 FIG. 1.—A typical Bessemer converter.
FIG. 1.—A typical Bessemer converter.
Project Gutenberg's The Working of Steel, by Fred H. Colvin and A. Juthe
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
Title: The Working of Steel
Annealing, Heat Treating and Hardening of Carbon and Alloy Steel
Author: Fred H. Colvin
A. Juthe
Release Date: January 4, 2007 [EBook #20282]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK THE WORKING OF STEEL ***
Produced by Robert J. Hall
ANNEALING, HEAT TREATING
AND
HARDENING OF CARBON AND ALLOY
STEEL
BY
Member American Society of Mechanical Engineers and Franklin Institute; Editor of the American Machinist, Author of "Machine Shop Arithmetic," "Machine Shop Calculations," "American Machinists' Hand Book."
AND
Chief Engineer, American Metallurgical Corp. Member American Society Mechanical Engineers, American Society Testing Materials, Heat Treatment Association, Etc.
SECOND EDITION
THIRD IMPRESSION
McGRAW-HILL BOOK COMPANY, Inc.
NEW YORK: 370 SEVENTH AVENUE
LONDON: 6 & 8 BOUVERIE ST., E. C. 4
Advantage has been taken of a reprinting to revise, extensively, the portions of the book relating to the modern science of metallography. Considerable of the matter relating to the influence of chemical composition upon the properties of alloy steels has been rewritten. Furthermore, opportunity has been taken to include some brief notes on methods of physical testing—whereby the metallurgist judges of the excellence of his metal in advance of its actual performance in service.
NEW YORK, N. Y.,
August, 1922.
The ever increasing uses of steel in all industries and the necessity of securing the best results with the material used, make a knowledge of the proper working of steel more important than ever before. For it is not alone the quality of the steel itself or the alloys used in its composition, but the proper working or treatment of the steel which determines whether or not the best possible use has been made of it.
With this in mind, the authors have drawn, not only from their own experience but from the best sources available, information as to the most approved methods of working the various kinds of steel now in commercial use. These include low carbon, high carbon and alloy steels of various kinds, and from a variety of industries. The automotive field has done much to develop not only new alloys but efficient methods of working them and has been drawn on liberally so as to show the best practice. The practice in government arsenals on steels used in fire arms is also given.
While not intended as a treatise on steel making or metallurgy in any sense, it has seemed best to include a little information as to the making of different steels and to give considerable general information which it is believed will be helpful to those who desire to become familiar with the most modern methods of working steel.
It is with the hope that this volume, which has endeavored to give due credit to all sources of information, may prove of value to its readers and through them to the industry at large.
July, 1921.
THE AUTHORS.
THE ABC OF IRON AND STEEL
In spite of all that has been written about iron and steel there are many hazy notions in the minds of many mechanics regarding them. It is not always clear as to just what makes the difference between iron and steel. We know that high-carbon steel makes a better cutting tool than low-carbon steel. And yet carbon alone does not make all the difference because we know that cast iron has more carbon than tool steel and yet it does not make a good cutting tool.
Pig iron or cast iron has from 3 to 5 per cent carbon, while good tool steel rarely has more than 1¼ per cent of carbon, yet one is soft and has a coarse grain, while the other has a fine grain and can be hardened by heating and dipping in water. Most of the carbon in cast iron is in a form like graphite, which is almost pure carbon, and is therefore called graphitic carbon. The resemblance can be seen by noting how cast-iron borings blacken the hands just as does graphite, while steel turnings do not have the same effect. The difference is due to the fact that the carbon in steel is not in a graphitic form as well as because it is present in smaller quantities.
In making steel in the old way the cast iron was melted and the carbon and other impurities burned out of it, the melted iron being stirred or "puddled," meanwhile. The resulting puddled iron, also known as wrought iron, is very low in carbon; it is tough, and on being broken appears to be made up of a bundle of long fibers. Then the iron was heated to redness for several days in material containing carbon (charcoal) until it absorbed the desired amount, which made it steel, just as case-hardening iron or steel adds carbon to the outer surface of the metal. The carbon absorbed by the iron does not take on a graphitic form, however, as in the case of cast iron, but enters into a chemical compound with the iron, a hard brittle substance called "cementite" by metallurgists. In fact, the difference between the hard, brittle cementite and the soft, greasy graphite, accounts for many of the differences between steel and gray cast iron. Wrought iron, Page x which has very little carbon of any sort in it, is fairly soft and tough. The properties of wrought iron are the properties of pure iron. As more and more carbon is introduced into the iron, it combines with the iron and distributes itself throughout the metal in extremely small crystals of cementite, and this brittle, hard substance lends more and more hardness and strength to the steel, at the expense of the original toughness of the iron. As more and more carbon is contained in the alloy—for steel is a true alloy—it begins to appear as graphite, and its properties counteract the remaining brittle cementite. Eventually, in gray cast iron, we have properties which would be expected of wrought iron, whose tough metallic texture was shot through with flakes of slippery, weak graphite.
But to return to the methods of making steel tools in use 100 years ago.
The iron bars, after heating in charcoal, were broken and the carbon content judged by the fracture. Those which had been in the hottest part of the furnace would have the deepest "case" and highest carbon. So when the steel was graded, and separated into different piles, a few bars of like kind were broken into short lengths, melted in fire-clay crucibles at an intense white heat, cast carefully into iron molds, and the resulting ingot forged into bars under a crude trip hammer. This melting practice is still in use for crucible steel, and will be described further on page 4.
Page 1 THE WORKING OF STEEL
ANNEALING, HEAT TREATING AND HARDENING
OF
CARBON AND ALLOY STEEL
STEEL MAKING
There are four processes now used for the manufacture of steel. These are: The Bessemer, Open Hearth, Crucible and Electric Furnace Methods.
The bessemer process consists of charging molten pig iron into a huge, brick-lined pot called the bessemer converter, and then in blowing a current of air through holes in the bottom of the vessel into the liquid metal.
The air blast burns the white hot metal, and the temperature increases. The action is exactly similar to what happens in a fire box under forced draft. And in both cases some parts of the material burn easier and more quickly than others. Thus it is that some of the impurities in the pig iron—including the carbon—burn first, and if the blast is shut off when they are gone but little of the iron is destroyed. Unfortunately sulphur, one of the most dangerous impurities, is not expelled in the process.
A bessemer converter is shown in Fig. 1, while Fig. 2 shows the details of its construction. This shows how the air blast is forced in from one side, through the trunnion, and up through the metal. Where the steel is finished the converter is tilted, or swung on its trunnions, the blast turned off, and the steel poured out of the top.
The open hearth furnace consists of a big brick room with a low arched roof. It is charged with pig iron and scrap through doors in the side walls.
 FIG. 1.—A typical Bessemer converter.
FIG. 1.—A typical Bessemer converter.
Through openings at one end of the furnace come hot air and gas, which burn in the furnace, producing sufficient heat to melt the charge and refine it of its impurities. Lime and other nonmetallic substances are put in the furnace. These melt, forming a "slag" which floats on the metal and aids materially in the refining operations.
In the bessemer process air is forced through the metal. In the open-hearth furnace the metal is protected from the flaming gases by a slag covering. Therefore it is reasonable to suppose that the final product will not contain so much gas.
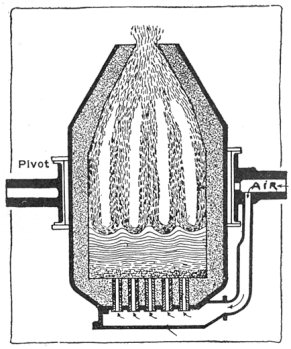 FIG. 2.—Action of Bessemer converter.
FIG. 2.—Action of Bessemer converter.
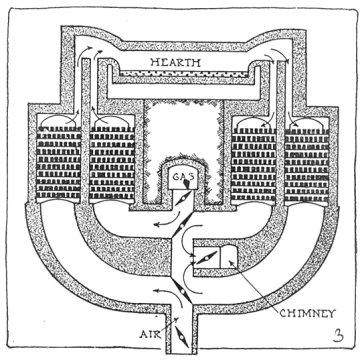 FIG. 3.—Regenerative open hearth furnace.
FIG. 3.—Regenerative open hearth furnace.
A diagram of a modern regenerative furnace is shown in Fig. 3. Page 3 Air and gas enter the hearth through chambers loosely packed with hot fire brick, burn, and exit to the chimney through another pair of chambers, giving to them some of the heat which would otherwise waste. The direction is reversed about every twenty minutes by changing the position of the dampers.
Crucible steel is still made by melting material in a clay or graphite crucible. Each crucible contains about 40 lb. of best puddled iron, 40 lb. of clean "mill scrap"—ends trimmed from tool steel bars—and sufficient rich alloys and charcoal to make the mixture conform to the desired chemical analysis. The crucible is covered, lowered into a melting hole (Fig. 4) and entirely surrounded by burning coke. In about four hours the metal is converted into a quiet white hot liquid. Several crucibles are then pulled out of the hole, and their contents carefully poured into a metal mold, forming an ingot.
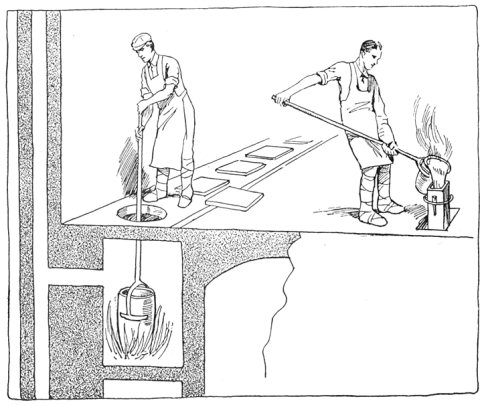 FIG. 4.—Typical crucible furnace.
FIG. 4.—Typical crucible furnace.
If modern high-speed steel is being made, the ingots are taken out of the molds while still red hot and placed in a furnace which keeps them at this temperature for some hours, an operation known as annealing. After slow cooling any surface defects are ground out. Ingots are then reheated to forging temperature, hammered down into "billets" of about one-quarter size, and 10 to 20 per cent of the length cut from the top. After reheating the billets are hammered or rolled into bars of desired size. Finished bars are packed with a little charcoal into large pipes, the ends sealed, Page 5 and annealed for two or three days. After careful inspection and testing the steel is ready for market.
The fourth method of manufacturing steel is by the electric furnace. These furnaces are of various sizes and designs; their size may be sufficient for only 100 lb. of metal—on the other hand electric furnaces for making armor-plate steel will hold 40 tons of steel. Designs vary widely according to the electrical principles used. A popular furnace is the 6-ton Heroult furnace illustrated in Fig. 5.
It is seen to be a squat kettle, made of heavy sheet steel, with a dished bottom and mounted so it can be tilted forward slightly and completely drained. This kettle is lined with special fire brick which will withstand most intense heat and resist the cutting action of hot metal and slag. For a roof, a low dome of fire brick is provided. The shell and lining is pierced in front for a pouring spout, and on either side by doors, through which the raw material is charged.
Two or three carbon "electrodes"—18-in. cylinders of specially prepared coke or graphite—extend through holes in the roof. Electrical connections are made to the upper ends, and a very high current sent through them. This causes tremendous arcs to form between the lower ends of the electrodes and the metal below, and these electric arcs are the only source of heat in this style of furnace.
Electric furnaces can be used to do the same work as is done in crucible furnaces—that is to say, merely melt a charge of carefully selected pure raw materials. On the other hand it can be used to produce very high-grade steel from cheap and impure metal, when it acts more like an open-hearth furnace. It can push the refining even further than the latter furnace does, for two reasons: first the bath is not swept continuously by a flaming mass of gases; second, the temperature can be run up higher, enabling the operator to make up slags which are difficult to melt but very useful to remove small traces of impurities from the metal.
Electric furnaces are widely used, not only in the iron industry, but in brass, copper and aluminum works. It is a useful melter of cold metal for making castings. It can be used to convert iron into steel or vice versa. Its most useful sphere, however, is as a refiner of metal, wherein it takes either cold steel or molten steel from open hearth or bessemer furnaces, and gives it the finishing touches.
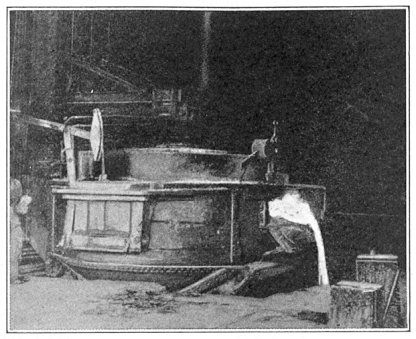 FIG. 5.—"Slagging off" an electric furnace.
FIG. 5.—"Slagging off" an electric furnace.
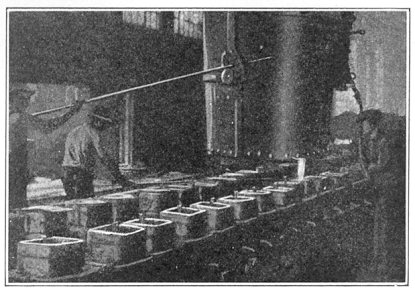 FIG. 6.—Pouring the ingots.
FIG. 6.—Pouring the ingots.
As an illustration of the furnace reactions that take place the following schedule is given, showing the various stages in the making of a heat of electric steel. The steel to be made was a high-carbon chrome steel used for balls for ball bearings:
| 11:50 A.M. | —Material charged: | |||||||||||||
| Boiler plate | 5,980 lb. | |||||||||||||
| Stampings | 5,991 lb. | |||||||||||||
| 11,971 lb. | ||||||||||||||
| Limestone | 700 lb. | |||||||||||||
| 12:29 P.M. | —Completed charging (current switched on). Page 7 | |||||||||||||
| 3:20 P.M. | —Charge melted down. | |||||||||||||
| Preliminary analysis under black slag. | ||||||||||||||
| Analysis: | ||||||||||||||
| ||||||||||||||
| Note the practical elimination of phosphorus. | ||||||||||||||
| 3:40 P.M. | —The oxidizing (black) slag is now poured and skimmed off as clean as possible to prevent rephosphorizing and to permit of adding carburizing materials. For this purpose carbon is added in the form of powdered coke, ground electrodes or other forms of pure carbon. | |||||||||||||
The deoxidizing slag is now formed by additions of lime, coke and fluorspar (and for some analyses ferrosilicon). The slag changes from black to white as the metallic oxides are reduced by these deoxidizing additions and the reduced metals return to the bath. A good finishing slag is creamy white, porous and viscous. After the slag becomes white, some time is necessary for the absorption of the sulphur in the bath by the slag.
The white slag disintegrates to a powder when exposed to the atmosphere and has a pronounced odor of acetylene when wet.
Further additions of recarburizing material are added as needed to meet the analysis. The further reactions are shown by the following:
| 3:40 P.M. | —Recarburizing material added: | |||||||||||
| 130 lb. | ground electrodes. | |||||||||||
| 25 lb. | ferromanganese. | |||||||||||
| Analysis: | ||||||||||||
| ||||||||||||
To form white slag there was added:
| 225 lb. | lime. | |||||||||||
| 75 lb. | powdered coke. | |||||||||||
| 55 lb. | fluorspar. | |||||||||||
| 4:50 P.M. | — | |||||||||||
| Analysis: | ||||||||||||
| ||||||||||||
During the white-slag period the following alloying additions were made:
| 500 lb. | pig iron. |
| 80 lb. | ferrosilicon. |
| 9 lb. | ferromanganese. |
| 146 lb. | 6 per cent carbon ferrochrome. |
Page 8 The furnace was rotated forward to an inclined position and the charge poured into the ladle, from which in turn it was poured into molds.
| 5:40 P.M. | —Heat poured. | |||||||||||
| Analysis: | ||||||||||||
| ||||||||||||
| Ingot weight poured | 94.0 per cent | |||||||||||
| Scull | 2.7 per cent | |||||||||||
| Loss | 3.3 per cent | |||||||||||
| Total current consumption for the heat, 4,700 kW.-hr. or 710 kw.-hr. per ton. | ||||||||||||
Electric steel, in fact, all fine steel, should be cast in big-end-up molds with refractory hot tops to prevent any possibility of pipage in the body of the ingot. In the further processing of the ingot, whether in the rolling mill or forge, special precautions should be taken in the heating, in the reduction of the metal and in the cooling.
No attempt is made to compare the relative merits of open hearth and electric steel; results in service, day in and day out, have, however, thoroughly established the desirability of electric steel. Ten years of experience indicate that electric steel is equal to crucible steel and superior to open hearth.
The rare purity of the heat derived from the electric are, combined with definite control of the slag in a neutral atmosphere, explains in part the superiority of electric steel. Commenting on this recently Dr. H. M. Howe stated that "in the open hearth process you have such atmosphere and slag conditions as you can get, and in the electric you have such atmosphere and slag conditions as you desire."
Another type of electric furnace is shown in Figs. 7 and 8. This is the Ludlum furnace, the illustrations showing a 10-ton size. Figure 7 shows it in normal, or melting position, while in Fig. 8 it is tilted for pouring. In melting, the electrodes first rest on the charge of material in the furnace. After the current is turned on they eat their way through, nearly to the bottom. By this time there is a pool of molten metal beneath the electrode and the charge is melted from the bottom up so that the roof is not exposed to the high temperature radiating from the open arc. The electrodes in this furnace are of graphite, 9 in. in diameter and the current consumed is about 500 kw.-hr. per ton.
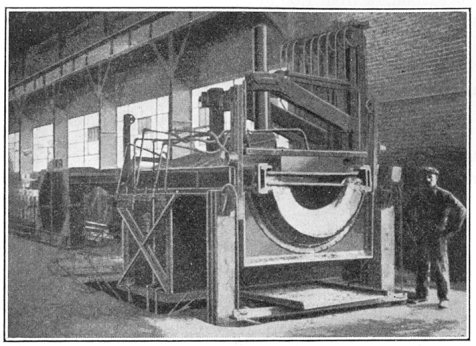 FIG. 7.—Ludlum electric furnace.
FIG. 7.—Ludlum electric furnace.
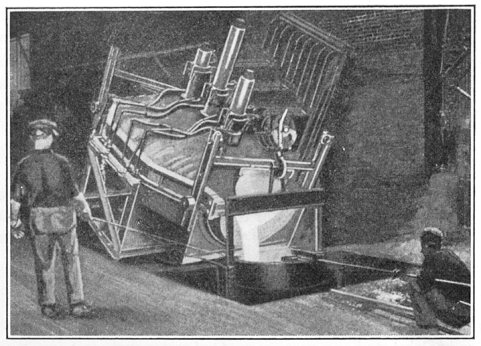 FIG. 8.—The furnace tilted for pouring.
FIG. 8.—The furnace tilted for pouring.
One of the things which sometimes confuse regarding the contents of steel is the fact that the percentage of carbon and the other alloys are usually designated in different ways. Carbon is usually designated by "points" and the other alloys by percentages. The point is one ten-thousandth while 1 per cent is one one-hundredth of the whole. In other words, "one hundred Page 10 point carbon" is steel containing 1 per cent carbon. Twenty point carbon, such as is used for carbonizing purposes is 0.20 per cent. Tool steel varies from one hundred to one hundred and fifty points carbon, or from 1.00 to 1.50 per cent.
Nickel, chromium, etc., are always given in per cent, as a 3.5 per cent nickel, which means exactly what it says—3½ parts in 100. Bearing this difference in mind all confusion will be avoided.
Among makers and sellers, carbon tool-steels are classed by "grade" and "temper." The word grade is qualified by many adjectives of more or less cryptic meaning, but in general they aim to denote the process and care with which the steel is made.
Temper of a steel refers to the carbon content. This should preferably be noted by "points," as just explained; but unfortunately, a 53-point steel (containing 0.53 per cent carbon) may locally be called something like "No. 3 temper."
A widely used method of classifying steels was originated by the Society of Automotive Engineers. Each specification is represented by a number of 4 digits, the first figure indicating the class, the second figure the approximate percentage of predominant alloying element, and the last two the average carbon content in points. Plain carbon steels are class 1, nickel steels are class 2, nickel-chromium steels are class 3, chromium steels are class 5, chromium-vanadium steels are class 6, and silico-manganese steels are class 9. Thus by this system, steel 2340 would be a 3 per cent nickel steel with 0.40 per cent carbon; or steel 1025 would be a 0.25 plain carbon steel.
Steel makers have no uniform classification for the various kinds of steel or steels used for different purposes. The following list shows the names used by some of the well-known makers:
| Air-hardening steel | Chrome-vanadium steel |
| Alloy steel | Circular saw plates |
| Automobile steel | Coal auger steel |
| Awl steel | Coal mining pick or cutter steel |
| Axe and hatchet steel | Coal wedge steel |
| Band knife steel | Cone steel |
| Band saw steel | Crucible cast steel |
| Butcher saw steel | Crucible machinery steel |
| Chisel steel | Cutlery steel |
| Chrome-nickel steel | Drawing die steel (Wortle) |
| Drill rod steel | Patent, bush or hammer steel |
| Facing and welding steel | Pick steel |
| Fork steel | Pivot steel |
| Gin saw steel | Plane bit steel |
| Granite wedge steel | Quarry steel |
| Gun barrel steel | Razor steel |
| Hack saw steel | Roll turning steel |
| High-speed tool steel | Saw steel |
| Hot-rolled sheet steel | Scythe steel |
| Lathe spindle steel | Shear knife steel |
| Lawn mower knife steel | Silico-manganese steel |
| Machine knife steel | Spindle steel |
| Magnet steel | Spring steel |
| Mining drill steel | Tool holder steel |
| Nail die shapes | Vanadium tool steel |
| Nickel-chrome steel | Vanadium-chrome steel |
| Paper knife steel | Wortle steel |
Passing to the tonnage specifications, the following table from Tiemann's excellent pocket book on "Iron and Steel," will give an approximate idea of the ordinary designations now in use:
| Grades | Approximate carbon range | Common uses |
| Extra soft (dead soft) |
0.08-0.18 | Pipe, chain and other welding purposes; case-hardening purposes; rivets; pressing and stamping purposes. |
| Structural (soft) (medium) | 0.08-0.18 | Structural plates, shapes and bars for bridges, buildings, cars, locomotives; boiler (flange) steel; drop forgings; bolts. |
| Medium | 0.20-0.35 | Structural purposes (ships); shafting; automobile parts; drop forgings. |
| Medium hard | 0.35-0.60 | Locomotive and similar large forgings; car axles; rails. |
| Hard | 0.60-0.85 | Wrought steel wheels for steam and electric railway service; locomotive tires; rails; tools, such as sledges, hammers, pick points, crowbars, etc. |
| Spring | 0.85-1.05 | Automobile and other vehicle springs; tools, such as hot and cold chisels, rock drills and shear blades. |
| Spring | 0.90-1.15 | Railway springs; general machine shop tools. |
COMPOSITION AND PROPERTIES OF STEEL
It is a remarkable fact that one can look through a dozen text books on metallurgy and not find a definition of the word "steel." Some of them describe the properties of many other irons and then allow you to guess that everything else is steel. If it was difficult a hundred years ago to give a good definition of the term when the metal was made by only one or two processes, it is doubly difficult now, since the introduction of so many new operations and furnaces.
We are in better shape to know what steel is than our forefathers. They went through certain operations and they got a soft malleable, weldable metal which would not harden; this they called iron. Certain other operations gave them something which looked very much like iron, but which would harden after quenching from a red heat. This was steel. Not knowing the essential difference between the two, they must distinguish by the process of manufacture. To-day we can make either variety by several methods, and can convert either into the other at will, back and forth as often as we wish; so we are able to distinguish between the two more logically.
We know that iron is a chemical element—the chemists write it Fe for short, after the Latin word "ferrum," meaning iron—it is one of those substances which cannot be separated into anything else but itself. It can be made to join with other elements; for instance, it joins with the oxygen in the air and forms scale or rust, substances known to the chemist as iron oxide. But the same metal iron can be recovered from that rust by abstracting the oxygen; having recovered the iron nothing else can be extracted but iron; iron is elemental.
We can get relatively pure iron from various minerals and artificial substances, and when we get it we always have a magnetic metal, almost infusible, ductile, fairly strong, tough, something which can be hardened slightly by hammering but which cannot be hardened by quenching. It has certain chemical properties, which need not be described, which allow a skilled Page 13 chemist to distinguish it without difficulty and unerringly from the other known elements—nearly 100 of them.
Carbon is another chemical element, written C for short, which is widely distributed through nature. Carbon also readily combines with oxygen and other chemical elements, so that it is rarely found pure; its most familiar form is soot, although the rarer graphite and most rare diamond are also forms of quite pure carbon. It can also be readily separated from its multitude of compounds (vegetation, coal, limestone, petroleum) by the chemist.
With the rise of knowledge of scientific chemistry, it was quickly found that the essential difference between iron and steel was that the latter was iron plus carbon. Consequently it is an alloy, and the definition which modern metallurgists accept is this:
"Steel is an iron-carbon alloy containing less than about 2 per cent carbon."
Of course there are other elements contained in commercial steel, and these elements are especially important in modern "alloy steels," but carbon is the element which changes a soft metal into one which may be hardened, and strengthened by quenching. In fact, carbon, of itself, without heat treatment, strengthens iron at the expense of ductility (as noted by the percentage elongation an 8-in. bar will stretch before breaking). This is shown by the following table:
| Class by use. | Class by hardness. |
Per cent carbon. |
Elastic limit lb. per sq. in. |
Ultimate strength lb. per sq. in. |
Percentage elongation in 8 inches. |
|---|---|---|---|---|---|
| Boiler rivet steel | Dead soft | 0.08 to 0.15 | 25,000 | 50,000 | 30 |
| Struc. rivet steel | Soft | 0.15 to 0.22 | 30,000 | 55,000 | 30 |
| Boiler plate steel | Soft | 0.08 to 0.10 | 30,000 | 60,000 | 25 |
| Structural steel | Medium | 0.18 to 0.30 | 35,000 | 65,000 | 25 |
| Machinery steel | Hard | 0.35 to 0.60 | 40,000 | 75,000 | 20 |
| Rail steel | Hard | 0.35 to 0.55 | 40,000 | 75,000 | 15 |
| Spring steel | High carbon | 1.00 to 1.50 | 60,000 | 125,000 | 10 |
| Tool steel | High carbon | 0.90 to 1.50 | 80,000 | 150,000 | 5 |
Just why a soft material like carbon (graphite), when added to another soft material like iron, should make the iron harder, has Page 14 been quite a mystery, and one which has caused a tremendous amount of study. The mutual interactions of these two elements in various proportions and at various temperatures will be discussed at greater length later, especially in Chap. VIII, p. 105. But we may anticipate by saying that some of the iron unites with all the carbon to form a new substance, very hard, a carbide which has been called "cementite." The compound always contains iron and carbon in the proportions of three atoms of iron to one atom of carbon; chemists note this fact in shorthand by the symbol Fe3C (a definite chemical compound of three atoms of iron to one of carbon). Many of the properties of steel, as they vary with carbon content, can be linked up with the increasing amount of this hard carbide cementite, distributed in very fine particles through the softer iron.
Sulphur is another element (symbol S) which is always found in steel in small quantities. Some sulphur is contained in the ore from which the iron is smelted; more sulphur is introduced by the coke and fuel used. Sulphur is very difficult to get rid of in steel making; in fact the resulting metal usually contains a little more than the raw materials used. Only the electric furnace is able to produce the necessary heat and slags required to eliminate sulphur, and as a matter of fact the sulphur does not go until several other impurities have been eliminated. Consequently, an electric steel with extremely low sulphur (0.02 per cent) is by that same token a well-made metal.
Sulphur is of most trouble to rolling and forging operations when conducted at a red heat. It makes steel tender and brittle at that temperature—a condition known to the workmen as "red-short." It seems to have little or no effect upon the physical properties of cold steel—at least as revealed by the ordinary testing machines—consequently many specifications do not set any limit on sulphur, resting on the idea that if sulphur is low enough not to cause trouble to the manufacturer during rolling, it will not cause the user any trouble.
Tool steel and other fine steels should be very low in sulphur, preferably not higher than 0.03 per cent. Higher sulphur steels (0.06 per cent, and even up to 0.10 per cent) have given very good service for machine parts, but in general a high sulphur steel is a suspicious steel. Screw stock is purposely made with up to 0.12 per cent sulphur and a like amount of phosphorus so it will cut freely.
Page 15 Manganese counteracts the detrimental effect of sulphur when present in the steel to an amount at least five times the sulphur content.
Phosphorus is an element (symbol P) which enters the metal from the ore. It remains in the steel when made by the so-called acid process, but it can be easily eliminated down to 0.06 per cent in the basic process. In fact the discovery of the basic process was necessary before the huge iron deposits of Belgium and the Franco-German border could be used. These ores contain several per cent phosphorus, and made a very brittle steel ("cold short") until basic furnaces were used. Basic furnaces allow the formation of a slag high in lime, which takes practically all the phosphorus out of the metal. Not only is the resulting metal usable, but the slag makes a very excellent fertilizer, and is in good demand.
Silicon is a very widespread element (symbol Si), being an essential constituent of nearly all the rocks of the earth. It is similar to carbon in many of its chemical properties; for instance it burns very readily in oxygen, and consequently native silicon is unknown—it is always found in combination with one or more other elements. When it bums, each atom of silicon unites with two atoms of oxygen to form a compound known to chemists as silica (SiO2), and to the small boy as "sand" and "agate."
Iron ore (an oxide of iron) contains more or less sand and dirt mixed in it when it is mined, and not only the iron oxide but also some of the silicon oxide is robbed of its oxygen by the smelting process. Pig iron—the product of the blast furnace—therefore contains from 1 to 3 per cent of silicon, and some silicon remains in the metal after it has been purified and converted into steel.
However, silicon, as noted above, burns very readily in oxygen, and this property is of good use in steel making. At the end of the steel-making process the metal contains more or less oxygen, which must be removed. This is sometimes done (especially in the so-called acid process) by adding a small amount of silicon to the hot metal just before it leaves the furnace, and stirring it in. It thereupon abstracts oxygen from the metal wherever it finds it, changing to silica (SiO2) which rises and floats on the surface of the cleaned metal. Most of the silicon remaining in the metal is an excess over that which is required to remove the dangerous oxygen, and the final analysis of many steels show enough silicon (from 0.20 to 0.40) to make sure that this step in the manufacture has been properly done.
Page 16 Manganese is a metal much like iron. Its chemical symbol is Mn. It is somewhat more active than iron in many chemical changes—notably it has what is apparently a stronger attraction for oxygen and sulphur than has iron. Therefore the metal is used (especially in the so-called basic process) to free the molten steel of oxygen, acting in a manner similar to silicon, as explained above. The compound of manganese and oxygen is readily eliminated from the metal. Sufficient excess of elemental manganese should remain so that the purchaser may be sure that the iron has been properly "deoxidized," and to render harmless the traces of sulphur present. No damage is done by the presence of a little manganese in steel, quite the reverse. Consequently it is common to find steels containing from 0.3 to 1.5 per cent.
Alloying Elements.—Commercial steels of even the simplest types are therefore primarily alloys of iron and carbon. Impurities and their "remedies" are always present: sulphur, phosphorus, silicon and manganese—to say nothing of oxygen, nitrogen and carbon oxide gases, about which we know very little. It has been found that other metals, if added to well-made steel, produce definite improvements in certain directions, and these "alloy steels" have found much use in the last ten years. Alloy steels, in addition to the above-mentioned elements, may commonly contain one or more of the following, in varying amounts: Nickel (Ni), Chromium (Cr), Vanadium (Va), Tungsten (W), Molybdenum (Mo). These steels will be discussed at more length in Chapters III and IV.
Steels are known by certain tests. Early tests were more or less crude, and depended upon the ability of the workman to judge the "grain" exhibited by a freshly broken piece of steel. The cold-bend test was also very useful—a small bar was bent flat upon itself, and the stretched fibers examined for any sign of break. Harder stiff steels were supported at the ends and the amount of central load they would support before fracture, or the amount of permanent set they would acquire at a given load noted. Files were also used to test the hardness of very hard steel.
These tests are still used to a considerable extent, especially in works where the progress of an operation can be kept under close watch in this way, the product being periodically examined by more precise methods. The chief furnace-man, or "melter," Page 17 in a steel plant, judges the course of the refining process by casting small test ingots from time to time, breaking them and examining the fracture. Cutlery manufacturers use the bend test to judge the temper of blades. File testing of case-hardened parts is very common.
However there is need of standardized methods which depend less upon the individual skill of the operator, and which will yield results comparable to others made by different men at different places and on different steels. Hence has grown up the art of testing materials.
Strength of a metal is usually expressed in the number of pounds a 1-in. bar will support just before breaking, a term called the "ultimate strength." It has been found that the shape of the test bar and its method of loading has some effect upon the results, so it is now usual to turn a rod 5½ in. long down to 0.505 in. in diameter for a central length of 2-3/8 in., ending the turn with 1/2-in. fillets. The area of the bar equals 0.2 sq. in., so the load it bears at rupture multiplied by 5 will represent the "ultimate strength" in pounds per square inch.
Such a test bar is stretched apart in a machine like that shown in Fig. 9. The upper end of the bar is held in wedged jaws by the top cross-head, and the lower end grasped by the movable head. The latter is moved up and down by three long screws, driven at the same speed, which pass through threads cut in the corners of the cross-head. When the test piece is fixed in position the motor which drives the machine is given a few turns, which by proper gearing pulls the cross-head down with a certain pull. This pull is transmitted to the upper cross-head by the test bar, and can be weighed on the scale arm, acting through a system of links and levers.
Thus the load may be increased as rapidly as desirable, always kept balanced by the weighing mechanism, and the load at fracture may be read directly from the scale beam.
This same test piece may give other information. If light punch marks are made, 2 in. apart, before the test is begun, the broken ends may be clamped together, and the distance between punch marks measured. If it now measures 3 in. the stretch has been 1 in. in 2, or 50 per cent. This figure is known as the elongation Page 18 at fracture, or briefly, the "elongation," and is generally taken to be a measure of ductility.
When steel shows any elongation, it also contracts in area at the same time. Often this contraction is sharply localized at the fracture; the piece is said to "neck." A figure for contraction in area is also of much interest as an indication of toughness; the diameter at fracture is measured, a corresponding area taken out from a table of circles, subtracted from the original area (0.200 sq. in.) and the difference divided by 0.2 to get the percentage contraction.
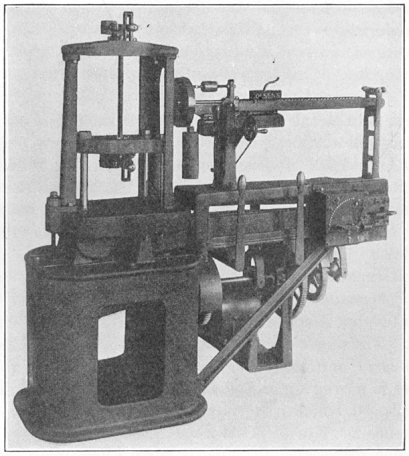 FIG. 9.—Olsen testing machine.
FIG. 9.—Olsen testing machine.
Quite often it is desired to discover the elastic limit of the steel, in fact this is of more use to the designer than the ultimate strength. The elastic limit is usually very close to the load where the metal takes on a permanent set. That is to say, if a delicate caliper ("extensometer," so called) be fixed to the side of the test specimen, it would show the piece to be somewhat longer under load than when free. Furthermore, if the load had not yet reached Page 19 the yield point, and were released at any time, the piece would return to its original length. However, if the load had been excessive, and then relieved, the extensometer would no longer read exactly 2.0 in., but something more.
Soft steels "give" very quickly at the yield point. In fact, if the testing machine is running slowly, it takes some time for the lower head to catch up with the stretching steel. Consequently at the yield point, the top head is suddenly but only temporarily relieved of load, and the scale beam drops. In commercial practice, the yield point is therefore determined by the "drop of the beam." For more precise work the calipers are read at intervals of 500 or 1,000 lb. load, and a curve plotted from these results, a curve which runs straight up to the elastic limit, but there bends off.
A tensile test therefore gives four properties of great usefulness: The yield point, the ultimate strength, the elongation and the contraction. Compression tests are seldom made, since the action of metal in compression and in tension is closely allied, and the designer is usually satisfied with the latter.
Impact tests are of considerable importance as an indication of how a metal will perform under shock. Some engineers think that the tensile test, which is one made under slow loading, should therefore be supplemented by another showing what will happen if the load is applied almost instantaneously. This test, however, has not been standardized, and depends to a considerable extent upon the type of machine, but more especially the size of the specimen and the way it is "nicked." The machine is generally a swinging heavy pendulum. It falls a certain height, strikes the sample at the lowest point, and swings on past. The difference between the downward and upward swing is a measure of the energy it took to break the test piece.
It has been known for fifty years that a beam or rod would fail at a relatively low stress if only repeated often enough. It has been found, however, that each material possesses a limiting stress, or endurance limit, within which it is safe, no matter how often the loading occurs. That limiting stress for all Page 20 steels so far investigated causes fracture below 10 million reversals. In other words, a steel which will not break before 10,000,000 reversals can confidently be expected to endure 100,000,000, and doubtless into the billions.
About the only way to test one piece such a large number of times is to fashion it into a beam, load it, and then turn the beam in its supports. Thus the stress in the outer fibers of the bar varies from a maximum stretch through zero to a maximum compression, and back again. A simple machine of this sort is shown in Fig. 10, where B and E are bearings, A the test piece, turned slightly down in the center, C and D ball bearings supporting a load W. K is a pulley for driving the machine and N is a counter.
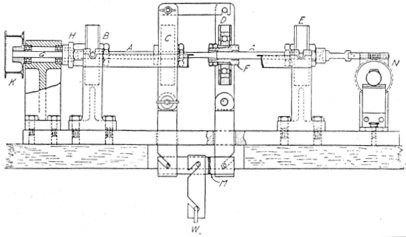 FIG. 10.—Sketch of rotating beam machine for measuring endurance
of metal.
FIG. 10.—Sketch of rotating beam machine for measuring endurance
of metal.
The word "hardness" is used to express various properties of metals, and is measured in as many different ways.
"Scratch hardness" is used by the geologist, who has constructed "Moh's scale" as follows:
| Talc | has a hardness of | 1 |
| Rock Salt | has a hardness of | 2 |
| Calcite | has a hardness of | 3 |
| Fluorite | has a hardness of | 4 |
| Apatite | has a hardness of | 5 |
| Feldspar | has a hardness of | 6 |
| Quartz | has a hardness of | 7 |
| Topaz | has a hardness of | 8 |
| Corundum | has a hardness of | 9 |
| Diamond | has a hardness of | 10 |
Page 21 A mineral will scratch all those above it in the series, and will be scratched by those below. A weighted diamond cone drawn slowly over a surface will leave a path the width of which (measured by a microscope) varies inversely as the scratch hardness.
"Cutting hardness" is measured by a standardized drilling machine, and has a limited application in machine-shop practice.
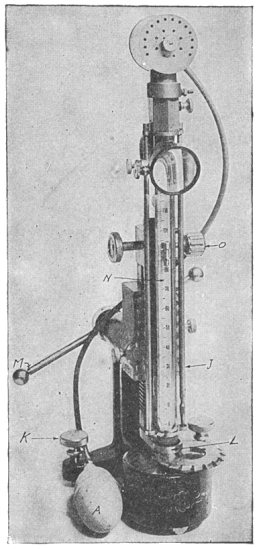 FIG. 11.—Shore scleroscope.
FIG. 11.—Shore scleroscope.
"Rebounding hardness" is commonly measured by the Shore scleroscope, illustrated in Fig. 11. A small steel hammer, ¼ in. in diameter, ¾ in. in length, and weighing about 1/12 oz. is dropped a distance of 10 in. upon the test piece. The height of rebound in arbitrary units represents the hardness numeral.
Should the hammer have a hard flat surface and drop on steel so hard that no impression were made, it would rebound about 90 per cent of the fall. The point, however, consists of a slightly spherical, blunt diamond nose 0.02 in. in diameter, which will indent the steel to a certain extent. The work required to make the indentation is taken from the energy of the falling body; the rebound will absorb the balance, and the hammer will now rise from the same steel a distance equal to about 75 per cent of the fall. A permanent impression is left upon the test piece because the impact will develop a force of several hundred thousand pounds per square inch under the tiny diamond-pointed hammer head, stressing the test piece at this point of contact much beyond its ultimate strength. The rebound is thus dependent upon the indentation hardness, for the reason that the less Page 22 the indentation, the more energy will reappear in the rebound; also, the less the indentation, the harder the material. Consequently, the harder the material, the more the rebound.
"Indentation hardness" is a measure of a material's resistance to penetration and deformation. The standard testing machine is the Brinell, Fig. 12. A hardened steel ball, 10 mm. in diameter, is forced into the test piece with a pressure of 3,000 kg. (3-1/3 tons). The resulting indentation is then measured.
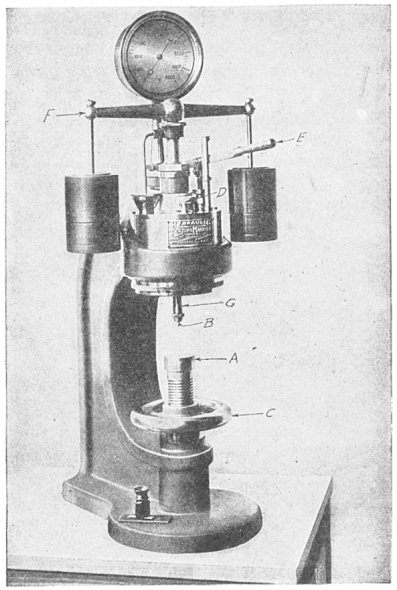 FIG. 12.—Hydraulic testing machine. (Brinell principle.)
FIG. 12.—Hydraulic testing machine. (Brinell principle.)
While under load, the steel ball in a Brinell machine naturally flattens somewhat. The indentation left behind in the test piece is a duplicate of the surface which made it, and is usually regarded as being the segment of a sphere of somewhat larger radius than Page 23 the ball. The radius of curvature of this spherical indentation will vary slightly with the load and the depth of indentation. The Brinell hardness numeral is the quotient found by dividing the test pressure in kilograms by the spherical area of the indentation. The denominator, as before, will vary according to the size of the sphere, the hardness of the sphere and the load. These items have been standardized, and the following table has been constructed so that if the diameter of the identation produced by a load of 3,000 kg. be measured the hardness numeral is found directly.
| Diameter of Ball Impression, mm. |
Hardness Number for a Load of 3,000 kg. |
Diameter of Ball Impression, mm. |
Hardness Number for a Load of 3,000 kg. |
|---|---|---|---|
| 2.0 | 946 | 4.5 | 179 |
| 2.1 | 857 | 4.6 | 170 |
| 2.2 | 782 | 4 7 | 163 |
| 2.3 | 713 | 4.8 | 156 |
| 2.4 | 652 | 4.9 | 149 |
| 2.5 | 600 | 5.0 | 143 |
| 2.6 | 555 | 5.1 | 137 |
| 2.7 | 512 | 5.2 | 131 |
| 2.8 | 477 | 5.3 | 126 |
| 2.9 | 444 | 5.4 | 121 |
| 3.0 | 418 | 5.5 | 116 |
| 3.1 | 387 | 5.6 | 112 |
| 3.2 | 364 | 5.7 | 107 |
| 3.3 | 340 | 5.8 | 103 |
| 3.4 | 321 | 5.9 | 99 |
| 3.5 | 302 | 6.0 | 95 |
| 3.6 | 286 | 6.1 | 92 |
| 3.7 | 269 | 6.2 | 89 |
| 3.8 | 255 | 6.3 | 86 |
| 3.9 | 241 | 6.4 | 83 |
| 4.0 | 228 | 6.5 | 80 |
| 4.1 | 217 | 6.6 | 77 |
| 4.2 | 207 | 6.7 | 74 |
| 4.3 | 196 | 6.8 | 71.5 |
| 4.4 | 187 | 6.9 | 69 |
ALLOYS AND THEIR EFFECT UPON STEEL
In view of the fact that alloy steels are coming into a great deal of prominence, it would be well for the users of these steels to fully appreciate the effects of the alloys upon the various grades of steel. We have endeavored to summarize the effect of these alloys so that the users can appreciate their effect, without having to study a metallurgical treatise and then, perhaps, not get the crux of the matter.
Nickel may be considered as the toughest among the non-rare alloys now used in steel manufacture. Originally nickel was added to give increased strength and toughness over that obtained with the ordinary rolled structural steel and little attempt was made to utilize its great possibilities so far as heat treatment was concerned.
The difficulties experienced have been a tendency towards laminated structure during manufacture and great liability to seam, both arising from improper melting practice. When extra care is exercised in the manufacture, particularly in the melting and rolling, many of these difficulties can be overcome.
The electric steel furnace, of modern construction, is a very important step forward in the melting of nickel steel; neither the crucible process nor basic or acid open-hearth furnaces give such good results.
Great care must be exercised in reheating the billet for rolling so that the steel is correctly soaked. The rolling must not be forced; too big reduction per pass should not be indulged in, as this sets up a tendency towards seams.
Nickel steel has remarkably good mechanical qualities when suitably heat-treated, and it is preeminently adapted for case-hardening. It is not difficult to machine low-nickel steel, consequently it is in great favor where easy machining properties are of importance.
Page 25 Nickel influences the strength and ductility of steel by being dissolved directly in the iron or ferrite; in this respect differing from chromium, tungsten and vanadium. The addition of each 1 per cent nickel up to 5 per cent will cause an approximate increase of from 4,000 to 6,000 lb. per square inch in the tensile strength and elastic limit over the corresponding steel and without any decrease in ductility. The static strength of nickel steel is affected to some degree by the percentage of carbon; for instance, steel with 0.25 per cent carbon and 3.5 per cent nickel has a tensile strength, in its normal state, equal to a straight carbon steel of 0.5 per cent with a proportionately greater elastic limit and retaining all the advantages of the ductility of the lower carbon.
To bring out the full qualities of nickel it must be heat-treated, otherwise there is no object in using nickel as an alloy with carbon steel as the additional cost is not justified by increased strength.
Nickel has a peculiar effect upon the critical ranges of steel, the critical range being lowered by the percentage of nickel; in this respect it is similar to manganese.
Nickel can be alloyed with steel in various percentages, each percentage having a very definite effect on the microstructure. For instance, a steel with 0.2 per cent carbon and 2 per cent nickel has a pearlitic structure but the grain is much finer than if the straight carbon were used. With the same carbon content and say 5 per cent nickel, the structure would still be pearlitic, but much finer and denser, therefore capable of withstanding shock, and having greater dynamic strength. With about 0.2 per cent carbon and 8 per cent nickel, the steel is nearing the stage between pearlite and martensite, and the structure is extremely fine, the ferrite and pearlite having a very pronounced tendency to mimic a purely martensite structure. Steel with 0.2 per cent carbon and 15 per cent nickel is entirely martensite. Higher percentages of nickel change the martensitic structure to austenite, the steel then being non-magnetic. The higher percentages, that is 30 to 35 per cent nickel, are used for valve seats, valve heads, and valve stems, as the alloy is a poor conductor of heat and is particularly free from any tendency towards corrosion or pitting from the action of waste gases of the internal-combustion engine.
Nickel steels having 3½ per cent nickel and 0.15 to 0.20 per cent carbon are excellent for case-hardening purposes, giving hard surfaces and tough interiors.
Page 26 To obtain the full effect of nickel as an alloy, it is essential that the correct percentage of carbon be used. High nickel and low carbon will not be more efficient than lower nickel and higher carbon, but the cost will be much greater. Generally speaking, heat-treated nickel alloy steels are about two to three times stronger than the same steel annealed. This point is very important as many instances have been found where nickel steel is incorrectly used, being employed when in the annealed or normal state.
Chromium when alloyed with steel, has the characteristic function of opposing the disintegration and reconstruction of cementite. This is demonstrated by the changes in the critical ranges of this alloy steel taking place slowly; in other words, it has a tendency to raise the Ac range (decalescent points) and lower the Ar range (recalescent points). Chromium steels are therefore capable of great hardness, due to the rapid cooling being able to retard the decomposition of the austenite.
The great hardness of chromium steels is also due to the formation of double carbides of chromium and iron. This condition is not removed when the steel is slightly tempered or drawn. This additional hardness is also obtained without causing undue brittleness such as would be obtained by any increase of carbon. The degree of hardness of the lower-chrome steels is dependent upon the carbon content, as chromium alone will not harden iron.
The toughness so noticeable in this steel is the result of the fineness of structure; in this instance, the action is similar to that of nickel, and the tensile strength and elastic limit is therefore increased without any loss of ductility. We then have the desirable condition of tough hardness, making chrome steels extremely valuable for all purposes requiring great resistance to wear, and in higher-chrome contents resistance to corrosion. All chromium-alloy steels offer great resistance to corrosion and erosion. In view of this, it is surprising that chromium steels are not more largely used for structural steel work and for all purposes where the steel has to withstand the corroding action of air and liquids. Bridges, ships, steel building, etc., would offer greater resistance to deterioration through rust if the chromium-alloy steels were employed.
Prolonged heating and high temperatures have a very bad effect upon chromium steels. In this respect they differ from Page 27 nickel steels, which are not so affected by prolonged heating, but chromium steels will stand higher temperatures than nickel steels when the period is short.
Chromium steels, due to their admirable property of increased hardness, without the loss of ductility, make very excellent chisels and impact tools of all types, although for die blocks they do not give such good results as can be obtained from other alloy combinations.
For ball bearing steels, where intense hardness with great toughness and ready recovery from temporary deflection is required, chromium as an alloy offers the best solution.
Two per cent chromium steels; due to their very hard tough surface, are largely used for armor-piercing projectiles, cold rolls, crushers, drawing dies, etc.
The normal structure of chromium steels, with a very low carbon content is roughly pearlitic up to 7 per cent, and martensitic from 8 to 20 per cent; therefore, the greatest application is in the pearlitic zone or the lower percentages.
A combination of the characteristics of nickel and the characteristics of chromium, as described, should obviously give a very excellent steel as the nickel particularly affects the ferrite of the steel and the chromium the carbon. From this combination, we are able to get a very strong ferrite matrix and a very hard tough cementite. The strength of a strictly pearlitic steel over a pure iron is due to the pearlitic being a layer arrangement of cementite running parallel to that of a pure iron layer in each individual grain. The ferrite i.e., the iron is increased in strength by the resistance offered by the cementite which is the simple iron-carbon combination known to metallurgists as Fe3C. The cementite, although adding to the tensile strength, is very brittle and the strength of the pearlite is the combination of the ferrite and cementite. In the event of the cementite being strengthened, as in the case of strictly chromium steels, an increased tensile strength is readily obtained without loss of ductility and if the ferrite is strengthened then the tensile strength and ductility of the metal is still further improved.
Nickel-chromium alloy represents one of the best combinations available at the present time. The nickel intensifies the physical characteristics of the chromium and the chromium has a similar effect on the nickel.
Page 28 For case-hardening, nickel-chromium steels seem to give very excellent results. The carbon is very rapidly taken up in this combination, and for that reason is rather preferable to the straight nickel steel.
With the mutually intensifying action of chromium and nickel there is a most suitable ratio for these two alloys, and it has been found that roughly 2½ parts of nickel to about 1 part of chromium gives the best results. Therefore, we have the standard types of 3.5 per cent nickel with 1.5 per cent chromium to 1.5 per cent nickel with 0.6 per cent chromium and the various intermediate types. This ratio, however, does not give the whole story of nickel-chromium combinations, and many surprising results have been obtained with these alloys when other percentage combinations have been employed.
Vanadium has a very marked effect upon alloy steels rich in chromium, carbon, or manganese. Vanadium itself, when combined with steel very low in carbon, is not so noticeably beneficial as in the same carbon steel higher in manganese, but if a small quantity of chromium is added, then the vanadium has a very marked effect in increasing the impact strength of the alloy. It would seem that vanadium has the effect of intensifying the action of chromium and manganese, or that vanadium is intensified by the action of chromium or manganese.
Vanadium has the peculiar property of readily entering into solution with ferrite. If vanadium contained is considerable it also combines with the carbon, forming carbides. The ductility of carbon-vanadium steels is therefore increased, likewise the ductility of chrome-vanadium steels.
The full effect of vanadium is not felt unless the temperatures to which the steel is heated for hardening are raised considerably. It is therefore necessary that a certain amount of "soaking" takes place, so as to get the necessary equalization. This is true of all alloys which contain complex carbides, i.e., compounds of carbon, iron and one or more elements.
Chrome-vanadium steels also are highly favored for case hardening. When used under alternating stresses it appears to have superior endurance. It would appear that the intensification of the properties due to chromium and manganese in the alloy steel accounts for this peculiar phenomenon.
Page 29 Vanadium is also a very excellent scavenger for either removing the harmful gases, or causing them to enter into solution with the metal in such a way as to largely obviate their harmful effects. Chrome-vanadium steels have been claimed, by many steel manufacturers and users, to be preferable to nickel-chrome steels. While not wishing to pass judgment on this, it should be borne in mind that the chrome-vanadium steel, which is tested, is generally compared with a very low nickel-chromium alloy steel (the price factor entering into the situation), but equally good results can be obtained by nickel-chromium steels of suitable analysis.
Where price is the leading factor, there are many cases where a stronger steel can be obtained from the chrome and vanadium than the nickel-chrome. It will be safe to say that each of these two systems of alloys have their own particular fields and chrome-vanadium steel should not be regarded as the sole solution for all problems, neither should nickel-chromium.
Manganese adds considerably to the tensile strength of steel, but this is dependent on the carbon content. High carbon materially adds to the brittleness, whereas low-carbon, pearlitic-manganese steels are very tough and ductile and are not at all brittle, providing the heat-treating is correct. Manganese steel is very susceptible to high temperatures and prolonged heating.
In low-carbon pearlitic steels, manganese is more effective in increasing ultimate strength than is nickel; that is to say, a 0.45 carbon steel with 1.25 per cent manganese is as strong as a 0.45 carbon steel with 1.5 per cent nickel. The former steel is much used for rifle barrels, and in the heat-treated condition will give 80,000 to 90,000 lb. per square inch elastic limit, 115,000 to 125,000 lb. per square inch tensile strength, 23 per cent elongation, and 55 per cent reduction in area.
Manganese when added to steel has the effect of lowering the critical range; 1 per cent manganese will lower the upper critical point 60°F. The action of manganese is very similar to that of nickel in this respect, only twice as powerful. As an instance, 1 per cent nickel would have the effect of lowering the upper critical range from 25 to 30°F.
Low-carbon pearlitic-manganese steel, heat-treated, will give dynamic strength which cannot be equaled by low-priced and Page 30 necessarily low-content nickel steels. In many instances, it is preferable to use high-grade manganese steel, rather than low-content nickel steel.
High-manganese steels or austenite manganese steels are used for a variety of purposes where great resistance to abrasion is required, the percentage of manganese being from 11 to 14 per cent, and carbon 1 to 1.5 per cent. This steel is practically valueless unless heat-treated; that is, heated to about yellow red and quenched in ice water. The structure is then austenite and the air-cooled structure of this steel is martensite. Therefore this steel has to be heated and very rapidly cooled to obtain the ductile austenite structure.
Manganese between 2 and 7 per cent is a very brittle material when the carbon is about 1 per cent or higher and is, therefore, quite valueless. Below 2 per cent manganese steel low in carbon is very ductile and tough steel.
The high-content manganese steels are known as the "Hadfield manganese steels," having been developed by Sir Robert Hadfield. Small additions of chrome up to 1 per cent increase the elastic limit of low-carbon pearlitic-manganese steels without affecting the steel in its resistance to shock, but materially decrease the percentage of elongation.
Vanadium added to low-carbon pearlitic manganese steel has a very marked effect, increasing greatly the dynamic strength and changing slightly the susceptibility of this steel to heat treatments, giving a greater margin for the hardening temperature. Manganese steel with added vanadium is most efficient when heat-treated.
Tungsten, as an alloy in steel, has been known and used for a long time. The celebrated and ancient damascus steel being a form of tungsten-alloy steel. Tungsten and its effects, however, did not become generally realized until Robert Mushet experimented and developed his famous mushet steel and the many improvement made since that date go to prove how little Mushet himself understood the peculiar effects of tungsten as an alloy.
Tungsten acts on steel in a similar manner to carbon, that is, it increases its hardness, but is much less effective than carbon in this respect. If the percentage of tungsten and manganese is Page 31 high, the steel will be hard after cooling in the air. This is impossible in a carbon steel. It was this combination that Mushet used in his well-known "air-hardening" steel.
The principal use of tungsten is in high-speed tool steel, but here a high percentage of manganese is distinctly detrimental, making the steel liable to fire crack, very brittle and weak in the body, less easily forged and annealed. Manganese should be kept low and a high percentage of chromium used instead.
Tools of tungsten-chromium steels, when hardened, retain their hardness, even when heated to a dark cherry red by the friction of the cutting or the heat arising from the chips. This characteristic led to the term "red-hardness," and it is this property that has made possible the use of very high cutting speeds in tools made of the tungsten-chromium alloy, that is, "high-speed" steel.
Tungsten steels containing up to 6 per cent do not have the property of red hardness any more than does carbon tool steel, providing the manganese or chromium is low.
When chromium is alloyed with tungsten, a very definite red-hardness is noticed with a great increase of cutting efficiency. The maximum red-hardness seems to be had with steels containing 18 per cent tungsten, 5.5 per cent chromium and 0.70 per cent carbon.
Very little is known of the actual function of tungsten, although a vast amount of experimental work has been done. It is possible that when the effect of tungsten with iron-carbon alloys is better known, a greater improvement can be expected from these steels. Tungsten has been tried and is still used by some steel manufacturers for making punches, chisels, and other impact tools. It has also been used for springs, and has given very good results, although other less expensive alloys give equally good results, and are in some instances, better.
Tungsten is largely used in permanent magnets. In this, its action is not well understood. In fact, the reason why steel becomes a permanent magnet is not at all understood. Theories have been evolved, but all are open to serious questioning. The principal effect of tungsten, as conceded by leading authorities, is that it distinctly retards separation of the iron-carbon solution, removing the lowest recalescent point down to atmospheric temperature.
A peculiar property of tungsten steels is that if a heating temperature of 1,750°F. is not exceeded, the cooling curves indicate Page 32 but one critical point at about 1,350°F. But when the heating temperature is raised above 1,850°F., this critical point is nearly if not quite suppressed, while a lower critical point appears and grows enormously in intensity at a temperature between 660 and 750°F.
The change in the critical ranges, which is produced by heating tungsten steels to over 1,850°F., is the real cause of the red-hard properties of these alloys. Its real nature is not understood, and there is no direct evidence to show what actually happens at these high temperatures.
It may readily be understood that an alloy containing four essential elements, namely: iron, carbon, tungsten and chromium, is one whose study presents problems of extreme complexity. It is possible that complex carbides may be formed, as in chromium steels, and that compounds between iron and tungsten exist. Behavior of these combinations on heating and cooling must be better known before we are able to explain many peculiarities of tungsten steels.
Molybdenum steels have been made commercially for twenty-five years, but they have not been widely exploited until since the war. Very large resources of molybdenum have been developed in America, and the mining companies who are equipped to produce the metal are very active in advertising the advantages of molybdenum steels.
It was early found that 1 part molybdenum was the equivalent of from 2 to 2½ parts of tungsten in tool steels, and magnet steels. It fell into disrepute as an alloy for high-speed tool steel, however, because it was found that the molybdenum was driven out of the surface of the tool during forging and heat treating.
Within the last few years it has been found that the presence of less than 1 per cent of molybdenum greatly enhances certain properties of heat-treated carbon and alloy steels used for automobiles and high-grade machinery.
In general, molybdenum when added to an alloy steel, increases the figure for reduction of area, which is considered a good measure of "toughness." Molybdenum steels are also relatively insensible to variations in heat treatment; that is to say, a chromium-nickel-molybdenum steel after quenching in oil from 1,450°F. may be drawn at any temperature between 900 and 1,100°F. with Page 33 substantially the same result (static tensile properties and hardness).
Silicon prevents, to a large extent, defects such as gas bubbles or blow holes forming while steel is solidifying. In fact, steel after it has been melted and before it has been refined, is "wild" and "gassy." That is to say, if it would be cast into molds it would froth up, and boil all over the floor. A judicious amount of silicon added to the metal just before pouring, prevents this action—in the words of the steel maker, silicon "kills" the steel. If about 1.75 per cent metallic silicon remains in a 0.65 carbon steel, it makes excellent springs.
Phosphorus is one of the impurities in steel, and it has been the object of steel makers for years to eliminate it. On cheap grades of steel, not subject to any abnormal strain or stress, 0.1 per cent phosphorus is not objectionable. High phosphorus makes steel "cold short," i.e., brittle when cold or moderately warm.
Sulphur is another impurity and high sulphur is even a greater detriment to steel than phosphorus. High sulphur up to 0.09 per cent helps machining properties, but has a tendency to make the steel "hot short," i.e., subject to opening up cracks and seams at forging or rolling heats. Sulphur should never exceed 0.06 per cent nor phosphorus 0.08 per cent.
Steel used for tool purposes should have as low phosphorus and sulphur contents as possible, not over 0.02 per cent.
We can sum up the various factors something as follows for ready reference.
| The ingredient | Its effect |
|---|---|
| Iron | The basis of steel |
| Carbon | The determinative |
| Sulphur | A strength sapper |
| Phosphorus | The weak link |
| Oxygen | A strength destroyer |
| Manganese | For strength |
| Nickel | For strength and toughness |
| Tungsten | Hardener and heat resister |
| Chromium | For resisting shocks Page 34 |
| Vanadium | Purifier and fatigue resister |
| Silicon | Impurity and hardener |
| Titanium | Removes nitrogen and oxygen |
| Molybdenum | Hardener and heat resister |
| Aluminum | Kills or deoxidizes steel |
The following table shows the percentages of carbon, manganese, nickel, chromium and vanadium in typical steel alloys for engineering purposes. It also gives the elastic limit, tensile strength, elongation and reduction of area of the various alloys, all being given the same heat treatment with a drawing temperature of 1,100°F. (600°C.). The specimens were one inch rounds machined after heat treatment.
Tungsten is not shown in the table because it is seldom used in engineering construction steels and then usually in combination with chromium. Tungsten is used principally for the magnets of magnetos, to some extent in the manufacture of hacksaws, and for special tool steels.
| Carbon, per cent | Manganese, per cent | Nickel, per cent | Chromium, per cent | Vanadium, per cent | Elastic limit, lb. per sq. in. | Tensile Strength, lb. per sq. in. | Elongation in 2 in., per cent | Reduction of area, per cent |
|---|---|---|---|---|---|---|---|---|
| 0.27 | 0.55 | 49,000 | 80,000 | 30 | 65 | |||
| 0.27 | 0.47 | 0.26 | 66,000 | 98,000 | 25 | 52 | ||
| 0.36 | 0.42 | 58,000 | 90,000 | 27 | 60 | |||
| 0.34 | 0.87 | 0.13 | 82,500 | 103,000 | 22 | 57 | ||
| 0.45 | 0.50 | 65,000 | 96,000 | 22 | 52 | |||
| 0.43 | 0.60 | 0.32 | 96,000 | 122,000 | 21 | 52 | ||
| 0.47 | 0.90 | 0.15 | 102,000 | 127,500 | 23 | 58 | ||
| 0.30 | 0.60 | 3.40 | 75,000 | 105,000 | 25 | 67 | ||
| 0.33 | 0.63 | 3.60 | 0.25 | 118,000 | 142,000 | 17 | 57 | |
| 0.30 | 0.49 | 3.60 | 1.70 | 119,000 | 149,500 | 21 | 60 | |
| 0.25 | 0.47 | 3.47 | 1.60 | 0.15 | 139,000 | 170,000 | 18 | 53 |
| 0.25 | 0.50 | 2.00 | 1.00 | 102,000 | 124,000 | 25 | 70 | |
| 0.38 | 0.30 | 2.08 | 1.16 | 120,000 | 134,000 | 20 | 57 | |
| 0.42 | 0.22 | 2.14 | 1.27 | 0.26 | 145,000 | 161,500 | 16 | 53 |
| 0.36 | 0.61 | 1.46 | 0.64 | 117,600 | 132,500 | 16 | 58 | |
| 0.36 | 0.50 | 1.30 | 0.75 | 0.16 | 140,000 | 157,500 | 17 | 54 |
| 0.30 | 0.50 | 0.80 | 90,000 | 105,000 | 20 | 50 | ||
| 0.23 | 0.58 | 0.82 | 0.17 | 106,000 | 124,000 | 21 | 66 | |
| 0.26 | 0.48 | 0.92 | 0.20 | 112,000 | 137,000 | 20 | 61 | |
| 0.35 | 0.64 | 1.03 | 0.22 | 132,500 | 149,500 | 16 | 54 | |
| 0.50 | 0.92 | 1.02 | 0.20 | 170,000 | 186,000 | 15 | 45 |
Certain steels have a very low rate of expansion and contraction in hardening and are very desirable for test plugs, gages, punches and dies, for milling cutters, taps, reamers, hard steel bushings and similar work.
It is recommended that for forging these steels it be heated slowly and uniformly to a bright red, but not in a direct flame or blast. Harden at a dull red heat, about 1,300°F. A clean coal or coke fire, or a good muffle-gas furnace will give best results. Fish oil is good for quenching although in some cases warm water will give excellent results. The steel should be kept moving in the bath until perfectly cold. Heated and cooled in this way the steel is very tough, takes a good cutting edge and has very little expansion or contraction which makes it desirable for long taps where the accuracy of lead is important.
The composition of these steels is as follows:
| Per cent | |
| Manganese | 1.40 to 1.60 |
| Carbon | 0.80 to 0.90 |
| Vanadium | 0.20 to 0.25 |
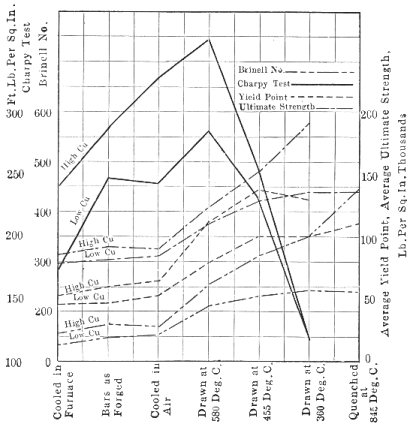 FIG. 13.—Effect of copper in steel.
FIG. 13.—Effect of copper in steel.
This shows the result of tests by C. R. Hayward and A. B. Johnston on two types of steel: one containing 0.30 per Page 36 cent carbon, 0.012 per cent phosphorus, and 0.860 per cent copper, and the other 0.365 per cent carbon, 0.053 per cent phosphorus, and 0.030 per cent copper. The accompanying chart in Fig. 13 shows that high-copper steel has decided superiority in tensile strength, yield point and ultimate strength, while the ductility is practically the same. Hardness tests by both methods show high-copper steel to be harder than low-copper, and the Charpy shock tests show high-copper steel also superior to low-copper. The tests confirm those made by Stead, showing that the behavior of copper steel resembles that of nickel steel. The high-copper steels show finer grain than the low-copper. The quenched and drawn specimens of high-copper steel were found to be slightly more martensitic.
High-chromium, or what is called stainless steel containing from 11 to 14 per cent chromium, was originally developed for cutlery purposes, but has in the past few years been used to a considerable extent for exhaust valves in airplane engines because of its resistance to scaling at high temperatures.
| Percentage | |
| Carbon | 0.20 to 0.40 |
| Manganese, not to exceed | 0.50 |
| Phosphorus, not to exceed | 0.035 |
| Sulphur, not to exceed | 0.035 |
| Chromium | 11.50 to 14.00 |
| Silicon, not to exceed | 0.30 |
The steel should be heated slowly and forged at a temperature above 1,750°F. preferably between 1,800 and 2,200°F. If forged at temperatures between 1,650 and 1,750°F. there is considerable danger of rupturing the steel because of its hardness at red heat. Owing to the air-hardening property of the steel, the drop-forgings should be trimmed while hot. Thin forgings should be reheated to redness before trimming, as otherwise they are liable to crack.
The forgings will be hard if they are allowed to cool in air. This hardness varies over a range of from 250 to 500 Brinell, depending on the original forging temperature.
Annealing can be done by heating to temperatures ranging from 1,290 to 1,380°F. and cooling in air or quenching in water or oil. After this treatment the forgings will have a hardness of Page 37 about 200 Brinell and a tensile strength of 100,000 to 112,000 lb. per square inch. If softer forgings are desired they can be heated to a temperature of from 1,560 to 1,650°F. and cooled very slowly. Although softer the forgings will not machine as smoothly as when annealed at the lower temperature.
Hardening.—The forgings can be hardened by cooling in still air or quenching in oil or water from a temperature between 1,650 and 1,750°F.
The physical properties do not vary greatly when the carbon is within the range of composition given, or when the steel is hardened and tempered in air, oil, or water.
When used for valves the following specification of physical properties have been used:
| Yield point, pounds per square inch | 70,000 |
| Tensile strength, pounds per square inch | 90,000 |
| Elongation in 2 in., per cent | 18 |
| Reduction of area, per cent | 50 |
The usual heat treatment is to quench in oil from 1,650°F. and temper or draw at 1,100 to 1,200°F. One valve manufacturer stated that valves of this steel are hardened by heating the previously annealed valves to 1,650°F. and cooling in still air. This treatment gives a scleroscope hardness of about 50.
In addition to use in valves this steel should prove very satisfactory for shafting for water-pumps and other automobile parts subject to objectionable corrosion.
|
|
| |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quenched in oil from degrees Fahrenheit | 1,600 | 1,600 | 1,650 | ||||||||||||||||
| Tempered at degrees Fahrenheit | 1,160 | 1,080 | 1,100 | ||||||||||||||||
| Yield point, pounds per square inch | 78,300 | 75,000 | 91,616 | ||||||||||||||||
| Tensile strength, pounds per square inch | 104,600 | 104,250 | 123,648 | ||||||||||||||||
| Elongation in 2 in., per cent | 25.0 | 23.5 | 14.5 | ||||||||||||||||
| Reduction of area, per cent | 52.5 | 51.4 | 33.5 |
| ||||||||||||||||||
| Hardening medium | Hardened from, degrees Fahrenheit | Tempered at, degrees Fahrenheit | Elastic limit, per lb. sq. in. | Tensile strength, lb. per sq. in. | Elongation in 2 in. per cent | Reduction of area, per cent | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Air | 1,650 | 930 | 158,815 | 192,415 | 13.0 | 40.5 | ||||||||||||
| 1,100 | 99,680 | 120,065 | 21.0 | 59.2 | ||||||||||||||
| 1,300 | 70,785 | 101,250 | 26.0 | 64.6 | ||||||||||||||
| 1,380 | 66,080 | 98,335 | 28.0 | 63.6 | ||||||||||||||
| 1,470 | 70,785 | 96,990 | 27.0 | 64.7 | ||||||||||||||
| Oil | 1,650 | 930 | 163,070 | 202,720 | 8.0 | 18.2 | ||||||||||||
| 1,100 | 88,255 | 116,480 | 20.0 | 56.9 | ||||||||||||||
| 1,300 | 77,950 | 105,505 | 25.5 | 63.8 | ||||||||||||||
| 1,380 | 88,255 | 98,785 | 27.0 | 66.3 | ||||||||||||||
| Water | 1,650 | 930 | 158,815 | 202,050 | 12.0 | 34.2 | ||||||||||||
| 1,100 | 90,270 | 120,735 | 22.0 | 59.8 | ||||||||||||||
| 1,300 | 66,080 | 102,590 | 25.8 | 64.8 | ||||||||||||||
| 1,380 | 67,200 | 97,890 | 27.0 | 65.2 | ||||||||||||||
This steel can be drawn into wire, rolled into sheets and strips and drawn into seamless tubes.
Corrosion.—This steel like any other steel when distorted by cold working is more sensitive to corrosion and will rust. Rough cut surfaces will rust. Surfaces finished with a fine cut are less liable to rust. Ground and polished surfaces are practically immune to rust.
When chromium content is increased to 16 to 18 per cent and silicon is added, from 2 to 4 per cent, this steel becomes rust proof in its raw state, as soon as the outside surface is removed. It does not need to be heat-treated in any way. These compositions are both patented.
The following steel specifications are considered standard by the Society of Automotive Engineers and represents automobile practice in this country. These tables give the S. A. E. number, the composition of the steel and the heat treatment. These are referred to by letter—the heat treatments being given in detail on pages 134 to 137 in Chap. 8. It should be noted that the percentage of the different ingredients desired is the mean, or halfway between the minimum and maximum.
| S. A. E. Specification no. | Carbon (minimum and maximum) |
Manganese (minimum and maximum) |
Phosphorus (maximum) | Sulphur (maximum) | Heat treatment |
|---|---|---|---|---|---|
| 1,010 | 0.05 to 0.15 | 0.30 to 0.60 | 0.045 | 0.05 | Quench at 1,500 |
| 1,020 | 0.15 to 0.25 | 0.30 to 0.60 | 0.045 | 0.05 | A or B |
| 1,025 | 0.20 to 0.30 | 0.50 to 0.80 | 0.045 | 0.05 | H |
| 1,035 | 0.30 to 0.40 | 0.50 to 0.80 | 0.045 | 0.05 | H, D or E |
| 1,045 | 0.40 to 0.50 | 0.50 to 0.80 | 0.045 | 0.05 | H, D or E |
| 1,095 | 0.90 to 1.05 | 0.25 to 0.50 | 0.040 | 0.05 | F |
| S. A. E. Specification no. |
Carbon | Manganese | Phosphorus (maximum) |
Sulphur |
|---|---|---|---|---|
| 1,114 | 0.08 to 0.20 | 0.30 to 0.80 | 0.12 | 0.06 to 0.12 |
| S. A. E. Specification no. | Carbon (minimum and maximum) |
Manganese (minimum and maximum) |
Phosphorus (maximum) | Sulphur (maximum) | Nickel (minimum and maximum) |
Heat treatment |
|---|---|---|---|---|---|---|
| 2,315 | 0.10 to 0.20 | 0.50 to 0.80 | 0.04 | 0.045 | 3.25 to 3.75 | G, H or K |
| 2,320 | 0.15 to 0.25 | 0.50 to 0.80 | 0.04 | 0.045 | 3.25 to 3.75 | G, H or K |
| 2,330 | 0.25 to 0.35 | 0.50 to 0.80 | 0.04 | 0.045 | 3.25 to 3.75 | H or K |
| 2,335 | 0.30 to 0.40 | 0.50 to 0.80 | 0.04 | 0.045 | 3.25 to 3.75 | H or K |
| 2,340 | 0.35 to 0.45 | 0.50 to 0.80 | 0.04 | 0.045 | 3.25 to 3.75 | H or K |
| 2,345 | 0.40 to 0.50 | 0.50 to 0.80 | 0.04 | 0.045 | 3.25 to 3.75 | H or K |
| S. A. E. Specification no. | Carbon (minimum and maximum) | Manganese (minimum and maximum) | Phosphorus (maximum) | Sulphur (maximum) | Nickel (minimum and maximum) | Chromium (minimum and maximum) | Heat treatment |
|---|---|---|---|---|---|---|---|
| 3,120 | 0.15 to 0.25 | 0.50 to 0.80 | 0.04 | 0.045 | 1.00 to 1.50 | 0.45 to 0.75* | G, H or D |
| 3,125 | 0.20 to 0.30 | 0.50 to 0.80 | 0.04 | 0.045 | 1.00 to 1.50 | 0.45 to 0.75* | H, D or E |
| 3,130 | 0.25 to 0.35 | 0.50 to 0.80 | 0.04 | 0.045 | 1.00 to 1.50 | 0.45 to 0.75* | H, D or E |
| 3,135 | 0.30 to 0.40 | 0.50 to 0 80 | 0.04 | 0.045 | 1.00 to 1.50 | 0.45 to 0 75* | H, D or E |
| 3,140 | 0.35 to 0.45 | 0.50 to 0.80 | 0.04 | 0.045 | 1.00 to 1.50 | 0.45 to 0.75* | H, D or E |
| 3,220 | 0.15 to 0.25 | 0.30 to 0.60 | 0.04 | 0.040 | 1.50 to 2.00 | 0.90 to 1.25 | G, H or D |
| 3,230 | 0.25 to 0.35 | 0.30 to 0.60 | 0.04 | 0.040 | 1.50 to 2.00 | 0.90 to 1.25 | H or D |
| 3,240 | 0.35 to 0.45 | 0.30 to 0.60 | 0.04 | 0.040 | 1.50 to 2.00 | 0.90 to 1.25 | H or D |
| 3,250 | 0.45 to 0.55 | 0.30 to 0.60 | 0.04 | 0.040 | 1.50 to 2.00 | 0.90 to 1.25 | M or Q |
| X3,315 | 0.10 to 0.20 | 0.45 to 0.75 | 0.04 | 0.040 | 2.75 to 3.25 | 0.60 to 0.95 | G |
| X3,335 | 0.30 to 0.40 | 0.45 to 0.75 | 0.04 | 0.040 | 2.75 to 3.25 | 0.60 to 0.95 | P or R |
| X3,350 | 0.45 to 0.55 | 0.45 to 0.75 | 0.04 | 0.040 | 2.75 to 3.25 | 0.60 to 0.95 | P or R |
| 3,320 | 0.15 to 0.25 | 0.30 to 0.60 | 0.04 | 0.040 | 3.25 to 3.75 | 1.25 to 1.75 | L |
| 3,330 | 0.25 to 0.35 | 0.30 to 0.60 | 0.04 | 0.040 | 3.25 to 3.75 | 1.25 to 1.75 | P or R |
| 3,340 | 0.35 to 0.45 | 0.30 to 0.60 | 0.04 | 0.040 | 3.25 to 3.75 | 1.25 to 1.75 | P or R |
* Another grade of this type of steel is available with chromium content of 0.15 per cent to 45 per cent. It has somewhat lower physical properties.
| S. A. E. Specification no. | Carbon (minimum and maximum) |
Manganese (minimum and maximum) |
Phosphorus (maximum) | Sulphur (maximum) | Chromium (minimum and maximum) |
Heat treatment |
|---|---|---|---|---|---|---|
| 5,120 | 0.15 to 0.25 | * | 0.04 | 0.045 | 0.65 to 0.85 | B |
| 5,140 | 0.35 to 0.45 | * | 0.04 | 0.045 | 0.65 to 0.85 | H or D |
| 5,165 | 0.60 to 0.70 | * | 0.04 | 0.045 | 0.65 to 0.85 | H or D |
| 5,195 | 0.90 to 1.05 | 0.20 to 0.45 | 0.03 | 0.03 | 0.90 to 1.10 | M, P or R |
| 51,120 | 1.10 to 1.30 | 0.20 to 0.45 | 0.03 | 0.03 | 0.90 to 1.10 | M, P or R |
| 5,295 | 0.90 to 1.05 | 0.20 to 0.45 | 0.03 | 0.03 | 1.10 to 1.30 | M, P or R |
| 52,120 | 1.10 to 1.30 | 0.20 to 0.45 | 0.03 | 0.03 | 1.10 to 1.30 | M, P or R |
—Two types of steel are available in this class, one with manganese 0.25 to 0.50 per cent (0.35 per cent desired), and silicon not over 0.20 per cent; the other with manganese 0.60 to 0.80 per cent (0.70 per cent desired), and silicon 0.15 to 0.50 per cent.
| S. A. E. Specification no. | Carbon (minimum and maximum) |
Manganese (minimum and maximum) |
Phosphorus (maximum) | Sulphur (maximum) | Chromium (minimum and maximum) |
Vanadium (minimum and maximum) |
Heat treatment |
|---|---|---|---|---|---|---|---|
| 6,120 | 0.15 to 0.25 | 0.50 to 0.80 | 0.04 | 0.04 | 0.80 to 1.10 | 0.15 | S |
| 6,125 | 0.20 to 0.30 | 0.50 to 0.80 | 0.04 | 0.04 | 0.80 to 1.10 | 0.15 | S or T |
| 6,130 | 0.25 to 0.35 | 0.50 to 0.80 | 0.04 | 0.04 | 0.80 to 1.10 | 0.15 | T or U |
| 6,135 | 0.30 to 0.40 | 0.50 to 0.80 | 0.04 | 0.04 | 0.80 to 1.10 | 0.15 | T or U |
| 6,140 | 0.35 to 0.45 | 0.50 to 0.80 | 0.04 | 0.04 | 0.80 to 1.10 | 0.15 | T or U |
| 6,145 | 0.40 to 0.50 | 0.50 to 0.80 | 0.04 | 0.04 | 0.80 to 1.10 | 0.15 | U |
| 6,150 | 0.45 to 0.55 | 0.50 to 0.80 | 0.04 | 0.04 | 0.80 to 1.10 | 0.15 | U |
| 6,195 | 0.90 to 1.05 | 0.20 to 0.45 | 0.03 | 0.03 | 0.80 to 1.10 | 0.15 | U |
| S. A. E. Specification no. | Carbon (minimum and maximum) |
Manganese (minimum and maximum) |
Phosphorus (maximum) | Sulphur (maximum) | Silicon (minimum and maximum) |
Heat treatment |
|---|---|---|---|---|---|---|
| 9,250 | 0.45 to 0.55 | 0.60 to 0.80 | 0.045* | 0.045 | 1.80 to 2.10 | V |
| 9,260 | 0.55 to 0.65 | 0.50 to 0.70 | 0.045* | 0.045 | 1.50 to 1.80 | V |
* Steel made by the acid process may contain maximum 0.05 phosphorus.
The requirements for materials for the Liberty motor connecting rods are so severe that the methods of securing the desired qualities will be of value in other lines. The original specifications called for chrome-nickel but the losses due to the difficulty of handling caused the Lincoln Motor Company to suggest the substitution of chrome-vanadium steel, and this was accepted by the Signal Corps. The rods were accordingly made from chromium-vanadium steel, containing carbon, 0.30 to 0.40 per cent; manganese, 0.50 to 0.80 per cent; phosphorus, not over 0.04 per cent; sulphur, not over 0.04 per cent; chromium, 0.80 to 1.10 per cent; vanadium, not less than 0.15 per cent. This steel is ordinarily known in the trade as 0.35 carbon steel, S. A. E., specification 6,135, which provides a first-rate quality steel for structural parts that are to be heat-treated. The fatigue resisting or endurance qualities of this material are excellent. It has a tensile strength of 150,000 lb. minimum per square inch; elastic limit, 115,000 lb. minimum per square inch; elongation, 5 per cent minimum in 2 in.; and minimum reduction in area, 25 per cent.
The original production system as outlined for the manufacturers had called for a heat treatment in the rough-forged state for the connecting rods, and then semi-machining the rod forgings before giving them the final treatment. The Lincoln Motor Company insisted from the first that the proper method would be a complete heat treatment of the forging in the rough state, and machining the rod after the heat treatment. After a number of trial lots, the Signal Corps acceded to the request and production was immediately increased and quality benefited by the change. This method was later included in a revised specification issued to all producers.
The original system was one that required a great deal of labor per unit output. The Lincoln organization developed a method of handling connecting rods whereby five workmen accomplished the same result that would have required about 30 or 32 by the original method. Even after revising the specification so as to allow complete heat treatments in the rough-forged state, the ordinary methods employed in heat-treating would have required 12 to 15 men. With the fixtures employed, five men could handle 1,300 connecting rods, half of which are plain and half, forked, in a working period of little over 7 hr.
 Fig. 14.—Rack for holding rods.
Fig. 14.—Rack for holding rods.
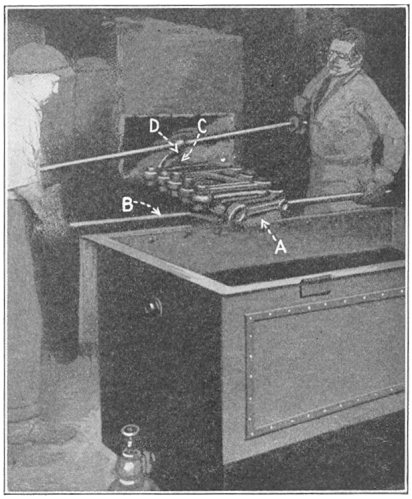 Fig. 15.—Sliding rods into tank.
Fig. 15.—Sliding rods into tank.
Page 44 The increase in production was gained by devising fixtures which enabled fewer men to handle a greater quantity of parts with less effort and in less time.
In heat-treating the forgings were laid on a rack or loop A, Fig. 14, made of 1¼-in. double extra-heavy pipe, bent up with parallel sides about 9 in. apart, one end being bent straight across and the other end being bent upward so as to afford an easy grasp for the hook. Fifteen rods were laid on each loop, there being four loops of rods charged into a furnace with a hearth area of 36 by 66 in. The rods were charged at a temperature of approximately 900°F. They were heated for refining over a period of 3 hr. to 1,625°F., soaked 15 min, at this degree of heat and quenched in soluble quenching oil.
In pulling the heat to quench the rods, the furnace door was raised and the operator pulls one of the loops A, Fig. 15 forward to the shelf of the furnace, supporting the straight end of the loop by means of the porter bar B. They swung the loop of rods around from the furnace shelf and set the straight end of the loop on the edge of the quenching tank, then raise the curved end C, by means of their hook D so that all the rods on the loop slide into the oil bath.
Before the rods cooled entirely, the baskets in the quenching tank were raised and the oil allowed to partly drain off the forgings, and they were stacked on curved-end loops or racks and charged into the furnace for the second or hardening heat. The temperature of the furnace was raised in 1½ hr. to 1,550°F., the rods soaked for 15 min. at this degree of heat and quenched in the same manner as above.
They were again drained while yet warm, placed on loops and charged into the furnace for the third or tempering heat. The temperature of the furnace was brought to 1,100°F. in 1 hr., and the rods soaked at this degree of heat for 1 hr. They were then removed from the furnace the same as for quenching, but were dumped onto steel platforms instead of into the quenching oil, and allowed to cool on these steel platforms down to the room temperature.
The forgings were then pickled in a hot solution of either niter cake or sulphuric acid and water at a temperature of 170°F., and using a solution of about 25 per cent. The solution was Page 45 maintained at a constant point by taking hydrometer readings two or three times a day, maintaining a reading of about 1.175. Sixty forked or one hundred single rods were placed in wooden racks and immersed in a lead-lined vat 30 by 30 by 5 ft. long. The rack was lowered or lifted by means of an air hoist and the rods were allowed to stay in solution from 1/2 to 1 hr., depending on the amount of scale. The rods were then swung and lowered in the rack into running hot water until all trace of the acid was removed.
The rod was finally subjected to Brinell test. This shows whether or not the rod has been heat-treated to the proper hardness. If the rods did not read between 241 and 277, they were re-treated until the proper hardness is obtained.
APPLICATION OF LIBERTY ENGINE MATERIALS TO THE AUTOMOTIVE INDUSTRY[1]
[Footnote 1: Paper presented at the summer meeting of the S. A. E. at Ottawa Beach in June, 1919.]
The success of the Liberty engine program was an engineering achievement in which the science of metallurgy played an important part. The reasons for the use of certain materials and certain treatments for each part are given with recommendations for their application to the problems of automotive industry.
The most important items to be taken into consideration in the selection of material for parts of this type are uniformity and machineability. It has been demonstrated many times that the ordinary grades of bessemer screw stock are unsatisfactory for aviation purposes, due to the presence of excessive amounts of unevenly distributed phosphorus and sulphide segregations. For this reason, material finished by the basic open hearth process was selected, in accordance with the following specifications: Carbon, 0.150 to 0.250 per cent; manganese, 0.500 to 0.800 per cent; phosphorus, 0.045 maximum per cent; sulphur, 0.060 to 0.090 per cent.
This material in the cold-drawn condition will show: Elastic limit, 50,000 lb. per square inch, elongation in 2 in., 10 per cent, reduction of area, 35 per cent.
This material gave as uniform physical properties as S. A. E. No. 1020 steel and at the same time was sufficiently free cutting to produce a smooth thread and enable the screw-machine manufacturers to produce, to the same thread limits, approximately 75 per cent as many parts as from bessemer screw stock.
There are but seven carbon-steel carbonized parts on the Liberty engine. The most important are the camshaft, the camshaft rocker lever roller and the tappet. The material used for parts of this type was S. A. E. No. 1,020 steel, which is of the following chemical analysis: Carbon 0.150 to 0.250 per cent; manganese, 0.300 to 0.600 per cent; phosphorus, 0.045 maximum per cent; sulphur, 0.050 maximum per cent.
Page 47 The heat treatment consisted in carbonizing at a temperature of from 1,650 to 1,700°F. for a sufficient length of time to secure the proper depth of case, cool slowly or quench; then reheat to a temperature of 1,380 to 1,430°F. to refine the grain of the case, and quench in water. The only thing that should limit the rate of cooling from the carbonizing heat is distortion. Camshaft rocker lever rollers and tappets, as well as gear pins, were quenched directly from the carbonizing heat in water and then case-refined and rehardened by quenching in water from a temperature of from 1,380 to 1,430°F.
The advantage of direct quenching from the carbonizing heat is doubtless one of economy, and in many cases will save the cost of a reheating. Specifications for case hardening, issued by the Society of Automotive Engineers, have lately been revised; whereas they formerly called for a slow cooling, they now permit a quenching from the pot. Doubtless this is a step in advance. Warpage caused by quenching can be reduced to a minimum by thoroughly annealing the stock before any machine work is done on it.
Another advantage obtained from rapid cooling from the carbonizing heat is the retaining of the majority of the excess cementite in solution which produces a less brittle case and by so doing reduces the liability of grinding checks and chipping of the case in actual service.
In the case of the camshaft, it is not possible to quench directly from the carbonizing heat because of distortion and therefore excessive breakage during straightening operations. All Liberty camshafts were cooled slowly from carbonizing heat and hardened by a single reheating to a temperature of from 1,380 to 1,430°F. and quenching in water.
Considerable trouble has always been experienced in obtaining uniform hardness on finished camshafts. This is caused by insufficient water circulation in the quenching tank, which allows the formation of steam pockets to take place, or by decarbonization of the case during heating by the use of an overoxidizing flame. Another cause, which is very often overlooked, is due to the case being ground off one side of cam more than the other and is caused by the roughing master cam being slightly different from the finishing master cam. Great care should be taken to see that this condition does not occur, especially when the depth of case is between 1/32 and 3/64 in.
Low-stressed, carbon-steel forgings include such parts as carbureter control levers, etc. The important criterion for parts of this type is ease of fabrication and freedom from over-heated and burned forgings. The material used for such parts was S. A. E. No. 1,030 steel, which is of the following chemical composition: Carbon, 0.250 to 0.350 per cent; manganese, 0.500 to 0.800 per cent; phosphorus, 0.045 maximum per cent; sulphur, 0.050 maximum per cent.
To obtain good machineability, all forgings produced from this steel were heated to a temperature of from 1,575 to 1,625°F. to refine the grain of the steel thoroughly and quenched in water and then tempered to obtain proper machineability by heating to a temperature of from 1,000 to 1,100°F. and cooled slowly or quenched.
Forgings subjected to this heat treatment are free from hard spots and will show a Brinell hardness of 177 to 217, which is proper for all ordinary machining operations. Great care should be taken not to use steel for parts of this type containing less than 0.25 per cent carbon, because the lower the carbon the greater the liability of hard spots, and the more difficult it becomes to eliminate them. The only satisfactory method so far in commercial use for the elimination of hard spots is to give forgings a very severe quench from a high temperature followed by a proper tempering heat to secure good machine ability as outlined above.
The important carbon-steel forgings consisted of the cylinders, the propeller-hubs, the propeller-hub flange, etc. The material used for parts of this type was S. A. E. No. 1,045 steel, which is of the following chemical composition: Carbon, 0.400 to 0.500 per cent; manganese, 0.500 to 0.800 per cent; phosphorus, 0.045 maximum per cent; sulphur, 0.050 maximum per cent.
All forgings made from this material must show, after heat treatment, the following minimum physical properties: Elastic limit, 70,000; lb. per square inch, elongation in 2 in., 18 per cent, reduction of area, 45; per cent, Brinell hardness, 217 to 255.
To obtain these physical properties, the forgings were quenched in water from a temperature of 1,500 to 1,550°F., followed by tempering to meet proper Brinell requirements by heating to a temperature of 1,150 to 1,200°F. and cooled slowly or Page 49 quenched. No trouble of any kind was ever experienced with parts of this type.
The principal carbon-steel pressed parts used on the Liberty engine were the water jackets and the exhaust manifolds. The material used for parts of this type was S. A. E. No. 1,010 steel, which is of the following chemical composition: Carbon, 0.05 to 0.15 per cent; manganese, 0.30 to 0.60 per cent; phosphorus, 0.045 maximum per cent; sulphur, 0.045 maximum per cent.
No trouble was experienced in the production of any parts from this material with the exception of the water jacket. Due to the particular design of the Liberty cylinder assembly, many failures occurred in the early days, due to the top of the jacket cracking with a brittle fracture. It was found that these failures were caused primarily from the use of jackets which showed small scratches or die marks at this joint and secondarily by improper annealing of the jackets themselves between the different forming operations. By a careful inspection for die marks and by giving the jackets 1,400°F. annealing before the last forming operation, it was possible to completely eliminate the trouble encountered.
The highly stressed parts on the Liberty engine consisted of the connecting-rod bolt, the main-bearing bolt, the propeller-hub key, etc. The material used for parts of this type was selected at the option of the manufacturer from standard S. A. E. steels, the composition of which are given in Table 11.
| Steel No | 2,330 | 3,135 | 6,130 |
| Carbon, minimum | 0.250 | 0.300 | 0.250 |
| Carbon, maximum | 0.350 | 0.400 | 0.450 |
| Manganese, minimum | 0.500 | 0.500 | 0.500 |
| Manganese, maximum | 0.800 | 0.800 | 0.800 |
| Phosphorus, maximum | 0.045 | 0.040 | 0.040 |
| Sulphur, maximum | 0.045 | 0.045 | 0.045 |
| Nickel, minimum | 3.250 | 1.000 | |
| Nickel, maximum | 3.750 | 1.500 | |
| Chromium, minimum | 0.450 | 0.800 | |
| Chromium, maximum | 0.750 | 1.100 | |
| Vanadium, minimum | 0.150 |
Page 50 All highly stressed parts on the Liberty engine must show, after heat treatment, the following minimum physical properties: Elastic limit, 100,000 lb. per square inch; elongation in 2 in., 16 per cent; reduction of area, 45 per cent; scleroscope hardness, 40 to 50.
The heat treatment employed to obtain these physical properties consisted in quenching from a temperature of 1,525 to 1,575°F., in oil, followed by tempering at a temperature of from 925 to 975°F.
Due to the extremely fine limits used on all threaded parts for the Liberty engine, a large percentage of rejection was due to warpage and scaling of parts. To eliminate this objection, many of the Liberty engine builders adopted the use of heat-treated and cold-drawn alloy steel for their highly stressed parts. On all sizes up to and including 3/8 in. in diameter, the physical properties were secured by merely normalizing the hot-rolled bars by heating to a temperature of from 1,525 to 1,575°F., and cooling in air, followed by the usual cold-drawing reductions. For parts requiring stock over 3/8 in. in diameter, the physical properties desired were obtained by quenching and tempering the hot-rolled bars before cold-drawing. It is the opinion that the use of heat-treated and cold-drawn bars is very good practice, provided proper inspection is made to guarantee the uniformity of heat treatment and, therefore, the uniformity of the physical properties of the finished parts.
The question has been asked many times by different manufacturers, as to which alloy steel offers the best machineability when heat-treated to a given Brinell hardness. The general consensus of opinion among the screw-machine manufacturers is that S. A. E. No. 6,130 steel gives the best machineability and that S. A. E. No. 2,330 steel would receive second choice of the three specified.
In the finishing of highly stressed parts for aviation engines, extreme care must be taken to see that all tool marks are eliminated, unless they are parallel to the axis of strain, and that proper radii are maintained at all changes of section. This is of the utmost importance to give proper fatigue resistance to the part in question.
The material used for all gears on the Liberty engine was selected at the option of the manufacturer from the following Page 51 standard S. A. E. steels, the composition of which are given in Table 12,
| Steel No | X-3,340 | 6,140 |
| Carbon, minimum | 0.350 | 0.350 |
| Carbon, maximum | 0.450 | 0.450 |
| Manganese, minimum | 0.450 | 0.500 |
| Manganese, maximum | 0.750 | 0.800 |
| Phosphorus, maximum | 0.040 | 0.040 |
| Sulphur, maximum | 0.045 | 0.045 |
| Nickel, minimum | 2.750 | |
| Nickel, maximum | 3.250 | |
| Chromium, minimum | 0.700 | 0.800 |
| Chromium, maximum | 0.950 | 1.100 |
| Vanadium, minimum | 0.150 |
All gears were heat-treated to a scleroscope hardness of from 55 to 55. The heat treatment used to secure this hardness consisted in quenching the forgings from a temperature of 1,550 to 1,600°F. in oil and annealing for good machineability at a temperature of from 1,300 to 1,350°F. Forgings treated in this manner showed a Brinell hardness of from 177 to 217.
At the option of the manufacturer, the above treatment of gear forgings could be substituted by normalizing the forgings at a temperature of from 1,550 to 1,600°F. The most important criterion for proper normalizing, consisted in allowing the forgings to cool through the critical temperature of the steel, at a rate not to exceed 50°F. per hour. For the two standard steels used, this consisted in cooling from the normalizing temperature down to a temperature of 1,100°F., at the rate indicated. Forgings normalized in this manner will show a Brinell hardness of from 177 to 217. The question has been repeatedly asked as to which treatment will produce the higher quality finished part. In answer to this I will state that on simple forgings of comparatively small section, the normalizing treatment will produce a finished part which is of equal quality to that of the quenched and annealed forgings. However, in the case of complex forgings, or those of large section, more uniform physical properties of the finished part will be obtained by quenching and annealing the forgings in the place of normalizing.
Page 52 The heat treatment of the finished gears consisted of quenching in oil from a temperature of from 1,420 to 1,440°F. for the No. X-3,340 steel, or from a temperature of from 1,500 to 1,540°F. for No. 6,140 steel, followed by tempering in saltpeter or in an electric furnace at a temperature of from 650 to 700°F.
The question has been asked by many engineers, why is the comparatively low scleroscope hardness specified for gears? The reason for this is that at best the life of an aviation engine is short, as compared with that of an automobile, truck or tractor, and that shock resistance is of vital importance. A sclerescope hardness of from 55 to 65 will give sufficient resistance to wear to prevent replacements during the life of an aviation engine, while at the same time this hardness produces approximately 50 per cent greater shock-resisting properties to the gear. In the case of the automobile, truck or tractor, resistance to wear is the main criterion and for that reason the higher hardness is specified.
Great care should be taken in the design of an aviation engine gear to eliminate sharp corners at the bottom of teeth as well as in keyways. Any change of section in any stressed part of an aviation engine must have a radius of at least 1/32 in. to give proper shock and fatigue resistance. This fact has been demonstrated many times during the Liberty engine program.
The material used for all connecting rods on the Liberty engine was selected at the option of the manufacturer from one of two standard S. A. E. steels, the composition of which are given in Table 13.
| Steel No. | X-3,335 | 6,135 |
| Carbon, minimum | 0.300 | 0.300 |
| Carbon, maximum | 0.400 | 0.400 |
| Manganese, minimum | 0.450 | 0.500 |
| Manganese, maximum | 0.750 | 0.800 |
| Phosphorus, maximum | 0.040 | 0.040 |
| Sulphur, maximum | 0.045 | 0.045 |
| Nickel, minimum | 2.750 | |
| Nickel, maximum | 3.250 | |
| Chromium, minimum | 0.700 | 0.800 |
| Chromium, maximum | 0.950 | 1.100 |
| Vanadium minimum | 0.150 |
Page 53 All connecting rods were heat-treated to show the following minimum physical properties; Elastic limit, 105,000 lb. per square inch: elongation in 2 in., 17.5; per cent, reduction of area 50.0; per cent., Brinell hardness, 241 to 277.
The heat treatment used to secure these physical properties consisted in normalizing the forgings at a temperature of from 1,550 to 1,600°F., followed by cooling in the furnace or in air. The forgings were then quenched in oil from a temperature of from 1,420 to 1,440°F. for the No. X-3,335 steel, or from a temperature of from 1,500 to 1,525°F. for No. 6,135 steel, followed by tempering at a temperature of from 1,075 to 1,150°F. At the option of the manufacturer, the normalizing treatment could be substituted by quenching the forgings from a temperature of from 1,550 to 1,600°F., in oil, and annealing for the best machineability at a temperature of from 1,300 to 1,350°F. The double quench, however, did not prove satisfactory on No. X-3,335 steel, due to the fact that it was necessary to remove forgings from the quenching bath while still at a temperature of from 300 to 500°F. to eliminate any possibility of cracking. In view of the fact that this practice is difficult to carry out in the average heat-treating plant, considerable trouble was experienced.
The most important criterion in the production of aviation engine connecting rods is the elimination of burned or severely overheated forgings. Due to the particular design of the forked rod, considerable trouble was experienced in this respect because of the necessity of reheating the forgings before they are completely forged. As a means of elimination of burned forgings, test lugs were forged on the channel section as well as on the top end of fork. After the finish heat treatment, these test lugs were nicked and broken and the fracture of the steel carefully examined. This precaution made it possible to eliminate burned forgings as the test lugs were placed on sections which would be most likely to become burned.
There is a great difference of opinion among engineers as to what physical properties an aviation engine connecting rod should have. Many of the most prominent engineers contend that a connecting rod should be as stiff as possible. To produce rods in this manner in any quantity, it is necessary for the final heat treatment to be made on the semi-machined rod. This practice would make it necessary for a larger percentage of the semi-machined rods to be cold-straightened after the finish heat Page 54 treatment. The cold-straightening operation on a part having important functions to perform as a connecting rod is extremely dangerous.
In view of the fact that a connecting rod functions as a strut, it is considered that this part should be only stiff enough to prevent any whipping action during the running of the engine. The greater the fatigue-resisting property that one can put into the rod after this stiffness is reached, the longer the life of the rod will be. This is the reason for the Brinell limits mentioned being specified.
In connection with the connecting rod, emphasis must be laid on the importance of proper radii at all changes of section. The connecting rods for the first few Liberty engines were machined with sharp corners at the point where the connecting-rod bolt-head fits on assembly. On the first long endurance test of a Liberty engine equipped with rods of this type, failure resulted from fatigue starting at this point. It is interesting to note that every rod on the engine which did not completely fail at this point had started to crack. The adoption of a 1/32-in. radius at this point completely eliminated fatigue failures on Liberty rods.
The crankshaft was the most highly stressed part of the entire Liberty engine, and, therefore, every metallurgical precaution was taken to guarantee the quality of this part. The material used for the greater portion of the Liberty crankshafts produced was nickel-chromium steel of the following chemical composition: Carbon, 0.350 to 0.450 per cent; manganese, 0.300 to 0.600 per cent; phosphorus, 0.040 maximum per cent; sulphur, 0.045 maximum per cent; nickel, 1.750 to 2.250 per cent; chromium, 0.700 to 0.900 per cent.
Each crankshaft was heat-treated to show the following minimum physical properties: Elastic limit, 116,000 lb. per square inch; elongation in 2 in., 16 per cent, reduction of area, 50 per cent, Izod impact, 34 ft.-lb.; Brinell hardness, 266 to 321.
For every increase of 4,000 lb. per square inch in the elastic limit above 116,000 lb. per square inch, the minimum Izod impact required was reduced 1 ft.-lb.
The heat treatment used to produce these physical properties consisted in normalizing the forgings at a temperature of from 1,550 to 1,600°F., followed by quenching in water at a temperature Page 55 of from 1,475 to 1,525°F. and tempering at a temperature of from 1,000 to 1,100°F. It is absolutely necessary that the crankshafts be removed from the quenching tank before being allowed to cool below a temperature of 500°F., and immediately placed in the tempering furnace to eliminate the possibility of quenching cracks.
A prolongation of not less than the diameter of the forging bearing was forged on one end of each crankshaft. This was removed from the shaft after the finish heat treatment, and physical tests were made on test specimens which were cut from it at a point half way between the center and the surface. One tensile test and one impact test were made on each crankshaft, and the results obtained were recorded against the serial number of the shaft in question. This serial number was carried through all machining operations and stamped on the cheek of the finished shaft. In addition to the above tensile and impact tests, at least two Brinell hardness determinations were made on each shaft.
All straightening operations on the Liberty crankshaft which were performed below a temperature of 500°F. were followed by retempering at a temperature of approximately 200°F. below the original tempering temperature.
Another illustration of the importance of proper radii at all changes of section is given in the case of the Liberty crankshaft. The presence of tool marks or under cuts must be completely eliminated from an aviation engine crankshaft to secure proper service. During the duration of the Liberty program, four crankshafts failed from fatigue, failures starting from sharp corners at bottom of propeller-hub keyway. Two of the shafts that failed showed torsional spirals running more than completely around the shaft. As soon as this difficulty was removed no further trouble was experienced.
One of the most important difficulties encountered in connection with the production of Liberty crankshafts was hair-line seams. The question of hair-line seams has been discussed to greater length by engineers and metallurgists during the war than any other single question. Hair-line seams are caused by small non-metallic inclusions in the steel. There is every reason to believe that these inclusions are in the greater majority of cases manganese sulphide. There is a great difference of opinion as to the exact effect of hair-line seams on the service of an aviation Page 56 engine crankshaft. It is the opinion of many that hair-line seams do not in any way affect the endurance of a crankshaft in service, provided they are parallel to the grain of the steel and do not occur on a fillet. Of the 20,000 Liberty engines produced, fully 50 per cent of the crankshafts used contain hair-line seams but not at the locations mentioned. There has never been a failure of a Liberty crankshaft which could in any way be traced to hair-line seams.
It was found that hair-line seams occur generally on high nickel-chromium steels. One of the main reasons why the comparatively mild analysis nickel-chromium steel was used was due to the very few hair-line seams present in it. It was also determined that the hair lines will in general be found near the surface of the forgings. For that reason, as much finish as possible was allowed for machining. A number of tests have been made on forging bars to determine the depths at which hair-line seams are found, and many cases came up in which hair-line seams were found 3/8 in. from the surface of the bar. This means that in case a crankshaft does not show hair-line seams on the ground surface this is no indication that it is free from such a defect.
One important peculiarity of nickel-chromium steel was brought out from the results obtained on impact tests. This peculiarity is known as "blue brittleness." Just what the effect of this is on the service of a finished part depends entirely upon the design of the particular part in question. There have been no failures of any nickel-chromium steel parts in the automotive industry which could in any way be traced to this phenomena.
Whether or not nickel-chromium-steel forgings will show "blue brittleness" depends entirely upon the temperature at which they are tempered and their rate of cooling from this temperature. The danger range for tempering nickel-chromium steels is between a temperature of from 400 to 1,100°F. From the data so far gathered on this phenomena, it is necessary that the nickel-chromium steel to show "blue brittleness" be made by the acid process. There has never come to my attention a single instance in which basic open hearth steel has shown this phenomena. Just why the acid open hearth steel should be sensitive to "blue brittleness" is not known.
All that is necessary to eliminate the presence of "blue brittleness" is to quench all nickel-chromium-steel forgings in water from their tempering temperature. The last 20,000 Liberty crankshafts that were made were quenched in this manner.
The piston pin on an aviation engine must possess maximum resistance to wear and to fatigue. For this reason, the piston pin is considered, from a metallurgical standpoint, the most important part on the engine to produce in quantities and still possess the above characteristics. The material used for the Liberty engine piston pin was S. A. E. No. 2315 steel, which is of the following chemical composition: Carbon, 0.100 to 0.200 per cent; manganese, 0.500 to 0.800 per cent; phosphorus, 0.040 maximum per cent; sulphur, 0.045 maximum per cent; nickel, 3.250 to 3.750 per cent.
Each finished piston pin, after heat treatment, must show a minimum scleroscope hardness of the case of 70, a scleroscope hardness of the core of from 35 to 55 and a minimum crushing strength when supported as a beam and the load applied at the center of 35,000 lb. The heat treatment used to obtain the above physical properties consisted in carburizing at a temperature not to exceed 1,675°F., for a sufficient length of time to secure a case of from 0.02 to 0.04 in. deep. The pins are then allowed to cool slowly from the carbonizing heat, after which the hole is finish-machined and the pin cut to length. The finish heat treatment of the piston pin consisted in quenching in oil from a temperature of from 1,525 to 1,575°F. to refine the grain of core properly and then quenching in oil at a temperature of from 1,340 to 1,380°F. to refine and harden the grain of the case properly, as well as to secure proper hardness of core. After this quenching, all piston pins are tempered in oil at a temperature of from 375 to 400°F. A 100 per cent inspection for scleroscope hardness of the case and the core was made, and no failures were ever recorded when the above material and heat treatment was used.
The information given on the various parts of the Liberty engine applies with equal force to the corresponding parts in the construction of an automobile, truck or tractor. We recommend as first choice for carbon-steel screw-machine parts material produced by the basic open hearth process and having the following chemical composition; Carbon, 0.150 to 0.250 per cent; manganese, 0.500 to 0.800 per cent; phosphorus, 0.045 maximum per cent; sulphur, 0.075 to 0.150 per cent.
Page 58 This material is very uniform and is nearly as free cutting as bessemer screw stock. It is sufficiently uniform to be used for unimportant carburized parts, as well as for non-heat-treated screw-machine parts. A number of the large automobile manufacturers are now specifying this material in preference to the regular bessemer grades.
As second choice for carbon-steel screw-machine parts we recommend ordinary bessemer screw stock, purchased in accordance with S. A. E. specification No. 1114. The advantage of using No. 1114 steel lies in the fact that the majority of warehouses carry standard sizes of this material in stock at all times. The disadvantage of using this material is due to its lack of uniformity.
The important criterion for transmission gears is resistance to wear. To secure proper resistance to wear a Brinell hardness of from 512 to 560 must be obtained. The material selected to obtain this hardness should be one which can be made most nearly uniform, will undergo forging operations the easiest, will be the hardest to overheat or burn, will machine best and will respond to a good commercial range of heat treatment.
It is a well-known fact that the element chromium, when in the form of chromium carbide in alloy steel, offers the greatest resistance to wear of any combination yet developed. It is also a well-known fact that the element nickel in steel gives excellent shock-resisting properties as well as resistance to wear but not nearly as great a resistance to wear as chromium. It has been standard practice for a number of years for many manufacturers to use a high nickel-chromium steel for transmission gears. A typical nickel-chromium gear specification is as follows: Carbon, 0.470 to 0.520 per cent; manganese, 0.500 to 0.800 per cent; phosphorus, 0.040 maximum per cent; sulphur, 0.045 maximum per cent; chromium, 0.700 to 0.950 per cent.
There is no question but that a gear made from material of such an analysis will give excellent service. However, it is possible to obtain the same quality of service and at the same time appreciably reduce the cost of the finished part. The gear steel specified is of the air-hardening type. It is extremely sensitive to secondary pipe, as well as seams, and is extremely difficult to forge and very easy to overheat. The heat-treatment range is very wide, but the danger from quenching cracks is very great. In regard to the machineability, this material is the hardest to machine of any alloy steel known.
If the nickel content of this steel is eliminated, and the percentage of chromium raised slightly, an ideal transmission-gear material is obtained. This would, therefore, be of the following composition: Carbon, 0.470 to 0.520 per cent; manganese, 0.500 to 0.800 per cent; phosphorus, 0.040 maximum per cent; sulphur, 0.045 maximum per cent; chromium, 0.800 to 1.100 per cent.
The important criterion in connection with the use of this material is that the steel be properly deoxidized, either through the use of ferrovanadium or its equivalent. Approximately 2,500 sets of transmission gears are being made daily from material of this analysis and are giving entirely satisfactory results in service. The heat treatment of the above material for transmission gears is as follows: "Normalize forgings at a temperature of from 1,5.50 to 1,600°F. Cool from this temperature to a temperature of 1,100°F. at the rate of 50° per hour. Cool from 1,100°F., either in air or quench in water."
Forgings so treated will show a Brinell hardness of from 177 to 217, which is the proper range for the best machineability. The heat treatment of the finished gears consists of quenching in oil from a temperature of 1,500 to 1,540°F., followed by tempering in oil at a temperature of from 375 to 425°F. Gears so treated will show a Brinell hardness of from 512 to 560, or a scleroscope hardness of from 72 to 80. One tractor builder has placed in service 20,000 sets of gears of this type of material and has never had to replace a gear. Taking into consideration the fact that a tractor transmission is subjected to the worst possible service conditions, and that it is under high stress 90 per cent of the time, it seems inconceivable that any appreciable transmission trouble would be experienced when material of this type is used on an automobile, where the full load is applied not over 1 per cent of the time, or on trucks where the full load is applied not over 50 per cent of the time.
The gear hardness specified is necessary to reduce to a minimum the pitting or surface fatigue of the teeth. If gears having a Brinell hardness of over 560 are used, danger is encountered, due to low shock-resisting properties. If the Brinell hardness is under 512, trouble is experienced due to wear and surface fatigue of the teeth.
For ring gears and pinions material of the following chemical composition is recommended: Carbon, 0.100 to 0.200 per cent; Page 60 manganese, 0.350 to 0.650 per cent; phosphorus, 0.040 maximum per cent; sulphur, 0.045 maximum per cent; chromium, 0.550 to 0.750 per cent; nickel, 0.400 to 0.600 per cent.
Care should be taken to see that this material is properly deoxidized either by the use of ferrovanadium or its equivalent. The advantage of using a material of the above type lies in the fact that it will produce a satisfactory finished part with a very simple treatment. The heat treatment of ring gears and pinions is as follows: "Carburize at a temperature of from 1,650 to 1,700°F. for a sufficient length of time to secure a depth of case of from 1/32 to 3/64 in., and quench directly from carburizing heat in oil. Reheat to a temperature of from 1,430 to 1,460°F. and quench in oil. Temper in oil at a temperature of from 375 to 425°F. The final quenching operation on a ring gear should be made on a fixture similar to the Gleason press to reduce distortion to a minimum."
One of the largest producers of ring gears and pinions in the automotive industry has been using this material and treatment for the last 2 years, and is of the opinion that he is now producing the highest quality product ever turned out by that plant.
On some designs of automobiles a large amount of trouble is experienced with the driving pinion. If the material and heat treatment specified will not give satisfaction, rather than to change the design it is possible to use the following analysis material, which will raise the cost of the finished part but will give excellent service: Carbon, 0.100 to 0.200 per cent; manganese, 0.350 to 0.650 per cent; phosphorus, 0.040 maximum per cent; sulphur, 0.045 maximum per cent; nickel, 4.750 to 5.250 per cent.
The heat treatment of pinions produced from this material consists in carburizing at a temperature of from 1,600 to 1,650°F. for a sufficient length of time to secure a depth of case from 1/32 to 3/64 in. The pinions are then quenched in oil from a temperature of 1,500 to 1,525°F. to refine the grain of the core and quenched in oil from a temperature of from 1,340 to 1,360°F. To refine and harden the case. The use of this material however, is recommended only in an emergency, as high-nickel steel is very susceptible to seams, secondary pipe and laminations.
The main criterion on rear-axle and pinion shafts, steering knuckles and arms and parts of this general type is resistance to fatigue and torsion. The material recommended for parts of Page 61 this character is either S. A. E. No. 6135 or No. 3135 steel, which have the chemical composition given in Tables 9 and 7.
Parts of this general type should be heat-treated to show the following minimum physical properties: Elastic limit, 115,000 lb. per square inch; elongation in 2 in., 16 per cent; reduction of area, 50 per cent; Brinell hardness, 277 to 321.
The heat treatment used to secure these physical properties consists in quenching from a temperature of from 1,520 to 1,540°F. in water and tempering at a temperature of from 975 to 1,025°F. Where the axle shaft is a forging, and in the case of steering knuckles and arms, this heat treatment should be preceded by normalizing the forgings at a temperature of from 1,550 to 1,600°F. It will be noted that these physical properties correspond to those worked out for an ideal aviation engine crankshaft. If parts of this type are designed with proper sections, so that this range of physical properties can be used, the part in question will give maximum service.
One of the most important developments during the Liberty engine program was the fact that it is not necessary to use a high-analysis alloy steel to secure a finished part which will give proper service. This fact should save the automotive industry millions of dollars on future production.
If the proper authority be given the metallurgical engineer to govern the handling of the steel from the time it is purchased until it is assembled into finished product, mild-analysis steels can be used and the quality of the finished product guaranteed. It was only through the careful adherence to these fundamental principles that it was possible to produce 20,000 Liberty engines, which are considered to be the most highly stressed mechanism ever produced, without the failure of a single engine from defective material or heat treatment.
Steel balls are made from rods or coils according to size, stock less than 9/16-in. comes in coils. Stock 5/8-in. and larger comes in rods. Ball stock is designated in thousandths so that 5/8-in. rods are known as 0.625-in. stock.
Page 62 Steel for making balls of average size is made up of:
| Carbon | 0.95 to 1.05 per cent |
| Silicon | 0.20 to 0.35 per cent |
| Manganese | 0.30 to 0.45 per cent |
| Chromium | 0.35 to 0.45 per cent |
| Sulphur and phosphorus not to exceed | 0.025 per cent |
For the larger sizes a typical analysis is:
| Carbon | 1.02 per cent |
| Silicon | 0.21 per cent |
| Manganese | 0.40 per cent |
| Chromium | 0.65 per cent |
| Sulphur | 0.026 per cent |
| Phosphorus | 0.014 per cent |
Balls 5/8 in. and below are formed cold on upsetting or heading machines, the stock use is as follows:
| Diameter of ball, inch |
Diameter of stock inch |
Diameter of ball, inch |
Diameter of stock inch |
|---|---|---|---|
| 1/8 | 0.100 | 5/16 | 0.235 |
| 5/32 | 0.120 | 3/8 | 0.275 |
| 3/16 | 0.145 | 7/16 | 0.320 |
| 7/32 | 0.170 | 1/2 | 0.365 |
| 1/4 | 0.190 | 9/16 | 0.395 |
| 9/32 | 0.220 | 5/8 | 0.440 |
For larger balls the blanks are hot-forged from straight bars. They are usually forged in multiples of four under a spring hammer and then separated by a suitable punching or shearing die in a press adjoining the hammer. The dimensions are:
| Diameter of ball, inch |
Diameter of die, inch |
Diameter of stock, inch |
|---|---|---|
| 3/4 | 0.775 | 0.625 |
| 7/8 | 0.905 | 0.729 |
| 1 | 1.035 | 0.823 |
Page 63 Before hardening, the balls are annealed to relieve the stresses of forging and grinding, this being done by passing them through a revolving retort made of nichrome or other heat-resisting substance. The annealing temperature is 1,300°F.
The hardening temperature is from 1,425 to 1,475°F. according to size and composition of steel. Small balls, 5/16 and under, are quenched in oil, the larger sizes in water. In some special cases brine is used. Quenching small balls in water is too great a shock as the small volume is cooled clear through almost instantly. The larger balls have metal enough to cool more slowly.
Balls which are cooled in either water or brine are boiled in water for 2 hr. to relieve internal stresses, after which the balls are finished by dry-grinding and oil-grinding.
The ball makers have an interesting method of testing stock for seams which do not show in the rod or wire. The Hoover Steel Ball Company cut off pieces of rod or wire 7/16 in. long and subject them to an end pressure of from 20,000 to 50,000 lb. A pressure of 20,000 lb. compresses the piece to 3/16 in. and the 50,000 lb. pressure to 3/32 in. This opens any seam which may exist but a solid bar shows no seam.
Another method which has proved very successful is to pass the bar or rod to be tested through a solenoid electro-magnet. With suitable instruments it is claimed that this is an almost infallible test as the instruments show at once when a seam or flaw is present in the bar.
THE FORGING OF STEEL
So much depends upon the forging of steel that this operation must be carefully supervised. This is especially true because of the tendency to place unskilled and ignorant men as furnace-tenders and hammer men. The main points to be supervised are the slow and careful heating to the proper temperature; forging must be continued at a proper rate to the correct temperature. The bar of stock from which a forging was made may have had a fairly good structure, but if the details of the working are not carefully watched, a seamy, split article of no value may easily result.
Heating.—Although it is possible to work steels cold, to an extent depending upon their ductility, and although such operations are commonly performed, "forging" usually means working heated steel. Heating is therefore a vital part of the process.
Heating should be done slowly in a soaking heat. A soft "lazy" flame with excess carbon is necessary to avoid burning the corners of the bar or billet, and heavily scaling the surface. If the temperature is not raised slowly, the outer part of the metal may be at welding heat while the inner part is several hundred degrees colder and comparatively hard and brittle.
The above refers to muffle furnaces. If the heating is done in a small blacksmith's forge, the fire should be kept clean, and remade at intervals of about two hours. Ashes and cinders should be cleaned from the center down to the tuyere and oily waste and wood used to start a new fire. As this kindles a layer of coke from the old fire is put on top, and another layer of green coal (screened and dampened blacksmiths' coal) as a cover. When the green coal on top has been coked the fire is ready for use. As the fuel burns out in the center, the coke forming around the edge is pushed inward, and its place taken by more green coal. Thus the fire is made up of three parts; the center where coke is burning and the iron heating; a zone where coke is forming, and the outside bank of green coal.
Steel Worked in Austenitic State.—As a general rule steel should be worked when it is in the austenitic state. (See page 108.) It is then soft and ductile.
Page 65 As the steel is heated above the critical temperature the size of the austenite crystals tends to grow rapidly. When forging starts, however, these grains are broken up. The growth is continually destroyed by the hammering, which should consequently be continued down to the upper critical temperature when the austenite crystals break up into ferrite and cementite. The size of the final grains will be much smaller and hence a more uniform structure will result if the "mother" austenite was also fine grained. A final steel will be composed of pearlite; ferrite and pearlite; or cementite and pearlite, according to the carbon content.
The ultimate object is to secure a fine, uniform grain throughout the piece and this can be secured by uniform heating and by thoroughly rolling it or working it at a temperature just down to its critical point. If this is correctly done the fracture will be fine and silky. Steel which has been overheated slightly and the forging stopped at too high a temperature will show a "granular" fracture. A badly overheated or "burned" steel will have iridescent colors on a fresh fracture, it will be brittle both hot and cold, and absolutely ruined.
Steel Can be Worked Cold.—As noted above, steel can be worked cold, as in the case of cold-rolled steel. Heat treatment of cold-worked steel is a very delicate operation. Cold working hardens and strengthens steel. It also introduces internal stresses. Heat-treatments are designed to eliminate the stresses without losing the hardness and strength. This is done by tempering at a low heat. Avoid the "blue" range (350 to 750°C.). Tempering for a considerable time just under the critical is liable to cause great brittleness. Annealing (reheating through the critical) destroys the effect of cold work.
High-speed Steel.—Heat very slowly and carefully to from 1,800 to 2,000°F. and forge thoroughly and uniformly. If the forging operation is prolonged do not continue forging the tool when the steel begins to stiffen under the hammer. Do not forge below 1,700°F. (a dark lemon or orange color). Reheat frequently rather than prolong the hammering at the low heats.
After finishing the forging allow the tool to cool as slowly as possible in lime or dry ashes; avoid placing the tool on the damp ground or in a draught of air. Use a good clean fire for heating. Do not allow the tool to soak at the forging heat. Do not heat Page 66 any more of the tool than is necessary in order to forge it to the desired shape.
Carbon Tool Steel.—Heat to a bright red, about 1,500 to 1,550°F. Do not hammer steel when it cools down to a dark cherry red, or just below its hardening point, as this creates surface cracks.
Oil-hardening Steel.—Heat slowly and uniformly to 1,450°F. and forge thoroughly. Do not under any circumstances attempt to harden at the forging heat. After cooling from forging reheat to about 1,450°F. and cool slowly so as to remove forging strains.
Chrome-nickel Steel.—Forging heat of chrome-nickel steel depends very largely on the percentage of each element contained in the steel. Steel containing from 1/2 to 1 per cent chromium and from 1½ to 3½ per cent nickel, with a carbon content equal to the chromium, should be heated very slowly and uniformly to approximately 1,600° F., or salmon color. After forging, reheat the steel to about 1,450° and cool slowly so as to remove forging strains. Do not attempt to harden the steel before such annealing.
A great deal of steel is constantly being spoiled by carelessness in the forging operation. The billets may be perfectly sound, but even if the steel is heated to a good forging heat, and is hammered too lightly, a poor forging results. A proper blow will cause the edges and ends to bulge slightly outwards—the inner-most parts of the steel seem to flow faster than the surface. Light blows will work the surface out faster; the edges and ends will curve inwards. This condition in extreme cases leaves a seam in the axis of the forging.
Steel which is heated quickly and forging begun before uniform heat has penetrated to its center will open up seams because the cooler central portion is not able to flow with the hot metal surrounding it. Uniform heating is absolutely necessary for the best results.
Figure 16 shows a sound forging. The bars in Fig. 17 were burst by improper forging, while the die, Fig. 18, burst from a piped center.
Figure 19 shows a piece forged with a hammer too light for the size of the work. This gives an appearance similar to case-hardening, the refining effect of the blows reaching but a short distance from the surface.
While it is impossible to accurately rate the capacity of steam hammers with respect to the size of work they should handle, on account of the greatly varying conditions, a few notes from the Page 67 experience of the Bement works of the Niles-Bement-Pond Company will be of service.
 FIG. 16.—A sound forging.
FIG. 16.—A sound forging.
 FIG. 17.—Burst from improper forging.
FIG. 17.—Burst from improper forging.
For making an occasional forging of a given size, a smaller hammer may be used than if we are manufacturing this same piece in large quantities. If we have a 6-in. piece to forge, such as a pinion or a short shaft, a hammer of about 1,100-lb. capacity would answer very nicely. But should the general work be as Page 68 large as this, it would be very much better to use a 1,500-lb. hammer. If, on the other hand, we wish to forge 6-in. axles economically, it would be necessary to use a 7,000- or 8,000-lb. hammer. The following table will be found convenient for reference for the proper size of hammer to be used on different classes of general blacksmith work, although it will be understood that it is necessary to modify these to suit conditions, as has already been indicated.
 FIG. 18.—Burst from a piped center.
FIG. 18.—Burst from a piped center.
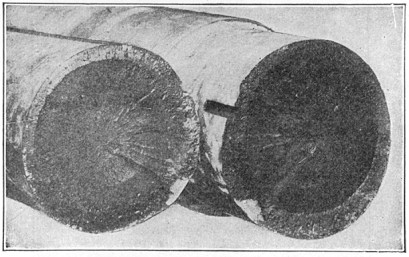 FIG. 19.—Result of using too light a hammer.
FIG. 19.—Result of using too light a hammer.
| Diameter of stock | Size of hammer Page 69 | |||
|---|---|---|---|---|
| 3½ | in. | 250 to | 350 lb. | |
| 4 | in. | 350 to | 600 lb. | |
| 4½ | in. | 600 to | 800 lb. | |
| 5 | in. | 800 to | 1,000 lb. | |
| 6 | in. | 1,100 to | 1,500 lb. | |
Steam hammers are always rated by the weight of the ram, and the attached parts, which include the piston and rod, nothing being added on account of the steam pressure behind the piston. This makes it a little difficult to compare them with plain drop or tilting hammers, which are also rated in the same way.
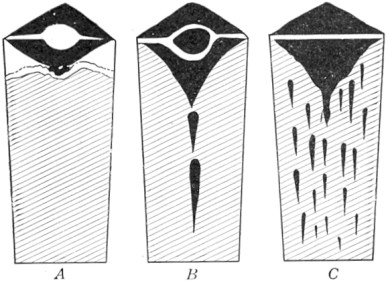 FIG. 20.—Good and bad ingots.
FIG. 20.—Good and bad ingots.
Steam hammers are usually operated at pressures varying from 75 to 100 lb. of steam per square inch, and may also be operated by compressed air at about the same pressures. It is cheaper, however, in the case of compressed air to use pressures from 60 to 80 lb. instead of going higher.
Forgings must, however, be made from sound billets if satisfactory results are to be secured. Figure 20 shows three cross-sections of which A is sound, B is badly piped and C is worthless.
The forging of rifle barrels in large quantities and heat-treating them to meet the specifications demanded by some of the foreign governments led Wheelock, Lovejoy & Company to establish a complete plant for this purpose in connection with their warehouse in Cambridge, Mass. This plant, designed and constructed Page 71 by their chief engineer, K. A. Juthe, had many interesting features. Many features of this plant can be modified for other classes of work.
 FIG. 21.—Cutting up barrels.
FIG. 21.—Cutting up barrels.
 FIG. 22.—Upsetting the ends.
FIG. 22.—Upsetting the ends.
The stock, which came in bars of mill length, was cut off so as to make a barrel with the proper allowances for trimming (Fig. 21). They then pass to the forging or upsetting press in the adjoining room. This press, which is shown in more detail in Fig. 22, handled the barrels from all the heating furnaces shown. The men changed work at frequent intervals, to avoid excessive fatigue.
 FIG. 23.—Continuous heating furnace.
FIG. 23.—Continuous heating furnace.
Then the barrels were reheated in the continuous furnace, shown in Fig. 23, and straightened before being tested.
The barrels were next tested for straightness. After the heat-treating, the ends are ground, a spot ground on the enlarged end and each barrel tested on a Brinell machine. The pressure used is 3,000 kg., or 6,614 lb., on a 10-millimeter ball, which is standard. Hardness of 240 was desired.
The heat-treating of the rifle blanks covered four separate operations: (1) Heating and soaking the steel above the critical temperature and quenching in oil to harden the steel through to the center; (2) reheating for drawing of temper for the purpose Page 72 of meeting the physical specifications; (3) reheating to meet the machine ability test for production purposes; and (4) reheating to straighten the blanks while hot.
A short explanation of the necessity for the many heats may be interesting. For the first heat, the blanks were slowly brought to the required heat, which is about 150°F. above the critical temperature. They are then soaked at a high heat for about 1 hr. before quenching. The purpose of this treatment is to eliminate any rolling or heat stresses that might be in the bars from mill operations; also to insure a thorough even heat through a cross-section of the steel. This heat also causes blanks with seams or slight flaws to open up in quenching, making detection of defective blanks very easy.
The quenching oil was kept at a constant temperature of 100°F., to avoid subjecting the steel to shocks, thereby causing surface cracks. The drawing of temper was the most critical operation and was kept within a 10° fluctuation. The degree of heat necessary depends entirely on the analysis of the steel, there being a certain variation in the different heats of steel as received from the mill.
Reheating for machine ability was done at 100° less than the drawing temperature, but the time of soaking is more than double. After both drawing and reheating, the blanks were buried in lime where they remain, out of contact with the air, until their temperature had dropped to that of the workroom.
For straightening, the barrels were heated to from 900 to 1,000°F. in an automatic furnace 25 ft. long, this operation taking about 2 hr. The purpose of hot straightening was to prevent any stresses being put into the blanks, so that after rough-turning, drilling or rifling operations they would not have a tendency to spring back to shape as left by the quenching bath.
A method that produces an even better machining rifle blank, which practically stays straight through the different machining operations, was to rough-turn the blanks, then subject them to a heat of practically 1,0000 for 4 hr. Production throughout the different operations is materially increased, with practically no straightening required after drilling, reaming, finish-turning or rifling operations.
 FIG. 24.
FIG. 24.
 FIG. 25.
FIG. 25.
FIGS. 24 and 25.—Roof system of cooling quenching oil.
Page 74 This method was tested out by one of the largest manufacturers and proved to be the best way to eliminate a very expensive finished gun-barrel straightening process.
 FIG. 26.—Details of the cooler.
FIG. 26.—Details of the cooler.
The heat-treating required a large amount of cooling oil, and the problem of keeping this at the proper temperature required considerable study. The result was the cooling plant on the roof, as shown in Figs. 24, 25 and 26. The first two illustrations show the plant as it appeared complete. Figure 26 shows how the oil was handled in what is sometimes called the ebulator system. The oil was pumped up from the cooling tanks through the pipe A to the tank B. From here it ran down onto the breakers or separators C, which break the oil up into fine particles that are caught by the fans D. The spray is blown up into the cooling tower E, which contains banks of cooling pipes, as can be seen, as well as baffies F. The spray collects on the cool pipes and forms drops, which fall on the curved plates G and run back to the oil-storage tank below ground.
The water for this cooling was pumped from 10 artesian wells at the rate of 60 gal. per minute and cooled 90 gal. of oil per minute, lowering the temperature from 130 or 140 to 100°F. The water as it came from the wells averaged around 52°F. The motor was of a 7½-hp. variable-speed type with a range of from 700 to 1,200 r.p.m., which could be varied to suit the amount of oil to be cooled. The plant handled 300 gal. of oil per minute.
ANNEALING
There is no mystery or secret about the proper annealing of different steels, but in order to secure the best results it is absolutely necessary for the operator to know the kind of steel which is to be annealed. The annealing of steel is primarily done for one of three specific purposes: To soften for machining purposes; to change the physical properties, largely to increase ductility; or to release strains caused by rolling or forging.
Proper annealing means the heating of the steel slowly and uniformly to the right temperature, the holding of the temperature for a given period and the gradual cooling to normal temperature. The proper temperature depends on the kind of steel, and the suggestions of the maker of the special steel being used should be carefully followed. For carbon steel the temperatures recommended for annealing vary from 1,450 to 1,600°F. This temperature need not be long continued. The steel should be cooled in hot sand, lime or ashes. If heated in the open forge the steel should be buried in the cooling material as quickly as possible, not allowing it to remain in the open air any longer than absolutely necessary. Best results, however, are secured when the fire does not come in direct contact with the steel.
Good results are obtained by packing the steel in iron boxes or tubes, much as for case-hardening or carbonizing, using the same materials. Pieces do not require to be entirely surrounded by carbon for annealing, however. Do not remove from boxes until cold.
Steel to be annealed may be classified into four different groups, each of which must be treated according to the elements contained in its particular analysis. Different methods are therefore necessary to bring about the desired result. The classifications are as follows: High-speed steel, alloy steel, tool or crucible steel, and high-carbon machinery steel.
For annealing high-speed steel, some makers recommend using ground mica, charcoal, lime, fine dry ashes or lake sand as a packing in the annealing boxes. Mixtures of one part charcoal, Page 76 one part lime and three parts of sand are also suggested, or two parts of ashes may be substituted for the one part of lime.
To bring about the softest structure or machine ability of high-speed steel, it should be packed in charcoal in boxes or pipes, carefully sealed at all points, so that no gases will escape or air be admitted. It should be heated slowly to not less than 1,450°F. and the steel must not be removed from its packing until it is cool. Slow heating means that the high heat must have penetrated to the very core of the steel.
When the steel is heated clear through it has been in the furnace long enough. If the steel can remain in the furnace and cool down with it, there will be no danger of air blasts or sudden or uneven cooling. If not, remove the box and cover quickly with dry ashes, sand or lime until it becomes cold.
Too high a heat or maintaining the heat for too long a period, produces a harsh, coarse grain and greatly increases the liability to crack in hardening. It also reduces the strength and toughness of the steel.
Steel which is to be used for making tools with teeth, such as taps, reamers and milling cutters, should not be annealed too much. When the steel is too soft it is more apt to tear in cutting and makes it more difficult to cut a smooth thread or other surface. Moderate annealing is found best for tools of this kind.
Crucible steel can be annealed either in muffled furnace or by being packed. Packing is by far the most satisfactory method as it prevents scaling, local hard spots, uneven annealing, or violent changes in shape. It should be brought up slowly to just above its calescent or hardening temperature. The operator must know before setting his heats the temperature at which the different carbon content steels are hardened. The higher the carbon contents the lower is the hardening heat, but this should in no case be less than 1,450°F.
The term alloy steel, from the steel maker's point of view, refers largely to nickel and chromium steel or a combination of both. These steels are manufactured very largely by the open-hearth process, although chromium steels are also a crucible product. It is next to impossible to give proper directions for the proper Page 77 annealing of alloy steel unless the composition is known to the operator.
Nickel steels may be annealed at lower temperatures than carbon steels, depending upon their alloy content. For instance, if a pearlitic carbon steel may be annealed at 1,450°C., the same analysis containing 2½ per cent nickel may be annealed at 1,360°C. and a 5 per cent nickel steel at 1,270°.
In order that high chromium steels may be readily machined, they must be heated at or slightly above the critical for a very long time, and cooled through the critical at an extremely slow rate. For a steel containing 0.9 to 1.1 per cent carbon, under 0.50 per cent manganese, and about 1.0 per cent chromium, Bullens recommends the following anneal:
|
The carbon content of this steel is above 30 points and is hardly ever above 60 points or 0.60 per cent. Annealing such steel is generally in quantity production and does not require the care that the other steels need because it is very largely a much cheaper product and a great deal of material is generally removed from the outside surface.
The purpose for which this steel is annealed is a deciding factor as to what heat to give it. If it is for machineability only, the steel requires to be brought up slowly to just below the critical and then slowly cooled in the furnace or ash pit. It must be thoroughly covered so that there will be no access of cool air. If the annealing is to increase ductility to the maximum extent it should be slowly heated to slightly over the upper critical temperature and kept at this heat for a length of time necessary for a thorough penetration to the core, after which it can be cooled to about 1,200°F., then reheated to about 1,360°F., when it can be removed and put in an ash pit or covered with lime. If the annealing is just to relieve strains, slow heating is not necessary, but the steel must be brought up to a temperature not much less than a forging or rolling heat and gradually cooled. Covering in this case is only necessary in steel of a carbon content of more than 40 points.
Steel and cast iron may both be annealed in granulated bone. Pack the work the same as for case-hardening except that it is not necessary to keep the pieces away from each other. Pack with bone that has been used until it is nearly white. Heat as hot as necessary for the steel and let the furnace cool down. If the boxes are removed from furnace while still warm, cover boxes and all in warm ashes or sand, air slaked lime or old, burned bone to retain heat as long as possible. Do not remove work from boxes until cold.
In general, all forgings of the components of the arms manufactured at the Armory and all forgings for other ordnance establishments are packed in charcoal, lime or suitable material and annealed before being transferred from the forge shop.
Except in special cases, all annealing will be done in annealing pots of appropriate size. One fire end of a thermo-couple is inserted in the center of the annealing pot nearest the middle of the furnace and another in the furnace outside of but near the annealing pots.
The temperatures used in annealing carbon steel components of the various classes used at the Armory vary from 800°C. To 880°C. or 1,475 to 1,615°F.
The fuel is shut off from the annealing furnace gradually as the temperature of the pot approaches the prescribed annealing temperature so as to prevent heating beyond that temperature.
The forgings of the rifle barrel and the pistol barrel are exceptions to the above general rule. These forgings will be packed in lime and allowed to cool slowly from the residual heat after forging.
CASE-HARDENING OR SURFACE-CARBURIZING
Carburizing, commonly called case-hardening, is the art of producing a high-carbon surface, or case, upon a low carbon steel article. Wrenches, locomotive link motions, gun mechanisms, balls and ball races, automobile gears and many other devices are thereby given a high-carbon case capable of assuming extreme hardness, while the interior body of metal, the core, remains soft and tough.
The simplest method is to heat the piece to be hardened to a bright red, dip it in cyanide of potassium (or cover it by sprinkling the cyanide over it), keep it hot until the melted cyanide covers it thoroughly, and quench in water. Carbon and nitrogen enter the outer skin of the steel and harden this skin but leave the center soft. The hard surface or "case" varies in thickness according to the size of the piece, the materials used and the length of time which the piece remains at the carburizing temperature. Cyanide case-hardening is used only where a light or thin skin is sufficient. It gives a thickness of about 0.002 in.
In some cases of cyanide carburizing, the piece is heated in cyanide to the desired temperature and then quenched. For a thicker case the steel is packed in carbon materials of various kinds such as burnt leather scraps, charcoal, granulated bone or some of the many carbonizing compounds.
Machined or forged steel parts are packed with case-hardening material in metal boxes and subjected to a red heat. Under such conditions, carbon is absorbed by the steel surfaces, and a carburized case is produced capable of responding to ordinary hardening and tempering operations, the core meanwhile retaining its original softness and toughness.
Such case-hardened parts are stronger, cheaper, and more serviceable than similar parts made of tool steel. The tough core resists breakage by shock. The hardened case resists wear from friction. The low cost of material, the ease of manufacture, and the lessened breakage in quenching all serve to promote cheap production.
Page 80 For successful carburizing, the following points should be carefully observed:
The utmost care should be used in the selection of pots for carburizing; they should be as free as possible from both scaling and warping. These two requirements eliminate the cast iron pot, although many are used, thus leaving us to select from malleable castings, wrought iron, cast steel, and special alloys, such as nichrome or silchrome. If first cost is not important, it will prove cheaper in the end to use pots of some special alloy.
 FIGS. 27 to 30.—Case-hardening or carburizing boxes.
FIGS. 27 to 30.—Case-hardening or carburizing boxes.
 FIG. 31.—A lid that is easily luted.
FIG. 31.—A lid that is easily luted.
The pots should be standardized to suit the product. Pots should be made as small as possible in width, and space gained by increasing the height; for it takes about 1½ hr. to heat the Page 81 average small pot of 4 in. in width, between 3 and 4 hr. to heat to the center of an 8-in. box, and 5 to 6 hr. to heat to the center of a 12-in. box; and the longer the time required to heat to the center, the more uneven the carburizing.
The work is packed in the box surrounded by materials which will give up carbon when heated. It must be packed so that each piece is separate from the others and does not touch the box, with a sufficient amount of carburizing material surrounding each. Figures 27 to 31 show the kind of boxes used and the way the work should be packed. Figure 31 shows a later type of box in which the edges can be easily luted. Figure 30 shows test wires broken periodically to determine the depth of case. Figure 28 shows the minimum clearance which should be used in packing and Fig. 29 the way in which the outer pieces receive the heat first and likewise take up the carbon before those in the center. This is why a slow, soaking heat is necessary in handling large quantities of work, so as to allow the heat and carbon to soak in equally.
While it has been claimed that iron below its critical temperature will absorb some carbon, Giolitti has shown that this absorption is very slow. In order to produce quick and intense carburization the iron should preferably be above its upper critical temperature or 1,600°F.,—therefore the carbon absorbed immediately goes into austenite, or solid solution. It is also certain that the higher the temperature the quicker will carbon be absorbed, and the deeper it will penetrate into the steel, that is, the deeper the "case." At Sheffield, England, where wrought iron is packed in charcoal and heated for days to convert it into "blister steel," the temperatures are from 1,750 to 1,830°F. Charcoal by itself carburizes slowly, consequently commercial compounds also contain certain "energizers" which give rapid penetration at lower temperatures.
The most important thing in carburizing is the human element. Most careful vigilance should be kept when packing and unpacking, and the operator should be instructed in the necessity for clean compound free from scale, moisture, fire clay, sand, floor sweepings, etc. From just such causes, many a good carburizer has been unjustly condemned. It is essential with most carburizers to use about 25 to 50 per cent of used material, in order to prevent undue shrinking during heating; therefore the necessity of properly screening used material and carefully inspecting it for foreign substances before it is used again. It is right here that the greatest carelessness is generally encountered.
Page 82 Don't pack the work to be carburized too closely; leave at least 1 in. from the bottom, ¾ in. from the sides, and 1 in. from the top of pots, and for a 6-hr. run, have the pieces at least 1/2 in. apart. This gives the heat a chance to thoroughly permeate the pot, and the carburizing material a chance to shrink without allowing carburized pieces to touch and cause soft spots.
Good case-hardening pots and annealing tubes can be made from the desired size of wrought iron pipe. The ends are capped or welded, and a slot is cut in the side of the pot, equal to one quarter of its circumference, and about 7/8 of its length. Another piece of the same diameter pipe cut lengthwise into thirds forms a cover for this pot. We then have a cheap, substantial pot, non-warping, with a minimum tendency to scale, but the pot is difficult to seal tightly. This idea is especially adaptable when long, narrow pots are desired.
When pots are packed and the carburizer thoroughly tamped down, the covers of the pot are put on and sealed with fire clay which has a little salt mixed into it. The more perfect the seal the more we can get out of the carburizer. The rates of penetration depend on temperature and the presence of proper gas in the required volume. Any pressure we can cause will, of course, have a tendency to increase the rate of penetration.
If you have a wide furnace, do not load it full at one time. Put one-half your load in first, in the center of the furnace, and heat until pots show a low red, about 1,325 to 1,350°F. Then fill the furnace by putting the cold pots on the outside or, the section nearest the source of heat. This will give the work in the slowest portion of the furnace a chance to come to heat at the same time as the pots that are nearest the sources of heat.
To obtain an even heating of the pots and lessen their tendency to warp and scale, and to cause the contents of the furnace to heat up evenly, we should use a reducing fire and fill the heating chamber with flame. This can be accomplished by partially closing the waste gas vents and reducing slightly the amount of air used by the burners. A short flame will then be noticed issuing from the partially closed vents. Thus, while maintaining the temperature of the heating chamber, we will have a lower temperature in the combustion chamber, which will naturally increase its longevity.
Sometimes it is advisable to cool the work in the pots. This saves compound, and causes a more gradual diffusion of the carbon Page 83 between the case and the core, and is very desirable condition, inasmuch as abrupt cases are inclined to chip out.
The most satisfactory steel to carburize contains between 0.10 and 0.20 per cent carbon, less than 0.35 per cent manganese, less than 0.04 per cent phosphorus and sulphur, and low silicon. But steel of this composition does not seem to satisfy our progressive engineers, and many alloy steels are now on the market, these, although more or less difficult to machine, give when carburized the various qualities demanded, such as a very hard case, very tough core, or very hard case and tough core. However, the additional elements also have a great effect both on the rate of penetration during the carburizing operation, and on the final treatment, consequently such alloy steels require very careful supervision during the entire heat treating operations.
According to Guillet, the absorption of carbon is favored by those special elements which exist as double carbides in steel. For example, manganese exists as manganese carbide in combination with the iron carbide. The elements that favor the absorption of carbon are: manganese, tungsten, chromium and molybdenum those opposing it, nickel, silicon, and aluminum. Guillet has worked out the effect of the different elements on the rate of penetration in comparison with steel that absorbed carbon at a given temperature, at an average rate of 0.035 in. per hour.
His tables show that the following elements require an increased time of exposure to the carburizing material in order to obtain the same depth of penetration as with simple steel:
| When steel contains | Increased time of exposure |
|---|---|
| 2.0 per cent nickel | 28 per cent |
| 7.0 per cent nickel | 30 per cent |
| 1.0 per cent titanium | 12 per cent |
| 2.0 per cent titanium | 28 per cent |
| 0.5 per cent silicon | 50 per cent |
| 1.0 per cent silicon | 80 per cent |
| 2.0 per cent silicon | 122 per cent |
| 5.0 per cent silicon | No penetration |
| 1.0 per cent aluminum | 122 per cent |
| 2.0 per cent aluminum | 350 per cent |
The following elements seem to assist the rate of penetration of carbon, and the carburizing time may therefore be reduced as follows:
| When steel contains | Increased time of exposure Page 84 |
|---|---|
| 0.5 per cent manganese | 18 per cent |
| 1.0 per cent manganese | 25 per cent |
| 1.0 per cent chromium | 10 per cent |
| 2.0 per cent chromium | 18 per cent |
| 0.5 per cent tungsten | 0 |
| 1.0 per cent tungsten | 0 |
| 2.0 per cent tungsten | 25 per cent |
| 1.0 per cent molybdenum | 0 |
| 2.0 per cent molybdenum | 18 per cent |
The temperature at which carburization is accomplished is a very important factor. Hence the necessity for a reliable pyrometer, located so as to give the temperature just below the tops of the pots. It must be remembered, however, that the pyrometer gives the temperature of only one spot, and is therefore only an aid to the operator, who must use his eyes for successful results.
The carbon content of the case generally is governed by the temperature of the carburization. It generally proves advisable to have the case contain between 0.90 per cent and 1.10 carbon; more carbon than this gives rise to excess free cementite or carbide of iron, which is detrimental, causing the case to be brittle and apt to chip.
T. G. Selleck gives a very useful table of temperatures and the relative carbon contents of the case of steels carburized between 4 and 6 hrs. using a good charcoal carburizer. This data is as follows:
| At 1,500°F., the surface carbon content will be 0.90 per cent |
| At 1,600°F., the surface carbon content will be 1.00 per cent |
| At 1,650°F., the surface carbon content will be 1.10 per cent |
| At 1,700°F., the surface carbon content will be 1.25 per cent |
| At 1,750°F., the surface carbon content will be 1.40 per cent |
| At 1,800°F., the surface carbon content will be 1.75 per cent |
To this very valuable table, it seems best to add the following data, which we have used for a number of years. We do not know the name of its author, but it has proved very valuable, and seems to complete the above information. The table is self-explanatory, giving depth of penetration of the carbon of the case at different temperatures for different lengths of time:
| Penetration | Temperature Page 85 | ||
|---|---|---|---|
| 1,550 | 1,650 | 1,800 | |
| Penetration after 1/2 hr. | 0.008 | 0.012 | 0.030 |
| Penetration after 1 hr. | 0.018 | 0.026 | 0.045 |
| Penetration after 2 hr. | 0.035 | 0.048 | 0.060 |
| Penetration after 3 hr. | 0.045 | 0.055 | 0.075 |
| Penetration after 4 hr. | 0.052 | 0.061 | 0.092 |
| Penetration after 6 hr. | 0.056 | 0.075 | 0.110 |
| Penetration after 8 hr. | 0.062 | 0.083 | 0.130 |
From the tables given, we may calculate with a fair degree of certainty the amount of carbon in the case, and its penetration. These figures vary widely with different carburizers, and as pointed out immediately above, with different alloy steels.
The simplest carburizing substance is charcoal. It is also the slowest, but is often used mixed with something that will evolve large volumes of carbon monoxide or hydrocarbon gas on being heated. A great variety of materials is used, a few of them being charcoal (both wood and bone), charred leather, crushed bone, horn, mixtures of charcoal and barium carbonate, coke and heavy oils, coke treated with alkaline carbonates, peat, charcoal mixed with common salt, saltpeter, resin, flour, potassium bichromate, vegetable fibre, limestone, various seed husks, etc. In general, it is well to avoid complex mixtures.
H. L. Heathcote, on analyzing seventeen different carburizers, found that they contained the following ingredients:
| Per cent | |||
|---|---|---|---|
| Moisture | 2.68 | to | 26.17 |
| Oil | 0.17 | to | 20.76 |
| Carbon (organic) | 6.70 | to | 54.19 |
| Calcium phosphate | 0.32 | to | 74.75 |
| Calcium carbonate | 1.20 | to | 11.57 |
| Barium carbonate | nil | to | 42.00 |
| Zinc oxide | nil | to | 14.50 |
| Silica | nil | to | 8.14 |
| Sulphates (SO3) | trace | to | 3.45 |
| Sodium chloride | nil | to | 7.88 |
| Sodium carbonate | nil | to | 40.00 |
| Sulphides (S) | nil | to | 2.80 |
Page 86 Carburizing mixtures, though bought by weight, are used by volume, and the weight per cubic foot is a big factor in making a selection. A good mixture should be porous, so that the evolved gases, which should be generated at the proper temperature, may move freely around the steel objects being carburized; should be a good conductor of heat; should possess minimum shrinkage when used; and should be capable of being tamped down.
Many "secret mixtures" are sold, falsely claimed to be able to convert inferior metal into crucible tool steel grade. They are generally nothing more than mixtures of carbonaceous and cyanogen compounds possessing the well-known carburizing properties of those substances.
It is considered good practice to quench alloy steels from the pot, especially if the case is of any appreciable depth. The texture of carbon steel will be weakened by the prolonged high heat of carburizing, so that if we need a tough core, we must reheat it above its critical range, which is about 1,600°F. for soft steel, but lower for manganese and nickel steels. Quenching is done in either water, oil, or air, depending upon the results desired. The steel is then very carefully reheated to refine the case, the temperature varying from 1,350 to 1,450°F., depending on whether the material is an alloy or a simple steel, and quenched in either water or oil.
 FIG. 32.—Case-hardening depths.
FIG. 32.—Case-hardening depths.
There are many possibilities yet to be developed with the carburizing of alloy steels, which can produce a very tough, tenacious Page 87 austenitic case which becomes hard on cooling in air, and still retains a soft, pearlitic core. An austenitic case is not necessarily file hard, but has a very great resistance to abrasive wear.
The more carbon a steel has to begin with the more slowly will it absorb carbon and the lower the temperature required. Low-carbon steel of from 15 to 20 points is generally used and the carbon brought up to 80 or 85 points. Tool steels may be carbonized as high as 250 points.
In addition to the carburizing materials given, a mixture of 40 per cent of barium carbonate and 60 per cent charcoal gives much faster penetration than charcoal, bone or leather. The penetration of this mixture on ordinary low-carbon steel is shown in Fig. 32, over a range of from 2 to 12 hr.
 FIGS. 33 to 37.
FIGS. 33 to 37.
Each of these different packing materials has a different effect upon the work in which it is heated. Charcoal by itself will give a rather light case. Mixed with raw bone it will carburize more rapidly, and still more so if mixed with burnt bone. Raw bone and burnt bone, as may be inferred, are both quicker carbonizers than charcoal, but raw bone must never be used where the breakage of hardened edges is to be avoided, as it contains phosphorus and tends to make the piece brittle. Charred leather mixed with charcoal is a still faster material, and horns and hoofs exceed even this in speed; but these two compounds are restricted by their cost to use with high-grade articles, usually of tool or high-carbon steel, that are to be hardened locally—that is, "pack-hardened." Page 88 Cyanide of potassium or prussiate of potash are also included in the list of carbonizing materials; but outside of carburizing by dipping into melted baths of this material, their use is largely confined to local hardening of small surfaces, such as holes in dies and the like.
Dr. Federico Giolitti has proven that when carbonizing with charcoal, or charcoal plus barium carbonate, the active agent which introduces carbon into the steel is a gas, carbon monoxide (CO), derived by combustion of the charcoal in the air trapped in the box, or by decomposition of the carbonate. This gas diffuses in and out of the hot steel, transporting carbon from the charcoal to the outer portions of the metal:
If energizers like tar, peat, and vegetable fiber are used, they produce hydrocarbon gases on being heated—gases principally composed of hydrogen and carbon. These gases are unstable in the presence of hot iron: it seems to decompose them and sooty carbon is deposited on the surface of the metal. This diffuses into the metal a little, but it acts principally by being a ready source of carbon, highly active and waiting to be carried into the metal by the carbon monoxide—which as before, is the principal transfer agent.
Animal refuse when used to speed up the action of clean charcoal acts somewhat in the same manner, but in addition the gases given off by the hot substance contain nitrogen compounds. Nitrogen and cyanides (compounds of carbon and nitrogen) have long been known to give a very hard thin case very rapidly. It has been discovered only recently that this is due to the steel absorbing nitrogen as well as carbon, and that nitrogen hardens steel and makes it brittle just like carbon does. In fact it is very difficult to distinguish between these two hardening agents when examining a carburized steel under the microscope.
One of the advantages of hardening by carburizing is the fact that you can arrange to leave part of the work soft and thus retain the toughness and strength of the original material. Figures 33 to 37 show ways of doing this. The inside of the cup in Fig. 34 is locally hardened, as illustrated in Fig. 34, "spent" or used bone being packed around the surfaces that are to be left soft, while cyanide of potassium is put around those which are desired hard. The threads of the nut in Fig. 35 are kept soft by carburizing the nut while upon a stud. The profile gage, Fig. 36, is made of high-carbon steel and is hardened on the inside by packing with charred leather, but kept soft on the outside by surrounding it with fireclay. The rivet stud shown in Fig. 37 is carburized Page 89 while of its full diameter and then turned down to the size of the rivet end, thus cutting away the carburized surface.
After packing the work carefully in the boxes the lids are sealed or luted with fireclay to keep out any gases from the fire. The size of box should be proportioned to the work. The box should not be too large especially for light work that is run on a short heat. If it can be just large enough to allow the proper amount of material around it, the work is apt to be more satisfactory in every way.
Pieces of this kind are of course not quenched and hardened in the carburizing heat, but are left in the box to cool, just as in box annealing, being reheated and quenched as a second operation. In fact, this is a good scheme to use for the majority of carburizing work of small and moderate size. Material is on the market with which one side of the steel can be treated; or copper-plating one side of it will answer the same purpose and prevent that side becoming carburized.
In some operations case-hardened work is quenched from the box by dumping the whole contents into the quenching tank. It is common practice to leave a sieve or wire basket to catch the work, allowing the carburizing material to fall to the bottom of the tank where it can be recovered later and used again as a part of a new mixture. For best results, however, the steel is allowed to cool down slowly in the box after which it is removed and hardened by heating and quenching the same as carbon steel of the same grade. It has absorbed sufficient carbon so that, in the outer portions at least, it is a high-carbon steel.
The quenching tank is an important feature of apparatus in case-hardening—possibly more so than in ordinary tempering. One reason for this is because of the large quantities of pieces usually dumped into the tank at a time. One cannot take time to separate the articles themselves from the case-hardening mixture, and the whole content of the box is droped into the bath in short order, as exposure to air of the heated work is fatal to results. Unless it is split up, it is likely to go to the bottom as a solid mass, in which case very few of the pieces are properly hardened.
 FIG. 38.—Combination cooling tank for case-hardening.
FIG. 38.—Combination cooling tank for case-hardening.
A combination cooling tank is shown in Fig. 38. Water inlet and outlet pipes are shown and also a drain plug that enables the tank to be emptied when it is desired to clean out the spent carburizing material from the bottom. A wire-bottomed tray, framed with angle iron, is arranged to slide into this tank from the top and rests upon angle irons screwed to the tank sides. Its function is to catch the pieces and prevent them from settling to the tank bottom, and it also makes it easy to remove a batch of work. A bottomless box of sheet steel is shown at C. This fits into the wire-bottomed tray and has a number of rods or wires running across it, their purpose being to break up the mass of material as it comes from the carbonizing box.
Below the wire-bottomed tray is a perforated cross-pipe that is connected with a compressed-air line. This is used when case-hardening for colors. The shop that has no air compressor may rig up a satisfactory equivalent in the shape of a low-pressure hand-operated air pump and a receiver tank, for it is not necessary to use high-pressure air for this purpose. When colors are desired on case-hardened work, the treatment in quenching is Page 91 exactly the same as that previously described except that air is pumped through this pipe and keeps the water agitated. The addition of a slight amount of powdered cyanide of potassium to the packing material used for carburizing will produce stronger colors, and where this is the sole object, it is best to maintain the box at a dull-red heat.
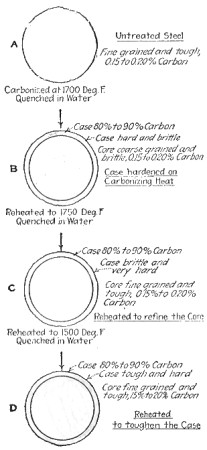 FIG. 39.—Why heat treatment of case-hardened work is necessary.
FIG. 39.—Why heat treatment of case-hardened work is necessary.
The old way of case-hardening was to dump the contents of the box at the end of the carburizing heat. Later study in the structure of steel thus treated has caused a change in this procedure, the use of automobiles and alloy steels probably hastening this result. The diagrams reproduced in Fig. 39 show why the heat treatment of case-hardened work is necessary. Starting at A with a close-grained and tough stock, such as ordinary machinery steel containing from 15 to 20 points of carbon, if such work is quenched on a carbonizing heat the result will be as shown at B. This gives a core that is coarse-grained and brittle and an outer case that is fine-grained and hard, but is likely to flake off, owing to the great difference in structure between it and the core. Reheating this work beyond the critical temperature of the core refines this core, closes the grain and makes it tough, but leaves the case very brittle; in fact, more so than it was before.
This is remedied by reheating the piece to a temperature slightly above the critical temperature of the case, this temperature corresponding ordinarily to that of steel having a carbon content of 85 points, When this is again quenched, the temperature, which has not been high enough to disturb the refined core, will have closed the grain of the case and toughened it. So, instead of but one heat and one quenching for this class of work, we have three of each, although it is quite possible and often profitable to omit the quenching after carburizing and allow the Page 92 piece or pieces and the case-carburizing box to cool together, as in annealing. Sometimes another heat treatment is added to the foregoing, for the purpose of letting down the hardness of the case and giving it additional toughness by heating to a temperature between 300° and 500°. Usually this is done in an oil bath. After this the piece is allowed to cool.
It is possible to harden the surface of tool steel extremely hard and yet leave its inner core soft and tough for strength, by a process similar to case-hardening and known as "pack-hardening." It consists in using tool steel of carbon contents ranging from 60 to 80 points, packing this in a box with charred leather mixed with wood charcoal and heating at a low-red heat for 2 or 3 hr., thus raising the carbon content of the exterior of the piece. The article when quenched in an oil bath will have an extremely hard exterior and tough core. It is a good scheme for tools that must be hard and yet strong enough to stand abuse. Raw bone is never used as a packing for this class of work, as it makes the cutting edges brittle.
Plain water, salt water and linseed oil are the three most common quenching materials for case-hardening. Water is used for ordinary work, salt water for work which must be extremely hard on the surface, and oil for work in which toughness is the main consideration. The higher the carbon of the case, the less sudden need the quenching action take hold of the piece; in fact, experience in case-hardening work gives a great many combinations of quenching baths of these three materials, depending on their temperatures. Thin work, highly carbonized, which would fly to pieces under the slightest blow if quenched in water or brine, is made strong and tough by properly quenching in slightly heated oil. It is impossible to give any rules for the temperature of this work, so much depending on the size and design of the piece; but it is not a difficult matter to try three or four pieces by different methods and determine what is needed for best results.
The alloy steels are all susceptible of case-hardening treatment; in fact, this is one of the most important heat treatments for such steels in the automobile industry. Nickel steel carburizes more slowly than common steel, the nickel seeming to have the effect of slowing down the rate of penetration. There is no Page 93 cloud without its silver lining, however, and to offset this retardation, a single treatment is often sufficient for nickel steel; for the core is not coarsened as much as low-carbon machinery steel and thus ordinary work may be quenched on the carburizing heat. Steel containing from 3 to 3.5 per cent of nickel is carburized between 1,650 and 1,750°F. Nickel steel containing less than 25 points of carbon, with this same percentage of nickel, may be slightly hardened by cooling in air instead of quenching.
Chrome-nickel steel may be case-hardened similarly to the method just described for nickel steel, but double treatment gives better results and is used for high-grade work. The carburizing temperature is the same, between 1,650 and 1,750°F., the second treatment consisting of reheating to 1,400° and then quenching in boiling salt water, which gives a hard surface and at the same time prevents distortion of the piece. The core of chrome-nickel case-hardened steel, like that of nickel steel, is not coarsened excessively by the first heat treatment, and therefore a single heating and quenching will suffice.
The process of carburizing by gas, briefly mentioned on page 88, consists of having a slowly revolving, properly heated, cylindrical retort into which illuminating gas (a mixture of various hydrocarbons) is continuously injected under pressure. The spent gases are vented to insure the greatest speed in carbonizing. The work is constantly and uniformly exposed to a clean carbonizing atmosphere instead of partially spent carbonaceous solids which may give off very complex compounds of phosphorus, sulphur, carbon and nitrogen.
Originally this process was thought to require a gas generator but it has been discovered that city gas works all right. The gas consists of vapors derived from petroleum or bituminous coal. Sometimes the gas supply is diluted by air, to reduce the speed of carburization and increase the depth.
Copper-plating has been found effective and must have a thickness of 0.0005 in. Less than this does not give a continuous coating. The plating bath used has a temperature of 170°F. A voltage of 4.1 is to be maintained across the terminals. Regions which are to be hardened can be kept free from copper by coating them with paraffin before they enter the plating tank. The operation is as follows:
| Operation No. |
Contents of bath | Purpose
Page 94 |
|---|---|---|
| 1 | Gasoline | To remove grease |
| 2 | Sawdust | To dry |
| 3 | Warm potassium hydroxide solution | To remove grease and dirt |
| 4 | Warm water | To wash |
| 5 | Warm sulphuric acid solution | To acid clean |
| 6 | Warm water | To wash |
| 7 | Cold water | Additional wash |
| 8 | Cold potassium cyanide solution | Cleanser |
| 9 | Cold water | To wash |
| 10 | Electric cleaner, warm sodium hydroxide case-iron anode | Cleanser to give good plating surface |
| 11 | Copper plating bath of copper sulphate and potassium cyanide solution warm | Plating bath |
There are also other methods of preventing case-hardening, one being to paint the surface with a special compound prepared for this purpose. In some cases a coating of plastic asbestos is used while in others thin sheet asbestos is wired around the part to be kept soft.
At the works of the Dayton Engineering Laboratories Company, Dayton, Ohio, they have a large quantity of small shafts, Fig. 40, that are to be case-hardened at A while the ends B and C are to be left soft. Formerly, the part A was brush-coated with melted paraffin but, as there were many shafts, this was tedious and great care was necessary to avoid getting paraffin where it was not wanted.
 FIG. 40.—Shaft to be coated with paraffin.
FIG. 40.—Shaft to be coated with paraffin.
To insure uniform coating the device shown in Fig. 41 was made. Melted paraffin is poured in the well A and kept liquid by setting the device on a hot plate, the paraffin being kept high Page 95 enough to touch the bottoms of the rollers. The shaft to be coated is laid between the rollers with one end against the gage B, when a turn or two of the crank C will cause it to be evenly coated.
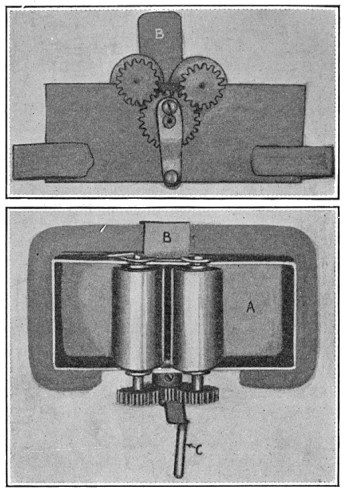 FIG. 41.—Device for coating the shaft.
FIG. 41.—Device for coating the shaft.
Carburized mild steel is used to a great extent in the manufacture of automobile and other parts which are likely to be subjected to rough usage. The strength and ability to withstand hard knocks depend to a very considerable degree on the thoroughness with which the carburizing process is conducted.
Many automobile manufacturers have at one time or another passed through a period of unfortunate breakages, or have found that for a certain period the parts turned out of their hardening shops were not sufficiently hard to enable the rubbing surfaces to stand up against the pressure to which they were subjected.
Page 96 So many factors govern the success of hardening that often this succession of bad work has been actually overcome without those interested realizing what was the weak point in their system of treatment. As the question is one that can create a bad reputation for the product of any firm it is well to study the influential factors minutely.
The matter to which these notes are primarily directed is the introduction of carbon into the case of the article to be hardened. In the first place the chances of success are increased by selecting as few brands of steel as practicable to cover the requirements of each component of the mechanism. The hardener is then able to become accustomed to the characteristics of that particular material, and after determining the most suitable treatment for it no further experimenting beyond the usual check-test pieces is necessary.
Although a certain make of material may vary in composition from time to time the products of a manufacturer of good steel can be generally relied upon, and it is better to deal directly with him than with others.
In most cases the case-hardening steels can be chosen from the following: (1) Case-hardening mild steel of 0.20 per cent carbon; (2) case-hardening 3½ per cent nickel steel; (3) case-hardening nickel-chromium steel; (4) case-hardening chromium vanadium. After having chosen a suitable steel it is best to have the sample analyzed by reliable chemists and also to have test pieces machined and pulled.
To prepare samples for analysis place a sheet of paper on the table of a drilling machine, and with a 3/8-in. diameter drill, machine a few holes about 3/8 in. deep in various parts of the sample bar, collecting about 3 oz. of fine drillings free from dust. This can be placed in a bottle and dispatched to the laboratory with instructions to search for carbon, silicon, manganese, sulphur, phosphorus and alloys. The results of the different tests should be carefully tabulated, and as there would most probably be some variation an average should be made as a fair basis of each element present, and the following tables may be used with confidence when deciding if the material is reliable enough to be used.
| Carbon | 0.15 to 0.25 per cent |
| Silicon | Not over 0.20 per cent |
| Manganese | 0.30 to 0.60 per cent |
| Sulphur | Not over 0.04 per cent |
| Phosphorus | Not over 0.04 per cent |
A tension test should register at least 60,000 lb. per square inch.
| Carbon | 0.12 to 0.20 per cent |
| Manganese | 0.65 per cent |
| Sulphur | Not over 0.045 per cent |
| Phosphorus | Not over 0.04 per cent |
| Nickel | 3.25 to 3.75 per cent |
| Carbon | 0.15 to 0.25 per cent |
| Manganese | 0.50 to 0.80 per cent |
| Sulphur | Not over 0.045 per cent |
| Phosphorus | Not over 0.04 per cent |
| Nickel | 1 to 1.5 per cent |
| Chromium | 0.45 to 0.75 per cent |
| Carbon | Not over 0.25 per cent |
| Manganese | 0.50 to 0.85 per cent |
| Sulphur | Not over 0.04 per cent |
| Phosphorus | Not over 0.04 per cent |
| Chromium | 0.80 to 1.10 per cent |
| Vanadium | Not less than 0.15 per cent |
Having determined what is required we now proceed to inquire into the question of carburizing, which is of vital importance.
The choice of a carburizing furnace depends greatly on the facilities available in the locality where the shop is situated and the nature and quantity of the work to be done. The furnaces can be heated with producer gas in most cases, but when space is of value illuminating gas from a separate source of supply has some compensations. When the latter is used it is well to install a governor if the pressure is likely to fluctuate, particularly where the shop is at a high altitude or at a long distance from the gas supply.
Many furnaces are coal-fired, and although greater care is Page 98 required in maintaining a uniform temperature good results have been obtained. The use of electricity as a means of reaching the requisite temperature is receiving some attention, and no doubt it would make the control of temperature comparatively simple. However, the cost when applied to large quantities of work will, for the present at least, prevent this method from becoming popular. It is believed that the results obtainable \with the electric furnace would surpass any others; but the apparatus is expensive, and unless handled with intelligence would not last long.
The most elementary medium of carburization is pure carbon, but the rate of carburization induced by this material is very low, and other components are necessary to accelerate the process. Many mixtures have been marketed, each possessing its individual merits, and as the prices vary considerably it is difficult to decide which is the most advantageous.
Absorption from actual contact with solid carbon is decidedly slow, and it is necessary to employ a compound from which gases are liberated, and the steel will absorb the carbon from the gases much more readily.
Both bone and leather charcoal give off more carburizing gases than wood charcoal, and although the high sulphur content of the leather is objectionable as being injurious to the steel, as also is the high phosphorus content of the bone charcoal, they are both preferable to the wood charcoal.
By mixing bone charcoal with barium carbonate in the proportions of 60 per cent of the former to 40 per cent of the latter a very reliable compound is obtained.
The temperature to which this compound is subjected causes the liberation of carbon monoxide when in contact with hot charcoal.
Many more elaborate explanations may be given of the actions and reactions taking place, but the above is a satisfactory guide to indicate that it is not the actual compound which causes carburization, but the gases released from the compound.
Until the temperature of the muffle reaches about 1,300°F. carburization does not take place to any useful extent, and consequently it is advisable to avoid the use of any compound from which the carburizing gases are liberated much before that temperature is reached. In the case of steel containing nickel slightly higher temperatures may be used and are really necessary Page 99 if the same rate of carbon penetration is to be obtained, as the presence of nickel resists the penetration.
At higher temperatures the rate of penetration is higher, but not exactly in proportion to the temperature, and the rate is also influenced by the nature of the material and the efficiency of the compound employed.
The so-called saturation point of mild steel is reached when the case contains 0.90 per cent of carbon, but this amount is frequently exceeded. Should it be required to ascertain the amount of carbon in a sample at varying depths below the skin this can be done by turning off a small amount after carburizing and analyzing the turnings. This can be repeated several times, and it will probably be found that the proportion of carbon decreases as the test piece is reduced in diameter unless decarburization has taken place.
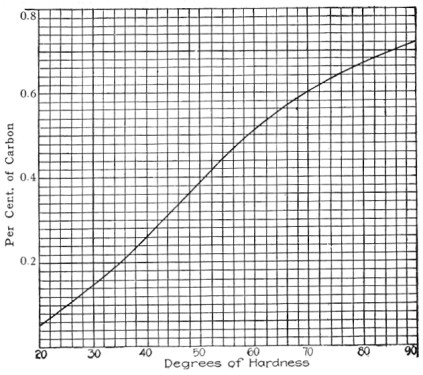 FIG. 42.—Chart showing penetration of carbon.
FIG. 42.—Chart showing penetration of carbon.
The chart, Fig. 42, is also a good guide.
In order to use the chart it is necessary to harden the sample we desire to test as we would harden a piece of tool steel, and then test by scleroscope. By locating on the chart the point on the horizontal axis which represents the hardness of the sample the curve enables one to determine the approximate amount of carbon present in the case.
Page 100 Should the hardness lack uniformity the soft places can be identified by etching. To accomplish this the sample should be polished after quenching and then washed with a weak solution of nitric acid in alcohol, whereupon the harder points will show up darker than the softer areas.
The selection of suitable boxes for carburizing is worthy of a little consideration, and there can be no doubt that in certain cases results are spoiled and considerable expense caused by using unsuitable containers.
As far as initial expense goes cast-iron boxes are probably the most expedient, but although they will withstand the necessary temperatures they are liable to split and crack, and when they get out of shape there is much difficulty in straightening them.
The most suitable material in most cases is steel boiler plate 3/8 or 1/2 in. thick, which can be made with welded joints and will last well.
The sizes of the boxes employed depend to a great extent on the nature of the work being done, but care should be exercised to avoid putting too much in one box, as smaller ones permit the heat to penetrate more quickly, and one test piece is sufficient to give a good indication of what has taken place. If it should be necessary to use larger boxes it is advisable to put in three or four test pieces in different positions to ascertain if the penetration of carbon has been satisfactory in all parts of the box, as it is quite possible that the temperature of the muffle is not the same at all points, and a record shown by one test piece would not then be applicable to all the parts contained in the box. It has been found that the rate of carbon penetration increases with the gas pressure around the articles being carburized, and it is therefore necessary to be careful in sealing up the boxes after packing. When the articles are placed within and each entirely surrounded by compound so that the compound reaches to within 1 in. of the top of the box a layer of clay should be run around the inside of the box on top of the compound. The lid, which should be a good fit in the box, is then to be pressed on top of this, and another layer of clay run just below the rim of the box on top of the cover.
A mixture of fireclay and sand will be found very satisfactory for closing up the boxes, and by observing the appearance of the Page 101 work when taken out we can gage the suitability of the methods employed, for unless the boxes are carefully sealed the work is generally covered with dark scales, while if properly done the articles will be of a light gray.
By observing the above recommendations reliable results can be obtained, and we can expect uniform results after quenching.
Although the advantages offered by the gas-fired furnace for carburizing have been generally recognized in the past from points of view as close temperature regulation, decreased attendance, and greater convenience, very little information has been published regarding the consumption of gas for this process. It has therefore been a matter of great difficulty to obtain authentic information upon this point, either from makers or users of such furnaces.
In view of this, the details of actual consumption of gas on a regular customer's order job will be of interest. The "Revergen" furnace, manufactured by the Davis Furnace Company, Luton, Bedford, England, was used on this job, and is provided with regenerators and fired with illuminating gas at ordinary pressure, the air being introduced to the furnace at a slight pressure of 3 to 4 in. water gage. The material was charged into a cold furnace, raised to 1,652°F., and maintained at that temperature for 8 hr. to give the necessary depth of case. The work consisted of automobile gears packed in six boxes, the total weight being 713 lb. The required temperature of 1,652°F. was obtained in 70 min. from lighting up, and a summary of the data is shown in the following table:
| Cubic Foot Per Pound of Load |
Total Number of Cubic Foot | |
|---|---|---|
| Gas to raise furnace and charge from cold to 1,652°F., 70 min. | 1.29 | 925 |
| Gas to maintain 1,652°F. for 1st hour | 0.38 | 275 |
| Gas to maintain 1,652°F. for 2nd hour | 0.42 | 300 |
| Gas to maintain 1,652°F. for 3rd hour | 0.38 | 275 |
| Gas to maintain 1,652°F. for 4th hour | 0.42 | 300 |
| Gas to maintain 1,652°F. for 5th hour | 0.49 | 350 |
| Gas to maintain 1,652°F. for 6th hour | 0.49 | 350 |
| Gas to maintain 1,652°F. for 7th hour | 0.45 | 325 |
| Gas to maintain 1,652°F. for 8th hour | 0.45 | 325 |
Page 102 The overall gas consumption for this run of 9 hr. 10 min. was only 4.8 cu. ft. per pound of load.
Of all the opportunities for practicing economy in the heat-treatment department, there is none that offers greater possibilities for profitable returns than the systematic cleaning, blending and reworking of artificial carburizers, or compounds.
The question of whether or not it is practical to take up the work depends upon the nature of the output. If the sole product of the hardening department consists of a 1.10 carbon case or harder, requiring a strong highly energized material of deep penetrative power such as that used in the carburizing of ball races, hub-bearings and the like, it would be best to dispose of the used material to some concern whose product requires a case with from 0.70 to 0.90 carbon, but where there is a large variety of work the compound may be so handled that there will be practically no waste.
This is accomplished with one of the most widely known artificial carburizers by giving all the compound in the plant three distinct classifications: "New," being direct from the maker; "half and half," being one part of new and one part first run; and "2 to 1," which consists of two parts of old and one part new.
During the pulling of the heat, the pots are dumped upon a cast-iron screen which forms a table or apron for the furnace. Directly beneath this table is located one of the steel conveyor carts, shown in Fig. 43, which is provided with two wheels at the rear and a dolly clevis at the front, which allows it to be hauled away from beneath the furnace apron while filled with red-hot compound. A steel cover is provided for each box, and the material is allowed to cool without losing much of the evolved gases which are still being thrown off by the compound.
 FIG. 43.—The cooling carts.
FIG. 43.—The cooling carts.
 FIG. 44.—Machine for blending the mixture.
FIG. 44.—Machine for blending the mixture.
As this compound comes from the carburizing pots it contains bits of fireclay which represent a part of the luting used for sealing, and there may be small parts of work or bits of fused material in it as well. After cooling, the compound is very dusty and Page 104 disagreeable to handle, and, before it can be used again, must be sifted, cleaned and blended.
Some time ago the writer was confronted with this proposition for one of the largest consumers of carburizing compound in the world, and the problem was handled in the following manner: The cooled compound was dumped from the cooling cars and sprinkled with a low-grade oil which served the dual purposes of settling the dust and adding a certain percentage of valuable hydrocarbon to the compound. In Fig. 44 is shown the machine that was designed to do the cleaning and blending.
Essentially, this consists of the sturdy, power-driven separator and fanning mill which separates the foreign matter from the compound and elevates it into a large settling basin which is formed by the top of the steel housing that incloses the apparatus. After reaching the settling basin, the compound falls by gravity into a power-driven rotary mixing tub which is directly beneath the settling basin. Here the blending is done by mixing the proper amount of various grades of material together. After blending the compound, it is ready to be stored in labeled containers and delivered to the packing room.
It will be seen that by this simple system there is the least possible loss of energy from the compound. The saving commences the moment the cooling cart is covered and preserves the valuable dust which is saved by the oiling and the settling basin of the blending machine.
Then, too, there is the added convenience of the packers who have a thoroughly cleaned, dustless, and standardized product to work with. Of course, this also tends to insure uniformity in the case-hardening operation.
With this outfit, one man cleans and blends as much compound in one hour as he formerly did in ten.
HEAT TREATMENT OF STEEL
Heat treatment consists in heating and cooling metal at definite rates in order to change its physical condition. Many objects may be attained by correct heat treatment, but nothing much can be expected unless the man who directs the operations knows what is the essential difference in a piece of steel at room temperature and at a red heat, other than the obvious fact that it is hot. The science of metallography has been developed in the past 25 years, and aided by precise methods of measuring temperature, has done much to systematize the information which we possess on metallic alloys, and steel in particular.
One of the most important means of investigating the properties of pure metals and their alloys is by an examination of their heating and cooling curves. Such curves are constructed by taking a small piece and observing and recording the temperature of the mass at uniform intervals of time during a uniform heating or cooling. These observations, when plotted in the form of a curve will show whether the temperature of the mass rises or falls uniformly.
The heat which a body absorbs serves either to raise the temperature of the mass or change its physical condition. That portion of the heat which results in an increase in temperature of the body is called "sensible heat," inasmuch as such a gain in heat is apparent to the physical senses of the observer. If heat were supplied to the body at a uniform rate, the temperature would rise continuously, and if the temperature were plotted against time, a smooth rising curve would result. Or, if sensible heat were abstracted from the body at a uniform rate, a time-temperature curve would again be a smooth falling curve. Such a curve is called a "cooling curve."
However, we find that when a body is melting, vaporizing, or otherwise suffering an abrupt change in physical properties, a quantity of heat is absorbed which disappears without changing the temperature of the body. This heat absorbed during a change of state is called "latent heat," because it is transformed Page 106 into the work necessary to change the configuration and disposition of the molecules in the body; but it is again liberated in equal amount when the reverse change takes place.
From these considerations it would seem that should the cooling curve be continuous and smooth, following closely a regular course, all the heat abstracted during cooling is furnished at the expense of a fall in temperature of the body; that is to say, it disappears as "sensible heat." These curves, however, frequently show horizontal portions or "arrests" which denote that at that temperature all of the heat constantly radiating is being supplied by internal changes in the alloy itself; that is, it is being supplied by the evolution of a certain amount of "latent heat."
In addition to the large amount of heat liberated when a metal solidifies, there are other changes indicated by the thermal analysis of many alloys which occur after the body has become entirely solidified. These so-called transformation points or ranges may be caused by chemical reactions taking place within the solid, substances being precipitated from a "solid solution," or a sudden change in some physical property of the components, such as in magnetism, hardness, or specific gravity.
It may be difficult to comprehend that such changes can occur in a body after it has become entirely solidified, owing to the usual conception that the particles are then rigidly fixed. However, this rigidity is only comparative. The molecules in the solid state have not the large mobility they possess as a liquid, but even so, they are still moving in circumscribed orbits, and have the power, under proper conditions, to rearrange their position or internal configuration. In general, such rearrangement is accompanied by a sudden change in some physical property and in the total energy of the molecule, which is evidenced by a spontaneous evolution or absorption of latent heat.
Cooling curves of the purest iron show at least two well-defined discontinuities at temperatures more than 1,000°F., below its freezing-point. It seems that the soft, magnetic metal so familiar as wrought iron, and called "alpha iron" or "ferrite" by the metallurgist, becomes unstable at about 1,400°F. and changes into the so-called "beta" modification, becoming suddenly harder, and losing its magnetism. This state in turn persists no higher than 1,706°C., when a softer, non-magnetic "gamma" iron is the stable modification up to the actual melting-point of the metal. These various changes occur in electrolytic Page 107 iron, and therefore cannot be attributed to any chemical reaction or solution; they are entirely due to the existence of "allotropic modifications" of the iron in its solid state.
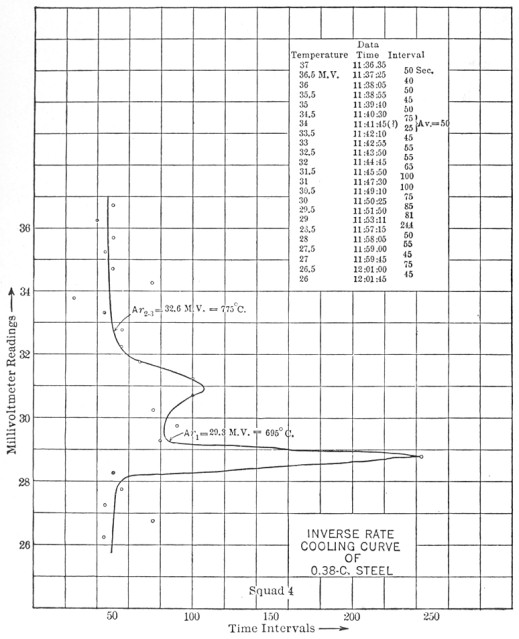 FIG. 45.—Inverse Rate Cooling Curve of 0.38 C Steel.
FIG. 45.—Inverse Rate Cooling Curve of 0.38 C Steel.
Steels, or iron containing a certain amount of carbon, develop somewhat different cooling curves from those produced by pure iron. Figure 45 shows, for instance, some data observed on a cooling piece of 0.38 per cent carbon steel, and the curve constructed therefrom. It will be noted that the time was noted when the needle on the pyrometer passed each dial marking. If the metal were not changing in its physical condition, the time between Page 108 each reading would be nearly constant; in fact for a time it required about 50 sec. to cool each unit. When the dial read about 32.5 (corresponding in this instrument to a temperature of 775°C. or 1,427°F.) the cooling rate shortened materially, 55 sec. then 65, then 100, then 100; showing that some change inside the metal was furnishing some of the steadily radiating heat. This temperature is the so-called "upper critical" for this steel. Further down, the "lower critical" is shown by a large heat evolution at 695°C. or 1,283°F.
Just the reverse effects take place upon heating, except that the temperatures shown are somewhat higher—there seems to be a lag in the reactions taking place in the steel. This is an important point to remember, because if it was desired to anneal a piece of 0.38 carbon steel, it is necessary to heat it up to and beyond 1,476° F. (1,427°F. plus this lag, which may be as much as 50°).
It may be said immediately that above the upper critical the carbon exists in the iron as a "solid solution," called "austenite" by metallographers. That is to say, it is uniformly distributed as atoms throughout the iron; the atoms of carbon are not present in any fixed combination, in fact any amount of carbon from zero to 1.7 per cent can enter into solid solution above the upper critical. However, upon cooling this steel, the carbon again enters into combination with a definite proportion of iron (the carbide "cementite," Fe3C), and accumulates into small crystals which can be seen under a good microscope. Formation of all the cementite has been completed by the time the temperature has fallen to the lower critical, and below that temperature the steel exists as a complex substance of pure iron and the iron carbide.
It is important to note that the critical points or critical range of a plain steel varies with its carbon content. The following table gives some average figures:
| Carbon Content. | Upper Critical. | Lower Critical. |
|---|---|---|
| 0.00 | 1,706°F. | 1,330°F. |
| 0.20 | 1,600°F. | 1,330°F. |
| 0.40 | 1,480°F. | 1,330°F. |
| 0.60 | 1,400°F. | 1,330°F. |
| 0.80 | 1,350°F. | 1,330°F. |
| 0.90 | 1,330°F. | 1,330°F. |
| 1.00 | 1,470°F. | 1,330°F. |
| 1.20 | 1,650°F. | 1,330°F. |
| 1.40 | 1,830°F. | 1,330°F. |
| 1.60 | 2,000°F. | 1,330°F. |
Page 109 It is immediately noted that the critical range narrows with increasing carbon content until all the heat seems to be liberated at one temperature in a steel of 0.90 per cent carbon. Beyond that composition the critical range widens rapidly. Note also that the lower critical is constant in plain carbon steels containing no alloying elements.
 FIG. 46.—Microphotograph of steel used in S. K. F. bearings,
polished and etched with nitric acid and magnified 1,000 times. Made
by H. O. Walp.
FIG. 46.—Microphotograph of steel used in S. K. F. bearings,
polished and etched with nitric acid and magnified 1,000 times. Made
by H. O. Walp.
This steel of 0.90 carbon content is an important one. It is called "eutectoid" steel. Under the microscope a properly polished and etched sample shows the structure to consist of thin sheets of two different substances (Fig. 46). One of these is pure iron, and the other is pure cementite. This structure of thin sheets has received the name "pearlite," because of its pearly appearance under sunlight. Pearlite is a constituent found in all annealed carbon steels. Pure iron, having no carbon, naturally would show no pearlite when examined under a microscope; only abutting granules of iron are delicately traced. The metallographist calls this pure iron "ferrite." As soon as a little carbon enters the alloy and a soft steel is formed, small angular areas of pearlite appear at the boundaries of the ferrite crystals (Fig. 47). With increasing carbon in the steel the volume of iron crystals becomes less and less, and the relative amount of pearlite increases, until arriving at 0.90 per cent carbon, the large ferrite crystals have been suppressed and the structure is all pearlite. Higher carbon steels show films of cementite outlining grains of pearlite (Fig. 48).
This represents the structure of annealed, slowly cooled steels. It is possible to change the relative sizes of the ferrite and cementite crystals by heat treatment. Large grains are associated with brittleness. Consequently one must avoid heat treatments which produce coarse grains.
 FIG. 47.—Structure of low carbon steel, polished, etched and
viewed under 100 magnifications. Tiny white granules of pure iron
(ferrite) have small accumulations of dark-etching pearlite
interspersed between them. Photograph by H. S. Rawdon.
FIG. 47.—Structure of low carbon steel, polished, etched and
viewed under 100 magnifications. Tiny white granules of pure iron
(ferrite) have small accumulations of dark-etching pearlite
interspersed between them. Photograph by H. S. Rawdon.
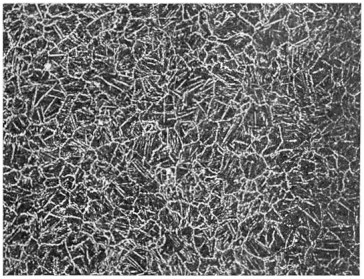 FIG. 48.—Slowly cooled high-carbon steel, polished, etched and
viewed at 100 magnifications. The dark grains are pearlite, separated
by white films of iron carbide (cementite). Photograph by H. S. Rawdon.
FIG. 48.—Slowly cooled high-carbon steel, polished, etched and
viewed at 100 magnifications. The dark grains are pearlite, separated
by white films of iron carbide (cementite). Photograph by H. S. Rawdon.
In general it may be said that the previous crystalline structure of a steel is entirely obliterated when it passes just through the critical range. At that moment, in fact, the ferrite, cementite or Page 110 pearlite which previously existed has lost its identity by everything going into the solid solution called austenite. If sufficient time is given, the chemical elements comprising a good steel distribute themselves uniformly through the mass. If the steel be then cooled, the austenite breaks up into new crystals of ferrite, cementite and pearlite; and in general if the temperature has not Page 111 gone far above the critical, and cooling is not excessively slow, a very fine texture will result. This is called "refining" the grain; or in shop parlance "closing" the grain. However, if the heating has gone above the critical very far, the austenite crystals start to grow; a very short time at an extreme temperature will cause a large grain growth. Subsequent cooling gives a coarse texture, or an arrangement of ferrite, cementite and pearlite grains which is greatly coarsened, reflecting the condition of the austenite crystals from which they were born.
It maybe noted in passing that the coarse crystals of cast metal cannot generally be refined by heat treatment unless some forging or rolling has been done in the meantime. Heat treatment alone does not seem to be able to break up the crystals of an ingot structure.
Steel is hardened by quenching from above the upper critical. Apparently the quick cooling prevents the normal change back to definite and sizeable crystals of ferrite and cementite. Hardness is associated with this suppressed change. If the change is allowed to continue by a moderate reheating, like a tempering, the hardness decreases.
If a piece of steel could be cooled instantly, doubtless austenite could be preserved and examined. In the ordinary practice of hardening steels, the quenching is not so drastic, and the transformation of austenite back to ferrite and cementite is more or less completely effected, giving rise to certain transitory forms which are known as "martensite," "troostite," "sorbite," and finally, pearlite.
Austenite has been defined as a solid solution of cementite (Fe3C) in gamma iron. It is stable at various temperatures dependent upon its carbon content, which may be any amount up to the saturated solution containing 1.7 per cent. Austenite is not nearly as hard as martensite, owing to its content of the soft gamma iron. Fig. 49 shows austenite to possess the typical appearance of any pure, crystallized substance.
In the most quickly quenched high carbon steels, austenite commonly forms the ground mass which is interspersed with martensite, a large field of which is illustrated in Fig. 50. Martensite is usually considered to be a solid solution of cementite in beta iron. It represents an unstable condition in which the metal Page 112 is caught during rapid cooling. It is very hard, and is the chief constituent of hardened high-carbon steels, and of medium-carbon nickel-steel and manganese-steel.
Troostite is of doubtful composition, but possibly is an unstable mixture of untransformed martensite with sorbite. It contains more or less untransformed material, as it is too hard to be composed entirely of the soft alpha modification, and it can also be tempered more or less without changing in appearance. Its normal appearance as rounded grains is given in Fig. 51; larger patches show practically no relief in their structure, and a photograph merely shows a dark, structureless area.
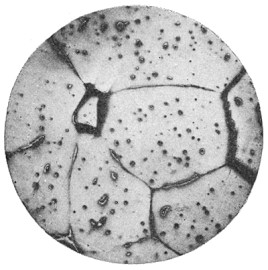 FIG. 49.—Coarse-grained martensite, polished and etched with
nitric acid and magnified 50 times. Made by Prof. Chas. Y. Clayton.
FIG. 49.—Coarse-grained martensite, polished and etched with
nitric acid and magnified 50 times. Made by Prof. Chas. Y. Clayton.
Sorbite is believed to be an early stage in the formation of pearlite, when the iron and iron carbide originally constituting the solid solution (austenite) have had an opportunity to separate from each other, and the iron has entirely passed into the alpha modification, but the particles are yet too small to be distinguishable under the microscope. It also, possibly, contains some incompletely transformed matter. Sorbite is softer and tougher than troostite, and is habitually associated with pearlite. Its components are tending to coagulate into pearlite, and will do so in a fairly short time at temperatures near the lower critical, which heat will furnish the necessary molecular freedom. The normal appearance, however, is the cloudy mass shown in Fig. 52.
Pearlite is a definite conglomerate of ferrite and cementite containing about six parts of the former to one of the latter. When pure, it has a carbon content of about 0.95 per cent. It represents the complete transformation of the eutectoid austenite accomplished by slow-cooling of an iron-carbon alloy through the transformation range. (See Fig. 46.)
 FIG. 50.—Quenched high-carbon steel, polished, etched and viewed
at 100 magnifications. This structure is called martensite and is
desired when maximum hardness is essential. Photograph by H. S. Rawdon.
FIG. 50.—Quenched high-carbon steel, polished, etched and viewed
at 100 magnifications. This structure is called martensite and is
desired when maximum hardness is essential. Photograph by H. S. Rawdon.
|

|
| FIG. 51.—Martensite (light needles) passing into troosite (dark patches). 130 X. From a piece of eutectoid steel electrically welded. | FIG. 52.—Sorbite (dark patches) passing into pearlite (wavy striations). Light Areas are Patches of Ferrite. 220 X. From a piece of hypo-eutectoid steel electrically welded. |
These observations are competent to explain annealing and toughening practice. A quickly quenched carbon steel is mostly martensitic which, as noted, is a solid solution of beta iron and Page 113 cementite, hard and brittle. Moderate reheating or annealing changes this structure largely into troostite, which is a partly transformed martensite, possessing much of the hardness of martensite, but with a largely increased toughness and shock resistance. This toughness is the chief characteristic of the next Page 114 material in the transformation series, sorbite, which is merely martensite wholly transformed into a mixture of ultramicroscopic crystals of ferrite (alpha iron) and cementite (Fe3C).
"Tempering" or "drawing" should be restricted to mean moderate reheating, up to about 350° C., forming troostitic steel. "Toughening" represents the practice of reheating hardened carbon steels from 350° C. up to just below the lower critical, and forms sorbitic steel; while "annealing" refers to a heating for grain size at or above the transformation ranges, followed by a slow cooling. Any of these operations not only allows the transformations from austenite to pearlite to proceed, but also relieves internal stresses in the steel.
Normalizing is a heating like annealing, followed by a moderately rapid quench.
While the use of a pyrometer is of course the only way to have accurate knowledge as to the heat being used in either forging or hardening steels, a color chart will be of considerable assistance if carefully studied. These have been prepared by several of the steel companies as a guide, but it must be remembered that the colors and temperatures given are only approximate, and can be nothing else.
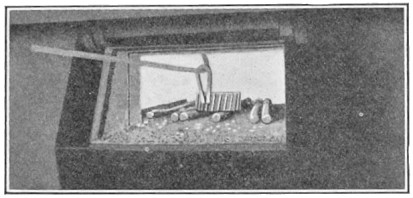 FIG. 53.—Finding hardening heats with a magnet.
FIG. 53.—Finding hardening heats with a magnet.
The Magnet Test.—The critical point can also be determined by an ordinary horse-shoe magnet. Touch the steel with a magnet during the heating and when it reaches the temperature at which steel fails to attract the magnet, or in other words, loses its magnetism, the critical point has been reached.
Figures 53 and 54 show how these are used in practice.
Page 115 The first (Fig. 53) shows the use of a permanent horse-shoe magnet and the second (Fig. 54) an electro-magnet consisting of an iron rod with a coil or spool magnet at the outer end. In either case the magnet should not be allowed to become heated but should be applied quickly.
 FIG. 54.—Using electro-magnet to determine heat.
FIG. 54.—Using electro-magnet to determine heat.
The work is heated up slowly in the furnace and the magnet applied from time to time. The steel being heated will attract the magnet until the heat reaches the critical point. The magnet is applied frequently and when the magnet is no longer attracted, the piece is at the lowest temperature at which it can be hardened properly. Quenching slightly above this point will give a tool of satisfactory hardness. The method applies only to carbon steels and will not work for modern high-speed steels.
This section is based on a paper read before the American Gear Manufacturers' Association at White Sulphur Springs, W. Va., Apr. 18, 1918.
Great advancement has been made in the heat treating and hardening of gears. In this advancement the chemical and metallurgical laboratory have played no small part. During this time, however, the condition of the blanks as they come to the machine shop to be machined has not received its share of attention.
There are two distinct types of gears, both types having their champions, namely, carburized and heat-treated. The difference between the two in the matter of steel composition is entirely in the carbon content, the carbon never running higher than 25-point in the carburizing type, while in the heat-treated gears the carbon is seldom lower than 35-point. The difference in the final gear is the hardness. The carburized gear is file hard on the surface, with a soft, tough and ductile core to withstand shock, while the heat-treated gear has a surface that can be touched by a file with a core of the same hardness as the outer surface.
Page 116 Annealing Work.—With the exception of several of the higher types of alloy steels, where the percentages of special elements run quite high, which causes a slight air-hardening action, the carburizing steels are soft enough for machining when air cooled from any temperature, including the finishing temperature at the hammer. This condition has led many drop-forge and manufacturing concerns to consider annealing as an unnecessary operation and expense. In many cases the drop forging has only been heated to a low temperature, often just until the piece showed color, to relieve the so-called hammer strains. While this has been only a compromise it has been better than no reheating at all, although it has not properly refined the grain, which is necessary for good machining conditions.
Annealing is heating to a temperature slightly above the highest critical point and cooling slowly either in the air or in the furnace. Annealing is done to accomplish two purposes: (1) to relieve mechanical strains and (2) to soften and produce a maximum refinement of grain.
Process of Carburizing.—Carburizing imparts a shell of high-carbon content to a low-carbon steel. This produces what might be termed a "dual" steel, allowing for an outer shell which when hardened would withstand wear, and a soft ductile core to produce ductility and withstand shock. The operation is carried out by packing the work to be carburized in boxes with a material rich in carbon and maintaining the box so charged at a temperature in excess of the highest critical point for a length of time to produce the desired depth of carburized zone. Generally maintaining the temperature at 1,650 to 1,700° F. for 7 hr. will produce a carburized zone 1/32 in. deep.
Heating to a temperature slightly above the highest critical point and cooling suddenly in some quenching medium, such as water or oil hardens the steel. This treatment produces a maximum refinement with the maximum strength.
Drawing to a temperature below the highest critical point (the temperature being governed by the results required) relieves the hardening strains set up by quenching, as well as the reducing of the hardness and brittleness of hardened steel.
Effect of Proper Annealing.—Proper annealing of low-carbon steels causes a complete solution or combination to take place between the ferrite and pearlite, producing a homogeneous mass of small grains of each, the grains of the pearlite being surrounded Page 117 by grains of ferrite. A steel of this refinement will machine to good advantage, due to the fact that the cutting tool will at all times be in contact with metal of uniform composition.
While the alternate bands of ferrite and pearlite are microscopically sized, it has been found that with a Gleason or Fellows gear-cutting machine that rough cutting can be traced to poorly annealed steels, having either a pronounced banded structure or a coarse granular structure.
Temperature for Annealing.—Theoretically, annealing should be accomplished at a temperature at just slightly above the critical point. However, in practice the temperature is raised to a higher point in order to allow for the solution of the carbon and iron to be produced more rapidly, as the time required to produce complete solution is reduced as the temperature increases past the critical point.
For annealing the simpler types of low-carbon steels the following temperatures have been found to produce uniform machining conditions on account of producing uniform fine-grain pearlite structure:
0.15 to 0.25 per cent carbon, straight carbon steel.—Heat to 1,650°F. Hold at this temperature until the work is uniformly heated; pull from the furnace and cool in air.
0.15 to 0.25 per cent carbon, 1½ per cent nickel, 1/2 per cent chromium steel.—Heat to 1,600°F. Hold at this temperature until the work is uniformly heated; pull from the furnace and cool in air.
0.15 to 0.25 per cent carbon, 3½ per cent nickel steel.—Heat to 1,575°F. Hold at this temperature until the work is uniformly heated; pull from the furnace and cool in air.
Care in Annealing.—Not only will benefits in machining be found by careful annealing of forgings but the subsequent troubles in the hardening plant will be greatly reduced. The advantages in the hardening start with the carburizing operation, as a steel of uniform and fine grain size will carburize more uniformly, producing a more even hardness and less chances for soft spots. The holes in the gears will also "close in more uniformly," not causing some gears to require excessive grinding and others with just enough stock. Also all strains will have been removed from the forging, eliminating to a great extent distortion and the noisy gears which are the result.
With the steels used, for the heat-treated gears, always of a Page 118 higher carbon content, treatment after forging is necessary for machining, as it would be impossible to get the required production from untreated forgings, especially in the alloy steels. The treatment is more delicate, due to the higher percentage of carbon and the natural increase in cementite together with complex carbides which are present in some of the higher types of alloys.
Where poor machining conditions in heat-treated steels are present they are generally due to incomplete solution of cementite rather than bands of free ferrite, as in the case of case-hardening steels. This segregation of carbon, as it is sometimes referred to, causes hard spots which, in the forming of the tooth, cause the cutter to ride over the hard metal, producing high spots on the face of the tooth, which are as detrimental to satisfactory gear cutting as the drops or low spots produced on the face of the teeth when the pearlite is coarse-grained or in a banded condition.
In the simpler carburized steels it is not necessary to test the forgings for hardness after annealing, but with the high percentages of alloys in the carburizing steels and the heat-treated steels a hardness test is essential.
To obtain the best results in machining, the microstructure of the metal should be determined and a hardness range set that covers the variations in structure that produce good machining results. By careful control of the heat-treating operation and with the aid of the Brinell hardness tester and the microscope it is possible to continually give forgings that will machine uniformly and be soft enough to give desired production. The following gives a few of the hardness numerals on steel used in gear manufacture that produce good machining qualities:
0.20 per cent carbon, 3 per cent nickel, 1¼; per cent chromium—Brinell 156 to 170.
0.50 per cent carbon, 3 per cent nickel, 1 per cent chromium—Brinell 179 to 187.
0.50 per cent carbon chrome-vanadium—Brinell 170 to 179.
The size of the piece influences the physical properties obtained in steel by heat treatment. This has been worked out by E. J. Janitzky, metallurgical engineer of the Illinois Steel Company, as follows:
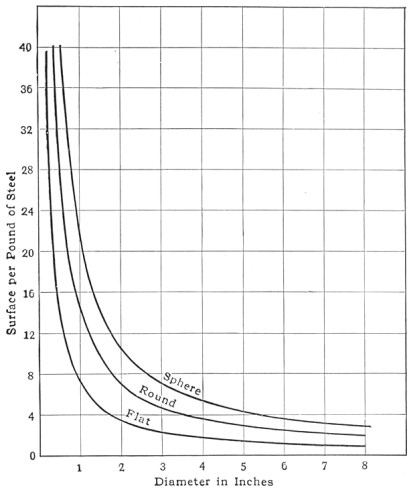 FIG. 55.—Effect of size on heating.
FIG. 55.—Effect of size on heating.
"With an increase in the mass of steel there is a corresponding decrease in both the minimum surface hardness and depth hardness, when quenched from the same temperature, under identical conditions of the quenching medium. In other words, the physical properties obtained are a function of the surface of the metal quenched for a given mass of steel. Keeping this primary assumption in mind, it is possible to predict what physical properties may be developed in heat treating by calculating the surface per unit mass for different shapes and sizes. It may be pointed out that the figures and chart that follow are not results of actual tests, but are derived by calculation. They indicate the mathematical relation, which, based on the fact that the physical properties of steel are determined not alone by the rate which heat is lost per unit of surface, but by the rate which heat is lost per unit of weight in relation to the surface exposed for that unit. The unit of weight has for the different shaped bodies and their sizes a certain surface which determines their physical properties.
"For example, the surface corresponding to 1 lb. of steel has been computed for spheres, rounds and flats. For the sphere with a unit weight of 1 lb. the portion is a cone with the apex at the center of the Page 120 sphere and the base the curved surface of the sphere (surface exposed to quenching). For rounds, a unit weight of 1 lb. may be taken as a disk or cylinder, the base and top surfaces naturally do not enter into calculation. For a flat, a prismatic or cylindrical volume may be taken to represent the unit weight. The surfaces that are considered in this instance are the top and base of the section, as these surfaces are the ones exposed to cooling."
The results of the calculations are as follows:
| Diameter of sphere |
Surface per pound of steel |
|---|---|
| X | Y |
| 8 in. | 2.648 sq. in. |
| 6 in. | 3.531 sq. in. |
| 4 in. | 5.294 sq. in. |
| 3 in. | 7.062 sq. in. |
| 2 in. | 10.61 sq. in. |
| XY = 21.185. | |
| Diameter of round |
Surface per pound of steel |
|---|---|
| X | Y |
| 8.0 in. | 1.765 sq. in. |
| 6.0 in. | 2.354 sq. in. |
| 5.0 in. | 2.829 sq. in. |
| 4.0 in. | 3.531 sq. in. |
| 3.0 in. | 4.708 sq. in. |
| 2.0 in. | 7.062 sq. in. |
| 1.0 in. | 14.125 sq. in. |
| 0.5 in. | 28.25 sq. in. |
| 0.25 in. | 56.5 sq. in. |
| XY = 14.124. | |
| Diameter of flat |
Surface per pound of steel |
|---|---|
| X | Y |
| 8.0 in. | 0.8828 sq. in. |
| 6.0 in. | 1.177 sq. in. |
| 5.0 in. | 1.412 sq. in. |
| 4.0 in. | 1.765 sq. in. |
| 3.0 in. | 2.345 sq. in. |
| 2.0 in. | 3.531 sq. in. |
| 1.0 in. | 7.062 sq. in. |
| 0.5 in. | 14.124 sq. in. |
| 0.25 in. | 28.248 sq. in. |
| XY = 7.062. | |
Page 121 Having once determined the physical qualities of a certain specimen, and found its position on the curve we have the means to predict the decrease of physical qualities on larger specimens which receive the same heat treatment.
When the surfaces of the unit weight as outlined in the foregoing tables are plotted as ordinates and the corresponding diameters as abscissæ, the resulting curve is a hyperbola and follows the law XY = C. In making these calculations the radii or one-half of the thickness need only to be taken into consideration as the heat is conducted from the center of the body to the surface, following the shortest path.
The equations for the different shapes are as follows:
| For flats | XY = 7.062 |
| For rounds | XY = 14.124 |
| For spheres | XY = 21.185 |
It will be noted that the constants increase in a ratio of 1, 2, and 3, and the three bodies in question will increase in hardness on being quenched in the same ratio, it being understood that the diameter of the sphere and round and thickness of the flat are equal.
Relative to shape, it is interesting to note that rounds, squares, octagons and other three axial bodies, with two of their axes equal, have the same surface for the unit weight.
For example:
| Size | Length | Surface | Weight | Surface for 1 lb. |
|---|---|---|---|---|
| 2 in. Sq. | 12 in. | 96.0 sq. in. | 13.60 lb. | 7.06 sq. in. |
| 2 in. Round | 12 in. | 75.4 sq. in. | 10.68 lb. | 7.06 sq. in. |
Although this discussion is at present based upon mathematical analysis, it is hoped that it will open up a new field of investigation in which but little work has been done, and may assist in settling the as yet unsolved question of the effect of size and shape in the heat treatment of steel.
The heat-treating department of the Brown-Lipe-Chapin Company, Syracuse, N. Y., runs day and night, and besides handling all the hardening of tools, parts of jigs, fixtures, special machines and appliances, carburizes and heat-treats every month between 150,000 and 200,000 gears, pinions, crosses and other components entering into the construction of differentials for automobiles.
The treatment of the steel really begins in the mill, where the steel is made to conform to a specific formula. On the arrival of the rough forgings at the Brown-Lipe-Chapin factory, the first of a long series of inspections begins.
Page 122 Annealing Method.—Forgings which are too hard to machine are put in pots with a little charcoal to cause a reducing atmosphere and to prevent scale. The covers are then luted on and the pots placed in the furnace. Carbon steel from 15 to 25 points is annealed at 1,600°F. Nickel steel of the same carbon and containing in addition 3½ per cent nickel is annealed at 1,450°F. When the pots are heated through, they are rolled to the yard and allowed to cool. This method of annealing gives the best hardness for quick machining.
The requirements in the machine operations are very rigid and, in spite of great care and probably the finest equipment of special machines in the world, a small percentage of the product fails to pass inspection during or at the completion of the machine operations. These pieces, however, are not a loss, for they play an important part in the hardening process, indicating as they do the exact depth of penetration of the carburizing material and the condition of both case and core.
Heat-treating Department.—The heat-treating department occupies an L-shaped building. The design is very practical, with the furnace and the floor on the same level so that there is no lifting of heavy pots. Fuel oil is used in all the furnaces and gives highly satisfactory results. The consumption of fuel oil is about 2 gal. per hour per furnace.
The work is packed in the pots in a room at the entrance to the heat-treatment building. Before packing, each gear is stamped with a number which is a key to the records of the analysis and complete heat treatment of that particular gear. Should a question at any time arise regarding the treatment of a certain gear, all the necessary information is available if the number on the gear is legible. For instance, date of treatment, furnace, carburizing material, position of the pot in the furnace, position of gear in pot, temperature of furnace and duration of treatment are all tabulated and filed for reference.
After marking, all holes and parts which are to remain uncarburized are plugged or luted with a mixture of kaolin and Mellville gravel clay, and the gear is packed in the carburizing material. Bohnite, a commercial carburizing compound is used exclusively at this plant. This does excellent work and is economical. Broadly speaking, the economy of a carburizing compound depends on its lightness. The space not occupied by work must be filled with compound; therefore) other things being Page 123 equal, a compound weighing 25 lb. would be worth more than twice as much as one weighing 60 lb. per cubic foot. It has been claimed that certain compounds can be used over and over again, but this is only true in a limited way, if good work is required. There is, of course, some carbon in the compound after the first use, but for first-class work, new compound must be used each time.
The Packing Department.—In Fig. 56 is shown the packing pots where the work is packed. These are of malleable cast iron, with an internal vertical flange around the hole A. This fits in a bell on the end of the cast-iron pipe B, which is luted in position with fireclay before the packing begins. At C is shown a pot ready for packing. The crown gears average 10 to 12 in. in diameter and weigh about 11 lb. each. When placed in the pots, they surround the central tube, which allows the heat to circulate. Each pot contains five gears. Two complete scrap gears are in each furnace (i.e., gears which fail to pass machining inspection), and at the top of front pot are two or more short segments of scrap gear, used as test pieces to gage depth of case.
 FIG. 56.—Packing department and special pots.
FIG. 56.—Packing department and special pots.
After filling to the top with compound, the lid D is luted on. Ten pots are then placed in a furnace. It will be noted that the pots to the right are numbered 1, 2, 3, 4, indicating the position they are to occupy in the furnace.
The cast-iron ball shown at E is small enough to drop through the pipe B, but will not pass through the hole A in the bottom of Page 124 the pot. It is used as a valve to plug the bottom of the pot to prevent the carburizing compound from dropping through when removing the carburized gears to the quenching bath.
Without detracting from the high quality of the work, the metallurgist in this plant has succeeded in cutting out one entire operation and reducing the time in the hardening room by about 24 hr.
Formerly, the work was carburized at about 1,700°F. for 9 hr. The pots were then run out into the yard and allowed to cool slowly. When cool, the work was taken out of the pots, reheated and quenched at 1,600°F. to refine the core. It was again reheated to 1,425°F. and quenched to refine the case. Finally, it was drawn to the proper temper.
Short Method of Treatment.—In the new method, the packed pots are run into the case-hardening furnaces, which are heated to 1,600°F. On the insertion of the cold pots, the temperature naturally falls. The amount of this fall is dependent upon a number of variables, but it averages nearly 500°F. as shown in the pyrometer chart, Fig. 61. The work and furnace must be brought to 1,600°F. Within 2½ hr.; otherwise, a longer time will be necessary to obtain the desired depth of case. On this work, the depth of case required is designated in thousandths, and on crown gears, the depth in 0.028 in. Having brought the work to a temperature of 1,600°F. the depth of case mentioned can be obtained in about 5½ hr. by maintaining this temperature.
As stated before, at the top of each pot are several test pieces consisting of a whole scrap gear and several sections. After the pots have been heated at 1,600°F. for about 5¼ hr., they are removed, and a scrap-section test-piece is quenched direct from the pot in mineral oil at not more than 100°F. The end of a tooth of this is then ground and etched to ascertain the depth of case. As these test pieces are of exactly the same cross-section as the gears themselves, the carburizing action is similar. When the depth of case has been found from the etched test pieces to be satisfactory, the pots are removed. The iron ball then is dropped into the tube to seal the hole in the bottom of the pot; the cover and the tube are removed, and the gears quenched direct from the pot in mineral oil, which is kept at a temperature not higher than 100°F.
The Effect.—The heating at 1,600°F. gives the first heat treatment which refines the core, which under the former high heat Page 125 (1,700°F.) was rendered coarsely crystalline. All the gears, including the scrap gears, are quenched direct from the pot in this manner.
The gears then go to the reheating furnaces, situated in front of a battery of Gleason quenching machines. These furnaces accommodate from 12 to 16 crown gears. The carbon-steel gears are heated in a reducing atmosphere to about 1,425°F. (depending on the carbon content) placed in the dies in the Gleason quenching machine, and quenched between dies in mineral oil at less than 100°F. The test gear receives exactly the same treatment as the others and is then broken, giving a record of the condition of both case and core.
Affinity of Nickel Steel for Carbon.—The carbon- and nickel-steel gears are carburized separately owing to the difference in time necessary for their carburization. Practically all printed information on the subject is to the effect that nickel steel takes longer to carburize than plain carbon steel. This is directly opposed to the conditions found at this plant. For the same depth of case, other conditions being equal, a nickel-steel gear would require from 20 to 30 min. less than a low carbon-steel gear.
From the quenching machines, the gears go to the sand-blasting machines, situated in the wing of the heat-treating building, where they are cleaned. From here they are taken to the testing department. The tests are simple and at the same time most thorough.
Testing and Inspection of Heat Treatment.—The hard parts of the gear must be so hard that a new mill file does not bite in the least. Having passed this file test at several points, the gears go to the center-punch test. The inspector is equipped with a wooden trough secured to the top of the bench to support the gear, a number of center punches (made of ¾-in. hex-steel having points sharpened to an angle of 120 deg.) and a hammer weighing about 4 oz. With these simple tools, supplemented by his skill, the inspector can feel the depth and quality of the case and the condition of the core. The gears are each tested in this way at several points on the teeth and elsewhere, the scrap gear being also subjected to the test. Finally, the scrap gear is securely clamped in the straightening press shown in Fig. 57. With a 3½-lb. hammer and a suitable hollow-ended drift manipulated by one of Sandow's understudies, teeth are broken out of the scrap gear at various points. These give a record confirming Page 126 the center-punch tests, which, if the angle of the center punch is kept at 120 deg. and the weight of the hammer and blow are uniform, is very accurate.
After passing the center-punch test the ends of the teeth are peened lightly with a hammer. If they are too hard, small particles fly off. Such gears are drawn in oil at a temperature of from 300 to 350°F., depending on their hardness. Some builders prefer to have the extreme outer ends of the teeth drawn somewhat lower than the rest. This drawing is done on gas-heated red-hot plates, as shown at A in Fig. 58.
 FIG. 57.—Press for holding test gears for breaking.
FIG. 57.—Press for holding test gears for breaking.
Nickel steel, in addition to all the tests given to carbon steel, is subjected to a Brinell test. For each steel, the temperature and the period of treatment are specific. For some unknown reason, apparently like material with like treatment will, in isolated cases, not produce like results. It then remains for the treatment to be repeated or modified, but the results obtained during inspection form a valuable aid to the metallurgist in determining further treatment.
Page 127 Temperature Recording and Regulation.—Each furnace is equipped with pyrometers, but the reading and recording of all temperatures are in the hands of one man, who occupies a room with an opening into the end of the hardening department. The opening is about 15 ft. above the floor level. On each side of it, easily legible from all of the furnaces, is a board with the numbers of the various furnaces, as shown in Figs. 59 and 60. Opposite each furnace number is a series of hooks whereon are hung metal numbers representing the pyrometer readings of the temperature in that particular furnace. Within the room, as shown in Fig. 60, the indicating instrument is to the right, and to the left is a switchboard to connect it with the thermo-couples in the various furnaces. The boards shown to the right and the left swing into the room, which enables the attendant easily to change the numbers to conform to the pyrometer readings. Readings of the temperatures of the carburizing furnaces are taken and tabulated every ten minutes. These, numbered 1 to 10, are shown on the board to the right in Fig. 59. The card shown in Fig. 61 gives such a record. These records are filed away for possible future reference.
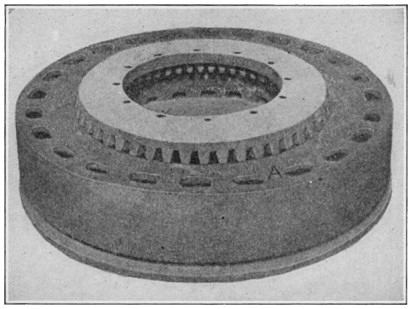 FIG. 58.—Gas heated drawing plate for tooth ends.
FIG. 58.—Gas heated drawing plate for tooth ends.
The temperatures of the reheating furnaces, numbered from 1 to 26 and shown on the board to the left in Fig. 59, are taken every 5 min.
Each furnace has a large metal sign on which is marked the temperature at which the furnace regulator is required to keep Page 129 his heat. As soon as any variation from this is posted on the board outside the pyrometer room, the attendant sees it and adjusts the burners to compensate.
 FIG. 59.—Pyrometer recording room.
FIG. 59.—Pyrometer recording room.
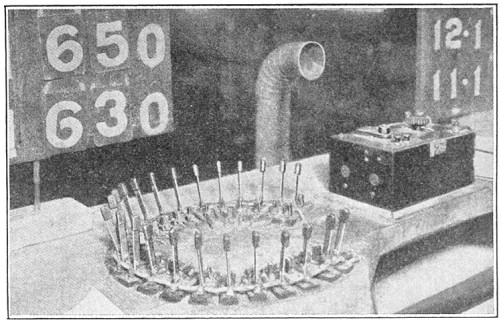 FIG. 60.—Inside of Pyrometer switch room.
FIG. 60.—Inside of Pyrometer switch room.
Dies for Gleason Tempering Machines.—In Fig. 62 is shown a set of dies for the Gleason tempering machine. These accurately made dies fit and hold the gear true during quenching, thus preventing distortion.
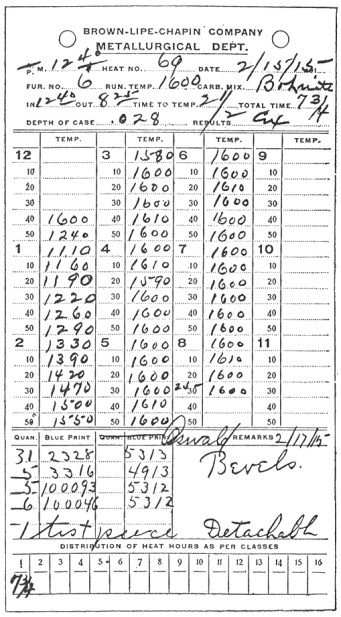 FIG. 61.—Carburizing furnace record.
FIG. 61.—Carburizing furnace record.
Referring to Fig. 62, the die A has a surface B which fits the face of the teeth of the gear C. This surface is perforated by a large number of holes which permit the quenching oil to circulate freely. The die A is set in the upper end of the plunger Page 130 A of the tempering machine, shown in Fig. 63, a few inches above the surface of the quenching oil in the tank N. Inside the die A are the centering jaws D, Fig. 62, which are an easy fit for the bore of the gear C. The inner surface of the centering jaws is in the shape of a female cone. The upper die is shown at E. In the center (separate from it, but a snug sliding fit in it) is the expander G, which, during quenching, enters the taper in the centering jaws D, expanding them against the bore of the gear C. The faces F of the upper die E fit two angles at the back of the gear and are grooved for the passage of the quenching oil. The upper die E is secured to the die carrier B, shown in Fig. 9, and inside the die is the expander G, which is backed up by compression springs.
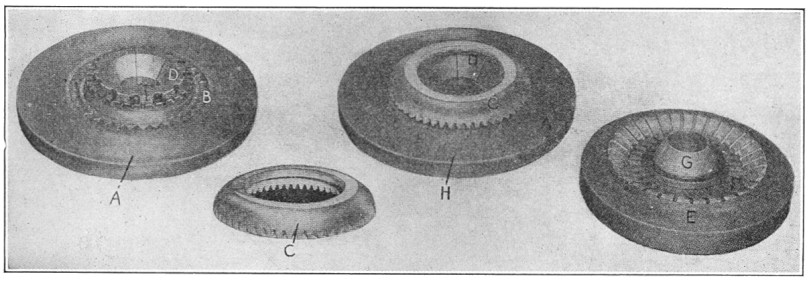 FIG. 62.—Dies for Gleason gear-hardening machine.
FIG. 62.—Dies for Gleason gear-hardening machine.
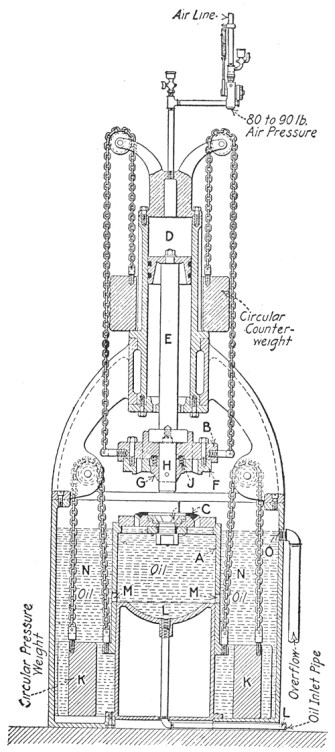 FIG. 63.—Gleason tempering machine.
FIG. 63.—Gleason tempering machine.
Hardening Operation.—Hardening a gear is accomplished as follows: The gear is taken from the furnace by the furnaceman and placed in the lower die, surrounding the centering jaws, as shown at H in Fig. 62 and C in Fig. 63. Air is then turned into the cylinder D, and the piston rod E, the die carrier B, the top die F and the expander G descend. The pilot H enters a hole in the center of Page 131 the lower die, and the expander G enters the centering jaws I, causing them to expand and center the gear C in the lower die. On further advance of the piston rod E, the expander G is forced upward against the pressure of the springs J and the upper die F comes in contact with the upper surface of the gear. Further downward movement of the dies, which now clamp the work securely, overcomes the resistance of the pressure weight K (which normally keeps up the plunger A), and the gear is submerged in the oil. The quenching oil is circulated through a cooling system outside the building and enters the tempering machine through the inlet pipe L. When the machine is in the position shown, the oil passes out through the ports M in the lower plunger to the outer reservoir N, passing to the cooling system by way of the overflow O. When the lower plunger A is forced downward, the ports M are automatically closed and the cool quenching oil from the inlet pipe L, having no other means of escape, passes through the holes in the lower die and the grooves in the upper, circulating in contact with the surfaces of the gear and passes to Page 132 the overflow. When the air pressure is released, the counterweights return the parts to the positions shown in Fig. 63, and the operator removes the gear.
The gear comes out uniformly hard all over and of the same degree of hardness as when tempered in an open tank. The output of the machine depends on the amount of metal to be cooled, but will average from 8 to 16 per hour. Each machine is served by one man, two furnaces being required to heat the work. A slight excess of oil is used in the firing of the furnaces to give a reducing atmosphere and to avoid scale.
 FIG. 64.—Hardening and shrinking sleeves.
FIG. 64.—Hardening and shrinking sleeves.
Carburizing Low-carbon Sleeves.—Low-carbon sleeves are carburized and pushed on malleable-iron differential-case hubs. Formerly, these sleeves were given two treatments after carburization in order to refine the case and the core, and then sent to the grinding department, where they were ground to a push fit for the hubs. After this they were pushed on the hubs. By the method now employed, the first treatment refines the core, and on the second treatment, the sleeves are pushed on the hub and at the same time hardened. This method cuts out the internal grinding time, pressing on hubs, and haulage from one department to another. Also, less work is lost through splitting of the sleeves.
Page 133 The machine for pushing the sleeves on is shown in Fig. 64. At A is the stem on which the hot sleeve B is to be pushed. The carburized sleeves are heated in an automatic furnace, which takes them cold at the back and feeds them through to the front, by which time they are at the correct temperature. The loose mandrel C is provided with a spigot on the lower end, which fits the hole in the differential-case hub. The upper end is tapered as shown and acts as a pilot for the ram D. The action of pushing on and quenching is similar to the action of the Gleason tempering machine, with the exception that water instead of oil is used as a quenching medium. The speed of operation depends on a number of variables, but from 350 to 500 can be heated and pressed on in 11 hr.
Cyanide Bath for Tool Steels.—All high-carbon tool steels are heated in a cyanide bath. With this bath, the heat can be controlled within 3 deg. The steel is evenly heated without exposure to the air, resulting in work which is not warped and on which there is no scale. The cyanide bath is, of course, not available for high-speed steel because of the very high temperatures necessary.
The kind of steel used in the die of course influences the heat treatment it is to receive, but this also depends on the kind of work the die is to perform. If the die is for a forging which is machined all over and does not have to be especially close to size, where a variation of 1/16 in. is not considered excessive, a low grade steel will be perfectly satisfactory.
In cases of fine work, however, where the variation cannot be over 0.005 to 0.01 in. we must use a fine steel and prevent its going out of shape in the heating and quenching. A high quality crucible steel is suggested with about the following analysis: Carbon 0.75 per cent, manganese 0.25 per cent, silicon 0.15 per cent, sulphur 0.015 per cent, and phosphorus 0.015 per cent. Such a steel will have a decalescent point in the neighborhood of 1,355°F. and for the size used, probably in a die of approximately 8 in., it will harden around 1,450°F.
To secure best results care must be taken at every step. The block should be heated slowly to about 1,400°F., the furnace closed tight and allowed to cool slowly in the furnace itself. It should not soak at the high temperature.
Page 134 After machining, and before it is put in the furnace for hardening, it should be slowly preheated to 800 or 900°F. This can be done in several ways, some putting the die block in front of the open door of a hardening furnace and keeping the furnace at about 1,000°F. The main thing is to heat the die block very slowly and evenly.
The hardening heat should be very slow, 7 hr. being none too long for such a block, bringing the die up gradually to the quenching temperature of 1,450°. This should be held for 1/2 hr. or even a little more, when the die can be taken out and quenched. There should be no guess work about the heating, a good pyrometer being the only safe way of knowing the correct temperature.
The quenching tank should be of good size and have a spray or stream of water coming up near the surface. Dip the die block about 3 in. deep and let the stream of water get at the face so as to play on the forms. By leaving the rest of the die out of the water, moving the die up and down a trifle to prevent a crack at the line of immersion, the back of the block is left tough while the face is very hard. To overcome the tendency to warp the face it is a good plan to pour a little water on the back of the die as this tends to even up the cooling. The depth to which the die is dipped can be easily regulated by placing bars across the tank at the proper depth.
After the scleroscope shows the die to be properly hardened, which means from 98 to 101, the temper should be drawn as soon as convenient. A lead pot in which the back of the die can be suspended so as to heat the back side, makes a good method. Or the die block can be placed back to the open door of a furnace. On a die of this size it may take several hours to draw it to the desired temper. This can be tested while warm by the scleroscope method, bearing in mind that the reading will not be the same as when cold. If the test shows from 76 to 78 while warm, the hardness when cold will be about 83, which is about right for this work.
The Society of Automotive Engineers have adopted certain heat treatments to suit different steels and varying conditions. These have already been referred to on pages 39 to 41 in connection with the different steels used in automobile practice. These treatments are designated by letter and correspond with the designations in the table.
Heat Treatment A
After forging or machining:
Heat Treatment B
After forging or machining:
Heat Treatment D
After forging or machining:
Heat Treatment E
After forging or machining:
Heat Treatment F
After shaping or coiling:
Heat Treatment G
After forging or machining:
Heat Treatment H
After forging or machining:
Page 136 Heat Treatment K
After forging or machining:
Heat Treatment L
After forging or machining:
Heat Treatment M
After forging or machining:
Heat Treatment P
After forging or machining:
Heat Treatment Q
After forging:
Heat Treatment R
After forging:
Heat Treatment S
After forging or machining:
Heat Treatment T
After forging or machining:
Page 137 Heat Treatment U
After forging:
Heat Treatment V
After forging or machining:
 FIG. 65.—Chart of changes due to heating and cooling.
FIG. 65.—Chart of changes due to heating and cooling.
The effect of heat treatment on overheated steel is shown graphically in Fig. 65 to the series of illustrations on pages 137 to 144. This was prepared by Thos. Firth & Sons, Ltd., Sheffield, England.
 FIG. 66.—The structure of overheated mild steel from which all
the pegs were made (magnified 25 diameters). The pegs withdrawn at
720°C., or earlier, had this structure and were quite soft.
FIG. 66.—The structure of overheated mild steel from which all
the pegs were made (magnified 25 diameters). The pegs withdrawn at
720°C., or earlier, had this structure and were quite soft.
 FIG. 67.—Peg withdrawn at 750°C. (magnified 25 diameters).
The structure is apparently unaltered, but the peg was hard and,
unlike the earlier ones, would not bend double.
FIG. 67.—Peg withdrawn at 750°C. (magnified 25 diameters).
The structure is apparently unaltered, but the peg was hard and,
unlike the earlier ones, would not bend double.
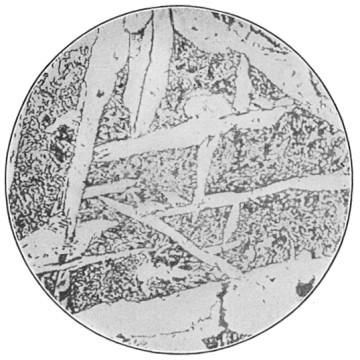 FIG. 68.—A portion of 66 magnified 200 diameters to show that
the dark (pearlite) areas are laminated.
FIG. 68.—A portion of 66 magnified 200 diameters to show that
the dark (pearlite) areas are laminated.
 FIG. 69.—A portion of 67 magnified 200 diameters,
showing that pearlite areas are no longer laminated and providing
reason for observed hardness
FIG. 69.—A portion of 67 magnified 200 diameters,
showing that pearlite areas are no longer laminated and providing
reason for observed hardness
 FIG. 70.—Peg withdrawn at 780°C. (magnified 25 diameters),
showing inter-diffusion of transformed pearlite and ferrite areas.
FIG. 70.—Peg withdrawn at 780°C. (magnified 25 diameters),
showing inter-diffusion of transformed pearlite and ferrite areas.
 FIG. 71.—Peg withdrawn at 800°C. (magnified
25 diameters), showing inter-diffusion so far advanced that the
original outline of the crystals is now only faintly suggested.
FIG. 71.—Peg withdrawn at 800°C. (magnified
25 diameters), showing inter-diffusion so far advanced that the
original outline of the crystals is now only faintly suggested.
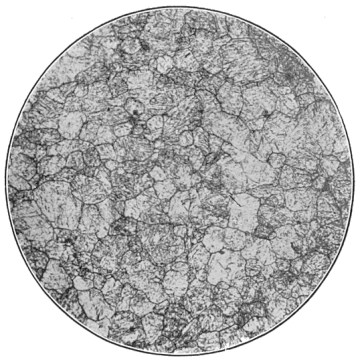 FIG. 72.—Peg withdrawn at 850°C. (magnified 100 diameters)
after inter-diffusion was completed. Note the regular outlines and
the small size of the crystals as compared with 67.
FIG. 72.—Peg withdrawn at 850°C. (magnified 100 diameters)
after inter-diffusion was completed. Note the regular outlines and
the small size of the crystals as compared with 67.
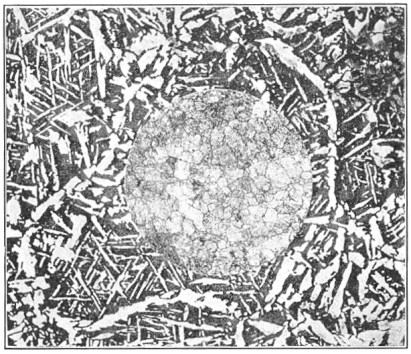 FIG. 73.—To facilitate comparison 67 was enlarged to the same
magnification as 62, and the one superimposed on the other. The
single large crystal occupied as much space as 8,000 of the smaller
ones.
FIG. 73.—To facilitate comparison 67 was enlarged to the same
magnification as 62, and the one superimposed on the other. The
single large crystal occupied as much space as 8,000 of the smaller
ones.
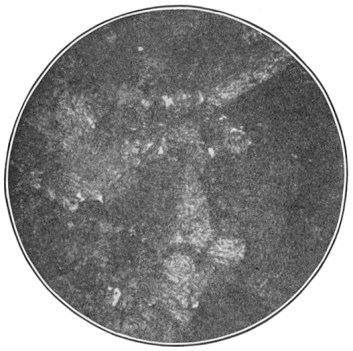 FIG. 74.—The peg withdrawn on cooling at 800°C. (magnified
100 diameters) shows the first reappearance of free ferrite. All pegs
withdrawn at higher temperatures were like Fig. 72.
FIG. 74.—The peg withdrawn on cooling at 800°C. (magnified
100 diameters) shows the first reappearance of free ferrite. All pegs
withdrawn at higher temperatures were like Fig. 72.
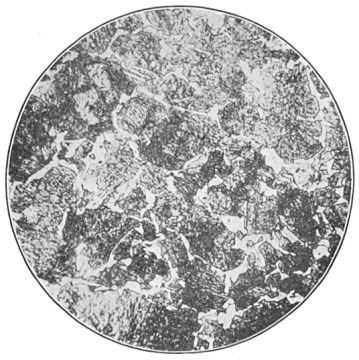 FIG. 75.—Peg withdrawn after cooling to 760°C.
The increased amount of free ferrite arranges itself about the
crystals as envelopes.
FIG. 75.—Peg withdrawn after cooling to 760°C.
The increased amount of free ferrite arranges itself about the
crystals as envelopes.
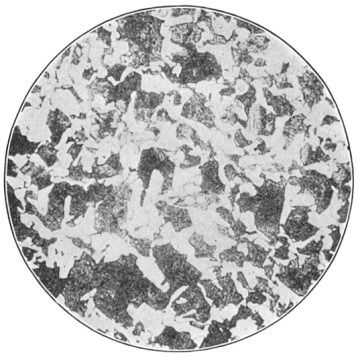 FIG. 76.-Peg withdrawn after cooling to 740°C.
FIG. 76.-Peg withdrawn after cooling to 740°C.
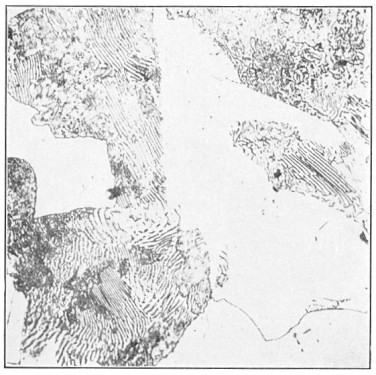 FIG. 77.—Peg withdrawn after cooling to 670°C. (magnified
800 diameters). Just at this moment the lamination of pearlite,
which now occupied its original area, was taking place. In some
parts the lamination was perfect, in other parts the iron and
iron-carbide were still dissolved in each other.
FIG. 77.—Peg withdrawn after cooling to 670°C. (magnified
800 diameters). Just at this moment the lamination of pearlite,
which now occupied its original area, was taking place. In some
parts the lamination was perfect, in other parts the iron and
iron-carbide were still dissolved in each other.
The center piece Fig. 65 represents a block of steel weighing about 25 lb. The central hole accommodated a thermo-couple which was attached to an autographic recorder. The curve is a copy of the temperature record during heating and cooling. Into the holes in the side of the block small pegs of overheated mild steel were inserted. One peg was withdrawn and quenched at Page 144 each of the temperatures indicated by the numbered arrows, and after suitable preparation these pegs were photographed in order to show the changes in structure taking place during heating and cooling operations. The illustrations here reproduced are selected from those photographs with the object of presenting pictorially the changes involved in the refining of overheated steel or steel castings. Figures 66 to 79 with their captions show much that is of value to steel users.
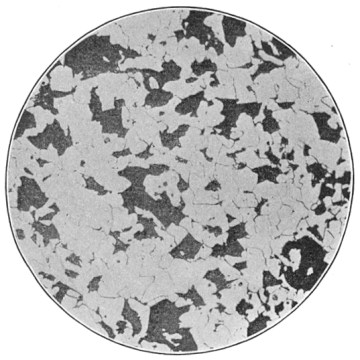 FIG. 78.—Any peg withdrawn after 670°C. on cooling
(magnified 100 diameters).
FIG. 78.—Any peg withdrawn after 670°C. on cooling
(magnified 100 diameters).
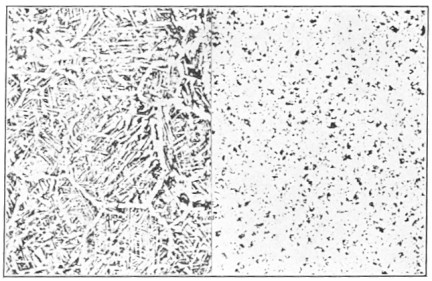 FIG. 79.—Structure of overheated steel before (left) and after
refining (right).
FIG. 79.—Structure of overheated steel before (left) and after
refining (right).
HARDENING CARBON STEEL FOR TOOLS
For years the toolmaker had full sway in regard to make of steel wanted for shop tools, he generally made his own designs, hardened, tempered, ground and usually set up the machine where it was to be used and tested it.
Most of us remember the toolmaker during the sewing machine period when interchangeable tools were beginning to find their way; rather cautiously at first. The bicycle era was the real beginning of tool making from a manufacturing standpoint, when interchangeable tools for rapid production were called for and toolmakers were in great demand. Even then, jigs, and fixtures were of the toolmaker's own design, who practically built every part of it from start to finish.
The old way, however, had to be changed. Instead of the toolmaker starting his work from cutting off the stock in the old hack saw, a place for cutting off stock was provided. If, for instance, a forming tool was wanted, the toolmaker was given the master tool to make while an apprentice roughed out the cutter. The toolmaker, however, reserved the hardening process for himself. That was one of the particular operations that the old toolmaker refused to give up. It seemed preposterous to think for a minute that any one else could possibly do that particular job without spoiling the tools, or at least warp it out of shape (most of us did not grind holes in cutters 15 to 20 years ago); or a hundred or more things might happen unless the toolmaker did his own hardening and tempering.
That so many remarkably good tools were made at that time is still a wonder to many, when we consider that the large shop had from 30 to 40 different men, all using their own secret compounds, heating to suit eyesight, no matter if the day was bright or dark, and then tempering to color. But the day of the old toolmaker has changed. Now a tool is designed by a tool designer, O.K.'d, and then a print goes to the foreman of the tool department, who specifies the size and gets the steel from the cutting-off department. After finishing the machine work Page 146 it goes to the hardening room, and this is the problem we shall now take up in detail.
The Modern Hardening Room.—A hardening room of today means a very different place from the dirty, dark smithshop in the corner with the open coal forge. There, when we wanted to be somewhat particular, we sometimes shoveled the coal cinders to one side and piled a great pile of charcoal on the forge. We now have a complete equipment; a gas- or oil-heating furnace, good running water, several sizes of lead pots, and an oil tank large enough to hold a barrel of oil. By running water, we mean a large tank with overflow pipes giving a constant supply. The ordinary hardening room equipment should consist of:
Gas or oil muffle furnace for hardening.
Gas or oil forge furnace.
A good size gas or oil furnace for annealing and case-hardening.
A gas or oil furnace to hold lead pots.
Oil tempering tank, gas- or oil-heated.
Pressure blower.
Large oil tank to hold at least a barrel of oil.
Big water tank with screen trays connected with large pipe from bottom
with overflow.
Straightening press.
The furnace should be connected with pyrometers and tempering tank with
a thermometer.
Beside all this you need a good man. It does not make much difference how completely the hardening department is fitted up, if you expect good work, a small percentage of loss and to be able to tackle anything that comes along, you must have a good man, one who understands the difference between low- and high-carbon steel, who knows when particular care must be exercised on particular work. In other words, a man who knows how his work should be done, and has the intelligence to follow directions on treatments of steel on which he has had no experience.
Jewelers' tools, especially for silversmith's work, probably have to stand the greatest punishment of any all-steel tools and to make a spoon die so hard that it will not sink under a blow from an 1,800-lb. hammer with a 4-ft. drop, and still not crack, demands careful treatment.
To harden such dies, first cover the impression on the die with paste made from bone dust or lampblack and oil. Place face down in an iron box partly filled with crushed charcoal, leaving back of die uncovered so that the heat can be seen at all times. Page 147 Heat slowly in furnace to a good cherry red. The heat depends on the quality and the analysis of steel and the recommended actions of the steel maker should be carefully followed. When withdrawn from the fire the die should be quenched as shown in Fig. 80 with the face of die down and the back a short distance out of the water. When the back is black, immerse all over.
 FIG. 80.—Quenching a die, face down.
FIG. 80.—Quenching a die, face down.
If such a tank is not at hand, it would pay to rig one up at once, although a barrel of brine may be used, or the back of the die may be first immersed to a depth of about 1/2 in. When the piece is immersed, hold die on an angle as in Fig. 81.
 FIG. 81.—Hold die at angle to quench.
FIG. 81.—Hold die at angle to quench.
This is for the purpose of expelling all steam bubbles as they form in contact with hot steel. We are aware of the fact that a great many toolmakers in jewelry shops still cling to the overhead bath, as in Fig. 82, but more broken pieces and more dies with soft spots are due to this method than to all the others combined, as the water strikes one spot in force, contracting Page 148 the surface so much faster than the rest of the die that the results are the same as if an uneven heating had been given the steel.
Take Time for Hardening.—Uneven heating and poor quenching has caused loss of many very valuable dies, and it certainly seems that when a firm spends from $75 to $450 in cutting a die that a few hours could be spared for proper hardening. But the usual feeling is that a tool must be hurried as soon as the hardener gets it, and if a burst die is the result from either uneven or overheated steel and quenching same without judgment, the steel gets the blame.
 FIG. 82.—An obsolete method.
FIG. 82.—An obsolete method.
Give the steel a chance to heat properly, mix a little common sense with "your 30 years experience on the other fellows steel." Remember that high-carbon steel hardens at a lower heat than low-carbon steel, and quench when at the right heat in the two above ways, and 99 per cent of the trouble will vanish.
When a die flies to pieces in quenching, don't rush to the superintendent with a "poor-steel" story, but find out first why it broke so that the salesman who sold it will not be able to harden piece after piece from the same bar satisfactorily. If you find a "cold short," commonly called "a pipe," you can lay the blame on the steelmaker. If it is a case of overheating and quenching Page 149 when too hot, you will find a coarse grain with many bright spots like crystals to the hardening depth. If uneven heating is the cause, you will find a wider margin of hardening depth on one side than on the other, or find the coarse grain from over-heating on one side while on the other you will find a close grain, which may be just right. If you find any other faults than a "pipe," or are not able to harden deep enough, then take the blame like a man and send for information. The different steel salesmen are good fellows and most of them know a thing or two about their own business.
For much work a cooling bath at from 50 to 75°F. is very good both for small hobs, dies, cutter plates or plungers. Some work will harden best in a barrel of brine, but in running cold water, splendid results will be obtained. Cutter plates should always be dipped corner first and if any have stripper holes, they should first be plugged with asbestos or fire clay cement.
In general it may be said that the best hardening temperature for carbon steel is the lowest temperature at which it will harden properly.
Carbon tool steel, or "tool steel" as it is commonly called, usually contains from 80 to 125 points (or from 0.80 to 1.25 per cent) of carbon, and none of the alloys which go to make up the high speed steels. This was formerly known also as crucible or "cast" steel, or crucible cast steel, from the way in which it was made. This was before the days of steel castings. The advent of these caused so much confusion that the term was soon dropped. When we say "tool steel," we nearly always refer to carbon-tool steel, high-speed steel being usually designated by that name.
For many purposes carbon-steel cutters are still found best, although where a large amount of material is to be removed at a rapid rate, it has given way to high-speed steels.
All users of tool steels should carefully study the different qualities of the steels they handle. Different uses requires different kinds of steel for best results, and for the purpose of designating different steels some makers have adopted the two terms "temper," and "quality," to distinguish between them.
In this case temper refers to the amount of carbon which Page 150 is combined with the iron to make the metal into a steel. The quality means the absence of phosphorous, sulphur and other impurities, these depending on the ores and the methods of treatment.
Steel makers have various ways of designating carbon steels for different purposes. Some of these systems involve the use of numbers, that of the Latrobe Steel Company being given herewith. It will be noted that the numbers are based on 20 points of carbon per unit. The names given the different tempers are also of interest. Other makers use different numbers.
The temper list follows:
| No. 3 | temper 0.60 to 0.69 per cent carbon |
| No. 3½ | temper 0.70 to 0.79 per cent carbon |
| No. 4 | temper 0.80 to 0.89 per cent carbon |
| No. 4½ | temper 0.90 to 0.99 pet cent carbon |
| No. 5 | temper 1.00 to 1.09 per cent carbon |
| No. 5½ | temper 1.10 to 1.19 per cent carbon |
| No. 6 | temper 1.20 to 1.29 per cent carbon |
| No. 6½ | temper 1.30 to 1.39 per cent carbon |
| No. 7 | temper 1.40 to 1.49 per cent carbon |
Die Temper.—No. 3: All kinds of dies for deep stamping, pressing and drop forgings. Mining drills to harden only. Easily weldable.
Smiths' Tool Temper.—No. 3½: Large punches, minting and rivet dies, nailmakers' tools, hammers, hot and cold sets, snaps and boilermakers' tools, various smiths' tools, large shear blades, double-handed chisels, caulking tools, heading dies, masons' tools and tools for general welding purposes.
Shear Blade Temper.—No. 4: Punches, large taps, screwing dies, shear blades, table cutlery, circular and long saws, heading dies. Weldable.
General Purpose Temper.—No. 4½: Taps, small punches, screwing dies, sawwebs, needles, etc., and for all general purposes. Weldable.
Axe Temper.—No. 5: Axes, chisels, small taps, miners' drills and jumpers to harden and temper, plane irons. Weldable with care.
Cutlery Temper.—No. 5½: Large milling cutters, reamers, pocket cutlery, wood tools, short saws, granite drills, paper and tobacco knives. Weldable with very great care.
Tool Temper.—No. 6: Turning, planing, slotting, and shaping tools, twist drills, mill picks, scythes, circular cutters, engravers' tools, surgical cutlery, circular saws for cutting metals, bevel and other sections for turret lathes. Not weldable.
Hard Tool Temper.—No. 6½: Small twist drills, razors, small and intricate engravers' tools, surgical instruments, knives. Not weldable.
Razor Temper.—No. 7: Razors, barrel boring bits, special lathe tools for turning chilled rolls. Not weldable.
The highest grades of carbon or tempering steels are to be recommended for tools which have to withstand shocks, such as for cold chisels or punches. These steels are, however, particularly useful where it is necessary to cut tempered or heat-treated steel which is more than ordinarily hard, for cutting chilled iron, etc. They are useful for boring, for rifle-barrel drilling, for fine finishing cuts, for drawing dies for brass and copper, for blanking dies for hard materials, for formed cutters on automatic screw machines and for roll-turning tools.
Steel of this kind, being very dense in structure, should be given more time in heating for forging and for hardening, than carbon steels of a lower grade. For forging it should be heated slowly and uniformly to a bright red and only light blows used as the heat dies out. Do not hammer at all at a black heat. Reheat slowly to a dark red for hardening and quench in warm water. Grind on a wet grindstone.
Where tools have to withstand shocks and vibration, as in pneumatic hammer work, in severe punching duty, hot or cold upsetting or similar work, tool steels containing vanadium or chrome-vanadium give excellent results. These are made particularly for work of this kind.
[Footnote 1: Abstract of paper by HENRY FOWLER, chief mechanical engineer of the Midland Ry., England, before the Institution of Mechanical Engineers.]
In the chief mechanical engineer's department of the Midland Ry., after considerable experimenting, it was decided to order chisel steel to the following specifications: carbon, 0.75 to 0.85 per cent, the other constituents being normal. This gives a complete analysis as follows: carbon, 0.75 to 0.85; manganese, 0.30; silicon, 0.10; sulphur, 0.025; phosphorus, 0.025.
The analysis of a chisel which had given excellent service was as follows: carbon, 0.75; manganese, 0.38; silicon, 0.16; sulphur, 0.028; phosphorus, 0.026. The heat treatment is unknown.
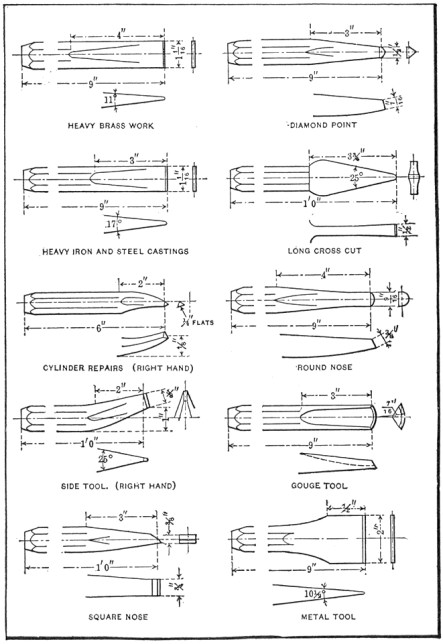 FIG. 83.—Forms of chisels standardized for the locomotive
shops of the Midland Ry., England.
FIG. 83.—Forms of chisels standardized for the locomotive
shops of the Midland Ry., England.
At the same time that chisel steel was standardized, the form of the chisels themselves was revised, and a standard chart of these as used in the locomotive shops was drawn up. Figure 83 shows the most important forms, which are made to stock orders Page 152 in the smithy and forwarded to the heat-treatment room where the hardening and tempering is carried out on batches of fifty. A standard system of treatment is employed, which to a very large extent does away with the personal element. Since the chemical composition is more or less constant, the chief variant is the section which causes the temperatures to be varied slightly. The chisels are carefully heated in a gas-fired furnace to a temperature of from 730 to 740°C. (1,340 to 1,364°F.) according to section. In practice, the first chisel, is heated to 730°C.; and Page 153 the second to 735°C. (1,355°F.); and a 1 in. half round chisel to 740°C., because of their varying increasing thickness of section at the points. Upon attaining this steady temperature, the chisels are quenched to a depth of 3/8 to 1/2 in. from the point in water, and then the whole chisel is immersed and cooled off in a tank containing linseed oil.
The oil-tank is cooled by being immersed in a cold-water tank through which water is constantly circulated. After this treatment, the chisels have a dead hard point and a tough or sorbitic shaft. They are then tempered or the point "let down." This is done by immersing them in another oil-bath which has been raised to about 215°C. (419°F). The first result is, of course, to drop the temperature of the oil, which is gradually raised to its initial point. On approaching this temperature the chisels are taken out about every 2°C. rise and tested with a file, and at a point between 215 and 220°C. (428°F.), when it is found that the desired temper has been reached, the chisels are removed, cleaned in sawdust, and allowed to cool in an iron tray.
No comparative tests of these chisels with those bought and treated by the old rule-of-thumb methods have been made, as no exact method of carrying out such tests mechanically, other than trying the hardness by the Brinell or scleroscope method, are known; any ordinary test depends so largely upon the dexterity of the operator. The universal opinion of foremen and those using the chisels as to the advantages of the ones receiving the standard treatment described is that a substantial improvement has been made. The chisels were not "normalized." Tests of chisels normalized at about 900°C. (1,652°F.) showed that they possessed no advantage.
Tools or pieces which have holes or deep depressions should be filled before heating unless it is necessary to have the holes hard on the inside. In that case the filling would keep the water away from the surface and no hardening would take place. Where filling is to be done, various materials are used by different hardeners. Fireclay and common putty seem to be favored by many.
Every mechanic who has had anything to do with the hardening of tools knows how necessary it is to take a cut from the surface of the bar that is to be hardened. The reason is that in the process of making the steel its outer surface has become decarbonized. This change makes it low-carbon steel, which will of Page 154 course not harden. It is necessary to remove from 1/16 to ¼ in. of diameter on bars ranging from 1/2 to 4 in.
This same decarbonization occurs if the steel is placed in the forge in such a way that unburned oxygen from the blast can get at it. The carbon is oxidized, or burned out, converting the outside of the steel into low-carbon steel. The way to avoid this is to use a deep fire. Lack of this precaution is the cause of much spoiled work, not only because of decarbonization of the outer surface of the metal, but because the cold blast striking the hot steel acts like boiling hot water poured into an ice-cold glass tumbler. The contraction sets up stresses that result in cracks when the piece is quenched.
It is especially important to prevent decarbonization in such tools as taps and form cutters, which must keep their shape after hardening and which cannot be ground away on the profile. For this reason it is well to put taps, reamers and the like into pieces of pipe in heating them. The pipe need be closed on one end only, as the air will not circulate readily unless there is an opening at both ends.
Even if used in connection with a blacksmith's forge the lead bath has an advantage for heating tools of complicated shapes, since it is easier to heat them uniformly and they are submerged and away from the air. The lead must be stirred frequently or the heat is not uniform in all parts of the lead bath. Covering the lead with powdered charcoal will largely prevent oxidization and waste of lead.
Such a bath is good for temperatures between 620 and 1,150°F. At higher temperatures there is much waste of lead.
Work quenched from a high temperature and not afterward tempered will, if complex in shape, contain many internal stresses which may later cause it to break. They may be eased off by slight heating without materially lessening the hardness of the piece. One way to do this is to hold the piece over a fire and test it with a moistened finger. Another way is to dip the piece in boiling water after it has first been quenched in a cold bath. Page 155 Such steps are not necessary with articles which will afterward be tempered and in which the strains are thus reduced.
In annealing steels the operation is similar to hardening, as far as heating is concerned. The critical temperatures are the proper ones for annealing as well as hardening. From this point on there is a difference, for annealing consists in cooling as slowly as possible. The slower the cooling the softer will be the steel.
Annealing may be done in the open air, in furnaces, in hot ashes or lime, in powdered charcoal, in burnt bone, in charred leather and in water. Open-air annealing will do as a crude measure in cases where it is desired to take the internal stresses out of a piece. Care must be taken in using this method that the piece is not exposed to drafts or placed on some cold substance that will chill it. Furnace annealing is much better and consists in heating the piece in a furnace to the critical temperature and then allowing the work and the furnace to cool together.
When lime or ashes are used as materials to keep air away from the steel and retain the heat, they should be first heated to make sure that they are dry. Powdered charcoal is used for high-grade annealing, the piece being packed in this substance in an iron box and both the work and the box raised to the critical temperature and then allowed to cool slowly. Machinery steel may be annealed in spent ground-bone that has been used in casehardening; but tool steel must never be annealed in this way, as it will be injured by the phosphorus contained in the bone. Charred leather is the best annealing material for high-carbon steel, because it prevents decarbonizing taking place.
Water annealing consists in heating the piece, allowing it to cool in air until it loses its red heat and becomes black and then immediately quenching it in water. This plan works well for very low-carbon steel; but for high-carbon steel what is known as the "double annealing treatment" must be given, provided results are wanted quickly. The process consists in heating the steel quickly to 200° or more above the upper critical, cooling in air down through the recalescence point, then reheating it to just above the critical point and again cooling slowly through the recalescence, then quenching in oil. This process retains in the steel a fine-grained structure combined with softness.
To secure proper hardness, the cooling of quenching of steel is as important as its heating. Quenching baths vary in nature, there being a large number of ways to cool a piece of steel in contrast to the comparatively few ways of heating it.
Plain water, brine and oil are the three most common quenching materials. Of these three the brine will give the most hardness, and plain water and oil come next. The colder that any of these baths is when the piece is put into it the harder will be the steel; but this does not mean that it is a good plan to dip the heated steel into a tank of ice water, for the shock would be so great that the bar would probably fly to pieces. In fact, the quenching bath must be sometimes heated a bit to take off the edge of the shock.
Brine solutions will work uniformly, or give the same degree of hardness, until they reach a temperature of 150°F. above which their grip relaxes and the metals quenched in them become softer. Plain water holds its grip up to a temperature of approximately 100°F.; but oil baths, which are used to secure a slower rate of cooling, may be used up to 500° or more. A compromise is sometimes effected by using a bath consisting of an inch or two of oil floating on the surface of water. As the hot steel passes through the oil, the shock is not as severe as if it were to be thrust directly into the water; and in addition, oil adheres to the tool and keeps the water from direct contact with the metal.
The old idea that mercury will harden steel more than any other quenching material has been exploded. A bath consisting of melted cyanide of potassium is useful for heating fine engraved dies and other articles that are required to come out free from scale. One must always be careful to provide a hood or exhaust system to get rid of the deadly fumes coming from the cyanide pot.
The one main thing to remember in hardening tool steel is to quench on a rising heat. This does not mean a rapid heating as a slow increase in temperature is much better in every way.
The Theory of Tempering.—Steel that has been hardened is generally harder and more brittle than is necessary, and in order to bring it to the condition that meets our requirements a treatment called tempering is used. This increases the toughness of the steel, i.e., decrease the brittleness at the expense of a slight decrease in hardness.
Page 157 There are several theories to explain this reaction, but generally it is only necessary to remember that in hardening we quench steel from the austenite phase, and, due to this rapid cooling, the normal change from austenite to the eutectoid composition does not have time to take place, and as a consequence the steel exists in a partially transformed, unstable and very hard condition at atmospheric temperatures. But owing to the internal rigidity which exists in cold metal the steel is unable to change into its more stable phase until atoms can rearrange themselves by the application of heat. The higher the heat, the greater the transformation into the softer phases. As the transformation takes place, a certain amount of heat of reaction, which under slow cooling would have been released in the critical range, is now released and helps to cause a further slight reaction.
If a piece of steel is heated to a certain temperature and held there, the tempering color, instead of remaining unchanged at this temperature, will advance in the tempering-color scale as it would with increasing temperature. This means that the tempering colors do not absolutely correspond to the temperatures of steels, but the variations are so slight that we can use them in actual practice. (See Table 23, page 158.)
Temperatures to Use.—As soon as the temperature of the steel reaches 100°C. (212°F.) the transformation begins, increasing in intensity as the temperature is raised, until finally when the lower critical range is reached, the steel has been all changed into the ordinary constituents of unhardened steels.
If a piece of polished steel is heated in an ordinary furnace, a thin film of oxides will form on its surface. The colors of this film change with temperature, and so, in tempering, they are generally used as an indication of the temperature of the steel. The steel should have at least one polished face so that this film of oxides may be seen.
An alternative method to the determination of temper by color is to temper by heating in an oil or salt bath. Oil baths can be used up to temperatures of 500°F.; above this, fused-salt baths are required. The article to be tempered is put into the bath, brought up to and held at the required temperature for a certain length of time, and then cooled, either rapidly or slowly. This takes longer than the color method, but with low temperatures the results are more satisfactory, because the temperature of the bath can be controlled with a pyrometer. The tempering Page 158 temperatures given in the following table are taken from a handbook issued by the Midvale Steel Company.
| Temperature for 1 hr. |
Color | Temperature for 8 min. |
Uses | ||
|---|---|---|---|---|---|
| Deg. F. | Deg. C. | Deg. F. | Deg. C. | ||
| 370 | 188 | Faint yellow | 460 | 238 | Scrapers, brass-turning tools, reamers, taps, milling cutters, saw teeth. |
| 390 | 199 | Light straw | 510 | 265 | Twist drills, lathe tools, planer tools, finishing tools |
| 410 | 210 | Dark straw | 560 | 293 | Stone tools, hammer faces, chisels for hard work, boring cutters. |
| 430 | 221 | Brown | 610 | 321 | Trephining tools, stamps. |
| 450 | 232 | Purple | 640 | 337 | Cold chisels for ordinary work, carpenters' tools, picks, cold punches, shear blades, slicing tools, slotter tools. |
| 490 | 254 | Dark blue | 660 | 343 | Hot chisels, tools for hot work, springs. |
| 510 | 265 | Light blue | 710 | 376 | Springs, screw drivers. |
It will be noted that two sets of temperatures are shown, one being specified for a time interval of 8 min. and the other for 1 hr. For the finest work the longer time is preferable, while for ordinary rough work 8 min. is sufficient, after the steel has reached the specified temperature.
The rate of cooling after tempering seems to be immaterial, and the piece can be cooled at any rate, providing that in large pieces it is sufficiently slow to prevent strains.
Knowing What Takes Place.—How are we to know if we have given a piece of steel the very best possible treatment?
The best method is by microscopic examination of polished and etched sections, but this requires a certain expense for laboratory equipment and upkeep, which may prevent an ordinary commercial plant from attempting such a refinement. It is highly recommended that any firm that has any large amount of heat treatment to do, install such an equipment, which can be purchased Page 159 for from $250 to $500. Its intelligent use will save its cost in a very short time.
The other method is by examination of fractures of small test bars. Steel heated to its correct temperatures will show the finest possible grain, whereas underheated steel has not had its grain structure refined sufficiently, and so will not be at its best. On the other hand, overheated steel will have a coarser structure, depending on the extent of overheating.
To determine the proper quenching temperature of any particular grade of steel it is only necessary to heat pieces to various temperatures not more than 20°C. (36°F.) apart, quench in water, break them, and examine the fractures. The temperature producing the finest grain should be used for annealing and hardening.
Similarly, to determine tempering temperatures, several pieces should be hardened, then tempered to various degrees, and cooled in air. Samples, say six, reheated to temperatures varying by 100° from 300 to 800°C. will show a considerable range of properties, and the drawing temperature of the piece giving the desired results can be used.
For drawing tempers up to 500°F. oil baths of fresh cotton seed oil can be safely and satisfactorily used. For higher temperature a bath of some kind of fused salt is recommended.
Do not hesitate to ask for information from the maker as to the best steel to use for a given purpose, mentioning in as much detail as possible the use for which it is intended.
Do not heat the steel to a higher degree than that fixed in the description of each class. Never heat the steel to more than a cherry red without forging it or giving it a definite heat treatment. Heating steel at even moderate temperature is liable to coarsen the grain which can only be restored by forging or by heat treating.
Let the forging begin as soon as the steel is hot enough and never let tool steel soak in the fire. Continue the hammering vigorously and constantly, using lighter blows as it cools off, and stopping when the heat becomes a very dull red or a faint brown.
Should welding be necessary care should be taken not to overheat in order to make an easy weld. Keep it below the sparkling point as this indicates that the steel is burnt.
Begin to forge as soon as the welds are put together, taking Page 160 care to use gentle strokes at first increasing them as the higher heat falls, but not overdoing the hammering when the steel cools. The hammering should be extended beyond the welding point and should continue until the dull red or brown heat is reached.
The blacksmith in the small shop, where equipment is usually very limited, often consisting of a forge, a small open hard-coal furnace, a barrel of water and a can of oil must have skill and experience. With this equipment the smith is expected to, and usually can, produce good results if proper care is taken.
In hardening carbon tool steel in water, too much cannot be said in favor of slow, careful heating, nor against overheating if cracks are to be avoided.
It is not wise to take the work from the hardening bath and leave it exposed to the air if there is any heat left in it, because it is more liable to crack than if left in the bath until cold. In heating, plenty of time is taken for the work to heat evenly clear through, thus avoiding strains caused by quick and improper heating, In quenching in water, contraction is much more rapid than was the expansion while heating, and strains begin the moment the work touches the water. If the piece has any considerable size and is taken from the bath before it is cold and allowed to come to the air, expansion starts again from the inside so rapidly that the chilled hardened surface cracks before the strains can be relieved.
Many are most successful with the hardening bath about blood warm. When the work that is being hardened is nearly cold, it is taken from the water and instantly put into a can of oil, where it is allowed to finish cooling. The heat in the body of the tool will come to the surface more slowly, thus relieving the strain and overcoming much of the danger of cracking.
Some contend that the temper should be drawn as soon as possible after hardening: but that if this cannot be done for some hours, the work should be left in the oil until the tempering can be done. It is claimed that forming dies and punch-press dies that are difficult to harden will seldom crack if treated in this way.
Small tools or pieces that are very troublesome because of peculiar shape should be made of steel which has been thoroughly annealed. It is often well to mill or turn off the outer skin of the bar, to remove metal which has been cold-worked. Then heat Page 161 slowly just through the critical range and cool in the furnace, in order to produce a very fine grain. Tools machined from such stock, and hardened with the utmost care, will have the best chance to survive without warping, growth or cracking.
Steel can be shrunk or enlarged by proper heating and cooling. Pins for forced fits can be enlarged several thousandths of an inch by rapid heating to a dull red and quenching in water. The theory is that the metal is expanded in heating and that the sudden cooling sets the outer portion before the core can contract. In dipping the piece is not held under water till cold but is dipped, held a moment and removed. Then dipped again and again until cold.
Rings and drawing dies are also shrunk in a similar way. The rings are slowly heated to a cherry red, slipped on a rod and rolled in a shallow pan of water which cools only the outer edge. This holds the outside while the inner heated portion is forced inward, reducing the hole. This operation can be repeated a number of times with considerable success.
A number of circular dies of carbon tool steel for use in tool holders of turret lathes were required. No proper tempering oven was available, so the following method was adopted and proved quite successful.
After the dies had been hardened dead hard in water, they were cleaned up bright. A pair of ordinary smiths' tongs was made with jaws of heavy material and to fit nicely all around the outside of the die, leaving a 3/32-in. space when the jaws were closed around the die. The dies being all ready, the tongs were heated red hot, and the dies were picked up and held by the tongs. This tempered them from the outside in, left the teeth the temper required and the outside slightly softer. The dies held up the work successfully and were better than when tempered in the same bath.
The following information has been supplied by Automatic and Electric Furnaces, Ltd., 6, Queenstreet, London, S. W.:
Two gages of ¾ in. diameter, 12 threads per inch, were heated in a Wild-Barfield furnace, using the pyroscopic detector, and Page 162 were quenched in cold water. They were subsequently tempered in a salt bath at various increasing temperatures, the effective diameter of each thread and the scleroscope hardness being measured at each stage. The figures are in 10,000ths of an inch, and indicate the change + or - with reference to the original effective diameter of the gages. The results for the two gages have been averaged.
| Thread | After quenching |
Tempering temperature, degrees Centigrade | |||||
|---|---|---|---|---|---|---|---|
| 220 | 260 | 300 | 340 | 380 | 420 | ||
| 1 | +25 | +19 | +17 | +15 | +13 | +11 | +11 |
| 2 | +18 | +12 | +11 | + 9 | + 6 | + 5 | + 5 |
| 3 | +12 | + 6 | + 5 | + 3 | 0 | 0 | 0 |
| 4 | +10 | + 4 | + 4 | + 2 | ... | 0 | - 1 |
| 5 | + 9 | + 4 | + 4 | + 2 | 0 | 0 | 0 |
| 6 | + 9 | + 4 | + 3 | + 2 | 0 | 0 | 0 |
| 7 | +10 | + 5 | + 5 | + 3 | + 2 | + 1 | +2 |
| 8 | + 8 | + 4 | + 3 | + 2 | 0 | 0 | + 1 |
| 9 | + 9 | + 4 | + 3 | + 2 | + 1 | + 1 | + 1 |
| 10 | + 9 | + 5 | + 5 | + 3 | + 2 | + 2 | + 2 |
| 11 | + 7 | + 4 | + 4 | + 2 | + 1 | + 1 | + 1 |
| 12 | + 9 | + 5 | + 5 | + 5 | + 4 | + 4 | + 3 |
| Scleroscope | 80 | 70 | 70 | 62 | 56 | 53 | 52 |
Had these gages been formed with a plain cylindrical end projecting in front of the screw, the first two threads would have been prevented from increasing more than the rest. The gages would then have been fairly easily corrected by lapping after tempering at 220°C. Practically no lapping would be required if they were tempered at 340°C. There seems to be no advantage in going to a higher temperature than this. The same degree of hardness could have been obtained with considerably less distortion by quenching directly in fused salt. It is interesting to note that when the swelling after water quenching does not exceed 0.0012 in., practically the whole of it may be recovered by tempering at a sufficiently high temperature, but when the swelling exceeds this amount the steel assumes a permanently strained condition, and at the most only 0.0014 in. can be recovered by tempering.
Opinions differ as to the temperature which is indicated by the various colors, or oxides, which appear on steel in tempering.
The figures shown are from five different sources and while the variations are not great, it is safer to take the average temperature shown in the last column.
| A | B | C | D | E | Average | |
|---|---|---|---|---|---|---|
| Faint yellow | 430 | 430 | 430 | 430 | 430 | 430 |
| Light straw | 475 | 460 | 450 | ... | 450 | 458 |
| Dark straw | 500 | 500 | 470 | 450 | 470 | 478 |
| Purple (reddish) | 525 | 530 | 520 | 530 | 510 | 523 |
| Purple (bluish) | ... | 555 | 550 | 550 | 550 | 551 |
| Blue | 575 | 585 | 560 | 580 | 560 | 572 |
| Gray blue | ... | 600 | ... | 600 | 610 | 603 |
| Greenish blue | ... | 625 | ... | ... | 630 | 627 |
| Degrees Fahrenheit |
High temperatures judged by color | |
|---|---|---|
| 430 | Very pale yellow | Visible in full daylight |
| 460 | Straw-yellow | |
| 480 | Dark yellow | |
| 500 | Brown-yellow | |
| 520 | Brown-purple | |
| 540 | Full purple | |
| 560 | Full blue | |
| 600 | Very dark blue | |
| 752 | Red heat, visible in the dark | |
| 885 | Red heat, visible in the twilight | |
| 975 | Red heat, visible in the daylight | |
| 1,292 | Dark red | |
| 1,652 | Cherry-red | |
| 1,832 | Bright cherry-red | |
| 2,012 | Orange-red | |
| 2,192 | Orange-yellow | |
| 2,372 | Yellow-white | |
| 2,552 | White welding heat | |
| 2,732 | Brilliant white | |
| 2,912 | Dazzling white (bluish-white) | |
Page 164 These differences might easily be due to the difference in the light at the time the colors were observed. It must also be remembered that even a thin coating of oil will make quite a difference and cause confusion. It is these possible sources of error, coupled with the ever present chance of human error, that makes it advisable to draw the temper of tools in an oil bath heated to the proper temperature as shown by an accurate high-temperature thermometer.
Another table, by Gilbert and Barker, runs to much higher temperatures. Beyond 2,200°, however, the eye is very uncertain.
| Approximate color and temperature |
Kind of tool |
|---|---|
| Yellow 430 to 450°F. |
Thread chasers, hollow mills (solid type) twist drills centering tools, forming tools, cut-off tools, profile cutters, milling cutters, reamers, dies, etc. |
| Straw-yellow 460°F. |
Thread rolling dies, counterbores, countersinks. Shear blades, boring tools, engraving tools, etc. |
| Brown-yellow 500°F. |
Taps, Thread dies, cutters, reamers, etc. |
| Light purple 530°F. |
Taps, dies, rock drills, knives, punches, gages, etc. |
| Dark purple 550°F. |
Circular saws for metal, augers, dental and surgical instruments, cold chisels, axes. |
| Pale blue 580°F. |
Bone saws, chisels, needles, cutters, etc. |
| Blue 600°F. |
Hack saws, wood saws, springs, etc. |
HIGH-SPEED STEEL
For centuries the secret art of making tool steel was handed down from father to son. The manufacture of tool steel is still an art which, by the aid of science, has lost much of its secrecy; yet tool steel is today made by practical men skilled as melters, hammer-men, and rollers, each knowing his art. These practical men willingly accept guidance from the chemist and metallurgists.
A knowledge of conditions existing today in the manufacture of high-speed steel is essential to steel treaters. It is well for the manufacturer to have steel treaters understand some of his troubles and difficulties, so that they will better comprehend the necessity of certain trade customs and practices, and, realizing the manufacturer's desire to cooperate with them, will reciprocate.
The manufacturer of high-speed steel knows and appreciates the troubles and difficulties that may sometimes arise in the heat-treating of his product. His aim is to make a uniform steel that will best meet the requirements of the average machine shop on general work, and at the same time allow the widest variation in heat treatment to give desired results.
High speed steel is one of the most complex alloys known. A representative steel contains approximately 24 per cent of alloying metals, namely, tungsten, chromium, vanadium, silicon, manganese, and in addition there is often found cobalt, molybdenum, uranium, nickel, tin, copper and arsenic.
The selection of a standard analysis by the manufacturer is the result of a series of compromises between various properties imparted to the steel by the addition of different elements and there is a wide range of chemical analyses of various brands. The steel, to be within the range of generally accepted analysis, should contain over 16 per cent and under 20 per cent tungsten; Page 166 if of lower tungsten content it should carry proportionately more chromium and vanadium.
The combined action of tungsten and chromium in steel gives to it the remarkable property of maintaining its cutting edge at relatively high temperature. This property is commonly spoken of as "red-hardness." The percentages of tungsten and chromium present should bear a definite relationship to each other. Chromium imparts to steel a hardening property similar to that given by carbon, although to a less degree. The hardness imparted to steel by chromium is accompanied by brittleness. The chromium content should be between 3.5 and 5 per cent.
Vanadium was first introduced in high-speed steel as a "scavenger," thereby producing a more homogeneous product, of greater density and physical strength. It soon became evident that vanadium used in larger quantities than necessary as a scavenger imparted to the steel a much greater cutting efficiency. Recently, no less an authority than Prof. J. O. Arnold, of the University of Sheffield, England, stated that "high-speed steels containing vanadium have a mean efficiency of 108.9, as against a mean efficiency of 61.9 obtained from those without vanadium content." A wide range of vanadium content in steel, from 0.5 to 1.5 per cent, is permissible.
An ideal analysis for high-speed steel containing 18 per cent tungsten is a chromium content of approximately 3.85 per cent; vanadium, 0.85 to 1.10 per cent, and carbon, between 0.62 and 0.77 per cent.
Detrimental Elements.—Sulphur and phosphorus are two elements known to be detrimental to all steels. Sulphur causes "red-shortness" and phosphorus causes "cold-shortness." The detrimental effects of these two elements counteract each other to some extent but the content should be not over 0.02 sulphur and 0.025 phosphorus. The serious detrimental effect of small quantities of sulphur and phosphorus is due to their not being uniformly distributed, owing to their tendency to segregate.
The manganese and silicon contents are relatively unimportant in the percentages usually found in high-speed steel.
The detrimental effects of tin, copper and arsenic are not generally realized by the trade. Small quantities of these impurities are exceedingly harmful. These elements are very seldom determined in customers' chemical laboratories and it is somewhat difficult for public chemists to analyze for them.
Page 167 In justice to the manufacturer, attention should be called to the variations in chemical analyses among the best of laboratories. Generally speaking, a steel works' laboratory will obtain results more nearly true and accurate than is possible with a customer's laboratory, or by a public chemist. This can reasonably be expected, for the steel works' chemist is a specialist, analyzing the same material for the same elements day in and day out.
The importance of the chemical laboratory to a tool-steel plant cannot be over-estimated. Every heat of steel is analyzed for each element, and check analyses obtained; also, every substance used in the mix is analyzed for all impurities. The importance of using pure base materials is known to all manufacturers despite chemical evidence that certain detrimental elements are removed in the process of manufacture.
The manufacture of high-speed steel represents the highest art in the making of steel by tool-steel practice. Some may say, on account of our increased knowledge of chemistry and metallurgy, that the making of such steel has ceased to be an art, but has become a science. It is, in fact an art; aided by science. The human element in its manufacture is a decided factor, as will be brought in the following remarks:
The heat treatment of steel in its broad aspect may be said to commence with the melting furnace and end with the hardening and tempering of the finished product. High-speed steel is melted by two general types of furnace, known as crucible and electric. Steel treaters, however, are more vitally interested in the changes that take place in the steel during the various processes of manufacture rather than a detailed description of those processes, which are more or less familiar to all.
In order that good high-speed steel may be furnished in finished bars, it must be of correct chemical analysis, properly melted and cast into solid ingots, free from blow-holes and surface defects. Sudden changes of temperature are to be guarded against at every stage of its manufacture and subsequent treatment. The ingots are relatively weak, and the tendency to crack due to cooling strains is great. For this reason the hot ingots are not allowed to cool quickly, but are placed in furnaces which are of about the same temperature and are allowed to cool gradually before being placed in stock. Good steel can be made only from good ingots.
Steel treaters should be more vitally interested in the important Page 168 changes which take place in high-speed steel during the hammering operations than that of any other working the steel receives in the course of its manufacture.
The quality of high-speed steel is dependent to a very great extent upon its structure. The making of the structure begins under the hammer, and the beneficial effects produced in this stage persist through the subsequent operations, provided they are properly carried out. The massive carbides and tungstides present in the ingot are broken down and uniformly distributed throughout the billet.
To accomplish this the reduction in area must be sufficient and the hammer blows should be heavy, so as to carry the compression into the center of the billet; otherwise, undesirable characteristics such as coarse structure and carbide envelopes will exist and cause the steel treater much trouble. Surface defects invisible in the ingot may be opened up under the hammering operation, in which event they are chipped from the hot billet.
Ingots are first hammered into billets. These billets are carefully inspected and all surface defects ground or chipped. The hammered billets are again slowly heated and receive a second hammering, known as "cogging." The billet resulting therefrom is known as a "cogged" billet and is of the proper size for the rolling mill or for the finishing hammer.
Although it is not considered good mill practice, some manufacturers who have a large rolling mill perform the very important cogging operation in the rolling mill instead of under the hammer. Cogging in a rolling mill does not break up and distribute the carbides and tungstides as efficiently as cogging under the hammer; another objection to cogging in the rolling mill is that there is no opportunity to chip surface defects developed as they can be under the trained eye of a hammer-man, thereby eliminating such defects in the finished billet.
The rolling of high-speed steel is an art known to very few. The various factors governing the proper rolling are so numerous that it is necessary for each individual rolling mill to work out a practice that gives the best results upon the particular analysis of steel it makes. Important elements entering into the rolling Page 169 are the heating and finishing temperatures, draft, and speed of the mill. In all of these the element of time must be considered.
High-speed steel should be delivered from the rolling mill to the annealing department free from scale, for scale promotes the formation of a decarbonized surface. In preparation of bars for annealing, they are packed in tubes with a mixture of charcoal, lime, and other material. The tubes are sealed and placed in the annealing furnace and the temperature is gradually raised to about 1,650°F., and held there for a sufficient length of time, depending upon the size of the bars. After very slow cooling the bars are removed from the tubes. They should then show a Brinnell number of between 235 and 275.
The inspection department ranks with the chemical and metallurgical departments in safeguarding the quality of the product. It inspects all finished material from the standpoint of surface defects, hardness, size and fracture. It rejects such steel as is judged not to meet the manufacturer's standard. The inspection and metallurgical departments work hand in hand, and if any department is not functioning properly it will soon become evident to the inspectors, enabling the management to remedy the trouble.
The successful manufacture of high-speed steel can only be obtained by those companies who have become specialists. The art and skill necessary in the successful working of such steel can be attained only by a man of natural ability in his chosen trade, and trained under the supervision of experts. To become an expert operator in any department of its manufacture, it is necessary that the operator work almost exclusively in the production of such steel.
As to the heat treatment, it is customary for the manufacturer to recommend to the user a procedure that will give to his steel a high degree of cutting efficiency. The recommendations of the manufacturer should be conservative, embracing fairly wide limits, as the tendency of the user is to adhere very closely to the manufacturer's recommendations. Unless one of the manufacturer's expert service men has made a detailed study of the customer's problem, the manufacturer is not justified in laying down set rules, for if the customer does a little experimenting he can probably modify the practice so as to produce results that are particularly well adapted to his line of work.
The purpose of heat-treating is to produce a tool that will Page 170 cut so as to give maximum productive efficiency. This cutting efficiency depends upon the thermal stability of the complex hardenites existing in the hardened and tempered steel. The writer finds it extremely difficult to convey the meaning of the word "hardenite" to those that do not have a clear conception of the term. The complex hardenites in high-speed steel may be described as that form of solid solution which gives to it its cutting efficiency. The complex hardenites are produced by heating the steel to a very high temperature, near the melting point, which throws into solution carbides and tungstides, provided they have been properly broken up in the hammering process and uniformly distributed throughout the steel. By quenching the steel at correct temperature this solid solution is retained at atmospheric temperature.
It is not the intention to make any definite recommendations as to heat-treating of high-speed steel by the users. It is recognized that such steel can be heat-treated to give satisfactory results by different methods. It is, however, believed that the American practice of hardening and tempering is becoming more uniform. This is due largely to the exchange of opinions in meetings and elsewhere. The trend of American practice for hardening is toward the following:
First, slowly and carefully preheat the tool to a temperature of approximately 1,500°F., taking care to prevent the formation of excessive scale.
Second, transfer to a furnace, the temperature of which is approximately 2,250 to 2,400°F., and allow to remain in the furnace until the tool is heated uniformly to the above temperature.
Third, cool rapidly in oil, dry air blast, or lead bath.
Fourth, draw back to a temperature to meet the physical requirements of the tool, and allow to cool in air.
It was not very long ago that the desirability of drawing hardened high-speed steel to a temperature of 1,100° was pointed out, and it is indeed encouraging to learn that comparatively few treaters have failed to make use of this fact. Many treaters at first contended that the steel would be soft after drawing to this temperature and it is only recently, since numerous actual tests have demonstrated its value, that the old prejudice has been eliminated.
High-speed steel should be delivered only in the annealed Page 171 condition because annealing relieves the internal strains inevitable in the manufacture and puts it in vastly improved physical condition. The manufacturer's inspection after annealing also discloses defects not visible in the unannealed state.
The only true test for a brand of high-speed steel is the service that it gives by continued performance month in and month out under actual shop conditions. The average buyer is not justified in conducting a test, but can well continue to purchase his requirements from a reputable manufacturer of a brand that is nationally known. The manufacturer is always willing to cooperate with the trade in the conducting of a test and is much interested in the information received from a well conducted test. A test, to be valuable, should be conducted in a manner as nearly approaching actual working conditions in the plant in which the test is made as is practical. In conducting a test a few reputable brands should be allowed to enter. All tools entered should be of exactly the same size and shape. There is much difference of opinion as to the best practical method of conducting a test, and the decision as to how the test should be conducted should be left to the customer, who should cooperate with the manufacturers in devising a test which would give the best basis for conclusions as to how the particular brands would perform under actual shop conditions.
The value of the file test depends upon the quality of the file and the intelligence and experience of the person using it. The file test is not reliable, but in the hands of an experienced operator, gives some valuable information. Almost every steel treater knows of numerous instances where a lathe tool which could be touched with a file has shown wonderful results as to cutting efficiency.
Modern tool-steel practice has changed from that of the past, not by the use of labor-saving machinery, but by the use of scientific devices which aid and guide the skilled craftsman in producing a steel of higher quality and greater uniformity. It is upon the intelligence, experience, and skill of the individual that quality of tool steel depends.
We will now take up the matter of hardening high-speed steels. The most ordinary tools used are for lathes and planers. The Page 172 forging should be done at carbon-steel heat. Rough-grind while still hot and preheat to about carbon-steel hardening heat, then heat quickly in high-speed furnace to white heat, and quench in oil. If a very hard substance is to be cut, the point of tool may be quenched in kerosene or water and when nearly black, finish cooling in oil. Tempering must be done to suit the material to be cut. For cutting cast iron, brass castings, or hard steel, tempering should be done merely to take strains out of steel.
On ordinary machinery steel or nickel steel the temper can be drawn to a dark blue or up to 900°F. If the tool is of a special form or character, the risk of melting or scaling the point cannot be taken. In these cases the tool should be packed, but if there is no packing equipment, a tool can be heated to as high heat as is safe without risk to cutting edges, and cyanide or prussiate of potash can be sprinkled over the face and then quenched in oil.
Some very adverse criticism may be heard on this point, but experience has proved that such tools will stand up very nicely and be perfectly free from scales or pipes. Where packing cannot be done, milling cutters, and tools to be hardened all over, can be placed in muffled furnace, brought to 2,220° and quenched in oil. All such tools, however, must be preheated slowly to 1,400 to 1,500° then placed in a high-speed furnace and brought up quickly. Do not soak high-speed steel at high heats. Quench in oil.
We must bear in mind that the heating furnace is likely to expand tools, therefore provision must be made to leave extra stock to take care of such expansion. Tools with shanks such as counter bores, taps, reamers, drills, etc., should be heated no further than they are wanted hard, and quench in oil. If a forge is not at hand and heating must be done, use a muffle furnace and cover small shanks with a paste from fire clay or ground asbestos. Hollow mills, spring threading dies, and large cutting tools with small shanks should have the holes thoroughly packed or covered with asbestos cement as far as they are wanted soft.
To cut a piece from an annealed bar, cut off with a hack saw, milling cutter or circular saw. Cut clear through the bar; do not nick or break. To cut a piece from an unannealed bar, cut right off with an abrasive saw; do not nick or break. If of large cross-section, Page 173 cut off hot with a chisel by first slowly and uniformly heating the bar, at the point to be cut, to a good lemon heat, 1,800 to 1,850°F. and cut right off while hot; do not nick or break. Allow the tool length and bar to cool before reheating for forging.
Forging.—Gently warm the steel to remove any chill, is particularly desirable in the winter, then heat slowly and carefully to a scaling heat, that is a lemon heat (1,800 to 2,000°F.), and forge uniformly. Reheat the tool for further forging directly the steel begins to stiffen under the hammer. Under no circumstances forge the steel when the temperature falls below a dark lemon to an orange color about 1,700°F. Reheat as often as is necessary to finish forging the tool to shape. Allow the tool to cool after forging by burying the tool in dry ashes or lime. Do not place on the damp ground or in a draught of air.
The heating for forging should be done preferably in a pipe or muffle furnace but if this is not convenient use a good clean fire with plenty of fuel between the blast pipe and the tool. Never allow the tool to soak after the desired forging heat has been reached. Do not heat the tool further back than is necessary to shape the tool, but give the tool sufficient heat. See that the back of the tool is flatly dressed to provide proper support under the nose of the tool.
Hardening High-speed Steel.—Slowly reheat the cutting edge of the tool to a cherry red, 1,400°F., then force the blast so as to raise the temperature quickly to a full white heat, 2,200 to 2,250°F., that is, until the tool starts to sweat at the cutting face. Cool the point of the tool in a dry air blast or preferably in oil, further cool in oil keeping the tool moving until the tool has become black hot.
To remove hardening strains reheat the tool to from 500 to 1,100°F. Cool in oil or atmosphere. This second heat treatment adds to the toughness of the tool and therefore to its life.
Grinding Tools.—Grind tools to remove all scale. Use a quick-cutting, dry, abrasive wheel. If using a wet wheel, be sure to use plenty of water. Do not under any circumstances force the tool against the wheel so as to draw the color, as this is likely to set up checks on the surface of the tool to its detriment.
Forging—Forge as before.—Annealing.—Place the steel in a pipe, box or muffle. Arrange the steel so as to allow at least 1 in. of packing, consisting of dry powder ashes, powdered charcoal, mica, etc., between the pieces and the walls of the box or pipe. If using a pipe close the ends. Heat slowly and uniformly to a cherry red, 1,375 to 1,450°F. according to size. Hold the steel at this temperature until the heat has thoroughly saturated through the metal, then allow the muffle box and tools to cool very slowly in a dying furnace or remove the muffle with its charge and bury in hot ashes or lime. The slower the cooling the softer the steel.
The heating requires from 2 to 10 hr. depending upon the size of the piece.
Hardening and Tempering.—It is preferable to use two furnaces when hardening milling cutters and special shape tools. One furnace should be maintained at a uniform temperature from 1,375 to 1,450°F. while the other should be maintained at about 2,250°F. Keep the tool to be hardened in the low temperature furnace until the tool has attained the full heat of this furnace. A short time should be allowed so as to be assured that the center of the tool is as hot as the outside. Then quickly remove the tool from this preheating furnace to the full heat furnace. Keep the tool in this furnace only as long as is necessary for the tool to attain the full temperature of this furnace. Then quickly remove and quench in oil or in a dry air blast. Remove before the tool is entirely cold and draw the temper in an oil bath by raising the temperature of the oil to from 500 to 750°F. and allow this tool to remain, at this temperature, in the bath for at least 30 min., insuring uniformity of temper; then cool in the bath, atmosphere or oil.
If higher drawing temperatures are desired than those possible with oil, a salt bath can be used. A very excellent bath is made by mixing two parts by weight of crude potassium nitrate and three parts crude sodium nitrate. These will melt at about 450°F. and can be used up to 1,000°F. Before heating the steel in the salt bath, slowly preheat, preferably in oil. Reheating the hardened high-speed steel to 1,000°F. will materially increase the life of lathe tools, but milling and form cutters, taps, dies, etc., should not be reheated higher than 500 to 650°F., unless extreme Page 175 hardness is required, when 1,100 to 1,000°F., will give the hardest edge.
Owing to the wide variations in the composition of high-speed steels by various makers, it is always advisable to follow the directions of each when using his brand of steel. In the absence of specific directions the following general suggestions from several makers will be found helpful.
The Ludlum Steel Company recommend the following:
Cutting-off.—To cut a piece from an annealed bar, cut off with a hack saw, milling cutter or circular saw. Cut clear through the bar; do not nick or break. To cut a piece from an unannealed bar, cut right off with an abrasive saw; do not nick or break. If of large cross-section, cut off hot with a chisel by first slowly and uniformly heating the bar, at the point to be cut, to a good lemon heat, 1,800°-1,850°F. and cut right off while hot; do not nick or break. Allow the tool length and bar to cool before reheating for forging.
To Forge.—Gently warm the steel to remove any chill is particularly desirable in the winter. Then heat slowly and carefully to a scaling heat, that is a lemon heat (1,800°-2,000°F.), and forge uniformly. Reheat the tool for further forging directly the steel begins to stiffen under the hammer. Under no circumstances forge the steel when the temperature falls below a dark lemon to an orange color: about 1,700°F. Reheat as often as is necessary to finish forging the tool to shape. Allow the tool to cool after forging by burying the tool in dry ashes or lime. Do not place on the damp ground or in a draught of air.
The heating for forging should be done preferably in a pipe or muffle furnace, but if this is not convenient use a good clean fire with plenty of fuel between the blast pipe and the tool. Never allow the tool to soak after the desired forging heat has been reached. Do not heat the tool further back than is necessary to shape the tool, but give the tool sufficient heat. See that the back of the tool is flatly dressed to provide proper support under the nose of the tool.
Page 176 Hardening.—Slowly reheat the cutting edge of the tool to a cherry red, 1,400°F., then force the blast so as to raise the temperature quickly to a full white heat, 2,200°-2,250°F., that is, until the tool starts to sweat at the cutting face. Cool the point of the tool in a dry air blast or preferably in oil; further cool in oil, keeping the tool moving until the tool has become black hot.
To remove hardening strains reheat the tool to from 500° to 1,100°F. Cool in oil or atmosphere. This second heat treatment adds to the toughness of the tool and therefore to its life.
Grinding.—Grind tools to remove all scale. Use a quick cutting, dry, abrasive wheel. If using a wet wheel, be sure to use plenty of water. Do not under any circumstances force the tool against the wheel so as to draw the color, as this is likely to set up checks on the surface of the tool to its detriment.
The Firth-Sterling Steel Company say:
Instead of printing any rules on the hardening and tempering of Firth-Sterling Steels we wish to say to our customers: Trust the steel to the skill and the judgement of your Toolsmith and Tool Temperer.
The steel workers of today know by personal experience and by inheritance all the standard rules and theories on forging, hardening and tempering of all fine tool steels. They know the importance of slow, uniform heating, and the danger of overheating some steels, and underheating others.
The tempering of tools and dies is a science taught by heat, muscle and brains.
The tool temperer is the man to hold responsible for results. The tempering of tools has been his life work. He may find suggestions on the following pages interesting, but we are always ready to trust the treatment of our steels to the experienced man at the fire.
Fire.—For these tools a good fire is one made of hard foundry coke, broken in small pieces, in an ordinary blacksmith forge with a few bricks laid over the top to form a hollow fire. The bricks should be thoroughly heated before tools are heated. Hard coal may be used very successfully in place of hard coke and will give a higher heat. It is very easy to give Blue Chip the proper heat if care is used in making up the fire.
Forging.—Heat slowly and uniformly to a good forging heat. Do not hammer the steel after it cools below a bright red. Avoid as much as Page 177 possible heating the body of the tool, so as to retain the natural toughness in the neck of the tool.
Hardening.—Heat the point of the tool to an extreme white heat (about 2,200°F.) until the flux runs. This heat should be the highest possible short of melting the point. Care should be taken to confine the heat as near to the point as possible so as to leave the annealing and consequent toughness in the neck of the tool and where the tool is held in the tool post.
Cool in an air blast, the open air or in oil, depending upon the tools or the work they are to do.
For roughing tools temper need not be drawn except for work where the edge tends to crumble on account of being too hard.
For finishing tools draw the temper to suit the purpose for which they are to be used.
Grind thoroughly on dry wheel (or wet wheel if care is used to prevent checking).
The Fire.—Gas and electric furnaces designed for high heats are now made for treating high-speed steels. We recommend them for treating all kinds of Blue Chip tools and particularly the above class. After tools reach a yellow heat in the forge fire they must not be allowed to touch the fuel or come in contact with the blast or surrounding air.
Heating.—Tools of this kind should be heated to a mellow white heat, or as hot as possible without injuring the cutting edges (2,000 to 2,200°F.). For most work the higher the heat the better the tool. Where furnaces are used, we recommend preheating the tools to a red heat in one furnace before putting them in a white hot furnace.
Cooling.—We recommend quenching all of the above tools in oil when taken from the fire. We have found fish oil, cottonseed oil, Houghton's No. 2 soluble oil and linseed oil satisfactory. The high heat is the important thing in hardening Blue Chip tools. If a white hot tool is allowed to cool in the open air it will be hard, but the air scales the tool.
Drawing the Temper.—Tools of this class should be drawn considerably more than water-hardening steel for the same purpose.
Heating.—The degree to which tools of the above classes should be heated depends upon the shape, size and use for which they are intended. Generally, they should not be heated to quite as high a heat Page 178 as lathe tools or milling cutters. They should have a high heat, but not enough to make the flux run on the steel (by pyrometer 1,900 to 2,100°F.).
Cooling.—Depending on the tools, some should be dipped in oil all over, some only part way, and others allowed to cool down in the air naturally, or under air blast. In cooling, the toughness is retained by allowing some parts to cool slowly and quenching parts that should be hard.
Drawing the Temper.—As in cooling, some parts of these tools will require more drawing than others, but, on the whole, they must be drawn more than water hardening tools for the same purpose or to about 500°F. all over, so that a good file will just "touch" the cutting or working parts.
Barium Chloride Process.—This is a process developed for treating certain classes of tools, such as taps, forming tools, etc. It is being successfully used in many large plants. Briefly the treatment is as follows:
In this treatment the tools are first preheated to a red heat, but small tools may be immersed without preheating. The barium chloride bath is kept at a temperature of from 2,000 to 2,100°F., and tools are held in it long enough to reach the same temperature. They are then dipped in oil. The barium chloride which adheres to the tools is brushed off, leaving the tools as dean as before heating.
The Latrobe Steel Company make a high-speed steel without tungsten, its red-hardness properties depending on chromium and cobalt instead of tungsten. It is known as P. R. K-33 steel. It does not require the high temperature of the tungsten steels, hardening at 1,830 to 1,850°F. instead of 2,200° or even higher, as with the tungsten.
This steel is forged at 1,900 to 2,000°F. and must not be worked at a lower temperature than 1,600°F. It requires soaking in the fire more than the tungsten steels. It can be normalized by heating slowly and thoroughly to 1,475°F., holding this for from 10 to 20 min. according to the size of the piece and cooling in the open air, protected from drafts.
A peculiarity of this steel is that it becomes non-magnetic at or above 1,960°F. and the magnetic quality is not restored by cooling. Normalizing as above, however, restores the magnetic qualities. This enables the user to detect any tools which have been overheated, with a horseshoe magnet.
Page 179 It is sometimes advantageous to dip tools, before heating for hardening, in ordinary fuel or quenching oil. The oil leaves a thin film of carbon which tends to prevent decarbonization, giving a very hard surface.
For other makes of high-speed steel used in lathe and planer tools the makers recommend that the tools be cut from the bar with a hack saw or else heated and cut with a chisel. The heating should be very slow until the steel reaches a red after which it can be heated more rapidly and should only be forged at a high heat. It can be forged at very high heats but care should be taken not to forge at a low heat. The heating should be uniform and penetrate clear to the center of the bar before forging is begun. Reheat as often as necessary to forge at the proper heat.
After forging cool in lime before attempting to harden. Do not attempt to harden with the forging heat as was sometimes done with the carbon tools.
For hardening forged tools, heat slowly up to a bright red and then rapidly until the point of the tool is almost at a melting heat. Cool in a blast of cold, dry air. For large sizes of steel, cool in linseed oil or in fish oil as is most convenient. If the tools are to be used for finishing cuts heat to a bright yellow and quench in oil. Grind for use on a sand wheel or grindstone in preference to an emery or an artificial abrasive wheel.
For hardening milling and similar cutters, preheat to a bright red, place the cutter on a round bar of suitable size, and revolve it quickly over a very hot fire. Heat as high as possible without melting the points of the teeth and cool in a cold blast of dry air or in fish oil.
Light fragile cutters, twist drills, taps and formed cutters may be heated almost white and then dipped in fish oil for hardening. Where possible it is better to give an even higher heat and cool in the blast of cold, dry air as previously recommended.
The following suggestions for handling high-speed steels are given by a maker whose steel is probably typical of a number of different makes, so that they will be found useful in other cases as well. These include hints as to forging as well as hardening, together with a list of "dont's" which are often Page 180 very useful. This applies to forging, hardening of lathe, slotting, planing and all similar tools.
 FIG. 84.—All-steel, 5/8 in. square, 1/2 × 1 in., and larger
is usually mild finished, and can be cut in a hack saw. If cut off
hot, be sure to heat the butt end slowly and thoroughly in a clean
fire. Rapid and insufficient heating invariably cracks the steel.
If you want to stamp the end with the name of the steel, it is
necessary that this is done at a good high orange color heat, as
it is otherwise apt to split the steel. (Take your time, do not
hurry.)
FIG. 84.—All-steel, 5/8 in. square, 1/2 × 1 in., and larger
is usually mild finished, and can be cut in a hack saw. If cut off
hot, be sure to heat the butt end slowly and thoroughly in a clean
fire. Rapid and insufficient heating invariably cracks the steel.
If you want to stamp the end with the name of the steel, it is
necessary that this is done at a good high orange color heat, as
it is otherwise apt to split the steel. (Take your time, do not
hurry.)
In forging use coke for fuel in the forge. Heat steel slowly and thoroughly to a lemon heat. Do not forge at a lower heat. Do not let the steel cool below a bright cherry red while forging. After the tool is dressed, reheat to forging heat to remove the forging strain, and lay on the floor until cold. Then have the tool rough ground on a dry emery wheel.
 FIG. 85.—Be sure to have a full yellow heat at the dotted
line. Remember this is a boring mill tool and will stand out in
the tool-post, and if you do not have a high thorough lemon heat,
your tool will snap off at the dotted line. (Ninety-five per cent
of all tools which break, have been forged at too low a heat or
at a heat not thorough to the center.)
FIG. 85.—Be sure to have a full yellow heat at the dotted
line. Remember this is a boring mill tool and will stand out in
the tool-post, and if you do not have a high thorough lemon heat,
your tool will snap off at the dotted line. (Ninety-five per cent
of all tools which break, have been forged at too low a heat or
at a heat not thorough to the center.)
 FIG. 86.—Keep your high lemon forging heat up. If you forge
under a steam hammer, take light blows. Do not jam your tool into
shape. Put frequently back into the fire. Never let the high lemon
color go down and beyond the dotted line.
FIG. 86.—Keep your high lemon forging heat up. If you forge
under a steam hammer, take light blows. Do not jam your tool into
shape. Put frequently back into the fire. Never let the high lemon
color go down and beyond the dotted line.
Page 181 For built-up and bent tools special care should be taken that the forging heat does not go below a bright cherry. For tools ¾ by 1½ or larger where there is a big strain in forging, such as bending at angles of about 45 deg. and building the tools up, they should be heated to at least 1,700°F. Slowly and without much blast. For a ¾ by 1½ tool it should take about 10 min. with the correct blast in a coke fire. Larger tools in proportion. They can then be bent readily, but no attempt should be made to forge the steel further without reheating to maintain the bright cherry red. This is essential, as otherwise the tools crack in hardening or while in use.
 FIG. 87.—Be sure that the tool is absolutely straight at the
bottom, so as to lie flat in the tool-post.
FIG. 87.—Be sure that the tool is absolutely straight at the
bottom, so as to lie flat in the tool-post.
 FIG. 88.—This is the finished forged tool, and let this grow
cold by itself, the slower the better. It is well to cool the tool
slowly in hot ashes, to remove all forging strain. You can now
grind the tool dry on a sharp emery wheel. The more you now finish
the tool in grinding, the less there is to come off after hardening.
FIG. 88.—This is the finished forged tool, and let this grow
cold by itself, the slower the better. It is well to cool the tool
slowly in hot ashes, to remove all forging strain. You can now
grind the tool dry on a sharp emery wheel. The more you now finish
the tool in grinding, the less there is to come off after hardening.
In hardening place the tool in a coke fire (hollow fire if possible) with a slow blast and heat gradually up to a white welding heat on the nose of the tool. Then dip the white hot part only into thin oil or hold in a strong cold air blast. When hardening in oil do not hold the tool in one place but keep it moving so that it cools as quickly as possible. It is not necessary to draw the temper after hardening these tools.
 FIG. 89.—This tool is ground, ready for hardening. Never
harden from the forging heat.
FIG. 89.—This tool is ground, ready for hardening. Never
harden from the forging heat.
 FIG. 90.—Heat the nose of the tool only up to dotted line,
very slowly and thoroughly to an absolutely white welding heat, so
that it shows a trifle fused around the edges, and be very sure that
this fusing has gone thoroughly through the nose, otherwise the
fusing effect will be taken off after the second grinding. Note
the difference of the nose between this and Fig. 86.
FIG. 90.—Heat the nose of the tool only up to dotted line,
very slowly and thoroughly to an absolutely white welding heat, so
that it shows a trifle fused around the edges, and be very sure that
this fusing has gone thoroughly through the nose, otherwise the
fusing effect will be taken off after the second grinding. Note
the difference of the nose between this and Fig. 86.
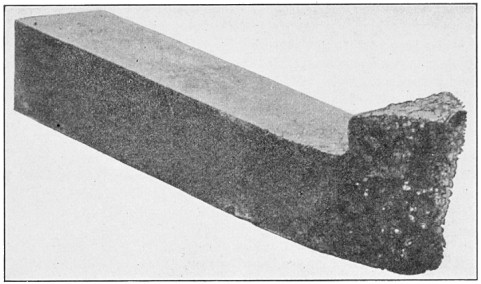 FIG. 91.—Shows unnecessary roasting and drossing. Such
hardening requires a great amount of grinding and is not good.
After hardening grind carefully on a wet emery wheel, and be sure
that the wheel is sharp with a plentiful supply of water. Do not
force the grinding, otherwise the cold water striking the steel
heated up by friction, will crack the nose. Be sure that the grinding
wheel is sharp.
FIG. 91.—Shows unnecessary roasting and drossing. Such
hardening requires a great amount of grinding and is not good.
After hardening grind carefully on a wet emery wheel, and be sure
that the wheel is sharp with a plentiful supply of water. Do not
force the grinding, otherwise the cold water striking the steel
heated up by friction, will crack the nose. Be sure that the grinding
wheel is sharp.
In grinding all tools should be ground as lightly as possible on a soft wet Page 183 sandstone or on a wet emery wheel, and care should be taken not to create any surface cracks, which are invariably the result of grinding too forcibly. The foregoing illustrations, Figs. 84 to 91, with their captions, will be found helpful.
Special points of caution to be observed when hardening high-speed steel.
Don't use a green coal fire; use coke, or build a hollow fire.
Don't have the bed of the fire free from coal.
Don't hurry the heating for forging. The heating has to be done very slowly and the forging heat has to be kept very high (a full lemon color) heat and the tool has to be continually brought back into the fire to keep the high heat up. When customers complain about seams and cracks, in 9 cases out of 10, this has been caused by too low a forging heat, and when the blacksmith complains about tools cracking, it is necessary to read this paragraph to him.
Don't try to jam the tool into shape under a steam hammer with one or two blows; take easy blows and keep the heat high.
Don't have the tool curved at the bottom; it must lie perfectly flat in the tool post.
Don't harden from your forging heat; let the tool grow cold or fairly cold. After forging you can rough grind the tool dry, but not too forcibly.
Don't, for hardening, get more than the nose white hot.
Don't get the white heat on the surface only.
Don't hurry your heating for hardening; let the heat soak thoroughly through the nose of the tool.
Don't melt the nose of the tool.
Don't, as a rule, dip the nose into water; this should be done only for extremely hard material. It is dangerous to put the nose into water for fear of cracking and when you do put the nose into water put just 1/2 in. only of the extreme white hot part into the water and don't keep it too long in the water; just a few seconds, and then harden in oil. We do not recommend water hardening.
Don't grind too forcibly.
Don't grind dry after hardening.
Don't discolor the steel in grinding.
Don't give too much clearance on tools for cutting cast iron.
Don't start on cast iron with a razor edge on the tool. Take an oil stone and wipe three or four times over the razor edge.
Don't use tool holder steel from bars without hardening the nose of each individual tool bit.
Air-hardening Steels.—These steels are recommended for boring, turning and planing where the cost of high-speed seems excessive. They are also recommended for hard wood knives, for roughing and finishing bronze and brass, and for hot bolt forging dies. This steel cannot be cut or punched cold but can be shaped and ground on abrasive wheels of various kinds.
It should be heated slowly and evenly for forging and kept Page 184 as evenly heated at a bright red as possible. It should not be forged after it cools to a dark red.
After the tool is made, heat it again to a bright red and lay it down to cool in a dry place or it can be cooled in a cold, dry air blast. Water must be kept away from it while it is hot.
FURNACES
There are so many standard furnaces now on the market that it is not necessary to go into details of their design and construction and only a few will be illustrated. Oil, gas and coal or coke are most common but there is a steady growth of the use of electric furnaces.
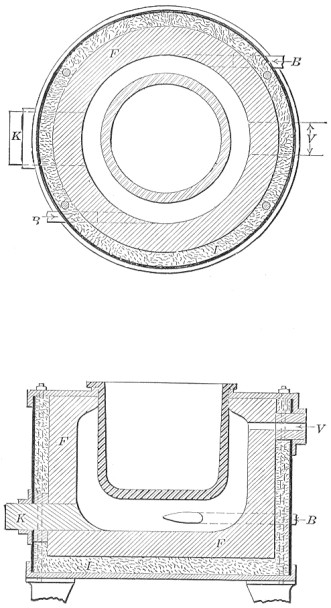 FIG. 92.—Standard lead pot furnace.
FIG. 92.—Standard lead pot furnace.
Page 186 Typical Oil-fired Furnaces.—Several types of standard oil-fired furnaces are shown herewith. Figure 92 is a lead pot furnace, Fig. 93 is a vertical furnace with a center column. This column reduces the cubical contents to be heated and also supports the cover.
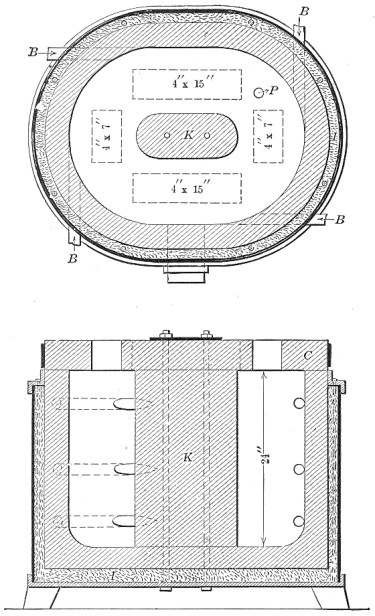 FIG. 93.—Furnace with center column.
FIG. 93.—Furnace with center column.
A small tool furnace is shown in Fig. 94, which gives the construction and heat circulation. A larger furnace for high-speed steel is given in Fig. 95. The steel is supported above the heat, the lower flame passing beneath the support.
For hardening broaches and long reamers and taps, the furnace shown in Fig. 96 is used. Twelve jets are used, these coming in radially to produce a whirling motion.
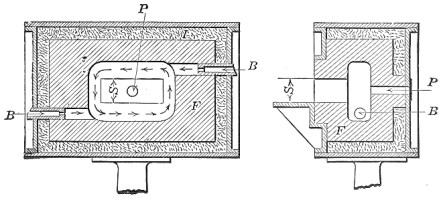 FIG. 94.—Furnace for cutting tools.
FIG. 94.—Furnace for cutting tools.
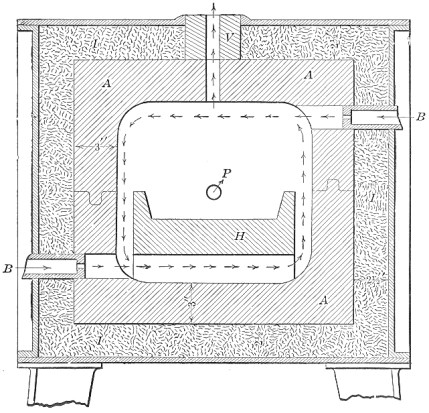 FIG. 95.—High-speed steel furnace.
FIG. 95.—High-speed steel furnace.
Oil and gas furnaces may be divided into three types: the open heating chamber in which combustion takes place in the chamber and directly over the stock; the semimuffle heating chamber in which combustion takes place beneath the floor of the chamber from which the hot gases pass into the chamber through suitable openings; and the muffle heating chamber in which the heat entirely surrounds the chamber but does not enter it. The open furnace is used for forging, tool dressing and welding. The muffle furnace is used for hardening dies, taps, cutters and similar Page 188 tools of either carbon or high-speed steel. The muffle furnace is for spring hardening, enameling, assaying and work where the gases of combustion may have an injurious effect on the material.
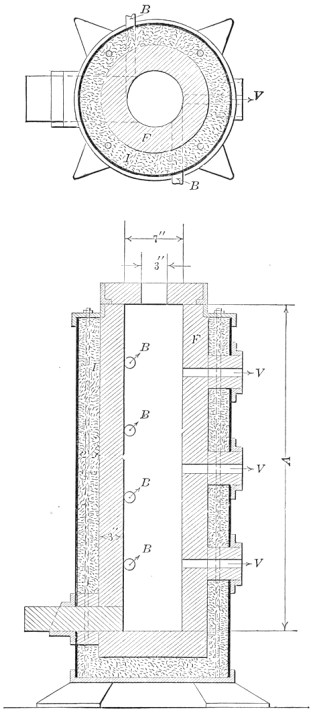 FIG. 96.—Furnace for hardening broaches.
FIG. 96.—Furnace for hardening broaches.
 FIG. 97.—Forging and welding furnace.
FIG. 97.—Forging and welding furnace.
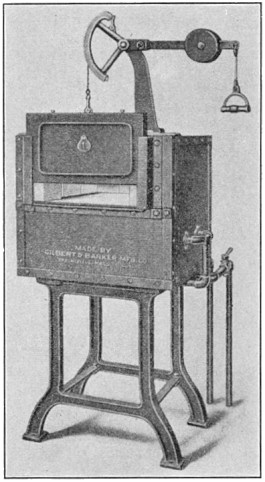 FIG. 98.—Semi-muffle furnace.
FIG. 98.—Semi-muffle furnace.
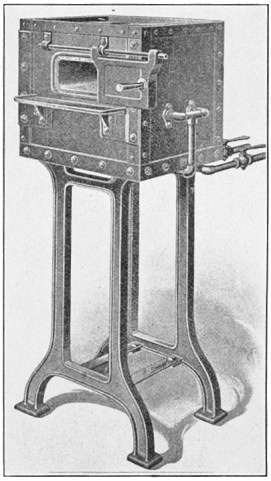 FIG. 99.—Muffle furnace.
FIG. 99.—Muffle furnace.
Furnaces of these types of oil-burning furnaces are shown in Figs. 97, 98, and 99; these being made by the Gilbert & Barker Manufacturing Company. The first has an air curtain formed Page 190 by jets from the large pipe just below the opening, to protect the operator from heat.
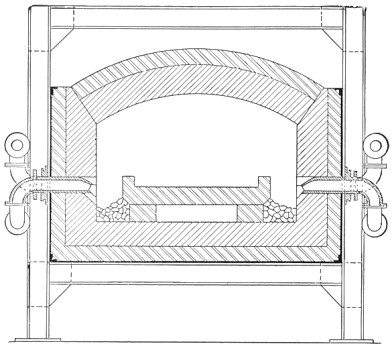 FIG. 100.—Gas fired furnace.
FIG. 100.—Gas fired furnace.
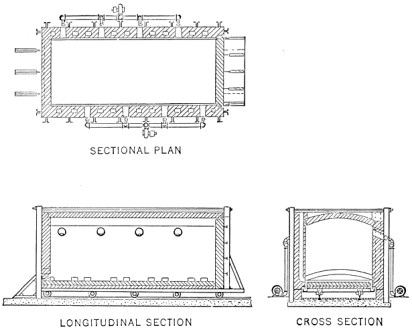 FIG. 101.—Car door type of annealing furnace.
FIG. 101.—Car door type of annealing furnace.
Oil furnaces are also made for both high- and low-pressure air, each having its advocates. The same people also make gas-fired furnaces.
Several types of furnaces for various purposes are illustrated Page 191 in Fig. 100 and 101. The first is a gas-fired hardening furnace of the surface-combustion type.
A large gas-fired annealing furnace of the Maxon system is shown in Fig. 101. This is large enough for a flat car to be run into as can be seen. It shows the arrangement of the burners, the track for the car and the way in which it fits into the furnace. These are from the designs of the Industrial Furnace Corporation.
Before deciding upon the use of gas or oil, all sides of the problem should be considered. Gas is perhaps the nearest ideal but is as a rule more expensive. The tables compiled by the Gilbert & Barker Manufacturing Company and shown herewith, may help in deciding the question.
| Heat units per thousand cubic feet 1,000,000 | |
|---|---|
| Natural gas | 1,000,000 |
| Air gas (gas machine) 20 cp | 815,500 |
| Public illuminating gas, average | 650,000 |
| Water gas (from bituminous coal) | 377,000 |
| Water and producer gas, mixed | 175,000 |
| Producer gas | 150,000 |
Since a gallon of fuel oil (7 lb.) contains 133,000 heat units, the following comparisons may evidently be made. At 5 cts. a gallon, the equivalent heat units in oil would equal:
| Heat units per thousand cubic feet at $0.375 | |
|---|---|
| Natural gas | at $0.375 |
| Air gas, 20 cp | at 0.307 |
| Public illuminating gas, average | at 0.244 |
| Water gas (from bituminous coal) | at 0.142 |
| Water and producer gas, mixed | at 0.065 |
| Producer gas | at 0.057 |
Comparing oil and coal is not always simple as it depends on the work to be done and the construction of the furnaces. The variation rises from 75 to 200 gal. of oil to a ton of coal. For forging and similar work it is probably safe to consider 100 gal. of oil as equivalent to a ton of coal.
Then there is the saving of labor in handling both coal and ashes, the waiting for fires to come up, the banking of fires and the dirt and nuisance generally. The continuous operation possible with oil adds to the output.
Page 192 When comparing oil and gas it is generally considered that 4½ gal. of fuel oil will give heat equivalent to 1,000 cu. ft. of coal gas.
The pressure of oil and air used varies with the system installed. The low-pressure system maintains a pressure of about 8 oz. on the oil and draws in free air for combustion. Others use a pressure of several pounds, while gas burners use an average of perhaps 1½ lb. of air to give best results.
The weights and volumes of solid fuels are: Anthracite coal, 55 to 65 lb. per cubic foot or 34 to 41 cubic feet per ton; bituminous coal, 50 to 55 lb. per cubic foot or 41 to 45 cubic feet per ton; coke, 28 lb. per cubic foot or 80 cubic feet per ton—the ton being calculated as 2,240 lb. in each case.
A novel carburizing furnace that is being used by a number of people, is built after the plan of a fireless cooker. The walls of the furnace are extra heavy, and the ports and flues are so arranged that when the load in the furnace and the furnace is thoroughly heated, the burners are shut off and all openings are tightly sealed. The carburization then goes on for several hours before the furnace is cooled below the effective carburizing range, securing an ideal diffusion of carbon between the case and the core of the steel being carburized. This is particularly adaptable where simple steel is used.
Workmen needlessly exposed to the flames, heat and glare from furnaces where high temperatures are maintained suffer in health as well as in bodily discomfort. This shows several types of shields designed for the maximum protection of the furnace worker.
Bad conditions are not necessary; in almost every case means of relief can be found by one earnestly seeking them. The larger forge shops have adopted flame shields for the majority of their furnaces. Years ago the industrial furnaces (particularly of the oil-burning variety) were without shields, but the later models are all shield-equipped. These shields are adapted to all of the more modern, heat-treating furnaces, as well as to those furnaces in use for working forges; and attention should be paid to their use on the former type since the heat-treating furnaces are constantly becoming more numerous as manufacturers find need of them in the many phases of munitions making or similar work.
The heat that the worker about these furnaces must face may be divided in general into two classes: there is first that heat due to the flame and hot gases that the blast in the furnaces forces out onto a man's body and face. In the majority of furnaces Page 193 this is by far the most discomforting, and care must be taken to fend it and turn it behind a suitable shield. The second class is the radiant heat, discharged as light from the glowing interior of the furnace. This is the lesser of the two evils so far as general forging furnaces are concerned, but it becomes the predominating feature in furnaces of large door area such as in the usual case-hardening furnaces. Here the amount of heat discharged is often almost unbearable even for a moment. This heat can be taken care of by interposing suitable, opaque shields that will temporarily absorb it without being destroyed by it, or becoming incandescent. Should such shields be so constructed as to close off all of the heat, it might be impossible to work around the furnace for the removal of its contents, but they can be made movable, and in such a manner as to shield the major portion of the worker's body.
First taking up the question of flame shields, the illustration, Fig. 102, is a typical installation that shows the main features for application to a forging machine or drop-hammer, oil-burning furnace, or for an arched-over, coal furnace where the flame blows out the front. This shield consists of a frame covered with sheet metal and held by brackets about 6 in. in front of the furnace. It will be noted that slotted holes make this frame adjustable for height, and it should be lowered as far as possible when in use, so that the work may just pass under it and into the furnace openings.
Immediately below the furnace openings, and close to the furnace frame will be noted a blast pipe carrying air from the forge-shop fan. This has a row of small holes drilled in its upper side for the entire length, and these direct a curtain of cold air vertically across the furnace openings, forcing all of the flame, or a greater portion of it, to rise behind the shield. Since the shield extends above the furnace top there is no escape for this flame until it has passed high enough to be of no further discomfort to the workman.
In this case fan-blast air is used for cooling, and this is cheaper and more satisfactory because a great volume may be used. However, where high-pressure air is used for atomizing the oil at the burner, and nothing else is available, this may be employed—though naturally a comparatively small pipe will be needed, in which minute holes are drilled, else the volume of air used will Page 195 be too great for the compressor economically to supply. Steam may also be employed for like service.
 FIGS. 102 to 108.—Protective devices for furnace fronts.
FIGS. 102 to 108.—Protective devices for furnace fronts.
The latest shields of this type are all made double, as illustrated, with an inner sheet of metal an inch or two inside of the front. In the illustration, A, Fig. 102, this inner sheet is smaller, but some are now built the same size as the front and bolted to it with pipe spacers between. The advantage of the double sheet is that the inner one bears the brunt of the flame, and, if needs be, burns up before the outer; while, if due to a heavy fire it should be heated red at any point, the outer sheet will still be much cooler and act as an additional shield to the furnace man.
Heavy Forging Practice.—In heavy forging practice where the metal is being worked at a welding heat, the amount of flame that will issue from an open-front furnace is so great that a plain, sheet-steel front will neither afford sufficient protection nor stand up in service. For such a place a water-cooled front is often used. The general type of this front is illustrated in Fig. 103, and appears to have found considerable favor, for numbers of its kind are scattered throughout the country.
In this case the shield is placed at a slight angle from the vertical, and along the top edge is a water pipe with a row of small holes through which sprays of water are thrown against it. This water runs down in a thin sheet over the shield, cooling it, and is collected in a trough connected with a run-off pipe at the bottom. The lower blast-pipe arrangement is similar to the one first described.
There are several serious objections to this form of shield that should lead to its replacement by a better type; the first is that with a very hot fire, portions in the center may become so rapidly heated that the steam generated will part the sheet of water and cause it to flow from that point in an inverted V, and that section will then quickly become red hot. Another feature is that after the water and fire are shut down for the night the heat of the furnace can be great enough to cause serious warping of the surface of the shield so that the water will no longer cover it in a thin, uniform sheet.
After rigging up a big furnace with a shield of this type several years ago, its most serious object was found in the increase of the water bill of the plant. This was already of large proportions, but it had suddenly jumped to the extent of several hundred Page 196 dollars. Investigation soon disclosed the fact that this water shield was one of the main causes of the added cost of water. A little estimating of the amount of water that can flow through a 1/2-in. pipe under 30-lb. pressure, in the course of a day, will show that this amount at 10 cts. per 1,000 gal., can count up rather rapidly.
Figure 103 is a section through a portion of the furnace front and shield showing all of the principal parts. This shield consists essentially of a very thin tank, about 2½ in. between walls, and filled with water. Like other shields it is fitted with an adjustment, that it may be raised and lowered as the work demands. The tank having an open top, the water as it absorbs heat from the flame will simply boil away in steam; and only a small amount will have to be added to make up for that which has evaporated. The water-feed pipe shown at F ends a short distance above the top of the tank so that just how much water is running in may readily be seen.
An overflow pipe is provided at O which aids in maintaining the water at the proper height, as a sufficient quantity can always be permitted to run in, to avoid any possibility of the shield ever boiling dry; at the same time the small excess can run off without danger of an overflow. The shield illustrated in Fig. 104 has been in constant use for over two years, giving greater satisfaction than any other of which the writer has known. It might also be noted that this shield was made with riveted joints, the shop not having a gas-welding outfit. To flange over the edges and then weld them with an acetylene torch would be a far more economical procedure, and would also insure a tight and permanent joint.
The water-cooled front shown in Fig. 105 is an absurd effort to accomplish the design of a furnace that will provide cool working conditions. This front was on a bolt-heating furnace using hard coal for fuel; and it may be seen that it takes the place of all of the brickwork that should be on that side. Had this been nothing more than a very narrow water-cooled frame, with brickwork below and supporting bricks above, put in like the tuyeres in a foundry cupola, the case would have been somewhat different, for then it would have absorbed a smaller proportion of the heat.
A blacksmith who knows how a piece of cold iron laid in a small welding furnace momentarily lowers the temperature, will appreciate the enormous amount of extra heat that must be maintained in the central portion of this furnace to make up Page 197 for the constant chilling effect of the cold wall. Moreover, since there would have been serious trouble had steam generated in this front, a steady stream of water had to be run through it constantly to insure against an approach to the boiling point. This is illustrated because of its absurdity, and as a warning of something to avoid.
Water-cooled, tuyere openings, as mentioned above, which support brick side-walls of the furnace, have proved successful for coal furnaces used for forging machine and drop-hammer heating, since they permit a great amount of work to be handled through their openings without wearing away as would a brick arch. Great care should be exercised properly to design them so that a minimum amount of the cold tuyere will be in contact with the interior of the furnace, and all interior portions possible should be covered by the bricks. However, a discussion of these points will hardly come in the flame-shield class, although they can be made to do a great deal toward relieving the excessive heat to be borne by the furnace worker.
Flange Shields for Furnaces.—Such portable flame shields as the one illustrated in Fig. 106 may prove serviceable before furnaces required for plate work, where the doors are often only opened for a moment at a time. This shield can be placed far enough in front of the furnace, that it will be possible to work under it or around it, in removing bulky work from the furnace, and yet it will afford the furnace tender some relief from the excessive glare that will come out the wide-opened door. To have this shield of light weight so that it may be readily pushed aside when not wanted, the frame may be made up of pipe and fittings, and a piece of thin sheet steel fastened in the panel by rings about the frame.
About the most disagreeable task in a heat-treating shop is the removal of the pots from the case-hardening furnaces; these must be handled at a bright red heat in order that their contents may be dumped into the quenching tank with a minimum-time contact with the air, and before they have cooled sufficiently to require reheating. Facing the heat before the large open doors of the majority of these furnaces, in a man-killing task even when the weather is moderately cool. The boxes soon become more or less distorted, and then even the best of lifting devices will not remove a hot pot without several minutes labor in front of the doors.
In Fig. 107 is shown a method of arranging a shield on one Page 198 type of charging and removing truck. This shield cannot afford more than a partial protection to the body of the furnace tender, because he must be able to see around it, and in some cases even push it partly through the door of the furnace, but even small as it is it may still afford some welcome protection. The great advantage in this case of having the shield on the truck instead of stationary in front of the furnace, is that it still affords protection as long as the hot pot is being handled through the shop on its way to the quenching tank.
It might be interesting to many engaged in the heat-treating or case hardening of steel parts, to make a special note of the design of the truck that is illustrated in connection with the shield; the general form is shown although the actual details for the construction of such a truck are lacking; these being simple, may be readily worked out by anyone wishing to build one. This is considered to be one of the quickest and easiest operated devices for the removal of this class of work from the furnace. To be sure it may only be used where the floor of the furnace has been built level with the floor of the room, but many of the modern furnaces of this class are so designed.
The pack-hardening pots are cast with legs, from two to three inches high, to permit the circulation of the hot gases, and so heat more quickly. Between these legs and under the body of the pot, the two forward prongs of the truck are pushed, tilting the outer handle to make these prongs as low as possible. The handle is then lowered and, as it has a good leverage, the pot is easily raised from the floor, and the truck and its load rolled out.
Heating of Manganese Steel.—Another form of heat-treating furnace is that which is used for the heating of manganese and other alloy steels, which after having been brought to the proper heat are drawn from the furnace into an immediate quenching tank. With manganese steel in particular, the parts are so fragile and easily damaged while hot that it is frequent practice to have a sloping platform immediately in front of the furnace door down which the castings may slide into a tank below the floor level. Such a furnace with a quenching tank in front of its door is shown in Fig. 108.
These tanks are covered with plates while charging the furnace and the cold castings are placed in a moderately cool furnace. Since some of these steels must not be charged into a furnace where the heat is extreme but should be brought up to their Page 199 final heat gradually, there is little discomfort during the charging process. When quenching, however, from a temperature of 1,800° to 1,900°, it is extremely unpleasant in front of the doors. The swinging shield is here adapted to give protection for this work. As will be noted it is hung a sufficient distance in front of the doors, that it may not interfere with the castings as they come from the furnace, and slide down into the tank.
To facilitate the work, and avoid the necessity of working with the bars outside the edges of the shield, the slot-like hole is cut in the center of the shield, and through this the bars or rakes for dragging out the castings are easily inserted and manipulated. The advantage of such a swinging shield is that it may be readily moved from side to side, or forward and back as occasion requires.
In order to give definite information concerning furnaces, fuels etc., the following data is quoted from a paper by Seth A. Moulton and W. H. Lyman before the Steel Heat Treaters Society in September, 1920.
This considers a factory producing 30,000 lb. of automobile gears per 24 hr. The transmission gears will be of high-carbon steel and the differential of low-carbon steel, carburized. The heat-treating equipment required is:
| 1. Annealing furnaces | 1,400 to | 1,600°F. |
| 2. Carburizing furnaces | 1,700 to | 1,800°F. |
| 3. Hardening furnaces | 1,450 to | 1,550°F. |
| 4. Drawing furnaces | 350 to | 950°F. |
All of the forging blanks are annealed before machining, about three-quarters of the machined gears and parts are carburized, all the carburized gears are given a double treatment for core and case, all gears and parts are hardened and all parts are drawn.
The possible sources of heat supply and their values are as follows:—
| 1. Oil | 140,000 | B.t.u. per gallon |
| 2. Natural gas | 1,100 | B.t.u. per cubic foot |
| 3. City gas | 650 | B.t.u. per cubic foot |
| 4. Water gas | 300 | B.t.u. per cubic foot |
| 5. Producer gas | 170 | B.t.u. per cubic foot |
| 6. Coal | 12,000 | B.t.u. per pound |
| 7. Electric current | 3,412 | B.t.u. per kilowatt-hour |
Page 200 For the heat treatment specified only comparatively low temperatures are required. No difficulty will be experienced in attaining the desired maximum temperature of 1,800°F. with any of the heating medium above enumerated; but it should be noted that the producer gas with a B.t.u. content of 170 per cubic foot and the electric current would require specially designed furnaces to obtain higher temperatures than 1800°F.
| Assuming | |
| Cost of oil- and gas-fired furnaces installed as | $100.00 per square foot of hearth |
| Cost of coal-fired furnace installed as | 150.00 per square foot of hearth |
| Cost of electric furnace 100 kw. capacity installed as | 90.00 per kilowatt |
| Cost of electric furnace 150 kw. capacity installed as | 70.00 per kilowatt |
Output 3,000 lb. charge, 8 hr. heat carburizing, 2 hr. heating only. Annual service 7,200 hr. Fixed charges including interest, depreciation, taxes, insurance and maintenance 15 per cent. Extra operating labor for coal-fired furnace 60 cts. per hour, one man four furnaces.
| Class fuel | Fuel per charge | Unit fuel cost | Installation cost | Efficiency per cent | Fixed charges | Cost per charge | |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Carburizing | |||||||
| 1 | Oil | 52.0 gal. | $0.15 gal. | $2,400.00 | 12.6 | $.40 | $8.20 |
| 2 | Natural gas | 4.4 M | 0.50 M | 2,400.00 | 18.8 | 0.40 | 2.60 |
| 3 | City gas | 8.3 M | 0.80 M | 2,400.00 | 17.0 | 0.40 | 7.04 |
| 4 | Water gas | 18.7 M | 0.40 | 2,400.00 | 16.4 | 0.40 | 7.88 |
| 5 | Producer gas | 37.3 M | 0.10 M | 2,400.00 | 14.5 | 0.40 | 4.13 |
| 6 | Coal | 814.0 lb. | 6.00 ton | 3,600.00 | 9.4 | 0.60 | 3.98 |
| 7 | Electricity | 500.0 kw-hr. | 0.015 kw. | 9,000.00 | 53.0 | 1.50 | 9.00 |
| Heating | |||||||
| 1 | Oil | 30.8 gal. | 0.15 gal. | 2,400.00 | 21.4 | 0.10 | 4.72 |
| 2 | Natural gas | 2.61 M | 0.50 M | 2,400.00 | 32.0 | 0.10 | 1.40 |
| 3 | City gas | 4.9 M | 0.80 M | 2,400.00 | 28.8 | 0.10 | 4.02 |
| 4 | Water gas | 11.1 M | 0.40 M | 2,400.00 | 27.6 | 0.10 | 4.54 |
| 5 | Producer gas | 22.1 M | 0.10 M | 2,400.00 | 24.6 | 0.10 | 2.31 |
| 6 | Coal | 348.0 lb. | 6.00 ton | 3,600.00 | 22.0 | 0.15 | 1.38 |
| 7 | Electricity | 329.0 kw-hr. | 0.015 kw. | 10,500.00 | 81.75 | 0.44 | 5.38 |
This shows but two of the operations and for a single furnace. The total costs for all operations on the 30,000 lb. of gears per 24 hr. is shown in Table 29.
| No. | Equipment | Installation cost | Annual operating expenses | Total | Cost per lb. metal, cents | ||
|---|---|---|---|---|---|---|---|
| Fixed charges | Heat | Labor | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| I | Oil | $179,000.00 | $26,850.00 | $156,000.00 | $105,000.00 | $287,850.00 | $3.19 |
| II | Oil and electric | 213,000.00 | 31,950.00 | 142,770.00 | 97,000.00 | 271,720.00 | 3.02 |
| III | Natural gas | 117,000.00 | 17,550.00 | 44,250.00 | 97,000.00 | 158,800.00 | 1.78 |
| IV | (A) Natural gas containing furnaces | 120,000.00 | 18,000.00 | 41,000.00 | 94,000.00 | 153,000.00 | 1.70 |
| V | Natural gas and electric | 181,000.00 | 27,150.00 | 73,820.00 | 90,000.00 | 190,970.00 | 2.13 |
| VI | City gas | 122,000.00 | 18,300.00 | 123,200.00 | 94,000.00 | 235,500.00 | 2.62 |
| VII | City gas and electric | 182,000.00 | 27,300.00 | 128,820.00 | 90,000.00 | 246,020.00 | 2.74 |
| VIII | Water gas | 214,000.00 | 18,600.00 | 104,000.00 | 94,000.00 | 216,600.00 | 2.41 |
| IX | Water gas and electric | 238,000.00 | 27,450.00 | 117,420.00 | 90,000.00 | 234,870.00 | 2.62 |
| X | Producer gas | 246,000.00 | 18,900.00 | 69,300.00 | 90,000.00 | 178,200.00 | 1.98 |
| XI | Producer gas and electric | 255,000.00 | 27,750.00 | 92,520.00 | 90,000.00 | 210,270.00 | 2.34 |
| XII | Coal and electric | 194,000.00 | 29,100.00 | 87,220.00 | 90,000.00 | 206,320.00 | 2.30 |
| XIII | Electric | 257,000.00 | 38,550.00 | 135,000.00 | 84,000.00 | 257,550.00 | 2.86 |
NOTE.—Producer plant fixed charges are included in the cost of gas and are charged as "heat" in column 5, so they are omitted from column 4.
PYROMETRY AND PYROMETERS
A knowledge of the fundamental principles of pyrometry, or the measurement of temperatures, is quite necessary for one engaged in the heat treatment of steel. It is only by careful measurement and control of the heating of steel that the full benefit of a heat-treating operation is secured.
Before the advent of the thermo-couple, methods of temperature measurement were very crude. The blacksmith depended on his eyes to tell him when the proper temperature was reached, and of course the "color" appeared different on light or dark days. "Cherry" to one man was "orange" to another, and it was therefore almost impossible to formulate any treatment which could be applied by several men to secure the same results.
One of the early methods of measuring temperatures was the "iron ball" method. In this method, an iron ball, to which a wire was attached, was placed in the furnace and when it had reached the temperature of the furnace, it was quickly removed by means of the wire, and suspended in a can containing a known quantity of water; the volume of water being such that the heat would not cause it to boil. The rise in temperature of the water was measured by a thermometer, and, knowing the heat capacity of the iron ball and that of the water, the temperature of the ball, and therefore the furnace, could be calculated. Usually a set of tables was prepared to simplify the calculations. The iron ball, however, scaled, and changed in weight with repeated use, making the determinations less and less accurate. A copper ball was often used to decrease this change, but even that was subject to error. This method is still sometimes used, but for uniform results, a platinum ball, which will not scale or change in weight, is necessary, and the cost of this ball, together with the slowness of the method, have rendered the practice obsolete, especially in view of modern developments in accurate pyrometry.
Armor plate makers sometimes use the copper ball or Siemens' water pyrometer because they can place a number of the balls or Page 203 weights on the plate in locations where it is difficult to use other pyrometers. One of these pyrometers is shown in section in Fig. 109.
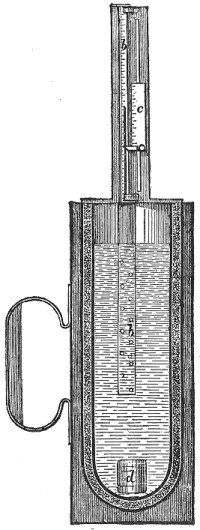 FIG. 109.—Siemens' copper-ball pyrometer.
FIG. 109.—Siemens' copper-ball pyrometer.
Siemens' Water Pyrometer.—It consists of a cylindrical copper vessel provided with a handle and containing a second smaller copper vessel with double walls. An air space a separates the two vessels, and a layer of felt the two walls of the inner one, in order to retard the exchange of temperature with the surroundings. The capacity of the inner vessel is a little more than one pint. A mercury thermometer b is fixed close to the wall of the inner vessel, its lower part being protected by a perforated brass tube, whilst the upper projects above the vessel and is divided as usual on the stem into degrees, Fahrenheit or Centigrade, as desired. At the side of the thermometer there is a small brass scale c, which slides up and down, and on which the high temperatures are marked in the same degrees as those in which the mercury thermometer is divided; on a level with the zero division of the brass scale a small pointer is fixed, which traverses the scale of the thermometer.
Short cylinders d, of either copper, iron or platinum, are supplied with the pyrometer, which are so adjusted that their heat capacity at ordinary temperature is equal to one-fiftieth of that of the copper vessel filled with one pint of water. As, however, the specific heat of metals increases with the temperature, allowance is made on the brass sliding scales, which are divided according to the metal used for the pyrometer cylinder d. It will therefore be understood that a different sliding scale is required for the particular kind of metal of which a cylinder is composed. In order to obtain accurate measurements, each sliding scale must be used only in conjunction with its own thermometer, and in case the latter breaks a new scale must be made and graduated for the new thermometer.
The water pyrometer is used as follows:
Page 204 Exactly one pint (0.568 liter) of clean water, perfectly distilled or rain water, is poured into the copper vessel, and the pyrometer is left for a few minutes to allow the thermometer to attain the temperature of the water.
The brass scale c is then set with its pointer opposite the temperature of the water as shown by the thermometer. Meanwhile one of the metal cylinders has been exposed to the high temperature which is to be measured, and after allowing sufficient time for it to acquire that temperature, it is rapidly removed and dropped into the pyrometer vessel without splashing any of the water out.
The temperature of the water will rise until, after a little while, the mercury of the thermometer has become stationary. When this is observed the degrees of the thermometer are read off, as well as those on the brass scale c opposite the top of the mercury. The sum of these two values together gives the temperature of the flue, furnace or other heated space in which the metal cylinder had been placed. With cylinders of copper and iron, temperatures up to 1,800°F. (1,000°C.) can be measured, but with platinum cylinders the limit is 2,700°F. (1,500°C.).
For ordinary furnace work either copper or wrought-iron cylinders may be used. Iron cylinders possess a higher melting point and have less tendency to scale than those of copper, but the latter are much less affected by the corrosive action of the furnace gases; platinum is, of course, not subject to any of these disadvantages.
The weight to which the different metal cylinders are adjusted is as follows:
| Copper | 137.0 grams |
| Wrought-iron | 112.0 grams |
| Platinum | 402.6 grams |
In course of time the cylinders lose weight by scaling; but tables are provided giving multipliers for the diminished weights, by which the reading on the brass scale should be multiplied.
With the application of the thermo-couple, the measurement of temperatures, between, say, 700 and 2,500°F., was made more simple and precise. The theory of the thermo-couple is simple; it is that if two bars, rods, or wires of different metals are joined Page 205 together at their ends, when heated so that one junction is hotter than the other, an electromotive force is set up through the metals, which will increase with the increase of the difference of temperature between the two junctions. This electromotive force, or voltage, may be measured, and, from a chart previously prepared, the temperature determined. In most pyrometers, of course, the temperatures are inscribed directly on the voltmeter, but the fact remains that it is the voltage of a small electric current, and not heat, that is actually measured.
There are two common types of thermo-couples, the first making use of common, inexpensive metals, such as iron wire and nichrome wire. This is the so-called "base metal" couple. The other is composed of expensive metals such as platinum wire, and a wire of an alloy of platinum with 10 per cent of rhodium or iridium. This is called the "rare metal" couple, and because its component metals are less affected by heat, it lasts longer, and varies less than the base metal couple.
The cold junction of a thermo-couple may be connected by means of copper wires to the voltmeter, although in some installations of base metal couples, the wires forming the couple are themselves extended to the voltmeter, making copper connections unnecessary. From the foregoing, it may be seen that accurately to measure the temperature of the hot end of a thermo-couple, we must know the temperature of the cold end, as it is the difference in the temperatures that determines the voltmeter readings. This is absolutely essential for precision, and its importance cannot be over-emphasized.
When pyrometers are used in daily operation, they should be checked or calibrated two or three times a month, or even every week. Where there are many in use, it is good practice to have a master pyrometer of a rare metal couple, which is used only for checking up the others. The master pyrometer, after calibrating against the melting points of various substances, will have a calibration chart which should be used in the checking operation.
It is customary now to send a rare metal couple to the Bureau of Standards at Washington, where it is very carefully calibrated for a nominal charge, and returned with the voltmeter readings of a series of temperatures covering practically the whole range of the couple. This couple is then used only for checking those in daily use.
Pyrometer couples are more or less expensive, and should be Page 206 cared far when in use. The wires of the couple should be insulated from each other by fireclay leads or tubes, and it is well to encase them in a fireclay, porcelain, or quartz tube to keep out the furnace gases, which in time destroy the hot junction. This tube of fireclay, or porcelain, etc., should be protected against breakage by an iron or nichrome tube, plugged or welded at the hot end. These simple precautions will prolong the life of a couple and maintain its precision longer.
Sometimes erroneous temperatures are recorded because the "cold end" of the couple is too near the furnace and gets hot. This always causes a temperature reading lower than the actual, and should be guarded against. It is well to keep the cold end cool with water, a wet cloth, or by placing it where coal air will circulate around it. Best of all, is to have the cold junction in a box, together with a thermometer, so that its temperature may definitely be known. If this temperature should rise 20°F. on a hot day, a correction of 20°F. should be added to the pyrometer reading, and so on. In the most up-to-date installations, this cold junction compensation is taken care of automatically, a fact which indicates its importance.
Optical pyrometers are often used where it is impracticable to use the thermo-couple, either because the temperature is so high that it would destroy the couple, or the heat to be measured is inaccessible to the couple of ordinary length. The temperatures of slag or metal in furnaces or running through tap-holes or troughs are often measured with optical pyrometers.
In one type of optical pyrometer, the observer focuses it on the metal or slag and moves an adjustable dial or gage so as to get an exact comparison between the color of the heat measured with the calor of a lamp or screen in the pyrometer itself. This, of course, requires practice, and judgment, and brings in the personal equation. With care, however, very reliable temperature measurements may be made. The temperatures of rails, as they leave the finishing pass of a rolling mill, are measured in this way.
Another type of optical pyrometer is focused on the body, the temperature of which is to be measured. The rays converge in the telescope on metal cells, heating them, and thereby generating a small electric current, the voltage of which is read an a calibrated voltmeter similar to that used with the thermo-couple. The best precision is obtained when an optical pyrometer is used each time under similar conditions of light and the same observer.
Page 207 Where it is impracticable to use either thermo-couples or optical pyrometers, "sentinels" may be used. There are small cones or cylinders made of salts or other substances of known melting points and covering a wide range of temperatures.
If six of these "sentinels," melting respectively at 1,300°, 1,350°, 1,400°, 1,450°, 1,500°, and 1,550°F., were placed in a row in a furnace, together with a piece of steel to be treated, and the whole heated up uniformly, the sentinels would melt one by one and the observer, by watching them through an opening in the furnace, could tell when his furnace is at say 1,500° or between 1,500° and 1,550°, and regulate the heat accordingly.
A very accurate type of pyrometer, but one not so commonly used as those previously described, is the resistance pyrometer. In this type, the temperature is determined by measuring the resistance to an electric current of a wire which is at the heat to be measured. This wire is usually of platinum, wound around a quartz tube, the whole being placed in the furnace. When the wire is at the temperature of the furnace, it is connected by wires with a Wheatstone Bridge, a delicate device for measuring electrical resistance, and an electric current is passed through the wire. This current is balanced by switching in resistances in the Wheatstone Bridge, until a delicate electrical device shows that no current is flowing. The resistance of the platinum wire at the heat to be measured is thus determined on the "Bridge," and the temperature read off on a calibration chart, which shows the resistance at various temperatures.
These are the common methods used to-day for measuring temperatures, but whatever method is used, the observer should bear in mind that the greatest precision is obtained, and hence the highest efficiency, by keeping the apparatus in good working order, making sure that conditions are the same each time, and calibrating or checking against a standard at regular intervals.
In the heat treatment of steel, it has become absolutely necessary that a measuring instrument be used which will give the operator an exact reading of heat in furnace. There are a number of instruments and devices manufactured for this purpose but any instrument that will not give a direct reading without any guess work should have no place in the heat-treating department.
A pyrometer installation is very simple and any of the leading Page 208 makers will furnish diagrams for the correct wiring and give detailed information as to the proper care of, and how best to use their particular instrument. There are certain general principles, however, that must be observed by the operators and it cannot be too strongly impressed upon them that the human factor involved is always the deciding factor in the heat treatment of steel.
A pyrometer is merely an aid in the performance of doing good work, and when carefully observed will help in giving a uniformity of product and act as a check on careless operators. The operator must bear in mind that although the reading on the pyrometer scale gives a measure of the temperature where the junction of the two metals is located, it will not give the temperature at the center of work in the furnace, unless by previous tests, the heat for penetrating a certain bulk of material has been decided on, and the time necessary for such penetration is known.
Each analysis of plain carbon or alloy steel is a problem in itself. Its critical temperatures will be located at slightly different heats than for a steel which has a different proportion of alloying elements. Furthermore, it takes time for metal to acquire the heat of the furnace. Even the outer surface lags behind the temperature of the furnace somewhat, and the center of the piece of steel lags still further. It is apparent, therefore, that temperature, although important, does not tell the whole story in heat treatment. Time is also a factor.
Time at temperature is also of great importance because it takes time, after the temperature has been reached, for the various internal changes to take place. Hence the necessity for "soaking," when annealing or normalizing. Therefore, a clock is as necessary to the proper pyrometer equipment as the pyrometer itself.
For the purpose of general work where a wide range of steels or a variable treatment is called for, it becomes necessary to have the pyrometer calibrated constantly, and when no master instrument is kept for this purpose the following method can be used to give the desired results:
An easy and convenient method for standardization and one which does not necessitate the use of an expensive laboratory equipment is that based upon determining the melting point of common table salt (sodium chloride). While theoretically salt Page 209 that is chemically pure should be used (and this is neither expensive nor difficult to procure), commercial accuracy may be obtained by using common table salt such as is sold by every grocer. The salt is melted in a clean crucible of fireclay, iron or nickel, either in a furnace or over a forge-fire, and then further heated until a temperature of about 1,600 to 1,650°F. is attained. It is essential that this crucible be clean because a slight admixture of a foreign substance might noticeably change the melting point.
The thermo-couple to be calibrated is then removed from its protecting tube and its hot end is immersed in the salt bath. When this end has reached the temperature of the bath, the crucible is removed from the source of heat and allowed to cool, and cooling readings are then taken every 10 sec. on the milli-voltmeter or pyrometer. A curve is then plotted by using time and temperature as coördinates, and the temperature of the freezing point of salt, as indicated by this particular thermocouple, is noted, i.e., at the point where the temperature of the bath remains temporarily constant while the salt is freezing. The length of time during which the temperature is stationary depends on the size of the bath and the rate of cooling, and is not a factor in the calibration. The melting point of salt is 1,472°F., and the needed correction for the instrument under observation can be readily applied.
It should not be understood from the above, however, that the salt-bath calibration cannot be made without plotting a curve; in actual practice at least a hundred tests are made without plotting any curve to one in which it is done. The observer, if awake, may reasonably be expected to have sufficient appreciation of the lapse of time definitely to observe the temperature at which the falling pointer of the instrument halts. The gradual dropping of the pointer before freezing, unless there is a large mass of salt, takes place rapidly enough for one to be sure that the temperature is constantly falling, and the long period of rest during freezing is quite definite. The procedure of detecting the solidification point of the salt by the hesitation of the pointer without plotting any curve is suggested because of its simplicity.
Complete Calibration of Pyrometers.—For the complete calibration of a thermo-couple of unknown electromotive force, the new couple may be checked against a standard instrument, placing the two bare couples side by side in a suitable tube and taking frequent readings over the range of temperatures desired.
Page 210 If only one instrument, such as a millivoltmeter, is available, and there is no standard couple at hand, the new couple may be calibrated over a wide range of temperatures by the use of the following standards:
| Water, boiling point | 212°F. |
| Tin, under charcoal, freezing point | 450°F. |
| Lead, under charcoal, freezing point | 621°F. |
| Zinc, under charcoal, freezing point | 786°F. |
| Sulphur, boiling point | 832°F. |
| Aluminum, under charcoal, freezing point | 1,216°F. |
| Sodium chloride (salt), freezing point | 1,474°F. |
| Potassium sulphate, freezing point | 1,958°F. |
A good practice is to make one pyrometer a standard; calibrate it frequently by the melting-point-of-salt method, and each morning check up every pyrometer in the works with the standard, making the necessary corrections to be used for the day's work. By pursuing this course systematically, the improved quality of the product will much more than compensate for the extra work.
The purity of the substance affects its freezing or melting point. The melting point of common salt is given in one widely used handbook at 1,421°F., although chemically pure sodium chloride melts at 1,474°F. as shown above. A sufficient quantity for an extended period should be secured. Test the melting point with a pyrometer of known accuracy. Knowing this temperature it will be easy to calibrate other pyrometers.
Placing of Pyrometers.—When installing a pyrometer, care should be taken that it reaches directly to the point desired to be measured, that the cold junction is kept cold, and that the wires leading to the recording instrument are kept in good shape. The length of these lead wires have an effect; the longer they are, the lower the apparent temperature.
When pyrometers placed in a number of furnaces are connected up in series, and a multiple switch is used for control, it becomes apparent that pyrometers could not be interchanged between furnaces near and far from the instrument without affecting the uniformity of product from each furnace.
Calibration can best be done without disturbing the working pyrometer, by inserting the master instrument into each furnace separately, place it alongside the hot junction of the working pyrometer, and compare the reading given on the indicator connected with the multiple switch.
Page 211 Protection tubes should be replaced when cracked, as it is important that no foreign substance is allowed to freeze in the tube, so that the enclosed junction becomes a part of a solid mass joined in electrical contact with the outside protecting tube. Wires over the furnaces must be carefully inspected from time to time, as no true reading can be had on an instrument, if insulation is burned off and short circuits result.
If the standard calibrating instrument used contains a dry battery, it should be examined from time to time to be sure it is in good condition.
The potentiometer pyrometer system is both flexible and substantial in that it is not affected by the jar and vibration of the factory or the forge shop. Large or small couples, long or short leads can be used without adjustment. The recording instrument may be placed where it is most convenient, without regard to the distance from the furnace.
Its Fundamental Principle.—The potentiometer is the electrical equivalent of the chemical balance, or balance arm scales. Measurements are made with balance scales by varying known weights until they equal the unknown weight. When the two are equal the scales stand at zero, that is, in the position which they occupy when there is no weight on either pan; the scales are then said to be balanced. Measurements are made with the potentiometer by varying a known electromotive force until it equals the unknown; when the two are equal the index of the potentiometer, the galvanometer needle, stands motionless as it is alternately connected and disconnected. The variable known weights are units separate from the scales, but the potentiometer provides its own variable known electromotive force.
The potentiometer provides, first, a means of securing a known variable electromotive force and, second, suitable electrical connections for bringing that electromotive force to a point where it may be balanced against the unknown electromotive force of the couple. The two are connected with opposite polarity, or so that the two e.m.f.s oppose one another. So long as one is stronger than the other a current will flow through the couple; when the two are equal no current will flow.
Figure 107 shows the wiring of the potentiometer in its simplest Page 212 form. The thermo-couple is at H, with its polarity as shown by the symbols + and -. It is connected with the main circuit of the potentiometer at the fixed point D and the point G.
 FIG. 110.—Simple potentiometer.
FIG. 110.—Simple potentiometer.
A current from the dry cell Ba is constantly flowing through the main, or so-called potentiometer circuit, ABCDGEF. The section DGE of this circuit is a slide wire, uniform in resistance throughout its length. The scale is fixed on this slide wire. The current from the cell Ba as it flows through DGE, undergoes a fall in potential, setting up a difference in voltage, that is, an electromotive force, between D and E. There will also be electromotive force between D and all other points on the slide wire. The polarity of this is in opposition to the polarity of the thermo-couple which connects into the potentiometer at D and at G. By moving G along the slide wire a point is found where the voltage between D and G in the slide wire is just equal to the voltage between D and G generated by the thermo-couple. A galvanometer in the thermo-couple circuit indicates when the Page 213 balance point is reached, since at this point the galvanometer needle will stand motionless when its circuit is opened and closed.
 FIG. 111.—Standard cell potentiometer.
FIG. 111.—Standard cell potentiometer.
The voltage in the slide wire will vary with the current flowing through it from the cell Ba and a means of standardizing this is provided. SC, Fig. 111, is a cadmium cell whose voltage is constant. It is connected at two points C and D to the potentiometer circuit whenever the potentiometer current is to be standardized. At this time the galvanometer is thrown in series with SC. The variable rheostat R is then adjusted until the current flowing is such that as it flows through the standard resistance CD, the fall in potential between C and D is just equal to the voltage of the standard cell SC. At this time the galvanometer will indicate a balance in the same way as when it was used with a thermo-couple. By this operation the current in the slide wire DGE has been standardized.
 FIG. 112.—Hand adjusted cold-end compensator.
FIG. 112.—Hand adjusted cold-end compensator.
Development of the Wiring Scheme of the Cold-end Compensator.—The net voltage generated by a thermo-couple depends upon the temperature of the hot end and the temperature of the cold end. Therefore, any method adopted for reading temperature by means of thermo-couples must in some way provide a means of correcting for the temperature of the cold end. The potentiometer may have either of two very simple devices for this purpose. In one form the operator is required to set a small index to a point on a scale corresponding to the known cold junction temperature. In the other form an even more simple automatic compensator is employed. The principle of each is described in the succeeding paragraphs, in which the assumption is made that the reader already understands the potentiometer principle as described above.
Page 214 As previously explained the voltage of the thermo-couple is measured by balancing it against the voltage drop DG in the potentiometer.
As shown in Fig. 111, the magnitude of the balancing voltage is controlled by the position of G. Make D movable as shown in Fig. 112 and the magnitude of the voltage DG may be varied either from the point D or the point G. This gives a means of compensating for cold end changes by setting the slider D. As the cold end temperature rises the net voltage generated by the couple decreases, assuming the hot end temperature to be constant. To balance this decreased voltage the slider D is moved along its scale to a new point nearer G. In other words, the slider D is moved along its scale until it corresponds to the known temperature of the cold end and then the potentiometer is balanced by moving the slider G. The readings of G will then be direct.
 FIG. 113.—Another type of compensator.
FIG. 113.—Another type of compensator.
The same results will be obtained if a slide wire upon which D bears is in parallel with the slide wire of G, as shown in Fig. 113.
Automatic Compensator.—It should be noted that the effect of moving the contact D, Fig. 113, is to vary the ratio of the resistances on the two sides of the point D in the secondary slide wire. In the recording pyrometers, an automatic compensator is employed. This automatic compensator varies the ratio on the two sides of the point D in the following manner:
The point D, Fig. 114, is mechanically fixed; on one side of D is the constant resistance coil M, on the other the nickel coil N. N is placed at or near the cold end of the thermo-couple (or couples). Nickel has a high temperature coefficient and the electrical proportions of M and N are such that the resistance Page 215 change of N, as it varies with the temperature of the cold end, has the same effect upon the balancing voltage between D and G that the movement of the point D, Fig. 114, has in the hand-operated compensator.
Instruments embodying these principles are shown in Figs. 115 to 117. The captions making their uses clear.
 FIG. 114.—Automatic cold-end compensator.
FIG. 114.—Automatic cold-end compensator.
 FIG. 115.—Potentiometer ready for use.
FIG. 115.—Potentiometer ready for use.
The following illustrations from the Taylor Instrument Company show different applications of the thermo-couples to furnaces of various kinds. Figure 118 shows an oil-fired furnace with a simple vertical installation. Figure 119 shows a method Page 216 of imbedding the thermo-couple in the floor of a furnace so as to require no space in the heating chamber.
Various methods of applying a pyrometer to common heat-treatment furnaces are shown in Figs. 120 to 122.
 FIG. 116.—Eight-point recording pyrometer-Carpenter Steel Co.
FIG. 116.—Eight-point recording pyrometer-Carpenter Steel Co.
The principles of this very popular method of measuring temperature are sketched in Fig. 123.
 FIG. 117.—Multiple-point thermocouple recorder—Bethlehem
Steel Co.
FIG. 117.—Multiple-point thermocouple recorder—Bethlehem
Steel Co.
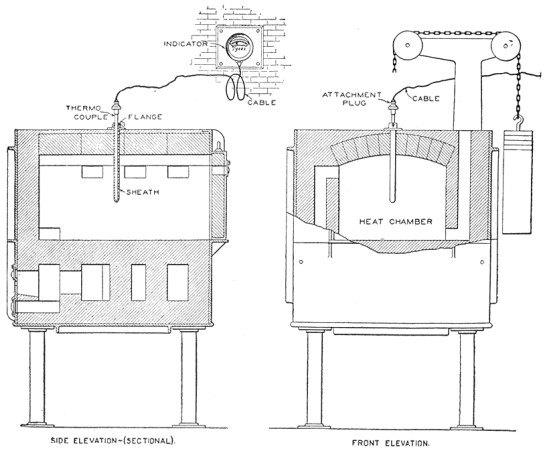 FIG. 118.—Tycos pyrometer in oil-fired furnace.
FIG. 118.—Tycos pyrometer in oil-fired furnace.
The instrument is light and portable, and can be sighted as Page 218 easily as an opera glass. The telescope, which is held in the hand, weighs only 25 oz.; and the case containing the battery, rheostat and milliammeter, which is slung from the shoulder, only 10 lb.
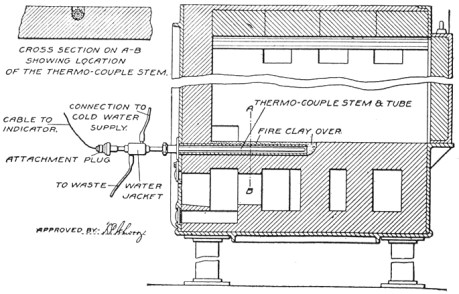 FIG. 119.—Thermocouple in floor of furnace.
FIG. 119.—Thermocouple in floor of furnace.
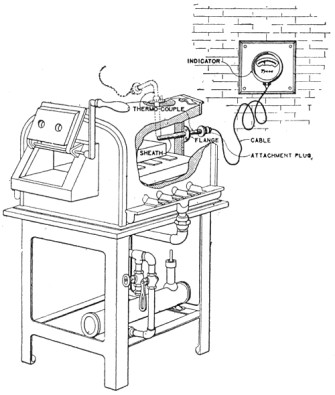 FIG. 120.—Pyrometer in gas furnace.
FIG. 120.—Pyrometer in gas furnace.
A large surface to sight at is not required. So long as the image formed by the objective is broader than the lamp filament, the temperature can be measured accurately.
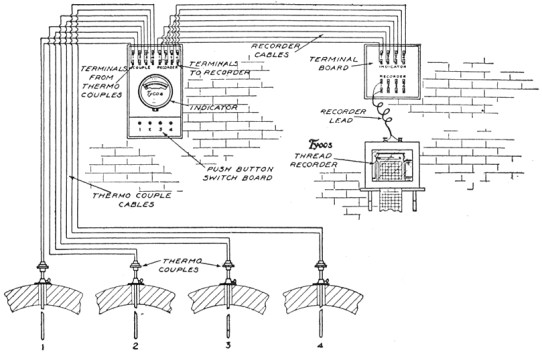 FIG. 121.—Tycos multiple indicating pyrometer and recorder.
FIG. 121.—Tycos multiple indicating pyrometer and recorder.
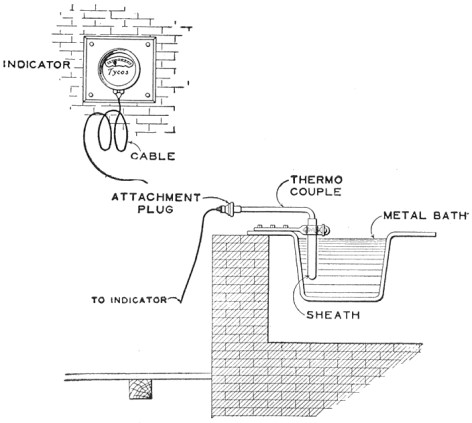 FIG. 122.—Pyrometer in galvanizing tank.
FIG. 122.—Pyrometer in galvanizing tank.
Page 220 Distance does not matter, as the brightness of the image formed by the lens is practically constant, regardless of the distance of the instrument from the hot object.
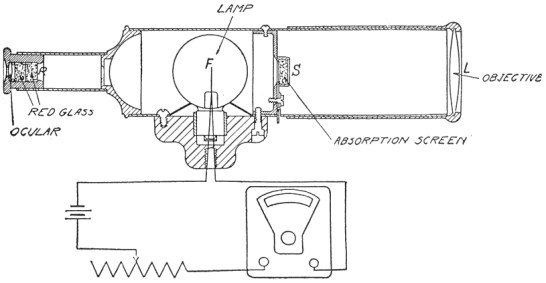 FIG. 123.—Leeds & Northrup optical pyrometer.
FIG. 123.—Leeds & Northrup optical pyrometer.
The manipulation is simple and rapid, consisting merely in the turning of a knurled knob. The setting is made with great precision, due to the rapid change in light intensity with change in temperature and to the sensitiveness of the eye to differences of light intensity. In the region of temperatures used for hardening steel, for example, different observers using the instrument will agree within 3°C.
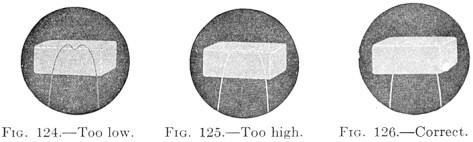
Only brightness, not color, of light is matched, as light of only one color reaches the eye. Color blindness, therefore, is no hindrance to the use of this method. The use of the instrument is shown in Fig. 127.
Optical System and Electrical Circuit of the Leeds & Northrup Optical Pyrometer.—For extremely high temperature, the optical pyrometer is largely used. This is a comparative method. By means of the rheostat the current through the lamp is adjusted Page 221 until the brightness of the filament is just equal to the brightness of the image produced by the lens L, Fig. 123, whereupon the filament blends with or becomes indistinguishable in the background formed by the image of the hot object. This adjustment can be made with great accuracy and certainty, as the effect of radiation upon the eye varies some twenty times faster than does the temperature at 1,600°F., and some fourteen times faster at 3,400°F. When a balance has been obtained, the observer notes the reading of the milliammeter. The temperature corresponding to the current is then read from a calibration curve supplied with the instrument.
 FIG. 127.—Using the optical pyrometer.
FIG. 127.—Using the optical pyrometer.
As the intensity of the light emitted at the higher temperatures becomes dazzling, it is found desirable to introduce a piece of red glass in the eye piece at R. This also eliminates any question of matching colors, or of the observer's ability to distinguish colors. It is further of value in dealing with bodies which do not radiate light of the same composition as that emitted by a black body, since nevertheless the intensity of radiation of any one color from Page 222 such bodies increases progressively in a definite manner as the temperature rises. The intensity of this one color can therefore be used as a measure of temperature for the body in question. Figures 124 to 126 show the way it is read.
The voltage generated by a thermo-couple of an electric pyrometer is dependent on the difference in temperature between its hot junction, inside the furnace, and the cold junction, or opposite end of the thermo-couple to which the copper wires are connected. If the temperature or this cold junction rises and falls, the indications of the instrument will vary, although the hot junction in the furnace may be at a constant temperature.
A cold-junction temperature of 75°F., or 25°C., is usually adopted in commercial pyrometers, and the pointer on the pyrometer should stand at this point on the scale when the hot junction is not heated. If the cold-junction temperature rises about 75°F., where base metal thermo-couples are used, the pyrometer will read approximately 1° low for every 1° rise in temperature above 75°F. For example, if the instrument is adjusted for a cold-junction temperature of 75°, and the actual cold-junction temperature is 90°F., the pyrometer will read 15° low. If, however, the cold-junction temperature falls below 75°F., the pyrometer will read high instead of low, approximately 1° for every 1° drop in temperature below 75°F.
With platinum thermo-couples, the error is approximately 1/2° for 1° change in temperature.
Correction by Zero Adjustment.—Many pyrometers are supplied with a zero adjuster, by means of which the pointer can be set to any actual cold-junction temperature. If the cold junction of the thermo-couple is in a temperature of 100°F., the pointer can be set to this point on the scale, and the readings of the instrument will be correct.
Compensating Leads.—By the use of compensating leads, formed of the same material as the thermo-couple, the cold junction can be removed from the head of the thermo-couple to a point 10, 20 or 50 ft. distant from the furnace, where the temperature is reasonably constant. Where greater accuracy is desired, a common method is to drive a 2-in. pipe, with a pointed closed end, some 10 to 20 ft. into the ground, as shown Page 223 in Fig. 128. The compensating leads are joined to the copper leads, and the junction forced down to the bottom of the pipe. The cold junction is now in the ground, beneath the building, at a depth at which the temperature is very constant, about 70°F., throughout the year. This method will usually control the cold-junction temperature within 5°F.
Where the greatest accuracy is desired a compensating box will overcome cold-junction errors entirely. It consists of a case enclosing a lamp and thermostat, which can be adjusted to maintain any desired temperature, from 50 to 150°F. The compensating leads enter the box and copper leads run from the compensating box to the instrument, so that the cold junction is within the box. Figure 129 shows a Brown compensating box.
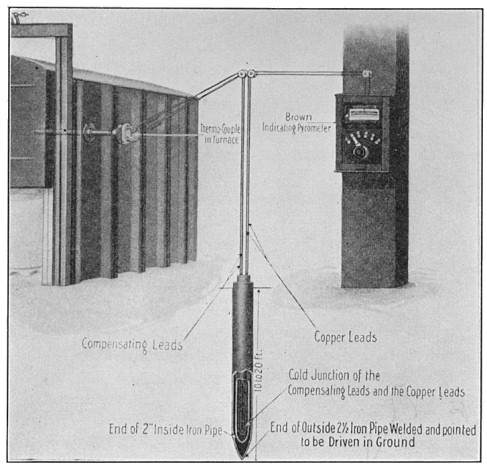 FIG. 128.—Correcting cold-junction error.
FIG. 128.—Correcting cold-junction error.
If it is desired to maintain the cold junction at 100°: the thermostat is set at this point, and the lamp, being wired to the 110- or 220-volt lighting circuit, will light and heat the box until 100° is reached, when the thermostat will open the circuit and Page 224 the light is extinguished. The box will now cool down to 98°, when the circuit is again closed, the lamp lights, the box heats up, and the operation is repeated.
 FIG. 129.—Compensating box.
FIG. 129.—Compensating box.
In large heat-treating plants it has been customary to maintain an operator at a central pyrometer, and by colored electric lights at the furnaces, signal whether the temperatures are correct or not. It is common practice to locate three lights above each furnace-red, white and green. The red light burns when the temperature is too low, the white light when the temperature is within certain limits—for example, 20°F. of the correct temperature—and the green light when the temperature is too high.
 FIG. 130.—Brown automatic signaling pyrometer.
FIG. 130.—Brown automatic signaling pyrometer.
Instruments to operate the lights automatically have been Page 225 devised and one made by Brown is shown in Fig. 130. The same form of instrument is used for this purpose to automatically control furnace temperatures, and the pointer is depressed at intervals of every 10 sec. on contacts corresponding to the red, white and green lights.
 FIG. 131.—Automatic temperature control.
FIG. 131.—Automatic temperature control.
Automatic temperature control instruments are similar to the Brown indicating high resistance pyrometer with the exception that the pointer is depressed at intervals of every 10 sec. upon contact-making devices. No current passes through the pointer which simply depresses the upper contact device tipped with platinum, which in turn comes in contact with the lower contact device, platinum-tipped, and the circuit is completed through these two contacts. The current is very small, about Page 226 1/10 amp., as it is only necessary to operate the relay which in turn operates the switch or valve. A small motor is used to depress the pointer at regular intervals. The contact-making device is adjustable throughout the scale range of the instrument, and an index pointer indicates the point on the instrument at which the temperature is being controlled. The space between the two contacts on the high and low side, separated by insulating material, is equivalent to 1 per cent of the scale range. A control of temperature is therefore possible within 1 per cent of the total scale range. Figure 131 shows this attached to a small furnace.
 FIG. 132.—Portable thermocouple testing molten brass.
FIG. 132.—Portable thermocouple testing molten brass.
Pyrometers for molten metal are connected to portable thermocouples as in Fig. 132. Usually the pyrometer is portable, as shown in this case, which is a Brown. Other methods of mounting for this kind of work arc shown in Figs. 133 and 134. The bent mountings are designed for molten metal, such as brass or copper and are supplied with either clay, graphite or carborundum tubes. Fifteen feet of connecting wire is usually supplied.
The angle mountings, Fig. 134, are recommended for baths such as lead or cyanide. The horizontal arm is usually about 14 in. long, and the whole mounting is easily taken apart making Page 227 replacements very easy. Details of the thermo-couple shown in Fig. 132 are given in Fig. 135. This is a straight rod with a protector for the hand of the operator. The lag in such couples is less than one minute. These are Englehard mountings.
Thermo-couples must be protected from the danger of mechanical injury. For this purpose tubes of various refractory materials are made to act as protectors. These in turn are usually protected by outside metal tubes. Pure wrought iron is largely used for this purpose as it scales and oxidizes very slowly. These tubes are usually made from 2 to 4 in. shorter than the inner tubes. In lead baths the iron tubes often have one end welded closed and are used in connection with an angle form of mounting.
 FIG. 133.—Bent handle thermocouple with protector.
FIG. 133.—Bent handle thermocouple with protector.
Where it is necessary for protecting tubes to project a considerable distance into the furnace a tube made of nichrome is frequently used. This is a comparatively new alloy which stands high temperatures without bending. It is more costly than iron but also much more durable.
When used in portable work and for high temperatures, pure nickel tubes are sometimes used. There is also a special metal tube made for use in cyanide. This metal withstands the intense penetrating characteristics of cyanide. It lasts from six to ten months as against a few days for the iron tube.
Page 228 The inner tubes of refractory materials, also vary according to the purposes for which they are to be used. They are as follows:
Marquardt mass tubes for temperatures up to 3,000°F., but they will not stand sudden changes in temperature, such as in contact with intermittent flames, without an extra outer covering of chamotte, fireclay or carborundum.
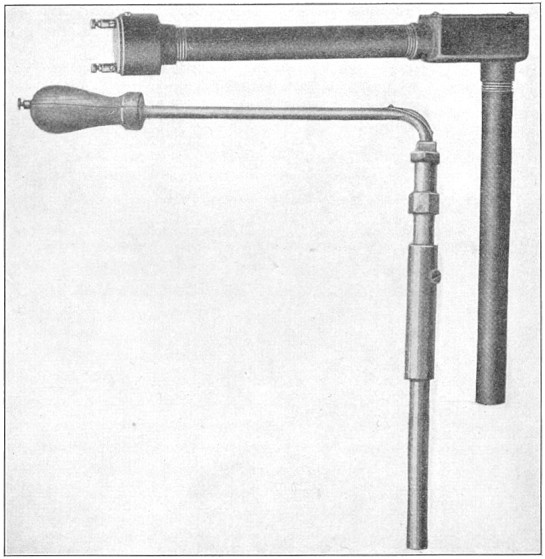 FIG. 134.—Other styles of bent mounting.
FIG. 134.—Other styles of bent mounting.
Fused silica tubes for continuous temperatures up to 1,800°F. and intermittently up to 2,400°F. The expansion at various temperatures is very small, which makes them of value for portable work. They also resist most acids.
Chamotte tubes are useful up to 2,800°F. and are mechanically strong. They have a small expansion and resist temperature Page 229 changes well, which makes them good as outside protectors for more fragile tubes. They cannot be used in molten metals, or baths of any kind nor in gases of an alkaline nature. They are used mainly to protect a Marquardt mass or silica tube.
Carborundum tubes are also used as outside protection to other tubes. They stand sudden changes of temperature well and resist all gases except chlorine, above 1,750°F. Especially useful in protecting other tubes against molten aluminum, brass, copper and similar metals.
Clay tubes are sometimes used in large annealing furnaces where they are cemented into place, forming a sort of well for the insertion of the thermo-couple. They are also used with portable thermo-couples for obtaining the temperatures of molten iron and steel in ladles. Used in this way they are naturally short-lived, but seem the best for this purpose.
 FIG. 135.—Straight thermocouple and guard.
FIG. 135.—Straight thermocouple and guard.
Corundite tubes are used as an outer protection for both the Marquardt mass and the silica tubes for kilns and for glass furnaces. Graphite tubes are also used in some cases for outer protections.
Calorized tubes are wrought-iron pipe treated with aluminum vapor which often doubles or even triples the life of the tube at high temperature.
These tubes come in different sizes and lengths depending on the uses for which they are intended. Heavy protecting outer tubes may be only 1 in. in inside diameter and as much as 3 in. outside diameter, while the inner tubes, such as the Marquardt mass and silica tubes are usually about ¾ in. outside and 3/8 in. inside diameter. The length varies from 12 to 48 in. in most cases.
Special terminal heads are provided, with brass binding posts for electrical connections, and with provisions for water cooling when necessary.
TABLE 32.—Temperature Conversion Tables.
TABLE 33.—Comparison Between Degrees Centigrade and Degrees Fahrenheit.
TABLE 34.—Weight of Round, Octagon and Square Carbon Tool Steel per Foot.
TABLE 35.—Weight of Round Carbon Tool Steel 12 In. in Diameter and Larger, per Foot.
TABLE 36.—Decimal Equivalents of a foot.
Page 232 TEMPERATURE CONVERSION TABLES
By ALBERT SAUVEUR
| -459.4 to 0 | 0 to 100 | 100 to 1000 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. | F. | C. | F. | C. | F. | C. | F. | C. | F. | |||||
| -273 | -459.4 | -17.8 | 0 | 32 | 10.0 | 50 | 122.0 | 38 | 100 | 212 | 260 | 500 | 932 | |
| -268 | -450 | -17.2 | 1 | 33.8 | 10.6 | 51 | 123.8 | 43 | 110 | 230 | 266 | 510 | 950 | |
| -262 | -440 | -16.7 | 2 | 35.6 | 11.1 | 52 | 125.6 | 49 | 120 | 248 | 271 | 520 | 968 | |
| -257 | -430 | -16.1 | 3 | 37.4 | 11.7 | 53 | 127.4 | 54 | 130 | 266 | 277 | 530 | 986 | |
| -251 | -420 | -15.6 | 4 | 39.2 | 12.2 | 54 | 129.2 | 60 | 140 | 284 | 282 | 540 | 1004 | |
| -246 | -410 | -15.0 | 5 | 41.0 | 12.8 | 55 | 131.0 | 66 | 150 | 302 | 288 | 550 | 1022 | |
| -240 | -400 | -14.4 | 6 | 42.8 | 13.3 | 56 | 132.8 | 71 | 160 | 320 | 293 | 560 | 1040 | |
| -234 | -390 | -13.9 | 7 | 44.6 | 13.9 | 57 | 134.6 | 77 | 170 | 336 | 299 | 570 | 1058 | |
| -229 | -380 | -13.3 | 8 | 46.4 | 14.4 | 58 | 136.4 | 82 | 180 | 358 | 304 | 580 | 1076 | |
| -223 | -370 | -12.8 | 9 | 48.2 | 15.0 | 59 | 138.2 | 88 | 190 | 374 | 310 | 590 | 1094 | |
| -218 | -360 | -12.2 | 10 | 50.0 | 15.6 | 60 | 140.0 | 93 | 200 | 392 | 316 | 600 | 1112 | |
| -212 | -350 | -11.7 | 11 | 51.8 | 16.1 | 61 | 141.8 | 99 | 210 | 410 | 321 | 610 | 1130 | |
| -207 | -340 | -11.1 | 12 | 53.6 | 16.7 | 62 | 143.6 | 100 | 212 | 413 | 327 | 620 | 1148 | |
| -201 | -330 | -10.6 | 13 | 55.4 | 17.2 | 63 | 145.4 | 104 | 220 | 428 | 332 | 630 | 1166 | |
| -196 | -320 | -10.0 | 14 | 57.2 | 17.8 | 64 | 147.2 | 110 | 230 | 446 | 338 | 640 | 1184 | |
| -190 | -310 | -9.44 | 15 | 59.0 | 18.3 | 65 | 149.0 | 116 | 240 | 464 | 343 | 650 | 1202 | |
| -184 | -300 | -8.89 | 16 | 61.8 | 18.9 | 66 | 150.8 | 121 | 250 | 482 | 349 | 660 | 1220 | |
| -179 | -290 | -8.33 | 17 | 63.6 | 19.4 | 67 | 152.6 | 127 | 260 | 500 | 354 | 670 | 1238 | |
| -173 | -280 | -7.78 | 18 | 65.4 | 20.0 | 68 | 154.4 | 132 | 270 | 518 | 360 | 680 | 1256 | |
| -169 | -273 | -459.4 | -7.22 | 19 | 67.2 | 20.6 | 69 | 156.2 | 138 | 280 | 536 | 366 | 690 | 1274 |
| -168 | -270 | -454 | -6.67 | 20 | 68.0 | 21.1 | 70 | 158.0 | 143 | 290 | 554 | 371 | 700 | 1292 |
| -162 | -260 | -436 | -6.11 | 21 | 69.8 | 21.7 | 71 | 159.8 | 149 | 300 | 572 | 377 | 710 | 1310 |
| -157 | -250 | -418 | -5.56 | 22 | 71.6 | 22.2 | 72 | 161.6 | 154 | 310 | 590 | 382 | 720 | 1328 |
| -151 | -240 | -400 | -5.00 | 23 | 73.4 | 22.8 | 73 | 163.4 | 160 | 320 | 608 | 388 | 730 | 1346 |
| -146 | -230 | -382 | -4.44 | 24 | 75.2 | 23.3 | 74 | 165.2 | 166 | 330 | 626 | 393 | 740 | 1364 |
| -140 | -220 | -364 | -3.89 | 25 | 77.0 | 23.9 | 75 | 167.0 | 171 | 340 | 644 | 399 | 750 | 1382 |
| -134 | -210 | -346 | -3.33 | 26 | 78.8 | 24.4 | 76 | 168.8 | 177 | 350 | 662 | 404 | 760 | 1400 |
| -129 | -200 | -328 | -2.78 | 27 | 80.6 | 25.0 | 77 | 170.6 | 182 | 360 | 680 | 410 | 770 | 1418 |
| -123 | -190 | -310 | -2.22 | 28 | 82.4 | 25.6 | 78 | 172.4 | 188 | 370 | 698 | 416 | 780 | 1436 |
| -118 | -180 | -292 | -1.67 | 29 | 84.2 | 26.1 | 79 | 174.2 | 193 | 380 | 716 | 421 | 790 | 1454 |
| -112 | -170 | -274 | -1.11 | 30 | 86.0 | 26.7 | 80 | 176.0 | 199 | 390 | 734 | 427 | 800 | 1472 |
| -107 | -160 | -256 | -0.56 | 31 | 87.8 | 27.2 | 81 | 177.8 | 204 | 400 | 752 | 432 | 810 | 1490 |
| -101 | -150 | -238 | 0 | 32 | 89.6 | 27.8 | 82 | 179.6 | 210 | 410 | 770 | 438 | 820 | 1508 |
| -95.6 | -140 | -220 | 0.56 | 33 | 91.4 | 28.3 | 83 | 181.4 | 216 | 420 | 788 | 443 | 830 | 1526 |
| -90.0 | -130 | -202 | 1.11 | 34 | 93.2 | 28.9 | 84 | 183.2 | 221 | 430 | 806 | 449 | 840 | 1544 |
| -84.4 | -120 | -184 | 1.67 | 35 | 95.0 | 29.4 | 85 | 185.0 | 227 | 440 | 824 | 454 | 850 | 1562 |
| -78.9 | -110 | -166 | 2.22 | 36 | 96.8 | 30.0 | 86 | 186.8 | 232 | 450 | 842 | 460 | 860 | 1580 |
| -73.3 | -100 | -148 | 2.78 | 37 | 98.6 | 30.6 | 87 | 188.6 | 238 | 460 | 860 | 466 | 870 | 1598 |
| -67.8 | -90 | -130 | 3.33 | 38 | 100.4 | 31.1 | 88 | 190.4 | 243 | 470 | 878 | 471 | 880 | 1616 |
| -62.2 | -80 | -112 | 3.89 | 39 | 102.2 | 31.7 | 89 | 192.2 | 249 | 480 | 896 | 477 | 890 | 1634 |
| -56.7 | -70 | -94 | 4.44 | 40 | 104.0 | 32.2 | 90 | 194.0 | 254 | 490 | 914 | 482 | 900 | 1652 |
| -51.1 | -60 | -76 | 5.00 | 41 | 105.8 | 32.8 | 91 | 195.8 | 488 | 910 | 1670 | |||
| -45.6 | -50 | -58 | 5.56 | 42 | 107.6 | 33.3 | 92 | 197.6 | 493 | 920 | 1688 | |||
| -40.0 | -40 | -40 | 6.11 | 43 | 109.4 | 33.9 | 93 | 199.4 | 499 | 930 | 1706 | |||
| -34.4 | -30 | -22 | 6.67 | 44 | 111.2 | 34.4 | 94 | 201.2 | 504 | 940 | 1724 | |||
| -28.9 | -20 | 4 | 7.22 | 45 | 113.0 | 35.0 | 95 | 203.0 | 510 | 950 | 1742 | |||
| -23.3 | -10 | 14 | 7.78 | 46 | 114.8 | 35.6 | 96 | 204.8 | 516 | 960 | 1760 | |||
| -17.8 | 0 | 32 | 8.33 | 47 | 116.6 | 36.1 | 97 | 206.6 | 521 | 970 | 1778 | |||
| 8.89 | 48 | 118.4 | 36.7 | 98 | 208.4 | 527 | 980 | 1796 | ||||||
| 9.44 | 49 | 120.2 | 37.2 | 99 | 210.2 | 532 | 990 | 1814 | ||||||
| 37.8 | 100 | 212.0 | 538 | 1000 | 1832 | |||||||||
| 1000 to 2000 | 2000 to 3000 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. | F. | C. | F. | C. | F. | C. | F. | ||||
| 538 | 1000 | 1832 | 816 | 1500 | 2732 | 1093 | 2000 | 3632 | 1371 | 2500 | 4534 |
| 543 | 1010 | 1850 | 821 | 1510 | 2750 | 1099 | 2010 | 3650 | 1377 | 2510 | 4552 |
| 549 | 1020 | 1868 | 827 | 1520 | 2768 | 1104 | 2020 | 3668 | 1382 | 2520 | 4560 |
| 554 | 1030 | 1886 | 832 | 1530 | 2786 | 1110 | 2030 | 3686 | 1388 | 2530 | 4588 |
| 560 | 1040 | 1904 | 838 | 1540 | 2804 | 1116 | 2040 | 3704 | 1393 | 2540 | 4606 |
| 566 | 1050 | 1922 | 843 | 1550 | 2822 | 1121 | 2050 | 3722 | 1399 | 2550 | 4622 |
| 571 | 1060 | 1940 | 849 | 1560 | 2840 | 1127 | 2060 | 3740 | 1404 | 2560 | 4640 |
| 577 | 1070 | 1958 | 854 | 1570 | 2858 | 1132 | 2070 | 3758 | 1410 | 2570 | 4658 |
| 582 | 1080 | 1976 | 860 | 1580 | 2876 | 1138 | 2080 | 3776 | 1416 | 2580 | 4676 |
| 588 | 1090 | 1994 | 866 | 1590 | 2894 | 1143 | 2090 | 3794 | 1421 | 2590 | 4694 |
| 593 | 1100 | 2012 | 871 | 1600 | 2912 | 1149 | 2100 | 3812 | 1427 | 2600 | 4712 |
| 599 | 1110 | 2030 | 877 | 1610 | 2930 | 1154 | 2110 | 3830 | 1432 | 2610 | 4730 |
| 604 | 1120 | 2048 | 882 | 1620 | 2948 | 1160 | 2120 | 3848 | 1438 | 2620 | 4748 |
| 610 | 1130 | 2066 | 888 | 1630 | 2966 | 1166 | 2130 | 3866 | 1443 | 2630 | 4766 |
| 616 | 1140 | 2084 | 893 | 1640 | 2984 | 1171 | 2140 | 3884 | 1449 | 2640 | 4784 |
| 621 | 1150 | 2102 | 899 | 1650 | 3002 | 1777 | 2150 | 3902 | 1454 | 2650 | 4802 |
| 627 | 1160 | 2120 | 904 | 1660 | 3020 | 1182 | 2160 | 3920 | 1460 | 2660 | 4820 |
| 632 | 1170 | 2138 | 910 | 1670 | 3038 | 1188 | 2170 | 3938 | 1466 | 2670 | 4838 |
| 638 | 1180 | 2156 | 916 | 1680 | 3056 | 1193 | 2180 | 3956 | 1471 | 2680 | 4854 |
| 643 | 1190 | 2174 | 921 | 1690 | 3074 | 1199 | 2190 | 3974 | 1477 | 2690 | 4876 |
| 649 | 1200 | 2192 | 927 | 1700 | 3092 | 1204 | 2200 | 3992 | 1482 | 2700 | 4892 |
| 654 | 1210 | 2210 | 932 | 1710 | 3110 | 1210 | 2210 | 4010 | 1488 | 2710 | 4910 |
| 660 | 1220 | 2228 | 938 | 1720 | 3128 | 1216 | 2220 | 4028 | 1493 | 2720 | 4928 |
| 666 | 1230 | 2246 | 943 | 1730 | 3146 | 1221 | 2230 | 4046 | 1499 | 2730 | 4946 |
| 671 | 1240 | 2264 | 949 | 1740 | 3164 | 1227 | 2240 | 4064 | 1504 | 2740 | 4964 |
| 677 | 1250 | 2282 | 954 | 1750 | 3182 | 1232 | 2250 | 4082 | 1510 | 2750 | 4982 |
| 682 | 1260 | 2300 | 960 | 1760 | 3200 | 1238 | 2260 | 4100 | 1516 | 2760 | 5000 |
| 688 | 1270 | 2318 | 966 | 1770 | 3218 | 1243 | 2270 | 4118 | 1521 | 2770 | 5018 |
| 693 | 1280 | 2336 | 971 | 1780 | 3236 | 1249 | 2280 | 4136 | 1527 | 2780 | 5036 |
| 699 | 1290 | 2354 | 977 | 1790 | 3254 | 1254 | 2290 | 4154 | 1532 | 2790 | 5054 |
| 704 | 1300 | 2372 | 982 | 1800 | 3272 | 1260 | 2300 | 4172 | 1538 | 2800 | 5072 |
| 710 | 1310 | 2390 | 988 | 1810 | 3290 | 1266 | 2310 | 4190 | 1543 | 2810 | 5090 |
| 716 | 1320 | 2408 | 993 | 1820 | 3308 | 1271 | 2320 | 4208 | 1549 | 2820 | 5108 |
| 721 | 1330 | 2426 | 999 | 1830 | 3326 | 1277 | 2330 | 4226 | 1554 | 2830 | 5126 |
| 727 | 1340 | 2444 | 1004 | 1840 | 3344 | 1282 | 2340 | 4244 | 1560 | 2840 | 5144 |
| 732 | 1350 | 2462 | 1010 | 1850 | 3362 | 1288 | 2350 | 4262 | 1566 | 2850 | 5162 |
| 738 | 1360 | 2480 | 1016 | 1860 | 3380 | 1293 | 2360 | 4280 | 1571 | 2860 | 5180 |
| 743 | 1370 | 2498 | 1021 | 1870 | 3398 | 1299 | 2370 | 4298 | 1577 | 2870 | 5198 |
| 749 | 1380 | 2516 | 1027 | 1880 | 3416 | 1304 | 2380 | 4316 | 1582 | 2880 | 5216 |
| 754 | 1390 | 2534 | 1032 | 1890 | 3434 | 1310 | 2390 | 4334 | 1588 | 2890 | 5234 |
| 760 | 1400 | 2552 | 1038 | 1900 | 3452 | 1316 | 2400 | 4352 | 1593 | 2900 | 5252 |
| 766 | 1410 | 2570 | 1043 | 1910 | 3470 | 1321 | 2410 | 4370 | 1599 | 2910 | 5270 |
| 771 | 1420 | 2588 | 1049 | 1920 | 3488 | 1327 | 2420 | 4388 | 1604 | 2920 | 5288 |
| 777 | 1430 | 2606 | 1054 | 1930 | 3506 | 1332 | 2430 | 4406 | 1610 | 2930 | 5306 |
| 782 | 1440 | 2624 | 1060 | 1940 | 3524 | 1338 | 2440 | 4424 | 1616 | 2940 | 5324 |
| 788 | 1450 | 2642 | 1066 | 1950 | 3542 | 1343 | 2450 | 4442 | 1621 | 2950 | 5342 |
| 793 | 1460 | 2660 | 1071 | 1960 | 3560 | 1349 | 2460 | 4460 | 1627 | 2960 | 5360 |
| 799 | 1470 | 2678 | 1077 | 1970 | 3578 | 1354 | 2470 | 4478 | 1632 | 2970 | 5378 |
| 804 | 1480 | 2696 | 1082 | 1980 | 3596 | 1360 | 2480 | 4496 | 1638 | 2980 | 5396 |
| 810 | 1490 | 2714 | 1088 | 1990 | 3614 | 1366 | 2490 | 4514 | 1643 | 2990 | 5414 |
| 1093 | 2000 | 3632 | 1649 | 3000 | 5432 | ||||||
| C. | F. | C. | F. | ||
|---|---|---|---|---|---|
| 0.56 | 1 | 1.8 | 3.33 | 6 | 10.8 |
| 1.11 | 2 | 3.6 | 3.89 | 7 | 12.6 |
| 1.67 | 3 | 5.4 | 4.44 | 8 | 14.4 |
| 2.22 | 4 | 7.2 | 5.00 | 9 | 16.2 |
| 2.78 | 5 | 9.0 | 5.56 | 10 | 18.0 |
NOTE.—The numbers in bold face type refer to the temperature either in degrees Centigrade or Fahrenheit which it is desired to convert into the other scale. If converting from Fahrenheit degrees to Centigrade degrees the equivalent temperature will be found in the left column, while if converting from degrees Centigrade to degrees Fahrenheit, the answer will be found in the column on the right. These tables are a revision of those by Sauveur & Boylston, metallurgical engineers, Cambridge, Mass. Copyright, 1920.
Page 234 Those using pyrometers will find this and the preceding conversion table of great convenience:
| Degrees | Degrees | Degrees | Degrees | Degrees | Degrees | Degrees | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F. | C. | F. | C. | F. | C. | F. | C. | F. | C. | F. | C. | F. | C. |
| -40 | -40.0 | 3 | -16.1 | 46 | 7.7 | 89 | 31.6 | 132 | 55.5 | 175 | 79.4 | 275 | 135.0 |
| -39 | -39.4 | 4 | -15.5 | 47 | 8.3 | 90 | 32.2 | 133 | 56.1 | 176 | 80.0 | 300 | 148.8 |
| -38 | -38.8 | 5 | -15.0 | 48 | 8.8 | 91 | 32.7 | 134 | 56.6 | 177 | 80.5 | 325 | 162.7 |
| -37 | -38.3 | 6 | -14.4 | 49 | 9.3 | 92 | 33.3 | 135 | 57.2 | 178 | 81.1 | 350 | 176.6 |
| -36 | -37.7 | 7 | -13.8 | 50 | 10.0 | 93 | 33.9 | 136 | 57.7 | 179 | 81.6 | 375 | 190.5 |
| -35 | -37.2 | 8 | -13.3 | 51 | 10.5 | 94 | 34.4 | 137 | 58.3 | 180 | 82.2 | 400 | 204.4 |
| -34 | -36.6 | 9 | -12.7 | 52 | 11.1 | 95 | 35.0 | 138 | 58.8 | 181 | 82.7 | 425 | 218.3 |
| -33 | -36.1 | 10 | -12.2 | 53 | 11.6 | 96 | 35.5 | 139 | 59.4 | 182 | 83.3 | 450 | 232.2 |
| -32 | -35.5 | 11 | -11.6 | 54 | 12.2 | 97 | 36.1 | 140 | 60.0 | 183 | 83.8 | 475 | 246.1 |
| -31 | -35.0 | 12 | -11.1 | 55 | 12.7 | 98 | 36.6 | 141 | 60.5 | 184 | 84.4 | 500 | 260.0 |
| -30 | -34.4 | 13 | -10.5 | 56 | 13.3 | 99 | 37.2 | 142 | 61.1 | 185 | 85.0 | 525 | 273.8 |
| -29 | -33.9 | 14 | -10.0 | 57 | 13.8 | 100 | 37.7 | 143 | 61.6 | 186 | 85.5 | 550 | 287.7 |
| -28 | -33.3 | 15 | -9.3 | 58 | 14.4 | 101 | 38.3 | 144 | 62.2 | 187 | 86.1 | 575 | 301.6 |
| -27 | -32.7 | 16 | -8.8 | 59 | 15.0 | 102 | 38.8 | 145 | 62.7 | 188 | 86.6 | 600 | 315.5 |
| -26 | -32.2 | 17 | -8.3 | 60 | 15.5 | 103 | 39.4 | 146 | 63.3 | 189 | 87.2 | 625 | 329.4 |
| -25 | -31.6 | 18 | -7.7 | 61 | 16.1 | 104 | 40.0 | 147 | 63.8 | 190 | 87.7 | 650 | 343.3 |
| -24 | -31.1 | 19 | -7.2 | 62 | 16.6 | 105 | 40.5 | 148 | 64.4 | 191 | 88.3 | 675 | 357.2 |
| -23 | -30.5 | 20 | -6.6 | 63 | 17.2 | 106 | 41.1 | 149 | 65.0 | 192 | 88.8 | 700 | 371.1 |
| -22 | -30.0 | 21 | -6.1 | 64 | 17.7 | 107 | 41.6 | 150 | 65.5 | 193 | 89.4 | 725 | 385.0 |
| -21 | -29.4 | 22 | -5.5 | 65 | 18.3 | 108 | 42.2 | 151 | 66.1 | 194 | 90.0 | 750 | 398.8 |
| -20 | -28.8 | 23 | -5.0 | 66 | 18.8 | 109 | 42.7 | 152 | 66.6 | 195 | 90.5 | 775 | 412.7 |
| -19 | -28.3 | 24 | -4.4 | 67 | 19.4 | 110 | 43.3 | 153 | 67.2 | 196 | 91.1 | 800 | 426.6 |
| -18 | -27.7 | 25 | -3.8 | 68 | 20.0 | 111 | 43.8 | 154 | 67.7 | 197 | 91.6 | 825 | 440.5 |
| -17 | -27.2 | 26 | -3.3 | 69 | 20.5 | 112 | 44.4 | 155 | 68.3 | 198 | 92.2 | 850 | 454.4 |
| -16 | -26.6 | 27 | -2.7 | 70 | 21.1 | 113 | 45.0 | 156 | 68.8 | 199 | 92.7 | 875 | 468.3 |
| -15 | -26.1 | 28 | -2.2 | 71 | 21.6 | 114 | 45.5 | 157 | 69.4 | 200 | 93.3 | 900 | 482.2 |
| -14 | -25.5 | 29 | -1.6 | 72 | 22.2 | 115 | 46.1 | 158 | 70.0 | 201 | 93.8 | 925 | 496.1 |
| -13 | -25.0 | 30 | -1.1 | 73 | 22.7 | 116 | 46.6 | 159 | 70.5 | 202 | 94.4 | 950 | 510.0 |
| -12 | -24.4 | 31 | -0.5 | 74 | 23.3 | 117 | 47.2 | 160 | 71.1 | 203 | 95.0 | 975 | 523.8 |
| -11 | -23.8 | 32 | -0.0 | 75 | 23.8 | 118 | 47.7 | 161 | 71.6 | 204 | 95.5 | 1,000 | 537.7 |
| -10 | -23.3 | 33 | +0.5 | 76 | 24.4 | 119 | 48.3 | 162 | 72.2 | 205 | 96.1 | 1,100 | 593.3 |
| -9 | -22.7 | 34 | 1.1 | 77 | 25.0 | 120 | 48.8 | 163 | 72.7 | 206 | 96.6 | 1,200 | 648.8 |
| -8 | -22.2 | 35 | 1.6 | 78 | 25.5 | 121 | 49.4 | 164 | 73.3 | 207 | 97.2 | 1,300 | 704.4 |
| -7 | -21.6 | 36 | 2.2 | 79 | 26.1 | 122 | 50.0 | 165 | 73.8 | 208 | 97.7 | 1,400 | 760.0 |
| -6 | -21.1 | 37 | 2.7 | 80 | 26.6 | 123 | 50.5 | 166 | 74.4 | 209 | 98.3 | 1,500 | 815.5 |
| -5 | -20.5 | 38 | 3.3 | 81 | 27.2 | 124 | 51.1 | 167 | 75.0 | 210 | 98.8 | 1,600 | 871.1 |
| -4 | -20.0 | 39 | 3.8 | 82 | 27.7 | 125 | 51.6 | 168 | 75.5 | 211 | 99.4 | 1,700 | 926.6 |
| -3 | -19.4 | 40 | 4.4 | 83 | 28.3 | 126 | 52.2 | 169 | 76.1 | 212 | 100.0 | 1,800 | 982.2 |
| -2 | -18.8 | 41 | 5.0 | 84 | 28.8 | 127 | 52.7 | 170 | 76.6 | 213 | 100.5 | 1,900 | 1,037.7 |
| -1 | -18.3 | 42 | 5.5 | 85 | 29.4 | 128 | 53.3 | 171 | 77.2 | 214 | 101.1 | 2,000 | 1,093.3 |
| 0 | -17.7 | 43 | 6.1 | 86 | 30.0 | 129 | 53.8 | 172 | 77.7 | 215 | 101.6 | 2,100 | 1,148.8 |
| +1 | -17.2 | 44 | 6.6 | 87 | 30.5 | 130 | 54.4 | 173 | 78.3 | 225 | 107.2 | 2,200 | 1,204.4 |
| 2 | -16.6 | 45 | 7.2 | 88 | 31.1 | 131 | 55.0 | 174 | 78.8 | 250 | 121.1 | 2,300 | 1,260.0 |
| Degrees Fahrenheit = | 9 x degrees C. | + 32 |
| 5 | ||
| Degrees Centigrade = | 5 x (degrees F. - 32) | |
| 9 | ||
Page 235 Three other useful tables are also given on the following pages.
| Size in inches |
Round | Octagon | Square | Size in inches |
Round | Octagon | Square |
|---|---|---|---|---|---|---|---|
| 1/16 | 0.010 | 0.011 | 0.013 | 2-1/2 | 16.79 | 17.71 | 21.37 |
| 1/8 | 0.042 | 0.044 | 0.053 | 2-5/8 | 18.51 | 19.52 | 23.56 |
| 3/16 | 0.094 | 0.099 | 0.120 | 2-3/4 | 20.31 | 21.42 | 25.86 |
| 1/4 | 0.168 | 0.177 | 0.214 | 2-7/8 | 22.20 | 23.41 | 28.27 |
| 5/16 | 0.262 | 0.277 | 0.334 | 3 | 24.17 | 25.50 | 30.78 |
| 3/8 | 0.378 | 0.398 | 0.481 | 3-1/8 | 26.23 | 27.66 | 33.40 |
| 7/16 | 0.514 | 0.542 | 0.655 | 3-1/4 | 28.37 | 29.92 | 36.12 |
| 1/2 | 0.671 | 0.708 | 0.855 | 3-3/8 | 30.59 | 32.27 | 38.95 |
| 9/16 | 0.850 | 0.896 | 1.082 | 3-1/2 | 32.90 | 34.70 | 41.89 |
| 5/8 | 1.049 | 1.107 | 1.336 | 3-5/8 | 35.29 | 37.23 | 44.94 |
| 11/16 | 1.270 | 1.339 | 1.616 | 3-3/4 | 37.77 | 39.84 | 48.09 |
| 3/4 | 1.511 | 1.594 | 1.924 | 3-7/8 | 40.33 | 42.54 | 51.35 |
| 13/16 | 1.773 | 1.870 | 2.258 | 4 | 42.97 | 45.34 | 54.72 |
| 7/8 | 2.056 | 2.169 | 2.618 | 4-1/4 | 48.51 | 51.17 | 61.77 |
| 15/16 | 2.361 | 2.490 | 3.006 | 4-1/2 | 54.39 | 57.37 | 69.25 |
| 1 | 2.686 | 2.833 | 3.420 | 4-3/4 | 60.60 | 63.92 | 77.16 |
| 1-1/8 | 3.399 | 3.585 | 4.328 | 5 | 67.15 | 70.83 | 85.50 |
| 1-1/4 | 4.197 | 4.427 | 5.344 | 5-1/4 | 74.03 | 78.08 | 94.26 |
| 1-3/8 | 5.078 | 5.356 | 6.646 | 5-1/2 | 81.25 | 85.70 | 103.45 |
| 1-1/2 | 6.044 | 6.374 | 7.695 | 5-3/4 | 88.80 | 93.67 | 113.07 |
| 1-5/8 | 7.093 | 7.481 | 9.031 | 6 | 96.69 | 101.99 | 123.12 |
| 1-3/4 | 8.226 | 8.674 | 10.474 | 7 | 131.61 | 138.82 | 167.58 |
| 1-7/8 | 9.443 | 9.960 | 12.023 | 8 | 171.90 | 181.32 | 218.88 |
| 2 | 10.744 | 11.332 | 13.680 | 9 | 217.57 | 229.48 | 277.02 |
| 2-1/8 | 12.129 | 12.793 | 15.443 | 10 | 268.60 | 283.31 | 342.00 |
| 2-1/4 | 13.598 | 14.343 | 17.314 | 11 | 325.01 | 342.80 | 413.82 |
| 2-3/8 | 15.151 | 15.981 | 19.291 | 12 | 386.79 | 407.97 | 492.48 |
High-speed steel, being more dense than carbon steel, weighs from 10 to 11 per cent more than carbon steel. This should be added to figures given in the table.
| Diameter, inches |
Weight per foot |
Diameter, inches |
Weight per foot |
Diameter, inches |
Weight per foot |
|---|---|---|---|---|---|
| 12 | 386.790 | 15-7/8 | 677.527 | 19-3/4 | 1,049.010 |
| 12-1/8 | 395.518 | 16 | 687.600 | 19-7/8 | 1,061.705 |
| 12-1/4 | 404.246 | 16-1/8 | 699.017 | 20 | 1,074.400 |
| 12-3/8 | 412.974 | 16-1/4 | 710.435 | 20-1/8 | 1,088.502 |
| 12-1/2 | 421.702 | 16-3/8 | 721.852 | 20-1/4 | 1,102.605 |
| 12-5/8 | 430.430 | 16-1/2 | 733.270 | 20-3/8 | 1,116.707 |
| 12-3/4 | 439.158 | 16-5/8 | 744.687 | 20-1/2 | 1,130.810 |
| 12-7/8 | 447.886 | 16-3/4 | 756.105 | 20-5/8 | 1,144.912 |
| 13 | 456.615 | 16-7/8 | 767.522 | 20-3/4 | 1,159.015 |
| 13-1/8 | 465.343 | 17 | 778.940 | 20-7/8 | 1,173.118 |
| 13-1/4 | 474.071 | 17-1/8 | 790.358 | 21 | 1,187.220 |
| 13-3/8 | 482.799 | 17-1/4 | 801.777 | 21-1/8 | 1,201.322 |
| 13-1/2 | 491.527 | 17-3/8 | 813.195 | 21-1/4 | 1,215.425 |
| 13-5/8 | 500.255 | 17-1/2 | 824.614 | 21-3/8 | 1,229.527 |
| 13-3/4 | 508.983 | 17-5/8 | 836.030 | 21-1/2 | 1,243.630 |
| 13-7/8 | 517.711 | 17-3/4 | 847.447 | 21-5/8 | 1,257.732 |
| 14 | 526.440 | 17-7/8 | 858.863 | 21-3/4 | 1,271.835 |
| 14-1/8 | 536.512 | 18 | 870.280 | 21-7/8 | 1,285.937 |
| 14-1/4 | 546.585 | 18-1/8 | 883.105 | 22 | 1,300.040 |
| 14-3/8 | 556.657 | 18-1/4 | 895.920 | 22-1/8 | 1,315.485 |
| 14-1/2 | 566.730 | 18-3/8 | 908.740 | 22-1/4 | 1,330.930 |
| 14-5/8 | 576.802 | 18-1/2 | 921.560 | 22-3/8 | 1,346.375 |
| 14-3/4 | 586.875 | 18-5/8 | 934.380 | 22-1/2 | 1,361.820 |
| 14-7/8 | 596.947 | 18-3/4 | 947.200 | 22-5/8 | 1,377.265 |
| 15 | 607.020 | 18-7/8 | 960.020 | 22-3/4 | 1,392.710 |
| 15-1/8 | 617.092 | 19 | 972.840 | 22-7/8 | 1,408.155 |
| 15-1/4 | 627.165 | 19-1/8 | 985.035 | 23 | 1,423.600 |
| 15-3/8 | 637.237 | 19-1/4 | 998.230 | 23-1/8 | 1,454.490 |
| 15-1/2 | 647.310 | 19-3/8 | 1,010.925 | 23-1/4 | 1,485.380 |
| 15-5/8 | 657.382 | 19-1/2 | 1,023.620 | 23-3/8 | 1,516.270 |
| 15-3/4 | 667.455 | 19-5/8 | 1,036.315 | 24 | 1,547.160 |
To find the weight of discs made of carbon steel, in diameters up to and including 12 in., without any allowance for finishing multiply the per foot weight of round bar steel, shown herewith by the decimal equivalent of a foot given in the following table:
| In. | 0 | 1/8 | 1/4 | 3/8 | 1/2 | 5/8 | 3/4 | 7/8 |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.000 | 0.010 | 0.021 | 0.031 | 0.042 | 0.052 | 0.063 | 0.073 |
| 1 | 0.083 | 0.094 | 0.104 | 0.115 | 0.125 | 0.135 | 0.146 | 0.156 |
| 2 | 0.167 | 0.177 | 0.188 | 0.198 | 0.208 | 0.219 | 0.229 | 0.240 |
| 3 | 0.250 | 0.260 | 0.270 | 0.281 | 0.292 | 0.302 | 0.313 | 0.323 |
| 4 | 0.333 | 0.344 | 0.354 | 0.364 | 0.375 | 0.385 | 0.396 | 0.406 |
| 5 | 0.416 | 0.427 | 0.437 | 0.448 | 0.458 | 0.469 | 0.479 | 0.480 |
| 6 | 0.500 | 0.510 | 0.520 | 0.531 | 0.542 | 0.552 | 0.563 | 0.573 |
| 7 | 0.583 | 0.594 | 0.604 | 0.615 | 0.625 | 0.635 | 0.646 | 0.656 |
| 8 | 0.666 | 0.677 | 0.687 | 0.698 | 0.708 | 0.719 | 0.729 | 0.740 |
| 9 | 0.750 | 0.760 | 0.770 | 0.781 | 0.792 | 0.802 | 0.813 | 0.823 |
| 10 | 0.833 | 0.844 | 0.854 | 0.865 | 0.875 | 0.885 | 0.896 | 0.906 |
| 11 | 0.916 | 0.927 | 0.937 | 0.948 | 0.953 | 0.969 | 0.979 | 0.990 |
EXAMPLE.—If the weight of a carbon steel disc 7 in. diameter, 1-5/8 in. thick is desired, turn to page 233, where the per foot weight of 7 in. round is given as 131.6 lb. Multiply this by the decimal equivalent of 1-5/8 in., or 0.135, as shown in the above table, and the product will be the net weight of the disc.
| 131.61 | lb. | = the weight of 1 ft. of 7 in. round. |
| 0.135 | = the per foot decimal equivalent of 1-5/8 in: | |
| 65805 | ||
| 39483 | ||
| 13161 | ||
| 17.76735 | lb. | = weight of disc 7 in. diam. 1-5/8 in. thick without any allowance for finishing. |
A
ADDIS, W H., 102
AMERICAN MACHINISTS' HANDBOOK, 69
AMERICAN STEEL TREARERS' SOCIETY,
119
AMERICAN GEAR MFRS. ASSO., 115
AUTOMATIC AND ELECTRIC FURNACES LTD.,
161
ARNOLD, PROF. J. O., 167
B
BURLEIGH, R. W.
BORDEN, B.
BOKER, HERMAN & Co.
BROWN INSTRUMENT Co., 224
BROWN-LIPE-CHAPLIN Co., 121
C
CAMPBELL, H. H.
CARHART, H. A., 42
CLAYTON, C. Y., 112
CURTIS AIRPLANE Co.
E
ENGLEHARD, CHARLES, 227
ENSAW, HOWARD, 79,
95
F
FIRTH-STERLING STEEL Co., 176
FIRTH, THOMAS & SONS, 137
FOWLER, HENRY, 151
G
H
HAYWAHD, C. R., 35
HOWE, DR. H. M., 8,
108
HOOVER STEEL BALL CO., 61
HEATHCOTE, H. L., 85
HARRIS, MATTHEW, 94
HUNTER, J. V., 192
J
JANITZKY, E. J., 119
JOHNSTON, A. B., 35
JUTHE, K. A., 1,
24, 65,
75, 79,
105, 145
L
LATROBE STEEL CO., 150,
178
LUDLUM STEEL CO., 175
LEEDS & NORTHRUP CO.,
211
LYMAN, W. H., 199
M
MANSFIELD, C. A.
MIDVALE STEEL Co.
McKENNA, ROY C., 164
MOULTON, SETH A., 199
N
NILES, BEMENT, POND, 67
P
PARKER, S. W.
POOLE, C. R.
R
S
S. A. E. (SOCIETY AUTOMOTIVE ENGINEERS),
39, 46,
49, 134
SAUVEUR, ALBERT, 105,
232
SPRINGFIELD ARMORY, 78
SELLACK, T. G.
SMITH, A. J., 101
SHIRLEY, ALFRED J.
T
TAYLOR INSTRUMENT Co., 215
THUM, E. E., 12-23;
105-121.
TIEMANN, H. P., 11
U
U. S. BALL BEARING Co.
UNITED STEEL Co.
UNDERWOOD, CHARLES N.
V
VAN DE VENTER, JOHN H., 86
W
WALP, H. O., 109
WOOD, HAROLD F., 46
WHEELOCK, LOVEJOY & Co., 69
A
ABC of iron and steel, ix
Absorption of carbon, rate of, 83
Air hardening steels, 183
Analysis of high speed steel, 165
Allotropic modifications, 107
Alloy steel, annealing, 76
properties of, 34
Alloys and their effect, 16,
24
in high speed steel, 166
in steel, value of, 16,
24
upon steel, 24
Alpha iron, 106
Annealing, 113,
115
care in, 154,
155
furnace, 190
high-chromium steel, 36
high speed tools, 174
in bone, 77
methods, 122
proper, 117
rifle components, 78
rust-proof steel, 36
steels, 75
temperature, 119
Arrests, 106
Austentite, 108, 111
Automotive industry, application of Liberty engine materials to,
46
temperature control, 225
Axles, heat treatment of, 61
B
Balls, making steel, 61
Barium chloride process, 178
Baths for tempering, 157
Bessemer converter, 2
Beta iron, 106
Blending compounds, 103
Blister steel, 81
Blue brittleness, 56
Bone, annealing in, 77
Boxes for case hardening or carburizing, 80
Breaking test gears, 126
Brinell hardness, 22
Broach hardening furnace, 188
Brown automatic pyrometer, 224
Burning, 65
C
Calorized tubes, 229
Carbon, 13
content at various temperatures, 84
content of case hardened work, 81
in cast iron, ix
in tool steel, 149-150
introduction of, 96
penetration of, 95
steel, 11
steel forgings, Liberty engine, 48
steel tools, 145
steels, S. A. E., 10, 39
steels, temper colors, 163
strengthens iron, 13
tool steel, forging, 65
Carbonizing, see Carburizing
Carborundum tubes, 229
Carburization, preventing, 93
Carburizing by gas, 88,
93
boxes, 80
compounds, 88,
102
gas consumption by, 101
local, 94
material, 85
nickel steel, 125
or case hardening, 79
pots for, 123
Page 240
Carburizing, process of, ix,
83, 116
short method, 124
sleeves, 132
with charcoal, 81,
88
See Case hardening
Car door type of furnace, 190
Case, depth of, 86
Case hardening boxes, 80
cast iron, 89
local, 94
or surface carburizing, 79
treatments for various steels, 92
see Carburizing
Cast iron, carbon in, ix
case hardening, 89
Cementite, ix, 14
Center column furnace, 186
Centigrade table, 232-234
Chamotte tubes, 228
Chart of carbon penetration, 97
heat treatment, 151
shape, 151
Chrome steel, 26-27
Chrome-nickel steel, 27-28
steel, forging, 66
Chrome-vanadium steel, 28
Chromium, 26-27
steels, S. A. E., 41
Chromium-cobalt steel, 178
Chromium-vanadium steel, S. A. E., 41
Classification of steel, 10
Clay tubes, 229
Cold end compensator, 213
junction errors, 222
shortness, 15,
166
worked steel, 65
Color in tempering, 157
Colors on carbon steels, 163
Combination tank, 90
Comparison of fuels, 191
Compensating leads, 222
Compensator for cold ends, 214
automatic, 214
Composition of steel, 13
Compound, blending, 103
separating from work, 102
Compounds for carburizing, 102
Connecting rods, Liberty motor, 42,
52
Continuous heating furnace, 71
Converter, Bessemer, 2
Cooling curves, 106,
107
Cooling quenching oil, roof system, 74
rate of, for gear-forgings, 51
Copper, effect of, in medium carbon steel, 35
Copper-plating to prevent carburizing, 93
Corrosion of high-chromium steel, 38
of rust-proof steel, 38
Corundite tubes, 229
Cost of operating furnaces, 200
Cracks in hardening, preventing, 106
Crankshaft, Liberty motor, 54
Critical point, 105
Crucible or tool steel, x,
4
Cutting off high speed steel, 172
Cyanide bath for tool steel, 133
D
Decarbonizing of outer surface, 153
preventing, 154
Depth of case, 86
Detrimental elements in steel, 166
Dies, drop forging, 133
quenching, 147
soft spots in, 147
tempering round, 161
Drawing, 114
ends of gear teeth, 127
Drop forging dies, 133
Ductility, 13, 18
E
Effect of alloys, 24
of different carburizing material,
87
of size of piece, 89,
119
of copper in medium carbon steel,
35
Elastic limit, 18
Electric process of steel making, 5
Electrode, 5
Elements, chemical, 12
Elongation, 18
Page 241
Endurance limit, 20
Energizer, 81, 88
Enlarging steel, 161
Equipment for heat treating, 121
Eutectoid, 109
F
Fahrenheit temperature table, 232
Fatigue test, 19
Ferrite, 106
File test, 16, 17
Flame shields, 193
Flange shields for furnaces, 197
Forging furnace, 189
high speed tools, 174
improper, 66
of steel, 64
practice, heavy, 195
rifle barrels, 69
Forgings, carbon steel Liberty engine,
48
Formed tools, high speed, 174
Fractures, examining by, 16,
159
Furnace, continuous heating, 71
crucible, 4
data, 199
electric, 5
Heroult, 6
open hearth, 3
records, 129
Furnaces, 185
annealing, 190
broach hardening, 188
car door type, 190
center column, 186
cost of operating, 200
data on, 199
forging, heavy, 195
fuels for, 199
gas fired, 190
high speed steel, 187
lead pot, 185
manganese steel, 198
muffle, 189
oil fired, 186
operating costs, 200
screens for, 192
tool, 187
Furnaces, water cooled fronts, 197
Fuels, comparison of, 191
for furnaces, 199
G
Gages, changes due to quenching, 162
tempering, 161
Gamma iron, 106
Gas, carburizing by, 93
consumption for carburizing,
101
fired furnace, 190
illuminating, for carburizing,
97
Gear blanks, heat treatment of, 115
forgings, rate of cooling for Liberty engine,
51
hardening machine, 130
steel, transmission, 59
teeth, drawing ends of, 127
Gears, Liberty engine, 50
Gleason tempering machine, 129
Grade of steel, 10
Grain, refining, 91,
110
size, 16
Graphitic carbon, ix
Grinding high speed steel, 176
H
Hair lines in forgings, 56
Hardening, 111
carbon steel for tools, 145
cracks, preventing, 160
dies, 146
gears, 130
high speed steel, 171
high speed tools, 177
of high-chromium steel, 37
of rust-proof steel, 37
room, modern, 146
Hardness tests, 20
Heating, effect of size, 119
for forging, 64
Heat, judging by color, 114
treating departments, 122
equipment, 121
forgings, 44
inspection of,
125
Liberty motor,
44
Page 242
Heat treating, of axles, 61
of chisels, 151
of gears, 131
of high speed steel, 170
of steel, 105
S. A. E., 134-137
Heat treatment, 105
Heroult furnace, 6
High-chromium steel, 36
annealing of, 36
corrosion of, 38
hardening of, 37
Highly stressed parts of Liberty engine,
49
High speed steel, analysis of, 166
annealing, 75
cutting off, 172
forging, 65
furnace, 187
hardening, 171
heat treatment of, 170
instructions for, 175,
180
manufacture, 166,
169
pack hardening, 172
structure of, 168
Hints for steel users, 159
I
Illuminating gas for carburizing, 97
Impact test, 19
Improper forging, 66
Influence of size on heating, 119
Inspection of heat treatment, 125
Internal stresses, relieving, 154
Introduction of carbon, 96
J
Jewelers' tools, 146
Judging heat of steel by color, 114
L
Latent heat, 105
Lathe and planer tools, 176
tools, high speed, 173
Latrobe temper list, 150
Lead bath, 154
pot furnace, 185
Leeds & Northrup potentiometer 211
optical pyrometer, 220
Liberty engine, highly stressed parts of, 49
Liberty engine materials, application to automotive industry,
46
motor connecting rods, 42,
52
motor, crankshaft, 54
motor piston pin, 57
Local case hardening, 94
Luting mixture, 100
M
Machineability of steel, 72
Machinery steel, annealing, 77
Magnet test, 114
Making steel in electric furnace, 6
Manganese, 16, 33,
107
steel, 29-30
furnace,
198
Manufacture of high speed steel, 169
Marquardt mass tubes, 228
Martensite, 111
Medium carbon steel, effect of copper on,
35
Metallography, 105
Microphotographs, 109 e. s.
Microscopic examination, 158
Milling cutters, high speed, 174
Mixture for luting, 100
Modern hardening room, 146
Molten metal pyrometers, 226
Molybdenum, 32
Muffle furnace, 189
N
Nickel, 24
Nickel-chromium steel, 27-28
steels, S. A. E., 40
Nickel, influence of, on steel, 25
steel, 24-26
affinity for carbon,
125
steels, S. A. E., 39
Non-homogeneous melting, 24
Non-shrinking steels, 35
Normalizing, 114
Page 243 O
Oil bath for tempering, 157
cooling on roof, 74
fired furnace, 186
hardening steel, forging,
66
steels, 35
temperature of quenching,
124
Open hearth furnace, 3
Operating costs of furnaces, 200
Outer surface decarbonizer, 153
Over-heated steel, restoring, 137
Overheating, 65
dies, 148
P
Pack-hardening, 87
high speed steel, 173
Packing work for carburizing, 123
Paste for hardening dies, 146
Pearlite, 109,
112
Penetration of carbon, 95
carbon, chart of, 97
in case hardening, 83
Phosphorus, 15, 33
Pickling Liberty motor forgings, 44
Pig iron, ix
Piston pin, Liberty motor, 57
Placing pyrometers, 210
Planer tools, high speed, 173
"Points" of carbon in steel, 9
Potentiometer, Leeds & Northrup,
211
Pots for carburizing, 123
Press for testing gears, 126
Preventing carburization, 93
cracks in hardening, 160
Properties of alloy steels, 34
of alloy steels, table, 34
of steel, 12
Protective screens for furnaces, 192
Puddled iron, ix
Punches and chisels, steels for, 151
Pyrometers, 202
calibration, 208
copper ball, 202
indicating, 219
inspection, 208
iron ball, 202
molten metal, 226
optical, 206,
220
placing, 210
recording, 216
Siemens, 202
testing, 209
water, 203
Q
Quality and structure of high speed steel,
168
of steel, 149
Quenching,
after carburizing, 86-88
dies in tank, 147
obsolete method, 148
oil, temperature of, 124
tank, 89
tool steel, 156
R
Rate of absorption of carbon, 83
Recording temperatures, 127
Red shortness, 14,
166
Refining the grain, 91,
111
Regenerative open hearth furnace, 3
Restoring overheated steel, 137
Rifle barrels, forging, 69
components, annealing, 78
Roof system of cooling oil, 74
Rust-proof steel, 36
annealing of, 36
corrosion of, 38
hardening of, 37
S
S. A. E. carbon steels, 10,
39
chromium steels, 41
chromium-vanadium, 41
heat treatments, 134-137
nickel-chromium steels, 40
nickel steels, 39
screw stock, 39
silico-manganese steel, 41
standard steels, 39
Page 244
Salt bath for tempering, 157
Scleroscope test, 21
Scratch hardness, 20
Screens for furnaces, 192
Screw stock, S. A. E., 14,
39
Sensible heat, 105
Sentinels, melting of, 207
Separating work from compound, 102
Shields for furnace doors, 193
Shore Scleroscope, 21
Short method of carburizing, 124
Shrinking steel, 161
Silica tubes, 228
Silico-manganese steels, S. A. E., 41
Silicon, 15, 33,
107
Silversmiths' tools, 146
Size of piece, effect of, 89,
119
Slags, 7
Sleeves, carburizing, 132
hardening and shrinking,
132
shrinking, 132
Solid solution, 106
Sorbite, 112
Specimens, test, 17
Standard S. A. E. steels, 39
Steel,
balls, stock for, 62
bolts, making, 61
composition of, 12
deoxidation, 15
for chisels and punches,
151
forging of, 64
give it a chance, 148
heat treatment of, 105
high speed, 165
making, 1,
6, 15
Bessemer process, 1
crucible process, 4
electric furnace process,
5
open hearth, 1
tools, carbon, in, 149
users' hints, 159
Structure of high speed steel, 168
Sulphur, 14, 33
T
Tables, air, oil and water hardened steel,
38
alloy steels, properties of,
34
carbon content, 84
carbon steels, 39
case hardening, 97
changes due to quenching,
162
chromium steels, 41
chromium-vanadium steels,
41
colors and temperature,
163
composition of steels,
51, 52
cost of furnaces, 200
effect of size, 119
fuels, comparison of,
191
high-chromium steel,
37
nickel-chromium steels,
40
nickel steels, 39
operating cost of furnaces,
200
production cost of furnaces,
201
S. A. E. steels, 49
screw stock, 39
silico-manganese steels,
41
stock for balls,
62
temperature conversion,
232-234
tempering temperatures,
158
weight of steel,
235-237
Tank for quenching, 89
dies, 147
Taylor instruments, 215
Temper, colors of, 157
list, Latrobe, 150
of steel, 10,
149
Temperature recorders, 127
tables, 232-234
Temperatures for tempering, 158
Tempering colors on carbon steels,
163
gages, 161
high speed tools, 177
machine, Gleason, 129
round dies, 161
temperatures, 158
theory of, 114,
156
Tempers of carbon steel, 10,
150
Tensile test, 17
Testing heat treatment, 125
Tests of steel, 16
Test specimens, 17
Theory of tempering, 114,
150
Thermocouple, 204
base metal, 205
Page 245
cold end, 206
placing, 218
protectors, 227
rare metal, 205
Time for hardening, 148
Tool furnace, small, 187
Tool or crucible steel, annealing, 76
Tool steel, cyanide bath for, 133
quenching, 150
Tools, carbon in different, 149
carbon steel, 145
of high speed steel, 173
sulphur in, 14
tempers of various, 150
transformation points,
106
Transmission gear steel, 59
Treatments for various steels, 92
Troosite, 112
Tubes, calorized, 229
carborundum, 229
Chamotte, 228
clay, 229
Marquardt mass, 228
silica, 228
Tungsten steel, 30
U
Ultimate strength, 17
Users of steel, hints for, 159
V
Vanadium steel, 28
W
Water annealing, 155
cooled furnace fronts,
197
Weight of steel bars, 235-237
Working instructions for high speed steel,
175
Wrought iron, ix
Y
Yield Point, 19
End of the Project Gutenberg EBook of The Working of Steel, by
Fred H. Colvin and A. Juthe
*** END OF THIS PROJECT GUTENBERG EBOOK THE WORKING OF STEEL ***
***** This file should be named 20282-h.htm or 20282-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/2/0/2/8/20282/
Produced by Robert J. Hall
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License (available with this file or online at
http://gutenberg.org/license).
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH F3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS' WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTIBILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need, is critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation web page at http://www.pglaf.org.
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Its 501(c)(3) letter is posted at
http://pglaf.org/fundraising. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at
809 North 1500 West, Salt Lake City, UT 84116, (801) 596-1887, email
business@pglaf.org. Email contact links and up to date contact
information can be found at the Foundation's web site and official
page at http://pglaf.org
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit http://pglaf.org
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: http://pglaf.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart is the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For thirty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
http://www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.