there is any sediment. Used to make pimento water, and by cooks and confectioners as a ‘flavouring.’
Essence of Al′monds. Syn. Essence of bitter almonds, E. of peach kernels, E. of ratafia, E. of noyeau, Quintessence of n., Almond flavour; Essentia amygdalæ, E. a. amaræ, L. Prep. 1. From essential oil of almonds, as the last.
2. (Pereira.) Essential oil of almonds, 1 fl. oz.; rectified spirit, 7 fl. oz.
Uses, &c. It is added to wine, cordials, perfumery, pastry, &c., to impart an agreeable nutty flavour or aroma. It is also employed to prepare cherry-laurel, peach-kernel, and bitter-almond water. A large quantity is consumed by the confectioners, and by wine merchants to ‘improve’ their sherries, and to give Cape wine a sherry flavour. It should be used in very small quantities, as it is very powerful, and, in quantity, poisonous. A few drops are sufficient for several pounds of pastry. The first formula is that used in trade. The second is sometimes used by the druggists, and is occasionally vended under the name of ‘CONCENTRATED ESSENCE OF BITTER ALMONDS,’ &c. The directions for purifying the almond oil from hydrocyanic acid before dissolving it in the spirit, given in more than one recent book of receipts, are absurd, as in this way the oil loses much of its characteristic odour and flavour, and by keeping gradually becomes nearly destitute of both. See Essential Oil.
Essence of Am′bergris. Syn. Essentia ambræ griseæ, E. a. simplex, Tinctura a. concentrata, L. Prep. 1. Ambergris (cut very small), 5 dr.; rectified spirit, 1 pint; place them in a strong bottle or tin can, secure the mouth very firmly, and expose it to the heat of the sun, or in an equally warm situation, for 1 or 2 months, frequently shaking it during the time; lastly, decant, and filter through paper.
2. (Guibourt.) Ambergris, 1 dr.; rectified spirit, 3 oz.; digest 10 or 12 days.
3. (Redwood.) Ambergris, 21⁄2 dr.; rectified spirit, 1 pint; macerate for 14 days. Chiefly used as an element in other perfumes. The first is the formula employed by the London houses.
Essence of Ambergris and Musk. Syn. Concentrated tincture of a. and m.; E. ambræ griseæ (odorata), E. a. et moschi, E. regia, L.; Essence royale, Fr. Prep. 1. Ambergris (cut small), 3⁄4 oz.; 1 or 2 fresh-emptied musk-pods650 (or musk, 12 gr.); rectified spirit, 1 pint; proceed as in No. 1 (above).
2. Ambergris, 21⁄2 oz.; bladder musk, 1⁄2 oz.; spirit of ambrette (purple sweet sultan), 1 gal.; as last.
3. Ambergris, 21⁄2 oz.; bladder musk, 1 oz.; spirit of ambrette, 1 gal.; as before. The fragrance of the above, especially of the last two, is very powerful, and is much esteemed.
4. Ambergris, 1⁄2 oz.; musk and lump sugar, of each 1⁄4 oz.; triturate together in a wedgwood-ware mortar, adding oil of cloves, 20 drops; true balsam of Peru, 30 drops, and enough essence of jasmine or tuberose to convert it into a perfectly smooth paste; then put it into a strong bottle with rectified spirit, 1 quart; observing, before adding the whole of the last, to rinse the mortar out well with it, that nothing may be lost; lastly digest for 6 or 8 weeks, as directed in No. 1 (above).
5. Ambergris, 4 dr.; musk, 11⁄2 dr.; sand, 3 oz.; triturate, then add, of oil of cinnamon, 1 dr.; oil of rhodium, 1⁄2 dr.; essence of roses and eau fleurs d’orange, of each 1⁄4 pint; rectified spirit, 11⁄2 pint; digest as before (or not less than 14 days), and decant and filter. The last two are very fine, though inferior to Nos. 2 and 3.
6. To the last (No. 5), add civet, 1 dr.; salt of tartar, 3 dr.; and an additional pint of rectified spirit. Inferior to the above, but cheaper.
Obs. Essence of ambergris is used as a perfume, and is added in small quantities to sweet-scented spirits and wines, to improve their flavour and aroma. A very small quantity of any one of them added to lavender water, eau de Cologne, tooth-powder, hair-powder, wash-balls, or a hogshead of claret, communicates a delicious fragrance. See Ambergris and Essence Royale (below).
Essence d’Ambrette. [Fr.] Syn. Essence of musk seed, Spirit of m. s.; Esprit d’ambrette, Fr. Prep. 1. Musk seed (ground in a clean pepper-mill), 11⁄4 lb.; rectified spirit, 3 pints; digest for 3 or 4 weeks in a warm place, and filter.
2. Musk seed, 4 lbs.; rectified spirit, 1 gal.; digest 10 days, add water, 2 quarts, and distil over 1 gal. Very fine.
Essence of Ammoni′acum. Syn. Concentrated tincture of ammoniacum; Essentia ammoniaci, Tinctura a. concentrata, L. Prep. 1. Ammoniacum (in tears), 1 lb., is bruised in a very cold marble mortar with half its weight of coarse and well-washed siliceous sand or powdered glass, and rectified spirit, 1⁄2 pint, gradually added; the trituration is continued until the whole is reduced to a smooth paste, and is then placed in a wide-mouthed bottle, and spirit of wine, 11⁄2 pint, further added; the whole is then digested together for a week with constant agitation, and after sufficient repose to settle, the supernatant transparent liquid is decanted into another bottle for use.
2. Gum ammoniacum, 1 lb., is reduced to a cream with boiling water, 3⁄4 pint; as soon as the mixture has cooled a little, it is placed in a strong bottle, and rectified spirit of wine, 11⁄4 pint, is cautiously added; the mixture is then corked down close, and the whole macerated for a few days; the bottle is next placed in a moderately warm situation, that the sediment may subside, after which the clearer portion is poured off through flannel into another bottle.
Obs. This preparation is used as a substitute for the gum in substance, for extemporaneously preparing emulsion of ammoniacum, mixture of a., &c. It is represented to possess fully the same amount of medicinal virtue as an equal weight of the solid gum, on which account it has a considerable sale. The product of the first formula, when well managed, is a beautiful pale brownish-coloured, transparent tincture; that of the second is milky and less sightly. The preparation generally sold under the name of ‘CONCENTRATED ESSENCE OF AMMONIACUM’ (ESSENTIA AMMONIACI CONCENTRATA, L.), and represented as twice as strong as the gum in substance, is generally prepared by the first formula given above for ESSENCE OF AMMONIACUM. A stronger article may be prepared by a similar process, by using 1 lb. of ammoniacum to a pint of the strongest rectified spirit. As, however, a clear liquid at this strength is somewhat difficult to produce, it is very seldom attempted by the druggists; they therefore generally content themselves with sending out the liquid at half the professed strength, leaving the label to confer the additional concentration. See Ammoniacum.
Essence of Anchov′ies. Syn. Essentia clupeæ, L. Prep. 1. Anchovies, 1 lb., are ‘boned,’ reduced to a pulp in a wedgwood-ware or marble mortar, and passed through a clean hair or brass-wire sieve: meanwhile the bones and other portion that will not pass through the sieve are boiled with water, 1 pint, for 15 minutes, and strained; to the strained liquor, salt, and flour, of each, 21⁄2 oz., together with the pulped anchovies are added, and the whole simmered for 3 or 4 minutes, when the vessel is removed from the fire, and as soon as the mixture has cooled a little, strong pickling vinegar, 1⁄2 pint, is mixed in; it is then bottled, and the corks tied over with bladder, and either ‘waxed’ or ‘capsuled.’ Product, 3 lbs. (nearly).
2. Anchovies, 7 lbs.; water, 9 pints; salt and flour, of each, 1 lb. Product, 20 lbs.
3. To the last add of Cayenne pepper, 1⁄4 oz.; the peel of a lemon (grated), and mushroom catsup, 4 oz. Very savoury.
4. From British anchovies (pickled sprats) or young pilchards, along with herring liquor, or the drainings of anchovy barrels.
Use, &c. As a sauce and condiment; when well prepared, it has a fine flavour. That of the shops is usually coloured with Venetian red or Armenian bole. An infusion of cochineal, or a little annotta, would form a more appropriate651 colouring, and would be perfectly harmless. See Anchovy and Sauce.
Essence of Angel′ica. Syn. Essentia angelicæ, L. Prep. (Van Mons.) Angelica root (bruised), 1 part; rectified spirit, 8 parts; water, 16 parts; digest, and distil over 6 parts; Stomachic, carminative, and alexipharmic.—Dose, 1 to 2 spoonfuls.
Essence of Ani′seed. Syn. Essentia anisi (B. P.), L.; Esprit d’anise, Fr. Oil of anise, 1 part; rectified spirit, 4 parts; mix (B. P.). Stimulant, aromatic, and carminative.—Dose, 10 to 20 minims. Used also to flavour liqueurs, and to make aniseed water. See Spirit.
Essence of An′odyne. Syn. Essentia anodyna, L. Prep. 1. Hard aqueous extract of opium (in powder), 1 dr.; powdered cinnamon, 1⁄2 dr.; rectified spirit, 1 fl. oz.; digest a week.—Dose, 5 to 20 drops.
2. Extract of henbane (recent), 5 dr.; rectified spirit, 2 fl. oz.; as last.—Dose, 10 to 30 drops. Narcotic, sedative, and antispasmodic. Both are excellent preparations.
Essence, Antihyster′ic. Syn. Essentia antihysterica, L. Prep. 1. Cyanuret of potassium, 3 gr.; powdered sugar, 1 dr.; rectified spirit and eau d’orange, of each 4 fl. dr.; agitate together until dissolved.—Dose, 10 to 20 drops, in pure water; in hysteria, gastrodynia, &c. See Draught (Antihysteric).
2. (P. Cod.) Resembles FETID SPIRIT OF AMMONIA (which see).
Essence of Ap′ple. Syn. Solution of valerianate of amyl; Essentia pomi odorata, L. Prep. From apple oil (valerianate of oxide of amyl), as ESSENCE OF ALMONDS. Used to flavour liqueurs and confectionery.
Essence of Ar′nica. Syn. Essentia arnicæ, E. a. florum, Tinctura a. e. concentrata, L. Prep. (Ph. Baden, 1841.) From arnica flowers, ESSENCE OF ACONITE. It represents half its weight of herb.
Essence, Aromat′ic. Syn. Essentia aromatica, L. Prep. From hay saffron, dr.; and rectified spirit, 6 fl. dr.; digested together; to the filtered tincture is added oil of cinnamon and powdered white sugar, of each 1 dr.; ether (rect.), 2 fl. dr.; oil of nutmeg and essence of ginger, of each 1⁄2 dr.; after agitation and a few days’ repose, the clear portion is decanted into a stoppered phial.—Dose, 5 to 15 drops, on sugar or in a glass of wine or weak spirit; in cholera, diarrhœa, spasms, &c.
Essence of Bark. Syn. Essentia cinchonæ, E. corticis c., L. Prep. 1. Resinous extract of yellow bark, 4 dr.; rectified spirit, 11⁄2 fl. oz.; tincture of orange peel, 1⁄2 fl. oz.; acetic acid (Ph. L.), 1 fl. dr.; digest a week.
2. Disulphate of quinine, 1⁄2 dr.; resinous extract of bark, 2 dr.; rectified spirit, 2 fl. oz.; as before.—Dose, 12 drops to a teaspoonful; as a febrifuge and tonic.
Essence of Beef. Syn. Essence of red meats, &c. Prep. 1. From lean beef (chopped small), 1 lb.; water, 1⁄2 pint; place them in a bottle, which they will only half fill, and agitate them violently for half an hour; then throw the whole on a sieve, and receive the liquid in a jug; next boil the undissolved portion in water, 1 pint, for 20 minutes; strain, mix the decoction with the cold infusion, evaporate the liquid to the consistence of a thin syrup, adding spice, salt, &c., to taste, and pour the essence, whilst boiling hot, into bottles, jars, or (still better) tin cans, which must then be at once hermetically corked, sealed, or soldered up, and stowed away in a cold place. In this state it will keep a long time. (Brande’s.)
2. (Ellis.) Take of lean beef (sliced), a sufficient quantity to fill the body of a porter bottle; cork it up loosely, and place it in a pot of cold water, attaching the neck, by means of a string, to the handle of the pot; boil for 11⁄2 to 2 hours, then decant the liquid and skim it. Spices, salt, wine, brandy, &c., may be added as before. Highly nutritious and sustaining.
Essence of Ber′gamot. See Oil (Volatile).
Essence, Bit′ter. Syn. Essentia amara, L. Prep. (Ph. Den.) Wormwood, 4 parts; gentian root, bitter orange peel, and blessed thistle, of each 1 part; rectified spirit, 45 parts; digest a week. Tonic and stomachic.—Dose, 1⁄2 dr. to 2 dr.
Essence of Calum′ba. Syn. Essentia calumbæ, L. See Infusion of Calumba.
Essence of Cam′phor. Syn. Camphor drops, Liquor of camphor, Concentrated essence of c., Concentrated solution of c., Conc. camphor julep; Essentia camphoræ, Liquor c., L. c. concentratus, L. Prep. 1. Camphor (clean), 41⁄2 oz.; rectified spirit, 1 gall.; dissolve. This forms the ‘ESSENCE OF CAMPHOR’ and ‘LIQUOR CAMPHORÆ’ of the wholesale houses. About 1⁄2 fl. dr., added to 71⁄2 fl. dr. of cold distilled water, forms (by agitation) a transparent aqueous solution of camphor, fully equal in strength to the filtered ‘MISTURA CAMPHORÆ’ (camphor julep) of the Ph. L. The above made with weaker spirit forms the ‘spirit of wine and camphor’ of the shops.
2. Camphor, 1 oz.; rectified spirit, 10 oz. (by weight); dissolve. This forms the ‘CONCENTRATED ESSENCE OF CAMPHOR’ of the wholesale druggists. 10 or 12 drops, added to 1 fl. oz. of pure cold water, make a transparent camphor julep, as before. There is a large quantity of these solutions of camphor sold by the London houses, who charge a considerable price for them. They are very convenient for preparing extemporaneous camphor julep or camphor mixture in dispensing.
3. (Fordred.) Tincture of camphor, 13 fl. dr.; tincture of myrrh, 1⁄2 fl. dr.; rectified spirit, 181⁄2 fl. dr.; mix. 1 fl. dr., added to 4 fl. oz. of water, forms camphor julep. It has been proposed to bleach the tincture of myrrh with animal charcoal, but this interferes with its proper action.
4. (Homœopathic.) See Cholera remedies, Nos. 6 and 7.
652
5. (Houlton.) Spirit of camphor (Ph. L.), 1 fl. oz.; proof spirit, 7 fl. oz.; 1 fl. dr. to 3 fl. oz. of water; forms ‘CAMPHOR JULEP,’
6. (Redwood.) Camphor, 1 dr.; rectified spirit, 21⁄2 oz.; dissolve, and add of water, 1⁄2 oz.
7. (Swediaur.) Powdered camphor, 1 dr.; water saturated with carbonic acid gas, 12 fl. oz.; dissolve. 1 part of this solution, added to 4 parts of water, forms ‘CAMPHOR MIXTURE,’ See Camphor.
Essence of Cap′sicum. See Essence of Cayenne.
Essence of Car′away. Syn. Essentia carui, L. Prep. From oil of caraway, as ESSENCE OF ALMONDS. Its applications and uses are similar. An inferior kind is prepared by macerating the seeds in proof spirit.
Essence of Car′damoms. Syn. Essentia cardamomi, E. c. concentrata, L. Prep. From lesser cardamom seeds (ground in a pepper mill), 51⁄2 lbs.; rectified spirit of wine, 1 gall.; digest for a fortnight, press, and filter.
Obs. This preparation is very convenient for flavouring cordials, pastry, &c., and is very powerful. In the laboratory it is frequently substituted for powdered cardamoms in making compound extract of colocynth, and has the advantage of adding no inert matter to the preparation, whilst it imparts the characteristic odour of the seeds in a remarkable degree. When used in this way it is not added to the extract until it is nearly cold and about to be taken from the pan. The testæ or shells of the seed should be separated from the kernels, as the former are quite inert, and if used occasion a loss of spirit for no purpose.
Essence of Cascaril′la. Syn. Essentia cascarillæ, L. Prep. 1. Cascarilla (bruised), 12 oz.; proof spirit, 1 pint; proceed either by digestion or percolation. The product is 8 times the strength of the infusion of cascarilla. (Ph. L.)
2. See Infusion (Concentrated).
Essence of Cas′sia. Syn. Essentia cassiæ, L. Prep. From oil of cassia, as essence of allspice or almonds.
Essence of Cayenne′. Syn. Essence of cayenne pepper, E. of capsicum, Concentrated e. of c.; Essentia capsici, Tinctura capsici concentrata, L. Prep. 1. Capsicum (recent dried pods, bruised), 3 lbs.; rectified spirit, 1 gall.; digest 14 days, press, and filter. Some persons prepare it by the method of displacement.
2. Capsicum, 1⁄4 lb.; proof spirit, 1 pint; digest as before. Weaker than No. 1.
3. (Kitchener’s.) Cayenne pepper, 1 oz.; brandy, 1 pint; digest, &c., as before.
Obs. The product of the first formula is a transparent, dark-coloured liquid, having an intensely burning taste. One drop is sufficient to deprive a person of the power of speech for several seconds; and a few drops will impart the rich pungency of cayenne to a large quantity of soup, sauce, or any other article. It forms the ‘ESSENCE OF CAYENNE’ and the ‘CONC. ESS. OF CAYENNE PEPPER’ of the London houses. It is principally used as a flavouring, and to make SOLUBLE CAYENNE PEPPER; also in dispensing. It is fully eight times as strong as the ‘TINCTURA CAPSICI’ (Ph. L.). The product of the third formula is used exclusively for culinary purposes. The pods or fruit of Capsicum annuum (capsicum chilly), C. baccatum (bird pepper), and C. fructescens (Guinea pods, red pepper), are indiscriminately used for this preparation, but the first are those preferred for medicinal purposes; the others have similar properties, but are more pungent and acrimonious; hence the preference given to them in the preparation of cayenne pepper. See Pepper.
Essence of Ce′drat. See Oil (Volatile).
Essence of Cel′ery. Syn. Essence of celery seed; Essentia apii, Ess. a. seminis, L. Prep. 1. From celery seed (bruised or ground), 41⁄2 oz.; proof spirit, 1 pint; digest a fortnight, and strain.
2. (Concentrated.) Celery seed, 7 oz.; rectified spirit, 1 pint; digest as before. Very fine. Both are used for flavouring.
Essence, Cephalic. See Essence for Headache.
Essence of Cham′omile. Syn. Chamomile drops; Essentia anthemidis, E. chamæmeli, E. c. alba, L. Prep. 1. From essential oil of chamomile, as essence of allspice. Stomachic and stimulant.—Dose, 5 to 30 drops; 1⁄2 fl. oz., shaken with about 1 pint of pure water, forms an excellent extemporaneous chamomile water.
2. Gentian root (sliced or bruised), 1 lb.; dried orange peel, 1⁄4 lb.; proof spirit, 1 gal.; essential oil of chamomile, 31⁄2 fl. oz.; macerate a week. Slightly coloured. Some persons use 1⁄2 lb. of quassia wood, instead of the gentian and orange peel. Both the above are stomachic and tonic, and are favourite remedies in loss of appetite, dyspepsia, &c.—Dose. As the last, on sugar, or in a wine-glassful of wine or beer.
Essence of Chiret′ta. See Infusion (Concentrated).
Essence of Cin′namon. Syn. Essentia cinnamomi, Spiritus c. concentratus, L. Prep. 1. From oil of cinnamon, as ESSENCE OF ALLSPICE or ALMONDS.
2. Cinnamon, 5 oz.; rectified spirit, 3⁄4 pint; water, 1⁄4 pint; digest a week, and strain. Inferior to the last. Essence of cassia is commonly sold for it.
Essence of Civ′et. Syn. Essentia zibethi, L. Prep. 1. Civet (cut small), 1 oz.; rectified spirit, 1 pint; as ESSENCE OF MUSK.
2. Instead of rectified spirit use spirit of ambrette. Both are used in perfumery; chiefly in combination with other substances.
Essence of Cloves. Syn. Essentia caryophilli, L. Prep. 1. (White.) From oil of cloves, as ESSENCE OF ALLSPICE. Used as a ‘flavouring.’
2. (Coloured.) Cloves (bruised), 31⁄2 oz.653 proof spirit, 3⁄4 pint; water, 1⁄4 pint; digest a week, and strain. Inferior to the last. It is 8 times as strong as INFUSION OF CLOVES (Ph. L.). Chiefly used in dispensing.
Essence of Cof′fee. See Coffee.
Essence of Co′gnac. (kōne′-yăk). Syn. Brandy essence. Prep. From brandy oil, 2 fl. oz.; rectified spirit, 18 fl. oz. For flavouring malt spirit to imitate brandy. See Oil.
Essence of Cologne. Syn. Concentrated eau de Cologne; Essentia Coloniensis, Aqua C. concentrata, L. Prep. 1. By taking 8 times the quantity of the ingredients ordered for Cologne water, and using the strongest rectified spirit.
2. Oils of lemon and cedrat, of each, 2 dr.; oil of rosemary, 1 dr.; oil of bergamotte, 1 oz.; spirit of neroli, 2 fl. oz.; purest rectified spirit, 5 fl. oz. Used as a condensed perfume.
Essence of Colts′foot. Prep. 1. (Ryan.) Balsam of tolu, 1 oz.; rectified spirit and compound tincture of benzoin, of each 3 oz.; dissolve, and in a few days decant the clear portion.
2. (Paris.) Equal parts of balsam of tolu and compound tincture of benzoin, with double the quantity of rectified spirit.
3. Tincture of tolu, 5 fl. oz.; compound tincture of benzoin, 3 fl. oz.; powdered sugar (quite dry), 1 oz.; hay saffron, 1 dr.; digest a week, with frequent agitation.
Obs. Pectoral and stimulant. A quack remedy for consumption and most other diseases of the lungs, but unless assisted by occasional aperients, and in the absence of fever, it is more likely to kill than cure in these complaints. The last is the best formula.
Essence of Cu′bebs. Syn. Concentrated essence of cubebs; Essentia cubebæ, E. c. concentrata, L. Prep. 1. Cubebs (bruised, or preferably ground in a pepper mill), 1⁄2 lb.; rectified spirit, 1 pint; digest 14 days, press, and filter.
2. (Wholesale.) Cubebs, 41⁄4 lbs.; rectified spirit, 1 gall. This essence has a very large sale, and if carefully prepared from a good sample of the drug, is a most excellent preparation. Every fl. oz. represents 21⁄2 dr. of cubebs.—Dose, 1 to 3 dr.
Essence of Cubebs (Oleo-resinous). Prep. (Dublanc.) Oleo-resinous extract of cubebs, 1 oz.; rectified spirit, 3 oz.; dissolve. A very active and concentrated form of administering cubebs, which must not be confounded with the preceding preparation, which is the one always meant when ‘Essence of Cubebs’ is ordered.—Dose, 1⁄2 dr. to 1 dr.
Essence of Dill. Syn. Dill drops; Essentia anethi, L. Prep. 1. From oil of dill, as ESSENCE OF ALLSPICE.
2. Oil of dill, extract of dill, and salt of tartar, of each 1⁄2 oz.; rectified spirit, 1 pint; digest, and strain. Both the above are aromatic and anti-flatulent. The first is commonly used as an adjunct to other medicines, especially to purgatives for children. The second is a popular tonic and stomachic in the flatulent colic, dyspepsia, &c., of women and children.—Dose. A few drops, on sugar.
Essence of Er′got. See Liquor or Ergot of Rye.
Essence of Ergot (Ethereal). Syn. Essentia ergotæ etherea, E. secalis cornuti e., L. Prep. 1. (Mr Lever.) Ergot (powdered), 2 oz.; rectified sulphuric ether, 2 fl. oz.; digest a week, express the tincture, filter, and abandon the liquid to spontaneous evaporation; lastly, dissolve the residuum in ether, 1 fl. oz. This is an expensive and troublesome formula. The following modification of it is both simpler and less expensive.
2. Ergot (ground), 8 oz.; ether, 16 fl. oz.; prepare a tincture as before, and by a gentle heat distil off the ether in a retort connected with a well-cooled refrigerator, until 15 fl. oz. shall have passed over; continue the evaporation at a reduced heat until the remainder of the ether has passed off; lastly, dissolve the residuum, as soon as cold, in ether, 4 fl. oz.
Obs. Each fl. oz. represents 2 oz. of ergot.—Dose, 10 to 30 drops as a parturifacient, taken on sugar; 3 to 5 drops as a hæmostatic and emmenagogue, in hæmorrhages, floodings, &c. It possesses all the acrid, narcotic principle of the ergot, but less of the hæmostatic principle than the ordinary essence, whilst it is much more costly.
Essence of Fen′nel. Syn. Essence of sweet fennel; Essentia fœniculi, L. Prep. From oil of fennel (Fœniculum dulce), as ESSENCE OF ALLSPICE.
Essence of Gen′tian. See Infusion of Gentian (Concentrated).
Essence of Gin′ger. Syn. Concentrated tincture of ginger, Essentia zingiberis, Tinctura z. concentrata, L. Prep. 1. Unbleached Jamaica ginger (bruised), 5 oz.; rectified spirit, 1 pint; digest a fortnight, press, and filter.
2. (Oxley’s ‘Concentrated essence of Jamaica ginger,’ The same as the preceding, with the addition of a very small quantity of essence of cayenne. The above possess only about 4 times the strength of tincture of ginger (Ph. L.); and though vended in the shops as essence of ginger, scarcely deserves the name.
3. As No. 1 (next article, below), but using double the quantity of spirit. Very fine.
4. (Kitchener’s.) Ginger (grated), 3 oz.; yellow peel of lemon (fresh), 2 oz.; brandy, 11⁄2 pint; digest 10 days. For culinary purposes, &c. See below.
Essence of Ginger (Concentrated). Syn. Essentia zingiberis concentrata. Prep. 1. Jamaica ginger (best unbleached, in coarse powder) and siliceous sand, equal parts, are sprinkled with rectified spirit of wine, q. s. to perfectly moisten them, and after 24 hours the654 mass is placed in a ‘percolator,’ and after returning the first runnings 2 or 3 times, the receiver is changed, and more rectified spirit poured on gradually, and at intervals, as required, until as much essence is obtained as there has been ginger employed.
Obs. The quality of the product of the above formula is excellent, but the process is somewhat difficult to manage. The mass remaining in the percolator is treated with fresh spirit until exhausted, and the tincture so obtained is employed, instead of spirit, for making more essence with fresh ginger. The last portion of spirit in the waste mass may be obtained by adding a little water. Coarsely powdered charcoal is frequently used instead of sand, in which case the product has less colour; at the same time, however, a little of the flavour is lost.
2. (Wholesale.)—a. Best unbleached Jamaica ginger (as last), 12 lbs.; rectified spirit, 21⁄2 galls., are digested together for 14 days, and the expressed and strained tincture reduced by distillation, in a steam or water bath, to exactly 1 gall.; it is next cooled, and transferred as quickly as possible into stoppered bottles, and the next day filtered.
Obs. The product of the last formula is a most beautiful article, of immense strength, and the richest flavour. The assertion made by a recent writer on pharmacy, that ‘the product is very strong, but has lost some of the flavour of the ginger,’ is evidently made in ignorance of the preparation. “We were the first to introduce and publish this formula, and have employed it for years on the most extensive scale, and can conscientiously assert that, for inexpensiveness, and the quality of the essence produced by it, it is unequalled by any other. The process, though apparently complicated is, in reality, easily performed. The spirit distilled over contains none of the fragrant or aromatic principles of the ginger; on the contrary, the little flavour it has received (apparently from a species of ethereal oil) is rather disagreeable than otherwise, and is better got rid of than retained in the essence. The spirit is used with advantage for preparing the common tincture of ginger, and several other articles. The cause of failure when this process is adopted is careless or awkward manipulation. When possible, hydraulic pressure should be employed to express the tincture, 2 oz. of this essence are regarded as equivalent to 3 oz. of the finest ginger, being fully twenty times as strong as the ‘TINCTURE OF GINGER’ (Ph. L.). A single drop, swallowed, will almost produce suffocation.” Cooley.
b. From ginger (as last), 24 lbs.; rectified spirit, 6 gall.; make a tincture, as before, and reduce it by distillation to 1 gall.; then cool as quickly as possible out of contact with the air and add, of the strongest rectified spirit of wine, 1 gall.; lastly, filter, if required. Quality resembles No. 2, a (nearly). “We are in the habit of applying the method developed in the last two formulæ to the preparation of the essences of several other substances, the active principles of which are not volatile at a low temperature.” Cooley.
Essence of Grape. Prep. From grape oil, as ESSENCE OF ALMONDS. It is used to flavour brandy and wines. See Oil (Volatile).
Essence of Guaiac′um. Syn. Fluid extract of guaiacum; Essentia guaiaci, Extractum guaiaci fluidum, L. Prep. Recent guaiacum shavings, from which the dust has been sifted, 3 cwt., are exhausted by coction in water, as in the preparation of an extract, using as little of that fluid as is absolutely necessary; the decoction is evaporated to exactly 13⁄4 gall.; it is next stirred until cold, to prevent the deposit of resinous matter, when it is put into a bottle, and spirit of wine, 5 pints, is added; the whole is then repeatedly agitated for a week, after which it is allowed to settle for 7 or 8 days, and the clear portion is decanted into another bottle.
Obs. This preparation is frequently substituted for guaiacum shavings in the preparation of compound decoction of sarsaparilla. 1 pint of this essence is considered equivalent to 19 lbs. of guaiacum in substance. See Decoction of Sarsaparilla (Comp.).
Essence for the Handkerchief. See Essentia Odorata, &c.
Essence for the Headache. Syn. Cephalic essence, Embrocation of ammonia, Dr Hawkins’ embrocation, Ward’s e., Ward’s essence for the headache; Embrocatio ammoniæ, Linimentum a., Essentia cephalica, L. Prep. 1. Oil of lavender (Mitcham), 1 dr.; camphor, 1 oz.; liquor of ammonia, 4 oz.; rectified spirit, 1 pint; dissolve. Very fragrant and powerful.
2. (Beasley.) Spirit of camphor, 2 lbs.; strong water of ammonia, 4 oz.; essence of lemon, 1⁄2 oz.
3. (Redwood.) Camphor and liquor of ammonia, of each 2 oz.; oil of lavender, 4 dr.; rectified spirit, 14 oz. Very fragrant. Stimulant and rubefacient. Used as a counter-irritant lotion in local pains, as headache, earache, colic, &c. Compound camphor liniment is usually sold for it. See Liniment.
Essence of Henbane. Syn. Essentia hyoscami, L. See Essence (Anodyne), No. 2.
Essence of Hop. Syn. Essentia lupuli, E. humuli, Tinctura lupuli concentrata, L. Prep. 1. New hops (rubbed small), 261⁄2 oz.; proof spirit, 1 quart; digest 24 hours, then distil over (quickly) 1 pint, and set the distillate (spiritus lupuli) aside in a corked bottle; to the residuum add water, 1 pint; boil 15 minutes, cool, express the liquor, strain, and evaporate it as quickly as possible to dryness by the heat of a water bath, powder the residuum, and add it to the distilled spirit; digest a week, and filter.
2. Lupulinic grains (yellow powder or lupulin of the strobiles), 5 oz.; rectified spirit, 1655 pint; digest 10 days; express, and filter. Both the above are powerfully bitter, and loaded with the aroma of the hop. They are fully 8 times as strong as the ‘TINCTURA LUPULI’ of the Ph. L. A few drops added to a glassful of ale or beer render it agreeably bitter and stomachic.
3. (Brewer’s E. of Hops.) Several noxious preparations under the name of extract of hops are sold by the brewer’s druggist. They are mostly semi-fluid extracts of quassia, gentian, and like powerful bitters. Of three of these articles which we have examined, one (for PALE ALE) consisted of the mixed extracts of quassia and chamomile; another was a preparation of picric acid; whilst a third (‘strongly recommended for PORTER’) consisted of about equal parts of the extracts of bitter aloes, cocculus indicus, and wormwood. A few years ago one of these vile compounds was publicly advertised, and ‘warranted’ as being equal to 100 times its weight in hops (1 oz. to 51⁄2 lbs.).
Essence of Jargonelle’ Pear. Syn. Pear essence, Esprit de Jargonelle, &c. Prep. From pear oil (acetate of oxide of amyl), as ESSENCE OF ALMONDS. This is now largely employed to flavour confectionery and liqueurs. See Amyl and Oil (Volatile).
Essence of Jas′mine. See Spirit and Oil (Volatile).
Essence of Jes′samine. See Spirit and Oil.
Essence of Jon′quil. See Spirit and Oil.
Essence of Lav′ender. Syn. Essentia lavandulæ (odorata), L. Prep. 1. Oil of lavender (Mitcham), 2 oz.; rectified spirit (strongest), 1 pint.
2. As the strongest Eau de lavende. See Spirit.
Essence of Lavender (Red). See Spirit and Tincture.
Essence of Lem′on. Syn. Essentia limonis, L. Prep. 1. See Oil (Volatile).
2. (W. Procter.) Fresh oil of lemons, 1 fl. oz.; deodorised alcohol (strongest flavourless rectified), 8 fl. oz.; exterior yellow rind of lemons (fresh), 1⁄2 oz.; digest 48 hours, and filter. Used for flavouring mixtures, pastry, &c.
3. From oil of lemons, as ESSENCE OF ALLSPICE. Used as the last.
Essence of Lemon Peel. Syn. Essence of lemon rind, Quintescence of l. p.; Essentia corticis limonis, L. Prep. 1. Yellow peel of fresh lemons, 1⁄2 lb.; spirit of wine, 1 pint; digest for a week, press, and filter. Very fragrant.
2. Yellow peel of fresh lemons, 1 lb.; boiling water, 1⁄2 gall.; infuse 1 hour, express the liquor, boil down to 1⁄2 pint, cool, and add oil of lemon, 1⁄4 oz., dissolved in spirit of wine, 11⁄2 pint; mix, and filter. Used as the preceding.
Essence of Lov′age. Syn. Essentia levistici, L. Prep. (Ph. Wurt.) Lovage root (levisticum officinale), 2 oz.; lovage seeds, 1 oz.; rectified spirit, 10 oz.; digest a week, and filter. Aromatic, stomachic, and diaphoretic.—Dose, 1⁄2 dr. to 1 dr.; in dyspepsia, dropsies, &c.
Essence, Madden’s. Concentrated infusion of roses.
Essence of Malt. See Colouring.
Essence of Mint. Syn. Essence of spearmint; Essentia menthæ, E. m. spicatæ, E. m. viridis, L. Prep. As ESSENCE OF PEPPERMINT.
Essence of Moss-Rose (from the ‘Chemist and Druggist’). Otto of rose, 11⁄2 dr.; essence of ambergris, 21⁄2 oz.; essence of musk, 1 oz.; alcohol, 15 oz.; concentrated rose water, 10 oz. Mix, and shake frequently for a week.
Essence of Musk. Syn. Essentia moschi, Tinctura m. concentrata, L. Prep. 1. Grain musk, 2 oz., and boiling water, 1 pint, are digested together in a close vessel until cold, when rectified spirit of wine, 7 pints, is added; the vessel (preferably a tin bottle) being corked close, and tied over with bladder, the whole is digested, with frequent agitation, for 2 months, in the sunshine (in summer), or in an equally warm situation in winter. At the end of the time the essence is decanted and filtered.
2. Grain musk, 1⁄4 oz.; rectified spirit of wine, 2 pints; essence of ambergris, 1 fl. oz.; digest as before.
3. Musk (from the bladder, rubbed very small), 5 oz.; civet, 1 oz.; essence of ambergris, 1 pint; spirit of ambrette, 1 gall.; as before.
Obs. All the preceding formulæ yield superior essences, but the product of the last is of the very finest quality, and such as is seldom sold, except by the most celebrated houses, when it fetches a very high price. It is powerfully and deliciously odorous, and has received the approval of royalty itself, both in these kingdoms and on the Continent. The second formula also produces a very fine article, but less choice than just referred to. The digestion should be long continued, and on no account less than 3 weeks, as otherwise much fragrant matter is left undissolved. The addition of 1 fl. dr. of either liquor of ammonia or liquor of potassia (the first is best) to each pint of the essence, vastly increases its fragrance. The essence of musk of the wholesale London druggists is generally made by merely digesting the freshly emptied musk pods in rectified spirit. Sometimes a little (a very little) grain musk is added. See Essence Royale and Essence of Ambergris.
4. (Guibourt.) Musk, 1 part; proof spirit, 12 parts; digest a fortnight, or longer. Used in dispensing, &c.
Essence of Musk Seed. See Essence d’Ambrette.
Essence of Mus′tard. Syn. Essentia sinapis, L. Prep. (Whitehead’s.) Black mustard seed (bruised), and camphor, of each 2 oz.; oil of rosemary, 3 dr.; balsam of tolu, 1 dr.; annatto, 1⁄2 dr.; digest a week, and filter.
656
Essence of Myr′tle. Syn. Essence of myrtle blossoms; Essence de myrte, Esprit de m., Fr. Prep. Myrtle tops (in blossom), 21⁄2 lbs.; proof spirit, 9 pints; digest 3 days, then distil 1 gall. A pleasant perfume. See Oil (Volatile).
Essence of Nero′li. Syn. Essence de fleurs d’oranges, Esprit de f. d’o., Fr. Prep. 1. Neroli, 3 dr.; rectified spirit of wine, 1 pint; mix. A delicious perfume.
2. Oil of orange, 2 dr.; orris root (bruised), 1⁄2 oz.; ambergris, 10 gr.; neroli, 35 drops; spirits of wine, 1 pint; digest 14 days, and filter. Very fragrant, but less ‘chaste’ than the last.
Essence of Nut′meg. Syn. Essentia myristicæ, E. m. moschatæ, E. nucis m., L. Prep. From essential oil of nutmeg, as ESSENCE OF ALLSPICE. Used as a flavouring or zest by cooks, liqueuristes, and confectioners.
Essence, Odontal′gic. See Essence, Toothache.
Essence d’Œillets. [Fr.] Prep. From cinnamon, 3 oz.; cloves, 11⁄4 oz. (both well bruised); rectified spirit, 1 quart; digest for a week. Oil of cloves and spirit of cloves also bear this name in some places.
Essence of O′pium. See Essence Anodyne, No. 1. Black drop and Rousseau’s laudanum have also been sometimes so called.
Essence of O′range. Syn. Essentia aurantii, L. Prep. As ESSENCE OF LEMON.
Essence of Orange Peel. Syn. Essentia corticis aurantii, L. Prep. 1. (Golden.) Fresh yellow rind of orange, 4 oz.; rectified spirit and water, of each 1⁄2 pint; digest for a week, press, filter, and add of sherry wine, 1 quart. A pleasant liqueur.
2. (Saccharated.) See Oleo-saccharum.
Essence d’Orient. [Fr.] A pearly-looking substance, found at the base of the scales of the blay or bleak, a small fish of the genus cyprinus.
Prep. The scales are scraped from the fish into a tub containing water, and after agitation and repose the fluid is poured off, and its place supplied with fresh water, and this in its turn, after agitation and repose, is also poured off. This part of the operation is repeated till the ‘essence’ and scales are perfectly freed from impurities, when the whole is thrown on a sieve, which retains the latter, but allows the former to flow through. After repose for a short time, the essence is obtained as a deposit at the bottom of the vessel.
Obs. Essence d’Orient has a bluish-white and pearly aspect, and is employed to cover the interior of glass bubbles and beads, in imitation of pearls and mother-of-pearl. Its tendency to putrefaction, while in the moist state, may be obviated by the addition of a little liquor of ammonia.
Essence of Patch′ouli. Syn. Essence de patchoulie, Esprit de pouchâ pât [Fr.]. Prep. 1. Indian patchouli (leaves or foliaceous tops), 21⁄2 lb.; rectified spirit, 9 pints; digest for a week; add of water, 1 gall.; oil of lavender (Mitcham), 3 dr.; common salt, 2 lbs.; agitate well together, distil over (rapidly) 1 gallon, and add of essence of musk, 31⁄2 fl. dr. A very fashionable perfume. Essence of patchouli, thus prepared, has been largely used, both at court and by the nobility generally.
2. Patchouli, 3 oz.; rectified spirit, 1 pint; digest a week, press, and filter. A still commoner kind is made with proof spirit.
Essence of Pear. Syn. Essence of jargonelle.
Essence of Pen′nyroyal. See Essentia pulegii, E. menthæ p., L. Prep. From pennyroyal (Mentha pulegium), as Essence of peppermint. Stimulant, carminative, and emmenagogue. Used in dispensing, especially to make extemporaneous pennyroyal water.
Essence of Pep′permint. Syn. Essentia menthæ piperitæ (B. P.) L. Prep. 1. (B. P.) Oil of peppermint, 1 part; rectified spirit, 4 parts. Mix.—Dose, 10 to 20 minims.
2. To the last add of herb peppermint, parsley leaves, or spinach leaves (preferably one of the first two), 1⁄2 oz., and digest for a week, or until sufficiently coloured. Sap green (10 or 12 gr., rubbed up with a teaspoonful of hot water) is also used for the same purpose. A delicate light green.
3. (Ph. U. S.) Oil of peppermint, 2 fl. oz.; rectified spirit, 16 fl. oz.
Obs. Essence of peppermint is not conceived to be good by the ignorant unless it has a pale-greenish tint, which they take as a proof of its being genuine. The most harmless way of tinging it is that indicated above. A little green mint or parsley will, indeed, be found to improve the flavour. These additions are quite harmless. The practice of using cupreous salts, adopted by some lazy and unprincipled makers, is unpardonable, and admits of no excuse, even a lame one, as not the least advantage, either of convenience, cost, or appearance, results from such a practice, while the colouring matter, though small in quantity, is nevertheless sufficient to impart a noxious quality to the liquid. This fraud may be detected by the addition of liquor of ammonia in excess, which will strike a bluish or greenish-blue colour when copper is present.
Essence of peppermint (like that of most of the other aromatic oils) is cordial, stimulant, and stomachic. A few drops (10 to 30) on sugar, or mixed with a little water or wine, is an excellent remedy in flatulence, colic, nausea, sickness, &c. It is also extensively used as a flavouring ingredient by cooks, confectioners, and druggists. A few drops, well agitated with half a pint of cold water, form an excellent extemporaneous peppermint water.
The formulæ 1 and 2, generally the latter, are those employed by the respectable portion of the London trade. The various published receipts for this and similar essences, ordering the essential oil in a larger proportion than657 that directed above, are never adopted in practice, and their products (often impossible combinations) exist only in the imaginations of the writers.
Essence of Pimen′to. See Essence of Allspice.
Essence of Pine-apple. From pine-apple oil (butyric ether, butyrate of ethyl), as ESSENCE OF ALMONDS. It forms a delicious flavouring for liqueurs, confectionery, rum, &c. See Ether and Oil (Volatile).
Essence of Quas′sia. Syn. Essentia quassiæ, L. Prep. 1. From quassia (sliced), 11⁄2 oz.; proof spirit, 1 pint; digest 10 days, and filter; 1⁄2 fl. dr. added to 71⁄2 fl. dr. of water, forms the infusion of quassia, of the Ph. L.—Dose, 1⁄2 dr. in water or wine, an hour before a meal, as a stomachic tonic, in dyspepsia, loss of appetite, &c., particularly when complicated with gout; 1 to 2 dr., three or four times daily, as a febrifuge, and antiseptic, in intermittents, putrid fevers, &c.
2. (Brewer’s).—a. From powdered quassia (sprinkled with a little rum) and “foots” (coarse moist sugar or sugar bottoms), equal parts, reduced to the consistence of a semi-fluid extract by the addition of a few spoonfuls of water. For ale.
b. From powdered quassia, 1 part; burnt sugar colouring, 2 parts; well stirred together. For porter and stout. Both are used by fraudulent brewers as substitutes for hops.
Essence of Quin′ine. Syn. Essentia quinæ, L. Prep. From disulphate of quinine, 11⁄2 oz.; rectified spirit, 1⁄2 pint; digest with warmth, gradually dropping in a little dilute sulphuric acid (avoiding excess), and employing constant agitation until the whole is dissolved. 1 fl. dr., added to 7 dr. of proof spirit, forms the ‘TINCTURE OF QUININE’ (Ph. L.). Every fl. dr. contains 8 gr. of disulphate of quinine, or about 10 gr. of the neutral sulphate. If more sulphuric acid is added than is sufficient to dissolve the salt (i. e. convert it into a neutral sulphate), the solution is apt to deposit part of it on keeping, owing to the gradual formation of ether, by the action of the excess of acid on the alcohol.
Essence of Rat′afia. The same as Essence of Almonds. So called from being used to flavour ratafias, noyeau, and other liqueurs.
Essence of Rhu′barb. Syn. Essentia rhei, L. Prep. From rhubarb (in powder) and siliceous sand, of each 5 oz.; proof spirit, 1 pint; by the method of displacement. Every fl. oz. represents the active virtues of 2 dr. of rhubarb.
Essence of Rondele′tia. Prep. 1. Essence (oil) of bergamotte, essence (oil) of lemon, and oil of cloves, of each 1 dr.; otto of roses, 10 drops; rectified spirit, 1 pint.
2. To the last add, of oil of lavender, 1 dr.; neroli, 15 drops. A very fashionable and agreeable perfume.
Essence of Rose′mary. Syn. Essentia rosemarini, L. Prep. From oil of rosemary, as ESSENCE OF ALLSPICE. Used as a perfume; also to make extemporaneous rosemary water.
Essence of Ro′ses. Syn. Essentia rosæ (odorata), L. Prep. 1. Attar of roses (genuine), 2 dr.; alcohol, 1 pint; agitate frequently until they unite.
2. Attar of roses, 1 oz.; rectified spirit, 1 gall.; mix in a close vessel, and assist the solution by placing it in a bath of hot water. (See Essence of Musk.) As soon as the spirit gets warm, take it from the water and shake it till quite cold; the next day filter.—Obs. Unless the spirit of wine is of more than the common strength, it will not retain the whole of the otto in solution in very cold weather.
3. To each pint of either of the preceding, add, of oil of bergamotte, 30 drops; neroli and essence of musk, of each 20 drops.
4. Petals of roses, 3 lbs., digest in spirit of wine, 5 quarts, for 24 hours; distil to dryness in a water bath; digest the distilled spirit on 2 lbs. of fresh rose petals, as before, and repeat the whole process of maceration and distillation, a third, fourth, fifth, and sixth time, or oftener, the last time only drawing over 1 gall., which is the essence. Each of the above is very superior. The last has a peculiar delicacy of flavour, when the spirit used to make it is pure.
Essence of Roses (Red). Syn. Essentia rosæ (rubra), Tinctura e. concentrata, L. Prep. From rose leaves, 1 lb.; proof spirit, 1 gall.; digest for 14 days, press, strain, add concentrated acetic acid, 21⁄2 fl. dr.; mix well, and the next day filter. Used to make extemporaneous SYRUP and HONEY OF ROSES, &c. Smells, colours, and tastes strongly of the flower. Concentrated infusion of roses is sold under the same name.
Essence Royale. [Fr.] Prep. 1. (Soubeiran.) Ambergris, 40 gr.; musk, 20 gr.; civet and carbonate of potassa, of each 10 gr.; oil of cinnamon, 6 drops; oil of rhodium and otto of roses, of each 4 drops; rectified spirit of wine, 4 fl. oz. (say 1⁄4 pint); macerate for 10 days or longer. Antispasmodic and aphrodisiac. A few drops on sugar, or in syrup of capillaire.
2. See Essence of Ambergris.
Essence of Sarsaparil′la. Syn. Concentrated essence of sarsaparilla; Essentia sarsæ; E. sarsaparillæ; L. Prep. 1. Sarsaparilla root (best red Jamaica), 23⁄4 lbs., is carefully decorticated, the bark reduced to coarse powder, and digested for a week or 10 days in sherry, 3⁄4 pint, and rectified spirit, 1⁄4 pint, with frequent agitation; after which the essence is expressed, and in a week the clear portion is decanted from the sediment. A very elegant preparation. 1⁄2 fl. dr. added to 7 fl. dr. of water forms 1 fl. oz. of a solution of equal strength to decoction of sarsaparilla of the Ph. L. Every fl. oz. represents the active principles of 2 oz. (= 2 oz. 85 gr. avoir.) of sarsaparilla root. In other words, it is twice658 as strong as the root, and 16 times as strong as the decoction.
2. Alcoholic extract of sarsaparilla, 7 oz.; sherry, 3⁄4 pint; rectified spirit, 1⁄4 pint; dissolve and filter. Strength as the last.
3. (Beral.) Alcohol extract, 4 oz.; sherry wine, 1 pint; dissolve and filter. About 3 fl. dr., added to water, 1 pint, form an extemporaneous decoction.
4. (Guibourt.) Alcoholic extract, 4 oz.; white wine, 1 lb. Strength the same as Nos. 1 and 2 (nearly).
5. (Hening.) Sarsaparilla (bruised), 10 oz.; distilled water, 6 pints; macerate at a temperature of 120° Fahr. for six hours and strain; repeat with the same quantity of fresh water; mix the liquors, and evaporate in china vessels at 160° Fahr. If reduced to 10 fl. oz. (or to 9 fl. oz., with 1 fl. oz. of rectified spirit added), 1 fl. dr., mixed with 7 fl. dr. of water, will be equal to the decoction of the usual strength. If reduced to 5 fl. oz. 1 fl. dr. will be equal to 2 fl. oz. of the decoction.
6. The bark separated from sarsaparilla root, 23⁄4 lbs., is exhausted with water as last; the liquid is evaporated as quickly as possible, in a water bath, to 16 fl. oz., and when cold, mixed with rectified spirit, 4 fl. oz. Strength same as No. 1.
7. The infusion in No. 6 is evaporated to 101⁄2 fl. oz., and when cold mixed with sherry, 1⁄2 pint; in a week the clear portion is decanted from the sediment. Strength same as No. 1.
Obs. The formulæ Nos. 1, 2, 6, and 7 have each in turn been extensively employed by us in the laboratory with the most satisfactory results. See Liquor of Sarsaparilla.
Essence of Sarsaparilla (Compound). Syn. Essentia sarsaparillæ composita, E. sarsæ c., L. Prep. 1. One pint of No. 1, 2, 6, or 7 (above), is triturated with the extract prepared from mezereon bark, 31⁄4 oz., and extract of liquorice, 4 oz.; when mixed it is returned to the bottle, and essence of guaiacum, 11⁄2 fl. dr., and oil of sassafras, 20 drops, are added, the whole is then well agitated for at least 15 minutes, and after a week’s repose the clear portion is decanted as before. 1⁄2 fl. dr., with 71⁄2 fl. dr. of water, forms extemporaneous compound decoction of sarsaparilla.
2. (Cadet.) Sarsaparilla (bruised), 8 oz.; hot water, q. s.; exhaust the root by successive macerations; unite the liquors, and evaporate to 10 fl. oz.; strain, and add, when cold, of alcohol (·842) and tinctures of guaiacum and mezereon, of each 4 fl. dr.; white wine, 1 fl. oz.; oil of sassafras, 12 drops; extract of liquorice, 2 dr.; agitate, and after repose decant as before. This is nearly 8 times as strong as ‘DEC. SARSÆ CO.’—Ph. L. The first is the best formula. See Liquor of Sarsaparilla (Compound).
Essence of Sa′′vory Spices. Prep. 1. Black pepper, 4 oz.; powdered turmeric, 3 dr.; coriander seeds, 11⁄2 dr. (all ground and genuine); oil of pimento, 11⁄2 fl. dr.; oils of nutmeg, cloves, cassia and caraway, of each 1⁄2 dr.; rectified spirit, 1 pint; digest, with agitation, for a fortnight. Very fine.
2. Black pepper, 3 oz.; allspice, 11⁄4 oz.; nutmegs and burnt sugar, of each 1⁄2 oz.; cloves, cassia, coriander, and caraway seeds, of each 1 dr. (all bruised or ground); rectified spirit, 1 pint; digest with agitation, as before, for 14 days, press, and filter. Used as a flavouring. When made with proof spirit or brandy, and only 1⁄2 the above weight of spice, it is called ‘TINCTURE OF SAVORY SPICES,’
Essence of Sen′na. See Liquor and Infusion (Concentrated).
Essence of Smoke. See Essence, Westphalian.
Essence of Soap. Syn. Spirit of soap, Shaving fluid; Esprit de savon, Essence de savon, Essence royale pour faire la barbe, Fr.; Essentia saponis, Tinctura saponis concentrata, L. Prep. 1. Castile soap (in shavings), 4 oz.; proof spirit, 1 pint; dissolve, and add a little perfume.
2. Venetian soap, 3⁄4 lb.; salt of tartar, 1 oz.; benzoin, 1⁄2 oz.; spirit of wine, 1 gall.
3. Best soft soap, 1⁄4 lb.; boiling water, 1 pint; dissolve, cool, and add, oils of cinnamon (cassia), verbena, and neroli, of each 6 drops; dissolved in rectified spirit, 1 pint; mix well, and if not perfectly transparent, add a little more strong spirit, or filter through blotting paper.
Obs. This alcoholic solution of soap is chiefly used for shaving, and is very convenient in travelling, as a good lather may be instantly produced without the trouble of employing a soap-box. Instead of the above perfumes, 15 drops of essence of musk or ambergris, or 30 drops of any of the perfumed spirits, or 3 drops of attar of roses, or 6 drops of any of the aromatic essential oils, may be added, when a corresponding name is given to the preparation, as esprit de savon, de la rose, &c.
4. (P. Cod.) White soap, 3 oz.; carbonate of potassa, 1 dr.; proof spirit, 12 oz.; dissolve. Used medicinally. They are all used as frictions, &c.
5. (Camphorated,—Guibourt.) White soap, 3 parts; camphor, 1 part; spirit of rosemary, 16 parts; dissolve. A variety of opodeldoc. Used as an embrocation in rheumatic pains, sore throat, &c.
Essence of Soup Herbs. Syn. Spirit of soup herbs, Conc. tincture of s. h., &c. Prep. (Kitchener’s.) Lemon thyme, winter savory, sweet marjoram, and sweet bazil, of each 1 oz.; lemon peel (grated), and eschalots, of each 1⁄2 oz.; bruised celery seed, 1⁄4 oz.; proof spirit or brandy, 1 pint; digest for 10 days or a fortnight. A superior flavouring essence for soups, gravies, &c. See Essence of Savoury Spices.
Essence of Spear′mint. See Essence of Mint.
Essence of Sprats. Syn. Essence of British anchovies. From pickled sprats659 (British anchovies), as Essence of anchovies, for which it is commonly sold.
Essence of Spruce. Syn. Fluid extract of spruce; Essentia abietis, Extractum a. fluidum, L. Prep. A decoction of the young tops of the black spruce-fir Abies nigra, evaporated to the consistence of a thick syrup. Used to make spruce beer, &c.
Essence, Toothache. Syn. Essentia odontalgia, L. Prep. 1. Acetate of morphia, 1⁄2 dr.; tincture of pellitory of Spain (made with rectified spirit), 2 fl. oz.; acetic acid (glacial), 4 fl. dr.; dissolve, and add of oil of cloves, 6 fl. dr.
2. (Redwood.) Pellitory, 1⁄2 lb.; extract of belladonna, 2 dr.; rectified spirit, 1 pint; digest 14 days, strain, and add, of hyponitrous ether, 1 oz.; oil of wine, 1⁄2 oz.; oil of cloves, 2 dr. See Drops (Odontalgic).
Essence of Tu′′berose. Prep. The flowers are stratified with sheep’s or cotton wool, impregnated with the purest oil of ben or of olives, in an earthen vessel, closely covered, and kept for 12 hours in a water bath; the flowers are then removed, and fresh ones substituted, and this is repeated until the oil (HUILE AU TUBEROSE) is sufficiently scented. The wool or cotton is then mixed with the purest spirit of wine, and distilled in a water bath; or it is first digested in a warm situation, and in a well-closed vessel, for several days, during the whole of which time frequent agitation is had recourse to. A similar plan is followed for the preparation of essences of jasmine, violets, and other like flowers. See Spirit.
Essence of Turtle. Syn. Essence of green turtle. Prep. From essence of anchovies and shallot wine, of each 3 oz.; basil wine, 1⁄2 pint; mushroom ketchup, 1⁄4 pint; the juice of 2 lemons; the yellow peel of 1 lemon; curry powder, 1⁄4 oz.; digest for a week. Used to impart the flavour of turtle to soups and gravies.
Essence of Tyre. See Hair Dye.
Essence of Vanil′la. Syn. Essentia vanillæ, Tincture v. concentrata, L. Prep. 1. Vanilla (cut small), 2 oz.; rectified spirit, 1 pint, digest a fortnight.
2. (Wholesale.) Vanilla, 2 lbs.; rectified spirit, 1 gall.; proceed as for ESSENCE OF MUSK. Very superior.
3. Vanilla (best), 3⁄4 lb.; spirit of ambrette, 1 quart; cloves, 30 gr.; grain musk, 7 gr.; as last. Much esteemed. It is chiefly used as a perfume and for flavouring.
Essence of Verbe′na. Syn. Essence of lemon-grass, E. of citronelle; Essentia verbenæ, L. Prep. 1. From oil of lemon-grass or verbena (Andropogon citratum), as ESSENCE OF ALLSPICE.
2. To the last add, of essences of ambergris and bergamotte (oil), of each 1 fl. dr.; neroli, 1⁄2 fl. dr.
3. To No. 1. add, of oils of lavender and bergamotte, of each 1⁄2 dr.; essence of vanilla, 2 fl. dr. A powerful and refreshing perfume.
Essence of Vio′let. Syn. Essentia violæ, L.; Essences des violettes, Fr. See Essence of Tuberose and Spirit.
Essence of Vittie Vayr. Syn. Essence of vetiver; Essence de vittie vayr double, Fr. Prep. 1. Vittie vayr or cuscus (the root of Andropogon muricatus, cut small and bruised), 3 lbs.; proof spirit, 9 pints; digest a week, add of water, 5 pints, and the next day distil over 1 gall. of essence.
2. To the last, before distillation, add, of otto of roses, 1⁄2 dr.; eau de melisse (spirit of balm), 1⁄2 pint; and proceed as before. Used as a perfume. In 1831 it was much employed in Paris as a prophylactic of cholera.
Essence, Volatile (Acetic). Syn. Pungent acetic essence; Essentia volatilis acetica, L. Aromatic vinegar.
Essence, Volatile (Ammoniacal). Syn. Pungent ammoniacal essence, Aromatic ammoniacal e.; Essentia volatilis, E. v. ammoniacalis, E. v. aromatica, &c., L. Prep. 1. Oil of cinnamon, 6 drops; otto of roses, 12 drops; oil of cloves, 1 fl. dr.; essence of bergamotte, 2 fl. dr.; oil of lavender (Mitcham), 4 fl. dr.; essence of musk, 5 fl. dr.; liquor of ammonia (strongest), 1 pint; mix in a cold place, and shake the bottle until the whole is combined.
2. Essence of violets and oil of cinnamon, of each 12 drops; neroli, essence of jasmine, and otto of roses, of each 1⁄2 dr.; oil of lavender, 1 dr.; essence royale and essence (oil) of bergamotte, of each 21⁄2 dr.; liquor of ammonia (strongest), 1 pint; as the last.
3. Oils of lemon and bergamotte, of each 5 fl. dr.; oil of lavender, 11⁄2 fl. dr.; otto of roses, 1 fl. dr.; oils of cassia, neroli, cloves, and cedrat, of each 1⁄2 fl. dr.; oil of sandal wood, 15 drops; liquor of ammonia (strongest), 1 pint.
4. Essence of bergamotte, 6 fl. dr.; oil of lavender, 4 fl. dr.; oil of cloves, 3 fl. dr.; oil of cassia, 11⁄2 fl. dr.; oil of verbena (lemon-grass), 1 fl. dr.; otto of roses, 30 drops; liquor of ammonia, 18 fl. oz.
5. (Redwood.) Oil of bergamotte, 3 oz.; essence of lemons, 2 oz.; oil of lavender, 6 dr.; essence of jasmine, 4 dr.; oil of sassafras, 3 dr.; oil of neroli, 2 dr.; otto of roses, 11⁄2 dr.; oil of origanum and essence of ambergris, of each 1 dr.; musk, 20 gr.; macerate for a week, and decant the clear portion. It is added to the strongest liquor of ammonia in proportion of 11⁄2 oz. to the pint.
Obs. The above are used to fill smelling-bottles. They are all very fragrant and refreshing.
Essence, Ward’s. See Essence Headache.
Essence of Water-fen′nel. Syn. Essentia phellandri aquatici, E. fœniculis a., L. Prep. (Cottereau.) Water-fennel seeds (fine-leaved water-hemlock, bruised), 1 oz.; proof spirit, 4 fl. oz.; digest. Narcotic and pectoral.—Dose, 5 to 25 drops, combined with bark; in phthisis, &c.
Essence, Westphalian. Essence of smoke,660 E. of wood-smoke, Cambrian essence, Smoking fluid; Essentia fuliginis, &c., L. Prep. 1. Crude or empyreumatic pyroligneous acid, 1 pint; sugar colouring, 2 oz.; dissolve, and in a week decant the clear portion.
2. Tar, 3 dr.; sugar colouring, 2 oz.; hot crude pyroligneous acid, 1 pint; agitate constantly for 1 hour, and after repose decant the clear portion.
3. Acetic acid (Ph. L.), 1 pint; creasote, 5 dr.; mix. White.
4. Barbadoes tar, 1⁄4 oz.; burnt sugar and common salt, of each 1 oz.; strong pickling vinegar, 3⁄4 pint; port or elder wine, 1⁄4 pint; digest as before. Inferior to the preceding. Used to impart a smoky flavour to meat, fish, &c., by brushing it over them, or adding a little to the brine in which they are pickled.
Essence of Worm′wood. Syn. Essentia amara, E. Absinthii, L. Prep. 1. Extract of wormwood, 4 oz.; oil of wormwood, 1 oz.; rectified spirit, 1 pint; digest a week and filter. Tonic, stomachic, and vermifuge.—Dose, 10 drops to a teaspoonful.
2. (Van Mons.) Tincture of wormwood, 1 pint; salt of wormwood, 5 dr.; extract of wormwood, 1 dr.; digest as before.—Dose, 1⁄2 to 11⁄2 fl. dr.
Essences, Fla′′vouring. Syn. Culinary essences, Spice e., Essences for the table, &c. Those used by cooks, confectioners, liqueurists, &c., are all made by either dissolving 1 fl. oz. of the essential oil of the particular substance in 1 pint of rectified spirit, or by digesting 4 to 6 oz. of the bruised spice, or 5 to 10 oz. of the dried herb, in a like quantity (1 pint) of spirit. The first method is preferable, from being the least troublesome, and yielding the finest product. They are commonly labelled ‘CONCENTRATED ESSENCE OF ——.’ An inferior article, vended under the names of ‘ESSENCES OF CULINARY HERBS,’ ‘CULINARY TINCTURES,’ ‘TINCTURES FOR KITCHEN USE,’ &c., are prepared from half the above quantity of oil or spice, infused in a pint of proof spirit or British brandy. The principal compounds of this class are the essences of allspice, caraway, cardamoms, cassia, cayenne, celery seed, cinnamon, cloves, coriander seed, fennel, garlic, ginger, lemon peel, mace, marjoram, nutmegs, orange peel, peppermint, spearmint, sweet basil, and the like. The whole of these are employed to flavour soups, gravies, sweetmeats, pastry, wines, mulled wines, liqueurs, &c.
Essences, Flower. Those for which separate formulæ are not given in this work may most of them be made from the essential oil of the flowers and rectified spirit, as the last; or by digesting the flowers (crushed or bruised), 3 to 5 lbs., in proof spirit, 2 galls., for a few days, and then drawing over, by distillation, 1 gall. For the essences of those flowers which are not strongly odorous, the spirit thus obtained is distilled from a like quantity of flowers, a second, and a third time, or even oftener. The essences of other organic substances, whose fragrant principles are volatile, may be prepared in the same manner. A small quantity of some other odorous essence is frequently added to the product, to enrich or modify the fragrance. See Flowers and Essences by Infusion.
Essences, Fra′grant. See Flower essences (above), Essentia Odorata, Perfumery, &c.
Essences, Fruit. See Essences of apple, Pine-apple, Jargonelle, &c.
Essences by Infu′sion. This term, among perfumers, is commonly applied to those essences, eaux, and esprits, which are prepared by digesting the ingredients in the spirit used as the vehicle for the aroma, in opposition to those obtained by ‘distillation,’ or by ‘contact,’ or ‘pressure.’ Thus, the ESSENCES OF AMBERGRIS, MUSK, and VANILLA, are of this class.
Essences, Vi′nous. Syn. Essentia vinosa, L. These are prepared in a similar way to the wines (VINA) of the pharmacopœia, by using 8 times the usual quantity of ingredients, and the very strongest sherry wine. 1 fl. dr., added to 7 fl. dr. of wine or water (properly the first only), forms an extemporaneous imitation of the officinal VINA MEDICATA. Some of the above are largely used in dispensing, and by travellers. See Liquor and WINE.
ESSENTIA BI′NA. See Colouring.
Essentia, Odora′ta. Prep. 1. Oil of lavender, 1 dr.; oils of cloves, cassia, and bergamot, of each 1⁄2 dr.; neroli, 20 drops; essence royale, 2 fl. dr.; rectified spirit, 1⁄2 pint; mix.
2. (Redwood.) English oil of lavender, 48 drops; oil of cloves, 32 drops; oil of orange peel, 16 drops; oil of bergamotte and sweet spirit of nitre, of each 8 drops; oil of yellow sandal-wood, neroli, and otto of roses, of each 2 drops; oil of cinnamon, 1 drop; rectified spirit, and essence of ambergris and musk, of each 1 oz.; honey water, 8 oz.; mix. Used as a perfume for the handkerchief, &c. The last form seems unnecessarily complicated and minute.
Essentia Odorif′era. Prep. 1. Grain musk and balsam of Peru, of each 10 gr.; civet, 4 gr.; oil of cloves, 5 drops; oil of rhodium, 3 drops; salt of tartar (dried by a dull-red heat and cooled), 1⁄2 dr.; rectified spirit (strongest), 21⁄2 fl. oz.; macerate for 14 days, and pour off the clear.
2. Oil of rhodium and balsam of Peru, of each 1⁄2 dr.; oil of cloves, 1 dr.; spirit of ammonia, 2 fl. dr.; essence of civet and vanilla, of each 2 fl. oz.; essence of musk, 5 fl. oz.; neroli, oils of lavender, verbena, and cassia, of each 6 drops. As last. Both are very pleasant, durable, and powerful perfumes for personal use.
ESSENTIAL OIL. See Oil (Volatile).
ESSENTIAL SALT OF BARK. See Bark and Extract.
ESSENTIAL SALT OF LEMONS. Syn. Salt of lemons; Sal limonum, L. The preparation sold under this name is made by661 mixing cream of tartar (bitartrate of potassa) with twice its weight of salt of sorrel (quadroxalate of potassa), both in fine powder. It is used to remove fruit stains, &c., from linen, by rubbing a little of it on the part moistened with warm water. It is poisonous, if swallowed in quantity.
ETCH′ING. A species of engraving, in which the design is formed on the plate by the action of an acid, or some other fluid, instead of being cut out by the graver.
Proc. In the ORDINARY PROCESS OF ETCHING the plate is covered with ‘etching ground’ (an acid-resisting varnish), and the design is scratched on the metal through the ground, by means of a pointed tool of steel called the ‘etching needle’ or ‘point.’ A border of wax is then placed round the plate, and the ‘biting’ fluid poured on, and allowed to remain till the ‘lights’ or finest portions of the design are sufficiently ‘bitten in,’ The etching fluid is then poured off, the plate washed, and the light parts ‘stopped out’ with Brunswick black or other varnish; the solvent is again poured on, and allowed to remain until the finest portion of the exposed lines are sufficiently deep, when the acid is again poured off, and the whole process is repeated till the very darkest lines or shadows are sufficiently ‘bitten in,’ The plate is then cleaned, and is ready to be printed from. Occasionally the etched design receives a few finishing touches with the ‘graver.’
There are several varieties of etching, of which the following are the principal:—Etching with a soft ground, when a coating of lard or tallow is employed, and the design is drawn on a piece of paper, laid evenly on the ground, by which means the fatty matter adheres to the paper, on the parts pressed on by the point or pencil, and the copper beneath becomes exposed, and is then acted on by the acid. The effect resembles that of chalk or pencil drawings.—Stippling, or executing the design in dots instead of lines.—Aquatinta or AQUATINT, a mode of etching on copper for producing an effect resembling a drawing in Indian ink. It is performed by sifting powdered asphaltum or lac resin on the plate, previously slightly greased, and, after shaking off the loose powder, gently heating it over a chafing dish; on cooling, the lights are covered with turpentine varnish coloured with lampblack, by means of a hair pencil, and a rim of wax being placed round the plate, a mixture of ‘aquafortis’ and water is poured on it, and allowed to remain for 5 or 6 minutes, when it is poured off, the plate dried, and recourse had to the pencil as before. The process of ‘stopping’ and ‘etching’ is repeated again and again, until the darkest shades are produced. Sometimes, instead of using asphaltum, an alcoholic solution of shell-lac or gum mastic is poured over the plate, placed in a slanting direction; this varnish forms a film, which, on drying, leaves innumerable cracks or minute fissures through which the acid acts on the plate. The fineness or coarseness of the grain depends entirely upon the condition of the powdered asphaltum, or on the quantity of matter dissolved in the spirit employed to form the ground.
The fluids employed for ‘biting in’ the designs vary considerably, almost every artist having his own receipt. Aquafortis, more or less diluted, is, however, generally employed for COPPER, and this, with the addition of pyroligneous acid, for etching on STEEL; but any fluid that rapidly dissolves the metal may be used for the purpose. The ‘etching ground’ may be formed of any substance capable of resisting the action of the etching fluid, and which is, at the same time, sufficiently soft to allow of the free use of the needle or point, and sufficiently solid to prevent an injury to the design during the ‘scratching in,’
In ETCHING ON GLASS, the ground is laid on, and the design ‘scratched in’ in the usual way, when liquid hydrofluoric acid is applied, or the glass is exposed to the action of hydrofluoric acid gas. The former renders the surface of the etching transparent, the latter opaque. A simple modification of the process is to wet the design with sulphuric acid, and then to sprinkle on some finely pulverised fluor spar (fluoride of calcium), by which means hydrofluoric acid is set free and attacks the glass. This method may be very easily applied to the graduation of glass vessels, thermometer tubes, &c.
Etching on Glass by Electricity. Planté (‘Ann. Chem. Phys.’ [5], xiii, 143-144). The author had previously drawn attention to the fact that when an electric current is passed through saline solutions in glass vessels, platinum wire serving as electrodes, the glass is immediately attacked, and he therefore proposes the following method for etching on glass:
The surface of the glass to be engraved is coated with a concentrated solution of potassium nitrate, and beneath the layer of liquid a platinum wire, connected with one of the poles of a battery, is stretched across the plate. With the other pole is connected another platinum wire, the whole of which, except the point, is insulated; with this the designs are drawn on the glass, which is engraved wherever the wire comes in contact with it, flashes of light being emitted at the same time.
The depth of engraving depends on the rate at which the platinum wire moves; the slower the rate the deeper the line.
A rapid method of etching on iron or steel, capable of very general application, is as follows:—“The metal is warmed until it is capable of melting a piece of beeswax, or ‘etching ground,’ which is then carefully rubbed over it, so as to form a thin and even coating; when cold, the design is ‘scratched in’ in the common way; a little powdered662 iodine is then sprinkled on the exposed parts, and at the same time a few drops of water are added, and the two worked into a liquid paste with a camel-hair pencil. The paste is then moved about over the intended etching, for a period varying from one to five minutes, according to the depth of the lines required to be produced. Afterwards the whole is removed, and reapplied, &c., as with the usual etching fluids. The same etching-paste, by being kept for a few days, again acquires the property of dissolving iron, and may be used again and again; but independently of this, the iodide of iron formed during the process, if rapidly evaporated to dryness in a clean iron vessel by a moderate heat, and placed in stoppered bottles, will sell for more than the original cost of the iodine. To travellers and amateurs who amuse themselves with the delightful art of etching, iodine, from its portability and convenience, will, doubtless, prove invaluable. We have adopted it with considerable success, and have found it especially useful in marking surgical instruments, razors, and other edge tools. We published this method many years ago. Several parties have since availed themselves of our suggestions and formulæ, but without the slightest acknowledgment of the source from which they obtained them.” (A. J. Cooley.)
Etching, Electro-. This mode of etching, which is in many respects superior to the ordinary mode, is based upon the destructive action of certain ‘anions’ during ‘electrolysis.’[281] If two plates of copper be connected with the opposite ends of a voltaic battery, and placed in a vessel containing very dilute sulphuric acid, the plate connected with the copper of the battery will be attacked by the anion oxygen which is released during the decomposition of the acid. This destructive action can be localised at pleasure by covering certain parts of the plate with a protecting stratum of varnish, ordinary ‘etching ground’ for instance. In the practice of electro-etching, the drawing is ‘scratched in’ in the usual way through an ordinary ground; a stout wire is then soldered to the plate, and this, as well as the back of the plate, is coated with sealing-wax varnish. Thus prepared, the plate is placed in a suitable ‘decomposition cell’ opposite a plate of somewhat similar size, and the two are connected respectively with the copper and zinc of a ‘Daniell’s cell,’ or the silver and zinc of a ‘Smee’s cell.’[282] After about ten minutes the plate is removed, washed, and dried; and when the ‘fine work’ has been stopped out with Brunswick black, it is returned for another space of ten minutes. By alternately exposing the plate to the action of the decomposing fluid, and ‘stopping out’ parts of the work, the required gradation in tints is obtained. The exact duration of the various exposures, as well as their number, must, of course, be regulated by circumstances. See Etching Fluids (below).
[281] See Electrolysis and Electrotype, pages 428 and 429.
[282] See Voltaic Electricity.
Etching Fluids. 1. (For COPPER.)—a. From ‘aquafortis,’ 21⁄2 fl. oz.; water, 5 fl. oz.; mix.
b. To the last add of verdigris, 1 oz.; water, 21⁄2 fl. oz.; dissolve. For light touches.
c. (Eau forte,—Callot and Piranesi.) Alum, sal-ammoniac, sea salt, and verdigris, of each 4 oz.; vinegar (pyroligneous acid), 8 fl. oz.; water, 16 fl. oz.; mix, dissolve, boil for 1 or 2 minutes in a glazed or stoneware vessel, cool, and decant the clear portion. Used as the last.
d. Water acidulated with sulphuric acid. Used in the process of electro-etching.
2. (For STEEL.)—a. From iodine, 1 oz.; iron filings or wire, 1⁄2 dr.; water, 4 fl. oz. It must be kept in a stoppered bottle, until required for use.
b. From iodine, 3 dr.; iodide of potassium, 1 dr.; proof spirit, 1 fl. oz.; water, 2 fl. oz. As the last.
c. (Mr Turrel.) Pyroligneous acid, 4 fl. oz.; alcohol (rectified spirit), 1 fl. oz.; mix, and add of nitric acid or double aquafortis (sp. gr. 1·28), 1 fl. oz.
d. From hydrochloric acid, 5 parts; water, 95 parts; mix, and add the liquid to a solution of chlorate of potassa, 1 part, in water, 50 parts.
e. A solution of common salt. Used in the process of electro-etching.
Etching Ground. Syn. Etching varnish. Prep. 1. Beeswax, 5 parts; linseed oil, 1 part; melted together.
2. (Callot’s Hard varnish, Florentine v., Florence v.) From linseed oil and mastic, equal parts, melted together.
3. (Callot’s Soft varnish.) From linseed oil, 4 oz.; gum benzoin and white wax, of each 1⁄2 oz.; boil to two thirds.
4. (Lawrence.) White wax, 2 oz.; black pitch and Burgundy pitch, of each 1⁄2 oz.; melt, add by degrees, of powdered asphaltum, 2 oz.; and boil together, until a piece, when thoroughly cold, will break by being bent double 2 or 3 times between the fingers; next pour it into warm water, make it into small balls, and place each of them in a piece of taffety for use.
Obs. The preceding compositions are applied to the surface of the plates, previously made sufficiently warm to melt them easily, their even diffusion being promoted by dabbing them with a wad of cotton. Those that are white are then generally blackened on the surface by skilfully passing them over the smoky flame of one or more candles, by which the marks of the etching point on the bright metal are rendered the more visible.
E′THER. Syn. Oxide of ethyl. Described under Ethyl, Oxide of. Several substances are known under the name of ethers besides the true ethers or salts of ethyl, and are given below.
663
Ether of Canthar′ides. Syn. Æther cantharidalis, L. Prep. (Œttinger.) From powdered cantharides, 1 part; ether, 2 parts; digested together for 3 or 4 days, and the tincture expressed. Used as a vesicant, &c.
Ether, Chlo′′ric. This name was applied by Dr T. Thomson to the CHLORIDE OF OLEFIANT GAS, or ‘Dutch liquid,’ and afterwards, by Guthrie and Silliman, to CHLOROFORM, which they took for an alcoholic solution of chloride of olefiant gas. It now forms one of the synonyms of chloroform. The medicinal ‘CHLORIC ETHER’ of the shops is a solution of chloroform, 1 part, in rectified spirit 8 parts; of which the dose is 20 or 30 drops in water, as an antispasmodic and anodyne. See Chloroform.
Ether, Chlorinet′ted. Formed by the action of dry chlorine on pure ether. When the action is long continued, a heavy, oily product (BICHLORINETTED ETHER), smelling like fennel, is formed. By the still further action of chlorine, aided by sunlight, a white, crystalline substance (PENTACHLORINETTED ETHER), a compound resembling sesquichloride of carbon, is obtained.
Ether, Cu′′preous. Syn. Tinctura cupri chloridi ætherea, L. Prep. (Van Mons.) Sulphate of copper, 6 parts, and chloride of barium, 5 parts, are triturated together, and the mixture digested in ether, 3 or 4 parts, until all the chloride of copper is dissolved.—Dose, 2 to 5 drops; in epilepsy, &c.
Ether, Methy′lic. Syn. Oxide of methyl, Wood-ether, Methyl-ether; Æther methylicus, L. Prep. From wood spirit, 1 part; concentrated sulphuric acid, 4 parts; mix in a retort, apply heat, pass the evolved gas (methylic ether) through a little strong solution of potassa, and then collect it over mercury. See Methyl.
Ether, Spirits of Nitrous. See Spirits.
Ether, Washed. Syn. Æther lotus, L. Ordinary ether, agitated first with 2 or 3 times its volume of distilled water, and a few grains of carbonate of potassa, or a few drops of milk of lime; and after decantation, again agitated with a like quantity of water only. Used for inhalations. For other purposes the washed ether is afterwards digested on chloride of calcium, to deprive it of retained water.
E′THERIN. Syn. Camphor of oil of wine. A volatile, white, crystalline substance, deposited by light oil of wine when left in a cold situation for some time. It is isomeric with etherole, and received its name from the assumption of its being the base of the ethereal compounds. According to this hypothesis, ether is a hydrate of etherin. Etherin forms brilliant prisms and plates; is tasteless; soluble in alcohol and ether; fuses at 230° Fahr., and boils at 500° Fahr.; and is a little lighter than water. The crystals are purified by pressure between the folds of bibulous paper, solution in ether, and evaporation.
E′THEROLE. The yellowish, oily liquid, forming the residual portion of light oil of wine, after it has deposited its etherin. It is lighter than water; is freely soluble in both alcohol and ether; and has a rather high boiling-point. See Etherin and Oil of Wine.
ETHION′IC AC′ID. Prep. An alcoholic solution of the crystals of sulphate of carbyle is diluted with water, the whole neutralised with carbonate of baryta, the filtered liquid evaporated by a very gentle heat to a small bulk, and a large quantity of alcohol added; the precipitate (ethionate of baryta) is treated (cautiously) with dilute sulphuric acid (avoiding excess), by which the baryta is withdrawn, and ethionic acid left in solution.
Prop., &c. Ethionic acid closely resembles sulphovinic acid. It is decomposed by heat. Its salts (ethionates), however, differ completely from the sulphovinates. They are all soluble in water, and are said to be anhydrous. The ethionates of ammonia, potassa, and soda crystallise readily; those of lead, baryta, lime, and the other earths are uncrystallisable. See Isethionic Acid, and below.
ETHION′IC ANHY′DRIDE. Prep. Pure and dry olefiant gas is passed over anhydrous sulphuric acid (‘sulphuric anhydride’) contained in a U-shaped tube.
Prop., &c. When thus produced, it is in white, milky crystals, which speedily deliquesce in the air, giving rise to ethionic acid. It is similar in appearance, and probably identical with, ‘sulphate of carbyle,’ which results from the absorption of the vapour of anhydrous sulphuric acid by absolute alcohol.
E′THIOPS. Syn. Æthiops, L. A name given by the older chemists to several black powders on account of their colour, and still occasionally employed in medical works.
Ethiops, Graphi′tic. Syn. Ethiops of plumbago; Æthiops graphiticus, L. From plumbago, 2 parts; quicksilver, 1 part; triturated together until the globules disappear.—Dose, 5 to 10 gr.; in herpes, and some other obstinate skin diseases.
Ethiops, Martial. Black oxide of iron, prepared by keeping iron filings under water, and occasionally shaking them. It is washed with water, dried as quickly as possible, and preserved from the air, to prevent further oxidation. Formerly much esteemed as a tonic.
Ethiops, Min′eral. Syn. Ethiops mineral; Æthiops mineralis, Hydrargyri sulphuretum cum sulphure, L. Black sulphuret of mercury, with excess of sulphur.
(Tyson’s.) Oxide of mercury (prepared by decomposing calomel with an equivalent proportion of liquor of potassa to which a little liquor of ammonia has been added) and flowers of sulphur, equal parts, triturated together. This is recommended as an efficient substitute for the old and uncertain preparation commonly sold under the name of Ethiops mineral. It is, however, of more than double664 the usual strength, and should therefore be taken in proportionate doses. See Mercury (Sulphide).
Ethiops, Veg′etable. Syn. Æthiops vegetabilis, Pulvus quercûs marinæ, L. Bladder wrack (Fucus vesiculosus), burned in a close vessel till it becomes black and friable. Used in bronchocele, scrofula, &c. Like burnt sponge, it owes its virtues to the presence of a very minute quantity of iodine.—Dose, 20 gr. to 1 dr., or more, made into an electuary with honey or sugar.
E′THYL. C2H5. Syn. Ethyle. The hydrocarbon assumed to be the radical of the ether-compounds (ethyl-series). A body containing carbon and hydrogen in the proportions indicated by the formula of ethyl, 2(C2H5), has been obtained by exposing dry iodide of ethyl in sealed tubes for several hours to the action of finely divided zinc, at a temperature of from 320° to 338° Fahr. In this reaction the iodine of the iodide of ethyl combines with the zinc, and the hydrocarbon supposed to be ethyl is set free. On opening the sealed tubes and allowing the gas (which is a mixture of the ‘ethyl’ and certain secondary products) to pass into a freezing mixture, the temperature of which is kept below -9° Fahr., the ‘ethyl’ condenses to a colourless, mobile liquid. Hitherto no compound ether has been produced from the ‘ethyl’ thus prepared.
According to the beautiful theory of Liebig, ethyl is a ‘salt-basyle,’ forming ‘haloid salts’ with chlorine, iodine, and bromine; its oxide is ether, and the hydrate of this oxide alcohol. The compound ethers may be compared with ordinary salts in which the metal is replaced by the radical ethyl.
Ethyl, Oxide of. (C2H5)2O. Syn. Ether, Sulphuric ether, Æther (B. P.), Æther sulphuricus (Ph. E. D. & U. S.), Æ. rectificatus, Æ. vitriolicus, Æ. spiritus vitrioli dulois, L. A colourless, highly volatile, fragrant, inflammable liquid, obtained by distilling a mixture of sulphuric acid and alcohol. It was not known before the 13th century.
Prep. There are two methods employed for the preparation of ether. The one is by mixing the whole of the ingredients at once, and immediately subjecting them to distillation at a proper temperature; the other is by adding the alcohol in a slender streamlet to the acid, previously heated to the etherifying point. The former, though less economical, is the one more generally employed.
1. Rectified spirit, 3 lbs.; sulphuric acid, 2 lbs.; carbonate of potassa (previously ignited), 1 oz.; pour 2 lbs. of the spirit into a glass retort, add the acid, and place the vessel on a sand bath, so that the liquor may boil as quickly as possible, and the ether, as it forms, pass over into a well-cooled receiver; continue the distillation until a heavier fluid begins to pass over, then lower the heat, add the remainder of the spirit, and distil as before; mix the distilled liquors together, pour off the supernatant portion, add the carbonate of potassa, and agitate occasionally for one hour; finally, distil the ether from a large retort, and keep it in a well-stoppered bottle. Sp. gr. ·750.
2. The strongest oil of vitriol, 3 parts, are mixed with alcohol, q. s. (about 2 parts at ·830) to reduce its sp. gr. to 1·780; an object which may be easily obtained by distilling off some of the ether if required. The still or retort is then connected with a vessel full of alcohol, of at least 90%, by means of a small syphon tube, furnished with a stop-cock; the longer limb of which should be of glass, and so arranged that it just dips into the mixture of acid and alcohol. Heat is next applied, and the contents of the still raised to the boiling-point as rapidly as possible, and as soon as full ebullition commences the stop-cock of the syphon is cautiously turned, so as to allow the alcohol to flow down in such a manner as to keep the boiling liquid exactly at the same level; or, in other words, to supply a quantity of alcohol exactly equal to that of the liquid which distils over. By careful manipulation the whole of the alcohol which enters the retort passes over as ether and water, and this decomposition proceeds for some time, and would continue for an unlimited period did not the sulphuric acid ultimately become too weak to form ether, from the gradual absorption of the superfluous water contained in the alcohol. Were it convenient or practicable to use absolute alcohol, a given weight of sulphuric acid of the proper strength, would maintain the power of producing ether for an indefinite period. In practice, the quantity of alcohol that may thus be etherified is twice or thrice as much as by the common process, while neither sulphurous acid, sulphovinic acid, nor sweet oil of wine is generated, and the residual liquid of the distillation continues limpid, and has only a pale-brown colour. This is termed the ‘continuous,’ or ‘Boullay’s’ method. (This process is similar to that given in the B. P.)
3. Alcohol of 90%, five parts are mixed with oil of vitriol, 9 parts, in a vessel of copper or iron immersed in cold water; the mixture is next introduced into a still or retort, and raised to a state of ebullition as rapidly as possible, as before. A fresh quantity of alcohol, equal in bulk to the liquid distilled over, is then added to the liquid in the still, and distillation again had recourse to. As much concentrated alcoholic solution of potassa as will give it a perceptible alkaline reaction is next added to distilled liquor, which is then rectified by the heat of a water bath, as long as the ether which distils over has the sp. gr. ·720 to ·725 at 80° Fahr. Instead of the potassa, a little milk of lime may be used, along with its own bulk of water. By allowing the product to stand for some days over chloride of calcium or quicklime, and again rectifying it along with one of these substances, perfectly pure ether may be obtained.
Obs. The mixture of alcohol with sulphuric665 acid requires some caution. It is best done by introducing the alcohol into a suitable vessel, and imparting to it a rapid whirling motion, by which a considerable conical cavity is formed in the centre, and into which the acid may be gradually poured with perfect safety. The mixed fluids should be brought to a state of rapid ebullition, as quickly as possible, as without this precaution much of the alcohol distils over before the liquor acquires the proper temperature for etherification. On the small scale, a tubulated retort, connected with a Liebig’s condensing tube, and two globular receivers surrounded with a freezing mixture, or ice-cold water, may be employed as the distillatory apparatus. The second receiver should be connected with the first one by means of a bent glass tube, reaching nearly to the bottom of the former; and the whole of the joints should be securely luted, as soon as the expanded air has been allowed to escape. We have employed the following convenient little apparatus for the preparation of small quantities of ether, and it will be found very suitable for the distillation of most other highly volatile liquids, and particularly for boiling mixtures of alcohol and organic acids. By connecting the neck of a flask or digester containing volatile fluids with the lower instead of the upper end of the refrigerator, ebullition may be carried on without loss, as the vapour will be condensed, and run back into the vessel from which it has distilled.
a. Condenser tube.
b, c Glass tube.
d. Funnel by which cold water runs in from the water bottle h.
e. Pipe by which water escapes through f into the bottle g.
i. Retort.
k. Adapter, connecting the retort with the condenser.
l. Adapter, connecting the condenser with the bottles t, t.
A. Wooden tressel, with movable arms n, o, for supporting and adjusting the heights of the condenser.
B. Wooden stool for supporting the water bottle.
q. Furnace.
r. Support for the furnace.
p. Gutter for carrying off water that overflows the funnel d, and preventing its escape along the pipe c.
s. Leg of syphon connected with bottle containing alcohol.
t, t. Glass globes, placed in the basins v, v, and surrounded with pounded ice or ice-cold water.
w. Safety tube, containing a little mercury at x.
For the rectification of ether, a water bath is employed along with the above simple refrigerator, and the receivers surrounded by ice or a freezing mixture.
Chem. comp., &c. Ether is generally regarded as the oxide of ethyl, and alcohol as the hydrate of this base. This view is borne out by analysis, which proves that ether differs from alcohol by the elements of water. Recent experiments have also shown that the relation existing between the two compounds is—if alcohol be expressed by the formula C2H6O, the true formula of ether will be (C2H5)2O. We cannot describe these experiments here, but we may remark that ether cannot be made to combine with water directly, nor can alcohol be converted into ether by the abstraction of water, pure and simple, without the aid of other substances.
The compound ethers may be compared to ordinary salts in which the metal is replaced by a radical termed ethyl, having the formula C2H5. This view is, of course, in accordance with the theory which regards ether as the oxide of ethyl.
According to theory, 1 equivalent, or 46 parts of absolute alcohol, should produce 1 eq., or 37 parts, of pure ether; but in practice, the greatest product obtained by operating according to Boullay’s method, which produces more ether than any other, does not exceed 33½ parts for the preceding quantity of alcohol, or 71·5%. A mixture of 9 parts of oil of vitriol, and 5 parts of alcohol of 90%, ceases to produce ether after 31 parts of such alcohol have been added.
The most economical method of etherification is that known as the continuous ether process, or the process of Boullay. When this is adopted, the retort or flask should be fitted with a sound cork, perforated by an aperture to receive a thermometer, and the application of the heat, and the flow of alcohol, should be so managed, that a temperature of 300° Fahr. and a state of rapid and violent ebullition (points of essential importance) are maintained.
Prop., Uses, &c. Pure ether is a colourless, transparent, and very limpid fluid, having a penetrating and agreeable smell, and a burning, sweetish taste; its evaporation produces the sensation of extreme cold; when prevented, a sensation of heat is experienced. Its specific gravity varies between ·712 and ·724. If it contains water it begins to crystallise in brilliant666 white plates when cooled to -24° Fahr., and become a white crystalline mass at -46° or -47° Fahr.; but if absolutely pure, ether cannot be solidified by any degree of cold that can be produced, it remaining fluid when placed in contact with solid carbonic acid, at a temperature of about -148° Fahr. Boils at 96° or 97° Fahr.; is very combustible; is soluble in about 10 parts of distilled water, and mixes with alcohol in all proportions. It abstracts corrosive sublimate, terchloride of gold, sesquichloride of iron, and many of the alkaloids, from their watery solutions, and is hence invaluable in analysis and pharmacy. It readily dissolves the volatile and fixed oils, and most fatty matters, as well as sulphur and phosphorus in small quantities. By exposure to light and air it absorbs oxygen, and water and acetic acid are gradually formed. It is decomposed by exposure to a high temperature. Its evaporation occasions intense cold. The greatest degree of cold yet produced (-166° Fahr.) has resulted from the admixture of ether with solid carbonic acid. Ether is powerfully stimulant, narcotic, and antispasmodic, and externally refrigerant, if allowed to evaporate, or stimulant and counter-irritant if its evaporation is prevented, and is used in various diseases. Applied to the forehead by means of the fingers or a strip of linen, it generally relieves simple cases of nervous headache. In pharmacy it is largely employed in the preparations of tinctures alkaloids, spirits, &c.; and in chemistry is invaluable in organic analyses. Its principal commercial application is as a solvent for pyroxyline, in the manufacture of collodion. It is also employed as a solvent of resins, india rubber, &c., in the preparation of varnishes, and for several other useful purposes.—Dose, 20 drops to 2 fl. dr.; in water or wine. Excessive doses of ether produce intoxication resembling that from alcohol, and require similar antidotes. Sulphuric ether is said to be taken largely in the north of Ireland as a stimulant, particularly in Antrim. Shortly before the discovery of chloroform, it was found that when the vapour of ether was inhaled it gradually produced insensibility to pain. It was therefore employed as an anæsthetic in surgical operations. Having been found less efficient than chloroform, and more troublesome to administer, its use for this purpose has been abandoned.
Tests. Ether may be recognised by its volatility, odour, taste, sparing solubility in water, admixture with alcohol in all proportions, great inflammability (burning with a yellowish-white flame), and its power of dissolving fats and resins. Its further identification can only be effected by ultimate analysis.
Pur. The ether of the shops generally contains alcohol, water, or acetic acid, and sometimes all of them. Its usual specific gravity fluctuates between ·733 and ·765. Exposed to the air, it volatises entirely. It turns litmus paper red; sometimes very slightly, and occasionally even not at all. 1⁄2 fl. oz. mixes completely with 1⁄2 pint of water. Pure ether should, however, be neutral to test-paper, although seldom so. When shaken in a minim measure with half its volume of concentrated solution of chloride of calcium, its volume should not lessen. 10 fluid ounces of water should only dissolve 1 fluid ounce of ether, and remain transparent.
Preserv. Ether rapidly evaporates at common temperatures when kept in corked bottles, and even in bottles secured with ground-glass stoppers and tightly tied over with bladder and leather; it also becomes sour by age. To prevent this waste, the stoppers should fit accurately, and the bottles should be placed in as cool a situation as possible. Bottles furnished with ground-glass caps, as well as stoppers, are frequently employed. (See engr.) Dewar’s ‘ether phial’ is formed on a similar principle. We have seen bottles of ether accurately stoppered, tied over with bladder, and thickly coated with wax, which have yet become quite empty by a voyage to the tropics, though they still appeared to be as closely secured as when they were first filled.
Caution. The vapour of ether is very inflammable, and when mixed with atmospheric air, it forms a violently explosive mixture. The density of this vapour is 2·586, that of air being 1; hence it rapidly sinks, and frequently accumulates in the lower parts of buildings, especially cellars which are badly ventilated, in the same way as water does. The only remedy is thorough ventilation. Many serious accidents have arisen from this cause, for no sooner is a light carried into an apartment where such vapour is present than an explosion takes place.
Ethyl, Acetate of. C2H5C2H3O2 Syn. Acetate of oxide of ethyl, Acetic ether, Pyroligneous ether; Æther aceticus, L. A compound discovered by the Count de Lauraguais in 1759.
Prep. 1. Acetate of potassa, 3 parts (or an equiv. quant. of acetate of soda), alcohol (85%), 3 parts, oil of vitriol (strongest), 2 parts, are mixed together and distilled, by the heat of a sand bath, from a glass or earthenware retort into a well-cooled receiver; the distillate is agitated with a little water to remove undecomposed alcohol, and then digested first with a little chalk to remove acidity, and afterwards with fused chloride of calcium, to absorb water; it is, lastly, rectified by a gentle heat.
2. Rectified spirit (sp. gr. ·84), 50 parts, acetic acid (sp. gr. 1·075), 33 parts, are mixed together, and oil of vitriol (strongest), 10 parts, added; the distillation is continued until 65 parts have passed over, and the distillate, after digestion for some hours on a little dry carbonate of potassium, is rectified as before, the first 50 parts only being kept for use.
667
Prop., &c. Acetic ether is colourless, and bears a considerable resemblance to ordinary ether, but it has a much more agreeable and refreshing odour. It boils at 165° Fahr.; has a sp. gr. of ·89 at 60° Fahr., dissolves in about 7 parts of water; and mixes in all proportions with alcohol and ether. It is decomposed by alkalies and the strong acids.
Acetic ether is diaphoretic, stimulant, antispasmodic, and narcotic.—Dose, 1⁄2 to 2 fl. dr.; in similar cases to those in which sulphuric ether is employed, and especially in nervous and putrid fevers, spasmodic vomitings, and diseases of the bowels and stomach, arising from debility, and not of an inflammatory character. Its principal consumption is in the manufacture of British brandy.
Ethyl, Benzoate of. C2H5C7H5O2. Syn. Benzoic ether, Benzoate of ether, B. of oxide of ethyl; Æther benzoicus, L. Prep. Alcohol (sp. gr. ·830), 4 parts; benzoic acid (cryst.), 2 parts; concentrated hydrochloric acid, 1 part, are distilled together; as soon as the product turns milky when mixed with water, the receiver is changed, and the liquid that distills over collected; to this liquid water is added, and the supernatant ether is decanted, and boiled with water, and a little oxide of lead (to separate benzoic acid); it is, lastly, freed from water by allowing it to stand over chloride of calcium.
Prop., &c. A colourless oily liquid, slightly heavier than water, and possessing an aromatic odour and taste. It boils at 410° Fahr., and is miscible with alcohol and ether.
Ethyl, Bromide of. C2H5Br. Syn. Æther hydrobromicus, L. A volatile, ethereal liquid, discovered by Serullas.
Prep. Bromine, 8 parts; alcohol, 32 parts; dissolve, place the mixture in a retort, add of phosphorus, 1 part, and distil by a gentle heat as soon as the liquid becomes cold. The ether is separated from the distillate by the addition of water.
Prop., &c. A very volatile liquid, with a penetrating taste and smell; boiling at 105° Fahr., and heavier than water.
Ethyl, Bu′tyrate of. C2H5C4H7O2. Syn. Butyric ether, Pine-apple oil; Æther butyricus, L. Prep. By passing hydrochloric acid gas into an alcoholic solution of butyric acid, and purifying the product from free acid.
Commercially, from crude butyric acid saponified with caustic potassa or baryta, and the resulting soap distilled along with alcohol and oil of vitriol.
Uses. Crude butyric ether forms the ‘pine-apple oil’ of commerce, and when largely diluted with rectified spirit, the ‘pine-apple essence’ so much employed as a flavouring substance by confectioners, liqueuristes, &c. It imparts a delicious flavour to sweetmeats, rum, arrack, punch, &c. The Germans add it to common rum, to form the flavouring for their ‘pine-apple ale.’
Ethyl, Carbonate of. (C2H5)2CO3. Syn. Carbonic ether, Carbonate of oxide of ethyl; Æther carbonicus, L. Prep. Fragments of potassium are added to oxalic ether, gently warmed, as long as bubbles of gas are formed; the excess of metal is removed from the semi-solid mass, some water added, and the whole distilled. The carbonic ether floats on the surface of the liquid in the receiver, and is collected, dried by contact with chloride of calcium, and rectified along with some potassium or sodium, till it ceases to yield acetate of potassa when acted on by caustic potassa.
Prop., &c. Colourless, limpid, and aromatic; tastes pungent and burning; boils at 259° to 260° Fahr. It greatly resembles oxalic ether. It is decomposed by alkalies.
Ethyl, Chlo′ride of. C2H5Cl. Syn. Light Hydrochloric e., Chloride of ethyl; Æther hydrochloricus, L. A highly volatile compound, formed of ethyl and chlorine.
Prep. Rectified spirit of wine is saturated with dry hydrochloric acid gas in the cold, and the product is distilled in a retort connected with a Wolfe’s apparatus, the first bottle of which should be two thirds filled with tepid water (70° to 75° Fahr.), and the remainder surrounded with a mixture of ice and salt. To render it perfectly anhydrous, it must be digested on a few fragments of fused chloride of calcium.
A mixture of oil of vitriol, 3 parts, and alcohol, 2 parts, is poured upon common salt (dried), 4 parts; and the whole distilled as before.
Prop., &c. This ether has a sweetish taste; is soluble in about 15 parts of water, and miscible in all proportions with alcohol; boils at 54° Fahr.; burns with a flame edged with green; is neutral to test paper; and does not affect a solution of nitrate of silver. Sp. gr. ·921, at 32° Fahr.—Dose, 10 to 30 drops, as an antispasmodic and a powerful diffusible stimulant. Owing to its extreme volatility it can only be taken dissolved in spirit.
Ethyl, Cy′anide of. C2H5CN. Syn. Æther hydrocyanicus. L. Prep. Cyanide of potassium and sulphovinate of baryta, equal parts, are mixed and distilled in a glass retort by a moderate heat. The product separates into two strata; the lighter one is impure hydrocyanic ether; this is decanted and agitated with 4 or 5 times its bulk of water at 120° to 140° Fahr., and the operation is repeated with about 2 parts of water; the ether is again decanted, and placed in contact with chloride of calcium for 24 hours, and then rectified.
Prop., &c. It boils at 190° Fahr. Sp. gr. ·788. In its therapeutical effects it resembles hydrocyanic acid, but is less active. Its odour is, however, more penetrating and offensive.—Dose, 2 to 6 drops, in mucilage or emulsion; in obstinate or convulsive coughs, gastrodynia, hysterical affections, &c.
Ethyl, Cy′anate of. C2H5CNO Syn. Cyanic ether, Cyanate of oxide of ethyl.668 Prep. By distilling a dry mixture of cyanate of potassa and sulphovinate of potassa in nearly equivalent proportions. A mixture of cyanic and cyanuric ethers passes over into the receiver. By distilling this mixture the two are readily separated; that which passes over by the heat of a water bath being the first, and the residuum in the retort the second.
Prop., &c. An ethereal, very mobile liquid, boiling at 140° Fahr.
Ethyl, Cyan′urate of. (C2H5)3C3N3O3. Syn. Cyanurate of oxide of ethyl. Prep. See Cyanic Ether.
Prop., &c. Tasteless, inodorous, colourless, transparent, needles and prisms; fusing at 185° Fahr.
Ethyl, I′odide of. C2H5I. Syn. Æther hydriodicus, L. Prep. Phosphorous, 4 parts, alcohol (sp. gr. ·84), 70 parts, and iodine, 100 parts, are gradually and cautiously mixed together, and distilled.
Prop., &c. A colourless liquid, possessing a strong ethereal odour, and boiling at 158° Fahr.; sp. gr. 1·92. It is reddened and decomposed by exposure to air and light.
Ether, Methy′lic. Syn. Oxide of methyl, Wood-ether, Methyl-ethyl. See Methyl.
Ether, Muriatic (Heavy). Syn. Æther muriaticus ponderosus, L. Prep. Alcohol, of 80 to 85%, is saturated, in the cold, with chlorine gas, water is next added, and the oily fluid that separates collected and washed with water, as long as any of it is dissolved.
Prop., &c. Heavy muriatic ether is a volatile, oily, colourless liquid, boiling at about 245° Fahr., and heavier than water. Its precise constitution is undetermined. This ether enters into the composition of the SPIRITUS MURIATICO-ETHEREUS, a remedy occasionally used on the Continent.
Ethyl, Nitrate of. C2H5NO3. Syn. Nitric ether, Nitrate of oxide of ethyl; Æther nitricus, L.
Prep. Nitric acid (sp. gr. about 1·375), 50 parts; nitrate of urea, a little (say 2 or 3 parts); dissolve, add alcohol, 50 parts, and distil with the usual precautions, until 7-8ths of the whole (of the liquid portion) have passed over; agitate the distillate with a little water to separate the ether, and preserve the heavier portion.
Prop., &c. Nitric ether possesses an agreeable sweetish taste and odour; it is insoluble in water; the alcoholic (but not the aqueous) solution of potassa decomposes it rapidly; sp. gr. 1·112. Its vapour is very apt to explode when strongly heated, and therefore a small quantity only should be prepared at a time.
Ethyl, Nitrite. C2H5NO2. Syn. Nitric ether, Hyponitrous ether, Nitrite of ether, Nitrite of oxide of ethyl, Hyponitrite of E.; Æther nitrosus, Æ. hyponitrosus, L. This is a compound, of which ‘sweet spirit of nitre’ is an impure alcoholic solution.
Prep. 1. Starch (potato farina), 1 part; nitric acid (sp. gr. 1·30), 10 parts; mix in a capacious retort, connected with a wide tube, 2 or 3 feet long, bent at right angles, and terminating near the bottom of a two-necked bottle, containing a mixture of alcohol (of 85%), 2 parts, and water, 1 part, and surrounded with a freezing mixture, pounded ice, or very cold water; the other neck of the bottle being connected by a long glass tube with a good refrigerator or condenser. All elevation of temperature must be avoided. The heat of a water bath only must be cautiously applied to the retort. The gas liberated passes into the alcohol, causing the ether to distil in a gentle stream. The tube connecting the retort and bottle must be cooled by means of rag or moist paper, kept wetted with ice-cold water; as, if the temperature of the tube and the alcohol rises only a little, the latter becomes spontaneously hot, and boils violently, by which the product is vitiated. This process is very productive and economical, and yields pure nitrous ether.
2. A mixture of oil of vitriol, 8 parts, and alcohol, 9 parts, is poured upon crystallised nitrate of ammonia, 11 parts, contained in any suitable distillatory vessel connected with a well-cooled receiver. Nitrous ether gradually distils over on the application of a gentle heat. An admirable process, but more expensive than the preceding. Even a common fire may be employed without danger, as the liberation of the ether proceeds gradually, and not almost instantaneously, as in operating in the usual way. Sulphate of ammonia is left in the retort. The product is scarcely inferior to that of the last formula.
3. Rectified spirit, 46 fl. oz.; pure nitric acid (sp. gr. 1·500), 7 fl. oz.; put 15 fl. oz. of the spirit, with a little clean sand, into a quart matrass, fitted with a cork, and a safety tube reaching to within an inch of the spirit, and a second tube leading to a refrigeratory. Fill the safety tube with pure nitric acid, then add through it, gradually and cautiously, 31⁄2 fl. oz. of the acid. When the violent action that ensues is nearly over, gradually add the remaining portion of the acid, 1⁄2 fl. oz. at a time, and at intervals. Agitate the ether that distils over, first with a little milk of lime, till it ceases to redden litmus paper, and then with half its volume of concentrated solution of chloride of calcium. The pure hyponitrous ether thus obtained should have a density of ·899.
4. (Mr John Williams.) Nitrite of ethyl is best made by passing nitrous acid gas into alcohol.
The nitrous acid gas is prepared by acting upon such bodies as starch, copper, mercury, or arsenious acid with nitric acid. The alcohol is generally recommended in the text-books to be diluted with half its bulk of water; this, however, I consider a decided mistake. The alcohol should be as concentrated as possible, even absolute alcohol is preferable. The main points to be attended to are that the current669 of nitrous acid gas should be slow and steady, so as to give time for the reaction to proceed properly, and that the vessel containing the alcohol should be kept as cool as possible; in this way much of the production of bye-products will be avoided, and the gas can be passed through the alcohol, as long as it continues to be absorbed.
The resulting liquid is anything but pure; it contains much nitrite of ethyl, some aldehyde, acid—it is even stated to contain malic acid. In fact it is well known that the reaction between the nitrous acid, and such products as alcohol, however pure, is not sharp, but is always accompanied by secondary products.
From the crude alcoholic solution obtained by the method I have described, the pure nitrite of ethyl can be obtained without difficulty. Nitrite of ethyl is an extremely volatile liquid; it boils at about 61° F., whereas aldehyde boils at 90° F., and alcohol at 180° F. Taking advantage of this fact, we are enabled to separate it from the crude liquid by distillation. Some precautions are, however, necessary, to ensure the purity of the product. The flask containing the crude product is placed in a water bath, and connected by bent tubes with several other flasks and bottles. The first tube should be passed into a small empty flask, this will condense most of the alcohol which may pass over during the operation. Then a second bent tube passes into a second flask containing a little water; this condenses any alcohol which may not have been stopped in the first flask, together with free acid, and nearly all the aldehyde.
From this wash bottle a third tube proceeds into a somewhat shallow flask, containing a strong solution of caustic potash; the gas, however, is not allowed to pass through this alkaline liquid, but simply over the surface. In this way the last portion of the aldehyde is absorbed, and the potash solution gradually assumes an amber colour. From this vessel, the gas (for such at the ordinary temperature of the laboratory, the nitrite of ethyl is—in very cold weather it would be necessary to gently warm the different flasks) is passed through a tube charged with anhydrous chloride of calcium to absorb moisture, and the pure and dry nitrite of ethyl thus produced, finally passes into alcohol, which readily absorbs it.
It is only necessary to note the weight of the alcohol used for absorbing the gas, and its weight at the end of the operation, to know the strength or per-centage of nitrite of ethyl which must be in solution. Thus, if 9 oz. of alcohol becomes 10 oz., it is evident we have a solution of 10 per cent.; if it becomes 12 oz., then the strength must be 25 per cent., and so on. Ordinary spirits will answer for condensing the nitrite of ethyl, but it is better to use absolute alcohol, as it is very desirable to avoid the presence of water in any form. The solutions made with weaker spirit soon turn acid; those made with absolute alcohol, on the other hand, keep a long time. It is very true the very strong solutions of 50 and 25 per cent. show traces of acidity when tested with moistened litmus paper, but the 10 per cent. solution is quite neutral.[283]
[283] The object of Mr Williams’ paper, which is published in the ‘Pharmaceutical Journal’ for Dec. 8th, 1877, is to give instructions for the preparation of a pure nitrite of ethyl, which when mixed with alcohol in definite proportions, shall supersede the variable compound sold under the name of “Sweet Spirits of Nitre.”
One point I have not mentioned; it is that the distillation must be conducted at the very lowest possible temperature; in fact, the water in the water-bath should only be kept gently warm, and the process should be continued only so long as the conducting tubes feel cool to the touch; when they become warm the distillation should be discontinued. By passing the gas into a tube in a freezing mixture, instead of into alcohol, the pure nitrite of ethyl is readily obtained in a liquid form; it is, however, necessary to seal the tube, otherwise the very volatile liquid would soon be lost.
Prop., &c. Pure nitrous ether has a pale-yellow colour, an agreeable odour of apples, and boils at 62° Fahr.; sp. gr. ·947 at 60° Fahr. Commercial nitrous ether contains aldehyde, boils at 70° Fahr., has a more or less suffocating odour combined with that of the pure ether, has a sp. gr. of ·886 at 40° Fahr., and turns brown when mixed with alcoholic solution of potassa, while the latter remains unaltered. It also acidifies by age, whilst pure nitrous ether remains neutral. They are both very inflammable, and burn with a white flame. Ordinary nitrous ether dissolves in about 48 parts of water, and mixes in all proportions with alcohol and sulphuric ether.
Nitrous ether is refrigerant, diaphoretic, and diuretic, but is seldom employed alone, though, when largely diluted with alcohol (sweet spirits of nitric, spirit of nitric ether), it is a common remedy in several diseases. It is also used to flavour malt spirit, in imitation of brandy (British brandy), although for this purpose it is vastly inferior to acetic ether. See Spirits (Medicinal).
Ethyl, Œnanthate of. Syn. Œnanthic ether, Pelargonic ether, Œnanthate of oxide of ethyl. Prep. 1. The oil obtained towards the end of the distillation of fermented liquors, especially wines, consists, in a great measure, of the crude ether. It is purified by agitation with a weak solution of carbonate of potassa, freed from water by a few fragments of chloride of calcium, and then re-distilled.
Prop., &c. Œnanthic ether is colourless; lighter than water; boils at about 500° Fahr.; and has a powerful, intoxicating vinous odour, resembling that of an empty wine cask or bottle that has been exposed to the air for some time. It is very sparingly soluble in water, but freely soluble in alcohol. Its sp. gr. is ·862. As obtained by distillation, it is670 united with a little ŒNANTHIC ACID. 2200 imperial gallons of wine (about 35 hogsheads) only yielded 21⁄5 lbs. of the mixed oil.
Ethyl, Oxalate of. (C2H5)2C2O4. Syn. Oxalic ether, Oxalate of oxide of ethyl; Æther oxalicus, L. Prep. Alcohol and dry oxalic acid, equal parts, are digested together in a glass flask furnished with a very long glass tube of small bore, so that the spirit, volatilised by the heat, may be condensed, and flow back into the flask. After 6 or 8 hours the process is generally complete, and the liquid contains merely a trace of free acid, from which it may be separated.
Prop., &c. A colourless, oily liquid, slightly heavier than water, boiling at 363° Fahr., only slightly soluble in water, and having an aromatic smell. Alkalies decompose it. Sp. gr. 1·09.
Ethyl, Sulphate of. (C2H5)2SO4.
Prep. The vapour of pure anhydrous sulphuric acid is passed (with the usual precautions) into perfectly anhydrous ether; the resulting syrupy liquid is agitated with a mixture of 4 volumes of water, and 1 volume of ether, and after repose the upper stratum, which is an ethereal solution of the sulphate of ethyl, is decanted, and the oxide of ethyl volatilised by a very gentle heat. The colourless liquid forming the residuum is the true sulphuric ether or sulphate of ethyl just referred to. It is a very unstable compound, and cannot be distilled without suffering decomposition.
Ethyl, Valerianate of. C2H5C5H9O2. Syn. Valerianic ether, Valerate of oxide of ethyl; Æther valerianicus, L. Prep. By passing dry hydrochloric acid gas into an alcoholic solution of valeric acid. It is a fragrant, volatile liquid, lighter than water, having a high boiling-point, and a rich fruity odour, said to closely resemble that of butyric ether or pine-apple oil. It is used to flavour liqueurs, &c.
ETHYL′AMINE. NH2(C2H5). Syn. Ethyl-ammonia. One of the bases of the ethyl-series, obtained by substituting one atom of hydrogen in ammonia NH3 by ethyl C2H5.
EUCALYP′TIN. A peculiar substance existing in Botany Bay kino. A substance exuded by several species of the Eucalyptus. It has been employed medicinally in diarrhœa.
EUCALYPTOL. See Eucalyptus.
EUCALYPTUS. The Eucalypti, of which there are many species, belong to the natural order Myrtaceæ, and are natives of Australia, where they are known under the names of “gum-trees,” or as “stringy-bark trees.” The most interesting and important characteristic of these plants is the power they undoubtedly possess of correcting, if not of removing, the pestilential exhalations which are regarded as the origin of the fevers that occur in marshy localities. This discovery is due to M. Ramel, and was made by him in 1856.
M. Gimbert, amongst other cases, cites one of a farm, twenty miles from Algiers, the atmosphere surrounding which was of a very pestilential character. In the spring of 1867 13,000 eucalyptus trees were planted on the farm, and M. Gimbert states that since then not a single case of fever has taken place, the freedom from disease occurring the same year the plants were placed in the ground, and the good effects commencing whilst the trees were only two or three mètres in height.
The following is extracted from ‘Les Mondes’ (1876):—“Between Nice and Monaco there is a locality so unhealthy that the Paris, Lyons, and Mediterranean Railway Company have been obliged to change every two or three months the watchman at a crossing there.
“Plantations of the eucalyptus have been formed there, and at present the same watchman has resided there for several months with his family without experiencing the least inconvenience.”
Again:—“In the Campagna, about three miles from Rome, there stand some deserted church buildings and a monastery, the latter having been abandoned because of the mortality amongst the monks caused by the noxious exhalations. Some six years since a company of French trappists, having obtained permission from the Italian Government, planted the grounds in and around the monastery with eucalyptus trees, and the result is stated to have been so total an immunity of the building from fever, although situated in the worst part of the Campagna, that the monastery is now tenanted, the health of the occupants being, it is said, unimpaired.”
The writer in the ‘Pharmaceutical Journal,’ who contributes this statement, adds:—“Whether this grand result has been obtained through the efficacy of the extract of the eucalyptus taken each morning with their cup of black coffee, or whether it is to be attributed to the effects of the plantations, I leave to scientific men to determine.”[284]
[284] Bentley.
It seems very probable that the effects above described are due to the eucalyptus having such extensive and far-spreading roots, which suck up and appropriate the moisture of the surrounding soil, the presence of which, aided by heat, giving rise to vegetable decomposition, is believed to be the cause of malarial poisoning.
The avidity of the plant for water is very great; it has been computed that one tree will absorb ten times its weight of moisture from the soil.[285] It is most likely owing, at any rate in very large measure, to this cause, rather than to the supposed antiseptic and disinfecting odours exhaled by its leaves, that the salubrious effects of the eucalyptus are due. The blue gum tree, or Eucalyptus globulus (so distinguished because of the rounded form of the lid which covers its unexpanded flower bud), has been successfully introduced into Asia,671 Africa, and Southern Europe. If, as asserted, it can only exist in a climate where the temperature is never lower than the freezing point, its domestication (save in hot-houses) is impossible in our own country.
[285] ‘Pharm. Journal,’ February 5th, 1876.
 Eucalyptus Globulus (from the ‘Archiv der Pharm.,’
1873, p. 129.)
Eucalyptus Globulus (from the ‘Archiv der Pharm.,’
1873, p. 129.)
The Eucalyptus globulus is a very rapidly-growing tree, and attains to great proportions. “In some cases it has been known to attain the colossal dimensions of 350 feet in height and a 100 in circumference.”[286]
[286] Bentley.
This magnitude is entirely out of proportion to the size of the seed, which is very minute; so minute that it has been computed one pound weight of the seed could produce 162,000 trees. Various preparations of the leaves and672 bark of the eucalypti have been introduced into medicine, which will be found under the respective pharmaceutical preparations. They were asserted to be specially serviceable in intermittent fevers and bronchitis. The idea that their efficacy in the former class of disease was due to the presence in the barks of the eucalypti of an alkaloid similar to, if not the same as, quinine, has been shown to be an erroneous one, from the experiments of the Government chemist of Ootacamund (Mr Broughton), who, after a most careful chemical analysis, failed to discover either quinine, quinidine, cinchonine, cinchonidine, or the least trace of any one of the cinchona alkaloids.
When the leaves of the Eucalyptus globulus are held to the light they reveal the presence of little semi-transparent dots, which are found to be receptacles for a volatile oil, that may be obtained in large quantity by submitting the plant to aqueous distillation.
This volatile oil has been examined by Cloez, who found it to consist chiefly of a substance allied in chemical characters to camphor, which substance he named eucalyptol.[287] Any therapeutic power possessed by the eucalyptus may be referred to this substance, since, as just stated, it cannot be due to a bark alkaloid.
[287] Messrs Faust and Homeyer state that Cloez’s “Eucalyptol” is a mixture of terpen and cymol.
Before finishing our notice of the reputed curative effects of the eucalyptus we may mention that Dr Gimbert employs the leaves instead of lint for dressing wounds and fetid ulcers, and says he has found them, when thus used, excellent deodorisers; that another method of employing the leaves of the eucalyptus consists in having them made into cigarettes, which are reported to be useful in asthma and bronchial complaints. Lastly, let us state that another species of eucalyptus exudes a very astringent substance, which, from its appearance and properties, being so analogous to kino, has been denominated Botany Bay kino. (See Eucalyptin.)
The essential oil of eucalyptus, which, according to the species of the plant from which it is obtained, varies in colour from light yellow to light blue, is now largely employed as a diluent for the more delicate volatile oils used in perfumery.
Many species of the eucalyptus yield excellent timber, possessed of great hardness and durability, and little affected by moisture. This timber has the power of resisting the attacks of insects. The wood of the eucalyptus is also very rich in potash. The maple and the elm, which are regarded as yielding a large per-centage of this substance, afford only about half as much as can be obtained from the eucalyptus, this latter tree yielding 21 per cent. of potash.
The barks of different species have also been advantageously utilised for paper making, as well as for tanning.
In this country eucalyptus seeds are reared in a greenhouse. They may be sown in a mixture of loam, peat, and ordinary soil, with a sprinkling of sand on the surface.
The following directions for the cultivation of the eucalyptus in England were communicated to the ‘Medical Times and Gazette’ of 1873 by Mr Bennett Stanford, of Pyt House, Tisbury:—“I have successfully reared from seed two dozen of these trees, and they are now growing well out of doors. I obtained the seed five years ago from South Australia, and forced it in a hothouse; in one year it was four feet high, and now, in its fifth year, it is growing rapidly in a sheltered position in the park, having attained a height of thirty feet. The first three years the tree must be taken under cover in the winter, and the fourth and fifth years should be protected for several feet up with wisps of hay or straw. When the trees are kept indoors in winter it should be in an orangery or very high greenhouse, with plenty of light and a little water.”
EUCHLO′′RINE. A bright-yellow gas, prepared by gently heating chlorate of potassa with hydrochloric acid. It is probably a mixture of chlorous acid and free chlorine. Prof. Stone, of Manchester, has found Euchlorine of a great service as an aerial disinfectant.
EUGLENÆ. These are ciliated infusorial animalcules inhabiting ponds and water-tanks. Sometimes they abound in water in quantities so enormous as to impart to that fluid a blood-red appearance. The principal species are the Euglena viridis and the Euglena pyrum. Their presence is supposed to indicate the existence in the water in which they are found, of decaying animal and vegetable matter upon which they are believed to feed.
EUPHOR′BIUM. Syn. Gum euphorbium; Euphorbium (Ph. E.), L. The concrete resinous juice of the Euphorbia canariensis, and other species of the same genus. It is a powerful acrid, purgative, rubefacient, sternutatory, and vesicant, and the violence of its action has led to its disuse.
EU′PIONE. An ethereal liquid forming the chief portion of the light oil of wood-tar, and which also exists in the tar obtained during the destructive distillation of animal substances, and in the fluid product of the distillation of rape oil. It is separated from these substances by agitating them with oil of vitriol, or a mixture of oil of vitriol and nitre, and subsequent cautious distillation. Pure eupione is tasteless, exceedingly thin, limpid, and aromatic; boils at 116° Fahr.; and is the lightest fluid known; sp. gr. ·655. It is very inflammable, burns with a very bright flame, and gives a transient greasy stain to paper. It is isomeric with hydride of amyl. Other volatile hydrocarbons of like origin are often confounded with eupione by chemical writers.
EUPYR′ION. Any contrivance for obtaining673 instantaneous light; as a lucifer match, &c.
EVAC′UANTS. Syn. Evacuantia, L. Medicines which augment the secretions or excretions. Cathartics, DIAPHORETICS, DIURETICS, EMETICS, ERRHINES, EXPECTORANTS, and SIALOGOGUES, belong to this class.
EVAPORA′TION. The conversion of a fluid into vapour by means of heat, diminished atmospheric pressure, or exposure to a dry atmosphere. Evaporation is had recourse to—1. For the vapour as a source of heat or power, as in the case of steam-boilers, &c.;—2. To separate volatile fluids from impurities or other bodies, which are either fixed or less volatile;—3. To recover solid bodies from their solutions, as in the preparation of extracts, chemical salts, &c.;—4. To concentrate or strengthen a solution by the expulsion of some of the fluid matter that forms the menstruum;—5. To purify liquids by the dissipation of the volatile matters which may contaminate them.
It is found that, under ordinary circumstances, evaporation is confined to the surface of the heated liquid, and is therefore slower or quicker, in proportion to the extension of that surface. Hence has arisen the adoption of wide, shallow vessels for containing fluids during their exposure to heat for this purpose. Evaporation proceeds most rapidly when a current of air (especially hot and dry air) is made to pass over the surface of the fluid; as, in this ease, the vapour is prevented from resting upon the surface, and impeding the process by its pressure. For a similar reason, liquids evaporate more rapidly in vessels partially covered than in open ones. In the former case the cool incumbent air condenses and throws back a portion of the vapour, which thereupon, besides its cooling action, offers mechanical resistance to the diffusion of the vaporous particles as they arrive at the surface of the liquid. In the latter case these obstacles are avoided, and the impetus of the vapour pouring forth from a contracted orifice (or pipe), not only readily overcomes the pressure of the atmosphere, but offers less surface for its cooling action, until it has passed much beyond the points at which it can exert any influence on the fluid from which it has escaped. In this way the chemical action of the atmosphere on the liquid operated on is also considerably lessened. On the small scale, shallow capsules of glass, wedgwood-ware, porcelain, or metal, are commonly employed as evaporating vessels, and these are exposed to heat by placing them over a lamp, or naked fire, or in a water bath, or sand bath, according to the temperature at which it is proper to conduct the process. On the large scale, high-pressure steam is usually employed as the source of heat. The term ‘spontaneous evaporation’ is applied to the dissipation of a fluid by mere exposure in open vessels, at the common temperature of the atmosphere, and without the application of artificial heat. The celerity of this species of evaporation wholly depends on the degree of humidity of the surrounding air, and differs from the former, in which the rate of evaporation is proportionate to the degree of heat at which the process is conducted, and the amount of pressure upon the surface of the liquid. Evaporation ‘in vacuo’ (as it is called) is conducted under the receiver of an air-pump, or in an attenuated atmosphere, produced by filling a vessel with steam, by which means the air is expelled, when all communication with the external atmosphere is cut off, and the vapour condensed by the application of cold. Fluids are also evaporated in air-tight receivers over sulphuric acid, by which they are continually exposed to the action of a very dry atmosphere. When such a receiver is connected with an air-pump in action, evaporation proceeds with increased rapidity, and intense cold is produced. It appears, from the experiments of Dr Ure, that “if the bottom of a pan, and the portion of the sides immersed in a hot fluid medium (solution of chloride of calcium, for example), be corrugated, so as to contain a double expanse of metallic surface, that pan will evaporate exactly double the quantity of water, in a given time, which a like pan, with smooth bottom and sides, will do, immersed equally deep in the same bath. If the corrugation contain three times the quantity of metallic surface, the evaporation will be threefold in the above circumstances. But if the pan, with the same corrugated bottom and sides, be set over a fire, or in an oblong flue, so that the current of flame may sweep along the corrugations, it will evaporate no more water from its interior than a smooth pan of like shape and dimensions placed alongside it in the same flue, or over the same fire.”
In the laboratory, steam heat is now almost exclusively employed. Copper, or tinned, glazed, or silvered coppered pans, boilers, and stills, are surrounded by a ‘jacket’ of cast iron, and high-pressure steam admitted between the two. By due management of the supply-cock, a range of temperature may be thus obtained extending from about 90° to 325° Fahr.
It is found that, under ordinary circumstances, 10 square feet of heated surface will evaporate fully 1 lb. of water per minute; and that a thin copper tube exposing 10 feet surface will condense about 3 lbs. of steam per minute, with a difference of temperature of about 90° Fahr. This is equal to 30° Fahr. per lb.; and, consequently, the heat of the steam employed to produce the evaporation should be 212° + 30° = 242° Fahr.
An attention to the facts and principles thus briefly explained above will be found of great value in the laboratory.
EXCIP′IENT. See Prescription.
EXCI′TANTS. See Stimulants.
EXCORIA′TIONS. Syn. Sprays, Chafings.
In surgery and pathology, superficial injuries or affections of the skin, consisting of the removal674 of the scarf-skin or cuticle, accompanied with more or less irritation and slight inflammations. When arising from rough friction or attrition, they are more commonly called abrasions. Young children are very apt to be chafed under the arms, behind the ears, between the thighs, and in the wrinkles and folds of the skin generally, unless great attention is paid to cleanliness, and wiping the skin perfectly dry after washing them. Whenever there is a tendency to excoriations of this kind, either in adults or children, a little finely powdered starch, or violet powder, applied by means of a puff, or a small bag of muslin, once or twice a day, will generally remove them, and prevent their occurrence in future. Mild unguents, as cold cream, or spermaceti cerate or ointment, may also be used with advantage. The preference should, however, be given to the remedies first named, from their not soiling the linen. See Abrasion.
EXCRETA. The excrementitious matter evacuated from the bowels varies of course in composition and quantity according to the food from which it is derived.
Berzelius found a sample analysed by himself to yield about seventy-five per cent. of water, the remainder being made up of alimentary waste, and biliary matter. A large amount of phosphates of calcium and magnesium was found in the ash remaining after the incineration of the solid matter. A specimen of fæcal matter examined by Playfair yielded 15 per cent. of nitrogen and 45 per cent. of carbon. Marcet states that he has obtained from excrement a crystallisable body possessing an alkaline reaction; to which he gives the name excretin; also a fatty substance, which he terms excretolic acid. To excretin he assigns the formula C78H156SO2; the composition of the acid has not been determined.
Hinterberger has succeeded in getting excretine (excretin), free from sulphur, and gives as its simplest formula C20H36O; which shows a close resemblance to cholesterin, C26H44O.
But cholesterin is less easily dissolved in vinegar than excretin, and the solution deposits crystals which, when viewed by the microscope, are found to be beautiful silky six-sided prisms, while the excretin solution yields round masses.
Treated with bromine, excretin gave a crystalline body having the formula C20H34Br2O; but the author did not succeed in preparing a chlorinated compound of excretin.
In the excreta of carnivorous animals no excretin has been discovered, although a substance resembling it has been found. Cholesterin has been obtained from the fæces of the crocodile, but no urates; whilst the excreta of the boa contain urates, but are destitute of cholesterin.
The fæces of animals that live on vegetables contain neither excretin, butyric acid, nor cholesterin.
The excreta of birds and serpents, which mixed with the secretion from the kidneys, are discharged from the animals by the cavities, are very similar to urine, and consist chiefly of alkaline urates and earthy phosphates.
The excrements of insects consist mainly of the remnants of the tissues, animal or vegetable which they have swallowed as food, mixed with constituents of the urine, provided the insect has no special urinary organs.
Briéger examined the fæces of healthy persons, and of convalescents, and found in addition to acetic, butyric, and isobutyric acids, small quantities of phenol and indol, and a new crystallisable body, which he terms skatol (skatos, fæces). It crystallises in irregular-dentate shining plates, resembling indol, which by frequent recrystallisation from hot water, can be obtained snow white. Skatol forms the chief constituent of the volatile aromatic components of human fæces. Fæces of dogs (whether fed on meat or bread diet) contained no skatol, but indol, and in addition a yellow oil, with a revolting and peculiarly irritating smell.
Briéger has not yet been able to analyse this yellow oil, although it forms the chief volatile constituent of dogs’ fæces. He has repeatedly obtained it from distillation from human pathological fluids. In the pancreas after putrefaction, and in the fæces of typhus patients, no skatol was found. The author considers skatol identical with the substance which Secretan obtained by the decomposition of egg albumen under water for six months.
Skatol injected under the skin of rabbits, passes out in the urine as a substance yielding colouring matter. Skatol is believed by the author to be the substance in human urine which, according to Jaffé, yields a red or violet colour on the addition of hydrochloric acid and chloride of lime.
Phenol, the author finds, is a constant component of human fæces. The above results show that specific products of decomposition are normal components of intestinal digestion.[288]
[288] ‘Deut. Chem. Ges. Berg.,’ x, 1027-1031.
Liebig calculated that the daily average amount of fæcal matter passed by a man is 51⁄2 oz; Lawes says that it averages in healthy male adults, 4·2 oz; Parkes estimates it (in Europe) at 4 oz. on the average; Letheby at 2·784, and Frankland at 3 oz. In India, a native on the average excretes as much as 12 oz., this increase over the above quantities being due to the large proportion of rice and farinaceous food of which the Hindoos’ diet consists.
The daily average amount of urine excreted by a human being has been given by Lawes at 46 oz.; Parkes places it at 50 oz. by measure for each male adult; Letheby at 31·851, and Frankland at nearly 40 oz. by measure. According to Parkes’ figures a population of a thousand persons, would thus void daily 156 lbs.675 of solids, and 260 gallons of urine; or 25 tons of fæces, and 91,250 gallons of urine per annum; whilst according to Letheby, from the same number of people, the daily discharge would be 2266 lbs. avoirdupois of urine and 177·5 lbs. of fæces.
Messrs Lawes and Gilbert estimate the manurial value of the urine and fæces together at 6s. 8d. per annum for every individual, which corresponds to a yearly produce of about 10 lbs. of ammonia; but Messrs Hoffman, Witt, and Thudichum assess it at 8s. 6d. for a mixed population of both sexes and of all ages, which they say represents about 13 lbs. of ammonia.
Fæcal matter decomposes much more rapidly when mixed with urine than it would otherwise do, ammonia and fetid gases being given off in considerable quantities. Should much water be also present, and the temperature moderately high, light carburetted hydrogen, carbonic anhydride, nitrogen, and sulphuretted hydrogen are likewise evolved.
Unless human excreta be effectually as well as speedily removed from the dwellings, streets, &c., of a community, that community will assuredly pay the penalty of their neglect in the shape of health seriously endangered and deteriorated. If this be so with healthy evacuations, the peril becomes considerably intensified when the excreta are discharged by patients labouring under contagious or many other diseases. See Urine, Sewage.
EX′ERCISE is essential to the healthy performance of the functions of both body and mind. Without it, the stomach acts feebly, the bowels become inactive, and the circulation of the blood languid and imperfect; the chest contracts, the respiration becomes impeded, the brain is insufficiently supplied with pure arterial blood, the mind grows lethargic, the complexion assumes a sickly and effeminate hue, and the features generally lack the energy and expression which they possess in perfect health. With exercise, the bodily functions are performed with vigour and regularity, the constitution is thereby strengthened, and the attacks of disease repelled. By exercise the mind too is excited to healthy action, its gloomy reveries are dispelled, and the fair face of creation is presented to the mind’s eye in its proper hues. It robs undue mental exertion of half its injurious effects upon the body, whilst it stimulates and directs it in its proper course. It improves the temper, and humanises the character. The disposition is refined, the passions restrained, violent emotions checked, the habits improved, and the personal charms promoted under the stimulus of judicious exercise.
To females, bodily exercise is even more necessary than to males. The disposition and education of females are such as tend to produce habits of sloth and indolence to a greater degree than in the other sex. Hence to them exercise is doubly important—it is inseparable from health. The more retiring dispositions of females lead them almost unconsciously into habits of inactivity, which, above all, they should endeavour to shake off and avoid. By so doing—by replacing habits of indolence and inactivity by liveliness and moderate exercise, the development of the body will be promoted, additional grace and elegance imparted to its natural movements, and the enjoyments arising from both mental and bodily health increased, whilst disease and deformity will be prevented by the removal of their cause.
The necessity of exercise exists equally in every grade of society and age of life. Those who are engaged in sedentary employments or in-door occupations, should particularly seek refreshing out-door exercise during the periods of relaxation from their diurnal duties. To the studious and delicate of both sexes, this is absolutely necessary to preserve the health and vigour of the body.
In infancy, exercise of a suitable kind should be almost the constant occupation of the little beings that claim our protection and care. It should, however, be always borne in mind, that the muscular exercise of very young children must be of the gentlest class. Prejudice and ignorance frequently induce nurses and parents to teach their children to walk, as they falsely call it, and thus their feeble limbs are urged to make premature efforts to totter along, before the bones and muscles have acquired sufficient strength to support the body in an erect position. From this course the legs and joints frequently become bent and misshapened, and severe injuries are often inflicted on the head and body by blows and falls. It should never be forgotten, that crawling and rolling are their first modes of progression, and require the least exertion. Next comes the sitting posture; from this the child gradually advances to the erect one; then to walk by slight assistance; and, lastly, to walk safely alone. All this should come naturally, and never be promoted, further than by laying the infant on the carpet or floor, for the full exercise of its little strength. As soon as a healthy child is able to walk instead of crawl, its own disposition induces it to do so. The faculty of imitation, the spirit of enterprise, and the pride of doing what others do, present even in infancy, is rather apt to lead the infant to over-exertion than the contrary. The practice of constantly ‘dolling’ children in the arms is most prejudicial to the early development of their feeble powers.
It is injudicious to take an infant out during the hottest part of the day in summer; such a proceeding tends to enervate and depress, rather than to strengthen him. Whenever he goes out his head should be protected from the direct rays of the sun by means of a large brimmed hat made of cotton or straw and an umbrella. The neglect of these precautions frequently gives rise to the disordered stomach,676 sickness, and diarrhœa, so prevalent during very hot weather. During other periods of the day, the weather being favorable and the locality healthy, an infant cannot be too much out of doors, especially during teething.
Infants of three or four months’ old may, under certain precautions, be sent out into the open air during the winter. They must be well wrapped up; they should be carried in the nurse’s arms, and not consigned to a perambulator; they should never go out in foggy nor wet weather; if the wind be neither in the east nor the north-east there will be no objection to their being sent out on a clear frosty day. Spring is a trying period for infants and children, because of the prevalence of east winds; hence the necessity of seeing that they are well and warmly clad when sent out during this season. There is much less danger of a child taking cold during the autumn than the spring, as in autumn the winds frequently blow from the south, or warm quarter.
In childhood the exercise should be regulated according to constitution and age; avoiding inactivity, on the one hand, and excessive exercise on the other. The out-door plays and pastimes of BOYS will generally be found sufficient, and in some cases will even require to be curbed, to prevent fatigue and the overtasking of the young frame. With girls it is frequently difficult to find sufficient exercise without trespassing on the prejudices of the ignorant, or the routine of their daily education. With them walking, and some healthy amusement, as skipping, hooping, or the like, should be indulged in for some hours daily. When this is impossible or inconvenient, they may be habituated to the practice of the more simply and cleanly portion of the domestic duties. In the performance of the latter, the health will be promoted, whilst the care and attention which is always due by a female to herself and others, at all periods of her life, will become an easy acquisition, and assist the cultivation of the best feelings of her nature.
In youth exercise matures and promotes the development of the frame; and in manhood it is equally necessary, as already noticed, to keep it in healthy action. In age it will be found to assist the vital functions, and put off decay. In fact, to all—young, old, rich, and poor, physical exercise is essential to the permanent enjoyment of health.
In a medical point of view, “exercise, employed moderately, has a tonic and stimulating influence on the system, and is calculated to prove beneficial in a great variety of complaints. Used immoderately, it exhausts both the mental and bodily powers, and produces great debility.” (Pereira.) Well-directed exercise favours the preservation of the general health, by calling into direct action the majority of the organs of the body; and it also acts powerfully on the skin, by stimulating its functions, increasing its temperature, awakening its tone, and subjecting it to a current of atmosphere favorable to its respiratory offices. But to be beneficial in the highest degree, exercise must be accompanied by feelings of present interest and enjoyment. The mind must direct and go with it; to ensure its full benefits, the “soul must be present.”
“During convalescence, properly regulated exertion is highly serviceable; but it should never be carried so far as to produce exhaustion, and should be pursued for some time in doors, before it be attempted in the open air; the latter, at first, should always take place in a carriage, that can be opened or closed at will; the patient may then attempt short walks in the open air; but, in all cases, it is of importance that he is not unduly fatigued, as, otherwise, injury instead of benefit will be the result. One of the most serious errors, committed with regard to exertion, is that of permitting a convalescent to sit up too frequently, or for too long a time, under the mistaken notion of giving him strength. A patient should never be allowed to sit up longer than is agreeable to his feelings, and never so long as to produce a sense of fatigue.” (Dr R. E. Griffith.)
The physiological effects of exercise have been studied by numerous scientific observers. The carefully conducted experiments of Dr Edward Smith have satisfactorily demonstrated that during bodily exertion the circulation of the blood through the lungs is much increased in velocity, that these latter inspire air and eliminate carbonic anhydride in quantities proportionate to the exercise taken, and that these quantities show an enormous increase over the amounts of these gases inhaled and exhaled during a state of rest.
Adopting the recumbent position as unity, Dr Edward Smith has given the following table, illustrating the quantities of air inhaled during various forms of exercise:
| Lying position | 1· |
| Sitting | 1·18 |
| Standing | 1·33 |
| Singing | 1·26 |
| Walking 1 mile per hour | 1·90 |
| Walking 2 miles per hour | 2·76 |
| Walking 3 miles per hour | 3·22 |
| Walking and carrying 34 lbs. | 3·50 |
| Walking and carrying 62 lbs. | 3·84 |
| Walking and carrying 118 lbs. | 4·75 |
| Walking at 4 miles per hour | 5· |
| Walking at 6 miles per hour | 7· |
| Riding and trotting | 4·05 |
| Swimming | 4·33 |
| Treadmill | 5·50 |
Since a man takes into his lungs 480 cubic inches of air per minute, in walking four miles an hour he draws in 2400 cubic inches, and if six miles 3260 cubic inches a minute.[289]
[289] Parkes.
677
Dr Smith estimated the amount of carbonic anhydride evolved under differing conditions, and found that—
| Carbonic acid exhaled per minute in grains. | |
| During sleep | 4·99 |
| Lying down, and almost asleep (average of three observations) | 5·91 |
| Walking at the rate of 2 miles an hour | 18·10 |
| Walking at the rate of 3 miles an hour | 25·83 |
| Working at the treadmill, ascending at the rate of 26·65 feet per minute (average of three observations) | 44·97 |
The relative amounts of carbonic anhydride eliminated from the lungs during periods of rest and exercise have also been investigated by Pettenkofer and Voit. The following table, which gives the results of their experiments, also records the quantities of oxygen absorbed, and of water and urea excreted at the same time:—
| Absorption of Oxygen in Grammes. | Elimination in Grammes of— | |||
| Carbonic Acid. | Water. | Urea. | ||
| Rest-day. | 708·9 | 911·5 | 828·0 | 37·2 |
| Work-day. | 954·5 | 1284·2 | 2042·1 | 37·0 |
| Excess on work-day (with exception of urea) | 246·6 | 372·7 | 1214·1 | 0·2 |
If the quantities in the above table be converted into ounces it will be found that nearly 83⁄4 oz. more oxygen were absorbed and 13 oz. more of carbonic anhydride eliminated by the lungs during a work-day than during a rest-day.[290] It must be stated that during the work-day an interval of rest was taken, and that the labour was by no means excessive.
[290] Parkes.
Hirn and Speck appear to have conclusively proved that the formation of the carbonic anhydride occurs in the muscles, and that it is rapidly carried off from them. In short, this latter result seems essential for the development of muscular energy. At any rate it is found that if the respiratory movements be in any way interfered with during exercise, and the elimination of carbonic anhydride in any degree checked, the muscular power rapidly diminishes.
An examination of Pettenkofer and Voit’s table shows that exercise gives rise to the escape of a large amount of water from the body, and to a slightly diminished quantity of urea.
Since the accumulation of the superfluous carbon supplied by the food gives rise to morbid and diseased states of the body,[291] we shall now be enabled to understand why deficient exercise should be a source of physical ill-being, and why, on the contrary, the proper use of the muscles should be so essential a condition for the maintenance of health, since it is in them that the great formation of the eliminated carbon is effected. We shall also not fail to see why, since during exercise the excretion of water is so largely increased, the blood necessarily becomes less diluted and richer in quantity.
[291] “Deficient exercise is one of the causes which produce those nutritional alterations in the lung which we class as tuberculosis.”—Parkes.
Whilst insufficiency of exercise gives rise to a weak action of the heart, and very frequently to fatty degeneration of that organ, exercise that is not excessive, although it increases the beats of the heart from ten to thirty beyond this acceleration, and imparting greater force to the pulsations, does not interfere with their regularity. Furthermore, muscular exercise, by considerably augmenting the flow of the blood through the whole body, the heart included, exercises a most beneficial function, “since it causes in all organs a more rapid outflow of plasma and absorption—in other words, a quicker renewal. In this way also it removes the products of their action, which accumulate in organs, and restores the power of action to various parts of the body.”[292]
[292] Parkes.
Palpitation, enlargement, and valvular disease of the heart result from excessive or injudicious exercise. Wherever, therefore, fatigue or embarrassment of the heart shows itself rest must be had recourse to. Persons having weak hearts suffer greatly in ascending mountain or other heights.
The effect of exercise upon the kidneys is to diminish the quantity of water, as well as the chloride of sodium and other chlorides in the urine. As we have seen, the urea is very slightly lower; but after much exertion the uric acid is increased. There is also a slight increase in the amounts of sulphates and carbonic anhydride. Parkes could find no alteration in the phosphates. The diminution of water and the chlorides is due to the excretion of these by the skin, the function of which is greatly augmented by exercise. No urea escapes by the skin, but many acids (probably fatty ones) are liberated by that organ. Speck has shown that during exercise the amount of fluid is nearly double what it is when the body is quiescent.
This escape of fluid by perspiration doubtless affords an explanation of a diminution in the quantity of the excreta from the bowels. The fæces exhibit no decrease in nitrogen.
Exercise increases the growth of the muscles, making them at the same time harder,678 and also causing them to obey more readily the behests of the will. Prolonged or excessive exertion, without sufficient rest, has been found to interfere with their nutrition, and to cause them to become soft.
There is a tolerably general impression that much exercise tends to cripple the development of the mental faculties, and this idea is said to have received support from the circumstance that the athletes at our universities seldom signalise themselves in contests of learning. But this fact, it has been suggested, may be explained by the athletic exercise being indulged in to such an extent as to leave no time for cultivating the mind. If an illustration were required to prove that great bodily energy is quite consonant with mental vigour it might be found in the life of the late Professor Wilson, of Edinburgh. On this point Dr Parkes says: “Considering that perfect nutrition is not possible except with bodily activity, we should infer that sufficient exercise would be necessary for the perfect performance of mental work.”
As regards the changes that take place in the muscles during exercise Dr Parkes writes: “The chief changes that take place in the muscles during action appear to be these: there is a considerable increase of temperature (Helmholtz), which, up to a certain point, is proportioned to the amount of work; it is also proportioned to the kind, being less when the muscle is allowed to shorten than if prevented from shortening (Heidenhain); the neutral or alkaline reaction of the tranquil muscle becomes acid from para-lactic acid and acid potassium phosphate; the venous blood passing from the muscles becomes much darker in colour, is much less rich in oxygen, and contains much more carbonic acid (Sczelkow); the extractive matters soluble in water lessen, those soluble in alcohol increase (Helmholtz, in frogs); the amount of water increases (in tetanus, J. Ranke), and the blood is consequently poorer in water; the amount of albumen in tetanus is less, according to Ranke, but Kühne has pointed out that the numbers do not justify the inference.”
Liebig stated that the creatin is increased (but this was an inference from old observations on the extractum carnis of hunted animals, and requires confirmation). Sarokin has stated the same fact in respect to the frog. The electro-motor currents show a decided diminution during contraction.
That great molecular changes go on in the contracting muscles is certain, but their exact nature is not clear; according to Ludimar Hermann there is a jelly-like separation and coagulation of the myosin, and then a resumption of its prior form, so that there is a continual splitting of the muscular structure into a myosin coagulum, carbonic acid, and a free acid, and this constitutes the main molecular movement. But no direct evidence has been given of this.
The increased heat, the great amount of carbonic acid, and the disappearance of oxygen, combined with the respiratory phenomena already noted, all seem to show that an active oxidation goes on; and it is very probable that this is the source of the muscular action. The oxidation may be conceived to take place in two ways—either during rest oxygen is absorbed and stored up in the muscles, and gradually acts there, producing a substance which, when the muscle contracts, splits up into lactic acid, carbonic acid, &c.; or, on the other hand, during the contraction an increased absorption of oxygen goes on in the blood, and acts on the muscles, or on the substances in the blood circulating through the muscles. The first view is strengthened by some of Pettenkofer and Voit’s experiments, which show that during rest a certain amount of storage of oxygen goes on, which no doubt in part occurs in the muscles themselves.
Indeed, it has been inferred that it is this stored-up oxygen, and not that breathed in at the time, which is used in muscular action. The increased oxidation gives us a reason why the nitrogenous food must be increased during periods of great exertion.
An increase in the supply of oxygen is a necessity for increased muscular action; but Pettenkofer and Voit’s observations have shown that the absorption of oxygen is dependent on the amount and action of the nitrogenous structures of the body, so that, as a matter of course, if more oxygen is required for increased muscular work, more nitrogenous food is necessary. But, apart from this, although experiments on the amount of nitrogenous elimination show no very great change on the whole, there is no doubt that, with constant regular exercise, a muscle enlarges, becomes thicker, heavier, contains more solid matter, and, in fact, has gained in nitrogen. This process may be slow, but it is certain; and the nitrogen must either be supplied by increased food, or be taken from other parts.
So that, although we do not know the exact changes going on in the muscles, it is regarded as certain that regular exercise produces in them an addition of nitrogenous tissue.
Whether this addition occurs, as usually believed, in the period of rest succeeding action, when in some unexplained way the destruction which it is presumed has taken place is not only repaired, but is exceeded (a process difficult to understand), or whether the addition of nitrogen is actually made during the action of the muscle, must be left undecided for the present.
The substances which are thus oxidised in the muscle or in the blood circulating through it, and from which the energy manifested as heat or muscular movement is believed to be derived, may probably be of different kinds. Under ordinary circumstances the experiments679 of Fick and Wislicenus and others, and the arguments of Traube, seem sufficient to show that the non-nitrogenous substances, and perhaps especially the fats, furnish the chief substances acted upon. But it is probable that the nitrogenous substances also furnish a contingent of force. The exact mode in which the energy thus liberated by oxidation is made to assume the form of mechanical motion is quite obscure.
There seems little doubt that the exhaustion of muscles is chiefly owing to two causes—first and principally to the accumulation in them of the products of their own action (especially para-lactic acid); and secondly, from the exhaustion of the supply of oxygen. Hence rest is necessary, in order that the blood may neutralise and carry away the products of action, so that the muscle may recover its neutrality and its normal electrical currents, and may again acquire oxygen in sufficient quantity for the next contraction.
In the case of all muscles these intervals of action and of exhaustion take place, in part even of the period which is called exercise; but the rest is not sufficient entirely to restore it. In the case of the heart the rest between the contractions (about two thirds of the time) is sufficient to allow the muscle to perfectly recover itself.
The foregoing remarks on the effects of muscular exercise will have prepared us for the inference which statistics abundantly support, viz. that, other conditions being favorable, the healthiest occupation is that which consists in the practice (of course within reasonable limits) of manual labour in the open air.
The Rev. Professor Haughton, in his work entitled ‘A New Theory of Manual Labour,’ has drawn up a table (which we append) of the amount of force expended during various kinds of work. It represents the number of tons lifted one foot per diem:—
| Labouring Force of Man. | |||
| Kind of Work. | Amount of Work. | Authority. | |
| Pile-driving | 312 | tons lifted 1 foot. | Coulomb. |
| Pile-driving | 352 | ” | Lamaude. |
| Turning a winch | 374 | ” | Coulomb. |
| Porters carrying goods, and returning unladen | 325 | ” | ” |
| Pedlars always loaded | 303 | ” | ” |
| Porters carrying wood up a stair, and returning unloaded | 381 | ” | ” |
| Paviours at work | 352 | ” | Haughton. |
| Military prisoners at shot drill (3 hours), and oakum-picking and drill | 310 | ” | ” |
| Shot drill alone (3 hours) | 160·7 | ” | ” |
Professor Haughton has devised a formula by means of which a certain amount of walking exercise may be made to represent its equivalent in manual labour. He points out that walking on a level surface is equivalent to raising one twentieth part of the weight of the body through the distance walked.
When ascending any height, the whole weight of the body is, of course, raised through the ascent. The formula is—
where W is the weight of the person; Wl the weight carried (if any); D the distance walked in feet; 20 the co-efficient of traction; and 2240 the number of pounds in a ton. The result is the number of tons raised one foot. To get the distance in feet 5280 must be multiplied by the number of miles walked.
Supposing a man to weigh 150 lbs. with his clothes, by the employment of the above formula we should arrive at the following results:—
| Kind of Exercise. | Work done in tons lifted 1 foot. |
| Walking 1 mile | 17·67 |
| Walking 2 miles | 35·34 |
| Walking 10 miles | 176·7 |
| Walking 20 miles | 353·4 |
| Walking 1 mile and carrying 60 lbs. | 24·75 |
| Walking 2 miles and carrying 60 lbs. | 49·5 |
| Walking 10 miles and carrying 60 lbs. | 247·5 |
| Walking 20 miles and carrying 60 lbs. | 495 |
From the above data something like a rough approximation may be formed of the daily amount of exercise requisite for a healthy male adult.
Since 500 tons lifted a foot is extremely hard work, the number of miles corresponding to this extreme amount of labour would, if persevered in, be objectionable.
Dr Parkes, regarding 300 tons lifted a foot as an average day’s work for a healthy man, thinks that walking exercise equivalent to half that amount should be taken daily.680 This, or a 150 tons, represents a nine miles’ walk. He, however, qualifies the suggestion by adding “that, as there is much exertion taken in the ordinary business of life, this amount may be in many cases reduced;” and concludes by saying, “It is not possible to lay down rules to meet all cases, but probably every man with the above facts before him could fix the amount necessary for himself with tolerable accuracy.”
For muscular exercise to be safe and efficient, it must be taken under certain conditions and precautions. We have noticed the evil effects of immoderate bodily exertion on the heart. The lungs are no less seriously affected by an excessive indulgence in it, which shows itself in spitting of blood and in congestion of the pulmonary vessels. Congestion of the lungs brought on by overtaxed bodily strength very frequently causes the death of horses in the hunting field.
These facts, therefore, not only point to the importance of avoiding undue or extreme exertion,[293] but also to the necessity of ensuring the full and uncramped play of the respiratory organs during exercise, and the consequent removal of any impediment in the way of tight clothing that in any manner interferes with their freedom of exercise. Laboured respiration and sighing are indications of pulmonary congestion, and counsel temporary rest and abstention from exercise.
[293] “There must be proper intervals of rest, or the store of oxygen, and of the material in the muscles which is to be metamorphosed during contraction, cannot take place.”
The great augmentation in the excretion of carbon which leaves the lungs in the form of carbonic anhydride during exercise has been already referred to. As this carbon is derived from the food, it follows that in the intervals of exercise an increase of carbonaceous diet is necessary. For this purpose physiologists prefer the fatty to the amylaceous varieties of diet. It has been already stated why at the same time the nitrogenous food must be increased during periods of great exertion. There seems little doubt that water is the best drink that can be taken during moderate as well as great exercise.... It is best taken in small quantities and frequently. Spirits are decidedly prejudicial, and indispose to bodily exertion. They are hurtful because they lessen the exhalation of carbonic anhydride from the lungs. Trainers never allow them, and but very little wine or beer.
The thirst that not unfrequently accompanies exercise is due to the great escape of water from the skin which has been already alluded to. This liberation of moisture, being also accompanied, as already explained, by a large excretion of the chlorides and, perhaps, by other salts. Dr Parkes advises the use of an additional supply of chloride of sodium to the diet of those taking much exercise; he suggests that probably potassium chloride and phosphate might be added with advantage.
The evaporation from the skin has the effect of reducing the bodily temperature and rendering it equable. This temperature, however, falls very rapidly after exertion is over; and hence at this time it is always advisable to guard against the chance of a chill by covering the body over. Flannel forms the best protection. Keeping the skin clean by daily ablution greatly aids in the escape of fluid during exercise.
The large amount of carbonic anhydride given off by the lungs during bodily exercise explains the advantages of open air exercise, and why walking in the fresh air produces such excellent effects in some forms of dyspepsia. This increased exhalation of carbonic anhydride also points to the importance of thorough ventilation when indoor exercise is taken, particularly by large bodies of men or women, as in riding schools and on the treadmill. The mortality amongst miners, whose labour is performed in confined and ill-ventilated spaces is very great. According to Mr Simon, with the exception of those who work in the well-ventilated mines of Durham and Northumberland, the 300,000 miners in England break down prematurely from bronchitis and pneumonia, caused by the atmosphere in which they are compelled to work.
EXPAN′SION. All substances, solid, liquid, and gaseous, when chemical change does not take place, expand by heat, and contract by cold. In some of them this property occurs in a greater degree than in others, but is constant for the same substance under the same circumstances. The chemist avails himself of this property in the construction of his thermometer; the wheelwright, in fixing on the tire of his wheels; the engineer, in restoring to the perpendicular the leaning walls of buildings, &c.
This expansion by heat is of great importance in the manufactures, as allowance has to be made of it in every purpose where metals are employed.
The following is a list of the expansion of the chief metals, &c., when heated from 32° to 212° Fahr., or from 0° to 100° Cent.:—
| Substance. | Expansion. | |
| In bulk. | In length. | |
| Glass | 1 in 384 | 1 in 1150 |
| Platinum | 1 in 377 | 1 in 1311 |
| Steel | 1 in 309 | 1 in 926 |
| Iron | 1 in 282 | 1 in 846 |
| Gold | 1 in 227 | 1 in 682 |
| Copper | 1 in 194 | 1 in 582 |
| Brass | 1 in 179 | 1 in 536 |
| Silver | 1 in 175 | 1 in 524 |
| Tin | 1 in 172 | 1 in 516 |
| Lead | 1 in 117 | 1 in 351 |
| Zinc | 1 in 113 | 1 in 340 |
Of the liquids, they expand as follows, when681 heated from 0° to 100° Cent., or from 32° to 212° Fahr.:—
| Mercury | 1 in 55 in bulk. |
| Water | 1 in 21 in bulk. |
Gases practically all expand alike; that is to say, for every degree Fahrenheit a gas expands 1⁄491 of its bulk at 32°, and for every degree Centigrade 1⁄273 of their volume at 0°C.
An example will show the importance of this. Suppose an iron bar, connecting two sides of a building, and of a length of about 85 feet. The increase in length by heat of this bar would make it 1 inch longer in summer than in winter; and it would, if no allowance be made, pull or thrust the walls to this extent each year.
EXPEC′TORANTS. Syn. Expectorantia, L. Medicines that promote the secretion of the trachial and bronchial mucus. According to Dr Good, true expectorants are “those medicines which rather promote the separation of the viscid phlegm with which the bronchiæ are loaded, than simply inviscate and dilute it; though these are also treated as expectorants by many writers.” Ammoniacum, antimonials, assafœtida, the balsams of Peru and tolu, benzoic acid, benzoin; the fumes of vinegar, tar, and several of the volatile oils; garlic, ipecacuanha, the oleo-resins, squills, tartarised antimony, and the smoke of tobacco and stramonium, are among the principal substances commonly called expectorants. Tartarised antimony, squills, chlorine, and ammoniacal gases, have also been used (diluted) to provoke the coughing and favour the expulsion of foreign bodies from the air-passages; and also to favour the expectoration of mucus, pus, and membranous concretions, when the local irritation is not sufficiently great. (Schwilgue.) Expectorants are commonly employed in pulmonary complaints and affections of the air tubes, attended by a vitiated state of the mucus, or an imperfect performance of the natural functions of the secretory vessels. “Of all classes of the materia medica, none are more uncertain in their action than expectorants.” (Pereira.) The act of ejecting matter from the chest is called EXPECTORATION.
EXPER′IMENTS are acts or operations intended to develop some unknown fact, principle, or effect; or to establish or demonstrate it, when discovered. Similar operations, performed merely for amusement, are also often, though incorrectly, called by this name. In rational experiments these two objects are combined. To experimental research is due the present high state of advancement and usefulness of the various sciences most intimately connected with our happiness and well-being. The danger of taking things for granted has been thus pleasantly and instructively pointed out by Archbishop Whately:—“It was objected to the system of Copernicus, when first brought forward, that if the earth turned on its axis, as he represented, a stone dropped from the summit of a tower would not fall at the foot of it, but at a great distance to the west; in the same manner as a stone dropped from the masthead of a ship in full sail does not fall at the foot of the mast, but towards the stern. To this it was answered, that a stone, being a part of the earth, obeys the same laws, and moves with it; whereas it is no part of the ship, of which, consequently, its motion is independent. The solution was admitted by some, but opposed by others; and the controversy went on with spirit; nor was it till one hundred years after the death of Copernicus that, the experiment being tried, it was ascertained that the stone, thus dropped from the head of the mast, does fall at the foot of it.”
EXPORTATION. (Exportation on Drawback.) By law, a certain allowance, or drawback of duty, is payable on certain articles, when exported from any part of the United Kingdom, either as merchandise or ship stores. Thus:—
Sugar, refined in the United Kingdom, from 4s. to 6s. per cwt., according to quality.
Tobacco, manufactured in the United Kingdom, 3s. 3d. per lb. The full drawback is only allowed on normal tobacco, which contains 13 per cent. of moisture. If the moisture exceeds 13 per cent., a proportionate reduction is made in the drawback; if it is found less than 13 per cent., a proportionate increase is granted.
Snuff is entitled to drawback at 3s. 3d. per lb., subject, however, to an increase if the moisture is less than 13 per cent., and the inorganic matter not over 18 per cent., and to a decrease if the moisture in organic matter exceeds these per-centages.
Beer. The amount of this drawback is proportional to the quantity of malt or sugar used in the brewing of the beer, and is nearly equivalent to the duty originally paid on such malt or sugar. It is computed according to the following scale:—For every barrel, or 36 gallons of beer, the original gravity of which was not less than 1040°, a drawback of 4s. 3d., and for every additional 5 degrees, from 1040° to 1125° inclusive, a further sum of 6d. per barrel.
Solidified Worts, made by a licensed brewer, from malt or sugar, or malt and sugar, a drawback of 2s. 105⁄100d. per 28 lbs.
Malt. Under certain restrictions, a drawback of the duty charged, after deducting 71⁄2 per cent. of the measured quantity.
Spirits, from 10s. to 10s. 3d. per proof gallon.
In all cases samples are taken by the Custom House officer, and forwarded to the Inland Revenue laboratory, where they are examined previous to the payment of the drawback.
EXPRES′SION. In the useful arts, the mechanical operation by which a fluid contained in the pores or cells of a solid is pressed out or expelled. Many of the fluid substances employed in pharmacy and chemistry are obtained682 by expression. Thus, the unctuous vegetable oils, as those of almonds, linseed, &c., are procured by submitting these substances to powerful pressure between iron plates, which are either made warm, or the bruised seeds are previously exposed in bags to the steam of boiling water. The juices of fresh vegetables are also obtained by expression. The substances are first bruised in a marble mortar, or, on the large scale, in a mill, and immediately submitted to the press, to prevent them passing into a state of fermentation, which would injure the quality of the product. Fruits which contain highly flavoured or fragrant seeds, or which have rinds containing essential oil, are generally deprived of them before being sent to the press. The subacid fruits are also allowed to lay together for some days before pressing them, as the quantity and quality of the product is thereby increased. The fluid matter absorbed by the ingredients employed in the preparation of tinctures, infusions, decoctions, extracts, &c., is generally obtained by powerful pressure. Expression is also frequently had recourse to for the purpose of obtaining solids in a state of purity, as in the expulsion of olein from stearin, water from bicarbonate of soda, &c. On the small scale, the common screw-press, or one of like construction, is usually employed; but the power thus obtained is insufficient to expel the whole of a fluid diffused through the pores of a solid. Hence has arisen the use of the hydraulic press, which is now almost alone employed on the large scale. In all these cases the substances are placed in bags made of haircloth, or coarse canvas, previously to their being submitted to pressure. For tinctures and like pharmaceuticals, a small screw-press (TINCTURE PRESS) made of ‘galvanised’ or tinned iron, and varying in capacity from 1 quart to several gallons, is employed.
EXSICCA′TION. See Desiccation.
EX′TRACT. Syn. Extractum, L. Among chemists this term is understood to apply to the residuum of the evaporation of aqueous decoctions or infusions of vegetable matter. In medicine and pharmacy, it has a less definite signification, being applied to various preparations obtained by evaporating the expressed juices, or the decoctions, infusions, or tinctures of vegetable substances, until a mass, of a solid or semi-solid consistence is formed. Extracts vary in their nature and composition with the substances from which they are prepared, and the fluids employed as solvents. When water is employed as the menstruum, the products (AQUEOUS EXTRACTS, WATERY E.; EXTRACTA AQUOSA, E. SIMPLICIORA, L.) usually consist of gum, starch, sugar, albumen, extractive and saline and other matter, along with the peculiar principles on which the medicinal virtue of the vegetable depends. When spirit is employed as the solvent, the products (ALCOHOLIC EXTRACTS; EXTRACTA ALCOHOLICA, L.) contain most of the substances above enumerated, except the gum and starch, together with several other substances which are soluble in spirit, but which are either wholly or nearly insoluble in water; as resins, essential oils, and the proximate principles of vegetables. These preparations, with scarcely an exception, are considerably more powerful than the aqueous extracts of the same vegetables. In some cases proof spirit or under-proof spirit is employed, when the extracts (SPIRITUOUS EXTRACTS; EXTRACTA SPIRITUOSA, L.) generally possess properties between those of the above. In other cases, dilute acetic acid or acidulated water is employed as the menstruum, when the products (ACETIC EXTRACTS; EXTRACTA ACETICA, L.) possess much greater activity than when prepared with water; and would in many cases prove fatal, if exhibited in doses as large as those of the aqueous extracts. Still more active extracts are obtained by a combination of the last two menstrua. According to Ferrari, plants treated with rectified spirit of wine, mixed with 1⁄36th part of acetic acid, yields extracts of remarkable activity. On the Continent ether is sometimes used as the menstruum for the active principles of certain substances, as cantharides, cubebs, worm-seed, &c. (ETHEREAL EXTRACTS; EXTRACTA ETHEREA, L.) The term ‘simple extract’ is applied to an extract prepared from a single plant or vegetable substance, and the term ‘compound extract’ to one prepared from two or more of such substances. The FLUID EXTRACTS (EXTRACTA FLUIDA, L.) of modern pharmacy are those which are only evaporated to the consistence of a thin syrup, and then mixed with 1-10th to 1-8th of their volume of rectified spirit.
Prep. The preparation of medicinal extracts may be conveniently considered under two divisions, viz.—1. The production of a solution of the soluble portion of the substances operated on; and, 2. The reduction of this solution by evaporation to the consistence of an extract.
1. Preparation of Solutions:—The preliminary operations in the manufacture of extracts are similar to those employed in the preparation of DECOCTIONS, INFUSIONS, and TINCTURES. The proper quantity of the ingredients being taken, the whole is well bruised or reduced to coarse powder, or otherwise divided by slicing with a knife, that every portion may be fully exposed to the solvent action of the fluid. In some few cases (as with gentian, &c.) the ‘slicing,’ or reduction to fragments, is often conveniently deferred until the action of the menstruum shall have so far softened the ingredients as to render them of easy division by the knife. Those substances (as sarsaparilla, chamomiles, &c.) whose medicinal principles reside in the cortical portion, of which are of easy solubility, are commonly subjected to the action of the menstruum without being subjected to any particular preparation.
683
In the preparation of AQUEOUS EXTRACTS, the ingredients are treated with water until all the soluble matter that it is desirable to obtain is dissolved out. There are several methods of effecting this object, depending upon the nature of the substances acted on. In some cases maceration in cold water is resorted to; in others percolation with that fluid in a ‘displacement apparatus.’ More generally, however, boiling water is poured on the substance, and is digested on it for some time, as in the preparation of infusions; or the substance is exhausted by boiling in water, as in the preparation of decoctions. After the ebullition or infusion has continued a sufficient time, the heat is removed, and the liquid portion drawn off. The ingredients are then pressed to extract the remaining liquid; or they are washed or ‘sparged’ with hot water, which expels it by displacement. According to the usual practice in the majority of cases, a second quantity of water is poured on after the first has been thoroughly drained off, and the effusion or decoction is repeated a second and even a third time, or until the ingredients are perfectly exhausted of their soluble portion. The liquor or liquors thus obtained being allowed to repose for 15 or 20 minutes, for the purpose of depositing the sand or other gritty and heavy matter that is mechanically mixed with them, are carefully decanted from the sediment, and, after being run through a fine hair-sieve, or flannel bag, are ready for concentration. In some instances, however, this method proves insufficient to render the liquid clear. In such cases, the solution may generally be rendered transparent by clarification with a little white of egg, removing the scum as it rises, straining the liquid through flannel, as before; or the liquid may be filtered through a bag made of fine ‘Welsh flannel,’ or of ‘tweeled cotton cloth’ (Canton flannel), both of which should be soaked in clean water for at least an hour before use. In the small way, filters of linen or paper are sometimes employed; but as all media sufficiently fine to render vegetable solutions transparent soon choke up, this filtration is objectionable, from the length of time it occupies. In some houses the aqueous infusion or decoction is allowed to repose for 24 hours, and then decanted and evaporated; but such a plan is objectionable, as, however smooth and glossy extracts so prepared may appear, their medicinal virtues are lessened by the lengthened exposure to the atmosphere.
When about one half of an aqueous solution has evaporated, it is often advantageous to repass it through a flannel or horsehair strainer, to remove the flocculi that generally form by the action of the heat and air. This is especially necessary with vegetable solutions prepared without boiling, and should be adopted whenever a smooth and slightly extract is desired.
II. Reduction of Solutions:—The reduction of the solution to the proper consistence is effected by evaporation. The mode in which this is performed varies for different extracts. The London College directs that, “unless otherwise ordered, the evaporation should be conducted as quickly as possible, in a broad, shallow pan, placed in a water bath, until a proper consistence is acquired for forming pills; stirring assiduously with a spatula towards the end of the operation.” The Dublin College orders that “all simple (aqueous) extracts (EXTRACTA SIMPLICIORA), unless otherwise ordered, are to be prepared by boiling the vegetable matter in 8 times its weight of water, till the liquid is reduced to one half; the liquor is then to be expressed, and, after a short time allowed for defecation, to be decanted, filtered, and evaporated in a steam or water bath, until it begins to thicken, and then finally inspissated by a reduced heat, with continual stirring, until a consistence for forming pills be attained.” The instructions of the Edinburgh College are similar, with the one important exception, however, of ordering the evaporation to be conducted in a water bath saturated with chloride of sodium.
Though the water bath has the sanction of the London College, it is ill adapted for the purpose to which it is here ordered to be applied, as from its low evaporative power the advantages which are derived from its equable temperature are vastly overbalanced by the lengthened exposure of the solution in a heated state to the action of the atmosphere. It has been shown that a vegetable extract so prepared is inferior in quality to a similar one formed by rapid evaporation in a shallow pan over a naked fire, or placed in a sand bath, provided proper care is taken, and assiduous stirring is adopted during the whole time of the exposure to heat. In practice, however, the use of a naked fire is perfectly inadmissible, as the least neglect on the part of the operator would probably lead to the incineration of the whole. These objections are obviated by the addition of the 1⁄5th part of salt to the water of the bath, which raises its boiling-point to 2183⁄4° Fahr., when the temperature of the contained extract is fully 212°; the remaining 6° being lost by the interposition of the substance of the evaporating vessel.
On the large scale, the evaporation of infusions or decoctions for extracts is usually conducted in very wide, shallow, copper or tinned-copper pans, having steam-tight jackets of cast iron, and heated by steam ‘playing’ between the two.
The rapid deterioration which vegetable juices and solutions undergo by exposure to the air, especially at high temperatures, has led to the introduction of apparatus, by which they may be concentrated without contact with the atmosphere, and at a less degree of heat than is required for that purpose in open vessels. Such is the method, commonly called ‘Barry’s process,’ in which the air is removed from certain684 air-tight refrigerators by the introduction of steam, which is then condensed by the application of cold, by which means a partial vacuum is obtained. Another process for attenuating the atmosphere over the surface of fluids during evaporation is by the action of an air-pump. This plan was introduced by Howard, and is commonly applied to the concentration of syrups in the sugar refineries. Extracts obtained by either of these methods are said to be prepared ‘in vacuo,’ and are found in practice to be immensely superior to the common extracts of the shops, and consequently require to be exhibited in proportionably small doses.
‘The American Journal of Pharmacy’ for September, 1877, contains a new process for the preparation of extracts without heat, by Professor Herrara. We extract the following from the Professor’s paper:—
“The results of my observations have satisfied me that, when the water partially congeals, the dissolved principles remain in solution in the mother liquors, and that two or three congelations are generally sufficient for obtaining the solutions concentrated enough to finish the extract by exposure upon plates to the heat of the sun, or of a drying closet, heated to about 30° cent. (86° Fahr.). The extracts prepared by this method accurately represent the properties of the plants, and the principles which are changed by the influence of heat remain unaltered; even the volatile constituents are not dissipated, though most of the water be removed by freezing. Owing to the small cost of the necessary apparatus, it appears to me that my process for preparing extracts should be preferable even in those countries where ice is less readily obtainable than combustibles.
“Extract of conium prepared with unpurified juice by the process mentioned, has preserved the characteristic odour of conia, and by dissolving it in water. I have obtained a solution exactly representing the juice of the plant in appearance and properties, and giving when heated an abundant coagulation, proving that even albumen had remained unaltered. 1750 grams of cow’s milk at 9° R., left, after three congelations, 750 grams of a liquid having a density of 148, and by evaporation in the sun this left a dry extract of milk, which again formed that liquid on being dissolved in water. A number of other liquids similarly treated, gave corresponding results, and it seems to me, therefore, that medicinal extracts are best prepared by congelation. It may be objected that the medical juices should be previously purified, but it should be remembered that coagulated albumen always encloses a considerable portion of the active principles, and that the heat necessary to effect the coagulation and the evaporation by means of a water-bath is sufficient to change many principles; also that the extracts thus prepared are sometimes inert or less active. The careful experiments made by Orfila and the clinical experience of others demonstrate that extracts prepared with unpurified juice are stronger.
“The apparatus employed by me is the so-called sorbetière;[294] for larger quantities the apparatus of Gougaud is preferable. The frigoric mixture is composed of ice and sodium chloride, or preferably of crystallised calcium chloride. After a large portion of the solution has congealed, the mass is enclosed in a cloth and subjected to pressure, the press-cake of ice is broken and again pressed, to separate the mother liquor as completely as possible, and the congelation is repeated two or three times, with the precaution that it is not carried far enough to cause the precipitation of the sparingly soluble principles. The mother liquor is then put into shallow dishes and exposed to the heat of the sun or of a drying room, the temperature of which does not exceed 30° C. (86° F.) until the extract has attained the desired consistence.”
[294] An apparatus similar to that used for ice-cream.
Obs. When water, acidulated with acetic acid, is employed in the preparation of extracts, the vegetable substances are usually macerated in it, in the cold, or the dilute acid is sprinkled over the bruised plant in the fresh or recent state, and the whole is then submitted to strong pressure, to expel the juice, which is strained and evaporated in the usual way, but preferably in a well-tinned or plated-copper pan.
Alcoholic and SPIRITUOUS EXTRACTS are prepared by evaporating a filtered concentrated tincture of the ingredients in any suitable vessel, by which the volatilized spirit may be saved. In general, rectified spirit is used as the menstruum; but in some cases proof spirit is employed; and, in others, the substances are first digested in proof spirit, and afterwards in water, and the mixed tincture and infusion evaporated in the usual manner.
Ethereal extracts are obtained in a similar manner to alcoholic ones; but being merely prepared in small quantities at a time, the process may be conveniently performed in glass vessels. When it is required to boil either of the above fluids (alcoholic or ethereal), or any other volatile liquid on the ingredients, a vessel fitted with a long tube, or a Liebig’s condenser reversed, as noticed under ether, may be used to prevent any loss of the menstruum.
The INSPISSATED VEGETABLE JUICES (JUICES, E.; SUCCI, L.) of the British Pharmacopœia are obtained by expressing the juices from the fresh plants, and preserving them by the addition of spirit. “By thus preserving the juice of the plant its properties are not impaired by the action of the air during the time necessary to dry the leaf for tincture, nor by the action of both air and heat during the time necessary to evaporate the juice to the consistence of an extract.”—Squire. The directions of the Edinburgh College for preparing their inspissated juices (SUCCI SPISSATI,685 L.) are—“Beat the fresh substance, and press it strongly through a canvas bag, in order to obtain the juice; which, being put into a wide, shallow vessel, and heated by means of boiling water saturated with sea-salt, is to be reduced to the consistence of honey. The mass, when cold, is to be put into glazed earthen vessels, and moistened with strong alcohol.” By operating in this way a considerable portion of the activity of narcotic vegetables is lost. Some of their juices, as that of aconite, are impaired in so short a time as scarcely to compensate for the trouble of preparing them. This deterioration does not, however, take place in any remarkable degree, if the expressed juice from the recent vegetable be evaporated by exposing it in a thin stratum to a current of very dry air, as adopted by Mr Squire. This may be managed by putting the juice into small, flat trays or dishes, placed on shelves in a suitably arranged apparatus, alternated with similar vessels of concentrated sulphuric acid; or by causing a current of very dry air, at the common temperature of the atmosphere, to pass over them. It has been shown that 10 gr. of extract, thus prepared, were more than equal to 20 gr. prepared in vacuo; and to more than 60 gr., and in some cases, 90 gr., of those prepared by the common process of boiling down the juice to an extract.
The concluding portion of the process of extract-making, technically termed ‘finishing-off,’ requires the most scrupulous attention. As the evaporation advances, the heat should be lessened, and as soon as the extract acquires the consistence of thick treacle it should be removed altogether, and the remainder of fluid matter evaporated by the heat retained by the copper pan, the escape of vapour being promoted by assiduous and laborious stirring with a suitably shaped wooden spatula. This part of the process should be continued until a proper consistence is attained and the extract is nearly cold. When high-pressure steam or a chloride of calcium bath is employed, care must be taken to withdraw the heat before stirring the semi-liquid mass; as, if an extract having a temperature of about the boiling-point of water, or even a few degrees below it, is agitated, it becomes full of bubbles, and appears rough and puffy, and this appearance cannot be removed by subsequent stirring, or by any method but redissolving it in water and re-evaporation. This is especially the case with the extracts of sarsaparilla (simple and compound), gentian, liquorice, and most others of a similar class. A good laboratory man knows from experience the proper time for the removal of the heat, but unpractised persons often fail in this particular. In such cases should the heat retained by the evaporating pan, and by the extract, prove insufficient to complete the process, a little more may be cautiously applied. Without assiduous and laborious stirring in the way described, a very smooth and glossy extract cannot be produced. To promote this artificial appearance, some persons add 3% or 4% each of olive oil and gum arabic, dissolved in water, with about 1% or 2% of spirit of wine.
The consistence of the ordinary extracts of the shops is the same as that of electuaries and confections, and is described in the Ph. E. as equal to that of “thick honey.” The instructions of the Ph. L. and D., to evaporate the mass “until it acquires a consistence proper for making pills,” except in 2 or 3 cases (as Ext. Colocynth. Comp., &c.), is not adopted, and, indeed, would be found inconvenient in practice. Extracts evaporated to such a consistence are commonly termed ‘pilular extracts,’ and when evaporated so that they are quite dry, and brittle when cold, they are called ‘hard extracts’ (EXTRACTA DURA, L.).
Pres. Extracts should be put into pots as soon as taken from the pan, and, after being carefully and securely tied over with bladder, should be ‘stored’ in a dry situation. The London College orders “a small quantity of rectified spirit to be sprinkled upon all the softer extracts, to prevent them becoming mouldy.” A better way is to employ a little spirit, holding in solution a few drops of oil of cloves, or a still less quantity of creasote. This should be added to them the last thing before removing them from the evaporating pan, and when they are nearly cold. The same object is effected by moistening the inside of the bladder (used to tie them over) with a few drops of oil of cloves or creasote. Hard extracts should be kept in bladders or gut skins, placed in stone pots, and well covered over. With care, extracts prepared from recent vegetable substances may be preserved twelve months, or from season to season; and those from dry ingredients, or such as are less inclined to spoil, for perhaps double that time; but beyond these periods their virtues cannot be relied on, and they should consequently be discarded, if remaining unused or unsold.
Pur., &c. The quality of an extract cannot be ascertained by mere inspection, nor is it readily discovered by chemical tests. A knowledge of these facts has induced the mercenary and fraudulent manufacturer to employ damaged and inferior drugs in their preparation, alike regardless of the welfare of the patient and the credit of the practitioner. A common practice with some manufacturers is, not only to pick out the least expensive variety of every drug for the preparation of their extracts, but the most inferior and often damaged and worthless portion of this already inferior article. The production of a smooth, bright, and glossy extract is all that is usually attempted by these individuals, and all that is sought after by the mass of purchasers, who mistake the simulation of the mere external signs of good quality for its actual existence. It is a fact, which we can verify from extensive686 experience in the laboratory, and from years of practical observation on this point, that extracts faithfully prepared from good materials do not possess the sightly and pleasing appearance of those commonly vended by the wholesale druggists. On comparing the extracts prepared by different metropolitan houses, we have found that those which have exhibited a remarkably bright and glossy appearance have been uniformly inferior, and sometimes nearly inert; whilst others, with a less prepossessing appearance, have been generally of good quality. These facts are well established by reference to the extracts of those houses and institutions that are remarkable for the superior quality of their preparations, and by comparing them with the common extracts of the shops supplied by the wholesale trade.
A good extract should be—1. Free from grit, and wholly soluble in 20 parts of the menstruum employed in its preparation, forming a nearly clear solution.—2. It should have a uniform texture and colour, and be of a proper consistence.—3. If a narcotic or active extract, it may be exhibited in proper doses, and its effects watched. Its activity may also be tested on any small animal.—4. An assay for the proximate vegetable principle (alkaloid, &c.) contained in the plant from which it has been prepared may be made. The extracts prepared from the expressed juices of plants, without straining off the coagulated albumen, are, of course, exceptions to the first test. Unfortunately, these tests are not always easily performed, and the last two are inapplicable to those extracts that exercise no very marked physiological action, unless when taken in repeated doses, long continued. This want of a ready means of accurately testing the qualities of extracts has enabled the fraudulent manufacturer to sell inferior articles with impunity, and often without the least fear or danger of detection.
In general, an extract more than six months old contains only half the activity of a similar one newly made. When more than twelve months old they should be rejected as worthless, and the stock renewed.
Uses, &c. The extracts of the shops are generally acknowledged to be the most varying, imperfect, and uncertain class of medicines belonging to modern pharmacy. They are mostly used in the same cases as the plants from which they are prepared, but in smaller doses.
Concluding Remarks. In the preparation of extracts the great desiderata to be aimed at are—to suit the menstrua and the methods of manipulating to the peculiar characteristics of the active constituents of the vegetable substances operated on. The pharmaceutist should always bear in mind that a perfect extract should be a concentrated, solid mass, representing, as near as possible, in medicinal efficacy, the materials from which it has been prepared, and capable of being redissolved, so as to form a solution closely resembling that from which it has been derived. An extract possessing equal strength to the whole mass of the ingredients from which it has been prepared is almost next to an impossibility, however desirable such a degree of perfection may be. The medicinal properties of all solutions of vegetable matter are injured by being reduced to the solid state; and this deterioration, more or less, takes place, whether the solvent be water, acetic acid, proof spirit, or alcohol. The volatile portions (the essential oils, the aroma, &c.) are nearly or wholly dissipated; and though these do not always form the principal or active ingredients of the vegetables from which extracts are prepared, yet they generally exercise a modifying and controlling influence over the other ingredients, which considerably alters their therapeutical action. The loss of aroma may often be a trifling deficiency, but in the extracts of aconite, henbane, hemlock, belladonna, and other narcotic plants, this is not the case. In these cases it is well known that the inert preparations are wholly deficient of the odour of the recent plant, and that in proportion as the odour is developed, so is their activity preserved. The powerful smell of the recently expressed juice of hemlock, with the scarcely perceptible odour of the extract (EXTRACTUM CONII, Ph. L.), offers an excellent example of this fact. The dose of the one often reaches 20 or 30 gr., whilst that of the other seldom exceeds 5 or 10 drops, or a portion equivalent in dry ingredients to considerably less than 1⁄2 gr.
When extracts are ordered in prescriptions, those of the ‘Pharmacopœia’ should be alone employed by the dispenser, as the substitution of others for them would not only be violating faith with the prescriber, but might also produce consequences alike injurious to the dispenser and the patient. Many medical gentlemen prefer extracts prepared by particular processes or persons, but such intention is always indicated in their prescriptions.
Extract of Ac′onite. Syn. Extract of wolfsbane, E. of monkshood, Inspissated juice of aconite; Extractum aconiti (B. P., Ph. L. E. & U. S.), Succus spissatus aconiti (Ph. D. 1826), L. Prep. 1. (B. P.) Take 112 lbs. of the fresh leaves and flowering tops, bruise them, press out the juice, heat it gradually to 130° F., and separate the green matter by a calico filter. Heat the strained liquor to 200° F. to coagulate albumen, and again filter. Evaporate the filtrate by a water bath to the consistence of a thin syrup; then add to it the green colouring matter previously separated, and stirring the whole together assiduously, evaporate at a temperature not exceeding 140° F. to a pill consistence.—Dose, 1 to 2 gr.
2. (Ph. L.) Take of fresh leaves of aconite, 1 lb.; bruise them in a stone mortar, express the juice, and evaporate it, unstrained, to a687 proper consistence. The formulæ of the Ph. D. & U. S. are similar.
3. (Ph. E.) Beat the fresh leaves of aconite to a pulp, and express the juice, then subject the residuum to percolation with rectified spirit until the latter passes through without being materially coloured; unite the expressed juice and the percolated tincture, filter, distil off the spirit, and evaporate in a vapour or a water bath to a proper consistence. Stronger than the preceding.
Obs. A variable and uncertain preparation. Numbness and tingling follow its application to the limbs or tongue when it is of good quality.—Product. 1 cwt. of fresh leaves yield between 5 lbs. and 6 lbs. of extract. Prop. Anodyne, sudorific, and narcotic; very poisonous.—Dose, 1⁄2 gr. to 2 gr., made into a pill with liquorice powder; once or twice a day, in neuralgic pains, chronic rheumatism, glandular swellings, &c., gradually and cautiously increased to 5 or 6 gr.
4. (Alcoholic; E. a. alcoholicum, L.)—a. (P. Cod.) Aconite (in coarse powder), 1 lb.; proof spirit, 31⁄2 lbs. (say 21⁄2 pints); proceed by the method of displacement, and when all the spirit has penetrated the powdered mass, keep this covered with distilled water, until the liquid begins to cause a precipitate in falling into that which has previously passed through; next distil the spirit from the tincture, and evaporate the residuum to the proper consistence.
b. (Ph. U. S.) Aconite 1 lb.; spirit, sp. gr. ·935 (= 13 u. p.), 1 quart, or q. s.; as last.
c. (Ph. Baden.) From the tincture prepared with rectified spirit, and by either maceration or displacement. Stronger than the last two.
d. (Ph. Bor.) The juice is expressed from the fresh herb, which is then sprinkled with about 1⁄3 of its weight of water, and again pressed; the mixed and strained liquid is evaporated in a vapour bath, at 122° to 140° Fahr., to about one half; to this, as soon as cold, an equal weight of spirit (sp. gr. ·900) is added, and after frequent agitation for 24 hours, the whole is filtered, with pressure; the marc is treated with fresh spirit (equal to about 1-4th that first used) and again pressed; the mixed liquors are next filtered, and are, lastly, evaporated, as before, to the proper consistence.
Obs. Resembles the simple extract, but is much more powerful. It has been exhibited internally in the form of pills, and used externally, combined with ointment or spread on simple plaster.—Dose, 1⁄12 to 1⁄6 gr. every three hours.
5. (Ammoniated; E. a. ammoniatum, L.—Dr Turnbull.) Extract of aconite, 1 dr.; liquor of ammonia (strongest), 10 or 12 drops; mix.
6. (Dried); E. a. siccum, L.—P. Cod.) The expressed juice, strained through a sieve or coarse linen, is at once, without depuration, exposed in earthen dishes, in layers of about 2 lines deep, in a stove or current of dry air, to a temperature ranging between 95° and 104° Fahr., until reduced to dryness. The dried extract is to be packed in bottles.
7. (Saccharated; E. a. saccharatum, L.) From extract of aconite (Ph. Bor.), 4 oz.; sugar of milk (in powder), 1 oz.; mix, and dry the mass in a warm place, adding sugar of milk, q. s. to make the whole equal in weight to that of the extract used (4 oz.). An excellent preparation, which keeps well. The other NARCOTIC EXTRACTS, as those of BELLADONNA, HEMLOCK, HENBANE, &c., are to be treated in a similar manner. See Aconite, and below.
Extract of Aconite Root. Syn. Extractum aconiti radicis alcoholicum, L. Prep. (Dr Fleming; Dr Turnbull.) From a tincture of the root made with rectified spirit. It is said to be 12 times as strong as the extract of the leaves.
Extract of Ag′aric. Syn. Extractum agarici, L. Prep. (P. Cod.) From the infusion of white agaric (Polyporus officinalis) prepared with cold water. Purgative.—Dose, 1 to 4 gr.
Extract of Alcorno′co. Syn. Extractum alcornocæ, L. Prep. From a decoction of alcornoco bark (South American). Astringent and tonic.—Dose, 5 to 20 gr. in phthisis, &c.
Extract of Al′oes. Syn. Purified aloes, Washed a.; Extractum aloës Barbadensis (B. P.), Extractum aloës (Ph. L.), E. a. aquosum (Ph. D.), L. Prep. 1. (B. P.) Barbadoes aloes, in small pieces, 1 lb.; treated with 1 gall. of boiling water for 12 hours, and the clear liquid evaporated.—Dose, 1 to 3 gr. B. P. 2 to 6 gr.
2. (B. P.) Socotrine aloes, 1 lb., treated with 1 gall. of boiling water for 12 hours, and the clear liquid evaporated to dryness.
3. (Ph. D.) Aloes (hepatic), 4 oz.; water, 1 quart; boil till dissolved; when cold, decant the clear liquid, and evaporate as before.
4. (Ph. Bor. 1847.) By macerating powdered aloes in cold water for 48 hours, with frequent agitation, and then evaporating in a water bath at a temperature not exceeding 150° to 165° Fahr., until a pilular consistence is attained.
Obs. The second is the form commonly adopted in the laboratory. When made with the juice of borage, burgloss, &c., it forms the old ‘ALOES INSUCCATA,’—Dose, 5 to 15 gr. See Aloes and Extract of Barbadoes aloes.
Extract of Aloes, prepared with Sulphuric Acid. Syn. Extractum aloës acido sulfurico correctum (Germ. Ph.). Prep. Dissolve extract of aloes, 8 ounces, in distilled water 32 ounces, then gradually add sulphuric acid, 1 oz. (by weight), and evaporate to a dry extract.
Extract of Anem′one. See Extract of Pasque Flower688.
Extract of Angel′ica. Syn. Extractum angelicæ, L. Prep. 1. (Ph. Baden.) From a tincture of the root, prepared with spirit sp. gr. ·944 (= 211⁄2 u. p.).
2. (Ph. Bor.) Angelica root and rectified spirit, of each 2 parts; water, 9 parts; digest, strain, and evaporate. Inferior to the preceding.
3. (Dr Moir.) Angelica root, 2 lbs.; rectified spirit, 1 gall.; make a tincture; to the ‘marc’ add proof spirit, 1 gall., and repeat the digestion; filter the two tinctures separately, mix, distil off the spirit, and evaporate. Balsamic, stomachic, and tonic.—Dose, 10 to 20 gr. The last is the most balsamic and agreeable.
Extract of Ap′ples. Syn. Chalybeated e. of a.; Extractum ferri pomatum, L. Prep. 1. (Ph. Bor.) Crab-apples (unripe), 6 lbs.; peel them and reduce them to a pulp; add iron wire (in small coils), 1 lb.; digest in a vapour bath for about a week, express, strain, decant, and evaporate in a porcelain vessel, with constant stirring, to the consistence of a soft extract; dissolve the residuum in water, 4 parts, strain and evaporate as before.—Dose, 5 to 10 gr.; as a chalybeate tonic. The formula of the Ph. Baden is nearly similar.
2. (Ph. Germ.) Reduce 5 lbs. of unripe apples to a pulp; mix them with cut straw, and press. To the strained juice after removal of the sediment add 11⁄2 oz. of reduced iron. When this has dissolved, to the cooled liquid add as much water as will make up 43⁄4 lbs. Filter, and reduce to a thick extract.
Extract of Ar′nica. Syn. Extract of arnica flowers; Extractum arnicæ florum, L. Prep. 1. (P. Cod.) From the dried flowers, as ALCOHOLIC EXTRACT OF ACONITE—P. Cod.
2. (Ph. Græca, 1837.) From a tincture of the flowers, prepared with rectified spirit, 3 parts, and water, 5 parts.—Dose, 2 to 6 gr.; as a stimulant in various diseases accompanied with debility, deficient nervous sensibility, paralysis, dropsies, diarrhœa, amenorrhœa, dysentery, &c.
Extract of Arnica-Root. Syn. Extract of arnica; Extractum arnicæ radicis, L. Prep. 1. (Ph. Baden.) As Extract of angelica—Ph. Baden.
2. (Ph. Græca.) From tincture of the root, prepared as No. 2 (above). The form of the Hamburg Codex is nearly similar.—Dose, &c. As the last.
Extract of Art′ichoke. Syn. Extractum Cynaræ, L. Prep. From the fresh leaves of the artichoke, as EXTRACT OF ACONITE—Ph. L.—Dose, 3 to 6 gr., twice or thrice daily; in rheumatism, &c.
Extract of Aspar′agus. Syn. Extractum asparagi, L. Prep. 1. (Soubeiran.) From the expressed juice of the shoots, clarified and evaporated by a gentle heat.
2. From the juice of the roots, as No. 1. Both are diuretic.—Dose, 15 gr. to 1⁄2 dr., or more.
Extract of Bael. Syn. Extractum belæ liquidum, L. B. P. Bael, 1; distilled water, 15; rectified spirit, 1⁄8; macerate for 12 hours in 5 of the water, pour off the liquid, repeat the operation twice for 1 hour; press, filter, and evaporate to 1, including the spirit. A fluid ounce is equal to a solid ounce.—Dose, 1 to 2 dr.
Extract of Balsam Apple. Syn. Extractum balsamimæ. The inspissated juice of the balsam apple.—Dose, 5 to 15 drops in dropsy.
Extract of Bark. See Extract of Cinchona.
Extract of Belladon′na. Syn. Extract of deadly nightshade, Inspissated juice of belladonna; Extractum belladonnæ (B. P., Ph. L. E. & D.), Succus spissatus belladonnæ, L. Prep. 1. (B. P.) Take 112 lbs. of fresh leaves and tender branches, bruise in a stone mortar or other suitable apparatus, and press out the juice, heat it gradually to 130° F., separate the green colouring matter by a calico filter, heat the strained liquor to 200° F. to coagulate the albumen, and again filter; evaporate the filtrate by a water bath to the consistence of a thin syrup, then add to it the green colouring matter previously separated, and, stirring the whole together assiduously, continue the evaporation at a temperature not exceeding 140°, until the extract is of a suitable consistence for forming pills.—Dose, 1⁄4 to 1⁄2 gr., gradually increased to 1 or 2 gr.
2. (Ph. E.) Express the juice from the bruised fresh plant, sprinkle the ‘marc’ with water, and again apply pressure; mix the expressed liquids, filter them, and evaporate the filtered liquor in a vapour bath to the consistence of an extract.
3. (Ph. D.) From the leaves, collected when the plant begins to flower. The expressed juice is allowed to stand for 24 hours, and the clear portion is decanted; the sediment is placed on a calico filter, washed with an equal bulk of cold water, and the filtrate mixed with the expressed juice. The mixed liquid is next heated in a water bath, to coagulate its albumen, and after being skimmed, and filtered through flannel whilst hot, the washed sediment is added, and the whole evaporated, as before.
4. (Ph. U. S.) The expressed juice is heated to the boiling-point, filtered and evaporated (see below).
Obs. The P. Cod. directs this extract to be made by two different formulæ. The product of the one resembles that of the Ph. L.; that of the other, that of the Ph. E. That of the Ph. L., from retaining the fecula, is the weakest preparation.—Dose, 1⁄2 gr. to 1 gr., gradually increased to 3 or 4 gr.; as an anodyne in neuralgia, tic-douloureux, &c.; as an antispasmodic to relieve rigidity and spasms, in various affections of the uterus, rectum, urethra, bladder, &c., and in hooping-cough; in various maladies of the eyes; and as a resolvent and discutient in several glandular diseases. It689 has been highly recommended as a preservative against scarlet fever. It is most frequently employed externally, under the form of a plaster, ointment, or lotion. It is poisonous.
5. (Alcoholic; E. b. alcoholicum, L.)—a. (P. Cod.) As ALCOHOLIC EXTRACT OF ACONITE—P. Cod.
b. (Ph. U. S.) As the last (nearly), using spirit of ·935 (=about 13 u. p.).
c. (Moir.) The expressed juice is coagulated by heat, cautiously applied, and filtered; the filtrate is reduced to the consistence of a syrup, and mixed with an equal volume of nearly anhydrous alcohol (say of 90%); the clear portion is lastly evaporated, as before.
Obs. The above is much more powerful than the common extract, and is chiefly used in external applications. See Belladonna, and below.
Extract of Belladonna Ber′ries. Syn. Extractum baccarum belladonnæ, L. Prep. (P. Cod.) From the expressed juice of the berries, evaporated to the consistence of thick honey.—Dose, 1 to 5 gr.
Extract of Bis′tort. Syn. Extractum bistortæ, L. Prep. 1. (P. Cod.) From the dried root of bistort or snake-weed (Polygonum Bistorta), by percolation with temperate distilled water.
2. From the infusion made with boiling water, or from the decoction. Astringent and tonic.—Dose, 10 gr. to 1⁄2 dr.
Extract of Bit′ter-sweet. Syn. Extract of woody nightshade; Extractum dulcamaræ, L. Prep. 1. From the decoction of the stalks.
2. (Ph. U. S.) From the dried stalks, by percolation with temperate water. Diaphoretic, diuretic, and narcotic.—Dose, 3 to 6 gr.; in chronic asthma, rheumatism, and chest diseases; and particularly in chronic skin diseases.
Extract, Black. See Extract of Cocculus.
Extract of Black Pepper. See Extract of Pepper.
Extract of Bladder-wrack. Syn. Extractum fuci vesiculosi. From the dried plant of the bladder-wrack. Given in obesity.
Extract of Bor′age. Syn. Extractum boraginis, L. Prep. 1. (P. Cod.) From the dried herb (Borago officinalis).
2. (Ph. Lusit.) From the clarified juice of the fresh plant. Exhilarating, restorative, and pectoral.—Dose, 10 to 30 gr., or more.
Extract of Box. Syn. Extractum buxi, E. corticis b., L. Prep. (P. Cod.) From the tincture of the root bark, prepared (with proof spirit) by displacement, as EXTRACT OF ACONITE—P. Cod.
Extract of Broom. Syn. Extract of broom tops; Extractum scoparii, E. spartii scoparii, L. From decoction of broom tops. Diuretic and cathartic; and, occasionally, emetic.—Dose, 20 gr. to 1 dr.; in dropsy, &c. It is now seldom used.
Extract of Bry′ony. Syn. Extractum bryoniæ, E. b. albæ, E. radicis b. a., L. Prep. From the infusion or decoction of the root of white bryony (Bryonia dioica). Purgative, diuretic, and emmenagogue.—Dose, 10 gr. to 1⁄2 dr. It was once a favourite remedy in asthma, dropsy, epilepsy, &c.
Extract of Bu′chu. Syn. Extractum buchu, E. diosmæ, L. Prep. 1. From buchu leaves, as the last.
2. (Ethereo-alcoholic; E. b. æthero-alcoholicum, L.—W. Procter.) Buchu (in coarse powder), 1 lb.; ether, 4 fl. oz.; alcohol (rectified spirit), 12 fl. oz.; percolate without digestion, adding dilute alcohol until a pint of ethereo-alcoholic tincture is obtained, and suffer this to evaporate spontaneously; treat the residue in the displacer with dilute alcohol, till 2 pints are obtained; evaporate to a syrup, add the product of the first tincture, mix, and complete the evaporation.—Dose, 5 to 10 gr.; in diseases of the urinary organs, &c.
3. (Fluid; E. b. fluidum, L.—W. Procter.) Buchu leaves, 8 oz.; rectified spirit, 16 fl. oz.; for a tincture by displacement, adding water, until 12 fl. oz. have passed through; allow this to evaporate spontaneously until reduced to one half; next digest the mass in the percolator with cold water, 1 pint, for 12 hours, express a pint, and evaporate this to 10 fl. oz.; lastly, add the 6 fl. oz. of residual tincture, agitate together, and in a few days filter, or decant the clear portion.—Dose, 1 to 2 teaspoonfuls. See Diosma.
Extract of Buck′bean. Syn. Extractum menyanthis, L. Prep. 1. (P. Cod.) From the expressed juice of the fresh plant.
2. (Ph. Bor.) From the infusion made with boiling water. Bitter, tonic and astringent.—Dose, 5 to 10 gr. In large doses it is purgative, cathartic, and even emetic.
Extract of Buck′thorn. Syn. Extractum rhamni, E. baccarum r., L. Prep. From the filtered expressed juice of buckthorn berries. Some persons allow it first to run into a state of fermentation; but the quantity of the product is thereby greatly lessened. Hydragogue and purgative.—Dose, 15 gr. to 1 dr., or more.
Extract of Bur′dock. Syn. Extractum bardanæ, L. Prep. 1. From the decoction of burdock root.
2. (P. Cod.) As EXTRACT OF BISTORT—P. Cod. In gout, rheumatism, skin diseases, &c.—Dose, 10 gr. to 1 dr. Sir Robert Walpole praised burdock root as a gout medicine; and others have considered it an excellent substitute for sarsaparilla. (Lindley.)
Extract of Butter-nut. Syn. Extractum juglandis, L. Prep. (Ph. U. S.) From the inner bark of the root of the butter-nut or white walnut (Juglanda alba), as EXTRACT OF BITTER SWEET690—Ph. U. S. A mild, yet efficacious aperient and vermifuge.—Dose. As a laxative, 5 to 10 gr.; as a purgative, 15 to 30 gr.
Extract of Cainca Root. Syn. Extractum caincæ. (P. Pharm.) Prep. Put 10 oz. of the dried root of cainca into a percolator, pour on it proof spirit q. s. to penetrate the powder in every part, and let it remain 12 hours; then let the liquid drain, and pass successively through the powder in the percolator as much proof spirit as will amount with that previously used to 60 oz. by weight. Distil off the spirit and evaporate to a soft extract.
Extract of Calabar Bean. Syn. Extractum physostigmatis. (B. P.) Calabar bean in coarse powder, 1; rectified spirit, 5; macerate the bean for 48 hours in one fourth of the spirit in a closed vessel, agitating occasionally, then transfer to a percolator, and when the fluid ceases to pass add the remainder of the spirit, so that it may slowly penetrate through the powder; subject the residue of the bean to pressure, adding the pressed liquid to the product of the percolation; distil off the spirit, and evaporate what is left to the consistence of a soft extract by a water bath.—Dose, 1⁄16 to 1⁄4 gr.
Extract of Calum′ba. Syn. Extractum calumbæ, E. radicis c., L. Prep. 1. (B. P.) Calumba cut small, 1; water, 5; macerate in half the water for 12 hours, strain, and press; macerate again with the remaining water, strain, and press; mix and filter the liquors, and evaporate with the heat of a water bath to pill consistency.—Dose, 2 to 10 gr.
2. (Alcoholic—Ph. Bor.) Nearly as No. 3 (below), but using stronger spirit; the evaporation is to be conducted at a heat not above 167° Fahr., until it acquires the consistence of a pill-mass, which, after being rendered quite dry by a very gentle heat, is to be reduced to fine powder. It should have a brownish-yellow colour, and give a turbid solution with water.—Dose, 4 to 12 gr. They are all tonic and stomachic.
3. (Spirituous—P. Cod.) As EXTRACT OF BOX. The Ph. Baden orders spirit of ·944 to be used.—Dose, 5 to 15 gr.
Extract of Cannabis Indicæ. See Extract of Indian Hemp.
Extract of Canthar′ides. Syn. Extract of Spanish flies; Extractum cantharides, E. lyttæ, L. Prep. 1. (P. Cod.) From the tincture, as EXTRACT OF BOX.
2. (Soubeiran.) From a tincture prepared with spirit of the sp. gr. ·923 (about 2 u. p.).
3. (Acetic; E. c. aceticum, L.) From a tincture prepared with acetic acid, sp. gr. 1·048.
4. (Ethereal; E. c. æthereum, L.) From the ethereal tincture.
Obs. The ether, acid, and spirit distilled from the above must be either thrown away or used to make fresh extract, as it is highly poisonous. They are all for external use only, and should have the consistence of soft butter.
Extract of Car′damoms. Syn. Ethereal e. of c.; Extractum cardamomi æthereum, L. Prep. (W. Procter.) By spontaneous evaporation of the ethereal tincture. It consists of the volatile and fixed oils of the seeds and is used to aromatise pills, powders, &c.
Extract of Ca′rob Beans. Syn. Extractum ceratoniæ, L. Prep. From the decoction of the pods (CAROB, or ALGAROBA BEANS) of the ‘Ceratonia siliqua,’ or ‘St. John’s bread tree.’ See Algaroba.
Extract of Caroli′na Pink. See Extract of Pink-root.
Extract of Car′rot. Syn. Extractum carotæ, E. radicis c., L. Prep. (Swediaur.) From the clarified expressed juice, evaporated to the consistence of honey. Recommended as an application to ulcerated cancers.
Extract of Cascaril′la. Syn. Extractum cascarillæ, E. corticis c., L. Prep. 1. (Guibourt.) From the alcoholic (rectified spirit) tincture.
2. (Ph. Baden.) As the last, but using spirit of the sp. gr. ·944.
3. (Ph. L. 1788.) As Extract of jalap—Ph. L.
Obs. This extract is tonic, aromatic, and stomachic.—Dose, 5 to 15 gr., or more, 2 or 3 times a day. 28 lbs. of bark yield about 51⁄4 lbs. of extract.
Extract of Cas′sia. Syn. Extractum cassiæ, L. See Cassia Pulp.
Extract of Cat′echu. Syn. Extractum catechu, L. Prep. 1. From decoction of catechu.
2. (P. Cod.) From the infusion in boiling water. Astringent and tonic.—Dose, 5 to 25 gr. See Catechu.
Extract of Cel′andine. Syn. Extractum chelidonii, L. Prep. 1. (Ph. Bor.) From the herb (Chelidonium majus), as ALCOHOLIC EXTRACT OF ACONITE—Ph. Bor.—Dose, 3 to 10 gr.
2. (Van Mons.) From the expressed juice, coagulated by heat, filtered, and evaporated, towards the end adding the coagulum.—Dose, 5 to 15 gr., or more. Used as a drastic hydragogue in dropsies; and in scrofula, &c.
Extract of Cen′taury. Syn. Extractum centaurii, L. Extracts under this name are prepared from ‘American centaury’ (Sabbatia angularis), and ‘common centaury’ (Erythræa Centaurium). Prep. 1. By evaporating the decoction, or the infusion made with hot water. The dose and properties resemble those of extract of gentian.
2. (Alcoholic; E. c. alcoholicum, L.) As EXTRACT OF BOX (see above).
Extract of Cevadil′′la. Syn. Alcoholic extract of sabadilla; Extractum sabadillæ, L. Prep. (Dr Turnbull.) From tincture of cevadilla seeds, made with rectified spirit. Employed by Dr Turnbull as a remedy in painful rheumatic and neuralgic affections, and, generally, as a substitute for VERATRIA.—Dose, 1⁄10 to 1⁄6 gr. It is extremely poisonous.
Extract of Cham′omile. Syn. Extractum anthemidis (Ph. E.), E. a. nobilis, L. Prep.691 By evaporating the decoction of the flowers to the proper consistence.
Obs. This extract contains only the bitter portion of the chamomile, the aromatic volatile oil being dissipated during the evaporation. This, however is remedied in the formulæ given by the British Pharmacopœia, which is as follows:—
Boil chamomile flowers 1 lb., in one gallon of distilled water, until the volume is reduced to one half; strain, press and filter. Evaporate by a water bath to a proper consistence, adding oil of chamomile, 15 minims at the end of the process.
It is usually prepared from old flowers that have lost their smell and colour, and are thus rendered unsaleable. The extract of chamomile that smells strongly of the flowers, frequently vended by the druggists, is prepared by adding 1 dr. of the essential oil of chamomile to every pound of extract, when nearly cold, and just before removing it from the evaporating pan. This addition, unlike many which are made in the laboratory, vastly increases the medicinal virtues of this article. The mass of extract of chamomile met with in the shops is nothing but extract of gentian scented with a little oil of chamomile. 1 cwt. of chamomiles yields about 48 lbs. of extract.
Extract of chamomile is bitter, tonic, and stomachic.—Dose, 10 to 20 gr., made into a pill, either alone or combined with a little rhubarb and ginger. See Pills, &c.
Extract of Chenopo′′dium. Syn. Extract of stinking goose-foot; Extractum chenopodii, L. Prep. 1. From the stinking orache or goose-foot (Chenopodium olidum), as EXTRACT OF ACONITE.—Ph. L.
2. (Mr Houlton.) From the expressed juice by spontaneous evaporation. A better plan is to expose it to heated air. Antihysteric, emmenagogue, and vermifuge.—Dose, 5 to 20 gr.
Extract (Fluid) of Wild Cherry. Syn. Extractum Pruni Virginianæ fluidum. (Ph. U. S.) Wild cherry in fine powder, 16 oz. (troy); glycerin, 4 oz. (old measure); water, 8 oz. (old measure). Mix the glycerin and the water, and digest the wild cherry in 8 oz. of the mixture for 4 days, then pack in a percolator and pour on the remaining 4 oz. of glycerin and water. When this has disappeared from the surface pour on rectified spirit (·817) until 12 oz. (old measure) of fluid have been obtained, and set this portion aside. Then percolate with spirit, until 20 oz. (old measure) more have been obtained; evaporate to 4 oz. (old measure), and mix with the reserved portion.
Extract of Cincho′na. Syn. Extract of bark. Three simple extracts, prepared respectively from YELLOW, PALE, and RED CINCHONA, are given in Ph. L.:—Prep. 1. (From CALISAYA or YELLOW BARK:—Extract of cinchona, E. of yellow c., E. of heart-leaved c.; Extractum cinchonæ, L.)—a. Extractum cinchonæ flavæ liquidum (B. P.). Yellow cinchona bark in coarse powder, 16; distilled water, a sufficiency; rectified spirit, 1; macerate the bark in 40 of water for twenty-four hours, then pack in a percolator, and add water until 240 have passed through, or until the bark is exhausted; evaporate the liquor to 20, at a temperature not exceeding 160°; then filter, and continue the evaporation to 3, or until the sp. gr. of the liquid is 1·200; when cold, add the spirit gradually, constantly stirring. Sp. gr. 1·100.—Dose, 10 to 30 minims.
b. (Ph. L.) Yellow cinchona (coarsely bruised), 3 lbs.; distilled water (temperate), 4 pints; macerate for 24 hours (constantly stirring), and strain through linen; what remains, again macerate in water, 1 quart, for 24 hours, and strain; evaporate the mixed liquids to a proper consistence.
Obs. The aqueous extracts of cinchona bark possess little medicinal virtue, owing to the insolubility of the alkaloids (quinine, cinchonine, &c.) in water, and also from the rapid oxidation of their extractive matter, when exposed in solution to the joint action of heat and atmospheric oxygen.—Dose, 5 gr. to 1⁄2 dr., in mixtures, faintly acidulated with sulphuric acid. Cinchona bark yields from 24% to 30% of aqueous extract.
2. (From PALE BARK:—Extract of pale cinchona, E. of pale bark, E. of lance-leaved b.; Extractum cinchonæ vallidæ, L.)—a. (Ph. L.) From pale bark, as EXTRACT OF CINCHONA—Ph. L. (above).
b. (Ph. L. 1836.) From the decoction.
Obs. This forms the EXTRACT OF BARK of the shops. The red and yellow cinchona barks are scarcely ever used for making extracts. Their richness in quinine leads to their almost, exclusive employment for the manufacture of that alkaloid, by which their value is greatly enhanced. As far as our knowledge extends, no other extract of bark than this is either employed or asked for.
3. (From RED BARK:—Extract of red cinchona, E. of red bark, E. of oblong-leaved b.; Extractum cinchonæ rubræ, L.)—a. (Ph. L.) From red bark, as EXTRACT OF CINCHONA—Ph. L. (above).
b. (Ph. L. 1836.) From the decoction.
Obs. These extracts are ordered to be kept in two states, the one (SOFT EXTRACT OF CINCHONA; EXTRACTUM CINCHONÆ MOLLE) for making pills, &c.; the other (HARD EXTRACT OF CINCHONA; EXTRACTUM CINCHONÆ DURUM) for powdering.—The dose, &c., of all the above are the same.
4. (Dry:—Essential salt of bark; Extractum cinchonæ siccum, L.)—a. (P. Cod.) From an aqueous infusion of pale bark (prepared by displacement with water at a temperature not above 77° Fahr.), evaporated to the consistence of a thick syrup, and then spread thinly and uniformly on earthenware dishes, or sheets of glass, and dried in a stove,692 by a very gentle heat. It is separated from the plates with a knife, and preserved in well-closed phials. Prior to spreading it out on the plates, about 4% or 5% of thick mucilage is commonly added.
b. (Ph. Bor.) As the above (nearly).
c. (Ph. Hann. 1831.) Similar to the above; but the liquid, when it acquires the consistence of treacle, is diluted with water, and again evaporated to a like consistence; and this dilution and evaporation is repeated until, on the addition of water, it forms a clear solution.—Dose, 5 to 25 gr. The product of the last formula is nearly inert, and that of the others possesses little activity.
5. (Fluid:—Extractum cinchonæ fluidum, L.)—a. See Liquor of Cinchona.
b. (Dr Neligan.) From yellow bark, as FLUID EXTRACT OF BUCHU.
6. (Resinous:—Alcoholic extract of bark; Extractum cinchonæ alcoholicum, E. cinchonæ, L.)—a. (Ph. E.) From any variety of cinchona bark (in powder), 4 oz.; proof spirit, 24 fl. oz.; prepare a tincture by displacement, distil off most of the spirit, and evaporate the residuum to the consistence of an extract. This is the only EXTRACTUM CINCHONÆ of the Edinburgh College.
b. (Ph. U. S.) Peruvian bark, 1 lb.; rectified spirit, 4 pints; make 4 pints of tincture by displacement; add water to the mass in the percolator, digest, and obtain 6 pints of infusion; distil off the spirit from the tincture, and evaporate the infusion to the consistence of syrup, then mix the two, and complete the evaporation. More active than the aqueous extract.—Dose, 5 to 20 gr.
c. (Ellis.) Yellow bark, 2 lbs.; hydrochloric acid, 4 fl. dr.; water, 1 gall.; boil, strain, and repeat the decoction with fresh water and acid; mix the decoctions, filter, and agitate it with fresh-slaked lime, 21⁄2 oz.; filter or decant; dry the residuum, and exhaust it with hot alcohol, q. s.; lastly, evaporate the alcoholic tincture to a pilular consistence.—Dose, 1 to 5 gr. Some persons have proposed to call this ‘ESSENTIAL SALT OF BARK,’
7. (Vinous:—Extractum cinchonæ vinosum, L.—Ph. Hesse.) Peruvian bark (in powder), 1 part; white wine (sherry), 8 parts; digest 3 days, express, filter, and evaporate.
Extract of Coc′culus. Syn. Extract of cocculus indicus, Black extract, Extract (Brewer’s), Beer strengthener, Hard multum; Extractum cocculi, E. c. indici, L. Prep. From cocculus indicus, by decoction. It is kept in two states—one having the consistence of thick treacle; the other, that of a pilular extract. The first is ‘put up’ in bladders; the last is made into 1⁄2-lb. rolls, like lead-plaster-or roll-chocolate. It is narcotic and poisonous, and is employed by fraudulent brewers and publicans to give a false strength to their liquors. See Cocculus Indicus, Beer, &c.
Extract of Col′chicum. Syn. Extract of meadow saffron, E. of the corms of colchicum; Extractum colchici (B. P.). Prep. 1. (B. P.) The expressed juice of fresh colchicum corms, cleared of deposit, boiled, strained, and evaporated to a proper consistency at a temperature of 160° Fahr.—Dose, 1 to 2 gr.
2. (Wholesale.) From the decoction of the dried corms. Prod. 50% to 55%.
Obs. This extract is given in the usual cases in which colchicum is employed.—Dose, 1 to 4 gr., every third or fourth hour. (Thomson.) “A favorite remedy of Dr Hue, of St. Bartholomew’s Hospital, in the early stages of acute rheumatism. The dose is 1 gr. every four hours.” (Pereira.)
3. (Acetic; Acetic extract of meadow saffron; Extractum colchici aceticum (B. P.)—a. (B. P.) Crushed fresh corms, previously peeled, 19; acetic acid, 1; stir together, press, boil, and strain through flannel, and evaporate to a soft extract.—Dose, 1 to 2 gr. with an equal weight of liquorice powder.
b. (Wholesale.) Dried corms, 14 lbs.; acetic acid (pyroligneous), 6 pints; distilled water, 51⁄4 gall.; digest for 14 days, express, filter, and evaporate. Product, 21⁄2 to 3 lbs.
Obs. The above extracts are generally prepared from the dried corms, and hence the very uncertain and inferior quality of those commonly met with. They also possess less activity than pharmacopœial preparations. They rapidly get dry and crumbly, and, unless a little spirit and oil of cloves are added, will scarcely keep a week in warm weather without becoming mouldy.—Dose, 1 to 3 gr. two or three times a day. It is much stronger than the common extract, and contains the acetate of colchicine. Sir C. Scudamore prefers the acetic extract prepared by the formula b (above).
4. (Alcoholic; Extractum colchici alcoholicum, L.—P. Cod.). As extract of Box. More active than even the acetic extract. All the preparations of colchicum are poisonous in large doses.
Extract of Colo′cynth. Syn. Extract of bitter apple; Extractum colocynthidis (Ph. L. & E.), E. c. simplex, E. c. molle, L. Prep. 1. (Ph. L.) From colocynth pulp (cut in pieces and the seeds removed), by simple maceration in cold water for 36 hours, frequently pressing it with the hands, and afterwards strongly pressing out the liquor, which must be strained before evaporating it.
2. (Ph. E.) From the decoction. This is the plan adopted at Apothecaries’ Hall, and in the laboratory generally. Many houses do not even remove the seeds.
Obs. This extract rapidly gets hard, crumbly, and mouldy by keeping. For the remedy, see observations on EXTRACT OF COLCHICUM693, above.—Dose, 5 gr. to 20 gr.; as a cathartic. Colocynth pulp yields above 65% of extract.
3. (Alcoholic; Extractum colocynthidis alcoholicum, L.)—a. (Ph. Baden.) As EXTRACT OF ANGELICA—Ph. Bad.)
b. (P. Cod.) From a tincture prepared with proof spirit. Much more active than the simple extract.—Dose, 2 to 7 gr.
4. (Dry; Extractum colocynthidis siccum, L.)—(Ph. Bor.) As the last, but using spirit of the sp. gr. ·900 (about 16 o. p.), digesting at a tepid heat, evaporating to dryness, and powdering.—Dose, 1 to 6 gr.
Extract of Colocynth (Compound). Syn. Compound extract of bitter apple, Cathartic extract; Extractum catharticum, E. colocynthidis compositum, B. P. Prep. 1. (B. P.) Colocynth free from seeds, 6; extract of Socotrine aloes, 12; scammony, or resin of scammony in powder, 4; hard soap in powder, 3; cardamoms free from capsules in fine powder, 1; proof spirit, 160. Macerate the colocynth in the spirit for four days, press out the tincture, distil off the spirit, and add to it the extract of aloes, the soap, and the scammony; then evaporate the residue by a water bath to a pilular consistence, adding the cardamoms towards the end of the process.—Dose, 2 to 5 gr., with 2 or 3 gr. of extract of hyoscyamus to prevent griping.
2. (Ph. L. 1836.) Colocynth pulp (sliced, without the seeds), 6 oz., proof spirit, 1 gal.; digest with a gentle heat for 4 days, express, strain, and add, of extract of aloes (Ph. L. 1836), 12 oz., powdered scammony, 4 oz., Castile soap (cut small), 3 oz., and evaporate (distil) to a proper consistence; adding, towards the last, powdered cardamoms, 1 oz.
3. (Wholesale.) The formulæ adopted by the wholesale druggists are mere modifications of that of the Ph. L. 1809; water being used instead of spirit as the menstruum, with actual benefit, as we honestly believe, to the quality of the preparation. The following are extensively employed by those who do most in this article, and we can speak highly of the quality of the products obtained by their use.
a. Turkey colocynth, 18 lbs., is boiled in about 20 times its weight of water for five or six hours; to the strained decoction is added hepatic aloes, 40 lbs., which are boiled until dissolved, when the solution is decanted. In the mean time the colocynth is exhausted with a second quantity of water (less than the first), and the strained liquor is added to the undissolved residuum of the aloes, and boiled for a few minutes; after which it is drawn off and mixed with the first decoction of aloes; the mixed liquors are then allowed to stand until quite cold (commonly until the next day), to deposit the resinous portion. The liquor is next decanted or drawn off, and set evaporating as quickly as possible; as soon as the consistence of treacle is arrived at, the whole is allowed to cool considerably, and moist sugar (clean), 4 lbs., and Castile soap, 10 lbs. (previously melted with a little water), are added; powdered scammony, 6 lbs., is next gradually sifted in, the extract all the time being assiduously stirred by a second person. Lastly, the heat is further moderated, and the stirring continued until a rather harder consistence is acquired than is proper for the extract, when the steam is wholly ‘shut off,’ or the vessel removed from the heat, and as soon as the whole has become sufficiently cool to prevent any considerable evaporation of the spirit, essence of cardamoms, 2 lbs. (say 1 quart), is expertly stirred in; and the extract at once (whilst still warm) put into stone jars or pots, and tied or covered over for store or use. The product is usually labelled ‘Ext. Colocynth. Comp. Opt.’ It looks well, and smells very aromatic, and is really an excellent preparation.
b. Turkey colocynth, 21⁄4 lbs.; hepatic aloes, 51⁄2 lbs.; powdered scammony, 11⁄2 lb.; powdered cardamoms, 6 oz. (or essence, 1⁄2 pint); Castile soap (genuine), 1 lb. 2 oz.; pale moist sugar, 1⁄2 lb.; proceed as last. This produces a beautiful article, and of unquestionable quality, equally effective, and milder in its action than the College preparation. It is labelled and sent out as Ext. Colocynth. Comp. Ph. L. (1836).
4. (Ph. L. 1809.) Colocynth, 6 dr. (6 parts); aloes, 11⁄2 oz. (12 parts); scammony, 1⁄2 oz. (4 parts); hard soap, 3 dr. (3 parts); cardamoms, 1 dr. (1 part); as No. 3, a (nearly).
Qual., &c. Compound extract of colocynth is often adulterated with acrid cathartics to make up for the deficiency or inferiority of its proper ingredients, and foreign matter often becomes mixed with it by the use of impure scammony. The presence of cape aloes may usually be detected by the nauseous odour; chalk (an article frequently present in bad scammony), by placing a little ball of the extract in a glass tube, and pouring over it some dilute hydrochloric or acetic acid, when an effervescence will ensue if that substance be present; jalap, scammony adulterated with fecula, and other starchy substances, by the filtered decoction of the extract turning blue on the addition of tincture of iodine; gamboge, by the decoction becoming deep red on the addition of liquor of potassa, and by a filtered alcoholic solution of the extract forming a yellow emulsion with water, which becomes transparent and assumes a deep-red colour on the addition of caustic potassa; and further, by this solution (if the alkali is not in excess) giving a yellow precipitate with acids and with acetate of lead, a brown precipitate with sulphate of copper, and a very dark brown one with the salts of iron; also by the ethereal solution of the extract dropped on water yielding an opaque yellow film, soluble in caustic potassa if it contains gamboge.
Dose, 3 gr. to 15 gr. It is a safe and mild, yet certain, purgative. It may be mixed with calomel without the latter being decomposed. 21⁄2 or 3 gr., mixed with an equal weight of blue pill and taken overnight forms an excellent694 aperient in dyspepsia, liver complaints, &c. See Abernethy Medicines.
Obs. There are few formulæ which have undergone so many alterations in the hands of the College as that for compound extract of colocynth. Before 1809, proof spirit was ordered to be employed as the menstruum, and, omitting the soap, the preparation resembled that of the Ph. L. 1836. In 1809, the College directed water to be used instead of spirit, and added a certain quantity of soap. In the next edition of the Pharmacopœia (1815), the soap was again omitted; but in the edition of 1824, the formula of 1809 was again adopted, substituting, however, proof spirit for the water. These directions were also continued in the edition of 1836. In the last London Pharmacopœia (1851) the formula for this extract is omitted altogether, and in its place a pill (PILULA COLOCYNTHIDIS COMPOSITA) is inserted.
The compound extract of colocynth and the simple and compound extracts of sarsaparilla are in greater demand in the wholesale trade, and are sold in larger quantities at a time, than all the other medicinal extracts put together. As a proof, if it were necessary, that honesty is the best policy, it may be mentioned that a certain metropolitan druggist, remarkable for the superiority of this preparation, has obtained no inconsiderable fortune by its sale alone; while the host of miserable vendors of the evaporated decoction of musty colocynth seed, Cape aloes, worthless scammony, and scentless cardamoms, sold under the name, attempt to ruin each other by offering their rubbish at a price that precludes the possibility of a large profit, or even of the establishment of a respectable connection.
Extract of Conia. See Extract of Hemlock.
Extract of Contrayer′va. Syn. Extractum contrayervæ, L. Prep. (Palat. Cod.) From contrayerva root, as EXTRACT OF CINCHONA—Ph. L.—Dose, 10 gr. to 1⁄2 dr.; as a diaphoretic tonic in low conditions of the system.
Extract of Copai′ba. Syn. Resinous extract of copaiba; Extractum copaibæ, E. c. resinosum, L. Prep. (Mr Thorn.) From balsam of copaiba, by distilling off the oil until the residuum assumes the consistence of an extract.—Dose, 10 to 20 gr., or more. One of the many useless preparations which encumber modern pharmacy. It may be taken in 3 dr. doses without any perceptible effect beyond a fit of indigestion.
Extract of Copal′che. Syn. Extractum copalchi, E. corticis c., L. Prep. From copalchi bark (Croton pseudo-China), as EXTRACT OF CASCARILLA, which it for the most part resembles.—Dose, 1 to 3 gr.
Extract (Fluid) of Cotton Root. Syn. Extractum gossypii radicis fluidum (Ph. U. S.). Cotton root in very fine powder, 16 oz. (troy); macerate with glycerin, 3 fluid oz. (old measure); rectified spirit, 8 oz. (old measure); water, 5 oz. (old measure), in closed percolator for 4 days; then let the percolation commence, and finish it by adding dilute alcohol (eq. vols. of alcohol ·835 and water) until 24 oz. (old measure) have been obtained; reserve the first 14 oz., and evaporate the remaining 10 oz. (to which previously add 1 fluid oz., old measure) of glycerin to 2 fluid oz. (old measure), and mix with the reserved portion.
Extract of Couch Grass. Syn. Extract of dog’s grass; Extractum graminis, L. Prep. 1. (P. Cod.) From the root of couch grass, or dog’s grass (Triticum repens), as EXTRACT OF BISTORT—P. Cod.
2. From the fresh root, as EXTRACT OF ACONITE—Ph. L.
3. (Fluid; Mellago graminis, Extractum graminis fluidum, L.—Ph. Hann. 1831.) From the decoction of the fresh root of couch grass, evaporated to the consistence of new honey. Pectoral.—Dose, 15 gr. to 1⁄2 dr., or more.
Extract of Cu′bebs. Syn. Extractum cubebæ, L. Prep. 1. From the alcoholic tincture evaporated by a very gentle heat.—Dose, 5 gr. to 30 gr.
2. (Mr Toller.) To the last add a little powdered Castile soap, when it begins to thicken, and evaporate to a pilular consistence.—Dose, 10 gr. to 30 gr.
3. (Fluid: Liquor cubebæ, Extractum c. fluidum, L.)—a. Cubebs (ground in a coffee-mill), 11⁄4 lb.; rectified spirit, 1 quart; prepare a tincture, either by displacement or by digestion for a week, and reduce it, by distillation at a very gentle heat, until the whole measures exactly 1 pint. Every fl. oz. represents 2 oz. of cubebs.—Dose, 20 to 40 drops.
b. (M. Puche.) From cubebs and proof spirit, equal parts, by percolation; without subsequent evaporation. Represents its own weight in cubebs.—Dose, 1⁄2 to 1 fl. dr.
c. (Ph. U. S. 1851.) Cubebs, 1 lb. (nearly); ether, q. s.; make 1 quart of tincture; then distil off 11⁄2 pint of the ether by the heat of a water bath, and expose the residuum in a shallow vessel until the remainder of the ether has evaporated.
d. (Ph. U. S.) Extractum cubebæ fluidum. Cubebs in moderately fine powder, 16 oz. (troy); alcohol (·817), 16 oz. (old measure). Macerate in a closed percolator for 4 days, and then let the percolation commence, and finish it by adding more menstruum until 24 oz. (old measure) have been obtained; reserve the first 14 oz., evaporate the remaining 10 oz. to 2 oz., and mix this with the reserved portion.
4. (Oleo-resinous; Extractum cubebæ, E. cubebrum, E. c. oleo-resinosum, L.)—a. (M. Dublanc.) The essential oil resulting from the careful distillation of any given quantity of cubebs, is mixed with the resinous extract obtained by evaporating a tincture of the dried residuum made with rectified spirit; the whole being reduced to the consistence of a thick695 syrup. 1 lb. of cubebs yields about 6 oz. of this extract.
b. (Labelonge.) Cubebs are first exhausted with ether, and then with proof spirit, in a displacement apparatus; the alcoholic tincture is evaporated to an extract over a water bath, and when cold, the ethereal tincture is mixed with it, and the mixture abandoned to spontaneous evaporation until the ether is volatilised.
c. (W. Procter.) An ethereal tincture (by displacement) is poured into a large retort, and 5-6ths is drawn over by the heat of a water bath; the evaporation of the residuum, to the proper consistence, is carried on at a heat not exceeding 120° Fahr. The formula of the Ph. Baden is nearly similar. Said to represent 6 to 8 times its weight in cubebs. 1 lb. yields 2 oz. of this extract.
d. (Hamb. Cod. 1845.) This resembles a (above).
Obs. This extract has a darkish brown colour, and tastes and smells strongly of cubebs. It is only slightly soluble in water.—Dose, 5 gr. to 20 gr.; made into an emulsion or pills, or enclosed in a capsule. See Cubebs.
Extract of Cu′cumber. See Elaterium.
Extract of Cuspa′′ria. Extract of angostura bark; Extractum cuspariæ, E. corticis c., E. angosturæ, L. Prep. 1. From angostura bark, as EXTRACT OF CINCHONA—Ph. L.
2. (Alcoholic.) As EXTRACT OF CINCHONA—Ph. E. Stronger than the last. Both are aromatic, bitter, tonic, and stimulant.—Dose, 10 gr. to 1⁄2 dr.; in dyspepsia, chronic diarrhœa, dysentery, &c.
Extract of Daff′odil. Syn. Extractum narcissi, L. Prep. 1. From the fresh flowers of daffodil or yellow narcissus (Narcissus pseudo-narcissus), as EXTRACT OF ANCONITE—Ph. L.
2. (Alcoholic.) From the dried flowers, as EXTRACT OF BOX. Both are pectoral and expectorant; and in large doses nauseant and emetic.—Dose, 1⁄2 gr. to 2 gr.; in hooping-cough, &c.
Extract of Dandeli′on. See Extract of Taraxacum.
Extract of Digita′lis. See Extract of Foxglove.
Extract of Dog’s Grass. See Extract of Couch Grass.
Extract of Dog′wood. Syn. Extractum cornûs, E. corni, L. Prep. From American or tree dogwood (Cornus Florida), as EXTRACT OF CINCHONA BARK.
Obs. In its general effects, American dogwood approaches the cinchonas, and is said to be not inferior to them in the cure of intermittents. (Bigelow.) It contains a peculiar bitter principle, called cornine. Several other varieties of the genus Cornus, as round-leaved dogwood (Cornus circinata), swamp dogwood (Cornus sericea), &c., are used in America, but are less valuable.
Extracts, Dried or Powdered. Syn. Extracta sicca vel pulverata (Ph. Prus.). These are made by mixing 4 parts of the extract with 1 part of powder of sugar of milk, and setting the mixture in a warm place till dry.
Triturate the mass to powder, adding more of the sugar of milk if necessary, to make the weight the same as the extract used. The German Pharmacopœia directs them to be mixed with dextrin, and then dried at a temperature of 122° Fahr., and, while still warm, triturated into a uniform powder, with dextrin q. s. to make the weight of the powder equal to twice the weight of the extract employed.
Extract of Dulcama′′ra. See Extract of Bitter Sweet.
Extract of Elate′rium. Syn. Inspissated juice of the squirting cucumber; Succus spissatus momordicæ elaterii. For preparation and recent synonyms, see Elaterium.
Extract of El′der Berries. Syn. Elder rob; Roob sambuci, Extractum sambuci, E. s. nigræ, E. Baccarum s., Succus sambuci inspissatus, L. Prep. 1. (Ph. L. 1788.) The expressed and depurated juice of elder berries, evaporated to the consistence of honey.
2. (Ph. E. 1744.) To the above, when it begins to thicken, add 1-5th part of sugar.
3. (Ph. Bor.) As the last (nearly), but adding only 1 oz. of white sugar to each pound of the extract whilst still warm.—Dose, 1 to 4 dr.; in rheumatism, gout, and various skin affections.
Extract of El′ecampane. Syn. Extractum inulæ, E. Radicum i. campanæ, E. Helenii, L. Prep. 1. (Ph. L. 1746.) From a decoction of the dried root.
2. (P. Cod.) As EXTRACT OF BISTORT—P. Cod.
3. (Ph. Suec 1845.) From a tincture prepared with proof spirit and water, equal parts.—Dose, 10 gr. to 1⁄2 dr.; as a diaphoretic, expectorant, and tonic; in asthma, hooping-cough, various skin diseases, &c.
Extract of Elm. Syn. Extractum ulmi, E. corticus u., L. Prep. 1. From the decoction of the bark of the common elm (Ulmus campestris).
2. (Soubeiran.) As EXTRACT OF BOX. Astringent and alterative.—Dose, 20 gr. to 1 dr.; in secondary syphilis, chronic skin affections, &c.
Extract of Er′got. Syn. Aqueous extract of ergot, Hæmostatic extract; Extractum ergotæ, E. e. aquosum, E. secalis cornuti, E. hæmostaticum, L. Prep. 1. (B. P.) Extractum Ergotæ Liquidum. Ergot in coarse powder, 16; ether, 20; distilled water, 70; rectified spirit, 8. Shake the ether in a bottle with half its bulk of the water, and, after separation, decant the ether. Place the ergot in a percolator, and free it from oil by passing the washed ether through it; remove696 the marc and digest it in the remainder of the water for twelve hours at 160° F. Press out the liquor, and evaporate it to 9, and when cold add the 8 of spirit; allow it to stand for an hour to coagulate, filter, and make up the quantity to 16.—Dose, 15 to 30 minims.
According to Squire, the amount of ether employed should be double the above, in two percolations, and the marc should be dried in the air before digesting with water. See Ergotine (Bonjeau’s).
2. (Alcoholic; Extractum ergotæ alcoholicum, L.) See Ergotine (Wigger’s).
Extract of Eucalyptus Globulus. Syn. Extractum eucalypti globuli. (Griffith.) Prep. Eucalyptus leaves cut at will. Distil the volatile oil with water; exhaust the residue in the still with water, prepare an extract, exhaust this with alcohol, evaporate to the consistence of an extract, and, while cooling, stir in the volatile oil.—Dose, 2 gr. to 8 gr.
Extract of Fern. Syn. Extractum filicis 1iquidum—B. P. see Extract of Male Fern.
Extract of Fleabane (Canadian). Syn. Extractum erigeronis. Prep. 1. From Canadian fleabane, by evaporating an aqueous infusion.—Dose, 5 to 10 gr.
2. (Extractum erigerontis Canadensis Fluidum—Ph. U. S.) Canadian erigeron in moderately coarse powder, 16 oz. (troy); rectified spirit, 16 oz. (old measure). Proceed as for fluid extract of cubebs (Ph. U. S.).
Extract of Flesh. See Extract of Meat, Essence of Beef, Tea (Beef), &c.
Extract of Fox′glove. Syn. Extractum Digitalis (Ph. E.), L. Prep. 1. (Ph. L. 1836.) From the leaves of Digitalis purpurea, as EXTRACT OF ACONITE—(Ph. L.)
2. (Ph. E.) From the filtered expressed juice, either evaporated in vacuo, with the aid of heat, or by exposure to a current of dry air.
3. (P. Cod.)—a. As EXTRACT OF BISTORT—P. Cod.
b. As EXTRACT OF BOX—P. Cod.
4. (Ph. Baden.) As ALCOHOLIC EXTRACT OF ACONITE—Ph. Bad.
Obs. The juice of foxglove is very readily injured by exposure to air and heat. The evaporation should therefore be conducted as rapidly as possible, but at a low temperature. It is narcotic, sedative, and is powerfully poisonous.—Dose, 1⁄2 gr., cautiously increased to 2 or 3 gr. It is principally given in fevers, dropsy, diseases of the heart, pulmonary consumption, epilepsy, scrofula, and asthma. This extract spoils by long keeping. The last two are stronger than the rest, and keep better. It is omitted in the present Ph. L.
Extract of Fu′mitory. Syn. Extractum Fumariæ, L. Prep. 1. From either the infusion or decoction of the dried leaves of common fumitory (Fumaria officinalis).
2. (B. Cod.) From the clarified juice of the fresh herb. Slightly aperient, diaphoretic, and alterative. It has been given in obstructions of the liver and cutaneous affections of the leprous kind.
Extract of Galls. Syn. Extractum gallæ, E. gallarum, L. Prep. 1. From the infusion by maceration or displacement with cold water.
2. From the hot infusion or decoction. The first is to be preferred. Astringent. Used chiefly in ointments and injections for piles, foul ulcers, &c., and, internally, in hæmorrhages, spitting of blood, &c.
Extract of Gen′tian. Syn. Extractum gentianæ (B. P.), L. Prep. 1. (Ph. L.) Gentian root (sliced), 3 lbs.; distilled water (temperate), 4 pints; macerate for 12 hours, and gently express the liquor; repeat the maceration with water, 1 quart, for 6 hours; and evaporate the mixed liquors.
2. (Ph. L. 1836.) From the ordinary infusion of the root made with 10 or 12 times its weight of boiling water, the maceration being continued for 24 hours.
3. (Ph. E.) From an infusion prepared by percolation with cold water. The formulæ of the Ph. Baden, Paris, and U. S. are similar.
4. (B. P.) Gentian, 1 lb.; water (boiling), 10; macerate for 2 hours, boil 15 minutes, strain, and evaporate to a soft pilular consistence.—Dose, 10 to 15 gr.
5. (Ph. D. 1826.) From the decoction.
Obs. On the large scale, this extract is almost universally prepared by exhausting the root by coction with water, as in the last formula. When well prepared it is one of the smoothest and brightest extracts of the Pharmacopœia. Good gentian root yields by infusion in hot water fully 50%, and by decoction about 60% of extract.—Dose, 10 gr. to 30 gr., two or three times daily, as a stomachic bitter and tonic; either alone or combined with rhubarb, ginger, or aloes. It is, however, more especially used as a vehicle for chalybeates and other metallic preparations. The principal consumption of extract of gentian is by the brewers, in lieu of hops.
6. (Hard e. of g.; E. d. durum, L.) The last dried by a gentle heat until brittle enough to powder.
Extract (Fluid) of Ginger. Syn. Extractum zingiberis fluidum. (Ph. U. S.) As fluid extract of cubebs, but using rectified spirit.
Extract (Fluid) of Golden Seal. Syn. (Ph. U. S.) Extractum hydrastis Fluidum. Prep. Hydrastis (Golden Seal) in very fine powder, 16 oz. (troy); macerate with 2 oz. (old measure) of glycerine; rectified spirit, 14 oz. (old measure), in closed percolator for 4 days; then let the percolation commence, and finish it by adding a mixture consisting of 2 parts of spirit and 1 of water, until 24 oz. (old measure) have been obtained. Remove the first 14 oz.; evaporate the remaining 10697 oz. to 2 oz. (old measure), and mix with the reserved portion.
Extract, Goulard’s. See Solution of Subacetate of Lead.
Extract of Guaiac′um. Syn. Extractum guaiaci, L. Prep. 1. (Ph. L. 1746.) From lignum vitæ shavings or sawdust, exhausted by coction with water; as soon as the mass becomes thick, 1-8th of rectified spirit is to be added.
2. As the last, omitting the spirit. Diaphoretic, diuretic, and alterative; in dropsy, gout, rheumatism, skin diseases, &c.
Extract of Guarana′. Syn. Extractum guaranæ, E. paulliniæ, L. Prep. (Dr Gavrelle.) From tincture of guarana (seeds of Paullinia sorbilis), prepared by coction with proof spirit. Tonic and alterative.—Dose, 2 to 5 gr., twice or thrice daily.
Extract, Hæmostat′ic. See Extract of Ergot.
Extract of Hedge Hyssop. Syn. Extractum gratiolæ, L. Prep. 1. (Ph. Bor.) From the herb (Gratiola officinalis), as ALCOHOLIC EXTRACT OF ACONITE—Ph. Bor.
2. (Ph. Baden.) As Extract of aconite—Ph. Baden.
3. (Vinous.) As VINOUS EXTRACT OF CINCHONA. Purgative, diuretic, and vermifuge.—Dose, 2 to 5 gr., gradually increased, watching its effects; in dropsy, jaundice, gout, &c. It has been said to be the basis of the celebrated ‘Eau Médicinale d’Husson,’
Extract of Hel′lebore. The extracts prepared from three different plants may be included under this head:—
1. (Extract of black hellebore; Extractum hellebori, E. h. nigri, L.)—a. (Ph. L., 1788.) From the infusion or decoction of black hellebore (Helleborus officinalis).—Dose, 5 to 12 gr.
b. (Alcoholic—P. Cod. & Ph. U. S.) As EXTRACT OF BOX (nearly). That of the Ph. Bad. is similar.—Dose, 3 to 8 gr.
c. (Vino-alcoholic—Cottereau.) Powdered black hellebore, 2 lbs.; salt of tartar, 1⁄2 lb.; dilute alcohol (sp. gr. ·935), 7 pints; digest 12 hours, and express the tincture; add to the marc, white wine, 7 pints; digest for 24 hours, express, mix the tincture, filter, and evaporate.—Dose, 2 to 6 gr.
Obs. When prepared by coction with water till exhausted of soluble matter, black hellebore root yields about 40% of extract. In small doses it is alterative, purgative, and resolvent; in larger ones, it is a drastic, hydragogue cathartic, and emmenagogue, dangerous unless combined and its effects carefully watched.
2. (Extract of green hellebore, E. of american h., E. of itch-wood; Extractum veratri viridis, L.) From the fresh root (rhizome) of the green hellebore (Veratrum viride), as EXTRACT OF ACONITE—Ph. L.—Dose, 1⁄6 to 1⁄2 gr. Used in America in the same cases as white hellebore.
3. (Extract of white hellebore; Extractum veratri, E. hellebori albi, L.) From the root (rhizome) of the white hellebore (Veratrum album), as EXTRACT OF BLACK HELLEBORE.—Dose, 1⁄12 gr. to 1⁄4 gr. Emetic, purgative, stimulant, and highly acrid. In gout, rheumatism, and nervous affections, mania, &c. See Veratrine.
Extract of Hem′lock. Syn. Inspissated juice of hemlock; Extractum conii (B. P.), Succus spissatus conii, L. Prep. 1. (B. P.) The inspissated juice of the fresh plant, prepared as directed for EXTRACTUM BELLADONNÆ.—Dose, 4 to 6 gr.
2. (Ph. L.) From the fresh plant (Conium maculatum), as EXTRACT OF ACONITE.—Ph. L.
3. (Ph. E.) As EXTRACT OF FOXGLOVE—Ph. E.
4. (Ph. D.) As EXTRACT OF BELLADONNA—Ph. D.
Obs. Of all the inspissated juices (not even excepting that of aconite), this is the one most readily injured by exposure to the air and heat, and which soonest loses its qualities by age. Its active principle is CONINE. Extract of hemlock has a greenish colour, and a strong odour of the fresh-bruised plant. It is “of good quality only when a very strong odour of conia (a ‘mouse-odour’) is disengaged by degrees, on its being carefully triturated with liquor of potassa.” (Ph. E.) “The extracts of hemlock may become feeble, if not inert, in one of two ways,—either by the heat being continued after the concentration has been carried to a certain extent, or by long keeping. On the one hand, I have always observed that from the point at which the extract attains the consistence of thin syrup, ammonia begins to be given off in abundance, together with a modified odour of conine; and, on the other hand, I have found extracts which were unquestionably well prepared at first, entirely destitute of conine in a few years.” (Christison.) “The most active extract is that which is procured by moderate pressure from the leaves only.” (Brande.) “The extract of the Ph. D., being freed from the inert albumen and chlorophyll, contains most of the active principle, and is nearly soluble in water.” (Royle.) On the large scale, the whole of the green portion of the plant is pressed for juice. 1 cwt. of hemlock yields from 3 to 5 lbs. of extract.—Dose, 2 gr., gradually increased to 5 gr., or more, until some obvious effect is produced; as an anodyne, alterative, and resolvent in various obstinate disorders, as glandular and visceral enlargements, foul and painful ulcers, scrofula, cancer, neuralgia, rheumatism, troublesome coughs, &c.
5. (Alcoholic; Extractum conii alcoholicum, L.)—a. (Ph. Baden.) As ALCOHOLIC EXTRACT OF ACONITE—Ph. Baden.
b. (P. Cod.) As the last, but using proof spirit.—Dose, 1⁄2 to 2 gr.
6. (Dried; Extractum conii siccum, L.)—a. As the DRIED EXTRACT OF ACONITE—P. Cod.
698
b. (Archer.) By drying the extract of the Dublin College with a continuous current of warm air.
7. (Extractum conii seminis alcoholicum.) (P. Cod.) Prep. Hemlock seeds in coarse powder, 1 lb; percolate with proof spirit until exhausted. Distil off most of the spirit, and evaporate residue in a water bath to pilular consistence.
Extract of Hemp. Syn. Extract of American hemp; Extractum apocyni, E. a. cannabini, L. Prep. From the root of the Apocynum cannabinum, as EXTRACT OF GENTIAN. A hydragogue cathartic.—Dose, 2 to 6 gr.; in dropsy, &c. The plant from which this extract is prepared is called ‘Indian HEMP’ in the United States of America, a practice which should be avoided, as this name is now almost exclusively appropriated to Cannabis Indica, a variety of the common hemp (Cannabis sativa, var. Indica) growing in India. See Extract of Indian Hemp.
Extract of Hen′bane. Syn. Extract of hyoscyamus; Extractum hyoscyami (B. P.), Succus spissatus hyoscyami, L. Prep. 1. (Ph. L.) From the fresh leaves and leafstems of common henbane (Hyoscyamus niger), as EXTRACT OF ACONITE—Ph. L.
2. (Ph. E.) As EXTRACT OF FOXGLOVE—Ph. E.
3. (Ph. P.) From the fresh leaves and young branches, as EXTRACT OF BELLADONNA.—Dose, 3 to 6 gr.
4. (Ph. U. S.) From the expressed juice coagulated by heat and strained.
Obs. In the Paris Codex Extracts are ordered to be prepared from henbane both by the processes Nos. 1 and 4 above.—Product (by the ordinary method):—1 lb. of the fresh leaves yielded fully 8 dr. of extract (Geiger); 1 cwt. yielded 4 to 5 lb. (Brande); 1 cwt. of the recent plant yielded, by an ordinary screw press, 591⁄2 lbs. of juice, and this evaporated in a water bath gave 5 lbs. 9 oz. of extract (Squire); 13⁄4 cwt. of the green herb yielded 11 lbs. of extract (Gray).—Dose, 2 to 10 gr.; as an anodyne, hypnotic, antispasmodic, sedative, and narcotic, more especially in those cases in which the use of opium is objectionable. Externally, as a topical application to sore or inflamed parts, either made into an ointment or spread on plaster.
5. (Alcoholic; Extractum hyoscyami alcoholicum, L.) The formulæ of the Ph. Bad., Par. & U. S. are similar to those for ALCOHOLIC EXTRACT OF ACONITE.
6. (E. of henbane seeds; Extractum seminum hyoscyami, L.—P. Cod.) An extract of the seeds made with spirit sp. gr. ·900 (=about 16 o. p.) is dissolved in 4 parts of cold water, and the solution filtered and evaporated. Stronger than the simple extract.—Dose, 1⁄4 to 3 gr.
Extract of Ho′ly This′tle. Syn. Extractum cardui benedicti, L. Prep. 1. (Ph. Baden.) From holy or blessed thistle (Carduus Benedictus) by displacement with cold water.
2. (Ph. Bor.) As EXTRACT OF GENTIAN—Ph. L. (nearly). Tonic, diaphoretic, febrifuge, often diuretic, and occasionally emetic.—Dose, 5 to 15 gr., as a tonic or stomachic chiefly.
Extract of Hops. Syn. Extractum lupuli (B. P., Ph. L. & E.), E. humuli (Ph. D.), L. Prep. 1. (B. P.) Hop, 8; rectified spirit, 15; distilled water, 80. Macerate the hop in the spirit for 7 days, press out the tincture, filter, and distil off the spirit, leaving a soft extract; boil the residual hop with the water for one hour, then express the liquor, strain, and evaporate on a water bath to the consistence of a soft extract. Mix the two extracts, and evaporate at a temperature not exceeding 160° to a pilular consistence.—Dose, 5 to 10 gr.
2. (Ph. L.) From commercial hops (the strobiles or catkins of Humulus Lupulus), 21⁄2 lbs.; boiling distilled water, 2 galls.; macerate for 24 hours, boil to a gallon, strain whilst hot, and evaporate to a proper consistence. The form of the Ph. E. is nearly similar.
3. (Ph. D.) As EXTRACT of ALOES—Ph. D. Tonic and stomachic, and slightly anodyne and hypnotic.—Dose, 5 gr. to 30 gr.; in dyspepsia, and cases that do not permit of the use of opium. 1 cwt. of ordinary hops yield about 40 lbs. of extract. (Brande.) The druggists usually employ hops 2 or more years old, called by the dealers ‘yearlings,’ ‘olds,’ or ‘old olds,’ because these may be purchased at 2⁄3 to 1⁄2 the price of those of the last season’s growth. The first of the above are estimated to have only 2⁄3 the strength of new hops; the second about 1⁄2; and the last little or none, at least in a medical point of view.
4. (Alcoholic; Extractum lupuli alcoholicum, L.—Cottereau.) By displacement with proof spirit. Stronger than the aqueous extract.
Extract of Hore′hound. Syn. Extractum marrubii, L. Prep. 1. From the fresh herb, as EXTRACT OF ACONITE.
2. From the infusion or decoction. Antispasmodic, pectoral, tonic, and emmenagogue.—Dose, 10 gr. to 1 dr.
3. (Ph. Baden.) By displacement with cold water.
4. (Alcoholic; Extractum marrubii alcoholicum, L.)—a. From a tincture made with proof spirit. Said by M. Thoriel to possess considerable power as a febrifuge.—Dose, 5 gr. to 20 gr.
b. (Ph. Lusit.) From a tincture made with a mixture of rectified spirit, 1 part, and water, 7 parts. Inferior to the last.
Extract of Ind′ian Hemp. Syn. Cannabine, Hemp resin, Alcoholic extract of Indian hemp; Extractum cannabis Indicæ, E. c. I. alcoholicum, Resina cannabis, L. Prep. 1. (B. P.) Indian hemp in coarse powder, 1;699 rectified spirit, 5; macerate seven days, press out the tincture, distil off the spirit, and evaporate.—Dose, 1⁄4 to 1 grain in pill.
2. (O’Shaughnessy.) The dried resinous tops of Indian hemp (‘GUNJAH’) are boiled in rectified spirit until all the resin is taken up, when most of the spirit is distilled off, and the evaporation completed by the heat of a water bath. 1 cwt. yields about 7 lbs.
3. (Robertson). By slowly acting on the ‘gunjah’ with the vapour of alcohol, by a species of percolation; the spirit of the resulting tincture is partly removed by distillation, and the rest by slow evaporation at a temperature not above 150° Fahr. 1 cwt. yields about 8 lbs.—Dose of the last two, 1 to 3 gr., gradually increased.
4. (Messrs Smith.) The bruised ‘gunjah’ is exhausted with tepid water, then with a solution of carbonate of soda (1 of carbonate to 2 of gunjah), and next with pure water; it is then pressed, dried, and exhausted by displacement with rectified spirit; the tincture is agitated with a milk of lime (containing 1 oz. of lime for every lb. of gunjah), and, after filtration or decantation, any retained lime is precipitated by a little sulphuric acid in slight excess; the tincture is next agitated with animal charcoal, and again filtered; most of the spirit is now removed by distillation, and 3 or 4 times its bulk of water being added, the remaining spirit is removed by a gentle heat; lastly, the remaining water is poured off, and the resin remaining washed with fresh water, and dried. Product, 6%.—Commencing dose, 1⁄4 gr.
3. (Purified; Extractum cannabis Indicæ purificatum, L.—Ph. D.) From the crude extract of Indian hemp, as imported (‘CHURRUS’), 1 oz.; rectified spirit, 4 fl. oz.; dissolve, and after defecation, decant, and evaporate.
Obs. The preparations of Indian hemp are said to be anæsthetic, anodyne, hypnotic, stimulant, phrenic, and aphrodisiac, and, in overdoses, to produce catalepsy. They have been recommended in hysteria, hydrophobia, cholera, rheumatism, chorea, convulsions, and various other painful spasmodic and nervous affections of a serious character. According to the observations of Dr O’Shaughnessy, 1 gr. of the extract produced catalepsy in a rheumatic patient. The extract prepared with the plant grown in our botanic gardens has quite a different effect to that of the Indian plant; and it also appears that the inhabitants of this country are less susceptible to its action than those of India, and consequently bear the drug in larger doses. This hemp is known in India as the ‘increaser of pleasure,’ the ‘exciter of desire,’ the ‘cementer of friendship,’ the ‘causer of a reeling gait,’ the ‘laughter-mover,’ &c. See Extract of Hemp (above), Hemp, &c.
Extract of Ipecac′uanha. Syn. Extractum ipecacuanha, L. Prep. 1. (P. Cod.) From ipecacuanha, as EXTRACT OF BOX.—P. Cod.
2. (Ph. Bor.) As Extract of henbane seeds. Expectorant and emetic.—Dose, 11⁄2 to 8 gr.
Extract of I′ron. Syn. Extractum ferri, E. martis, L. Prep. 1. From tincture of tartarised iron.—Dose, 2 to 10 gr.; as a chalybeate tonic.
2. (Compound.) See Extract of Apple.
Extract of Jabor′andi (Fluid). (F. V. Greene, ‘Amer. Journ. Pharm.,’ 1877.) Prep. Jaborandi leaves in moderately fine powder, 16 troy oz.; alcohol (50 per cent.), a sufficient quantity. Moisten the powder thoroughly with the menstruum, pack in a conical glass percolator, place a layer of two inches of well-washed sand on the top of the cloth covering the material, add menstruum until the liquid begins to drop from the percolator, when the lower orifice is to be closed with a cork, and the percolator securely covered; set aside in a moderately warm place for four days. At the expiration of this time remove the cork, and add more menstruum by degrees until the material is exhausted. The first 14 ounces (old measure) of the percolate are to be reserved, and the remainder evaporated on a water bath, with constant stirring towards the close, to 2 fluid ounces (old measure), which are to be added to the reserved portion. If the percolation and evaporation have been properly performed the fluid extract will not be required to be filtered.
Extract of Jal′ap. Syn. Extractum jalapæ (B. P.), E. sive resina jalapæ (Ph. E.), L. Prep. 1. (B. P.) Jalap in coarse powder, 1; rectified spirit, 5; distilled water, 10; macerate the jalap in the spirit for seven days, press out the tincture, then filter, and distil off the spirit, leaving a soft extract; again macerate the residual jalap in the water for four hours, express, strain through flannel, and evaporate by a water bath to a soft extract; mix the two extracts, and evaporate at a temperature not exceeding 140° F. to a proper consistence for forming pills.—Dose, 5 to 15 gr.
2. (Ph. L.) Jalap (powdered) 21⁄2 lbs.; rectified spirit, 1 gall.; digest four days, and express the tincture; boil the ‘marc’ in water, 2 galls.; until reduced to 1⁄2 gall.; filter the tincture and decoction separately, and let the one distil and the other evaporate until each thickens; lastly, mix the two and complete the evaporation.—Product. About 66% = 16% of alcoholic and 50% of aqueous extract. (Brande.) 18 lbs. yield 12 lbs. of extract. (Lab. Journ.)—Dose, 6 to 15 gr.
3. (Ph. E.) From tincture of jalap prepared by displacement with rectified spirit. It consists of impure resin of jalap. It is more active than the last.—Prod. 16%.—Dose, 2 to 6 gr.
4. (Ph. Ed. 1744, Extractum jalapæ alkalinum.) As extract jalap (B. P.) adding for every pound of jalap, 1 oz., or q. s. of carbonate of potash.
Obs. Extract of jalap is an active purgative.700 It should be well beaten up with a little sulphate of potassa, sugar, or some aromatic powder, to prevent it griping. The substance commonly sold as extract of jalap in the shops is prepared by boiling jalap root for 3 or 4 hours in water, when it is taken out, and well bruised or sliced, and again boiled with water until exhausted of soluble matter. The mixed decoctions are then allowed 12 or 14 hours for defecation, after which the supernatant portion is decanted and evaporated.
Extract of jalap “should be kept in the soft state (EXTRACTUM JALAPÆ, E. J. MOLLE), so as to form pills; and in the hard state (HARD EXTRACT OF JALAP; EXTRACTUM JALAPÆ DURUM), that it may be rubbed to powder.” (Ph. L.)
Extract of Jasmine (Yellow). Syn. Extractum Gelsemii fluidum. (Ph. U. S.) Prep. Yellow jasmine in very fine powder, 16 oz. (troy); rectified spirit, 16 oz. (old measure). Proceed as for fluid extract of cubebs. (Ph. U. S.)
Extract of Ju′niper. Syn. Extractum juniperi, E. baccarum j., L. Prep. (P. Cod.) Macerate juniper berries in water at 77° to 86° Fahr., for 24 hours, strain, repeat the process with a fresh quantity of water, mix the liquors, filter, and evaporate.—Dose, 20 gr. to 1 dr.; as a stimulant diuretic, in dropsy, &c.; and also as a pill-basis.
Extract of Kalada′na. Syn. Extractum kaladanæ, L. Prep. (Bengal Disp.) From the tincture of the seeds of kaladana (Pharbitis Nil). Purgative said to be equal to EXTRACT OF JALAP, and of double the strength.
Extract of Lettuce. Syn. Inspissated juice of lettuce; Extractum lactucæ (B. P.), L. Prep. 1. (B. P.) The inspissated juice evaporated to a pillular consistence, according to the directions given for EXTRACTUM BELLADONNÆ.
2. (Ph. L.) From the fresh leaves of garden lettuce (Lactuca sativa), as EXTRACT OF ACONITE—Ph. L. Anodyne, sedative, hypnotic, and antispasmodic.—Dose, 3 to 10 gr. 1 cwt. of lettuce yields 4 lbs. to 5 lbs. of extract.
3. (Probait.) From the external parts of the stalks and the old and yellow leaves, after the plants have flowered, by maceration in water for 24 hours, and decoction for 2 hours; the expressed liquid is first evaporated by a gentle heat, and afterwards spread on shallow dishes, and dried by exposure to a current of air. Stronger than the last.—Dose, 1 to 5 gr.
4. (E. of wild lettuce, Inspissated juice of w. l.; Extractum lactucæ virosæ, Succus spissatus l. v.—Ph. E., L.)—a. (Ph. E.) From the leaves of strong-scented wild lettuce (Lactuca virosa).[295]
[295] See general instructions, p. 682.
b. (P. Cod.) As ALCOHOLIC EXTRACT OF ACONITE.—P. Cod.
c. (Ph. Baden.) As EXTRACT OF FOXGLOVE—Ph. Baden. See Lactucarium.
Extract of Liq′uorice. Syn. Extractum glycyrrhizæ (B. P.), L. Prep. 1. (Soft e. of l.; Extractum glycyrrhizæ molle, L.)—a. (Ph. L.) From fresh liquorice root, as EXTRACT OF HOPS—Ph. L.
b. (Ph. E.) From the fresh root, cut into slices, dried, and powdered, as EXTRACT OF GENTIAN—Ph. E. The form of the Ph. Baden is very similar.
c. (Ph. D.) As ordered for simple extracts (EXTRACTA SIMPLICIORA—Ph. D.).
d. (B. P.) Liquorice root in coarse powder, 1; cold distilled water, 5; macerate the root in half the water for twelve hours, strain, and press; again macerate the pressed marc with the remainder of the water for 6 hours, strain and press, mix the strained liquors, heat to 212° F., strain, and evaporate to a pill consistence.—Dose, 1⁄2 to 1 dr.
e. (B. P. Extractum glycyrrhizæ liquidum.) Prep. Liquorice root in coarse powder, 1 lb.; distilled water, 4 pints. Macerate the liquorice with two pints of water for twelve hours, strain, and press; again macerate the pressed marc with the remainder of the water for six hours, strain, and press. Mix the strained liquors, heat to 212° Fahr., and strain through flannel; then evaporate by a water bath until it has acquired, when cold, a specific gravity of 1·160; add to this one eighth of its volume of rectified spirit; let the mixture stand for twelve hours and filter.
f. (U. S. Disp.) Crude liquorice (Spanish juice), q. s. is dissolved in water, and the solution filtered and evaporated. To produce a good article (EXTRACTUM GLYCYRRHIZÆ PURIFICATUM) in this way, the solution should be allowed some hours for defecation, and should not be decanted and strained until quite cold.
Obs. Soft extract of liquorice is often employed as a pill-basis, and the hard extract (Spanish juice, &c.) is used as a lozenge to allay tickling cough. The principal portion of the latter is, however, consumed by the porter brewers and brewers’ druggists. The product of the last formula, evaporated until it is quite solid when cold, and made into small pipes, sticks, or rolls, forms the BEST REFINED LIQUORICE or REFINED JUICE of the shops.
2. (Hard e. of l., Spanish juice, S. liquorice, Glycyrrhizin, Black sugar; Extractum glycyrrhizæ simplex, E. g. durum, Succus g., S. g. spissatus, L.) This is seldom prepared by the English druggists, being principally imported in the dry state from Spain and Italy. That from Solazzi (Solazzi juice) is the most esteemed. A great deal of the foreign extract is mixed with fecula, or the pulp of plums, hence its inferior quality. It also frequently contains copper, derived from the boilers in which it is prepared. The extract prepared from the fresh root is usually preferred to the best foreign, as the latter has a less sweet and agreeable taste. Refined juice is prepared by dissolving the foreign701 juice in water, filtering and evaporating. See Liquorice, and above.
Extract of Lobe′lia. Syn. Acetic extract of Indian tobacco; Extractum lobeliæ, E. l. inflatæ, L. Prep. (W. Proctor.) Lobelia seeds (bruised), 8 oz.; dilute alcohol (sp. gr. ·935), 4 pints; acetic acid, 1 fl. oz.; by maceration for 24 hours, and subsequent displacement. Expectorant and diaphoretic, in small doses; emetic and narcotic, in larger ones. It is principally used in asthma and other chest diseases.—Dose, 1⁄4 gr. to 5 gr.
Extract of Log′wood. Syn. Extractum hæmatoxyli (B. P.), E. h. Campechiani, (Ph. E.), L. Prep. 1. (B. P.) Logwood in chips, 1; boiling distilled water, 10; macerate 24 hours, boil to 5, strain, and evaporate to an extract, but not in iron vessels.
2. (Ph. L.) From cut logwood (logwood chips), as EXTRACT OF HOPS—Ph. L.
3. (Ph. E.) As the last (nearly).
4. (Ph. D. 1826 and Wholesale.) From the decoction.
Obs. The Ph. U. S. 1841 orders the wood to be rasped. The Ph. Baden directs displacement with cold water. On the large scale, this extract is prepared solely by decoction. 1 cwt. of wood yields about 20 lbs. of extract (Brande); 80 lbs. yield 14 lbs. of extract (Gray.) It is kept in two states—hard (EXTRACTUM HÆMATOXYLI DURUM) and soft (E. H. MOLLE). The dose of the first is 10 to 20 gr., dissolved in wine, or any cordial water; as an astringent, after each motion in diarrhœa; the second is often employed as a lozenge in the same disease, and is an inexpensive and agreeable remedy for simple relaxation of the bowels.
Extract of Lov′age. Syn. Extractum levistici, L. Prep. (Ph. Baden.) From lovage (Levisticum officinale), as EXTRACT OF BISTORT—P. Cod. Aromatic, stomachic, and diaphoretic.—Dose, 5 to 15 gr.
Extract of Lu′puline. Syn. Extractum lupulinæ, L. Prep. 1. From lupuline (the powder separated from hops by rubbing and sifting), by infusion in cold water, or by displacement.
2. (Extractum lupulinæ coctione paratum.) From the decoction. Both similar to extract of hops, but stronger. The first is the most aromatic; the last the most bitter.
Extract of Mad′der. Syn. Extractum rubiæ. L. Prep. (Ph. Hamb.) From the tincture of dyer’s madder (Rubia tinctorum), made with rectified spirit, 1 part, and water, 3 parts.—Dose, 10 gr. to 30 gr.; as a diuretic, emmenagogue, and parturifacient; and in jaundice, &c.
Extract of Mahog′any. Syn. Extractum swieteniæ, L. From the chips and sawdust of mahogany (Swietenia Mahogoni). It is astringent, and is frequently sold for kino. It is also employed in tanning.
Extract of Male Fern. Syn. Alcoholic extract of male fern; Extractum filicis, B. P. Prep. 1. (Dr Ebers.) From the tincture of the dried root of male fern (Lastræa Filix-mas), made with rectified spirit. In tape-worm.—Dose, 20 gr. to 1⁄2 dr., twice a day, made into an electuary with powdered sugar, and followed by a strong dose of castor oil.
2. (Ethereal.) B. P. (Extractum filicis liquidum.) Fern root, in coarse powder, 1; ether, 21⁄2, or a sufficiency. Pack closely in a percolator with 1 of the ether, add the rest at intervals, until it passes through colourless, distil off the ether, and the liquid extract remains.—Dose, 30 to 60 minims. See Oil of Male Fern.
Extract of Malt. Syn. Extractum malti, E. Bynes, L. Prep. 1. From the infusion made with water at a temperature ranging between 160° and 170° Fahr., drained off, without pressure, and evaporated to the consistence of honey. Nutritious and laxative.—Dose. A table-spoonful, or more, ad libitum.
2. Extract of malt, ferrated. (Ph. G.) Extract of malt, 471⁄2 oz., mixed with 1 oz. of pyrophosphate of iron and citrate of ammonia, dissolved in 11⁄2 oz. of water.
Extract of Ma′′rygold. Syn. Extractum calendulæ, L. Prep. 1. (Guibourt.) By maceration of the herb and flowers of the common marygold (Calendula officinalis) in tepid water for 24 hours, and subsequent coction for 15 or 20 minutes.
2. (Ph. Baden.) As EXTRACT OF ANGELICA—Ph. Baden.—Dose, 2 to 10 gr.; cordial, diaphoretic, alterative, and emmenagogue; in dyspepsia, and scirrhous and cancerous affections.
Extract of May-apple. Syn. Extractum podophylli. L. Prep. (Ph. U. S. 1841.) From the tincture of the root (rhizomes) of may-apple (Podophyllum peltatum).—Dose, 5 to 15 gr.; as a substitute for extract of jalap. See Podophyllin.
Extract of Mea′dow Saf′fron. See Extract of Colchicum.
Extract of Meat. Syn. Extract of flesh; Extractum carnis, L. Prep. (Liebig.) The lean of recently killed meat (chopped very small), 1 part; cold water, 8 parts; agitate it well together for 10 minutes; then heat it gradually to the boiling-point, let it simmer gently for a few minutes, and strain through a hair sieve whilst still hot; lastly, evaporate to a soft mass. 1 lb. of meat yields barely 1 oz. See Essence of Beef, Tea (Beef), &c.
Extract of Mezere′on. Syn. Mezereon resin; Extractum mezerei, Resina mezerei, L. Prep. 1. (Alcoholic; E. m. alcoholicum, L.)—a. (Ph. Hamb.) By distilling off 5⁄6ths of the tincture made with rectified spirit, and filtering the residue, retaining what is left on the filter.
b. By the simple distillation of the tincture.
Obs. Green or brownish green; insoluble in water. 1⁄2 oz. mezereon root-bark yielded702 11⁄2 oz. (Hamb. Disp.) It is chiefly used in preparing blistering ointments and plasters.
2. (Ethereal; Green oil of mezereon; Extractum mezerei æthereum. B. P.)—a. B. P. Mezereon bark cut small, 1 lb.; rectified spirit, 8 pints; ether, 1 pint. Macerate the mezereon in 6 pints of the spirit for 3 days, with frequent agitation, strain, and press. To the residue of the mezereon add the remainder of the spirit, and again macerate for 3 days, with constant agitation, strain, and press. Mix and filter the strained liquors, recover the greater part of the spirit by distillation, evaporate what remains to the consistence of a soft extract, put this in a stoppered bottle with the ether, and macerate for 24 hours, shaking them frequently; decant the etherial solution, recover part of the ether by distillation, and evaporate what remains to the consistence of a soft extract.
b. (Ph. Bor.) By digesting alcoholic extract of mezereon in ether for some days with agitation, reducing the tincture to 1-4th by careful distillation, and evaporating the residuum by a gentle heat to the consistence of an extract.
c. (Ph. U. S. Extractum mezerei fluidum.) Mezereon in moderately coarse powder, 16 oz. (troy); alcohol (·817), 16 oz. (old measure); proceed as for fluid extract of cubebs. (Ph. U. S.)
Obs. Both the alcoholic and ethereal extract of mezereon must be kept in stoppered bottles. The latter, like the former, is used as an external irritant.
Extract of Mil′foil. Syn. Extractum millefolii, E. Achiliæ m., L. Prep. From the herb milfoil or yarrow (Achillea millefolium), as EXTRACT OF HOPS—Ph. L. Astringent, tonic, and alterative.—Dose, 10 gr. to 1⁄2 dr.
Extract of Mimo′sa Bark. Syn. Extractum corticis mimosæ, L. Prep. From the bark of several Australian species of Acacia or Mimosa (Acacia mollissima, A. decurrens, A. melanoxylon, &c.). It is chiefly imported from Van Diemen’s Land. Astringent. Said to be superior to oak bark for tanning.
Extract of Mone′sia Bark. Syn. Extractum monesiæ, E. m. purificatum, L. Prep. From the monesia or buranheim bark (bark of Chrysophyllum Buranheim); or from commercial monesia, as EXTRACT OF CATECHU. Astringent.—Dose, 4 to 8 gr.
Extract of Mug′wort. Syn. Extractum artemisiæ, L. Prep. From the tops of the common mugwort (Artemisia vulgaris), as EXTRACT OF BOX,—P. Cod. An active emmenagogue.
Extract of Myrrh. Syn. Extractum myrrhæ, L. Prep. 1. (Aqueous; Extractum myrrhæ aquosum, L.)—a. From the strained decoction.
b. (Ph. Bor.) As EXTRACT OF ALOES—Ph. Bor., afterwards reducing it to a fine powder. The formula of the Ph. Baden is similar.—Dose, 6 to 15 gr., or more.
2. (Alcoholic; Resin of myrrh; Extractum myrrhæ alcoholicum, E. m. resinosum, L.) From the tincture. Tonic and stimulant.—Dose, 5 to 10 gr., or more.
3. (Compound; Extractum myrrhæ compositum, L.—Swediaur.) Myrrh, 2 oz.; gum arabic (in powder), 2 dr.; triturate, add water, q. s. to form a thick emulsion, and add extract of couch grass, 4 oz. Much recommended in phthisis and uterine ulcerations.—Dose, 1 to 2 dr. in water, twice or thrice daily.
Extracts, Narcotic, with Sugar. Syn. Extractum narcotica cum saccharo. (Guager.) Prep. Dissolve 6 oz. of alcoholic extract of the plant in 14 dr. or 2 oz. of strong alcohol by trituration in a porcelain mortar, and mix with it 30 oz. of powdered white sugar, gradually added, with constant stirring. Set the mixture in a warm situation until dry. Add sugar q. s. to make up 36 oz. These preparations are less liable to lose their efficacy than the simple extracts. 6 gr. represent one of the extract.
Extract of Net′tles. Syn. Extractum urticæ, L. Prep. (P. Cod.) From the juice of nettles (Urtica dioica), as EXTRACT OF ACONITE—Ph. L. Antiscorbutic, diuretic, and narcotic.
Extract of Nose′gay. Syn. Essence of nosegay; Extrait de bouquet, Fr. Prep. Flowers of benzoin, 1 dr.; essence of ambergris, 2 fl. oz.; spirits of jasmine and extract (esprit) of violets, of each 1 pint; spirits of cassia, roses, orange flowers and gillyflowers, of each 1⁄2 pint; mix. A delightful perfume.
Extract of Nux Vom′ica. Syn. Extract of koochla nuts; Extractum nucis vomicæ, B. P. Prep. 1. (Aqueous; E. n. v. Aquosum, L.—Ph. Bor.) From the powdered nut, as EXTRACT OF HOPS—Ph. L. (nearly.)—Dose, 1 to 5 gr.
2. (Alcoholic; Extractum nucis vomicæ—Ph. L. E. and D., E. n. v. alcoholicum, L.)—a. (B. P.) Soften nux vomica by steam, dry rapidly, and reduce to fine powder; boil with rectified spirit until exhausted, strain, distil off the spirit, and evaporate to the consistence of a soft extract.—Dose, 1⁄2 to 2 gr.
b. (Ph. L.) Koochla or poison nuts (seed or fruit of Strychnos Nux-vomica), 8 oz.; rectified spirit, 3 pints; expose them to steam until softened, then bruise, slice, and dry them, and macerate them in 2-3rds of the spirit for 7 days; express the tincture, and repeat the maceration with the remaining 1-3rd of the spirit; again express the liquid; lastly, filter the mixed tinctures, distil off the greater part of the spirit, and complete the evaporation by a gentle heat. The formula of the P. Cod. is similar, but using spirit sp. gr. ·863 (=41 o. p.).
c. (Ph. E.) The sliced and dried nuts are to be ground in a coffee-mill, and either exhausted703 by percolation with rectified spirit or by boiling the powder in repeated portions of the menstruum. The formulæ of the Ph. Baden and U. S. are similar.
d. (Ph. D. 1826.) From a tincture of the rasped nut made with proof spirit, observing that the extract, whilst thickening, should be properly stirred.
Obs. This extract consists chiefly of impure igasurate of strychnia, and is exhibited in similar cases to that alkaloid. Used as a stimulant of the nervous system in paralysis.—Dose, 1⁄2 gr., gradually and cautiously increased to 2 gr. It is very poisonous. On the large scale, the nuts are ground in a drug-mill.
Extract of Oak Bark. Syn. Extractum quercûs, E. corticis quercûs, L. Prep. (Ph. D. 1826.) From the decoction. Astringent.—Dose, 10 gr. to 1⁄2 dr. Now seldom used.
Extract of O′pium. Prep. 1. (Aqueous extract of opium, Simple e. of o.; Extractum opii (B. P.), E. o. aquosum (Ph. D.), E. o. purificatum, L.)—a. (B. P.) Opium in thin slices, 1 lb.; distilled water, 6 pints. Macerate the opium in 2 pints of the water for 24 hours, and express the liquor. Reduce the residue of the opium to a uniform pulp, macerate again in 2 pints of the water for 24 hours, and express. Repeat the operation a third time. Mix the liquors, strain through flannel, and evaporate by a water bath to a proper consistence for forming pills.—Dose, 1⁄2 to 1 gr., or more.
b. (Ph. L.) Opium (powdered), 11⁄2 lb.; distilled water (cold), 21⁄2 pints; mix gradually, and macerate for 24 hours, frequently stirring with a spatula; (press), strain, and repeat the maceration for 24 hours with a fresh quantity (21⁄2 pints) of water; lastly, evaporate the (mixed) strained liquors to a proper consistence. The formulæ of the Ph. E. D. and Baden, and P. Cod., are essentially the same.
c. (Ph. D. 1826.) As the last, but using boiling water, and exposing the mixed liquors to the air for 2 days, before filtering, and evaporating them. Inferior to the last.
d. (Purified.) The extract, prepared with cold water, is evaporated to dryness, powdered, and redissolved in cold water; after 48 hours’ exposure, and defecation, it is decanted from the dregs, filtered, and gently evaporated, as before. Superior to any other extract of opium made.
Obs. Good opium yields from 60% to 70% of its weight of extract, but much depends upon the variety used.—Dose, 1⁄4 gr. to 2 gr., as an anodyne, sedative, and hypnotic. It is less stimulating than ordinary opium. That prepared by the third formula is, indeed, scarcely inferior in its action to the salts of morphine.
This extract is kept in both the hard and soft state (EXTRACTUM OPII DURUM, E. O. MOLLE). A solution of the former, in distilled water, with the addition of a little spirit to keep it, forms Battley’s ‘LIQUOR OPII SEDATIVUS,’ The Dublin formula is that adopted by the wholesale druggists.
Besides the aqueous extract, there are the following preparations:—
2. (Extractum opii liquidum, B. P.; Extract of opium.) Distilled water, 16; rectified spirit, 4. Digest the extract of opium in the water for an hour, stirring frequently; filter, and add the spirit. The product should measure 20.—Dose, 10 to 30 minims.
3. (Acetic; Extractum opii aceticum, L.—Soubeiran.) Opium, 1 oz.; distilled vinegar, 1 quart; digest 2 days (with heat), decant, filter, and evaporate.
4. (Alcoholic; Extractum opii alcoholicum, L.—Ph. Antwerp.) From the tincture.
5. (Aqueo-alcoholic; E. opii aquo-alcoholicum, L.—Taddei.) The opium, exhausted by spirit, is digested in warm water, and the infusion and tincture, separately filtered, are mixed and evaporated.
6. (De-narcotised; Extractum opii absque narcotina, L.—a. (P. Cod.) The aqueous extract is reduced with hot water to the consistence of a syrup, and when cold this is mixed with 8 times its volume of ether, and the whole is frequently shaken for a day or two; the ethereal solution is then decanted, and the process repeated with fresh ether, as long as it dissolves anything. The original form of Robiquet is similar. Said to consist entirely of impure MURIATE OF MORPHINE, GUM, and EXTRACTIVE.
b. (M. Lamousin-Lamothe.) Aqueous extract, 4 parts; resin, 1 part; beat well together; add boiling water, 16 parts; boil to one half; add cold water, 8 parts, filter, and evaporate.
7. (By fermentation; Extractum opii per fermentationem, L.)—a. Opium and sugar, of each 4 oz.; water, 1 quart; rub together, and keep the mixture, loosely covered, in a warm situation (about 70° Fahr.), for 10 days or more; then add of cold water, 1 quart, and the next day filter and evaporate.
b. (Duyeux.) From an unstrained mixture of opium, 1 part, with water, 8 parts, and a little yeast; left for a week at a temperature of 68° to 77° Fahr., and then diluted, filtered, and evaporated. Some parties have recommended quince juice as the menstruum.
8. (Roasted; Extractum opii torrefacti, L.—Guibourt.) Powdered opium is heated on a flat dish over a moderate fire, with constant stirring, as long as fumes are given off; it is then exhausted by treating it twice with 6 times its weight of water, and the mixed liquor, after filtration, is evaporated.
9. (Vinous; Extractum opii vino paratum, L.—P. Cod.) From wine of opium. The above extracts of opium (excepting the alcoholic) are regarded as less exciting than the other preparations of the drug. The dose of704 each is similar to that of the aqueous extract.
Extract of Or′ange Peel. Syn. Extractum corticis aurantii, L. Prep. 1. From the thin yellow peel, as EXTRACT OF MADDER.
2. See Aurantii.
Extract of Ox-gall. Syn. Inspissated ox-gall; Extractum fellis bovini, L. Prep. 1. (P. Cod.) From ox-gall, strained, and evaporated in a water bath.—Dose, 5 to 15 gr.; in pills.
2. (Hunter Lane.) As the last, but reducing the gall to dryness, and then powdering it. It must be preserved in well-corked bottles.—Dose, 3 to 12 gr.
Extract of Parei′ra. Syn. Extractum pareiræ (B. P.), L. Prep. 1. (B. P.) Pareira root in coarse powder, 1; boiling distilled water, 10, or a sufficiency; digest the pareira with 11⁄2 of water for twenty-four hours, then pack in a percolator, and water till by slow percolation 10 has passed through; evaporate in a water bath to a pilular consistence.—Dose, 13 to 20 gr.
2. (Ph. L.) From the root of velvet leaf or pareira brava (Cissampelos Pareira), as EXTRACT OF HOPS—Ph. L.
3. (P. E.) As EXTRACT OF LIQUORICE—Ph. E. The P. Cod. formula is similar. Alterative, tonic, and diuretic.—Dose, 10 gr. to 1⁄2 dr.; chiefly in affections of the bladder.
4. Extractum pareira liquidum (B. P.). Pareira in coarse powder, 16; boiling distilled water, 160, or a sufficiency; rectified spirit, 3; macerate in 20 of water for twenty-four hours; pack in a percolator, adding more of the water; allow the liquor slowly to pass until 160 has been collected, or the pareira is exhausted; evaporate to 13, and, when cold, add the spirit; filter and make up to 16.—Dose, 1⁄2 dr. to 2 dr.
Extract of Par′sley. Syn. Extractum Petroselini, L. Prep. (P. Cod.) From the root, as EXTRACT OF BISTORT—P. Cod.
2. (M. Peraibe.) From the fresh leaves, as EXTRACT OF ACONITE. Febrifuge and tonic.—Dose, 5 to 10 gr.
Extract of Pasque Flower. Syn. Extractum anemonis, L. Prep. (P. Cod.) From the recent or dried flower, as either of the EXTRACTS OF ACONITE—P. Cod.—Dose, 1 to 4 gr.
Obs. Several species of Anemone have been used in medicine, especially Anemone pratensis and A. pulsatilla. According to Baron Stoerck, the former is resolvent, and is an effectual remedy in various chronic diseases, particularly in amaurosis, cataract, opacity of the cornea, nocturnal pains, suppressions, &c. 1⁄2 to 1 gr., combined with sugar of milk, has been highly recommended in hooping-cough.
Extract of Pa′tience Dock. Syn. Extractum patientiæ, L. Prep. From the root of Rumex Patientia or garden patience, as EXTRACT OF HOPS. Aperient and stomachic. Used in double doses in lieu of extract of rhubarb.
Extract of Paullin′ia. See Extract of Guarana.
Extract of Peach Blos′som. Prep. From essence of lemons, 1 oz.; pure balsam of Peru, 2 dr.; essence (oil) of bitter almonds, 1 dr.; rectified spirit, 3 pints; spirit of orange flowers, 1 pint; spirit of jasmin, 1⁄4 pint; mix. A pleasant and powerful perfume.
Extract of Pel′litory. Syn. Extractum pyrethri, E. p. æthero-alcoholicum, L. Prep. (W. Procter.) Alcohol (rectified spirit), 1 pint; ether, 1⁄2 pint; mix, and pour it gradually on root of pellitory (Anacyclus Pyrethrum), 1 lb., placed in a percolator; afterwards pour on alcohol, 1 pint; and subsequently sufficient dilute alcohol (proof spirit) to displace 21⁄2 pints of tincture (ESSENCE OF PELLITORY, TOOTHACHE ESSENCE); the latter is either suffered to evaporate spontaneously, or by a very gentle heat, until a soft extract is attained. Used to destroy the sensibility of the nerves of the teeth, previous to plugging, and for toothache.
Extract of Pep′per. Syn. Extractum piperis, E. p. nigri, L. Prep. 1. From decoction of black pepper (bruised). Stimulant; stronger tasted than the berries, but less aromatic.—Dose, 10 gr. to 1 dr.; in agues.
2. (Fluid; Extractum piperis fluidum, L.)—Ph. U. S. From black pepper, as FLUID EXTRACT OF CUBEBS—Ph. U. S., separating the PIPERINE by expression through a cloth, and keeping the fluid portion for use.
Extract of Pimpinel′la. Syn. Extractum pimpinellæ, L. Prep. From the root of burnet saxifrage (Pimpinella saxifraga), as EXTRACT OF HOPS. Astringent.—Dose, 10 to 20 gr.
Extract of Pink′root. Syn. Extract of worm grass, E. of wormseed root; Extractum spigeliæ, L. Prep. 1. From Carolina pinkroot (Spigelia Marylandica), as EXTRACT OF BOX—P. Cod.—Dose, 5 to 20 gr.
2. (Fluid; Essence of pinkroot, Liquor of p.; Extractum spigeliæ fluidum, L.) Pinkroot, 1 lb.; proof spirit, 3 pints; make a tincture, evaporate to 10 fl. oz., add sugar, 3⁄4 lb., and rectified spirit, q. s. to make the whole measure exactly a pint.—Dose. For a child, beginning with 1⁄2 a teaspoonful.
3. (Compound; Compound liquor of pinkroot; Extractum spigeliæ fluidum comp., L.)—a. (Estlack.) Carolina pinkroot or spigelia (bruised), 4 oz.; senna, 3 oz.; savine, 1 dr.; pour on boiling water, 1 quart; when cold, add rectified spirit, 1⁄2 pint; digest 24 hours, express (or percolate), filter, evaporate to 12 fl. oz., in which dissolve, manna, 1 oz.; sugar, 8 oz. Every fl. oz. is equal to 2 dr. of pinkroot and 11⁄2 dr. of senna.—Dose. For a child, 1⁄2 to 1 teaspoonful; for an adult, a tablespoonful.
b. (W. Procter.) Pinkroot, 16 oz.; senna, 8 oz. (both in coarse powder); dilute alcohol (sp. gr. ·935), 2 pints; macerate for 2 days, then proceed by displacement, adding fresh705 spirit, until 4 pints have passed through; filter, evaporate to 20 fl. oz., and add carbonate of potassa, 1 oz.; next add oils of caraway and aniseed, of each 1⁄2 dr.; (previously triturated with) powdered sugar, 24 oz.; lastly, apply a gentle heat to dissolve the sugar.
c. (Extractum spigeliæ et sennæ fluidum—Ph. U. S.) As the last (nearly).—Dose. As above. All the above preparations of pinkroot are regarded as powerful and certain anthelmintics; particularly the last two.
Extract of Pipsis′sewa. See Extract of Winter-green.
Extract of Poi′son Oak. Syn. Extractum rhois toxicodendri, L. Prep. (P. Cod.) From the expressed juice of the leaves of Rhus toxicodendron. Narcotic, stimulant, and alterative.—Dose, 1⁄2 gr. to 1 gr., gradually increased; in chronic rheumatism, obstinate skin diseases, &c.
Extract of Pomegran′ate. Syn. Extractum granati, L. Prep. 1. (Soubeiran, & P. Cod.) From the root-bark of pomegranate, as EXTRACT OF BOX. In tapeworm.—Dose, 10 to 20 gr.; followed by a purgative.
2. (E. g. corticis fructus, L.) From the decoction of the fruit-rind. As the last.
Extract of Pop′pies. Syn. Extractum papaveris (B. P.), E. p. albi, L. Prep. 1. (B. P.) Capsules coarsely powdered, 16; rectified spirit, 2; distilled water, a sufficiency; mix the poppy capsules with 40 of the water, stirring them frequently during 24 hours; then pack in a percolator, and pass water slowly through them until about 160 have passed through; evaporate the liquor by a water bath to 20; when cold, add the spirit. After 24 hours filter the liquor, and evaporate to a pilular consistence.—Dose, 2 or 5 grains.
2. (Ph. L.) Bruised poppy-heads (without the seeds), 15 oz.; boiled distilled water, 1 gall.; macerate 24 hours, boil to one half, strain, and complete the evaporation.
3. (Ph. E.) As the last, with “capsules not quite ripe.”
Obs. The medical action of extract of poppies, for the most part, resembles that of opium; but it is considerably weaker, and is generally regarded as less prone to produce headache and delirium.—Dose, 2 to 12 gr. It is usually prepared by the large manufacturers, by exhausting the capsules, by coction with water; hence the inferior quality of the extract of the shops, which contains a considerable quantity of inert matter.
The principal consumption of this extract is among the brewers, brewers’ druggists, and wine merchants. For this purpose it is evaporated until it becomes hard on cooling, when it is formed into half-pound rolls, and covered with paper, like lead plaster. One of these rolls added to a hogshead of ale, stout, or sherry, materially increases the ‘headiness’ or apparent strength of these beverages.
Extract of Pota′to. Syn. Extractum Solani tuberosi, L. Prep. (Dr J. Latham.) From the stem and leaves of the potato plant, as EXTRACT OF ACONITE—Ph. L. Narcotic.—Dose, 2 to 10 gr.
Extract of Pur′ging Flax. Syn. Extractum lini cathartici, L. Prep. (Dr B. Lane.) From the dried herb, as EXTRACT OF HOPS—Ph. L. Aperient and diuretic—Dose, 5 to 10 gr.; 14 lbs. yielded 21⁄4 lbs. of extract.
Extract of Quas′sia. Syn. Extractum quassiæ (B. P.), Ex. q. ligni, L. Prep. 1. (B. P.) Quassia scraped, 1 lb.; distilled water, a sufficiency; macerate the quassia in 8 oz. of water for 12 hours; pack in a percolator; add water till the quassia is exhausted; evaporate, filter before it becomes thick, and again evaporate in a water bath to a proper consistence for pills.—Dose, 2 to 5 gr.
2. From the decoction of quassia chips. Product, 5% to 6%.
3. (Ph. E.) From the rasped wood, as EXTRACT OF BISTORT—P. Cod. Bitter and stomachic.—Dose, 5 to 10 gr., or more.
Obs. This extract is almost universally prepared by coction, and is principally consumed by the brewers, who employ it as a substitute for hops, in large quantities. The bark is frequently substituted for the wood, but is considerably less bitter. The Ph. Baden has an extract prepared with spirit of ·944.
Extract of Quince Seeds. Syn. Extractum cydoniæ, E. c. seminum. Prep. From the decoction. Sucked as a lozenge, in hoarseness, &c.
Extract of Ragwort. Syn. Extractum JacobϾ. The inspissated juice of rag-wort.
Extract of Rha′tany. Syn. Extractum rhataniæ; E. krameriæ (B. P.), L. Prep. 1. (B. P.) Rhatany in coarse powder, 1; cold distilled water, 15; macerate 24 hours in 2 of the water, then percolate the whole; evaporate by water bath to dryness.—Dose, 5 to 20 grains.
2. (Ph. E. Baden and U. S.) From dried rhatany root (Krameria triandria), as EXTRACT OF BISTORT—P. Cod.
3. (Ph. Bor.) By two successive macerations in boiling water of 24 hours each, and evaporating at a temperature not exceeding 165° Fahr.
Obs. Extract of rhatany is astringent and tonic.—Dose, 10 to 20 gr. A large quantity of this extract, of very inferior quality, is imported from Brazil, and other parts of South America. It is kept in two states, hard and soft; the former resembles KINO, and is often sold for it; the latter is chiefly consumed by the manufacturers and ‘improvers’ of port wine.
Extract of Rhu′barb. Syn. Extractum rhei (B. P.), L. Prep. 1. (B. P.) Rhubarb (sliced or bruised), 8 oz.; rectified spirit, 5 oz.; distilled water, 50 oz.; macerate 4 days, strain, and set it aside, that the fæces may subside; next decant the clear portion, strain, mix, and evaporate to a proper consistence over a water bath at 160° F.
706
2. (Ph. L.) As EXTRACT OF CINCHONA—Ph. L. (nearly). “The extract is obtained of finer quality by evaporation in a vacuum with a gentle heat.” The Baden formula is similar.
Obs. This extract is usually prepared by decoction from inferior and damaged rhubarb, picked out from the chests on purpose; hence the inferior quality of the extract of the shops. When made of good Turkey, or even East India rhubarb, it is a very valuable preparation.—Dose. As a stomachic, 5 to 10 gr.; as a purgative, 10 gr. to 1⁄2 dr. It is seldom exhibited alone. Product. 5%.
3. (Fluid; Liquor of rhubarb, Essence of r.; Liquor rhei, Extractum rhei fluidum, L.)—a. (W. Procter.) Rhubarb (in coarse powder), 8 oz.; mix it with an equal bulk of coarse sand, and moisten it with dilute alcohol (sp. gr. ·935, = 13 u. p.) to form a pasty mass; in a short time introduce it into a percolator, shake it until uniformly settled, and cover it with cloth or paper; then pour on the rest of the spirit (the remainder of 2 pints) until the product has little odour or taste of the root; next gently evaporate the tincture to 51⁄2 fl. oz., and add sugar, 5 oz., when the whole should measure 8 fl. oz.—Dose, 15 to 30 drops.
b. (Ph. U. S.) As the last, adding of oils of fennel and anise, of each 4 drops; (dissolved in) tincture of ginger, 4 fl. dr.
4. (Compound; Extractum rhei compositum, E. panchymagogum, L.—Ph. Bor.) Extract of rhubarb, 3 dr.; extract of aloes, 1 dr. (softened with) water, 4 dr.; mix, and add of soap of jalap, 1 dr. (dissolved in) proof spirit, 4 dr.; lastly, evaporate to an extract, dry this in a warm place, and powder. Stomachic and purgative.—Dose, 4 to 20 gr.
Extract of Rue. Syn. Extractum rutæ, E. foliorum rutæ, L. Prep. 1. From rue leaves (Rutæ graveolens), as EXTRACT OF HOPS—Ph. L.
2. (Alcoholic—P. Cod.) As ALCOHOLIC EXTRACT OF ACONITE—P. Cod. (nearly.) The formula of the Ph. Wert. is similar.
Obs. This extract is stomachic, carminative and emmenagogue.—Dose, 10 to 20 gr., twice a day. It is usual to add a little of the essential oil to the extract, just before taking it out of the evaporating-pan, and when nearly cold. The first is the form adopted in trade in this country.
Extract of Saf′fron. Syn. Polychroite, Extractum croci, L. Prep. 1. From hay saffron, as EXTRACT OF COLOCYNTH—Ph. L.
2. (P. Cod.) From the tincture. Superior to the last.
Obs. The first is used chiefly as a colouring and flavouring substance by cooks, confectioners, wine and cordial brewers, &c.—Dose, 5 to 15 gr.; as an excitant, antispasmodic, and emmenagogue.
Extract of Sarsaparil′la. Syn. Extractum sarzæ, E. sarsaparillæ, L.; Extrait de salsepareille, Fr. Prep. 1. (Ph. L. 1836.) From sarsaparilla, as EXTRACT OF HOPS—Ph. L. The Ph. D. 1826 is similar.—Dose, 10 gr. to 1 dr. Product. (From Jamaica sarsaparilla) 32% to 36%.
2. (Alcoholic; Extractum sarzæ alcoholicum, L.)—a. From a tincture of the root-bark, prepared with proof spirit, either by digestion or percolation.
b. (P. Cod. and Ph. U. S.) From sarsaparilla root (powdered), as ALCOHOLIC EXTRACT OF ACONITE—P. Cod. Superior to the aqueous extract.—Dose, 10 to 20 gr.
3. (Fluid; Liquor of sarsaparilla, Essence of s.; Liquor sarzæ, Essentia sarsaparillæ, Extractum sarzæ liquidum—Ph. L., E. s. fluidum—Ph. E. & D., L.); Extractum sarzæ liquidum—(B. P.) a. Jamaica sarsaparilla cut transversely, 16; distilled water (temp. 160° Fahr.), 280; rectified spirit, 1. Macerate in half the water for 6 hours, and decant the liquor; digest the residue in the remainder of the water for 6 hours more, mix the liquors, express and filter; evaporate by water bath to 7, or until it has a sp. gr. of 1·130; when cold add the spirit. Sp. gr. should be about 1·025.—Dose, 1 to 4 dr.
b. (Ph. L.) Sarsaparilla, 31⁄2 lbs.; distilled water, 3 galls.; boil to 12 pints, pour off the liquor, and strain whilst hot; again boil the sarsaparilla in water, 2 galls., to one half, and strain; evaporate the mixed liquors to 18 fl. oz.; and when cold, add of rectified spirit, 2 fl. oz. Each fl. oz. represents 21⁄8 oz. of the root (nearly).
c. (Ph. E.) Sarsaparilla, 1 lb.; boiling water, 4 pints; digest 2 hours, then bruise the root, boil it for 2 hours, filter, and express the liquid; repeat the coction with water, 2 pints, as before; evaporate the mixed liquors to the consistence of a thin syrup, and, when cold enough, add of rectified spirit q. s. to make up 16 fl. oz. Each fl. oz. represents 6 dr. of the root, and 6 fl. oz. of the decoction.
d. (Ph. D.) Sarsaparilla, 1 lb. (avoir.); proceed as before, and add of rectified spirit, q. s. to make the product up to 20 fl. oz. Strength, as the last (nearly). In the Ph. D. 1826 the decoction of sarsaparilla, 1 lb. (troy), was ordered to be evaporated to 30 oz., which with the spirit (2 oz.) made the preparation only half the strength of the present one.
4. (Compound; Extractum sarzæ compositum, E. sarsaparillæ comp., L.) There is no form for this preparation in the Pharmacopœias, but it is nevertheless in immense demand, from its great convenience in dispensing. The following formulæ are employed by one of the wholesale houses that does largest in this preparation:—
a. Guaiacum shavings (from which the small has been sifted), 30 lbs., Italian juice, 24 lbs., mezereon root, 6 lbs., are boiled with water707 q. s., for 1 hour; the decoction is then drawn off, and the boiling repeated with fresh water a second and a third time; the mixed decoctions are allowed to deposit for 6 or 8 hours, or longer, and the clear portion decanted and strained through flannel; the liquid is now reduced to the consistence of treacle, when extract of sarsaparilla, 9 lbs., is added, and the evaporation conducted at a considerably lower temperature until near its completion, when the source of heat is removed, and the remaining evaporation conducted at the expense of that retained by the metal of the ‘pan,’ when nearly cold, and just before removing the extracts to the ‘pots’ or ‘jars,’ essential oil of sassafras, 2 dr., dissolved in rectified spirit, 1 quart, is added, and quickly but completely stirred in. The product is a very showy article, if well managed, and weighs about 45 lbs., the precise quantity depending on the quality of the juice employed. It is labelled ‘Ext. Sarzæ Comp.’
b. As the last, but only using 15 lbs. of juice, and that Solazzi. Prod. About 35 lbs. It is labelled and sent out as ‘Ext. Sarzæ Co. Opt.’
c. By any of the forms given under Compound Decoction of Sarsaparilla, either common or concentrated, by continuing the evaporation.—Dose. Same as that of the simple extract.
5. (Fluid Compound; Compound liquor of sarsaparilla.)—a. From any of the preceding formulæ by arresting the evaporation when the fluid has acquired the consistence of a thin syrup, and adding to each pint, when cold, rectified spirit, 4 fl. oz.
b. (Alcoholic—W. Hodgson.) Sarsaparilla (bruised), 16 oz.; liquorice root (bruised), guaiacum wood (rasped), and sassafras bark (sliced), of each 2 oz.; mezereon (sliced), 6 dr.; spirit, sp. gr. ·935 (= 13 u. p.) 7 pints; digest 14 days, express, filter, evaporate to 12 fl. oz.; add of sugar, 8 oz., and as soon as this is dissolved, withdraw the heat. Stronger than the last.—Dose, 1 fl. dr.
c. (Ph. U. S. Extractum Sarsaparillæ Compositum fluidum.) Prep. Sarsaparilla in moderately fine powder, 16 oz. (troy); liquorice root in moderately fine powder, 2 oz. (troy); sassafras in moderately fine powder, 2 oz. (troy); mezereon in moderately fine powder, 360 grains; glycerin, 4 oz. (old measure); rectified spirit, 8 oz. (old measure); water, 4 oz. (old measure). Macerate in a closed percolator for 4 days, and then let the percolation commence, and finish it by adding diluted alcohol (equal volumes of alcohol at ·835, and water), until 2 pints (old measure) have been obtained. Reserve the first 12 oz., having added 4 oz. (old measure) of glycerin to the remainder of the percolate, which evaporate to 6 oz. (old measure), and mix with the reserved portion.
6. (From the root-bark; Extractum corticis sarzæ, L.) From the decoction or tincture of the root-bark. The cortical portion of sarsaparilla yields fully 50% of aqueous extract. “Five times as much as the meditullium.” (Pope.)
Obs. Each of the above extracts of sarsaparilla (simple, fluid, and compound), when of good quality, dissolves in water, forming a deep reddish-brown solution, perfectly transparent, and depositing little sediment, even by standing some days. See Sarsaparilla.
1. Extract of Savine. Syn. Extractum Sabinæ. (Ph. L. 1788.) By evaporating a decoction of dry savine.
2. (Ph. U. S. Extractum Sabinæ fluidum.) As fluid extract of cubebs. (Ph. U. S.)
Extract of Scam′mony. Syn. Resin of scammony; Resina Scammonii, E. s. alcoholicum, E. sive resina scammonii (Ph. E.), L. Prep. 1. From powdered scammony, exhausted with proof spirit, and the resulting tincture distilled until little but water passes over; the remaining water is then poured from the resin, which is next well washed in boiling water and dried at a temperature below 240° Fahr. Brown; impure.
2. As the last, but using either alcohol of 90% or ether, and animal charcoal. White; pure.
Obs. Scammony resin is translucent, fusible, and combustible; and freely soluble in alcohol, ether, and oil of turpentine. It is frequently adulterated with jalap resin, a fraud readily detected by its insolubility in the last two menstrua.—Dose, 5 to 10 gr. “When pure or virgin scammony can be procured it is an unnecessary preparation.” (Pereira.)
Extract of Scurvy-grass. Syn. Extractum cochleariæ, L. Prep. (P. Cod.) From the clarified juice of fresh scurvy-grass, by exposure to warm air. Anti-scorbutic, stimulant, anti-rheumatic, and diaphoretic.—Dose, 1 to 2 dr. The valuable principles of the juice are dissipated by much heat.
Extract of Sen′ega. Syn. Extractum senegæ, L. Prep. 1. (P. Cod.) From seneka or snake-root (Polygala Senega), as EXTRACT OF BOX—P. Cod.
2. Compound; Extractum senegæ, COMPOSITUM, E. s. et scillæ, L.—Ecky.) From equal parts of squills and senega, as the last, but by displacement. Both the above are stimulant, expectorant, sudorific, and diuretic.—Dose, 1 to 12 gr.
3. (Ph. U. S. Extractum senegæ fluidum.) As EXTRACT OF COTTON-ROOT. (Ph. U. S.)
Extract of Sen′na. Syn. Extractum sennæ, L. Prep. 1. (Extractum sennæ aquosum, L.)—a. As EXTRACT OF COLOCYNTH—Ph. L.
b. (P. Cod.) As EXTRACT OF BISTORT—P. Cod.
c. (Ph. Bor.) From senna leaves, by maceration in tepid water (104° Fahr.) for 24708 hours, and expression and filtration; the operation is repeated with fresh water, and the strained liquors evaporated to a thick extract (at 149° to 157° Fahr.), which is dissolved in water, 4 parts, the solution filtered, and again evaporated.—Dose, 10 to 20 gr. It is principally used as a basis for purgative pill. When prepared by decoction it is nearly inert. A better extract is prepared from the common tincture made with proof spirit.
2. Alcoholic; Extractum sennæ alcoholicum, L.—(Guibourt.) Senna (in powder), 1 part; rectified spirit, 5 parts; heat gradually to boiling, let it cool; in 24 hours express, strain, and repeat the process with fresh spirit; lastly, distil and evaporate. Proof spirit answers for this purpose.
3. (Fluid; Extractum sennæ fluidum, L.—Ph. (U. S.) Senna (in coarse powder), 21⁄2 lbs.; spirit (at or near proof), 64 fl. oz.; macerate 24 hours, then act by displacement, subsequently adding weak spirit (1 of rectified spirit to 3 of water) until 10 pints of tincture are obtained; evaporate to 1 pint, filter, add sugar, 20 oz., and oil of fennel, 1 fl. dr. (dissolved in) compound spirit of ether, 2 fl. dr. Every fl. oz. represents 1 oz. of senna.
Extract of Smoke. Syn. Extractum fuliginis, L. Prep. 1. (Aqueous.) Wood-soot, 2 oz.; water, 1 pint; boil to 16 fl. oz., filter, and evaporate.
2. (Acetic.) Wood-soot, 2 oz.; distilled vinegar and water, of each, 1⁄2 pint; as the last. Formerly reputed antispasmodic, alterative, &c.—Dose, 3 to 6 gr., 2 three times a day; in dyspepsia, hysteria, cancer, scrofula, and various syphilitical affections.
Extract of Snake-root. See Extract of Senega.
Extract of Black Snake-root (fluid). Syn. Extractum cimicifugæ fluidum (Ph. U. S.). Prep. As FLUID EXTRACT OF CUBEBS. (Ph. U. S.)
Extract of Soap′wort. Syn. Extractum saponariæ, L. Prep. (P. Cod. & Ph. Bad.) From the dried roots of soapwort (Saponaria officinalis), as EXTRACT OF BISTORT—P. Cod. Aperient and alterative.—Dose, 15 gr. to 1⁄2 dr.
Extract of Spruce. See Essence of Spruce.
Extract of Squills. Syn. Extractum scillæ, L. Prep. 1. (Aqueous; E. s. aquosum.—a. (Ph. Baden.) From squills, as EXTRACT OF COLOCYNTH—Ph. L. (nearly).
b. (Ph. Bor.) From squills, as EXTRACT OF SENNA—Ph. Bor. (nearly), but using boiling water, avoiding ebullition during the evaporation, and powdering the residuum.—Dose, 1 to 5 gr.
2. (Alcoholic; Extractum scillæ alcoholicum, L.—P. Cod.) From the tincture prepared with proof spirit, by distillation and evaporation.—Dose, 1⁄2 to 3 gr., as an expectorant and diuretic, twice or thrice a day. In larger doses it is nauseant and emetic.
3. (Acetic; Extractum scillæ aceticum.) Digest powder of squills, 1 lb., in acetic acid, 3 oz.; and distilled water, 1 pint, with a gentle heat, for 48 hours.
Express strongly, and without straining; evaporate to a proper consistence. (One grain of this is said to equal three of the powder.)
Extract (fluid) of Stillingiæ. Syn. Extractum stillingæ fluidum (Ph. U. S.). Prep. Stillingia, in fine powder, 16 oz. (troy); macerate with 12 oz. (old measure) of rectified spirit; 3 oz. (old measure) of glycerin; and 1 oz. (old measure) of water, for four days in a closed percolator, and proceed as for FLUID EXTRACT OF COTTON-ROOT. (Ph. U. S.)
Extract of Stor′ax. See Styrax.
Extract of Stramo′′nium. Syn. Extract of thorn-apple; Extractum Stramonii, (Ph. L. & D.), L. Prep. 1. (B. P.) Pack stramonium seeds, coarsely powdered, in a percolator, and pass about their own weight of washed ether slowly through them, remove the ether, and set aside. Now pour over them proof spirit until the seeds are exhausted; distil off the spirit, and evaporate the residue by a water bath to a proper pill consistence.—Dose, 1⁄4 gr., gradually increasing.
2. (Ph. L.) Seeds of thorn-apple (Datura stramonium), 15 oz.; boiling distilled water, 1 gall.; macerate for 4 hours in a vessel lightly covered, near the fire; afterwards take out the seeds, bruise them in a stone mortar, and return them to the liquor; then boil down to 4 pints, strain whilst hot, and evaporate. The Ph. D. is similar. Product. (About) 12%, Anodyne and narcotic.—Dose, 1⁄4 gr. to 1⁄2 gr., gradually increased, twice or thrice a day; neuralgia, rheumatism, tic doloureux, spasmodic asthma, epilepsy, worms, &c.
3. (P. Cod. & Ph. U. S.) From the expressed juice of the fresh leaves, heated to boiling, and filtered. The P. Cod. also orders it to be prepared as EXTRACT OF ACONITE—Ph. L. Anodyne and narcotic.—Dose, 1⁄2 gr. to 1 gr.
Obs. On the large scale, this extract is prepared by expressing the juice of the fresh herb, and boiling the remainder in water; the juice and decoction are then mixed, filtered, and evaporated. 11⁄2 cwt. of stramonium yielded 37 lbs. of juice, and this, with the decoction, gave 31 lbs. of extract. (Gray.)
4. (Alcoholic; Extractum stramonii—Ph. E., E. s. alcoholicum, L.)—a. (Ph. E. & Ph. U. S.) From the seeds (ground in a coffee-mill), by percolation with proof spirit. Product. (About) 14%; 1 lb. yielded 21⁄4 oz. (Recluz.)
b. (P. Cod.) From the leaves, as EXTRACT OF ACONITE—P. Cod.—Dose, 1⁄4 gr. gradually increased. (See above.)
Extract of Suc′cory. Syn. Extractum chicorii, L. Prep. (Guibourt.) From the fresh root, as EXTRACT OF ACONITE709—Ph. L. Aperient, deobstruent, and tonic.—Dose, 10 gr. to 1⁄2 dr.
Extract of Sweet Flag. Syn. Extractum acidi, E. calami aromatici, L. Prep. From the rhizomes, as EXTRACT OF RHUBARB—Ph. L. See Sweet Flag.
Extract of Tan′sy. Syn. Extractum tanaceti, L. Prep. 1. From the herb (Tanacetum vulgaris), as EXTRACT OF HOP—Ph. L.
2. (Giordano.) As EXTRACT OF HOREHOUND—Ph. Lusitan.
Obs. This extract is said to be tonic, stomachic, anthelmintic, emmenagogue, and febrifuge. Dr Clark says that in Scotland it was found to be serviceable in various cases of gout. The infusion is, however, preferable.—Dose, 5 gr. to 20 gr.
Extract of Taraxacum. Syn. Extract of dandelion; Extractum taraxaci (Ph. L. & E.), E. t. herbæ et radicis (Ph. D. 1826), L. Prep. 1. (B. P.) Crush fresh dandelion root, press out the juice, and allow it to deposit; heat the clear liquor to 212° F., and maintain the temperature for 10 minutes; then strain and evaporate by a water bath, at a temperature not exceeding 160° F. to a proper consistence.—Dose, 5 to 15 grains.
2. (Ph. L.) From the recent root of dandelion (Leontodon Taraxacum), as EXTRACT OF HOP—Ph. L. The formulæ of the Ph. E. & U. S. are nearly similar.
3. (Ph. D.) From the herb and root, as the other simple extracts (EXTRACTA SIMPLICIORA).
4. (P. Cod.) From the expressed juice, as EXTRACT OF STRAMONIUM—P. Cod.)
5. (Ph. Bor.) As EXTRACT OF SENNA—Ph. Bor. (nearly).
6. (Ph. Baden.) By displacement with cold water.
7. (Wholesale.) From the decoction.
8. (Fluid.) See Liquor of Taraxacum.
Obs. The extract of the shops is usually prepared by exhausting the root by coction with water. The products of the first two of the above formulæ, when recent, have a faint and agreeable odour, and a sweet bitter taste; those of Nos. 4, 5, and 6, smell strongly of the recent root, have a pale and lively brownish-yellow colour, and a bitter acidulous taste, without any trace of sweetness; that of the last one is devoid of odour, and possesses a coffee-brown colour, and a sweetish, burnt taste, not much unlike a solution of burnt sugar. The medicinal virtue of this extract is greatest when the aroma and bitter taste of the recent root is well developed; and when sweet, its efficacy as a remedy is impaired. (Squire.)
Taraxacum root should be gathered during the winter months, when the quantity of the product is looked at; as then a given weight of the juice yields more extract; but in summer and autumn it possesses more bitterness and aroma. 4 lbs. of juice from roots gathered in November and December yielded 1 lb. of extract, while it took from 6 to 9 lbs. of juice from the root, gathered in spring or summer, to yield a like quantity. (Squire.) The herb yields by the evaporation of its expressed juice about 5% of extract. According to Mr Jacob Bell, the average yield of 1 cwt. of root is about 71⁄8 lbs. (‘Pharm Journ.,’ x, 446.)
Good extract of taraxacum should be wholly soluble in water.—Dose, 10 gr. to 1⁄2 dr.; as a resolvent, aperient, and tonic, in liver and stomach complaints, &c.
Extract of Tea. Syn. Extractum theæ, L. Prep. 1. From an infusion of any of the rougher kinds of black tea. Astringent. Has been recommended in diarrhœa; formed into pills.—Dose, 10 gr. to 1⁄2 dr. A hard, black-looking substance, smelling and tasting faintly of tea, is imported under the same name from China.
2. (Pidding’s.) The joint products of distillation and infusion combined. Proposed to be made in China, and exported as a condensed preparation of tea. (Essence of tea; Essentia theæ); to be used as a substitute for the leaves, in order to save the expense of freight, &c.
Extract of Thorn-Apple. See Extract of Stramonium.
Extract of Tobac′co. Syn. Extractum tabaci, E. Nicotianæ, L. Prep. 1. (Chippendale.) From decoction of tobacco. Proposed as an external application in neuralgia, &c.
2. (Alcoholic; Extractum tabaci alcoholicum, L.—Ph. Bor.) Tobacco leaves, 1 lb.; spirit (sp. gr. ·900), 2 lbs.; digest in a warm place for some days, express strongly, and again digest in a mixture of water and spirit (·900), of each, 1 lb., for 24 hours; again press out the liquor, and evaporate the strained and mixed liquors into a vapour bath, at a temperature not exceeding 167° Fahr.
Extract of Tor′mentil. Syn. Extractum tormentillæ, L. Prep. (Ph. Amst.) From the root of Potentilla Tormentilla, as EXTRACT OF HOPS—Ph. L. The Ph. Baden directs its preparation by displacement with cold water. Astringent and febrifuge.—Dose, 15 to 30 gr.; in diarrhœa. It was formerly regarded as a specific in syphilis. (Lindley.)
Extract of U′va Ur′si. See Extract of Whortleberry.
Extract of Valer′ian. Syn. Extractum valerianæ, L. Prep. 1. From valerian root, as EXTRACT OF HOP—Ph. L.; but using a covered vessel.
2. (Ph. Bor. and Baden.) As EXTRACT OF CINCHONA—Ph. L. (nearly), employing strong force in the expression of the liquor, and only evaporating to the consistence of syrup.
Obs. It is usual to add to this extract a little of the ESSENTIAL OIL OF VALERIAN, dissolved in a small quantity of rectified spirit, just before removing it from the evaporating-pan, and when nearly cold. Anti-spasmodic and nervine.—Dose, 10 gr. to 1⁄2 dr. In hysteric710 and spasmodic diseases. Valerian yields about 40% of soft extract.
3. (Alcoholic; Extractum valerianæ alcoholicum, L.—P. Cod.) As EXTRACT OF BOX.—P. Cod.
4. (Fluid; Extractum valerianæ fluidum, L.—Ph. U. S.). Rectified spirit, 12 fl. oz.; mix, add of valerian (in coarse powder), 8 oz. digest and percolate, adding, subsequently, spirit (at or near proof) until 16 fl. oz. of tincture have passed through; let this evaporate spontaneously, in a shallow vessel, until reduced to 5 fl. oz.; in the meantime add fresh spirit to the mass in the percolator, until 10 fl. oz. more of tincture are obtained, which add to the above residuum of the evaporation, observing to dissolve any oleo-resinous deposit in a little rectified spirit, and add to it to the rest; lastly, filter, and add of rectified spirit, q. s. to make the whole measure 16 fl. oz.
Extract of Vanil′la. See Liquor of Vanilla.
Extract of Wall Pel′litory. Syn. Extractum parietariæ, L. Prep. From fresh wall-pellitory (Parietaria officinalis), as EXTRACT OF ACONITE—Ph. L. Aperient, diuretic, and pectoral.—Dose, 10 gr. to 1⁄2 dr.
Extract of Wal′nut. Syn. Extractum juglandis immaturæ, L. Prep. 1. From unripe walnuts (Juglans regia), as EXTRACT OF ACONITE—Ph. L.
2. From the decoction of the green shells. Vermifuge.—Dose, 20 to 30 gr. in cinnamon water.
Extract of Walnut Leaves. Syn. Extractum juglandis foliorum, L. Prep. 1. From the decoction of dried walnut leaves.
2. (Soubeiran.) By displacement with tepid water. Diaphoretic and alterative.—Dose, 2 to 4 gr., twice or thrice a day; in scrofula, scirrhus, &c.
3. (Alcoholic; Extractum juglandis foliorum alcoholicum, L.—Ph. Bor.) From walnut leaves (cut), as ALCOHOLIC EXTRACT OF TOBACCO—Ph. Bor. (nearly).
Extract of Wa′ter-dock. Syn. Extractum rumicis aquatici, L. Prep. From the root, as EXTRACT OF HOPS, Ph. L. Astringent and antiscorbutic.—Dose, 15 gr. to 1 dr.; in skin diseases, &c.
Extract of Whor′tleberry. Syn. Extract of bearberry; Extractum uvæ ursi. (Ph. L.), L. Prep. 1. From the dried leaves of the bearberry (Arctostaphylos Uva-Ursi), as EXTRACT OF HOPS—Ph. L.—Dose, 5 to 15 gr., twice or thrice a day; in chronic diseases of the bladder and kidneys, attended with increased secretion of mucus, without inflammation.
2. (Ph. U. S. Extractum uvi-ursi eluidum.) As fluid extract of cotton-root. (Ph. U. S.)
Extract of Willow Bark. Syn. Extractum salicis. (Ph. Par.) From Powdered willow bark, as EXTRACT OF RHATANY.
Extract of Win′ter Cher′ry. Syn. Extractum alkekengi, L. Prep. From the berries of Physalis alkekengi, as EXTRACT OF ELDER. Aperient, detergent, and diuretic. Dose, 2 to 4 dr.
Extract of Win′ter-green. Syn. Extract of pipsissewa; Extractum chimaphilæ, L. Prep. From the herb winter-green or pipsissewa (Chimaphila umbellata), as EXTRACT OF HOPS—Ph. L.—Dose, 10 gr. to 1⁄2 dr.; in dropsy, scrofula, and chronic affections of the urinary organs.
Extract of Wood Sor′rel. Syn. Extractum acetosellæ, L. Prep. (Pideret.) From the expressed juice of the fresh herb (Oxalis acetosella.) Acid, bitter, and antiscorbutic.—Dose, 15 gr. to 1⁄2 dr.
Extract of Worm Grass. See Extract of Pinkroot.
Extract of Worm′seed. Syn. Extractum cinæ æthereum, E. seminum c. æ., L. Prep. (Hamb. Cod. 1845.) Wormseed, 1 oz.; ether, 4 oz.; digest 3 or 4 days, press, filter, distil off 4-5ths, and evaporate the residuum to a proper consistence. Prod. 25% to 30%. Vermifuge.—Dose, 3 to 10 gr., night and morning, for 2 or 3 successive days, followed by a brisk purge.
Extract of Worm-wood. Syn. Extractum absinthii; Extractum artemesiæ absinthii, L. Prep. 1. (Ph. D., 1826.) From the dried flowering tops of wormwood, as the other simple extracts (EXTRACTA SIMPLICIORA—Ph. D.)
2. (Ph. Bor.) As EXTRACT OF RHATANY—Ph. Bor.
3. (P. Cod. and Ph. Baden.) By displacement by cold water.
Obs. Bitter, stomachic, tonic, and vermifuge.—Dose, 10 gr. to 20 gr., 2 or 3 times daily; in dyspepsia, loss of appetite, gout, &c. It is usual to add a few drops of the oil of wormwood to the extract before taking it from the pan.
4. (Alcoholic; Extractum absinthii alcoholicum, L.— Guibourt.) From a tincture prepared from the dried tops of wormwood boiled in proof spirit. More active than the last.
Extract of Yew. Syn. Extractum taxi, L. Prep. 1. (Loder.) From the inspissated juice of the fresh leaves of the yew (Taxus baccata). Its action on the circulation greatly resembles that of digitalis, but is more manageable.—Dose, 1 to 7 gr.; in epilepsy, &c.
2. (Alcoholic,—Ph. Baden.) From the dried leaves, as ALCOHOLIC EXTRACT OF ACONITE—Ph. Baden.
Obs. In addition to the preparations given above, there are many others which are often called ‘EXTRACTS,’ These may be grouped under the following heads:—
Extracts, Concentra′ted. Syn. Resinoids. Pharmaceutical preparations of more or less value, largely employed by the American physicians who style themselves ‘ECLECTICS,’ They are supposed to present in the most711 concentrated form the medicinal virtues of the plants from which they are derived. See Resinoids.
Extracts, Fluid. Syn. Extracta fluida, Extracta liquida, L. This name has been applied in modern pharmacy to various preparations differing materially from each other in their degree of fluidity and concentration. Some of these have been already noticed, and others will be found under one or other of their synonyms. Much confusion would be avoided by confining the name ‘FLUID EXTRACT’ to those preparations only which differ from the ordinary officinal extracts in being in the liquid form; whilst others of a like character, but of less consistence or concentration, might be conveniently classed under the general denomination of ‘LIQUORS’ (LIQUORES, L.). The various condensed preparations of vegetable substances, now common in trade, professedly several times stronger than the common DECOCTIONS, INFUSIONS, and TINCTURES, might be simply and advantageously distinguished by the addition of ‘CONCENTRATED’ to their names. Tinctures made with rectified spirit, and of (say) at least 8 times the usual strength, might be appropriately termed ‘ESSENCES,’ See Decoction, Essence, Extract, Infusion, Oleo-resin, Syrup, Tincture, &c.
Extracts, Perfu′matory. See Extrait.
Extracts, Pulver′ulent. Syn. Dried extracts, Desiccated e.; Saccharated e.; Extracta pulverata, E. sicca, E. cum saccharo, L. Prep. 1. Ordinary soft extract of the drug, 4 parts; white sugar (in powder), 1 part; mix, and dry by exposure in a warm situation; lastly, reduce the mass to powder, and if it weighs less than 4 parts, triturate it with more powdered sugar until its weight is equal to the original weight of the extract used in its preparation. The strength of the extract thus continues unchanged.
2. (Ph. Bor.) As the last, but using powdered sugar of milk, in lieu of cane sugar.
3. (Gauger.) Alcoholic extract, 3 parts, rectified spirit, 1 part, are triturated together in a porcelain mortar until thoroughly incorporated, when white sugar (in powder), 15 oz., is gradually added, and the two carefully and completely blended together; the mixture is dried as before, and more sugar added until the whole weighs exactly 18 oz. Six grains represent one grain of the unprepared extract.
Obs. The above are admirable preparations, intended chiefly to render the perishable extracts of the narcotic plants (EXTRACTA NARCOTICA) less liable to suffer by age. See Extract of Aconite (Saccharated), &c.
EXTRAC′TIVE. Syn. Extractive principle. Fourcroy entertained the belief that all vegetable extracts contained a common basis of definite composition, to which he gave the name of extractive. Chevreul and other chemists have shown, however, that Fourcroy’s extractive is not a chemical compound but a heterogeneous mixture, varying in composition with the plant from which it is obtained. Extractive has a brown colour, or one becoming so in the air; it speedily putrefies, and becomes oxidised, and is rendered insoluble by long exposure to air, and by repeated solutions and evaporations. In its unaltered state it is soluble in water and in alcohol, is nearly insoluble in ether, and is precipitated from its solutions by the acids and metallic oxides. With alumina it forms the basis of several brown dyes.
EXTRAIT. [Fr.] Literally an extract. Among perfumers, extraits are mostly spirituous solutions of the essential oils or odorous principles of plants and other fragrant substance. The French commonly apply the term to any concentrated spirit, either simple or compound. In the shops of the Parisian perfumers upwards of 60 preparations of the kind are distinguished by this name. The extracts of JASMINE, JONQUIL, MAY-LILY, ORANGE BLOSSOMS, VIOLETS, and other like flowers of delicate perfume, are obtained by agitating and digesting the ‘huiles’ and ‘pomades’ of the flowers with the purest rectified spirit in the manner described under Scented Spirits (‘esprits’). This process is repeated with fresh oil or pomade until the spirit is rendered sufficiently fragrant. The other extracts (both simple and compound) are made by the common methods of infusion and distillation. See Essence, Extract, Spirit, &c.
EYE. In anatomy and physiology, the organ of vision. In order that vision may be distinct, it is necessary that the pencil of rays diverging from each point of the object and entering the pupil should converge to a focus on the retina. Near-sightedness (‘MYOPIA,’ L.) is due to the too great convexity of either the ‘lens’ or ‘cornea,’ causing the rays to converge to a focus before reaching the retina. The spectacles worn by myopic persons have concave glasses, which, by increasing the divergence of the rays falling upon the eye, have the effect of carrying back each focal point towards the retina. In the long sight of old people (‘PRESBYOPIA,’ L.) the foci of the refracted pencils are situated behind the retina, the ‘lens’ or the ‘cornea’ being not sufficiently convex. This defect is corrected by convex glasses, which increase the convergence of the incident rays.
Foreign Bodies in the Eye.—Particles of dust, small insects, hairs, and such like minute bodies frequently get under the eyelid, and thus become a source of considerable discomfort, and very frequently of great pain. Hence the necessity of their prompt removal. In order to effect this the inside of the lids should be so exposed as to reveal the intruding substance. The lower lid may be easily turned down so as to show the inner surface, but the upper lid cannot be so easily manipulated. The end, however, may be attained by taking firm hold of the lid with the finger712 and thumb, drawing it downward and forward, placing a quill or a small pencil-case on the outer upper part, and turning the lid backwards over it. When the annoying particle is seen it should be removed by gently drawing over it, with a wiping motion, a piece of rag or linen handkerchief, wrapped round the finger, or by means of a camel-hair brush, if this latter be at hand.
If these means fail to remove it, and it should be imbedded too firmly in the membrane, it may be picked off with a tooth-pick, the end of a pair of tweezers, a fine ivory paper-knife, or with a stiff hair from a clothes-brush bent at right angles. If lime-dust has blown into the eye it is only the larger particles that can be removed in this manner; the finer particles may be dissolved out by washing the eye with a lotion made of one part of common vinegar and two parts of water. A drop or two of pure sugar syrup will also frequently dissolve the lime. When a powerfully destructive substance, such, for instance, as sulphuric acid or oil of vitriol, is, as sometimes happens, thrown by some person into the eye, the best course is to wash it out with a solution containing four grains of washing soda in an ounce of water. This should be done as quickly as possible, and pending the time the soda lotion is being got ready, the eye, being kept open, should be diligently washed with cold water. Grains of gunpowder should be carefully removed. Hot fluid, such as melted fat or pitch, may be got rid of by putting into the eye a few drops of almond or olive oil.
Upon removal of the foreign body the pain generally subsides; but it sometimes happens that the membranes may be lacerated, in which case more or less inflammation may ensue. Under these circumstances a medical practitioner should be consulted. For animals the same treatment may be followed. See Blindness, Colour Blindness, Vision, &c.
Eye Balsam, Vegetable (Martin Reichel, Würzburg). Opium, 5 parts; oxide of mercury, 5 parts; camphor, 2 parts; wax cerate, 52 parts. (Hager.)
Eye Drops. See Water (Eye).
Eye Essence (Dr Romershausen). A tincture prepared from fennel seeds and fresh young fennel. (Hager.)
Eye Powder (Laeyson, Paris), also known as Odorous Powder. For the strengthening, restoration, and preservation of the sight. A powder composed of—Burnt chalk, 100 parts; ammonia, 50 parts; charcoal, 6 parts; oxide of iron, 2 parts; cinnamon bark, 2 parts. (P. L. Geiger.)
Eye Pow′ders. See Collyria.
Eye Salt. Powdered alum. (G. Graefe.)
Eye Salve. See Ointment (Eye).
Eye Snuff. See Snuff.
Eye Water (Biedermann, Annaberg). 2 grms. sulphate of zinc in 60 grms. distilled water, with a little infusion of cloves.
Eye Water (Brun) is a solution of 4 parts of aloes in 32 parts of white wine, with 32 parts of rose water, and 11⁄2 part of tincture of saffron.
Eye Water (Chantomelanus) “makes spectacles superfluous.” A turbid yellow-brownish liquid, consisting of a weak extract of lavender flowers in diluted spirit, in which some oil of lavender has also been dissolved. (Opwyrda.)
Eye Water, Dr Graefe’s (L. Roth, Berlin). Sulphate of zinc, 1·5 grms.; fennel water, 100 grms., slightly coloured with fennel seed tincture. (Schädler.)
Eye Water (J. P. H. Hette). A solution of ethereal oils of lavender, bergamot, rosemary, and tincture of opium in spirits of wine, 50 per cent. (Wittstein.)
Eye Water (Bernhard Kraft, Calbe) for acute inflammation of the eyes and for strengthening the sight. Seven grammes of an impure muddy sediment-leaving spring water containing half a gramme of native sulphate of zinc containing iron. (Schädler.)
Eye Water (Inspector Stroinski, Neisse). One part of sulphate of zinc dissolved in 500 parts of common river water. (Schreiber.)
Once a trace of patchouli perfume was added to this water. (Hager.)
Eye Water, Dr White’s (T. Ehrhard, Altenfeld, Thuringia). Four cloves, a piece of cinnamon the size of a large pea, 2 teaspoonfuls of rose water, 1 drop of vinegar, 10 drops of arnica tincture. Digest for an hour and filter. Dissolve in the filtrate some white vitriol of the size of a pea. (Hager.)
Sulphate of zinc, 3 parts; honey, 4 parts; water, 80 parts; perfumed with oil of cloves and a trace of mustard oil. (Wittstein.)
Eye Waters. See Water.
FACE A′GUE. The common name for the intermittent form of facial NEURALGIA or TIC DOULOUREUX. See Neuralgia.
FACE PAINTS. Syn. Fards, Fr. See Bloom, Carmine, Pearl White, Rouge, &c.
FAC-SIM′ILE. An exact imitation of an original in all its traits and peculiarities. The term is chiefly used in relation to copies of old manuscripts, or of the handwriting of famous men, or of interesting documents, produced by engraving or lithography. See Signatures.
FACTI′′TIOUS. Syn. Factitius, L. Artificial; made by art, in distinction from that produced by nature. Numerous illustrations of the application of this word occur in the pages of the present work.
FÆCES. Excrement. In the laboratory, the ‘settling’ or sediment deposited by a liquor. See Defecation, Excreta.
FAINT′ING. Syn. Swooning; Syncope, Deliquium animi, L. In pathology, a state in which the respiration and circulation are apparently suspended for a time, or are extremely feeble. The symptoms are too well known to require description. The causes are supposed to be—diminished energy of the brain, and organic affections of the heart713 or neighbouring vessels. This has led nosologists to divide syncope into two varieties:—
1. Occasional (syncope occasionalis, s. accidentalis, L.), primitively induced by sudden and violent emotions of the mind, powerful odours, derangement of the stomach or bowels, constrained position of the body, tight-lacing, pressure, loss of blood, debility from disease, &c. This variety is frequently followed by vomiting, and, occasionally, by convulsions or epileptic fits. The recovery is accelerated by the horizontal position, without the head being the least elevated, by which the arterial blood is more vigorously thrown upon the brain, and thereby stimulates it to resume its usual functions. Pungent substances (smelling-bottle, vinaigrette, &c.) may be applied to the nostrils, and cold water sprinkled on the face and chest. In all cases the dress (corset, waist-band, neck-cloth, &c.) should be instantly loosened, and indeed this is the first assistance which should be given, either in syncope or apoplexy. As soon as the patient can swallow, a little brandy-and-water, or wine, or a few drops of ether or spirit of sal volatile, may be given.
2. Cardiac (syncope cardiaca, L.), arising without any apparent cause, with violent palpitation during the intervals, and altogether of a more formidable character than the preceding. The subsequent treatment must here be directed to the cure or alleviation of the original disease.
FAINTS. The first and last runnings of the whiskey-still. The one is technically termed the ‘strong faints,’ the other, the ‘weak faints.’ They are both purified by rectification, &c. See Distillation.
FAITH. Dr Pereira remarks, that “faith in the beneficial agency of remedies, and confidence in the skill of the medical attendant, are important adjuvants in the treatment of disease. To them both the physician and empiric owe part of their success.”
FAL′LING SICK′NESS. See Epilepsy.
FAMILIENSALBE, Family Ointment (Göring). 16 grammes of a hard yellow salve in a round box; a mixture of 9 parts wax, 3 parts fat; 2 parts turpentine, 2 parts inspissated juice of Ornithogalium scilloides Jacquin, or O. candatum Aiton. These plants are known to the public as Meerzwiebel (sea onion or squill), but they are only related to that plant in appearance. (Hager.)
FAR′CY. See Glanders.
FARDEL-BOUND. Syn. Clue-bound, Wood-evil. An affection of the third stomach of cattle, induced by their unduly partaking of coarse indigestible food. Cattle are most commonly attacked by fardel in summer and autumn, when they are able to get at tough, strong, and hard grass. It is also frequently caused by rye-grass in seed and ripe vetches, as well as by eating largely of the shoots of trees or the cuttings of hedges, a circumstance which has given rise to the disease being called ‘wood-evil.’ Sometimes an attack may be brought on through over-feeding, combined with a deficiency of water. The symptoms vary greatly in intensity, and are often some days before they definitely manifest themselves. The animal ceases to ruminate, refuses food, and, if a cow, the secretion of milk is stopped. Then, after a day or two fever (indicated by heat and dryness in the nose and mouth) comes on, with somewhat quickened circulation and breathing, the breathing by the second or third day being accompanied by a grunt at the beginning and end of respiration, which is very noticeable when an attempt is made to move the animal.
In all attacks the animal suffers from obstinate constipation. The first stomach is frequently much distended, and if any fæces are passed they are caked, dark coloured, and of variable consistence.
When the disease is attended with most of these symptoms, the animal may live ten days or a fortnight; but unless relief is afforded, nausea very frequently sets in, and continues to increase, the pulse at the same time getting gradually weaker, and the strength failing. In some instances the animal has an epileptic fit, and in others death is preceded by great stupor; whilst in others, again, if it be a horse, it is attacked with stomach staggers.
“The treatment consists in removing the obstinate constipation by powerful purgatives, advantage being taken to gain their utmost efficacy by combining several together, and giving them along with plenty of fluid.
“Three-quarters of a pound each of Epsom and of common salt, twenty croton beans, and a drachm of calomel, will suffice for a full-grown, middle-sized ox or cow, and must be administered in three or four bottles of water or very thin gruel. In this disease there is little fear of giving too much medicine.
“The action of the purgatives is greatly expedited by the use of occasional stimulants, which in diseases of the digestive organs of cattle may be given without fear of engendering or aggravating inflammation. Every encouragement must be used to get the animal to drink, for large quantities of fluid are obviously most essential in washing out the obstruction which causes the evil. The cessation of the grunt, the passage of some hard cakes of dung, with the subsequent abatement of the fever, are the signs of amendment for which we watch; but even after the first movement of the bowels considerable attention, a sloppy diet, and several doses of purgative medicine, are requisite to empty the canal and prevent the recurrence of the obstruction. If twenty hours elapse after the administration of the above combination without any action of the bowels, the same dose may be repeated, along with a good quantity of some stimulant, such as a bottle of ale, with two ounces of oil of turpentine and two ounces of ginger. Half714 the quantity of the purgative may be given at the end of a like interval, if no effect be produced; but the further employment of purgatives is injurious, inasmuch as it increases the nausea without expediting the action of the bowels.
“A week will sometimes elapse without any alvine evacuation; in some cases I have known ten or eleven days, and in some fifteen days. Yet even in these recovery took place; and so long as stupor and frenzy are staved off, there is always hope of a cure. After the prompt and energetic adoption of the treatment recommended, little further remains to be done except to withhold all solid indigestible food, administer frequent quantities of water, or any simple fluid, which must be horned over if the beast will not take it; allow also plenty of treacle, and encourage the action of the medicine by clysters, scalding the belly, and occasional exercise. Blood-letting is not only useless, but even injurious.” (Finlay Dun.)
FAR′INA. The flour of any species of corn, pulse, tuber, or starchy root. The most important kinds of farina are noticed under their respective heads. The following dietetic articles of a farinaceous character are extensively advertised:—
Baker’s Alimentary Compound. Fine flour (pastrycook’s), 2 parts; finely ground rice, 1 part.
Baster’s Compound Farina. Wheat flour, 14 oz.; white sugar, 2 oz.
Braden’s Farinaceous Food. Similar to Hard’s (below).
Bright’s Nutritious Farina. Rice flour and potato starch, equal parts.
Bright’s Breakfast Powder. Chocolate, 1 part; nutritious farina (Bright’s) 2 parts.
Bullock’s Semola. Wheat flour, from which a portion of the starch has been removed, so as to leave an excess of gluten.
Denham’s Farinaceous Food. Wheat flour, 3 parts; barley meal, 1 part; the mixture is slightly baked, and again ground and sifted. Said to be slightly laxative.
Duryea’s Maizena. Indian corn starch prepared for food.
Gardiner’s Alimentary Preparation. Pure rice flour, very finely ground.
Hard’s Farinaceous Food. Wheat flour, slightly baked, and resifted.
Kingsford’s Oswego Prepared Corn. An excellent preparation of Indian corn.
Leath’s Alimentary Farina. Wheat flour (baked), with some sugar, Indian corn meal, and tapioca. According to some, it also contains potato starch.
Maidman’s Nutritious Farina. Potato starch tinged with beet-root or other pink colouring matter.
Plumbe’s Farinaceous Food. South-sea arrow-root, with about 1-3rd its weight of pea flour.
Polson’s Corn Flour. The starch of Indian corn or maize prepared with great care. It is much used as a substitute for arrow-root, and for custards, puddings, &c.
Smith’s Nursing Farina. Equal parts of baked wheat flour and rice flour.
Obs. Many of the above compounds are deficient in the nitrogenous elements of nutrition, and all of them nearly destitute of the mineral and saline matters which are absolutely necessary to the formation of the bones and tissues, and the support of the body in health, and are consequently utterly unsuitable as an exclusive article of diet, especially for young children. Unfortunately, it has been too much the fashion of medical men of late years to recommend these compounds, and even to furnish testimonials as to their excellence, apparently relying solely on the representation of their proprietors or vendors. We deem it, however, to be a public duty to caution parents and nurses against their injudicious use. As mere adjuvants or auxiliaries, when the natural food supplied by the mother may be insufficient for the nutrition of the infant, some of them may doubtless be of value; but in all other cases they should be largely combined with pure cow’s milk, beef tea, meat broths or gravies, eggs, or other substances rich in the nitrogenous and saline elements of nutrition.
FARM′ING. The business or management of a FARM. Formerly farming was looked upon as a profession easily understood, and successfully pursued only by an empiric. It is now, however, regarded in a different light, and the farmer, to succeed, not only requires perseverance and observation, but also a sound knowledge of natural sciences. See Butter, Cheese, Implements, Manures, Soils, &c.
FARMS, SEWAGE. See Sewage Farms.
FAT. Syn. Adeps, L. The fat of animals is a concrete oil contained in the cellular membrane of their bodies, more especially round the kidneys, in the folds of the omentum, at the base of the heart, upon the surface of the intestines, and among many of the muscles. Fat varies in consistence, colour, and odour, with the animal from which it is obtained. That of the carnivora is usually soft and rank-flavoured; that of the ruminantia solid and nearly scentless. It is generally whitest and most copious in the well-fed young animal, and yellowish and more scanty in the old. That under the skin and surrounding the kidneys (suet) is also more solid than that in the neighbourhood of the movable viscera. In the cetacea, or whale tribe, the fatty secretion assumes the form of oil. These variations in consistency depend upon the relative proportions of solid stearin and liquid olein present in the fat.
The vegetable fats are found in various parts of certain plants, but are generally most abundant in the seeds. They are extracted by simple pressure or else by boiling. Two kinds715 of vegetable fat, namely, palm-oil and cocoa-nut oil, are extensively employed in the useful arts.
All fats are lighter than water. They are all soluble in ether, benzol, and turpentine, and may be mixed with each other in any proportion.
In former times the fats of many animals were employed in pharmacy, but at present those principally used are lard and suet. In perfumery, in addition to these, beef marrow and bear’s grease are employed. For both these purposes the crude material is cut into small pieces, and freed as much as possible from all extraneous membranes; after which it is placed in a boiler with water, and heated until it is completely fused, when the whole is strained, and allowed to cool very slowly. By this means a cake of cleansed fat is obtained, which may be readily separated from any adhering water.
Fats and the fat oils are best preserved by being run into glazed jars, and secluded from the action of the air. A little benzoic acid or gum-benzoin, dissolved in them by heat, will generally prevent, and in all cases greatly defer, the accession of rancidity. We introduced this method into the laboratory in our early days of manipulation, and ourselves, and others to whom we have made it known, have since employed it with undeviating advantage in the manufacture of cerates, ointments, and other preparations containing fatty matter or the fixed oils. It has been shown by Dr Griesler that nitric ether, and its alcoholic solution, act in the same manner. A few drops are not only sufficient to prevent rancidity, but, it is said, will even destroy the disagreeable odour of rancid fat. When heated to remove the alcohol, they immediately become bright, clear, and scentless. See Oil, Glycerin, Olein, Palmitin, Stearin, Tallow, &c., also below.
Fat, to melt down. Let all the small pieces of fat cut off joints, &c., be collected, divided into small pieces, put in a stew pan (a little water being added to prevent their burning), and placed on the fire. This must be stirred carefully at intervals to prevent any of the pieces of fat sticking to the bottom.
When thoroughly melted (which it will be in about an hour and a half) pour through a strainer into a basin with some cold water in it. Thus prepared, dripping or fat may be used instead of suet, and there are few who would know any difference between them. Dripping, if clarified as above, may be used over and over again for frying, provided it has not been previously employed in dressing fish, in which case it will impart a fishy taste. But it can be used repeatedly for fish if it is kept for that purpose only. The skimmings off the top of the saucepans, while a piece of meat is boiling, will also do capitally for light puddings.[296]
[296] ‘Artisan Cookery.’ Griffith and Farran.
FAT′TY ACIDS. In chemistry, compounds having acid properties derived from the various fats and oils. The radicals of these acids exist in the natural fats combined with a base called glyceryl. When fats are saponified by an alkali, stearate, palmitate, and oleate of potassa or soda, as the case may be, are produced and glycerin is set free. On decomposing either of these compounds with sulphuric acid a sulphate of the alkali is formed, and the fatty acid is precipitated. Some of the fatty acids, as stearic, cerotic, palmitic, and lauric, are solid at ordinary temperatures; others, as oleic, are liquid. The hard fatty acids are extensively used as candle materials, being superior in every respect to the natural fats from which they are derived.
FAT′TENING. Until comparatively a recent date, the plan used to fatten domestic animals was to prevent their taking exercise, and to gorge them with food. The excessive fat produced by these means was, however, found to be far from wholesome, and was less delicate than that arising in the natural way. This system was therefore gradually abandoned in favour of the present one, which consists in supplying the animal with abundance of wholesome food, and with the means of taking exercise as far as the disposition or feelings dictate. Hence the farmers “in the most enlightened districts, such as Berwickshire, East Lothian, &c., instead of tying up their fattening cattle in stables like horses, and placing their food before them, put two or three together in small yards with sheds attached, in which they can run about, eat when they choose, and take shelter from the rain, or cold, or the sun, at pleasure, under the open shed. Swine are treated in the same manner, and also spring lambs that are fattened for the market. Poultry are no longer kept in coops and crammed, or rabbits in hutches; but the former are allowed to take exercise in fields sown with various herbs, and the latter are kept in a species of artificial warren, where they can take exercise by burrowing.” (Loudon.)
FAVOURITE PRESCRIPTION (Dr Pierce’s) for the cure of those chronic weaknesses and complaints peculiar to females. 280 grammes of a turbid greenish-brown fluid with a bulky deposit of the same colour, made according to the following recipe:—Savin tops, 10 grammes; larch agaric and cinnamon, of each 5 grammes; China Jaën (ash cinchona bark), 10 grammes; boil with sufficient water to make 220 grammes when strained. Dissolve in the filtrate gum Arabic, 10 grammes; white sugar, 5 grammes; and add tinct. digitalis and tinct. opii, of each 2 grammes; star anise oil, 8 drops; 90 per cent. spirit, 45 grammes. (Hager.)
FEAR. Although fear is a depressing and debilitating emotion, and sometimes acts prejudicially on the health, it frequently acts as a curative or preventive of disease. It is a well-known716 fact that females who are the most faint-hearted and desponding during the period of their sex’s trial, generally experience a more rapid convalescence than those who are more confident and resolute. During the raging of an epidemic fear generally induces temperance, cleanliness, and the adoption of other precautions which tend powerfully to prevent disease. Boerhaave, according to Pereira, is said to have prevented the occurrence of epileptic attack (brought on by the sight of a person falling down in a fit in the sight of the hospital patients), by directing a red-hot iron to be applied to the person who should next be affected.
FEA′THERS. Ostrich feathers are those most esteemed as articles of personal decoration, and goose feathers for beds; but the feathers of other birds are commonly used for both purposes.
Feathers are prepared for ornamental purposes by scouring them with white soap-and-water (1 oz. to the pint), used hot; they are next well rinsed in several successive portions of pure water, and after being drained and shaken, are, lastly, passed through water slightly blued with pure indigo, and dried out of the dust. When dry, the ribs are generally rubbed with a piece of glass, having a curved notch in it, for the purpose of increasing their pliancy, and the filaments are curled by drawing them, between the edge of a blunt knife and the ball of the thumb of the hand which holds it.
Feathers, Bleaching of:—
A new trade has sprung up within the past ten years, by which black, brown, or grey feathers are bleached sufficiently to enable them to be dyed any required colour.
The process is as follows:—The feathers are first thoroughly washed with soap-and-water, to free them from any oil they may contain. They are next transferred to a bath composed of bichromate of potash dissolved in water, to which has been added a few drops of nitric or sulphuric acid. In this bath they rapidly lose their black, brown, or grey colour, and become almost white. On being removed from this bath they are well rinsed in water, and are then fit to be dyed, even the most delicate colour. Great care is required in the process, as the flue of the feather is apt to be destroyed, if kept too long in the bath. A bleached feather may be readily known by the yellow colour of its stem.
Other methods have been adopted, such as a bath of chloride of lime, peroxide of hydrogen, or sulphurous acid, &c., but the bichromate bath gives the best results.
Feathers, Dyeing of:—
Black. By immersion for 2 or 3 days in a bath (at first hot) of logwood, 8 parts, and copperas or acetate of iron, (about) 1 part.
Blue. With the indigo vat.
Brown. By any of the brown dyes for silk or woollen.
Crimson. A mordant of alum, followed by a hot bath of brazil wood, and afterwards by a weak one of cudbear.
Pink or Rose. With safflower and lemon juice.
Plum. The red dye, followed by alkaline bath.
Red. A mordant of alum, followed by a hot brazil-wood bath.
Yellow. From an alum mordant, followed by a bath of turmeric or weld. Other shades may be obtained by a mixture of the above dyes.
Feathers may also be dyed by simple immersion, for two or three minutes, in a bath of any of the aniline colours.
Goose feathers for BEDS are generally PURIFIED by simply exposing them to the sun or in a stove until perfectly dry, and then beating them to remove loose dirt. When carelessly collected and dirty, they are sometimes first cleansed with lime water, or, better still, with a weak solution of carbonate of soda, or water to which a little solution of chloride of lime has been added; after which they are rinsed in clean water, and dried or stoved as before. Old feathers are cleansed or purified in the same way.
FEB′RIFUGES. Syn. Febrifuga, L. In pharmacy, substances or agents which cure or alleviate fever. The term is more particularly applied to medicines used against the ague, as CINCHONA BARK and ARSENIOUS ACID, and their preparations. The extreme value of cold water, as a drink in ardent fever, has been known in all ages. In 1723 Dr Hancocke published a work entitled—‘Febrifugum Magnum, or Common Water the best Cure for Fevers, and probably for the Plague,’ which in a short time ran through several large editions, but appears to have been overlooked by the hydropaths of the present day.
FEC′ULA. Syn. Fæcula, L. The matter which subsides from cold water in which bruised or rasped vegetable substances have been washed. The fecula obtained from the seeds of the cereals and leguminosæ, and from tuberous or bulbous roots, consists of nearly pure STARCH. In some cases the starch is associated with the green colouring matter (CHLOROPHYLL) and the narcotic principles of the vegetables which yield it. The green fecula obtained by straining the expressed juices of the leaves and herbaceous parts of plants is of this character.
The fecula of all the amylaceous roots, rhizomes, and tubers, may be easily obtained, on the small scale, by rasping them, pressing, and working the pulp in cold water, and after straining the resulting milky liquid through a hair sieve, allowing it to settle. The sediment may be again washed by diffusion through clean cold water, and must be, lastly, collected, and dried out of the dust, and, without artificial heat.
The fecula of narcotic plants for medicinal purposes is obtained by allowing the expressed717 juice to repose for 24 hours, and then decanting the clear portion, and drying the residue. Sometimes heat is employed. See Arrow-root, Starch, &c.
FEEDING BOTTLES. We extract from ‘The Sanitary Record’ the following valuable paper on ‘Feeding Bottles,’ by Dr Eustace Smith, assistant-physician to the City of London Hospital for Diseases of the Chest, and Physician to the East London Hospital for Children:—“In the artificial rearing of infants it is of importance that food should be given to them from a feeding-bottle. By this means the natural method of taking nourishment is imitated; the muscles of the mouth and cheeks are brought into play; and the secretion of saliva—a secretion which, very scanty at birth, becomes gradually more copious and takes so active a part in digestion—is encouraged and increased.
“Almost all babies will take their food more readily by this method, their instinct teaching them to suck everything that is put into their mouths. Even in cases where a deficiency in the hard palate presents so great an obstacle to sucking, on account of the impossibility of creating the necessary vacuum in the mouth, the difficulty can be overcome by a simple mechanical contrivance. Therefore, in every case of hand-feeding, a suitable bottle is the first thing to be desired.
“To be satisfactory a feeding-bottle must fulfil three indispensable conditions: it must be simple in construction and easily manageable; it must be capable of being readily cleaned; and in its use the milk must flow easily and without great effort on the part of the infant. The ordinary feeder in use at the present time consists of a flattened glass flask, closed at the mouth by a cap, which fits over the neck. A caoutchouc tube passes through the cap, and is connected inside the bottle with a straight glass pipe. The other end of the elastic tube is attached to the teat, or mouth-piece, by means of a short hollow cylinder called the ‘union-joint.’ The teat is firmly fixed to this by means of the shield. In the construction of the cap and union-joint, metal, earthenware, or wood, is employed. The metal used by the best makers is tin, and this, if cleanliness be properly attended to, is not objectionable. In cheaper bottles, sold in the shops for sixpence, the mouth is closed by a perforated cork, through which the flexible tube passes. Here there is no cap, but in all essential points the construction is the same as in the more expensive articles.
“In this apparatus it is important that the channel through the tubes should be perfectly free. The point at which the channel is narrowest is the union-joint, which connects the mouth-piece with the flexible tube. In a badly made bottle an impediment may exist at this point from carelessness in the manufacture, and may present a great obstacle to the ready passage of the fluid. Care also should be taken that the flexible tube passes completely through the cap, before it becomes connected with the glass pipe. This is very important. In the early feeding-bottles constructed upon this model by O′Connel, the glass pipe passed from within the bottle through the cap, and was attached outside this to the caoutchouc tube. It was thus held rigidly in the centre of the bottle, and as a natural consequence, when the apparatus was in use, unless the bottle was held upright during the whole meal, long before its contents were exhausted the milk ceased to flow, as the end of the pipe soon came to be above the surface of the fluid, which necessarily gravitated to the lowest part as the bottle lay on its side.
“When, however, the connection between the two tubes is made within instead of outside the bottle, this disadvantage no longer exists, for the glass tube being free to move, its end is able to sink to whichever side of the bottle is undermost, and therefore always remains below the level of the fluid. The best bottles have a small cylindrical stop, i.e. a thick ring of metal or wood placed within the flexible tube, just above its junction with the glass pipe. The object of this is to prevent the latter being drawn through the cap, and thus held rigidly in the centre of the bottle.
“The method of connection of the cap with the neck of the bottle is not unimportant. It should not be too tight or air will be prevented from entering the bottle to supply the place of the milk which is withdrawn. A common plan is to line the interior of the cap with cork, but this substance, besides its risk of being broken and detached by careless handling, has the further disadvantage of absorbing milk, which turns sour and may afterwards set up fermentation in fresh milk put into the bottle for a subsequent meal. In the best bottles the cap is constructed to screw on to the neck, as in the ‘Alexandra’ Feeding bottle made by the Messrs Maw; or is united to it by an application of the ‘bayonet catch,’ as in the ‘Improved’ feeding-bottle made by Messrs Lynch and Son. In this very admirable apparatus three grooves in the inside of the cap pass over corresponding projections on the neck of the bottle; the cap is then turned to the right, with a slight screwing motion, and becomes securely fastened.
“With badly made bottles infants often have very great difficulty in drawing up the milk, and can only do so by violent efforts, which soon exhaust their strength or their patience. There are two reasons why milk in these cases may not flow easily—either the cap fits too tightly, so that air cannot enter with sufficient facility in proportion as the liquid contents become diminished, as has just been mentioned; or the caoutchouc forming the flexible tube is too thin, so that it collapses when suction is applied. In the first case a small hole should be made through the cap, so as to allow a free admission of air, or if the718 bottle be a simpler one, closed at the mouth by a perforated cork, this may be slightly eased at the neck of the bottle, so as to fit less closely. In the second case, stouter caoutchouc should be used in the construction of the tube. In weakly infants, or those much reduced in strength by acute disease, special attention should be paid to these points, as such children will often refuse to take the bottle, if they find any difficulty in drawing up the milk.
“Infants born with a cleft palate cannot suck from an ordinary bottle, as the deficiency in the hard palate prevents the necessary vacuum being formed in the mouth. Such children are, therefore, usually brought up with a spoon, and often waste and die through insufficient nourishment. An ingenious contrivance first suggested by Mr Oakley Coles will, however, entirely remove the difficulty, and enable them to suck with as much ease as if they suffered from no such congenital difficulty. The plan is a very simple one, and consists in attaching to the nipple of any ordinary feeding-bottle a flap of sheet elastic, cut to fit the roof of the mouth. This flap must be of the shape and about the size of the bowl of a teaspoon, and is to be sewn to the upper part of the stalk of the teat, where this projects from the shield. In the mouth of the child the flap forms an artificial palate, which if the sheet elastic chosen be sufficiently stout, offers firm resistance to the tongue pressing against in sucking, and prevents fluid from passing into the nose in the act of swallowing.
“The closest attention must be paid to the cleaning of feeding-bottles. Each time after being used the whole apparatus should be well washed out with water containing a little soda in solution.
“The inside of the cap must be carefully cleaned, and the brush should be carried several times through the whole length of the tubing. Afterwards the bottle and tubes should be laid in cold water until again wanted. An objection to the common brush usually supplied with each feeder is, that after a few days’ use the softened bristles are apt to get detached and be caught in the joints of the tubing, whence they may afterwards be washed by the stream of fluid and be swallowed by the child. Accordingly, a new cleaner has been manufactured by Messrs Maw and Sons, in which bristles are entirely dispensed with. They are replaced by a thin strip of caoutchouc, which is wound round in a spiral form, at the end of the ordinary wire handle. This instrument answers all the purposes of a brush, without the disadvantages alluded to, and is besides far more durable.
“Excellent feeding-bottles are now made by many different manufacturers, and are sold at prices which place them within the reach of the poorest. These cannot all be mentioned, but some of the bottles more commonly met with, may be shortly referred to. The six-penny feeder made by Messrs Maw, Son, and Thompson, can be recommended for its simplicity of construction, and at the same time for its perfect efficiency. In this instrument there is no cap, instead the mouth of the bottle is closed by a cork, which is perforated for the passage of the flexible tube. In all other respects the construction of this apparatus is the same as in the more expensive instruments. The ‘Alexandra’ feeding-bottle, price half-a-crown, by the same makers, is an admirable bottle. The cap screws on to the neck, and is furnished with a small hole for the admission of air. A ‘stop’ in the lower part of the flexible tube prevents the glass pipe being drawn into the cap, and the instrument is supplied with all the latest improvements. The bottles made by Messrs Maw are all furnished with the new patent cleaner just described. The improved feeding-bottle made by Messrs Lynch and Son, at one shilling and eighteen pence, has been before referred to. The material used for the cap is boxwood. It is a capital bottle, and will give the fullest satisfaction to the purchaser. Mr Lang’s ‘Alma Mater’ feeding-bottle can also be recommended. In this instrument the cap is made of earthenware and is lined with cork. A good bottle is made by Mr Elam, of Oxford Street, price two shillings; the cap is formed of britannia metal, and screws on to the neck. A cheaper bottle, but one which for elegance of design and accuracy of detail cannot be surpassed, is Mr Mather’s ‘Princess’ feeding-bottle. A tin cup screws on the neck, and is pierced by a small hole for the admission of air. The opening is fitted with a ‘cone valve’ of simple and ingenious construction, which allows air to enter freely when suction is applied to the tube, but closes firmly against any escape through the air-hole of the fluid contents of the bottle. The bottle itself has a double curve towards the neck to provide against too sudden bending of the flexible tube against the tap. This is apt to happen when the curve is single, if the bottle lie with the convexity downwards, and partial obstruction of the tube may be the result. The ‘Princess’ feeding-bottle is sold in the shops for eighteen pence.
“All bottles bear their name in raised letters upon the glass, but a report which has obtained currency that these letters are hollow in the interior, and difficult to cleanse is without any foundation in fact. Any one may test this for himself by placing a finger within the bottle underneath the letters, when the internal surface will be found perfectly plain and uniform. In all cases where cork enters into the construction of a feeding-bottle, especial care should be taken in cleansing the apparatus, and the cork should be well soaked in soda and water in order that any sour milk it may contain may be neutralised at once.”
FEET (The). To preserve the feet in a proper condition, they should be frequently soaked, and well washed in warm or tepid719 water. The nails of the toes should be pared, to prevent their becoming inconveniently long, and from growing into the flesh, soaked, and well washed in warm or tepid water. Many persons suffer severely from TENDER FEET. This generally arises from the use of thin cotton or silk stockings, and boots or shoes that are either too tight or stiff, or not sufficiently porous to permit of the escape of the perspiration. Waterproof boots and shoes which are also air-tight (as those of gutta percha and India rubber), are common causes of tender feet, and even of headaches, dyspepsia, and apoplexy. The best treatment of tender feet is the immediate adoption of worsted stockings or socks, and light, easy shoes of buckskin, goatskin, or some other equally soft kind of leather. It is highly necessary for the preservation of health to preserve the feet DRY; persons who are, therefore, exposed to the wet, or who are frequently passengers through the public streets in bad weather, should regard sound and good boots and shoes as of the first importance. In fact, in a hygienic point of view, a wet back should be less shunned than wet feet. Many persons frequently experience EXTREME COLDNESS and NUMBNESS OF THE FEET. The best and most natural remedy for this is active exercise or friction, the former being always adopted when possible. In such cases the use of warm woollen stockings is absolutely necessary, and the debilitated and aged may advantageously keep them on throughout the night, or at all events until the feet acquire a comfortable degree of warmth. The DISAGREEABLE ODOUR which is evolved by the feet of some individuals in hot weather may be removed by the observance of extreme cleanliness, and by occasionally soaking the feet in warm water, to which a small quantity of chloride of lime or sal ammoniac has been added. A good deodoriser for unpleasant smelling feet is said to be the following, invented by M Paulcke:—A mixture of equal parts of salicylic acid, soap, talc, and starch, to be applied in the form of powder.
Distortion of the feet is not uncommon in childhood, being sometimes congenital, but as frequently the result of weakness or bad nursing. “A child with its feet turned inwards is called VARUS; when they are turned outwards it is styled VALGUS. The proper use of bandages, early applied, will generally correct these deformities; but if they be neglected in infancy they become incurable.” (‘Med. Lex.’) Clubfoot, of which there are several varieties, may also be frequently relieved by a simple surgical operation. See Boots and Shoes, Distortions.
FELT′ING. This is a process by which various species of fur, hair, and wool, are blended into a compact texture, in many respects resembling cloth. It depends on the peculiar anatomical construction of these substances, enabling them to interlace and intertwine with each other, by which they become permanently matted together. Felt was formerly chiefly employed for hats. It is now commonly used for mill-bands, filters, &c.; and when varnished or japanned, or saturated with asphalte or bitumen, is a durable substitute for japanned leather, and for roofing.
FENNEL. Syn. Fœniculum (Ph. L.), L. The fruit (seed) of Fœniculum dulce, or sweet fennel; the oil distilled from the fruit (OIL OF FENNEL; OLEUM FŒNICULI, L.) as well as a distilled water (FENNEL WATER; AQUA FŒNICULI, L.), are officinal in the Pharmacopœias. They are stimulant and carminative; but are now seldom employed.
FEN′UGREEK. The seeds of Trigonella Fœnum Græcum. Resolvent and stomachic. The seeds dye yellow; formerly roasted for coffee; now chiefly employed in veterinary medicine.
FER′MENT. Syn. Fermentum, L. A substance which induces fermentation. According to one view ferments are compounds whose decomposition proceeds simultaneously with that of the body undergoing metamorphosis. They all contain albuminous or azotised principles, which in a moist state putrefy and suffer decomposition. According to Pasteur, however, fermentation is excited by living organisms—fungi and infusoria. See Fermentation and Yeast.
FERMENTA′TION. Syn. Fermentatio, L. In chemistry, a peculiar metamorphosis of a complex organic substance, by a transposition of its elements under the agency of an external disturbing force. Fermentation, according to the theory proposed by Liebig, is a metamorphosis, by which the elements of a complex molecule group themselves so as to form more intimate and stable compounds. It is excited by the contact of all bodies the elements of which are in a state of active decomposition or fermentation. “In nitrogenised substances of a very complex constitution, putrefaction or fermentation is spontaneously established when water is present, and the temperature sufficiently high, and it continues till the original compounds are wholly destroyed. Substances destitute of nitrogen, on the contrary, require, in order to their undergoing this metamorphosis, the presence of a nitrogenised substance, already in a state of putrefaction (fermentation).” (Liebig.) The substances which promote this change are termed FERMENTS, and among these the principal are gliadin, gluten, vegetable albumen, and all nitrogenous substances in a state of spontaneous decomposition or fermentation. “It is imagined that when these substances, in the act of undergoing change, are brought into contact with neutral ternary compounds of small stability, as sugar, the molecular disturbance of the body, already in a state of decomposition, may be, as it were, propagated to the other, and bring about the destruction of the equilibrium of forces to which it owes its being. The complex body, under these circumstances, breaks720 up into simpler products, which possess greater permanence.” (Fownes.) Yeast, the ferment most commonly employed for inducing the vinous fermentation, is such a substance in an active state of putrefaction, and whose atoms are in continual motion. Putrefying animal substances are equally capable of exciting the same action. “If we add to a solution of pure sugar an albuminous substance, a caseous or fleshy matter, the development of yeast becomes manifest, and an additional quantity of it is found at the end of the operation. Thus, with nourishment, ferment engenders ferment. It is for this reason that a little fermenting must, added to a body of fresh grape juice, excite fermentation in the whole mass. These effects are not confined to alcoholic (vinous) fermentation. The smallest portion of sour milk, of sour dough, or sour juice of beet-root, of putrefied flesh and blood, occasions like alterations in fresh milk, dough, juice of beet-root, flesh, and blood. But further, and which is a very curious circumstance, if we put into a liquid containing any fermenting substance another in a sound state, the latter would suffer decomposition under the influence of the former. If we place urea in the presence of beer-yeast, it experiences no change; while if we add it to sugar-water in a fermenting state, the urea is converted into carbonate of ammonia. “We thus possess two modes of decomposition; the one direct, the other indirect.” (Ure.)
A very remarkable circumstance connected with fermentation is that it is always accompanied by the development of microscopic living organism—fungi and infusoria. “So constantly, indeed, is this the case, that many chemists and physiologists regard these organisms as the existing cause of fermentation and putrefaction; and this view appears to be corroborated by the fact that each particular kind of fermentation takes place most readily in contact with certain living organisms.” (Fownes.) Thus the vinous or alcohol-producing fermentation is accompanied, or caused, by two fungi, called Torula cerevisiæ and Penicillium glaucum; the acetous or vinegar-producing fermentation by Torula aceti; the lactous fermentation (souring of milk) by Penicillium glaucum.
The butyric fermentation by an animal—an infusorium, which cannot exist in free oxygen, but flourishes in an atmosphere of hydrogen, &c.
Of late years these latter views as to the cause of fermentation have been accepted by most of the scientific world, notwithstanding the opposition they experienced from so powerful an antagonist as Liebig.
From the researches of Pasteur, the distinguished author of the modern theory of fermentation, as opposed to the chemico-physical theory of Liebig, it appears that when yeast is placed in a solution of sugar and water, or in a solution of sugar and water containing albuminous substances, under proper conditions as to temperature, the fermentation that ensues is due to the process of growth taking place in the yeast plant; the new cells of which, in assimilating part of the sugar and converting it into cellulose and fat, cause, at the same time, the breaking up of the sugar molecule, and resolve it into the more stable combinations of alcohol and carbonic acid.
In order that the ferment or fungus should grow it is essential that, in addition to the cellulose and fat, it should be supplied with ammoniacal salts and soluble phosphates. These are generally present in the liquid about to be fermented; but when yeast is added to pure sugar and water “it lives at the expense of the sugar, and of the nitrogenous and mineral substances contained within itself.”[297]
[297] Pasteur.
Speaking of the influence of oxygen on the development of yeast on alcoholic fermentation, Pasteur states that ready-formed yeast can germinate and grow in a liquid containing sugar and albuminous matters, even when oxygen is completely excluded. The quantity of yeast formed, however, in this case, is but small, and the fermentation goes on slowly; nevertheless, a large quantity of sugar disappears (sixty to eighty parts to one part of yeast). If the air has access to a large surface the fermentation goes on quickly, and a much larger quantity of yeast is formed in proportion to the quantity of sugar which disappears.
In this case, also, oxygen is absorbed by the yeast, which grows quickly, but does not act so decidedly as a ferment, inasmuch as only four to ten parts of sugar disappear for one part of yeast produced.
When the air is excluded the same yeast again acts as a powerful ferment. Pasteur, therefore, infers that yeast which acts as a ferment in the absence of air abstracts oxygen from the sugar, and that upon this deoxidising power its action as a ferment depends. The violent activity of the yeast at the commencement of the fermentation is due to oxygen dissolved in the liquid. In liquids containing albumen (yeast and water, &c.) yeast likewise grows, though sparingly, even if the solution does not contain a trace of sugar, provided there is a sufficient access of air. But if the air is excluded this does not take place, even though the liquid may contain, besides albumen, a non-fermentable sugar, such as milk sugar. The yeast formed in a liquid not containing sugar possesses all the properties of a ferment, and excites fermentation in a solution of sugar excluded from the air.[298]
[298] ‘Bull. Soc. Chem.,’ 1861, pp. 61, 79.
Similarly, Pasteur regards putrefaction as a kind of fermentation, set up and maintained by an animal organism, or ferment belonging to the genus Vibrio. Putrefaction, when taking place in contact with the air, is always721 accompanied by decay or EREMACAUSIS. The abandonment of the old theory as to the nature of eremacausis, viz. that it consisted in the gradual combustion of decaying organic matters by atmospheric oxygen, has been necessitated by the experiments of Pasteur, Schröder, and others, which have conclusively established the facts that organic substances are not oxidised by perfectly pure air, and that their decomposition and subsequent destruction are due to the presence in the air of the sporules or seeds of certain low organisms. Pasteur cites numerous instances corroborative of the statement that perfectly pure oxygen fails to affect, save to a very limited extent, organic substances.
In one case an aqueous infusion of yeast mixed with sugar was enclosed in a sealed flask with double its volume of air, which had been previously depurated by being made to pass through a red-hot tube. At the end of three years the liquid (which had during part of the time been kept at a temperature of from 25° to 30° Cent.) was found to be perfectly fresh and transparent, and the air when examined gave 18·1 vols. of oxygen, 80·5 vols. of nitrogen, and 1·4 of carbonic acid. Under the same conditions urine and milk, whether fresh or previously boiled, showed minute traces only of oxidation; crystals of uric acid and phosphates formed in the urine, but the milk was unaltered, having preserved its alkaline reaction, and showed no disposition to curdle.
Very different, however, was the result when either of the above substances was enclosed with ordinary air. It was then found that in a few days the whole of the oxygen was absorbed, carbonic acid being at the same time simultaneously formed. A certain quantity of moistened oak sawdust kept in contact with ordinary air for a fortnight was found at the end of that time to have absorbed 140 cubic centimètres of oxygen; whilst the same amount of sawdust enclosed with an equal volume of purified air had removed only a few cubic centimètres of the gas in a month. In the former experiment a microscopic film of mycelia and spores of Mucidineæ formed on the sawdust.
From numerous experiments of a like nature with the above, and attended with analogous results, chemists and physiologists now generally regard eremacausis as effected by agencies similar in character to those which produce fermentation and putrefaction.
“The observations of Schröder upon the processes of fermentation and putrefaction are remarkable. He has shown that any organic liquid may be prevented from fermenting or putrefying if it be heated under pressure to about 266° F. (130° C.), then transferred to a flask and boiled, the mouth of the flask being plugged whilst boiling with a pellet of cotton wool, which is left in the neck of the flask. In this way he preserved, during a hot summer, various liquids, including freshly-boiled wort, blood, white of egg, whey, urine, broth, and milk; but when afterwards the plug of cotton wool was withdrawn these liquids in a few days began to undergo decomposition. He explains these results by supposing that the spores of some organism must find access to the substance in order to set up the process of decomposition. By a temperature of 260° F. (126·7° C.) any such spores which the substance itself might contain are destroyed, and as the air is filtered through the cotton wool before it reaches the interior of the flask, none of these organic germs can afterwards gain access to the body under experiment. I have repeated some of these experiments with complete success.[299]
[299] The Editor of this work has also repeated Schröder’s experiments on milk, and obtained the same results.
“If air be transmitted with suitable precautions slowly through narrow ignited platinum tubes, so as to destroy all suspended organic particles, no fermentation or putrefaction will take place on admitting such air into contact with putrescible substances previously heated to 260° for an hour.”[300]
[300] Miller.
Pasteur has shown the existence of these floating germs in the air by drawing a large volume of atmospheric air, by means of an aspirator, through a narrow tube obstructed by collodion wool. On subsequently dissolving this wool in a mixture of alcohol and ether various microscopic sporules were left undissolved.
The entire absence of the exciting causes—warmth, air, and moisture—leaves even those substances which under ordinary circumstances are most liable to change, in a state in which they may remain for an almost indefinite period without perceptible alteration. Thus, animal substances in a frozen or dry state do not undergo decomposition, nor does a solution of sugar or the juice of grapes (must) when perfectly excluded from the air; but on the mere exposure of these substances to warmth, moisture, or atmospheric air, putrefaction or fermentation immediately commences. Remove the cork from the bottle of ‘capillaire’ on the parlour sideboard, or pierce the skin of one of the grapes on the dessert table with a needle, and these bodies, which would have otherwise suffered no change for weeks, or even months, will soon exhibit symptoms of spontaneous decomposition. The knowledge of this fact has been practically applied to the preservation of animal and vegetable substances for food. Even the most putrescible of these may be preserved for an unlimited period by enclosure in metallic cases, or glass bottles, from which the air has been completely removed and excluded.
The important duties which fermentation or putrefaction performs in the economy of our globe, and in several of the arts of life and civilisation, have long rendered the development of its principles an object of the highest interest and importance, both in a scientific722 and practical point of view. In its most extended sense, this subtile process of nature, though occasionally productive of injurious effects, may be regarded as one of the most necessary and beneficial with which we are acquainted. Like the labours of a scavenger, it speedily removes from the surface of our globe those matters which would otherwise remain for some time without undergoing decomposition. It either dissipates in air, or reduces to more fixed and useful forms of matter, those organic substances which, by their presence, would prove noxious, or, at all events, useless to the animal and vegetable kingdoms. It is the giant power that cleans the Augean stable of nature, at the same time that it provides some of the most esteemed articles of utility and luxury for the well-being and enjoyment of man.
Chemists have distinguished fermentation into different varieties, which, in general, are named after the more important products of its action. Of late years, the number of these varieties has been greatly increased by the extension of the term to several operations besides those formerly included under it. See Acetification, Bread, Putrefaction, Brewing, &c.
FERN (Male). Syn. Male shield fern, Filix mas, Radix filicis, L. The root (rhizome) of the Lastræa Filix-mas, or male fern. It is bitter, astringent, or vermifuge.—Dose, 1 to 3 dr. in powder, or made into a decoction, repeated for 3 or 4 days, and followed by a purge. It is chiefly given in tapeworm. In Switzerland it is deemed almost infallible, but has proved less successful in these countries. See Oils.
FERRICY′ANIDE. Syn. Ferridcyanide, Ferridcyanuret. A compound of ferricyanogen with a metal or other basic radical. The FERRICYANIDE OF POTASSIUM, or ‘RED PRUSSIATE OF POTASH,’ as it is often improperly called, is a well-known example. The ferricyanides of AMMONIUM and the ALKALIES and ALKALINE EARTHS are soluble; those of most of the METALS, insoluble. See below.
FERRICYAN′OGEN. Syn. Ferridcyanogen, Ferric-cyanogen. The peculiar salt-radical which exists in the so-called red prussiate of potash. It is isomeric with ferrocyanogen, from which it differs in capacity of saturation (being tribasic), and in the behaviour of its compounds with solutions of the metals. It has not been isolated. See Potassium (Ferricyanide).
FERROCY′ANIDE. Syn. Ferrocyanuret, Prussiate; Ferrocyanidum, Ferrocyanuretum, L. A compound of ferrocyanogen with a metal or other basic radical. The principal substance of this kind is the FERROCYANIDE OF POTASSIUM or ‘YELLOW PRUSSIATE OF POTASH,’ as it is often called. See the respective basis—Ammonium, Potassium, Sodium, &c., and below.
FERROCYAN′OGEN. Syn. Ferrocyanogenium, L. A bibasic salt radical, composed of the elements of 3 equivalents of CYANOGEN and 1 equivalent of the metal IRON. It has never been isolated. It unites with the various bases to form FERROCYANIDES. See Cyanogen, Hydroferrocyanic Acid, Iron, &c.
FERRU′GO. [L.] Rust of iron. See Iron (Sesquioxide).
FE′VER. Syn. Febris, Pyrexia, L. In pathology a condition characterised by loss of appetite, thirst, languor, debility, unwillingness to move, accelerated pulse, increased heat of surface, and general disturbance of all the functions. A large number of diseases in which all or some of these symptoms appear are called FEVERS. They have been divided by nosologists into intermittent (INTERMITTENTES), remittent (REMITTENTES), and continued fevers (CONTINUÆ). The first of these are generally known as AGUES; the second differ from agues in there being one or more marked exacerbations and remissions of the symptoms every 24 hours, but without any entire intermission. The terms ‘hectic,’ ‘nervous,’ ‘bilious,’ ‘inflammatory,’ &c., have also been applied to particular varieties of fever; and names indicative of certain cutaneous appearances connected with them have been given to others; as ‘scarlet’ fever, ‘yellow’ fever, &c.
The usual symptoms of incipient fever (febrile symptoms) are—chilliness (varying from a simple shiver to a sensation of cold water running down the back), a quick pulse, hot and dry skin or flushing, languor, often evinced by yawning, depression of spirits, alternate fits of shivering and heat, hurried and uneasy respiration, flying pains in various parts of the body, as the head, back, and loins; loss of appetite, nausea or vomiting, dry mouth, furred tongue, costiveness, urine small in quantity, and usually of a deep colour, &c. When any of these symptoms appear, their progress may often be arrested by the timely exhibition of an emetic, followed by a saline purgative, and diaphoretics; at the same time promoting the action of these remedies by a low diet and drinking copiously of diluents, and carefully avoiding animal food, spirits, fermented liquors, or anything at all stimulant. Whenever symptoms of fever become established, medical advice should be sought and implicitly followed. In parts where it cannot be obtained the treatment recommended under Ague, Inflammation, Remittent Fever, and Typhus, may be followed with advantage.
In visiting or attending persons labouring under fevers, it is advisable to avoid immediate contact with them or their clothing, or standing near them in such a position as to inhale their breath, or the effluvia evolved (in some cases) by their bodies; and when remaining for some time in the apartment it is preferable to sit or stand near the fireplace, or between723 the window and door, as such parts of the room are generally better ventilated than the other portions. The greatest purifier of the atmosphere of a sick chamber is a good fire, because it occasions a continual current of the impure air up the chimney, and a corresponding influx of fresh air from without. Chloride of lime, or chloride of zinc, or their solutions, are also good purifiers. The first, however, should not be used in quantity, as the evolved chlorine might in that case impede the respiration of the patient. It is also advisable to avoid entering the room of a patient labouring under contagious diseases of any class when the stomach is empty or the spirits depressed; and it has been recommended to clear the mouth of the saliva immediately after quitting the chamber. See Ablution, &c.
FEVER DROPS (C. Warburg’s Vegetable). Camphor and aloes, 21⁄2; orange peel, 10; elecampane root, 12; digest with 90 per cent. spirit 240, mixed with ac. sulphuric dil. 24. To the tincture add quinine sulphate 9; tinct. opii crocatæ, 21⁄2. (Ragsky.)
FEVER POWDERS (James’s, also called James’s Powder and Pulvis Jacobi). It consists essentially of phosphate and antimoniate of lime with free antimonic acid.
FEVERSTONE—Lapis Anti-febrilis—Fieber Stein. Lead oxide, 54 parts; arsenic acid, 46 parts; melted together. (Winckler.)
FI′BRIN. Syn. Fibrine. An azotised substance, forming the coagulable portion of fresh-drawn blood, and the principal constituent of the muscular or fleshy parts of animals. It is eminently nutritious, and capable of yielding in the animal body albumen, caseine, and the tissues derived from them. (Liebig.)
Prep. Fibrin is easily obtained in a nearly pure state, by agitating or beating newly drawn blood with a small bundle of twigs, when it attaches itself to the latter under the form of long reddish filaments, which become white when worked with the hands in a stream of cold water. It may also be procured by washing the coagulum of blood, tied up in a cloth, in cold water, until all the soluble portions are removed. A small quantity of fat, which it still contains, may be removed by digesting it in ether.
Prop., &c. Pure fibrin occurs as long, white, elastic filaments, which are tasteless, inodorous, and insoluble in both hot and cold water. Wetted with acetic acid, it forms, after a time, a transparent jelly, which is slowly soluble in pure water. Very dilute solutions of the caustic alkalies dissolve it completely, and the new solution greatly resembles liquid albumen. Dried by a gentle heat it loses about 80% of water.
FICHTENNADEL-BRUSTZUCKER (Pine-needle Pectoral Sugar). (L. Morgenthau, Mannheim.) For irritable cough, hoarseness, tightness of the chest, asthma, stubborn lung affections, chronic catarrh, &c. Little sticks of bonbon, containing a very little opium, and wrapped in tinfoil. (Hager.)
FICHTENNADEL-TABAK (Pine-Needle Tobacco. (L. Morgenthau.) Is said to be patented in England. Ordinary tobacco moistened or sprinkled with a weak spirituous solution of wood wool extract and wood wool oil and dried; made up in cigars for smoking. (Hager.)
FIG. Syn. Ficus (B. P., Ph. L. E. & D.), Carica, Caricæ fructus, L. The figs of commerce are the dried fruit of Ficus Carica, the common fig-tree. They are demulcent, emollient, laxative, and pectoral. Roasted and boiled figs are occasionally employed as poultices to gumboils and other affections of the mouth.
FILARIA DRACUNCULUS. The Guinea worm. The female of this parasite is to be met with in tropical climates only, infesting the subcutaneous cellular tissue of man and some animals. In appearance it resembles a piece of white whip-cord of uniform thickness. According to Mr Ewart it varies in length from twelve and three quarters to forty inches, and is on an average twenty-five and a half inches long. It usually contains only one young worm, although rare instances have occurred in which as many as fifty of its progeny have been discovered in the same parent. In almost every case when this creature leaves the body, it does so by the lower extremities; occasionally, however, it does so by the mouth, the cheeks, or below the tongue. When the young of the guinea worm are placed in pure water they survive only four or five days; in foul water they will exist for three weeks. It appears that immersion in water, of the body of the person afflicted with the parasite, sometimes has the effect of inducing the creature to leave his human quarters, since Dr Lorimer states “that many people belonging to the bazaars in the vicinity of the lines, affected with the parasite, come, for the express purpose of extracting the worm, to the same tank where the men of the regiment bathe. The people so infested swim about in the water, with the worm hanging loose, drawing the limb quickly backwards and forwards, and from side to side, until the expulsion is affected.” Outside the body the guinea worm is generally found beneath organic débris in wells, tanks, and other reservoirs for water, from whence it appears to be now pretty universally admitted it effects an entrance through the skin during bathing or wading.
FILARIA SANGUINIS HOMINIS. In 1872, Dr T. R. Lewis, in examining microscopically the blood and urine of some of his patients in India, discovered a worm enveloped in an extremely delicate tube, closed at both ends, within which it could either elongate or shorten itself. This parasite (called from its principal habitat the Filaria Sanguinis Hominis)724 is about 1⁄75th of an inch in length, and about 1⁄35000th of an inch in diameter. When removed from the body with a small quantity of blood, it is described as being in a state of incessant motion, unceasingly coiling and uncoiling itself, lashing the blood-corpuscles in all directions, and insinuating itself between them.
The worms are said, when first taken from the body, to present a translucent appearance; the larger specimens, however, frequently exhibit an aggregation of granules towards the junction of the lower and middle half. Occasionally a bright spot, suggestive of a mouth, is seen at the thicker extremity. It is stated that they continue active from six to thirty hours. Mr Lewis does not believe they are able to perforate the tissues.
“These parasites,” says Mr Lewis, “are so persistently ubiquitous, as to be obtained day after day by simply pricking any portion of the body, even to the tips of the fingers and toes of both hands and both feet of one and the same person, with a finely pointed needle. On one occasion six excellent specimens were obtained in a single drop of blood by merely pricking the lobule of the ear.”
Dr Lewis estimates, from the number of the Filaria found in one drop of the blood of one patient, that his body must have contained more than 140,000. The presence of these creatures in the blood is believed to be the cause of chylous urine, which is a very common disease in the East. It seems probable they gain admission into the body from being present in drinking water.
FIL′BERT. Syn. Filberd. The fruit of the cultivated hazel or nut-tree (Corylus Avellana). Filberts are distinguished from common nuts by their lengthened figure and larger size. The best are imported from Spain.
FILES. The manufactures of these articles do not come within the limits of this work. It may, however, be useful to mention that FILES, FLOATS, and RASPS, which “cut dull” from age, dirt, or being much worn, are greatly improved by being kept wet, immersed in water for some hours, or even for a day or two.
Mr Ernest Spon recommends the following method for renovating files:—The file to be first cleansed from all foreign matter, and then dipped in a solution of one part of nitric acid, three parts of sulphuric acid, and seven parts of water; the time of immersion will be according to the extent the file has been worn, and the fineness of the teeth, varying from five seconds to five minutes. On taking it out of the mixture, wash in water, then dip in milk of lime, wash off the lime, dry by a gentle heat, rub over equal parts of olive oil and turpentine, and finally brush over with powdered coke.
FIL′TER. Syn. Filtrum, L. An instrument or apparatus for straining or filtering liquids.
FIL′TERING POWDERS. Prep. 1. Fuller’s earth washed, dried without heat, and reduced to coarse powder.
2. Pipe clay or potter’s clay, as the last. Both the above are used to filter and bleach oils.
3. Clay or fuller’s earth, 1 part; fine siliceous sand, 2 parts; the two are separately washed, after which they are drained, and mixed together, and dried as before. Used for GLUTINOUS OILS.
4. Granulated animal charcoal, sifted and fanned from the dust. Used to filter and bleach SYRUPS and VEGETABLE SOLUTIONS.
Obs. Filtering powders are prepared of several degrees of coarseness, and should be chosen with reference to the degree of fluidity of the liquid to be filtered through them. In no case should they be reduced to fine powder, as not only is the process of filtration thereby rendered unnecessarily tedious, but in some cases (as when charcoal dust is mixed with glutinous vegetable solutions and syrups) the filtrate carries off a portion of the powder, which can afterwards be separated from it only with considerable difficulty. See Charcoal, Filtration, Oil, &c.
FILTRA′TION. Syn. Filtratio, L. The separation of liquids from substances mechanically suspended in them, by passing them through media having pores sufficiently fine to retain or keep back the solid matter. Filtration is one of the most common and useful of the chemico-mechanical operations of the arts, and its successful performance in an economical and expeditious manner is therefore a matter of the highest importance in the laboratory, and, indeed, in almost every branch of human skill and industry, in which liquids are employed. Simple in principle, and apparently easily performed, it is, nevertheless, one of those operations which require no less of care than of tact and experience to conduct it with certainty and success. The losses sustained in the laboratory, by defective manipulation in this particular, often exceed those arising from ignorance and accidents in every other department conducted in it.
Filtration is generally resorted to for the purpose of freeing liquids from feculence, dirt, and other foreign matter, and for obtaining them in a clear or transparent state; but, in some cases, it has for its object the collection of the suspended substances, as precipitates, &c., and in others both these intentions are combined. The word ‘filtration’ is absolutely synonymous with ‘straining,’ but in the language of the laboratory it is usually applied to the operation of rendering liquids transparent, or nearly so, by passing them through fine media, as filtering paper, sand, and the like; whilst the term ‘straining’ is employed to designate the mere separation of the grosser portion, by725 means of coarse media, flannel, horsehair cloth, &c., through which they flow with considerable rapidity. Filtration is distinguished from ‘clarification’ by its mere mechanical action, whereas the latter operates by depuration, or the subsidence of the suspended substances or fæces, arising from their gravity being naturally greater than the fluid with which they are mixed, or being rendered so by the application of heat, or by the addition of some foreign substance.
The apparatus, vessels, or media, employed for filtration, are called ‘FILTERS,’ and are technically distinguished from ‘STRAINERS’ by the superior fineness of their pores.
Both strainers and filters act on the same principles as the common sieve on powders; they all, in like manner, retain or hold back the coarser matter, and permit the liquid or smaller and more attenuated particles to pass through. The term ‘medium’ (pleural ‘media’) is applied to the substance or substances through the pores of which the liquid percolates.
 Fig. 1.
Fig. 1.
The form of filters, and the substances of which they are composed, are various, and depend upon the nature of the liquids for which they are intended. On the small scale, funnels of tin, zinc, copper, wedgwood-ware, earthenware, glass, or porcelain, are commonly employed as the containing vessels. (See engr.) The filtering medium may be any substance of a sufficiently spongy or porous nature to allow of the free percolation of the liquid, and whose pores are, at the same time, sufficiently small to render it limpid or transparent. Unsized paper, flannel, linen, calico, cotton wool, felt, sand, coarsely powdered charcoal, porous stone, or earthenware, and numerous other substances of a similar kind, are employed for this purpose.
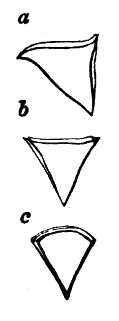 Fig. 2.
Fig. 2.
For many liquids that filter easily, and in which the suspended matter is of a coarse and porous nature, it is often sufficient merely to place a little cotton wool or tow, or a small piece of sponge, in neck of the funnel, as at (a, fig. 1) in the above engr.; but such an apparatus, from the small extent of the filtering surface, acts either slowly or imperfectly, and soon gets choked up. Filters of unsized paper are well suited for all liquids that are not of a corrosive or viscid nature, and are universally employed for filtering small quantities of liquids in the laboratory. A piece of the paper is taken of a size proportionate to the quantity of the liquid to be filtered, and is first doubled from corner to corner into a triangle (see engr. a), which is again doubled into a smaller triangle b, and the angular portion of the margin being rounded off with a pair of scissors c, it constitutes a paper cone, which is placed on a funnel of proportionate capacity, and is then nearly filled with the liquid. A piece of paper so cut, when laid flat upon the table, should be nearly circular. Filtering paper is now sold ready cut in circles of various sizes, which simply require doubling for use. Another method of forming a paper filter, preferred by some persons, is to double the paper once, as above, and then to fold it in a similar way to a fan, observing so to open it and lay it on the funnel that a sufficient interval be left between the two to permit of the free passage of the filtered liquid on its descent towards the receiver. The ‘plaited filter,’ as thus formed, is exceedingly useful for general purposes; it exposes the entire surface of the paper to the liquid, and allows filtration to proceed more rapidly than a ‘plain filter’ does. (See Fig. 3.)
 Fig. 3.
Fig. 3.
Mr Rother takes objection to the ordinary plain paper filter employed in the laboratory, because of the superfluous fold which in two thicknesses lies under one half of the extended surface of the filter. He says the interposition of these two extra layers compels the liquid to pass through three thicknesses of paper on the half side of the extended filter, whilst the other half side presents only a single thickness. It is evident that the two hidden layers are a very appreciable impediment to the current, aside from the more important fact that the liquid will traverse this side less rapidly than the other, and thus occasion an imperfect washing of the precipitate, or at least prolong the operation beyond reasonable limits. Recognising these objections to the old filter, Mr Rother has invented a very simple modification of the plain filter which, whilst saving 50 per cent. of the paper, he states, removes all the defects of the old form. This new filter practically presents but a single thickness of paper to penetrate, at the same time preserving an even surface, equal in all other advantages to the plain filter.
The filtrations are said to be more rapid than with the usual form, and the absence of the superfluous half sheet admits of more rapid drying.
To make the new filter:—Cut the circular disk of filtering paper in two through the line of its diameter, take either half disk, and fold it across the line of the radius, then turn down the double edge of the cut side and fold it over several times—finally, run a hard smooth surface along the seam thus produced, to compress it, and spread the finished filter into an appropriate funnel, first moistening it with water before the liquid to be filtered is poured in.
726
In reference to funnels, it may be remarked that those employed for filtering rapidly should be deeply ribbed on the inside, or small rods of wood or glass, or pieces of straw, or quills, should be placed between them and the paper. The neck or tubular part of the funnel should, in like manner, be deeply ribbed or fluted on the outside, to permit of the free passage of the air, when it is placed in a narrow-mouthed bottle or receiver. When this is not the case, filtration proceeds but slowly, and the filtered liquid is apt to be driven up the outside of the neck of the funnel by the confined air, and to be continually hissing and flowing over the mouth of the vessel. The breadth of a funnel, to filter well, should be about three fourths its height, reckoning from the throat (a). When deeper, the paper is liable to be continually ruptured, from the pressure of the superincumbent fluid; and when shallower, filtration proceeds slowly, and an unnecessarily large surface of the liquid is exposed to the atmosphere, and is lost by evaporation. To lessen this as much as possible, the upper edge of the glass is frequently ground perfectly smooth, and a piece of smooth plate-glass is laid thereon. When paper filters are of large dimensions, or employed for aqueous fluids that rapidly soften the texture of the paper, or for collecting heavy powders, or metallic precipitates, it is usual to support them on linen or calico, to prevent them breaking. This is best done by folding the cloth up with the paper, and cutting the filter out of the two, in the same way as would be done with doubled paper, observing so to place it in the funnel that the paper and calico may remain close together, especially towards the bottom.
The filtration of small quantities of liquid, as in chemical experiments, may often be conveniently performed by merely placing the paper on the circular top of a recipient (see engr.), or on a ring of glass or earthenware laid on the top of any suitable vessel. A filter of this kind that will hold one fluid ounce will filter many ounces of some liquids in an hour.
Good filtering paper should contain no soluble matter, and should not give more than 1⁄250 to 1⁄230 of its weight of ashes. The soluble matter may be removed by washing it, first, with very dilute hydrochloric acid, and secondly, with distilled water.
The ‘Munktell’ Swedish filtering paper[301] is composed of flax fibres very much crushed and broken, and owes its value to the broken pieces of the fibres filling up the pores, and thus preventing solids from passing through the paper. Rhenish filtering paper is also made from flax, but in consequence of the more perfect condition of its fibres, is more porous than Munktell’s, and therefore inferior to it for filtering purposes. Another kind of Rhenish paper, also of flax, in which the fibres are much torn, is manufactured and is said to be a useful article, and to allow the rapid passage of fluids through it. The white filtering papers of English make have a small quantity of cotton mixed with the flax; and the fibres are much torn and crushed; hence they make serviceable filters.
[301] Dr F. Mohr says that Swedish filtering paper is now undeserving its traditional reputation, and that it contains soluble alumina.
The grey, circular cut filtering paper of varying sizes, of foreign make, as well as the grey sheet filtering paper of Dutch and English manufacture, contains a large quantity of wool, much of which is coloured; as well as jute and esparto grass, both of these latter in an unbleached state. The amount of ash in the Munktell paper has of late increased in quality.[302]
[302] Greenish.
For filtering a larger quantity of a liquid than can be conveniently managed with a funnel, and also for substances that are either too viscid or too much loaded with feculence to allow them to pass freely through paper, conical bags made of flannel, felt, tweeled cotton cloth or Canton flannel, linen or calico, and suspended to iron-hooks by rings or tapes, are commonly employed. The first two of the above substances are preferable for saccharine, mucilaginous, and acidulous liquors; the third for oily ones; and the remainder for tinctures, weak alkaline lyes, and similar solutions. These bags have the disadvantage of sucking up a considerable quantity of the fluid poured into them, and are therefore objectionable, except for large quantities, or when they are to be continued in actual use as filters for some time. On the large scale, a number of them are usually worked together, and are generally enclosed in cases to prevent evaporation, and to exclude dirt from the filtered liquor that trickles down their sides. These arrangements will be noticed further on.
A simple mode of filtering aqueous fluids, which are not injured by exposure to the air, is to draw them off from one vessel to another, by means of a number of threads of loosely twisted cotton or worsted, arranged in the form of a syphon. (See engr.) The little cotton rope at once performs the operations of decantation and filtration. This method is often convenient for sucking off the water from a small quantity of a precipitate.
For fuller information on the subject of laboratory filtration, the reader is referred to the following papers (which are too long727 for quotation here) in ‘The Chemical News’:—
“On a New Mode of Filtration,” by J. B. Cooke, May 30th, 1873; “Filtering Apparatus,” by John F. Kerr, February 6th, 1874; “Implements for Filtration,” by P. Casamajor, July 23rd, 1875, and 30th, 1875; Ibid, by W. Jago, February 4th, 1876; “On Rapid Filtration,” by E. C. H. Hildebrand, August 11th, 1876; also to ‘Journal of the Chemical Society,’ for papers on:—“Simple Suction arrangement for Rapid Filtering,” by C. Holthof, vol. xxxii, part 2, p. 508; “Employment of Compressed Air on Filtering Solutions,” by W. Leübe, vol. xxxii, part 1, p. 270.
When solid substances, as porous stone or earthenware, are used as the media for filtrations, vessels of metal, wood, or stone-ware, are employed to contain them and the supernatant liquid. In these cases the filtering medium is usually arranged as a shelf or diaphragm, and divides the vessel into two compartments; the upper one being intended to contain the dirty liquid, and the under one to receive the same when filtered. Such an apparatus is set in operation by merely filling the upper chamber, and may at any time be readily cleared out by reversing it, and passing clean water through it in an opposite direction. Small arrangements of this kind, intended to be screwed on to the water supply-pipe by either end, and which answer the purpose intended in the most satisfactory manner, have been manufactured and vended under the name of ‘REVERSIBLE’ or ‘SELF-CLEANING FILTERS,’ When pulverulent substances, as sand, coarsely powdered charcoal, &c., are employed, a similar arrangement is followed; but in this case the shelf or diaphragm must consist of any convenient substance pierced with numerous holes, over which must be placed, first a stratum of coarse pebbles, next some of a finer description, and on this a proper quantity of the sand, charcoal, or other medium. Over the whole should be placed another layer of pebbles, or a board or plate of metal or earthenware, pierced with a number of holes, to allow the liquid to be poured into the filter without disturbing its arrangement. Apparatus of this kind, of a permanent description, and arranged for filtering large quantities of liquids, are properly denominated ‘FILTERING MACHINES,’
Among the liquids usually submitted to filtration, the following may be mentioned as the principal—water, oils, syrups, tinctures, vegetable juices, infusions, and decoctions.
The filtration of water may now be considered. The water of our wells is presented by nature ready filtered to the hand of man, and often exhibits an admirable degree of transparency and purity. It acquires this state by percolating through the mineral strata of the earth, which deprives it of the organic matter it derives from the soil and subsoil, but, at the same time, it dissolves a portion of the saline and earthy media through which it passes, and hence acquires that peculiar ‘hardness’ which is constantly found in spring water. On the large scale, this natural system of filtration has been imitated by some of the commercial companies that supply our cities and towns with water. Extensive beds of sand and gravel have been employed, with variable success, as the filtering media; and were it not that filters gradually lose their porosity by the accumulation of the retained matter in their pores, such a method would be excellent. But the great expense of such filters precludes the possibility of frequently cleaning or renewing them, by which means they can alone be kept in an efficient state.
A filter which possesses the advantages of being easily and cheaply cleaned when dirty, and which frees water from mechanical impurities with immense rapidity, may be formed by placing a stratum of sponge between two perforated metallic plates, united by a central screw, and arranged in such a manner as to permit of the sponge being compressed to any required degree. Water, under gentle pressure, flows with such rapidity through the pores of compressed sponge, that it is said that a few square feet of this substance will perfectly filter several millions of gallons of water daily. This method of filtration has been made the subject of a patent, and has been favorably noticed by the legislature.
A few barrels or hogsheads of water may be easily filtered daily, by the arrangement represented in the engraving.
A. A common water-pipe or cock.
b. A false bottom fitting in perfectly water-tight.
c. A perforated wooden or metallic vessel or box covered with a bag of felt or other filtering substance (not shown in the engraving). d. A small tube, fitting water-tight into the false bottom and uniting the interior of the filter with the lower portion of the cask.
It is evident that when water is poured into the upper portion B of a vessel, so arranged, it will sink through the filter c, and pipe d, into the lower chamber C, and this filtration will go on as long as the supply continues, and water is drawn from the cock e. By uniting the cock e with a tank or casks, and by keeping the upper portion B always full by means of a ball-cock, a considerable quantity of water may be thus filtered. The advantage of this plan is, that the filter c can be always readily got at, and easily cleaned or renewed.
For filtering water on the small scale, and for domestic use, ‘alcarazzas,’ diaphragms of porous earthenware and filtering-stone and layers of sand and charcoal, &c., already referred to, are commonly employed as filtering728 media. The filtering power of porous stone or earthenware may be greatly increased by adopting the arrangement represented in the margin, which consists in making the diaphragm of the shape of a disc (d), supporting plates of the same material, the whole forming but one piece. The ‘PLATYLITHIC WATER-FILTERS,’ which are formed of porous stone cut on this plan, present 200 to 300 square inches of filtering surface. A cheap, useful form of portable filter, is the following, given in the ‘Proceedings of the British Association,’ “Take any common vessel, perforated below, such as a flower-pot, fill the lower portion with coarse pebbles, over which place a layer of finer ones, and on these a layer of clean coarse sand. On the top of this a piece of burnt clay, perforated with small holes, should be put, and on this again a stratum of three or four inches thick, of well burnt pounded animal charcoal. A filter thus formed will last a considerable time, and will be found particularly useful in removing noxious and putrescent substances held in solution by water.”[303] The ‘PORTABLE-FILTERS,’ set up in stone-ware, that are commonly sold in the shops, contain a stratum of sand, or coarsely-powdered charcoal;[304] before, however, having access to this, the water has to pass through a sponge, to remove the coarser portion of the impurities. Among the many new kinds of portable filters now offered for sale, which claim special notice, are the following, viz.—
[303] A very similar filter to this was invented by the late Mr George Robins, the celebrated auctioneer. Mr Robins’ filter differed from the above in having a lid with a hole in the centre in which a sponge was placed; an arrangement which by keeping back the suspended matter contained in the water, prevented the filter from being clogged up.
[304] Frankland and Byrne have shown that animal is greatly superior to vegetable charcoal when employed for water-filters.
1. The MOULDED CARBON FILTER, consisting of a spherical or cylindrical vessel formed of compressed carbon.
2. The SILICATED CARBON FILTER, in which the medium is a compact substance, formed of animal charcoal and the ashes of Boghead coal.
Of the many forms of this filter, we may mention the ‘Syphon Filter for Travellers,’ by means of which wholesome water may be drunk from any pond or stream by simply immersing the filter therein and drawing the water through the tube by suction. Of the ‘Silicated Carbon Filter,’ Professor Wanklyn says that it will render river water containing a considerable amount of free and albuminoid ammonia as pure as deep spring water.
3. Bischoff’s Patent Spongy-iron filter.—This differs from one invented many years ago by Dr Medlock, in bringing the water into contact with spongy iron instead of thin iron rods, and thus effecting filtration much more rapidly. Medlock believed that the iron rods brought about the oxidation of the nitrogenous organic matter and its consequent conversion into nitrites and nitrates. Bischoff states that he has experimentally investigated the properties of spongy iron, and finds that it—
a. Decomposes even distilled water, which has been previously boiled.
b. That it reduces nitric acid to ammonia.
c. That the amounts of organic nitrogen and albuminoid ammonia are always much reduced after filtration through spongy iron.
d. That a minute quantity of iron is dissolved by the carbonic acid contained in the water, ferrous bicarbonate being formed. The latter being soon oxidised and precipitated is easily removed by filtration.
e. That the action of spongy iron on impure water is two fold, viz. chemical and mechanical. “The chemical action is clearly indicated by the decomposition of water. The readiest explanation for the decomposition of water, is, the intimate contact between the electro-positive and electro-negative bodies, such as metallic iron and carbon, or even metallic iron and any ferric oxide, which has escaped reduction, or which has been reoxidised by exposure to air or water; and it may well be supposed that, consequent to the galvanic current thus produced, the atmospheric oxygen dissolved in water is ozonised, and caused to act as a powerful oxidising agent in organic matter.”
We extract the tables on the next page from the Sixth Report of the Royal Commission on Rivers’ Pollution. The Commissioners, we may here state, speak in high terms of this filter.
4. The so-called Magnetic carbide of iron filter. In this, the filtering material is said to be prepared by heating hæmatite with sawdust. This filter has a good repute.
⁂ The Royal Commission “on Rivers Pollution” strongly recommend filters of animal charcoal to be recharged every three to six months, “since they found that myriads of minute worms were developed in the animal charcoal, and passed out with the water when these filters were used for Thames water, and when the charcoal was not renewed at sufficiently short intervals.”
Cleansing of Filters.—Every two or three months (according to the kind of water) air should be blown through, and if the charcoal be in the block form it should be brushed. Then four to six ounces of the pharmacopœial solution of potassium permanganate, or twenty to thirty grains of the solid permanganate in a quart of distilled water, and ten drops of strong sulphuric acid, should be poured through, and subsequently a quarter to half an ounce of pure hydrochloric acid in two to four gallons of distilled water. This plan would be useful on foreign stations where the filter cannot be sent home, or taken to pieces; if it can be taken to pieces, the charcoal should be spread out in a thin layer, and exposed for some time to air or sun, or heated in an oven.
729
| Description. | Dissolved Matters. | |||||
| Total solid impurity. | Organic carbon. | Organic nitrogen. | Ammonia. | Nitrogen, as nitrates and nitrites. | Total combined nitrogen. | |
| As delivered from Chelsea Waterworks | 28·04 | ·198 | ·042 | ·0009 | ·117 | ·220 |
| The same water filtered through spongy iron | 16·8 | ·069 | ·018 | ·019 | ·018 | ·049 |
| The mean of the 14th and 15th taken after the spongy iron filter had been in operation in the Rivers Commission Laboratory for upwards of eight months.[305] As supplied from Waterworks | 24·47 | ·170 | ·055 | ·001 | ·098 | ·154 |
| After filtration through spongy iron | 14·26 | ·083 | ·016 | 0 | 0 | ·016 |
[305] The figures demonstrate that the purifying action of spongy iron, if at all altered, has been increased, as regards the most important impurities of water, viz., nitrogenous matters and hardness.
| Description. | Dissolved Matters. | |||||
| Previous Sewage or Animal contamination. | Chlorine. | Hardness. | No. of samples analysed. | |||
| Temporary. | Permanent. | Total. | ||||
| As delivered from Chelsea Waterworks | 1·464 | 2·01 | 15·5 | 6·2 | 21·7 | 15 |
| The same filtered through spongy iron | ·177 | 2·00 | 6·8 | 4·9 | 11·7 | 15 |
| The mean of the 14th and 15th samples taken after the spongy iron filters had been in operation in the Rivers Commission Laboratory for upwards of eight months. As supplied from Waterworks | ·675 Analysis of the 15th sample. | 1·95 | — | — | 19·1 | — |
| After filtration through spongy iron | 0 | 1·95 | — | — | 9·6 | — |
If sponges are at all used, they should be removed from time to time, and thoroughly washed in hot water.[306]
[306] Parkes ‘Practical Hygiene.’
Oils are filtered, on the small scale, through cotton-wool, or unsized paper, arranged in a funnel; and on the large scale, through long bags, made of tweeled cotton-cloth (Canton flannel). These bags are usually made about 12 or 15 inches in diameter, and from 4 to 8 feet long (see engr.), and are inclosed in bottomless casings, or bags of coarse canvas, about 5 to 6 or 8 inches in diameter, for the purpose of condensing a great extent of filtering surface into the smallest possible space. A number of these double bags (from 1 to 50 or 60) are connected with corresponding holes730 in the bottom of a block-tin or tinned-copper cistern, into which the oil to be filtered is poured. The mode in which these bags are fastened to the cistern is of the utmost importance, as on the joint being close and secure depends the integrity of the apparatus. Three methods of doing this are figured in the engraving, which, with the references, will explain themselves, the same letters referring to the same parts of each.

Filtering-bag of cotton-cloth.
Cotton filtering-bag, ‘creased,’ or enclosed in its canvas envelope, ready for fixing.
The second of the above arrangements is the least expensive, and certainly the most convenient in practice; and when the cylinder l fits the hole closely (allowing for the bag), is as safe, or safer, than an ordinary screw.
a. Bottom of cistern.
b. Filtering-bag.
c. Screw of the conical nozzle fitting into the cistern.
d. Binding cord connecting bag and nozzle.
e. Binding cord connecting bag and lower nozzle.
f. Bayonet-catch, connecting the lower portion of the nozzle fastened to the bag with the upper and fixed part, g.
i. The thick hem at the top of the bag (purposely made large by enclosing a piece of thick cord therein), resting on the shoulders, k.
l. A metallic cylinder, loosely fitting the hole in the cistern, and over which the top of the bag is drawn, before being put into its place; when fitted, as in the engraving, it retains the hem i securely in its place above the shoulder k.
The bags are surrounded by a wooden screen fitted up with doors for the purpose of keeping off the dust; and the bottom of the apartment is furnished with large steam-pipes, by which a proper temperature may be kept up in cold weather. The use of heat should, however, never be had recourse to when it can be avoided, as although it vastly increases the rate of filtration, the oil so filtered is more apt to become opaque in cold weather than when the process is conducted at the natural temperature of the atmosphere. This is particularly the case with castor oil and sperm oil. In the United States of America, where the latter is consumed in enormous quantities for illumination, the best is always ‘winter strained,’ as it is popularly called. In practice, it is more convenient to have a number of small cisterns at work (say 50 or 100 galls. each), than one or two larger ones, as any accident that may occur is more easily remedied, and that without stopping the whole operation.
When cotton-cloth bags are employed without being ‘creased,’ or enclosed in others of canvas, they should not be longer than about 3 or 4 feet, and not wider than about 5 or 6 inches when filled. When larger they are dangerous.
A convenient method of filtering a single cask of oil is, to insert the pipe of a two-way patent filter into the cork-hole, by which means the whole will be filtered as drawn off, without any trouble on the part of the operator. This filter consists of a porous bag stretched over a perforated metallic vessel, nearly the shape and size of the exterior casing, and its edge is tightly screwed between the sides and bottom of the latter, so as to be quite water-tight. The cock communicates with the interior of the perforated plate and filter, and the supply-pipe with the exterior. By this means the interior chamber, which occupies 5⁄6ths of the vessel, rapidly fills with filtered oil, and continues full as long as any liquor remains in the cask. This arrangement is also well adapted to the filtration of wines, beer, cordials, porter, and various other liquors. It is unequalled in simplicity and usefulness. The same filter may be removed from cask to cask, with the facility of a common cock.
The filtration of SYRUPS is now generally effected on the large scale by passing them through the ‘CREASED BAG FILTER’ just described. On the small scale, as employed by confectioners and druggists, they are usually passed through CONICAL FLANNEL BAGS. (See page 726.) The filtration of thick syrups is, however, attended with some difficulty, and it is therefore a good plan to filter them in a somewhat dilute state, and afterwards to reduce them to a proper consistence by evaporation in clean vessels of tinned copper, by steam heat. Syrups, when filtered in a heated state, run well for a time, but the pores of the fabric rapidly get choked, from the thickening of the syrup and partial crystallization of the sugar, occasioned by the evaporation of the aqueous portion from the surface of the bag. This may be partially prevented by enclosing the bag in a metallic casing. On the whole clarification is preferable for syrups to filtration on the small scale. They need only be well beaten up while cold with a little white of egg, and then heated; a scum rises, which must be removed as soon as it becomes consistent, and the skimming continued until the liquid becomes clear. Any floating portions of scum that may have escaped notice are easily removed by running the syrup through a coarse flannel strainer, whilst hot. The most extensive application of the process of731 filtration in the arts is in the refining of sugars.
Tinctures and dilute spirits are usually filtered, on the small scale, through BIBULOUS or UNSIZED PAPER placed on a funnel; and on the large scale, through thin and fine COTTON BAGS. In general, however, tinctures clarify themselves by the subsidence of the suspended matter, when allowed to repose for a few days. Hence it is the bottoms alone that require filtering; the supernatant clear portion need only be run through a small hair sieve, a piece of tow or cotton placed in the throat of a funnel, or some other coarse medium, to remove any floating substances, as pieces of straw, &c. Spirits which are largely loaded with essential oil, as those of ANISEED, &c., run rapidly through paper or calico, but usually require the addition of a spoonful or two of magnesia before they will flow quite clear. When possible, tinctures, spirits, and all similar volatile fluids, are better and more economically cleared by subsidence or clarification than by filtration, as, in the latter way, a portion is lost by evaporation, and the strength of the liquid is thereby altered.
Vegetable juices should be allowed to deposit their feculous portion before filtration. The supernatant liquid will then be often found quite clear. It is only when this is not the case that filtration should be had recourse to. A small quantity may be filtered through coarse or woollen filtering paper, supported on a piece of coarse calico placed on a funnel; when the quantity is large, one of the CONICAL BAGS before described should be employed. The bottoms from which the clear portion has been decanted should be placed on a separate filter, or else not added until the whole of the other portion has drained through. Vegetable juices are often rendered clear by simply heating them to about 180° or 200° Fahr., by which their albumen is coagulated; they are also frequently clarified by the addition of a little white of egg and heat, in the same way as syrups. Many of them (as those of hemlock, henbane, aconite, &c.) are greatly injured by heat, and must consequently be filtered, or only simply decanted after repose. In all cases they should be exposed to the air as little as possible, as they rapidly suffer decomposition.
Vegetable infusions and decoctions may be cleared by defecation followed by filtration. The conical bags of flannel before described are usually employed for this purpose. When the liquid is to be evaporated to an extract, they are commonly suspended by a hook over the evaporating pan. A convenient method of straining these fluids, practised in the laboratory, is to stretch a square of flannel on a frame or ‘horse,’ securing it at the corners by pieces of string. (See engr.) Such a frame, laid across the mouth of a pan, is more easily fed with fresh liquid than a bag, whose mouth is 40 or 50 inches higher. The same purpose, for small quantities of liquid, is effected by laying the flannel across the mouth of a coarse hair sieve. The concentrated infusions and decoctions being usually weak tinctures, may be filtered in the same way as the latter. (See above.) Many vegetable solutions, that from the viscidity of the suspended matter can scarcely be filtered, may be readily clarified with white of egg in the cold, or pass the filter rapidly if a very small quantity of acetic, tartaric, sulphuric, or other strong acid, is previously added.
Corrosive liquids, as the STRONG ACIDS, are filtered through powdered glass, or SILICEOUS SAND, supported on pebbles in the throat of a glass funnel, or through asbestos or gun-cotton placed in the same manner. Charcoal has also been employed for the same purpose, but is not fit for some acids. Strong caustic alkaline lyes are also filtered through powdered glass or sand. Weak alkaline lyes may be filtered through fine calico, stretched across the mouth of a funnel. Many corrosive liquids, as solution of potassa, &c., require to be excluded from the air during filtration. The simplest apparatus that can be employed for this purpose is that figured in the margin:—(a) is a globular bottle fitted with the ground stopper (d), and having a perforated neck (f) ground to the bottle (b); (c) is a small tube, wrapped round with as much asbestos, linen, or calico, as is required to make it fit the under neck of the bottle through which it passes. The tube (c) may also be fixed by placing pebbles and powdered glass or sand round it, as before mentioned. For use, the solution to be filtered is poured into the bottle (a) nearly as high as the top of the tube (c), and the stopper is replaced. The liquid then descends into (b), and a similar quantity of air passes up the tube into (a). Liquor potassæ may be always obtained fine by depuration in close vessels, when the sediment of lime only need be filtered, which may be effected with calico fixed across the mouth of a funnel.
When a precipitate, or the suspended matter in a liquid, is the object of the filtration, the filter should be of such a nature that the powder may be easily separated from it, when dry, and that with the least loss possible. Linen filters are for this reason preferable for large quantities, and those of smooth bibulous paper for small ones. The powder should be washed down the sides of the filter, and collected, by means of a small stream of732 water, in one spot at the bottom, assisting the operation with a camel-hair pencil; and, when the whole is dry, it should be swept off the paper or cloth with a similar pencil or brush, and not removed by a knife, as is commonly done, when it can be possibly avoided.
The ‘first runnings’ of liquid from a filter are commonly foul, and are pumped back or returned until the fluid runs perfectly limpid and transparent, when it is ‘turned into’ the ‘filtered liquor cistern,’ or proper receiver. In many cases the liquid does not readily become transparent by simply passing through the filter; hence has arisen the use of FILTERING POWDERS, or substances which rapidly choke up the pores of the media in a sufficient degree to make the fluid pass clear. In the employment of these powders care should be taken that they are not in too fine a state of division, nor used in larger quantities than are absolutely necessary, as they are apt to choke up the filter, and to absorb a large quantity of the liquid. The less filtering powder used, the more rapid will be the progress of the filtration, and the longer will be the period during which the apparatus will continue in effective action. For some liquids these substances are employed for the double purpose of decolouring or whitening, as well as rendering them transparent. In such cases it is preferable first to pass the fluid through a layer of the substance in coarse powder, from which it will ‘run’ but slightly contaminated into the filter; or, if the powder is mixed with the whole body of the liquid, as in bleaching almond oil, &c., to pass the mixture through some coarser medium to remove the cruder portion before allowing it to run into the filter. Another plan is, after long agitation and subsequent repose, to decant the clearer portion from the grosser sediment, and to employ separate filters for the two. Granulated animal charcoal is used according to the first method, to decolour syrups, oils, &c.; and filtering powder by the second and third, to remove a portion of the colour, and to clarify castor and other oils. The common plan of mixing large quantities of filtering powder with castor oil, and throwing the whole into the filter, as adopted by the druggists, is injudicious. When simple filtration is required, it is better to use little or no powder, and to continue returning the oil that ‘runs’ through, until, by the swelling of the fibres of the filter bags, it flows quite clear. By this plan the same filters may be used for a long period of time (for many years), and will continue to work well; whilst, by the usual method, they rapidly decline in power, and soon deliver their contents slowly, and after a short time scarcely at all.
It is often of great advantage to render a filter ‘self-acting,’ or to construct it in such a way that it may ‘feed itself,’ so that it may continue full and at work without the constant attention of the operator. On the small scale, this may be readily effected on the principle of the common fountain lamp (see engr.); and on the large scale, by placing the vessel containing the unfiltered liquid on a higher level than the filter, and by having the end of the supply-pipe fitted with a ball-cock, to keep the liquid in the filter constantly at the same height.
The rapidity of filtration depends upon—the porosity of the filtering medium—the extent of the filtering surface—the relative viscidity or mobility of the filtering liquid—the pressure or force by which the liquid is impelled through the pores of the filter, and—the porosity and fineness of the substances it holds in suspension. The most efficient filter is produced when the first two or the first three are so graduated to the others that liquid filters rapidly, and is at the same time rendered perfectly transparent.
In the common method of filtration no pressure is exerted beyond that of the weight of the column of the liquid resting on the filtering medium, but in some cases additional pressure is employed. This is had recourse to for the purpose of producing a more rapid filtration, and more especially for filtering liquids that, from their viscidity, will scarcely pass through the pores of substances sufficiently fine to remove their impurities in the ordinary way.
One of the easiest means of employing pressure in filtration is to increase the height of the column of the filtering liquid. From the peculiar properties of fluids, by which they transmit pressure in an equal degree in all directions, this column need not be of equal diameter throughout, but may be conveniently contracted to the size of a small pipe, as in the accompanying engraving, which represents a small filter on this construction at work. (a) Is the funnel or reservoir of foul liquid; (b) a small pipe conveying the liquid to the filter; (c c) a chamber, of which the upper portion (d) is filled with the descending liquid, and the lower portion (e) with the filtering media; (i i) are screws by which the bottom plate is fastened on, which plate is removed to clean out or renew the filter. For use, the cocks (k) and (l) are closed, and the liquid poured into the funnel (a); the cock (k) is next opened, and, in a few minutes733 after, the cock (l), when an uninterrupted flow of filtered liquor will be obtained as long as any fluid remains in the funnel (a) and the tube (b). The length of the tube determines the degree of pressure. Care must be taken first to pass the foul liquid through a hair sieve, or some other strainer, to remove any substance that might choke up the pipe (b).
Another method of employing pressure in filtration is the withdrawal of the air from the receiving vessel, as in the vacuum filter, by which a pressure of about 141⁄2 lbs. to the square inch becomes exerted on the surface of the liquid by the atmosphere. The vacuum in the receiving vessel may be produced by the air-pump, by steam, or by the Bunsen or Sprengel pump.
A commoner method of applying pressure than either of those already mentioned is to condense the air over the surface of the liquid by means of a forcing-pump, or by steam.
On the small scale, pressure may be applied to filtration by means of a syphon, whose shorter leg has its mouth blown into the shape of a bell or funnel, over which filtering paper or fine calico may be stretched.
The application of pressure to filtration is not always advantageous, and beyond a certain limit is generally attended with inconvenience, if not with absolute disadvantage. It is found in practice that fluids under pressure take a longer period to run clear than without pressure, and that ruptures of the media more frequently take place in the former case, or with pressure, than in the latter. Great pressure is in no case advantageous.
The filters already noticed are those that act by the fluid descending through the media; but in some cases the reverse method is employed, and the liquid filters upwards, instead of downwards. These are called ascending filters, and are often preferable to those on the descending principle, because the suspended matters that require removal by filtration usually sink, and thus a portion escapes being forced into the pores of the filter. They are also more convenient when pressure is employed. The construction depends upon the same principles as the common filter, and merely requires that the feeding vessel should be higher than the upper surface of the filtering media. Oils are conveniently filtered in this way, because of their little specific gravity. By fixing a small filter on this principle into the head of a cask, and pouring in water through a funnel, whose neck reaches nearly to the bottom of the cask, the oil will float up and pass the filter, leaving the sediment behind. In cold weather hot water may be employed.
In some cases the upward and downward systems of filtration are united in the same apparatus, and this plan is advantageous where the space for operating is limited. For this purpose it is merely necessary to connect the bottom of an ascending filter with the top of a descending one, or the reverse; the proper pressure being in either case applied.
Filtration, the Laws of. The ‘Revue Universelle des Mines,’ 1874, pp. 469, 551 contains a paper by M. Paul Havre recording his investigations on the rapidity of the filtration of water through sand, wool, &c., which resulted in ascertaining and measuring the influences which may modify the flow of water. In all cases of filtration, the influences which are exerted are:—the pressure and temperature of the water, the thickness of the filtering medium, compression in the case of fibrous filters, the size of the grains and their mixture in the case of a filtering medium analogous to sand. The influence of obstruction, due to the dirtiness of the filter, depends on circumstances too variable to be taken into account. The delivery of a filter per square mètre per 24 hours is equal to two cubic mètres multiplied by the pressure of water in mètres, divided by the thickness of the filtering medium in mètres. An application of this formula is made to existing filter beds, including those at Southwark and at the Chelsea waterworks.
The first experiments for ascertaining the influence of a head of water on the delivery led to the following results:—The delivery increases in a higher ratio than the square root of the pressure, due to the height (Torrecelli’s Law); the delivery increases in direct ratio to the height of the column of water above the filter, admitting a previous initial delivery, due solely to the pressure of water above the filter; the co-efficient of the increase of delivery is constant, and in this case of a filtering substance 8·662 inches (22 centimètres) thick, is equal to 0·106 pint (6 centilitres) for sand to 0·528 pint (30 centilitres) for compressed wool, and to 0·792 pints (45 centilitres) for wool only slightly compressed.
The subsequent experiments were made with graduated transparent cylinders, 3·28 feet (1 mètre) high, with the ends perfectly level, the filtering substances being kept in place by a thick double cloth tied tightly under the bottom of the tube. This apparatus presented no other obstacle to the running of the water than the layer of filtering substance; it permitted experiments to be made at all temperatures, and the thickness of the filtering medium to be measured exactly.
In these experiments sand is ‘taken as the type of pulverulent substances,’ but an unexpected difficulty was encountered in the settling or partial agglomeration of the large and small grains of the unsifted sand, thus diminishing the delivery of water to one half, one third, and ultimately to one fifth of its previous volume. This led to the adoption of734 sand—the grains of which were uniform in size, and to the discovery of the fact that, other tissues being equal, the resistance of filtration is constant when the sand is coarse, when the grains of fine sand are of nearly equal size, and when there is but little fine sand mixed with the coarse. From experiments in filtering through a layer of coarse sand approximately 4 inches (10 centimètres) thick, it was found that the higher the temperature the more rapid was the delivery, and by filtering through a layer of coarser sand 11·8 inches (30 centimètres) thick, the conclusion was arrived at that the temperature exerts an influence in proportion to the thickness of the layer.
See Air-Pump, Bunsen’s Water-Air Pump; Clarification; Defecation; Finings, &c.
FI′NINGS. Substances used by publicans, brewers, wine merchants, &c., to clarify their liquors.
Prep. 1. (Brewer’s finings; Cooper’s f.) Isinglass (finely shredded), 1 lb., and sour beer or cider or vinegar, 3 or 4 pints, are macerated together, and more of the sour liquor added as the isinglass swells, until about a gallon has been used, agitation with a whisk or a small bundle of twigs being occasionally had recourse to, for the purpose of promoting the solution. As soon as the whole of the isinglass is dissolved, the mixture is reduced to the consistence of thin syrup, with weak mild beer, or cider, or any other liquid that the finings are intended for. The whole is next strained through a tammy cloth or a hair sieve, and at once reduced to a proper state of dilution, by the addition of more liquor. Product, 61⁄2 to 7 galls. “A pound of good isinglass will make about 12 galls. of finings.” (Ure.) Used to clarify fermented liquors, especially beer. 1 to 11⁄2 pint is the usual dose for a barrel of ale or porter; and a quart for a hogshead of cider or wine.
2. (Spirit finings.)—a. Alum (ord. cryst.), 1 lb.; powder, and divide it into 12 equal portions, which are to be separately wrapped in blue paper, and marked No. 1. Next take of carbonate of soda (sesquicarbonate of the shops), 6 oz.; divide this as the last, wrap it in white paper, and mark each parcel No. 2. Keeps dry anywhere.
b. From alum, 1 lb.; salt of tartar (dry), 1⁄4 lb.; proceed as before. The white papers containing the salt of tartar must be kept in a dry, well-corked, wide-mouthed bottle or jar. Both of the last two are used to clarify gin and cordials. The contents of one of the blue papers are dissolved in about a pint of hot water, and the resulting solution is well ‘rummaged up’ with the liquor. A solution of the contents of one of the white papers, in about 1⁄2 pint of hot water, is then added, and the agitation continued for some minutes longer; after which the cask is ‘bunged’ close and the whole allowed to repose until the next day. This is sufficient for a barrel (say 30 to 36 galls.), but many persons use double the quantity. The effect is not only to clarify, but also to ‘blanch’ the liquor.
Obs. Good liquors, either fermented or spirituous, need no artificial ‘fining,’ as they always clarify themselves by repose. With those, however, which are out of ‘condition,’ or of inferior quality, it is often necessary, as, without such a proceeding, they remain unsaleable. This is particularly the case with malt liquor. “Attempts to clarify it in the cask seldom fail to do harm. The only thing that can be used with advantage for fining foul or muddy beer is isinglass.” (Ure.) The disadvantages resulting from the artificial clarification of fermented liquors are—that they do not afterwards ‘stand well on draught,’ that much of the conservative astringent matter which they contain is precipitated with the ‘finings,’ that their piquancy and flavour is more or less diminished, and that they are more than usually liable to become flat and vapid, whether in cask or bottle. The larger the proportion of ‘finings’ used, the more marked are their injurious effects, and the shorter the interval which elapses before the accession of the several symptoms referred to. We have seen the most disastrous consequences follow the injudicious use of ‘finings,’ more especially in respect to those liquors in which a certain amount of piquancy, astringency, and briskness, is an essential condition. In one instance which came under our notice upwards of 30 barrels of ‘underground’ (a very strong old ale) was thus reduced in value to less than 1-3rd its original cost; and in another, a large bottled stock of the ‘finest old Burton’ was found to be utterly unsaleable. In both cases the ‘spoiled liquor’ was got rid of by mixing it in and selling it with 3d. and 4d. beer.
Liquors which ‘refuse to fine’ or become clear, when treated with ‘finings’ in the usual manner, are called ‘stubborn’ by coopers and cellarmen. See Brewing, Gin, Malt, Liquors, Wines, &c.
FIRE. The calamities resulting from this destructive agent are of such frequent occurrence, as to justly claim a notice of the subject here. The causes of fires are numerous, and of a varied character, and, in most instances, difficult to determine, because it is the interest of those concerned to suppress all evidence connected with the matter. Accident, that convenient word given to the imaginary hack to which so many fires are referred, if truthfully interpreted, will, in general, be found to be equivalent to carelessness, recklessness, or guilt. We believe that there are few fires which have happened that might not have been prevented by the exercise of common prudence, and that a vast number have been caused by direct negligence, arising from sheer laziness and indifference, to use no harsher terms. As familiar instances, may be mentioned—allowing735 sparks to fall on the ground and remain there without extinguishing them; carrying a naked candle into rooms containing inflammable substances; smoking carelessly and in dangerous places, as workshops, warehouses, on shipboard, &c.; keeping instantaneous light matches in improper places, and neglecting to pick up those that may happen to fall on the ground, &c. &c. The list might easily be extended, but we believe every reflecting reader can do so for himself. The great increase in the number of fires since the introduction of lucifer matches, and the almost general use of tobacco, cannot fail to have attracted the attention of every one. The danger of matches falling about might be avoided by the use of those which can only be ignited by rubbing them on the prepared surface of the box. These ‘safety matches’ are coming into general use, and must eventually supersede all the more dangerous kinds.
The late Mr Braidwood classes the causes of fires under the following heads:—1. Inattention in the use of fires and lights. 2. Improper construction of buildings, &c. 3. Furnaces or close fires, for heating buildings, or for mechanical purposes. 4. Spontaneous ignition. 5. Incendiarism.
Amongst many other causes of fire, too numerous to specify, may be noticed—incautiously approaching window- and bed-curtains with a candle or lamp, airing linen before the fire, allowing children to play with fire, women’s dresses taking fire, and taking off the burning coals from a fire and laying them on the hearth. Another very common cause of fire is covering up a fire-place when not in use with wood, or paper and canvas, &c. The soot falls either from the flue itself or an adjoining one into the grate; a neighbouring chimney takes fire, a spark from this falls down the blocked-up flue, ignites the soot in the grate, which smoulders until the covering is burnt through, and thus sets the building on fire.
Another cause of fire, and one which cannot be too strongly condemned, is the dangerous practice of reading in bed by candle-light. A very serious annual loss of property is also caused by want of proper care in hanging up or removing the goods in linendrapers’ shop windows when the gas is burning. Another frequent cause of fire is the employment of young children in lighting fires, from their propensity to play with flame.
The employment of close fires with brick flues is also a frequent source of danger. Frequently, from various causes, the furnace almost always cracks, thus giving egress to smoke and flame. When this occurs no time should be lost in thoroughly repairing the defect, or building a new furnace; merely plastering over the surface will be found an ineffective and dangerous remedy.
To guard against the dangers arising from the ignition of wearing apparel many methods have been suggested for rendering fabrics flame proof, all of them consisting in soaking the dress in a weak solution of a non-inflammable substance, such as chloride of zinc, alum, tungstate of sodium, sulphate of ammonia, &c. Of these alum has the advantage of greatly improving the appearance of the fabrics, especially if they be coloured.
Fire-guards, particularly where there are children, ought to be adopted much more generally than they appear to be.
Prev. This consists of the exercise of those ordinary precautions which the good sense of every careful and trustworthy man, be he taskmaster or servant, cannot fail to suggest. It would be useless to enumerate them.
Immediately on the fire being discovered, secure an alarm being given to the nearest of the fire escape stations, not delaying an instant; do not wait “to see if it is wanted.” Life is more valuable than property, and events have often proved how fatal even a moment’s hesitation is in sending for the fire-escape.[307]
[307] ‘Handbook for Emergencies,’ Cassell.
The late Mr Braidwood’s advice was, “that if the fire appears at all serious, and there are fire-engines within a reasonable distance, that it is best to wait until they arrive; many buildings have been destroyed from opening doors, and trying to extinguish fires with insufficient means. If no engines are within reach it is advisable to keep a hand-pump. If that is not to be had, the next best thing is to collect as many buckets outside the room on fire as can be obtained, keeping the door shut; then to creep into the room on hands and knees (if the heat and smoke are considerable), and throw the water as nearly in the direction of the fire as possible, keeping the door shut while more water is being collected.
“The police of the metropolis understand shutting up fires so well, that they have in many instances kept fires two or three miles distant from the engine-stations, shut up till the fireman arrived in time to extinguish them.”
Fires might often be readily extinguished when first discovered by the timely application of a few buckets of water. When an apartment is found to be on fire, the door, chimney, and windows should be immediately closed, if possible, and only opened for the purpose of projecting water on the flames. By this means the supply of air will be cut off, and rapid combustion prevented. The same applies to the lower doors and windows of a house (especially the shop window), which are often injudiciously kept open or removed, under the pretence of rendering assistance. The neglect of this precaution has often caused a mere smouldering fire, that might have been easily put out, to burst into an unextinguishable mass of flame.
It has been proposed at various times to make certain additions to the water used for the purpose of extinguishing fires, in order to render its action more certain and effective.736 It is found that sal ammoniac (5 oz. to the gall.) exerts this property in a remarkable degree. Several other articles, as common salt, pearlash, and kitchen soda, act in the same way, though less effectively. A few buckets of such water will speedily arrest the progress of a fire before it has much extended itself. Such a plan is easily applied, by adding the saline matter to the buckets of water, which are either used by hand, or to feed the engine for the first few minutes of its working. When, however, a fire has made much progress, the action of such substances becomes scarcely perceptible.
Chimneys on fire are readily extinguished in several ways, without having recourse to throwing water down them from the top, by which much damage is frequently done to the furniture in the rooms. One of the simplest methods is, to cautiously scatter a handful of flowers of sulphur over the dullest part of the burning coals; the sulphurous vapours, being incapable of supporting combustion, rapidly extinguish the flames. Another method is, to shut the doors and windows, and to stop up the bottom of the chimney with a piece of wet carpet or blanket, throwing a little water or flowers of sulphur, or even common salt, on the fire immediately before doing so. By this means the draught is stopped, and the burning soot extinguished for want of air. In many of the first-class houses recently erected, ‘fire-place shutters’ are provided, which, when partly drawn down, act as powerful bellows or ‘blowers’ and which, when wholly drawn down, so as to touch the hearth-stone, entirely close up the fireplace, and instantly extinguish the combustion of the fuel in the grate, or that of the soot in the chimney. This simple arrangement, the advantages of which were pointed out in an early edition of this work, renders fires in chimneys of little moment, as it is only necessary to draw down the shutter to put them out. If a chimney is stopped at top, instead of at the bottom, the whole of the smoke must, of necessity, be driven into the apartment.
In France, M. Marateuh has successfully applied the principle of Davy’s safety lamp for the prevention of fires in chimneys. He places fire-frames of iron work near the base of the chimney, one above the other, about one foot apart; no flame passes through them, whilst the draught in the chimney is not interfered with, the result being that no fire can happen in the chimney.
Escape from apartments on fire may be best effected by creeping on the hands and knees. In this way the window or door may be reached. It is found that the atmosphere of a room so full of smoke as to produce suffocation to a person standing upright, may generally be safely breathed on nearly a level with the floor. A damp cloth, or handkerchief, tied over the mouth and nostrils, or, still better, over the whole face and head, will enable a person to effect a passage through the densest smoke, and, in many cases, to escape from buildings on fire, when otherwise it would be impracticable. Should descent by the staircase be found impossible, then the window should be immediately sought, and a ladder or fire-escape waited for. In the absence of either, if the danger is imminent, a rope should be made by tying the sheets and blankets of the bed together, one end of which should be firmly secured to a chair or table, or preferably to one of the bed-posts, and with this apparatus descent should be cautiously attempted. Jumping out of the window should be avoided, as persons who have not been brought up as clowns, or harlequins, run just as much danger in performing such an exploit as they do by remaining in the burning building. When it is impossible to escape from a burning building by the stairs or windows, retreat may be sometimes secured by a trap door opening on to the roof, or by a skylight, when, unless it be an isolated house, the roof of one of the adjoining buildings may probably be gained with safety.
Fire-escapes of various kinds have been employed of late years in the metropolis, and have proved of the greatest value in rescuing persons from burning buildings.
It is said that there is no instance on record of a person being burnt to death in a dwelling-house in Edinburgh, where the houses are usually high; yet in London, where fire-engines and fire-escapes are provided in greater numbers, deaths are very frequent from this cause. The reason of this difference is, that in the former city the stairs are all made of stone, by which means a road of escape is secured.
The clothes of females and children, when on fire, may be most readily extinguished by rolling the sufferer in the carpet, hearth-rug, table-cover, a great-coat, cloak, or any other woollen article at hand. If this be expertly done, the flames may be rapidly put out, unless the skirts of the dress be distended by hoops or crinoline, when there is great difficulty in staying the progress of the flames. Should assistance not be at hand, the person whose clothes are on fire should throw herself on the ground, and roll the carpet round her, as before described; or if such a thing is not in the room, she should endeavour to extinguish the flames with her hands, and by rapidly rolling over and over on the floor. In this way the fire will be stifled, or at least the combustion will proceed so slowly that less personal injury will be experienced before assistance arrives. The advantage of assuming the horizontal position is manifest from the fact that nine times out of ten it is the lower parts of the dresses of females that first catch fire.[308]
[308] For the mode of rendering muslin and other inflammable articles of ladies’ apparel fire-proof, see Incombustible fabrics.
737
The extinction of fires on board ships by means of carbonic-acid gas was some years since suggested to the Admiralty by Mr J. R. Hancorn. He proposes that a simple and economical apparatus should be attached to every decked vessel capable of supplying this gas, which is a well-known non-supporter of combustion, and will extinguish fire at the very instant of coming in contact with the burning matter. Chalk with sulphuric acid diluted with water (vinegar with any other acid will do) yields 44% of the gas; hence, a ton of chalk, and a fourth part of that quantity of sulphuric acid, will be found sufficient to extinguish any fire on board a ship. Mr Hancorn also proposed this as a method of destroying vermin in ships, such as rats and cockroaches, for which purpose it is more easily applied and more effectual than that usually adopted. This plan was rejected by the Admiralty, from a fear that the destructive action of the gas might extend to the crew as well as the fire. But “it surely is possible by mechanical means to expel the gas before again entering the ship’s hold. At any rate, the grand point would be obtained of extinguishing the fire, though the crew might have only the deck to stand on.”
Precautions to be taken against a Fire amongst Farming Stock.—The following are the suggestions of Mr Beaumont, the secretary of the County Fire Office:—
“Forbid your men to use lucifer matches, to smoke or light pipes or cigars, destroy wasp nests, or fire off guns in or near the rickyard, or to throw hot cinders into or against any wooden out-building on the farm, on pain of instant dismissal.
“Place your ricks in a single line, and as far distant from each other as you conveniently can. Place hayricks and cornstacks alternately; the hayrick will check the progress of the fire. Keep the rickyard, and especially the spaces between the stacks and ricks, clear of all loose straw, and in all respects in a neat and clean state. The loose straw is more frequently the means of firing than the stack itself. Have a pond close to the rickyard, although there may be a bad supply of water. When a steam thrashing machine is to be used, place it on the lee-side of the stack or barn, so that the wind may blow the sparks away from the stacks. Let the engine be placed as far from the machine as the length of the strap will allow. Have the loose straw continually cleared away from the engine; see that two or three pails of water are kept close to the ashpan, and that the pan itself is kept constantly full of water.”
It is often difficult to get horses out of buildings on fire, but it is said that they will readily come out if, after being blindfolded, the saddle and bridle, or the harness, &c., to which they are accustomed, are thrown over them as usual.
We learn from the last report issued by Captain Shaw that the actual number of fires in the year 1877 in London was 1533. Of these fires 1374, or 90 per cent., were slight, no persons being endangered, and no considerable destruction of property taking place. The number of really serious conflagrations was 150; in 88 of these life was endangered, and in 24 cases there was loss of life. The actual number of persons whose lives were in danger was 165; but of these 136 were saved, and the lives eventually lost amounted only to 29. The smallness of the loss is due in great degree to the courage of the members of the Brigade, seven of whom have been commended for special efforts for saving life during the year. Even of the twenty-nine persons who perished fourteen were taken alive out of the burning buildings, and died in hospital of their wounds. It is very satisfactory in view of the vast height of buildings used in business, and the flimsy character of so many London houses, that the risk of death from fire should be so small. It is one of the very slightest risks to which we are exposed in modern London. The fire-escapes must of course be credited with much of this security. There are now 108 stations of these useful machines; and instances of their utility in rescuing the inmates of burning houses are constantly occurring.
The various tables which Captain Shaw appends to his report give some very curious details as to the character of London fires. The hours at which they most commonly break out are by no means those which are popularly supposed to be the most dangerous. No considerable proportion occur after people have gone to bed. From seven o’clock in the evening till eleven o’clock there are more alarms of fire than in an equal portion of the twenty-four hours. Not a third of the number which occur in these evening hours take place in the small hours of the morning, which are in fact less destructive than the same period in the afternoon. There are, moreover, in the detailed list of fires some curious statistics, illustrating the comparative security of private houses over places of business. A very large part of the half million houses in London must come under the description of private dwellings, yet the alarms of fire in this class of buildings were only 316 in the year, and only in five of those were there serious conflagrations. In the lists of business premises nearly every trade in the metropolis is mentioned; and next to houses let out in lodgings, public-houses seem to suffer most. The causes of fires tell the old story of carelessness. They were instances of the almost inconceivable folly of seeking for an escape of gas with a lighted candle. The throwing down of lights is responsible for a considerable number of fires. Ordinary cases of chimneys on fire are not included in Captain Shaw’s summary; but they give the brigade a good deal of work. The number of calls of this kind was 3744, of738 which 1256 proved to be false alarms. The number of these false alarms will probably be reduced when the stations at which men with hose are situated are more numerous.
Fire Anni′hilator (Phillips’s). This is essentially a gaseous fire engine, which at any moment can be made to discharge a stream of mixed gases and vapours having the power of checking combustion. When first introduced it was generally regarded as a most important invention, but it has not proved an effective substitute for the common water engine. For extinguishing fires on board ship and in close apartments it is undoubtedly well adapted, but as a street engine it is comparatively useless, owing to the unmanageable nature of its fire-annihilating vapours.
The composition with which the ‘Fire Annihilator’ is charged is a mixture of dried ferrocyanide of potassium, sugar, and chlorate of potassa. It is set in action by a blow on a glass vessel containing oil of vitriol, which, being fractured, permits the acid to flow over the ‘charge,’ when the anti-combustion gas is liberated, and rushes forth with great impetuosity.
Fire-damp. See Hydrogen (Light Carburetted).
Fire-engine. The common fire-engine is a compound forcing-pump, consisting of two ‘forcing-pumps’ placed on opposite sides of an ‘air-vessel,’ with which both communicate. The ‘fulcrum’ of the ‘lever’ by which both pumps are worked is placed midway between them; consequently they act alternately in charging the air-vessel. In order to obtain a very forcible jet it is necessary to prevent the escape of any portion of the contents of the air-vessel until the confined air is considerably compressed. The lever is connected with handrails on each side of the engine, and these are alternately raised and depressed by the workers. Engines worked by steam power are now common in London and most of our large towns.
Fire-Extinguishing Powder (Feuerloschpulver), Bucher Leipzig. Nitre, 59 parts; sulphur, 36 parts; coal, 4 parts; iron oxide, 1 part. (Wittstein.)
Fire, how to light a. In a close stove the first thing is to empty the fireplace. Take out the larger cinders and half-burnt coal with your fingers, and lay them on one side for lighting the fire; then rake out all the ashes (this can be done with the lids on, then it will not make so much dust). Next take off all the lids, and sweep all the soot carefully out; once or twice a week the flue pipe must be taken off and cleared out, also the flues under the oven. The soot should be carried away at once, as it blows about. Then blacklead the stove; put in a few cinders, lay on them a piece of paper and a few sticks crossing each other; on these lay very lightly some pieces of half-burnt coal and a few cinders, leaving space for the draught.
Do not fill the grate full; put the lids on, draw out the damper, light the fire, and shut the front door. An open fire is lighted in much the same way. There are no flues to clean out; but the chimney, as high as one can reach and behind the register door, should be cleared from soot daily.[309]
[309] ‘Household Management, &c.,’ by W. T. Tegetmeier.
Fire-proofing. See Incombustibility, &c.
Fireworks. See Pyrotechny, and below.
FIRES. (In pyrotechny.) Coloured fires may be termed, not inaptly, the chefs-d’œuvre of the pyrotechnist’s art, since on their excellence the attractions of most other varieties of fireworks depend. The following forms, under judicious management, yield fires of remarkable beauty.
Blue Fire. Prep. 1. From metallic antimony, 1 part; sulphur, 2 parts; nitre, 5 parts.
2. From realgar, 2 parts; charcoal, 3 parts; chlorate of potassa, 5 parts; sulphur, 13 parts; nitrate of baryta, 77 parts.
3. (Mr A. Bird.) Charcoal and orpiment, of each 1 part; black sulphuret of antimony, 16 parts; nitre, 48 parts; sulphur, 64 parts.
4. (Fownes.) Tersulphuret of antimony, a part; sulphur, 2 parts; dry nitre, 6 parts. This is the composition used for the Bengal or blue signal light employed at sea.
5. (Prof. Marchand.) Sulphur, sulphate of potassa, and ammonio-sulphate of copper, of each 15 parts; nitre, 27 parts; chlorate of potassa, 28 parts. For theatrical illuminations. This may be rendered either lighter or darker coloured by lessening or increasing the quantities of the sulphate of potassa and ammonio-sulphate of copper.
6. (Light blue—Marchand.) Sulphur, 16 parts; calcined alum, 23 parts; chlorate of potassa, 61 parts.
7. (Dark blue—Marchand.) Calcined alum and carbonate of copper, of each 12 parts; sulphur, 16 parts; chlorate of potassa, 60 parts.
8. (Marsh.) Sulphate of copper, 7 parts; sulphur, 24 parts; chlorate of potassa, 69 parts.
9. (Ruggieri.) Nitre, 2 parts; sulphur and zinc, of each 3 parts; gunpowder, 4 parts.
10. From sulphur, 1 part; dried verdigris, 2 parts; chlorate of potassa, 9 parts.
Fire, Crimson. Prep. 1. (Marsh.) Chlorate of potassa, 41⁄4 parts; charcoal (alder or willow), 53⁄4 parts; sulphur, 221⁄2 parts; nitrate of strontia, 671⁄2 parts. For pots.
2. (Marsh.) Charcoal, 41⁄4 parts; sulphuret of antimony, 51⁄2 parts; chlorate of potassa, 171⁄4 parts; sulphur, 18 parts; nitrate of strontia, 55 parts. For boxes and stars.
3. (Marchand.) Sulphur, 16 parts; chalk (dry), 23 parts; chlorate of potassa, 61 parts. Turns on the purple. See Red Fire (below).
Fire, Green. Prep. 1. Nitrate of baryta, 77 parts; chlorate of potassa, 8 parts; fine charcoal, 3 parts; sulphur, 13 parts.
739
2. From metallic arsenic, 2 parts; charcoal, 3 parts; chlorate of potassa, 5 parts; sulphur, 13 parts; nitrate of baryta, 77 parts. Very beautiful, particularly when burnt before a reflector.
3. (Mr A. Bird.) Charcoal and black sulphuret of antimony, of each 2 parts; chlorate of potassa, 5 parts; sulphur, 6 parts; nitrate of baryta, 80 parts.
4. (Fownes.) Lampblack, 1 part; chlorate of potassa, 4 parts; sulphur, 6 parts; dry nitrate of baryta, 18 parts.
5. (Marchand.) Boracic acid, 10 parts; sulphur, 17 parts; chlorate of potassa, 73 parts. Very beautiful.
6. (Marchand.) Chlorate of potassa, 18 parts; sulphur, 22 parts; nitrate of baryta, 60 parts. For theatrical illuminations.
7. (Light green—Marchand.) Sulphur, 16 parts; carbonate of baryta, 24 parts; chlorate of potassa, 60 parts. Extremely delicate.
8. (Marsh.) Charcoal and sulphuret of arsenic, of each 13⁄4 parts; sulphur, 101⁄2 parts; chlorate of potassa, 231⁄4 parts; nitrate of baryta, 621⁄2 parts. For pots or stars.
Fire, Lilac. Prep. 1. (Marsh.) Black oxide of copper, 6 parts; dry chalk, 20 parts; sulphur, 25 parts; chlorate of potassa, 49 parts. For pans.
2. (Marsh.) From black oxide of copper, 3 parts; dried chalk, 22 parts; sulphur, 25 parts; chlorate of potassa, 50 parts. For stars.
Fire, Orange. See Red Fire, No. 8 (below).
Fire, Pink. Prep. (Marchand.) Charcoal, 1 part; chalk and sulphur, of each 20 parts; chlorate of potassa, 27 parts; nitre, 32 parts. For theatrical illuminations. See Red Fire, No. 10 (below).
Fire, Purple. Prep. 1. From lampblack, realgar, and nitre, of each 1 part; sulphur, 2 parts; chlorate of potassa, 5 parts; fused nitrate of strontia, 16 parts.
2. (Marsh.) Sulphuret of antimony, 23⁄4 parts; black oxide of copper, 10 parts; sulphur and nitrate of potassa, of each 223⁄4 parts; chlorate of potassa, 42 parts. For pans.
3. (Marsh.) Sulphate of copper, 93⁄4 parts; sulphur, 13 parts; chlorate of potassa, 771⁄4 parts. For stars.
4. From sulphur, 12 parts; black oxide of copper, 12 parts; chlorate of potassa, 30 parts. See Crimson Fire, No. 3 (above), and Red Fire, No. 9 (below).
Fire, Red. Prep. 1. From sulphur, sulphuret of antimony, and nitre, of each 1 part; dried nitrate of strontia, 5 parts.
2. (Mr A. Bird.) Charcoal, 1 part; black sulphuret of antimony, 4 parts; chlorate of potassa, 5 parts; sulphur, 13 parts; dried nitrate of strontia, 40 parts.
3. (Fownes.) Lampblack, 2 parts; chlorate of potassa, 8 parts; sulphur, 9 parts; dried nitrate of strontia, 32 parts.
4. (Marchand.) Sulphur, 16 parts; carbonate of strontia, 23 parts; chlorate of potassa, 61 parts.
5. (Marchand.) Chlorate of potassa, 20 parts; sulphur, 24 parts; nitrate of strontia, 56 parts. For theatrical illuminations.
6. (Marsh.) Coaldust, 2 parts; gunpowder, 6 parts; sulphur, 20 parts; dried nitrate of strontia, 72 parts.
7. (Ruggieri.) Sulphuret of antimony, 4 parts; chlorate of potassa, 5 parts; sulphur, 13 parts; fused nitrate of strontia, 40 parts. A little charcoal or lampblack makes it burn quicker.
8. (Orange red—Marchand.) Sulphur, 14 parts; chalk, 34 parts; chlorate of potassa, 52 parts.
9. (Purple red—Marchand.) Sulphur, 16 parts; chalk, 23 parts; chloride of potassa, 61 parts.
10. (Rose-red—Marchand.) Sulphur, 16 parts; dried chloride of calcium, 23 parts; chlorate of potassa, 61 parts. See Pink Fire.
11. From charcoal, 2 parts; chlorate of potassa, 6 parts; sulphur, 13 parts; dried nitrate of strontia, 40 parts.
Fire, Violet. Prep. 1. From charcoal, 8 parts; sulphur, 10 parts; metallic copper, 15 parts; chlorate of potassa, 30 parts.
2. (Dark violet—Marchand.) Alum and carbonate of potassa, of each 12 parts; sulphur, 16 parts; chlorate of potassa, 60 parts.
3. (Pale violet—Marchand.) Sulphur, 14 parts; alum and carbonate of potassa, 16 parts; chlorate of potassa, 54 parts.
Fire, White. Prep. 1. From nitre, 60 parts; sulphur, 20 parts; black antimony, 10 parts; meal powder, 6 parts; powdered camphor, 4 parts. For either pans or stars.
2. (Mr A. Bird.) White arsenic, 1 part; charcoal, 2 parts; black antimony, 16 parts; nitre, 48 parts; sulphur, 64 parts.
3. (Marchand.) Charcoal, 2 parts; sulphur, 22 parts; nitre, 76 parts. For theatrical illuminations.
4. (Marchand.) Gunpowder, 15 parts; sulphur, 21 parts; nitre, 64 parts. As the last.
5. (Marsh.) Gunpowder, 121⁄2 parts; zinc filings, 18 parts; sulphur, 23 parts; nitre, 461⁄2 parts. For pans.
6. (Marsh.) Zinc dust or filings, 15 parts; sulphur, 28 parts; nitre, 57 parts. For stars.
7. (Ruggieri.) Sulphur, 131⁄4 parts; sulphuret of antimony, 171⁄4 parts; nitre, 48 parts.
8. (Ruggieri.) From realgar, 2 parts; sulphur, 7 parts; nitre, 24 parts.
9. (Ruggieri.) Charcoal, 1 part; sulphur, 24 parts; nitre, 75 parts.
10. (Ruggieri.) Iron or zinc borings, 25 parts; gunpowder, 100 parts.
Fire, Yellow. Prep. 1. From sulphur, 16 parts; dried carbonate of soda, 23 parts; chlorate of potassa, 61 parts.
2. (Marchand.) Gunpowder, 14 parts; sulphur, 16 parts; dried soda, 20 parts; nitre, 50 parts.
3. (Marchand.) Charcoal, 11⁄2 parts; sulphur, 171⁄2 parts; dried soda, 20 parts; nitre, 61 parts.
740
| No. | Potassium Chlorate, per cent. | Barium Nitrate, per cent. | Sulphur, per cent. |
| 1 | 36 | 40 | 24 |
| 2 | 29 | 48 | 23 |
| 3 | 24 | 53 | 23 |
| 4 | 21 | 57 | 22 |
| 5 | 18 | 60 | 22 |
| 6 | 16 | 62 | 22 |
| 7 | 14 | 64 | 22 |
| 8 | 13 | 66 | 21 |
| 9 | 12 | 67 | 21 |
| 10 | 11 | 68 | 21 |
| 11 | 10 | 69 | 21 |
| 12 | 9·5 | 69·5 | 21 |
| 13 | 9 | 70 | 21 |
| 14 | 8·5 | 70·5 | 21 |
| 15 | 8 | 71 | 21 |
[310] Kern (‘Chemical News,’ September 29th, 1876).
| No. | Potassium Chlorate, per cent. | Strontium Nitrate, per cent. | Sulphur, per cent. | Carbon Powder, per cent. |
| 1 | 40 | 39 | 18 | 3 |
| 2 | 32 | 46 | 19 | 2 |
| 3 | 27 | 51 | 20 | 2 |
| 4 | 23 | 55 | 20 | 2 |
| 5 | 20 | 58 | 20·5 | 1·5 |
| 6 | 18 | 60 | 21 | 1 |
| 7 | 16 | 61·6 | 21·2 | 1·2 |
| 8 | 15 | 63 | 21 | 1 |
| 9 | 13 | 64 | 22 | 1 |
| 10 | 12 | 65 | 22 | 1 |
| 11 | 11 | 66 | 22 | 1 |
| 12 | 10 | 67 | 22 | 1 |
| 13 | 10 | 67·25 | 22 | 0·75 |
| 14 | 9·25 | 68 | 22 | 0·75 |
| 15 | 9 | 68·35 | 22 | 0·65 |
| No. | Potassium Chlorate, per cent. | Calcium Carbonate, per cent. | Malachite powdered, per cent. | Sulphur, per cent. |
| 1 | 52 | 29 | 4 | 15 |
| 2 | 52 | 28 | 5 | 15 |
| 3 | 52 | 26 | 7 | 15 |
| 4 | 52 | 24 | 9 | 15 |
| 5 | 52 | 23 | 10 | 15 |
| 6 | 52 | 21 | 13 | 15 |
| 7 | 51 | 20 | 14 | 15 |
| 8 | 51 | 18 | 16 | 15 |
| 9 | 51 | 16 | 18 | 15 |
| 10 | 51 | 15 | 19 | 15 |
| 11 | 51 | 13 | 21 | 15 |
| 12 | 51 | 11 | 23 | 15 |
| 13 | 51 | 10 | 24 | 15 |
| 14 | 51 | 8 | 26 | 15 |
| 15 | 51 | 6 | 28 | 15 |
741
4. (Marsh.) Charcoal, 6 parts; sulphur, 191⁄2 parts. For pans. Very beautiful.
In preparing coloured fires for fireworks according to the usual formulæ given in manuals of pyrotechny, it is often important to know the speed at which they burn; as in some cases, such as decorations and lances, they should burn slowly; whereas in others, such as wheels, stars for rockets, and Roman candles, they ought to burn quicker. The foregoing tables are so arranged that every formula with a higher number yields a slower burning mixture than one with a lower number. Thus No. 5 burns quicker than No. 6, and slower than No. 4.
Obs. The ingredients in the above compounds are to be separately reduced to powder and sifted through lawn, after which they should be kept in well-corked wide-mouthed bottles until the time of mixing them for use. The chlorate of potassa, more especially, must be separately treated and cautiously handled, in order to prevent the possibility of explosion from friction whilst it is in contact with combustible matter. The requisite quantity of each of the ingredients being weighed out and placed on a clean sheet of white paper, the whole is to be thoroughly but carefully mixed together with a light hand, by means of a bone or wooden knife. The compound is next lightly packed into small cups or pans for illuminations, or into small pill-boxes for stars and trains, a little priming and quick-match being lastly attached to each. To ensure success the several ingredients must be dry and commercially pure; and though reduced to the state of a uniform powder, care must be taken that they are not absolutely ‘dusty,’ or too finely pulverised. The nitrate of strontia, alum, saltpetre, carbonate of soda, &c., before being weighed, require to be gently heated in an iron pot or pan until they fall to powder, and lose their hygrometric moisture, or water of crystallisation. To ensure the perfect admixture of the ingredients, the whole, after they have been stirred together on paper, as before directed, may be passed through a hair or perforated zinc or brass sieve. Further, as coloured fires rapidly deteriorate by keeping, and even sometimes inflame spontaneously, to prevent disappointment and accidents they should not be prepared long before they will be required for use, and should be stored in some situation in which their spontaneous combustion would be productive of no disastrous consequences.
Of the above formulæ, those bearing the name of the late Mr Marsh, of Woolwich, more especially deserve the attention of the pyrotechnist. To guard against the danger sometimes arising from the spontaneous combustion of coloured fires containing sulphur and chlorate of potash, Mr Saunders recommends intimately mixing 120 grains of bicarbonate of potash with each pound of sulphur before using it in the manufacture of any composition into which chlorates enter. See Flame, Pyrotechny, &c.
FISH. Syn. Pisces, L. Fishes form the fourth class of vertebrate animals (VERTEBRATA) in the Cuvierian arrangement of the animal kingdom, and in the variety of their genera and species are second only to the INSECTA, whilst in prolificness and number they probably exceed all other animated beings that reach a size equal to that of even the smallest member of their prodigious race. Besides their value to man as food, they furnish him with oil, isinglass, and various other articles of utility and luxury, and provide, either directly or indirectly, an inexhaustible supply of manure for the fertilisation of his fields. As food fish are undoubtedly wholesome and nutritious, although less so than the flesh of animals or the grains of the cereals. Of all the various substances used as aliments by man, fish are, however, the most liable to run into a state of putrefaction, and should therefore be only eaten when perfectly fresh or, if not recently taken, then only when their perfect preservation has been ensured by any of the ordinary methods employed for the purpose. Those that are the whitest and most flaky when cooked, as cod, flounders, haddock, hake, soles, turbot, whiting, &c., are the most easily digested; and those abounding in oily matter, as eels, herrings, mackerel, salmon, &c., are most nutritious, though the most likely to offend the stomach. Salt-water fish have been said to be more wholesome than river fish, but without sufficient reason. Salted fish are hard of digestion, unless when carefully cooked and well masticated. Skin diseases are said to be more common among those who live continually on fish than among those who abstain from it; but this probably arises from their use being unaccompanied by a proper quantity of fresh vegetables or fruit, both of which are scarcer on the sea-coast than further inland. As one of the components of a mixed diet, the value of fish is indisputable. Acid sauces and pickles are the proper additions to fish, from their power of retarding the progress of putrefaction, and of correcting the relaxing tendency of large quantities of oil and butter.
Artificial Propagation. The fecundity of fish is positively marvellous. According to the recent observations of Mr Frank T. Buckland, salmon yield about 1000 ova or eggs to every lb. of their weight; a trout weighing 1 lb. produced upwards of 1000; a mackerel (1 lb.), 86,120; a herring (1⁄2 lb.), 19,840; a sole (1 lb.), 134,466; a turbot (8 lbs.), 385,200; and a cod (20 lbs.), 4,872,000. The ova here spoken of form what is commonly called the ‘hard roe’ of the female fish; the ‘soft roe’ is ‘the milt’ of the male fish. To protect the spawn, and the fry, when hatched, is the object of the art of fish culture, which has made great progress during late years. When the spawn is not artificially protected, the greater portion is always wasted, being swept away by the742 stream, and devoured by fish, birds, and insects. The natural enemies of the newly hatched fish are, again, so numerous, that it is really surprising that any should escape destruction. According to given data and accurate calculations of the returns of fisheries made by Messrs Ashworth and Buist, only one salmon egg out of every thousand deposited ever becomes a fish fit for human food. Other fish, both fresh and salt water, suffer in proportion. The hatching of fish by artificial means has been carried out on a large scale in France, and has been commenced in Scotland and Ireland, and on a small scale in England. The spawning fish, having been caught by a net, is made to deposit her eggs by gently pressing on the abdomen; these are impregnated by ‘milt’ expressed from the male fish in a similar manner, and mixed with them in a shallow tub or other vessel prepared for the purpose. The impregnated eggs are placed in long shallow boxes, bottomed with gravel and pebbles, and so arranged that a small stream of water from a reservoir may flow from one to another. The time of hatching depends entirely upon the temperature of the water; from 40° to 45° Fahr. seems to be the healthiest temperature. After about 50 days (in the case of salmon), when all goes well, the young fish makes its appearance as a misshapen creature about an inch long, with a bag containing the yolk of the egg attached to its abdomen. At 3 days old the fry is about 2 gr. in weight; at 16 months it has increased to 2 oz. To preserve the young fish in health, the boxes must be covered with shades of slate or zinc. The French fish-breeders generally feed the young fry with boiled frogs powdered fine. The Scotch give boiled liver. Mr Buckland prescribes a diet of roe of sole, or plaice, or whiting. As to the age at which it is advisable to turn the young fish out of the nursery, there is much difference of opinion. Some breeders recommend turning them out as soon as the ‘umbilical bag’ is absorbed; others think they should be taken care of till they are older and stronger, and better able to defend themselves or escape from attack. For full details respecting the artificial propagation of fish, the reader is referred to Mr Buckland’s recent work, entitled ‘Fish-Hatching.’
Nutritive Value of Fish.—The white varieties of fish, such as whiting, cod, haddock, sole, plaice, flounder, and turbot, according to Letheby, contain only about twenty-two per cent. of solid matter, of which eighteen is nitrogenous. To increase their nutritive value, therefore, these fish should be eaten with butter.
According to the same authority mackerel, eels, and salmon are richer in fat than the above kinds; mackerel containing about seven per cent., and salmon about six, whilst the oily matter of eels amount to nearly fourteen per cent. The same is the case with the sprat, the herring, and the pilchard, as well as with most of our fresh-water fish.
As regards shell-fish, all the different varieties of them afford about the same amount of nutrition. They contain about thirteen per cent. of solid matter, which in composition is similar to that of white fish. Shell-fish vary in digestibility; mussels, limpets, and whelks being rather difficult of digestion, whilst scallops, cockles, periwinkles, lobsters, and crabs are a trifle more easy of digestion, and oysters still more so. All shell-fish are unsuited for delicate stomachs; although they are largely eaten by the poorer dwellers on the coast.
On the Continent, vineyard snails, and in China, slugs, are eaten, and are said to possess a delicate flavour and nutritive properties.[311]
[311] Letheby.
Choice, &c. “The flesh of any fish is always in the highest perfection, or in season, as it is called, during the period of the ripening of the milt and roe. After the fish has deposited the spawn, the flesh becomes soft, and loses a great deal of its peculiar flavour. This is owing to the disappearance of the oil or fat from the flesh, it having been expended in the function of reproduction.” (Fleming’s ‘Phil. Zoology.’) Fish should be dressed as soon after being caught as possible, as much of their peculiar delicacy and flavour is lost by keeping, even for a few hours. Turbot and salmon are said by the fishmongers to be improved in flavour when 2 or 3 days old, but this is surely a mistake, as the former, when dressed immediately after being caught, possesses a fine creamy taste, which it afterwards loses; whilst the latter, by the loss of a single tide, loses a portion of the fine white curd which is previously found between the flakes, and by longer keeping, this curd, with the larger flakes, disappear altogether. In the eyes of some epicures the richness, is however, increased by this change. Mackerel, and some other fish suffer so much from keeping only a few hours, that they become quite unwholesome. Herrings offer a remarkable example of the advantage of dressing fish as fresh as possible. When cooked soon after being caught, they possess considerable delicacy and flavour, but after being kept for only a few hours, the oil separates from the flesh, and they become soft, greasy, and strong-flavoured.
In the choice of every kind of fish, stiffness, brightness of the eyes, and redness of the gills, may be regarded as invariable signs of freshness. A peculiar elasticity will also be perceived in fish recently caught, little or no permanent impression being made by the ordinary pressure of the fingers, from the flesh immediately rising when the pressure is withdrawn. Fresh fish also lie in a partly curled position, and never quite straight, as in the case when they have been kept for some time.743 Thickness and fleshiness are deemed marks of the good condition of all fish.
Cleaning, dressing, &c. On the proper cleaning of fish preparatory to dressing it, depends much of its delicacy and flavour. Ordinary cooks seldom do this well, from not slitting the fish sufficiently open to permit the inside to be thoroughly washed, and seldom using sufficient water. The superior flavour of fish cleaned by the fishmongers arises from their performing the operation more completely, and from the large quantity of water they employ about them. The flavour of all fish is improved by adding a little salt or vinegar to the last water in which they are washed. The sound, milt, and roe, should be carefully cleaned and preserved.
Fish is preferably ‘dressed’ by simple boiling, broiling, or frying; in fact, the finer kinds of fish are often injured by the excessive interference of the cook. When boiled, “all large fish, with the skin whole, must be placed on the fire in cold water; if crimped, or cut into slices or pieces, in boiling water; if whole, it must not be covered with more than two or three inches of water, or the skin will crack, and not only spoil the appearance of the fish, but will diminish the gelatine and gluten it contains, and instead of eating firm and full of flavour, it will be soft and woolly, especially if over-boiled.” (Soyer.) As soon as a scum rises from boiling, it should be removed by the skimmer. The addition of a little salt or vinegar to the water improves the flavour of most fish, and renders the flesh firmer. The proportions should be “two teaspoonfuls of salt to every quart of water.” “If the fish be whole, as soon as it begins to boil remove the cover on one side, and let it simmer gently until done. (Soyer.) A fish is known to be sufficiently dressed by the flesh in the thicker parts separating easily from the bone. “If a large fish I generally try it by gently pushing a wooden skewer through the thickest part; if it goes in easily it is done.” (Soyer.) When this is the case it should be removed from the kettle, as by soaking in the water fish loses its firmness, and becomes soddened. Sole, skate, and mackerel, are usually put into boiling water, whether whole or sliced. Fish for broiling should be well washed in strong vinegar, wiped dry with a towel, and floured before placing them on the gridiron; and the bars of the latter should be hot, and well buttered. (Rundell.) Fish for frying should be prepared as for broiling, and the butter, oil, or lard should be allowed to boil for a minute or two before putting them into the frying-pan. The latter should be perfectly smooth and bright, and the butter or oil in abundance, to prevent the fish sticking to it and burning. As the fish are cooked solely by the heat of the melted fat, to fry them in the highest perfection there should be enough of it to cover them. Butter or oil is the best for the purpose. To avoid loss, the contents of the frying-pan, after the fish is removed, should be poured into a clean jelly-jar or basin, and reserved for another occasion. The fish being removed from the pan, the superfluous fat should be drained from them preparatory to ‘serving’ them. When fish is divided into fillets or cutlets before being cooked, it is usual to take out the bones, and to dress it with force-meat, &c.
In serving fish of the finer kinds, no other additions are required than melted butter and the ordinary fish sauces and pickles. The dishes are commonly garnished with raw parsley, for the sake of appearance, but boiled parsley, chopped small, should accompany it. All kinds of fish should be served on a napkin.
Caution. It sometimes happens that a fishbone accidentally swallowed remains in the œsophagus, and occasions serious inconvenience; in fact, instances have been known where so much irritation has arisen that death has followed. In such cases it is advisable, as soon as possible, to take of tartar emetic, 4 gr., dissolved in warm water, 1⁄2 pint; and immediately afterwards the whites of six eggs. The coagulated mass will not remain in the stomach more than two or three minutes, and the remedy has been known to “remove no less than 24 pins at once.”
FISH GLUE. See Glue and Isinglass.
FISH POISONING. See Accidents.
FISH SKIN. Syn. Shark skin. The skin of the spotted dog-fish or rough hound (chien de mer, Fr.), stretched and dried. Used for polishing wood and ivory. Several other varieties of fish skin are employed in the arts. The dressed skin of the ‘rousette’ (peau de rousette, F.), is transparent, and very beautiful. Cemented on green paper, and rubbed down and polished, it is used as veneer for fancy boxes. The skins of several varieties of Squalus are also used for both the above purposes. See Shagreen.
FIVE HERBS. See Species.
FIX′ATURE Syn. Bandoline, Clysphitique, Eau collante, Fixateur, Fr. This consists of any of the simple vegetable mucilages, combined with a little spirit, to preserve it, and with a little perfume, to render it more agreeable.
Prep. 1. From carrageen, Irish, or pearl moss, soaked in cold water for an hour or two, and after being drained, and pressed dry in a clean napkin, dissolved by boiling in soft water, q. s. The decoction is strained through cambric, and when nearly cold is mixed with about 1⁄3rd or 1⁄4th of its volume of eau de Cologne or other scented spirit, with the further addition of a few drops (5 or 6) of oil of cloves. Sometimes a little brandy is added to the mucilage, and when it is intended for present use, as is common with home manufactures, the spirit is frequently omitted altogether. 1⁄4 oz.744 of the prepared moss is fully enough for 3⁄4 pint of strained decoction, if rightly managed.
2. From quince seed boiled in water, as the last. 1⁄4 oz. yields nearly 3⁄4 pint of strained decoction.
3. Pale gum arabic (picked), 11⁄2 oz.; rose water, 2 fl. oz.; pure water, 3 fl. oz.; dissolve.
4. Gum arabic, 31⁄4 oz.; water, 1⁄2 pint; dissolve, and drop in eau de Cologne, gradually, until the cloudiness at first occasioned ceases to be removed by agitation; the next day decant the clear portion. All of the above are very superior, and keep well.
5. (Redwood.) Gum tragacanth, 11⁄2 dr.; water, 7 oz.; proof spirit, 3 oz.; otto of roses, 10 drops; macerate 24 hours, and strain.
6. Malt, 7 oz.; hot water (that will barely permit the finger to be held in it without pain), 1⁄2 pint; infuse in a covered jug or basin, gently press out the liquid, and as soon as cold add of proof spirit (or brandy or Cologne water), 21⁄2 fl. oz. and strain.
Obs. Bandoline is used by ladies and by hairdressers for stiffening the hair, and to make it curl firmly and remain in place. It is applied either by moistening the fingers and passing the hair through them, or by means of a small sponge. See Pommade.
FIXED AIR. See Carbonic Acid.
FIXED OILS. See Fat and Oils.
FLAKE WHITE. See White Pigments.
FLAME. Gas or vapour in an incandescent state. The light emitted from pure flame is exceedingly feeble; illuminating power being almost entirely dependent upon the presence of solid matter. See Illumination, and below.
Flame Colours. The vapours of metallic compounds communicate colours to flames. The characteristic colours of some metals are very beautiful, and their exhibition forms a favorite experiment of chemical lecturers. The coloured flames are generally produced by the combustion of alcohol or rectified spirit upon certain salts in fine powder. In this way a GREEN colour is communicated by boracic acid or chloride of copper; a RED one by the nitrates of iron, lime, or strontia; a VIOLET, by potassa and its salts; and a YELLOW, by nitrate of soda. Messrs Church and Crookes have recently described a mode of exhibiting the characteristic flames of the metals which is admirably adapted for the lecture-table.[312] ‘Gun-paper,’ made in the same way as ‘gun-cotton,’ is to be soaked in solutions of the chlorates of the different metals, dried with care, and kept dry. A good ‘gun-paper’ for the purpose is prepared by soaking strips of Swedish filtering-paper for ten minutes in a mixture of 4 parts oil of vitriol with 5 parts strong nitric acid, both by measure. The strips, when taken out of the acid, should be washed first with cold, and then with hot rain or distilled water, till the washings are no longer sour to the taste. The solutions of the metallic salts need not be very strong; but if they are warm, the strips of ‘gun-paper’ will be more easily and completely saturated with them. Since some of the chlorates attract moisture from the air, it is better to dry the papers prepared with them before the fire previous to lighting them. They are shown to best advantage when a strip is loosely crumpled up into a pellet, lighted quickly at one corner, and thrown up into the air against a dark back ground. They leave after burning, if properly prepared, no ash whatever. Paper prepared with the salt of potassa gives a flash of VIOLET flame, that prepared with the soda salt the characteristic YELLOW flame, and that with chlorate of baryta a very beautiful GREEN light. The chlorates of strontium, lithium, and calcium, when thus ignited, give intense colours. The VIOLET-BLUE flame of copper is well seen, even with the chloride of that metal, while paper soaked in nitrate of potassa shows the potassium flame better than if the chlorate be used. ‘Gun-paper’ prepared with a very weak solution of chloride or chlorate of thallium shows the characteristic SPRIG-GREEN flame of that metal with great distinctness. Chlorate of barium, being an article of commerce, may be employed for the preparation of the other chlorates, it being merely necessary to add to this salt in solution an exactly equivalent quantity of the sulphate or carbonate of the metal whose chlorate is desired. For instance, in order to make ‘chlorate of copper,’ 15·1 gr. of chlorate of barium being dissolved in hot distilled water, a boiling solution containing 12·5 gr. of pure crystallised sulphate of copper is to be added to it. Insoluble white ‘sulphate of baryta’ falls, while the solution, filtered and evaporated, yields the new chlorate in crystals. See Fires, Pyrotechny, &c.
[312] See ‘Intellectual Observer,’ April, 1863.
FLAN′NEL. It has been shown by the experiments of Count Rumford that the conducting power of the different materials employed for clothing varies considerably. A thermometer surrounded with cotton wool, and heated by immersion in boiling water, took 1046 seconds to lose 135° Fahr., when plunged into a bath of melting ice; but, under the same circumstances, when sheep’s wool was employed, 1118 seconds elapsed before a like sinking of the thermometer took place (‘Phil. Trans.,’ 1792); thus showing the greater conducting power of the former, and consequently the superiority of the latter substance for the manufacture of warm clothing. But the chief advantage of wool, as an article of underclothing, depends less upon its actual power of conducting heat than its peculiar texture. Flannel acts as a gentle stimulus on the skin, and exercises the most beneficial action, by keeping the pores clean, and in a state most favorable to perspiration. This action is a species of friction similar in character, although inferior in degree, to that of the common flesh-brush or horse-hair glove, so long employed as a skin stimulant. Flannel has also the advantage745 of absorbing the perspiration as soon as emitted, and allowing its watery portion to pass off into the atmosphere almost as soon as formed, but this is not the case with cotton and linen fabrics. The different effects of flannel and linen are particularly susceptible during brisk exercise. When the body is covered with the former, though perspiration be necessarily increased, the perspired matter freely passes off through the flannel, and the skin remains dry and warm. If the same exercise be taken in linen shirts, perspiration, as in the former case, is indeed also increased, but the perspired matter, instead of being dispersed into the atmosphere, remains upon the linen, and not only clogs the pores of the skin, but gives a disagreeable sensation. From this property of flannel, persons who wear it next the skin seldom catch cold from changes of temperature, even though perspiring profusely; but in similar cases, when linen or calico shirts are worn, chilliness immediately comes on, followed by sniffling, sneezing, and cough, and all the other symptoms of severe catarrh.
The common objections raised against the use of flannel are founded on vulgar prejudices, ignorance, obstinacy, or bravado, and are undeserving of the notice of sensible people. In a fickle and moist climate like that of England, every person should wear a robe of flannel next the skin, or at all events a waistcoat of flannel reaching below the loins; and this should not be discarded as soon as the cold weather has passed, but its use should be continued all the year round; for in reality flannel is, if possible, even more required in summer than in winter, because persons perspire more freely in hot than in cold weather, and are consequently more susceptible of cold, while at that period of the year their clothing is less capable of protecting them from the effects of sudden changes of temperature and draughts of cold air, moisture, &c. Females, children, persons of delicate constitutions, and all others who from their habits of body or life perspire freely, or are much exposed, should wear flannel.
In washing flannels it is recommended that they should only be put into warm water, by which method their colour will be preserved, and they will be prevented from shrinking.
FLASH. Prep. From burnt-sugar colouring, 1 gall.; fluid extract of capsicum or essence of Cayenne, 3⁄4 pint, or enough to give a strong fiery taste. Used to colour spirits, and to give them a false strength. It is made by the brewers’ druggists, and labelled ‘ISINGLASS AND BURNT SUGAR,’
FLASKS. The late lamented and ingenious Mr Fownes suggested the employment of Florence oil-flasks as cheap substitutes for retorts, receivers, digesters, and some other vessels used for chemical purposes. His plan was to cut the neck smoothly round with a hot iron, and softening it in the flame of a good argand gas-lamp, to turn over the edge so as to form a lip, or border. The neck will then bear a tight-fitting cork without splitting.
FLATULENCE. Syn. Flatulency, Wind. In pathology, a morbid collection of gas in the stomach and bowels. Its most common cause is indigestion. When the natural fluids of the stomach are secreted in a healthy state, they exercise an antiseptic and digestive action on the food, by which it is speedily reduced to a magma that is little liable to spontaneous change whilst in the body; but when the reverse is the case, fermentation soon commences, and the stomach and associated viscera become distended with gas, and all the well-known symptoms of flatulency are developed in rapid succession. The quantity of gas thus accumulated in the ‘primæ viæ’ is often enormous. An ordinary apple during fermentation yields about 600 times its bulk of gas, and many vegetables yield much more. (Dr Hales.) It is, therefore, not at all surprising that so much inconvenience should be felt when the food, instead of being digested and assimilated, runs into the state of active fermentation.
The treatment of flatulency consists mainly in the selection of proper articles of food. Oleraceous vegetables, peas, beans, under-dressed potatoes, and indigestible fruits should be especially avoided, as well as the use of large quantities of weak or warm liquids. The diet should consist principally of animal food, carefully but not over-cooked, with a sufficient quantity of good mealy potatoes (mashed, not whole), and good wheaten meal-bread, moderately seasoned with common salt and spices. The most suitable beverages are toast-and-water, and a little good brandy largely diluted with water. The healthy tone of the stomach may be re-established by the proper use of tonics, bitters, and mild aperients.
To relieve the fit of flatulency, carminatives and aromatics, as black pepper, mustard, peppermint, ginger, cinnamon, lavender, and most spices, may be had recourse to. A glass of peppermint cordial, or of brandy strongly flavoured with peppermint or ginger, is a popular and efficient remedy. A few drops (15 to 30) of ether, with a little tincture of capsicum or spirit of sal volatile, seldom fail to give relief. See Dyspepsia.
FLAVOURING SUBSTANCES. See Essence, Oil (Volatile), Spice, Wine, &c.
FLAX. See Linen, Linseed, and Oil.
FLEA. This troublesome little animal is the Pulex irritans of Linnæus, and belongs to the Suctoria, or fourth order of the Insecta. Its favorite haunts are our warm underclothing, and its most productive breeding-places are in the ‘flue’ which careless servants allow to accumulate underneath our beds. Cold, light, perfumes, and ventilation, are inimical to its propagation.
FLECHTENKAPSELN (Tetter Capsules, or Dr Berkeley’s Antiherpetic Capsules for Skin746 Diseases, Tetter, &c.) Capsules filled with tar. (Hager.)
FLECHTENMITTEL—Tetter Cure. (Paris). 1. A Washing Fluid.—Common water, containing 11⁄2 per cent. sulphuric acid. 2. A Salve. A mixture of lard and spermaceti, with 1⁄24 of their weight of calomel. (X. Schmidt.)
FLECHTENPULVER—Tetter Powder (St Lube’s, France). Nitre, 100; antimony chloride, 10; antimony oxide, 200, 1·5 grammes for a dose. (Wittstein.)
FLECHTENSALBE—Tetter Salve (Fontaine, Paris). For all skin diseases. Olive oil and white wax, with 1⁄16 of white precipitate. (Wittstein.)
Flechtensalbe (Bruno Reichel, Apolda). A mixture of wax and lard coloured green. (Schädler.)
Flechtensalbe (F. Schwarzlose, Berlin, and S. G. Schwartz, Breslau). For salt-flux, tetters, and similar skin diseases. Peru balsam, 1; carbolic acid, 2; yellow wax, 10; lard, 30. (Schädler.)
Flechtensalbe (Surbi, Paris). For all kinds of skin diseases. A mixture of beef tallow, 30; olive oil, 10; zinc oxide, 2; steatite, 2. (Wittstein.)
FLECHTENSEIFE, Tetter Soap (Dr Berkeley). Ordinary tar soap. (Hager.)
FLECHTENWASSER. The wonderful wholesome mineral vegetable tetter-water (Dr A. von S.). Corrosive sublimate, ·25 grammes; water, 180 grammes; benzoin tincture, 6 grammes. (Weber.)
FLECKENWASSER (Bronner). Cleansing fluid (literally spot or stain water) for the removal of grease and dirt spots. Benzine only.
Fleckenwasser, Englisches (English cleansing fluid for removing acid, resin, wax, tar, and grease spots.) A mixture of 95 per cent. alcohol, 100 grammes; liq. ammon., sp. gr. ·875, 30 grammes; benzine, 4 grammes. (Artus.)
FLEISCH-EXTRACT-LIQUEUR (Eau de Vie Alimenteuse—Extract of Meat Liqueur—Aqua Vitæ Incarnativa) (A. Hensel, Berlin). A beautiful red spicy liquor, leaving, when distilled, 32 per cent. of solid matter. This residue contains in 100 parts (besides anilin-red), resin and extractive (partly from ginger and partly from cinnamon), 31⁄4; sugar, 271⁄2; extract of meat, 11⁄4. (Hager.)
Fleisch-Extract-Syrup (Syrup of Extract of Meat) (Meyer, Berk). Blood-serum made into a syrup with sugar. (Hager.)
FLEISCHFASER-ZWIEBACK FÜR HUNDE—Fibrin Dog Biscuits (New York). Said to be made of pure meal, fibrin, dates, and other ingredients, and recommended as an excellent food for dogs. According to the prospectus its use makes all other foods unnecessary, as it gives the animals peculiar endurance, strong muscles, and sound bones. The directions for use say that it is most advantageously given in its unprepared form, as dry, heavy, hard cakes, and only in case it is refused should it be softened for a short time in cold water. According to the analysis performed in the laboratory of the Poppelsdorf Agricultural Academy the proportion of nitrogenous to non-nitrogenous ingredients is 1 to 3·70. Microscopic analysis detects the presence of dried fibrin, and also a considerable admixture of structureless hyaline cartilaginous matter. From this it follows that the nitrogen revealed by analysis does not all represent protein or fibrin, and that the proportion which arises from indigestible gelatinous matter will be of smaller value. (Dr E. Kern.)
FLESH. Syn. Caro, L. The muscular substances of animals; the softer, solid portions of the body, as distinguished from the bones and fluids. See Fibrin, Food, &c.
Flesh-brush. This simple instrument is used for exciting the cutaneous circulation. Those which have the bristles set on a leather back are esteemed the best. The flesh-glove or hair flesh-rubber is a useful modification of the common flesh-brush. Those manufactured by Messrs Savory and Moore, in imitation of the Indian kheesah or mitten, are superior to all others. In the absence of both flesh-brush and glove, a rough towel wound round the hand is no bad substitute. See Friction.
FLIEGENPAPIER, GIFTFREIES—Non-Poisonous Fly Papers (Bergmann & Co., Rochlitz). Contains abundance of arsenious acid. (Hager.)
FLIEGENPULVER—Fly Powder (Baumann, now Markel, Austria). 93 to 94 per cent. of dry sandy ferruginous clay (ordinary loam) saturated with a decoction of some bitter substance, as quassia or gentian. (Hager.)
FLIES. See Fly.
FLIP. See Egg Flip.
FLÖHEMITTEL—Flea Powder (Leipsic). Powdered soap. (Fischer.)
FLÖHEWASSER—Flea Water (Koch, veterinary surgeon, Vienna). 7 brandy, 1 benzine, 1 black soap. (Hager.)
FLORILINE—Vegetable Tooth Paste made by John Yates (Albin Müller, Brünn). It is contained in a quadrangular china pot, and is a red, dry, rather hard mass made from prepared chalk, 20 grammes; starch powder, 10 grammes; glycerin, 8 grammes; pellitory tincture, 3 grammes; peppermint oil, 10 drops; and water q. s., coloured with Florentine lac. (Hager.)
FLOUN′DER. A flat fish, very like the plaice, but smaller, and of more obscure colour. It is very common about the British coast, and is found in the Northern, Baltic, and Mediterranean seas. Its flesh is very wholesome.
FLOUR. Syn. Farina, L. The finely ground and ‘dressed’ meal of bread corn, and of the seeds of some of the leguminosæ. That known specifically as ‘flour’ in this country is747 obtained from spring varieties of Triticum vulgare (the common wheat).
Var., &c. Of varieties of flour there are several, depending chiefly on the amount of bran which they contain, and the relative fineness of the sieves through which they are passed:—
Fine wheat flour, Pastry flour; Farina, F. tritici, F. seminis tritici. The finest flour, obtained from the meal produced in the first grinding of wheat between sharp stones, by means of a sieve of 64 wires to the inch. Used for pastry.
Middlings. The remainder of the flour of the first grinding, obtained by means of a slightly coarser sieve. Used for making household bread, but is mostly reground for the next variety.
Seconds. The finest part of the flour, obtained by regrinding ‘middlings’ between blunt stones. Used by the bakers for their finest wheaten bread.
Pollard. The coarse flour, from which the seconds has been sifted. Used for making sea biscuits and gingerbread, and to fatten poultry and hogs.
Country household flour. This is usually ground only once, and sifted to 4⁄5ths of the weight of the wheat.
Ammunition flour is ground and sifted to nearly 5⁄6ths the weight of the wheat.
According to Mr Accum, thirty-two pecks of wheat in the London mills yield, of flour 381⁄2 parts; pollard, 8 parts; and bran (furfur tritici), 12 parts; the bulk of the wheat being doubled by grinding.
According to Mr Hard, miller, of Dartford, quoted by Dr Pereira, the wheat having been ground in the usual way, is allowed to remain in the state of meal for some time before ‘dressing,’ which removes the heat caused by the process, and enables the miller to obtain more flour, and the baker a better quality, than if ‘dressed’ immediately it is ground.
“The process of dressing is by a wire cylinder containing a certain number of sheets of different texture or fineness, which cylinder contains eight hair brushes attached to a spindle passing through the centre of the cylinder, and laid out so as to gently touch the wire. This cylinder is fed by a ‘shoe’ with the meal; then the ‘flour’ and ‘offal,’ after passing through the wire in this way, are divided by wooden partitions fixed close to the outside of the cylinder.” “The produce of the wheat-meal dressed through the wire machine consists of—1, Flour;—2, White Stuff, or Boxings, or Sharps;—3, Fine Pollard;—4, Coarse Pollard, or Horse Pollard;—5, Bran. The 2nd product (i. e. the white stuff) is then submitted to another ‘dressing’ through a fine cloth machine, and produces—1, Fine Middlings, for biscuits;—2, Toppings, or Specks;—3, Dustings;—4, Best Pollard, Turkey Middlings, or Coarse Middlings.
| Flour | 392 | lbs. |
| Biscuit or fine middlings | 10 | ” |
| Toppings or specks | 8 | ” |
| Best pollard, Turkey p., or twenty-penny | 15 | ” |
| Fine pollard | 18 | ” |
| Bran and coarse pollard | 50 | ” |
| Loss by evaporation and waste | 11 | ” |
| —— | ||
| 504 | ” | |
| —— |
| Peligot. Mean of 14 Analyses. | Letheby. | Payen. | Wanklyn. Fine Wheaten Flour. | |
| Per cent. | Per cent. | Per cent. | Per cent. | |
| Water | 14·0 | 15·0 | 14·22 | 16·5 |
| Fat | 1·2 | 2·0 | 1·25 | 1·2 |
| Nitrogenous matters, gluten, &c. | 12·8 | 10·8 | 14·45 | 12·0 |
| Ditto, soluble in water | 1·8 | — | — | — |
| Non-nitrogenised substances, dextrin, sugar, &c. | 7·2 | 70·5 | 68·48 | 69·6 |
| Starch | 59·7 | |||
| Cellulose | 1·7 | |||
| Salts (ash) | 1·6 | 1·7 | 1·6 | 0·74 |
According to Vauquelin, French wheat flour contains about 10% of water, 11% of gluten, 71% of starch, 5% of sugar, and 3% of gum; and the water of the dough amounts to about 50%. The quantity of the bran in wheat ranges under 2%.
Pur. This article of food is very frequently adulterated both by the miller and the baker, as has been before alluded to in the article on bread. The principal physical characteristics of wheat flour of good quality are the following—it has a dull white colour, somewhat inclining to yellow;—it exhibits no trace of bran, even when pressed smooth with the hand,748 or with a polished surface;—its cohesiveness is so great that, on being squeezed in the hand, the lump is some time before it loses its shape;—it has a homogeneous appearance, and does not lose more than from 6% to 12% by being carefully dried in a stove. The smaller the loss in this way the finer is the quality, other matters being equal, and the more economical in use.[313] (See below.)
[313] See also Bread, Adult, and Exam.
Tests. 1. Solution of ammonia turns pure wheat flour yellow; but if any other corn has been ground with it, pale brown; or if peas or beans have been ground with it, a still darker brown.
2. Solution of potassa, containing about 12% of caustic alkali, dissolves pure wheat-flour almost completely; but when it is adulterated with the flour of the leguminous seeds (beans, peas, &c.), the cellulosæ of these substances remains undissolved, and its hexagonal tissue is readily identified under the microscope. Mineral substances (chalk, plaster of Paris, bone dust, &c.) are also insoluble in this test, and appear as a heavy white sediment.
3. Boiling water poured on the sample causes the evolution of the peculiar odour of pea or bean flour when these substances are present. Bread made with such flour evolves a like odour on being toasted.
4. Pure hydrochloric acid poured on potato flour, or on wheat flour adulterated with it, develops a smell of rushes; it also dissolves starch, but changes the colour of pure wheat-flour to a deep violet.
5. Nitric acid turns wheat flour of an orange-yellow colour, but forms a stiff and tenacious jelly with potato fecula, the colour of which it does not alter.
6. A portion of the suspected sample submitted to dry distillation in a stoneware retort, and the distillate collected in a receiver containing a little water, the latter is found to remain perfectly neutral if the wheat flour is pure, but acquires a distinctly alkaline reaction when beans, pulse, or pea meal is present. (Rodrigues.)
7. Triturate 300 gr. of the sample with an equal weight of clean siliceous sand, and after five minutes form a homogeneous paste with water; afterwards further adding more water, until about 2 fl. oz. have been used. The filtered liquid, treated with an equal quantity of a strong and pure aqueous solution of iodine, develops a pink colour, which gradually disappears when the specimen examined consists of pure wheat flour; but assumes a deep-purple colour, which disappears much more slowly, if the flour is adulterated with even 10% of fecula or potato flour. This test succeeds, not only with flour and meal, but also with macaroni, vermicelli, &c. (M. Chevallier.)
8. The milky liquid holding the starch in suspension (see Anal., page 749) is poured into a small conical glass, and left at rest for some time; the clear liquid is then decanted, and any remaining water carefully sucked up with a pipette, and the whole left for some time, in order that the deposit may harden. The upper gray layer is next removed with a teaspoon, and the harder and stiffer second layer left undisturbed until it becomes quite solid by drying. When in this state, it may be upset in the form of a cone, upon a lump of dry plaster. The fecula or potato starch (if any is present), being heavier than that of wheat, forms the apex of the cone, and its quantity may be estimated in the following manner:—The operator cuts from the apex of the little cone above mentioned a slice, which he triturates only for a short time in an agate mortar (one of glass, or porcelain, or wedgwood-ware, will not do), and he tests that with aqueous solution of iodine. If it turns blue, it is fecula. Another slice is treated in the same manner, until the operator comes to the wheat starch, which, in the present instance, is not affected by the aqueous solution of iodine. This difference of behaviour of the two species of starch with iodine is due to the friction of the pestle and mortar, which is sufficient to divide or tear the envelopes of the particles of the potato starch, which then become blue when treated by solution of iodine. The particles of wheat starch, on the contrary, are not disaggregated by that treatment, and being therefore protected by their envelope, are not acted upon by the solution of iodine, or, at most, assume only a brown tinge. (M. Robine.)
9. Wheat flour adulterated with plaster of Paris, ground bones, chalk, and potato flour, has a higher specific gravity than a sample of the pure flour. This may be readily ascertained by any person by filling a small vessel with some pure flour, and then with the given sample. “A vessel which will contain 1 lb. of wheat flour will contain 11⁄2 lb. of fecula” (potato flour), and hence “the proportion of this adulteration may be easily estimated.” (Ure.)
10. If to a sample of wheat flour is added a solution of potassa, containing about 13⁄4% of the pure alkali, the granules of potato farina, or of bean meal, or pea meal, present (if any), will acquire 4 or 5 times their original volume, while those of the pure wheat starch will be scarcely affected by it. This change is very perceptible under a microscope of small power. 2 parts of liquor of potassa (Ph. L.) and 5 parts of distilled water form a mixture that answers for the above purpose.
11. By means of the microscope the admixture of the cheaper feculas and meals with wheat flour is readily detected by the characteristic appearance of the starch grains; and when the adulteration exceeds 9% or 10%, its extent may be readily estimated with considerable accuracy. As the range of adulteration is generally from 12% to 27%, this method is applicable in the greater number of cases.
749
Analysis. The value of wheat flour as an aliment depends upon the quantity of gluten, sugar, starch, and phosphate of lime, which it contains; and its superiority over the flour of the grains of the other cereals is referred to its containing a larger proportion of the first and last of these substances than they do. The quantitative analysis of flour is very simple, and may be easily made by persons unacquainted with chemistry, by attending to the instructions below:—
a. Make 1000 gr. of the sample into a dough with a little water, let it rest an hour and then gently knead it in successive water, until the starchy particles are perfectly removed. Collect the portion (GLUTEN) left in the hand, drain off the water, place it on a piece of filtering or blotting paper, several times doubled, and set it aside.
b. Mix the several waters employed in the preceding process, and set them aside in a tall vessel, to deposit the suspended portion (STARCH). After a sufficient time pour off the clear liquid, and throw the whole of the sediment on a weighed paper filter, placed in a funnel, observing to remove the portion adhering to the bottom of the vessel by means of a little clean water, that none may be lost.
c. Evaporate the decanted liquid, as well as what runs from the filter, until it becomes curdy, then filter it through a piece of weighed blotting paper, and preserve the sediment (ALBUMEN); next evaporate the residuum to the consistence of a syrup, agitate it with 10 times its weight of alcohol, and filter, observing to wash the paper filter clean with a little alcohol after the solution has passed through it. The substance on the paper is PHOSPHATE OF LIME and GUM, and must be set aside. By subsequent digestion in water, filtration, and evaporation, the two may be obtained separately.
d. Evaporate or distil off the spirit from the solution and washings, as above; the residuum is SUGAR.
e. Dry the substances educed as above, by a gentle heat, and weigh them. The weight of the albumen may be taken with that of the gluten, as it possesses about the same nutritive value, and also because it has been asserted by some persons that the former substance is in reality gluten, and not albumen. By dividing the given weights by 10, the per-centage value of the sample is obtained. The pieces of filtering paper employed should be carefully dried and weighed before using them, and the same degree of heat should be employed for this purpose as that to which they will be afterwards exposed in the drying of the substances resulting from the operations.
Obs. The above method of ascertaining the actual value of any sample of flour as an article of food, though not strictly accurate, approximates sufficiently to the truth for all practical purposes, and is well adapted to the wants of the baker and large purchaser. In many cases it will only be necessary to perform the first part of the process (a), which will give the per-centage of the most important constituent of the flour; the rest being of minor consequence.
In addition to what has been already stated in the article on Bread, it may be useful to mention that a pound of the best flour, from thoroughly dried wheat, will take 10 fl. oz. of water to form it into ordinary dough, or 9 fl. oz. to form it into bread dough. Under the old parliamentary acts, a sack of flour (280 lbs.) was presumed to produce 80 loaves (quartern or quarter-peck), the weight of which, within 48 hours after being baked, was to be 4 lbs. 51⁄2 oz. each. At the present time fully 92 loaves, weighing 4 lbs. each, are produced by the London bakers from one sack of flour, when honest weight is given; but as the latter is rarely the case, and the bread is frequently ‘slack’ or ‘under-baked,’ and thus contains more water than good bread ought to do, a much larger product is commonly obtained. The dough loses about 1⁄7th of its weight in baking, if in batches; but fully 1⁄6th, if baked in small loaves, and placed in the oven separately. The best bread contains about 1⁄4-1⁄6th of its weight of added water; and common bread, often much more than 1⁄4th. The proportion of water in the London bread has greatly increased during the last few years, owing to the introduction of the fraudulent plan of making the dough with rice jelly or moss jelly. This is the reason why the bread of some bakers suffers such a loss of weight in a few hours after being taken from the oven. A 4 lbs. loaf of bread purchased from a baker at Lambeth, after remaining on the sideboard of a sitting-room for 24 hours, was found to have lost no less than 61⁄2 oz. by evaporation, and in two days longer its interior cells were covered with green mould, and the whole was unfit for food. The bakers, aware of these facts, are particularly careful not to bake more bread than they can dispose of whilst ‘new,’ and are in the habit of refusing to weigh their bread before selling it, when it is more than 10 or 12 hours old, although they are liable to be ‘fined’ for such a refusal. See Bread, Cakes, Farina, &c., also below.
Flour, Baked. Syn. Farina tosta, F. tritici tosta, L. Prep. From wheat flour, carefully baked in a ‘slack’ oven, until it acquires a pale-buff hue. Astringent; used to make food for infants troubled with diarrhœa. See Farina.
Flour, Barley (Prepared). Syn. Farina hordei preparata, L. Prep. (Ph. Bor.) From barley flour, compressed into a tin cylinder until the vessel is 2-3rds full, which is then suspended at the upper part of a still 2-3rds filled with water, and after the ‘head’ is fitted on, the water is kept boiling for 30 hours (2 days of 15 hours each). Lastly, the upper layer being removed, the rest is reduced to powder, and kept in a dry place.
750
Flour, Boiled. Syn. Triticina, Farina Preparata, L. Prep. From fine flour, tied up in a linen cloth as tight as possible, and after it has been frequently dipped into cold water, the outside of the cloth is dredged over with flour, until a crust is formed round it, to prevent the water soaking into it whilst boiling; it is then boiled for a long time, and when cold, it is divided into small oblong pieces. For use, it is reduced to powder, either by grinding or grating it, and is then prepared like arrow-root. It forms a good diet for children, in diarrhœa, &c.; and as it may be easily prepared at home, it has the advantage of being free from adulteration.
Flour, Jones’s Patent. Prep. From kiln-dried flour, 1 cwt.; tartaric acid, 101⁄2 oz.; mix thoroughly; after 2 or 3 days, add, of bicarbonate of soda, 12 oz.; lump sugar, 1⁄2 lb.; common salt, 11⁄2 lb.; mix, and pass the compound through the ‘dressing-machine.’ It is necessary that the whole of the ingredients should be perfectly dry, and separately reduced to fine powder before adding them to the flour. By simply mixing it with cold water, and at once baking it, it produces light, porous bread.
Obs. We have already had occasion to pay a passing tribute to the excellence and usefulness of Jones’s Patent Flour.[314] It is, indeed, invaluable to every household, as furnishing the means of producing, with great economy, and extemporaneously, not merely cakes, puddings, pastry, and fancy bread, but the ‘staff of life’ itself, household bread, of a purity, flavour, and lightness, seldom, if ever, met with in that purchased of the bakers.
Flour, Sewell’s Patent. a. (No. 1.) Flour, 1 sack (280 lbs.); hydrochloric acid (sp. gr. 1·14), 45 oz.; mix, by adding the acid in a ‘spray,’—b. (No. 2.) To the last, add (expertly) bicarbonate of soda, 39 oz.; mix thoroughly, and pass the whole through a sieve or ‘dressing machine.’
Obs. This flour is used as the last, to which, however, it is inferior in quality. No. 1 will keep 5 weeks. No. 2 will keep a month. Jones’s flour will keep good in a dry place for years. If No. 1 is alone employed for the dough, to each pound of the flour, 65 gr. of bicarbonate of soda, with salt q. s., must be added. The patentee claims for his invention the merit of the soda and acid being converted into culinary salt in the process of making up the flour and baking the dough.[314]
[314] See Unfermented Bread.
FLOWER DEW (F. J. Weber, successor of Rau, Bamberg). A flat bottle with the name of Rau moulded on it; its gross weight is more than 80 grammes, but it contains scarcely 22 grammes of a nearly colourless but slightly yellow fluid, consisting of a pleasant aromatic solution of oils of bergamot, lemon, orange flowers, and rose in strong spirit.
FLOWERS. Syn. Flores, L. These beautiful and fragrant ornaments of our gardens and our dwellings are too highly esteemed by all classes of the community to require anything in favour of their cultivation to be said here. Our remarks will, therefore, chiefly refer to their collection, improvement, and preservation.
‘Full’ or ‘double flowers,’ or those in which the internal organs become petals, are so much more beautiful than the ‘single flowers’ of the corresponding species and varieties, that their production, with tolerable ease and certainty, has long been a desideratum with both the professional and amateur florist. Various plans have been proposed having this object in view, among which are the following:—1. The use of the best seed only, but not before it is at least 3 or 4 years old. 2. The selection of the outer row of seed only, and its careful preservation intact for at least 2 seasons before sowing it. We are assured that this method is particularly successful with dahlias. 3. The removal of the plants to a shady situation as soon as the flower-buds begin to develop themselves, and stinting them with water and nourishment for a few weeks. In this method a few only of the buds are permitted to mature; the rest being snipped off with a pair of scissors as early as possible. 4. The use of small pots and a scanty supply of water until the flowers are partly developed, when water is supplied in abundance, with or without the addition of a little liquid manure.
To hasten the blooming of flowers, it is a common practice with some gardeners to grow them in as small pots as is consistent with their healthy existence, and carefully to avoid transplanting them to larger pots, for several weeks before their usual time of blossoming. A plant on the point of flowering, if transferred to a larger pot and a richer soil, immediately commences making roots and leaves, whilst the embryo flowers either wholly decay, or their development is checked until the usual season of their production has passed over.
The following liquid has been used with great advantage to promote the vigorous growth and the early flowering of plants:—Sulphate or nitrate of ammonia, 4 oz.; nitrate of potassa, 2 oz.; sugar, 1 oz.; hot water, 1 pint; dissolve and keep it in a well-corked bottle. For use, put 8 or 10 drops of this liquid into the water of a hyacinth glass or jar, for bulbous-rooted plants, changing the water every 10 or 12 days. For flowering plants in pots, a few drops must be added to the water employed for them. The preference should be given to rain water for this purpose. The fluid sold under the name of liquid guano may be used in the same manner.
Flowers may be preserved in a fresh state for a considerable time, by keeping them in a moist atmosphere. When growing on the parent stem, the large amount of evaporation from the surface of their leaves is compensated for by an equivalent proportion of moisture751 supplied by the roots; but when they are plucked, the evaporation from the surface continues, while the supply of moisture is cut off. To supply, in part, this loss of moisture by evaporation, has arisen the almost universal practice of placing flowers in water; but their mutilated stems possess a far inferior power of sucking up fluids to that of the roots, and thus their decay is only deferred for a time. To preserve them more effectually, or at least to render their existence less ephemeral, we may surround them with a moist atmosphere, by which the loss of water from the surface of their leaves will be reduced to the smallest possible amount. “It is now eighteen years ago since we first saw, in the drawing-room of a gentleman, in the hot dry weather of the dog-days, flowers preserved day after day in all their freshness by the following simple contrivance—A flat dish of porcelain had water poured into it. In the water a vase of flowers was set; over the whole a bell-glass was placed, with its rim in the water. This was a ‘Ward’s case’ in principle, although different in its construction. The air that surrounded the flowers being confined beneath the bell-glass, was kept constantly moist with the water that rose into it in the form of vapour. As fast as the water was condensed it ran down the sides of the bell glass back into the dish; and if means had been taken to inclose the water on the outside of the bell-glass, so as to prevent its evaporating into the air of the sitting-room, the atmosphere around the flowers would have remained continually damp. We recommend those who love to see plenty of fresh flowers in their sitting-rooms in dry weather to adopt this method. The experiment can be tried by inverting a tumbler over a rose-bud in a saucer of water.” (‘Gardener’s Chron.’)
Another method by which some flowers may be preserved for many months is to carefully dip them, as soon as gathered, in perfectly limpid gum water, and after allowing them to drain for 2 or 3 minutes, to set them upright, or arrange them in the usual manner in an empty vase. The gum gradually forms a transparent coating on the surface of the petals and stems, and preserves their figure and colour long after they have become dry and crisp.
Yet another method (given in the ‘Pharmaceutical Journal’) is as follows:—“A vessel with a movable cover is provided, and having removed the cover from it, a piece of metallic gauze of moderate fineness is fixed over it, and the cover replaced. A quantity of sand is then taken sufficient to fill the vessel, and passed through a sieve into an iron pot, where it is heated with the addition of a small quantity of stearin, carefully stirred so as to thoroughly mix the ingredients.
“The quantity of stearin to be added is at the rate of half a part to 100 parts of sand. Care must be taken not to add too much, as it would sink to the bottom and injure the flowers. The vessel with its cover on and the gauze beneath it is then turned upside down, and the bottom being removed, the flowers to be operated upon are carefully placed on the gauze and the sand gently poured in, so as to cover the flowers entirely, the leaves being thus prevented from touching each other. The vessel is then put in a hot place, such, for instance, as the top of a baker’s oven, where it is left for 48 hours. The flowers thus become dried, and they retain their natural colours. The vessel still remaining bottom upwards, the lid is taken off, and the sand runs away through the gauze, leaving the flowers uninjured.
Faded flowers may be generally more or less restored by immersing them half-way up their stems in very hot water, and allowing them to remain in it until it cools, or they have recovered. The coddled portion of the stems must then be cut off, and the flowers placed in clean cold water. In this way a great number of faded flowers may be restored, but there are some of the more fugacious kinds on which it proves useless.
Flowers may be produced in winter by taking up the plants, trees, or shrubs in the spring, at the time when they are about to bud, with some of their own soil carefully preserved around the roots, and placing them upright in a cellar till Michaelmas; when, with the addition of fresh earth, they are to be put into proper tubs or vessels, and placed in a stove or hot-house, when they must be treated in the usual manner. By this method, in the month of February, fruits or roses will appear. Flowers sown in pots about Michaelmas may thus be made to bloom at Christmas.
The apparently instantaneous flowering of plants, exhibited a few years ago by M. Herbert to an astonished audience, was, we believe, effected by the heat generated by fragments of quicklime concealed in the mould close to, but not in immediate contact with, the roots. The plants selected by M. Herbert—a group of geraniums and a rose tree—were planted in two rather deep boxes of garden mould, and were covered with glass shades. The operator commenced by pouring over the roots, from a small watering-pot, a liquid which, uniting to the ingredients already in the earth, caused a great heat, as was shown by an intense steam or vapour, which was evolved within the shades, and allowed, to some extent, to escape through a small hole in the top, which at first was kept closed. The effect upon the geraniums was certainly almost instantaneous; the buds beginning to burst in about five or six minutes, and the plants being in full bloom within ten minutes, when the blossoms were gathered by M. Herbert, and distributed amongst the ladies present. With the rose tree the exhibitor was less fortunate. The invention may prove useful where ladies require to decorate their drawing-rooms or boudoirs with the beauties of the floral world752 somewhat earlier in the season than they can otherwise be obtained. It must not, however, be forgotten that the plants are, as it were, parboiled during the process, and die after a few days.
As regards the sanitary value of flowers, Mantegazza, of Pavia, states that ozone is generated in larger quantities by certain plants possessing spicy aromatic odours, than by the action of electricity upon the air. He says that in some plants this ozone is developed by the direct rays of the sun, whilst in others the action, once begun in solar light, is continued in darkness; and that cherry-laurel, clove, lavender, mint, lemon, fennel, narcissus, heliotrope, hyacinth, mignonette, &c., all produce ozone largely on exposure to the rays of the sun.[315] He also finds that whilst the ozonigenic properties of flowers reside mainly in their perfumes, the most odoriferous yielding the largest amount of ozone, certain others possessing no particular perfume, have extraordinary ozonigenic power; as, for instance, the sunflower, broad belts of which were planted by the late Commodore Maury around the grounds of the national observatory at Washington, to the effect of which he attributed the after immunity of his family from intermittent fevers.[316]
[315] The experiments of Mr Kingzetti on the limited oxidation of essential oils, lead to the inference that instead of ozone, peroxide of hydrogen is the body evolved.—Ed.
[316] Recent researches seem to have shown that the hygienic properties of the sun-flower, like those of the Eucalyptus, are chiefly, if not wholly due to the power the plant possesses of abstracting enormous quantities of moisture from the soil, and thus removing from certain localities an active element in the production of malaria.—Ed.
The collection and preservation of flowers for medicinal purposes and distillation, will be found noticed under Vegetables.
Flowers, Artificial. The beauty and value of these pleasing articles of personal decoration mainly depend upon the taste and ingenuity of the maker. The delicate fingers of woman, and her ready powers of imitation and invention, combined with her natural affection for the chaste and beautiful, have enabled her the more especially to excel in this manufacture. The productions of the female artificial florists of the French capital are justly admired everywhere.
The French employ velvet, kid, and fine cambric for the petals, and taffeta for the leaves. Very recently thin plates of bleached whalebone have been used with great success for some portions of artificial flowers.
As colours and stains, the following are employed in Paris:—
Blue. Indigo dissolved in oil of vitriol, and the acid partly neutralised with salt of tartar or whiting.
Green. A solution of distilled verdigris.
Lilac. Liquid archil.
Red. Carmine dissolved in a solution of salt of tartar, or in spirits of hartshorn.
Violet. Liquid archil, mixed with a little salt of tartar.
Yellow. Tincture of turmeric.
The above colours are usually applied to the petals with the fingers.
Flowers. Syn. Flores, L. Among chemists, this term is applied to various pulverulent substances obtained by sublimation, as flowers of antimony, benzoin, zinc, sulphur, &c. The term has been discarded from modern chemical nomenclature, but is still commonly employed in familiar language and trade.
FLUID CAM′PHOR. Prep. (Sir J. Murray.) From camphor (in powder), 1 dr.; freshly precipitated carbonate of magnesia, 2 dr.; cold distilled water, 1 pint; the solution is effected by forcing in carbonic acid gas under pressure. Each fl. oz. contains 3 gr. of camphor, and 6 gr. of carbonate of magnesia. See Essence of Camphor.
FLUID MAGNE′SIA. Syn. Liquor Magnesiæ carbonatis, L. M. BICARBONATIS, L. The preparations sold under this name are mere solutions of freshly precipitated carbonate of magnesia in water, formed by means of carbonic acid gas, under powerful pressure, and long agitation. Those best known are Sir J. Murray’s and Mr Dinneford’s, each fl. oz. of which is said to contain about 171⁄2 gr. of the carbonate, but their actual richness in the latter seldom exceeds 10 or 12 gr., and by the time they reach the consumer is often as low as 5 or 6 gr. Recently precipitated carbonate of magnesia placed in a bottle or other suitable vessel, which is then filled by means of a soda-water apparatus with water fully charged with carbonic acid gas, readily dissolves on slight and cautious agitation, and the aërated water becomes saturated with magnesia. A scruple of carbonate of magnesia put into a soda-water bottle, and thus treated, is all taken up in from 20 minutes to half an hour, and the beverage continues beautifully clear.
FLUID-OZON (J. Krohn, Munich, with a certificate from Justus von Liebig). A mouth wash and toilet water. An aqueous solution of permanganate of soda, 1 in 9, contaminated with traces of sodium sulphate and chloride. (Wittstein.)
FLUM′MERY. A species of thick hasty-pudding made with oatmeal or rice, flavoured with milk, cream, almonds, orange flowers, lemons, &c., according to fancy.
Prep. 1. (Dutch flummery.) From blancmange and eggs, flavoured with lemon peel and sweetened with sugar.
2. (French flummery.) From equal parts of blancmange and cream, sweetened, and flavoured. The above are poured into forms, and served cold, to eat with wine, spirit, cider, &c.
3. (A. T. Thomson.) Take oatmeal or groats, 1 quart; rub it for a considerable time with hot water, 2 quarts; and let the mixture stand until it becomes sour; then add another quart of hot water, and strain through a hair sieve. Let stand till a white sediment is deposited,753 decant the fluid portion, and wash the sediment with cold water. This is now to be boiled with fresh water, until it forms a mucilage, stirring the whole time. A light and nutritious food, during early convalescence.
FLUOBORIC ACID. Syn. Borofluoric acid. This may be easily prepared by saturating hydrofluoric acid with boracic acid, keeping the mixture cool, and then concentrating it in platinum vessels till dense fumes arise.
FLUOHYDRIC ACID. See Fluoride of hydrogen.
FLU′ORIDE OF HYDROGEN. HF. Syn. Fluohydric acid; Hydrofluoric acid; A. hydrofluoricum, L. An acid composed of hydrogen and fluorine. It was discovered by Scheele, but was first obtained in a pure state by Gay-Lussac and Thénard, in 1810.
Prep. Pour concentrated sulphuric acid on half its weight of fluor spar, carefully separated from siliceous earth, and reduced to fine powder. The mixture must be made in a capacious leaden retort, and a gentle heat only applied, and the evolved gas must be collected in a leaden receiver, surrounded by ice.
Prop., &c. A colourless fluid below 59° Fahr., which speedily evaporates in dense white fumes when exposed to the air. Its affinity for water exceeds that of sulphuric acid, and its combination with that fluid is accompanied with a hissing noise, and a considerable increase of its sp. gr. up to a certain point. It attacks glass and silica, for which reason it cannot be preserved in glass vessels. Bottles of lead, silver, platinum, or pure gutta percha, are used to keep it in. It is highly corrosive, instantaneously destroying the skin on contact, and producing deep and serious ulcerations; its vapour is pungent, irritating, irrespirable, and poisonous. With the bases it unites to form FLUORIDES.
In the arts, hydrofluoric acid is used for etching on glass.
FLU′ORIDES. Compounds of fluorine with metals and other basic radicals. The fluorides of the metals are, with the exception of those of the alkaline metals, insoluble in water, while the fluorides of hydrogen, boron, and silicon, are gaseous, condensing at a low temperature to volatile liquids.
FLU′ORINE. F. Syn. Fluorinium, L. An element that has not yet been isolated, owing to its attacking and combining with every element or compound that at present has been exposed to it, except oxygen. It is presumably gaseous, and of a pale greenish-yellow colour.
FLU′OSILICIC ACID. Syn. Fluoride of silicon and hydrogen; Hydrofluosilic acid. Prep. From powdered fluor spar, and siliceous sand or powdered glass, of each 1 part; concentrated sulphuric acid, 2 parts: mix in a glass retort, apply a gentle heat, and pass the evolved gas into water through a layer of mercury. Decomposition ensues, silica being deposited in a gelatinous state, and hydrofluosilicic acid or fluosilic acid remains in solution. The acid liquor is used as a test for potassium and barium, with whose salts it yields nearly insoluble precipitates.
FLUX. Syn. Fluxus, Fluor, L. In medicine, a term formerly applied to several diseases attended with a copious discharge, as diarrhœa (FLUX), dysentery (BLOODY FLUX), English cholera (BILIOUS FLUX), fluor albus (WHITE FLUX), &c. These terms are still current among the vulgar.
Flux. In metallurgy, &c., a term applied to various substances of easy fusibility, which are added to others which are more refractory, to promote their fusion.
Prep. 1. (Black flux.) Nitre, 1 part; crude tartar or cream of tartar, 2 parts; mix, and deflagrate, by small quantities at a time, in a crucible, heated to dull redness. The product consists of carbonate of potassa, mixed with charcoal in a finely divided state. Used for smelting metallic ores. It exercises a reducing action, as well as promotes the fusion. It must be kept in a dry corked bottle.
2. (Christison’s flux.) Carbonate of soda (cryst.), 8 parts; charcoal (in fine powder), 1 part; heat the mixture gradually to redness. For reducing arsenic.
3. (Cornish reducing flux.) Crude tartar, 10 parts; nitre, 4 parts; borax, 3 parts; triturate together.
4. (Cornish refining flux, White flux.) Crude tartar and nitre, equal parts, deflagrated together. See Black Flux.
5. (Crude flux.) Same as BLACK FLUX, omitting the deflagration. Reducing.
6. (Fresenius’s flux.) Carbonate of potassa (dry), 3 parts; cyanide of potassium, 1 part. For the arsenical compounds.
7. (Liebig’s flux.) Carbonate of soda (dry) and cyanide of potassium, equal parts. As the last. See Arsenious Acid.
8. (Morveau’s reducing flux.) Powdered glass (free from lead), 8 parts; calcined borax and charcoal, of each, 1 part; all in fine powder, and triturated well together. Used as BLACK FLUX.
9. (White flux.) See above.
10. (Fluxes for enamels.) See Enamels.
11. (Various.) Borax, tartar, nitre, sal-ammoniac, common salt, limestone, glass, fluor spar, and several other substances, are used as fluxes in metallurgy.
Obs. On the large scale, crude tartar is employed in the preparation of fluxes; on the small scale, commercial cream of tartar or bitartrate of potassa.
FLY. The common house-fly (Musca domestica) causes considerable annoyance to the person in hot weather, as well as damage to handsome furniture, especially to picture frames, gilding, and the like. The best way to exterminate them is to expose on a plate754 one or other of the mixtures given under Fly Poison (below). The blow-fly (Musca vomitoria), and other insects, may be kept from attacking meat by dusting it over with black pepper, powdered ginger, or any other spice, or by skewering a piece of paper to it on which a drop or two of creasote has been poured. The spices may be readily washed off with water before dressing the meat.
It is a fact not generally known, that flies will not pass through a netting made of fine silk, thread, or wire, even though the meshes may be an inch apart, unless there is a window or light behind it. This affords us a ready means of excluding these insects from all our apartments which have windows only on one side of them, without keeping the latter closed. It is merely necessary to have an ornamental netting stretched across the opening, when, although flies may abound on the outside, none will venture into the room so protected. If, however, there is a window on the other side of the room, they will fly through the netting immediately. See below.
Fly-blow in Sheep. Oil of turpentine, 3 oz.; oil of amber, 1 oz.; corrosive sublimate, 1 dr. The sublimate must be first dissolved in a pint of whey, and then mixed with the oils.
Fly Papers. Those papers which, a few years ago, were sold about the streets of London by harsh-voiced cries of “Catch ′em alive-oh!” and which might be seen in many shop-windows covered with dead and dying flies, were prepared by rubbing factitious birdlime over sheets of paper. It would be difficult to conceive a more cruel or more offensive mode of catching flies than that of glueing their living bodies to an adhesive surface. A preferable kind of fly-paper is that called ‘PAPIER MOURE,’ which contains a large quantity of arsenic in its substance.[317] This paper is kept wet when in use, and the flies, by sipping the moisture, are poisoned.
[317] Mr Plowman, in a letter to the ‘Pharm. Journ.,’ June 22nd, 1878, says that in a specimen of “Papier Moure” examined by him he failed to detect the least trace of arsenic.
Fly Poison. Prep. 1. A strong solution of white arsenic (say 1 dr. to the pint), sweetened with moist sugar, treacle, or honey. Sold under the name of ‘Fly water,’
2. Treacle, honey, or moist sugar, mixed with about 1⁄12th their weight of King’s yellow or orpiment.
Obs. Both the above are dangerous preparations, and should never be employed where there are children.
3. (Redwood.) Quassia chips (small), 1⁄4 oz.; water, 1 pint; boil 10 minutes, strain, and add of treacle, 4 oz. “Flies will drink this with avidity, and are soon destroyed by it.”
4. Black pepper, 1 teaspoonful; brown sugar, 2 teaspoonfuls; cream, 4 teaspoonfuls. See below.
Fly Powder. The dark grey-coloured powder (so-called ‘sub-oxide’) obtained by the free exposure of metallic arsenic to the air. Mixed with sweets, it is used to kill flies.
Fly Water. See Fly Poison (above).
FOG. The influence of very intense foggy weather upon the death-rate is well illustrated by a reference to the Registrar-General’s returns for 1873. From the 8th to the 12th of December of that year an unprecedently thick fog prevailed in London. The mortality in the metropolis for the week ending December 6th was twenty-three persons per thousand; in the week following, during which the fog occurred, the death-rate rose to twenty-seven; and in the week after that, when the full effects of the fog could be estimated, the deaths were found to be thirty-eight in the thousand. In the same periods the deaths from phthisis and diseases of the respiratory organs were respectively 520, 764, and 1112. That this increased death-rate was not the result of the inclement weather by which the fog was accompanied is evidenced by the circumstance that in large provincial towns, where the weather was equally severe, but in which no fog occurred, the increase in the mortality, when compared to London, was slight.
The mean of the deaths registered in London, in the two weeks ending December 20th, showed an increase of 41 per cent. upon the number returned in the first week of the month; whilst during the same date the deaths in seventeen large English towns were only 8 per cent. This fatal fog occurred during the London cattle-show week, and killed a great number of the animals sent for exhibition.
In a specimen of the air of Manchester, obtained during the visitation of that city by a very dense fog, Dr Angus Smith discovered it contained a diminished amount of oxygen when compared with a favorable sample of air.
FOILS. These are thin leaves of polished metal, placed under precious stones and pastes, to heighten their brilliancy, or to vary the effect. Foils were formerly made of copper, tinned copper, tin, and silvered copper, but the last is the one wholly used for superior work at the present day.
Foils are of two descriptions:—white, for diamonds and mock diamonds, and—coloured, for the coloured gems. The latter are prepared by varnishing or lacquering the former. By their judicious use the colour of a stone may often be modified and improved. Thus, by placing a yellow foil under a green stone that turns too much on the blue, or a red one under a stone turning too much on the crimson, the hues will be brightened and enriched in proportion.
Prep. 1. (Crystal, Diamond, or White foil.)—a. This is made by coating a plate of copper with a layer of silver, and then rolling it into sheets in the flatting mill. The foil is755 then highly polished, or covered with crystal varnish.
b. The inside of the socket in which the stone or paste is to be set is covered with tin foil, by means of a little stiff gum or size; when dry, the surface is polished and the socket heated, and whilst it is warm, filled with quicksilver; after repose for two or three minutes the fluid metal is poured out, and the stone gently fitted in its place; lastly, the work is well fitted round the stone, to prevent the alloy being shaken out.
c. The bottom of the stone is coated with a film of real silver, by precipitating it from a solution of the nitrate in spirit of ammonia, by means of the oils of cassia and cloves.[318] This method vastly increases the brilliancy both of real and factitious gems, and the work is very permanent.
[318] See Silvering.
2. (Coloured foils.) The following formulæ produce beautiful coloured effects, when judiciously employed:—
a. (Amethyst.) Lake and Prussian blue, finely ground in pale drying oil.
b. (Blue.) Prussian blue (preferably Turnbull’s), ground with pale, quick-drying oil. Used to deepen the colour of sapphires.
c. (Eagle marine.) Verdigris tempered in shell-lac varnish (alcoholic), with a little Prussian blue.
d. (Garnet.) Dragon’s blood dissolved in rectified spirit of wine.
e. (Vinegar garnet.) Orange lake finely tempered with shell-lac varnish.
f. (Green.)—α. From pale shell-lac, dissolved in alcohol (lacquer), and tinged green by dissolving verdigris or acetate of copper in it.
β. From sesquiferrocyanide of iron and bichromate of potassa, of each 1⁄2 oz.; ground to an impalpable powder, first alone, and then with gum mastic (clean and also in fine powder), 2 oz.; a little pyroxilic spirit is next added, gradually, and the whole again ground until the mass becomes homogeneous and of a fine transparent green. The beauty increases with the length of the grinding. The predominance of the bichromate turns it on the yellowish green; that of the salt of iron on the bluish green. For use it is to be thinned with pyroxilic spirit. (‘Chem.,’ iii, 231.) Used for emeralds.
g. (Red.) Carmine, dissolved in spirit of hartshorn, or in a weak solution of salt of tartar, and a little gum (dissolved) added.
h. (Ruby.)—α. From lake or carmine, ground in isinglass.
β. Lake ground in shell-lac varnish. Both are used when the colour turns on the purple.
γ. From bright lake ground in oil. Used when the colour turns on the scarlet or orange.
i. (Yellow.)—α. Various shades of yellow may be produced by tinging a weak alcoholic solution of shell-lac or mastic, by digesting turmeric, annotta, saffron, or socotrine aloes in it. The former is the brightest, and is used for topazes.
β. From hay saffron digested in 5 or 6 times its weight of boiling water until the latter becomes sufficiently coloured, and a little solution of gum or isinglass added to the filtered liquor. When dry, a coating of spirit varnish is applied.
Obs. By the skilful use of the above varnishes, good imitations of the gems may be cheaply made from transparent white glass or paste; and by applying them to foils set under coloured pastes (FACTITIOUS GEMS), a superior effect may be produced. The pigments employed must be reduced to the finest state possible by patient grinding, as without this precaution transparent and beautiful shades cannot be formed. The palest and cleanest mastic and lac, dissolved in alcohol, and also the palest and quickest drying oil should alone be used when these substances are ordered. In every case the colour must be laid on the foil with a broad soft brush; and the operation should be performed, if possible, at once, as no part should be crossed, or twice gone over, whilst wet. If the colour turns out too pale, a second coat may be given when the first one has become quite dry, but this practice should be avoided if possible.
FOMENTA′TION. Syn. Fomentatio, FOMENTUM, Fotus, L. A liquid, either simple or medicated, used for local bathing. Fomentations are distinguished from lotions chiefly in being applied in a heated state, and in larger quantities, and for a longer period at a time.
Fomentations are chiefly employed to allay pain or irritation, or to promote suppuration or the healthy action of the parts. As the intention is to convey heat, combined with moisture, to the part fomented, the utmost care must be taken to manage the application so as to promote the object in view as much as possible. Flannel cloths wrung out of the hot or boiling liquid, by means of two sticks, turned in opposite directions, form the best vehicles for fomentations. If they are shaken up, and laid lightly over the part, they involve a considerable quantity of air, which, being a bad conductor, retains the heat in them for a considerable time. “In every process of fomenting there should be two flannels, each (say) three yards long, with the ends sewed together, to admit of the boiling water being wrung out of them; and the one flannel should be got ready whilst the other is applied. The fineness or the coarseness of the flannel is not a matter of indifference. The coarser it is the less readily does it conduct heat, and the longer it retains its warmth; therefore it is more efficient for fomenting. White flannel also retains the heat longer than coloured flannel.” (Dr R. E. Griffith.) More harm than good is frequently done by allowing the patient to become chilled during the application. “If only one (flannel) is used, the756 skin becomes chilled during the time occupied in removing the flannel, soaking it in the water, wringing it out, and reapplying it; but if two are used, one of them is ready, and can be applied the moment the other is taken off, by which means the part is never exposed to the air, no matter how long the fomentation is continued. In some diseases (rheumatism, peritonitis, &c.), the patient is scarcely conscious of a degree of heat which scalds the nurse’s hands. In this case the fomenting flannels should be put in a towel, by which means they may be wrung out without being handled by the nurse, and may be applied far hotter than can be done by any other method.” (Dr J. B. Nevins.)
The quantity of liquid forming a fomentation, as well as the size of the cloths employed, must entirely depend upon circumstances. In some cases (as in slight affections of the face, &c.) the application may be effectually made by holding the part in the steam of the hot liquid, and bathing it continually by means of a sponge or cloth. In some instances 1⁄2 pint to a pint of liquid may be found a sufficient quantity; whilst in others several quarts will be required. Under all circumstances, care must be taken to keep the fomentation as near as possible at the temperature ordered, during the whole time of its application; and, as soon as the operation is finished, to quickly wipe the part dry, and to cover it with ample clothing, in order that the reaction set up may not be prematurely checked.
Fomentations usually consist of simple water, or the decoction of some simple vegetable substance, as chamomiles, elder flowers or mallows; but, occasionally, the leaves and flowers of aromatic and narcotic plants, and saline matter, are employed under this form. The following formulæ are given as examples:—
Fomentation, Acetic. Syn. Fotus aceticus (Paris Codex). Fomentations of vinegar are sometimes prepared with white, with rose, or with aromatic vinegar (Paris Codex) in the proportion of one of vinegar to four of water.
Fomentation, An′odyne. Syn. Fotus anodynus, Fomentatio anodyna, Fomentum anodynum, L. Prep. 1. Simple decoction of poppy-heads.
2. (Hosp. F.) Poppy-heads (without the seeds), 11⁄2 oz.; water, 31⁄2 pints; boil to 21⁄2 pints; add of elder flowers, 3⁄4 oz.; boil to a quart and strain. Used to allay pain.
3. (Pierquin.) Opium, 1 oz.; wine, 1 quart; boil to a pint and strain. Used in severe gouty, rheumatic, neuralgic, and syphilitic pains.
4. Opium, 1 oz.; water, 1 quart; boil to 3⁄4 pint, add pyroligneous acid, 2 fl. oz.; boil for 10 minutes longer, then further add of sherry wine, 3⁄4 pint; and as soon as the whole again boils, strain it for use. Superior to the last, and cheaper.
Fomentation, Antineural′gic. Syn. Fomentatio antineuralgica, L. Prep. 1. (Mialhe.) Acetate of morphia, 2 gr.; acetic acid, 2 or 3 drops; eau de Cologne, 2 or 3 dr.; dissolve. In facial neuralgia.
2. (Trousseau and Reveil.) Cyanide of potassium, 1 dr.; distilled water, 6 fl. oz.; dissolve and keep it in a well-closed bottle in the dark. Used in neuralgia, especially in that of the face (tic douloureux). A compress of lint or soft linen is dipped in it and applied to the part. It must not be used internally or applied to a wounded surface, as it is very poisonous. See Fomentations, anodyne, Nos. 3 and 4 (above), also Fomentation, Stimulant.
Fomentation, Antiseptic. Syn. Fomentatio antiseptica, L. Prep. 1. Decoction of mallows, 4 pints; sal ammoniac, 2 oz.; dissolve, and add of disulphate of quinine, 20 gr., dissolved in camphorated spirit, 4 fl. oz.
2. (Hosp. F.) Decoction of bark, 1 quart; infusion of chamomile, 1 pint; camphorated spirit, 2 fl. oz.; hydrochloric acid, 1 fl. dr. Both are used when there is a tendency to gangrene or putrescence.
Fomentation of Ar′nica. Syn. Fomentatio arnicæ, L. Prep. 1. Flowers of arnica, 1 oz.; water, 3 pints; boil to a quart, and strain. Used in contusions.
2. (Graefe.) Flowers of arnica, 2 oz.; rue (leaves), 1 oz.; boiling water, q. s. to strain 12 fl. oz. of infusion after an hour’s maceration at nearly the boiling temperature. Used in contusions and extravasations, especially as an application to black eyes.
3. (Radius.) Flowers of arnica, 1⁄2 oz.; boiling vinegar, q. s. to strain 6 fl. oz. of infusion, in which dissolve of carbonate of ammonia, 2 dr. Used in œdema of the scrotum.
Fomentation, Aromat′ic. Syn. Fomentatio aromatica, Fotus aromaticus, L. Prep. 1. Sea wormwood, southernwood, and chamomiles, of each 1 oz.; laurel leaves, 1⁄2 oz.; water 5 pints; boil to half gall., and strain. In rheumatism, cutaneous affections, colic, &c.
2. (Augustin.) Rosemary, 1⁄2 oz.; red wine, and water, of each 3 fl. oz.; infuse and strain with expression. In contusions, especially black eyes.
3. (Hosp. F.) Cloves and mace, of each 1 oz.; opium, 20 gr.; red wine (boiling), 1 pint; digest at near boiling for 1 hour, and strain. Used as both the last.
4. (Rideau.) Bay leaves, rosemary, southernwood, and wormwood, of each 1 oz.; water, 2 quarts; boil 5 minutes, and strain. As No. 1.
Fomentation, Astrin′gent. Syn. Fotus astringens, F. roborans, L. Prep. 1. Decoction of oak bark.
2. To each quart of the last add of alum 1 dr.
3. (Ph. Chirur.) Bruised galls, 1 oz.; boiling water, 21⁄2 pints; digest 1 hour, and strain.
4. (Ricord.) Tannin, 21⁄2 dr.; aromatic wine (hot), 1⁄2 pint; dissolve.
757
5. Bistort and pomegranate peel, of each 2 oz.; sal ammonia, 1⁄4 oz.; red wine, 1 pint.; infuse at a gentle heat. The above are used in hæmorrhages, piles, prolapsus, &c.
Fomentation of Belladon′na. Syn. Fotus belladonnæ, L. Prep. (Ophthalmic Hosp.) Extract of belladonna, 1 dr.; boiling water, 1 pint. Used to dilate the pupil in certain affections of the eye; it is usually applied on the forehead.
Fomentation of Bitter-sweet. Syn. Decoctum dulcamaræ, L. Prep. Bitter-sweet stalks, 10 dr.; water, 11⁄2 pint; boil to a pint, and strain.
Fomentation of Cham′omile. Syn. Fomentatio anthemidis, L. Prep. Chamomiles, 2 oz.; water, 3 pints; boil 10 minutes, and strain with expression. Emollient.
Fomentation, Com′mon. Syn. Fotus communis. (L. 1744.) Prep. Dried southernwood, sea wormwood, chamomile, of each 1 oz.; dried bay leaves, 1⁄2 oz.; water, 5 pints; boil slightly, and strain.
Fomentation, Compound of Hemlock. (Guy’s Hosp.) Syn. Fomentum conii compositum. Prep. Dried hemlock, 2 oz.; dried chamomiles, 1⁄2 oz.; boiling water, 11⁄2 pint; macerate for 2 hours, strain, and press.
Fomentation, Diuret′ic. Syn. Fomentatio diuretica, L. Prep. (Trousseau.) Tinctures of squills and foxglove, of each 2 oz.; hot water, 6 fluid oz.; mix. Applied by lint or linen compresses to the insides of the thighs, in dropsies, when the stomach will not bear diuretics.
Fomentation of El′der Flowers. Syn. Fotus sambuci, L. Prep. From elder flowers, 1 oz.; boiling water, 2 quarts; digest in a hot place for 1 hour, and express the liquor. Emollient.
Fomentation, Emol′lient. Syn. Fomentatio emolliens. L. Prep. 1. Marshmallow root and poppy heads, of each 1 oz.; water, 3 pints; boil to a quart, and strain.
2. (P. Cod.) Emollient herbs, 1 oz.; boiling water, 1 quart; infuse 1 hour, and strain with expression. (See above.)
Fomentation, Foxglove. Syn. Fomentum digitalis. Dried foxglove, 1 oz.; boiling water, 11⁄2 pint; infuse, and strain.
Fomentation of Galls. Syn Fomentum gallæ. Prep. Bruised galls, 1⁄2 oz.; boiling water, 2 lbs.; macerate for an hour and strain.
Fomentation, Narcot′ic. Syn. Fomentatio narcotica, L. Prep. (P. Cod.) Narcotic herbs, 1 oz.; boiling water, 11⁄2 pint; infuse as last.
Fomentation, Poppy. Syn. Fomentum papaveris. As Decoction of Poppies.
Fomentation, Resol′vent. Syn. Fotus resolvens, L. Prep. (Richard.) Fomentation of elder flowers, 8 fl. oz.; liquor of diacetate of lead, 1⁄2 fl. dr.; mix. Used to discuss tumours, &c.
Fomentation, Stim′ulant. Syn. Fomentatio stimulans, L. Prep. 1. Sesquicarbonate of ammonia, 1 oz.; tincture of cantharides, 2 fl. oz.; warm water, 1 pint.
2. Household mustard, 4 oz.; hot water, 11⁄2 pint; mix. Both the above are rubefacient and counter-irritant, and excellent in rheumatism, neuralgia, &c.
Fomentation, Tannin. Syn. Fomentum tannini. (Ricord.) Prep. Tannin, 2 dr.; aromatic wine, 8 oz.
Fomentation, Ver′mifuge. Syn. Fomentatio vermifuga, Fotus anthelminticus, L. Prep. Leaves and flowers of tansy, wormwood, and chamomile, of each 3 oz.; water, 1 quart; boil to 11⁄2 pint, and strain. Applied to the abdomen, &c., in worms.
Fomentation, Wine. Syn. Fotus vinosus (Par. Cod.) Prep. Red wine, 2 pints; honey 4 oz.
FOOD. Syn. Cibus, Materia alimentaria, L. Anything which feeds or promotes the natural growth of organic bodies, by supplying them with materials which, by assimilation, may be converted into the substances of which they are composed; or which, by its decomposition or slow combustion, maintains the temperature, or some other essential condition of life, at the proper standard. The numerous articles employed as food are all compounds; and in many cases they consist of mechanical mixtures or chemical combinations of two or more compounds. Organized matter, or that which has possessed either animal or vegetable life, or which has been produced by living organs, seems to be alone capable of assimilation, to any extent, by the animal system; and hence it is from the organic kingdom that our aliments are necessarily derived. Water, iron, earthy phosphates, chloride of sodium, and other salts, which form the inorganic constituents of the body, though not of themselves nourishing, are also assimilated when taken in conjunction with organic aliments, and then contribute essentially to nutrition. In the animal and vegetable substances employed as food, these inorganic compounds are provided in small but sufficient quantities to meet the requirements of the healthy body, and in this state of combination alone can they be regarded in the light of aliments. A complete consideration of this subject embraces, not only all the substances used as food, but also those things which when taken with them improve their flavour, promote their digestion, and render them more wholesome and nutritive; and also their preparation for the table in its various relations with health and disease.
The following ‘BILLS OF FARE,’ for which we are indebted chiefly to Soyer, Rundell, and others, exhibit the various articles in season at different periods of the year.
First quarter. January.—Poultry and game: Pheasants, partridges, hares, rabbits, woodcocks, snipes, turkeys, capons, pullets, fowls, chickens, and tame pigeons.—Fish:758 Carp, tench, perch, lampreys, eels, cray-fish, cod, soles, flounders, plaice, turbot, thornback, skate, sturgeon, smelts, whitings, lobsters, crabs, prawns, and oysters.—Vegetables: Cabbage, savoys, colewort, sprouts, leeks, onions, beet, sorrel, chervil, endive, spinach, celery, garlic, scorzonera, potatoes, parsnips, turnips, brocoli (white and purple), shalots, lettuces, cresses, mustard, rape, salsafy, and herbs of all sorts (some dry and some green); cucumbers, asparagus, and mushrooms are also to be had, though not in season.—Fruit: Apples, pears, nuts, walnuts, medlars, and grapes.
February and March.—Meat, fowls, and game, as in January, with the addition of ducklings and chickens.—Fish: As the last two months (cod is not thought so good from February to July, although it is still sold at the fishmonger’s).—Vegetables: The same as the previous months, with the addition of kidney-beans.—Fruit: Apples, pears, and forced strawberries.
Second quarter. April, May, and June.—Meat: Beef, mutton, veal, lamb, and venison (in June).—Poultry: Pullets, fowls, chickens, ducklings, pigeons, rabbits, and leverets.—Fish: Carp, tench, soles, smelts, eels, trout, turbot, lobsters, chub, salmon, herrings, cray-fish, mackerel, crabs, prawns, and shrimps.—Vegetables: As before; and in May, early potatoes and cabbages, peas, radishes, kidney-beans, carrots, turnips, cauliflowers, asparagus, artichokes, and numerous salads (forced).—Fruit: (in June) strawberries, cherries, melons, green apricots, and currants and gooseberries for tarts; pears, grapes, nectarines, peaches, and some other fruit.
Third quarter. July, August, and September.—Meat, as before.—Poultry, &c.: Pullets, fowls, chickens, rabbits, pigeons, green geese, leverets, and turkey poults. Two former months, plovers and wheat-ears (in September), partridges, geese, &c.—Fish: Cod, haddocks, flounders, plaice, skate, thornback, mullets, pike, carp, eels, shellfish (except oysters), and mackerel (during the first two months of the quarter, but they are not good in August).—Vegetables: Of all sorts, beans, peas, French beans, &c.—Fruit: (In July)—Strawberries, gooseberries, pine-apples, plums (various), cherries, apricots, raspberries, melons, currants, and damsons. (In August and September)—Peaches, plums, figs, filberts, mulberries, cherries, apples, pears, nectarines, and grapes. (During the latter months)—Pines, melons, strawberries, medlars, and quinces. (In September)—Morella cherries, damsons, and various plums.
Fourth quarter. October, November, and December.—Meat, as before, and doe venison.—Poultry and Game: Domestic fowls, as in first quarter; pheasants (from the 1st of October); partridges, larks, hares, dotterels (at the end of the month), wild-ducks, teal, snipes, widgeon, and grouse.—Fish: Dories, smelts, pike, perch, halibuts, brills, carp, salmon-trout, barbel, gudgeons, tench, and shellfish.—Vegetables: (As in January), French-beans, last crops of beans, &c.—Fruit: Peaches, pears, figs, bullace, grapes, apples, medlars, damsons, filberts, walnuts, nuts, quinces, services, and medlars. (In November)—Meat, &c.: Beef, mutton, veal, pork, house-lamb, doe venison, and poultry and game as in the last month.—Fish: As the last month.—Vegetables: Carrots, turnips, parsnips, potatoes, skirrets, scorzonera, onions, leeks, shalots, cabbage, savoys, colewort, spinach, chardbeats, chardoons, cresses, endive, celery, lettuces, salad-herbs, and various pot-herbs.—Fruit: Pears, apples, nuts, walnuts, bullace, chestnuts, medlars, and grapes. (In December)—Meat, &c.: Beef, mutton, veal, house-lamb, pork, and venison.—Poultry and Game: Geese, turkeys, pullets, pigeons, capons, fowls, chickens, rabbits, hares, snipes, woodcocks, larks, pheasants, partridges, sea-fowls, guinea-fowls, wild ducks, teal, widgeon, dotterels, dun-birds, and grouse.—Fish: Cod, turbot, halibuts, soles, gurnets, sturgeon, carp, gudgeons, codlings, eels, dories, and shell-fish.—Vegetables: As in last month. Asparagus, &c., forced.—Fruit: As before, except bullace.
Food, Inspection of. The Public Health Act enacts that—
“Any medical officer of health or inspector of nuisances may at all reasonable times, inspect and examine any animal, carcase, meat, poultry, game, flesh, fish, fruit, vegetables, corn, bread, flour, or milk exposed for sale, or deposited in any place for the purpose of sale, or of preparation for sale, and intended for the food of man, the proof that the same was not exposed or deposited for any such purpose, or was not intended for the food of man, resting with the party charged; and if any such animal, carcase, meat, poultry, game, flesh, fish, fruit, vegetables, corn, bread, flour, or milk, appears to such medical officer or inspector to be diseased, or unsound, or unwholesome, or unfit for the food of man, he may seize and carry away the same himself or by an assistant, in order to have the same dealt with by a justice.” (P. H., s. 116.)
“If it appears to the justice that any animal, carcase, meat, poultry, game, flesh, fish, fruit, vegetables, corn, bread, flour, or milk so seized is diseased, or unsound, or unwholesome, or unfit for the food of man, he shall condemn the same and order it to be destroyed, or so disposed of, as to prevent it from being exposed for sale, or used for such food; and the person to whom the same belongs or did belong at the time of sale, or of exposure for sale, or in whose possession, or on whose premises the same was found, shall be liable to a penalty not exceeding £20 for every animal, carcase, or fish, or piece of meat, flesh, or fish, or any poultry or game, or for the parcel of fruit, vegetables, corn, bread, or flour, or for the milk so condemned, or at the discretion of the justice, without the infliction759 of a fine, to imprisonment for a term of not more than three months.
“The justice who, under this section, is empowered to convict the offender, may be either the justice who may have ordered the article to be disposed of or destroyed, or any other justice having jurisdiction in the place.” (P. H., s. 117.)
“Any person who in any manner prevents any medical officer of health or inspector of nuisances from entering any premises and inspecting any animal, carcase, meat, poultry, game, flesh, fish, fruit, vegetables, corn, bread, flour, or milk exposed or deposited for the purpose of sale, or of preparation for sale, and intended for the food of man, or who obstructs or impedes any such officer or inspector, or his assistant, when carrying into execution the provisions of this Act, shall be liable to a penalty, not exceeding £5.” (P. H., s. 118.)
“Any complaint made on oath, by a medical officer of health, or by an inspector, or other officer of a local authority, any justice may grant a warrant to any such officer, to enter any building, or part of a building in which any such officer has reason for believing that there is kept or concealed any animal, carcase, meat, poultry, game, flesh, fish, fruit, vegetables, corn, bread, flour, or milk which is intended for sale for the food of man, and is diseased, unsound or unwholesome, or unfit for the food of man and to search for, seize, and carry away any such animal, or other article, in order to have the same dealt with by a justice under the provisions of this Act.
Any person who obstructs any such officer in the performance of his duty, under such warrant shall, in addition to any other punishment to which he may be subject, be liable to a penalty not exceeding £20.” (P. H., s. 119.)
FOOL. Cooks give this name to a species of jam made of boiled and crushed fruit, mixed with milk or cream, and sweetened.
Fool, Ap′ple. From the peeled and cored fruit, placed in a jar, with moist sugar, q. s. to render it palatable, and a very little cider or perry; the jar is set in a saucepan of water over the fire, and the heat continued until the apples become quite soft, when they are pulped through a colander, and a sufficient quantity of milk, a little cream, and some sugar, added to bring them to the proper ‘palate.’
Fool, Goose′berry. From gooseberries, as the last. Those which are unripe are generally preferred. These preparations, when nicely made, are very pleasant and wholesome.
FOOT (Human). See Feet.
FOOTS. Coarse moist sugar. The scrapings of the sugar hogsheads, refuse sugar, waste, and dirt, is also sold to the publicans under this name, who use it in the adulteration of their beer; chiefly to make it stand more water, and to impart ‘briskness.’
FORCE′MEAT. Syn. Farce, Stuffing. A species of sausage meat, either served up alone, or employed as an ingredient in other dishes.
Mrs Rundell truly remarks that “at many tables, where everything else is done well, it is common to find very bad forcemeat or stuffing.” To avoid this error, care should be taken to so proportion the ingredients that “no one flavour should predominate; yet if several dishes be served the same day, there should be a marked variety in the tastes of the forcemeats as well as of the gravies. A general fault is, that the tastes of lemon peel and thyme overcome all others; therefore they should only be used in small quantities.” Forcemeats should be just consistent enough to cut with a knife, but not dry and heavy. Herbs are very essential ingredients; and it is the copious and judicious use of them that chiefly gives the cookery of the French its superior flavour. “To force fowls, meat, &c., is to stuff them.” (Mrs Rundell.)
FOR′CING. Horticulturists apply this term to the art of accelerating the growth of plants, so as to obtain fruits or flowers at unusual seasons. Dung-beds, bark-beds, and frames, pits, and houses, with glass roofs, are commonly employed by the gardeners for this purpose.
FORGERIES, PROTECTION FROM. See Paper, Protective.
FORMATE. Syn. Formiate. Salts, in which one atom of hydrogen in formic acid is replaced by a metal or other basic radical. They are best obtained either by direct saturation of the acid, or by double decomposition; most of them are very soluble, and are decomposed by hot oil of vitriol. Formate of ammonium crystallises in square prisms; formate of sodium, in rhombic prisms; formate of potassium is deliquescent, and crystallises with difficulty; the formates of barium, calcium, magnesium, and strontium, form small prismatic crystals; formate of lead assumes the shape of small colourless needles, soluble in 40 parts of water; the formates of cobalt, iron, manganese, nickel, and zinc, are easily crystallisable, whilst that of copper forms very beautiful, large, bright-blue rhombic prisms; formate of silver is less soluble than the salt of lead, and is decomposed at a gentle heat.
FORMIC ACID. HCHO2. Syn. Hydrogen Formiate. An organic acid, obtained by oxidizing many organic substances, and found in the red ant.
Prep. Sugar, 1 part; water, 2 parts; binoxide of manganese, 3 parts; mix in a retort capable of holding fully 10 times the bulk of the ingredients, and add, cautiously, oil of vitriol, 3 parts, diluted with an equal weight of water; as soon as the first violent effervescence has subsided, heat may be applied, and the product collected and purified, as below.
Formate of lead in fine powder is introduced into a long glass tube, one end of which is connected with an apparatus evolving sulphuretted hydrogen, and the other with a760 receiver. As soon as the salt is entirely decomposed (blackened) a very gentle heat is applied, and the distilled liquid collected; the product is, lastly, boiled for a minute or less, to expel any adhering sulphuretted gas. This furnishes chemically pure formic acid.
From wood spirit, 1 part; bichromate of potassium and sulphuric acid, of each 3 parts; the sulphuric acid, diluted with an equal weight of water, being gradually added last. A portion of wood spirit distils over with the acid, and may be again treated with bichromate of potassium and sulphuric acid, when a fresh portion of formic acid will be produced. This process yields a large product.
Prop., purific., &c. The products of the above processes are limpid and colourless; the stronger ones fume slightly in the air, and possess an extremely penetrating odour. The acid obtained by the second process boils at 209° Fahr., crystallises in brilliant scales below 32°, and has the sp. gr. 1·2353. Its vapour is inflammable, and burns with a blue flame. It is extremely corrosive, and rapidly destroys the texture of living organic substances. The products of the other processes are very dilute, and possess the above properties in only a minor degree. They may all be purified and concentrated by saturating them with pure carbonate of sodium or of potassium, and after subjecting the liquid to a gentle heat for a short time, and liberating the formic acid from the salt by means of dilute sulphuric acid, finally submitting the mixture to distillation, when the hydrated acid will come over perfectly pure.
Formic acid reduces the salts of mercury and silver, and forms salts with the bases termed formiates.
Formic acid is readily distinguished from acetic acid, which in many points it resembles, by heating it with a little solution of oxide of silver or mercury; the metal is reduced, and precipitated in a pulverulent state, while carbonic acid is extricated. The odours of the two acids also vary.
FORMICA. Syn. The Ant. The following are the principal species of the genus Formica. F. flava, the yellow ant. Many careful observers say this species keeps in its nest the Aphis radicans, which when its abdomen is touched by the ant, excretes a saccharine substance on which the ants feed. F. rufa, or large red ant, F. fusca; or brown ant, Polyergus rufescens, and F. sanguinea. These two latter are sometimes called amazon ants, from their pugilistic propensities. They have been known to make regular raids against other species of ants, and to carry off their larvæ and pupæ to their own nests, where they rear the insects that come of them, and afterwards employ them as slaves, causing them to excavate passages, to collect food, to carry larvæ and (so dependent do the masters become on the exertions of their bondsman) even to feed them; it being a well-attested fact that the slave holders would starve if left to themselves. See Ant.
FORMOBENZOIC ACID. (HC7H6O,CHO2). Syn. Formiate of Hydride of Benzoyle. Mandelic acid. When the distilled water of bitter almonds (containing hydrocyanic acid and the essential oil) is boiled with hydrochloric acid, a curious reaction occurs; the hydrocyanic acid is decomposed—into ammonia which unites with the hydrochloric acid, and formic acid which enters into combination with the oil of almonds—producing a new body possessed of acid properties, and termed Formobenzoic acid. On evaporating the solution the acid may be obtained in mixture with ammonia hydrochlorate, from which it may be separated by ether; the ethereal solution deposits it in rhomboidal tables. It has a sour taste and is easily soluble in alcohol. When heated it fuses at a low temperature, emitting an agreeable odour of hawthorn blossoms. (Miller.)
FORM′ULA. [L.] In pharmacy and medicine, a short form of prescription; a recipe. By chemists the term is applied to a grouping of symbols, expressing the composition of a body; thus, HCl (standing for 1 atom of hydrogen united to 1 atom of chlorine) is the formula for hydrochloric acid. A chemical formula is termed empirical when it merely gives the simplest possible expression of the composition of the substance to which it refers. A rational formula, on the contrary, aims at describing the exact composition of molecule, or combining weight of the substance, but stating the absolute number of atoms of such of the elements essential to that object, as well as the mere relations existing between them. The empirical formula is at once deduced from the analysis of the substance, reckoned to 100 parts; the rational formula requires, in addition, a knowledge of its combining quantity, which can only be obtained by direct experiment, by synthesis, or by the careful examination of one or more of its most definite compounds. Thus, the composition of acetic acid is expressed by the formula CH2O, which exhibits the simplest relations of the three elements; if we want to express the quantities of these, in atoms required to make up one molecule of acetic acid, we have to adopt the formula C2H4O2 or HC2H3O2.
FOR′MYL. Syn. Formyle. A hypothetical organic radical, having the composition C2H. Its existence was inferred from the constitution of certain organic compounds which are now referred to the methyl-series. Formic acid was supposed to be an oxide of formyl; and chloroform, the terchloride of formyl.
FOXGLOVE. Syn. Digitalis (B. P.), L. A genus of plants belonging to the natural order Scrophulariacæ. The leaves of the uncultivated ‘Digitalis purpurea,’ or purple foxglove, are officinal in our pharmacopœias. They must be gathered before the terminal flowers have expanded. “The petiole and761 midrib of the leaf being cut off, dry the lamina.” (Phar. L.) The seeds (DIGITALIS SEMINA), which were ordered, as well as the leaves, in former pharmacopœias, are said to be in many points preferable to them. When good, the leaves are of a dull-green colour, and possess a feeble narcotic odour, and a bitter, unpleasant taste. Both the dried leaves and the powder should be preserved in corked bottles covered with dark-coloured paper, or in well-closed tin canisters, and kept in a dark cupboard; and the stock should be renewed yearly, as age considerably diminishes the medicinal activity of digitalis.
Action, uses, &c. Foxglove is diuretic, sedative, and antispasmodic, and exerts a specific action over the cerebro-spinal system, promoting the functions of the absorbents, and reducing the force of the circulation in a remarkable manner. It is administered in fevers and inflammations, to reduce the frequency of the pulse, and to allay excessive vascular excitement; in dropsy (unless the habit is full and pulse tight and cordy), as a diuretic, either alone, or combined with squills, calomel, salines, or bitters; in internal hæmorrhages, as a sedative, when the pulse is full, hard, and throbbing; in diseases of the heart and great vessels, and in phthisis, to reduce the force and velocity of the circulation; in epilepsy and insanity, to repress vascular excitement; and in spasmodic asthma, scrofula, and several other diseases, with one or other of the above intentions.
The greatest caution is required in the use of foxglove, as its effects accumulate in the system, and the unwary practitioner is occasionally surprised at the sudden demise of his patient, even after he has left off the use of this drug.—Dose, 1⁄2 gr. to 11⁄2 gr., in powder, every 6 hours. See Extract, Infusion, Tincture, &c.
FOX′ING. See Malt Liquors.
FRACT′URE. Syn. Fractura, L. The breaking or disrupture of a bone. When the bone is nearly divided into two parts, it is called a SIMPLE FRACTURE; when the integuments are also lacerated, a COMPOUND FRACTURE; and when the bone is splintered, a COMMINUTED FRACTURE.
FRAGRANT PAIN-CURER (Five-minute). Dr Walter Scott, New York. A remedy to remove all kinds of pain in five minutes. A clear colourless fluid containing ether, 6 grammes; glycerin, 21 grammes; common salt, 3·4 grammes; distilled water, 170 grammes. (Hager.)
FRANK′INCENSE. Syn. Common frankincense; Thus (Ph. L.), L. The turpentine which exudes from the bark of Abies excelsa (Norway spruce fir) and Pinus palustris (pitch or swamp pine), hardened by the air. (Ph. L.) The gum-resin olibanum, which is the produce of the Boswellia thurifera, is the ‘odorous frankincense’ of commerce.
Prepared Frankincense. Syn. Thus præparatum (Ph. L.), L. Prep. (Ph. L.) Frankincense, 1 lb.; water, q. s. to cover it; boil until the resin is melted, and strain through a hair sieve; when the whole has cooled, pour off the water, and keep the frankincense for use. Resembles common resin in its general properties.
FRAX′ININ. Syn. Fraxin; Fraxina, L. A peculiar bitter, neutral, and crystallisable substance, soluble in boiling water, extracted from the bark of Fraxinus excelsior, or common ash. It is febrifuge.
FREC′KLES. These are round or oval-shaped yellowish spots, similar to stains, developed on the skin. There are two varieties—Freckles, or SUMMER FRECKLES, resulting from the action of the sun and heat during the summer season, and disappearing with the hot weather or exposure; and—Cold freckles, which occur at all times of the year. The former are chiefly confined to persons of fair complexion, whilst the latter attack persons of all complexions indifferently, and sometimes assume a lively yellow or greenish colour.
Treatment. Common freckles may generally be removed by the frequent application of dilute spirits, acids or alkaline solutions; the last two just strong enough to prick the tongue. Cold freckles commonly occur from disordered health, or some general disturbance of the system, to which attention should be chiefly directed. In both varieties the solution of bichloride of mercury (Ph. L.), or Gowland’s lotion, will be found a most useful external application. See below.
Freckles, Lotion for. Prep. 1. Bichloride of mercury, 5 gr.; hydrochloric acid, 30 drops; lump sugar, 1 oz.; rectified spirit of wine, 2 oz.; rose water, 7 oz.; agitate together until the whole is dissolved.
2. Petals or leaves of red roses, 1 oz.; hot water, 12 fl. oz.; infuse an hour, and strain, with expression, 1⁄2 pint; add of citric acid, 30 gr.; dissolve, and in a few hours, decant and clear.
3. Rose leaves (dried), 1⁄4 oz.; lemon juice (freshly expressed) and rum or brandy, of each 1⁄4 pint; digest 24 hours, and squeeze out the liquor for use.
4. (Kittoe’s.) Sal ammoniac, 1 dr.; spring water, 1 pint; lavender water or eau de Cologne, 1⁄4 oz; mix. The above are applied with the fingers night and morning, or oftener.
Freckles, Pomade for. Prep. 1. Citrine ointment, 1 dr.; simple ointment, 7 dr.; otto of roses, 3 drops.
2. Elder flower ointment, 1 oz.; sulphate of zinc (levigated), 20 gr.; mix by porphyrization, or by trituration in a wedgwood-ware mortar. Both the above, applied night and morning, are excellent for either cold or summer freckles.
FREEZ′ING MIXTURES. See Ice and Refrigeration.
FRENCH BER′RIES. Syn. Persian berries,762 Avignon b.; Graines d’Avignon, Fr. The unripe berries or fruit of the Rhamnus infectorius. They are imported from France and Persia; those from the latter country being esteemed the best. Some writers state that the Persian berries are the product of a distinct species, namely, R. amygdalinus. They are chiefly used for dyeing morocco leather yellow. Their decoction dyes cloth, previously mordanted with alum, tartar, or protochloride of tin, of a yellow colour; with sulphate of copper, an olive; and with red sulphate of iron, an olive-green colour.
FRENCH POL′ISH. Several varnishes are used under this name. That most generally employed is a simple solution of pale shell-lac in either methylated spirit or wood naphtha. Sometimes a little mastic, sandarac, or elemi, or copal varnish, is added to render the polish tougher.
Prep. 1. From pale shell-lac, 51⁄2 oz.; finest wood naphtha, 1 pint; dissolve.
2. Pale shell-lac, 3 lbs.; wood naphtha, 1 gall. Methylated spirit (68 o. p.) may be substituted for the naphtha in each of the above formulæ.
3. Pale shell-lac, 5 oz.; gum sandarac, 1 oz.; spirit (68 o. p.), 1 pint.
4. Pale shell-lac, 51⁄2 oz.; gum elemi, 3⁄4 oz.; spirit, 1 pint.
5. Pale shell-lac, 11⁄4 lbs.; mastic, 1⁄4 lb.; spirit, 2 quarts.
6. Pale shell-lac, 21⁄4 lbs.; mastic and sandarac, of each 3 oz.; spirit, 1 gall.; dissolve, add copal varnish, 1 pint, and mix by roughly agitating the vessel. All the above are used in the manner described below.
7. Shell-lac, 12 oz.; wood naphtha, 1 quart; dissolve, and add of linseed oil, 1⁄2 pint.
8. Shell-lac, 1⁄2 lb.; gum sandarac, 1⁄4 lb.; spirit, 1 quart; dissolve, add of copal varnish, 1⁄4 pint; mix well, and further add of linseed oil, 1⁄2 pint. The last two require no oil on the rubber.
Obs. The preparation of French polish is precisely similar to that of other spirit or naphthalic varnishes. Sometimes it is coloured, in order to modify the character of the wood. A REDDISH TINGE is given with dragon’s blood, alkanet root, or red sanders wood; and a YELLOWISH TINGE, by turmeric root or gamboge. When it is simply desired to DARKEN the wood, brown shell-lac is employed to make the polish; and when the object is to keep the wood LIGHT COLOURED, a little oxalic acid (2 to 4 dr. to the pint) is commonly added. These substances are either steeped in or agitated with the polish, or with the solvent, before pouring it on the ‘gums,’ until they dissolve, or a sufficient effect is produced. French polish is not required to be so clear and limpid as other varnishes, and is, therefore, never artificially clarified. See Varnish, and below.
FRENCH POL′ISHING. This process, now so generally employed for furniture and cabinet work, is performed as follows:—The surface to be operated on being finished off as smoothly as possible with glass paper, and placed opposite the light, the ‘rubber’ being made as directed below, and the polish (see above) being at hand, and preferably contained in a narrow-necked bottle, the workman moistens the middle or flat face of the rubber with the polish, by laying the rubber on the mouth of the bottle and shaking up the varnish against it, once, by which means the rubber imbibes the proper quantity to cover a considerable extent of surface. He next encloses the rubber in a soft linen cloth, doubled, the rest of the cloth being gathered up at the back of the rubber to form a handle. The face of the linen is now moistened with a little raw linseed oil, applied with the finger to the middle of it, and the operation of polishing immediately commenced. For this purpose the workman passes his rubber quickly and lightly over the surface uniformly in one direction, until the varnish becomes dry, or nearly so, when he again charges his rubber as before, omitting the oil, and repeats the rubbing, until three coats are laid on. He now applies a little oil to the rubber, and two coats more are commonly given. As soon as the coating of varnish has acquired some thickness, he wets the inside of the linen cloth, before applying the varnish, with alcohol, or wood naphtha, and gives a quick, light, and uniform touch over the whole surface. The work is, lastly, carefully gone over with the linen cloth, moistened with a little oil and rectified spirit or naphtha, without varnish, and rubbed, as before, until dry.
The RUBBER for French polishing is made by rolling up a strip of thick woollen cloth (list) which has been torn off, so as to form a soft elastic edge. It should form a coil, from 1 to 3 inches in diameter, according to the size of the work.
FRES′CO-PAINTING. See Painting.
FRICANDEAU. [Fr.] Among cooks, a ragoût, or fricassée of veal. The same term is also sometimes applied to stewed beef, highly seasoned.
FRICASSEE. [Fr.] A dish prepared by stewing or semi-frying, highly flavoured with herbs, spices, or sauce. Small things, as chickens, lamb, &c., and cold meat, are usually formed into fricassees.
FRIC′TION. In a general sense, the act of rubbing one body against another; attrition.
Friction. In mechanics this is the resistance which the surface of a moving body meets with from the surface of the body on which it moves. To lessen the amount of friction in machines, various unctuous substances, as oil, tallow, soap, black-lead, &c., are used by engineers. These substances act by imparting smoothness to the points of contact, and thus reduce their resistance to each other. The full consideration of the subject belongs to engineering.
763
Friction. In medicine, friction, whether simple or conjoined with liniments, is a therapeutical agent of considerable power. By it the circulation is promoted in debilitated parts, and medicinal substances (iodine, mercurials, opium, &c.) are made to penetrate the pores of the skin. “The benefit of friction, which consists of motion and heat, whether or not the same be raised by rubbing the body with a coarse cloth or the flesh-brush, has advantages inconceivable and scarcely credible, by which the obstructions of the pores and cutaneous glandules are opened, their stagnating juices broken into small particles, dissolved, and rendered fit to be carried off in perspiration, in the room of which, as my Lord Verulam well observes, new juice will succeed with new vigour to the body; and longevity, saith that great naturalist, is this way most certainly promoted” (Daniel Turner).
Simple friction is performed by the hand alone, or with a piece of flannel, a hair glove, or a flesh-brush. “If it be properly performed—namely, by short, brisk strokes with the tips of the fingers, and with great celerity, when the naked hand is the agent; and if it be continued for an hour or upwards, and repeated several times a day—its influence in reducing swelled glands and swellings of the joints, as well as in alleviating rheumatic pains, is very great; but, besides being well performed, the friction should be continued for (at least) half an hour, in order to render it useful.” (Dr R. E. Griffith.)
Gentle, slow, and equable friction, by producing a continued repetition of an agreeable impression on the nervous system, acts both as an anodyne and hypnotic. For this purpose “the operator should sit by the side of the bed, and introducing the hand under the bedclothes, rub the legs or the arms (or other parts) gently with equally lengthened but slow movements. When the invalid is a child, its influence is more powerful when aided by a monotonous, but a soft tune, which, although it operates upon a distinct sense, yet, by combination, renders the friction more soporific.” (Griffith.)
When the friction is accompanied with the use of any acrid or irritating substance, or is intended to introduce any active remedy into the system, the rubbing should be brisk, and of sufficient force to slightly abrade and inflame the cuticle; and should be continued until the substance, which is usually in the form of an ointment, either wholly or partially disappears, owing to its absorption by the skin. The hand of the operator should, in most cases, be guarded by a glove; otherwise he is likely to share with the patient the effects of the medicine, a result not always agreeable or even safe.
FRIGORIFIC MIXTURES. See Refrigeration.
FRIT. The pulverulent materials of glass, heated until they coalesce without melting. See Enamel, Glass, &c.
FRIT′TERS. Fried batter. A species of pancake, containing fruit, sweetmeats, poultry, meat, or fish.
Prep. 1. (M. Alexis Soyer.) “The following is thirty receipts in one:”—Soak crum of bread, 1 lb., in cold water, q. s.; take the same quantity of any kind of boiled or roasted meat (a little fat), and chop it into fine dice; press the water out of the bread; put into the pan butter, lard, or dripping, 2 oz., with chopped onions, two teaspoonfuls; fry two minutes, add the bread, stir with a wooden spoon until rather dry, then add the meat, and season with salt, 1 teaspoonful, pepper, 1⁄2 do., and a little grated nutmeg if handy; stir till quite hot; then further add two eggs, one at a time, mix very quickly, and pour it on a dish to cool; next roll it into the shape of small eggs, then in flour, ‘egg’ them, and bread-crum them; lastly, fry in abundance of fat to a nice yellow colour, and serve either plain or with any sharp or other savory sauce you fancy. Innumerable dishes can be made in this way; in fact, from everything that is eatable, and at any season of the year—from the remains of meat, poultry, game, fish, vegetables, &c. The same can be done with chopped, dried, or preserved fruits, simply using a 1⁄4 lb. more bread, and sifting powdered sugar and cinnamon over them. Cream may also be used for fruit, or curds.
Fritters are also (and more commonly) fried in ordinary batter, instead of bread-crumbs. “There is no end to what may be done with these receipts.” “They can be ornamented and made worthy the table of the greatest epicure if the bread be soaked in cream, and spirits or liquor introduced into them.” (Soyer.)
2. Mrs Rundell:—a. (Apple fritters.) See Fruit fritters.
b. (Buckwheat fritters, B. cakes, Bockings.) Made by beating up buckwheat flour to a batter with some warm milk, adding a little yeast, letting it rise before the fire for 30 or 40 minutes, then beating in some eggs and milk or warm water, as required, and frying them like pancakes. Buckwheat fritters, when well prepared, are excellent. Made without eggs and served up with molasses, they form a common dish in almost every breakfast in North America.
c. (Curd fritters.) From dried curd, beaten with yolk of egg and a little flour, and flavoured with nutmeg.
d. (French fritters.) Common pancakes, beaten up with eggs, almonds, and flavouring sugar, orange-flower water, and nutmeg, and the paste dropped into a stew- or frying-pan half full of boiling lard, so as to form cakes the size of large nuts, which are cooked till brown.
e. (Fruit fritters.) From the sliced fruits, with rich batter.
764
f. (Soufflé fritters.) Rich pancakes, flavoured with lemon.
g. (Spanish fritters.) From slices of French rolls soaked in a mixture of cream, eggs, sugar, and spices, and fried brown.
FROG. The esculent variety, in Europe, is the common green or gibbous frog, the Rana esculenta of Linnæus. As an aliment, it is much esteemed on the Continent, the hind legs only being eaten. Its liver is among the simples of the Ph. L. 1618, and was once considered a useful remedy in certain forms of ague.
The Americans eat the bull-frog (the Rana taurina). This variety of the edible frog, which is a native of the Northern States and is much prized as a table delicacy, has been lately introduced into France by the Société d’Acclimatisation. Its flesh, when cooked, is said to have a taste very like that of turtle. In South Africa, a large frog called Matlamétlo is eaten. Frogs are also favourite food with the natives of China and Australia.
FROG OINTMENT or Thrush Mixture. Brown syrup, 90 grammes; verdigris, 6 grammes; strong acetic acid, 10 grammes; solution of perchloride of iron, 2 grammes. (Hager.)
FROST-BITES. When those parts of the body in which the circulation of the blood is most languid are exposed to extreme cold, they quickly become frozen, or, as it is called, ‘frost bitten.’ The fingers, toes, ears, nose, and chin are most liable to this attack. The remedy is long-continued friction with the hands or cold flannel, avoiding the fire, or even a heated apartment.
FROSTBEULENTINCTUR, FROSTBEULENWASSER—Chilblain Tincture, Chilblain Water. Manufactured by a chiropodist of Munich. It is a solution of 2 grammes zinc sulphate in 60 grammes water. (Wittstein.)
FROSTSALBE—Frost Ointment (Wahler, Kupferzell). Mutton tallow, 24; hog’s lard, 24; iron oxide, 4; heat it in an iron vessel, stirring continually with an iron rod until the whole has become black; then add 4 parts Venice turpentine, 2 parts bergamot oil, and 2 parts Armenian bole rubbed smooth with olive oil.
FRUIT. Syn. Fructus, L. Among botanists this is the mature ovary or pistil, containing the ripened ovules or seeds. In familiar language, the term is applied to any product of a plant containing the seed, more especially those that are eatable.
Fruits are extensively employed as articles of diet by man, both as luxuries and nutriment. The fruit of the cereals furnishes our daily bread; that of the vine gives us the well-known beverage, wine, whilst other varieties enrich our desserts, and provide us with some of our most valuable condiments and aromatics. The acidulous and subacid fruits are antiseptic, aperient, attenuant, diuretic, and refrigerant. They afford little nourishment, and are apt to promote diarrhœa and flatulency. They are, however, occasionally exhibited medicinally, in putrid affections, and are often useful in bilious and dyspeptic complaints. The farinaceous fruits (grain), as already stated, furnish the principal and most useful portion of the food of man. The oleo-farinaceous (nuts, &c.) are less wholesome and less easy of digestion than those purely farinaceous. The saccharine fruits, or those abounding in sugar, are nutritious and laxative, but are apt to ferment and disagree with delicate stomachs when eaten in excess. Stone fruits are more difficult of digestion than the other varieties, and are very apt to disorder the stomach and bowels.
As a rule, fruit should never be eaten in large quantities at a time, and only when quite ripe. It then appears to be exceedingly wholesome, and to be a suitable corrective to the grossness of animal food. It also exercises a powerful action on the skin, and is a specific for scurvy in its early stages. Many cutaneous diseases may likewise be removed by the daily use of a moderate quantity of fruit, or other fresh vegetable food. Cases are not uncommon which, after resisting every variety of ordinary medical treatment, yield to a mixed fruit or vegetable diet.
Fruits should be gathered in dry weather, and preferably about noon, because the dew and moisture deposited on them during the night and earlier part of the morning has then evaporated. They should be quite ripe when gathered, but the sooner they are removed from the tree after this point is arrived at, the better. Immature fruit never keeps so well as that which has ripened on the tree; and overripe fruit is liable to be bruised and to lose flavour. The less fruit is handled in gathering the better. Some of them, as PEACHES, NECTARINES, GRAPES, PLUMS, &c., require to be treated with great delicacy, to avoid bruising them or rubbing off the bloom. Some fruit, as a few varieties of APPLES, PEARS, and ORANGES, &c., are gathered before they are fully ripe, in order that they may the better undergo the perils of transit and storage.
Pres. Ripe fruits are commonly preserved in the fresh state by placing them in a cool dry situation, on shelves, so that they do not touch each other; or by packing them in clean, dry sand, sawdust, straw, bran, or any similar substance, with like care, to preserve them from the action of air and moisture. An excellent plan, commonly adopted for dessert fruit in this country, is to wrap each separately in a piece of clean, dry paper, and to fill small, wide-mouthed jars or honey-pots with them. The filled pots are then packed one upon another (see engr.) in a dry and cold place (as a cellar), where the frost cannot reach them. The space (a) between the two pots may be advantageously filled up with plaster of Paris made into a paste with water. The joint is thus rendered air-tight, and the fruit will keep765 good for a long time. The mouth of the top jar is covered with a slate. For use, the jars should be taken one at a time from the store-room as wanted, and the fruit exposed for a week or ten days in a warm dry room before being eaten, by which the flavour is much improved.
Fruit is preserved on the large scale for the London market by placing in a cool situation first a layer of straw or paper, and so on alternately, to the height of 20 or 25 inches, which cannot be well exceeded, as the weight of the superincumbent fruit is apt to crush or injure the lower layers. Sometimes alternate layers of fruit and paper are arranged in baskets or hampers, which are then placed in the cellar or fruit-room. The baskets admit of being piled one over the other without injury to the fruit. The use of brown paper is inadmissible for the above purposes, as it conveys its peculiar flavour to the fruit. Thick white-brown paper is the cheapest and the best.
Fruit Essences (Artificial). These remarkable products first attracted attention at the Exhibition of 1851. To speak somewhat generally, they are mixtures of amylic, butyric, pelargonic, valerianic, and other ethers, in alcohol. By judicious mixture, the flavour of almost any fruit can be more or less perfectly imitated. The artificial essences are generally coloured to represent the juice of the fruit from which they are supposed to be derived. The ESSENCE OF JARGONELLE PEAR and the ESSENCE OF APPLE, which are, perhaps, the best of all the artificial essences, are respectively formed from the ACETATE and VALERIANATE OF AMYLE. See Amyle, Essence, &c.
FRU′MENTY. Wheat boiled in water until quite soft, then taken out, drained, thinned with milk, sweetened with sugar, and flavoured with nutmeg. When currants and eggs are added, it forms ‘Somersetshire frumenty,’ Some persons boil the wheat like rice. “Eaten with milk, in the evening, for some time, it will often relieve costiveness.” (Griffith.)
FRY′ING. “The frying-pan is, without doubt, the most useful of all kitchen implements, and, like a good-natured servant, is often imposed upon and obliged to do all the work, while its companion, the gridiron, is quietly reposing in the chimney corner.” “The usual complaint of food being rendered greasy by frying is totally remedied by sautéing the meat in a small quantity of fat, butter, or oil, which has attained a proper degree of heat, instead of placing it in cold fat, and letting it soak while melting.” “According to the (common) mode in which all objects are cooked which are called fried, it would answer to the French word ‘sauté,’ or the old English term ‘frizzle,’ but to fry any object, it should be immersed in very hot fat, oil, or butter.” “To frizzle, sauté, or, as I will now designate it, semi-fry, is to place in the pan any oleaginous substance, so that, when melted, it shall cover the bottom of the pan by about two lines; and when hot, the article to be cooked is to be placed therein. To do it to perfection requires a little attention, so that the pan shall never get too hot. It should also be perfectly clean—a great deal depends on this.” (Soyer.)
According to the writer quoted above, a chop or steak, for frying, should be chosen 3⁄4 of an inch thick, and should “never exceed one inch, nor be less than half an inch, and to be as near as possible of the same thickness all over.” “An ill-cut chop (or steak) never can be but ill-cooked; you can always equalise them (when badly cut) by beating them out with a chopper.”
“The motive of semi-frying food is to have it done quickly; therefore, to fry a whole fowl, or even half (for example), is useless, as it could be cooked in a different way in the same time; but to semi-fry a fowl (in joints or pieces), with the object of having it quickly placed on the table, in order to satisfy a good, and perhaps fastidious appetite, it should be done in a similar way to that practised in Egypt some 3000 years since, and of late years for the great Napoleon—that is, cooked in oil. In France this dish is called ‘Poulet à la Marengo,’ It is related that the great conqueror, after having gained that celebrated victory, ate three small chickens at one meal done in this way, and his appetite and taste were so good, and he approved of them so highly, that he desired that they may always be served in the same way during the campaign.”
“For many objects I prefer the frying-pan to the gridiron; that is, if the pan is properly used. As regards economy, it is preferable, securing all the fat and gravy, which is often lost when the gridiron is used.” “This simple batterie de cuisine” may be employed “equally as well in the cottage as in the palace, or in the bachelor’s chamber as in the rooms of the poor.” (Soyer.)
FUCH′SIN. See Tar Colours.
FUCUS′AMID, FU′CUSINE, and FU′CUSOL. Compounds obtained by Dr Stenhouse from several varieties of FUCUS by treatment with sulphuric acid, as in the preparation of FURFURINE (which see).
FU′EL. Matter used for the production of heat by burning. The principal substances employed as fuel are—ANTHRACITE, CHARCOAL, COAL GAS, COKE, OIL, SPIRIT, PITCOAL, TURF, and WOOD.
The heating power of almost every description of fuel has been determined by the direct experiments of Lavoisier, Regnault, Andrews, and others; the general principle of their methods consisting in the use of an apparatus wherein the entire heat of combustion was absorbed by a known weight of water, the whole arrangement being protected from the766 influence of external changes of temperature, and the increase of the temperature of the water being known by the simultaneous indication of several delicate thermometers suspended in it. The real value of such determinations is simply relative. The imperfect character of most boiler and furnace arrangements, and the large quantity of fuel which passes into the ‘ash-pit’ unconsumed, together with the irregular ‘draught,’ and the amount of heat absorbed by excess of cold air, result practically in an enormous loss of heating power, even under the most careful management. The mechanical condition of a fuel must be considered in estimating its value. In a series of trials instituted by the Government it was a sine quâ non that the toughness of each kind of coal must be such, for naval use, as to resist, without crumbling, the constant friction in the ship’s hold, at the same time that its ‘fracture’ must be such that it packs into the smallest possible space.[319]
[319] For full information on coal and other fuels, refer to Ure’s ‘Dict. of Arts, Manufactures, &c.,’ Percy’s ‘Metallurgy,’ and Watt’s ‘Dict. of Chemistry.’
In the chemical laboratory COAL GAS is now generally employed as fuel. It is cheap and manageable, and, with proper apparatus, may be made to supply almost any amount of heat. Where gas cannot be conveniently procured, OIL and SPIRIT are used as fuel for lamps. See Anthracite, Charcoal, Coke, Furnace, Pitcoal, &c., also below.
Fuel, Econom′ical. Various mixtures have been recommended under this name. The following is one of the best:—
Prep. Small coal, charcoal, or sawdust, 1 part; clay, loam, or marl, 1 part; sand, or ashes, 2 parts; water, q. s.; make the mass up wet into balls. For use, these balls are piled on an ordinary fire to a little above the top bar. They are said to produce a heat considerably more intense than that of common fuel, and ensure a saving of one half the quantity of coals, whilst a fire thus made up will require no stirring, nor fresh fuel for ten hours. The quantity of the combustible ingredient in them should be doubled, when they are intended to be used with a very little foundation of coal.
Obs. Of late years simple FIRE-CLAY BALLS have been much used for radiating heat from parlour-grates, and so effecting saving in the consumption of fuel. They are very useful for partially filling up those roomy, old-fashioned, badly-constructed grates, which are still to be found in many private houses.
Peat and TURF, both recent and charred, are commonly used as fuel by the lower classes, in neighbourhoods where they are plentiful. Fir cones or TOPS contain a great quantity of solid woody in addition to the resinous matter, and are well adapted for domestic fires.
Fuel, Prepared. Syn. Compressed fuel, Patent f., Steam f. Many artificial fuels are now in use. The greater number have one character in common—they are composed of small coal cemented by some bituminous matter. The following are the principal kinds:—
Fuel, Aberdare Patent Steam. From the ‘small’ of the South Wales Steam Coal mixed with coal, pitch, and compressed by hydraulic machinery. The pitch is broken up, and thoroughly mixed with the small coal over a furnace, in iron pans, in which shafts with obliquely attached blades are continually revolving. The mixture is afterwards pressed into iron moulds by a force equal to about 21⁄2 tons per inch. The weight of a cubic foot of this excellent fuel is 80 lbs.; the space occupied by 1 ton, 28 cubic feet.
Fuel, Case and Morris’s Patent. From the ‘small’ of the ‘best steam coal,’ ground moderately fine, treated so as to absorb a certain portion of liquid coal tar, and then pressed by machinery into blocks. It is said to occupy less space by about 10% than ordinary coal.
Fuel, Grant’s Patent. This is formed of coal dust, 1 cwt., and coal-tar pitch, 20 lbs., melted together by a heat of 220° Fahr., and moulded into blocks the size of common bricks, under a pressure of 5 or 6 tons. These are, lastly, whitewashed. It is heavier than common steam coal, and is said to go fully one third further, by which facility of transport and economy is combined.
Fuel, Purified Block. This is prepared by the torrefaction of washed coal dust, and is said to possess in a remarkable degree the advantages of both coke and steam coal.
FU′LIGOKA′LI. Prep. (Dr Polya.) Caustic potassa, 1 part; water, q. s.; dissolve; add of wood soot, 5 parts; boil 1 hour, dilute with water, filter, evaporate the filtrate to dryness, and put the product at once into warm, dry bottles.—Dose, 2 to 3 gr., thrice a day, made into pills, which must be coated with gum and kept from the air. (See below.)
Fuligokali, Sulphuretted. Syn. Fuligokali sulphuretum, L. Prep. (Dr Polya.) Caustic potassa, 7 parts; sulphur, 2 parts; water, q. s.; dissolve with heat, add of fuligokali, 30 parts, evaporate to dryness, and preserve it in well-corked dry bottles.—Dose, &c. As the last.
Obs. M. Gibert states that he has tried both fuligokali and sulphuretted fuligokali on his patients at the Hospital Saint-Louis, both internally and externally, with manifest advantage in various obstinate chronic skin diseases. He made a pomade of 30 grammes (say 1 oz.) of lead ointment, and 1 or 2 grammes (say 20 to 25 gr.) of fuligokali, in which he recognised positive resolvent, detersive, and stimulant properties. See Anthracokali.
FULLER’S EARTH. Syn. Cimolia, C. terra, L. A soft, unctious, friable, greenish or yellowish-grey species of clay, containing 53% of silica, 10% of alumina, and about 9% of oxide of iron. After being dug out of the767 earth it is thoroughly dried in ovens, and then thrown into cold water, where it soon falls to powder, and is purified by the common process of edulcoration or washing-over. It is extensively used to extract oil and grease from cloth in the process of ‘fulling,’ it forms an excellent filtering powder for oils, and is applied as a cooling and healing dressing by the poor to inflamed breasts, excoriations, &c.
FUL′MINATING COMPOUNDS. These are numerous, and are scattered through several distinct classes of bodies. Among the most powerful and dangerous are the chloride and iodide of nitrogen and the fulminates of silver and mercury.
Fulminating An′timony. Syn. Pyrophorus of antimony, L. Prep. Tartar emetic (dried), 100 parts; lampblack or charcoal powder, 3 parts; triturate together, put it into a crucible that it will three fourths fill (previously rubbed inside with charcoal powder), cover it with a layer of dry charcoal powder, and lute on the cover; after 3 hours’ exposure to a strong heat in a ‘reverberatory furnace,’ and 6 or 7 hours’ repose to allow it to cool, &c., cautiously transfer the solid contents of the crucible, as quickly as possible, without breaking it, to a wide-mouthed stoppered phial, where, after some time, it will spontaneously crumble down into a powder.
Obs. When the above process is properly conducted, the resulting powder contains potassium, and fulminates violently on contact with water. A piece the size of a pea introduced into a mass of gunpowder explodes it on being thrown into water, or on its being moistened in any other manner.
Fulminating Bis′muth. Prep. From bismuth, 120 parts; carburetted cream of tartar, 60 parts; nitre, 1 part. Very rich in potassium.—Prop., &c., resemble those of the last. We have been assured that this is the compound used by the late Capt. Warner for some of his secret fusees.
Fulminating Cop′per. Syn. Fulminate of copper. Prep. Digest copper (in powder or filings) with fulminate of mercury or of silver, and a little water. It forms soluble green crystals that explode with a green flame.
Fulminating Gold. Prep. Recently precipitated peroxide of gold is digested in strong liquor of ammonia for 24 hours, and the resulting product is dried in the open air or at a temperature below 180° Fahr., care being taken to avoid the slightest friction, lest it should explode. A deep olive-coloured powder.
Obs. This compound can only be safely made in very small quantities at a time, as without great care it explodes with extreme violence. This is caused by the slightest friction or sudden increase of heat. Its fulminating property may be destroyed by boiling it in pearlash lye, or weak oil of vitriol; and by heating the residuum after washing it in water, pure gold will be obtained.
Fulminating Mer′cury. Syn. Fulminate, Fulminate of mercury. Prep. 1. Mercury, 1 part; nitric acid (sp. gr. 1·375), 12 parts; dissolve, add at intervals, to this solution, alcohol (sp. gr. ·850), 16·3 parts; apply heat till the effervescence and cloud of gas disappears, adding, gradually, on the action becoming violent, 16·3 parts more of alcohol.—Product. 112% of the mercury employed.
2. Mercury, 100 parts; nitric acid (sp. gr. 1·4), 1000 parts (or 740 parts by measure); dissolve by a gentle heat, and when the solution has acquired the temperature of 130° Fahr., slowly pour it through a glass funnel tube into alcohol (sp. gr. ·830), 830 parts (or 1000 parts by measure); as soon as the effervescence is over, and white fumes cease to be evolved, filter through double paper, wash with cold water, and dry by steam (not hotter than 212°) or hot water. The fulminate is then to be packed in 100 gr. paper parcels, and these stored in a tight box or corked bottle.—Product, 130% of the weight of mercury employed.
Prop., &c. Small brownish-grey crystals, which sparkle in the sun; entirely soluble in 130 parts of boiling water, and deposited as the solution cools under the form of beautiful pearly spangles. It greatly resembles fulminate of silver in its appearance and general properties. It explodes violently by both friction and percussion, but unlike the silver-salt, merely burns with a sudden and almost noiseless flash when kindled in the open air.
Obs. The second formula is not only the cheapest, but the best and safest. The first is more expensive and dangerous. There is no little hazard in pouring the alcohol into the nitric solution; for at each effusion an explosive blast takes place; whereas, by pouring the solution into the alcohol, no danger whatever is incurred. This preparation is used for priming the copper percussion caps for fowling-pieces, muskets, &c. Dr Ure, in his first report to the Board of Ordnance, recommended the use of a spirituous solution of gum sandarac, as the best substance for diluting the fulminate, and fixing it in the caps; but in a subsequent report to the same Board, he stated that a solution of mastic in spirit was to be preferred. At the present time the following composition is applied to the interior of percussion caps in quantities varying from ·2 to ·3 of a grain:—Chlorate of potassium, 26 parts; nitre, 30; fulminate of mercury, 12; sulphur, 17; ground glass, 14; gum, 1, making altogether 100 parts. (Watts.)
Caution. Fulminate of mercury should only be dried in small parcels at a time, and these should be placed at a distance from each other. The dreadful explosion which occurred some years ago at Apothecaries’ Hall, and by which Mr Hennel, the talented chemist of the Apothecaries’768 Company, lost his life, was occasioned by the spontaneous detonation of this substance.
Fulminating Plat′inum. Syn. Platinum fulminans, L. Prep. By acting on binoxide of platinum with pure ammonia. It is analogous to the gold and silver ammonio-compound.
Fulminating Powder. Syn. Detonating powder; Pulvis fulminans, L. Prep. 1. Nitre, 3 parts; carbonate of potash (dry), 2 parts; flowers of sulphur, 1 part; reduce them separately to fine powder, before mixing them. A little of this compound (20 to 30 gr.), slowly heated on a shovel over the fire, first fuses and becomes brown, and then explodes with a deafening report.
2. Sulphur, 1 part; chlorate of potassa, 3 parts. When triturated, with strong pressure, in a marble or wedgwood-ware mortar, it produces a series of loud reports. It also fulminates by percussion.
3. Chlorate of potassa, 6 parts; pure lampblack, 4 parts; sulphur, 1 part. A little placed on an anvil detonates with a loud report, when struck with a hammer. No. 1 is the substance commonly known as ‘FULMINATING POWDER,’ See Blasting Powder.
Fulminating Sil′ver. Syn. Argentum fulminans, L. Two very distinct compounds are known by this name, the one containing oxide of silver and ammonia, and the other being a true fulminate of silver.
Prep. 1. (Ammonia-compound of silver, Berthollet’s fulminating silver.)—a. Digest oxide of silver (recently precipitated and dried by pressure between bibulous paper) in concentrated liquor of ammonia, for 12 or 15 hours, pour off the liquid, and cautiously dry the black powder in the air, in divided portions. The decanted ammoniacal liquor, when gently heated, yields, on cooling, small crystals, which possess a still more formidable power of detonation than the black powder, and will scarcely bear touching, even whilst under the liquid.
b. Dissolve chloride of silver in strong liquor of ammonia, cautiously add pure potassa (in fragments), and when effervescence ceases, decant the fluid portion, and wash and dry the powder, as before.
2. (Fulminate of silver, Brugnatelli’s fulminating silver; Argenti fulminas, L.)—a. Pour alcohol, 1 oz., on nitrate of silver (in fine powder), 100 gr., previously placed in a capacious flask or beaker glass, and shortly afterwards add strong nitric acid, 1 oz.; as soon as all the powdered nitrate assumes the form of white clouds, add cold distilled water, q. s. to suspend the ebullition, and next collect the powder on a filter, and otherwise proceed as with the ammonia-compound (above).
b. (Fownes.) Metallic silver, 40 to 50 gr.; nitric acid (sp. gr. 1·37), 3⁄4 fl. oz.; dissolve by the aid of a gentle heat, add, whilst the solution is still hot, alcohol, 2 fl. oz., and again apply heat until reaction commences; the fulminate slowly separates from the hot liquid under the form of small, brilliant, white, crystalline plates, which, after being slightly washed with a little cold distilled water, are to be distributed upon separate pieces of filtering paper, in portions not exceeding 1 or 2 gr. each, and left to dry in the air. When dry, the papers are to be folded up, and carefully preserved in a box or bottle. A sixpence and the strongest commercial nitric acid and rectified spirit answer for the above purpose.
c. (Liebig.) Grain silver, 1 part; nitric acid (sp. gr. 1·36 to 1·38), 10 parts; dissolve at a gentle heat, and add the solution to alcohol of 85%, 23 parts; apply a gentle heat till the liquid begins to boil, then remove it from the fire and set it aside to cool; the fulminate of silver is deposited in lustrous, snow-white, acicular crystals, and when washed and dried, equals in weight that of the silver employed.
Prop., &c. The properties of both compounds are very similar. Those of the true FULMINATE OF SILVER (No. 2) need only be considered here. This dissolves in 36 parts of boiling water, but the solution deposits the greater portion of the fulminate as it cools. It is one of the most dangerous substances for which we are indebted to modern chemistry. It explodes with unparalleled violence by friction or percussion, or when strongly heated, or when touched with strong sulphuric acid; the metal is reduced, and a large volume of gaseous matter suddenly liberated. Strange to say, though its explosive tendency is so great that it can be hardly made, handled or kept, with safety, it may, when very cautiously mixed with oxide of copper, be burned in a tube to determine its composition, in a similar manner to that employed in the analysis of other organic substances. Many frightful accidents have happened from the spontaneous explosion of this substance. 1 or 2 gr. are the most that can be exploded with safety in a building or confined space.
Fulminating Zinc. Syn. Fulminate of zinc; Zincum fulminans, Zinci fulminas, L. Prep. From fulminate of silver, zinc filings, and a little water, digested together, as FULMINATING COPPER.
FULMINA′TION. Syn. Fulminatio, L. Detonation. A sudden explosion, accompanied with a loud report and extreme violence. Some chemists, without sufficient reason, have endeavoured to confine the application of the term to the explosion of a fulminate.
FUMIGA′TION. Syn. Fumigatio, Suffumigatio, L. Fumigations (FUMIGATIONES) are vapours of gases extemporaneously extricated for the purpose of destroying contagious or noxious miasmata or effluvia, or to mask unpleasant odours, or to produce a medicinal action on those parts of the body with which they are brought in contact.
Fumigations, for the purpose of obviating or769 masking unpleasant odours in the sick chamber, must never be employed to the neglect of cleanliness and ventilation; for most of them, instead of purifying the air, actually render it less fit for respiration. The common practice of burning scented paper, pastilles, sugar, juniper berries, benzoin, cascarilla, &c., so as to create an odoriferous smoke, is of this character. As disinfecting agents, they are probably useless, and are relics of an ancient custom of burning frankincense and other odorous substances in vitiated air, to overcome the fetor which is more or less present. The fumes thus diffused through the atmosphere “disguise unpleasant odours; but they accomplish nothing more. The infection remains not only unaltered by the diffusion of the most powerful aromatic vapours, but its deleterious properties are sometimes augmented by them.”[320]
[320] We deem it right to remark that a different opinion respecting the disinfecting power of odoriferous smoke is now held by many scientific men. According to this opinion, the minute particles of aromatic substances do really destroy or render inert the noxious miasmata.
Among the various substances used as DISINFECTING FUMIGATIONS, chlorine, by almost general consent, holds the first place. Dr Carmichael Smyth recommended nitrous acid, which is even now preferred by Dr Christison to chlorine; whilst Prof. Graham regarded the fumes of burning sulphur as more efficacious than either of these substances. The vapours of hydrochloric acid and of vinegar, and the smoke of gunpowder, which once had their advocates, have now justly sunk into disfavour.
No apartment should be submitted to fumigation until it is vacated; as until then its thorough disinfection is impossible, and but little benefit or immunity from contagion is conferred by any aërial disinfecting agent, the presence of which fails to cause discomfort to the patient.
Of all common diseases, scarlet fever appears to be the one most requiring fumigation. For this purpose, chlorine gas or heat should be employed. The infectious matters of certain diseases, especially scarlet fever, are either dissipated or destroyed at a heat slightly above that of boiling water. (Dr Henry.) Contagious diseases are very commonly propagated in this metropolis by persons having their linen washed by laundresses who perform their operations in the same sinks of dirt and misery in which they live. See Cigars (in pharmacy), Disinfectant, Inhalation, &c., and below.
Fumigation, Ace′tic. Syn. Fumigatio acetica, L. The fumes of strong vinegar or acetic acid, obtained by heating the liquid over a lamp, or by sprinkling it on a hot shovel. Aromatic vinegar in this way yields very refreshing fumes, and was formerly thought more efficacious than simple acetic acid.
Fumigation, An′odyne. Syn. Fumigatio anodyna, L. Prep. (Trousseau & Reveil.) Stramonium and sage, equal parts, sufficient to fill a small pipe. Smoked in spasmodic asthma, irritating coughs, &c.
Fumigation, Aromat′ic. See Balsamic fumigation.
Fumigation, Balsam′ic. Syn. Aromatic fumigation; Fumigatio aromatica, F. balsamica, L. Prep. 1. From gum benzoin, either alone or mixed with olibanum or styrax, thrown on hot cinders or a heated shovel.
2. (Dr Dohrn.) Gum olibanum, 4 parts; gum benzoin, styrax, and flowers of roses and lavender, of each 1 part; to be reduced to powder, and used as before.
3. Amber, mastic, and olibanum, of each 3 oz.; benzoin and styrax, of each 1 oz.; camphor, 1 dr. As last. The above are used in hooping-cough, asthma, &c.; a small quantity only being employed at a time.
Fumigation, Belladon′na. Syn. Fumigatio belladonnæ, L. Prep. (M. Schroeder.) From dried belladonna leaves, 1 to 2 dr.; as before. In spitting of blood, asthma, tickling cough, &c.
Fumigation, Chlorine. Syn. Disinfecting fumigation, Guyton-morveau’s f.; Fumigatio chlorinii, L. Prep. 1. (P. Cod.) Common salt, 3 parts; water and sulphuric acid, of each 2 parts; black oxide of manganese 1 part; mix in a shallow vessel, placed in the centre of the apartment. This is used to disinfect unoccupied rooms.
2. Hydrochloric acid and powdered black oxide of manganese mixed in proportions so as to make a thin paste. Used as directed under 1.
3. Chloride of lime, either sprinkled on the floor (if uncarpeted) or (if carpeted) placed about the room in shallow dishes. Used for inhabited rooms, and on shipboard, &c.
4. A solution of chloride of lime (1 oz. of the chloride to each quart of water). Used as the last but more freely.
Obs. Chlorine fumigations, although so popular, and so much relied on by many medical practitioners, are apparently useless in preventing the progress of certain contagious diseases. “In Moscow, chlorine was extensively tried and found unavailing, nay, even injurious, in cholera.” (Dr Pereira.) “At the time that the cholera hospital was filled with clouds of chlorine, then it was that the greatest number of the attendants were attacked.” (Dr Albers.) At the Smallpox Hospital, where chlorine was tried, with the view of arresting the progress of erysipelas, “all offensive smell was removed, but the power of communicating the disease remained behind.” (‘Lond. Med. Gaz.’) Notwithstanding these marked failures, the confidence of many eminent members of the profession continues unabated. “As a fumigating agent, disinfectant and antiseptic, chlorine, I believe, stands unrivalled.” “For destroying miasmata, noxious effluvia, and putrid odours, it is the most powerful agent known.” (Dr Pereira.) Our own experience leads us to the conclusion that chlorine is more useful in neutralising the contagious or morbific matter of fevers (especially770 of scarlet fevers) and putrid diseases generally, than of the other diseases in which it has been employed.
Fumigation, Hydrochlo′′ric. Syn. Muriatic fumigation; Fumigatio muriatica, F. acidi hydrochlorici, L. Prep. From common salt placed in a cup or saucer, and an equal weight of sulphuric acid poured over it. Now seldom used. It rapidly neutralises ammoniacal fumes.
Fumigation, I′odine. Syn. Fumigatio iodinii, L. Prep. 1. From iodine, 5 to 25 gr., or more, according to extent of surface, placed on a heated iron contained in a box or case in which the limb is enclosed. In the usual skin diseases in which the use of iodine is indicated. Iodine may be readily diffused through the atmosphere by placing a small quantity on a hot plate. Duroy says iodine powerfully arrests putrefaction.
2. (Compound; Fumigatio iodinii composita—Sellers.) Iodine, 20 gr.; red sulphide of mercury, 40 gr.; sulphur, 6 dr.; mix, and divide into 12 powders. One to be used, as the last, 3 times daily; in lepra, psoriasis, &c.
Fumigation, Mercu′′rial. Syn. Fumigatio Mercurialis, L. Prep. (Bouchardat.) Olibanum (in powder), 2 parts; red sulphide of mercury, 3 parts. A little is sprinkled on red-hot coals or a heated shovel held beneath the part; or the fumes are inhaled. Obs. Abernethy used the black oxide of mercury (11⁄2 to 2 dr.), and applied it to the whole body, excepting the head, in a similar way to the sulphur bath, and continued the application for about a quarter of an hour. See Candles, (Mercurial), and No. 2 (above).
Fumigation, Mu′riatic. See Hydrochloric F. (above).
Fumigation, Ni′trous. Syn. Fumigatio nitrosa. Prep. (P. Cod.) Sulphuric acid, diluted with half its weight of water, is placed in a porcelain cup (any shallow vessel of glass or earthenware will do), placed over heated cinders, and small quantities of powdered nitre added to it from time to time.
Obs. Heat causes the gas to be evolved more rapidly, and thus renders the fumes more offensive, without increasing their efficacy. Equal weights of oil of vitriol and water are the proportions usually employed, 1⁄4 oz. of nitre is said to be sufficient for a small room. (Dr Bateman.) The vessel containing the ingredients should be placed in an elevated position in the centre of the apartment.
Dr Carmichael Smith, who introduced nitrous acid gas as a fumigation (1799), received a reward of £5000 from Parliament for publishing his formula.
Fumigation, Sulphu′rous. Syn. Fumigatio sulphurosa, F. sulphuris, L. Prep. 1. The gas produced by burning sulphur, sulphurous anhydride, or, as Mr Keates has suggested, by burning bisulphide of carbon.
To guard against the danger arising from fire, when sulphur is burnt for the purposes of fumigation, the operator is advised to proceed as follows:—Having closed the fireplace, windows, &c., of the apartment to be disinfected, procure a common pail or a large earthenware pan, and place it in the centre of the room; then into the middle of the pail or pan put upside down an ordinary flower-pot. Then pour water into the pail or pan (as the case may be) until it nearly reaches to the top of the inverted flower-pot. Now stand on the flower-pot a plate or saucer of earthenware or common crockery, sufficiently large to hold the quantity of sulphur required; place this quantity of sulphur in the plate or saucer, and put on it a few live coals; then close the door of the apartment, and leave it undisturbed for six or eight hours. At the expiration of this time the door may be opened, as well as the windows, the barricade being at the same time removed from the fireplace; a thorough draught of air being thus established, the sulphurous smell will soon disappear. During the fumigation all articles within the room should be spread out so as to expose as great a surface as possible. “The cubic space to be thus disinfected should be calculated by multiplying the length, height, and breadth together, and taking an ounce and a half of sulphur for every 100 cubic feet. For a small bedroom one pound of sulphur would be sufficient. Indeed, eighteen ounces would suffice for a room measuring 12 ft. × 10 ft. × 10 ft.”[321]
[321] ‘Water, Air, and Disinfectants,’ by Noel Hartley.
2. Flowers of sulphur, 7 parts; nitre, 4 parts; benzoin and olibanum, of each 2 parts; camphor, 1 part; pressed into the bowls of tobacco-pipes, and lighted with a quick-match. See Bath and Disinfectant.
Fumigation, Tar. Syn. Fumigatio picea, Suffumigatio picis liquidæ, L. Prep. 1. Vegetable tar, 1 part; water, 7 or 8 parts; mix, and let it simmer in an open vessel set over a spirit lamp placed near the centre of the apartment.
2. (Sir A. Crichton.) Norway tar, 1 lb.; powdered carbonate of potash, 1⁄2 oz. or 1 oz.; mix, and heat it as last. The potash is added to neutralise any volatile acid. Formerly highly thought of in bronchitis and pulmonary consumption.
Fumigation, Tooth′ache. Syn. Fumigatio odontalgica, F. anti-neuralgica, L. Prep. 1. From henbane seeds, powdered and thrown into a basin of boiling water, and the affected part held in the steam. Sometimes a little of the seed is placed on a heated iron spoon, and the part exposed to the fumes.
2. (Beasley.) A popular remedy is to throw henbane seed on hot cinders, inverting a cup over them to receive the smoke and empyreumatic oil produced. The cup is then filled with hot water, and the steam conveyed to the affected side of the mouth.
FU′′MING LIQUORS. See Ammonium Sulphydrate, Arsenic Trichloride, Tin Bichloride, 771&c.
FUNG′I. In botany, a natural order of cellular plants, producing their fructification in the air; growing in or upon decaying or living organic substances, and nourished through their vegetative structure called the spawn or mycelium. Fungi have very variable properties. Some are medical, others edible, others are deadly poisons. The various diseases of plants known as blight, mildew, rust, smut, vine-mildew, potato-disease, ergot, &c., are either caused by or accelerated by the agency of fungi. See Agaric, Mushroom, &c.
FUR′NACE. An enclosed fireplace for obtaining a high degree of heat. Furnaces vary much in construction and size, according to the particular manufacture in which they are employed. They may be broadly divided into two classes—WIND-FURNACES and BLAST FURNACES. In the former a high temperature is produced without the aid of bellows by means of a powerful draught. In the latter heated air is blown in through a pipe or pipes at the bottom. For many metallurgic and large chemical operations REVERBERATORY FURNACES are employed. A furnace of this kind is usually long, with a low roof to keep down the flame and hot air upon the ‘hearth’ or space between the fireplace and the flue.[322] For the smaller operations in chemistry, a variety of furnaces have been invented, and the introduction of coal-gas as a fuel by Develle, Griffin, Gore, Fletcher, and others, has wrought a complete change in the arrangements of the laboratory. The GAS-FURNACES of Mr J. J. Griffin are adapted for almost every operation performed by the aid of heat. Those more recently introduced by Mr W. Gore are very compact and portable, and will rapidly produce a ‘white heat,’ without the help of bellows or high chimney, by means of ordinary coal-gas and atmospheric air. The first and smallest size consumes 33 cubic feet of gas (value seven farthings) per hour, and is suitable for assayers, jewellers, analytical chemists, experimentalists, dentists, and others. It is capable of fusing eight ounces of copper or six ounces of cast iron, copper begins to melt in it in about twelve minutes from the time of lighting. The second-sized one consumes about twice that quantity of gas, is suitable for manufacturing jewellers generally, and for a great variety of practical persons who require to melt small quantities of gold, silver, copper, german silver, brass, cast iron, glass, and other substances, or require a small crucible heated to high temperatures. It is capable of melting 45 ounces of copper, or 40 ounces of cast iron, and with its heat up it melts one pound of copper in eight minutes; copper begins to melt in about twenty minutes from the time of lighting.
[322] For an illustration of this kind of furnace, see Sodium, Carbonate of.
Fletcher’s[323] Universal Furnaces for high temperatures, which are said to require neither blast nor attention, are intended for laboratory purposes, enamel burning, heating soldering irons, and for jewellers’ and dentists’ work. These furnaces are made in two distinct types; one with a perforated cover to the crucibles and muffles to attain the maximum heat; the other with a slide chimney and a double lid over the crucible.
[323] Manufactured by Thos. Fletcher, Museum Street, Warrington.
The power and rapidity of working depend in each case on the length of the chimney used. A furnace with a four-feet chimney will melt a crucible of cast-iron in thirty-five minutes; a furnace with an eight-feet chimney will melt the same quantity of iron in about twenty minutes, starting with the furnace cold. The stove with the side chimney, although more convenient in use, is slower in working, taking about twice as long to obtain the same temperature.
The following are varieties of Fletcher’s Universal Furnace:—
1. Small Laboratory Furnace for crucibles, with nickel-plated burner tubes. This takes crucibles up to 21⁄2 by 21⁄4 inches outside, and with a three-feet chimney, as supplied with the furnace, will, it is stated, melt copper, gold, silver, &c., in about ten minutes, or cast-iron in thirty-five minutes from the time the gas is lighted. Small muffle fittings, with muffles 21⁄4 by 3 by 21⁄2 inches inside, can be supplied with this furnace.
2. Small Crucible Furnace, with fixed chimney. This furnace is more especially designed for gold, silver, copper, &c., and, as sent out with a four-feet chimney and a single lid, is amply powerful, and practically of a very convenient form.
3. Small Muffle Furnace, with three feet chimney. This requires about eighteen inches longer chimney than the small crucible furnace to obtain the same temperature in the same time, owing to a slight loss of heat by radiation from the stoppers.
4. a. Large Muffle Furnace. This is identical in design and construction with the smaller one. The clear working space inside the muzzle is 37⁄8 by 5 inches, by about 3 inches deep. This is recommended as a useful furnace for watch dial enamellers, assayers, photo-enamel burning, and for all purposes where exact temperatures are required not exceeding the fusing point of cast iron.
The burner of this furnace is twice the size of the small laboratory furnace, and requires a gas supply from a pipe and tap of half-an-inch bore. The burner is the same shape as the muffle, and is unfit for crucible work.
b. Extra Large Muffle Furnace 41⁄2 by 33⁄4 by 7 inches clear inside working space. This will take a No. 3 plumbago pot, and with half an inch gas pipe, giving a supply of about 35 feet per hour, will, it is affirmed, melt 3 or 4 lbs. of brass in about 25 minutes, and the same quantity of cast iron in 60 or 70 minutes772 from the time the gas is first lighted, without the slightest trouble or attention.
5. Ladle Furnace. This takes ladles up to 61⁄2 inches diameter, and will melt 6 or 8 lbs. of zinc in about 15 minutes, or the same quantity of lead, tin, &c., in about half the time. It is said to be a convenient and powerful arrangement for dentists, heating soldering-irons, making granulated zinc, sand baths, &c.
6. Small Laboratory Furnace, complete for crucibles, muffles, ladles, and sand baths.
7. Fletcher’s Injector Gas Furnace (with Blast). This furnace is intended for general purposes, and for the treatment of refractory substances at high temperatures. The patentee states “that it will burn perfectly in the same space any available gas supply from 10 to 50 feet per hour, or more, if required, giving temperatures in exact proportion; and any operation may be repeated at any time by taking a note of the position of the air slide which governs the combustion of the gas.”
Mr Fletcher gives the power of the small furnace as follows:—With an 1⁄2 inch gas supply-pipe, day pressure, starting with the furnace cold, it will melt silver in 3 minutes, cast iron in 8 minutes, cast steel in 25 minutes.
With a supply of 50 feet per hour, the same results are stated to be obtained in a little over half the time, and so on in proportion with a greater or less gas supply. It is also said to work satisfactorily for gold, &c., melting it with a supply of gas too small for any other furnace, and the maximum temperatures obtained are limited, only by the available gas supply and the fusibility of the casing. The highest temperature as obtained by measuring by Wedgwoods’ Pyrometer, is said to be 9000 Fahrenheit. This furnace is stated to be particularly suited for gold and silver melting, and refining, iron assays, and general crucible work, and safe in the hands of the most careless workman. It is adapted for crucibles not exceeding 4 inches by 21⁄4, 5 inches by 31⁄2, 71⁄2 inches by 5. For further information respecting furnaces intended for use in the laboratory and assay office, the reader is referred to ‘Watt’s Dictionary of Chemistry,’ also to ‘Ure’s Dictionary of Arts, Manufactures, and Mines,’ for description of the furnaces employed in the different metallurgical operations; and to the ‘Chemical News’ (June 30th, 1876, and February 2nd, 1877), for a description of a new decomposing furnace. See Assaying, Chimneys, Copper, Crucible, Fuel, &c.
FUR′NISHING. It is essential for the sake of neatness, and for a pleasing effect to the eye, that there should be a harmony of colours, and also a similarity of style, in the main articles of furniture. The tints of the carpet, of the paper or paint of the walls, and of the window-curtains, should be all in harmony in each room; that is, either possess a general resemblance of colour, or various colours in pleasing contrast and harmony with each other. If the preponderating colour of the curtains is scarlet, and the colour of the walls or carpet blue, a most inharmonious and unpleasing effect is produced; but brown and green, or green and gold, will be in harmony, and may, therefore, be placed together. Carpets being the most expensive articles, it is safest to buy them first, and then to let their colour guide us in the tone and style of the curtains, paper-hangings, chair-covers, hearth-rugs, and the various minor articles. It is also economical to buy carpets of the same pattern for several rooms, because in the event of removal to a house with different sized apartments, a piece of one carpet may be taken to alter the size of another.
FUR′NITURE. See French Polishing, Oil, Polish, Varnish, &c.
FURS. Of these the most valuable are Ermine and Sable. Fur skins, when unprepared, or merely dried, go under the name of ‘Peltry.’ (Brande.)
Furs may be preserved from moths and other insects by placing a little colocynth pulp (bitter apple), or spice (cloves, pimento, &c.), wrapped in muslin, among them; or they may be washed in a very weak solution of corrosive sublimate in warm water (10 to 15 gr. to the pint), and afterwards carefully dried. As well as every other species of clothing, they should be kept in a clean, dry place, from which they should be taken out occasionally, well beaten, and exposed to the air, and re-turned.
FU′SEL-OIL. Syn. Fousel oil, Potato-oil, Oil of potato spirit, Grain oil, Grain-spirit oil, Marc-brandy oil, Crude hydrated oxide of amyl. Source. An offensive, strong-smelling oil, produced along with alcohol during the fermentation of grain, potatoes, &c., on the large scale, and which gives the peculiar and disagreeable flavour and odour to raw whiskey. It is found chiefly in the last portion of the spirit which passes over, called the ‘faints,’ to which it imparts its characteristic odour and flavour. By rectifying the faints at a very gentle heat, most of the alcohol and water first pass over together with only a little fusel oil, whilst the latter forms the residuum in the still. Various names (as above) are given to the crude oil thus obtained, according to its source. In each case it essentially consists of hydrated oxide of amyl, but trifling and variable quantities of other organic compounds are mixed with it, which slightly modify its character, more particularly its odour and flavour. The oil of potato spirit is the purest form of crude fusel oil.
Obs. The exertions of the distiller are directed, as much as possible, to lessen the formation of fusel oil during the fermentation of his ‘worts,’ and to eliminate, during the distillation and rectification of his liquors, the greatest possible proportion of that with which they may be contaminated.
Prop., &c. Fusel oil is a nearly colourless773 volatile liquid, with a rather high boiling point, a durable, penetrating, offensive smell, and an acrid, burning taste; when swallowed, it occasions nausea, giddiness, headache, &c.; in slightly larger quantities, vomiting, delirium, oppressive respiration, and lessened sensibility to pain; its vapour also produces these effects. In quantity, it is a narcotic poison. The greater intoxicating power of whiskey, more especially that from raw grain, than other spirit, is due to the larger quantity of fusel oil which it contains. This appears to be well known to the lower class of whiskey drinkers in these countries, and to the consumers of corn brandy in some of the northern parts of Europe. The last are said to frequently demand to be served with “a glass of good fusel.” In England fusel oil is chiefly used for lamps and varnishes.
Purific. The AMYLIC ALCOHOL (ALCOHOL AMYLICUM) of the Dublin College is thus prepared. Introduce the ordinary fusel oil of the distilleries into a small still or retort, connected with a condenser, and apply heat; as soon as the oil begins to flow over, unmixed with water, the receiver should be changed, and the distillation resumed, and carried nearly to dryness; the product in the second receiver, and the oily matter which separates from the water in the first receiver, are to be reserved for use. It is employed in the preparation of VALERIANATE OF SODA. See Amyl.
FU′SIBLE ALLOY′. Syn. Fusible metal. Prep. 1. Bismuth, 2 parts; lead, 5 parts; tin, 3 parts. Melts in boiling water.
2. (D’Arcet’s.) Bismuth, 8 parts; lead, 5 parts; tin, 3 parts. Melts below 212° Fahr.
3. (Walker.) Bismuth 8, tin 4, lead 5 parts; antimony, 1 part. The metals should be repeatedly melted and poured into drops, until they are well mixed.
4. (Onion’s.) Lead, 3 parts; tin, 2 parts; bismuth, 5 parts. Melts at 197° Fahr.
5. To the last, after removing it from the fire, add of quicksilver (warm), 1 part. Liquid at 172°, solid at 140° Fahr.
Obs. The first four of the above are used to make TOY-SPOONS, to surprise children by their melting in hot liquors. A little mercury may be added to lower their melting points. Nos. 2 and 3 are specially adapted for making ELECTROTYPE MOULDS. The beautiful casts of the French medals known to all electrotypers as Clichée moulds are in the alloy No. 3. The above alloys are also used to form PENCILS for writing on asses’ skin, or paper prepared by rubbing burnt hartshorn into it, &c.; also as a METAL BATH in the laboratory. The last is used for ANATOMICAL INJECTIONS.
FU′SION. Syn. Fusio, L. The liquefaction of solid bodies by the action of heat. The term AQUEOUS FUSION has been applied to the melting of salts in their combined water when heated; and the term IGNEOUS FUSION, to the liquefaction of bodies by heat alone.
The vessels in which substances are fused are formed of various materials and shapes, according to the properties of the solid operated on, and principally with reference to the degree of heat required for its fusion. In every case the containing vessel should be capable of sustaining the proper degree of heat, without either melting or cracking, and should also be unacted on by the substances melted in them. See Crucible, Furnace, &c.
FÜRSTENBALSAM, Bamberger für Frauen—Bamberg Prince’s Balsam for Women. An embrocation for strengthening women after confinement. A hexagonal eau de Cologne bottle containing about 100 grammes of a clear reddish-brown fluid, which is a filtered mixture of equal parts of spirit of lavender (Sp. Lavand. Co.) and spirit of soap, mixed with a little camphor and ammonia. (Hager.)
FUS′TIC. Syn. Fustic wood. Two distinct dye-stuffs are known by this name, but are distinguished by the adjectives ‘old’ and ‘young.’
Fustic, Old. Syn. Bois jaune, Fr. The wood of the Maclura tinctoria. Its decoction dyes woollens yellow of different shades, according to the ‘mordant.’ Alum, tartar, and spirits of tin brighten the tint; acetate and sulphate of iron and common salt darken it; with sulphate of iron it gives olives and browns; with the indigo vat and sulphate of indigo green. These colours are very permanent. Its yellow turns on the lemon when pale, and on the orange when darker. 1 lb. of old fustic will dye 3 to 5 lbs. of wool.
Fustic, Young. Syn. Yellow fustic; Fustet, Fr. The wood of the Rhus Cotinus or Venice sumach. It gives a yellow turning on the green, but its colours are not very permanent. It is chiefly used in combination with other dye-stuffs.
GAL′BANUM. Syn. Gum galbanum; Galbanum (B. P.), L. “A gum-resin derived from an unascertained umbelliferous plant. In irregular tears about the size of a pea, usually agglutinated into masses; of a greenish-yellow colour, translucent, having a strong disagreeable odour, and an acrid bitter taste.” (B. P.) Its properties are similar to the other fetid antispasmodic gum-resins. It ranks between ASSAFŒTIDA and AMMONIACUM.
Galbanum, Strained. Syn. Prepared galbanum; Galbanum colatum, G. præparatum (Ph. L.), L. From crude galbanum, as prepared ammoniacum. Formerly the common practice was to melt it in the dry state, by heat cautiously and quickly applied, and to strain it through a piece of coarse canvas stretched across a wooden frame or ‘horse.’ The ‘strained galbanum’ of the shops is seldom pure. The following forms are current in the trade for its ‘reduction,’ as this species of adulteration is technically termed:—
1. Galbanum (true), 9 lbs.; strain as above, then add, towards the end black resin (clean),774 3 lbs.; and when the whole is melted, further add of Venice turpentine, 2 lbs.—Product. 12 lbs.
2. Strained galbanum and black resin, of each 6 lbs.; melt, and add, of strained assafœtida, 2 oz.; Venice turpentine, 3 lbs.—Prod. 141⁄2 lbs.
Galbanum, Facti′′tious Strained. Syn. Galbanum colatum factitium, L. Prep. 1. From black resin, 4 lbs.; melt, and add of Venice turpentine, 2 lbs.; assafœtida, 21⁄2 oz.; oils of juniper and fennel, of each 11⁄2 dr.; water, 1⁄2 pint.
2. As the last, adding soft soap, 5 oz. Sometimes the small and ‘waste’ of the chests are added to the above to improve them.
GALÈNE-EINSPRITZUNG—Galen’s Injection (J. F. Schwarzlose Söhne, Berlin). According to Hager:—Gum Arabic, 25 grammes; water, 65·5 grammes; sugar of lead, 4·5 grammes; tinct. opii with saffron, 5 grammes. According to Schädler:—Sulphocarbolate of zinc, 3 grammes; gum Arabic, 20 grammes; tinct. opii, 2 grammes; water, 100 grammes.
GALL. Syn. Bile; Bilis, Chole, Fel, L. A bitter fluid secreted by the liver; in part flowing into the intestines, and in part regurgitating into the gall-bladder. Its uses in the animal economy appears to be—to separate the chyle from the chyme, to promote digestion of oleaginous substances, and to assist in exciting the peristaltic action of the intestines. The fæces appear to owe their colour chiefly to the presence of bile, since, without, they appear of a dirty pipe-clay colour.
The gall of various animals was formerly used in medicine. From whatever source it was obtained, it was believed to be calefacient, desiccant, detergent, discutient, and parturifacient; but besides these properties, each variety was conceived to possess virtues peculiarly its own. Thus, bear-gall (fel ursi) was reputed anti-epileptic; eel-gall (fel anguillarum), parturifacient; hare-gall (fel leporis), “good in cataract;” and ox-gall (fel bovis), “sovereign against stiff joints, rheumatics, angry ulcers, and stomach colics.” The gall of the bat, goat, hen, hog, partridge, silurus, &c., were also employed as remedies. At the present time ox-gall is the only one used in medicine and the arts.
Ox-gall has been recently reintroduced into medicine by Dr Allnatt and others, and in certain cases of dyspepsia and biliary derangement appears to be a valuable remedy.
Crude ox-gall is extensively employed by the scourers of woollen cloth, clothes renovators, &c. It rapidly extracts grease and oil from textile fabrics without injuring the colour. See Constipation, Dyspepsia, Ox-gall, &c.
Gall, Glass. See Saniver.
GAL′LATE. Syn. Gallas. L. A salt of gallic acid. The alkaline gallates are soluble. They rapidly suffer decomposition in the presence of excess of the base, and the liquor gradually acquires a blackish colour. The gallates of most of the other metallic oxides are insoluble.
GALLEN-MIXTUR FÜR PFERDE—Gall Mixture for Horses (F. Barth, veterinary surgeon, Freibach-by-Altenhofen, Carinthia). A clear decanted solution of 8 parts wood tar in 92 parts common kienöl (ol. pini). (Hager.)
Gallen-Mixtur—Gall Mixture (Ph. Barth, Marburg in Steiermark). The same preparation as the above, coloured with 3⁄4 per cent. of dragon’s blood. (Wittstein.)
Gallen-Tinctur—Gall Tincture (Dr G. Krieger, Garz). 5 parts wood tar, 10 parts water, 30 parts spirit, 1 part corrosive sublimate, and 1⁄20 part rosanilin, mixed with a gentle heat, allowed to deposit, and filtered. (Hager.)
GAL′LIC ACID. H3C7H3O5.Aq. Syn. Acidum gallicum (B. P.), L. “A crystalline acid prepared from galls.” (B. P. L.) It may be also obtained from other vegetable substances. It appears to be a product of the oxidation of tannic acid, and probably does not exist ready formed in recent vegetables.
Prep. 1. (Dumas.) Nut-galls, reduced to powder, are moistened with water, and exposed to the action of the air, in a warm situation (say 70° to 80° Fahr.), for two or three months, adding more water, from time to time, to make up for that lost by evaporation. At the end of the above period the mouldy, dark-coloured mass is strongly pressed in a cloth, and the solid portion boiled in a considerable quantity of water. The solution (filtered whilst hot) deposits, on cooling, crystals of gallic acid, which, after being thoroughly drained and pressed dry between bibulous paper, are purified by boiling them along with about 1⁄6th of their weight of prepared animal charcoal in 8 parts of water, and filtering, &c., as before.
2. (Graham.) A strong infusion or decoction of galls is precipitated with sulphuric acid in the cold; the resulting thick mass is mixed with dilute sulphuric acid (cold), and the liquid expressed; the ‘marc’ is next treated with sulphuric acid diluted with twice its weight of water, and after boiling the mixture for some minutes the whole is allowed to cool; the resulting crystals are purified as before.
3. (Liebig.) A strong aqueous solution of tannic acid (tannin) is added to sulphuric acid as long as a precipitate falls; the powder is collected, washed, and dissolved by the aid of heat in dilute sulphuric acid; the solution, after being boiled for a few minutes, deposits, on cooling, crystals of gallic acid in considerable quantity.
4. (Scheele.) A filtered decoction of galls is exposed for some months in an open vessel; after a time it grows mouldy, and becomes covered with a thick, glutinous pellicle; in two or three months the sides of the vessel and the under portion of the pellicle are found775 to be covered with small yellow crystals of gallic acid, which are purified as directed above. (See No. 1.)
5. (Ph. D., B. P.) The Dublin contains two formulæ for gallic acid, the one being based on that of Dumas or Scheele, the other on that of Graham or Liebig.—a. From galls (in coarse powder), 1 lb.; water, q. s. to make a stiff paste; a porcelain dish is ordered, and the exposure in the moistened condition is to be continued for 6 weeks; the solution of the first crop of crystals is to be made in 10 fl. oz. of boiling water, and when the filtrate has cooled to 80° Fahr., it is to be poured off from the crystals which have formed, which are then to be washed with ice-cold water, 3 fl. oz., and dried—first in blotting paper, and finally by a steam or water heat. By boiling the undissolved portion of the galls with 45 fl. oz. of fresh water, more crystals may be obtained.
b. Powdered gall-nuts, 1 lb., are steeped for 24 hours in water, 1 pint, and after being placed in a porcelain displacement apparatus, are treated with water, 11⁄2 pint, added in successive portions; oil of vitriol, 5 fl. oz., diluted with an equal volume of water, and allowed to cool, is now added to the percolated infusion, and after thorough admixture the liquid is filtered from the viscid precipitate which forms; oil of vitriol, 5 fl. oz. (diluted as before), is then added to the filtrate, the precipitates, enveloped in calico, are submitted to powerful pressure, and subsequently dissolved in oil of vitriol, 16 fl. oz., previously diluted with water, 56 fl. oz.; the solution is boiled for 20 minutes, and set aside for a week; at the end of this time the deposit which forms is dissolved in three times its weight of boiling water, and the solution treated as before.
Prop. Gallic acid forms small, feathery, and nearly colourless crystals, which have a beautiful silky lustre; that of commerce is usually of a pale-yellow colour; it is soluble in 100 parts of cold water, and in 3 parts of boiling water; it is also soluble in alcohol, and slightly so in ether; the aqueous solution is decomposed by exposure to the air; dissolved in hot oil of vitriol, it forms a deep, rich, red solution, which, when thrown into water, drops the gallic acid, deprived of some of its water. This substance is soluble in the alkalies, and dyes cloth like madder. When strongly heated, gallic acid is converted into metagallic acid, or into pyrogallic acid, according to the manner in which the heat is applied.
Tests. Gallic acid is distinguished from tannic acid by not affecting solutions of gelatin, the protosalts of iron, or the salts of the alkaloids, and by giving a deep bluish-black precipitate with the sesquisalts of iron, which disappears when the liquid is heated. It is distinguished from pyrogallic acid by its inferior insolubility in water, and by its not affecting the solutions of the protosalts of iron. To detect gallic acid mixed with tannic acid, the latter should be removed, either by digesting the substance in ether, or by immersing for some time in its solution a piece of skin depilated by lime, previously to applying the tests.
Pur. Free from colour; decomposed by heat; soluble in water and in rectified spirit. It turns preparations of the sesquioxide of iron, dissolved in water, of a bluish black colour, but throws down nothing from a solution of isinglass.
Uses, &c. The principal use of pure gallic acid is in the art of photography. It has recently been employed in medicine, as an internal astringent, in doses of 3 to 10 gr., thrice a day, or oftener; in hæmorrhage and fluxes, as well as for checking the night sweats in phthisis. Dr Todd says, that in all cases of internal hæmorrhage, or hæmorrhagic tendency, it is the best astringent or styptic we possess. As an external astringent, it is greatly inferior to tannic acid. It has been given in doses of 15 to 30 gr. in tape-worm, “but without any benefit.” (Pereira.)
Purification. Gallic acid, as obtained by either of the above forms, is never quite pure; but it may be rendered absolutely pure by combining it with oxide of lead, and decomposing the compound (gallate of lead) by sulphuretted hydrogen. The sulphuret of lead acts like animal charcoal in removing the colour. (Liebig.) Commercial gallic acid “may be rendered nearly white by dissolving it in 20 times its weight of boiling distilled water, and causing the solution to traverse a stratum of prepared animal charcoal, spread upon a calico filter. When the liquid passes through colourless, it should be evaporated to 1-6th its volume, and then suffered to cool, in order to the separation of the crystallised acid.” (Ph. D.)
GALLIC FERMENTATION. This name has been given to the peculiar process by which tannic acid is converted into gallic acid, under the joint influence of moisture and atmospheric oxygen. According to the researches of M. Antoine Larocque, the peculiar ferment of nut-galls which operates this change also converts sugar into alcohol and carbonic acid, in the same way as yeast does; whilst beer yeast, muscular flesh, and caseous matter change tannin into gallic acid. The similarity of the gallic and vinous fermentation may hence be reasonably inferred.
GALLIUM. A new metal discovered in August, 1875, by means of the spectroscope, by M. Lecoq Boisbaudran, in a specimen of blende from the mines of Pierrefitte, in the Pyrenees. The new element was named gallium in honour of France, the discoverer’s native country.
Gallium gives a spectrum composed of two bands in the violet, one of the bands being brilliant, and of wave length 417, and the other, a feeble one of wave length, 403·3.
776
The Pierrefitte blende contains one part of gallium in four hundred thousand. It is, however, found much more abundantly in a black blende from Bensberg, on the Rhine, one hundred thousand parts of this latter yielding one part of gallium.
Gallium resembles lead in appearance, but is less blue in colour. Exposed to moist air it tarnishes slightly. It is a little harder than lead, is flexible, malleable, and may be easily cut with a knife. If melted and poured upon glass, it adheres to it, and forms a mirror which is whiter than that caused by mercury. A red heat fails to volatilise it to any appreciable extent, and it is only slightly oxidised at that temperature; therefore it is not tarnished when exposed to the air. Hot nitric acid dissolves it, but the cold acid has scarcely any action on it. It melts at 30·15 C. When once fused, it preserves the liquid condition even for several months at 0° C.,[324] until it is touched by some solid body, or by a piece of solid gallium, when it congeals to a crystalline solid, having a specific gravity of 5·93; when fused it has a specific gravity of 6·08. It crystallises in square octohedra. In properties gallium is more or less intermediate between the metals aluminium and indium.
[324] In consequence of this curious property gallium was first described as a liquid metal.
Chemical reactions of gallium:—The following are the chief reactions of the salts of gallium when in solution. With ammonia they give a white gelatinous precipitate, soluble, but not readily in excess of the precipitant; potash gives a similar precipitate, soluble in excess; acetate of ammonia, on boiling in a solution free from excess of acid, precipitates a basic compound; barium carbonate readily precipitates gallium salts in the cold. A sulphate and a chloride of gallium have already been obtained. These salts are both very soluble; the sulphate is a non-deliquescent substance, the chloride, on the contrary, is excessively so, and decomposed by a large excess of water. Gallium also forms an alum consisting of the double sulphate with ammonium. Gallium alum is a beautifully crystalline body, more soluble in cold than in hot water.
At a meeting of the ‘Academie des Sciences’ in March, 1878, M. de Baubradon stated that he had determined the atomic weight of gallium. The mean of two experiments showed it to be 69·9.
The ‘Comptes Rendus’ for February, 1878 (No. 7), contains a communication from MM. Lecoq de Boisbaudran and E. Jungfleisch, on the extraction of gallium from the ores in which it is found associated with indium.
The following is the process given by the authors:—The blende of Bendsberg is pulverised and then roasted in a Perret furnace, by which treatment the greater part of the indium is volatilised. The residue is treated with sulphuric acid in quantity sufficient to dissolve almost all the zinc, and there is thus obtained a residue which is treated with excess of sulphuric acid.
The persalts of iron present are then reduced by means of metallic zinc, and the filtrate fractionally precipitated with carbonate of sodium; the precipitates are redissolved in sulphuric acid, and the reduction with zinc and the fractional precipitation repeated, the latter operation being in both cases watched by the spectroscope.
The precipitate containing the gallium concentrated in a small bulk, is redissolved in acid, and the excess of the latter reagent removed by evaporation, after which it is boiled with much water. The filtrate separated from the sediment containing titanic acid, which form is treated with sulphuretted hydrogen, then mixed with acetate of ammonium and again treated with sulphuretted hydrogen, which throws down the galliferous sulphide of zinc free from alumina. Again the precipitate is dissolved in sulphuric acid, and the solution fractionally precipitated with carbonate of sodium, which operation, guided as it is by spectral examination, entirely removes the zinc. By once more dissolving in the exactly necessary amount of sulphuric acid, and treating with sulphuretted hydrogen, cadmium, lead, indium and zinc are removed, and the filtrate is then largely diluted with water and boiled. The bulky sub-salt of gallium which separates at this temperature is treated with potash, which leaves iron, indium, &c., undissolved, and the alkaline liquor when treated with sulphuretted hydrogen, and subsequently with sulphuric acid to slight acidity, yields a deposit consisting mainly of sulphide of indium.
The slightly acid liquid is then boiled with much water, and the deposit of sub-salt of gallium thus obtained is dissolved in potash, and the solution subjected to electrolysis, by which means a metallic deposit of gallium is obtained.
It is interesting to note how accurately many of the chemical physical properties to gallium had, previously to its discovery, been predicted by the Russian chemist, Mendelejeff, by reasoning on the so-called “periodic law,” which he thus defines:—“The properties of the simple bodies, as also the properties and constitution of their combinations, are periodic functions of the atomic weights of the elements.”
In 1864 an English chemist named Mr Newlands, observing certain relations existing between the atomic weights of many of the elements, was the first to arrange them in such a manner or serial form as to suggest that when certain gaps were observed in the atomic weights of a series, new elements might be assumed to exist. Guided by this theory, Mendelejeff affirmed that the “periodic law” not only indicates vacancies in the classificatory scheme of the known elements, but enables us to predict the properties of elements as yet undiscovered, and of their compounds.777 Thus, of one of the vacancies observable in the table of the elements arranged according to his classification, Mendeljeff asserted, that should the element (which he named Eka aluminium) with the corresponding atomic number be discovered, it would possess the following characteristics:—It would most probably, like indium and thallium, be discovered by the aid of spectrum analysis. Gallium, as we have seen, was found by this means. The formula of its oxide would be El2O3; the oxide of gallium is best represented by Ga2O3.
The salts would have the general formula ElX3; the salts of gallium have most probably the general formula GaX3. It will form an alum isomorphous with common alum, this we have seen gallium does. Its salts would be precipitable by barium carbonate; the gallium salts are thrown down by this reagent. It would not oxidise in the air; gallium does not tarnish upon exposure to the air. It would decompose water at a red heat; gallium readily does this at high temperatures. Its specific gravity (and this is very remarkable) would be about 5·9; gallium has a specific gravity of 5·93. Its atomic weight would be about 68; that of gallium is 69·9.
The hypothetical Eka aluminium of Mendelejeff appears therefore to correspond with the gallium of Boisbaudran.
GALLS. Syn. Gall-nuts, Nut-galls; Galla (B. P.); Gallæ (Ph. E.). “Excrescences on Quercus infectoria caused by the puncture and deposited ova of Diplolepis Gallæ tinctoriæ.” The best galls are blueish-black, heavy, and not yet perforated; intensely astringent. They are imported from Aleppo, and are known in commerce as black or blue galls (GALLÆ NIGRÆ, G. CŒRULÆ). The next quality is termed, from their colour, green galls (GALLÆ VIRIDES). Both are gathered before the insect has escaped, and are styptic and powerfully astringent. White galls (GALLÆ ALBÆ) are lighter, less astringent, and inferior.
Uses, &c. Galls are extensively employed in the art of dyeing, and constitute one of the principal ingredients in all the shades of black, and are also employed to fix or improve several other colours. A decoction of galls, to which a little green copperas and gum Arabic has been added, forms common writing ink. In medicine they are used as an astringent, in hæmorrhages and fluxes, in doses of 10 to 20 gr.; and topically, under the form of infusion or decoction, as a gargle in relaxation of the uvula; as an injection in gleet and leucorrhœa; as a lotion or fomentation in flabby ulcers, prolapsus ani, &c.; and as an ointment in piles, watery ulcers, &c. The infusion or decoction is also used as an antidote to poisoning by the alkaloids, and was formerly given as a tonic in intermittents. See Gallic Acid, Ink, &c.
GALL′STONE. Syn. Calculus biliosus, C. cysticus bovinus, L. Formed in the gall-bladder of neat cattle in winter, when they are fed upon dry food. Used as a yellow pigment, and in medicine—Dose, 1 gr.; in dyspepsia and flatulency. Man is also subject to gall-stone, the presence or passage of which is attended with the most acute pain, frequently accompanied with nausea and sickness. In no case should a patient suffering from a paroxysm such as above described delay to seek immediate medical aid. The following treatment is recommended for the benefit of those only who, like emigrants and others, may be unable to obtain professional assistance.
The pain and spasm should be endeavoured to be alleviated by full doses of laudanum, given in soda water. If there be much sickness, the laudanum should be given in the form of an enema. If the paroxysm be excessive, the cautious inhalation of ether or chloroform should be tried. When the pain is of long duration, leeching should be had recourse to. Ice applied freely to the pit of the stomach has sometimes been found to give relief. See Calculus.
GALV′ANIZED IRON. See Iron and Zincing.
GAM′BOGE. Syn. (Camboge; Cambogia, L. B. P.) Gambogia, L. “A gum-resin obtained from Garcinia Morella.” (B. P.) Gamboge is an active hydragogue and drastic purgative, which occasionally proves useful in torpor of the abdominal and pelvic viscera; but which is highly dangerous in an irritable or inflammatory state of the stomach or bowels, and during pregnancy. It is very apt to induce nausea and vomiting. In large quantities it is a violent poison. “The deaths which have occurred from the use of enormous quantities of Morrison’s pills are mainly ascribable to the gamboge contained in those medicines.” (Pereira.)—Dose, 1 to 5 gr., made into pills or mixture, every 4 to 6 hours; in obstinate constipation, in dropsies, in apoplexy and like cerebral affections, and in worms (especially tape-worm), either alone or combined with other cathartics. See Compound Extract of Colocynth.
GAME. The flesh of game is believed to possess strengthening qualities superior to that of poultry. It also contains less fat. Game is tender and easy of digestion, and it has a delicate and marked flavour. It forms a valuable diet for the invalid, by reason of its easy digestibility.
Respecting the choice and preservation of game, Eliza Acton writes—“Buck venison, which is in season only from June to Michaelmas, is considered finer than doe venison, which comes into the market in October, and remains in season through November and December; neither should be cooked at any other part of the year.
“The greater the depth of fat upon the haunch the better the quality of the meat will be, provided it be clear and white, and the lean of a dark hue.
778
“If the cleft of the hoof, which is always left on the joint, be small and smooth, the animal is young; but it is old when the marks are the reverse of these.[325] Although the haunch is the prime and favourite joint of venison, the neck and shoulder are also excellent, dressed in various ways, and make much-approved pasties. A free current of air in a larder where venison is kept is always a great advantage.
[325] Venison is not in perfection when young.
“All moisture should be wiped daily, or even more frequently, from the venison with soft cloths, when any appears upon the surface, and every precaution must be taken to keep off the flies when the venison is not hung in a wire safe. Black pepper thickly powdered on it will generally answer the purpose.
“Hares and rabbits are stiff when freshly killed, and if young the ears tear easily, and the claws are smooth and sharp. A hare in cold weather will remain good for ten or fourteen days; care only must be taken to prevent the inside from becoming musty, which it will do if it has been emptied in the field. Pheasants, partridges, and other game, may be chosen by nearly the same tests as poultry—by opening the bill the staleness will be detected easily if they have been kept too long by the hardness of the bill. With few exceptions game depends almost entirely for the fine flavour and the tenderness of its flesh, on the time which it is allowed to hang before it is cooked, and it is never good when very fresh; but it does not follow that it should be sent to table in a really offensive state.”
Game, Hashed. Ingredients.—The remains of cold game, one onion stuck with three cloves, a few whole peppers, a strip of lemon peel, salt to taste, thickening of butter and flour, one glass of port wine, one tablespoonful of lemon juice, one tablespoonful of ketchup, and one pint of water or weak stock.
Proceed as follows:—Cut the remains of cold game into joints, reserve the best pieces, and put the inferior ones and the trimmings into a stewpan with the onion, pepper, lemon peel, salt, and water or weak stock; stew these for about an hour, and strain the gravy; thicken it with butter and flour; add the wine, lemon juice, and ketchup; lay in the pieces of game, and place them by the side of the fire until they are warmed through, avoiding boiling, otherwise the game will become too hard. Just on the point of simmering serve, and garnish the dish with sippets of toasted bread. Time. Altogether, an hour and a quarter.
⁂ The above recipe applies to any kind of game.
If desirable, the flavour may be varied by adding flavoured vinegars, curry powder, &c.; these, however, cover the gamey taste of the dish, and are, therefore, not to be recommended.
Grouse, to Roast. Ingredients.—Grouse, butter, a thick slice of toasted bread. Mode.—Let the birds hang as long as possible; pluck and draw them; wipe (but do not wash them) inside and out, and truss them without the head, the same as for a roast fowl. Put them down to a sharp clear fire, keep them well basted the whole of the time they are cooking, and serve them on buttered toast, soaked in the dripping-pan, with a little melted butter poured over them, or with bread sauce and gravy. Time. Half an hour; if liked thoroughly done, thirty-five minutes. Seasonable from the 12th of August to the beginning of December. (Mrs Beeton.)
Hare, Jugged. Ingredients.—One hare, a bunch of sweet herbs, two onions, each stuck with three cloves, six whole allspice, half a teaspoonful of black pepper, a strip of lemon peel, thickening of butter and flour, two table-spoonfuls of mushroom ketchup, quarter of a pint of port wine. Mode.—Wash the hare nicely, cut it up into joints (not too large), and flour and brown them; then put them into a stewpan with the herbs, onions, cloves, allspice, pepper, and lemon peel; cover them with hot water, and when it boils carefully remove all the scum, and let it simmer gently till tender, which will be in about 13⁄4 hour, or longer should the hare be very old. Take out the pieces of hare, thicken the gravy with flour and butter, add the ketchup and port wine, let it boil for about ten minutes, strain it through a sieve over the hare, and serve. A few fried forcemeat balls should be added at the moment of serving, or, instead of frying them, they may be stewed in the gravy, about ten minutes before the hare is wanted for use. Do not omit to serve red-currant jelly with it. Time. Altogether, two hours. Seasonable from September to the end of February. (Mrs Beeton.)
Hare, to Roast. Ingredients.—Hare, forcemeat, a little milk, and butter. To be eaten in perfection, the hare must hang for some time. After it is skinned wash it well, and soak it for an hour in warm water to draw out the blood. Make a forcemeat, wipe the hare dry, fill the belly with it, and sew it up. Bring the hind and fore legs close to the body towards the head, run a skewer through each, fix the head between the shoulders by means of another skewer, and be careful to leave the ears on. Put a string round the body from skewer to skewer and tie it above the back. Mode.—The hare should be kept at a distance from the fire when it is first laid down. Baste it well with milk for a short time, and afterwards with butter; and particular attention must be paid to the basting, so as to preserve the meat on the back juicy and nutritive. When it is almost roasted enough, flour the hare, and baste well with butter. When nicely frothed dish it, remove the skewers, and send it to table with a little gravy in the dish, and a tureen of the same. Red-currant jelly must be served with779 it. If the liver is good it may be parboiled, minced, and mixed with the stuffing; but it should not be used unless quite fresh. Time. A middling-sized hare an hour and a quarter; a large hare one and a half to two hours. (Mrs Beeton.)
Partridges, to Roast. Let the birds hang as long as they can possibly be kept without becoming offensive; pick them carefully, draw and singe them, wipe the insides thoroughly with a clean cloth, truss them with the head turned under the wing and the legs drawn close together, not crossed. Flour them when first laid to the fire, and baste them plentifully with butter. Serve them with bread sauce and good brown gravy; a little of this last should be poured over them. Time. 30 or 40 minutes. In preparing them for the spit the crop must be removed through a slit cut in the back of neck, the claws clipped close, and the legs held in boiling water for a minute, that they may be skinned more easily. (Eliza Acton.)
Pheasant, to Roast. Let it hang as many days as possible without becoming tainted. Pluck off the feathers carefully, cut a slit in the back of the neck to remove crop, then draw the bird in the usual way, and either wipe the inside very clean with a damp cloth, or pour water through it; wipe the outside also, but with a dry cloth; cut off the toes, turn the head of the bird under the wing, with the bill laid straight along the breast; skewer the legs, which must not be crossed; flour the pheasant well, lay it to a brisk fire, and baste it constantly and plentifully with well-flavoured butter. Send bread sauce and good brown gravy to table with it. Time. Three quarters of an hour, a few minutes less if liked very much underdone, five or ten more for thorough roasting, with a good fire in both instances. In season from October to the end of January. (Eliza Acton.)
Rabbit, to Boil. Rabbits that are three parts grown, or, at all events, which are still quite young, should be chosen for boiling. Wash them well, truss them firmly, with the heads turned and skewered to the sides, drop them into sufficient boiling water to keep them quite covered until they are cooked, and simmer them gently from thirty to forty-five minutes; when very young they will require even less time than this. Cover them with rich white sauce mixed with livers parboiled, finely pounded, and well seasoned with cayenne and lemon juice; or with white onion sauce, or with parsley and butter, made with milk or cream instead of water (the livers, minced, are often added to the last of these), or with good mushroom sauce. Time. 30 to 45 minutes. (Eliza Acton.)
Rabbit, to Roast. This is much improved by having the backbone taken out. When this is done line the inside with thin slices of bacon, fill it with forcemeat, sew it up, truss, and roast it at a clear, brisk fire, and baste it constantly with butter. Flour it well soon after it is laid down. Serve it with good brown gravy, and with currant jelly, when this last is liked. Time. 3⁄4 hour to 1 hour; less if small. (Eliza Acton.)
Venison, Haunch of, to Roast. To prepare the venison for the spit wash it slightly with tepid water, or merely wipe it thoroughly with damp cloths, and dry it afterwards with clean ones; then lay over the fat side a large sheet of thickly-buttered paper, and next a paste of flour and water about three quarters of an inch thick; cover this again with two or three sheets of stout paper, secure the whole well with twine, and lay the haunch to a sound, clear fire; baste the paper immediately with butter or clarified dripping, and roast the joint from three hours and a half to four and a half, according to its weight and quality. Doe venison will require half an hour less time than buck. Twenty minutes before the joint is done remove the paste and paper, baste the meat in every part with butter, and dredge it very lightly with flour; let it take a pale-brown colour, and send it to table as hot as possible, with gravy in a tureen and good currant jelly. Time. 31⁄2 to 41⁄2 hours. The kind of gravy appropriate to venison is a matter on which individual taste must decide. (Eliza Acton.)
Venison, Hashed. Ingredients.—The remains of roast venison, its own or mutton gravy, thickening of butter and flour. Mode.—Cut the meat from the bones in neat slices, and, if there is sufficient of its own gravy left, put the meat into this, as it is preferable to any other. Should there not be enough put the bones and trimmings into a stewpan with about a pint of mutton gravy; let them stew gently for an hour, and strain the gravy. Put a little flour and butter into the stewpan, keep stirring until brown, then add the strained gravy, and give it a boil up; skim and strain again, and when a little cool put in the slices of venison. Place the stewpan by the side of the fire, and when on the point of simmering serve. Do not allow it to boil. Send red-currant jelly to table with it. Time. Altogether, an hour and a half. A small quantity of Harvey sauce, ketchup, or port wine, may be added to enrich the gravy.
GAN′GRENE. See Mortification.
GAN′TEINE. A composition used to clean kid and other leather gloves.
Prep. 1. (M. Buhan.) Curd soap (in small shavings), 1 part; water, 3 parts; mix with heat, and stir in of essence of citron, 1 part.
2. (Saponine,—Duvignau.) Soap (in powder), 250 parts; water, 155 parts; dissolve with heat, cool, and add, of eau de javelle, 165 parts, solution of ammonia, 10 parts, and rub the whole to a smooth paste. Patent. A small portion of either of the above is rubbed over the glove with a piece of flannel (always in one direction), until it is sufficiently clean. See Gloves.
780
GARAN′CINE. See Madder Red.
GAR′DENING. See Horticulture.
GAR′GLE. Syn. Gargarism, Throatwash; Gargarisma, Gargarismus, Gargarismum, L. A liquid medicine applied to the back part of the mouth or upper part of the throat. Gargles are applied by allowing a small mouthful to run as much as possible over the affected part, by holding the head backwards and breathing through it, by which means the liquid is agitated and its action promoted.
Gargles are not to be swallowed. It often happens, however, that patients, either by accident or from negligence, do swallow a certain quantity, notwithstanding the instructions given them to the contrary. Care should therefore be taken to avoid making gargles of such substances as may occasion unpleasant symptoms in small doses, though they may not, perhaps, amount to poisoning.
Gargles usually have for their basis either simple water, or milk, wine, or vinegar, diluted with water, to which, in both cases, sugar, honey, or syrup is generally added. Their other ingredients vary with the indication, but must, in all cases, be either in the liquid form, or soluble in the liquid used as the excipient.
Gargles are commonly dispensed in mixture bottles. The quantity used at a time, under ordinary circumstances, may be about 2-3rds of a wine-glassful.
Gargle. Syn. Gargarisma, G. commune, G. simplex, L. Prep. 1. (St. B. Hosp.) Honey or honey of roses, 11⁄2 fl. oz.; strong vinegar, 21⁄2 fl. oz.; barley water, 1 pint.
2. (St. George’s.) Oxymel, 1 fl. dr.; decoction of barley, 5 fl. dr. In common sore throats, &c. The formulæ of several other hospitals are similar.
Gargle of Ac′etate of Ammo′′nia. Syn. Gargarisms ammoniæ acetatis, L. Prep. (Wendt.) Solution of acetate of ammonia and honey of roses, of each 1 fl. oz.; elder-flower water, 8 fl. oz.; mix. In the ulcerated sore throat of scarlet fever.
Gargle of Acetate of Manganese. Syn. Gargarisma Manganesii Acetatis. Prep. Acetate of Manganese, 1 drachm; water, 7 fluid ounces; clarified honey, 1 oz. The chloride and sulphate of manganese are also used, about 1⁄2 drachm or 2 scruples to 6 oz. of barley water.
Gargle of Ace′tic Acid. Syn. Oxymel gargle; Gargarisma acidi acetici, L. Prep. 1. (St. B. Hosp.) Acetic acid, 1 dr.; oxymel, 2 fl. dr.; water to make up 4 fl. oz.
2. Barley water, 12 fl. oz.; acetic acid, 11⁄2 fl. oz.; honey, 6 dr. Antiseptic. For sore throat.
Gargle of Aluminium Chloride. Syn. Gargarisma Aluminii chloridi. Prep. (Throat Hosp.) Solution of chloride of aluminium 12 minims, water 1 fl. oz. Astringent and antiseptic.
Gargle of Al′um. Syn. Gargarisma aluminis, L. Prep. 1. (Augustin.) Oak-bark (in powder), 1 oz.; water, 11⁄2 pint; boil to a pint, filter, cool, and add, of alum, 1⁄2 dr.; brandy, 2 fl. oz. In inflammation of the mouth and throat.
2. (Cavarra.) Alum, 3 dr.; water, 6 fl. oz.; dissolve. In offensive breath.
3. (Foy.) Alum, 1 dr.; tincture of myrrh, 2 fl. dr.; tincture of bark, 4 fl. dr.; honey of roses, 2 oz.; laudanum, 20 drops; wine, 2⁄3 pint. In scurvy.
4. (Grant.) Alum, 1 oz.; tincture of myrrh, 1⁄2 fl. oz.; peppermint water, 7 fl. oz. In relaxation of the uvula, &c.
5. (Mid. Hosp.) Alum, 1 dr.; honey, 2 dr.; water to make 6 fl. oz. As No. 4.
6. (P. Cod.) Alum, 40 gr.; honey of roses, 1 oz.; infusion of roses, 6 fl. oz. As the last.
7. (Ratier.) Alum, 1 oz.; infusion of red roses and barley water, of each 3 fl. oz.; honey of roses, 2 oz. As No. 4.
8. (Westm. Hosp.) Alum, 1 dr.; dilute sulphuric acid, 1 fl. dr.; treacle, 4 dr.; water to 15 fl. oz.
9. (Ph. Wirtem.) Alum and nitre, of each 3 oz.; cream of tartar, 4 oz.; dilute acetic acid, 4 lbs.; dissolve, evaporate to dryness, and powder the residuum. For use, 1⁄2 oz. of the powder is dissolved in water, 8 fl. oz. Highly recommended in inflammation of the fauces and tonsils. This forms Zobel’s ‘Specific for quinsy,’
Gargle, Antiscorbu′tic. Syn. Gargarisma antiscorbuticum, L. Prep. (P. Cod.) Bitter species, 1 dr.; boiling water, 8 oz.; macerate 1 hour, strain, and add, syrup of honey, 2 oz.; antiscorbutic tincture, 1 oz.
Gargle, Antisep′tic. Syn. Gargarisma antisepticum, L. Prep. (Fr. Hosp.) Decoction of bark, 6 oz.; camphor, 20 gr.; sal-ammoniac, 12 gr. In putrid sore throat, &c.
Gargle, Astrin′gent. Syn. Gargarisma astringens, L. Prep. 1. (Collier.) Tincture of galls, 2 fl. dr.; honey, 1⁄2 oz.; water, 6 fl. oz. In relaxation of the uvula and fauces.
2. (Collier.) Honey, 4 dr.; tincture of myrrh, 3 dr.; powdered alum, 40 gr.; compound infusion of roses, 51⁄2 fl. oz. As the last, and in fetid sore throat.
3. (Sir A. Cooper.) Alum, 2 dr.; decoction of bark, 12 oz.; honey of roses, 11⁄2 oz.
4. (Dr A. T. Thomson.) Infusion of roses, 7 fl. oz.; dilute sulphuric acid, 1 fl. dr.; tincture of catechu, 6 fl. dr.; laudanum, 11⁄2 fl. dr. For relaxation of the uvula. See Gargle of Alum.
Gargle of Bo′′rax. Syn. Gargarisma boracis, L. Prep. 1. (Ellis.) Borax, 1 dr.; tincture of myrrh, 4 fl. dr.; clarified honey, 1 fl. oz.; rose water, 4 fl. oz.
2. (Fr. Hosp.) Borax, 2 dr.; honey or capillaire, 1 oz.; rose water, 7 fl. oz.
3. (Guy’s Hosp.) Borax, 2 dr.; honey of roses, 1 oz.; barley water, 7 fl. oz.
781
4. (Mid. Hosp.) Borax, 1 dr.; simply oxymel, 2 dr.; water to make 3 fl. oz. The above are used in thrush or aphthous sore mouth, ptyalism, &c.
Gargle of Bromide of Potassium. Syn. Gargarisma potassii bromidi. Prep. (Throat Hosp.) Bromide of potassium, 10 grains; water, 1 fl. oz. Sedative.
Gargle of Cap′sicum. Syn. Gargle of cayenne pepper; Gargarisma capsici, L. Prep. 1. (Dr Griffith.) Tincture of capsicum, 1⁄2 fl. oz.; rose water, 8 fl. oz.
2. (St. B. Hosp.)—a. Capsicum, 3 dr.; common salt, 1 oz.; boiling water, 1 pint; macerate for 12 hours, strain, and add of distilled vinegar, 1 pint.
b. Tincture of capsicum, 1 fl. dr.; compound infusion of roses, 8 fl. oz.
3. (U. C. Hosp.) Tincture of capsicum, 1 fl. dr.; honey, 6 dr.; water to 4 fl. oz. Used in ulcerated sore throat, scarlet fever, &c.
Gargle of Carbolic Acid. Syn. Gargarisma acidi carbolici. Prep. (Throat Hosp.). Carbolic acid, 2 gr.; glycerin, 25 minims; water, 1 fluid ounce. Stimulant and antiseptic.
Gargle of Chlo′′rate of Potas′sa. Syn. Gargarisma potassæ chloratis, L. Prep. (Beasley.) Chlorate of potassa, 1 dr.; honey of roses, 1 oz.; water, 7 oz. Used in malignant sore throat, scarlatina, &c.
Gargle of Chlo′′ride of Lime. Syn. Gargarisma calcis chlorinatæ, L. Prep. From chloride of lime, 1 dr.; water, 1⁄2 pint; agitate together for 10 minutes, filter through linen, and add of simple syrup, 1 fl. oz. Used in putrid sore throat, scarlet fever, &c.
Gargle of Chloride of So′da. Syn. Gargarisma sodæ chlorinatæ, L. Prep. 1. (Copland.) Liquor of chloride of soda, 12 fl. dr.; honey, 1⁄2 oz.; water, 6 fl. oz.
2. (Hosp. Form.) Chlorinated solution of soda, 4 fl. dr.; water to 4 fl. oz. Used as the last.
Gargle of Chlo′′rine Water. Syn. Gargarisma chlorinii, L. Prep. 1. (Fr. Hosp.) Chlorine water, 1⁄2 fl. oz.; syrup, 1 fl. oz.; water, 4-1·2 fl. oz.
2. (Mid. Hosp.) Chlorine water, 2 fl. oz.; distilled water, 10 fl. oz. Use. As the last.
Gargle of Cincho′na Bark. Syn. Gargarisma cinchonæ, L. Prep. 1. From decoction of cinchona, 7 fl. oz.; simple oxymel, 1 fl. oz. Antiseptic and astringent in relaxation, &c.
2. (Acidulated; Gargarisma cinchonæ acidus, L.) Hydrochloric acid, 11⁄2 fl. dr.; honey, 11⁄2 oz.; decoction of bark to make up 8 fl. oz.
Gargle, Com′mon. Syn. Gargarisma commune, L. Prep. 1. (Ed. Hosp.) Water, 6 fl. oz.; nitre, 1 dr.; honey of roses, 1 oz. For ordinary sore throat, &c.
2. (Lond. Hosp.) Alum, 1 dr.; dilute sulphuric acid, 2 fl. dr.; tincture of myrrh, 4 fl. dr.; water to 12 fl. oz.
Gargle of Cy′anide of Mercury. Syn. Gargarisma hydrargyri cyanidi, L. Prep. 1. (Brera.) Cyanide of mercury, 10 gr.; honey of roses, 1 oz.; barley water, 1 pint.
2. (Cullerier.) Cyanide of mercury, 10 gr.; linseed tea, 1 pint. Used in the same cases as mercurial gargle.
Gargle, Deter′gent. Syn. Gargarisma detergens, L. Prep. 1. (P. Cod.) Alcoholised sulphuric acid, 1 fl. dr.; honey of roses, 2 oz.; barley water, 8 oz.
2. (Dr A. T. Thomson.) Nitre, 2 dr.; honey of roses, 4 fl. dr.; infusion of roses, 51⁄2 fl. oz. In inflammatory sore throat.
Gargle, Emol′lient. Syn. Gargarisma emolliens, L. Prep. 1. (Buchan.) Marshmallow root, 1 oz.; figs, 2 oz.; water, 1 quart; boil to a pint and strain. Demulcent, soothing.
2. (Trousseau & Reveil.) Barley water, 8 oz.; honey, 11⁄2 oz. Both are used in inflammatory affections of the throat and mouth.
Gargle of Horserad′ish. Syn. Gargarisma armoraciæ, L. Prep. (Collier.) Compound spirit of horseradish, 1 fl. oz.; honey, 2 oz.; water, 4 fl. oz. A good gargle for scurvy of the fauces and pharynx, vulgarly called the ‘inward scurvy.’
Gargle of Hydrochlo′′ric Acid. Syn. Muriatic acid gargle; Gargarisma acidi hydrochlorici. L. Prep. 1. (Guy’s Hosp.) Hydrochloric acid, 30 drops; honey of roses, 2 oz.; barley water, 6 fl. oz.
2. (Ratier.) Hydrochloric acid, 2 fl. dr.; clarified honey, 2 fl. oz.; barley water, 1 pint.
3. (St. B. Hosp.) Red-rose leaves, 2 dr.; boiling water, 1 pint; hydrochloric acid, 11⁄2 fl. dr.; digest 1 hour, and strain. In inflammatory sore throat, ulcerations of the mouth, scarlet fever, &c.
Gargle of I′odine. Syn. Gargarisma iodinii, L. Prep. 1. Iodine, 10 gr.; iodide of potassium, 12 gr.; rectified spirit and simple syrup, of each, 1 fl. oz.; water, 5 fl. oz. In chronic enlargement of the tonsils, in scrofulous habits.
2. (Dr Ross.) Tincture of iodine, 11⁄2 fl. dr.; tincture of opium, 1 fl. dr.; water, 6 fl. oz.
3. (St. T. Hosp.) Compound tincture of iodine, 2 fl. dr.; water, 5 fl. oz. In ulceration of the tonsils.
Gargle, Mercu′′rial. Syn. Gargarisma hydrargyri, G. mercuriale, L. Prep. 1. (G. hyd. bichloridi.) Corrosive sublimate, 2 to 5 gr.; barley water, 1 pint; honey of roses, 2 fl. oz. For syphilitic ulcers in the throat.
2. (Plenck.) Calomel, 6 gr.; quicksilver, 30 gr.; powdered gum, 3 dr.; syrup of poppies, 1⁄2 oz.; triturate till the globules of metal disappear, and add of decoction of clematis, 26 fl. oz.; honey of roses, 1 oz.; essence of myrrh, 1 dr. (or tincture of myrrh, 1 fl. oz.). In syphilitic and putrid sore throat.
Gargle of Mustard. Syn. Gargarisma sinapis. Prep. (Fleury). Black mustard782 seed, bruised 4 ounces; salt, 4 scruples; vinegar, 8 scruples; warm water, 7 ounces. Digest and filter.
Gargle of Myrrh. Syn. Gargarisma myrrhæ, L. Prep. 1. (Ainslie.) Tincture of myrrh, 6 fl. dr.; vinegar, 1 fl. dr.; honey of roses, 11⁄2 fl. oz.; barley water, 12 fl. oz.
2. (Ph. Chirur.) Tincture of myrrh, 1⁄2 oz.; honey of roses, 11⁄2 oz.; lime water, 6 fl. oz. In scarlatina and putrid sore throat. See Astringent Gargle, &c.
Gargle of Ni′tre. Syn. Gargarisma salis nitri, G. potassæ nitratis, L. Prep. 1. Nitre, 2 dr.; honey or syrup, 1⁄2 oz.; rose water, 51⁄2 fl. oz.
2. (Brande.) Nitre, 2 dr.; oxymel, 1 fl. oz.; barley water, 7 fl. oz. In inflammatory sore throat. See Common Gargle.
Gargle of Oak-Bark. Syn. Gargarisma corticis quercûs, L. Prep. 1. Oak-bark, 2 dr.; boiling water, 6 fl. oz.; macerate 1 hour, and strain.
2. (Ellis.) Decoction of oak-bark, 1 pint; alum, 1⁄2 dr.; brandy, 2 fl. oz. In chronic sore throat, relaxation of the uvula, &c.
Gargle of Oxide of Manganese. Syn. Gargarisma manganesii oxidi. Prep. (Pareira). Black oxide of manganese, 2 dr.; decoction of barley, 5 fl. oz.
Gargle of Pel′litory. Syn. Gargarisma pyrethri. Prep. 1. Pellitory root, 4 dr.; water, 16 fl. oz.; boil to 8 fl. oz., and add of liquor of ammonia 2 fl. dr.
2. (Swediaur.) Infusion of pellitory, 1 pint; vinegar, 3 fl. oz.; sal-ammoniac, 3 dr. To promote the maturation and healing of throat ulcers.
Gargle of Permanganate of Potash. Syn. Gargarisma Potassæ Permanganatis. Prep. (Throat Hosp.) Solution of permanganate of potash (B. P.), 6 minims; distilled water, 1 fl. oz. Antiseptic.
Gargle of Ro′′ses. Syn. Gargarisma rosæ, G. rosarum, L. Prep. (Kendrick.) Conserve of roses, 3 oz.; boiling water, 16 fl. oz.; infuse 1 hour; add of dilute sulphuric acid, 2 fl. dr., and strain. Antiseptic, astringent; used in several indications.
Gargle of Subacetate of Lead. Syn. Gargarisma plumbi subacetatis. Prep. (Ratier.) Liquid subacetate of lead, 1⁄2 dr.; barley water, 1 lb.; syrup, 1 fl. oz.
Gargle of Turpentine. Syn. Gargarisma Terebinthinatum. Prep. (Geddings.) Oil of turpentine, 2 dr.; mucilage, 61⁄2 fl. oz. In salivation.
Gargle, Spirit. Syn. Gargarisma spirituosum, G. spiritûs vini, L. Prep. 1. (Dr Watson.) French brandy, 1 fl. oz.; water, 1⁄4 pint.
2. (St. George’s.) Proof spirit, 1 fl. oz.; oxymel, 5 fl. dr.; decoction of barley, to make up 6 fl. oz. In relaxations and salivation.
Gargle, Stim′ulant. Syn. Gargarisma stimulans, L. Prep. (Dr Copland.) Infusion of roses, 61⁄2 fl. oz.; dilute hydrochloric acid, 40 drops; tincture of capsicum, 11⁄2 fl. dr.; honey, 3 dr. See Gargle of Capsicum.
Gargle of Tan′nin. Syn. Gargarisma acidi tannici, L. Prep. 1. (Beral.) Tannin, 1 dr.; honey of roses, 2 oz.; rose water, 2 fl. oz.; distilled water, 8 fl. oz.
2. (Jannart.) As the last, but using only half the quantity of tannin. In salivation and aphthous ulcerations.
Gargle of Verd′igris. Syn. Gargarisma æruginus, G. cupri acetatis, L. Prep. (Guy’s Hosp.) Oxymel of verdigris, 4 dr.; honey of roses, 2 oz.; barley water, 31⁄2 fl. oz. Used as a detergent for ulcers in the throat. If swallowed it produces violent vomiting. The addition of 21⁄2 oz. of water to the above produces a gargle sufficiently strong for most cases.
Gargle of Vin′egar. See Gargle of Acetic Acid.
Gargle of Zinc. Syn. Gargarisma zinci, G. z. sulphatis, L. Prep. (Dr Copland.) Sulphate of zinc, 20 gr.; oxymel, 1 fl. oz.; rose water, 7 fl. oz. In aphthous sores, relaxations, ulceration of the tonsils, &c.
GAR′LIC. Syn. Allium, L. The Allium sativum of botanists. It is diaphoretic, diuretic, expectorant, stimulant, and tonic; and externally, irritant, rubefacient, and even vesicant.—Dose, 1⁄2 dr. to 11⁄2 dr.; in enfeebled digestion, chronic diarrhœa, old chronic coughs, atonic dropsies, and worms. An antispasmodic and counter-irritant liniment is made of the juice, which was formerly esteemed in chest diseases and infantile convulsions. A small clove of garlic, or a few drops of the juice, was formerly introduced into the ear in certain forms of deafness. As a condiment its properties resemble those of the onion, than which it is much more powerful.
GAR′NET. In mineralogy, one of the precious stones or gems. The finest specimens of noble garnet (Syrian or Oriental garnet) are brought from Pegu. According to chemical analysis, the garnet is a double silicate of alumina and lime, coloured with iron and manganese.
Garnet, Facti′′tious. See Pastes.
GA′RUM. [L.] A species of pickle or sauce prepared of fish, in a state of incipient putrefaction, strongly salted and seasoned with aromatics. According to Pliny, the Romans used a species of lobster for this purpose.
GAS. Syn. Gaz, Fr. A permanently elastic aëriform fluid. In English the term ‘air’ is now usually restricted to the gaseous mixture forming the atmosphere, but it was formerly used as a synonym for ‘gas.’ The principal gases are the elementary bodies hydrogen, chlorine, oxygen and nitrogen, and the compounds ammonia, carbonic acid, carbonic oxide, carburetted hydrogen, hydrochloric acid, phosphoretted hydrogen, protoxide of nitrogen, sulphuretted hydrogen, and sulphurous acid.783 See these substances under their respective heads.
Gas. Syn. Coal gas, Illuminating g. The term ‘gas’ is popularly applied to the important mixture of hydrocarbons produced by the destructive distillation of pit-coal, and now employed as a source of artificial light in most of large towns of Europe and America. Although artificial illumination by means of coal-gas was, previous to 1819, used in Great Britain in isolated cases, and had been employed for the occasional lighting up of the mansion of Culrose Abbey in Scotland, by Lord Dundonald, as far back as 1787; and by Murdoch, in 1798, for lighting the foundry of Boulton and Watts in Soho, it does not appear to have been generally adopted in London, and the other large towns of England and Scotland until that year; since which time to the present artificial gas illumination has steadily progressed, and increased to so enormous an extent, that some works are now delivering millions of cubic feet of coal-gas a day. The apparatus used in the manufacture of gas on the large scale consists essentially of a system of closed retorts (a) of cast iron or fire-clay, generally having the form of a flattened cylinder, and arranged in sets of three or five, and heated by the same coal fire, as shown in the accompanying drawing.
The quantity of coal required to charge each retort is about two bushels, and it takes about four hours for the coal to give off all its gas. When it has done this the resulting coke is removed from the retort, and a fresh charge of coal is thrown into it, the mouth of the retort being then closed with a thick iron plate, and luted with clay. An iron pipe ascends from the upper side of the front of the retort, projecting from the furnace, and after describing a curve at its upper extremity, this iron pipe opens into a much wider tube, called the hydraulic main (b), which latter passes horizontally along the front of the range of furnaces, the tubes from all the retorts dipping into it. The hydraulic main is always kept half full of the water and the tar which condenses from the ascending gas; owing to which arrangement the opening into each retort is effectually closed by a water-valve, and thus permits a fresh charge of coals to be thrown in, and of coke to be withdrawn in any one or more of the retorts, without interfering with the distillation going on in the others.
The aqueous portion of the liquid deposited in the hydraulic main, which is known as the ammoniacal liquor, and forms the principal source of the commercial salts of ammonia, consists principally of solution of carbonate of ammonium, but contains also sulphide, cyanide, and sulphocyanide of ammonium. After it leaves the hydraulic main, the gas passes into the condenser (e), which is composed of a series of bent iron tubes (shown in the plate), these being kept cool either by the large surface they expose to the air, or, if necessary, by means of a stream of cold water applied to the outside.
Any of the volatile hydrocarbons or salts of ammonia escaping condensation in the hydraulic main are arrested in the condensers, but not always; hence it is necessary to afterwards carry the gas through a scrubber (not figured in the plate) or case containing pieces of coke, over which a stream of water being made to trickle, absorbs any remaining ammoniacal vapours. The gas next passes through the lime purifier (f), an iron784 box fitted with shelves, on which is placed slaked lime, which absorbs the carbonic acid, and part, but not the whole of, the sulphuretted hydrogen contained in the gas. Of the many methods devised for the removal of the sulphuretted hydrogen, none appears to be so successful and economical as that which consists in passing the gas over a mixture of sulphate of iron, slaked lime, and sawdust.
The gas, after it has become purified by the foregoing processes, is passed into the gasometer (g) (part of which is represented in the plate), whence it passes into the mains, by which it is conveyed to the various condensers. Another prejudicial impurity formed in gas is carbon disulphide, which when burned gives rise to small quantities of sulphuric acid, and this in time attacks certain kinds of furniture, as well as the bindings of books.
Dr Angus Smith effects the removal of the disulphide by passing the gas through a solution of plumbic oxide in caustic soda, diffused through sawdust.
The quality of coal-gas is largely dependent upon the temperature employed in its manufacture. If the retorts are insufficiently heated, the result will be the formation of certain easily-condensable hydrocarbons, which not only diminish the bulk of the gas, but cause considerable inconvenience by collecting in and blocking up the pipes. On the contrary, should too much heat be used, the gas becomes partially decomposed by contact with the red-hot retort, and deposits on its sides the substance known as “gas carbon,” thus not only removing to a certain extent the constituent to which the gas owes its illuminating power, but impoverishing its lighting qualities still more, by diluting it with an unnecessary quantity of liberated hydrogen. These latter effects are forcibly illustrated in the following analysis of the gas collected from Wigan cannel coal at different periods of the distillation.
The best gas is said to be produced when the retorts are heated to a bright cherry red.
| In 100 Volumes. | 1st Hour. | 5th Hour. | 10th Hour. |
| Olefiant gas and volatile hydro-carbons | 13·0 | 7·0 | 0·0 |
| Marsh gas | 82·5 | 56·0 | 20·0 |
| Carbonic oxide | 3·2 | 11·0 | 10·0 |
| Hydrogen | 0·0 | 21·3 | 60·0 |
| Nitrogen | 1·3 | 4·7 | 10·0 |
“The value of gas as an illuminating agent may be said to depend on the amount of hydrocarbons present, and on the relation which the carbon bears to the hydrogen in these substances. In marsh gas, CH4, which is, practically speaking, non-luminous, the per-centage composition is, carbon 75, and hydrogen 25. In olefiant gas, C2H4, the carbon is 85·7, and the hydrogen 14·3, and the gas possesses a correspondingly greater amount of illuminating value. In acetylene, C2H2, we have a gas of still greater illuminating value, the proportion of carbon to hydrogen being also greater, the per-centage composition being, carbon 92·3, and hydrogen 7·7. In benzol, C6H6, we have the same per-centages; while in naphthalene, C10H8, a still higher ratio between the carbon and hydrogen exists, and a corresponding increased value in light-giving power. It was formerly taken as an axiom that the illuminating value of a mixture of gases was also proportionate to the relation between the carbon and hydrogen, but although this is very good as a rough criterion in practice, the statement must not be accepted as strictly true. The illuminating power of a mixture of gases is known now to depend far more on the nature of the particular compounds present, than upon the absolute proportion between the hydrogen and carbon; for while on the one hand it is possible to have a gas (marsh gas) containing as much as 75·4 per cent. of carbon, and yet which is valueless for illuminating purposes; it is also possible to have a mixture of gases in which the per-centage of carbon is far less, although the illuminating value is much greater.”[326]
[326] ‘Chemistry, Theoretical, Practical, and Analytical,’—Mackenzie.
Coal gas consists of a mixture of the following bodies:
The yield of gas, and also the illuminating power of the product, vary greatly with different kinds of coal. The average yield may be roughly estimated at 10,000 cubic feet of gas per ton of coal.[327]
[327] For practical details respecting the manufacture of this product, see the article Coal gas in ‘Ure’s Dictionary of Arts, Manufactures, &c.,’ ‘Wagner’s Chemical Technology,’ and ‘Chemistry, Theoretical, Practical, and Analytical.’
Anthracite is by no means suited for a gas coal. The best coals for this purpose are those which are bituminous; they comprise caking coal, parrot coal, and certain varieties of cannel coal. London gas (which is generally deficient in785 illuminating power) is manufactured principally from Durham and Newcastle coal.
In addition to the elementary composition of the coal, the amount and nature of the volatile matter contained in it is an important factor in its value as a source for gas. It should also yield a small amount of ash, and be as free as possible from sulphur, besides which its ultimate analysis should show a comparatively small proportion of oxygen. If there be an excess of this latter element, the production of the hydrocarbon illuminants will be diminished, since the hydrogen which would go to their formation would unite with the oxygen to form useless water.
The late Charles Mansfield proposed to increase the illuminating power of ordinary coal-gas, and to render water gas or even atmospheric air luminiferous, by passing them through sponges or over trays containing mineral naphtha or benzole; and a patent was taken out for this purpose. The gas so treated imbibes or dissolves a portion of the liquid, and burns with increased brilliancy. The method of saturating the gas with the liquid hydrocarbon is as follows:—“The apparatus consists of a brass reservoir or chamber attached to the end of the gas-pipe, near the burner. This reservoir may be in the shape of an oil-flask, made air-tight, with a screw-joint, or other means of supplying any highly volatile oil, turpentine, or mineral naphtha, and should be kept about half full. Into this reservoir the gas-pipe ascends a little above the surface of the oil; a very small jet-pipe of gas, regulated by a stop-cock, is branched off below this chamber, to supply a minute flame, so as to cause a sufficient evaporation from the oil to unite with the gas in the flask receiver. The whole is, of course, surmounted with the usual burner and lamp-glass.”
The naphthalising of gas did not work well on a large scale. Recently, however, an attempt was made to get up a company in England to work a French patented process, which differed only from that of Mansfield’s in the substitution of another hydrocarbon (probably a petroleum product) for benzol. The chemical and technical journals exposed this invention, and prevented the sinking of capital in a worthless undertaking. On a small scale, simple ‘naphthalisers’ appear to work very well.
The illuminating power of gas, as well as of other sources of light, may be directly ascertained by what is termed the ‘comparison of shadows,’ or indirectly, and more conveniently, by chemical analysis.[328] See Air Gas, Illumination.
[328] See ‘Watts’s Dictionary of Chemistry,’ vol. i.
GASTROPHAN (Apotheker J. Fürst, Prague). For strengthening the digestion and improving the appetite. Quassia, 30 grammes; orange berries, 15 grammes; galangal, 4 grammes; cardamoms, 2 grammes; star anise oil, 10 drops; orange-peel oil, 10 drops; spirit, 180 grammes; water, 120 grammes; digested and filtered. (Hager.)
GASTROPHILE (Dr Borchard). There are several numbers of this preparation. Soda water, containing common salt, perhaps in some of the numbers mixed with Glauber’s salts.
GAZ′OGENE. [Fr.] Syn. Aërating Machine. A portable apparatus for aërating water and other liquids. Many forms have been given to this instrument, but in all the principle is the same. Powders for generating carbonic acid gas are placed in a separate compartment, and the liquid to be aërated in another. The two compartments are connected by a suitable tube, and a second tube, furnished with a spring tap, affords an exit for the aërated liquid. By the aid of the gazogene, water, wine, ale, &c., may in a few minutes be fully saturated with carbonic acid gas, and so rendered brisk and piquant. By using fruit syrups, manufactured from English and foreign fruits, the most delicious aërated summer beverages can be made, resembling those so much esteemed by travellers in the South of Europe and the sea-board cities of the Western world.
The following are the proportions of soda and acid required for charging gazogenes:
For 2 pints, powdered tartaric acid, 280 grains; bicarbonate of soda, 340 grains.
For 3 pints, powdered tartaric acid, 340 grains; bicarbonate of soda, 420 grains.
For 5 pints, tartaric acid, 620 grains; carbonate of soda, 760 grains.
Put the acid and soda in different coloured papers.
GEDACHTNISS-LIMONADE—Mnemonic Lemonade (manufactured by G. M. Raufer, Vienna). A mixture of 15 parts phosphoric acid, 15 parts glycerin, 70 parts water. (Schädler.)
GEHOR INSTRUMENT. Instrument for deafness (Apotheker F. Brunner, Troppau). A little tube of silver plate, 2 centimètres long and as thick as a straw, with a small mussel-shaped widening at one end, which is wrapped in cotton wool, to be inserted in the ear.
GEHOR LIQUOR, Schweizer—Swiss Cure for Deafness (Raudnitz). Water mixed with a little coarse brandy. (Wittstein.)
GEHOROL—Oil for Deafness (C. Brockelmann, Soest). Provence oil adulterated with sunflower oil and mixed with very small traces of camphor and cajeput, sassafras, and rosemary oils. (Hager.)
GEL′ATIN. Syn. Gelatine; Gélatine, Fr.; Gelatina, L. Animal jelly, obtained by the prolonged action of boiling water on the organic tissue of the bones, tendons, and ligaments, the cellular tissue, the skin, and the serous membranes. Glue and size are coarse varieties of gelatin, prepared from hoofs, hides, skins, &c.; and isinglass is a purer kind, obtained from the air bladders of some other membranes of fish.
786
Prop., &c. Gelatin is insoluble in cold water, but dissolves with greater or less readiness on the application of heat, according to the source where it is obtained, and in this state forms a tremulous and transparent jelly on cooling; it is insoluble in both alcohol and ether, and is decomposed by the strong alkalies and acids; with tannic acid it forms an insoluble compound of a buff colour, which is the basis of leather; when acted on by cold concentrated sulphuric acid, it yields glycocoll or gelatin sugar; and when boiled with strong alkalies, it yields glycocoll and leucine. Chlorine passed into a solution of gelatin occasions a dense white precipitate (chlorite of gelatin), which ultimately forms a tough, elastic, pearly mass, somewhat resembling fibrin.
Tests. Its aqueous solution is recognised as follows:—1. It gelatinises on cooling. 2. It is precipitated by alcohol. 3. Bichloride of mercury gives a whitish flocculent precipitate. 4. Tannic acid or infusion of galls gives a copious yellowish-white, curdy precipitate, which, on being stirred, coheres into an elastic mass, insoluble in water, and incapable of putrefaction, and which, when dried, assumes the appearance of over-tanned leather. 5. The gelatinising property is destroyed by nitric acid. 6. It is not affected by either alum or acetate of lead. In this respect it differs from chondrin.
Qual. The goodness of commercial gelatin intended for food is readily proved by pouring boiling water over it, and digesting the two together for a short time. If it is pure and wholesome, its colour remains unaltered, and during its solution it continues entirely free from smell. The resulting solution and jelly are also odourless, neutral to test-paper, free from unpleasant taste, and perfectly transparent. If it forms a yellow gluey-looking mass, and evolves an offensive odour, it should be rejected as of inferior quality, and unfit for culinary purposes.
Uses, &c. Gelatin is largely employed as an article of food, as in soups, jellies, &c.; but its value in this respect has been, perhaps, overrated.[329] Animals fed exclusively on gelatin die of starvation. But when mixed with other food, especially with substances abounding in albumen, casein, or fibrin, gelatin may be useful as an aliment, and serve directly to nourish the gelatinous tissues. (Liebig.) Hence gelatin is a fitting substance to form part (but only a part) of the diet of convalescents, as it conveys nutrition directly to these tissues, without tasking the diminished powers of life for its conversion; but its use should be accompanied by a proper quantity of azotised animal food to supply the elements to the blood, for the support and increase of the muscular tissue, or fleshy portion of the body. In France gelatin obtained from bones is employed as a part of the diet in hospitals with the best effect, materially abridging the period of convalescence; but when given alone, all animals soon become disgusted with it, and die if not supplied with other food. (D’Arcet.) See Glue, Isinglass, and below.
[329] The reader interested in this subject should consult a paper by Carl Voit in the ‘Zeitschrift für Biologie,’ viii, 297-388.
Gelatin, Bone. Obtained from crushed bones by boiling with water, or by the action of steam and water successively, either with or without pressure; or by maceration in dilute hydrochloric acid, to extract the phosphate of lime, the remaining gelatinous mass being well washed in cold water, and afterwards dissolved in boiling water in the usual manner. A little carbonate of soda is commonly added to the last water. Gelatin has even been extracted from fossil bones. “A soup was prepared from one of the bones of the great mastodon by the préfet of one of the departments of France.” (Pereira.) Butchers’ meat contains, on an average, 24% of dry flesh, 56% of water, and 20% of bone. The last will yield, by proper treatment, nearly 1-3rd of its weight of dry gelatin, or a quantity equal to about 6% of the meat from which it is cut. This, as well as other varieties of gelatin, is frequently blanched by sulphurous acid or animal charcoal, and tinged of various colours with the ordinary vegetable dyes. Thus, blue is given with sulphate of indigo or the juice of blue berries; green, with the juice of spinach; and red, with juice of red-beet.
Gelatin, French. Syn. Cake gelatin. Gelatin made up into small thin cakes, like the finer sorts of glue. A good deal of it is prepared in Paris from the cuttings of the skins used in making kid gloves and slippers.
Gelatin, Patent. Various qualities of gelatin are manufactured from glue pieces, or cuttings of the hides of beasts and skins of calves, and from inferior isinglass. According to Mr Nelson’s specification, the crude materials, freed from hair, wool, flesh, and fat, after being thoroughly washed and ‘scored,’ are macerated for 10 days in a lye of caustic soda, and are then placed in covered vessels at a temperature varying from 60° to 70° Fahr., until they become tender; they are next washed to free them from alkali, and are then exposed to the vapour of burning sulphur until they acquire a sensibly acid reaction; they are now dissolved in water contained in earthen vessels heated to 150° Fahr., and the solution, after being strained, is put into ‘settling vessels,’ and heated to 100° to 120° Fahr., for 8 or 9 hours; at the end of this time the clear liquor is drawn off, and poured on the ‘cooling slabs,’ to the depth of about 1⁄2 an inch. As soon as the jelly is cold, it is cut into pieces, and washed in water until perfectly free from acid. It is then redissolved in water at about 85°, the solution poured out on slabs as before, and when cold, it is cut up, and, lastly, dried on nets.
According to another specification (Rattray’s787 Patent) glue-pieces are steeped in water until they begin to putrefy, then washed with water, drained, and put from 12 to 24 hours into water strongly soured with sulphurous acid; they are afterwards washed first with cold water, and then in water at 120° Fahr., and are lastly converted into size by digestion for 24 hours in water at 120° Fahr., the resulting solution being filtered through bags of double woollen-cloth.
Patent gelatins are often sold cut up in imitation of ‘picked isinglass,’ to which, for the preparation of jellies, soups, and blancmanges, they are not much inferior.
Gelatin, Rough. Syn. Gelatine Brut, Fr. From the skulls of oxen, the spongy insides of the horns and ribs, and from several other soft bony parts (deprived of fat), by washing them in water, digesting in an equal weight of hydrochloric acid of 6° Baume, in cold weather, and 4° or 5° in summer, for 10 days, then in acid of only 1° Baume for 24 hours longer; afterwards soaking and washing in successive portions of cold water until all the acid is washed out, adding an ounce of carbonate of soda to the last water. Used to make glue, &c. A similar article is prepared from the bones of sheep. The pieces, after being treated as above, are steeped in boiling water for a few minutes, wiped dry, and shaken together in a bag to remove the internal pellicle; after which they are cut into squares or dice to disguise them, and finally dipped into a hot solution of gelatin to varnish them. In this state the article is called ‘GELATINE BRUT FIN,’ Used to make soup. It keeps better than the cakes of portable soup. When less carefully prepared, it is also used to make glue for fine work. See Bone gelatin.
GELEE (pour le Goitre). See Liniment of Iodide of Potassium.
GELSEMIUM SEMPERVIRENS. Syn. Gelsemium nitridum, Gelsemium sempervirens, Gelsemium lucidum, Anonymus sempervirens, Bignonia sempervirens; Lisanthus sempervirens. The Yellow jasmine, or WOODBINE. The Carolina jasmine.
Different botanists have placed the plant in different natural orders. De Candolle assigns it to the Loganiaceæ; Decaisne to the Apocynaceæ; Chapman to the Rubiaceæ.
The root, which is the only part of this plant employed in medicine, and of which a fluid extract has been introduced into the United States Pharmacopœia, as met with in English commerce occurs in two states; either in packets prepared by the shakers of New Lebanon, which contain the root in small pieces, formed into a compact mass by hydraulic pressure, and in which state it is difficult to powder; or it is simply sold cut up into pieces varying from two to eight inches in length, and one-third to three fourths of an inch in diameter. It is frequently mixed with about half its bulk of long, wiry, pale-brown rootlets.
The so-called gelsemium root consists chiefly of subterranean stem with a small proportion of true root, occasionally a slender piece of the aërial stem may be found intermixed, and is readily distinguished by its purplish colour and hollow centre, and by the silky and tow-like fibre, rendered visible when the epidermis is peeled off (fig. 1 e).
The true root is hard and woody, slightly undulated in outline, very sparingly branched, except in the slender pieces, externally of a pale brown colour, nearly smooth, and furnished with a thin scurfy cuticle, which is slightly cracked longitudinally. When a transverse section is examined with a lens, the bark of the root is seen to be very thin, and to consist of two layers, the inner one being usually almost as pale as the woody portion, and of somewhat soft texture, the outer one is darker and more compact (fig. 1 b, c).
The meditullium, or woody portion of the root occupies nearly its whole diameter, is of a pale yellowish bright colour, the yellow tint becoming much more distinct when the root is wetted. The medullary rays are white and very distinct, and the woody tissue between the rays is very porous, the pores being very small, but visible to the unaided eye, especially when the root is broken instead of cut (fig. 1 d). There is no pith or central cavity in the root. The root has a bitter taste and pleasant flavour, somewhat between those of senega and green tea; this is more readily perceived in the tincture.
The subterranean stem (fig. 1 a) is also furnished with rootlets, but is easily distinguished from the root by the presence of a small, dark coloured, central cavity representing the pith, and by the external surface being rougher, and frequently variegated with dark longitudinal lines, which are the remains of the same purplish cuticle which presents so marked a feature in the aërial stem. The bark is thicker than that of the true root, and the inner layer is usually dark brown. If the subterranean stem is broken slowly and carefully, a thin row of silky fibres projects fully a quarter of an inch from the broken edge. The fibres do not appear when the bark of the root is broken, and thus serve to distinguish the stem of this drug from the root. Experiments as to the relative value of the bark of the root and stem are wanting. The bark of the stem has the same bitter taste as that of the root, and if it be hereafter shown that it is equally active, the above character of scattered strong fibres, taken in conjunction with the flavour of the drug and its porous structure, will serve to distinguish it from all other roots and stems used in materia medica.[330]
[330] Holmes.
Medicinal properties.—The American medical journals record the successful administration of gelsemium in a great number and variety of diseases, including intermittent, remittent, typhoid, and yellow788 fevers, the irritative fevers of childhood, inflammation of the lungs and pleura, dysentery, rheumatism, and other inflammatory affections, neuralgia, obstinate menstruation, delirium tremens, morbid wakefulness, St. Vitus’ dance, hysteria, epilepsy, spasmodic stricture of the urethra, and gonorrhœa. Dr Hurd, an American physician, reports very favorably of the drug as a cardiac sedative, and considers it more efficient than any other remedy in the palpitation and the difficult breathing that accompany heart disease; and Dr Hill, of Maine, finds it when combined with bromide of potassium useful in irritable bladder.

Its principal use, however, in American medical practice has been as a febrifuge. In periodic fevers it has been employed with great advantage, as well as in cases of intermittent fever, which having failed to yield to quinine alone, succumbed, when this latter medicine was combined with gelsemium.
In England gelsemium has been successfully employed for the relief of facial neuralgia, or of the pain caused in the face and jaws by decayed teeth; as well as in obscure nervous affections and severe headaches. It is given principally in the form of tincture; but sometimes in powder in doses of from one to two grains.
The therapeutic action of gelsemium is believed to be due to the sedative effect it exercises on the nervous and arterial systems—hence its power in controlling the nervous irritability so prevalent during fever. In moderate doses it causes a sensation of agreeable langour, accompanied with muscular relaxation; in larger doses, dizziness, dilated pupil, double vision, general muscular debility and prostration; these symptoms being accompanied by a diminution in the force and frequency of the pulse as well as in the respiration. At the same time the patient becomes insensible to pain; but is free from stupor and delirium. These symptoms are said to pass off, after a time, and to be attended with no unpleasant results.
The ‘Lancet’ as well as many of the American medical journals record several cases of poisoning arising from giving an overdose of this drug. The symptoms are a great prostration of nervous energy, accompanied by paralysis of sensation and motion. When death occurs it is probably owing to syncope. The antidotes are, first, an emetic, and after this has acted, stimulants, such as carbonate of ammonia with brandy, or aromatic spirits of ammonia. In cases accompanied with insensibility, recourse should be had to electricity.
Kollock, in the ‘American Journal of Pharmacy’ for 1855, states that he found the root on analysis to yield volatile oil, dry acrid resin, fatty resin, fixed oil, gallic acid, starch, pectic acid, albumen, extractive matter, lignin, gum, a yellow colouring matter, mineral matter (chiefly salts of potassium, calcium, magnesium, iron and silica), and an alkaloid, to which the789 name gelseminine or gelsemia has been given. Kollock also states that the leaves and flowers contain the same ingredients as the root, although in much smaller quantities.
Eberle, in the ‘American Journal of Pharmacy’ for 1864, says he failed to obtain gelseminine from the root. In a paper contributed to the ‘American Journal of Pharmacy,’ for January, 1870, by Dr Wormley, the author stated that he said he not only succeeded in obtaining pure gelseminine from the root, but also a peculiar acid which he calls gelseminic, or gelsemic[331] acid; which he regards as existing in combination with the gelsemia, forming the gelsemate of gelsemia.
[331] Professor Sonneschien, having submitted the so-called Gelseminic Acid to analysis thinks there can be no doubt that it is perfectly identical with æsculin, a glucoside obtained from the bark of the horse-chesnut—the Esculus hippocastanum.
Probably the alkaloid gelseminine may at some future time be introduced into medicine, since it would appear to be the chief ingredient to which the root owes its activity. It is strongly poisonous. Dr Wormley injected one eighth of a grain under the skin of a large cat, which in 40 minutes exhibited great prostration, and died in an hour and a half from the time of the injection of the poison. The properties of the gelseminic acid, the resin, the volatile oil, and other ingredients of the root, have not been fully investigated. See Tincture of gelsemium, GELSEMININE.
In the ‘American Journal of Pharmacy’ for April, 1877, Dr Wormley gives the following directions for the preparations of gelseminic acid, and gelsemine:—A given volume of fluid extract, acidulated with acetic acid, is slowly added with constant stirring to about eight volumes of water; after the separated resinous matter has completely deposited, the liquid is filtered, and the filtrate concentrated on a water bath, to something less than the volume of fluid extract employed. The gelseminic acid is then extracted from the concentrated fluid by ether, after which the liquid is treated with slight excess of carbonate of sodium, and the gelsemine extracted with ether or chloroform. For the extraction of the first of these principles, it is not essential that the liquid should be acidulated, but in the presence of a free acid the results are more satisfactory.
GEMS. Syn. Jewels; Gemmæ, L. “Gems are precious stones, which, by their colour, limpidity, lustre, brilliant polish, purity, and rarity, are sought after as objects of dress and decoration. They form the principal part of the crown jewels of kings, not only from their beauty, but because they are supposed to comprise the greatest value in the smallest bulk; for a diamond, no larger than a nut, or an acorn, may be the representative sign of the territorial value of a whole country, the equivalent in commercial exchange for a hundred fortunes, acquired by severe toils and privations.” “Among these beautiful minerals mankind have agreed in forming a select class, to which the title of gems or jewels has been appropriated; while the term precious stone is more particularly given to substances which often occur under a more considerable volume than fine stones ever do. Diamonds, sapphires, emeralds, rubies, topazes, hyacinths, and chrysoberyls, are reckoned the most valuable gems;—crystalline quartz, pellucid, opalescent, or of various hues, amethyst, lapis lazuli, malachite, jasper, agate, &c., are ranked in the much more numerous and inferior class of ornamental stones.” (Ure.)
Tests. The only tests applicable to gems and precious stones are the determination of their relative hardness and their specific gravity. By the first test, pastes or factitious gems are readily detected; but beyond this, owing to the difficulty of applying it, it ceases to be useful to persons unconnected with the trade. The determination of the specific gravity is, however, of more general application, as gems are generally dismounted when offered for sale, or are so set that they may be removed from their ‘mountings’ without injury or inconvenience. See Specific Gravity, and below.
Obs. The relative hardness of the different substances is measured by the power they possess of cutting or scratching the other substances having a smaller number attached to them in the table. Thus, no gem but the DIAMOND (20) will scratch either the RUBY (17) or the SAPPHIRE (16); and, for the same reason, a blue stone that will cut the EMERALD or the TOPAZ can be no other than the SAPPHIRE. The sp. gr. is ascertained in the usual manner, and will be found sufficiently indicative of the true nature of the stone when considered in connection with its other characteristics. The index of refraction is a certain key to the quality of the stone, in the hands of those who are capable of determining it, and may be applied to either mounted or unmounted gems. The most convenient instrument for the purpose is Wollaston’s ‘REFLECTING-GONIOMETER,’
Gems, Facti′′tious. These, with few exceptions, are made of very pure, fusible, highly transparent, and dense glass, usually termed ‘PASTE’ or ‘STRASS,’ which is generally formed of oxide of lead, potassa, and silica, with small quantities of other ingredients to increase the brilliancy and clearness. The characteristic tints are imparted by the addition of metallic oxides. The beauty of artificial stones and gems depends, chiefly, upon the tint of the real stones being exactly imitated, and upon proper care and skill being exercised in the cutting, polishing, and mounting them. All the coloured glasses, and enamels, may be worked up into artificial gems.
| Name. | Relative Hardness. | Specific Gravity. | Index of Reflection. |
| Agate | 12 | 2·6 | |
| Amethyst (occidental) | 11 | 2·7 | |
| Calcareous spar | 6 | 2·7 | |
| Chalk | 3 | 2·7 | |
| Chrysolite | 10 | 3·7 | |
| Cornelian | 11 | 2·7 | |
| Crystal | 11 | 2·6 | |
| Diamond (bluish) | 19 | 3·3 | 2·439 |
| Diamond (cubic) | 18 | 3·2 | |
| Diamond (from Ormus) | 20 | 3·7 | |
| Diamond (pink) | 19 | 3·4 | |
| Diamond (yellowish) | 19 | 3·3 | |
| Diamond (average colourless) | 19 to 20 | 3·3 to 3·55 | |
| Emerald | 12 | 2·8 | |
| Fluor spar | 7 | 3·5 | 1·434 |
| Garnet | 12 | 4·4 | 1·815 |
| Glass | various | 2·3 to 3·62 | 1·525 to 2·028 |
| Glass (crystal or flint) | 3·0 to 3·6 | 1·830 to 2·028 | |
| Glass (plate) | 2·5 to 2·6 | 1·514 to 1·542 | |
| Gypsum | 5 | 2·3 | |
| Jasper (green) | 11 | 2·7 | |
| Jasper (reddish yellow) | 9 | 2·6 | |
| Onyx | 12 | 2·6 | |
| Opal | 10 | 2·6 | |
| Quartz | 10 | 2·7 | 1·548 |
| Ruby | 17 | 4·2 | 1·779 |
| Ruby (pale, from Brazil) | 17 | 3·5 | |
| Ruby (spinelle) | 13 | 3·4 | 1·764 |
| Sapphire (deep blue) | 16 | 3·8 | 1·794 |
| Sapphire (paler) | 17 | 3·8 | |
| Sardonyx | 12 | 2·6 | |
| Schoerl | 10 | 3·6 | |
| Topaz | 15 | 4·2 | |
| Topaz (Bohemian) | 11 | 2·8 | |
| Topaz (whitish) | 14 | 3·5 | |
| Tourmaline | 10 | 3·0 | |
| Zeolite | 8 | 2·1 | |
| Zircon | — | — | 1·961 |
MM. Fremy and Feil have lately succeeded790 in manufacturing artificial corundum, ruby and topaz, having a composition the same as the natural stones. The process by which they have effected this consists in fusing together at a red heat, in the furnace of a glass works for a considerable time, a fusible aluminate (such as aluminate of lead), and some silicious body.
The silica is found to unite with the lead, and to liberate the alumina in the crystalline form. When equal weights of alumina and red lead are heated together in a crucible made of some refractory silicious substance, the above conditions if the temperature has been maintained sufficiently long and high ensue, and there is found in the crucible at the end of the operation a layer of silicate of lead, and very frequently another of pure crystallised alumina or corundum.
The ruby colour is given by adding to the mixture in the crucible two or three per-cent. of bichromate of potash, the blue being produced by the addition of a small quantity of oxide of cobalt, with a trace only of bichromate of potash. A film of silicate of lead very frequently adheres to the ruby crystals, and this has to be removed.
In some instances, however, the crystals occur nearly pure, and are precisely similar to the natural gems in crystalline form, composition, hardness, and lustre.
Upon being heated, the artificial ruby, like the natural one, loses its rose colour, and recovers it again on cooling. It is said that the factitious gems hitherto obtained are not, as a rule, equal in lustre to the natural ones, and are consequently not so well suited for jewellers’ work; also that they do not present to791 the lapidary conditions favorable to cleavage or cutting. They are, however, very well adapted for the works of watches. See Enamels, Pastes, &c.
GENE′VA. See Gin and Hollands.
GEN′TIAN ROOT. Syn. Gentinæ Radix, L. The dried root of Gentiana lutea, or ‘yellow gentian.’ Dose, 10 to 30 gr.; as a simple bitter tonic, and stomachic, in dyspepsia, loss of appetite, gout, &c. It was formerly a favourite remedy in agues. “Joined with galls or tormentil, and given in sufficient quantity, it has not failed in any intermittents in which I have tried it.” (Dr Cullen.) In excessive doses it is apt to relax the bowels and disturb the system. When taken for some time, it imparts its bitter flavour to the perspiration and urine. See Decoction, Extract, &c.
GEN′TIANIN. Syn. Gentianine; Gentianina, L. A substance obtained by MM. Henry and Caventou from the root of common gentian.
Prep. 1. Gentian root (in powder) is digested for 2 or 3 days in cold ether, with agitation, and the filtered tincture evaporated to dryness; the residuum is dissolved in rectified spirit, and the solution is again evaporated; the semi-crystalline mass is, lastly, redissolved in either alcohol or ether, and crystallised by careful evaporation.
2. (Magendie.) The ethereal extract is exhausted with cold alcohol (rectified spirit), as before, and the resulting tincture is evaporated to dryness; the residuum is dissolved in water, calcined magnesia added in excess, and the whole boiled and filtered; the sediment is digested in ether, and the ethereal tincture allowed to crystallise by slow evaporation.
Prop., &c. Gentianin forms golden-yellow needles, scarcely soluble in cold water, but very soluble in alcohol and ether. It is a powerful bitter and stomachic.—Dose, 1⁄2 gr. to 2 gr.
GER′MAN PASTE. Prep. From pea-meal, 2 lbs.; sweet almonds (blanched), 1 lb.; fresh butter or lard, 1⁄4 lb.; moist sugar, 5 oz.; hay saffron, 1⁄2 dr.; beat to a smooth paste, adding cold water q. s., granulate the mass by passing it through a colander, and expose the product to the air in a warm place, until quite hard and dry. The addition of 2 or 3 eggs improves it. Used to feed larks, nightingales, and other insectivorous birds. It will keep good for 12 months in a dry place.
GER′MAN SILVER. Syn. Albata, Argentan, Electrum, Nickel Silver, Tutenag, Virginian Plate, White Copper. A well-known alloy, the finer varieties of which nearly equal silver in whiteness and susceptibility of receiving a high polish, whilst they surpass it in hardness and durability. The following formulæ are from the highest authorities, or are the results of actual analysis of the finest commercial samples:—
Prep. 1. Copper, 50 parts; nickel, 20 parts; zinc, 30 parts. Very malleable, and takes a high polish.
2. Copper, 50 parts; nickel, 26 parts; zinc, 24 parts. Closely resembles silver; an excellent sample.
3. Copper and zinc, of each 41 parts; nickel, 18 parts. Rather brittle.
4. (M. Gersdorff.) Copper, 50 parts; nickel and zinc, of each 25 parts. Very white and malleable, and takes a high polish. Recommended as a general substitute for silver.
5. (Gersdorff.) Copper, 60 parts; nickel and zinc, of each 20 parts. For castings, as bells, candlesticks, &c.
6. (Gersdorff.) Copper, 60 parts; nickel, 25 parts; zinc, 20 parts. For rolling and wire. Very tough and malleable.
7. (Sample made from the ore of Hilburghausen.) Copper, 401⁄2 parts; nickel, 311⁄2 parts; iron, 21⁄2 parts; zinc, 251⁄2 parts. Equal to the best Chinese sample.
8. (Pelouze.) Copper and nickel, equal parts. Recommended by M. Pelouze as superior to any of the alloys containing zinc.
9. (Pelouze.) Copper, 2 parts; nickel, 1 part. Not so white as the last, but more malleable.
10. (White Copper from China.)—a. Copper, 30 parts; nickel, 36 parts; zinc, 34 parts.
b. (Said to be prepared from native ore.) Copper, 41 parts; nickel, 32 parts; iron, 21⁄2 parts; zinc, 241⁄2 parts. Silvery white, takes a high polish, very sonorous, malleable both cold and at a dull-red heat, and may be rolled into leaves or formed into wire.
11. (White metal spoon, sold as ‘German Plate.’) Copper, 55 parts; nickel, 24 parts; zinc, 16 parts; tin, 3 parts; iron, 2 parts.
Anal. This may be briefly described as follows:—a. 100 gr. of the alloy is digested in nitric acid q. s., diluted with a little water. If the sample is unequally attacked by the acid, and a white external shell is observed which dissolves more slowly than the internal portion, it is ‘plated’ on those parts with silver. If this silver shell or casing has a polished surface on both sides, the article has been ‘electro-plated,’ if the contrary is the case, it has most probably been plated in the usual way.
b. The solution being completed, heat is applied to expel the excess of acid, and the remainder is largely diluted with distilled water; dilute hydrochloric acid is now dropped in as long as it occasions a precipitate, and the whole, after being moderately heated for a short time, and cooled, is thrown upon a small paper filter; the precipitate on the filter is next washed with distilled water, carefully dried, and ignited in a small porcelain crucible, the filter itself being separately burnt on the cover of the crucible, and the ashes added to its contents prior to ignition. Every 1431⁄2 gr. of the resulting fused chloride is equal to 108 gr. of metallic silver.
c. The filtered liquid (see b) is next treated792 with a stream of sulphuretted hydrogen, and the black precipitate is collected, washed, and digested in strong nitric acid; when the solution is complete sulphuric acid is dropped in to precipitate the lead (if any is present); if a precipitate is formed, the whole is evaporated to dryness, and the excess of sulphuric acid expelled by a rather strong heat applied towards the end; the dry mass is now collected on a filter, washed with a mixture of water and alcohol, dried, and exposed to slight ignition in a porcelain crucible. Every 152 gr. of the resulting dry sulphate is equal to 104 gr. of lead.
d. The liquor filtered from the sulphate of lead, or (in its absence) the nitric solution of the precipitate produced by the sulphuretted hydrogen (see c), is next treated with potassa, &c., as described under the analysis of brass. Every 40 gr. of the dry protoxide thus obtained represents 32 gr. of pure copper.
e. The liquor which was filtered from the precipitate produced by the sulphuretted hydrogen (see c) is boiled until it loses its offensive odour, and is then precipitated with carbonate of soda, in slight excess, and again boiled for a few minutes; the precipitate (mixed oxides of nickel and zinc) is collected, washed, and redissolved in dilute acetic or nitric acid, in excess; a current of sulphuretted hydrogen is next passed through the solution, the precipitate collected on a filter, washed, redissolved in hydrochloric acid, and the solution again treated with carbonate of soda; the last precipitate (oxide of zinc) is washed, dried, and gently ignited. Every 40 gr. of this oxide is equivalent to 32 gr. of metallic zinc.
f. The washings of the precipitated oxides and the liquid filtered from the precipitate occasioned by the sulphuretted hydrogen (see e) are mixed together, pure solution of ammonia added in considerable excess, and the mixture agitated for some time; the undissolved portion of the precipitate is then collected on a filter, washed with distilled water, redissolved in dilute nitric acid, again precipitated with solution of potassa, and this last precipitate (ferric oxide) washed, dried, ignited, and weighed. Every 80 gr. represents 50 gr. of metallic iron.
g. The ammoniacal solution filtered from the precipitate of sesquioxide of iron (see f) is precipitated with pure solution of potassa, boiled for a few minutes, and, when cold, thrown on a filter; the precipitate is, lastly, washed with hot water, dried, ignited, and weighed. Every 371⁄2 gr. of the oxide thus obtained is equal to 291⁄2 gr. of metallic nickel.
Obs. The manufacture of nickel or German silver has of late acquired an importance which is second only to that of silver plate itself. The superior quality of this alloy, and the graceful patterns which it is often made to assume in the hands of the accomplished artist cannot fail to have attracted the admiration of the majority of our readers. The value of correct information regarding the preparation of this alloy, and of a ready method of determining the composition of the most improved commercial samples will, therefore, be fully appreciated by every metallurgist who wishes to throw his wares into the arena of public competition. Much that is vended under the name of German silver is little better than the Britannia metal or PLATE PEWTER formerly so plentiful in every establishment in this country. German silver has quite superseded copper as the basis of ‘electro-plated goods.’
[332] See Electrotype.
The union of the metals in the above formulæ is effected by heat with the usual precautions. When iron is ordered, it is generally added under the form of ‘tin plate.’ See Alloy, Brass, Britannia Metal, Bronze, &c.
GER′MAN TIN′DER. See Amadou.
GERMINA′TION. The growth or vegetation of a seed by which a young plant is produced. The conditions essential to germination are the presence of warmth, air, and moisture. The most favorable temperature is between 60° and 85° Fahr., according to the habitat of the respective plants. Below 40° Fahr. most of the more perfect seeds either refuse to vegetate, or vegetate slowly and feebly; and at or near the freezing-point none of them undergo this change. At a temperature above 100° Fahr. the young germ is usually injured, and at about 125°, if it forms, it soon withers and dies. See Malting, Seed, &c.
GERMS. The ‘germ theory of disease’ may be briefly stated to be that which supposes the cause of epidemic and contagious maladies to be due to the agency of specific, inconceivably small germs,—different germs giving rise to different diseases.
These disease germs gaining an entrance by means of air, water, or food into the healthy body, and being possessed of extraordinary powers of increase and subdivision, are supposed to set up the particular disease, and at the same time to multiply to an incredible extent by feeding upon the tissues best suited for their support. Further, they are conceived to be thrown off into the atmosphere from the body of the patient, whence they are conveyed as before described into other healthy animal organism, in which, comporting themselves as in the previous case, they set up a similar disease. See Bacteria as originators of disease.
GHEE. A sort of butter used by the natives of India. Prep. Milk is boiled in large earthen pots for an hour or two, then allowed to cool, a little curdled milk called ‘dhye’ being added, in order to make the whole coagulate. After a lapse of some hours the contents of each to the depth of 5 or 6 inches are removed and placed in a larger earthenware utensil, in793 which they are churned by means of a piece of split bamboo for about half an hour; then hot water is poured in, and the churning continued for half an hour longer, after which time the butter is found to be formed. When this becomes rancid, it is melted in an earthen vessel, and boiled until all the water has evaporated; after which a little salt or betel-leaf is put into it, and finally it is poured off into suitable vessels in which it can be preserved from the air. Bottles are commonly used for this purpose. See Butter.
GHER′KINS. Syn. Gir′kins. Small cucumbers adapted for pickling. See Pickles.
GILD′ING. Syn. Dorure, Fr. The art or process of covering the surfaces of bodies with a thin film of gold, for the purpose of increasing their durability or improving their appearance. For the sake of brevity we shall briefly notice the leading varieties of gilding, and their applications, in alphabetical order.
Gilding, Burnished. This is distemper gilding to which a ‘face’ has been given with the ‘burnisher.’ It is chiefly employed for the polished portions of the frames of pictures and mirrors, the more prominent parts of statuettes, &c.
Gilding, Chemical. Those varieties in which the film of gold is formed on the surface through the agency of chemical affinity, in opposition to mechanical gilding, in which the gold is made to adhere by the intervention of some glutinous substance.
Gilding, Cold. The articles (copper or brass) to be gilded, after being softened, annealed, and polished in the usual manner, are rubbed with a little gilding powder by means of a piece of cork moistened with a solution of salt in water; after which the work is burnished with a piece of hematite or polished steel. (See below.)
Gilding, Distemper. This is applied to wood, plaster, marble, &c. It is commonly performed in this country by giving the wood, first, a coating of good size, and next, several successive coats of size thickened with finely powdered whiting, Spanish white, or plaster of Paris until a good face is produced; observing to let each coat become quite dry, and to rub it perfectly smooth with fine glass paper, before the application of the following one. When the proper ‘face’ is obtained, the surface is thinly and evenly gone over with gold size, and when this is nearly dry, the gold leaf is applied, and afterwards burnished with an agate or dog’s tooth. The process, as adopted by the Parisian artists, who greatly excel in this species of gilding, is very complicated, and is divided into at least 17 distinct operations, each of which they declare to be essential to its excellence.
Gilding, Electro-. See Electrotype.
Gilding, Grecian. In this variety sal-ammoniac and corrosive sublimate, equal parts, are dissolved in nitric acid, and a solution of gold made with this menstruum; after slight concentration the liquid is applied to the surface of silver, which immediately becomes black, but on being heated exhibits a rich gilded surface.
Gilding, Japanner’s. The surface is covered with oil size thinned with spirits of turpentine, and gold, in powder, is gently dabbed on with a puff of wash leather. This gives the appearance of ‘frosted gold.’ A coating of varnish is next given, followed by exposure to a gentle heat in the ‘stove.’
Gilding, Leaf. This term is commonly applied to the gilding of paper, vellum, &c., by applying leaf gold to the surface, previously prepared with a coating of gum water, size, or white of egg. It is usually burnished with an agate or dog’s tooth.
Gilding, Mechanical. See Chemical gilding (above).
Gilding, Mercurial. See Wash gilding (below).
Gilding, Oil. This species of gilding may be divided into several operations. The following are the abridged instructions of a Parisian artist on the subject:—1. The surface is prepared by a coating of white lead in drying oil.—2. Another coat is given, made with calcined white lead or massicot, ground in linseed oil and turpentine. 3 or 4 coats of this mixture are often given, at intervals of at least 23 hours, observing to carefully smooth off each coat with pumice stone or shave grass before the application of the following ones.—3. The ‘Gold Colour,’ or paint, is next applied. It is usually very adhesive gold size, or the bottom of the pot or dish in which painters wash their brushes. For this purpose it is thoroughly ground and strained.—4. When the gold colour becomes partially dry and sufficiently tenacious, the gold leaf is applied, and pressed on with a wad of cotton-wool or a soft brush. It is now left for several days to harden.—5. A coat of spirit varnish is next given, and the object is cautiously passed over a chafing-dish of charcoal, observing to avoid stopping the motion of the piece whilst doing so, as the work would then become discoloured and blistered.—6. The work is ‘finished off’ with pale oil varnish. For out-door gilding and common work the varnishing process is generally omitted. This species of gilding is applied to woodwork, plaster, metal, &c.
Gilding, Varnish. This is a mere variety of oil gilding, applied to equipages, furniture, mirror and picture frames, &c., the surface being highly varnished and polished before it receives the size or gold colour; and after the gilding has become quite dry, a coat of spirit varnish, fumed with the chafing dish as above, is applied, followed by 2, 3, or more coats of the best copal varnish, at intervals of 3 or 4 days each. The whole is, lastly, carefully polished with tripoli and water.
Gilding, Wash, Amalgam g., Mercurial g., Water g. This consists in the application794 of a thin coating of amalgam of gold to the metallic surface (brass, bronze, or copper) to be gilded, and the subsequent volatilisation of the mercury by heat. It is the usual method of gilding articles of copper and its alloys, and possesses great beauty and durability when skilfully executed. The occupation is, however, an unhealthy one, owing to the continual exposure of the workman to the fumes of mercury. The furnace invented by M. D’Arcet obviates this evil, as the whole of the volatilised mercury is carried off, and again condensed for further use. It should, therefore, be adopted by every water-gilder who studies economy and the health of those in his employ.
The process of water gilding consists in several distinct operations, and can only be successfully performed by those who have been schooled in the art by an apprenticeship to the trade. It would, therefore, be waste of space to enter into details here. Formulæ for several of the articles employed for the purpose will be found in the alphabetical places in this work.
Gilding, Water. See above.
Among the applications of the process of gilding that deserve a separate notice are the following:—
The gold letters and figures on the covers of BOOKS are thus formed:—Gum mastic, in fine powder, is dusted over the surface to be gilded; an iron or brass tool bearing the design upon its face is then heated to a proper temperature, and gently pressed upon a piece of leaf gold, which slightly adheres to it; the two are then transferred to the cover, and the tool is gently pressed on it, by which means the mastic softens and retains the gold. The loose gold and powdered mastic are then dusted off with a brush. Gold leaf will adhere to leather without the use of mastic, but not so firmly as when it is employed.
The edges of the leaves of books and paper are first cut perfectly smooth, and then washed over with a solution of isinglass in weak spirit, or with a varnish made of Armenian bole, 4 parts, and powdered sugar-candy, 1 part, mixed up to a proper consistence with strained white of egg. The coating is allowed to dry, and is then smoothed with a wet rag, after which the gold leaf is applied and polished with the burnisher.
Brass buttons, formerly so much in demand, are covered by a rough species of wash gilding. The buttons are polished in the lathe and thrown into a pan with a little amalgam of gold, and as much aquafortis diluted with water as will wet them all over. Here they are well stirred up, until they assume a silvery appearance, when they are washed with clean water. They are then submitted to a sufficient heat in a suitable apparatus, until the mercury is volatilised. The buttons are next cooled, and well tossed and rubbed about with a painter’s brush; and are, lastly, burnished by washing them well with beer or ale grounds.
Twelve dozen (1 gross) of buttons, of 1 inch in diameter, may be perfectly gilded on both sides with only 5 gr. of gold. By an Act of Parliament, which is still unrepealed, this is the smallest quantity of gold permitted to be used for a gross of buttons of the above size.
Glass, PORCELAIN, and EARTHENWARE, are gilded by blending powdered gold with gum-water and a little borax, and applying the mixture by means of a camel-hair pencil; the article is then heated in an oven or furnace, by which means the gum is burnt, and the borax, vitrifying, cements the gold to the surface. It is afterwards polished with a burnisher. Names, dates, or any fancy device, may thus be permanently and easily fixed on glass, china, earthenware, &c.
Japanned work is gilded by the method explained as ‘Japanner’s gilding’ (above).
Leather is gilded in the same way as the covers of books. (See above.) For common work, silver leaf, or even tin foil, is applied to the surface, previously covered with size or white of egg, and after being burnished down and dried, is washed over with gold-coloured lacquer.
The LETTERS of sign-boards and the ornamental gilding for out-door work are done by first covering the design with yellow paint, then with oil gold-size, and when this is nearly dry applying the leaf gold, observing to shield it properly from the wind, lest it be blown away or become crumpled before being properly attached. The work is, lastly, varnished.
Polished metals may be gilded by one or other of the methods already noticed. Articles in silver, copper, brass, and bronze, are usually coated by the process of wash or water gilding; or, directly, by the application of gold leaf, as follows:—The piece or article is heated to a bluish tint, and gold leaf pressed gently and carefully on it with the burnisher; heat is again applied, and the process repeated with fresh leaves of gold until the gilding has acquired the proper thickness and tone. The surface is lastly polished with the burnisher, or is coloured in the usual manner at the stove. This succeeds with iron, steel, silver, copper and its alloys, &c. Another method for polished articles in iron and steel, which, however, is less durable than the preceding, is to apply an ethereal solution of gold to the surface with a camel-hair pencil. The ether flies off and leaves the surface coated with gold, which is then polished as before. In this way, any fancy device or writing may be executed on steel or iron with extreme facility.
Silks, SATINS, WOOLLENS, IVORY, BONE, &c., may be readily gilded by immersing them in a solution of neutral terchloride of gold (1 of the salt, and 3 to 6 of water), and then exposing them to the action of hydrogen gas. The latter part of the process may readily be performed by pouring some dilute sulphuric795 acid on zinc or iron filings, in a wide-mouthed bottle, and placing it under a jar or similar vessel, inverted, at the top of which the articles to be gilded are suspended. Flowers or other ornamental designs may be produced by painting them on the surface with a camel-hair pencil dipped in the solution. The design, after a few minutes’ exposure to the hydrogen, shines with all the splendour of the purest gold, and will not tarnish on exposure to the air, or in washing.
Gilded thread or GOLD THREAD is merely a thread of yellow silk covered with a very thin flatted wire of gold, by means of a revolving wheel.
Wire (copper, silver, or brass) is occasionally gilded, in coils, by a similar process to that adopted for BUTTONS; but more frequently as follows:—Rods (usually of silver) are covered with gold foil of a thickness proportionate to the quality of the intended wire, and the compound bar is then drawn into wire, in the usual way. 100 gr. of gold was formerly the lowest legal quantity that could be employed for 1 lb. of silver.
Patents. Among the varieties of chemical gilding may be mentioned
1. (Elkington’s patent—German gilding, Bonnet’s GILDING PROCESS.) The articles to be gilded, after being perfectly cleaned from scale or grease, and receiving a proper ‘face,’ are suspended, by means of wires, in the gilding liquid (boiling hot), and moved about therein for a period varying from a few seconds to a minute, or longer; the precise time required depending on the newness and strength of the liquid. When sufficiently gilded, the articles are withdrawn from the ‘solution of gold,’ washed in clean water, and dried; after which they undergo the usual operation of ‘colouring,’ &c. A dead gold appearance is produced by the application to the articles of a weak solution of nitrate of mercury previously to the immersion in the gilding liquor; or the deadening may be given by applying a solution of the nitrate to the newly gilded surface, and then expelling the mercury by heat.
The gilding liquor.—Take of fine gold, 5 oz. (troy); nitro-muriatic acid, 52 oz. (avoirdupois); dissolve by heat, and continue the heat until red or yellow vapours cease to be evolved; decant the clear liquid into a suitable vessel; add of distilled water, 4 galls.; pure bicarbonate of potassa, 20 lbs.; and boil for 2 hours. The nitro-muriatic acid is made with pure nitric acid (sp. gr. 1·45), 21 oz.; pure muriatic acid (sp. gr. 1·15), 17 oz.; and distilled water, 14 oz.
This process, though patented by Mr Elkington in England, was in reality discovered and first practised by M. Bonnet, a foreigner. Articles thus gilded do not bear friction and the operations of being put in colour (mise en couleur) so well as those gilded by the mercurial process, or by the methods of cold or leaf gilding as applied to polished metals.
2. (Talbot’s patent.) By this process polished metallic articles are gilded by simple immersion in a solution of gallic acid in water, ether, or alcohol, to which a solution of gold has been previously added. Silvering and PLATINISING may be effected in the same manner, by using a solution of either of these metals instead of one of gold.
⁂ These and other chemical processes have been almost completely superseded by the certain and economical process of ELECTRO-GILDING. See Electrotype.
Gilding Amalgam. See Amalgam.
Gilding Liquor. This name has been given to various solutions of gold, and to other liquids employed in gilding. The former are noticed elsewhere. Among the latter are the following:
Deading aquafortis. From mercury, 1 part; aquafortis (sp. gr. 1·33), 3 parts; dissolve, and add of soft water, 7 parts. Used to produce a dead-gold effect. It is applied (diluted) to the articles, before spreading the amalgam over them, in water gilding; or before placing them in the ‘gilding liquor,’ in the chemical processes.
Mercurial solution. From mercury, 10 parts, dissolved in aquafortis (sp. gr. 1·33), 11 parts, and the solution diluted with 25 times its weight of water. Used to moisten the scratch brush before drawing it over the amalgam, in mercurial gilding; also to deaden the gilded surface, by moistening the latter with it, and then exposing the piece to a heat sufficiently high to drive off the mercury.
Gilder’s pickle. From alum and common salt, of each 1 oz.; nitre, 2 oz.; dissolved in water, 1⁄2 pint. Used to impart a rich colour to gold surfaces, especially of trinkets. Its application should not be too long continued, as it dissolves a small portion of the gold. For common purposes it is best used largely diluted with water.
Vermeil, Vermeil coating, Or-molu c. From annotta and salt of tartar, of each 1 oz.; dragon’s blood, 1⁄2 oz.; water, 1 quart; simmer down to about one fourth, add saffron, 20 gr., and when merely tepid, strain through fine muslin into a bottle. Used to give lustre and fire to distemper gilding. A little is floated over the surface with a very soft, flat, camel-hair brush.
Gilding Metal. The metal employed as a base for gilding is usually brass, or a mixture of brass and copper. The following proportions have been recommended:—
Gilding Powder. Prep. 1. Pure gold, 5 dr.; pure copper, 1 dr.; aqua regia, 10 oz.; dissolve, moisten clean linen rags with the solution, dry them, and burn them to ashes. The latter contain the gold in a state of minute division, and must be carefully collected.
796
2. Grain gold, 1 dr.; rose copper, 15 gr.; aqua regia, 2 fl. oz.; proceed as last. Used in ‘Gold Gilding.’
3. See Gold (in powder).
Gilding Shells. See Gold Shells.
Gilding Size. See Gold Size.
Gilding Wax. Syn. Gilding varnish, Gilder’s wax. Prep. 1. From beeswax, 4 oz.; verdigris and sulphate of copper, of each 1 oz.; melted together.
2. Beeswax, 4 oz.; verdigris, red ochre, and alum, of each 1 oz. Used to give a red gold colour to water gilding.
GIN. Syn. Gene′va. Corn spirit flavoured with either oil of juniper or oil of turpentine.
Gin was originally and, for some time, wholly imported from Holland, and was a rich, soft spirit, flavoured, chiefly, with juniper berries; on which account it had obtained the name of ‘GENEVA,’ from ‘GENIÈVRE,’ the French for juniper. After a time the distillation of an imitation geneva sprung up in this country, when the foreign spirit came to be called ‘Hollands,’ or ‘Hollands geneva,’ to distinguish it from the spirit of home manufacture. The English monosyllable ‘GIN’ is a corruption of geneva, the primary syllable of which, as in numerous other instances, was seized on by the vulgar, and adopted as a short and convenient substitute for the whole word.
The liquor at present known by the name of ‘gin’ in this country is a very different article to that imported from Holland, and consists of plain corn-spirit, flavoured with oil of turpentine and small quantities of certain aromatics. The thousand and one receipts for this article, which have from time to time been printed in books, produce a flavoured spirit bearing no resemblance to the more esteemed samples of English gin; and, if possible, the products are even more unlike genuine Hollands. Any persons may easily satisfy himself of the truth of this assertion by actual experiment on the small scale. The cause of this incongruity has arisen chiefly from the writers not being practically acquainted with the subject, and from the disinclination of well-informed practical men to divulge, gratuitously, what they conceive to be valuable secrets. Hence the utter failure of any attempts to produce either gin or Hollands from the receipts usually published. The authors appear to have all imbibed a juniper-berry mania—probably from the imbibition of their favourite beverage. Oil of juniper, in the hands of these gentlemen, appears to be a perfect aqua mirabilis, that readily converts whisky into gin, and imparts the rich creamy flavour of ‘Schiedam Hollands’ to crude corn or molasses spirit. But theory and experiment sometimes disagree. In practice, it is found that the true flavour of foreign geneva cannot be imparted to spirit by juniper alone, and that the English gin of the present day depends for its flavour on no such a substance. The following formulæ are merely given as specimens; and it is proper to remark, that every distiller has his own receipt for this notorious beverage. Hence it is that the gins of no two distillers are of precisely the same flavour; and this difference is still more marked when the distillers reside in parts of the country remote from each other. Booth’s, Smith’s, and Nicholson’s gins have each a characteristic flavour, readily perceived by their respective votaries; whilst the difference between ‘Plymouth’ or ‘Bristol gin,’ and the ‘gin of the metropolis,’ is as remarkable as that between ‘Barclay’s XXX’ and ‘Guinness’s bottled stout.’ These variations in flavour generally depend on the use of more or less flavouring matter, or of a spirit more or less clean or free from taint; and, less frequently, on the addition of a small quantity of some peculiar aromatic, which exercises a modifying influence on the chief flavouring ingredient. In many cases the flavour has originated from accident, but the consumers having become accustomed to, and hence relishing, that particular ‘palate,’ it is found to be unwise or commercially impossible to alter it. Any change in these matters is therefore looked upon in every distillery as a dangerous innovation, which would prove more prejudicial to the prosperity of its exchequer than the repeal of the duty on French wines and brandy, or even a frightful conflagration. The distillers, like the brewers, are thorough conservatives in all matters connected with the flavour of their liquors.
In the preparation of gin, both sweetened and unsweetened, and indeed of liquors generally, the greatest possible care must be taken to avoid an excess of flavouring. The most esteemed samples are those that consist of very pure spirit, slightly flavoured.
Prep. 1. Clean corn spirit, at proof, 80 galls.; newly rectified oil of turpentine, 11⁄4 pint; mix well by violent agitation, add culinary salt, 14 lbs., dissolved in water, 40 galls.; again well agitate, and distil over 100 galls., or until the faints begin to rise. Product. 100 galls. of gin 22 u. p., besides 2 galls. contained in the faints. If 100 galls. at 17 u. p. are required, 85 galls. of proof spirit, or its equivalent at any other strength, must be employed.
2. Proof spirit (as above), 8 galls.; oil of turpentine, 1 fl. oz.; salt, 11⁄2 lb., dissolved in water, 4 galls.; draw over 10 galls., as before. 22 u. p.
3. Clean corn spirit, 80 galls.; oil of turpentine, 1 pint; pure oil of juniper, 3 fl. oz.; salt, 21 lbs.; water, 35 galls.; draw over 100 galls., as before. 22 u. p.
4. To the last, before distillation, add, of oil of caraway, 1⁄2 fl. oz.; oil of sweet fennel, 1⁄4 fl. oz.; cardamoms (ground), 8 oz.
5. To No. 3 add, of essential oil of almonds, 1 dr.; essence of lemon, 4 dr.
797
6. To No. 1, before distillation, add of creasote, 3 fl. dr.
7. To No. 3 add of creasote, 2 dr.
8. Proof spirit, 80 galls.; oil of turpentine, 3⁄4 pint; oil of juniper, 1⁄4 pint; creasote, 2 dr.; oranges and lemons, sliced, of each 9 in no.; macerate for a week, and distil 100 galls. 22 u. p.
9. To No. 1 add of rectified fusel oil, 1⁄2 pint.
10. To No. 1 add of oil of juniper, 1⁄2 pint.
Concluding Remarks. The oil of turpentine for this purpose should be of the best quality, and not that usually vended for painting, which always contains resin and often fixed oil. Juniper berries, bitter almonds, and the aromatic seeds, may be used instead of the essential oils; but the latter are the most convenient. Turpentine conveys a plain-gin flavour,—juniper berries or oil gives a Hollands flavour,—creasote imparts a certain degree of smokiness, or whiskey flavour,—lemon and the other aromatics, a creaminess, fulness, and richness. The flavour imparted by cardamoms, when used judiciously, is peculiarly agreeable and appropriate. That from caraways is also in general esteem. Cassia in extremely small proportions also tells well. Fusel oil gives a whiskey-gin flavour; and in conjunction with creasote or crude pyroligneous acid, a full whiskey flavour. The only danger in the employment of all these articles is using too much of them. When this misfortune happens, the remedy is to add sufficient plain spirit to reduce the flavour to the proper standard. The creaminess and smoothness so much admired in ‘foreign geneva’ results chiefly from age. The English rectifier endeavours to imitate this by the addition of a little sugar. A rich mellowness, that combines well with gins turning on the ‘Hollands flavour,’ is given by a very small quantity of garlic, and with Canadian balsam or Strasburg turpentine. The peculiar piquancy, or the property of ‘biting the palate,’ regarded as a proof of strength and quality by the ignorant gin-drinker, is imparted to the liquor by the addition of a little caustic potassa. Sliced horseradish gives piquancy as well as mellowness. Grains of paradise, cayenne pepper, and sulphate of zinc, are also commonly added by fraudulent dealers.
Although gin is always prepared on the large scale by distillation, it may also be made by the simple solution or digestion of the flavouring ingredients in the spirit; but it is, of course, better for distillation. If made in the former way, no salt must be employed. The gin produced by the above formulæ is that denominated in the trade ‘UNSWEETENED GIN,’ ‘GROG GIN,’ &c.; but the gin usually sold in the metropolis is a sweetened spirit, and hence is technically distinguished by the terms ‘SWEETENED,’ or ‘MADE UP,’ The generality of London gin-drinkers prefer the latter article, even when weaker and inferior, which it usually is, as the addition of sugar permits adulteration and watering to an enormous extent with absolute impunity. Sweetened spirit cannot be easily tested for its strength, and is taken by the Excise at the strength which it is declared to possess by the dealer. To ascertain whether gin is sweetened or not, a little may be evaporated in a spoon, over a hot coal or a candle, when, if it is pure, it will leave the spoon scarcely soiled; but if, on the contrary, it has been sweetened, a small quantity of syrupy liquid, or sugar, will be obtained, the sweetness of which may be easily recognised by tasting it.
The whole of the casks and utensils employed for gin should be perfectly clean, and properly prepared, so as not to give colour; as, if this spirit acquires the palest coloured tint, its value is lessened, and if much coloured it is rendered unsaleable. When gin has once become much stained, the only remedy is to re-distil it; when it is only slightly stained, the addition of a few lbs. of acetic acid (B. P.) to a pipe or butt, a spoonful or two to a gallon, or a few drops to a decanterful, will usually decolour it, either at once or as soon as it is mixed with water to make grog. See Alcoholometry, Casks, Distillation, Hollands, Spirits, &c., and below.
Gin, Cor′dial. This is gin sweetened with sugar, and slightly aromatised.
Prep. Good gin (22 u. p.), 90 galls.; oil of almonds, 1 dr.; oils of cassia, nutmeg, and lemon, of each 2 dr.; oils of juniper, caraway, and coriander, of each 3 dr.; essences of orris root and cardamoms, of each 5 fl. oz.; orange-flower water, 3 pints; lump sugar, 56 to 60 lbs.; dissolved in water, 4 galls. The essences are dissolved in 2 quarts spirit of wine, and added gradually to the gin until the requisite flavour is produced, when the sugar (dissolved) is mixed in, along with a sufficient quantity of soft water, holding 4 oz. of alum in solution, to make up 100 galls. When the whole is perfectly mixed, 2 oz. of salt of tartar, dissolved in 2 or 3 quarts of hot water, are added, and the liquor is again well rummaged up; after which the cask is bunged up, and allowed to repose. In a week, or less, it will have become brilliant, and may be either ‘racked,’ or drawn from the same cask. Product. 100 galls., about 30 u. p.
Gin, Sweetened. Prep. From unsweetened gin (22 u. p.), 95 galls.; lump sugar, 40 to 45 lbs., dissolved in clear water, 3 galls.; mix well, and fine it down as above. Product. 100 galls., at 26 u. p. This, as well as the last, is usually ‘permitted’ at 22 or 24 u. p., which is also done when the gin has been further lowered with water so as to be even 30 or 35 u. p. See Spirits, and above.
GIN′GER. Syn. Ginger root; Zingiberis radix, Zingiber (B. P.), L. “The scraped and dried rhizome” (rootstock or underground stem) of “Zingiber officinale”—(B. P.). Ginger is an aromatic stimulant and798 stomachic, very useful in flatulence and spasms of the stomach and bowels, and in loss of appetite and dyspepsia, arising from debility, or occurring in old or gouty subjects. A piece chewed an hour before dinner tends to provoke the appetite; as a masticatory, it often relieves toothache, relaxation of the uvula, tender gums, and paralytic affections of the tongue. Made into a paste with warm water, and spread on paper, it forms a useful and simple ‘headache-plaster,’ which frequently gives relief when applied to the forehead or temples. As a condiment and flavouring ingredient, it is perhaps one of the most wholesome of the aromatic kinds, and is less acrid than the peppers.—Dose, 10 gr. to 1⁄2 teaspoonful, stirred up in any simple liquid.
Pur., &c. The best is that known in commerce as ‘unbleached Jamaica ginger,’ which is an uncoated pale variety, occurring in large, bold, fleshy pieces (‘RACES’), which cut soft, bright, and pale-coloured. The inferior varieties occur in smaller pieces, and are darker-coloured, flinty, and shrivelled. The dealers frequently ‘dress up’ the common dark-coloured gingers by washing them in water, drying them, and then ‘rouncing’ them in a bag with a little calcined whiting or magnesia (WASHED GINGER); or they bleach them by dipping them into a solution of chloride of lime, or by exposing them to the fumes of burning sulphur (BLEACHED GINGER); or they dip them into a milk formed of quicklime or whiting and water (WHITE-WASHED GINGER). The last has a chalk-white surface, which cannot be mistaken for the natural one. Powdered ginger is with difficulty obtained pure and good. The common adulterants are wheat-flour, or East Indian arrow-root, and plantain-meal. The first may be detected by the microscope, the others by the flavour and action of hot water. See Lozenges, &c.
GINGERIN. Syn. Oleoresina Zingiberis. Prep. (Pharm., U. S.) Put 1 lb. (Troy) of ginger in fine powder into a percolator, and pour on it 12 ounces (old measure) of pure ether. When this has been absorbed, add rectified spirit until 12 ounces (old measure) have been obtained. Recover the greater part of the ether by distillation over a water bath, and expose the residue in a porcelain dish until the volatile part has evaporated. Keep it in a stoppered bottle.
GINSENG. The root of the Panax Schinseng (Ginseng) is greatly esteemed in China, where it is regarded as a panacea for nearly all diseases, and where it realises a high price in consequence. This opinion of its therapeutic value is not shared by British and American practitioners, who look upon it as a comparatively inert substance. An allied species, the Panax quinquefolium, is sold in America, less for the sake of its very feeble demulcent properties, than to supply the demand of those who have acquired a taste for it. “The root has a somewhat bitter taste, and is somewhat mucilaginous. It occurs in pieces usually about three or four inches long, often partially divided, being joined together at the base; when clean it has a semi-transparent appearance.”[333]
[333] ‘Gardener’s Chronicle.’
Preserved Ginger. Syn. Conditum zingiberis, L. An excellent stomachic sweetmeat or preserve. It is chiefly imported from the West Indies and China. See Candying, &c.
A Factitious Preserved Ginger is sometimes met with, prepared from the stalks of lettuces just going to seed, using a concentrated syrup, strongly flavoured with Jamaica ginger. See Candy, &c.
GIN′GER BEER. See Beer.
GIN′GERBREAD. Prep. 1. (Dr Colquhoun.) Flour, 1 lb.; carbonate of magnesia, 1⁄4 oz.; mix; add, of treacle, 1⁄2 lb.; moist sugar, 1⁄4 lb.; melted butter, 2 oz.; tartaric acid (dissolved in a little water), 1 dr.; make a stiff dough, then add of powdered ginger and cinnamon (cassia), of each 1 dr.; grated nutmeg, 1 oz.; set it aside for half an hour or an hour before putting it into the oven. Obs. It should not be kept longer than two or three hours at the utmost, before being baked.
2. Flour and treacle, of each 1 lb.; butter, 11⁄2 oz.; carbonate of magnesia, 1 oz.; add spices (ginger, cinnamon, nutmeg, allspice, cayenne, corianders, &c.) to taste; mix as last. Obs. Fit for baking in from four to six hours.
3. Flour, 2 lbs.; carbonate of magnesia, 1⁄2 oz.; mix; add, treacle, 11⁄2 lb.; butter, 2 oz.; spice, q. s.; tartaric acid, 1⁄4 oz.; mix quickly, and make it into forms. Obs. Ripe for the oven in half an hour to one hour.
4. Instead of tartaric acid in the last formula, use cream of tartar (dissolved in water), 2 oz. Obs. Ripens in 40 or 50 minutes.
5. Flour or fine pollard, 1 lb.; treacle, 3⁄4 lb.; salt of tartar, 1⁄2 oz., dissolved in water, q. s., butter, 1 oz.; spices, to palate. Obs. Takes several days to ripen; sometimes a fortnight.
6. (Extemporaneous.)—a. From flour, 11⁄4 lb.; moist sugar and treacle, of each 1⁄2 lb.; butter, 21⁄2 oz.; baker’s salt (carbonate of ammonia), 1⁄4 oz., dissolved in cold water, q. s.; ginger, 3 dr.; nutmeg, 2 dr.; cassia, 1 dr.; cayenne pepper (best), 1⁄2 dr.
b. From flour, 6 lb.; powdered ginger, 21⁄2 oz.; caraway seeds, 1 oz. (and other spices to palate); candied lemon and orange peels, of each 2 oz.; moist sugar and melted butter, of each 1⁄2 lb.; treacle, 4 lb.; volatile salt, 2 oz.; water, q. s.; mix as above. May be baked at once.
c. From Jones’s patent flour, 2 lbs.; treacle, 1 lb.; moist sugar, 3⁄4 lb.; butter, 21⁄2 oz.; spice, q. s.; mix as quickly as possible, and bake it instantly. If the dough is expertly mixed up, the quality of the product is fully equal, if not superior, to that of any of the preceding formulæ.
Obs. Gingerbread is either rolled out into799 thin sheets and cut into cakes or nuts (GINGERBREAD NUTS) with the top of a wine-glass or canister, or is formed into thick cakes, which are baked in ‘batches’ (ordinary GINGERBREAD). Both varieties require a pretty brisk oven; the thinner kinds (nuts, &c.), especially, must be baked as crisp as possible, without being burnt. The varieties called LEMON GINGERBREAD, CARAWAY G., &c., have a perceptible predominance of these flavouring ingredients. The addition of a little alum, dissolved in water, makes the bread both lighter and crisper, and causes it to ripen quicker, but at the same time lessens its wholesomeness.
GIN′GER CAN′DY. See Candying.
GIN′GER DROPS. See Drops (Confectionery).
GLAIRE. White of egg. See Albumen and Egg.
GLAN′DERS. Syn. Farcinoma, L. A contagious disease, generally confined to the horse, ass, and mule, but communicable to man, in whom it assumes a highly malignant and often fatal character. This disease appears under two forms—1. Simple acute glanders, marked by copious discharge of foul mucous matter from the nostrils and adjacent parts; and—2. Farcy, Farcin, or Farcy glanders, when it attacks the lymphatics of the skin, either generally, producing a distended appearance of the vessels, like moles or buttons (LEAD or BUTTON FARCY), or locally, when it takes the form of dropsical accumulations in the legs (WATER FARCY).
Treat. Mr Youatt considers it useless to attempt the cure of glandered horses; but that farcy in its earlier stages and milder forms may be often successfully treated. “All the mercurials have been used with benefit in farcy; but they must be discontinued as soon as the mouth is sufficiently affected, or sickness, loss of appetite, and like symptoms, are produced.” (Blaine.) Feeding the animal entirely on green food appears to be the best mode of treatment in both varieties. The buttons are generally removed with caustic or a red-hot iron.
“Glanders is quite incurable, but by generous diet, good stabling, and mineral tonics, life, except in extremely acute cases, may be prolonged for many weeks. This, however, is not desirable; for it involves great risk, not only to other horses, but also to the attendants.” (Finlay Dun.)
GLASS. Syn. Vitrum, L. This well-known substance is essentially a mixture of silicates with an excess of silica or silicic acid. It generally contains the silicates of potassa, soda, lime, baryta, magnesia, alumina, and lead, coloured by small portions of iron, manganese, cobalt, uranium, copper, or gold. In its usual form it is brittle, transparent, noncrystalline, insoluble, and fusible; but it sometimes exhibits other properties.
The manufacture of glass is one of the highest beauty, and, considering the comparative worthlessness of the materials of which it is made, and the various purposes of a useful, ornamental, and scientific nature which it subserves, it may be regarded as, perhaps, the most important in the history of inventions. The principle of its production is very simple, although great skill and experience are necessary to ensure its excellence. Silica (commonly under the form of sand) is heated with carbonate of potassa or of soda, and slaked lime or oxide of lead, until the mixture fuses, and combination takes place. After a time the melted mass becomes perfectly limpid and free from air-bubbles, when it is allowed to cool until it assumes the peculiar tenacious condition proper for working. The operation of fusion is conducted in large crucibles of refractory fire-clay, which, in the case of ‘lead-glass,’ are covered with a dome at the top, and have an opening at the side by which the materials are introduced, and the melted glass withdrawn.
The manufacture of glass is only conducted on the large scale, and the precise character and proportions of the ingredients used by the glass-maker must necessarily greatly depend upon the nature of the raw materials furnished by his locality, or otherwise at his command. The attention of the manufacturer should be directed to the use of his materials in such proportions as will furnish, in the melting-pot, the proper quantities of the essential ingredients, as determined from the known composition of the best commercial samples. The purity of the raw materials and the accuracy of his proportions and quantities are proved or disproved by the excellence of the product; and the cause of error (if any) may be at once determined by carefully ascertaining the quality of the ingredients employed, and the composition of the defective glass.
A writer (in ‘Chem. Centr.,’ 1872, 528) points out that very generally the soda used in glass making, contains sulphate, and that when this is so a poor glass is produced. The addition of ·75-1 part of wood charcoal for every 100 parts of true soda—improves the quality of the glass.
Prep. The following formulæ exhibit the composition of the leading commercial glasses, as shown by chemical analysis, together with the proportions of the raw materials used in their production.
Bottle glass. Sp. gr. 2·700 to 2·735.—
a. Composition by analysis:—
1. Silica, 53·55%; lime, 29·22%; mixed alkali, 5·48%; alumina, 6·01%; oxide of iron, 5·74%. Dark green.
2. Silica, 52%; baryta, 21·6%; soda, 26·1%; oxides of iron and manganese, ·3%. Pale green; very superior.
b. Raw materials used:—
1. Yellow sand, 20%; kelp, 8%; lixiviated wood-ashes, 30%; fresh wood-ashes, 8%; pale clay, 16%; ‘cullet’ (broken glass), 18%. This is the common mixture for coarse bottles, in Belgium, France, and Germany.
800
2. To the last add of black oxide of manganese, 21⁄2 to 3%. Has a rich yellowish colour; used for Rhenish-wine bottles.
3. Pale sand, 51%; lixiviated wood-ashes, 33%; pearl-ashes (dried), 8%; common salt, 71⁄2%; white arsenic, 1⁄2%; charcoal, q. s. Very pale green.
4. Siliceous sand (pale), 681⁄2%; potash (or its equiv.), 4%; lime, 231⁄2%; heavy spar, 21⁄2%; peroxide of manganese, 11⁄2%. This forms the celebrated ‘flask-glass’ of St. Etienne.
Glass, Broad, Spread window glass. Sp. gr. 2·642.—
a. By analysis:—
Silica, 69·70%; lime, 13·30%; soda, 15·25%; oxide of iron (and loss), 1·75%.
b. Materials used:—
1. White sand, 50%; dried sulphate of soda, 22%; charcoal (in powder), 9%; ‘cullet,’ 41%; peroxide of manganese, a little. Pale.
2. White sand, 60%; potashes (good), 24%; common salt, 10%; nitre, 5%; white arsenic, 1%; peroxide of manganese, a little (1⁄12 to 1⁄10%); pale ‘cullet,’ at will (10 to 30%). Very pale. This is the ‘spread’ or ‘sheet window-glass’ in common use.
Glass, Chemical. Sp. gr. 2·390 to 2·396.—
a. By analysis:—
1. Silica, 72·80%; potassa, 16·80%; lime (with a trace of alumina), 9·68%; magnesia, 40%; traces of oxide of manganese and iron (and loss) ·32%. This is the difficultly fusible ‘Bohemian tube-glass,’ so valuable in chemical manipulations.
2. Silica, 69·3%; potassa, 15·8%; soda, 3%; lime, 7·6%; alumina, 1·2%; magnesia, 2%; oxide of iron, ·5%; oxide of manganese (and loss), ·6%. English chemical glass (without lead). More fusible than the last.
b. Materials used:—
1. Quartz (hyalin, in powder), 60%; calcined purified pearlash, 30%; fresh-burnt lime (very pure), 9%; nitre (dried), 3⁄4%; arsenious acid or peroxide of manganese, 1⁄4%. Said to be the proportions used in the production of a, 1 (above).
2. (M. Peligot.) Quartz, 711⁄2%; carbonate of potassa (or its equiv., dry), 20%; quicklime, 81⁄2%; (manganese, a little). Said to be the formula for the hardest and least fusible ‘Bohemian tube-glass.’ It is very intractable and infusible, except at a very high temperature; but the addition of an exceedingly small quantity of boracic acid, borax, or arsenious acid, causes it to flow into a glass possessing great brilliancy and hardness, and capable of being wrought at the highest heat of the ordinary furnace.
Glass, Crown, White window-glass. Sp. gr. 2·486 to 2·488.—
a. By analysis:—
1. Silica, 62·8%; potassa, 22·1%; lime, 15·5%; alumina (with traces of oxide of iron and manganese), 2·6%. Crown-glass of Bohemia, according to Dumas. Very beautiful.
2. Silica, 72·5%; soda, 17·75%; lime, 9·75%. English crown-glass; excellent quality, but not so white as the last.
b. Materials used:—
1. Finest white siliceous sand, 64%; purified potashes (dry), 23%; lime, 12%; white arsenic, 3⁄4%; oxide of manganese, 1⁄4%. Said to be used in Bohemia.
2. (Schweigger.) Pure sand, 57%; dry sulphate of soda, 281⁄2%; quicklime, 111⁄2%; powdered charcoal, 3 or 4%. Corresponds to a, 2, above (nearly).
3. Pure sand, 40%; soda ash, 24%; lime, 5%; white ‘cullet,’ 31%, Rather superior to the last.
Crystal, Crystal glass. The ‘crystal glass’ of England is flint glass’ of superior quality; that of Bohemia is noticed under Table glass.
Glass, Flint, Crystal. Sp. gr. 3·000 to 3·620.—
a. By analysis:—
1. (Berthier.) Silica, 59·19%; oxide of lead, 28·68%; potassa, 12·13%; oxides of iron and manganese, traces. Finest colourless English crystal.
2. (Brande; Faraday.) Silica, 52%; oxide of lead, 34%; potassa, 34%. Crystal.
3. (Faraday.) Silica, 44·30%; oxide of lead, 43·05%; potassa, 11·75%; alumina, ·50%; oxides of iron and manganese, ·12%; (loss 28%). Heaviest of three samples of flint glass examined.
b. Materials used:—
1. Finest Lynn-sand (calcined, sifted, and washed), 51%; litharge (purest), 28% (or red lead, 29%), refined pearlashes (calcined before being weighed), 16%; nitre (purified), 43⁄4% arsenious acid and peroxide of manganese, of each, 1⁄8%. Very fine crystal.
2. (M. Payen.) Fine sand, 46%; red lead, 31%; purified carbonate of potash, 23%. French crystal.
3. (Geddes.) White Lynn-sand, 51%; red lead or litharge, 33%; refined pearlashes, 13%; nitre, 3%; a very little arsenious acid and peroxide of manganese. Ordinary English flint-glass. Crystal ‘cullet’ may be added at will to the above. This glass was originally prepared from powdered flints, a fact to which it owes its common name.
Glass, Optical. 1. (Crown glass.) Purest siliceous sand, 55%; carbonate of soda (dry), 12%; chalk (dry), 11%; carbonate of baryta, 22%.
2. (Flint glass.)—
a. By analysis:—
Silica, 44·30%; oxide of lead, 43·05%; potassa, 11·75%. This is Guinand’s ‘dense optical glass.’
b. Materials used:—
1. Purest quartz, 42%; red lead (finest), 42%; purified potash, 143⁄4%; purified nitre, 11⁄4%. These are the proportions used for the last.
2. (Korner.) Finest quartz (reduced to powder, treated with hydrochloric acid, washed, and dried), 471⁄2%; red lead, 381⁄4%; cream of801 tartar, 141⁄2%. The above are used by opticians in the construction of achromatic object-glasses.
Glass, Plate. Sp. gr. 2·488 to 2·600.—
a. By analysis:—
1. (Dumas.) Silica, 75·9%; soda, 17·5%; lime, 3·8%; alumina, 2·8%. French mirror-glass.
2. (Mitscherlich.) Silica, 60%; potassa, 25%; lime, 12·5%; loss, 2·5%(?). Finest Bohemian plate.
b. Materials used:—
1. Finest siliceous sand, 45%; dried carbonate of soda, 25%; lime, 5%; nitre (purified), 2%; plate-glass cullet, 23%; peroxide of manganese and cobalt azure, a very little. Ordinary English plate.
2. Whitish quartz sand, 60%; purified carbonate of soda (dried), 20%; lime (slaked by exposure to the air), 9%; plate-glass cullet, 11% (or more). Sometimes as much cullet as sand is used; but in all cases 1% to 11⁄2% of its weight in carbonate of soda is added with it, besides that ordered in the formula, to compensate for loss of alkali by remelting. Used at the celebrated plate-glass works at Saint-Gobain, France. The product possesses an amount of excellence which British manufacturers have yet failed to equal.
Glass, Table Bohemian crystal. Sp. gr. 2·6 to 2·8.—
a. By analysis:—
1. (M. Berthier.) Silica, 71·7%; potassa, 12·7%; soda, 2·3%; lime, 10·3%; alumina, ·4%; oxides of iron and manganese (and loss), 2·6%. Very white, hard, and beautiful table glass.
2. (Dumas.) Silica, 70%; potassa, 20%; lime, 4%; alumina, 5%; oxide of iron, ·6%; peroxide of manganese, ·4%. A beautiful white wineglass.
b. Materials used:—
1. Finest sand, 50%; purified potashes, 25%; chalk, 10%; nitre, 2%; crystal cullet, 27%; manganese, a little (say 1⁄16%). Used in England recently for table glass.
2. Quartz (hyalin, in powder), 63%; purified potashes, 26%; slaked lime (carefully sifted), 11%; manganese, a little; crystal cullet, at will. Used in Bohemia.
3. (M. Perdonnet.) Powdered quartz, 44%; carbonate of potassa, 33%; quicklime (in fine powder), 22%; nitre, 1%; and a very small quantity of arsenious acid and peroxide of manganese. Said to be the formula used at Neuwelt for the glass a, 1 (above).
Qual., &c. These are denoted by its hardness, transparency, homogeneity, strength, and power of resisting the action of water, air, light, and the stronger acids and alkalies. The power of glass to resist the action of menstrua is readily tried by exposing it to boiling oil of vitriol, and hot but dilute solution of caustic potassa. Neither of these tests should cause the glass to lose its transparency or to become dim.
Swallowed glass. Glass and enamel, both in fragments and in powder, have occasionally been swallowed, with different results. These bodies are insoluble in the fluids of the body, and, consequently, any injurious action they may exert upon the system whilst they are retained in it must entirely depend upon mechanical attrition or irritation. As treatment, we must administer an emetic, and assist its action by thick mucilaginous liquids, and afterwards have recourse to antiphlogistics, if necessary.
Anal.—a. A portion of the sample for examination is heated to dull redness, and then suddenly thrown, whilst still hot, into a vessel of cold water. It is next dried, and reduced to fine powder in an agate or hardened-steel mortar.
b. 100 gr. of the prepared powder is thoroughly mixed with 200 gr. of pure potassic hydrate, and the whole is exposed to heat in a silver or platinum crucible or capsule until perfect fusion takes place; when cold, the crucible and its contents are boiled in about half a pint of distilled water; nitric acid is added to the resulting solution, in excess, and the mixture, together with any sediment, is evaporated to dryness, after which the heat is gradually increased to 400° or 500° Fahr.; the dry residuum is next reduced to powder, and digested in water acidulated with nitric acid, until exhausted of soluble matter; the insoluble portion is then carefully dried, gently ignited and weighed. The weight in grains represents the per-centage of silica in the sample examined.
c. The mixed liquid and washings of b is next acidulated with nitric acid, and treated to a stream of sulphuretted hydrogen, which, if it produces a precipitate, is continued for some time; the precipitate is collected on a very small filter, washed, and dried; the filter with the precipitate next placed in a beaker glass, and strong fuming nitric acid is cautiously added, drop by drop, until complete solution takes place; after boiling the solution for a few minutes, diluting with distilled water, and allowing it to cool, it is precipitated with sulphuric acid, in excess; this precipitate (sulphate of lead) is washed, dried, slightly ignited in a porcelain crucible, and weighed. The weight in grains, multiplied by ·7369, gives the per-centage of oxide of lead or litharge.
d. The filtered liquid from c is evaporated to dryness, and redissolved in water acidulated with hydrochloric acid, and treated with a solution of ammonium chloride, and afterwards with ammonia, in excess; the precipitate (alumina and oxide of iron) is collected, washed, and boiled in a solution of potassium hydrate; the undissolved portion is collected on a filter, washed with boiling water, ignited, and weighed. This gives the per-centage of peroxide of iron.
e. The liquid filtered from the oxide of iron holds the alumina (if any) in solution; a solution802 of carbonate of ammonium is dropped in; the resulting precipitate is washed, dried, ignited, and weighed. This gives the per-centage of alumina.
f. The filtrate from the alumina and oxide of iron (see d), after being evaporated to dryness, is redissolved in a large quantity of distilled water, and is treated with a solution of oxalic acid (a solution of oxalate of ammonium is preferable when no baryta is present); the precipitate is washed, dried, gently ignited, and weighed. The weight of the resulting carbonate of calcium, in grains, multiplied by ·56292, gives the per-centage of lime required.
g. The filtrate from f is now mixed with carbonate of potassium, in considerable excess, and boiled for a long time; the resulting precipitate (if any) is then collected on a filter, slightly washed with hot water, dried, and exposed to a full red heat for some time (say 2 hours); the residuum of the calcination is then weighed. This furnishes the per-centage value of the sample in magnesia.
h. The filtrate from f is treated with dilute sulphuric acid or the solution of a sulphate, as long as a precipitate falls; the precipitate (sulphate of barytum,) is washed, dried, gently ignited, and weighed. The weight, in grains, multiplied by ·6589, gives the per-centage of baryta in the sample.
The above may be varied by gently concentrating the liquid filtered from the precipitate of alumina and oxide of iron (see d), and precipitating it with dilute sulphuric acid; the mixed precipitate is exhausted by digestion in water holding chloride of ammonium in solution; the undissolved residuum (sulphate of barytum,) is washed, dried, and otherwise treated as before; whilst the solution with the washings is treated with a solution of carbonate of ammonium; the precipitate is carbonate of calcium, which is to be washed, &c., as directed under f. The liquor, &c., filtered from the lime, is lastly tested for magnesia. (See g.)
i. A second 100 gr. of the powdered glass (see a) is mixed with 200 gr. of fluor spar, also in powder; the compound is placed in a platinum or leaden capsule, 500 gr. of strong sulphuric acid are added, and the whole cautiously stirred together with a silver stirrer or spoon, care being taken to avoid inhaling the fumes; the heat of a spirit lamp is next applied, and at first is kept at about 212° Fahr., but towards the conclusion of the process is raised to 300° Fahr., or even higher, and is continued for at least 2 hours, or until fumes entirely cease to be evolved; 5 or 6 fl. oz. of distilled water are next poured on the residuary mass, and, after thorough agitation, the whole is thrown on a filter, more water being at last poured on to wash out any remains of soluble matter; to the filtrate, carbonate of ammonium is added in excess, and after a time the earthy salts are removed by filtration; the filtered liquor is now evaporated to dryness, and ignited to dull redness for 2 or 3 minutes; the residuum (sulphate of potassium or sodium, or of both), after being weighed (the weight being carefully noted down), is redissolved in distilled water; a solution of chloride of barium is then added as long as it disturbs the liquor, and after a time the whole is again filtered; the filtrate is concentrated by evaporation, and solution of bichloride of platinum added in excess; the whole is now gently evaporated to dryness, mixed with alcohol, collected on a filter, carefully washed with weak alcohol, dried at a temperature under 212° Fahr., and weighed. The weight, in grains, multiplied by ·1940, gives the per-centage of potassa sought.
k. The weight of sulphate of potassium in the ignited residuum in i is calculated from that of the potassium last found (47 parts of the one being equal to 87 parts of the other), and this weight is deducted from the gross weight of the ignited sulphates; the remainder represents the quantity of sulphate of sodium present. The weight of the latter, in grains, multiplied by ·4367, gives the per-centage of pure soda required.
Concluding Remarks. One of the chief points to which the skilful glass manufacturer directs his attention, is the quality of the materials. Great care is exercised in the selection of the sand for all the finer varieties of glass. The usual practice is to test it before using it, by exposing it to a very high temperature. The purest sand is that which is the whitest and freest from iron, and which, consequently, suffers the least alteration by this treatment. The alkalies (potash, soda) employed are purified by solution and crystallisation. The red lead and litharge must be pure and absolutely free from oxide of copper (a common contamination), which gives a green tint to the glass. The former, which is the most costly, is preferable to the finest crystal. Care must also be taken that the lime, clay, &c., are respectively of proper purity; and that the ‘cullet,’ or broken glass, which is almost always remelted with the other materials, is of proper quality, and of the same kind as that to which it is added. Potassa produces a better glass than soda, although the latter is now very generally employed, from its lower price. It is, however, quite inadmissible as an ingredient in the manufacture of the better class of crystal and plate glass, as, however pure it may be, it imparts to the product a slight greenish tinge more or less destructive of its beauty. When sulphate of soda (Glauber salt) is used as a source of soda, it is gently calcined to dissipate its water of crystallisation, and requires the addition of about 8% of charcoal to effect its reduction in the melting-pot. Common salt is also employed as a source of soda in the same manner. Sometimes native sulphide of lead (galena) is used to decompose the sulphate of soda, and in lieu of part of the803 oxide of lead; in which case about 5 parts of the sulphuret are taken for every 9 parts of the calcined sulphate.
To anticipate the results of his processes, and to carry out with certainty his various intentions, the glass manufacturer, perhaps more than any other person, requires the aid of science and experience. All his most essential operations depend on chemical principles. The products of his furnaces are not formed by the mere mechanical admixture of their several ingredients whilst in the state of fusion, but result from the play of delicate affinities which only act under certain conditions, and when the materials are presented to each other in uniform and definite proportions. Chemically speaking, the glasses are mixed super-silicates of the respective bases which enter into their composition (potassium, calcium, lead, &c.), and, like all other compounds which are formed by elective attraction, obey the common laws of combination, as developed by Dalton, and now so successfully applied in almost every department of industrial art. It has been shown by the most careful analysis, that in all the more valuable and beautiful commercial glasses the relative proportions of the materials are conformable to these laws, and that several of them are true atomic compounds, as perfect in this respect as the crystalline bodies commonly denominated salts. In some of the harder glasses of Bohemia the number of atoms or equivalents of silica are to each of the bases with which it is united, nearly as 5 to 1; whilst in a softer glass of German manufacture the proportions of the two are found to be as 4 to 1. The celebrated plate glass of St. Gobain is an atomic compound formed of 1 equivalent of trisilicate of soda united to 1 equivalent of trisilicate of lime, with a small per-centage of alumina in combination with silicic acid, also in atomic proportion. Glasses in which the ingredients bear no atomic ratio to each other are never homogeneous, but always more or less striated and of unequal colour and refractive power. The absence of atomic proportion between the substances entering into its composition appears to be the only reason why the best English plate and mirror glass is so greatly inferior to that of France and Germany, that comparison of the two becomes absurd. The only variety of glass in the production of which the English manufacturer excels is flint glass or crystal, and here he certainly surpasses all his numerous competitors. The subject is doubtless involved in difficulty, owing to the precise temperature necessary to effect the perfect combination of the bases with the silicic acid, varying with the character of the compound, and not being satisfactorily settled by observation or experience. The modifying influence of temperature is shown by the fact that the lower the heat employed in the process, the smaller the quantity of silica which enters into the composition of the resulting glass; whilst at higher temperatures a part of the base is dissipated in fumes, until such proportions of base and acid result as are required to produce a permanent atomic compound corresponding to the temperature employed. If the heat is excessive or improperly continued, the loss of base produces an opposite effect, and an opaque, semi-vitrified mass is formed, resembling ‘Reaumur’s porcelain.’ The quality of the resulting glass depends on this change being more or less complete. If the furnace yields the right temperature, and the duration of the exposure to its action is neither too short nor too prolonged, nature makes up for the unskilful conduct of the operative, and removes the stumbling blocks which his ignorance had placed in the way of his own attempts at excellence. The proceedings and their results are accidental; but being once obtained, the first are repeated without further trouble or inquiry. This accounts for the same mixture of materials yielding products of different qualities at different times, and in different works, which the operative contents himself with referring to the ‘going of the furnace.’ The common plan in this country is to regulate the proportions and firing by experience only, rather than by theory and practice combined. Now, although the chemist has much yet to learn on the precise constitution of the glasses, and although theory may not be able to ensure unvarying success, it is nevertheless certain that, in all cases, it can afford much valuable assistance in that direction. Indeed, it has been asserted by one of the leading Continental chemists, that ingredients that will yield the proper equivalent proportions in the melting pot cannot produce a bad glass, if exposed to such a temperature as to permit of perfect combination taking place.
It is found that those glasses which contain a predominance of alkali are acted on by water, and when this is in great excess they are perfectly soluble in that fluid. Ordinary flint glass is affected by long coction in water, whilst crown glass, which contains less alkali, is unaltered by that trial. Glass which contains any considerable quantity of lead is acted on by sulphuretted hydrogen. This is the cause of the surface of flint glass, under certain circumstances, becoming opaque and iridescent. Glasses made of silica and alkali alone are incapable of permanently resisting the action of water. The addition of lime or oxide of lead appears to be necessary to give them this quality. Glasses that have a slight greenish or bluish tint may be often whitened, or rendered colourless, by exposure to light and air. This arises from the peroxidation of the iron, to whose protoxide they owe their tint. Other glasses become purpled by exposure, owing to the peroxidation of the manganese.
Different colours are communicated to glass by the addition of metallic oxides. Thus, oxide of manganese gives an amethyst; oxide804 of cobalt, a blue; oxide of iron, a brown; black oxide of copper, a green; oxide of gold, a purple; suboxide of copper, a ruby-red; oxide of tin, a white; oxide of silver, a yellow, &c. These substances are either added to the melted contents of the glass-pot, as in preparing artificial gems, &c., or they are applied in a thin layer to the surface of the object, which is then heated until fusion of the coloured compound occurs, as in enamelling and painting on glass.
Glass is FORMED or FASHIONED into articles by the processes of blowing, casting, drawing, rolling, or spreading. In the process of BLOWING GLASS the workman begins by collecting a proper quantity of glass in a soft, pasty state, at the end of his blow-pipe (an iron tube, five or six feet in length, terminated by a mouth-piece of wood), which he then commences blowing through, by which the lump is expanded into a kind of flask, susceptible of having its form modified by the position in which it is held, and the velocity of rotation continually given to the iron tube. If an open-mouthed vessel is to be made, an iron rod, called a ‘pontil’ or ‘puntil,’ is dipped into the glass-pot and applied to the bottom of the flask, to which it thus serves as a handle, the blow-pipe being removed by the application of a cold iron to the neck. The vessel is now re-heated, and the aperture enlarged, and the vessel otherwise altered in figure by the aid of a few simple tools until completed. It is then detached, and carried to the ‘annealing oven,’ where it undergoes slow and gradual cooling during many hours. In this way bottles, flasks, carboys, and an almost infinite variety of other articles, are formed. The large circular tables of CROWN-GLASS are made by a joint process of BLOWING and SPREADING. The globular flask at first produced, transferred from the blow-pipe to the ‘pontil,’ is suddenly made to assume the form of a flat disc by the centrifugal force of the rapid rotatory movement given to the rod. Spread or BROAD GLASS is formed into sheets in a nearly similar manner. Plate-glass is cast upon a flat metal table, and, after very careful annealing, is ground and polished by suitable machinery. Tubes are made by rapidly drawing out a hollow cylinder; and from these a great variety of useful small apparatus are constructed with the help of a lamp and blowpipe, or, still better, the bellows-table of the barometer-maker. Glass beads are made from small tubes chopped into pieces of suitable lengths, which are stirred first in a mixture of sand and wood-ashes, in the cold, and afterwards in an iron pan over the fire until they assume a rounded form. Small tubes are bent in the flame of a spirit lamp or gas-jet, and cut by a file, a scratch being made, and the two portions pulled or broken asunder in a way easily learned by a few trials. Large tubes require the heat of a powerful blowpipe and lamp, or that of a furnace.
The following hints respecting the MANAGEMENT OF GLASS may prove useful to the inexperienced:—
Annealing. The process of annealing glass has been briefly referred to before. The extreme brittleness of imperfectly annealed wrought glass may generally be remedied on the small scale by immersing the articles in a bath of oil, or a concentrated solution of chloride of calcium, or common salt, and heating the whole gradually and cautiously to the boiling-point, and letting it again cool—the slower the better. By this treatment the glass will be enabled to bear any alterations of temperature between the two extremes to which it has been exposed.
Blowing. By the ingenious art of GLASS-BLOWING and GLASS-DRAWING, as practised on the small scale, with a blowpipe lamp furnace, a variety of articles of ornament and utility may be made, their number being limited only by the ingenuity of the artist. The details of the various operations are, however, too lengthy to describe here.
Cleaning. 1. Windows, looking-glasses, &c., may be quickly cleaned as follows:—Dip a slightly moistened rag or flannel into whiting, fuller’s earth, wood-ashes, or rotten-stone, in impalpable powder, with which smear the glass, and wipe it off with a dry, soft cloth. This does well when the surface is very dirty. In other cases, a little thumb blue, whiting, or chalk, in fine powder, tied up in muslin, may be dusted on the glass, which should then be cleaned off with chamois leather. This gives a fine polish.
2. The vessel to be cleansed, is filled, or, if large, rinsed, with a moderately dilute solution of permanganate of potash, contact being prolonged till a film of hydrated manganic oxide has been deposited; the solution is then poured away, and the glass vessel rinsed with some strong hydrochloric acid.
Cutting. Glass may be easily cut with a common well-hardened steel file, provided it be moistened with oil of turpentine, or plunged under water. It may be also perforated with a common steel brad-awl in the same way. Glass vessels, as bottles and tubes, may be readily cut or shortened by placing a heated iron ring over the spot, or a piece of loose string or cotton dipped in oil of turpentine and set on fire, and immediately on the withdrawal of either applying cold water to the part. Glass vessels or tubes thus treated will generally crack round, and may be readily divided into two parts. In this manner a common Florence oil-flask may be converted into an evaporating dish and a funnel. By a little practice a crack may be led in almost any direction, or a new one made, by the point of a red-hot poker or a spring coal (an ignited crayon of prepared charcoal). The parts may then be separated by a little force or a smart rap, and the divided edges smoothed by the flame of a blowpipe, or by grinding805 them with powdered emery and water on a flat stone. In this way many broken articles in glass may be converted into others scarcely less useful.
Etching on glass has been already noticed under the head of Etching.
Gilding of glass. Gold chloride is dissolved in boiling water; the solution is filtered, and the filtrate so far diluted, that 200 cubic centimètres contain 0·0648 gram of the metal, and it is then made alkaline with soda. The reducing agent is alcohol saturated with marsh gas; this is diluted with its own volume of water. 25 cubic centimetres of this solution are mixed with the alkaline gold chloride solution, and this mixture is poured between the perfectly well-cleaned plate to be gilded, and another sheet of glass placed at a distance of 3 mm. under the first. After two to three hours’ rest the gilding is effected. The plate is removed and washed. (‘Dingler’s Journal.’)
Grinding. This, on the large scale, like glass-cutting, forms a distinct occupation. On the small scale, glass may be roughed or ground by friction with powdered emery and water and a flat rubber of wood; care being taken that the article, if a plate, is laid on a perfectly flat surface, or, if hollow, is supported by a core of cement or plaster. The frosted appearance of ground glass is given to the panes of windows by gently dabbing the glass over with a piece of glazier’s putty, stuck on the ends of the fingers. When applied with a light and even touch, the resemblance is considerable. Another method is to dab the glass over with thin white paint, or flour paste, by means of a brush, but the effect is much inferior to the above.
Glass, packing. This subject will be considered under the general head of Packing.
Writing on glass may be performed by a piece of French chalk or crayons prepared for the purpose; or even with a common pen held nearly perpendicular. Indian ink, or, when the article will be exposed to damp, shell-lac ink or varnish, thickened with a little Vermillion, or lampblack, is best adapted to this purpose. Common ink is not sufficiently opaque.
Glass, to prevent the cracking of, by boiling water. When new, all glass and earthenware should be placed in cold water in a saucepan, and after some hours the saucepan containing the vessel or vessels, should be placed over the fire, until the water reaches the boiling point.
Glass. This term was applied by the older chemists to various substances to which a vitreous appearance has been given by heat. Thus we have ‘GLASS OF ANTIMONY,’ ‘GLASS OF BORAX,’ &c. It is now obsolete.
Glass, Iridescent. The inventor of the process by which this beautiful variety of glass is made is M Clémandot.
The ‘Chemical News’ states that the principle observed in its manufacture consists in submitting the glass articles to the action of dilute hydrochloric, sulphuric, or other acid, under a pressure of from two to six atmospheres. M Clémandot claims to be able to imitate the nacreous films which are seen on ancient glass which has been exposed to combined atmospheric influences for thousands of years.
Glass, Pow′dered. Syn. Vitrum pulverisatum, L. Prep. Heat the glass red hot, throw it into cold water, dry, and powder it. Used to filter acids, and glued upon paper as a polishing powder; also to wear down corns upon the feet, after the feet have been well soaked, and dried.
Glass, Sol′uble. Syn. Water glass; Vitrum solubile, L. An impure alkaline silicate. Prep. Silica, 1 part; carbonate of potassium or of sodium, 2 parts; fused together.
Carbonate of sodium (dry), 54 parts; carbonate of potassium (dry), 70 parts; silica, 192 parts; as last. Soluble in boiling water, yielding a fine, transparent, semi-elastic varnish.
Carbonate of potassium (dry), 10 parts; powdered quartz (or sand free from iron and alumina), 15 parts; charcoal 1 part; fused together. Soluble in 5 or 6 times its weight of boiling water; and the filtered solution, evaporated to dryness, yields a transparent glass, permanent in the air.
M. F. Capitaine, who, acting upon a suggestion made by Liebig, some twenty years since, has recently taken up the subject of the manufacture of soluble glass, and silicate of potash, from farine fossile (an infusorial earth), has published an account of his researches in ‘Dingler’s Polytechnic Journal.’[334]
[334] See ‘The Journal of the Society of Arts’ for January 11th, 1878.
Although M. Capitaine does not think that the farine will be able to compete in cheapness with flint (where this latter is abundant) for the preparation of the alkaline silicates, he states that it possesses the advantage over flint of being much more soluble, and of yielding a far more neutral glass; added to which the production of the silicate is said to be effected with much less trouble than when flint is employed. An important condition is, that the farine must be first well calcined, since if the least trace of organic matter be left in it, the resulting solution will have a yellowish or brownish tint, which will make it unsaleable.
“The lyes being prepared partly with caustic soda, and partly with carbonate of soda, had densities ranging from 1·22 to 1·24 which were found to be most advantageous. A reservoir furnished with mechanical agitators, was about two thirds filled with lye, and the necessary quantity of calcined farine added, the stirring being kept up continually. The proportion of farine is easily calculated on the datum, that one part of hydrate of soda dissolves about 2·8 parts of chemically pure farine, the quality of which varies but little. Lye of the806 density indicated produces a rather light solution, which presents little resistance to the agitators. If steam is afterwards introduced the solution becomes very rapid, when the pressure reaches about three atmospheres, and at the end of about three hours the silica is completely dissolved.
“For the preparation of silicate of potash for surgical purposes the farine fossile is said to be peculiarly adapted. In this case the boiling must be continued for one or two hours longer than in the case of soluble glass, with an addition of 10 to 15 per cent. of farine.”
Uses. &c. Soluble glass, in solution, has been used to render textile fabrics less combustible, as a varnish to protect stone, and as a vehicle in fresco-painting, The soda compound (silicate of sodium) is largely used as a dung-substitute in calico-printing, and by soap manufacturers in place of the resinates formerly in use. 10 or 12 tons are produced weekly in the district of South Lancashire. The potassa compound (silicate of potassium) has been recommended as a remedy for gouty concretions by Mr Ure.—Dose, 10 to 15 gr., in 6 or 8 fl. oz. of water twice a day. See Dunging, Varnish, &c.
Glass, Toughened. Syn. Verre Trempé. M. de la Bastie’s process for converting ordinary, into toughened, tempered, or hardened glass, may in general terms be said to consist in heating the glass to a certain temperature, and then plunging it into an oleaginous bath. For the process, however, to be successful, the observance of a number of minute details is essential; if these be neglected failure is certain to ensue. Thus it is found, that if the glass be insufficiently heated it will, when immersed in the bath, fail to be affected by it, and will consequently experience no alteration in properties. Again, if overheated, it will then get out of shape; or, further it may be heated to the right temperature, and yet be spoilt as it is being transferred to the bath. Moreover, the exact composition of the bath itself, and its temperature constitute very important conditions, the most trifling departure from which may give rise to unsatisfactory results. All these obstacles appear to have been overcome by M. de la Bastie, who has designed plant in the shape of furnaces and baths, by means of which the tempering process can be carried out, without chance of failure. When the glass is brought to the required temperature, all that is necessary is that they should be plunged into the bath, and instantly withdrawn. The cost of the operation is stated to be very small.
“The process as carried out at New York is thus described:—The glass after being run from the furnaces and moulded as usual, instead of being put into annealing pans, is immersed in a hot bath consisting of three parts of flaxseed oil, and one part of tallow. The bath stands at about 320°; and after remaining in this the ware is removed to a second, and similar bath, by which it is cooled down to about 200°. Finally the pieces are immersed in a water bath, and then dipped into a quantity of ordinary refined burning oil. They are then cleaned, ready for packing, with plaster of Paris powder. The work is but in its infancy, and but one small furnace is used in the experiments. Improvements will doubtless be made, by which the cleaning can be done more rapidly than by the powdered plaster, probably some chemical being used for the purpose. It is supposed that the oil works into the pores of the hot glass, and thus toughens it. Great care has to be exercised in the final cooling by water, as too long a contact with the air in changing from one bath to another, makes the ware crack. Articles cooled entirely in oil retain the oil on the surface, but are thus rendered stronger than otherwise.
This new process is very much employed in the manufacture of lamp chimneys, though they have the disadvantage of flying into small pieces, and with violence when they do break, which sometimes does occur.”[335]
[335] Supplement to ‘Ure’s Dictionary of Arts, Manufactures, &c., 1878.
The results so far obtained when glass is subjected to M. de la Bastie’s process are variable. In some cases the articles subjected to it possess great toughness, and the glass bears a blow without experiencing any fracture. In other instances, however, a slight fall or blow shivers it to atoms. When the toughened glass under any circumstances breaks, it possesses a disadvantage over ordinary broken glass, in distributing itself into a great number of small, sharply angular fragments.
Another process for toughening glass, which has been patented by Herr F. Siemens, consists in heating, and then pressing, and suddenly cooling the glass to be hardened; but when the articles are such as are usually moulded, the hardening and tempering are accomplished at the same time as the pressing; thus the molten glass is run into suitable moulds, and while still highly heated, is squeezed, the moulds effecting the necessary cooling, a proceeding which renders the employment of the oleaginous bath unnecessary. Mr Bauer’s method for toughening glass consists in heating ordinary glass plates so strongly that they begin to bend from softening, and then plunging them into a liquid paraffin bath having a temperature of 200°.
Toughened glass is liable to rupture under circumstances that have not yet been accounted for.
M. de la Bastie conceives that the fragile nature of glass is due to the weakness of the cohesion of its particles, and that if this cohesive power can be increased, the strength of the material will be improved in proportion. M. de la Bastie first tried to obtain this end by forcibly compressing the glass while in a plastic807 or fluid condition, but without success; and it was only after various experiments that he was enabled to harden the glass, by dipping it into oil or any other liquid that permitted of being heated to a temperature considerably above that of water.
GLAZE. Syn. Glazing. Any coating or varnish applied to a surface to render it smooth and glassy; any factitious, shining exterior. The following applications of this term are the following:—
Glaze. In cookery, is commonly understood to be gravy or clarified soups boiled until it gelatinises on cooling. It is used as a species of varnish to cover various dishes for the table, and may be spiced and flavoured according to the fancy of the cook. White of egg is generally used as a glaze for pastry.
Glaze. In the porcelain and earthenware manufacture, the vitreous coating which is so essential to the beauty and utility of potter’s ware. Glazes are either white or coloured. The former, by the addition of the colouring ingredients used for enamels, are converted into the latter.
a. For Earthenware:—
Prep. 1. (With lead.) White lead (pure), 53 parts; quartz or ground flints, 36 parts; Cornish stone, or felspar, 16 parts; white flint glass, 5 parts; reduce the whole to an impalpable powder. For common earthenware.
2. (Without lead.) Fine washed sand, 10 parts; purified potash, 8 parts; nitre, 1 part; slaked lime, 2 parts; nitre, 43⁄4%; powder, mix, heat the mixture in a blacklead crucible in a reverberatory furnace, till the mass flows into a clear glass; let this cool, then reduce it to fine powder. For glazing pharmaceutical and chemical vessels.
b. For Porcelain:—
Prep. (Rose.) Felspar, 27 parts; borax, 18 parts; finest siliceous sand, 4 parts; nitre, soda, and purest china clay (Cornish), 3 parts; mix, heat to a ‘frit,’[336] powder, and add of calcined borax, 3 parts.
[336] A technical term for the half-fused mass formed by heating together the materials of which glass is composed.
c. For Stoneware:—
1. (Ure.) White felspar, 26 parts; soda, 6 parts; nitre, 2 parts; borax, 1 part; ‘frit’ together as last. Of the product take 13 parts; red lead, 50 parts; white lead, 40 parts; flints, 12 parts; reduce the whole to powder as before. For painted stoneware.
2. From common salt, which is thrown into the heated furnace containing the ware. It is volatilised and decomposed by the joint agency of the silica of the ware and of the vapour of water always present; hydrochloric acid and soda are produced, the latter forming a silicate, which fuses over the surface of the ware, and gives a thin but excellent glaze. ‘Salt-glazed stoneware’ is now generally used for large chemical vessels, drain-pipes, &c.
Obs. Glazes must be reduced to very fine powder. For use, they are ground with water to a very thin paste or smooth cream, into which the articles, previously baked to the state called ‘biscuit,’ are then dipped; they are afterwards exposed to a sufficient heat in the kiln to fuse the glaze. Another method of applying them is to immerse the biscuit in water for a minute or so, and then to sprinkle the dry powder over the moistened surface.
GLI′ADIN. Syn. Glutin, Vegetable gelatin. One of the proximate principles of wheat gluten, soluble in alcohol.
GLIADINPFLASTER (A. L. Klose, Berlin). For rheumatism. A thin paper on which is spread a solution of gelatin containing spirit and some acrid substance, such as cantharides or euphorbium.—Hager.
GLOB′ULIN. Syn. Crystallin. An albumenoid body existing in the crystalline lens of the eye.
GLOVE POW′DER. Prep. 1. From Castile soap, dried by exposure to a warm dry atmosphere for a few days, and then reduced to fine powder in a mortar. Used to clean gloves.
2. Pipe-clay, coloured with yellow ochre, umber, or Irish slate q. s., and afterwards scented with a little powdered orris root or cloves. Used to colour gloves made of doe-skin and similar leather.
GLOVES. Syn. Gants, Fr. Although gloves constitute a less costly article of dress at the present day than they did during the Middle Ages, the following information may nevertheless be sometimes found of value to their wearers:—
Glove cleaning. 1. (Kid gloves.)—a. Damp them slightly, stretch them gently over a wooden hand of appropriate size, and clean them with a sponge dipped in benzol, recently rectified oil of turpentine, or camphine; as soon as they are dry, withdraw them gently from the stretcher, and suspend them in a current of air for a few days, or until they cease to smell of the cleaning liquid used. The smell of benzol passes off very quickly. Heat must be avoided. The cleaning liquid should be used liberally, and the first dirty portion should be sponged off with clean liquid.
b. By employing a saponaceous compound. See Ganteine.
2. (Doe-skin and WASH-LEATHER GLOVES.)—a. Stretch them on a hand, or lay them flat on a table, and rub into them a mixture of finely powdered fuller’s earth and alum; sweep it off with a brush, sprinkle them with a mixture of dry bran and whiting, and, lastly, dust them well off. This will not do if they are very dirty.
b. Wash them in lukewarm soft water, with a little Castile or curd soap, ox-gall, or bran tea; then stretch them on wooden hands, or pull them into shape without wringing them; next rub them with pipe-clay and yellow ochre, or umber, or a mixture of them in any required shade made into a paste with808 ale or beer; let them dry gradually, and, when about half dry, rub them well, so as to smooth them and put them into shape; when they are dry, brush out the superfluous colour, cover them with paper, and smooth them with a warm (not hot) iron.
Glove dyeing. Leather gloves, if not greasy, may be dyed with any of the ordinary dyes by brushing the latter over the gloves stretched out smooth. The surface alone should be wetted, and a second or third coat may be given after the former one has become dry. When the last coat has become thoroughly dry, the superfluous colour should be well rubbed out, a smooth surface given them by rubbing them with a polished stick or piece of ivory, and the whole gone over with a sponge dipped in white of egg.
Gloves, Cosmetic. Syn. Gants cosmetiques. These are mock kid or lambskin gloves rubbed over, on the inside, with the following composition:—Spermaceti cerate, 3 oz.; melt, add of balsam of Peru, 1⁄2 dr., stir for 5 minutes, and, after a few minutes’ repose, pour off the clear portion; to this add of oil of nutmeg, 15 drops; oil of cassia and essence of ambergris, of each 6 drops; and stir until cold. Used by ladies to soften the hands and to prevent or cure chilblains and chaps. They are commonly worn all night in bed.
GLUCI′NUM. Gl. Syn. Beryl′lium. The metallic base of glucina. It was first obtained by Wöhler, in 1828, by a similar process to that adopted for aluminum, a metal which it greatly resembles. See Aluminum.
Gluci′num, Oxide of. Syn. Glucina, Beryllia. A pulverent white substance, found as silicate in the beryl, emerald, &c.
Prep. The beryl, in fine powder, 1 part; carbonate of potassium, 3 parts; expose the mixture to a strong red heat for half an hour, dissolve the calcined mass in hydrochloric acid, and evaporate the solution to dryness; redissolve the residuum in very dilute hydrochloric acid, and precipitate with pure ammonia; wash the precipitate well, digest it with a large quantity of carbonate of ammonium, filter, and boil the solution as long as carbonate of glucinum subsides. By exposure to a red heat the carbonic acid may be expelled, and the earth rendered anhydrous.
Prop., &c. Glucina closely resembles alumina, from which, however, it is distinguished by its solubility when freshly precipitated in a cold solution of carbonate of ammonia, from which it is again thrown by boiling. Glucina is classed with the earths. The beryl contains 14% of this substance.
GLU′COSE. See Sugar (Grape).
GLUE. Syn. Gluten, Glutinum, L.; Colle, Colle forte, Fr. Inspissated animal jelly, or gelatin, used as a cement.
Prep. Glue is principally prepared from the parings and waste-pieces of hides and skins, the refuse of tanneries, and the tendons and other offal of slaughter-houses. These substances, when intended for the glue-maker, are steeped for 14 or 15 days in milk of lime, then drained, and dried by exposure to the air. This constitutes what is termed the ‘cleansing’ or ‘preparation,’ and in this state the ‘glue pieces,’ as they are called, may be kept for a long time, and transported to any distance without suffering decomposition. Before conversion into glue, they are usually again steeped in weak milk of lime, and next well washed and exposed to the air for 24 to 30 hours. They are then placed in a copper boiler two thirds filled with water, and furnished with a perforated false bottom, to prevent them from burning, and as much is piled on as will fill the vessel and rest on the top of it. Heat is next applied, and the whole gently boiled or simmered together, until the liquor on cooling forms a firm gelatinous mass. The clear portion is then run off into another vessel, and a very small quantity of alum (dissolved) added; here it is kept hot by a water bath, and allowed to repose for some hours to deposit its impurities, after which it is run into the ‘congealing boxes,’ and placed in a cool situation. The next morning the cold gelatinous masses are turned out upon boards wetted with water, and are cut horizontally into thin cakes with a stretched piece of brass wire, and then into smaller cakes with a moistened flat knife. The latter are placed on nettings to dry. The dry cakes of glue are next dipped one by one into hot water, and slightly rubbed with a brush wetted with boiling water, to give them a gloss; they are, lastly, stove-dried for sale. This furnishes the palest and best glue.
As soon as the liquor of the first boiling has drained off, the undissolved portion of skins, &c., left in the copper is treated with fresh water, and the whole operation is repeated again and again, as long as any gelatinous matter is extracted. In this way a second and other inferior qualities of glue are obtained. The product from dried glue-pieces is about 50%.
Var. These chiefly depend on the care with which the process is conducted. Hatmakers’ glue is prepared from the tendons of the legs of neat cattle and horses. It is brown, opaque, and soft; and grows moist in damp weather, but it does not render felt brittle like the other varieties. Fish glue is made in like manner from various membranous and solid parts of fishes. Parchment glue is prepared from shreds or shavings of parchment, vellum, white leather, &c., dissolved by boiling them in water. It is scentless, and nearly colourless.
Qual. The best glue is transparent, nearly colourless, and tasteless, has very little smell, even when melted, and is extremely adhesive. The presence of more than a trace of alum is objectionable; an undue quantity may be easily detected by the usual tests. The strongest glue is that obtained from skins, more especially from the hides of oxen and cows. That809 obtained from the bones, cartilages, and tendons, is weaker.
Glue, Liq′uid. Prep. (Dumoulins.) Soft water, 1 quart; best pale glue, 2 lbs.; dissolve in a covered vessel by the heat of a water bath, cool, and add, gradually, of nitric acid (sp. gr. 1·335), 7 oz.; when cold put it into bottles. Very strong, and does not gelatinise. For the ‘LIQUID GLUE’ sold in the shops, see Chinese cement.
Glue, Marine. Prep. 1. India rubber (cut small), 1 part; coal tar or mineral naphtha, 12 parts; digest in a covered vessel with heat and agitation, and when the solution is complete, add of powdered shell-lac, 20 parts; continue the heat and stirring until perfect liquefaction has taken place, and pour the fused mass, whilst still hot, on slabs of polished metal or stone, so as to form thin sheets. For use, it is heated to its melting-point (248° to 250° Fahr.) in an iron vessel, and applied in the liquid state with a brush. Employed in ship-building, &c.
2. Caoutchouc, 15 to 20 gr.; chloroform, 2 fl. oz.; dissolve, and add of powdered mastic, 1⁄2 oz. It must be kept well corked and in a cool place, to prevent loss by evaporation. Used for small, fine work.
Glue, a New. Ordinary glue is dissolved in nitric ether, and a little bit of caoutchouc added. This solution forms a very strong glue, and does not get thick or pasty. (‘Dengler’s Journal.’)
Glue, Port′able. Syn. Bank-note glue. Mouth g., Indian g.; Colle à bouche, Fr. Prep. From the best pale glue, 1 lb.; water, q. s.; dissolve in a double glue-pot or water bath, and of pale-brown sugar, 1⁄2 lb., continue the heat until the mixture is complete, and pour it into moulds; or pour it on a marble slab, and when cold cut it into small pieces and dry them in the air. This glue is very useful to draughtsmen, architects, &c., as it dissolves almost immediately in warm water, fastens paper, &c., without the process of damping, and may be softened for many purposes with the tongue. When great strength not required, 4 oz. more of sugar may be used.
GLU′TEN. Syn. Glutin. A peculiar substance found in the grain of wheat. It is composed of true vegetable fibrin and a small quantity of gliadin. It is prepared by washing paste made of the flour of wheat or rye in successive waters until all starchy matter is removed. The paste may be conveniently enclosed in a bag of fine linen during the washing.
Prop., Uses. Gluten is believed to be eminently nutritious. It is the presence of gluten in wheaten flour that imparts to it its viscidity or tenacity, and confers upon it its peculiar excellence for the manufacture of MACARONI, VERMICELLI, and similar pastes. The superiority of wheaten over other bread depends upon the greater tenacity of its dough, which during the fermentation is puffed up by the evolved carbonic acid, and retained in its vesicular texture so as to form a light loaf.
Gluten is greyish coloured, and extensible whilst fresh and moist, like caoutchouc. It turns blue when mixed with guaiacum resin.
Gluten Bread. Prep. 1. From wheat flour which has been deprived of about 2-3rds of its starch by washing it with water.
2. From gluten flour. Recommended in diabetes.
Gluten Choc′olate. (Gentile’s.) A mixture of cocoa and gluten flour. As a nutritious and appropriate food in diabetes.
Gluten Flour. Prep. 1. From the waste gluten of the starch works, washed, dried, and ground.
2. (Gentile’s.) From the last, mixed with about an equal weight of wheat flour.
GLYC′ERIN. C3H3O3. Syn. Glycerin, Hydrated oxide of glyceryl; GLYCERINUM, L. A sweet syrupy liquid formed during the saponification of oils and fats.
Prep. 1. Olive oil (or other suitable oil), protoxide of lead, and water are heated together until an insoluble soap of lead (lead plaster) is formed. The glycerin remains in the aqueous liquid. As this crude solution of glycerin is produced in great quantities in the manufacture of lead plaster, the operative chemist has only to purify it. This may be done as follows:—
The water and washings from lead plaster are mixed together, filtered, and submitted to the action of a stream of sulphuretted hydrogen to throw down the lead; the supernatant liquor is decanted from the precipitate, filtered, and evaporated to the consistence of a syrup in a water bath. To render it quite pure it is diluted with water, decoloured with a little animal charcoal, filtered, and again evaporated to the consistence of a thin syrup, after which it is further evaporated in vacuo, or over sulphuric acid, until it acquires the sp. gr. 1·265.
2. (M. Bruère-Perrin.) From the sweet liquor of the stearine works (a product of the process of lime-saponification). The quantity of lime present in the sample is first determined by means of oxalic acid, and the proportion of sulphuric acid necessary for its saturation at once calculated and added; the crude liquor is then concentrated in a tinned-copper vessel, evaporation being promoted by brisk agitation, until the sp. gr. sinks to 10° Baumé; it is next cooled and filtered, and accurately neutralised (if it is required) with carbonate of potassa, after which it is evaporated to the sp. gr. 24° Baumé; on cooling, it deposits gelatinous sulphate of potassa; the whole is now filtered, the deposit on the filter washed with a little very weak spirit and water, the filtrate and washings mixed together and evaporated, as before, with agitation, until the sp. gr. 28° Baumé, whilst hot (36° cold), is attained, when the whole is allowed to cool; the clear liquid is, lastly, decanted810 and filtered. In this state it has an amber colour, but may be rendered colourless and odourless by rediluting it with water, treating it with animal charcoal, filtering, and again evaporating to a proper consistence.
3. By saponifying olive oil with caustic alkali, decomposing the resulting soap with dilute sulphuric or tartaric acid, evaporating the aqueous portion to dryness (nearly), dissolving out the glycerin with cold rectified spirit, and filtering and evaporating the solution as before.
4. The residuary liquor of a soap manufactory is evaporated, and treated with alcohol to dissolve out the glycerin. The spirit is then evaporated off, the glycerin diluted with water, and finally boiled repeatedly with animal charcoal until all colour and odour are removed.
Obs. The products of the above processes are nearly pure, but that of Price’s patent process, described below, is to be preferred to any of them.
5. (Commercial.) From sweet stearin-liquor, by precipitating the lime by a stream of carbonic acid gas, or by a solution of carbonate of soda, carefully avoiding adding the latter in excess; the liquor is then boiled a little, filtered, evaporated to a syrupy consistence, and again filtered. This is the common glycerin of the shops. It may be further purified as above.
6. (Price’s glycerin—Patent dated 1854.) Superheated steam of from 550° to 600° Fahr.) is introduced into a distillatory apparatus containing palm oil or other fatty body. The action of the steam effects the decomposition of the fat, and glycerin and the fatty acids distil over together but no longer in combination. In the receiver the condensed glycerin, from its higher specific gravity, sinks below the fatty acids. Sufficient steam must be supplied, and the temperature nicely regulated. The glycerin is concentrated by evaporation, and if discoloured, it is redistilled. It is usually prepared with sp. gr. 1·24, and then contains 94% of anhydrous glycerin. It can, however, be concentrated to sp. gr. 1·26 when it contains 98%.
Prop. Pure glycerin is a colourless, odourless, uncrystallisable liquid, sweet to the taste, and of a syrupy consistence; it mixes with water in all proportions; it is unctuous and emollient, and softens bodies, like oil, but without greasing them; it does not evaporate or change in the air at ordinary temperatures, and is not susceptible of rancidity or spontaneous fermentation; mixed with yeast and kept in a warm place, it is gradually converted into propionic acid; a strong heat decomposes it, with the production of acrolein; it is neutral to test-paper, and possesses neither basic nor acid properties; it is easily charged with the aroma of the essential oils, and may be combined with soap, and many other substances, without undergoing change. Sp. gr., 1·27 (see above).
MM. Champion and Pellet recommend the following methods for testing the purity of glycerin, as being convenient in application, and giving accurate results.
Qualitative Test. The glycerin diluted with twice its weight of water is treated in the cold.
(1.) With tribasic acetate of lead. If an abundant precipitate be formed, and rapidly deposited, the presence of a proportion of foreign matters may be assumed which would make it unsuitable for use in various applications, such as the manufacture of nitro-glycerin, &c. The crude glycerin obtained in treating fats with sulphuric acid is frequently thus contaminated. These foreign matters result from the action of sulphuric acid at a high temperature (about 110° C.) upon the fatty matter itself or on the impurities it may contain.
(2.) Glycerin obtained by calcareous saponification, also may contain oleate of lime. This may be detected with oxalate of ammonia, which throws down the lime as a clearly perceptible precipitate.
The colour of glycerin is in no way an index of the purity of the product. In all cases it is useful to be assured of the neutrality of the glycerin.
The preceding tests are suited for glycerins more or less impure, but not adulterated. According to the authors’ experiments the tribasic acetate of lead separates all the foreign substances due to normal impurity of the product or alteration in the glycerin during its manufacture. Any addition of glucose may be detected by Fehlings’ solution.
Quantitative Test. This test should comprehend the determination of the water, the foreign organic matter, the lime, and the glycerin.
In the following table the authors have given the density of various mixtures of water and glycerin, comparatively with the degrees Baumé, and also the proportions of water corresponding to the densities. They state, that these determinations have been verified by means of pure anhydrous glycerin, prepared by keeping glycerin for several hours at a temperature of 160° C, and terminating the operation in vacuô. The density found was in accord with that given by Berthelot, namely, 1·264.
Estimation of Organic Matter. Fifty grams of glycerin diluted with water are treated with an excess of tribasic acetate of lead, and the precipitate collected on two tared filters, and the lead compound weighed. The whole is then calcined, the residue treated with nitric acid, and then with sulphuric acid, and from the sulphate of lead is calculated the quantity of oxide of lead, that was in combination with organic matters, and consequently the proportion of the latter, which rarely exceeds 1 to 1·5 per cent.
Lime may be estimated in the usual manner by oxalate of ammonia.
| Hydrometer Weight of Litre. | Areometer Degrees, Baumé. | Water, per Cent. |
| 1264·0 | 31·2 | 0·0 |
| 1262·5 | 31·0 | 0·5 |
| 1261·2 | 30·9 | 1·0 |
| 1260·0 | 30·8 | 1·5 |
| 1258·5 | 30·7 | 2·0 |
| 1257·2 | 30·6 | 2·5 |
| 1256·0 | 30·4 | 3·0 |
| 1254·5 | 30·3 | 3·5 |
| 1253·2 | 30·2 | 4·0 |
| 1252·0 | 30·1 | 4·5 |
| 1250·5 | 30·0 | 5·0 |
| 1249·0 | 29·9 | 5·5 |
| 1248·0 | 29·8 | 6·0 |
| 1246·5 | 29·7 | 6·5 |
| 1245·5 | 29·6 | 7·0 |
| 1244·0 | 29·5 | 7·5 |
| 1242·7 | 29·3 | 8·0 |
| 1241·2 | 29·2 | 8·5 |
| 1240·0 | 29·0 | 9·0 |
| 1239·0 | 28·9 | 9·5 |
| 1237·5 | 28·8 | 10·0 |
| 1236·2 | 28·7 | 10·5 |
| 1235·0 | 28·6 | 11·0 |
| 1233·5 | 28·4 | 11·5 |
| 1232·2 | 28·3 | 12·0 |
| 1230·7 | 28·2 | 12·5 |
| 1229·5 | 28·0 | 13·0 |
| 1228·0 | 27·8 | 13·5 |
| 1227·0 | 27·7 | 14·0 |
| 1225·5 | 27·6 | 14·5 |
| 1224·2 | 27·4 | 15·0 |
| 1223·0 | 27·3 | 15·5 |
| 1221·7 | 27·2 | 16·0 |
| 1220·2 | 27·0 | 16·5 |
| 1219·0 | 26·9 | 17·0 |
| 1217·7 | 26·8 | 17·5 |
| 1216·5 | 26·7 | 18·0 |
| 1215·0 | 26·5 | 18·5 |
| 1213·7 | 26·4 | 19·0 |
| 1212·5 | 26·3 | 19·5 |
| 1211·2 | 26·2 | 20·0 |
| 1210·0 | 26·0 | 20·5 |
| 1208·5 | 25·9 | 21·9 |
The authors consider that industrially the811 tribasic acetate of lead might be used for the removal of organic matter from crude glycerin.
After separation of the precipitate, excess of the lead salt could be removed by a current of sulphuretted hydrogen, and during the concentration of the glycerin, the acetic acid set free would be volatilized with injury to the product. The lead salt might be regenerated by calcination, and again converted into acetate.[337]
[337] ‘Moniteur Scientifique,’ Quesneville [3], vol. iii, p. 1033.
The following quantitative test which it is said will detect upon concentration of the fluids, one-tenth per cent. of glycerin in beer; one per cent. in sherry, one per cent. in milk, and five per cent. in treacle, is based upon a fact observed by Iles, viz. that borax when treated with glycerin, gives to a Bunsen flame the green colour characteristic of boracic acid. The method of its application as given by Messrs Senier and Lowe is as follows:—The suspected solution is rendered alkaline by dilute soda, and a borax bead placed in it for a short time. The bead is then held in a Bunsen flame, and if the solution contains one per cent. of glycerin a distinct reaction is observed. Erythrite and glycol give the same colour.
If a small quantity of glycerin from which the fatty acids have not been removed, be poured into the palm, and rubbed between the hands, a peculiar fetid, mouse-like odour will be perceived.
Uses, &c. Glycerin is extensively employed as an excipient for medicines (see Glyceroles), also, either alone, or in lotions, baths, &c., as a soothing emollient, and is added to poultices and dressings instead of oil, to prevent their hardening. Diluted with water, it often succeeds in allaying itching and irritation of the skin when all other means fail. As a cosmetic, either made into a lotion or added to soap (glycerin soap), or used in small quantities (along with the water employed in washing), it imparts a healthy clearness and a sensation of softness and coolness to the skin, which is very agreeable and refreshing. It is the best remedy known for chapped nipples, hands, lips, &c.; all of which may be prevented by its use as an article of the toilet. Glycerin is sometimes used as a sweetening agent, as a substitute for syrup.
Glycerin is employed for a great variety of purposes other than medicinal; such, for example, as for:—Keeping clay moist for the modeller, for preventing mustard from drying up, for keeping snuff damp, for the preservation of fruit, for sweetening liqueurs, wine, beer, and malt extracts. It is also used as a lubricant for some kinds of machinery, more especially for watch and chronometer works, because it is unaffected by contact with the air, does not thicken at a low temperature, and is without action on such metals as copper, brass, &c. Glycerin is also an ingredient in copying inks. It renders printing ink soluble in water; indeed it is an excellent solvent for many substances, including the Tar-colours (aniline blue, cyanine, aniline violet, and alizarine), and arsenious acid. It is also added to the pulp of paper in order to render it soft and pliable. It is said that leather driving-belts made as they usually are of weakly tanned leather, when kept in glycerin for twenty-four hours are not so liable to fray. A solution812 of glycerin in water is now largely used instead of water alone for the purpose of filling gas metres, as such a solution does not freeze in winter nor evaporate in summer. It has also been used for the compasses on board screw-steamers, in order to protect the inner compass-box, against the vibrations caused by the motion of the propeller. It is also employed for the preservation of anatomical preparations, and for mounting microscopic specimens; as well as for rendering wooden casks impervious to petroleum or other oils; as well as for the preparation of artificial oil of mustard, or sulpho-cyan-allyl, which is made by treating glycerin with iodide of phosphorus, whereby iodide of allyl is formed, which on being dissolved in alcohol, and next distilled with sulpho-cyanide of potassium, yields sulpho-cyan-allyl. When treated with concentrated nitric acid, glycerin yields nitro-glycerin.[338]
[338] Wagner’s ‘Chemical Technology.’
Even the above long list does not exhaust the many useful purposes to which glycerin is now applied.
Glycerin Cream for Chilblains. Equal parts of glycerin, soft soap, and cherry-laurel water, mixed together.
Glycerin Cream with Camphor. Glycerin, 2 parts; camphor, 1 part; rectified spirit, 1 part. Mix. For chilblains.
Glycerin Jelly for Microscopic Mounting. (‘Ed. Pharm. Journal.’) Soak any quantity of good clean gelatine in cold water for three or four hours. Pour off the superfluous water, and melt the gelatine at a gentle heat; when melted filter through flannel, and to the filtrate add an equal quantity of Price’s gelatin.
The above forms a good firm jelly, requiring little trouble in securing the cover.
Glycerin Ointment. Glycerin, 8 parts; spermaceti, 4 parts; white wax, 1 part; oil of almonds (fixed), 16 parts. Add the glycerin to the melted ingredients, and stir briskly till cold. For chaps and excoriations.
GLYCEROLE. A pharmaceutical preparation, in which glycerin is employed as the excipient.
Glycerole of Belladonna. Syn. Glycerinum belladonnæ. Prep. (Par. Codex.) Extract of belladonna, 1 oz., glycerole of starch, 10 oz. (by weight); rub together until perfectly smooth. Glyceroles of hemlock, henbane, and opium are ordered by the Paris Codex to be prepared in the same manner.
Glycerole of Borax. (B. P.) Syn. Glycerinum boracis, L. 1 of borax in 41⁄2 of glycerin.
Glycerole of Carbolic Acid. (B. P.) Syn. Glycerinum acidi carbolici, L. 1 of acid in 41⁄2 of glycerin.
Glycerole of Gallic Acid. (B. P.) Syn. Glycerinum acidi gallici, L. 1 of acid in 41⁄2 of glycerin.
Glycerole of Iodine. Syn. Glycerinum cum iodinio. Prep. (Par. Codex.) Dissolve 5 parts of iodide of potassium and 1 part of iodine in their own weight of water, and add to 40 parts of glycerin (by weight). Applied in skin diseases.
Glycerole of Iodide of Potassium. Syn. Glycerinum potassii iodidi. Prep. (Par. Codex.) Iodide of potassium, 2 parts, glycerole of starch, 15 parts (by weight); dissolve the iodine in its own weight of water, and add to this glycerole of starch.
Glycerole of Starch. (B. P.) Syn. Glycerinum amyli, L. 1 of starch in 81⁄2 of glycerin.
Glycerole of Tannic Acid. (B. P.) Syn. Glycerinum acidi tannici, L. 1 of acid in 41⁄2 of glycerin.
Glycerole of Tar. Syn. Glycerinum picis liquidæ. Prep. (Par. Codex.) Purified tar, 1 oz. (by weight), glycerole of starch, 3 oz. (by weight).
GLYCOARNICIN. A radical cure for gangrene and tubercle (Zeller). 40 grammes clarified honey, with 35 grammes of a tincture of fresh arnica herb, made with weak brandy. (Hager.)
GLYCOBLASTOL (Professor Kletzinsky, Vienna). An extract of the pericarps of cayenne pepper, made with glycerine, diluted with a little water, and perfumed with a trace of pleasant-smelling oil containing a suspicion of patchouli. (Hager.)
GLYCOCINE. Syn. Glycoll. Sugar of gelatin. (C2H5NO2). This is one of the products of the decomposition of gelatin when boiled with dilute sulphuric acid; after the acid is removed by means of barium carbonate, the glycocine may be procured in crystals by evaporating the solution.
It may also be obtained by heating gelatin with a solution of potash or of soda. It is, however, most easily separated in a state of purity by boiling hippuric acid for half an hour with hydrochloric acid; as the liquid cools benzoic acid is separated in abundance, and glycocine remains in combination with hydrochloric acid; on the addition of absolute alcohol, after the solution has been concentrated by evaporation and super-saturated with ammonia, pure glycocine is deposited in minute crystals.
Pure glycocine has a sweet taste, inferior to that of cane sugar. It is soluble in about 400 parts of cold water, less soluble in rectified spirit, and insoluble in absolute alcohol and in ether. It is not susceptible of the alcoholic fermentation.
GLYCYR′RHIZIN. Syn. Liquorice sugar. An uncrystallisable variety of sugar obtained from the root of common liquorice (Glycyrrhiza glabra). It is yellow, transparent, soluble in both water and alcohol, and is not susceptible of the vinous fermentation.
GLYSTER. See Enema.
GNATS and MOS′QUITOES. Smoke and strong fumes of any kind will drive away these insects. If you only burn a piece of brown paper in an enclosed space where they are, they soon after ‘settle,’ and appear to become so stupefied as to remain inactive for some813 time after. In those parts of the New World where mosquitoes abound, tobacco smoke is commonly had recourse to in-doors, and large fires made of brush-wood or under-wood out-of-doors. Old travellers, when compelled to bivouac during the season in which they are troublesome, are very careful to keep close on the ‘lee’ of these fires.
GOA POWDER. See Araroba.
GOITRE. Syn. Derbyshire neck; Bronchocele, Tracheocele; Hernia bronchialis, L. A tumour on the fore part of the neck. It sometimes occurs in Derbyshire, and is endemic in the Alps and several other mountainous districts. Iodine and the iodides appear to be the only substances capable of curing or even arresting the progress of this disease.
There seems little doubt that goitre arises from drinking water rendered hard by the presence of magnesian and lime salts.
The disease called cretinism, which is a peculiar form of idiocy, is in some countries more particularly frequently associated with goitre. Both these maladies prevail in Wurtemberg, Saxony, Silesia, the Tyrol, Carynthia, Galicia, Austria, and Switzerland. In England, goitre seems principally confined to the magnesian limestone district extending from Nottingham to the Tyne; it also prevails in a smaller degree in Derbyshire, Norfolk, Cambridge, and Somersetshire, where a few scattered cases of cretinism are to be met with. Goitre is very much more general than is usually supposed in France. In Asia, it is to be found amongst the inhabitants of Chinese Tartary, Thibet, and Ceylon, and in India amongst the dwellers in the valleys and extensive plains that lie at the foot of the Himalayan mountains.
The disease is likewise known to exist in many parts of Africa; goitre is also far from uncommon in certain districts of North America; whilst in South America it is met with amongst the people inhabiting the plateaus of New Grenada, which comprise localities differing so greatly in climatic conditions, as deep and humid valleys, and arid plains almost or entirely destitute of verdure.
Goitre is a disease that may be very rapidly and readily set up. Bally says he has known certain waters in Switzerland produce it even in eight or ten days; and the French medical journals contain many similar instances of its early development.
GOLD. Au. Syn. Aurum; Or, Fr.; Gold, Ger.) Gold is the most valuable and, probably, the longest known of all the metals. From the remotest period it has been esteemed for its beauty and permanence, and has been taken as the standard measure of value amongst all civilised nations. An account of the uses of gold in the arts, and its influence on society in all ages, as a symbol of wealth and an article of ornament and utility, would embrace the whole history of mankind. At the present day it alike contributes to the conveniences, comforts, and luxuries of life; as often exciting the baser passions of the human heart as promoting the cause of benevolence and virtue.
Gold is found almost invariably in the metallic state. It occurs as gold dust in the sands of various rivers, and in the alluvial soil of auriferous districts, from both of which it is obtained by the simple process of washing. Traces of it are constantly found in the iron and other pyrites of the more ancient rocks. Sometimes it occurs beautifully crystallised in the cubic form, associated with quartz, oxide of iron, and other substances, in regular veins. In the gold fields of California and Australia lumps of nearly pure gold have been discovered in abundance during the last few years. In the former country a mass of gold weighing 28 pounds was found, whilst in our own colonies one weighing 106 pounds was dug out of a quartz rock, near Bathurst. The latter contained upwards of 91% of pure gold, and nearly 81⁄2% of silver; being as pure as the English sovereign, or, in trade language, ‘22 carats fine.’
Prep. This consists merely in the separation of the gold and its subsequent purification. Formerly, the auriferous sulphides, if very poor, were first roasted, then fused into ‘mattes’ and again roasted; they were next melted with lead, and the alloy thus obtained was refined by cupellation. When the ores were very rich, the preliminary calcination and fusion were omitted, and the alloy of lead at once formed. This method (by fusion) does not answer well with auriferous copper pyrites or ores very poor in gold. At the present time the method of amalgamation is principally followed. When a ‘vein-stone’ is to be wrought for gold, it is reduced to powder (on the small scale by hand, on the large scale in stamping mills), and is shaken in a suitable apparatus with water and mercury; an amalgam of gold is formed, which is then separated from the mixture, and its mercury removed by distillation. The gold is next cast into ‘ingots.’
Refining.—Gold obtained by the first method usually contains a little copper and silver, and frequently tin or iron. Tin may be removed by adding a little corrosive sublimate or nitre to the gold melted in a crucible. The process by amalgamation commonly leaves no other alloy than silver. This metal is removed either in the ‘dry way,’ by fusing the gold with sulphur or sulphide of antimony; or in the ‘wet way,’ by ‘quartation’ and ‘parting.’ At the Royal Mint, “when gold ingots contain a certain quantity of silver” (say 2% or 3%), “instead of leaving it, as formerly, to constitute a part of the standard alloy, it pays to extract it, and to substitute copper in its place. To get the silver out of the said ingots, they are melted with about 3 parts of silver—the resulting alloy is granulated and boiled with sulphuric acid—the gold remains untouched, and all the silver is dissolved and converted into sulphate.... The sulphate of silver is then decomposed by the immersion814 of copper plates; the silver is precipitated in a fine, crystalline powder, washed, pressed into masses, and melted, and so affords PURE SILVER, which is afterwards made standard by alloying it with copper, and is used for coinage. The resulting sulphate of copper (which exists in the solution) is then crystallised, and sold.” (Brande.) “By first exhausting the gold with nitric acid, and then boiling it in sulphuric acid, some two or three thousandth part of silver which escaped the action of the nitric acid is dissolved out, and perfectly pure gold is obtained.” (Ure.)
By a foreign invention, patented in 1851 by Mr W. E. Newton, the operations of ‘separations’ and ‘refining’ are conducted by one process. The argentiferous substance, whether in the state of ore or bullion, is reduced to a granulated or spongy state, by fusion along with zinc, or some other metal cheaper than silver, and the zinc is subsequently removed, by digesting the resulting granulated, laminated, or pulverulent alloy, in dilute sulphuric acid, or other acid. The zinc, &c., is recovered by the usual means. This process, carefully conducted, produces metal of great ductility and purity, containing 99% to 991⁄2% of pure gold.
Chemically pure gold is obtained by dissolving the metal in nitro-hydrochloric acid, adding a solution of protosulphate of iron, and collecting and washing the precipitate. In this state it is a brown powder, which acquires a metallic lustre by friction or heat.
Prop. The most marked properties of gold are its rich yellow colour, its ductility, malleability, insolubility in all menstrua except ‘aqua regia’ (nitro-hydrochloric acid), aqueous chlorine, and hydrofluoric acid, and its very slight affinity for oxygen. It melts at a bright red heat (2316° Fahr.—Daniell), and the fused metal has a brilliant green colour. It forms compounds with chlorine, iodine, oxygen, sulphur, &c. Sp. gr. of native gold, 13·3 to 17·7; of pure gold, 19·3 (average); its greatest density is 19·5.
Tests. Metallic gold is characterised by its yellow colour, insolubility in nitric acid, and its ready solubility in aqua regia, forming a rich yellow or amber-coloured liquid, which stains the skin purple. Solutions of gold exhibit the following reactions:—Protosulphate of iron gives a brown precipitate, which acquires a metallic lustre when rubbed;—Protochloride of tin (preferably containing a little perchloride) gives a violet, purple, or blackish precipitate, insoluble in hydrochloric acid;—Sulphuretted hydrogen and hydrosulphide, of ammonia give a black precipitate, insoluble in simple acids;—Ammonia gives a reddish-yellow precipitate (‘fulminating gold’), with tolerably concentrated solutions, either at once or on boiling the liquid;—Liquor of potassa gives a reddish-yellow precipitate with neutral solutions of gold, insoluble in excess.
Estim. 1. In the dry way;—
The quantity of gold in an ALLOY is usually estimated by ‘assaying’ the sample. Before proceeding to the assay, it is necessary to form some estimate of the quantity of other metals (copper or silver, or both) in the specimen to be examined, in order to employ the proper proportion of lead in the ‘cupellation.’ The experienced assayer commonly does this by the ‘assay of the touch,’ and, in certain cases, by a rough preliminary assay. The quantity of lead employed may be about 16 times the weight of the copper present in the sample, and when the alloy contains silver an additional allowance of lead, equal to 1⁄10th of its weight, is made on that account. When no silver is present, or it is not required to be estimated, a much larger proportion of lead may be employed. The weight taken for the assay (‘assay pound’) is usually 12 or 6 gr. The alloy and dose of lead being accurately weighed and separately wrapped in small pieces of paper, the assay may be at once proceeded with.
α. Cupellation. This operation, the most important of the whole, has been already described. Unlike silver, gold will bear the highest heat of the furnace without ‘vegetating,’ ‘fuming,’ or being absorbed by the cupel. The loss of weight gives the amount of copper in the alloy.
β. Quartation. The cupelled sample is fused with three times its weight of pure silver (called the ‘witness’), by which the gold is reduced to one fourth of the mass, or less, and in this state may be easily removed.
γ. Parting. The alloy, after quartation, is hammered or rolled out into a thin strip or leaf, curled into a spiral form, and boiled for a quarter of an hour, in a small flask, with about 21⁄2 to 3 oz. of nitric acid (sp. gr. 1·3); and the fluid being poured off, it is again boiled in a similar manner with 11⁄2 to 2 oz. more of nitric acid (sp. gr. 1·2), after which the gold is carefully collected, washed in pure water, and dried. When the operation of ‘parting’ is skilfully conducted, and the acid not too strong, the metal preserves its spiral form; otherwise, it falls into the state of flakes or powder. The second boiling or digestion is technically termed the ‘reprise.’ The loss of weight by ‘parting,’ after deducting that of the ‘witness,’ corresponds to the quantity of silver originally in the specimen.
δ. Annealing. This consists in putting the pure gold obtained by the last process into a small porous crucible or cupel, and heating it to redness in the muffle.
ε. Weighing. This must be done with the utmost accuracy. The weight, in grains troy, doubled or quadrupled, as the case may be, gives the number of carats fine of the alloy examined, without calculation.
According to the ‘old French method’ of assaying gold, the following quantities are taken:—For the assay pound, 12 gr.; fine silver, 30 gr.; lead, 108 gr. These having been cupelled together, the (perfect) button is815 rolled into a leaf (11⁄2 × 5 inches), twisted on a quill, and submitted to parting with 21⁄2 oz. and 11⁄2 oz. of nitric acid, sp. gr. 1·16 (20° Baumé). The remainder of the process is similar to that above described. Two assays are made in the same manner, with a third on pure gold or gold of a known fineness; and no conclusion is drawn, unless the assay of the latter comes out accurately, and that of the first two correspond to each other.
For alloys containing platinum, which usually consist of copper, silver, platinum, and gold, the method of assaying is as follows:—The alloy is ‘cupelled’ in the usual way, the loss of weight expresses the amount of copper; and the button, made into a riband and treated with sulphuric acid, indicates, by the portion dissolved, that also of the silver present. By submitting the residuum to quartation, the platinum becomes soluble in nitric acid. The loss after digestion in this menstruum expresses the weight of that metal, and the weight of the portion now remaining is that of the pure gold. Gold containing palladium may be assayed in the same manner.
2. In the wet way:
The richness in gold of any substance, whether liquid or solid, when the quantity is small (and indeed in all other cases), is most simply and economically performed by the common method of chemical analysis. The gold may be thrown down from its solution by adding a solution of protosulphate of iron; the precipitate, after being washed, dried, and gently heated, may be weighed as pure gold.
Pois., &c. The soluble preparations of gold (chlorides) are violent poisons. The symptoms resemble those occasioned by corrosive sublimate, but are somewhat less violent. Metallic gold in a minute state of division is also capable of producing very unpleasant consequences, and even endangering life. The antidote is iron filings or a solution of sulphate of iron, given conjointly with an emetic.
Uses. The numerous applications of gold in the arts and the daily transactions of life need only be alluded to here. In medicine, gold has been given in the form of powder, in scrofula and syphilis, by Chrestein, Niel, and others, with apparent advantage.—Dose, 1⁄4 gr. to 1 gr., 3 or 4 times a day, in pills, or as a friction on the tongue and gums. An ointment made of 1 gr. of powdered gold and 30 gr. of lard has been applied by Niel to the skin deprived of the epidermis (endermically) in the above diseases.
The more important chemical compounds containing gold, the alloys and commercial forms of the metal, together with certain factitious substances popularly called ‘gold’, are noticed in alphabetical order below:—
Gold, Alloys and Preparations of:—
Gold, Dutch. Mannheim gold, Mosaic g., Or-molu, Pinchbeck, Prince’s metal, Red brass, Similor, Tombac. These names are applied to several varieties of fine gold-coloured brass, differing slightly in tint and in the proportions of copper and zinc. The terms tombac, prince’s metal, similor, and Mannheim gold, are used by some authors to designate alloys consisting of about 85% of copper and 15% of zinc; whereas, according to other authors, prince’s metal and Mannheim gold are synonymous, and are composed of 75% copper and 25% zinc; according to another author, similor consists of about 711⁄2% copper and 281⁄2% zinc, and Mannheim gold of 80% copper and 20% zinc; and, again, according to another author, similor and Mannheim gold are synonymous, and are applied to alloys of copper containing from 10 to 12% zinc and from 6 to 8% tin. Seeing that such inextricable confusion exists in the employment of the terms above mentioned, it is desirable to discard them altogether. At the celebrated works of Hegermühl, near Potsdam, the proportions copper, 11 parts, to zinc, 2 parts, are employed to produce a metal which is afterwards rolled into sheets for the purpose of making Dutch leaf-gold. This alloy has a very rich, deep gold colour. Its malleability is so remarkable that it may be beaten out into leaves not exceeding 1⁄52900 inch in thickness.
Gold, Facti′′tious. Prep. From copper, 16 parts; platinum, 7 parts; zinc, 1 part; fused together. This alloy resembles in colour gold of 16 carats fine, or 2⁄3, and will resist the action of nitric acid, unless very concentrated and boiling.
Gold, Grain. Syn. Aurum granulatum, L. Prep. From cupelled gold, 1 part; silver, 3 parts; melted together, and poured in a small stream into water; the silver being afterwards dissolved out by digestion in boiling nitric acid, and the grains, after being well washed in water, heated to redness in a crucible or cupel. Used to make preparations of gold.
Gold, Jew′eller’s. This term is applied to alloys of gold used for trinkets and inferior articles of jewelry, ranging from 3 or 4 carats fine upwards; or which are too inferior to receive the ‘Hall mark’. The lowest alloy of this class is formed of copper, 16 parts; silver, 1 to 11⁄2 part; gold, 2 to 3 parts; melted together. This is worth only from 8s. 6d. to 9s. 6d. the oz.
It has recently been found that gold of the quality of 12 carats, or less, if alloyed with zinc instead of the proper quantity of silver, presents a colour very nearly equal to that of a metal at least 21⁄2 to 3 carats higher, or of 8s. or 10s. an ounce more value; and the consequence has been that a large quantity of jewellery has been made of gold alloyed in this manner; and the same has been purchased by some shopkeepers, very much to their own loss as well as that of the public; inasmuch as a galvanic action is produced, after a time, upon gold so alloyed, by means of which the metal is split into several pieces, and the articles rendered perfectly useless.
Gold, Leaf. Syn. Gold-leaf. Gold reduced816 to leaves by hammering it between thin animal membrane. Its preparation constitutes the trade of the goldbeater. These leaves are only 1⁄28200 of an inch in thickness. Gilt silver is hammered in the same way, but the leaves are thicker. The latter is called party gold. Both are used by artists and gilders, and by druggists to gild pills, &c.
Gold, Powdered. Syn. Divided gold, Gilding powder, Gold bronze, Gold colour; Auri pulvis. Prep. Gold, 1 part; mercury, 7 parts; form an amalgam, and expose it to heat until all the mercury is volatilised; or the mercury may be dissolved out with hot nitric acid. In either case the residuum is to be powdered, washed, and dried. If the quantity operated on is considerable, the process should be so conducted as to save the mercury.
From gold leaf and honey ground together, as the last, by means of a stone and muller. This is the plan commonly adopted in the small way by artists.
From a solution of gold in aqua regia precipitated by protosulphate of iron, the resulting powder being washed, dried, and gently heated, This gives pure gold.
Uses, &c. Powdered gold is employed in gilding by japanners and by artists. It is either sold in powder (gold in powder), or made up into shells (gold in shells). Its use in medicine has been already noticed.
Gold, Standard. The standard gold of this country is an alloy of pure gold, 11 parts, with pure copper, 1 part. Formerly the alloy consisted partly of silver, as found in some of the older coins now in circulation. It is often spoken of as 22 carats fine.
Gold, Chlorides of:
1. Monochloride. AuCl. Syn. Aurous chloride, Protochloride of gold. A yellowish-white mass, formed when a solution of trichloride of gold is evaporated to dryness, and the residuum is exposed to a heat of about 440° Fahr., until fumes of chlorine cease to be evolved. It is insoluble in water, which decomposes it, slowly when cold, but rapidly by the aid of heat, into metallic gold and the trichloride.
2. Trichloride. AuCl3. Syn. Auric chloride, Terchloride of gold, Trichloride of gold, Auri chloridum. Prep. Gold, 1 part, dissolved by aid of heat in nitro-hydrochloric acid, 8 parts, and evaporated down to near dryness, and allowed to crystallise.
Prop. Orange-red crystalline needles, or ruby-red prismatic crystals; deliquescent; soluble in water, ether, and alcohol, forming a deep-yellow solution; at the heat of 500° Fahr. it suffers decomposition, chlorine being given off and pure gold left behind. It is reduced by ferrous sulphate, oxalic, sulphurous, formic and phosphorous acids, as well as by most of the metals, to metallic gold. It combines with several of the metallic chlorides, forming a series of double salts, which are mostly yellow when in crystals, and red when deprived of water.
Uses, &c. It has been employed by Duportal, Chrestien, Niel, Cullerier, Legrand, and others, as a substitute for mercury, in scrofula, bronchocele, chronic skin diseases, &c.; also as a caustic.—Dose, 1⁄20 gr., dissolved in distilled water, or made into a pill with starch; or, in frictions on the gums, in quantities of 1⁄16 to 1⁄10 gr. Its most important use, however, is as a reagent in photography, large quantities being manufactured for use as a chief agent in toning photographic prints.
To some extent it is also used for electro-gilding, and mixed with excess of bicarbonate of potassium, it forms a good yielding solution for small articles of copper. These are to be first cleaned with dilute nitric acid, and then boiled for some time in the mixture.
The above is the salt generally referred to under the name of the ‘chloride of gold,’ or in commerce occasionally as the ‘muriate of gold.’
Gold, Chloride of, and Sodium. AuCl3. NaCl. 2Aq. Syn. Aurochloride of sodium; Sodii aurochloridum. Prep. Auric chloride, 85 parts; chloride of sodium, 16 parts; dissolve in a little distilled water, evaporate until a pellicle forms, then put it aside to crystallise. It forms beautiful orange-coloured rhombic prisms.
Dose, &c. 1⁄20 to 1⁄12 gr., made into a pill with starch or lycopodium, in the same cases in which the terchloride is ordered. Mixed with 2 or 3 times its weight of orris powder, it has been used in frictions on the tongue and gums, and an ointment made with 1 gr. of the salt, mixed with 36 gr. of lard, has been applied to the skin deprived of the epidermis by a blister.
Gold, Cyanide of. AuCy3. Syn. Auric cyanide. Prep. Add a solution of pure cyanide of potassium to a solution of pure auric chloride as long as a precipitate forms, carefully avoiding any excess; wash, and dry the precipitate.
Prop., Uses, &c. The salt is a pale-yellow powder, insoluble in water, but very soluble in a solution of cyanide of potassium, forming the double cyanide of gold and potassium so largely used in the electrotype process. Cyanide of gold is employed to a certain extent in medicine.—Dose, 1⁄12 to 1⁄10 gr., made into a pill, in the usual cases in which gold is administered. The first formula is essentially similar to that of the French Codex.
Gold, Extraction of, by Sodium Amalgam. (Crookes’ Method, Patented.) In the extraction of gold by amalgamation serious difficulties are often occurring through the ‘flouring’ or ‘sickening’ of the mercury employed, and the prevention of the amalgamation by a coating of tarnish on the gold. So much is this the case that losses of from 30 to 60 per cent. of the gold are usually incurred, and, in many cases a still more serious loss of mercury.
When certain minerals, as tellurium compounds, pyrites, &c., occur in the gold ore, the mercury is apt, on trituration, to become subdivided into excessively minute globules, which, owing to their tarnished condition, refuse817 to unite, and are consequently washed away, it being almost impossible to separate them from the heavier portions of the ore. This is technically called ‘flouring,’ ‘granulating,’ &c. Besides this, certain of these minerals affect the mercury in another way, that is, by ‘sickening’ it, or causing it to lose its bright surface and fluidity, and prevents its amalgamating with the gold. Besides the inconvenience and loss thus caused, a further loss of gold takes place from the inability of the ordinary mercury to touch or amalgamate tarnished gold, unless it is ground with it, for a more lengthy period than is found practicable in most cases.
Mr Crookes, F.R.S., has, by means of the addition of a certain proportion of sodium, in the form of an amalgam, to the mercury, effectually prevented this serious loss of gold and mercury. By adding certain quantities of amalgams B and C, an amount which, differing from each ore, is ascertained by experiment, the ‘flouring’ and ‘sickening’ of the mercury is effectually prevented, the mercury remaining throughout in the best condition. The addition of about one tenth per-centage of amalgam A, at intervals of some hours, increases most powerfully the affinity of the mercury for the precious metals, and secures a more thorough amalgamation.
This invention has met with general approval, and experiments conducted at many mines show its great practical value, giving an increase of from 5 to 30 per cent. in the yield of gold, and, in fact, with many pyrites that yielded no gold to the ordinary amalgamation process, gave a considerable yield of gold to the sodium amalgamation process. This has led to its use in most mines, both silver and gold, in America.
Gold, Iodide of. AuL3. Syn. Auric iodide, Tri-iodide of gold, Gold teriodide, Auri iodum. Prep. Add a solution of trichloride of gold to one of iodide of potassium. The resulting precipitate is at first redissolved on agitation, a soluble double iodide being formed; subsequently the iodide of gold is precipitated, leaving the supernatant liquor free of colour.
Prop., Uses, &c. A dark-green powder, easily soluble in hydriodic acid. It is occasionally employed as a medicine, and, like other preparations of gold, is of an alterative character.—Dose. About 1⁄16th of a grain.
Gold, Oxides of:—
1. Monoxide. Au2O. Syn. Aurous oxide, Protoxide of gold. Prep. Formed by treating the aurous chloride with strong potassium hydrate. Green powder, somewhat soluble in potassium hydrate solution, and readily decomposing into metallic gold and auric oxide.
2. Trioxide. Au2O3. Syn. Auric oxide, Oxide of gold, Peroxide of gold, Auric acid, Auri oxidum. Prep. Magnesic oxide, 4 parts; auric chloride, 1 part; water, 40 parts; mix, boil, and wash the precipitate with water, dilute nitric acid, and again with water. It must be dried in the shade.
Reddish-yellow powder, easily decomposed by heat; readily soluble in hydrochloric and hydrobromic acids and strong nitric acid, but insoluble in water and the other acids. Forms unstable salts with the alkalies.
Uses, &c. Trioxide of gold has been given in scrofula, &c., in doses of 1⁄12 to 1⁄2 gr., or 1 gr., in scrofula, syphilis, &c., made into a pill with extract of mezereon.
Gold, Ammoni′uret of*. Syn. Aurate of ammonia, Berthollet’s fulminating gold; Aurum ammoniatum, Ammoniæ auras, L. Prep. By adding ammonia to a solution of gold in aqua regia (trichloride), as long as a reddish-yellow precipitate (fulminating gold) forms; the latter must be collected, washed, and dried with the greatest possible caution.
Obs. Ammonia fails to precipitate trioxide of gold from solutions which are not tolerably concentrated, and in those containing free acid or ammoniacal salts the precipitate only forms upon boiling the solution. Before adding the ammonia, it is, therefore, proper to drive off the excess of acid, if any, by the application of heat. See Fulminating Compounds.
Gold, Sul′phide of. Au2S3. Syn. Sulphuret of gold, Tersulphuret of g.; Auri sulphuretum, L. Prep. Transmit a current of sulphuretted hydrogen gas through a solution of terchloride of gold in water; or add hydrosulphuret of ammonia to the same solution; collect the precipitate, wash it with cold distilled water, and dry it in the shade.
GOLD DETER′GENT. Prep. (Upton.) Take quicklime, 1 oz.; sprinkle it with a little hot water to slake it, then gradually add boiling water, 1 pint, so as to form a milk. Next dissolve pearlash, 2 oz., in boiling water, 11⁄2 pint; mix the two solutions, cover up the vessel, agitate occasionally for an hour, allow it to settle, decant the clear, put it into flat half-pint bottles, and well cork them down.
Use. To clean gilding, &c., either alone or diluted with water. It is applied with a soft sponge, and then washed off with clean water. It is essentially a weak solution of potassa, and may be extemporaneously prepared by diluting solution of potassa (Ph. L.) with about 5 times its volume.
GOLD SHELLS. Gold leaf or powdered gold ground up with gum-water, and spread upon the insides of shells. Used by artists.
GOLD SIZE. Syn. Gilding size, Gilder’s s., Gold colour. Prep. 1. (Oil size.) Drying or boiled oil thickened with yellow ochre or calcined red ochre, and carefully reduced to the utmost smoothness by grinding. It is thinned with oil of turpentine. Improves by age. Used for oil gilding.
2. (Water size.) Parchment or isinglass size, mixed with finely ground yellow ochre. Used in burnished or distemper gilding.
GOLD-BEAT′ER’S SKIN is prepared from818 the peritoneal membrane of the cæcum of the ox. It is used to separate the leaves of gold whilst under the hammer, as a nearly invisible defensive dressing for cuts, as a fabric for court plaster, &c.
GOLDEN SEAL. See Hydrastis canadensis.
GONG METAL. See Bell Metal.
GONIOM′ETRY. The art of measuring the angles of crystals, by means of a GONIOMETER; a most important matter in chemistry and mineralogy. The only accurate and simple instrument of this kind is the REFLECTING GONIOMETER invented by Dr Wollaston. Facility in using this instrument is readily acquired by a few trials.
GOOSE. This bird, the Anser domesticus, is a favourite article of food almost everywhere, and may fairly claim a similar position amongst poultry to that occupied by “good Sir Loin” among joints of meat. The vulgar inuendos occasionally heard to its prejudice should be directed against the cook rather than the bird, as it is only when it is unskilfully dressed and too highly seasoned that it is apt to disagree with that “irascible member of the interior,” a delicate or overloaded stomach. Undue susceptibility in that quarter may, however, be generally allayed by an oblation, in the shape of a little ‘eau de vie,’ used as sauce or gravy. Formerly, almost miraculous virtues were attributed to this bird. Its flesh was said to promote longevity, to cure hydrophobia, and to be aphrodisiac. The fat (GOOSE GREASE; ADEPS ANSERIS), mixed with honey, was supposed to be “good against the bitings of a mad dog.” At the present day it is occasionally used in clysters, and, when scented, as a pomade to make the hair grow, for which purpose it is said to be superior to bear’s grease. In quantity it is an emetic of very easy action. The large feathers of the wings (quills) are used for writing. The small feathers form the common stuffing of our beds.
GOOSE′BERRY. The fruit or berry of Ribes grossularis. Unripe fruit, cold and acidulous; ripe fruit, wholesome and slightly laxative; but the seeds and skins should not be eaten, as they are very indigestible; the juice of the green fruit is made into wine (English champagne); the seeds, washed and roasted, were formerly used as a substitute for coffee (GOOSEBERRY COFFEE). Gooseberries are preserved by simply bottling them, and keeping them in a very cold place. See Cheese, Fool, Fruit, &c.
GOULARD. Syn. Goulard’s extract. See Solution of Diacetate of Lead.
GOUT. Syn. Arthritis, L. A painful disease that chiefly attacks the male sex, particularly those of a corpulent habit and robust frame. Persons who live temperately and take much exercise are seldom troubled with gout. Indolence, inactivity, luxurious habits of life, and free living, are the chief exciting causes of this disease; but excessive study, grief, watchfulness, exposure to cold, and the too free use of acidulous liquors, also occasionally bring it on. In some persons it is an hereditary disease.
Symp. Gout is generally preceded by unusual chilliness of the feet and legs, and a numbness or a sensation of prickling along the lower extremities; the appetite fails, flatulency, indigestion, torpor, and languor ensue, and extreme lassitude and fatigue follow the least bodily exercise; the bowels become costive, and the urine pallid. The fits usually come on in the night; the patient is awakened by the severity of the pain, generally in the first joint of the great toe, or occasionally in the heel, whole foot, or calf of the leg. The pain resembles that of a dislocated joint, accompanied by a sensation resembling the effusion of cold water; the pain increases, rigors and febrile symptoms ensue, accompanied with local throbbing and inflammation. Sometimes both feet and legs are attacked; at others, only one. Towards morning the patient generally falls asleep, and sinks into a state of copious perspiration, from which he awakes comparatively recovered. This constitutes what is called a ‘fit of gout.’ These fits or paroxysms are apt to return at intervals, commonly every evening, with more or less violence; and when frequent, the disease usually extends its action, the joints become affected, and concretions of a chalky nature (chalk stones, gout stones) are formed upon them, and they become stiff and nearly immovable.
Treat. A plain or vegetable diet, moderate exercise, and the use of warm laxatives, gentle tonics, diaphoretics, and diuretics, are among the best preventives. The moderate use of alkaline remedies, as potassa and magnesia, has also been recommended. To relieve the fit of gout, or to check it at its commencement, the affusion of cold water will be often found effective. The use of the ‘eau médicinale’, or the ‘vinum colchici’ of the Pharmacopœia, may also be had recourse to; a due dose of which taken at bedtime will frequently carry off the paroxysm, and nearly always mitigate the symptoms. The effect of the above remedies do not greatly differ from each other. The action of both medicines is accompanied with great languor, and a deadly nausea or sickness, which terminates in vomiting or a discharge from the bowels, or both. These symptoms have often reached an alarming extent, and in some constitutions follow even a moderate dose. This method of cure should not, therefore, be unadvisedly and incautiously adopted.
Another remedy which has been recommended for gout is lemon juice, but experience has proved that this agent is not to be depended on. The dose proposed by Dr O. Rees, who originated this treatment, was 2 or 3 fl. oz., twice or thrice a day.
To ensure the efficacy of lemon juice, it must819 be expressed from the fruit into the glass shortly before being taken. That purchased at the shops is generally stale and disagreeable, and is often worse than useless. In some cases it is advisable to take the juice undiluted, but the more common practice is to mix it with about an equal quantity of water. See Rheumatism, Colchicum, Draught (Anti-arthritic), Lemon Juice, Vinegar of Colchicum, Wine of Colchicum, &c.
Gout Cor′dial. Prep. Rhubarb, senna, coriander seed, sweet-fennel seed, and cochineal, of each 2 oz.; liquorice root and saffron, of each 1 oz.; raisins, 21⁄2 lbs.; rectified spirit of wine, 2 gals.; digest for 14 days, press, and filter. Used in gout and rheumatism. Aromatic and slightly laxative.—Dose, 1 to 3 table-spoonfuls.
Gout Med′icine. (Duncan’s.) A mixture of wine of colchicum, wine of opium, and tincture of saffron.
Gout Rem′edy. (Alexander’s.) According to Dr Paris, this contains—aniseed, cumin seed, ginger, hermodactyles, pepper, and scammony.
Gout Specific. (Murray’s.) A mixture of iodide of potassium, sulphate of magnesia, and wine of colchicum, disguised with an aromatic tincture.
GOUTTES AMERES. [Fr.] See Drops (Bitter).
GRAD′UATOR. See Vinegar.
GRAFTING COM′POST. Clay tempered with water, to which a little linseed oil is sometimes added. Used to cover the joint formed by the scion and stock in grafting.
GRAINS OF HEALTH, Dr Franck’s—Gesundheitspillen—Grains de Santé, ou Grains de Vie, du Docteur Franck. Silvered pills, containing 1 part gamboge and 4 parts aloes. (Hager.)
GRAINS OF PAR′ADISE. Syn. Guinea grains, Meleguetta pepper. The seeds of the Ammomum meleguetta. Grains of paradise are hot, acrid, and aromatic, and in general properties similar to the other peppers. In some parts of the world they are used as a condiment. They are principally employed in these countries to impart a false strength to wine, beer, spirits, and vinegar.
GRANIL′LA. A small inferior variety of cochineal (which see).
GRANULA′TION. The act or process of forming, or breaking into, grains or small masses.
The granulation of MEDICINES has of late years received considerable attention from both foreign and British pharmaceutists. In France, granulated powders (POUDRES GRANULÉES) are coming into general use in place of impalpable powders, the most unpleasant of all forms of medicine. The French process consists in enveloping the particles of medicines in syrup by means of heat and constant stirring. Mr Banner, of Liverpool, has lately introduced a method of granulating medicines far preferable to that of the French pharmaceutists. The powder to be granulated is placed in a mortar, and mucilage of gum acacia is gradually added until a crumbly mass is made; this is then rubbed through a wire sieve (about 12 meshes to the inch), and the granules produced are spread out on paper, and left to dry spontaneously, or they are placed in a copper pan, and kept in constant motion over a stove until dry; when perfectly dry, they are placed in a mortar, and sufficient quantity of strong tincture of tolu (3 dr. to 1 oz.) is added to them, until by constant stirring they all appear glossy and shining; they are then dried again by a gentle heat, being kept in constant motion. The granules thus formed keep well, are tasteless, and are much more elegant and agreeable preparations than pills or ordinary powders. Many saline substances are granulated by the simple process of dissolving the salt in water, and evaporating to dryness with constant stirring.
Metals are granulated (reduced to drops, grains, or coarse powder) by pouring them, in the melted state, into water. In many cases they are allowed to run through the holes of a species of colander or sieve to produce minute division; and in order to render the drops spherical, they are allowed to fall from a sufficient height to permit of their acquiring the solid state before striking the water. Lead shot is made in this way. Shot towers are often upwards of 100 feet in height. See Copper, Gunpowder, Powder, Zinc, &c.
GRAPES. Syn. Uvæ, L. The fruit of Vitis vinifera, or the common grape vine. Ripe grapes are cooling and antiseptic, and in large quantities diuretic and laxative. They are very useful in bilious affections and dyspepsia, and in all febrile, putrid, and inflammatory complaints. The skin and seed, which are indigestible, should be rejected. “Grapes which contain a large quantity of sugar are, if taken without the husks, the safest and most nutritive of summer fruits.” (Cullen.) “The subjects of pulmonary affections, who pass the summer in Switzerland, may try the effects of a course of grapes, ‘cure de raisins,’ a remedy held in high estimation in several parts of the Continent.” (Sir J. Clark.)
Grapes, in bunches are preserved by wrapping them in silver paper, and packing them in dry bran. Each bunch is suspended by the stem with the fingers of one hand, whilst the bran is poured round it with the other; the jar being occasionally gently shaken as the process of packing proceeds. Some paper is then laid over the top of the jar, the mouth or cover of which is, lastly, tied firmly over with bladder, to exclude the air and moisture. See Fruit, &c.
GRAPH′ITE. See Plumbago.
GRATE. A frame of iron bars used for burning coal as fuel. In the construction of a grate it is desirable to make the perpendicular height of the fuel as great as is consistent820 with safety. A stratum of burning coal will radiate considerably more heat into an apartment if placed vertically than if arranged horizontally; besides which a great saving of fuel will be effected in proportion to the heat radiated. Hence the faulty construction of the old-fashioned wide grates. The fuel should also be so divided in a fireplace as to be easy of ignition, and so placed as to give free access of air to all its parts, as the smoke is then more likely to be burnt.
GRAV′EL. A collection of small pebbles commonly mixed with sand or clay, or both. Gravel for garden walks is chosen for its fine colour and binding properties. The gravel of Kensington and Wimbledon is esteemed the finest in the world. Gravel walks when once in order, may be rendered nearly equal to asphalt by pouring over them tar or a mixture of tar and pitch, absorption being promoted, if required, by the application of a hot iron.
Gravel. In pathology, a term popularly applied to calculous matter formed in the kidneys, and passing off in the urine; and sometimes to distinct calculi or concretions in the bladder itself.
An attack of gravel, as commonly understood, is accompanied by a deposit of red, gritty, sand-like particles in the urine, which do not dissolve when the urine is heated. The deposit consists of uric acid. Pains in the loins are a common accompaniment of gravel, and there is also sometimes pain in passing water.
Treat. Give twenty minims of solution of potash (of the B. P.) three times a day in barley water; or twenty grains of bicarbonate of soda, also three times a day. If the attack be attended with much pain a brisk dose of Gregory’s powder should be additionally taken every morning. Vichy Water will also be found a useful remedy. See Calculus.
GRAVIM′ETER. See Hydrometer.
GRAV′ITY. Syn. Gravitation. The attractive force by which bodies fall towards the centre of the earth. Weight is the measure of gravity. The determination of the relative weight of bodies with reference to a given standard, is explained under Specific Gravity.
GRA′VY. The juice or liquid matter that drains from dressed meat after it is placed on the dish for serving. The common practice among cooks is to pour a spoonful or two of boiling water or broth over the joint, to increase the quantity. The natural gravy that oozes from the meat after it is cut is the richest and most wholesome. Made gravies are prepared by adding spice and flavouring to the foregoing, or to strong meat soup.
The gravy for roast meat is usually made by sprinkling a little salt on the joint after it is placed in the dish, and then pouring some boiling water over it; this washes off some of the brown, and makes a coloured liquid in the dish.
Another method for making gravy for roast meat is the following:—Take any bones, scraps of cold meat, or trimmings of the joint, put them in a half pint of water, with a little salt and half an onion, let them stew all the time the meat is roasting; colour with a little burnt sugar. When the meat is done, pour the dripping from it carefully into a basin, leaving the gravy at the bottom of the tin; strain the gravy you have made into this, let it boil, and pour round (not over) the meat. If the gravy is liked thick, put a dessert-spoonful of flour, mixed into a smooth paste, with two of cold water, into the saucepan five minutes before you strain it. See Sauce.
GRAY. Syn. Grey; Gris, Fr. A mixture of black and white. Delicate grays result from mixture of the three elementary colours, red, yellow, and blue, in which the blue preponderates to a greater or less extent.
GRAY DYE. Syn. Teinte grise. Gray is dyed with the same materials as black, but both the bath and mordaunt are used in a more diluted state. Cotton goods may be worked in sumach and then in copperas; this gives rather a bluish grey, which may be modified to any particular hue by the addition of suitable colouring matter. To make it yellowish, a small amount of fustic and alum are employed; to make it ‘fuller,’ peachwood and Lima-wood with alum are used. The methods of obtaining grey on SILK and WOOL are very numerous; they are similar in principle to the above, all depending on the blending of the three primary colours, or on the modification of weak blacks. See Black Dye.
GREASE. A general term applied to soft animal fats; as BEAR’S GREASE, GOOSE GREASE, &c.
Grease. An inflammatory affection of the heels of horses, which produces dryness, scurfiness and stiffness. The treatment consists of emollient poultices, accompanied with physic and diuretic balls, to subdue the inflammation, followed by mild astringent lotions or ointments.
GREAVES. Syn. Graves. The sediment of melted tallow, consisting chiefly of animal membranes mixed with fat, made up into cakes. Used as a coarse food for dogs.
GRE′CIAN WATER. See Hair Dyes.
GREEN. Syn. Viridis, L.; Vert, Fr. Of the colour of the leaves of growing plants; subst. a green colour.
GREEN DYE. Syn. Teinte verte, Fr. All the green dyes in use, with the practically unimportant exception of Chinese green and oxide of chromium green, are compounded of blue and yellow. The goods, in practice, are generally dyed blue first, observing to regulate the shade according to that of the intended green; they are then dried, rinsed, and passed through a yellow bath, with the like precautions, until the proper shade is obtained. See Blue Dye, Yellow Dye, &c.
GREEN PIG′MENTS. Several of the green821 pigments of commerce are obtained from copper. Oxide of chromium furnishes some which are very beautiful. Many are formed by the mere mechanical admixture of blue and yellow pigments. The bright blues and yellows, when mixed in this way, produce the liveliest greens; orange, or red and blue, and the yellowish browns and blue, the more dingy greens. In this way are produced all the extemporaneous greens of the artist. Nickel and titanium also furnish green colours, but these are not in common use. The following list embraces all the best-known and most useful green pigments:—
Green Arsen′ical. Arsenite and aceto-arsenite of copper. See Green, Scheele’s and Schweinfurt (below).
Green, Barth’s. From yellow lake, Prussian blue, and clay, ground together.
Green, Bice. Same as mountain green.
Green, Bremen. This is properly green verditer, but other preparations are frequently sold under the name.
Green, Brighton. A mixture of impure acetate of copper and chalk, prepared as follows:—
To sulphate of copper, 7 lbs., add sugar of lead, 3 lbs.; each separately dissolved in water, 5 pints; mix the solutions, stir in of whiting, 24 lbs., set the resulting paste on chalk stones, and when dry grind it to powder.
Green, Brunswick. This is probably a crude chloride of copper, but a mixture of carbonate of copper and alumina or chalk is now commonly sold under the name in the shops.
Prep. 1. A saturated solution of sal ammoniac, 3 parts, is poured over copper filings or shreds, 2 parts, contained in a vessel capable of being closed up, and the mixture is kept in a warm place for some weeks, when the newly formed green pigment is separated from the unoxidised copper, by washing the mixture on a sieve; it is then edulcorated with water, and slowly dried in the shade. Colour very deep and rich. The lighter shades are produced by the addition of sulphate of baryta.
2. A solution of crude carbonate of ammonia or bone spirit is added to a mixed solution of alum and blue vitriol, as long as it affects the liquor; in a short time the precipitate is collected, washed and dried. The various shades of green are produced by using different quantities of alum, which pales and cheapens it.
Green, Chrome. The superb green pigment used by enamellers under this name is the green oxide or sesquioxide of chromium. A hydrated oxide of chromium forms the emerald green of Pannetier; it is prepared by melting in a crucible equivalent quantities of anhydrous boracic acid and bichromate of potassium, and treating the fused mass with water. The hydrated oxide thus produced is washed and finely triturated.
The chrome green of the oil and colour shops is a mixture of chrome yellow and Prussian green.
Green, Cop′per. Green bice or mountain green, Brunswick green, emerald green, verditer, and several other well-known pigments, may be thus named.
Green, Em′erald. This term is commonly applied to the aceto-arsenite of copper, as prepared in England. It is the same compound, chemically speaking, as Schweinfurt green (which see).
Prep. A pulp is formed with verdigris, 1 part, and boiling water q. s., and after being passed through a sieve, to remove lumps, is added gradually to a boiling solution of arsenious acid, 1 part, in water, 10 parts, the mixture being constantly stirred until the precipitate becomes a heavy, granular powder, when it is collected on a calico filter, and dried on chalk stones.
Green, Frise. Syn. Friezland green. This resembles Brunswick green.
Green, Gellart’s. A mixture of cobalt blue and flowers of zinc with some yellow pigment.
Green, Impe′rial. Schweinfurt green (see below).
Green, Iris. A pigment prepared by grinding the juice of the petals of the blue flag with quicklime. It is very fugitive.
Green Lake. See Lake.
Green, Min′eral. This is the same as mountain green.
Green, Mitis. Another of the many synonyms of Schweinfurt green.
Green, Mountain. This pigment is properly the native green carbonate or bicarbonate of copper (malachite) ground to powder, either with or without the addition of a little orpiment or chrome yellow. That of the shops is commonly prepared by adding a solution of carbonate of soda, or of potassa, to a hot mixed solution of sulphate of copper and alum. Green verditer is commonly sold for this article. According to Watts, mountain green is the same as Neuwieder green.
Green, Neuwieder. Schweinfurt green mixed with gypsum or sulphate of baryta.
Green, Prussian. The sediment of the process of making Prussian blue from bullock’s blood or horns, before it has had the hydrochloric acid added to it. It is also prepared by pouring liquid chloride upon freshly precipitated Prussian blue. As now sold, this pigment is generally a mixture of Prussian blue and gamboge.
Green, Rinman’s. This resembles that of Gellert.
Green, Sap. A very fugitive pigment, prepared from the juice of buckthorn berries. The berries are allowed to ferment for a week or eight days in a wooden tub. The juice is then pressed out, strained, a little alum added, and the whole evaporated to a proper consistence;822 it is next run into pigs’ bladders, and hung up in a dry situation to harden. An inferior article is made from the juice of black alder, and of evergreen privet. It is a common practice to add 3⁄4 pint of lime water and 1⁄2 oz. of gum Arabic to every pint of either of the above juices.
Green, Scheele’s. This is arsenite of copper.
Prep. 1. White arsenic (in powder), 1 part; commercial potash, 2 parts; boiling water, 35 parts; dissolve, filter, and add the solution gradually, whilst still warm, to a filtered solution of sulphate of copper (cryst.), 2 parts, as long as a precipitate falls; lastly, wash the newly formed pigment with warm water, and dry it.
2. (Ure.) Powdered arsenious acid, 11 oz.; carbonate of potassa, 11⁄2 lb.; boiling water, 1 gall.; dissolve, filter, and add the solution, as before, to another solution of crystallised sulphate of copper, 2 lbs., in water, 3 galls. Prod. 11⁄2 lb. A very fine grass-green colour.
Green, Schweinfurt. This splendid green pigment is the aceto-arsenite of copper.
Prep. 1. Acetate of copper and arsenious acid, equal parts, are each dissolved separately in the least possible quantity of boiling water, and the solutions mixed whilst still as hot as possible; an olive-green precipitate falls, which, by being boiled in the liquor 5 or 6 minutes, changes to a dense granular powder of a superb green colour.
2. Instead of boiling the solution containing the precipitate, it is allowed to cool and stand for several hours, or until the powder assumes a granular and beautiful tint. Very rich.
3. (Kastner.) Arsenious acid, 8 lbs., is dissolved in water as before, and added to verdigris, 9 or 10 lbs., diffused through water q. s., at 120° Fahr., the pap of the other being first passed through a sieve; the mixed ingredients are then set aside till the mutual reaction produces the proper shade.
4. (Dr Ure.) Sulphate of copper, 50 lbs., and lime, 10 lbs., are dissolved in good vinegar, 20 galls., and a boiling hot solution of white arsenic, 50 lbs., is conveyed as quickly as possible into the liquor; the mixture is stirred several times, and then allowed to subside, after which it is collected on a filter, dried and powdered. The supernatant liquor is employed the next time for dissolving the arsenic.
5. See Green, Emerald (above).
Obs. This is a very fine, permanent green pigment. “A great deal of needless alarm has been excited about its supposed deleterious effects. It is extensively employed for staining wall-papers, and persons inhabiting rooms thus papered are said to have had their health seriously deranged by the arsenical fumes evolved from it. Now, it is utterly impossible that arsenic could volatilise from such a compound at ordinary temperatures; it does not decompose at any temperature below redness.” (Watts.) [It is, however, probable that the air of such apartments is sometimes charged with the poisonous pigment through its becoming mechanically detached from the paper. To breathe an atmosphere so impregnated would be dangerous. The use of papers coloured with Scheele’s green, especially of the kind called ‘flock,’ should, therefore, be carefully avoided.—Ed.]
Verd′igris. See Copper (Acetates) and Verdigris.
Green, Verd′iter. This is essentially a mixture of oxide and carbonate of copper in uncertain proportions, with chalk. Factitious green bice and mountain green have a like composition. See Verditer.
Green, Verona. The mineral called green earth.
Green, Vienna. The same as Schweinfurt green.
GREEN SICKNESS. See Chlorosis.
GREEK FIRE. This compound, so much used in ancient warfare, is believed to have had naphtha for its chief ingredient. According to some authorities, it was a mixture of asphalt, nitre, and sulphur.
GREGORY’S SALT. The crude hydrochlorate of morphia, prepared by Gregory’s process. It is a double hydrochlorate of morphia and codeia.
GRINDELIA ROBUSTA. A perennial plant belonging to the natural order Compositæ; a native of California, in which state it is largely used against poisoning by the “poison oak” (the Rhus toxicodendron). Of late years it is said to have been in American medical practice used with excellent effect in asthma and kindred diseases. Dr Q. C. Smith, writing to the ‘Pacific Medical and Surgical Journal’ for April, 1875, states one patient to whom pills made of the solid extract were administered, had suffered from severe and frequent attacks of asthma since childhood, and had found no relief from various remedies. Dr Smith gave his patient the extract of the grindelia in pills of three grains each, one three times a day for two or three days, then a pill at bedtime only, for eight or ten days longer. Under this mode of treatment the attacks are said to have been much less severe, and less frequent; the patient not only gaining in strength and general health in the meantime, but having experienced an immunity from attack for four months. The parts of the plant used are the selected leaves and tops.
GRIND′ING. The operation of reducing substances to powder by attrition or friction. In the laboratory, the term is chiefly applied to powdering by means of a mill or by mechanical power, in opposition to simple pounding or trituration in a mortar or with a slab and muller. All the principal powders, paints, &c., sold by the druggist, drysalter, and colourman, are reduced in the drug or colour mill. Recently machinery has even been applied to the common mortar. An ingenious and very useful823 contrivance of this kind is the ‘mechanical mortar’ of Mr H. Goodhall, of Derby.
GRIND′STONES. (Artificial). Washed siliceous sand, 3 or 4 parts; shell-lac, 1 part; melt together, and form the mass into the proper shape whilst warm, with strong pressure. The fineness of the sand must depend on the work the stone is intended for. The same composition is formed upon pieces of wood, as corn rubbers, and for the purpose of sharpening knives, and cutting stones, shells, &c. See Emery.
GROATS. Syn. Grits; Grutellum, Avena decorticata, Avenæ semina, Avena (Ph. L.), L. Common oats, deprived of their exterior integuments or husks. This is generally effected in a mill, which, at the same time, cuts them into two or three pieces. When crushed flat, they are denominated Embden groats.
GROUT. Mortar reduced to a thin paste with water, used to fill up the joints of masonry and brickwork. A finer kind is used to ‘finish off’ the best ceilings.
GRUEL. Syn. Oatmeal gruel, Water g.; Decoctum avenæ, L. Oatmeal or groats boiled with water to a proper consistence, and strained. It is variously flavoured to suit the palate; but the addition of a little white sugar, and finely powdered Jamaica ginger, with or without a glass of wine, is the least likely to offend the stomach. Nutmegs, cinnamon, &c., frequently disagree with invalids. Sometimes milk or butter is added. Embden groats require less boiling than the common groats. Of oatmeal, the Scotch is commonly said to be the best.
The following directions for making gruel from oatmeal are given by Dr A. T. Thomson: “Oatmeal, 2 oz.; cold water, 11⁄2 pint; rub the meal in a basin, with the back of a spoon, in some of the water, pouring off the fluid after the grosser particles have subsided, but whilst the milkiness remains; repeat this with fresh water, unite the washings, and boil until a soft, thick mucilage is formed.”
GUA′IACIN. Syn. Guaiacic acid, Pure guaiacum resin. A substance having the nature of an acid, discovered by Trommsdorff in the wood and bark of Guaiacum officinale.
Prep. The tincture of guaiacum is treated with hydrate of lime, and the guaiacate of lime thus formed is decomposed with dilute sulphuric acid; it is purified by dissolving it in alcohol.
Prop., &c. Insoluble in water; soluble in alcohol and ether; it unites with the caustic alkalies, forming alkaline guaiacates (guaiacum soaps); air and light turn it green; gluten, mucilage of gum Arabic, &c., turn it blue; nitric acid and chlorine turn it successively green, blue, and brown; tincture of guaiacin, added to hydrocyanic acid and sulphate of copper, produces an intense blue colour. (Pagenstecher.) A delicate photographic paper may be formed by washing unsized paper with an alcoholic solution of guaiacum resin, and afterwards with one of neutral acetate of lead. (Johnston.)
GUA′IACUM. Syn. Guaiac, Gum guaiacum, Guaiacum resin; Guaiacum (Ph. L.), (Guaiac resin, Guaiaca resina, B. P.). The resin prepared by means of fire from the wood of Guaiacum officinale, by natural exudation, by incision, or by heat. (B. P.) This substance is often adulterated. When pure, its “fresh fracture is red, slowly passing to green; the tincture slowly strikes a lively blue colour on the inner surface of a thin paring of raw potato.” (B. P.) Adulteration with resin may be generally discovered by the odour evolved when the guaiacum is heated. An alcoholic tincture of guaiacum, rendered milky with water, recovers its transparency on the addition of caustic potassa in excess; but this is not the case when resin is present.
Guaiacum is stimulant, sudorific, and alterative.—Dose, 10 to 30 gr., either in powder or pills; in chronic rheumatism, gout, obstinate chronic skin disease, scrofula, syphilis, &c. It forms the active ingredient of the once celebrated ‘Chelsea Pensioner,’ and the ‘GOUT SPECIFIC’ of Mr Emerigon. The latter was made by digesting 2 oz. of guaiacum resin in 48 fl. oz. of rum, for seven or eight days. The dose of this was a table-spoonful every morning, fasting, for a twelvemonth. Its other properties are similar to those of GUAIACIN, but are less marked. Sp. gr. 1·20 to 1·22.
Guaiacum Wood. Syn. Lignum vitæ, Guaiaci lignum (Ph. L.), L. The wood of Guaiacum officinale. This is employed under the form of shavings, raspings, and sawdust, in decoctions only. See Decoction and Balsam.
GUA′NO. Syn. Huanho, Peruv. This substance, now so extensively used in agriculture, is the partially decomposed excrement of certain aquatic birds, chiefly the common penguin, which congregate in countless numbers on the barren and uninhabited islets and rocks on the western coasts of South America and the coasts of Africa. It abounds in ammonia and the phosphates, and is undoubtedly the richest natural manure known. Under judicious application the increase of the crops of grain, turnips, potatoes, and grass consequent upon its use is said to be about 33%. “Guano is peculiarly adapted to horticultural and floricultural improvement, by its relative cleanliness and facility of application.” (Ure.)
“According to Denham Smith,[339] South American guano, as imported, presents itself in three distinct states, the three varieties being not unfrequently mixed together in the same bag; the first variety is damp and pulverulent; the second exists as large concretions, presenting various aspects when broken; the third is heavy and crystalline, and is termed ‘stone’ by the labourers. These three varieties differ widely in composition, as the following comprehensive analysis, by Smith, will show:—
[339] ‘Proceedings of the Chem. Soc.,’ vol. ii.
824
| Soluble in Water. | |||
| I. Pulverulent. | II. Concrete. | III. Saline. | |
| Water | 222·00 | 250·00 | 97·00 |
| Chloride of ammonium | 25·50 | — | 30·30 |
| Sulphate of potash | 80·00 | — | — |
| Sulphate soda | traces | 258·44 | 191·77 |
| Oxalate of ammonia | 74·00 | 93·90 | — |
| Oxalate soda | — | — | 105·63 |
| Phosphate of ammonia | 63·30 | 61·24 | — |
| Phosphate potash | — | 77·32 | 49·47 |
| Phosphate soda | 1·20 | — | 3·60 |
| Chloride of sodium | — | 29·22 | 286·31 |
| Chloride of potassium | — | — | 41·63 |
| Organic matter | 15·00 | 6·68 | 25·53 |
| Urate of ammonia | 154·18 | — | — |
| Uric acid | 25·16 | — | — |
| Ammonia phosphate of magnesia | 5·64 | 7·84 | 1·33 |
| Animal matter | 11·80 | 8·60 | 7·56 |
| Insoluble in Water. | |||
| I. Pulverulent. | II. Concrete. | III. Saline. | |
| Oxalate of lime | 25·60 | 109·58 | — |
| Phosphate of lime | 199·30 | 62·70 | 132·23 |
| Phosphate of magnesia | 20·30 | 8·74 | 25·80 |
| Oxide of iron | — | — | 1·56 |
| Humus and organic matters | 60·92 | 8·00 | 18·36 |
| Sand | 15·60 | 7·20 | 4·20 |
| Loss | ·50 | 10·54 | 7·78 |
| ——— | ——— | ——— | |
| 1000·00 | 1000·00 | 1000·00 | |
“Several of the South American guano beds are now exhausted, but new varieties are constantly being introduced; and although the qualities are continually varying, guanos, on the whole, may be divided into two classes, the one characterised by the abundance of ammonia, the other by that of phosphates, the Peruvian and Angamos being characteristic of the former, and the Saldanha Bay and Bolivian of the latter. In selecting a guano, the following points (Anderson) ought to be attended to by the farmer:—
“1st. The guano should be light coloured and dry, colouring very slightly when squeezed together, and not gritty.
“2nd. It should not have too powerful an ammoniacal smell, and should contain lumps which, when broken, appear of a paler colour than the powder.
“3rd. A bushel should not weigh more than from 56 to 60 pounds.
“These characters are, however, imitated with great skill, so that they cannot be implicitly relied upon, and they are applicable to Peruvian guano only.”[340]
[340] The above particulars are from an elaborate paper by Dr H. M. Noad, in the ‘Chemist and Druggist,’ vol. ii.
Purity, Adulteration. Guano, owing to its high price, is very commonly adulterated, or is in an advanced stage of decomposition when sold. Much of what is vended under the name is altogether a fictitious article. These artificial mixtures are made to look so like genuine guano, that the mere practical man, who goes only by their appearance, is very often deceived by them, and, owing to the failure of his crops in consequence, is led to distrust the efficacy of guano as a manure. A sample of pretended guano examined by Johnstone was found to contain, in the state in which it was sold, more than half its weight of gypsum, the rest being peat or coal ashes, with a little common salt, crude sulphate of ammonia, and either dried urine or the refuse of the glue manufactories, to give it a smell. “I could not satisfy myself that it825 contained a particle of real guano.”[341] Vessels which sail hence for the guano stations are now very commonly ballasted with rough gypsum or plaster of Paris. This substance is mixed with the guano as it is loaded, and enables the importers to deliver from the vessel a “nice-looking, light-coloured article.” Purchasers of guano are very desirous of having it delivered from the vessel, as they believe they thus obtain it pure. The favourite material for the adulteration of guano, at the present moment, is a variety of umber, which is brought from Anglesea in large quantities. The rate of admixture is said to be about 15 cwt. of umber to about 5 cwt. of Peruvian guano, from which an excellent-looking article is manufactured, which is sold under the name of ‘African guano.’
[341] ‘Elem. of Agric. Chem.’
Pure guano has a pale-brown colour, a more or less offensive odour, and the average sp. gr. of 1·63 to 1·64. If the sp. gr. exceed 1·75, it is either damaged or adulterated; and if it is less than 1·62, it contains an undue quantity of moisture. The best is neutral to test-paper, and sometimes has even an acid reaction; but that of commerce has generally an alkaline reaction, owing to the presence of free ammonia, and, in consequence, turns turmeric paper brown, and gives white fumes when a glass rod dipped in hydrochloric acid is held over it. Triturated with quicksilver or caustic potassa, good guano evolves a powerful odour of ammonia; digested in water, fully one half of it is dissolved; dried by the heat of boiling water, it does not lose more than from 7 to 9% in weight; and burned upon a red-hot shovel, it leaves a white ash, not a red or dark-coloured one. (See directions for selecting guano given above, also below.)
Analysis or assay. The quantitative analysis of guano, so as to exhibit the names and proportions of all its numerous component substances, is an extremely tedious and difficult matter in the hands of persons unaccustomed to chemical manipulations. As, however, its value to the agriculturist depends chiefly on its richness in ammonia, potassa, and phosphoric acid, the analysis of guano for practical purposes may be reduced to an assay for these articles. Indeed, the presence of ammonia (the most valuable of them), in the proper quantity, may be fairly taken as evidence of the presence of the rest. The following methods of testing guano are both simple and accurate, and are so arranged as to permit its per-centage richness in one or more of its leading constituents to be determined without much trouble or expense.
1. a. 100 gr. of the sample for examination (fairly selected) are crushed to a powder, and placed on a small, weighed, and perfectly dry paper filter, and then desiccated, by exposure for 2 or 3 hours to the heat of boiling water. The loss in weight, taken in grains, after deducting 9, indicates the quantity per cent. of water or moisture which the sample contains in excess of that present in good or pure guano.[342]
[342] According to Dr Noad, the proportion of water in genuine guanos ranges from 7 to 20%.
b. The paper filter, with its contents, is next suspended for some time over concentrated sulphuric acid (oil of vitriol) contained in a wide-mouthed bottle or jar, by means of a thread attached to the cork or stopper, care being taken to exclude the external air. The exposure in this way is continued until the guano ceases to diminish in weight, which is ascertained by weighing it at intervals after the first 3 or 4 hours. When this point is arrived at, the filter and its contents are very carefully weighed. The difference between its present weight and its original weight (before the desiccation in a), taken in grains, gives the gross quantity of water per cent.
c. The dried guano from b is next placed in a weighed, smooth crucible or capsule, and exposed to a low red heat until all the organic matter is completely destroyed, and the whole is reduced to a white ash, which is weighed as soon as it has become cold. This weight, in grains, gives the gross weight per cent. of non-volatile matter (fixed alkaline and earthy chlorides, phosphates, and sulphates); the total loss of weight by combustion denotes the gross per-centage of combustible and volatile matter (urea, uric acid, ammoniacal salts, and organic matter). The latter should not be less than 55 to 60%.
2. a. A second 100 gr. of the guano, selected as before, is distilled along with about 75 gr. of fresh-slaked quicklime, and a little water, in a small matrass connected with a tubular, triple bulb-condenser, containing cold distilled water, and immersed in a basin of ice-cold water. (See engr.) The condenser is charged by plunging one of its extremities into the water, and sucking at the other, until the liquid reaches the level indicated in the margin. A very gentle heat only, cautiously increased, need be employed. After the process is over, the strength of the solution of ammonia found in the condenser is tested, either by taking its density in a small specific-gravity bottle, or by determining its saturating826 power in the manner described under Alkalimetry. This furnishes the per-centage of ready formed ammonia sufficiently accurate for all ordinary purposes, provided proper care is taken.
When extreme accuracy is required, the condenser is charged with a weighed quantity of dilute hydrochloric acid of a known strength, instead of water, and after the process is over, this is tested as before. The quantity of ammoniacal test-liquor (see Alkalimetry) now taken to saturate it, deducted from what it would have taken before the exposure in the condenser, gives the per-centage sought.
Another method, giving very accurate results, is to use a rather strong hydrochloric acid (sp. gr. about 1·13) for the condenser; after the operation is over, the contents of the latter are poured into a glass or porcelain capsule, a solution of bichloride of platinum is added, in excess, and the whole is then gently evaporated to dryness; the residuum is rubbed to powder, and exhausted with a mixture of two measures of alcohol and one measure of ether; the undissolved portion is next dried at a heat not exceeding 212° Fahr., and weighed. The weight, in grains, of the ammonia chloride of platinum thus obtained, multiplied by ·0763, gives the per-centage of ready-formed ammonia, as before. When hydrochloric acid is used for the condenser, a simple U-tube and beaker glass may be employed, if a bulb-condenser is not at hand. (See engr.) The advantages resulting from the use of acid instead of water for the condenser is, that with the former no ammonia can possibly escape being absorbed, whilst little care is required to keep the condenser cool.

b. 25 gr. of the guano are next weighed, and after being slightly moistened with a little dilute hydrochloric acid, are thoroughly dried by the heat of boiling water; the dried sample is then mixed in a warm unglazed porcelain mortar with 10 times its weight of a mixture of 2 parts of quicklime to 1 part of hydrate of soda (both quite dry). This mixture is introduced into a combustion tube of hard Bohemian glass, about 16 or 18 inches long, and 3⁄4 of an inch in diameter (see engr.) The mortar is rubbed out with a little of the soda-lime mixture, which is also introduced into the tube with that already put there; a little plug of ignited asbestos is then loosely placed over the whole, and the tube is immediately connected with a tubular bulb-condenser, containing moderately strong hydrochloric acid, great care being taken that the joints are made air-tight, which may be determined by the operator sucking a few bubbles out of the apparatus. If, after suction, the liquid remains at a higher level in the furthest bulb (b), it is a sign that the connection is sound. This being done, heat is applied to the combustion-tube, by means of spirit-lamps; or, more conveniently, by means of the furnace now usually employed in organic analysis (see engr.) The tube is next gradually surrounded with red-hot charcoal, by shifting by degrees the screen (c), and adding more charcoal, so as to gradually expel the ammonia. The disengagement of gas should take place uninterruptedly, but not too rapidly, in order that the acid may not ascend into the combustion-tube and spoil the experiment. The non-condensable volatile matters which pass off furnish a key to the progress of the operation. The heat is at length increased to a full red. When gas ceases to be evolved, and the mixture in the tube has become quite white, the experiment is at an end. The point (a) of the combustion-tube is broken off, and the ammonia which remains in the tube is expelled by sucking gently at the extremity (b) of the bulb-condenser. The latter is then disconnected with the apparatus, and emptied into a glass or porcelain capsule, in order to be tested, as directed under 2, a. The quantity of ammonia, in grains, thus found, multiplied by 4, gives the WHOLE QUANTITY of AMMONIA per cent., both actual and potential, producible from the sample of guano examined.
c. The quantity of ready-formed ammonia (see 2, a) deducted from the quantity last found (see 2, b) gives the quantity of LATENT or POTENTIAL AMMONIA that will be slowly827 developed by the decomposition of the guano in the soil, and become available for the food of plants. This is the most valuable product of this substance as a manure, and can only be obtained in quantity from well-preserved, dry guano.
3. a. A third quantity of 100 gr. of the guano, selected as before, is triturated and digested for some time with 12 times its weight of hot distilled water, and the whole being thrown on a filter, the undissolved portion is washed with a little warm distilled water; the solution and ‘washings’ are then mixed together, and acidulated with nitric acid; a solution of pernitrate of iron is next added, and afterwards solution of ammonia, in excess; the liquid is next heated for a short time, and the bulky reddish-brown precipitate is collected, washed with hot water, dried, ignited, and weighed. The weight, in grains, less the weight of the peroxide of iron in the pernitrate consumed, gives the weight of PHOSPHORIC ACID present in the soluble phosphates contained in the sample. The pernitrate of iron is made by direct solution in hot strong nitric acid, of twice as much pure iron wire as there is phosphoric acid suspected to be present in the liquid. A slight excess will not alter the result. The number of grains of metallic iron used to form the solution, multiplied by 1·4286, gives the weight of the peroxide of iron which is to be deducted from the gross weight of the precipitate.
b. The filtrate and ‘washings’ left from 3 a are mixed, and treated with a little oxalate of ammonia to throw down any lime, and then carefully evaporated to dryness and ignited; the residuum of the ignition, when cold, after being carefully weighed, is treated with the smallest portion of water that will dissolve it; the solution is acidified with hydrochloric acid, and a solution of bichloride of platinum added, in excess; some strong alcohol is next poured in, the precipitate carefully collected on a filter, washed with rectified spirit, dried at 212° Fahr., and weighed. The weight, in grains, multiplied by ·1940, gives the per-centage of POTASSA sought.
c. The weight of the potassa multiplied by 1·852, and deducted from the weight of the ignited residuum in 3 b already found (see above), gives the quantity of CHLORIDE OF SODIUM or COMMON SALT (nearly).
4. a. The insoluble residuum from 3 a, dried, and ignited, or the ash from 1 c, is digested for 10 or 12 hours in 600 times its weight of water (to which a little common salt or sal-ammoniac may be added), after which the whole is thrown upon a filter; a solution of chloride of barium is then added to the filtrate as long as a precipitate (if any) forms; the latter is collected, washed, dried, ignited, and weighed. The weight, in grains, multiplied by ·5843, denotes the quantity of GYPSUM or SULPHATE OF LIME which has been used to adulterate the sample.
b. The insoluble residuum last left on the filter is digested for some time in warm dilute hydrochloric acid; the whole is then thrown upon a filter, and the undissolved portion (SILICA or SAND, with, perhaps, a trace of ALUMINA) is washed, dried, ignited, and weighed. It should not weigh more than 3 to 31⁄2 gr. (3 to 31⁄2%).
c. The filtrate and ‘washings’ from b are next mixed together; the mixed liquid is acidified with dilute sulphuric acid and heated until all the hydrochloric acid is expelled, and the whole reduced to a soft pasty mass; rectified spirit is now poured in, and after active stirring for some time, the mixture is thrown on a filter, and the solid portion washed with a little more rectified spirit; it is then dried, ignited, and weighed. The weight, in grains, multiplied by ·7650, gives the quantity of PHOSPHATE OF LIME per cent. required.
d. The filtrate from c is diluted with water, and after being boiled for a few minutes, ammonia is added in slight excess, followed by a solution of sulphate of magnesia (previously mixed with as much sal-ammoniac as will prevent ammonia producing a precipitate in it), slowly dropped in as long as it disturbs the liquor; the whole is now allowed to rest for 10 or 12 hours, when the precipitate is collected on a filter, and washed with water alkalised with ammonia, as long as the filtering liquid is rendered turbid by chloride of barium; it is next dried, submitted to intense ignition for some time in a covered platinum crucible, and, when cold, carefully weighed. The weight, in grains, multiplied by ·6429, indicates the per-centage of PHOSPHORIC ACID in the insoluble phosphates (phosphates of lime, magnesia, &c.) in the sample examined.
5. A fourth 100 gr, of guano is weighed, and exhausted by trituration and digestion with hot water (see 3 a); the solution is evaporated to dryness by a gentle heat, and the residuum of the evaporation, after being weighed, is powdered and enclosed in a stout phial with 8 times its volume of alcohol, sp. gr. ·825 (63 o. p.); the plant is next securely corked and guarded, and exposed for some time, with agitation, to the heat of 212° Fahr., the whole is then allowed to cool, the contents of the phial filtered, the undissolved portion washed with hot alcohol, and both the filtrate and the ‘washings’ gently evaporated to dryness, and weighed. This gives the richness of the sample in UREA, one of the most valuable constituents of the best guano. Its presence is “a certain proof of its entire soundness.” (Ure.)
6. a. Another 100 gr. of the guano is taken, and, after being exhausted with water, is dried at 212° Fahr., and weighed; it is then digested with heat in 20 times its weight of borax-water (containing 1% of borax), or in a solution of caustic potassa, and after a time the whole is thrown on a weighed filter, washed with a little cold distilled water, dried by a heat not828 higher than that of boiling water, and again carefully weighed. The loss, in grains, indicates the proportion per cent. of URIC ACID.
The accuracy of the result may be verified by adding dilute hydrochloric acid, in slight excess, to the filtrate, collecting the bulky, crystalline precipitate of uric acid which forms, washing it carefully with a little rectified spirit, drying it, and weighing it, as before. This weight, which in general is a very little under that denoted above, is the more accurate of the two. The precipitate is shown to be uric acid by its assuming a rich crimson colour when treated with a little nitric acid, which turns to a rich purple (murexide) when it is moistened with ammonia water.
b. The quantity of uric acid last obtained, multiplied by 1·1012, gives the per-centage of URATE OF AMMONIA.
Obs. Amongst the numerous constituents of guano, none are so valuable in an agricultural point of view as the three substances referred to in the last two sections. Indeed, almost all the ammonia furnished by this substance to the soil, after the latter, manured with it, has been exposed to the air and rain, is derived from the slow decomposition of urea, or urate of ammonia. It is these substances from which the store of latent, or, as Dr Ure terms it, potential ammonia, is derived. The ammonia existing in the guano under the form of carbonate, or of soluble salts (ready formed ammonia), is either soon dissipated in air or is washed away by heavy rains, and, therefore, forms the least valuable and durable portion of this manure. It may be even added artificially, a matter almost impossible with the former. An assay, therefore, for the latent ammonia, or the urea, or the urate of ammonia, any one of them singly, at once furnishes us, as we have already hinted, with evidence of the quality of the guano examined, without the expense and trouble of a complete analysis of this substance. Urea and uric acid are only to be found in the very best samples of guano, and their presence is a positive proof of entire soundness and superior quality. The other valuable portions of guano are potassa and phosphoric acid (phosphate of lime chiefly); the rest are of little importance. (See 2 c, above.)
GUARANA (Grimault & Co., Paris). 12 migrain powders, each weighing 1·75 grammes, consisting of guarana, but perhaps also containing an admixture of cocoa seeds, neither prepared nor roasted. (Hager.)
GUARANA′. Syn. Paullinia, Brazilian cocoa. An alimentary and medicinal substance prepared from the seeds of Paullinia sorbilis, a Brazilian tree. The dried seeds, deprived of their aril, are pounded and kneaded into a mass, which is afterwards made into oblong or rounded cakes (GUARANA BREAD). These cakes are used as we use chocolate—mixed with water and sugar, and drank as a beverage. In Brazil this beverage is largely consumed, both on account of its nutritive qualities, and for its stomachic, febrifugal, and aphrodisiac effects. See Chocolate, &c., also below.
GUARANINE′. A crystalline substance discovered by M. Martius in guarana. It appears to be identical with caffeine, the active principle of coffee and tea.
GUD′GEON. The Cyprinus gobeo (Linn.), a small fresh-water fish, common almost everywhere. The white is considered the best. It was formerly used in medicine.
GUM. Syn. Gummi, L. The general term for an important class of vegetable products. Gums are more or less soluble in cold water, but insoluble in alcohol, ether, and oils. They are obtained from certain plants in amorphous masses; most of them exude spontaneously, or on puncturing the bark. The most perfect type of this class is the substance called GUM ARABIC, or GUM ACACIA. The gums are employed as demulcents in medicines, and are used as cements, and for giving stiffness and gloss to textile fabrics. Among the vulgar the term is often incorrectly applied to the resins and gum resins.
Gum Acacia. Syn. Gum Arabic; Acaciæ gummi (B. P.); G. arabicum, G. acacia, Acacia (Ph. L.), L. “From various species” (of Acacia) “yielding gum” (Ph. L. & E.), chiefly Acacia arabica and A. vera. “Whitish or yellowish, transparent or cracked on the surface, and opaque; brittle; it dissolves freely in water.” (Ph. L.) It is scentless, and may be bleached by exposure to the sun and air, at the temperature of boiling water. Sp. gr. 1·355. (Ure.) The pure soluble principle of gum Arabic is termed ARABIN (which see). Barbary or Morocco gum, Gum Senegal, and East India gum, are inferior commercial varieties of the same substance from other species of Acacia (see below).
Powdered gum Arabic (PULVIS ACACIÆ) is frequently adulterated with flour or farina, or with Senegal or other inferior gums. The first may be detected by agitating a little of the powder with cold water; the pure gum dissolves rapidly, whilst the starch or flour falls to the bottom of the vessel. Or, a little of the powder may be mixed with boiling water, and when cold, tested with tincture of iodine; if it contains starch or flour, the paste will assume a blue colour. If it contains cherry-tree gum or tragacanth, it will be only partly soluble in cold water, and the paste will be partly coloured, and more or less interspersed with gelatinous clots.
For the detection of dextrin in gum Arabic Hager finds that when some of the adulterated article is placed in a glass dish, with vertical sides, and a solution of ferric chloride, density 1·48, diluted with an equal volume of water, is poured over it until the grains are just covered, in the course of a minute or so that particles of gum Arabic will adhere to the bottom of the vessel, whilst the grains of dextrin do not.
829
Much of the white gum Arabic of the shops is formed by bleaching gum Senegal, by what is called ‘Picciotto’s process.’ The gum is dissolved in water, and sulphurous acid gas passed through the solution. The liquid is afterwards boiled to expel the sulphurous acid, a little of which, however, still remains behind. To obtain the gum in a still whiter state, carbonate of baryta is added, and after agitation the mixture is filtered; it is afterwards shaken with gelatinous alumina, again filtered, and evaporated. The product (BLEACHED GUM) is very white, but lacks the peculiar toughness and adhesiveness of the best gum acacia.
Gum, Barbary. Syn. Morocco gum. An inferior product, consisting of a mixture of several Acacia gums. It is exported from Mogador.
Gum, Bassora. A solution of yellowish gum brought from the neighbourhood of Bassora. It differs from most gums in being nearly insoluble in water. The plant yielding it is believed to be a species of Mimosa. It contains the principle BASSORIN, which also exists in gum tragacanth.
Gum, Bleached. See Gum Arabic (above).
Gum, Brit′ish. Syn. Dextrin, Starch gum. Starch converted by the action of acids, diastase, or heat, into a soluble substance resembling gum.
Prep. 1. Malt (crushed small), 1 lb.; warm water, 2 galls.; mix, heat the whole to 145° Fahr., add of potato starch 5 lbs., raise the heat to 160° or 165° Fahr., and mash for about 25 minutes, or until the liquid becomes thin and clear; it must then be instantly run off, and raised to the boiling point to prevent the formation of sugar; after boiling for 3 or 4 minutes the whole must be filtered, and evaporated to dryness by a steam heat.
2. By exposing dry potato starch, in a stove, to a heat of about 400° Fahr. Yellow and inferior.
3. (M. Payen.) Dry starch, 1 ton, is moistened uniformly with concentrated nitric acid, 41⁄2 lbs. (diluted with), water, q. s., and the paste or dough is made up into small bricks or loaves, and dried in a stove; it is next reduced to coarse powder, and exposed in a stove-room for some time to a current of air at 160° to 165° Fahr.; it is next ground, sifted, and exposed, as before, to a heat of about 228° Fahr.; it is, lastly, ground, and passed through the ‘bolting machine.’ Very white and superior. This process has been patented in France by M. Henzé.
4. (Pinel.) Water, 100 galls., nitric acid, 1⁄2 gall., and hydrochloric acid, 1⁄2 pint, are mixed together, and so much potato starch is mixed as will form a thin paste; in two hours the liquid is drained off, and the solid matter is made up into lumps, which are dried by a gentle heat in a stove-room; they are next coarsely pulverised, and the powder is exposed on three successive days to the respective temperatures of 100°, 150°, and 190° Fahr.; the whole is then sifted, and, lastly, exposed to a heat ranging from 300° to 350° Fahr. Darker coloured than the last. To give it the appearance of gum Arabic, it is made into a paste with water containing 1% of nitric acid, and after being spread on copper plates in layers 3⁄4 to 1 inch thick, it is exposed to a stove heat ranging from 240° to 300° Fahr.
Prop., &c. White; insipid; transparent; friable; soluble in cold water, and in dilute spirit; insoluble in alcohol and ether; its solution yields a precipitate with acetate of lead. Iodine commonly turns commercial dextrin blue, but does not affect the colour of pure dextrin. It is distinguished from ordinary gum by its right-handed polarization of light, and by yielding oxalic but not mucic acid, when treated with nitric acid.
Dextrin is nutritive, emollient, and agglutinant. In France it is largely employed by the pastry-cooks and confectioners, and in medicine as a substitute for gum. The French surgeons also commonly employ it as a ‘stiffening’ for the splints used for fractured limbs. In this country it is chiefly used as a fine dressing for muslins, silk, and other textile fabrics, and in calico printing. Recently it has been made up into tear-like masses, and sold for gum Arabic, to which, however, it is vastly inferior as an agglutinant. See Dextrin.
Gum, Cherry-tree. Syn. Fruit-tree gum, Plum-tree g.; Gummi cerasi, G. pruni, L. An exudation from the stems of cherry, plum, and some other of the Rosaceæ. It is only partly soluble in water. It contains CERASIN (which see).
Gum, East India. This product, which consists of inferior kinds of gum acacia, is chiefly exported from Bombay, having been previously conveyed there from the coast of Arabia. It varies greatly in quality. Some samples are quite unfitted for making gum-water.
Gum, Insoluble. See Bassora Gum, Cherry-tree Gum, and Gum Tragacanth.
Gum, Seed. Syn. Gummi seminum, L. A species of soluble gum extracted from the seed of the flax (linseed), quince, &c.
Gum, Senegal. This product, which is largely exported from Portendie, Sierra Leone, and the French settlements on the Senegal, ranks next in quality to gum acacia, and for many purposes, as calico-printing for instance, it answers equally well. The transparent and light-coloured pieces are frequently picked out and sold as gum Arabic.
Gum Trag′acanth. Syn. Tragacanth, Gum dragon; Gummi tragacantha, G. draconis, Tragacantha (Ph. L.), L. The gummy exudation of the Astragalus verus, hardened by the air. When digested in water, it swells considerably, a portion is dissolved, and the whole combines to form a thick mucilage. It is totally soluble in boiling water, when some change is supposed to take place830 in it; a great portion, however, afterwards separates. Sp. gr. 1·384. It is chiefly employed in calico-printing, and by shoemakers and lozenge-makers; by the latter to give toughness to the saccharine mass.
Powdered tragacanth is often adulterated with flour of starch, and not unfrequently with the commoner varieties of gum Arabic. According to M. Planche, a mixture of pulverised tragacanth and gum Arabic forms, with water, a thinner mucilage than the same quantity of either of these gums alone. This fraud may be detected as follows:—Make a mucilage of the suspected gum, and add thereto a few drops (2 or 3 to the dr.) of alcoholic tincture of guaiacum, taking care to stir it all the while. If the sample contains any gum Arabic, the mixture, in the course of a few minutes, assumes a fine blue colour, whilst it does not change colour if the gum tragacanth is pure, 5% of gum arabic can be thus detected. When the quantity is very small, one to four hours may elapse before the colour is developed. Starch and flour are detected in the manner noticed under Gum Arabic.
Gum, Turkey. Various qualities of gum acacia are sold under this name.
GUM RES′INS. Syn. Gummi resinæ, L. Vegetable products in which the properties of gum and resin are combined. They are partly soluble in water, and partly in alcohol. Many of them form a species of emulsion when triturated with the former fluid. The principal gum resins are AMMONIACUM, ASSAFŒTIDA, BDELLIUM, GALBANUM, GAMBOGE, MYRRH, OLIBANUM, OPOPONAX, SAGAPENUM, and SCAMMONY.
GUN BAR′RELS. See Browning.
GUN COT′TON. See Pyroxylin.
GUN MET′AL. An alloy containing 90·5% of copper and 9·5% of tin, used for casting pieces of ordnance (erroneously termed ‘brass guns’), also those parts of machinery which are subjected to considerable friction. See Alloys, Bronze, Stereo-metal, &c.
GUN′POWDER. This substance is a mechanical mixture of saltpetre, charcoal, and sulphur. It is seldom prepared on the small scale.
Prep. The saltpetre having been trebly refined, by boiling, skimming, filtering, and crystallising, is melted into cakes, which are then brushed to remove any adhering grit or dirt, broken into pieces with a mallet, ground to a fine powder in a mill, and sifted through a fine bolting sieve of brass wire. The charcoal is that of the alder or willow, and is carefully burnt, as already described, and is then reduced to powder. The sulphur is refined by distillation, and ground to the same fineness as the charcoal and saltpetre. The ingredients are weighed out in the proper proportions, and mixed together in a machine consisting of a wooden drum, having a shaft passing through its centre, to which numerous ‘flyers’ in the shape of knife-blades are attached, the drum and flyers revolving in a contrary direction. When mixed, the charge is carried to the ‘incorporating mill,’ where it is ground under vertical iron ‘mill-stones,’ with a small quantity of distilled water, until the ingredients are thoroughly incorporated. The product of this operation is then pressed into a hard cake, which is next broken into pieces, granulated by means of sieves, and after being ‘glazed’ by friction, and the dust separated, is dried, with proper precautions, in a stove heated to about 130° by steam pipes.
The proportions of saltpetre, charcoal, and sulphur, used for different kinds of powder, differ very slightly. In ‘sporting powders’ the proportion of saltpetre is generally from 1 to 3% greater than in the Government powders. In ‘miners’ powders’ it is about 10% less, an excess of sulphur being used. The following are the proportions adopted by European powers:
| Saltpetre. | Charcoal. | Sulphur. | |
| England | 75 | 15 | 10 |
| France | 75 | 12·5 | 12·5 |
| Austria | 75 | 15 | 10 |
| Prussia | 75 | 13·5 | 11·5 |
| Russia | 73·78 | 13·59 | 12·63 |
| Spain | 76·47 | 10·78 | 12·75 |
| Sweden | 76 | 15 | 9 |
| (Capt. Jervis-White Jervis.) | |||
Obs. The quality of gunpowder is best estimated by actual trial of its power and cleanliness in use. It should be dry, hard, and free from dust; the grains should be of a uniform size, and glossy, and the colour a dark-grey or brownish-grey, not perfectly black. A very little placed on a piece of paper and fired should instantly explode with a flash, and neither leave an appreciable residue on the paper nor burn it. Dried by the heat of boiling water, or in vacuo, it should not lose more than 1⁄2 to 1% of its weight. Damp powder rapidly ‘fouls’ the gun. Gunpowder, containing more than 7% of water, does not recover its strength by simply drying it. The sp. gr. ranges between 1·795 and 1·800.
Karolyi succeeded in analysing the gases of gunpowder which had been fired in conditions closely resembling those which occur in artillery practice. For this purpose he enclosed a charge of powder in an iron cylinder of such strength that it just burst when the powder was fired by means of the electric spark. This charged cylinder was suspended in a hollow spherical bomb, from which the air was exhausted before firing.
After the explosion had been produced, the gases and the solid residue of the powder were submitted to analysis. The results obtained were the following:[343]
[343] ‘Phil. Mag.,’ 1863.
831
| Ordnance Powder. | Small Arms Powder. | ||
| Nitre | 73·78 | 77·15 | |
| Sulphur | 12·80 | 8·63 | |
| Charcoal. | Carbon | 10·88 | 11·78 |
| Hydrogen | 0·38 | 0·42 | |
| Oxygen | 1·82 | 1·79 | |
| Ash | 0·31 | 0·28 | |
| ——— | ——— | ||
| 99·97 | 100·05 | ||
| Ordnance Powder. | Small Arms Powder. | ||||
| Gaseous. | Nitrogen | 9·77 | 30·58 | 10·06 | 34·18 |
| Carbonic anhydride | 17·39 | 21·79 | |||
| Carbonic oxide | 2·64 | 1·47 | |||
| Hydrogen | 0·11 | 0·14 | |||
| Sulph. hydrogen | 0·27 | 0·23 | |||
| Marsh gas | 0·40 | 0·49 | |||
| Solid. | Ammonic sesquicarbonate | 2·68 | 69·25 | 2·66 | 65·14 |
| Potassic sulphate | 36·95 | 36·17 | |||
| Potassic carbonate | 19·40 | 20·78 | |||
| Potassic hyposulphite | 2·85 | 1·77 | |||
| Potassic sulphide | 0·11 | 0·00 | |||
| Charcoal | 2·57 | 2·60 | |||
| Sulphur | 4·69 | 1·16 | |||
| Loss. | 0·17 | 0·68 | |||
| 100·00 | 100·00 | ||||
| Ordnance Powder. | Small Arms Powder. | |||
| Nitrogen | 37·58 | 100 | 35·33 | 100 |
| Carbonic anhydride | 42·74 | 48·90 | ||
| Carbonic oxide | 10·19 | 5·18 | ||
| Hydrogen | 5·93 | 6·90 | ||
| Sulphuretted hydrogen | 0·86 | 0·67 | ||
| Marsh gas | 2·70 | 3·02 | ||
It will be seen from the above figures that in addition to the generation of a considerable amount of carbonic anhydride (carbonic acid) by the combustion of gunpowder, there is liberated at the same time a large quantity of solid matter, in the form of sulphate and carbonate of potash, sulphide of potassium, sulphur, charcoal, &c. This will explain why the air of mines is so prejudicial to the health of the miner, particularly when he is engaged in blasting operations, these being carried on in a more or less confined space. See Air, vitiated.
Gunpowder, Schultze. The subjoined account of Schultze gunpowder is a transcription of a report communicated to the editor of the ‘Field’ newspaper by Mr F. Toms, A.I.C., F.C.S. After referring to a previous communication on the same subject Mr Toms proceeds as follows:—I have carried out some further experiments, with the aid (by Dr Frankland’s kind permission) of apparatus more suited to my requirements than that previously at my disposal; and I now proceed to lay before you the results of these experiments, and the conclusions to which they have led me, respecting the powders formerly received and the new Schultze powder, with a sample of which you have since favoured me.
The main constituent of the Schultze gunpowder, as you are aware, is wood fibre, which, having first been purified, is then subjected to the action of strong nitric acid (intensified by mixture with sulphuric acid), and thus is converted into a kind of nitro-cellulose or pyroxylin, the ordinary form of which is gun-cotton. The wood fibre undergoes no change in appearance by this treatment; but a change takes place in its chemical composition, which may thus be exemplified:
| Cellulose (unconverted cotton or wood fibre). | Nitro-cellulose (cotton or wood fibre treated with nitric acid). | |
| Carbon | 6 parts | 6 parts. |
| Oxygen | 5 parts | 5 parts |
| Hydrogen | 10 parts | 7 parts or more. |
| Nitroxyl (NO2) | none | 3 parts or less. |
It will thus be seen that the sole difference between gun-cotton or Schultze powder and ordinary cotton or wood fibre is, that some of the hydrogen is abstracted and has its place supplied by nitroxyl—a substance contained in nitric acid, and composed of one part of nitrogen united with two parts of oxygen. Under the most favorable circumstances, it is possible to replace three of the ten parts of hydrogen by three of the nitroxyl, when the832 substance produced is explosive, and is called from its composition tri-nitro-cellulose. This is the purest form of gun-cotton. If weaker acid is used, less hydrogen is displaced, and the product is called di-nitro-cellulose or mono-nitro-cellulose, according as it contains two or only one part of nitroxyl. These derivatives are either feebly explosive or not explosive at all. Such are the compounds known as photographic collodion and soluble gun cotton—the latter name distinguishing it from pure gun-cotton, which is not soluble in a mixture of ether and alcohol.
The Schultze powder contains both the explosive and the non-explosive varieties of nitro-cellulose.
If the wood fibre, after being carefully purified according to the method described in Schultze’s patent of 1864, were thoroughly desiccated and allowed to cool out of contact with air, and then dipped in acid of the strength mentioned in the specification, there seems no theoretical reason why an explosive powder containing at least 90% of true tri-nitro-cellulose should not be produced. As, however, I find on experiment that nothing like that per-centage is arrived at, I can only conclude that, in order to moderate the violence of the explosion, the Schultze Company secure the formation of a large per-centage of “soluble” or less explosive nitro-compounds by merely air-drying their wood.
If this supposition be generally true, it seems probable that the sample of Schultze powder supplied by Messrs Blissett may owe its extra explosive force to exceptional care being taken, during the interval between the drying and the dipping, to prevent the absorption of moisture—with the addition, perhaps, of an increased length of exposure to the action of the acid.
That some such variation of the ordinary procedure was carried out seems evident from the different proportions of soluble and insoluble gun-cotton in the specimens of Schuitze powder supplied by Messrs Blissett and Messrs Bland; for it was found that on the washed wood fibre from each being submitted to the action of a mixture of alcohol and ether, about one half of the former powder and two thirds of the latter were dissolved out. This shows that while the “Blissett” specimen contained about one half its weight of insoluble or explosive nitro-cellulose, the “Bland” contained only about one third—a difference which confirms the result obtained by analysis as stated below.
The soluble gun-cotton, ordinarily non-explosive, may, however, be rendered explosive by saturating it with bodies rich in oxygen, which promote the decomposition and complete the combustion of the fibre. Nitre is used for that purpose, because it parts with its oxygen readily; and nitrate of baryta is also used, because, being more stable than the nitre, it renders the combustion more gradual than would be the case if nitre were alone employed. When both are used, the nitre, I should think, would start, and the nitrate of baryta continue and finish the combustion of the powder. The amount used is, I suppose, the result of calculation and experiment; but a powder containing little true tri-nitro-cellulose should require more of these salts than one containing much tri-nitro-cellulose; and an excess of the salts would lower the rate of burning of the powder.
I will now give my analysis in full of the three powders, viz.—(1) the ordinary powder issued last season, being part of a supply obtained from Messrs Bland, gunmakers, of the Strand; (2) some powder furnished by Messrs Blissett, of Holborn, and alluded to in their letter in the ‘Field’ of Jan. 19th last, as having damaged a gun made by them; and (3) some of the new powder of 1878, as used at the ‘Field’ trial of explosives in May last.
| 1877 Bland’s. | Blissett’s. | 1878 Trial or New. | ||
| Moisture, per cent. | 2·18 | 2·39 | 2·97 | |
| Extracted by water. | Nitrate of baryta, per cent. | 21·50 | 16·59 | 22·32 |
| Nitrate of potash, per cent. | 11·46 | 10·46 | 6·47 | |
| Yellow coloured organic substance, trace of chlorides, &c., undetermined | ||||
| Insoluble in water. | The converted wood fibre (nitro-cellulose) then remaining contained the following per-centage of mineral matter | 5·0 | 6·0 | 2·95 |
The converted wood fibre (after allowing for extraneous mineral matter) possessed the following per-centage composition. I place for comparison Professor Abel’s determination of the composition of tri-nitro-cellulose, and two of the impurities found along with it, in a parallel column.
| Bland’s. | Blissett’s. | Trial or New. | Tri-nitro- Cellulose. | Impurities. | ||
| Carbon | 28·75 | 28·07 | 28·12 | 24·24 | 29·20 | 30·50 |
| Hydrogen | 3·49 | 3·65 | 3·54 | 2·36 | — | 2·91 |
| Nitrogen | 10·80 | 15·60 | 11·66 | 14·14 | 11·85 | — |
| Oxygen | 56·06 | 52·68 | 56·68 | 59·26 | — | — |
These powders exploded at a temperature of833 about 190° C. (374° F.), the different samples varying but slightly. Pure gun-cotton is stated by Professor Abel to explode at 150° C. (302° F.); and black powders are said, by different authorities, to explode at various temperatures between 500° and 600° F., according to the variation in their composition and manufacture.
In addition to the difference in chemical composition of these Schultze powders, I would point out that there is a difference in density—the Blissett being heaviest, the Bland next, and the New the lightest of the three. I think this fact also has some bearing on the violence of the explosion. In black powders, I believe, a dense powder, speaking generally, is stronger than a lighter one; and the Schultze patent states that hard woods make more explosive powders—not, I take it, because the composition is thereby altered, but because a denser powder is produced. It would appear to me, from the above analyses, that the new trial powder should contain rather more explosive force than the Bland variety, though considerably less than the Blissett. The result may, however, be modified by the difference in density of the powders; and your practical experiments will show how far this agrees with the results of the shooting.
I have hitherto only spoken of the explosive force of the powder; now I will touch on another point—its tendency to spontaneous decomposition. Knowing that, in the case of gun-cotton, its stability is injured by a small proportion of resin and other organic impurities, and by the presence of free mineral acids. I did not expect to find this powder (made from a less pure kind of cellulose, from which also it must be somewhat difficult to wash all traces of acid) equal in stability to gun-cotton; and on subjecting the three kinds of Schultze powder to the Government ‘heat test’ of 150° F. (with a minimum of 10 minutes’ duration), it was found that the
This shows that the ‘new’ powder is very stable, as it stood the test for two minutes beyond the Government minimum, while the other two samples were a good way below it. The officials at Waltham Abbey would accept no gun-cotton which did not stand the test for ten minutes; and I have seen the best gun-cotton stand it for fifteen.
Whether the loose granulated condition of the Schultze powder, when stored, is sufficient to neutralise this inferiority in purity, and render a sample of Schultze, which only stands the test of seven minutes, as little liable to spontaneous combustion as gun-cotton which stands the test for ten minutes, there is at present no evidence to show.
To carry out this ‘heat test’ properly, some practice is required; so, in order to put the matter beyond doubt, I called in the assistance of my friend Mr Arthur Linnell, F.C.S., chemist to the Gun-Cotton Company, Stowmarket, a gentleman who uses the test daily, and who carried out the above three experiments strictly after the manner adopted by himself and by the Government officials.
In addition to Mr Linnell’s experiments, I noted that the aqueous extract of ‘Blissett’ was very faintly acid; that when heated in a chest at 195° F. moist blue litmus was very quickly reddened.
I think this serious defect (want of stability) is due to want of care in the washing; and I base this opinion on the following facts:
(1) The ‘Bland’ and ‘Blissett’ samples (the powders of least stability) are of a deeper tint than the ‘new’ (due to the soluble yellow impurity before mentioned). By continued washing in warm water they become pale, like the more carefully prepared new powder, and the yellow substance is dissolved away. Hence the lighter colour of the ‘new’ (and most stable) indicates it has less of this organic impurity.
(2) Sulphuric and nitric acids are used in the dipping of the powder, but should be entirely washed out, as they promote spontaneous decomposition. If left in, the sulphuric acid will, when the salts are added, decompose the nitrate of baryta, forming insoluble baric sulphate and free nitric acid.
On experiment I ascertained that the abnormally large quantity of mineral matter or ash (5 and 6 per cent.) found in the insoluble part of the ‘Bland’ and ‘Blissett’ powders is due to baric sulphate, and I think the acidity of the aqueous extract is due to the nitric acid thus set free.
Had this baric sulphate been present in the new powder, I should have thought it was purposely formed in all to prevent access of moisture; but, not finding this substance in this carefully prepared sample, I attribute its presence in the other cases to carelessness on the part of the workmen.
I should state that all these powders consisted of a granulated and consolidated pulp. This improvement must, I think, have considerable advantages over the sawdust form previously adopted by the Schultze Company in as much as it facilitates a more thorough purification being carried out, and produces a more homogeneous and equal powder. It is possible, too, that working with pulp may be of advantage, inasmuch as the company may now, by varying the pressure in forming the cake, obtain grains of any required density.
In conclusion, I may say that, in my opinion the most difficult task which the Schultze Company834 have had to encounter is that of obtaining uniformity of strength in their explosive; and the ‘Blisset’ sample of their powder may he looked upon as an experimental batch in which (by altering the mode of procedure in some such manner as I have indicated) they made a powder with a large per-centage of tri-nitro-cellulose, thus producing a more rapidly burning substance, and consequently a more violent explosion.
Taking all things into consideration, I think the Schultze Company, in manufacturing a nitro-explosive which gives the uniformity of shooting power shown in your recent experiments, have worked out a most troublesome problem with remarkable success. The difficulty of obtaining such results is evidenced by the fact that so many inventions of a somewhat similar character have been abandoned for sporting purposes from a deficiency in this respect.
But, however difficult it may be to manufacture a powder giving uniform shooting, it is evidently possible, with suitable care to produce (as the ‘new’ Schultze shows) a wood powder which is perfectly safe and stable, as far as spontaneous decomposition is concerned. The company, therefore, if they have not already done so, ought to take means to prevent powder of the low stability of the ‘Bland’ and ‘Blissett’ samples being again issued from their works.
P.S.—Since writing the above I have examined cursorily a sample of the ‘Dittmar’ wood powder, an American variety of ‘Schultze,’ used by Captain Bogardus in some of his recent shooting competitions. The powder is somewhat darker in tint, and of slightly larger grain, than the Schultze. In density it is intermediate between ‘Bland’s’ and the ‘new’ powder; and the charge in a twenty-bore cartridge was forty-two grains. This powder would seem to be made from solid cubes of wood (not a pulped mass like the present ‘granulated’ Schultze, or of sawdust splinters like the old so-called ‘cube’ Schultze). It contains no nitrate of baryta, but has a small quantity of nitrate of potash and soda. Possessing, as it would seem, therefore, a much smaller proportion of oxidizing salts than the English Schultze, it should contain, to make up for this loss of force, a larger proportion of explosive pyroxylin; but this is a point I have not experimentally determined. (‘Field,’ August 3rd, 1878, No. 1336, p. 143.)
Gunpowder, White. Syn. Blasting powder. Prep. 1. See Blasting Powder, No. 3.
2. Yellow prussiate of potash and white sugar, of each 1 part; chlorate of potassa, 2 parts; powder each separately, and mix them well, but carefully, with a bone or wooden knife. It may be granulated like gunpowder, by making the powder into a paste with a little water, and pressing the mass through a parchment sieve.[344]
[344] See the precautions noticed under Blasting Powder, page 230.
GUN′JAH. See Hemp (Indian).
GUT. Syn. Fishing gut, Silkworm g. This is obtained from the Bombyx mori (Linn.) or silkworm caterpillar. Prep. The silkworms, when just ready to spin, are steeped in strong vinegar for 12 hours in warm weather, or 2 or 3 in cold weather, and are then broken in half, and stretched out as far as possible on a board, furnished with slits or pegs to hold them; in this state they are allowed to dry in the sun or a warm place.
Obs. Used by anglers. The worms may be known to be going to spin by refusing food, and by having a fine silken thread hanging from the mouth.
GUT′TA PERCHA. The concrete juice of the Isonandro Gutta, a tree growing only in the Malayan Archipelago, and of other species of the same genus. The stem of the gutta-percha tree grows to the diameter of 5 or 6 feet, and on being notched yields a milky juice, which, after exposure to the air for some time, solidifies, forming the gutta percha of commerce. It arrives in this country in irregular blocks of some pounds in weight, usually containing a large portion of impurities in the form of pieces of wood, stones, and earth. To prepare this crude product for manufacturing into useful articles, the blocks are first cut into slices, and then torn into shreds. These are softened by hot water, and kneaded in a ‘masticator,’ the stones, earth, and other impurities, being gradually washed away by water. After several hours the gutta percha is found to be kneaded into a perfectly homogeneous mass, which is rolled or drawn into sheets, bands, or tubes, as required.
Prop., &c. Gutta percha is a tough, inelastic substance, becoming soft and plastic at 212° Fahr., at which temperature two pieces may be firmly welded together. It is one of the best insulators of electricity, is impervious to moisture, and resists the action of acids and alkalies to a great extent. Its best solvents are benzol, chloroform, bisulphuret of carbon, rectified mineral naphtha, and rectified oil of turpentine. All these dissolve it readily. According to the analysis of Payen, the purified gutta percha of commerce consists of 75 to 828 of chemically pure gutta percha, which is insoluble in ether and alcohol, and a white and a yellow resin, soluble in boiling alcohol.
Uses. These are numerous and varied. No substance, perhaps, with the exception of caoutchouc, has been ‘tortured’ to so many different purposes. Its perfect plasticity when warm, and its capability of receiving the most delicate impressions, render it invaluable in many cases where india rubber would be useless. Beautiful mouldings, picture frames,835 and a number of ornamental articles, are made from it. To the chemist and photographer it is of great use as a material for making bottles, carboys, photographic baths, and voltaic battery cells. One of the most important uses to which it has been applied is for enclosing the metallic wires used for telegraphic purposes. Its indestructibility by water, its plasticity, and high insulating power, have rendered it particularly valuable for this purpose. At the International Exhibition of 1862 the Gutta Percha Company exhibited one mile of covered wire perfectly insulated, which was hardly thicker than common sewing cotton. Gutta percha may be rolled into thin transparent sheets, which, being perfectly impervious to moisture, are well adapted for surgical purposes. Again, a solution of gutta percha in chloroform forms an excellent dressing for incised wounds, and a protection for abraded surfaces, burns, &c. It is used in the same way as collodion.
Gutta Percha, Purified. Dr Cattell, of London, has succeeded in purifying gutta percha so perfectly from all extraneous matter, that it presents the appearance of ivory. The raw material is dissolved in a certain solvent, and the solution most carefully filtered until it leaves on evaporation the gutta percha in a pure milk-white condition.
GYP′SUM. This is native sulphate of lime. When baked, to deprive it of water, and ground, it forms plaster of Paris. Gypsum is an excellent manure for certain soils.
HAARBALSAM, Vegetabilischer—Vegetable Hair Balsam (Joh. Andr. Hauschild, Leipsic). A decoction of burdock root, containing a little spirit and coloured green with indigo. (König.) Hager analysed a turbid brownish fluid, which deposited a brown precipitate on standing, and when filtered consisted of a decoction of burdock root with 20 per cent. of spirit.
Haarbalsam Mailandischer—Mailand’s Hairbalsam (Kreller, Nuremberg). Beef marrow, 40 parts; cinchona extract, 5 parts; balsam of Peru, 1 part; storax, 1 part; oil of bergamot, 1 part; oil of lemons, 1⁄2 part. (Hager.)
Haarbalsam Ostindischer—East Indian Hairbalsam (Dr Ayer). Contains sugar of lead, sulphur, glycerin, oil of lavender, and water.
Haarbalsam (J. F. Sehwarzlose Söhne, Berlin). A brownish-yellow spirituous aromatic fluid, having nearly the composition of eau de Cologne, with liquid storax, carbonate of potash, and a fat—perhaps derived from cantharides. (Hager.)
Haarbalsam (A. Marquart, Leipsic). A mixture of 83 grammes water perfumed with eau de Cologne, with 12 grammes glycerin, 4·25 grammes milk of sulphur, and 1·2 gramme lead nitrate.
HAD′DOCK. A small sea-fish, allied to the cod, and esteemed an excellent article of food. It is the Gadus æglefinus of Linnæus. Split, smoked, and dried, it is common in the smaller shops of London.
HÆMATEM′ESIS. In pathology, vomiting of blood. See Stomach Affections.
HÆM′ATITE. Syn. Hematite. In mineralogy, one of the most important iron ores. Two kinds are distinguished, the red, which is an anhydrous peroxide of iron, and the brown, which is the hydrated peroxide.
HÆMATOCRYS′TALLIN. A crystalline substance obtained by the action of oxygen and afterwards carbonic acid on the ‘clot’ of blood.
HÆMATOS′IN. Syn. Hæmatin, Red pigment of blood. The red colouring principle of the blood. It is not known in a state of purity. It differs from the other animal principles in containing, as an essential ingredient, the sesquioxide of iron.
HÆMATOX′YLIN. A principle obtained by Chevreul from common logwood (Hæmatoxylon campechianum), and on which its colour appears to depend.
Prep. 1. Infuse logwood chips in water, at a temperature of about 130° Fahr., for 12 hours, filter, and evaporate to dryness in a water bath; digest the residuum in rectified spirit for 24 hours, again filter and evaporate; then add a little water; again gently evaporate and set aside the solution in a cold place that crystals may form; these must be washed in rectified spirit and dried.
2. Digest powdered hard extract of logwood in rectified spirit, and proceed as last.
3. Powdered logwood is mixed with sand and digested for several days in pure ether; the resulting liquid is filtered, evaporated to a syrup, and set aside to crystallise.
Prop., &c. Brilliant reddish-white or straw-yellow crystals, soluble in boiling water, forming an orange-red solution which turns yellow as it cools, but resumes its former colour on being heated. Alkalies in excess change its colour successively into purple, violet, and brown; acids brighten it; with the metallic oxides it forms compounds having a blue, purple, or violet colour.
HÆMOP′TYSIS. In pathology, spitting of blood. It generally arises from extreme fulness of the blood-vessels of the lungs, or the rupture of blood-vessels, as a consequence of ulceration; but sometimes it is induced by excessive exertion or external violence. Depletion, aperients, acidulous and astringent drinks, and nauseants, are the usual remedies. Acetate of lead, in small doses, has been recommended for this affection. When this substance is given, it should be accompanied with a sufficient quantity of free acetic acid, to prevent its being converted into the poisonous carbonate of lead in the system.
HÆM′ORRHAGE. Syn. Hemorrhage; Hæmorrhagia, L. A bleeding or flow of836 blood. Bleeding may be divided into active, passive, and accidental.—Active hæmorrhage is that arising from a full state of the vessels, or plethora.—Passive hæmorrhage, from general debility of the system, and of the blood-vessels in particular.—Accidental hæmorrhage, from external violence, as blows, wounds, &c. The first generally requires depletion, and the second the usual treatment to establish the general health and vigour of the body. The bleeding from wounds, if extensive, should be arrested by tying the ruptured blood-vessels; or where this cannot be done, and in less important cases, by the application of styptics, as creasote, sulphate of iron, infusion of galls, compound tincture of benzoin, &c.
HAIR. Syn. Capillus, Pilus, L. The hair of the human head has continually formed a subject for the chisel of the sculptor, the pencil of the artist, and the lay of the poet. Nor is this surprising, since all the features of the face, as well as the head it covers, derive from it additional finish and unequalled grace. The hair is, indeed, one of the greatest auxiliaries of personal beauty, and imparts to it some of its principal charms. All nations, in all ages of the world, have been unanimous in their admiration of luxuriant and flowing or gracefully arranged hair.
Of all organic substances, hair is the one least liable to suffer spontaneous change. It is also less affected by aqueous liquids than most other substances. Hence its value in various branches of the useful arts.
The preservation of the hair of the head, independently of its connection with personal beauty, is a matter of the utmost importance in relation to hygiene. In other parts of this work, we have referred to its management under various conditions, but a few observations may be added here.
When the hair is in a weakly state, and either falls off or grows feebly, frequently cutting it will be found of the greatest service. “In the arrangement of the hairs, on the surface of the body, it might be inferred that little existed to excite attention; but this is not the fact, if we are to judge by the careful investigations to which the subject has given rise. The hair-tubes are not placed perpendicularly, but obliquely, in the skin; hence the direction of the hairs, after their escape from the tubes, is in the same sense inclined towards the surface; and the ‘set’ of the hair, from the root to the point, is governed by a law as precise as that which regulates any other of the secondary vital functions. Thus, on the head, the hair radiates from a single point, the crown, to every part of the circumference, making a gentle sweep, behind towards the left and in front to the right. The direction of this sweep is naturally indicated on the heads of children, and is that in which the hair is turned,” (Eras. Wilson.) The same occurs on the face and other parts of the body. In making our toilet, this natural arrangement of the hair should be interfered with as little as possible. Combing it or banding it in an opposite direction to that which it naturally assumes, is highly prejudicial to its healthy growth, and if long persevered in, leads to its premature and rapid decay. The practice now common among ladies, of throwing the hair from the forehead towards the back of the head, is of this reprehensible character.
In addition to our remarks elsewhere, we may here observe, that all the various systems proposed for strengthening or restoring the hair depend for their efficacy upon simple excitation or stimulation of the skin. Friction with the hair-brush, and the use of the ordinary hair-oils, pomades, and washes, are of this kind. The various advertised nostrums for reproducing or restoring the hair are either stimulants or rubefacients of more or less activity, or are emollients, which are directed to be applied by friction, in such a manner as to set up a considerable amount of irritation. When the affection depends on the languid circulation of blood in the part, this treatment often succeeds; but when the hair-bulbs are withered or decayed, or the scalp much attenuated, the restoration of the hair is an impossibility. See Baldness.
HAIR COSMETICS. Under this head are included all preparations which are used for beautifying, preserving, or restoring the hair. These are fully described in different parts of this work, and we shall here merely name the principal heads under which they will be found. The hard pomatums used for keeping the hair, moustache, and whiskers, in form, and sometimes to colour them at the same time, are noticed under Cosmetique; the mucillaginous preparations for stiffening the hair, under Fixature; the compounds for removing superfluous hairs, under Depilatory; the applications for the cure and prevention of baldness, under Pomades and Washes; and those employed to cleanse or beautify the hair under the last two heads, and under Hair Dyes and Oils.
HAIR DYES. Syn. Tinctura cappillorum, L. The practice of dyeing the hair is of great antiquity; and though not so common as formerly, it is still far from infrequent at the present day. The numerous preparations vended for this purpose have generally a basis of lead or silver. Bismuth, pyrogallic acid, and certain astringent vegetable juices, are also occasionally thus employed. The following list embraces all those of any value:
Prep. 1. Litharge, 1 part; fresh-slaked lime and starch, of each 2 parts; all in fine powder, and perfectly dry; mix, and keep the compound in well-corked bottles. This powder is to be made into a thin paste or cream with water (for black), or milk (for brown), and applied to the hair (previously freed from grease with soap and water, and dried), by837 means of a sponge or brush, or the fingers; observing to rub it well into the roots, and to pass a comb for some time through it, to ensure its coming in contact with every part. The whole must be then covered with a moist leaf of cotton wadding, or some brown paper several times doubled and well damped with hot water, and allowed to remain so for 3 or 4 hours, or even longer; or an oil-silk cap, or a bladder, may be worn, the object being simply to prevent the evaporation of the moisture. After a sufficient time has elapsed, the powder may be removed by rubbing it off with the fingers, and afterwards washing it out with warm soap-and-water. A little pomatum or hair-oil will restore the usual gloss to the hair. Another method of operating is to apply the cream or paste as before, and then to keep rubbing it about the hair with a brush as long as may be required, occasionally adding a few drops of hot water to preserve the whole moist. In this way the action of the dye is facilitated, and the process concluded in a much shorter time.
2. Lime (slaked in the air), 2 parts; carbonate of lead (pure white lead), 1 part; mixed and applied as the last.
3. (Aqua Orientalis.) From grain silver, 2 dr.; steel filings, 4 dr.; nitric acid, 1 oz.; soft water, 11⁄2 fl. oz.; digested together, the solution being afterwards diluted with water, 31⁄2 fl. oz., and filtered. Applied by means of a fine-toothed comb, or a half-worn tooth-brush to the hair, previously well cleaned with soap and water, and dried.
4. (Argentan tincture.) From nitrate of silver, 1 dr.; eau de rose, 1 fl. oz.; nitrate of copper, 2 gr., or q. s. to impart a slight greenish tint. Used as the last.
5. (Dr Cattell.) Nitrate of silver, 11 dr.; nitric acid, 1 dr.; distilled water, 1 pint; sap green, 3 dr.; gum arabic, 11⁄4 dr.; digest together. Used as No. 3.
6. No. 1 Solution. Gallic acid, 71⁄2 gr.; acetic acid, 20 min.; distilled water, 1 fl. oz.
No. 2 Solution. Nitrate of silver, in crystals, 301⁄2 gr.; distilled water, 1 fl. oz.; ammonia sufficient to form the precipitate formed at first.
7. (Chestnut hair dye.) “We have met with the following, but do not guarantee it:— Permanganate of potash gives the hair a beautiful chestnut-brown colour, varying according to the strength of the solution of the salt. A good formula is permanganate of potash, 1 dr.; powdered gum Arabic, 2 dr.; rose water, 3 oz.; mix. Apply carefully with a tooth brush so as to avoid staining the skin. (‘Chemist and Druggist.’)
8. (Hair restorer.) This is in reality a dye. Sulphur, 45 gr.; acetate of lead, 20 gr.; glycerin, 1⁄2 oz.; water to make up 10 oz.
9. (Golden hair dye, Aureoline.) A solution of peroxide of hydrogen in water; containing from 3 to 6 per cent. of the peroxide.
10. (Brown hair dye.) Acetate of lead, 2 dr.; hyposulphate of soda, 1 dr.; rose water, 14 oz.; glycerin, 2 oz. Dissolve the acetate of lead and the hyposulphite in separate portions of the rose water; filter separately, mix the solutions, and add the glycerin.
11. (A harmless hair dye. Dr Hager.) Ten parts of subnitrate of bismuth, and 150 parts of glycerin are mixed in a glass vessel and heated in a water bath; solution of potash is then added in small portions, and with continued agitation, until a clear solution has been obtained, to which a concentrated solution of citric acid is added until merely a slight alkaline reaction is observed. Enough orange-flower water is added to make the whole liquid weigh 300 parts; the addition of a small quantity of a solution of an aniline colour completes the preparation.
12. (Chevallier.) Fresh-slaked lime, 5 dr.; water, 11⁄2 oz.; mix, strain through gauze, and pour the milk into a four-ounce bottle. Next dissolve sugar of lead, 5 dr., in water, 3 fl. oz.; add to this solution, dry slaked lime, 1 dr., stir well together, wash the precipitate with a little soft water, drain off the water, then add it to the milk of lime in the bottle, and shake the whole well together, and again before use. Applied as No. 1; but it acts much more quickly.
13. (Delcroix.) From acetate of lead, 2 oz.; prepared chalk, 3 oz.; quicklime, 4 oz.; each in an impalpable powder. Used as No. 1.
14. (Eau d’Afrique—Hopekirk.)—a. Nitrate of silver (cryst.), 11⁄2 dr.; distilled water, 2 fl. oz.; dissolve, and pour the solution into the bottles labelled ‘Solution No. 1,’—b. Liquor of potassa, 3 dr.; sulphydrate of ammonium, 7 dr.; water, 1 fl. oz.; mix, and pour the liquid into the bottles labelled ‘Solution No. 2,’ For use, the hair is moistened by means of a small-toothed comb or tooth-brush, with the Solution No. 1, either alone or diluted with a little water; care being taken to avoid touching the skin, if possible. After the lapse of 8 or 10 minutes the Solution No. 2, diluted with at least 5 times its measure of water, is applied in the same manner, and any spots on the skin removed by rubbing them with the corner of a napkin wetted with the liquid. The skin is then sponged clean with a little warm water, and wiped dry, and the hair is arranged with the comb as usual. It is better to avoid rubbing it or washing it for a few hours. Sometimes the process is reversed, and the liquid No. 2 applied first. In this way the stains on the skin are more readily removed, but the dye is less permanent than when the other plan is adopted.
15. (Eau d’Egypte.) Resembles No. 4 (above).
16. (Essence of Tyre.) Resembles the last.
17. (Grecian water.) Resembles No. 3, or 4.
18. (Dr Hanmann.) Litharge, 275 gr.838 (say 1 part); quicklime, 1875 gr. (or 63⁄4 parts); hair powder (or starch), 930 gr. (or 31⁄2 parts): all in fine powder. Used as No. 1.
19. (Hewlet’s.) Resembles Spencer’s (No. 28).
20. (Instantaneous.) Moisten the hair first with a solution of nitrate of silver in water (1 to 7 or 8), and then with a weak solution of sulphydrate of ammonium. The colour of the hair, unaltered by the silver solution, instantly turns black when moistened with the sulphuret. See Eau d′Afrique.
21. (La Forest’s.) See Washes.
22. (Orfila’s.) From litharge, 3 parts; quicklime, 2 parts; starch, 1 part. The original form for this article is as follows:—Sulphate of lead, 4 parts; dry fresh-slaked lime, 5 parts; water, 30 parts; boil 1 hour, collect the paste on a piece of calico, and apply it in a similar manner to No. 1.
23. (Pomade dye.)—a. Nitrate of silver, 1 part; nitric acid, 2 parts; iron filings, 2 parts; mix, and let them stand together for 4 or 5 hours, then pour them on oatmeal, 2 parts; next add, lard, 3 parts; and mix well together.
b. From nitrate of silver and cream of tartar, of each 1 dr.; liquor of ammonia, 2 dr.; dissolve, add of lard, 4 dr.; and mix well together.
24. (Poudre d’Italie.) Resembles Orfila’s (No. 22.)
25. (Pyrogallic stain.) A weak solution of crude pyrogallic acid. Another article sold under this name is prepared by distilling nutgalls (coarsely powdered) in a retort, dissolving the solid acid which sublimes in a little hot water, and after mixing this with the acid liquid which also passes over, adding a little rectified spirit. The floating oil is then separated and the solution filtered.
26. (Redwood.) Litharge, 2 oz.; slaked lime and powdered starch, of each 1 oz.; liquor of potassa, 2 dr.; water, q. s. to form a thick cream. Used as No. 1.
27. (Redwood.) Liquor of potassa and distilled water, of each 1 pint; mix, and pass sulphuretted hydrogen through the liquid until it is saturated. Of this solution take 20 oz.; liquor of potassa, 4 oz.; mix, and label it ‘Solution No. 1,’ Next dissolve nitrate of silver, 1 dr., in distilled water, 2 oz.; and label the liquid ‘Solution No. 2,’ Used in the same manner as No. 8 and 20.
28. (Spencer’s.) From sap green, 1⁄2 dr.; nitrate of silver, 1 dr.; hot water, 1 oz. Applied as No. 3.
29. (Tincture of walnut.) A strong tincture of the shells of green walnuts, scented with oil of lavender.
30. (Ure.) Litharge, fresh-slaked lime, and bicarbonate of potassa, mixed in various proportions, according to the shade of colour desired. Used like No. 1.
31. (Warren’s.) From litharge, 1 oz.; white lead, 2 oz.; quicklime (in fine powder), 16 oz.; mix, sift through lawn, and at once bottle the mixture. Used like No. 1. Mixed with water, it is said to dye the hair black; with milk, brown.
32. White lead, 1 oz.; fresh slaked lime, 11⁄2 oz.; litharge and oxide of bismuth, of each 1⁄2 oz.; water, 1 pint; mix, boil 15 minutes, with frequent agitation, cool, pour it into a bottle, add of solution of ammonia, 1⁄4 fl. oz., shake the whole frequently for some hours and the next day pour off the liquid portion from the white sediment which forms the dye. Used like No. 1. It is applied for 8 or 10 minutes for a brown; 30 minutes, or longer, for a black. For the first, it is washed off with water containing a little common soda.
33. The juice of the bark or shell of green walnuts, applied with a sponge. (Paulus Ægineta.)
34. A leaden comb used daily is said to darken the hair, but we have known persons persevere in its use for months without any perceptible change occurring. Premature baldness is a frequent consequence of its use.
Obs. It is right to inform the reader that all those compounds which contain nitrate of silver stain the skin as well as the hair. These stains may be removed, when quite recent, by rubbing them with water containing a little sulphydrate of ammonium (see above) or iodide of potassium in solution; but as this is attended with some trouble and inconvenience, the best way is to avoid the necessity of doing so. The hair-dressers adopt the plan of smearing hard pomatum over the skin immediately surrounding the hair, to protect it from the dye. By very skilful manipulation, and the observance of due precautions, the hair may be thoroughly moistened with the above fluids, without touching the adjacent skin, but this can only be done, in the case of the hair of the head, by a second person. This has led to a preference being given by many to the compounds containing lead, as the colouring matter formed in them does not stain the skin. The hue given by the latter (when pale) is very apt to possess an unnatural redness, but all the shades of colour given by the preparation of silver are rich and unexceptionable. Pyrogallic acid, and the juice of walnuts, also stain the skin, although less intensely and permanently than nitrate of silver.
The detection of dyed hair is often a matter of importance in medico-legal research. The presence of silver may be shown by digesting the hair in a little weak chlorine water or hydrochloric acid, when the resulting chloride of silver may be dissolved out with liquor of ammonia, and submitted to the usual tests. Hair containing lead, when digested in dilute nitric acid, gives a solution of nitrate of lead, in which form it is readily detected. See Lead and Silver.
All the preceding compounds are for dyeing living hair (human); horse-hair, bristles, &c., and other dead hair, may be readily stained by839 steeping them in any of the ordinary liquid dyes, more especially those employed for wool and silk. See Pomades, Washes, &c.
HAIRWASH, Golden, or Auricomus, is a clear inodorous fluid, which is said to dye hair blond or yellowish red, and really does so. Sold in bottles containing 250 grammes. When exposed to the air the fluid decomposes with time. This hair-dye is an aqueous solution of hydroxyl contaminated with traces of baryta, and can be prepared as follows:—17 parts crystallised caustic baryta and 3 parts potassium chlorate, intimately mixed in fine powder, are melted by a gentle heat. The mass must be washed with cold water to remove the potassium chloride, and the residue shaken in the cold with a solution of 8 parts glacial phosphoric acid in 25 parts water, the whole being cooled with ice. When the peroxide of barium is decomposed, the fluid should be decanted from the precipitate. (Hager.)
HALL MARKS. The ‘Hall Marks’ on articles in gold and silver not only inform us of their fineness, but furnish us with other important particulars.
The Hall Mark (proper) denotes the place of manufacture or assay, being an anchor, for Birmingham; a dagger or 3 wheat sheaves, for Chester; Hibernia, for Dublin; castle and lion for Edinburgh; castle with 2 wings, for Exeter; tree and salmon with a ring in its mouth, for Glasgow; leopard’s head for London; 3 castles, for Newcastle-on-Tyne; a crown, for Sheffield; and five lions’ heads and a cross, for York.
The Duty Mark is the head of the Sovereign, showing that the duty is paid.
The Date Mark is a letter of the alphabet, which varies every year, and with the different companies, thus: the Goldsmith’s Company of London have used from 1716 to 1755, Roman capital letters; from 1756 to 1775, small Roman letters; from 1766 to 1795 old English letters; from 1796 to 1815, Roman capital letters, from A to U, omitting J; from 1816 to 1835, small Roman letters, a to u, omitting j; from 1836, old English letters.
The Standard Mark for gold is, for England, a lion passant; Edinburgh, a thistle; Glasgow, a lion rampant; Ireland, a harp crowned. For silver, a figure of Britannia. If under 22 carats, gold has the figures 18.
The Manufacturer’s Mark is the initials of the maker, as S. H., W. T., C. E., &c.
HAL′OGENS. In chemistry, a name given by Berzelius to chlorine, bromine, iodine, and fluorine. These elements unite with metals to form compounds called ‘haloid salts.’
HAMBURGH POWDER. The material known under this name is used to adulterate chicory. It is composed of roasted and ground peas, coloured with Venetian red.
HAMS. These are usually prepared from the legs of bacon pigs, but those of the sheep are also sometimes used for the same purpose. Smoked ham is strong eating, and rather fit for a relish than for diet, and should be particularly avoided by the dyspeptic and by convalescents.
Choice. A sharp knife thrust under the bone should have a pleasant smell when withdrawn. The recently cut fat should be hard and white, the lean fine-grained, and of a lively red. Those short in the hock are the best.
Curing. An ordinary sized ham requires nearly three weeks, if wet salted, and about a month if dry salted, to cure it perfectly. At the expiration of this time they are ready for smoking. Mutton hams should not lie in pickle longer than 12 or 14 days.
Cooking. Hams should be put into the water cold, and should be gradually heated. A ham of 14 lbs. will take about 4 hours, one of 16 lbs. will take 61⁄2 hours, and one of 20 lbs. about 51⁄2 hours, to dress it properly. “If it is an old ham, it should be soaked for 12 hours previously.” (Soyer.)
Pres. Most grocers and dealers in hams enclose them, after being smoked, in canvas, for the purpose of defending them from the attacks of the little insect, the Dermestes lardarius, which, by laying its eggs in them, soon fills them with its larvæ; or maggots. This troublesome and expensive process may be altogether superseded by the use of pyroligneous acid, applied by means of a painter’s brush.
HANDS. Dirty and coarse hands are no less the marks of slothfulness and low breeding, than clean and delicate hands are those of cleanliness and gentility. To promote the softness and whiteness of the skin, mild emollient soaps, or those abounding in oil, should alone be used, by which means CHAPS AND CHILBLAINS will generally be avoided. The coarse, strong kinds of soap, or those abounding in alkali, should for a like reason be rejected, as they tend to render the skin rough, dry, and brittle. The immersion of the hands in alkaline lyes, or strongly acidulated water, has a like effect. When the hands are very dirty, a little good soft soap may be used with warm water, which will rapidly remove oily and greasy matter. Fruit and ink stains may be taken out by immersing the hands in water slightly acidulated with oxalic acid or a few drops of oil of vitriol, or to which a little pearlash or chloride of lime has been added; observing afterwards to well rinse them in clean water, and not to touch them with soap for some hours, as any alkaline matter will bring back the stains, after their apparent removal by all the above substances, except the last. The use of a little chloride of lime and warm water, or Gowland’s lotion, imparts a delicate whiteness to the skin; but the former should be only occasionally used, and should be well washed off with a little clean water to remove its odour. Glycerine employed in the same manner renders the skin840 soft, white, and supple. The use of a little sand or powdered pumice stone with the soap will generally remove the roughness of the skin frequently induced by exposure to cold. The hands may be preserved dry, for delicate work, by rubbing a little club moss (LYCOPODIUM), in fine powder, over them. A small quantity of this substance sprinkled over the surface of a basin of water will permit the hand to be plunged to the bottom of the basin without its becoming wet.
HANG′ING. In cases of suspended animation from hanging, the assistance must be prompt and energetic. The body on its discovery should be instantly relieved from the state of suspension and all pressure about the throat. The remedial treatment chiefly consists, in the severer cases, in cupping the temples or opening the jugular vein, and so relieving the head of the blood which is accumulated in its superficial veins in consequence of strangulation. When the body is cold, friction, and the other means used for restoring the animal heat in drowned persons, should be resorted to. See Asphyxia and Drowning.
HARD′NESS. Compactness; solidity; the power of resisting abrasion. Mineral substances are frequently distinguished and identified by their relative hardness. This is ascertained by their power to scratch or be scratched by one another. A valuable table on this subject will be found in the article on Gems.
HAR′MALINE. Syn. Harmalina. An alkaloid, forming yellow-brown crystals, discovered in the seeds of Peganum harmala. It has a bitter astringent and acrid taste, is soluble in alcohol, and forms yellow, soluble salts with the acids. It has been proposed as a yellow dye. By oxidation it yields another compound (harmine), which is a magnificently red dye-stuff, easily prepared and applied. The seeds are produced abundantly in Southern Russia.
HAR′NESS POLISH. See Blacking, &c.
HARTS′HORN. Syn. Cornu cervi, C. Cervinum, Cornu (Ph. L.) L. The “horn of the Cervus elephas” (Ph. L.) or stag.
Hartshorn, Burnt. Syn. Cornu ustum (Ph. L.), Cornu cervi ustum, L. Prep. (Ph. L. 1836.) Burn pieces of harts’ horns until perfectly white, then grind and prepare them in the same way as directed for prepared chalk.
Obs. Finely powdered bone-ash is usually sold for burnt hartshorn, and possesses exactly the same properties.—Dose, 10 to 30 gr., or more 2 or 3 times a day, in rickets, &c
Hartshorn Shavings. Syn. Hartshorn raspings; Rarura cornu cervi, Ramenta c. c., L. Obtained from the turners. Boiled in water, it yields a nutritive jelly. Used by straw-plait workers to stiffen bonnets, &c.
HATCH′ING. See Incubation.
HATS. Those should be chosen possessing a short, smooth, fine nap, and a good black colour; and sufficiently elastic to resist ordinary wear and tear, without breaking or giving way. The HAT BRUSH for daily use should be made of soft hairs, but a stiffer one should be employed occasionally, to lay the nap smooth and close. Grease may be removed by means of porous brown paper, and pressure with a hot iron.
HAY-FEVER. Syn. Hay-asthma, Catarrhus æstivas. Dr Aitken defines this affection as “a variety of asthma or catarrh, occurring generally during the summer months, especially during the inflorescence of the hay crop, or during the drying or conversion of the newly-mown grass into hay, in May and June.” The disease is distinguished by extreme irritation of the eyes, nose, and the whole of the air-passages, these symptoms giving rise in succession to troublesome itching of the eyes and nose, frequent paroxysms of sneezing, with copious discharge from the nostrils, pricking sensation in the throat, cough, tightness of the chest and difficulty of breathing, accompanied sometimes with, and sometimes without, great mucous expectoration. The inhalation of the powder of ipecacuanha sets up similar symptoms with some persons.
Dr Aitken’s definition of hay-fever seems to point to what is pretty generally accepted as its cause, viz. the inhalation of minute and impalpable emanations from certain grasses given off during the period of their flowering and subsequent conversion into hay. This supposition as to the origin of this disease derives support from the circumstance, that it always takes place during the hay season, and at no other; and also that it may be cured by the avoidance of hay-fields and hay-stacks. “Hence going to the sea-coast, and especially to those parts of the coast that are barren of grass, offers a means of protection; and when this cannot be done, such persons obtain refuge in some measure from the cause of irritation, by remaining within doors and shutting out as much as possible the external air during the hay-crop.”[345]
[345] Sir Thomas Watson.
Furthermore, those whom the disease attacks are not particularly subject to catarrh at other times.
Treatment.—Numerous remedies have been proposed and employed for hay-asthma. Dr Elliotson suggests the mild fumigation of the patient’s apartment by means of the solutions of the chlorides of lime or soda; and further advised the sufferers using a smelling bottle containing one or the other of the chlorides. He also employed with success the sulphate of quinine and iron. Mr Gordon recommends the tincture of Lobelia inflata, with the use of the cold shower-bath. Tincture of nux vomica is also said to have been used with good results, as also has Fowler’s solution of arsenic, with very decided advantage, by Dr Mackenzie.
These potent remedies, however, should only be administered under the supervision of a qualified medical practitioner. An esteemed841 medical friend assures us he has employed the new remedy, Quinetum (the alkaloid of the East India red bark), with the happiest effects. He gives four grains of the quinetum three times a day. The use of an ori-nasal respirator of cotton wool has also been suggested. Great relief has, we know, in a great number of cases, been experienced by snuffing from a smelling bottle containing the following ingredients:— Pure crystallised carbolic acid, 1 dr.; sesquicarbonate of ammonia, 1 oz.; wood charcoal, 1 oz.; oil of lavender, 1⁄2 dr.; compound tincture of benzoin, 1⁄2 oz.; all reduced to fine powder, and thoroughly mixed.
HEAD′ACHE. Syn. Cephalalgia, L. In pathology, pain in the head. The symptoms of this very general complaint are too well known to require any description. According to pathologists, headache arises either from a sympathy with the stomach and chylopoietic (chyle-forming) viscera, or from a weakness or exhaustion of the power of the encephalon. The former may be called SYMPATHETIC HEADACHE, and the latter NERVOUS HEADACHE. When it attacks only one side of the head it is called HEMICRANIA. The treatment of the first form should consist in restoring the healthy action of the stomach, by the administration of aperients, and by the use of proper food and exercise; or when that viscus is overloaded with undigested food, by the exhibition of an emetic. For this purpose 1⁄4 to 1⁄2 an oz. of ipecacuanha wine may be taken in a cupful of warm water, which will generally relieve the stomach, especially if its action is assisted by drinking copiously of warm water. Headache is a common accompaniment of indigestion and stomach disease, and in general it will be found that whatever will remove the one will also cure the other. Nervous headaches are relieved by nervous tonics and stimulants, as bark, cascarilla, calumba, and gentian, camphor, ammonia, ether, and wine, the latter in a state of considerable dilution. A cup of strong coffee or strong green tea often acts like a charm in removing this species of headache. Small doses of tincture of henbane have also often a like effect. 20 or 30 drops of laudanum, or, preferably, half that number of liquor opii sedativus, may be taken with advantage as an anodyne, and to induce sleep. Amongst popular remedies may be mentioned ‘nasal stimulants,’ as snuff (cephalic), smelling salts, and aromatic vinegar, the use of which is familiar to every one; and local applications, as very cold water, ether, vinegar, strong spirits, Cologne water, &c., all of which are rubbed over the part of the head affected, with the fingers, or a linen rag dipped in them is laid thereon instead. Pressure on the head has also been used with advantage. Silence, darkness, and repose, are powerful remedies, alike suitable to every variety of headache; and change of air, scene, and occupation, are especially beneficial to those resulting from excessive mental anxiety or exertion. Blisters are extensively applied behind the ears in cases of violent headache.
Headache is often symptomatic of other diseases, especially those of the inflammatory and nervous kind, rheumatism, &c. In all these the primary disease should be sought out and attempted to be cured. In many cases these attacks rapidly yield to a few doses of compound decoction of sarsaparilla containing a little iodide of potassium. Headache in pregnancy may generally be removed by proper attention to the bowels; observing to assist their action, should they require it, by the use of some mild aperient, as castor oil, lenitive electuary, seidlitz powders, &c. When the constitution is very robust, blood may be taken. Headache in bed may frequently be relieved by washing the head with cold water, and discontinuing the use of a nightcap, at the same time preserving the feet warm by wearing worsted socks or stockings.
HEAD′ING. Syn. Beer heading, Cauliflower H. Prep. 1. Alum and green copperas, equal parts, in fine powder.
2. Alum, copperas, and common salt, equal parts.
Used by brewers to make their beer keep its head or froth.
HEALTH. That state of the living body in which all its functions are duly performed. See Hygiene.
HEALTH, GOOD—Gut-Heil (Aust). A liquor containing the extractive matters of calamus root, rhubarb, cinnamon, orange peel, &c., with 35 per cent. of sugar. (Hager.)
HEAR′ING. See Deafness, Ear, &c.
HEART′BURN. Syn. Cardialgia, L. Anxiety and pain about the region of the stomach, generally attended by a sense of gnawing and heat; hence its popular name. Faintness, nausea, and eructation of a thin, acidulous, watery liquid, especially in the morning, are common symptoms of this complaint. The usual causes of heartburn are excess in eating and drinking, the use of improper food, and sedentary habits. A good remedy is a teaspoonful of carbonate of magnesia, or carbonate of soda, in a glass of peppermint or cinnamon water, to which a little powdered ginger may be added with advantage. This dose may be taken 2 or 3 times daily until the disease is removed. Articles of food that easily undergo fermentation should at the same time be avoided, and a dry diet had recourse to as much as possible. Soda water, toast-and-water, and weak spirit-and-water, are the most suitable beverages in this complaint.
HEAT. Syn. Caloric; Caloricum, L. The consideration of this subject belongs to physics and chemistry. Much useful information, in connection with it, will, however, be found in this work under the heads Ebullition, Evaporation, Expansion, Refrigeration, &c.
HEAVY SPAR. Native sulphate of barium. See Baryta.
842
HED′ERIN. Syn. Hederina, L. From the decoction of the ground seeds of ivy (Hedera helix), boiled in water, along with a little slaked lime or magnesia, the precipitate being afterwards digested in rectified spirit, and the filtered tincture evaporated. Febrifuge and sudorific.
HEIGHT, Average of Man. The ‘Boston Journal of Chemistry’ gives the following particulars of the average height of man:—“The Yankee would appear to be the tallest of civilised men, if we may trust some statistics given in foreign journals as the result of the measurement of over half a million men. The mean height of the American Indian is 67·934 inches; of the American white man, 67·672; Scotch, 67·066; English, 66·575; Russian, 66·393; French, 66·277; Mexican, 66·110.” If the Yankee carries off the palm as the tallest of men, he also does so for his tallest tales; but if weight were introduced into the calculation, we think our Transatlantic cousins would rank last.
HEIGHTS. The following table, calculated by Regnault, gives the temperature at which water boils at the corresponding heights of the barometric column. The figures have been confirmed by direct observation.
| Boiling Point. Deg. Fahr. | Barometer. Inches. | Boiling Point. Deg. Fahr. | Barometer. Inches. | Boiling Point. Deg. Fahr. | Barometer. Inches. |
| 184 | 16·676 | 195 | 21·124 | 206 | 26·529 |
| 185 | 17·047 | 196 | 21·576 | 207 | 27·068 |
| 186 | 17·421 | 197 | 22·030 | 208 | 27·614 |
| 187 | 17·803 | 198 | 22·498 | 209 | 28·183 |
| 188 | 18·196 | 199 | 22·965 | 210 | 28·744 |
| 189 | 18·593 | 200 | 23·454 | 211 | 29·331 |
| 190 | 19·992 | 201 | 23·937 | 212 | 29·922 |
| 191 | 19·407 | 202 | 24·441 | 213 | 30·516 |
| 192 | 19·822 | 203 | 25·014 | 214 | 31·120 |
| 193 | 20·254 | 204 | 25·468 | 215 | 31·730 |
| 194 | 20·687 | 205 | 25·992 | 216 | 32·350 |
HEL′ENIN. See Inulin.
HELIOG′RAPHY. See Photography.
HEL′LEBORE. Syn. Black hellebore; Helleborus (Ph. L.), L. “The rhizome and root” of “Helleborus niger” (Ph. L.) or black hellebore. It is alterative and emmenagogue, in small doses (2 to 8 gr.); and a drastic hydragogue purgative and anthelmintic in larger ones (10 to 20 gr.) See White Hellebore.
HELLEBOR′IE. Syn. Soft resin of hellebore. An odourless, acrid substance, extracted by alcohol from black hellebore, and on which, according to Vauquelin, the activity of that drug depends.
HEM′LOCK. Syn. Conii Folia (B. P.); Conium (Ph. L. E. & D.), L. In pharmacy, “the fresh and dried leaf of the wild herb Conium maculatum,” or spotted hemlock. The first is used to make the extract; the last, the tincture and powder.
Hemlock is a powerful narcotic acrid poison, occasioning stupor, delirium, paralysis, convulsions, coma, and death. In small doses it is anodyne, alterative, resolvent, antispasmodic, and anaphrodisiac, and has been exhibited in cancer, dropsy, epilepsy, rheumatism, scrofula, syphilis, and other diseases.—Dose, 3 or 4 gr. of the powder, twice or thrice daily, until some obvious effect is produced.
Hemlock, whether in leaf (conii folia) or powder (pulvis conii) rapidly deteriorates by keeping. When good, the powder, triturated with solution of potassa, exhales a powerful odour of conia.
In cases of poisoning by hemlock, the treatment is similar to that noticed under Aconite. See Conia, Extract, Tincture, &c.
HEMP. Syn. Cannabis, L. In botany the typical genus of the natural order Cannabinaceæ. The common hemp, from the fibres of which cordage is made, is the species Cannabis sativa. The fruit of this plant (hemp seed) is demulcent and oleaginous. It is said that the plumage of bullfinches and goldfinches fed on it for too long a time, or in too large a quantity, changes from red and yellow to black.[346]
[346] Burnett, ‘Outlines of Botany.’
Hemp, Indian. Syn. Hashish, Cannabis Indica. This plant, now so largely used in medicine, is a variety of Cannabis sativa, or, perhaps, the same simply rendered more active by climate. The parts employed in Asia for the purposes of intoxication, and in Europe as medicine, are the herb or leaves and the resin. The ‘gunjah’ sold in the bazaars in the East Indies is the plant, just after flowering, dried, and pressed together. ‘Bang,’ ‘bhang,’ ‘subjee,’ or ‘sidhee,’ consists of the larger leaves and capsules without the stalk. The concrete resinous exudation from the leaves, stems, and flowers, is called ‘churrus,’ and in this country ‘resin of Indian hemp.’ ‘Hashish’ seems843 to be a general term for the preparation of hemp.
Dr Preobraschensky, has lately subjected hashish to a chemical analysis, and states that he has found an alkaloidal body, not only in the commercial substance, but also in the flower-tops of the hemp itself, and the pure extract prepared from it, which was recognised as nicotine.
Indian hemp is anæsthetic, anodyne, exhilarant, antispasmodic, hypnotic, and narcotic. In the East it is commonly used as an intoxicant, either by smoking it, like tobacco, or swallowing it. The inebriation produced by it is of an agreeable or cheerful character exciting the party under its influence to laugh, dance, sing, and to commit various extravagancies. It also acts as an aphrodisiac, augments the appetite for food, and, in some cases, occasions a kind of reverie and catalepsy. In this country its action is less marked. It has here been chiefly administered under the form of alcoholic or resinous extract. See Extract of Indian Hemp.
HEN′BANE. Syn. Hyoscyami Folia (B. P.); Hyoscyamus (Ph. L. E. and D.), L. In pharmacy, “the fresh and dried stalk-leaf of the biennial herb, Hyoscyamus niger” (Ph. L.), or common biennial or black henbane. The first is used for preparing the extract; the last, for the powder and tincture.
Henbane is anodyne, hypnotic, antispasmodic, and sedative. It differs from opium in not being stimulant, and by not confining the bowels; and hence may be administered in cases in which that drug would be improper. In large doses it acts as a powerful narcotic poison, producing obscurity of vision, dilatation of the pupils, delirium, phantasms, coma, &c.—Dose, 3 to 10 gr., in powder. It is usually given in the form of extract or tincture. The antidotes, &c., are the same as those noticed under Opium.
HEN-COOPS, Fumigator for. Consisted wholly of coal-tar.
HE′PAR. Syn. Liver. A name given by the older chemists to various combinations of sulphur, from their brownish or liver colour; as ‘hepar antimonii,’ ‘hepar sulphuris,’ &c. See Antimony (Liver of), Potassium (Sulphide), &c.
HERBAR′IUM. [Eng., L.] Syn. Hortus siccus, L. A collection of dried specimens of plants; hence called HORTUS SICCUS, or dry garden. Plants for the herbarium should be gathered on a dry day, and carried home in a tin-box (‘VASCULUM’), or other convenient receptacle which will preserve them fresh for a time. Those which have collected moisture in their leaves should be allowed to dry, their stalks being placed in water to keep them alive. Plants with very thick, succulent leaves or stems must be killed by immersion in hot water before they can be safely placed in the drying press. The press consists simply of a few stout boards with a screw—or, still better, a number of heavy weights, bricks, or stones—for pressing them together. The specimens of plants, when all superficial moisture has been removed, are placed between layers of bibulous paper (BOTANICAL PAPER), care being taken that the parts of each are arranged in a natural manner. The sheets containing the specimens are then placed between the boards, and pressure is applied. This must be very gentle at first, and should be gradually increased as the plants become dry. The paper is changed every day or every second day, and the damp sheets are dried for use at a future time. When properly dried, the specimens are placed on sheets of writing paper, and fixed by a few stitches of thread, a little gum, or strips of gummed paper. The name of the genus and species, and the locality where found, &c., are then marked beside each. Camphor or a little corrosive sublimate may be used to preserve herbaria from the ravages of insects. The preparation of an herbarium offers an almost endless source of amusement to the ingenious, whilst the specimens so collected, if well preserved, are almost as useful to the botanist as the living plants.
HERBS. Syn. Herbæ, L. The collection and drying of herbs for medicinal purposes and perfumery are noticed under Vegetable Substances.
Amongst cooks, several aromatic herbs, either fresh or dried, are used for seasoning. “In many receipts is mentioned a bunch of sweet herbs, which consists, for some stews and soups, of a small bunch of parsley, two sprigs of thyme, and one bayleaf; if no parsley, then of four sprigs of winter savory, six of thyme, and one bayleaf.” (Soyer.)
HER′NIA. See Rupture.
HER′RING. A well-known small sea-fish, belonging to the family of Clupeidæ, a branch of the order Malacopterygii. As an article of food, herrings are of a vast importance to a large proportion of the population of Europe. When recently caught and dressed by broiling or boiling, they are wholesome and agreeable; but if fried, or long kept, they become strong and oily, and are then apt to offend the stomach. The preparation of salted and dried of smoked herrings (bloaters, red herrings) furnishes employment for thousands, both in these countries and Holland. Real Yarmouth bloaters and Dutch herrings are highly esteemed by many as a relish. Salted herrings are said to be diuretic. The pickle was formerly used in clysters, dropsies, &c. M. Soyer calls this fish “the poor man’s friend,” and tells us that, after being “cleaned and scaled, and the head removed,” it should be “opened in the back, and the gut taken out.” Also that “the way to ascertain if a herring is too salt is to take the fish in the left hand, and pull out a few of the fins from the back, and to taste them. You may thus find out the844 quality and flavour. This plan is adopted by the large dealers.”
HESPER′IDIN. A peculiar substance obtained from the white portion of the rind of oranges, lemons, &c. It forms crystalline silky needles, is odourless, tasteless, fusible, soluble in alcohol and ether, less soluble in water. Hesperidin is a glucoside.
HIC′COUGH (hĭk′-ŭp). Syn. Hiccup; Singultus, L. A convulsive motion of the diaphragm and parts adjacent. The common causes are flatulency, indigestion, acidity, and worms. It may generally be removed by the exhibition of warm carminatives, cordials, cold water, weak spirits, camphor julep, or spirits of sal-volatile. A sudden fright or surprise will often produce the like effect. An instance is recorded of a delicate young lady that was troubled with hiccough for some months, and who was reduced to a state of extreme debility from the loss of sleep occasioned by it, that was cured by a fright, after medicines and topical applications had failed. A pinch of snuff, a glass of iced soda water, or an ice-cream, will also frequently remove this affection.
HI′ERA-PI′CRA. See Powder of Aloes and Canella.
HIP′POCRAS. An aromatic medicated wine, formerly much used in England, and still employed on the Continent.
Prep. Lisbon and Canary wine, of each 12 pints; cinnamon, 2 oz.; white canella, 1⁄2 oz.; cloves, mace, nutmeg, ginger, and galangal, or cardamoms, of each 1 dr.; bruise the spices, and digest them in the wine for three or four days; strain, and add of lump sugar, 21⁄2 lbs.
HIPPU′RIC ACID. HC9H8NO3. Syn. Acidum hippuricum, L. A compound discovered by Liebig in the urine of the horse, cow, and other graminivora, in which it exists as hippurate of potassium or sodium.
Prep. Concentrate fresh cow’s urine by a gentle heat to about 1⁄10th its bulk, filter from deposit, mix the liquid with excess of hydrochloric acid, and set it aside to crystallise. It may be decoloured by redissolving it in boiling water, and treating it with animal charcoal, or with a little chloride of lime along with some hydrochloric acid, and re-crystallising it.
Obs. Hippuric acid, when pure, forms long, slender, milk-white, square prisms; it is soluble in 400 parts of cold water; it also dissolves in hot alcohol. When strongly heated, it yields benzoic acid, benzoate of ammonia, and benzonitrile, with a coaly residue. The urine of horses or cows, left to itself for some time, or evaporated at a boiling temperature, yields not a trace of hippuric acid, but only benzoic acid. Nitric acid and hot oil of vitriol convert it into benzoic acid. Boiling hydrochloric acid converts it into benzoic acid and glycocoll. With the bases it forms salts, which are called hippurates. See Benzoic Acid.
HIPS. Syn. Heps; Rosa canina (Ph. L.). The fresh fruit of the dog rose (Rosa canina), or wild briar. Used to make a conserve.
HOL′LANDS. Syn. Geneva, Schiedam, Hollands gin, Dutch g. Prep. 1. The materials employed in the distilleries of Schiedam, in the preparation of this excellent spirit, are 2 parts of the best unmalted rye and 1 part of malted bigg, reduced to the state of coarse meal by grinding. About a barrel (36 galls.) of water, at a temperature of from 162° to 168° Fahr., is put into the mash-tun for every 11⁄2 cwt. of meal, after which the malt is introduced and stirred, and, lastly, the rye is added. Powerful agitation is next given to the magma till it becomes quite uniform, when the mash-tun is covered over with canvas, and left in this state for two hours. Agitation is then again had recourse to, and the transparent ‘spent wash’ of a preceding mashing is added, followed by as much cold water as will reduce the temperature of the whole to about 85° Fahr. The gravity of the wort at this point varies from 33 to 38 lbs. A quantity of the best pressed Flanders yeast, equal to 1 lb. for every 100 galls. of the mashed materials, is next stirred in, and the whole is fermented in the mash-tun for about 3 days, or until the attenuation is from 7 to 4 lbs. (sp. gr. 1·007 to 1·004). During this time the yeast is occasionally skimmed off the fermenting wort. The wash, with the grains, is then transferred to the still, and converted into ‘low wines.’ To every 100 galls. of this liquor, 2 lbs. of juniper berries (3 to 5 years old), and about 1 lb. of salt, are added, and the whole is put into the low-wine still, and the fine spirit drawn off by a gentle heat, one receiver only being employed. The product per quarter varies from 18 to 21 galls. of spirit, 2 to 3 o. p.
2. (Best Hollands.) Hollands rectified to the strength of 24° Baumé (sp. gr. ·9125, or about 6 o. p.).
3. (English-made.)—a. From juniper berries (at least a year old, and crushed in the hands), 3 lbs.; rectified spirit, 11⁄2 gall. (or proof spirit, 21⁄2 galls.); digest, with agitation, for a week, and then express the liquor; after 24 hours’ repose, decant the clear portion, add it to good corn spirit, at 2 or 3% overproof, 90 or 100 galls., and mix them well together.
b. From juniper berries, 21⁄2 lbs.; sweet fennel seed, 5 oz.; caraway seed, 31⁄2 oz.; proof spirit, 2 galls.; corn spirit, 90 or 100 galls.
c. As the last, with the addition of Strasburg turpentine or Canadian balsam, 1 lb.
d. To either of the last two or three add a very small quantity of ground cardamoms or horse-radish. Some compounders also add 4 or 5 cloves of garlic, or about 15 gr. of assafœtida, with 1 gr. of ambergris rubbed to a powder with a little white sand or lump sugar. Good plain gin may be advantageously employed in lieu of the corn spirit ordered above, when expense is no object.
845
Obs. The last four forms, which are only given as examples, produce a very pleasant spirit, if it is kept for some time to ‘mellow.’ Age is one of the principal causes of the ‘creaminess’ of foreign gin, which usually lies in bond for some time before being consumed. The product is, however, much superior if the ingredients are rectified along with 20 galls. of water, and about 14 lbs. of salt, by a gentle heat.
It will be seen from the above that the superior flavour of Hollands spirit depends more on the peculiar mode of its manufacture than on the quantity of juniper berries employed; 2 lbs. of them, when new, being barely equivalent to 1 oz. of the essential oil; and when old, to less than 1⁄2 oz., a quantity wholly insufficient to flavour 100 gallons of spirit. The Dutch distillers, most noted for this liquor, add a little pure Strasburg turpentine and a handful or two of hops to the spirit, along with the juniper berries, before rectification. The former substance has a pale yellowish-brown colour, and a very fragrant and agreeable smell, and tends materially to impart that fine aroma for which the best geneva is distinguished. At Rotterdam sweet fennel seed is commonly added as a flavouring; and at Weesoppe Strasburg turpentine and fennel seeds, or the essential oil of fennel, are frequently substituted for a large portion of the juniper berries.
Schiedam Hollands is considered the best; the next quality is that of Rotterdam; after these comes that of Weesoppe.
Attempts have been made by Mr Robert Moore, and others to introduce into general consumption in this country a home-made liquor, resembling and prepared in the same manner as foreign geneva, “but the palates of our gin-drinkers were too corrupted to relish so pure a spirit.”
HOMŒOP′ATHY. Syn. Homœopathia, L. A medical hypothesis promulgated at the commencement of the present century by the late Dr Hahnemann, of Leipsic, according to which diseases may be cured by the administration of minute doses of medicines capable of producing in healthy persons affections similar to those it is intended to remove. The doctrine that “similia similibus curantur” had long previously been practically acted on, to a limited extent, in certain cases, in legitimate medicine (allopathy, heteropathy), although not verbally recognised as belonging to its system. The administration of infinitesimal doses is an absurdity which homœopathy, however, alone can claim. According to this method, the millionth of a grain is often an excessive dose; whilst billionths and decillionths, quantities so small as to be vastly beyond human perception, form the common doses. This reduces the whole practice of homœopathy to a system of doing nothing beyond regulating the diet and habits of the patient. “All judicious practitioners have long been agreed that there are many cases which are best treated in the manner just mentioned, and in which physic does more harm than good; in which, in short, a sensible physician endeavours to amuse the patient, whilst nature cures the disorder; so that the frequent success of homœopathic treatment may be explained, without admitting the principle upon which it is presumed to be founded.” (Brande.)
HON′EY. Syn. Mel (B. P.), L. The sweet substance elaborated by the domestic bee from the juices of the nectaries of flowers, and deposited in the cells of wax forming the honeycomb.
Var. Pure honey consists of a syrup of uncrystallisable sugar and crystalline saccharine grains, resembling grape sugar.—‘Virgin honey’ is that which flows spontaneously from the comb.—‘Ordinary honey,’ that obtained by heat and pressure. The former is pale and fragrant; the latter darker, and possessing a less agreeable taste and smell.—‘English honey’ is chiefly collected from furze and broom flowers, and is more waxy than that from the South of Europe;—‘Narbonne honey,’ chiefly from rosemary, and other labiate flowers, very fine;—‘Poisonous honey’ is found near Trebizond, in Asia, its toxic effects being due to the bees having collected it from a poisonous plant, the Azalea pontica.
Pur. Honey is frequently adulterated with treacle, potato-sugar syrup, potato farina, starch, and wheat flour. The first may be detected by the colour and odour; the second in the way noticed under Sugar; and the others by the honey not forming a nearly clear solution with cold water, and striking a blue colour with iodine. When it contains wheat flour, and is heated, it at first liquefies, but on cooling it becomes solid and tough. The absence of starchy matter or flour is easily proved by the following test:—Boiled with water for five minutes, and allowed to cool, it should not become blue with iodine water—indicating absence of flour.
Uses, &c. Honey is nutritive and laxative, but rather apt to gripe. It is employed in the preparation of OXYMELS and GARGLES, and also to cover the taste of nauseous medicines, which it does better than sugar. Clarified honey is alone ordered to be used in medicine.
Honey, Clarified. Syn. Refined honey, Strained h.; Mel depuratum (Ph. D.), Mel præparatum, L. The honey is simply melted by the heat of a water bath, and strained whilst hot through flannel (Ph. D.); or—it is melted as last, and the scum removed (Ph. U. S.); or—it is melted with 1-3rd its weight of water, skimmed, strained through flannel, and evaporated until it reaches the sp. gr. 1·261. (P. Cod.) Honey is not to be employed without being desquamated. (Ph. L.)
Obs. Clarified honey is less agreeable than846 raw honey, and has lost the crystalline character of the latter; but it is less liable to ferment and gripe. The use of copper and iron vessels or implements should be avoided, as honey acquires a dark colour by contact with them. Berlin-ware, stone-ware, or well-silvered or tin copper pans, should alone be used. On the large scale, one or other of the following plans are adopted:—
1. The honey is mixed with an equal weight of water and allowed to boil up 5 or 6 times without skimming; it is then removed from the fire, and after being cooled, brought on several strong linen strainers, stretched horizontally, and covered with a layer of clean and well-washed sand, an inch in depth; the sand is rinsed with a little cold water, and the mixed liquor is finally evaporated to the thickness of syrup.
2. Dissolve the honey in water, as last, clarify with white of egg, and evaporate to a proper consistence.
3. Dissolve in water, add 11⁄2 lb. of animal charcoal to every 1⁄4 cwt. of honey, gently simmer for 15 minutes, add a little chalk to saturate excess of acid, if required, strain or clarify, and evaporate.
4. Honey, 1 cwt.; water, 9 galls.; fresh burnt animal charcoal, 7 lbs.; simmer for 15 minutes, add a little chalk to saturate free acid (if required), strain or clarify, and evaporate as before.
HONEYS. (In pharmacy.) Syn. Melita, L. These are minor preparations, now almost superseded by ‘syrups’ (Syrupi). The mellita of the Ph. L., including two ‘oxymels,’ are only four in number.
Honey of Bo′′rax. Syn. Mel boracis (B. P. Ph., L. E. & D.), L. Prep. (B. P.) Finely powdered borax, 1; clarified honey, 7; mix. Astringent, detersive, and cooling. It is employed in aphthæ of the mouth, excessive salivation, &c. A great improvement would be to dissolve 1 of borax in 1 of glycerin, and then add 6 of honey.
Honey of Col′chicum. Syn. Mel colchici, L. Prep. (Beasley.) Dried colchicum 1 part; water (at 140°), 16 parts; infuse for 12 hours, strain, let it settle, and boil the clear liquor with white honey, 12 parts, to the consistence of a syrup. See Colchicum.
Honey of Liq′uorice. Syn. Mel glycyrrhizatum, L. Prep. (Ph. Hamb.) Honey and a strong infusion of liquorice boiled to a proper consistence. Emollient, pectoral, and laxative.
Honey of Male Fern. Syn. Mel filicis, L. Prep. (Dunglison.) Ethereal extract of male fern, 30 gr.; honey of roses, 4 dr.; mix. In tapeworm.—Dose. One half at bedtime, followed by the remainder in the morning.
Honey of Mercury. Syn. Mel hydrargyri, L. Prep. (Bell.) Mercury, 1 dr.; honey, 1 oz.; triturate till the globules disappear. Allard adds of oil of cloves, 1⁄2 dr. Properties similar to those of mercurial pill. It is chiefly used as an application to ulcers of the throat.
Honey of Ro′′ses. Syn. Mel rosæ (Ph. L. and E.), L. Prep. 1. (Ph. L.) Dried petals of the red rose (the leaves separated), 4 oz.; boiling water, 16 fl. oz.; macerate for 2 hours; lightly press them in the hand, and strain; then add 8 fl. oz. more of boiling water to the roses, macerate for a short time, and again gently express the liquor; to this add the other half; next add to the mixed liquors, honey, 5 lbs.; and evaporate in a water bath, so that, the infusion which was set aside being added, it may become of a proper consistence.
2. (Ph. E.) Dried rose petals, 4 oz.; boiling water, 21⁄2 pints; infuse for 6 hours, and gently squeeze out the liquor; after the impurities have subsided, decant the clear, add of honey, 5 lbs., and evaporate as before, to a proper consistence, removing the scum which forms. Used to make astringent gargles. It must not be boiled in a copper or iron vessel, as they will spoil the colour. The last form is that commonly adopted in trade.
Honey of Squills. Syn. Mel scillæ, L. Prep. 1. Thick clarified honey, 3 lbs.; tincture of squills, 2 lbs.; mix.
2. (Soubeiran.) Dried squills, 1 oz.; boiling water, 3⁄4 pint; infuse 2 hours, strain, add of honey, 12 oz.; and evaporate to a proper consistence. Resembles OXYMEL OF SQUILLS (nearly).
Honey of Verdigris. Egyptiacum.
Honey of Vi′olets. Syn. Mel violæ; L. Prep. From clarified honey, 2 parts; expressed and depurated juice of violets, 1 part. Resembles syrup of violets.
HON′EY DEW. Syn. Ros mellitus, L. A sweetish matter ejected upon the leaves of plants by certain aphides.
HOOP′ING COUGH. See Whooping Cough.
HOPS. Syn. Lupulus (B. P.), L. “The catkins of the female plant of the Humulus lupulus” or common hop. (B. P.) “The dried strobiles.” (Ph. D.) The hops of commerce are the strobiles or catkins (LUPULI STROBILI, L. AMENTA) of the hop plant. The yellow powder or small lupulinic grains or glands (LUPULIN), which are attached to the strobiles, are the portion on which their characteristic qualities chiefly depend.
The hop is tonic, stomachic, and moderately narcotic. It is used in diseases of local debility with morbid vigilance and other nervous derangement, producing sleep where opiates are objectionable. Hops may be used topically as a fomentation or a poultice, as a resolvent or discutient in painful swellings and tumours. The golden dust attached to the scale of the hop is sometimes administered in doses of from 5 to 10 grains. Very freshly dried hops, made into a pillow, procure sleep.
In the choice of hops, care should be taken to select those that have large cones or strobiles, that are the most powerfully odorous847 and most free from leaves, stems, scaly fragments, and sticks, and which, when rubbed between the hands, impart, in the greatest degree, a yellowish tint and glutinous feeling to the skin. The tightness with which they are packed should also be noticed; as, without being very firmly pressed together, and quite solid they soon spoil by keeping. The finest flavoured hops are the ‘GOLDINGS,’ grown chiefly in middle and east Kent; the ‘WHITEBINES’ of Farnham and Canterbury; and the Worcester hops, grown on the red soils of the vale of the Severn. These are principally employed for the finer class of ales. Mid Kent and Sussex hops are also used for ale, but have an inferior colour and flavour. The best hops are packed in sacks of fine canvas, termed ‘pockets,’ weighing from 11⁄4 cwt. to 13⁄4 cwt. each; and the inferior qualities in coarse ‘bags,’ of about double the size. The former are mostly purchased by the ale brewers, and the latter by the porter brewers. When hops are older than of last season’s growth they are termed ‘yearlings,’—when of the second season’s growth, ‘old,’—and when three years, or older, ‘old olds.’ See Brewing, Extract, Humulin, Lupulin, Tincture, &c.
HOOSE. Young cattle, especially calves, as well as sheep and lambs, are frequently liable to attacks of a species of bronchitis, caused by the presence in the bronchial tubes of minute worms. They are mostly so attacked in autumn.
Treatment. For a calf of six months old give half an ounce of oil of turpentine in two ounces of linseed oil, to be repeated once or twice after an interval of two days. Half this dose may be given to sheep. The mixture should be administered by the mouth, and not by the nostrils, as usually recommended. Calves should additionally be comfortably housed at night, and be fed with a little oil cake and other good food.
HORE′HOUND. Syn. White horehound; Marrubium vulgare (Linn.), L. This herb has long been a popular remedy in chronic pulmonary complaints, especially catarrh, and in uterine and liver affections. Horehound tea (THEA MARUBII, INFUSUM MARUBII) is prepared by infusing 1 oz. of the herb in boiling water, 1 pint, for an hour;—syrup of horehound (SYRUPUS MARUBII), by thickening the infusion of tea with sugar;—candied horehound (MARUBIUM CONDITUM), by mixing 1 pint of horehound juice with 8 or 10 lbs. of white sugar, boiling the mixture to a candy height, and pouring it, whilst warm, into moulds, or small paper cases, well dusted with finely powdered lump-sugar; or by pouring it out on a dusted slab, and cutting it into squares. See Candying.
HORN. For the purposes of the turner and comb-maker, horns of the goat and sheep are preferred on account of their superior whiteness and transparency. For medical purposes, those of the stag (HARTSHORN) are ordered to be employed.
Horn is dyed with the same dyes, and in a similar manner to bones and ivory.
Horn is softened, bent, and moulded, by means of heat and pressure. For these purposes boiling water and a screw press are commonly employed.
Horn is reduced to plates or sheets by sawing it, and then exposing it to powerful pressure between hot iron plates; the pith having been previously removed, and its texture softened by soaking for some days in water, and subsequent boiling in that liquid.
Surfaces and edges may be united or cemented together by softening the horn by the heat of boiling water, placing the parts in contact under strong pressure, and exposing the whole thus arranged to the heat of boiling water.
Horn is stained or party-coloured to imitate tortoise-shell, by a solution of terchloride of gold, for the red portion; nitrate of silver, for the dark brown and black; and nitrate of mercury (hot), or a paste made of red lead, and potash or quicklime, for the brown. When the last is used the horn must be heated and exposed to its action for some hours.
Horn Silver. (Ag. Cl.) A native chloride of silver, which occurs either crystallized in cubes, or as a compact semi-transparent mass.
HORS-D’ŒUVRES. [Fr.] Syn. Assiettes, Fr. Small entrées, as ‘aiguillettes,’ ‘ragouts,’ plates of sardines, anchovies, or other relishes, served at dinner between the leading dishes. ‘Assiettes volantes’ (flying plates) are dishes handed round to the guests, but not placed on the table.
HORSE. Syn. Equus, L. This most useful quadruped belongs to the family Equidæ, distinguished by a single digit and hoof on each foot. The horse can scarcely be said to exist at the present day in its natural wild state, as the so-called ‘wild-horses’ of America and Asia are but the progeny of horses which have escaped from the haunts of civilisation. Of all animals the horse is most useful to man. Independently of its value as a beast of burden and draught, its skin, its hide, intestines, and bones, furnish us with leather, the thongs of whips, gut, grease, bone-black, manure, &c. The excrement, fat, and hoof were included in the Materia Medica of the Ph. L. 1618. The flesh is eaten in some countries, and was formerly esteemed to possess many virtues.
Injuries of a serious character, and even death, are often occasioned by horses running away, or becoming unmanageable. Various methods have been proposed to prevent accidents of this kind, and to place the animal entirely under the power of its rider or driver. In Russia, around the horse’s neck, near the neck strap, is placed a cord with a running knot. To this slip-noose is attached a pair of848 reins, which always lie thrown over the dashboards, ready to be seized at once. When the horse starts, and becomes unruly, the gentleman takes up this cord, and tightens the horse’s throat, so that he cannot take breath. The most furious horse stops instantly, and will not fall or kick. See Bedding, Bran Mash, Broken Knees, Broken Wind, Clipping, Canker, Catarrh, Choking, Chorea, Cholic, Constipation, Corns, Crib-biting, Curb.
HORSE BALLS. See Veterinary Medicine.
HORSES, Condition Powder for. The principal ingredients were: Fenugreek, liquorice root, resin, brimstone, common salt, nitrate of potash, and a green powder, probably senna. It contained traces of calcium and magnesium carbonates; alumina, silica, and iron.
HORSE POW′ER. This term was first employed by James Watt to express a power capable of raising 33,000 lbs. one foot high per minute. The effective pressure on the surface of the piston was estimated at 7 lbs. to the square inch, and hence the area of the piston, in square inches, multiplied by 7, gave the gross effective moving pressure, and the space passed over by this piston in a minute gave the distance through which the pressure was exerted, or the weight was raised. From these data the horse power was easily calculated. In process of time improvements in the formation of boilers and steam engines increased the effective pressure on the piston, and, consequently, the power of the engine. In modern engines the actual power is commonly from 2 to 4 times greater than the nominal power, which is, however, still retained as the unit of power in commercial calculations.
HORSERAD′ISH. Syn. Armoracia radix. (B. P.). “The fresh root of Cochlearia Armoracia” (B. P.). Horseradish is pungent, acrid, stimulant, and rubefacient. It is also regarded as diaphoretic, diuretic, and antiscorbutic. It forms a useful masticatory in hoarseness, sore throat, and toothache. As a condiment, it provokes the appetite and assists digestion. Reduced to shreds (scraped horseradish), it forms a common and excellent accompaniment to roast beef. The root of aconite or wolfsbane, which somewhat resembles it in appearance, has occasionally been mistaken for it, with fatal results; the two are, however, readily distinguished from each other, as the taste of horseradish is warm and pungent, approaching that of mustard, whilst aconite is bitter, and its odour is earthy and disagreeable, and after a few minutes’ contact with the lips, tongue, and fauces, produces a sensation of numbness, and tingling. See Aconitum Napellus; under which article will be found engravings of the two roots. The root may be kept fresh for some time if buried in sand in a cool place. Horseradish powder is prepared from the roots gathered in November or December, and dried by a gentle heat or exposure to a current of dry air. It is used as a condiment.
HOR′TICULTURE. Syn. Gardening. The art of cultivating gardens. According to Loudon, horticulture differs from agriculture, chiefly in the comparatively limited space over which it extends, and in being conducted by manual labour; whilst the latter is performed jointly by human and animal labour, in fields, or on an extensive tract of land called a farm.
HOR′TUS-SICCUS. See Herbarium.
HOS′PITAL GAN′GRENE. Syn. Phagedæna gangrenosa. L. A species of ulcerating mortification, particularly characterised by its infectious nature, and its tendency to attack wounds and ulcers in crowded hospitals, so that often the most trifling operation cannot be performed with safety. Under its influence the parts are rapidly destroyed, not by the formation of ordinary sloughs, as in common mortification, but by their conversion into an ash-coloured viscid substance interspersed with bloody specks. The treatment is similar to that noticed under Mortification, but here, above all things, thorough ventilation must be established, and persevered in, and, when possible, change of situation sought.
HUILE. [Fr.] Oil; a term applied to various substances and preparations on account of their smoothness, consistence, or real or imaginary emollient or oleaginous nature. See Liqueur, Oil, &c.
Huile Acoustique. Prep. From garlic and bay leaves, of each, 1⁄2 oz.; olive oil, 1⁄2 lb.; boiled together for 15 minutes, and strained. Used in ear-ache and deafness. A little is dropped on cotton wool and placed in the ear.
Huile, Antique. See Oils (Hair).
Huile Liqueureuse. Prep. 1. (De la Rose.) From eau de rose, 1 part; simple syrup, 2 parts; mixed together.
2. (Des fleurs d’oranges.) From orange-flower water and syrup, as No. 1.
3. (De vanille.) From essence of vanilla, 1 dr.; simple syrup, 1 pint.
Obs. The above are kept in small decanters, and used to flavour water, grog, liqueurs, &c., instead of sugar or capillaire; also to perfume the breath. Other flavoured syrups, for the same purposes, are prepared in a similar manner.
HU′MIC ACID. Syn. Ulmic acid. See Humus.
HUMULIN. The name given to a beautiful extract or essence of hops, made as follows:—
A concentrated tincture of hops is prepared by percolation with rectified spirit; the same hops are then exhausted with water; the spirit is removed from the tincture by careful distillation, and the upper aqueous portion is skimmed off, and added to the infusion, which latter is then evaporated to the consistence of a soft extract; the oleo-resinous residuum of the tincture is next added, and well mixed in;849 after which the whole is put into pots and carefully tied over for sale. The product possesses all the fragrant, tonic, and bitter qualities of the hop in a highly condensed form. See Hops, Lupulin, &c.
HU′MUS. Syn. Ulmin. When wood, or woody fibre, is exposed to the joint action of air and moisture, it suffers eremacausis or decay, and crumbles down into a dark-brown or black powder commonly called ‘mould,’ and to which chemists have given the name of ‘humus.’ In this state it exists in fertile soils, in which it is derived from the decay of plants. A powder of similar composition is produced by the action of powerful chemical reagents on sugar, lignin, &c. When acted upon by dilute boiling solution of caustic potassa, this substance yields a deep-brown solution, from which acids precipitate a flocculent brown substance generally called ‘ulmic’ or ‘humic acid.’ Both bodies require further investigation, as they are supposed to vary exceedingly in composition.
HUNGER. The peculiar sensation arising from the want of food. When severe, it increases to actual pain, the coats of the stomach are acted on by its own juices, the respiration becomes less frequent, the circulation languid, and there is a general diminution of the heat of the body and of the secretions. The return of hunger is accelerated by exercise and labour, and by the exposure of the body to a low temperature. Long fasting is injurious, more particularly to the young and the debilitated. See Appetite, Nutrition, &c.
HUS′BANDRY. The business of the farmer; by some the term is restricted to the joint operations of farming and gardening on the small scale. It is also sometimes used synonymously with agriculture.
HY′ACINTH. In botany, the English name for the genus Hyacinthus. There are numerous varieties of the garden hyacinth, all very beautiful. The bulbs are largely imported from Holland, and are often grown in water contained in suitable glass vessels (hyacinth glasses). In mineralogy, the term is applied to crystallised yellow or brown zircon. See Gems.
HYDRAC′IDS. Syn. Hydrogen acids. A name formerly given to those acids which do not contain oxygen, as hydrochloric, &c. It is still occasionally employed.
HY′DRAGOGUES. Syn. Hydragoga, L. Medicines which cause the removal of water from any of the cavities of the body. Many cathartics, as gamboge, jalap, &c., are classed under this head.
HYDRAS′TIN. The name given to a concentrated remedy much employed by the medical eclectics of America.
Prep. Treat the powdered root of golden-seal (Hydrastis Canadensis) with cold water by percolation; acidulate the infusion with hydrochloric acid; collect the precipitate on a filter; then dry it, dissolve the dried mass in alcohol, filter, and set aside to crystallise.
Prop. Yellow, acicular crystals, insoluble in cold alcohol, ether, and water.—Dose, 3 to 5 gr., 3 to 6 times a day; as a tonic in dyspepsia, inflammation of the stomach, &c.—Obs. According to the most recent investigations, hydrastin contains berberine, and another alkaline called hydrastia or hydrastina.
HYDRASTIS CANADENSIS. Syn. The Golden Seal. This is a small herbaceous perennial North American plant, belonging to the natural order, Ranunculaceæ. The rhizome, which is the officinal part, though yellow in the recent root, becomes of a dark yellowish-brown by age. It contains albumen, starch, fatty matter, resin, yellow colouring matter, sugar, lignin, and various salts; also a peculiar nitrogenous crystallisable substance, to which Dr Durand, the discoverer, proposed the provisional name of hydrastin, which substance will be found described below. The root of the golden-seal, as well as the alkaloids obtainable from it, are largely used in American medical practice, and are stated to possess valuable tonic, aperient, diuretic, and deobstruent powers. They have been employed in dyspepsia, jaundice, and functional disorders of the liver. They are also regarded as one of the best substitutes for quinine in intermittents.
Golden seal has been given in the form of infusion, decoction, tincture, and extract, and the fluid extract is now officinal in the United States’ Pharmacopœia.
HY′DRATES. Compounds of hydroxyl (HO) with other bodies, e.g. KHO—hydrate of potassium. The term hydrate is also given to chemical combinations of water (H2O) with other substances, e.g. C2HCl3O.H2O—hydrate of chloral.
HY′DRIDE. A compound of hydrogen with another radical, e.g. hydride of methyl—CH3H.
HYDRIO′DATE. A name formerly given to the salts now termed iodides. See Iodides.
HYDRIO′DIC ACID. Syn. Iodhydric acid; Acidum hydriodicum, L. An acid compound of iodine and hydrogen. See Iodine.
Prep. 1. By heating iodine in hydrogen, the volume of the gas becomes doubled, and a colourless acid gas is produced; it is, however, never prepared for use by this means. 2. Place 10 parts of potassic iodide in a small retort with 5 parts of water, and add 20 of iodide; then drop in cautiously one part of phosphorus, cut into small fragments, and apply a gentle heat. The gas will be given off abundantly and may be collected, by displacement, in dry bottles.
A solution of hydriodic acid may be prepared by suspending iodine in water, and passing a current of sulphuretted hydrogen through the mixture until the brown colour of the850 iodine disappears; sulphur is deposited in abundance, and hydriodic acid formed.
HYDRO′BENZANIDE. White crystalline mass, obtained from oil of bitter almonds by treatment with ammonia.
HYDROBRO′MIC ACID. See Bromide.
Hydrobromic Acid. (HBr.) Syn. Hydric Bromide, Hydrogen Bromide.
Prep. This very powerfully acid gaseous body may be prepared as follows:—1. By decomposing bromide of potassium with a concentrated solution of phosphoric acid. 2. By decomposing bromide of phosphorus by means of a small quantity of water.
Hydrobromic acid gas is colourless and non-inflammable; it extinguishes flame. It is extremely irritating to the lungs when breathed. It is very soluble in water.
HYDROBRO′MIDE. Syn. Bromide (which see).
HYDROCAR′BON. A compound of carbon and hydrogen. The hydrocarbons constitute a most important series of organic compounds.
HYDROCHLORIC ACID. (HCl = 36·5.) Syn. Muriatic Acid, Hydric Chloride, Hydrogen Chloride. This important gaseous compound was discovered by Priestly in 1772. In nature it is given off with other gases from active volcanoes, and is occasionally to be met with in the springs and rivers of volcanic districts. When hydrogen and chlorine are mixed in equal volumes, they are without action upon each other if kept in the dark, but if exposed to direct sunlight, chemical combination, accompanied by a loud explosion, instantly takes place between them, the result of their union being the colourless gaseous, intensely sour hydrochloric acid. If, instead of bright sunshine, the mixed gases are exposed to diffused daylight, chemical union also ensues between them, but the process is then a slow and gradual one; the passage through them, however, of the electric spark, or the application of a lighted match or taper instantly causes their explosion and combination.
One volume of chlorine unites with one volume of hydrogen, forming two volumes of hydrochloric acid; no condensation occurs in the act of union.
Hydrochloric acid may also be formed by transmitting moist chlorine through a red-hot porcelain tube; oxygen being at the same time liberated.
Prep. Hydrochloric acid, save for the purposes of illustrative experiment, is never obtained by any of the above processes. An easy mode of procuring it, when required for laboratory use, is to heat the ordinary aqueous solution of the acid in a flask, and to collect the gas, which is given off by displacement. It may also be readily got by introducing pieces of common salt (which should have been previously fused in a crucible at a red-heat and allowed to cool) into a glass retort, and pouring over them about twice their weight of oil of vitriol. The hydrochloric acid, which escapes very abundantly, must be collected either by displacement or over mercury.
Prop. Hydrochloric acid is a colourless gas, very acid to the taste, and irritating to the eyes; and induces coughing even if breathed in small quantities, or when largely diluted. It is very destructive to vegetation, on which account the soda manufacturer is compelled by law to condense and thus prevent the escape of its fumes. It has a specific gravity of 1·261. When subjected to a pressure of 40 atmospheres at 50° F., it becomes a colourless fluid capable of dissolving bitumen, and having a specific gravity of 1·27. It has never been frozen. Hydrochloric acid neither burns, nor supports combustion. The white fumes which it forms when exposed to the air, are due to its condensing the atmospheric moisture, and thus giving rise to a body less volatile than water. This gas is greedily and instantly absorbed by water. A fragment of ice placed in a jar of the gas absorbs it, and becomes immediately dissolved.
Hydrochloric Acid, Solution of. The hydrochloric acid of commerce is a solution of the above gas in water. When exposed to the air it emits grey fumes. Water at 40° F. absorbs about 480 times its bulk of hydrochloric acid, increasing in volume about one third in doing so, acquiring a density of 1·2109, and then containing nearly forty-three per cent. of the acid.
Strength of Solution of Hydrochloric Acid, 77° Fahr. (E. Davy.)
| Sp. Gravity. | Hydrochloric acid in 100 parts. | Sp. Gravity. | Hydrochloric acid in 100 parts. |
| 1·21 | 42·43 | 1·10 | 20·20 |
| 1·20 | 40·40 | 1·09 | 18·18 |
| 1·19 | 38·38 | 1·08 | 16·16 |
| 1·18 | 36·36 | 1·07 | 14·14 |
| 1·17 | 34·34 | 1·06 | 12·12 |
| 1·16 | 32·32 | 1·05 | 10·10 |
| 1·15 | 30·30 | 1·04 | 8·08 |
| 1·14 | 28·28 | 1·03 | 6·06 |
| 1·13 | 26·26 | 1·02 | 4·04 |
| 1·12 | 24·24 | 1·01 | 2·02 |
| 1·11 | 22·22 |
In the laboratory, solution of hydrochloric acid is in constant use. It may be easily prepared from chloride of sodium and sulphuric acid. The retort should be connected with a couple of Woulfe’s bottles; into the first of which a small quantity of water should be poured, to detain any impurities mechanically carried over with the gas;851 the second bottle should contain four parts of water, and should be placed in a vessel of cold water, as the gas in becoming condensed, disengages a large amount of heat. The gas comes off and is absorbed readily by the water upon applying a gentle heat to the retort.
It is by this last method that solution of hydrochloric acid is obtained in such enormous quantities[347] for the various purposes in which it is used, in the arts and manufactures.
[347] In South Lancashire alone, more than 1000 tons of hydrochloric acid in solution are made weekly.
Hydrochloric acid is, in fact, a by-product in the manufacture of carbonate of soda, and is generated during the first stage of the operation, known as the salt-cake process, which consists in the decomposition of salt by sulphuric acid, and is accomplished in a furnace called the salt-cake furnace.
The hydrochloric acid gas which is given off escapes from the furnace through a flue with the products of combustion into high brick towers filled with coke or stones, over which a stream of water trickles down, the whole of the acid vapours are thus condensed, the smoke passing off by a chimney connected with the towers. The diluted acid solution thus formed is concentrated by the aid of the apparatus shown in section in figs. 1, 2, and 3.
This apparatus consists of several cast-iron cylinders, 57 feet long by 27 feet in diameter, closed in the same manner as gas retorts, by lids luted with clay. One of the lids has an opening o, into which is fitted the stoneware or leaden pipe a, conveying the hydrochloric acid to the condensing apparatus. The other, or posterior lid, is also provided with an opening d, through which is passed the tube of a leaden funnel, so that after the retort is filled with salt sulphuric acid may be poured in. The construction of the furnace in which two retorts are usually placed, permits the flame of the fire at O to play round the cylinders before reaching the flue leading to the chimney F. B is an arch over the furnace. The first stage of the operation consists in filling each cylinder with 330 lbs. of salt. The lids or covers are then luted on, and the fire is kindled. The requisite quantity of strong sulphuric acid is next poured into the retort, and the funnel having been withdrawn from D, the hole is covered by a clay plug.
 Fig. 1.
Fig. 1.
As soon as the reaction is over, the 396 lbs. of sulphate of soda produced are removed, and the operation repeated.
The condensation apparatus 1 and 3 is composed of rows of Woulfe’s bottles, partly filled with water, care being taken to place the first pairs of these bottles in a tank of cold water.
The condensation of the last portions of hydrochloric acid gas is effected either by the aid of the coke columns, or in leaden chambers, into which fine jets of cold water are injected on all sides.
“A saturated solution of hydrochloric acid in water has the specific gravity of 1·21; and when heated in a retort, loses at first hydrochloric acid gas, but after a time an aqueous acid distils over, at the ordinary atmospheric pressure, containing 20·22 per cent. of hydrochloric acid, and boiling constantly at 110° C. If the distillation be conducted under diminished pressure, the liquid boils at a lower temperature, and attains a composition which is different for each boiling point; hence the dilute acids thus obtained by boiling the solution of hydrochloric acid gas in water, cannot be considered as definite compounds of hydrochloric acid and water.”[348]
[348] Roscoe and Dittmar.
852
 Fig. 2.
Fig. 2.
 Fig. 3.
Fig. 3.
Commercial hydrochloric acid is usually of a yellow colour owing to its being contaminated with iron. It also very frequently contains sodium, arsenic, sulphuric and sulphurous acids, and free chlorine.
Pure aqueous solution of hydrochloric acid should leave no residue upon evaporation; it should give no precipitation of ferric oxide when saturated with ammonia, sulphuretted hydrogen should cause no turbidity in it; if diluted with three or four times its volume of water, and chloride of barium be added, no white cloud or precipitate should form in the mixture; nor should the acid, if pure, discolour a fluid made faintly blue with iodide of starch.
Hydrochloric acid is largely consumed in the manufacture of chlorine, sal ammoniac, chloride antimony, glue, phosphorus, in the preparation of carbonic acid for the manufacture of artificial mineral waters, in beet-root sugar works, hydro-metallurgy, and alone, or mixed with nitric acid, for dissolving various metals.[349] See Acids, Effects of Vegetation on, Chlorine.
[349] Wagner.
HYDROCHLORIC ETHER. (C2H5Cl.) Syn. Ethyl Chloride, Chloride of Ethyl. This ether may be obtained either by saturating alcohol with hydrochloric acid gas, and then distilling at a gentle heat, or by distilling a mixture of three parts of oil of vitriol, two of alcohol, and four of fused chloride of sodium; the retort is in either case connected with a tubulated receiver, surrounded by water at a temperature of about 68° Fahr., in which most of the alcohol and water which pass over during the operation become condensed, whilst the ether escapes in the form of vapour through a bent tube, which is inserted into the tubulure of the receiver, and passes to the bottom of a flask kept cool with ice. The liquid which is condensed in the flask must be rectified from calcic chloride.
Hydrochloric ether is a colourless liquid, having a specific gravity at 32° Fahr. of 0·921, and a boiling point of 51·9° Fahr. The specific gravity of its vapour is 2·219. It has an ethereal, penetrating, somewhat garlicky odour. It is sparingly soluble in water, but readily so in alcohol. These solutions fail to give a precipitate with argentic nitrate.
HYDROCYANIC ACID. (HCN HCy.) Syn. Prussic acid, Hydric cyanide, Cyanhydric acid. Hydrocyanic acid was discovered by Scheele; but its nature and chemical properties were first investigated by Gay-Lussac.
Sources. This acid is found in water distilled from the kernels of the apricot, the peach, the plum, and cherry, the leaves of the laurel, and some other shrubs. The kernels of the bitter almond also yield it by distillation, mixed with an essential oil. The juice of the tapioca plant (the Jatropha manihot) likewise contains it. Many nitrogenous substances,853 when submitted to destructive distillation, also evolve hydrocyanic acid. Crystallised ammonic formiate subjected to heat in a retort yields a vapour which, passed through a red-hot tube, decomposes into this acid and water. Another method by which it may be obtained, consists in sending a current of dry sulphuretted hydrogen gas through a long tube filled with cyanide of mercury; and very recently it has been obtained by the direct combination of nitrogen and acetylene gas, by adding one volume of the former to two of the latter, and passing a series of electric sparks through the mixture, the gases combining without condensation. Lastly, it is yielded when a metallic cyanide or ferrocyanide is decomposed by an acid, this latter being the means by which it is invariably procured.
1. Anhydrous hydrocyanic acid may be prepared by Wöhler’s plan, which is as follows:—A crude potassium cyanide is prepared by fusing eight parts of the dried potassium ferrocyanide with three of potassium carbonate and one of charcoal.
The fused mass is treated with six times its weight of water in a well-closed vessel; the clear liquid is decanted from the iron, which it is the object of this operation to separate, and is poured into a retort: sulphuric acid, diluted with an equal weight of water, is gradually added in the proportion of one part of oil of vitriol to two parts of the cyanide. At first the distillation proceeds spontaneously from the heat developed by the admixture of sulphuric acid with the water. In order to condense the acid, the products are made to pass through a long U-shaped tube, immersed in cold water and filled with calcic chloride, with the exception of the first fourth of the tube, which contains fragments of the crude potassium cyanide; to the bent tube is attached a second delivery tube, which passes to the bottom of a bottle cooled with ice and salt. The calcic chloride in the syphon tube retains the moisture, and the potassic cyanide any sulphuric acid that might chance to pass over, whilst the hydrocyanic acid collects in the anhydrous state in the cooled receiver.
Trautwein recommends it to be prepared by the dehydration of the strong aqueous acid, by means of fused and pulverised chloride of calcium.[350]
[350] The details of this process are given in ‘Watt’s Chemical Dictionary.’
⁂ The observance of the greatest care and caution are necessary in the preparation of this most potent poison. The operation is most safely performed in winter. The apparatus should be so arranged as to allow of any vapours given off being carried from the operator by a brisk current of air.
Prop. At ordinary temperatures anhydrous hydrocyanic acid is a colourless liquid, having a specific gravity of 0·7058 at 44·6° Fahr. It is very inflammable, burning with a violet flame resembling that of cyanogen, but somewhat whiter in colour. It is soluble in all proportions in water, the resulting mixture being lighter than that fluid, and miscible with alcohol. It is very feebly acid; potassic cyanide always having an alkaline reaction. Red oxide of mercury is readily dissolved by it, and when added to a solution of argentic nitrate it precipitates white flocculi of cyanide of silver. Anhydrous hydrocyanic acid is an extremely volatile liquid; if a drop be let fall on a glass plate, part of it becomes frozen by the cold produced by its own evaporation.
2. Preparation of aqueous hydrocyanic acid.
a. From hydrated ferrocyanide of potassium.—By heating it in a glass retort with oil of vitriol and water, Everitt states that the best proportions are nearly ten parts of the salt to seven of oil of vitriol (diluted with any convenient amount of water). Adopting these proportions, 422·4 parts of ferrocyanide of potassium yield 81 parts of hydrocyanic acid. The greater part of the hydrocyanic acid passes over at the beginning of distillation, at a temperature a little above 212° Fahr.; and when the residual liquid reaches a higher temperature, the water (which then contains but little hydrocyanic acid) is then carried over. It is therefore necessary to employ a good condensing apparatus, or the hydrocyanic acid which passes over at first will for the most part be dissipated in vapour mixed with the air of the apparatus. This loss may also be obviated by placing water in the receiver. The residue need not be boiled down to dryness; it will be found best to distil off from two thirds to three fourths of the liquid, according to the amount of water present.
It is not necessary to dissolve the ferrocyanide in water previous to adding the sulphuric acid, as it readily dissolves in the water during the process of distillation.
Three conditions are important to be observed in the arrangements of the apparatus:—1. The mixture in the retort should not be allowed to spirt over. 2. It should contain but little air. 3. It should present the greatest possible amount of surface to be cooled.
If sulphate of potassium and prussian blue are spirted over into the distillate, this must be carefully rectified over a small quantity of magnesia, chalk, or carbonate of barium.
b. From cyanide of potassium (without distillation).—To a solution of nine parts of tartaric acid in sixty parts of water, contained in a well-stoppered bottle nearly filled with it, four parts of pure cyanide of potassium are added; the vessel is shaken, frequently dipped into cold water, and then left in the cold for twelve hours; and the aqueous hydrocyanic acid, which contains but a very small quantity of tartrate of potassium, is poured off from the crystallised tartrate.[351] This acid contains 3·6 per cent. of anhydrous hydrocyanic acid.
[351] ‘London Med. Surg. Journ.,’ vi, 524.
854
c. From cyanide of mercury.—126 (accurately weighed) parts of cyanide of mercury are agitated with at least 28 parts of iron filings, in a well-stoppered bottle, containing 49, or rather more, parts of oil of vitriol, diluted with a considerable quantity of water. The agitation is continued until a portion of the liquid taken out is not blackened by sulphuretted hydrogen.
The solution, decanted from the iron and mercury, is then placed in a retort and distilled; the acid coming over at a gentle heat. Excess of iron accelerates the decomposition.
d. From cyanide of silver.—Everitt recommends 200 parts of pure cyanide of silver to be shaken up with 240 parts of hydrochloric acid of specific gravity of 1·129, and when the decomposition is complete, the hydrocyanic acid to be separated from the chloride of silver by decomposition.
This hydrocyanic acid may contain a small quantity of hydrochloric acid, a not very objectionable admixture, since it retards decomposition. It possesses the advantage of definite strength.
Prop. The aqueous is very similar in properties to the anhydrous acid, differing in taste, odour, poisonous and combustible properties, according to its degree of concentration. Like the anhydrous, the aqueous acid decomposes, but not so readily; when perfectly pure, becoming brown, and at last black. As before stated, a little free mineral acid assists to preserve it. It should be always kept in a dark place.
Detection and estimation of hydrocyanic acid and soluble cyanides.—The presence of hydrocyanic acid, indicated by the characteristic smell, which is given off by the contents of the stomach, or of any fluid containing it (provided this is not disguised by any substance of stronger odour) may be confirmed by the following tests:
1. To the filtered suspected fluid add a slight excess of caustic potash, and then a solution containing ferrous and ferric sulphates. If hydrocyanic acid, or a soluble cyanide be present, upon the addition of an excess of hydrochloric acid the liquid turns to a blue colour (more or less intense according to the quantity of acid present), owing to the formation of Prussian blue.
2. Add the suspected fluid solution of nitrate of silver; if hydrocyanic acid be present, a white cyanide of silver is formed, which is nearly insoluble in cold nitric acid, but is soluble in ammonia and cyanide of potash, and which, when heated to redness, gives off the inflammable violet-flamed cyanogen.
3. Acidulate a small quantity of the suspected liquid with a few drops of hydrochloric acid, and place it in a watch-glass; then invert a second watch-glass, moistened with a drop of solution of ammonic sulphide over this. After a few minutes remove the upper watch-glass, and evaporate the liquid to dryness over a water bath; let the dry residue be treated with a drop of a weak solution of ferric chloride. If hydrocyanic acid be present, a blood-red colour is produced, owing to the formation of red ferric hydrocyanide, which may be discharged by chloride of mercury; a reaction which distinguishes it from a similar colour given by meconic acid.
Where large quantities of material have to be examined, it is desirable that the acid should be distilled off by the heat of a water-bath, acidulating the liquid with tartaric acid if it be alkaline. The distillate is then to be tested by any of the above methods.
Antidotes.—Give a scruple of carbonate of potash dissolved in about an ounce of distilled water, and directly afterwards, ten grains of sulphate of iron, also dissolved in the same quantity of distilled water, to which should be added one drachm of tincture of perchloride of iron. Whilst this is being prepared, and subsequently, apply cold affusion to the head and neck, artificial respiration, and, if practicable, give strong coffee and brandy. A more ready remedy is ammonia, given both internally and applied to the nostrils.
Hydrocyanic Acid, Diluted. Syn. Acidum Hydrocyanicum Dilutum. (B. P.) The ‘British Pharmacopœia’ gives the following simple directions for the preparation of this acid:—
Dissolve two and a quarter ounces (avoir.) of ferro-cyanide of potassium in ten fluid ounces of distilled water, then add one fluid ounce of sulphuric acid, previously diluted with four fluid ounces of distilled water, and cooled. Put them into a retort and adapt this to a receiver, containing eight fluid ounces of water, which must be kept carefully cold. Distil with a gentle heat until the fluid in the receiver measures seventeen fluid ounces. Add to this three fluid ounces of the water, or as much as may be sufficient to bring the acid to the required strength of two per cent. by weight.
Prop. Specific gravity, ·997. 100 grains, or 110 minims, precipitated with a solution of nitrate of silver, give a precipitate of cyanide of silver, which when dried, weighs 10 grains. 270 grains rendered alkaline by liquor sodæ, require a 1000 grain measures of volumetric solution of nitrate of silver, before a permanent precipitate begins to form.
Antidotes. See Hydrocyanic Acid.
Hydrocyanic Acid, Scheele’s. Syn. Acidum Hydrocyanicum Scheelii. The original process of Scheele does not yield an acid of uniform strength, and is probably never followed. It is therefore impossible to state precisely what is intended when Scheele’s acid is prescribed, or to understand why it should be preferred by certain physicians to the British Pharmacopœia preparation, which is of a known and definite strength. As prepared by different makers it has been found to contain855 from three to five per cent. of anhydrous acid. The following is Scheele’s process:—
Mix two ounces of Prussian blue, with six ounces of red precipitate of mercury, and add six ounces of water. Boil for some minutes, constantly agitating; pour the whole on a filter and wash the residuum on the filter with two ounces of hot water, which is to be added to the filtered liquor. Add to this an ounce and a half of clean iron filings, and three drachms of sulphuric acid; shake well and let it settle; then pour the clear liquor into a retort, and distil a fourth part into a receiver well luted and kept cold.
HYDROFLUORIC ACID. (H. F.) Syn. Hydric Fluoride, Hydrogen Fluoride. Prep. 1. From fluor-spar (free from silica and metallic sulphides) and oil of vitriol. The fluor-spar being reduced to fine powder and placed in a leaden retort, is mixed with twice its weight of concentrated oil of vitriol, and on applying heat, an acid and highly acid vapour distils over, which condenses to a liquid if passed into a receiver of the same metal, standing in a freezing mixture at a temperature of 4° Fahr. Louyet has shown that the liquid acid, obtained as above, is not (as once believed) anhydrous.
2. From the double fluoride of potassium and hydrogen. Fremy first renders the salt anhydrous by careful drying; and by the subsequent application of a strong heat, expels the equivalent of hydrofluoric acid contained in it; condensing it into a colourless, mobile, very volatile liquid by the application of a freezing mixture of ice and salt.
3. By decomposing plumbic fluoride by dry hydrogen.
Prop. The strong, aqueous, hydrofluoric acid obtained by the action of oil of vitriol on fluor-spar, is a densely fuming, volatile, colourless liquid, which boils at 60° F., and remains unfrozen at 4°. It combines with water so greedily, and evolves so much heat in doing so, as to give rise to a hissing noise like that produced when a red-hot iron is plunged into cold water. In a concentrated form it has a specific gravity of 1·060. Brought into contact with animal matter of any kind it instantly destroys it, the smallest drop on the skin producing a deep and painful wound; hence the necessity of the greatest care in its preparation. With the exception of platinum, gold, silver, mercury and lead, hydrofluoric acid, when diluted, dissolves the metals, the metal when it undergoes solution, displacing hydrogen. Potassium decomposes the strong acid with explosion.
In both the gaseous and fluid form hydrofluoric acid is largely consumed for etching on glass; and this property constitutes one of its most available and reliable tests. The test may be conveniently applied as follows:—
Cover a small piece of window glass or a watch glass with a thin layer of wax, scraping away a very small portion by means of a sharply pointed instrument, and then expose the glass for a short time to the vapour of the acid, given off when the materials are heated in a small leaden saucer or platinum crucible; on removing the wax with a little turpentine, the marks on the glass caused by the hydrofluoric acid will be distinctly perceived.
HYDROFLUOSILIC′IC ACID. (4HF.SiF4.) Fluoride of silicon and hydrogen. Prep. From powdered fluor-spar, and siliceous sand or powdered glass, of each 1 part; concentrated sulphuric acid, 2 parts; mix in a glass retort, apply a gentle heat, and pass the evolved gas (fluoride of silicon) into water. Decomposition ensues, silica being deposited in a gelatinous state, and hydrofluosilicic acid remaining in solution. This acid liquor, which is a double fluoride of silicon and hydrogen, is used as a test for barium and potassium, with which it forms nearly insoluble precipitates.
HY′DROGEN. (H.) Syn. Hydrogenium, L. An elementary body discovered by Cavendish in 1766. It has been found existing in an uncombined state in the gases evolved from the solfataras of Iceland. Combined with oxygen, it constitutes water, and in this form is extensively distributed through earth, air, and ocean. It is an important constituent of all organised tissues.
Prep. Hydrogen is always obtained for experimental purposes by the deoxidation of water, by one or other of the following methods:—
1. A tube of iron or porcelain (a gun-barrel, for instance) containing a quantity of iron turnings or scraps of iron, is fixed across a furnace, so that its middle portion may be made red-hot; to the one end is attached a retort or other vessel containing water, and to the other a bent tube connected with a pneumatic trough or gasometer. The tube being now heated to redness, and the water in the retort brought into a state of brisk ebullition, the evolved steam suffers decomposition; the oxygen being absorbed by the iron, and the hydrogen escaping into the gas receiver.
2. Sulphuric acid (oil of vitriol), diluted with 6 or 8 times its bulk of water, is poured on granulated zinc (or scraps of iron) placed in a retort or gas bottle; hydrogen is evolved and is collected, as before.
Obs. This is the most convenient method of preparing hydrogen, and the one usually adopted in the laboratory. To ensure the gas being quite pure, distilled zinc is employed, and the gas is passed, first through a concentrated solution of pure potassa, then through a solution of nitrate of silver, and, lastly, through strong oil of vitriol, or over fragments of chloride of calcium. When hydrogen is prepared from crude zinc, it has a slight smell; and when from iron, its odour is often strong and disagreeable.
Prop. Gaseous; colourless; tasteless; odourless (when pure); combustible; sp. gr. ·06935,856 being 16 times lighter than oxygen gas, and 14·4 times lighter than atmospheric air. 100 cubic inches, at 60° Fahr. and 30 inches of the barometer, weigh 2·1371 (say 2·14) gr.; 1 gr. occupies 46·6 inches. It is very readily inflamed, even by a red-hot wire. It burns with a scarcely visible flame. Mixed with atmospheric air or oxygen, it explodes with extreme violence on the approach of flame, or sudden compression. One measure of hydrogen and 5 of atmospheric air, and 2 of hydrogen and 1 of oxygen, are the proportions that explode with the greatest violence. The combination of hydrogen and oxygen, when mixed, is brought about by the heat of a red-hot solid or a flame, by the electric spark, and by the presence of spongy platinum, the black powder of platinum, clean platinum foil, and some other substances. A jet of hydrogen burnt in oxygen gas, or a jet of these gases (mixed) burnt in the air, with proper precautions, produces a most intense heat. Water absorbs about 2% by volume of hydrogen.
Hydrogen has recently been liquefied and even solidified.
Tests. It is recognised by—its combustibility;—the pale colour of its flame;—producing water only when burnt in air or oxygen;—extinguishing the flame of other bodies; and—exploding when mixed with half its volume of oxygen, and fired.
Uses, &c. Pure and uncombined hydrogen is not employed in the arts. Inhalations of this gas have, however, been occasionally used in medicine. Dr Beddoes recommended them in phthisis. In combination, the uses of hydrogen are almost numberless. Combined with oxygen, it forms water; with chlorine, hydrochloric acid; with fluorine, hydrofluoric acid; with cyanogen, hydrocyanic acid; with carbon, innumerable hydrocarbons; with nitrogen, ammonia; with sulphur, sulphuretted hydrogen—in fact, an enumeration of the valuable compounds which it enters into would occupy many pages of this work. From its extreme lightness it has been used to fill balloons, but coal-gas is now commonly employed for this purpose. On its property of inflaming in contact with spongy platinum is arranged the popular little instrument for the production of instantaneous light (Dobereiner’s Lamp) sold by the philosophical instrument makers. The chemist avails himself of the great heat developed by its combustion in oxygen in the formation of the OXYHYDROGEN BLOWPIPE.
Some of the compounds of hydrogen are noticed below; the others under their respective names.
Hydrogen, Antimo′′niuretted. (SbH3.) Syn. Antimonetted hydrogen, Hydride of antimony, Stibamine; Hydrogenium antimoniatum, L. A gaseous compound of antimony and hydrogen, prepared by dissolving an alloy of antimony with a large excess of zinc in hydrochloric or dilute sulphuric acid. It has never been obtained pure, a variable proportion of free hydrogen being always present. It burns with a bluish-white flame, giving rise to dense fumes of teroxide of antimony, and when conducted through a red-hot tube, or the flame is thrown on a cold surface, as a porcelain plate, metallic antimony is deposited. This gas is a deadly poison when inhaled. See Arsenious acid.
Hydrogen, Arsen′′iuretted. (AsH3.) Syn. Arsenetted hydrogen, Hydride of arsenic, Arsenamine; Hydrogenium arseniuratum, L. A gaseous compound of arsenic and hydrogen.
Prep. Arsenide of zinc (made by fusing together equal weights of zinc and arsenic) is acted upon by strong hydrochloric acid or by sulphuric acid diluted with three parts of water.
Obs. This gas is produced whenever arsenious or arsenic acid, or any of their salts, is in presence of nascent hydrogen. The properties of arsenetted hydrogen are fully described in the tests for ARSENIOUS ACID. This gas is a deadly poison when inhaled.
Hydrogen, Car′buretted. Syn. Carbonetted hydrogen. This term is specially applied to two of the numerous compounds of carbon and hydrogen (CARBIDES OF HYDROGEN, HYDROCARBONS):—
1. Light Carburetted Hydrogen. (CH4.) Syn. Marsh gas, Fire-damp, Gas of the acetates. This is often abundantly disengaged in coal mines, and its combustion occasions those fearful explosions which are so destructive to human life. The mud at the bottom of stagnant pools, on being stirred, suffers bubbles of gas to escape, which, when collected and examined, are found to be a mixture of light carburetted hydrogen and carbonic acid. The latter is easily removed by passing the gas through a solution of caustic potassa or milk of lime.
Prep. (Dumas.) A mixture of acetate of soda (cryst.) and hydrate of potassa (dry), of each 2 parts, and quicklime (in powder), 3 parts, is strongly heated in a flask or retort. The gas in a state of absolute purity is disengaged in great abundance, and may be collected over water.
Prop. Colourless; neutral; nearly inodorous; burns with a yellow flame, producing pure water and carbonic acid; explodes when kindled in contact with air or oxygen.
2. Heavy Carburetted Hydrogen. (C2H4.) See Olefiant Gas.
Obs. Coal gas, OIL GAS, and RESIN GAS, consist, for the most part, of mixtures of these two gaseous hydrocarbons in uncertain proportions, obtained respectively from coal, oil, and resin, by the action of heat, and used for the purposes of illumination. See Gas.
Hydrogen, Oxides of. There are two well-defined compounds of hydrogen and oxygen:—
1. Subox′ide of Hydrogen. (H2O.) Water (which see).
2. Perox′ide of Hydrogen. (HO.) Syn. Hydroxyl, Binoxide of hydrogen, Deutoxide of H., Oxygenated water; Hydrogeniibinoxydum,857 L. This singular fluid was discovered by M. Thénard in 1818.
Prep. (Odling.) A known quantity of pure hydrochloric acid, diluted with 8 or 10 times its volume of distilled water, is placed in a glass beaker surrounded with ice or a freezing mixture. A quantity of binoxide of barium rather less than sufficient to neutralise the acid is then ground to a fine paste with distilled water, and added gradually to the acid in which it should dissolve without effervescence. Diluted sulphuric acid is next added cautiously, to precipitate the barium, and reproduce hydrochloric acid to act upon a fresh quantity of peroxide. The liquid having been filtered from the insoluble sulphate of baryta, a second proportion of binoxide of barium paste is added gradually, as before. The treatment with sulphuric acid, filtration and addition of binoxide, is repeated 6 or 7 times. Sulphate of silver is then very carefully added, so as exactly to precipitate in the form of chloride of silver the whole of the chlorine. After filtration, pure baryta, first as a paste and then in solution, is cautiously added, to precipitate exactly the sulphuric acid set free from the sulphate of silver. Filtration is again resorted to, and the clear liquid (aqueous solution of peroxide of hydrogen) is placed in a dish over oil of vitriol in vacuo, in order that the water mixed with it may evaporate.
Prop., &c. A colourless, transparent, somewhat syrupy liquid, of sp. gr. 1·452. It has a metallic taste, and corrodes the skin. It is easily resolved into oxygen and water. It mixes freely with water, and becomes more permanent by the dilution. It bleaches organic substances, and acts as a powerful oxidating agent. Under certain circumstances, however, it plays the part of a reducing agent. To the chemist, peroxide of hydrogen and its analogue, binoxide of barium, have been of great service as instruments of research. Binoxide of hydrogen has been applied in the arts to restore the blackened lights of paintings which have become darkened by sulphuretted hydrogen; it is also sold by hair-dressers for bleaching human hair.
Hydrogen, Phosphuret′ted. See Phosphorus.
Hydrogen, Sulphides of. See Sulphur.
HYDROMEL. Syn. Hydromeli, L. An aqueous solution of honey. Prep. (P. Cod.) Honey, 2 oz.; boiling water, 32 oz.; dissolve, and strain. A refreshing and slightly laxative drink; in fevers, hoarseness, sore throats, &c.
HYDROM′ETER. Syn. Areometer, Gravimeter; Hydrometrum, L. An instrument for ascertaining the specific gravities of liquids, and hence their strength, the latter being either in inverse or direct proportion to the former. Hydrometers are of two kinds:—1. Those which are always immersed to the same depth in distilled water, and the liquid to be tried, small weights being used for the purpose, as in Fahrenheit’s and Nicholson’s hydrometers; and 2nd, those which are suffered to rise or sink freely in the liquid, until they come to a state of rest, as in Syke’s, Baumé’s, &c. In both cases a correction must be made for any variation in temperature.
Of the two kinds, the first give the most accurate results, and have the great advantage of being applicable to liquids either lighter or heavier than water, but the second are the readier in practice, requiring less time and less skill to use them. The following are those best known:—
Baumé’s hydrometer or AREOMETER, which is very generally employed on the Continent, consists of two distinct instruments, the one for liquids heavier than water, the other for liquids lighter than that fluid. The first floats at the 0, or ‘zero,’ of the scale, in distilled wafer, at the temperature of 58° Fahr., and each degree, marked downwards, indicates a density corresponding to one per cent. of common salt. The hydrometer for liquids lighter than water is poised so that the 0 of the scale is at the bottom of the stem, when it is floating in a solution of 1 oz. of common salt in 9 oz. of water, and the depth to which it sinks in distilled water shows 10°; the space between these fixed points being equally divided, and the graduation continued upwards to the top of the scale.
The temperature at which these instruments were originally adjusted by Baumé was 12·5° Centigrade (54·5° Fahr.). They are now commonly adjusted in this country at 58° or 60° Fahr. Hence arise the discrepancies observable in the published tables of the “correspondence between degrees of Baumé and real specific gravities.”
Cartier’s hydrometer, which is much used in France for light liquids, has the same point for the zero of its scale as Baumé’s, but its degrees are rather smaller, 30° Baumé being equal to 32° Cartier.
Fahrenheit’s hydrometer consists of a hollow ball, with a counterpoise below, and a very slender stem above, terminating in a small dish. The middle, or half-length of the stem, is distinguished by a fine line across it. In this instrument every division of the stem is rejected, and it is immersed in all experiments to the middle of the stem, by placing proper weights in the little dish above. Then, as the part immersed is constantly of the same magnitude, and the whole weight of the hydrometer is known, this last weight, added to the weights in the dish, will be equal to the weight of fluid displaced by the instrument, as all writers on hydrostatics prove. And accordingly, the specific gravities for the common form of the tables will be had by the proportion—
As the whole weight of the hydrometer and its load, when adjusted in distilled water, is to the number 1000, so is the whole weight when adjusted in any other fluid, to the number expressing its specific gravity.
858
Gay-Lussac’s alcoholometer is used to determine the strength of spirituous liquors. It, at once, indicates on the stem, the per-centage of absolute alcohol in the liquid examined. The original experiments of Gay-Lussac having been made on liquids at a temperature of 59° Fahr., all examples examined by the alcoholometer, must either be brought to that temperature previous to being tested, or a correction made in the strength found.
Nicholson’s hydrometer is constructed on the same principle as Fahrenheit’s. It has in addition to the small dish for weights above, a little cup attached below, for holding any solid body whose weight in water is required. It is chiefly intended for taking the sp. gr. of minerals.
Richter’s hydrometer resembles, for the most part, Gay-Lussac’s.
Syke’s hydrometer is that adopted by the Revenue authorities in England for ascertaining the strength of spirits, and has been already fully noticed.
Tralles’s hydrometer resembles Gay-Lussac’s (nearly).
Twaddell’s hydrometer is much used in the bleaching establishments of Scotland, and in some part of England. According to this scale, 0 is equal to 1000 or the sp. gr. of distilled water, and each degree is equal to ·005; so that, by multiplying this number by the number of degrees marked on the scale, and adding 1·, the real specific gravity is obtained.
Obs. Hydrometers, unless manufactured with great care and skill, merely afford approximate results; but which are nevertheless sufficiently correct for all ordinary purposes. They also require several ounces of liquid to float them, and hence cannot be used for very small quantities. Those of Fahrenheit, Nicholson, and Sykes are the most accurate, both in principle and application. They are all employed with a tall glass cylinder termed a sample, test, or hydrometer glass, in the way already noticed; but the thermometer for ascertaining the temperature must be covered with a glass case, or arranged with a folding scale to allow of its immersion in corrosive liquids.
Alcoholometers, Elaiometers, Saccharometers, Urinometers, &c., are simply hydrometers so weighted and graduated as to adapt them for testing spirits, syrups, urine, &c. See Alcoholometry, Alonholmetry, Areometer, Specific Gravity, &c.
HYDROM′ETRY. Syn. Areometry. The art of determining the specific gravity of liquids, and hence their strength and commercial value. The instruments used are noticed above; their action depends upon the fact that a floating body displaces a bulk, equal to itself in weight, of the fluid in which it floats, and consequently that a body of a given weight sinks deeper in a lighter than in a heavier fluid. In hydrometric determinations the temperature of the samples must be carefully attended to, for fluids expand as their temperature is increased. The hydrometers used in England are generally adjusted to the standard temperature of 60° Fahr., and when ‘Hydrometer Tables,’ giving the corrections for the variations of the thermometer, are not accessible, the fluids to be examined should be brought to this standard temperature by applying heat directly to the vessel, when the temperature is below the standard, or by surrounding the vessel, with cold water, when it is above the standard. The principal applications of hydrometry are described in different parts of this work. See Acetimetry, Alcoholometry, Chlorometry, Specific Gravity, &c.
HYDROP′ATHY. Syn. Water cure; Hydropathia, L. A mode of curing diseases by the copious use of pure cold water, both internally and externally, together with dry sweating, and the due regulation of diet, exercise, and clothing. This “treatment of diseases undoubtedly includes powerful therapeutic agents, which, in the hands of the educated and honourable practitioner, might be most beneficially resorted to as remedial agents.” (Pereira.)
HYDROPHO′BIA. Syn. Canine madness; Rabies canina, L. A disease which is generally considered as the result of a morbid poison being introduced into the system by the bite of a rabid animal. A clear case of idiopathic or spontaneous hydrophobia has never yet been known to occur in the human subject.
The common symptoms of hydrophobia are great excitability and horror at the sight of water, or the attempt to drink, fever, vomiting, excessive thirst, spitting of viscid saliva, difficult respiration, irregular pulse, convulsions, syncope, delirium, and death.
The whole materia medica has been, unfortunately, unsuccessfully sought without the discovery of a single remedy for this disease, or even a palliative of its severer symptoms. See Curarine.
The treatment of recent bites of venomous animals has been fully explained, and need not be repeated here. To prevent secondary or constitutional effects arising, the use of lemon juice, or arsenical solution, has long been popular. (Graham, and others.) Dr Buchan remarks that “vinegar is of considerable use, and should be taken freely.”
HYDROSULPHU′RIC ACID. See Sulphur.
HYGIENE′. Syn. Hygiene, Fr. Health; its preservation, promotion, and restoration. That department of medicine and civil government which relates to health. See Air, Bath, Exercise, Flannel, Food, Nutrition, Sleep, Ventilation, &c.
HYGROMETER. An instrument for measuring the amount of moisture in the atmosphere.
Amongst the various contrivances for accomplishing859 this end are Daniel’s dew-point hygrometer; and the wet bulb hygrometer.
By the first, the quantity of moisture in the atmosphere, is determined by noting with a sensitive thermometer, the temperature at which a film of dew mass, to deposit on one of the bulbs of a species of cryophorus, disappears; the tension of the aqueous vapour present in the air at that period, being readily ascertained from tables constructed for the purpose, the corresponding proportion of moisture can thus be readily ascertained.
The wet bulb hygrometer consists of two small thermometers placed side by side, the bulb of them being surrounded by cotton films kept constantly damp by a simple contrivance. According to the rate of evaporation of the bulb so moistened, with the fall of the thermometer to which the moistened bulb belongs, the depression, of course, being greater the further the surrounding atmosphere is from the saturation point, and tables are furnished for determining the degree of saturation for all differences of temperature within the ordinary atmospheric range.
HYOCHO′LIC ACID. C25H40O4. Syn. Glycohyocholalic acid. A compound peculiar to the gall of pigs, discovered by Strecker and Gundelach.
Prep. The fresh gall of pigs is mixed with a solution of sulphate of sodium; the precipitate is dissolved in absolute alcohol, and decolourised by animal charcoal. From this solution ether throws down hyocholate of sodium, which, on the addition of sulphuric acid, yields hyocholic acid as a resinous mass, which is dissolved in alcohol, re-precipitated by water, and dried. When heated with alkaline solutions, glycocine and a new crystalline acid (hyocholalic acid) are formed. When boiled with acids, it yields glycocine and hyodyslysin.
HYOSCY′AMINE. Syn. Hyoscyamia, Hyoscyamina, Daturine, Daturia. An alkaloid obtained from common henbane (Hyoscyamus niger), and also from the thorn apple (Datura stramonium). See Datura.
HYPNOT′ICS. Syn. Hypnotica, L. Agents or medicines which induce sleep, as opium, morphia, henbane, Indian hemp, lactucarium, &c. Agents which prevent sleep are called agrypnotics (Agrypnotica, L.), or anthypnotics (Anthypnotica, L.).
HYPOCHLO′RIC ACID. See Chlorine.
HYPOCHONDRI′ASIS. Syn. Hypochondriacism. The ‘hip’ or ‘hyp,’ the ‘vapours,’ depression of spirits, ‘blue devils.’ This disease chiefly affects persons of the melancholic temperament, and is commonly induced by hard study, irregular habits of life, want of proper social intercourse, living in close apartments, and insufficient out-of-door exercise. The treatment may, in most cases be similar to that recommended for DYSPEPSIA, observing, however, that success depends more on amusing and engaging the mind, and in gradually weaning it from old conceits, than in the mere administration of medicine. When the patient is tormented with a visionary or exaggerated sense of pain, or of some concealed disease, or a whimsical dislike of certain persons, places, or things, or groundless apprehensions of personal danger or poverty, or the conviction of having experienced some dreadful accident or misfortune, the better way is to avoid any direct attempts to alter his opinions, but to endeavour to inspire confidence in some method of relief. Greding mentions the case of a medical man who conceived that his stomach was full of frogs, which had been successively spawning ever since he had bathed, when a boy, in a pool in which he had perceived some tadpoles; and he had spent his life in endeavouring to get them removed. One patient, perhaps, fancies himself a giant; another as heavy as lead; a third a feather, in continual danger of being blown away by the wind; and a fourth a piece of glass, and is hourly fearful of being broken. Marcellus Dentatus mentions a baker of Ferrara who thought himself a lump of butter, and durst not sit in the sun, or come near the fire, for fear of being melted. The writer of this article once knew a man who always put on his coat the wrong side in front, because he conceived his face looked behind him. In such cases it is useless to argue with the patient, as it only causes irritation, and increases the malady. The restoration of the bodily health, and a sudden surprise or change of scene, will often effect a cure.
HYPONI′TRIC ACID. See Nitrogen.
HYPONI′TROUS ACID. See Nitrogen.
HYPOPHOS′PHITES. See Phosphorus.
HYPOPHOS′PHITE. A salt of hypophosphorous acid.
HYPOSUL′PHATE. Syn. Dithionate; Hyposulphas, L. A salt of hyposulphuric acid.
HYPOSUL′PHITE. Syn. Thiosulphate; Hyposulphis, L. A salt of hyposulphurous acid.
HYPOSUL′PHUROUS ACID. See Sulphurous Acid.
HYRA′CEUM. A substance produced by the Cape badger (Hyrax Capensis), and proposed as a substitute for CASTOREUM. Pereira considered it to be inert and useless.
HYSTERICS. Syn. Hysteria, Passio hysterica, L. In pathology, a nervous affection peculiar to women, attacking in paroxysms or fits, preceded by dejection; tears, difficult breathing, sickness, and palpitation of the heart. The treatment of this disease varies with the causes and the symptoms. Bleeding, cupping, and depletives, are generally had recourse to in robust and plethoric habits, and stimulants and tonics in those of a weakly or relaxed constitution. Affusion of cold water and nasal stimulants will frequently remove the fit in mild cases. Exercise, proper amusements and regular hours and diet, are the best860 preventives. See Draught (Antihysteric and Hydrocyanic), &c.
ICE. Syn. Glacies, L. Water in the solid state. On being cooled, water gradually contracts until the temperature has fallen to 39·9° Fahr., when it begins to expand. At the freezing-point, 32° Fahr., under ordinary conditions, water crystallises or freezes, and in consequence of the continued expansion, the sp. gr. of ice, as compared with that of water at 39·9°, is as ·94 to 1·00. Ice has the peculiar property of reuniting by the contact of adjoining surfaces after having been broken into fragments (REGELATION). Coloured water and salt water, by freezing, produce colourless and fresh ice; and clean solid ice, when thawed, furnishes water equal in purity to that which has been distilled.
The use of ice in the preparation of ICE-CREAMS, ICED-LIQUORS, &c., is noticed elsewhere. The confectioner collects his ice as early as possible during the winter, and stores it in a well-drained well or excavation, somewhat of the form of an inverted sugar-loaf, contained in a small shed or building called an ICE-HOUSE. This building should always be situated on a dry sandy soil, and, if possible, on an eminence. The door should be on the north side, and the roof should be conical and thickly thatched with straw.
In medicine, ice is frequently employed externally in inflammation of the brain, to resolve inflammation, to stop hæmorrhage, to constringe relaxed parts, and an anodyne, to deaden pain. For these purposes it is pounded small, in a cloth, and placed in a bladder or bag of gauze (ICE-CAP, ICE-POULTICE) before applying it. Internally, ice or ice-cold water has been given with advantage in heartburn, typhus, inflammation and spasms of the stomach, to check the vomiting in cholera, and to arrest hæmorrhage, whether bronchial, gastric, nasal, or uterine. Very recently, ice has been proposed as a remedy in the treatment of diphtheria. Small lumps of ice, or a small glassful of pounded ice-and-water, will often temporarily restore the tone of the stomach and nervous system during hot weather, when all other means fail. Ice-creams, taken in moderation, act in the same way.
In the warmer climates of Europe an ICE-HOUSE or an ICE-SAFE (a REFRIGERATOR) is a necessary appendage to every respectable dwelling, not merely for the purpose of pleasing the palate with iced beverages, but to enable the residents to preserve their provisions (fish, meat, game, milk, butter, &c.) in a wholesome state from day to day. In addition to large cargoes of ice imported yearly from Norway, and principally consumed in England, Germany and France, ice is now manufactured to no inconsiderable amount, in these three countries artificially, the principal consumption of the factitious article being by brewers, who use it for the cooling of their worts. The artificial manufacture of ice is effected by the means of the condensation of elastic vapours in machines expressly made for the purpose. In Siebe’s ice-making machine the vapour of ether is made to traverse metallic tubes surrounded with a concentrated solution of common salt, by which it becomes recondensed to the liquid state, to be again utilised in the production of the vapour; the solution of salt becoming at the same time so reduced in temperature, as to convert into ice, water, contained in proper vessels, placed in it. In Carré’s machine the same end is accomplished by means of ammoniacal gas, a solution of calcic chloride being used for absorbing the cold instead of common salt. Reece’s is a modification, (he states an improvement) of Carré’s. Ice machines are also made, in which ice is produced, by bringing water into contact with air, which has been greatly reduced in temperature by cooling it when in the compressed state, and subsequently allowing it to expand. Liquid carbonic and sulphurous acids have likewise been used in the preparation of artificial ice, but not when it has been required in any considerable quantity. See Refrigeration.
Ice, Medicated. Mr Martin, of Weston-super-Mare, writing to the ‘Lancet,’ says:—“Every practitioner has at times to face the difficulties of the scarlatinal throat in young children. It may sadly want topical medication; but how is he to apply it? Young children cannot gargle, and to attempt the brush or the spray fills them with terror. In many cases neither sternness nor coaxing avails. Yet these little ones in almost every case will greedily suck bits of ice. This has long been my chief resource where I could not persuade the child to submit to the sulphurous acid spray. Lately, I have been trying an ice formed of the frozen solution of the acid (or some other antiseptic). Though, of course, not so tasteless as pure ice, the flavour is so much lessened by the low temperature, and probably also through the parched tongue, very little appreciating any flavour, that I find scarcely any complaint on that score from the little sufferers; they generally take to it very readily. The process of making it is very simple. A large test-tube immersed in a mixture of ice and salt is the only apparatus required, and in this the solution is easily frozen. When quite solid a momentary dip of the tube in hot water enables one to turn out the cylinder of ice, as the cook turns out her mould of jelly. I have tried the three following formulæ, all of which answer, although I think I prefer the first.
“1. Sulphurous acid, 1⁄2 dr.; water, 71⁄2 dr.; mix and freeze.
“2. Chlorate of potass, 1 scruple; water, 1 oz.; dissolve, and freeze.
“3. Solution of chlorinated soda, 1⁄2 dr.; water, 1 oz.; mix and freeze.
861
“However, the form is of secondary importance, as each practitioner can construct his own. Boracic acid, salicylic acid, or any other harmless antiseptic with not too much taste, would doubtless be as useful as those indicated.”
ICE′LAND MOSS. Syn. Cetraria (B. P.), Lichen Islandicus, L. The lichen termed Cetraria Islandicus. It is much employed, both as a nutritious food and as a mild mucilaginous tonic, in catarrh and consumption. It may be purified from its bitter principle by a little cold solution of potassa.
Iceland Moss, Saccharated. Syn. (P. C.), Saccharum lichenis. Iceland moss, 1 lb.; refined sugar 1 lb.; macerate the moss in water to extract the bitterness, express, boil in water for an hour, strain, let settle, decant, add the sugar, evaporate to dryness with a gentle heat, constantly stirring, and finally reduce to powder.
ICES. (In confectionery.) These are commonly composed of cream or sweetened water, variously flavoured, and congealed by ice or a freezing mixture. Sometimes, instead of cream, the materials of a custard are used. The mixed ingredients are placed in a tin furnished with a handle at top, called a ‘freezer,’ or ‘freezing-pot,’ which is then plunged into a bucket containing ice broken small, and mixed with about half its weight of common salt, and is kept in rapid motion, backwards and forwards, until its contents are frozen. As the cream congeals and adheres to the sides, it is broken down with the ice-spoon, so that the whole may be equally exposed to the cold. As the salt and ice in the tub melt, more is added, until the process is finished. The ‘ice-pot,’ with the cream in it, is next placed in a leaden ‘ice-stand,’ is at once surrounded with a mixture of ice and salt, and closely covered over. In this state it is carried into the shop. The glasses are filled as required for immediate use, and should have been previously made as cold as possible.
Plain ice-cream, or CREAM FOR ICING, is commonly made by one or other of the following formulæ:—
1. New milk, 2 pints; yolks of 6 eggs; white sugar, 4 oz.; mix, strain, heat gently and cool gradually.
2. Cream 1 pint; sugar, 4 oz.; mix as above.
3. Cream and milk, of each 1 pint; white sugar, 1⁄2 lb.
Flavoured ice-creams are made by mixing cream for icing with half its weight of mashed or preserved fruit, previously rubbed through a clean hair sieve; or, when the flavour depends on the juice of fruit or on essential oil, by adding a sufficient quantity of such substances. Raspberry and STRAWBERRY ICES are made according to the former method; LEMON, ORANGE, NOYEAU, and ALMOND ICES, by the latter method. In the same way any other article besides cream may be frozen.
Chocolate for icing is made by rubbing 1 oz. of chocolate to a paste with a tablespoonful of hot milk, and then adding ‘cream for icing,’ 1 pint.
Coffee for icing is made of cream for icing, 1 quart, to which a small teacupful of the strongest possible clarified coffee has been added together with 2 oz. of sugar and the yolks of 3 or 4 eggs. See Icing (below).
I′CING. (For cakes.) Syn. Sugar ice. The covering of concreted sugar with which the confectioners adorn their cakes. Prep. Beat the white of eggs to a full froth, with a little rose or orange-flower water; then add gradually, as much finely powdered sugar as will make it thick enough, beating it well all the time. For use, dust the cakes over with flour, then gently rub it off, lay on the icing with a flat knife, stick on the ornaments while it is wet, and place it in the oven for a few minutes to harden, but not long enough to discolour it. It may be tinged of various shades by the addition of the proper ‘stains.’
ID′RIALIN. A fusible, inflammable substance, found associated with the native cinnabar of the mines of Idria, in Carniola. It is extracted from the ore by means of oil of turpentine. It is only slightly soluble in alcohol and ether. When pure, it is white and crystalline.
ID′RYL. A hydrocarbon generally found associated with idrialin.
IGASU′RIC ACID. Syn. Acidum igasuricum, L. An acid associated with strychnine in the St. Ignatius’ bean and in nux vomica. It may be obtained by digesting the rasped or ground beans first in ether and then in boiling alcohol, evaporating the latter decoction to dryness, diffusing the residuum through water, adding a little carbonate of magnesium, again boiling for some minutes, filtering, washing the powder with cold water, and digesting it in alcohol, and filtering. The igasurate of magnesium thus obtained is dissolved in boiling water, the solution decomposed by acetate of lead, and the precipitate (igasurate of lead), after being washed and diffused through distilled water, is decomposed by sulphuretted hydrogen. The solution thus obtained yields crystals (igasuric acid) on being evaporated. It is soluble in both water and alcohol.
IGNI′′TION. In the laboratory this term is commonly applied to the act of heating to redness or luminousness. See Calcination.
ILLICIN. Boil a clear decoction of holly with animal charcoal; let it settle, collect the deposited charcoal, wash it with cold water, dry it, and treat it with boiling alcohol; let the filtered liquid be evaporated to dryness. Febrifuge.—Dose, 6 to 24 gr.
ILLUMINA′TION. The act of illuminating or making luminous. For supplying artificial light to streets and the interiors of houses coal gas and oils and fats are generally employed. These illuminating agents are compounds rich in carbon, upon the presence of which the brightness of their flames depends. Flame is gas or vapour heated to incandescence during862 the process of combustion. A flame containing no solid particles emits but a feeble light, even if its temperature is the highest possible. Pure hydrogen, for instance, burns with a pale, smokeless flame, though with the production of considerable heat. On the other hand, wax, paraffin, coal-gas, &c., while undergoing combustion, give out considerable light, because their flames contain innumerable solid particles of carbon, which act as radiant points. To give the greatest degree of luminosity to flame, the supply of air must be proportioned to the character of the burning substance, and be insufficient for the instantaneous combustion of the evolved gases; in which case the hydrogen takes all the oxygen, and the larger portion of the carbon is precipitated, and burnt in the solid form, at some little distance within the outer surface of the flame. When the supply of air is sufficient for the immediate and complete combustion of the whole of the combustible matter, no such precipitation takes place, and the flame is neither white nor brilliant. The richest coal-gas, mixed with sufficient air to convert all its hydrogen and carbon into water and carbonic acid, explodes with a pale blue flash; yet the same gas, when consumed in the ordinary way, burns with a rich white flame. Every one must have noticed the effect of a gust of wind upon the flaring gas-jets of a butcher’s shop; the plentiful supply of air causes complete combustion, and so converts the bright white flames into dull blue streaks of fire. When the supply of air is insufficient to cause the combustion of the newly formed solid carbon at the instant of its development, and whilst it is in an incandescent state, the flame becomes red and smoky, and unburnt sooty particles are thrown off. The same occurs when the temperature of any portion of the hydrogen is reduced below that intensity required for the combustion of the newly separated charcoal. Solid bodies, as tallow, oils, and fats, which burn with flame, are converted into the state of gas by the heat required to kindle them, and it is this gaseous matter which suffers combustion, and not the substance which produces it.
The relative value of the ordinary illuminating agents has been accurately determined by Dr Frankland. According to his experiments, the quantities of various substances required to give the same amount of light as would be obtained from 1 gallon of Young’s Paraffin oil are as follows:—
| Young’s Paraffin oil | 1·00 | gall. |
| American rock oil[352] | 1·26 | ” |
| Paraffin candles | 18·6 | lbs. |
| Sperm | 22·9 | ” |
| Wax | 26·4 | ” |
| Stearic | 27·6 | ” |
| Composite | 29·5 | ” |
| Tallow | 39·0 | ” |
[352] Acknowledged to be an inferior sample.
The following table exhibits the comparative cost of the light of 20 sperm candles, each burning 10 hours at the rate of 120 gr. per hour; also the amount of carbonic acid produced and heat evolved per hour, in obtaining this quantity of light:—
| Cost. | ||||
| s. | d. | Carb. acid per hour in cub. feet. | Units of heat per hour. | |
| Wax | 7 | 21⁄2 | 8·3 | 82 |
| Spermaceti | 6 | 8 | ||
| Paraffin candles | 3 | 10 | 6·7 | 66 |
| Tallow | 2 | 8 | 10·1 | 100 |
| Rock oil | 0 | 71⁄2 | 3·0 | 29 |
| Paraffin oil | 0 | 6 | ||
| Coal gas | 0 | 41⁄2 | 5·0 | 47 |
| Cannel gas | 0 | 3 | 4·0 | 32 |
These figures prove that coal-gas and the mineral oils are the cheapest and best illuminating agents, producing the largest amount of light with the least development of heat.
The light emitted by incandescent lime (Drummond light, HYDRO-OXYGEN LIGHT, LIME LIGHT, OXYHYDROGEN LIGHT) is intensely brilliant, and is often made use of to enable workmen to continue operations at night. It is obtained by directing the flame produced by the combustion of a mixture of hydrogen (or coal-gas) and oxygen upon a small cylinder of lime. In the improved form of this light the lime is protected from crumbling by a cage of platinum wire, and is caused to rotate slowly by means of clockwork, so as constantly to expose a fresh surface to the flame. When reflected from a ‘parabolic mirror’ in a pencil of parallel rays, the Drummond light has been recognised during daylight at a distance of 108 miles. The lime light produced with coal-gas and oxygen is used for the MAGIC LANTERN and GAS MICROSCOPE.
The most powerful illuminator is the ELECTRIC LIGHT, which is now being subjected to trial in many cities for street illuminations, &c., in place of coal-gas. It is usually produced by the passage of a strong current of electricity between two pencils of hard carbon. The electric light has been successfully applied to lighthouse illumination. Hitherto it has been found too intense and too costly for application to domestic purposes. See Candles, Flame, Gas, Photometry, &c.
ILLU′TATION. See Bath (Mud).
IMAGINA′TION. The influence of the imagination, both in the production and cure of disease, has been long admitted by medical practitioners. It is probably the most powerful therapeutic agent known. “Extraordinary cures have been ascribed to inert and useless means, when, in fact, they were referable to the influence of the imagination.” (Dr Pereira.)
IMPE′RIAL. Syn. Potus imperialis, Ptisana i., L. Prep. 1. Cream of tartar, 1⁄4 oz.; 1 lemon, sliced; lump sugar, 2 oz.; boiling water, 1 quart; infuse, with occasional stirring until cold, then pour off the clear portion for use.
863
2. A lemon, sliced; sugar, 1 oz.; boiling water, 1 pint.
3. Yellow rind and juice of lemon; citric acid, 1 dr.; sugar, 21⁄2 oz.; hot water (which has been boiled), 1 quart; as No. 1. Refrigerant and slightly diuretic. Used as a common drink in fevers, dropsy, &c., and as a summer beverage.
IM′PLEMENTS (Agricultural). “Almost all the operations of agriculture may be performed by the plough, the harrow, the scythe, and the flail; and these are the sole implements in the primitive agriculture of all countries. With the progress of improvement, many other implements (and machines) have been introduced, the more remarkable of which are the Drill plough, the HORSE HOE, the WINNOWING MACHINE, the THRESHING MACHINE, the HAY-MAKING MACHINE, and the REAPING MACHINE. The object of all these implements and machines is to abridge human labour, and to perform the different operations to which they are applied with a greater degree of rapidity, and in a more perfect manner than before.” (Loudon.) On the perfection of agricultural implements and machines depends much of the improvement of which this art is susceptible. See Agriculture, &c.
IMPROV′ING. The trade name for ‘doctoring,’ ‘adulterating,’ or ‘lowering,’ the quality of any substance, with the view of cheapening it or increasing its bulk. See Wine, &c.
IN′CENSE. Prep. 1. Olibanum, 2 or 3 parts; gum benzoin, 1 part.
2. Olibanum, 7 parts; gum benzoin, 2 parts; cascarilla, 1 part. Placed on a hot plate or burned, it exhales an agreeable perfume. Used in some of the rituals of the Roman Catholic church.
3. Benzoin and storax, of each 4 oz.; labdanum and myrrh, of each 6 oz.; cascarilla 3 oz.; oil cinnamon, 8 minims; oils bergamot and lavender, of each 20 minims; oil cloves, 10 minims; mix, and pass through a coarse sieve.
INCINERA′TION. The reduction of organic substances to ashes by combustion. See Calcination.
INCOMBUSTIBIL′ITY. The property of being incapable of being kindled, or of being consumed by fire. Substances possessing this property are said to be ‘incombustible’ or ‘fire-proof.’
INCOMBUST′IBLE FAB′RICS. Syn. Non-inflammable fabrics. The fashion of wearing light gauzy dresses extended by hoops or crinoline has made death from fire a common casualty. With a view of diminishing the danger to which women expose themselves, chemists have lately devoted considerable attention to the problem of rendering muslin and other light fabrics non-inflammable. This object may be attained by steeping the fabric in almost any saline solution. Thus, cotton or linen stuffs prepared with a solution of borax, phosphate of soda, phosphate of ammonia, alum, or sal ammoniac, may be placed in contact with ignited bodies without their suffering active combustion or bursting into flame. The salts act by forming a crust of incombustible matter on the surface of the fibres. They do not, however, prevent carbonisation taking place, when the temperature is sufficiently high. It is by a knowledge of this property of culinary salt that jugglers are enabled to perform the common trick of burning a thread of cotton while supporting a ring or a small key, without the latter falling to the ground. The cotton is reduced to a cinder, but from the action of the salt its fibres still retain sufficient tenacity to support a light weight.
The addition of about 1 oz. of alum or sal ammoniac to the last water used to rinse a lady’s dress, or a set of bed furniture, or a less quantity added to the starch used to stiffen them, renders them uninflammable, or at least so little combustible that they will not readily take fire; and if kindled, are slowly consumed without flame. None of the above-named salts are adapted for fine soft muslins, which mostly require chemical treatment, because they injure the texture, rendering the fabric harsh and destroying all its beauty. The salt which is found to answer most completely all the required conditions is TUNGSTATE OF SODA. “Muslin steeped in a solution containing 20% of this salt is perfectly non-inflammable when dry, and the saline film left on the surface is smooth and of a fatty appearance like talc, and therefore does not interfere with the process of ironing, but allows the hot iron to pass smoothly over the surface. The non-fulfilment of this latter condition completely prevents the use of many other salts—such as sulphate or phosphate of ammonia, which are otherwise efficacious in destroying inflammability—for all fabrics which have to be washed and ironed.” (Watts.)
The addition of a little phosphoric acid or phosphate of soda to the tungstate is recommended, for without this addition a portion of the tungstate is apt to undergo a chemical change and become comparatively insoluble. Messrs Versmann and Oppenheim, the introducers of tungstate of soda, give the following formula for a solution of minimum strength:—
Dilute a concentrated solution of neutral tungstate of soda with water to 28° Twaddell (sp. gr. 1·14), and then add 3% of phosphate of soda. This solution is found to keep and to answer its purpose very well; it is now constantly used in the Royal Laundry.
Paper, WOOD, &c., may be also rendered comparatively incombustible by soaking them in saline solutions. See Asbestos, Fire, &c.
INCOMPAT′IBLES. In medicine and pharmacy, substances which exert a chemical action on each other, and cannot, therefore, with propriety, be prescribed together in the same864 formula or prescription. The principles on which we should act to avoid prescribing or dispensing incompatibles, are briefly developed under the heads Affinity and Decomposition. To this we may add that, if a substance is endowed with well-marked therapeutical or poisonous properties, independent of those which may exert a chemical effect upon the tissues, its mode of action will neither be changed nor destroyed by the combinations which it forms, provided always that the new compounds are not insoluble in water.
“It is not necessary to give two incompatible medicines at the same time, in order to produce decomposition; it is sufficient if they are given within a very short interval of each other. Thus, a sick person, who has been treated with lead externally, or even internally, will present a discoloration of the skin, if he takes a sulphur bath four or five days after the lead treatment has been discontinued. If a person is rubbed with iodide of potassium shortly after having applied Vigo’s plaster (plaster of ammoniacum with mercury), or the Neapolitan ointment (mercurial ointment), iodide of mercury and caustic potash will be formed, which will cause vesication. So also vomiting occurs if lemonade made with tartaric acid is taken five or 6 days after the administration of white oxide of antimony.” (Trousseau and Reveil.)
Lists of incompatibles are published in many pharmaceutical and medical works, but are, in reality, of little use beyond illustrating rules and principles which are familiar to every chemist, and which every prescriber should also be intimately acquainted with.
INCRUSTATION, Prevention of, in Steam Boilers. With all qualities of water commonly used for feeding steam boilers there is a tendency to the production of hard calcareous deposits or layers of incrustation within the boilers, due to the separation of lime salts (particularly the carbonate and sulphate, or mixtures of these with a certain amount of carbonate of magnesia) as the direct consequence of the accumulation of these impurities from large quantities of water evaporated. The sparing solubility of the sulphate of lime (gypsum) in hot water fully accounts for its deposition in the boiler, and the carbonate of lime (chalk) is thrown down, not only as the result of direct evaporation, but by the ebullition expelling free carbonic acid, which holds this body to some extent in solution. Rain water, which of itself is too pure to give rise to these incrustations, cannot be used alone for boiler purposes, for it has been found to exert a highly corrosive action upon the iron plates and fittings. It can, however, be advantageously employed in conjunction with ‘hard’ spring or river waters, and has the effect of diminishing the incrustation merely as the result of dilution. The drain pipes leading from the roof of the factory may be placed in connection with the tank or well from which the supply of water is drawn for the boilers. It will be seen hereafter that the self-same remedy is efficient both as a means of preventing incrustation and obviating corrosion, and that by using one of the alkaline substances about to be specified this twofold advantage may be secured. Iron will not rust when immersed in water containing a mere trace of caustic alkali, and it is a common observation that the iron vessels used in the preparation of potash and soda remain for any length of time free from all appearance of rust. This singular property is, no doubt, susceptible of important applications, amongst them may be mentioned the better protection of iron ships from the attack of bilge water, of hydraulic rams, moulding boxes, smith’s tools, and other objects liable to be placed at times under the influence of water. Some forms of surface condensers become quickly corroded in consequence of the purity of the water accumulating in them by the process of distillation, and a small dose of caustic alkali is then useful as a means of protection; the engine-cylinders also to some extent are preserved when alkaline anti-incrustation fluids are introduced into the boilers, for the minute quantity which is carried forward mechanically in the form of spray mixed with the steam, suffices to preserve the iron. Whilst a tendency to ‘priming’ undoubtedly results from a too liberal use of soda or other alkali in the boiler, it will in practice be found easy to adjust the proportion of this ingredient, so as to secure immunity from corrosion and incrustation, and at the same time, avoid the tumultuous kind of ebullition known as ‘priming.’ In all cases it is advisable to carry out a rigid system of inspection, and it is only in the way of saving fuel and labour that the application of boiler fluids is to be recommended.
Much benefit has often resulted from a coating of coal-tar or ‘dead oil’ applied to the interior surfaces below the water line, when the boiler is opened for cleaning and inspection. These will tend very considerably to lessen the adhesion of calcareous crusts, and are not in any way affected by the boiler fluids in common use. Soda crystals and caustic soda may be used with great success in boilers to effect the immediate precipitation of the lime salts, and they act by throwing down a finely divided form of carbonate of lime, which in time furnishes nuclei for the deposition of subsequent accretions both of the carbonate and sulphate, so that they are prevented from crystallising upon the walls of the boiler. A granular mud is thus formed, which subsides quickly and may be for the most part got rid of through the ‘blow-off cock,’ which should be opened for this purpose two or three times every day, and run out with as little water as possible.
The use of caustic soda has undergone a thorough trial at the hands of Mr J. Spiller, F.C.S., in the boilers of the Royal Arsenal,865 Woolwich, and we are favoured with the following general instructions regarding its use, which are based upon an experience of upwards of ten years. The caustic soda should be dissolved in water so as to make a concentrated solution of specific gravity 1·300. This, being perfectly miscible with water, may be introduced into the boiler with the feed-water at any time when, from the pressure of steam, it may not be convenient to pour it through the safety valve or other openings in the boiler. But when the steam is down there is no difficulty in introducing the prescribed dose by using a tin funnel with flattened aperture to pass it through the safety valve; or a tubular arrangement with double cocks will answer at all times. Half a gallon per diem is the average quantity found sufficient for a 20-horse stationary boiler, working with Thames water for ten hours daily. If the water should happen to be unusually hard a larger dose may be employed, but it would not be expedient to add in one charge more than the amount required for the day’s consumption. Locomotive and multitubular boilers have been worked successfully with caustic soda, and it is here that the importance of using anti-incrustation fluids makes itself most apparent.
Many other methods have at various times been proposed to prevent the formation of deposits in steam boilers. Dr Ritterband’s method consists in simply throwing a little sal ammoniac into the boiler, by which carbonate of ammonia is formed, which passes off with the steam, and chloride of calcium, which remains in solution. In Holland this plan has been used with satisfaction for locomotive boilers. About 2 oz. of the salt may be placed in the boiler twice a week. The chloride of tin is equal to sal ammoniac, and is similar in its action. Carbonate of soda has been recommended by Kuhlmann and Fresenius of Germany, and by Crace Calvert of England. It is now employed generally in the boilers of engines in Manchester. The common plan adopted by working engineers to prevent incrustations from either variety of water is, on each occasion of cleaning out the boiler, to introduce some substance which, by its mechanical action, shall prevent the precipitated earthy matter caking together, or adhering to the boiler plates. Some common tar, bitumen, or pitch, appears to answer well under most circumstances. Mr Ira Hill recommends the use of 3 or 4 shovelfuls of course sawdust. He states that, after adopting the use of this article, he never had any difficulty from lime, although using water strongly impregnated with it, and has always found the inside of his boilers as smooth as if just oiled. Mr De Haen recommends the sulphate and bicarbonate of calcium to be decomposed by adding barium chloride and milk of lime in the proper proportion; when the water is at a temperature of 35°-45° C. the whole becomes clear in about ten minutes, a precipitate consisting of a mixture of barium sulphate and calcium carbonate deposits; if the water be cold, the greater part separates in ten minutes, but a little turbidity is noticeable for some hours due to suspended matter.
Protzen recommends the introduction of a piece of zinc into the boiler, this determines a galvanic current, which protects the iron against oxidation and corrosion, and causes the mineral ingredients of the water to be deposited as a fine loose mud, entirely preventing the formation of incrustation.
Slippery elm bark, and spent bark from the tan works have also been suggested. We (A. J. Cooley) have worked a powerful boiler daily for months without opening the ‘man-hole,’ after throwing a few pounds of potatoes into it. In all cases, when the earthy matter can be kept in a state of solution, or precipitated in a pulverulent form, it is easily removed from the boiler by what engineers term ‘priming,’ which is allowing the hot water to be blown over with the steam, so that, after a sufficient time, the whole original contents of the boiler are removed, and replaced by fresh water. Before doing so, however, it is of consequence to cut off the communication with the cylinders, and to open the waste-steam cock. Consult a pamphlet on ‘Boiler Incrustation and Corrosion’ by F. J. Rowan, published by Spon, London.
INCUBA′TION (Artificial). The hatching of eggs by artificial heat. This has been practised by the Egyptians from a very remote period. M. Bonnemain has the honour of having introduced this art to Western Europe, in 1775, and having been the first to pursue it successfully on the commercial scale. The source of heat employed by him was a circulatory hot-water apparatus, and the temperature maintained by it 100° Fahr. His plan was to introduce, daily, 1-20th only of the eggs the apparatus was capable of receiving, so that on the 21st day the first chickens were hatched, and a like number every day afterwards as long as the supply of eggs was kept up. Among the trays containing the eggs he placed saucers of water, to compensate for the absence of moisture derived in natural incubation by transpiration from the body of the hen. The chickens, as soon as hatched, were transferred to a ‘nursery’ or ‘chick-room,’ also artificially heated, and were fed with crushed millet seed. Several attempts have been made of late years to introduce artificial incubation into this country, with variable success.
IN′CUBUS. See Nightmare.
IN′DIA RUB′BER. See Caoutchouc.
INDIGES′TION. See Dyspepsia.
IN′DIGO. Syn. Indicum, Pigmentum Indicum, L. A blue dyestuff extracted from several plants growing in India and America, especially from the leguminous species Indigofera tinctoria and I. cœrulea. It exists in the plant as a colourless juice. The method of manufacture consists in steeping the plant866 in water until fermentation sets in; the colouring matter dissolves in the water, forming a yellow solution, which is drawn off from the rest of the vegetable matter, and agitated and beaten to bring it freely into contact with the air for about 2 hours; this treatment causes the indigo to form and settle down as a blue precipitate; this is cut, while soft, into cubical cakes, and dried by artificial heat. To hasten the formation of the indigo, a little lime water is sometimes added to the yellow solution. The indigo of commerce contains INDIGO-BLUE or INDIGOTIN, its most important constituent, INDIGO-RED, and many other substances, some of which must be regarded as accidental impurities or adulterations.
Prop. Tasteless; scentless; of an intense blue colour, passing into purple; when rubbed with a smooth hard body, it assumes a coppery hue; insoluble in water, cold alcohol, ether, alkalies, hydrochloric acid, dilute sulphuric acid, and the cold fixed and volatile oils; slightly soluble in boiling alcohol and oils; freely soluble in concentrated sulphuric acid, and, when decoloured or reduced by contact with deoxidising substances, in alkaline lyes; soluble in creasote; its colour is destroyed by chromic acid, nitric acid and chlorine; when suddenly heated, it gives off rich purple fumes, which condense into brilliant copper-coloured needles.
Pur. The best indigo is that which has the deepest purple colour, that assumes the brightest coppery hue when rubbed with the nail; its fracture is homogeneous, compact, fine-grained, and coppery; its powder is of an intensely deep blue tint, and light enough to swim on water; and it leaves only a fine streak when rubbed upon a piece of white paper. In general, when indigo is in hard, dry lumps of a dark colour, it is considered of bad or inferior quality. Indigo, when in hard or brittle lumps, or in dust or small bits, is often adulterated with sand, pulverised slate, and other earthy substances.
Estimation. Various methods for estimating the value of samples of indigo have been proposed, but none of them can be depended upon to give perfectly accurate results. The plan recommended by O′Neill[353] is perhaps the best; it is performed as follows:—
[353] See ‘Dictionary of Calico Printing and Dyeing.’
Weigh 25 gr. of a fair sample of the indigo finely ground; and to soften or disintegrate it still further, boil it for a short time with weak caustic soda, and then, if there be any soft lumps or clots, strain through calico; mix this with 3 quarts of water in a narrow-necked bottle which it will nearly fill, and add 400 gr. of quicklime, which has been slaked as perfectly as possible; shake well up and add a 1000 gr. measure of solution of green copperas (protosulphate of iron) at 30° Twaddell; cork the bottle closely, and leave it for three days, frequently shaking it in the interval. The indigo will be dissolved by this time; 1 quart of the clear solution is drawn off, shaken up in a bottle to oxidise it, acidified with acetic acid, and the pure indigo (INDIGOTIN) collected upon a filter, dried, and weighed. Four times the weight of the pure indigo is the per-centage of indigo in the sample.
Uses. As a dye stuff indigo is of great importance, both from the beauty and permanence of the colour it yields, and from the ease with which it is applied to fabrics of all materials. As a medicine it has been employed in various affections of a spasmodic character, as chorea, convulsions, epilepsy, hysteria, &c. In large quantities it often induces giddiness, vomiting, and diarrhœa; and when continued for sometime, muscular twitchings, resembling those arising from strychnine.—Dose. Beginning at about 15 gr., and gradually increased to 1, 2, or even 3 dr., at which it should be continued for 3 or 4 months; made into an electuary with honey or sugar, to which some aromatic may be added. See Indigo Dye, Indigotin, &c.
Indigo, Sul′phate of. Syn. Sulphindylic acid, Sulphindigotic a., Saxony blue, Soluble indigo.
Prep. By gradually adding indigo (in fine powder), 1 part, to fuming sulphuric acid (Nordhausen sulphuric acid), 5 parts, or oil of vitriol, 8 parts contained in a stone-ware vessel placed in a tub of very cold water, to prevent the mixture heating; the ingredients are stirred together with a glass rod at short intervals until the solution is complete, after which the whole is allowed to repose for about 48 hours, by which time it becomes a homogeneous pasty mass of an intense blue colour, which in a dull light appears nearly black.
Obs. In this state it forms ‘Barth’s blue,’ or the ‘CHEMIC BLUE’ or ‘INDIGO COMPOSITION’ of the dyer. Diluted with about twice its weight of soft water, it is converted into the ‘Saxony blue’ or ‘LIQUID BLUE’ of the shops, also used for dyeing. When commercial sulphate of indigo is diffused through a large quantity of water, nearly boiling, and wool (old white flannel rags, &c.) is macerated in it for some time, the latter absorbs the whole of the sulphate and is dyed blue, whilst the liquor assumes a greenish-blue colour. Wool, so prepared, when well rinsed in cold water, and boiled for some minutes in a large quantity of that liquid containing 1% or 2% of carbonate of potassa, or a quantity equal to about 1-3rd that of the indigo originally employed, gives up its blue colour, and becomes of a dull brown. The liquid is now a rich blue-coloured solution of sulphindylate of potassa, from which the salt may be obtained by cautious evaporation. This compound is prepared on the large scale, by diluting sulphate of indigo with about 12 times its weight of soft water, and imperfectly saturating the solution with carbonate of potassa; the sulphindylate falls down as a dark-blue867 coppery-looking powder, soluble in 140 parts of cold water and in about 90 parts of boiling water. This substance is kept both in the moist and dry state, and is known in commerce under the respective names of ‘DISTILLED INDIGO,’ ‘PRECIPITATED INDIGO,’ ‘SOLUBLE INDIGO,’ ‘INDIGO PASTE,’ ‘BLUE CARMINE,’ ‘DISTILLED BLUE,’ ‘SOLUBLE BLUE,’ &c. It is extensively used in dyeing; and when mixed with starch, whilst in the moist state, and made into cakes or knobs, it constitutes the finest variety of the ‘BLUE’ used by laundresses for tinging linen. The ammonia and soda salts may be prepared in the same way as the potassa salt, by substituting the carbonates of those bases for carbonate of potassa. The ammonia salt is very soluble.
INDIGO BLUE. See Indigotin.
INDIGO DYE. There are two methods of preparing solutions of indigo for dyeing.—1. By deoxidising it, and then dissolving it in alkaline menstrua.—2. By dissolving it in sulphuric acid. The former method is used in preparing the ordinary INDIGO VAT of the dyers.
1. a. (Cold vat.) Take of indigo, in fine powder, 1 lb.; green copperas (clean cryst.), 21⁄2 to 3 lbs.; newly slaked lime, 31⁄2 to 4 lbs.; triturate the powdered indigo with a little water or an alkaline lye, then mix it with some hot water, add the lime, and again well mix, after which stir in the solution of copperas, and agitate the whole thoroughly at intervals for 24 hours. A little caustic potassa or soda is frequently added, and a corresponding portion of lime omitted. For use, a portion of this ‘preparation vat’ is ladled into the ‘dyeing vat,’ as wanted. After being employed for some time, the vat must be refreshed with a little more copperas and fresh-slaked lime, when the sediment must be well stirred up, and the whole thoroughly mixed together. This is the common vat for cotton.
b. (Potash vat.) Take indigo, in fine powder, 12 lbs.; madder, 8 lbs.; bran, 9 lbs.; ‘potash,’ 24 lbs.; water at 125° Fahr., 120 cubic feet; mix well; at the end of about 36 hours add 14 lbs. more potash, and after 10 or 12 hours longer further add 10 lbs. of potash, and rouse the whole up well; as soon as the fermentation and reduction of the indigo are well developed, which generally takes place in about 72 hours, add a little fresh-slaked lime. This vat dyes very quickly, and the goods lose less of their colour in alkaline and soapy solutions than when dyed in the common vat. It is well adapted for woollen goods.
c. (Wood vat.) As the last, but employing wood instead of madder; the vat is ‘set’ at 160° Fahr., and kept at that temperature until the deoxidation and solution of the indigo has commenced. The last two are also called the ‘warm vat.’
d. (Pastel vat.) This is ‘set’ with a variety of wood which grows in France, and which is richer in colouring matter than the plant commonly known as ‘wood.’
e. (Schützenberger and De Lalande’s vat.) It is known that the low stage of oxidation of sulphur obtained on the reduction of sulphurous acid with zinc, dissolves indigo. On this reaction the following proceedings for dyeing and printing with indigo are founded:—To prepare the reducing liquid, a solution of bisulphite of soda at 35° B. is brought in contact with sheet zinc in a closed vessel, of which the liquid should occupy only one fourth. After the lapse of an hour the zinc is precipitated from the clear liquid by means of milk of lime. It is then diluted or decanted, or filtered with exclusion of air.
The clear liquid is then poured upon the ground indigo, with the addition of the needful soda and lime. One kilo of indigo yields in this manner a very concentrated vat of from 10 to 15 litres. Cotton is dyed cold, and wool with the aid of heat. A vat is filled with water, and a suitable quantity of the above indigo mixture introduced, when the dyeing can be performed at once. The excess of the low sulphur acid dissolves the froth which appears on the surface. During the process of dyeing, further quantities of indigo can be added as required. Cotton can be rapidly and easily dyed in this manner; and in the case of wool, the dyer escapes the many disadvantages of the hot vat and obtains brighter and clearer shades. To print a fast blue the alkaline solution of the reduced indigo is printed on with an excess of the reducing agent, aged for twelve to twenty-four hours, washed and soaped. In comparison with the old process there is a saving of indigo to the extent of 50 to 60 per cent.; the shades are richer and the impressions sharper. The colour requires no subsequent treatment, and can therefore be printed on simultaneously with most other colours.
Obs. Wool, silk, linen, and cotton, may each be dyed blue in the indigo vat. The goods, after being passed through a weak alkaline solution, are subjected to the action of the vat for about fifteen minutes; they are then freely exposed to the air; the immersion in the vat and the exposure are repeated until the colour becomes sufficiently deep. Wood and madder improve the richness of the dye. Other deoxidising substances, besides those above mentioned, may be used to effect the solution of the indigo; thus a mixture of caustic soda, grape sugar, indigo, and water, is often employed on the Continent for this purpose; and orpiment lime, and pearlash are also occasionally used. When properly prepared, the indigo vat may be kept in action for several months by the addition of one or other of its constituents, as required. An excess of either copperas or lime should be avoided.
868
2. Solution of sulphate of indigo is added to water, as required, and the goods, previously boiled with alum, are then immersed in it, and the boiling and immersion are repeated until the wool becomes sufficiently dyed.
Obs. With this every shade of blue may be dyed, but it is most commonly employed to give a ground to logwood blues. The colouring matter has affinity for woollen and silk with or without ‘mordant,’ but none for cotton. A solution of soluble indigo (sulphindylate of potassa or soda), in water very slightly acid with sulphuric acid, imparts a very fine blue to cloth, superior in tint to that given by the simple sulphate. See Dyeing, &c.
INDIGO PUR′PLE. Syn. Phœnicine. The name given by Mr Crum to the purple precipitate obtained by filtration from a solution of indigo in fuming sulphuric acid, when largely diluted with water.
INDIGO RED. Syn. Indigo resin, Red resin of indigo. This is prepared by boiling alcohol (sp. gr. ·830), on powdered indigo previously exhausted by digestion in dilute acids and in a strong alkaline solution. When heated, it is converted into a white sublimate (deoxidised indigo red), but recovers its red colour by the action of nitric acid.
INDIGO WHITE. Syn. Indigogene, Indicyle, Reduced indigo, Hydrogenised i., Hydrate of i. Reduced or deoxidised indigo blue.
Prep. The yellow alkaline solution obtained by one or other of the processes noticed under Indigotin is carefully protected from the air, both before and after precipitation with hydrochloric acid; and the precipitate, after being rapidly washed with recently boiled distilled water, or with very dilute sulphurous acid, is drained on a filter, dried in vacuo, and then at once transferred to a well-stoppered bottle.
Prop., &c. A greyish-white mass of minute crystals, generally light blue on the surface, and rapidly turning blue on exposure to the air; soluble in alkalies, alcohol, and ether, to which it imparts a yellow colour. These solutions deposit indigo blue on exposure to the air. A solution of this substance constitutes the indigo vat of the dyer (see above).
INDIGO′TIN. Syn. Cerulin, Indigo blue. This is the pure blue principle of indigo. It appears to be the oxide of the same organic radical of which indigo white is probably the hydrate.
Prep. 1. Indigo (in fine powder) is digested successively in dilute hydrochloric acid, solution of potassa, and alcohol; the dried residuum is crude indigotin.
2. Indigo (in fine powder), 1 part; green sulphate of iron, 2 parts; hydrate of lime, 3 parts; water, 15 parts; mix, agitate occasionally until the colour is destroyed, then decant the clear portion, precipitate with dilute hydrochloric acid, and wash the powder first with water, and then with boiling alcohol, until the latter ceases to acquire a yellow colour.
3. Caustic soda and grape sugar, of each 1 part; water, 20 parts; powdered indigo, 5 parts; mix, and proceed as last. The above are essentially the same as the indigo vat, but on the small scale.
4. The process for estimating the value of indigo given under Indigo is a good process for obtaining Indigotin.
Obs. The product from all the above exceeds 50% of the indigo operated on.
5. (Taylor.) Powdered indigo, 2 parts; plaster of Paris, 1 part; water, q. s. to reduce the mixture to a thin paste; spread the mass evenly upon an oblong iron plate to the depth of about 1⁄8 inch, and dry it by a gentle heat. It must then be held over the flame of a spirit lamp, when a disgusting odour will be evolved, the mass will begin to smoke, and in a few minutes will be covered with a heavy purple vapour, which will condense into brilliant flattened prisms or plates of an intense copper colour, forming a thick velvety coating over the surface immediately exposed to the heat. Should the mass catch fire, it may be instantly extinguished by a drop of water let fall upon it. Prod. 15 to 18%. See Indigo, &c.
INDIUM. In. = 113·4. This very rare metal was discovered by means of the spectroscope by Messrs Reich and Richter in a specimen of zinc-blende from Freiberg, in 1863. Since then it has been found in the flue dust of some zinc furnaces worked in the Hartz mountains, also in a black blende, known as christophite, occurring in Saxony; in the Wolfranc of Zinnwald, associated with zinc, as well as in Wolfranc alone; and also in the blende met within steatite, near Schlaggenwald. In all of these substances the indium is present in very minute quantity,[354] and is more or less associated with lead, arsenic, cadmium, iron, and copper; its separation from which is matter of no inconsiderable labour and difficulty.
[354] In the flue dust of the zinc furnaces it is present to the amount of about 0·1 per cent.; in christophite in the proportion of 0·0062 per cent.
The following process for the detection of indium in zinc-blende, and its extraction from the same source, is given by Winkler. Precipitate the hydrochloric acid solution of the roasted ore with metallic zinc at the boiling heat; dissolve the precipitate in nitro-hydrochloric acid; remove the arsenic, cadmium, &c., by sulphuretted hydrogen, and precipitate the indium as oxide by barium carbonate. Should this precipitate contain any iron, it must be removed by resolution, heating with sodium sulphate, and digestion with barium carbonate in a closed vessel. The indium may also be precipitated from the original solution, either directly by barium carbonate, or from a solution containing sulphuric acid, by neutralisation with sodium carbonate, till a precipitate begins to form, and869 addition of sodium acetate; it is then precipitated as a basic sulphate containing zinc.”[355]
[355] Various other processes are given in ‘Watts’ Dictionary.’
Indium may be obtained in the metallic state from the reduction of its oxide by means of hydrogen; charcoal or carbonaceous fluxes are not good reducing agents, as their employment necessitates a very high temperature, and loss from volatilisation occurs. Sodium is found to be the best reducing agent when large quantities of the metal are required.
Böttger’s method is to precipitate the indium by zinc, to press the spongy metal so obtained in hot water, then to submit it to pressure in a screw press between filtering paper, and finally to melt it with cyanide of potassium.
Prop. Indium is a soft, white, durable metal, somewhat resembling cadmium, wholly destitute of crystalline structure. Its specific gravity, which is 7·421 at 16·8° C., is not altered by rolling or hammering. When heated in the air to 176° C., it melts without becoming oxidised; at a temperature above this, however, it becomes covered with a coating of suboxide, becoming gradually changed into the yellow sesquioxide. Indium is less volatile than either cadmium or zinc. It dissolves slowly in dilute sulphuric and hydrochloric acids, hydrogen being given off. In strong hydrochloric acid it dissolves rapidly. Nitric acid oxidises it, evolving at the same time nitric oxide; whilst sulphuric acid converts it into anhydrous sulphate.
When examined by means of the spectroscope, the flame of indium reveals two brilliant bands—a violet and a blue one.
Indium is completely precipitated from a solution of its acetate, as well as from neutral solutions of its salts in general, by sulphuretted hydrogen. Ammonia, neutral sodium carbonate, and acid sodium carbonate, throw down white precipitates insoluble in excess of the precipitant; caustic potash and soda produce a white precipitate of indium hydrate, soluble in excess. Barium carbonate precipitates it completely. Potassium ferrocyanide gives a white precipitate.
Estim. “The most convenient method of estimating indium is by precipitating it as hydrate with ammonia, dissolving the washed precipitate in hot dilute nitric acid, evaporating, igniting, and weighing the oxide thus obtained. Precipitation with sulphuretted hydrogen does not give exact results on account of the solubility of the indium sulphide.”[356]
[356] Watts.
Indium forms compounds with bromine, chlorine, iodine, oxygen, and with several of the organic and inorganic acids.
INDURA′TION. In pathology, an increase in the consistence of any portion of the body, usually resulting from chronic inflammation, pressure, or friction.
IN′FANCY. “The domestic treatment of infants and children is comprised in the application of the laws of health to the mother as well as to the child. The position of parent is one of serious responsibility, both morally and physically, and the edict has gone forth that ‘the sins of the parent shall be visited on the children.’ If we could ensure good mothers, we could vastly improve the race of men. The nursing mother of a sick infant must, by following faithfully the rules of health in respect of the four great hygienic principles—food, clothing, exercise, and ablution—give health with her milk to her offspring; she must also pay close attention to her mind, avoid all sources of irritation and anxiety, and remember that an angry mother sours her milk, and produces a fractious and often a diseased infant. I am quite of opinion that if mothers were sound in constitution, and bestowed the requisite care upon the maintenance of their health, we should hear little of diseases of children. In children, as well as in parent, the rules of health must be carried out,” and their neglect cannot fail to bring with it a heavy retribution. (Eras. Wilson.) See Exercise, Nursing, &c.
INFANT DEATH-RATE. In England, according to Dr Farr, out of 1000 infants born, 149 die annually before reaching their first year; and the same authority tells us that 311 out of every 1000 die during the first month in the same period. Amongst illegitimate children, the lives of one half never exceed the first month.
The above figures represent the yearly average of infantile deaths throughout the whole of England, when we come to the large cities the mortality is notably higher. In Liverpool, for instance, out of 1000 children born, 239 died in their first year.
When we examine into the infant mortality prevailing amongst different classes, we find the proportion existing between the death-rate of the children of the nobility, and the general death-rate up to one year, to be as 3 to 8.
In 1874, Mr Charles Ansell, jun., published a work entitled ‘Statistics of Families of the Upper and Professional Classes,’ in which he showed, from investigations into the deaths occurring amongst 48,000 children of the wealthy, professional, and titled classes, that in the first year of life, about 80 only of such children die out of every 1000. According to Dr Farr, the northern countries of Europe show a much lower infant death-rate than the southern ones. Infant mortality is lowest in Norway, and highest in High Bavaria, where 404 infants per 1000 die in their first year. In New York, in 1869, the mortality amongst infants under one year old was 27·4 per cent. and in 1873, 31·0 per cent.
Both in France and England the mortality prevailing amongst illegitimate children up to the age of one year is very large. In 1860, the death-rate amongst the foundlings of the870 Loire-Inférieure, was as much as 876 in the 1000, and it averages between 500 and 700 in France. In Wakefield, amongst the same class of children, it was 26·22 per cent.; in Coventry, 40; in Padstow, 50; and in Bantry, 80; in manufacturing towns the average is 35 per cent. In London the number of illegitimate children who die annually under the age of a year is probably about 75 per cent.[357]
[357] ‘Proceedings of the Obstetrical Society for 1870.’
In the Montreal Foundling Asylum, out of 4060 infants, only 7 per cent. lived one year. In the rural districts of England and also in Bavaria, the average of deaths at one year is about the same for the illegitimate as for the legitimate children.
In the ‘Transactions of the Obstetrical Society for 1870’ there is a valuable and interesting report, throwing much light on the condition and treatment of the children of the poor in England. The following is the substance of this report, the information contained in which was collected by various members of the society:—It was found that amongst the poorer populations of villages 30 to 90 per cent. are attended by midwives, and that this custom prevails to an almost equal extent in the large provincial and manufacturing towns. Thus in Glasgow, 75 per cent., in Coventry, 90 per cent.; and in Leeds and Sheffield, equally large numbers of the population employ the services of these women. In Edinburgh the midwife is rarely called on; neither is she in the West End of London; but in the East End 30 to 50 per cent. of the accouchements are undertaken by women.
Except in Glasgow, Sheffield, and London, the women are asserted to be totally ignorant and incompetent to meet any difficulty that might arise. In country districts the pernicious custom of giving an aperient to a newly born babe was very general, but less prevalent in London and the large towns. Amongst the married poor, suckling was found to be the rule; and it seemed to be pretty conclusively proved that it is often unreasonably prolonged for eighteen months or even two years, as a preventive to renewed pregnancy. It was found, however, that illegitimate children were rarely suckled, but almost always fed on artificial food. Amongst the married poor also it appears universal to give the children artificial food as well as the breast, and this from a very early and tender age. Further, it was found that the food was generally unsuited to the child both in quantity and quality. It consisted chiefly of bread soaked in water and milk, and sweetened, of arrow-root, sago and corn flour, and such like objectionable substances.
In one case the mother admitted to giving her infant (who was a few months old only) a diet similar to that she herself partook of, viz.—cheese, bread, meat, salt fish, heavy pastry, vegetables and beer. Amongst the upper classes it was ascertained that there is an increasing tendency amongst mothers to discontinue lactation, and to employ instead the services of a wet nurse; and where this was not done, it was found that the food partaken of by the babe was much more judiciously chosen than is the case amongst the poorer women. Mr Curquiven and others observed that a large number of women in London do not suckle their offspring, because of a deficient secretion of milk. It is satisfactory to find that both in the manufacturing and agricultural districts, the children of the poor are so constantly in the open air; but equally unsatisfactory to learn that at night they sleep in ill-ventilated and over crowded dormitories. The infant is encouraged to sleep to the utmost, and should it fail in securing renewed slumber (its waking being attributed to a desire for food), is very often dosed with gin, syrup of poppies, and paregoric. Another plan adopted for keeping infants quiet, consisted in letting them remain in the cradles and allowing them to suck the nipple attached to the empty feeding bottle, long after the food had been consumed; a practice that gives rise to infantile dyspepsia.
As regards cleanliness, it was learnt that poor people’s children are tolerably well attended to in this respect, or rather that the baby generally comes in for a larger share of ablution than the elder ones, who are sometimes much neglected in the matter of soap and water. The clothing of the little ones was much too scanty, and this was the case no matter what the season of the year. A prevalent practice, except in the agricultural villages, was that of giving the children cordials, spirits, and medicines.
A still worse custom was found to be the systematic administration of opiates. At Long Sutton, in Lincolnshire, which has a population of 6000, one chemist alone sold 251⁄2 gallons yearly of Godfrey’s cordial (a mixture principally consisting of treacle and opium), whilst 61⁄2 pints were got rid of weekly by another chemist in the same town. This administration of anodynes was mostly confined to illegitimate infants, and factory children placed out to nurse. A habit not unusual was that of an intentional deferring sending for a doctor when the child was first seized with illness, medical advice being only sought when in many cases it could be of no avail. Desertion by fathers and mothers of their children, especially of illegitimate ones, as well as concealment of birth and infanticide, were found to be much more general in London and the larger cities than in the country.
The causes of the high rate of infant mortality prevailing amongst the poor, seem very clearly indicated in the above abstract. For instance, the deaths of nearly half the children under one year of age are referable to diarrhœa, convulsions, atrophy, mesenteric871 disease, and allied disorders; all of which maladies are caused by the grossly erroneous and unsuitable diet upon which the children are fed. In the article “Infants’ Food,” we have already pointed out that the proper and only safe aliment for an infant up to the age of eight or nine months is the maternal milk, and failing this, the pure and unadulterated milk of the cow; and we have furthermore shown that the admission into the dietary of infants even above nine months old, of farinaceous foods, should be regulated with great caution. Yet we learn from the above report, that in the early days of the infants’ existence, amongst the poor the breast milk is in most cases supplemented by large quantities of these very farinaceous matters, which we have shown to be so prejudicial and dangerous. That the deaths from these causes are clearly preventable, in a large measure, if not wholly, is proved by the very much less extent in which they occur amongst the higher classes, who use much greater judgment in the selection of their children’s food. Another important factor in the high infant death-rate is the extensive use of narcotics, a practice there is no doubt which yearly carries off a large number of infants, by poisoning, more or less prolonged. Inadequate clothing is likewise another source of mortality amongst the very young, whose tender frames easily succumb to inclement weather, and the sudden changes of temperature occurring in our variable climate. Hence we shall have no difficulty in understanding why pneumonia, bronchitis, &c., should be so prevalent among poor children. The habits of overcrowding and bad ventilation which prevail amongst the poor must also be fertile sources of disease amongst their offspring—the more immediate effect of such violations of sanitary principles resulting in bronchitis. That the extensive recourse by the poor to uneducated and unskilful midwives, also adds to infant mortality, seems indisputable.
In the report already alluded to, beyond reference to the fact that infanticide was rare, we find no mention made as to the number of violent deaths occurring amongst infants. It appears that about a sixteenth of the mortality amongst infants under one year old is due to violence, mostly accidental, the great majority of such deaths being caused by the mother lying upon and smothering her babe. These misadventures are said to occur mostly on Saturday nights, and raise the question as to whether a large proportion of such deaths are not due to the drunkenness of the mothers, who retire to rest in a state of alcoholic stupor.[358]
[358] Blythe.
The inference, we think, to be drawn from the above statements is, that the preponderating mortality prevailing amongst the children of the poor is due to ignorance and poverty, and not to intentional neglect or want of parental affection. Bearing in mind that poor women much more frequently suckle their babes than rich ones, it might perhaps be argued that this was evidence of greater maternal solicitude, and that, therefore, the poor mother exhibits more natural affection than the lady who consigns her offspring to the arms of the wet-nurse; but, possibly, were the circumstances of the two inverted, the lady might be found giving her infant the breast, whilst the humbler wife might call in the services of the wet-nurse; neither of them perhaps reflecting that, in choosing the latter alternative, they were depriving another little human unit of the maternal sustenance, and exposing it to the dangers of vicarious nurture and supervision. What these dangers are that beset the children of the poor when removed from their mother, the revelations of baby-farming and the appalling statistics of illegitimate infant mortality serve very forcibly to illustrate. For every 311 legitimate children of all classes 500 illegitimate out of every 1000 die each year under one month old, this large increase being due to cruel neglect and substitution of bad, insufficient, and improper food for the maternal milk. That in their own homes they are exposed to the serious hardships arising from errors in diet has already been shown, but in their own homes they die at little more than half this rate; hence the deduction is unavoidable that half of these poor little waifs perish because they are deprived of the care and solicitude of the mother.
Writing on this subject M. Hanon says that of 59,927 infants born in Paris, 20,049 are sent into the country to nurses. Of those children that remain in Paris, and that are so rarely suckled by their mothers, no less than 8250 die from 0-1 year, which gives a death-rate of 243 per 1000 births. As to the mortality of the unfortunate infants, 20,049 in number, sent off to nurse, this amounts to 500 or 700 per 1000 in the first year of life.
We extract the following from the ‘Echo’ of October 9th, 1878:
“Dr Hardwicke, the coroner for Central Middlesex, held an inquest at the Islington Coroner’s Court, Holloway Road, this morning, relative to the death of the female child of Emily Corley, a servant at 49, Gower Street, Euston Road. Mr Baby, the inspector under the Infants Life Protection Act, watched the case. The mother of the child had gone to service as a wet nurse after coming out of the workhouse, leaving it with one Ann Leach, who said it was a very delicate child. She added that she had for a time two children under one year of age with her, and had been told that this was contrary to the Act. The inspector under the Infant Life Protection Act pointed out that persons having more than one child to nurse under a year old had to obtain a licence. The coroner characterised the Act872 as a mere farce. It left children to take care of themselves after they were twelve months old—the most critical time of their existence. He also remarked on how prolific a source of prostitution such cases as the present were, where mothers had to forsake their own illegitimate offspring, depriving them of breast milk in order that they might sell it to the rich. The jury found that the child died from exhaustion following from diarrhœa, accelerated by want of breast milk.”
INFANTS, Food for. For the newly-born and very young of all mammiferous animals, no food is so expressly and admirably adapted as that drawn from the mother. In the nourishment of the babe from the maternal breast lies the soundest condition for its physical well-being and growth, subject to the qualification that the mother must be in good health, which, of course, implies that she must be well fed. This latter essential fulfilled, it is very wonderful to note how nature makes provision for the proper nourishment of the offspring by converting even a weakly and frequently ailing mother into a strong one during the period of suckling.
There may be, and doubtless are, many circumstances in which lactation cannot be practised with safety either to mother or child; but, where such circumstances do not exist, the practice of seeking the vicarious services of the wet-nurse, or of having recourse to other than the maternal milk, for many reasons, calls for remonstrance and reproof.
We may emphasise this by the following quotation from Dr West’s admirable work, ‘Diseases of Infancy and Childhood.’ He says: “The infant whose mother refuses to perform towards it a mother’s part, or who by accident, disease, or death is deprived of the food that nature destined for it, too often languishes and dies. Such children you may see with no fat to give plumpness to their limbs—no red particles in their blood to impart a healthy hue to the skin, their face wearing in infancy the lineaments of age; the voice a constant wail; their whole aspect an embodiment of woe. But give to such children the food that nature destined for them, and if the remedy do not come too late to save them, the mournful cry will cease, the face will assume a look of content, by degrees the features will disclose themselves, the limbs will grow round, the skin pure red and white.”
But although the maternal aliment (or, failing this, that supplied from the breast of a young and healthy wet-nurse, who has been recently confined) is undoubtedly the best adapted for infantile nutrition, it fortunately happens that in circumstances where the infant is unable to be fed from either of these sources, we have a very valuable substitute in the milk of the cow, the similarity of which, in composition to woman’s milk, will be seen at once by studying the following table arranged by Dr Letheby:
| Woman’s Milk. | Cow’s Milk. | |||
| Max. | Min. | Average. | Average. | |
| Casein | 4·36 | 2·97 | 3·52 | 3·64 |
| Butter | 5·18 | 4·45 | 4·02 | 3·55 |
| Sugar of Milk | 4·43 | 3·29 | 4·27 | 4·70 |
| Various salts | 0·26 | 0·38 | 0·28 | 0·81 |
| Total solids | 14·20 | 11·09 | 12·09 | 12·70 |
| Water | 85·80 | 88·91 | 87·91 | 87·30 |
| Total | 100·00 | 100·00 | 100·00 | 100·00 |
The milk of the cow being rather richer in solids than that of woman, it is considered desirable to somewhat dilute the former when it is used as food for the infant. Dr Letheby recommends the addition to it of a third of water, with a little sugar to sweeten it, and to render it more acceptable to the baby palate. It cannot be too forcibly insisted upon that immeasurably the best and safest food for an infant, next to human milk, is the milk of the cow, and nothing else, until it reaches the age of eight or nine months. It is perhaps needless to state that the milk must be perfectly pure and unadulterated, and that it will fail of being this if yielded by an unhealthy cow. The animal’s food and habitat also exercise an important influence on the quality of the milk, that given by grass-fed cows roaming in open pastures undoubtedly being the best and richest.
Different cows yield different qualities of milk; hence, when milk from any particular cow suits an infant, it has been found desirable not to change it.
The newer and fresher the milk the better is it adapted for the child’s use; that which has in the least become soured should be especially rejected.
Sometimes even fresh and good milk is found to disagree with a child. When this is the case it may be remedied by adding a little lime water to it previous to its being drunk. If it were practicable, and within the means of every family to keep their own cow, so that the infant could be fed with the milk directly it came from the animal, nature’s example in giving it direct from the mother’s breast might be followed. The writer remembers, some years ago, the Princess of Wales travelling with her baby on a voyage to and from Denmark, and being accompanied by her bovine purveyor in the shape of an Alderney.
In hot weather, more particularly, if milk be kept even for a short time it is liable to become acid, or “to turn,” as it is called. It is, therefore, always desirable to keep it in a cool cellar till required for use, and in very hot weather it should be stood in ice.
The daily allowance of milk for a child during the first month of its life is two or three pints. M. Guillot says 21⁄2 lbs. avoirdupois is873 the least the babe can properly subsist on. He weighed several infants before and after they had taken the breast, and found that they had gained in weight, in quantities varying from 2 oz. to 5 oz.
Opinion is divided as to the value of condensed cows’ milk as a food for infants. Its chief merit seems to be that it affords a substitute for the natural milk in cases where this latter is not obtainable, or where, in consequence of disease amongst the cows of a neighbourhood, it cannot with safety be consumed. Since the maternal fluid, without undergoing alteration or modification, forms so perfect and model a food for infants, it does not seem an unreasonable inference that the milk from the cow, which so nearly approaches it in composition and qualities, should prove most advantageous when partaken of under similar conditions. It has been asserted that condensed milk is inferior in strengthening qualities to the natural cows’ milk. If this be the case it is certainly not due, according to Mr Wanklyn, to any removal of the constituents of the latter. In his useful little work on ‘Milk Analysis’ Mr Wanklyn says: “A year ago a report was spread that these preserved milks were preserved skim-milk, and not preserved new milk. I have myself examined the principal brands of preserved and condensed milk, which are in the London market, and find that the milk which has been condensed, or condensed and preserved, had been charged with its due proportion of fat.”
The physiological facts that in an early stage of infancy the digestion is very feeble, and that until an infant has cut its first teeth there is but little, if any, secretion of saliva, which latter is essential for the conversion in the system of starch into sugar, point, therefore, to the imprudence of feeding very young infants upon so-called “infants’ foods,” where these consist of amylaceous substances. The starch of which these latter are composed not only fails to become assimilated, and therefore to produce no nutrient effect, but clogs up the lower parts of the bowels, and thus gives rise to a train of evils, amongst which may be included indigestion, diarrhœa, vomiting, and not infrequently convulsions and death.
The difference in the mortality between infants under one year of age who annually die of convulsions in England and Scotland is attributed to the fact that whereas the English mother feeds her offspring on thick spoon-food, the Scotch woman nourishes hers from the breast. In ‘The Fourteenth detailed Annual Report of the Registrar-General of Scotland’ it is stated that “The English practice of stuffing their babes with spoon-meat occasioned the death by convulsions of 23,198 children under one year of age during the year 1868, out of 786,858 births; in other words, caused 1 death from convulsions in every 34 of the children born during the year in England. In Scotland, during the same year, only 312 infants under one year of age fell victims to convulsions out of 115,514 children born during the year; in other words, 1 death from convulsions in every 370 born during the year.”
When a child has reached the age of eight or nine months the judicious use of farinaceous foods is not only unobjectionable but desirable; but even then it is most important to increase the quantity of the food very cautiously with the age, as well as to see that it has been well baked and afterwards boiled before being partaken of. In all cases it should be mixed with the milk.
When the child has reached the age of twenty months Dr Letheby advises the quantity of farinaceous food to be still further increased, and with a little egg given in the form of pudding until it attains its third year. At this period the child’s diet may also include bread and butter, and at the end of it well-boiled potato with a little meat gravy.
From the third to the fifth year he prescribes a small quantity of meat, and at the end of the ninth year the usual food of the family. During all these periods the use of milk as an important article of the dietary is enforced.
The following table by the late Dr Edward Smith, exhibiting the proportions between the daily quantities of carbon and nitrogen required at different periods of human existence, illustrates the great preponderance of nitrogen demanded by the infant over those who succeed him in the scale of age:
| Carbon. | Nitrogen. | |
| In infancy | 69 | 6·72 |
| At ten years of age | 48 | 2·81 |
| At sixteen years of age | 30 | 2·16 |
| At adult life | 23 | 1·04 |
| In middle life | 25 | 1·13 |
See Milk.
IN′FANTS′ PRESER′VATIVE. (Atkinson’s.) Carbonate of magnesia, 6 dr.; white sugar, 21⁄2 oz.; oil of aniseed, 20 drops; compound spirit of ammonia and rectified spirit, of each 21⁄2 fl. dr.; laudanum, 1 fl. dr.; syrup of saffron, 1 oz.; caraway water, q. s. to make the whole measure 1 pint. Antacid, anodyne, and hypnotic.
INFEC′TION. Syn. Contagion. The communication of disease, either by personal contact with the sick or by means of effluvia arising from their bodies. Attempts have been made to restrict the term contagion to the former, and infection to the latter, but this distinction is now discarded by the majority of writers. The following are the principal diseases which are commonly regarded as contagious:—Chicken-pox, cholera, cow-pox, dysentery, erysipelas, glanders, gonorrhœa, hooping-cough, hydrophobia, itch, measles, mumps, ophthalmia (purulent), plague, scald-head, scarlet fever, smallpox, syphilis, yaws. See Disinfectant, 874&c.
INFLAM′MABLE AIR. See Hydrogen.
INFLAMMA′TION. Syn. Inflammatio, L. In pathology, a certain state of disease. The common symptoms of inflammation are pain, swelling, heat, and redness, attended with fever, and general constitutional derangement when severe.
The treatment of inflammations, whether trifling; or serious, is essentially the same in principle, and only differs in degree. This consists in the adoption of the usual means for lowering the force of the circulation and the frequency of the pulse; of which leeching, purging, a low diet, and the use of refrigerant drinks and lotions, form the most important part. The constitutional derangement or symptomatic inflammatory fever, and inflammatory condition of the blood always accompany local inflammation, and progress with its intensity. In inflammations of a more purely local character, cupping or leeching the part immediately affected, or the parts adjacent to it, is in general more appropriate and successful. In these cases the application of refrigerant or sedative lotions, baths, &c., generally proves of much advantage. In cases in which there is induration or dryness of the part, the use of warm embrocations is indicated.
Inflammation often arises from apparently very trifling causes, particularly in persons of a full or bad habit of body, or who indulge in the free use of malt liquors. In some persons a very trifling local injury, as a slight abrasion, cut, prick, or sprain, produces a considerable amount of tumefaction, attended with severe constitutional excitement. Punctured wounds, sprains, and dislocations commonly furnish the most serious cases of inflammation that depend on mere external injury.[359] See Abscess, Fever, Tumour, &c.
[359] In all inflammatory cases of a serious nature, the reader is strongly advised to commit himself to the care of a medical practitioner.
Inflammation of the Bowels. The common causes are incautious exposure to cold, the use of improper food, and the presence of acrid substances or hardened fæces in the bowels. The more constant symptoms are pain over the abdomen, thirst, heat, and extensive restlessness and anxiety; sickness, obstinate constipation, and a hard, small, quick pulse. In the later stages the pain and tenderness of the abdomen, especially around the navel, become excessive, and there is difficult micturition. In some cases the pain suddenly ceases, the belly becomes tumid, the pulse scarcely perceptible, the countenance ghastly, and the patient dies in a few hours. The treatment consists in blisters, leeches to the abdomen, hot bath and fomentations, aperient clysters, and mercurial purges; with effervescing draughts and opium to allay sickness, followed by diaphoretic salines and gentle aperients. See Stomach affections, &c.
INFLAMMATORY FE′VER. See Fever and Inflammation.
INFLUEN′ZA. See Catarrh.
INFU′SION. Syn. Infusum, Infusio, L. A liquid medicine, prepared by macerating vegetable or animal substances in water, at any temperature below that of ebullition.
The mode of preparing infusions is, with most substances, precisely similar to that pursued for making the almost universal beverage—TEA. The ingredients are commonly placed in a stoneware pot or vessel (an ‘infusion pot’), previously made hot; boiling water is then poured over them, and the cover being placed on, the whole is allowed to digest together, at first, for a short time, in a warm situation, as on the hob or the fender, and afterwards (the vessel being removed from the heat) until the whole becomes cold. The liquid is then poured from the ingredients, and the latter, being slightly pressed, if necessary, the infusion is strained through a piece of clean linen or a hair sieve for use. During the digestion the ingredients should be occasionally stirred, an important matter often neglected, and not even referred to by most pharmaceutical writers.
The substances employed for making infusions receive the same preliminary treatment as those intended for making DECOCTIONS. Shavings, leaves, and flowers require no previous preparation beyond being pulled asunder; but roots, woods, and other solid substances must be bruised or sliced, if in the green or recent state, or bruised or coarsely pulverised, if dry, for the purpose of exposing as large a surface as possible to the action of the menstruum.
The substances extracted by water from vegetables by infusion are chiefly gum, mucus extractive, tannin, certain vegetable acids, the bitter and narcotic principles, gum-resin, essential oil, and alkaloids. Some of these substances are only sparingly soluble in water at ordinary temperatures; but more readily so in hot water, and freely soluble in boiling water. The temperature of the water should be therefore proportioned to the nature of the vegetable matter operated on. For mere ‘demulcent infusions,’ in which starch and gum are the chief substances sought to be dissolved out, and when the active principle is scarcely soluble in water, unless at nearly the boiling temperature, boiling water alone should be employed; but when the medicinal virtues of vegetables are soluble in water at lower temperatures, it is better to employ hot water (165° to 175° Fahr.), and to allow a little longer period for the digestion. In many cases temperate water (from 60° to 70° Fahr.), or tepid water (from 80° to 90° Fahr.), may be used with advantage, especially in the preparation of ‘aromatic bitter infusions,’ and in most cases where it is wished that the product should contain as little inert matter as possible; but when water at low temperatures is875 employed, the period of the maceration must be proportionately increased. By adopting the method of maceration in vacuo, or in an atmosphere of carbonic acid, the menstruum may be allowed to lay in contact with the vegetable matter for an unlimited period, without decomposition taking place.
Infusions, like decoctions, are liable to undergo spontaneous decomposition by keeping, especially in warm weather, when a few hours are often sufficient for their passage into a state of active fermentation; they should, therefore, when possible, be prepared for use daily, as beyond twenty-four hours they cannot be depended on. The London College directs a pint only to be made at a time, thus very properly regarding them as extemporaneous preparations.
Concentrated infusions, now so common in the shops, and, unfortunately, so generally used in dispensing, are either made by taking 8 times the quantity of the ingredients ordered in the pharmacopœia, and then proceeding in the usual manner, or by the method of displacement; or, by carefully and rapidly concentrating the simple infusions, by evaporation in a steam or salt-water bath, until reduced to about 1-7th of the original quantity. In either case the liquid is put into a strong bottle, without being filtered, and 10 to 12% of rectified spirit added to it, whilst still hot. The cork is then put in and secured down, and the whole agitated for some minutes, after which it is set aside for a week, when the clear portion is carefully decanted from the sediment for sale. Another method, which answers well with the aromatic bitter vegetables, is to take 8 times the usual quantity of the ingredients, and to exhaust them with a mixture of rectified spirits, 1 part, and distilled water, 3 parts; by digestion, or, better still, by percolation. Concentrated infusions made in this way keep well, and deposit scarcely any sediment. Many houses that are remarkable for the ‘brilliancy’ and beauty of these preparations, employ 1⁄3 spirit of wine and 2⁄3 water as the menstruum. It may, however, be taken, as a general rule, that for vegetable substances that abound in woody fibre, and contain little extractive matter soluble in water (as quassia, for instance), 1⁄6 to 1⁄5 part of spirit is sufficient for their preservation; whilst for those abounding in mucilage or fecula, or that readily soften and become pulpy and glutinous in weak spirit (as rhubarb), 1⁄5 to 1⁄3 is required.
By adopting the method originally suggested by Mr Alsop, infusions may be preserved, uninjured, for a year or longer, without the addition of spirit or any other substance. The only precaution necessary is to keep them in bottles, perfectly filled and hermetically sealed.[360] Our own plan is to put a few bruised cloves or seeds of black mustard into the bottles, which must be only 2-3rds filled, then completely fill them with a condensed atmosphere of carbonic acid gas; and, lastly, to stopper them and seal them over, so as to perfectly exclude the air. A pint of decoction of sarsaparilla and 1⁄2 pint of infusion of calumba, treated in this way, kept good for fully 9 years. By simply macerating in the infusion as much bruised mustard seed as can be added without flavouring the liquor, along with a little bruised cloves, we find that most vegetable infusions may be preserved in bottles which are occasionally uncorked, without either fermenting or becoming mouldy, by the use of very little spirit (1⁄9 or 1⁄10).
[360] ‘Pharm. Journ.,’ i, 57.
Before adding the spirit to infusions made with cold water, or with water which is only tepid, it is advisable to heat the liquid to about 185° Fahr., in a water bath, and after keeping it at that temperature for a few minutes, and allowing it again to become cold, to separate it from the precipitated matter, either by filtration or decantation.
It is often very difficult to render vegetable infusions and decoctions perfectly transparent, a quality always expected in the concentrated preparations. Defecation by repose is always better than filtration, owing to the more or less viscidity of the suspended matter. When this is not sufficient, they may be clarified with white of egg (2 or 3 to the gall.), previously beaten up with 5 or 6 fl. oz. of water. Most of the vegetable infusions and decoctions will readily pass the filter, after a very small quantity of acetic, nitric, or sulphuric acid has been added to them. The most obstinate may be rendered ‘brilliant,’ or ‘candle bright,’ as the ‘cellarmen’ call it, by shaking them up, first with about a drachm of dilute sulphuric acid, and afterwards with the whites of 3 or 4 eggs, previously mixed with a few ounces of water, for each gallon of the liquid. This plan is, however, objectionable for many medicinal preparations.
As many infusions which are occasionally employed in medicine must necessarily escape being separately noticed in this work, it may be as well to remark that the infusions of all vegetables that do not exert a very powerful action on the human frame as ordinary herbs and roots may be made by pouring 1 pint of boiling water on 1 oz. of the vegetable matter, and allowing it to macerate for 1⁄2 an hour to an hour. The decoctions of the same vegetables may be made by simply boiling the above ingredients, in the same proportions, for 10 or 15 minutes, instead of operating by mere infusion. With substances of somewhat greater activity, only half the above quantity should be taken; whilst, with the narcotic plants and those possessing great activity, 1 to 2 dr. to water, 1 pint, will be the proper quantity. The ordinary dose of such infusions and decoctions is 1⁄2 to 1 wine-glassful (1 to 2 fl. oz.), two, three, or four times a day, as the case may indicate.
Infusion is preferred for all bodies of a delicate texture,876 which readily yield their active principles to water; and especially when these are either volatile or liable to be injured by the heat of ebullition.
The simple infusions are now less frequently made by the druggist than formerly. In most cases he merely furnishes the ingredients, and the infusions are prepared by either the nurse or patient, by whom they are commonly called ‘TEAS,’
⁂ The following list embraces most of the infusions used in prescribing or noticed in books. Where the proportions of the ingredients are not given, 1 oz. of the medicinal substance and 1 pint of boiling water are to be taken, and the dose is that referred to above.
Infusion of Agrim′ony. Syn. Agrimony tea; Infusum Agrimonii, L. From the fresh tops before the flowers are formed. Vermifuge.—Dose. A teacupful 3 or 4 times a day; also used as an astringent gargle and lotion. For internal use, an equal weight of liquorice root (sliced) is commonly added.
Infusion of Al′kaline. Syn. Infusum alkalinum, L. Prep. (Beasley.) Hickory ash, 1 pint; wood soot, 1⁄4 pint; boiling water, 1 gall.; in 24 hours decant the clear. “A popular remedy in America for dyspepsia with acidity.”
Infusion of Alkaline. Syn. Infusum alkalinum. Prep. Hickory ash, 1 lb.; wood soot, 1⁄4 lb.; boiling water, 1⁄2 gall. Let them stand 24 hours, and decant. A wine-glassful three or four times a day. This is simply another form of the previous preparation.
Infusion of Al′oes. Syn. Infusum aloës, D. Prep. 1. From hepatic or Socotrine aloes (in powder), 2 dr.; carbonate of potassa, 11⁄2 dr.; boiling water, 1 pint.
2. (Compound; Infusum aloës compositum, L.)—a. As the COMPOUND DECOCTION OF A. (Ph. L.), but using only a pint of boiling water.
b. (Fothergill.) Calumba and rhubarb, of each, 1 oz.; aloes, 2 dr.; lime water, 16 fl. oz.; spirit of horseradish, 1 fl. oz.; macerate in the cold for 12 hours, and strain. The last three, like the decoction, are aperient, antacid, stomachic, tonic, and emmenagogue.—Dose, 1 tablespoonful to a small wine-glassful, in water. The last one is an admirable medicine in dyspepsia, loss of appetite, and troublesome constipation.
Infusion of Amer′ican Calum′ba. Syn. Infusum Fraseræ, L. From the dried root of American calumba (Frasera Carolinensis). A pure, powerful, and excellent bitter, destitute of aroma, and fully equal to gentian. (Lindley.)
Infusion of Amer′ican Cen′taury. Syn. Infusum sabatii, L. From the herb (Sabbatia angularis). A pure bitter tonic, without astringency or aroma.
Infusion of Amer′ican Sen′na. Syn. Infusum cassiæ Marylandicæ, L. Prep. (Martin.) Leaves of American or wild senna (Cassia Marylandica), 11⁄2 oz.; coriander seed, 1 dr.; boiling water, 1 pint. Purgative.
Infusion of Angel′ica. Syn. Infusum angelicæ, L. From the root of garden angelica. A warm stomachic and diaphoretic; and, in large doses, aperient. It is a popular remedy in dyspepsia, flatulent colic, and heartburn.
Infusion of Aniseed. Syn. Aniseed tea; Infusum anisi, L. Carminative; an excellent adjunct to purgatives, to prevent griping; given to infants to relieve colic, &c. Dr Prout recommends the use of water at 120° or 125° Fahr.
Infusion, Antiscorbu′tic. Syn. Infusum antiscorbuticum, Mistura antiscorbutica, L. Prep. Water trefoil (Menyanthes trifoliata), 1 oz.; orange peel, 2 dr.; boiling water, 1 quart; infuse for 8 or 10 hours, strain, and add of compound spirit of horseradish, 5 fl. oz. In scurvy.
Infusion of Ar′nica. Syn. Infusum arnicæ, L. 1. From the flowers of mountain arnica or German leopard’s bane (Arnica montana). Cottereau orders 1 oz., Dr Pereira 1⁄2 oz., and Dr A. T. Thomson, 1⁄4 oz. of the flowers to the pint. The first is the usual quantity. The dose of the first is a tablespoonful; of the second, 1⁄2 to 1 fl. oz.; of third, 1⁄2 to 1 wine-glassful.
2. (Compound; Infusum arnicæ compositum, L.—Ph. Copenh.) Flowers of arnica, 1 dr.; peppermint, 2 dr.; chamomiles, 1⁄2 oz.; boiling water, 1⁄2 pint.—Dose, 1 fl. oz. As the last.
Infusion of Arnica-root. Syn. Infusum arnicsæ radicis, L. Prep. (Ph. Castr. Ruth.) Arnica root, 40 gr.; water, 1 lb.—Dose, 1 fl. oz. As the above.
Infusion, Astrin′gent. Syn. Infusum astringens, Mistura a., L. Prep. 1. From oak-bark.
2. Infusion of cusparia, 17 fl. oz.; tincture of catechu or kino, 1 fl. oz.; powdered ipecacuanha, 1 dr.; powdered opium, 12 gr.; mix. In diarrhœa, &c. It must be well shaken before pouring out the dose.
Infusion of Balm. Syn. Infusum melissæ, L. Prep. (Plenck.) Fresh herb, 5 dr.; boiling water, 1 pint; infuse for fifteen minutes.
Infusion of Aya-pana, Compound (Dr Camera). Syn. Infusum ayæ-panæ compositum. Prep. Leaves of Brazilian aya-pana, 2 dr.; aniseed, 1 dr.; boiling water, 2 pints.
Infusion of Bar′berry. Syn. Infusum barberis, L. Prep. (Dr Copland.) From the bark of the barberry shrub (Berberis vulgaris). In jaundice, biliary fluxes, and other cases where heat and acrimony prevail; either alone or combined with a little carbonate of soda or potassa, and tincture of calumba.
Infusion of Bark. See Infusion of Cinchona.
Infusion of Bay-leaves. Syn. Infusum lauri, I. lauri nobilis, L. From the leaves or the berries of the sweet bay (Laurus nobilis).877 Aromatic, stimulant, and emmenagogue; in very large doses, emetic and poisonous. It is chiefly given in colic, flatulence, paralysis of the extremities, and obstructed menstruation.
Infusion of Bearberry (B. P.) Syn. Infusum uvæ ursi. Prep. Infuse bearberry leaves, bruised, 1⁄2 oz.; in boiling distilled water, 10 oz.; in a covered vessel for 2 hours, and strain.
Infusion of Beef. See Essence, Tea, &c.
Infusion of Belladon′na. Syn. Infusum belladonnæ. L. Prep. 1. (Dr Paris.) Leaves of deadly nightshade (dried), 4 gr.; boiling water, 2 fl. oz.; for a dose.
2. (Compound;—Dr Saunders.) Leaves (dried), 1⁄2 dr.; boiling water, 12 fl. oz.; infuse, strain, and to every 7 fl. oz. of the infusion add of compound tincture of cardamoms, 1 fl. oz.
Infusion of Bis′tort. Syn. Infusum Bistortæ, L. Prep. (Radius.) Bistort or snake-weed root (Poligonum Bistorta), 1⁄2 oz.; boiling water, 1 pint; infuse 2 hours, and strain. In passive hæmorrhages.
Infusion of Black Snake-root. Syn. Infusum cimicifugæ racemosæ, L. In dropsy, rheumatism, and chest complaints.
Infusion of Blessed Thistle. Syn. Infusum cardui benedicti, L. From the whole herb. In small doses it is diaphoretic; in larger ones, tonic, stomachic, and deobstruent; taken warm, it is occasionally given to promote the action of emetics. The properties of carduus benedictus “are such as to lead us to the belief that it has been superseded by other not more efficacious remedies.” (Lindley.)
Infusion of Blood-root. Syn. Infusion of puccoon; Infusum sanguinariæ, L. Prep. Blood-root (Sanguinaria Canadensis), 1⁄2 oz.; boiling water, 1 pint. Stimulant and emetic.
Infusion of Blue Flag. Syn. Infusum iridis versicoloris, L. Prep. 1. From the flowers of blue flag (Iris versicolor).—2. From the root of rhizomes. The first is used chiefly for its rich colour, as a test, &c.; the second is diuretic and cathartic, and apt to produce distressing nausea and prostration.
Infusion of Bone′set. Syn. Infusum eupatorii, L. Prep. 1. (Ph. U. S.) From the dried leaves and flowers of boneset or thorough-wort (Eupatorium perfoliatum). Diaphoretic, nauseant, and emetic when warm; tonic when cold.
2. (Compound; Infusum eupatorii compositum, L.—Ellis.) Boneset and sage, of each 1⁄2 oz.; cascarilla, 1 dr.; boiling water, 11⁄2 pint; infuse until cold, and strain. In hectic fever. A wine-glassful of either of the above, given hourly, in these diseases, until perspiration and nausea are induced, has been highly recommended in influenza.
Infusion of Braz′il-wood. Syn. Infusum ligni Brasilinsis, L. From ground or rasped Brazil wood. When wanted to keep, rectified spirit, 3 fl. oz., is added to every pint. Used for colouring, and as a test.
Infusion of Broom. Syn. Infusum scoparii, L. See Decoction of Broom.
Infusion of Bu′chu. Syn. Infusum buchu (B. P.), I. bucku (Ph. E.), I. diosmæ, L. Prep. 1. (B. P.) From bruised buchu leaves, 1 oz.; boiling distilled water, 1 pint; infuse for an hour and strain. Diuretic, sudorific, tonic; in dyspepsia, &c.; but chiefly in chronic affections of the bladder and urethra attended with copious secretion.—Dose, 1 to 2 oz.
2. (Compound; Infusum buchu compositum, I. diosmæ c., L.—(Radius.) Leaves of buchu and whortleberry, of each 1⁄2 oz.; boiling water, 8 oz. (say 1⁄2 pint); digest for half an hour, strain, and add of syrup of senega, 1⁄2 fl. oz.—Dose, 1 or 2 table-spoonfuls every hour; in atony of the bladder and mucous discharges.
Infusion of Buck′bean. Syn. Infusum menyanthis, L. From the herb or root of buckbean or marsh trefoil (Menyanthes trifoliata). Bitter, stomachic, tonic, and diuretic; in large doses, purgative, vermifuge, and emetic. It has been recommended in agues, gout, dropsy, scurvy, worms, &c. The chief consumption of this plant is by the brewers; “2 oz. being equal to 1 lb. of hops.” (Gray.)
Infusion of Bur′dock. Syn. Infusum bardanæ, L. From the root of common burdock. Aperient, diuretic, diaphoretic, and tonic; in gout, rheumatism, skin diseases, &c. See Decoction and Extract.
Infusion of Calum′ba. Syn. Infusum calumbæ (B. P.) L. Prep. 1. (B. P.) Calumba, in coarse powder, 1 oz.; cold distilled water, 2 oz.; macerate one hour, and strain. Infusion of calumba is a good tonic and stomachic bitter.—Dose, 1 to 3 fl. oz.; in dyspepsia, &c., and for restraining vomiting and diarrhœa during pregnancy or dentition. It is preferably joined with small doses of the carbonates of soda, potassa, ammonia, or magnesia, when there is acidity; or with chalybeates, when there is paleness and a low pulse; with all of which substances it may be mixed without suffering any sensible alteration.
2. (Concentrated; Infusum calumbæ concentratum, L.)—a. Calumba, in coarse powder, 51⁄2 oz.; cold distilled water, 12 fl. oz.; digest with frequent agitation, for 3 or 4 hours, then express the liquor, and repeat the digestion with 51⁄2 fl. oz. more of tepid water; after another hour, express this portion also, using as much force as possible; next mix the liquors, heat them quickly to the boiling-point in a shallow vessel, and pour the infusion, whilst still hot, into a strong bottle, and when it has cooled a little add of rectified spirit, 4 fl. oz., secure down the stopper or cork, and agitate well for a few minutes; the bottle must now be set aside for a week, after which the clear portion is to be decanted from the dregs. Very superior.
878
b. (Wholesale.) From calumba (reduced to coarse powder), 51⁄4 lbs.; rectified spirit, 5 pints; (diluted with) water, 12 pints; digest for a week, or precede by displacement. Should there be any difficulty in obtaining it free from cloudiness, the whites of 4 or 5 eggs, previously mixed with about a 1⁄4 pint of cold water, may be added to the infusion, which, after being well agitated for about ten minutes, must be allowed to repose for 7 or 8 days, and then decanted from the dregs. Should it not be perfectly transparent, it may be filtered through blotting paper.—Product, 20 lbs.
Obs. The concentrated infusion produced by the above formulæ is of very superior quality, and has acquired an extensive sale in the wholesale trade. 1 part added to 51⁄4 parts of water makes a perfectly transparent liquid, possessing exactly similar virtues to the INFUSION OF CALUMBA—B. P.
Infusion of Canthar′ides. Syn. Infusion of Spanish flies; Infusum cantharidis, I. lyttæ, L. Prep. (Soubeiran.) Spanish flies (powdered) 20 gr.; boiling water, q. s. (about 31⁄2 fl. oz.) to yield 3 fl. oz., after expression and filtration.
Infusion of Cap′sicum. Syn. Infusum capsici, L. Prep. 1. (Pereira.) Capsicum (powdered), 1⁄2 oz.; boiling water, 1 pint.—Dose, 1⁄2 fl. oz.
2. (Stephen’s ‘Pepper Medicine’—Pereira.) Red pepper (Capsicum fructescens), 2 table-spoonfuls (or 3 of cayenne pepper); common salt, 2 teaspoonfuls; boiling water, 1⁄2 pint; to the strained liquor, when cold, add of very sharp vinegar, 1⁄2 pint.—Dose, 1 table-spoonful, slowly swallowed, every half hour, in cholera, malignant sore throat, scarlatina, &c.
Infusion of Car′away. Syn. Caraway tea; Infusum carui, L. Prep. From bruised caraway seed, 3 dr.; boiling water, 1 pint. In the flatulent colic of infants, and as an adjunct to aperient medicine.
Infusion of Car′rot Seed. Syn. Infusum dauci, I. carotæ, L. Diuretic; in dropsy and nephritic complaints; 1⁄2 to 1 pint being taken daily.
Infusion of Cascaril′la. Syn. Infusum cascarillæ (B. P.), L. Prep. 1. (B. P.) Cascarilla, in coarse powder, 1 oz.; boiling distilled water, 10 oz.; infuse for one hour in a closed vessel and strain.—Dose. 1 to 2 oz., usually combined with carbonate of soda and tincture of cascarilla. It is an excellent medicine in dyspepsia, debility, diarrhœa, &c.
2. (Concentrated; Infusum cascarillæ concentratum, L.)—a. Cascarilla (good and fragrant, bruised), 61⁄2 lbs.; rectified spirit of wine, 3 pints; cold water, 6 pints; macerate in a close vessel for 14 days, express the liquor, and filter.
b. As the last, but proceeding by the process of percolation.
Obs. If the preceding processes are well managed, the product is 10 lbs., and resembles brandy in colour and transparency, and is delightfully fragrant. 1 part of this infusion mixed with 61⁄2 parts of water makes a preparation exactly resembling the INFUSION OF CALUMBA—B. P.
3. (Alkaline; Infusum cascarillæ alkalisatum, L.—Ph. Palat.) Cascarilla, 3 oz.; carbonate of potassa, 2 dr.; boiling water, 16 fl. oz. Antacid and tonic.—Dose, l tablespoonful.
Infusion of Cas′sia. Syn. Cassia tea; INFUSUM CASSIA FISTULÆ, L.; Eau de casse, Fr. Prep. (Soubeiran.) Cassia pods (bruised), 4 oz.; boiling water, 11⁄2 pint. Laxative.
Infusion of Cate′chu. Syn. Compound infusion of catechu; Infusum catechu (B. P.), L. Prep. (B. P.) Catechu in coarse powder, 160 gr., cinnamon, bruised, 40 gr., boiling water, macerate for half an hour in a covered vessel, and strain. Astringent in diarrhœa.—Dose, 1 to 2 oz. three or four times a day, or after every liquid dejection.
Infusion of Catmint. Syn. Infusum catariæ. Prep. Dry catmint, 2 oz.; boiling water, 1 pint.
Infusion of Cayenne Pep′per. See Infusion of capsicum.
Infusion of Cen′taury. Syn. Infusum centauri, L. From the flowering tops of common or lesser centaury (Erythæa centaurium). Bitter, febrifuge, stomachic, and vermifuge. A popular remedy in obstructions, jaundice, debility, dyspepsia, &c.; and externally, for the itch, and to destroy pediculi. An infusion is also made of the root, which is about one half more powerful than the tops. The plant is “a valuable native medicine; in the places where it grows it is carefully collected for use in rustic pharmacy.” (Lindley.)
Infusion, Cephal′ic. Syn. Infusum cephalicum, L. Prep. (Edin. Hosp.) Valerian root, 2 oz.; rosemary tops, 4 dr.; boiling water, 1 quart, infuse 12 hours, strain, and add aromatic water, 4 fl. oz. As an antispasmodic, and in various affections of the head.
Infusion of Cham′omile. Syn. Chamomile tea; Infusum anthemidis (B. P.) I. chamæmeli, L. Prep. 1. (B. P.) Chamomile flowers, 1⁄2 oz.; boiling water, 10 oz.; infuse for fifteen minutes, and strain.
Tonic, bitter, and stomachic; also emetic. It should be drunk cold, as it is emetic when warm.—Dose. As a stomachic, 1 to 3 oz.; as an emetic, 5 to 10 oz.
2. (Concentrated; Infusum anthemidis concentratum, L. From chamomiles, 51⁄2 oz., water; 1 pint; boil till the mixture weighs exactly 21 oz., express the liquor by means of a powerful tincture-press, cool, and add of essential oil of chamomile, 15 drops, dissolved in rectified spirit, 5 fl. oz. agitate well, let it repose until the next day, then decant the clear, and filter. Strongly bitter and odorous, and beautifully transparent. 51⁄2 times as strong as the ordinary INFUSION—B. P.
Infusion of Chamomile and Orange (Dr Percival). Syn. Infusum anthemidis et aurantii.879 Prep. Chamomile flowers, 1 oz.; dried orange peel, 1⁄2 oz.; cold water, 3 lbs.; macerate for 24 hours.
Infusion of Cher′ry-laurel. Syn. Infusum lauro-cerasi, L. Prep. (Dr Cheston.) Fresh leaves of the common or cherry-laurel (Cerasus Lauro-cerasus). 21⁄2 oz.; boiling water, 1 pint; infuse, strain, and add of clarified honey, 21⁄2 oz. As a lotion in cancer of the lip, and as a wash for malignant ulcers.
Infusion of Chiret′ta. Syn. Infusum chiratæ, L. Prep. 1. (B. P.) Chiretta, cut small, 1 oz.; distilled water, at 120° F., 40 oz.; infuse half an hour, and strain.—Dose, 1 to 2 oz.
Obs. Chiretta is a pure tonic bitter, closely allied to gentian, and has been long esteemed in the East Indies as a remedy for acidity, flatulence, and dyspepsia, especially when occurring in gouty or debilitated habits. It is usually given in combination with carbonate of soda or salts of iron. The whole of the plant is employed.
2. (Concentrated; Infusum chirettæ concentratum, L.) From Chiretta, 4 oz.; for each pint of the product, prepared as either CONC. INFUSION OF CALUMBA or CASCARILLA. Eight times as strong as the common infusion.
Infusion of Cincho′na. Syn. Infusion of bark, Infusum cinchonæ, L. Prep. 1. (B. P.) Yellow cinchona (calisaya) bark, in coarse powder, 1 oz.; boiling distilled water, 1 pint; infuse for two hours in a covered vessel, and strain.
Obs. Infusion of bark is tonic and stomachic, and in very large doses febrifuge. It is an extremely useful medicine in dyspepsia, debility, and during convalescences, and is often a valuable adjunct to more active remedies. Like the decoction, it is most energetic when strained whilst hot. The addition of 1 fl. dr. of diluted sulphuric acid to the water before pouring it on the bark increases its solvent power, and, consequently the strength of the infusion.—Dose, 1 to 3 fl. oz.
2. (Concentrated; Infusum cinchonæ concentratum, L.)—a. Yellow bark (coarsely powdered), 4 lbs.; boiling water, 8 lbs.; digest for 12 hours, express the liquid, add rectified spirit, 2 lbs., and after 24 hours’ repose decant the clear portion.
b. Yellow bark (in coarse powder), 4 lbs.; cold water, 8 lbs.; rectified spirit, 2 lbs.; dilute sulphuric acid, 4 fl. oz.; mix the fluids, and either macerate the bark in them for a week in a closed vessel, or proceed by the method of displacement. Very superior.
Obs. 1 fl. dr. of either of the above, added to 7 fl. dr. of water, produces an extemporaneous infusion of cinchona resembling that of the pharmacopœia. The concentrated preparation of the Ph. L. being more than 8 times the usual strength, is placed amongst Liquors.
3. From PALE BARK:—a. (Ph. L., Infusion of pale cinchona; Infusum cinchonæ pallidæ—Ph. L.) From pale bark, as INFUSION OF CINCHONA—Ph. L.
b. (Ph. D.; Infusum cinchonæ—Ph. D.) Crown or pale bark, 1 oz.; boiling water, 1⁄2 pint; infuse 1 hour in a covered vessel, and strain through paper.
Obs. “This infusion is inferior to the preceding” (from yellow bark) “in activity, and is a very unnecessary one. It is said to oppress the stomach less than that of the other cinchona bark; the reason is obvious—it is weaker.” (Pereira.)
c. Concentrated; Infusum cinchonæ pallidæ concentratum, L. As CONCENTRATED INFUSION OF CINCHONA, but using pale bark. The concentrated preparation of the Ph. L. will be found under Liquors.
Infusion of Cin′namon. Syn. Cinnamon tea; Infusum Cinnamomi, L. In flatulence, dyspepsia, and nervous colics.
Infusion of Cloves. Syn. Clove tea; Infusum caryophyllorum, I. cariophylli (B. P.), L. Prep. 1. (B. P.) Cloves (bruised), 1 oz.; boiling distilled water, 20 oz.; infuse for half an hour, and strain. Aromatic, stimulant, and stomachic, either alone or in combination; in colic, dyspepsia, gout, &c.—Dose, 1 to 2 oz.
2. (Concentrated: Infusum caryophylli concentratum, L.)—a. Bruised cloves, 3 oz.; boiling water, 16 fl. oz.; infuse as above and strain; when cold, add of rectified spirit 1⁄4 pint, and filter.
b. Bruised cloves, 13⁄4 lb.; rectified spirit, 1 quart; cold water, 3 quarts; macerate for 7 days, and express the liquid; sprinkle the marc with water, 12 fl. oz., and after the lapse of an hour again submit it to the press; lastly filter the mixed liquors. Very fine. The above are about eight times the strength of the INFUSION OF CLOVES.—Ph. L.
Infusion of Cof′fee. Syn. Infusum caffei, L. Prep. (Dr McBride.) Unroasted coffee berries (bruised), 30 in no.; cold water, 1 quart; macerate 2 or 3 hours. In calculus, &c.—Dose, 1⁄2 pint every morning.
Obs. Sir J. Floyer and Sir J. Pringle cured asthma with a strong solution of roasted coffee. M. Bouchardat prescribes a strong infusion made by displacement (percolation), and mixed with a little brandy, in poisoning by opium and other like narcotics, after the administration of emetics and ioduretted water. M. Honore also employs very strong-made coffee in albuminuria. Clausen gives it in gout, and Parker employs it as a nervous stimulant in lieu of ammonia and wine, for persons of a slightly sensitive and excitable temperament.
Infusion of Contrayer′va. Syn. Infusum contrayervæ, L. Prep. (Pereira.) Contrayerva (in powder), 1 oz.; boiling water, 12 fl. oz. Stimulant, tonic, and diaphoretic; in low fevers, &c.
Infusion of Copal′che Bark. Syn. Infusum copalchi corticis, L. Prep. (Dr Stark.) Bark of copalche bush (Croton pseudo-China),880 1⁄2 oz.; boiling water, 1 pint; digest 2 hours, and strain. A warm bitter and stomachic.
Infusion of Cor′sican Moss. Syn. Infusum helminthocorti, L. Prep. (Farr.) Corsican moss, 5 dr., boiling water, 1 pint; macerate for 10 or 12 hours, and strain. Ad libitum in cancer. See Decoction.
Infusion of Cotula. Syn. Infusum cotulæ. From dried flowers of Anthemio cotula, as infusion of chamomiles.
Infusion of Cuspa′′ria. Syn. Infusion of angostura bark; Infusum cuspariæ (B. P.), I. angusturæ, L. Prep. (B. P.) Cusparia, in coarse powder, 1 oz.; distilled water, at 120°, 20 oz.; infuse 2 hours, and strain. Stimulant and tonic; in typhus fever, bilious diarrhœa, dysentery, &c.
Infusion of Daf′′fodil. Syn. Infusum narcissi pseudo-narcissi, L. Prep. (Dufresnoy.) Flowers of daffodil (Narcissus pseudo-Narcissus), 3 to 16 in no.; boiling water, 1 pint. Expectorant, nauseant, and emetic. In hooping-cough.
Infusion of Dah′′lia Pe′tals. From the violet or blue varieties. Used for its colour and as a test.
Infusion of Dandeli′on. Syn. Infusion of taraxacum; Infusum taraxaci, L. 1. From the sliced root. Stimulant, resolvent, and tonic.
2. (Concentrated; Infusum taraxaci concentratum, L.) From the root (sliced), 1 lb.; exposed to a current of warm dry air until crisp, then coarsely pulverised, and digested for a week in a mixture of rectified spirit, 12 fl. oz.; cold water, 11⁄2 pint. 8 times the usual strength.
3. (Compound; Infusum taraxaci compositum, L.—Meigs.) Infusion of dandelion, 4 fl. oz.; extract of do., 2 dr.; sesquicarbonate of soda, 1⁄2 dr.; tartrate of potassa, 3 dr.; tincture of rhubarb, 3 fl. dr.; tincture of henbane, 20 drops. In dropsical and visceral affections.—Dose. One third part thrice daily. See Decoction, Extract, &c.
Infusion of Digita′lis. See Infusion of Foxglove.
Infusion, Diuret′ic. Syn. Infusum diureticum, L. Prep. 1. Broom tops, 1 oz.; boiling water, 1 pint; infuse 1 hour, strain, cool, and add of sweet spirits of nitre, 3 fl. dr.—Dose. A wine-glassful every other hour.
2. Infusion of foxglove, 1 fl. oz.; tincture of foxglove, 1⁄2 fl. dr.; acetate of potassa, 1 dr.; laudanum, 10 drops.—Dose, 1 table-spoonful twice or thrice a day, carefully watching the effects.
3. Juniper berries, 2 oz.; aniseed, 1⁄4 oz.; boiling water, 1 pint; infuse 1 hour; strain, and when cold, add of compound spirit of juniper, 2 fl. oz.; tincture of squills, 1 fl. dr.; nitre, 1 dr.—Dose, 1⁄2 a teacupful frequently. All the above are used as diuretics in dropsy. See Infusions of Broom, Foxglove, and Juniper.
Infusion of Dog′wood. Syn. Infusum cornus Floridæ, L. From the bark of American dogwood (Cornus Florida). See Decoction.
Infusion of Dulcamara (B. P.). Syn. Infusum dulcamaræ. Prep. Infuse bruised dulcamara, 1 oz.; in 10 fluid ounces of boiling water in a covered vessel for 1 hour; and strain.—Dose, 1 oz. to 2 oz.
Infusion of El′der Flowers. Syn. Elder-flower tea; Infusum sambuci florum, L. From the picked flowers, 1⁄2 oz.; boiling water, 1 pint. Pectoral, expectorant, and diaphoretic, either alone or sweetened with honey.
Infusion of Elecampane. Syn. Infusum inulæ. Prep. Elecampane root, 5 dr.; boiling water, 1 pint; infuse for two hours, and strain.
Infusion of Elm-bark. Syn. Compound inulæ, L. Diaphoretic, expectorant, and tonic. FUSION OF ELM-BARK; Infusum ulmi compositum, L. Prep. (Cadet.) Elm-bark, bitter-sweet, burdock, and fumitory, of each 2 dr.; boiling water, 1 pint; digest for 4 hours, strain, and add of syrup of sarsaparilla, 1 oz. The whole to be taken in 24 hours, in divided doses in the chronic exanthemata. See Decoction.
Infusion of Er′got of Rye. Syn. Infusum ergotæ (B. P.). L. Prep. 1. (B. P.) Ergot, 1, in coarse powder, 1 oz.; boiling distilled water, 40 oz.; infuse 1⁄2 an hour in a covered vessel, and strain. Should be made fresh when required.—Dose, 1 to 2 oz. every 1⁄2 hour or hour, as a parturient. Also as an injection for gleet.
2. (Concentrated.) See Liquor of Ergot.
Infusion of Eucalyptus. (Griffiths.) Syn. Infusum Eucalypti globuli. Prep. Cut leaves of Eucalyptus globulus, 2 dr.; boiling water, 4 oz.; infuse and strain. Take morning and evening.
Infusion of Fen′nel. Syn. Fennel tea; Infusum fœniculi, L. Prep. From sweet fennel-seeds, 1⁄2 oz.; boiling water, 1 pint. In griping and windy colic of infants; a few drops to 1⁄2 a teaspoonful for a dose, or a little by way of enema.
Infusion of Flax-seed. See Infusion of Linseed.
Infusion of Fleabane. Syn. Infusum erigeromis canadensis. Prep. Canadian fleabane, 1 oz.; boiling water, 16 oz. Diuretic and astringent.
Infusion of Fox′glove. Syn. Infusum digitalis (B. P.), L. Prep. 1. (B. P.) Digitalis, dried, 30 gr.; distilled water, 10 oz.; infuse 1 hour, and strain.—Dose, 1⁄4 to 1⁄2 oz.
2. (Ph. E.) Foxglove (dried), 2 dr.; boiling water, 18 fl. oz.; spirit of cinnamon, 2 fl. oz.
3. (Ph. D.) Foxglove (dried and reduced to a coarse powder), 1 dr.; boiling water, 9 fl. oz.; infuse 1 hour. The product should measure about 8 fl. oz. The last two are of double the strength of the infusion Ph. L., and the dose must consequently be only 2 to881 4 fl. dr. “I believe this, when properly made, to be the most effectual of the preparations of foxglove.” (Pereira.) See Foxglove.
Infusion of Fu′mitory. Syn. Infusum fumariæ, L. From the herbaceous portion of common fumitory (Fumaria officinalis). Aperient and diaphoretic; in obstinate skin diseases and chronic obstructions of the liver.
Infusion of Galls. Syn. Infusum gallæ, L. 1. From Aleppo galls, coarsely powdered. In diarrhœa, hæmorrhages, &c.; also freely, in cases of poisoning by the alkaloids; and diluted with 3 or 4 times its volume of water, for injections, embrocations, gargles, &c.
2. (Compound; Infusum gallæ compositum, Mistura gallæ, L.—Ellis.) Infusion of galls, 4 fl. oz.; prepared chalk, 1⁄2 oz.; powdered gum, 1 dr.; tincture of opium, 1⁄2 fl. dr.—Dose, 1 table-spoonful every 2 hours, in diarrhœa, &c.
Infusion of Gar′lic. Syn. Infusum allii, L. Prep. (White.) Garlic (recent), 1⁄2 lb.; water, 4 lbs.; place them in a covered pot, set it in a very slow oven for 3 or 4 hours, and when cold, express the fluid portion.—Dose. In epilepsy, 2 teaspoonfuls before and after every meal; in chronic diarrhœa, a teaspoonful after every motion.
Infusion of Gen′tian. Syn. Infusum gentianæ, L. Prep. 1. (Beral.) Gentian (bruised), 2 dr.; boiling water, 1 pint; infuse 5 or 6 hours, and strain. Stomachic.
2. (Compound; Infusum gentianæ compositum—B. P.)
Prep. a. (B. P.) Gentian, sliced, 1 oz.; orange peel, cut small, 1 oz.; lemon peel (fresh), 2 oz.; boiling distilled water, 1 pint; infuse for an hour in a covered vessel, and strain.—Dose, 1 to 2 oz.
b. (Ph. E.) Sliced gentian root, 1⁄2 oz.; bitter orange peel (dried and bruised) and coriander seeds, of each 1 dr.; proof spirit, 4 fl. oz.; digest for 3 hours, then add of cold water, 16 fl. oz., and in 12 hours more strain.
c. (Ph. D.) Gentian and dried orange peel, of each 2 dr.; boiling water, 1⁄2 pint; macerate 1 hour, and strain.—Dose of the last two, 1⁄2 to 1 fl. oz.
3. (Concentrated Compound; Infusum gentianæ comp. concentratum, L.)—a. Gentian root (bruised), 41⁄2 lbs.; boiling water, q. s. to cover it; infuse with occasional agitation for 2 hours, express the liquor, wash the marc with a little boiling water, and evaporate to 13 quarts; when cold, strain through flannel, add of rectified spirit, 1 gall., and pour the mixed fluids on dried orange peel, 41⁄2 lbs., and fresh lemon peel, 9 lbs.; macerate for 1 week, then express the liquor in a powerful press, and filter through paper.
b. Gentian and dried orange peel, of each 41⁄2 lbs.; fresh lemon peel, 9 lbs.; cold distilled water, 13 quarts; rectified spirit, 1 gall.; macerate for 14 or 15 days, with frequent agitation, then express the liquid, add 1 dr. each of the essential oils of lemon and orange, agitate well, and filter through paper.
c. Gentian, 11⁄4 lb.; essence of lemon, 1 dr.; essence of orange, 1⁄2 dr.; essence of cedrate, 15 drops; rectified spirit, 1 quart; cold water, 3 quarts; digest for 10 days and filter.
4. (With Rhubarb; Infusum gentianæ et Rhei, Mistura stomachica, L.) From gentian and rhubarb (bruised), of each 2 dr.; boiling water, 1 pint; digest 1 hour, and strain; to the cold infusion add of sesquicarbonate of ammonia, 1 dr. An admirable medicine in dyspepsia, hysteria, loss of appetite, constipation, chronic rheumatism, &c.
Infusion of Gin′ger. Syn. Ginger tea; Infusum zingiberis, L. From the best unbleached Jamaica ginger, freshly bruised or grated. In flatulence, colic, and indigestion.
Infusion of Gin′seng. Syn. Ginseng tea; Infusum ginseng, I. radicis g., L. Prep. Ginseng (the root of Panax Schinseng), 1⁄2 oz.; ginger (grated), 1 dr.; boiling water, 1 pint; macerate 1 hour, then add of cinnamon (bruised), 1⁄2 dr.; infuse for another hour, and strain. Ginseng tea, made according to the above formula, has a wonderful reputation in China, as a stimulant, restorative, and aphrodisiac. In Europe, however, it is merely regarded as an aromatic demulcent.
Obs. American ginseng (the root of Panax quinquefolium) may be substituted for the Asiatic product.
Infusion of Gold′thread. Syn. Infusum coptis, L. From the root of Coptis trifolia. Bitter, stomachic; in dyspepsia, and as a mouth-wash in thrush.
Infusion of Gua′co. Syn. Infusum guaco, L. From the bruised leaves and stems of guaco or huaco (Mikania guaco). Sudorific and vulnerary; reputed in South America to be a powerful remedy for the bites of venemous serpents and for hydrophobia, but the trials in this country do not show it to be of any value in such cases.
Infusion of Guaiac′um. Syn. Compound infusion of guaiacum, I. of the woods; Infusum guaiaci compositum, Aqua benedicta composita, L. Prep. (Ph. D. 1826.) Guaiacum shavings, 6 oz.; bruised liquorice root, 1 oz.; sassafras bark, 1⁄2 oz.; coriander seeds, 3 dr.; lime water, 96 fl. oz. (say 5 pints); infuse for 2 days, and strain. Dose, 3 to 4 fl. oz., twice or thrice a day, in scrofula, rheumatism, gout, eruptions, &c.
Infusion of Gum. Syn. Infusum acaciæ, L. From gum acacia and lump sugar, of each 2 oz.; boiling water, 1 pint; macerate until dissolved, then cool, and add of orange-flower water, 1⁄2 fl. oz. A pleasant demulcent in coughs, hoarseness, &c.
Infusion of Hedge Hys′sop. Syn. Infusum gratiolæ, L. Prep. (A. T. Thomson.) Hedge hyssop (Gratiola officinalis), dried, 2 dr.; boiling water, 8 fl. oz. Cathartic, diuretic, emetic, and vermifuge.—Dose, 3 to 6 fl.882 dr.; in dropsies, gout, jaundice, &c. See Extract.
Infusion of Hem′lock. Syn. Infusum conii, I. conii maculati, L. Prep. (Guy’s Hosp.) Dried leaves of hemlock, and coriander seeds, of each 2 dr.; boiling water, 8 oz.; infuse for 2 hours. Combined with acetate of ammonia, tincture of henbane, and syrup of poppies, in pulmonary complaints, &c.
Infusion of Henbane. Syn. Infusum hyoscyami, L. Prep. 1. From fresh leaves, 1⁄2 oz.; boiling water, 1 pint. As a lotion for painful ulcers, swelled face, &c.
2. (Compound; Henbane fomentation; Infusum hyoscyami compositum, L.—Radius.) Henbane leaves, poppy heads, and mallows, of each 1 oz.; boiling water, 2 quarts. For painful ulcers, and in facial neuralgia, &c.
Infusion of Hops. Syn. Hop tea; Infusum humuli, I. lupuli (Ph. L.), L. Prep. (Ph. L.) Hops, 6 dr.; boiling distilled water, 1 pint; macerate for 4 hours in a covered vessel (press), and strain. Tonic and anodyne. Well-hopped mild ale is a good substitute.
Infusion of Hore′hound. Syn. Horehound tea; Infusum marrubii, L. From the leaves; demulcent, pectoral; a popular remedy in coughs, colds, hoarseness, and chest affections generally, taken freely.
Infusion of Horserad′ish. Syn. Infusum armoraciæ, L. 1. From horseradish alone. Diuretic and stomachic.
2. (Compound; Infusum armoraciæ compositum, L.—Ph. L.) Horseradish (sliced) and mustard seed (bruised), of each 1 oz.; boiling distilled water, 1 pint; macerate for 2 hours in a covered vessel, strain, and add of compound spirit of horseradish, 1 fl. oz. Stimulant, stomachic, and diuretic; in dropsies, paralysis, scurvy, chronic rheumatism, &c.
Infusion of Hys′sop. Syn. Hyssop tea; Infusum hyssopi, L. 1. From the leaves of Hyssopus officinalis (Linn.) Stimulant, stomachic, emmenagogue, and expectorant; in dyspepsia, flatulency, hysterical affections, &c.; also used by boxers as a wash for black eyes.
2. (Compound; Infusum hyssopi compositum, L.—Ratier). Hyssop leaves, 21⁄2 dr.; liquorice, 2 dr.; boiling water, 1 quart. As a demulcent drink in catarrhal affections.
Infusion of Indian Sarsaparil′la. Syn. Infusum hemidesmi, L. From Indian or scented sarsaparilla (Hemidesmus Indicus). Dr Ashburner orders it to be made with lime water (cold); but this plan is seldom followed.—Dose and uses, same as those of infusion of sarsaparilla.
Infusion of I′ron (Bitter). Syn. Infusum ferri amarum, L. Prep. (Dr R. E. Griffith.) Iron filings, 3 oz.; gentian and ginger, of each bruised, 1 oz.; orange peel, 1⁄2 oz.; strong old cider, 1 pint; infuse for a month, frequently stirring, and filter.—Dose, 1⁄2 to 1 dr., 3 or 4 times daily, as a chalybeate tonic.
Infusion of Ju′niper. Syn. Infusum juniperi, I. baccæ j., L. 1. From the berries alone. As a stimulant diuretic, in dropsies &c.
2. (Compound; Infusum juniperi compositum, L.)—a. (Guy’s Hosp.) Juniper berries, 21⁄2 oz.; boiling water, 1 pint; to the strained solution, when cold, add, of compound spirit of juniper, 10 fl. dr.; bitartrate of potassa, 1 dr.
b. (Parrish.) Ginger, juniper berries, and mustard, of each bruised, 1⁄2 oz.; horseradish and parsley root, of each bruised, 1 oz.; cider, 1 quart; infuse, and strain with expression. All the above are used in dropsies.
Infusion of Ki′no. Syn. Infusum kino, L. From kino, 5 dr.; boiling water, 1 pint. In diarrhœa, and diluted with 4 or 5 times its bulk of water, as an injection in chronic gonorrhœa.
Infusion of Justitia. Syn. Infusum justiciæ. Prep. Root of painted justicia, 2 dr.; boiling water, 1 pint; infuse for 1 hour.
Infusion of Kousso (B. P.) Syn. Infusum cusso. Prep. Infuse kousso in fine powder, 1⁄2 oz.; in boiling distilled water, 8 fl. oz., in a covered vessel for 15 minutes. Must not be strained.
Infusion of Lime Flowers. Syn. Linden-flower tea; Infusum tillæ, L. 1. From the flowers of the lime or linden tree (Tilia Europæa). Antispasmodic, diaphoretic, and cephalic.
2. (Compound; Infusum tillæ compositum, L.—Foy.) Chamomiles, linden flowers, and orange leaves, of each 2 dr.; boiling water, 1 quart; infuse, strain, and add of syrup, 2 fl. oz. In nervous headaches, &c. The above are much used on the Continent.
Infusion of Lin′seed. Syn. Linseed tea, Flaxseed t.; Infusum lini (B. P.), L. Prep. (B. P.) Linseed (bruised). 160 gr.; fresh liquorice root (sliced), 60 gr.; boiling distilled water, 10 oz.; infuse for 4 hours and strain. A cheap and useful demulcent in pulmonary and urinary irritation; especially in catarrhs, gonorrhœa, &c.; ad libitum. Dr Pereira recommends the addition of sliced lemon and sugar-candy, to render it more palatable. See Decoction.
Infusion of Liq′uorice. Syn. Infusum glycyrrhizæ, L. From the fresh root, sliced. Demulcent and laxative; taken ad libitum.
Infusion of Lit′mus. Syn. Infusum lacmi, L. Used for its colour, and as a liquid test, and to make test-paper.
Infusion of Lobelia. Syn. Infusum lobeliæ, I. l. inflatæ. From lobelia or Indian tobacco. In asthmas chiefly.—Dose, 1 to 2 table-spoonfuls every half-hour, until it occasions nausea.
Infusion of Log′wood. Syn. Logwood tea; Infusum hæmatoxyli, L. From logwood chips. One of the best remedies known for simple diarrhœa arising from weakness; also used as a colour and test. See Decoction, Extract883, &c.
Infusion (Maiden-hair). Syn. Infusum adianti, L. From either common maiden-hair (Adiantum capillus Veneris), or Canadian maiden-hair (Adiantum pedatum). They are both slightly bitter, aromatic, and pectoral. The infusion forms an excellent demulcent drink in catarrhs.
Infusion of Malam′bo Bark. Syn. Infusum corticis malambo, L. Prep. (Ure.) Bark (from Croton Malambo), 2 dr.; boiling water, 1 pint. An aromatic tonic and astringent.
Infusion of Mallow Flowers. Syn. Infusum malvæ florum, L. Pectoral and laxative. Chiefly used as a test.
Infusion of Malt. Syn. Malt tea, Sweet wort; Infusum bynes, I. malti, L. Prepared with hot water (165° to 170° Fahr.). Demulcent and laxative. A useful drink in sore throat, inflammatory fevers, &c. Some persons flavour it with sliced lemon.
Infusion of Ma′′rygold. Syn. Infusum calendulæ, L. From the flowers of the common marygold (Calendula officinalis). Carminative, diaphoretic, and emmenagogue. It has been recently recommended in cancerous affections, both internally and as a lotion. Radius adds syrup of orange peel to flavour it.
Infusion of Mat′ico. Syn. Infusum maticonis, I. maticæ, I. matico, L. 1. From the leaves of the matico plant (Artanthe elongata). Aromatic, bitter, stimulant, and reputed hæmostatic; in internal hæmorrhages and mucous discharges. The Indians of South America use it as an aphrodisiac. (Martius.)
2. Compound; Infusum maticonis compositum, L.—Watmough.) Matico and senna, of each 2 dr.; boiling water, 1 pint. In hæmorrhagic and other discharges, piles, &c.; a wine-glassful repeatedly.
Infusion of May-weed. Syn. Infusum cotulæ, L. From the dried flowers of may-weed or stinking chamomile (Anthemis cotula). Bitter, stomachic, and diaphoretic; in large doses, emetic and sudorific; chiefly in hysterical affections, scrofula, &c.
Infusion of Mea′dow Rue. Syn. Infusum thalictri flavi, L. From the herb meadow rue (Thalictrum flavum). In hydrophobia, taken plentifully.
Infusion of Mil′foil. Syn. Yarrow tea; Infusum millefolii, L. In dropsies, and as a fomentation to bruises. See Extract, &c.
Infusion of Mint. Syn. Mint tea. 1. (Ph. D.—Infusum menthæ simplex.) From the dried leaves of green or spearmint. Carminative and stomachic; chiefly used as a vehicle for other medicines. A wine-glassful ad libitum.
2. (Compound; Infusum menthæ compositum.) To mint tea 6 fl. oz., add of oil of spearmint, 3 drops, previously triturated with lump sugar, 2 dr., and dissolved in compound tincture of cardamoms, 1⁄2 fl. oz. A useful remedy in colic, flatulence, &c.; as the last.
Infusion of Mu′dar. Syn. Infusion of mudar-bark; Infusum corticis mudaris, L. From the root bark of Calotropis gigantea. Resembles infusion of ipecacuanha.—Dose, 1 to 3 teaspoonfuls, as an alterative; a wine-glassful as an emetic. In the East Indies it is highly esteemed in epilepsy, hysteria, syphilis, convulsions, and various spasmodic diseases.
Infusion of Net′tle Seed. Syn. Infusum urticæ seminum, L. Prep. (Garde.) Seed of common nettle (Urtica dioica), 21⁄2 dr.; boiling water, 18 fl. oz.; infuse 3 hours, strain, and add of syrup, 2 fl. oz. Astringent, diuretic, and pectoral.
Infusion of Nux Vom′ica. Syn. Infusum nucis vomicæ, L. Prep. (Hosp. F.) Nux vomica (ground or rasped), 1 dr.; boiling water, 1 pint; digest 3 hours, and strain. It must be taken with caution, and the effects watched. See Extract, Nux Vomica, and Strychnine.
Infusion of Or′ange Peel. Syn. Infusum aurantii, B. P. Prep. 1. Dried bitter orange peel, cut small, 1 oz.; boiling water, 20 oz.; infuse for 15 minutes, and strain.—Dose, 1 to 2 oz. Bitter and stomachic.
2. (Compound; Infusum aurantii—Ph. E., I. a. compositum—Ph. L. & D., L.)—a. (Ph. L. & E.) Dried bitter orange peel, 1⁄2 oz.; fresh lemon peel, 2 dr.; cloves (bruised), 1 dr.; boiling distilled water, 1 pint; macerate for 15 minutes in a covered vessel, and strain.
b. (Ph. D.) Dried orange peel, 3 dr.; cloves, 1⁄2 dr.; boiling water, 1⁄2 pint; macerate half an hour. An agreeable stomachic. It is chiefly employed as a vehicle for other medicines.
c. (B. P.) Dried bitter orange peel, cut small, 1⁄2 oz.; fresh lemon peel, 120 gr.; cloves (bruised), 60 gr.; boiling water, 20 oz. Infuse for 15 minutes, and strain.—Dose, 1 to 2 oz.
3. (Concentrated Compound; Infusum aurantii concentratum, I. a. com. conc., L.)—a. Seville orange peel (dried), 31⁄4 lbs.; fresh lemon peel, 11⁄2 lb.; bruised cloves, 3⁄4 lb.; boiling water, 9 pints; infuse for 20 minutes, press out the liquor, and, when cold, add of rectified spirit, 1 quart, and filter.
b. Dried orange peel, 18 oz.; fresh lemon peel, 1⁄2 lb.; bruised cloves, 1⁄4 lb.; rectified spirit, 1 pint; cold water, 3 pints; macerate for 1 week, press, and filter. Very superior.
Obs. 1 fl. dr. of either of the above, added to 7 fl. dr. of water, makes a similar (preferable) preparation to the COMPOUND INFUSION OF ORANGE PEEL.—Ph. L.
Infusion of Parei′ra. Syn. Infusum pareiræ (Ph. E. & D.), I. p. bravæ, L. Prep. 1. (Ph. E.) Velvet leaf or pareira brava root, 6 dr.; boiling water, 1 pint; macerate for 2 hours in a lightly covered vessel, and strain.
2. (Ph. D.) Pareira (bruised and torn), 1⁄2 oz.; boiling water, 9 fl. oz.; macerate 1 hour, and strain. In irritation and mucous discharges from the urinary organs. The corresponding884 preparation of the Ph. L. will be found among the Decoctions.
Infusion of Pars′ley Root. Syn. Infusum petroselini, L. From the root of garden parsley. Aromatic, diuretic, and slightly aperient. It has been highly recommended by Dr Chapman and others in dropsy, in the strangury arising from blisters, &c.; taken freely, either alone or combined with a little sweet spirit of nitre.
Infusion of Peach Leaves. Syn. Infusum persicæ, I. p. folii, L. Prep. (Pereira.) Peach leaves (dried), 1⁄2 oz.; boiling water, 1 pint; macerate an hour, and strain.—Dose, 1 to 2 table-spoonfuls, twice or thrice a day; to allay irritation of the bladder and urethra, and as a vermifuge.
Infusion, Pectoral. Syn. Infusum pectorale, L. Prep. (Hosp. F.) Linseed (bruised), 3⁄4 oz.; coltsfoot leaves, 1⁄2 oz.; liquorice root (sliced) and poppy-heads, of each 1⁄4 oz.; boiling water, 1 pint; digest two hours, and strain. In coughs, colds, hoarseness, &c., accompanied with a dose of aperient medicine. See Species, &c.
Infusion of Pennyroy′al. Syn. Pennyroyal tea; Infusum pulegii, I. menthæ pulegii, L. A popular remedy for nausea, flatulence, colds, hooping-cough, hysterical affections, obstructed menstruation, &c.
Infusion of Pep′permint. Syn. Peppermint tea; Infusum menthæ piperitæ, L. In flatulence, colic, griping, &c., and as a vehicle for other medicines.
Infusion of Periwin′kle. Syn. Infusum vincæ minoris, L. From the leaves of lesser periwinkle (Vinca minor). Astringent and tonic; in diarrhœa, dysentery, &c. Mr Weathers employs it in passive hæmorrhages, and others have recommended it as an external tonic applied to the perinæum, &c., in piles, relaxation of the genitals, &c.
Infusion of Persim′mon. Syn. Infusum persimmonis, L. From the bark of persimmon (Diospyrus Virginiana). Astringent; very valuable in diarrhœa, hæmorrhages, agues, &c.; and as a gargle in ulcerated sore throat.
Infusion of Peru′vian Bark. See Infusion of bark.
Infusion of Pink′root. Syn. Pinkroot tea, Worm t.; Infusum spigiliæ, L. 1. From Indian pinkroot. Vermifuge; either combined with or followed by a purge after the third or fourth dose. The dose for a child 3 to 5 years old is 1 to 2 table-spoonfuls.
2. (Compound; Infusum spigiliæ compositum, I. s. cum sennâ, L.—Ellis.) Pinkroot, 1⁄2 oz., senna, 2 dr.; fennel seed, 3 dr.; manna, 1 oz.; boiling water, 1 pint.—Dose, 1⁄2 wine-glassful to a child 2 or 3 years old; in worms. See Extract.
Infusion of Pleu′risy Root. Syn. Infusum asclepiadis tuberosæ, L. From the root of butterfly weed or pleurisy root (Asclepias tuberosa). Expectorant and diuretic; in large doses, purgative; in colds, pleurisy, pneumonia, &c. According to Bigelow, it is a valuable mild tonic and stimulant.
Infusion of Pois′on-oak. Syn. Infusum rhois toxicodendri, L. Prep. From the dried leaves of the poison-oak (Rhus toxicodendron), 3 dr.; boiling water, 1 pint. Stimulant and narcotic; chiefly in palsy and mania.
Infusion of Pop′py-heads. Syn. Poppy tea; Infusum papaveris, L. From poppy-heads (capsules of Papaver somniferum). Soothing, anodyne. Sweetened with honey, it is a popular remedy for tickling cough, restlessness, &c.; also used hot, as an embrocation, in painful tumours, inflammations, &c. See Infusion of Red Poppy.
Infusion of Pur′ging Flax. Syn. Infusum lini cathartici, L. From the dried leaves of purging flax (Linum catharticum). Cathartic. The dose should be repeated at intervals of an hour or an hour and a half, until it operates.
Infusion of Quas′sia. Syn. Quassia tea; Infusum quassiæ (B. P., Ph. L. E. & D.), L. Prep. 1. (B. P.) Quassia, in chips, 60 gr.; cold distilled water, 10 oz.; infuse for half an hour, and strain.—Dose, 1 to 2 oz.
2. (Ph. L.) Quassia (sliced), 40 gr.; boiling distilled water, 1 pint; infuse for 2 hours in a covered vessel, and strain.
3. (Ph. E.) Quassia, 1 dr.; boiling water, 1 pint.
4. (Ph. D.) Quassia (rasped), 1 dr.; boiling water, 81⁄2 fl. oz.
5. (Ph. U. S.) Quassia, 2 dr.; cold water, 16 fl. oz.; macerate for 12 hours, and strain. As a bitter tonic, in loss of appetite, dyspepsia, &c.; either combined with alkaline carbonates or chalybeates. Sweetened with moist sugar or honey, it forms a common FLY-WATER or FLY-POISON.
6. (Compound; Infusum quassiæ compositum, L.—Ellis.) Quassia, serpentary, and dried orange peel, of each 1⁄4 oz.; boiling water, 1 pint. A stimulant stomachic.
Infusion of Red Cab′bage. Syn. Infusion of blue cabbage. Used as a colour, and to make test-paper. It will not keep without the addition of about 1-10th of its weight of rectified spirit.
Infusion of Red Pop′py. Syn. Red-Poppy tea; Infusum rhœados, L. From the petals of the red or corn poppy. Anodyne and pectoral. Sweetened with sugar or honey, it is a popular remedy in catarrhal affections: but the use of this, as well as of INFUSION OF POPPY-HEADS, should be accompanied by a dose of aperient medicine.
Infusion of Rhat′any. Syn. Infusum krameriæ (B. P.), Infusum rhataniæ, I. krameriæ (Ph. L. & D.), L. Prep. 1. (B. P.) Rhatany, bruised, 1 oz.; boiling distilled water, 20 oz.; infuse 1 hour, and strain.—Dose, 1 to 2 oz.
2. (Ph. L.) Rhatany root, 1 oz.; boiling distilled water, 1 pint; macerate for 4 hours in a covered vessel, and strain.
885
3. (Ph. D.) Rhatany, 1⁄2 oz.; boiling water, 9 fl. oz.; macerate 1 hour, and strain. Astringent and tonic; chiefly in chronic diarrhœa.
4. (Concentrated; Infusum krameriæ concentratum, L.) From 8 times the usual quantity of ingredients, as INFUSION OF CASCARILLA.
Infusion of Rhododen′dron. Syn. Infusum rhododendri, L. From the leaves of yellow rhododendron (Rhododendron chrysanthum), 1⁄2 oz.; boiling water, 1⁄2 pint. Highly recommended by Pallas and Koelpin in gout, chronic rheumatism, and syphilis. It has marked narcotic properties.
Infusion of Rhubarb. Syn. Infusum rhei (B. P., Ph. L. E. & D.), L. Prep. 1. (B. P.) Rhubarb (in thin slices), 1 oz.; boiling distilled water, 40 oz.; infuse for 1 hour, and strain.—Dose, 1 to 2 oz.
2. (Ph. L.) Rhubarb (sliced), 3 dr.; boiling distilled water, 1 pint; macerate for 2 hours in a covered vessel, and strain.
3. (Ph. D.) Rhubarb, 2 dr.; boiling water, 9 fl. oz.; macerate 1 hour.
4. (Ph. E.) Rhubarb (in coarse powder). 1 oz.; boiling water, 18 fl. oz.; infuse for 12 hours, add of spirit of cinnamon, 2 fl. oz,; and strain through linen or calico. Stomachic and purgative; along with neutral salts or aromatics.
Obs. The infusion of the Ph. E. being fully double as strong as that of the Ph. L. & D., must be taken in proportionate doses.
5. (Concentrated; Infusum rhei concentratum, L.)—a. Rhubarb (in coarse powder), 10 oz.; rectified spirit, 1 pint; cold distilled water, 1 quart; digest 10 days, with frequent agitation, then express the liquor, and filter it; or proceed by the method of displacement.
b. Rhubarb, 3 lbs. 5 oz.; cold distilled water, 11 pints; rectified spirit, 51⁄2 pints; as the last.
Obs. 1 fl. dr. of either of the above, added to 7 fl. dr. of water, forms 1 fl. oz. of liquid, resembling, and in many points preferable to, the infusion of the Ph. L. The above is the only way a fine, rich-coloured, and transparent concentrated preparation can be made, that will keep well. Should it not prove perfectly limpid, it may be clarified in the way already mentioned.
6. (Alkaline; Infusum rhei alkalinum, I. r. cum potassâ, L.—Copland.) Rhubarb, 2 dr.; carbonate of potassa, 1 dr.; boiling water, 1⁄2 pint; macerate for 4 hours, strain, and add of tincture of cinnamon, 1⁄2 fl. oz. In dyspepsia, acidity, heartburn, &c.
Infusion of Ro′ses. Syn. Infusum rosæ, L., 1. (Simple.) From the petals of red roses. Used as colouring and for a test; mixed with vinegar and sweetened with honey, it forms a popular gargle in sore throat.
2. (Compound; Infusum rosæ—Ph. E. I. rosæ compositum—Ph. L., I. r. acidum—B. P., Ph. D.) Prep.—a. Red rose petals (broken up), 1 oz.; dilute sulphuric acid, 1⁄2 oz.; boiling distilled water, 40 oz.; infuse for half an hour with the acid and water, and strain.—Dose, 1 to 2 oz.
b. (Ph. L.) Petals of the red or damask rose (dried and pulled asunder), 3 dr.; boiling water, 1 pint; mix, and add of dilute sulphuric acid, 11⁄2 fl. dr.; macerate for 2 hours, strain off the liquor, and dissolve in it white sugar, 6 dr. The Edinburgh form is nearly similar.
c. (Ph. D.) Petals, 2 dr.; boiling water, 1⁄2 pint; infuse 1 hour, strain, and add of dilute sulphuric acid, 1 fl. dr.
Obs. A vessel or glass of stoneware should be used to make the infusion in, as metallic vessels injure the colour of the liquid, and are also attacked by the acid. The best plan is to add the dilute sulphuric acid to the water before pouring it on the leaves. The infusion may be squeezed out of the leaves with the hands.
The COMPOUND INFUSION OF ROSES is principally used as a vehicle for sulphate of quinine, saline purgatives, and some other medicines. It is astringent and refrigerant, and, when diluted with water, forms a pleasant drink in febrile disorders, phthisical sweats, hæmorrhages, diarrhœa, &c. It also makes a very useful astringent gargle.—Dose, 1 to 4 fl. oz.; either alone or diluted with water. It is incompatible with the alkalies and earths, and their carbonates and their bicarbonates.
3. (Concentrated; Infusum rosæ concentratum, L.)—a. Rose petals, 10 oz.; boiling distilled water, 3 pints; infuse for 2 hours, with frequent agitation, express the liquid, strain through a clean hair sieve, and add of dilute sulphuric acid, 41⁄2 fl. oz.; after agitation for 5 or 6 minutes, and repose for 2 or 3 hours, decant the clear portion, and filter through paper supported on calico; next, dissolve in the liquid 11⁄4 lb. of the finest white sugar, broken up into small lumps, but perfectly free from dust and dirt; lastly, pour the infusion into clean, stoppered, green-glass bottles, and, as much as possible, keep them from the light, and in a cool place.
b. Rose petals, 31⁄4 lbs.; boiling water, 2 gall.; diluted sulphuric acid, 24 fl. oz.; finest white sugar, 61⁄2 lbs.; as the last.
c. The same quantity of dilute sulphuric acid and cold water, as before; mix, and infuse the rose leaves in the liquid for 48 hours, then express, filter, and add the sugar. Product very fine, and keeps well without becoming gelatinous.
Obs. This preparation is 8 times as strong as that of the Ph. L. (2, a). Great care should be taken that the utensils are perfectly clean, especially the press, if one is employed; and earthenware glazed with lead should be avoided. The pressing should also be conducted as rapidly as possible, to avoid the colour being injured by the iron. Clean wrought iron does not readily injure the colour of infusion of roses before the addition of the acid. When the last formula is adopted, strong pressure of the886 leaves with the hands can alone be safely had recourse to. If the infusion does not filter quite clear through paper, it should be set aside for a few days, when, in general, it will be found to filter more readily and satisfactorily. Should it be wanted for immediate sale, the addition of the whites of 2 or 3 eggs, diluted with 2 or 3 ounces of water, followed by violent agitation of the liquid for a few minutes, and repose for an hour or two, will usually render it ‘fine,’ when it may be either decanted, or filtered should it require it. It will now pass rapidly through ordinary filtering paper, and at once run clear.
Infusion of Rue. Syn. Rue tea; Infusum rutæ, L. Carminative, antispasmodic, emmenagogue, and vermifuge. It is a popular and useful remedy in flatulent colic, infantile convulsions, epilepsy, hysteria, suppressed menstruation, &c.
Infusion of Rupture-wort. Syn. Infusum herniariæ. Prep. Rupture wood, 2 dr.; boiling water, 1 pint.
Infusion of Safflower. Syn. Infusum carthami. Prep. Safflower, 2 dr.; boiling water, 16 fl. oz.; infuse for an hour.—Dose. A wine-glassful, as a diaphoretic.
Infusion of Sage. Syn. Sage tea; Infusum salviæ, L. 1. From the leaves of common garden sage. Carminative and stomachic. In flatulence and dyspepsia, and diluted with water as a drink, to lessen the nightsweats in phthisis and fever, and to stop the secretion of milk after weaning.
2. (Compound; Infusum salviæ compositum, L.—Ellis.) Sage and boneset, of each 1⁄2 oz.; cascarilla, 1 dr.; boiling water, 11⁄2 pint; infuse until cold. A wine-glassful every 3 or 4 hours in hectic fever.
Infusion of Sarsaparil′la. Syn. Infusum sarzæ, I. sarsaparillæ (Ph. U. S.), L. 1. From the bruised root. Dr Hancock adds 1⁄2 fl. dr. of hydrochloric acid to each pint of the water employed, as a menstruum, by which he says the efficacy of the infusion is greatly increased. At St. George’s Hospital a little liquorice root and solution of potassa is added for the same purpose.
2. (Compound; Infusum sarsaparillæ compositum, L.—Ph. D. 1826.) Sarsaparilla root (washed clean with a little cold water, and sliced), 1 oz.; lime water (cold), 16 fl. oz.; macerate for 12 hours, and strain. Inferior to the simple infusion, since both earths and alkalies lessen the solvent action of water on sarsaparilla. Use of both the above, similar to that of the DECOCTION.
Infusion of Sas′safras. Syn. Sassafras tea; Infusum sassafras, L. From sassafras chips. Alterative, stimulant, and sudorific; a popular remedy in various cutaneous, rheumatic, scrofulous, and syphilitical affections. Hufeland recommends the addition of a little liquorice root.
Infusion of Sav′ine. Syn. Savine tea; Infusum sabinæ, L. Prep. (Pereira.) Fresh savine leaves or herb, 1 dr.; boiling water, 8 fl. oz.; infuse in a covered vessel. Stimulant, emmenagogue, and vermifuge; in chlorosis, and suppressed menstruation depending on a torpid action of the uterine vessels; in chronic rheumatism, worms, &c.—Dose, 1 to 2 table-spoonfuls, cautiously administered.
Infusion of Sax′ifrage. Syn. Saxifrage tea; Infusum pimpinellæ, L. From the root of burnet saxifrage (Pimpinella Saxifraga). Astringent; in diarrhœa, and externally as a wash to remove freckles.
Infusion of Scutella′′ria. Syn. Infusum scutellariæ, L. Prep. (Dr Spalding.) Dried herb of Scutellaria lateriflora, in powder, 11⁄2 teaspoonful; boiling water, 1 pint. By teacupfuls, thrice daily, to prevent hydrophobia.
Infusion of Sen′ega. Syn. Infusion of rattle-snake root, Seneka tea; Infusum senegæ (B. P., Ph. E.), I. polygalæ (Ph. D.), L. Prep. 1. (B. P.) Senega, bruised, 1 oz.; boiling distilled water, 20 oz.; infuse 1 hour, and strain.—Dose, 1 to 2 oz.
2. (Ph. E.) Senega snake-root (bruised), 10 dr.; boiling water, 1 pint; infuse for 4 hours in a covered vessel, and strain.
3. (Ph. D.) Polygala root, 1⁄2 oz.; boiling water, 9 fl. oz. Stimulant, expectorant, and diuretic, either alone or combined with ammonia; in catarrhs, &c. See Decoction, Extract, &c.
Infusion of Sen′na. Syn. Senna tea; Infusum sennæ (B. P., Ph. E.), I. sennæ compositum (Ph. L. & D.), L. Prep. 1. (B. P.) Senna, 1 oz.; ginger, sliced, 30 gr.; boiled distilled water, 10 oz.; infuse 1 hour, and strain.—Dose, 1 to 2 oz.
2. (Ph. L.) Senna, 15 dr.; ginger (bruised), 4 scruples; boiling water, 1 pint; macerate for an hour in a covered vessel, and strain.
3. (Ph. E.) Senna, 11⁄2 oz.; ginger, 4 scrup.; boiling water, 1 pint. (See No. 9, below.)
4. (Ph. D.) Senna, 1⁄2 oz.; ginger, 1⁄2 dr.; boiling water, 1⁄2 pint. Purgative.—Dose, 1 to 2 wine-glassfuls. It is usually given in doses of 1 to 11⁄2 fl. oz., combined with 3 to 6 dr. of Epsom salts, or other saline purgative, under the name of ‘BLACK DRAUGHT,’
Obs. This infusion is very apt to spoil in warm weather, to prevent which Mr Squire recommends the addition of 1 gr. of nitrate of potassa to each ounce.
5. (Concentrated; Infusum sennæ concentratum, L.)—a. Senna, 2 lbs. 1 oz.; tepid water, 1 quart, macerate for 12 hours, frequently stirring with a stick, and express the liquor; to the ‘marc,’ add of tepid water 11⁄4 pint, repeat the maceration for 3 hours, and again express the liquor with powerful pressure; mix the infusions, and after 2 hours’ repose decant the clear portion, and evaporate it as rapidly as possible, by steam or a chloride of sodium bath, until it measures 11⁄2 pint; pour this into a strong bottle, and when887 nearly cold, add of rectified spirit, 1⁄2 pint; bruised ginger, 31⁄2 oz.; macerate a week with frequent agitation, and after repose for a few days decant the clear portion, and add dilute spirit (1 to 4), q. s. to make the whole measure exactly a quart.
b. Take 8 times the quantity of senna and ginger ordered in the Ph. L., put them into a displacement apparatus, either alone or mixed with clean washed sand, and transmit water, mixed with 1⁄4th part of rectified spirit, through the mass, until the proper quantity of infusion is obtained.
c. (Wholesale.) Alexandrian senna (best), 7 lbs.; unbleached Jamaica ginger (finest, bruised), 3 lbs.; rectified spirit and water, of each 1 gall.; macerate for 14 days, press out the fluid, filter, and set it aside in a well-corked bottle; then take of good East India senna, 25 lbs.; and the ‘pressings’ or ‘marc’ from the tincture, and macerate in the least possible quantity (10 or 12 galls.) of cold distilled water, for 12 or 14 hours, employing frequent agitation with a wooden spatula; next press out the liquid, and again macerate the ‘marc’ in cold distilled water (5 or 6 galls.) for 2 hours; press, mix the two liquors, strain, heat gradually to the boiling point, carefully separate the coagulated albumen and afterwards evaporate as quickly as possible to exactly 9 quarts; put the liquid at once into a vessel capable of holding 5 gallons, bung close to exclude the air, and when nearly cold add the ‘tincture’ obtained from the Alexandrian senna and the ginger; the whole must now be well agitated together, and allowed to stand for a week, when the clear portion must be carefully decanted into bottles (Winchester quarts) for sale.
d. As the last, but employing hot water, and limiting the period of the infusions to 2 hours and 1 hour.
Obs. The preceding formulæ are at present employed in the wholesale trade, by nearly all those houses that are most noted for the superior quality of their ‘CONCENTRATED INFUSIONS,’ The products of the whole are excellent. That from c is very beautiful, and contains all the valuable active matter that it is possible to extract from the ingredients, under the circumstances. It also keeps well. The last one, like all preparations of senna made with hot water, is apt to drop a large deposit on standing, from which the last portion of the infusion is obtained with difficulty. They each furnish a liquid, of which 1 fl. dr. added to 7 fl. dr. of pure water forms 1 fl. oz. of a preparation precisely similar in medicinal qualities to the INFUSUM SENNÆ COMP.—Ph. L.
From the extreme bulkiness of senna, it has become a practice with certain unprincipled druggists to employ only 1⁄3 or 1⁄4 of the proper quantity of that drug, and to add burnt sugar or treacle to bring up the consistence and colour, and alkaline solution of gamboge to impart the necessary purgative quality. Concentrated infusion of senna, as generally met with, is nearly worthless. This arises from either the employment of inferior senna, or the destruction of its active principle, by the lengthened exposure to heat and atmospheric oxygen during its manufacture.
6. (With COFFEE; Infusum sennæ cum caffeâ, L.)—a. (Foy.) Senna, 2 dr.; roasted coffee (ground), 1 dr.; boiling water and hot milk, of each 3 fl. oz.; infuse for 12 hours (4?), and strain. For an adult; to be taken in the morning fasting.
b. (Guersand and Blake.) Senna, 10 to 30 gr. (according to age); hot coffee and hot milk at will; infuse, and when cold strain, and sweeten it with sugar, q. s. As a purge for children.
7. (With LEMON JUICE; Infusum sennæ) limoniatum, L.) From senna, 11⁄2 oz.; fresh lemon peel, 1 oz.; lemon juice, 1 fl. oz.; boiling water, 16 fl. oz.; infuse.
8. (With RHUBARB; Infusum sennæ et rhei, L.—Ellis.) Senna, 6 dr.; manna, 1 oz.; rhubarb and cardamoms, of each (bruised), 2 dr.; boiling water, 1 pint; infuse 1 hour and strain.
9. (With TAMARINDS; Infusum sennæ compositum—Ph. E., Sennæ cum tamarindis, L.—Ph. E.) Senna, 3 dr.; tamarinds, 1 oz.; coriander seeds, 1 dr.; sugar, 1⁄2 oz. (if brown, 1 oz.); boiling water, 8 fl. oz.; infuse for four hours, with agitation, and then strain through calico. Pleasanter than the ordinary infusion of senna.
10. (With TARTAR; Infusum sennæ tartarizatum, L.) From senna, 11⁄2 oz.; coriander seeds, 4 dr.; cream of tartar, 2 dr.; boiling water, 16 fl. oz.
Infusion of Ser′pentary. Syn. Infusum serpentariæ (B. P., Ph. L. & E.), L. Prep. 1. (B. P.) Serpentary, bruised, 1 oz.; boiling distilled water, 40 oz.; infuse 2 hours, and strain.—Dose, 1 to 2 oz.
2. (Ph. L.) Serpentary or Virginian snake-root, 1⁄2 oz.; boiling distilled water, 1 pint; macerate for 4 hours in a closed vessel, and strain. The form of the Ph. E. is similar. As a stimulating expectorant and diaphoretic; in chronic catarrhs, low fevers, agues, &c.
3. (Compound; Infusum serpentariæ compositum, L.—Guy’s Hosp.) Virginian snake-root and contrayerva, of each 5 dr.; boiling water, 1 pint; macerate 2 hours, strain; and when cold add of tincture of serpentary 2 fl. oz. As the last.
Infusion of Simaru′ba. Syn. Infusum simarubæ (B. P., Ph. E. & D.), L. Prep. 1. (B. P.) Simaruba, bruised, 3 dr.; boiling water, 1 pint; infuse 2 hours, and strain.—Dose, 1 to 2 oz.
2. (Ph. E. & Ph. L., 1836.) Bark of the bitter simaruba or mountain damson, 3 dr.; boiling water, 1 pint; macerate 2 hours, and strain.
888
3. (Ph. D.) Simaruba bark, 2 dr.; boiling water, 9 fl. oz. Tonic, and, in large doses, emetic; in chronic diarrhœa and dysentery, either alone or combined with opium; and in agues, dyspepsia, &c.
4. (Compound; Infusum simarubæ compositum, L.—Foy.) Simaruba bark and wormwood, of each 2 dr.; boiling water, 1 pint; infuse for 15 minutes, strain, and add of syrup of gentian, 1 fl. oz. In agues and dyspepsia.
Infusion of Slip′pery Elm. Syn. Infusum ulmi (Ph. U. S.), I. u. Fulvæ, L. Prep. (Ph. U. S.) Inner bark of slippery elm (Ulmus fulva), 1 oz.; boiling water, 16 fl. oz.; infuse for 2 hours, and strain. Demulcent.
Infusion of Soap-wort. Syn. Infusum saponariæ, L. From soap-wort root (Saponaria officinalis). Aperient and demulcent; also reputed alterative and antisyphilitic.
Infusion of South′ernwood. Syn. Southernwood tea; Infusum abrotani, L. From the herb southernwood or old man (Absinthium Abrotanum). Antispasmodic, tonic, and vermifuge; in hysteria, difficult and painful menstruation, worms, &c.
Infusion, Stim′ulant. Syn. Infusum stimulans, L. Prep. (Dr Paris.) Black mustard seed (bruised), and dittander, of each 1⁄2 oz.; boiling water, 16 fl. oz.; macerate for 1 hour, strain, and when cold add of spirit of sal-volatile, 1 fl. dr.; spirit of pimento, 1⁄2 fl. oz.—Dose, 2 table-spoonfuls 3 times a day; in palsy.
Infusion of Stink′ing Hel′lebore. Syn. Infusum hellebori fœtidi, L. Prep. (Woodville.) Dried leaves of setter-wort or Helleborus fœtidum, 1⁄2 dr. (or green herb, 2 dr.); boiling water, 16 fl. oz.; macerate 1 hour, and strain. Aperient and vermifuge; and emetic, in large doses. It is chiefly used against the large round worms of children and females, taken fasting.
Infusion of Suc′cory. Syn. Chicory tea; Infusum chicorii, L. From the dried root. Aperient, deobstruent, and tonic; either alone or sweetened with honey or sugar.
Infusion of Sweet Flag. Syn. Calamus tea, Sweet-flag t.; Infusum acori, I. Calami aromatici, L. An aromatic stimulant, tonic, and stomachic. See Sweet Flag.
Infusion of Tam′arinds. Syn. Infusum tamarindi, L. Cooling and laxative; in sore throat, febrile affections, &c., taken ad libitum. See Infusion of Senna.
Infusion of Tan′sy. Syn. Tansy tea; Infusum tanaceti, L. From the dried herb, or the green herb using double the quantity. Aromatic, bitter, tonic, and vermifuge.
Infusion of Tar. Syn. Tar water, Tar tea; Infusum picis liquidæ, Aqua p. l. (Ph. D.), L. Prep. 1. (Bishop Berkeley.) Wood tar, 1 quart; cold water, 1 gall.; stir with a stick for 15 minutes, then allow the tar to subside, strain, and keep it in well-stoppered jars.
2. (Ph. D.) As the last. Taken to the extent of a pint daily in chronic catarrhal and nephritic affections; also used as a lotion in chronic cutaneous diseases, especially those of the scalp in children. See Decoction.
Infusion of Tarax′acum. See Infusion of Dandelion.
Infusion of Tobac′co. Syn. Tobacco water; Infusum tabaci, L. Prep. (Ph. D. 1826.) Tobacco leaves, 1 dr.; boiling water, 16 fl. oz.; macerate for an hour. Used for enemas; in strangulated hernia, obstinate colic, &c., observing not to administer more than one half at a time; also as a wash to kill pediculi.
Infusion, Ton′ic. See Infusions of Calumba, Cascarilla, Gentian, &c., also Mixtures.
Infusion of Sessamum. (Dr Wood.) Syn. Infusum sesami. Prep. Two fresh leaves of sessamum (Venne) infused in 8 oz. of cold water, form a mucilaginous demulcent drink. Dried leaves require hot water.
Infusion of Silk-weed. Syn. Infusum asclepiadis. Prep. Bark of the common silk-weed, 1 oz.; boiling water, 1 pint.-Dose, 1 oz. to 11⁄2 oz. In cough and dyspnœa.
Infusion of Tre′foil. See Infusion of Buckbean.
Infusion of Tu′lip-tree Bark. Syn. Infusum liriodendri, L. From the bark of the tulip tree (Liriodendron tulipifera). Diaphoretic, stimulant, stomachic, and tonic; in dyspepsia, fevers, &c.; also used to flavour liquors.
Infusion of Tur′meric. Syn. Infusion curcumæ, L. Used as a test and to prepare test-paper. When required for keeping, about 1-7th of its volume of rectified spirit must be added.
Infusion of Valer′ian. Syn. Infusum valeriane (B. L., Ph. L. & D.), L. Prep. 1. (B. P.) Valerian, bruised, 120 gr.; boiling distilled water, 10 oz.; infuse 1 hour and strain.—Dose, 1 to 2 oz.
2. (Ph. L.) Valerian root, 1⁄2 oz.; boiling distilled water, 1 part; infuse for an hour in a covered vessel, and strain.
3. (Ph. D.) Valerian, 2 dr.; boiling water, 9 fl. oz. Antispasmodic and nervine; in hysteria, hypochondriasis, epilepsy, and low fevers.
4. (Compound; Infusum valerianæ compositum, L.) Yellow cinchona bark, 1 oz.; valerian, 1⁄2 oz.; boiling water, 1 pint; as before. In debilitated nervous habits.
Infusion of Vanil′la. Syn. Vanilla tea; Infusum vanillæ, L. Prep. Vanilla, 11⁄2 dr.; boiling water, 1 pint. A stimulant antispasmodic; in hysteria, rheumatism, anaphrodisia, &c.; but chiefly used as a flavouring for liqueurs, confectionery, &c.
Infusion of Vittie Vayr. Syn. Vittie vayr tea; Infusum vetiveriæ, L. From the roots of Andropogon muricatus (VETIVER, VITTIE VAYR, or CUSCUS). Antispasmodic, diaphoretic, and stimulant, and, when warm, diaphoretic and emmenagogue; in rheumatism889 gout, slight febrile cases, &c.; and as a prophylactic of cholera. See Essence.
Infusion of Wall-pel′litory. Syn. Infusum parietariæ, L. From the dried herb (Parietaria officinalis). Aperient, diuretic, and pectoral; in asthmas, dropsies, calculous affections, &c.
Infusion of Wal′nut Leaves. Syn. Walnut-leaf tea; Infusum juglandis, L. From the fresh leaves of the common walnut (Juglans regia); also from the inner wood-bark, and the green rind of the fruit. See Decoction and Extract.
Infusion of Water-fen′nel. Syn. Infusum phellandri, L. Prep. (Bird.) Seeds of water-fennel, 5 dr.; boiling water, 1 pint.—Dose, 3 to 4 fl. dr.; to check excessive expectoration.
Infusion of Whor′tleberry. Syn. Infusum uvæ ursi, L. With alkalies, henbane, or opium, in diseases of the urinary organs; and with sulphuric acid and foxglove, in affections of the lungs. See DECOCTION and Extract.
Note.—Infusum Uvæ Ursi of the Brit. Pharmacopœia.
Infusion of Wild-cherry Bark. Syn. Infusum pruni Virginianæ (Ph. U. S.), L. Prep. (Ph. U. S.) Wild cherry-tree bark (Prunus Virginiana or Cerasus Serotina), 1⁄2 oz.; cold water, 16 fl. oz.; infuse 24 hours, and strain. A valuable tonic and febrifuge. Wild-cherry bark also exercises a sedative action on the circulatory and nervous system, and is much used in America in a variety of diseases.
Infusion of Wild Gin′ger. Syn. Infusum asari Canadensis, L. From the root of wild ginger or Canada snake-root (Asarum Canadense). A warm stimulant diaphoretic, in the same cases as infusion of Virginian snake-root.
Infusion of Wil′low Bark. Syn. Infusum salicis, L. From the bark of the white or common willow (Salix alba). Astringent, tonic, and febrifuge; often used instead of INFUSION OF CINCHONA.
Infusion of Win′ter Green. Syn. Infusum pyrolæ, I. chimaphilæ, L. Astringent, tonic, and diuretic; in dropsy, nephritic pains, and chronic affections of the urinary organs. It blackens the urine, like uva ursi. See Decoction.
Infusion of Wood Soot. Syn. Soot tea; Infusum fuliginis ligni, L. Antacid and stimulant. A similar preparation is also made from coal-soot, which is reputed antispasmodic and vermifuge.
Infusion of Worm′wood. Syn. Wormwood tea, Infusum absinthii, L. From the fresh tops of the plant, or from only half the quantity of the dried herb. In loss of appetite, dyspepsia, amenorrhœa, leucorrhœa, gout, worms, &c. See Bitters.
INHALA′TION. Syn. Inhalatio, L. In medicine, the drawing in or inspiring of vapour with the breath. Inhalations (INHALATIONES) are vapours or gases imbibed for the purpose of medicating the mucous membrane of the air-passages. The substances that are to furnish the vapours or fumes are put into a vessel called an ‘inhaler’ (see Inhaler), which may be simply a small covered pot or mug of metal or glass, furnished with a short flexible tube, terminating in a small mouth-piece. In many cases even this simple apparatus may be dispensed with, and the fumes inhaled by holding the head over a vessel containing a little of the substance furnishing them; or, as with chloroform, a little may be dropped on a handkerchief or napkin, which is then held to the nose.
The following are the principal substances that are employed for inhalations at the present day:—
1. Carbonic acid gas and nitrous oxide; occasionally used in phthisis, by means of a bladder and mouth-piece.
2. Chlorine gas; exhibited by adding 5 or 6 drops of aqueous chlorine to the water (tepid) of the inhaler, which should be, in this case, of glass; employed in France for phthisis, but seldom used in England.
3. Chloroform; as an anæsthetic.
4. Vapour of iodine, administered in the same way as chlorine; occasionally used in phthisis.
5. Oxygen and hydrogen gases, either alone or diluted with air; employed in asthma and phthisis, by means of a bladder and mouth-piece.
6. Tar vapour, obtained by heating tar, mixed with a little carbonate of potash, over a spirit lamp, occasionally employed in bronchitis, and recommended by Sir A. Crighton in phthisis, but appears of little value in the latter.
7. Steam of hot water; in bronchitis, and to allay the cough in phthisis; small quantities of the seeds of henbane, opium, poppy-heads, &c., are frequently added to produce an anodyne effect. See Cigars (in pharmacy), Disinfectants, Fumigation, Vapours, &c.
INJEC′TION. Syn. Injectio, L. In medicine, any liquid medicine thrown into a cavity of the body by means of a syringe or an elastic bag. Those thrown into the rectum are commonly called ‘clysters’ or ‘enemata,’ and are noticed under the head of Enema. The following are the principal injections employed in medical practice at the present day:—
Injection of Ac′etate of Ammo′′nia. Syn. Injectio ammoniæ acetatis, L. Prep. (Ph. Chirur.) Solution of acetate of ammonia (Ph. L.), 1 part; water, 3 parts. Refrigerant.
Injection of Ac′etate of Cop′per. Syn. Injectio cupri acetatis, L. Prep. From verdigris, 10 gr.; oil of almonds (hot), 41⁄2 oz.; triturate until dissolved, and strain. Detergent.
Injection of Ac′etate of Lead. Syn. Injectio plumbi acetatis, L. Prep. 1. Sugar of lead, 1⁄2 dr.; distilled water, 1⁄2 pint.
890
2. (Dr Collier.) Acetate of lead, 40 gr.; rose water, 8 fl. oz. Astringent and sedative. See Sedative injection.
Injection of Ac′etate of Zinc. Syn. Injectio zinci acetatis, L. Prep. 1. (Ellis.) Acetate of zinc, 8 gr.; rose water, 4 fl. oz.
2. (Brodie.) Sulphate of zinc, 1 dr.; sugar of lead, 80 gr.; water, 1 pint; dissolve separately, mix, and filter. Astringent.
Injection, Alkaline. Syn. Injectio alkalina, I. lithontriptica, L. Prep. (Chevallier.) Carbonate of soda, 1 dr.; Castile soap, 2 dr.; water, 12 fl. oz.; dissolve. In certain forms of calculus.
Injection of Aloes. (Bories.) Syn. Injectio aloes. Prep. Aloes, 10 gr.; muriate of ammonia, 10 gr.; honey of roses, 1 oz.; fennel water, 6 oz.
4. (Dr Reece). Alum, 1 dr.; acetate of lead, 11⁄2 dr.; triturate with 6 oz. of boiling water, and in an hour filter.
Injection of Al′um. Syn. Injectio aluminis, L. Prep. 1. (Dr Collier.) Alum, 18 gr.; rose-water, 6 fl. oz.; dissolve. For the urethra.
2. (Collier.) Alum, 3 dr.; water, 1 quart. For the vagina.
3. (Ph. Ch.) Alum, 4 gr.; rose-water, 4 fl. oz. The above are all astringent.
Injection of Ammo′′nia. Syn. Injectio ammoniæ, L. Prep. 1. (Dr Ashwell.) Liquor of ammonia, 1 to 2 fl. dr.; milk, 1 pint. In obstructed menstruation.
2. (Lavagna.) Liquor of ammonia, 8 to 20 drops; milk, 2 fl. oz. As the last, thrice daily, beginning with the least quantity of ammonia.
3. Liquor of ammonia, 1 fl. dr.; mucilage, 1 oz.; water, 9 fl. oz. As the last.
Injection of Ammo′′nio-Sulphate of Cop′per. Syn. Injectio cupri ammoniati, L. Prep. (Swediaur.) Ammonio-sulphate of copper, 5 gr.; rose-water, 8 fl. oz. In chronic gonorrhœa.
Injection of Bichlo′′ride of Mer′cury. Syn. Injectio hydrargyri bichloridi, L. Prep. 1. Corrosive sublimate, 2 gr.; rose water, 5 fl. oz.; hydrochloric acid, 1 drop.
2. Corrosive sublimate and sal ammoniac, of each 5 to 10 gr.; water, 1 pint.
3. Sublimate, 5 gr.; rose water, 21⁄2 fl. oz. Used to promote healthy action, and to prevent infection.
Injection of Cal′omel. Syn. Injectio calomelanos, I. hydrargyri, CHLORIDI, L. Prep. (St. B. Hosp.) Calomel, 1 dr.; mucilage, 1 fl. oz.; water, 1⁄2 pint. Some persons order ‘quince mucilage.’
Injection of Carbolic Acid. (Throat Hosp.) Syn. Injectio acidi carbolici. Prep. Carbolic acid, 5 gr.; water, 1 oz.; mix. Antiseptic.
Injection of Car′bonate of Lead. Syn. Injectio cerussæ, I. plumbi carbonatis, L. Prep. (Hosp. F.) Carbonate of lead (finely levigated), 1⁄2 dr.; sulphate of zinc, 8 gr.; mucilage, 1 oz.; rose water, 5 oz. Cooling and astringent.
Injection of Chlo′′ride of Lime. Syn. Injectio calcis hypochloris, L. Prep. 1. Chloride of lime, 1⁄2 dr.; water, 1⁄2 pint; agitate well together, and filter. To prevent infection.
2. (Detmold.) Chloride of lime, 2 dr.; decoction of rhatany, 13 fl. oz.; dissolve, and filter. In foul discharges, especially in ozæna, or fœtid ulceration of the nose.
3. (Rousse.) Chloride of lime, 20 gr.; water, 7 fl. oz.; wine of opium, 1 fl. oz. In foul discharges, and to allay irritation.
Injection of Chlo′′ride of So′da. Syn. Injectio sodæ hypochloris, L. Prep. From solution of chloride of soda, 1 fl. dr.; rose water, 3 fl. oz. As the last.
Injection of Chlo′′ride of Zinc. Syn. Injectio zinci chloridi, L. Prep. From chloride of zinc, 2 gr.; rose water, 3 fl. oz.; hydrochloric acid, 1 drop. In gonorrhœa.
Injection of Copai′ba. Syn. Injectio copaibæ, L. Prep. 1. (Abernethy.) Copaiba 2 dr.; thick mucilage, 5 dr.; lime water, 6 fl. oz.; make an emulsion.
2. (Plenck.) Copaiba, 1⁄2 oz.; yolk of egg, q. s.; lime water, 6 fl. oz.; honey of roses, 3 oz. As the last.
3. (Ricord.) Copaiba, 6 dr.; yolk of egg, q. s.; decoction of poppies, 3 to 4 fl. oz. In ulcers of the rectum, vagina, and urethra; and in gonorrhœa.
Injection of Cre′asote. Syn. Injectio creasoti, L. Prep. (Dr Allnatt.) Creasote, 20 drops; white sugar, 2 dr.; liquor of potassa, 2 fl. dr.; triturate, and add of water, 8 fl. oz. In leucorrhœa and piles.
Injection of Cu′bebs. Syn. Injectio cubebæ, L. Prep. (Soubeiran.) Cubebs (in powder), 1 oz.; extract of belladonna, 1 dr.; boiling water, 16 fl. oz.; infuse in a covered vessel, and strain. Stimulant and narcotic. In gonorrhœa and leucorrhœa.
Injection for the Ear. Syn. Injectio acoustica, L. Prep. 1. Ox-gall, 3 dr.; balsam of Peru, 1 dr.; mix. In hardened wax, dryness of membranes, &c.
2. Oil of almonds or cloves, 2 oz.; oil of amber, 20 drops; tincture of castor, 1 fl. dr.; spirit of camphor, 1⁄2 dr.; laudanum, 3 drops; mix. In ear-ache and chronic deafness.
3. (Alibert.) Balsam of Peru, 2 dr.; tincture of musk, 4 or 5 drops; otto of roses, 1 or 2 drops; decoction of St. John’s wort (warm), 16 fl. oz.; agitate together, and after repose decant the clear. In discharges from the ear.
Obs. Mr Yearsley states that drops and injections for the ear should be used with very great caution, and only under proper advice, as they otherwise often aggravate the ailment, instead of curing it.
Injection of Er′got. Syn. Injectio ergotæ, I. secalis cornuti, L. Prep. 1. (Boudin.) Ergot, 1 dr.; boiling water, 8 fl. oz.; infuse891 until cold. When the urethra is highly sensitive.
2. (Descrolles.) Powdered ergot, 1 oz.; boiling water, 1 pint. Both the above are used in chronic inflammation of the vagina, and in gonorrhœa.
Injection of Gal′lic Acid. Syn. Injectio acidi gallici, L. Prep. (Dunglison.) Gallic acid, 1⁄2 dr.; water, 1 pint. In leucorrhœa.
Injection of Galls. Syn. Injectio gallæ, L. Prep. From galls (bruised), 2 dr.; boiling water, 1 pint; infuse 1 hour, and strain. Astringent; in leucorrhœa.
Injection of Hydrochlo′′ric Acid. Syn. Injectio acidi hydrochlorici, L. Prep. From hydrochloric acid, 10 drops; soft water, 5 fl. oz. To prevent and to remove recent infection; also to remove particles of lime and iron from the eye.
Injection of Hydrocyan′ic Ac′id. Syn. Injectio acidi hydrocyanici, L. Prep. Medicinal hydrocyanic acid, 1 fl. dr.; soft water or almond emulsion, 1 pint. Anodyne; to allay excessive irritability, both in chronic ophthalmia and gonorrhœa, and to relieve chordee; but in all cases it must be used with caution, and at first largely diluted with water.
Injections, Hypodermic. Syn. Injectiones hypodermicæ, Injectiones subcutaneæ.
1. Hypodermic Injection of Ergotine. (Dr Hildebrandt.) Aqueous extract of ergotine, 3 parts; distilled water and glycerin, of each 71⁄2 parts; for uterine fibroid tumours (Dr Drasch), 5 gr. of ergotine in 1 dr. of glycerin; 1⁄5th to be injected, according to circumstances, once or twice a day, in the region of the pectoral muscles; in internal hæmorrhage, hæmoptysis, and epistaxis.
2. Hypodermic Injection of Iodic Acid. Dr Luton uses this in goitre, 1⁄2 dr. of solution containing 1⁄5th of acid injected at once into the midst of the tumour.
3. Hypodermic Injection of Perchloride of Mercury. (Dr Staub.) Perchloride of mercury and chloride of ammonium, of each 20 gr.; chloride of sodium about 62 gr.; distilled water, 20 gr. After filtration the whole is mixed with solution of the white of one egg, and 41⁄2 dr. of water. The solution contains 1⁄33rd of a gr. of perchloride to every 20 drops. 1⁄6th of a grain of perchloride to be injected each day.
4. Hypodermic Injection of Morphia. (B. P.) Hydrochlorate of morphia 88 gr.; solution of ammonia, acetic acid, distilled water of each, q. s. Dissolve the hydrochlorate in 2 oz. of distilled water by a gentle heat, then add the solution of ammonia, so as to precipitate the morphia, and render the liquid slightly alkaline; allow it to cool; collect the precipitate on a filter, wash with distilled water, and allow it to drain; then transfer the morphia to a porcelain dish, and add acetic acid until the morphia is dissolved, and a very slightly acid solution is formed. Now add distilled water, q. s. to make the solution measure 2 fl. oz. For subcutaneous injection, 1 to 6 minims.
5. Sulphate of morphia is a very good soluble salt.
6. Hypodermic Injection of Quinine. Three to 6 gr. of neutral sulphate of quinine placed on a watch glass, previously warmed, without acid; to this add 12 minims of distilled water, and apply a moderate heat by a spirit lamp for a second, or two. The syringe should be warmed before being used.
Dr Rosenthal advocates the use of glycerin as a medium for the solution of various substances used for subcutaneous injection. The glycerin must be very pure. By gradual elevation of temperature it can be made to take up a large number of certain alkaloids and salts, and will retain them dissolved for a year. 1 fl. dr. will dissolve one scruple of sulphate of quinine, and 10 gr. of hydrochlorate of morphia. Dr Rosenthal states that the injection of quinine has been found very useful in intermittents.
Injection of Io′dide of I′ron. Syn. Injectio ferri iodidi, L. Prep. 1. (Ricord.) Iodide of iron, 6 gr.; water, 5 fl. oz. In gonorrhœa, gradually increasing the quantity of iodide.
2. (Soubeiran.) Iodide of iron, 3 to 4 dr.; water, 1 pint. In suppressed and painful menstruation, leucorrhœa, &c. Both are astringent and well adapted to scrofulous patients.
Injection of I′odide of Potas′sium. Syn. Injectio potassii iodidi, L. Prep. (Foy.) Iodide of potassium, 3 gr.; pure water, 1 pint. As a stimulant to fistulous sinuses and ulcers in persons of scrofulous habits.
Injection of I′odine. Syn. Ioduretted injection; Injectio iodureta, I. iodinii, L. Prep. 1. (M. Ameuille.) Tincture of iodine, 1 part; water, 5 or 6 parts. In refractory fistulæ.
2. (M. Bonnet.) Iodine, 1 part; iodide of potassium, 2 parts; water, 10 parts. In scrofulous hydrarthrosis, &c.
3. (Bransby Cooper.) Compound tincture of iodine, 2 fl. dr.; water, 6 fl. dr. In hydrocele.
4. (Guibourt.) Iodine, 4 gr.; iodide of potassium, 8 gr.; water, 1 pint. To stimulate fistulous sinuses.
5. (Velpeau.) Tincture of iodine, 1 fl. dr.; water, 3 fl. dr. In hydrocele.
Injection, Lithontrip′tic. Syn. Injectio lithontriptica, I. vesicalis, L. Prep. (Dr Hoskins.) Nitro-saccharate of lead, 1 gr.; saccharic acid, 5 drops; rub together, then add of distilled water, 1 fl. oz. As a solvent for phosphatic calculi. See Injection alkaline.
Injection, Mercu′′rial. Syn. Injectio mercurialis, I. hydrargyri, L. Prep. 1. Quicksilver, 1 dr.; gum mucilage, 11⁄2 oz.; triturate until the globules disappear, and gradually add of water, 11⁄2 fl. oz.
2. (Hosp. F.) Quicksilver and balsam of copaiba, of each 4 dr.; yolk of an egg; rose892 water, 1⁄2 pint. An awkward and useless preparation.
Injection of Mor′phia. Syn. Injectio morphiæ, L. Prep. (Brera.) Morphia, 2 gr.; oil of almonds (warm), 1 oz.; triturate together until united. Anodyne and emollient. To ease the pain in ear-ache, acute gonorrhœa, piles, &c.
Injection of Night Shade, Black. (P. C.) Syn. Injectio foliarum solani nigrum. Prep. Dried leaves of black night shade, 13⁄4 oz.; boiling water, 36 oz. Infuse 1 hour, and strain.
Injection of Nitrate of Sil′ver. Syn. Injectio argenti nitratis, L. Prep. 1. (Acton.) Nitrate of silver, 3 gr.; distilled water, 1⁄2 pint; dissolve.
2. (Dr Arnott.) Nitrate, 12 gr.; water, 1 fl. oz.
3. (Dr Collier.) Nitrate, 2 gr.; rose water, 1 fl. oz.
4. (Dr Culverwell.) Nitrate, 20 to 30 gr.; water, 1 fl. oz.
5. (Dr Jewell.) Nitrate, 12 gr.; water, 6 fl. oz.
6. (Ricord.) Nitrate, 8 gr.; water, 1 fl. oz.
7. (West. Hosp.) Nitrate, 11⁄2 gr.; diluted nitric acid, 11⁄4 minim; distilled water, 1 fl. oz.
Obs. The weaker solutions are used in chronic gonorrhœa, gleet, and leucorrhœa; those of an intermediate strength to prevent an attack of gonorrhœa following the incipient symptoms of that disease; and the strongest, chiefly in spermatorrhœa. Their use requires great caution.
Injection of Oak Bark. Syn. Injectio quercus. Prep. (Univ. Hosp.) Alum, 6 gr.; decoction of oak bark, 1 fl. oz. For the vagina. Astringent.
Injection, Oleaginous. Syn. Injectio oleosa. Prep. Oil of almonds, 4 oz.; liquid subacetate of lead, 8 drops.
Injection of O′′pium. Syn. Injectio opii, I. opiata, L. Prep. 1. Tincture of opium or wine of opium, 1 to 2 fl. dr. (according to circumstances); water, 5 fl. oz. As an anodyne, in gonorrhœa.
2. (Foy.) Extract of opium, 6 gr.; extract of belladonna, 11⁄2 dr.; decoction of wild lettuce, 16 fl. oz. In neuralgia and hæmorrhages.
Injection of Opium with Lead. (Wendt.) Syn. Injectio plumbi opiata. Prep. Extract of opium, 11⁄2 gr.; distilled water, 2 oz.; mucilage, 2 dr.; liquid subacetate of lead, 4 drops.
Injection of Pancreas. (Merkel.) Syn. Injectio pancreatini. Prep. One bullock’s pancreas; glycerin, 8 oz. Rub the finely minced pancreas with the glycerin, mix one third of this mixture with from 4 to 5 oz. of finely minced meat, and inject into the rectum. Said to be easily digested.
Injection of Platino-Chloride of Soda. (Hœffer.) Syn. Injectio platino-chloridi Sodii. Prep. Decoction of poppy, 8 oz.; chloride of platinum and sodium, 1⁄2 dr.
Injection, Sed′ative. Syn. Injectio sedativa, L. Prep. (Hosp. F.) Oil of almonds, 1 oz.; solution of diacetate of lead, 20 drops. Cooling, sedative, and emollient.
2. (Wendt.) Aqueous extract of opium, 11⁄2 gr.; mucilage, 2 dr.; solution of diacetate of lead, 4 drops; water, 2 fl. oz. Cooling, sedative, and anodyne.
3. (Gassincourt.) Simple emulsion, 5 fl. oz.; decoction of poppies, 16 fl. oz.; white of 1 egg; mix. In acute gonorrhœa.
Injection, Stim′ulating. Syn. Injectio stimulans, L. Prep. (St. Marie.) Myrrh, 1 oz.; quicklime, 2 oz.; water, 1 quart; digest for 2 or 3 days, and decant the clear portion. In fistulous ulcers.
Injection of Sul′phate of Cop′per. Syn. Injectio cupri sulphatis, L. Prep. 1. Sulphate of copper, 5 gr.; rose water, 4 fl. oz. In chronic gonorrhœa.
2. (Hunter.) Sulphate of copper, 3 gr.; water, 4 fl. oz. As the last.
3. (Swediaur.) Sulphate of copper, 6 gr.; water, 4 fl. oz.; dissolve, and add solution of diacetate of lead, 20 drops. In phimosis.
Injection of Sul′phate of Ir′on. Syn. Injectio ferri sulphatis, L. Prep. (Berends.) Sulphate of iron and mucilage, of each 1⁄2 dr.; sage water, 4 fl. oz.; dissolve. In nasal and uterine hæmorrhages.
Injection of Sul′phate of Zinc. Syn. Injectio zinci sulphatis. Prep. 1. (Hosp. F.) Sulphate of zinc, 2 gr.; water, 1 fl. oz.
2. (King’s Coll.—Injectio communis.)—a. Sulphate of zinc, 3 gr.; solution of lead, 20 drops; water, 1 fl. oz. For a man. b. Sulphate of zinc, 10 gr.; alum, 10 gr.; decoction of oak bark, 1 fl. oz. For a woman.
Injection of Sul′phuret of Potas′sium. Syn. Injectio potassii sulphureti, L. Prep. (Wedekind.) Sulphuret of potassium, 1 dr.; water, 1⁄2 pint. In gonorrhœa.
Injection of Tan′nic Acid. Syn. Injectio tannini, I. acidi tannici, L. Prep. (Béral.) Tannin, 1⁄2 dr.; distilled water, 8 fl. oz. (or better, 1⁄2 pint). In gleet and leucorrhœa.
Injection of Tea. Syn. Injectio theæ, L. Prep. (Hosp. F.) Green tea (or rough black tea), 1 dr. (say 2 teaspoonfuls); boiling water, 1⁄2 pint. Astringent; in gleet and fluor albus.
Injection of Turpentine. (St. Bart.’s Hosp.) Syn. Injectio terebinthinæ. Prep. Oil of turpentine, 11⁄2 fl. oz.; olive oil, 12 fl. oz.
Injection, Vi′nous. Syn. Injectio vini rubri, I. vinosa, L. Prep. (Earle.) Red wine, 1 part; water, 2 or 3 parts. In hydrocele.
Injection of Wood-soot. Syn. Injectio Fuliginis. (Rognetta). Decoction of wood-soot, 16 oz.; alum 1⁄2 oz.; water, 6 oz. In leucorrhœa.
Ink. Syn. Atramentum, L. Coloured liquid employed for writing with a pen. Ink is made of various substances and colours; but893 at present we shall confine our attention to the tanno-gallic compounds, to which the term, when standing alone, is almost exclusively applied.
Prep. 1. Aleppo galls (well bruised), 4 oz.; clean soft water, 1 quart; macerate in a clean corked bottle for 10 days or a fortnight, or even longer, with frequent agitation, then add of gum Arabic (dissolved in a wine-glassful of water), 11⁄4 oz.; lump sugar, 1⁄2 oz.; mix well, and afterwards further add of sulphate of iron (green copperas, crushed small), 11⁄2 oz.; agitate occasionally for 2 or 3 days, when the ink may be decanted for use, but is better if the whole is left to digest together for 2 or 3 weeks. When time is an object, the whole of the ingredients may at once be put into a bottle, and the latter agitated daily until the ink is made; and boiling water instead of cold water may be employed. Product. 1 quart of excellent ink, writing pale at first, but soon turning intensely black.
2. Aleppo galls (bruised), 12 lbs.; soft water, 6 galls.; boil in a copper vessel for 1 hour, adding more water to make up for the portion lost by evaporation; strain, and again boil the galls with water, 4 galls.; for 1⁄2 an hour; strain off the liquor, and boil a third time with water, 21⁄2 galls., and strain; mix the several liquors, and while still hot, add of green copperas (coarsely powdered), 41⁄2 lbs.; gum arabic (bruised small), 4 lbs.; agitate until dissolved, and after defecation strain through a hair sieve, and keep it in a bunged-up cask for use. Product. 12 galls.; very fine and durable.
3. Aleppo galls (bruised), 14 lbs.; gum, 5 lbs; put them in a small cask, and add of boiling soft water, 15 galls.; allow the whole to macerate, with frequent agitation, for a fortnight, then further add of green copperas, 5 lbs., (dissolved in) water, 7 pints; again mix well, and agitate the whole once daily for 2 or 3 weeks. Prod. Fully 15 galls. Resembles No. 1.
4. Galls (bruised), 9 lbs.; logwood chips (best Campeachy), 3 lbs.; boil as in No. 2; to the strained mixed liquors, add of gum arabic and green copperas, of each (bruised small), 4 lbs.; simmer or digest until dissolved, and at once strain through a hair sieve into the store-cask or jars. Prod. 161⁄2 galls. Excellent, but inferior to the preceding.
5. Galls (bruised), 2 lbs.; logwood chips, green copperas, and gum, of each 1 lb.; water, 7 galls.; boil 2 hours, and strain. Prod. 5 galls. A superior ink for retail.
6. Galls (bruised), 1 lb.; logwood, 2 lbs.; gum (common), 1 lb.; green copperas, 3⁄4 lb.; water, 8 galls.; proceed as last. Prod. 6 galls. Common, but fit for all ordinary purposes.
The following formulæ are for some of the advertised inks, or are those recommended by the authorities whose names are attached to them:—
7. (Anti-corrosive.) Same as ‘Asiatic ink.’
8. (Asiatic.) Galls, 4 lbs.; logwood, 2 lbs.; pomegranate peel, 2 lb.; soft water, 5 galls; boil as in No. 2, then add to the strained and decanted liquor, when cold, of gum Arabic, 1 lb.; lump sugar or sugar candy, 1⁄4 lb.; dissolved in water, 3 pints. Product. 41⁄2 galls. Writes pale, but flows well from the pen, and soon gets black.
9. (Brande.) Galls, 6 oz.; green copperas and gum Arabic, of each 4 oz.; soft water, 3 quarts; by decoction.
10. (Chaptal.) As No. 4 (nearly), adding sulphate of copper, 1⁄2 lb. Full coloured, but less durable and anticorrosive than the preceding.
11. (Desormeaux.) Galls, 1 lb.; logwood chips, 4 oz.; water, 6 quarts; boil 1 hour, strain 5 quarts, add of sulphate of iron (calcined to whiteness), 4 oz.; brown sugar, 3 oz.; gum, 6 oz.; acetate of copper, 1⁄4 oz.; agitate twice a day for a fortnight, then decant the clear, bottle, cork up for use. Writes a full black, and otherwise resembles No. 10.
12. (Elsner.) Galls (powdered), 42 oz.; gum Senegal (powdered), 15 oz.; distilled or rain water, 18 quarts; sulphate of iron (free from copper), 18 oz.; liquor of ammonia, 3 dr.; spirit of wine, 24 oz.; mix these ingredients in an open vessel, stirring frequently until the ink attains the desired blackness. This formula is said to give a deep black, neutral ink that does not corrode steel pens.
13. (Exchequer.) Galls (bruised), 40 lbs. (say 4 parts); gum, 10 lbs. (say one part); green sulphate of iron, 9 lbs. (say one part); soft water, 45 galls., (say 45 parts); macerate for 3 weeks, employing frequent agitation. “This ink will endure for centuries.”
14. (Guibourt.) Galls (in powder), 50 parts; hot water, 800 parts; digest 24 hours, strain, and add of green sulphate of iron and gum Arabic, of each 25 parts; when dissolved, add the following solution and mix well:—Sal ammoniac, 8 parts; gum, 2 parts; oil of lavender, 1 part; boiling water, 16 parts. Said to be indelible.
15. (Japan.) This is a black and glossy kind of ink, which may be prepared from either of the above receipts by calcining the copperas until white or yellow, or by sprinkling it (in powder) with a little nitric acid before adding it to the decoction (preferably the former), by which the ink is rendered of a full black as soon as made. The glossiness is given by using more gum. It flows less easily from the pen than other inks, and is less durable than ink that writes paler and afterwards turns black. It is unfitted for steel pens.
16. (Lewis.) Bruised galls, 3 lb.; gum and sulphate of iron, of each 1 lb.; vinegar, 1 gall.; water, 9 quarts; macerate with frequent agitation for 14 days. To produce 3 galls. Fine quality, but apt to act on steel pens.
17. (Prerogative Court.) Galls, 1 lb.; gum Arabic, 6 oz.; alum, 2 oz.; green vitriol, 7 oz.; kino, 3 oz.; logwood raspings, 4 oz.;894 soft water, 1 gall.; macerate at last. Said to write well on parchment.
18. (Ribaucourt.) Galls, 1 lb.; logwood chips and sulphate of iron, of each 1⁄2 lb.; gum 6 oz.; sulphate of copper and sugar candy, of each, 1 oz.; boil the first two in soft water, 21⁄2 galls., to one half, then add the other ingredients. Full coloured, but somewhat corrosive, as No. 10.
19. (Dr Ure.) Galls, 12 lbs.; green copperas and gum Senegal, of each 5 lbs.; as No. 2 (nearly). To produce 12 galls.
20. (Dr Wollaston.) Galls, 1 oz.; sulphate of iron, 3 dr.; gum, 1⁄4 oz.; cold water 1⁄2 pint; put into a bottle and shaken together every day for a fortnight or longer. A good durable ink, which will bear diluting.
21. (Pharmaceutische Zeitung.) By adding ferrocyanide of potassium to ordinary ink, an indelible writing ink may be obtained. The removal of such an ink by an acid would result in the production of Prussian blue.
General Commentary. According to the most accurate experiments on the preparation of black ink, it appears that the quantity of sulphate of iron should not exceed 1⁄3rd part of that of the galls, by which an excess of astringent vegetable matter, which is necessary for the durability of the colour, is preserved in the liquid. Gum, by shielding the writing from the action of the air, tends to preserve the colour; but if much is employed, the ink flows languidly from quill pens, and scarcely at all from steel pens. The latter require a very limpid ink. The addition of sugar (especially of moist sugar) increases the flowing property of the liquid, but makes it dry more slowly, and frequently to pass into an acetous state, in which condition it acts injuriously on the pen. Vinegar, for a like reason, is not calculated for the menstruum, as it rapidly softens quill or horn, and corrodes iron and steel.
To ensure the permanency of the colour of the tanno-gallic inks, the best Aleppo or blue nut-galls must alone be used. No second or inferior quality should be employed. A contrary practice, often adopted for the sake of economy, is nearly always followed by unpleasant results and often by considerable loss.
The only improvement of importance which has been made in the manufacture of writing ink from the common materials, during the last few years, is the practice of first roasting the gall-nuts, which is now adopted by a few of the houses most celebrated for their COPYING INK. In this way a portion of pyrogallic acid is formed, which is very soluble in water, and strikes an intense bluish-black colour with the protosulphate or green sulphate of iron. From galls so treated an ink may be made to write black at once. Care must, however, be taken to avoid any loss of materials by volatilisation.
To prevent any tendency to mouldiness in ink, a few bruised cloves, or a little oil of cloves, or, still better, a few drops of creasote (carbolic acid) may be added. The last two should be previously dissolved in a small quantity of strong vinegar, or rectified spirit. With the same intention some of the large makers allow the ink to become covered with a skin of ‘mould’ in the cask, to render it less liable to undergo the same change when subsequently bottled. Formerly the practice was to add a little spirit for the same purpose.
Sumach, logwood, and oak-bark are frequently substituted for galls in the preparation of common ink. When such is the case, only about 1⁄6 or 1⁄7th of their weight of copperas should be employed. Inks so made possess little durability.
The very general use of steel pens of late years has caused a corresponding demand for easy-flowing inks, many of which are now vended under the titles of WRITING-FLUIDS, STEEL-PEN INK, ANTICORROSIVE INK, &c. The greater number of these are prepared from galls in the preceding manner; but a less quantity of gum is employed, and greater attention is paid than heretofore to avoid every source of ‘greasiness’ among which smoke and dirty utensils are, perhaps, the principal. The blue ‘writing fluids,’ which either maintain their colour or turn black by exposure to the air, are, in general, prepared from ferrocyanide of potassium, or from indigo, and are fully noticed in another place. Copying ink, another variety of ink of recent introduction, is characterised by its suitableness to metallic pens, and by furnishing a transcript by means of the ‘copying press’ or ‘copying machine.’ (See below.)
The inks prepared by the first four of the above formulæ are very durable and limpid, and will bear dilution with nearly an equal bulk of water, and still be superior in quality to the ordinary inks of the shops. See Galls, Iron, Writing fluid, and below.
Ink, Blue and Blue black. See Writing fluid.
Ink, Brown. 1. A strong decoction of catechu; the shade may be varied by the cautious addition of a little weak solution of bichromate of potash.
2. A strong decoction of logwood, with a very little bichromate of potash.
Ink, Carbon. Dissolve real Indian ink in common black ink, or add a small quantity of lampblack previously heated to redness, and ground perfectly smooth, with a small portion of the ink.
Ink, Carmine. Heat a scruple of carmine with 3 oz. of water of ammonia for some minutes, a little below boiling, and add 15 to 20 gr. of gum. (The inkstand must be kept well closed.)
Ink, Chrome. See Green ink and Writing fluid.
Ink, Coloured. Inks of various colours may be made from a strong decoction of the ingredients used in dyeing, mixed with a little895 alum or other substance used as a mordant, and gum Arabic. Any of the ordinary water-colour cakes employed in drawing, diffused through water, may also be used as coloured ink. See Brown, Green, and Red inks, &c.
Ink, Copying. This is usually prepared by adding a little sugar or other saccharine matter to ordinary black ink, which for this purpose should be very rich in colour, and preferably made from galls prepared by heat, as noticed above. Writing executed with this ink may be copied within the space of 5 or 6 hours, by passing it through a press (COPYING PRESS) in contact with thin unsized paper (BANK-POST), slightly damped, enclosed between two sheets of thick oiled or waxed paper, when a reversed transcript will be obtained, which will read in proper order when the back of the copy is turned upwards. In the absence of a press a copy may be taken, when the ink is good and the writing very recent, by rolling the sheets, dully arranged on a ruler, over the surface of a flat smooth table, employing as much force as possible, and avoiding any slipping or crumbling of the paper. Another method is to pass a warm flat-iron over the paper laid upon the writing. The following proportions are employed:
1. Sugar candy or lump sugar, 1 oz.; or treacle or moist sugar, 11⁄4 oz.; rich black ink, 11⁄2 pint; dissolve.
2. Malt wort, 1 pint; evaporate it to the consistence of a syrup, and then dissolve it in good black ink, 11⁄4 pint.
3. Solazza juice, 2 oz.; mild ale, 1⁄2 pint; dissolve, strain, and triturate with lampblack (previously heated to dull redness it a covered vessel), 1⁄4 oz.; when the mixture is complete, add of strong black ink, 11⁄2 pint, mix well, and in 2 or 3 hours decant the clear.
Obs. After making the above mixtures, they must be tried with a common steel pen, and if they do not flow freely, some more unprepared ink should be added until they are found to do so.
Ink, Gold. From gold in the state of a impalpable powder, ground up with a little gum water. The brilliancy of the writing performed with this ink is considerable, and may be increased by burnishing.
Ink, Green. 1. From sap green dissolved in very weak alum water.
2. A strong solution of binacetate of copper in water, or of verdigris in vinegar.
3. (Klaproth.) Verdigris, 2 oz.; cream of tartar, 1 oz.; water, 1⁄2 pint; boil to one half, and filter.
4. (Winckley.) Bichromate of potassa, 3 parts; hot water, 8 parts; dissolve, add of rectified spirits, 4 parts, mix, and further add of sulphuric acid, q. s. to liberate the chromic acid, avoiding excess; next evaporate to one half, dilute with water, filter, and add to the filtrate rectified spirit, 4 parts together with 3 or 4 drops of sulphuric acid (if required), to precipitate any remaining potash salt; lastly, decant and preserve the liquid until it assumes a rich green colour.
5. A solution of recently precipitated hydrated oxide of chromium in liquor of ammonia, diluted with distilled water, q. s. A magnificent dark-green liquid, perfectly anti-corrosive.
Ink, Horticultural. Prep. Chloride of platinum, 1⁄4 oz.; soft water, 1 pint; dissolve, and preserve it in glass. Used with a clean quill to write on zinc labels. It almost immediately turns black, and cannot be removed by washing. The addition of gum and lampblack, as recommended in certain books, is unnecessary, and even prejudicial to the quality of the ink.
2. Verdigris and sal ammoniac, of each 1⁄2 oz.; levigated lampblack, 1⁄2 oz.; common vinegar, 1⁄4 pint; mix thoroughly. Used as the last, for either zinc, iron, or steel.
3. Blue vitriol, 1 oz.; sal ammoniac, 1⁄2 oz. (both in powder); vinegar, 1⁄4 pint; dissolve. A little lampblack, or vermilion, may be added, but it is not necessary. As No. 1; for iron, tin, or steel plate. Some of the preparations described below under ‘Incorrodible ink’ are also used by gardeners and nurserymen.
Ink, Incorro′′dible. This name has been given to several preparations of a resinous character, capable of resisting the action of damp and acids.
Prep. 1. Boiled linseed oil, ground with lampblack and Prussian blue, of each q. s. to impart a deep black colour. It may be thinned with oil of turpentine.
2. Good copal or amber varnish, coloured with either plumbago or vermilion.
3. Trinidad asphaltum (genuine), 1 part; oil of turpentine, 4 parts; colour (as last) q. s.
4. (Close.) Cobalt (in powder), 25 gr.; oil of lavender, 200 gr.; dissolve by a gentle heat, and add of lampblack, 3 gr.; indigo 1 gr. (both in impalpable powder); or vermilion, q. s.
5. (Hausmann.) As No. 3 (nearly). Resists the action of iodine, chlorine, alkalies, and acids.
6. (Sheldrake.) Asphaltum dissolved in amber varnish and oil of turpentine, and coloured with lampblack.
Coarsely powdered anacardium nuts (the fruit of the Anacardium orientale) are macerated in a well-closed bottle with petroleum ether, for some time. Upon allowing the latter to evaporate spontaneously, a syrupy residue is left, and this, when applied to linen or cotton cloth, imparts to them a brownish-yellow colour, which instantly changes to a deep black on the addition of ammonia or lime water. (Böttger.)
Obs. The above are also frequently called ‘indelible’ or ‘indestructible inks.’ They are employed for writing labels on bottles containing strong acids and alkaline solutions.896 The last five are very permanent, and are capable of resisting the action of iodine, chlorine, alkaline lyes, and acids, together with all the operations of dyeing and bleaching, and at once offer a cheap and an excellent material for marking linen, &c., as they cannot be dissolved off by any menstrua that will not destroy the fabric. They must be employed with stamps, types, or stencil plates, by which greater neatness will be secured than can be obtained with either a brush or pen. See Horticultural Ink, Indelible Ink, &c.
Ink, Indel′ible. Syn. Indestructible ink. Prep. 1. Lampblack (previously heated to dull redness in a covered vessel), 1⁄4 oz.; triturate with good black ink (gradually added), 1 pint. Resists chlorine, weak acids, and weak alkaline lyes, in the cold.
2. (Bezanger.) Lampblack ground in a lye of caustic soda, combined with a mixture of gelatin and caustic soda. Said to be indelible, and to resemble genuine China ink.
3. (Braconnot.) Dantzic potash, 4 parts; tanned leather parings, 2 parts; sulphur, 1 part; water, 20 parts; boil them in an iron vessel to dryness, then raise the heat (constantly stirring with an iron rod) until the whole forms a soft mass, observing that it does not ignite; next dissolve the mass in water, q. s., and filter the solution through a cloth. Flows freely from a pen, and resists the action of many chemical substances.
4. (Carbon ink.) Genuine Indian ink, rubbed down with good black ink until it will flow easily from a pen. Resists chlorine, oxalic acid, and ablution with a hair pencil or sponge.
5. (Coathupe.) Borax, 1 oz.; shell-lac, 2 oz., water, 18 fl. oz.; boil in a covered vessel until dissolved, strain, add of thick mucilage, 1 oz., and triturate it with levigated indigo and lampblack, of each q. s., to give a good colour. After 2 hours’ repose, decant it from the dregs, and bottle for use. Resists moisture, chlorine, and acids.
6. (French.)—a. From Indian ink, diffused through water acidulated with hydrochloric acid. For quills.—b. From Indian ink diffused through water slightly alkalised with liquor of potassa. For metallic pens.
7. (Herberger.) Wheat gluten (free from starch), q. s., is dissolved in weak acetic acid of good pure vinegar, 4 fl. oz.; lampblack (best), 10 or 12 gr.; indigo, 2 or 3 gr.; and oil of cloves, 1 or 2 drops, are then added, and the whole is thoroughly incorporated together. The product is inexpensive, has a beautiful black colour, and resists the action of water, chlorine, and weak acids.
Obs. The products of the above formulæ, though called ‘indelible ink’ and ‘indestructible ink,’ are in reality only indelible as compared with common writing ink, as they may all be removed with more or less facility by chemical reagents, assisted by mechanical means. They are intended chiefly for paper, pasteboard, and parchment. No 5 is also used for glass and metal. See Marking ink.
Ink, In′dian. Syn. China ink; Atramentum indicum, L. Prep. 1. Lampblack (finest) is ground to a paste with very weak liquor of potassa, and this paste is then diffused through water slightly alkalised with potassa, after which it is collected, washed with clean water, and dried; the dry powder is next levigated to a smooth, stiff paste, with a strong filtered decoction of carrageen or Irish moss, or of quince seed, a few drops of essence of musk, and about half as much essence of ambergris being added, by way of perfume, towards the end of the process; the mass is, lastly, moulded into cakes, which are ornamented with Chinese characters and devices, as soon as they are dry and hard.
2. A weak solution of fine gelatin is boiled at a high temperature in a Papin’s digester for 2 hours, and then in an open vessel for 1 hour more; the liquid is next filtered and evaporated to a proper consistence, either in a steam or salt-water bath; it is, lastly, made into a paste, as before, with pure lampblack which has been previously heated to dull redness in a well-closed crucible. Neither of the above gelatinise in cold weather, like the ordinary imitations.
3. (Gray.) Pure lampblack made up with asses′-skin glue, and scented with musk.
4. (Merimée.) Dissolve superfine glue in water, add a strong solution of nut-galls, and wash the precipitate in hot water; then dissolve it in a fresh solution of glue, filter, evaporate to a proper thickness, and form it into a paste as before, with purified lampblack.
5. (Proust.) As No. 1 (nearly).
6. Seed-lac, 1⁄2 oz.; borax, 11⁄2 dr.; water, 1⁄2 pint; boil to 8 oz., filter, and make a paste with pure lampblack, as before. When dry, it resists the action of water.
Obs. The Chinese do not use glue in the preparation of their ink, but an infusion or decoction of certain seeds abounding in a glutinous transparent mucilage, which at once imparts brilliancy and durability to the colour. Starch converted into gum by means of sulphuric acid, or ‘British gum,’ has been recommended as a substitute. (M. Merimée.) Indian ink is chiefly employed by artists, but it has been occasionally given as a medicine, dissolved in water or wine, in hæmorrhages and stomach complaints.—Dose, 1 to 2 dr.
[For continuation of the article on Inks, see Vol. II.]
END OF VOL. I.
Transcriber’s Note: Blank pages have been deleted. On pages that remain, some unnecessary page numbers may have been deleted when they fall in the middle of lists. Some illustrations may have been moved. The publisher’s inadvertent omissions of important punctuation have been corrected. Some wide tables have been rearranged or re-formatted to narrower equivalents with some words replaced with commonly known abbreviations and possibly a key. Some ditto marks have been replaced with the words represented. The author often skipped the period in ‘Dr,’, ‘Mr,’, Messrs. and ‘Mrs.’ so all such instances are formatted here without the period. The author switched from using ‘B. P.’ to ‘B.P.’ for British Pharmacopœia part of the way through the text so all such instances have been changed to ‘B.P.’. Duplicative front matter has been removed.
Other changes are listed below. The listed source publication page number also applies in this reproduction except possibly for footnotes since they have been moved. Words in italics are indicated like _this_. Text emphasized with bold characters or other treatment is shown like =this=.
Page Change
ix which I should display my acquisitions to mankind.[”]
x at last to wake a “Cyclopædist[”].
3 Lon. or Long., Longtitude[Longitude].
4 (often expressed by the sign o/o{‘o/o’ graphic not replicable})
5 Cyath., cyathus, vel[vel deleted]
8 that has been so indiscrimately[indiscriminately]
8 the employment of castille[Castile] soap,
12 {Illustration caption:} [Added: Meal mite.]
17 Adopter[Adapter] connecting retort and globes.
21 earthenware are also frequenly[frequently] employed;
21 per-oxide[peroxide] of manganese, or red
29 as well as ‘test-solutions’ containng[containing]
33 weezers[tweezers]. A long piece of thick
33 Ger. Monkshood; wolfsban[wolfsbane]. In
34 Some specimes[specimens] are white and spongy;
34 A white, colourless, semicrystalline[semi-crystalline] mass
39 MAGNESIA, MAGNESIA SULPHATE[entry rearranged],
44 with the result of their adminsistration[administration]
54 article with a paste of freshly slacked[slaked] lime
63 1·43% ” ” ·29%[2·9%]
73 information in connection with Alcholometry[Alcoholometry]
95 of the exaet[exact] temperature at which
96 into lumps like resin, it is SALICIN[SALICINE];
98 other, different, and παφος[παθος]
109 allum[alum] (dissolved in gum-water),
119 It has a a[delete] smell resembling
126 (three-daggers)[⁂] The sp. gr. of any sample of liquid ammonia,
132 conical mass, whieh[which] is technically called
132 untll[until] the liquor has remained
132 as eady[already] mentioned,
136 in the neighbeurhood[neighbourhood] of London.
136 made with lemon-juice and drank[drunk] effervescing,
137 the ctlear[clear] portion of the liquid,
137 after repose decaned[decanted],
143 form the body under examination;[1][del]
147 and litanium[titanium]. Hydrogen also
154 [he created] a new world for future naturalists
161 Pottsviile[Pottsville], Sharp Mount 1·412 2382
165 smelting is the common sulphide kuown[known] as
170 OXIDUM, L.). Prpe.[Prep.] (B. P.)
173 ANTISBORBU′TICA[ANTISBORBU′TICA], L.), used in scurvy.
175 COMPOSIT[COMPOSITÆ]. A plant growing in the North
175 and the head and leck[neck] laid bare.
177 APFEL, Ger.; APPLE[APPEL], Dut.;
178 A. NIVA′LIS or D.[A.] EX NI′VE,
179 fit for or under tillage or aration[aëration];
181 it was an ocult[occult] power of nature,
185 A spirituous liqur[liquor] imported
188 Trii′odide of.= AsT[I]_{3}.
198 succession, and pre ise[precise] character
198 Hydrated sulphide of iron (as 10[the] last).
201 An[By an] Act of Parliament[1] it is provided
208 Sesquioixde[Sesquioxide]
219 [Symbols in table replaced with numeric equivalents.]
219 In the foregoing table {symbol} represents one volume.[deleted all]
219 10 pints; slacked[slaked] lime, 1 oz.;
220 once agitating 6 or 8 vlumes[volumes] of water
245 and is sufficciently[sufficiently] pure for making
246 abundantly columnar crsytals[crystals]
255 melt the materia1[l] in a reverberatery[reverberatory]
257 vary[very] liable to crack when filled with hot water
261 L. [1] Green sulphate of iron, 1 to 2
272 form of mattrass[mattress] than one made of horsehair
272 the horsehair mattrass[mattress] calls even more imperatively
272 Both pillows and mattrass[mattress] should be taken to pieces
273 These are less than those of eather[feather] beds
281 and Mr Denison[Dennison] differ from him in opinion.
284 Goood[Good] samples of benzoin yield
286 and bronchial aflections[affections], when
288 can only be accounted for by aamples[samples]
289 the fluid expresssed[expressed] made up to 26
290 the precipitate thereby produeed[produced].
300 petroleum may bementioned[be mentioned] as examples.
300 much of the asphalt and bituminou[bituminous] pavement
304 1/3rd of this docoction[decoction] is then
307 through the str ets[streets] of this great metropolis;
308 (see 1[I], 4, above), but dissolving
308 6. (Without Vitriol.) As 1[I], 5
308 The final addition of the 3 lbs.[footnote given a number]
310 by which they bcome[become] considerably larger;
312 About A.D. 60.[Footnote not referenced in text; deleted.]
312 of Barthollet[Berthollet] on chlorine, in 1784,
312 {Transcriber’s note: Footnote omitted by publisher.}
313 1[I]. BLEACHING of =Cotton=:
313 The pieces are bucked or boiled in mllk[milk] of lime[5]
319 may be mentioned the folowing[following]:——
319 n[in] breadth, is observed round the glass,
319 [i]t is certain that vesication is effected.
323 and MAGNESIA are indicated, the latter fine[five] when added
331 staranise[star-anise] oil, 40 drops
337 ·941 to [·]942 (18 to 20 u. p.). The common
338 processes detailed under ALCOHOMETRY[ALCOHOLOMETRY].
338 and examining the later or[for] heavier products.
346 a wineglassful[wine-glassful] of this solution added
355 The whole of the water nsed[used]
356 a littie[little] more ‘lobb’ is generally added,
360 2. To the last add of sugar or treacle, 1/2 b[lb].
361 the mixture haying[having] been placed in a retort
362 These vessels are furrounded[surrounded] with belts of iron
369 Oil-paintin[g] a wall
373 nothwithstanding[notwithstanding] the assertions
377 not exceeding 180[°] Fahr.,
377 other butter ever possesees[possesses].
380 =Cadmium, Car′bonate of.= CdCo_{3}[CdCO_{3}].
384 flour, 1-1/2 lb.; curants[currants], 2 lbs.;
399 India rubber or cauotchouc[caoutchouc],
420 When applied let it [be] heated.
427 CERATE PLASTER (Emplastru m[Emplastrum] Cerati Saponis)
429 let it rest for a night in a closed mattrass[matrass];
430 =Chalk, Browu[Brown].= A familiar name for umber.
431 A more economical methed[method]
456 prepares a standard solution as fellows[follows]:
457 Langour[Languor], listlessness, fatigue after the least exercise,
460 a pint of cold water, and drank[drunk] ad libitum.
462 any of the iron preparation[preparations] will
468 CINCHONA[CINCHONÆ] FLAVÆ CORTEX.
476 into a very considerable and importa[important]
477 it gives a head[bead] of a magnificent
478 between the best and the lowest quantity[quality]
484 Compound of caffein with potash 35[3·5] to 5·000
484 treated with a per-salt[persalt] of iron;
487 to about 1/3rd or 1/4th of it[its] bulk
487 iu[in] a cool place. This preparation
487 It differs frem[from] veratria in being soluble
491 =Iodide and Bromide Solution.= Iodid[Iodide] of cadmium
496 boil the saffron [and] turmeric in
499 Dr. Gardner.[Footnote not referenced in text; deleted.]
501 In the caes[case] of a dangerous attack
504 Its activity is considerd[considered] equal
506 thus described in the ‘Encyclopœdia[Encyclopædia] Brittanica.’
507 Copper has a brilliant yellowish-ed[yellowish-red] colour
508 the soluble part to spontaneous evaportion[evaporation].
510 thin film of copper by merely immersing is[it]
512 corns may be removed by ayplying[applying] ivy leaf,
518 requires practice as to the heat of ihe[the] water;
520 at the Hôpital des Enfans[Enfants],
521 are excedingly[exceedingly] useful for many operations,
525 (See table at bottom of previous column.)[(See previous table.)]
531 Davids-Thee, Echter Karolinenthaler——Genuine Karoin’s[Karolin’s]
534 properly qualified medical practioner[practitioner],
536 thould [should] be well bruised
545 sassafras, guaiacmu[guaiacum] turnings,
547 dysentery, dirrrhœa[diarrhœa], and excoriations
549 kind than other persous[persons].
549 in moderarely[moderately] large doses,
549 Lassaigne and Feneulle ln[in] Delphinium staphysagria
550 after rincing[rinsing] the mouth out with water.
555 tne[the] only property which the bodies
560 camphor, fragrant pastiles[pastilles], cascarilla,
560 until the temperature has fallen [to] 39·9° Fahr.,
562 FFRRALUM[FERRALUM] is a mixture of ferrous
563 distinguiished[distinguished] by a mouldy or musty odour
564 if we desire to makx[make] our sanitary surroundings
567 Disinfections of the apartments[apartment]
569 is conected[connected] with the chimney
569 for the purification of mattrasses[mattresses], linen,
571 For heating mattrasses[mattresses] another apparatus
572 surrounding the too[top] of the capital of the still
573 distiller is allowed to produce worst[worts] from any substance
574 Dr. Druitt.[footnote renumbered.]
583 complete disconnection betweeen[between] the pipe
590 =Rasp′berry.= See DROPS, FRUIT (aboue[above].)
594 or convulsive twitching, a vein may be epened[opened].
594 use shall be commercialls[commercially] pure
596 a final wince[rinse] in cow-dung before dyeing is advantageous.
599 dependent for his purples on orchil or cudbeer[cudbear],
603 originally a mixture of siver[silver] nitrate,
605 Liquid sulphurous acid ×[+] 17·6 ”
606 by the ravages of the Ecchinococcus[Echinococcus] is the
607 lines connecting f, g, k, represents[represent]
607 Does[Dose], 1/2 to 2 gr.
607 ASCLEPEDIN. From Asclepiastuberosa[Asclepias tuberosa].
607 Stimulant, astringent, and antispadmodic[antispasmodic].
609 whilst bad or wortless[worthless] ones will swim
610 and stirred ne[one] way all the time,
611 with potasssa[potassa] and solution of silver
611 dried by exposure to the air and a gentle head[heat]
612 and distinguised[distinguished] as negative and positive,
613 instrument is to [be] placed upon the resinous plate
613 Syn. ELECTRO-MET′ALURGY[ELECTRO-MET′ALLURGY],
616 The double chloride of platinum and potassum[potassium],
617 =Elecutary[Electuary], Bath.= Syn.
618 honey and syrup of wormwo[wormwood],
618 In intermittents occurring in crofulous[scrofulous] subjects.
620 A teaspooonful[teaspoonful], as a mild stomachic
620 Dose, 1 to 2 teaspoonsfuls[teaspoonfuls],
622 2 teaspoonfuls; in an enorrhœa[amenorrhœa], constipation,
624 bitter almonds (bruised), 8 in on.[no.];
628 gradually added, the ematine[emetine] is dissolved,
628 however, that ematine[emetine] is alone
629 other remedies can be optained[obtained] and applied.
634 and that to exert a liked[like] absorbent action
634 coats of the intestines, and to allay irritatation[irritation];
638 Fluor[Flour, {in table}]
638 but its energy must be develoyed[developed] in the place
642 the diptheria[diphtheria] affecting oxen,
642 tend to sssume[assume] a typhoid form,
642 parasite that multiply[multiplies] so rapidly as speedly[speedily]
643 Titanum[Titanium {in table}]
643 Lanthanium[Lanthanum]
651 =Essence, Anthysteri′ic[Antihyster′ic].=
657 From pine-apple oil (buytric[butyric] ether,
657 rectfied[rectified] spirit, 1/2 pint; digest
657 the solution is apt to depoit[deposit] part of it
657 3.[2.] Attar of roses, 1 oz.; rectified spirit,
654 in the last two formulæ to th[the] preparation of
658 at 160[°] Fahr. If reduced
658 and neroli, of each 6 drops; dissoved[dissolved]
659 or verbenæ[verbena] (Andropogon citratum),
663 a heavy, oily pro-product[product]
666 Having been found ls[less] efficient than
667 does not effect[affect] a solution of nitrate
669 it continues to be absorded[absorbed].
669 the nitrite of ethel[ethyl] is——in very
672 Eugleda[Euglena] viridis and the
672 and the Eugleda[Euglena] pyrum.
673 the fluid matter that forms the menstrnum[menstruum];
673 To purify liqnids[liquids] by the dissipation of
673 the vapour is prevented [from] resting upon the
679 W is the weight of the person, W[W_{l}] the weight carried
682 SOLIDFIED[SOLIDIFIED] WORTS, made by a licensed brewer,
682 In medi cne[medicine] and pharmacy,
686 unless when taken in repeated does[doses], long continued.
687 q. s. to make che[the] whole equal in weight [to] that
694 to be employed as the mentruum[menstruum],
699 fluid extract will not [be] required
698 exclusively appropriated to Cannabic[Cannabis] sativa
700 pill consistence.——Dose, 1/2 [to] 1 dr.
701 by maceration or[for] 24 hours,
701 (EXTRACTUM LUBULINAE[LUPULINÆ] COCTIONE PARATUM.)
702 c. (Ph. U. S. EXTRACTUM MEZEREIFLUIDUM[MEZEREI FLUIDUM].)
704 L. Prep. [1.] (P. Cod.) From ox-gall,
708 Guibort[Guibourt].) Senna (in powder), [1] part;
708 by distillation and vaporation[evaporation].——Dose,
708 In arger[larger] doses it is nauseant and emetic.
711 flowers with the purest recitfied[rectified] spirit
712 When a powerfully destructive substence[substance],
712 etherial[ethereal] oils of lavender,
712 intermitent[intermittent] form of facial NEURALGIA
717 simple in construction and easily managable[manageable]; it
718 instead the mouth of the botttle[bottle] is closed
722 in the so-called red prusstate[prussiate] of potash
722 PUTREFACTION, BBEWING[BREWING], &c.
726 from one vesssel[vessel] to another,
727 first a stratum of course[coarse] pebbles,
728 is said to be prepared by heating hœmatite[hæmatite]
729 washed in hot water.[Footnote tag renumbered.]
729 Commision[Commission]{in table}
733 because of of[del 2nd of] their little specific gravity.
733 of this formulæ[formula] is made to existing
738 invention, bnt[but] it has not proved
738 contents of the air-vesel[vessel] until
741 Artficial[Artificial] Propagation.
742 when dressed immediately after being caught, possessess[possesses]
743 spirit is frequently omitted altogther[altogether].
746 Microscropic[Microscopic] analysis detects the presence
746 =FLEIGENPULVER[FLIEGENPULVER]——Fly Powder=
748 See also BREAD,[No footnote reference in text; added one.]
749 The best bread contains about 1/4-1/6ths[1/6th] of
749 than they can dipose[dispose] of whilst ‘new,’
755 (Eagle marine.) Vedigris[Verdigris] tempered in
757 from the organic kingdom that our ailments[aliments]
758 Beef, mutton, veal, lamb, and vension[venison]
759 is diseased, unound[unsound] or unwholesome,
761 5[4]. (Kittoe’s.) Sal ammoniac, 1 dr.; spring
762 stewing or semi frying[semi-frying], highly flavoured
762 namely, R. amydalinus[amygdalinus].
764 Brown syrup, 90 grammes; verdegris[verdigris], 6 grammes;
764 the perils of transit and storeage[storage].
767 property may be destoyed[destroyed] by boiling it
770 Syn. FUMIGATIO MURCURIALIS[MERCURIALIS], L.
771 jewellers, analytical chemists, expermentalists[experimentalists],
774 two or three months the sides of the vesse[vessel]
777 to his classification, Mendeljeff[Medelejeff] asserted, that
777 The hypothetical Eka aluminium of Mendeljeff[Mendelejeff]
776 when with treated[when treated with] sulphuretted hydrogen,
776 The per-salts[persalts] of iron present are then
779 for a minute, that that[del 2nd that] they may be skinned
782 =GAR′NET.= In mineraloyy[mineralogy],
782 carburetted hydrogen, hyrochloric[hydrochloric] acid,
786 in a ley[lye] of caustic soda,
787 GELSEMINUM[GELSEMIUM] NITRIDUM,
792 {Footnote 332:} See ELECTROTYPS[ELECTROTYPE].]
794 gross of buttons of the abve[above] size.
795 DEADING AUQAFORTIS[AQUAFORTIS]. From mercury,
801 lime, 3·8%; alumina, 2 8%[2·8%]. French mirror-glass.
814 solutions of gold, insoluble in excesss[excess].
818 scented, as a pommade[pomade] to make the hair grow,
819 rectified spirit of wine, 2 galls[gals.];
819 GUINEA GRAINS, MALAGUETTA[MELEGUETTA] PEPPER.
819 The seeds of the Ammomum melaguetta[meleguetta].
826 ammonia chloride o[of] platinum thus obtained,
826 the per-centage of ready-formed amm onia[ammonia],
829 Prop., &c. White; inspid[insipid]; transparent;
837 For use, the air[hair] is moistened by means
839 When the hand[hands] are very dirty,
846 macerate for 2 2[del 2nd 2] hours;
846 Dried petals of the the[del 2nd the] red rose
847 A native chloride or[of] silver,
851 and the fuunel[funnel] having been withdrawn
858 =HYGIENE′.= Syn. HXGIENE[HYGIENE], Fr.
859 direct attempts to alter his opinons[opinions],
859 fancies himsef[himself] a giant;
860 A fusible, inflammable sub stance[substance],
864 white ozide[oxide] of antimony.
864 but by the ebulition[ebullition] expelling free carbonic acid
865 Several attemps[attempts] have been made of late years
870 Loire-Inferiéure[Inférieure],
872 no less than 8250 die from 0·1[0-1] year,
872 having recourse to other than the maternal mik[milk]
875 regarding them as extemporaneous preparatious[preparations].
876 A pure bitter tonic, without a-astringency[astringency]
879 Concentrated: INFUSUM CARYPHYLLI[CARYOPHYLLI]
887 active matter that it it[del 2nd it] is possible
889 Canada snake-root (Assarum[Asarum] Canadense)
890 Carbonate of lead (finely evigated[levigated]),
894 the add then[then add the] other ingredients.
895 and preferably made [from] galls prepared by heat,
895 Used us[as] the last, for either zinc,
End of the Project Gutenberg EBook of Cooley's Cyclopædia of Practica
Receipts and Collateral Informa, by Arnold Cooley and Richard Tuson
*** END OF THIS PROJECT GUTENBERG EBOOK COOLEY'S CYCLOPÆDIA ***
***** This file should be named 39733-h.htm or 39733-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/3/9/7/3/39733/
Produced by Chris Curnow, Leonora Dias de Lima, Henry
Gardiner and the Online Distributed Proofreading Team at
http://www.pgdp.net
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation information page at www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at 809
North 1500 West, Salt Lake City, UT 84116, (801) 596-1887. Email
contact links and up to date contact information can be found at the
Foundation's web site and official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For forty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.