
VIEW OF THE COMMERCIAL SALT COMPANY’S BRINE RESERVOIRS AT RODE HEATH, CHESHIRE,
Showing the Brine being pumped up from a depth of 250 feet
Frontispiece
The Project Gutenberg eBook of Salt and the salt industry, by Albert F. Calvert
Title: Salt and the salt industry
Author: Albert F. Calvert
Release Date: December 20, 2022 [eBook #69590]
Language: English
Produced by: deaurider, Charlie Howard, and the Online Distributed Proofreading Team at https://www.pgdp.net (This book was produced from images made available by the HathiTrust Digital Library.)
Transcriber’s Note
Larger versions of most illustrations may be seen by right-clicking them and selecting an option to view them separately, or by double-tapping and/or stretching them.
i

VIEW OF THE COMMERCIAL SALT COMPANY’S BRINE RESERVOIRS AT RODE HEATH, CHESHIRE,
Showing the Brine being pumped up from a depth of 250 feet
Frontispiece
PITMAN’S COMMON COMMODITIES
AND INDUSTRIES
BY
ALBERT F. CALVERT, F.C.S.
AUTHOR OF “SALT IN CHESHIRE”;
“THE SALT DEPOSITS OF THE WORLD”; ETC.
London
Sir Isaac Pitman & Sons, Ltd., 1 Amen Corner, E.C.4
Bath, Melbourne and New York
Printed by Sir Isaac Pitman
& Sons, Ltd., London, Bath,
Melbourne and New York
iii
The fact that salt is almost universally distributed over the surface of the globe, and has been worked in a number of countries from time immemorial, will explain the impossibility, in the limited space at my disposal, to consider the mineral and its manufacture comprehensively as the staple of a world-industry. The salt deposits of China, India, Russia, Japan, and Austria would each require a volume of the size of this if the subject was to be even adequately represented. I have, therefore, dared to assume that the public will accept a book practically restricted to one phase of the matter, and allow me to concentrate upon our Cheshire salt district and its industry.
Caesar’s salinators, who found the natives of Cheshire procuring brine from little natural springs in the neighbourhoods of Northwich and Nantwich, taught them to boil the brine and precipitate the salt crystals in open pans set over open fires, and in the following 1,700 years all the salt of Cheshire was manufactured by that process. With the discovery of rock salt in 1670, mining was introduced, and for another 200 years both rock salt and brine salt were produced. But from causes which I have described, the mines collapsed in rapid succession from about the middle of the nineteenth century, and fresh water breaking into the abandoned workings converted them into the brine reservoirs from which the salt-men have since obtained their inexhaustible supplies of brine.
But, although the salt industry is one of the oldest in the country, it has received scant treatment at the hands of authors, and this is accounted for by the factiv that the trade has been conducted by a comparatively small group of men who have resisted all attempts of outsiders to participate in either their secrets or their profits. The desire for information has been consistently rebuked, and practical details relating to borings, working expenses, levels of brine, and quantities raised have been jealously concealed. It was my good fortune to be able to prosecute most of my researches on the spot, and to supplement the knowledge gained from books, pamphlets, scientific papers and periodicals, with material contained in private records and documents placed at my disposal, and information obtained by word of mouth.
There is romance in every industry, and a modicum of it enters into the development of the Cheshire salt trade; but for the most part the story is a chronicle of bitter struggles to maintain a monopoly, of money thrown away, of produce sold at ruinous loss, of obsolete methods stubbornly persisted with, and of hardship and injustice callously inflicted—in a word, of the sordid determination of the salt magnates to crush competition and control prices. The methods of the Dark Ages survived both in the manufacture and the marketing of the produce, and the industry has more than once been reduced almost to ruin through the war of extermination in which for so many years the salt-men were engaged. It is not a pretty story, but it is one of unusual interest; and I have endeavoured in the telling of it to retain the interest and preserve the essential facts.
ALBERT F. CALVERT.
Royston,
Eton Avenue, N. W.
vi
| CHAP. | PAGE | |
| PREFACE | iii | |
| I. | THE CHEMISTRY AND PROPERTIES OF SALT | 1 |
| II. | THE BEGINNINGS OF THE SALT INDUSTRY | 8 |
| III. | THE CHESHIRE WICHES | 32 |
| IV. | DEVELOPMENT OF BRINE PROCESSES | 56 |
| V. | FORMATION AND EXTENT OF THE CHESHIRE DEPOSITS | 83 |
| VI. | THE CHESHIRE SUBSIDENCES | 97 |
| VII. | LATEST METHODS OF SALT-MAKING | 125 |
| VIII. | THE SALT MARKET | 142 |
vii
viii
| PAGE | |
| VIEW OF THE COMMERCIAL SALT COMPANY’S BRINE RESERVOIRS AT RODE HEATH, CHESHIRE Frontispiece | |
| ANCIENT SALT WORKS | 13 |
| ANCIENT SALT WORKS | 19 |
| WIELIEZKA SALT MINES | 21 |
| SLANICU, RUMANIA, INTERIOR OF SALT MINE | 25 |
| WIELIEZKA SALT MINES | 29 |
| SUBSIDENCE OF LAND, NORTHWICH | 41 |
| DUNKIRK SUBSIDENCE, NORTHWICH | 49 |
| THE CANAL-BURST AND LANDSLIP NEAR NORTHWICH IN 1907 | 59 |
| A SALT STORE-SHED | 67 |
| WITTON BROOK, SUBMERGENCE OF AGRICULTURAL LAND | 75 |
| WORKING IN DANGEROUS GROUND AFTER SUBSIDENCE, DUNKIRK LAKE, NORTHWICH | 81 |
| STREET-RAISING IN PROGRESS—HIGH STREET, NORTHWICH | 89 |
| THIS ROAD WAS RAISED TWENTY FEET IN TWENTY YEARS. NONE OF THESE BUILDINGS IS NOW STANDING—NORTHWICH | 93 |
| INTERIOR PENNY’S LANE MINE, NORTHWICH | 99 |
| REMARKABLE SUBSIDENCE IN NORTHWICH | 111 |
| A ROW OF OPEN PANS | 119 |
| ILLUSTRATION OF FOUR SCOTT PATENT DOUBLE EFFECT SALT EVAPORATORS, WITH AUTOMATIC SALT DISCHARGERS, SALT CONVEYORS, AND HYDRO-EXTRACTORS | 131 |
| THE HODGKINSON PATENT SALT-MAKING PLANT | 137 |
1
ix
“Salt” was the name which was given in the first place to the residue left by the evaporation of sea-water, but the designation was subsequently employed to include the other substances held in solution in the sea, and, at a still later period, the name was still further extended by chemists to cover all the combinations of a base and an acid which are now classed as “salts.” Sodium, or sodic chloride Na Cl, which is now distinguished as “common salt,” is an example of the simplest type of chemical salt, its molecule consisting of one atom of the metal sodium combined with one atom of the gas chlorine, both sodium and chlorine being mono-valent elements, i.e., one atom of each being able to unite with, or displace from a compound, one atom of hydrogen.
Rock-salt is rarely found in an absolutely pure anhydrous state, in which it is colourless and perfectly transparent. In most rock-salt mines such specimens are regarded as curiosities, but in the deposits of Nevada and of Wieliezka, in Hungary (where the salt, containing 100 per cent. NaCl, is the purest in the world), large masses of quite transparent salt are encountered. The white opaque mass which the ordinary person is accustomed to think of as rock-salt, is the purified product2 of commerce. The colour of sea-water is affected by its percentage of salt, the colour changing from blue to green as the quantity of salt decreases; but sea-salt is generally white, although not transparent owing to the presence of minute particles of water, air, etc., in its intercrystalline spaces. But rock-salt is never more than whitish inclining to grey, and, as a general rule, it is coloured by earth or mineral impurities. The Salt Range in the Panjab yields a substance that varies from pink to red, according to the different quantities of iron present as impurities. That found at Marston, in Cheshire, varies from yellow to red and brownish-red in colour. Small blocks of transparent salt of a deep sapphire blue are occasionally found in the Wieliezka mines. The colour disappears on heating, and when the salt is ground to powder. It is attributed by some chemists to the presence of subchloride of sodium, by others to the presence of thin cavities having parallel surfaces with gas inclusions.
Common salt, which is classed as “sweet” to distinguish it from the bitter-tasting salts of magnesium, has a peculiar saline taste which gains in pungency with refinement, and in its pure state is odourless. In solution, the smallest quantity perceptible to the taste is about 15 grains to the litre, roughly, 68 grains to the gallon.
Common salt is highly soluble in cold water, and rather more so in hot water, but while it dissolves slightly in alcohol, neither ether nor oil has any effect upon it. One hundred parts of distilled water at 60° F. (15·5° C.) will dissolve 35·9 parts of chemically pure NaCl. A saturated solution of common salt, therefore, contains 26·42 per cent. NaCl. The increase of solubility of NaCl in proportion to the rise in temperature, calculated by Gay Lussac and Poggiale, is3 particularly marked between 100 deg. and 110 deg., when boiling point is passed, the increase amounting to ·74 parts of 10 deg., as compared with an increase of one 1·09 parts between freezing and boiling points. In a double solution of NaCl and some other more soluble salt, as sodium or magnesium sulphate or magnesium chloride, the solubility of sodium chloride is very greatly reduced.
The evaporation of brine is slightly less rapid than that of ordinary pure water, and the boiling point of brine varies with the amount of NaCl present in solution, from 100·21 deg. when only 1 per cent. NaCl is present, to 108·99 deg. when the solution contains 29·4 per cent. of NaCl. A saturated solution of refined table-salt (i.e., a solution containing 26·4 per cent. NaCl) has, at normal temperatures, specific gravity 1·2. Salt crystals have specific gravity 2·167 at a temperature of 17°. The salt which separates at high temperature contains no water of crystallization. But when the thermometer falls much below -15° C. the crystals have the composition NaCl.2H₂O and are prismatic in shape. When heated, these give up their water of crystallization and take the simple composition NaCl.
Pure sodium chloride is not deliquescent (i.e., it does not dissolve and become liquid by absorbing moisture from the air), but, owing to the presence of minute quantities of magnesium chloride (one of the most deliquescent substances known), all except the most refined table-salt appears to be so to a slight extent. Even the finest table-salt is slightly hygroscopic, its crystals absorbing as much as ·6 per cent. moisture from a damp atmosphere. In some of the mines of Cheshire and Austria the very fine saline dust that is diffused through the atmosphere is found by the miners to be extremely irritating to the eyes and lungs, but4 all the more usual kinds of salt are sufficiently hygroscopic to indicate plainly the condition of the atmosphere.
Sodic chloride melts at a very high temperature, and at a still higher temperature it evaporates, while at white heat it forms thick clouds.
It would be supposed that in the same ocean areas, the proportion of the salt contents, except where marked differences in temperature occur, would be fairly constant, but it has been demonstrated that, even where masses of water of varying densities are superimposed upon each other, no very complete process of diffusion takes place between them, and practical salt-makers are familiar with differences in density which occur in different parts of the same salt pan.
The hardness of a mineral depends upon the degree of cohesion of its particles; but although no unit of hardness has been determined upon, and therefore no accurate method of measuring hardness has been arrived at, minerals have been approximately classed in a comparative table of ten substances, of which talc is placed at one end and diamond at the other. In this table, rock salt appears in the second place, and its hardness is estimated at 2·5. Its cohesion or power of supporting pressure is, therefore, about twice as great as that of bricks, and the practical advantage of this property is fully employed in rock-salt mines, where galleries and roofs are supported upon pillars of salt.
Common salt is a crystalline substance which crystallizes in the Isometric, Monometric, or Tesseral system. That is to say, each crystal has three equal perpendicular planes of symmetry and six equal diagonal planes of symmetry. The crystals generally form cubes having5 six rectangular and equilateral faces. When these form on the surface of brine the sides often collapse, giving the distinctive “hopper-shaped” forms. More rarely the crystals form in octahedra, having eight equal, equilateral triangular faces, or in long needles under certain modifying conditions.
The hollow quadrangular pyramidal form with an irregular inner surface arranged in steps, which manufactured salt generally takes, is the result of continuous depositions of crystals from a constantly saturated solution of brine during a considerable period, being superimposed layer after layer upon each other.
In his exhaustive explanation of these phenomena, given in his Principles of Chemistry, Mendeléeff says: “If a solution of sodium chloride be slowly heated from above, where the evaporation takes place, the upper layer will become saturated before the lower and cooler layers, and therefore crystallization will begin on the surface, and the crystals first formed will float—having also dried from above—on the surface until they become quite soaked. Being heavier than the solution the crystals are partially immersed in it, and the following crystallization, also proceeding on the surface, will only form crystals by the side of the original crystals. A funnel is formed in this manner. It will be borne on the surface like a boat (if the liquid be quiescent) because it will grow more from the upper edges. We can thus understand this, at first sight, strange funnel-form of crystallized salt. To explain why the crystallization under the above conditions begins at the surface and not at the lower edges, it must be mentioned that the specific gravity of a crystal of sodium chloride is 2·16, and that a solution saturated at 25° contains 26·7 per cent. of salt and has a specific gravity 1·2004 at 25°; at 15° a saturated solution6 contains 26·5 per cent. of salt and has a specific gravity 1·203 at 15°. Hence, a solution saturated at a higher temperature is specifically lighter, notwithstanding the greater amount of salt it contains. With many substances, surface crystallization cannot take place, because their solubility increases more rapidly with the temperature than their specific gravity decreases. In this case the saturated solution will always be in the lower layers, where also the crystallization will take place.”
The acoustic properties of common salt render it an excellent medium for the transmission of sound, and as it possesses in a high degree the power of staying decomposition in dead organisms, it is, perhaps, the commonest of all preservatives. It is largely owing to its preservative property that common salt is an absolute necessity to the life of man and the higher animals, from a quarter to half an ounce a day being sufficient to prevent the putrefaction of food in the digestive tract in the case of an adult. In agriculture, salt is not only valuable as a destroyer of weeds and insect life, but used sparingly and with knowledge, it forms an excellent manure; while its more strictly chemical value in the manufacture of soda, chlorine, etc., causes it to play an important part in many branches of industry.
Even at the highest temperatures, heat cannot effect the decomposition of common salt. At a red heat, pure sodic chloride melts and becomes liquid, and if cooled again, a solid crystalline mass is formed. Ordinary salt fuses at a lower temperature and volatilizes when heated in an open vessel. But even in a closed vessel the purest salt will volatilize at a white heat. When gases or fluids are present in the crystalline cavities, heat causes decrepitation.
7
On the subject of the composition of brine, it is only necessary to add that it is so extremely variable that no two districts produce brine springs of the same strength and density, while the composition of ocean brine varies not only from ocean to ocean, but also for different parts and different depths in the same plane of water, and with the different distances from the mouths of large rivers. In the Cheshire district, the Brine test or Salinometer is graduated to show ounces in the gallon; but the gallon is the old Winchester Gallon of 231 cub. in. and not the Imperial Gallon of 277·274 cub. in. These are related to each other in the proportion of 10 to 12, therefore the Imperial Gallon will contain ⅕ more than the old gallon. Fully saturated brine by the Salinometer contains 42 oz. (2 lb. 10 oz.), therefore, in the Imperial Gallon 50·4 oz. As brines vary from 2 lb. 8 oz., or 40 oz. old measure, or 3 lb. or 48 oz. Imperial to 2 lb. 10 oz., or 3 lb. 2 oz. Imperial, so 1,000 gallons, which has been chosen as the measure for assessing brine-pumpers—under the Brine Pumping Compensation for Subsidence Act of 1891—will contain under the old measurement 2,625 lb. and under the Imperial 3,125 lb. of salt.
8
Salt, being existent in all animal and vegetable life, is coeval with life itself; it was present in the first herbage which gave nourishment to the first beast that, in its turn, became food for the first omnivorous man. In the beginning, man consumed the saline essences that were required to preserve his body in health, in the form of sodium chloride, which he absorbed in the uncooked flesh of animals, birds, and fishes, and in raw green-foods. The herbivorous animals were equally dependent upon salt, and, finding it in only infinitesimal quantities in the grasses upon which they fed, instinct directed them to the sea swamp pasturage and to the outcropping salt deposits. So long as man’s diet consisted of uncooked foods, his fresh meat provided him with a sufficiency of salt, but directly he employed a cook-pot in the preparation of his food, the boiling processes denuded it of 70 per cent. of its natural salt, and it became necessary for him to make up the deficiency. It must have been at this period that his herds directed his attention to the “salt licks” from which they satisfied their own saline wants, and enabled him to secure salt as a distinct and separate condiment.
It is probable that, from the Palaeolithic Age down to the time of the early Roman writers, man was content to season his victuals by the simple process of licking a piece of rock-salt, and we have no record to indicate the period when salt was first employed in the cooking of food. From varieties of grain and9 fragments of pottery that have been discovered in the dwellings of the cave-men of Belgium, it is supposed that salt was employed in the cooking of wheat and barley some five thousand years ago. Thirteen centuries before Christ, fish preserved in salt was eaten in Ancient Troy, and, according to Herodotus, the Egyptians not only salted ducks, quails, and a species of sardine which inhabited the Nile, but also employed salt or brine as an antiseptic in preparing the bodies of the illustrious dead for the process of embalming.
We cannot determine the period in which salt came to be regarded as a symbol of sanctity or entered into the religious ceremonials of the ancients. We know that in the Levitical Law, promulgated in 1500 B.C., every meat-offering was seasoned with salt, and salt is referred to in the “Verbal Instructions” which were enunciated by the founder of Buddhism, five centuries later. By the time of Pythagoras, about 600 B.C., salt was regarded as the emblem of justice, but who shall say when the Arabs first employed it as a token of friendship, or the Chinese offered their first oblation to Phelo, the salt deity of Celestial worship? We read in Herodotus that caravans brought salt from North Africa, and Schleiden tells us that the priests of Egypt preferred the salt of Hammomen to that evaporated from sea-water; but these references do not help us to fix the date when salt became an article of commerce, or tell us when or where or by whom it was first produced in a manufactured form. It was rock-salt which the Egyptians procured from the salt basin of the Sahara, and rock-salt from the margin of the Red Sea was the variety that is referred to by the compilers of Biblical history. But, although the natural crude product was probably the sole form in which it was known in the Western world by the Ancients, and through the10 vaunted golden epochs of Babylon, Byzantium, and Greece, the Chinese—who had invented explosives before the Romans had perfected the catapult, and had learnt to navigate by the compass while yet the mariners of the Mediterranean were dependent upon the stars and their wits—had probably been familiar for ages with a salt manufactured by a process, the origin of which they had forgotten, but the practice of which was to remain in operation, almost without revision, for further thousands of years.
The first mention of salt in the Chinese language is found in the annals of the Emperor Yu (2205–2197 B.C.), who ordered the province of Shantung to supply the Court with that commodity. During the Chow dynasty (1122–249 B.C.) the administration of the salt industry was conducted by Court officials, but the Crown monopoly of salt was not instituted until the days of Kuan Chung, who died 645 B.C. Between A.D. 561 and A.D. 583, references to various taxes on salt lead us to the conclusion that salt was produced at that period from sea-water, salt marshes, and salt springs, and at the present day salt is produced in China in three varieties—sea-salt, lake-salt, and well-salt. As the success of the boiling operation (which antedated by unnumbered centuries the comparatively modern industry of extracting salt from sea-water by evaporation in the sun) depends mainly on the condition of the brine and the time allowed in each stage of the process, the details were the subject of many series of experiments in the pursuit of the perfect system, but since about the twelfth century the following method has been consistently followed by the Chinese salt-makers. The whole of the sea-shore in the neighbourhood of the salt works is measured out and divided into a number of small, regular squares; the surface layer in each is11 dug out; the bottom of each pit thus formed is then strewn with straw, and the earth that has been removed is thrown back upon it. When these brine ovens, as they were called—which are shaped like chests, 9 ft. long, 2 ft. broad, and 3 ft. deep—are prepared, they are soaked with sea-water. The sea-water in the interior of the ovens forms brine, and flows through little ditches into wells which have been dug for its reception. From these wells, which are about 8 ft. deep, the brine is drawn out and carried to the boiling ovens. These brine ovens are furnished with large evaporating pans, three to five of which are attached to each oven. The boiling takes place at once and is continued without interruption, from 11 p.m. until 10.30 on the following morning, and during this period the salt is taken out six times. As soon as the salt begins to harden, pods of the tsao-chio tree are thrown into the pans, in order that the particles of salt may combine more quickly, and as soon as it is precipitated, it is removed and the pans are refilled with fresh brine. On an average, 600 cathés of the best brine yield 140 cathés of pure salt, which is produced in three qualities and colours—white, dark, and yellow. The white is the best, the dark is less esteemed, and the yellow, which is much inferior, has a bitter taste.
Since the fifteenth century, the Chinese have produced salt by solar evaporation of salt water, according to a simple but satisfactory process. Pits are dug on the sea-shore and bamboos are laid crosswise over them. The whole is covered with double mats, and sand is strewn over the top. Every morning and evening the covering of sand is soaked with sea-water by the tide, and the salt liquor finds its way into the pits. As soon as the water has receded, the salt workers appear with flaming bundles of straw, to test the saline12 character of the moisture, which is not regarded as fully impregnated unless the salt vapour arising from the pits extinguishes the fire. The brine thus produced is drawn off and run into secondary or crystallizing ponds, the level of which is set a foot or so below the first series of pits. The secondary ponds, which are smaller and of less depth, are provided with carefully-rolled, hard clay bottoms. When a sufficiently thick crystalline deposit has been formed at the bottom of the secondary ponds, workmen, starting at the centre, scrape the bottoms, working outward spirally and finishing at the corner of the pond, where the coarse crystalline product is collected and allowed to drain. When drained and dried, the salt is ready for transfer to the market.
In Japan, where the manufacture of sea-salt by boiling or by spontaneous evaporation was introduced more than two thousand years ago, the process is similar to that employed in China, but in some parts of the kingdom the evaporation basin generally employed in solar evaporation is dispensed with. In the latter method, a level field is formed close to the sea and sprinkled over with fine sand. Sea-water is then poured into the field, and, after evaporation of the water, the salt crystallizes and adheres to the sand. The mixture of salt and sand is next thrown into a kind of extracting apparatus and sea-water is poured upon it, whereupon the salt is dissolved and filtered in the form of a thick salt liquid. In other Japanese salt fields the concentrated liquor is poured into a crystallization basin prepared for the purpose, and, upon evaporation of the water by the sun’s heat, the salt crystallizes.

ANCIENT SALT WORKS
A. Wooden Ladle. B. Cask. C. Tub. D. The Master. E. Assistant. F. The Master’s Wife. G. Wooden Spade. H. Boards. I. Salt-baskets. K. Hoe. L. Rake. M. Straw. N. Bowls. O. Bucket for Blood. P. Beer Tankard.
From an Old Print
Published in 1556·
In Italy most of the salt is made by solar evaporation. The salt grounds, which occupy extensive areas, are14 furnished with reservoirs for the preparation of the sea-water by saturation and for the deposit of salt. The former are known as condensers and the latter as crystallizing beds, and in both the work is carried on by solar evaporation only. Every salt-ground, or salt-garden, as it is called, has a feeding channel for the inflow of sea-water, a drainage channel, and a network of internal channels at low and high levels, as are required for immission or drainage purposes. In Portugal and Spain, salt is made by solar evaporation from sea-water, and although there are differences between the several methods, they apply only to details regarding the areas of the salt-grounds or the sizes of the reservoirs.
Let it be clearly understood that all commercial salt is produced either from the sea or from rock-salt. Sea-water is evaporated to precipitate its salt either by the heat of the sun or by artificial heat. Rock-salt is mined and refined for market purposes, and it is resolved into brine from which the salt is extracted by solar heat or the process of boiling, but whether the salines are obtained from salt lakes or from natural brine springs, or are prepared by flooding salt deposits with water and pumping it out in the form of fully saturated brine, rock-salt is the foundation for them all. And in all the processes of manufacture the basic principle is the same, and consists of applying heat to drive off the liquid which contains the salt and collecting the crystalline deposit which remains.
The principle of what is described as the boiling process is fundamental and unalterable, and for thousands of years the plant and utensils employed in the process underwent no material change. Since the sixteenth century in England, variations in the shape, size, and capacity of the pans have been introduced, and experiments have been made in the re-arrangement15 of the receptacles and redistribution of the furnaces, while coal fuel has been substituted for straw and wood, but it is only in the past twenty-five years that any material success has been achieved in the matter of economizing and accelerating the process of production, controlling the heat in order to regulate the grain of the salt, producing more than one grade of salt in one operation, or of automatically and continuously collecting the salt as it is precipitated from the brine.
The earliest exact and detailed description that we have of salt-making appears in De Re Metallica, a famous work by Georgius Agricola, of Saxony, which was published in 1556, and which for the following 180 years, remained the standard text-book on mining and metallurgy. In Chapter XII of this work, the preparation of which occupied Agricola for a quarter of a century, he gives the exhaustive particulars relating to the boiling process from which the ensuing account is compiled.
After explaining the method by which sea-water is received into the first series of prepared trenches, in which the first stage of evaporation takes place and is thereafter carried into the second basins, where it is thickened by further evaporation to the constituency in which it is ready to be converted into salt, Agricola tells us that the liquor is then boiled in pans placed in sheds arranged for the purpose. Each shed is divided into three parts. In the first part is stored the firewood or straw, and in the second is the fireplace on which is placed the caldron. To the right of the caldron is a tub for the brine that is to be converted into salt, and on the left is a bench upon which the salt is placed before being removed to the third compartment, where it is moulded into cones or tablets and left to dry in the warm air.
16
The fireplaces are made 8½ ft. long and 7¾ ft. wide; if wood is burned in them they are nearly 4 ft. high, but if straw fuel is used, they are 6 ft. in height. The caldrons are rectangular, 8 ft. long and 7 ft. wide, and 6 in. deep. They are made of sheets of iron or lead, “not very thick so that the water is heated more quickly by the fire and is boiled away rapidly.” To prevent the brine from leaking out at the points where the metal plates are fastened with rivets, the caldrons are smeared over with a cement of ox-liver, or ox-blood, mixed with ashes. As soon as the first dipperful of brine is poured from the brine tub into the caldron, the wood or straw is ignited in the fireplace. If the firewood consists of faggots or brushwood, the salt will be white, but if straw is burned the salt is not infrequently blackish from the sparks which rise with the smoke and settle upon the water.
In order to accelerate the condensation of the brine, the salt-maker adds and mixes into it bullock’s blood, or calf’s blood, or buck’s blood, which dissolves and is distributed into all the corners of the caldron. When the boiling water seems to be mixed with scum, it is skimmed with a ladle, and from the firing of the furnace to the skimming of the boiling scum is the work of half an hour. After this it boils down for another quarter of an hour, and thereafter it begins to condense into salt. When the brine commences to thicken with the heat, it is stirred assiduously with a wooden spatula, and then allowed to boil for an hour. At this stage beer is added to the contents of the caldron, which is protected from the wind by boards, and the salt is then withdrawn with a shovel and thrown into baskets. The remaining brine is allowed to boil for another three-quarters of an hour, when the salt is again removed and placed in the drying compartment. In17 this manner the salters alternately boil the brine and collect the salt, “day and night, with the exception only of the annual feast days.” No caldron is able to stand the fire for more than half a year. New caldrons are washed out three times in the first two weeks, and afterwards once a week. In this manner the incrustations fall from the bottom of the caldron, and if this is not done the salt would have to be made more slowly over a fiercer fire, which not only requires more brine but burns the plates of the caldron. If any cracks make their appearance in the caldron, they are filled up with cement. The salt made during the first two weeks in a new caldron is usually inferior in quality, being stained by the rust at the bottom where incrustations have not yet adhered.
Agricola’s description is full of technical exactness in regard to those parts of the apparatus and the process which are of comparatively insignificant interest, but it is, unfortunately, silent about details on which fuller information would be useful. He tells us the capacity of the tubs in which the brine is conserved, but not of the caldrons in which it is boiled, and we cannot calculate the quantity by the dimensions of the receptacles, since he omits to mention the depth to which they are filled. He explains that it takes half an hour to fill the baskets with the salt that is drawn from the caldron, but as he does not give us the dimensions of the baskets employed, or the amount of wood or straw consumed, we cannot determine the length of time required to make a certain quantity of salt, or the cost in fuel. But, condensed and simplified by the elimination of extraneous particulars and complex technicalities, the foregoing enables us to obtain a fair idea of the methods employed by the salter of Halle, in Saxony, assisted by his wife as helper and a18 youthful stoker—working naked, on account of the great heat, save for a straw cap and a breech cloth—in the first half of the sixteenth century.
The subject of the formation of rock-salt deposits will be treated in a later chapter, in which a description will be given of rock-salt mining in Cheshire. The primitive methods that characterized the brine industry have been adhered to with equal tenacity in the winning of rock-salt. It is extraordinary that, in the manufacture and in the mining of salt, each successive generation of salt-men, in inheriting their methods from their forefathers, or adapting them from the miners of another country, have always preserved the intense conservatism that appears to be inseparable from the industry, and have resisted all innovations that have promised to simplify or expedite their labours.

ANCIENT SALT WORKS
A. Sheds. B. Painted Signs. C. First Room. D. Second Room. E. Third Room. F. Windows. G. Window in Roof. H and I. Wells. K. Casks. L. Pole. M. Forked Resting Sticks
From an Old Print
Published in 1556·
It would be an interminable and unprofitable undertaking to conduct the reader upon a tour of the salt mines of the world, and explain the different methods that are adopted to conform with the local and geological conditions which obtain in the various salt regions. The systems followed in most countries are governed by traditions that have their origin in immemorial times, and the disposition to perpetuate the operations without change through succeeding ages is, perhaps, traceable to the races that work the mines rather than to the deposits in which they work. The process of solar evaporation which is employed to-day on the shores of the Mediterranean and the Adriatic is practically the same as it was when the civilization of China was in its infancy; the implements and methods in present use in the salt mines of Austria, Russia, and Rumania were introduced by the discoverers of the lodes in the darkest ages. We cannot even fix the comparatively recent period in which it was decreed20 that the Rumanian mines of Tirgu-Ocna and Ocnele-Mari should be exploited by convict labour, while the Slavic mine was to find employment for free workers only. Every country, every salt district, and almost every mine has its peculiar and distinguishing rules, customs, and methods of work, which are interesting in themselves but of insufficient importance to warrant detailed consideration in a treatise of this scope. There are, however, certain salt regions and mines which, by reason of their magnitude and the possession of unprecedented features, have obtained rank among the lesser wonders of the world, and for this reason we must devote a little space to “the Great Salt” of Wieliezka, in Hungary, and to the great Rumanian salt deposits.
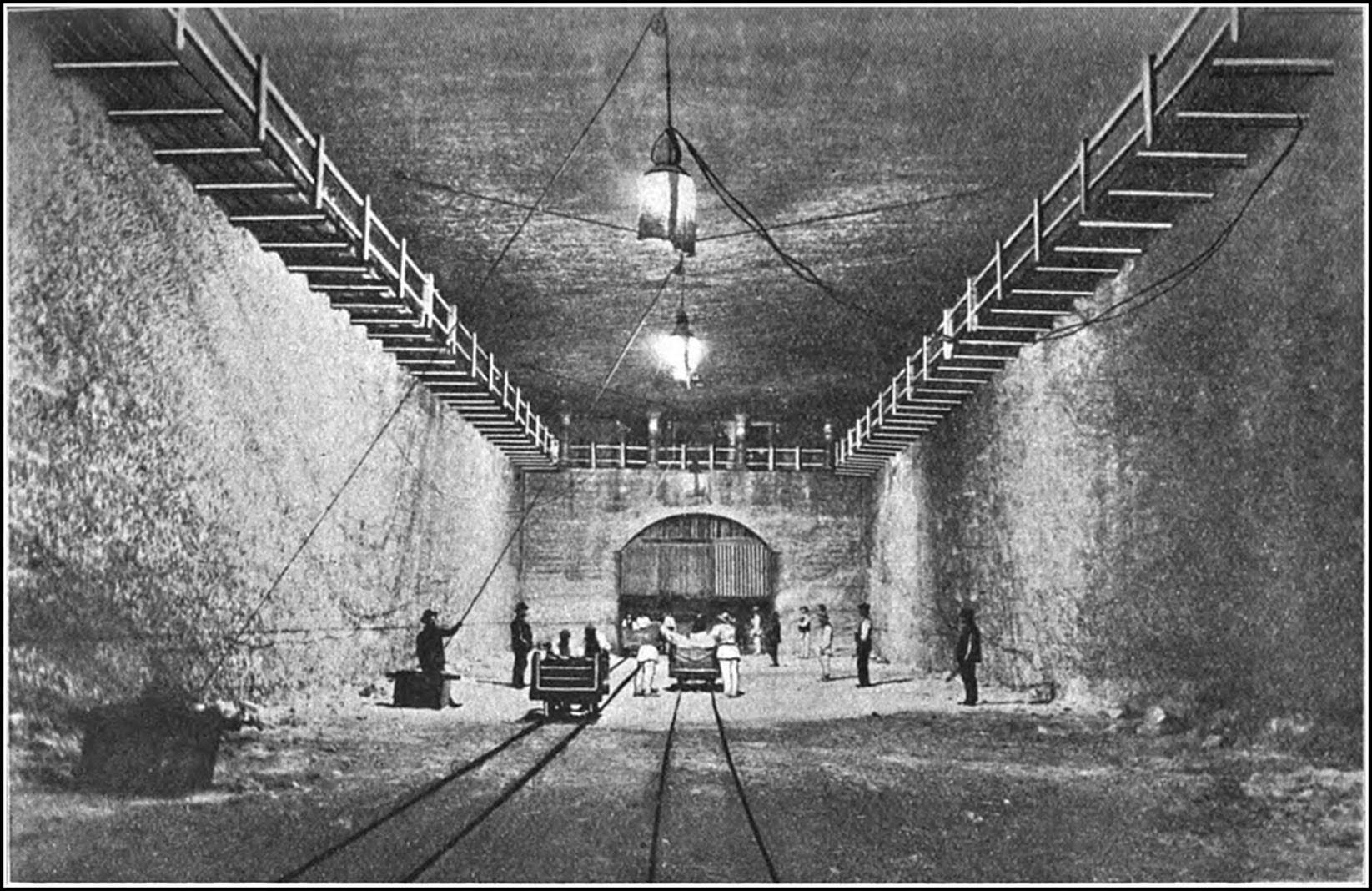
The famous mines of Wieliezka, in the lower Carpathians, about eight miles from the city of Cracow—with their underground roads, houses, and monuments; their churches, ball-rooms, and restaurants; their lakes, bridges, and railway stations—constitute a city commemorative of the art and industry of bygone periods, and present a spectacle, weird and splendid, that reminds one of the marvels of the Thousand-and-One Nights. The Wieliezka system, which has been in operation since the thirteenth century, extends over an area of about twelve square miles, and reaches a maximum depth of some 12,000 ft. The various galleries at present accessible have an aggregate length of 65 miles, and the total length of mining railways is about thirty miles. Each mine consists of five storeys. The first storey is about 200 ft. below the surface, and between the different storeys a body of earth or salt from 80 ft. to 100 ft. thick is left. As in Northwich, many of the old workings in Wieliezka have fallen in, and whole chambers and streets have been engulfed in the holes. Broad staircases connect the various22 storeys, each of which boasts its distinctive chambers and thoroughfares. The air in the upper levels is much more moist than in the lower excavations, with the result that the salt statues in these apartments are gradually losing their shape. The head of one is nearly gone, the arms of another are wasted; while the deeper furrows, which are observable upon the sculptured bodies, give them a grotesque appearance. The smoke of lamps and wicks adds to the moisture of the air and darkens the surface of the statues, which might be carved in black marble. Onward and downward one proceeds, the stairways appear to be innumerable; the visitor loses all sense of depth, distance, and direction; chambers and passages lead to further chambers and passages, until the tour of the workings leaves one with a dominating impression of limitless repetition. Everything is of solid salt, except where some insecure roof is supported by huge timbers or a wooden bridge is thrown over some vast chasm. As depth is attained the air grows drier and purer, and the points and faces of the rock become more crystalline and beautiful. Onward and downward still, through labyrinths of shafts, galleries, and chambers, up crooked passages, and under vaulted archways, that lead into innumerable, unnamed smaller apartments.
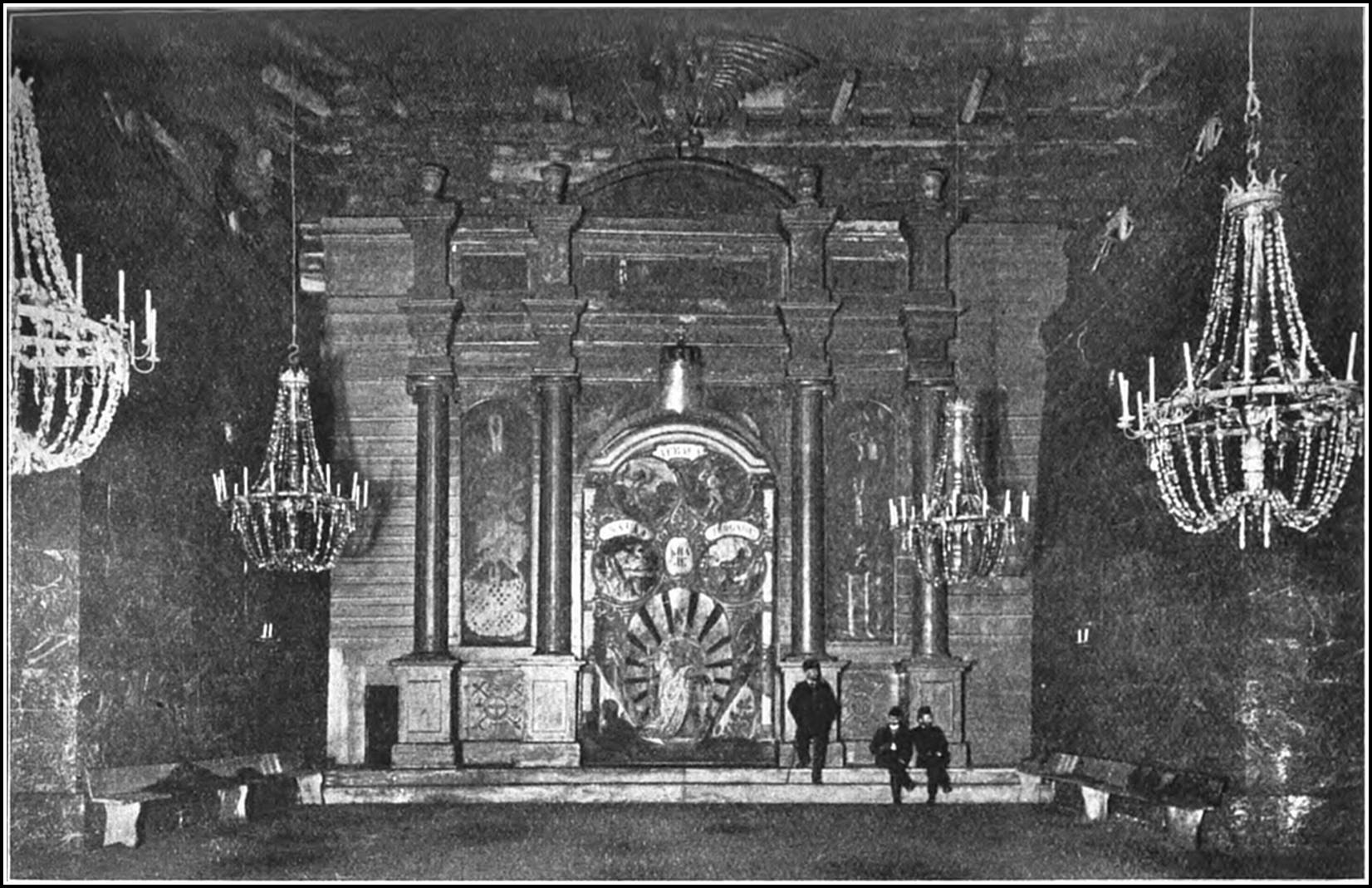
Groups and gangs of miners, naked to the hips, are everywhere busy with pick, mallet, and wedge, with which they block out and separate the salt slabs from the solid mass. The process has the simplicity of the age in which it was first employed. The blocks are marked out on the surface of the rock by grooves. One side is then deepened to the required thickness, and the face is split off by wedges inserted under the block. It is then divided into pieces of 100 lb. each and removed to the shafts, where it is hoisted, stage23 after stage, to the surface. The number of labourers continually engaged is from one to two thousand. The miners, who are muscular, healthy-looking men, are divided into gangs. The work is carried on in shifts of six hours each, and in each shift a gang will quarry out about 1,000 lb. weight of salt.
The Letow ball-room, which lies at a depth of 216 ft. below the surface, dates from 1750, and has been the scene of many Royal visits and splendid entertainments. One end of the spacious chamber is adorned with a colossal Austrian eagle, and in an alcove at the opposite end is set up a crystal throne. The giant chamber which bears the name of Michalowice, a fearsome and stupendous excavation, was completed in 1701, as the result of forty years of continuous labour. It is 59 ft. long by 92 ft. broad, and the roof, supported by a wooden framework, has a height of 118 ft. The chamber is lit by a salt chandelier furnished with 300 electric bulbs. The Francis Joseph ball-room is another of the wonders of this subterranean city. It is an immensely large and immensely lofty apartment, lit by six large chandeliers fashioned of crystalline rock-salt. Salt statues of Vulcan and Neptune, which adorn the hall, reflect the electric light from myriad brilliant points and angles, and contribute to the general impression of flashing splendour which the scene conveys. Beneath these great reception rooms, are smaller halls, each beautiful in itself, bearing the names of royal or princely personages. Massive pyramids of salt and sculptured monuments, with carved inscriptions, perpetuate the memories of Emperors and Empresses of Austria, or commemorate their visits to the mines. Near to the Letow ball-room is the celebrated St. Anthony’s Chapel, which was hewn in 1698, and for upwards of two centuries has been the resort of24 thousands of the devout. The vestibule in the chapel consists of a symmetrical archway with figures at the sides. The interior is beautified by an altar bearing a sculptured representation of the Crucifixion, and flanked by salt effigies of kneeling monks. Hard by St. Anthony’s Chapel a magnificent shrine is hewn in one of the passages, peopled with figured saints, which leads to the Queen’s Chapel, with the superbly-chiselled altar and its view of Bethlehem carved in the solid salt.
The central railway station in the third storey, and the great restaurant, with its ponderous pillars and its long vista of latticed galleries, are among the many marvels of the mines, but nothing it contains is so wonderful as the subterranean lake, lying 700 ft. below the surface of the earth. The waters of the lake are dark, thick, and heavy, and as the boat glides over its surface the slumberous wavelets roll up against the sides of the grotto with a ghost-like swish. A ponderous solitude over weighs all. The Styx alone of all the legendary rivers of death could rival this in stillness. The boat is guided through the Stephanie and Rudolf grottoes by ropes running on pulleys along the sides of the curious craft, and the boatman, with his hands resting on the stern, pushes it with his feet braced against the rope. Of the sixteen lakes in different parts of the mine, this is the only one upon which visitors are allowed to go. The report of a gun fired in the centre of the lake fills the vault with long and lingering echoes, and the voice of the boatman sounds like a giant’s voice uprising from the depths of chaos.
The illumination of the mine is arranged according to a regular tariff based on the number of visitors sharing the expense. For any number of persons up to twenty, the illumination, which comprehends the employment of over a thousand candles and electric26 lamps, costs about ninety shillings, but for an additional sovereign, which is charged when a party numbers over thirty persons, the whole mine becomes a blaze of light.
INTERIOR OF SALT MINE AT SLANICU, RUMANIA
This famous mine has been worked since the time of the Romans.
Serious calamities at Wieliczka are now practically unknown, owing to the care exercised by the officials, but minor accidents are unavoidable. Some few years ago a huge mass of rock-salt, weighing some 200 tons, fell from the roof of one of the chambers; in 1868 the mines were flooded by the bursting of a subterranean salt lake; and a fire in 1815 resulted in the loss of several hundred lives. The early history of the mines contains the record of several terrible disasters, including an incendiary fire in 1510, which caused a great number of deaths, and another fire in 1644, which raged for over a year, and consumed all the people, horses, and mules who were in the mine when the fire occurred.
The working of the three great Rumanian salt deposits present other examples of the persistent survival of ancient methods, but it must be admitted that an attempt was made at one time to introduce modern machinery. It was demonstrated that the machine produced more salt in a given time, and that the waste of about 25 per cent. of the salt attendant upon manual labour and the use of picks was saved, but as the supply of salt is practically inexhaustible, and there is no limit set upon the time of winning it, and as man-power, especially convict man-power, is cheaper than machinery, the authorities soon reverted to the old system. In the Slanic mine, in which the salt is crystalline, white, and almost absolutely pure, the free labourers, of whom about 500 are employed, are divided into gangs of six men. Each man takes an oblong piece of the floor of the mine, about as big as an ordinary tombstone, and, using his pick, scoops round it a27 narrow groove about 5 in. deep. This done, he summons the rest of his gang, and, standing beside him on the slab, they raise and bring down their picks simultaneously at the word of command. Force is necessary, but rhythmical accuracy in the planting of the blow is more essential, and by long practice the men have become so extraordinarily expert that they scarcely ever diverge a hair’s breadth from the point at which they aim. In a few minutes the persistent blows detach the slab, which the six men raise with the aid of a lever. The gang proceed from slab to slab until all six have been detached and lifted, after which each man breaks his own slab into chunks and loads it into a truck for removal to the shaft, through which it is hauled to the surface. An expert miner’s earnings at this work range from half a crown to three shillings a day.
The convicts employed in the Tirgu-Ocna and Ocnele-Mari mines are paid from sixpence to eightpence a day for their work, and, save that liberty and the hospitality of the local taverns are denied them, their condition is little worse than that of the free labourers. As capital punishment does not obtain in Rumania, the convict miners include murderers, brigands, and the worst class of criminals, and armed soldiers escort them to and fro between the prison and the mine, and remain on guard while they are at work. Dashes for liberty used to be common, and organized attempts to escape have also been attempted, but now, on the first sign of suspicious behaviour on the part of the convicts, the order is given for the whole gang to throw themselves flat upon the ground. As those who disobey the order are immediately shot, instantaneous compliance with the command is usually observed. On one occasion a body of disaffected convicts had28 recourse to a form of passive resistance, and when the day’s work was over they refused to leave the mine. The guards and overseers thereupon withdrew and left the mutineers to reflect in an intolerably salt atmosphere upon the virtues of fresh water, of which they had no supply. After two days of torture, the men capitulated. But the work of superintending the convicts in the mines is a delicate and dangerous task. The overseers are compelled to mix with the men, and it is but the work of a few silent minutes for a gang to overpower an unpopular official and squeeze the breath out of his body. As the murder is a communal affair, and the practice of making an example of one man pour l’encourager les autres is not adopted in Rumania, the extent of punishment inflicted upon the whole gang is less than would be meted out to individual offenders. As the salt reserves in the three principal mines of Rumania are estimated at 8,774,000,000 tons, and the annual extraction has never exceeded 150,000 tons, it follows that, at the present rate of progress, the deposits cannot be exhausted for several millenniums.
Where the salt deposits are composed of a mineral that is white, odourless, and practically pure, as in the Wieliezka system and the mines of Rumania, and particularly if labour is abundant and cheap, and the industry is a monopoly of the State, rock-salt mining will always hold its own.

Even in this country, when the old open-pan system of evaporating salt from brine produced only two tons of salt for the consumption of a ton of fuel, rock-salt could be raised, purified, and marketed in competition with white salt, but the modern boiling processes have effected such substantial improvements and consequent economical advantages, that the rock-salt industry appears to be doomed to decay. Rock-salt, as quarried30 from its native bed, is found in many variations of colour, from grey and yellow to green and brick red, according to the nature of the impurities of the locality in which the deposit lies, and such salt must be cleansed from all traces of iron, clay, gypsum, or bitumen before it is fit for domestic use. Many processes have been experimented with for the removal of impurities. One of the most plausible methods was based on the fact that salt fuses at a temperature of about 1,750 degrees, and the theory was to remove all impurities from the fluid mass by the agency of compressed air. The principle was unsuccessfully experimented with in Würtemberg nearly half a century ago, but a modern adaptation of the process claimed to be more successful. The molten material, in this case, ran into rotating pans and gradually overflowed; and it was then shovelled into another receptacle and, while subjected to the action of compressed air, raised by small buckets to a certain height and emptied into inclined screens, through which it was automatically graded. It was claimed that from the time of casting the crude material into the furnace, until the perfect white salt appeared, the process occupied only fifteen minutes, and that rock-salt could be broken in the mine, transported, fused, and packed ready for table use in less than two hours.
At the time when the master-patent for this process was taken out, the latest brine-evaporating systems were unperfected, and there was some possibility that the invention might be capable of taking the rock-salt direct from the mine, eliminating at one stroke all its impurities, and in the course of an hour or two delivering into the warehouse an anhydrous salt “at a fraction of the cost of the ordinary process” of evaporating salt from brine. But by the time that this bold claim was31 put forward on behalf of the process, the admitted total cost of production had been advanced from 4s. to 5s. 8d. per ton, while the latest patent brine-evaporating system was producing the manufactured article at a total inclusive cost of 3s. 6d. per ton. Since then, this rock process was installed in Mexico, persevered with for a while, and finally discarded because, in the words of Mr. W. L. Bonney, the United States Consul, “the experiment proved a failure.” Even if the latest brine process has not “relegated rock-salt mining into the limbo of extinct enterprises,” it appears certain that it will never be able to be worked in competition with the process by which salt is manufactured direct from brine where brine is available.
32
If we turn from the study of salt as one of the staples of world industry to the history of the salt industry in England, we find that it is practically comprised in the records of the development of the trade in rock-salt and brine in the county of Cheshire. The first documentary reference to the existence of saline deposits in this country, as well as the earliest mention of the method of native manufacture and of the introduction of the open-pan system of salt-making, dates from the time of the Roman occupation. The Caesarean soldiers, who penetrated as far north as the Northwich district, found the people obtaining salt by the process of pouring brine upon faggots of charcoal and scraping away the resultant crystalline formation. A little spring which existed at that period in Sheath Street, Northwich, furnished the Romans with a limited supply of brine, and from this source, with the crude plant improvised on the spot, they produced the first salt ever manufactured in England by the boiling of brine in open pans.
The Britons named the brine spring at Nantwich “Hellath Wen,” or the White Pit, on account of the whiteness of the salt produced from its waters; while the spring at Northwich received the name of “Hellath Du,” or the Black Pit. The suffix “wich” may have been introduced into Cheshire direct from the Vikings of the North, or brought there by way of the south-eastern counties. In Camden’s Britannia (published in Latin in 1607, and translated by Philemon Holland,33 1610), we read that the word Wiccij “may seeme to have beene derived of those salt pittes that the old Englishmen in their language named Wiches ,” and William Smith, a Cheshire Man and author of a work which is known as King’s Vale Royal (1656 edition), says: “The house in which the salt is boiled is called the Wychhouse; whence may be guessed what wych signifies, and why all those towns where there are salt-springs or salt made are called by the name of wych , viz., Namptwych , Northwych , Middlewych , Droitwych .” But the Norse word wig and the Anglo-Saxon wic signified, in the original, a dwelling-place, and in the latter form of wich , it is seen in the names of Woolwich, Norwich, Harwich, Sandwich, etc. The Norse and Danish pirates who visited our coasts to pillage and procure salt, established wigs —afterwards wiches or hamlets—on the bays and inlets, and wherever they located themselves they proceeded to make bay-salt. The word wich , in course of time, became identified not with the village but with the salt manufacture that was carried on there, and when the Cheshire towns developed the industry they may easily have adopted the nomenclature that was already regarded as indicative of the manufacture.
In the records of Droitwich, which was also called Durt-wich “by reason of the wettish ground on which it stands,” we learn that in the year 816, Kenulph, King of the Mercians, gave Hamilton and ten houses in Wich together with their salt-furnaces, to the church of Worcester, and that in 906 the same church was endowed by Edwy, King of England, with Fepstone and five salt-furnaces; but the next earliest references to the Cheshire Wiches must be searched for among the entries in Domesday Book, which was prepared between 1084 and 1086. William the Conqueror’s34 authorized inquiry as to the several places in which salt was being made, and the persons who had held proprietorial rights in them since the time of Edward the Confessor, was productive of much detailed information. From the zincograph reproduction of the original made by Mr. William Beaumont in 1863, it would appear that the Cheshire brine-springs and salt works were strictly held, and were subject to certain well-defined customs. In several localities the existence of solitary salt-houses is mentioned, and it would seem safe to infer that the supply of brine was obtained in the vicinity and the salt was only made for local consumption. Salt-making for commercial purposes was confined to Nantwich, in Warmundestron Hundred, and Northwich and Middlewich in the Hundred of Mildestvic, and, although no figures relating to output or revenues are given, the laws governing the trade, the prices charged, and the method of dividing the moneys accruing from rents and sales are concisely set forth in the following paragraphs—
“Mildestvic hundred. Hugh and William held of the Earl Rode Godric and Ravesa held it for two manors and were free men.”
“In the same hundred of Mildestvic there was a third Wich called Norwich (Northwich), which was in farm at eight pounds. In it there were the same laws and customs as in the other Wiches, and the King and the Earl divided the receipts in the like manner. All the thanes who held salt-houses in this Wich gave no Friday’s boilings of salt the year through. Whoever brought a cart, with two or more oxen, from another shire, gave 4 pence for the toll. A man from the same shire gave for his cart 2 pence within the third night after his return home. If he allowed the third night to pass, he was fined 40 shillings. A man from another35 shire paid 1 penny for a horse load. But a man from the same shire paid 1 styca within the third night after his return, as aforesaid. A man living in the same hundred, if he carted salt about through the same county to sell, gave a penny for every cart, for as many times as he loaded it. If he carried salt on a horse to sell, he gave 1 penny at Martinmas. Whoso did not pay it at that time was fined 40 shillings. All the other customs in the Wiches are the same. This manor was waste when Earl Hugh received it. It is now worth 35 shillings.”
“Nantwich.—In King Edward’s time there was a Wich in Warmundestron hundred, in which there was a well for making salt, and between the King and Earl Edwin there were 8 salt-houses, so divided that of all their issues and rents the King had two parts and the Earl the third. But besides these, the Earl had one salt-house adjoining his manor of Acatone (Acton) which was his own. From this salt-house the Earl had sufficient salt for his house throughout the year. But if he sold any from thence, the King had twopence, and the Earl a third penny, for the toll. In the same Wich many men from the country had salt-houses, of which this was the custom—
“From our Lord’s Ascension to Martinmas, anyone having a salt-house might carry home salt for his own house. But if he sold any of it either there, or elsewhere in the county of Chester, he paid toll to the King and the Earl. Whoever after Martinmas carried away salt from any salt-house except the Earl’s, under his custom aforesaid, paid toll, whether the salt was his own or purchased. These aforesaid 8 salt-houses of the King and the Earl, in every week that salt was boiled or they were used on a Friday, rendered 16 boilings of salt, of which 15 made a horse-load. From36 our Lord’s Ascension to Martinmas, the salt-houses of the other men did not give these Friday’s boilings. But from Martinmas to our Lord’s Ascension, these boilings were given according to custom, as from the salt-houses of the King and the Earl. All these salt-houses, both of the lord and other people, were surrounded on one part by a certain river, and on the other part by a ditch. Whosoever committed a forfeiture within these bounds, might make amends, either by the payment of 2 shillings, or by 30 boilings of salt, except in the case of homicide, or of a theft, for which the thief was adjudged to die. These last, if done here, were dealt with as in the rest of the shire. If out of the prescribed circuit of the salt-houses, any person within the county withheld the toll, and was convicted thereof, he brought it back and was fined 40 shillings, if a free man; or if not free, 4 shillings. But if he carried the toll into another shire, where it was demanded the fine was the same. In King Edward’s time, this Wich, with all pleas in the same hundred, rendered 21 pounds in farm. When Earl Hugh received it, except only one salt-house, it was waste. William Maldebeng now holds of the Earl the same Wich, with all the customs thereto belonging, and all the same hundred, which is rated at 40 shillings, of which 30 shillings are put on the land of the said William, and 10 shillings on the land of the Bishop, and the lands of Richard and Gilbert which they have in the same hundred, and the Wich is let to farm at 10 pounds.”
“Middlewich.—In Mildestvich hundred there is another Wich between the King and the Earl. There, however, the salt-houses were not the lord’s, but they had the same laws and customs that have been mentioned in the above-mentioned Wich, and the customs were divided between the King and the Earl in the37 same manner. This Wich was let to farm for 8 pounds and the hundred wherein it was, for 40 shillings. The King had two parts, and the Earl the third. When Earl Hugh received it, it was waste. The Earl now holds it, and it is let to farm for 25 shillings, and two wain-loads of salt. But the hundred is worth 40 shillings. From these two Wiches, whoever carried away bought salt in a wain drawn by four oxen or more, paid 4d. for the toll; but if by two oxen, 2 pence if the salt were two horse-loads. A man from another hundred gave 2d. for a horse-load. But a man of the same hundred gave only a halfpenny for a horse-load. Whoever loaded his wain so that the axle broke within a league of either Wich, gave 2 shillings to the King’s or the Earl’s officers, if he were overtaken within the league. In like manner, he who loaded his horse, so as to break its back, gave 2 shillings if overtaken within the league, but nothing if overtaken beyond it. Whoever made two horse-loads of salt out of one, was fined 40 shillings if the officers overtook him. If he was not found, nothing was to be exacted from any other. Men on foot from another hundred buying salt, paid 2d. for eight men’s loads. Men of the same hundred paid 1d. for the same number of such loads.”
The first private record relating to salt appears in the foundation deed of Combermere Abbey, dated 1132, in which Hugh Malbane, the founder, caused it to be written: “And I also grant to the same monks the fourth part of the town of Wych, and tythe of my salt and of the salt pits that are mine, and salt of Blessed Mary the Virgin, and salt on Friday, and salt for the Abbot’s table as freely as I have it at my table.”
Ancient Deeds in the Record Office contain occasional reference to salt properties in the thirteenth and fifteenth centuries which show that salt was made38 in limited quantities in Cambridgeshire and at Rye, Mimera, and Brembre (formerly Hayerskys), in the County of Sussex.
Protests against the importation of salt from abroad, and of salt-making by foreigners contrary to the liberties and ancient customs of the borough of Northwich, are recorded in the Harleian MSS. In response to a complaint made on behalf of the burgesses and inhabitants of Northwich concerning the mischievous irregularities committed in the making of salt by “p’sons forrayne and not inhabiting w/thin the Sḍ towne,” King Henry VIII issued an Order to the Justice and Chamberlaine of the County Palatine of Chester to the effect: “WHEREFORE we will and command you that in any case such forrayne p’son or p’sons not inhabiting within the s/d towne, do, or hereafter at any time shall attempt to use makeing of salt contrary to the lib/erties and ancient customes of the same within the same towne without lycence of the burgess and the rulers thereof. THAT then without delay ye and ether of you from tyme to tyme upon complaynt or of the rulers and govnors of the same towne do send for all and every such forrayne p’sons as do or hereafter shall attempt to make any salt within the s/d towne of Northwich contrary to the libties and ancient customes of the same, without the assent and agreem/t of the s/d Burgess and ruler by o/r writts of subp: to appear before you in o/ Castle of Chester at there appearance to punish and reforme them: And also further to order them as right and good conscience shall require according to the lawes and customes heretobefore used now in other wyches there abts w/thin o/r s/d County Palatyne, for the reformacon of such transgressions fayle ye not hereof as ye maye intend to please us.”
In the time of the Tudors, the salt-makers of Cheshire39 were composed of natives and “forrayners,” or residents born outside the boundaries of the county, and in the Northwich Book of Orders is given a list of ten “outliers” in the town of Northwich who occupied between them no fewer than eighty-nine salt pans or leads. Although we have no information as to the exact size and capacity of the evaporating pans of the period, it is evident that they were made to a regulation scale, and we read that it was the business of an officer of the Court Leet to examine the leads and see that they conformed with the standard dimensions. If the prescribed measurements were exceeded, the official cut a piece out of the corner of the pan with a pair of shears with which he was furnished for the purpose, so as to reduce its capacity to the legal limit.
Only three of these old salt-pans have been recovered, and, of these, one was cut up and sold as old lead. One which was drawn out of the river at Northwich in 1866 was forwarded by the River Weaver Trustees to the Warrington Exhibition, and was transferred subsequently to the Northwich Museum. This pan measures 3 ft. 8 in. long on one side, and 3 ft. 4½ in. on the other; it has a width of 2 ft. 8 in., and is 4 in. deep. The thickness of the lead is about half an inch. and the weight of the pan is 2 cwt., 1 qr. 18 lb. There are raised patterns on each end of the pan, which was evidently cast, and the sides are rounded up from the bottom. In 1878, in the vicinity of Ashton’s Salt Works at Witton, was found a smaller pan made out of a sheet of lead 2 ft. 8 in. square. The sheet was bent up to form a pan and the corners were hammered together. This lead is 25 in. square by 3 in. in depth, and has a capacity of about 7 gallons.
In the early years of the reign of James I we have particulars of the salt districts in Camden’s Britannia,40 and in a letter received in February, 1605, from Chomley written by one George Johnson. Camden explains that the Cheshire Wiches were so-called because “there bee here very notable salt pits and many salt springs often-time have been found which notwithstanding are stopped up, because it was provided (as wee read) that for the saving of woods, salt should not be boiled but in certain places.”

Meagre as these accounts are in exact particulars, they constitute the only information we have concerning the supply and treatment of brine in England in the early days of the industry, and, consequently, they invite attention. Camden is responsible for the following details—
“At Northwich there is a deep and plentiful brine pit with stairs about it, by which, when they have drawn the water in their leathern buckets, they ascend, half naked, to their troughs and fill them, from whence it is conveyed to the wich-houses about which there stand on every side many stakes and piles of wood.
“Nantwich.—There is but one salt pit here (they call it the brine pit) distant about 14 ft. from the river. From this brine pit they convey water by wooden troughs into the houses adjoining, where there stand ready little barrels, fixed in the ground, which they fill with that water; and at the notice of a bell, they presently make a fire under their leads, whereof they have six in every house for boiling the water. These are attended by ‘Wallers’—a name probably derived from the Anglo-Saxon weallere , a boiler; German, wallen , to boil—who with little wooden rakes, draw the salt out of the bottom of them and put it in baskets, out of which the liquor runs, but the salt remains and settles....
“The depth of the salt springs is in some places not above three or four yards. In Nantwich the pit is42 full 7 yards (deep) from the footing about the pit: which is guessed to be the natural height of the ground, though the bank be 6 foot higher, accidentally raised by rubbish of long making salt or “walling,” as they call it. In two places within our Township, the spring breaks up so in the meadows as to fret away not only the grass, but part of the earth, which lies like a breach at least half a foot or more lower than the turf of the meadow: and hath a salt liquid ousing (oozing) as it were out of the meed but very gently.
“Droitwich possesses three fountaines yielding plenty of water to make salt of, divided asunder by a little brooke of fresh water passing betweene, by a peculiar gift of nature spring out: out of which most pure white salt is boiled for six months every yeare, to wit, from Midsommer to Midwinter, in many set fornaces round about: wherewith a mighty deal of wood is consumed, Fakenham Forest (where trees grew sometime thicker), and the woods round about, if men hold their peace, will by their thinness, make manifest more and more....”
Of the two wells of salt-water at Middlewich, which are separated by a small brook, we are only told that “one stands not open but at certain set times, because folke willingly steale the watere thereof, as being of great vertue and efficacie.”
More informative on essential points is the unknown correspondent of George Johnson, who writes as follows—
“Namptwich.
“There is in the town of Namptwich two hundred and sixteen salt-houses of six leads apeece, and every of the said houses doth spend in wood per annum eight pounds so as there is spent in wood yearly within the said town in omnibus annis.... £1728
43
“Middlewich.
“There is, in the said town, one hundred and seven salt houses of six leads apeece, and one of four leads and every of the said houses doth spend yearly in wood the sum of £13. 6. 8, so as there is spent every year within the said town, £1435. 4. 0.
“Northwich.
“The said Northwich is a Burrow and holden of the Earle of Chester by the service of twelve armed men to serve at the Watergate in Chester in the time of wars betwixt England and Wales. There is, in the same towne or Burrow, one hundred and thirteen salt houses, every one containing four leads apeece, and one odd lead and one four leads which was given to the Earl of Derby by the Burgesses, occupiers of the said Town, for the portion of his house, and no land in the Town for it, and every four leads must have in provision of wood, nine quarters and so rateable, whether it be four leads or six leads, so that there is spent in wood in the said town 1026 quarters and a peece after the rate of five score to the hundred and after the rate of forty shillings per Quarter comes to £2056. 10. Spent in the wich houses yearly in wood, £5219. 14.”
The particulars which are given of the salt manufacture in the Wiches in 1605 and 1607 by George Johnson’s correspondent and by Camden, are repeated with only the slightest variation half a century later in King’s Vale Royal . But in the latter account we are able to glean a little more information about the towns themselves. Concerning Northwich, we are told that it had the mischance to be burnt in July, Anno 1438, and was “most part miserably consumed with fire,” in December, 1583. “But through the Benevolence gathered throughout the Realm, it is new builded, and is in as good case or rather better than before.” The44 town in 1656 was divided into two parts, one of which was called the Cross, while a “very fair church of stone,” called Northwich Church, stood “without the Town’s-end.” But although it was called Northwich Church, we are told that it was only a chapel and its proper name was Witton; a combination of coincidences which caused the chronicler to conclude “that the town was named first Northwich, after the finding of the salt.” Of Nantwich, we are only informed that the town was visited in 1617 by the gracious King’s Most Excellent Majestie, who, with his own eyes beheld the manner of the brine well and the labours of the drawers of brine—who, in the course of their work, “spend the coldest day in frost and snow, without any clothing more than a shirt with great cheerfulnesse”—and “with his own hand most princely rewarded them.” Middlewich is described by the same authority as no market town: “yet may it pass amongst them, as well for the bigness thereof, as also it hath Burgesses and other privileges, as the other wiches have, yet it hath a small market of flesh and other things every Saturday, and yearly two fairs: that is to say on Ascension Day and St. Luke’s Day. It hath divers streets and lanes, as King Street, Kinderton Street, Wich House Street, Lewis Street, Wheelock Street: Pepper Lane: Cow Lane and Dog Lane. But the chiefest place of all is a broad place in the middest of the Town, in manner of a market place, called the King’s Mexon.”
A large accumulation of matter of great local and antiquarian interest is to be found in the Northwich Book of Orders, the Court Rolls, and the Walling Booke of Northwich, which consist of documents and records relating to the government of the town and the regulation of its salt industry about the middle of the seventeenth century. The “Ancient Customes of the45 Burrow and Town of Northwich,” the inventory of “The Liberties and Priviledges of Burgesses,” and the Orders “concerning the making of salt,” were collected and set down by Peter Warburton, of Chester, Esquire, Steward of Northwich, and afterwards a Justice of the Court of Common Pleas at Westminster. At a Court held on 18th December, 1608, this compilation, “so full of interest and instruction,” was ratified and confirmed by Thomas Berrington, Gentleman, Steward of the said Court, and a jury of Burgesses, and Thomas Poole, Gentleman, Clerk of the said Court, was instructed to write them into a Booke “to the end the same may remain upon record to future ages.”
The Nine Customes, numbered 10 to 18, which were written in 1638, were supplemented in 1641 by other Nine Customes, numbered 1 to 9, which had been “heretofore omitted merely through forgetfulness.” Of the eighty-four Orders relating to salt-making which appear in these records, the first sixty-one were agreed upon by “The Steward and Jury at Diverse Courts” up to 1629, the seven following were added in 1630, and seven more appeared on the rolls before Master Poole made a fair copy of the Orders in 1638. In the following year eight further regulations were issued. Order No. 84 bears the date of December, 1656, and only three subsequent unnumbered enactments were included up to 1666, when the record comes to an end.
Although these old Orders (1629–1666) include directions relating to the general behaviour of the townspeople, injunctions concerning the sales of liquor and butchers’ meat, the malpractices of begging at men’s doors, piking or stealing wood, “scoulding or chideing ... to the trouble or disquietness of the good and honest neighbours,” and rules for the maintenance of cleanliness in the streets and public places and the46 publication and preservation of Proclamations put forth by the King, the bulk of the laws are framed in the interests of the staple industry of the district. No detail connected with salt-making, from the drawing of the brine to the transport of the manufactured product, is left to chance or the discretion of the individual. The rights and privileges of Burgesses, and particularly of such as occupy salt-houses or wallings, are set forth in the Ancient Customes, but in all particulars relating to the making of salt, the Orders are paramount and precise. Space does not permit of the reproduction here of the whole of the regulations, but a few of the Items may be quoted as evidence of the care and thoroughness with which they were framed.
“7. Item. It is ordered that no man shall enter into the Lead-looker’s book any more walling or occupation for one Wich-house than six leads walling upon paine for every offence ... 10s.”
“15. Item. That if any Person or Persons receive into their Houses any Wood by Night or by day by the way of Exchange for Candles, Meat or Drink every such Person as well the Changer as the Receiver shall pay fine to the Lord for every default 5s. or to be punished by the Steward.”
“17. Item. That every Waller shall sell the salt she maketh by the Walme or Cranock and not by the sack or load, and at the price which the officers sett down to be the com’on price of the Towne upon pains for every default 3s. and also to make up the full price to her Mr. upon her wages.”
“18. Item. That no Waller nor no other Person shall make any fire in the Wich-house streets in the night time, and every such offence to be presented by the Bailiffe at any single Court and punished by the Steward according to his discretion.”
47
“22. Item. That no person shall deliver any bryne to be carryed out of this Towne either in Hodge heads or Barrels (except upon Woemen’s heads) upon paine to forfeit to the Lord for every such offence ... 20s.”
“24. Item. That there should be left at every pile made at the end of any Wich-house or Wood roome a yard and a halfe between the said pile and the Crest of the Pavement to the intent that waynes may have better passage upon paine of 6s. 8d. presentable at any single Court.”
“26. Item. It is ordered that no Person from henceforth shall be suffered to wall or occupy any Odd Lead as 3, 5 or 7, but 2, 4 or 6 Leads for avoiding of trouble to the officers except in such case as cannot be remedied upon paine of ... 10s.”
“27. Item. It is ordered that henceforth no Person shall occupie Walling unless they first continue a householder for the space of three years and after such time expired to be allowed by the Steward or his Deputy, and the Lead-lookers (except he be a Burgess) upon paine to forfeit for every lead ... 13s. 4d.”
“33. Item. That all Inhabitants and Occupiers of the Towne do aide and assist lawfully every Officer of the Towne in Executing their office lawfully upon paine every one that offendeth to pay for every offence ... 10s.”
“40. Item. It is ordered that if any Waller be found making of Course Salt when they might make it better if they would, the Lead-lookers or Salt-viewers so finding them and making presentment thereof e’ry such Waller so offending shall fine to yr Lord for e’ry offence therein ... 2d.”
“43. Item. We do also order that every Occupiers’ Leads of this town shall henceforth be made Tenn stone weight a peece to the pan before they be cast, upon paine of the Lead-casters forfeiture to the Lord of this Towne48 for every default in casting any Leads contrary to this order the sum of ... 10s.”
“63. Item. It is also ordered that every occupier of Walling or his Waller, or his Servts shall weekly make cleane ye pavemt agt their Wich-Houses one yard and a half from the middle of the pavement upon paine to forfeit for every such offence ... 12d.”
Duly set forth in these records are the forms of oaths to be administered by the Court to those who “shall well and truly execute the office” of Constable, Lead-looker, Overseer, Salt-viewer, Assessor, Killer of Salt, Market Looker, Sealer and Searcher of Leather, Ale-Taster; Skavinger, Gutter Viewer, Wood Tender, or Pan Cutter. Each of these important officers in the prescribed form must “swear by the holy Contents of this Booke,” to “spare no man for any love, favour or affection” in the fulfilment of his several duties but “of all Defaults and Defects that you find in the execution of yor office you shall present at every Single Court to be holden after such Default made—So help you God.”
The compiler of Vale Royal (1656) does not admit that he is indebted to the Northwich Book of Orders for his information, but he alludes in general terms to the “authentique rules and customes” which regulate the manner of making salt in the Cheshire wiches, and adds: “All these things I leave to be read other where, knowing well their jealous love to be such towards this their beloved commodity as I should soon incur some reprehension for being too busie to look narrowly upon such a beauty.”
In “A Copie of The Walling booke of Northwch,” amongst the Harleian MSS., the earliest list of occupiers of wich-houses with the number of leads, together with the names of such persons as had wich-houses of Inheritance50 in the town, with their number of leads, was compiled in 1565, and gives a total of “five score and thirteene Salt houses and one lead.” A list of owners and of salt-houses arranged in the form of a street directory was drawn up in 1593, and, about 1600, a revised list, compiled in accordance with the location of the houses, and giving the number of leads in each, was supplemented by a street plan of the town. In the list of 1589 it is recorded that—
“Our Soveraigne lady the queene hath two salt-houses of free occupa’ion, and toulfree wth all and one is Judger of Cogshall.”
In the list of 1604, the King appears as the owner of two salt-houses, and it is assumable that His Majesty acquired an additional half of a salt-house in the following year, since, in the more detailed compilation drawn up in 1605, we read that—
“Our Soveraigne Lord the Kings Majty hath two Salt-house and a halfe which be both towle free and ffine free and is Judger of Cockshall.”
The King’s name as the owner of “2½ towle free and fine free” salt-houses heads the list for 1619 and that for 1636–1638. This last contains the names of forty-six lords and owners of salt-houses, having an aggregate of over 400 leads.
It was the custom to repeat the legend either at the beginning or the end of each succeeding list that—
“There is and tyme out of mynd hath been within the Towne of Northwich 112 four leads and one odde lead and noe more; and four leads called the Running Wich-house. Soe the totall is 113 four leads and one odde lead.”
This formula was evidently only a fable. The discrepancy between the figures and the statement was pointed out by a scribe in 1630, who, having cast51 up the number of leads tabulated in the list of 1589, appended the following note: “These leads answere but unto 308 leads whereas there is 453 leads yearly walled for ut pateat ante: soe that there wanted 145 leads to make up the full accoumpt for 308 leads and 145 leads make but just 453.”
There are about 450 leads accounted for in the next list, which was drawn up in 1593, but the clerk persisted in the assumption that what had been “time out of mynd” could suffer no change, and he formally declared, despite his own figures to the contrary, that “the totall some is 113 four leads and one odde lead, which stand in the Towne rowe as is before written and declared.”
On folio 61 of one of the Harleian MSS., following the list of Northwich salt-owners for the years 1636–38, are undated lists of salt-owners of Middlewich and Nantwich. The clerk admits the incompleteness of the list of twenty-two owners in Middlewich, but he explains that the names he gives are “as manie as I can learne for the p’sent,” and he adds, “But the number of their sev’all and respective howses and leads I cannot learne.” Only five owners appear in the returns for Nantwich, and the meagre particulars that the clerk has been able to acquire respecting the other salt districts of Cheshire are contained in the following note: “There is another Wiche where there is a great store of Salt made in Cheshire And wch is of greate Antiquitie called Fulwich, also Durtwich, and my Lo: Brereton is an owner of sev’all wich-houses theire. But whoe are owners of the rest I cannot learne.”
Nantwich was long famous among the Wiches for its production of the finest and best white salt. The Welsh named it Hellath Wen, and the London Magazine, in 1750, translated the words as the “White Salt Town,”52 but there is no reference to the quality or colour of its output in the present name, which is derived from the Welsh word “nant,” a vale, and the Saxon “wyche.” That its salt was good, plentiful, and of considerable commercial value would seem to be shown by the fact that under the Saxons the supplies were in the hands of the princes and nobles, and William the Conqueror had not been in England more than a year before he divided the salt production of Nantwich between himself and Earl Edwin, who owned some salt-houses in the district.
According to Leland, there were 400 salt works at Nantwich in the reign of Henry VIII, but the number was reduced to 216 under Elizabeth, and in 1624 only 108 were in existence. Nantwich was described in the London Magazine of 1750 as the largest and most considerable town in the county next to Chester, but its salt industry at that period was fast declining. An Act of Parliament which had been obtained in 1734 to extend the navigation of the river Weaver from Winsford to Nantwich, was never put into operation. In 1778 the salt works had been reduced to two, each containing five large pans of wrought iron. The Nantwich salt industry was practically moribund in 1849, but some twenty-five tons per week were produced by one maker until 1856, which is the last year in which salt was made in the district. In 1891 a company was registered for the purpose of acquiring property in Nantwich and manufacturing salt from brine, but the necessary financial support was not forthcoming and the project was abandoned. The decline of the Nantwich salt industry is ascribed in Poole’s History of Cheshire (1778) to various causes, including the frequent destruction by fire of the works in the town—“fourteen of which in the memory of persons living lately, having53 been destroyed in one day”; to the discovery and exploitation of new salt springs in adjacent localities; and to the superior advantages in the matter of accessibility which were possessed by Northwich and Winsford.
Northwich, described by the Welsh as Hellath-du, became the chief of the Cheshire salt towns in the seventeenth century, and its output of brine is still greater than that of any other district. In 1605, Northwich had 449 leads, against 642 leads at Middlewich and 1,296 leads at Nantwich, but the comparative superiority of the brine pumped at Nantwich over that of her rivals is demonstrated by the relative amount of boiling required to precipitate the salt. In Northwich, the annual expenditure for wood fuel was £2,056; Middlewich, with nearly one-third more leads, consumed wood fuel to the amount of £1,435 yearly; while Nantwich, working twice as many leads as Middlewich, and nearly three times the number operated at Northwich, had an annual wood bill of only £1,728.
In 1670, Winsford, which had only just started as a salt producer, had two salt works in operation on a small scale. In 1675, Lord Brereton ignored the output of Winsford in his calculation of the total annual salt production of the Cheshire works at 26,927 tons. In 1878, or practically two centuries later, the Cheshire output of salt was calculated at 2,055,000 tons, made up as follows: Winsford and District, 1,036,000 tons; Northwich and District, 880,000 tons; Middlewich and District, 21,000 tons; and the newly-developed Sandbach District, 118,000 tons. But while Winsford has surpassed her older competitors in the matter of salt production, Northwich is still the commercial centre of the industry and the greatest producer of brine; whereas, in the case of the other districts, the brine is54 converted into salt on the spot, the Northwich brine, to the amount of hundreds of millions of gallons annually, is pumped out of the neighbourhood through the Marbury pipe, to be employed in the chemical works of Brunner, Mond & Co., and be manufactured into salt at the Salt Union’s works at Weston Point.
Compared with the other salt-making centres, the record of Middlewich is of slight importance, and although the ancient town boasts an honourable place in the history of the Cheshire Wiches, it now takes a secondary position among the salt-producing districts.
Lawton, in the south-eastern corner of the Cheshire salt region, is a comparatively modern entrant into the local industry, for although the place is of historic importance as the scene of the discovery of the bottom bed of salt in 1779, white salt has only been manufactured there for something over 130 years. The deposits, which are found at a considerable height above sea-level, are of great but undefined magnitude, as the lowest strata has been bored through for a thickness of 72 feet, without penetrating the formation. The rock salt here was acknowledged to be purer than any previously encountered in Cheshire, and the brine derived therefrom, containing 26·100 chloride sodium by weight, yields on evaporation an exceptionally high class of white salt. The Commercial Salt Company, Ltd., which was formed to work the Hodgkinson Patent Salt-making Process, to which further reference must be made later, have their works at Lawton, where they are most conveniently situated in the important matters of transport and fuel, being on the canal which brings them nearer to the markets of the Midland Counties than any other salt works in the country, and obtaining their coal from workings within two miles of the property. The rock salt formation is so vast that the supply of55 brine, if not actually inexhaustible, will allow of an enormous production of salt for many generations to come. The output of white salt at Lawton for nearly a century and a half has not appreciably depleted the deposits and is not at present being drawn upon, as the Commercial Salt Company are pumping from an excellent “brine run” which is pumped without the damage to property and subsidence of land that have occurred in other parts of the Cheshire salt districts.
The chronicle of the salt industry of Winsford is one of the romances of commerce. Until the river Weaver was made navigable, the Winsford salt manufacture was limited to the output of only four pans of unrecorded dimensions, which were probably worked by Middlewich makers. In 1758, the first year in which the Winsford shippings were recorded separately, the export of white salt was 1,055 tons. By the end of the century, Winsford sent 44,384 tons down the river Weaver, and, in the year 1850, their shipments had increased to 324,249 tons. This output had risen in 1880 to 794,824 tons of white salt. In the ensuing ten years there was a slight increase, followed by a sharp decline (in 1890) to 501,548 tons, or a fall from the high-water mark of 834,306 tons in 1881, of no less than 332,758 tons. The decline in the Winsford make of salt was not arrested by the formation of the Salt Union in 1888, and ten years later the output of white salt had decreased to 403,455 tons, and the export of rock-salt from Winsford, which had recommenced with an output of 141 tons in 1856 and risen to 28,236 tons in 1886, ceased in 1898.
56
It has been said that De Re Metallica of Georgius Agricola, published in 1556, was regarded as the standard text-book on the subject for nearly two centuries, and in that long period the method he describes of salt-making by the artificial evaporation of brine underwent no material change. But from the last half of the seventeenth century, various attempts were made to effect improvements in the open-pan process in this country, and the history of these endeavours is set forth in a sequence of interesting publications. Among the most important of these is an article, which was printed in the Philosophical Transactions of the Royal Society of England, in 1669, in which Dr. William Jackson, in the form of a catechism, gives a number of particulars concerning the salt springs of Nantwich and the ways of salt-making as practised in that town. It appears from this account that each of the salt houses was still furnished with six leads, but one learns that this number of leads had, in the case of the majority of the salt-houses, been converted into four iron-pans, rather more than 3 ft. square by about 6 in. deep, and containing the same quantity of brine as was previously distributed among the six leads; while still more recently the four pans had been again changed into two larger pans, and some salt-makers had re-fashioned these two receptacles into one great pan. The description which Dr. Jackson gives of the process is so concise and lucid that it may be reproduced here without the alteration of a word. The question that he propounds to himself is—
57
“What is the manner of their (the salt-makers) work? or what time of boiling the salt water? Whether they use any peculiar thing to make it granulate, and, if so, what that is?”
In the course of his reply, he says: “They use for their fuel pitcoals brought out of Staffordshire. These pans are set upon iron bars, bricked in very close. They first fill their pans with brine out of the pit: which comes to them in several wooden gutters: then they put into their pans amongst the brine, a certain mixture, made of about 20 gallons of brine, and two quarts of calves’, cows’, and, chiefly, sheep’s blood. Of this mixture they put about 2 quarts into a pan that holds about 360 quarts of brine: this bloody brine at the first boiling of the pan brings up a scum which they are careful to skim off: they continue their fire as quick as they can till half the brine be wasted, and this they call boiling upon the fresh. But when it is half boiled away, they fill their pans again with new brine out of the ship (so they call a great cistern by their pan sides, into which their brine runs through the wooden gutters from the pump, that stands in the pit) then they put into the pan two quarts of the mixture following: they take a quart of white of eggs, beat them with as much brine, as before was done with the blood; and thus that which they call the whites is made. As soon as this is in, they boil sharply till the second scum arise: then scum it off as before, and boil very gently till it corne; to procure which, when part of the brine is wasted they put into each pan of the size aforesaid, about a quarter of a pint of the best and strongest ale they can get: this makes a momentary ebullition, which is soon over, and then they abate their fires yet not so but that they keep it boiling all over though gently: for the workmen say that if they boil fast here, it wastes their salt. After58 all their leach brine is in, they boil gently till a kind of scum comes on it like a thin ice: which is the first appearance of the salt: then that sinks and the brine everywhere gathers into cornes at the bottom to it, which they gently rake together with their loots, this they continue till there is but very little brine left in the pan: then with their loots they take it up, the brine dropping from it, and throw it into their barrows, which are cases made with flat cleft wickers, in the shape almost of a sugar loaf, the bottom uppermost. When the barrow is full they let it stand so for an hour and a half in the trough where it drains out all the leach brine, then they remove it into their hothouse behind their works made there by two tunnels under their pans, carried back for that purpose. The leach brine that runs from the barrows they put into the next boiling, for it is to their advantage being salt melted and wanting only hardening.
“This work is performed in two hours in the smaller pans, which are shallower, and generally boil their brine more away: wherefore their salt will last better, though it does not granulate so well, because when the brine is wasted, the fire and stirring breaks the cornes. But this salt weighs heavier and melts not so soon: and therefore is bought for many sales to a distance. But in the greater pans, which are usually deeper, they are above half an hour longer in boiling; but because they take their salt out of their brine, and only harden it in their hothouse, it is apter to melt away in a moist air: yet of this sort of salt the longer the grain is, the longer it endures: and generally this is the better granulated and the clearer, though the other be the whiter. And I think it is rather the taking of the salt out of the brine before it is wasted, that causes the granulating of it, than the ale, to which the workmen impute it.
60
“They never cover their pans at all, during the whole time of boiling. They have their houses like barns open up to the thatch with a cover-hole or two to vent the steam of the pans.”

On the subject of the supply and quality of the brine obtained at Nantwich and Middlewich, Dr. Jackson explains that the springs are rich or poor in a double sense, as a spring may be rich in salt but poor in the quantity of brine it affords. Thus, the chief pit at Middlewich contained a rich brine yielding a full fourth part of salt, but the supply was so meagre that the inhabitants were “limited to their proportions out of it,” and their requirements were made up out of pits furnishing a weaker brine. The pit at Nantwich was so plentiful as to supply all the salters, but while the brine contained only a sixth part of salt, “such quick use of it extremely strengthens the brine, perhaps to a degree little less than that of Middlewich pit.” In support of this statement that freshly drawn brine is richer than the liquor that has stood for some days in the pit, Dr. Jackson testified, as the result of personal experiment, that “a quart of brine, when the pit has been drawn off three or four days first, to supply five or six wich-houses, has yielded an ounce and a half more of salt than at another time, when it has had a rest of a week or thereabouts.”
In the Droitwich locality of Worcestershire, the quality of the brine closely resembled that of the Cheshire salt springs. In the account by Dr. Thomas Rastel, published in 1678 in the Philosophical Transactions, the writer says: “In the great pit at Upwich, we have at once three sorts of brine, which we call by the names of first-man, middle-man, and last-man, these sorts being of different strengths. The brine is drawn by a pump: that which is in the bottom is first61 pumped out; which is that we call first-man, etc. A quart measure of this brine weighs 29 ounces troy, but of distilled water only 24 ounces. This brine yields about a fourth part of salt; so that four tons of brine make about a ton of salt. The other two sorts less, or 28 ounces. And the pit yields 450 bushels of salt per day. In the best pit at Netherwich a quart of brine weighs 28 ounces and a half; this pit is 18 feet deep and 4 broad, and yields as much brine every 24 hours as makes about 40 bushels of salt. The worst pit at Netherwich is of the same breadth and depth as the former: a quart of brine out of which weighs 27 ounces and yields as much brine daily as makes about 30 bushels of salt.”
Although Dr. Rastel’s account of the salt-making methods in use at Droitwich coincides with that employed about the same period in Cheshire, he explains one or two minor variants and the reason of their adoption. “The vats we boil the brine in,” he writes, “are made of lead, cast into a flat plate 5 feet and a half long and 3 feet over: having the side and ends beaten up, and a little raised in the middle, which are set upon brickwork called ovens, in which is a grate to make the fire on, and an ash-hole which we call a trunk. In some seals are 6 of these pans, in some 5, some 4, some 3, some 2. In each of these pans is boiled at a time as much brine as makes 3 pecks of white salt. For clarifying the salt we should have little need, were it not for dust accidentally falling into the brine. The brine of itself being so clear that nothing can be clearer. For clarifying it, we use nothing but the whites of eggs, of which we take a quarter of a white, and put it into a gallon or two of brine, which being beaten with the hand, lathers as if it were soap, a small quantity of which froth put into each vat raises all the scum, the62 white of one egg clarifying 20 bushels of salt, by which means our salt is as white as anything can be: neither has it any ill savour, as that salt has that is clarified with blood. For granulating it we use nothing at all, for the brine is so strong of itself, that unless it be often stirred, it will make salt as large grained as bay-salt. I have boiled brine to a candy height, and it has produced clods of salt as clear as the clearest alum, like Isle of May salt: so that we are necessitated to put a small quantity of rosin into the brine, to make the grain of the salt small.”
“If it is asked why we use not iron pans as in Cheshire,” Dr. Rastel concludes, “I answer there have been trials made of both forged iron pans and cast iron. The former the strength of the brine so corrodes, that it quickly wears them out, the latter the brine breaks.”
The first serious attempt to effect a real improvement in the making of salt from brine was communicated to the Lords of the Admiralty in 1746 by Thomas Lowndes, and under the title of “Brine Salt Improved or the Method of Making Salt from Brine, that shall be as good or better than French Bay-Salt.” It was published in the same year in a handsomely printed, block-type brochure of 40 pages by S. Austin, of Newgate Street, Lowndes, who had spent his infancy in Middlewich and had acquired in his youth a thorough acquaintance with the Cheshire manner of salt-making, employed several years in travelling in France, during which he studied the process employed in the making of salt by solar evaporation from sea-water in the neighbourhood of Rochelle. At this time the bay-salt of Rochelle was regarded by merchants, victuallers, and fishermen as the best in Europe. He afterwards visited Holland for the purpose of ascertaining why the Dutch white herrings were superior to those cured in England, and63 he learned that the cause was explained by the method employed by the Dutch in purifying their salt. Armed with the knowledge he had acquired in France and Holland, and allowing for the difference between the French, Dutch, and English brines, Lowndes offered to enter into an agreement with the Admiralty to supply them with a better article than the French bay-salt, made by the following process—
“Let a Cheshire salt-pan (which commonly contains about eight hundred gallons) be filled with Brine, to within about an inch of the top; then make and light the fire; and when the Brine is just lukewarm, put in about an ounce of blood from the butcher’s, or the whites of two eggs; let the pan boil with all possible violence; as the scum rises take it off; when the fresh or watery part is pretty well decreased, throw into the pan the third part of a pint of new ale, or that quantity of bottoms of malt-drink; upon the Brine’s beginning to grain, throw into it the quantity of a small nutmeg of fresh butter; and when the liquor has sailed for about half an hour, that is, has produced a good deal of Salt, draw the pan, in other words, take out the Salt. By this time the fire will be greatly abated, and so will the heat of the liquor. Let no more fewel be thrown on the fire; but let the Brine gently cool, till one can just bear to put one’s hand into it; keep the Brine of that heat as near as possible; and when it has worked for some time, and is beginning to grain, throw in the quantity of a small nutmeg of fresh butter; and about two minutes after that, scatter throughout the pan, as equally as may be, an ounce and three quarters of clean common Allom pulverized very fine; and then instantly, with the common iron-scrape-pan stir the Brine very briskly in every part of the pan, for about a minute; then let the pan settle, and constantly feed the fire, so that the Brine may never be quite scalding hot, nor near so64 cold as lukewarm; let the pan stand working thus, for about three days and nights, and then draw it.
“The Brine remaining will by this time be so cold, that it will not work at all; therefore fresh Coals must be thrown upon the fire, and the Brine must boil for about half an hour, but not near so violently as before the first drawing; then, with the usual instrument, take out such Salt as is beginning to fall, (as they term it) and put it apart; now let the pan settle and cool. When the Brine becomes no hotter, than one can just bear to put ones hand into it, proceed in all respects as before; only let the quantity of Allom not exceed an ounce and a quarter. And in about eight and forty hours after draw the pan. ”
This process, as will be seen, involved the use of much slower fires than were usually employed in Cheshire, and allowed the liquor to simmer instead of boiling for a longer period. For this purpose, Mr. Lowndes proposed to use a large proportion of cinders in his furnaces, “since long boiling with great fires not only deprives salt of its spirit and strength, but causes its grain to become loose and soft, since cinders are better than coals in preserving a constant, equal, and gentle heat.” In order to correct the ill-effects suffered by the salt through being made in an enclosed, intensely hot room, filled with steam and smoke, he had recourse to the use of alum, which, he claimed, would restore to the salt its “natural cubical shoot and give it a proper hardness.” He further claimed that by this process the hot-houses or drying-houses could be dispensed with, waste in carriage would be avoided, and the pans would last three times as long; while, in order to anticipate the inevitable objections of the salt-makers and dispel the pretended difficulties that the workmen would find in executing his directions, the65 inventor explained that he had been careful to accommodate his process, as near as possible, “to the present practice in Cheshire.”
At the request of the Admiralty, the College of Physicians conducted several examinations of salt made by the Lowndes process, and reported that it was “in all respects, a strong and pure salt, equal at least, if not preferable to any we are acquainted with.” On the strength of this testimonial, Mr. Lowndes applied to the Admiralty to allow him a six months’ trial to prove the goodness of his salt for domestic purposes, twelve months to prove its excellence for the purpose of the Fishery of America, and two years in which to prove its efficacy in preserving beef and pork for the Royal Navy. If in this series of tests it should be proved that salt made by his process equalled French bay-salt, he proposed that they should pay him a total sum of £7,000, and should the trials demonstrate the slightest inferiority, he would be content to make his country a present of his labours. When the Admiralty declined to enter into negotiations with him, Mr. Lowndes laid his scheme before the House of Commons, which petitioned the King to instruct the Admiralty to make the tests on the inventor’s terms, but the sudden death of Lowndes in 1748 closed the controversy.
But the determination to bring the art of salt-making to “greater perfection” was not abandoned, although, as Dr. William Brownrigg admitted, the success achieved by Thomas Lowndes was thought by some people “to supersede the necessity of any further attempts for improving or extending our salt manufacture.” Brownrigg commended Lowndes’s method and testified to the purity and strength of his salt which had been exhibited before the College of Physicians, but he maintained that by other methods a purer and stronger salt might66 be made at a less expense. In point of fact, Dr. Brownrigg’s objection to the Lowndes’ method was that it was applied only to salt made from brine, or a solution of English rock-salt often prepared with impure water, and that the salt so produced, in his opinion, was inferior to marine salt. Brownrigg, only half realizing Lowndes’s intention, would appear to have grasped the fact that his process aimed at economy of fuel combined with uniformity in the degree and distribution of heat, but he does not seem to have appreciated the value of the improvement anticipated therefrom.
It must by this time have become evident to scientific investigators and practical salt-men that the solution of the problems of economical manufacture and increased output lay in the application and regulation of heat. Christopher Chrysel, of Leipsic, after fourteen years of “great industry, much pains, and cost” spent in the practice and study of salt-making in Cheshire, published the result of his labours in 1787. Chrysel claimed that by his method “with the least Fire and Coal the most Salt can be made and the greatest Profit received such as in no other way can possibly happen,” but curiously enough the improvement for which he obtained a Royal Patent, was primarily based upon a more advantageous arrangement of the brine pans, while the improvement effected in the furnace was treated as a matter of only subsidiary importance. Chrysel demonstrated his method at Bye Flat, near Northwich, in Cheshire, in June, 1776. The experiment was carried out in the presence of witnesses, the same pan was used in testing both the old and the new methods, the same two salt-boilers were employed in conducting both operations, and the amounts of fuel consumed and salt produced were carefully weighed and attested. The results were recorded in the following report furnished by68 the Liverpool Agent of Mr. Richard Kent’s salt-works at Bye Flat—

“In three ‘firings’ of 2 Furnaces under a salt pan set up on the old plan ten years ago and constantly worked till the present time—24 feet long: 15 feet broad and 12 inches deep—filled with Brine three times in a half week, and boiled down each time in 24 hours and the salt drawn out there was burnt 5½ tons of Coal and made 7 tons 31 bushels, or 155½ cwts. of salt.
“After the experiment the Patentee, Mr. Christopher Chrysel, set up the same pan on his improved Patent Method, and then in three similar firings in half a week as before there was only burnt 3 tons 5 cwt. of coal and made 8 tons 2 cwts. or 162 cwts. of salt=2 tons 10 cwts. of salt per ton of fuel.”
Chrysel says in his treatise that the pan mentioned in his experiments—24 ft. long, 15 ft. broad, and 12 in. deep—will be regarded by his German readers as of phenomenal bigness, but he explains that in England it is looked upon as only a medium-sized receptacle. The pans in use in Cheshire at this period were of various sizes, but the tendency was to introduce pans of increasing dimensions. “Indeed I can with all truth say,” he writes, “that in England I have seen with my own eyes, pans two, three and four times as big (as the one he used at Bye Flat) and have measured them with my own hands, and have proved each one designedly and have seen and marked and become persuaded that from large salt pans the greater advantage and the most noted cheapness in the manufacture of salt depend and proceed.”
In the course of his experiments with pans of all sizes, he proved that in a small pan, 8 ft. square and 9 in. deep, heated with one furnace, he obtained in69 five weeks a clear profit of £35 15s. 2d., while in one pan, compounded out of five of the small pans, and heated with two furnaces, the profit of one week’s working was £42 15s. 5d., or a net additional profit of £7 0s. 3d., and the saving of four weeks in time and labour.
He further experimented with three of the largest pans for one week, with the following results—
“The first—36 by 25 feet and 13 inches deep holding 975 cubic feet of Brine—burnt in 3 Furnaces in one week 12 tons of coal and made 32 tons 2 cwts. of salt.
“The second—40 by 27 ft. and 13 inches deep holding 1170 cubic feet of brine—burnt in 3 Furnaces in a week 15 tons 18 cwts. of coal and made 34½ tons of salt.
“The third—52 by 26 feet and 13 inches deep holding 1464 cubic feet of Brine—burnt in 4 Furnaces in one week, 24 tons of coal and made 62 tons of salt.”
Chrysel is himself amazed that pans containing 360, 900, and even 1,400 cubic feet of brine can be boiled into salt in the same space of time, and he is feign to admit that “up to now, nobody, to my knowledge, has proved what length, breadth, and depth of pan is calculated to make the most salt with the least consumption of coal. Consequently everywhere are to be found many different pans, and other varieties are continually being tested. And I myself cannot feel that I am capable of deciding the question, nevertheless I will, from my experience and conscientious conviction, say what I consider is the best, cheapest, and most reliable pan for this purpose.”
After long search, and close inquiry in numerous salt-works, and as the result of his study of salt-making in pans of every size, Chrysel came to the conclusion70 that a single pan—“26 feet long, 18 feet broad, and 12 inches deep, with two furnaces, in a roomy salt-works with sufficient room for the workmen and baskets on both sides of the pan”—was to be preferred to all others. But this considered judgment was amended after further application to the problem by advocating an increase in length without changing the breadth of the pan. His ultimate verdict was in favour of a pan 52 ft. long, 18 ft. broad, and 1 ft. deep, with a capacity of 936 cubic feet of brine, equipped with two furnaces, and he declared that this pan, producing about 638 cwt. of salt per week, at a cost of £10 5s. 6d. for fuel, and selling for £127 15s. 6d., and giving a profit of £117 10s. was “the perfect article.”
Although, as I have pointed out, Chrysel’s patent was principally concerned with the arrangement of the brine pans, which were so arranged as to obtain the maximum amount of heat from the fuel consumed in the furnaces, in the course of his experiments he evolved an improved, if by no means a perfect, furnace. The peculiar nature of the superiority effected was based on the common knowledge that it is the natural tendency of fire heat and smoke to escape into the open air and disappear. He proceeds: “If, however, they are confined and shut up in a furnace under a salt pan they still require an opening to escape to the chimney else the fire cannot burn and is extinguished. If however the opening and place of exit into the draughts and chimney is too large and wide, as it is generally, and particularly under salt pans, not only will the draught of Air cause Wood and Coal to be more rapidly consumed and changed into Ashes which will choke the fire but also the fireheat and smoke will, by the draught of the air, hasten into the draughts and chimney, and the bottom of the pan will hardly be touched and71 scarcely half the work be done. On the contrary, if the opening and exit into the draughts and chimney has a proper proportion, according to the different sizes of the Pans and to the requisite Fire in the Furnace under the pan, the Fireheat and Smoke will be longer contained under the pan and that, steadily coming from the Furnace, will be increased and strengthened, so that double work under the pan will result, and wood or coal will not so rapidly be burnt to ashes but last longer and consequently do more work. All that is required in this is to calculate the mathematical proportion between the different sizes of the pans, the Furnace and the Fires and between the opening and Exit into the draughts and chimney, and to apply it.”
It will be recognized that both Lowndes and Chrysel were on the way to the solution of the problem of the perfect salt-making plant when they devoted themselves to the improvement of the furnace, but another century and a half was to elapse before the secret that eluded their efforts should be revealed. The luckless Furnival, some fifty years later than Chrysel, approached nearly to the goal to which they were all striving, and he, in common with his forerunners, had his share of the savage jealousy and persecution that the salt-men have ever visited upon those who venture into the lists with them. “No malice has been wanting to bring a disreputation upon my salt; and every wicked art will be practised to render its virtues ineffectual. The Salt Commissioners are my avowed enemies; for the miscarrying of my attempts will be their gain.” Thus wrote poor Lowndes, and Chrysel had similar grounds for complaint. “Before the above proof (the result of his experiment at Bye Fleet) was made openly, nobody believed in the anticipated saving,” he says, “but everybody doubted and some declared it to be72 impossible. After, however, the thing was made known, everybody on the contrary was in a state of wonderment. In a short time wonder was changed into envy, ill-will and malice, and many attempts were made to suppress me and destroy my patent, although it was not possible for any one to point out any failures or errors.” We shall see presently how the salt-men dealt with their successor, William Furnival.
Henry Holland, writing in 1808 on “The Production of Salt Brine,” furnishes some reliable details concerning the manufacture of brine-salt as it was conducted in Cheshire at the beginning of the nineteenth century. According to this authority: “The pans used in Cheshire, for the evaporating of the brine, are now made of wrought iron. The dimensions of these vary very much; but, in general, those of modern erection are considerably larger than what were in use a few years ago; and they usually contain from 600 to 800 superficial feet. One or two pans of still larger dimensions have been erected, each containing nearly 1,000 feet. Their usual form is that of an oblong square, and their depth from 12 to 16 inches. To a pan containing 600 to 800 superficial feet, there are usually three furnaces, from six and a half to seven feet long, and 20 to 24 inches wide. The grates are from two and a half to three feet from the bottom of the pan. The furnace-doors are single, and there are no doors to the ash-pits.
“The different pans are usually partitioned out from each other, and there is a separate pan-house to each pan. Within this pan-house, at one end is the coal-hole; the chimney occupies the other end, there is a walk along the two remaining sides of the pan, five or six feet wide; and between these walks and the sides of the pan-house, which are generally of wood, long benches four or five feet wide, are fixed, on which the salt is73 placed in conical baskets to drain after it has been taken out of the pan; a wooden or slated roof is placed over the pan-house, with louvres to allow the steam to pass freely out.
“The manufacture is conducted in several different ways, or rather heat is applied in various degrees, to effect the evaporation of the water of solution; and according to these different degrees of heat, the product is the stoved or lump salt; common salt; the large grained flaky; and large grained or fishery salt.”
In the making of stoved salt, the brine was brought to a boiling heat—which in brine fully saturated is 226 degrees of Fahrenheit—and the pan was twice filled in the course of twenty-four hours. In the making of common salt, the brine was first brought to boiling heat, for the double purpose of expediting saturation and clearing the brine of any earthy contents, and then, moderating the fires, the process of crystallization was completed with the brine heated to 160 or 170 degrees of Fahrenheit. The pan in which common salt was made was filled only once in twenty-four hours. The large grained, flaky salt was made with an evaporation conducted at the heat of 130 or 140 degrees, and the pan was filled once in every forty-eight hours; while in the case of fishery salt, the brine was brought to a heat of from 100 to 110 degrees of Fahrenheit, and five or six days were required to evaporate the water of solution. In the course of these several processes, various additions were often made to the brine, with the view of promoting the separation of any earthy mixture, or the more ready crystallization of the salt. These additions varied in different works, and many of them seem to have been made from ill-founded prejudices without any exact idea as to their probable effects. The principal additions made at various times74 were acids, animal jelly and gluten, vegetable mucilage, new or stale ale, wheat-flour, resin, butter, and alum.
Holland believed that the addition of acids to the brine was an innovation based upon the mistaken idea that the use of acid accounted for the superiority of the Dutch salt, but at the time at which he wrote the practice had been discontinued in Cheshire. Animal jelly and gluten for clearing the brine and promoting the separation of the earthy contents, were much used in preference to blood, which, while excellent for the purpose when fresh, was difficult to procure in sufficient quantity and to preserve from putrefaction. White of eggs, glue, and jelly procured by boiling cows’ and calves’ feet, were also found to answer perfectly well for the purpose of clarifying brine, but the use of new or stale ale and beer grounds as a brine clarifier, had been abandoned as inefficacious by Cheshire salt-men. Dr. Brownrigg was of opinion that salt-boilers had little to plead in favour of the addition of butter during the evaporation process, beyond immemorial custom, but Holland considered the salt-makers had ample grounds for their belief that butter assisted the granulation of the salt and made the brine “work more kindly.” On the question of the addition of alum opinions varied. Lowndes ascribed the superiority of his salt to the use of alum, but Brownrigg declared that “the goodness of Mr. Lowndes’ salt does not seem to be owing to the alum with which it is mixed, but may be attributed chiefly to the gentle heat used in the preparation.”

Holland combated the general impression obtaining at the time, that salt formed from the same brine varied by the application of different degrees of heat, not only in external appearance but also in quality, and the equally prevalent idea that salt formed from76 natural brine was inferior in its power of preserving animal flesh to bay-salt. He proved by quotation and experiment that such prejudices were entirely unfounded, and proceeded to show that the action of bay-salt is exactly similar to that of the large-grained salt, and that neither variety has any advantage over the salt prepared by a boiling heat except in the size and compactness of its crystals and in its containing a somewhat smaller proportion of the water of crystallization; and as the large-grained fishery salt is more than equal to the bay salt in these important points, it at least equals the latter in its power of preserving animal flesh or provisions.
The first person who introduced steam heat into the manufacture of salt, and, in so doing, anticipated the revolutionary improvements which were achieved some three-quarters of a century later by the Vacuum System and the Hodgkinson Patent Salt-Making Process, was William Furnival. For our knowledge of the intentions and achievements of this bold and persevering innovator we have to rely almost entirely upon his “Statement of Facts, Humbley and Respectfully submitted to the Consideration of His Majesty, His Majesty’s Ministers, and Both Houses of Parliament.” In this document we have a story of oppression, conspiracy, and persecution which the author describes as “unparalleled in free England,” and since his narration of the treatment he endured has never been refuted, we must conclude that the gist of what he writes is substantially true. It is to be regretted that in this only available account of his activities, Furnival is so intent upon exposing the wrongs to which he had been subjected that he omits to furnish us with a detailed description of his process. We know that in 1823 Furnival erected works at Droitwich and commenced making salt, and we have77 his assurance that his patent answered every expectation he had formed of it. Moreover, its working was investigated by Messrs. S. Fowler, Fardon & Co., who, on 17th April, 1824, certified that the advantages of the Furnival method over all existing processes, consisted—
“Firstly.—In the saving of fuel which may be stated at about one-half.
“Secondly.—In the production of twice the quantity of salt, as usually made in vessels of the same size, in a given space of time.
“Thirdly.—In the superior quality of the salt, arising out of the regular distribution of heat to the bottom of the brine pan.”
In April, 1825, Furnival disposed of his salt property at Anderton, and three years later, to a month, he bought property at Marston for £1,550. On this ground he erected works covering an area of about twelve acres, and installed some three miles of pannage at a cost of upwards of £135,000, capable of producing some 130,000 tons of salt per annum. He subsequently bought and started to erect works intended, when finished, to occupy nearly six acres of ground at Marston. He asserted that these Wharton and Marston properties were the only two in the kingdom possessing the peculiar advantages of inexhaustible supplies of fully saturated brine and dry rock-salt on the same premises, and he claimed that he could not only deliver rock-salt at fully 25 to 30 per cent. less than any other mine in the country, but, further, that the salt made on his principle was admitted to be superior in quality, owing to the regular distribution of heat, by which more uniform and superior crystals were produced. In the autumn of 1829, he opened negotiations in two separate quarters to lease on royalty certain portions of his salt-works78 at Wharton, and two committees, each consisting of three men, were appointed by the prospective tenants to investigate the system. On 22nd August, 1829, the two committees drew up a joint report, from which I extract the following—
“The first committee entered upon the investigation on the 15th August, 1829; remained on duty eight hours; was then relieved by the second for the like period, and so continued the investigation, alternately superintending the weighing and delivery of the coals and salt, and taking note of the temperatures every hour.
“The following is the result of working for 162 hours, a steam boiler, constantly fed with brine, the specific gravity from 23 to 25·100ths.
| Length of the boiler, 20 ft.; width, 8 ft. | |
| A triangular flue pan, 80 ft.; width, 8 ft. | |
| A triangular steam pan, 101·6 in.; width, 8 ft. forming a surface of 1,612 superficial ft. of brine. | |
| The quantity of coal consumed was 8½ tons. | |
| The quantity of fine salt produced was | 356 cwt. |
| Ditto of common and fishery | 404 „ |
| Making | 760 cwt. |
“Being a product of four and a half tons of salt for every ton of coals consumed.”
It will be convenient here to explain what became of these several Furnival properties, and then describe very briefly the stages which led to the inventor’s incarceration in Horsemonger Lane Gaol and caused him to address his Statement of Facts to the Government. In April, 1825, he sold his works at Anderton to the British Rock and Salt Company, which continued to ship salt until 1829. The Marston property appears to have been worked until 1847. The Wharton works were managed by Trustees until 1839, when they were taken over by the National Patent Salt Company,79 which became one of the most important firms in the Winsford trade. In 1875, Justice Manisty, the surviving leaseholder, transferred his interests to Stubbs Brothers, who, in 1888, disposed of the business to the Salt Union.
Furnival had no sooner established himself as a salt-maker at Anderton than the old salt proprietors, “who had contrived in the past to ruin all, or any one who should dare to enter the lists against them,” became seriously alarmed at the apparent magnitude of his plans and the great improvements which threatened both their exorbitant profits and their hitherto unchallenged monopoly. Furnival, to employ a colloquialism, proceeded to “back himself both ways.” Having more than once proved his strength by breaking up “the Coalition” (which the old proprietors had formed to regulate the output and price of salt), and bringing down the price of common salt from 20s. to 8s. per ton, he offered to erect his patent apparatus at his own cost and risk on the works of his wealthy rivals, and to allow them two-thirds of the saving effected by its application. According to Furnival’s unsupported but uncontradicted account, they ridiculed his offer, declaring that they wanted none of his patents, that they could command their own profits in defiance of him, and that they would never sanction any improvements or innovations in the trade.
Furnival, who had secured patents for France and the Netherlands, thereafter gave his English competitors a rest and proceeded to erect salt-works at Rotterdam and Ghent capable of taking nearly 60,000 tons of rock salt annually from the British market. But the English salt refiners prompted their Dutch and Belgian confrères to bring official discredit upon the enterprise, and Furnival and his partner were compelled80 to abandon their works, which had cost them £13,000 to erect, and to forgo the £33,000 they were to have received for their patent rights.
Furnival returned to England and set up his salt-works at Wharton, where he “produced some of the finest rock-salt in the kingdom.” The old proprietors decided that no sacrifice was too great that would have the effect of crushing this competitor. They lowered the price of rock-salt 50 per cent., and kept manufactured salt so low that every establishment was worked at a loss. A meeting of proprietors, convened to consider the situation, resolved that while “they deeply lamented the low price of salt, they considered, at the same time, that it would not be prudent to raise the price until Mr. Furnival was disposed of.” The salt manufacturers admitted, in a circular published in 1829, that this cutting-out operation had, in four and a half years, involved the trade in a loss of £282,194 14s., but it had the effect of frightening away Furnival’s financial supporters, and landed him in further misfortunes.

In 1826, Furnival had entered into a contract with a Peter Bouvain to erect a salt-works in the Isle of Rhé, but the month that was required to prove the capabilities of the patent plant was sufficient to demonstrate the commercial worthlessness of the Frenchman, and Furnival cut his loss and returned to England. Bouvain brought a claim against him for the loss of prospective profits and obtained a judgment for £8,000, against which Furnival appealed to the Court of Cassation in Paris. Before the case was heard, Furnival was inveigled to the Netherlands by a forged invitation, purporting to come from a wealthy Belgian salt-refiner, driven over the frontier, arrested in France at the suit of Bouvain, and thrown into gaol. Finding that82 legal redress was unobtainable, Furnival escaped from prison after four months’ incarceration, and in January, 1830, was again in Cheshire. He was engaged in a bitter and protracted altercation with his two sets of tenants in the Wharton salt-works in August, 1832, when he was arrested for the non-payment of his debt of £8,180 to Bouvain, and lodged in Horsemonger Lane Gaol. In January, 1833, he brought an action for perjury against Bouvain, who fled to France to escape the warrant that was issued for his arrest, but this moral victory brought Furnival neither release nor amelioration of his lot, and he found himself “foully and unjustly charged by a band of conspirators, defeated in every attempt to obtain justice, and left without a hope or prospect of being able to vindicate himself, or extricate himself from a confinement more close than that awarded to a felon.” The end of Furnival need not occupy us; he came into the salt trade in 1822 with a sufficiency of financial backing, an unusual stock of confidence and energy, and a patent which “created a sensation through the whole salt trade”; we take our leave of him eleven years later in a debtors’ gaol—a victim to the methods which the Cheshire salt proprietors invariably adopted in ridding themselves of an obtrusive competitor.
83
The theories propounded and the conclusions arrived at on the subject of the formation of the Cheshire salt beds do not differ in any important particular from those which have been put forward, investigated, and accepted with regard to rock-salt deposits in all parts of the world, but, because of the enormous geologic and climatic changes that have occurred in the English county since a salt basin was in course of formation there, scientists were slower in accepting those conclusions in respect of our home deposits than in the case of the salt areas which are found in the Runn of Kutch, at Lake Elton, or Black Gulph on the eastern side of the Caspian Sea.
The facts that the chief accompaniment of every known deposit of rock-salt is clay, and that clay is deposited in water, formed the basis of the erroneous theory that because salt is a deposit out of water, and sea-water contains salt, all salt beds must have been deposited in the sea. But salt does not mix mechanically with water and has not been deposited like sedimentary rocks; it forms a solution, and not until the solution becomes super-saturated does it crystallize out. Now sea-water rarely contains more than 3½ per cent. of salt, and since the solution must contain at least 26 per cent. of salt before the salt will crystallize out, and, provided it is left from contact with the air, a solution of this strength may be left for an indefinite length of time without a single particle of salt depositing, the old theory that all salt beds were deposited in the sea had to be abandoned.
84
The theory of the sea-water deposition of salt beds having been disposed of, it was long a popular idea that the beds of rock-salt owed their formation to volcanic action. Professor C. Thompson was of opinion that some enormous electrical force had been at work in its crystallization; Professor Silvestri found quantities of chloride of sodium varying from 50 to 90 per cent. in different sublimations in the lava which was erupted from Etna in 1863; Bunsen discovered a considerable but less important sublimation of chloride of sodium in the lava erupted from Hekla in 1854; and G. F. Rodwell and H. M. Elder also recognized small traces of sodic chloride as one of the products of volcanic action. In a paper contributed to the Manchester Geological Society, in 1842, on “An Inquiry into the Origin of the Salt Field of Cheshire,” so respected an authority as Ormerod stated his conclusions as follows—
“(a) That from the lithological character of the accompanying beds and partings, and from the regularity in the thickness of the respective beds, as far as the same were now known, these salt beds were, in his opinion, deposited from an aqueous menstruum, and had not been injected.
“(b) That from the absence of marine remains, from the salt deposits containing matter not found in the ocean, and from similar beds of salt not being in any place known to have been formed from the ocean, he considered that there were not satisfactory reasons for ascribing the origin of the salt found in the new red sandstone of England to marine deposits.
“(c) That from the minerals found associated with the salt, and adjoining red sandstone rocks, being similar to those found together with it in volcanic districts in other parts of the world; that from former or present volcanic action being apparent at localities85 in various parts of the globe, at which beds of salt of similar character are found, and the origin of which can be evidently traced to that cause, and from the salt beds in England being always found accompanied by neighbouring traces of volcanic action, he considered that there were satisfactory reasons for ascribing the origin of the salt fields of England to volcanic agency.”
Ormerod was not only convinced that the Cheshire deposits were the result of volcanic action which had impregnated neighbouring lagoons and formed the aqueous menstruum from which those beds were precipitated, but that these lakes lay in depressions of the upper New Red Sandstone, and that the alternation of the strata of rock and salt had arisen from subsidences, followed or accompanied by fresh discharges of the same impregnating matter.
This theory is untenable, for beyond the fact that salt has been ejected in volcanic eruptions there is practically nothing to support it. Volcanic action is always accompanied by intense heat, and the fact that the pure rock crystal is one of slow growth in a cool liquid, and is not of rapid formation in a hot fluid, conclusively disposes of the volcanic theory. Particles of chloride of sodium in volcanic ejections were no explanation of the formation of huge deposits of rock-salt, and since it was realized that salt in large quantity can only be obtained from salt water, and that it cannot be got naturally from the sea, it became evident that what man does in isolating tracts of sea-water to produce salt by solar evaporation, must have been practised by nature on an extensive scale in all ages. And as an isolated tract of salt water is a salt lake, we are directed to the obvious conclusion that all rock-salt formations have been deposited in salt lakes.
In support of this theory we have the evidence of86 the salt-forming process that is now in operation in Southern Russia, America, and India. It is evident that at one time the low-lying country to the west and north of the Caspian Sea was part of that inland sea, and that, when its surface was contracted by shrinkage, the retreating water left behind it numerous swamps, which now form salt lakes, and tracts of intervening land which, in the dry season, are covered with a saline afflorescence. The large quantities of salt which, in ordinary seasons are deposited in these salt lakes, are collected by the Russian Government. In India there are many salt lakes, such as Lake Sambhur, in Rajpootana, which in the rainy season has a length of from fifteen to twenty miles, but in the dry season is only three or four miles long, the remainder of its course consisting of a succession of small salt pools alternating with stretches of salt-encrusted ground. In the great desert of Mongolia many square miles of country are spread with salt incrustations; and in America similar tracts are found which once formed the beds of considerable lakes. In Nevada, at the sink of the Carson River, is an area of five square miles which was once the bed of a salt lake. The famous Great Salt Lake, between the Wahsatch Mountains and the Nevadas in America, is the remains of a large inland sea which once covered the district, and should the climate become drier than it is now, the shrinkage, which went on for ages, will be resumed, and a huge salt deposit will be formed.
The salt lakes in rainless districts soon dry up, and the salt, being quickly deposited, is almost pure, but such instances are not usual, and, in dealing with existing salt-depositing lakes, we find continual references to the salt and clay mixtures, or alternations of the deposits. Herr Cech tells us that the yearly layers87 of salt in Lake Elton are separated from one another by a layer of black mud; beneath the fourth layer is found black clay, and beneath this are further layers of salt of a more solid quality. Schleiden, in speaking of Lake Elton, says: “On this old salt is deposited a blackish mud layer (salt clay) which separates the salt from the next succeeding layer. In 1805 Göbel bored, in the very shallow lake, about 1½ miles from the shore. He found forty-two distinctly separated layers of rock salt, the uppermost from 1 to 4 inches thick, the lowest 9 inches thick. The deeper he bored the more solid the salt was, and the more pure. At the hundredth layer the salt was so hard that the iron tool broke.”
From the foregoing, which are among a great collection of accepted data, it will be seen that, in whatever quarter of the world salt lakes occur, the same characteristics are encountered, viz., salt depositing on mud and covered by mud. Every shower of rain creates a certain amount of mud or sand, and every brook and stream running into the salt lakes during the rainy season brings in a certain quantity of the same material. The mud represents the wet season of the year, and the salt the dry season. The geological conditions must have been the same when salt was deposited in Cheshire, and with the instances of modern salt-forming regions before us, and the strata of the Cheshire salt country to guide us, it must be concluded that the genesis of rock-salt, modified by local circumstances, must have been the same in every case. Indeed, in the face of the evidence, it seems certain that the Cheshire beds of rock-salt have been crystallized out of the saturated waters of salt lakes, and that their admixture of marl has been caused by streams running into the lakes during the wet seasons, and that the peculiar amorphous mixture of marl and salt known as rock-salt is the88 result of the continual growth of pure salt crystals, and their partial destruction by mud-bearing fresh waters.
This conclusion on the subject, which is now generally accepted, is based on the theory that the Cheshire salt lake was situated in a desert, or more probably a salty steppe , such as are found in the region of the Caspian Sea, and that the climate was divided into wet and dry seasons. The presence of rock-salt supports these ideas, because the marls could only be formed in periods of heavy rainfall, and the salt could only crystallize out in dry, water-evaporating periods. It is further evident that the lake, though extensive in area, was shallow, and that the dry seasons produced extensive shrinkages and caused salt to form in the saturated water that remained in the deeper parts, while the occurrence of the deep deposits in a shallow lake is explained by the constantly varying elevation and depression of the earth’s surface. The difficulty of explaining how the salt in this lake could be renewed to enable the waters to go on depositing for a geologic age is recognized, but it is no greater than that which is presented by scores of existing salt lakes out of which thousands of tons of salt are taken annually without causing any apparent diminution in the salt which forms year by year. And when it is considered that, in a lake having a probable area of from 500 to 1,000 square miles, the known salt deposits do not occupy 50 square miles, and in many portions contain 50 per cent. of marl, the difficulty does not seem to be insuperable. It is, moreover, safe to conclude that, when the bar rose that eventually cut off the Cheshire lake from the sea, it would be many years before the high tides ceased to wash over it and replenish the lake, and Dr. Ball’s theory as to the enormity of the tides that occurred in past ages—owing to the moon being nearer to the90 earth than at present—reveals a means by which the lake might continue to receive fresh accessions of sea-water for many generations.

Irrespective of all theories, the outstanding fact remains that enormous beds of salt were deposited in the Cheshire salt lake, and an examination of the strata in the appended Northwich section will enable the salt to tell its own history.
| Depth. | Thickness. | |||
| Ft. | in. | Ft. | in. | |
| 1 | 6 | 1 | 6 | Soil. |
| 9 | 0 | 7 | 6 | Drift composed of brown sand mixed with clay varying from 1 to 100 ft. in thickness. |
| 27 | 0 | 18 | 0 | Brown clay with greenstone, etc., boulders. |
| 132 | 0 | 105 | 0 | Marl in thin bands, brown and blue with thin beds and streaks of gypsum to the rock head. |
| 216 | 0 | 84 | 0 | Rock-salt, top bed. |
| 222 | 0 | 6 | 0 | Upper blue marlstone mixed with brown, which falls on exposure. |
| 229 | 0 | 7 | 0 | Brown marl and marlstone, with vein of red rock-salt. |
| 234 | 0 | 5 | 0 | Lower blue marlstone, very compact, hard, and does not fall on exposure. (This forms the foundation for the wedging-curb of the shaft cylinders.) |
| 246 | 0 | 12 | 0 | Marl and rock-salt mixed in about equal parts. |
| 330 | 0 | 84 | 0 | Rock-salt, bottom bed. |
| 334 | 0 | 4 | 0 | Brown and blue marlstone, with rock-salt. |
| 417 | 0 | 83 | 0 | Ditto with thin veins of rock-salt, ramifying in various directions. |
| 320 | 0 | 3 | 0 | Rock-salt, almost pure. |
| 501 | 0 | 81 | 0 | Brown and blue marlstone, with thin veins of rock-salt. |
| 507 | 0 | 6 | 0 | Rock-salt, almost transparent. |
| 525 | 0 | 18 | 0 | Hard blue marlstone, not sunk through. |
The formation has only been bored through to a depth of 525 ft., where we find an unpierced stratum, 18 ft. thick, of hard marl. Above it are 6 ft. of pure rock-salt, then 81 ft., of marl with thick veins of rock-salt, then 3 ft. of nearly pure salt, then 83 ft. of marl91 with thin veins of salt, and above it 4 ft. of marl and salt. So far it is evident that the wet seasons predominated, and that marl was deposited far more extensively than salt. For a time, a cycle of dry seasons prevailed; a great change occurred, and a bed of rock-salt, 84 ft. in thickness, was deposited. In other parts, the bed of rock-salt varies from 80 ft. to over 100 ft. in thickness, none of which is perfectly pure, and not more than 20 ft. of it is sufficiently pure to be of commercial use. The greatly changed seasons are indicated by these formations. A portion near the bottom, containing less clay, shows a less copious or less protracted rainfall, and these periods were followed by wet seasons and the presence of much clay. After a time, so much rain fell that for a period sufficiently long for about 30 ft. of marl to deposit, practically no salt formed. Here and there in this deposit are veins of salt, and as these are perpendicular and run as if deposited in rifts or cracks of the marl, the salt doubtless belongs to the next period, when another change occurred and another bed of salt, varying from 50 to 80 ft. in thickness, was deposited. The whole of this bed is fairly full of marl, and, for an untold period, marls were deposited, covering up the rock-salt.
The cycles of greater or less rainfalls are traceable in the varying preponderance of marls, in the crystallization of salt, and in the form in which the rock-salt is found. Each minute cube starts as a crystal from some independent point of rock salt, and these increase in numbers until they form a mass of crystallization possessing no distinct lines or features. Had the dry season continued for a long period a thick mass of rock-salt would have been formed. The floor of the lake would have been covered with salt crystals, like the crystal floor of a mine, and the moment the rainy92 season commenced, and the brooks began to bring in fresh water and mud, these crystals, being attacked by non-saturated water, immediately lost their sharp angles and became covered with a fine layer of mud. As soon as the crystals became completely covered they ceased to dissolve, but the angles and cubes disappeared, and a shapeless mass of mixed salt and mud was formed. With the next dry season, crystallization again set in and another crystal floor was produced, to be again destroyed by the succeeding wet season. This constant growth and destruction of crystals went on for ages, until the salt beds were formed and the water ceased to become super-saturated.
Scientific exploration work and a great number of borings have enabled us to form a fairly accurate estimate of the area of the Cheshire salt-beds, except in the region to the north of the deposits, where systematic examination has still to be undertaken. Without quoting the exact locations of bore-holes and distances between them—particulars which would convey little or nothing to the general reader—it may be broadly stated that the proved salt area in the Northwich district is about four square miles, while the increasing quantity of marl that is mixed with the salt to the northward favours the probability that the beds soon die out in that direction. The Winsford salt district comprises an area of six square miles, while it is calculated, with less preciseness, that the Middlewich, Nantwich, and Lawton districts all contain large quantities of rock-salt. At the bore-hole at Marston, which appears to be on the highest proved portion of the salt-bed, the salt is found at 47 ft. below ordnance datum, and from this central point the surface of the salt falls away gently in every direction. Mr. James Thompson, a recognized local authority upon salt and94 salt-mining, writing on the subject nearly fifty years ago, gave the thickness of the upper bed of rock-salt at about twenty-five yards, but that thickness was only maintained within a circle of about three miles in circumference, beyond which he found that it thinned off rapidly on the upper surface. The extent of the second or bottom bed, from which all the rock-salt produced in Cheshire since 1780 has been extracted, is less clearly defined, but it is known to underlie not only the whole of the upper bed, but a further considerable area in all directions.

Professor Thompson, in calculating the period of time that was required to lay the salt contents comprised in these deposits, fixed upon an inch in ten years as a fair estimate of the rate of progress at which it was accumulated, and found that it must have taken 21,000 years to lay 60 yds. of rock-salt. With this figure before us, it is interesting to study the following calculation of the salt contents of the Cheshire deposits and of the quantity of mineral that is extracted from the interior of the earth in the form of brine to produce the salt that is made in the Cheshire districts.
Calculating the Northwich salt area at 3 square miles or 1,920 acres or 9,292,800 square yards, and
Taking the upper bed of rock-salt at an average of 25 yds. thick, we have 232,320,000 cubic yds. of rock-salt.
Taking the specific gravity of rock-salt at 2·125, a cubic yard of rock-salt weighs 32 cwts., therefore weight of rock-salt in
upper bed
232,020,000 × 32 tons / 20 = 371,702,000 tons.
95
Taking the bottom bed as extending over the same area, but having a thickness of 35 yds., we find in it—
9,292,800 × 35 × 32 / 20 = 520,396,800 tons,
or, in both beds together, 892,108,800 tons.
The Winsford district, taking the beds of rock-salt at an average thickness of 65 yds., which is 5 yds. less than the figure given by Dickinson, we have 1,932,902,400 tons.
As the whole of the white salt has been manufactured from brine derived from the rock-salt, it represents so many tons of rock-salt pumped up. Now, as the specific gravity of rock-salt is 2·125, a cubic yard contains 32 cwts. This being the case, we find the cubic yards of rock-salt pumped up annually in each district to be, viz.—
In Winsford District—
687,000 × 20 / 32 = 429,375 cubic yds.
In Northwich District—
587,000 × 20 / 32 = 366,875 cubic yds.
In Middlewich District—
14,000 × 20 / 32 = 8,750 cubic yds.
In Sandbach District—
78,000 × 20 / 32 = 48,750 cubic yds.
96
Making a total of 853,750 cubic yds. This represents 176·5 acres of 1 yd. thick.
This is entirely independent of the rock-salt, which, at a low estimate, equals 120,000 tons per annum, or, say, 75,000 cubic yds., or 15·5 acres of 1 yd. thick.
In these calculations no allowance has been made for wastage, and this is very large. During the year every pan requires picking from six to twelve times, the stoved oftener than the common. This necessitates the pan being swept out and an enormous quantity of brine wasted. Besides this, the pan scale contains a large percentage of salt. Again, in drawing the salt out of the pans a large quantity of brine is wasted. Add to this also the leakage in pipes, overflow of cisterns, leakage through defective pans, etc., and the total of wastage will be very large. It is scarcely possible to estimate this, but if we calculate 10 per cent. we shall be under the mark. Thus, for waste, we may set down 136,600 tons. This would represent 85,075 cubic yds., or 17·65 acres 1 yd. thick.
We thus see that 209·65 acres of rock-salt 1 yd. thick is every year consumed in the Cheshire salt district.
97
The salt industry of Cheshire may be divided into three periods, viz.: the natural brine period, the rock-salt period, and the prepared brine period. From Saxon times up to the last quarter of the seventeenth century the manufacture of white salt from brine had been continued without interruption, but the output had never been large. In 1675 the production of the three “Wiches” was returned at 20,000 tons, and all the evidence shows that the total annual make had never exceeded 30,000 tons. In 1670, rock-salt was discovered in the county, and for the next hundred years, although brine continued to be worked, rock-salt mining was the chief producing industry. With the collapse of the mines, the salt proprietors turned once more to the brine supply, upon which Cheshire has since risen to its present commercial eminence as one of the great salt-making centres of the world.
In 1670, a rock of natural salt was discovered on the Marbury Estate, about one mile north of Northwich, by one John Jackson, of Halton, who was engaged at the time in “searching for coals on behalf of the Lord of the Soil (or Manor, I should say), William Marbury, of Marbury, Esquire.” The event was communicated to the Royal Society by Mr. Adam Martindale in a letter dated 12th December, 1670. He added that the liquid issuing from the rock was “a vigorous sharp brine beyond any of the springs made use of in our salt works,” and, being asked by the Royal Society to visit the place and send a further report, he subsequently wrote: “The rock of salt, by the relation of the workmen, is between 33 and 34 yards distant from the surface98 of the earth, about 30 whereof are already digged and they hope to be at the Flagg which covers the salt rock about three weeks hence.... That piece of natural salt which the instrument brought up (divers saw it, a pure ore) was as hard as alum and as pure.”
The records of the rock-salt mining period are singularly incomplete, inexact, and disappointing. It is not known for certain which was the first mine sunk after the discovery of the salt-bed in 1670. It may have been the one which is described as “very near to a small brook which drains Marbury Mere and joins the Witton Brook, near the Buttevant Bridge on the Marbury Estate.” Or it may have been another early mine which was situated “close to a small runnel or gutter which runs into this small brook near the Dairy House Farm but passes across the land of Mr. Lyons and over the old Marston mine.” If the curious inquirer is not yet satisfied with these conjectures, he is further informed that there is yet another subsidence of an old mine, “close to the Forge Lane or road leading to Budworth across the Fields, where the road branches off at the cottages and salt-works of Mr. Lyons’ property ... and this mine is probably the earliest sunk.”
But if little is known about the beginning of the salt-mining industry in Cheshire, there is not much more to be learnt about its development and ultimate decay. To-day, only the Adelaide Marston Mine at Northwich is working, and of the nineteen mines that were open in Cheshire in 1881, only nine were at work, while from an undated plan and key showing the rock-salt mines in the Northwich district, which was probably published a few years earlier, we learn that of the fifty rock-salt mines that had been abandoned, twelve had been sunk to the bottom bed and the rest had been worked as top-bed mines.
99
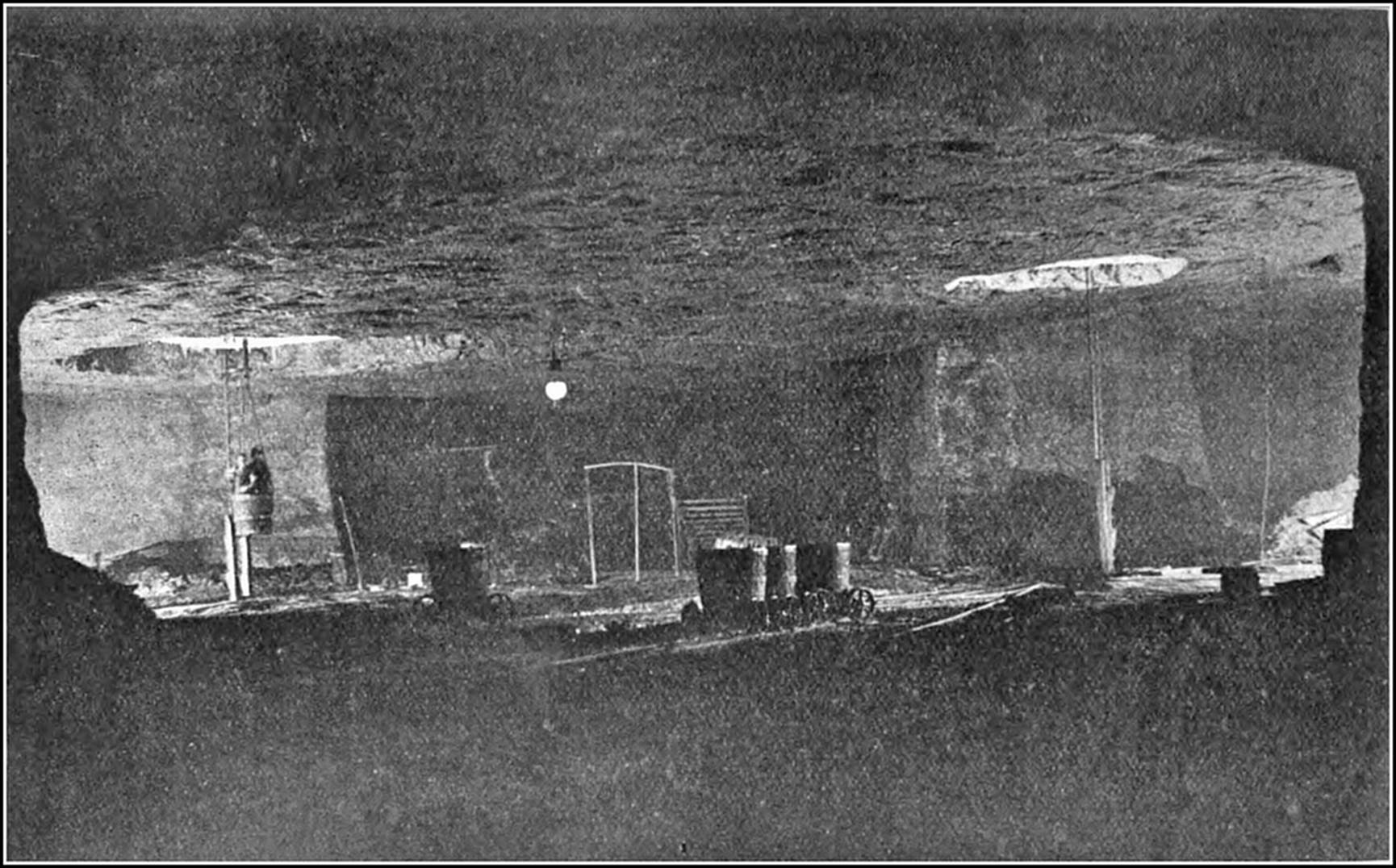
100
The story of the exploitation of the top and bottom beds is one that is soon told. The top bed was worked until the mines began to fall in and the subsequent breaking in of fresh water converted the old workings into brine reservoirs. In 1779, the discovery of the lower bed of rock-salt at Lawton prompted the owners of the Marston Mine at Northwich to sink below the top bed in which they were working, and, in 1781, a trial shaft which was sunk from the top mine by means of a horse gin, demonstrated the existence of the bottom deposit in that district. Other owners transferred their operations from the top to the bottom bed, and for the next fifty years practically all the rock-salt was excavated from that source. In 1830 the roofs in these workings began to crack, and attention was directed to the insufficiency of the pillars by which they were supported. A competent surveyor, who did not hesitate to declare that the workings were in a dangerously insecure condition, was regarded as an alarmist by the old salt proprietors, who commissioned other “experts” to examine the pits, and were satisfied with their assurance that they considered each pit to be entirely free from any danger, and that they should not hesitate to work in any of them. Three years later the roof of the first bottom-bed mine fell in, others collapsed in rapid succession, and by 1840 some twenty mines had collapsed, let in water, and become filled with brine. In 1881, only nine rock-salt mines were at work, and eight of these had a combined area of 123 acres.
Rock-salt mining in England is a dead industry, but it will be of interest to outline very briefly the methods that were employed in Cheshire during the comparatively short period of its existence. The old top-bed mines were operated, in the first place, with one shaft to each mine, and they were ventilated by means of101 an air-pipe and a fan. A horse gin was used for winding, but the winding-shaft in which the gin rope worked did not go into the rock-salt, but only to within a short distance of it, and it was out of this shaft, at a distance of 2 or 3 yds. from the bottom, that a side drift was driven. From this side drift a windlass pit was sunk into the rock-salt, and it was up this windlass pit that the rock-salt was drawn to the drift and thence taken to and up the gin shaft, the part of the gin shaft below the drift being used as a sump or lodgment for water. These top-bed workings did not usually extend more than 100 yds. from the shaft, but, as the number of the mines increased, the workings from adjoining shafts occasionally become connected. In this way one shaft became a downcast and the other an upcast, and the air-pipe and fan at each were able to be dispensed with. The thickness of rock-salt worked averaged from 30 to 36 ft., and pillars of natural rock-salt, usually about 5 yds. square, were left to support the roof and superincumbent strata.
Although the bottom-bed mines were worked upon the same plan, the inadequacy of the supports employed in the top-mines was rectified by an increase in the size of the supporting pillars and in the thickness of the rock-salt roof that was left between them. Steam engines with direct shafts to the bottoms of the mines were substituted for the horse-gins and windlasses, and improved methods were introduced for preventing water from breaking into the shafts. Two winding-shafts were sunk, placed about 10 to 15 yds. apart, and a pump-shaft was sunk to the depth to which the surface water penetrated. One of the earliest precautions taken in the rock-salt shafts, and afterwards in brine shafts when they came to be sunk through rock-salt, was to protect the sides from the ravages of fresh water.102 All the shafts were roofed over to keep out rain or snow, and the wood casing, which was originally used, was replaced, in 1845, by cast-iron tubings, similar in construction to those used in colliery shafts.
As soon as the miners had sunk the shaft to the depth of the sole or floor of the mine and had made an opening large enough for their purpose, they proceeded to blast off enough rock to form a chamber about 5 ft. high. This formed, they advanced by blasting off the rock-salt from the face of the seam. The salt was loaded into waggons, which ran along small railways to the mouth of the shaft. The men engaged in blasting the rock and squaring the walls and pillars (for these were left quite square and well hewn) were called miners ; those who loaded the trucks and conveyed them to the shaft were ferriers. They were a fine set of men, and their occupation, compared with coal-mining, was a very healthy one. The mines were of an equable temperature, and were sufficiently warm for the men to dispense with their shirts. Being lofty, the air was pure, except when excessive blasting was undertaken. The greatest number of men employed in one pair of shafts was about eighty, and the quantity of blasting powder used by that number in the course of a day averaged 1 cwt. Safety fuses were seldom used, the charge being fired by a straw filled with fine powder, which was lighted from a candle.
Many of the mines were of considerable size, and some of them increased at the rate of about an acre annually. The quantity of rock-salt mined was small compared with coal. No mine in the district yielded above 40,000 tons per annum.
Rock-salt is more free from danger than most kinds of mining; no explosions occur, for there are no deleterious gases, and accidents are rare. In a general way103 the rock-salt strata are remarkably free from carbonic acid gas, and in only one instance in Northwich, and twice at Meadow Bank, Winsford, does fire-damp appear to have been met with, and then only at pipe veins and in very small quantity. There are no falls of earth, as in coal mines, for the rock-salt is extremely tenacious, and the miners never undermine it but blast it, which is a much safer operation. The two great dangers to which rock-salt mining is exposed, though they rarely result in loss to human life, are the falling in of the mine bodily, or of the shafts and neighbouring earths, and the breaking in of brine either at the head of the top-rock shaft or from old mines, long disused, and full of brine.
Neither the name of the first mine that fell in, nor the date of its collapse, is recorded. We know that a mine in Witton fell in in 1750, and another to the north of the Northwich Town Bridge followed in 1759, and that many others collapsed before 1770. Lakes, or “flashes” as they are called locally, have formed over the larger of these sinkings, but the sites are more commonly marked by what are known as rock-pit holes, and large tracts of country are scored with these funnel-shaped indentations. There can be no doubt that a number of these old mines were worked with pillars that were too few and slender for the purpose, and these supports gradually weakened to their ultimate collapse under the pressure of the superincumbent earths. As the sinking did not take place evenly all over the mine, but most frequently occurred near the shafts and at the greatest distance from the sides of the cavity, the roof would curve down towards the sinking centre and the falling-in formed the V-shaped apertures on the surface which are described as rock-pit holes. But, while in a percentage of cases the collapse of the mine could be traced104 to the crashing of the pillars, the destruction of the majority of the mines was caused directly by the influx of water, although this water, having become saturated with salt, would, if undisturbed, cause no further havoc in the interior of the mine.
But the manufacture of white salt from brine, which was temporarily surpassed in importance by the rock-salt industry, was not discontinued, and a copious supply of brine flowing over the rock head of the upper bed, was tapped by shafts and pumped to the surface. When, about 1850, this supply showed signs of failing, attention was directed to the enormous reservoirs of brine in the old inundated mines, into which had drained a great quantity of the rock-head brine. The attempt to pump brine out of the abandoned workings was successful, and for some years an abundant supply was obtained. After 1870 the pumping operations caused further collapses, the land overlying the mines subsided, and lakes were formed which, at intervals, broke into the partially exhausted reservoirs, and, pouring through the top-rock workings into the mines in the bottom bed of salt, replenished the supply of brine. A great collapse which occurred in the Dunkirk district in 1880 let down the waters of Cranage Brook and the Wadebrook, together with a huge quantity from the river Weaver. The subsidence resulted in the formation of a large lake, which, following upon a later subsidence in the same area, suddenly disappeared into the earth and literally flooded all the underlying strata.
Surprise has frequently been expressed that in a salt country in which brine has been manufactured for over twenty centuries, the existence of the rock-salt deposits should only have been discovered in the last two hundred and fifty years, but it must be borne in mind that not105 only was the brine the best custodian of the secret of its own source, but that, when the problem of the supply had been solved, the danger of tapping and controlling it had still to be overcome. When the supply of brine in many of the springs was cut into, it proved so copious that the sinkers had to flee for their lives and to ascend the shaft among the brine. The fact that the depth at which the brine would be encountered was unknown, explained the inability to provide a safeguard against the sudden inrush of brine, but subsequent observation showed that when the workmen met with the “flag,” or bed of hard marlstone that existed above the top of the rock-salt in many districts, the brine might be expected to be found at high pressure. It was then the practice to case the shaft sides down to the flag to prevent the entrance of surface water, and either to blow through the flag with powder or pierce it with boring rods. At a later period, the shaft was sunk to the approximate point of encounter with the brine, and cased with iron cylinders, the bottom cylinder being furnished with an iron bottom pierced with two pipe holes. A column of pipes was erected in the cylinder, and a set of boring rods was let down each pipe, so that when the flag was bored through, the brine rose until it attained its level in the pipes, while by means of a tap attached to each pipe it was possible to stop the entry of the brine and to empty the shaft.
In the brine-shafts employed in the case of the old rock-salt mines, in which the brine was met with at a much higher pressure than in the rock-head brine-shafts, the tapping operation was attended with extraordinary difficulties. The brine in the old workings rose to a height corresponding with that attained by the brine found at the rock-head, and as it had to be tapped106 through a pillar near the bottom of the old workings, the pressure was proportionately higher. When the holeing was first effected into the brine in the old bottom-bed workings, the rush of the incoming brine was so strong that it passed through the two 5 in. bore holes and rose up a 4½ ft. shaft to a height of 67 yds. in eight minutes. The shaft for tapping this supply of brine was sunk in a pillar of rock-salt, and a drift, fitted with two 5 in. bore-holes, was worked through the intervening face and into the brine. When these bore-holes were knocked through, the brine entered with the report of a cannon, and the engineer and his assistant, leaving their tools behind them, leapt into the bucket and were hastily drawn up the shaft, closely pursued by the rising brine.
An improved process for tapping the brine, which entirely removed the danger attending the operation, was subsequently introduced. This was effected by boring the last part of the main bore-hole through a stuffing-box at the other end—an innovation which prevented brine from escaping during the boring. A drift, with the usual ⅝ in. bore-hole in advance, was driven 61 yds. into the barrier, until the small bore-hole showed that only 10 yds. remained between the face and the brine that was known to be present in the old workings. Into this remaining 10 yds. of barrier a hole 11 in. in diameter was bored until nearly through, and a closely-fitted pipe was inserted into the hole for a distance of 7 ft. The pipe was 10 ft. long, but at 7 ft. from the inner end was a disc 3 ft. in diameter to rest against the face of the drift, leaving the remaining 3 ft. of pipe in the drift. About midway between the disc and the outer end of the pipe, were placed two strong iron uprights, let into a trench cut 1 ft. deep in solid rock-salt in the roof and floor to secure the pipe107 against the pressure. These two uprights were placed close together at the top and bottom, but in the middle they were curved so as to form a circle for the pipe to pass between them. The face of the drift against which the disc had to rest, having been carefully dressed, and a disc of india-rubber covered with red lead having been placed between the iron disc and the dressed face of rock-salt, the iron disc was secured up tight against the face by means of six set screws. A stop-valve was then fitted to the outer end of the pipe, and to this, for the temporary purpose only of completing the bore-hole, was attached an end piece with a stuffing box and a hole in it large enough for the bore rod to be worked through. The bore rock was then withdrawn and, the valve being closed, the stuffing box and the temporary end piece were removed. A range of pipes was attached to the stop-valve and, in this range, the brine was taken through the old workings and up one of the shafts to the surface.
Many geologists have subscribed to the theory that the Cheshire meres were formed by subsidences which occurred in pre-historic times, but the evidence based on the phenomena attending the modern subsidences proves that the latter were the result of artificial and readily-identified causes. Leland, in 1533, reports a sinking near Combermere and the formation of a pit containing salt-water; in 1657 a small sinking occurred at Bickley, near Malpas; and a third took place in 1713 at Weaver Hall, to the south of Winsford. No traces of any of these subsidences now remain, but, from the descriptions handed down to us, these sinkings belonged to the class of funnel-shaped holes and were of limited diameter and no great depth.
Of the modern subsidences, which are of three kinds, we have no documentary evidence prior to 1777, and108 the earliest distinct record belongs to the year 1790. From that time to the present day this class of sinking has continued to increase in extent year by year. In 1790 the sinking portion along the Witton Brook was recorded as being 130 yds. long by 90 yds. wide. In 1837, the subsidence had obtained an area of 1,230 yds. long by 130 yds. wide. In 1811, about 20 statute acres in Witton commenced sinking, and in the ensuing thirty-three years some portions of this area had sunk 24 ft. In 1880, the piece of water called the Top of the Brook had subsided over an area of 4,370 ft. by 1,470 ft., and in the same year it was estimated that no less than 2,700 acres of land in Northwich and Winsford were inundated.
These modern subsidences usually consist of funnel-shaped holes caused by the falling-in of top-rock mines, and of trough-shaped hollows which cannot be connected with rock-salt mining, and are frequently found in places far removed from the localities of the old workings. Of two dozen subsidences, two are nearly four miles distant from the nearest old workings or from the brine shafts, fifteen are upwards of two miles, and only one is less than a mile from either a mine or a pumping station. The subsidences could not be caused by volcanic action or the shock of earthquakes, as nothing of the kind has occurred in the districts, and it is impossible to explain them by the action of natural brine springs running to waste in the brooks or rivers, because it is known that no such springs now exist, while evidence accumulated from all parts of the world confirms the conclusion that where brine springs escape into the streams, no subsidence has ever occurred. Yet it is evident in Cheshire that some subterranean denudation must be taking place which is removing portions of the lower strata and allowing the109 super-incumbent earths to sink into the excavations thus made. Many theories have been advanced to explain the phenomena, but even those people whose interests have caused them to seek for alternative causes must realize that it can only be attributed to the simple and most obvious agency.
When the number of brine pits was multiplied and the natural springs of a weak solution of salt decreased in volume, it was necessary to sink down to the rock-head brine, which was a highly-saturated solution consisting of one part salt to three parts water. When this supply is pumped up, its place is taken by fresh water, which, flowing over the rock-beds, takes up its quota of salt on its way to the pumping shafts, and is raised to the surface in the form of brine.
It is not the presence of water over the beds of salt or in the old salt workings which causes the damage, because when such water has taken up salt to the extent of a fourth of its bulk, it remains inactive and makes no further ravages upon the mineral earths with which it is in contact. But when the saturated brine is pumped up and its place is taken with a new supply of water which collects its tribute from the salt strata, and that water, in its turn, is raised, to be replaced by more, and when it is known that each 100 tons of water that traverses the salt-bed to the pumps carries away with it 25 tons of solid earth, the work of destruction that is continually going on is explained.
It may be convenient to explain at this point that the subsidences caused by this simple operation of removing rock-salt from the earth in the form of brine are divided into three classes, viz.—
1. Shallow troughs, with sides not terraced or broken up.
2. Very shallow depressions extending over considerable areas.
110
3. Deep troughs, much broken up, and with stepped or terraced sides.
With these three classes in mind, it is easy to follow the results of the action of the subterranean brine and associate the causes with the effects produced. At first the water flowed over the salt in irregular channels and reached the pumping centres by devious routes, but after a time it made defined courses for itself exactly as the rainfall carves out for itself channels on the surface of the earth. These underground streams of brine all gravitate towards the pumps, widening and deepening as the continually renewed water takes up its supply of salt. Where the earths overlying these brine “runs” are not too tenacious, they soon follow the hollow or trough formed on the surface of the salt bed, and a corresponding hollow or trough is formed on the surface of the ground. Where the hollow forms at an early stage, it rarely attains any considerable depth, for the sinking earths impede the course of the flowing brine stream and cause the fluid to spread and be diffused over a wider area. These subsidences are the shallow troughs, not stepped or terraced on the sides, and are best seen in streets and roads where the weight of the houses and the constant passage of traffic cause the earths to gradually follow the wasting surface of the salt. Where, at a considerable distance from the shafts, the water has not formed for itself a definite channel, it percolates over a wide area. The denudation in such cases is more generally spread, and a very extensive shallow trough or basin is formed. Again, where the pumping stations are close together, or in the same line, the various rivulets or streams of brine converge into one broad and deep channel, in which the denudation proceeds with great rapidity. The magnitude of these channels causes the112 super-incumbent ground to subside swiftly, forming deep troughs with stepped or terraced sides, where the earths have broken away in huge masses. Where the earth consists of strong marls and a kind of flagstone they are very tenacious and remain suspended for a considerable time over these deeper cavities. When they will bear no longer, a sudden fall occurs in one spot, and tens of thousands of tons of suspended earths fall into the trough below, forcing out the stream of brine at the weaker places and leaving a huge, crater-shaped hole on the surface, which fills with water.

In addition to the three classes of subsidences already mentioned, there is another which is the result of a combination of collapses of the surface earth caused by the rock-salt mining operations, and the denudation of subterranean strata caused by the pumping of brine. The pumping from the reservoirs formed by the flooding of the old mines does not empty these huge receptacles, as the place of the brine is continuously retaken by fresh water, which naturally gravitates to these centres and proceeds to dissolve and take up its quota of rock-salt. When a subsidence occurs on the site of these old workings it is of the most destructive nature, and as all the top-rock mines were in the neighbourhood of streams and brooks, the surface waters flow into the cavity until it is filled to the level of the earth and allows the streams to pursue their proper course. But as fast as the fresh water becomes saturated and is pumped to the surface, the overlying stream or brook lets in further supplies of fresh water to fill the vacuum, and the work of internal destruction is followed by further subsidences of the suspended earths.
The immense bodies of water in the neighbourhood of Northwich and Winsford, locally called “Flashes,” which cover a total area of many hundreds of acres,113 are the work of subsidences. The Flashes are not shallow swamps, but lakes varying in depth over many acres, from a few yards to 50 ft. The largest Flash, known as the Top of the Brook and resembling the letter L in shape, has a length in each arm of about half a mile, an average breadth of a quarter of a mile, and attains a depth of 150 ft. In an account of these subsidences, written in 1879, we read: “The whole of the surrounding district still sinks rapidly, and year by year the water covers more ground. The land subsides gradually here; but when we go a quarter of a mile to the north-east of the Top of the Brook, we come across a subsidence of a still more alarming character. Here the ground sinks bodily in immense masses to a great depth. A tiny brook or ditch that a child could skip across passed over flat fields some five years ago. Gradually the land began to sink, and cracks opened in the surface right across the course of the brook. The water went down the crevices. The land immediately sank more rapidly; huge cracks, wide enough for a man to slip down, formed, and very soon a district extending fully one thousand feet in length by as many in breadth, sank rapidly to a depth of forty or fifty feet in the centre, and was filled up to a certain height with water, which covered the hedges and trees. At times cracks opened in the bottom of this lake, and the whole of the water rushing rapidly below, caused still more extensive sinking.”
One of the most extraordinary subsidences, which was described in Chambers’s Journal, occurred in Dunkirk, on the outskirts of Northwich, in December, 1880. The earliest intimation of impending disturbance on an unusual scale was a rumbling subterranean noise, the violent bubbling of the water in all the surrounding pools, and the uprushing of air and foul gas through114 rifts which its passage tore in the ground. It was quickly discovered that Wincham Brook, a channel of water nearly 20 ft. in width, had broken into the earth about 1,000 ft. from its entrance into the Top of the Brook, and the uprush of air from the old mines, was caused by the force of the descending waters. A series of alarming, but comparatively small, subterranean displacements caused extensive rifts in the ground about Ashton Salt-works, and these were followed by a sudden explosion in a neighbouring pool, which ejected a geyser of mud and water some 30 ft. into the air. In the ruin that ensued, stacks of timber, an engine and boiler, a salt pan, and other material disappeared into the gaping earth, and a massive chimney stack, some 90 ft. high and 9 ft. square at the base, tilted towards the centre of subsidence and collapsed with a terrible crash. Scarcely had this subsidence ceased, says the writer in Chambers’s Journal, “when an enormous sinking of the whole of Ashton’s Old Rock Pit Hole and the surrounding land, for an area of over five hundred feet in diameter, took place, leaving two very deep holes. The land was riven and cracked all round, and fell in steps of two feet. Over ten thousand tons of water went down into the subterranean cavities. A huge brine cistern was riven in two, and the brine all lost; and two large brick kilns cut completely in halves, and the bricks scattered about. The whole surface of the Weaver and the Top of the Brook was lowered fully a foot over one hundred and sixty acres in about four hours; and if we add to this the whole of the water of the Wincham Brook for twelve hours, we shall find, on a careful computation, that not less than six hundred thousand tons of water rushed below.”
From the time of the “Great Subsidence,” as this115 event is described, the sinking has been continuous throughout the locality. In some places meadows have been converted into swamps, roads have sunk fully 30 ft. below their original level, and small brooks have become lakes of many acres in extent; sunken and distorted fences, roads, and streams are common objects of the country-side, the tenure of pastoral lands is precarious, and property is valueless for building purposes; and nothing but its inexhaustible reserves of brine saves the district from abandonment as a place accursed.
The shallow, gradual, almost imperceptible subsidences which occurred in the neighbourhood of the towns of Northwich and Winsford were at first infrequent and of comparative unimportance, but as time went on the damage to property increased so rapidly that, in 1860, the house-owners of Northwich combined in an unsuccessful attempt to obtain legal redress. By 1880, many parts of the towns were rendered unfit for habitation. In Northwich alone, nearly 400 houses and other property to the value of over £100,000 were more or less seriously affected, while water-mains, sewers, and gas-pipes were being continually repaired; houses were condemned, pulled down and rebuilt, and bridges had to be raised. The rents of many lots of property were absorbed in keeping them in repair, and in some districts property had been raised and rebuilt three times in eleven years. “The area of the mischief is extending yearly,” wrote Mr. Thomas Ward in 1881, “and a larger proportion of property is becoming affected, and more and more land is sinking beneath the water and increasing the area of the already existing extensive lakes. Very few, except those conversant with the district, have the slightest knowledge of the amount of suffering caused to property owners by this subsiding of the land.”
116
For over half a century the appearance of Northwich, with its undulating streets, its ramshackle, dilapidated houses, its fissured walls, and its system of shoring and bolting-up of property to postpone as long as possible its inevitable condemnation and demolition, has presented a tragico-comic spectacle. “If a stranger were to be set down some morning in the town of Northwich,” wrote a Times correspondent, “without any previous knowledge of its peculiarities, he would be struck with a startling and novel spectacle. He would see buildings of every sort, from the humble, two-storeyed cottage of the artisan to the solidly built church or chapel, standing many degrees out of the perpendicular, and suggestive, all of them, were it not for the props and iron stays with which they are secured, of some recent convulsion of nature. In main thoroughfares and back streets alike there are houses whose sloping floors and cracked walls would lend considerable colour to such an effort of the imagination. The inhabitants seem to take this tumble-down state of their dwellings quite as a matter of course. They have, in fact, to make the best of a condition of things from which there is absolutely no escape. The effects described are produced, not indeed by any sudden catastrophe, but by a slow, though equally effective process of subsidence, which may be detected in continuous operation over nearly the whole area of the Cheshire salt field, and which will continue to operate so long as the earth yields its vast stores of salt for human consumption.”
But although newspaper representatives could philosophize upon the matter-of-fact spirit in which the inhabitants of the salt towns faced existence in their tumble-down surroundings, and the salt proprietors desired that they should make the best of a condition of things from which they wished them to believe there117 was absolutely no amelioration or escape, a feeling of resentment was rapidly growing in the neighbourhood. The people of Northwich and Winsford were being pumped out of their houses and out of their lands, and the future held every promise of a continuation and extension of the damage. Lord Delamere, who, as an owner and letter of salt lands, benefited by the brine industry and suffered from the depredation it wrought, admitted the damage and the cause thereof. Indeed, nobody but the salt proprietors doubted that the pumpers were wholly responsible for the destruction, and most people recognized that their wrong-doing was twofold in character. Standing on the ancient assumption in law that everything beneath a man’s property belongs to the owner, the owners of property in the affected districts contended that they were not only being deprived of the rock-salt which legally belonged to them, but were further despoiled by having their land made worthless by the abstraction of the salt for which they received no payment. The justice of the protest was obvious, and it became a public question how far these operations, useful in themselves, but involving consequences of a disastrous nature, should be allowed to proceed. In December, 1880, the Daily News asked who was to compensate the sufferers, who had neither caused nor contributed to the disaster.
Following the failure of the property owners to obtain compensation from the salt proprietors for the damage attributable to the pumping operations, an application was made to the Trustees of the River Weaver to devote a portion of their surplus revenue for compensation purposes. The application was refused, and an appeal to Quarter Sessions failed. The evil was allowed to drag on until 1871, when the Board of Trade, in response to representations made to them by the Northwich118 Salt Chamber of Commerce, instructed Mr. Joseph Dickinson to report upon the salt districts of Cheshire. Mr. Dickinson, one of the most eminent Inspectors of Mines in the service of the Government, after a prolonged investigation, reported his conclusions that the subsidences and the resulting damages to property were caused by the pumping of brine, which constituted a public danger and inflicted heavy losses upon many persons totally unconnected with the salt industry. A further report by Colonel Cox, corroborating the conclusions arrived at by Mr. Dickinson, came before Parliament in 1879, and upon the recommendation of the Local Government Board, the local Boards decided to promote the Cheshire Salt Districts Compensation Bill, “to make provision for the assessment, levy, and application for compensation for damage by subsidence of land in the salt districts of the County of Cheshire, and for other purposes.”
The salt proprietors exerted every effort to frustrate the plans of the promoters of the Bill; they declared that a tax upon salt would cripple the trade and ruin the entire neighbourhood; they endeavoured to create local ill-feeling by insisting that the movement was an attempt of the property-owners to saddle the ratepayers with the expense of the proposed measure. Briefly stated, the case that the promoters were asked to make required them to prove (a) the subsidence in the salt district; (b) that the subsidence was caused by the pumping of brine for the manufacture of salt; (c) that the subsidence was of a most extensive and serious character, and affected the property of persons deriving no benefit either from the manufacture of salt in the form of compensation from the salt manufacturers for the salt extracted, or for damage done to the property by such abstraction; (d) that there was no legal remedy120 for the injury suffered; and, finally, (e) that the moneys required to adequately compensate for the injury done, if levied upon the manufacture of salt, would not injuriously affect the salt industry.
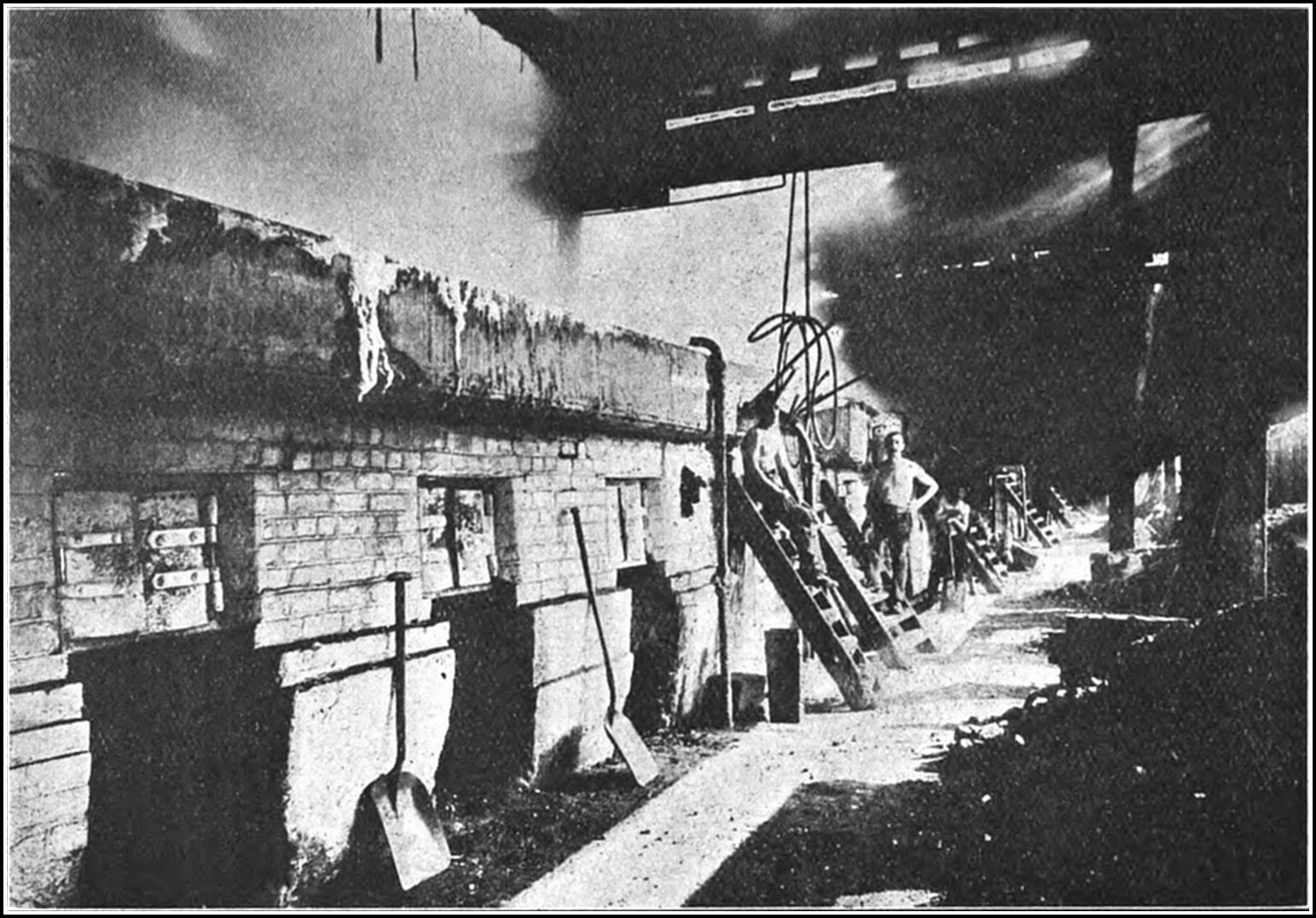
A copy of the Bill was lodged in December, 1880; it was read a first and second time on 21st January and 4th February, 1881, and referred to a Select Committee, which commenced sitting on 5th May, and on the 20th of the same month announced their unanimous opinion that the preamble of the Bill had not been proved. In the preamble of the Bill it was estimated that a contribution not exceeding threepence for every ton of salt in brine in the district covered by the Act would be sufficient to provide the required compensation. The opponents of the Bill declared that a compensation tax upon the salt trade would severely injure the industry and act as a restraint upon trade; they put forward expert witnesses to contend that if the brine—which they contended was produced by rainfall percolating through the superincumbent strata and reaching the salt—was not pumped out, it would run away to the sea, and the consequent subsidence of land and injury to property would not be arrested. The theory that brine, in quantity sufficient for the manufacture of 1,600,000 tons of salt per annum would, without pumping, have been carried away into the rivers by natural agency and deposited in the sea, was supported by such ingenious misstatement and misrepresentation, and the fictitious instances of brine springs overflowing and causing damage in other parts of the world were quoted with so much specious authority that they succeeded in wrecking the Bill.
After a further ten years of continued subsidences and attendant damage to public and private property, the Brine Pumping (Compensation for Subsidence) Bill121 was introduced in 1891, to authorize the formation of Compensation Districts and Boards, with power to levy a yearly rate not exceeding threepence per 1,000 gallons of brine pumped. Shortly after the passing of the Bill, the action of Northwich, which memorialized for the formation of the whole of the County of Cheshire into one district for purposes of compensation, led to an inquiry by the Local Government Board, as the result of which Middlewich and Sandbach were excluded. The Provisional Order uniting Northwich and Winsford in one area was opposed by Winsford, and a Select Committee of the House, in 1893, quashed the Provisional Order and made Northwich an independent compensation district.
The next great struggle in the salt district, known locally as the Battle of the Brine, arose out of the action of the Salt Union, which, in 1909, enlarged its works at Weston Point with the intention of manufacturing salt at that place from brine pumped at Marston, near Northwich, 11 miles distant. In pursuance of their policy of stalling off competition and safeguarding their monopoly, the Salt Union, in 1890, had successfully petitioned against the Bill that was promoted to obtain powers to convey brine from Cheshire to be made into salt at Widnes, in Lancashire, and at Middlewich they had obtained an injunction to restrain trade competitors from laying pipes under one of the streets of the town for the conveyance of brine from their own pumps to their own salt-pans. In 1766, 1833, and 1861, the Trent and Mersey Canal, the Grand Junction Railway, and the West Cheshire Railway, respectively, received authorizations from Parliament, but in each instance a clause was inserted prohibiting the several companies from conveying or permitting to be conveyed in or upon any part of their properties, any brine for the122 making of salt to any district beyond the district in which salt was then made. In 1884, when the London and North-Western Railway sought to gain the repeal of the brine clause in order to enable brine to be carried from one salt township to another adjoining, Parliament refused to sanction even such a limited modification of the prohibition. The logical objection which the salt districts opposed to the removal from the several pumping centres of the brine upon which the prosperity of the towns entirely depended, had thus consistently been upheld by Parliament, but in the face of these facts, and of their previous attitude on the subject, the Salt Union insisted upon their right to carry brine from Marston to Weston Point, and announced their intention to defend their position to the utmost of their power.
It must be explained that the Marbury Pipe Line had been laid in 1882 by the Mersey Salt & Brine Company, who carried it, by agreement, across lands belonging to private landowners and over a canal belonging to the North Stafford Railway. The railway made a formal protest, but an amicable settlement was ultimately reached by which the Mersey Salt Company agreed to pay the North Stafford Company £5 a year and to remove the pipe on receipt of a three months’ notice. The railway company appear to have persisted with their opposition in order to force an admission from the Mersey Company that they possessed no permanent right to carry the pipe across their canal, but the concern was of such trifling importance that it was practically ignored by the people of the district, and for twenty years after the property of the Mersey Salt Company and the Marbury Brine Pipe had been acquired by the Salt Union, the question of the removal of brine from the neighbourhood in which it was raised123 had found all classes of the salt community united against such proposals. But with the completion of the works at Weston Point, and the enlargement of the Marbury Pipe and the installation of powerful engines, capable of driving millions of gallons of brine from Marbury to be converted into salt at the sea-board, a new menace was organized against which the Urban Authorities and Local Councils made a long and spirited, if fruitless, resistance.
In the autumn of 1910, the North Stafford Railway served the Salt Union with a notice to remove their pipe line from the Trent and Mersey Canal by the end of the following March, and the Salt Union proving obdurate, the towns of Northwich, Winsford, and Middlewich promoted the Brine Pumping (Cheshire) Bill, “to regulate the conveyance of brine pumped, raised and gotten” in the county. The original draft, which proposed to permit the removal of brine by pipe to a distance of three miles within the county from the place at which it was raised, was amended to permit manufacturers to carry brine by pipe from one set of works to another in their own occupation, and they further attempted to meet the alleged rights of the Salt Union by the insertion of a clause allowing the Marbury Pipe to be used for the conveyance of brine to the extent of 250 million gallons a year. But the Salt Union declined all conciliatory overtures, and combated the Bill before the Select Committee on the grounds that it was a proposal to alter the common law of England and interfere with the sacred rights of property. The injury that was sought to be done, not only to the Salt Union but to the export trade of the country, was enlarged upon, and the Committee may have been impressed by the assurance that the Union, so far from intending to leave Winsford and124 Northwich, expected to do an even greater trade in those districts in the future than had been done there in the past. In the result, the Salt Union’s insistence upon the legality of a course of action which they had previously denounced and opposed as totally illegal, carried so much weight with the Select Committee, that they made an unsavoury meal of the Parliamentary decisions of 1766, 1833, 1861, 1890, 1891, and 1893, and announced that the Bill could not proceed.
125
In tracing the development of the salt-making industry in this country, it will be observed that, until the last quarter of a century, the old open-pan system defied improvement, and the salt-makers from generation to generation successfully resisted the endeavours of all who suggested innovations or hinted that better methods could be introduced in the manufacture. It is true that experiments were made with the sizes and arrangement of the pans, that coal replaced wood and straw as fuel, that the locomotive superseded the wain as a means of transporting salt from the works to the markets, and that pumps were employed instead of buckets to raise the brine and deposit it in the cisterns which supplied the pans; but these several developments produced no change in the system of manufacture, which consisted of lighting a fire beneath a pan of brine, driving off the water in the form of vapour, and collecting the salt crystals that form and sink to the bottom of the pan. The salt-men were devoted to their primitive, rule-of-thumb methods, and the most enterprising among them regarded the process as unimprovable. In the construction of salt-works there was no attempt at engineering exactness; the size of the pans was regulated roughly by the dimensions of the plates of which they were made; and the heights of the brickwork of the furnaces, etc., was usually reckoned by courses of bricks.
The fireman, the real salt-maker, whose business it was to attend to the fires and see that the proper degree of heat was maintained to produce the variety126 of salt required, did his work almost entirely by rule-of-thumb. It was only rarely that a thermometer was used. The technical knowledge acquired by experience enabled a man to see at a glance whether the pan was working properly, and the quantity and quality of the salt showed whether his work had been well or ill done. The late Thomas Ward was a greatly respected authority, one of the most reliable experts of the Salt Union, and a voluminous writer and indefatigable lecturer on every aspect of the subject of salt, but he failed to persuade himself that it was even thinkable that the open-pan system should ever be abandoned in favour of a more scientific, more rapid, or more economical process.
Mr. Ward admitted that the process was archaic, but he was at pains to demonstrate that the trade was justified in desiring it to remain so. He argued that the price of salt was so low, and the product was so bulky, that costly and elaborate apparatus was both inappropriate and ineffective. He compared the life of an ordinary open salt-pan with that of any of the innumerable patented pans that had been tried, and found that the ancient article produced salt at less cost than the patent contraptions, and was far easier to repair. “The chief business of the salt manufacturer,” Mr. Ward wrote in 1894, “is to utilize to the best purpose, for the production of salt, the heat obtained from the fuel. To this end, innumerable patents have been taken out, but few have been so successful as the simple application of direct heat to open pans. The method seems a very primitive one, and most visitors to salt-works think they can improve upon what they consider a rude, antiquated system. I have had brought before me, and have seen working, scores of patented plans. In all, or nearly all, the idea was to economize heat; and if the whole of salt manufacturing127 consisted in evaporating the greatest quantity of water with the least quantity of fuel, doubtless many of the schemes would succeed instead of fail, as they do now.”
Since the open-pan system of manufacturing salt from brine was in general and uninterrupted use in this country from the time of Julius Caesar to within a few years ago, we must study the interim developments from direct-fire to vacuum pan evaporation in the industry of the State of New York. The salt springs in New York State were discovered by Jesuit missionaries about the middle of the seventeenth century, but the manufacture of salt on a commercial scale was not begun until 1788, when the industry was established in the vicinity of Syracuse. Solar salt is still manufactured in large quantities at Syracuse, where the evaporating surface covers an area of over 12,000,000 square feet, and the season’s output amounts to about 3,500,000 bushels of salt, but between the solar and the vacuum processes the American salt-men have exploited the Pan and the Kettle processes of direct-fire evaporation, and the Steam Kettle and the Grainger processes of steam evaporation; all of which methods are employed to-day in the State of New York.
In the Pan process, several pans, having a width of 20 to 24 ft., a length of 100 ft. in two sections, and a depth of 12 in., are placed under one roof. Adjoining this front row of pans at the back are arranged a second row of pans, 20 to 24 ft. wide, 30 ft. long, and 12 in. deep, set from 12 to 16 in. higher than the front pans, to enable the easy transfer of brine by syphon from the back to the front pan. The grates are 3 to 4 ft. wide, by 5 to 6 ft. long, and the pan bottoms, which are directly over the fires are protected from a too intense heat by fire-brick arches, which decrease in width from the front to the back of the pan, while the air spaces128 between the arches increase in width in the same direction. Beyond 20 ft. from the front of the first section of the pan they cease altogether. To convey the heat as close to the pan bottom as possible, beyond the last arch, the flues are usually filled in with earth or plaster, and thus the distance between the pan and flue bottom is between 3 and 4 ft., or even less, at the end of the first pan, where a perpendicular wall, called a bridge wall, reduces the space to about 1½ to 2 ft., through which the products of combustion pass under the back pan and finally into a common chimney.
After the pans are properly cleansed they are white-washed with a thin milk of lime to prevent their rusting before they become thoroughly heated; the fires are started, and the pans are filled by syphons to a depth of about 6 in. with brine from the back pans. The former are so inserted that a constant flow of brine passes from the back pans into the last section of the front pans, and from these under the partition into the first section. Into the back pan flows a constant stream from the outside cistern, until the front pans are sufficiently full, when the flow is stopped. After a sufficient amount of salt has collected in the first section of the front pan it is removed to the “drip” for drainage. This is called drawing or raking the pans. The front pans are refilled from the back pan in which the brine has become considerably heated, and thus is prevented a too rapid cooling of the brine in the front pan, which would seriously interfere with the formation of a properly grained salt. For the same reason, the partition is placed in the front pan, since it prevents any cold brine from coming suddenly into the first section, but is compelled to enter at the bottom of the pan, where the temperature is at the highest.
For the purpose of aiding the formation of fine129 grained salt, butter, specially prepared soft soap, gelatine, or white glue are added, and when this variety of salt is made the pans are drawn every 45 to 60 minutes. In the manufacture of coarser grained salt, the drawing of the pans take place at intervals of from two to twelve hours, while the temperature is reduced from 229° F. to as low as 148° F., according to the size of the grain.
The Kettle process, which is exclusively employed on the Onondaga Salt Reservation, consists of from 60 to 100 hemispherical cast-iron kettles suspended or hung on “lugs” or pins in two parallel flues, called arches, ending in one chimney, which has a height of 50 to 100 ft., according to the length of the flues. In front the arches are provided with cast-iron, flat-topped grates, 3 ft. in width and 5 ft. long, perforated with holes ⅜ in. in diameter and 1 in. apart. These are well adapted for the burning of anthracite dust, which is now exclusively used for the purpose. The necessary artificial draught is furnished by a pressure blower. The kettles are from 23 to 26 in. in depth, and from 3 ft. 10 in. to 4 ft. 2 in. in diameter, with a capacity of 100 to 150 gallons. The distance from the bottom of the kettle to the top of the grate is 3 ft. 6 in., with a solid fire-brick arch in each, extending somewhat beyond the length of the grate. The distance from the bottom of the kettle to the crown of this arch is 10 to 12 in. Beyond the grate the fire-brick arches are constructed in sections, the air spaces between the arches increasing in size with the advancing distance from the grates. This construction allows the heated gases to pass through these spaces without striking the kettle bottoms directly. While the distance between the bottom of the front kettle and the top of the grate is 3 ft. 6 in., these flues decrease in depth as they advance130 towards the chimney, so that under the last kettle the distance is but 6 or 8 in. The kettles are hung as close as possible with their rims against each other, and the space between the walls and kettles above the lugs is properly covered by masonry, etc., for the purpose of confining all the heat as much as possible within the two arches.
The system of kettles is fed by means of a conduit connected with large wooden cisterns situated outside the building and sufficiently elevated to supply the brine contained therein by gravity to the kettles in the block.
The manufacture proper of salt is commenced by lighting the fires under the kettles and filling them partly with brine as soon as they become warm, and from within 3 to 4 in. of the top when evaporation has well commenced. When salt commences to separate, the pan is withdrawn and the evaporation is allowed to go on undisturbed till a sufficient amount of salt has separated, when the contents of the kettle are well stirred with the ladle and dipped into the basket resting on the so-called basket-sticks laid across the rim of the kettle. While the process of taking the salt from the kettle is going on, the workman opens the faucet for a few minutes to add some fresh brine to the concentrated pickle of the kettle, and washes the salt, so to speak, with this mixture, thereby freeing it as much as possible from the adhering calcium sulphate and the calcium and magnesium chlorides.
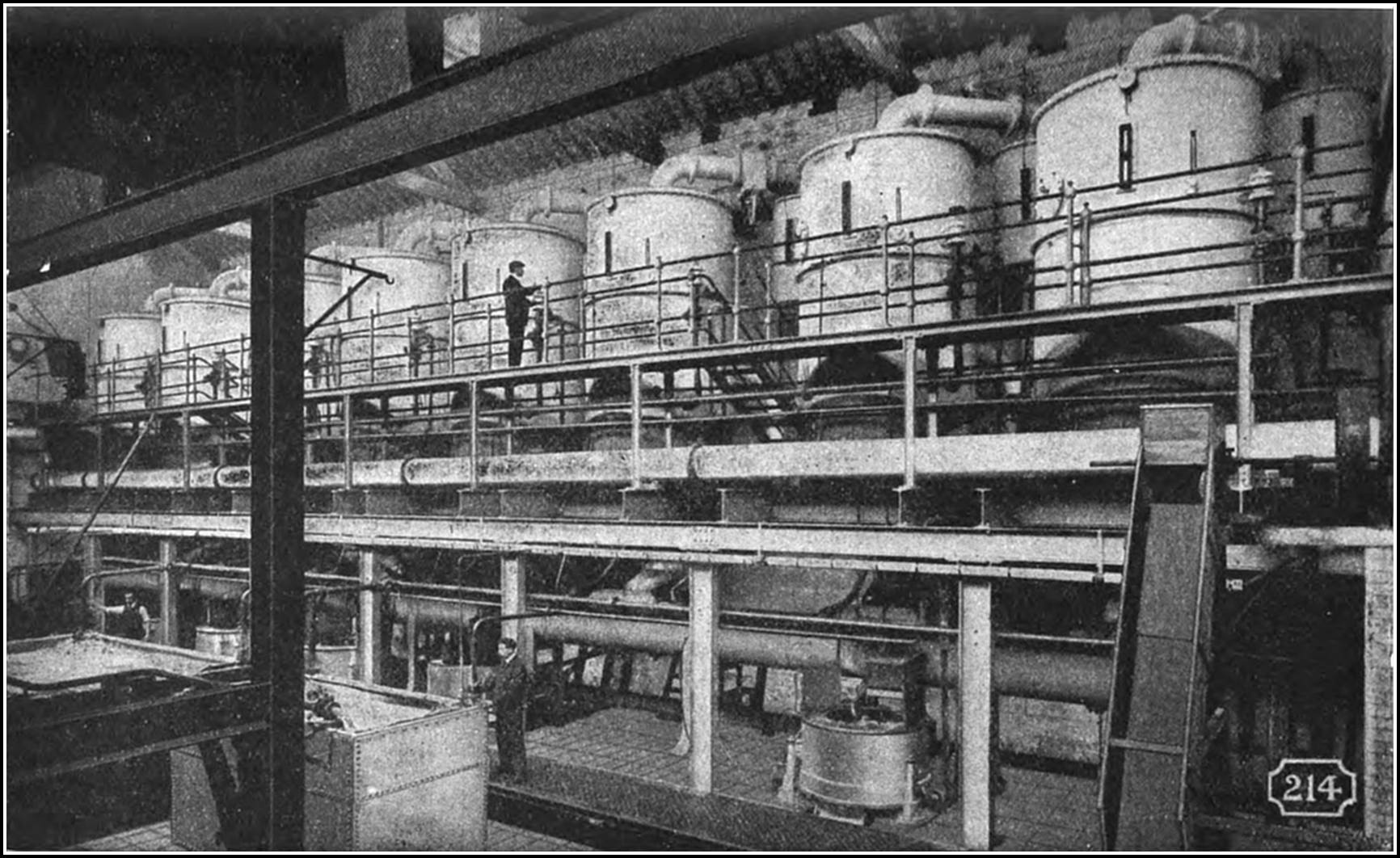
The panning process, though carried out in the best possible manner, will not completely remove from the kettle all the separated calcium sulphate, but some of it, together with separated salt, will bake on the bottom and sides, forming an incrustation constantly increasing in thickness, though at every refilling of the kettle with132 fresh brine much of this adhering salt re-dissolves. This incrustation increases much more rapidly in the front kettles than in those nearer to the chimney, since, a front kettle is usually drawn every 4 or 5 hours, while a back kettle often requires from 24 to 36 hours before a sufficient amount of salt has separated. Moreover, a front kettle holds 150 gallons of brine, while those nearest the chimney contain but 100 gallons. Usually, in 5 or 6 days the incrustation becomes so thick that it interferes very materially with the evaporation, causing a great loss of fuel, as gypsum is one of the poorest conductors of heat. The workman therefore draws the salt from the kettle, removes the remaining brine to within a few gallons, and refills the kettle with fresh water. After a continuous boiling of about half an hour, the greater part of the adhering salt has dissolved and the rest of the incrustation can easily be removed.
The time a salt block is in operation is between 10 and 15 days, and the manufactured salt, according to the State laws, remains undisturbed for 14 days for drainage. A salt block usually cools sufficiently in 24 hours for the kettles, grates, arches, etc., to be properly cleaned and made ready for the next run, so that about two runs can be accomplished per month. The quantity of salt produced in 24 hours in a good salt block, with average good coal dust and brine, is from 500 to 600 bushels of 56 lbs. each, and the amount obtainable by the burning of 1 ton of 2,000 lb. of this fuel varies from 45 to 50 bushels.
There are two salt blocks at the Wyoming Valley, at Warsaw, in which the Onondaga kettles are heated by steam instead of direct fire. Here, in place of the brick arches in which the kettles are hung at Syracuse, they are supported by a framework, and each kettle133 is surrounded by a steam jacket covered with a non-conductor. Moreover, the kettle is made much thinner for the better transmission of the heat. The steam enters the jacket at the upper end of the kettle at one side, and the condensed water escapes by a valve below it, to be returned to the steam boiler. The method of manufacture of the salt does not differ in any particular from the Onondaga method.
The grainer or Michigan process is, like the “kettle method,” a purely American invention, and consists in passing live or exhaust steam through a set of iron pipes immersed in long, shallow wooden or iron vats. These vats rest on a strong wooden frame. They are from 100 to 150 ft. long, usually 12 ft. wide, and from 20 to 24 in. deep; provided with four or six steam pipes having a diameter of 4 to 5 in., and hung on pendants 4 to 6 in. above the bottom of the vats. These pipes are within a few feet of the same length as the grainer, and so arranged that the salt can be conveniently removed towards the outer side of the grainer.
To obtain the best effect in a grainer system, the temperature of the heated brine is kept at or near the boiling-point when no lifting or removal of salt is in progress. To do this an abundance of high-pressure steam must first be supplied to the grainers, and, secondly, the constant supply of brine required for the grainers while evaporation is going on, must enter at a temperature but little lower than that of the brine in the grainer. For this purpose two large tanks, called settlers, are employed, which are usually as long and wide as the grainers, but 6 ft. deep, and provided with four rows of steam pipes about 1 ft. above the floor to heat the cold brine drawn into them from the outside cisterns as required. Although the six rows of steam pipes in the grainer have an entire length of from134 550 to 750 ft. (suspended in the brine 4 to 6 in. above the bottom of the grainer and with 8 to 10 in. of brine above them) and a heating surface of from 700 to 1,000 square feet, a great deal of the steam supplied to them is not condensed, and, therefore, passes from the grainer pipes into the settler pipes (sometimes passing through a steam trap to separate the condensed water) to heat the brine of the settlers.
The main difficulty with which the manufacturers of New York State have to contend is the calcium sulphate. In fact, it is this impurity which causes the interruption of the process, and the laborious cleaning out, whether the kettle, the pan, the grainer, or the vacuum pan is used. It not only entails a great loss of heat in consequence of its slow conductivity, but it also causes the overheating of the metal exposed to direct fire, wherever this is employed. Suggestions and experiments have been made to overcome this difficulty, involving the expenditure of great sums of money, but without any practical results as far as mechanical means are concerned.
From the time of the introduction of the open-pan system in Cheshire, until the beginning of the present century it was found impossible, owing to the nature of the furnaces employed in the process, to maintain a sufficiently high and uniform temperature to produce salt which, without grinding, is marketed as finest table salt, or to make more than 2 tons of salt from the consumption of 1 ton of coal. Experiments for the purpose of economizing fuel appeared destined to perpetual failure, and the hand-stoking of the furnaces entailed so many variations of temperature that the production of salt crystals of uniform size was impossible. Then, within the same decade, two processes were invented which, between them, solved the problems that135 had hitherto eluded all the efforts of the scientist, the engineer, and the practical salt-man.
In order to understand the advantages secured by the operation of the Vacuum System, which comes to us from the United States, it must be remembered that, under atmospheric pressure, brine boils at a temperature of 226° F., whereas in a vacuum of 28 in. mercury, the boiling temperature is reduced to about 100° F. It will thus be seen that evaporation in vacuo renders it possible to use multiple effect apparatus without causing unduly high pressure in the first vessel, and it has this further advantage, that the low-pressure steam, in passing through the evaporation gives up its latent heat, whereas if the steam went to the condenser direct from the engine, the heat employed in the steam engine would be only the difference between the heat contained in steam at 170 lb. and the steam at 5 lb. pressure. By multiple effect evaporation, a great economy in the amount of steam required is effected. Between the evaporation of brine and that of other liquors, the chief difference to be noted is that in the multiple effect system, each pan or unit is supplied with its brine independently of the others, and graining goes on in the pans, whereas in concentrating other liquors the pans are fed from the first to the second and from the second to the third. The removal of the salt from each pan has, therefore, to be arranged for. The method of working a triple-effect plant may be briefly described as follows—
Each of the three pans having been charged with brine to the proper level, exhaust steam from the engines is admitted to the calandria of the first pan in which the highest temperature is maintained. The brine in this pan becomes quickly heated, and the steam given off enters the calandria of the second pan, where it136 serves to raise the temperature of the brine. After doing its work in the second stage, the steam is condensed, and thus creates a partial vacuum in the first pan. The atmospheric pressure being thus reduced, violent ebullition of the brine in the first pan results. The same process takes place in the second pan, owing to the calandria of the third pan acting as a condenser of the vapour and producing a vacuum. The vapour given off by the brine in the third pan is condensed by means of a jet condenser. It will, therefore, be seen that the highest vacuum and the lowest temperature exist in the third pan, while the highest temperature and lowest vacuum are found in the first pan. As the salt is precipitated it falls to the bottom of the pans. The bottom of each vacuum pan is connected with the boot of a continuous bucket elevator, which is carried in a cast-iron, water-tight casing to a level sufficiently above that of the brine in the pans to ensure that they shall be brine-sealed. The salt is delivered into waggons and the brine drainage returns to the pans. The further treatment of the salt crystals varies with the purpose for which they are required. For table salt they are subjected to grinding, but for export they are simply allowed to drain.
The general aim of the Vacuum apparatus is to divide the boiling process into two stages, in order to prepare the brine beforehand by purification, and out of the purified brine to produce the purest salt possible—chiefly by boiling under atmospheric pressure—and to acquire another liquor of the highest content in medium salt. Balzberg, in his Die Erdesalz Erzeugung, has to admit that the process results in the most complete purification of the common salt, but in the conclusion of his critical summary of the vacuum plant, he says: 138“At the same time it must be admitted that a137 complicated machine, which only gains, at a high cost, advantages that can be achieved by more economical and simpler means is of no use in practical business. The question then arises as to whether it is necessary, for the production of domestic or table salt, to have pure chloride of sodium, and whether it pays to use complicated machinery to attain this end.”
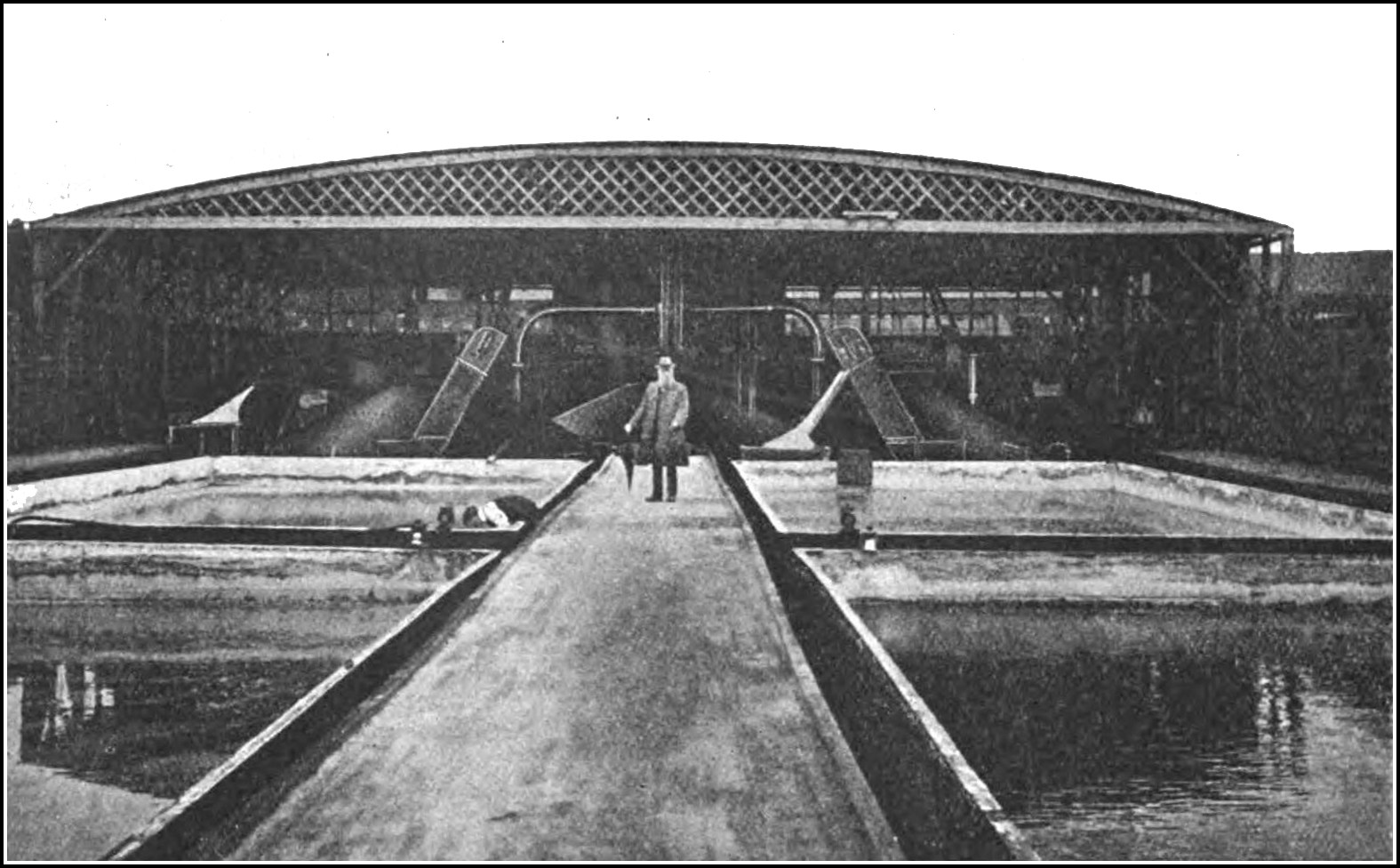
While the largest size triple-effect vacuum plants are capable of turning out 1,000 tons of salt per diem, with brine at or near saturation, and produce about 6 tons of salt for the combustion of 1 ton of coal, it is a very expensive process to operate as well as to install. The cost of the plant ranges from £26,000 to £100,000, and a large percentage of skilled labour is required in its manipulation. But, despite the high initial cost, and the fact that it only makes one grade of salt, it is extremely complicated, and has to be stopped for 4 hours in each 24 for the purpose of boiling out and cleaning up the pans, the vacuum plant is a highly efficient piece of mechanism, and for a while it remained the best and most economic system on the market.
But the Vacuum process was not destined to remain long without a rival. In point of fact, the merits of the American invention had scarcely obtained recognition when a new furnace was designed which, when applied to the open-pan system and subjected to practical tests, proved an entire success. The late James Hodgkinson, the patentee, was not a salt-man, but the head of a Manchester firm of engineers and machinery manufacturers, and it was a professional visit to a salt-works which revealed to him the crudity of the brine-boiling operation and gave him the idea of adapting to the salt furnaces a mechanical stoker of his own invention, which was already being operated139 for other manufacturing purposes. In the development of his idea, and with his mechanical stoker as its foundation, he perfected the Hodgkinson Patent Salt-Making Process, the advantages of which over all other processes for the manufacture of salt from brine have been summarized by Sir Thomas H. Holland, D.Sc., F.R.S., under the following six heads—
1. Complete utilization of the heat derived from the fuel employed.
2. The absolute maintenance uniformly of this heat.
3. The fact that finely-divided first-quality table salt can be produced in the dry form fit for dispatch to the market without grinding or other preparation.
4. The fact that coarsely crystallized salt can be produced at the same time as the finest table salt.
5. That the proportion of the different grades of salt can be varied at will, as well as maintained constantly, to suit the varying requirements of the market.
6. The automatic and continuous removal of the salt as fast as it is precipitated from the brine.
The essential features of the Hodgkinson plant consist of (a) a mechanically-stoked furnace for the production of heat; (b) a primary closed evaporating pan, 30 ft. in diameter; (c) two secondary circular pans, 25 ft. in diameter; (d) four open rectangular pans, 60 ft. by 25 ft.; (e) a series of folded steam-jacketed pipes for heating the inflowing brine by the waste steam; and (f) a condensing arrangement to produce a partial vacuum in the closed pans.
The Hodgkinson furnace is not placed under the pan, as in the old system, but in front of the plant, and the heated gases pass under the primary pan, where the temperature ranges between 1,800 and 2,000°F. In this primary pan is made a finer and better salt than can be manufactured by any other system140 in the world. Moreover, by means of the mechanically-stoked furnace, and the consequent uniform high temperature, it is possible, for the first time, to control the character of the salt produced. Where the temperature varies, as in the open-pan system, crystals of varying shapes and sizes are produced, and this mixed salt must be ground to make it suitable for table purposes. Where steam heat is employed, as in the vacuum process, the temperature is not high enough to make crystals of the smallest size. By the Hodgkinson system the primary pan produces a precipitation which requires no grinding, which flows in a cascade of salt from the pan, and can be delivered to the consumer without having come into contact with the hand of man in the whole course of the operation.
The heated gases, having passed under the primary pan, are then divided and sent under the two secondary pans, and from thence they pass under the open rectangular pans, the gases being distributed by the broken columns of brickwork on which the pans stand. The temperature of the gases passing under the open pans commences at about 600° F., and gradually decreases to about 200° F. under the farthest pans. By the automatic regulation of the temperature, the waste gases are utilized to produce salts of the various degrees of coarseness required for the dairy, the stock-yard, and fishery purposes. In the two secondary closed pans, finely divided table salt is also produced, but it is possible, by opening the manhole traps in the covers, to increase the size of the crystal and make dairy salt in these pans. The coarser crystals and flake salts are made in the open pans in which the crystallization is at the lowest rate. The grain of the salt can be altered at will. In order to meet any change in the market requirements, coarser salt can be produced at a moment’s141 notice in the secondary pans. One very marked superiority of the whole system over all other processes is seen in the fact that a change in the type of salt produced can be immediately effected, and a constant and uniform output of any combination of products can be absolutely guaranteed.
The improvements which the Hodgkinson plant has effected in the open-pan system are: the increased production of from 2 to 7 tons of salt from the combustion of 1 ton of coal, the production of the finest table salt without grinding, and of every grade of salt from the flour-fine table to the coarsest fishery salt, in one and the same operation, and the saving of time that is required in all other processes for scraping and cleaning the pans. Its superiority over the Vacuum system lies in the facts that its initial cost is about £4,000, as against anything from £26,000 to £100,000; that the majority of the work being automatic, the expense of specially trained, skilled labour is dispensed with; that it is operated for 24 hours a day as against 20; requires no grinding process in the manufacture of table salt; and produces every grade of salt simultaneously. Sir Thomas Holland, while studying the Hodgkinson process in operation, is said to have exclaimed: “This is not an improvement, it is a revolution”; and in his subsequent report upon the process, he has declared that it “has an enormous advantage over any known process for the production of salt.”
142
Although no purpose would be served by dealing in detail with other of the many schemes that have been elaborated in the past three hundred years for the improvement of brine salt manufacture, the complete list of patents that have been taken out for the purpose constitutes a record of almost unrelieved failure which would occupy many pages. It has always been obvious to every intelligent investigator outside the little circle of salt proprietors, that the open-pan process was a survival of the dark ages, but the principle governing the precipitation of salt from brine is so simple that the equal difficulty presented itself to the practical salt-men, of either effecting further simplification or of securing further economies by the elaboration of the process. Individuals in every generation recognized that the methods of mediaevalism cried aloud for revision, but the salt trade resolutely and consistently set their faces, and their hands, against every suggested innovation. The salt-men were the avowed enemies of Thomas Lowndes, they drove Chrysel back to Saxony, they loaded Furnival with misfortune and landed him in gaol. In 1890, an official of the Salt Union reflected with grim complaisance that, although no trade had had more patents applied to it than the salt trade, no trade could show so large a percentage of failures in the matter of reformed methods, and since all the companies that had brought forward new plants and processes in competition with the Salt Union had come short of success, he piously concluded that the system which had survived the trial of generations must be the fittest.
143
The opposition of the salt trade to the introduction of new methods of manufacture is explained by the fact that the profits accruing from the old, clumsy, crude, and wasteful process were so large that the proprietors could see no possible reason for welcoming innovations. Moreover, the manufacture under established conditions was in the hands of a comparatively small number of makers, who could not adopt new measures without letting in more men, and the long tenure of their monopoly made the salt-men intolerant of a competitive system. Opposition was so abominable to them that, while they would combine as one man to keep out the daring intruder, or to crush such an one if he succeeded in getting in, they were not at all averse from employing similar tactics for the purpose of exterminating one another. Although it had cost them over a quarter of a million sterling to dispose of William Furnival, the game of price-cutting was not discontinued after 1833. In order to safeguard themselves against the periodical falls in prices, which, if persisted in, would mean wholesale ruin, all sorts of associations, syndicates, trusts, committees, and pools were formed for the regulation of stocks and prices, but each successive combination was successively abandoned, and was followed by another period of bitter jealousy and trading loss. Between 1846 and 1880, the trade was being continually reorganized for offensive and defensive commercial purposes, but, in 1881, it was admitted that, in spite of all attempts to encourage a better feeling among the leading manufacturers, “the spirit of envy, hate, malice, and all uncharitableness, which has so long been the bane of the salt trade, has again become rampant,” with the result that the price of common salt—4s., less the brokers’ discount of 5 per cent.—was the lowest that it had touched since the American144 Civil War. Two years later it was declared that the trade, instead of being ruled by common sense and business experience, was being ruined by personal animosities and trade jealousies.
The files of the Northwich Guardian at this period chronicle the development of a state of affairs which must be almost without parallel in any other trade. During 1883 the violent competition continued, resulting in heavy loss, the closing of many works, and a large increase in bankruptcies. In 1884, the Guardian declared that “the battle is fast becoming a war of giants.... Capital is showing itself, almost everywhere, a remorseless Juggernaut, crushing thousands of victims beneath its ponderous wheels.” In that year a proposal to form the trade into one huge company was frustrated by the bitterness of internal jealousies. Further attempts to bring all the salt proprietors into a combination for mutual protection and profit were made and abandoned from the same cause. “It is easy to make laws and regulations and to carry them out successfully when men are governed by the ordinary laws of business and common sense,” commented the Guardian in 1886, “but when sentiment or passion is allowed to interfere, it is impossible either to make sensible laws or carry them out successfully when made.”
In March, 1888, we read that “the great struggle for mastery still goes on”; in April, “the process of exhaustion is not yet complete,” and the deplorable state of the salt trade was attributed to “a few men who seem to have made more money than they know what to do with, and are spending it in seeing what amount of injury they can do to each other, and as a necessary consequence to numbers of others who are innocent of offence.” In May, a correspondent of the same journal deplored the material damage the trade was145 suffering through the perversity and selfishness of the salt proprietors, and he came to the conclusion that their object was “not really to do business but to kill one another out.” “Is there more morality,” he asked, “in the man of means starving out the man without means by selling, below cost of make, than there would be in stopping him on the highway and picking his pocket?... When the intention in the two cases is the same—the plunder or ruin of the opponent—how can the morality differ? It does seem a most grievous thing that when the greater number in the trade are anxious to do business at a profit to themselves ... they should be prevented because a few—a very few—should think only of themselves, and care nothing for the sufferings of others, and carry on the fight to the bitter end, causing enormous suffering and distress....” “We can see very clearly,” he concluded, “that if something is not done shortly to bring about a better state of affairs, some defensive action must be taken by those firms outside the present strife which will result in no good to the parties now responsible for the mischief.”
The “defensive action” referred to was already being formulated, and in October, 1888, was issued the prospectus of the Salt Union Limited, which was formed with a capital of £4,000,000 for the purpose of consolidating the undertakings of the Salt Proprietors in the United Kingdom, “with a view to ending reckless competition which injuriously affects the salt industry without conferring any adequate advantage on the public.” By virtue of the sixty-four agreements, covering the purchase of properties involving the inclusive payment of £3,704,519, the Union became the greatest salt proprietors in the world, and the success of the flotation was described as “almost unprecedented.” Apparently the only two newspapers146 that had the least dubiety concerning the success of the venture were The Times and the Northwich Guardian. The Times, while recognizing that the primary object of the movement, viz., that of “curtailing supply and creating an artificial scarcity”—would be gained if an effective monopoly could be secured, pointed out that: “The Syndicate has not acquired the control of all the mines or works at which salt is produced, and unless they do this they will not have an absolute monopoly.” The Guardian admitted that with careful management the company would prosper, but, speaking from its intimate knowledge of the spirit which animated the salt-trade, it cautiously predicted that the first few months’ operations would show whether the enterprise could go on successfully. “The scheme is a gigantic one, and may prove either a great blessing or a great curse, according to the principles on which it is conducted. Let us hope that a spirit of justice and fairness towards shareholders, servants, and the public at large will make the scheme a blessing.”
The warning voiced by The Times with regard to the Salt Union’s inefficient monopoly was justified almost immediately by the issue of prospectuses of rival salt schemes, and although opposition of this kind was treated by the Union with affected contempt, and the public was assured that the insignificant salt lands secured by rash outsiders were “such as to break the hearts of all investors who might visit them,” the fact remained, as was noted in November, 1889, that “the most remarkable thing in connection with salt has been the continuous fall in the price of Salt Union shares.” The principle on which the valuation of the Union’s acquisitions was made did not transpire, but The Times understood that “the selling price has been quite satisfactory to the vendors,” and the Chairman of the147 Union, in 1896, was feign to confess that “never were covenants so ingeniously framed as to cause lawsuits.” It is not overstating the case to say that the terms upon which the Salt Union purchased their properties provides one of the most amazing instances of reckless optimism in the history of comparatively modern finance, and the subsequent administration of the Company’s affairs was as unfortunate as the preliminary settlements had been disastrous. In one law case with a vendor from whom they had purchased for £600,000 a property which their own representative valued at £400,000, they had to pay a further £60,000, and they settled another action by selling for £125,000 a tract of land which they had originally acquired for £372,000. In 1895, they increased their capital to £4,200,000 by issue of further debentures to the amount of £200,000; and, in 1901, the capitalization of the Union was reduced to £2,600,000. Up to 1913, they had paid away £117,451 for directors’ fees, travelling expenses, etc., £99,236 for preliminary and Parliamentary expenses, law charges, etc., and £723,985 for administration charges, and from 1896 to 1914 they had only paid (in 1907) one dividend of ½ per cent. on the ordinary shares.
149
THE END
Printed by Sir Isaac Pitman & Sons, Ltd., Bath, England
Punctuation, hyphenation, and spelling were made consistent when a predominant preference was found in the original book; otherwise they were not changed.
Archaic spellings were retained.
Simple typographical errors were corrected; unbalanced quotation marks were remedied when the change was obvious, and otherwise left unbalanced.
Illustrations in this eBook have been positioned between paragraphs and outside quotations. In versions of this eBook that support hyperlinks, the page references in the List of Illustrations lead to the corresponding illustrations.
The index was not checked for proper alphabetization or correct page references.
This eBook is for the use of anyone anywhere in the United States and most other parts of the world at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org. If you are not located in the United States, you will have to check the laws of the country where you are located before using this eBook.