
Title: The Cambridge natural history, Vol. 01 (of 10)
Editor: S. F. Harmer
Author: Marcus Hartog
Sydney J. Hickson
E. W. MacBride
Igerna Brünhilda Johnson Sollas
Editor: Sir A. E. Shipley
Release date: September 18, 2023 [eBook #71677]
Language: English
Original publication: New York: MacMillan & Co, 1906
Credits: Keith Edkins, Peter Becker and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
THE
CAMBRIDGE NATURAL HISTORY
EDITED BY
S. F. HARMER, Sc.D., F.R.S., Fellow of King's College, Cambridge; Superintendent of the University Museum of Zoology
AND
A. E. SHIPLEY, M.A., F.R.S., Fellow of Christ's College, Cambridge; University Lecturer on the Morphology of Invertebrates
VOLUME I
PROTOZOA
By Marcus Hartog, M.A., Trinity College (D.Sc. Lond.), Professor of Natural History in the Queen's College, Cork
PORIFERA (SPONGES)
By Igerna B. J. Sollas, B.Sc. (Lond.), Lecturer on Zoology at Newnham College, Cambridge
COELENTERATA & CTENOPHORA
By S. J. Hickson, M.A., F.R.S., formerly Fellow and now Honorary Fellow of Downing College, Cambridge; Beyer Professor of Zoology in the Victoria University of Manchester
ECHINODERMATA
By E. W. Macbride, M.A., F.R.S., formerly Fellow of St. John's College, Cambridge; Professor of Zoology in McGill University, Montreal
London
MACMILLAN AND CO., Limited
NEW YORK: THE MACMILLAN COMPANY
1906
All rights reserved
And pitch down his basket before us,
All trembling alive
With pink and grey jellies, your sea-fruit;
You touch the strange lumps,
And mouths gape there, eyes open, all manner
Of horns and of humps.
Browning, The Englishman in Italy
| PAGE | |
| Scheme of the Classification adopted in this Book | ix |
| PROTOZOA | |
| CHAPTER I | |
| Protozoa—Introduction—Functions of Protoplasm—Cell-division—Animals and Plants | 3 |
| CHAPTER II | |
| Protozoa (continued): Spontaneous Generation—Characters of Protozoa—Classification | 42 |
| CHAPTER III | |
| Protozoa (continued): Sarcodina | 51 |
| CHAPTER IV | |
| Protozoa (continued): Sporozoa | 94 |
| CHAPTER V | |
| Protozoa (continued): Flagellata | 109 |
| CHAPTER VI | |
| Protozoa (continued): Infusoria (Ciliata and Suctoria) | 136 |
| {vi}
PORIFERA (SPONGES) |
|
| CHAPTER VII | |
| Porifera (Sponges)—Introduction—History—Description of Halichondria Panicea as an Example of British marine Sponges and of Ephydatia Fluviatilis from Fresh Water—Definition—Position in the Animal Kingdom | 165 |
| CHAPTER VIII | |
| Porifera (continued): Forms of Spicules—Calcarea—Homocoela—Heterocoela—Hexactinellida—Demospongiae—Tetractinellida—Monaxonida—Ceratosa—Key to British Genera of Sponges | 183 |
| CHAPTER IX | |
| Porifera (continued): Reproduction, Sexual and Asexual—Physiology—Distribution—Flints | 226 |
| COELENTERATA | |
| CHAPTER X | |
| Coelenterata—Introduction—Classification—Hydrozoa—Eleutheroblastea—Milleporina—Gymnoblastea—Calyptoblastea—Graptolitoidea—Stylasterina | 245 |
| CHAPTER XI | |
| Hydrozoa (continued): Trachomedusae—Narcomedusae—Siphonophora | 288 |
| CHAPTER XII | |
| Coelenterata (continued): Scyphozoa = Scyphomedusae | 310 |
| CHAPTER XIII | |
| Coelenterata (continued): Anthozoa = Actinozoa—General Characters—Alcyonaria | 326 |
| CHAPTER XIV | |
| Anthozoa (continued): Zoantharia | 365 |
| {vii}
CTENOPHORA |
|
| CHAPTER XV | |
| Ctenophora | 412 |
| ECHINODERMATA | |
| CHAPTER XVI | |
| Echinodermata—Introduction—Classification—Anatomy of a Starfish—Systematic Account of Asteroidea | 427 |
| CHAPTER XVII | |
| Echinodermata (continued): Ophiuroidea = Brittle Stars | 477 |
| CHAPTER XVIII | |
| Echinodermata (continued): Echinoidea = Sea-Urchins | 503 |
| CHAPTER XIX | |
| Echinodermata (continued): Holothuroidea = Sea-Cucumbers | 560 |
| CHAPTER XX | |
| Echinodermata (continued): Pelmatozoa—Crinoidea = Sea-Lilies—Thecoidea—Carpoidea—Cystoidea—Blastoidea | 579 |
| CHAPTER XXI | |
| Echinodermata (continued): Development and Phylogeny | 601 |
| INDEX | 625 |
The names of extinct groups are printed in italics.
| PROTOZOA (pp. 1, 48). | |||||
| SARCODINA (p. 51) | Rhizopoda (p. 51) |
Lobosa (p. 51). Filosa (p. 52). |
|||
| Foraminifera (p. 58) |
Allogromidiaceae (p. 58). Astrorhizidaceae (p. 59). Lituolidaceae (p. 59). Miliolidaceae (p. 59). Textulariaceae (p. 59). Cheilostomellaceae (p. 59). Lagenaceae (p. 59). Globigerinidae (p. 59). Rotaliaceae (p. 59). Nummulitaceae (p. 59). |
||||
| Heliozoa (p. 70) |
Aphrothoraca (p. 70). Chlamydophora (p. 71). Chalarothoraca (p. 71). Desmothoraca (p. 71). |
||||
| Radiolaria (p. 75) | Porulosa = Holotrypasta (p. 76) | Spumellaria = Peripylaea (pp. 76, 77) | Collodaria (p. 77) |
Colloidea (p. 77). Beloidea (p. 77). |
|
| Sphaerellaria (p. 77) |
Sphaeroidea (p. 77). Prunoidea (p. 77). Discoidea (p. 77). Larcoidea (p. 77). |
||||
| Acantharia = Actipylaea (pp. 76, 78) |
Actinelida (p. 78). Acanthonida (p. 78). Sphaerophracta (p. 78). Prunophracta (p. 78). |
||||
| Osculosa = Monotrypasta (p. 76) | Nassellaria = Monopylaea (pp. 76, 78) |
Nassoidea (p. 78). Plectoidea (p. 78). Stephoidea (p. 78). Spyroidea (p. 78). Botryoidea (p. 79). Cyrtoidea (p. 79). |
|||
| Phaeodaria = Cannopylaea = Tripylaea (pp. 76, 79) |
Phaeocystina (p. 79). Phaeosphaeria (p. 79). Phaeogromia (p. 79). Phaeoconchia (p. 79). |
||||
| Proteomyxa (p. 88) | Myxoidea (p. 89) |
Zoosporeae (p. 89). Azoosporeae (p. 89). |
|||
| Catallacta (p. 89). | |||||
| Mycetozoa (p. 90) |
Acrasieae (p. 90). Filoplasmodieae (p. 90). |
||||
| SPOROZOA (p. 94) | Telosporidia (p. 97) | Gregarinidaceae (pp. 97, 98) |
Schizogregarinidae (p. 97). Acephalinidae (p. 97). Dicystidae (p. 97). |
||
| Coccidiaceae (pp. 97, 99) | |||||
| Neosporidia (p. 97) |
Myxosporidiaceae (pp. 98, 106). Actinomyxidiaceae (p. 98). |
||||
| FLAGELLATA (p. 109) | Pantostomata (p. 109). | ||||
| Protomastigaceae (p. 110) |
Distomatidae (p. 110). Oikomonadidae (p. 111). Bicoecidae (p. 111). Craspedomonadidae (pp. 111, 121). Phalansteridae (p. 111). Monadidae (p. 111). Bodonidae (p. 111). Amphimonadidae (p. 111). Trimastigidae (p. 111). Polymastigidae (p. 111). |
||||
| Chrysomonadaceae (pp. 110, 125) | Coccolithophoridae (p. 114). | ||||
| Cryptomonadaceae (p. 110). | |||||
| Volvocaceae (pp. 110, 111) | |||||
|
Chloromonadaceae (p. 110). Silicoflagellata (pp. 110, 114). |
|||||
|
INFUSORIA (p. 136) |
Ciliata (p. 137) |
Gymnostomaceae (pp. 137, 152). Aspirotrichaceae (pp. 137, 153). Heterotrichaceae (pp. 137, 153). Oligotrichaceae (pp. 137, 155). |
|||
| Suctoria = Tentaculifera (p. 158). | |||||
| PORIFERA (p. 163). | |||||
| Class. | Sub-Class. | Order. | Family. | Sub-Family. | |
| MEGAMASTICTORA (pp. 183, 184) | Calcarea (p. 184) | Homocoela (p. 185) |
Leucosoleniidae (p. 185). Clathrinidae (p. 185). |
||
| Heterocoela (p. 187) |
Sycettidae (p. 187). Grantiidae (p. 192). Heteropidae (p. 192). Amphoriscidae (p. 192). |
||||
| Pharetronidae (p. 192) |
Dialytinae (p. 192). Lithoninae (p. 193). |
||||
| Astroscleridae (p. 194). | |||||
| MICROMASTICTORA (pp. 183, 195) | Myxospongiae (p. 196). | ||||
| Hexactinellida (p. 197) |
Amphidiscophora (p. 203). Hexasterophora (p. 203). |
||||
| Receptaculitidae (p. 207). | |||||
|
Octactinellida (p. 208). Heteractinellida (p. 208). |
|||||
| Demospongiae (p. 209) | Tetractinellida (pp. 211, 212) |
Choristida (p. 212). |
|||
| Monaxonida (pp. 211, 216) |
Halichondrina (p. 217). Spintharophora (p. 217). |
||||
| Ceratosa (pp. 211, 220) | Dictyoceratina (p. 220) |
Spongidae (p. 220). Spongelidae (p. 220). |
|||
| Dendroceratina (pp. 220, 221). | |||||
| COELENTERATA (p. 243). | |||||
| Class. | Order. | Sub-Order. | Family. | Sub-Family. | |
| HYDROZOA (p. 249) |
Eleutheroblastea (p. 253). Milleporina (p. 257). |
||||
| Gymnoblastea (Anthomedusae) (p. 262) |
Bougainvilliidae (p. 269). Podocorynidae (p. 270). Clavatellidae (p. 270). Cladonemidae (p. 270). Tubulariidae (p. 271). Ceratellidae (p. 271). Pennariidae (p. 272). Corynidae (p. 272). Clavidae (p. 272). Tiaridae (p. 273). Corymorphidae (p. 273). Hydrolaridae (p. 273). Monobrachiidae (p. 274). Myriothelidae (p. 274). Pelagohydridae (p. 274). |
||||
| Calyptoblastea (Leptomedusae) (p. 275) |
Aequoreidae (p. 278). Thaumantiidae (p. 278). Cannotidae (p. 278). Sertulariidae (p. 278). |
||||
| Plumulariidae (p. 279) |
Eleutheroplea (p. 279). Statoplea (p. 279). |
||||
|
Hydroceratinidae (p. 279). Campanulariidae (p. 280). Eucopidae (p. 280). Dendrograptidae (p. 281). |
|||||
| Graptolitoidea (p. 281) |
Monoprionidae (p. 282). Diprionidae (p. 282). Retiolitidae (p. 282). |
||||
| Stromatoporidae (p. 283). | |||||
| Stylasterina (p. 283) | Stylasteridae (p. 285). | ||||
| Trachomedusae (p. 288) |
Olindiidae (p. 291). Petasidae (p. 294). Trachynemidae (p. 294). Pectyllidae (p. 294). Aglauridae (p. 294). Geryoniidae (p. 295). |
||||
| Narcomedusae (p. 295) |
Cunanthidae (p. 296). Peganthidae (p. 296). Aeginidae (p. 296). Solmaridae (p. 296). |
||||
| Siphonophora (p. 297) | Calycophorae (p. 305) | Monophyidae (p. 306) |
Sphaeronectinae (p. 306). Cymbonectinae (p. 306). |
||
|
Diphyidae (p. 306) |
Amphicaryoninae (p. 306) Prayinae (p. 306) Desmophyinae (p. 307) Stephanophyinae (p. 307) |
Oppositae (p. 306) | |||
|
Galeolarinae (p. 307) Diphyopsinae (p. 307) Abylinae (p. 307) |
Superpositae (p. 307) | ||||
| Polyphyidae (p. 307). | |||||
| Physophorae (p. 307) | Physonectidae (p. 307) |
Agalminae (p. 307). Apoleminae (p. 307). Physophorinae (p. 308). |
|||
|
Auronectidae (p. 308). Rhizophysaliidae (p. 308). Chondrophoridae (p. 308). |
|||||
| SCYPHOZOA = SCYPHOMEDUSAE (pp. 249, 310) | Cubomedusae (p. 318) |
Charybdeidae (p. 318). Chirodropidae (p. 319). Tripedaliidae (p. 319). |
|||
| Stauromedusae (p. 320) |
Lucernariidae (p. 320). Depastridae (p. 321). Stenoscyphidae (p. 321). |
||||
| Coronata (p. 321) |
Periphyllidae (p. 322). Ephyropsidae (p. 322). Atollidae (p. 322). |
||||
| Discophora (p. 323) | Semaeostomata (p. 323) |
Pelagiidae (p. 323). Cyanaeidae (p. 324). Ulmaridae (p. 324). |
|||
| Rhizostomata (p. 324) | Cassiopeidae (p. 324) | = Arcadomyaria (p. 324). | |||
| Cepheidae (p. 324) | = Radiomyaria (p. 324). | ||||
|
Rhizostomatidae (p. 325) Lychnorhizidae (p. 325) Leptobrachiidae (p. 325) Catostylidae (p. 325) |
= Cyclomyaria (p. 325). | ||||
| Class. | Sub-Class. | Grade. | Order. | Sub-Order. | Family. |
| ANTHOZOA = ACTINOZOA (pp. 249, 326) | Alcyonaria (p. 329) | Protoalcyonacea (p. 342) | Haimeidae (p. 342). | ||
| Synalcyonacea (p. 342) | Stolonifera (p. 342) |
Cornulariidae (p. 344). Clavulariidae (p. 344). Tubiporidae (p. 344). Favositidae (p. 344). |
|||
| Coenothecalia (p. 344) |
Heliolitidae (p. 346). Helioporidae (p. 346). Coccoseridae (p. 346). Thecidae (p. 346). Chaetetidae (p. 346). |
||||
|
Alcyonacea (p. 346) |
Xeniidae (p. 348). Telestidae (p. 348). Coelogorgiidae (p. 349). Alcyoniidae (p. 349). Nephthyidae (p. 349). Siphonogorgiidae (p. 349). |
||||
| Gorgonacea (p. 350) | Pseudaxonia (p. 350) |
Briareidae (p. 350). Sclerogorgiidae (p. 351). Melitodidae (p. 351). Coralliidae (p. 352). |
|||
| Axifera (p. 353) |
Isidae (p. 353). Primnoidae (p. 354). Chrysogorgiidae (p. 355). Muriceidae (p. 355). Plexauridae (p. 356). Gorgoniidae (p. 356). Gorgonellidae (p. 357). |
||||
| Pennatulacea (p. 358) | Pennatuleae (p. 361) |
Pteroeididae (p. 361). Pennatulidae (p. 361). Virgulariidae (p. 362). |
|||
| Spicatae (p. 362) |
Funiculinidae (p. 362). Anthoptilidae (p. 362). Kophobelemnonidae (p. 362). Umbellulidae (p. 362). |
||||
| Verticilladeae (p. 363) | |||||
| Renilleae (p. 363) | Renillidae (p. 363). | ||||
| Veretilleae (p. 364) | |||||
| Zoantharia (pp. 329, 365) | Edwardsiidea (p. 375) |
Edwardsiidae (p. 377). Protantheidae (p. 377). |
|||
| Actiniaria (p. 377) | Actiniina (p. 380) |
Halcampidae (p. 380). Actiniidae (p. 381). Sagartiidae (p. 381). Aliciidae (p. 382). Phyllactidae (p. 382). Bunodidae (p. 382). Minyadidae (p. 383). |
|||
|
Stichodactylina (p. 383) |
Corallimorphidae (p. 383). Discosomatidae (p. 383). Rhodactidae (p. 383). Thalassianthidae (p. 383). |
||||
| Madreporaria (p. 384) |
Cyathophyllidae (p. 394). Cyathaxoniidae (p. 394). Cystiphyllidae (p. 394). |
||||
| Entocnemaria (p. 394) |
Madreporidae (p. 395). Poritidae (p. 396). |
||||
| Cyclocnemaria (p. 397) |
Aporosa (p. 397). Turbinoliidae (p. 398) Oculinidae (p. 399) Astraeidae (p. 399) A. Gemmantes (p. 400) A. Fissiparantes (p. 400) Trochosmiliacea [Sub-Fam.] (p. 401) Pocilloporidae (p. 401) |
||||
|
Fungacea (p. 402). Plesiofungiidae (p. 403) Fungiidae (p. 403) Cycloseridae (p. 404) Plesioporitidae (p. 404) Eupsammiidae (p. 404) |
|||||
| Zoanthidea (p. 404) |
Zoanthidae (p. 404). Zaphrentidae (p. 406). |
||||
| Antipathidea = Antipatharia (p. 407) |
Antipathidae (p. 408). Leiopathidae (p. 409). Dendrobrachiidae (p. 409). |
||||
| Cerianthidea (p. 409). | |||||
| CTENOPHORA (p. 412). | ||
| Class. | Order. | Family. |
| TENTACULATA (p. 417) | Cydippidea (p. 417) |
Mertensiidae (p. 417). Callianiridae (p. 417). Pleurobrachiidae (p. 418). |
| Lobata (p. 418) |
Lesueuriidae (p. 419). Bolinidae (p. 419). Deiopeidae (p. 419). Eurhamphaeidae (p. 419). Eucharidae (p. 420). Mnemiidae (p. 420). Calymmidae (p. 420). Ocyroidae (p. 420). |
|
| Cestoidea (p. 420) | Cestidae (p. 420). | |
| Platyctenea (p. 421) |
Ctenoplanidae (p. 421). Coeloplanidae (p. 422). |
|
| NUDA (p. 423) | Beroidae (p. 423). | |
| ECHINODERMATA (p. 425). | |||||
| Sub-Phylum. | Class. | Order. | Sub-Order. | Family. | Sub-Family. |
| ELEUTHEROZOA (p. 430) | Asteroidea (pp. 430, 431) | Spinulosa (pp. 461, 462) |
Echinasteridae (p. 462). Solasteridae (p. 462). Asterinidae (p. 463). Poraniidae (p. 464). Ganeriidae (p. 464). Mithrodiidae (p. 464). |
||
| Velata (pp. 461, 464) |
Pythonasteridae (p. 464). Myxasteridae (p. 464). Pterasteridae (p. 466). |
||||
| Paxillosa (pp. 461, 466) |
Archasteridae (p. 466). Astropectinidae (p. 467). Porcellanasteridae (p. 470). |
||||
| Valvata (pp. 461, 471) |
Linckiidae (p. 471). Pentagonasteridae (p. 471). Gymnasteridae (p. 471). Antheneidae (p. 471). Pentacerotidae (p. 471). |
||||
| Forcipulata (pp. 462, 473) |
Asteriidae (p. 473). Heliasteridae (p. 474). Zoroasteridae (p. 474). Stichasteridae (p. 474). Pedicellasteridae (p. 474). Brisingidae (p. 474). |
||||
| Ophiuroidea (pp. 431, 477) | Streptophiurae (p. 494) | ||||
| Zygophiurae (pp. 494, 495) |
Ophiolepididae (p. 495). Amphiuridae (p. 497). Ophiocomidae (p. 499). Ophiothricidae (p. 499). |
||||
| Cladophiurae (pp. 494, 500) |
Astroschemidae (p. 501). Trichasteridae (p. 501). Euryalidae (p. 501). |
||||
| Echinoidea (pp. 431, 503) | Endocyclica (pp. 529, 530) |
Cidaridae (p. 533). Echinothuriidae (p. 535). Saleniidae (p. 537). Arbaciidae (p. 538). Diadematidae (p. 538). |
|||
| Echinidae (p. 539) |
Temnopleurinae (p. 539). Echininae (p. 539). |
||||
| Clypeastroidea (pp. 529, 542) | Protoclypeastroidea (p. 548). | ||||
| Euclypeastroidea (p. 549) |
Fibularidae (p. 549). Echinanthidae = Clypeastridae (p. 549). Laganidae (p. 549). Scutellidae (p. 549). |
||||
|
Echinonidae (p. 553) Nucleolidae (p. 554) Cassidulidae (p. 554) |
Asternata (p. 554). | ||||
|
Ananchytidae (p. 554) Palaeostomatidae (p. 554) Spatangidae (p. 554) Brissidae (p. 556) |
Sternata (p. 554). | ||||
|
Archaeocidaridae (p. 557). Melonitidae (p. 557). Tiarechinidae (p. 557). Holectypoidea (p. 558). Echinoconidae (p. 558). Collyritidae (p. 559). |
|||||
| Holothuroidea (pp. 431, 560) |
Aspidochirota (p. 570). Elasipoda (p. 571). Pelagothuriida (p. 572). Dendrochirota (p. 572). Molpadiida (p. 575). Synaptida (p. 575). |
||||
| PELMATOZOA (pp. 430, 579) | Crinoidea (p. 580) |
Hyocrinidae (p. 590). Rhizocrinidae (p. 590). Pentacrinidae (p. 591). Holopodidae (p. 592). Comatulidae (p. 594). |
|||
|
Inadunata (p. 595). Articulata (p. 595). Camerata (p. 595). |
|||||
BY
MARCUS HARTOG, M.A., Trinity College (D.Sc. Lond.)
Professor of Natural History in the Queen's College, Cork.
PROTOZOA—INTRODUCTION—FUNCTIONS OF PROTOPLASM—CELL-DIVISION—ANIMALS AND PLANTS
The Free Amoeboid Cell.—If we examine under the microscope a fragment of one of the higher animals or plants, we find in it a very complex structure. A careful study shows that it always consists of certain minute elements of fundamentally the same nature, which are combined or fused into "tissues." In plants, where these units of structure were first studied, and where they are easier to recognise, each tiny unit is usually enclosed in an envelope or wall of woody or papery material, so that the whole plant is honeycombed. Each separate cavity was at first called a "cell"; and this term was then applied to the bounding wall, and finally to the unit of living matter within, the envelope receiving the name of "cell-wall." In this modern sense the "cell" consists of a viscid substance, called first in animals "sarcode" by Dujardin (1835), and later in plants "protoplasm"[1] by Von Mohl (1846). On the recognition of its common nature in both kingdoms, largely due to Max Schultze, the latter term prevailed; and it has passed from the vocabulary of biology into the domain of everyday life. We shall now examine the structure and behaviour of protoplasm and of the cell as an introduction to the detailed study of the Protozoa, or better still Protista,[2] the lowest types of living beings, and of Animals at large.
It is not in detached fragments of the tissues of the higher animals that we can best carry on this study: for here the cells are in singularly close connexion with their neighbours during life; the proper appointed work of each is intimately related to that of the others; and this co-operation has so trained and specially modified each cell that the artificial severance and isolation is detrimental to its well-being, if not necessarily fatal to its very life. Again, in plants the presence of a cell-wall interferes in many ways with the free behaviour of the cell. But in the blood and lymph of higher animals there float isolated cells, the white corpuscles or "leucocytes" of human histology, which, despite their minuteness (1⁄3000 in. in diameter), are in many respects suitable objects. Further, in our waters, fresh or salt, we may find similar free-living individual cells, in many respects resembling the leucocytes, but even better suited for our study. For, in the first place, we can far more readily reproduce under the microscope the normal conditions of their life; and, moreover, these free organisms are often many times larger than the leucocyte. Such free organisms are individual Protozoa, and are called by the general term "Amoebae." A large Amoeba may measure in its most contracted state 1⁄100 in. or 250 µ in diameter,[3] and some closely allied species (Pelomyxa, see p. 52) even twelve times this amount. If we place an Amoeba or a leucocyte under the microscope (Fig. 1), we shall find that its form, at first spherical, soon begins to alter. To confine our attention to the external changes, we note that the outline, from circular, soon becomes "island-shaped" by the outgrowth of a promontory here, the indenting of a bay there. The promontory may enlarge into a peninsula, and thus grow until it becomes a new mainland, while the old mainland dwindles into a mere promontory, and is finally lost. In this way a crawling motion is effected.[4] The promontories are called "pseudopodia" (= {5}"false-feet"), and the general character of such motion is called "amoeboid."[5]
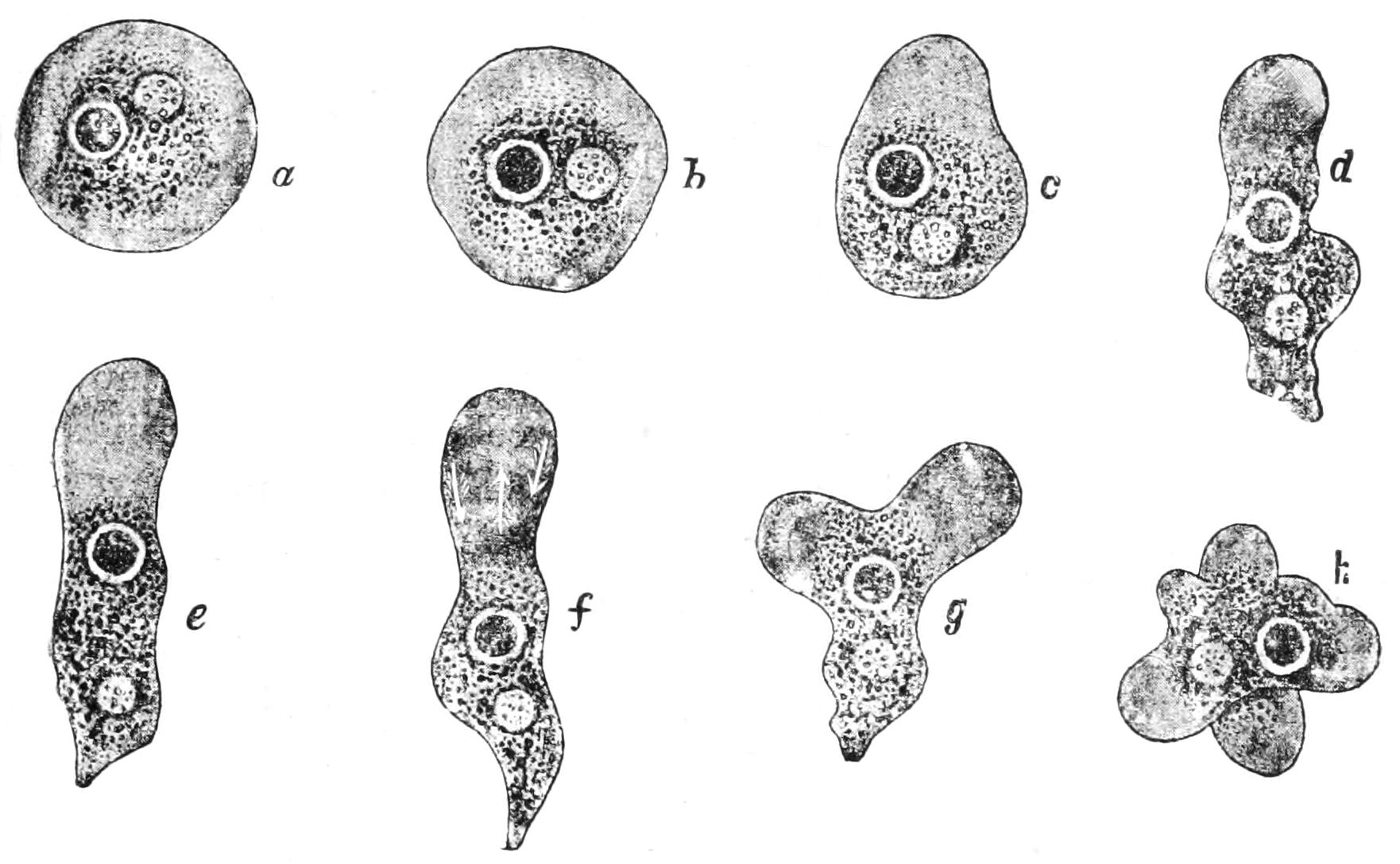
Fig. 1.—Amoeba, showing clear ectoplasm, granular endoplasm, dark nucleus, and lighter contractile vacuole. The changes of form, a-f, are of the A. limax type; g, h, of the A. proteus type. (From Verworn.)
The living substance, protoplasm,[6] has been termed a "jelly," a word, however, that is quite inapplicable to it in its living state. It is viscid, almost semi-fluid, and may well be compared to very soft dough which has already begun to rise. It resembles it in often having a number of spaces, small or large, filled with liquid (not gas). These are termed "vacuoles" or "alveoles," according to their greater or their lesser dimensions. In some cases a vacuole is traversed by strands of plasmic substance, just as we may find such strands stretching across the larger spaces of a very light loaf; but of course in the living cell these are constantly undergoing changes. If we "fix" a cell (i.e. kill it by {6}sudden heat or certain chemical coagulants),[7] and examine it under the microscope, the intermediate substance between the vacuoles that we have already seen in life is again found either to be finely honeycombed or else resolved into a network like that of a sponge. The former structure is called a "foam" or "alveolar" structure, the latter a "reticulate" structure. The alveoles are about 1 µ in diameter, and spheroidal or polygonal by mutual contact, elongated, however, radially to any free surface, whether it be that of the cell itself or that of a larger alveole or vacuole. The inner layer of protoplasm ("endoplasm," "endosarc") contains also granules of various nature, reserve matters of various kinds, oil-globules, and particles of mineral matter[8] which are waste products, and are called "excretory." In fixed specimens these granules are seen to occupy the nodes of the network or of the alveoli, that is, the points where two or three boundaries meet.[9] The outermost layer ("ectoplasm" or "ectosarc") appears in the live Amoeba structureless and hyaline, even under conditions the most favourable for observation. The refractive index of protoplasm, when living, is always well under 1.4, that of the fixed and dehydrated substance is slightly over 1.6.
Again, within the outer protoplasm is found a body of slightly higher refractivity and of definite outline, termed the "nucleus" (Figs. 1, 2). This has a definite "wall" of plasmic nature, and a substance so closely resembling the outer protoplasm in character, that we call it the "nucleoplasm" (also "linin"), distinguishing the outer plasm as "cytoplasm"; the term "protoplasm" including both. Within the nucleoplasm are granules of a substance that stains well with the commoner dyes, especially the "basic" ones, and which has hence been called "chromatin." The linin is {7}usually arranged in a distinct network, confluent into a "parietal layer" within the nuclear wall; the meshes traversing a cavity full of liquid, the nuclear sap, and containing in their course the granules; while in the cavity are usually found one or two droplets of a denser substance termed "nucleoles." These differ slightly in composition from the chromatin granules[10] (see p. 24 f.).
The movements of the leucocyte or Amoeba are usually most active at a temperature of about 40° C. or 100° F., the "optimum." They cease when the temperature falls to a point, the "minimum," varying with the organism, but never below freezing-point; they recommence when the temperature rises again to the same point at which they stopped. If now the temperature be raised to a certain amount above 40° they stop, but may recommence if the temperature has not exceeded a certain point, the "maximum" (45° C. is a common maximum). If it has been raised to a still higher point they will not recommence under any circumstances whatever.
Again, a slight electric shock will determine the retraction of all processes, and a period of rest in a spherical condition. A milder shock will only arrest the movements. But a stronger shock may arrest them permanently. We may often note a relation of the movements towards a surface, tending to keep the Amoeba in contact with it, whether it be the surface of a solid or that of an air-bubble in the liquid (see also p. 20).
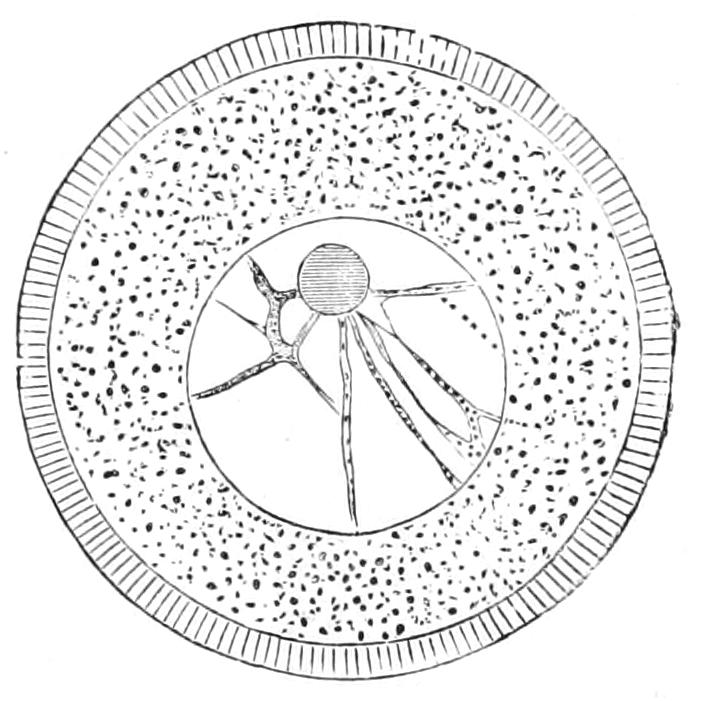
Fig. 2.—Ovum of a Sea-Urchin, showing the radially striated cell-membrane, the cytoplasm containing yolk-granules, the large nucleus (germinal vesicle), with its network of linin containing chromatin granules, and a large nucleole (germinal spot). (From Balfour's Embryology, after Hertwig.)
If a gentle current be set up in the water, we find that the movements of the Amoeba are so co-ordinated that it moves upstream; this must of course be of advantage in nature, as keeping the being in its place, against the streams set up by larger creatures, etc. (see also p. 21).
If substances soluble in water be introduced the Amoeba will, {8}as a rule, move away from the region of greater concentration for some substances, but towards it (provided it be not excessive) for others. (See also pp. 22, 23.) We find, indeed, that there is for substances of the latter category a minimum of concentration, below which no effect is seen, and a maximum beyond which further concentration repels. The easiest way to make such observations is to take up a little strong solution in a capillary tube sealed at the far end, and to introduce its open end into the water, and let the solution diffuse out, so that this end may be regarded as surrounded by zones of continuously decreasing strength. In the process of inflammation (of a Higher Animal) it has been found that the white corpuscles are so attracted by the source of irritation that they creep out of the capillaries, and crowd towards it.
We cannot imagine a piece of dough exhibiting any of these reactions, or the like of them; it can only move passively under the action of some one or other of the recognised physical forces, and that only in direct quantitative relation to the work that such forces can effect; in other words, the dough can have work done on it, but it cannot do work. The Amoeba or leucocyte on the contrary does work. It moves under the various circumstances by the transformation of some of its internal energy from the "potential" into the "kinetic" state, the condition corresponding with this being essentially a liberation of heat or work, either by the breaking down of its internal substances, or by the combination of some of them with oxygen.[11] Such of these changes as involve the excretion of carbonic acid are termed "respiratory."
This liberation of energy is the "response" to an action of itself inadequate to produce it; and has been compared not inaptly to the discharge of a cannon, where foot-tons of energy are liberated in consequence of the pull of a few inch-grains on the trigger, or to an indefinitely small push which makes electric contact: the energy set free is that which was stored up in the charge. This capacity for liberating energy stored up within, in response to a relatively small impulse from without, is termed "irritability"; the external impulse is termed the "stimulus." The responsive act has been termed "contractility," because it so often means an obvious contraction, but is better termed {9}"motility "; and irritability evinced by motility is characteristic of all living beings save when in the temporary condition of "rest."
Again, in the case of the cannon, the gunner after its discharge has to replenish it for future action with a fresh cartridge; the Amoeba or leucocyte can replenish itself—it "feeds." When it comes in contact with a fragment of suitable material, it enwraps it by its pseudopodia (Fig. 3), and its edges coalesce where they touch on the far side as completely as we can join up the edges of dough round the apple in a dumpling. It dissolves all that can be dissolved—i.e. it "digests" it, and then absorbs the dissolved material into its substance, both to replace what it has lost by its previous activity and to supply fuel for future liberation of energy; this process is termed "nutrition," and is another characteristic of living beings.
Again, as a second result of the nutrition, part of the food taken in goes to effect an increase of the living protoplasm, and that of every part, not merely of the surface—it is "assimilated"; while the rest of the food is transformed into reserves, or consumed and directly applied to the liberation of energy. The increase in bulk due to nutrition is thus twofold: part is the increase of the protoplasm itself—"assimilative growth," part is the storage of reserves—"accumulative growth": these reserves being available in turn by digestion, whether for future true growth or for consumption to liberate energy for the work of the cell.
We can conceive that our cannon might have an automatic feed for the supply of fresh cartridges after each shot; but not that it could make provision for an increase of its own bulk, so as to gain in calibre and strength, nor even for the restoration {10}of its inner surface constantly worn away by the erosion of its discharges. Growth—and that growth "interstitial," operating at every point of the protoplasm, not merely at its surface—is a character of all living beings at some stage, though they may ultimately lose the capacity to grow. Nothing at all comparable to interstitial growth has been recognised in not-living matter.[12]

Fig. 4.—Amoeba polypodia in successive stages of equal fission; nucleus dark, contractile vacuole clear. (From Verworn, after F. E. Schulze.)
Again, when an Amoeba has grown to a certain size, its nucleus divides into two nuclei, and its cytoplasmic body, as we may term it, elongates, narrows in the middle so as to assume the shape of a dumb-bell or finger-biscuit, and the two halves, crawling in opposite directions, separate by the giving way of the connecting waist, forming two new Amoebas, each with its nucleus (Fig. 4). This is a process of "reproduction"; the special case is one of "equal fission" or "binary division." The original cell is termed the "mother," with respect to the two new ones, and these are of course with respect to it the "daughters," and {11}"sisters" to one another. We must bear in mind that in this self-sacrificing maternity the mother is resolved into her children, and her very existence is lost in their production. The above phenomena, IRRITABILITY, MOTILITY, DIGESTION, NUTRITION, GROWTH, REPRODUCTION, are all characteristic of living beings at some stage or other, though one or more may often be temporarily or permanently absent; they are therefore called "vital processes."
If, on the other hand, we violently compress the cell, if we pass a very strong electric shock through it, or a strong continuous current, or expose it to a temperature much above 45° C., or to the action of certain chemical substances, such as strong acids or alkalies, or alcohol or corrosive sublimate, we find that all these vital processes are arrested once and for all; henceforward the cell is on a par with any not-living substance. Such a change is called "DEATH," and the "capacity for death" is one of the most marked characters of living beings. This change is associated with changes in the mechanical and optical properties of the protoplasm, which loses its viscidity and becomes opaque, having undergone a process of de-solution; for the water it contained is now held only mechanically in the interstices of a network, or in cavities of a honeycomb (as we have noted above, p. 5), while the solid forming the residuum has a refractive index of a little over 1.6. Therefore, it only regains its full transparency when the water is replaced by a liquid of high refractive index, such as an essential oil or phenol. A similar change may be effected by pouring white of egg into boiling water or absolute alcohol, and is attended with the same optical results. The study of the behaviour of coagulable colloids has been recently studied by Fischer and by Hardy, and has been of the utmost service in our interpretation of the microscopical appearances shown in biological specimens under the microscope.[13]
The death of the living being finds a certain analogy in the breaking up or the wearing out of a piece of machinery; but in no piece of machinery do we find the varied irritabilities, all conducive to the well-being of the organism (under ordinary conditions), or the so-called "automatic processes"[14] that enable the living being to go through its characteristic functions, to grow, and as we shall see, even to turn conditions unfavourable for active life and growth to the ultimate weal of the species (see p. 32). At the same time, we fully recognise that for supplies of matter and energy the organism, like the machine, depends absolutely on sources from without. The debtor and creditor sheet, in respect of matter and energy, can be proved to balance between the outside world and Higher Organisms with the utmost accuracy that our instruments can attain; and we infer that this holds for the Lower Organisms also. Many of the changes within the organism can be expressed in terms of chemistry and physics; but it is far more impossible to state them all in such terms than it would be to describe a polyphase electrical installation in terms of dynamics and hydraulics. And so far at least we are justified in speaking of "vital forces."
The living substance of protoplasm contains a large quantity of water, at least two-thirds its mass, as we have seen, in a state of physical or loose chemical combination with solids: these on death yield proteids and nucleo-proteids.[15] The living protoplasm {13}has an alkaline reaction, while the liquid in the larger vacuoles, at least, is acid, especially in Plant-cells.[16]
Metabolism.—The chemical processes that go on in the organism are termed metabolic changes, and were roughly divided by Gaskell into (1) "anabolic," in which more complex and less stable substances are built up from less complex and more stable ones with the absorption of energy; and (2) "catabolic" changes in which the reverse takes place. Anabolic processes, in all but the cells containing plastids or chromatophores (see p. 36) under the influence of light, necessarily imply the furnishing of energy by concurrent catabolic changes in the food or reserves, or in the protoplasm itself.
Again, we have divided anabolic processes into "accumulative," where the substances formed are merely reserves for the future use of the cell, and "assimilative," where the substances go to the building of the protoplasm itself, whether for the purpose of growth or for that of repair.
Catabolic processes may involve (1) the mere breaking of complex substances into simpler ones, or (2) their combination with oxygen; in either case waste products are formed, which may either be of service to the organism as "secretions" (like the bile in Higher Animals), or of no further use (like the urine). When nitrogenous substances break down in this way they give rise to "excretions," containing urea, urates, and allied substances; other products of catabolism are carbon dioxide, water, and mineral salts, such as sulphates, phosphates, carbonates, oxalates, etc., which if not insoluble must needs be removed promptly from the organism, many of them being injurious or even poisonous. The energy liberated by the protoplasm being derived through the breakdown of another part of the same or of the {14}food-materials or stored reserves, must give rise to waste products. The exchange of oxygen from without for carbonic acid formed within is termed "respiration," and is distinguished from the mere removal of all other waste products called "excretion." In the fresh-water Amoeba both these processes can be studied.
Respiration,[17] or the interchange of gases, must, of course, take place all over the general surface, but in addition it is combined in most fresh-water Protista with excretion in an organ termed the "contractile" or "pulsatile vacuole" (Figs. 1, 4, etc.). This particular vacuole is exceptional in its size and its constancy of position. At intervals, more or less regular, it is seen to contract, and to expel its contents through a pore; at each contraction it completely disappears, and reforms slowly, sometimes directly, sometimes by the appearance of a variable number of small "formative" vacuoles that run together, or as in Ciliata, by the discharge into it of so-called "feeding canals." As this vacuole is filled by the water that diffuses through the substance, and when distended may reach one-third the diameter of the being, in the interval between two contractions an amount of water must have soaked in equal to one-twenty-seventh the bulk of the animal, to be excreted with whatever substances it has taken up in solution, including, not only carbon dioxide, but also, it has been shown, nitrogenised waste matters allied to uric acid.[18]
That the due interchanges may take place between the cell and the surrounding medium, it is obvious that certain limits to the ratio between bulk and surface must exist, which are disturbed by growth, and which we shall study hereafter (p. 23 f.).
The Protista that live in water undergo a death by "diffluence" or "granular disintegration" on being wounded, crushed, or sometimes after an excessive electric stimulation, or contact with alkalies or with acids too weak to coagulate them. In this process the protoplasm breaks up from the surface inwards into a mass of granules, the majority of which themselves finally dissolve. If the injury be a local rupture of the external pellicle or {15}cuticle, a vacuole forms at the point, grows and distends the overlying cytoplasm, which finally ruptures: the walls of the vacuole disintegrate; and this goes on as above described. Ciliate Infusoria are especially liable to this disintegration process, often termed "diffluence," which, repeatedly described by early observers, has recently been studied in detail by Verworn. Here we have death by "solution," while in the "fixing" of protoplasm for microscopic processes we strive to ensure death by "desolution," so as to retain as much of the late living matter as possible. It would seem not improbable that the unusual contact with water determines the formation of a zymase that acts on the living substance itself.
We have suggested[19] that one function of the contractile vacuole, in naked fresh-water Protists, is to afford a regular means of discharge of the water constantly taken up by the crystalloids in the protoplasm, and so to check the tendency to form irregular disruptive vacuoles and death by diffluence. This is supported by the fact that in the holophytic fresh-water Protista, as well as the Algae and Fungi, a contractile vacuole is present in the young naked stage (zoospore), but disappears as soon as an elastic cell-wall is formed to counterbalance by its tension the internal osmotic pressure.
Digestion is always essentially a catabolic process, both as regards the substance digested and the formation of the digesting substance by the protoplasm. The digesting substance is termed a "zymase" or "chemical ferment," and is conjectured to be produced by the partial breakdown of the protoplasm. In presence of suitable zymases, many substances are resolved into two or more new substances, often taking up the elements of water at the same time, and are said to be "dissociated" or "hydrolysed" as the case may be. Thus proteid substances are converted into the very soluble substances, "proteoses" and "peptones," often with the concurrent or ultimate formation of such relatively simple bodies as leucin, tyrosin, and other amines, etc. Starch and glycogen are converted into dextrins and sugars; fats are converted into fatty acids and glycerin. It is these products of digestion, and not the actual food-materials (save certain very simple sugars), that are really taken up by the protoplasm, {16}whether for assimilation, for accumulation, or for the direct liberation of energy for the vital processes of the organism.
Not only food from without, but also reserves formed and stored by the protoplasm itself, must be digested by some zymase before they can be utilised by the cell. In all cases of the utilisation of reserve matter that have been investigated, it has been found that a zymase is formed by the cell itself (or sometimes, in complex organisms, by its neighbours); for, after killing the cell in which the process is going on by mechanical means or by alcohol, the process of digestion can be carried on in the laboratory.[20] The chief digestion of all the animal-feeding Protista is of the same type as in our own stomachs, known as "peptic" digestion: this involves the concurrent presence of an acid, and Le Dantec and Miss Greenwood have found the contents of food-vacuoles, in which digestion is going on, to contain acid liquid. The ferment-pepsin itself has been extracted by Krukenberg from the Myxomycete, "Flowers of tan" (Fuligo varians, p. 92), and by Professor Augustus Dixon and the author from the gigantic multinucleate Amoeba, Pelomyxa palustris (p. 52).[21] The details of the prehension of food will be treated of under the several groups.
The two modes of Anabolism—true "assimilation" in the strictest sense and "accumulation"—may sometimes go on concurrently, a certain proportion of the food material going to the protoplasm, and the rest, after allowing for waste, being converted into reserves.
Movements all demand catabolic changes, and we now proceed to consider these in more detail.
The movements of an Amoeboid[22] cell are of two kinds: "expansion," leading to the formation and enlargement of {17}outgrowths, and "contraction," leading to their diminution and disappearance within the general surface.[23] Expansion is probably due to the lessening of the surface-tension at the point of outgrowth, contraction to the increase of surface-tension. Verworn regards these as due respectively to the combination of the oxygen in the medium with the protoplasm in diminishing surface-tension, and the effect of combination with substances from within, especially from the nucleus in increasing it. Besides these external movements, there are internal movements revealed by the contained granules, which stream freely in the more fluid interior. Those Protista that, while exhibiting amoeboid movements, have no clear external layer, such as the Radiolaria, Foraminifera, Heliozoa, etc., present this streaming even at the surface, the granules travelling up and down the pseudopodia at a rate much greater than the movements of these organs themselves. In this case the protoplasm is wetted by the medium, which it is not where there is a clear outer layer: for that behaves like a greasy film.
Motile organs.—Protoplasm often exhibits movements much more highly specialised than the simple expansion or retraction of processes, or the general change of form seen in Amoeba. If we imagine the activities of a cell concentrated on particular parts, we may well suppose that they would be at once more precise and more energetic than we see them in Amoeba or the leucocyte. In some free-swimming cells, such as the individual cells known as "Flagellata," the reproductive cells of the lower Plants, or the male cells ("spermatozoa") of Plants as high as Ferns, and even of the Highest Animals, there is an extension of the cell into one or more elongated lash-like processes, termed "flagella," which, by beating the water in a reciprocating or a spiral rhythm, cause the cell to travel through it; or, if the cell be attached, they produce currents in the water that bring food particles to the surface of the cell for ingestion. Such flagella may, indeed, be seen in some cases to be modified pseudopodia. In other cases part, or the whole, of the surface of the cell may be covered with regularly arranged short filaments of similar activity (termed "cilia," from their resemblance to a diminutive eyelash), which, however, instead of whirling round, bend sharply {18}down to the surface and slowly recover; the movement affects the cilia successively in a definite direction in waves, and produces, like that of flagella, either locomotion of the cell or currents in the medium. We can best realise their action by recalling the waves of bending and recovery of the cornstalks in a wind-swept field; if now the haulms of the corn executed these movements of themselves, they would determine in the air above a breeze-like motion in the direction of the waves (Fig. 5).[24] Such cilia are not infrequent on those cells of even the Highest Animals that, like a mosaic, cover free surfaces ("epithelium cells"). In ourselves such cells line, for instance, the windpipe. One group of the Protozoa, the "Ciliata," are, as their name implies, ciliated cells pure and simple.
The motions of cilia and of flagella are probably also due to changes of surface tension—alternately on one side and the other in the cilium, but passing round in circular succession in the flagellum,[25] giving rise to a conical rotation like that of a weighted string that is whirled round the head. This motion is, however, strongest at the thicker basal part, which assumes a spiral form like a corkscrew of few turns, while the thin lash at the tip may seem even to be quietly extended like the point of the corkscrew. If the tip of the flagellum adhere, as it sometimes does, to any object, the motions induce a jerking motion, which in this case is reciprocating, not rotatory. When the organism is free, the flagellum is usually in advance, and the cell follows, rotating at the same time round its longitudinal axis; such an anterior flagellum, called a "tractellum," is the common form in Protista that possess a single one (Figs. 29, 7, 8; 30, C). In the spermatozoa of Higher Animals (and some Sporozoa) the flagellum is posterior, and is called a "pulsellum."
The cilium or flagellum may often be traced a certain distance into the substance of the cytoplasm to end in a dot of denser, {19}readily-staining plasm, which corresponds to a "centrosome" or centre of plasmic forces (see below, pp. 115, 121, 141); it has been termed a "blepharoplast."[26]
Again, the cytoplasm may have differentiated in it definite streaks of specially contractile character; such streaks within its substance are called "myonemes"; they are, in fact, muscular fibrils. A "muscle-cell," in the Higher Animals, is one whose protoplasm is almost entirely so modified, with the exception of a small portion of granular cytoplasm investing the nucleus, and having mainly a nutritive function.
Definite muscular fibrils in action shorten, and at the same time become thicker. It seems probable that they contain elongated vacuoles, and that the contents of these vary, so that when they have an increased osmotic equivalent, the vacuoles absorb water, enlarge, and tend to become more spherical, i.e. shorter and thicker, and so the fibril shortens as a whole. The relaxation would be due to the diffusion outwards of the solution of the osmotically active substances which induced expansion.[27]
The Motile Reactions of the Protozoa[28] require study from another point of view: they are either (1) "spontaneous" or "arbitrary," as we may say, or (2) responsive to some stimulus. The latter kind we will take first, as they are characteristic of all free cells. The stimuli that induce movements of a responsive character are as follows:—(i.) MECHANICAL: such as agitation and contact; (ii.) force of GRAVITY, or CENTRIFUGAL FORCE; (iii.) CURRENTS in the water; (iv.) RADIANT ENERGY (LIGHT); (v.) changes in the TEMPERATURE of the medium; (vi.) ELECTRIC CURRENTS through the medium; (vii.) the presence of CHEMICAL SUBSTANCES in the medium.
These, or some of them, may induce one of three different results, or a combination thereof: (1) a single movement or an arrest of motion; (2) the assumption of a definite position; (3) movement of a definite character or direction.
(i.) Mechanical stimuli.—Any sudden touch with another body tends to arrest all motion; and if the shock be protracted or severe, the retraction of the pseudopodia follows. It is to this reaction that we must ascribe the retracted condition of the pseudopodia of most Rhizopods when first placed on the slide and covered for microscopic examination. Free-swimming Protista may, after hitting any body, either remain in contact with it, or else, after a pause, reverse their movement, turn over and swim directly away. This combination of movements is characteristic as a reaction of what we may term "repellent" stimuli in general.[29] Another mechanical reaction is that to continuous contact with a solid; and the surface film of water, either at the free surface or round an air-bubble, may play the part of a solid in exciting it; we term it "thigmotaxy" or "stereotaxy." When positive it determines a movement on to the surface, or a gliding movement along it, or merely the arrest of motion and prolongation of contact; when negative, a contact is followed by the retreat of the being. Thus Paramecium (Fig. 55, p. 151) and many other Ciliates are led to aggregate about solid particles or masses of organic débris in the water, which indeed serve to supply their food. On contact, the cell ceases to move its cilia except those of the oral groove; as these lash backwards, they hold the front end in close contact with the solid, at the same time provoking a backward stream down the groove, which may bring in minute particles from the mass.
(ii.) Most living beings are able to maintain their level in water by floating or crawling against Gravity, and they react in virtue of the same power against centrifugal force. This mode of irritability is termed (negative) "geotaxy" or "barotaxy." We can estimate the power of resisting such force by means of a whirling machine, since when the acceleration is greater than the resistance stimulated thereby in the beings, they are passively sent to the sides of the vessel. The Flagellates, Euglena and Chlamydomonas, begin to migrate towards the centre when exposed to a centrifugal force about equal to ½ G (G = 32.2 feet or 982 cm. per second); they remain at the centre until the centrifugal force is increased to 8 G; above that they yield to the force, and are driven passively to the sides. The reaction ceases or is reversed at high temperatures.
(iii.) Rheotaxy.—This is the tendency to move against the stream in flowing water. It is shown by most Protists, and can be conveniently studied in the large amoeboid plasmodia of the Myxomycetes, which crawl against the stream along wet strips of filter paper, down which water is caused to flow. Most animals, even of the highest groups, tend to react in the same way; the energetic swimming of Fishes up-stream being in marked contrast with their sluggishness the other way; and every student of pond-life knows how small Crustacea and Rotifers, no less than Ciliates, swim away from the inrush of liquid into the dipping-tube, and so evade capture. (See Vol. II. p. 216.)
(iv.) The movements of many Protozoa are affected greatly by Light. These movements have been distinguished into "photopathic," i.e. to or from the position of greatest luminosity; and "phototactic," along the direct path of the rays.[30] Those Protozoa that contain a portion of their cytoplasm, known as a "plastid" or "chromatophore" (see pp. 36, 39), coloured by a green or yellow pigment are usually "phototactic." They mostly have at the anterior end a red pigment spot, which serves as an organ of sight, and is known as an "eye-spot." In diffused light of low intensity they do not exhibit this reaction, but in bright sunlight they rise to the surface and form there a green or yellow scum.
Most of the colourless Protista are negatively phototactic or photopathic; but those which are parasitic on the coloured ones are positively phototactic, like their hosts.
Here, as in the case of other stimuli,[31] the absolute intensity of the light is of importance; for as it increases from a low degree, different organisms in turn cease to be stimulated, and {22}then are repelled instead of being attracted. The most active part of the spectrum in determining reactions of movement are the violet and blue rays of wave-length between 40 µ/10 and 49 µ/10, while the warmer and less refractive half of the spectrum is inert save in so far as it determines changes in the temperature of the medium.
(v.) The movements of many Protozoa are rendered sluggish by cold, and active by a rise of Temperature up to what we may term the "optimum"; the species becomes sluggish again as the temperature continues to rise to a certain point when the movements are arrested, and the being is said to be in a state of "heat-rigor." Most Protozoa, again, tend to move in an unequally heated medium to the position nearest to their respective optimum temperature. This is called "thermotaxy." The temperature to which Amoeba is thermotactic is recorded as 35° C. (95° F.); that of Paramecium is 28° C. (82° F.).
(vi.) Most active Protozoa tend to take up a definite position in respect to a current of Electricity passing through the medium, and in the majority of cases, including most Ciliates, Amoeba, and Trachelomonas, they orient their long diameters in the direction of the lines of force and swim along these to assemble behind the cathode. The phenomenon is called "galvanotaxy," and this particular form is "negative." Opalina (Fig. 41, p. 123), however, and most Flagellates are "positively galvanotactic," and move towards the anode. H. H. Dale[32] has shown that the phenomenon may be possibly in reality a case of chemiotaxy, for the direction of motion varies with the nature and concentration of the medium. It would thus be a reaction to the "ion" liberated in contact with the one or other extremity of the being. Induction shocks, as we have seen, if slight, arrest the movements of Protozoa, or if a little stronger determine movements of contraction; if of sufficient intensity they kill them. No observation seems to have been made on the behaviour of Protista in an electric field. A magnetic field of the highest intensity appears to be indifferent to all Protista.
(vii.) We have already referred to the effect of dissolved Chemical Substances present in the water. If the substance is in itself not harmful, and the effect varies with the concentration, we term the reaction one of "tonotaxy," which combines {23}with that of "chemiotaxy" for substances that in weak solution are attractive or repellent to the being. Paramecium, which feeds on bacteria, organisms of putrefaction, is positively chemiotactic to solutions of carbon dioxide, and as it gives this off in its own respiration, it is attracted to its fellows. The special case of reaction to gases in solution is termed "aerotaxy," or "pneumotaxy," according as the gas is oxygen or carbon dioxide. We find that in this respect there are degrees, so that a mixed culture of Flagellates in an organic infusion sorts itself out, under the cover of a microscopic preparation, into zones of distinct species, at different distances from the freely aerated edge, according to the demands of each species for oxygen and CO2 respectively.
Finally, we must note that the apparently "spontaneous movements" of Protists can hardly be explained as other than due either to external stimuli, such as we have just studied, or to internal stimuli, the outcome of internal changes, such as fatigue, hunger, and the like. Of the latter kind are the movements that result in REPRODUCTION.
Reproduction.—We have noted above that the growth of an organism which retains its shape alters the ratio of the surface area to the whole volume, so necessary for the changes involved in life. For the volume of an organism varies as the cube of any given diameter, whereas the surface varies with the square only. Without going into the arithmetical details, we may say that the ratio of surface to volume is lessened to roughly four-fifths of the original ratio when the cell doubles its bulk. As Herbert Spencer and others have pointed out, this must reduce the activities of the cell, and the due ratio is restored by the division of the cell into two.[33] This accounts for what we must look on as the most primitive mode of reproduction, as it is the simplest, and which we term "fission" at Spencer's "limit of {24}growth." Other modes of reproduction will be studied later (p. 30), after a more detailed inquiry into the structure of the nucleus and of its behaviour in cell-division. All cell-division is accompanied by increased waste, and is consequently catabolic in character, though the anabolic growth of living protoplasm, at the expense of the internal reserves, may be concurrent therewith.
Cell-Division
In ordinary cases of fission of an isolated cell the cell elongates, and as it does so, like other viscid bodies, contracts in the middle, which becomes drawn out into a thread, and finally gives way. In some cases (e.g. that of the Amoeba, Fig. 4) the nucleus previously undergoes a similar division by simple constriction, which is called direct or "amitotic" division. But usually the division of the nucleus prior to cell-division is a more complex process, and involves the co-operation of the cytoplasm; and we must now study in detail the nucleus and its structure in "rest" and in fission.[34]
We have noted above (p. 6, Fig. 2) the structure of the so-called "resting nucleus,"[35] when the cell is discharging the ordinary functions of its own life, with its wall, network of linin, chromatin-granules, and nucleole or nucleoles. The chromatin-granules are most abundant at two periods in the life of the cell, (1) when it is young and fresh from division, and (2) at the term of its life, when it is itself preparing for division. In the interim they are fewer, smaller, and stain less intensely. In many Protista the whole or greater part of the chromatin is densely aggregated into a central "nuclein-mass" or karyosome {25}suspended in the linin network (long regarded as a mere nucleole). Such a nucleus is often termed a "vesicular nucleus".[36]
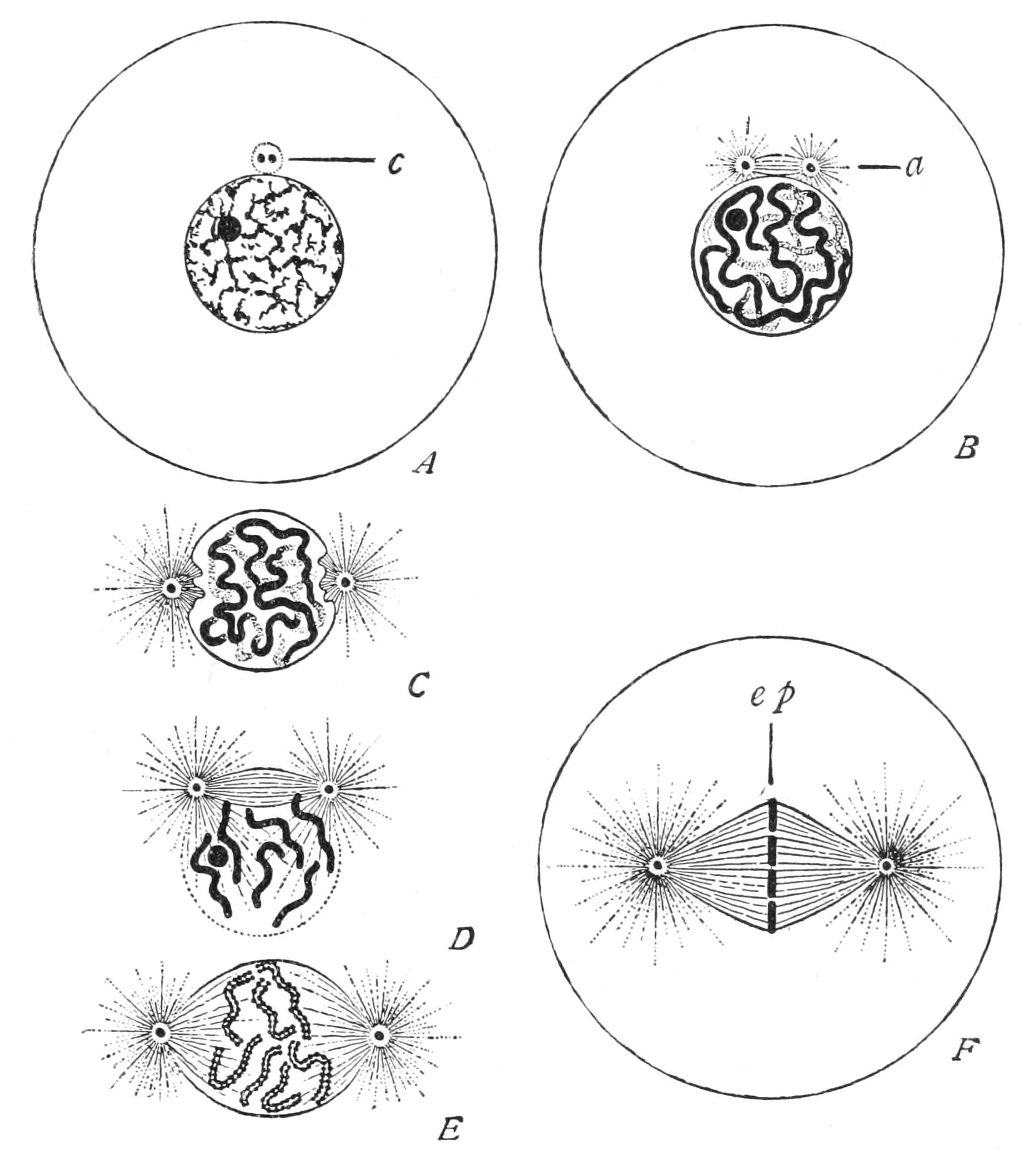
Fig. 6.—Changes in nucleus and cell in indirect (mitotic) nuclear division. A, resting nucleus with two centrioles[37] in single centrosphere (c); B, centrosphere divided, spindle and two asters (a) forming; C, centrospheres separated, nuclear wall disappearing; D, resolution of nucleus into chromosomes; E, mature plasmic spindle, with longitudinal fission of chromosomes; F, chromosomes forming equatorial plate (ep) of spindle. (From Wilson.)
When cell-division is about to take place the linin, or at least the greater part of it, assumes the character of a number of distinct threads, and the whole of the chromatin granules are distributed at even distances along these (Fig. 6, A, B, C), so as to appear like so many strings of beads. Each such thread is called a "chromosome." Then each bead divides longitudinally into two. The thread flattens into a ribbon, edged by the two lines of chromatin beads. Finally, the ribbon splits longitudinally into two single threads of beads (Fig. 6, E). During these changes the nucleole or nucleoles diminish, or even disappear, as if they had contributed their matter to the growth of the chromatin proper. In Higher Animals and Plants the nuclear wall next disappears, and certain structures become obvious, especially in the cytoplasm of Metazoa. Two minute spheres of plasm (themselves often showing a concentric structure), the "centrosomes,"[38] which hitherto lay close together at the side of the nuclear wall, now separate; but they remain connected by a spindle of clear plasmic threads (Fig. 6, B-E) which, as the centres diverge, comes to lie across the spot the nucleus occupied, and now the chromosomes lie about the equator of this spindle (Fig. 6, F). Moreover, the surrounding cytoplasm shows a radiating structure, diverging from the centrosome, so that spindle and external radiations together make up a "strain-figure," like that of the "lines of force" in relation to the poles of a magnet. Such we can demonstrate in a plane by spreading or shaking iron filings on a piece of paper above the poles of a magnet, or in space by suspending finely divided iron in a thick liquid, such as mucilage or glycerin, and bringing the vessel with the mixture into a strong magnetic field;[39] the latter mode has the advantage {27}of enabling us to watch the changes in the distribution of the lines under changing conditions or continued strain.
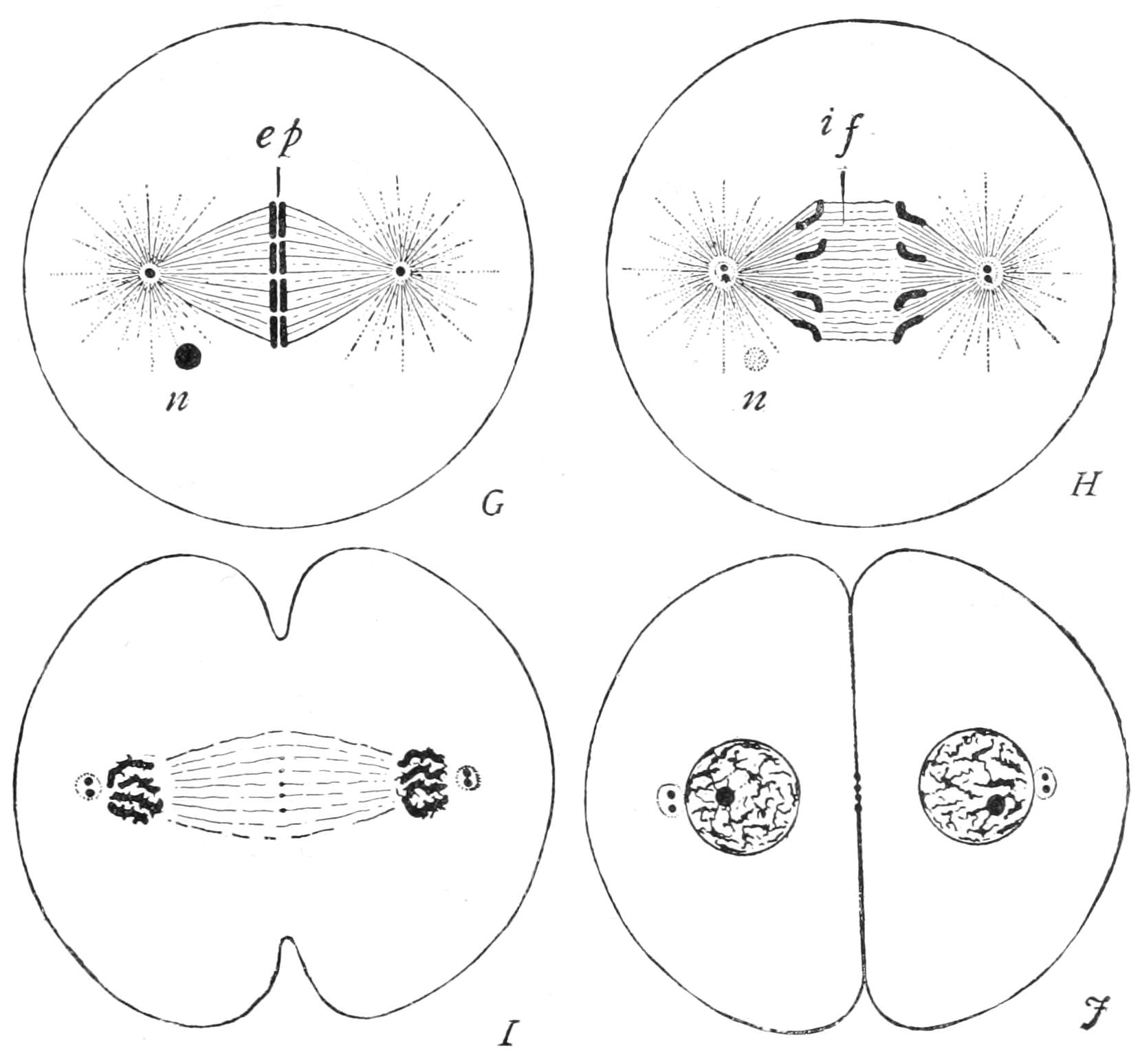
Fig. 7.—Completion of mitotic cell-division. G, splitting of equatorial plate (ep); H, recession of daughter chromosomes; I, J, reconstitution of these into new nuclei, fission of the centrioles and of the cytoplasm. if, Central fibres of spindle; n, remains of old nucleole. (From Wilson.)
The chromosomes are now completely split, each into its two daughter-segments, which glide apart (Fig. 7, G, ep), and pass each to its own pole of the spindle, stopping just short of the centrosome (I). Thus, on the inner side of either centrosome is found an aggregation of daughter-segments, each of which is sister to one at the opposite pole, while the number at either pole is identical with that of the segments into which the old nucleus had resolved itself at the outset. The daughter-segments shorten and thicken greatly as they diverge to the poles, and on their arrival crowd close together.
A distinct wall now forms around the aggregated {28}daughter-chromosomes (J), so as to combine them into a nucleus for the daughter-cell. The reorganisation of the young nucleus certainly varies in different cases, and has been ill-studied, probably because of the rapidity of the changes that take place. The cytoplasm now divides, either tapering into a "waist" which finally ruptures, or constricting by the deepening of a narrow annular groove so as to complete the formation and isolation of the daughter-cells.
We might well compare the cell-division to the halving of a pumpkin or melon, of which the flesh as a whole is simply divided into two by a transverse cut, while the seeds and the cords that suspend them are each singly split to be divided evenly between the two halves of the fruit; the flesh would represent the cytoplasm, the cords the linin threads of the nucleus, and the seeds the chromatin granules. In this way the halving of the nucleus is much more complete and intimate than that of the cytoplasm; and this is the reason why many biologists have been led to regard the nuclear segments, and especially their chromatic granules, as the seat of the hereditary properties of the cell, properties which have to be equally transmitted on its fission to each daughter-cell.[40] But we must remember that the linin is also in great part used up in the formation of these segments, like the cords of our supposed melon; and it is open to us to regard the halving in this intimate way of the "linin" as the essence of the process, and that of the chromatin as accessory, or even as only part of the necessary machinery of the process. The halving or direct splitting lengthwise of a viscid thread is a most difficult problem from a physical point of view; and it may well be that the chromatin granules have at least for a part of their function the facilitation of this process. If such be the case, we can easily understand the increase in number, and size and staining power of these granules as cell-division approaches, and their atrophy or partial disappearance during their long intervening periods of active cell life. Hence we hesitate to accept the views so commonly maintained that the chromatin represents a {29}"germ-plasm" or "idioplasm" of relatively great persistence, which gives the cell its own racial qualities.[41]
The process we have just examined is called "mitosis," "karyomitosis," or "karyokinesis"; and the nucleus is said to undergo "indirect" division, as compared to "direct" division by mere constriction. In an intermediate mode, common to many Protista, the nuclear wall persists throughout the whole process, though a spindle is constituted within, and chromosomes are formed and split: the division of the nucleus takes place, however, by simple constriction, as seen in the Filose Rhizopod Euglypha (Fig. 8).
Fig. 8.—Fission with modified karyokinesis in the Filose Rhizopod Euglypha. A, outgrowth of half of the cytoplasm, passage of siliceous plates for young shell outwards; B, completion of shell of second cell, formation of intra-nuclear spindle; C, D, further stages. (From Wilson, after Schewiakoff.)
In many Sarcodina and some Sporozoa the nucleus gives off small fragments into the cytoplasm, or is resolved into them; {30}they have been termed "chromidia" by E. Hertwig. New nuclei may be formed by their growth and coalescence, the original nucleus sometimes disappearing more or less completely.
In certain cases the division of the nucleus is not followed by that of the cytoplasm, so that a plurinucleate mass of protoplasm results: this is called an "apocyte"; and we find transitional forms between this and the uninucleate or true cell. Thus in one species of Amoeba (A. binucleata) there are always two nuclei, which divide simultaneously to provide for the outfit of the daughter-cells on fission. Again, we find in some cases that similar multinucleate masses may be formed by the union of two or more cells by their cytoplasm only: such a union is termed "permanent plastogamy," and the plurinucleate mass a "plasmodium."[42] Here again we find intermediate forms between plasmodium and apocyte, for the nuclei of the former may divide and so increase in number, without division of the still growing mass. Both kinds of plurinucleate organisms are termed "coenocytes" without reference to their mode of origin.
The rhythm of cell-life that we have just studied is called the "Spencerian" rhythm. Each cell in turn grows from half the bulk of its parent at the time it was formed to the full size of that parent, when it divides in its own turn. Rest is rare, and assumed only when the cell is exposed to such unfavourable external conditions as starvation, drought, etc.; it has no necessary relation to fission.
Multiple fission or brood-formation.—We may now turn to a new rhythm, in strong contrast to the former: a cell after having attained a size, often notably greater than its parents, divides: without any interval for growth, the daughter-cells again divide, and this may be repeated as many as ten times, or even more, so as to give rise to a number of small cells—4, 8, 16—1024,[43] etc., respectively. Such an assemblage of small cells so formed is called a brood, and well deserves this name, for they never separate until the whole series of divisions is completed. By this process the number of individuals is rapidly {31}increased, hence it has received the name of "sporulation." The term spores is especially applied to the reproductive bodies of Cryptogams, such as Mosses, Fungi, etc.: the resulting cells are called "spores," "zoospores" if active ("amoebulae" if provided with pseudopodia, "flagellulae" if flagellate), "aplanospores," if motionless. We prefer to call them by the general term "brood-cells," the original cell the "brood-mother-cell," and the process, "multiple fission" or "brood-formation." As noted, the brood-mother-cell usually attains an exceptionally large size, and it in most cases passes into a state of rest before entering on division: thus brood-formation is frequently the ultimate term of a long series of Spencerian divisions. Two contrasting periods of brood-formation may occur in the life cycle of some beings, notably the Sporozoa.[44]
Colonial union.—In certain cases, the brood-cells instead of separating remain together to form a "colony"; and this may enlarge itself again by binary division of its individual cells at their limit of growth. Here, certain or all of the cells may (either after separation, or in their places) undergo brood-formation: such cells are often termed "reproductive cells" in contrast with the "colonial cells."
Some such colonial Protista must have been the starting-points for the Higher Animals and Plants; probably apocytial Protista were the starting-points of the Fungi. In the Higher Animals and Plants, the spermatozoa and the oospheres (the male and female pairing-cells) are alike the offspring of brood-formation: and the coupled-cell (fertilised egg) starts its new life by segmentation, which is a brood-formation in which the cells do not separate, but remain in colonial union, to differentiate in due course into the tissue-cells of the organism.
Retarded brood-formation.—The nuclear divisions may alternate with cell-divisions, as above stated, or the former may be {32}completed before the cytoplasm divides; thus the brood-mother-cell becomes temporarily an apocyte,[45] which is then resolved simultaneously into the 1-nucleate brood-cells.
A temporary apocytial condition is often passed through in the formation of the brood of cells by repeated divisions without any interval for enlargement; for the nuclear divisions may go on more rapidly than those of the cytoplasm, or be completed before any cell-division takes place (Figs. 31, 34, 35, pp. 95, 101, 104), the nuclear process being "accelerated" or the cytoplastic being "retarded," whichever we prefer to say and to hold. Thus as many as thirty-two nuclei may have been formed by repeated binary subdivisions before any division of the cytoplasm takes place to resolve the apocyte into true 1-nucleate cells.
In many cases of brood-formation the greater part of the food-supply of the brood-mother-cell has been stored as reserve-products, which accumulate in quantity in the cell; this is notably seen in the ovum or egg of the Higher Animals. How great such an accumulation may be is indeed well seen in the enormous yolk of a bird's egg, gorged as it were to repletion. When a cell has entered on such course of "miserly" conduct, it may lose all power of drawing on its own supplies, and finally that of accumulating more, and passes into the state of "rest." To resume activity there is needed either a change in the internal conditions—demanding the lapse of time—or in the external conditions, or in both.[46] We may call this resumption "germination."
Very often in the study of a large and complex organism we are able to find processes in action on a large scale which, depending as they must do on the protoplasmic activities of its individual cells, reveal the nature of similar processes in simple unicellular beings: such a clue to the utilisation of reserves by a cell which has gorged itself with them so as to pass into a state of rest is to be found in that common multicellular organism, the Potato. This stores up reserves in its underground stems (tubers); if we plant these immediately on the completion of their growth, they will not start at once, even under what would outwardly seem to be most appropriate conditions. A certain lapse of time is an essential factor for sprouting. It would appear that in the Potato the starch can only be digested by a definite ferment, which does not exist when it is dug, but which is only formed very slowly, and not at all until a certain time has supervened; and that sprouting can only {33}take place when soluble material has been provided in this way for the growth of the young shoots. We have also reason to believe that these ferments are only formed by the degradation of the protoplasm itself. Now obviously this degradation must be very slow in a resting organism; and any external stimulus that will tend to protoplasmic activity will thereby tend to form at the same time the digestive ferments and dissolve the stored supplies, to render them available for the life-growth and reproduction of the being. We now see why inactive "miserly" cells so often pass into a resting state before dividing, and why they go on dividing again and again when once they re-enter upon an active life, the living protoplasm growing at the expense of the reserves.[47] Resting cells of this type occur of course only at relatively rare intervals in the animal-feeding Protozoa, that have to take into their substance the food they require for their growth and life-work, and cannot therefore store up much reserves. For they are constantly producing in the narrow compass of their body those very ferments that would dissolve the reserves when formed. Internal parasites and "saprophytes," that is, beings which live on dead and decayed organic matter, on the other hand, live surrounded by a supply of dissolved food; and rarely do we find larger cells, richer in reserves, than in the parasitic Sporozoa, which owe their name to the importance of brood-formation in their life-history. In Radiolaria (p. 75 f.) a central capsule separates off an inner layer of protoplasm; the outer layer is the one in which digestion is performed, while the inner layer stores up reserves; and here brood-formation appears to be the rule. But the largest cells of all are the eggs of the Metazoa, which in reality lead a parasitic life, being nurtured by the animal as a whole, and contributing nothing to the welfare of it as an individual. Their activity is reduced to a minimum, and the consequent need for a high ratio of surface to volume is also reduced, which accounts for their inordinate size. But directly the reserve materials are rendered available by the formation of a digestive ferment, then protoplasmic growth takes place, and the need for an extended surface is felt; cell-division follows cell-division with scarcely an interval in the process of segmentation.
Syngamy.[48]—The essence of typical syngamy is, that two cells ("pairing-cells," "gametes") of the same species approach one another, and fuse, cytoplasm with cytoplasm, and nucleus with nucleus, to form a new cell ("coupled-cell," "zygote "). This process is called also "conjugation" or "cytogamy." In the simplest cases the two cells are equal and attract one another equally ("isogamy"), and have frequently the character of zoospores.
In an intermediate type, the one cell is larger and more sluggish (female), "megagamete," "oogamete," "oosphere," "egg"; the other smaller, more active (male), "microgamete," "spermogamete," "spermatozoon," "sperm"; and in the most specialised {34}cases which prevail among the Higher Animals and Plants, the larger cell is motionless, and the smaller is active, ciliate, flagellate, or amoeboid: the coupled-cell or zygote is here termed the "oosperm."[49] It encysts immediately in most Protista except Infusoria, Acystosporidae, Haemosporidae, and Trypanosomatidae.
As the size of the two gametes is so disproportionate in most cases that the oosphere may be millions of times bigger than the sperm, and the latter at its entrance into the oosphere entirely escape unaided vision, the term "egg" is applied in lax speech, both (1) to the female cell, and (2) to the oosperm, the latter being distinguished as the "fertilised egg," a survival from the time when the union of two cells, as the essence of the process, was not understood.
We know that in many cases, and have a right to infer that in all, chemiotaxy plays an important part in attracting the pairing-cells to one another. In Mammals and Sauropsida there seems also to be a rheotactic action of the cilia lining the oviducts, which work downwards, and so induce the sperms to swim upwards to meet the ovum, a condition of things that was most puzzling until the nature of rheotaxy was understood. A remarkable fact is that equal gametes rarely appear to be attracted by members of the same brood, though they are attracted by those of any other brood of the same species.[50] It may well be that each brood has its own characteristic secretion, or "smell," as it were, slightly different from that of other broods, just as every dog has his, so easily recognisable by other dogs; and that the cells only react to different "smells" to their own. Such a secretion from the surface of the female cell would lessen its surface tension, and thereby render easier the penetration of the sperm into its substance.
As a rule, one at least of the pair-cells is fresh from division, and it would thus appear that the union of the nuclei is facilitated when one at least of them is a "young" one. Of the final mechanism of the union of the nuclei, we know nothing: they may unite in any of the earlier phases of mitosis, or even in the "resting state." A fibrillation of the cytoplasm during the process, radiating around a centrosome or two centrosomes indicates a strained condition.[51]
Regeneration.—Finally, experiments have been made by several observers as to the effects of removing parts of Protozoa, to see how far regeneration can take place. The chief results are as follows:—
1. A nucleated portion may regenerate completely, if of sufficient size. Consequently, multinucleate forms, such as Actinosphaerium (Heliozoa, Fig. 19, p. 72), may be cut into a number of fragments, and regenerate completely. In Ciliata, such as Stentor (Fig. 59, p. 156), each fragment must possess a portion of the meganucleus, and at least one micronucleus (p. 145), and, moreover, must possess a certain minimum size. A Radiolarian "central-capsule" (p. 75) with its endoplasm and nucleus may regenerate its ectoplasm, but the isolated ectoplasm being non-nucleate is doomed. A "central capsule" of one species introduced into the ectoplasm of another, closely allied, did well. All non-nucleate pieces may exhibit characteristic movements, but appear unable to digest; and they survive only a short time.[52]
"Animals" and "Plants"
Hitherto we have discussed the cell as if it were everywhere an organism that takes in food into its substance, the food being invariably "organic" material, formed by or for other cells; such nutrition is termed "holozoic." There are, however, limits to the possibilities in this direction, as there are to the fabled capacities of the Scillonians of gaining their precarious livelihood by taking in one another's washing. For part of the food material taken in in this way is applied to the supply of the energies of the cell, and is consequently split up or oxidised into simpler, more stable bodies, no longer fitted for food; and of the matter remaining to be utilised for building up the organism, a certain proportion is always wasted in by-products. Clearly, then, the supply of food under such conditions is continually lessening in the universe, and we have to seek for a manufactory of food-material from inorganic materials: this is to be found in those cells that are known as "vegetal," in the widest sense of {36}the word. In this, sense, vegetal nutrition is the utilisation of nitrogenous substances that are more simple than proteids or peptones, together with suitable organic carbon compounds, etc., to build up proteids and protoplasm. The simplest of organisms with a vegetal nutrition are the Schizomycetes, often spoken of loosely as "bacteria" or "microbes," in which the differentiation of cytoplasm and nucleus is not clearly recognisable. Some of these can build up their proteids from the free uncombined nitrogen of the atmosphere, carbon dioxide, and inorganic salts, such as sulphates and phosphates. But the majority of vegetal feeders require the nitrogen to be combined at least in the form of a nitrate or an ammonium salt—nay, for growth in the dark, they require the carbon also to be present in some organic combination, such as a tartrate, a carbohydrate, etc. Acetates and oxalates, "aromatic" compounds[53] and nitriles are rarely capable of being utilised, and indeed are often prejudicial to life. In many vegetal feeders certain portions of the protoplasm are specialised, and have the power of forming a green, yellow, or brown pigment; these are called "plastids" or "chromatophores." They multiply by constriction within the cell, displaying thereby a certain independent individuality. These plastids have in presence of light the extraordinary power of deoxidising carbon dioxide and water to form carbohydrates (or fats, etc.) and free oxygen; and from these carbohydrates or fats, together with ammonium salts or nitrates, etc., the vegetal protoplasm at large can build up all necessary food matter. So that in presence of light of the right quality[54] and adequate intensity, such coloured vegetal beings have the capacity for building up their bodies and reserves from purely inorganic materials. Coloured vegetal nutrition, then, is a process involving the absorption of energy; the source from which this is derived in the bacteria being very obscure at present. Nutrition by means of coloured plastids is {37}distinguished as "holophytic," and that from lower substances, which, however, contain organically combined carbon, as "saprophytic," for such are formed by the death and decomposition of living beings. The third mode of nutrition (found in some bacteria) from wholly inorganic substances, including free nitrogen, has received no technical name. All three modes are included in the term "autotrophic" (self-nourishing).
Vegetal feeders have a great tendency to accumulate reserves in insoluble forms, such as starch, paramylum, and oil-globules on the one hand, and pyrenoids, proteid crystals, aleurone granules on the other.
When an animal-feeding cell encysts or surrounds itself with a continuous membrane, this is always of nitrogenous composition, usually containing the glucosamide "chitin." The vegetal cell-wall, on the contrary, usually consists, at least primarily, of the carbohydrate "cellulose"—the vegetal cell being richly supplied with carbohydrate reserves, and drawing on them to supply the material for its garment. This substance is what we are all familiar with in cotton or tissue-paper.
Again, not only is the vegetal cell very ready to surround itself with a cell-wall, but its food-material, or rather, speaking accurately, the inorganic materials from which that food is to be manufactured, may diffuse through this wall with scarcely any difficulty. Such a cell can and does grow when encysted: it grows even more readily in this state, since none of its energies are absorbed by the necessities of locomotion, etc. Growth leads, of course, to division: there is often an economy of wall-material by the formation of a mere party-wall dividing the cavity of the old cell-wall at its limit of growth into two new cavities of equal size. Thus the division tends to form a colonial aggregate, which continues to grow in a motionless, and, so far, a "resting" state. We may call this "vegetative rest," to distinguish it from "absolute rest," when all other life-processes (as well as motion) are reduced to a minimum or absolutely suspended.
The cells of a plant colony are usually connected by very fine threads of protoplasm, passing through minute pores where the new party-wall is left incomplete after cell-division.[55] In a few plants, such as most Fungi, the cell-partitions are {38}in abeyance for the most part, and there is formed an apocyte with a continuous investment, sometimes, however, chambered at intervals by partitions between multinucleate units of protoplasm. We started with a purely physiological consideration, and we have now arrived at a morphological distinction, very valid among higher organisms.
Higher Plants consist of cells for the most part each isolated in its own cell-cavity, save for the few slender threads of communication.
Higher Animals consist of cells that are rarely isolated in this way, but are mostly in mutual contact over the greater part of their surface.
Again, Plants take in either food or else the material for food in solution through their surface, and only by diffusion through the cell-wall. Insectivorous Plants that have the power of capturing and digesting insects have no real internal cavity. Animal-feeding Protista take in their food into the interior of their protoplasm and digest it therein, and the Metazoa have an internal cavity or stomach for the same purpose. Here again there are exceptions in the case of certain internal parasites, such as the Tapeworms and Acanthocephala (Vol. II. pp. 74, 174), which have no stomachs, living as they do in the dissolved food-supplies of their hosts, but still possessing the general tissues and organs of Metazoa.
Corresponding with the absence of mouth, and the absorption instead of the prehension of food, we find that the movements of plant-beings are limited. In the higher Plants, and many lower ones, the colonial organism is firmly fixed or attached, and the movements of its parts are confined to flexions. These are produced by inequalities of growth; or by inequalities of temporary distension of cell-masses, due to the absorption of liquid into their vacuoles, while relaxation is effected by the cytoplasm and cell-wall becoming pervious to the liquid. We find no case of a differentiation of the cytoplasm within the cell into definite muscular fibrils. In the lower Plants single naked motile cells disseminate the species; and the pairing-cells, or at least the males, have the same motile character. In higher Cryptogams, Cycads, and Ginkgo (the Maiden-hair Tree), the sperms alone are free-swimming; and as we pass to Flowering Plants, the migratory character of the male cells is restricted to the smallest limits. {39}though never wholly absent. Intracellular movements of the protoplasm are, however, found in all Plants.
In Plants we find no distinct nervous system formed of cells and differentiated from other tissues with centres and branches and sense-organs. These are more or less obvious in all Metazoa, traces being even found in the Sponges.
We may then define Plants as beings which have the power of manufacturing true food-stuffs from lower chemical substances than proteids, often with the absorption of energy. They have the power of surrounding themselves with a cell-wall, usually of cellulose, and of growing and dividing freely in this state, in which animal-like changes of form and locomotion are impossible; their colonies are almost always fixed or floating; free locomotion is only possible in the case of their naked reproductive cells, and is transitory even in these. The movements of motile parts of complex plant-organisms are due to the changes in the osmotic powers of cells as a whole, and not to the contraction of differentiated fibrils in the cytoplasm of individual cells. Plants that can form carbohydrates with liberation of free oxygen have always definite plastids coloured with a lipochrome[56] pigment, or else (in the Phycochromaceae) the whole plasma is so coloured. Solid food is never taken into the free plant-cell nor into an internal cavity in complex Plants. If, as in insectivorous Plants, it is digested and absorbed, it is always in contact with the morphological external surface. In the complex Plants apocytes and syncytes are rare—the cells being each invested with its own wall, and, at most, only communicating by minute threads with its neighbours. No trace of a central nervous system with differentiated connexions can be made out.
Animals all require proteid food; their cyst-walls are never formed of cellulose; their cells usually divide in the naked condition only, or if encysted, no secondary party-walls are formed between the daughter-cells to unite them into a vegetative colony. Their colonies are usually locomotive, or, if fixed, their parts largely retain their powers of relative motion, and are often provided on their free surfaces with cilia or flagella; and many cells have differentiated in their cytoplasm contractile muscular fibrils. Their food (except in a few parasitic groups) is always taken {40}into a distinct digestive cavity. A complex nervous system, of many special cells, with branched prolongations interlacing or anastomosing, and uniting superficial sense-organs with internal centres, is universally developed in Metazoa. All Metazoa fulfil the above conditions.
But when we turn to the Protozoa we find that many of the characters evade us. There are some Dinoflagellates (see p. 130) which have coloured plastids, but which differ in no other respect (even specific) from others that lack them: the former may have mouths which are functionless, the latter have functional mouths. Some colourless Flagellates are saprophytic and absorb nutritive liquids, such as decomposing infusions of organic matter, possibly free from all proteid constituents; while others, scarcely different, take in food after the fashion of Amoeba. Sporozoa in the persistence of the encysted stage are very plant-like, though they are often intracellular and are parasitic in living Animals. On the other hand, the Infusoria for the most part answer to all the physiological characters of the Animal world, but are single cells, and by the very perfection of their structure, all due to plasmic not to cellular differentiation, show that they lie quite off the possible track of the origin of Metazoa from Protozoa. Indeed, a strong natural line of demarcation lies between Metazoa and Protista. Of the Protozoa, certain groups, like the Foraminifera and Radiolaria and the Ciliate and Suctorial Infusoria are distinctly animal in their chemical activities or metabolism, their mode of nutrition, and their locomotive powers. When we turn to the Proteomyxa, Mycetozoa, and the Flagellates we find that the distinction between these and the lower Fungi is by no means easy, the former passing, indeed, into true Fungi by the Chytridieae, which it is impossible to separate sharply from those Flagellates and Proteomyxa which Cienkowsky and Zopf have so accurately studied under the name of "Monadineae." Again, many of the coloured Flagellates can only (if at all) be distinguished from Plants by the relatively greater prominence and duration of the mobile state, though classifiers are generally agreed in allotting to Plants those coloured Flagellates which in the resting state assume the form of multicellular or apocytial filaments or plates.
On these grounds we should agree with Haeckel in distinguishing the living world into the Metazoa, or Higher Animals, which {41}are sharply marked off; the Metaphyta, or Higher Plants, which it is not so easy to characterise, but which unite at least two or more vegetal characters; and the Protista, or organisms, whose differentiation is limited to that within the cell (or apocyte), and does not involve the cells as units of tissues. These Protista, again, it is impossible to separate into animal and vegetal so sharply as to treat adequately of either group without including some of the other: thus it is that every text-book on Zoology, like the present work, treats of certain Protophyta. The most unmistakably animal group of the Protista, the Ciliata, is, as we have seen, by the complex differentiation of its protoplasm, widely removed from the Metazoa with their relatively simple cells but differentiated cell-groups and tissues. The line of probable origin of the Metazoa is to be sought, for Sponges at least, among the Choanoflagellates (pp. 121 f. 181 f.).
PROTOZOA (CONTINUED): SPONTANEOUS GENERATION—CHARACTERS OF PROTOZOA—CLASSIFICATION
The Question of Spontaneous Generation
From the first discovery of the Protozoa, their life-history has been the subject of the highest interest: yet it is only within our own times that we can say that the questions of their origin and development have been thoroughly worked out. If animal or vegetable matter of any kind be macerated in water, filtered, or even distilled, various forms of Protista make their appearance; and frequently, as putrefaction advances, form after form makes its appearance, becomes abundant, and then disappears to be replaced by other species. The questions suggested by such phenomena are these: (1) Do the Protista arise spontaneously, that is, by the direct organisation into living beings of the chemical substances present, as a crystal is organised from a solution: (2) Are the forms of the Protista constant from one generation to another, as are ordinary birds, beasts, and fishes?
The question of the "spontaneous generation" of the Protista was readily answered in the affirmative by men who believed that Lice bred directly from the filth of human skins and clothes;[57] and that Blow-flies, to say nothing of Honey-bees, arose in rotten flesh: but the bold aphorism of Harvey "omne vivum ex ovo" at once gained the ear of the best-inspired men of science, and set them to work in search of the "eggs" that gave rise to the organisms of putrefaction. Redi (ob. 1699) showed that Blow-flies never arise save when other Blow-flies gain access to meat and deposit their very visible eggs thereon. Leeuwenhoek, his {43}contemporary, in the latter half of the seventeenth century adduced strong reasons for ascribing the origin of the organisms of putrefaction to invisible air-borne eggs. L. Joblot and H. Baker in the succeeding half-century investigated the matter, and showed that putrefaction was no necessary antecedent of the appearance of these beings: that, as well as being air-borne, the germs might sometimes have adhered to the materials used for making the infusion; and that no organisms were found if the infusions were boiled long enough, and corked when still boiling. These views were strenuously opposed by Needham in England, by Wrisberg in Germany, and by Buffon, the great French naturalist and philosopher, whose genius, unballasted by an adequate knowledge of facts, often played him sad tricks. Spallanzani made a detailed study of what we should now term the "bionomical" or "oecological" conditions of Protistic life and reproduction in a manner worthy of modern scientific research, and not attained by some who took the opposite side within living recollection. He showed that infusions kept sufficiently long at the boiling-point in hermetically sealed vessels developed no Protistic life. As he had shown that active Protists are killed at much lower temperatures, he inferred that the germs must have much higher powers of resistance; and, by modifying the details of his experiments, he was able to meet various objections of Needham.
Despite this good work, the advocates of spontaneous generation were never really silenced; and the widespread belief in the inconstancy of species in Protista added a certain amount of credibility to their cause. In 1845 Pineau put forward these views most strongly; and from 1858 to 1864 they were supported by the elder Pouchet. Louis Pasteur, who began life as a chemist, was led from a study of alcoholic fermentation to that of the organisms of fermentation and of putrefaction and disease. He showed that in infusions boiled sufficiently long and sealed while boiling, or kept at the boiling-point in a sealed vessel, no life manifested itself: objections raised on the score of the lack of access of fresh air were met by the arrangement, so commonly used in "pure cultures" at the present day, of a flask with a tube attached plugged with a little cotton-wool, or even merely bent repeatedly into a zigzag. The former attachment filtered off all germs or floating solid particles from the air, the latter brought about the settling of such particles in the elbows {44}or on the sides of the tube: in neither case did living organisms appear, even after the lapse of months. Other observers succeeded in showing that the forms and characters of species were as constant as in Higher Animals and Plants, allowing, of course, for such regular metamorphoses as occur in Insects, or alternations of generations paralleled in Tapeworms and Polypes. The regular sequences of such alternations and metamorphoses constitute, indeed, a strong support of the "germ-theory"—the view that all Protista arise from pre-existing germs. It is to the Rev. W. H. Dallinger and the late Dr. Charles Drysdale that we owe the first complete records of such complex life-histories in the Protozoa as are presented by the minute Flagellates which infest putrefying liquids (see below, p. 116 f.). The still lower Schizomycetes, the "microbes" of common speech, have also been proved by the labours of Ferdinand Cohn, von Koch, and their numerous disciples, to have the same specific constancy in consecutive generations, as we now know, thanks to the methods first devised by De Bary for the study of Fungi, and improved and elaborated by von Koch and his school of bacteriologists.
And so to-day the principle "omne vivum ex vivo" is universally accepted by men of science. Of the ultimate origin of organic life from inorganic life we have not the faintest inkling. If it took place in the remote past, it has not been accomplished to the knowledge of man in the history of scientific experience, and does not seem likely to be fulfilled in the immediate or even in the proximate future.[58]
PROTOZOA
Organisms of various metabolism, formed of a single cell or apocyte, or of a colony of scarcely differentiated cells, whose organs are formed by differentiations of the protoplasm, and its secretions and accretions; not composed of differentiated multicellular tissues or organs.[59]
This definition, as we have seen, excludes Metazoa (including Mesozoa, Vol. II. p. 92) sharply from Protozoa, but leaves an uncertain boundary on the botanical side; and, as systematists share with nations the desire to extend their sphere of influence, we shall here follow the lead of other zoologists and include many beings that every botanist would claim for his own realm. Our present knowledge of the Protozoa has indeed been largely extended by botanists,[60] while the study of protoplasmic physiology has only passed from their fostering care into the domain of General Biology within the last decade. The study of the Protozoa is little more than two centuries old, dating from the school of microscopists of whom the Dutchman Leeuwenhoek is the chief representative: and we English may feel a just pride in the fact that his most important publications are to be found in the early records of our own Royal Society.
Baker, in the eighteenth century, and the younger Wallich, Carter, Dallinger and Drysdale, Archer, Saville Kent, Lankester, and Huxley, in the last half-century, are our most illustrious names. In France, Joblot, almost as an amateur, like our own Baker, flourished in the early part of the eighteenth century. Dujardin in the middle of the same century by his study of protoplasm, or sarcode as he termed it, did a great work in laying the foundations of our present ideas, while Balbiani, Georges Pouchet, Fabre-Domergue, Maupas, Léger, and Labbé in France, have worthily continued and extended the Gallic traditions of exact observation and careful deduction. Otto Friedrich Müller, the Dane, in the eighteenth century, was a pioneer in the exact study and description of a large number of forms of these, as of other microscopic forms of life. The Swiss collaborators, Claparède and Lachmann, in the middle of the nineteenth century, added many facts and many descriptions; and illustrated them by most valuable figures of the highest merit from every point of view. Germany, with her large population of students and her numerous universities, has given many names to our list; among these, Ehrenberg and von Stein have added {46}the largest number of species to the roll. Ehrenberg about 1840 described, indeed, an enormous number of forms with much care, and in detail far too elaborate for the powers of the microscope of that date: so that he was led into errors, many and grave, which he never admitted down to the close of a long and honoured life. Max Schultze did much good work on the Protozoa, as well as on the tissues of the Metazoa, and largely advanced our conceptions of protoplasm. His work was continued in Germany by Ernst Haeckel, who systematised our knowledge of the Radiolaria, Greeff, Richard Hertwig, Fritz Schaudinn, and especially Bütschli, who contributed to Bronn's Thier-Reich a monograph of monumental conception and scope, and of admirable execution, on which we have freely drawn. Cienkowsky, a Russian, and James-Clark and Leidy, both Americans, have made contributions of high quality.
Lankester's article in the Encyclopædia Britannica was of epoch-making quality in its philosophical breadth of thought.
Delage and Hérouard have given a full account of the Protozoa in their Traité de Zoologie Concrète, vol. i. (1896); and A. Lang's monograph in his Vergleichende Anatomie, 2nd ed. (1901), contains an admirable analysis of their general structure, habits, and life-cycles, together with full descriptions of well-selected types. Calkins has monographed "The Protozoa" in the Columbia University Biological series (1901). These works of Bütschli, Delage, Lang, and Calkins contain full bibliographies. Doflein has published a most valuable systematic review of the Protozoa parasitic on animals under the title Die Protozoen als Parasiten und Krankheitserreger (1901); and Schaudinn's Archiv für Protistenkunde, commenced only four years ago, already forms an indispensable collection of facts and views.
The protoplasm of the Protozoa (see p. 5 f.) varies greatly in consistency and in differentiation. Its outer layer may be granular and scarcely altered in Proteomyxa, the true Myxomycetes, Filosa, Heliozoa, Radiolaria, Foraminifera, etc.; it is clear and glassy in the Lobose Rhizopods and the Acrasieae; it is continuous with a firm but delicate superficial pellicle of membranous character in most Flagellates and Infusoria; and this pellicle may again be consolidated and locally thickened in some members of both groups so as to form a coat of mail, even with definite spines and hardened polygonal plates (Coleps, Fig. 54, {47}p. 150). Again, it may form transitory or more or less permanent pseudopodia,[61] (1) blunt or tapering and distinct, with a hyaline outer layer, or (2) granular and pointed, radiating and more or less permanent, or (3) branching and fusing where they meet into networks or perforated membranes. Cilia or flagella are motile thread-like processes of active protoplasm which perforate the pellicle; they may be combined into flattened platelets or firm rods, or transformed into coarse bristles or fine motionless sense-hairs. The skeletons of the Protozoa, foreign to the cytoplasm, will be treated of under the several groups.
Most of the fresh-water and brackish forms (and some marine ones) possess one or more contractile vacuoles, often in relation to a more or less complex system of spaces or canals in Flagellates and Ciliates.
The Geographical Distribution of Protozoa is remarkable for the wide, nay cosmopolitan, distribution of the terrestrial and fresh-water forms;[62] they owe this to their power of forming cysts, within which they resist drought, and can be disseminated as "dust." Very few of them can multiply at a temperature approaching freezing-point; the Dinoflagellates can, however, and therefore present Alpine and Arctic forms. The majority breed most freely at summer temperatures; and, occurring in small pools, can thus achieve their full development in such as are heated by the sun during the long Arctic day as readily as in the Tropics. In infusions of decaying matter, the first to appear are those that feed on bacteria, the essential organisms of putrefaction. These, again, are quickly followed and preyed upon by carnivorous species, which prefer liquids less highly charged with organic matters, and do not appear until the liquid, hitherto cloudy, has begun to clear. Thus we have rather to do with "habitat" than with "geographical {48}distribution," just as with the fresh-water Turbellaria and the Rotifers (vol. ii. pp. 4 f., 226 f.). We can distinguish in fresh-water, as in marine Protista, "littoral" species living near the bank, among the weeds; "plankton," floating at or near the surface; "zonal" species dwelling at various depths; and "bottom-dwellers," mostly "limicolous" (or "sapropelic," as Lauterborn terms them), and to be found among the bottom ooze. Many species are "epiphytic" or "epizoic," dwelling on plants or animals, and sometimes choice enough in their preference of a single genus or species as host. Others again are "moss-dwellers," living among the root-hairs of mosses and the like, or even "terrestrial" and inhabiting damp earth. The Sporozoa are internal parasites of animals, and so are many Flagellates, while many Proteomyxa are parasitic in plant-cells. The Foraminifera (with the exception of most Allogromidiaceae) are marine, and so are the Radiolaria; while most Heliozoa inhabit fresh water. Concerning the distribution in time we shall speak under the last two groups, the only ones whose skeletons have left fossil remains.
Classification.—The classification of the Protozoa is no easy task. We omit here, for obvious reasons, the unmistakable Plant Protists that have a holophytic or saprophytic nutrition; that are, with the exception of a short period of locomotion in the young reproductive cells, permanently surrounded with a wall of cellulose or fungus-cellulose, and that multiply and grow freely in this encysted state: to these consequently we relegate the Chytridieae, which so closely allied to the Proteomyxa and the Phycomycetous Fungi; and the Confervaceae, which in the resting state form tubular or flattened aggregates and are allied to the green Flagellates; besides the Schizophyta. At the opposite pole stand the Infusoria in the strict sense, with the most highly differentiated organisation found in our group, culminating in the possession of a nuclear apparatus with nuclei of two kinds, and exhibiting a peculiar form of conjugation, in which the nuclear apparatus is reorganised. The Sporozoa are clearly marked off as parasites in living animals, which mostly begin life as sickle-shaped cells and have always at least two alternating modes of brood-formation, the first giving rise to aplanospores, wherein is formed the second brood of sickle-shaped, wriggling zoospores. The remainder, comprising the Sarcodina, or Rhizopoda in the old wide sense (including all {49}that move by pseudopodia during the great part of their active life), and the Flagellata in the widest sense, are not easy to split up; for many of the former have flagellate reproductive cells, and many of the latter can emit pseudopodia with or without the simultaneous retraction of their flagella. The Radiolaria are well defined by the presence in the body plasm of a central capsule marking it off into a central and a peripheral portion, the former containing the nucleus, the latter emitting the pseudopodia. Again, on the other hand, we find that we can separate as Flagellata in the strict sense the not very natural assemblage of those Protista that have flagella as their principle organs of movement or nutrition during the greater part of their active life. The remaining groups (which with the Radiolaria compose the Sarcodina of Bütschli), are the most difficult to treat. The Rhizopoda, as we shall limit them, are naked or possess a simple shell, never of calcium carbonate, have pseudopodia that never radiate abundantly nor branch freely, nor coalesce to form plasmatic networks, nor possess an axial rod of firmer substance. The Foraminifera have a shell, usually of calcium carbonate, their pseudopodia are freely reticulated, at least towards the base; and (with the exception of a few simple forms) all are marine. The Mycetozoa are clearly united by their tendency to aggregate more or less completely into complex resting-groups (fructifications), and by reproducing by unicellular zoospores, flagellate or amoeboid, which are not known to pair. The Heliozoa resemble the Radiolaria in their fine radiating pseudopodia, but have an axial filament always present in each, and lack the central capsule; and are, for the most part, fresh-water forms. Finally, the Proteomyxa forms a sort of lumber-room for beings which are intermediate between the Heliozoa, Rhizopoda, and Flagellata, usually passing through an amoeboid stage, and for the most part reproducing by brood-formation. Zoospores that possess flagella are certainly known to occur in some forms of Foraminifera, Rhizopoda, Heliozoa, and Radiolaria, though not by any means in all of each group.[63]
| A. Pseudopodia the principal means of locomotion and feeding; flagella absent or transitory | I. Sarcodina |
| (1) Plastogamy only leading to an increase in size, never to the formation of "fructifications." | |
| (a) Pseudopodia never freely coalescing into a network nor fine to the base | Rhizopoda. |
| (*) Ectoplasm clear, free from granules; pseudopodia, usually blunt | Rhizopoda Lobosa |
| (**) Ectoplasm finely granular; pseudopodia slender, branching, but not forming a network, passing into the body by basal dilatation | Rhizopoda Filosa |
| (b) Pseudopodia branching freely and coalescing to form networks; ectoplasm granular; test usually calcareous or sandy | Foraminifera |
| (c) Pseudopodia fine to the very base; radiating, rarely coalescing. | |
| (i.) Pseudopodia with a central filament | Heliozoa |
| (ii.) Pseudopodia without a central filament. | |
| (*) Body divided into a central and a peripheral part by a "central capsule" | Radiolaria |
| (**) Body without a central capsule | Proteomyxa |
| (2) Cells aggregating or fusing into plasmodia before forming a complex "fructification" | Mycetozoa |
| B. Cells usually moving by "euglenoid" wriggling or by excretion of a trail of viscid matter; reproduction by alternating modes of brood-formation, rarely by Spencerian fission | II. Sporozoa |
| C. Flagella (rarely numerous) the chief or only means of motion and feeding | III. Flagellata |
| D. Cilia the chief organs of motion, in the young state at least; nuclei of two kinds | IV. Infusoria |
PROTOZOA (CONTINUED): SARCODINA
I. Sarcodina.
Protozoa performing most of their life-processes by pseudopodia; nucleus frequently giving off fragments (chromidia) which may play a part in nuclear reconstitution on division; sometimes with brood-cells, which may be at first flagellate; but never reproducing in the flagellate state.[64]
1. Rhizopoda
Sarcodina of simple form, whose pseudopodia never coalesce into networks (1),[65] nor contain an axial filament (2), which commonly multiply by binary fission (3), though a brood-formation may occur; which may temporarily aggregate, or undergo temporary or permanent plastogamic union, but never to form large plasmodia or complex fructifications as a prelude to spore-formation (4); test when present gelatinous, chitinous, sandy, or siliceous, simple and 1-chambered (5).
Classification.[66]
| I. Ectoplasm distinct, clear; pseudopodia blunt or tapering, but not branching at the apex | Lobosa |
| Amoeba, Auctt.; Pelomyxa, Greeff; Trichosphaerium, A. Schneid.; Dinamoeba, Leidy; Amphizonella, Greeff; Centropyxis, Stein; Arcella, {52}Ehr.; Difflugia, Leclercq; Lecqueureusia, Schlumberger; Hyalosphenia, Stein; Quadrula, F. E. Sch.; Heleopera, Leidy; Podostoma, Cl. and L.; Arcuothrix, Hallez. | |
| II. Ectoplasm undifferentiated, containing moving granules; pseudopodia branching freely towards the tips | Filosa |
| Euglypha, Duj.; Paulinella, Lauterb.; Cyphoderia, Schlumb.; Campascus, Leidy; Chlamydophrys, Cienk.; Gromia, Duj. = Hyalopus, M. Sch. | |
We have defined this group mainly by negative characters, as such are the only means for their differentiation from the remaining Sarcodina; and indeed from Flagellata, since in this group zoospores are sometimes formed which possess flagella. Moreover, indeed, in a few of this group (Podostoma, Arcuothrix), as in some Heliozoa, the flagellum or flagella may persist or be reproduced side by side with the pseudopodia. The subdivision of the Rhizopoda is again a matter of great difficulty, the characters presented being so mixed up that it is hard to choose: however, the character of the outer layer of the cytoplasm is perhaps the most obvious to select. In Lobosa there is a clear layer of ectosarc, which appears to be of a greasy nature at its surface film, so that it is not wetted. In the Filosa, as in most other Sarcodina, this film is absent, and the ectoplasm is not marked off from the endoplasm, and may have a granular surface. Corresponding to this, the pseudopodia of the Lobosa are usually blunt, never branching and fraying out, as it were, at the tip, as in the Filosa; nay, in the normal movements of Amoeba limax (Fig. 1, p. 5) the front of the cell forms one gigantic pseudopodium, which constantly glides forward. Apart from this distinction the two groups are parallel in almost every respect.
There may be a single contractile vacuole, or a plurality; or none, especially in marine and endoparasitic species. The nucleus may remain single or multiply without inducing fission, thus leading to apocytial forms. It often gives off "chromidial" fragments, which may play an important part in reproduction.[67] In Amoeba binucleata there are constantly two nuclei, both of which divide as an antecedent to fission, each giving a separate nucleus to either daughter-cell. Pelomyxa palustris, the giant of the group, attaining a diameter of 1‴ (2 mm.), has very blunt pseudopodia, an enormous number of nuclei, and no contractile vacuole, though {53}it is a fresh-water dweller, living in the bottom ooze of ponds, etc., richly charged with organic débris. It is remarkable also for containing symbiotic bacteria, and brilliant vesicles with a distinct membranous wall, containing a solution of glycogen.[68] Few, if any, of the Filosa are recorded as plurinuclear.
The simplest Lobosa have no investment, nor indeed any distinction of front or back. In some forms of Amoeba, however, the hinder part is more adhesive, and may assume the form of a sucker-like disc, or be drawn into a tuft of short filaments or villi, to which particles adhere. Other species of Lobosa and all Filosa have a "test," or "theca," i.e. an investment distinct from the outermost layer of the cell-body. The simplest cases are those of Amphizonella, Dinamoeba, and Trichosphaerium, where this is gelatinous, and in the two former allows the passage of food particles through it into the body by mere sinking in, like the protoplasm itself, closing again without a trace of perforation over the rupture. In Trichosphaerium (Fig. 9) the test is perforated by numerous pores of constant position for the passage of the pseudopodia, closing when these are retracted; and in the "A" form of the species (see below) it is studded with radial spicules of magnesium carbonate. Elsewhere the test is more consistent and possesses at least one aperture for the emission of pseudopodia and the reception of food; to avoid confusion we call this opening not the mouth but the "pylome": some Filosa have two symmetrically placed pylomes. When the test is a mere pellicle, it may be recognised by the limitation of the pseudopodia to the one pylomic area. But the shell is often hard. In Arcella (Fig. 10, C), a form common among Bog-mosses and Confervas, it is chitinous and shagreened, circular, with a shelf running in like that of a diving-bell around the pylome: there are two or more contractile vacuoles, and at least two nuclei. Like some other genera, it has the power of secreting carbonic acid gas in the form of minute bubbles in its cytoplasm, so as to enable it to float up to the surface of the water. The chitinous test shows minute hexagonal sculpturing, the expression of vertical partitions reaching from the inner to the outer layer.
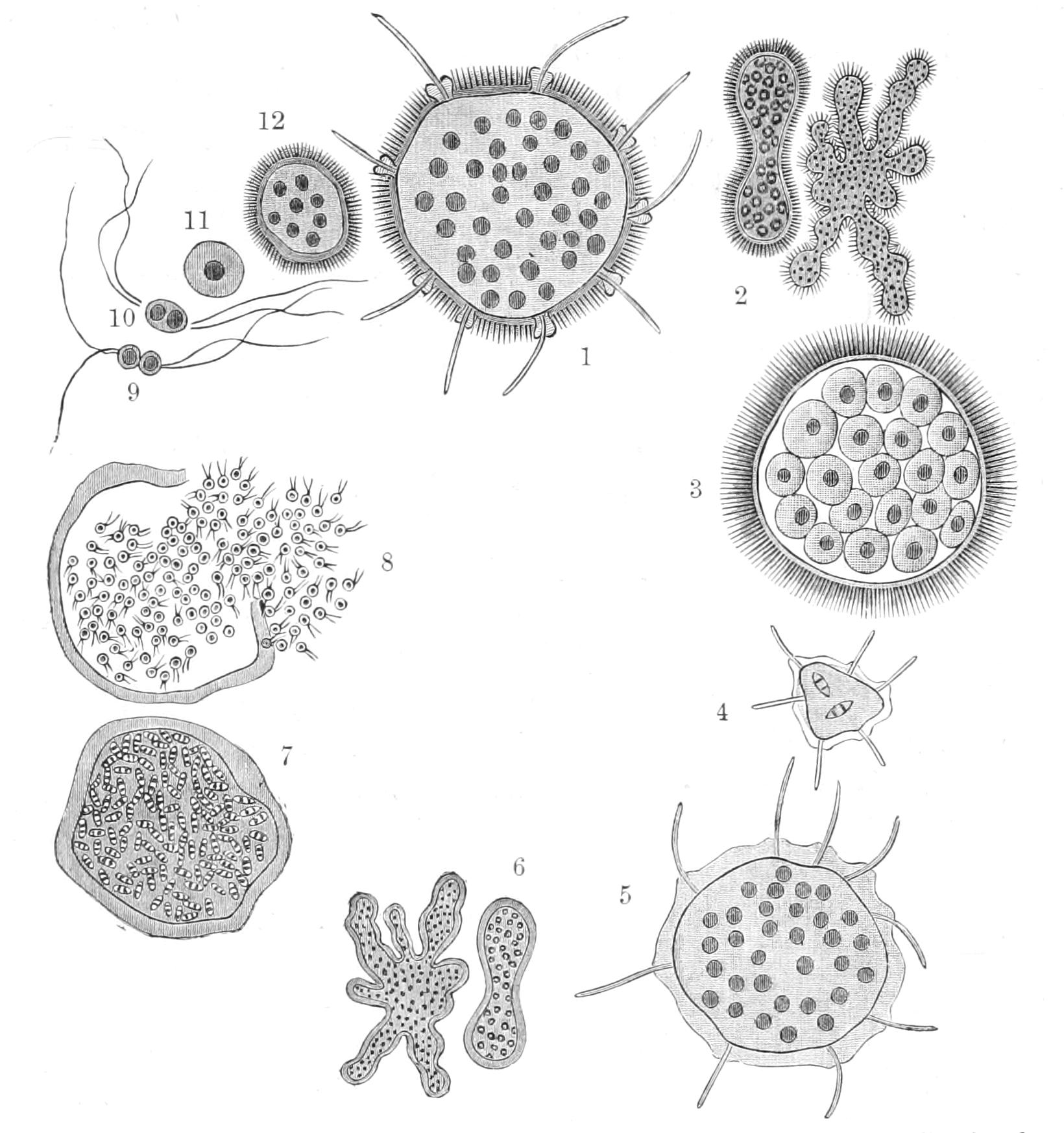
Fig. 9.—Trichosphaerium sieboldii. 1, Adult of "A" form; 2, its multiplication by fission and gemmation; 3, resolution into 1-nucleate amoeboid zoospores; 4, development (from zoospores of "A") into "B" form (5); 6, its multiplication by fission and gemmation; 7, its resolution after nuclear bipartition into minute 2-flagellate zoospores or (exogametes); 8, liberation of gametes; 9, 10, more highly magnified pairing of gametes of different origin; 11, 12, zygote developing into "A" form. (After Schaudinn.)
Several genera have tests of siliceous or chitinous plates, formed in the cytoplasm in the neighbourhood of the nucleus, and connected by chitinous cement. Among these Quadrula (Fig. 10, A) is Lobose, with square plates, Euglypha (Fig. 8, p. 29), and Paulinella[69] are Filose, with hexagonal plates. In the latter they are in five longitudinal rows, with a pentagonal oral plate, perforated by the oval pylome. In other genera again, such as Cyphoderia (Filosa), the plates are merely {55}chitinous. Again, the shell may be encrusted with sand-grains derived directly from without, or from ingested particles, as shown in Centropyxis, Difflugia (Fig. 10, D), Heleopera, and Campascus when supplied with powdered glass instead of sand. The cement in Difflugia is a sort of organic mortar, infiltrated with ferric oxide (more probably ferric hydrate). In Lecqueureusia spiralis (formerly united with Difflugia) the test is formed of minute sausage-shaped granules, in which may be identified the partly dissolved valves of Diatoms taken as food; it is spirally twisted at the apex, as if it had enlarged after its first formation, a very rare occurrence in this group. The most frequent mode of fission in the testaceous Rhizopods (Figs. 8, 10) is what Schaudinn aptly terms "bud-fission," where half the protoplasm protrudes and accumulates at the mouth of the shell, and remains till a test has formed for it, while the other half retains the test of the original animal. The materials for the shell, whether sand-granules or plates, pass from the depths of the original shell outwards into the naked cell, and through its cytoplasm to the surface, where they become connected by cementing matter into a continuous test. The nucleus now divides into two, one of which passes into the external animal; after this the two daughter-cells separate, the one with the old shell, the other, larger, with the new one.
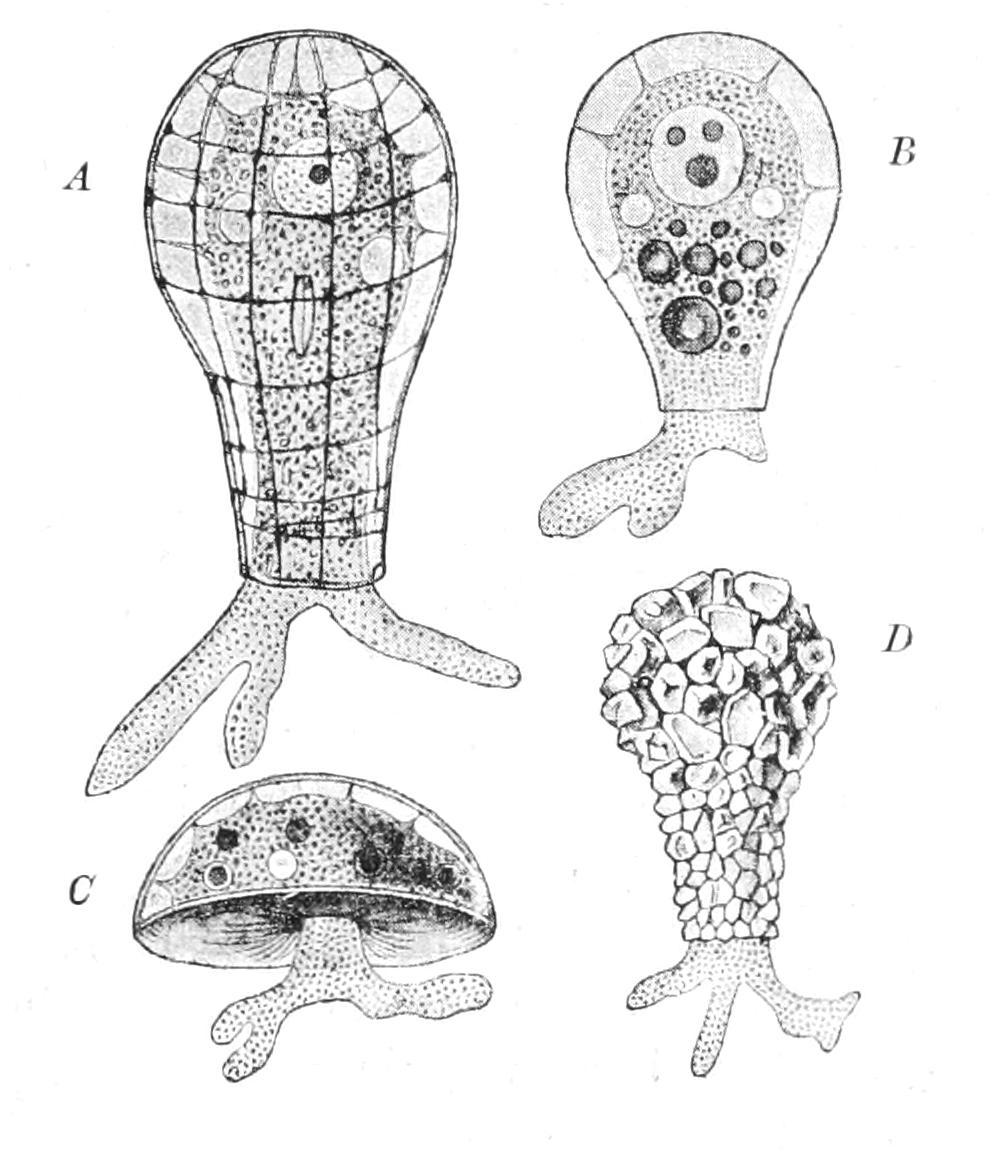
Fig. 10.—Test-bearing Rhizopods. A, Quadrula symmetrica: B, Hyalosphenia lata; C, Arcella vulgaris; D, Difflugia pyriformis. (From Lang's Comparative Anatomy.)
If two individuals of the shelled species undergo bud-fission in close proximity, the offspring may partially coalesce, so that a monstrous shell is produced having two pylomes.
Reproduction by fission has been clearly made out in most members of the group; some of the multinucleate species often abstrict a portion, sometimes at several points simultaneously, so that fission here passes into budding[70] (Fig. 9, 2, 6).
Brood-division, either by resolution in the multinucleate species, or preceded by multiple nuclear division in the habitually 1-nucleate, though presumably a necessary incident in the life-history of every species, has only been seen, or at least thoroughly worked out, in a few cases, where it is usually preceded by encystment, and mostly by the extrusion into the cyst of any undigested matter.[71]
In Trichosphaerium (Fig. 9) the cycle described by Schaudinn is very complex, and may be divided into two phases, which we may term the A and the B subcycles. The members of the A cycle are distinguished by the gelatinous investment being armed with radial spicules, which are absent from the B form. The close of the A cycle is marked by the large multinucleate body resolving itself into amoeboid zoospores (3), which escape from the gelatinous test, and develop into the large multinucleate adults of the B form. These, like the A form, may reproduce by fission or budding. At the term of growth, however, they retract their pseudopodia, expel the excreta, and multiply their nuclei by mitosis (7). Then the body is resolved into minute 2-flagellate microzoospores (8), which are exogamous gametes, i.e. they will only pair with similar zoospores from another cyst. The zygote (9-11) resulting from this conjugation is a minute amoeboid; its nucleus divides repeatedly, a gelatinous test is formed within which the spicules appear, and so the A form is reconstituted. In many of the test-bearing forms, whether Lobose or Filose, plastogamic unions occur, and the two nuclei may remain distinct, leading to plurinucleate monsters in their offspring by fission, or they may fuse and form a giant nucleus, a process which has here no relation to normal syngamy, as it is not associated with any marked change in the alternation of feeding and fission, etc. In Trichosphaerium also plastogamic unions between small individuals have for their only result the increase of size, enabling the produce to deal with {57}larger prey. Temporary encystment in a "hypnocyst" is not infrequent in both naked and shelled species, and enables them to tide over drought and other unfavourable conditions.
Schaudinn has discovered and worked out true syngamic processes, some bisexual, some exogamous, in several other Rhizopods. In Chlamydophrys stercorea the pairing-cells are equal, and are formed by the aggregation of the chromidia into minute nuclei around which the greater part of the cytoplasm aggregates, while the old nucleus (with a little cytoplasm) is lost. These brood-cells are 2-flagellate pairing-cells, which are exogamous: the zygote is a brown cyst; if this be swallowed by a mammal, the original Chlamydophrys appears in its faeces.[72]
Centropyxis aculeata, a species very common in mud or moss, allied to Difflugia, also forms a brood by aggregation around nuclei derived from chromidia. The brood-cells are amoeboid, and secrete hemispherical shells like those of Arcella; some first divide into four smaller ones, before secreting the shell. Pairing takes place between the large and the small forms; and the zygote encysts. Weeks or months afterwards the cyst opens and its contents creep out as a minute Centropyxis. Finally, Amoeba coli produces its zygote in a way recalling that of Actinosphaerium (pp. 73-75, Fig. 21): the cell encysts; its nucleus divides, and each daughter divides again into two, which fuse reciprocally. Thus the cyst contains two zygote nuclei. After a time each of these divides twice, so that the mature cyst contains eight nuclei. Probably when swallowed by another animal they liberate a brood of eight young amoebae. Thus in different members of this group we have exogamy, both equal and bisexual, and endogamy.
Most of the Rhizopoda live among filamentous Algae in pools, ponds, and in shallow seas, etc.; some are "sapropelic" or mud-dwellers (many species of Amoeba, Pelomyxa, Difflugia, etc.), others frequent the roots of mosses. Amoeba coli is often found as a harmless denizen of the large intestine of man. Amoeba histolytica, lately distinguished therefrom by Schaudinn, is the cause of tropical dysentery. It multiplies enormously in the gut, and is found extending into the tissues, and making its way into the abscesses that so frequently supervene in the liver and other organs. Chlamydophrys stercorea is found in the {58}faeces of several mammals. The best monograph of this group is that of Penard.[73]
2. Foraminifera[74]
Sarcodina with no central capsule or distinction of ectosarc; the pseudopodia fine, branching freely, and fusing where they meet to form protoplasmic networks, or the outermost in the pelagic forms radiating, but without a central or axial filament: sometimes dimorphic, reproducing by fission and by rhizopod or flagellate germs in the few cases thoroughly investigated: all marine (with the exception of some of the Allogromidiaceae), and usually provided with a test of carbonate of lime ("vitreous" calcite, or "porcellanous" aragonite?), or of cemented particles of sand ("arenaceous"); test-wall continuous, or with the walls perforated by minute pores or interstices for the protrusion of pseudopodia.
The classification of Carpenter (into Vitreous or Perforate, Porcellanous or Imperforate, and Arenaceous), according to the structure of the shell, had proved too artificial to be used by Brady in the great Monograph of the Foraminifera collected by the "Challenger" Expedition,[75] and has been modified by him and others since then. We reproduce Lister's account of Brady's classification.[76] We must, however, warn the tyro that its characterisations are not definitions (a feature of all other recent systems), for rigid definitions are impossible: here as in the case, for instance, of many Natural Orders of Plants, transitional forms making the establishment of absolute boundaries out of the question. In the following classification we do not think it, therefore, necessary to complete the characterisations by noting the extremes of variation within the orders:—
1. Allogromidiaceae: simple forms, often fresh-water and similar to Rhizopoda; test 0, or chitinous, gelatinous, or formed of cemented particles, whether secreted platelets or ingested granules. Biomyxa, Leidy = Gymnophrys, Cienk.; {59}Diaphorodon, Archer; Allogromia, Rhumbl. (= Gromia, auctt.[77] nec Duj.) (Fig. 14, 1); Lieberkühnia, Cl. and Lachm. (Fig. 12); Microgromia, R. Hertw. (Fig. 11); Pamphagus, Bailey.
2. Astrorhizidaceae: test arenaceous, often large, never truly chambered, or if so, asymmetrical. Astrorhiza, Sandahl; Haliphysema, Bowerb.; Saccammina, M. Sars (Fig. 13, 1); Loftusia, Brady.
3. Lituolidaceae: test arenaceous, often symmetrical or regularly spiral, isomorphous with calcareous forms: the chambers when old often "labyrinthine" by the ingrowth of wall-material. Lituola, Lam.; Reophax, Montf.; Ammodiscus, Reuss; Trochammina, Parker and Jeffreys.
4. Miliolidaceae: test porcellanous, imperforate, spirally coiled or cyclic, often chambered except in Cornuspira: simple in Squamulina. Cornuspira, Max Sch.; Peneroplis, Montf.; Miliolina, Lam. (incl. Biloculina (Fig. 15), Triloculina, Quinqueloculina (Figs. 14, 4; 15, B), Spiroloculina (Fig. 13, 5) of d'Orb.); Alveolina, d'Orb.; Hauerina, d'Orb.; Calcituba, Roboz; Orbitolites, Lam.; Orbiculina, Lam.; Alveolina, Park. and Jeffr.; Nubecularia, Def.; Squamulina, Max Sch. (Fig. 14, 3).
5. Textulariaceae: test calcareous, hyaline, perforated; chambers increasing in size in two alternating rows, or three, or passing into a spiral. Textularia, Def.; Bulimina, d'Orb.; Cassidulina, d'Orb.
6. Cheilostomellaceae: test vitreous, delicate, finely perforated, chambered, isomorphic with the spiral forms of the Miliolidaceae. Cheilostomella, Reuss.
7. Lagenaceae: Test vitreous, very finely perforate, chambers with a distinct pylome projecting (ectosolenial), or turned in (entosolenial), often succeeding to form a necklace-like shell. Lagena, Walker and Boys (Fig. 13, 2); Nodosaria, Lam. (Fig. 13, 3); Cristellaria, Lam.; Frondicularia, Def. (Fig. 13, 4); Polymorphina, Lam.; Ramulina, Wright.
8. Globigerinidae: test vitreous, perforate; chambers few, dilated, and arranged in a flat or conical spiral, usually with a crescentic pylome to the last. Globigerina, d'Orb. (Figs. 13, 6; 16, 2); Hastigerina, Wyv. Thoms.; Orbulina, d'Orb. (Fig. 16, 1).
9. Rotaliaceae; test vitreous, perforate, usually a conical spiral (like a snail), chambers often subdivided into chamberlets, and with a proper wall, and intermediate skeleton traversed by canals. Rotalia, Lam. (Fig. 14, 2); Planorbulina, d'Orb. (Fig. 13, 9); Polytrema, Risso; Spirillina, Ehr. (non-septate); Patellina, Will.; Discorbina, P. and J. (Fig. 13, 7).
10. Nummulitaceae: test usually a complex spiral, the turns completely investing their predecessors: wall finely tubular, often with a proper wall and intermediate skeleton. Fusulina, Fisch.; Polystomella, Lam.; Nummulites, d'Orb. (Fig. 13, 11); Orbitoides, d'Orb.
The Allogromidiaceae are a well-marked and distinct order, on the whole resembling the Rhizopoda Filosa, and are often found with them in fresh water, while all other Foraminifera are marine. The type genus, Allogromia (Fig. 14, 1), has an oval chitinous shell. Microgromia socialis (Fig. 11) is often found in aggregates, the pseudopodia of neighbours fusing where they meet into a {60}common network. This is due to the fact that one of the two daughter-cells at each fission, that does not retain the parent shell, remains in connexion with its sister that does: sometimes, however, it retracts its pseudopodia, except two which become flagella, wherewith it can swim off. The test of Pamphagus is a mere pellicle. In Lieberkühnia (Fig. 12) it is hardly that; though the body does not give off the fine pseudopodia directly, but emits a thick process or "stylopodium"[78] comparable to the protoplasm protruded through the pylome of its better protected allies; and from this, which often stretches back parallel to the elongated body, the reticulum of pseudopodia is emitted. Diaphorodon has a shell recalling that of Difflugia (Fig. 10, D, p. 55), formed of sandy fragments, but with interstices between them through which as well as through the two pylomes the pseudopodia pass. In all of these the shell is formed as in the Rhizopods once for all, and does not grow afterwards; and the fresh-water forms, which are the majority, have one or more contractile vacuoles; in Allogromia they are very numerous, scattered on the expanded protoplasmic network.
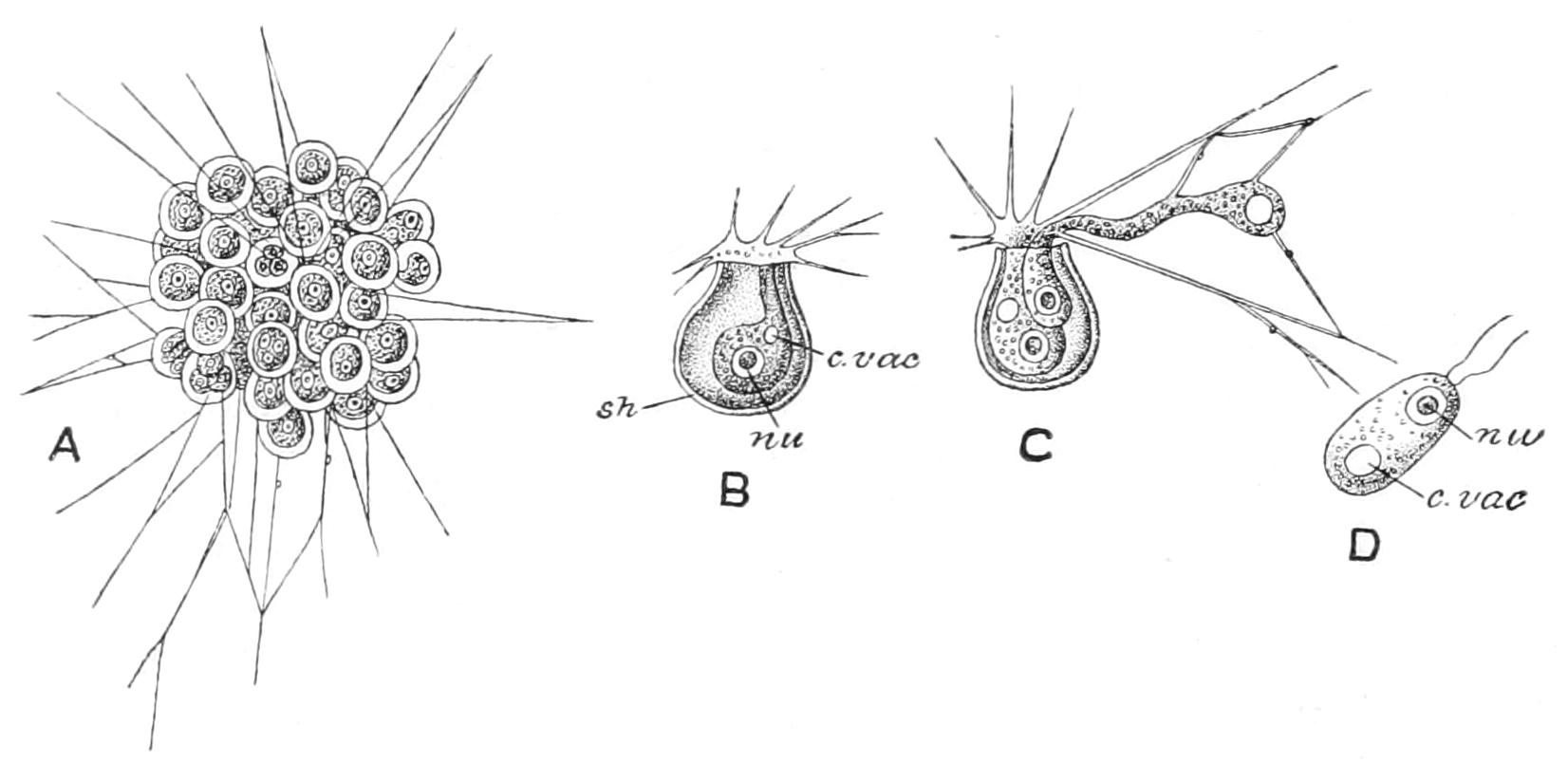
Fig. 11.—Microgromia socialis. A, entire colony; B, single zooid; C, zooid which has undergone binary fission, with one of the daughter-cells creeping out of the shell; D, flagellula. c.vac, Contractile vacuole; nu, nucleus; sh, shell. (From Parker and Haswell, after Hertwig and Lesser.)

Fig. 12.—Lieberkühnia, a fresh-water Rhizopod, from the egg-shaped shell of which branched pseudopodial filaments protrude. (From Verworn.)
The remaining marine families may all be treated of generally, before noting their special characters. Their marine habitat is variable, but in most cases restricted. A few extend up the brackish water of estuaries: a large number are found between tide-marks, or on the so-called littoral shelf extending to deep water; they are for the most part adherent to seaweeds, or lie among sand or on the mud. Other forms, again, are pelagic, such as Globigerina (Figs. 13, 6, 16, 17) and its allies, and float as part of the plankton, having the surface of their shells extended by delicate spines, their pseudopodia long and radiating, and the outer part of their cytoplasm richly vacuolated ("alveolate"), and probably containing a liquid lighter than sea water, as in the Radiolaria. Even these, after their death and the decay of the protoplasm, must sink to the bottom (losing the fine spines by solution as they fall); and they accumulate there, to form a light oozy mud, the "Globigerina-ooze" of geographers, at depths where the carbonic acid under pressure is not adequate to dissolve the more solid calcareous matter. Grey Chalk is such an ooze, consolidated by {62}the lapse of time and the pressure of superincumbent layers. Some Foraminifera live on the sea bottom even at the greatest depths, and of course their shell is not composed of calcareous matter. Foraminifera may be obtained for examination by carefully washing sand or mud, collected on the beach at different levels between tide-marks, or from dredgings, or by carefully searching the surface of seaweeds, or by washing their roots, or, again, by the surface or deep-sea tow-net. The sand used to weight sponges for sale is the ready source of a large number of forms, and may be obtained for the asking from the sponge-dealers to whom it is a useless waste product. If this sand is dried in an oven, and then poured into water, the empty shells, filled with air, will float to the surface, and may be sorted by fine silk or wire gauze.
From the resemblance of the shells of many of them to the Nautilus they were at first described as minute Cephalopods, or Cuttlefish, by d'Orbigny,[79] and their true nature was only elucidated in the last century by the labours of Williamson, Carpenter, Dujardin, and Max Schultze. At first they possess only one nucleus, but in the adult stage may become plurinucleate without dividing, and this is especially the case in the "microsphaeric" states exhibited by many of those with a complex shell; the nucleus is apt to give off fragments (chromidia) which lie scattered in the cytoplasm. At first, too, in all cases, the shell has but a single chamber, a state that persists through life in some. When the number of chambers increases, their number has no relation to that of the nuclei, which remains much smaller till brood-formation sets in.
The shell-substance, if calcareous, has one of the two types, porcellanous or vitreous, that we have already mentioned, but Polytrema, a form of very irregular shape, though freely perforated, is of a lovely pink colour. In the calcareous shells sandy particles may be intercalated, forming a transition to the Arenacea. In these the cement has an organic base associated with calcareous or ferruginous matter; in some, however, the cement is a phosphate of iron. The porcellanous shells are often deep brown by transmitted light.
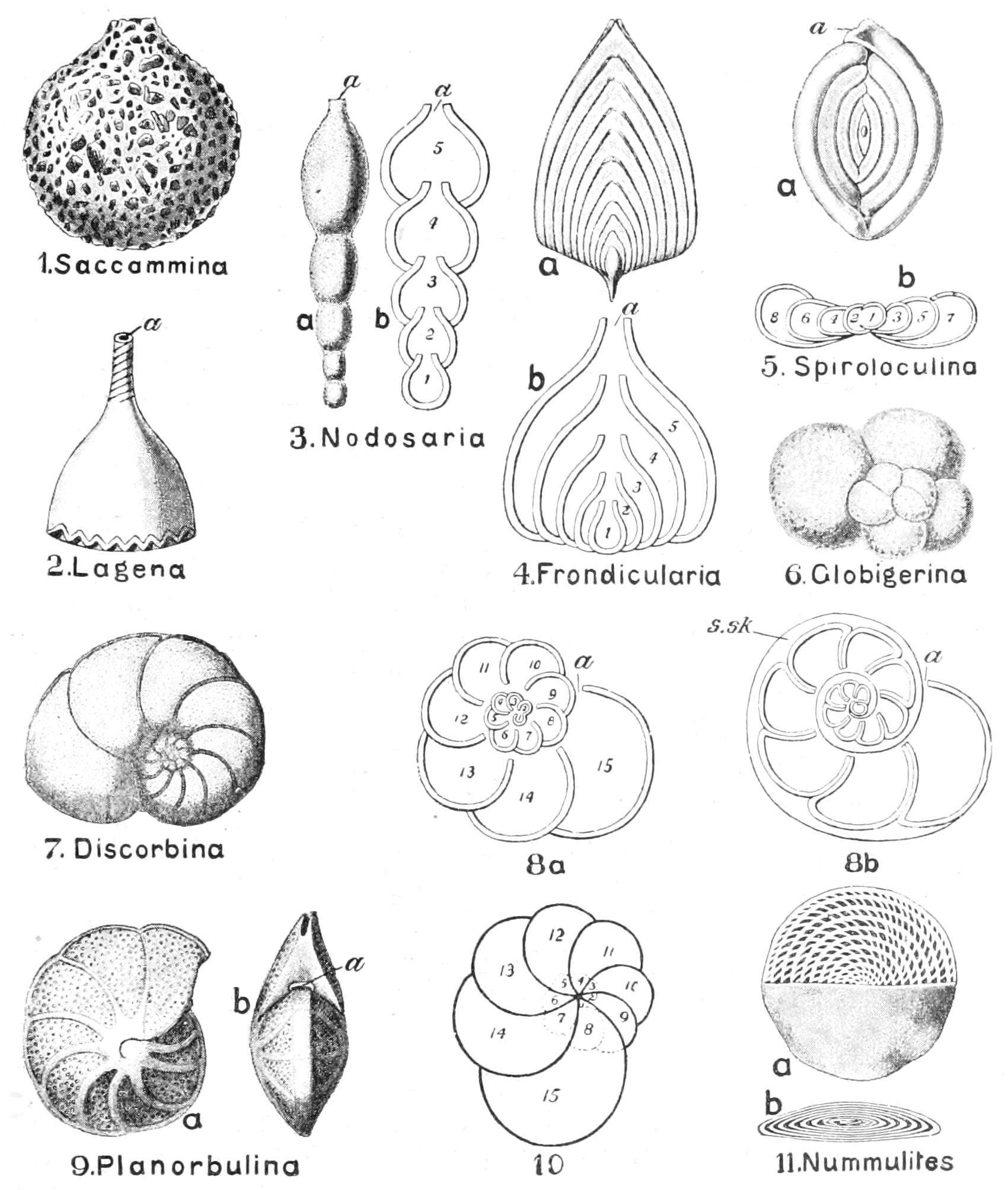
Fig. 13.—Shells of Foraminifera. In 3, 4, and 5, a shows the surface view, and b a section; 8a is a diagram of a coiled cell without supplemental skeleton; 8b of a similar form with supplemental skeleton (s.sk); and 10 of a form with overlapping whorls; in 11a half the shell is shown in horizontal section; b is a vertical section; a, aperture of the shell; 1-15, successive chambers, 1 being always the oldest or initial chamber. (From Parker and Haswell, after other authors.)
Despite the apparent uniformity of the protoplasmic body in this group, the shell is infinitely varied in form. As Carpenter writes, in reference to the Arenacea, "There is nothing more wonderful in nature than the building up of these elaborate and symmetrical structures by mere jelly-specks, presenting no traces {64}whatever of that definite organisation which we are accustomed to regard as necessary to the manifestations of conscious life.... The tests (shells) they construct when highly magnified bear comparison with the most skilful masonry of man. From the same sandy bottom one species picks up the coarsest quartz grains, unites them together with a ferruginous cement, and thus constructs a flask-shaped test, having a short neck and a single large orifice; another picks up the finer grains and puts them together with the same cement into perfectly spherical tests of the most extraordinary finish, perforated with numerous small pores disposed at pretty regular intervals. Another species selects the minutest sand grains and the terminal portions of sponge-spicules, and works them up together—apparently with no cement at all, but by the mere laying of the spicules—into perfect white spheres like homoeopathic globules, each showing a single-fissured orifice. And another, which makes a straight, many-chambered test, the conical mouth of each chamber projecting into the cavity of the next, while forming the walls of its chambers of ordinary sand grains rather loosely held together, shapes the conical mouths of the chambers by firmly cementing together the quartz grains which border it." The structure of the shell is indeed variable. The pylome may be single or represented by a row of holes (Peneroplis, Orbitolites), or, again, there may be several pylomes (Calcituba); and, again, there are in addition numerous scattered pores for the protrusion of pseudopodia elsewhere than from the stylopodium, in the whole of the "Vitrea" and in many "Arenacea"; and, as we shall see, this may exercise a marked influence on the structure of the shell.
In some cases the shell is simple, and in Cornuspira and Spirillina increases so as to have the form of a flat coiled tube. In Calcituba the shell branches irregularly in a dichotomous way, and the older parts break away as the seaweed on which they grow is eaten away, and fall to the bottom, while the younger branches go on growing and branching. The fallen pieces, if they light on living weed, attach themselves thereto and repeat the original growth; if not, the protoplasm crawls out and finds a fresh weed and forms a new tube. In the "Polythalamia" new chambers are formed by the excess of the protoplasm emerging and surrounding itself with a shell, organically united with the existing chamber or chambers, and in a space-relation which follows definite laws characteristic of the species or of its stage of growth, so as to give rise to circular, spiral, or irregular complexes (see Fig. 13).
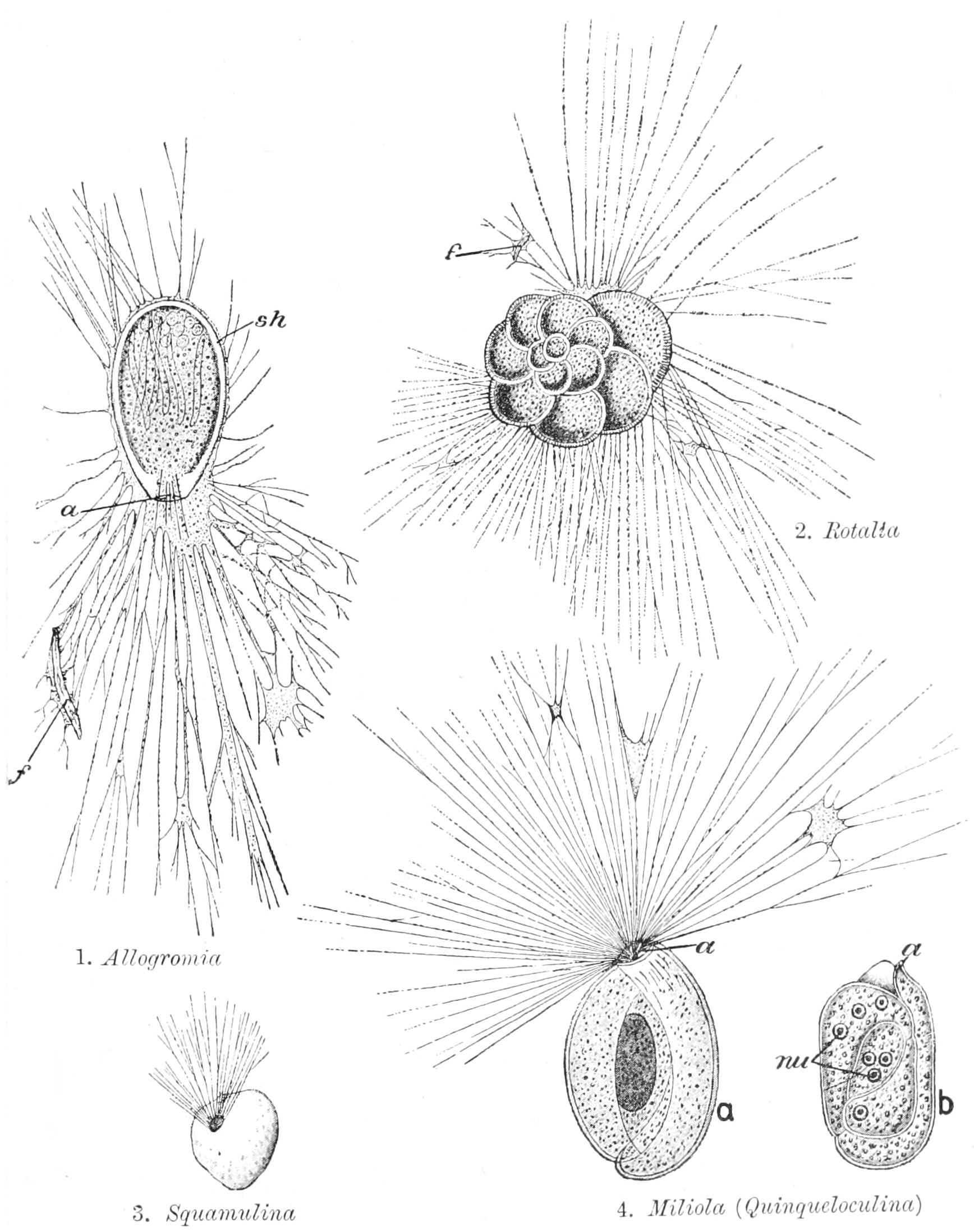
Fig. 14.—Various forms of Foraminifera. In 4, Miliola, a, shows the living animal; b, the same killed and stained; a, aperture of shell; f, food particles; nu, nucleus; sh, shell. (From Parker and Haswell, after other authors.)
In most {66}cases the part of the previously existing chamber next the pylome serves as the hinder part of the new chamber, and the old pylome becomes the pore of communication. But in some of the "Perforata" each new chamber forms a complete wall of its own ("proper wall," Fig. 13, 8b), and the space between the two adjacent walls is filled with an intermediate layer traversed by canals communicating with the cavities of the chambers ("intermediate skeleton"), while an external layer of the same character may form a continuous covering. The shell of the Perforata may be adorned with pittings or fine spines, which serve to increase the surface of support in such floating forms as Globigerina, Hastigerina, and the like (Fig. 17). In the "Imperforata" the outer layer is often ornamented with regular patterns of pits, prominences, etc., which are probably formed by a thin reflected external layer of protoplasm. In some of the "Arenacea" a "labyrinthine" complex of laminae is formed.
A very remarkable point which has led to great confusion in the study of the Foraminifera, is the fact that the shell on which we base our characters of classification, may vary very much, even within the same individual. Thus in the genus Orbitolites the first few chambers of the shell have the character of a Milioline, in Orbiculina of a Peneroplis. The arrangements of the Milioline shell, known as Triloculine, Quinqueloculine, and Biloculine respectively, may succeed one another in the same shell (Figs. 14 4, 15). A shell may begin as a spiral and end by a straight continuation: again, the spherical Orbulina (Fig. 16 1) is formed as an investment to a shell indistinguishable from Globigerina, which is ultimately absorbed. In some cases, as Rhumbler has pointed out, the more recent and higher development shows itself in the first formed chambers, while the later, younger chambers remain at a lowlier stage, as in the case of the spiral passing into a straight succession; but the other cases we have cited show that this is not always the case. In Lagena (Fig. 13 2) the pylome is produced into a short tube, which may protrude from the shell or be turned into it, so that for the latter form the genus Entosolenia was founded. Shells identical in minute sculpture are, however, found with either form of neck, and, moreover, the polythalamial shells (Nodosaria, Fig. 13 3), formed of a nearly straight succession of Lagena-like chambers, may have these chambers with their {67}communications on either type. Rhumbler goes so far as to suggest that all so-called Lagena shells are either the first formed chamber of a Nodosaria which has not yet become polythalamian by the formation of younger ones, or are produced by the separation of an adult Nodosaria into separate chambers.
Fig. 15.—A, Megalospheric; B, microspheric shell of Biloculina. c, The initial chamber. The microspheric form begins on the Quinqueloculina type. (From Calkins' Protozoa.)
Many of the chambered species show a remarkable dimorphism, first noted by Schlumberger, and finally elucidated by J. J. Lister and Schaudinn. It reveals itself in the size of the initial chamber; accordingly, the two forms may be distinguished as "microspheric" and "megalospheric" respectively (Fig. 15), the latter being much the commoner. The microspheric form has always a plurality of nuclei, the megalospheric a single one, except at the approach of reproduction. Chromidial masses are, however, present in both forms. The life-history has been fully worked out in Polystomella by Schaudinn, and in great part in Polystomella, Orbitolites, etc., by Lister; and the same scheme appears to be general in the class, at least where the dimorphism noted occurs. The microspheric form gives birth only to the megalospheric, but the latter may reproduce megalospheric broods, or give rise to swarmers, which by their (exogamous) {68}conjugation produce the microspheric young. The microspheric forms early become multinucleate, and have also numerous chromidia detached from the nuclei, which they ultimately replace. These collect in the outer part of the shell and aggregate into new nuclei, around which the cytoplasm concentrates, to separate into as many amoeboid young "pseudopodiospores" as there are nuclei. These escape from the shell or are liberated by its disintegration, and invest themselves with a shell to form the initial large central chamber or megalosphere.
Fig. 16.—1, Orbulina universa. Highly magnified. 2, Globigerina bulloides. Highly magnified. (From Wyville Thomson, after d'Orbigny.)
In the ordinary life of the megalospheric form the greater part of the chromatic matter is aggregated into a nucleus, some still remaining diffused. At the end of growth the nucleus itself disintegrates, and the chromidia concentrate into a number of small vesicular nuclei, each of which appropriates to itself a small surrounding zone of thick plasm and then divides by mitosis twice; and the 4-nucleate cells so formed are resolved into as many 1-nucleate, 2-flagellate swarmers, which conjugate {69}only exogamously.[80] The fusion of their nuclei takes place after some delay: ultimately the zygote nucleus divides into two, a shell is formed, and we have the microsphere, which is thus pluri-nucleate ab initio. As we have seen, the nuclei of the microsphere are ultimately replaced by chromidia, and the whole plasmic body divides into pseudopodiospores, which grow into the megalospheric form.
Fig. 17.—Shell of Globigerina bulloides, from tow-net, showing investment of spines. (From Wyville Thomson.)
In the Perforate genera, Patellina and Discorbina, plastogamy precedes brood formation, the cytoplasms of the 2-5 pairing individuals contracting a close union; and then the nuclei proceed to break up without fusion, while the cytoplasm aggregates around the young nuclei to form amoebulae, which acquire a shell and separate. In both cases it is the forms with a single nucleus, corresponding to megalospheric forms that so pair, and the brood-formation is, mutatis mutandis, the same as in these forms. Similar individuals may reproduce in the same way, in both genera, without this plastogamic pairing, which is therefore, though probably advantageous, not essential. If pseudopodiospores form their shells while near one another, they may coalesce to form monsters, as often happens in Orbitolites.[81]
The direct economic uses of the Foraminifera are perhaps greater than those of any other group of Protozoa. The Chalk is {70}composed largely of Textularia and allied forms, mixed with the skeletons of Coccolithophoridae (pp. 113-114), known as Coccoliths, etc. The Calcaire Grossier of Paris, used as a building stone, is mainly composed of the shells of Miliolines of Eocene age; the Nummulites of the same age of the Mediterranean basin are the chief constituent of the stone of which the Pyramids of Egypt are built. Our own Oolitic limestones are composed of concretions around a central nucleus, which is often found to be a minute Foraminiferous shell.
The palaeontology of the individual genera is treated of in Chapman's and Lister's recent works. They range from the Lower Cambrian characterised by perforated hyaline genera, such as Lagena, to the present day. Gigantic arenaceous forms, such as Loftusia, are among the Tertiary representatives; but the limestones formed principally of their shells commence at the Carboniferous. The so-called Greensands contain greenish granules of "glauconite," containing a ferrous silicate, deposited as a cast in the chambers of Foraminifera, and often left exposed by the solution of the calcareous shell itself. Such granules occur in deep-sea deposits of the present day.[82]
3. Heliozoa
Sarcodina with radiate non-anastomosing pseudopodia of granular protoplasm, each with a stiff axial rod passing into the body plasma; no central capsule, nor clear ectoplasm; skeleton when present siliceous; nucleus single or multiple; contractile vacuole (or vacuoles) in fresh-water species, superficial and prominent at the surface in diastole; reproduction by fission or budding in the active condition, or by brood-formation in a cyst, giving rise to resting spores; conjugation isogamous in the only two species fully studied; habitat floating or among weeds, mostly fresh water.
1. Naked or with an investment only when encysted.
Aphrothoraca.—Actinolophus F.E. Sch.; Myxastrum Haeck.; Gymnosphaera Sassaki; Dimorpha (Fig. 37, 5, p. 112) Gruber; Actinomonas Kent; Actinophrys Ehrb.; Actinosphaerium St.; Camptonema Schaud; Nuclearia Cienk.
2. Invested with a gelatinous layer, sometimes traversed by a firmer elastic network.
Chlamydophora.—Heterophrys Arch.; Mastigophrys Frenzel; Acanthocystis, Carter.
3. Ectoplasm with distinct siliceous spicules.
Chalarothoraca.—Raphidiophrys Arch.
4. Skeleton a continuous, fenestrated shell, sometimes stalked.
Desmothoraca.—Myriophrys Penard; Clathrulina Cienk.; Orbulinella Entz.
This class were at first regarded and described as fresh-water Radiolaria, but the differences were too great to escape the greatest living specialist in this latter group, Ernst Haeckel, who in 1866 created the Heliozoa for their reception. We owe our knowledge of it mainly to the labours of Cienkowsky, the late William Archer, F. E. Schulze, R. Hertwig, Lesser, and latterly to Schaudinn, who has monographed it for the "Tierreich" (1896); and Penard has published a more recent account.
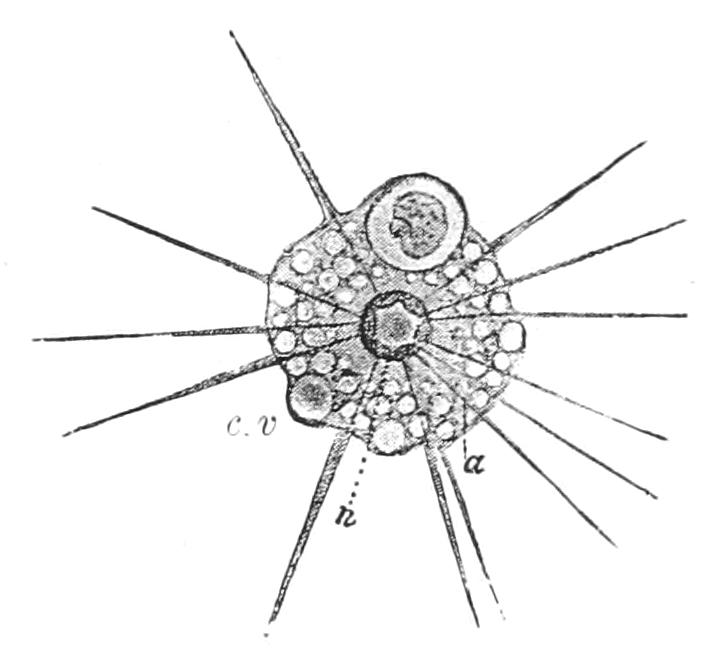
Fig. 18.—Actinophrys sol. a, Axial filament of pseudopod; c.v, contractile vacuole; n, nucleus. (From Lang's Comparative Anatomy, after Grenacher.)
Actinophrys sol Ehrb. (Fig. 18) is a good and common type. It owes its name to its resemblance to a conventional drawing of the sun, with a spherical body and numerous close-set diverging rays. The cytoplasm shows a more coarsely vacuolated outer layer, sometimes called the ectosarc, and a denser internal layer the endosarc. In the centre of the figure is the large nucleus, to which the continuations of the rays may be seen to converge; the pseudopodia contain each a stiffish axial filament,[83] which is covered by the fine granular plasm, showing currents of the granules. The axial filament disappears when the pseudopodia are retracted or bent, and is regenerated afterwards. This bending occurs when a living prey touches and adheres to a ray, all its neighbours bending in like the tentacles of a Sundew. The prey is carried down to the surface of the ectoplasm, and {72}sinks into it with a little water, to form a nutritive vacuole. Fission is the commonest mode of reproduction, and temporary plastogamic unions are not uncommon. Arising from these true conjugations occur, two and two, as described by Schaudinn. A gelatinous cyst wall forms about the two which are scarcely more than in contact with their rays withdrawn. Then in each the nucleus divides into two, one of which passes to the surface, and is lost (as a "polar body"), while the other approaches the corresponding nucleus of the mate, and unites with it, while at the same time the cytoplasms fuse. Within the gelatinous cyst the zygote so formed divides to produce two sister resting spores, from each of which, after a few days, a young Actinophrys escapes, as may take place indeed after encystment of an ordinary form without conjugation.
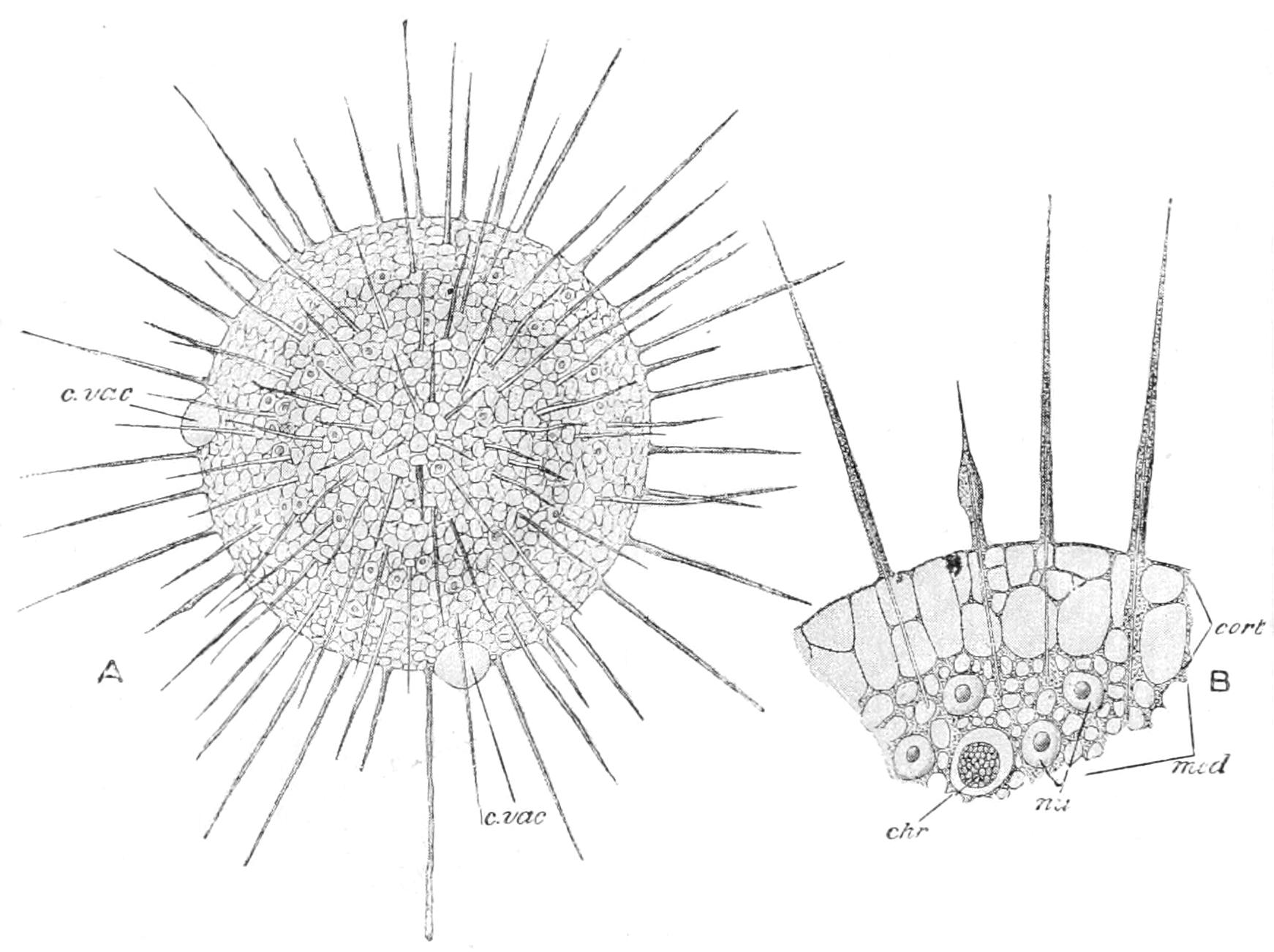
Fig. 19.—Actinosphaerium eichornii. A, entire animal with two contractile vacuoles (c.vac); B, a portion much magnified, showing alveolate cytoplasm, pseudopodia with axial rods, non-nucleate cortex (cort), multiple nuclei (nu) of endoplasm (med), and food-vacuole (chr). (From Parker and Haswell.)
The axial rods of the pseudopodia may pass either to the circumference of the nucleus or to a central granule, corresponding, it would appear, to a centrosome or blepharoplast; or again, {73}in the plurinucleate marine genus Camptonema, each rod abuts on a separate cap on the outer side of each nucleus. The nucleus is single in all but the genera Actinosphaerium, Myxastrum, Camptonema, and Gymnosphaera. The movements of this group are very slow, and are not well understood. A slow rolling over on the points of the rays has been noted, and in Camptonema they move very decidedly to effect locomotion, the whole body also moving Amoeba-fashion; but of the distinct movements of the species when floating no explanation can be given. The richly vacuolate ectoplasm undoubtedly helps to sustain the cell, and the extended rays must subserve the same purpose by so widely extending the surface. Dimorpha (Fig. 37, 5, p. 112) has the power of swimming by protruding a pair of long flagella from the neighbourhood of the eccentric nucleus; and Myriophrys has an investment of long flagelliform cilia. Actinomonas has a stalk and a single flagellum in addition to the pseudopodia; these genera form a transition to the Flagellata.
Several species habitually contain green bodies, which multiply by bipartition, and are probably Zoochlorellae, Chlamydomonadidae of the same nature as we shall find in certain Ciliata (pp. 154, 158) in fresh-water Sponges (see p. 175), in Hydra viridis (p. 256), and the marine Turbellarian Convoluta (Vol. II. p. 43).
Reproduction by fission is not rare, and in some cases (Acanthocystis) the cell becomes multinuclear, and buds off 1-nucleate cells. In such cases the buds at first lack a centrosome, and a new one is formed first in the nucleus, and passes out into the cytoplasm. These buds become 2-flagellate before settling down. In Clathrulina the formation of 2-flagellate zoospores has long been known (Fig. 20, 3). In Actinosphaerium (Figs. 19, 21), a large species, differing from Actinophrys only in the presence of numerous nuclei in its endoplasm, a peculiar process, which we have characterised as endogamy, results in the formation of resting spores. The animal retracts its rays and encysts; and the number of nuclei is much reduced by their mutual fusion, or by the solution of many of them, or by a combination of the two processes. The body then breaks up into cells with a single nucleus, and each of these surrounds itself with a wall to form a cyst of the second order.
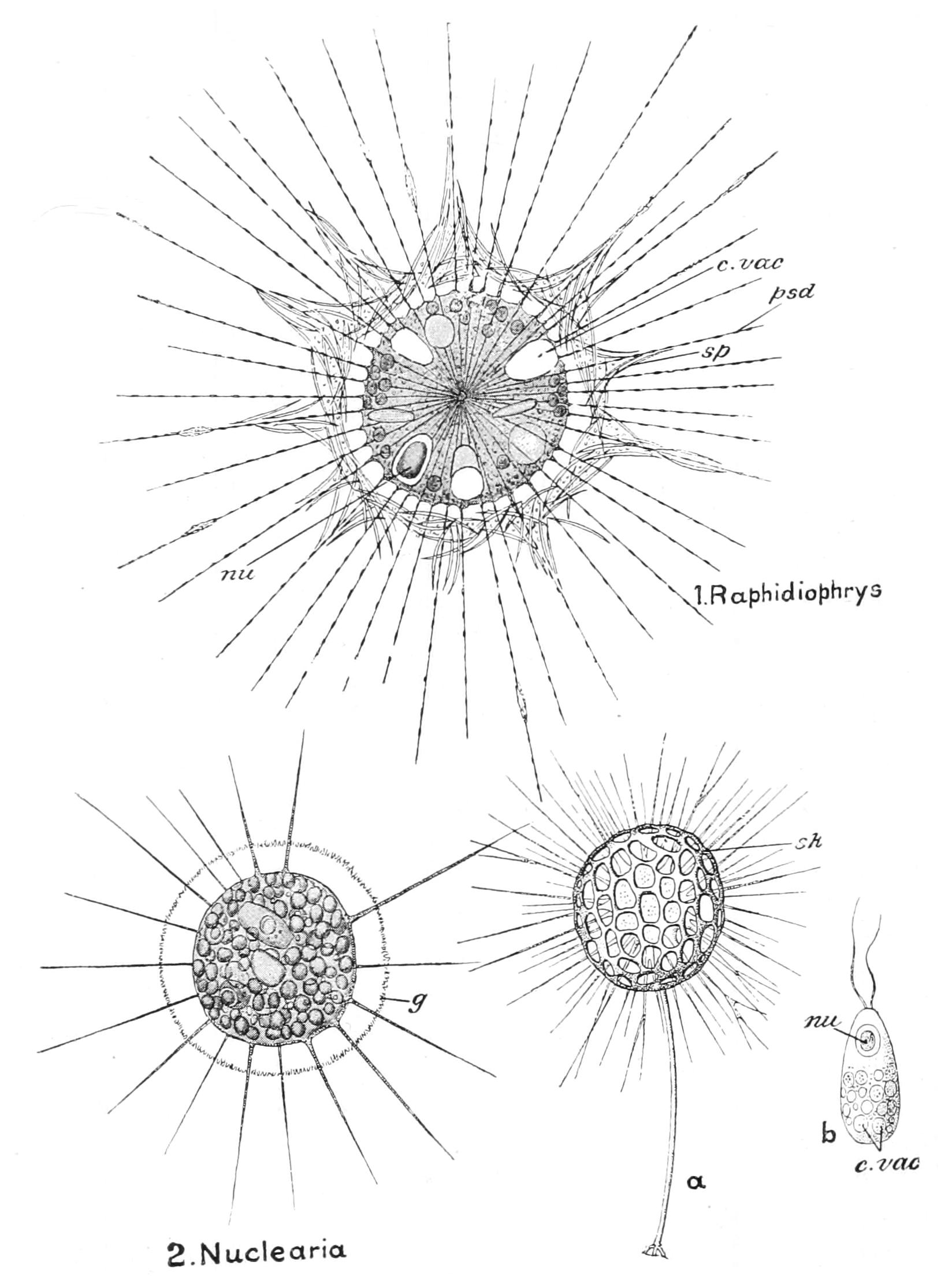
Fig. 20.—Various forms of Heliozoa. In 3, a is the entire animal and b the flagellula; c.vac, contractile vacuole; g, gelatinous investment; nu, nucleus; psd, pseudopodia; sk, siliceous skeleton; sp, spicules. (From Parker and Haswell, after other authors.)
Each of these divides, and the two sister cells then conjugate after the same fashion as in Actinophrys, but the nuclear divisions to form the coupling nucleus are two in number, i.e. the nucleus divides into two, one of which goes to the surface as the first polar body, and the sister of this again divides to form a second polar body (which also passes to {75}the surface) and a pairing nucleus.[84] The two cells then fuse completely, and surround themselves with a second gelatinous cyst wall, separated from the outer one by a layer of siliceous spicules. The nucleus appears to divide at least twice before the young creep out, to divide immediately into as many Actinophrys-like cells as there were nuclei; then each of these multiplies its nuclei, to become apocytial like the adult form.
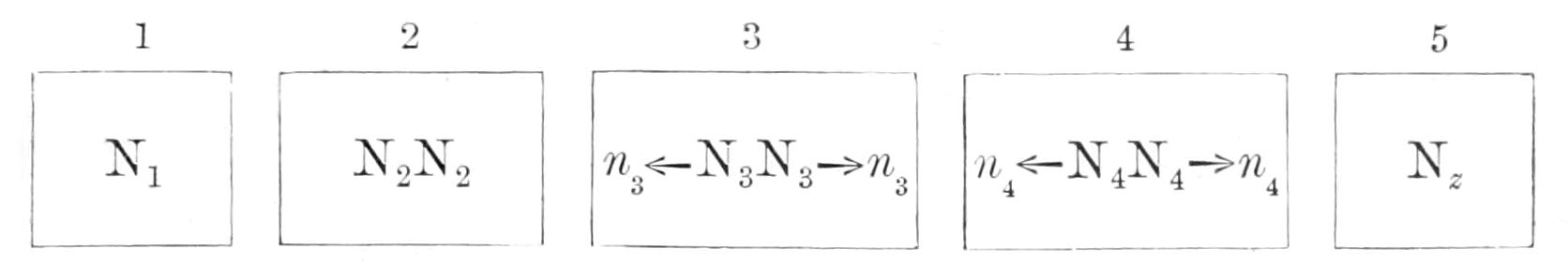
Fig. 21.—Diagram illustrating the conjugation of Actinosphaerium. 1, Original cell; 2, nucleus divides to form two, N2N2; 3, each nucleus again divides to form two, N3 and n3, the latter passing out with a little cytoplasm as an abortive cell; 4, repetition of the same process as in 3; 5, the two nuclei N4 have fused in syngamy to form the zygote nucleus Nz.
Schaudinn admits 24 genera (and 7 doubtful) and 41 species (and 18 doubtful). None are known fossil. Their geographical distribution is cosmopolitan, as is the case with most of the minute fresh-water Protista; 8 genera are exclusively marine, and Orbulinella has only been found in a salt-pond; Actinophrys sol is both fresh-water and marine, and Actinolophus has 1 species fresh-water, the other marine. One of the 14 species of Acanthocystis is marine; the remaining genera and species are all inhabitants of fresh water.[85]
4. Radiolaria
Sarcodina with the protoplasm divided by a perforated chitinous central capsule into a central mass surrounding the nucleus, and an outer layer; the pseudopodia radiate, never anastomosing enough to form a marked network; skeleton either siliceous, of spicules, or perforated; or of definitely arranged spicules of proteid matter (acanthin), sometimes also coalescing into a latticed shell; reproduction by fission and by zoospores formed in the central capsule. Habitat marine, suspended at the surface (plankton), at varying depths (zonarial), or near the bottom (abyssal).
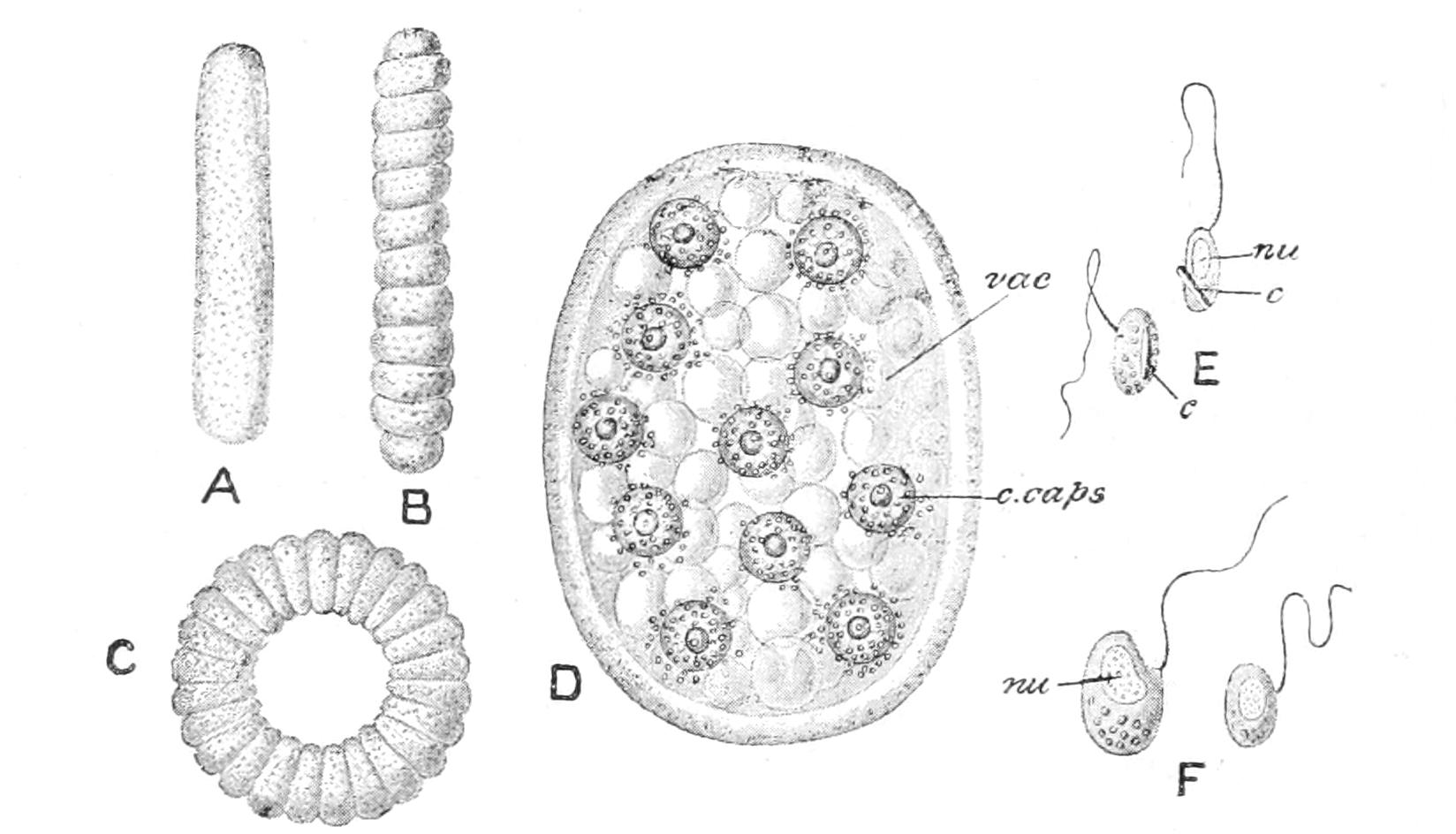
Fig. 22.—Collozoum inerme. A, B, C, three forms of colony; D, small colony with central capsules (c.caps), containing nuclei, and alveoli (vac) in ectoplasm; E, isospores, with crystals (c); F, anisospores; nu, nucleus. (From Parker and Haswell.)
The following is Haeckel's classification of the Radiolaria:—
I. Porulosa (Holotrypasta).—Homaxonic, or nearly so. Central capsule spherical in the first instance; pores numerous, minute, scattered; mostly pelagic.
A. Spumellaria (Peripylaea).—Pores evenly scattered; skeleton of solid siliceous spicules, or continuous, and reticulate or latticed, rarely absent; nucleus dividing late, as an antecedent to reproduction.
B. Acantharia (Actipylaea).—Pores aggregated into distinct areas; skeleton of usually 20 centrogenous, regularly radiating spines of acanthin, whose branches may coalesce into a latticed shell; nucleus dividing early.
II. Osculosa (Monotrypasta).—Monaxonic; pores of central capsule limited to the basal area (osculum), sometimes accompanied by two (or more) smaller oscula at apical pole, mostly zonarial or abyssal.
C. Nassellaria (Monopylaea).—Central capsule ovoid, of a single layer; pores numerous on the operculum or basal field; skeleton siliceous, usually with a principal tripod or calthrop-shaped spicule passing, by branching, into a complex ring or a latticed bell-shaped shell; nucleus eccentric, near apical pole.
D. Phaeodaria (Cannopylaea, Haeck.; Tripylaea, Hertw.).—Central capsule spheroidal, of two layers, in its outer layer an operculum, with radiate ribs and a single aperture, beyond which protrudes the outer layer; osculum basal, a dependent tube (proboscis); accessory oscula, when present, simpler, usually two placed symmetrically about the apical pole; skeleton siliceous, with a combination of organic matter, often of hollow spicules; nucleus sphaeroidal, eccentric; extracapsular protoplasm containing an accumulation of dusky pigment granules ("phaeodium").
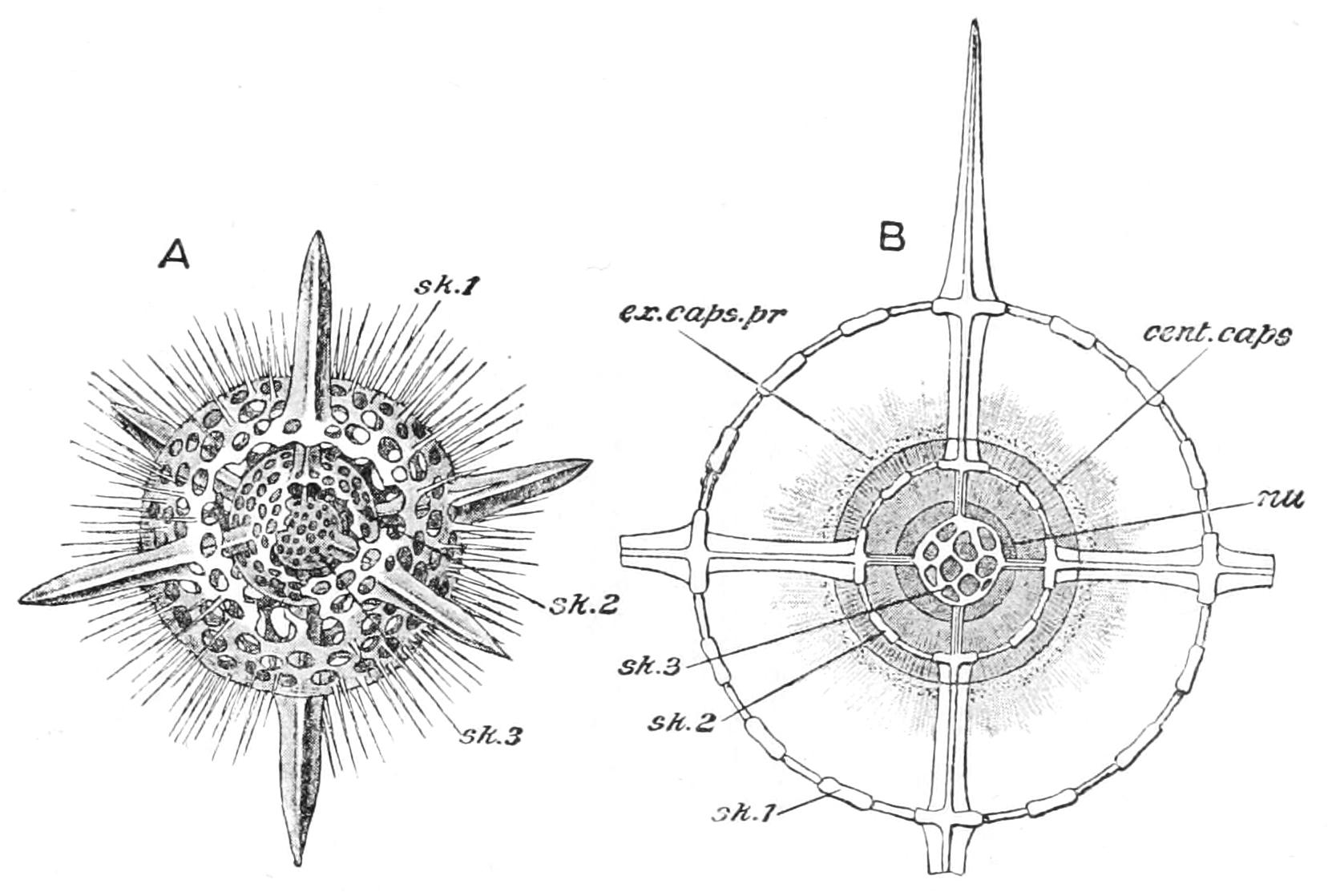
Fig. 23.—Actinomma asteracanthion. A, the shell with portions of the two outer spheres broken away; B, section showing the relations of the skeleton to the animal, cent.caps, Central capsule; ex.caps.pr, extra-capsular protoplasm: nu, nucleus; sk.1, outer, sk.2, middle, sk.3, inner sphere of skeleton. (From Parker and Haswell, after Haeckel and Hertwig.)
A. Spumellaria.
Sublegion (1). Collodaria.[86]—Skeleton absent or of detached spicules; colonial or simple.
Order i. Colloidea.—Skeleton absent. (Families 1, 2.) Thalassicolla Huxl.; Thalassophysa Haeck.; Collozoum Haeck.; Collosphaera J. Müll.; Actissa Haeck.
Order ii. Beloidea.—Skeleton spicular. (Families 3, 4.)
Sublegion (2). Sphaerellaria.—Skeleton continuous, latticed or spongy, reticulate.
Order iii. Sphaeroidea.—Skeleton of one or several concentric spherical shells; sometimes colonial. (Families 5-10.) Haliomma Ehrb.; Actinomma Haeck. (Fig. 23).
Order iv. Prunoidea.—Skeleton a prolate sphaeroid or cylinder, sometimes constricted towards the middle, single or concentric. (Families 11-17.)
Order v. Discoidea.—Shell flattened, of circular plan, simple or concentric, rarely spiral. (Families 18-23.)
Order vi. Larcoidea.—Shell ellipsoidal, with all three axes unequal or irregular, sometimes becoming spiral. (Families 24-32.)[87]
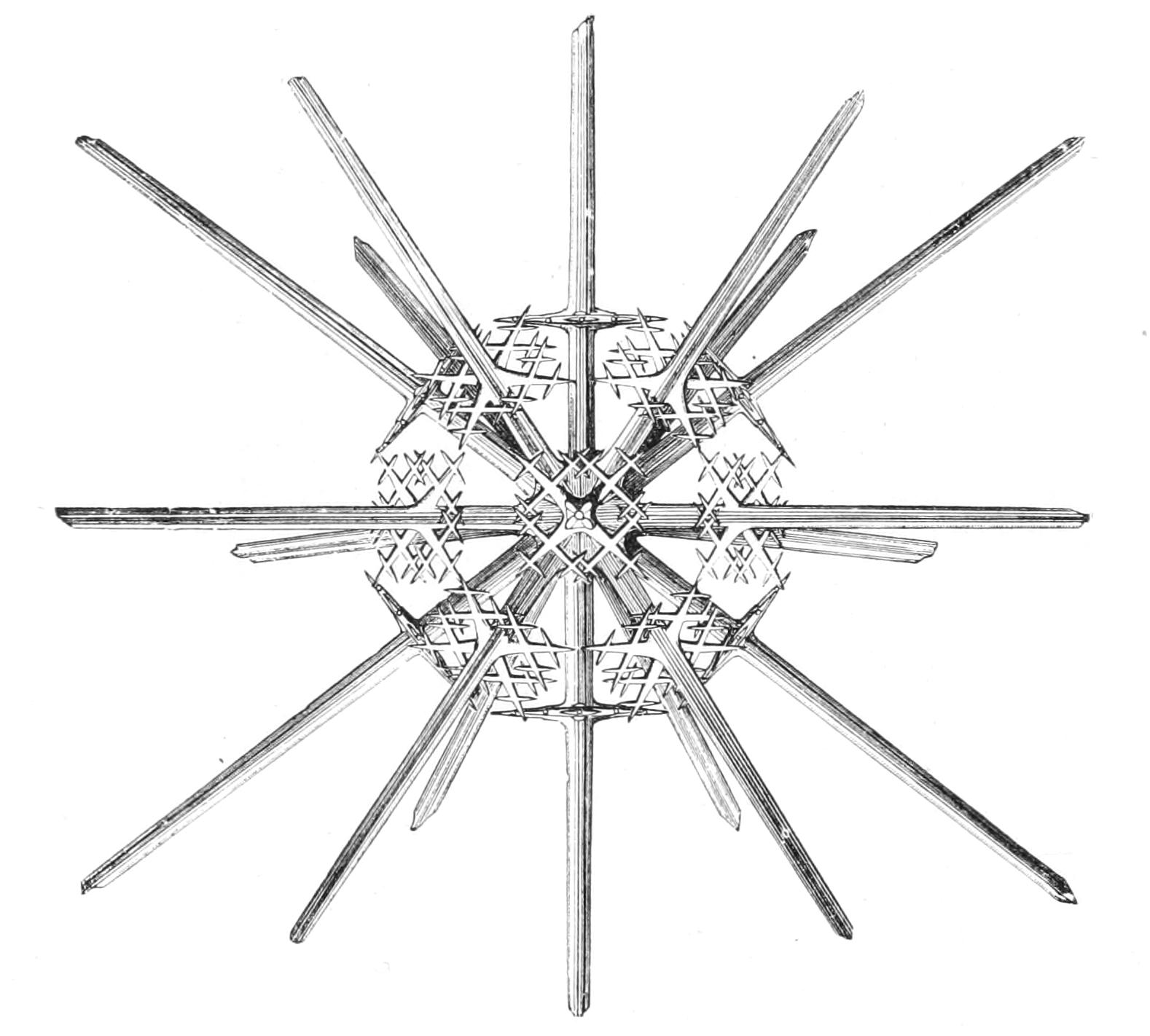
Fig. 24.—Xiphacantha (Acantharia). From the surface. The skeleton only, × 100, (From Wyville Thomson.)
B. Acantharia.
Order vii. Actinelida.—Radial spines numerous, more than 20, usually grouped irregularly. (Families 33-35.) Xiphacantha Haeck.
Order viii. Acanthonida.—Radial spines equal. (Families 36-38.)
Order ix. Sphaerophracta.—Radial spines 20, with a latticed spherical shell, independent of, or formed from the reticulations of the spines. (Families 39-41.) Dorataspis Haeck. (Fig. 25, A).
Order x. Prunophracta.—Radial spines 20, unequal; latticed shell, ellipsoidal, lenticular, or doubly conical. (Families 42-44.)
C. Nassellaria.
Order xi. Nassoidea.—Skeleton absent. (Family 45.)
Order xii. Plectoidea.—Skeleton of a single branching spicule, the branches sometimes reticulate, but never forming a latticed shell or a sagittal ring. (Families 46-47.)
Order xiii. Stephoidea.—Skeleton with a sagittal ring continuous with the branched spicule, and sometimes other rings or branches. (Families 48-51.) Lithocercus Théel (Fig. 26, A).
Order xiv. Spyroidea.—Skeleton with a latticed shell developed around the sagittal ring (cephalis), and constricted in the sagittal plane, with a lower chamber (thorax) sometimes added. (Families 52-55.)
Order xv. Botryoidea.—As in Spyroidea, but with the cephalis 3-4 lobed; lower chambers, one or several successively formed. (Families 56-58.)
Order xvi. Cyrtoidea.—Shell as in the preceding orders, but without lobing or constrictions. (Families 59-70.) Theoconus Haeck. (Fig. 25, B).
D. Phaeodaria.
Order xvii. Phaeocystina.—Skeleton 0 or of distinct spicules; capsule centric. (Families 71-73.) Aulactinium Haeck. (Fig. 26, B).
Order xviii. Phaeosphaeria.—Skeleton a simple or latticed sphere, with no oral opening (pylome); capsule central. (Families 74-77.)
Order xix. Phaeogromia.—Skeleton a simple latticed shell with a pylome at one end of the principal axis; capsule excentric, sub-apical. (Families 78-82.) Pharyngella Haeck.; Tuscarora Murr.; Haeckeliana Murr. (Fig. 28).
Order xx. Phaeoconchia.—Shell of two valves, opening in the plane ("frontal") of the three openings of the capsule. (Families 83-85.)
We exclude Haeckel's Dictyochida, with a skeleton recalling that of the Stephoidea, but of the impure hollow substance of the Phaeodaria (p. 84). They rank now as Silicoflagellates (p. 114).
The Radiolarian is distinguished from all other Protozoa by the chitinous central capsule, so that its cytoplasm is separated into an outer layer, the extracapsular protoplasm (ectoplasm), and a central mass, the intracapsular, containing the nucleus.[88]
The extracapsular layer forms in its substance a gelatinous mass, of variable reaction, through which the plasma itself ramifies as a network of threads ("sarcodictyum"), uniting at the surface to constitute the foundation for the pseudopodia. This gelatinous matter constitutes the "calymma." It is largely vacuolated, the vacuoles ("alveoli"), of exceptional size, lying in the nodes of the plasmic network, and containing a liquid probably of lower specific gravity than seawater; and they are especially abundant towards the surface, where they touch and become polygonal. On mechanical irritation they disappear, to be formed anew after an interval, a fact that may explain the sinking from the surface in disturbed water. This layer may contain minute pigment granules, but the droplets of oil and of albuminous matter frequent in the central layer are rare here. {80}The "yellow cells" of a symbiotic Flagellate or Alga, Zooxanthella, are embedded in the jelly of all except Phaeodaria, and the whole ectosarc has the average consistency of a firm jelly.
The pseudopodia are long and radiating, with a granular external layer, whose streaming movements are continuous with those of the inner network. In the Acantharia they contain a firm axial filament, like that of the Heliozoa, which is traceable to the central capsule; and occasionally a bundle of pseudopodia may coalesce to form a stout process like a flagellum ("sarcoflagellum"). Here, too, each spine, at its exit from the jelly, is surrounded by a little cone of contractile filaments, the myophrisks, whose action seems to be to pull up the jelly and increase the volume of the spherical body so as to diminish its density.
The intracapsular protoplasm is free from Zooxanthella except in the Acantharia. It is less abundantly vacuolated, and is finely granular. In the Porulosa it shows a radial arrangement, with pyramidal stretches of hyaline plasma separated by intervals rich in granules. Besides the alveoli with watery contents, others are present with albuminoid matter in solution. Oil-drops, often brilliantly coloured, occur either in the plasma or floating in either kind of vacuole; and they are often luminous at night. Added to these, the intracapsular plasm contains pigment-granules, most frequently red or orange, {81}passing into yellow or brown, though violet, blue, and green also occur. The "phaeodium,"[89] however, that gives its name to the Phaeodaria, is an aggregate of dark grey, green, or brown granules which are probably formed in the endoplasm, but accumulate in the extracapsular plasm of the oral side of the central capsule. Inorganic concretions and crystals are also found in the contents of the central capsule, as well as aggregates of unknown composition, resembling starch-grains in structure.
In the Monopylaea, or Nassellaria (Figs. 25, B, 26, A), the endoplasm is differentiated above the perforated area of the central capsule into a cone of radiating filaments termed the "porocone," which may be channels for the communication between the exoplasm and the endoplasm, or perhaps serve, as Haeckel suggests, to raise, by their contraction, the perforated area: he compares them to the myophane striae of Infusoria. In the Phaeodaria (Fig. 26, B), a radiating laminated cone is seen in the outermost layer of the endoplasm above the principal opening ("astropyle"), and a fibrillar one around the two accessory ones ("parapyles"); and in some cases, continuous with these, the whole outer layer of the endoplasm shows a meridional striation.
The nucleus is contained in the endoplasm, and is always at first single, though it may divide again and again. The nuclear wall is a firm membrane, sometimes finely porous. If there are concentric shells it at first occupies the innermost, which it may actually come to enclose, protruding lobes which grow through the several perforations of the lattice-work, finally coalescing outside completely, so as to show no signs of the joins. In the Nassellaria a similar process usually results in the formation of a lobed nucleus, contained in an equally lobed central capsule. The chromatin of the nucleus may be concentrated into a central mass, or distributed into several "nucleoli," or it may assume the form of a twisted, gut-like filament, or, again, the nuclear plasm may be reticulated, with the chromatin deposited at the nodes of the network.
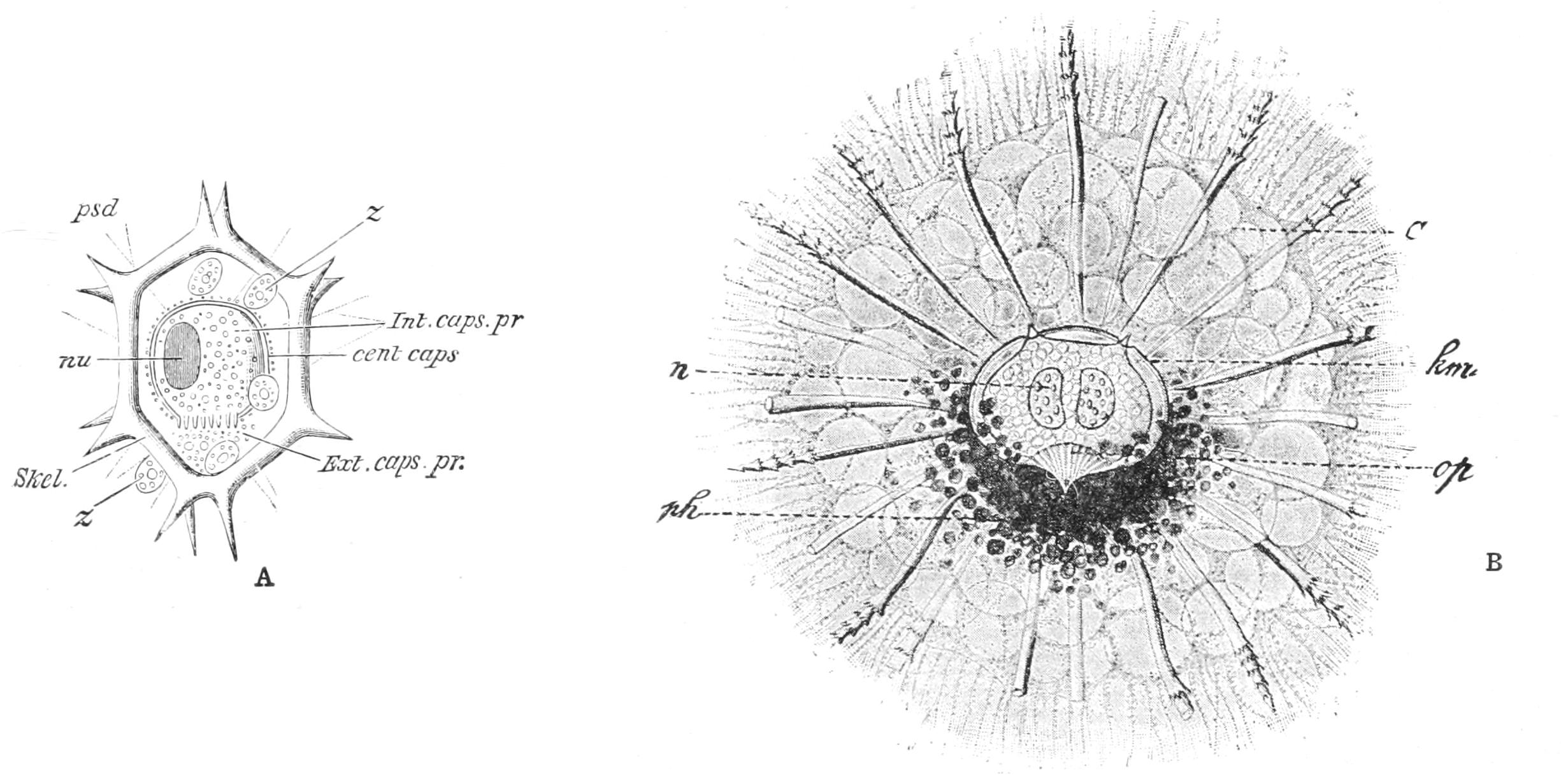
Fig. 26.—A, Lithocercus annularis, with sagittal ring (from Parker and Haswell). B, Aulactinium actinastrum. C, calymma; cent.caps., km, central capsule; Ext.caps.pr., Extracapsular, and Int.caps.pr., intracapsular protoplasm; n, nu, nucleus; op, operculum; ph, phaeodium; psd, pseudopodium; Skel., skeleton; z, Zooxanthella. (From Lang's Comparative Anatomy, after Haeckel.)
The skeleton of this group varies, as shown in our conspectus, in the several divisions.[90] The Acantharia (Figs. 24, 25, A) have a skeleton of radiating spines meeting in the centre of figure of the endoplasm, and forcing the nucleus to one side. The spines are typically 20 in number, and emerge from the surface of the regular spherical forms (from which the others may be readily derived) radially, in five sets of four in the regions corresponding to the equator and the tropics and polar circles of our world. {83}The four rays of adjacent circles alternate, so that the "polar" and "equatorial" rays are on one set of meridians 90° apart, and the "tropical" spines are on the intermediate meridians, as shown in the figures. By tangential branching, and the meeting or coalescence of the branches, reticulate (Figs. 23, 24, 25) and latticed shells are formed in some families, with circles of openings or pylomes round the bases of the spines. In the Sphaerocapsidae the spines are absent, but their original sites are inferred from the 20 circles of pylomes.
In the Spumellaria the simplest form of the (siliceous) skeleton is that of detached spicules, simple or complex, or passing into a latticed shell, often with one or more larger openings (pylomes). Radiating spines often traverse the whole of the cavity, becoming continuous with its latticed wall, and bind firmly the successive zones when present (Fig. 23).
Calcaromma calcarea was described by Wyville Thomson as having a shell of apposed calcareous discs, and Myxobrachia, by Haeckel, as having collections of the calcareous Coccoliths and Coccospheres. In both cases we have to do with a Radiolarian not possessing a skeleton, but retaining the undigested shells of its food, in the former case (Actissa) in a continuous layer, in the latter (Thalassicolla) in accumulations that, by their weight, droop and pull out the lower hemisphere into distinct arms.
The (siliceous) skeleton of the Nassellaria is absent only in the Nassoidea, and is never represented by distinct spicules. Its simplest form is a "tripod" with the legs downward, and the central capsule resting on its apex. The addition of a fourth limb converts the tripod into a "calthrop," the central capsule in this case resting between the upturned leg and two of the lower three regarded as the "anterolateral"; the odd lower leg, like the upturned one, being "posterior." Again, the skeleton may present a "sagittal ring," often branched and spiny (Fig. 26, A), or combined with the tripod or calthrop, or complicated by the addition of one or more horizontal rings. Another type is presented by the "latticed chamber" surrounding the central capsule, with a wide mouth ("pylome") below. This is termed the "cephalis"; it may be combined in various ways with the sagittal ring and the tripod or calthrop; and, again, it may be prolonged by the addition of one, two, or three chambers below, {84}the last one opening by a pylome (Fig. 25, B). These are termed "thorax," "abdomen," and "post-abdomen" respectively.
In the Phaeodaria the skeleton may be absent, spicular (of loose or connected spicules) or latticed, continuous or bivalve. It is composed of silica combined with organic matter, so that it chars when heated, is more readily dissolved, and is not preserved in fossilisation. The spicules or lattice-work are hollow, often with a central filament running in the centre of the gelatinous contents. The latticed structure of the shell of the Challengeridae (Fig. 28) is so fine as to recall that of the Diatomaceae. In the Phaeoconchida the shell is in two halves, parted along the "frontal" plane of the three apertures of the capsule.

Fig. 27.—Scheme of various possible skeletal forms deposited in the meshes of an alveolar system, most of which are realised in the Radiolaria. (From Verworn, after Dreyer.)
The central capsule (rarely inconspicuous and difficult, if not impossible to demonstrate) is of a substance which resembles chitin, though its chemical reactions have not been fully studied hitherto, and indeed vary from species to species. It is composed of a single layer, except in Phaeodaria, where it is double. The operculum in this group, i.e. the area around the aperture, is composed of an outer layer, which is radially thickened, and a thin inner layer; the former is produced into the projecting tube ("proboscis").
Reproduction in the Radiolaria may be simple fission due to the binary fission of the nucleus, the capsule, and the ectoplasm in succession. If this last feature is omitted we have a colonial organism, composed of the common ectoplasm containing numerous central capsules; and the genera in which this occurs, all belonging to the Peripylaea, were formerly separated (as Polycyttaria) from {85}the remaining Radiolaria (Monocyttaria). They may either lack a skeleton (Collozoidae, Fig. 22), or have a skeleton of detached spicules (Sphaerozoidae), or possess latticed shells (Collosphaeridae) one for each capsule, and would seem therefore to belong, as only differentiated by their colonial habit, to the several groups having these respective characters. Fission has been well studied in Aulacantha (a Phaeodarian) by Borgert.[91] He finds that in this case the skeleton is divided between the daughter-cells, and the missing part is regenerated. In cases where this is impossible one of the daughter-cells retains the old skeleton, and the other escapes as a bud to form a new skeleton.
Fig. 28.—Shells of Challengeridae: A, Tuscarora; B, Pharyngella; C, Haeckeliana. (From Wyville Thomson.)
Two modes of reproduction by flagellate zoospores have been described (Fig. 22). In the one mode all the zoospores are alike—isospores—and frequently contain a crystal of proteid nature as well as oil-globules. In the Polycyttaria alone has the second mode of spore-formation been seen, and that in the same species in which the formation of isospores occurs. Here "anisospores" are formed, namely, large "mega-," and small "micro-zoospores." They probably conjugate as male and female respectively; but neither has the process been observed, nor has any product of such conjugation (zygote) been recognised. In every case the formation of the zoospores only involves the {86}endoplasm: the nucleus first undergoes brood division, and the plasma within the capsule becomes concentrated about its offspring, and segregates into the spores; the extracapsular plasm disintegrates.[92]
The Yellow Cells (Zooxanthella), so frequently found in the Radiolaria were long thought to be constituents of their body. Cienkowsky found that when the host died from being kept in unchanged water, the yellow cells survived and multiplied freely, often escaping from the gelatinised cell-wall as biflagellate zoospores. The cell-wall is of cellulose. The cell contains two chloroplastids, or plates coloured with the vegetal pigment "diatomin." Besides ordinary transverse fission in the ordinary encysted state in the ectoplasm of the host, when free they may pass into what is known as a "Palmella-state," the cell-walls gelatinising; in this condition they multiply freely, and constitute a jelly in which the individual cells are seen as rounded bodies. They contain starch in two forms—large hollow granules, not doubly refractive, and small solid granules which polarise light. We may regard them as Chrysomonadaceae (p. 113). Similar organisms occur in many Anthozoa (see pp. 261, 339, 373 f., 396). Diatomaceae (yellow Algae with silicified cell-walls) sometimes live in the jelly of certain Collosphaera. Both these forms live in the state known as "symbiosis" with their host; i.e. they are in mutually helpful association, the Radiolarian absorbing salts from the water for the nutrition of both, and the Alga or Flagellate taking up the CO2 due to the respiration of the host, and building up organic material, the surplus of which is doubtless utilised, at least in part, for the nutrition of the host. A similar union between a Fungus and a coloured vegetal ("holophytic") organism is known as a Lichen.
The Suctorian Infusorian Amoebophrya is parasitic in the ectoplasm of certain Acantharia, and in the peculiar genus Sticholonche which appears to be intermediate between this group and Heliozoa.
The Silicoflagellate family Dictyochidae are found temporarily {87}embedded in the ectoplasm of some of the Phaeocystina, and have a skeleton of similar nature. Their true nature was shown by Borgert.
The Amphipod crustacean Hyperia[93] may enter the jelly of the colonial forms, and feed there at will on the host.[94]
Haeckel, in his Monograph of the Radiolaria of the Challenger enumerated 739 genera, comprising 4318 species; and Dreyer has added 6 new genera, comprising 39 species, besides 7 belonging to known genera. Possibly, as we shall see, many of the species may be mere states of growth, for it is impossible to study the life-histories of this group; on the other hand, it is pretty certain that new forms are likely to be discovered and described. The Radiolaria are found living at all depths in the sea, by the superficial or deep tow-net; and some appear to live near the bottom, where the durable forms of the whole range also settle and accumulate. They thus form what is known as Radiolarian ooze, which is distinguished from other shallower deposits chiefly through the disappearance by solution of all calcareous skeletons, as they slowly fell through the waters whereon they originally floated at the same time with the siliceous remains of the Radiolaria. The greatest wealth of forms is found in tropical seas, though in some places in cold regions large numbers of individuals of a limited range of species have been found.
Radiolaria of the groups with a pure siliceous skeleton can alone be fossilised, even the impure siliceous skeleton of the Phaeodaria readily dissolving in the depths at which they live: they have been generally described by Ehrenberg's name Polycystineae. Tripolis (Kieselguhr) of Tertiary ages have been found in many parts of the globe, consisting largely or mainly of Radiolaria, and representing a Radiolarian ooze. That of the Miocene of Barbados contains at least 400 species; that of Gruppe at least 130. In Secondary and Palaeozoic rocks such oozes pass into Radiolarian quartzites (some as recent as the Jurassic). They occur also in fossilised excrement (coprolites), and in flint or chert concretions, as far down as the lowest fossiliferous rocks, {88}the Cambrian. The older forms are simple Sphaerellaria and Nassellaria. From a synopsis of the history of the order in Haeckel's Monograph (pp. clxxxvi.-clxxxviii.) we learn that while a large number of skeletal forms had been described by Ehrenberg, Huxley in 1851 published the first account of the living animal. Since then our knowledge has been extended by the labours of Haeckel, Cienkowsky, R. Hertwig, Karl Brandt, and A. Borgert.
5. Proteomyxa
Sarcodina without a clear ectoplasm, whose active forms are amoeboid or flagellate, or pass from the latter form to the former; multiplying chiefly, if not exclusively, by brood-formation in a cyst. No complete cell-pairing (syngamy) known, though the cytoplasms may unite into plasmodia; pseudopodia of the amoeboid forms usually radiate or filose, but without axial filaments. Saprophytic or parasitic in living animals or plants.
This group is a sort of lumber-room for forms which it is hard to place under Rhizopoda or Flagellata, and which produce simple cysts for reproduction, not fructifications like the Mycetozoa. The cyst may be formed for protection under drought ("hypnocyst"), or as a preliminary to spore-formation ("sporocyst"). The latter may have a simple wall (simple sporocyst), or else two or three formed in succession ("resting cyst"), so as to enable it to resist prolonged desiccation, etc.: both differing from the hypnocyst in that their contents undergo brood formation. On encystment any indigestible food materials are extruded into the cyst, and in the "resting cysts," which are usually of at least two layers, this faecal mass lies in the space between them. The brood-cells escape, either as flagellate-cells, resembling the simpler Protomastigina, called "flagellulae," and which often become amoeboid (Fig. 29); or already furnished with pseudopodia, and called "amoebulae," though they usually recall Actinophrys rather than Amoeba. In Vampyrella and some others the amoebulae fuse, and so attain a greater size, which is most probably advantageous for feeding purposes. But usually it is as a uninucleate cell that the being encysts. They may feed either by ingestion by the pseudopodia, by the whole surface contained in a living host-cell, or by passing a pseudopodium into a host-cell (Fig. 29 5). They may be divided as follows:—
A. Myxoidea.—Flagella 1-3; zoospores separating at once.
1. Zoosporeae.—Brood-cells escaping as flagellulae, even if they become amoeboid later. Ciliophrys Cienk.; Pseudospora Cienk. (Fig. 29).
2. Azoosporeae.—Cells never flagellate. Protomyxa Haeckel; Plasmodiophora Woronin; Vampyrella Cienk.; Serumsporidium L. Pfeiffer.
B. Catallacta.—Brood-cells of cyst on liberation adhering at the centre to form a spherical colony, multiflagellate; afterwards separating, and becoming amoeboid. Magosphaera Haeckel (marine).[95]
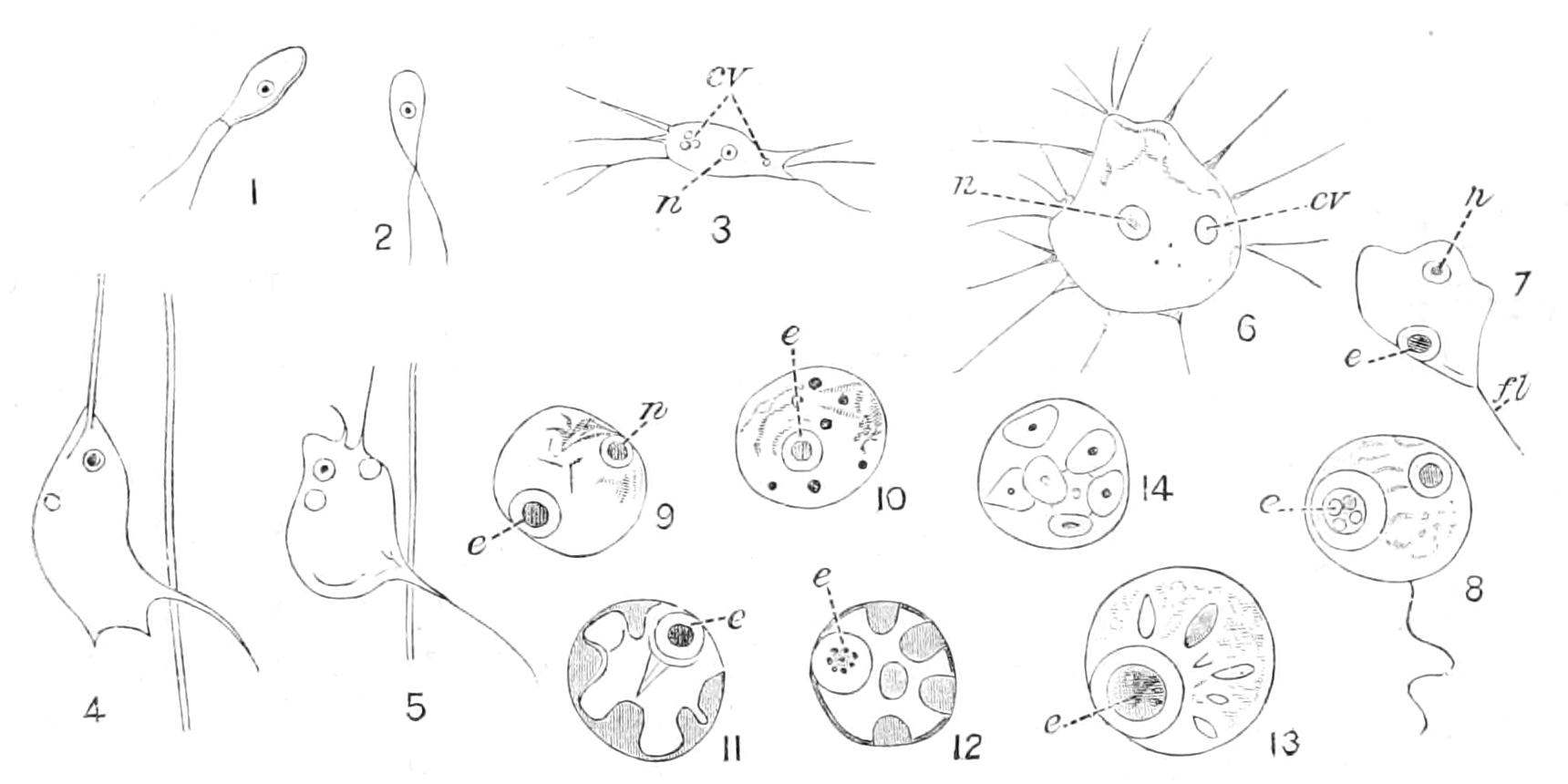
Fig. 29.—Pseudospora lindstedtii. 1, 2, Flagellate zoospores; 3, young amoebula, with two contractile vacuoles, one being reconstituted by three minute formative vacuoles; 4, 5, an amoebula migrating to a fungus hypha through the wall of which it has sent a long pseudopodium; 6, amoebula full-grown; 7, 8, mature cells rounded off, protruding a flagellum, before encysting; 9, young sporocyst; 10, the nucleus has divided into a brood of eight; 11-14, stages of formation of zoospores. cv, Contractile vacuole; e, mass of faecal granules; fl, flagellum; n, nucleus, × about 750⁄1.
Plasmodiophora infests the roots of Crucifers, causing the disease known as "Hanburies," or "fingers and toes," in turnips, etc. Serumsporidium dwells in the body cavity of small Crustacea. Many of this group were described by Cienkowsky under the name of "Monadineae" (in Arch. Mikr. Anat. i. 1865, p. 203). Zopf has added more than anyone else since then to our knowledge. He monographed them under Cienkowsky's name, as a subordinate group of the Myxomycetes, "Pilzthiere oder Schleimpilze," in Schenk's Handb. d. Bot. vol. iii. pt. ii. (1887). To Lankester (Encycl. Brit., reprint 1891) we owe the name here adopted. Zopf has successfully pursued their study in recent {90}papers in his Beitr. Nied. Org. The Chytridieae, usually ascribed to Fungi, are so closely allied to this group that Zopf proposes to include at least the Synchytrieae herein.
This group is very closely allied to Sporozoa; for the absence of cytogamy, and of sickle-germs,[96] and of the complex spores and cysts of the Neosporidia, are the only absolute distinctions.
6. Mycetozoa (Myxomycetes, Myxogastres)
Sarcodina moving and feeding by pseudopodia, with no skeleton, aggregating more or less completely into complex "fructifications" before forming 1-nucleate resting spores; these may in the first instance liberate flagellate zoospores, which afterwards become amoeboid, or may be amoeboid from the first; zoospores capable of forming hypnocysts from which the contents escape in the original form.
| 1. Aggregation taking place without plastogamy, zoospores amoeboid, with a clear ectosarc | Acrasieae. |
| Copromyxa Zopf; Dictyostelium Brefeld. | |
| 2. Aggregation remaining lax, with merely thread-like connexions, except when encystment is to take place; cytoplasm finely granular throughout; complete fusion of the cytoplasm doubtful | Filoplasmodieae |
| Labyrinthula Cienk.; Chlamydomyxa Archer; Leydenia (?) Schaud. | |
| 3. Plasmodium formation complete, eventuating in the formation of a complex fructification often traversed by elastic, hygroscopic threads, which by their contraction scatter the spores; zoospores usually flagellate at first | Myxomycetes. |
| Fuligo Hall.; Chondrioderma Rostaf.; Didymium Schrad. (Fig. 30). | |
I. The Acrasieae are a small group of saprophytes, often in the most literal sense, though in some cases it has been proved that the actual food is the bacteria of putrefaction. In them, since no cell-division takes place in the fructification, it is certain that the multiplication of the species must be due to the fissions of the amoeboid zoospores, which often have the habit of Amoeba limax (Fig. 1, p. 5).
II. Filoplasmodieae.—Chlamydomyxa[97] is a not uncommon inhabitant of the cells of bog-mosses and bog-pools, and its nutrition may be holophytic, as it contains chromoplasts; but it {91}can also feed amoeba-fashion. Labyrinthula is marine, and in its fructification each of the component cells forms four spores. Leydenia has been found in the fluid of ascitic dropsy, associated with malignant tumour.
III. Myxomycetes.—The fructification in this group is not formed by the mere aggregation of the zoospores, but these fuse by their cytoplasm to form a multinucleate body, the "plasmodium," which, after moving and growing (with nuclear division) for some time like a great multinucleate Reticularian, passes into rest, and develops a fructification by the formation of a complex outer wall; within this the contents, after multiplication of the nuclei, resolve themselves into uninucleate spores, each with its own cyst-wall. The fructifications of this group are often conspicuous, and resemble those of the Gasteromycetous fungi (e.g., the Puffballs), whence they were at first called Myxogastres. De Bary first discovered their true nature in 1859, and ever since they have been claimed by botanist and zoologist alike.
The spore on germination liberates its contents as a minute flagellate, with a single anterior lash and a contractile vacuole (Fig. 30, C). It soon loses the lash, becomes amoeboid, and feeds on bacteria, etc. (Fig. 30, D, E). In this state it can pass into hypnocysts, from which, as from the spores, it emerges as a flagellula. After a time the amoeboids, which may multiply by fission, fuse on meeting, so as to form the plasmodium (Fig. 30, F). This contains numerous nuclei, which multiply as it grows, and numerous contractile vacuoles. When it attains full size it becomes negatively hydrotactic, crawls to a dry place, and resolves itself into the fructification. The external wall, and sometimes a basal support to the fruit, are differentiated from the outer layer of protoplasm; while the nuclei within, after undergoing a final bipartition, concentrate each around an independent portion of plasma, which again is surrounded as a spore by a cyst-wall. Often the maturing plasmodium within the wall of the fruit is traversed by a network of anastomosing tubes filled with liquid, the walls of which become differentiated into membrane like the fruit-wall, and are continuous therewith. As the fruit ripens the liquid dries, and the tubes now form a network of hollow threads, the "capillitium," often with external spiral ridges (Fig. 30, A, B). These are very hygroscopic, and by their expansion and contraction {92}determine the rupture of the fruit-wall and the scattering of the spores.
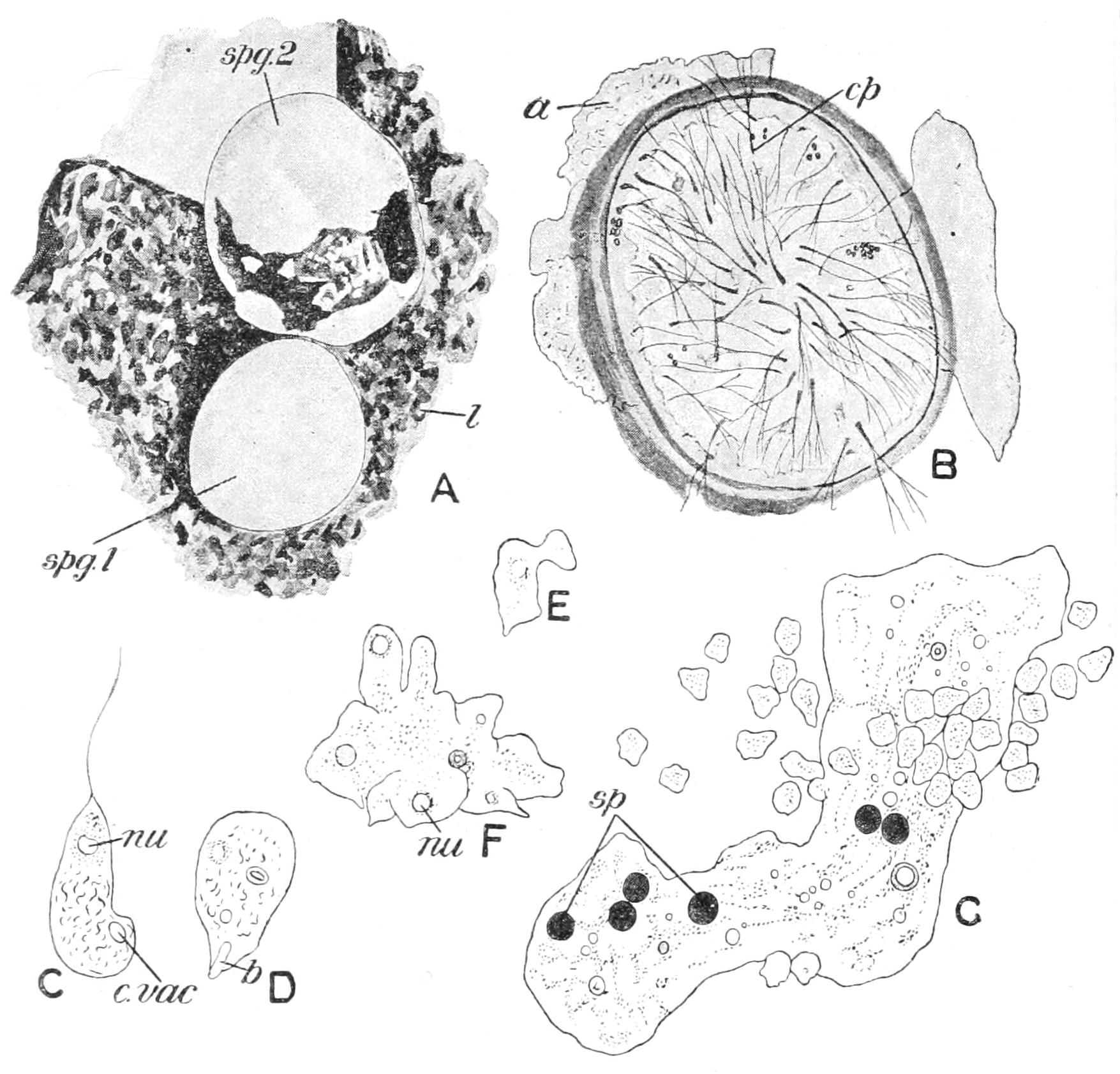
Fig. 30.—Didymium difforme. A, two sporangia (spg 1 and 2) on a fragment of leaf (l); B, section of sporangium, with ruptured outer layer (a), and threads of capillitium (cp); C, a flagellula with contractile vacuole (c.vac) and nucleus (nu); D, the same after loss of flagellum; b, an ingested bacillus; E, an amoebula; F, conjugation of amoebulae to form a small plasmodium; G, a larger plasmodium accompanied by numerous amoebulae; sp, ingested spores. (After Lister.)
Again, in some cases the plasmodia themselves aggregate in the same way as the amoeboids do in the Acrasieae, and combine to form a compound fruit termed an "aethalium,"[98] with the regions of the separate plasmodia more or less clearly marked off. The species formerly termed Aethalium septicum is now known as Fuligo varians. It is a large and conspicuous species, common on tan, and is a pest in the tanpits. Its aethalia may reach a {93}diameter of a foot and more, and a thickness of two inches. Chondrioderma diffusum, often utilised as a convenient "laboratory type," is common on the decaying haulms of beans in the late autumn. The interest of this group is entirely biological, save for the "flowers of tan."[99]
PROTOZOA (CONTINUED): SPOROZOA[100]
II. Sporozoa.
Protozoa parasitic in Metazoa, usually intracellular for at least part of their cycle, rarely possessing pseudopodia, or flagella (save in the sperms), never cilia; reproduction by brood-formation, often of alternating types; syngamy leading up to resting spores in which minute sickle-germs are formed, or unknown (Myxosporidiaceae).
This group, of which seven years ago no single species was known in its complete cycle, has recently become the subject of concentrated and successful study, owing to the fact that it has been recognised to contain the organisms which induce such scourges to animals as malarial fevers, and various destructive murrains. Our earliest accurate, if partial knowledge, was due to von Siebold, Kölliker, and van Beneden. Thirty years ago Ray Lankester in England commenced the study of species that dwell in the blood, destined to be of such moment for the well-being of man and the animals in his service; and since then our knowledge has increased by the labours of Manson, Ross and Minchin at home, Laveran, Blanchard, Thélohan, Léger, Cuénot, Mesnil, Aimé Schneider in France, Grassi in Italy, Schaudinn, Siedlecki, L. and R. Pfeiffer, Doflein in Central Europe, and many others.

Fig. 31.—Lankesteria ascidiae, showing life-cycle. a, b, c, Sporozoites in digestive epithelium cells of host; d, e, growth stages; f, free gregarine; g, association; h, encystment; i, j, brood-divisions in associated mates; k, pairing-cells; l, syngamy; m, zygote; n, o, p, nuclear divisions in spores; q, cyst with adult spores, each containing 8 sickle-germs. (After Luhe, modified from Siedlecki.)
As a type we will take a simple form of the highest group, the Gregarinidaceae, Monocystis, which inhabits the seminal vesicles of the earthworm. In its youngest state, the "sporozoite," it is a naked, sickle-shaped cell, which probably makes its way from the gut into one of the large radial cells of the seminal funnel, where it attains its full size, and then passes out into the vesicles or reservoirs of the semen, to lie among the sperm morulae and young spermatozoa. The whole interior is formed of the opaque endosarc, which contains a large central nucleus, and is full of refractive granules of paramylum or paraglycogen,[101] a carbohydrate allied to glycogen or animal starch, so common in the liver and {96}muscles of Metazoa; besides these it contains proteid granules which stain with carmine, and oil-drops. The ectosarc is formed of three layers: (1) the outer layer or "cuticle"[102] is, in many cases if not here, ribbed, with minute pores in the furrows, and is always porous enough to allow the diffusion of dissolved nutriment; (2) a clear plasmatic layer, the "sarcocyte"; (3) the "myocyte," formed of "myonemes," muscular fibrils disposed in a network with transverse meshes, which effect the wriggling movements of the cell. The endosarc contains the granules and the large central nucleus. The adult becomes free in the seminal vesicles; here two approximate, and surround themselves with a common cyst: a process which has received the name of "association" (Fig. 31, g-i). Within this, however, the protoplasms remain absolutely distinct. The nucleus undergoes peculiar changes by which its volume is considerably reduced. When this process of "nuclear reduction" is completed, each of the mates undergoes brood-divisions (j), so as to give rise to a large number of rounded naked 1-nucleate cells—the true pairing-cells. These unite two and two, and so form the 1-nucleate spores (k-m), which become oat-shaped, form a dense cyst-wall, and have been termed "pseudonavicellae" from their likeness to the Diatomaceous genus Navicella. Some of the cytoplasm of the original cells remains over unused, as "epiplasm," and ultimately degenerates, as do a certain number of the brood-cells which presumably have failed to pair. It is believed that the brood-cells from the same parent will not unite together. The contents of each spore have again undergone brood-division to form eight sickle-shaped zoospores, or "sporozoites" (n-q), and thus the developmental cycle is completed. Probably the spores, swallowed by birds, pass out in their excrement, and when eaten by an earthworm open in its gut; the freed sickle-germs can now migrate through the tissues to the seminal funnels, in the cells of which they grow, ultimately becoming free in the seminal vesicles.[103]
We may now pass to the classification of the group.
| A. Telosporidia.—Cells 1-nucleate until the onset of brood-formation, which is simultaneous. | |
| 1. Gregarinidaceae.—Cells early provided with a firm pellicle and possessing a complex ectosarc; at first intracellular, soon becoming free in the gut or coelom of Invertebrates. Pairing between adults, which simultaneously produce each its brood of gametes, isogamous or bisexual, which pair within the common cyst; zygotospores surrounded by a firm cyst, and producing within a brood of sickle-shaped zoospores. | |
| (i.) Schizogregarinidae.—Multiplying by simple fission in the free state as well as by brood-formation; the brood-cells conjugating in a common cyst, but producing only one pairing nucleus in each mate (the rest aborting), and consequently only one spore. | |
| Ophryocystis A. Schn. | |
| (ii.) Acephalinidae.—Cell one-chambered, usually without an epimerite for attachment. | |
| Monocystis F. Stein; Lankesteria Mingazzini. | |
| (iii.) Dicystidae.—Cell divided by a plasmic partition; epimerite usually present. | |
| Gregarina Dufour; Stylorhynchus A. Schn.; Pterocephalus A. Schn. | |
| 2. Coccidiaceae.—Cells of simple structure, intracellular in Metazoa. Pairing between isolated cells usually sexually differentiated as oosphere and sperm, the latter often flagellate. Brood-formation of the adult cell giving rise to sickle-shaped zoospores (merozoites), or progamic and producing the gametes. Oosperm motile or motionless, finally producing a brood of spores, which again give rise to a brood of sickle-spores. | |
| (i.) Coccidiidae.—Cell permanently intracellular, or very rarely coelomic, encysting or not before division; zoospores always sickle-shaped; oosperm encysting at once, producing spores with a dense cell-wall producing sickle-germs. | |
| (ii.) Haemosporidae.—Cells parasitic in the blood corpuscles or free in the blood of cold-blooded animals, encysting before brood-formation; zoospores sickle-shaped; oosperm at first motile. | |
| Lankesterella Labbé; (Drepanidium Lank.;) Karyolysus Labbé; Haemogregarina Danilewski. | |
| (iii.) Acystosporidae.—Cells parasitic in the blood and haematocytes of warm-blooded Vertebrates; never forming a cyst-wall before dividing; zoospores formed in the corpuscles, amoeboid. Gametocytes only forming gametes when taken into the stomach of insects. Oosperm at first active, passing into the coelom, producing naked spores which again produce a large brood of sickle zoospores, which migrate to the salivary gland, and are injected with the saliva into the warm-blooded host. | |
| Haemamoeba Grassi and Feletti; Laverania Grassi and Feletti; Haemoproteus Kruse; Halteridium Labbé.[104] | |
| B. Neosporidia.—Cells becoming multinucleate apocytes before any brood-formation occurs. Brood-formation progressive through the apocyte, not simultaneous. | |
| {98}
1. Myxosporidiaceae.—Naked parasites in cold-blooded animals. Spore-formation due to an aggregation of cytoplasm around a single nucleus to form an archespore, which then produces a complex of cells within which two daughter-cells form the spores and accessory nematocysts. |
|
| Myxidium Bütsch.; Myxobolus Bütsch.; Henneguya Thélohan; Nosema Nageli (= Glugea Th.). | |
| 2. Actinomyxidiaceae.[105]—Apocyte resolved into a sporange, containing eight secondary sporanges (so-called spores), of ternary symmetry and provided with three polar nematocysts. | |
| 3. Sarcosporidiaceae.—Encysted parasites in the muscles of Vertebrates, with a double membrane; spores simple. | |
| Sarcocystis Lankester. | |

Fig. 32.—Gregarina blattarum Sieb. A, two cephalonts, embedded by their epimerite (ep), in cells of the gut-epithelium; deu, deutomerite; nu, nucleus; pr, protomerite; B1, B2, two free specimens of an allied genus; the epimerite is falling off in B2, which is on its way to become a sporont; C, cyst (cy) of A, with sporoducts (spd) discharging the spores (sp), surrounded by an external gelatinous investment (g). (From Parker and Haswell.)
Monocystis offers us the simplest type of Gregarinidaceae. In most Gregarines (Figs. 31, 32) the sporozoite enters the epithelium-cell of the gut of an Arthropod, Worm or Mollusc, and as it enlarges protrudes the greater part of its bulk into the lumen, and may become free therein, or pass into the coelom. The attached part is often enlarged into a sort of grapple armed with spines, the "epimerite"; this contains only sarcocyte, the other layers being absent. The freely projecting body is usually divided by an ingrowth of the myocyte into a front segment ("protomerite"), and a rear one ("deutomerite"), with the nucleus usually in the latter. In this state the cell is termed a "cephalont." Conjugation is frequent, but apparently is not always connected with {99}syngamy or spore-formation; sometimes from two to five may be aggregated into a chain or "syzygy." The number of cases in which a syngamic process between two cells has been observed is constantly being increased. In Stylorhynchus (Fig. 33) the conjugation at first resembles that of Monocystis, but the actual pairing-cells are bisexually differentiated into sperms in the one parent, and oospheres in the other; it is remarkable that here the pear-shaped sperms are apparently larger than the oospheres. In Pterocephalus the chief difference is that the sperms are minute.[106] In all cases of spore-formation the epimerite is lost and the septum disappears; in this state the cell is termed a sporont. Sometimes the epiplasm of the sporont forms tubes ("sporoducts"), which project through the cyst-wall and give exit to the spores, as in Gregarina (Fig. 32, C), a parasite in the beetle Blaps.
Gregarines infest most groups of Invertebrates except Sponges and perhaps Coelenterates, the only exception cited being that of Epizoanthus glacialis, a Zoantharian (p. 406). They appear to be relatively harmless and are not known to induce epidemics.
The Coccidiaceae never attain so high a degree of cellular differentiation as the Gregarines, which may be due to their habitat; for in the growing state they are intracellular parasites. Their life-history shows a double cycle, which has been most thoroughly worked out in Coccidiidae by Schaudinn and Siedlecki in parasites of our common Centipedes. We take that of Coccidium schubergi (in Lithobius forficatus[107]), beginning with the sporozoite, which is liberated from the spores taken in with the food, in the gut of the Centipede. This active sickle-shaped cell (Fig. 34, l) enters an epithelial cell of the mid-gut, and grows therein till it attains its full size (a), when it is termed a "schizont"; for it segments (Gk. σχίζω, "I split") superficially into a large number of sickle-shaped zoospores, the "merozoites" (c), resembling the sporozoites. The segmentation is superficial, so that there may remain a large mass of residual epiplasm. The merozoites are set free by the destruction of the epithelium-cell in which they were formed, and which becomes disorganised, like the residual epiplasm. Each merozoite may repeat the {100}behaviour of the sporozoite, so that the disease spreads freely, and becomes acute after several reinfections. After a time the adult parasites, instead of becoming schizonts and simply forming merozoites by division, differentiate into cells that undergo a binary sexual differentiation. Some cells, the "oocytes" (d, e), escape into the gut, and the nucleus undergoes changes by which some of its substance (or an abortive daughter-nucleus) is expelled to the exterior (f), such a cell is now an "oogamete" or oosphere. Others, again, are spermatogones (h): each when full grown on escaping into the gut commences a division (i, j), like that of the schizonts. The products of this division or segment-cells are the flagellate sperms (s): they are more numerous and more minute than the merozoites produced by the schizonts, and are attracted to the oosphere by chemiotaxy (p. 23), and one enters it and fuses with it (g). The oosperm, zygote or fertilised egg, thus formed invests itself with a dense cyst-wall, as a "oospore" (k), its contents form one or more (2, 4, 8, etc.) spores; and each spore forms again one, two, or four sickle-shaped zoospores ("sporozoites"), destined to be liberated for a fresh cycle of parasitic life when the spores are swallowed by another host.
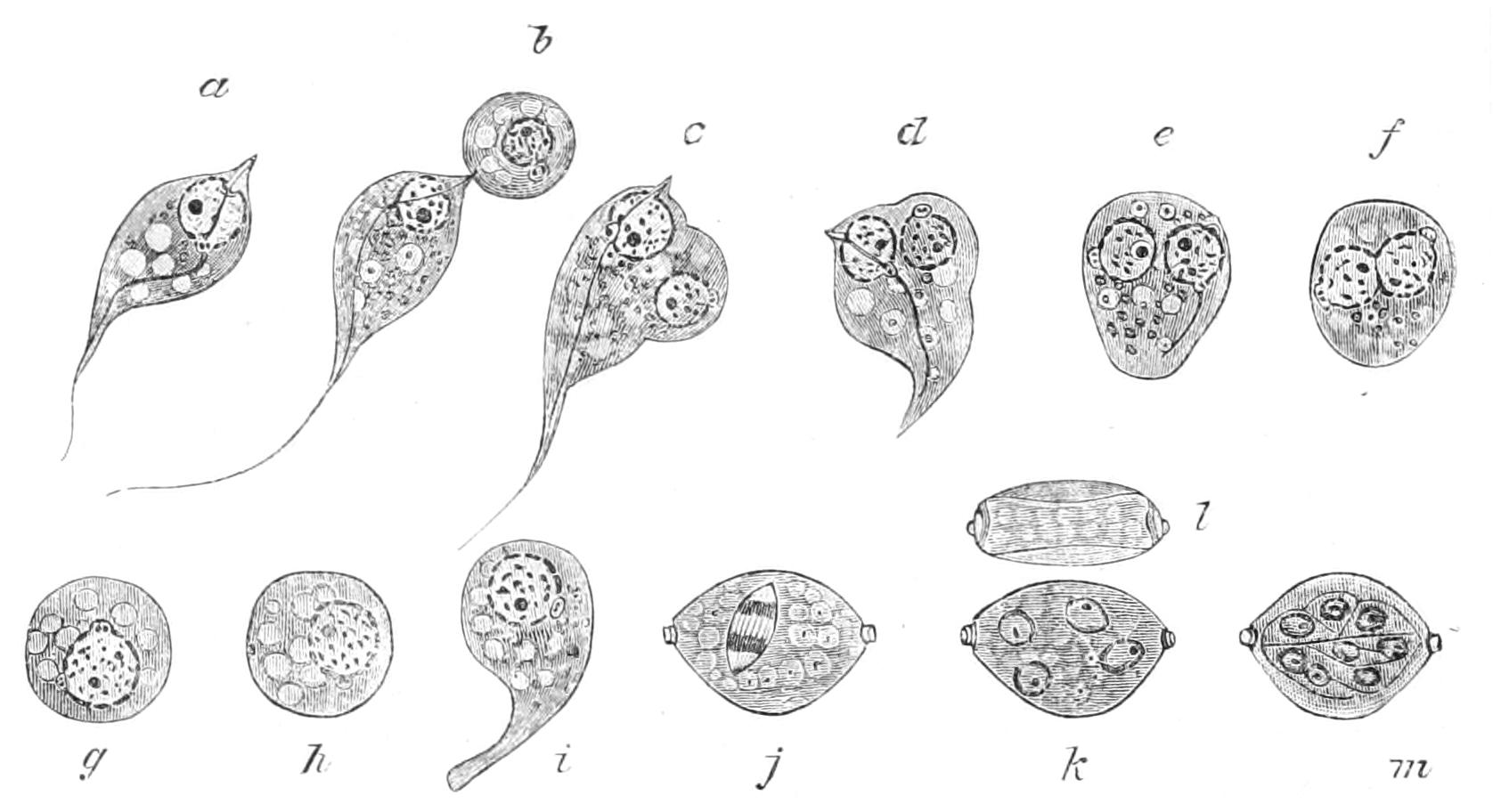
Fig. 33.—Bisexual pairing of Stylorhynchus. a, Spermatozoon; b-e, fusion of cytoplasm of spermatozoon and oosphere; f, g, fusion of nuclei; h-j, development of wall to zygote; k, l, formation of four sporoblasts; l, side view of spore; m, mature sporozoites in spore. (After Léger.)

Fig. 34.—Life-history of Coccidium schubergi. a, Penetration of epithelium-cell of host by sporozoite; b-d, stages of multiple cell-formation in naked state (schizogony); e, f, formation of oogamete; g, conjugation; h-j, formation of sperms (s); k, development of zygote (fertilised ovum) to form four spores; l, formation of two zoospores (or sickle germs) in each spore. (From Calkins's Protozoa, after Schaudinn.)
In some cases the oogametes are at first oblong, like ordinary merozoites, and round off in the gut. The microgametocyte, or spermatogone, has the same character, but is smaller; it applies itself like a cap to one pole of the oogamete, which has rounded off; it then divides into four sperms, whose cytoplasm is not sharply separated; one of these then separates from the common mass, enters the oogamete, and so conjugation is effected, with an oosperm as its result. This latter mode of conjugation is that of Adelea ovata and Coccidium lacazei: the former is probably the more primitive and the commoner. The sperms {102}of Coccidiidae, when free, usually possess two long flagella, either both anterior, or a very long one in front and a short one behind, both turned backwards.
The genus Coccidium affects many animals, and one species in particular, C. cuniculi Rivolta, attacks the liver of young rabbits,[108] giving rise to the disease "coccidiosis." Coccidium may also produce a sort of dysentery in cattle on the Alpine pastures of Switzerland; and cases of human coccidiosis are by no means unknown. Coccidium-like bodies have been demonstrated in the human disease, "molluscum contagiosum," and the "oriental sore" of Asia; similar bodies have also been recorded in smallpox and vaccinia, malignant tumours and even syphilis, but their nature is not certainly known; some of these are now referred to Flagellata (see p. 121).
Closely allied to the Coccidiidae are the Haemosporidae, dwellers in the blood of various cold-blooded Vertebrates,[109] and entering the corpuscles as sporozoites or merozoites to attain the full size, when they divide by schizogony; they are freed like those of the next family by the breaking up of the corpuscle. The merozoites were described by Gaule (1879) as "vermicles" ("Würmchen"), and regarded by him as peculiar segregation-products of the blood; though Lankester had described the same species in the Frog's blood as early as 1871, with a full recognition of its true character. His name, Drepanidium, has had to give way, having been appropriated to another animal, and has been aptly replaced by that of Lankesterella. The sexual process of Karyolysus has been found to take place in a Tick, that of Haemogregarina in a Leech, thus presenting a close analogy to the next group, which only differs in its less definite form in the active state, and in the lack of a cell-wall during brood-formation.
Laveran was the first to describe a member of the {103}Acystosporidae, in 1880, as an organism always to be found in the blood of patients suffering from malarial fever; this received the rather inappropriate name of Plasmodium, which, by a pedantic adherence to the laws of priority, has been used by systematists as a generic name. Golgi demonstrated the coincidence of the stages of the intermittent fever with those of the life-cycle of the parasite in the patient, the maturation of the schizont and liberation of the sporozoites coinciding with the fits of fever. Manson, who had already shown that the Nematodes of the blood that give rise to Filarial haematuria (see Vol. II. p. 149) have an alternating life in the gnats or mosquitos of the common genus Culex,[110] in 1896 suggested to Ronald Ross that the same might apply to this parasite, and thus inspired a most successful work. The hypothesis had old prejudices in its favour, for in many parts there was a current belief that sleeping under mosquito-netting at least helped other precautions against malaria. Ross found early in his investigations that Culex was a good host for the allied genus Haemoproteus or Proteosoma, parasitic in birds, but could neither inoculate man with fever nor be inoculated from man. He found, however, that the malaria germs from man underwent further changes in the stomach of a "dappled-wing mosquito," that is, as we have since learned, a member of the genus Anopheles. Thenceforward the study advanced rapidly, and a number of inquirers, including Grassi, Koch, MacCallum (who discovered the true method of sexual union in Halteridium[111]), and Ross himself, completed his discovery by supplying a complete picture of the life-cycles of the malaria-germs. Unfortunately, there has been a most unhappy rivalry as to the priority of the share in each fragment of the discovery, whose history is summarised by Nuttall, we believe, with perfect fairness.[112]
The merozoite is always amoeboid, and in this state enters the blood corpuscle; herein it attains its full size, as a schizont, becoming filled with granules of "melanin" or black pigment, probably a decomposition product of the red colouring matter (haemoglobin).
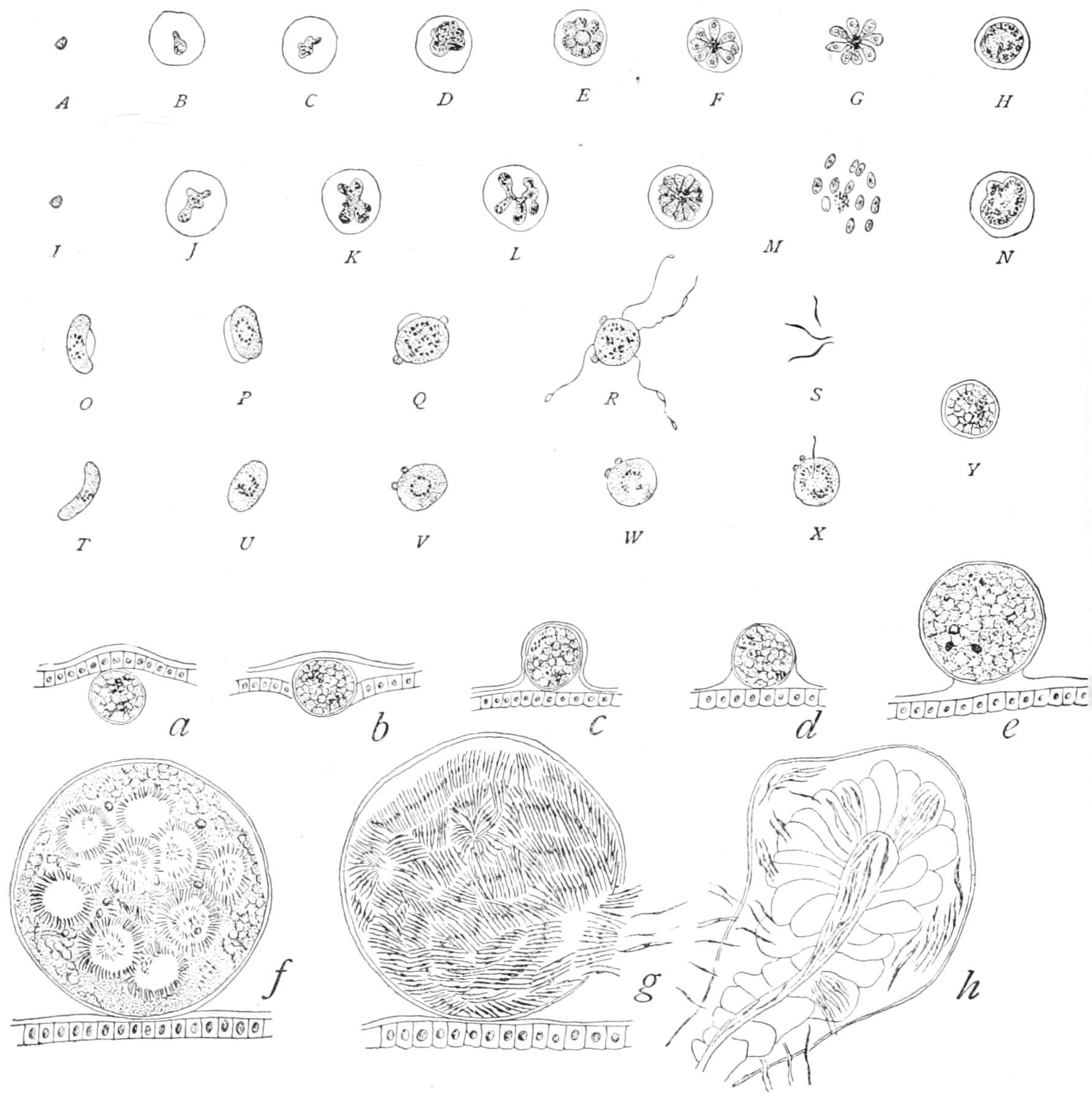
Fig. 35.—Life-history of Malarial Parasites. A-G, Amoebula of quartan parasite to sporulation; H, its gametocyte; I-M, amoebula of tertian parasite to sporulation; N, its gametocyte; O, T, "crescents" or gametocytes of Laverania; P-S, sperm-formation; U-W, maturation of oosphere; X, fertilisation; Y, zygote. a, Zygote enlarging in gut of Mosquito; b-e, passing into the coelom; f, the contents segmented into naked spores; g, the spores forming sickle-germs or sporozoites; h, sporozoites passing into the salivary glands. (From Calkins's Protozoa, after Ross and Fielding Ould.)
The nucleus of the schizont now divides repeatedly, and then the schizont segments into a flat brood of germs (merozoites), relatively few in the parasite of quartan fever (Haemamoeba malariae, Fig. 35, E-G), many in that of tertian (H. vivax, Fig. 35, M). These brood-cells escape and behave for the most part as before. But after the disease has persisted for some time we find that in the genus Haemamoeba, {105}which induces the common malarial fevers of temperate regions, certain of the full-grown germs, instead of behaving as schizonts, pass, as it were, to rest as round cells; while in the allied genus Laverania, (Haemomenas, Ross) these resting-cells are crescentic, with blunt horns, and are usually termed half-moons (Fig. 35, O, T), characteristic of the bilious or pernicious remittent fevers of the tropics and of the warmer temperate regions in summer. These round or crescent-shaped cells are the gametocytes, which only develop further in the drawn blood, whether under the microscope, protected against evaporation, or in the stomach of the Anopheles: the crescents become round, and then they, like the already round ones of Haemamoeba, differentiate in exactly the same way as the corresponding cells of Coccidium schubergi. The female cell only exhibits certain changes in its nucleus to convert it into an oosphere: the male emits a small number of sperms, long flagellum-like bodies, each with a nucleus; and these, by their wriggling, detach themselves from the central core, no longer nucleated. The male gametogonium with its protruded sperms was termed the "Polymitus form," and was by some regarded as a degeneration-form, until MacCallum discovered that a "flagellum" regularly undergoes sexual fusion with an oosphere in Halteridium, as has since been found in the other genera. The oosperm (Y) so formed is at first motile ("ookinete"), as it is in Haemosporidae, and passes into the epithelium of the stomach of the gnat and then through the wall, acquiring a cyst-wall and finally projecting into the coelom (a-e). Here it segments into a number of spheres ("zygotomeres" of Ross) corresponding to the Coccidian spores, but which never acquire a proper wall (f). These by segmentation produce at their surface an immense quantity of elongated sporozoites (the "zygotoblasts" or "blasts" of Ross, Fig. 35, g), these are ultimately freed by the disappearance of the cyst-wall of the oosperm, pass through the coelom into the salivary gland (h), and are discharged with its secretion into the wound that the gnat inflicts in biting. In the blood the blasts follow the ordinary development of merozoites in the blood corpuscle, and the patient shows the corresponding signs of fever. This has been completely proved by rearing the insect from the egg, feeding it on the blood of a patient in whose blood there were ascertained to be the germs of a definite species of {106}Haemamoeba, sending it to England, where it was made to bite Dr. Manson's son, who had never had fever and whose blood on repeated examination had proved free from any germs. In the usual time he had a well-defined attack of the fever corresponding to that germ, and his blood on examination revealed the Haemamoeba of the proper type. A few doses of quinine relieved him of the consequences of his mild martyrdom to science. Experiments of similar character but of less rigorous nature had been previously made in Italy with analogous results. Again, it has been shown that by mere precautions against the bites of Anopheles, and these only, all residents who adopted them during the malarious season in the most unhealthy districts of Italy escaped fever during a whole season; while those who did not adopt the precautions were badly attacked.[113]
Anopheles flourishes in shallow puddles, or small vessels such as tins, etc., the pools left by dried-up brooks and torrents, as well as larger masses of stagnant water, canals, and slow-flowing streams. Sticklebacks and minnows feed freely on the larvae and keep down the numbers of the species; where the fish are not found, the larvae may be destroyed by pouring paraffin oil on the surface of the water and by drainage. A combination of protective measures in Freetown (Sierra Leone) and other ports on the west coast of Africa, Ismailia, and elsewhere, has met with remarkable success during the short time for which it has been tried; and it seems not improbable, that as the relatively benign intermittent fevers have within the last century been banished from our own fen and marsh districts, so the Guinea coast may within the next decade lose its sad title of "The White Man's Grave."
So closely allied to this group in form, habit, and life-cycle are some species of the Flagellate genus Trypanosoma, that in their less active states they have been unhesitatingly placed here (see p. 119). Schaudinn has seen Trypanosomic characters in the "blasts" of this group, which apparently is the most primitive of the Sporozoa and a direct offshoot of the Flagellates.
The Myxosporidiaceae (Fig. 36) are parasitic in various {107}cold-blooded animals. They are at least binucleate in the youngest free state, and become large and multinucleate apocytes, which may bud off outgrowths as well as reproduce by spores. The spores of the apocyte are not produced by simultaneous breaking up, but by successive differentiation. A single nucleus aggregates around itself a limited portion of the cytoplasm, and this again forms a membrane, becoming an archespore or a "pansporoblast," destined to produce two spores; within this, nuclear division takes place so as to form about eight nuclei, two of which are extruded as abortive, and of the other six, three are used up in the formation of each of the two spores. Of these three nuclei in each spore, two form nematocysts, like those of a Coelenterate (p. 246 f.), at the expense of the surrounding plasm; while the third nucleus divides to form the two final nuclei of the reproductive body. The whole aggregate of the reproductive body and the two nematocysts is enveloped in a bivalve shell. In what we may call germination, the nematocysts eject a thread that serves for attachment, the valves of the shell open, and the binucleate mass crawls out and grows afresh. Nosema bombycis Nägeli (the spore of which has a single nematocyst) is the organism of the "Pébrine" of the silkworm, which was estimated to have caused a total loss in France of some £40,000,000 before Pasteur investigated the malady and prescribed the effectual cure, or rather precaution against its spread. This consisted in crushing each mother in water after it had laid its eggs and seeking for pébrine germs. If the mother proved to be infected, her eggs were destroyed, as the eggs she had laid were certain to be also tainted. Balbiani completed the study of the organism from a morphological standpoint. Some Myxosporidiaceae produce destructive epidemics in fish.
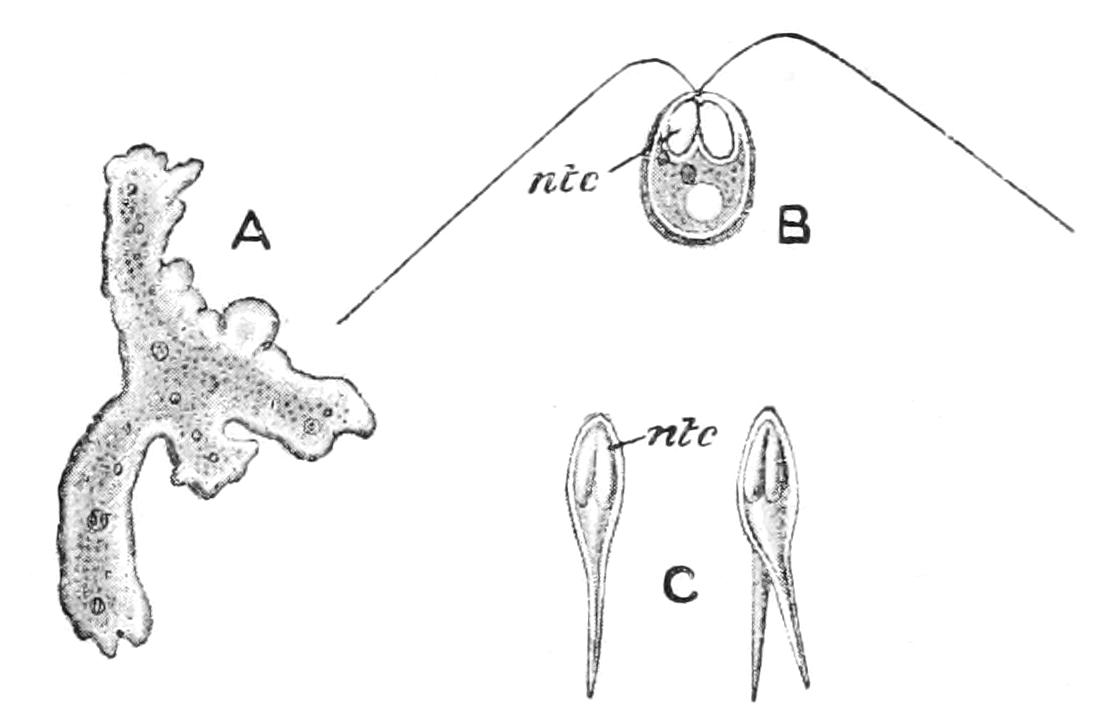
Fig. 36.—A, Myxidium lieberkühnii, amoeboid phase; B, Myxobolus mülleri, spore with discharged nematocysts (ntc); C, spores (psorosperms) of a Myxosporidian. ntc, nematocysts. (From Parker and Haswell.)
The Dolichosporidia or Sarcosporidiaceae are, in the adult state, elongated sacs, often found in the substance of the voluntary muscles, and known as "Rainey's" or "Miescher's Tubes"; they are at first uninucleate, then multinucleate, and then break up successively into uninucleate cells, the spores, in each of which, by division, are formed the sickle-shaped zoospores.[114]
PROTOZOA (CONTINUED): FLAGELLATA
III. Flagellata.
Protozoa moving (and feeding in holozoic forms) by long flagella: pseudopodia when developed usually transitory: nucleus single or if multiple not biform: reproduction occurring in the active state and usually by longitudinal fission, sometimes alternating with brood-formation in the cyst or more rarely in the active state: form usually definite: a firm pellicle or distinct cell-wall often present.
The Flagellates thus defined correspond to Bütschli's group of the Mastigophora. The lowest and simplest forms, often loosely called "Monads," are only distinguishable from Sarcodina (especially Proteomyxa) and Sporozoa by the above characters: their artificial nature is obvious when we remember that many of the Sarcodina have a flagellate stage, and that the sperms of bisexual Sporozoa are flagellate (as are indeed those of all Metazoa except Nematodes and most Crustacea). Even as thus limited the group is of enormous extent, and passes into the Chytridieae and Phycomycetes Zoosporeae on the one hand, and by its holophytic colonial members into the Algae, on the other.[115]
Classification.
| A. Fission usually longitudinal (transverse only in a cyst), or if multiple, radial and complete: pellicle absent, thin, or if armour-like, with not more than two valves. | |
| I. Food taken in at any part of the body by pseudopodia | 1. PANTOSTOMATA |
| Multicilia Cienk.; Mastigamoeba F. E. Sch. (Fig. 37, 4). | |
| {110}
II. Food taken in at a definite point or points, or by absorption, or nutrition holophytic. |
|
| 1. No reticulate siliceous shell. Diameter under 500 µ (1⁄50"). | |
| * Contractile vacuole simple (one or more). | |
| (α) Colourless: reserves usually fat: holozoic, saprophytic or parasitic | 2. Protomastigaceae |
| (β) Plastids yellow or brown: reserves fat or proteid: nutrition variable: body naked, often amoeboid in active state (C. nudae), or with a test, sometimes containing calcareous discs ("coccoliths," "rhabdoliths") of peculiar form (C. loricatae) | 3. Chrysomonadaceae |
| Chromulina Cienk.; Chrysamoeba Klebs; Hydrurus Ag. Dinobryon Ehrb. (Fig. 37, 11); Syncrypta Ehrb. (Fig. 37, 12); Zooxanthella Brandt; Pontosphaera Lohm.; Coccolithophora Lohm.; Rhabdosphaera Haeck. | |
| (γ) Green, (more rarely yellow or brown) or colourless: reserves starch: fission longitudinal | 4. Cryptomonadaceae |
| Cryptomonas Ehrb. (Fig. 37, 9); Paramoeba Greeff. | |
| (δ) Green (rarely colourless): fission multiple, radial | 5. Volvocaceae |
| ** System of contractile vacuoles complex, with accessory formative vacuoles or reservoir, or both. | |
| (ε) Pellicle delicate or absent: pseudopodia often emitted: excretory pore distinct from flagellar pit: reserves fat | 6. Chloromonadaceae |
| Chloramoeba Lagerheim; Thaumatomastix, Lauterborn. | |
| (ζ) Pellicle dense, tough or hard, often wrinkled or striate: contractile vacuole discharging by the flagellar pit. Nutrition variable | 7. Euglenaceae |
| Euglena Ehrb.; Astasia Duj. (Fig. 37, 3); Anisonema Duj.; Eutreptia Perty (Fig. 42, p. 124); Trachelomonas Ehrb. (Fig. 37, 1); Cryptoglena Ehrb. | |
| 2. Skeleton an open network of hollow siliceous spicules. Plastids yellow. Diameter under 500 µ. | 8. Silicoflagellata |
| Dictyocha Ehrb. | |
| 3. Diameter over 500 µ. Mouth opening into a large reticulate endoplasm: flagella 1, or 2, very unequal. | 9. Cystoflagellata |
| Noctiluca Suriray (Fig. 48); Leptodiscus R. Hertw. | |
| B. Fission oblique or transverse: flagella two, dissimilar, the one coiled round the base of the other or in a traverse groove; pellicle often dense, of numerous armour-like plates | 10. Dinoflagellata |
| Ceratium Schrank; Gymnodinium Stein; Peridinium Ehrb. (Fig. 46); Pouchetia Schütt; Pyrocystis Murray (Fig. 47); Polykrikos Bütschli. | |
The Protomastigaceae and Volvocaceae are so extensive as to require further subdivision.
Protomastigaceae
| I. Oral spots 2. Flagella distant in pairs. | Distomatidae |
| II. Oral spot 1 or 0. | |
| {111}
A. Flagellum 1. |
|
| (a) No anterior process: often parasitic | Oikomonadidae |
| Oikomonas K. (Figs. 37, 2, 8); Trypanosoma Gruby (Fig. 39, a-f); Treponema Vuill. (Fig. 39, g-i). | |
| (b) Anterior process unilateral or proboscidiform: cell often thecate | Bicoecidae |
| Bicoeca Clark; Poteriodendron St. | |
| (c) Anterior process a funnel, surrounding the base of the flagellum: cells often thecate. | |
| (i.) Funnel free | Craspedomonadidae |
| Codosiga Clark; Monosiga Cl.; Polyoeca Kent; Proterospongia Kent; Salpingoeca Cl. | |
| (ii.) Funnel not emerging from the general gelatinous investment | Phalansteridae |
| B. Flagella 2, unequal or dissimilar in function, the one sometimes short and thick. | |
| (a) Both flagella directed forwards | Monadidae |
| Monas St.; Anthophysa Bory (Fig. 37, 13). | |
| (b) One flagellum, usually the longer, turned backwards | Bodonidae |
| Bodo St. (Fig. 38). | |
| C. Flagella 2, equal and similar | Amphimonadidae |
| Amphimonas Duj.; Diplomita K. (Fig. 37, 10); Rhipidodendron St. (Fig. 37, 14). | |
| D. Flagella 3 | Trimastigidae |
| Dallingeria K. (Fig. 37, 6); Costia Leclercq. | |
| E. Flagella 4 or more: mostly parasitic in Metazoa | Polymastigidae |
| Trichomonas Donne; Tetramitus Perty (Fig. 37, 7); Hexamitus Duj.; Lamblia Blanchard. | |
| F. Flagella numerous, sometimes constituting a complete ciliiform investment, and occasionally accompanied by an undulating membrane: parasitic in Metazoa. | |
| (a) Flagella long: nucleus single: parasitic in insects | Trichonymphidae |
| Dinenympha Leidy; Joenia Grassi; Pyrsonympha Leidy; Trichonympha Leidy; Lophomonas St.; Maupasia Schew. | |
| (b) Flagella short, ciliiform, uniformly distributed: nuclei very numerous, all similar: parasitic in Amphibia | Opalinidae |
| Opalina Purkinje and Valentin (Fig. 41). | |
Volvocaceae
| A. Cells usually isolated, separating after fission or brood-formation. Usually green (sometimes red), more rarely colourless saprophytes | Chlamydomonadidae |
| Chlamydomonas Ehrb.; Phacotus Perty; Polytoma Ehrb.; Sphaerella Sommerf. (Fig. 43); Zoochlorella. | |
| B. Cells multiplying in the active state by radial divisions in the same plane and usually incurving to form a spherical colony, united in a gelatinous investment, sometimes traversed by plasmic threads | Volvocidae |
| Gonium O.F.M.; Eudorina Ehrb.; Pandorina Bory (Fig. 45); Stephanosphaera Cohn; Volvox L. (Fig. 44). | |
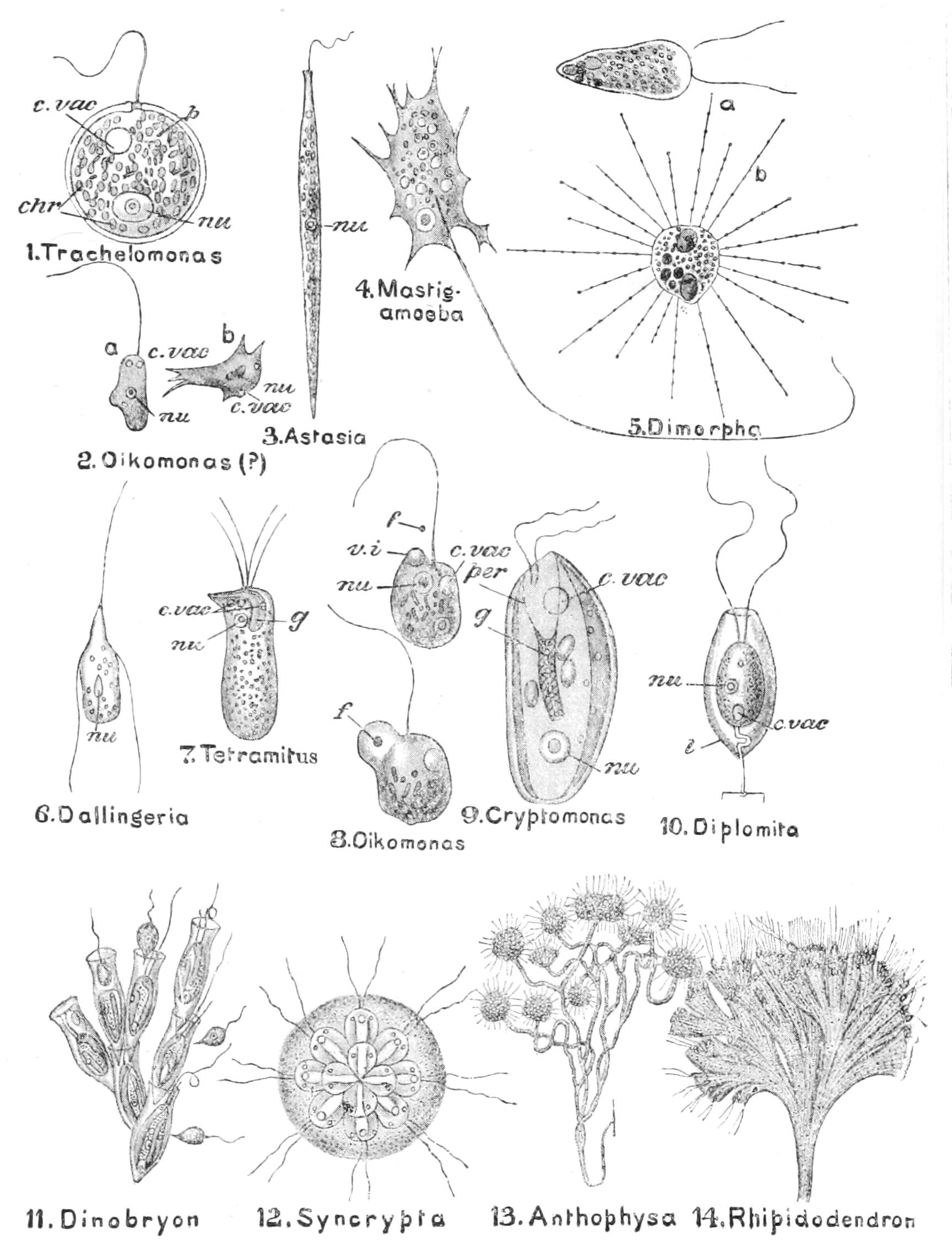
Fig. 37.—Various forms of Flagellata. 2, 6-8, 10, 13, 14, Protomastigaceae; 11, 12, Chrysomonadaceae; 9, Cryptomonadaceae; 1, 3, Euglenaceae; 4, Pantostomata: note branched stalk in 13; branched tubular theca in 14; distinct thecae in 11; stalk and theca in 10. In 2, flagellate (a) and amoeboid (b) phases are shown; in 5, flagellate (a) and Heliozoan (b) phases[116]; in 8 are shown two stages in the ingestion of a food particle (f); chr, plastoids; c.vac, contractile vacuole; f, food particle; g, gullet; l, theca; nu, nucleus; p, protoplasm; per, peristome; v.i, vacuole of ingestion. (From Parker and Haswell, mostly from Bütschli's Protozoa.)
The modes of nutrition are threefold: the simplest forms live in liquids containing decaying organic matter which they absorb through their surface ("saprophytic"): others take in food either Amoeba fashion, or into a vacuole formed for the purpose, or into a definite mouth ("holozoic"): others again have coloured plastids, green or brown or yellow ("holophytic"), having the plant's faculty of manufacturing their own food-supply. But we meet with species that show chromatophores at one time and lack them at another; or, again, the same individual (Euglena) may pass from holozoic life to saprophytic (Paramoeba, some Dinoflagellates) as conditions alter.
Many secrete a stalk at the hinder end: by "continuous" formation of this, without rupture at fission, a branching colony is formed (Polyoeca). This stalk may have a varying consistency. In Anthophysa (Fig. 37, 13) it appears to be due to the welding of excrementitious particles voided at the hinder end of the body with a gelatinous excretion; but the division of the stalk is here occasional or intermittent, so that the cells are found in tufts at the apex of the branches. A corresponding secretion, gelatinous or chitinous, around the body of the cell forms a cup or "theca," within which the cell lies quite free or sticking to it by its surface, or attached to it by a rigid or contractile thread. The theca, again, may assume the form of a mere gelatinous mass in which the cell-bodies may be completely plunged, so that only the flagella protrude, as in Volvocidae, Proterospongia (Fig. 75, p. 182), and Rhipidodendron (Fig. 37, 14). Often this jelly assumes the form of a fan (Phalansterium), the branching tubes of which it is composed lying for some way alongside, and ultimately diverging. In Hydrurus, the branching jelly assumes the form of a branching Confervoid.[117]
The cell-body may be bounded by an ill-defined plasmatic layer in Chrysomonadaceae and some Protomastigaceae,[118] or it may form a plasmatic membrane or "pellicle," sometimes very firm and tough, or striated as in Euglenaceae, or it may have a separate "cuticle" (in the holophytic species formed of cellulose), or even a bivalve or multivalve shell of distinct plates, hinged or overlapping (Cryptoglena, Phacotus, Dinoflagellates). The wall of the {114}Coccolithophoridae, a family of Chrysomonadaceae, is strengthened by embedded calcareous spicules ("coccoliths," "cyatholiths," "rhabdoliths"), which in the most complex forms (cyatholiths) are like a shirt-stud, traversed by a tube passing through the stem and opening at both ends. These organisms[119] constitute a large proportion of the plankton; the spicules isolated, or in their original state of aggregation ("coccospheres," "rhabdospheres"), enter largely into the composition of deep-sea calcareous oozes. They occur fossil from Cambrian times (Potsdam sandstone of Michigan and Canada), and are in some strata extremely abundant, 800,000 occurring to the mm. cube in an Eocene marl.
The Silicoflagellates have siliceous skeletons resembling that of many Radiolaria, to which they were referred until the living organism was described (see pp. 79, 86 f.).
The flagellum has been shown by Fischer to have one of two forms: either it is whip-like, the stick, alone visible in the fresh specimen, being seen when stained to be continued into a long lash, hitherto invisible; or the whole length is fringed with fine ciliiform lateral outgrowths. If single it is almost always protruded as a tugging organ ("tractellum");[120] the chief exceptions are the Craspedomonads, where it is posterior and acts as a scull ("pulsellum"), and some Dinoflagellates, where it is reversible in action or posterior. In addition to the anterior flagellum there may be one or more posterior ones, which trail behind as sense organs, or may anchor the cell by their tips. Dallingeria has two of these, and Bodo saltans a single anterior anchoring lash, by which they spring up and down against the organic débris among which they live, and disintegrate it. The numerous similar long flagella of the Trichonymphidae afford a transition in the genus Pyrsonympha to the short abundant cilia of Opalina, usually referred to the Ciliate Infusoria.
An undulating membrane occurs, sometimes passing into the flagellum in certain genera, all parasitic, such as Trypanosoma (incl. Herpetomonas), Trichomonas, Hexamitus, and Dinenympha.
In some cases the flagellum (or flagella) is inserted into a definite pit, which in allied forms is the mouth-opening. The contractile vacuole is present in the fresh-water forms, but not in all the marine ones, nor in the endoparasites. It may be single or surrounded by a ring of minute "formative" vacuoles or discharge into a permanently visible "reservoir." This again may discharge directly to the surface or through the pit or canal in which the flagellum takes origin (Euglena).
The "chromatophore" may be a single or double plate, or multiple.[121] In the peculiar form Paramoeba the chromatophore may degenerate and be reproduced anew. It often encloses rounded or polygonal granules of uncoloured plasma, very refractive, known as "pyrenoids." These, like the chromatophores, multiply by direct fission. The "reserves" may be (1) fat-globules; (2) granules of a possibly proteid substance termed "leucosin"; (3) a carbohydrate termed "paramylum," differing slightly from starch (see p. 95); (4) true starch, which is usually deposited in minute granules to form an investment for the pyrenoid when such is present.
A strongly staining granule is usually present in the plasma near the base of the flagellum. This we may term a "blepharoplast" or a "centrosome" in the wider sense.
Fission is usually longitudinal in the active state; a few exceptions are recorded. Encystment is not uncommon; and in the coloured forms the cyst-wall is of cellulose. Division in the cyst is usually multiple;[122] in the coloured forms, however, vegetative growth often alternates with division, giving rise to plant-like bodies. Polytoma and other Chlamydomonadidae multiply by "brood-formation" in the active state; the blepharoplast, as Dangeard suggests, persisting to continue the motion of the flagella of the parent, while the rest of the plasm divides to form the brood. Conjugation has been observed in many species. In some species of Chlamydomonas it takes place after one or both of the two {116}cells have come to rest, but in most cases it occurs between active cells. We find every transition between equal unions and differentiated sexual unions, as we shall see in discussing the Volvocaceae.[123] The "coupled-cell" differs in behaviour in the different groups, but almost always goes to rest and encysts at once, whatever it may do afterwards.
The life-history of many Flagellates has been successfully studied by various observers, and has shed a flood of light on many of the processes of living beings that were hitherto obscure. The first studies were carried through by the patient labours of Drysdale and Dallinger. A delicate mechanical stage enabled the observer to keep in the field of view a single Flagellate, and, when it divided into two, to follow up one of the products. A binocular eye-piece saved much fatigue, and enabled the observers to exchange places without losing sight of the special Flagellate under observation; for the one who came to relieve would put one eye to the instrument and recognise the individual Flagellate under view as he passed his hand round to the mechanism of the stage before the first watcher finally relinquished his place at the end of the spell of work. Spoon-feeding by Mrs. Dallinger enabled such shifts to be prolonged, the longest being one of nine hours by Dr. Dallinger.
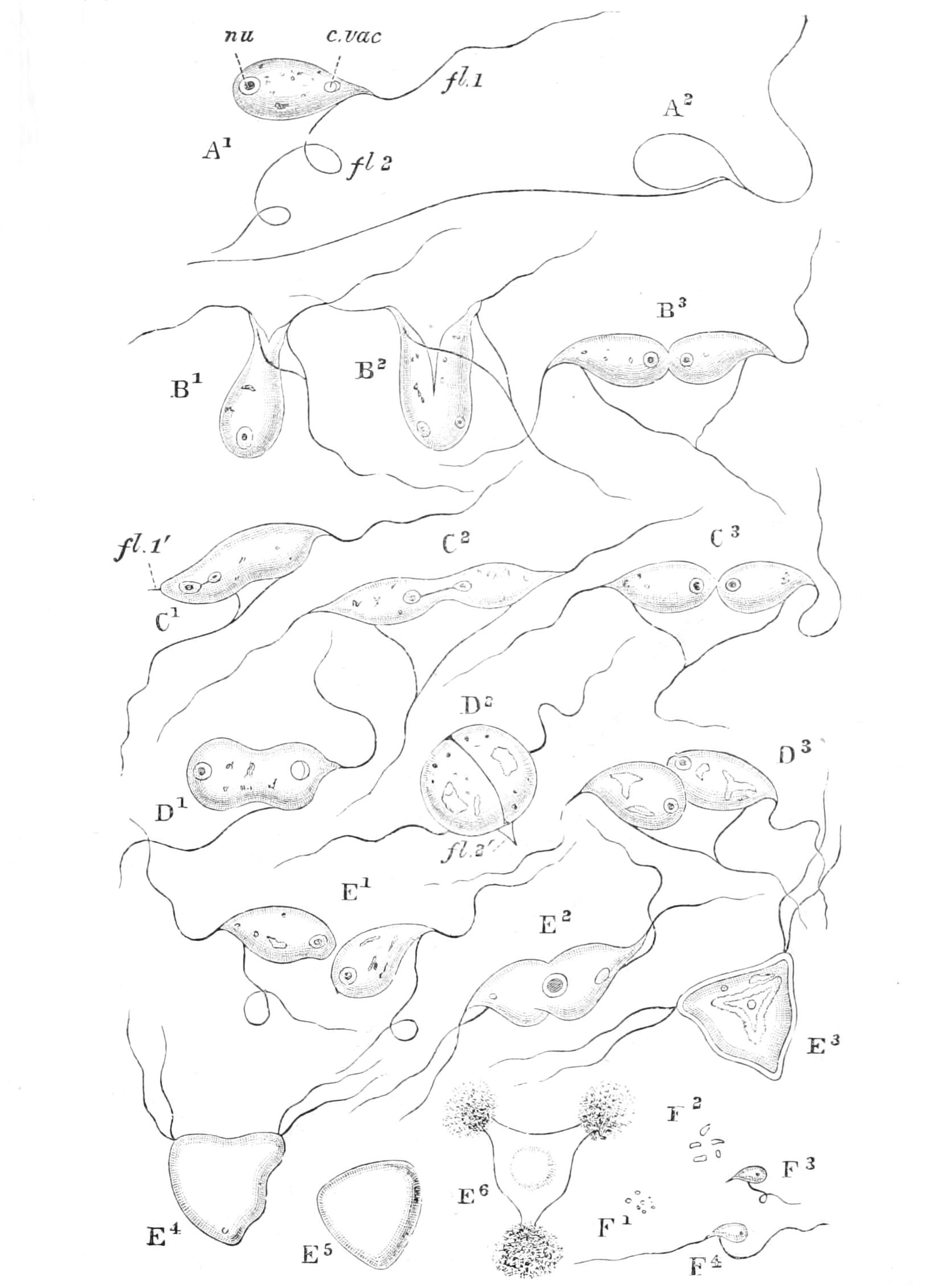
Fig. 38.—Bodo saltans. A, the positions assumed in the springing movements of the anchored form; B, longitudinal fission of anchored forms; C, transverse fission of the same; D, fission of free-swimming form; E1-E4, conjugation of free-swimming with anchored form; E5, zygote; E6, emission of spores from zygote; F, growth of spores: c.vac, contractile vacuole; fl.1, anterior; fl.2, ventral flagellum; nu, nucleus. (From Parker's Biology, after Dallinger.)
The life-cycles varied considerably in length. It was in every case found that after a series of fissions the species ultimately underwent conjugation (more or less unequal or bisexual in character);[124] the zygote encysted; and within the cyst the protoplasmic body underwent brood-formation, the outcome of which was a mass of {118}spores discharged by the rupture of the cyst (Fig. 38). These spores grow from a size too minute for resolution by our microscopes into the ordinary flagellate form. They withstand the effects of drying, if this be effected immediately on their escape from the ruptured cyst; so that it is probable that each spore has itself a delicate cyst-wall and an aplanospore, from which a single zoospore escapes. The complex cycle, of course, comprises the whole course from spore-formation to spore-formation. Such complete and regular "life-histories," each characteristic of the species, were the final argument against those who held to the belief that spontaneous generation of living beings took place in infusions of decomposing organic matter.
Previous to the work of these observers it had been almost universally believed that the temperature of boiling water was adequate to kill all living germs, and that any life that appeared in a closed vessel after boiling must be due to spontaneous change in its contents. But they now showed that, while none of the species studied resisted exposure in the active condition to a temperature of 138°-140° F., the spores only succumbed, in liquid, to temperatures that might even reach 268° F., or when dry, even 300° F. or more. Such facts explain the constant occurrence of one or more such minute species in liquids putrefying under ordinary conditions, the spores doubtless being present in the dust of the air. Very often several species may co-exist in one infusion; but they separate themselves into different zones, according to their respective need for air, when a drop of the liquid is placed on the slide and covered for examination. Dallinger[125] has made a series of experiments on the resistance of these organisms in their successive cycles to a gradual rise of temperature. Starting with a liquid containing three distinct species, which grew and multiplied normally at 60° F., he placed it under conditions in which he could slowly raise the temperature. While all the original inmates would have perished at 142° F., he succeeded in finally producing races that throve at 158° F., a scalding heat, when an accident put an end to that series of experiments. In no instance was the temperature raised so much as to kill off the beings, so that the increased tolerance of their descendants was due not, as might have been anticipated, to selection of those that best resisted, but to the inheritance of {119}an increased toleration and resistance from one generation or cycle to another.
As we noted above (p. 40), the study of the Flagellates has been largely in the hands of botanists. After the work of Bütschli in Bronn's Thier-Reich, Klebs[126] took up their study; and the principal monographs during the last decade have appeared in Engler and Prantl's Pflanzenfamilien, where Senn[127] treats the Flagellates generally, Wille[128] the Volvocaceae, and Schütt the "Peridiniales" or Dinoflagellata;[129] while only the Cystoflagellata, with but two genera, have been left to the undisputed sway of the zoologists.[130]
Among this group the majority are saprophytes, found in water containing putrefying matter or bacteria. The forms so carefully studied by Dallinger and Drysdale belong to the genera Bodo, Cercomonas, Tetramitus, Monas, and Dallingeria. Many others are parasites in the blood or internal cavities of higher animals, some apparently harmless, such as Trichomonas vaginalis, parasitic in man, others of singular malignity. Costia necatrix, infesting the epithelial scales of fresh-water fish, often devastates hatcheries. The genus Trypanosoma, Gruby, contributes a number of parasites, giving rise to deadly disease in man and beast.[131] T. lewisii is common in Rodents, but is relatively harmless. T. evansii is the cause of the Surra disease of Ruminants in India, and is apparently communicated by the bites of "large brown flies" (almost certainly Breeze Flies or Tabanidae, Vol. VI. p. 481). T. brucei, transferred to cattle by the Tsetse Fly, Glossina morsitans (see Vol. VI. Fig. 244, p. 513) in Equatorial Africa, is the cause of the deadly Nagana disease, which renders whole tracts of country impassable to ox or horse. Other Trypanosomic diseases of animals are, in Algeria and the Punjab, "dourine," infecting horses and dogs; in South America, Mal de Caderas (falling-sickness), an epidemic paralysis of cattle. During the printing of this book, much additional knowledge has been gained on this genus and the diseases it engenders. The Trypanosomic {120}fever recently recognised on the West Coast has been found to be the early stage of the sleeping-sickness, that well-known and most deadly epidemic of Tropical Africa. Through the researches of Castellani, Nabarro, and especially Colonel and Mrs. Bruce, we know now that the parasite T. gambiense is transferred by an intermediate host, a kind of Tsetse Fly (Glossina palpalis). Schaudinn's full study of a parasite of the blood corpuscles of the Owl has shown that while in its intracorpuscular state it resembles closely the malarial parasites in behaviour, and in its schizogenic multiplication, so that it was considered an Acystosporidian, under the name of Halteridium, it is really a Trypanosoma;[132] for the accomplishment of successful sexual reproduction it requires transference to the gut of a gnat (Culex). The germs may infect the ovary, and give the offspring of the insect the innate power of infecting Owls. Thus a new light is shed on the origin of the Coccidiaceae, whose "blasts" in the insect host resemble Trypanosoma in their morphology.
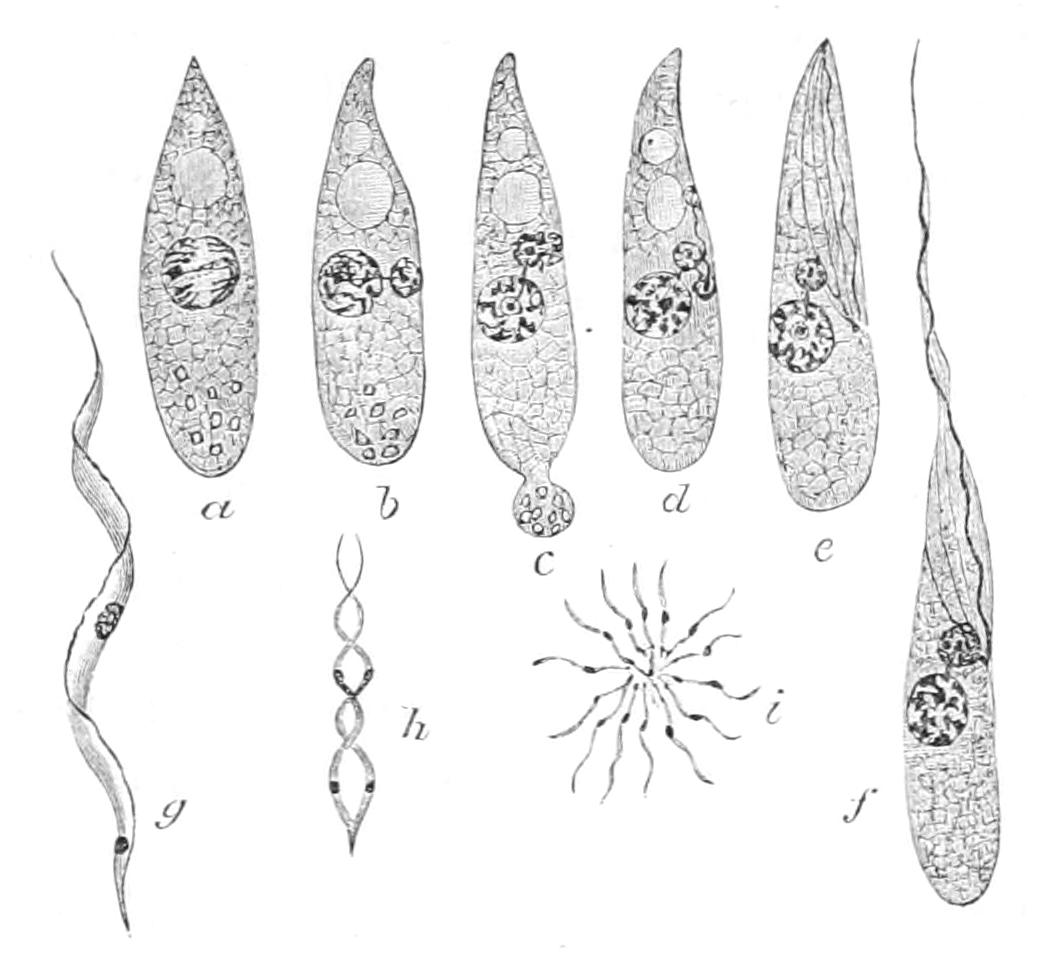
Fig. 39.—Morphology of Trypanosoma. a-f, Stages in development of Trypanosoma noctuae from the active zygote ("ookinete"); b, first division of nucleus into larger (trophic) and smaller (kineto-) nucleus; c, d, division of smaller nucleus and its transformations to form "blepharoplast" and myonemes; f, adult Trypanosoma; g, h, i, Treponema zeemannii of Owl; g, Trypanosome form; h, Spirochaeta form; i, rosette aggregate. (After Schaudinn.)
The human Tick fever of the Western United States and the epizootic Texas fever are known to be due to blood parasites of the genus Piroplasma (Babesia), of which the free state is that of a Trypanosome. It appears certain that Texas fever, though due to Tick bites, is not transferred directly from one beast to another by the same Tick; but the offspring of a female Tick that has sucked an infected ox contains Trypanosome germs, and will by their bites infect other animals. {121}It would seem probable that the virulence of the Persian Tick (Argas persica) is due to similar causes. The Indian maladies known as "Kala Azar" and "Oriental Sore" are characterised by blood parasites, at first called after their discoverer the "Leishman bodies," which have proved to be the effects of a Piroplasma.
Trypanosoma is distinguished by the expansion of its flagellum into an undulating membrane, that runs down the edge of the body, and may project behind as a second lash. In this membrane run eight fine muscular filaments, or myonemes, four on either surface, within the undulating membrane; at their lower end they are all connected with a rounded body, the "blepharoplast," which is here in its origin, as well as in its behaviour in reproductive processes, a true modified nucleus, comparable in some respects, as was first noted by Plimmer and Rose Bradford,[133] with the micronucleus of the Infusoria. Part of the segmentation spindle persists in the form of a filament uniting the blepharoplast with the large true functional nucleus (Fig. 39, a-f).
The blood of patients suffering from relapsing fever contains a fine wriggling parasite, which was described as a Schizomycete, allied to the bacteria, and hitherto termed Spirochaeta obermeieri. Schaudinn has shown that this and other similar blood parasites are closely allied to Trypanosoma; and since the original genus was founded on organisms of putrefaction which are undoubtedly Schizomycetes, Vuillemin has suggested the name Treponema. T. pallidum is found in syphilitic patients, and appears to be responsible for their illness.[134]
The Craspedomonadidae (often called Choanoflagellates, Fig. 40) are a group whose true nature was elucidated some forty years ago by the American zoologist, H. James-Clark. They are attached either to a substratum, by a stalk produced by the base of the cell, or to other members of the same colony; they are distinguished by the protrusion of the cytoplasm around the base of the single flagellum into a pellucid funnel,[135] in which the plasma is in constant motion, though the funnel retains its shape and size, except when, as sometimes happens, it is retracted.
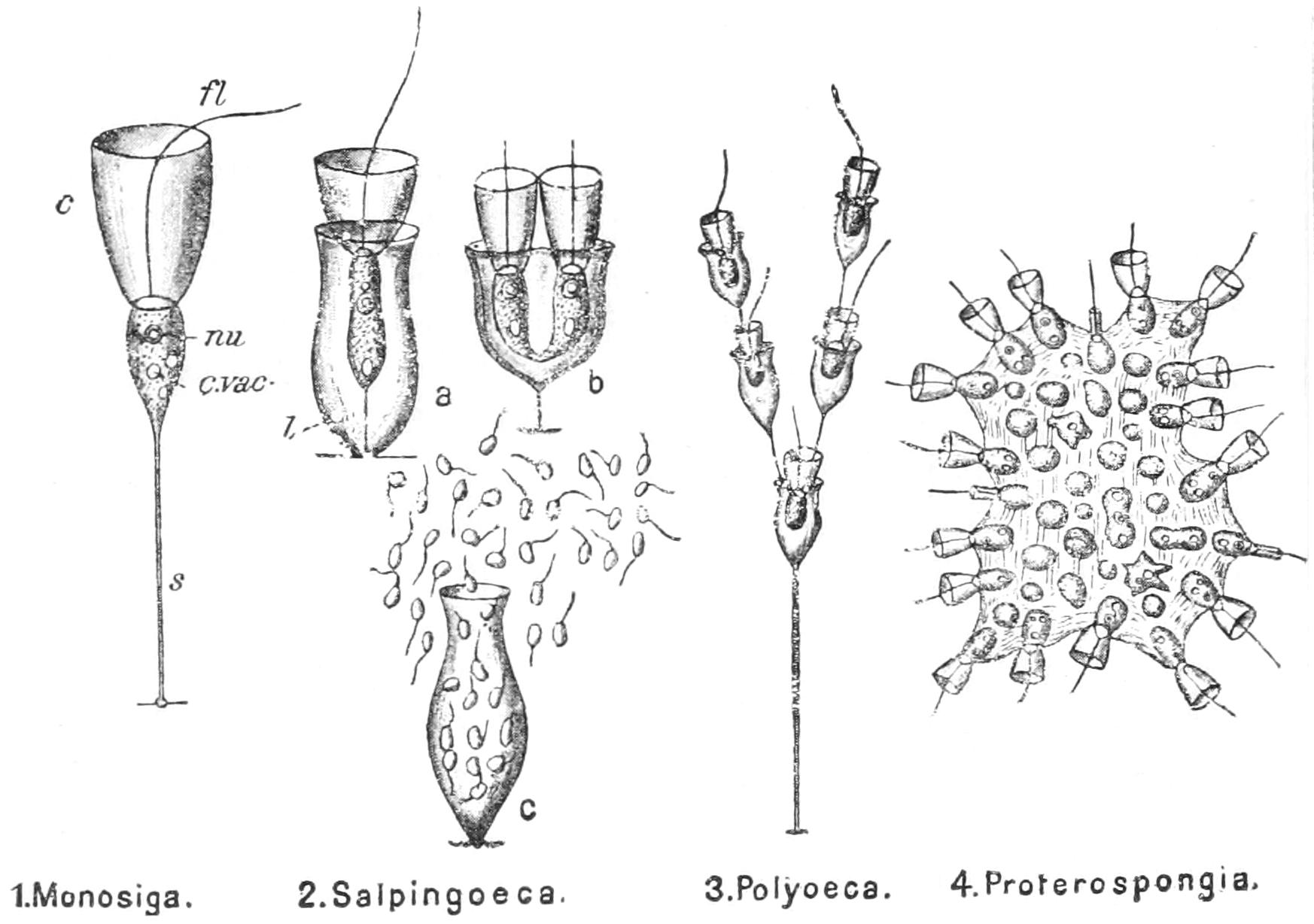
Fig. 40.—Various forms of Craspedomonadidae. 2, a, Adult cell; 2, b, longitudinal fission; 2, c, the production of flagellulae by brood-formation; c, collar; c.vac, contractile vacuole; fl, flagellum; l, theca; nu, nucleus; s, stalk. (After Saville Kent.)
The agitation of the flagellum determines a stream of water upwards along the outer walls of the funnel; and the food-particles brought along adhere to the outside of the funnel, and are carried by its streaming movement to the basal constriction, where they are swallowed by the plasma, which appears to form a swallowing vacuole at that point. Longitudinal fission is the ordinary mode of reproduction, extending up through the funnel. If the two so formed continue to produce a stalk, the result is the formation of a tree-like stem, whose twigs bear at the ends the funnelled cells, or "collar-cells" as they are usually called. In Salpingoeca, as in so many other Flagellates, each cell forms a cup or theca, often of most graceful vase-like outline, the rim being elegantly turned back. Proterospongia (Fig. 75, p. 182) secretes a gelatinous investment for the colony, which is attached to solid bodies. In this species, according to Saville Kent, the central members of the colony retract their collar, lose their flagellum, become amoeboid, and finally undergo brood-formation to produce minute zoospores. This is the form which by its differentiation recalls the Sponges, and has been regarded as a {123}transition towards them; for the flagellate, nutritive cells of the Sponges are provided with a collar, which exists in no other group of Metazoa (see pp. 171, 181, and Fig. 70, p. 176). The most recent monographer of the family is Raoul Francé, but James-Clark and Saville Kent did the pioneering work.
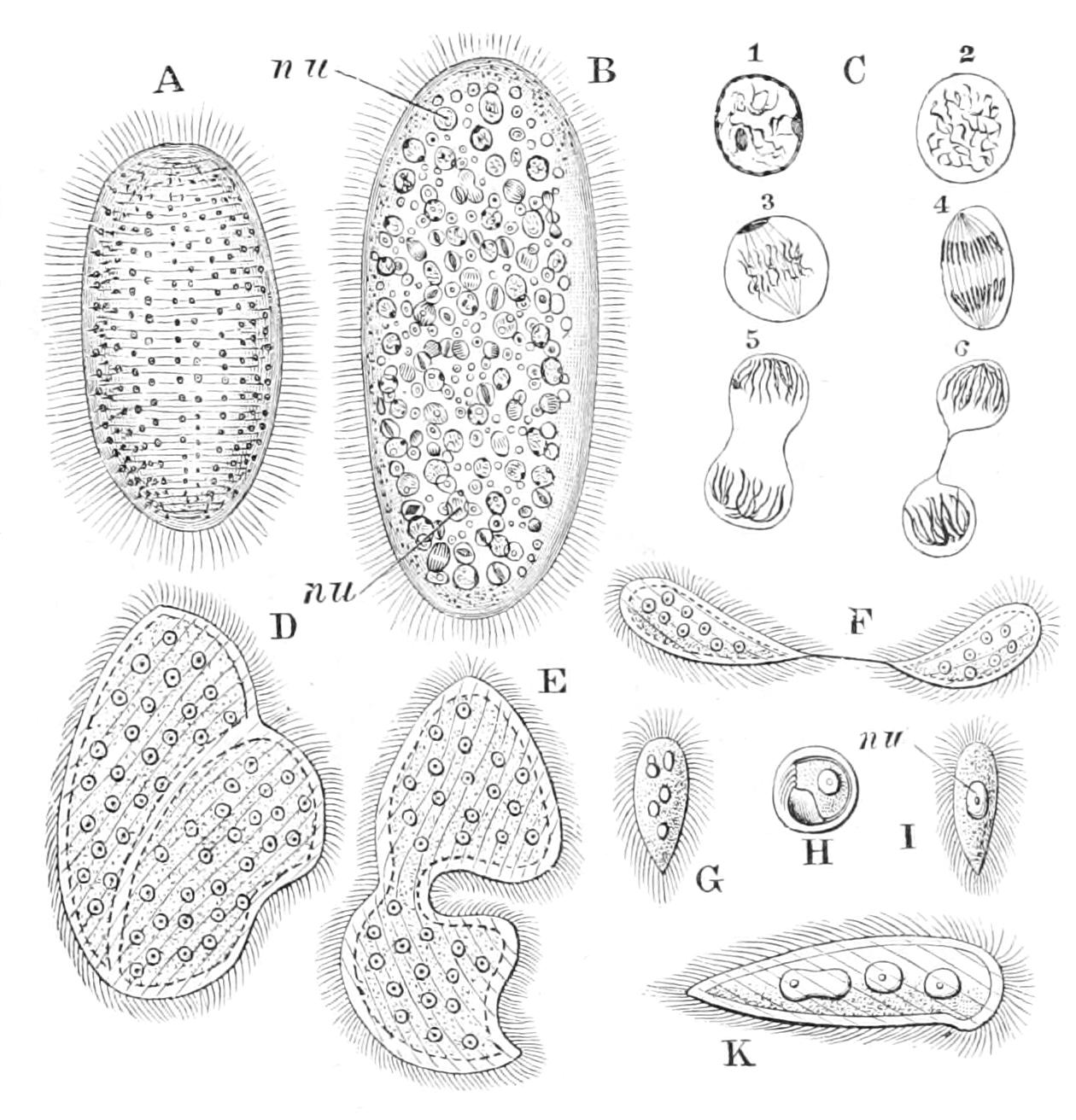
Fig. 41.—Opalina ranarum. A, living specimen; B, stained specimen showing nuclei; C, stages in nuclear division; D-F, stages in fission; G, final product of fission; H, encysted form; I, young form liberated from cyst; K, the same after multiplication of the nucleus has begun. nu, Nucleus. (From Parker's Biology, after Saville Kent and Zeller.)
Of the life-history of the Trichonymphidae,[136] all of which are parasitic in the alimentary canal of Insects, especially Termites or White Ants (Vol. V. p. 356), nothing is known. Some of them have a complete investment of motile flagella, like enormously long cilia, which in Dinenympha appear to coalesce into four longitudinal undulating membranes. Lophomonas inhabits the gut of the Cockroach and Mole-cricket. The Opalinidae have also a complete investment of cilia, which are short, and give the aspect of a Ciliate to the animal, which is common in the rectum of Amphibia, and dies when transferred to water. But despite the outward resemblance, the nuclei, of which there may be as many as 200, are all similar, and consequently this group cannot be placed among the Infusoria at all. Opalina has no mouth nor contractile vacuole. It multiplies by dividing {124}irregularly and at intervals, resolving finally into 1-nucleate fragments, which encyst and pass into the water. When swallowed the cyst dissolves, its contents enlarge, and ultimately assume the adult form.[137]
Maupasia has a partial investment of cilia, a single long flagellum and mouth, a contractile vesicle, and a single simple nucleus. It seems to find an appropriate place near the two above groups, though it is free, and possesses a mouth.
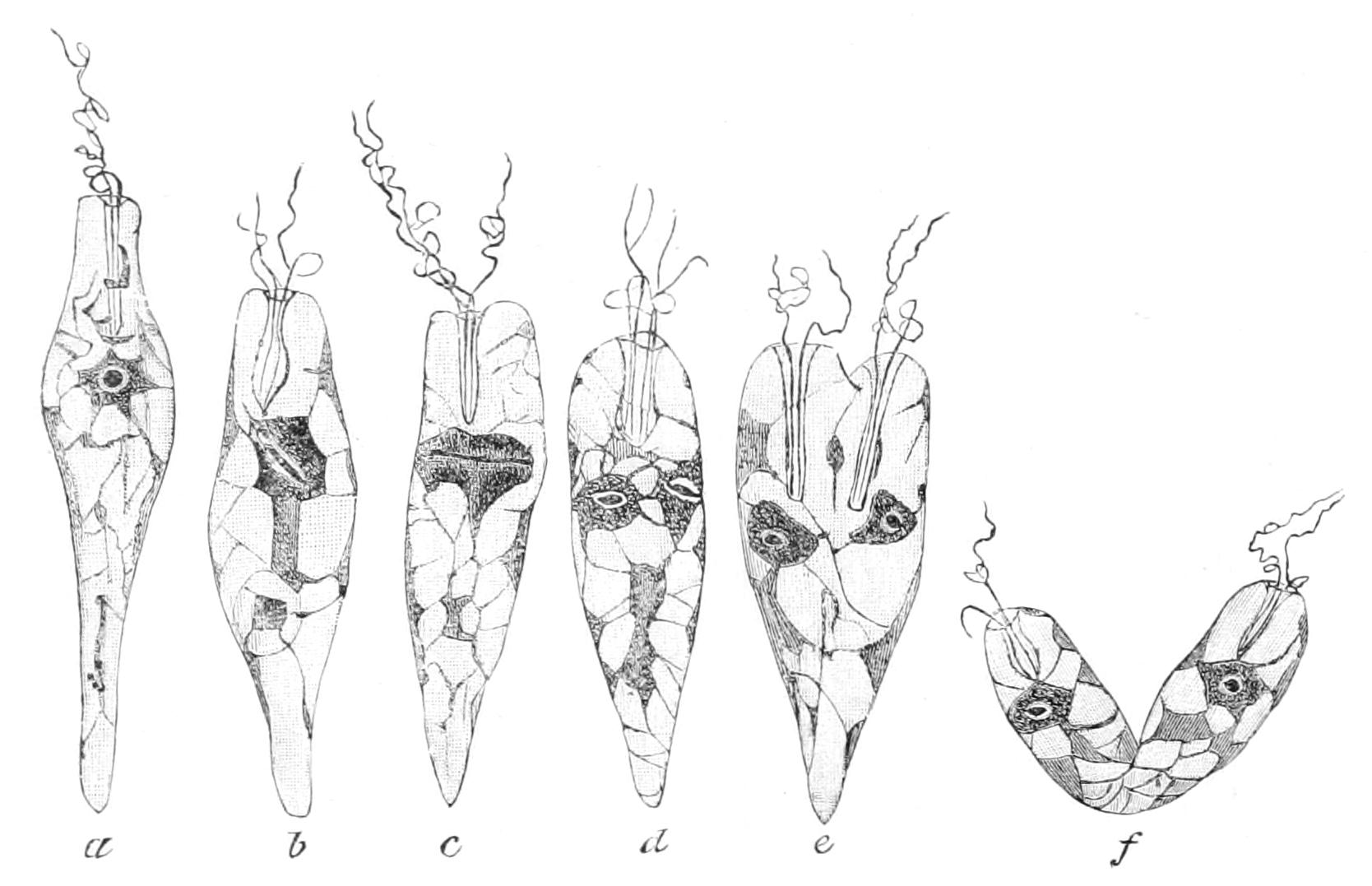
Fig. 42.—Longitudinal Fission of Eutreptia viridis (Euglenaceae), showing chloroplasts, nucleus, and flagella arising from pharynx-tube. (After Steuer.)
Among the Euglenaceae, Euglena viridis is a very common form, giving the green colour to stagnant or slow-flowing ditches and puddles in light places, especially when contaminated by a fair amount of dung, as by the overflow of a pig-sty, in company with a few hardy Rotifers, such as Hydatina senta (Vol. II. Fig. 106, p. 199) and Brachionus. Euglena is about 0.1 mm. in length when fully extended, oval, pointed behind, obliquely truncate in front, with a flagellum arising from the pharyngeal pit. It shows a peculiar wriggling motion, waves of transverse constriction passing along the body from end to end, as well as flexures in different meridians. Such motions are termed "euglenoid." The front part is colourless, but under a low {125}power the rest of the cell is green, owing to the numerous chlorophyll bodies or chloroplasts. The outermost layer of the cytoplasm shows a somewhat spiral longitudinal striation, possibly due to muscular fibrils. The interior contains many laminated plates of paramylum, and a large single nucleus. At the front of the body at the base of the flagellum is a red "eye-spot" on the dorsal side of the pharynx-tube or pit, from which the flagellum protrudes. Wager has shown that this tube receives, also on its dorsal side, the opening of a large vacuole, sometimes called the reservoir, for into it discharges the contractile vacuole (or vacuoles). The eye-spot is composed of numerous granules, containing the vegetal colouring matter "haematochrome." It embraces the lower or posterior side of the communication between the tube and the reservoir. The flagellum has been traced by Wager through the tube into the reservoir, branching into two roots where it enters the aperture of communication, and these are inserted on the wall of the reservoir at the side opposite the eye-spot. But on one of the roots near the bifurcation is a dilatation which lies close against the eye-spot, so that it can receive the light reaction. Euglena is an extremely phototactic organism. It shows various wrigglings along the longitudinal axis, and transverse waves of contraction and expansion may pass from pole to pole.[138]
Among the Chrysomonadaceae the genus Zooxanthella, Brandt, has already been described under the Radiolaria (p. 86), in the jelly of which it is symbiotic. It also occurs in similar union in the marine Ciliates, Vorticella sertulariae and Scyphidia scorpaenae, and in Millepora (p. 261) and many Anthozoa (pp. 373 f., 396).
Of the Chlamydomonadidae, Sphaerella (Haematococcus, Ag.) pluvialis (Fig. 43), and S. nivalis, in which the green is masked by red pigment, give rise to the phenomena of "red snow" and "bloody rain." The type genus, Chlamydomonas, is remarkable for the variations from species to species in the character and behaviour of the gametes. Sometimes they are equal, at other times of two sizes. In some species they fuse immediately on approximation, in the naked active state; in others, they encyst on approaching, and unite by the emission of a fertilising tube, {126}as in the Algal Conjugatae. Zoochlorella is symbiotic in green Ciliata (pp. 153 f., 158), Sponges (p. 175), Hydra (p. 256), and Turbellaria (Vol. II. p. 43).
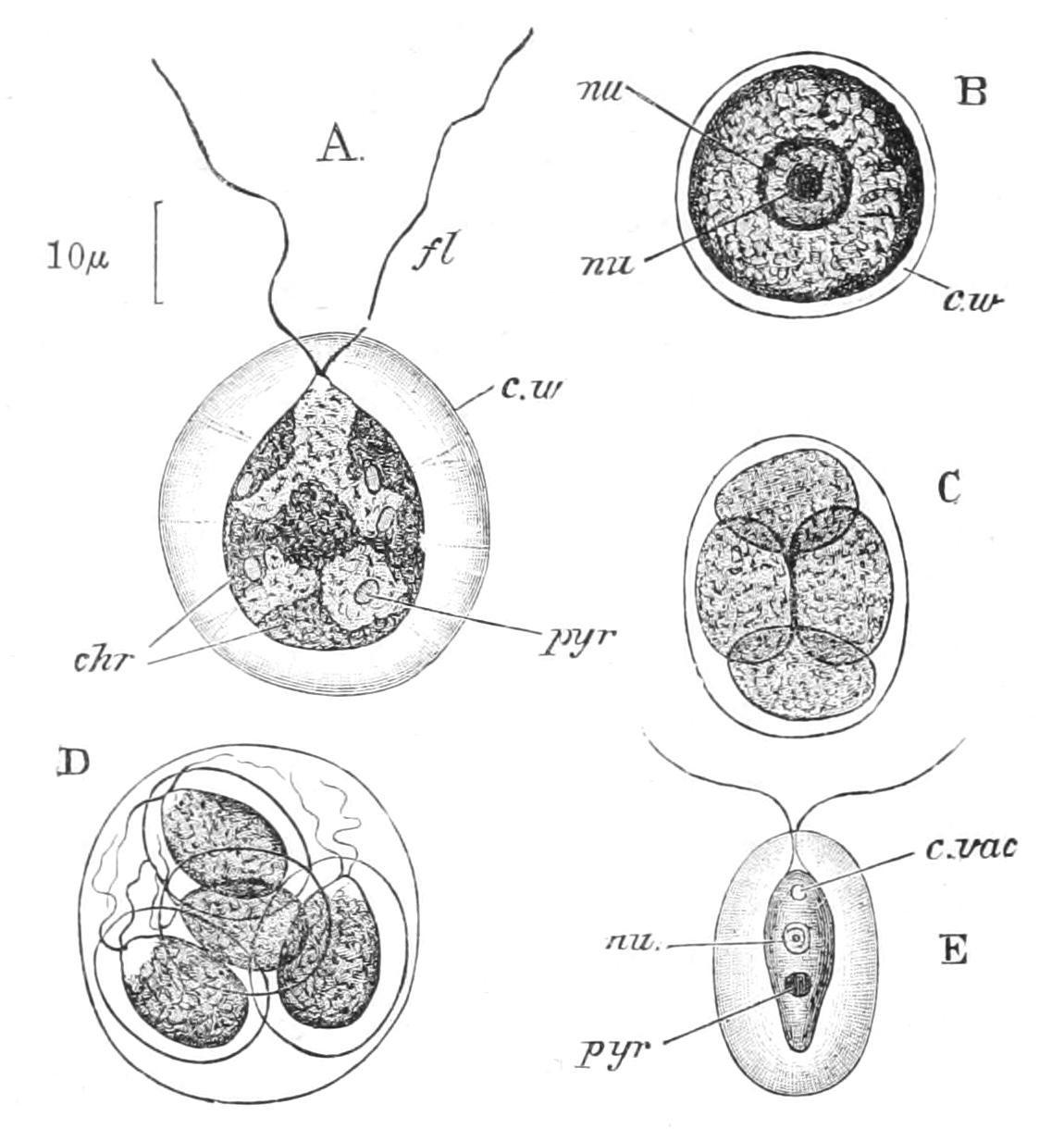
Fig. 43.—Sphaerella pluvialis. A, motile stage; B, resting stage; C, D, two modes of fission; E, Sphaerella lacustris, motile stage. chr, Chromatophores; c.vac, contractile vacuole; c.w, cell-wall; fl, flagella; nu, nucleus; nu', nucleolus; pyr, pyrenoids. (From Parker's Biology.)
Of the Volvocidae, Volvox (Fig. 44) is the largest and most conspicuous genus. Its colony forms a globe the size of a pin's head, floating on the surface of ponds, drains, or even puddles or water-barrels freely open to the light. It has what may be called a skeleton of gelatinous matter,[139] condensed towards the surface into a denser layer in which the minute cells are scattered. These have each an eye-spot, a contractile vacuole, and two flagella, by the combined action of which the colony is propelled. Delicate boundary lines in the colonial wall mark out the proper investment of each cell. The cells give off delicate plasmic threads which meet those of their neighbours, and form a bond between them. In that half of the hemisphere which is posterior in swimming, a few (five to eight) larger cells ("macrogonidia" of older writers) are evenly distributed, protruding as they increase in size into the central jelly. These as they grow segment to form a new colony.
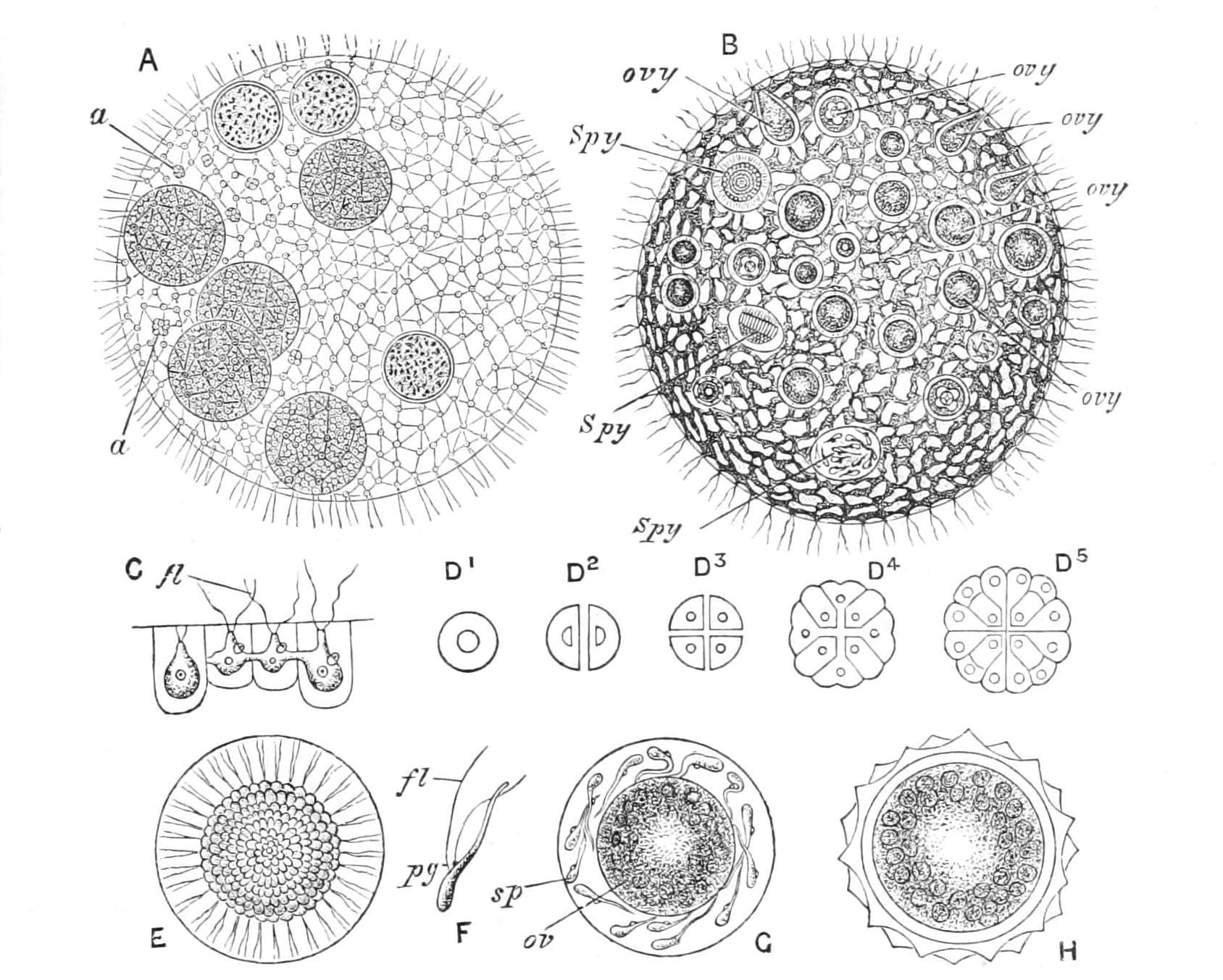
Fig. 44.—Volvox globator. A, entire colony, enclosing several daughter-colonies; B, the same during sexual maturity; C, four zooids in optical section; D1-D5, development of parthenogonidium; E, ripe spermogonium; F, sperm; G, ovum; H, oosperm. a, Parthenogonidia; fl, flagellum; ov, ovum; ovy, ovaries; pg, pigment spot; sp, sperms; Spy, spermogonia dividing to form sperms. (From Parker's Biology, after Cohn and Kirchner.)
The divisions are only in two planes at right angles, so that the young colony is at first a plate, but as the cells multiply the plate bends up (as in the gastrulation of the double cellular plate of the Nematode Cucullanus, Vol. II. p. 136), and finally forms a hollow sphere bounded by a single layer of cells: the site of the original orifice may be traced even in the adult as a blank space larger than exists elsewhere. Among the cells of the young colony some cease to divide, but continue to grow at an early period, and these are destined to become in turn the mothers ("parthenogonidia") of a new colony; they begin segmenting before the colony of which they are cells is freed. The young colonies are ultimately liberated by the rupture of the sphere as small-sized spheres, which henceforth only grow by enlargement of the sphere as a whole, and the wider separation of the vegetative cells. Thus the vegetative cells soon cease to grow; all the supply of food material due to their living activities goes to the nourishment of the parthenogonidia, or the young colonies, as {128}the case may be. These vegetative cells have therefore surrendered the power of fission elsewhere inherent in the Protist cell. Moreover, when the sphere ruptures for the liberation of the young colonies, it sinks and is doomed to death, whether because its light-loving cells are submerged in the ooze of the bottom, or because they have no further capacity for life. When conjugation is about to take place, it is the cells that otherwise would be parthenogonidia that either act as oospheres or divide as "spermogonia" to form a flat brood of minute yellow male cells ("sperms"). These resemble vegetative cells, in the possession of an eye-spot and two contractile vacuoles, but differ in the enormously enlarged nucleus which determines a beaked process in front. After one of these has fused with the female cell ("oosphere") the product ("oosperm") encysts, passes into a stage of profound rest, and finally gives rise to a new colony. The oospheres and sperm-broods may arise in the same colony or in distinct ones, according to the species.
Before we consider the bearings of the syngamic processes of Volvox, we will study those presented by its nearer allies, which have the same habitat, but are much more minute. Three of these are well known, Stephanosphaera, Pandorina, and Eudorina, all of which have spherical colonies of from eight to thirty-two cells embedded at the surface of a sphere, and no differentiation into vegetative cells and parthenogonidia (or reproductive cells).
Stephanosphaera has its eight cells spindle shaped, and lying along equidistant meridians of its sphere; in vegetative reproduction each of these breaks up in its place to form a young colony, and the eight daughter-colonies are then freed. In conjugation, each cell of the colony breaks up into broods of 4, 8, 16, or 32 small gametes, which swim about within the general envelope, and pair and fuse two and two: this is "isogamous," "endogamous" conjugation. In Pandorina (Fig. 45) the cells are rounded, and are from 16 to 32 in each colony. The vegetative reproduction in this, as in Eudorina, is essentially the same as in Stephanosphaera. In conjugation the cells are set free, and are of three sizes in different colonies, small (S), medium (M), and large (L). The following fusions may occur: S × S, S × M, S × L, M × M, M × L. Thus the large are always female, as it were, the medium may play the part of male to the large, female to the small; the small are males to the medium and to the large. The medium {129}and small are capable, each with its like, of equal, undifferentiated conjugation; so that we have a differentiation of sex far other than that of ordinary, binary sex. Eudorina, however, has attained to "binary sex," for the female cells are the ordinary vegetative cells, at most a little enlarged, and the male cells are formed by ordinary cells producing a large flat colony of sixty-four minute males or sperms. In some cases four cells at the apex of a colony are spermogonia, producing each a brood of sperms, while the rest are the oospheres. The transition to Volvox must have arisen through the sterilisation of the majority of cells of a colony for the better nutrition of the few that are destined alone for reproduction.
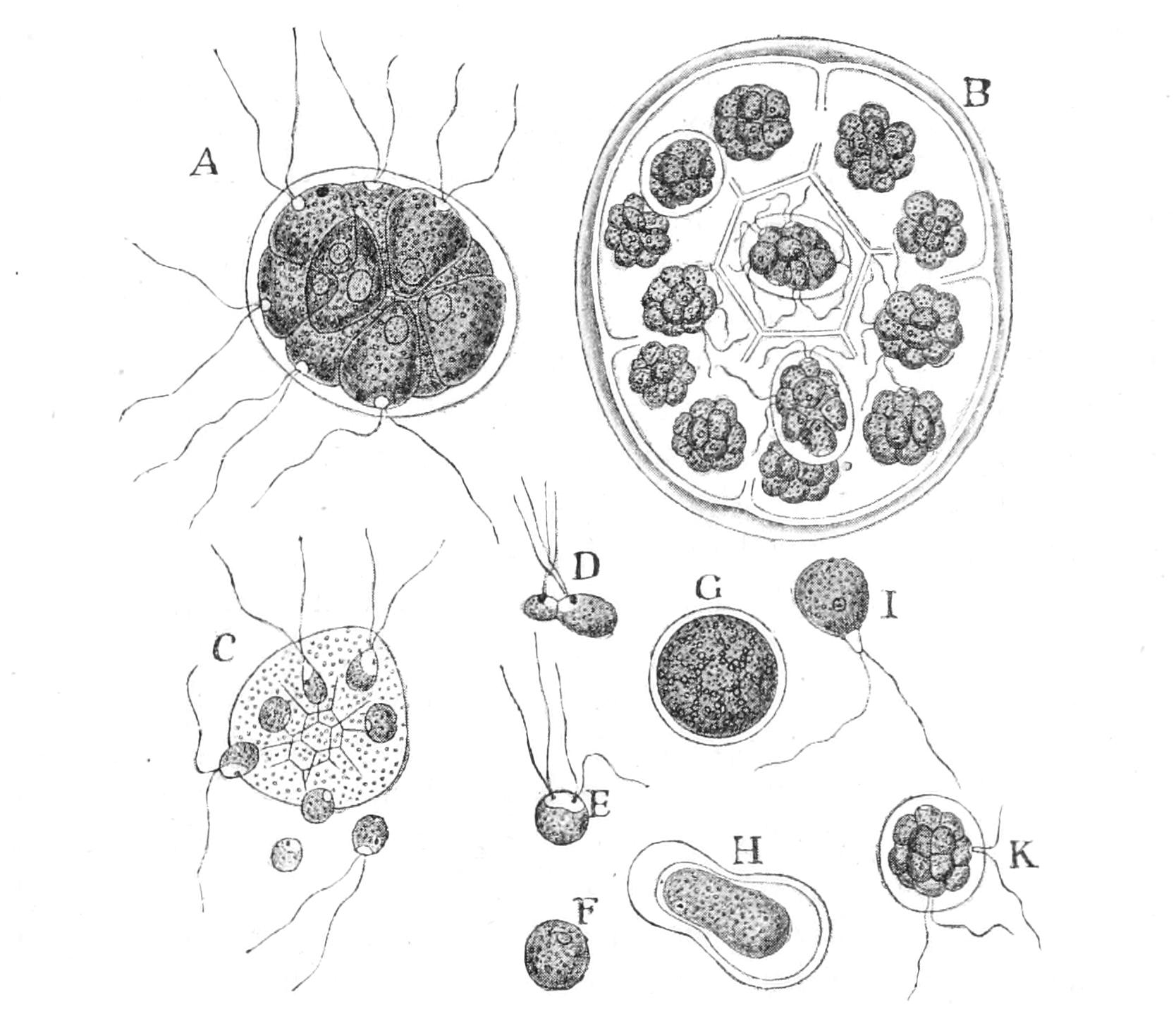
Fig. 45.—Pandorina morum. A, entire colony; B, asexual reproduction, each zooid dividing into a daughter-colony; C, liberation of gametes; D-F, three stages in conjugation of gametes; G, zygote; H-K, development of zygote into a new colony. (From Parker's Biology, after Goebel.)
Volvox, as we have seen, has attained a specialisation entirely comparable to that of a Metazoon, where the segmentation of the fertilised ovum results in two classes of cells: those destined {130}to form tissues, and condemned to ultimate death with the body as a whole, and those that ultimately give rise to the reproductive cells, ova, and sperms. But this is a mere parallelism, not indicating any sort of relationship: the oospores of the Volvocaceae show that tendency to an encysted state, in which fission takes place, that is so characteristic of Algae, and these again show the way to Cryptogams of a higher status. Thus, Volvox, despite the fact that in its free life and cellular differentiation it is the most animal of all known Flagellates, is yet, with the rest of the Volvocaceae, inseparable from the Vegetable Kingdom, and is placed here only because of the impossibility of cleaving the Flagellates into two.
The Dinoflagellata (Figs. 46, 47) are often of exceptionally large dimensions in this class, attaining a maximum diameter of 150 µ (1⁄160") and even 375 µ (1⁄67") in Pyrocystis noctiluca. The special character of the group is the presence of two flagella; the one, filiform, arises in a longitudinal groove, and extending its whole length projects behind the animal, and is the conspicuous organ of motion: the other, band-like, arises also in the longitudinal groove, but extends along a somewhat spiral transverse groove,[140] and never protrudes from it in life, executing undulating movements that simulate those of a girdle of cilia, or a continuous undulating membrane (Fig. 46). This appearance led to the old name "Cilioflagellata," which had of course to be abandoned when Klebs discovered the true structure.[141] There is a distinct cellulose membrane, sometimes silicified, to the ectoplasm, only interrupted by a bare space in the longitudinal groove, whence the flagella take origin. This cuticle is usually hard, sculptured, and divided into plates of definite form, bevelled and overlapping at their junction; occasionally the cell has been seen to moult them.
A large vacuolar space, traversed by plasmic strings, separates the peripheral cytoplasm from the central, within which is the large nucleus. There are in most species one or more chromatophores, coloured by a yellowish or brownish pigment, which is a mixture of lipochromes, distinct from diatomin. In a few species the presence of these is not constant, and these species {131}show variability as to their nutrition, which is sometimes holozoic. Under these conditions the cell can take in food-particles as bulky as the eggs of Rotifers and Copepods, by the protrusion of a pseudopod at the junction of the two grooves. As in most coloured forms an eye-spot is often present, a cup-shaped aggregation of pigment, with a lenticular refractive body in its hollow. A contractile vacuole, here termed a "pusule," occurs in many species, communicating with the longitudinal groove by a canal. Nematocysts (see p. 246 f.) are present in Polykrikos, trichocysts (see p. 142) in several genera.
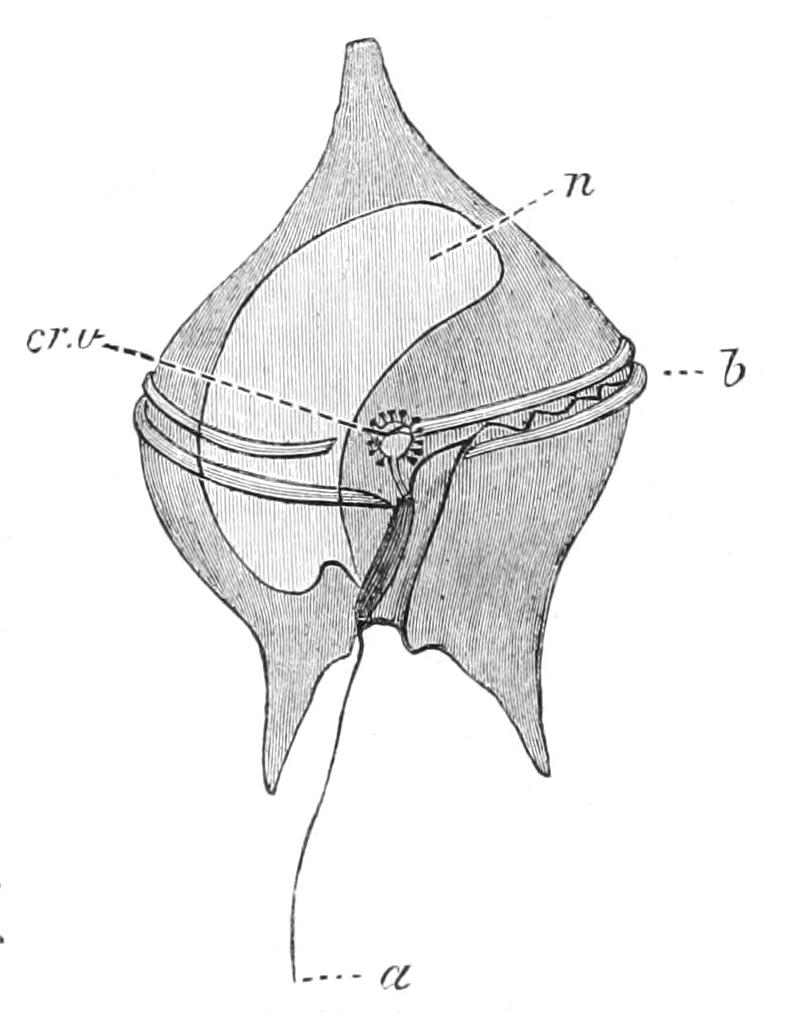
Fig. 46.—Peridinium divergens. a, Flagellum of longitudinal groove; b, flagellum of transverse groove; cr.v, contractile vacuole surrounded by formative vacuoles; n, nucleus. (After Schütt.)
Division is usually oblique, dividing the body into two dissimilar halves, each of which has to undergo a peculiar growth to reconstitute the missing portion, and complete the shell. The incomplete separation of the young cells leads to the formation of chains, notably in Ceratium and Polykrikos, the latter dividing transversely and occurring in chains of as many as eight. The process of division may take place when the cell is active, or in a cyst, as in Pyrocystis (Fig. 47). Again, encystment may precede multiple fission, resulting in the formation of a brood of minute swarmers. It has been suggested that these are capable of playing the part of gametes, and conjugating in pairs.[142]
The Dinoflagellates are for the most part pelagic in habit, floating at the surface, and when abundant tinge the water of fresh-water lakes or even ponds red or brown. Peridinium (Fig. 46) and Ceratium (the latter remarkable for the horn-like backward prolongations of the lower end) are common genera both in the sea {132}and fresh-waters. Gymnodinium pulvisculus is sometimes parasitic in Appendicularia (Vol. VII. p. 68). Polykrikos[143] has four transverse grooves, each with its flagellum, besides the terminal one. Many of the marine species are phosphorescent, and play a large part in the luminosity of the sea, and some give it a red colour.
Several fossil forms have been described. Peridinium is certainly found fossil in the firestone of Delitzet, belonging to the Cretaceous. A full monograph of the group under the name "Peridiniales" was published by Schütt.[144]
Fig. 47.—Pyrocystis fusiformis, Murray. × 100. From the surface in the Guinea Current. (From Wyville Thomson.)
The Cystoflagellates contain only two genera,[145] Noctiluca, common at the surface of tranquil seas, to which, as its name implies, it gives phosphorescence, and Leptodiscus, found by R. Hertwig in the Mediterranean. Noctiluca is enormous for a Flagellate, for with the form of a miniature melon it measures about 1 mm. (1⁄25") or more in diameter. In the depression is the "oral cleft," from one end of which rises, by a broad base, a large coarse flagellum, as long as the body or longer and transversely striated. In front of the base of the flagellum are two lip-like {133}prominences, of which one, a little firmer than the other, and transversely ridged, is called the tooth; at the junction of the two is a second, minute, flagellum, usually called the cilium. Behind these the oral groove has an oval space, the proper mouth; behind this, again, the oral groove is continued for some way, with a distinct rod-like ridge in its furrow. The whole body, including the big flagellum, is coated by a strong cuticular pellicle, except at the oblong mouth, and the lips and rod are mere thickenings of this. The cytoplasm has a reticulate arrangement: the mouth opens into a central aggregate, from which strands diverge branching as they recede to the periphery, where they pass into a continuous lining for the cuticular wall, liquid filling the interspaces. The whole arrangement is not unlike that found in many plant-cells, but the only other Protists in which it occurs are the Ciliata Trachelius (Fig. 56, p. 153) and Loxodes. The central mass contains the large nucleus. Noctiluca is an animal feeder, and expels its excreta through the mouth. The large flagellum is remarkable for the transverse striation of its plasma, especially on the ventral side. The cuticle may be moulted as in the Dinoflagellates. As a prelude to fission the external differentiations disappear, the nucleus divides in the plane of the oral groove, and a meridional constriction parts the two halves, the new external organs being regenerated. Conjugation occurs also, the two organisms fusing by their oral region; the locomotive organs and pharynx disappear; the conjoined cytoplasms unite to form a sphere, and the nuclei fuse to form a zygote or fertilisation nucleus. This conjugation is followed by sporulation or brood-formation.[146]
The nucleus passes towards the surface, undergoes successive fissions, and as division goes on the numerous daughter-nuclei occupy little prominences formed by the upgrowth of the cytoplasm of the upper pole. The rest of the cytoplasm atrophies, and the hillocks formed by the plasmic outgrowths around the final daughter-nuclei become separate as so many zoospores (usually 256 or 512); each of these is oblong with a dorsal cap-like swelling, from the edge of which arises a flagellum pointing backwards; parallel to this the cap is prolonged on one side into a style also extending beyond the opposite pole of the animal.[147] In this state the zoospore is, to all outward view, a naked Dinoflagellate, whence it seems that the Cystoflagellates are to be regarded as closely allied to that group. Leptodiscus is concavo-convex, circular, with the mouth central on the convex face, 1-flagellate, and attains the enormous size of 1.5 mm. (1⁄16") in diameter.
The remarkable phosphorescence of Noctiluca is not constant. It glows with a bluish or greenish light on any agitation, but rarely when undisturbed. A persistent stimulus causes a continuous, but weak, light. This light is so weak that several teaspoonsful of the organism, collected on a filter and spread out, barely enable one to read the figures on a watch a foot away. As in other marine phosphorescence, no rise of temperature can be detected. The luminosity resides in minute points, mostly crowded in the central mass, but scattered all through the cytoplasm. A slight irritation only produces luminosity at the point touched, a strong one causes the whole to flash. Any form of irritation, whether of heat, touch, or agitation, electricity or magnetism, is stated to induce the glow. By day, it is said, Noctiluca, when present in abundance, may give the sea the appearance of tomato soup.
The earliest account of Noctiluca will be read with interest. Henry Baker writes in Employment for the Microscope:[148]—"A curious Enquirer into Nature, dwelling at Wells upon the Coast of Norfolk, affirms from his own Observations that the Sparkling of Sea Water is occasioned by Insects. His Answer to a Letter wrote to him on that Subject runs thus, 'In the Glass of Sea Water I send with this are some of the Animalcules which cause the Sparkling Light in Sea Water; they may be seen by holding {135}the Phial up against the Light, resembling very small Bladders or Air Bubbles, and are in all Places of it from Top to Bottom, but mostly towards the Top, where they assemble when the Water has stood still some Time, unless they have been killed by keeping them too long in the Phial. Placing one of these Animalcules before a good Microscope, an exceeding minute Worm may be discovered, hanging with its Tail fixed to an opake Spot in a Kind of Bladder, which it has certainly a Power of contracting or distending, and thereby of being suspended at the Surface, or at any Depth it pleases in the including Water.'"
"The above-mentioned Phial of Sea Water came safe, and some of the Animalcules were discovered in it, but they did not emit any Light, as my Friend says they do, upon the least Motion of the Phial when the Water is newly taken up. He likewise adds, that at certain Times, if a Stone be thrown into the Sea, near the Shore, the Water will become luminous as far as the Motion reacheth: this chiefly happens when the Sea hath been greatly agitated, or after a Storm." Obviously what Mr. Sparshall, Baker's correspondent, took for a worm was the large flagellum.
The chief investigators of this group have been Huxley, Cienkowski, Allman, Bütschli, and G. Pouchet, while Ischikawa and Doflein have elucidated the conjugation.
PROTOZOA (CONTINUED): INFUSORIA (CILIATA AND SUCTORIA)
IV. Infusoria.
Complex Protozoa, never holophytic save by symbiosis with plant commensals, never amoeboid, with at some period numerous short cilia, of definite outline, with a double nuclear apparatus consisting of a large meganucleus and a small micronucleus (or several),[149] the latter alone taking part in conjugation (karyogamy), and giving rise after conjugation to the new nuclear apparatus.
The name Infusoria was formerly applied to the majority of the Protozoa, and included even the Rotifers. For the word signifies organisms found in "infusions" of organic materials, including macerations. Such were made with the most varied ingredients, pepper and hay being perhaps the favourites. They were left for varying periods exposed to the air, to allow the organisms to develop therein, and were then examined under the microscope.[150] With the progress of our knowledge, group after group was split off from the old assemblage until only the ciliate or flagellate forms were left. The recognition of the claims of the Flagellates to independent treatment left the group more natural;[151] while it was enlarged by the admission of the Acinetans (Suctoria), which had for some time been regarded as a division of the Rhizopoda.
I. Ciliata
Infusoria, with a mouth, and cilia by which they move and feed; usually with undulating membranes, membranellae, cirrhi, or some of these. Genera about 144: 27 exclusively marine, 50 common to both sea and fresh water, 27 parasitic on or in Metazoa, the rest fresh water. Species about 500.
We divide the Ciliata thus:[152]—
| (I.) Mouth habitually closed, opening by retraction of its circular or slit-like margin; cilia uniform | Order 1. Gymnostomaceae. |
| Lacrymaria, Ehrb.; Loxodes, Ehrb.; Loxophyllum, Duj.; Lionotus, Wrez.; Trachelius, Schrank; Amphileptus, Ehrb.; Actinobolus, St.; Didinium, St.; Scaphiodon, St; Dysteria, Huxl.; Coleps, Nitzsch.; Dileptus, Duj.; Ileonema, Stokes; Mesodinium, St. | |
| (II.) Mouth permanently open, usually equipped with one or more undulating membranes, receiving food by ciliary action (Trichostomata, Bütschli) | |
| (a) Cilia nearly uniform, usually extending over the whole body, without any special adoral wreath of long cilia or membranellae; mouth with one or two undulating membranes at its margin or extending into the short pharynx. | Order 2. Aspirotrichaceae. |
| Paramecium, Hill; Colpoda, O. F. Müll.; Colpidium, St.; Leucophrys, Ehrb.; Cyclidium, Cl. and L.; Lembadion, Perty; Cinetochilum, Perty; Pleuronema, Duj.; Ancistrum, Maup.; Glaucoma, Ehrb.; Uronema, Duj.; Lembus, Cohn; Urocentrum, Nitzsch; Icthyophtheirius, Fouquet. | |
| (b) Strong cilia or membranellae forming an adoral wreath, and bounding a more or less enclosed area, the "peristome," at one point of which the mouth lies. | |
| (i.) Body more or less equally covered with fine cilia; adoral wreath an open spiral | Order 3. Heterotrichaceae |
| Spirostomum, Ehrb.; Bursaria, O. F. Müll.; Stentor, Oken; Folliculina, Lamk.; Conchophtheirus, St.; Balantidium, Cl. and L.; Nyctotherus, Leidy; Metopus, Cl. and L.; Caenomorpha, Perty; Discomorpha, Levander; Blepharisma, Perty. | |
| (ii.) Body cilia limited in distribution or absent; peristome anterior, nearly circular, sinistrorse. | Order 4. Oligotrichaceae. |
| Halteria, Duj.; Maryna, Gruber; Tintinnus, Schrank; Dictyocystis, Ehrb.; Strombidium, Cl. and L. (= Torquatella, Lank.). | |
| (iii.) Peristome extending backwards along the ventral face, which alone is provided with motile cirrhi, etc.; dorsal cilia fine, motionless. | Order 5. Hypotrichaceae. |
| {138}
Stylonychia, Ehrb.; Kerona, O. F. Müll.; Oxytricha, Ehrb.; Euplotes, Ehrb.; Stichotricha, Perty; Schizotricha, Gruber. |
|
| (iv.) Body cilia reduced to a posterior girdle, or temporarily or permanently absent; peristome anterior, nearly circular, edged by the adoral wreath,[153] bounded by a gutter edged by an elevated rim or collar. | Order 6. Peritrichaceae. |
| Lichnophora, Cl.; Trichodina, Ehrb.; Vorticella, L.; Zoothamnium, Bory; Carchesium, Ehrb.; Epistylis, Ehrb.; Opercularia, Lamk.; Vaginicola, Lamk.; Pyxicola, Kent; Cothurnia, Ehrb.; Scyphidia, Lachmann; Ophrydium, Bory; Spirochona, St. | |
The Ciliata have so complex an organisation that, as with the Metazoa, it is well to begin with the description of a definite type. For this purpose we select Stylonychia mytilus, Ehrb. (Fig. 49), a species common in water rich in organic matter, and relatively large (1⁄75" = ⅓ mm.). It is broadly oval in outline, with the wide end anterior, truncate, and sloping to the left side behind; the back is convex, thinning greatly in front; the belly flat. It moves through the water either by continuous swimming or by jerks, and can either crawl steadily over the surface of a solid or an air surface such as an air bubble, or advance by springs, which recall those of a hunting spider. The boundary is everywhere a thin plasmic pellicle, very tender, and readily undergoing diffluence like the rest of the cell. From the pellicle pass the cilia, which are organically connected with it, though they may be traced a little deeper; they are arranged in slanting longitudinal rows, and are much and variously modified, according to their place and function. On the edge of the dorsal surface they are fine and motionless, probably only sensory (s.h.); except three, which protrude well over the hinder end (c.p.), stout, pointed, and frayed out at the ends, and possibly serving as oars or rudders for the darting movements. These are distinguished from simple cilia as "cirrhi."

Fig. 49.—Ventral view of Stylonychia mytilus. a.c, Abdominal cirrhi; an, anus discharging the shell of a Diatom; c.c, caudal cirrhi; c.p, dorsal cirrhi; cv, contractile vacuole; e, part of its replenishing canal; f.c, frontal cirrhi; f.v, food vacuoles; g, internal undulating membrane; l, lip; m, mouth or pharynx; mc, marginal cirrhi; N, N, lobes of meganucleus; n, n, micronuclei; o, anterior end; per, adoral membranellae; poc, preoral cilia; p.om, preoral undulating membrane; s.h, sense hairs. (Modified from Lang.)
At the right hand of the frontal area there begins, just within the dorsal edge, a row of strong cilium-like organs (Fig. 49, per); these, on careful examination, prove to be transverse triangular plates, which after death may fray into cilia.[154] They are the "adoral membranellae." This row passes to the left blunt angle, and there crosses over the edge of the body to the ventral aspect, and then curves inwards towards the median line, which it reaches about half-way back, where it passes into the pharynx (m). It forms the front and left-hand boundary of a wedge-shaped depression, the "peristomial area," the right-hand boundary being the "preoral ridge" or lip (l), which runs nearly on the median line, projecting downward and over the depression. This ridge bears on its inner and upper side a row of fine "preoral cilia" (poc) and a wide "preoral undulating membrane" (p.om), which extends horizontally across, below the peristomial area. The roof of this area bears along its right-hand edge an "internal undulating membrane" (g), and then, as we pass across to the left, first an "endoral membrane" and then an "endoral" row of cilia. In some allied genera (not in Stylonychia), at the base and on the inner side of each adoral membranella, is a "paroral" cilium. {140}All these motile organs, with the exception of the preoral cilia, pass into the pharynx; but the adoral membranellae soon stop short for want of room. There are some seventy membranellae in the adoral wreath.
The rest of the ventral surface is marked by longitudinal lines, along which the remaining appendages are disposed. On either side is a row of "marginal cirrhi" (mc.), which, like the membranellae, may fray out into cilia, but are habitually stiff spine-like, and straight in these rows; these are the chief swimming organs. Other cirrhi, also arranged along longitudinal rows, with so many blank spaces that the arrangement has to be carefully looked for, occur in groups along the ventral surface. On the right of the peristome are a group which are all curved—the "frontal cirrhi" (f.c.). Behind the mouth is a second group—the "abdominal cirrhi" (a.c.), also curved hooks; and behind these again the straight spine-like "caudal" or "anal" cirrhi (c.c), which point backwards. These three sets of ventral cirrhi are the organs by which the animal executes its crawling and darting movements. Besides the mouth there are two other openings, both indistinguishable save at the very moment of discharge; the anus (an) which is dorsal, and the pore of the contractile vacuole, which is ventral.
The protoplasm of the body is sharply marked off into a soft, semi-fluid "endoplasm" or "endosarc," and a firmer "ectoplasm" or "ectosarc." The former is rich in granules of various kinds, and in food-vacuoles wherein the food is digested. The mode of ingestion, etc., is described below (p. 145). The ectoplasm is honeycombed with alveoli of definite arrangement, the majority being radial to the surface or elongated channels running lengthwise; inside each of these lies a contractile plasmic streak or myoneme. The contractile vacuole (cv) lies in this layer, a little behind the mouth, and is in connexion with two canals, an anterior (e) and a posterior, from which it is replenished.
The nuclear apparatus lies on the inner boundary of the ectoplasm; it consists of (1) a large "meganucleus" formed of two ovoid lobes (N, N), united by a slender thread; and (2) two minute "micronuclei" (n, n), one against either lobe of the meganucleus.
Stylonychia multiplies by transverse fission, the details of which are considered on pp. 144, 147.
The protoplasm of Ciliata is the most differentiated that we {141}find in the Protista, and we can speak without exaggeration of the "organs" formed thereby.
The form of the body, determined by the firm pellicle or plasmic membrane, is fairly constant for each species, though it may be subject to temporary flexures and contractions. The pellicle varies in rigidity; where the cilia are abundant it is proportionately delicate, and scarcely differs from the ectoplasm proper, save for not being alveolate. In the Peritrichaceae it is especially resistant and proof against decay. In Coleps (Gymnostomaceae) it is hardened and sculptured into the semblance of plate-armour, and the prominent points of the plates around the mouth serve as teeth to lacerate other active Protista, its prey; but, like the rest of the protoplasm, this disappears by decay soon after the death of the Coleps. Where, as in certain Oligotrichaceae, cilia are absent over part of the body, the pellicle is hardened; and on the dorsal face and sides of Dysteria it even assumes the character of a bivalve shell, and forms a tooth-like armature about the mouth.
From the pellicle protrude the cilia, each of which is continued inwards by a slender basal filament to end in a "basal granule" or "blepharoplast." The body-cilia are fine, and often reversible in action, which is exceptional in the organic world. They may be modified or combined in various ways. We have seen that in Stylonychia some are motionless sensory hairs. The cirrhi and setae sometimes fray out during life, and often after death, into a brush at the tip, and have a number of blepharoplasts at their base. The same holds good for the membranellae and undulating membranes. They are thus comparable to the "vibratile styles" of Rotifers (Vol. II. p. 202) and the "combs" or "Ctenophoral plates" of the Ctenophora (p. 412 f.).[155]
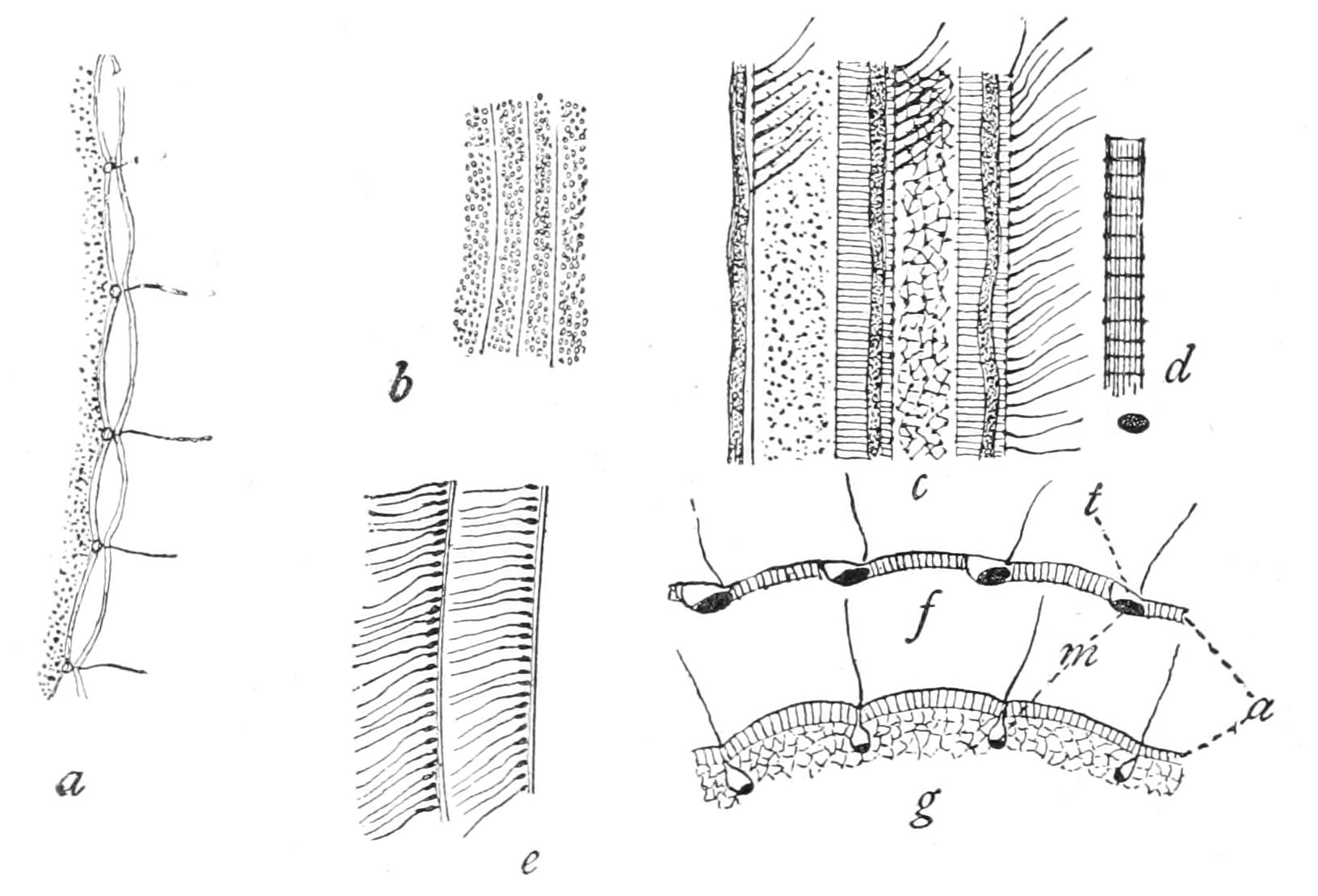
Fig. 50.—Ectosarc of Ciliata. a-f, from Stentor coeruleus; g, Holophrya discolor. a, Transverse section, showing cilia, pellicle, canals, and myonemes; b, surface view below pellicle, showing myonemes alternating with blue granular streaks; c, more superficial view, showing rows of cilia adjacent to myonemes; d, myoneme, highly magnified, showing longitudinal and transverse striation; e, two rows of cilia; f, g, optical sections of ectosarc, showing pellicle, alveolar layer (a), myonemes (m), and canals in ectosarc. (From Calkins, after Metschnikoff, Bütschli, and Johnson.)
The ectosarc has a very complex structure. Like other protoplasm it has a honeycombed or alveolate structure, but in this case the alveoli are permanent in their arrangement and position. Rows of these alveoli run under the surface; and the cilia are given off from their nodal points where the vertical walls of several unite, and wherein the basal granule or blepharoplast is contained. Longitudinal threads running along the inner walls of the alveoli of the superficial layer are differentiated into muscular fibrils or "myonemes," to which structures so many owe their marked longitudinal striation on the one hand, and their power of sudden contraction on the other. The appearance of transverse striation may be either due to transverse myonemes, or produced by the folds into which the contraction of longitudinal fibrils habitually wrinkles the pellicles, when it is fairly dense (Peritrichaceae); circular muscular fibrils, however, undoubtedly exist in the peristomial collar of this group. Embedded in the ectosarc are often found trichocysts,[156] analogous {143}to the nematocysts of the Coelenterata (p. 247), and doubtless fulfilling a similar purpose, offensive and defensive. A trichocyst is an oblong sac (4 µ long in Paramecium) at right angles to the surface, which on irritation, chemical (by tannin, acids, etc.) or mechanical, emits or is converted into a thread several times the length of the cilia (33 µ), often barbed at the tip. In the predaceous Gymnostomaceae, such as Didinium, the trichocysts around (or even within) the mouth are of exceptional size, and are ejected to paralyse, and ultimately to kill, the active Infusoria on which they feed. In most of the Peritrichaceae they are, when present, limited to the rim around the peristome, while in the majority of species of Ciliata they have not been described. Fibrils, possibly nervous,[157] have been described in the deepest layer of the ectosarc in Heterotrichaceae.
The innermost layer of the ectosarc is often channelled by a system of canals,[158] usually inconspicuous, as they discharge continuously into the contractile vacuole; but by inducing partial asphyxia (e.g. by not renewing the limited supply of air dissolved in the drop of water on the slide under the cover-glass), the action of the vacuole is slackened, and these canals may be more readily demonstrated. The vacuole, after disappearance, forms anew either by the coalescence of minute formative vacuoles, or by the enlargement of the severed end of the canal or canals. The pore of discharge to the surface is visible in several species, even in the intervals of contraction.[159] The pore is sometimes near that of the anus, but is only associated with it in Peritrichaceae, where it opens beside it into the vestibule or first part of the long pharynx, often through a rounded reservoir (Fig. 60, r) or elongated canal.
The endosarc, in most Ciliates well differentiated from the ectosarc, is very soft; though it is not in constant rotation like that of a Rhizopod, it is the seat of circulatory movements alternating with long periods of rest. Thus it is that the food-vacuoles, after describing a more or less erratic course, come to discharge their undigested products at the one point, the anus. {144}In a few genera (Didinium, for instance) the course from mouth to anus is a direct straight line, and one may almost speak of a digestive tract. In Loxodes and Trachelius (Fig. 56) the endosarc, as in the Flagellate Noctiluca (Fig. 48, p. 133), has a central mass into which the food is taken, and which sends out lobes, which branch as they approach and join the ectoplasm. The endosarc contains excretory granules, probably calcium phosphate, droplets of oil or dissolved glycogen, proteid spherules, paraglycogen grains, etc.
The nuclear apparatus lies at the inner boundary of the ectoplasm. The "meganucleus" may be ovoid, elongated, or composed of two or more rounded lobes connected by slender bridges (Stentor, Stylonychia). The "micronucleus" may be single; but even when the meganucleus is not lobed it may be accompanied by more than one micronucleus, and when it is lobed there is at least one micronucleus to each of its lobes.[160] The meganucleus often presents distinct granules of more deeply staining material, varying with the state of nutrition; these are especially visible in the band-like meganuclei of the Peritrichaceae (Figs. 51, 60). At the approach of fission it is in many cases distinctly fibrillated.[161] But all other internal differentiation, as well as any constriction, then disappears; and the ovoid or rounded figure becomes elongated and hour-glass shaped, and finally constricts into two ovoid daughter-meganuclei, which, during and after the fission of the cell, gradually assume the form characteristic of the species. The micronuclei (each and all when they are multiple) divide by modification of karyokinesis (or "mitosis") as a prelude to fission: in this process the chromatin is resolved into threads which divide longitudinally, but the nuclear wall {145}remains intact. If an Infusorian be divided into small parts, only such as possess a micronucleus and a fragment of the meganucleus are capable of survival. We shall see how important a part the micronuclei play in conjugation, a process in which the old meganuclei are completely disorganised and broken up and their débris expelled or digested.
The mouth of the Gymnostomaceae is habitually closed, opening only for the ingestion of the living Protista that form their prey. It usually opens into a funnel-shaped pharynx, strengthened with a circle of firm longitudinal bars, recalling the mouth of an eel-trap or lobster-pot ("Reusenapparat" of the Germans); and this is sometimes protrusible. In Dysteria the rods are replaced by a complicated arrangement of jaw- or tooth-like thickenings, which are not yet adequately described. We have above noted the strong adoral trichocysts in this group.
In all other Ciliates[162] the "mouth" is a permanent depression lined by a prolongation of the pellicle, and containing cilia and one or more undulating membranes, and when adoral membranellae are present, a continuation of these. In some species, such as Pleuronema (Fig. 57), one or two large membranes border the mouth right and left. In Peritrichaceae the first part of the pharynx is distinguished as the "vestibule," since it receives the openings of the contractile vacuole or its reservoir and the anus. The pharynx at its lower end (after a course exceptionally long and devious in the Peritrichaceae; Figs. 51, 60) ends against the soft endosarc, where the food-particles accumulate into a rounded pellet; this grows by accretion of fresh material until it passes into the endosarc, which closes up behind it with a sort of lurch. Around the pellet liquid is secreted to form the food-vacuole. If the material supplied be coloured and insoluble, like indigo or carmine, the vacuoles may be traced in a sort of irregular, discontinuous circulation through the endosarc until their remains are finally discharged as faeces through the anus. No prettier sight can be watched under the microscope than that of a colony of the social Bell-animalcule (Carchesium) in coloured water—all producing food-currents brilliantly shown up by the wild eddies of the pigment granules, and the vivid blue or crimson colour of {146}the food-vacuoles, the whole combining to present a most attractive picture. Ehrenberg fancied that a continuous tube joined up the vacuoles, and interpreted them as so many stomachs threaded, as it were, along a slender gut; whence he named the group "Polygastrica."
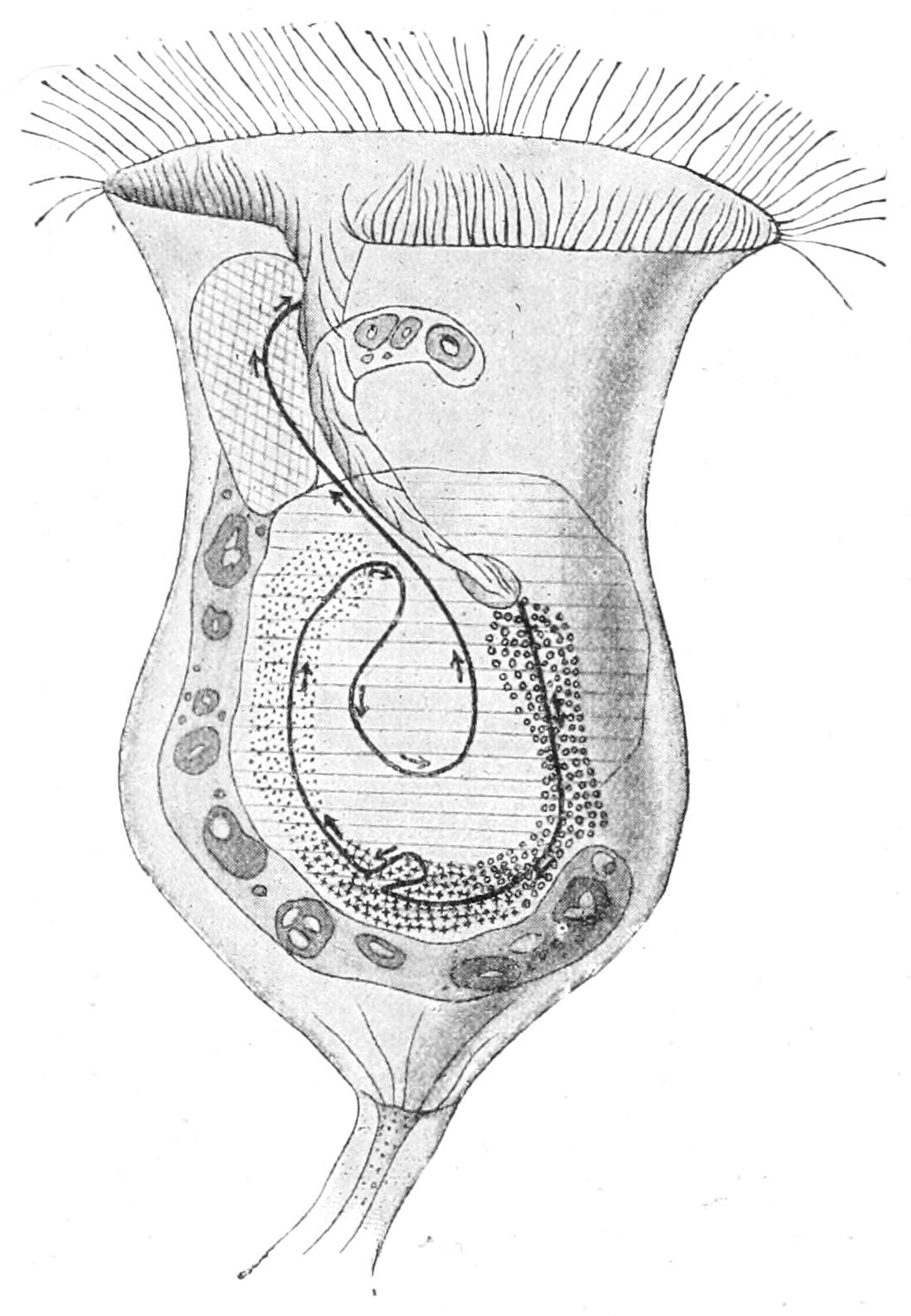
Fig. 51.—Carchesium polypinum. Scheme of the path taken by the ingested food in digestion and expulsion of the excreta. The food enters through the pharynx and is transported downward (small circles), where it is stored in the concavity of the sausage-shaped meganucleus (the latter is recognised by its containing darker bodies). It remains here for some time at rest (small crosses). Then it passes upward upon the other side (dots) and returns to the middle of the cell, where it undergoes solution. The excreta are removed to the outside, through the vestibule and cell mouth. The black line with arrows indicates the direction of the path. (From Verworn, after Greenwood.)
We owe to Miss Greenwood[163] a full account of the formation and changes of the food-vacuoles in Carchesium polypinum. The vacuole passes steadily along the endosarc for a certain time after its sudden admission into it, and then enters on a phase of quiescence. A little later the contents of the vacuole aggregate together in the centre of the vacuole, where they are surrounded by a zone of clear liquid; this takes place in the hollow of the meganucleus, in this species horseshoe-shaped. The vacuole then slowly passes on towards the peristome, lying deep in the endosarc, and the fluid peripheral zone is absorbed. {147}For some time no change is shown in the food-material itself: this is the stage of "storage." Eventually a fresh zone of liquid, the true digestive vacuole, forms again round the food-pellet, and this contains a peptic juice, of acid reaction. The contents, so far as they are capable of being digested, liquefy and disappear. Ultimately the solid particles in their vacuole reach the anal area of the vestibule, and pass into it, to be swept away by the overflow of the food-current. The anus is seated on a transverse ridge about a third down the tube, the remaining two-thirds being the true pharynx.
Fission is usually transverse; but is oblique in the conical Heterotrichaceae, and longitudinal in the Peritrichaceae. It involves the peristome, of which one of the two sisters receives the greater, the other the lesser part; each regenerates what is missing. When there are two contractile vacuoles, as in Paramecium, either sister receives one, and has to form another; where there is a canal or reservoir divided at fission, an extension of this serves to give rise to a new vacuole in that sister which does not retain the old one. In some cases the fission is so unequal as to have the character of budding (Spirochona). We have described above (p. 144) the relations of the nuclear apparatus in fission.
Several of the Ciliata divide only when encysted, and then the divisions are in close succession, forming a brood of four, rarely more. This is well seen in the common Colpoda cucullus. In the majority, however, encystment is resorted to only as a means of protection against drought, etc., or for quiet rest after a full meal (Lacrymaria).
Maupas[164] has made a very full study of the life-cycles of the Ciliata. He cultivated them under the usual conditions for microscopic study, i.e. on a slide under a thin glass cover supported by bristles to avoid pressure, preserved in a special moist chamber; and examined them at regular intervals.
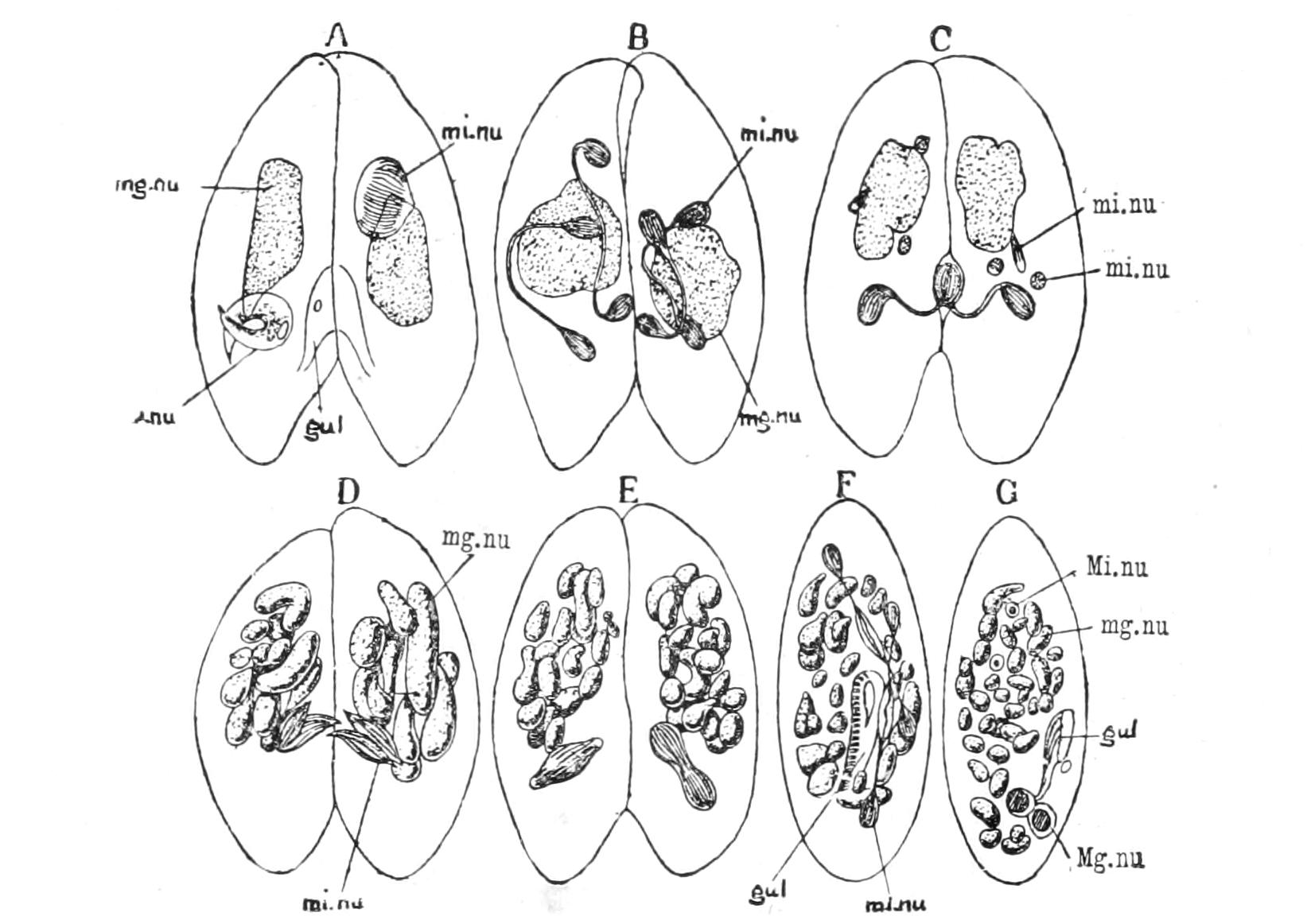
Fig. 52.—Paramecium caudatum, stages in conjugation. gul, Gullet; mg.nu, meganucleus; Mg.nu, reconstructed meganucleus; mi.nu, micronucleus; Mi.nu, reconstructed micronucleus; o, mouth. (From Parker and Haswell, after Hertwig.)
The animals collect at that zone where the conditions of aeration are most suitable, usually just within the edge of the cover, and when well supplied with food are rather sluggish, not swimming far, so that they are easily studied and counted. When well supplied with appropriate food they undergo binary fission at frequent intervals, dividing as often as five times in the twenty-four hours at a temperature of 65-69° F. (Glaucoma scintillans), so that in this period a single individual has resolved itself into a posterity of 32; but such a rapid increase is exceptional. At a minimum and a maximum temperature multiplication is arrested, the optimum lying midway. If the food-supply is cut off, encystment occurs in those species capable of the process; but when there is a mixture of members of different broods of the same species, subject to the limitations that we shall learn, conjugation ensues. Under the conditions of Maupas' investigations he found a limit to the possibilities of continuous fissions, even when interrupted by occasional encystment. The individuals of a series ultimately dwindle in size, their ciliary apparatus is reduced, and their nuclear apparatus degenerates. Thus the ultimate members of a fission-cycle show a progressive decay, notably in the nuclear apparatus, which Maupas has aptly compared to "senility" or "old age" in the Metazoan. If by the timely mixture of broods conjugation be induced, these senile degenerations do not occur.[165] In Stylonychia {149}mytilus the produce of a being after conjugation died of senility after 336 fissions; in Leucophrys after 660.
Save in the Peritrichaceae (p. 151) conjugation takes place between similar mates, either of the general character and size of the species, or reduced by fissions, in rapid succession, induced by the same conditions as those of mating. The two mates approach, lying parallel and with their oral faces or their sides (Stentor) together, and partially fuse thereby; though no passage of cytoplasm is seen it is probable that there is some interchange or mixture.[166]

Fig. 53.—Diagram of conjugation in Colpidium colpoda. Horizontal line means degeneration; parallel vertical lines, separation of gametes; broken lines (above), boundary between pairing animals; (below), first fission; single vertical line, continuity or enlargement. M, Meganucleus; µ, micronucleus; Z, zygote-nucleus.

Fig. 54.—Four individuals of Coleps hirtus (Gymnostomaceae) swarming about and ingesting a Vorticella (?) (From Verworn.)
The meganucleus lengthens, becomes irregularly constricted, and breaks up into fragments, which are ultimately extruded or partially digested. The micronucleus enlarges (Fig. 52, A) and undergoes three successive divisions, or, strictly speaking, two fissions producing four nuclei, of which one only undergoes the third. The other three nuclei of the second fission degenerate like the meganucleus.[167] Of the two micronuclei of this last division one remains where it is as a "stationary" pairing nucleus, while its sister passes as a "migratory" pairing-nucleus into the other mate, and fuses with its stationary pairing-nucleus. Thus in either mate is formed a "zygote-nucleus," or "fusion-nucleus." All these processes are simultaneous in the two mates; and the migratory nuclei cross one another on the bridge of junction of the two mates (Fig. 52, C). Each mate now has its original cytoplasm (subject to the qualification above), {151}but its old nuclear apparatus is replaced by the fusion-nucleus. This new nucleus undergoes repeated fissions; its offspring enlarge unequally, the larger being differentiated as mega-, the smaller as micro-nuclei. The mates now separate (Fig. 52, F, G), and by the first (or subsequent) fission of each, the new mega- and micro-nuclei are distributed to the offspring. Colpidium colpoda offers the simplest case, on which we have founded our diagram showing the nuclear relations. During conjugation the oral apparatus often atrophies, and is regenerated; and in some cases the pellicle and ciliary apparatus are also "made over."

Fig. 55.—Paramecium caudatum (Aspirotrichaceae). A, The living animal from the ventral aspect; B, the same in optical section, the arrows show the course taken by food-particles. buc.gr, Buccal groove; cort, cortex; cu, cuticle; c.vac, contractile vacuole; f.vac, food vacuole; gul, gullet; med, medulla; mth, mouth; nu, meganucleus; pa.nu, micronucleus; trch, trichocysts discharged. (From Parker's Biology.)
In the Peritrichaceae the mates are unequal; the larger is the normal cell, and is fixed; the smaller, mobile, is derived from an ordinary individual by brood-divisions, which only occur under the conditions that induce conjugation (Fig. 60). Here, though the two pairs of nuclei are formed, it is only the migratory {152}nuclei that unite, the stationary ones aborting in both mates. During the final processes of conjugation the smaller mate is absorbed into the body of the larger, and so plays the part of male there. But this process, though one of true binary sex, is clearly derived from the peculiar type of equal reciprocal conjugation of the other Infusoria.
The Ciliata are almost all free-swimming animals with the exception of most of the Peritrichaceae, and of the genera we now cite. Folliculina forms a sessile tube open at either end; and Schizotricha socialis inhabits the open mouths of a branching gelatinous tubular stem, obviously secreted by the hinder end of the animal, and forking at each fission to receive the produce. A similar habit to the latter characterises Maryna socialis; all three species are marine, and were described by Gruber.[168] Stentor habitually attaches itself by processes recalling pseudopodia, and often forms a gelatinous sheath.
The majority of the Oligotrichaceous Tintinnidae inhabit free chitinous tests often beautifully fenestrated, as in Dictyocystis.
Many genera are parasitic in the alimentary canal of various Metazoa, but none appear to be seriously harmful except Ichthyophtheirius, which causes an epidemic in fresh-water fish. Quite a peculiar fauna inhabit the paunch of Ruminants. Nyctotherus and Balantidium are occasionally found in the alimentary canal of Man.[169]
The Gymnostomaceae are predaceous, feeding for the most part on smaller Ciliates. We have described the peculiar character of the mouth and pharynx in this group, and the mail-like pellicle of Coleps (Fig. 54). Loxophyllum is remarkable for the absence of cilia from one of the sides of its flattened body, and the tufts of trichocysts studding its dorsal edge at regular intervals. Actinobolus has numerous tentacles, exsertile and retractile, each bearing a terminal tuft of trichocysts, which serve to paralyse such active prey as Halteria. Ileonema has one tentacle overhanging the mouth; and Mesodinium has four short sucker-like projections around it.[170] It has only two girdles {153}of cilia, which are stout and resemble fine-pointed cirrhi. In Dysteria the cilia are exclusively ventral, and the naked dorsal surface has its pellicle condensed into a bivalve shell; a posterior motile process ("foot") and a complex pharyngeal armature add to the exceptional characters of the genus.
The Aspirotrichaceae are well known to every student of "Elementary Biology" by the "type" Paramecium (Fig. 55), so common in infusions, especially when containing a little animal matter. P. bursaria often contains in its endosarc the green symbiotic Flagellate Zoochlorella. Colpoda cucullus, very frequent in vegetable infusions, usually only divides during encystment, and forms a brood of four. Pleuronema chrysalis (Fig. 57) is remarkable for its habit of lying for long periods on its side and for its immense undulating membrane, forming a lip on the left of its mouth; Glaucoma has two, right and left.
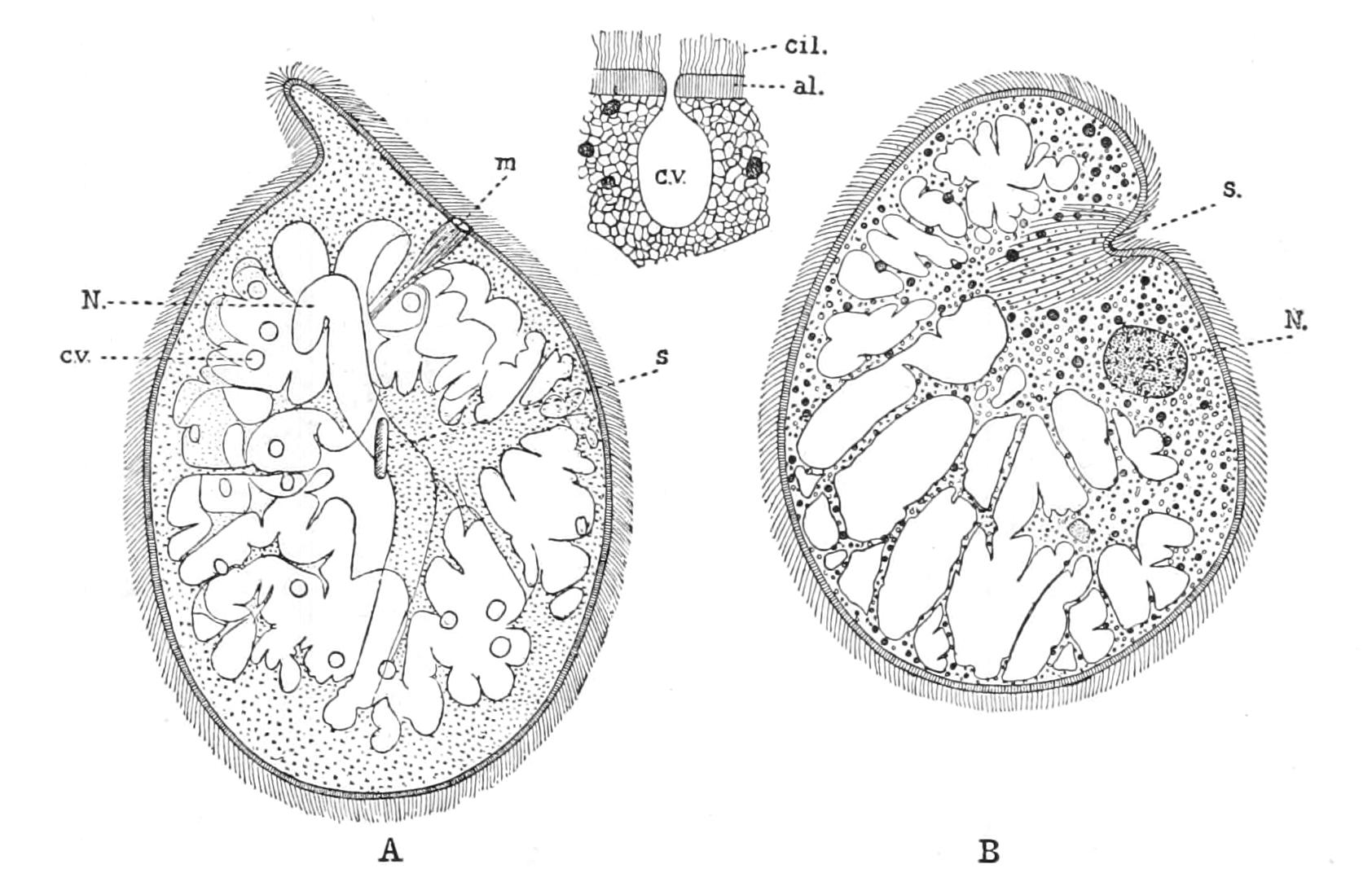
Fig. 56.—Trachelius ovum. A, general view; B, section through sucker; C, section through contractile vacuole and its pore of discharge. al, Alveolar layer of ectoplasm; cil, cilia; c.v, contractile vacuole; m, mouth; N, meganucleus; s, sucker, from which pass inwards retractile myonemes. (After Clara Hamburger.)
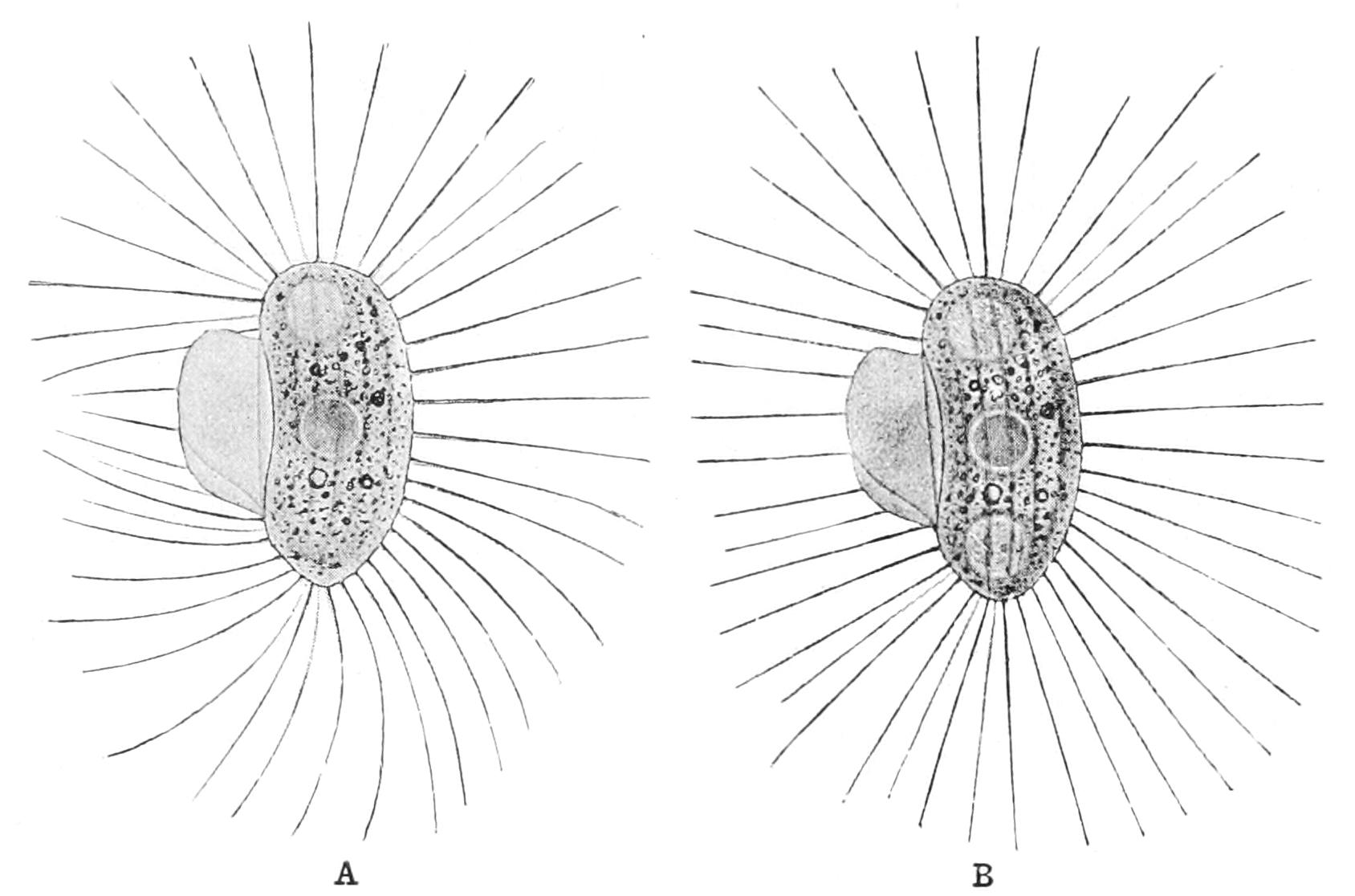
Fig. 57.—Pleuronema chrysalis (Aspirotrichaceae). A, Unstimulated, lying quiet; B, stimulated, in the act of springing by the stroke of its cilia. (From Verworn.)
The Heterotrichaceae present very remarkable forms. Spirostomum is nearly cylindrical, and, a very giant, may attain a length of 4 mm. (1⁄6"). Stentor can attach itself by its hinder end, which is then finely tapered and prolonged into a few pseudopodia; its body is trumpet-shaped, with a spiral peristome forming a coil round its wide end, and leading on the left side into the mouth. Many species when attached secrete a gelatinous sheath or tube. S. polymorphus is often coloured green by Zoochlorella (p. 125); S. coeruleus[171] and S. igneus owe their names to the brilliant pigment, blue or scarlet, deposited in granules in lines between the conspicuous longitudinal myonemes. From their large size and elongated meganucleus accompanied by numerous micronuclei, these two genera have frequently been utilised for experiments on regeneration. In Metopus sigmoides the peristomial area forms a dome above its wreath of membranellae; and in M. pyriformis this is so great as to form the larger part of the cell, which is top-shaped, tapering behind to a point. Caenomorpha (Fig. 58) has the same general form, with a peg-like tail, and possesses a girdle of cirrhi.[172] The converse occurs in {155}Bursaria; the cell is a half ellipse, something like a common twin tobacco-pouch when closed: a deep depression thus occupies the whole ventral surface, and opens by a wide slit extending along the anterior end. The peristomial area occupies the dorsal side of the pocket so formed, and the mouth is in the hinder left-hand corner. Blepharisma sp. is parasitic in the Heliozoon Raphidiophrys viridis (Fig. 20, 1, p. 74).

Fig. 58.—Caenomorpha uniserialis. crh, Zone of cirrhi; c.t, cilia of tail; c.v, contractile vacuole; c.w, ciliary wreath; g, granular aggregate; m, zone of membranellae; N, meganucleus; n, micronucleus; oe, pharynx; t, tail-spine; t1, accessory spine; u.m, undulating membrane; v, vacuole; z, precaudal process. (After Levander.)
Among Oligotrichaceae, Halteria, common among the débris at the bottom of pools in woods containing dead leaves, is remarkable for an equatorial girdle of very long fine setae, and for its rapid erratic darting movements, alternating with a graceful bird-like hover. The Tintinnidae are mostly marine, pelagic, with the general look of a stalkless Vorticella; some have a latticed chitinous shell.[173]

Fig. 59.—Stentor polymorphus. I, Young individual attached, extended; II, adult in fission, contracted; cv in I, afferent canal of contractile vacuole; in II, contractile vacuole; N, moniliform meganucleus (micronuclei omitted); o, mouth; the fine lines are the myoneme fibrils. (From Verworn.)
Among Peritrichaceae, Vorticella (Fig. 60) and its allies have long been known as Bell-animalcules to every student of pond-life. The body has indeed the form of an inverted bell, closed at its mouth by the "peristome," or oral disc; this is a short, inverted truncate cone set obliquely so that its wide base hardly projects at one side, but is tilted high on the other; the edge of the bell is turned out into a rim or "collar," separated from the disc by a deep gutter. The collar, habitually everted, or even turned down, contracts over the retracted disc when the animal is retracted (E2), which is brought about by any sort of shock, or when it swims freely backwards. For the latter purpose a posterior ring of cilia (or rather membranellae) is developed round the hinder end of the bell (A, cr, E3). The cilia of the adoral wreath are very strong, united at the base into a continuous membrane, and indeed themselves partake of the composite nature of membranellae. The wreath forms more than one turn of a right-handed spiral, the innermost turn ending abruptly on the disc, the outer leading down into the mouth at the point where the disc is most tilted and the groove deepest.[174] The pharynx (p) is long, and contains an undulating membrane (u.m) on its inner side projecting out through the mouth, and numerous cilia; it leads deep into the body (p). The first part is distinguished as the "vestibule" (v), as into it opens the anus, and the contractile vacuole (c.v.), the latter sometimes opening by a reservoir (r). The body contains in the ectoplasm {157}myonema-fibrils which, by their contraction, withdraw the disc, and at the same time circular fibrils close the peristome over it. In the type-genus the pellicle is continued into a long, slender elastic stalk (s), of which the longitudinal myoneme fibrils of the ectoplasm converge to the stalk, and are prolonged into it as a spirally winding fibre, sometimes transversely striated.[175] The effect of the contraction of this is to pull the stalk into a helicoid spiral (like a coil-spring), with the line of insertion of the muscle along the inner side of the coils, which is, of course, the shortest path from one end to the other (Fig. 60, B).
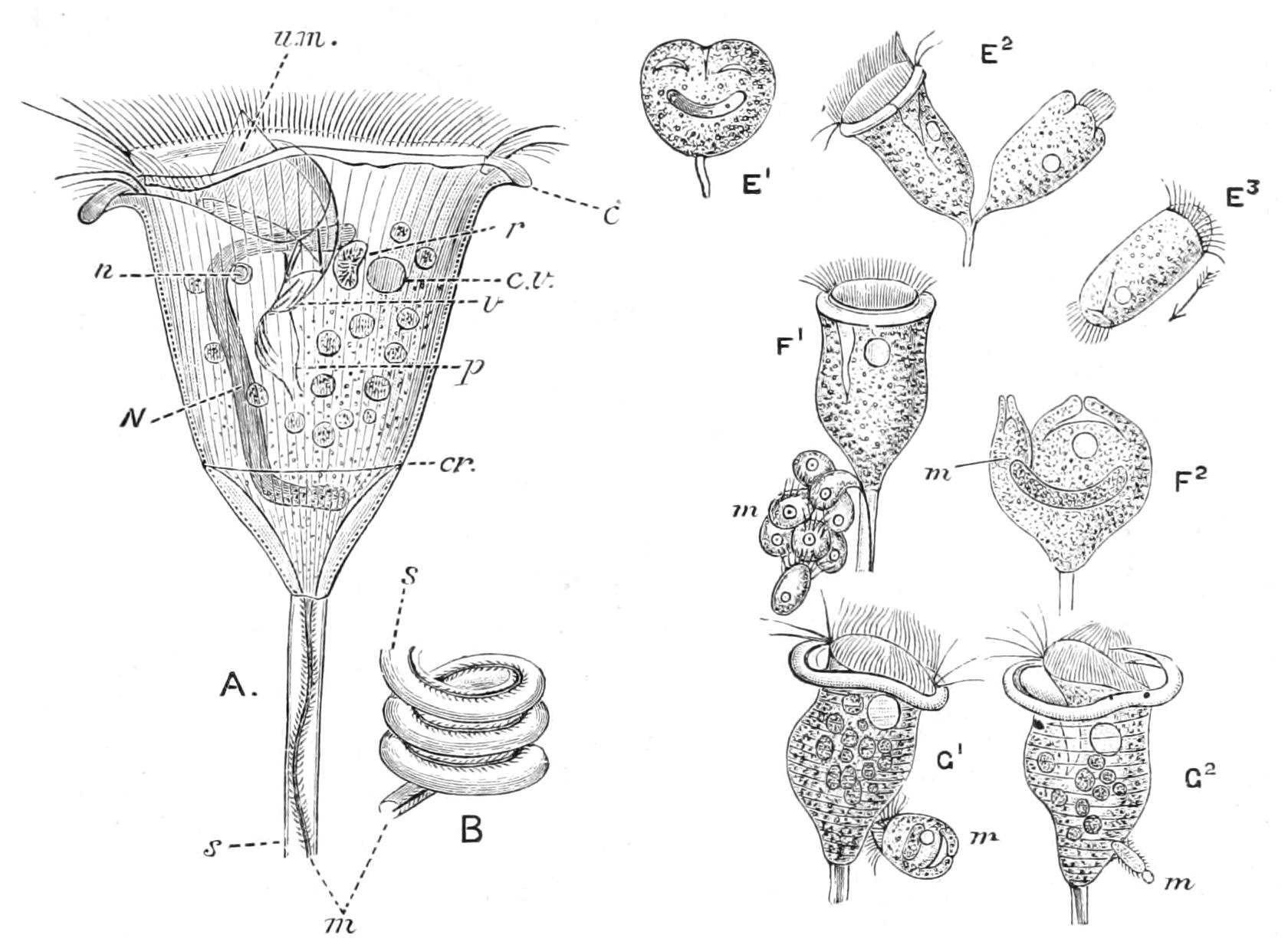
Fig. 60.—Vorticella. A, expanded; B, stalk in contraction; c, eversible collar below peristome; cr, line of posterior ciliary ring; c.v, contractile vacuole; m, muscle of stalk; N, meganucleus; n, micronucleus; p, pharynx; r, reservoir of contractile vacuole; s, tubular stalk; u.m, undulating membrane in vestibule; v, hinder end of vestibule. E1, E2, two stages in binary fission; E3, free zooid, with posterior wreath; F1, F2 division into mega- and micro-zooids (m); G1, G2, conjugation; m, microzooid. (Modified from Bütschli, from Parker and Haswell.)
The members of the Vorticellidae are very commonly attached to weeds or to various aquatic Metazoa, each species being more or less restricted in its haunts. Vorticella, the type, is singly {158}attached to a contractile stalk; fission takes place in the vertical plane, and one of the two so formed retains the original stalk, while the other swims off (Fig. 60, E1-E3), often to settle close by, so that the individuals are found in large social aggregates, side by side, fringing water-weeds with a halo visible to the naked eye, which disappears on agitation by the sudden contraction of all the stalks. Carchesium and Zoothamnium differ from Vorticella in the fact that the one daughter-cell remains attached by a stalk coming off a little below the body of the other, so as to give rise to large branching colonies.
In Carchesium (Fig. 51) the muscular threads of each cell are separate, while in Zoothamnium they are continuous throughout the colony. Epistylis has a solid, rigid stalk, and may give rise to branching colonies, which often infest the body of the Water-Fleas (Copepoda) of the genus Cyclops. Opercularia is characterised by the depth of the gutter, the height of the collar, and the tapering downward of the elongated disc. Vaginicola, Pyxicola, Cothurnia, Scyphidia, all inhabit tubes, some of extreme elegance. Ophrydium is a colonial form, found in ponds and ditches, resembling Opercularia, but inhabiting tubes of jelly[176] that coalesce by their outer walls into a large floating sphere; it usually contains the green symbiotic Flagellate Zoochlorella. Trichodina is free, short, and cylindrical, with both wreaths permanently exposed, and is provided with a circlet of hooks within the aboral wreath. It is often parasitic, or perhaps rather epizoic, on the surface of Hydra (see p. 254), gliding over its body[177] with a graceful waltzing movement; it occurs also in the bladder and genito-urinary passages of Newts, and even in their body-cavity and kidneys.
II. Suctoria = Tentaculifera
Infusoria with cilia only in the young state,[178] without mouth or anus, but absorbing food (usually living Ciliates) by one or more tentacles, perforated at the apex; mostly attached, frequently epizoic, rarely parasitic in the interior of other Protozoa.
Acineta, Ehrb. (Fig. 61, 2); Amoebophrya, Koppen; Choanophrya, Hartog (Fig. 62); Dendrocometes, St. (Fig. 61, 4); Dendrosoma, Ehrb. (Fig. 61, 9); Endosphaera, Engelm.; Ephelota, Str. Wright (Fig. 61, 5, 8); Hypocoma, Gruber; Ophryodendron, Cl. and L. (Fig. 61, 7); Podophrya, Ehrb. (Fig. 61, 1); Rhyncheta, Zenker (Fig. 61, 3); Sphaerophrya, Cl. and L. (Fig. 61, 6), Suctorella, Frenzel; Tokophrya, Bütschli.
This group, despite a superficial resemblance to the Heliozoa, show a close affinity to the Ciliata; the nuclear apparatus is usually double though a micronucleus is not always seen; the young are always ciliated, and the mode of conjugation is identical in all cases hitherto studied. Most of the genera are attached by a chitinous stalk (Fig. 61), continued in Acineta into a cup or "theca" surrounding the cell. The pellicle is firm, often minutely shagreened or "milled" in optical section by fine radial processes, whether superficial rods or the expression of the meeting edges of radial alveoli is as yet uncertain. The pellicle closely invests the ectosarc, is continued down into a tubular sheath, from the base of which the tentacle rises, and upwards to invest the tentacle, and is even prolonged into its cavity in Choanophrya, the only genus where the tentacles are large enough for satisfactory demonstration. These organs may be one or more, and vary greatly in character. They may be (1) pointed for prehension, puncture, and suction (Ephelota, Fig. 61, 5); (2) nearly cylindrical, with a slightly "flared" truncate apex (Podophrya, Fig. 61, 1a); (3) filiform with a terminal knob; (4) "capitate" (Acineta, Fig. 61, 2); (5) bluntly truncate and capable of opening into a wide funnel for the suction of food[179] (Choanophrya, Fig. 62; Rhyncheta, Fig. 61, 3). Their movements, too, are varied, including retraction and protrusion, and a degree of flexion which reaches a maximum in Rhyncheta (Fig. 61, 3), whose tentacle is as freely motile as an elephant's trunk might be supposed to be were it as slender in proportion to its length. They are continued into the body, and in Choanophrya may extend right across it. In Podophrya trold the pellicle rises into a conical tube about the base of the tentacle, which is retracted through it completely with the prey in deglutition. In Dendrocometes, Dendrosoma, and Ophryodendron (Fig. 61, 4, 9, 7), the tentacles arise from outgrowths of the cell-body.
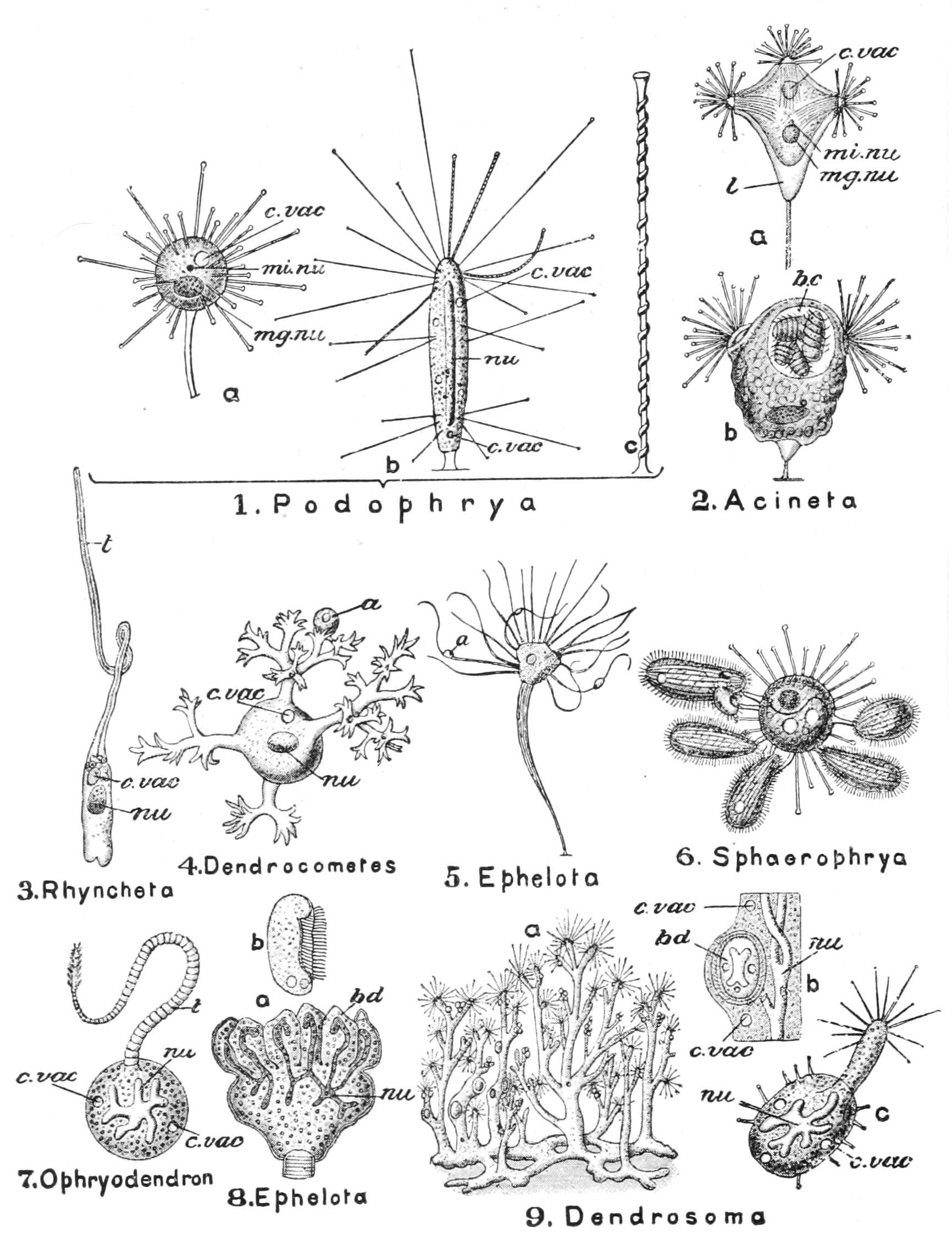
Fig. 61.—Various forms of Suctoria. 1, a and b, two species of Podophrya; c, a tentacle much enlarged; 2, a, Acineta jolyi; 2, b, A. tuberosa, with four ciliated buds; in 6 the animal has captured several small Ciliata; 8, a, a specimen multiplying by budding; 8, b, a free ciliated bud; 9, a, the entire colony; 9, b, a portion of the stem; 9, c, a liberated bud. a, Organism captured as food; b.c, brood-cavity; bd, bud; c.vac, contractile vacuole; l, test; mg.nu, meganucleus; mi.nu, micronucleus; nu, nucleus; t, tentacle. (From Parker and Haswell, after Bütschli and Saville Kent.)
The mechanism of suction is doubtful; but from the way particles from a little distance flow into the open funnels of Choanophrya, it may be the result of an increase of osmotic pressure. The external pellicle of the tentacles is marked by a spiral constriction,[180] which may be prolonged over the part included in the sheath. The endosarc is rich in oil-drops, often coloured, and in proteid granules which sometimes absorb stains so readily as to have been named "tinctin bodies." It usually contains at least one contractile vacuole.
In Dendrocometes (and perhaps others) the whole cell may become ciliated, detach itself and swim off; this it does when its host (Gammarus) moults its cuticle.
In fission or budding we have to distinguish many modes. (1) In the simplest, after the nuclear apparatus has divided, the cell divides transversely; the distal half acquires cilia and swims off to attach itself elsewhere, while the proximal remains attached. The tentacles have previously disappeared and have to be formed afresh in both. (2) More commonly fission passes into budding on the distal face; a sort of groove deepens around a central prominence which becomes the ciliated larva (Fig. 62, em); the tentacles of the "parent" are retained. This process passes into (3) "internal budding," where a minute pit leads into a bottle-shaped cavity.[181] (4) Again, the budding may be multiple, the meganucleus protruding a branch for each bud, while the micronucleus, by successive divisions, affords the supply requisite. Sphaerophrya (Fig. 61, 6) and Endosphaera multiply freely by fission within their Ciliate hosts, and were indeed described by Stein as stages in their life-cycle. Conjugation of the same type as in most Ciliates has been fully worked out in Dendrocometes alone, by Hickson,[182] who has found the meganuclei (though destined to disorganisation) conjugate for a short time by the bridge of communication before the reciprocal conjugation of the micronuclei.
We have referred to the endoparasitism of two genera. Amoebophrya lives in several Acanthometrids, and in the aberrant Radiolarian Sticholonche (see p. 86). The attached species are {162}some of them indifferent to their base; others are only found on Algae, or again only epizoic on special Metazoan hosts, or even on special parts of these. Thus Rhyncheta is only found on the couplers of the thoracic limbs of Cyclops, and Choanophrya on the ventral surface of its head and the adjoining appendages.
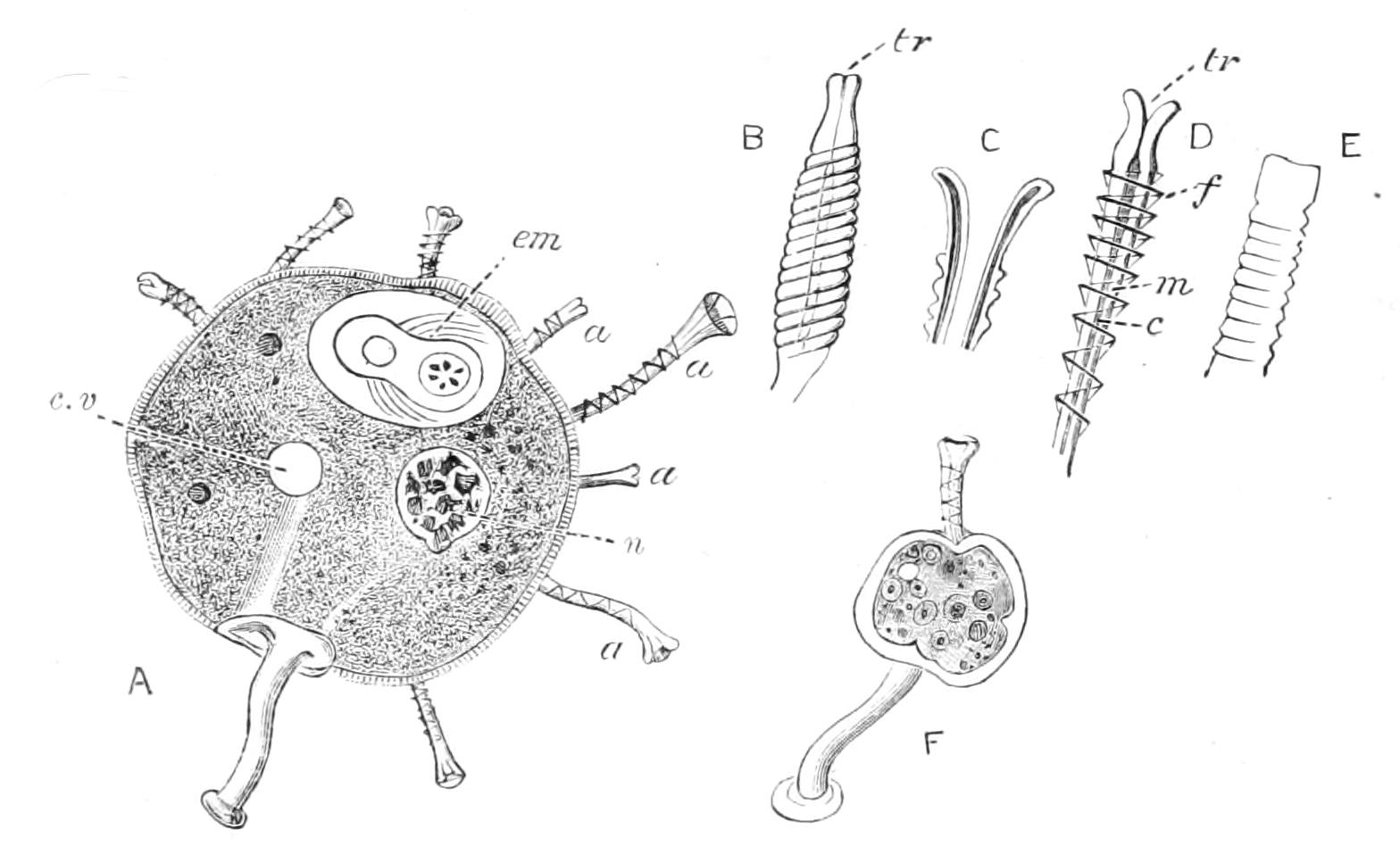
Fig. 62.—Choanophrya infundibulifera. A, adult; B-D, tentacles in action in various stages; E, tentacle at rest; F, young, just settled down, a, a, a, Tentacles in various stages of activity; c, central cavity; c.v, contractile vacuole; em, ciliated embryo showing contractile vacuole and nucleus; f, spiral ridge; m, muscular wall of funnel; n, nucleus; tr, opening of funnel. (A-D, F, modified after Zenker; E, original.)
We owe our knowledge of this group to the classical works of Ehrenberg, Claparède and Lachmann, Stein, R. Hertwig, and Bütschli. Plate has shed much light on Dendrocometes, and Hickson has studied its conjugation. Ischikawa[183] has utilised modern histological methods for the cytological study of Ephelota bütschliana. René Sand has written a useful, but unequal, and not always trustworthy monograph of the Order,[184] containing an elaborate bibliography.
BY
IGERNA B. J. SOLLAS, B.Sc. (Lond.)
Lecturer on Zoology at Newnham College, Cambridge.
PORIFERA (SPONGES)[185]
INTRODUCTION—HISTORY—DESCRIPTION OF HALICHONDRIA PANICEA AS AN EXAMPLE OF BRITISH MARINE SPONGES AND OF EPHYDATIA FLUVIATILIS FROM FRESH WATER—DEFINITION—POSITION IN THE ANIMAL KINGDOM.
Sponges occupy, perhaps, a more isolated position than any other animal phylum. They are not only the lowest group of multicellular animals, but they are destitute of multicellular relatives. They are all aquatic and—with the exclusion of a few genera found in fresh water—marine, inhabiting all depths from between tide marks to the great abysses of the ocean. They depend for their existence on a current of water which is caused to circulate through their bodies by the activity of certain flagellated cells. This current contains their food, it is their means of respiration, and it carries away effete matters. Consequently sponges cannot endure deprivation of oxygenated water except for short periods, and only the hardiest inhabit regions where the supply is intermittent, as between tide marks. This also renders useless attempts to keep specimens in tanks, unless the water is frequently renewed.
The outward appearance of sponges has an exceptionally wide range, so that it is difficult to give a novice any very definite picture of what he is to expect when searching for these animals. This diversity is in part due to the absence of organs of sufficient size to determine the shape of the whole or limit its variation, {166}that is to say, the separate organs are of an order of size inferior to that of the entire body. The animals are fixed or lie loose on the sea bottom; there are in no case organs of locomotion, and again no sense-organs, no segregated organs of sex, and as a rule no distinction into axis and lateral members. It is by these negative characters that the collector may easily recognise a sponge.
History.—Sponges are, then, in many of their characters unique; and they present a variety of problems for solution, both of special and general interest, they are widely distributed in time and space, and they include a host of forms. It therefore causes no little surprise to learn that they have suffered from a long neglect, even their animal nature having been but recently established. Though known to naturalists from the time of Aristotle, sponges have been left for modern workers as a heritage of virgin soil: it has yielded to them a rich harvest, and is as yet far from exhausted.
The familiar bath sponge was naturally the earliest known member of the phylum. It is dignified by mention in the Iliad and in the Odyssey, and Homer, in his choice of the adjective "full of holes," πολύτρητος, shows at least as much observation as many a naturalist of the sixteenth and seventeenth centuries. Aristotle based his ideas of sponges entirely upon the characters of the bath sponge and its near allies, for these were the only kinds he knew. With his usual perspicuity he reached the conclusion that sponges are animals, though showing points of likeness to plants.
The accounts of sponges after Aristotle present little of scientific interest until the last century. Doubtless this is in part due to the absence of organs which would admit of dissection, and the consequent necessity of finer methods of study. Like other attached forms, sponges were plant or animal as it pleased the imagination of the writer, and sometimes they were "plant animals" or Zoophyta: those who thought them animal were frequently divided among themselves as to whether they were "polypous" or "apolypous." An opinion which it is somewhat difficult to classify was that of Dr. Nehemiah Grew,[186] who says: "No Sponge hath any Lignous Fibers, but is wholly composed of those which make the Pith and all the pithy parts {167}of a Plant, ... So that a Sponge, instead of being a Zoophyton, is but the one-half of a Plant."
Sponges figure in herbals beside seaweeds and mushrooms, and Gerarde says:[187] "There is found growing upon rockes near unto the sea a certaine matter wrought together of the foame or froth of the sea which we call Spunges ... whereof to speak at any length would little benefit the reader ... seeing the use thereof is so well known." About the middle of the eighteenth century, authors, especially Peyssonnel, suggested that sponges were but the houses of worms, which built them much as a bee or wasp builds nests and cells. This was confuted by Ellis in 1765,[188] when he pointed out that the sponge could not be a dead structure, as it gave proof of life by "sucking and throwing out water." To Ellis, then, is due the credit of first describing, though imperfectly, a current set up by sponges. He mentions that Count Marsigli[189] had already made somewhat similar observations.
It was not till 1825 that attention was again turned to the current, when Robert Grant approached the group in a truly scientific manner, and was ably supported by Lieberkühn. It would be impossible to do justice to Grant in the brief summary to which we must limit ourselves. The most important of his contributions was the discovery that water enters the sponge by small apertures scattered over the surface, and leaves it at certain larger holes, always pursuing a fixed course. He made a few rough experiments to estimate the approximate strength of the current, and, though he failed to detect its cause, he supposed that it was probably due to ciliary action. Grant's suggestion was afterwards substantiated by Dujardin (1838), Carter (1847), Dobie (1852), and Lieberkühn (1857). These five succeeded in establishing the claims of sponges to a place in the animal kingdom, claims which were still further confirmed when James-Clark[190] detected the presence of the protoplasmic collar of the flagellated cells (see pp. 171, 176). Data were now wanted on which to base an opinion as to the position of sponges within the animal kingdom. In 1878 Schulze[191] furnished valuable embryological facts, in a description agreeing with an earlier one of Metschnikoff's, of the amphiblastula larva (p. 226) and its metamorphosis. {168}Then Bütschli[192] (1884) and Sollas[193] on combined morphological and embryological evidence (1884) concluded that sponges were remote from all the Metazoa, showing bonds only with Choanoflagellate Protozoa (p. 121). This the exact embryological work of Maas, Minchin, and Delage has done much to prove, but it has to be admitted that unanimity on the exact position of the phylum has not yet been attained, some authorities, such as Haeckel, Schulze, and Maas still wishing to include sponges in the Metazoa.
In this short history we have been obliged to refer only to work helping directly to solve the problem of the nature of a sponge, hence many names are absent which we should have wished to mention.
Halichondria panicea.
One of the commonest of British sponges, which may be picked up on almost any of our beaches, and which has also a cosmopolitan distribution, is known by the clumsy popular name of the "crumb of bread sponge," alluding to its consistency; or by the above technical name, with which even more serious fault may be found.[194]
In its outward form H. panicea affords an excellent case of a peculiarity common among sponges. Its appearance varies according to the position in which it has lived. In fact, Bowerbank remarks that it has no specific form. It may grow in sheets of varying thickness closely attached to a rock, when it is "encrusting," or it is frequently massive and lying free on the sea bottom; again, it may be fistular, consisting of a single long tube, or it may be ridge-like, apparently in this case consisting of a row of long tubes fused laterally. In this last form it used to be called the "cockscomb sponge," having been taken for a distinct species.
Bidder has proposed to call the different forms of the same species "metamps" of the species. Figures of the metamps of H. panicea will be found in Bowerbank's useful Monograph.[195]
The colour of the species is as inconstant as its form, ranging from green to light brown and orange. MacMunn concludes from spectroscopic work that H. panicea, contains at least three pigments, a chlorophyll, a lipochrome, and a histohaematin.[196] Lipochromes vary from red to yellow, chlorophyll is always associated with one or more of them. Histohaematin is a respiratory pigment. Proof has not yet been adduced that the chlorophyll is proper to the sponge and is not contained in symbiotic algae.
In spite of all this inconstancy H. panicea, is one of the most easily determined species. It is only necessary to dry a small fragment, including the upper surface; a beautiful honeycomb-like structure is then visible on this surface, and among British sponges this is a property peculiar to the species (Bowerbank). Whatever the form of the sponge, one or more large rounded apertures are always present on the exterior; these are the "oscula." In the encrusting metamp the oscula are flush with the general surface, while in the other cases they are raised on conical projections; fistular specimens carry the osculum at the distal end, and the cockscomb has a row of them along its upper edge. Much more numerous than the oscula are smaller apertures scattered over the general surface of the sponge, and known as "ostia."
Fig. 63.—Portion of the surface of H. panicea, from dried specimen. A, natural size; B, magnified. The large shaded patches are ostia.
If the sponge be placed in a shallow glass dish of sea water the function of the orifices can be made out with the naked eye, especially if a little powdered chalk or carmine be added to the water. If the specimen has been gathered after the retreating tide has left it exposed for some time, this addition is unnecessary, for as soon as it is plunged into water its current bursts vigorously forth, and is rendered visible by the particles of detritus that have accumulated in the interior during the period of {170}exposure and consequent suspended activity. The oscula then serve for the exit of currents of water carrying particles of solid matter, while the entrance of water is effected through the ostia.
Sections show that the ostia lead into spaces below the thin superficial layer or "dermal membrane"; these are continued down into the deeper parts of the sponge as the "incurrent canals," irregular winding passages of lumen continually diminishing as they descend. They all sooner or later open by numerous small pores—"prosopyles"—into certain subspherical sacs termed flagellated chambers. Each chamber discharges by one wide aperture—"apopyle"—into an "excurrent canal." This latter is only distinguishable from an incurrent canal by the difference in its mode of communication with the chambers.

Fig. 64.—H. panicea: the arrows indicate the direction of the current, which is made visible by coloured particles. (After Grant.)
The excurrent canals convey to the osculum the water which has passed through the ostia and chambers. All the peripheral parts of the sponge from which chambers are absent are termed the "ectosome," while the chamber-bearing regions are the "choanosome."
The peculiar crumb-of-bread consistency is due to the nature of the skeleton, which is formed of irregular bundles and strands of minute needles or spicules composed of silica hydrate, a substance familiar to us in another form as opal: they are clear and transparent like glass. They are scattered through the tissues in great abundance.
The classes of cellular elements in the sponge are as follows: Flattened cells termed "pinacocytes" cover all the free surfaces, that is to say, the external surface and the walls of the {171}excurrent and incurrent canals. The flagellated chambers are lined by "choanocytes" (cf. Fig. 70, p. 176); these are cells provided at their inner end with a flagellum and a collar surrounding it. They resemble individuals of the Protozoan sub-class Choanoflagellata, and the likeness is the more remarkable because no other organisms are known to possess such cells. Taken together the choanocytes constitute the "gastral layer," and they are the active elements in producing the current. The tissue surrounding the chambers thus lying between the excurrent and incurrent canals consists of a gelatinous matrix colonised by cells drawn from two distinct sources. In the first place, it contains cells which have a common origin with the pinacocytes, and which together with them make up the "dermal layer"; these are the "collencytes" and "scleroblasts"; secondly, it contains "archaeocytes," cells of independent origin.
Collencytes are cells with clear protoplasm and thread-like pseudopodial processes; they are distinguished as stellate or bipolar, according as these processes are many or only two. Scleroblasts or spicule cells are at first rounded, but become elongated with the growth of the spicule they secrete, and when fully grown are consequently fusiform.
Fig. 65.—Diagrammatic section of a siliceous Sponge. a.p, Apopyle; d.o, dermal ostia; ex.c, excurrent, or exhalant canal; in.c, incurrent canal; o, osculum. (Modified from Wilson.)
Each spicule consists of an organic filamentar axis or axial fibre around which sheaths of silica hydrate are deposited successively by the scleroblast. Over the greater length of the spicule the sheaths are cylindrical, but at each end they taper to a point. The axial canal in which the axial fibre lies is open at both ends, and the fibre is continuous at these two points with an organic sheath, which invests the entire spicule. From this structure we may conclude that the spicule grows at both ends—i.e. it grows in two opposite directions along one line—it has two rays lying in one axis, and is classed among uniaxial diactinal spicules. Being {172}pointed at both ends it receives the special name oxea. The lamination of the spicule is rendered much more distinct by heating or treatment with caustic potash.[197]
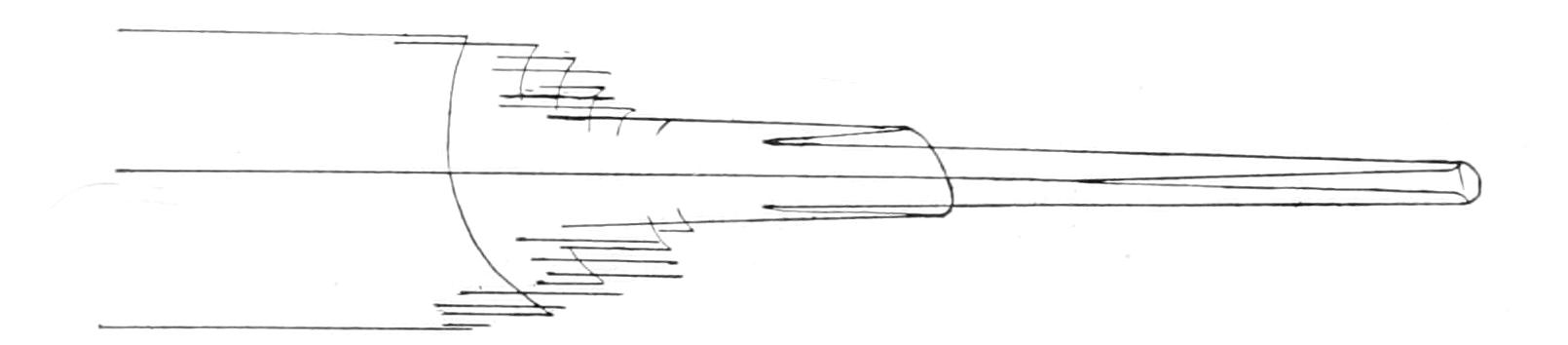
Fig. 66.—Cut end of a length of a siliceous spicule from Hyalonema sieboldii, with the lamellar structure revealed by solution. × 104. (After Sollas.)
The archaeocytes are rounded amoeboid cells early set apart in the larva; they are practically undifferentiated blastomeres. Some of them become reproductive elements, and thus afford a good instance of "continuity of germ plasm," others probably perform excretory functions.[198]

Fig. 67.—Free-swimming larva of Gellius varius, in optical section. a, Outer epithelium; pi, pigment; x, hinder pole. (After Maas.)
The reproductive elements are ova and spermatozoa, and are to be found in all stages in the dermal jelly. Dendy states that the eggs are fertilised in the inhalant canals, to which position they migrate by amoeboid movements, and there become suspended by a peduncle.
The larva has unfortunately not been described, but as the course of development among the near relatives of H. panicea is known to be fairly constant, it will be convenient to give a description of a "Halichondrine type" of larva based on Maas' account of the development of Gellius varius.[199] The free-swimming larvae escape by the osculum; they are minute oval bodies moving rapidly by means of a covering of cilia. The greater part of the body is a dazzling white, while the hinder pole is of a brown violet colour. This coloured patch is non-ciliate, the general covering of cilia ending at its edge in a ring of cilia twice the length of the others. Forward {173}movement takes place in a screw line; when this ceases the larva rests on its hinder pole, and the cilia cause it to turn round on its axis.
Sections show that the larva is built up of two layers:—
1. "The inner mass," consisting of various kinds of cells in a gelatinous matrix.
2. A high flagellated epithelium, which entirely covers the larva with the exception of the hinder pole.
Fig. 68.—Longitudinal section through the hinder pole of the larva of G. varius. a, Flagellated cells; ma1, undifferentiated cell; ma2, differentiated cell; pi, pigment; x, surface of hinder pole. (After Maas.)
The cells in the inner mass are classified into (1) undifferentiated cells, recognised by their nucleus, which possesses a nucleolus; these are the archaeocytes; (2) differentiated cells, of which the nucleus contains a chromatin net; these give rise to pinacocytes, collencytes, and scleroblasts. Some of them form a flat epithelium, which covers the hinder pole. Some of the scleroblasts already contain spicules. Fixation occurs very early. The front pole is used for attachment, the pigmented pole becoming the distal end (Fig. 69). The larva flattens out, the margin of the attached end is produced into radiating pseudopodial processes. The flagellated cells retreat to the interior, leaving the inner mass exposed, and some of its cells thereupon form a flat outer epithelium. This is the most important process of the metamorphosis; it is followed by a pause in the outward changes, coinciding in time with rearrangements of the internal cells to give rise to the canal system; that is to say, lacunae arise in the inner mass, pinacocytes pass to the surface of the lacunae, and form their lining; the flagellated cells, which have lain in confusion, become grouped in small clusters. These become flagellated chambers, communications are established between the various portions of the canal system, and its external apertures arise. There is at first only one osculum. The larvae may be obtained by keeping the parent sponge in a dish of sea water, shielded from too bright a light, and surrounded by a second dish of water to keep the temperature constant. They will undergo {174}metamorphosis in sea water which is constantly changed, and will live for some days.
We have said that the young sponge has only one osculum. This is the only organ which is present in unit number, and it is natural to ask whether perhaps the osculum may not be taken as a mark of the individual; whether the fistular specimens, for example, of H. panicea may not be solitary individuals, and the cockscomb and other forms colonies in which the individuals are merged to different degrees. Into the metaphysics of such a view we cannot enter here. We must be content to refer to the views of Huxley and of Spencer on Individuality.
But it is advisable to avoid speaking of a multi-osculate sponge as a colony of many individuals, even in the sense in which it is usual to speak of a colony of polyps as formed of individuals. The repetition of oscula is probably to be regarded as an example of the phenomenon of repetition of parts, the almost universal occurrence of which has been emphasised by Bateson.[200] Delage[201] has shown that when two sponge larvae fixed side by side fuse together, the resulting product has but one osculum. This, though seeming to bear out our point of view, loses weight in this connexion, when it is recalled that two Echinoderm larvae fused together give rise in a later stage to but one individual.
Fig. 69.—Larva of Gellius varius shortly after fixation. The pigmented pole, originally posterior, is turned towards the reader. R, Marginal membrane with pseudopodia; x, hinder pole. (After Maas.)
Ephydatia fluviatilis.
In the fresh water of our rivers, ponds, and lakes, sponges are represented very commonly by Ephydatia (Spongilla) fluviatilis, a cosmopolitan species. The search for specimens is most likely {175}to be successful if perpendicular timbers such as lock-gates are examined, or the underside of floating logs or barges, or overhanging branches of trees which dip beneath the surface of the water.
The sponge is sessile and massive, seldom forming branches, and is often to be found in great luxuriance of growth, masses of many pounds weight having been taken off barges in the Thames. The colour ranges from flesh-tint to green, according to the exposure to light. This fact is dealt with in a most interesting paper by Professor Lankester,[202] who has shown not only that the green colour is due to the presence of chlorophyll, but that the colouring matter is contained in corpuscles similar to the chlorophyll corpuscles of green plants, and, further, that the flesh-coloured specimens contain colourless corpuscles, which, though differing in shape from those which contain the green pigment, are in all probability converted into these latter under the influence of sufficient light. The corpuscles, both green and colourless, are contained in amoeboid cells of the dermal layer;[203] and in the same cells but not in the corpuscles are to be found amyloid substances.
The anatomy of Ephydatia fluviatilis is very similar to that of Halichondria panicea, differing only in one or two points of importance. The ectosome is an aspiculous membrane of dermal tissue covering the whole exterior of the sponge and forming the roof of a continuous subdermal space. This dermal membrane is perforated by innumerable ostia, and is supported above the subdermal cavity by means of skeletal strands, which traverse the subdermal cavity and raise the dermal membrane into tent-like elevations, termed conuli. The inhalant canals which arise from the floor of the subdermal cavity are as irregular as in H. panicea, and interdigitate with equally irregular exhalant canals; these latter communicate with the oscular tubes. Between the two sets of canals are the thin folds of the choanosome with its small subspherical chambers provided with widely open apopyles (Fig. 70). The soft parts are supported on a siliceous skeleton of oxeas, which may have a quite smooth surface or may {176}be covered in various degrees with minute conical spines (Fig. 72, a, b). These spicules are connected by means of a substance termed spongin deposited around their overlapping ends, so as to form an irregular network of strands, of which some may be distinguished as main strands or fibres, others as connecting fibres. In the main fibres several spicules lie side by side, while in the connecting fibres fewer or frequently single spicules form the thickness of the fibre. The fibres are continuous at the base with a plate or skin of spongin, which is secreted over the lower surface of the sponge and intervenes between it and the substratum. Of the chemical composition of spongin we shall speak later (see p. 237). It is a substance which reaches a great importance in some of the higher sponges, and forms the entire skeleton of certain kinds of bath sponge. Lying loose in the soft parts and hence termed flesh spicules, or microscleres, are minute spicules of peculiar form. These are the amphidiscs, consisting of a shaft with a many-rayed disc at each end (Fig. 72).
Fig. 70.—Ephydatia fluviatilis. Section of flagellated chamber, showing the choanocytes passing through the apopyle. (After Vosmaer and Pekelharing.)
In addition to its habitat the fresh-water sponge is worthy of attention on account of its methods of reproduction, which have arisen in adaptation to the habitat. A similar adaptation is widespread among fresh-water members of most aquatic invertebrates.[204]
Ephydatia fluviatilis normally produces not only free-swimming larvae of sexual origin, but also internal gemmules arising asexually. These bodies appear in autumn, distributed throughout the sponge, often more densely in the deeper layers, and they come into activity only after the death of the parent, an event which happens in this climate at the approach of winter.
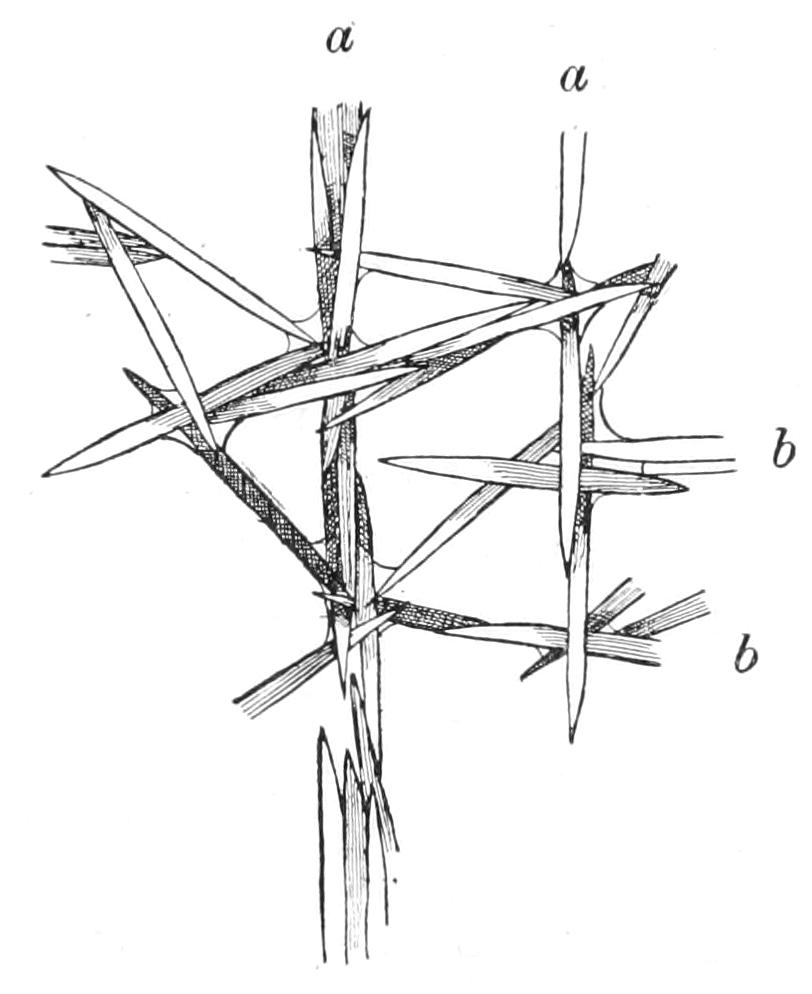
Fig. 71.—Portion of the skeletal framework of E. fluviatilis. a, Main fibres; b, connecting fibres. (After Weltner.)
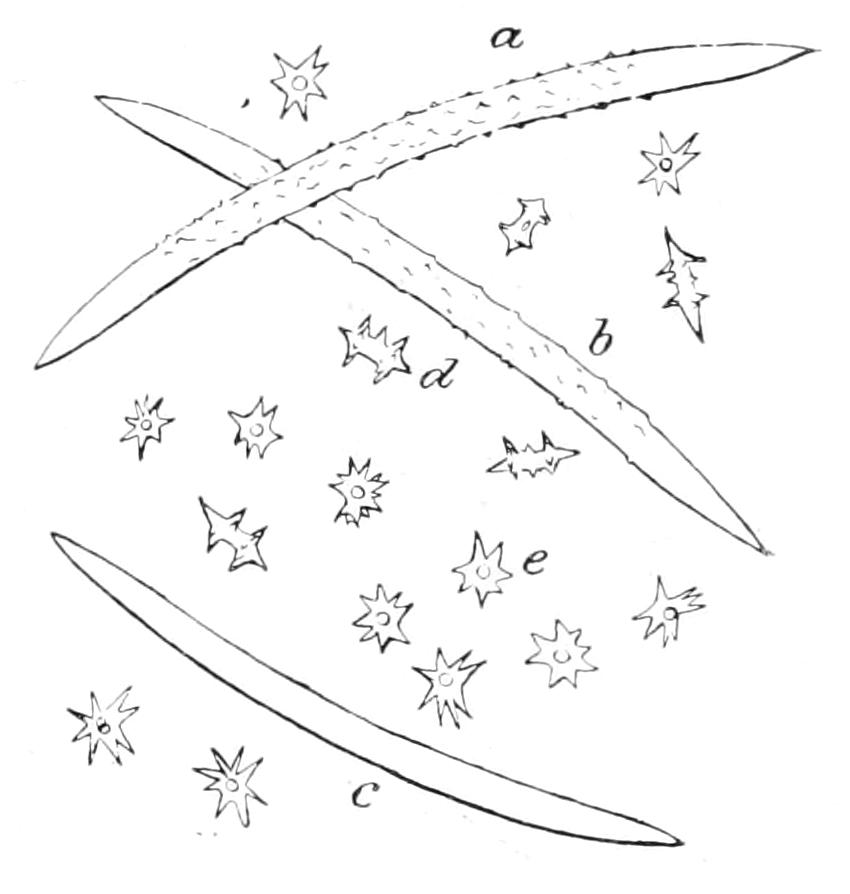
Fig. 72.—Spicules of E. fluviatilis. a. b. c. Oxeas, spined and smooth; d. e, amphidiscs, side and end views. (After Potts.)
Weltner[205] has shown that on the death and disintegration of the mother sponge some of the gemmules remain attached to the old skeleton, some sink and some float. Those which remain attached are well known to reclothe the dead fibres with living tissue. They inherit, as it were, the advantages of position, which contributed to the survival of the parent, as one of the selected fittest. The gemmules which sink are doubtless rolled short distances along the bottom, while those which float have the opportunity of widely distributing the species with the risk of being washed out to sea. But even these floating gemmules are exposed to far less dangers than the delicate free-swimming larvae, for their soft parts are protected from shocks by a thick coat armed with amphidiscs.
The gemmules are likewise remarkable for their powers of {178}resistance to climatic conditions, powers which must contribute in no small way to the survival of a species exposed to the variable temperatures of fresh water. Thus, if the floating gemmules or the parent skeleton with its attached and dormant offspring should chance to be included in the surface layer of ice during the winter, so far from suffering any evil consequences they appear to benefit by these conditions. Both Potts and Weltner have confirmed the truth of this statement by experiments. Weltner succeeded in rearing young from gemmules which had suffered a total exposure of 17 days to a temperature "under 0° C."
Of important bearing on the question of the utility of the gemmules are certain instances in which E. fluviatilis has been recorded as existing in a perennial condition.[206] The perennial individuals may or may not bear gemmules, which makes it evident that, with the acquisition of the power to survive the winter cold, the prime necessity of forming these bodies vanishes.
The perennial specimens are described as exhibiting a diminished vegetative activity in winter, the flagellated chambers may be absent (Lieberkühn), or present in unusually small numbers (Weltner), the entire canal system may be absent (Metschnikoff), or, on the other hand, it may be complete except for the osculum.
In tropical countries gemmulation occurs as a defence against the ravages caused by the dry season when the waters recede down their banks, exposing all or most of their sponge inhabitants to the direct rays of the sun. The sponges are at once killed, but the contained gemmules being thoroughly dried, become efficient distributing agents of the species; they are light enough to be carried on the wind. It is probable that those individual sponges which escape desiccation survive the dry season without forming gemmules.
It has been shown experimentally that gemmules are not injured by drying—Zykoff found that gemmules kept dry for a period of two years had not lost the power of germination.
The mature gemmules consist of a more or less spherical mass of cells, which we shall refer to as yolk cells, and of a complex coat. The latter is provided with a pore or pore tube (Fig. 74) which is closed in winter by an organic membrane.
There are three layers in the coat: an inner chitinous layer surrounded by an air-chamber layer, which is finely vesicular, showing a structure recalling plant tissue, and containing amphidiscs arranged along radii passing through the centre of the gemmule. One of the discs of each amphidisc lies in the inner chitinous coat, while the other lies in a similar membrane which envelopes the air-chamber layer and is termed the outer chitinous coat.
Marshall has suggested that one function of the amphidiscs is to weight the gemmules and thus protect them against the force of the river current; and no doubt the sinking or floating of individual gemmules depends on the relative degree of development of the air-chambers and of the amphidiscs.
A study of the development of Ephydatia gemmules vividly illustrates various characters of the inner processes of sponges. Specially noteworthy are the migrations of cells and the slight extent to which division of labour is carried: one and the same cell will be found to perform various functions.
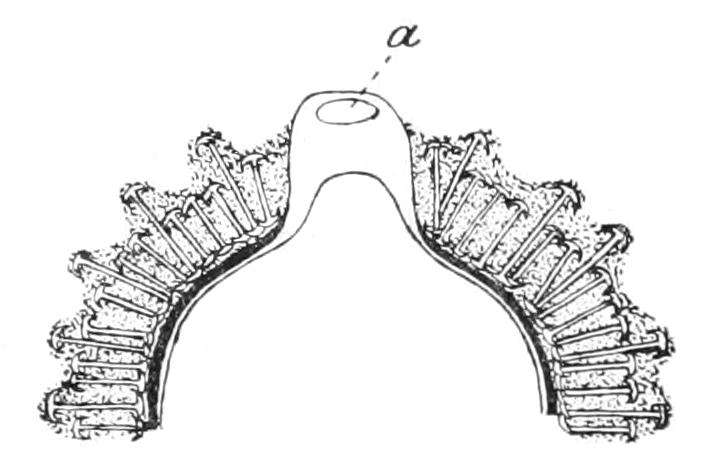
Fig. 74.—Part of a longitudinal section of a gemmule of Ephydatia sp. passing through the pore (a). (After Potts.)
The beginning of a gemmule is first recognisable[207] as a small cluster of amoeboid archaeocytes in the dermal membrane. These move into the deeper parts of the sponge to form larger groups. They are the essential part of the gemmule, the yolk cells, which, when germination takes place, give rise to a new sponge. They are followed by two distinct troops of actively moving cells. Those forming the first troop arrange themselves round the yolk cells and ultimately assume a columnar form so that they make an epithelioid layer. They then secrete the inner chitinous coat. The cells of the second troop are entrusted with the nutrition of the gemmule. Consequently they pass in among the yolk cells, distribute their food supplies, and make their escape {180}by returning into the tissues of the mother sponge, before the columnar cells have completed the chitinous coat. Yet another migration now occurs, the cells—"scleroblasts"—which have been occupied in secreting amphidiscs at various stations in the sponge, carry the fully formed spicules to the gemmules and place them radially round the yolk cells between the radially lying cells of the columnar layer. The scleroblasts themselves remain with the amphidiscs, and becoming modified, contribute to the formation of the air-chamber layer. The columnar cells now creep out between the amphidiscs till their inner ends rest on the outer ends of these spicules. They then secrete the outer chitinous coat and return to the mother sponge.
Carter gives directions[208] for obtaining young sponges from the gemmules. The latter should be removed from the parent, cleaned by rolling in a handkerchief, and then placed in water in a watch-glass, protected with a glass cover and exposed to sunlight. In a few days the contents of the gemmule issue from the foramen and can be seen as a white speck. A few hours later the young sponge is already active and may be watched producing aqueous currents. At this age the sponge is an excellent object for studying in the living condition: being both small and transparent it affords us an opportunity of watching the movements of particles of carmine as they are carried by the current through the chambers.
Potts[209] describes how he has followed the transportal of spicules by dermal cells, the end of each spicule multiplying the motion, swaying like an oscillating rod.
In E. fluviatilis reproduction also occurs during the warmer months in this climate by means of sexual larvae. These are interesting for certain aberrant features in their metamorphosis.[210] While some of the flagellated chambers are formed in the normal way from the flagellated cells of the larva, others arise each by division of a single archaeocyte. This, it is suggested, is correlated with the acquisition of the method of reproduction by gemmules, the peculiarities (i.e. development of organs from archaeocytes) of which are appearing in the larvae.
Definition.—We may now define sponges as multicellular, {181}two-layered animals; with pores perforating the body-walls and admitting a current of water, which is set up by the collared cells of the "gastral" layer.
Position in the Animal Kingdom.—Sponges are the only multicellular animals which possess choanocytes, and their mode of feeding is unique. Since they are two-layered it has been sought to associate them with the Metazoan phylum Coelenterata, but they are destitute of nematocysts or any other form of stinging cell, and their generative cells arise from a class of embryonic cells set apart from the first, while the generative cells of Coelenterata are derived from the ectoderm, or in other cases from the endoderm. These weighty differences between sponges and that group of Metazoa to which they would, if of Metazoan nature at all, be most likely to show resemblance, suggest that we should seek a separate origin for sponges and Metazoa. We naturally turn to the Choanoflagellate Infusorian stock (see p. 121) as the source of Porifera, leaving the Ciliate stock as the progenitors of Metazoa.
That both Porifera and Metazoa are reproduced by ova and spermatozoa is no objection to this view, seeing that the occurrence of similar reproductive cells has been demonstrated in certain Protozoa (see pp. 100, 128).
Let us now see which view is borne out by facts of embryology. Suppose, for the moment, we regard sponges as Metazoa, then if the sponge larva be compared with the Metazoan larva we must assign the large granular cells to the endoderm; the flagellated cells to the ectoderm; and we are led to the anomalous statement that the digestive cells in the adult are ectodermal, the covering, outer cells endodermal; or conversely, if we start our comparisons with the adults, then it follows that the larval ectoderm has the characters of an endoderm, and the larval endoderm those of an ectoderm.
Thus both embryology and morphology lead us to the same point, they both show that in the absence of any fundamental agreement between Porifera and Metazoa it is necessary to regard the two stocks as independent from the very first, and hence the name Parazoa (Sollas) has been given to the group which contains the Porifera as its only known phylum.
Interesting in connexion with the phylogeny of Parazoa is the Choanoflagellate genus Proterospongia (Fig. 75), described by {182}Saville Kent, and since rediscovered both in England and abroad.[211] This is a colony of unicellular individuals embedded in a common jelly. The individuals at the surface are choanoflagellate, while in the interior the cells are rounded or amoeboid, and some of them undergo multiple fission to form reproductive cells. This is just such a creature as we might imagine that ancestral stage to have been of which the free-swimming sponge larva is a reminiscence: for we have seen that the flagellated cells of the larva are potential choanocytes.

Fig. 75.—Proterospongia haeckeli. a, Amoeboid cell; b, a cell dividing; c, cell with small collar; z, jelly. × 800. (After S. Kent.)
PORIFERA (CONTINUED): FORMS OF SPICULES—CALCAREA—HOMOCOELA—HETEROCOELA—HEXACTINELLIDA—DEMOSPONGIAE—TETRACTINELLIDA—MONAXONIDA—CERATOSA—KEY TO BRITISH GENERA OF SPONGES
Sponges fall naturally into two branches differing in the size of their choanocytes: in the Megamastictora these cells are relatively large, varying from 5µ to 9µ in diameter; in Micromastictora they are about 3µ in diameter.[212] For further subdivision of the group the spicules are such important weapons in the hands of the systematist that it is convenient to name them according to a common scheme. This has been arrived at by considering first the number of axes along which the main branches of the spicules are distributed, and secondly whether growth has occurred in each of these axes in one or both directions from a point of origin.[213]
I. Monaxons.—Spicules of rod-like form, in which growth is directed from a single origin in one or both directions along a single axis. The axis of any spicule is not necessarily straight, it may be curved or undulating. The ray or rays are known as actines.
Biradiate monaxon spicules are termed "rhabdi" (Fig. 76, a). A rhabdus pointed at both ends is an "oxea," rounded at both ends a "strongyle," knobbed at both ends a "tylote." By branching a rhabdus may become a "triaene" (Fig. 110, k, l).
Uniradiate monaxon spicules are termed "styli."
II. Tetraxons.—Spicules in which growth proceeds from an {184}origin in one direction only, along four axes arranged as normals to the faces of a regular tetrahedron. Forms produced by growth from an origin in one direction along three axes lying in one plane are classed with tetraxons.
III. Triaxons.—Spicules in which growth is directed from an origin in both directions along three rectangular axes. One or more actines or one or two axes may be suppressed.
IV. Polyaxons.—Spicules in which radiate growth from a centre proceeds in several directions.
V. Spheres.—Spicules in which growth is concentric about the origin.
A distinction more fundamental than that of form is afforded by the chemical composition: all sponges having spicules composed of calcium carbonate belong to a single class, Calcarea, which stands alone in the branch Megamastictora.
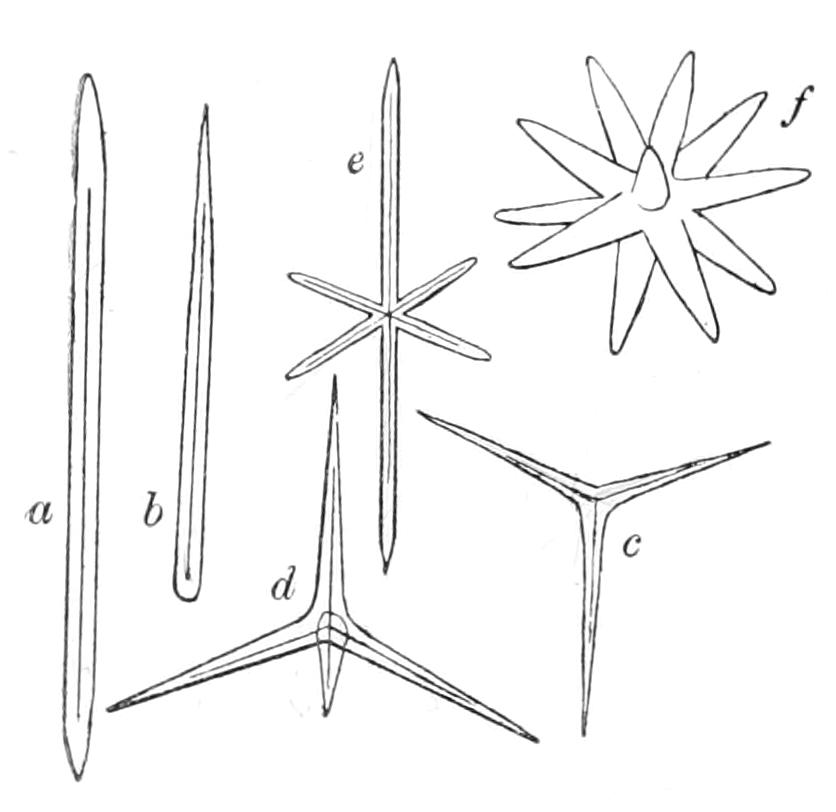
Fig. 76.—Types of megascleres. a, Rhabdus (monaxon diactine); b, stylus (monaxon monactine); c, triod (tetraxon triactine); d, calthrop (tetraxon tetractine); e, triaxon hexactine; f, euaster.
BRANCH I. MEGAMASTICTORA
CLASS CALCAREA
Calcarea are marine shallow-water forms attached for the most part directly by the basal part of the body or occasionally by the intervention of a stalk formed of dermal tissue. They are almost all white or pale grey brown in colour. Their spicules are either monaxon or tetraxon or both. The tetraxons are either quadriradiate and then called "calthrops," or triradiate when the fourth actine is absent. The triradiates always lie more or less tangentially in the body-wall; similarly three rays of a calthrop are tangentially placed, the fourth lying across the thickness of the wall. It is convenient to include the triradiate and the three tangentially placed rays of a calthrop under the common {185}term "triradiate system" (Minchin). The three rays of one of these systems may all be equal in length and meet at equal angles: in this case the system is "regular." Or one ray or one angle may differ in size from the other rays or angles respectively, which are equal: in either of these two cases the system is bilaterally symmetrical and is termed "sagittal." A special name "alate" is given to those systems which are sagittal in consequence of the inequality in the angles. Thus all equiangular systems whether sagittal or not are opposed to those which are alate. This is the natural classification.[214]
Sub-Class I. Homocoela.
The Homocoela or Ascons possess the simplest known type of canal system, and by this they are defined. The body is a sac, branched in the adult, but simple in the young; its continuous cavity is everywhere lined with choanocytes, its wall is traversed by inhalant pores, and its cavity opens to the exterior at the distal end by an osculum. The simple sac-like young is the well-known Olynthus of Haeckel—the starting-point from which all sponges seem to have set out. Two processes are involved in the passage from the young to the adult, namely, multiplication of oscula and branching of the original Olynthus tube or sac. If the formation of a new osculum is accompanied by fission of the sac, and the branching of the latter is slight, there arises an adult formed of a number of erect, well separated main tubes, each with one osculum and lateral branches. Such is the case in the Leucosoleniidae. In the Clathrinidae, on the other hand, branching of the Olynthus is complicated, giving rise to what is termed reticulate body form, that is, a sponge body consisting of a network of tubules with several oscula, but with no external indication of the limits between the portions drained by each osculum. These outward characters form a safe basis for classification, because they are correlated with other fundamental differences in structure and development.[215]
As in Halichondria, and in fact all sponges, the body-wall is formed of two layers; the gastral layer, as we have said, forming a continuous lining to the Ascon tube and its branches. The {186}dermal layer includes a complete outer covering of pinacocytes, which is reflected over the oscular rim to meet the gastral layer at the distal end of the tube; a deeper gelatinous stratum in which lie scleroblasts and their secreted products—calcareous spicules; and finally porocytes.[216] These last are cells which traverse the whole thickness of the thin body-wall, and are perforated by a duct or pore. The porocytes are contractile, and so the pores may be opened or closed; they are a type of cell which is known only in Calcarea. It will be noticed that the fusiform or stellate "connective tissue cells" are absent. The layer of pinacocytes as a whole is highly contractile, and is capable of diminishing the size of the sponge to such an extent as quite to obliterate temporarily the gastral cavity.[217]
The choanocytes show certain constant differences in structure in the families Clathrinidae and Leucosoleniidae respectively. In the former, the nucleus of the choanocyte is basal; in the latter, it is apical, and the flagellum can be traced down to it (Fig. 77).
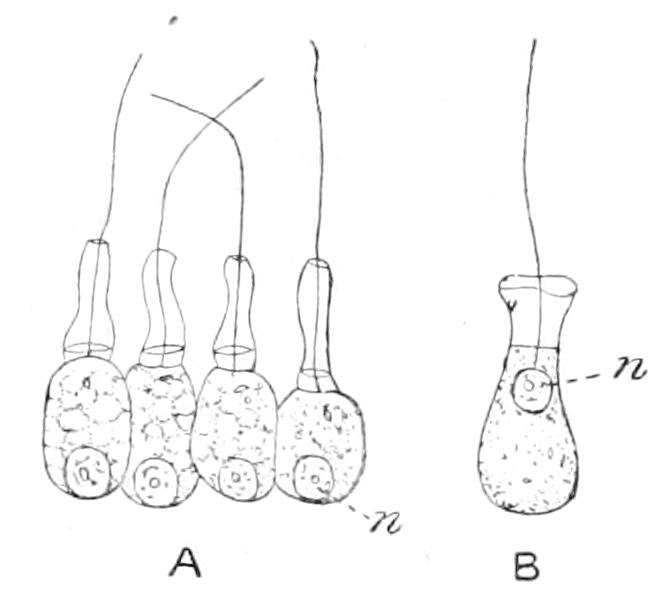
Fig. 77.—The two types of Asconid collar cells. A, of Clathrina, nucleus basal; B, of Leucosolenia, nucleus not basal, flagellum arising from the nuclear membrane. (A, after Minchin; B, after Bidder.)
The tetraxon spicules have "equiangular" triradiate systems in the Clathrinidae, while in Leucosoleniidae they are "alate." Finally, the larva of Clathrinidae is a "parenchymula" (see p. 226), that of Leucosoleniidae an "amphiblastula."
The fact that it is possible to classify the Calcarea Homocoela largely by means of histological characters is in accordance with the importance of the individual cell as opposed to the cell-layers generally throughout the Porifera, and is interesting in serving to emphasise the low grade of organisation of the Phylum. The organs of sponges are often unicellular (pores), or the products of the activity of a single cell (many skeletal elements); and even in the gastral layer, which approaches nearly to an epithelium, comparable with the epithelia of Metazoa, the component cells {187}still seem to assert their independence, the flagella not lashing in concert,[218] but each in its own time and direction.
Sub-Class II. Heterocoela.
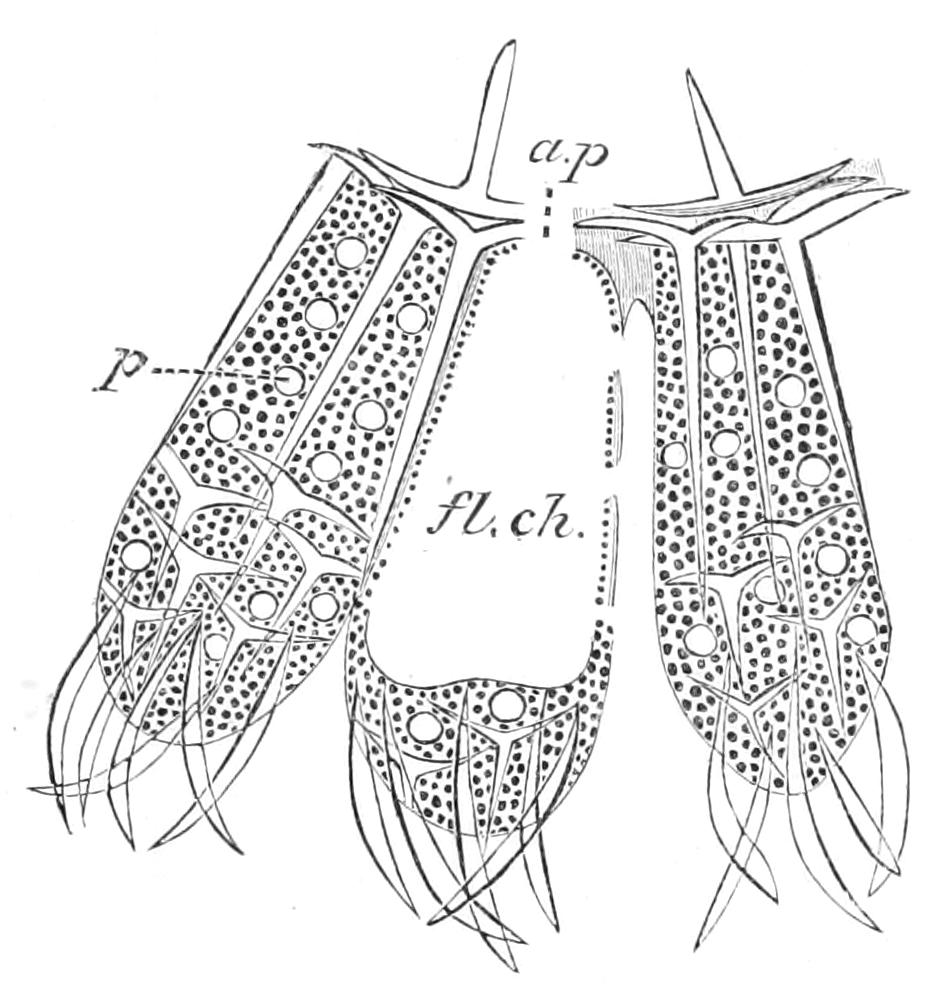
Fig. 78.—Transverse section of the body-wall of Sycon carteri, showing articulate tubar skeleton, gastric ostia (a.p), tufts of oxeas at the distal ends of the chambers (fl.ch), and pores (p). (After Dendy.)
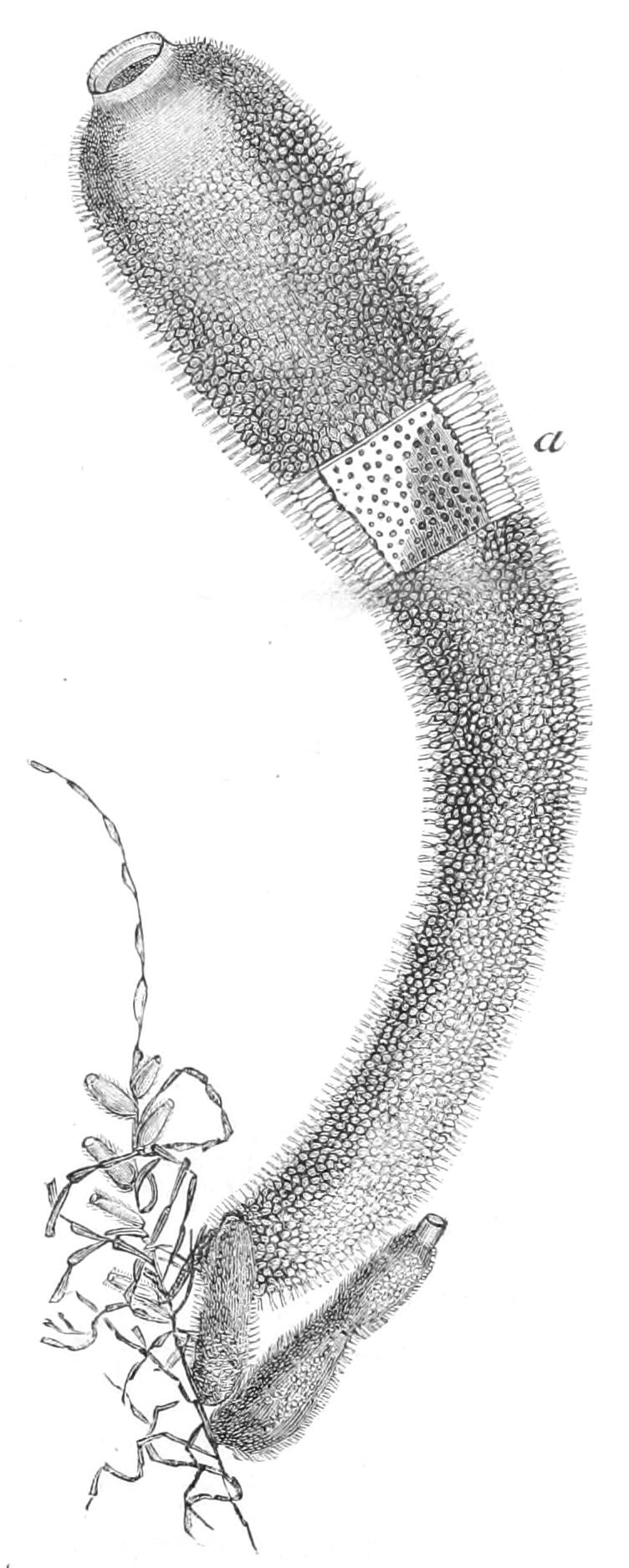
Fig. 79.—Sycon coronatum. At a a portion of the wall is removed, exposing the paragaster and the gastric ostia of the chambers opening into it.
The Heterocoela present a series of forms of successive grades of complexity, all derivable from the Ascons, from which they differ in having a discontinuous gastral layer. The simplest Heterocoela are included in the family Sycettidae, of which the British representative is Sycon (Fig. 79). In Sycon numerous tubular flagellated chambers are arranged radially round a central cavity, the "paragaster," into which they open (Figs. 78, 79). The chambers, which are here often called radial tubes, are close set, leaving more or less quadrangular tubular spaces, the {188}inhalant canals, between them; and where the walls of adjacent chambers come in contact, fusion may take place. Pores guarded by porocytes put the inhalant canals into communication with the flagellated chambers. The paragaster is lined by pinacocytes; choanocytes are confined to the flagellated chambers.
The skeleton is partly defensive, partly supporting; one set of spicules strengthens the walls of the radial tubes and forms collectively the "tubar skeleton." It is characteristic of Sycettidae that the tubar skeleton is of the type known as "articulate"—i.e. it is formed of a number of successive rings of spicules, instead of consisting of a single ring of large spicules which run the whole length of the tube.
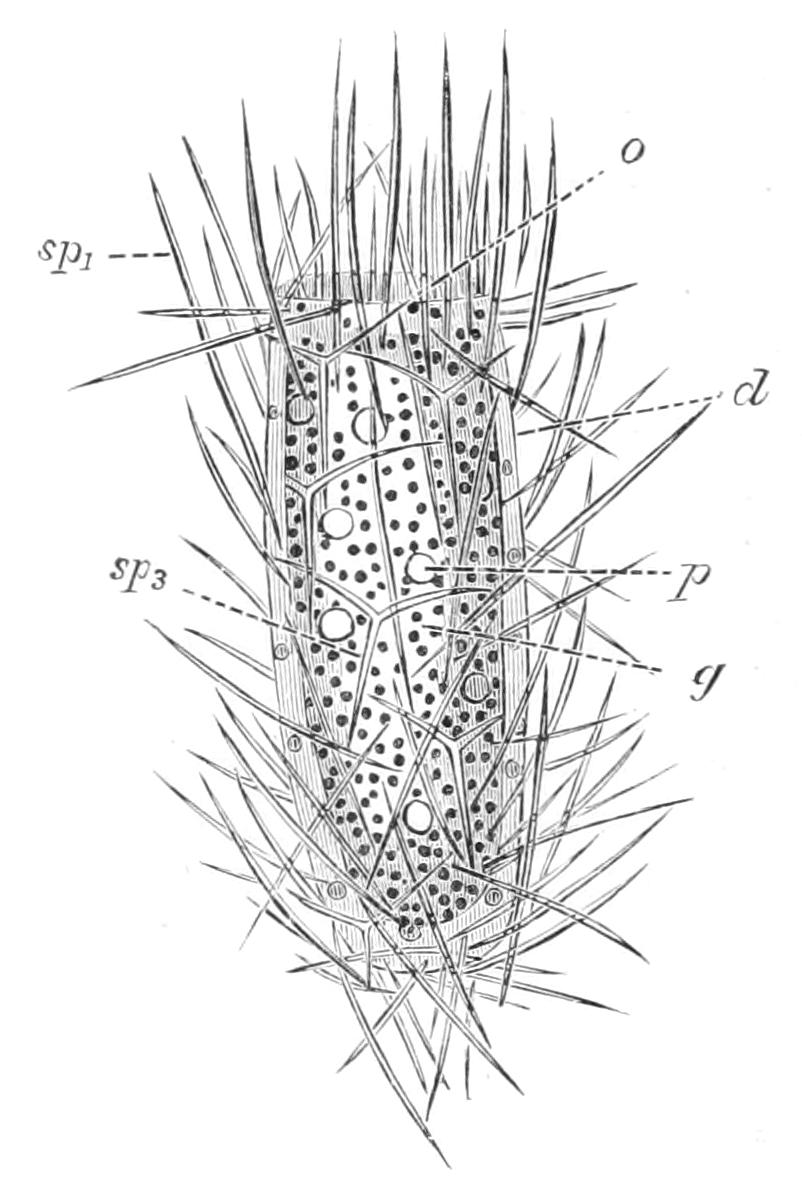
Fig. 80.—Sycon setosum. Young Sponge. × 200. d, Dermal cell; g, gastral cell; o, osculum; p, pore cell; sp1, monaxon; sp3, triradiate spicule. (After Maas.)
The walls of the paragaster are known as the "gastral cortex"; they contain quadriradiate spicules, of which the triradiate systems lie tangentially in the gastral cortex, while the apical ray projects into the paragaster, and is no doubt defensive. The distal ends of the chambers bristle with tufts of oxeate spicules, and the separate chambers are distinguishable in surface view. It is interesting to notice that in some species of Sycon, the gaps between the distal ends of the chambers are covered over by a delicate perforated membrane, thus leading on, as we shall see presently, to the next stage of advance.[219] The larva of Sycon is an amphiblastula (see p. 227). Fig. 80 is a drawing of the young sponge soon after fixation; it would pass equally well for an ideally simple Ascon or, neglecting the arrangement of the spicules, for an isolated radial tube of Sycon. Figs. 81, 82 show the same sponge, somewhat older. From them it is seen that the Sycon type is produced from the young individual, in what {189}may be called its Ascon stage, by a process of outgrowth of tubes from its walls, followed by restriction of choanocytes to the flagellated chambers. Minute observation has shown[220] that this latter event is brought about by immigration of pinacocytes from the exterior. These cells creep through the jelly of the dermal layer and line the paragaster as fast as its original covering of choanocytes retreats into the newly formed chambers.
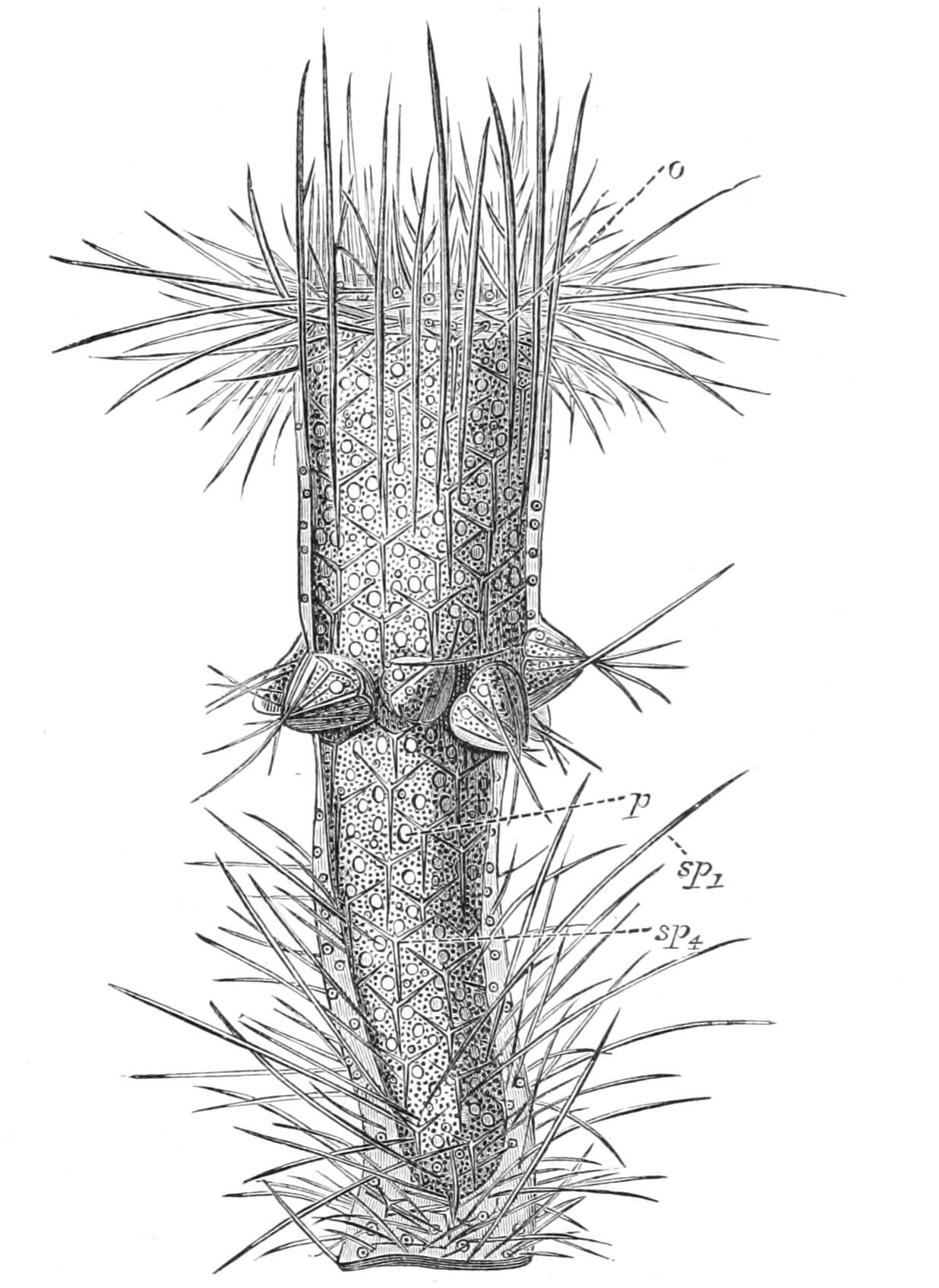
Fig. 81.—S. setosum. Young Sponge, with one whorl of radial tubes. o, Osculum; p, pore; sp1, monaxon; sp4, quadriradiate spicule. (After Maas.)
With a canal system precisely similar to that of Sycon, Ute (Fig. 83) shows an advance in structure in the thickening of the dermal layers over the distal ends of the chambers. The dermal thickenings above neighbouring chambers extend laterally and {190}meet; and there results a sheet of dermal tissue perforated by dermal ostia, which open into the inhalant canals, and strengthened by stout spicules running longitudinally. This layer is termed a cortex; it covers the whole sponge, compacting the radial tubes so that they form, together with the cortex, a secondary wall to the sponge, which is once more a simple sac, but with a complex wall. The cortex may be enormously developed, so as to form more than half the thickness of the wall (Fig. 84). The chambers taken together are spoken of as the chamber layer.
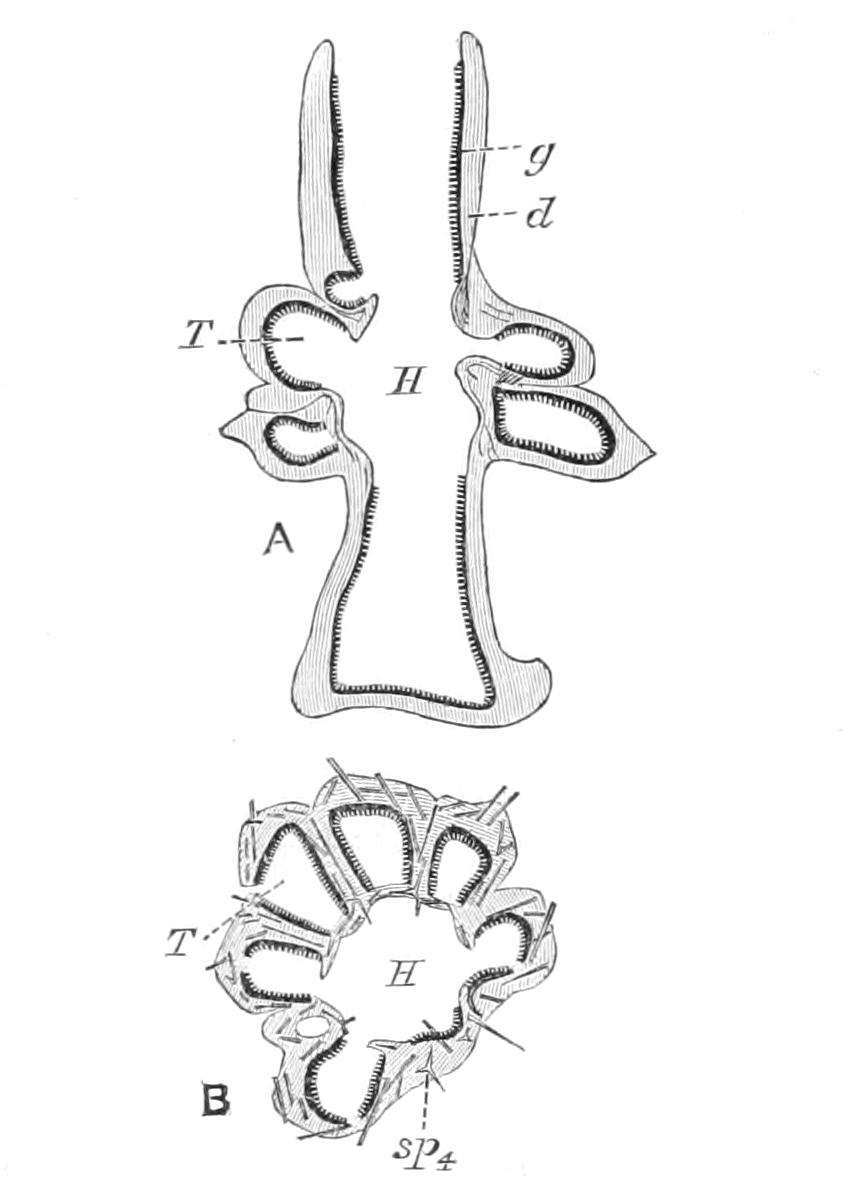
Fig. 82.—Sycon raphanus. A, Longitudinal section of young decalcified Sponge at a stage somewhat later than that shown in Fig. 81. B, Transverse section of the same through a whorl of tubes. d, Dermal membrane; g, gastral membrane; H, paragaster; sp4, tetraradiate spicule; T, radial tube. (After Maas.)
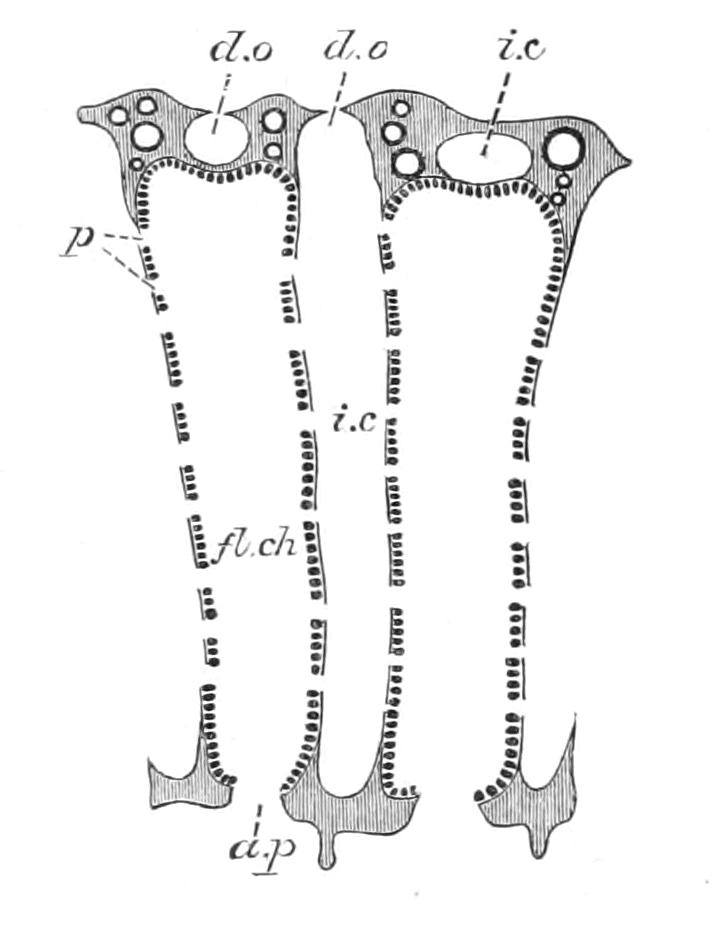
Fig. 83.—Transverse section of the body-wall of Ute, passing longitudinally through two chambers. a.p, Apopyle; d.o, dermal ostium; fl.ch, flagellated chamber or radial tube; i.c, inhalant canal; p, prosopyle. (After Dendy.)
We have already alluded to the resemblance between a young Ascon person and a radial tube of Sycon—a comparison which calls to mind the somewhat strange view of certain earlier authors, that the flagellated chambers are really the sponge individuals. If now we suppose each Ascon-like radial tube of Sycon to undergo that same process of growth by which the {191}Sycon itself was derived from the Ascon, we shall then have a sponge with a canal system of the type seen in Leucandra among British forms, but more diagrammatically shown in the foreign genus Leucilla (Fig. 85). The foregoing remarks do not pretend to give an account of the transition from Sycon to Leucilla as it occurred in phylogeny. For some indication of this we must await embryological research.
In Leucandra the fundamental structure is obscured by the irregularity of its canal system. It shows a further and most important difference from Leucilla in the smaller size and rounded form of its chambers. This change of form marks an advance in efficiency; for now the flagella converge to a centre, so that they all act on the same drop of water, while in the tubular chamber their action is more widely distributed and proportionately less intense (see p. 236).
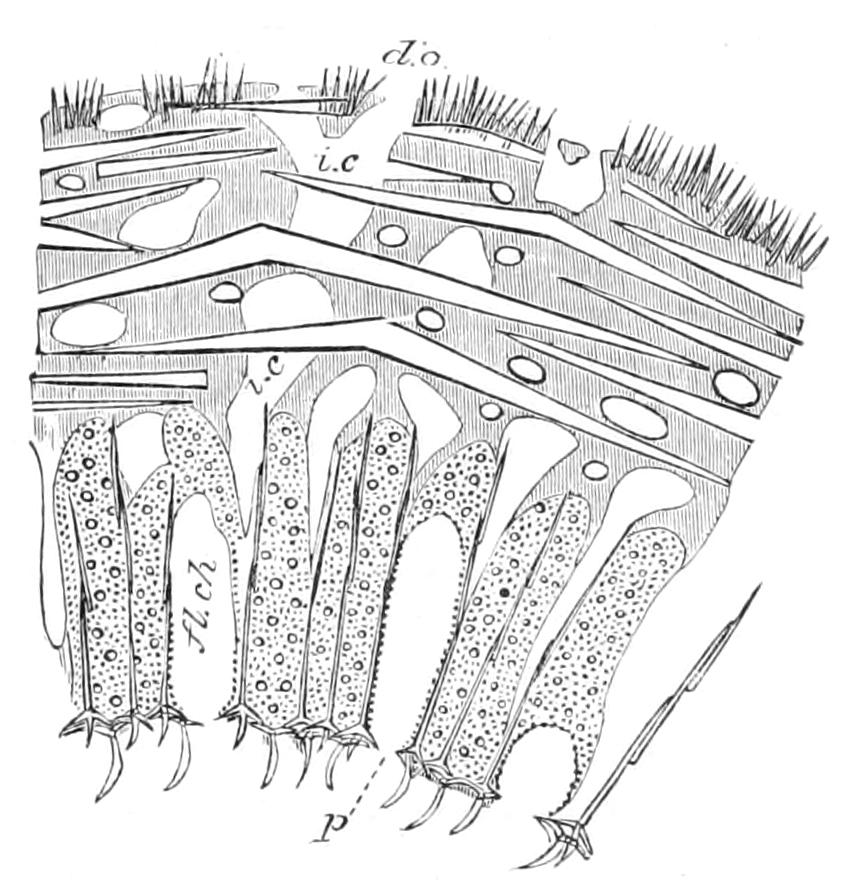
Fig. 84.—Transverse section through the body-wall of Grantiopsis. d.o, Dermal ostium; fl.ch, flagellated chamber; i.c, long incurrent canal traversing the thick cortex to reach the chamber layer; p, apopyle. (After Dendy.)
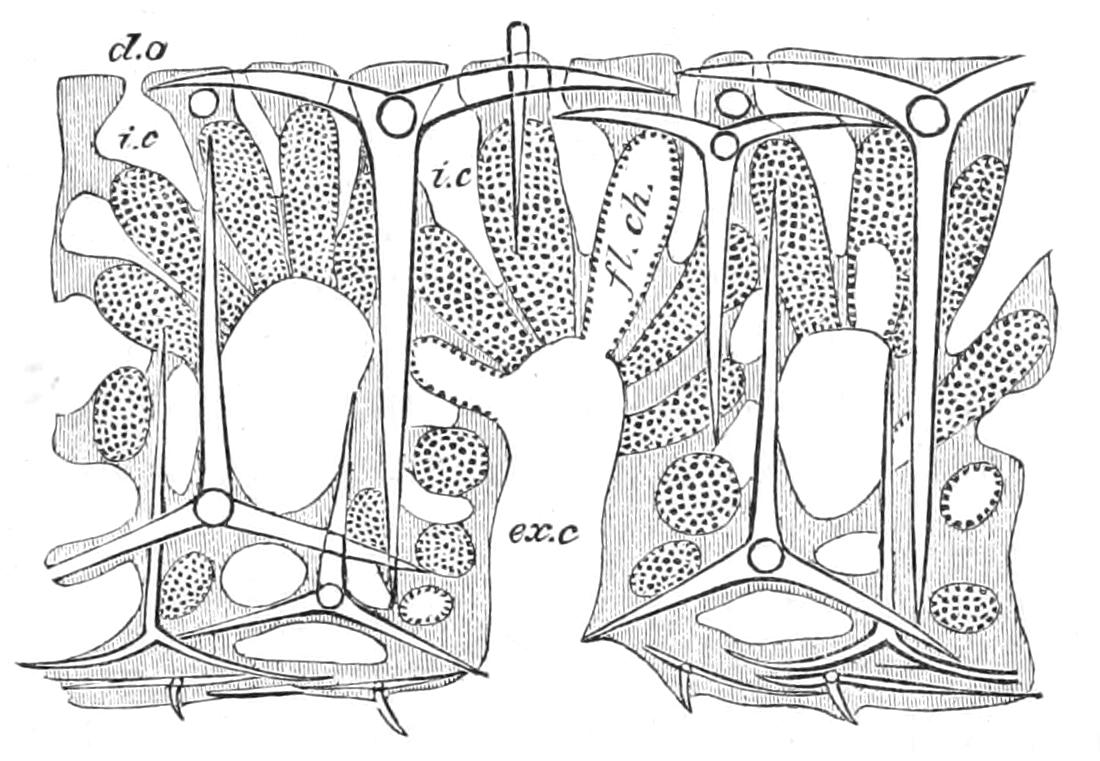
Fig. 85.—Transverse section through the body-wall of Leucilla. d.o, Dermal ostium; ex.c, exhalant canal; fl.ch, chamber; i.c, inhalant canal. (After Dendy.)
Above are described three main types of canal system—that of Homocoela, of Sycon, and of Leucandra and Leucilla. These are conveniently termed the first, second, and third types respectively, and may be briefly described as related to one another somewhat in the same way as a scape, umbel, and compound umbel among {192}inflorescences. These types formed the basis of Haeckel's famous classification.[221] It has, however, been concluded[222] that the skeleton is a safer guide in taxonomy, at any rate for the smaller subdivisions; and in modern classifications genera with canal systems of the third type will be found distributed among various families; while in the Grantiidae, Ute and Leucandra stand side by side. This treatment implies a belief that the third type of canal system has been independently and repeatedly evolved within the Calcarea—an example of a phenomenon, homoplasy, strikingly displayed throughout the group. It is, remarkably enough, the case that all the canal systems found in the remainder of the Porifera are more or less modified forms of one or other of the second two types of canal system above described.
The families Grantiidae, Heteropidae, and Amphoriscidae, all possessing a dermal cortex, are distinguished as follows:—The Grantiidae by the absence of subdermal sagittal triradiate spicules and of conspicuous subgastral quadriradiates; the Heteropidae by the presence of sagittal triradiates; the Amphoriscidae by the presence of conspicuous subgastral quadriradiates.
Two families of Calcarea, possibly allied, remain for special mention—the Pharetronidae, a family rich in genera, and containing almost all the fossil forms of the group, and the Astroscleridae.
The Pharetronidae are with one, or perhaps two exceptions, fossil forms, having in common the arrangement of the spicules of their main skeletal framework in fibres. The family is divided into two sub-families:—
I. Dialytinae.—The spicules are not fused to one another; the exact mode of their union into fibres is unknown, but an organic cement may be present.
Lelapia australis, a recent species, should probably be placed here as the sole living representative. Dendy has shown[223] that this remarkable species has a skeleton of the same fibrous character as is found in typical Dialytinae, and that the triradiate spicules in the fibres undergo a modification into the "tuning-fork" type (Fig. 86, C), to enable them to be compacted into smooth fibres. {193}"Tuning-forks," though not exclusively confined to Pharetronids, are yet very characteristic of them.

Fig. 86.—Portions of the skeleton of Petrostroma schulzei. A, Framework with ensheathing pellicle; B, quadriradiate spicules with laterally fused rays; C, a "tuning-fork." (After Doederlein.)
II. Lithoninae.—The main skeletal framework is formed of spicules fused together, and is covered by a cortex containing free spicules.
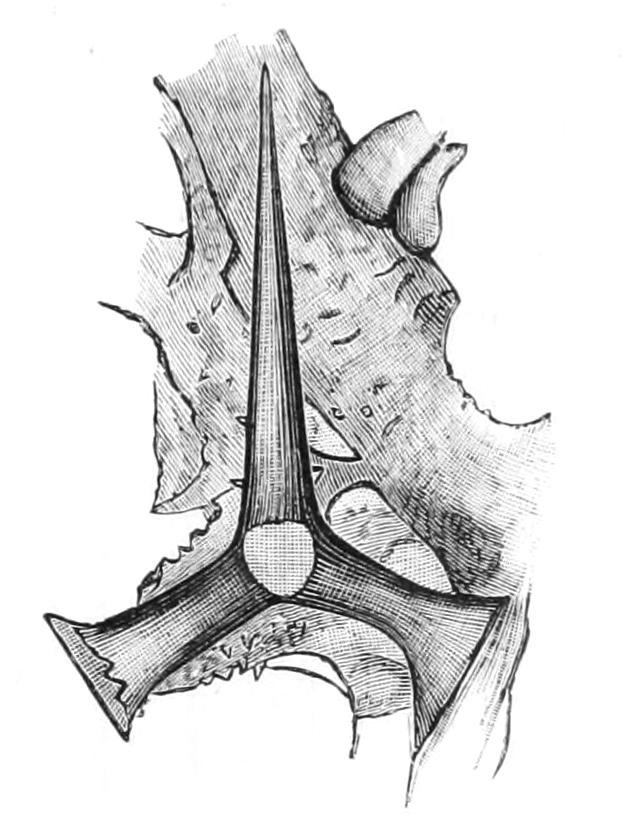
Fig. 87.—A spicule from the skeleton framework of Plectroninia, showing the terminally expanded rays. (After Hinde.)
The sub-family contains only one living genus and a few recently described fossil forms. Petrostroma schulzei[224] lives in shallow water near Japan; Plectroninia halli[225] and Bactronella were found in Eocene beds of Victoria; Porosphaera[226] long known from the Chalk of England and of the Continent, has recently been shown by Hinde[226] to be nearly allied to Plectroninia; finally, Plectinia[227] is a genus erected by Počta for a sponge from Cenomanian beds of Bohemia. Doederlein, in 1896, expressed his opinion that fossil representatives of Lithoninae would most surely be discovered. The fused spicules are equiangular quadriradiates; they are united in Petrostroma by lateral fusion of the rays, in Plectroninia (Fig. 87) and Porosphaera by {194}fusion of apposed terminal flat expansions of the rays, and in some, possibly all, genera a continuous deposit of calcium carbonate ensheaths the spicular reticulum. Thus they recall the formation of the skeleton on the one hand of the Lithistida and on the other of the Dictyonine Hexactinellida (see pp. 202, 211). "Tuning-forks" may occur in the dermal membrane.
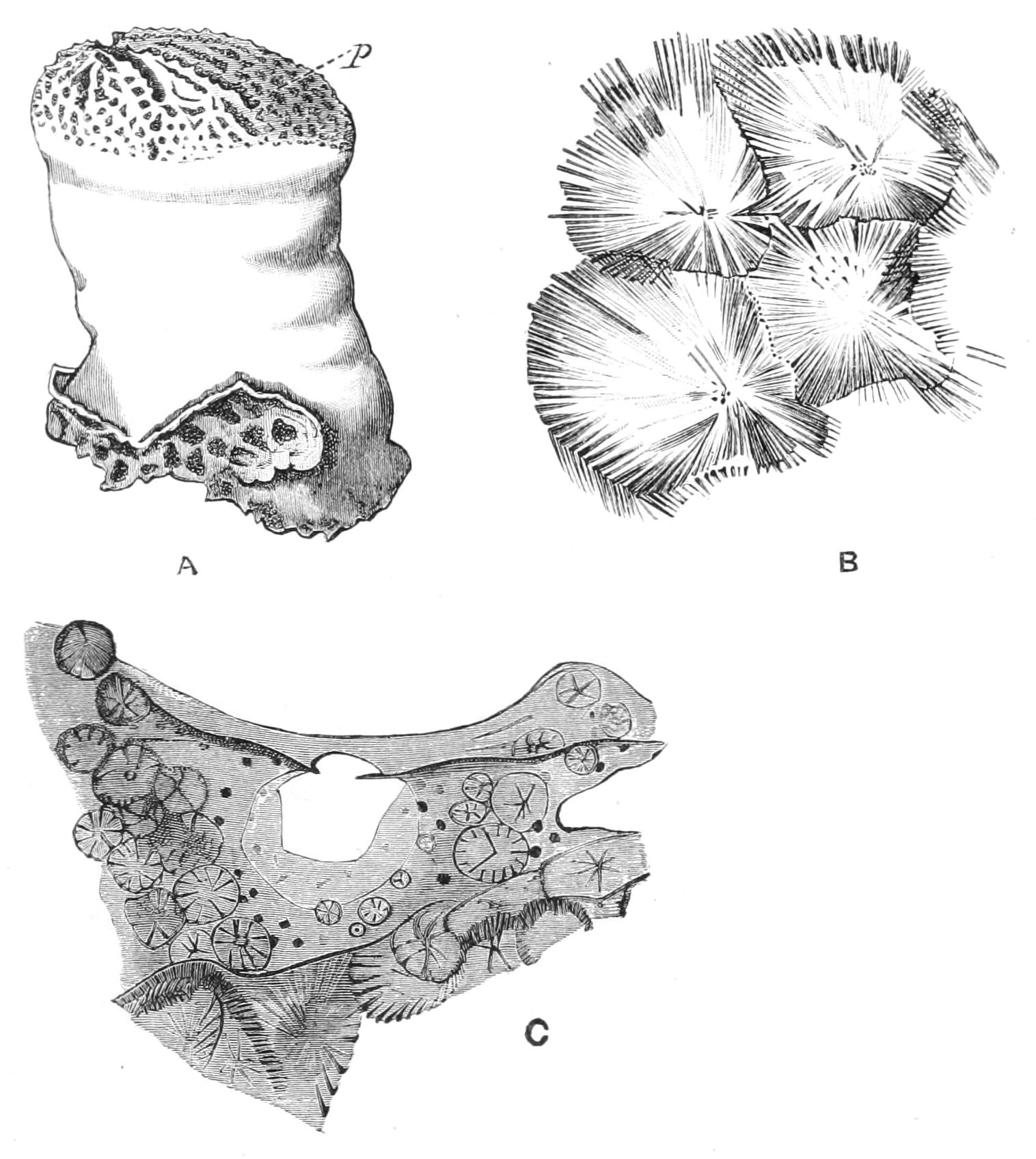
Fig. 88.—Astrosclera willeyana, Lister. A, the Sponge, × about 3. p, The ostia on its distal surface. B, a portion of the skeleton showing four polyhedra with radiating crystalline fibres. C, an ostium; the surrounding tissue contains young stages of polyhedra. (After Lister.)
The Astroscleridae, as known at present, contain a single genus and species, apparently the most isolated in the phylum. Astrosclera willeyana[228] was brought back from the Loyalty Islands, and from Funafuti of the Ellice group. Its skeleton is both chemically and structurally aberrant. In other Calcarea the calcium carbonate of the skeleton is present as calcite, in Astrosclera as aragonite, and the elements are solid polyhedra, {195}united by their surfaces to the total exclusion of soft parts (Fig. 88). Each element consists of crystalline fibres radially disposed around a few central granules, and terminating peripherally in contact with the fibres of adjacent elements. Young polyhedra are to be found free in the soft parts at the surface. The chambers are exceptionally minute, especially for a calcareous sponge, comparing with those of other sponges as follows:—
Astrosclera chambers, 10µ × 8µ to 18µ × 11µ.
Smallest chambers in Silicea, 15µ × 18µ to 24µ × 31µ.
Smallest chambers in Calcarea, 60µ × 40µ.
In its outward form Astrosclera resembles certain Pharetronids. The minute dimensions of the ciliated chambers relegate Astrosclera to the Micromastictora, and the fortunate fact that the calcium carbonate of its skeleton possesses the mineral characters not of calcite, but of aragonite, renders it less difficult to conceive that its relations may be rather with the non-calcareous than the calcareous sponges.
BRANCH II. MICROMASTICTORA
All sponges which do not possess calcareous skeletons are characterised by choanocytes, which, when compared with those of Calcarea, are conspicuous for their smaller size. The great majority (Silicispongiae) of the non-calcareous sponges either secrete siliceous skeletons or are connected with siliceous sponges by a nicely graded series of forms. The small remainder are entirely askeletal. All these non-calcareous sponges are included, under the title Micromastictora, in a natural group, opposed to the Megamastictora as of equal value.
The subdivision of the Micromastictora is a matter of some difficulty. The Hexactinellida alone are a well circumscribed group. After their separation there remains, besides the askeletal genera, an assemblage of forms, the Demospongiae, which fall into two main tribes. These betray their relationship by series of intermediate types, but a clue is wanting which shall determine decisively the direction in which the series are to be read. The askeletal genera are the crux of the systematist. It is perhaps safest, while recognising that many of them bear a likeness of {196}one kind or another to various Micromastictora, to retain them together in a temporary class, the Myxospongiae.
CLASS I. MYXOSPONGIAE
The class Myxospongiae is a purely artificial one, containing widely divergent forms, which possess a common negative character, namely, the absence of a skeleton. As a result of this absence they are all encrusting in habit.
One genus, Hexadella, has been regarded by its discoverer Topsent[229] as an Hexactinellid. The same authority places Oscarella with the Tetractinellida; it is more difficult to suggest the direction in which we are to seek the relations of the remaining type, Halisarca.
Hexadella, from the coast of France, is a remarkable little rose-coloured or bright yellow sponge, with large sac-like flagellated chambers and a very lacunar ectosome.
Oscarella is a brightly coloured sponge, with a characteristic velvety surface; it is a British genus, but by no means confined to our shores. Its canal system has been described by some authors as diplodal, by others as eurypylous. Topsent[230] has shown, and we can confirm his statement, that though the chambers have usually the narrow afferent and efferent ductules of a diplodal system, yet since each one may communicate with two or three canals, the canal system cannot be described as diplodal. The hypophare attains a great development, and in it the generative products mature. The pinacocytes, like those of Plakinidae, and perhaps of Aplysilla, are flagellated.
Halisarca, also British, is easily distinguished from Oscarella by the presence of a mucus-like secretion which oozes from it, and by the absence of the bright coloration characteristic of Oscarella. It naturally suggests itself that the coloration in the one case and the secretion in the other are protective, and in this respect perform one of the functions of the skeleton of other sponges. The chambers are long, tubular, and branched. There is no hypophare.
CLASS II. HEXACTINELLIDA[231]
Silicispongiae, defined by their spicules, of which the rays lie along three rectangular axes. The canal system is simple, with thimble-shaped chambers. The body-wall is divided into endosome, ectosome, and choanosome.
Some authors would elevate the Hexactinellida to the position of a third main sub-group of Porifera, thus separating them from other siliceous sponges. In considering this view it is important to realise at the outset that they are deep-water forms. They bear evident traces of the influence of their habitat, and like others of the colonists of the deep sea, are impressed with marked archaic features. Yet they are still bound to other Micromastictora, first by the small size of their choanocytes, and secondly by the presence of siliceous spicules. This second character is really a double link, for it involves not merely the presence of silica in the skeleton, but also the presence in each spicule of a well-marked axial filament. Now this axial filament is a structure which is gaining in importance, for purposes of classification, in proportion as its absence in Calcarea is becoming more probable. The Hexactinellida are the only sponges, other than the bath sponge, which are at all generally known. They have won recognition by their beauty, as the bath sponge by its utility, and, like it, one of their number—the Venus's Flower-Basket—forms an important article of commerce, the chief fishery being in the Philippine Islands. This wonderful beauty belongs to the skeleton, and is greatly concealed when the soft parts are present.
We have said that the Hexactinellids are deep-sea forms; they are either directly fixed to the bottom or more often moored in the ooze by long tufts of rooting spicules. In the "glass-rope sponge," the rooting tuft of long spicules, looking like a bundle of spun glass, is valued by the Japanese, who export it to us. In Monorhaphis the rooting tuft is replaced by a single giant spicule,[232] three metres in length, and described as "of the thickness of a little finger"! Probably it is as a result of their fixed life in the calm waters of the deep sea[233] that {198}Hexactinellids contrast with most other sponges by their symmetry. It should not, however, be forgotten that many of the Calcarea which inhabit shallow water exhibit almost as perfect a symmetry.
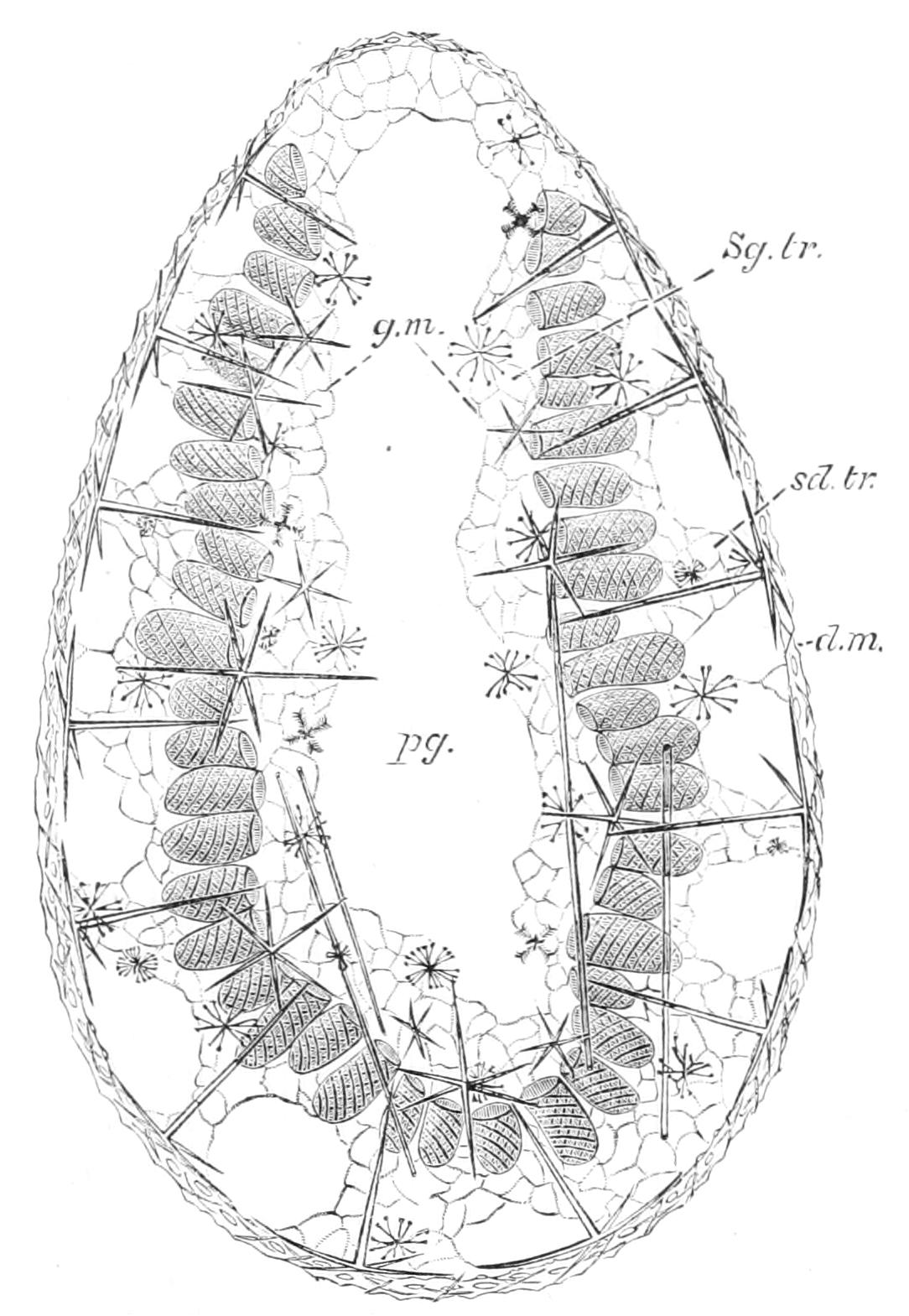
Fig. 89.—Longitudinal section of a young specimen of Lanuginella pupa O.S., with commencing formation of the oscular area. × 35. d.m, Dermal membrane; g.m, gastral membrane; pg, paragaster; sd.tr, subdermal trabeculae; Sg.tr, subgastral trabeculae. (After F. E. Schulze.)
The structure of the body-wall in Hexactinellida is so constant as to make it possible to give a general description applicable to all members of the group. It is of considerable thickness, but a large part is occupied by empty spaces, for the actual tissue is present in minimum quantity. In the wall the chamber-layer is suspended by trabeculae of soft tissue, between a dermal membrane on the outside and a similar gastral membrane on the inner side (Fig. 89). Thus the water entering the chambers through their numerous pores has first passed through the ostia in the dermal membrane and traversed the subdermal trabecular space; on leaving the chambers it flows through the subgastral trabecular space and the ostia in the gastral membrane, to enter the paragaster and leave the body at the osculum. The trabeculae and the dermal and gastral membranes together constitute the dermal layer. This conclusion is based on comparison with adults of the other groups, for in the absence of embryological knowledge no direct evidence is available. According to {199}the Japanese investigator, Isao Ijima,[234] the dermal and gastral membranes are but expansions of the trabeculae, and the trabeculae themselves are entirely cellular, containing none of the gelatinous basis met with in the dermal layer of all other sponges. There is no surface layer of pinacocytes, the cells forming the trabeculae being all of one type, namely, irregularly branching cells, connected with one another by their branches to form a syncytium. In the trabeculae are found scleroblasts and archaeocytes.
The chambers have a characteristic shape: they are variously described as "thimble-shaped," "tubular," or "Syconate," and they open by wide mouths into the subgastral trabecular space. Their walls have been named the membrana reticularis from the fact that, when preserved with only ordinary precautions, they are seen as a regular network of protoplasmic strands, with square meshes and nuclei at the nodes. This appearance recently found an explanation when Schulze, for the first time, succeeded in preserving the collared cells of Hexactinellids.[235] Schulze was then able to show that the choanocytes are not in contact with one another at their bases, where the nuclei are situated, but communicate with one another by stout protoplasmic strands. The form of the choanocyte can be seen in Fig. 91.

Fig. 90.—Portion of the body-wall of Walteria sp., showing the thimble-shaped flagellated chambers, above which is seen the dermal membrane. (After F. E. Schulze.)
To Schulze's description of the chamber, Ijima has added the important contributions that every mesh in the reticulum functions as a chamber pore or prosopyle; and that porocytes, such as are found in Calcarea, are wanting. This structure of the chamber-walls, the absence of gelatinous basis in the dermal layer, and the slight degree of histological differentiation in {200}the same layer, added to the more obvious character of thimble-shaped chambers, are the chief archaic features of Hexactinellid morphology.
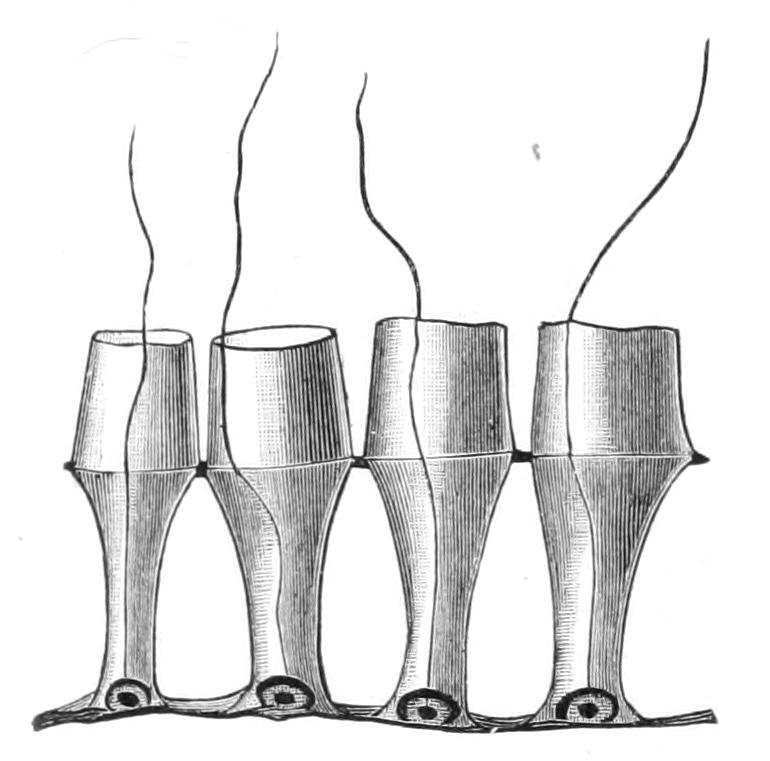
Fig. 91.—Portion of a section of the membrana reticularis or chamber-wall of Schaudinnia arctica, × 1500. (After F. E. Schulze.)
The skeleton which supports the soft parts is, like them, simple and constant in its main features. It is secreted by scleroblasts, which lie in the trabeculae, and is made up of only one kind of spicule and its modifications. This is the hexactine, a spicule which possesses six rays disposed along three rectangular axes. Each ray contains an axial thread, which meets its fellow at the centre of the spicule, where they together form the axial cross. Modifications of the hexactine arise either by reduction or branching, by spinulation or expansion of one or more of the rays. The forms of spicule arising by reduction are termed pentactines, tetractines, and so on, according to the number of the remaining rays. Those rays which are suppressed leave the proximal portion of their axial thread as a remnant marking their former position (Fig. 94). Octactine spicules seem to form an exception to the above statements, but Schulze has shown that they too are but modifications of the hexactine arising by (1) branching of the rays of a hexactine, followed by (2) recombination of the secondary rays (Fig. 92).
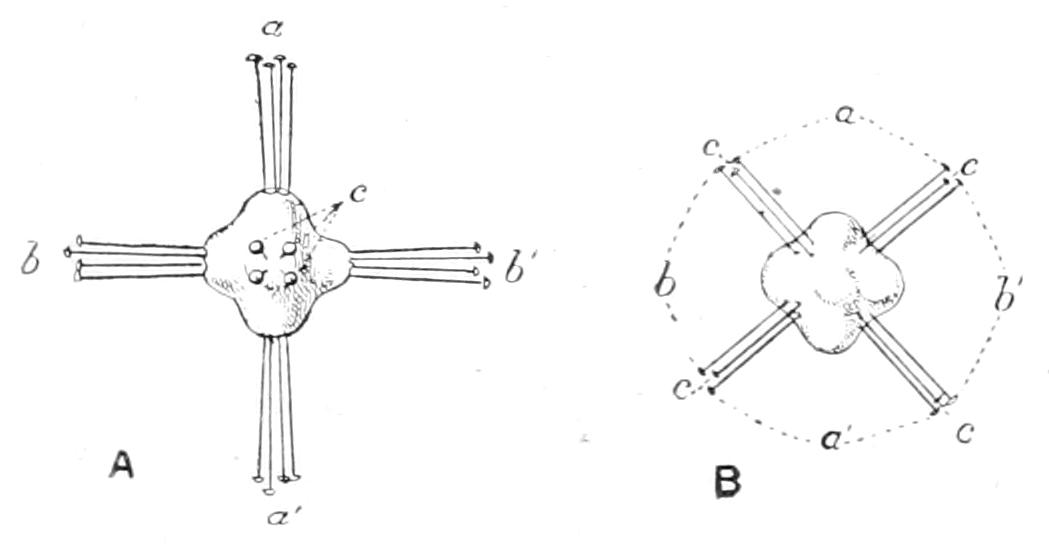
Fig. 92.—A, discohexaster, in which the four cladi a, a', b, b', c of each ray start directly from a central nodule. B, disco-octaster, resulting from the redistribution of the twenty-four cladi of A into eight groups of three. (After Schulze, from Delage.)
The various spicules are named, irrespective of their form, according to their position and corresponding function. The {201}arrangement of the spicules is best realised by means of a diagram (Fig. 93).
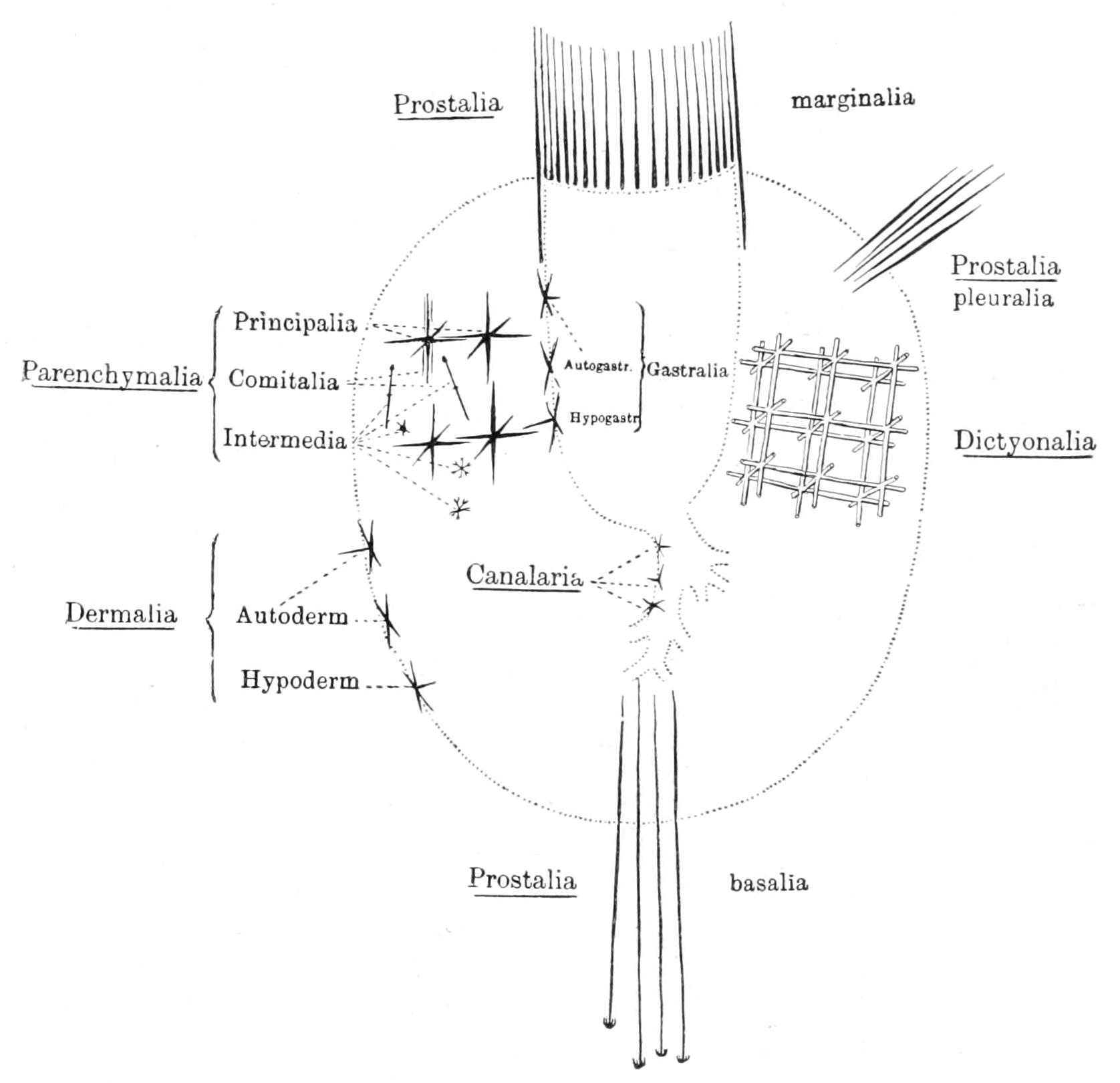
Fig. 93.—Scheme to show the arrangement of spicules in the Hexactinellid skeleton. Canalaria, microscleres in the walls of the excurrent canals; Dermalia Autoderm[alia], microscleres in the dermal membrane; D. Hypoderm[alia], more deeply situated dermalia; Dictyonalia, parenchymalia which become fused to form the skeletal framework of Dictyonina; Gastralia Autogastr[alia], microscleres in the gastral membrane; Gastralia Hypogastr[alia], more deeply situated gastralia; Parenchymalia Principalia, main supporting spicules between the chambers; P. Comitalia, slender diactine or triactine spicules accompanying the last; P. Intermedia, microscleres between the P. principalia; Prostalia, projecting spicules; P. basalia, rooting spicules, from the base; P. marginalia, defensive spicules, round the oscular rim; P. pleuralia, defensive spicules, from the sides. (From Delage and Hérouard, after F. E. Schulze.)
The deviations from this ground-plan of Hexactinellid structure are few and simple. They are due to folding of the chamber-layer, or to variations in the shape of the chambers, and to increasing fusion of the spicules to form rigid skeletons. A simple condition of the chamber-layer, like that of the young sponge of Fig. 89, {202}occurs also in some adult Hexactinellids, e.g. in Walteria of the Pacific Ocean (Fig. 90). Thus is represented in this order the second type of canal system described among Calcarea. More frequently, however, instead of forming a smooth sheet, the chamber-layer grows out into a number of tubular diverticula, the cavities of which are excurrent canals; these determine a corresponding number of incurrent canals which lie between them. In this way there arises a canal system resembling the third type of Calcarea. By still further pouching so as to give secondary diverticula, opening into the first, a complicated canal system is formed, as, for example, in Euplectella suberea.
To return to the skeleton, the most complete fusion is attained by the deposit of a continuous sheath of silica round the apposed parallel rays of neighbouring spicules. This may be termed the dictyonine type of union, for it occurs in all those forms originally included under the term Dictyonina, in which the cement is deposited pari passu with the formation of the spicules. In other cases connecting bridges of silica unite the spicules, or there may be a connecting reticulum of siliceous threads, or, again, rays crossing obliquely may be soldered together at the point of contact. These more irregular methods occur in species where the spicules are free at their first formation. Spicules originally free may later be united in a true Dictyonine fashion. The terms Lyssacina and Dictyonina are useful to denote respectively: the former all those Hexactinellida in which the spicules are free at their first formation, and the latter those in which the deposit of the cementing layer goes hand in hand with the formation of the spicules. But the terms do not indicate separateness of origin of the groups denoted by them, for there is evidence that Dictyonine types have been derived repeatedly from Lyssacine types, and that in fact every Dictyonine was once a Lyssacine.
The real or natural cleft in the class lies between those genera possessing amphidiscs (Figs. 94, 97) among their microscleres, and all the remainder of the Hexactinellida which bear hexasters (Fig. {203}96). The former set of genera constitute the sub-class Amphidiscophora, the latter the Hexasterophora.
Sub-Class 1. Amphidiscophora.—Amphidiscs are present, hexasters absent. A tuft of rooting spicules or basalia is always present. The ciliated chambers deviate more or less from the typical thimble shape, and the membrana reticularis is continuous from chamber to chamber (Figs. 94, 95, 97).
Sub-Class 2. Hexasterophora.—Hexasters are present, amphidiscs absent. The chambers have the typical regular form, and are sharply marked off from one another (Figs. 90, 96).
All the Amphidiscophora have Lyssacine skeletons; in the Hexasterophora both types of skeleton occur. The subdivision of the Hexasterophora is determined by the presence or absence of uncinate spicules. An "uncinatum" is a diactine spicule, pointed at both ends and bearing barbs all directed towards one end. This method of classification gives us a wholly Dictyonine order, Uncinataria, and an order consisting partly of Dictyonine, partly of Lyssacine genera, which may be distinguished as the Anuncinataria. {204}Ova have rarely been found, and sexually produced larvae never; but Ijima has found archaeocyte clusters in abundance, and his evidence is in favour of the view that they give rise asexually to larvae, described by him in this class for the first time (see p. 231).
Both sub-classes are represented in British waters: the Amphidiscophora by Hyalonema thomsoni and Pheronema carpenteri; the Hexasterophora by Euplectella suberea and Asconema setubalense, and of course possibly by others.
Hyalonema thomsoni, one of the glass-rope sponges, was dredged by the Porcupine off the Shetland Islands in water of about 550 fathoms. The spindle-shaped body of the sponge is shown in Fig. 97. Its long rooting tuft is continued right up its axis, to end in a conical projection, which is surrounded by four apertures leading into corresponding compartments of the paragaster.

Fig. 97.—Hyalonema thomsoni. A, Whole specimen with rooting tuft and Epizoanthus crust; B, pinulus, a spicule characteristic of but not peculiar to the Amphidiscophora, occurring in the dermal and gastral membranes; C, amphidisc with axial cross; D, distal end of rooting spicule with grapnel. (After F. E. Schulze.)
The crust of Anthozoa of the genus Epizoanthus (p. 406) on the rooting tuft is a constant feature in this as in other species of Hyalonema. It contributed to make the sponge a puzzle, which long defied interpretation. The earliest diagnosis the genus received was the "Glass Plant." Then the root tuft was thought to be part of the Epizoanthus, which was termed a "most aberrant Alcyonarian with its base inserted in a sponge"; next we hear of the sponge as parasitic {205}on the Sea Anemone. Finally, the root tuft was shown to be proper to the sponge, which was, however, figured upside down, till some Japanese collectors described the natural position, or that in which they were accustomed to find it.
Pheronema carpenteri was found by the Lightning off the north of Scotland in 530 fathoms. The goblet shaped, thick walled body and broad, ill-defined root tuft are shown in Fig. 98, but no figure can do justice to the lustre of its luxuriant prostalia and delicate dermal network with stellate knots at regular intervals. The basalia are two-pronged and anchor-like.
Both the Hexasterophoran genera were dredged off the north of Scotland, and both conform to the Lyssacine type without uncinates. Euplectella suberea is a straight, erect tube, anchored by a tuft {206}of basalia. The upper end of the tube is closed by a sieve plate, the perforations in which are oscula, while the beams contain flagellated chambers, so that the sieve is simply a modified portion of the wall. It is a peculiarity of this as of one or two other allied genera that the lateral walls are perforated by oscula. They are termed parietal gaps, and are regularly arranged along spiral lines encircling the body.
Ijima, who has dredged Euplectellids from the waters near Tokyo, finds that in young specimens oscula are confined to the sieve plate; parietal gaps are secondary formations. The groundwork of the skeleton is a lattice similar to that shown in Fig. 100. The chamber-layer is much folded. Various foreign species of Euplectella afford interesting examples of association with a Decapod Crustacean, Spongicola venusta, of which a pair lives in the paragaster of each specimen. The Crustacean is light pink, the female distinguished by a green ovary, which can be seen through the transparent tissues. It is not altogether clear what the prisoner gains, nor what fee, if any, the host exacts.
Ijima relates that the skeleton of Euplectella is in great demand in Japan for marriage ceremonies. He also informs us that the Japanese name means "Together unto old age and unto the same grave," while by a slight alteration it becomes "Lobsters in the same cell," and remarks that the Japanese find this an amusing pun.
The same Spongicola lives in pairs in Hyalonema sieboldi. Another case of apparently constant association is that of the Hydroid stocks which inhabit Walteria. F. E. Schulze describes Stephanoscyphus mirabilis (see p. 318) in a specimen of Walteria flemmingi; the presence of the polyp causes the sponge to grow out into little dome-shaped elevations, each of which shelters one polyp; while in W. leuckarti Ijima finds a similar association in every specimen examined.
Fossil Hexactinellida.
This group has the distinction of including among its Lyssacine members the oldest known sponge, Protospongia fenestrata, of Cambrian age (Salter). As preserved it consists of a single layer of quadriradiate, or possibly quinqueradiate spicules, which, arranged as a square meshed lattice, supported the superficial layer of the sponge (Fig. 101). Whether or not the fossil represents the whole of the sponge-skeleton does not appear.[236]
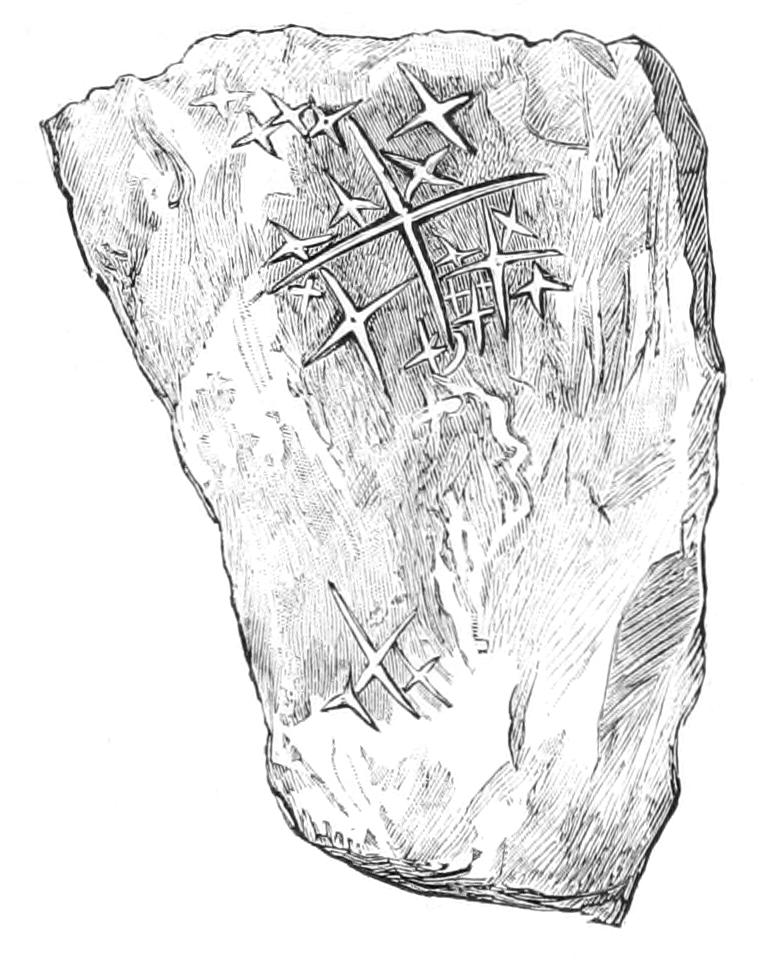
Fig. 101.—Part of the specimen of Protospongia fenestrata in the Sedgwick Museum, Cambridge. Nat. size. (After Sollas.)
Fig. 102.—A portion of the outer surface of a Receptaculitid, Acanthoconia barrandei, in which the expanded outer rays of the spicules are partially destroyed, revealing the four tangential rays beneath, × 3. (After Hinde.)
The extraordinary Receptaculitidae are probably early Lyssacine forms: they are cup- or saucer-shaped fossils, abundant in Silurian and above all in Devonian strata, and have been "assigned in turn to pine cones, Foraminifera, Sponges, Corals, Cystideans," and Tunicata. Hinde[237] brings forward important arguments for retaining them among Hexactinellida. The only elements in the skeleton of the simpler genera, e.g. Ischadites, are structures comparable to Hexactinellid spicules. The surface of the fossil presents a series of lozenges forming a regular mosaic. Each lozenge is the expanded end of one of the rays of a spicule; it conceals four rays in one plane, tangential to the wall of the cup-shaped fossil, while the sixth ray projects vertically to the wall into the cavity of the cup. In the genus Receptaculites itself there is an inner layer of plates abutting against the inner {208}ends of the sixth rays, and at present problematic. An axial canal is present in each of the rays—the six canals meeting at the centre of the spicule. Special chinks between the spicules appear to have provided a passage for the water current.
The beautiful Ventriculites, so common in the Chalk and present in the Cambridge Greensand, are historically interesting, for the fact that they are fossil Hexactinellida of which the general and skeletal characters were very minutely described by Toulmin Smith long before recent representatives of the group were known. In common with a number of fossil Dictyonine species they are distinguished by the perforation of the nodes, a character due to the fact that the siliceous investment which unites the spicules together stops short before reaching the centre of each spicule, and bridges across the rays so as to form a skeleton octahedron. This character is rare in recent Hexactinellids, but, as first pointed out by Carter, it is presented by one or two forms, of which Aulocystis grayi Bwk is best known. The majority of the fossil Hexactinellida belong to the Dictyonine section, a fact attributable to the greater coherence of their skeleton. The "Dictyonina" are to be reckoned among the rock-builders of Jurassic and Cretaceous times.
The Octactinellida and Heteractinellida are two classes created by Hinde[238] to contain certain little-known Devonian and Carboniferous sponges, possessing in the one case 8-rayed spicules, of which 6 rays lie in one plane and 2 are perpendicular to this plane; in the other case, spicules with a number of rays varying from 6 to 30. Bearing in mind the manner in which octactine spicules are known to arise in recent Hexactinellida (p. 200), it is clearly possible to derive these 8-rayed spicules from hexactines by some similar method; while the typical {209}spicule of the Heteractinellida is a euaster. Hence we may refer the Octactinellid fossils to the class Hexactinellida, and the Heteractinellid forms either to the Monaxonida or Tetractinellida.
CLASS III. DEMOSPONGIAE
Silicispongiae in which triaxonid spicules are absent.
This class has attained the highest level of organisation known among Porifera; the most efficient current-producing apparatus is met with here, so, too, are protective coverings, stout coherent skeletons, and the highest degree of histological differentiation found in the phylum.
Correspondingly it is the most successful group, the majority of existing sponges coming within its boundaries. A few genera and species are exceedingly specialised, for example, Disyringa dissimilis (p. 215). These, however, contribute only a very small contingent to the Demosponge population, those species which are really prolific and abundant being, as we should expect, the less exaggerated types.
Canal System.—With a few exceptions the representatives of the Demospongiae may be said to have taken up the evolution of the canal system at the stage where it was left in Leucandra aspera—a stage which the ancestral Demosponges must have reached quite independently of the Calcarea. These commoner members are thus already gifted with the advantages pertaining to a spherical form of ciliated chamber, and so, too, is the Rhagon (Fig. 105), an immature stage noteworthy as the simplest form of Demosponge, and thus the starting-point for the higher types of canal system. The exceptions above alluded to are not without interest: they are the Dendroceratina, of doubtful affinities, (p. 220), which possess small tubular Syconate chambers. They may be regarded either as of independent origin from other Demospongiae, thus making the group polyphyletic, or more simply as representing the ancestral condition, and in this case we must look on the possession of spherical chambers by the Rhagon as a secondary feature. Occupying as it does the important position above indicated, the Rhagon merits a brief description. It is a small discoid or hemispherical body attached by a flat base. It contains a central paragaster, with a single osculum at the free end. Into the paragaster open directly a {210}few spherical flagellated chambers, which lie in the lateral walls of the body. The basal wall of the paragaster, the parts of its lateral walls between the openings of neighbouring chambers, and the entire outer surface of the body are covered with pinacocytes. It is convenient to call the basal part of the sponge from which chambers are absent the hypophare, the upper chamber-bearing part the spongophare. In some of the deeper dermal cells spicules may be already present. In the Rhagon, then, the canal system is of the second type, but all the adult Demosponges have advanced to the third type, and the further evolution in this system is in the direction of improving the mode of communication of the chambers with the canal system. The changes involved go hand in hand with increasing bulk of the dermal layer. A glance at the accompanying figures will show at once the connexion between the phenomena. The increase in the dermal layer (1) greatly reduces the extent of the lumen of the excurrent canals; and (2) results in the intervention of a narrow tube or aphodus between the mouth of each chamber and the excurrent canal. The chamber system is then converted from an "eurypylous" to an "aphodal" type. When the incurrent canal also opens into the chamber by way of narrow tubes, one proper to each chamber and termed "prosodus," the canal system is of the "diplodal" type.
Fig. 104.—Diagram of (A) eurypylous and (B) aphodal canal systems. a, Apopyle; a', aphodus; E, excurrent canal; I, incurrent canal; p, prosopyle; p', short prosodus. (After Sollas.)
Cortex.—All the stages in the formation of a cortex are to be seen among the adult members of the group. Certain species (e.g. Plakina monolopha, F.E.S.) are destitute even of an ectosome, {211}others have a simple dermal membrane (Halichondria panicea, Tetilla pedifera) and various others are provided with a cortex, either of simple structure or showing elaboration in one or more particulars. Thus a protective armature of special spicules may be present in the cortex, e.g. in Geodia, or to a less extent in Tethya, or there may be an abundance of contractile elements, and these may be arranged in very definite ways, forming valve-like apparatus that will respond to stimuli.
Everywhere among sponges the goal of the skeleton appears to have been coherence. We have seen how in Calcarea and in Hexactinellida this has been attained by the secretion around the separate elements of a continuous mineral sheath, calcareous in the one case and siliceous in the other. Here we had an excellent instance of the attainment of one end by similar means in two different groups, after their separation from the common stock, and therefore independently. In Demospongiae, on the other hand, the same end—coherence—has been secured by two new methods, each distinct from the former: first the spicules may be united in strands by an organic deposit, spongin; secondly, the spicules may assume irregular shapes and interlock closely with one another, forming dense and stout skeletons. The latter method is that characteristic of the Lithistid Tetractinellida.
Classification.—It is not of great moment which scheme of classification we maintain, seeing that all hitherto proposed are confessedly more or less artificial, and sufficient data for framing a natural one are not yet forthcoming. For convenience, we accept three subdivisions and define them thus:—
I. Tetractinellida.—Demospongiae possessing tetraxon or triaene spicules or Lithistid desmas.
II. Monaxonida.—Demospongiae possessing monaxon but not tetraxon spicules.
III. Ceratosa.—Demospongiae in which the main skeleton is formed of fibres of spongin. The fibres may have a core of sand-grains or of foreign spicules, but not of spicules proper to the sponge.
But at the same time we admit that some of the Ceratosa are probably descended from some of the families of Monaxonida, so that we should perhaps be justified in separating these families of Monaxonida from the rest, and associating them with the allied families of Ceratosa—a method of classification due to {212}Vosmaer. Again, some Monaxonida approximate to Tetractinellida, and we might, with Vosmaer, unite them under the title Spiculispongiae. This proceeding, though it has the advantage of being at least an attempt to secure a natural classification, involves too much assumption when carried out in detail to be wholly satisfactory.
Sub-Class I. Tetractinellida.[239]
Tetractinellida appear to flourish best in moderate depths from 50 to 200 fathoms, but they are found to be fairly abundant also in shallower water right up to the coast line, and in deep water up to and beyond the 1000 fathom line. Occasionally they lie free on the bottom, but are far more commonly attached; fixation may be direct or by means of rooting spicules; the occurrence of a stalk is rare. There is great variety in the root tuft, which may be a long loose wisp of grapnel-headed spicules, as in many species of Tetilla, or a massive tangle, as in Cinachyra barbata; in these cases the sponge is merely anchored, so that it rests at the level of the surface of the ooze; in other cases, e.g. Thenea wyvillei, the root tuft consists of a number of pillars of spicules which raise the sponge above the level of the ooze, into which they descend and there become continuous with a large dense and confused mass of spicules. The parachute-like base of Tetilla casula invites comparison with the "Crinorhiza" forms of some Monaxonids (p. 216).
Two Orders are distinguished thus:—
I. Choristida.—Tetractinellida with quadriradiate spicules, which are never articulated together into a rigid network.
II. Lithistida.—Tetractinellida with branching scleres (desmas), which may or may not be modified tetrad spicules, articulated together to form a rigid network. Triaene spicules may or may not be present in addition.
Order I. Choristida.
Plakina monolopha, from the Adriatic and Mediterranean, furnishes a connecting link between the Rhagon stage and other Tetractinellida. The choanosome is simply folded; there is no distinct ectosome; the chambers are eurypylous. The skeleton {213}consists of microcalthrops and their derivatives. The hypophare is well developed. Plakina thus shows a certain amount of resemblance to Oscarella (p. 196), with which it shares the very remarkable possession of flagellated pinacocytes.
One of the species of Tetilla, T. pedifera, continues the series. The folds of its choanosome are more complicated than in P. monolopha, and their outer ends are bridged together by a thin layer of ectosome (cf. species of Sycon among Calcarea); the chambers are still eurypylous.
The skeleton reaches a high level: it includes oxeas and triaenes radiately disposed and microscleres (sigmata) scattered throughout the dermal layer. The British Poecillastra compressa from the north of Scotland and Orkney and Shetland is at about the same stage of development, being without cortex and having eurypylous chambers, but it is not so good an example, as the folds of its choanosome are confused.
From T. pedifera we pass to the other species of Tetilla and all the higher genera of Choristida; these possess a cortex not of homologous origin in the various cases, but probably to be classified under one of two heads, typified by Stelletta and Craniella respectively (Fig. 106).

Fig. 106.—A, Craniella type; B, Stellettid type. ch, Chone; co, collenchyma; d.o, dermal ostia; fb, fibrous tissue; i.c, intercortical cavity; sd, subdermal cavity; sp, sphincter. (After Sollas.)
In the Stellettids the cortex arises by the centrifugal growth of a dermal membrane such as that of Tetilla pedifera; in Craniella directly from the dermal tissue of the distal ends of the choanosomal folds.
In both cases the end result, after completion of cell differentiation, is a cortex either fibrous throughout or collenchymatous in its outer portion and fibrous in the deeper layers. In the Stellettid type the centrifugal growth of the dermal membrane involves the addition of secondary distal portions to the ends of the inhalant passages. These are the intercortical cavities or canals. Their most specialised form is the "chone." A chone is a passage through the cortex opening to the exterior by one or more ostia, and communicating with the deeper parts of the inhalant system by a single aperture provided with a sphincter (Fig. 106, B).
In the Craniella type the intercortical cavities are parts of the primary inhalant system. They communicate with its deeper parts by sphinctrate apertures. Without any knowledge of the development one would certainly have supposed that the subdermal cavity, pore-sieve and sphinctrate passages of Craniella represented a number of chones, of which the outer portions had become fused (Fig. 106, A).

Fig. 107.—Disyringa dissimilis. Diagrammatic longitudinal section of the Sponge. × ½. a, b, c, Transverse sections at the levels indicated to show subdivision of the lumina of the excurrent and incurrent tubes; e.t, excurrent tube; i.t, incurrent tube; o, osculum. (After Sollas.)
In both Craniella and Stelletta the chamber system is aphodal, and these genera may fairly be taken as representatives of the average level reached by Tetractinellida. The skeleton is of the radiate type: the type which prevails in the Choristida, but which has an erratic distribution, appearing in some genera of {215}each family but not in others. The genus Pachymatisma, of which we have the species P. johnstonia and P. normani in these islands, exemplifies this; it belongs to the highly differentiated family Geodiidae, possesses an elaborate cortex with chones, but its main skeleton is non-radiate.
Disyringa dissimilis is remarkable for the perfection of its symmetry, and for the absence of that multiplication of parts which is so common among sponges. It possesses a single inhalant tube and a single osculum (Fig. 107). Until quite recently it stood alone in the restriction of its inhalant apertures to a single area. Kirkpatrick, however, has now described a sponge—Spongocardium gilchristi[240]—from Cape Colony, in which the dermal ostia are concentrated in one sieve-like patch at the opposite pole to the single osculum. Disyringa is still without companions in the possession of an inhalant tube. The concentration of ostia into sieve areas occurs again in Cinachyra, each sponge possessing in this case several inhalant areas with or without scattered ostia also.
Order II. Lithistida.
The characteristic spicule of Lithistida—the desma—may be a modified calthrop (tetracrepid desma), or it may be produced by the growth of silica over a uniaxial spicule (rhabdocrepid desma) (Fig. 110, q), or it may be of the polyaxon type. It is probable that the group is polyphyletic,[241] and that some of its members should remain associated with Tetractinellida, while others should be removed to Monaxonida. Forms with tetracrepid desmas, and those forms with rhabdocrepid desmas which possess triaenes, have Tetractinellid affinities, while forms possessing rhabdocrepid desmas but lacking triaenes, and again those in which the desmas are polyaxon, are probably descendants of Monaxonida.
Owing to the consistency of the skeleton Lithistida are frequently found as fossils. The commonest known example is Siphonia.[242] As in the case of so many other fossil sponges the skeleton is often replaced by carbonate of lime, a fact which {216}misled some of the earlier investigators but was established by the researches of Sollas and Zittel.
Sub-Class II. Monaxonida.[243]
The Monaxonida inhabit for the most part shallow water, but they also extend through deep water into the abysses, thirteen species having been dredged from depths of over 2000 fathoms by the "Challenger" Expedition alone. In some cases, e.g. Cladorhiza, Chondrocladia, all the species of a genus may live in deep water, while in others the genus, or in others, again, the species, may have a wide bathymetrical range. Thus Axinella spp. occur in shallow water and in various depths down to 2385 fathoms, Axinella erecta ranges from 90 to 1600 fathoms, Stylocordyla stipitata from 7 to 1600, and so on. The symmetry of the deep-water forms contrasts strikingly with the more irregular shape of their shallow-water allies.[244] The shallow-water species are almost always directly attached, some few are stalked; those from deep water have either a long stalk or some special device to save them from sinking in the soft ooze or mud. Thus the deep-sea genus Trichostemma has the form of a low inverted cone, round the base of which a long marginal fringe of spicules projects, continuing the direction of the somal spicules, and so forming a supporting rim. The same form has been independently evolved in Halicnemia patera, and an approach to it in Xenospongia patelliformis. A similar and more striking case of homoplasy is afforded by the Crinorhiza form, which has been attained in certain species of the deep-sea genera Chondrocladia, Axoniderma, and Cladorhiza; here the sub-globular body is supported by a vertical axis or root, and by a whorl of stout processes radiating outwards and downwards from it, and formed of spicular bundles together with some soft tissue.
There is recognisable in the order Monaxonida a cleft between one set of genera, typically corticate, and suggesting by their structure a relationship, whether of descent or parentage, with the Tetractinellida, and a second set typically non-corticate: these latter are the Halichondrina, the former are the Spintharophora.
Order I. Halichondrina.
We have already seen typical examples of the Halichondrina in Halichondria panicea and Ephydatia fluviatilis. Within the Halichondrina the development of spongin reaches its maximum among spiculiferous sponges, and accordingly the Ceratosa take their multiple origin here (p. 220). Among Halichondrina spongin co-operates with spicules to form a skeleton in various ways, but always so as to leave some spicules bare or free in the flesh. It may bind the spicules end to end in delicate networks (as in Reniera or Gellius), or into strands, sometimes reaching a considerable thickness (as in Chalina and others). In a few cases there appears to be a kind of division of labour between the spicules and spongin, the latter forming the bulk of the fibre, i.e. fulfilling the functions of support, while the spicules merely beset its surface as defensive organs, rendering the sponge unfit for food. Fibres formed on this pattern are called plumose, and are typical of Axinellidae. The distinctive fibre of the Ectyoninae is as it were a combination of the Axinellid and Chalinine types: a horny fibre both cored with spicules and beset with them. Spicules besetting the surface of a fibre are termed "echinating." Whenever its origin has been investigated, spongin has proved to be the product of secretion of cells; in the great majority of cases it is poured out at the surface of the cell, and Evans showed,[245] at any rate in one species of Spongilla, that the spongin fibres are continuous with a delicate cuticle at the surface of the sponge. In Reniera spp. occurs a curious case of formation of spongin as an intracellular secretion. A number of spherical cells each secrete within themselves a short length of fibre; they then place themselves in rows, so orientated that their contained rods lie end to end in one line. The rods then fuse and make up continuous threads; the cells diminish in breadth, ultimately leaving the fibre free.[246]
Order II. Spintharophora.
These corticate forms are further characterised by the arrangement of their megascleres, which is usually, like that of most {218}Tetractinellida, radial, or approximating to radial. The microscleres are, when present, some form of aster. The cortex resembles that of Tetractinellida, and v. Lendenfeld has described chones in Tethya lyncurium.[247]
The existence of the above points of resemblance between Spintharophora and Tetractinellida suggests a relationship between the two groups as its cause. In judging this possibility the following reflections occur to us. A cortex exists in various independent branches of Tetractinellida. It has in all probability had a different phylogenetic history in each—why not then in these Monaxonida also? Within single genera of Tetractinellida some species are corticate, others not, witness Tetilla. The value of a cortex for purposes of classification may easily be overestimated. If we are to uphold the relationship between these two groups, we must base our argument on the conjunction of similar characters in each.
The genus Proteleia[248] is interesting for its slender grapnel-like spicules, which project beyond the radially disposed cortical spicules, and simulate true anatriaenes of minute proportions. That they are not anatriaenes is shown by the absence of an axial thread in their cladi. It is not surprising that a form of spicule of such obvious utility as the anatriaene should arise more than once.
Of exceptional interest, on account of their boring habit, are the Clionidae. How the process of boring is effected is not known; the presence of an acid in the tissues was suspected, but has been searched for in vain. The pieces of hard substance removed by the activity of the sponge take their exit through the osculum and have a fixed shape[249] (Fig. 108).
As borers into oyster shells, Clionidae may be reckoned as pests of practical importance, and in some coasts they even devastate the rocks, penetrating to a depth of some feet, and causing them to crumble away.[250]
Sponges, however, as agents in altering the face of the earth do not figure as destroyers merely. On the contrary, it has {219}been calculated[251] that sponge skeletons may give rise with considerable rapidity to beds of flint nodules; in fact, it appears that a period so short as fifty years is sufficient for the formation of a bed of flints out of the skeletons of sponges alone.
Suberites domuncula is well known for its constant symbiosis with the Hermit crab. The young sponge settles on a Whelk or other shell inhabited by a Pagurus, and gradually envelops it, becoming very massive, and completely concealing the shell, without however closing its mouth. The aperture of this always remains open to the exterior, however great the growth of the sponge, a tubular passage being left in front of it, which continues the lumen of the shell and maintains its spiral direction. When the crab has grown too big for the shell, it merely advances a little down this passage. The shell is never absorbed, as was once supposed.[252] The crab, besides being provided with a continually growing house, and being thus spared the great dangers attending a shift of lodgings, benefits continually by the concealment and protection afforded by the massive sponge; the latter in return is conveyed to new places by the crab.

Fig. 108.—A, calcareous corpuscle detached by Cliona; B, view of the galleries excavated by the Sponge. (After Topsent.)
Ficulina ficus is sometimes, like S. domuncula, found in symbiosis with Pagurus, but the constancy of the association is wanting in this case. The sponge has several metamps, one of which, from its fig-like shape, gives it its name.
Sub-Class III. Ceratosa.
The Ceratosa are an assemblage of ultimate twigs shorn from the branches of the Monaxonid tree. They are therefore related forms, but many of them are more closely connected with their Monaxonid relatives than with their associates in their own sub-class.
The genera Aulena and Phoriospongia, placed by v. Lendenfeld among Ceratosa, by Minchin among Monaxonida, show each in its own way how close is the link between these two sub-classes.
Aulena possesses in its deeper parts a skeleton of areniferous spongin fibres, in fact a typical Ceratose skeleton; but this is continuous with a skeleton in the more superficial parts, which is composed of spongin fibres echinated by spicules proper to the sponge, and precisely comparable to the ectyonine fibres of some Monaxonida.
Phoriospongia, as far as its main skeleton is concerned, is a typical Ceratose sponge, with fibres of the areniferous type, but it possesses sigmata free in the flesh.
The sub-class is confined to shallow water, no horny sponge having been dredged from depths greater than 410 fathoms.[253] The greatest number occur at depths between 10 and 26 fathoms.
In the majority of the Ceratosa the skeletal fibres are homogeneous, formed of concentric lamellae of spongin, deposited by a sheath of spongoblasts around a filiform axis. In others, however, the axis attains a considerable diameter, so as to form a kind of pith to the fibre, which is then distinguished as heterogeneous. In one or two cases some of the spongoblasts of a heterogeneous fibre are included in the fibre between the spongin lamellae. Ianthella is the best-known example in which this occurs.
Ceratosa are divided into Dictyoceratina and Dendroceratina, distinguished, as their names express, by the nature of the skeleton—net-like, with many anastomoses, in the one; tree-like, without anastomoses between its branches, in the other.
The Dictyoceratina comprise by far the larger number of Ceratosa. They fall into two main families, the Spongidae and Spongelidae, both represented in British waters. The Spongidae {221}are characterised by a granular ground substance and aphodal chamber system; the Spongelidae by a clear ground substance and sac-like eurypylous chambers.
The bath sponge, Euspongia officinalis, belongs to the Spongidae. The finest varieties come from the Adriatic, the coarser ones from the Dalmatian and North African coasts of the Mediterranean, from the Grecian Archipelago, from the West Indies, and from Australian seas. The softer species of the genus Hippospongia also form a source of somewhat inferior bath sponges.
Among Dendroceratina, Darwinella is unique and tempts to speculation, in that it possesses isolated spongin elements, resembling in their forms triaxon spicules.
Key to British Genera of Sponges.
|
1. |
(a) | Skeleton calcareous | 2 |
| (b) | Skeleton siliceous | 6 | |
| (c) | Skeleton horny, or without free spicules | 53 | |
| (d) | Skeleton absent | 55 | |
|
2. |
(a) | Gastral layer continuous | 3 |
| (b) | Gastral layer discontinuous, confined to chambers | 4 | |
|
3. |
(a) | Equiangular triradiate systems present | Clathrina |
| (b) | Triradiate systems all alate | Leucosolenia | |
|
4. |
(a) | Chambers tubular, radially arranged | 5 |
| (b) | Chambers spherical, irregularly scattered | Leucandra | |
|
5. |
(a) | Tufts of oxeate spicules at the ends of the chambers | Sycon |
| (b) | Oxeate spicules lying longitudinally in the cortex | Ute | |
|
6. |
(a) | All the spicules hexradiate or spicules easily derived from hexradiate type | 7 |
| (b) | Some of the spicules calthrops or triaenes | 10 | |
| (c) | Megascleres uniaxial | 15 | |
|
7. |
(a) | Amphidiscs present | 8 |
| (b) | Amphidiscs absent | 9 | |
|
8. |
(a) | Rooting spicules a well-defined wisp; four apertures lead into the gastric cavity | Hyalonema thomsoni |
| (b) | Rooting tuft diffuse; sponge oval; osculum single | Pheronema carpenteri | |
|
9. |
(a) | Sponge tubular, dermal and gastral pinuli absent | Euplectella suberea |
| (b) | Sponge a widely open cup; dermal and gastral pinuli present | Asconema setubalense | |
|
10. |
(a) | Tetractine spicule, a calthrop or triaene with short rhabdome; microsclere a spined microxea | Dercitus bucklandi |
| (b) | Triaenes with fully developed rhabdome | 11 |

Fig. 109.—Microscleres of Demospongiae. a, b, Sigmaspires viewed in different directions; c, d, bipocilli viewed in different directions; e, toxaspire; f, f', spiraster; g, sanidaster; h, amphiaster; i, sigma; j, diaucistra; k, isochela; l, m, anisochelae viewed in different directions; n, cladotyle; o, toxa; p, forceps; q, oxyaster; r, spheraster; s, oxyaster with 6 actines; t, another with 4 actines; u, another with rays reduced to two (centrotylote microxea); v, tylote microrhabdus; w, trichodragmata; x, oxeate microrhabdus or microxea.
|
11. |
(a) | Microscleres sigmata | Craniella cranium |
| (b) | Sigmata absent, asters present | 12 | |
|
12. |
(a) | Microscleres include spirasters | Poecillastra compressa |
| (b) | Microscleres include sterrasters | 14 | |
| (c) | Microscleres include euasters: spirasters and sterrasters absent | 13 | |
|
13. |
(a) | Two kinds of euaster present | Stelletta |
| (b) | Microscleres include a euaster and a sanidaster or amphiaster | Stryphnus ponderosus | |
|
14. |
(a) | Microscleres include microrhabdi | Pachymatisma johnstonia |
| (b) | Microscleres include many-rayed euasters | Cydonium milleri | |
|
15. |
(a) | Some of the microscleres asters | 16 |
| (b) | Microscleres absent, or not asters | 17 | |
|
16. |
(a) | Skeleton radiate; asters of more than one kind | Tethya |
| (b) | Sponge encrusting; asters of one kind only | Hymedesmia | |
| (c) | Skeleton fibrous | Axinella spp. | |
|
17. |
(a) | Megascleres all diactinal; chelae present | Desmacidon |
| (b) | Megascleres all diactinal; chelae absent | 18 | |
| (c) | Some or all of the megascleres monactinal | 19 | |
| {223}
18. |
(a) | Habitat fresh water | 56 |
| (b) | Habitat marine | 22 | |
|
19. |
(a) | Megascleres include cladotyles | Acarnus |
| (b) | Megascleres include dumb-bell or sausage-shaped spicules forming the main reticulum | Plocamia | |
| (c) | Microscleres include bipocilli | 20 | |
| (d) | Microscleres include diancistra | Hamacantha | |
| (e) | Megascleres include forceps | Forcepia | |
| (f) | Skeleton formed of isolated monactines vertically placed | Hymeraphia | |
| (g) | None of the above peculiarities present | 21 | |
|
20. |
(a) | Skeleton fibre not echinated | Iophon |
| (b) | Skeleton fibre echinated | Pocillon | |
|
21. |
(a) | Skeleton with echinating spicules | 28 |
| (b) | Skeleton without echinating spicules | 30 | |
|
22. |
(a) | Spongin abundant | 23 |
| (b) | Spongin scanty | 25 | |
|
23. |
(a) | Fibre not echinated | 24 |
| (b) | Fibre echinated | Diplodemia | |
|
24. |
(a) | Fibre with a single axial series of spicules | Chalina |
| (b) | Fibres with numerous spicules arranged polyserially | Pachychalina | |
|
25. |
(a) | Microscleres absent | 26 |
| (b) | Microscleres sigmata and/or toxa | 27 | |
|
26. |
(a) | Skeleton confused | Halichondria |
| (b) | Skeleton reticulate | Reniera | |
|
27. |
(a) | Rind and fistulous appendages present; microscleres sigmata | Oceanapia |
| (b) | No rind; skeleton reticulate; microscleres sigmata and/or toxa | Gellius | |
|
28. |
(a) | Skeleton confused or formed of bundles of spicules with echinating spined styles | 29 |
| (b) | Skeleton fibrous or reticulate, or formed of short columns | 45 | |
| (c) | Skeleton formed of a dense central axis, and columns radiating from it to the surface | 52 | |
|
29. |
(a) | Spicules of the ectosome styles | Pytheas |
| (b) | Spicules of the ectosome oxeas or absent | Clathrissa | |
| (c) | Main skeleton confused. Special ectosomal skeleton absent | Spanioplon | |
|
30. |
(a) | Megascleres of the choanosome not differing from those of the ectosome | 31 |
| (b) | Megascleres of the choanosome differing from those of the ectosome | 32 | |
|
31. |
(a) | Chelae absent. | 33 |
| (b) | Chelae present | 44 | |
|
32. |
(a) | Trichodragmata present | Tedania |
| (b) | Trichodragmata absent | 42 |

Fig. 110.—Megascleres. a-l and q-s, Modifications of monaxon type. a, Strongyle; b, tylote; c, oxea; d, tylotoxea; e, tylostyle; f, style; g, spined tylostyle; h, sagittal triod (a triaxon form derived from monaxon); j, oxytylote; k, anatriaene; l, protriaene; m, sterraster (polyaxon); n, radial section through the outer part of m, showing two actines soldered together by intervening silica; o, desma of an Anomocladine Lithistid (polyaxon); q, crepidial strongyle, basis of rhabdocrepid Lithistid desma; r, young form of rhabdocrepid desma, showing crepidial strongyle coated with successive layers of silica; s, rhabdocrepid desma.
|
33. |
(a) | Skeleton reticulate or fibrous | 34 |
| (b) | Skeleton radiate or diffuse | 37 | |
| (c) | Skeleton with radiating fibres forming a reticulum with others crossing them at right angles | Quasillina | |
|
34. |
(a) | No microscleres | 35 |
| (b) | Microscleres sigmata and/or toxa with or without trichodragmata | Desmacella | |
|
35. |
(a) | Sponge fan- or funnel-shaped | 36 |
| (b) | Sponge not fan- or funnel-shaped | Hymeniacidon | |
|
36. |
(a) | Megascleres slender and twisted | Phakellia |
| (b) | Megascleres somewhat stout, not twisted | Tragosia | |
|
37. |
(a) | Sigmata present, skeleton diffuse | Biemma |
| (b) | Sigmata absent | 38 | |
|
38. |
(a) | Skeleton more or less radiate | 39 |
| (b) | Skeleton diffuse; sponge boring | Cliona | |
|
39. |
(a) | Sponge discoid with marginal fringe | Halicnemia |
| (b) | Sponge massive or stipitate without marginal fringe | 40 | |
|
40. |
(a) | Sponge body prolonged into mammiform projections | Polymastia |
| (b) | Sponge body without mammiform projections | 41 | |
|
41. |
(a) | No microscleres. Megascleres tylostyles with or without styles | Suberites |
| (b) | Microscleres centrotylote. Megascleres styles or tylostyles | Ficulina | |
|
42. |
(a) | Choanosomal megascleres smooth | 43 |
| (b) | Choanosomal megascleres spined | Dendoryx | |
|
43. |
(a) | Microscleres chelae and sigmata of about the same size | Lissodendoryx |
| (b) | Chelae, if present, smaller than the sigmata | Yvesia | |
| {225}
44. |
(a) | Isochelae | Esperiopsis |
| (b) | Anisochelae | Esperella | |
|
45. |
(a) | Fibres or columns plumose | 46 |
| (b) | Fibres or columns ectyonine | 47 | |
|
46. |
(a) | Microscleres toxa | Ophlitaspongia |
| (b) | Microscleres absent | Axinella | |
|
47. |
(a) | Skeleton reticulate | 48 |
| (b) | Skeleton not reticulate | 49 | |
|
48. |
(a) | Microscleres present. Spicules of the fibre core spined | Myxilla |
| (b) | Microscleres absent. Spicules of the fibre core smooth | Lissomyxilla | |
|
49. |
(a) | Main skeleton formed of plume-like columns | 50 |
| (b) | Main skeleton formed of horny fibres (ectyonine). Special dermal skeleton wanting | Clathria | |
|
50. |
(a) | Dermal skeleton contains styles only | Microciona |
| (b) | Dermal skeleton contains diactine spicules with or without styli | 51 | |
|
51. |
(a) | Main skeleton columns with a core of smooth oxeas | Plumohalichondria |
| (b) | Main skeleton columns with a core of spined styles | Stylostichon | |
|
52. |
(a) | Central axis contains much spongin. Echinating spined styli present | Raspailia |
| (b) | Central axis with little or no spongin. Spined styles absent. Pillars radiating from the axis support dermal skeleton | Ciocalypta | |
|
53. |
(a) | Ground substance between chambers clear; chambers pear-shaped or oval; eurypylous | Spongelia |
| (b) | Ground substance granular. Chambers spherical with aphodi | 54 | |
|
54. |
(a) | Fibres not pithed; sponge fan-shaped | Leiosella |
| (b) | Fibres pithed; sponge massive | Aplysina | |
|
55. |
(a) | Chambers long, tubular, branched | Halisarca |
| (b) | Chambers not much longer than broad; not branched | Oscarella | |
|
56. |
(a) | Amphidiscs present | Ephydatia |
| (b) | Amphidiscs absent | Spongilla |
PORIFERA (CONTINUED): REPRODUCTION, SEXUAL AND ASEXUAL—PHYSIOLOGY—DISTRIBUTION—FLINTS
The reproductive processes of Sponges are of such great importance in leading us to a true conception of the nature of a sponge that we propose to treat them here in a special section. Both sexual and asexual methods are common; the multiplication of oscula we do not regard as an act of reproduction (p. 174).
Fig. 111.—A, amphiblastula larva of Sycon raphanus; B, later stage, showing invagination of the flagellated cells. c.s, Segmentation cavity; ec, ectoderm; en, endoderm. (After F. E. Schulze, from Balfour.)
A cursory glance at a collection of sponge larvae from different groups would suggest the conclusion that they are divisible into two wholly distinct types. One of these is the amphiblastula, and the other the parenchymula. This was the conclusion accepted by zoologists not long ago. We are indebted to Delage, Maas, and Minchin for dispelling it, and showing that {227}these types are but the extreme terms of a continuous series of forms which have all the same essential constitution and undergo the same metamorphosis.
The amphiblastula of Sycon raphanus (Fig. 111) consists of an anterior half, formed of slender flagellated cells, and a posterior half, of which the cells are large, non-flagellate, and rounded. These two kinds of cell are arranged around a small internal cavity which is largely filled up with amoebocytes. The flagellated cells are invaginated into the dome of rounded cells during metamorphosis, in fact, become the choanocytes or gastral cells; the rounded cells, on the other hand, become the dermal cells—an astonishing fact to any one acquainted only with Metazoan larvae.
A typical parenchymula is that of Clathrina blanca (Fig. 112). When hatched it consists of a wall surrounding a large central cavity and built up of flagellated cells interrupted at the hinder pole by two cells (p.g.c)—the mother-cells of archaeocytes. Before the metamorphosis, certain of the flagellated cells leave the wall and sink into the central cavity, and undergoing certain changes establish an inner mass of future dermal cells. By subsequent metamorphosis the remaining flagellated cells become internal, not this time by invagination, but by the included dermal cells breaking through the wall of the larva, and forming themselves into a layer at the outside.
Fig. 112.—Median longitudinal section of parenchymula larva of Clathrina blanca. p.g.c, Posterior granular cells—archaeocyte mother-cells. (After Minchin.)
In the larva of C. blanca, after a period of free-swimming existence, the same three elements are thus recognisable as in that of Sycon at the time of hatching; in the newly hatched larva of C. blanca, however, one set of elements, the dermal cells, are not distinguishable. The difference, then, between the two newly hatched larvae is due to the earlier cell differentiation of the Sycon larva.[254]
Now consider the larva of Leucosolenia. It is hatched as a {228}completely flagellated larva; its archaeocytes are internal (as in Sycon); future dermal cells, recognisable as such, are absent. They arise, as in C. blanca, by transformation of flagellated cells; but (1) this process is confined to the posterior pole, and (2) the internal cavity is small and filled up with archaeocytes. Consequently the cells which have lost their flagella and become converted into dermal cells cannot sink in as in C. blanca: they accumulate at the hinder pole, and thus arises a larva half flagellated, half not; in fact, an amphiblastula. Or, briefly, in Leucosolenia the larva at hatching is a parenchymula, and when ready to fix is an amphiblastula; and, again, the difference between the newly hatched larva and that of Sycon is due to the earlier occurrence of cell differentiation in the latter. What completer transitional series could be desired?
Turning to the Micromastictora, the developmental history already sketched is fairly typical (p. 172). The differences between Mega- and Micro-mastictoran larvae are referable mainly to the fact that the dermal cells in the latter become at once differentiated among themselves to form the main types of dermal cell of the adult.[255] The metamorphosis is comparable to that of C. blanca. Among Tetractinellida and Hexactinellida sexually produced larvae have not been certainly identified.
Asexual reproduction takes place according to one of three types, which may be alluded to as (1) "budding," (2) "gemmulation," (3) formation of "asexual larvae."
By budding (Fig. 113) is meant the formation of reproductive bodies, each of which contains differentiated elements of the various classes found in the parent. A simple example of this is described by Miklucho Maclay in Ascons, where the bud is merely the end of one of the Ascon tubes which becomes pinched off and so set free.
In Leucosolenia botryoides[256] Vasseur describes a similar process; in this, however, a strikingly distinctive feature is present (Fig. 114), namely, the buds have an inverse orientation with respect to that of the parent, so that the budding sponge presents a contrast to a sponge in which multiplication of oscula has occurred. In fact, the free distal end of the bud becomes the base of the young sponge, and the osculum is formed at the opposite extremity, where the bud is constricted from the parent.
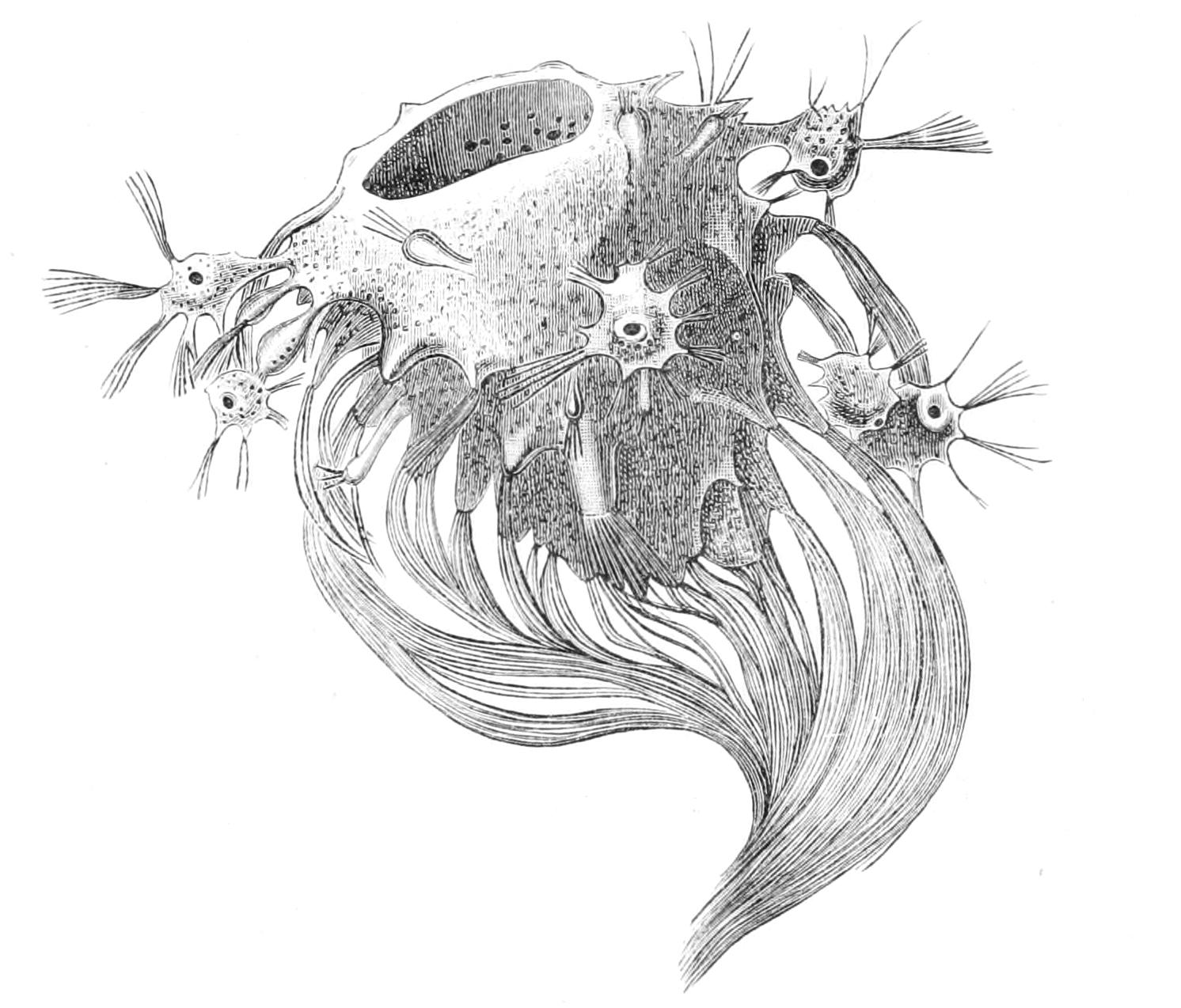
Fig. 113.—Lophocalyx philippensis. The specimen bears several buds attached to it by long tufts of spicules. (After F. E. Schulze.)
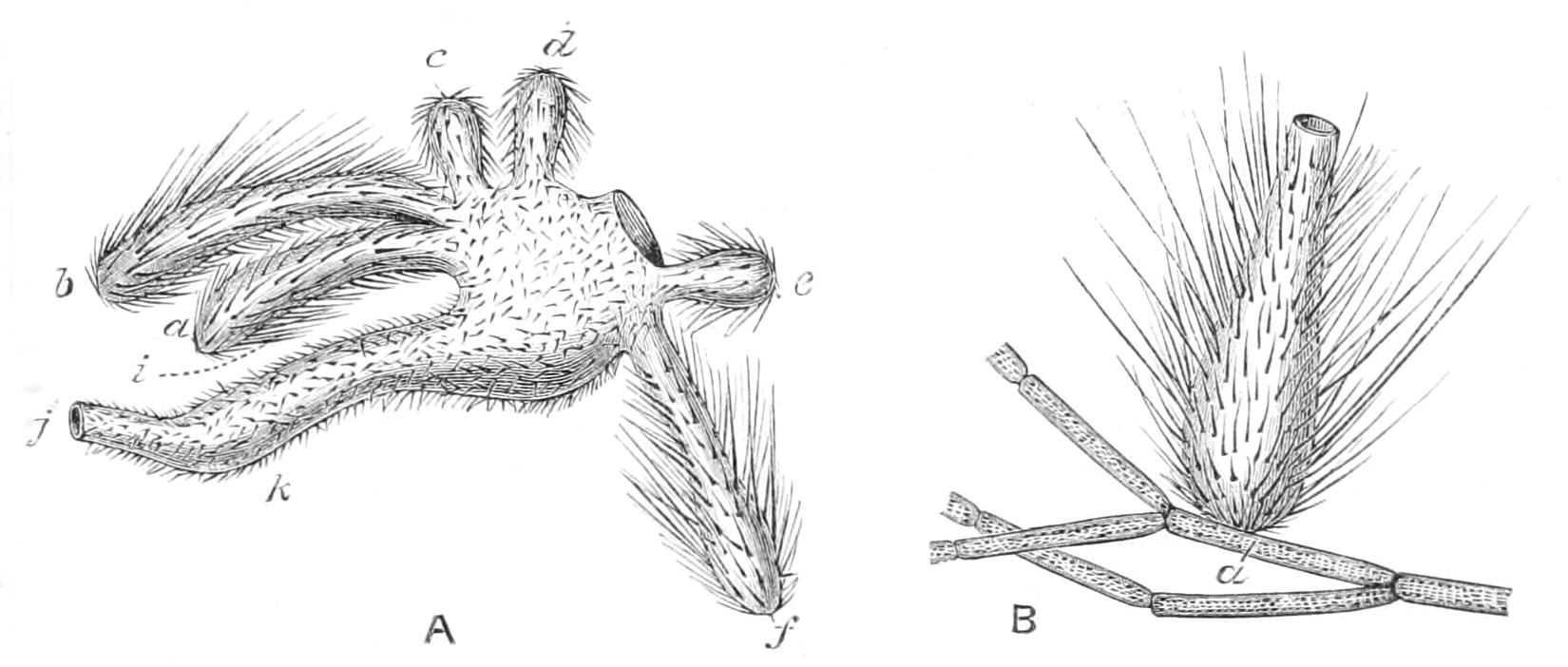
Fig. 114.—Leucosolenia botryoides. A, a piece of the Sponge laden with buds, a-f; i, the spicules of the buds directed away from their free ends; k, the spicules of the parent directed towards the osculum, j. B, a bud which has been set free and has become fixed by the extremity which was free or distal in A. (After Vasseur.)
Such a reversal of the position of the bud is noteworthy in view of its rarity, and the case is worth reinvestigating, for in other animal groups a bud or a regenerated part retains so constantly the same orientation as the parent that Loeb,[257] after experimenting on the {230}regeneration of Coelenterata and other forms, concluded that a kind of "polarity" existed in the tissues of certain animals.
In Oscarella lobularis[258] the buds are transparent floating bladders, derived from little prominences on the surface of the sponge. Scattered in the walls of the bladders are flagellated chambers, which open into the central cavity. The vesicular nature of the buds is doubtless an adaptation, lessening their specific gravity and so enabling them to float to a distance from the parent.
Gemmulation.—Spongilla has already afforded us a typical example of this process. Gemmules very similar to those of Spongilla are known in a few marine sponges, especially in Suberites and in Ficulina. They form a layer attached to the surface of support of the sponge—a layer which may be single or double, or even three or four tiers deep. A micropyle is sometimes present in the spongin coat, sometimes absent; possibly its absence may be correlated with the piling of one layer of gemmules on another, as this, by covering up the micropyle, would of course render it useless. Presumably when a micropyle is present the living contents escape through it and leave the sponge by way of the canal system (Fig. 115).

Fig. 115.—Gemmules of Ficulina. A, vertical section of gemmules in situ; B, vertical section of upper portion of one gemmule. m, Micropyle.
The only case besides Spongilla in which the details of development from gemmules have been traced is that of Tethya.[259] Mere microscopic examination of a Tethya in active reproduction would suggest that the process was simple budding, but Maas has shown that the offspring arise from groups of archaeocytes in the cortex, that is to say, they are typical gemmules. As they develop they migrate outwards along the radial spicule-bundles {231}and are finally freed, like the buds of the Hexactinellid Lophocalyx (Fig. 113).
The comparison of the process of development on the one hand by gemmules, and on the other by larval development, is of some interest.[260] In both cases two cell layers—a dermal and a gastral—are established before the young sponge has reached a functional state. Differences of detail in the formation of the chambers occur in the gemmule; these find parallels in the differences in the same process exhibited by the larvae of various groups of sponges. On the other hand, the order of tissue differentiation is not the same in the gemmule as in the larva.
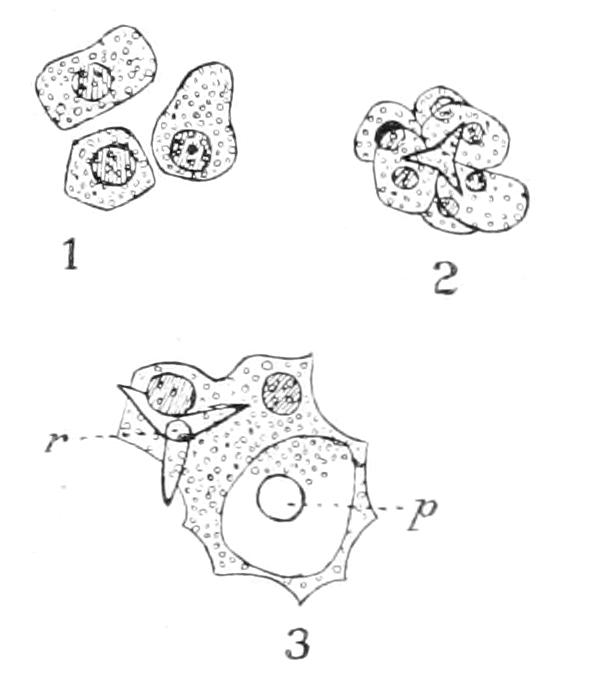
Fig. 116.—Development of the triradiate and quadriradiate spicules of Clathrina. (1) Three scleroblasts; (2) each has divided: the spicule is seen in their midst; (3) addition of the fourth ray by a porocyte. p, Dermal aperture of pore; r, fourth ray. (After Minchin.)
Of the reproduction of Tetractinellida extremely little is known. Spermatozoa occur in the tissues in profusion and are doubtless functional, but larvae have been seldom observed.
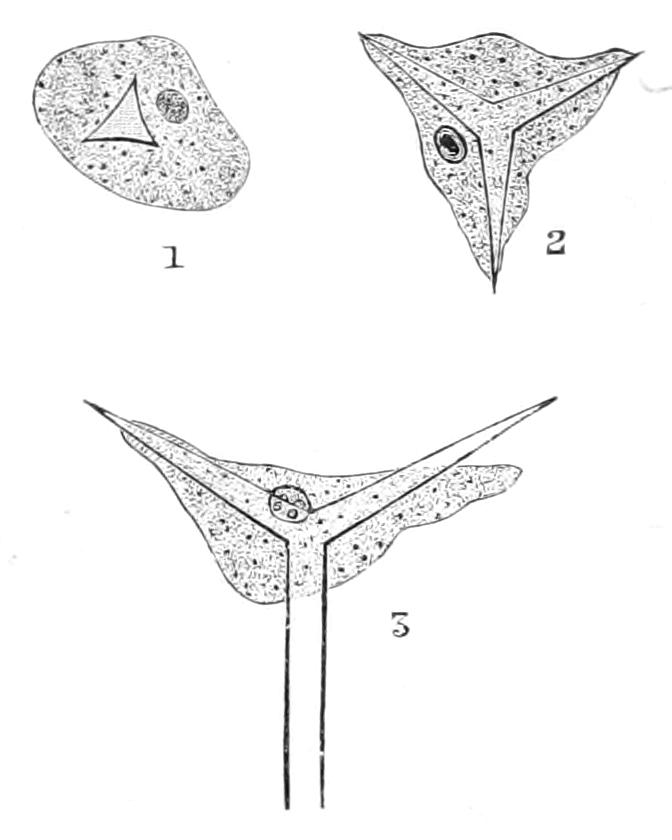
Fig. 117.—Three stages in the development of the triradiate spicules of Sycon setosum. × 1200. (After Maas.)
In Hexactinellida the place of sexually produced larvae is taken by bodies of similar origin to gemmules but with the appearance of parenchymulae. Ijima has indeed seen a few egg-cells in Hexactinellids.[261] He finds, however, that archaeocyte congeries occur in abundance, and there is good reason to believe with him that these are responsible for the numerous parenchymula-like asexually produced larvae he has observed. The discovery of "asexual larvae" was first made by Wilson in the Monaxonid Esperella; in this case the asexual larva is, as far as can be detected, identical with that developed from the fertilised egg. A similar phenomenon, the production {232}of apparently identical larvae by both sexual and asexual methods, has been observed in the Coelenterate Gonionema murbachii.[262]
Artificially, sponges may be reproduced with great advantage to commerce by means of cuttings. Cuttings of the bath sponge are fit to gather after a seven years' growth.
The development of the various forms of spicules is a subject about which little is yet known. Most spicules of which the development has been traced originate in a single dermal cell. The triradiate and quadriradiate spicules of Homocoela (Clathrinidae), as Minchin[263] has most beautifully shown, form an exception. Three cells co-operate to form the triradiate; these three divide to give six before the growth of the spicule is complete. A quadriradiate is formed from a triradiate spicule by addition of the fourth ray, which, again, has a separate origin in an independent cell, in fact a porocyte. The triradiate spicules of the Sycettidae, on the other hand, originate in a single cell,[264] but the quadriradiate spicules are formed from these by the addition of a fourth ray in a manner similar to that which has just been described for Clathrinidae.
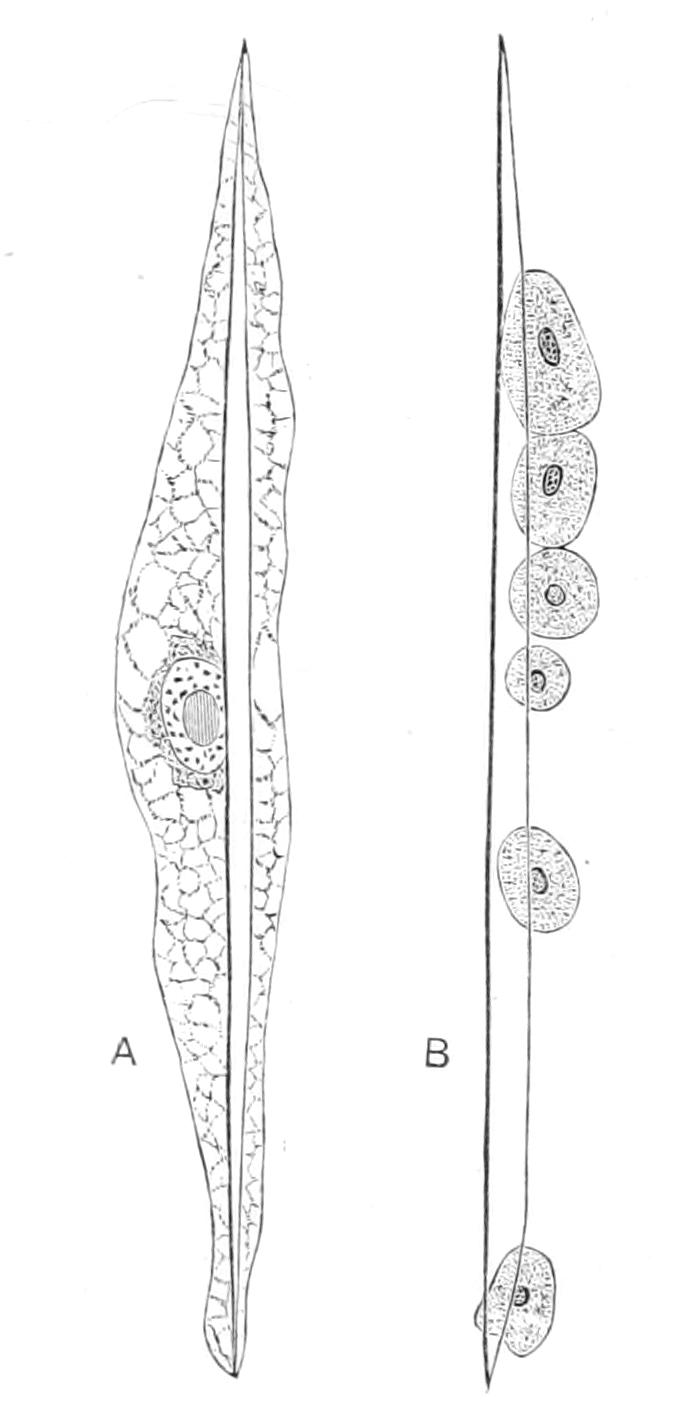
Fig. 118.—Development of monaxon spicules. A, from Spongilla lacustris, showing the single scleroblast. (After Evans.) B, a very large monaxon, from Leucosolenia, on which many scleroblasts are at work. (After Maas.)
Monaxon spicules if not of large size undergo their entire development within a single scleroblast (Fig. 118, A). In some cases if their dimensions exceed certain limits, several cells take part in their completion; some of these are derived from the {233}division of the original scleroblast, others are drawn from the surrounding tissue. In Tethya, for example, and in Leucosolenia[265] the scleroblasts round the large monaxon spicules are so numerous as to have an almost epithelioid arrangement.
The large oxeas of Tetilla, Stelletta, and Geodia, however, are formed each within a single scleroblast.[266]
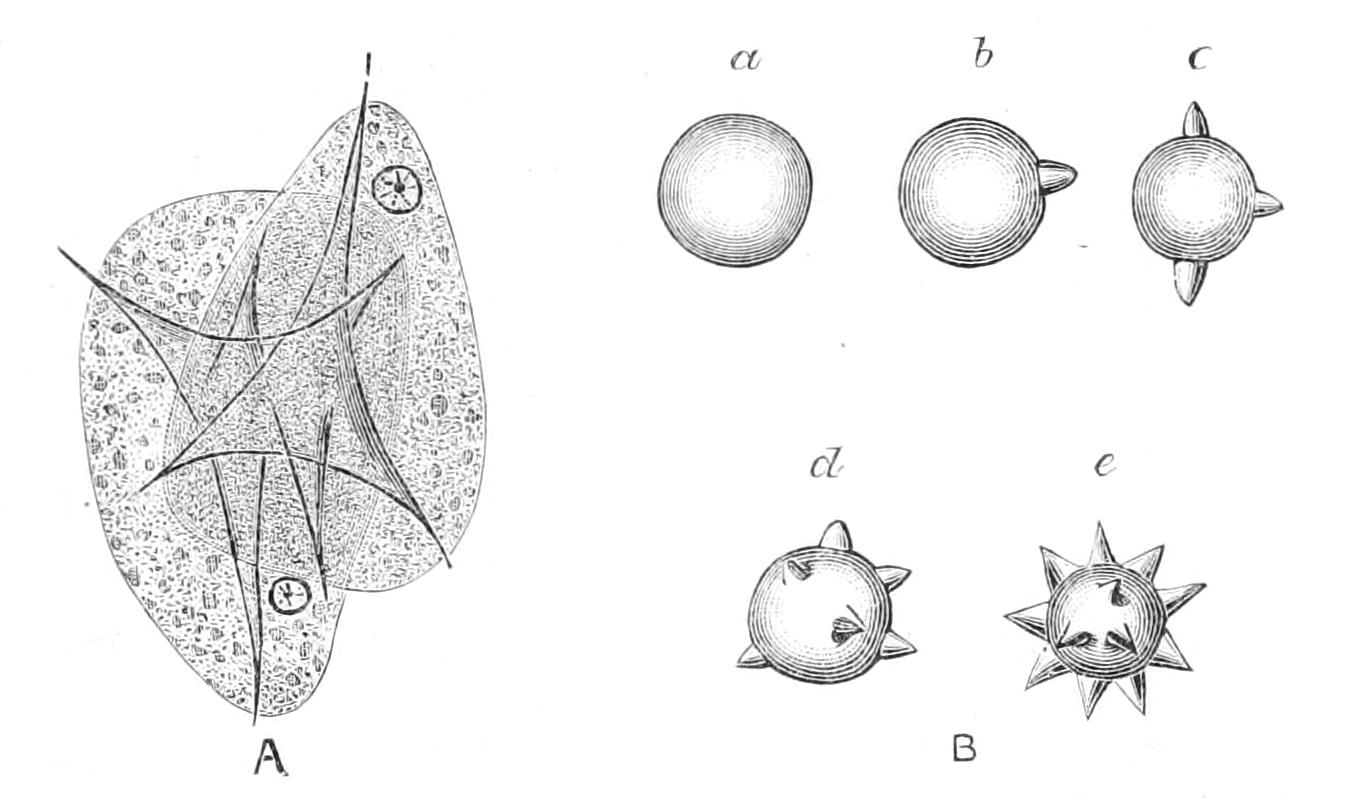
Fig. 119.—Development of spheraster. A, of Tethya, from union of two quadriradiate spicules. (After Maas.) B (a-e), of Chondrilla, from a spherical globule. (After Keller.)
Triaenes have been shown[267] to originate as monaxons with one swollen termination, from which later the cladi grow out. Information as to the scleroblasts in this case is needed.
The value of a knowledge of the ontogeny of microscleres might be great. Maas believes that he has shown that the spherasters of Tethya are formed by the union of minute tetractine calthrops (Fig. 119, A). If this view should be confirmed, it would afford a very strong argument for the Tetractinellid affinities of Tethya.
Keller,[268] on the other hand, finds that the spherasters of the Tetractinellid Chondrilla {234}originate as spheres (Fig. 119, B); and spheres have been observed in the gemmule of a Tethya; no spherasters were as yet present in the gemmule, and spheres were absent in the adult.[269]
In the genus Placospongia certain spicules are present which outwardly closely resemble the sterrasters so characteristic of certain Tetractinellidae. Their development, however, as will be seen from Fig. 120, shows that they are not polyaxon but spiny monaxon spicules. Placospongia is consequently transferred to the Monaxonida Spintharaphora.
Sterrasters originate within an oval cell as a number of hairlike fibres[270] (trichites), which are united at their inner ends. The outer ends become thickened and further modified. The position occupied by the nucleus of the scleroblast is marked in the adult spicule by a hilum.

Fig. 121.—Three stages in the development of an anisochela. al, Ala; al', lower ala; f, falx; f', lower falx; r, rostrum; r', lower rostrum. (After Vosmaer and Pekelharing.)
The anisochela has been shown repeatedly to originate from a C-shaped spicule.[271]
What little is known of the development of Hexactinellid spicules we owe to Ijima.[272] Numerous cells are concerned in certain later developmental stages of the hexaster; a hexaster passes through a hexactin stage, and—a fact "possibly of importance for the phylogeny of spicules in Hexactinellida"—in two species the first formed spicules are a kind of hexactin, known as a "stauractin," and possessing only four rays all in one plane (cf. Protospongia, p. 207).
Physiology
Production of the Current.—It is not at first sight obvious that the lashing of flagella in chambers arranged as above {235}described, between an inhalant and an exhalant system of canals, will necessarily produce a current passing inwards at the ostia and outwards at the osculum. And the difficulty seems to be increased when it is found[273] that the flagella in any one chamber do not vibrate in concert, but that each keeps its own time. This, however, is of less consequence than might seem to be the case. Two conditions are essential to produce the observed results: (1) in order that the water should escape at the mouth of the chamber there must be a pressure within the chamber higher than that in the exhalant passages; (2) in order that water may enter the chamber there must be within it a pressure less than that in the inhalant passages. But the pressure in the inhalant and exhalant passages is presumably the same, at any rate before the current is started, therefore there must be a difference of pressure within the chamber itself, and the less pressure must be round the periphery. Such a distribution of pressures would be set up if each flagellum caused a flow of water directed away from its own cell and towards the centre of the chamber; and this would be true whether the flagellum beats synchronously with its fellows or not.
The comparative study of the canal systems of sponges[274] acquires a greater interest in proportion as the hope of correlating modifications with increase of efficiency seems to be realised. In a few main issues this hope may be said to have been realised. The points, so to speak, of a good canal system are (1) high oscular velocity, which ensures rapid removal of waste products to a wholesome distance; (2) a slow current without eddies in the flagellated chambers, to allow of the choanocytes picking up food particles (see below), and moreover to prevent injury to the delicate collars of those cells; (3) a small area of choanocytes, and consequent small expenditure of energy in current production.
It is then at once clear at what a disadvantage the Ascons are placed as compared with other sponges having canal systems of the second or third types. Their chamber and oscular currents can differ but slightly, the difference being obtained merely by narrowing the lumen of the distal extremity of the body to form the oscular rim. Further, the choanocytes are {236}acting on a volume of water which they can only imperfectly control, and it is no doubt due to the necessity of limiting the volume of water which the choanocytes have to set in motion that the members of the Ascon family are so restricted in size. The oscular rim is only a special case of a device adopted by sponges at the very outset of their career, and retained and perfected when they have reached their greatest heights; the volume of water passing per second over every cross-section of the path of the current is of course the same, therefore by narrowing the cross-sectional area of the path at any point, the velocity of the current is proportionally increased at that point. The lining of the oscular rim is of pinacocytes; they determine a smooth surface, offering little frictional resistance to the current, while choanocytes in the same position would have been a hindrance, not only by setting up friction, but by causing irregularities in the motion.
Canal systems of the second type show a double advance upon that of the Ascons, namely, subdivision of the gastral cavity and much greater length of the smooth walled exhalant passage. The choanocytes have now a task more equal to their strength, and, further, there is now a very great inequality between the total sectional areas of the flagellated chambers and that of the oscular tube.
Canal systems of the third type with tubular chambers are an improvement on those of the second, in that the area of choanocytes is increased by the pouching of the chamber-layer without corresponding increase in the size of the sponge. However, the area of choanocytes represents expenditure of energy, and the next problem to be solved is how to retain the improved current and at the same time to cut down expense. The first step is to change the form of the chamber from tubular to spherical. Now the energy of all the choanocytes is concentrated on the same small volume of water. The area of choanocytes is less, but the end result is as good as before. At the wide mouth of the spherical chamber there is nevertheless still a cause of loss of energy in the form of eddies, and it is as an obviation of these that one must regard the aphodi and prosodi with which higher members of the Demospongiae are provided. The correctness of this view receives support, apart from mechanical principles, from the fact that the mass of the body of any one of these sponges is greater relatively to the total flagellated area than in those sponges with eurypylous chambers; that is to say, a few {237}aphodal and diplodal chambers are as efficient as many of the eurypylous type.
It is manifest that the current is the bearer of the supply of food; but it requires more care to discover (1) what is the nature of the food; (2) by which of the cells bathed by the current the food is captured and by which digested. The answer to the latter question has long been sought by experimenters,[275] who supplied the living sponge with finely powdered coloured matters, such as carmine, indigo, charcoal, suspended in water. The results received conflicting interpretations until it became recognised that it was essential to take into account the length of time during which the sponge had been fed before its tissues were subjected to microscopic examination. Vosmaer and Pekelharing obtained the following facts: Spongilla lacustris and Sycon ciliatum, when killed after feeding for from half an hour to two hours with milk or carmine, contain these substances in abundance in the bodies of the choanocytes and to a slight degree in the deeper cells of the dermal tissue; after feeding for twenty-four hours the proportions are reversed, and if a period of existence in water uncharged with carmine intervenes between the long feed and death then the chambers are completely free from carmine. These are perhaps the most conclusive experiments yet described, and they show that the choanocytes ingest solid particles and that the amoeboid cells of the dermal layer receive the ingested matter from them. In all probability it is fair to argue from these facts that solid particles of matter suitable to form food for the sponge are similarly dealt with by it and undergo digestion in the dermal cells.
Choanocytes are the feeding organs par excellence; but the pinacocytes perform a small share of the function of ingestion, and in the higher sponges where the dermal tissue has acquired a great bulk the share is perhaps increased.
In the above experiments is implied the tacit assumption that sponges take their food in the form of finely divided solids. Haeckel[276] states his opinion that they feed on solid particles derived from decaying organisms, but that possibly decaying substances in solution may eke out their diet. Loisel, in 1898,[277] {238}made a new departure in the field of experiment by feeding sponges with coloured solutions, and obtained valuable results. Thus solutions, if presented to the sponge in a state of extreme dilution, are subjected to choice, some being absorbed, some rejected. When absorbed they are accumulated in vacuoles within both dermal and gastral cells, mixed solutions are separated into their constituents and collected into separate vacuoles. In the vacuoles the solutions may undergo change; Congo red becomes violet, the colour which it assumes when treated with acid, and similarly blue litmus turns red. The contents of the vacuoles, sometimes modified, sometimes not, are poured out into the intercellular gelatinous matrix of the dermal layer, whence they are removed partly by amoeboid cells, partly, so Loisel thinks, by the action of the matrix itself. It adds to the value of these observations to learn that Loisel kept a Spongilla supplied with filtered spring-water, to which was added the filtered juice obtained from another crushed sponge. This Spongilla lived and budded, and was in good health at the end of ten days.
Movement.—Sponges are capable of locomotion only in the young stage; in the adult the only signs of movement are the exhalant current, and in some cases movements of contraction sufficiently marked to be visible to the naked eye. Meresjkowsky was one of the early observers of these movements. He mentions that he stimulated a certain corticate Monaxonid sponge by means of a needle point: a definite response to each prick inside the oscular rim was given by the speedy contraction of the osculum.[278]
Pigments and Spicules.—Various reasons lead one to conclude that the spicules have some function other than that of support and defence, probably connected with metabolism. For the spicules are cast off, sometimes in large numbers, to be replaced rapidly by new ones, a process for which it is difficult to find an adequate explanation if the spicules are regarded as merely skeletal and defensive.[279] Potts remarks upon the striking profusion with which spicules are secreted by developing Spongillids from water in which the percentage of silica present must have been exceedingly small. The young sponges climbed {239}up the strands of spicules as they formed them, leaving the lower parts behind and adding to the upper ends.
Of the physiology of the pigments of sponges not much is yet known: a useful summary of facts will be found in Von Fürth's text-book.[280]
Spongin.—Von Fürth[280] points out that this term is really a collective one, seeing that the identity of the organic skeletal substance of all sponge species is hardly to be assumed. Spongin is remarkable for containing iodine. The amount of iodine present in different sponges varies widely, reaching in certain tropical species of the Aplysinidae and Spongidae the high figure 8 to 14 per cent. Seaweeds which are specially rich in iodine contain only 1.5 to 1.6 per cent.
In view of the fact that iodine is a specific for croup, it is of interest to observe that the old herb doctors for many centuries recognised the bath sponge as a cure for that disease.
Fig. 122.—The ordinals measure (i.) the number of species, a-f, and (ii.) the number of stations, a'-f', at which successful hauls were made. The abscissae measure the depth: thus at I. the depth is from 0 to 50 fathoms; at II. from 51 to 200; at III. from 201 to 1000; at IV. from 1001 upwards. a, a', are the curves for Sponges generally; b, b', for Monaxonida; c, c', for Hexactinellida; d, d', for Tetractinellida; e, e', for Calcarea; f, f', for Ceratosa.
Distribution in Space.—All the larger groups of Sponges are cosmopolitan. Each group has, however, its characteristic bathymetrical range: the facts are best displayed by means of curves, as in Fig. 122, which is based wholly on the results obtained by the "Challenger" Expedition. The information as to littoral species is consequently inadequate, and we have not the data requisite for their discussion.
Sponges generally (a) and Monaxonida in particular (b) are more generally distributed in water of depths of 51 to 200 fathoms than in depths of less than 50 fathoms; but localities in shallow water are {240}richer, for the station curve (a') rises abruptly from I. to II., while the species curve (a) in the same region is almost horizontal.
The Hexactinellid curve (c) culminates on III., showing that the group is characteristically deep water. That for Tetractinellida (d) reaches its greatest height on II., i.e. between 51 and 200 fathoms. Even here, in their characteristic depths, the Tetractinellida fall below the Hexactinellida, and far below the Monaxonida in numbers. Again, the Monaxonida are commoner than Hexactinellida in deep water of 201 to 1000 fathoms, and it is not till depths of 1000 fathoms are passed that Hexactinellida prevail, finally preponderating over the Monaxonida in the ratio of 2:1.
The Calcarea and Ceratosa are strictly shallow-water forms. It is a fact well worth consideration that the stations at which sponges have been found are situated, quite irrespective of depth, more or less in the neighbourhood of land. In the case of Calcarea and Ceratosa this is to be expected, seeing that shallow water is commonest near land, but it is surprising that it should be true also of the Hexactinellida and of the deep-water species of Tetractinellida and of Monaxonida.
While the family groups are cosmopolitan, this is not true of genera and species. The distribution of genera and species makes it possible to define certain geographical provinces for sponges as for other animals. That this is so, is due to the existence of ocean tracts bare of islands; for ocean currents, can act as distributing agents with success only if they flow along a coast or across an ocean studded with islands. It is, of course, the larval forms which will be transported; whether they will ever develop to the adult condition depends on whether the current carrying them passes over a bottom suitable to their species before metamorphosis occurs and the young sponge sinks. If such a bottom is passed over, and if the depth is one which can be supported by the particular species in question, then a new station may thus be established for that species.
The distance over which a larva may be carried depends on the speed of the current by which it is borne, and on the length of time occupied by its metamorphosis. Certain of the ocean currents accomplish 500 miles in six days; this gives some idea of the distance which may intervene between the birthplace and {241}the final station of a sponge; for six days is not an excessive interval to allow for the larval period of at any rate some species.
Distribution in Time.—All that space permits us to say on the palaeontology of sponges has been said under the headings of the respective classes. We can here merely refer to the chronological table shown in Fig. 123:[281]—
Flints.—The ultimate source of all the silica in the sea and fresh-water areas is to be found in the decomposition of igneous rocks such as granite. The quantity of silica present in solution in sea water is exceedingly small, amounting to about one-and-a-half parts in 100,000; it certainly is not much more in average fresh water. This is no doubt due to its extraction by diatoms, which begin to extract it almost as soon as it is set free from the parent rock. It is from this small quantity that the siliceous sponges derive the supply from which they form their spicules. Hence it would appear that for the formation of one {242}ounce of spicules at least one ton of sea water must pass through the body of the sponge. Obviously from such a weak solution the deposition of silica will not occur by ordinary physical agencies; it requires the unexplained action of living organisms. This may account for the fact that deposits of flint and chert are always associated with organic remains, such as Sponges and Radiolaria. By some process, the details of which are not yet understood, the silica of the skeleton passes into solution. In Calcareous deposits, a replacement of the carbonate of lime by the silica takes place, so that in the case of chalk the shells of Foraminifera, such as Globigerina and Textularia and those of Coccoliths, are converted into a siliceous chalk. Thus a siliceous chalk is the first stage in the formation of a flint.
A further deposition of silica then follows, cementing this pulverulent material into a hard white porous flint. It is white for the same reason that snow is white. The deposition of silica continues, and the flint becomes at first grey and at last apparently black (black as ice is black on a pond). Frequently flints are found in all stages of formation: siliceous chalk with the corroded remains of sponge spicules may be found in the interior, black flint blotched with grey forming the mass of the nodule, while the exterior is completed by a thin layer of white porous flint. This layer must not be confused with the white layer which is frequently met with on the surface of weathered flints, which is due to a subsequent solution of some of the silica, so that by a process of unbuilding, the flint is brought back to the incompleted flint in its second stage. In the chalk adjacent to the flints, hollow casts of large sponge spicules may sometimes be observed, proving the fact, which is however unexplained, of the solution of the spicular silica. The formation of the flints appears to have taken place, to some extent at least, long after the death of the sponge, and even subsequent to the elevation of the chalk far above the sea-level, as is shown by the occurrence of layers of flints in the joints of the solid chalk.[282]
BY
S. J. HICKSON, M.A., F.R.S.
Formerly Fellow and now Honorary Fellow of Downing College, Beyer Professor of Zoology in the Victoria University of Manchester.
COELENTERATA
INTRODUCTION—CLASSIFICATION—HYDROZOA—ELEUTHEROBLASTEA—MILLEPORINA—GYMNOBLASTEA—CALYPTOBLASTEA—GRAPTOLITOIDEA—STYLASTERINA
The great division of the animal kingdom called Coelenterata was constituted in 1847 by E. Leuckart for those animals which are commonly known as polyps and jelly-fishes. Cuvier had previously included these forms in his division Radiata or Zoophyta, when they were associated with the Starfishes, Brittle-stars, and the other Echinodermata.
The splitting up of the Cuvierian division was rendered necessary by the progress of anatomical discovery, for whereas the Echinodermata possess an alimentary canal distinct from the other cavities of the body, in the polyps and jelly-fishes there is only one cavity to serve the purposes of digestion and the circulation of fluids. The name Coelenterata (κοῖλος = hollow, ἔντερον = the alimentary canal) was therefore introduced, and it may be taken to signify the important anatomical feature that the body-cavity (or coelom) and the cavity of the alimentary canal (or enteron) of these animals are not separate and distinct as they are in Echinoderms and most other animals.
Many Coelenterata have a pronounced radial symmetry, the body being star-like, with the organs arranged symmetrically on lines radiating from a common centre. In this respect they have a superficial resemblance to many of the Echinodermata, which are also radially symmetrical in the adult stage. But it cannot be insisted upon too strongly that this superficial resemblance of the Coelenterata and Echinodermata has no genetic significance. {246}The radial symmetry has been acquired in the two divisions along different lines of descent, and has no further significance than the adaptation of different animals to somewhat similar conditions of life. It is not only in the animals formerly classed by Cuvier as Radiata, but in sedentary worms, Polyzoa, Brachiopoda, and even Cephalopoda among the Mollusca, that we find a radial arrangement of some of the organs. It is interesting in this connexion to note that the word "polyp," so frequently applied to the individual Coelenterate animal or zooid, was originally introduced on a fancied resemblance of a Hydra to a small Cuttle-fish (Fr. Poulpe, Lat. Polypus).
The body of the Coelenterate, then, consists of a body-wall enclosing a single cavity ("coelenteron"). The body-wall consists of an inner and an outer layer of cells, originally called by Allman the "endoderm" and "ectoderm" respectively. Between the two layers there is a substance chemically allied to mucin and usually of a jelly-like consistency, for which the convenient term "mesogloea," introduced by G. C. Bourne, is used (Fig. 125).
The mesogloea may be very thin and inconspicuous, as it is in Hydra and many other sedentary forms, or it may become very thick, as in the jelly-fishes and some of the sedentary Alcyonaria. When it is very thick it is penetrated by wandering isolated cells from the ectoderm or endoderm, by strings of cells or by cell-lined canals; but even when it is cellular it must not be confounded with the third germinal layer or mesoblast which characterises the higher groups of animals, from which it differs essentially in origin and other characters. The Coelenterata are two-layered animals (Diploblastica), in contrast to the Metazoa with three layers of cells (Triploblastica). The growth of the mesogloea in many Coelenterata leads to modifications of the shape of the coelenteric cavity in various directions. In the Anthozoa, for example, the growth of vertical bands of mesogloea covered by endoderm divides the peripheral parts of the cavity into a series of intermesenterial compartments in open communication with the axial part of the cavity; and in the jelly-fishes the growth of the mesogloea reduces the cavity of the outer regions of the disc to a series of vessel-like canals.
Another character, of great importance, possessed by all Coelenterata is the "nematocyst" or "thread-cell" (Fig. 124). {247}This is an organ produced within the body of a cell called the "cnidoblast," and it consists of a vesicular wall or capsule, surrounding a cavity filled with fluid containing a long and usually spirally coiled thread continuous with the wall of the vesicle. When the nematocyst is fully developed and receives a stimulus of a certain character, the thread is shot out with great velocity and causes a sting on any part of an animal that is sufficiently delicate to be wounded by it.
The morphology and physiology of the nematocysts are subjects of very great difficulty and complication, and cannot be discussed in these pages. It may, however, be said that by some authorities the cnidoblast is supposed to be an extremely modified form of mucous or gland cell, and that the discharge of the nematocyst is subject to the control of a primitive nervous system that is continuous through the body of the zooid.
There is a considerable range of structure in the nematocysts of the Coelenterata. In Alcyonium and in many other Alcyonaria they are very small (in Alcyonium the nematocyst is 0.0075 mm. in length previous to discharge), and when discharged exhibit a simple oval capsule with a plain thread attached to it. In Hydra (Fig. 124) there are at least two kinds of nematocysts, and in the larger kind (0.02 mm. in length previous to discharge) the base of the thread is beset with a series of recurved hooks, which during the act of discharge probably assist in making a wound in the organism attacked for the injection of the irritant fluid, and possibly hold the structure in position while the thread is being discharged. In the large kind of nematocyst of Millepora and of Cerianthus there is a band of spirally arranged but very minute thorns in the middle of the thread, but none at the base. In some of the Siphonophora the undischarged nematocysts reach their maximum size, nearly 0.05 mm. in length.

Fig. 124.—Nematocyst (Nem) of Hydra grisea, enclosed within the cnidoblast. CNC, Cnidocil; f, thread of nematocyst; Mf, myophan threads in cnidoblast; N, nucleus of cnidoblast. (After Schneider.)
When a nematocyst has once been discharged it is usually {248}rejected from the body, and its place in the tissue is taken by a new nematocyst formed by a new cnidoblast; but in the thread of the large kind of nematocyst of Millepora there is a very delicate band, which appears to be similar to the myophan thread in the stalk of a Vorticella. Dr. Willey[283] has made the important observation that in this coral the nematocyst threads can be withdrawn after discharge, the retraction being effected with great rapidity. The "cnidoblast" is a specially modified cell. It sometimes bears at its free extremity a delicate process, the "cnidocil," which is supposed to be adapted to the reception of the special stimuli that determine the discharge of the nematocyst. In many species delicate contractile fibres (Fig. 124, Mf) can be seen in the substance of the cnidoblast, and in others its basal part is drawn out into a long and probably contractile stalk ("cnidopod"), attached to the mesogloea below.
There can be little doubt that new nematocysts are constantly formed during life to replace those that have been discharged and lost. Each nematocyst is developed within the cell-substance of a cnidoblast which is derived from the undifferentiated interstitial cell-groups. During this process the cnidoblast does not necessarily remain stationary, but may wander some considerable distance from its place of origin.[284] This habit of migration of the cnidoblast renders it difficult to determine whether the ectoderm alone, or both ectoderm and endoderm, can give rise to nematocysts. In the majority of Coelenterates the nematocysts are confined to the ectoderm, but in many Anthozoa, Scyphozoa, and Siphonophora they are found in tissues that are certainly or probably endodermic in origin. It has not been definitely proved in any case that the cnidoblast cells that form these nematocysts have originally been formed in the endoderm, and it is possible that they are always derived from ectoderm cells which migrate into the endoderm.
It is probably true that all Coelenterata have nematocysts, and that, in the few cases in which it has been stated that they are absent (e.g. Sarcophytum), they have been overlooked. It cannot, however, be definitely stated that similar structures do not occur in other animals. The nematocysts of the Mollusc Aeolis are not the product of its own tissues, but are introduced {249}into the body with its food.[285] The nematocysts that occur in the Infusorian Epistylis umbellaria and in the Dinoflagellate Polykrikos (p. 131) require reinvestigation, but if it should prove that they are the product of the Protozoa they cannot be regarded as strictly homologous with those of Coelenterata. In many of the Turbellaria, however, and in some of the Nemertine worms, nematocysts occur in the epidermis which appear to be undoubtedly the products of these animals.
The Coelenterata are divided into three classes:—
1. Hydrozoa.—Without stomodaeum and mesenteries. Sexual cells discharged directly to the exterior.
2. Scyphozoa.—Without stomodaeum and mesenteries. Sexual cells discharged into the coelenteric cavity.
3. Anthozoa = Actinozoa.—With stomodaeum and mesenteries. Sexual cells discharged into the coelenteric cavity.
The full meaning of the brief statements concerning the structure of the three classes given above cannot be explained until the general anatomy of the classes has been described. It may be stated, however, in this place that many authors believe that structures corresponding with the stomodaeum and mesenteries of Anthozoa do occur in the Scyphozoa, which they therefore include in the class Anthozoa.
Among the more familiar animals included in the class Hydrozoa may be mentioned the fresh-water polyp Hydra, the Hydroid zoophytes, many of the smaller Medusae or jelly-fish, the Portuguese Man-of-war (Physalia), and a few of the corals.
Included in the Scyphozoa are the large jelly-fish found floating on the sea or cast up on the beach on the British shores.
The Anthozoa include the Sea-anemones, nearly all the Stony Corals, the Sea-fans, the Black Corals, the Dead-men's fingers (Alcyonium), the Sea-pens, and the Precious Coral of commerce.
CLASS I. HYDROZOA
In this Class of Coelenterata two types of body-form may be found. In such a genus as Obelia there is a fixed branching colony of zooids, and each zooid consists of a simple tubular body-wall composed of the two layers of cells, the ectoderm and the {250}endoderm (Fig. 125), terminating distally in a conical mound—the "hypostome"—which is perforated by the mouth and surrounded by a crown of tentacles. This fixed colony, the "hydrosome," feeds and increases in size by gemmation, but does not produce sexual cells. The hydrosome produces at a certain season of the year a number of buds, which develop into small bell-like jelly-fish called the "Medusae," which swim away from the parent stock and produce the sexual cells. The Medusa (Fig. 126) consists of a delicate dome-shaped contractile bell, perforated by radial canals and fringed with tentacles; and from its centre there depends, like the clapper of a bell, a tubular process, the manubrium, which bears the mouth at its extremity. This free-swimming sexual stage in the life-history of Obelia is called the "medusome."
It is difficult to determine whether, in the evolution of the Hydrozoa, the hydrosome preceded the medusome or vice versâ. By some authors the medusome is regarded as a specially modified sexual individual of the hydrosome colony. By others the medusome is regarded as the typical adult Hydrozoon form, and the zooids of the hydrosome as nutritive individuals arrested in their development to give support to it. Whatever may be the right interpretation of the facts, however, it is found that in some forms the medusome stage is more or less degenerate and the hydrosome is predominant, whereas in others the hydrosome is degenerate or inconspicuous and the medusome is predominant. Finally, in some cases there are no traces, even in development, of a medusome stage, and the life-history is completed in the hydrosome, while in others the hydrosome stages are lost and the life-history is completed in the medusome.
If a conspicuous hydrosome stage is represented by H, a conspicuous medusome stage by M, an inconspicuous or degenerate hydrosome stage by h, an inconspicuous or degenerate medusome stage by m, and the fertilised ovum by O, the life-histories of the Hydrozoa may be represented by the following formulæ:—
| 1. | O — H — O | (Hydra) |
| 2. | O — H — m — O | (Sertularia) |
| 3. | O — H — M — O | (Obelia) |
| 4. | O — h — M — O | (Liriope) |
| 5. | O — M — O | (Geryonia) |
The structure of the hydrosome is usually very simple. It {251}consists of a branched tube opening by mouths at the ends of the branches and closed at the base. The body-wall is built up of ectoderm and endoderm. Between these layers there is a thin non-cellular lamella, the mesogloea.
In a great many Hydrozoa the ectoderm secretes a chitinous protective tube called the "perisarc." The mouth is usually a small round aperture situated on the summit of the hypostome, and at the base of the hypostome there may be one or two crowns of tentacles or an area bearing irregularly scattered tentacles. The tentacles may be hollow, containing a cavity continuous with the coelenteric cavity of the body; or solid, the endoderm cells arranged in a single row forming an axial support for the ectoderm. The ectoderm of the tentacles is provided with numerous nematocysts, usually arranged in groups or clusters on the distal two-thirds of their length, but sometimes confined to a cap-like swelling at the extremity (capitate tentacles). The hydrosome may be a single zooid producing others asexually by gemmation (or more rarely by fission), which become free from the parent, or it may be a colony of zooids in organic connexion with one another formed by the continuous gemmation of the original zooid derived from the fertilised ovum and its asexually produced offspring. When the hydrosome is a colony of zooids, specialisation of certain individuals for particular functions may occur, and the colony becomes dimorphic or polymorphic.
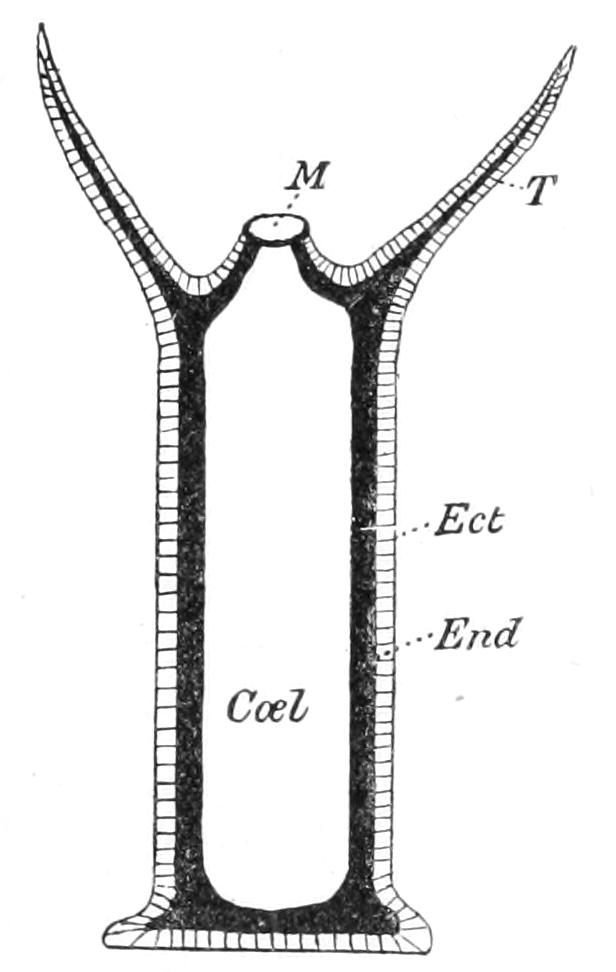
Fig. 125.—Diagram of a vertical section through a hydrosome. Coel, Coelenteron; Ect, ectoderm; End, endoderm. Between the ectoderm and the endoderm there is a thin mesogloea not represented in the diagram. M, mouth; T, tentacle.
The medusome is more complicated in structure than the hydrosome, as it is adapted to the more varied conditions of a free-swimming existence. The body is expanded to form a disc, "umbrella," or bell, which bears at the edge or margin a number of tentacles. The mouth is situated on the end of a hypostome, called the "manubrium," situated in the centre of the radially symmetrical body. The surface that bears the manubrium is {252}called oral, and the opposite surface is called aboral. The cavity partly enclosed by the oral aspect of the body when it is cup- or bell-shaped is called the "sub-umbrellar cavity."
In the medusome of nearly all Hydrozoa there is a narrow shelf projecting inwards from the margin of the disc and guarding the opening of the sub-umbrellar cavity, called the "velum."
The mouth leads through the manubrium into a flattened part of the coelenteric cavity, which is usually called the gastric cavity, and from this a number of canals pass radially through the mesogloea to join a circular canal or ring-canal at the margin of the umbrella.
A special and important feature of the medusome is the presence of sense-organs called the "ocelli" and "statocysts," situated at the margin of the umbrella or at the base of the tentacles.
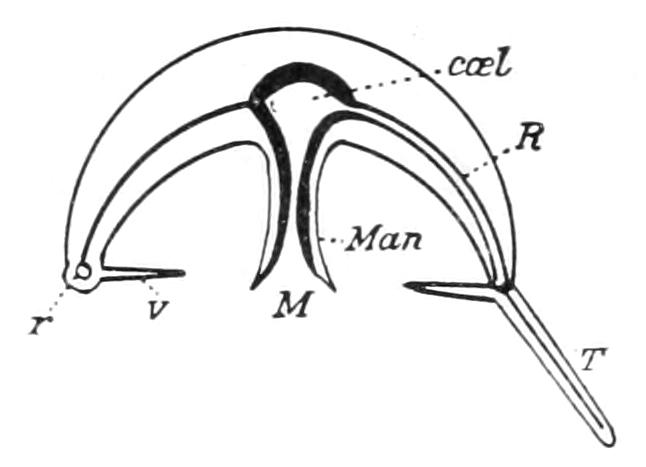
Fig. 126.—Diagram of a vertical section through a medusome. coel, Coelenteron; M, mouth; Man, manubrium; R, radial canal; r, ring or circular canal; T, tentacle; v, velum.
The ocelli may usually be recognised as opaque red or blue spots on the bases of the tentacles, in marked contrast to their transparent surroundings. The ocellus may consist simply of a cluster of pigmented cells, or may be further differentiated as a cup of pigmented cells filled with a spherical thickening of the cuticle to form a lens. The exact function of the ocelli may not be fully understood, but there can be little doubt that they are light-perceiving organs.
The function of the sense-organs known as statocysts, however, has not yet been so satisfactorily determined. They were formerly thought to be auditory organs, and were called "otocysts," but it appears now that it is impossible on physical grounds for these organs to be used for the perception of the waves of sound in water. It is more probable that they are organs of the static function, that is, the function of the perception of the position of the body in space, and they are consequently called statocysts. In the Leptomedusae each statocyst consists of a small vesicle in the mesogloea at the margin of the umbrella, containing a hard, stony body called the "statolith." In Geryonia and some other Trachomedusae the statolith is carried by a short tentacular process, the "statorhab," {253}projecting into the vesicle; in other Trachomedusae, however, the vesicle is open, but forms a hood for the protection of the statorhab; and in others, but especially in the younger stages of development, the statorhab is not sunk into the margin of the umbrella, and resembles a short but loaded tentacle. Recent researches have shown that there is a complete series of connecting links between the vesiculate statocyst of the Leptomedusae and the free tentaculate statorhab of the Trachomedusae, and there can be little doubt of their general homology.
In the free-swimming or "Phanerocodonic" medusome the sexual cells are borne by the ectoderm of the sub-umbrellar cavity either on the walls of the manubrium or subjacent to the course of the radial canals.
Order I. Eleutheroblastea.
This order is constituted mainly for the well-known genus Hydra. By some authors Hydra is regarded as an aberrant member of the order Gymnoblastea, to which it is undoubtedly in many respects allied, but it presents so many features of special interest that it is better to keep it in a distinct group.
Hydra is one of the few examples of exclusively fresh-water Coelenterates, and like so many of the smaller fresh-water animals its distribution is almost cosmopolitan. It occurs not only in Europe and North America, but in New Zealand, Australia, tropical central Africa, and tropical central America.
Hydra is found in this country in clear, still fresh water attached to the stalks or leaves of weeds. When fully expanded it may be 25 mm. in length, but when completely retracted the same individual may be not more than 3 mm. long. The tubular body-wall is built up of ectoderm and endoderm, enclosing a simple undivided coelenteric cavity. The mouth is situated on the summit of the conical hypostome, and at the base of this there is a crown of long, delicate, but hollow tentacles. The number of tentacles is usually six in H. vulgaris and H. oligactis,[286] and eight in H. viridis, but it is variable in all species.
During the greater part of the summer the number of individuals is rapidly increased by gemmation. The young Hydras produced by gemmation are usually detached from their parents {254}before they themselves produce buds, but in H. oligactis the buds often remain attached to the parent after they themselves have formed buds, and thus a small colony is produced. Sexual reproduction usually commences in this country in the summer and autumn, but as the statements of trustworthy authors are conflicting, it is probable that the time of appearance of the sexual organs varies according to the conditions of the environment.
Individual specimens may be male, female, or hermaphrodite. Nussbaum[287] has published the interesting observation that when the Hydras have been well fed the majority become female, when the food supply has been greatly restricted the majority become male, and when the food-supply is moderate in amount the majority become hermaphrodite. The gonads are simply clusters of sexual cells situated in the ectoderm. There is no evidence, derived from either their structure or their development, to show that they represent reduced medusiform gonophores. The testis produces a number of minute spermatozoa. In the ovary, however, only one large yolk-laden egg-cell reaches maturity by the absorption of the other eggs. The ovum is fertilised while still within the gonad, and undergoes the early stages of its development in that position. With the differentiation of an outer layer of cells a chitinous protecting membrane is formed, and the escape from the parent takes place.[288] It seems probable that at this stage, namely, that of a protected embryo, there is often a prolonged period of rest, during which it may be carried by wind and other agencies for long distances without injury.
The remarkable power that Hydra possesses of recovery from injury and of regenerating lost parts was first pointed out by Trembley in his classical memoir.[289]
A Hydra can be cut into a considerable number of pieces, and each piece, provided both ectoderm and endoderm are represented in it, will give rise by growth and regeneration to a complete zooid. There is, however, a limit of size below which fragments of Hydra will not regenerate, even if they contain {255}cells of both layers. The statement made by Trembley, that when a Hydra is turned inside out it will continue to live in the introverted condition has not been confirmed, and it seems probable that after the experiment has been made the polyp remains in a paralysed condition for some time, and later reverts, somewhat suddenly, to the normal condition by a reversal of the process. There is certainly no substantial reason to believe that under any circumstances the ectoderm can undertake the function of the endoderm or the endoderm the functions of the ectoderm.
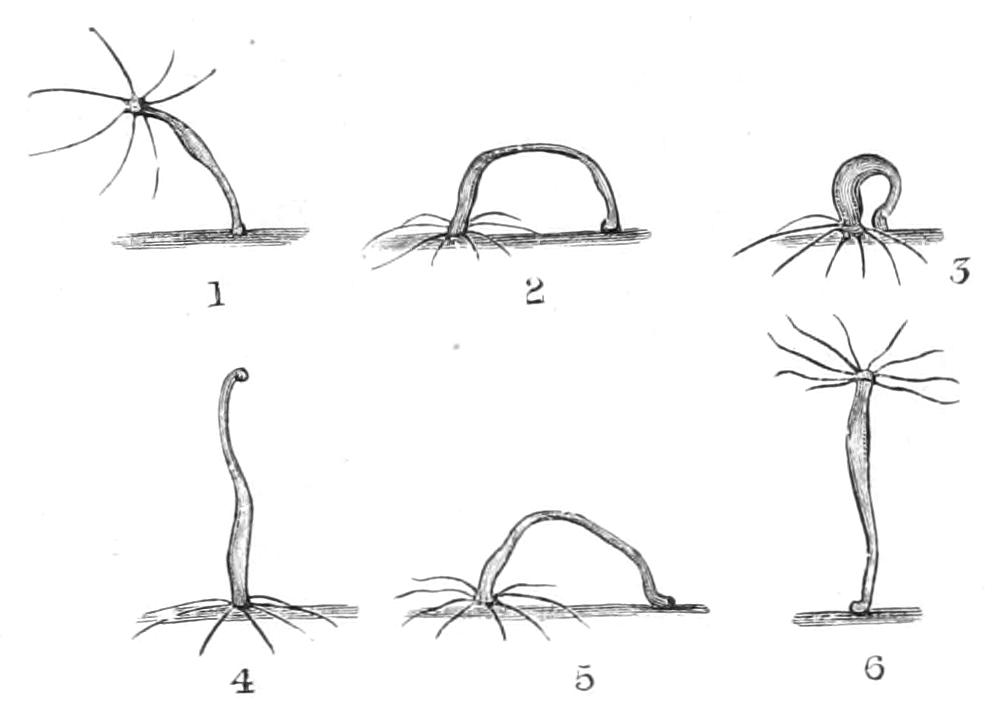
Fig. 127.—A series of drawings of Hydra, showing the attitudes it assumes during one of the more rapid movements from place to place. 1, The Hydra bending over to one side; 2, attaching itself to the support by the mouth and tentacles; 3, drawing the sucker up to the mouth; 4, inverted; 5, refixing the sucker; 6, reassuming the erect posture. (After Trembley.)
One of the characteristic features of Hydra is the slightly expanded, disc-shaped aboral extremity usually called the "foot," an unfortunate term for which the word "sucker" should be substituted. There are no root-like tendrils or processes for attachment to the support such as are found in most of the solitary Gymnoblastea. The attachment of the body to the stem or weed or surface-film by this sucker enables the animal to change its position at will. It may either progress slowly by gliding along its support without the assistance of the tentacles, in a manner similar to that observed in many Sea-anemones; or more rapidly by a series of somersaults, as originally described by Trembley. The latter mode of locomotion has been recently described as follows:—"The body, expanded and with expanded tentacles, bends over to one side. As soon as the tentacles touch the bottom they attach themselves and contract. Now one of two things happens. The foot may loosen its hold on the bottom and the body contract. In this manner the animal comes to stand on its tentacles with the foot pointing upward. The body now bends over again until the foot attaches itself close to the attached tentacles. These loosen in their turn, and so the Hydra is again {256}in its normal position. In the other case the foot is not detached, but glides along the support until it stands close to the tentacles, which now loosen their hold."[290]
Hydra appears to be purely carnivorous. It will seize and swallow Entomostraca of relatively great size, so that the body-wall bulges to more than twice its normal diameter. But smaller Crustacea, Annelid worms, and pieces of flesh are readily seized and swallowed by a hungry Hydra. In H. viridis the chlorophyll corpuscles[291] of the endoderm may possibly assist in the nourishment of the body by the formation of starch in direct sunlight.
Three species of Hydra are usually recognised, but others which may be merely local varieties or are comparatively rare have been named.[292]
H. viridis.—Colour, grass-green. Average number of tentacles, eight. Tentacles shorter than the body. Embryonic chitinous membrane spherical and almost smooth.
H. vulgaris, Pallas (H. grisea, Linn.).—Colour, orange-brown. Tentacles rather longer than the body, average number, six. Embryonic chitinous membrane spherical, and covered with numerous pointed branched spines.
H. oligactis, Pallas (H. fusca, Linn.).—Colour, brown. Tentacles capable of great extension; sometimes, when fully expanded, several times the length of the body. Average number, six. Embryonic chitinous membrane plano-convex, its convex side only covered with spines.
The genera Microhydra (Ryder) and Protohydra (Greeff) are probably allied to Hydra, but as their sexual organs have not been observed their real affinities are not yet determined. Microhydra resembles Hydra in its general form and habits, and in its method of reproduction by gemmation, but it has no tentacles. It was found in fresh water in North America.
Protohydra[293] was found in the oyster-beds off Ostend, and resembles Microhydra in the absence of tentacles. It multiplies by transverse fission, but neither gemmation nor sexual reproduction has been observed.
Haleremita is a minute hydriform zooid which is also marine. {257}It was found by Schaudinn[294] in the marine aquarium at Berlin in water from Rovigno, on the Adriatic. It reproduces by gemmation, but sexual organs have not been found.
Another very remarkable genus usually associated with the Eleutheroblastea is Polypodium. At one stage of its life-history it has the form of a spiral ribbon or stolon which is parasitic on the eggs of the sturgeon (Acipenser ruthenus) in the river Volga.[295] This stolon gives rise to a number of small Hydra-like zooids with twenty tentacles, of which sixteen are filamentous and eight club-shaped. These zooids multiply by longitudinal fission, and feed independently on Infusoria, Rotifers, and other minute organisms. The stages between these hydriform individuals and the parasitic stolon have not been discovered.
Order II. Milleporina.
Millepora was formerly united with the Stylasterina to form the order Hydrocorallina; but the increase of our knowledge of these Hydroid corals tends rather to emphasise than to minimise the distinction of Millepora from the Stylasterina.
Millepora resembles the Stylasterina in the production of a massive calcareous skeleton and in the dimorphism of the zooids, but in the characters of the sexual reproduction and in many minor anatomical and histological peculiarities it is distinct. As there is only one genus, Millepora, the account of its anatomy will serve as a description of the order.
The skeleton (Fig. 128) consists of large lobate, plicate, ramified, or encrusting masses of calcium carbonate, reaching a size of one or two or more feet in height and breadth. The surface is perforated by numerous pores of two distinct sizes; the larger—"gastropores"—are about 0.25 mm. in diameter, and the smaller and more numerous "dactylopores" about 0.15 mm. in diameter. In many specimens the pores are arranged in definite cycles, each gastropore being surrounded by a circle of 5-7 dactylopores; but more generally the two kinds appear to be irregularly scattered on the surface.
When a branch or lobe of a Millepore is broken across and examined in section, it is found that each pore is continued as a {258}vertical tube divided into sections by horizontal calcareous plates (Fig. 129, Tab). These plates are the "tabulae," and constitute the character upon which Millepora was formerly placed in the now discarded group of Tabulate corals.
The coral skeleton is also perforated by a very fine reticulum of canals, by which the pore-tubes are brought into communication with one another. In the axis of the larger branches and in the centre of the larger plates a considerable quantity of the skeleton is of an irregular spongy character, caused by the disintegrating influence of a boring filamentous Alga.[296]
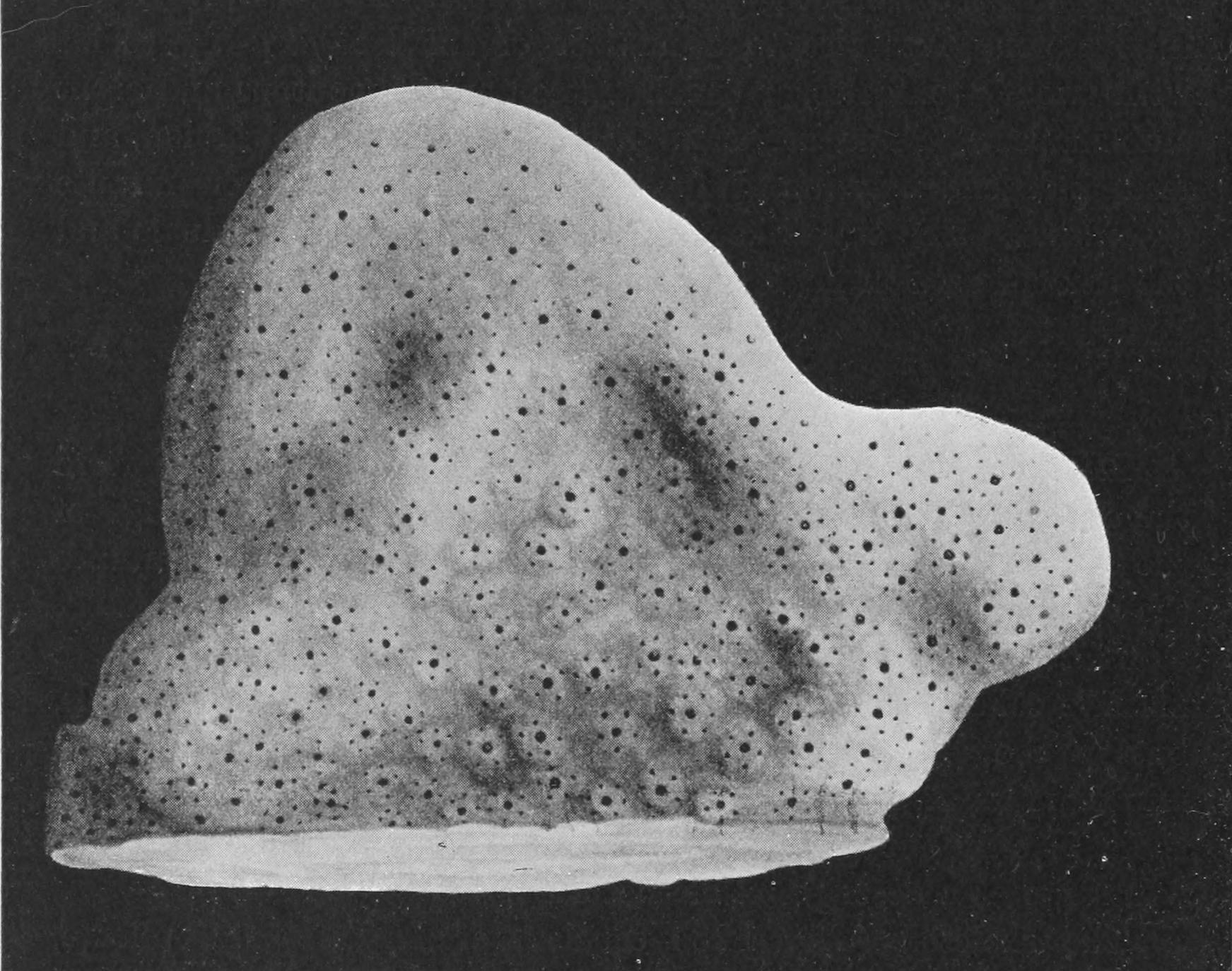
Fig. 128.—A portion of a dried colony of Millepora, showing the larger pores (gastropores) surrounded by cycles of smaller pores (dactylopores). At the edges the cycles are not well defined.
The discovery that Millepora belongs to the Hydrozoa was made by Agassiz[297] in 1859, but Moseley[298] was the first to give {259}an adequate account of the general anatomy. The colony consists of two kinds of zooids—the short, thick gastrozooids (Fig. 129, G) provided with a mouth and digestive endoderm, and the longer and more slender mouthless dactylozooids (D)—united together by a network of canals running in the porous channels of the superficial layer of the corallum. The living tissues of the zooids extend down the pore-tubes as far as the first tabulae, and below this level the canal-system is degenerate and functionless. It is only a very thin superficial stratum of the coral, therefore, that contains living tissues.
The zooids of Millepora are very contractile, and can be withdrawn below the general surface of the coral into the shelter of the pore-tubes. When a specimen is examined in its natural position on the reef, the zooids are usually found to be thus contracted; but several observers have seen the zooids expanded in the living condition. It is probable that, as is the case with other corals, the expansion occurs principally during the night.
The colony is provided with two kinds of nematocysts—the small kind and the large. In some colonies they are powerful enough to penetrate the human skin, and Millepora has therefore received locally the name of "stinging coral." On each of the dactylozooids there are six or seven short capitate tentacles (Fig. 129, t), each head being packed with nematocysts of the small kind; similar batteries of these nematocysts are found in the four short capitate tentacles of the gastrozooids. The nematocysts of the larger kind are found in the superficial ectoderm, some distributed irregularly on the surface, others in clusters round the pores. The small nematocysts are about 0.013 mm. in length before they are exploded, and exhibit four spines at the base of the thread; the large kind are oval in outline, 0.02 × 0.025 mm. in size, and exhibit no spines at the base, but a spiral band of minute spines in the middle of the filament. There is some reason to believe that the filament of the large kind of nematocysts can be retracted.[299]
At certain seasons the colonies of Millepora produce a great number of male or female Medusae. The genus is probably dioecious, no instances of hermaphrodite colonies having yet been found. Each Medusa is formed in a cavity situated above the last-formed tabula in a pore-tube, and this cavity, the "ampulla," having a greater diameter than that of the gastrozooid tubes, can be recognised even in the dried skeleton.
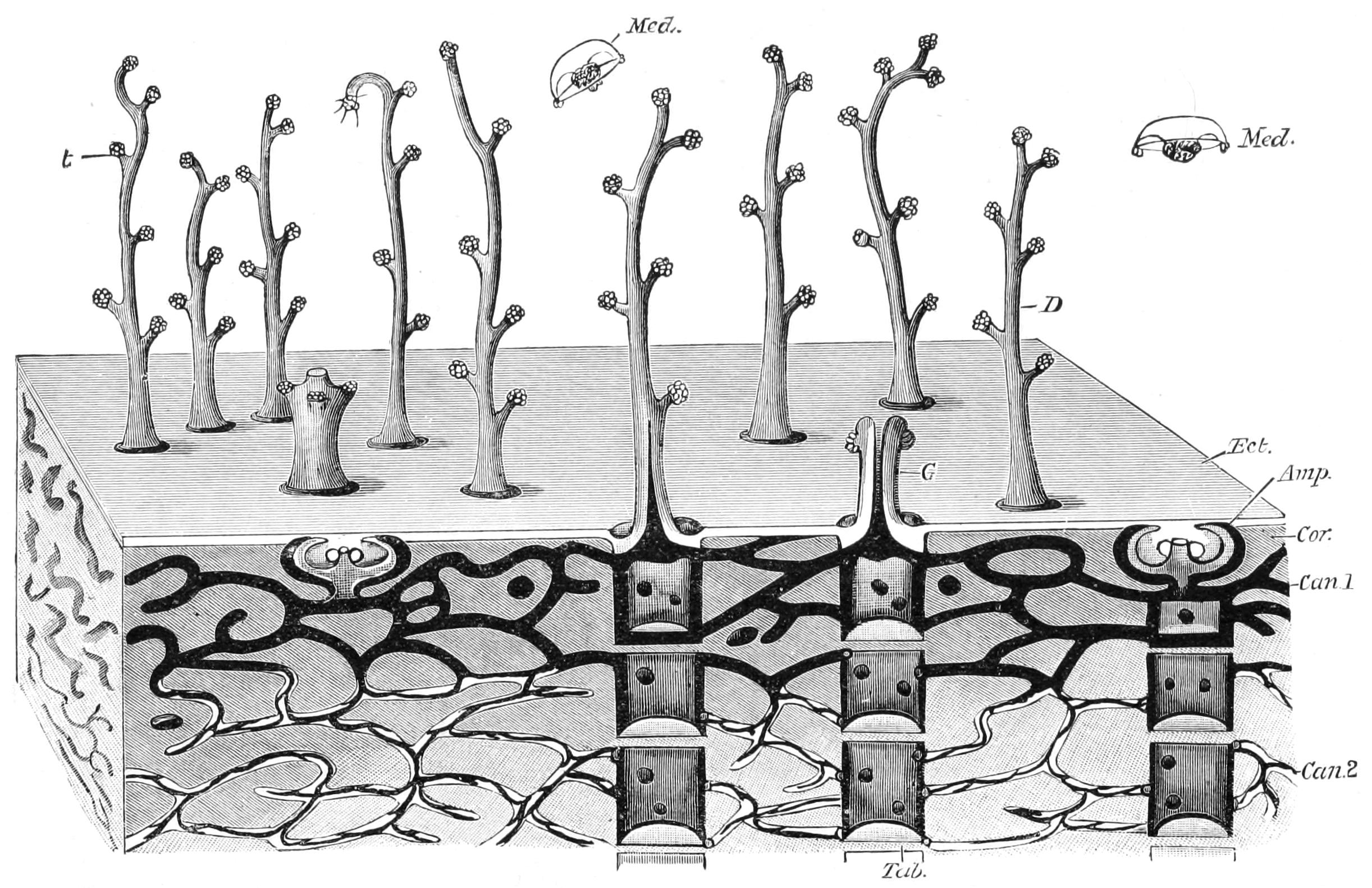
Fig. 129.—Diagrammatic sketch to show the structure of Millepora. Amp, an ampulla containing a medusa; Can.1, canal system at the surface; Can.2, canal system degenerating in the lower layers of the corallum; Cor, corallum; D, an expanded dactylozooid with its capitate tentacles; Ect, the continuous sheet of ectoderm covering the corallum (Cor); G, a gastrozooid, seen in vertical section; Med, free-swimming Medusae; t, tentacle; Tab, tabula in the pore-tubes. (Partly after Moseley.)
It is not known how frequently the sexual seasons occur, but from the rarity in the {261}collections of our museums of Millepore skeletons which exhibit the ampullae, it may be inferred that the intervals between successive seasons are of considerable duration.
The Medusae of Millepora are extremely simple in character. There is a short mouthless manubrium bearing the sexual cells, an umbrella without radial canals, while four or five knobs at the margin, each supporting a battery of nematocysts, represent all that there is of the marginal tentacles. The male Medusae have not yet been observed to escape from the parent, but from the fact that the spermatozoa are not ripe while they are in the ampullae, it may be assumed that the Medusae are set free. Duerden, however, has observed the escape of the female Medusae, and it seems probable from his observations that their independent life is a short one, the ova being discharged very soon after liberation.
Millepora appears to be essentially a shallow-water reef coral. It may be found on the coral reefs of the Western Atlantic extending as far north as Bermuda, in the Red Sea, the Indian and Pacific Oceans. The greatest depth at which it has hitherto been found is 15 fathoms on the Macclesfield Bank, and it flourishes at a depth of 7 fathoms off Funafuti in the Pacific Ocean.
Millepora, like many other corals, bears in its canals and zooids a great number of the symbiotic unicellular "Algae" (Chrysomonadaceae, see pp. 86, 125) known as Zooxanthellae. All specimens that have been examined contain these organisms in abundance, and it has been suggested that the coral is largely dependent upon the activity of the "Algae" for its supply of nourishment. There can be no doubt that the dactylozooids do paralyse and catch living animals, which are ingested and digested by the gastrozooids, but this normal food-supply may require to be supplemented by the carbohydrates formed by the plant-cells. But as the carbohydrates can only be formed by the "Algae" in sunlight, this supplementary food-supply can only be provided in corals that live in shallow water. It must not be supposed that this is the only cause that limits the distribution of Millepora in depth, but it may be an important one.
The generic name Millepora has been applied to a great many fossils from different strata, but a critical examination of their structure fails to show any sufficient reason for including many of them in the genus or even in the order. Fossils that are {262}undoubtedly Millepora occur in the raised coral reefs of relatively recent date, but do not extend back into Tertiary times. There seems to be no doubt, therefore, that the genus is of comparatively recent origin. Among the extinct fossils the genus that comes nearest to it is Axopora from the Eocene of France, but this genus differs from Millepora in having monomorphic, not dimorphic, pores, and in the presence of a minute spine or columella in the centre of each tube. The resemblances are to be observed in the general disposition of the canal system and of the tabulation. Whether Axopora is or is not a true Milleporine, however, cannot at present be determined, but it is the only extinct coral that merits consideration in this place.
Order III. Gymnoblastea—Anthomedusae.
This order was formerly united with the Calyptoblastea to form the order Hydromedusae, but the differences between the two are sufficiently pronounced to merit their treatment as distinct orders.
In many of the Gymnoblastea the sexual cells are borne by free Medusae, which may be recognised as the Medusae of Gymnoblastea by the possession of certain distinct characters. The name given to such Medusae, whether their hydrosome stage is known or not, is Anthomedusae. The Gymnoblastea are solitary or colonial Hydrozoa, in which the free (oral) extremity of the zooids, including the crown of tentacles, is not protected by a skeletal cup. The sexual cells may be borne by free Anthomedusae, or by more or less degenerate Anthomedusae that are never detached from the parent hydrosome. The Anthomedusae are small or minute Medusae provided with a velum, with the ovaries or sperm-sacs borne by the manubrium and with sense-organs in the form of ocelli or pigment-spots situated on the margin of the umbrella.
The solitary Gymnoblastea present so many important differences in anatomical structure that they cannot be united in a single family. They are usually fixed to some solid object by root-like processes from the aboral extremity, the "hydrorhiza," or are partly embedded in the sand (Corymorpha), into which long filamentous processes project for the support of the zooid. The remarkable species Hypolytus peregrinus[300] from Wood's Holl, {263}however, has no aboral processes, and appears to be only temporarily attached to foreign objects by the secretion of the perisarc. Among the solitary Gymnoblastea several species reach a gigantic size. Corymorpha is 50-75 mm. in length, but Monocaulus from deep water in the Pacific and Atlantic Oceans is nearly 8 feet in length. Among the solitary forms attention must be called to the interesting pelagic Pelagohydra (see p. 274).
The method of colony formation in the Gymnoblastea is very varied. In some cases (Clava squamata) a number of zooids arise from a plexus of canals which corresponds with the system of root-like processes of the solitary forms. In Hydractinia this plexus is very dense, and the ectoderm forms a continuous sheet of tissue both above and below. The colony is increased in size in these cases by the gemmation of zooids from the hydrorhiza. In other forms, such as Tubularia larynx, new zooids arise not only from the canals of the hydrorhiza, but also from the body-walls of the upstanding zooids, and thus a bushy or shrubby colony is formed.
In another group the first-formed zooid produces a hydrorhiza of considerable proportions, which fixes the colony firmly to a stone or shell and increases in size with the growth of the colony. This zooid itself by considerable growth in length forms the axis of the colony, and by gemmation gives rise to lateral zooids, which in their turn grow to form the lateral branches and give rise to the secondary branches, and these to the tertiary branches, and so one; each branch terminating in a mouth, hypostome and crown of tentacles. Such a method of colony formation is seen in Bougainvillia (Fig. 130). A still more complicated form of colony formation is seen in Ceratella, in which not a single but a considerable number of zooids form the axis of the colony and of its branches. As each axis is covered with a continuous coat of ectoderm, and each zooid of such an axis secretes a chitinous fenestrated tube, the whole colony is far more rigid and compact than is usual in the Gymnoblastea, and has a certain superficial resemblance to a Gorgoniid Alcyonarian (Fig. 133, p. 271).
The branches of the colony and a considerable portion of the body-wall of each zooid in the Gymnoblastea are usually protected by a thin, unjointed "perisarc" of chitin secreted by the ectoderm; but this skeletal structure does not expand distally to {264}form a cup-like receptacle in which the oral extremity of the zooid can be retracted for protection.
The zooids of the Gymnoblastea present considerable diversity of form and structure. The tentacles may be reduced to one (in Monobrachium) or two (in Lar sabellarum), but usually the number is variable in each individual colony. In many cases, such as Cordylophora, Clava, and many others, the tentacles are irregularly scattered on the sides of the zooids. In others there may be a single circlet of about ten or twelve tentacles round the base of the hypostome. In some genera the tentacles are arranged in two series (Tubularia, Corymorpha, Monocaulus), a distal series round the margin of the mouth which may be arranged in a single circlet or scattered irregularly on the hypostome, and a proximal series arranged in a single circlet some little distance from the mouth. In Branchiocerianthus imperator the number of tentacles is very great, each of the two circlets consisting of about two hundred tentacles.
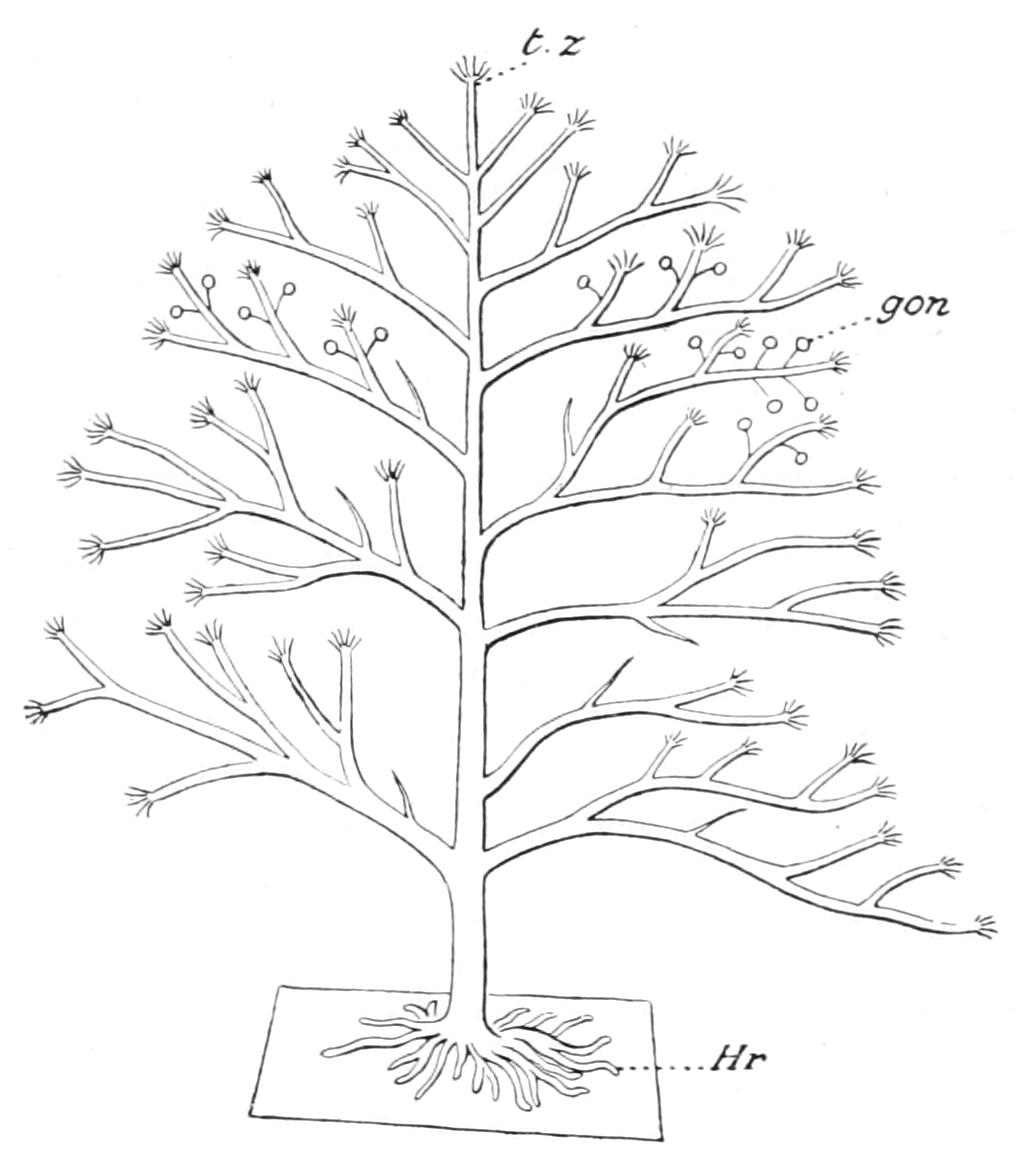
Fig. 130.—Diagrammatic sketch to show the method of branching of Bougainvillia. gon, Gonophores; Hr, hydrorhiza; t.z, terminal zooid.
The zooids of the hydrosome are usually monomorphic, but there are cases in which different forms of zooid occur in the same colony. In Hydractinia, for example, no less than four different kinds of zooids have been described. These are called gastrozooids, dactylozooids, tentaculozooids, and blastostyles respectively. The "gastrozooids" are provided with a conical hypostome bearing the mouth and two closely-set circlets of some ten to thirty tentacles. The "dactylozooids" are longer than the gastrozooids and have the habit of actively coiling and {265}uncoiling themselves; they have a small mouth and a single circlet of rudimentary tentacles. The "tentaculozooids" are situated at the outskirts of the colony, and are very long and slender, with rudimentary tentacles and no mouth. The "blastostyles," usually shorter than the gastrozooids, have two circlets of rudimentary tentacles and a mouth. They bear on their sides the spherical or oval gonophores.
The medusome stage in the life-history of these Hydrozoa is produced by gemmation from the hydrosome, or, in some cases, by gemmation from the medusome as well as from the hydrosome. In many genera and species the medusome is set free as a minute jelly-fish or Medusa, which grows and develops as an independent organism until the time when the sexual cells are ripe, and then apparently it dies. In other Gymnoblastea the medusome either in the female or the male or in both sexes does not become detached from the parent hydrosome, but bears the ripe sexual cells, discharges them into the water, and degenerates without leading an independent life at all. In these cases the principal organs of the medusome are almost or entirely functionless, and they exhibit more or less imperfect development, or they may be so rudimentary that the medusoid characters are no longer obvious. Both the free and the undetached medusomes are gonophores, that is to say, the bearers of the sexual cells, but the former were described by Allman as the "phanerocodonic" gonophores, i.e. "with manifest bells," and the latter as the "adelocodonic" gonophores. The gonophores may arise either from an ordinary zooid of the colony (Syncoryne), from a specially modified zooid—the blastostyle—as in Hydractinia, or from the hydrorhiza as in certain species of Perigonimus. The free-swimming Medusa may itself produce Medusae by gemmation from the manubrium (Sarsia, Lizzia, Rathkea, and others), from the base of the tentacles (Sarsia, Corymorpha, Hybocodon), or from the margin of the umbrella (Eleutheria).
The free-swimming Medusae or phanerocodonic gonophores of the Gymnoblastea are usually of small size (1 or 2 mm. in diameter) when first liberated, and rarely attain a great size even when fully mature. They consist of a circular, bell-shaped or flattened disc—the umbrella—provided at its margin with a few or numerous tentacles, and a tubular manubrium bearing the mouth depending from the exact centre of the under (oral) {266}side of the umbrella (Fig. 132, A). The mouth leads into a shallow digestive cavity, from which radial canals pass through the substance of the umbrella to join a ring-canal at the margin (Fig. 131).
The sense-organs of the Medusae of the Gymnoblastea are in the form of pigment-spots or very simple eyes (ocelli), situated at the bases of the tentacles. The orifice of the umbrella is guarded by a thin shelf or membrane, as in the Calyptoblastea, called the velum. The sexual cells are borne by the manubrium (Figs. 131 and 132, A).
There are many modifications observed in the different genera as regards the number of tentacles, the number and character of the radial canals, the minute structure of the sense-organs, and some other characters, but they agree in having a velum, ocellar sense-organs, and manubrial sexual organs. The tentacles are rudimentary in Amalthea; in Corymorpha there is only one tentacle; in Perigonimus there are two; and in Bougainvillia they are numerous; but the usual number is four or six. The radial canals are usually simple and four in number, but there are six in Lar sabellarum, which branch twice or three times before reaching the margin of the umbrella (Fig. 132, B).
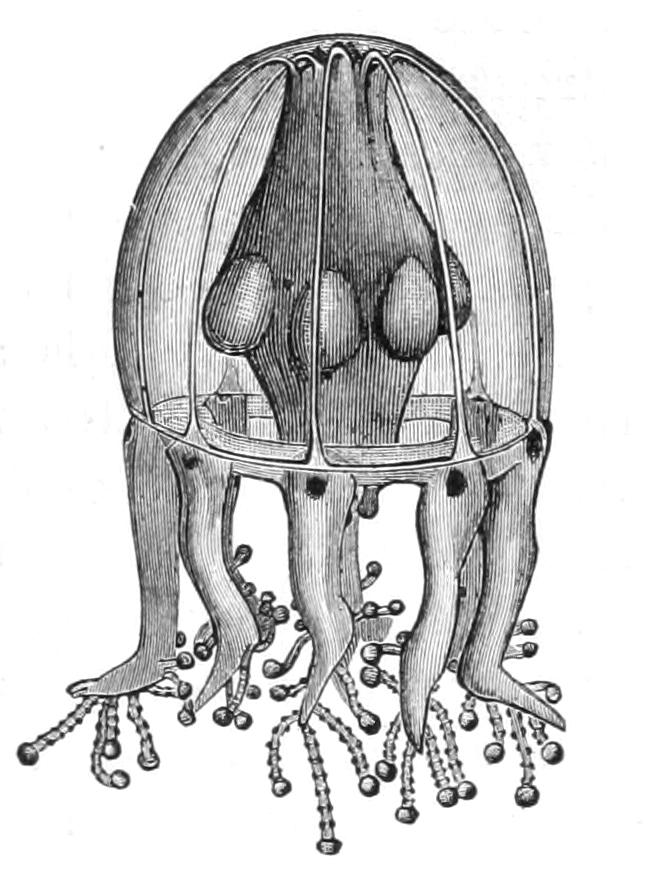
Fig. 131.—Medusa of Cladonema, from the Bahamas, showing peculiar tentacular processes on the tentacles, the ocelli at the base of the tentacles, the swellings on the manubrium that mark the position of the gonads, and the radial and ring-canals of the umbrella. (After Perkins.)
There can be no doubt that the Medusae of many Gymnoblastea undergo several important changes in their anatomical features during the period of the ripening of the sexual cells. Thus in Lar sabellarum the six radial canals are simple in the first stage of development (A); but in the second stage (B) each radial canal bifurcates before reaching the margin, and in the adult stage shows a double bifurcation. The life-history has, however, been worked out in very few of the Anthomedusae, and there can be little doubt that as our knowledge grows several forms which are now known as distinct species {267}will be found to be different stages of growth of the same species.

Fig. 132.—Two stages in the development of the Medusa of Lar sabellarum (Willsia stellata). A, first stage with six canals without branches; B, third stage with six canals each with two lateral branches. The developing gonads may be seen on the manubrium in A. (After Browne.)
The movements of the Medusae are well described by Allman[301] in his account of Cladonema radiatum:—"It is impossible to grow tired of watching this beautiful medusa; sometimes while dashing through the water with vigorous diastole and systole, it will all at once attach its grapples to the side of the vessel, and become suddenly arrested in its career, and then after a period of repose, during which its branched tentacles are thrown back over its umbrella and extended into long filaments which float, like some microscopic sea-weed in the water, it will once more free itself from its moorings and start off with renewed energy." The Medusa of Clavatella, "in its movements and mode of life, presents a marked contrast to the medusiform zooid of other Hydrozoa. The latter is active and mercurial, dancing gaily through the water by means of the vigorous strokes of its crystalline swimming-bell. The former strides leisurely along, or, using the adhesive discs as hands, climbs amongst the branches of the weed. In the latter stage of its existence it becomes stationary, fixing itself by means of its suckers; and {268}thus it remains, the capitate arms standing out rigidly, like the rays of a starfish, until the embryos are ready to escape."[302]
Among the Gymnoblastea there are many examples of a curious association of the Hydroid with some other living animals. Thus Hydractinia is very often found on the shells carried by living Hermit crabs, Dicoryne on the shells of various Molluscs, Tubularia has been found on a Cephalopod, and Ectopleura (a Corymorphid) on the carapace of a crab. There is but little evidence, however, that in these cases the association is anything more than accidental. The occurrence of the curious species, Lar sabellarum, on the tubes of Sabella, of Campaniclava cleodorae on the living shells of the pelagic Mollusc Cleodora cuspidata, and of a Gorgonia on the tubes of Tubularia parasitica, appear to be cases in which there is some mutual relationship between the two comrades. The genus Stylactis, however, affords some of the most interesting examples of mutualism. Thus Stylactis vermicola is found only on the back of an Aphrodite that lives at the great depth of 2900 fathoms. S. spongicola and S. abyssicola are found associated with certain deep-sea Horny Sponges. S. minoi is spread over the skin of the little rock perch Minous inermis, which is found at depths of from 45 to 150 fathoms in the Indian seas.
In many cases it is difficult to understand what is the advantage of the Hydroid to the animal that carries it, but in this last case Alcock[303] suggests that the Stylactis assists in giving the fish a deceitful resemblance to the incrusted rocks of its environment, in order to allure, or at any rate not to scare, its prey. Whether this is the real explanation or not, the fact that in the Bay of Bengal and in the Laccadive and Malabar seas the fish is never found without this Hydroid, nor the Hydroid without this species of fish, suggests very strongly that there is a mutual advantage in the association.
Cases of undoubted parasitism are very rare in this order. The remarkable form Hydrichthys mirus,[304] supposed to be a Gymnoblastic Hydroid, but of very uncertain position in the system, appears to be somewhat modified in its structure by its parasitic habits on the fish Seriola zonata. Corydendrium {269}parasiticum is said to be a parasite living at the expense of Eudendrium racemosum. Mnestra is a little Medusa which attaches itself by its manubrium to the Mollusc Phyllirhoe, and may possibly feed upon the skin or secretions of its host.
Nearly all the species of the order are found in shallow sea water. Stylactis vermicola and the "Challenger" specimen of Monocaulus imperator occur at a depth of 2900 fathoms, and some species of the genera Eudendrium and Myriothela descend in some localities to a depth of a few hundred fathoms. Cordylophora is the only genus known to occur in fresh water. From its habit of attaching itself to wooden piers and probably to the bottom of barges, and from its occurrence in navigable rivers and canals, it has been suggested that Cordylophora is but a recent immigrant into our fresh-water system. It has been found in England in the Victoria docks of London, in the Norfolk Broads, and in the Bridgewater Canal. It has ascended the Seine in France, and may now be found in the ponds of the Jardin des Plantes at Paris. It also occurs in the Elbe and in some of the rivers of Denmark.
The classification of the Gymnoblastea is not yet on a satisfactory basis. At present the hydrosome stage of some genera alone has been described, of others the free-swimming Medusa only is known. Until the full life-history of any one genus has been ascertained its position in the families mentioned below may be regarded as only provisional. The principal families are:—
Fam. Bougainvilliidae.—The zooids of the hydrosome have a single circlet of filiform tentacles at the base of the hypostome. In Bougainvillia belonging to this family the gonophores are liberated in the form of free-swimming Medusae formerly known by the generic name Hippocrene. In the fully grown Medusa there are numerous tentacles arranged in clusters opposite the terminations of the four radial canals. There are usually in addition tentacular processes (labial tentacles) on the lips of the manubrium. Bougainvillia is a common British zoophyte of branching habit, found in shallow water all round the coast. The medusome of Bougainvillia ramosa is said to be the common little medusa Margelis ramosa.[305] Like most of the Hydroids it has a wide geographical distribution. Other genera are Perigonimus, which has a Medusa with only two tentacles; and {270}Dicoryne, which forms spreading colonies on Gasteropod shells and has free gonophores provided with two simple tentacles, while the other organs of the medusome are remarkably degenerate. In Garveia and Eudendrium the gonophores are adelocodonic, in the former genus arising from the body-wall of the axial zooids of the colony, and in the latter from the hydrorhiza. Stylactis is sometimes epizoic (p. 268). Among the genera that are usually placed in this family, of which the medusome stage only is known, are Lizzia (a very common British Medusa) and Rathkea. In Margelopsis the hydrosome stage consists of a single free-swimming zooid which produces Medusae by gemmation.
Fam. Podocorynidae.—The zooids have the same general features as those of the Bougainvilliidae, but the perisarc does not extend beyond the hydrorhiza.
In Podocoryne and Hydractinia belonging to this family the hydrorhiza forms an encrusting stolon which is usually found on Gasteropod shells containing a living Hermit crab. In Podocoryne the gonophores are free-swimming Medusae with a short manubrium provided with labial tentacles. Hydractinia differs from Podocoryne in having polymorphic zooids and adelocodonic gonophores.
A fossil encrusting a Nassa shell from the Pliocene deposit of Italy has been placed in the genus Hydractinia, and four species of the same genus have been described from the Miocene and Upper Greensand deposits of this country.[306] These are the only fossils known at present that can be regarded as Gymnoblastic Hydroids.
The Medusa Thamnostylus, which has only two marginal tentacles and four very long and profusely ramified labial tentacles, is placed in this family. Its hydrosome stage is not known.
Fam. Clavatellidae.—This family contains the genus Clavatella, in which the zooids of the hydrosome have a single circlet of capitate tentacles. The gonophore is a free Medusa provided with six bifurcated capitate tentacles.
Fam. Cladonemidae.—This family contains the genus Cladonema, in which the zooids have two circlets of four tentacles, the labial tentacles being capitate and the aboral filiform. The gonophore is a free Medusa with eight tentacles, each provided with a number of curious capitate tentacular processes (Fig. 131).
Fam. Tubulariidae.—This important and cosmopolitan family is represented in the British seas by several common species. The zooids of the hydrosome of Tubularia have two circlets of numerous filiform tentacles. The gonophores are adelocodonic, and are situated on long peduncles attached to the zooid on the upper side of the aboral circlet of tentacles. The larva escapes from the gonophore and acquires two tentacles, with which it beats the water and, assisted by the cilia, keeps itself afloat for some time. In this stage it is known as an "Actinula."[307]
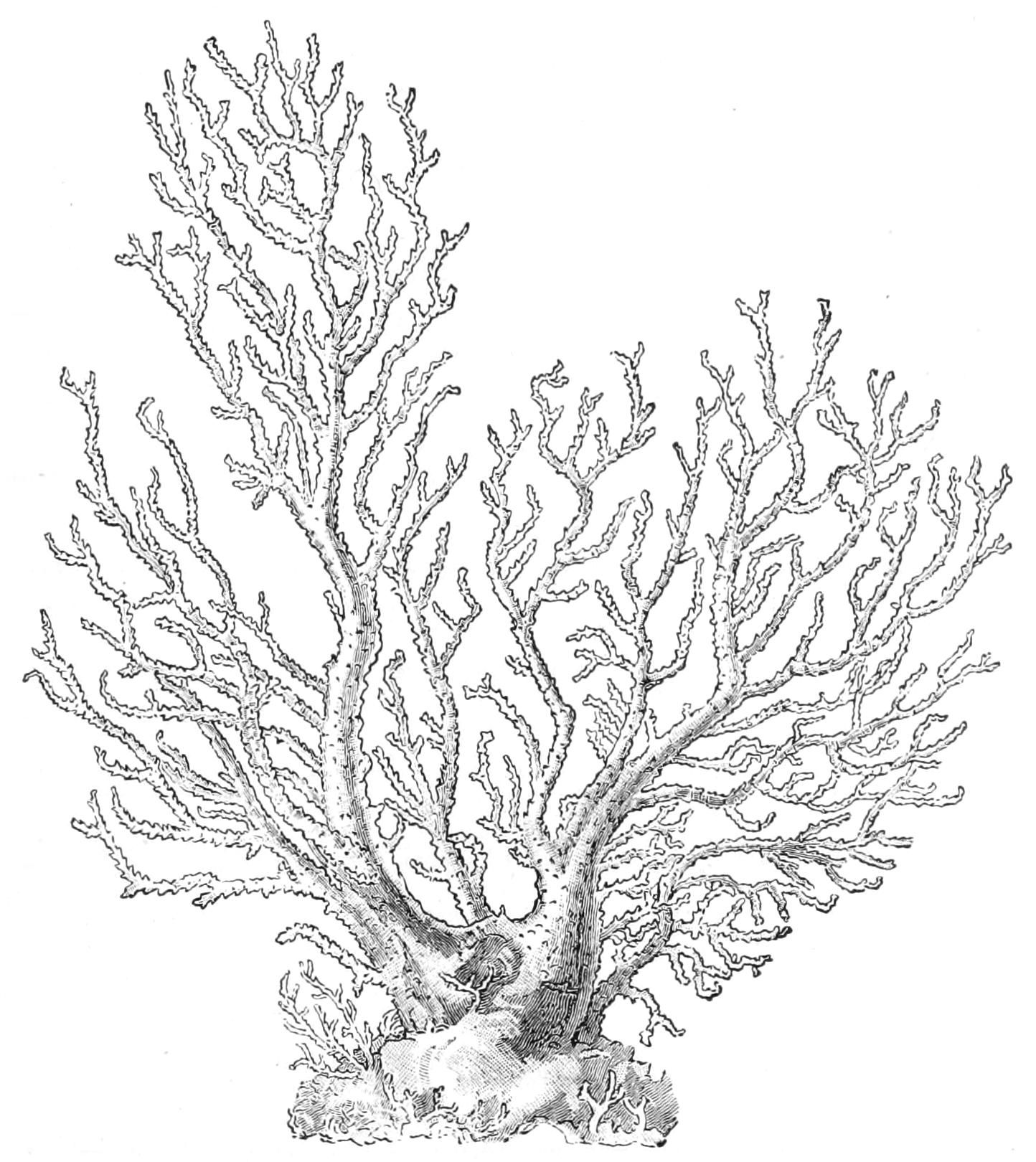
Fig. 133.—Ceratella fusca. About nat. size. (After Baldwin Spencer.[308])
Fam. Ceratellidae.—The colony of Ceratella may be five inches in height. The stem and main branches are substantial, and consist of a network of branching anastomosing tubes supported by a thick and fenestrated chitinous perisarc. The {272}whole branch is enclosed in a common layer of ectoderm. The zooids have scattered capitate tentacles. The Ceratellidae occur in shallow water off the coast of New South Wales, extend up the coast of East Africa as far as Zanzibar, and have also been described from Japan.
Fam. Pennariidae.—In the hydrosome stage the zooids have numerous oral capitate tentacles scattered on the hypostome, and a single circlet of basilar filiform tentacles. The medusa of Pennaria, a common genus of wide distribution, is known under the name Globiceps.
Fam. Corynidae.—In the hydrosome stage the zooids of this family possess numerous capitate tentacles arranged in several circlets or scattered.
In Cladocoryne the tentacles are branched. Syncoryne is a common and widely distributed genus with numerous unbranched capitate tentacles irregularly distributed over a considerable length of the body-wall of the zooid. In many of the species the gonophores are liberated as Medusae, known by the name Sarsia, provided with four filiform tentacles and a very long manubrium. In some species (S. prolifera and S. siphonophora) the Medusae are reproduced asexually by gemmation from the long manubrium. A common British Anthomedusa of this family is Dipurena, but its hydrosome stage is not known. In the closely related genus Coryne the gonophores are adelocodonic, and exhibit very rudimentary medusoid characters.
Fam. Clavidae.—This is a large family containing many genera, some with free-swimming Medusae, others with adelocodonic gonophores. In the former group are included a number of oceanic Medusae of which the hydrosome stage has not yet been discovered. The zooids of the hydrosome have numerous scattered filiform tentacles. The free-swimming Medusae have hollow tentacles.
Clava contains a common British species with a creeping hydrorhiza frequently attached to shells, and with adelocodonic gonophores. Cordylophora is the genus which has migrated into fresh water in certain European localities (see p. 269). It forms well-developed branching colonies attached to wooden gates and piers or to the brickwork banks of canals. Several Anthomedusae, of which the hydrosome stage is not known, appear to be related to the Medusae of this family, but are sometimes separated as {273}the family Tiaridae. Of these Tiara, a very brightly coloured jelly-fish sometimes attaining a height of 40 mm., is found on the British coasts, and Amphinema is found in considerable numbers at Plymouth in September. Turritopsis is a Medusa with a hydrosome stage like Dendroclava. For Stomatoca, see p. 415.
Fam. Corymorphidae.—This family contains the interesting British species Corymorpha nutans. The hydrosome stage consists of a solitary zooid of great size, 50-75 mm. in length, provided with two circlets of numerous long filiform tentacles. The free-swimming Medusae are produced in great numbers on the region between the two circlets of tentacles. These Medusae were formerly known by the name Steenstrupia, and are noteworthy in having only one long moniliform tentacle, opposite to one of the radial canals.
The gigantic Monocaulus imperator of Allman was obtained by the "Challenger" at the great depth of 2900 fathoms off the coast of Japan. It was nearly eight feet in length. More recently Miyajima[309] has described a specimen from 250 fathoms in the same seas which was 700 mm. (27.5 in.) in length. Miyajima's specimen resembles those described by Mark from 300 fathoms off the Pacific coast of North America as Branchiocerianthus urceolus in the remarkable feature of a distinct bilateral arrangement of the circlets of tentacles. Owing to the imperfect state of preservation of the only specimen of Allman's species it is difficult to determine whether it is also bilaterally symmetrical and belongs to the same species as the specimens described by Mark and Miyajima. These deep-sea giant species, however, appear to differ from Corymorpha in having adelocodonic gonophores.
Fam. Hydrolaridae.—This family contains the remarkable genus Lar, which was discovered by Gosse attached to the margin of the tubes of the marine Polychaete worm Sabella. The zooids have only two tentacles, and exhibit during life curious bowing and bending movements which have been compared with the exercises of a gymnast. The Medusae (Fig. 132, A and B) have been known for a long time by the name Willsia, but their life-history has only recently been worked out by Browne.[310]
Fam. Monobrachiidae.—Monobrachium, found in the White Sea by Mereschkowsky, forms a creeping stolon on the shells of Tellina. The zooids of the hydrosome have only one tentacle.
Fam. Myriothelidae.—This family contains the single genus Myriothela. The zooid of the hydrosome stage is solitary and is provided, as in the Corynidae, with numerous scattered capitate tentacles. The gonophores are borne by blastostyles situated above the region of the tentacles. In addition to these blastostyles producing gonophores there are, in M. phrygia, supplementary blastostyles which capture the eggs as they escape from the gonophores and hold them until the time when the larva is ready to escape. They were called "claspers" by Allman. In some of the Arctic species Frl. Bonnevie[311] has shown that they are absent. Each zooid of M. phrygia is hermaphrodite.
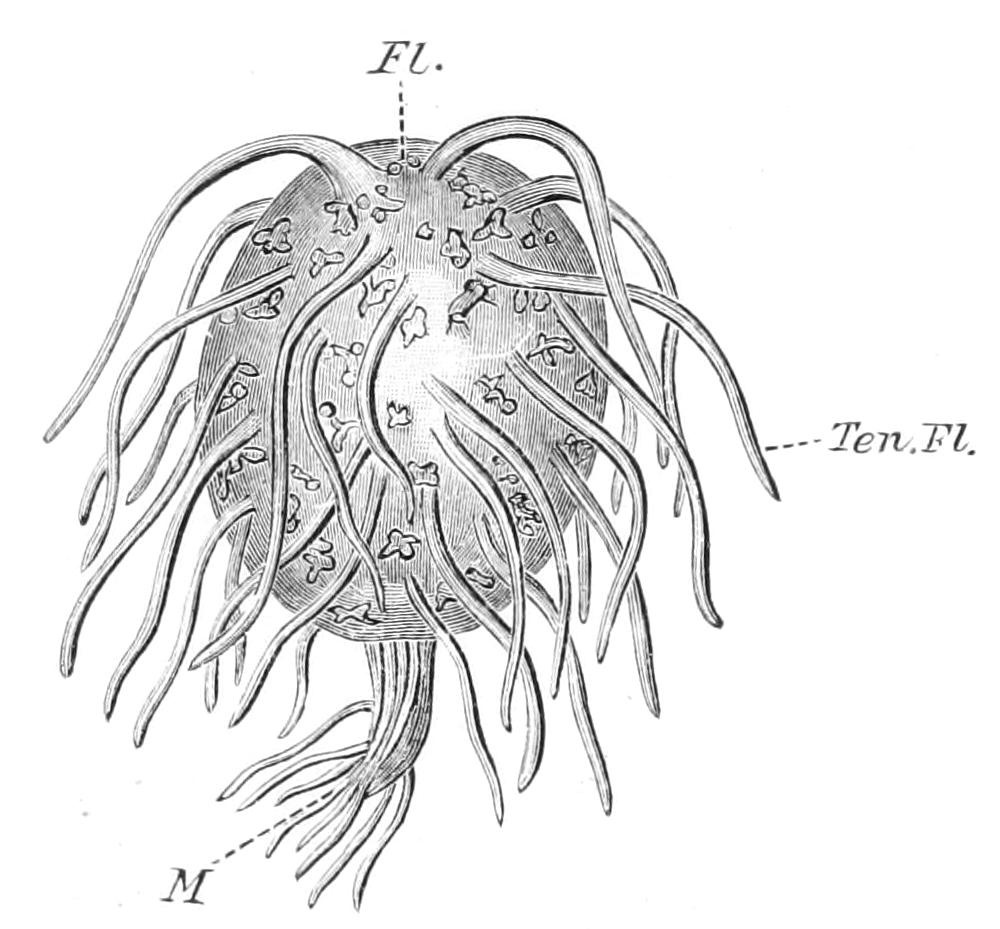
Fig. 134.—Pelagohydra mirabilis. Fl, The float; M, position of the mouth; Ten.Fl, filamentous tentacles of the float. (After Dendy.)
Fam. Pelagohydridae.—This family was constituted by Dendy[312] for the reception of Pelagohydra mirabilis, a remarkable new species discovered by him on the east coast of the South Island of New Zealand. The hydrosome is solitary and free-swimming, the proximal portion of the body being modified to form a float, the distal portion forming a flexible proboscis terminated by the mouth and a group of scattered manubrial tentacles. The tentacles are filiform and scattered over the surface of the float. Medusae are developed on stolons between the tentacles of the float. They have tentacles arranged in four radial groups of five each, at the margin of the umbrella.
As pointed out by Hartlaub,[313] Pelagohydra is not the only genus in which the hydrosome floats. Three species of the genus Margelopsis have been found that have pelagic habits, and two {275}of them have been shown to produce numerous free-swimming Medusae by gemmation; but at present there is no reason to suppose that in these forms there is any extensive modification of the aboral extremity of the zooid to form such a highly specialised organ as the float of Pelagohydra.
The affinities of Pelagohydra are not clear, as our knowledge of the characters of the Medusa is imperfect; but according to Dendy it is most closely related to the Corymorphidae. Margelopsis belongs to the Bougainvilliidae.
Order IV. Calyptoblastea—Leptomedusae.
The hydrosome stage is characterised by the perisarc, which not only envelops the stem and branches, as in many of the Gymnoblastea, but is continued into a trumpet-shaped or tubular cup or collar called the "hydrotheca," that usually affords an efficient protection for the zooids when retracted. No solitary Calyptoblastea have been discovered. In the simpler forms the colony consists of a creeping hydrorhiza, from which the zooids arise singly (Clytia johnstoni), but these zooids may give rise to a lateral bud which grows longer than the parent zooid.
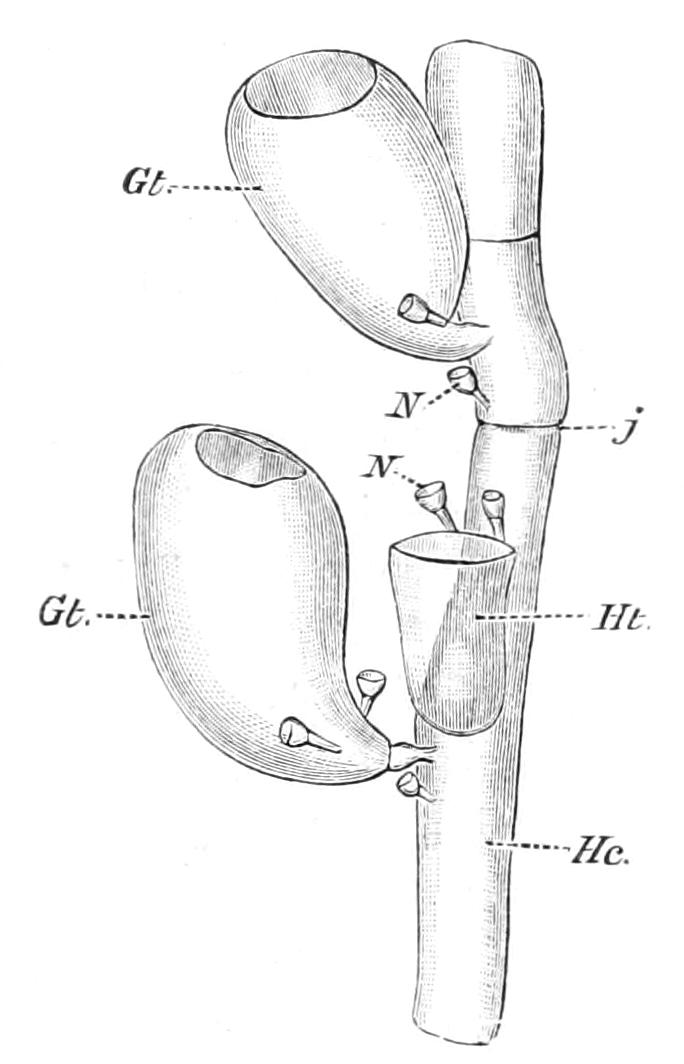
Fig. 135.—Part of a hydrocladium of a dried specimen of Plumularia profunda. Gt, Gonotheca; Hc, the stem of the hydrocladium with joints (j); Ht, a single hydrotheca; N, nematophores. Greatly enlarged. (After Nutting.)
The larger colonies are usually formed by alternate right and left budding from the last-formed zooid, so that in contrast to the Gymnoblast colony the apical zooid of the stem is the youngest, and not the oldest, zooid of the colony. In the branching colonies the axis is frequently composed of a single tube of perisarc, which may be lined internally by the ectoderm and endoderm tissues formed by the succession of zooids that have given rise to the branches by gemmation. Such a stem is said to be monosiphonic.
In some of the more complicated colonies, however, the stem is composed of several tubes, which may or may not be surrounded by a common sheath of ectoderm and perisarc, as they are in Ceratella among the Gymnoblastea. Such stems are said to be "polysiphonic" or "fascicled." The polysiphonic stem may arise in more than one way, and in some cases it is not quite clear in what manner it has arisen.[314]
In many colonies the zooids are only borne by the terminal monosiphonic branches, which receive the special name "hydrocladia." The gonophores of the Calyptoblastea are usually borne by rudimentary zooids, devoid of mouth and tentacles (the "blastostyles"), protected by a specially dilated cup of perisarc known as the "gonotheca" or "gonangium." The shape and size of the gonothecae vary a good deal in the order. They may be simply oval in shape, or globular (Schizotricha dichotoma), or greatly elongated, with the distal ends produced into slender necks (Plumularia setacea). They are spinulose in P. echinulata, and annulated in P. halecioides, Clytia, etc.
In some genera there are special modifications of the branches and hydrocladia, for the protection of the gonothecae. The name "Phylactocarp" is used to designate structures that are obviously intended to serve this purpose. The phylactocarp of the genera Aglaophenia and Thecocarpus is the largest and most remarkable of this group of structures, and has received the special name "corbula." The corbula consists of an axial stem or rachis, and of a number of corbula-leaves arising alternately from the rachis, bending upwards and then inwards to meet those of the other side above, the whole forming a pod-shaped receptacle. The gonangia are borne at the base of each of the corbula-leaves. There is some difference of opinion as to the homologies of the parts of the corbula, but the rachis seems to be that of a modified hydrocladium, as it usually bears at its base one or more hydrothecae of the normal type. The corbula-leaves are usually described as modified nematophores (vide infra), but according to Nutting[315] there is no more reason to regard them as modified nematophores than as modified hydrothecae, and he regards them as "simply the modification of a structure originally intended to {277}protect an indefinite person, an individual that may become either a sarcostyle[316] or a hydranth."
The other forms of phylactocarps are modified branches as in Lytocarpus, and those which are morphologically appendages to branches as in Cladocarpus, Aglaophenopsis, and Streptocaulus.
The structures known as "nematophores" in the Calyptoblastea are the thecae of modified zooids, comparable with the dactylozooids of Millepora. They form a well-marked character of the very large family Plumulariidae, but they are also found in species of the genera Ophiodes, Lafoëina, Oplorhiza, Perisiphonia, Diplocyathus, Halecium, and Clathrozoon among the other Calyptoblastea. The dactylozooids are usually capitate or filiform zooids, without tentacles or a mouth, and with a solid or occasionally a perforated core of endoderm. They bear either a battery of nematocysts (Plumularia, etc.), or of peculiar adhesive cells (Aglaophenia and some species of Plumularia). The functions of the dactylozooids are to capture the prey and to serve as a defence to the colony. In the growth of the corbula of Aglaophenia the dactylozooids appear to serve another purpose, and that is, as a temporary attachment to hold the leaves together while the edges themselves are being connected by trabeculae of coenosarc.
In a very large number of Calyptoblastea the gonophore is a reduced Medusa which never escapes from the gonotheca, but in the family Eucopidae the gonophores escape as free-swimming Medusae, exhibiting certain very definite characters. The gonads are situated not on the manubrium, as in the Anthomedusae, but on the sub-umbrellar aspect of the radial canals. The marginal sense-organs may be ocelli or vesiculate statocysts. The bell is usually more flattened, and the velum smaller than it is in the Anthomedusae, and the manubrium short and quadrangular. Such Medusae are called Leptomedusae.
Leptomedusae of many specific forms are found abundantly at the surface of the sea in nearly all parts of the world, but with the exception of some genera of the Eucopidae and a few others, their connexion with a definite Calyptoblastic hydrosome has not been definitely ascertained. It may be an assumption that time will prove to be unwarranted that all the Leptomedusae pass through a Calyptoblastic hydrosome stage.
Fam. Aequoreidae.—In this family the hydrosome stage is not known except in the genus Polycanna, in which it resembles a Campanulariid. The sense-organs of the Medusae are statocysts. The radial canals are very numerous, and the genital glands are in the form of ropes of cells extending along the whole of their oral surfaces. Aequorea is a fairly common genus, with a flattened umbrella and a very rudimentary manubrium, which may attain a size of 40 mm. in diameter.
Fam. Thaumantiidae.—The Medusae of this family are distinguished from the Aequoreidae by having marginal ocelli in place of statocysts. The hydrosome of Thaumantias alone is known, and this is very similar to an Obelia.
Fam. Cannotidae.—The hydrosome is quite unknown. The Medusae are ocellate, but the radial canals, instead of being undivided, as in the Thaumantiidae, are four in number, and very much ramified before reaching the ring canal. The tentacles are very numerous. In the genus Polyorchis, from the Pacific coast of North America, the four radial canals give rise to numerous lateral short blind branches, and have therefore a remarkable pinnate appearance.
Fam. Sertulariidae.—In this family the hydrothecae are sessile, and arranged bilaterally on the stem and branches. The general form of the colony is pinnate, the branches being usually on opposite sides of the main stem. The gonophores are adelocodonic. Sertularia forms more or less arborescent colonies, springing from a creeping stolon attached to stones and shells. There are many species, several of which are very common upon the British coast. Many specimens are torn from their attachments by storms or by the trawls of fishermen and cast up on the sand or beach with other zoophytes. The popular name for one of the commonest species (S. abietina) is the "sea-fir." The genus has a wide geographical and bathymetrical range. Another common British species frequently thrown up by the tide in great quantities is Hydrallmania falcata. It has slender spirally-twisted stems and branches, and the hydrothecae are arranged unilaterally.
The genus Grammaria, sometimes placed in a separate family, is distinguished from Sertularia by several characters. The stem and branches are composed of a number of tubes which are considerably compressed. The genus is confined to the southern seas.
Fam. Plumulariidae.—The hydrothecae are sessile, and arranged in a single row on the stem and branches. Nematophores are always present. Gonophores adelocodonic. This family is the largest and most widely distributed of all the families of the Hydrozoa. Nutting calculates that it contains more than one-fourth of all the Hydroids of the world. Over 300 species have been described, and more than half of these are found in the West Indian and Australian regions. Representatives of the family occur in abundance in depths down to 300 fathoms, and not unfrequently to 500 fathoms. Only a few species have occasionally been found in depths of over 1000 fathoms.
The presence of nematophores may be taken as the most characteristic feature of the family, but similar structures are also found in some species belonging to other families (p. 277).
The family is divided into two groups of genera, the Eleutheroplea and the Statoplea. In the former the nematophores are mounted on a slender pedicel, which admits of more or less movement, and in the latter the nematophores are sessile. The genera Plumularia and Antennularia belong to the Eleutheroplea. The former is a very large genus, with several common British species, distinguished by the terminal branches being pinnately disposed, and the latter, represented by A. antennina and A. ramosa on the British coast, is distinguished by the terminal branches being arranged in verticils.
The two most important genera of the Statoplea are Aglaophenia and Cladocarpus. The former is represented by a few species in European waters, the latter is only found in American waters.
Fam. Hydroceratinidae.—The colony consists of a mass of entwined hydrorhiza, with a skeleton in the form of anastomosing chitinous tubes. Hydrothecae scattered, tubular, and sessile. Nematophores present. Gonophores probably adelocodonic.
This family was constituted for a remarkable hydroid, Clathrozoon wilsoni, described by W. B. Spencer from Victoria.[317] The zooids are sessile, and spring from more than one of the numerous anastomosing tubes of the stem and branches. The whole of the surface is studded with an enormous number of small and very simple dactylozooids, protected by tubular nematophores. Only {280}a few specimens have hitherto been obtained, the largest being 10 inches in height by 4 inches in width. In general appearance it has some resemblance to a dark coloured fan-shaped Gorgonia.
Fam. Campanulariidae.—The hydrothecae in this family are pedunculate, and the gonophores adelocodonic.
In the cosmopolitan genus Campanularia the stem is monosiphonic, and the hydrothecae bell-shaped. Several species of this genus are very common in the rock pools of our coast between tide marks. Halecium is characterised by the rudimentary character of its hydrothecae, which are incapable of receiving the zooids even in their maximum condition of retraction. The genus Lafoea is remarkable for the development of a large number of tightly packed gonothecae on the hydrorhiza, each of which contains a blastostyle, bearing a single gonophore and, in the female, a single ovum. This group of gonothecae was regarded as a distinct genus of Hydroids, and was named Coppinia.[318] Lafoea dumosa with gonothecae of the type described as Coppinia arcta occurs on the British coast.
Perisiphonia is an interesting genus from deep water off the Azores, Australia, and New Zealand, with a stem composed of many distinct tubes.
The genus Zygophylax, from 500 fathoms off the Cape Verde, is of considerable interest in having a nematophore on each side of the hydrotheca. According to Quelch it should be placed in a distinct family.
Ophiodes has long and very active defensive zooids, protected by nematophores. It is found in the Laminarian zone on the English coast.
Fam. Eucopidae.—The hydrosome stage of this family is very similar to that of the Campanulariidae, but the gonophores are free-swimming Medusae of the Leptomedusan type.
One of the best-known genera is Obelia, of which several species are among the commonest Hydroids of the British coast.
Clytia johnstoni is also a very common Hydroid, growing on red algae or leaves of the weed Zostera. It consists of a number of upright, simple, or slightly branched stems springing from a creeping hydrorhiza. When liberated the Medusae are globular in form, with four radial canals and four marginal tentacles, but {281}this Medusa, like many others of the order, undergoes considerable changes in form before it reaches the sexually mature stage.
Phialidium temporarium is one of the commonest Medusae of our coast, and sometimes occurs in shoals. It seems probable that it is the Medusa of Clytia johnstoni.[319] By some authors the jelly-fish known as Epenthesis is also believed to be the Medusa of a Clytia.
Fam. Dendrograptidae.—This family includes a number of fossils which have certain distinct affinities with the Calyptoblastea. In Dictyonema, common in the Ordovician rocks of Norway, but also found in the Palaeozoic rocks of North America and elsewhere, the fossil forms fan-shaped colonies of delicate filaments, united by many transverse commissures, and in well-preserved specimens the terminal branches bear well-marked uniserial hydrothecae. In some species thecae of a different character, which have been interpreted to be gonothecae and nematophores respectively, are found.
Other genera are Dendrograptus, Thamnograptus, and several others from Silurian strata.
Order V. Graptolitoidea.
A large number of fossils, usually called Graptolites, occurring in Palaeozoic strata, are generally regarded as the skeletal remains of an ancient group of Hydrozoa.
In the simpler forms the fossil consists of a delicate straight rod bearing on one side a series of small cups. It is suggested that the cups contained hydroid zooids, and should therefore be regarded as the equivalent of the hydrothecae, and that the axis represents the axis of the colony or of a branch of the Calyptoblastea. In some of the forms with two rows of cups on the axis (Diplograptus), however, it has been shown that the cups are absent from a considerable portion of one end of the axis, and that the axes of several radially arranged individuals are fused together and united to a central circular plate. Moreover, there is found in many specimens a series of vesicles, a little larger in size than the cups, attached to the plate and arranged in a circle at the base of the axes. These vesicles are called the gonothecae.
The discovery of the central plate and of the so-called {282}gonothecae suggests that the usual comparison of a Graptolite with a Sertularian Hydroid is erroneous, and that the colony or individual, when alive, was a more or less radially symmetrical floating form, like a Medusa, of which only the distal appendages (possibly tentacles) are commonly preserved as fossils.
The evidence that the Graptolites were Hydrozoa is in reality very slight, but the proof of their relationship to any other phylum of the animal kingdom does not exist.[320] It is therefore convenient to consider them in this place, and to regard them, provisionally, as related to the Calyptoblastea.
The order is divided into three families.
Fam. 1. Monoprionidae.—Cups arranged uniserially on one side of the axis.
The principal genera are Monograptus, with the axis straight, curved, or helicoid, from many horizons in the Silurian strata; Rastrites, with a spirally coiled axis, Silurian; Didymograptus, Ordovician; and Coenograptus, Ordovician.
Fam. 2. Diprionidae.—Cups arranged in two or four vertical rows on the axis.
Diplograptus, Ordovician and Silurian; Climacograptus, Ordovician and Silurian; and Phyllograptus, in which the axis and cups are arranged in such a manner that they resemble an ovate leaf.
Fam. 3. Retiolitidae.—Cups arranged biserially on a reticulate axis.
Retiolites, Ordovician and Silurian; Stomatograptus, Retiograptus, and Glossograptus, Ordovician.
Fossil Corals possibly allied to Hydrozoa.
Among the many fossil corals that are usually classified with the Hydrozoa the genus Porosphaera is of interest as it is often supposed to be related to Millepora. It consists of globular masses about 10-20 mm. in diameter occurring in the Upper Cretaceous strata. In the centre there is usually a foreign body around which the coral was formed by concentric encrusting growth. Running radially from pores on the surface to the centre, there are numerous tubules which have a certain general resemblance to the pore-tubes of Millepora. The monomorphic {283}character of these tubes, their very minute size, the absence of ampullae, and the general texture of the corallum, are characters which separate this fossil very distinctly from any recent Hydroid corals. Porosphaera, therefore, was probably not a Hydrozoon, and certainly not related to the recent Millepora.
Closely related to Porosphaera apparently are other globular, ellipsoidal, or fusiform corals from various strata, such as Loftusia from the Eocene of Persia, Parkeria from the Cambridge Greensand, and Heterastridium from the Alpine Trias. In the last named there is apparently a dimorphism of the radial tubes.
Allied to these genera, again, but occurring in the form of thick, concentric, calcareous lamellae, are the genera Ellipsactinia and Sphaeractinia from the Upper Jurassic.
Another important series of fossil corals is that of the family Stromatoporidae. These fossils are found in great beds of immense extent in many of the Palaeozoic rocks, and must have played an important part in the geological processes of that period. They consist of a series of calcareous lamellae, separated by considerable intervals, encrusting foreign bodies of various kinds. Sometimes they are flat and plate-like, sometimes globular or nodular in form. The lamellae are in some cases perforated by tabulate, vertical, or radial pores, but in many others these pores are absent. The zoological position of the Stromatoporidae is very uncertain, but there is not at present any very conclusive evidence that they are Hydrozoa.
Stromatopora is common in Devonian and also occurs in Silurian strata. Cannopora from the Devonian has well-marked tabulate pores, and is often found associated commensally with another coral (Aulopora or Syringopora).
Order VI. Stylasterina.
The genera included in this order resemble Millepora in producing a massive calcareous skeleton, and in showing a consistent dimorphism of the zooids, but in many respects they exhibit great divergence from the characters of the Milleporina.
The colony is arborescent in growth, the branches arising frequently only in one plane, forming a flabellum. The calcareous skeleton is perforated to a considerable depth by the gastrozooids, dactylozooids, and nutritive canals, and the {284}gastropores and dactylopores are not provided with tabulae except in the genera Pliobothrus and Sporadopora. The character which gives the order its name is a conical, sometimes torch-like projection at the base of the gastropore, called the "style," which carries a fold of the ectoderm and endoderm layers of the body-wall, and may serve to increase the absorptive surface of the digestive cavity. In some genera a style is also present in the dactylopore, in which case it serves as an additional surface for the attachment of the retractor muscles. The pores are scattered on all aspects of the coral in the genera Sporadopora, Errina, and Pliobothrus; in Spinipora and Steganopora the scattered dactylopores are situated at the extremities of tubular spines which project from the general surface of the coral, the gastropores being situated irregularly between the spines. In Phalangopora the pores are arranged in regular longitudinal lines, and in Distichopora they are mainly in rows on the edges of the flattened branches, a single row of gastropores being flanked by a single row of dactylopores on each side. In the remaining genera the pores are arranged in definite cycles, which are frequently separated from one another by considerable intervals, and have, particularly in the dried skeleton, a certain resemblance to the calices of some of the Zoantharian corals.
In Cryptohelia the cycles are covered by a lid-like projection from the neighbouring coenenchym (Fig. 136, l 1, l 2). The gastrozooids are short, and are usually provided with a variable number of small capitate tentacles. The dactylozooids are filiform and devoid of tentacles, the endoderm of their axes being solid and scalariform.
The gonophores of the Stylasterina are situated in large oval or spherical cavities called the ampullae, and their presence can generally be detected by the dome-shaped projections they form on the surface of the coral. The female gonophore consists of a saucer-shaped pad of folded endoderm called the "trophodisc," which serves the purpose of nourishing the single large yolk-laden egg it bears; and a thin enveloping membrane composed of at least two layers of cells. The egg is fertilised while it is still within the ampulla, and does not escape to the exterior until it has reached the stage of a solid ciliated larva. All the Stylasterina are therefore viviparous. The male gonophore has a very much smaller trophodisc, which is sometimes (Allopora) prolonged into a columnar process or spadix, penetrating the {285}greater part of the gonad. The spermatozoa escape through a peculiar spout-like duct which perforates the superficial wall of the ampulla. In some genera (Distichopora) there are several male gonophores in each ampulla.
The gonophores of the Stylasterina have been regarded as much altered medusiform gonophores, and this view may possibly prove to be correct. At present, however, the evidence of their derivation from Medusae is not conclusive, and it is possible that they may have had a totally independent origin.
Distichopora and some species of Stylaster are found in shallow water in the tropics, but most of the genera are confined to deep or very deep water, and have a wide geographical distribution. No species have been found hitherto within the British area.
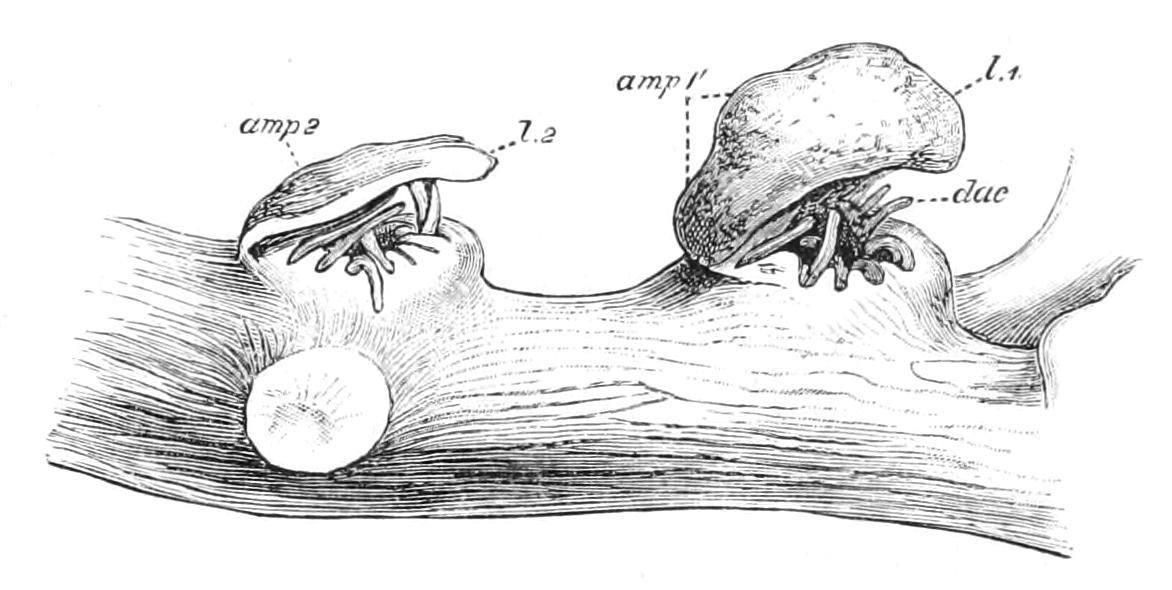
Fig. 136.—A portion of a branch of Cryptohelia ramosa, showing the lids l 1 and l 2 covering the cyclosystems, the swellings produced by the ampullae in the lids amp1, amp2, and the dactylozooids, dac. × 22. (After Hickson and England.)
A few specimens of a species of Stylaster have been found in Tertiary deposits and in some raised beaches of more recent origin, but the order is not represented in the older strata.
Fam. Stylasteridae.—All the genera at present known are included in this family.
Sporadopora is the only genus that presents a superficial general resemblance to Millepora. It forms massive, branching white coralla, with the pores scattered irregularly on the surface, and, like many varieties of Millepora, not arranged in cyclosystems. It may, however, be distinguished at once by the presence of a long, brush-like style in each of the gastropores. The ampullae are large, but are usually so deep-seated in the coenenchym that their presence cannot be detected from the surface. It was found off the Rio de la Plata in 600 fathoms of water by the "Challenger."
In Errina the pores are sometimes irregularly scattered, but in E. glabra they are arranged in rows on the sides of the branches, while in E. ramosa the gastropores occur at the angles of the branches only. The dactylopores are situated on nariform projections of the corallum. The ampullae are prominent. There are several gonophores in each ampulla of the male, but only one in each ampulla of the female. This genus is very widely distributed in water from 100 to 500 fathoms in depth.
Phalangopora differs from Errina in the absence of a style in the gastropore; Mauritius.—Pliobothrus has also no style in the gastropore, and is found in 100-600 fathoms of water off the American Atlantic shores.
Distichopora is an important genus, which is found in nearly all the shallow seas of the tropical and semi-tropical parts of the world, and may even flourish in rock pools between tide marks. It is nearly always brightly coloured—purple, violet, pale brown, or rose red. The colony usually forms a small flabellum, with anastomosing branches, and the pores are arranged in three rows, a middle row of gastropores and two lateral rows of dactylopores on the sides of the branches. There is a long style in each gastropore. The ampullae are numerous and prominent, situated on the anterior and posterior faces of the branches. Each ampulla contains a single gonophore in the female colony and two or three gonophores in the male colony.
Spinipora is a rare genus from off the Rio de la Plata in 600 fathoms. The branches are covered with blunt spines. These spines have a short gutter-like groove at the apex, which leads into a dactylopore. The gastropores are provided with a style and are situated between the spines.
Steganopora[321] from the Djilolo Passage, in about 600 fathoms, is very similar to Spinipora as regards external features, but differs from it in the absence of styles in the gastropores, and in the wide communications between the gastropores and dactylopores.
Stylaster is the largest and most widely distributed genus of the family, and exhibits a considerable range of structure in the many species it contains. It is found in all the warmer seas of the world, living between tide marks at a few fathoms, and extending to depths of 600 fathoms. Many specimens, but especially those from very shallow water, are of a beautiful rose {287}or pink colour. The corallum is arborescent and usually flabelliform. The pores are distributed in regular cyclosystems, sometimes on one face of the corallum only, sometimes on the sides of the branches, and sometimes evenly distributed. There are styles in both gastropores and dactylopores.
Allopora is difficult to separate from Stylaster, but the species are usually more robust in habit, and the ampullae are not so prominent as they are on the more delicate branches of Stylaster. It occurs at depths of 100 fathoms in the Norwegian fjords. A very large red species (A. nobilis) occurs in False Bay, Cape of Good Hope, in 30 fathoms of water. In this locality the coral occurs in great submarine beds or forests, and the trawl that is passed over them is torn to pieces by the hard, thick branches, some of which are an inch or more in diameter.
Astylus is a genus found in the southern Philippine sea in 500 fathoms of water. It is distinguished from Stylaster by the absence of a style in the gastropore.
Cryptohelia is an interesting genus found both in the Atlantic and Pacific Oceans at depths of from 270 to about 600 fathoms. The cyclosystems are covered by a projecting lid or operculum (Fig. 136, l 1, l 2). There are no styles in either the gastropores or the dactylopores. The ampullae are prominent, and are sometimes situated in the lids. There are several gonophores in each ampulla of the female colony, and a great many in the ampulla of the male colony.
HYDROZOA (CONTINUED): TRACHOMEDUSAE—NARCOMEDUSAE—SIPHONOPHORA
Order VII. Trachomedusae.
The orders Trachomedusae and Narcomedusae are probably closely related to one another and to some of the families of Medusae at present included in the order Calyptoblastea, and it seems probable that when the life-histories of a few more genera are made known the three orders will be united into one. Very little is known of the hydrosome stage of the Trachomedusae, but Brooks[322] has shown that in Liriope, and Murbach[323] that in Gonionema, the fertilised ovum gives rise to a Hydra-like form, and in the latter this exhibits a process of reproduction by gemmation before it gives rise to Medusae. Any general statement, therefore, to the effect that the development of the Trachomedusae is direct would be incorrect. The fact that the hydrosomes already known are epizoic or free-swimming does not afford a character of importance for distinction from the Leptomedusae, for it is quite possible that in this order of Medusae the hydrosomes of many genera may be similar in form and habits to those of Liriope and Gonionema.
The free border of the umbrella of the Trachomedusae is entire; that is to say, it is not lobed or fringed as it is in the Narcomedusae. The sense-organs are statocysts, each consisting of a vesicle formed by a more or less complete fold of the surrounding wall of the margin of the umbrella, containing a reduced clapper-like tentacle loaded at its extremity with a statolith.
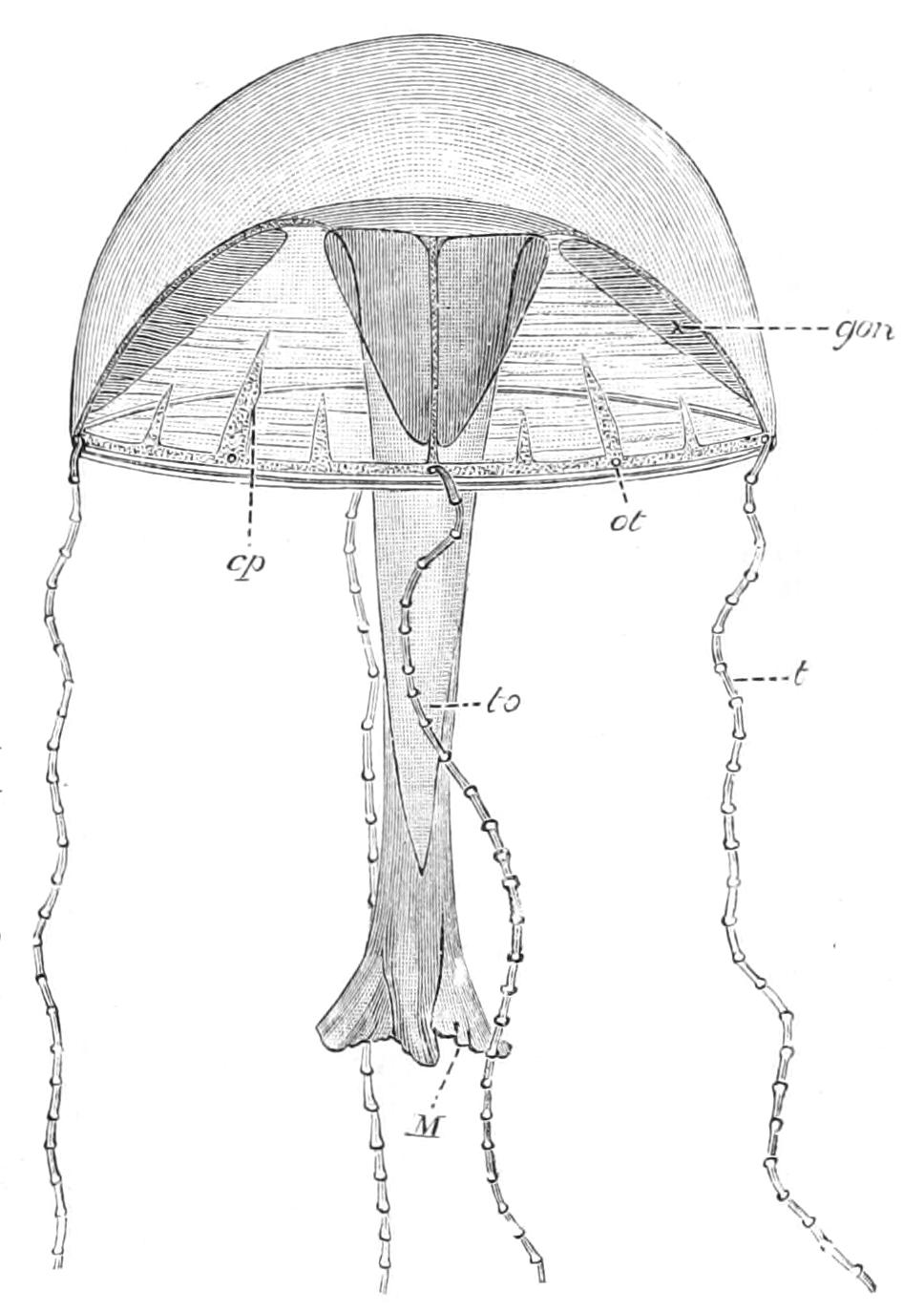
Fig. 137.—Liriope rosacea, one of the Geryoniidae, from the west side of North and Central America. Size, 15-20 mm. Colour, rose. cp, Centripetal canal; gon, gonad; M, mouth at the end of a long manubrium; ot, statocyst; t, tentacle; to, tongue. (After Maas.)
This statocyst is innervated by the outer nerve ring. There appears to be a very marked difference between these marginal sense-organs in some of the best-known examples of Trachomedusae and the corresponding organs of the Leptomedusae. The absence of a stalk supporting the statolith and the innervation of the otocyst by the inner instead of by the outer nerve ring in the Leptomedusae form characters that may be of supplementary value, but cannot be regarded as absolutely distinguishing the two orders. The statorhab of the Trachomedusae is probably the more primitive of the two types, and represents a marginal tentacle of the umbrella reduced in size, loaded with a statolith and enclosed by the mesogloea. Intermediate stages between this type and an ordinary tentacle have already been discovered and described. In the type that is usually found in the Leptomedusae the modified tentacle is still further reduced, and all that can be recognised of it is the statolith attached to the wall of the statocyst, but intermediate stages between the two types are seen in the family Olindiidae, in which the stalk supporting the statolith passes gradually into the tissue surrounding the statolith on the one hand and the vesicle wall on the other. The radial canals are four or eight in number or more numerous. They communicate at the margin of the umbrella with a ring canal from which a number of short blind tubes run in the umbrella-wall towards the centre of the Medusa (Fig. 137, cp). These "centripetal canals" are subject to {290}considerable variation, but are useful characters in distinguishing the Trachomedusae from the Leptomedusae. The tentacles are situated on the margin of the umbrella, and are four or eight in number or, in some cases, more numerous. The gonads are situated as in Leptomedusae on the sub-umbrella aspect of the radial canals.
In Gonionema murbachii the fertilised eggs give rise to a free-swimming ciliated larva of an oval shape with one pole longer and narrower than the other. The mouth appears subsequently at the narrower pole. The larva settles down upon the broader pole, the mouth appears at the free extremity, and in a few days two, and later two more, tentacles are formed (Fig. 138).
At this stage the larva may be said to be Hydra-like in character, and as shown in Fig. 138 it feeds and lives an independent existence. From its body-wall buds arise which separate from the parent and give rise to similar Hydra-like individuals. An asexual generation thus gives rise to new individuals by gemmation as in the hydrosome of the Calyptoblastea. The origin of the Medusae from this Hydra-like stage has not been satisfactorily determined, but it seems probable that by a process of metamorphosis the hydriform persons are directly changed into the Medusae.[324]
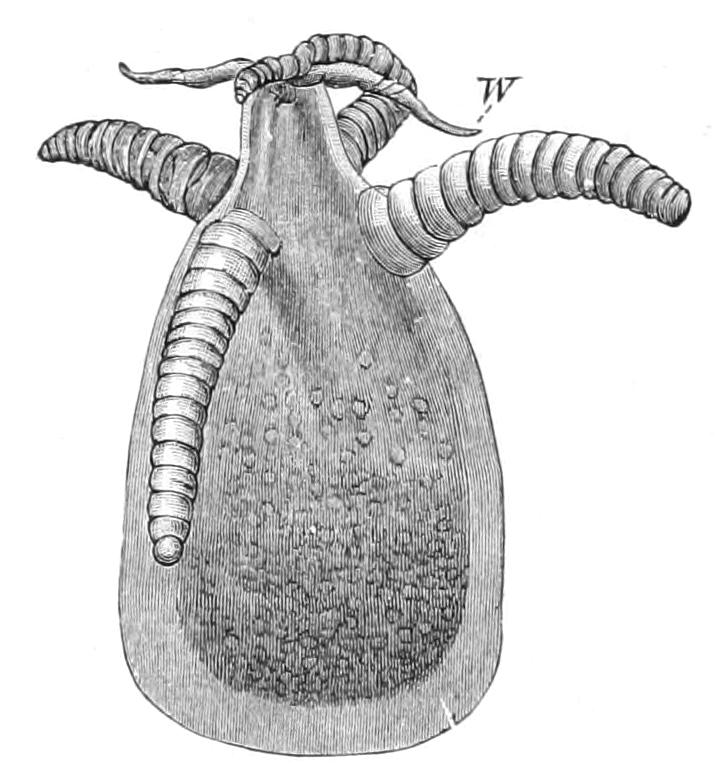
Fig. 138.—Hydra-like stage in the development of Gonionema murbachii. One of the tentacles is carrying a worm (W) to the mouth. The tentacles are shown very much contracted, but they are capable of extending to a length of 2 mm. Height of zooid about 1 mm. (After Perkins.)
In the development of Liriope the free-swimming larva develops into a hydriform person with four tentacles and an enormously elongated hypostome or manubrium; and, according to Brooks, it undergoes a metamorphosis which directly converts it into a Medusa.
There can be very little doubt that in a large number of Trachomedusae the development is direct, the fertilised ovum giving rise to a medusome without the intervention of a hydrosome stage. In some cases, however (Geryonia, etc.), the tentacles {291}appear in development before there is any trace of a sub-umbrella cavity, and this has been interpreted to be a transitory but definite Hydroid stage. It may be supposed that the elimination of the hydrosome stage in these Coelenterates may be associated with their adaptation to a life in the ocean far from the coast.
During the growth of the Medusa from the younger to the adult stages several changes probably occur of a not unimportant character, and it may prove that several genera now placed in the same or even different families are stages in the development, of the same species. In the development of Liriantha appendiculata,[325] for example, four interradial tentacles appear in the first stage which disappear and are replaced by four radial tentacles in the second stage.
As with many other groups of free-swimming marine animals the Trachomedusae have a very wide geographical distribution, and some genera may prove to be almost cosmopolitan, but the majority of the species appear to be characteristic of the warmer regions of the high seas. Sometimes they are found at the surface, but more usually they swim at a depth of a few fathoms to a hundred or more from the surface. The Pectyllidae appear to be confined to the bottom of the sea at great depths.
The principal families of the Trachomedusae are:—
Fam. Olindiidae.—This family appears to be structurally and in development most closely related to the Leptomedusae, and is indeed regarded by Goto[326] as closely related to the Eucopidae in that order. They have two sets of tentacles, velar and exumbrellar; the statocysts are numerous, two on each side of the exumbrellar tentacles. Radial canals four or six. Manubrium well developed and quadrate, with distinct lips. There is an adhesive disc on each exumbrellar tentacle.
Genera: Olindias, Olindioides, Gonionema (Fig. 139), and Halicalyx.
As in other families of Medusae the distribution of the genera is very wide. Olindias mülleri occurs in the Mediterranean, Olindioides formosa off the coast of Japan, Gonionema murbachii is found in abundance in the eel pond at Wood's Holl, United States of America, and Halicalyx off Florida.
Two genera may be referred to in this place, although their {292}systematic position in relation to each other and to other Medusae has not been satisfactorily determined.
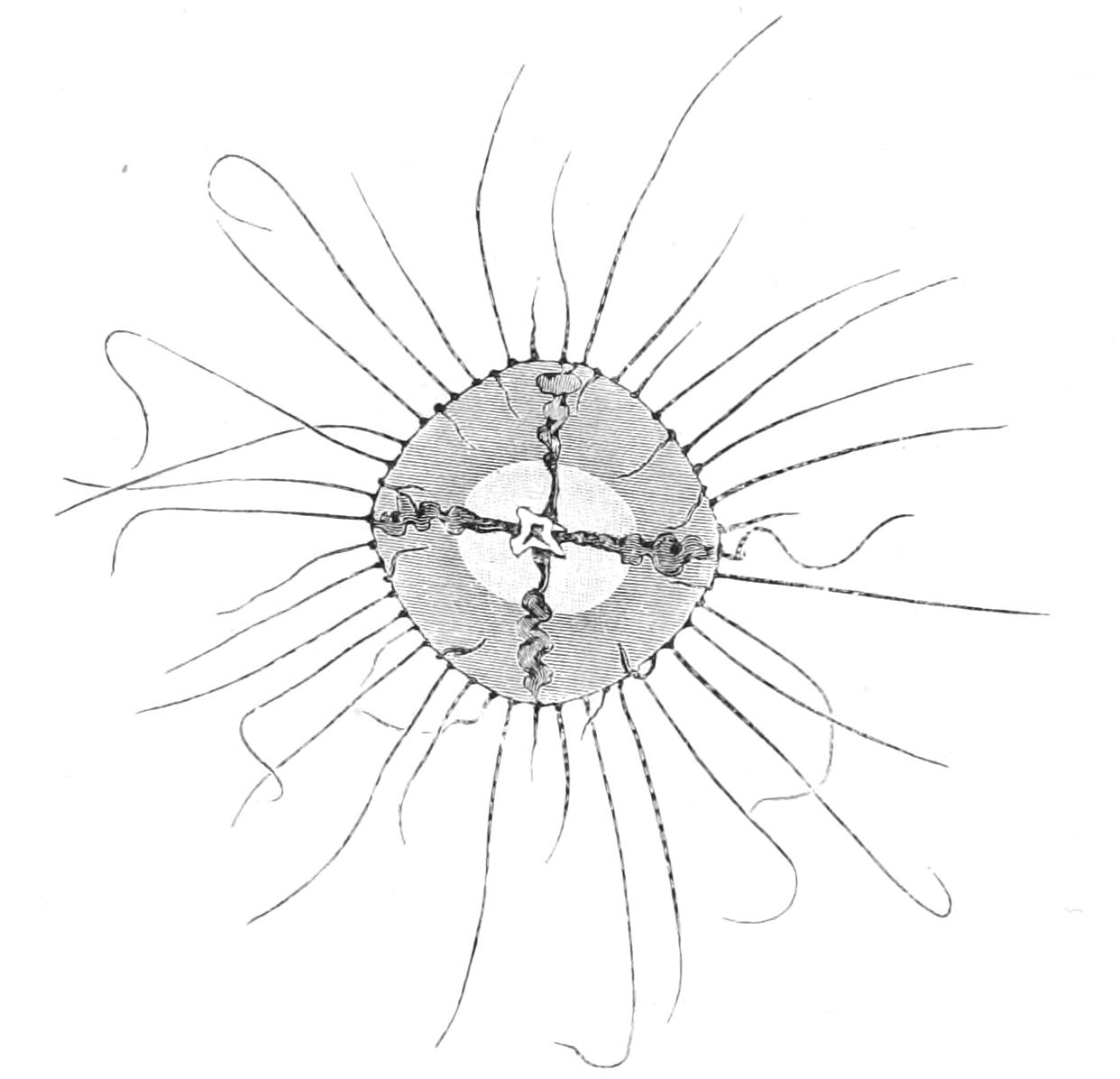
Fig. 139.—Gonionema murbachii. Adult Medusa, shown inverted, and clinging to the bottom. Nat. size. (After Perkins.)
Limnocodium sowerbyi is a small Medusa that was first discovered in the Victoria regia tanks in the Botanic Gardens, Regent's Park, London, in the year 1880. It has lately made its appearance in the Victoria regia tank in the Parc de la Bête d'Or at Lyons.[327] As it was, at the time of its discovery, the only fresh-water jelly-fish known, it excited considerable interest, and this interest was not diminished when the peculiarities of its structure were described by Lankester and others. It has a rather flattened umbrella, with entire margin and numerous marginal tentacles, the manubrium is long, quadrate, and has four distinct lips. There are four radial canals, and the male gonads (all the specimens discovered were of the male sex) are sac-like bodies on the sub-umbrellar aspect of the middle points of the four radial canals. In these characters the genus shows general affinities with the Olindiidae. The difficult question of the origin of the statoliths from the primary germ layers of the embryo and some other points in the minute anatomy of the Medusa have {293}suggested the view that Limnocodium is not properly placed in any of the other orders. Goto,[328] however, in a recent paper, confirms the view of the affinities of Limnocodium with the Olindiidae.
The life-history of Limnocodium is not known, but a curious Hydroid form attached to Pontederia roots was found in the same tank as the Medusae, and this in all probability represents the hydrosome stage of its development. The Medusae are formed apparently by a process of transverse fission of the Hydroid stock[329] similar in some respects to that observed in the production of certain Acraspedote Medusae. This is quite unlike the asexual mode of formation of Medusae in any other Craspedote form. The structure of this hydrosome is, moreover, very different to that of any other Hydroid, and consequently the relations of the genus with the Trachomedusae cannot be regarded as very close.
Limnocodium has only been found in the somewhat artificial conditions of the tanks in botanical gardens, and its native locality is not known, but its association with the Victoria regia water-lily seems to indicate that its home is in tropical South America.
Limnocnida tanganyicae is another remarkable fresh-water Medusa, about seven-eights of an inch in diameter, found in the lakes Tanganyika and Victoria Nyanza of Central Africa.[330] It differs from Limnocodium in having a short collar-like manubrium with a large round mouth two-thirds the diameter of the umbrella, and in several other not unimportant particulars. It produces in May and June a large number of Medusa-buds by gemmation on the manubrium, and in August and September the sexual organs are formed in the same situation.
The fixed hydrosome stage, if such a stage occurs in the life-history, has not been discovered; but Mr. Moore[331] believes that {294}the development is direct from ciliated planulae to the Medusae. The occurrence of Limnocnida in Lake Tanganyika is supposed by the same authority to afford a strong support to the view that this lake represents the remnants of a sea which in Jurassic times spread over part of the African continent. This theory has, however, been adversely criticised from several sides.[332]
The character of the manubrium and the position of the sexual cells suggest that Limnocnida has affinities with the Narcomedusae or Anthomedusae, but the marginal sense-organs and the number and position of the tentacles, showing considerable similarity with those of Limnocodium, justify the more convenient plan of placing the two genera in the same family.
Fam. Petasidae.—The genus Petasus is a small Medusa with four radial canals, four gonads, four tentacles, and four free marginal statorhabs. A few other genera associated with Petasus show simple characters as regards the canals and the marginal organs, but as very little is known of any of the genera the family may be regarded as provisional only. Petasus is found in the Mediterranean and off the Canaries.
Fam. Trachynemidae.—In this family there are eight radial canals, and the statorhabs are sunk into a marginal vesicle. Trachynema, characterised by its very long manubrium, is a not uncommon Medusa of the Mediterranean and the eastern Atlantic Ocean. Many of the species are small, but T. funerarium has sometimes a disc two inches in diameter. Homoconema and Pentachogon have numerous very short tentacles.
Fam. Pectyllidae.—This family contains a few deep-sea species with characters similar to those of the preceding family, but the tentacles are provided with terminal suckers. Pectyllis is found in the Atlantic Ocean at depths of over 1000 fathoms.
Fam. Aglauridae.—The radial canals are eight in number and the statorhabs are usually free. In the manubrium there is a rod-like projection of the mesogloea from the aboral wall of the gastric cavity, covered by a thin epithelium of endoderm, which occupies a considerable portion of the lumen of the manubrium. This organ may be called the tongue. Aglaura has an octagonal umbrella, and a manubrium which does not project beyond the velum. It occurs in the Atlantic Ocean and Mediterranean Sea.
Fam. Geryoniidae.—In this family there are four or six radial canals, the statorhabs are sunk in the mesogloea, and a tongue is present in the manubrium. Liriope (Fig. 137) is sometimes as much as three inches in diameter. It has a very long manubrium, and the tongue sometimes projects beyond the mouth. There are four very long radial tentacles. It is found in the Atlantic Ocean, the Mediterranean Sea, and the Pacific and Indian Oceans. Geryonia has a wider geographical distribution than Liriope, and is sometimes four inches in diameter. It differs from Liriope in having six, or a multiple of six, radial canals. Carmarina of the Mediterranean and other seas becomes larger even than Geryonia, from which it differs in the arrangement of the centripetal canals.
Liriantha appendiculata sometimes occurs on the south coast of England during September, October, or at other times.
Order VIII. Narcomedusae.
The Narcomedusae differ from the Trachomedusae in having the margin of the umbrella divided into a number of lobes, and in bearing the gonads on the sub-umbrellar wall of the gastral cavity instead of upon the radial canals. The tentacles are situated at some little distance from the margin of the umbrella at points on the aboral surface corresponding with the angles between the umbrella lobes. Between the base of the tentacle and the marginal angle there is a tract of modified epithelium called the "peronium." The manubrium is usually short, and the mouth leads into an expanded gastral chamber which is provided with lobular diverticula reaching as far as the bases of the tentacles. The marginal sense-organs are in the form of unprotected statorhabs. Very little is known concerning the life-history of any of the Narcomedusae. In Cunoctantha octonaria the peculiar ciliated larva with two tentacles and a very long proboscis soon develops two more tentacles and creeps into the bell of the Anthomedusan Turritopsis, where, attached by its tentacles, it lives a parasitic life. Before being converted into a Medusa it gives rise by gemmation to a number of similar individuals, all of which become, in time, Medusae. The parasitic stage is often regarded as the representative of the hydrosome stage reduced and adapted to the oceanic habit of the adult.
In Cunina proboscidea, and in some other species, a very remarkable method of reproduction has been described by Metschnikoff, called by him "sporogony." In these cases young sexual cells (male or female) wander from the gonad of the parent into the mesogloea of the umbrella, where they develop parthenogenetically into ciliated morulae. These escape by the radial canals into the gastric cavity, and there form a stolon from which young Medusae are formed by gemmation. In C. proboscidea these young Medusae are like the genus Solmaris, but in C. rhododactyla they have the form of the parent. In some cases the ciliated larvae leave the parent altogether and become attached to a Geryonia or some other Medusa, where they form the stolon.
This very interesting method of reproduction cannot be regarded as a primitive one, and throws no light on the origin of the order. It might be regarded as a further stage in the degeneration of the hydrosome stage in its adaptation to a parasitic existence.
The Narcomedusae have a wide geographical distribution. Species of Aeginopsis occur in the White Sea and Bering Strait, but the genera are more characteristic of warmer waters. Some species occur in moderately deep water, and Cunarcha was found in 1675 fathoms off the Canaries, but they are more usually found at or near the surface of the sea.
Fam. Cunanthidae.—Narcomedusae with large gastral diverticula corresponding in position with the bases of the tentacles. Cunina and Cunoctantha, occurring in the Mediterranean and in the Atlantic and Pacific Oceans, belong to this family. In Cunina the tentacles may be eight in number, or some multiple of four between eight and twenty-four. In Cunoctantha the number of tentacles appears to be constantly eight.
Fam. Peganthidae.—There appear to be no gastral pouches in this family. The species of Pegantha are found at depths of about 80 fathoms in the Indian and Pacific Oceans.
Fam. Aeginidae.—The large gastral pouches of this family alternate with the bases of the tentacles. Aegina occurs in the Atlantic and Pacific Oceans. Aeginopsis.
Fam. Solmaridae.—In this family the gastral pouches are variable, sometimes corresponding with, sometimes alternating with, the bases of the tentacles. The circular canal is represented {297}in some genera by solid cords of endoderm. Solmaris sometimes appears in the English Channel, but it is probably a wanderer from the warmer regions of the Atlantic Ocean. It is found in abundance during November on the west coast of Ireland.
Order IX. Siphonophora.
In this order the naturalist finds collected together a number of very beautiful, delicate transparent organisms to which the general term "jelly-fish" may be applied, although their organisation is far more complicated and difficult to describe than that of any of the Medusae. In several of the Hydrozoa the phenomenon of dimorphism has already been noticed. In these cases one set of individuals in a colony performs functions of stinging and catching food and another the functions of devouring and digesting it. In many of the Siphonophora there appears to be a colony of individuals in which the division of labour is carried to a much further extent than it is in the dimorphic Hydrozoa referred to above. Not only are there specialised gastrozooids and dactylozooids, but also gonozooids, zooids for propelling the colony through the water ("nectocalyces"), protective zooids ("hydrophyllia"), and in some cases a specialised zooid for hydrostatic functions; the whole forming a swimming or floating polymorphic colony. But this conception of the construction of the Siphonophora is not the only one that has met with support. By some zoologists the Siphonophoran body is regarded not as a colony of individuals, but as a single individual in which the various organs have become multiplied and dislocated.
The multiplication or repetition of organs that are usually single in each individual is not unknown in other Hydrozoa. In the Medusa of the Gymnoblast Syncoryne, usually known as Sarsia, for example, there is sometimes a remarkable proliferation of the manubrium, and specimens have been found with three or four long manubria attached by a tubular stalk to the centre of the umbrella. Moreover, this complex of manubria may become detached from the umbrella and live for a considerable time an independent existence.[333]
If we regard the manubrium of a Medusa as an organ of the {298}animal's body, it might be thought obvious that the phenomenon observed in the Medusae of Syncoryne is a case of a simple repetition of the parts of an individual; but the power that the group of manubria possesses of leading an independent existence renders its interpretation as a group of organs a matter of some inconvenience. If we can conceive the idea that an organ may become detached and lead an independent existence, there is no reason why we should not regard the Medusa itself of Syncoryne as an organ, and we should be driven to the paradoxical conclusion that, as regards several genera and families of Hydrozoa, we know nothing at present of the individuals, but only of their free-swimming organs, and that in others the individual has degenerated, although one of its organs remains.
There is, however, no convincing argument to support either the conception that the Siphonophoran body is a colony of individuals, or that it is an individual with disjointed organs. These two conceptions are sometimes called the "Poly-person" and "Poly-organ" theories respectively. The difficulty is caused by the impossibility of giving any satisfactory definition in the case of the Hydrozoa of the biological terms "organ" and "individual." In the higher animals, where the correlation of parts is far more complex and essential than it is in Coelenterata, a defined limit to the scope of these terms can be laid down, but in the lower animals the conception of what is termed an organ merges into that which is called an individual, and no definite boundary line between the two exists in Nature. The difficulty is therefore a permanent one, and, in using the expression "colony" for the Siphonophoran body, it must be understood that it is used for convenience' sake rather than because it represents the only correct conception of the organisation of these remarkable Coelenterates.
Regarding the Siphonophora as polymorphic colonies, then, the following forms of zooids may be found.
Nectocalyces.—The nectocalyces are in the form of the umbrella of a medusa attached to the stolon of the colony by the aboral pole. They are provided with a velum and, usually, four radial canals and a circular canal. There is no manubrium, and the marginal tentacles and sense-organs are rudimentary or absent. There may be one or more nectocalyces in each colony, {299}and their function is, by rhythmic contractions, to propel the colony through the water (Fig. 142, N).
Gastrozooids.—These are tubular or saccular zooids provided with a mouth and attached by their aboral extremity to the stolon (Fig. 142, G). In some cases the aboral region of the zooid is differentiated as a stomach. It is dilated and bears the digestive cells, the oral extremity or hypostome being narrower and more transparent. In some cases the mouth is a simple round aperture at the extremity of the hypostome, but in others it is dilated to form a trumpet-like lip.
Dactylozooids.—In Velella and Porpita the dactylozooids are similar in general characters to the tentacles of many Medusae. They are arranged as a frill round the margin of the colony, and each consists of a simple tube of ectoderm and endoderm terminating in a knobbed extremity richly provided with nematocysts.
In many other Siphonophora, however, the dactylozooids are very long and elaborate filaments, which extend for a great distance from the colony into the sea. They reach their most elaborate condition in the Calycophorae.
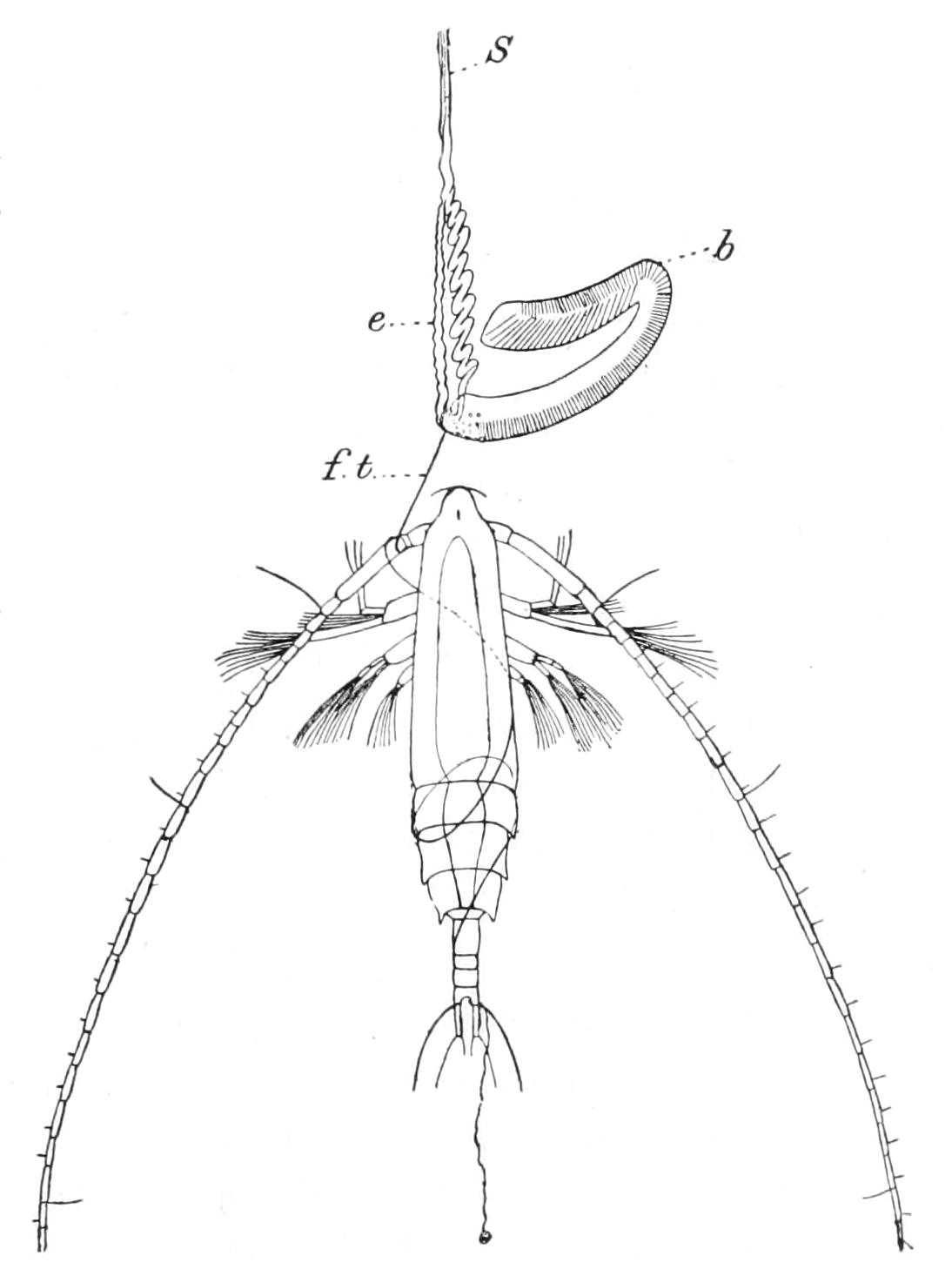
Fig. 141.—A small Crustacean (Rhinocalanus) caught by a terminal filament (f.t) of a battery of Stephanophyes. b, The proximal end of the battery with the most powerful nematocysts; e, elastic band; S, stalk supporting the battery on the dactylozooid. (After Chun.)
The dactylozooid in these forms has a hollow axis, and the lumen is continuous with the cavity of the neighbouring gastrozooid. Arranged at regular intervals on the axis is a series of tentacles ("tentilla"), and each of these supports {300}a kidney-shaped swelling, the "cnidosac," or battery, which is sometimes protected by a hood. Each battery contains an enormous number of nematocysts. In Stephanophyes, for example, there are about 1700 nematocysts of four different kinds in each battery. At the extremity of the battery there is a delicate terminal filament. The action of the battery in Stephanophyes is, according to Chun,[334] a very complicated one. The terminal filament lassos the prey and discharges its somewhat feeble nematocysts at it (Fig. 141). If this kills it, the dactylozooid contracts and passes the prey to a gastrozooid. If the animal continues its struggles, it is drawn up to the distal end of the battery and receives the discharge of a large number of nematocysts; and if this also fails to put an end to its life, a membrane covering the largest and most powerful nematocysts at the proximal end of the whole battery is ruptured, and a final broadside of stinging threads is shot at it.
The larger nematocysts of these batteries in the Siphonophora are among the largest found in Coelenterata, being from 0.5 to 0.1 mm. in length, and they are frequently capable of inflicting painful stings on the human skin. The species of Physalia, commonly called "Portuguese Men-of-War," have perhaps the worst reputation in this respect, the pain being not only intense but lasting a long time.
Hydrophyllia.—In many Siphonophora a number of short, mouthless, non-sexual zooids occur, which appear to have no other function than that of shielding or protecting other and more vital parts of the colony. They consist of an axis of firm mesogloea, covered by a layer of flattened ectoderm, and they may be finger-shaped or triangular in form. In Agalma and Praya an endoderm canal perforates the mesogloea and terminates in a little mouth at the free extremity. In Athoria and Rhodophysa the hydrophyllium terminates in a little nectocalyx.
Pneumatophore.—In all the Siphonophora, with the exception of the Calycophorae, there is found on one side or at one extremity of the colony a vesicle or bladder containing a gas,[335] which serves as a float to support the colony in the water. {301}This bladder or pneumatophore is probably in all cases a much modified nectocalyx. It shows great variations in size and structure in the group. It is sometimes relatively very large, as in Physalia and Velella, sometimes very small, as in Physophora. It is provided with an apical pore in some genera (Rhizophysa), or a basal pore in others (Auronectidae), but it is generally closed. In the many chambered pneumatophore of the Chondrophoridae there are several pores.
In many forms two distinct parts of the pneumatophore can be recognised—a distal region lined by chitin,[336] probably representing the sub-umbrellar cavity of the nectocalyx, and a small funnel-shaped region lined by an epithelium, the homology of which is a matter of dispute. It is believed that the gas is secreted by this epithelium. In the Auronectidae the region with secretory epithelium is relatively large and of a more complicated histological character. It is remarkable also that in this family the pore communicates, not with the chitin-lined region, but directly with the epithelium-lined region.
There is no pneumatophore in the Calycophorae, but in this sub-order a diverticulum of an endoderm canal secretes a globule of oil which may serve the same hydrostatic function.
The stolon is the common stem which supports the different zooids of the colony. In the Calycophorae the stolon is a long, delicate, and extremely contractile thread attached at one end to a nectocalyx, and bearing the zooids in discontinuous groups. These groups of zooids arranged at intervals on the stolon are called the "cormidia." The stolon is a tube with very thick walls. Its lumen is lined by a ciliated endoderm with circular muscular processes, and the surface is covered with an ectoderm, also provided with circular muscular processes. Between these two layers there is a relatively thick mesogloea showing on the outer side deep and compound folds and grooves supporting an elaborate system of longitudinal muscular fibres. In many Physonectidae the stolon is long and filamentous, but not so contractile as it is in Calycophorae, but in others it is much reduced in length and relatively stouter. The reduction {302}in length of the stolon is accompanied by a complication of structure, the simple tubular condition being replaced by a spongy complex of tubes covered by a common sheath of ectoderm. In the Auronectidae the stolon is represented by a conical or hemispherical spongy mass bearing the zooids, and in the Rhizophysaliidae and Chondrophoridae it becomes a disc or ribbon-shaped pad spreading over the under side of the pneumatophore.
Gonozooids.—The gonozooids are simple tubular processes attached to the stolon which bear the Medusae or the degenerate medusiform gonophores. In the Chondrophoridae the gonozooids possess a mouth, but in most Siphonophora they have neither mouth nor tentacles. In some cases, such as Anthophysa, the colonies are bisexual—the male and female gonophores being borne by separate gonozooids—but in others (e.g. Physalia) the colonies appear to be unisexual.
As a general rule the gonophores of Siphonophora do not escape from the parent colony as free-swimming Medusae, but an exception occurs in Velella, which produces a number of small free-swimming Medusae formerly described by Gegenbaur under the generic name Chrysomitra. This Medusa has a velum, a single tentacle, eight to sixteen radial canals, and it bears the gonads on the short manubrium. The Medusa of Velella has, in fact, the essential characters of the Anthomedusae.
Our knowledge of the life-history of the Siphonophora is very incomplete, but there are indications, from scattered observations, that in some genera, at least, it may be very complicated.
The fertilised ovum of Velella gives rise to a planula which sinks to the bottom of the sea, and changes into a remarkable larva known as the Conaria larva. This larva was discovered by Woltereck[337] at depths of 600-1000 metres in great numbers. It is very delicate and transparent, but the endoderm is red (the colour so characteristic of animals inhabiting deep water), and it may be regarded as essentially a deep-sea larva. The larva rises to the surface and changes into the form known as the Ratarula larva, which has a simple one-chambered pneumatophore containing a gas, and a rudiment of the sail. In contrast to the Conaria, the Ratarula is blue in colour. With the development of the zooids on the under side of this {303}larva (i.e. the side opposite to the pneumatophore), a definite octoradial symmetry is shown, there being for some time eight dactylozooids and eight definite folds in the wall of the pneumatophore. This octoradial symmetry, however, is soon lost as the number of folds in the pneumatophore and the number of tentacles increase.
It is probable that in the Siphonophora, as in many other Coelenterata, the production of sexual cells by an individual is no sign that its life-history is completed. There may possibly be two or more phases of life in which sexual maturity is reached.
An example of a complicated life-history is found in the Calycophoran species Muggiaea kochii. The embryo gives rise to a form with a single nectocalyx which is like a Monophyes, and this by the budding of a second nectocalyx produces a form that has a remarkable resemblance to a Diphyes, but the primary nectocalyx degenerates and is cast off, while the secondary one assumes the characters of the single Muggiaea nectocalyx. The stolon of the Muggiaea produces a series of cormidia, and as the sexual cells of the cormidia develop, a special nectocalyx is formed at the base of each one of them, and the group of zooids is detached as an independent colony, formerly known as Eudoxia eschscholtzii. In a similar manner the cormidia of Doramasia picta give rise to the sexual free-swimming monogastric forms, known by the name Ersaea picta (Fig. 142). In these cases it seems possible that the production of ripe sexual cells is confined to the Eudoxia and Ersaea stages respectively, but it is probable that in other species the cormidia do not break off from the stolon, or may escape only from the older colonies.

Fig. 142.—Free-swimming Ersaea group of Doramasia picta. B, B, batteries of nematocysts borne by the tentilla; D, dactylozooid; G, gastrozooid; H, hydrophyllium; N, nectocalyx; O, oleocyst; f.t, terminal filament of a battery; t, t, tentilla. The gonozooid is hidden by the gastrozooid. × 10. (After Chun.)
The Siphonophora are essentially free-swimming pelagic {304}organisms. Some of them (Auronectidae) appear to have become adapted to a deep-sea habit, others are usually found in intermediate waters, but the majority occur with the pelagic plankton at or very near the surface of the open sea. Although the order may be said to be cosmopolitan in its distribution, the Siphonophora are only found in great numbers and variety in the sub-tropical and tropical zones. In the temperate and arctic zones they are relatively rare, but Galeolaria biloba and Physophora borealis appear to be true northern forms. The only British species are Muggiaea atlantica and Cupulita sarsii. Velella spirans occasionally drifts from the Atlantic on to our western shores, and sometimes great numbers of the pneumatophores of this species may be found cast up on the beach. Diphyes sp., Physalia sp., and Physophora borealis are also occasionally brought to the British shores by the Gulf Stream.
The Calycophorae are usually perfectly colourless and transparent, with the exception of the oil-globule in the oleocyst, which is yellow or orange in colour. Many of the other Siphonophora, however, are of a transparent, deep indigo blue colour, similar to that of many other components of the plankton.
Most of the Siphonophora, although, strictly speaking, surface animals, are habitually submerged; the large pneumatophores of Velella and Physalia, however, project above the surface, and these animals are therefore frequently drifted by the prevailing wind into large shoals, or blown ashore. At Mentone, on the Mediterranean, Velella is sometimes drifted into the harbour in countless numbers. Agassiz mentions the lines of deep blue Velellas drifted ashore on the coast of Florida; and a small species of blue Physalia may often be seen in long lines on the shore of some of the islands of the Malay Archipelago.
The food of most of the Siphonophora consists of small Crustacea and other minute organisms, but some of the larger forms are capable of catching and devouring fish. It is stated by Bigelow[338] that a big Physalia will capture and devour a full-grown Mackerel. The manner in which it feeds is described as follows:—"It floats on the sea, quietly waiting for some heedless individual to bump its head against one of the tentacles. The fish, on striking, is stung by the nettle-cells, and fastened probably by them to the tentacle. Trying to run away the fish pulls on the {305}tentacle. The tension on its peduncle thus produced acts as a stimulus on apparently some centre there which causes it to contract. The fish in this way is drawn up so that it touches the sticky mouths of the squirming siphons [i.e. gastrozooids]. As soon as the mouths, covered as they are with a gluey substance and provided with nettle-cells, touch the fish they stick fast, a few at first, and gradually more. The mouths open, and their lips are spread out over the fish until they touch, so that by the time he is dead the fish is enclosed in a tight bag composed of the lips of a dozen or more siphon mouths. Here the fish is digested. As it begins to disintegrate partially digested fragments are taken into the stomachs of the attached siphons (gastrozooids). When they have become gorged they detach themselves from the remains of the fish, the process of digestion is completed in the stomachs, and the nutrient fluid is distributed...."
In consequence of the very unsatisfactory state of our knowledge of the life-history of the Siphonophora the classification of the order is a matter of unusual difficulty.
Sub-Order I. Calycophorae.
The character which distinguishes this sub-order is the absence of a pneumatophore.
The colony usually consists of a long, slender, contractile stolon, provided at one end with one, two, or several nectocalyces. Upon the stolon are arranged several groups ("cormidia") of polymorphic zooids.
The nectocalyces have a well-developed velum, four radial canals, and a muscular umbrella-wall. A special peculiarity of the nectocalyx of this sub-order is a diverticulum (oleocyst) from one of the radial canals, containing a coloured globule of oil. The function of this oil-globule is probably similar to that of the pneumatophore, and assists the muscular efforts of the nectocalyces in keeping the colony afloat. One of the nectocalyces of each colony exhibits on one side a deep ectodermic fold, which is frequently converted into a pit. At the bottom of this pit is attached the end of the stolon, the whole of which with its numerous cormidia can be withdrawn into the shelter of the pit when danger threatens. The cormidia consist of at least four {306}kinds of zooids: a gastrozooid with a trumpet-shaped mouth armed with nematocysts, a long dactylozooid provided with a series of tentilla, and a rudimentary gonozooid bearing numbers of male or female medusiform gonophores. These three kinds of zooids are partially covered and protected by a bent shield-shaped phyllozooid or hydrophyllium.
Each of the cormidia is unisexual, but the colony as a whole is usually hermaphrodite, the male and female cormidia regularly alternating, or the male cormidia being arranged on the nectocalycine half and the female cormidia on the opposite half of the stolon.
The families of the Calycophorae are:—
Fam. 1. Monophyidae.—In this family there is a single conical or mitre-shaped nectocalyx. The cormidia become detached as free-swimming Eudoxia or Ersaea forms.
Sub-Fam. 1. Sphaeronectinae.—The primary nectocalyx persists throughout life—Monophyes and Sphaeronectes.
Sub-Fam. 2. Cymbonectinae.—The primary nectocalyx is thrown off, and is replaced by a secondary and permanent nectocalyx—Cymbonectes, Muggiaea, and Doramasia.
Fam. 2. Diphyidae.—The primary mitre-shaped nectocalyx is thrown off and replaced by two secondary rounded, prismatic, or pyramidal, heteromorphic nectocalyces.
This family contains several sub-families, which are arranged in two groups: the Diphyidae Oppositae, in which the two secondary bells are opposite one another, and do not exhibit pronounced ridges; and the Diphyidae Superpositae, in which one of the two secondary nectocalyces is situated in front of the other, and each nectocalyx is provided externally with very definite and often wing-like ridges. In all the Diphyidae Oppositae the cormidia remain attached, whereas in most of the Diphyidae Superpositae they become free-swimming, as in the Monophyidae.
The sub-families of the Diphyidae Oppositae are:—
Sub-Fam. 1. Amphicaryoninae.—One of the two secondary nectocalyces becomes flattened above to form a shield, and at the same time its sub-umbrellar cavity is atrophied, and its radial canals reduced. Mitrophyes, Atlantic Ocean.
Sub-Fam. 2. Prayinae.—The colony exhibits a pair of large, obtuse nectocalyces, with a relatively small sub-umbrellar cavity. Praya, Mediterranean and Atlantic.
Sub-Fam. 3. Desmophyinae.—The colony bears a large number of reserve or tertiary nectocalyces arranged in two rows. Desmophyes, Indian Ocean.
Sub-Fam. 4. Stephanophyinae.—There are four nectocalyces arranged in a horizontal plane. Each one of the cormidia bears a nectocalyx, which is periodically replaced. This sub-family is constituted for Stephanophyes superba from the Canary Islands. It attains a length of 25 cm., and is probably the largest and most beautiful of all the Calycophoridae.[339]
The group Diphyidae Superpositae contains the following:—
Sub-Fam. 1. Galeolarinae.—Galeolaria.
Sub-Fam. 2. Diphyopsinae.—Diphyes.
Sub-Fam. 3. Abylinae.—Abyla.
These sub-families differ from one another in the character and shape of the nectocalyces and in other characters. They have a world-wide distribution, Diphyes and Galeolaria extending north into the Arctic Seas. Diphyes is British.
Fam. 3. Polyphyidae.—The nectocalyces are numerous, and superposed in two rows. The cormidia remain attached.
The family contains the genera Polyphyes and Hippopodius, both probably cosmopolitan in warm waters.
Sub-Order II. Physophorae.
In this sub-order the primary nectocalyx gives rise to a definite pneumatophore. There are four families.
Fam. 1. Physonectidae.—In this, the largest family of the sub-order, there is a monothalamic pneumatophore supporting a stolon, which in some forms is of great length, but in others is reduced to a stump or pad, on which there are usually found several nectocalyces, hydrophyllia, gastrozooids, gonozooids, and tentilla.
The principal sub-families are:—
Agalminae.—With a long stolon, bearing at the upper end (i.e. the end next to the pneumatophore) two rows of nectocalyces. The other zooids are arranged in cormidia on the stolon, each covered by a hydrophyllium. Dactylozooids with tentilla. Agalma and Cupulita, Mediterranean Sea.
Apoleminae.—Similar to the above, but without tentilla. {308}Apolemia—this genus attains a length of two or three metres. Mediterranean Sea. Dicymba, Indian Ocean.
Physophorinae.—The pneumatophore larger in proportion than it is in the preceding families. The stolon is short, and bears rows of nectocalyces at the upper end. The gastrozooids, dactylozooids, and gonozooids are arranged in verticils on the lower expanded part of the stolon. Hydrophyllia absent. Physophora, cosmopolitan in the areas of warm sea water.
Fam. 2. Auronectidae.—The pneumatophore is large. The stolon is reduced to a spongy mass of tissue on the under side of the pneumatophore, and this bears numerous cormidia arranged in a helicoid spiral. Projecting from the base of the pneumatophore there is a peculiar organ called the "aurophore," provided with an apical pore. This organ has been described as a specially modified nectocalyx, but it is probably a specialised development of the epithelium-lined portion of the pneumatophore of other Physophorae. The Auronectidae are found only at considerable depths, 300 to 1400 fathoms, and are probably specially adapted to that habitat. Rhodalia, Stephalia, Atlantic Ocean.
Fam. 3. Rhizophysaliidae.—The pneumatophore is large, or very large, in this family. The zooids are arranged in horizontal rows on the under side of the pneumatophore (Physalia), or in a helicoid spiral on a short stolon (Epibulia). There are no nectocalyces nor hydrophyllia.
The genus Physalia is the notorious "Portuguese Man-of-War." The pneumatophore is a large bladder-like vesicle, sometimes attaining a length of 12 cm. One species described by Haeckel under the generic name Caravella has a pneumatophore 30 cm. and more in length, and dactylozooids attaining a length of 20 metres. It is a curious fact that only the male colonies of Physalia are known, and it is suggested that the female may have quite a different form.[340] Epibulia has a much smaller bladder than Physalia. Both genera have a cosmopolitan distribution at the surface of the warm seas.
Fam. 4. Chondrophoridae.—This family stands quite by itself in the sub-order Physophorae, and is placed in a separate division of the sub-order by Chun, who gives it the name Tracheophysa. The essential distinguishing characters of the family are {309}the large polythalamic pneumatophore and the single large central gastrozooid.
The colony is disc-shaped, and has a superficial resemblance to a Medusa. On the upper side is the flattened pneumatophore, covered by a fold of tissue continuous with that at the edge of the disc. In Velella a vertical triangular sail or crest rises from the upper side, but this is absent in Porpita.
The mouth of the gastrozooid opens into a large digestive cavity, and between this and the under surface of the pneumatophore there is a glandular spongy tissue called the liver. The liver extends over the whole of the under side of the pneumatophore, and sends processes round the edge of the disc into the tissues of its upper surface. Intimately associated with the liver, and penetrating its interstices, is an organ which appears to be entirely composed of nematocysts, derived from the ectoderm, and called the central organ. At the margin of the disc there is a fringe of simple digitiform dactylozooids, and between the dactylozooids and the centrally placed gastrozooid are numerous gonozooids. Each of the gonozooids is provided with a distinct mouth, and bears the gonophores, which escape before the ripening of the gonads as the free-swimming Medusae called Chrysomitra. The pneumatophore consists of a number of annular chambers arranged in a concentric manner round the central original chamber formed from a modified zooid. These annular chambers are in communication with one another, and have each two pores (pneumatopyles) opening above to the exterior. The most remarkable feature, however, of the system is a series of fine branching tubes ("tracheae"), which pass from the annular chambers of the pneumatophore downwards into the hepatic mass and ramify there.
There are two well-known genera: Velella with a sail, and Porpita without a sail. They are both found at the surface of the warmer regions of the great oceans and in the Mediterranean. Velella sometimes drifts on to British coasts from the Atlantic.
The genus Discalia has a much more simple octoradial structure. It was found at depths of 2600 and 2750 fathoms in the Pacific Ocean.
COELENTERATA (CONTINUED): SCYPHOZOA = SCYPHOMEDUSAE
CLASS II. SCYPHOZOA = SCYPHOMEDUSAE
The Scyphozoa are jelly-fishes, usually found floating at or near the surface of the sea. A few forms (Stauromedusae) are attached to rocks and weeds by a stalked prolongation of the aboral region of the umbrella. With this exception, however, they are all, in the adult stage, of the Medusa type of structure, having a bell-shaped or discoid umbrella, from the under surface of which depends a manubrium bearing the mouth or (in Rhizostomata) the numerous mouths.
Although many of the species do not exceed an inch or a few inches in diameter, others attain a very great size, and it is among the Scyphozoa that we find the largest individual zooids of the Coelenterata. Some Discophora have a disc three or four feet in diameter, and one specimen obtained by the Antarctic Expedition of 1898-1900 weighed 90 lbs.[341] The common jelly-fish, Aurelia, of our coasts belongs to a species that appears to be very variable in general characters as well as in size. Specimens obtained by the "Siboga" in the Malay Archipelago ranged from 6 to 64 cm. in diameter. The colour is very variable, shades of green, blue, brown, and purple being conspicuous in many species; but a pale milky-blue tint is perhaps the most prevalent, the tissues being generally less transparent than they are in the Medusae of the Hydrozoa. The colour of the Cubomedusae is usually yellow or brown, but Charybdea xaymacana is colourless and transparent. The deep-sea species, particularly the Periphyllidae, have usually an opaque brown or dark red colour. The surface-swimming {311}forms, such as the common Aurelia, Pelagia, Cyanaea, are usually of a uniform pale milky-blue or green colour. Generally the colour is uniformly distributed, but sometimes the surface of the umbrella is freckled with irregular brown or yellow patches, as in Dactylometra and many others. There is frequently a special colour in the statorhabs which renders them conspicuous in the living jelly-fish, and the lips, or parts of the lips, of the manubrium have usually a different colour or tone to that of the umbrella.
There is no reason to believe that the general colour of any of these jelly-fishes has either a protective or a warning significance. Nearly all the larger species, whether blue, green, or brown in colour, can be easily seen from a considerable distance, and the colours are not sufficiently bright or alarming to support the belief that they can serve the purpose of warning either fish or birds of the presence of a dangerous stinging animal. It is possible, however, that the brighter spots of colour that are often noticed on the tips of the tentacles and on the lips may act as a lure or bait in attracting small fish and Crustacea.
Some of the Scyphozoa are phosphorescent, but it is a singular fact that there are very few recorded observations concerning the phosphorescence or the absence of it in most of the species. The pale blue light of Pelagia noctiluca or P. phosphora can be recognised from the deck of a ship in the open ocean, and they are often the most brilliant and conspicuous of the phosphorescent organisms.
The food of the Scyphozoa varies a good deal. Charybdea and Periphylla, and probably many others with large mouths, will capture and ingest relatively large fish and Crustacea; but Chrysaora isosceles[342] apparently makes no attempt to capture either Copepoda or small fish, but preys voraciously upon Anthomedusae, Leptomedusae, Siphonophora, Ctenophora, and pelagic worms. Very little is known about the food of the Rhizostomata, but the small size of the mouths of these forms suggests that their food must also be of minute size. The frequent association of small fish with the larger jelly-fish is a matter of some interest that requires further investigation. In the North Sea young whiting are the constant guests of Cyanaea capillata.[343] Over a {312}hundred young horse-mackerel (Caranx trachurus) may be found sheltering under the umbrella of Rhizostoma pulmo. As the animal floats through the water the little fishes hover round the margin, but on the slightest alarm dart into the sub-umbrella cavity, and ultimately seek shelter in the sub-genital pits.[344]
Two species of fish accompany the American Medusa Dactylometra lactea, one a Clupeoid, the other the young of the Butter-fish (Stromateus triacanthus). According to Agassiz and Mayer[345] this is not an ordinary case of mutualism, as the fish will tear off and devour fragments of the tentacles and fringe of the Medusa, whilst the Medusa will in its turn occasionally capture and devour one of the fish.
A great many of the Scyphozoa, particularly the larger kinds, have the reputation of being able to sting the human skin, and in consequence the name Acalephae[346] was formerly used to designate the order. Of the British species Aurelia aurita is almost harmless, and so is the rarer Rhizostoma pulmo; but the nematocysts on the tentacles of Cyanaea, Chrysaora, and Pelagia can inflict stings on the more delicate parts of the skin which are very painful for several hours, although the pain has been undoubtedly greatly exaggerated in many popular works.
The soft structure of the Medusae does not favour their preservation in the rocks, but the impressions left by several genera, all belonging apparently to the Rhizostomata, have been found in Cambrian, Liassic, and Cretaceous deposits.
There is reason to believe that many Scyphozoa exhibit a considerable range of variation in the symmetry of the most important organs of the body. Very little information is, however, at hand concerning the variation of any species except Aurelia aurita, which has been the subject of several investigations. Browne[347] has found that in a local race of this species about 20 per cent exhibit variations from the normal in the number of the statorhabs, and about 2 per cent in the number of gastric pouches.
The Scyphozoa are not usually regarded as of any commercial or other value, but in China and Japan two species of Rhizostomata (Rhopilema esculenta and R. verrucosa) are used as food. {313}The jelly-fish is preserved with a mixture of alum and salt or between the steamed leaves of a kind of oak. To prepare the preserved food for the table it is soaked in water, cut into small pieces, and flavoured. It is also stated that these Medusae are used by fishermen as bait for file-fish and sea-bream.[348]
In general structure the Scyphozoa occupy an intermediate position between the Hydrozoa and the Anthozoa. The very striking resemblance of the body-form to the Medusa of the Hydrozoa, and the discovery of a fixed hydriform stage in the life-history of some species, led the older zoologists to the conclusion that they should be included in the class Hydrozoa. Recently the finer details of development have been invoked to support the view that they are Anthozoa specially adapted for a free-swimming existence, but the evidence for this does not appear to us to be conclusive.
They differ from the Hydrozoa and resemble the Anthozoa in the character that the sexual cells are matured in the endoderm, and escape to the exterior by way of the coelenteric cavity, and not directly to the exterior by the rupture of the ectoderm as in all Hydrozoa. They differ, on the other hand, from the Anthozoa in the absence of a stomodaeum and of mesenteries.
The view that the Scyphozoa are Anthozoa is based on the belief that the manubrium of the former is lined by ectoderm, and is homologous with the stomodaeum of the latter; and that the folds of mesogloea between the gastric pouches are homologous with the septa.[349]
The Scyphozoa, notwithstanding their general resemblance to the Medusae of Hydrozoa, can be readily distinguished from them by several important characters. The absence of a velum in all of them (except the Cubomedusae) is an important and conspicuous character which gave to the class the name of Acraspeda. The velum of the Cubomedusae can, however, be distinguished from that of the Craspedote Medusae (i.e. the Medusae of the Hydrozoa) by the fact that it contains endodermal canals.
Sense-organs are present in all Scyphozoa except some of the Stauromedusae, and they are in the form of statorhabs (tentaculocysts), bearing statoliths at the extremity, and in many species, {314}at the base or between the base and the extremity, one or more eyes. These organs differ from the statorhabs of the Hydrozoa in having, usually, a cavity in the axial endoderm; but as they are undoubtedly specially modified marginal tentacles, they are strictly homologous in the two classes. In nearly all the Scyphozoa these organs are protected by a hood or fold formed from the free margin of the umbrella, and this character, although not of great morphological importance, serves to distinguish the common species from the Craspedote Medusae. It was owing to this character that Forbes gave the name Steganophthalmata, or "covered-eyed Medusae," to the class.
Another character of some importance is the presence in the coelenteric cavity of all Scyphozoa of clusters or rows of delicate filaments called the "phacellae." These filaments are covered with a glandular epithelium, and are usually provided with numerous nematocysts. They have a considerable resemblance to the acontia of certain Anthozoa, and are probably mainly digestive in function. These three characters, in addition to the very important character of the position and method of discharge of the sexual cells already referred to, justify the separation of the Scyphozoa from the Medusae of the Hydrozoa as a distinct class of Coelenterata.
The umbrella of the Scyphozoa varies a good deal in shape. It is usually flattened and disc-like (Discophora), but it may be almost globular (Atorella), conical (some species of Periphylla), or cubical (Cubomedusae). It is divided into an aboral and a marginal region by a circular groove in the Coronata. The margin may be almost entire, marked only by notches where the statorhabs occur, or deeply lobed as in the Coronata and many Discophora. Marginal tentacles are present in all but the Rhizostomata, and may be few in number, four in Charybdea, eight in Ulmaris (Fig. 143), or very numerous in Aurelia and many others. The tentacles may be short (Aurelia), or very long as in Chrysaora isosceles, in which they extend for a length of twenty yards from the disc.
The manubrium of the Scyphozoa is usually quadrangular in section, and in those forms in which the shape is modified in the adult Medusa the quadrangular shape can be recognised in the earlier stages of development. The four angles of the manubrium are of importance in descriptive anatomy, as the planes drawn {315}through the angles to the centre of the manubrium are called "perradial," while those bisecting the perradial planes and passing therefore through the middle line of the flat sides of the manubrium are called "interradial."
The free extremity of the manubrium in many Scyphozoa is provided with four triangular perradial lips, which may be simple or may become bifurcated or branched, and have frequently very elaborate crenate edges beset with batteries of nematocysts. In Pelagia and Chrysaora and other genera these lips hang down from the manubrium as long, ribbon-like, folded bands, and according to the size of the specimen may be a foot or more in length, or twice the diameter of the disc.
In the Rhizostomata a peculiar modification of structure takes place in the fusion of the free edges of the lips to form a suture perforated by a row of small apertures, so that the lips have the appearance of long cylindrical rods or tubes attached to the manubrium, and then frequently called the "oral arms." The oral arms may be further provided with tentacles of varying size and importance. In many Rhizostomata branched or knobbed processes project from the outer side of the upper part of the oral arms. These are called the "epaulettes."
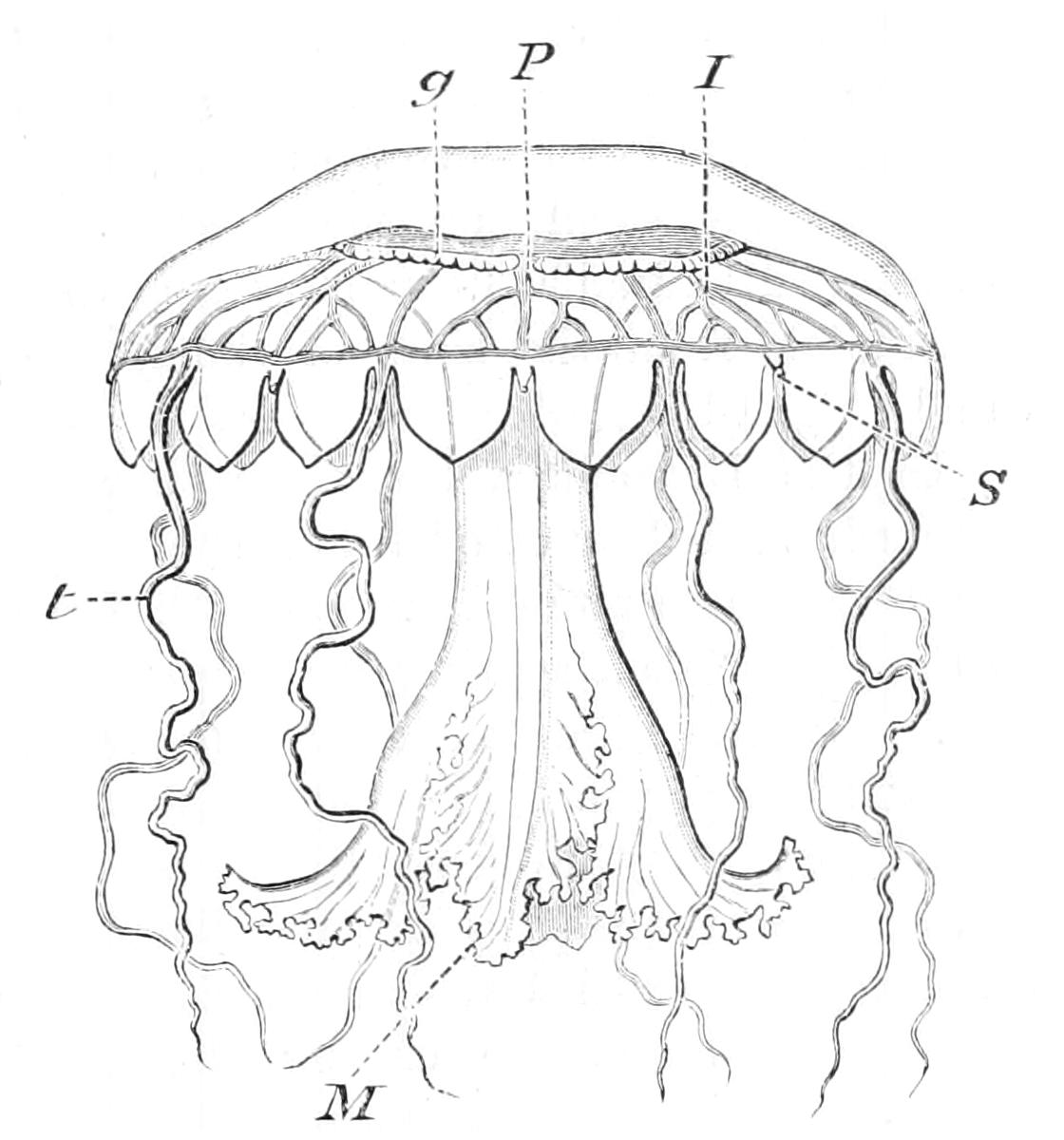
Fig. 143.—Ulmaris prototypus. g, Gonad; I, interradial canal; M, the fringed lip of the manubrium; P, perradial canal; S, marginal sense-organ; t, tentacle. × 1. (After Haeckel.)
The lumen of the manubrium leads into a large cavity in the disc, which is usually called the gastric cavity, and this is extended into four or more interradial or perradial gastric pouches. The number of these pouches is usually four, but in this, as in {316}other features of their radial symmetry, the jelly-fish frequently exhibit duplication or irregular variation of the radii.[350]
The gastric pouches may extend to the margin of the disc, where they are united to form a large ring sinus, or they may be in communication at the periphery by only a very narrow passage (Cubomedusae). In the Discophora the gastric pouches, however, do not extend more than half-way to the margin, and they may be connected with the marginal ring-canal by a series of branched interradial canals. Between the gastric pouches in these forms branched perradial canals pass from the gastric cavity to the marginal ring canal, and the system of canals is completed by unbranched "adradial" canals passing between the perradials and interradials from the sides of the gastric pouches to the ring-canal (Fig. 143).
In the Discophora there are four shallow interradial pits or pouches lined by ectoderm on the under side of the umbrella-wall. As these pits correspond with the position of the gonads in the gastric pouches they are frequently called the "sub-genital pits." In the Stauromedusae and Cubomedusae they are continued through the interradial gastric septa to the aboral side of the disc, and they are generally known in these cases by the name "interradial funnels." The functions and homologies of these ectodermic pits and funnels are still uncertain.
The Scyphozoa are usually dioecious, but Chrysaora and Linerges are sometimes hermaphrodite. The female Medusae can usually be distinguished from the male by the darker or brighter colour of the gonads, which are band-shaped, horseshoe-shaped, or circular organs, situated on the endoderm of the interradial gastric pouches. They are, when nearly ripe, conspicuous and brightly coloured organs, and in nearly all species can be clearly seen through the transparent or semi-transparent tissues of the disc. The reproductive cells are discharged into the gastric cavity and escape by the mouth. The eggs are probably fertilised in the water, and may be retained in special pouches on the lips of the manubrium until the segmentation is completed.[351] Asexual reproduction does not occur in the free-swimming or adult stage of any Scyphozoa. In some cases (probably exceptional) the development is direct. In Pelagia, for example, it is known that the fertilised egg gives {317}rise to a free-swimming Medusa similar in all essential features to the parent.
In many species, however, the planula larva sinks to the bottom of the sea, develops tentacles, and becomes attached by its aboral extremity to a rock or weed, forming a sedentary asexual stage of development with a superficial resemblance to a Hydra. This stage is the "Scyphistoma," and notwithstanding its simple external features it is already in all essential anatomical characters a Scyphozoon.
The Scyphistoma may remain as such for some time, during which it reproduces by budding, and in some localities it may be found in great numbers on seaweeds and stones.[352]
In the course of time, however, the Scyphistoma exhibits a ring-like constriction of the body just below the crown of tentacles, and as this deepens the general features of a Scyphomedusa are developed in the free part above the constriction. In time this free part escapes as a small free-swimming jelly-fish, called an "Ephyra," while the attached part remains to repeat the process. In many species the first constriction is followed by a second immediately below it, then a third, a fourth, and so on, until the Scyphistoma is transformed into a long series of narrow discs, each one acquiring, as it grows, the Ephyra characters. Such a stage has been compared in form to a pile of saucers, and is known as the "Strobila."
The Ephyra differs from the adult in many respects. The disc is thin and flat, the manubrium short, the margin of the umbrella deeply grooved, while the statorhabs are mounted on bifid lobes which project outwards from the margin. The stabilisation of the Scyphistoma is a process of reproduction by transverse fission, and in some cases this is supplemented by gemmation, the Scyphistoma giving rise to a number of buds which become detached from the parent and subsequently undergo the process of strobilisation.

Fig. 144.—The perisarc tubes of a specimen of Spongicola fistularis (N) ramifying in the skeleton of the Sponge Esperella bauriana (Sp.), as seen in a macerated specimen, × 1. (After Schulze.)
The Scyphistoma of Nausithoe presents us with the most {318}remarkable example of this mode of reproduction (Fig. 144), as it forms an elaborate branching colony in the substance of certain species of sponges. The ectoderm secretes a chitinous perisarc, similar to that of the hydrosome stage of many of the Hydrozoa, and consequently Stephanoscyphus (Spongicola), as this Scyphistoma was called, was formerly placed among the Gymnoblastea. It is remarkable that, although the Scyphozoan characters of Spongicola were proved by Schulze[353] in 1877, a similar Scyphistoma stage has not been discovered in any other genus.
Order I. Cubomedusae.
Scyphozoa provided with four perradial statorhabs, each of which bears a statolith and one or several eyes. There are four interradial tentacles or groups of tentacles. The stomach is a large cavity bearing four tufts of phacellae (Fig. 145, Ph), situated interradially. There are four flattened perradial gastric pouches in the wall of the umbrella which communicate with the stomach by the gastric ostia (Go). These pouches are separated from one another by four interradial septa; and the long leaf-like gonads are attached by one edge to each side of the septa. In many respects the Cubomedusae appear to be of simple structure, but the remarkable differentiation of the eyes and the occurrence of a velum (p. 313) suggest that the order is a highly specialised offshoot from a primitive stock.
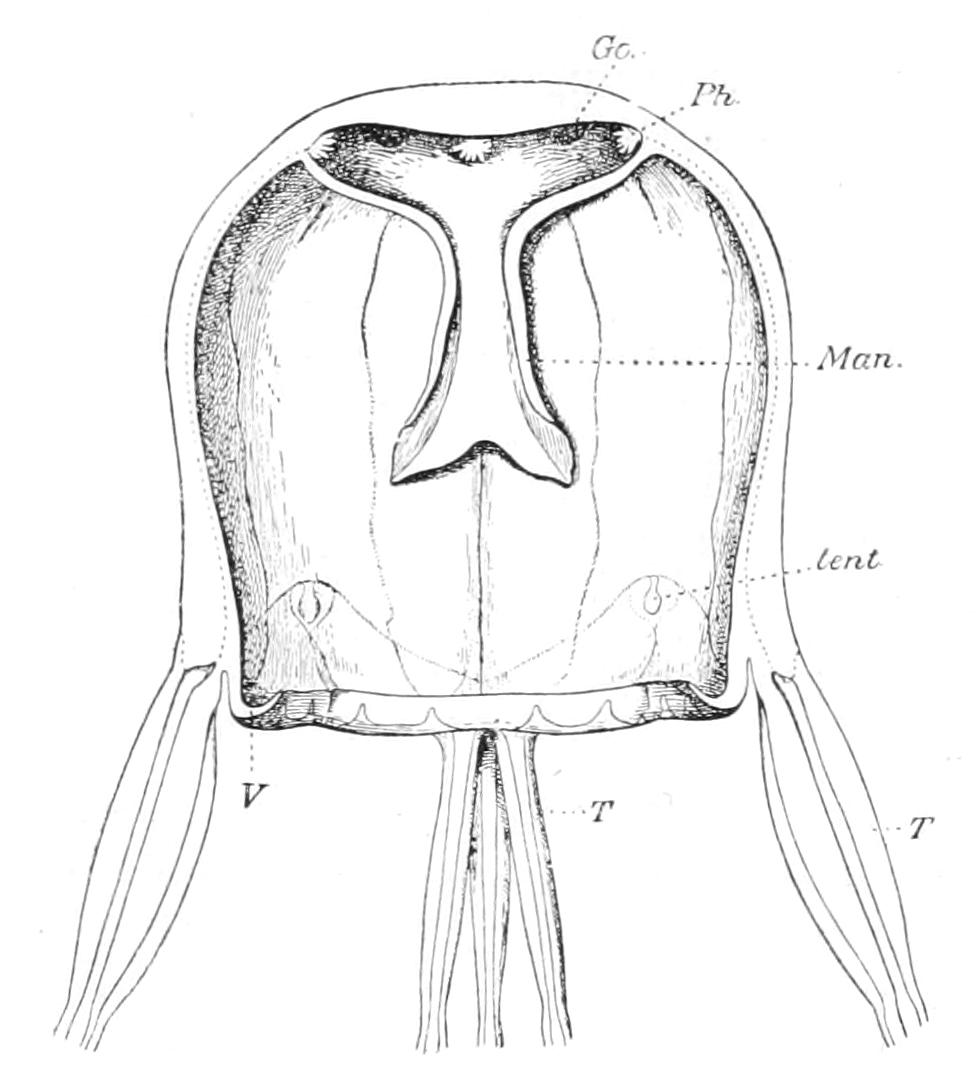
Fig. 145.—Vertical section in the interradial plane of Tripedalia cystophora. Go, Gastric ostia; Man, manubrium; Ph, group of phacellae; T, tentacles in four groups of three; tent, perradial sense-organs; V, velum. (After Conant.)
Fam. 1. Charybdeidae.—Cubomedusae with four interradial tentacles.
Charybdea appears to have a very wide geographical distribution. Some of the species are usually found in deep water and come to the surface only occasionally, but others (C. xaymacana) are only found at the surface of shallow water near the shore. The genus can be easily recognised by the four-sided prismatic shape of the bell and the oral flattened expansion of the base of the tentacles. The bell varies from 2-6 cm. in length (or height) in C. marsupialis, but a giant form, C. grandis,[354] has recently been discovered off Paumotu Island which is as much as 23 cm. in height. The colour is usually yellow or brown, but C. grandis is white and C. xaymacana perfectly transparent.
"Charybdea is a strong and active swimmer, and presents a very beautiful appearance in its movements through the water; the quick, vigorous pulsations contrasting sharply with the sluggish contractions seen in most Scyphomedusae." It appears to be a voracious feeder. "Some of the specimens taken contained in the stomach small fish, so disproportionately large in comparison with the stomach that they lay coiled up, head overlapping tail."[355]
Very little is known of the development, but it is possible that Tamoya punctata, which lacks gonads, phacellae, and canals in the velum, may be a young form of a species of Charybdea.
Fam. 2. Chirodropidae.—Cubomedusae with four interradial groups of tentacles.
This family is represented by the genera Chirodropus from the Atlantic and Chiropsalmus from the Indian Ocean and the coast of North Carolina.
Fam. 3. Tripedaliidae.—Cubomedusae with four interradial groups of three tentacles.
The single genus and species Tripedalia cystophora has only been found in shallow water off the coast of Jamaica. Specimens of this species were kept for some time by Conant in an aquarium, and produced a number of free-swimming planulae which settled on the glass, and quickly developed into small hydras with a mouth and four tentacles. The further development of this sedentary stage is unfortunately not known.
Order II. Stauromedusae.
This order contains several genera provided with an aboral stalk which usually terminates in a sucker, by means of which the animal is temporarily fixed to some foreign object. There can be little doubt that this sedentary habit is recently acquired, and the wide range of the characteristic features of the order may be accounted for as a series of adaptations to the change from a free-swimming to a sedentary habit.
It is difficult to give in a few words the characters of the order, but the Stauromedusae differ from other Scyphozoa in the absence or profound modification in structure and function of the statorhabs. They are absent in Lucernaria and the Depastridae, and very variable in number in Haliclystus.
The statorhab of Haliclystus terminates in a spherical knob, which is succeeded by a large annular pad or collar bearing a number of glandular cells which secrete a sticky fluid. At the base of the organ there is a rudimentary ocellus. The number is very variable, and sometimes they are abnormal in character, being "crowned with tentacles." There can be little doubt that the principal function of these organs is not sensory but adhesive, and hence they have received the names "colletocystophores" and "marginal anchors," but they are undoubtedly homologous with the statorhabs of other Scyphozoa.
The tentacles are short and numerous, and are frequently mounted in groups on the summit of digitate outgrowths from the margin of the umbrella. They are capitate, except in Tessera, the terminal swelling containing a battery of nematocysts.
Very little is known concerning the life-history and development of the Stauromedusae.
Fam. 1. Lucernariidae.—Marginal lobes digitate, bearing the capitate tentacles in groups. Haliclystus auricula is a common form on the shores of the Channel Islands, at Plymouth, and other localities on the British coast. It may be recognised by the prominent statorhabs situated in the bays between the digitate lobes of the margin of the umbrella. Each of the marginal lobes bears from 15 to 20 capitate tentacles. It is from 2 to 3 cm. in length. The genus occurs in shallow water {321}off the coasts of Europe and North America, extending south into the Antarctic region.
Lucernaria differs from Haliclystus in the absence of statorhabs. It has the same habit as Haliclystus, and is often found associated with it. L. campanulata is British.
Halicyathus is similar in external features to Haliclystus, but differs from it in certain important characters of the coelenteric cavities. It is found off the coasts of Norway, Greenland, and the Atlantic side of North America.
In Capria, from the Mediterranean, the tentacles are replaced by a denticulated membrane bearing nematocysts.
The rare genus Tessera, from the Antarctic Ocean, differs from all the other Stauromedusae in having no stalk and in having only a few relatively long non-capitate tentacles. If Tessera is really an adult form it should be placed in a separate family, but, notwithstanding the presence of gonads, it may prove to be but a free-swimming stage in the history of a normally stalked genus.
Fam. 2. Depastridae.—The margin of the umbrella is provided with eight shallow lobes bearing one or more rows of tentacles. Statorhabs absent.
Depastrum cyathiforme occurs in shallow water at Plymouth, Port Erin, and in other localities on the coasts of Britain and Norway. The tentacles are arranged in several rows on the margin of the umbrella. In Depastrella from the Canaries there is only one row of marginal tentacles.
Fam. 3. Stenoscyphidae.[356]—Stauromedusae with simple undivided umbrella margin. The eight principal tentacles are converted into adhesive anchors. Secondary tentacles arranged in eight adradial groups. Stenoscyphus inabai, 25 cm., Japan.
Order III. Coronata.[357]
The external surface of the umbrella is divided into two regions, an aboral region and a marginal region, by a well-marked circular groove (the coronal groove). The aboral region is usually smooth and undivided, but it is an elongated dome, {322}thimble- or cone-shaped, in marked contrast to the flattened umbrella of the Discophora. The margin is divided into a number of triangular or rounded lobes, and these are continued as far as the coronal groove as distinct areas delimited by shallow grooves on the surface of the umbrella. The tentacles arise from the grooves between the marginal areas, and are provided with expanded bases called the pedalia. The manubrium may be short or moderately long, but it is never provided with long lips.
Fam. 1. Periphyllidae.[358]—Coronata with four or six statorhabs.
In Pericolpa (Kerguelen) there are only four tentacles and four statorhabs. In Periphylla, a remarkable deep-sea genus from 700 to 2000 fathoms in all seas, but occasionally found at the surface, there are twelve tentacles and four statorhabs. The specimens from deep water have a characteristic dark red-brown or violet-brown colour. They are usually small Medusae, but the umbrella of P. regina is over 21 cm. in diameter. Atorella has six tentacles and six statorhabs.
Fam. 2. Ephyropsidae.—Coronata with eight or more than eight statorhabs.
Nausithoe punctata is a small, transparent jelly-fish, not exceeding 10 mm. in diameter, of world-wide distribution. Its Scyphistoma stage is described on p. 317. N. rubra, a species of a reddish colour found at a considerable depth in the South Atlantic and Indian Oceans, is probably an abysmal form. Palephyra differs from Nausithoe in having elongated instead of rounded gonads. Linantha and Linuche differ from the others in having subdivided marginal lobes.
Fam. 3. Atollidae.—Atolla is a deep-sea jelly-fish of very wide geographical distribution. It is characterised by the multiplication of the marginal appendages, but the number is very irregular. There may be double or quadruple the usual number of marginal lobes, or an indefinite number. There may be sixteen to thirty-two statorhabs, and the number of tentacles is quite irregular. Some of the species attain a considerable size, the diameter of the umbrella of A. gigantea being 150 mm., of A. valdiviae sometimes 130 mm., and of A. bairdi 110 mm.
Order IV. Discophora.
This order contains not only by far the greater number of the species of Scyphozoa, but those of the largest size, and all those that are familiar to the seaside visitor and the mariner under the general term jelly-fish.
They may be distinguished from the other Scyphozoa by several well-marked characters. The umbrella is flattened and disc-shaped or slightly domed, but not divided by a coronary groove. The perradial angles of the mouth are prolonged into long lips, which may remain free (Semaeostomata) or fuse to form an elaborate proboscis (Rhizostomata).
Sub-Order I. Semaeostomata.
In this sub-order the mouth is a large aperture leading into the cavity of the manubrium, and is guarded by four long grooved and often tuberculated lips. The margin of the umbrella is provided with long tentacles.
Fam. 1. Pelagiidae.—Semaeostomata with wide gastric pouches, which are not united by a marginal ring sinus. Pelagia, which forms the type of this family, has eight long marginal tentacles. It develops directly from the egg, the fixed Scyphistoma stage being eliminated.[359] It is probably in consequence of this peculiarity of its development and independence of a shore for fixation that Pelagia has become a common and widespread inhabitant of the high seas. In the Atlantic and Indian Oceans P. phosphora occurs in swarms or in long narrow lines many miles in length. It is remarkable for its power of emitting phosphorescent light. In the Atlantic it extends from 50° N. to 40° S., but is rare or absent from the colder regions. P. perla is found occasionally on the west coast of Ireland. Chrysaora differs from Pelagia in the larger number of tentacles. There are, in all, 24 tentacles and 8 statorhabs, separated by 32 lobes of the margin of the umbrella. C. isosceles is occasionally found off the British coast. It passes through a typical Scyphistoma stage in development. Dactylometra, a very {324}common jelly-fish of the American Atlantic shores, differs from Chrysaora in having sixteen additional but small tentacles arranged in pairs at the sides of the statorhabs.
Fam. 2. Cyanaeidae.—Semaeostomata with eight radial and eight adradial pouches, which give off ramifying canals to the margin of the umbrella; but these canals are not united by a ring-canal. The tentacles are arranged in bundles on the margin of the deeply lobed umbrella.
The yellow Cyanaea capillata and the blue C. lamarcki are commonly found on the British coasts.
Fam. 3. Ulmaridae.—The gastric pouches are relatively small, and communicate with a marginal ring-canal by branching perradial and interradial canals and unbranched adradial canals.
In Ulmaris prototypus (Fig. 143, p. 315) there are only eight long adradial tentacles, and the lips of the manubrium are relatively short. It is found in the South Atlantic.
Aurelia is a well-known and cosmopolitan genus, which may be recognised by the eight shallow lobes of the umbrella-margin beset with a fringe of numerous small tentacles.
Sub-Order II. Rhizostomata.
In this sub-order the lips are very much exaggerated in size, and are fused together by their margin in such a manner that the mouth of the animal is reduced to a number of small apertures situated along the lines of suture. Tentacles are absent on the margin of the umbrella. This sub-order contains some of the largest known jelly-fishes, and exhibits a considerable range of structure. The families are arranged by Maas[360] in three groups.
Group I. Arcadomyaria.—Musculature of the disc arranged in feather-like arcades. Oral arms pinnate.
Fam. Cassiopeidae.—There are no epaulettes on the arms. Labial tentacles present. Cassiopea is common in the Indo-Pacific seas, and extends into the Red Sea. It includes a great many species varying in size from 4 to about 12 cm. in diameter.
Group II. Radiomyaria.—Musculature arranged in radial tracts. Oral arms bifid.
Fam. Cepheidae.—The genera included in this family differ {325}from the Cassiopeidae in the characters of the group. Cephea is found in the Indo-Pacific Oceans and Red Sea. Cotylorhiza is common in the Mediterranean Sea and extends into the Atlantic Ocean.
Group III. Cyclomyaria.—The group contains the majority of the Rhizostomata. Musculature arranged in circular bands round the disc. Oral arms primarily trifid, but becoming in some cases very complicated. The principal families are:—
Fam. Rhizostomatidae.—With well-marked epaulettes, and sixteen radial canals passing to the margin of the umbrella.
Rhizostoma pulmo (= Pilema octopus), a widely distributed species, is often found floating at the surface off the western coasts of Scotland and Ireland, and sometimes drifts up the English Channel into the German Ocean in the autumn. The umbrella is about two feet in diameter, and the combined length of the umbrella and arms is four feet. The colour varies considerably, but that of a specimen obtained off Valencia in 1895 was described as follows: "The colour of the umbrella was pale green, with a deep reddish margin. Arms bright blue."[361]
The family includes Stomolophus, of the Pacific and Atlantic coasts of America, in which the oral arms are united at the base, and Rhopilema, the edible Medusa of Japan and China.
Fam. Lychnorhizidae.—Here there are only eight radial canals reaching as far as the margin of the umbrella, and eight terminating in the ring-canal. There are no epaulettes, and the oral tentacles are often very long. The family includes Lychnorhiza from the coast of Brazil, Crambione from the Malay Archipelago, and Crambessa from the Atlantic shores of France and Spain and from Brazil and Australia. The last-named genus has been found in brackish water at the mouth of the Loire.
In the families Leptobrachiidae and Catostylidae there are eight radial canals reaching the margin of the umbrella, and between them a network of canals with many openings into the ring-canal. In a few of the Leptobrachiidae the intermediate canal-network has only eight openings into the ring-canal, as in the Lychnorhizidae.
COELENTERATA (CONTINUED): ANTHOZOA = ACTINOZOA—GENERAL CHARACTERS—ALCYONARIA
CLASS III. ANTHOZOA = ACTINOZOA
Among the familiar objects included in this class are the Sea-anemones, the Stony Corals (Madrepores), the Flexible Corals, the Precious Coral, and the Sea-pens. With the exception of a few species of Sea-anemone, Anthozoa are not commonly found on British sea-shores; but in those parts of the tropical world where coral reefs occur, the shore at low tide is carpeted with various forms of this class, and the sands and beaches are almost entirely composed of their broken-down skeletons.
The majority of the Anthozoa are colonial in habit, a large number of individuals, or zooids as they are called, being organically connected together by a network of nutritive canals, and forming a communal gelatinous or stony matrix for their protection and support. Whilst the individuals are usually small or minute, the colonial masses they form are frequently large. Single colonies of the stony corals form blocks of stone which are sometimes five feet in diameter, and reach a height of two or three feet from the ground. From the tree or shrub-like form assumed by many of the colonies they were formerly included in a class Zoophyta or animal-plants.
But whether the individual polyps are large or small, whether they form colonies in the adult condition or remain independent, they exhibit certain characters in common which distinguish them not only from the other Coelenterata, but from all other animals. When an individual zooid is examined in the living and fully expanded condition, it is seen to possess a cylindrical {327}body, attached at one end (the aboral end) to the common colonial matrix or to some foreign object. At the opposite or free extremity it is provided with a mouth surrounded by a crown of tentacles. In these respects, however, they resemble in a general way some of the Hydrozoa. It is only when the internal anatomy is examined that we find the characters which are absolutely diagnostic of the group.
In the Hydrozoa the mouth leads directly into the coelenteric cavity; in the Anthozoa, however, the mouth leads into a short tube or throat, called the "stomodaeum," which opens into the coelenteric cavity. Moreover, this tube is connected with the body-wall, and is supported by a series of fleshy vertical bands called the mesenteries (Fig. 146). The mesenteries not only support the stomodaeum, but extend some distance below it. Where the mesenteries are free from the stomodaeum their edges are thickened to form the important digestive organs known as the mesenteric filaments (mf). It is in the possession of a stomodaeum, mesenteries, and mesenteric filaments that the Anthozoa differ from all the other Coelenterata. There is one character that the Anthozoa share with the Scyphozoa, and that is, that the gonads or sexual cells (G) are derived from the endoderm. They are discharged first into the coelenteric cavity, and then by way of the mouth to the exterior. In the Anthozoa the gonads are situated on the mesenteries.
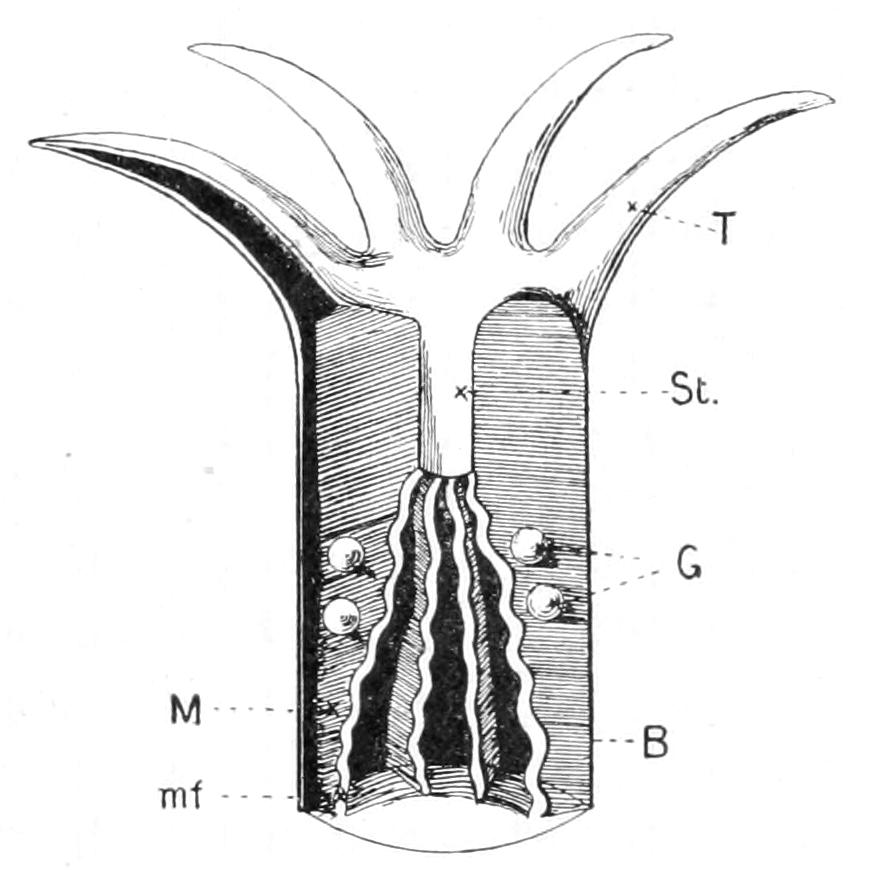
Fig. 146.—Diagram of a vertical section through an Anthozoan zooid. B, Body-wall; G, gonads; M, mesentery; mf, mesenteric filament; St, stomodaeum; T, tentacle.
Nearly all the Anthozoa are sedentary in habit. They begin life as ciliated free-swimming larvae, and then, in a few hours or days, they become attached to some rock or shell at the bottom and immediately (if colonial) start the process of budding, which gives rise to the colonies of the adult stage. Many of the Sea-anemones, however, move considerable distances by gliding {328}over the rocks or seaweeds, others habitually burrow in the sand (Edwardsia, Cerianthus), and one family (the Minyadidae) are supported by a gas bladder, and float at the surface of the sea. The Sea-pens, too, although usually partly buried in the sand or mud, are capable of shifting their position by alternate distension and contraction of the stalk.[362] The Anthozoa are exclusively marine. With the exception of a few Sea-anemones that are found in brackish or almost fresh water in river estuaries, they only occur in salt sea water. The presence of a considerable admixture of fresh water, such as we find at the mouths of rivers, seems to interfere very materially with the development and growth of all the reef-forming Corals, as will be noticed again in the chapter on coral reefs. A few genera descend into the greatest depths of the ocean, but the home of the Anthozoa is pre-eminently the shallow seas, and they are usually found in great abundance in depths of 0-40 fathoms from the shores of the Arctic and Antarctic lands to the equatorial belt.
The only Anthozoa of any commercial importance are the Precious Corals belonging to the Alcyonarian family Coralliidae. The hard pink axis of these corals has been used extensively from remote times in the manufacture of jewellery and ornaments. Until quite recently the only considerable and systematic fishery for the Precious Corals was carried on in the Mediterranean Sea, and this practically supplied the markets of the world. In more recent times, however, an important industry in corals has been developed in Japan. In 1901 the value of the coral obtained on the coasts of Japan was over £50,000, the greater part of which was exported to Italy, a smaller part to China, and a fraction only retained for home consumption. The history of the coral fishery in Japan is of considerable interest. Coral was occasionally taken off the coast of Tsukinada in early times. But in the time of the Daimyos the collection and sale of coral was prohibited, for fear, it is said, that the Daimyo of Tosa might be compelled to present such precious treasure to the Shogun. After the Meiji reform, however (1868), the industry revived, new grounds were discovered, improved methods employed, and a large export trade developed.
There is evidence, however, in the art of Japan, of another {329}coral fishery in ancient times, of which the history is lost. Coral was imported into Japan at least two hundred years ago, and used largely in the manufacture of those exquisite pieces of handicraft for which that country is so justly famous. On many of the carved "Netsukes" and other ornaments, however, the coral branches are represented as the booty of dark-skinned, curly-headed fishermen, "kurombo," and never of Japanese fishermen. The coral used in this art-work can hardly be distinguished from Mediterranean coral, and there are some grounds for believing that Japan imported coral from the far West in very early times. But this does not account for the "kurombo." The only coast-dwelling people of the type that is so clearly carved on these ornaments within the area of the Pacific Ocean at the present time are the Melanesians and Papuans, and the suggestion occurs that a coral fishery existed at one time in the Southern Pacific, which has since been lost.[363]
The class Anthozoa is divided into two sub-classes:—I. Alcyonaria; II. Zoantharia.
In the Alcyonaria the fully developed zooids have always eight tentacles and eight mesenteries. In the Zoantharia the number of tentacles and the number of mesenteries in the fully developed zooids may be six, twelve, twenty-four, or an indefinite number, but individuals with eight mesenteries and only eight tentacles are not known to occur.
Sub-Class I. Alcyonaria.
This sub-class includes a large number of genera living in shallow sea-water and a few genera that extend down into deep water. With a few doubtful exceptions (Protoalcyonacea) they all form colonies composed of a large number of zooids. These zooids may be connected together by basal plates or a network of basal strands (stolons), or by stolons with additional connecting bars (Clavularia viridis, Syringopora) or by plates (Tubipora). In the majority of the genera the individual zooids are for the greater part of their length, from the base upwards, united together to form a continuous spongy, colonial mass, which determines the shape of the colony as a whole.
In this last-named group of genera there may be {330}distinguished the free distal portions of the zooids bearing the mouths and tentacles (the "anthocodiae") from the common colonial mass perforated by the coelenteric cavities of the individual zooids. The coelenteric cavities are separated by a considerable amount of a substance called the "mesogloea," usually gelatinous in consistency but chemically more closely related to mucin than to gelatin, which is traversed by endodermal canals, rods of endoderm cells and a number of free amoeboid cells. In this substance, moreover, there are found in nearly all cases numerous spicules of carbonate of lime formed by the "scleroblasts" (spicule-forming cells) which have wandered from the superficial ectoderm of the common colonial mass. This common colonial mesogloea with its spicules, endoderm cells, and superficial covering of ectoderm is called the "coenenchym." The form assumed by the colonies is very varied. In some species of Clavularia they form encrusting plates following the irregularity of the rock or stones on which they grow, in Alcyonium they construct lobed masses of irregular form, in Sarcophytum they are usually shaped like a mushroom, in Juncella they are long whip-like rods, in most of the Gorgonacea they are branched in all directions like shrubs or in one plane to form fan-shaped growths, and in many of the Pennatulacea they assume that graceful feather form which gives the order its name.
The consistency and texture of the colonies also varies considerably. In some cases where the spicules are few or very small, the substance of the colony is soft to the touch, and frequently slimy at the surface, in other cases the great number of the spicules makes the colony hard but brittle, whilst in a few genera (Sclerophytum, Heliopora) the colony is so hard that it can only be broken by the hand with difficulty. In some genera (Spongodes and the Muriceidae) projecting spicules cause the surface to be rough or thorny, and in the Primnoidae the zooids and the surface of the general coenenchym are protected by a series of overlapping scales or plates.
In all the Alcyonaria the nematocysts are very minute, and although they can undoubtedly paralyse minute organisms they are unable to penetrate the human skin. None of the Alcyonaria have been described as stinging-corals except the Pennatulid Virgularia rumphii.
Zooids.—The fully formed zooids of the Alcyonaria exhibit {331}a remarkable uniformity of structure. They have eight intermesenteric tentacles containing a cavity continuous with the coelenteron. Each of these tentacles bears at least two rows of simple pinnules, and they are therefore said to be "pinnate" tentacles. In some species of Xenia the tentacles may have three or four rows of pinnules, which give them a much more feathery appearance than is usually the case. In the great majority of species a single row of from eight to fourteen pinnules is found disposed laterally on each side of the tentacle. The mouth is usually small and slit-like with a slight rounded gape at the ventral extremity. The stomodaeum is usually very short, but in Xenia and in the autozooids of some Pennatulids it is relatively much longer. It is not known how far the stomodaeum is of importance in the digestion of the food. In Xenia[364] it has probably some importance, as shown by its unusual length and the numerous large goblet cells (mucus cells) which it exhibits, associated with the fact that the mesenteric filaments are relatively very small. In Alcyonium and other Alcyonaria gland cells also occur in the stomodaeum, and it is probable that they secrete a fluid capable of digesting to some extent the food as it passes through. The most important part of the digestion, however, is performed by the six "ventral" mesenteric filaments.
Attention has already been drawn to the fact (p. 330) that two regions of the zooids of the colonial Alcyonaria can be recognised. At the oral end there is a region, which in the fully expanded condition consists of a crown of eight tentacles surrounding the mouth, and a body-wall free from its immediate neighbours. This region is called the "anthocodia." The anthocodia is continuous with a region which forms a part of the common colonial mass. Some genera seem to have very little power of contracting the tentacles or of withdrawing the anthocodiae. The zooids of Stereosoma, of Xenia, of Umbellula, and of a few other genera may be described as non-retractile. In many cases, however, the tentacles can be considerably contracted, bent over the mouth, and withdrawn into the shelter of the subjacent body-wall. In such a condition the surface of the colony exhibits a number of tubular, conical, or convex protuberances, called "verrucae," and the colony is said to be partially retractile. In many genera, however, the whole of the {332}anthocodiae can be withdrawn below the general surface of the coenenchym, so that the position of the zooids in the colony is indicated only by star-like holes, or simple key-hole slits in the superficial coenenchym. Such colonies are said to be completely retractile (Fig. 147).
It is often very difficult to determine whether a particular species is or is not completely retractile, unless observations can be made upon the living colony; and there are many instances of confusion in the work of systematists due to a species being described as partially retractile in one instance, and completely retractile in another. The complete retraction of the anthocodiae may be effected very slowly, and after continuous irritation only. If the colony is killed too quickly, the anthocodiae remain in a state of partial retraction. An example of this may be found in the common British Alcyonium digitatum. Specimens of this species which are put into a bucket of sea water and allowed to roll about with the movements of a small boat in a rough sea, undergo complete retraction; but if the same specimens be allowed to expand in the aquarium, and then plunged into spirit, or allowed to dry in the sun, they will die in a condition of partial retraction.
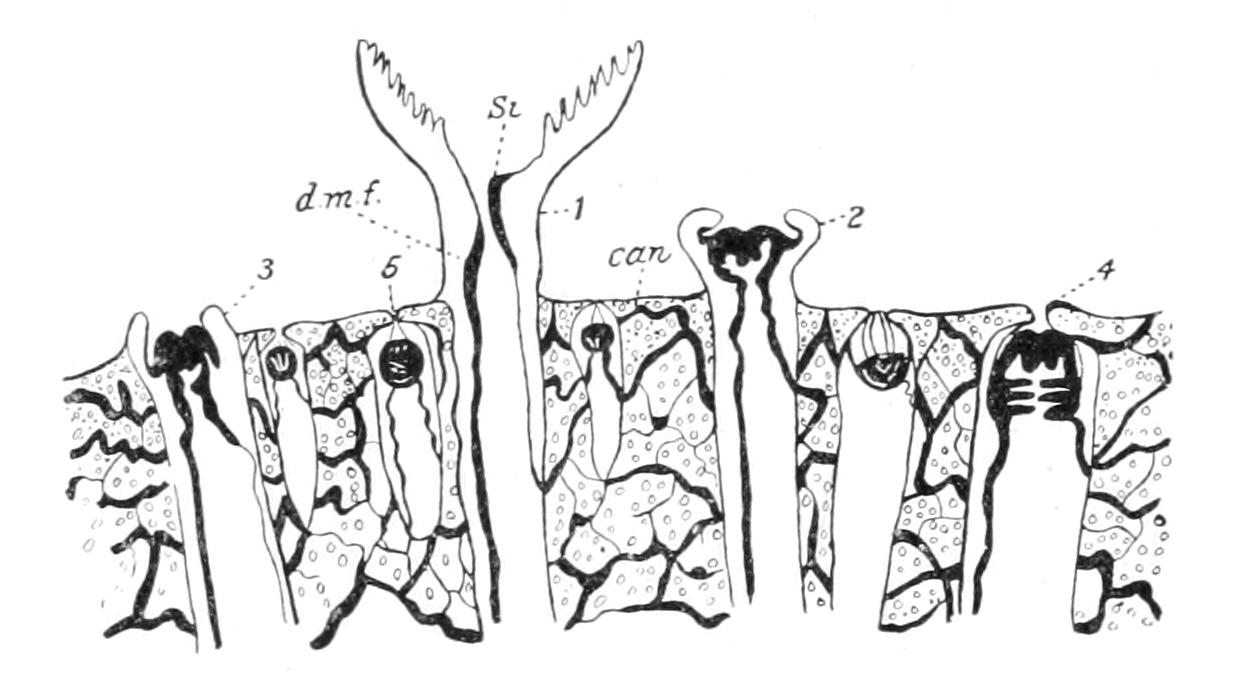
Fig. 147.—Diagram of a vertical section of a portion of a lobe of Alcyonium to show the mode of retraction of the anthocodiae. 1, Anthocodia of a zooid fully expanded; 2, in the first stage of retraction; 3, in the second stage; 4, in the third stage, leaving a shallow prominence or "verruca" on the surface; 5, final stage, the verruca flattened down and the coenenchym closed. can, Canal system; d.m.f, dorsal mesenteric filament of a zooid; si, siphonoglyph.
The phenomenon of dimorphism occurs in some Alcyonaria. A certain number of the zooids of a colony are arrested in their development, and are known as the "siphonozooids." They may be distinguished from the fully formed zooids, which, in these {333}cases, are called the "autozooids," by the absence of tentacles, by the absence of the six ventral and lateral mesenteric filaments, and by the incomplete development of the muscles on the mesenteries, and of the mesenteries themselves. They are, moreover, frequently distinguished by the greater development and extent of the ciliated groove or siphonoglyph on the ventral side of the stomodaeum.
It is often difficult to distinguish between true siphonozooids and young autozooids, and consequently dimorphism has been attributed to some genera in which it almost certainly does not occur. Simple dimorphism undoubtedly occurs in the genera Heteroxenia, Sarcophytum, Anthomastus, Lobophytum, Acrophytum, and Paragorgia. It has also been said to occur in Corallium (Moseley and Kishinouye), Melitodes (Ridley), and some species of Dasygorgiidae.
The Pennatulacea are trimorphic. The main shaft of these colonies is the much modified first formed or axial zooid, adapted for the support of all the other zooids. It usually exhibits no mouth, no tentacles, and only four of the original eight mesenteries. It has no mesenteric filaments and no stomodaeum, and bears no sexual cells. The other zooids of the colony are similar in structure to the autozooids and siphonozooids of the dimorphic Alcyonaria.
There are eight mesenteric filaments in all Alcyonarian zooids. They have the appearance of thickenings of the free edges of the mesenteries. Two of them, called the "dorsal" mesenteric filaments, are straight when the anthocodia is expanded, and extend from the edge of the stomodaeum for a long distance down into the coelenteron of the zooid; the other six, called the "ventral" mesenteric filaments (i.e. the ventral and ventro-lateral and dorso-lateral), are usually short and are almost invariably slightly convoluted. The dorsal filaments are built up of columnar cells provided with long cilia, and have usually no gland cells, the others may show a few cilia but are principally composed of non-ciliated gland cells. When the bolus of food has passed through the stomodaeum it is seized by these ventral filaments and rapidly disintegrated by the secretion of its cells. The function of the dorsal mesenteric filaments is mainly respiratory. During life their cilia produce a current which flows towards the stomodaeum. On the ventral side of the {334}stomodaeum itself there is a groove called the "siphonoglyph" composed of a specialised epithelium bearing long powerful cilia. But the current produced by the siphonoglyph flows from the mouth downwards into the coelenteric cavity and is thus in the opposite direction to that produced by the dorsal mesenteric filaments. It is very probable that these two currents on the opposite sides of the zooids maintain the circulation of water in the deep-seated parts of the colony which is necessary for the respiration of the tissues.
On each of the eight mesenteries there is a longitudinal ridge due to the presence of a band of retractor muscles. The position of these muscles on the ventral surfaces of the mesenteries only is one of the characteristic features of the sub-class (Fig. 148, and p. 329). They vary considerably in thickness and extent according to the power of retractility possessed by the zooids, but they never vary in their position on the mesenteries.

Fig. 148.—Diagrammatic transverse sections of an Alcyonarian. A, through the stomodaeum; B, below the level of the stomodaeum. DD, Dorsal directive; dlmf, dorso-lateral mesenteric filament; dmf, dorsal mesenteric filament; gon, gonad; Si, siphonoglyph; V.D, ventral mesentery; V.L, ventro-lateral mesentery. The upper half of the section in B is taken at a higher level than the lower half.
The skeleton of Alcyonaria may consist of spicules of calcium carbonate, of a horny substance frequently impregnated with calcium carbonate and associated with spicules of the same substance, or in Heliopora alone, among recent forms, of a continuous crystalline corallum of calcium carbonate.
The spicules constitute one of the most characteristic features of the Alcyonaria. They are not found in Cornularia, Stereosoma, in a recently discovered genus of Gorgoniidae (Malacogorgia), in certain Pennatulacea and in Heliopora; and it is probable that they may be absent in some local varieties of certain species of Clavularia.
The spicules of Alcyonaria consist of an organic matrix {335}supporting a quantity of crystalline calcium carbonate. In some cases (Xenia) the amount of inorganic salt is so small that the spicule retains its shape after prolonged immersion in an acid; but generally speaking the relative amount of calcium carbonate is so great that it is only by the careful decalcification of the spicules in weak acetic acid that the delicate fibrous organic matrix can be demonstrated.
The spicules vary in size from minute granules to long spindles 9 mm. in length (Spongodes, sp.). They exhibit so many varieties of shape that an attempt must be made to place them in groups. The most prevalent type perhaps is that called the spindle. This is a rod-shaped spicule with more or less pointed extremities. They are usually ornamented with short simple or compound wart-like tubercles (Fig. 149, 5). Spicules belonging to this type are found in all the principal subdivisions of the group except the Pennatulacea.
In the Pennatulacea a very characteristic form of spicule is a long rod or needle marked with two or three slightly twisted ridges, frequently a little knobbed or swollen at the extremities. In the same group, in Xenia and Heteroxenia among the Alcyonacea, and in the family Chrysogorgiidae the spicules are in the form of minute discs or spheres, and in some genera the discs may be united in couples (twins) or in threes (triplets) by short connecting bars (Fig. 149, 10). More irregular calcareous corpuscles of minute size are found in some genera of Pennatulacea.
Other characteristic spicules are the warted clubs of Juncella, the torch-like spicules of Eunicella (Fig. 149, 3), the clubs with irregular leaf-like expansions at one extremity ("Blattkeulen") of Eunicea, and the flat but very irregular scales of the Primnoidae. There are also many genera exhibiting spicules of quite irregular form (Fig. 149, 8).
In the greater number of cases the spicules lie loosely in the mesogloea and readily separate when the soft tissues of the colony decay or are dissolved in a solution of potash. In a few noteworthy examples the spicules become in their growth tightly wedged together to form a compact skeleton, which cannot subsequently be disintegrated into its constituent elements. In the Precious corals (Coralliidae) the spicules of the axial region fuse together to form a solid mass of lime almost as hard and compact as the substance of a pearl.

Fig. 149.—Spicules of Alcyonaria. 1, Club of Juncella; 2, warted cross of Plexaurella; 3, torch of Eunicella; 4, needle of Renilla; 5, warted spindle of Gorgonella; 6, spicule of Pennatula; 7, foliate club of Eunicea; 8, irregular spicule of Paramuricea; 9, scale of Primnoa; 10, spicules of Trichogorgia. (5 and 10 original, the remainder after Kölliker.)
In Paragorgia and some other closely related genera the spicules of the axis of the colony also become tightly wedged together, but the core thus formed is far more porous and brittle than it is in the Coralliidae. In Tubipora (the organ-pipe coral) and in Telesto rubra the spicules of the body-walls of the zooids fuse to form perforated calcareous tubes. In some species of Sclerophytum the large spicules of the coenenchym become so closely packed that they form dense stony masses, almost as hard as a Perforate Madreporarian coral. The horny substance, allied chemically to keratin, plays an {337}important part in the building up of skeletal structures in many Alcyonaria. In Clavularia viridis and in Stereosoma a change in the chemical character of the mesogloea of the body-walls of the polyps leads to the formation of a horny tube, which in the former case is built up of interlacing fibres, and in the latter is formed as a homogeneous sheath. In many of the Alcyonacea which have a compact axial skeleton the spicules are cemented together by a horny matrix.
In the Gorgonellidae and some others the hard axis is formed of a horny substance impregnated with a crystalline form of calcium carbonate; but in the Gorgoniidae, many of the Pennatulacea and some other genera very little or no carbonate of lime is found in the horny axis.
The skeleton of the genus Heliopora differs from that of all the other Alcyonaria in its development, structure, and form. In the words of Dr. G. C. Bourne,[365] "the calcareous skeleton of Heliopora is not formed from spicules developed within cells but is a crystalline structure formed by crystallisation of carbonate of lime, probably in the form of aragonite, in an organic matrix produced by the disintegration of cells which I have described as calicoblasts." It is further characterised by its blue colour. A peculiar form of the axial skeleton (Fig. 155), consisting of alternate nodes mainly composed of keratin, and internodes mainly composed of calcium carbonate, is seen in the families Isidae and Melitodidae. In the Melitodidae the nodes contain a considerable number of loose spicules, and the internodes are mainly composed of spicules in close contact but firmly cemented together by a sparse horny matrix. In the Isidae the scanty calcareous substance of the nodes, and the bulk of the substance of the internodes, is formed of amorphous crystalline limestone.
The Alcyonaria exhibit a great variety of colour. Very little is known at present of the chemistry of the various pigments found in the group, but they may conveniently be arranged in two sections, the soluble pigments and the insoluble pigments. To the former section belong various green and brown pigments found in the anthocodiae and superficial coenenchym of many genera. These are related to chlorophyll, and may be very largely the product, not of the Alcyonarians themselves, but of the {338}symbiotic "Algae" (cf. p. 261) they carry. A diffuse salmon-pink colour soluble in spirit occurs in the living Primnoa lepadifera of the Norwegian fjords, and a similar but paler pink colour occurs in some varieties of the common Alcyonium digitatum. Gilchrist[366] states that when he was preserving specimens of Alcyonium purpureum from Cape waters a considerable quantity of a soluble purple pigment escaped.
But the predominant colour of Alcyonarians is usually due to the insoluble pigments of the calcareous spicules. These may be of varying shades of purple, red, orange, and yellow. The colours may be constant for a species or genus, or they may vary in different specimens of one species, or even in different parts of a single colony. Thus the skeletons of Tubipora musica from all parts of the world have a red colour, the species of the genus Anthomastus have always red spicules. On the other hand, we find in Melitodes dichotoma red and yellow varieties in the same locality, and in M. chamaeleon some of the branches of a colony are red and others yellow. In Chironephthya variabilis the colour of the spicules in any one specimen varies considerably, but in a collection of several specimens from a single locality a kaleidoscopic play of colours may be seen, no two specimens being exactly the same in the arrangement of their colour pattern. The influences that determine the colour of the spicules is at present quite unknown, and in view of the great variability that occurs in this respect, colour must be regarded as a most uncertain guide for the determination of species. The blue colour of the genus Heliopora is due to a peculiar pigment which shows characteristic bands in the spectrum.[367]
Phosphorescence.—A great many Alcyonaria are known to be phosphorescent. Moseley says that "All the Alcyonarians dredged by the 'Challenger' in deep water were found to be brilliantly phosphorescent when brought to the surface." The phosphorescence of the common British Pennatula phosphorea has attracted more attention than that of any other species, and has been well described by Panceri, Forbes, and others. Forbes[368] says, "The pen is phosphorescent only when irritated by touch; the phosphorescence appears at the place touched, and {339}proceeds thence in an undulating wave to the extremity of the rachis, but never in the opposite direction; it is only the parts at and above the point of stimulation that show phosphorescence, the light is emitted for a longer time from the point of stimulation than from the other luminous parts; detached portions may show phosphorescence. When plunged in fresh water, the Pennatula scatters sparks about in all directions—a most beautiful sight."
Panceri was of opinion that the mesenteric filaments were the organs of phosphorescence, but the whole question of the cause and localisation of the light in these colonies requires further investigation.
Food.—Very little is known about the food of Alcyonaria, but it is very probable that it consists entirely of minute larvae and other living organisms. When the coelenteric cavities of preserved Alcyonaria are examined, food is very rarely found in them, although fragments of Crustacean appendages have occasionally been seen in the neighbourhood of the mesenteric filaments. Experimenting upon Alcyonium digitatum, Miss Pratt[369] has found that the zooids seize and swallow various small organisms of a surface-net gathering, and that they will also swallow finely minced fragments of the muscle of fish, but that they reject many kinds of fish ova. In many tropical and some extra-tropical species the superficial canal systems and the inter-mesenterial spaces of the zooids contain a large number of Zooxanthellae, and their presence seems to be associated in some cases with a decided degeneration of the digestive organs. It has been suggested that these symbiotic "Algae" prepare food materials after the manner of plants, and that these are absorbed by the hosts, but it appears improbable that in any case this source of food supply is sufficient. It must probably be supplemented in some degree by food obtained by the mouth, and digested in the coelenteric cavity.
The question whether the Alcyonaria can form an important part of the dietary of fish or other carnivorous animals may be economically important. Fragments of the Pennatulid Virgularia have been found in the stomachs of cod and other fish, but with this exception there is no evidence that any genus is systematically or even occasionally preyed upon by any animal. With a very {340}few exceptions Alcyonaria show no signs of having been torn, bitten, or wounded by carnivorous animals. It is improbable that the presence of nematocysts in the tentacles can account for this immunity, as it is known that some predaceous animals do feed upon Coelenterates provided with much larger nematocysts than any Alcyonarian possesses. All Alcyonaria, however, have a characteristic disagreeable odour, and it is possible, as in many other cases, that this is accompanied by an unpleasant taste. But if the Alcyonaria themselves are immune, it is possible that their large yolk-laden eggs may form a not unimportant source of food supply. In places where large colonies flourish, an immense number of eggs or embryos must be discharged into the water during the spawning season, and of these only a minute fraction can survive long enough to found a new colony.
Reproduction.—The formation of colonies by gemmation has frequently been mentioned above. The young buds of a colony arise from the endoderm canals in the body-wall of the zooids, in the general coenenchym, or in the stolon. They never arise from evagination of the coelenteric cavities of the zooids. There is no evidence that fission of a colony to form secondary colonies ever occurs. Gemmation leads to the increase in the number of zooids forming a colony, but not to an increase in the number of colonies.
Fission of the zooids is of extremely rare occurrence; a single case, however, has been recorded by Studer in the genus Gersemia. Sexual reproduction usually occurs once in a year; it is doubtful whether it ever occurs continuously. The colonies appear to be nearly always dioecious, only one case of hermaphroditism having yet been recorded.[370] The ova and sperm sacs are usually formed and matured on the six ventral mesenteries, rarely on the dorsal pair of mesenteries (Fig. 148, B) as well. The spawning season varies with the locality. Alcyonium digitatum spawns at Plymouth at the end of December, and somewhat later at Port Erin. The Pennatulid Renilla and the Gorgonid Leptogorgia spawn in the summer months on the coast of North America. In the Mediterranean Alcyonium palmatum spawns in September and October (Lo Bianco), Gorgonia cavolinii in May and June.
It is not known for certain when the fertilisation of the ova is effected, but in Alcyonium digitatum, and in the majority of the Alcyonarians, it probably takes place after the discharge of the ova from the zooids. A few forms are, however, certainly viviparous, the larvae of Gorgonia capensis being retained within the coelenteric cavity of the parent zooid until they have grown to a considerable size. The other viviparous Alcyonarians are Corallium nobile (de Lacaze Duthiers), the "Clavulaires petricoles," and Sympodium coralloides (Marion and Kowalevsky), and three species of Nephthya found at depths of 269 to 761 fathoms (Koren and Danielssen). The general features of the development are very similar in all Alcyonarians that have been investigated. The egg contains a considerable amount of yolk, and undergoes a modified form of segmentation. The free-swimming larva is called a "sterrula." It consists of an outer layer of clear ciliated ectoderm cells, surrounding a solid endodermic plasmodium containing the yolk. As the yolk is consumed a cavity appears in the endoderm, and the larva is then called a "planula" (Fig. 150). The mouth is subsequently formed by an invagination of the ectoderm at the anterior pole. The development of the mesenteries has not yet been fully described.
Classification.—The sub-class Alcyonaria may conveniently be classified as follows:—
Grade A. Protalcyonacea.
Grade B. Synalcyonacea.
Order 1. Stolonifera.
Order 2. Coenothecalia.
Order 3. Alcyonacea.
Order 4. Gorgonacea.
Order 5. Pennatulacea.
Grade A. Protoalcyonacea.
This Grade includes those genera which, like many sea-anemones, do not reproduce by continuous gemmation to form colonies.
Several genera have been described, and they have been placed together in one family called the Haimeidae.
Haimea funebris, M. Edwards, was found off the coast of Algeria; H. hyalina, Koren and Danielssen, in Norway; Hartea elegans, Wright, from the Irish coast; Monoxenia darwinii, Haeckel, from the Red Sea, and a large new species found by the "Siboga" Expedition in deep water off Ceram. All these species, however, are very rare, and there is no satisfactory evidence at present that they remain solitary throughout life.
Grade B. Synalcyonacea.
The sub-division of the Synalcyonacea into orders presents many difficulties, and several different classifications have been proposed. Only two orders of the five that are here recognised are clearly defined, namely, the Coenothecalia, containing the single living genus Heliopora, and the Pennatulacea or Sea-pens; the others are connected by so many genera of intermediate characters that the determination of their limits is a matter of no little difficulty.
Order I. Stolonifera.
These are colonial Alcyonaria springing from a membranous or ribbon-like stolon fixed to a stone or some other foreign object. The body-walls of the individual zooids may be free or connected by a series of horizontal bars or platforms (autothecalous); never continuously fused as they are in other orders (coenothecalous).
In the simplest form of this order, Sarcodictyon catenatum Forbes, the ribbon-like strands of the stolon meander over the surface of stones, forming a red or yellow network, from the upper surface of which the clear transparent anthocodiae of the zooids protrude. When retracted the anthocodiae are drawn down below the surface of the general coenenchym, and their position is indicated by small cushion-like pads on the stolon. {343}Sarcodictyon is found in depths of 10 to 22 fathoms in the Irish Sea, off the west coast of Scotland, the Shetlands, and off the Eddystone Lighthouse, South Devon.
Another very important genus is Tubipora, in which the tubular body-wall of each zooid is very much longer in proportion to its diameter than it is in Sarcodictyon, and the anthocodia is retracted not into the stolon, but into the basal part of the body-wall. The zooids are connected together by horizontal platforms on which new zooids are formed by gemmation. Both horizontal platforms and the body-walls of the zooids are provided with a skeleton of fused spicules of a red colour.
This genus is the well-known Organ-pipe coral, and is found sometimes in immense quantities on the coral reefs of both the old and new world.
It may be seen in pools on the edge of the reefs at low tides in colonies frequently a foot or more in diameter. The tentacles are often of a bright emerald green colour, and as the anthocodiae stand expanded in the clear water they contribute a brilliant patch of colour to the many beauties of their surroundings. When the coral is disturbed, or the water shallows and the anthocodiae are retracted, the dull red colour of the skeleton gradually takes the place of the bright green of the tentacles.
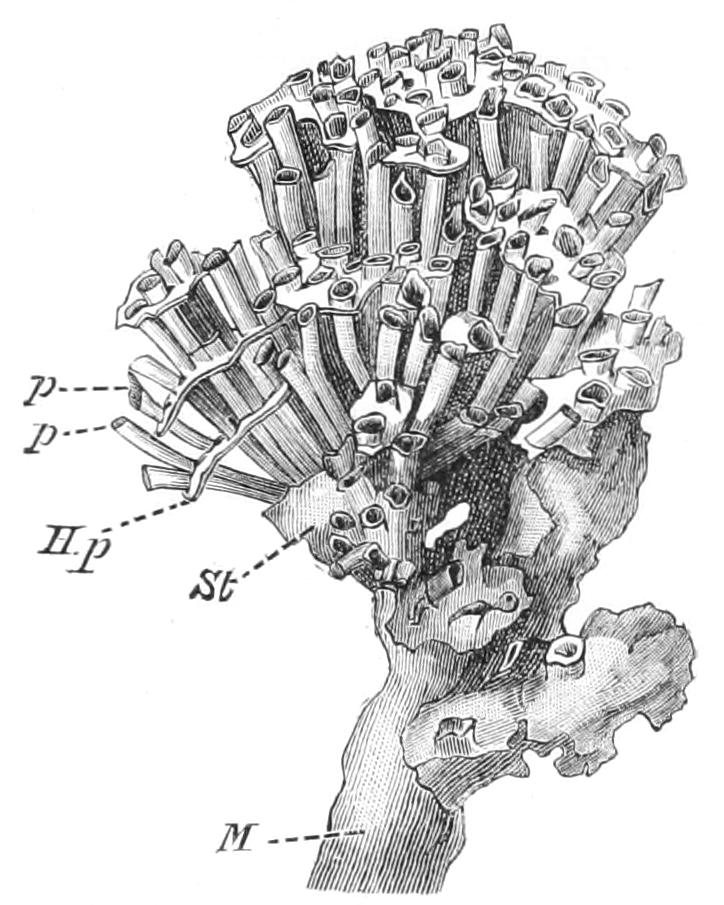
Fig. 151.—Tubipora musica, a young colony growing on a dead Madrepore branch (M). Hp, The connecting horizontal platforms; p, p, the skeletal tubes of the zooids; St, the basal stolon.
It is probable that this order of Alcyonaria was better represented on the reefs of some of the earlier periods of the world's history than it is at present. The fossil Syringopora, which is found abundantly in the carboniferous limestone and other strata, was probably an Alcyonarian belonging to this order. It resembles Tubipora in its mode of growth, but in place of the horizontal platforms connecting the zooids there are rods or bars from which new zooids spring (Fig. 152). Similar connecting bars are found in the recent Clavularia (Hicksonia, Delage) {344}viridis of the East Indian reefs (Fig. 153). Other fossil forms belonging to the order are Favosites, a very abundant coral of the Upper Silurian rocks, and possibly Columnaria.
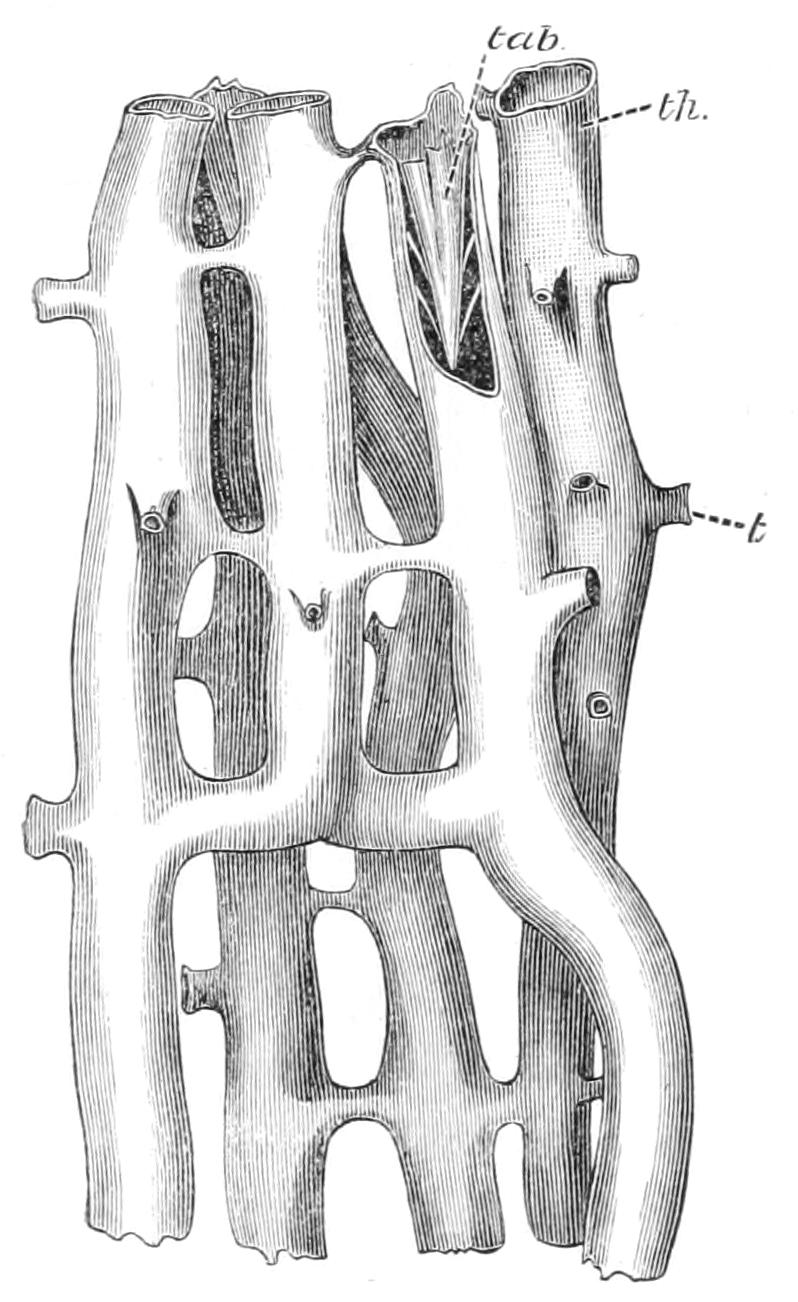
Fig. 152.—Syringopora, a fossil, showing autothecalous tubes (th), funnel-shaped tabulae (tab), and tubular cross-bars (t).
The principal families of the Stolonifera are:—
Fam. 1. Cornulariidae.—Without spicules; Cornularia, Lamarck, Mediterranean; Stereosoma, Hickson, Celebes.
Fam. 2. Clavulariidae.—Clavularia, Quoy and Gaimard; Sarcodictyon, Forbes, British; Sympodium, Ehrb.; Syringopora, Goldfuss, fossil.
Fam. 3. Tubiporidae.—Tubipora, Linnaeus, tropical shallow water.
Fam. 4. Favositidae.—Favosites, Lamarck; Syringolites, Hinde; Stenopora, King.
Order II. Coenothecalia.
This order contains the single genus and species Heliopora coerulea among recent corals, but was probably represented by a large number of genera and species in earlier periods.
It is found at the present day in many localities in the warm shallow waters of the tropical Pacific and Indian Oceans. It usually flourishes on the inside of the reef, and may form masses of stone five or six feet in diameter. The coral may easily be recognised, as it is the only one that exhibits a blue colour. This colour usually penetrates the whole skeleton, but in some forms is absent from the superficial layers.
The skeleton consists of a number of parallel tubes with imperforate walls, which are fused together in honey-comb fashion. On making a vertical section through a branch of the coral it is found that the tubes are divided into a series of chambers by transverse partitions or "tabulae." The soft living tissues of the coral, the zooids and coenosarc, are confined to the terminal chambers, all the lower parts being simply dead calcareous skeleton supporting the living superficial layer. Among the parallel tubes there may be found a number of larger chambers that seem to have been formed by the destruction of the adjacent walls of groups of about nineteen tubes. These chambers are provided with a variable number of pseudo-septa, and have a remarkable resemblance to the thecae of some Zoantharian corals. That Heliopora is not a Zoantharian coral was first definitely proved by Moseley, who showed that each of these larger chambers contains an Alcyonarian zooid with eight pinnate tentacles and eight mesenteries. The zooids arise from a sheet of coenosarc that covers the whole of the living branches of the coral mass, and this sheet of coenosarc bears a plexus of canals communicating on the one hand with the zooids, and on the other with a series of blind sacs, each of which occupies the cavity of one of the skeletal tubes as far down as the first tabula. The zooids of Heliopora are very rarely expanded during the day-time, and it has been found very difficult to get them to expand in an aquarium. The coral, however, is frequently infested with a tubicolous worm allied to the genus Leucodora, which freely expands and projects from the surface. So constant and so numerous are these worms in some localities that it has actually been suggested that Heliopora should be regarded as a Polychaete worm and not as an Alcyonarian. According to Mr. Stanley Gardiner, however, these worms do not occur in association with the Heliopora found on the reefs of the Maldive Archipelago.
There is very strong reason to believe that certain fossil corals were closely related to Heliopora; that Heliopora is in fact the solitary survivor of a group of Alcyonarian corals that in past times was well represented on the reefs, both in numbers and in species. The evidence is not so convincing that other fossil corals are closely related to Heliopora, and their true zoological position may remain a matter for surmise. The order may be classified as follows:—
Fam. 1. Heliolitidae.[371]—Coenothecalia with regular, well-developed septa, generally twelve in number, in each calicle.
Heliolites, Dana, Silurian and Devonian. Cosmiolithus, Lindström, Upper Silurian. Proheliolites, Klaer, Lower Silurian. Plasmopora, Edwards and Haime, Upper Silurian. Propora, E. and H., Upper Silurian. Camptolithus, Lindström, Upper Silurian. Diploëpora, Quenst, Upper Silurian. Pycnolithus, Lindström, Upper Silurian.
Fam. 2. Helioporidae.[372]—Coenothecalia with small irregularly arranged coenosarcal caeca, and a variable number of septa or septal ridges. Heliopora, de Blainville, recent, Eocene and Upper Cretaceous. Polytremacis, d'Orbigny, Eocene and Upper Cretaceous. Octotremacis, Gregory, Miocene.
The family Coccoseridae is regarded by Lindström as a sub-family of the Heliolitidae, and the families Thecidae and Chaetetidae are probably closely related to the Helioporidae.
Order III. Alcyonacea.
This order contains a large number of genera of great variety of form. The only characters which unite the different genera are that the body-walls of some groups of zooids, or of all the zooids, are fused together to form a common coenenchym penetrated by the coenosarcal canals, and that the spicules do not fuse to form a solid calcareous, or horny and calcareous, axial skeletal support.
The affinities with the order Stolonifera are clearly seen in the genera Xenia and Telesto. Some species of Xenia form flattened or domed colonies attached to stones or corals, with non-retractile anthocodiae and body-walls united for only a {347}short distance at the base. Young Xenia colonies are in fact Stolonifera in all essential characters. In Telesto prolifera we find a network of stolons encrusting coral branches and other objects after the manner of the stolons of many species of Clavularia, although the zooids do not arise from these stolons singly, but in groups, with their body-walls fused together for a certain distance. In Telesto rubra the spicules of the body-walls are fused together to form a series of perforated tubes very similar in some respects to the tubes of Tubipora.
A remarkable genus is Coelogorgia. Here we find a branching colony arising from a basal stolon, and the axis of the main stem and of each branch consists of a single very much elongated zooid bearing on its thickened walls the branches of the next series and other zooids. It is true that in this genus there is very little fusion of neighbouring zooids, and the amount of true coenenchym is so small that it can hardly be said to exist at all. Bourne[373] has united this genus with Telesto into a family Asiphonacea, which he joins with the Pennatulida in the order Stelechotokea; but their affinities seem to be closer with the Alcyonacea than with the Pennatulacea, from which they differ in many important characters.
The genus Alcyonium not only contains the commonest British Alcyonarian (A. digitatum), but it is one of the most widely distributed genera of all Alcyonaria that occur in shallow water.
The genera Sarcophytum and Lobophytum occur in shallow water in the tropics of the old world. The former frequently consists of huge toad-stool shaped masses, soft and spongy in {348}consistency, of a green, brown, or yellow colour. On some reefs the colonies of Sarcophytum form a very conspicuous feature, and from their very slimy, slippery surface, add to the minor dangers of wading in these regions. Both genera are dimorphic. Some species of the genus Sclerophytum,[374] which occur in the Indian Ocean, are so hard and brittle that they might readily be mistaken for a Zoantharian coral. This character is due to the enormous number of tightly packed spicules borne by the coenenchym. Some of these spicules in S. querciforme are 7 mm. × 1.7 mm.; the largest, though not the longest (vide p. 335) of any spicules occurring in the order.
Another very important genus occurring on coral reefs, and of very wide distribution, is Spongodes. This genus forms bushy and rather brittle colonies of an endless variety of beautiful shapes and colours. Arising from the neck of each anthocodia there are one or two long, sharp, projecting spicules, which give the surface a very spiny or prickly character.
The genera Siphonogorgia and Chironephthya form large brittle, branching colonies which might readily be mistaken for Gorgonians. The strength of the branches, however, is mainly due to the large, densely packed, spindle-shaped spicules at the surface of the coenenchym, the long coelenteric cavities of the zooids penetrating the axis of both stem and branches. Siphonogorgia is usually uniformly red or yellow in colour. Chironephthya, on the other hand, exhibits a great variety of colour in specimens from the same reef, and indeed in different branches of the same colony.
Fam. 1. Xeniidae.—Alcyonacea with non-retractile zooids. Spicules very small discs, usually containing a relatively small proportion of lime.
Xenia, Savigny; Indian Ocean and Torres Straits. Heteroxenia, Kölliker; Red Sea, Cape of Good Hope, and Torres Straits.
Fam. 2. Telestidae.—Colonies arising from an encrusting membranous or branching stolon. The erect stem and branches are formed by the body-walls of two or three zooids only, from which secondary zooids and branches of the next order arise.
Telesto, Lamouroux, widely distributed in warm waters of the Atlantic, Pacific, and Indian Oceans. The genus Fascicularia, Viguier, from the coast of Algiers, seems to be related to Telesto, {349}but the groups of zooids are short, and do not give rise to branches.
Fam. 3. Coelogorgiidae.—The colony arborescent, attached by stolon-like processes. The stem formed by an axial zooid with thickened body-walls. Branches formed by axial zooids of the second order, and branchlets by axial zooids of the third order, borne either on two sides or in spirals by the main stem. Genus Coelogorgia, Zanzibar.
Fam. 4. Alcyoniidae.—The colonies of this family are usually soft and fleshy, and the spicules, evenly distributed throughout the coenenchym, do not usually fuse or interlock to form a continuous solid skeleton. They may be unbranched or lobed, never dendritic in form. The principal genera are:—Alcyonium, Linnaeus, cosmopolitan, but principally distributed in temperate and cold waters. Alcyonium digitatum is the commonest British Alcyonarian. It is found in shallow water, from the pools left at low spring tides to depths of 40 or 50 fathoms, at most places on the British shores. It is stated by Koehler to descend into depths of over 300 fathoms in the Bay of Biscay. There are two principal varieties; one is white or pale pink in the living condition, and the other yellow. In some localities the two varieties may be found in the same pools. Another species, Alcyonium glomeratum, placed in a distinct genus (Rhodophyton) by Gray, and distinguished from the common species by its red colour and long digitate lobes, is found only off the coast of Cornwall. Paralcyonium, Milne Edwards; Mediterranean. Sclerophytum, Pratt; sometimes dimorphic, Indian Ocean. Sarcophytum, Lesson; dimorphic, principally tropical. Lolophytum, Marenzeller; dimorphic, tropical. Anthomastus, Verrill; dimorphic, Atlantic Ocean, deep water. Acrophytum, Hickson; dimorphic, Cape of Good Hope.
Fam. 5. Nephthyidae.—Colonies dendritic. Usually soft and flexible in consistency. Nephthya, Savigny; Indian and Pacific Oceans. Spongodes, Lesson; widely distributed in the Indian and Pacific Oceans.
Fam. 6. Siphonogorgiidae.—Colonies often of considerable size. Dendritic. Spicules usually large and abundant, giving a stiff, brittle consistency to the stem and branches. Siphonogorgia, Kölliker; Red Sea, Indian, and Pacific tropics. Chironephthya, Wright and Studer; Indian and Pacific Oceans. Lemnalia, {350}Gray; Zanzibar. Agaricoides, Simpson;[375] Indian Ocean, 400 fathoms.
Order IV. Gorgonacea.
This order contains a very large number of dendritic and usually flexible corals occurring in nearly all seas and extending from shallow waters to the very great depths of the ocean. A large proportion of them are brightly coloured, and as the principal pigments are fixed in the spicules, and are therefore preserved when the corals are dead and dried, they afford some of the most attractive and graceful objects of a natural history museum.
The only character that separates them from the Alcyonacea is that they possess a skeletal axis that is not perforated by the coelenteric cavities of the zooids. The coelenteric cavities are usually short. The order may conveniently be divided into two sub-orders.
Sub-Order 1. Pseudaxonia.
The axis in this sub-order consists of numerous spicules tightly packed together, or cemented together by a substance which is probably allied to horn in its chemical composition. This substance may be considerable in amount, in which case it remains after decalcification as a spongy, porous residue; or it may be so small in amount, as in Corallium, that the axis appears to be composed of solid carbonate of lime. The statement is usually made that the axis is penetrated by nutritive canals in certain genera, but the evidence upon which this is based is unsatisfactory and in some cases unfounded. There can be no doubt, however, that in some genera the axis is porous and in others it is not, and this forms a useful character for the separation of genera.
Fam. 1. Briareidae.—The medullary substance consists of closely packed but separate spicules embedded in a soft horny matrix, which is uniform in character throughout its course. Nearly all the genera form dendritic colonies of considerable size.
The principal genera are:—Solenocaulon, Gray; Indian Ocean and North Australia. Many of the specimens of this genus have fistulose stems and branches. The tubular character of the stem and branches is probably caused by the activity of a Crustacean, {351}Alpheus, and may be regarded as of the nature of a gall-formation.[376] Paragorgia, M. Edwards; Norwegian fjords, in deep water. This genus forms very large tree-like colonies of a ruby-red or white colour. It is perhaps the largest of the dendritic Alcyonarians. It is dimorphic. Spongioderma, Kölliker; Cape of Good Hope. The surface of this form is always covered by an encrusting sponge. Iciligorgia, Ridley; Torres Straits. The stem and branches are compressed and irregular in section.
Fam. 2. Sclerogorgiidae.—The medullary mass forms a distinct axis consisting of closely packed elongate spicules with dense horny sheaths.
Suberogorgia, Gray, has a wide distribution in the Pacific Ocean, Indian Ocean, and the West Indies. Keroeides, W. and S., comes from Japan.
Fam. 3. Melitodidae.—The axis in this family exhibits a series of nodes and internodes (Fig. 155), the former consisting of pads formed of a horny substance with embedded spicules, the latter of a calcareous substance with only traces of a horny matrix. The internodes are quite rigid, the nodes however give a certain degree of flexibility to the colony as a whole. Neither the nodes nor the internodes are penetrated by nutritive canals, but when dried the nodes are porous.
The principal genera are:—Melitodes, Verrill; widely distributed in the Indian and Pacific Oceans, Cape of Good Hope, etc. This genus is in some localities extremely abundant and exhibits great brilliancy and variety of colour. The branching is usually dichotomous at the nodes. Wrightella, Gray. This is a delicate dwarf form from Mauritius and the coast of South Africa. Parisis, Verrill; Pacific Ocean from Formosa to Australia but not very common. One species from Mauritius. The branches arise from the internodes.
Fam. 4. Coralliidae.—The axis is formed by the fusion of spicules into a dense, solid, inflexible, calcareous core.
Corallium, Lamarck. Corallium nobile, Pallas, the "precious coral," occurs in the Mediterranean, chiefly off the coast of North Africa, but also on the coasts of Italy, Corsica, Sardinia, and it extends to the Cape Verde Islands in the Atlantic Ocean. C. japonicum, Kishinouye, called Akasango by the fishermen, occurs off the coast of Japan, and C. reginae, Hickson, has recently been described from deep water off the coast of Timor.[377] The genus Pleurocorallium, Gray, is regarded by some authors as distinct, but the characters that are supposed to distinguish it, namely, the presence of peculiar "opera-glass-shaped spicules," and the occurrence of the verrucae on one side of the branches only, are not very satisfactory. The following species are therefore placed by Kishinouye[378] in the genus Corallium:—C. elatius, Ridley (Momoirosango); C. konojoi, Kishinouye (Shirosango); C. boshuensis, K.; C. sulcatum, K.; C. inutile, K.; and C. pusillum, K.,—all from the coast of Japan. Of the coral obtained from these species, the best kinds of Momoirosango vary in price from £30 per pound downwards according to the quality. The Shirosango is the least valuable of the kinds that are brought into the market, and is rarely exported.[379] Three species of Corallium (Pleurocorallium) have been described from Madeira,[380] and one of these, C. johnsoni, has recently been found in 388 fathoms off the coast of Ireland.[381] Other species are C. stylasteroides, from Mauritius; C. confusum, Moroff,[382] from Sagami Bay in Japan; and an undescribed species obtained by the "Siboga," off Djilolo. These corals range from shallow water to depths of 300-500 fathoms. Pleurocoralloides, Moroff, differs from the others in having very prominent verrucae and in the character of the large spindle-shaped and scale-like spicules. It was found in Sagami Bay, Japan. Specimens attributed to the genus Pleurocorallium have been found fossil in the white chalk of France, but Corallium has been found only in the tertiaries.[383]
Sub-Order 2. Axifera.
The axis in this sub-order may be horny, or horny with a core of calcium carbonate, or composed of horn impregnated with calcium carbonate, or of nodes of horn alternating with internodes of calcium carbonate. It may be distinguished from the axis of the Pseudaxonia by the fact that in no case have definite spicules been observed to take part in its formation. It has been suggested that as the Axifera represent a line of descent distinct from that of the Pseudaxonia they should be placed in a separate order. Apart from the character of the axis, however, the two sub-orders show so many affinities in their general anatomy that it is better to regard the two lines of descent as united within the Gorgonacean limit. It is very improbable that the two groups sprang independently from a stoloniferous ancestor.
Fam. 1. Isidae.—This family includes all those Axifera in which the axis is composed of alternate nodes of horn and internodes of calcareous substance.
There can be little doubt of the close affinities of many of the genera of this family with the Melitodidae among the Pseudaxonia. In both the coenenchym is thin and the coelenteric cavities short. No important differences have been observed between the structure of the zooids of the two families, and now that we know that the "nutritive canals" of Melitodes do not perforate the nodes there is no important difference left between the coenosarcal canal systems. The structure and method of calcification of the internodes of the two families are very similar. The main difference between them is that the nodes of the Isidae are purely horny, whereas in the Melitodidae the horny substance of the nodes contains calcareous spicules.
The principal genera are:—Isis, Linnaeus; Pacific Ocean. This genus forms substantial fan-shaped colonies with, relatively, a thick coenenchym, short stout internodes and black horny nodes. Mopsea, Lamouroux; Coast of Australia. The verrucae are club-shaped and are arranged in spiral rows round the stem. Acanella, Gray; principally found in deep water in the Atlantic Ocean but also in the Pacific. The internodes are long and the branches arise from the nodes. Most of the species occur in deep water, some in very deep water (A. simplex, 1600 to 1700 fathoms). In this and the following genera the coenenchym is {354}thin and the zooids imperfectly or not retractile. Ceratoisis, Wright; Atlantic Ocean, extending from shallow to deep water. The branches arise from the nodes. Chelidonisis, Studer; deep water off the Azores. Isidella, Gray; Mediterranean Sea. Bathygorgia, Wright; off Yokohama, 2300 fathoms. This genus is unbranched, with very long internodes and short nodes. The zooids are arranged on one side only of the stem.
Fam. 2. Primnoidae.—This is a well-marked family. The axis of the colonies is horny and calcareous. The coenenchym and the non-retractile zooids are protected by scale-like spicules, which usually overlap and form a complete armour for the protection of the soft parts. On the aboral side of the base of each tentacle there is a specialised scale, and these fit together, when the tentacles are folded over the peristome, to form an operculum.
The principal genera are:—Primnoa, Lamouroux; Atlantic Ocean, occurring also in the Norwegian fjords. This genus is usually found in moderately deep water, 100 to 500 fathoms. Primnoella, Gray. This genus seems to be confined to the temperate seas of the southern hemisphere. It is unbranched. The zooids are arranged in whorls round the long whip-like stem. Plumarella, Gray; southern hemisphere, in moderately deep water. This is branched pinnately in one plane. The zooids are small and arise at considerable intervals alternately on the sides of the branches. Stenella, Gray; widely distributed in deep water. The zooids are large and are arranged in whorls of three situated at considerable distances apart. Stachyodes, W. and S.; Fiji, Kermadecs, Azores, in deep water. Colony feebly branched. Zooids in regular whorls of five. Other genera belonging to this group of Primnoidae are Thouarella, Gray, and Amphilaphis, Antarctic seas.
The following genera are placed in separate sub-families:—Callozostron, Wright; Antarctic Sea, 1670 fathoms. The axis is procumbent and the zooids are thickly set in rows on its upper surface. The zooids are protected by large imbricate scales, of which those of the last row are continued into long spine-like processes. Calyptrophora, Gray; Pacific Ocean, in deep water. The base of the zooids is protected by two remarkably large scales. Primnoides, W. and S.; Southern Ocean. The opercular scales are not distinctly differentiated and the calyx is therefore imperfectly protected.
Fam. 3. Chrysogorgiidae.[384]—The axis in this family is composed of a horny fibrous substance with interstratified calcareous particles, and it springs from a calcareous plate, which sometimes gives off root-like processes. It may be unbranched or branched in such a way that the branches of the second, third, and subsequent orders assume in turn the direction of the base of the main axis. The axis is frequently of a metallic iridescent appearance. The zooids usually arise in a single straight or spiral row on the branches, and are not retractile. The coenenchym is thin. The spicules vary considerably, but in a very large proportion of the species they are thin, oval, or hour-glass plates (Fig. 149, 10, p. 336).
By some authors this family is considered to be the simplest and most primitive of the Axifera; but the delicate character of the axis of the main stem and branches, the thinness of the coenenchym, the position of the zooids on one side of the branches only, and the tenuity of the calcareous spicules may be all accounted not as primitive characters, but as special adaptations to the life in the slow uniform currents of deep water.
The principal genera are:—Lepidogorgia, Verrill; Atlantic and Pacific Oceans, 300 to 1600 fathoms. Axis unbranched. Zooids large and arranged in a single row. Trichogorgia, Hickson; Cape of Good Hope, 56 fathoms. Colony branching in one plane. Zooids numerous and on all sides of the branches. Chrysogorgia, D. and M.; deep water. Axis branched. Spicules on the zooids always large. Metallogorgia, Versluys; Atlantic Ocean, 400 to 900 fathoms. Basal part of the stem unbranched (monopodial). Iridogorgia, Verrill. Spiral stem and branches. Pleurogorgia, Versluys. Axis branched in one plane. Coenenchym thick. Riisea, D. and M. Monopodial stem and thick coenenchym.
Fam. 4. Muriceidae.—This is a large family, exhibiting very great variety of habit. The spicules are often very spiny, and project beyond the surface of the ectoderm, giving the colony a rough appearance. A great number of genera have been described, but none of them are very well known. The family requires careful revision.
The more important genera are:—Acanthogorgia, Gray; principally in deep water in the Atlantic Ocean. The calices are {356}large, cylindrical, and spiny. Villogorgia, D. and M.; widely distributed. Delicate, graceful forms, with thin coenenchym. Echinomuricea, Verrill; Muricea, Lamouroux; Paramuricea, Köll; Acamptogorgia, W. and S.; Bebryce, Philippi.
Fam. 5. Plexauridae.—In this family we find some of the largest and most substantial Gorgonids. The axis is usually black, but its horny substance may be impregnated with lime, particularly at the base. The coenenchym is thick, and the zooids are usually completely retractile, and the surface smooth. The species of the family are principally found in shallow water in warm or tropical regions.
The principal genera are:—Eunicea, Lamouroux. The calices are prominent, and not retractile. Plexaura, Lamouroux; Euplexaura, Verrill. Eunicella, Verrill. With an outer layer of peculiar torch-shaped spicules. The only British species of this order is Eunicella cavolini (formerly called Gorgonia verrucosa). It is found in depths of 10 to 20 fathoms off the coast of the English Channel and west of Scotland. Occasionally specimens are found in which a gall-like malformation with a circular aperture is seen, containing a Barnacle. Such gall formations, common enough in some species of Madreporaria, are rarely found in Alcyonaria.
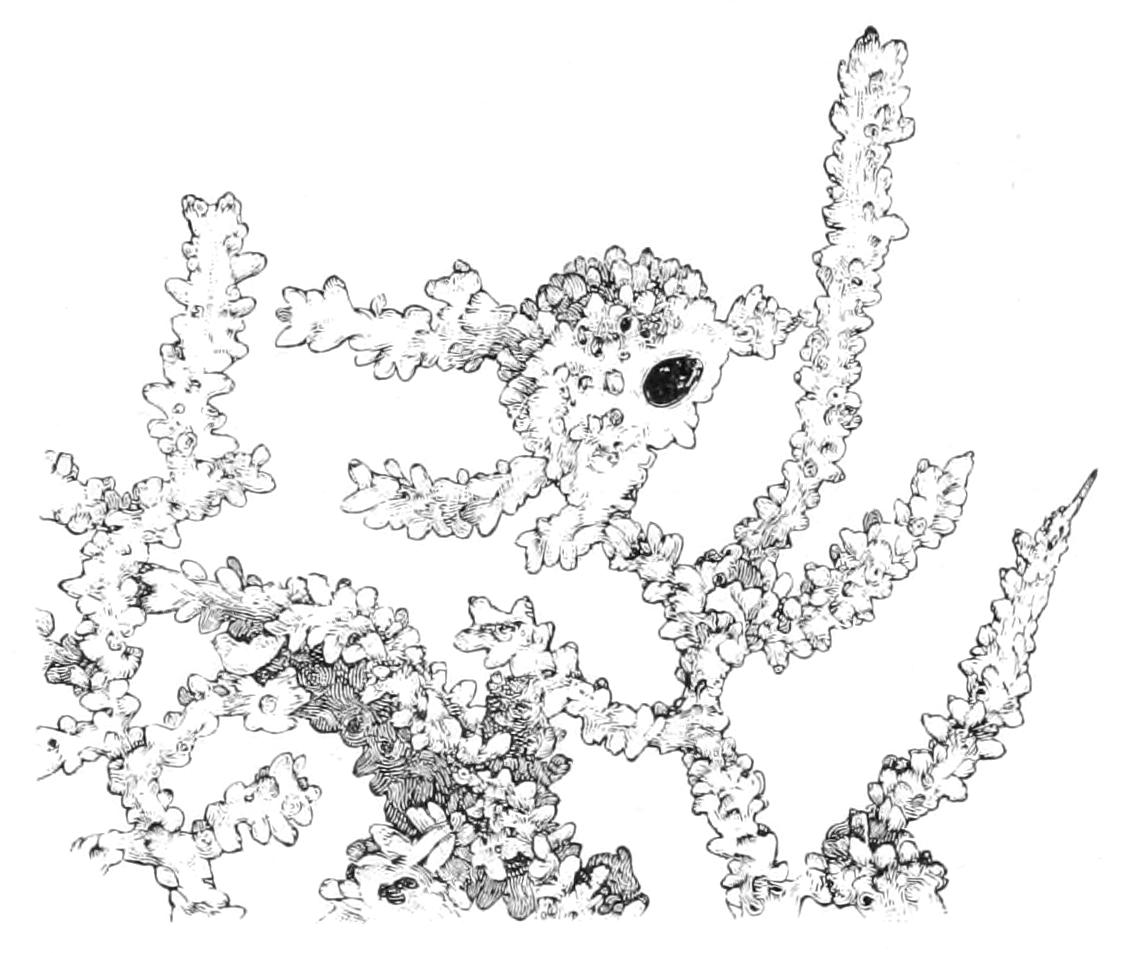
Fig. 156.—Eunicella cavolini. Some branches of a large dried specimen, showing a gall formed by a Cirripede.
Fam. 6. Gorgoniidae.—This family contains some of the commonest and best-known genera of the order. They usually form large flexible branched colonies with delicate horny axes and thin coenenchym. The zooids are usually completely retractile.
The principal genera are:—Gorgonia, Linn. This genus {357}includes Gorgonia (Rhipidogorgia) flabellum, the well-known fan Gorgonia with intimately anastomosing branches, from the warm waters of the Atlantic Ocean. The genera Eugorgia, Verrill, and Leptogorgia, Milne Edwards, differ from Gorgonia in the character of the spicules. In Xiphigorgia, Milne Edwards, from the West Indies, the branches are much compressed, forming at the edges wing-like ridges, which bear the zoopores in rows. Malacogorgia, Hickson, has no spicules. Cape of Good Hope.
Fam. 7. Gorgonellidae.—In this family the horny axis is impregnated with lime. The surface of the coenenchym is usually smooth, and the spicules small. The colonies are sometimes unbranched (Juncella). In the branching forms the axis of the terminal branches is often very fine and thread-like in dimensions.
The principal genera are:—Gorgonella, with a ramified flabelliform axis; Ctenocella, with a peculiar double-comb manner of branching; and Juncella, which forms very long unbranched or slightly branched colonies, with club-shaped spicules. All these genera are found in shallow water in the tropical or semi-tropical regions of the world. Verrucella is a genus with delicate anastomosing branches found principally in the shallow tropical waters of the Atlantic shores. Like many of the Gorgonacea, with branches disposed in one plane (flabelliform) Verrucella frequently carries a considerable number of epizoic Brittle stars, which wind their flexible arms round the branches, and thus obtain a firm attachment to their host. There is no reason to suppose that these Brittle stars are in any sense parasitic, as a specimen that bears many such forms shows no sign of injury or degeneration, and it is possible they may even be of service to {358}the Verrucella by preying upon other organisms that might be injurious. An interesting feature of the association is that the Brittle stars are of the same colour as the host, and the knob-like plates on their aboral surface have a close resemblance to the verrucae (Fig. 157).
Order V. Pennatulacea.
The Sea-pens form a very distinct order of the Alcyonaria. They are the only Alcyonarians that are not strictly sedentary in habit, that are capable of independent movement as a whole, and exhibit a bilateral symmetry of the colony. No genera have yet been discovered that can be regarded as connecting links between the Pennatulacea and the other orders of the Alcyonaria. Their position, therefore, is an isolated one, and their relationships obscure.
The peculiarities of the order are due to the great growth and modification in structure of the first formed zooid of the colony. This zooid (Oozooid, Hauptpolyp, or Axial zooid) increases greatly in length, develops very thick fleshy walls, usually loses its tentacles, digestive organs, and frequently its mouth, exhibits profound modification of its system of mesenteries, and in other ways becomes adapted to its function of supporting the whole colony.
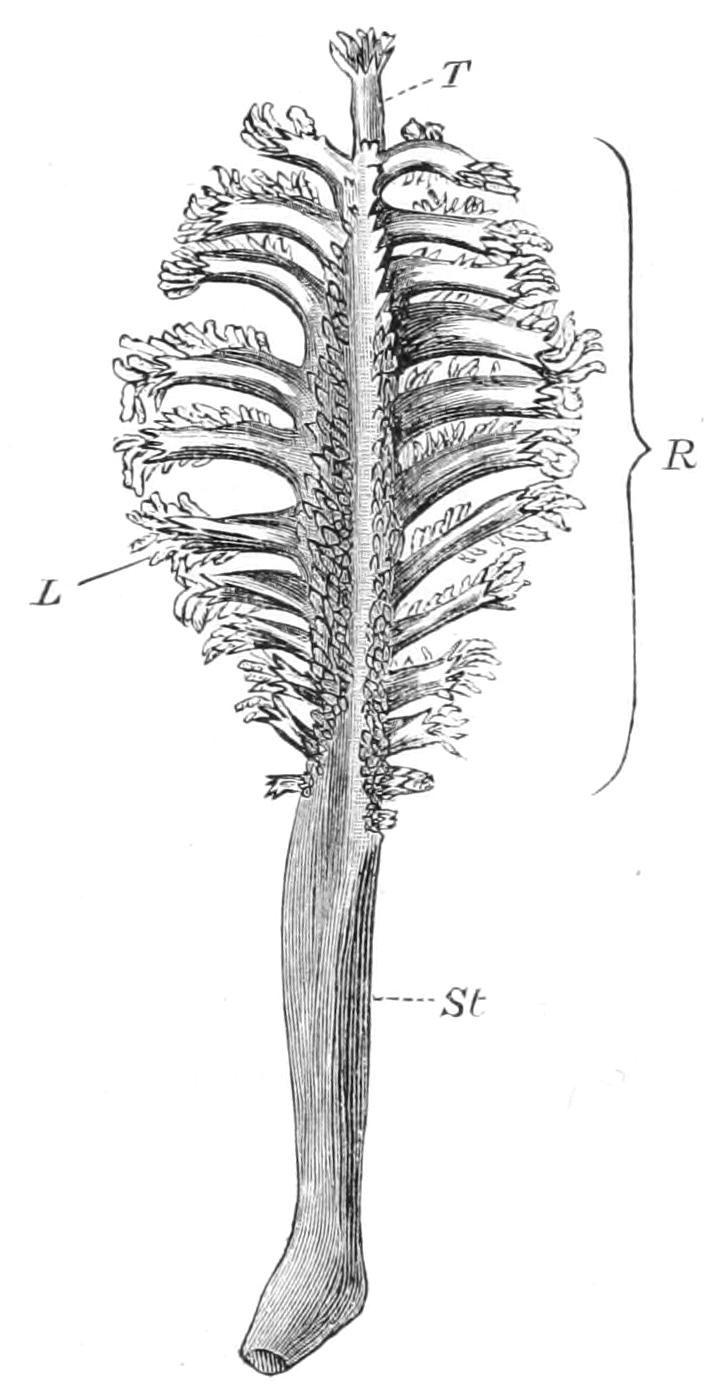
Fig. 158.—Diagram of a Sea-pen. L, leaves composed of a row of autozooids; R, rachis; St, stalk; T, anthocodia of the axial zooid, usually suppressed. (After Jungersen.)
The axial zooid shows from an early stage of development a division into two regions: a distal region which produces by gemmation on the body-wall numerous secondary zooids, and becomes the rachis of the colony; and a proximal region which becomes the stalk or peduncle, and does not produce buds (Fig. 158). The secondary zooids are of two kinds: {359}the autozooids and the siphonozooids. The former have the ordinary characters of an Alcyonarian zooid, and produce sexual cells; the latter have no tentacles, a reduced mesenteric system, and a stomodaeum provided with a very wide siphonoglyph.
The arrangement of the autozooids and siphonozooids upon the axial zooid is subject to great modifications, and affords the principal character for the classification of the order. In the Pennatuleae the autozooids are arranged in two bilaterally disposed rows on the rachis, forming the leaves or pinnae of the colony (Fig. 158). The number in each leaf increases during the growth of the colony by the addition of new zooids in regular succession from the dorsal to the ventral side of the rachis[385] (Fig. 159). In other Pennatulacea the autozooids are arranged in rows which do not unite to form leaves (Funiculina), in a tuft at the extremity of a long peduncle (Umbellula), scattered on the dorsal side of the rachis (Renilla, Fig. 160), or scattered on all sides of the rachis (Cavernularia, Fig. 161). In those forms in which the autozooids are scattered the bilateral symmetry of the colony as a whole becomes obscured. The siphonozooids may be found on the leaves (Pteroeides), but more frequently between the leaves or rows of autozooids, or scattered irregularly among the autozooids. Usually the siphonozooids are of one kind only, but in Pennatula murrayi there is one specially modified siphonozooid at the base of each leaf,[386] which appears to have some special but unknown function.
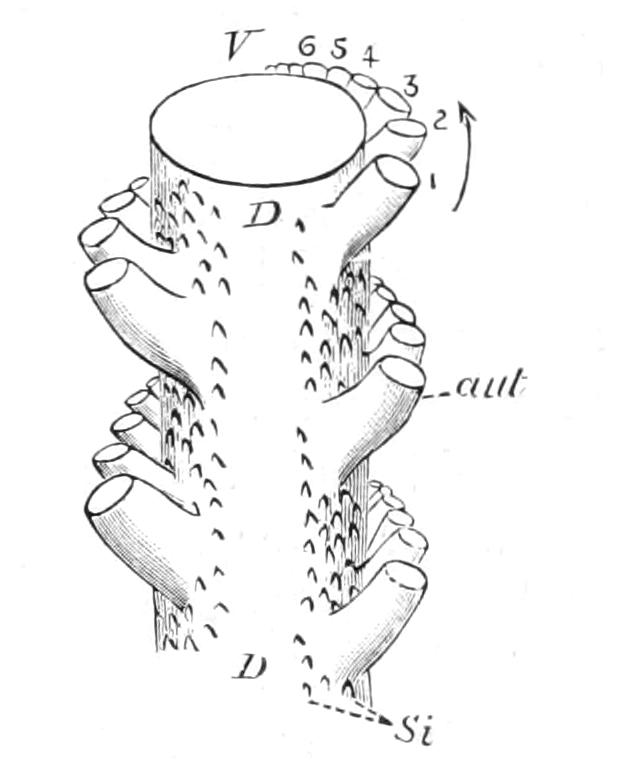
Fig. 159.—Diagram of a portion of a rachis of a Sea-pen, aut, The rows of autozooids; 1-6, the order of age of the autozooids composing a leaf; D, the dorsal side of the rachis; Si, the siphonozooids; V, the ventral side of the rachis. (After Jungersen.)
In Umbellula gracilis each siphonozooid bears a single pinnate tentacle, and in some other species of the same genus there is a tentacle which is not pinnate.[387]
The zooids and coenenchym are usually protected by a crust of coloured or colourless, long, smooth, needle-like, calcareous spicules, situated principally in the superficial layer, so as to leave the subjacent tissues soft and spongy in texture. In some cases the spicules are smooth double clubs, rods, discs, or irregular granules, and in Sarcophyllum, Chunella, some species of Umbellula and others, there is no calcareous skeleton. The tuberculated spindles, so common in other Alcyonaria, are not found in any species. In most genera a horny, or calcified horny rod is embedded in the central part of the axial polyp, serving as a backbone or support for its muscles. It is absent, however, in Renilla, and reduced or absent in Cavernularia.
The sexual organs are borne by the mesenteries of the autozooids only, and each colony is either male or female. There is no record of hermaphroditism in the order. The eggs contain a considerable amount of yolk, and fertilisation is effected in the sea-water after their discharge. The segmentation is irregular, and the free-swimming ciliated larva (of Renilla) shows the rudiments of the first buds from the axial polyp before it settles down in the mud.
The Sea-pens are usually found on muddy or sandy sea-bottoms, from a depth of a few fathoms to the greatest depths of the ocean. It is generally assumed that their normal position is one with the peduncle embedded in the mud and the rachis erect. Positive evidence of this was given by Rumphius, writing in 1741, in the case of Virgularia rumphii and V. juncea at Amboina,[388] and by Darwin in the case of Stylatula darwinii at Bahia Blanca.[389]
"At low water," writes Darwin, "hundreds of these zoophytes might be seen projecting like stubble, with the truncate end upwards, a few inches above the surface of the muddy sand. When touched or pulled they suddenly drew themselves in with force so as nearly or quite to disappear."
It is not known whether the Pennatulids have the power of moving from place to place when the local conditions become unfavourable. It is quite probable that they have this power, but the accounts given of the Sea-pens lying flat on the sand do not appear to be founded on direct observation. The fable of {361}Pennatula swimming freely "with all its delicate transparent polypi expanded, and emitting their usual brilliant phosphorescent light, sailing through the still and dark abyss by the regular and synchronous pulsations of the minute fringed arms of the whole polypi," appears to be based on a statement made by Bohadsch in 1761, and picturesque though it be, is undoubtedly erroneous.
The brilliant phosphorescence of many species of Pennatulacea has been observed by many naturalists, and it is very probable that they all exhibit this property to some degree. The phosphorescence appears to be emitted by the mesenteric filaments of the autozooids, but it is not yet determined whether the phenomenon is confined to these organs or is more generally distributed.
The Pennatulacea are usually devoid of epizoites, but occasionally the parasitic or semi-parasitic Entomostracan Lamippe is found in the zooids. A small crab is also frequently found between the large leaves of species of Pteroeides. The most remarkable case of symbiosis, however, has recently been observed in the form of an encrusting Gymnoblastic Hydroid[390] living on the free edge of the leaves of a species of Ptilosarcus.
The order Pennatulacea is divided into four sections.
Sect. 1. Pennatuleae.—In this section the colony is distinctly bilaterally symmetrical, and the autozooids are arranged in rows with their body-walls fused to form leaves.
The genus Pteroeides, the representative genus of the family Pteroeididae, is a fleshy Sea-pen found in shallow sea water in the warm waters of the Pacific Ocean and in the Mediterranean. It has large leaves with long spiny, projecting spicules, and the siphonozooids are borne by the leaves. Pennatula, the representative genus of the family Pennatulidae, has a wider distribution in area and in depth. Pennatula phosphorea is a common British species, found in depths of 10 to 20 fathoms in many localities off our coasts. It is about 5 inches in length. There are several varieties of this species distributed in Atlantic waters. Pennatula grandis is a magnificent species found in Norwegian fjords, in the Faeroe Channel, and off the northern coasts of N. America, in depths of from 50 to 1255 fathoms. Specimens have been {362}obtained no less than 2½ feet in length. P. murrayi and P. naresi are species of the genus found at depths of a few hundred fathoms in tropical seas.
The genus Virgularia, belonging to the family Virgulariidae, is represented in the British seas by V. mirabilis, a long slender Sea-pen found in many localities off the Scottish coasts.
Sect. 2. Spicatae.—This section includes those Sea-pens in which the autozooids are arranged bilaterally on the axial zooid in rows or more irregularly, but do not unite to form leaves. It is a large section and contains many widely divergent genera.
The family Funiculinidae is represented on our coasts by Funiculina quadrangularis, a long and slender Sea-pen 2 to 3 feet in length. The autozooids are arranged in oblique rows, and the siphonozooids are on the ventral side of the rachis. There is one point of special interest in this genus. The siphonozooids appear to change as the colony grows and to become autozooids. If this is the case it may be more correct to describe the genus as devoid of true siphonozooids.
The family Anthoptilidae contains the species Anthoptilum grandiflorum, which has a wide distribution in depths of 130 to 500 fathoms in the N. and S. Atlantic Ocean. It is perhaps the largest of all the Pennatulacea, specimens having been obtained from the Cape of Good Hope over 4 feet long with expanded autozooids, each more than half an inch in length.
The family Kophobelemnonidae contains a number of forms with remarkably large autozooids arranged in irregular rows on the two sides of the rachis. The siphonozooids are numerous and scattered, and their position is indicated by small papilliform calices on the coenenchym. The surface of these pens is usually rough, owing to the presence of numerous coarse projecting spicules. Kophobelemnon occurs in the Mediterranean in deep water, off the coasts of Ireland and Scotland, and in other regions.
The family Umbellulidae contains some of the most remarkable and interesting examples of the deep-sea fauna. The peduncle is very long and the rachis stunted and expanded. The autozooids are of great size, non-retractile, and arranged in a cluster or rosette on the terminal rachis. There is a wide structural range between the species. Some species have numerous large spicules, others have none. In some species the siphonozooids have a single pinnate or digitate tentacle, in others the siphonozooids {363}are of the usual type. Umbellula appears to be a somewhat rare but cosmopolitan genus in deep water, extending from the Arctic to the Antarctic region in water ranging from 200 to 2500 fathoms.
The interesting genus Chunella was discovered by the German "Valdivia" Expedition at a depth of about 420 fathoms off the coast of E. Africa, and subsequently by the Dutch "Siboga" Expedition at a depth of about 500 fathoms in the Malay Archipelago. According to Kükenthal,[391] this genus with another closely allied genus Amphianthus should form a new section of Pennatulacea, the Verticilladeae. Chunella has a long and very delicate rachis and peduncle, and the former terminates in a single autozooid and has five or six whorls of three autozooids, situated at considerable distances from one another. Spicules are absent. The full description of this genus has not yet been published, but it is clear that it occupies a very isolated position in the order.
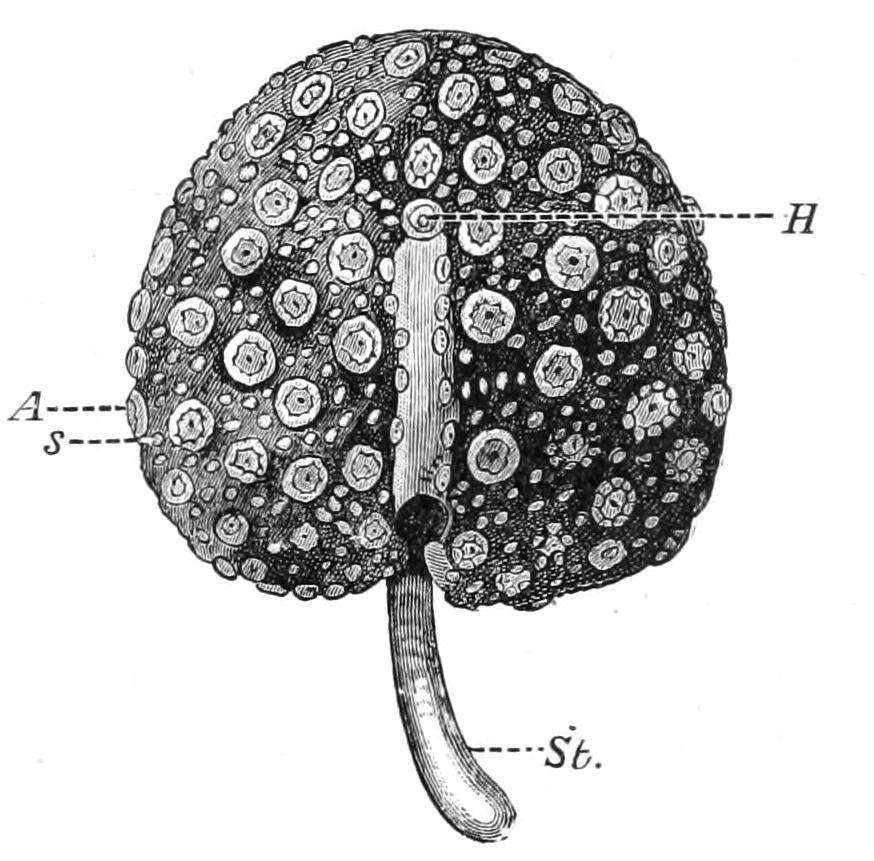
Fig. 160.—Renilla reniformis, a small specimen (34 mm.), showing the dorsal side of the expanded rachis. A, autozooid; H, the mouth of the axial zooid; s, siphonozooid; St, the short stalk. (After Kölliker.)
Sect. 3. Renilleae.—This section contains a single family Renillidae and a single genus Renilla (Fig. 160). The rachis is expanded into a flattened cordate form set at an angle to the peduncle, and the zooids are confined to the dorsal surface, which is uppermost in the natural position of the colony. The peduncle is short and does not contain an axial skeleton. The colour of {364}this Sea-pen is usually violet when dried or preserved. Specimens of Renilla are very abundant in shallow water in some localities on the Atlantic and Pacific coasts of N. America, but the genus has also been obtained from the Red Sea and the coast of Australia. A popular name for this genus is "Sea pansy."
Sect. 4. Veretilleae.—This section contains a number of genera in which the bilateral arrangement of the zooids is obscured by their gradual encroachment on the dorsal side of the axial polyp. The rachis and peduncle are thick and fleshy, and the autozooids and siphonozooids are irregularly distributed all round the rachis. The genus Cavernularia is not uncommonly found in moderate depths of water in the Indian and Pacific Ocean, and is distinguished from the other genera by the reduction of the skeletal axis. Other genera are Veretillum, Mediterranean and Atlantic Ocean, and Lituaria, Indian Ocean.
ANTHOZOA (CONTINUED): ZOANTHARIA
Sub-Class II. Zoantharia.
The Zoantharia exhibit a great deal more diversity of form and structure than the Alcyonaria. The sub-class is consequently difficult to define in a few words, and it may be taken to include all the Anthozoa which do not possess the typical Alcyonarian characters.
All the orders, with the exception of the Antipathidea and Zoanthidea, contain genera of solitary zooids, and the orders Edwardsiidea and Cerianthidea contain no genera that form colonies. In the Madreporaria, Zoanthidea, and Antipathidea, on the other hand, colonies are formed composed of a very large number of individuals which frequently attain to a very great size. The term "Sea-anemone" is commonly used in writing about the solitary Zoantharia which do not form any skeletal structures, and the term "Coral" is applied to all those Zoantharia which do form a skeleton.
In a scientific treatise, however, these popular terms can no longer be satisfactorily employed. The "Sea-anemones" exhibit so many important differences in anatomical structure that they must be placed in at least three distinct orders that are not closely related, and the organisms to which the term Coral has been applied belong to so many organisms—such as Alcyonaria, Hydrozoa, Polyzoa, and even Algae—that its use has become indeterminate.
Whilst these terms must disappear from the systematic part of Zoology, they may still be employed, however, in the description of a local fauna or coral reef to signify the soft solitary zooids on {366}the one hand, and the organisms, animals or plants, which form large, massive skeletons of carbonate of lime, on the other.
The form of the solitary zooids and of the colony of zooids in the Zoantharia, then, may be very divergent. In the Actiniaria we find single soft gelatinous zooids of considerable size adherent to rocks or half-buried in the sand. Among the Madreporaria we find great branching colonies of thousands of zooids supported by the copious skeleton of carbonate of lime that they have secreted. Among the Antipathidea, again, we find a dendritic skeleton of a dark horny substance, formed by a colony of small zooids that cover it like a thin bark. The majority of the Zoantharia are, like other zoophytes, permanently fixed to the floor of the ocean. Where the embryo settles, there must the adult or colony of adults remain until death. Some of the common Sea-anemones can, however, glide slowly over the surface on which they rest, and thus change their position according to the conditions of their surroundings. Others (the Minyadidae) float upside down in the sea, and are carried hither and thither by the currents. Others, again (Cerianthus, Edwardsia, Peachia), burrow in the sand or mud at the sea-bottom.
The structure of the zooid varies considerably, but in the following characters differs from the zooid of the Alcyonaria. The tentacles are usually simple finger-like processes, and when they bear secondary pinnae these can readily be distinguished from the rows of secondary pinnules of the Alcyonarian tentacle. The number of tentacles is very rarely eight (young Halcampa), and in these cases they are not pinnate. The number of tentacles may be six (many Antipathidea and some zooids of Madrepora), twelve (Madrepora), some multiple of six, or an indefinite number. In the Thalassianthidae and some other families of Actiniaria the tentacles are plumose, but do not exhibit the regular pinnate form of the tentacles of Alcyonaria.
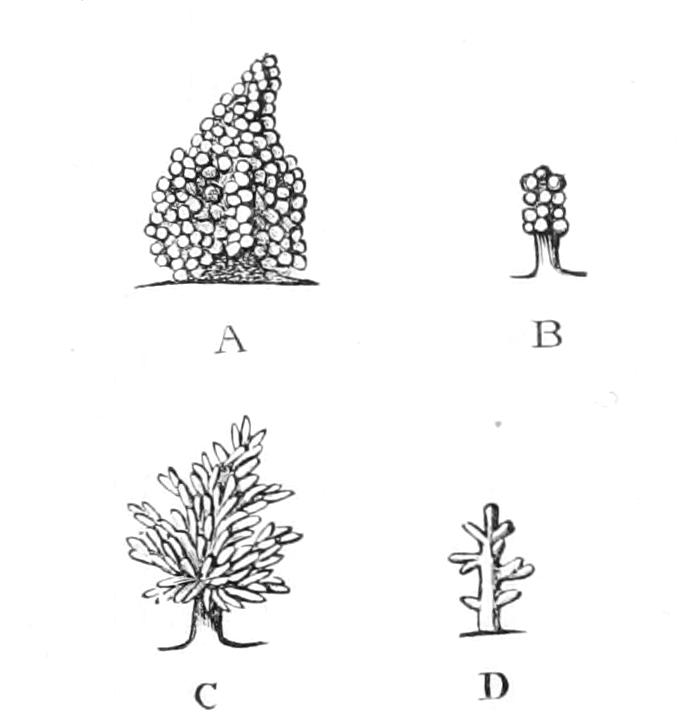
Fig. 162.—Large (A) and small (B) plumose tentacles of Actinodendron plumosum. Large (C) and small (D) plumose tentacles of A. glomeratum. (After Haddon.)
As regards the number of mesenteries, the Zoantharia exhibit {367}very great variety. It has been shown that there is frequently a stage in their development during which there are only eight mesenteries. This stage is usually called the Edwardsia stage. These eight mesenteries are arranged in bilateral pairs as follows:—One pair is attached to the body-wall and reaches to the dorsal side of the stomodaeum, and is called the pair of dorsal directives; a corresponding pair attached to the ventral side of the stomodaeum is called the pair of ventral directives. The other two pairs are the lateral mesenteries. To these four pairs are added, at the close of the Edwardsia stage, two additional pairs, making in all twelve mesenteries (cf. Fig. 163).
These six primary pairs of mesenteries, conveniently called the "protocnemes" by Duerden, may be traced in the development and recognised in the adult of the majority of Zoantharia. But the number of the mesenteries is usually increased in the later stages by the addition of other mesenteries called the "metacnemes." The metacnemes differ from the protocnemes in that they usually appear in unilateral pairs, that is to say, in pairs of which both members arise on the same side of the stomodaeum, and the number is very variable throughout the group. The space enclosed by a pair of mesenteries is called an "entocoele," and the space between two pairs of mesenteries is called an "ectocoele."
The twelve protocnemes are usually complete mesenteries, that is to say, they extend the whole distance from the body-wall to the stomodaeum, while the metacnemes may be complete or incomplete; in the latter case extending only a part of the distance from the body-wall towards the stomodaeum.
We find, therefore, in making a general survey of the anatomy of the Zoantharia that there is no general statement to be made, concerning the number or arrangement of the mesenteries, which holds good for the whole or even for a considerable portion of the genera.
The bands of retractor muscles are, as in the Alcyonaria, situated on one face only of the mesenteries (except in the Antipathidea and Cerianthidea), but an important character of the Zoantharia is that the muscle bands on the ventral pair of directives are situated on the dorsal faces of these mesenteries, and not on the ventral faces as they are in Alcyonaria.
In the Edwardsiidea there are only eight complete mesenteries, {368}but a variable number of other rudimentary and incomplete mesenteries have recently been discovered by Faurot.[392] In the Zoanthidea the mesenteries are numerous, but the order is remarkable for the fact that the dorsal directives are incomplete, and that, of the pairs of metacnemes that are added, one mesentery becomes complete and the other remains incomplete. In most of the genera of the Antipathidea there are only ten mesenteries, but in Leiopathes there are twelve, and as they bear no bands of retractor muscles it is difficult to determine accurately their true relation to the mesenteries of other Zoantharia.
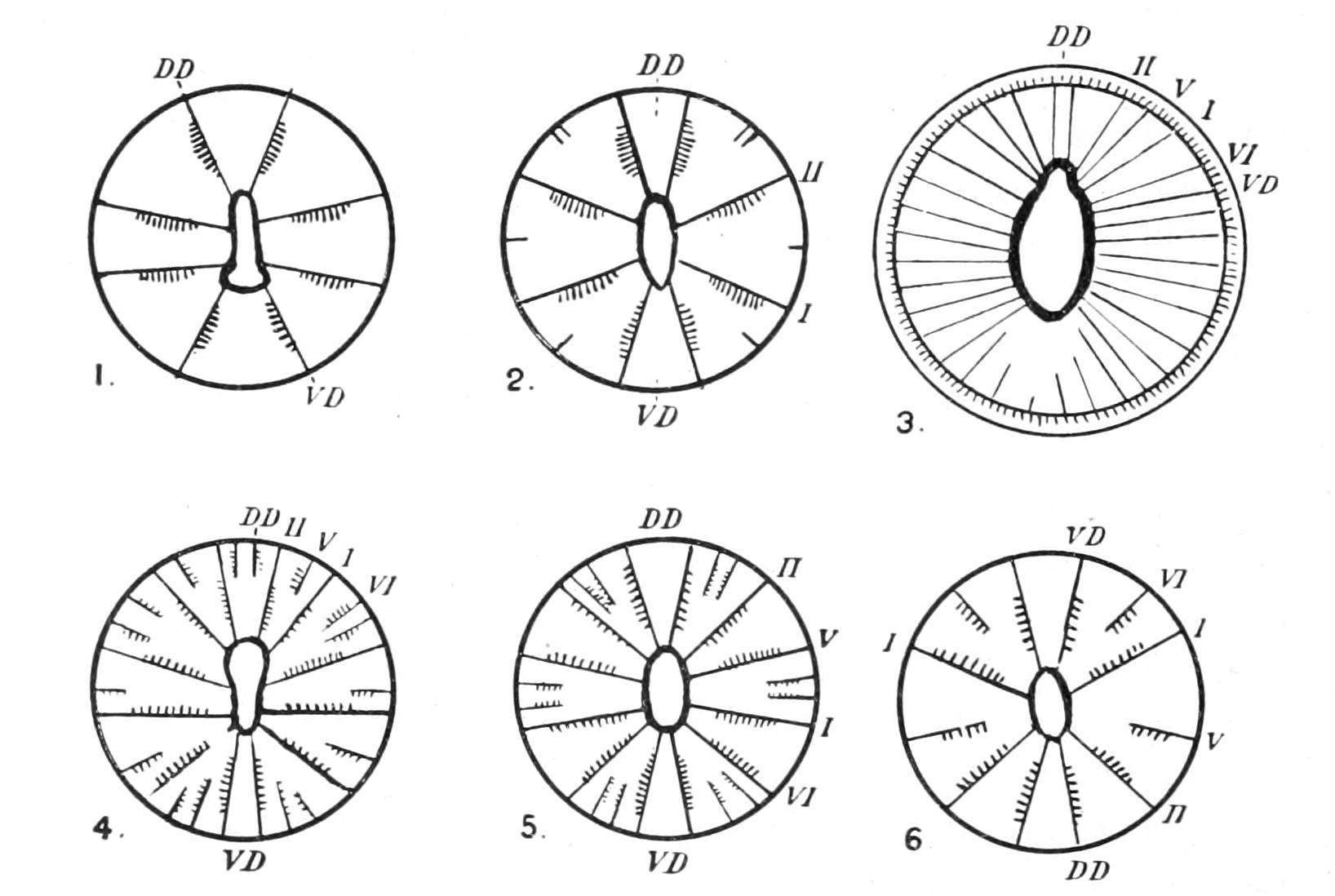
Fig. 163.—Diagrams of transverse sections of 1, Alcyonarian; 2, Edwardsia; 3, Cerianthus; 4, Zoanthus; 5, Favia; 6, Madrepora. DD, the dorsal directive mesenteries; VD, the ventral directives; I-VI, the protocnemes in order of sequence.
In the Cerianthidea the mesenteries are very numerous, and increase in numbers by the addition of single mesenteries alternately right and left in the ventral inter-mesenteric chamber throughout the life of the individual. These mesenteries do not bear retractor muscles.
In the Actiniaria and Madreporaria, with the exception of the genera Madrepora, Porites, and a few others, there are also very many mesenteries. The two pairs of directives are usually present, but they may not occur in those zooids that are produced {369}asexually by fission (see p. 388). The metacnemes are frequently formed in regular cycles, and in many genera appear to be constantly some multiple of six (Fig. 163, 5).
In Madrepora and Porites[393] the two pairs of directives and two pairs of lateral protocnemes are complete; the other two pairs of protocnemes are, however, incomplete; and metacnemes are not developed (Fig. 163, 6).
The stomodaeum is usually a flattened tube extending some distance into the coelenteric cavity and giving support to the inner edges of the complete mesenteries; in many of the Madreporaria, however, it is oval or circular in outline. In most of the Actiniaria there are deep grooves on the dorsal and ventral sides of the stomodaeum, but in Zoanthidea the groove occurs on the ventral side only and in the Cerianthidea on the dorsal side only. In the Madreporaria these grooves do not occur or are relatively inconspicuous.[394] In the Alcyonaria the siphonoglyph exhibits a very marked differentiation of the epithelium (see Fig. 148, p. 334), and the cilia it bears are very long and powerful. It has not been shown that the grooves in the Zoantharia show similar modifications of structure, and they are called by the writers on Zoantharia the sulci. There is no difference in structure, and rarely any difference in size, between the dorsal sulcus and the ventral sulcus in the Actiniaria, and the use of the word—sulculus—for the former is not to be commended.
The mesenteries bear upon their free edges the mesenteric filaments. These organs are usually more complicated in structure than the corresponding organs of the Alcyonaria, and the dorsal pair of filaments is not specialised for respiratory purposes as it is in that group.
In many genera the mesenteric filaments bear long, thread-like processes—the "acontia"—armed with gland cells and nematocysts which can be protruded from the mouth or pushed through special holes (the "cinclides") in the body-wall.
The gonads in the Zoantharia are borne upon the sides of the mesenteries and are usually in the form of long lobed ridges instead of being spherical in form, and situated at the edges of the mesenteries as they are in the Alcyonaria.
Nearly all the zooids and even the colonies of the Zoantharia are unisexual, but some species, such as Manicina areolata (Wilson), Meandrina labyrinthica (Duerden), Cerianthus membranaceus, and others, are hermaphrodite. Mr. J. S. Gardiner has recently given reasons for believing that the genus Flabellum is protandrous.
Skeleton.—The soft tissues of the Zoantharian zooids may be supported or protected by hard skeletal structures of various kinds. In the Zoanthidea and the Actiniaria there are many species that have no skeletal support at all, and are quite naked. These seem to be sufficiently well protected from the attacks of carnivorous animals by the numerous nematocysts of the ectoderm, and perhaps in addition by a disagreeable flavour in their tissues. Anemones do not seem to be eaten habitually by any fish, but cases have been described of Peachia hastata being found in the stomach of the Cod, and of Edwardsia in the stomach of the Flounder.[395] On the Scottish coasts Anemones are occasionally used with success as a bait for cod.[396] The body-wall of Edwardsia, however, is protected to a certain extent by the secretion of a mucous coat in which grains of sand and mud are embedded. Some Anemones, such as Urticina, Peachia, and others, lie half-buried in the sand, and others form a cuticle, like that of Edwardsia, to which foreign bodies are attached.
Cerianthus is remarkable for constructing a long tube composed of a felt-work of discharged nematocysts mixed with mud and mucus, into which it retires for protection. In the Zoanthidea the body-wall is frequently strengthened by numerous and relatively large grains of sand, which are passed through the ectoderm to lie in the thick mesogloea.
In the Madreporaria a very elaborate skeleton of carbonate of lime is formed. In the solitary forms it consists of a cup-shaped outer covering for the base and column of the zooid called the "theca," of a series of radial vertical walls or "septa" projecting into the intermesenteric chambers carrying the endodermal lining of the coelenteric cavity with them, and in some cases a pillar, the "columella," or a series of smaller pillars, the "pali" projecting upwards from the centre of the base of the {371}theca towards the stomodaeum. In the colonial forms the theca of the individual zooids is continuous with a common colonial skeleton called the "coenosteum." This is solid in the Imperforate corals, and it supports at the surface only a thin lamina of canals and superficial ectoderm. In the Perforate corals, however, the coenosteum envelopes and surrounds the canals during its formation, and thereby remains perforated by a network of fine channels. In the colonial Madreporaria the skeletal cups which support and protect the zooids are called the "calices."
The skeleton of the Antipathidea is of a different nature. It is composed of a horny substance allied to keratin. When it is old and thick, it usually has a polished black appearance, and is commonly termed "black coral." The surface of this kind of coral is ornamented with thorny or spiny projections, but it is never perforated by calices or canal systems. It forms a solid axis for the branches of the corals, and all the soft parts of the zooids and coenosarc are superficial to it.
It was formerly considered that this type of coral, which shows no trace of the shape and form of the living organisms that produce it, is of a different character to the calcareous skeleton which exhibits calices, septa, pores, and other evidence of the living organism, and it was called a "sclerobase" to distinguish it from the "scleroderm" of the Madreporaria.
It is now known that both the sclerobasic skeleton and the sclerodermic skeleton are products of the ectoderm, and consequently these expressions are no longer in general use.
Asexual reproduction in the Zoantharia may be effected by continuous or discontinuous fission or gemmation.
In the Edwardsiidea, Actiniaria, and Cerianthidea, that is to say in the animals popularly known as Sea-anemones, asexual reproduction does not commonly occur, but nevertheless a good many instances of it are now known in individual genera. In Actinoloba (Metridium), for example, Parker has described a case of complete longitudinal fission, and Duerden states that it occurs in the West Indian Anemones Actinotryx and Ricordea. A still more remarkable form of asexual reproduction known as transverse fission has been described in the genus Gonactinia.[397] In this case, the body of the Anemone becomes constricted in {372}the middle, a circlet of tentacles is formed below the constriction, and division takes place. The upper half floats away with the original tentacles and stomodaeum and becomes attached by the base in another place; the lower half remains behind and develops a new stomodaeum, mesenteric filaments, and sexual organs. In some of the Actiniaria another form of asexual reproduction occurs, known as "Pedal laceration." In the common British Actinoloba, for example, so often kept in aquaria, the pedal disc sometimes spreads on the glass or rock upon which the animal rests, in the form of a thin membrane or film of an irregular circular shape, nearly twice the diameter of the column. As the Anemone glides along, the film remains behind and breaks up into a number of hemispherical droplets, which in a few days develop tentacles, a mouth, mesenteries, and the other organs of a complete and independent Anemone. A similar method of reproduction has been observed in several species of Sagartia. A true process of discontinuous gemmation has also been observed in Gonactinia, in Corynactis, and in Actinoloba.
In the Madreporaria, Zoanthidea and Antipathidea, the usual method of reproduction to form the colonies is continuous gemmation. The new zooids that are added to the colony as it grows arise as buds, either from the superficial canals of the coenenchym, or from the base or body-wall of the older zooids. In these cases the young zooids acquire the same number of mesenteries, and the same characters of the stomodaeum as the original parent. Some further particulars of asexual reproduction in the Madreporaria are given on p. 387.
The sexual reproduction of a great many species of Zoantharia has now been observed. The eggs are, as a general rule, ripened in batches, and fertilisation is effected before their discharge from the body. In some cases the sexual condition is seasonal. In temperate climates the generative organs ripen in the spring and {373}summer months, and remain small and relatively inconspicuous in the colder weather; but British Sea-anemones, when kept in an aquarium and regularly fed, will breed nearly all the year round. The corals of the tropics living in warmer water of a more regular temperature show considerable variety in their breeding habits. Thus Duerden found that colonies of Favia, Manicina, Siderastraea and Porites are fertile at nearly all times, whereas colonies of Madrepora, Orbicella and Cladocora were rarely so. In nearly all cases the fertilisation is effected, and segmentation of the ovum occurs within the body of the parent, the young Zoantharian beginning its independent life as an oval or pear-shaped ciliated larva.
There are a great many cases among the Actiniaria in which the embryos are retained within the coelenteron, or in special brood pouches of the parent (p. 379), until a stage is reached with twelve or more tentacles.
The oval or pear-shaped larva swims about for a few days or hours, and then settles down on its aboral end. In swimming, the aboral end is always turned forwards. In the larva of Lebrunia coralligens and Rhodactis sancti-thomae, a distinct sense organ has been observed upon the aboral extremity, and a similar but less distinct organ on the larva of Actinia equina. These organs are of considerable interest, as they are probably the only specialised sense organs known to occur in the Zoantharia.
The larvae of Zoantharia present, as a rule, very little variation from the type described, and live but a short time if they fail to find a suitable place for fixation. The colour is usually white and opaque, but in some species the endoderm may be coloured yellow by Zooxanthellae (cf. pp. 86, 125).
The larvae of the Cerianthidea, however, are remarkable and exceptional. After the larva of these animals has passed through the gastrula stage, a certain number of mesenteries and tentacles are formed, and it rises in the water to live a pelagic life of some duration. This larva is known as Arachnactis, and is not unfrequently found in the plankton.
The character of the food of the Zoantharia varies with the size of the zooids, the occurrence of Zooxanthellae in the endoderm, and local circumstances; but in general it may be said to consist mainly of small living animals.
Sea-anemones kept in an aquarium will readily seize and devour pieces of raw beef or fragments of mussel that are offered to them; but they may also be observed to kill and swallow the small Crustacea that occur in the water. When a living animal of a relatively small size comes within range of the tentacles, it appears to be suddenly paralysed by the action of the nematocysts and held fast. The tentacles in contact with it, and others in the neighbourhood but to a lesser extent, then bend inwards, carrying the prey to the mouth. The passage of the food through the stomodaeum is effected partly by ciliary, and partly by muscular action, and the food is then brought to the region of the mesenteric filaments where it is rapidly disintegrated by the digestive fluids they secrete. Any unsavoury or undigested portions of the food are ejected by the mouth.
Very little is known concerning the food of the Madreporarian Corals. Many investigators have noticed that the zooids of preserved specimens very rarely contain any fragments of animal or plant bodies that could possibly be regarded as evidence of food. It is possible that many Corals derive a part, perhaps in some cases a considerable part, of their nourishment from the symbiotic Zooxanthellae (pp. 86, 125) which flourish in the endoderm; but it is improbable that in any case this forms the only source of food supply. The absence of food material in the cavities of the zooids may perhaps be accounted for by the fact that nearly all the Corals are fully expanded, and therefore capable of catching their food only at night. Corals are usually collected during the daytime, and therefore during the period of rest of the digestive organs.
It is true that nearly all Corals do exhibit Zooxanthellae in their endoderm, but there are some species from which they are nearly or wholly absent, such as Astrangia solitaria and Phyllangia americana on the West Indian reefs,[398] and the Pocilloporidae. The absence of any signs of degeneration in the tentacles or digestive organs of those corals with Zooxanthellae as compared with those without them suggests, at any rate, that the Zooxanthellae do not supply such a large proportion of the food necessary for the support of the colonies as to warrant any relaxation of the efforts to obtain food by other means. Mr. Duerden found that when living Annelids are placed upon the {375}tentacles of a living Siderastraea—a genus with Zooxanthellae, the tentacles at once close upon them and prevent their escape. The general conclusion seems to be, therefore, that the Madreporarian Corals feed upon small animals in much the same way as the Sea-anemones, whether they have Zooxanthellae or not, but that in general they feed only at night.
Age.—It is known that Sea-anemones kept in an aquarium and regularly fed will live for a considerable number of years without showing signs of weakness or failing health. Dalyell kept in an aquarium a specimen of Actinia mesembryanthemum, which lived for sixty-six years and then died a natural death; and specimens of Sagartia, still living, are known to be about fifty years old.[399] The unnatural conditions of life in an aquarium may have favoured the longevity of these specimens, and it would not be reasonable to conclude from these records that the average life of a full-grown Anemone on the rocks is more than thirty or thirty-five years, and perhaps it is a good deal less.
As regards the Madreporarian Corals, we know but little concerning their duration of life. An examination of any living coral reef is sufficient to convince an observer that the power of asexual reproduction of the colonial forms is not unlimited; that colonies, like individuals, have a definite span of life, and that they grow old, senile, and then die a natural death if spared in their youth from accident and disease. Mr. Gardiner has calculated that the duration of life in solitary Corals like Flabellum is about twenty-four years, in colonial forms such as Goniastraea, Prionastraea, Orbicella, and Pocillopora, from twenty-two to twenty-eight years.
Order I. Edwardsiidea.
This order contains only a few genera and species of small size living in shallow water in various parts of the world. In external features they closely resemble several genera of the Actiniaria, particularly those belonging to the family Halcampidae. The distinguishing character of the order is to be found in the system of mesenteries. In all the species only eight mesenteries are complete, namely, the first two pairs of protocnemes, and the two pairs of directives (Fig. 163, 2), {376}and these usually support such large and powerful muscle-bands that they appear to be the only mesenteries present. A careful examination of transverse sections, however, reveals the fact that other mesenteries are present. The fifth and sixth pairs of protocnemes seem to be invariably represented, and two or three pairs of metacnemes can also be traced in some species.
The tentacles are variable in number. In Edwardsia beautempsii, for example, they may be 14-16 in number, arranged in a single row round the oral disc. In E. timida they vary from 20 to 24. The normal number appears to be eight tentacles of the first cycle, corresponding to the eight primary inter-mesenteric chambers, plus 6 or 12 tentacles, corresponding with the chambers limited by the more rudimentary mesenteries,—making a total of 14 or 20 tentacles; but by the suppression of the two primary dorso-lateral tentacles, or by the addition of tentacles of another cycle, the actual number is found to vary considerably. The Edwardsiidea are not fixed to the bottom, but are usually found deeply embedded in sand, the aboral extremity being pointed and used for burrowing purposes. The general colour of the body is yellow or yellowish brown, but it is partly hidden by a short jacket of mud or sand and mucous secretion. The oral crown frequently shows beautiful colours. De Quatrefages relates that in Edwardsia beautempsii the oral cone is golden yellow, and the tentacles, transparent for the greater part of their extent, terminate in opaque points of a beautiful yellowish red colour.
Fam. 1. Edwardsiidae.—Several species of this family have been found in the British area. They are very local in their distribution, but sometimes occur in great numbers.
Edwardsia beautempsii occurs in shallow water near the shores of the English Channel and has been found in Bantry Bay; and E. carnea and E. timida have also been found in the Channel. E. tecta is a recently described species from the S. Irish coast, and E. allmani and E. goodsiri are found in Scottish waters.
Fam. 2. Protantheidae.—This family, constituted for the reception of three remarkable genera, is now usually included in the order Edwardsiidea on the ground that not more than eight mesenteries are complete.
The genus Gonactinia exhibits the very exceptional character of having a thick layer of muscles in the body-wall (cf. Cerianthidea, p. 409), and it is also remarkable for the frequency with which it reproduces itself asexually by longitudinal and, more rarely, by transverse fission. It has been found in Norway, the Mediterranean, and on the reefs of New Caledonia. The other genera of the family are Oractis from California, and Protanthea from the coast of Sweden.
Order II. Actiniaria.
This order contains nearly all the animals popularly known as Sea-anemones. They are usually found in shallow water, attached by a broad basal disc to shells, stones, or sea-weeds. In the Halcampidae, however, the aboral extremity ends in a blunt point as in the Cerianthidea and Edwardsiidea, and the animals live half-buried in sand or mud. The Minyadidae of the southern oceans are pelagic in habit, floating near the surface of the sea with the mouth turned downwards. They are supported in the water by a bladder, formed by an involution of the pedal disc, and filled with gas.
Many of the Sea-anemones are found in symbiotic association with other animals. The common Adamsia of the British coasts is found on whelk shells containing hermit crabs. The crab is probably protected from the attacks of some of its enemies by the presence of the Anemone, which in its turn has the advantage of securing some fragments of the food captured and torn to {378}pieces by the crab. The association, therefore, seems to be one of mutual advantage to the messmates. It is a noteworthy fact that in these associations the species of Sea-anemone associated with a particular hermit crab is nearly always constant. Thus in the English Channel, Adamsia palliata is almost invariably found associated with Eupagurus prideauxii, and Adamsia rondeletii with Eupagurus bernhardus. But, perhaps, the most remarkable association of this kind is to be seen in the case of the little shore crab of the Indian Ocean, Melia tesselata, which invariably holds in each of its large claws a small Sea-anemone. Möbius, who originally described this case, relates that when the crab is robbed of its Anemone it appears to be greatly agitated, and hunts about on the sand in the endeavour to find it again, and will even collect the pieces, if the Anemone is cut up, and arrange them in its claw.[400]
Another very interesting association is that of certain fish and Crustacea with the large Sea-anemones of the tropical Australian coast.[401] Thus Stoichactis kenti almost invariably contains two or more specimens of the Percoid fish Amphiprion percula. This fish is remarkable for its brilliant colour, three pearly white cross-bands interrupt a ground plan of bright orange-vermilion, and the ends of the cross-bands as well as the fins are bordered with black. In another species a prawn of similar striking colours is found. These companions of the giant Anemones swim about among the tentacles unharmed, and when disturbed seek refuge in the mouth. It has been suggested that these bright and attractive animals serve as a lure or bait for other animals, which are enticed into striking distance of the stinging threads of the Anemone, but how the commensals escape the fate of the animals they attract has yet to be explained.
In a considerable number of Sea-anemones, such as Actinoloba marginata and A. dianthus, some species of Sagartia, Actinia cari, Anemonia sulcata, and Calliactis parasitica, the fertilisation of the eggs and their subsequent development take place in the sea water.[402] In a great many others, such as Bunodes (several species), Cereactis aurantiaca, Sagartia troglodytes, Bunodactis {379}gemmacea, etc., the embryos are discharged into the water from the body-cavity of the parent, at a stage with six or twelve tentacles. In the Arctic species of the genera Urticina and Actinostola, however, the embryos are retained within the body of the parent until several cycles of tentacles are developed, and in Urticina crassicornis the young have been found with the full number of tentacles already formed. In Epiactis prolifera from Puget Sound, the young Anemones attach themselves to the body-wall of the parent after their discharge, and in Epiactis marsupialis, Pseudophellia arctica, Epigonactis fecunda, and other species from cold waters, the young are found in numerous brood sacs opening in rows on the body-wall. It is not known for certain how these embryos enter the brood sacs, but it is possible that each sac is formed independently for a young embryo that has settled down from the outside upon the body-wall of the parent. The most specialised example of this kind of parental care in the Sea-anemones is seen in Marsupifer valdiviae from Kerguelen, in which there are only six brood sacs, but each one contains a great many (50-100) embryos.
The wonderful colours of our British Sea-anemones are familiar to most persons who have visited the sea-side. The common Actinia mesembryanthemum of rock pools, for example, is of a purple red colour. The base is usually green with an azure line. Around the margin of the disc there are some twenty-five turquoise blue tubercles. On each side of the mouth there is a small purple spot, and the numerous tentacles forming a circlet round the mouth are of a pale roseate colour. Nothing could be more beautiful than the snowy-white Actinoloba dianthus or the variegated Urticina crassicornis.
Similar wonderful variety and beauty of colour are seen in the Sea-anemones of other parts of the world. Thus Saville Kent[403] in describing a species of the gigantic Stoichactis of the Australian Barrier Reef says, "the spheroidal bead-like tentacles occur in irregularly mixed patches of grey, white, lilac, and emerald green, the disc being shaded with tints of grey, while the oral orifice is bordered with bright yellow."
The order Actiniaria contains a large number of families, presenting a great variety of external form and of detail in general anatomy. The definitions of the families and their {380}arrangement in larger groups have presented many difficulties, and have led to considerable differences of opinion; and even now, although our anatomical knowledge has been greatly extended, the classification cannot be regarded as resting on a very firm basis. The families may be grouped into two sub-orders:—
Sub-Order 1. Actiniina.—The tentacles are simple and similar, and there is one tentacle corresponding to each intermesenteric chamber (endocoel).
Sub-Order 2. Stichodactylina.—The tentacles are simple and similar, or provided with teat-like or ramified pinnules. One or more tentacles may correspond with an endocoel, and there may be two kinds of tentacles (marginal and accessory) in the same genus.
Sub-Order 1. Actiniina.
Fam. 1. Halcampidae.—This family is clearly most closely related to the Edwardsiidea. There are, however, twelve complete mesenteries of the first cycle, and a second cycle of more or less incomplete mesenteries. The tentacles are usually twelve in number, but may be twenty or twenty-four. There is no pedal disc, but the base is swollen and rounded or pointed at the end.
The genus Halcampa includes a considerable number of small species occurring in the shallow waters of the temperate northern hemisphere, and of the Kerguelen Islands in the south. Three British species have been described, of which Halcampa chrysanthellum alone is common. The larva with eight tentacles and eight mesenteries has been found living on the Medusa Thaumantias.
Peachia is a genus containing Anemones of much larger size (10-25 cm.). It is remarkable for the very large siphonoglyph on the ventral side of the stomodaeum, prolonged into a papillate lip projecting from the mouth called the "conchula." The genera Scytophorus from 150 fathoms off Kerguelen and Gyractis from Ceylon, although showing some remarkable peculiarities of their mesenteric system, appear to be closely related to this family.
Ilyanthus mitchellii is a large Anemone with a vesicular base, forty-eight tentacles and mesenteries, occurring in the English Channel, but it is not very common. It is usually {381}placed in a separate family, but is in many respects intermediate in character between the Halcampidae and the Actiniidae.
Fam. 2. Actiniidae.—This family contains some of the commonest British Sea-anemones. There is a large flat pedal disc by which the body is attached to stones and rocks. The body-wall is usually smooth, and not perforated by cinclides. The edge of the disc is usually provided with coloured marginal tubercles. There are no acontia.
Actinia.—This genus contains the widely distributed and very variable species Actinia mesembryanthemum, one of the commonest of the Sea-anemones found in rock pools on the British coast. The colours of this species are often very beautiful (see p. 379) but variable.
Anemonia is a genus with remarkably long tentacles which are not completely retractile. A. sulcata (sometimes called Anthea cereus) is very common in the rock pools of our southern coasts.
Bolocera tuediae is, next to Actinoloba dianthus, the largest of the British Anemones. It has very much the same colour as the common varieties of Actinia mesembryanthemum, but the body-wall is studded with minute, rounded warts. It is found between tide marks in the Clyde sea-area, but usually occurs in deeper water.
Fam. 3. Sagartiidae.—This family includes several genera with a contractile pedal disc, with the body-wall usually perforated by cinclides, and provided with acontia.
The genera may be arranged in several sub-families distinguished by well-marked characters. Among the well-known Sea-anemones included in the family may be mentioned:—
Sagartia troglodytes, a very common British species found in hollows in rocks. It is usually of an olive green or olive brown colour, and the upper third or two-thirds of the body-wall is beset with numerous pale suckers. Adamsia palliata has a white body-wall spotted with bright red patches, and is associated with the hermit crab Eupagurus prideauxii.
Actinoloba (frequently called Metridium) dianthus is considered the handsomest of all the British Sea-anemones. It has a lobed disc frilled with numerous small tentacles, and is uniformly coloured, creamy-white, yellow, pale pink, or olive brown. It lives well in captivity, and sometimes reaches a length of 6 inches with a diameter of 3 inches (Fig. 164).
Aiptasia couchii is a trumpet-shaped Anemone, found under stones at low-water mark in Cornwall and the Channel Islands, with relatively slight power of retraction.
Gephyra dohrnii is an interesting species with twelve tentacles, which was supposed at one time to form a connecting link between the Actiniaria and the Antipathidea. It is found attached to the stems and branches of various Hydrozoa and Alcyonaria, sometimes in such numbers and so closely set that it gives the impression of having formed the substance of its support. Haddon[404] has described specimens found on the stems of Tubularia from deep water off the south and south-west coasts of Ireland. It also occurs in the Mediterranean and the Bay of Biscay.
Fam. 4. Aliciidae.—The members of this family have a large flat contractile base and simple tentacles. The body-wall is provided with numerous simple or compound outgrowths or vesicles, usually arranged in vertical rows. Alicia mirabilis is a rare Anemone from Madeira with a very broad base, capable of changing its position with considerable activity, and of becoming free and floating upside down at the surface of the sea. Other genera of the family are Bunodeopsis and Cystiactis. The genus Thaumactis, described by Fowler,[405] from the Papeete reefs, has many peculiarities, but is probably capable of crawling rapidly and of floating at the surface like other members of the family. The remarkable Anemone Lebrunia from the West Indies may be included in this family.
Fam. 5. Phyllactidae.—These are distinguished by the presence of a broad collar of foliaceous or digitate processes outside the circle of tentacles. The processes have some resemblance to the foliaceous tentacles of the Stichodactylinae. They are found in the Mediterranean, Red Sea, and on the shores of the Atlantic Ocean, but have not yet been found in the British area.
Fam. 6. Bunodidae.—This family is characterised by prominent verrucae and tubercles of the body-wall. It contains several British species, of which Bunodes gemmacea found between tide marks on our southern shores is fairly common. The very common British species Urticina (Tealia) crassicornis is usually placed in this family, but exhibits some peculiarities which seem {383}to warrant its removal to another division of the Actiniaria. It is found in tide pools attached to rocks, but is usually partially hidden by adherent sand or small stones.
Fam. 7. Minyadidae.—This family contains a number of floating Anemones. The basal disc is folded over to form a gas bladder lined by a cuticular secretion. The species are principally found in the seas of the southern hemisphere.
Sub-Order 2. Stichodactylina.
Fam. 1. Corallimorphidae.—In this family the marginal cycle of tentacles and accessory tentacles are all of the same kind. The accessory tentacles are arranged in radial rows. All the tentacles are knobbed at the extremity. The musculature is weak. Capnea sanguinea, Corynactis viridis, and Aureliania heterocera belong to the British fauna. They are all small Anemones of exquisite colours, but are not very common. The genus Corallimorphus is principally found in the southern hemisphere.
Fam. 2. Discosomatidae.—The tentacles are all of one kind and are very numerous. The mesenteries are also very numerous. The sphincter muscle is strong.
This family includes a rather heterogeneous assembly of forms, and will probably require some rearrangement as our knowledge increases. Nearly all the species are found in the shallow waters of the tropics, and among them are to be found some of the largest Anemones of the world. Stoichactis kenti, from the Barrier Reef, is from one to four feet in diameter across the disc. In the West Indies these Anemones do not attain to such a great size, but Homostichanthus anemone from Jamaica is sometimes 8 inches in diameter.
Fam. 3. Rhodactidae.—In this family the body-wall is smooth and the oral disc greatly expanded. The tentacles are of two kinds. On the margin there is a single cycle of minute tentacles, while on the disc there are numerous tuberculate or lobed tentacles. Many of the species of this family are quite small, but Actinotryx mussoides from Thursday Island has an oral disc 8 inches in diameter. The genera and species are widely distributed in the warm, shallow waters of the world.
Fam. 4. Thalassianthidae.—The tentacles are simple or {384}ramified (Fig. 166), and in some cases very long (Actinodendron arboreum). Many of the specimens of A. plumosum and Megalactis griffithsi are of very large size, 8 to 12 inches in diameter. Of the former of these two species Saville Kent remarks: "The colours are lacking in brilliancy, being chiefly represented by varying shades of light brown and white, which are probably conducive to its advantage by assimilating it to the tint of its sandy bed. When fully extended the compound tentacles are elevated to a height of 8 or 10 inches, and bear a remarkable resemblance to certain of the delicately branching, light brown sea-weeds that abound in its vicinity." The same author calls attention to their stinging, which is "nearly as powerful as the ordinary stinging nettle."
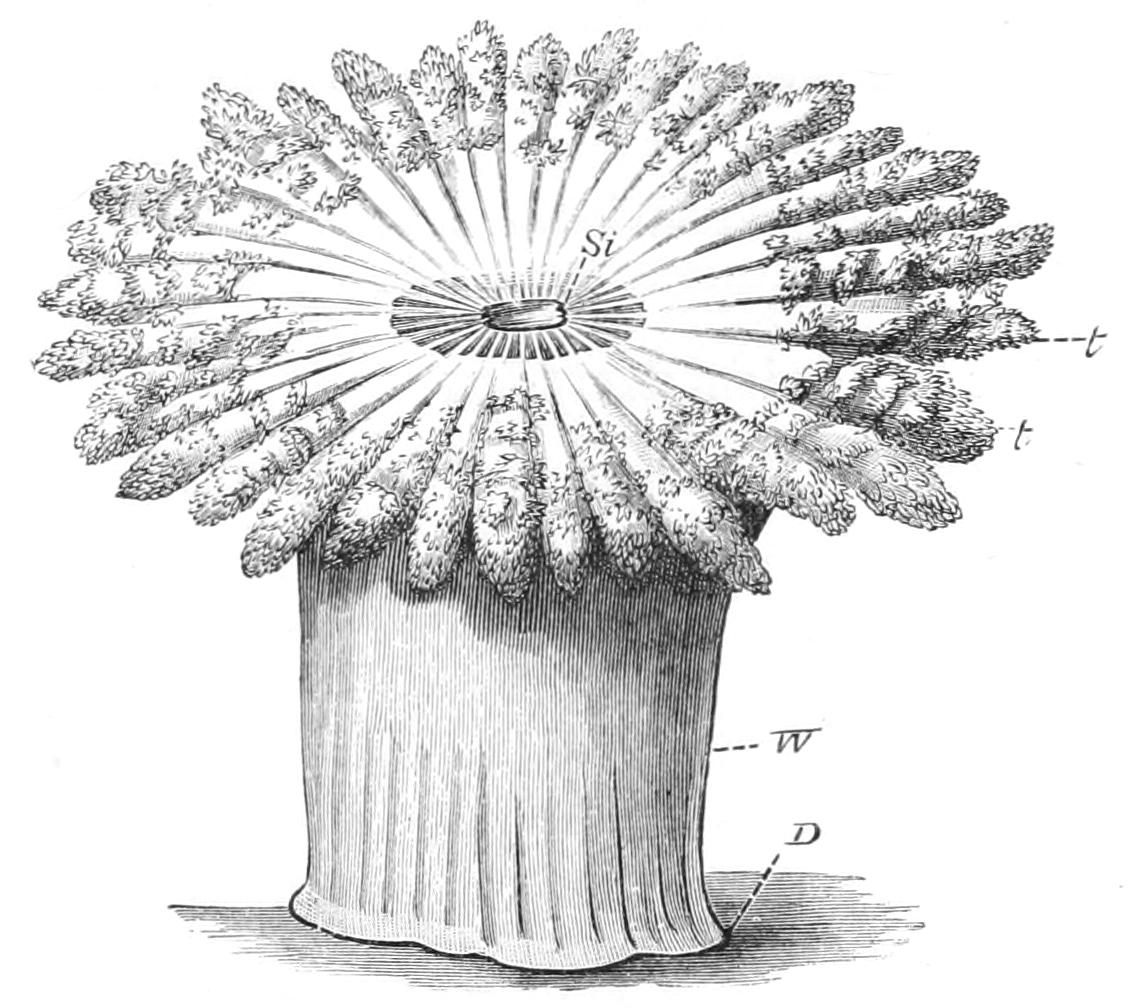
Fig. 166.—Actinodendron plumosum. D, disc of attachment; Si, siphonoglyph; t, t, lobes of the marginal disc bearing the tentacles; W, body-wall. Height of the column 200 mm. (After Haddon.)
Order III. Madreporaria.
The Madreporaria form a heterogeneous group of Zoantharia characterised by a single common feature, the formation of an extensive skeletal support of carbonate of lime. In a great many cases the skeleton exhibits cups or "calices" into which the zooids may be completely or partially retracted, and these calices usually exhibit a series of radially disposed vertical laminae, the "septa," corresponding with the inter-mesenteric spaces of the zooids. Calices and structures simulating septa also occur in Heliopora, which is an Alcyonarian, and in certain fossil corals which are probably not Zoantharians. The anatomy of the zooids of a great many Madreporaria is now known, and, {385}although a great deal of work yet remains to be done, it may be said that the Madreporaria exhibit close affinities in structure with the Actiniaria. The chief points in the anatomy of the zooids are described under the different sub-divisions, but a few words are necessary in this section to explain the principal features exhibited by the skeleton.
There is no more difficult task than the attempt to explain upon any one simple plan the various peculiarities of the Madreporarian skeleton.[406] The authorities upon the group are not agreed upon the use of the terms employed, nor are the current theories of the evolution of the skeleton consistent. It is necessary, however, to explain the sense in which certain terms are employed in the systematic part that follows, and in doing so to indicate a possible line of evolution of the more complicated compound skeletons from the simple ones.

Fig. 167.—Series of diagrams to illustrate the structure of the Madreporarian skeleton. A, young stage of a solitary coral with simple protheca (p.t). B, solitary coral, with theca (th), epitheca (e.t), and prototheca (p.t). C, young stage of colonial coral, showing coenosteum (coe) and theca (th), and the formation of the theca of a bud (b). D, two zooids of a more advanced stage of a colonial coral. coe, Coenosteum; th, theca. The black horizontal partitions are the tabulae. E, transverse section of a calyx. c, Costa; col, columella; d, dissepiment; g, septum; p, pali.
There can be no doubt whatever that the whole of the skeleton of these animals is formed by the ectoderm, and is external to their bodies. If we could get rid of the influence of tradition upon our use of popular expressions we should call this skeleton a shell. There can be little doubt, moreover, that this skeleton is formed by a single layer of specialised ectoderm cells called the "calicoblasts."
The calicoblasts form, in the first instance, a skeletal plate at the aboral end of the coral embryo, which becomes turned up at the edges to form a shallow saucer or cup. This cup is called the "prototheca."[407] At this stage the body-wall of the living zooid may or may not overflow the edge of the prototheca. In the former case the growth of the rim of the prototheca is brought about by the calicoblasts of an inner and outer layer of epiblast, and the cup is then called the "theca." In the latter case, the growth of the rim of the prototheca is continued by the calicoblasts of one layer of epiblast only, and it is called the "epitheca" (Flabellum). With the continued growth of the theca the tissues that have overflowed—the "episarc"—retreat from the base, and in doing so the ectoderm of the edge and, to some extent, the outer side of the episarc secrete a layer of epitheca which becomes more or less adherent to the theca. Thus the cup may have a double wall, the theca and the epitheca (Caryophyllia).
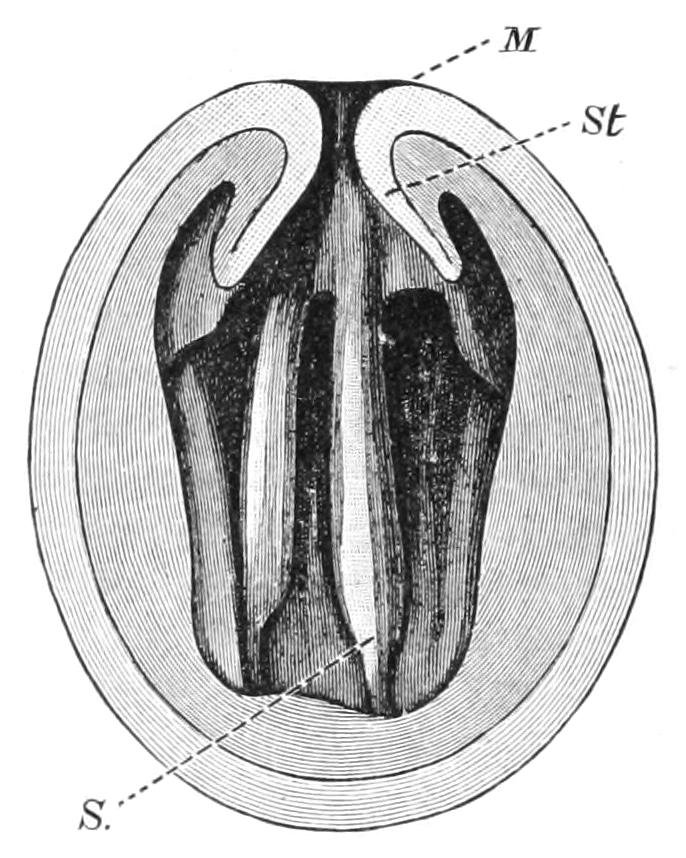
Fig. 168.—Diagram of a vertical section of a young Caryophyllia, showing the septa (S) covered with endoderm projecting into the coelenteric cavity. M, mouth; St, stomodaeum. (After G. von Koch.)
Fig. 169.—A young Caryophyllia, viewed from above, showing the tentacles (t) and the stomodaeum (St). The letter m points to a space between a pair of mesenteries, and the darker shading in this place shows a septum projecting radially from the wall of the theca. (After G. von Koch.)
With the growth of the theca and epitheca a certain number of radially disposed laminae of lime rise from the walls and grow centripetally. These are the "septa." Additional ridges on {387}the inner wall of the cup between the septa are called the "dissepiments." Corresponding with the septa there may be a circle of columns or bands rising from the basal parts of the prototheca—the "pali"; and from the actual centre a single column called the "columella." The longitudinal ridges on the outside of the theca, corresponding in position with the septa inside, are called the "costae" (Fig. 167, E, c).
We may imagine that in the primitive forms that gave rise to colonies, the episarc of the primary zooid overflowed on to the substance to which it was attached, and gave rise to successive layers of epithecal skeleton, which may be called the "coenosteum." The ectoderm at the base of the original prototheca is in some corals periodically dragged away from the skeleton, and forms another cup or platform of lime at a little distance from it—the "tabula." New zooids are developed at some distance from the primary one by a process of gemmation in the episarc, and independent thecae, septa, etc., are formed in it; the skeleton of the new zooid thus originated being connected with that of the primary zooid by the coenosteum.
There are many modifications of this simple description of skeleton formation to be considered before a thorough knowledge of coral structure can be understood, but sufficient has been said to explain the use of the terms that it is necessary to employ in the description of the families. When it is necessary to speak of the cup in which the zooid is situated without expressing an opinion as to the homology of its wall, it is called the calyx.
There are many forms of asexual reproduction observed in the Madreporaria. Of these the most frequent is gemmation. The buds are formed either on the episarc or on the canals running between zooids at the surface of the coenenchym. When the young zooids that have been formed by gemmation reach maturity they have the same characters as their parents. Fission occurs in the production of a great many colonies of Madreporaria. It occurs occasionally in such genera as Madrepora and Porites, where reproduction by gemmation prevails, but it is said that gemmation never occurs in those forms such as the Astraeidae Fissiparantes where fission is the rule. In fission a division of the zooid takes place in a vertical plane passing through the stomodaeum and dividing the zooid into two equal parts. In some cases these two parts become separated during the further {388}growth of the coral. In other cases, however, further divisions of the stomodaeum occur before the separation of the zooids, and then elongated, serpentine polyps are produced (as in Meandrina, etc.), which consist of a number of imperfectly separated zooids, each with a distinct mouth and stomodaeum but with continuous coelenteric cavities. Two kinds of fission must be distinguished from each other. In Madrepora and Porites the plane of fission passes dorso-ventrally through the zooids, that is, between the dorsal and ventral pairs of directive mesenteries. In these cases the zooids produced by fission are similar to the parent form. In most Madreporaria, however, the plane of fission appears to be more or less at right angles to this, and the resulting zooids are unlike the original parent form in having either no directive mesenteries at all or only one pair of them.

Fig. 170.—Diagrammatic transverse sections of Porites to illustrate the process of fission. A, before division; B, fission nearly completed. In A four bilateral pairs (a, b, c, d) of mesenteries have appeared in the entocoele of the ventral directives (VD). These are increased to six pairs and then fission commences as seen in B, the plane of fission passing through the entocoeles of the last pair of secondary mesenteries (f) and of the dorsal directives (DD). I, II, V, VI, the protocnemes in the order of their development. (After Duerden.)
The section Fungacea presents us with some exceptional and remarkable forms of asexual reproduction. The embryo Fungia gives rise to a conical fixed coral called a "trophozooid." The upper part of the calyx of this trophozooid expands and becomes disc-shaped. This is called the "anthocyathus," and after it has reached a certain size it breaks away from the rest of the trophozooid as an adult Fungia. Several anthocyathi may be formed in succession from one trophozooid. This may be described as a process of successive transverse fission. In Diaseris the disc divides into four quadrants, and each quadrant appears to be capable of acquiring the shape and size of the undivided parent.
Without doubt a process of sexual reproduction occurs in all Madreporaria. In some genera sexual reproduction appears to be almost continuous throughout the year; in others the sexual organs are formed only at periods separated by considerable intervals of sterility. According to the researches of Duerden the Madreporaria appear to be usually viviparous, the early stages of development are passed through within the body of the parent, and the young coral is discharged into the water as a free-swimming ciliated larva. The larvae are spheroidal, oval, or pear-shaped, but change their shape a good deal, and sometimes become elongated, straight, or spirally twisted rods. The larvae are at first dense and opaque, but subsequently they become distended by the absorption of water, and more nearly transparent. They swim about for one or two days, and then settle down by the aboral pole and become fixed. The tentacles are not formed, in any species that has yet been observed, during the free-swimming stage of existence.

Fig. 171.—A fixed stage in the development of Fungia. The trophozooid has become differentiated into a discoid crown, the anthocyathus (Cy) and a pedicle, the anthocaulus (Ca). (After G. C. Bourne.)
Distribution of Reef Corals.—The principal reef-forming corals reach their greatest size and grow with greatest rapidity in the warm, shallow waters of the world, but they are not confined to this habitat. A species of Madrepora has been found in the very cold waters of Archangel, and Manicina areolata occurs in Simon's Bay, Cape of Good Hope, many degrees south of the region of the East African coral reefs. As regards the distribution of these corals in depth, very little is known at present. The face of the growing coral reef that is turned towards the open sea is so steep that it has been found impossible to determine to what depth the living reef corals actually extend.
The survey of the Macclesfield bank proved that a considerable number of reef corals are to be found alive at depths {390}ranging from 30 to 50 fathoms.[408] To give one example:—In the dredging No. 50, depth 32 to 35 fathoms, living examples of the following genera of corals were obtained: Madrepora, Montipora, Psammocora, Pavonia, and Astraeopora.
Coral Reefs and Atolls.—In many regions of the tropical seas, banks and islands are found which are built up of blocks of coral, coral detritus, and altered or modified limestone. These are the famous coral reefs of which so much has been said and written during the last half-century. There can be little doubt that the superficial strata of these formations are entirely due to the action of coral-forming animals and plants living in warm, shallow sea-water.

Fig. 172.—Plan of Minikoi Atoll in Laccadive Archipelago. A, the land elevated above the level of high-water mark; Ch, the boat channel; 5 fm, the five fathom line; 2 fm, the two fathom line; L, the lagoon with a maximum depth of 7 fathoms; R, the reef continuing the circle on the east side of the atoll, awash at high tides. (After Stanley Gardiner.)
Three classes of coral reefs are usually recognised: the "fringing reefs" which follow the contour of the coast at a distance of a few hundred yards, and are separated from the beach at low tide by sand flats or a shallow lagoon; the "barrier reefs," following the contour of the coast less regularly than the fringing reefs, but at a much greater distance, and separated from the beach by a lagoon of sufficient depth to serve as a harbour for ships of great size; and, finally, the "atolls," which are ring-shaped, or broken circlets of low islands enclosing a lagoon which is, in some cases, of considerable depth.
It was observed by the early surveyors that in many cases the sea-bottom slopes downwards steeply or almost precipitously from the outer edge of the barrier reefs and atolls to very great {391}depths—to depths, in fact, at which reef-forming corals do not live.
It seems obvious, therefore, that the atolls and barrier-reefs are resting upon some stratum which could not possibly have been formed by reef-building organisms at the same relative position it has now, and the questions arose, What is the substratum and how was it formed?
If this stratum is a coral rock, it is clear that it must have been formed at a time when it was nearer to the surface of the sea than it is now, and that it must have subsided subsequently to greater depths. If, on the other hand, it is a primitive rock, we must assume that in such regions as the Indian Ocean and the South Pacific, where the archipelagoes of atolls extend for hundreds of miles, there are chains of mountain ranges with peaks reaching to a uniform level beneath the surface of the sea. "But we cannot believe that a broad mountain summit lies buried at the depth of a few fathoms beneath every atoll, and nevertheless that throughout the immense areas above named not one point of rock projects above the level of the sea. For we may judge of mountains beneath the sea by those on land, and where can we find a single chain, much less several such chains many hundred miles in length, and of considerable breadth, with broad summits attaining the same height from within 120 to 180 feet?"[409]
To account for the observed facts of the atolls and barrier-reefs, Darwin conceived and expounded the subsidence theory. According to this theory, the regions where atolls now occur were at one time dry land, or an archipelago of volcanic islands surrounded by fringing reefs of the ordinary type. A gradual subsidence of the land took place, and the area of the land diminished; but the area enclosed by the coral reefs did not diminish in a corresponding degree, and the young corals growing on the débris of the older ones as they sank continued the growth of the reef in a direction nearly vertical to the sea-bottom. The fringing reefs thus became barrier reefs, and they were separated from the land by a lagoon of considerable depth. Finally, when the mountain peaks disappeared beneath the waves, a ring-shaped reef or atoll was all that was left to mark the position of the former land.
The fundamental assumption in the subsidence-theory is that {392}the substratum of the coral reefs and islands is coral-formed limestone. To test the truth of this assumption an expedition was sent out to obtain, by boring, evidence of the character of the substratum of a typical atoll. The island of Funafuti in the Ellice group of the Pacific Ocean was selected, and after several attempts a successful boring was made to a depth of 1114 feet. The material from the boring was found to consist of rocks or sands entirely derived from the calcareous skeletons of marine Invertebrate animals and calcareous Algae.[410] Moreover, in the cores from various depths down to the lowermost the fossilised skeletons of the common genera of recent corals, and very few or no representatives of genera of corals now extinct were discovered.

Fig. 173.—Section of the outer edge of one of the Maldive Atolls. A, foundation of primitive rock cut down by the currents; B, upgrowth of the rim by the deep-sea-, intermediate depth- and (B') reef-organisms; C, extension outwards by means of the talus slope; D, lagoon. Scale in fathoms. (After Stanley Gardiner.)
These facts, therefore, prove the justice of Darwin's assumption as to the nature of the substratum—and give support to the subsidence-theory as applied to this particular island. A strong opinion has, however, been expressed by several authors of recent years that the subsidence-theory cannot account for the formation of all the atolls and barrier reefs that have now been investigated, and alternate hypotheses have been put forward to account for particular cases. The main chain of the Maldive Archipelago in the Indian Ocean, for example, presents special difficulties to the acceptance of the subsidence-theory as one of general application.[411] The main chain of these islands is more than 300 miles long, and lies at right angles to the monsoon currents of the {393}Indian Ocean. Here the action of the currents appears to have cut down a great tract of land to form a plateau more than 100 fathoms in depth. The outer rim of this plateau may have grown in height by the deposit of the skeletons of surface-swimming animals, and the skeletons of deep-sea corals, until it reached a level where reef-forming corals can thrive. A certain number of channels would be retained and even deepened as the rim grew up, and thus the coral would eventually reach the surface not as a single large atoll, but as a series of coral islands. When the coral reef has thus reached the surface and cannot grow farther in height, it spreads radially like a fairy ring on the talus formed by broken corals that have fallen down the slopes. The central parts, no longer protected by living organisms, are continually subject to the solvent action of the sea water penetrating the porous substratum, and sink to form the lagoon.
It is not only in the reefs of the Indian Ocean, however, but in many of the archipelagoes of the Pacific Ocean, where there is evidence of very extensive elevation of the land areas in the neighbourhood of atolls and barrier reefs, that the subsidence-theory does not satisfactorily account for all the observed facts. It appears probable, therefore, that although a gradual subsidence of the land may have been the primary cause of coral reef formation in some areas, similar reefs may have been formed in other areas by other natural methods.
Fossil Corals.—A great number of the genera of corals found in the newer Tertiary deposits, and a smaller number of those occurring in the older Tertiary and Cretaceous strata clearly belong to families now represented by recent corals. In the earlier strata, however, fossils are found which cannot be placed in our system with any degree of certainty. Attempts have been made from time to time to arrange these corals in their proper positions by the careful study and comparison of their skeletal features, but the reasons given are not convincing. The genus Syringopora, and the families Favositidae, Heliolitidae, and Coccoseridae have been noticed in the chapter on Alcyonaria (pp. 343-346). The family Zaphrentidae will be noticed when dealing with the order Zoanthidea.
Among the families of fossil corals of uncertain position which may still be included in the order Madreporaria, the more important are:—
Cyathophyllidae, a family of solitary and colonial corals with numerous radially arranged septa, extending from the Silurian to the Carboniferous limestone. It includes the genera Cyathophyllum, which was very abundant in Devonian times, and Lithostrotion, which, in the times of the formation of the Carboniferous limestone, occurred in continuous masses extending over great areas of the sea-bottom. The Cyathophyllidae may possibly be ancestral to the representatives of both Astraeidae and Fungiidae, which appeared in the Triassic strata.
The Cyathaxoniidae form a family of solitary turbinate or horn-shaped corals, with septa showing a regular, radial arrangement, and may have been the ancestors of the modern family Turbinoliidae. They have the same geological range as the Cyathophyllidae.
The Cystiphyllidae.—This family consists of solitary corals with very thin septa; the interseptal spaces are filled with an abundant vesicular substance called the "stereoplasm." The systematic position of this family is very doubtful, as the structure is evidently much destroyed, but by some authors it is supposed to be ancestral to the family Eupsammiidae.
These three families, together with the Zaphrentidae (p. 406), were formerly grouped together as the Tetracoralla or Rugosa.
Sub-Order 1. Entocnemaria.
Madreporaria forming perforate coralla, with calices that do not project above, or project only slightly above the surface of the coenosarc. The zooids of each colony are usually small and crowded. The mesenteries arise in bilateral pairs, and the increase in their number takes place in the chamber between the ventral or the dorsal pairs of directives. The corals included in this order are among the most important of the reef-builders. On many of the recent coral reefs they occur in enormous numbers and of great individual size. But although so prevalent upon recent reefs, they appear to have played a far less important part in the formation of the reefs of the early Tertiary times, and in the reefs of times antecedent to the Tertiary they were rare or absent.
Judging from the structure of the skeleton and the palaeontological history alone it might be thought that the Entocnemaria {395}represent the most recent types of Madreporarian structure, but the anatomy of the zooids points to a contrary conclusion. The zooids are of very simple structure; the mesenteries are found only in bilateral pairs, and all the new mesenteries formed after the protocnemes originate in one of the directive chambers. These are characters indicating a very ancient history, suggesting affinities with the Edwardsiidea on the one hand, and some ancient type of Cerianthidea on the other. There can be little doubt that it was owing to the evolution of a porous skeleton of rapid growth that these corals have caught up and passed the Astraeidae and other more specialised forms in the struggle for predominance on the coral reefs.
Fam. 1. Madreporidae.—The calices of the corallum are small and contain a few perfectly distinct septa. The coenosteum is porous and contains a plexus of the coenosarcal canals, which connects the cavities of neighbouring zooids. This family is divided into a number of sub-families, but it is only necessary here to mention the peculiarities of a few of the well-known genera.
Madrepora.—This genus is represented by an immense number of forms on the coral reefs of both the old and new world. Attempts have been made at various times to divide these forms into specific groups, and a large number of species have been defined and named. The differences between these species, however, are such as may be due to varying conditions of life upon the reefs and not to characters transmitted from generation to generation by heredity. There can be no doubt that when our knowledge of the soft tissues of these corals is extended the number of species will be greatly reduced. There are, however, three principal forms of growth or facies in the genus.
1. The flabellate or palmate colonies with large flat or concave fronds, radiating from an encrusting base: Forma palmata.
2. Much branched colonies, several branches radiating obliquely from a common centre: Forma prolifera.
3. Large and more erect colonies, less branched except towards the periphery: Forma cervicornis.
On some reefs one of these forms of growth predominates, and for miles the reef seems to be built up mainly of corals of this shape. On other reefs two or sometimes all three of these forms may be found within a stone's throw of one another. {396}Notwithstanding the difficulty of distinguishing the species, the genus itself is quite well defined. The calices project slightly from the surface of the branches and contain six septa, of which the pair that is parallel with the axis of the branch is the strongest. This strong pair of septa can usually be well seen when a slender branch of a Madrepore is examined by a lens by transmitted light. At the apex of each branch there is a terminal zooid and in the skeleton an apical calyx. The terminal zooid is (in some species at least) different from the lateral or radial zooids. The former is radially symmetrical and has six long equal digitiform tentacles, the latter have usually twelve tentacles, of which six are larger than the others. These tentacles alternate, but they are so arranged on the disc as to give a distinctly bilateral appearance to the zooids.
The colour of the West Indian Madrepores appears to be entirely due to Zooxanthellae (pp. 86, 125). They are lighter or darker shades of brown, sometimes becoming green, yellow, or orange. On the Australian barrier reef and other reefs of the eastern seas the growing points of the branches are variable and often brilliantly coloured, emerald green, violet, or red; giving some of the most wonderful colour effects for which the reef pools are famous. The cause of these brilliant apical colours has not yet been ascertained.
The genus is found in shallow water of all seas of the tropical belt except on the western side of the continent of America.
Montipora.—In this genus the calices are small and situated in depressions in the coenosteum, and there are six, sometimes twelve, septa of approximately equal size. There is no terminal calyx at the apex of the branches. This is a genus of very variable form and wide distribution in all tropical seas except on the shores of the Atlantic Ocean.
Turbinaria.—This genus is usually cup-shaped or foliaceous and twisted in form. The septa may be six to thirty in number. Some of the species of this genus attain to a very great size in favourable localities. There is a specimen in the British Museum that is 16 feet in circumference and weighed, when dried, 1500 lbs.
Fam. 2. Poritidae.—The corallum is usually encrusting, foliaceous, lobed or tufted, rarely dendritic. The whole skeleton is built up of a system of trabeculae and stout cross bars, and in {397}section the limits of the calices are not well defined. The septa are represented by twelve trabeculae. The zooids are small and are usually provided with twelve tentacles. The most important genus is Porites, which is so abundant on many reefs that it may be said to rival Madrepora itself in the luxuriance of its growth. On the Australian barrier reef a species of Porites builds up coralla over twenty feet in length and as many in height. According to Saville Kent they are usually found on the outer side of the reef and form a basis of support for the high-level Madreporas and other corals.[412]
The colours of Porites are very variable and often beautiful. In Jamaica[413] the prevailing colours are bright blue, pale yellow, and yellowish green. In Australia the colours are less brilliant perhaps, but among the prevailing tints are light or bright lilac, a delicate pink, dark yellow, and brown. The genus Porites occurs in Eocene and Miocene deposits, and is now found on all the more important coral reefs of the world.
The genus Alveopora is usually placed with the Poritidae. According to Bernard,[414] however, its affinities with this family are remote, and it is more closely related to the Favositidae (see p. 344). The walls of the calices are contiguous and the septa are reduced to rows of spines, as in the Favositidae. It is found in shallow water in the Pacific, the Indian Ocean, and the Red Sea.
Sub-Order 2. Cyclocnemaria.
Madreporaria forming perforate or imperforate coralla. Solitary or colonial. The zooids have usually a large number of mesenteries arranged in two or more cycles. The mesenteries beyond the protocnemic pairs arise in unilateral pairs in chambers other than those between the directives.
Sect. 1. Aporosa.—Cyclocnemaria in which the theca and septa are not perforated. The zooids of the colonial forms may communicate by means of superficial canals of the coenosarc, or they may be in contact with one another only at their edges.
Several families are included in this section, of which the more important are:—
Fam. 1. Turbinoliidae.—The corals included in this family are mostly solitary forms attached to foreign objects, or living partly embedded in sand. In some cases a small colony is formed by gemmation.
The genus Flabellum is a solitary coral of a compressed top shape. It has a large number of septa arranged radially on the cup-wall. This cup-wall is not a true theca but an epitheca. In some forms root-like tubes grow out from the sides of the cup near its base and may serve to support the coral on solid objects. In some remarkably fine specimens recently obtained from the Persian Gulf these tubes served to attach the coral to a telegraph cable. Flabellum seems to be cosmopolitan in its distribution. It is usually found in deep or moderately deep water, but some specimens have been dredged in water of 2 to 9 fathoms.

Fig. 174.—Side view of Trochocyathus hastatus, with exsert septa, well-marked costae (c), and with three spinous projections (Sp) at the base formed by outgrowths from primary costae. (After G. C. Bourne.)
Caryophyllia is a conical coral fixed by a slightly expanded base. The cup-wall is a true theca covered below by an epitheca. There is a spongy columella surrounded by a single circle of pali. There is one British species, C. smithii. It is found attached to shells at a depth of about thirty fathoms near the Eddystone Lighthouse and in other localities in the English Channel. It also occurs between tide marks in the Scilly Islands, and is found off the Shetlands, on the west coast of Scotland, and the south-west of Ireland. The genus is widely distributed and extends from shallow water to depths of 1500 fathoms. Caryophyllia sometimes occurs in clusters which have the appearance of an incipient colony. This may be due to the embryos fixing themselves upon the epitheca of existing individuals and developing there. It is doubtful whether the species ever reproduce asexually either by gemmation or by fission. When the zooid is fully expanded it projects some distance above the corallum and shows a very transparent body-wall with a crown of some fifty tentacles. Each tentacle terminates in a globose head (Fig. 169) charged with nematocysts. The general colour is pale pink, and there is a broad brown circle {399}round the mouth. Large specimens may be three-quarters of an inch in diameter.
Turbinolia is a common Eocene fossil genus found in England and France, and is stated to occur in the Caribbean Sea. The columella stands up like a stylet and the septa are "exsert," i.e. project above the rim of the theca.
Trochocyathus is a genus with well-marked "costae" occurring in tropical shallow water (Fig. 174).
Fam. 2. Oculinidae.—Colonial forms, dendritic or encrusting, with relatively large and rather prominent calices separated by considerable stretches of compact coenosteum. The zooids bear a crown of ten to forty-eight or more capitate tentacles.
Neohelia has a fistulose stem lined internally by a horny membrane. There seems to be some reason for supposing that this membrane is formed by the zooids themselves. A similar membrane is found in the fistulose stems of Amphihelia and perhaps other Oculinidae. If this membrane is really formed by the activity of the corals it forms an exception to the general rule that the skeleton of the Madreporaria is entirely calcareous. Others maintain, however, that this membrane is formed by the Chaetopod worms which are found in the tubes, and that the fistulose stem of the coral is formed by folding round and encrusting the horny tubes of the worm. Neohelia is found in the Pacific Ocean.[415]
Lophohelia is a genus forming dendritic colonies of considerable size. The calices have thick walls and are very deep. Lophohelia prolifera has been found in deep water off the island of Skye and in other localities off the west coast of Scotland. It is also not uncommon in some of the Norwegian fjords and in other parts of the world.
Oculina is another widely distributed genus found in the shallow tropical waters of the West Indies, the Indian and Pacific Oceans. It forms dendritic colonies of considerable size. The calices are usually arranged in a spiral manner on the branches. The colour of the West Indian species is stated to be light or dark brown when alive. The tentacles are arranged in three cycles, and are usually twenty-four in number. Asexual reproduction takes place by budding at the apex of the branches.
Fam. 3. Astraeidae.—This is a very large family, and {400}authorities are not agreed as to its limits or classification. Excluding the simple forms for the present, the family may be said to be distinguished by having the calices so closely crowded that there is little or no coenosteum between them. The corallum is compact and massive, unless bored and perforated by algae, worms, and other coral-destroying organisms.
The genera of Astraeidae that form colonies may be divided into two groups: the Gemmantes and the Fissiparantes. In the group Gemmantes asexual reproduction is effected by gemmation, and each zooid of a colony is a distinct individual with two pairs of directive mesenteries. Among the best known of recent corals included in this group may be mentioned Galaxea. In this genus there is a good deal more coenosteum between the calices than there is in most of the Astraeidae. The calices are long and project some distance above the coenosteum. The septa are exsert. In Galaxea esperi examined by Fowler[416] there are twelve septa, twelve pairs of mesenteries, and twenty-four tentacles, of which twelve are very small and twelve rather larger. The colour is green or brown. The genus is found in shallow water in the tropics of the old world.
In Astrangia solitaria the zooids are either isolated or more generally united by thin strands of perithecal tissue to form encrusting colonies. The septa are not exsert as in Galaxea. Six are prominent and belong to the first cycle, six smaller ones form a second cycle, and an incomplete third and fourth cycle may be seen. Corresponding with each septum there is a tentacle. The tentacles of the innermost cycle are the longest (3 mm. in length). All the tentacles terminate in a knobbed apex. The living zooids are colourless throughout, or display only very delicate tints within restricted areas.[417] This genus occurs principally on the coasts of the American continent, extending as far south as the Straits of Magellan. Other well-known genera of Astraeidae Gemmantes are Orbicella, Cladocora, Phyllangia.
In the group Fissiparantes asexual production takes place by fission without the production of morphologically complete zooids. The tentacles, mesenteries, and septa, when fission is established, are not arranged in regular hexameral cycles, and no {401}new directive mesenteries arise. In some cases very large corals are formed, and, if our conception is correct, these must be regarded, not as a colony of zooids, but as a single individual zooid divided into a considerable number of incompletely separated parts. Among the well-known genera belonging to this group are Euphyllia, Mussa, Meandrina, Coeloria, Favia, and Goniastraea.
In such genera as Euphyllia the parts of the colony become separated by deep grooves, and have the superficial appearance of being distinct individuals; but in the Brain-coral Coeloria and others the surface of the coral presents a series of more or less bent or curved grooves, each with a row of slit-shaped mouths and bordered by rows of tentacles.
A number of genera of solitary corals united in the subfamily Trochosmiliacea are generally included in the family Astraeidae. The study of their skeletal characters has suggested[418] that they are more closely allied to the Turbinoliidae. The principal genera thus transferred would be Trochosmilia, Placosmilia, Parasmilia, and Asterosmilia. As these genera and their allies are nearly all extinct, and nothing is known of the structure of the living zooids, their removal from the Astraeidae may be regarded as not fully justified.
Fam. 4. Pocilloporidae.—The general anatomy of the zooids of this family of corals has some resemblance to that of the Entocnemaria, and it is possible that they will eventually find a place in our classification near to, if not actually within that group. The fact, however, that the skeleton is imperforate is sufficient for the present to justify the inclusion of the family in the section Aporosa. There are but two genera at present known, and in both of them the zooids have twelve tentacles, twelve mesenteries, and only two mesenterial filaments. The zooids are connected together by an elaborate system of canals running in the superficial coenosarc. The calices are bilaterally symmetrical, and in Seriatopora the septa which are parallel with the axis of the branch are united in the centre of the calyx, and are very much larger than the others, as in Madrepora. In all these characters the family shows affinities with the Entocnemaria. In the characters of the skeleton, which is imperforate and tabulate, the affinities are rather with the {402}Cyclocnemaria. The two genera are widely distributed on the coral reefs of the old world, and in some localities are very abundant. Neither genus is found in the West Indies. They are both of recent origin, but Pocillopora occurs in the Miocene. It is a remarkable feature of the family that both genera may be attacked by the gall-forming crab Hapalocarcinus. From some reefs nearly all the Pocilloporidae show crab-galls on a large number of their branches, whereas other Madreporaria are free from them.

Fig. 176.—A single calyx of Pocillopora septata, showing Co, the columella; S, S, the septa; Th, the theca wall. (After Gardiner.)
Pocillopora is a coral that forms encrusting masses, rising into lobes or branches of considerable size, terminating in blunt apices. Seriatopora is much more slender and ramified, the branches terminating in sharp points.
Sect. 2. Fungacea.—This section of Cyclocnemaria contains a number of solitary and colonial corals of very varied form united in the possession of a number of cross-bars called "synapticula" connecting the septa, and thereby giving strength to the calyx apart from any increase in the thickness of the calyx-wall. The family Fungiidae shows many peculiarities which separate it very distinctly from both the Cyclocnemaria and the Aporosa. The Eupsammiidae, however, approach the {403}Cyclocnemaria in many respects, and the Plesiofungiidae form a connecting link with the Astraeidae. It is very probable that this section had a dual origin, and therefore does not represent a single line of descent.
Fam. 5. Plesiofungiidae.—This family is related to the Aporosa in the possession of septa that are generally solid and imperforate, and to the Astraeidae in particular in the possession of dissepiments. They differ from them, however, in the presence of synapticula and in certain peculiarities of the tentacles.
The genus Siderastraea has recently been studied by Duerden.[419] The colony is usually massive and encrusting in habit. The zooids when expanded do not rise much above the level of the corallum. The tentacles are short and are arranged in irregular cycles on the disc. They terminate in knobbed extremities, and those of the inner cycles are bifurcated. The colour of S. sideraea is reddish-brown when alive. Siderastraea is found in shallow water on the coral reefs, and is widely distributed.
In Agaricia the colony is more foliaceous. The tentacles are rudimentary or small. The colour of the living zooids is very similar to that of Siderastraea. Epistrelophyllum is a solitary coral, from the Jurassic series, belonging to the family.
Fam. 6. Fungiidae.—Fungia is an unattached solitary coral of a flat disc-like shape with very numerous exsert imperforate septa. It is frequently of considerable size (six to twelve inches in diameter). On many of the coral reefs of the old world it is extremely abundant, and consequently it is one of the commonest corals of our collections. When alive the corallum is almost hidden by the disc, which is studded all over with very numerous long tentacles.[420] The colour varies in different species, but is usually brown. One species on the Australian barrier reef, F. crassitentaculata, is of a dark olive green colour, the tentacles terminating in white knobs.
The free adult Fungias are derived from a fixed stock called the trophozooid, from which the young Fungias are detached by transverse fission (see p. 388). The thecal wall of the young Fungia when detached from the trophozooid is perforated, but {404}the pores become largely filled up during the later growth of the coral.
There are several genera of colonial Fungiidae of less frequent occurrence, such as Halomitra, Herpetolitha, and Cryptabacia.
Fam. 7. Cycloseridae.—These are solitary or colonial Fungacea with an imperforate theca. Bathyactis occurs at great depths. Diaseris, shallow water on coral reefs.
Fam. 8. Plesioporitidae.—The septa in this family are trabeculate and perforate, resembling in this respect the septa of Poritidae. Leptophyllia, Microsolena, extinct.
Fam. 9. Eupsammiidae.—This family of perforate corals is usually placed with the Madreporidae and Poritidae in the old group Perforata. The researches of Fowler and Gardiner have shown that the arrangement of the mesenteries is that of the Cyclocnemaria, and the presence of synapticula connecting the septa suggests affinities with the Fungacea. The synapticula of the Eupsammiidae, however, are peculiar in being arranged, not in a vertical series, but alternately with one another or quite irregularly in position. The members of this family are solitary or colonial in habit.
Stephanophyllia is a flattened disc-shaped coral, with perforate and dentate septa, found in the Pacific Ocean and as a fossil in various strata since Cretaceous times.
In Leptopenus, from depths of about 1500 fathoms, the perforations are much larger than in the last-named genus, and the skeleton is reduced to a system of slender trabeculae.
Rhodopsammia has a conical shape, and gives rise by gemmation to a number of young zooids, which remain attached for some time to the parent form before becoming free.
Among the colonial genera are Dendrophyllia, Coenopsammia, and the well-known Mediterranean genus Astroides.
Order IV. Zoanthidea.
This order of Zoantharia consists of a number of solitary or colonial Anemones that do not form a skeleton of horn or carbonate of lime, and are distinguished from the Actiniaria by the peculiar arrangement of their mesenteries.
Fam. 1. Zoanthidae.—Sphenopus is a solitary coral and terminates aborally in a small sucker-like base, by which it may {405}be attached to foreign bodies. The genera Gemmaria and Isaurus include solitary forms.
In the majority of the species of Zoanthids, however, a basal encrusting stolon is formed, which may be thick and fleshy or membranous, or may consist of a plexus of bands from which several zooids rise and on which the new buds are formed.
The tentacles are numerous, simple, usually short, and arranged in one or two circles on the margin of the disc. Most Zoanthidae are encrusted with sand, shell fragments, or sponge spicules, but Zoanthus and Isaurus are naked. The foreign particles that form the incrustation are firmly attached to the ectoderm, and as a rule many of them sink down into the mesogloea to give additional support to the body-wall. It is the presence of so much incorporated sand that frequently gives these Zoantharia such a very brittle character. The stomodaeum usually exhibits a well-marked ventral siphonoglyph. The mesenteries consist of a pair of complete ventral directives, a pair of incomplete dorsal directives, while of the remaining protocnemes the lateral mesenteries which are first and second in the order of appearance are complete, the sixth is incomplete, whereas the fifth is complete in the Macrocneminae and incomplete in the Brachycneminae. Duerden[421] has found in specimens of three species that the arrangement of the mesenteries is "brachycnemic" (the sixth protocneme imperfect) on one side and "macrocnemic" (the sixth protocneme perfect) on the other. The metacnemes appear in the spaces between the sixth protocnemes and the ventral directives in unilateral pairs, of which one becomes complete and the other always remains incomplete (Fig. 163, 4, p. 368).
The Zoanthidae are usually dioecious, but hermaphroditism undoubtedly occurs in the genera Zoanthus and Isaurus. Little is known of their development, but a larval form discovered by Semper off the Cape of Good Hope, of cylindrical shape, with an opening at each end and distinguished by a longitudinal band of cilia running from one end to the other, is probably the larva of a Zoanthid. It is commonly known as Semper's larva. Other larvae provided with a ring of cilia have also been attributed to this group.
A great many Zoanthidae are epizoic in habit. Thus several {406}species of Epizoanthus form colonies on the shells of Gasteropods inhabited by hermit crabs. Parazoanthus tunicans is found on the stem of a Plumularia; Parazoanthus separatus, from Jamaica, is associated with a sponge. The base of the bundle of long spicules of the Sponge Hyalonema (p. 204) is almost invariably sheathed by a colony of Epizoanthus stellaris.
The only genera occurring within the British area are Epizoanthus (with six species), Parazoanthus (with four species), and Zoanthus sulcatus.
Of the species of Epizoanthus, E. incrustatus is fairly common, in depths of twenty to eighty fathoms on all our coasts, and is frequently commensal with different species of hermit crabs, while E. paguriphilus is found in much deeper water off the west coast of Ireland and is always commensal with hermit crabs. Parazoanthus anguicomus is found at depths of a hundred fathoms off the Shetlands and west of Ireland, and is usually associated with various species of Sponges.
Gerardia savalia is the largest "black coral" of the Mediterranean. The colony begins by encrusting the stem of one of the Gorgoniidae, but soon surpassing its support in growth, it forms a basal horny skeleton of its own and builds up very large branching colonies. A specimen in the British Museum,[422] from twenty fathoms off the island Negropont, is two metres high and two metres wide. The genus appears to be related anatomically to Parazoanthus.

Fig. 177.—Zoanthus macgillivrayi, a small colony. The tentacles are shown somewhat contracted by the preservative. Each zooid is about 25 mm. in length. (After Haddon.)
Fam. 2. Zaphrentidae.—This family of Palaeozoic corals is usually placed with the Turbinoliidae or in the separate group Tetracoralla. Recently Duerden[423] has given reasons, based on the method of increase of the septa in Lophophyllum, for believing that their affinities lie rather with the Zoanthidae than {407}with the Madreporaria. They are solitary turbinate corals, with numerous septa exhibiting a distinct bilateral symmetry in arrangement. Zaphrentis, Lophophyllum.
Order V. Antipathidea = Antipatharia.
The members of this order can readily be distinguished from all other Zoantharia by the presence of a horny axial skeleton (sclerobase) and the absence of any spicules of calcium carbonate. The skeleton is covered by a thin bark which consists of a number of simple, naked zooids united at their edges. The zooids bear six tentacles, or if there are more than six, six large prominent tentacles. In most genera there are but ten mesenteries, in others twelve. In Cladopathes only six mesenteries are found. The skeleton of the Antipathidea is simple in Stichopathes and Cirripathes, but in all other genera it is ramified. The ramification is usually profuse and irregular. The horny substance of which it is composed is free from any deposit or infiltration of lime. The surface of the younger branches is beset with numerous short spines, the number and arrangement of which are characters largely used in the determination of species. The basal parts of the main axis and the thicker branches are frequently bare, the zooids having died and become disintegrated. In these cases the spines wear away and the skeleton appears to be smooth. The presence of spines on some of the branches is, however, generally sufficient to enable the naturalist to distinguish a dried Antipathid from the axis of a Gorgonid, with which alone it might be confounded.
There are six complete mesenteries in each zooid, but as they bear no retractor muscles it is not certain that they represent the first six protocnemes of other Zoantharia. In a great many species the zooids are oval in shape, the longer diameter being parallel with the axis of the branch. The mouth and stomodaeum are compressed and at right angles to this diameter. It is usually assumed that the mesenteries attached to the angles of the stomodaeum are the directives, and that the remaining pair, which is axial in direction, corresponds with the first pair of protocnemes. The axial pair of mesenteries is frequently very well developed and alone bears the gonads. When other mesenteries are formed they always arise in bilateral pairs between the axial mesenteries {408}and the directives. The tentacles correspond with the intermesenteric chambers. In some genera there is a constriction of the zooid between the pairs of the tentacles on each side of the axial mesenteries and the directive tentacles. This gives them the appearance of a division into three zooids with two tentacles apiece, one with a mouth and two without a mouth; and as the mouthless parts alone bear the gonads on the single axial mesentery, they have been called the "gastrozooids" and "gonozooids" respectively. This must not be regarded, however, as a case of true dimorphism, as the cavities of the so-called gastrozooid and gonozooids are continuous.
The Antipatharia are widely distributed in nearly all the great seas of the world. Some species are found in shallow water in the tropics, but most of them occur in depths of fifty to five hundred fathoms. The genus Bathypathes is only found at enormous depths ranging from 1070 to 2900 fathoms. Specimens of Cirripathes spiralis, Antipathella gracilis, and another species have recently been obtained in deep water off the west coast of Ireland,[424] but these are the only Antipatharia known to occur within the British area.
The very simple structure of the Antipatharia is usually attributed to degeneration. On this view the Antipathidae with only six complete mesenteries are the most modified, whereas the Leiopathidae with twelve mesenteries are more closely related to the ancestral forms, and Gephyra dohrnii (see p. 382) is a link connecting the order with the Actiniaria.
There is no reason, however, for supposing that Gephyra is specially related to this order, and, as pointed out recently by Roule,[425] the simple structure of the zooids of the Antipathidea is more easily explained if they are regarded as primitive forms.
Gerardia (p. 406), from the Mediterranean, forms a horny axial skeleton like that of the Antipathidea, but this genus is probably a Zoanthid.
Fam. 1. Antipathidae.—In this family the zooids have six tentacles and six or ten mesenteries. It includes nearly all the familiar genera, such as Stichopathes, Cirripathes, Antipathes, Antipathella, Cladopathes, and Bathypathes. Schizopathes and {409}its allies occurring in deep water are the forms regarded by Brook as dimorphic.

Fig. 178.—A portion of a branch of Antipathes ternatensis, showing three zooids and the horny axis beset with thorn-like projections. (After Schultze.)
Fam. 2. Leiopathidae.—This family includes the single genus Leiopathes of the Mediterranean Sea. It is distinguished from the others by the presence of twelve mesenteries.
Fam. 3. Dendrobrachiidae.—This family also consists of a single genus, Dendrobrachia, from 400 fathoms in the South Atlantic. It is distinguished by having pinnate retractile tentacles.
Order VI. Cerianthidea.
This order contains the remarkable Sea-anemone called Cerianthus. Two of the species have been placed in separate genera, but they do not appear to be of more than sub-generic rank. Cerianthus has a long cylindrical body with a double crown of numerous long tentacles at the oral extremity and tapering to a blunt point or rounded at the aboral extremity.
There are numerous mesenteries, which increase in number by the addition of bilateral pairs, arising only in the ventral inter-mesenteric space throughout the greater part, if not the whole, of the life of the zooid. The right mesentery of each young pair is always more advanced than the left, so that the mesenteries have the appearance of arising alternately right and left. None of the mesenteries bear conspicuous bands of retractor muscles. The movements of the body are effected by a thick band of longitudinal fibres lying between the ectoderm and the mesogloea in the body-wall.
The absence or very slight development of muscles on the mesenteries renders it difficult to recognise the homologues of the protocnemes of other Zoantharia in the adult. From the evidence of embryology, however, it seems certain that the six dorsal pairs of mesenteries represent the protocnemes (Fig. 163, 3, p. 368) and the others are metacnemes.
The stomodaeum exhibits a single long deep siphonoglyph, which is probably dorsal in position.
There are two tentacles to each inter-mesenteric space, one being marginal and the other circumoral. The gonads are borne upon alternate mesenteries, and both ova and spermatozoa are produced by the same individual.

Fig. 179.—Cerianthus membranaceus. Colour pink, with tentacles annulated pink and brown. About 35 cm. in length. (After Andres.)
The ectoderm of Cerianthus is remarkable for the immense number of nematocysts and gland cells. The latter secrete a quantity of mucus which binds the threads of the discharged nematocysts into a sticky feltwork and this secures particles of sand and mud, the whole forming a long tube in which the animal freely moves. This tube is often of considerable thickness. It is tough and resistant, smooth inside but ragged and muddy outside. It is often many times the length of the animal's body.
The embryo of Cerianthus is set free before the completion of segmentation, and it gives rise to a floating pelagic larva known as Arachnactis. It has a variable number of tentacles and mesenteries according to its age, but when it reaches a size of {411}about 15 mm. in length it has developed characters which are sufficient to determine its position as a Cerianthid.
The genus Cerianthus appears to be widely distributed. C. membranaceus is the common species in the Mediterranean Sea, but a smaller species has been described from Naples under the name C. oligopodus by Cerfontaine. C. americanus occurs on the eastern coasts of North America. The British and North European species is C. lloydii, but another species, C. vogti, has been found at a depth of 498 fathoms in the North Sea. C. nobilis is a gigantic species supposed to be about 1 foot in length when complete, from Torres Straits.
C. bathymetricus of Moseley, placed by Andres in the genus Bathyanthus, is a species of small size (25 mm.), obtained by the "Challenger" from a depth of 2750 fathoms in the North Atlantic. It exhibits a remarkable prolongation of the stomodaeum into the coelenteron in the form of a sack which contained food. Moseley described a species of Cerianthus, 6 inches long, living on the coral reef at Zebu in the Philippines fully expanded in the tropical sunshine.
Several species of Arachnactis larvae have been described. Of these Arachnactis lloydii appears to be undoubtedly the larva of C. lloydii. The adult forms of Arachnactis albida from various stations in the Atlantic Ocean and of Arachnactis americana are not known. The larva of Cerianthus membranaceus has been called Dianthea nobilis, and is characterised by the great length of the column, by the general opacity of all parts of the body, and by the precocious appearance of the median marginal tentacle. A considerable number of remarkable pelagic larvae have been described by van Beneden[426] from the Atlantic Ocean, and provisionally assigned by him to five different genera. The adult forms of these larvae are not known, but they are probably members of this order.
CTENOPHORA
The Ctenophora are spherical, lobed, thimble-shaped, or band-like animals, usually very transparent and gelatinous in structure. They are exclusively marine, and are found floating at or near the surface of the sea.
Although they are generally classified with the Coelenterata, they are regarded by some authors as having closer affinities with the Polyclad Turbellaria (cf. Vol. II. p. 7). They agree, however, with neither of these divisions in their essential characters, and the only way to indicate and emphasise their unique position is to place them in a separate Phylum.
They differ from all the Coelenterata in the absence of nematocysts, and in the presence in development of a definite mesoblast. The character from which they derive their name, Ctenophora, is the presence on the surface of bands of swimming plates. The plates are called the "combs" (κτείς, gen. κτενός = a comb) or "ctenophoral plates." They occur in all genera included in the Phylum except in Coeloplana (Fig. 183, p. 422).
Another peculiarity of all Ctenophora (except the Beroidae) is the presence, at some stage in the life-history, of two long and extremely contractile tentacles. There is also a well-developed sense-organ (statocyst) in the centre of the aboral area of the body.
The Ctenophora differ from the Turbellaria in the presence of the combs and of the two long tentacles, in the position and relative importance of the statocyst, and, with the exception of Coeloplana, in the general characters of the alimentary canal.
Shape.—Several of the Ctenophora are conical or spherical in shape, but exhibit at the pole where the mouth is situated {413}(Fig. 180, M) a slight conical projection, and at the opposite pole where the sense-organ is placed a slight depression (Ab). In others, the sides of the body are drawn out into a pair of wing-like lobes (Lobata), and the body is considerably flattened or compressed (Fig. 181). The Cestoidea have a long flattened ribbon- or band-shape (Fig. 182), and the Platyctenea (Fig. 183) are flattened in the oro-apical axis and exhibit a well-marked distinction between the dorsal and ventral surfaces. The shape of Beroe is that of a hollow cone or thimble.

Fig. 180.—Hormiphora plumosa. Ab, position of the aboral sense-organ; Ct, rib of ctenophoral plates; M, mouth; t, tentacle, with two kinds of pinnae. (After Chun.)
Ctenophoral plates.—In many Ctenophora eight lines can be traced, like the lines of longitude on a globe, from the area of the sense-organ to the base of the mouth-cone or hypostome. In the course of these lines are situated the ctenophoral plates. In some species they extend along the greater part of these lines of longitude, but in others they are more restricted. That part of the line that bears the plates is called the "rib" or "costa." These plates or combs form the principal organs of locomotion of the Ctenophores. They consist of a row of cilia fused at the base (cf. p. 141) to form the plate, but free at the extremity where they form the comb-like edge. They are alternately raised, by a rapid contractile action, and then slowly flattened down again. The plates are raised in succession from the aboral to the oral end of each rib, and the appearance given to the bands in the living animal is that of a series of waves travelling down the lines of longitude from the sensory area towards the mouth. The effect of these rhythmic movements of the combs is to {414}drive the animal slowly through the water with the oral cone forwards. In some Ctenophores the costæ are phosphorescent.[427]
Tentacles.—In all the Ctenophora, except the Beroidae and the adult stages of Lobata and Cestoidea, there is a single pair of tentacles. They are attached to the base of a blind funnel-shaped pit which opens to the exterior near the equator of the animal's body. The pits are on opposite sides of the body, and the plane which passes through them both vertically divides the body into approximately equal parts. It is called the "tentacular" or "transverse" plane (Fig. 180). The plane at right angles to this, which also passes through the mouth and statocyst, is called the "sagittal" plane.
The tentacles are solid, and in the Cydippidae, of considerable length. During life they are usually extended, and trail behind the animal as it progresses through the water. But they are extremely contractile, and when the animal is alarmed are suddenly withdrawn into the shelter of the tentacular pits. Each tentacle usually bears a row of short pinnae. The surfaces of the tentacles and of their pinnae are crowded with remarkable cells which carry little globules of an adhesive secretion, and are called the glue-cells or "colloblasts." These cells stick to any foreign body they touch, and may be drawn out some distance from the tentacle, but they remain attached to it by a long spiral thread which unwinds as the cell is pulled out. Although the colloblasts have the function of catching prey similar to that of the nematocysts of Coelenterata, they are true animal cells and are not therefore homologous with nematocysts, which are the cell products of the cnidoblasts.[428]
The Lobata and Cestoidea pass through a stage in development called the Cydippiform or Mertensia stage, when they possess a single pair of long tentacles similar to those described above. In the adult condition, however, these tentacles are absent, and their functions are performed by numerous small accessory tentacles or tentilla arranged in rows on definite lines along the body-wall.
Sense-organ.—At the aboral pole of the Ctenophore there is a hard granulated calcareous body, the "statolith." This is {415}supported by four tufts of fused cilia, and is usually covered by a dome of delicate protoplasmic texture, which is believed to be formed by a fusion of cilia. The dome enclosing the statolith is called the "statocyst."
Supporting the statocyst there is a circular or oval area of ciliated epithelium which is usually supposed, but on insufficient evidence, to be specially sensory in function. Extending from this area in the sagittal plane there are two strips of ciliated epithelium called the "polar fields."
The aboral sense-organ of the Ctenophora is one of the most characteristic organs of the Phylum. The aboral pole of the Medusae of Coelenterata is usually devoid of any special modification of the ectoderm of the bell, and in the Tiarid genus Stomatoca the little tassel at the aboral pole of the Medusa cannot in any sense be regarded as a homologue of the sense-organ of the Ctenophore. If the aboral sense-organ of the Ctenophora can be compared with that of any other group of animals, it would be with the statocyst of many of the Turbellaria, such as that of Convoluta, but it is far more satisfactory to regard it as an organ peculiar to the Ctenophora and as having no true relationship with any sense-organ found in other animals.
Alimentary Canal.—The mouth of the Cydippiform Ctenophores opens into a sac-like chamber called the "stomodaeum," flattened in the sagittal plane and stretching from the oral pole as far as the centre of the body. The stomodaeum passes into a chamber flattened in the transverse plane called the "infundibulum." From the infundibulum a narrow tube passes in the direction of the aboral pole called the "intestine," and from the extremity of this four short tubes pass to the sides of the polar fields at the place where these fields join the sensory area. Two, or, in some cases, all four of these tubes open to the exterior; but they do not appear to serve the purpose of ejecting the undigested portions of the food, which usually pass to the exterior by the mouth as in Coelenterata and Turbellaria.
From the lateral extremities of the infundibulum four pairs of tubes pass to the equatorial region of the body, where each one joins a longitudinal vessel which runs immediately beneath the epithelium supporting the ribs. These are called the longitudinal or "sub-costal" canals. From the infundibulum there also {416}passes a single pair of blind canals, the "paragastric canals," one on each side of the stomodaeum, to end in the oral cone.
In the Lobata the paragastric canals communicate with the longitudinal canals under the transverse costae,[429] and send long blind processes into the lobes. In the Cestoidea the arrangement of the canals is considerably modified in adaptation to the needs of the ribbon-like body. In the Beroidae the paragastric and longitudinal canals are in communication by a peripheral network of canals, and in the Platyctenea there is also a network of canals but without any definite longitudinal vessels.
Sexual Organs.—Most of the Ctenophora are undoubtedly hermaphrodite, but Willey was unable to find ova in some of his specimens of Ctenoplana that were producing spermatozoa. In the Cydippidea the ova are produced on one side of the longitudinal canal and the spermatozoa on the other. Each longitudinal canal therefore performs the functions of a hermaphrodite gland. When the sexual cells are ripe they escape into the infundibulum and are discharged by the mouth. In Ctenoplana there are definite and direct male genital ducts.
The ova are very small when discharged and undergo complete segmentation in the sea water. The development of the Cydippidea is really direct, but there is a stage passed through in which the tentacles are relatively very prominent and situated close to the aboral pole, and this stage is very different in appearance from the adult. In the Lobata and Cestoidea there is, however, a definite larval stage, of the general appearance of a Mertensia, and during this stage fertile eggs and spermatozoa are formed and set free.
Distribution.—Ctenophora are found at the surface of nearly all seas, and many of the genera have a cosmopolitan distribution. Some of the Lobata, the Cestoidea, and the Platyctenea are more commonly found in the warmer regions of the world. Pleurobrachia pileus, Bolina infundibulum, Beroe ovata, and B. cucumis occur off the British coast.
Most of the Ctenophora are from 5 to 20 mm. in diameter, but Beroe reaches the length of 90 mm., Eucharis multicornis {417}a height of 250 mm., and Cestus veneris has been found no less than 1½ metres from one extremity to the other.
Ctenophores usually go about in shoals, and in the case of Beroe cucumis and Eucharis multicornis the shoals may be of very great extent. Pleurobrachia pileus of the British coasts is often found at the end of the season (July) as a series of isolated individuals; but in June they occur in small shoals, swimming so close together that they will choke a tow-net in a very short space of time.
CLASS I. TENTACULATA
Ctenophora provided with a pair of tentacles in the larval stages only or in both larval and adult stages.
Order I. Cydippidea.
This order includes a number of spherical or oval Ctenophores, with a pair of tentacles retractile into deep tentacular pits in the adult stage.
Fam. 1. Mertensiidae.—The body is compressed in the transverse plane, and the ribs on the transverse areas are longer than those on the sagittal areas. The family includes the genus Euchlora, which occurs in the Mediterranean and in the northern part of the Atlantic Ocean. In Charistephane there are only two enormous ctenophoral plates in each of the longitudinal tracts. These plates are so broad that they almost meet laterally to form two continuous circlets round the body of the animal. This genus is found in the Mediterranean, but a few specimens have also been obtained in the Atlantic.
In Tinerfe the body is almost cylindrical, and there is a pair of kidney-shaped swellings at the sides of the aboral pole. It has a pale blue colour, and is found in the Guinea and south equatorial currents of the Atlantic Ocean.
The name Mertensia has been given to several forms that are undoubtedly the young stages of genera belonging to the Lobata, but Chun retains the name M. ovum for a species which is very abundant in the Arctic currents of the North Atlantic.
Fam. 2. Callianiridae.—Two or four wing-like processes, into {418}which the longitudinal canals extend, are found at the aboral pole. Callianira has two of these processes arranged in the transverse plane, and Lophoctenia has four. Callianira is found in the Mediterranean and in the Atlantic from the Arctic to the Antarctic waters.
Fam. 3. Pleurobrachiidae.—The body is almost spherical in form, and the eight ribs are equal in length.
This family includes the genus Pleurobrachia, in which the ribs extend for a considerable distance along the lines of longitude of the spherical body, but do not reach either the oral or the aboral areas. P. pileus is the commonest British Ctenophore, and may be found in shoals in May, June, and July at the surface of the sea or cast up on the sand as the tide ebbs. It is widely distributed in the North Atlantic waters. P. rhodopis of the Mediterranean has rather shorter ribs than P. pileus. Two new species have recently been described from the Malay Archipelago.[430] Hormiphora (Fig. 180, p. 413) differs from Pleurobrachia in having much shorter ribs, and in possessing two kinds of pinnae on the tentacles, those of the ordinary kind and others much larger and sometimes palmate in character. This genus has a world-wide distribution.
In Lampetia and Euplokamis the body is more cylindrical in shape than it is in the other genera, but the ribs and subjacent longitudinal canals extend up to the margin of the aboral field. Both these genera occur in the Mediterranean, but Lampetia is also found in the Malay Archipelago.
Order II. Lobata.
The body is considerably flattened in the transverse plane, and the sagittal areas are extended into the form of two wide peristomial lobes. The oral ends of the areas between the transverse and sagittal ribs are extended to form four flaps, called the "auricles." There are no tentacles nor tentacle-sheaths of the ordinary kind in the adult form; but numerous tentilla, similar in some respects to the pinnae of the tentacles of other Ctenophora, form a fringe round the margin of the auricles and the peristome. A single pair of long, filamentous, non-retractile tentacles arise from the sides of the peristomium in Eucharis {419}multicornis. These tentacles have no sheaths, and do not bear pinnae. They are probably not homologous with those of other Ctenophora.
The characters that separate the families of Lobata are chiefly those of varying size, shape, and position of the peristomial lobes and auricles. In the Lesueuriidae the peristomial lobes are rudimentary; in the other families they are moderately or very large. In the Bolinidae the auricles are short, but in most of the other families they are long and ribbon-like. In Eucharis they can be spirally twisted in repose.
The modifications of the external form seen in the Lobata are accompanied by some modifications of the internal structure. Among these, perhaps the most interesting is a communication between the transverse longitudinal and the paragastric canals, and the long convoluted tubes given off to the peristomial lobes by the sagittal longitudinal canals. Very little is known about the life-history and development of most of the Lobata, but Chun has shown that in Eucharis and Bolina there is a Cydippiform larval stage which produces ripe ova and spermatozoa. This is followed by a period of sterility, but when the adult characters are developed they become again sexually mature. To this series of sexual phenomena the name "Dissogony" is given.

Fig. 181.—Ocyroe crystallina. Ab, aboral sense-organ; au, auricle; Can, diverticulum from the paragastric canal passing into peristomial lobe; Ct, costae; M, mouth; Par, paragastric canal passing outwards to join one of the transverse subcostal canals; P.L, peristomial lobe; w, wart-like tubercles on the lobe. (After Mayer.)
The order contains only fifteen genera, but they are usually arranged in the following eight families:—
1. Lesueuriidae. Lesueuria.
2. Bolinidae. Bolina, Bolinopsis.
3. Deiopeidae. Deiopea.
4. Eurhamphaeidae. Eurhamphaea.
{420}5. Eucharidae. Eucharis.
6. Mnemiidae. Mnemia, Mnemiopsis.
7. Calymmidae. Calymma.
8. Ocyroidae. Ocyroe.
Most of these Ctenophores occur in the warm and tropical seas; but Bolina is found occasionally at Plymouth in the month of May, on the west coast of Ireland, and at other stations on the British coasts. Eucharis is regarded as one of the most beautiful of the Phylum. A swarm, some miles in length, of large specimens of E. multicornis was met by the Plankton Expedition in the south equatorial current of the Atlantic during the month of September.
Order III. Cestoidea.
In this order the body is so much compressed in the transverse plane and elongated in the sagittal plane that it assumes the shape of a long narrow band or ribbon. The tentacular sheaths are present but the tentacles are degenerate in the adult. The tentacular functions are performed by numerous tentilla situated in long grooves extending along the whole length of the oral side of the band-like body. The transverse ribs are reduced; the sagittal ribs extend along the whole of the aboral side.
Fam. Cestidae.—This is the only family of the order. Cestus veneris, the Venus's girdle of the Mediterranean Sea, is also found in the Atlantic Ocean, and specimens belonging to the same genus, but probably to a different species, occur as far north as the White Sea. Some of the larger specimens are considerably over 1 metre in length.

Fig. 182.—Cestus pectenalis. Ab, aboral sense-organ; Ct, the sagittal ribs; M, mouth. (After Bigelow.)
C. pectenalis was found in abundance off one of the Maldive Islands [431] and differs from C. veneris in having a large and {421}prominent orange patch at each end of the body. It is said to be extremely graceful in the water, moving with slow, ribbon-like undulations, and shining in the sunlight with a violet iridescence. Vexillum, from the Mediterranean Sea and Canary Islands, is rather more pointed at the extremities than Cestus, and differs from it in some important anatomical characters.
Order IV. Platyctenea.
This order has been constituted for two remarkable genera, in which the oro-apical axis is so much reduced that distinct dorsal and ventral surfaces can be distinguished.
There is a single pair of long milky-white tentacles capable of complete retraction into tentacular sheaths.
Fam. 1. Ctenoplanidae.—Ctenoplana was discovered by Korotneff in 1886 floating with the Plankton off the coast of Sumatra. In 1896 Willey [432] discovered four specimens on a cuttle-bone floating off the coast of New Guinea. To these authors we are indebted for the only accounts of this animal that have been published.
When the Ctenoplana is creeping on the bottom of a dish or with its dorsal side downwards on the surface film of the water, it has the form of a flattened disc with a notch on each side. On the upper or dorsal surface eight short rows of ctenophoral plates may be seen, and in a position corresponding with the two notches in the margin of the body are situated the two sheaths from which the long pinnate tentacles protrude. In the exact centre of the dorsal surface is situated the statolith, supported by stiff processes from adjacent cells; and forming a circlet round the statolith there is a row of short ciliated tentacles. These tentacles, however, when examined carefully in the living animal, are found to be arranged in two sets of about nine in each, separated by narrow gaps on each side, the gaps corresponding in position with the axis through the tentacles.
When the animal is swimming it assumes a helmet-shape by depressing the sides of the body like a pair of flaps on the tentacular axis, and then the ctenophoral plates come into play and produce the progressive movements of the animals. The pinnate tentacles are opaque white in colour, and have peculiar serpentine {422}movements. Very little is known at present concerning many details of the internal anatomy, but there is one point of considerable theoretical interest—namely, the presence of definite male genital ducts.
Three of Dr. Willey's specimens were mottled with a green pigment, whereas his fourth specimen and Korotneff's only specimen were mottled with a red pigment. It has yet to be determined whether the differences which have been observed in the individual specimens are of specific value.
Fam. 2. Coeloplanidae.—Coeloplana was originally discovered by Kowalevsky in the Red Sea, but has recently been found by Abbott [433] on the coast of Japan.

Fig. 183.—Coeloplana mitsukurii, floating at the surface of the sea with the dorsal side downwards. T, T, the tentacles expanded. (After Abbott.)
The Japanese species are found principally on encrusting Algae, Zostera, Melobesia, etc., which they resemble very closely in colour. The Red Sea species is, according to Kowalevsky, ciliated all over, but the Japanese species are ciliated only on the ventral surface. As in Ctenoplana, the body of Coeloplana is a flattened disc with a notch at each end of the tentacular axis, when creeping; but Coeloplana does not swim, nor at any time does it assume a helmet-shape. The tentacles are very long and of a chalky-white colour. They can be retracted into tentacle-sheaths. When the animal is excited it throws out the whole tentacle in a cloud of white filaments, "and to watch it at such a time, shooting out and retracting the tentacles, moving along the side of the aquarium like a battleship in action is truly a remarkable spectacle."[434] On the dorsal side of the body there is a series of processes which are called the dorsal tentacles. The statolith is very small, and is not surrounded by sensory processes as it is in Ctenoplana. There are no ctenophoral plates. The colours of the Japanese {423}species are scarlet or carmine red and dirty brown or brownish yellow. They are from 1 to 2 centimetres in diameter.
CLASS II. NUDA
Ctenophora without tentacles.
Fam. Beroidae.—Beroe, the only genus of this family and class, differs from other Ctenophora in several important particulars. There are no tentacles, and the stomodaeum is so large that the body-form assumes that of a thimble with moderately thick walls. The infundibulum is small. The paragastric and longitudinal canals give rise to numerous ramifications which form a network distributed throughout the surface of the body. The statolith is unprotected by a dome, and the polar fields are bordered by a number of small branching papillae. The eight ribs extend for nearly the whole length of the body. Beroe is almost cosmopolitan, and is frequently found at the surface of the sea in great numbers. B. ovata is found off the Shetlands, Hebrides, and west coast of Ireland, but is rare on the east coast of the British Islands and in the English Channel. At Valencia it is common in August and September, and sometimes reaches the great size of 90 mm. in length by 50 mm. in breadth. It is usually of a pale pink colour.
Appendix to Ctenophora
Hydroctena salenskii has recently been discovered by Dawydoff[435] floating with the Plankton off the island Saparua in the Malay Archipelago. It is claimed to be a connecting link between the Ctenophora and the Medusae of the Hydrozoa.
In external features it is like one of the Narcomedusae, having a transparent jelly-like bell with a wide bell-mouth guarded by a velum (Fig. 184, V). There are only two simple but solid tentacles (t), provided with tentacle-sheaths, but inserted on opposite sides of the bell—not on the margin, but, as in the Ctenophore, at a level not far removed from the aboral pole. At the aboral pole there is a minute pore surrounded by a high ciliated epithelium bearing an orange pigment. This leads into {424}a short blind canal, which terminates in an ampulla bearing two statoliths supported by elastic processes from the ampullar epithelium.
The sub-umbrellar cavity extends for a distance of about one-half the height of the bell. The mouth (M), which opens into this cavity, leads into a wide cavity that gives off a short blind canal to the side of each tentacular sheath, and a straight tube that leads straight to the statocyst, where it also ends blindly. There are no radial canals and no ring canal at the margin of the umbrella. There are also no ctenophoral plates. In the absence of any information concerning the position of the genital glands, the character of the epithelium of the tentacles and the development, we are not justified in regarding Hydroctena either as a Ctenophore or as a connecting link between the Ctenophora and the Hydromedusae. It may be regarded simply as a Craspedote Medusa, probably related to the Narcomedusae, with a remarkable aberrant aboral sense-organ.

Fig. 184.—Hydroctena salenskii. ab, Aboral organ; M, manubrium; t, tentacle; V, velum. (After Dawydoff.)
BY
E. W. MacBRIDE, M.A., FRS.
Formerly Fellow of St. John's
College
Professor of Zoology in McGill University, Montreal.
ECHINODERMATA—INTRODUCTION—CLASSIFICATION—ANATOMY OF A STARFISH—SYSTEMATIC ACCOUNT OF ASTEROIDEA
The name Echinodermata[436] means literally "spiny-skinned," and thus brings into prominence one very conspicuous feature of most of the animals belonging to this phylum. All, it is true, do not possess spines; but with one or two doubtful exceptions, all have calcareous plates embedded in the skin, and these plates, in many cases, push out projections which raise the skin into corresponding elevations, which are called the spines. The spines are, like the other plates, inside the skin, and to speak of an Echinoderm living in its shell, as we speak of a Snail, is a serious error. The shell of a Mollusc is fundamentally a secretion poured forth from the skin, and is thus entirely external to the real living parts; but the plates and spines of an Echinoderm may be compared to our own bones, which are embedded deeply in the flesh. Hence the name ossicle (little bone) is used to designate these organs.
Besides the possession of these spines, Echinoderms are characterised by having their organisation pervaded by a fundamental radial symmetry. The principal organs of the body are repeated and are arranged like the spokes of a wheel round a central axis instead of being, as, for example, in Chaetopoda, arranged behind one another in longitudinal series.
In addition to these striking peculiarities, Echinoderms possess a most interesting internal organisation, being in this respect almost exactly intermediate between the Coelenterata {428}and the higher Invertebrata. Like so many of the latter, the Echinodermata have an anus, that is, a second opening to the alimentary canal through which indigestible material is rejected; like them also, they have a body-cavity or coelom surrounding the alimentary canal—from the lining of which the genital cells are developed. On the other hand, there is no definite circulatory system, nor any specialised excretory organ, and the nervous system exhibits no concentration which could be called a brain, and is, moreover, in close connexion with the skin. In all these points the Echinodermata resemble the Coelenterata.
One of the most characteristic features of the internal anatomy of Echinodermata is the presence of a peculiar series of organs, known collectively as the water-vascular system or hydrocoel. This is really a special division of the coelom or body-cavity which takes on the form of a ring-shaped canal embracing the mouth, from which are given off long radial canals, usually five in number, running to the more peripheral parts of the body.[437] Each radial canal carries a double series of lateral branches, which push out the skin so as to appear as appendages of the body. These appendages are known as tentacles or tube-feet; they are both sensory and respiratory in function, and often in addition, as the name tube-foot indicates, assist in locomotion. As a general term for these appendages, to be applied in all cases without reference to their function, the name podium has been suggested and will be employed here. A system of canals, in many ways resembling the water-vascular system, is found in Brachiopoda, Gephyrea and Polyzoa, but the peculiarity of Echinodermata is the way in which it is kept filled with fluid. From the ring-canal in the interval (or interradius) between two radial canals, a vertical canal, termed the stone-canal, is given off, which communicates with the exterior by means of a sieve-like plate, the madreporite, pierced by fine canals. These canals and the stone-canal itself are lined with powerful cilia, which produce a strong inward current, and keep the water-vascular system tensely filled with sea water.
The phylum includes the familiar Starfish and Sea-urchins, which in sheltered spots are found between tide-marks; the {429}Brittle Stars and Sea-cucumbers, which can be dredged up from below low-water mark, and lastly the beautiful Feather-stars, of which there are comparatively few species still living, although huge beds of limestone are composed of the remains of fossil Feather-stars.
One species of Sea-cucumber (Synapta similis)[438] is said to enter brackish water in the mangrove swamps of the tropics; but, with this exception, the whole phylum is marine. A few species can endure partial exposure to the air when left bare by the receding tide, but the overwhelming majority are only found beneath low-water mark, and a considerable number live in the deepest recesses of the ocean.
Their distribution is, no doubt, partly determined by food, a number of species being strictly confined to the neighbourhood of the shore. On the other hand, since a very large number of species live on the layer of mud impregnated with animal remains which forms the superficial layer of the deposit covering the sea-floor, it is not surprising to learn that many have an exceedingly wide range, since this deposit is very widely distributed. Another equally important factor in determining distribution is wave-disturbance, and it is surprising to learn to what a depth this extends. Off the west coast of Ireland a large wave literally breaks on a submerged rock 15 fathoms beneath the surface. Speaking generally, it is useless to look for Echinoderms on an exposed coast, and the same species, which in the sheltered waters of the Clyde are exposed at low water, must be dredged up from 20 to 30 fathoms outside Plymouth Sound.
The ordinary collector is attracted to the group chiefly by the regularity and beauty of the patterns produced by the radial symmetry, but to the scientific zoologist they are interesting from many other points of view. Differing widely nevertheless from the higher Invertebrata in their symmetry when adult, they have as larvae a marked bilateral symmetry, and the secondary development of the radial symmetry constitutes one of the most remarkable life-histories known in the animal kingdom.
Then again, owing to the possession of ossicles, the Echinodermata are one of the few groups of Invertebrata of which abundant remains occur fossilised. In attempting, therefore, to {430}decipher the past history of life from the fossil record, it is necessary to have an exact and detailed knowledge of Echinoderm skeletons and their relation to the soft parts. Lastly, the internal organisation of Echinoderms throws valuable light on the origin of the complicated systems of organs found in the higher animals.
Echinodermata are divided into two great sub-phyla, which must have very early diverged from one another. These are:—
(1) Eleutherozoa,
(2) Pelmatozoa.[439]
The sub-phylum Pelmatozoa, to which the living Feather-stars (Crinoidea) and the majority of the known fossil species belong, is characterised by the possession of a fixing organ placed in the centre of the surface opposite the mouth—the aboral surface as it is called. Ordinarily this organ takes on the form of a jointed stalk, but in most modern species it is a little knob with a tuft of rooting processes, termed cirri. In the other sub-phylum, the Eleutherozoa, no such organ is found, and the animals wander about freely during their adult life, though for a brief period of their larval existence they may be fixed by a stalk-like protuberance arising from the oral surface.
SUB-PHYLUM I. ELEUTHEROZOA
The Eleutherozoa are divided into four main classes, between which no intermediate forms are found amongst the living species, though intermediate types have been found fossil.
The four classes into which the Eleutherozoa are divided are defined as follows:—
(1) Asteroidea (Starfish).—"Star"-shaped or pentagonal Eleutherozoa with five or more triangular arms, not sharply marked off from the central disc. The mouth is in the centre of one surface, called from this circumstance the "oral"; the anus is in the centre of the opposite surface, termed the "aboral." From the mouth a groove runs out on the under surface of each {431}arm towards its tip, termed the "ambulacral" groove. Projecting from the ambulacral groove are found the podia or tube-feet, the organs of movement and sensation of the animal.
(2) Ophiuroidea (Brittle Stars).—Eleutherozoa, in which the body consists of a round disc with long worm-like arms inserted in grooves on its under surface. No anus is present, and the ambulacral grooves are represented by closed canals. The podia are merely sensory and respiratory, locomotion being effected by muscular jerks of the arms.
(3) Echinoidea (Sea-urchins).—Globular or disc-shaped Eleutherozoa, in which the skeleton forms a compact cuirass except for a short distance round the mouth (peristome) and round the anus (periproct). The ambulacral grooves are represented by canals which, like meridians of longitude on a school-globe, run from the neighbourhood of the mouth to near the aboral pole of the body. The spines are large and movably articulated with the plates. The animals move by means of podia and spines, or by means of the latter only. The anus is usually situated at the aboral pole, but is sometimes displaced towards the side, or even on to the ventral surface.
(4) Holothuroidea (Sea-cucumbers).—Sausage-shaped Eleutherozoa, in which the skeleton is represented only by isolated nodules of calcium carbonate, and in which the body-wall is highly muscular. The mouth and anus are situated at opposite ends of the body, and the ambulacral grooves (represented by closed canals) run from near the mouth to the proximity of the anus. Movement is accomplished by means of the podia, aided by worm-like contractions of the body.
CLASS I. ASTEROIDEA[440] (Starfish)
The Starfish derive their name from their resemblance in shape to the conventional image of a star. The body consists of broad triangular arms (generally five in number) which coalesce in the centre to form a disc. The skin is soft and {432}semi-transparent, permitting the skeleton to be easily detected; this consists of a mesh-work of rods or plates, leaving between them intervals of soft skin. In a living Starfish it can be seen that many of these soft places are raised up into finger-like outgrowths, which are termed "papulae" or "dermal gills," through the thin walls of which an active interchange of gases with the surrounding water takes place, and the animal obtains in this way the oxygen necessary for its respiration.
Very few and feeble muscle-fibres exist in the body-wall, and the movements of the arms, as a whole, are very slow and limited in range. There is a membranous lip surrounding the mouth, from which five broad grooves run outwards, one on the underside of each arm. These are termed the "ambulacral grooves." Each groove is Λ-shaped, and its sides are stiffened by a series of rod-like ossicles called the "ambulacral ossicles."
The animal progresses by the aid of a large number of translucent tentacles, termed "tube-feet" or "podia," which are attached to the walls of the ambulacral grooves.
Anatomy of a Starfish.—As an introduction to the study of the anatomy not only of Starfish but of Echinodermata as a whole, we select Asterias rubens, the common Starfish of the British coasts, which in many places may be found on the beach near low-water mark.
External Features.—In this species (Fig. 185) the skeleton is a net-work of rod-like plates, leaving wide meshes between them, through which protrude a perfect forest of transparent papulae. From the points of junction of the rods arise short blunt spines surrounded by thick cushions of skin. The surfaces of these cushions are covered with a multitude of whitish specks, which, on closer inspection, are seen to have the form of minute pincers, each consisting of two movable blades crossing each other below and articulated to a basal piece. These peculiar organs are termed "pedicellariae" (Fig. 186), and their function is to keep the animal clean by seizing hold of any minute organisms which would attempt to settle on the soft and delicate skin. When irritated the blades open and then snap together violently, and remain closed for a long time.[441] These actions are brought about by appropriate muscles attaching the blades to the basal piece.
The last-named ossicle increases the certainty of the grip by fixing the lower parts of each blade in the same vertical plane, and preventing lateral slipping, so that it serves the same purpose as the pivot in a pair of scissors. Each blade, in fact, fits into a groove on the side of this piece. The muscles which close the blades arise from the lower ends (handles) of the blades, and are united below to form a common muscular string which attaches the whole organ to one of the plates of the skeleton. An attempt of the victim to tear the pedicellaria out is resisted by the contraction of this string, which thus brings about a closer grip of the blades. In order that the blades may open they must first be lifted out of the grooves on the basal piece—this is effected by special lifting muscles. The opening is {434}brought about by muscles extending from the "handle" of one blade to the upper part of the other.
Scattered about amongst the papulae between the cushions are other pedicellariae of a larger size in which the blades do not cross one another (Fig. 186, B).
In the space or "interradius" between two arms, on the aboral surface, there is found a button-shaped ossicle. This is covered with fine grooves, and from a fancied resemblance between it and some forms of coral it has received the name "madreporite" (Fig. 185, mad). The bottoms of the grooves are perforated by capillary canals lined by flagella, through the action of which water is constantly being introduced into the water-vascular system.
The anus is situated near the centre of the upper surface of the disc, but it is so minute as to require careful inspection in order to discover its position (Fig. 185).

Fig. 186.—View of pedicellariae of A. glacialis. A, Crossed form, × 100. 1, Ectoderm covering the whole organ; 2, basal piece; 3, auxiliary muscle closing the blades; 4, muscle lifting right blade out of the groove; 5, handle of left blade; 6, muscles closing the blades, and uniting to form 7, the muscular string attaching the pedicellaria to the skeleton. B, straight form, × 10. 1, Basal piece; 2, blades; 3 and 4, muscles closing the blades; 5, muscle opening the blades. (From Cuénot.)
On the under side of the animal the most conspicuous features are the five ambulacral grooves which radiate out from the "peristome," a thin membranous area surrounding the central mouth. The grooves are filled with the tube-feet, which are closely crowded together and apparently arranged in four rows.
Skeleton.—The sides of the ambulacral grooves are stiffened by the rod-like "ambulacral ossicles." To the outer ends of these are articulated a set of shorter rods termed the "adambulacral ossicles" which carry each two or three rod-like spines, the "adambulacral spines," the skin covering which bears numerous pedicellariae (Fig. 187, B). When the animal is irritated the edges of the groove are brought together, and these {435}spines then form a trellis-work covering and protecting the delicate tube-feet; the numerous pedicellariae are then in a position to make it unpleasant for any intruder. The closure of the groove is effected by means of powerful muscles connecting each ambulacral ossicle with its fellow. There are also feebler muscles connecting these plates with their successors and predecessors, which enable the arm to be bent downwards in a vertical plane. It is raised by a muscular band running along the dorsal wall of the coelom to the point of the arm.

Fig. 187.—A, Asterias rubens, seen from the oral surface, drawn from a living specimen, × 1. B, an adambulacral spine, showing three straight pedicellariae; C, a tube-foot expanded and contracted.
When the series of ambulacral and adambulacral ossicles is followed inwards towards the mouth it is seen that the first ambulacral ossicle is closely fixed to the second, but is widely {436}separated from its fellow, remaining, however, connected with the latter by a powerful adductor muscle. In consequence of the separation of this pair of ossicles each is brought into closer contact with the corresponding ossicle in the adjacent radius, to which it is connected by a muscle called the abductor. The first adambulacrals in adjacent radii are also brought into closer contact and carry long spines which, when the ambulacral grooves are contracted, project like a grating over the mouth. In the order of Asteroidea to which Asterias belongs, the adambulacrals themselves do not project much, but in all other cases they form prominent mouth-angles, so that the opening of the mouth becomes star-shaped (Fig. 211, p. 483).
Except in the case of the ambulacral and adambulacral plates little regular arrangement is to be detected in the ossicles of the skeleton which, as has already been mentioned, form a mesh-work. If, however, the arm be cut open and viewed from the inside it will be seen that the edge is strengthened above and below by very thick, powerful, rod-like plates. These are called the "supero-marginal" and "infero-marginal" ossicles; they are not visible from the outside, since they are covered by a thick layer of the body-wall containing other smaller plates (Fig. 190, marg). In many genera, however, they are exposed, and form a conspicuous edging to the arm above and below. In many genera, also, there are three conspicuous series of plates on the back of each arm, viz. a median row, called "carinals" (car., Fig. 191), and two lateral rows, termed "dorso-laterals" (d.lat., Fig. 191). These three rows, with the two rows of marginals, one of ambulacrals, and one of adambulacrals on each side (11 rows in all), constitute the primitive skeleton of the arm, and appear first in development.
The structure of all these elements of the skeleton is the same. They may be described as scaffoldings of carbonate of lime, interpenetrated by a mesh-work of cells fused with one another, by which the carbonate of lime has been deposited. The matrix in which the ossicles lie is a jelly-like substance traversed by a few bands of fibres which connect the various rods with one another. This jelly is almost fluid in the fresh state, but when heated forms a hard compound, possibly allied to mucin, which will turn the edge of a razor.
When the covering of the back is dissected off the coelom is {437}opened. This is a spacious cavity which apparently surrounds the alimentary canal and extends into the arms. It has, however, its own proper wall, which is called the "peritoneum," both on the outer side, where it abuts on the skin, and on the inner side, where it comes in contact with the wall of the alimentary canal. The outer wall is called the "somatic peritoneum," and it is possible to dissect off the rest of the body-wall and leave it intact; the inner wall, from its close association with the alimentary canal, is termed the "splanchnic peritoneum." This wall can only be distinguished in microscopic sections from that of the alimentary canal, to which it is closely applied.
The coelom is filled with a fluid, which is practically sea water with a little albuminous matter in solution. Through the thin walls of the papulae oxygen passes into this fluid, whence it easily reaches the inner organs, since they are all in contact with some part of the coelomic wall. Similarly CO2 is absorbed by the coelomic fluid from all parts of the body, and diffuses through the papulae to the surrounding water.
The Starfish possesses no definite kidney for getting rid of nitrogenous waste. In most of the higher animals with a well-developed coelom it has been proved that the kidney is simply a specialised portion of the coelom, and in many cases some parts of the coelomic wall still retain their excretory functions, which apparently the whole originally possessed. In the Starfish and in Echinodermata generally this primitive state of affairs is still retained. From the cells forming the coelomic wall, cells are budded off into the fluid, where they swim about. These cells from their movements are called amoebocytes. If a substance such as indigo-carmine, which when introduced into the tissues of the higher animals is eliminated by the kidney, is injected into the Starfish, it is found soon after to be vigorously absorbed by the amoebocytes. These later accumulate in the dermal branchiae, through the thin walls of which they make their way[442] to the outside, where they degenerate.
The coelom is indented by five folds, which project inwards from the interradii. These folds are called the "interradial septa"; they are stiffened by a calcareous deposit, which is not, however, sufficiently dense to constitute a plate. In one of the {438}septa the axial sinus and stone-canal (see below) are embedded. These septa are to be regarded as areas of lateral adhesion between the arms.

Fig. 188.—View of upper half of a specimen of Asterias rubens, which has been split horizontally into two halves. ax.c, Axial sinus; g.d, genital duct; oe, cut end of the oesophagus, the narrow neck of the stomach; py, pyloric sac; py.c, pyloric caeca; r, rectum; r.c, rectal caeca; sept, interradial septum; st.c, stomach lobe.
The alimentary canal consists of several distinct portions. The mouth leads by a narrow neck called the "oesophagus" into a voluminous baggy sac termed the "stomach," which is produced into ten short pouches, two projecting into each arm. The stomach leads in turn by a wide opening into a pentagonal flattened sac, the "pyloric sac," which lies above it. Each angle of the pyloric sac is prolonged into a tube—the so-called "pyloric duct"—running out into the arm, where it immediately bifurcates into two forks, each beset by a large number of small pouches {439}and attached to the dorsal wall of the coelom by suspensory bands of membrane called mesenteries. These ten forks are called "pyloric caeca"; they are of a deep green colour owing to the pigment in their wall. Beyond the pyloric sac the alimentary canal is continued as the slender "rectum" to the anus. The rectum gives off two small branched pouches of a brown colour called "rectal caeca." This comparatively complicated form of alimentary canal is related to the nature of the food of the animal and the method it employs to capture its prey.
The favourite food[443] of Asterias consists of the common bivalves of the coast, notably of the Mussel (Mytilus edulis). There is, however, no animal which it will not attack if it is fortunate enough to be able to catch it. The Starfish seizes its prey by the tube-feet, and places it directly under its mouth, folding its arms down over it in umbrella fashion. The muscles which run around the arms and disc in the body-wall contract, and the pressure thus brought to bear on the incompressible fluid contained in the coelom, forces out the thin membranous peristome and partially turns the stomach inside out. The everted edge of the stomach is wrapped round the prey.
Soon the bivalve is forced to relax its muscles and allow the valves to gape. The edge of the stomach is then inserted between the valves and applied directly to the soft parts of the prey which is thus completely digested. When the Starfish moves away nothing but the cleaned shell is left behind. If the bivalve is small it may be completely taken into the stomach, and the empty shell later rejected through the mouth.
It was for a long time a puzzle in what way the bivalve was forced to open. Schiemenz[444] has, however, shown that when the Starfish folds itself in umbrella-like form over the prey it holds on to the substratum by means of the tube-feet of the distal portions of the arms, whilst, by means of the tube-feet belonging to the central portions, it drags apart the valves by main force. He has shown experimentally: (1) that whilst a bivalve may be able to resist a sudden pull of 4000 grammes it will yield to a pull of 900 grammes long continued; (2) that a Starfish can exert a pull of 1350 grammes; (3) that a Starfish is unable to open a bivalve unless it be allowed to raise itself into a hump, so that the pull of the central tube-feet is at right angles to the prey. A Starfish confined between two glass plates walked about all day carrying with it a bivalve which it was unable to open.
The lining of the stomach is found to consist very largely of mucus-forming cells, which are swollen with large drops of mucus or some similar substance. It used to be supposed that this substance had some poisonous action on the prey and paralysed it, but the researches of Schiemenz show that this is incorrect. If when an Asterias is devouring a bivalve another be offered to it, it will open it, but will not digest it, and the victim shows no sign of injury but soon recovers. The cells forming the walls of the pyloric sac and its appendages are tall narrow cylindrical cells crowded with granules which appear to be of the nature of digestive ferment. This substance flows into the stomach and digests the captured prey.
A very small amount of matter passes into the rectum and escapes by the anus, as the digestive powers of the Starfish are very complete. The rectal caeca are lined by cells which secrete from the coelomic fluid a brown material, in all probability an excretion, which is got rid of by the anus.
When the meal is finished the stomach is restored to its former place by the action of five pairs of retractor muscles, one pair of which originates from the upper surface of the ambulacral ossicles in each arm and extends to the wall of the stomach, where they are inserted (Fig. 190, ret).
The tube-feet, which are at once the locomotor and the principal sensory organs of the Starfish, are appendages of that peculiar system of tubes known as the water-vascular system, which is derived from a part of the coelom cut off from the rest during the development of the animal. This system, as already mentioned, consists of (1) a narrow "ring-canal," encircling the mouth and lying on the inner surface of the membranous peristome; (2) a radial canal leaving the ring-canal and running along the under surface of each arm just above the ambulacral groove; (3) a vertical stone-canal running from the madreporite downwards to open into the ring-canal in the interspace between two arms. The madreporite is covered externally by grooves lined with long cilia, and is pierced with narrow canals of excessively fine calibre, the walls of which are also lined by powerful cilia. Most of these narrow canals open below into a main collecting canal, the stone-canal, but some open into a division of the coelom termed the axial sinus, with which also the stone-canal communicates by a lateral opening. The cavity of the stone-canal is reduced by the outgrowth from its walls of a peculiar Y-shaped projection, the ends being rolled on themselves in a complicated way (Fig. 190, B). The walls of the canal consist of a layer of very long narrow cells, which carry powerful flagella, and outside this of a crust of calcareous deposit, which gives rigidity to the walls and has suggested the name stone-canal.
The tube-feet are covered externally by ectoderm, inside which is a tube in connexion with the radial water-vascular canal. This latter is lined by flattened cells, which in the very young Starfish are prolonged into muscular tails; in the older animal these tails are separated off as a distinct muscular layer lying between the ectoderm and the cells lining the cavity of the tube. The tube-foot is prolonged inwards into a bulb termed the "ampulla," which projects into the coelom of the arm and in consequence is covered outside by somatic peritoneum. Just where the ampulla passes into the tube-foot proper the organ passes downwards between two of the powerful ambulacral ossicles which support {442}the ambulacral groove, and a little below this spot a short transverse canal connects the tube-foot with the radial canal which lies beneath these ossicles (Fig. 191).

Fig. 190.—A, view of the under half of a specimen of Asterias rubens, which has been horizontally divided into two halves. B, enlarged view of the axial sinus, stone-canal and genital stolon cut across. amb.oss, Ambulacral ossicle; amp. ampullae of the tube-feet; ax.s, axial sinus; gon, gonad; g.stol, genital stolon; marg, marginal ossicle; nerv.circ, nerve ring; oe, cut end of oesophagus; pst, peristome; ret, retractor muscle of the stomach; sept, interradial septum; stone c, stone-canal; T, Tiedemann's body; w.v.r, water-vascular ring-canal.
The tube-feet are, therefore, really a double row of lateral branches of the radial canal. The appearance of being arranged in four rows is due to the fact that the transverse canals connecting them with the radial canal are alternately longer and shorter so as to give room for more tube-feet in a given length of the arm. Each tube-foot ends in a round disc with a slightly thickened edge. The radial canal terminates in a finger-shaped {443}appendage, called the median tentacle, at the base of which is the eye.
The manner in which this complicated system acts is as follows:—When the tube-foot is to be stretched out the ampulla contracts and drives the fluid downwards. The contraction of the ampulla is brought about by muscles running circularly around it. The tube-foot is thus distended and its broad flattened end is brought in contact with the surface of the stone over which it is moving and is pressed close against it. The muscles of the tube-foot itself, which are arranged longitudinally, now commence to act, and the pressure of the water preventing the tearing away of the sucker from the object to which it adheres, the Starfish is slowly drawn forward, whilst the fluid in the tube-foot flows back into the ampulla.

Fig. 191.—Diagrammatic cross-section of the arm of a Starfish. adamb, Adambulacral ossicle; amb, ambulacral ossicle; amp, ampulla of tube-foot; branch, papula; car, carinal plate; d.lat, dorso-lateral plate; inf.marg, infero-marginal plate; p.br, peribranchial space; ped, pedicellaria; s.marg, supero-marginal plate. The nervous ridge between the bases of the tube-feet and the two perihaemal canals above this ridge are shown in the figure but not lettered.
If each tube-foot were practically water-tight, then each would be entirely independent of all the rest, and it would not be easy to suggest a reason for the presence of the complicated system of radial canals and stone-canal. Just at the spot, however, where the transverse canal leading from the radial canal enters the tube-foot there is a pair of valves which open inwards and allow fluid to pass from the radial canal into the tube-foot but prevent any passing outwards in the reverse direction. The presence of these valves renders it probable that the tube-foot is not quite water-tight; that when it is distended under the pressure produced by the contraction of the muscles of the ampulla, some fluid escapes through the permeable walls; and {444}that the loss thus suffered is made up by the entry of fresh fluid from the radial canal. The radial canal in turn draws from the ring-canal, and this last is supplied by the stone-canal, the cilia of which keep up a constant inward current.
In the fluid contained in the water-vascular system, as in the coelomic fluid, there are amoebocytes floating about. These are produced in short pouches of the ring-canal, nine in number, which are called after their discoverer "Tiedemann's bodies" (Fig. 190, T). From the cells lining these the amoebocytes are budded off.
The nervous system of the Starfish is in a very interesting condition. The essential characteristic of all nervous systems is the presence of the "neuron," a cell primitively belonging to an epithelium but which generally has sunk below the level of the others and lies amongst their bases. This type of cell possesses a round body produced in one direction into a long straight process, the "axon," whilst in the other it may have several root-like processes, or "dendrites," which may spring from a common stem, in which case the neuron is said to be "bipolar." The axon is often distinguished as a "nerve-fibre" from the round body which is termed the "nerve-cell." This is due to the fact that for a long time it was not recognised that these two structures are parts of a whole.
Now at the base of the ectoderm all over the body of the Starfish there is to be found a very fine tangle of fibrils; these are to be found partly in connexion with small bipolar neurons lying amongst them and partly with isolated sense-cells scattered amongst the ordinary ectoderm cells. This nervous layer is, however, very much thickened in certain places, so as to cause the ectoderm to project as a ridge. One such ridge is found at the summit of each ambulacral groove running along the whole under surface of the arm and terminating in a cushion at the base of the median tentacle of the water-vascular system. This ridge is called the radial nerve-cord. The five radial nerve-cords are united by a circular cord, the nerve-ring, which appears as a thickening on the peristome surrounding the mouth.
The sense-organs of the Starfish are chiefly the discs of the tube-feet. Round the edges of these there is a special aggregation of sense-cells; elsewhere, as in the skin of the back, only {445}isolated sense-cells are found, and it becomes impossible to speak of a sense-organ.
A prolongation of the radial nerve-cord extends outwards along one side of each tube-foot. This is often spoken of as the "pedal nerve," but the term nerve is properly retained for a mere bundle of axons such as we find in the higher animals, whereas the structure referred to contains the bodies of nerve-cells as well as their outgrowths or cell-fibres and is therefore a prolongation of the nerve-cord.

Fig. 192.—Diagrammatic longitudinal section through a young Asteroid passing through the tip of one arm and the middle of the opposite interradius. This diagram is generalised from a section of Asterina gibbosa. ab, Aboral sinus; ax, axial sinus; ax1, basal extension of axial sinus forming the inner perihaemal ring-canal; br, branchia = gill = papula; g.r, genital rachis; mp, madreporite; musc.tr, muscle uniting a pair of ambulacral ossicles; nerv.circ, nerve-ring; n.r, radial nerve-cord; oc, eye-pit; oss, ossicles in skin; p.br, peribranchial sinus; p.c, pore canal; perih (on the right), perihaemal radial canal, (on the left), outer perihaemal ring-canal; py, pyloric caecum; rect, rectum; rect.caec, rectal caeca; sp, spines; st.c, stone-canal; t, median tentacle terminating radial canal; w.v.r, water-vascular radial canal. The genital stolon (not marked by a reference line) is seen as an irregular band accompanying the stone-canal, its upper end projects into a small closed sac, also unmarked, which is the right hydrocoele or madreporic vesicle.
At the base of the terminal tentacle the radial nerve-cord ends in a cushion. This cushion is called the "eye," for it is beset with a large number of cup-shaped pockets of the ectoderm. Each pocket is lined partly by cells containing a bright orange pigment and partly by visual cells each of which ends in a small clear rod projecting into the cavity of the pit (Fig. 193, A, vis.r). The pit is apparently closed by a thin sheet of cuticle secreted by the most superficial cells.
An exposed nervous system and simple sense-organs such as the Starfish possesses lend themselves admirably to the purposes {446}of physiological experiment, and so Starfish have been favourite "corpora vilia" with many physiologists.

Fig. 193.—A, longitudinal section of a single eye-pit of Asterias. s.n, Nucleus of supporting cell; vis.n, nucleus of visual cell; vis.r, visual rod. B, view of the terminal tentacle showing the eye-pits scattered over it. (After Pfeffer.)
The light-perceiving function of the eye is easily demonstrated. If a number of Starfish be put into a dark tank which is illuminated only by a narrow beam of light they will be found after an interval to have collected in the space reached by the beam of light.[445] If all the median tentacles but one be removed this will still be the case; if, however, they are all removed the Starfish will exhibit indifference to the light.
If the under surface of a Starfish be irritated by an electric shock or a hot needle, or a drop of acid, the tube-feet of the affected area will be strongly retracted, and this irritation will be carried by the pedal nerves to the radial nerve-cord, with the result that finally all the tube-feet in the groove will be retracted and the groove closed by the action of the transverse muscle connecting each ambulacral ossicle with its fellow. If, on the other hand, the back of a Starfish be irritated this may produce a contraction of the tube-feet if the irritation be strong, but this will be followed by active alternate expansions and contractions, in a word, by endeavours to move. Preyer[446] by suspending a Starfish ventral surface upward, by {447}means of a small zinc plate to which a string was attached which passed through a hole bored in the back and through the mouth, caused movements of this description which lasted for hours. Irritation of the back causes also activity of the local pedicellariae, which open their valves widely and then close them with a snap in the endeavour to seize the aggressor.
The uninjured Starfish in moving pursues a definite direction, one arm being generally directed forwards, but this may be any one of the five. The tube-feet of this arm are directed forwards when they are stretched out, by the slightly unequal contraction of the longitudinal muscles of opposite sides of the foot, which persists even when the circular muscles of the ampulla are contracting. They thus may be said to swing parallel to the long axis of the arm. The tube-feet of the other arms assist in the movement, and hence swing obliquely with reference to the long axis of the arm to which they belong, although they move parallel to the general direction in which the Starfish is moving. A change in the direction of the swing of the tube-feet will bring about a change in the direction of the movement of the animal as a whole. If now the connexion of each radial nerve-cord with the nerve-ring be cut through, each arm will act as a separate Starfish and will move its tube-feet without reference to the movement of those in the other arms, so that the animal is pulled first one way and then another according as the influence first of one arm and then of another predominates. Similarly, when a Starfish is placed on its back, it rights itself by the combined action of the tube-feet of all the arms, extending them all as widely as possible, those which first catch hold being used as the pivot for the turning movement. If, however, the radial nerve-cords are cut through, each arm tries to right itself and it is only by chance that the efforts of one so predominate as to turn the whole animal over. From these experiments it is clear that the nerve-ring acts as co-ordinator of the movements of the Starfish, that is to say as its brain.
If a section be taken across the arm of a Starfish (Fig. 191), it will be seen that between the V-shaped ridge constituting the radial nerve-cord and the radial water-vascular canal there are two canals lying side by side and separated from one another by a vertical septum. These canals are not mere splits in the {448}substance of the body-wall, but have a well-defined wall of flattened cells. They are termed, for reasons which will be explained subsequently, perihaemal canals, and they open into a circular canal called the "outer perihaemal ring," situated just beneath the water-vascular ring-canal (Fig. 192, perih). These canals originate as outgrowths from the coelom. From their upper walls are developed the muscles which connect the pairs of ambulacral ossicles and close the groove, and also those which connect each ossicle with its successor and predecessor and help to elevate or depress the tip of the arm.
In most of the higher animals the processes of many of the ganglion-cells are connected together in bundles called "motor nerves," which can be traced into contact with the muscles, and thus the path along which the stimulus travels in order to evoke movement can clearly be seen. No such well-defined nerves can be made out in the case of the Starfish, and it is therefore interesting when exceptionally the paths along which stimuli travel to the muscles can be traced. This can be done in the case of the muscles mentioned above. Whereas they originate from the dorsal walls of the perihaemal canals, ganglion-cells develop from the ventral walls of these canals, which are in close contact with the nerve-cord, so that the nervous system of the Starfish is partly ectodermic and partly coelomic in origin. Stimuli reaching the ectodermic ganglion-cells are transmitted by them to the nervous part of the wall of the perihaemal canal and from that to the muscular portion of the same layer of cells.
Besides the radial perihaemal canals and their connecting outer perihaemal ring there are several other tubular extensions of the coelom found in the body-wall. These are:—
(1) The "inner perihaemal canal," a circular canal in close contact with the inner side of the outer perihaemal canal (Fig. 192, ax1).
(2) The "axial sinus" (ax) a wide vertical canal embedded in the body-wall outside the stone-canal. This canal opens into the inner perihaemal canal below; above it opens into several of the pore-canals and into the stone-canal. The separation of the axial sinus from the rest of the coelom is the remains of a feebly marked metamerism in the larva.
(3) The "madreporic vesicle," a closed sac embedded in the dorsal body-wall just under the madreporite. This sac by its {449}history in the larva appears to be a rudimentary counterpart of the water-vascular system, since this organ in correspondence with the general bilateral symmetry of the larva is at first paired. Into this a special process of the genital stolon projects.
(4) The "aboral sinus" (Fig. 192, ab), a tube embedded in the dorsal body-wall running horizontally round the disc. The aboral sinus surrounds the genital rachis (see p. 452) and gives off into each arm two branches, the ends of which swell so as to surround the genital organs. It has no connexion with the axial sinus though the contrary has often been stated by Ludwig.[447]
(5) The "peribranchial spaces," circular spaces which surround the basal parts of the papulae (Fig. 192, p.br).
Besides these, large irregular spaces have been described as existing in the body-wall by Hamann[448] and other authors, but for various reasons and especially because they possess no definite wall they appear to be nothing more than rents caused by the escape of CO2 gas during the process of decalcifying, to which the tissues of the Starfish must be subjected before it is easy to cut sections of them.
The question as to whether or not there is a blood system in the Starfish has an interesting history. It must be remembered that the examination of the structure of Echinodermata was first undertaken by human anatomists, who approached the subject imbued with the idea that representatives of all the systems of organs found in the human subject would be found in the lower animals also. So the perihaemal canals were originally described as blood-vessels. Later, Ludwig[449] discovered a strand of strongly staining material running in each septum which separates the two perihaemal canals of the arm. Each of these radial strands could be traced into connexion with a circular strand interposed between the outer and the inner perihaemal ring-canals. This circular strand again came into connexion with a brown, lobed organ, lying in the wall of the axial sinus, and this in turn {450}joined at its upper end a circular cord of pigmented material adhering to the dorsal wall of the coelom (lying in fact within the aboral sinus), from which branches could be traced to the generative organs. Ludwig concluded that he had at last discovered the true blood-vessels, though the facts that the radial strands and the oral circular strand absorbed neutral carmine strongly and that the vertical and aboral strands were pigmented, constituted a very slender basis on which to found such a conclusion. The colour apparently appealed to the imagination, and it is undoubtedly true that the "plasma" or blood-fluid of other animals often absorbs stain strongly.
The strands were accordingly named "radial blood-vessels," "oral blood-ring," "aboral blood-ring"; and the brown vertical strand was called the "heart," although no circulation or pulsations had ever been observed. When later investigations revealed the fact that the so-called heart was practically solid, the term "central blood-plexus" was substituted for heart, although it was still regarded as the central organ of the system. The name "perihaemal" was given to the spaces so called because they surrounded the supposed blood-vessels.
In order to come to a satisfactory conclusion on the matter some general idea as to the fundamental nature and function of the blood-vessels in general must be arrived at. Investigations made on various groups of animals, such as Annelida, Mollusca, Crustacea, Vertebrata, show that at an early period of development a considerable space intervenes between the alimentary canal and the ectoderm, which is filled with a more or less fluid jelly. Into this cavity, the so-called "primary body-cavity" or "archicoel," amoebocytes, budded from the ectoderm or endoderm or both, penetrate. In this jelly with its contained amoebocytes is to be found the common rudiment both of the connective tissue and of the blood system. The resemblance of the archicoele and its contents to the jelly of a Medusa is too obvious to require special insistence on, and therefore in the Coelenterata it may be stated that there is to be found a tissue which is neither blood system nor connective tissue but is the forerunner of both.
In the higher animals as development proceeds the jelly undergoes differentiation, for some of the amoebocytes become stationary and connected with their pseudopodia so as to form a protoplasmic network. A portion of this network becomes {451}altered into tough fibres, but a portion of each strand remains living, and in this way the connective tissue is formed. In the interstices of the network of fibres a semi-fluid substance (the unaltered jelly) is found, and this is traversed by free, wandering amoebocytes. In other places the jelly becomes more fluid and forms the plasma, or liquid of the blood, whilst the amoebocytes form the blood corpuscles. The blood system thus arises from regions of the archicoel where fibres are not precipitated.
Now in the Starfish the whole substance of the body-wall intervening between the ectoderm and the coelomic epithelium really represents the archicoel. The formation of fibres has, it is true, proceeded to a certain extent, since there are interlacing bundles of these, but there are left wide meshes in which amoebocytes can still move freely. Apart from the skeleton, therefore, the tissues of the body-wall of the Starfish do not exhibit much advance on those of a Jellyfish. If anything is to be compared to the blood system of the higher animals it must be these meshes in the connective tissue. From observations made on other Echinoderms it appears probable that the colour of the skin is due to amoebocytes loaded with pigment wandering outwards through the jelly of the body-wall and disintegrating there. The strands regarded as blood-vessels by Ludwig are specially modified tracts of connective tissue in which fibres are sparse, and in which there are large quantities of amoebocytes and in which the "jelly" stains easily. Cuénot[450] suggests that they are placed where new amoebocytes are formed; this is quite possible, and in this case they ought to be compared to the spleen and other lymphatic organs of Vertebrates, and not to the blood-vessels.[451]
The organ regarded as the heart, however, belongs to a different category: it is really the original seat of the genital cells and should be termed the "genital stolon." Careful sections show that at its upper end it is continuous with a strand of primitive germ-cells which lies inside the so-called aboral {452}blood-vessel, and is termed the "genital rachis" (Fig. 192, g.r). The germ-cells are distinguished by their large nuclei and their granular protoplasm. The genital organs are only local swellings of the genital rachis, and from the shape of some of the germ-cells it is regarded as highly probable that the primitive germ-cells wander along the rachis and accumulate in the genital organs. The genital rachis itself is an outgrowth from the genital stolon, and this latter originates as a pocket-like ingrowth of the coelom into the wall separating it from the axial sinus; when fully formed it projects into and is apparently contained in this latter space.
Not all the cells forming the genital stolon become sexual cells. Many degenerate and become pigment-cells, a circumstance to which the organ owes its brown colour. In very many species of Starfish many of the cells of the genital rachis undergo a similar degeneration, and hence is produced the apparent aboral blood-vessel. Further, the rachis is embedded in connective tissue which has undergone what we may call the "lymphatic" modification, and this for want of a better name we call the "aboral" blood-ring.
The size of the genital organs varies with the season of the year; they are feather-shaped, and attached to the genital rachis by their bases, but project freely into the coelom of the arm. From their great variation in size and also from the shape of some of the cells in the genital rachis, Hamann concludes that as each period of maturity approaches fresh germ-cells are formed in the rachis and wander into the genital organ and grow there in size. It is probable that the aboral end of the genital stolon is the seat of the formation of new germ-cells.
In the Starfish, therefore, as in other animals with a well-defined coelom, the genital cells ultimately originate from the coelomic wall.
The genital ducts are formed by the burrowing outwards of the germ-cells. When it is remembered that the fundamental substance of the body-wall is semi-fluid jelly, this process will be better understood.
When the ova and spermatozoa are ripe, they are simply shed out into the sea and fertilisation occurs there. The development is described in Chapter XXI. The free-swimming larval period lasts about six weeks.
Having described a single species with some degree of fulness, we must now give some account of the range of variation of structure met with in the group.
Number of Arms.—In the overwhelming majority of Starfish the number of arms is 5, but deviations from this rule are met with not only as individual variations, but as the characteristics of species, genera, and even families.
The number 5 is rarely diminished, but amongst a large collection of specimens of Asterina gibbosa, belonging to the author, some 4-rayed individuals are met with. One species of Culcita, C. tetragona, is normally 4-rayed.
On the other hand the number 5 is often exceeded. The families Heliasteridae and Brisingidae are characterised by possessing numerous (19-25) arms. In the normally 5-rayed family Asteriidae Pycnopodia has 22 arms; and in the Solasteridae the genera Rhipidaster and Solaster are characterised by possessing 8 and 11-15 arms respectively; whilst Korethraster and Peribolaster have only 5. The common Starfish of the Gulf of St. Lawrence, Asterias polaris, is 6-rayed, whilst most of the other species of the same genus are 5-rayed, though 6 rays are often met with as a variation.
In some species the fact that the number of arms exceeds 5 seems to be connected with the power of multiplication by transverse fission. Thus Ludwig[452] has shown that in Asterias tenuispina the number of arms is usually 7, but sometimes 5, 6, or 8, and that in most cases the arms are arranged in two groups—one consisting of small arms, the other of large.
Shape.—Apart from the varying number of arms, differences in the shape of the Starfish are due to two circumstances:—
(1) The proportion of breadth to length of arm; and
(2) The amount of adhesion between adjacent arms.
The adhesion can go so far that the animal acquires the shape of a pentagonal disc. This is the case for instance in Culcita. The fact that the body of this animal is really composed of adherent arms is at once made clear when the coelom is opened. This space is found to be divided up by inwardly projecting folds called interradial septa, which are stiffened by calcareous deposits and represent the conjoined adjacent walls of two arms.
In the family Heliasteridae the mutual adhesion between the arms has gone on merely to a slight extent, for the interradial septa are still double.
Skeleton.—Most of the schemes of classification have been founded on the skeleton, largely because the greater number of species have only been examined in the dried condition, and little is known of their internal anatomy or habits. There is, however, this justification for this procedure, that the habits and food of the species (with the exception of the Paxillosa) which have been observed in the living condition appear to be very uniform, and that it is with regard to the skeleton that Asteroidea seem to have split into divergent groups through adopting different means of protecting themselves from their foes.
The description of the various elements of the skeleton will be arranged under the following heads:—(a) Main framework; (b) Spines; (c) Pedicellariae; (d) Ambulacral skeleton.
(a) Main Framework.—The type of skeleton which supports the body-wall of Asterias is called reticulate. As already indicated it consists of a series of rods bound together by bundles of connective-tissue fibres so as to form a mesh-work. This is a very common type of aboral skeleton, but in a large number of Starfish a different type occurs, consisting of a series of plates which may fit edge to edge, leaving between them only narrow interstices, as in the Zoroasteridae, or which may be placed obliquely (as in Asterina) so that they imbricate or overlap one another. In a very large number of Asteroidea the supero- and infero-marginal ossicles are represented by squarish plates even when the rest of the skeleton is reticulate; this is the so-called "phanerozonate" structure, the term "cryptozonate" being used when the marginals are rod-like and inconspicuous. In other cases (Ganeriidae) the whole skeleton of the ventral surface is made of tightly fitting plates, whilst the aboral skeleton is either reticulate or made of imbricating plates. Lastly, the skeleton may be represented only by nodules forming the bases of paxillae (see p. 455), as in the Astropectinidae, or may be entirely absent over wide areas (Brisingidae).
(b) Spines.—The spines vary more than any other part of the skeleton. They may be close set and small, or few and large, and often bear spines of the second order, or spinelets, attached to them. In Asterias and its allies they are {455}comparatively short, blunt tubercles, covered with thick skin. In the Echinasteridae and Asterinidae they are short and blunt, but they are very numerous and thick set. In the Solasteridae they are long, and arranged in bundles diverging from a common base. Such bundles may be termed sheaves, and starting from an arrangement like this, two distinct lines of modification may be traced. Thus (1) the members of a sheaf become connected by a web of skin, so that the sheaf becomes an umbrella, and successive umbrellas may adhere, so that a supra-dorsal tent is formed (a structure characteristic of the Pterasteridae), or (2) the members of a sheaf may become arranged in a circle round a central vertical axis so that a structure like a capstan is produced, which is called a "paxilla" (characteristic of Astropectinidae, Porcellanasteridae, and Archasteridae). The axis,[453] as shown by its development, represents the plate which bore the bundle of spines. Again, the skeleton may consist of plates with a close covering of granules (Pentagonasteridae, etc.). Lastly, in Porania spines are absent, the plates being deeply embedded in a thick leathery skin.

Fig. 194.—Views of portions of the aboral surface of different genera of Asteroidea in order to show the main varieties of skeleton. A, Solaster, showing spines arranged in sheaves; B, Pteraster, showing webs forming supra-dorsal membrane supported by diverging spines; C, Astropecten, showing paxillae; D, Nardoa, showing uniform plating of granules. × 8. (After Sladen.)
(c) Pedicellariae.—These are to be looked on as spines of the second order. In Asterina and its allies they are not present, but groups of little spines arranged in twos and threes, each group being attached to a special small plate, are scattered over the aboral surface; and these on irritation approach one another, and represent the rudiment out of which pedicellariae have been developed. The most perfect form, termed "forcipulate," in which there is a basal ossicle, is found in Asteriidae, Brisingidae, Heliasteridae, Pedicellasteridae, Zoroasteridae, Stichasteridae. There are two varieties of forcipulate pedicellariae, the "crossed" and the "straight," which have been described on p. 432. In all other cases the pedicellariae are devoid of the basal ossicle, and the two or more spinelets forming the jaws are directly attached to one of the main plates of the skeleton.

Fig. 195.—Different forms of pedicellariae (excluding the forcipulate form, for which see Fig. 186). A, pectinate; B, pectinate; C, valvate; D, pincer-shaped; E, alveolate, from the side; F, alveolate, from above. × 10. (After Sladen.)
The simplest variety is termed "pectinate"; these pedicellariae are composed of two parallel rows of small spines opposed to each other. They are found in the Archasteridae, and are hardly more advanced in structure than the groups of spines found in Asterina. In Leptogonaster and its allies there are pincer-shaped pedicellariae composed of two curved rods articulating with one of the plates of the skeleton, and also "alveolate" pedicellariae, composed of two short prongs which are implanted on a concave tubercle borne on one of the plates of the skeleton. In the Antheneidae every plate of the ventral surface bears a large "valvate" pedicellaria consisting of two horizontally elongated ridges, which can meet one another. It is possible that valvate pedicellariae have been derived from a pectinate form in which successive spinules of one row have become adherent.
(d) Ambulacral Skeleton.—In every case, whether spines are developed elsewhere or not, the adambulacral plates bear spines. Where the spines are elsewhere represented by granules (Nardoa and its allies) (Fig. 194, D) the adambulacral spines are {457}short and blunt. The terms "monacanthid" and "diplacanthid" are used to express the occurrence of one or two rows of spines respectively on each adambulacral plate.
In the Zoroasteridae the adambulacral plates are curved, and are alternately convex and concave towards the ambulacral groove, so that this groove presents a wavy outline.
In the description of Asterias it was pointed out that the first adambulacral plates in adjacent radii are closely approximated to one another, and bear spines which can to some extent form a trellis-work over the mouth. In very many species not only is this the case, but the plates themselves project inwards over the mouth so as to form prominent "mouth-angles." This is not the case in the Asteriidae or the allied families.
Papulae.—In Asteriidae and many allied families these organs are found both on the upper and under surface of the disc, but in another large group consisting of Astropectinidae, Pentacerotidae, and allied families, papulae are only borne on the dorsal surface, and, in some cases, are restricted to a few groups at the base of the arms. In most Asteroidea the papulae are arranged singly, that is to say, each occupies one of the interspaces between the plates of the skeleton, but in Asterias and some other genera they are arranged in tufts of two or three.
Water-vascular System.—In its general structure this system of organs is very constant, the two most important variations being found, one, in Asteriidae and a few allied families, and the other, in the Astropectinidae and the families allied to them.
The first of the variations alluded to concerns the number of the tube-feet in a radius. In Asterias and its allies these are so numerous that there is not room for them one behind the other, but they follow one another in a zigzag line, the transverse canals connecting them with the radial canals being alternately longer and shorter. In this way the appearance of four rows of tube-feet is produced, and the advantage of this increase in number can be recognised by any one who has compared the quick movements of Asterias and the slow ones of a Cribrella, for instance.
The second important variation referred to is the complete loss of the sucker of the tube-foot, and, concomitantly, the loss {458}of the power of climbing. Starfish which have undergone this change live on sandy bottoms and run over the surface of the sand. They are also incapable of forcing asunder the valves of Molluscs, and hence are compelled to swallow their prey whole.
"Polian vesicles," or stalked sac-like outgrowths of the water-vascular ring, are absent from the Asteriidae, but are found in many families—the Asterinidae, Solasteridae, Astropectinidae, for example. They project outwards from the water-vascular ring in the interradii; when there are several present in one interradius they often arise from a common stalk. Cuénot believes that their sole function, like that of Tiedemann's bodies, is to produce amoebocytes, but this appears unlikely. It is more probable that they act as store-houses of fluid for the water-vascular ring.

Fig. 196.—Dissection of Ctenodiscus to show the Polian vesicles. amp, Ampullae of the tube-feet; nerv.circ, nerve-ring; Pol, Polian vesicle; sept, interradial septum; stone c, stone-canal; T, Tiedemann's body; w.v.r, water-vascular ring. × 1.
The stone-canal is rarely repeated, but this occurs in the aberrant genus Acanthaster, where there may even be several in one interradius, and each stone-canal has an axial sinus, genital stolon, and madreporite annexed to it. According to Cuénot, in Asterias, when 6-rayed specimens occur in a species normally 5-rayed, there are two stone-canals, suggesting that the repetition of stone-canals is a suppressed effort at multiplication by division. This is also true of Echinaster, but in Ophidiaster two madreporites may occur in an individual with five arms. In the Asterinidae the Y-shaped fold which projects into the cavity of the stone-canal is feebly developed, whereas in the Pentacerotidae it meets the opposite side of the stone-canal, and in Culcita gives out branches which reduce the cavity of the canal to a series of channels. In Echinasteridae and some Asterinidae, and in Astropectinidae and Pentacerotidae the ampullae become so deeply indented as to be almost divided into two, so that each tube-foot has virtually two ampullae.
The alimentary canal has a remarkably constant structure. {459}The only important variation from the type, as described in Asterias, is found amongst the Astropectinidae and Porcellanasteridae, where the anus is wanting. In Astropecten the rectum and the rectal caeca still persist, but in Luidia even these have disappeared. The rectal caeca are remarkably variable structures. In Asterias there are two, but in Pentacerotidae there are five forked caeca, in Asterina five simple caeca, and in the Echinasteridae and Astropectinidae one large flat slightly 5-lobed caecum. In the Asterinidae the pyloric caeca are remarkable for the size of the enlarged basal portion in each radius, which serves as a reservoir for the juices secreted by the branched forks of the caecum. In Porcellanaster pacificus the pyloric caeca are vestigial, and in Hyphalaster moseri they are absent.[454]
The genital organs are, as we have seen, outgrowths from radial branches of the genital rachis. In most species, as in Asterias, they are limited to a single cluster of tubes on each branch of the rachis, but in the Astropectinidae and Pentacerotidae each branch gives rise to a large number of clusters, arranged in longitudinal series, each cluster having its independent opening to the exterior.
Asexual reproduction, as a regular occurrence, is not common amongst Asteroidea. If, however, a Starfish loses some of its arms, it has the power of regenerating the missing members. Even a single arm will regenerate the whole Starfish. Now in some cases (Astropectinidae, Linckiidae) Starfish will readily snap off their arms on irritation. In Linckia this occurs at regular intervals and the separated arm forms a new individual. In one of the Asterinidae, Asterina wega, a small Starfish with seven arms, transverse fission regularly occurs, a portion with three arms separating from one with four. The same is believed to occur in two species of Asterias, and as has already been pointed out, the repetition of the madreporite and stone-canal is, in many cases, possibly connected with this tendency to transverse fission.
Classification of Asteroidea.
Whilst there is considerable agreement amongst the authorities as to the number of families, or minor divisions of unequivocal {460}relationship, to be found in the class Asteroidea, there has been great uncertainty both as to the number and limits of the orders into which the class should be divided, and also as to the limits of the various species. The difficulty about the species is by no means confined to the group Echinodermata; in all cases where the attempt is made to determine species by an examination of a few specimens of unknown age there is bound to be uncertainty; the more so, as it becomes increasingly evident that there is no sharp line to be drawn between local varieties and species. In Echinodermata, however, there is the additional difficulty that the acquisition of ripe genital cells does not necessarily mark the termination of growth; the animals can continue to grow and at the same time slightly alter their characters. For this reason many of the species described may be merely immature forms. In proportion, however, as the collections from various localities increase in number and size, difficulties connected with species will tend to disappear.
The disputes, however, as to the number of orders included in the Asteroidea proceed from a different cause. The attempt to construct detailed phylogenies involves the assumption that one set of structures, which we take as the mark of the class, has remained constant, whilst others which are regarded as adaptive, may have been developed twice or thrice. As the two sets of structures are often of about equal importance it will be seen to what an enormous extent the personal equation enters in the determination of these questions.
Where, as in Asteroidea, the internal organisation is very uniform, the best method of classification is to take as our basis the different methods in which the demands of the environment have been met. It is in this way, we hold, that divergence of character has been produced, for whilst species may differ in trifling details, families and orders differ in points of functional importance. The fact that one of the orders may have sprung from several allied species instead of one may be admitted, and at the same time the hopelessness of trying to push phylogenetic inference into details asserted.
Sladen, in his Monograph of the Asteroidea collected by the "Challenger" expedition, took for the basis of his system the presence or absence of distinct pavement-like marginal plates along the edges of the arms and the restriction of the papulae to {461}the aboral surface, or their distribution over the whole surface of the body. What connexion, if any, the presence of these pavement-like plates has with the habits it is impossible to say, but it is unlikely to be of the high importance with which it was regarded by Sladen, for in the same family we have genera with inconspicuous marginals (Asterina) and others with conspicuous marginals (Palmipes). The restriction of the papulae to the back also varies within the same family (Linckiidae), and whilst, on the whole, it is perhaps a primitive arrangement, it is in many cases connected with burrowing habits, which can scarcely be deemed to have been the original mode of life of the class.
A far better basis is supplied by the system of Perrier,[455] who divides the Asteroidea into five orders according to the character of the dorsal skeleton; and this classification really corresponds with the different habits assumed by groups of Asteroidea in order to meet what must be regarded as one of their chief dangers, viz. assaults by other animals, especially parasites, on their soft and delicate skins. Since the food (so far as is known) of all Asteroidea is more or less similar, the great differentiating factor in their development must have been the means they adopt to shelter themselves from their enemies. Perrier's classification, which we shall adopt, is as follows:—
Order 1. Spinulosa.—Asteroidea in which the plates of the dorsal skeleton bear spines arranged singly or in groups. The tube-feet have suckers and there are no pedicellariae. Marginals sometimes conspicuous, sometimes rod-like.
Order 2. Velata.—Asteroidea in which the dorsal surface of the animal is concealed from view by a false membrane composed of the webs of skin stretched between diverging groups of spines united at the base with one another. No pedicellariae. Tube-feet with suckers.
Order 3. Paxillosa.—Asteroidea in which the dorsal surface is beset with paxillae (upright spines bearing two or three circles of horizontal spinelets). Pedicellariae, when present, few, and never of the forcipulate variety; often absent. Marginals large. Papulae only on dorsal surface. Tube-feet mostly devoid of suckers.
Order 4. Valvata.—Asteroidea in which the dorsal surface {462}is protected by plates covered with a mail of minute granules. Pedicellariae of the valvate or alveolate type. Marginals large.
Order 5. Forcipulata.—Asteroidea in which the dorsal surface is beset with small spines surrounded by numerous forcipulate pedicellariae. Tube-feet with suckers and arranged in four rows. Marginals rod-like and inconspicuous.
Order I. Spinulosa.
This is by far the most primitive order of Asteroidea. The tube-feet are arranged in two rows only, and there is no special means of protecting the back, other than the small close-set plates bearing spines, with which it is covered. In some cases, as Asterina, these spines have a tendency to converge when irritated, and thus act somewhat like pedicellariae. This circumstance suggests strongly the manner in which pedicellariae have been developed from small groups of spines. The order is divided into six families, of which four have common representatives on the British coast.
Fam. 1. Echinasteridae.—Spinulosa in which the aboral skeleton is composed of close set plates bearing comparatively small spines. This family is represented on the British coasts by the beautiful scarlet Starfish Cribrella (Henricia) sanguinolenta. It is also found on the Norwegian coast and on the east coast of North America. On the Pacific coast it is replaced by a larger species, C. laeviuscula. The narrow ambulacral grooves and sluggish movements at once distinguish it from the Starfish described as the type. Indeed, all the Spinulosa seem to be slow in their movements in contrast to the comparatively active Asterias and its allies. Cribrella is remarkable for its large eggs, which have a rapid development. The larva never swims at the surface but glides only for a short time over the bottom. Echinaster is an allied genus in which each plate bears a single somewhat enlarged spine. It possesses on the skin of the aboral surface numerous pits lined by glandular walls, which probably secrete a poisonous fluid which defends it. Acanthaster has thorny spines, more than ten arms, and several stone-canals and madreporites.
Fam. 2. Solasteridae.—Spinulosa in which the aboral skeleton is a network of rods. Spines arranged in diverging bundles {463}(sheaves) attached to a basal button. This family includes the well-known "Sun-stars," with numerous arms and a wide peristome. There are two species found on both sides of the Atlantic. Solaster papposus, with thirteen or fourteen arms and long bundles of spines on the dorsal surface, which is of an orange colour variegated with yellow, and S. endeca with eleven rays and shorter spines and of a reddish violet colour. Rhipidaster has eight arms. Some genera have, however, only five arms, as, for instance, Peribolaster and Korethraster (Fig. 197). In this family there are conspicuous "Polian vesicles" attached to the water-vascular ring.
Fam. 3. Asterinidae.—Spinulosa in which the aboral skeleton consists of overlapping plates, each bearing a few small spines. The common British representative of this family is the small Asterina gibbosa, in which the arms are short and stout and of somewhat unequal length. This Starfish differs from most of its allies in being littoral in its habit. At low tide on the south and west coasts of England it can be found on the underside of stones feeding on the Sponges and Ascidians with which they are covered. Like Cribrella sanguinolenta this species has a modified development. The larva resembles that of Cribrella, and the larval stage only lasts about a week. Owing to the fact that {464}Asterina lays its eggs in accessible localities, its development has been more thoroughly worked out than that of any other species. Palmipes membranaceus, an animal of extraordinary thinness and flatness, is sometimes dredged up off the coast of Britain in deeper water. Its arms are so short that the general form is pentagonal. The infero-marginal plates are long and rod-like, and form a conspicuous border to the body when viewed from below.
Fam. 4. Poraniidae.—Spinulosa allied to the Asterinidae but possessing a thick gelatinous body-wall in which the plates and spines are buried, the marginals forming a conspicuous border to the body. This family is represented in British waters only by Porania pulvillus, a cushion-shaped Starfish with very short arms and of a magnificent reddish-purple colour. It is occasionally, but rarely, exposed at low tide.
Fam. 5. Ganeriidae.—Spinulosa allied to the Asterinidae but distinguished by the large marginals and by the fact that the skeleton of the oral surface consists of plates each bearing a few large spines. Ganeria, Marginaster.
Fam. 6. Mithrodiidae.—Spinulosa with a reticulate aboral skeleton. The spines are large and blunt, covered with minute spinules. Mithrodia, sole genus.
These last two families are not represented in British waters.
Order II. Velata.
This is a very extraordinary group of Starfish, about the habits of which nothing is known, since they all live at very considerable depths. Their nearest allies amongst the Spinulosa must be looked for amongst the Solasteridae. If the sheaves of spines with which the latter family are provided were to become adherent at their bases, and connected with webs of skin so as to form umbrella-like structures, and if then these umbrellas were to become united at their edges, we should have a supra-dorsal membrane formed such as is characteristic of the order.
Fam. 1. Pythonasteridae.—Velata in which each sheaf of spines is enveloped in a globular expansion of the skin and is not united with the neighbouring sheaves. Pythonaster, sole genus.
Fam. 2. Myxasteridae.—Velata with numerous arms in which the sheaves of spines are long and form with their connecting "umbrellas" web-like expansions which do not fuse with one another. Myxaster, sole genus.
Fam. 3. Pterasteridae.—Velata in which the membranes supported by the sheaves of spines are united so as to form a continuous supra-dorsal tent. The Pterasteridae are represented in British waters by a single species, Pteraster militaris, which is occasionally dredged in deep water off the British coast, and is found also in the Norwegian fjords and off the east coast of Canada. This interesting Starfish has five short, blunt arms, and its general appearance at first sight recalls that of Asterina. Closer inspection reveals the "false back." The anus is surrounded by five fan-like valves, supported by spines (Fig. 198), underneath which is a space in which the young complete their development, Pteraster being one of the genera in which the normal larval form is not developed. The tendency towards the union of adjacent spines by webs is deeply rooted in the organisation of the animal. It is seen on the under side where the spines borne by the ventral plates are united so as to form transverse combs. In Hymenaster (Fig. 199) the spines borne by the ventral plates are long and free.
Order III. Paxillosa.
This is an exceedingly well-marked order. The armature of the upper surface consists of paxillae. These organs as already mentioned are probably to be traced back to sheaves of spines like those of the Solasteridae. The same end as that striven after in the case of the Velata has been attained, but in a different way. The horizontal spinelets of the paxillae meet one another and form a close-fitting mail which is almost as efficient a protection as the webs and umbrellas of the Velata. Pedicellariae are occasionally present, but they are always of the pectinate or pincer variety, never forcipulate.
Fam. 1. Archasteridae.—Paxillosa in which the anus is still retained and in which the tube-feet have suckers.
The Archasteridae are a most interesting family. Thus Pararchaster has no true paxillae, but only small isolated groups of spines. The pectinate pedicellariae are composed each of two parallel rows of somewhat smaller spines. The members of this family are to some extent intermediate in structure between the {467}Spinulosa, such as Echinasteridae, and the other families of the Paxillosa—some genera, indeed, might almost be classed as Spinulosa. At the same time they are apparently closely allied with the more primitive Valvata such as Astrogonium and its allies, some of which have paxillae on the upper surface; although the retention of the anus and of the suckers on the tube-feet (in which characters they agree with the Archasteridae) distinguishes them from the more typical Paxillosa, in which both anus and suckers are lost. Archaster (Figs. 200, 201). Leptogonaster.
Fam. 2. Astropectinidae.—Paxillosa which have lost the anus, but which possess neither aboral protuberance nor interradial grooves. The marginal plates are thick, covered with spinules and placed horizontally. The tube-feet have no suckers.
This family is the only one of the order which occurs in British waters, where it is represented by two genera, Astropecten and Luidia. In Astropecten the inferior marginal plate is in {468}immediate contact with the adambulacral, whilst in Luidia it is separated from it by a small intermediate plate.
Astropecten irregularis is a very common species on the coast of Britain, and a study of its habits when in captivity has thrown a great deal of light on many obscure points in the anatomy of the Paxillosa. Owing to the loss of suckers it is unable to climb over rocks and stones like the ordinary species, but it runs over the surface of the hard sand in which it lives by means of its pointed tube-feet. The arms are highly muscular, and the animal when laid on its back rights itself by throwing the arms upwards and gradually overbalancing itself. The loss of suckers has also rendered Astropecten and its allies incapable of feeding in the manner described in the case of Asterias rubens. They are unable forcibly to open the valves of shell-fish, and the only resource left to them is to swallow their prey whole. The mouth is consequently wide, and the {469}unfortunate victims, once inside the stomach, are compelled by suffocation to open sooner or later, when they are digested.[456]

Fig. 202.—Oral view of Psilaster acuminatus. × 4⁄3. adamb, Adambulacral spines; pax, paxillae; pod, pointed tube-feet devoid of sucker. (After Sladen.)
Many interesting experiments have been made on Astropecten by Preyer and other investigators, but one important fact[457] has escaped their notice, that Astropecten, when at rest, lies buried in the sand, whilst the centre of the aboral surface is raised into a cone which projects above the surface. On the sides of this cone the few papulae which this species possesses are distributed. This raising of the aboral surface is obviously an expedient to facilitate respiration. It loosens the sand over the region of the papulae, and thus allows the water to have access to them. We can thus understand how the restriction of the papulae to the dorsal surface, so characteristic of the Paxillosa, is not always as Sladen imagined, a primitive characteristic, but often an adaptation to the burrowing habits which in all probability are characteristic of the whole order. In both Luidia and Astropecten Cuénot has described short spines covered with cilia in {470}the interspaces between the marginal plates, these also subserve respiration by drawing a current of water over the gills. Psilaster (Fig. 202).
Fam. 3. Porcellanasteridae.—Paxillosa which have lost the anus. There is a conical prominence in the centre of the dorsal surface termed the epiproctal cone, and in the interradial angles there are vertical grooves bordered by folds of membrane produced into papillae, the so-called "cribriform organs." The marginal plates are thin and form the vertical border of the thick disc. The tube-feet have no suckers.
Comparing the Porcellanasteridae with the Astropectinidae we see at once that the "epiproctal cone" is a permanent representative of the temporary aboral elevation in Astropecten, and we are inclined to suspect that the cribriform organs are grooves lined with cilia which keep up a respiratory current like the ciliated {471}spines of Luidia. In all probability the Porcellanasteridae are more habitual burrowers than even the Astropectinidae.
Ctenodiscus (Fig. 196), a genus in which there is a short epiproctal cone and numerous feeble cribriform organs in each interradius, is found in deep water north of the Shetland Islands. Porcellanaster (Fig. 203) is a more typical genus, with one large cribriform organ in each interradius. Hyphalaster has long arms, on which the supero-marginal plates meet above.
Order IV. Valvata.
The Starfish included in this order are characterised by the absence of prominent spines and by the superficial covering of minute granules. The skeleton consists, in most cases, of plates, and these plates with their covering of granules probably represent the first stage in the evolution of paxillae.
The tube-feet possess well-developed suckers. No members of this order can properly be said to be British.
Fam. 1. Linckiidae.—Valvata with long arms, the marginals being developed equally throughout the whole length. These Starfish are distinguished by their long narrow arms and small disc. It is possible that these forms, so different in many respects from the other families of the order, have been directly derived from the long-armed Echinasteridae. Ophidiaster, Nardoa, Linckia.
Fam. 2. Pentagonasteridae.—Valvata with short arms, the marginals being especially developed at the base and in the interradial angles. The aboral skeleton consists of close-fitting plates. Pentagonaster (Fig. 204), Astrogonium.
Fam. 3. Gymnasteridae.—Valvata allied to the foregoing but distinguished by possessing a very thick skin in which the plates are completely buried. Dermasterias, Asteropsis.
Fam. 4. Antheneidae.—Valvata with short arms. The dorsal skeleton is reticulate and each ventral plate bears one or several large valvular pedicellariae (Fig. 195, C). Hippasterias, Goniaster.
Fam. 5. Pentacerotidae.—Valvata with arms of moderate length. The dorsal skeleton is reticulate but the ventral plates bear only small pedicellariae or none. The upper marginals are smaller than the ventral ones.
The Pentacerotidae include both short-armed and long-armed {472}forms. Amongst the former is Culcita, in which the body is a pentagonal disc, all outer trace of the arms being lost; Pentaceros is a long-armed form.
The family Pentagonasteridae furnishes the key to the understanding of most of the forms contained in this order. It contains genera such as Astrogonium which possess on the back unmistakable paxillae, whilst on the under surface they have the characteristic covering of granules; these genera seem to be closely allied to the short-armed species of the Archasteridae, from which they are distinguished chiefly by the granular covering of the marginals. From a study of these cases it seems clear that the plates of the dorsal skeleton of the Valvata correspond to the supporting knobs of the paxillae much broadened out, and the granules correspond to the spinelets of the paxillae increased in number and diminished in size.
As mentioned above, Ludwig has proved that the paxillae develop in the life-history of the individual out of ordinary plates, the axis of the paxilla representing the plate.
Order V. Forcipulata.
This order, which includes the most highly developed members of the class Asteroidea, is at once distinguished by the possession of forcipulate pedicellariae which, as we have seen, possess a well-marked basal piece with which the two plates articulate. The pedicellariae are consequently sharply marked off from the spinelets, and no intermediate forms occur. The first conjoined adambulacrals, which in other orders form the "teeth" or mouth-angles, do not here project beyond the first pairs of ambulacral plates.
Fam. 1. Asteriidae.—Forcipulata in which the tube-feet are apparently arranged in four rows. Aboral skeleton a loose reticulum.
The general features of the family Asteriidae have been explained in the description of Asterias rubens (p. 432). There are five well-marked species of the genus found on the British coasts. Of these A. glacialis is found chiefly in the south-western parts of the English Channel. It is a large Starfish of a purplish-grey colour, with large spines surrounded by cushions of pedicellariae arranged in one or two rows down each arm. A. muelleri resembles the foregoing species, but is of much smaller size, and is further distinguished by having straight pedicellariae in the neighbourhood of the ambulacral groove only. It is found on the east coast of Scotland, and carries its comparatively large eggs about with it until development is completed. A. rubens is the commonest species, and is found on both east and west coasts. Its colour is a bright orange, but varies to almost a straw colour. It is at once distinguished from the foregoing species by the spines of the dorsal surface, which are small and numerous, an irregular line of somewhat larger ones being sometimes seen down the centre of each arm. A. murrayi is a peculiar species restricted to the west coast of Scotland and Ireland. It has flattened arms, with vertical sides, and only three rows of small spines on the dorsal surface. It is of a violet colour. A. hispida is also a western species. It is a {474}small Starfish with short stout arms; there are no straight pedicellariae, and only a few sharp spines on the dorsal surface.
On the eastern coast of North America there are several species of Asterias, of which the most noteworthy is the 6-rayed A. polaris of the Gulf of St. Lawrence. This species exhibits a marvellous range of colour-variation, ranging from bluish-violet through purple to red and straw-coloured. This variation seems to show that colour, as such, is of no importance to the animal, but probably depends on some compound of slightly varying composition which is being carried by the amoebocytes towards the exterior. On the Pacific coast there is a rich fauna of Starfish, among which we may mention as members of this family Asterias ochracea, a large violet species, so strong that it requires a severe wrench to detach it from the rock, and Pycnopodia with twenty-two arms.
Fam. 2. Heliasteridae.—Forcipulata allied to the Asteriidae, but with very numerous arms and double interradial septa. Heliaster.
Fam. 3. Zoroasteridae.—Forcipulata with the tube-feet in four rows at the base of the arm, in two rows at the tip. Aboral skeleton of almost contiguous plates bearing small spines or flattened scales. Zoroaster, Pholidaster.
Fam. 4. Stichasteridae.—Forcipulata with the tube-feet in four rows. Aboral skeleton of almost contiguous plates covered with granules. Stichaster, Tarsaster.
The Stichasteridae and Zoroasteridae have acquired a superficial resemblance to some of the long-armed Valvata, from which they are at once distinguished by their pedicellariae. It would be exceedingly interesting if more could be found out concerning the normal environment of these animals; it might then be possible to discover what is the cause of the assumption of this uniform mail of plates.
Fam. 5. Pedicellasteridae.—Forcipulata with two rows of tube-feet. The aboral skeleton bears projecting spines surrounded by cushions of straight pedicellariae. Pedicellaster, Coronaster.
Fam. 6. Brisingidae.—Forcipulata with numerous arms and only two rows of tube-feet. Aboral skeleton largely rudimentary and confined to the base of the arms. The small blunt spines are contained in sacs of skin covered with pedicellariae.
The Brisingidae, including Brisinga and Odinia, are a very {475}remarkable family, chiefly on account of the smallness of the disc and of the extraordinary length of the arms. The arms have what we must consider to have been the primitive arrangement, since there is no lateral adhesion between them, and interbrachial septa are consequently entirely absent. The reduction of the skeleton is a very marked peculiarity and, like the tendency to the reduction of the skeleton of deep-sea fish, may stand in some relation to the great pressure under which the animals live.
Fossil Asteroidea.
The Asteroidea occur somewhat plentifully as fossils. In the Lower Jurassic Asterias, Astropecten, Luidia, Solaster, and Goniaster have already made their appearance. In the Cretaceous {476}Pentaceros appears. In the older rocks occur a number of forms of different character from any now existing. Of these Aspidosoma (Fig. 206), with short lancet-shaped arms sharply distinguished from the disc and continued along its under surface, seems to be intermediate between Asteroidea and Ophiuroidea. The skeleton of the arm is composed of alternating ambulacral ossicles bordered by adambulacral ossicles, which are at the same time marginals and sharply distinguished from the marginals forming the edge of the disc. Palaeaster, on the other hand, is a true Asteroid; there are marginals distinct from the adambulacrals, but the disc is reduced to its smallest dimensions, there being only one plate on the ventral side of each interradius. There are a number of genera (Palaeocoma, for instance) with a large disc and very short arms and very shallow ambulacral grooves; all have alternating ambulacral plates. Some genera appear to have had the madreporite on the ventral surface of an interradius. On the other hand, in the Devonian occurs Xenaster, which was a fairly normal Asteroid, with pavement-like marginals, deep ambulacral grooves, and broad arms.

Fig. 206.—Three views of Aspidosoma, a fossil Asteroid. A, oral view; B, aboral view of one arm; C, enlarged view of a portion of the ambulacral groove. adamb, Adambulacral plate; amb, ambulacral plate; marg, marginal plate; pod, aperture for extension of tube-foot.
Thus it will be seen that already in Jurassic times the three orders, Forcipulata, Paxillosa, and Spinulosa were differentiated from each other, but how these are related to the older Palaeozoic forms it is at present impossible to say.
ECHINODERMATA (CONTINUED): OPHIUROIDEA = BRITTLE STARS
CLASS II. OPHIUROIDEA
The second class of Eleutherozoa are familiarly known as "Brittle Stars," on account of their tendency, when seized, to escape by snapping off an arm, although this habit is by no means confined to them, but is shared in a marked degree by many Asteroidea, such as Luidia, for instance. Like the Asteroidea, they are "starfish," that is to say, they consist of a disc and of arms radiating from it; but the scientific name Ophiuroidea really expresses the great dominating feature of their organisation. Literally it signifies "Snake-tail" (ὄφις, snake; οὐρά, tail), and thus vividly describes the wriggling, writhing movements of the long thin arms, by means of which the Ophiuroid climbs in and out of the crevices between the stones and gravel in which it lives. This feature, viz. the effecting of movement by means of muscular jerks of the arms, instead of by the slow protrusion and retraction of the tube-feet, is the key to the understanding of most of the points wherein the Brittle Stars differ from the true Starfish.
Asteroidea and Ophiuroidea agree in the common ground-plan of their structure, that is, they both possess arms; but the most obvious difference in their outer appearance is that whereas in Asteroidea the arms merge insensibly into the disc, in Ophiuroidea the disc is circular in outline and is sharply marked off from the arms. Closer inspection shows that in the Ophiuroid the arms are continued inwards along grooves, which run on the under surface of the disc, and that they finally coalesce to form a buccal framework surrounding the mouth. In {478}the very young Ophiuroid the arms melt into a small central disc, as in the Starfish, but the disc of the adult is made up of a series of interradial dorsal outgrowths which meet one another above the arms.

Fig. 208.—Oral view of the disc of Ophiothrix fragilis. g.b, Opening of the genital bursa; m.p, madreporite; pod, podia; t.p, tooth-papillae; v.p, ventral plates of the arms. × 1.
One of the commonest British Ophiuroids is Ophiothrix fragilis (Figs. 207, 208), which is found in swarms in shallow water off the west coast of England and Scotland. We may therefore select it as the type, and, since the arm is the most characteristic organ of an Ophiuroid, we may commence by studying it. Speaking generally, an Ophiuroid either drags itself forward by two arms and pushes itself by the other three (Fig. 207),[458] or else it drags itself by one and pushes with the other four (Fig. 217). The arms during this process are bent into characteristic curves, by the straightening of which in the posterior arms the animal is pushed onwards, whilst the intensification of these curves in the anterior arms causes the animal to be dragged forwards. The grip of the arm on the substratum is chiefly in the distal portion of the curve. The alteration of the curvature is due to the contraction of the muscles on one side of the arms. There is no ambulacral groove such as is found on the under side of the arms of all Asteroidea, for the arm is completely ensheathed by four series of plates, an upper row of dorsal plates, an under row of ventral plates, and two lateral rows of lateral plates. The last named, which in all probability correspond to the adambulacral plates of Starfish, bear each a transverse row of seven spines with roughened surfaces; these enable the animal to get a grip on the substratum over which it moves. The podia in Ophiuroidea are termed "tentacles"; they are totally devoid of suckers, being simple conical papillae used as sense-organs, and are of little, if any, service in locomotion. They issue from openings called "tentacle-pores" situated between the edges of the {480}ventral and lateral plates, guarded each by a valve-like plate called the "tentacle-scale." In Ophiothrix they are covered with sense-organs, each consisting of a hillock-like elevation of the ectoderm, in which are cells carrying long stiff sense-hairs. In most Ophiuroids such organs are not present, though abundant scattered sense-cells occur, and the outer surface of the tube-feet and the lining of certain pockets called "genital bursae" (Fig. 208, g.b) are the only portions of the surface where the ectoderm persists. Everywhere else, although present in the young, it disappears, leaving as remnants a few nuclei here and there attached to the under side of the cuticle.[459]

Fig. 209.—Diagrammatic transverse section of the arm of an Ophiuroid. coe, Dorsal coelomic canal; ect, ectoderm covering the tube-foot; ep, epineural canal; gang.p, pedal ganglion; L, nerve-cord; musc, longitudinal muscles attaching one vertebra to the next; nerv.rad, radial nerve-cord; perih, radial perihaemal canal; pod, podium (tube-foot); sp, lateral spines; w.v.r, radial water-vascular canal.
The greater part of the section of the arm is occupied by a disc-like ossicle called the "vertebra." Each vertebra articulates with its predecessor and successor by cup-and-ball joints, and it is connected to each of them by four powerful longitudinal muscles. Above, its outline is notched by a groove, in which lies an extension of the coelom of the disc (Fig. 209, coe), but contains no outgrowth of the alimentary canal, as is the case in Asteroidea. The vertebra is also grooved below, and in this lower groove are contained the radial water-vascular canal {481}(Fig. 209, w.v.r), and below it perihaemal canals as in Asteroidea; below this again the radial nerve-cord (L), and beneath this again a canal called the "epineural canal" (ep), which represents the missing ambulacral groove. This canal in the very young Brittle Star is an open groove, but becomes closed by the approximation of its edges. The vertebra, which has a double origin, represents a pair of fused ambulacral ossicles. In Ophiohelus these are only slightly adherent to one another (Fig. 216).

Fig. 210.—Proximal and distal views of the three types of vertebra found amongst Ophiuroidea. A, Ophioteresis, a type of the Streptophiurae (after Bell), × 24; B, Astroschema, a type of the Cladophiurae (after Lyman), × 10; C, Ophiarachna, a type of the Zygophiurae (after Ludwig), × 3. The upper figure in all cases represents the distal aspect, the lower the proximal aspect of the vertebra. v.g, Ventral groove.
When the surface of a vertebra is examined it is found that it can be divided into a thin border, to which are attached the four muscles by which it is connected to its successor and predecessor, and a central portion, on which are situated the knobs and pits, by means of which it articulates with the next vertebra.
The simultaneous contraction of the two upper muscles causes the arm to bend upwards. The contraction of the two lower bend it downwards, whilst a sideward movement is effected by the contraction of the upper and lower muscle of the same side. On the proximal surface of the central portion of the vertebra there is a central knob and two ventro-lateral knobs, {482}a median ventral pit and two dorso-lateral pits, and on the distal surface there are pits corresponding to the knobs on the proximal side and vice versa (Fig. 210, C). These knobs and pits restrict the movement of one vertebra on the next, so that although the arms can undergo an unlimited amount of flexion from side to side, they cannot be rolled up in the vertical plane. When the under surface of the vertebra is examined there is seen on each side of the central groove two round holes, a distal and a proximal. The distal pair are for the passage of the canals connecting the radial water-vessel with the tentacles, these canals traversing the substance of the vertebra for a part of their course; the proximal pair are for nerves going to the longitudinal muscles, which likewise perforate part of the ventral border of the vertebra.
In order to understand the anomalous circumstance that the canals going to the tentacles actually perforate the vertebrae, it must be clearly borne in mind that the basis of the body-wall in all Echinoderms is a mass of jelly with amoebocytes in it, to which we must assign the power of secreting carbonate of lime, and all we have to assume in the case of Ophiuroids is that calcification spread outwards from the original ambulacral ossicles into the surrounding jelly, enclosing any organs that happened to traverse it.
When the ossicles of the arm are followed inwards towards the mouth, they are seen to undergo a profound modification, so as to form, by union with the corresponding ossicles of adjacent arms, a structure called the mouth-frame. The general character of this modification is similar to that affecting the first ambulacral and adambulacral ossicles in the arms of an Asteroid, but in the Ophiuroid the change is much more profound. The first apparent vertebra consists of two separated halves, and each is fused with the first adambulacral (lateral) plate, which in turn is firmly united with the corresponding plate in the adjoining arm. Thus is formed the "jaw," as the projection is called. The extensions of the mouth-cavity between adjacent jaws are termed "mouth-angles." To the apex of each jaw is attached a plate bearing a vertical row of seven short blunt spines called "teeth" (Fig. 212, p). The plate is called the "torus angularis" (Fig. 211, T), and on its ventral edge there is a tuft of spines which are termed "tooth-papillae" (Fig. 208, t.p). On the upper aspect of the jaw {483}are a pair of plates termed "peristomial plates." These discs—of which there are two in each radius, one on each jaw which flanks the radius—possibly represent the separated halves of the first vertebra, the apparent first vertebra being really the second. On the flank of the jaw there is dorsally a groove for the water-vascular ring and nerve-ring (Fig. 212, n.r), and beneath this a groove for the first tentacle and a pore for the second, both of which spring directly from the ring-canal; below these, in most Ophiuroidea, but not in Ophiothrix, there is a row of blunt triangular spines called "mouth-papillae" (Fig. 212, p1).

Fig. 211.—Diagrams to show the modification of the ambulacral and adambulacral ossicles to form the armature of the mouth. A, Asteroid; B, Ophiuroid. A1-A4, the first four ambulacra ossicles; Ad1-Ad4, the first four adambulacral ossicles; J1, the first plate of the interradius (in the Ophiuroid the scutum buccale); P, the spines borne by the jaw (in the Ophiuroid the teeth); T, the torus angularis; W, the water-vascular ring; Wr, the radial water-vessel; I, II, the first two pairs of tube-feet. (After Ludwig.)
The words "jaw" and "tooth" are misleading. There is no evidence that the jaws of a Brittle Star are ever used for crushing food, but by means of the muscles attaching them to the first {484}complete vertebra in the arm they can be rotated downwards so as greatly to enlarge the mouth, and again rotated upwards and inwards, when they form an excellent strainer to prevent the entrance of coarse particles. To permit this extensive movement the articulatory facets on the proximal surface of the first vertebra have been much modified; the median knob and pit have disappeared, and the dorso-lateral pits are raised on to the surface of processes, so that there are in all four processes, two of which articulate with one half of a jaw.

Fig. 212.—Lateral view of mouth-frame of Ophiarachna incrassata. × 4. A1?, peristomial plate, possibly the half of the first vertebra; A2, the half of the second vertebra; A3, the third vertebra; F1, pores for pair of tentacles; gen, genital scale lying beside opening of genital bursa; musc, longitudinal muscles connecting vertebrae; n.r, groove for nerve-ring; p, tooth; p1, mouth-papilla; t, torus angularis. (After Ludwig.)
The mouth can be narrowed and the jaws forced inwards towards the centre by the simultaneous contraction of five muscles (musc. tr, Fig. 213) each, which unite the two halves of a jaw.
Turning now to the skeleton of the disc, we notice that dorsally it consists of a closely-fitting mosaic of small plates, which are usually concealed from view by a covering of minute spines. Opposite the insertion of each arm there are, however, a pair of large triangular plates ("radials"), which extend outwards to the periphery and strengthen it, much as the ribs do in an umbrella. These radial plates are always exposed, in Ophiothrix, even when the rest of the dorsal plates are concealed by spines. On the under surface there is a similar plating; but adjoining the jaws are five large, more or less rhomboidal, plates {485}termed "scuta buccalia" (Fig. 211, J1), on one of which open the few madreporic pores which the animal possesses. Attached to the sides of the scuta buccalia are the "lateral mouth shields," which are in fact the adambulacral plates belonging to the second pair of ambulacral plates which form the main mass of the jaws. Further out, on the under side of the disc, there is, on each side of each arm, a long narrow slit—the opening of the genital bursa (Fig. 208, g.b), so that there are ten genital bursae. The "genital bursa" (Fig. 214) is a sac lined by ciliated ectoderm projecting into the interior of the disc. It is called genital because the openings of the genital organs are situated on its surface; its main function, however, is respiratory, the cilia bringing about a constant inward current of fresh sea-water, the oxygen contained in which diffuses through the thin wall of the sac into the coelomic fluid. The opening of the bursa is strengthened on its radial side by a rod-like ossicle, the "genital plate," and on its interradial side by an ossicle called the "genital scale" (Fig. 212, gen), and in Ophiothrix the outer end of the radial plate articulates with the outer end of the genital plate. Muscles connect the two plates running on either side of the articulation.
Observations on Ophiothrix[460] show that in this species at any rate the radial plates can be raised or lowered. When they are raised the centre of the disc is lifted into a cone and water is sucked into the genital bursae, whereas when they are lowered the bursae are compressed and water is expelled. This forced respiration appears to come into play when the supply of oxygen is getting scanty.
The alimentary canal of Ophiothrix is a simple flattened sac (Fig. 213). It is devoid of an anus and cannot be everted through the mouth. There is a horizontal pouch given off into each interradial lobe of the disc. The sac is attached to the dorsal wall of the coelom by numerous mesenteries, fibrous cords traversing the coelomic cavity and clothed on the outer side by coelomic epithelium. To the mouth-frame it is attached by a circular membrane, which we have reason for believing is a {486}functionless remnant of the retractor muscles of the stomach of Asteroidea. In the young Asteroid there is a similar sheet of membrane, which later becomes resolved into the ten retractor bands.
The simple structure of the alimentary canal appears to be correlated with the exceedingly simple character of the food. Ophiothrix feeds on the most superficial layer of mud at the bottom of the sea. This deposit consists partly of microscopic Algae and partly of decaying organic matter, and is much more easily disposed of than the living animals on which the Starfish preys. The food is shovelled into the mouth by the first two or "buccal" pairs of tube-feet in each ray.

Fig. 213.—Longitudinal section through the disc of a young Ophiuroid passing along one arm and the middle of the opposite interradius. (Diagrammatised from an actual section of Amphiura squamata.) ab, Aboral sinus (dorsal in the radius, ventral in the interradius); ax, axial sinus; coe, dorsal coelomic canal of the arm; ep, epineural canal; gang.rad, ganglion of the radial nerve; gen.r, genital rachis contained in the aboral sinus; gen.st, genital stolon; mp, madreporic pore; musc.long, longitudinal muscle of the arm; musc.tr, transverse muscle uniting the two halves of each jaw; mv, madreporic vesicle; nerv.r, nerve-ring; p.c, pore-canal; perih, perihaemal canal; vert, vertebra; w.vr, radial water-vessel.
The water-vascular system has undergone a most interesting set of modifications, which can be explained by noticing the fact that the tube-feet have almost, if not quite, lost their locomotor function and are now used as tactile organs. The ampulla, or swollen inner end of the tube-foot, has disappeared, and the upper end of the organ is directly connected with the radial canal by means of a curved canal, which traverses the outermost flange of the vertebra, appearing on its {487}surface in a groove on the outer side of the dorsal lateral knob on the distal side of the ossicle. As in Asteroidea there are valves, which regulate the entrance of fluid into the tube-foot. The stone-canal is a curved tube of simple circular section and excessively narrow bore which extends from the water-vascular ring downwards to the madreporite (Fig. 213, mp) situated on one of the scuta buccalia. The madreporite, in Ophiothrix as in most Brittle Stars, is an exceedingly rudimentary structure, consisting of one or two pores leading into as many pore-canals. From each interradius, except that in which the stone-canal lies, a large Polian vesicle hangs down from the water-vascular ring into the coelom.
We saw that in the Asteroid the ampulla was used like the bulb of a pipette to force the fluid in the tube-foot down into the tip, so as to press the sucker against the substratum. But when the tube-foot is used as a sense-organ, a few circular fibres around its upper end suffice to bring about all the extension that is needed. Since the extension is no longer a very vigorous act, the loss of fluid by transudation has probably been rendered insignificant, and hence the stone-canal and madreporite, whose function it is to repair the loss, have been reduced in size. The curious ventral curvature of the stone-canal is, however, due to another cause. In the very young Ophiuroid the madreporite is on the edge of the disc, and the stone-canal extends horizontally outwards; and in some Asteroidea there is a similar outward direction in its course. As development proceeds the dorsal interradial areas of the disc of the young Ophiuroid grow out into lobes, building up the conspicuous adult disc and forcing the madreporite, and with it the stone-canal, downwards towards the ventral surface.
The pores of the madreporite in Ophiothrix, like some of those in the Asteroid, open not directly into the stone-canal but into the axial sinus (Fig. 213, ax). This is a large ovoid sac, lined with thin epithelium, lying between the stone-canal and the mouth-frame, since of course it has shared in the ventral rotation of the stone-canal. Its open connexion with the stone-canal was easily recognised by Ludwig, who termed it, on this account, the "ampulla."[461] The name "axial sinus" was bestowed {488}mistakenly on another cavity, which will be mentioned in connexion with the genital organs.
The radial perihaemal spaces of the arms open into a "perihaemal ring" representing the outer perihaemal ring of Asteroids; but the axial sinus does not have any such extension as constitutes the inner perihaemal ring in Starfish. So-called oral circular and radial blood strands are to be found in similar positions to the corresponding structures in Asteroidea.
The nervous system might have been expected to have become very much modified, since the activities of the Brittle Stars are so different from those of the Starfish. It is indeed a universal rule in the Animal Kingdom that, concomitantly with the increase in size and activity of a muscle, there is a corresponding increase in the number of ganglion-cells which control it. An accurate radial section of an arm shows that there is, corresponding to the interspaces between the two vertebrae, a ganglionic swelling of the nerve-cord. As in Asteroids, there are not only ectodermic ganglion-cells on the under surface of the cord abutting on the epineural canal, but also coelomic ganglion-cells derived from the floor of the radial perihaemal canal. Both these categories of cells are largely increased in number in the ganglion. From the dorsal-cells arise a pair of large nerves which pass directly up and supply the great intervertebral muscles. From the interspace between the ganglia a direct prolongation of the ventral part of the nerve-cord, the so-called pedal nerve, extends out along the side of the tentacle, as in Asteroids. In Ophiuroids it swells out into a ganglion, completely surrounding the tentacle and giving off nerves to the surfaces of the arm which terminate in the cuticle.
There is a large ganglion where the radial cord joins the nerve-ring, and, owing to the more specialised condition of the nervous system, a severed arm in an Ophiuroid is much more helpless than an arm of an Asteroid. It will not carry out "escape movements," and is for a long time rigid under the shock of section; at last it simply gives reflex movements on stimulation.
Preyer[462] endeavoured to test the "intelligence" of Ophiuroids by observing how they would adapt themselves to circumstances which it might be fairly assumed they had never encountered {489}in their ordinary experience. To this end he passed over the arm of a specimen a piece of indiarubber tubing, which clung to it tightly. He found that the animal first tried walking off, pressing the encumbered arm against the ground, so that the piece of tubing was rubbed off. It was then replaced more tightly than before; the animal, having tried the first method without result, waved the arm to and fro in the water till the rubber floated off. In a third experiment the animal held the rubber against the ground by a neighbouring arm, and drew the encumbered arm out. When the rubber was replaced a fourth time, the animal kicked it off by alternately pressing neighbouring arms against it. Finally, when the rubber was put on so firmly that all the above-mentioned methods failed, the arm was broken off. Preyer concludes from this that Ophiuroids have a high degree of intelligence; but this may be doubted, and the reader is referred to the account of Uexküll's experiments given in the next chapter. There is, however, no doubt at all that Ophiuroidea are by far the most active of all Echinoderms, and one would naturally correlate this with higher psychic development.
The radial nerve ends in a terminal tentacle sheltered by a median plate at the end of the arm; but eyes, such as are found in Asteroids, are wanting, and the animal does not appear to be sensitive to light.
The reproductive system in Ophiuroids consists of a genital stolon giving rise at its distal end to a genital rachis, which extends in a circular course round the disc, ensheathed in an "aboral sinus" (Fig. 213, ab) and swelling out so as to form the gonads (testes or ovaries), where it passes over the inner side of the genital bursae. The genital stolon (Fig. 213, gen.st) is a compact ovoid organ, often termed on account of its shape the "ovoid gland." It is situated close to the stone-canal, and, as in Starfish, it indents the outer wall of the axial sinus; but, unlike the stolon of the Asteroid, it is separated from the general coelom by a space, of which it forms the inner wall, but whose outer wall is formed by a sheet of membrane. This cavity must be carefully distinguished from the axial sinus of Asteroidea, to which it was supposed at one time to correspond; it is really formed by a pocket-like ingrowth of the general coelom into the septum dividing it from the axial sinus. The cells forming the inner side of this pocket form the primitive germ-cells, which {490}constitute the main mass of the ovoid gland; those of the outer side remain thin. The cavity of the ingrowth is shut off from the general coelom, but persists throughout life. In Asteroids a similar ingrowth takes place, but both walls thicken and become converted into germ cells, and the cavity disappears, and, as in Asteroidea, a considerable number of the germ-cells in the stolon degenerate.

Fig. 214.—Diagram of a tangential section through the edge of the disc of an Ophiuroid to show the relations of the disc, arm, and genital bursae. ep, Epineural canal; musc, longitudinal muscle of the arm; nerv.rad, radial nerve cord; ov, ovary; perih, radial perihaemal canal; w.v.r, radial water-vessel.
The genital rachis (Fig. 213, gen.r) is an outgrowth of the distal end of the genital stolon, which extends in a complete circle round the disc. The rachis does not, however, lie everywhere in the same plane, but by its undulating course bears witness to the distortion which the disc has undergone. In the radii it is, as in the Asteroid, dorsal; but in the interradii it is ventral, this ventral portion having, like stone-canal and axial sinus, been carried down by the preponderant growth of the dorsal parts of the disc. It is everywhere ensheathed by the aboral sinus, which, as in Asteroids, is an outgrowth of the coelom. The rachis is embedded in a strand of modified connective tissue, to which we may (as in the case of Asterias) apply the name "aboral blood-ring." Both on the central and peripheral sides of this sinus are vertical muscles connecting the genital and the radial plates, which bring about the respiratory movements already referred to. Just above the madreporite, at the end of the genital stolon, is a small, completely closed space, which by its position corresponds with the madreporic vesicle of Asteroids and represents the right hydrocoel (Fig. 213, mv). As the rachis passes over the genital bursa it gives off branches, which swell up to form the genital organs. In Ophiothrix there is {491}one such organ on each side of each bursa, but in other genera (cf. Ophiarachna) a large number of small ones. The genital products are shed into the water through the bursae.
Classification of Ophiuroidea.
Before proceeding to study the classification of Brittle Stars, it is necessary to give some account of the range of structure met with in the group.
Number of Radii.—The number of arms is rarely increased, and hardly ever exceeds six; a few species (each an isolated one in its genus) have six arms, and in one case (Ophiactis virens), at any rate, this is associated with the power of transverse fission. In many Cladophiurae the arms fork repeatedly, so that although there are only five radii, there is quite a crowd of terminal branches.
Vertebrae.—The vertebrae differ in the manner in which they articulate with one another. In Ophiothrix fragilis taken as the type, which in this respect resembles the vast majority of species (Zygophiurae), the knobs and pits on the faces of the vertebrae prevent the arms from being coiled in the vertical plane. In Ophioteresis (Fig. 210, A) and some allied genera (Streptophiurae) the knobs are almost obsolete, and the arms are free to coil in the vertical plane; whilst in Gorgonocephalus and Astrophyton (Cladophiurae) the arms are repeatedly branched and the vertebrae have saddle-shaped articulating surfaces, so that they have quite a snake-like capacity for coiling themselves round external objects. In Ophiohelus (Fig. 216) each vertebra consists of two rod-like plates placed parallel with the long axis of the arm and fused at both ends, but divergent in the middle, leaving a hole between them.
Covering Plates of the Arms.—The upper arm-plates are the most variable. They may be surrounded by small supplementary plates (Ophiopholis) or double (Ophioteresis). In all (?) Cladophiurae and most Streptophiurae they are absent, being replaced by minute calcareous granules. Under arm-plates are absent in Ophioteresis and in the distal portion of the arms in many Cladophiurae. Side arm-plates are constantly present, and in most Cladophiurae meet in the middle line below.
Arm-Spines.—The spines borne by the lateral covering plates of the arms vary greatly in character. In Ophiura and its {492}allies they are short and smooth, and are borne by the hinder edge of the arm and directed backwards; but in the larger number of genera they are borne nearer the centre of the plate, and are directed outwards at right angles to the arm. They may be covered by small asperities, as in Ophiothrix (Fig. 215, C), when they are said to be rough; or these asperities may become secondary spines, as in Ophiacantha (Fig. 215, B), when they are said to be thorny. In Ophiopteron all the spines borne by a single plate are united by a web of skin so as to constitute a swimming organ. The small plates guarding the ends of the tentacles (tentacle-scales) may be absent, or more rarely double. In Cladophiurae there is a regular transition from tentacle-scale to arm-spine; the tentacle-scale being merely the smallest of the series of lateral spines.
True pedicellariae are unknown amongst Ophiuroidea, since there is no longer a soft ectoderm to protect, but in some cases, as for instance in Ophiohelus, small hooks movable on a basal piece attached to the arms are found which may represent the vestiges of such organs (Fig. 216). Similar hooks are found in the young Ophiothrix fragilis just after metamorphosis and in all Cladophiurae, replacing in the latter case the arm-spines in the distal portion of the arm.

Fig. 215.—Three types of mouth-frame found in Zygophiurae. A, Ophioscolex, × 10; B, Ophiacantha, × 6; C, Ophiothrix, × 6. (After Lyman.)
Mouth-Frame.—In its broad outlines there is practically no variation in this organ throughout the group, but in respect of {493}the spines, which are borne on the flanks of the jaws (mouth-papillae) and on their apices (teeth and tooth-papillae) there is very great variation. Teeth are always present. Mouth-papillae are very frequently present, tooth-papillae are rarer, and it is only in a restricted number of genera (Ophiocoma and its allies) that both mouth-papillae and tooth-papillae are present at the same time.

Fig. 216.—A portion of an arm of Ophiohelus umbella, near the distal extremity, treated with potash to show the skeleton, × 55. The vertebrae are seen to consist of two curved rods united at their ends. The triangular side-plates bear a row of movable hooks which articulate with basal outgrowths of the plate. (After Lyman.)
Skeleton of the Disc.—This is typically composed of a mosaic of plates of different sizes, but in some cases (Ophiomyxa, most Streptophiurae, and Cladophiurae) these, with the exception of the radials and genitals, are entirely absent, and the disc is then quite soft and covered with a columnar epithelium, the persistent ectoderm. Even the scuta buccalia may disappear. Radial shields are absent in Ophiohelus. In many cases (Ophiothrix and Ophiocoma) all the dorsal plates except the radials are concealed from view by a covering of small spines. In some genera (Ophiopyrgus) there are five large plates in the centre of the upper part of the disc, which have been termed "calycinals" from a mistaken comparison with the plates forming the cup or calyx of the Pelmatozoa, but there is no connexion between the two sets of structures.
The madreporite is usually quite rudimentary, but in Cladophiurae there may be five madreporites, each with about 200 pores, and, of course, five stone-canals.
The number of genital organs varies very much. In the small Amphiura squamata there are two gonads, an ovary and a testis, attached to each bursa, but in the larger species there may be very many more.
We follow Bell's classification,[463] according to which the Ophiuroidea are divided, according to the manner in which the vertebrae move on one another (cf. Fig. 210), into three main orders, since these movements are of prime importance in their lives.
(1) Streptophiurae, in which the faces of the vertebrae have rudimentary knobs and corresponding depressions, so that the arms can be coiled in the vertical plane. These are regarded as the most primitive of Ophiuroidea.
(2) Zygophiurae, in which the vertebral faces have knobs and pits which prevent their coiling in a vertical plane.
(3) Cladophiurae, in which the arms can be coiled as in (1) and are in most cases forked. No teeth; the arm-spines are papillae, the covering plates of the arms are reduced to granules.
Order I. Streptophiurae.
This is not a very well defined order; it includes a few genera intermediate in character between the Cladophiurae and the Zygophiurae, and believed to be the most primitive Ophiuroids living. It is not divided into families. The vertebrae have rudimentary articulating surfaces, there being two low bosses and corresponding hollows on each side, and so they are capable of being moved in a vertical plane, as in the Cladophiurae; the arms never branch, and further, they always bear arm-spines and lateral arm-plates at least. No species of this order are found on the British coast, but Ophiomyxa pentagona, in which the dorsal part of the disc is represented only by soft skin, is common in the Mediterranean.
Ophioteresis is devoid of ventral plates on the arms, and appears to possess an open ambulacral groove, though this point has not been tested in sections. Ophiohelus and Ophiogeron have vertebrae in which traces of the double origin persist (see p. 491).
Order II. Zygophiurae.
This group includes all the common and better-known British forms. They are divided into five families, all of which are represented in British waters.
Fam. 1. Ophiolepididae.[464]—Arm inserted in a definite cleft {496}in the disc, or (expressing the same fact in another way) the interradial lobes out of which the disc is composed are not completely united. Radial shields and dorsal plates naked. Arm-spines smooth and inserted on the posterior border of the lateral arm-plates.
This family includes all the Brittle Stars of smooth porcelanous aspect and provided with only short spines. Forbes[465] called them Sand-stars, since their short spines render these animals incapable of burrowing or of climbing well, and hence they appear to move comparatively rapidly over firm ground, sand, gravel, or muddy sand, and they are active enough to be able to capture small worms and Crustacea. The prey is seized by coiling one of the arms around it.
One genus, Ophiura, is fairly common round the British coast, {497}being represented by O. ciliaris and O. albida; the former is the commoner. An allied species dredged by H.M.S. "Challenger" is represented in Figs. 217 and 218.
Ophiomusium (Fig. 219) is a very peculiar genus. The mouth-papillae on each side of each mouth-angle are confluent, forming a razor-like projection on each side of each mouth-angle (Fig. 220). The arms are short, and the podia are only developed at the bases of the arms. Ophiopyrgus has the dorsal surface raised into a conical elevation protected by a central plate surrounded by five large plates.
In the remaining four families the arms are inserted on the under surface of the disc; in other words, the interradial lobes which make up the disc have completely coalesced dorsally; and the spines stand out at right angles to the arm.
Fam. 2. Amphiuridae.—Mouth-papillae present, but no tooth-papillae; radial shields naked; small scuta buccalia.
The most interesting Brittle Star belonging to this family is Amphiura squamata (elegans), a small form, with a disc about ¼ inch in diameter covered with naked plates. It is hermaphrodite and viviparous, the young completing their development inside the bursae of the mother. Occasionally the whole disc, with the exception of the mouth-frame, is thrown off and regenerated. This appears to be a device to enable the young to escape. Three other species of Amphiura are found in British waters.
Ophiactis is another genus belonging to this family, distinguished from Amphiura by its shorter arms and smoother arm-spines. It lives in the interstices of hard gravel. The British species, O. balli, presents no special features of interest, but the Neapolitan O. virens is an extraordinary form. It has six arms, three of which are usually larger than the other three, for it is always undergoing a process of transverse division, each half regenerating the missing part. It has from 1 to 5 stone-canals, the number increasing with age; numerous long-stalked Polian-vesicles in each interradius, and in addition a number of long tubular canals which spring from the ring-canal, and entwine themselves amongst the viscera.[466] All the canals of the water-vascular system, except the stone-canals, contain non-nucleated {499}corpuscles, carrying haemoglobin,[467] the respiratory value of which compensates for the loss of the genital bursae, which have entirely disappeared.
Ophiopholis is distinguished from the foregoing genera by the granular covering of its dorsal plates; whilst in Ophiacantha these granules develop into prominent spinelets, and the arm-spines are also thorny. Ophiopholis aculeata occurs in swarms in the branches of the Firth of Clyde, and presents a most remarkable series of variations in colour. Ophiopsila is a closely allied form, distinguished by its large peristomial plates.
Fam. 3. Ophiocomidae.—Both mouth-papillae and tooth-papillae are present;[468] the arm-spines are smooth, and the disc is covered with granules.
Ophiocoma nigra is the only common British representative of this family. In this species the plates of the dorsal surface are completely hidden from view by a covering of granules. Ophiarachna.
Fam. 4. Ophiothricidae.—Tooth-papillae alone present, mouth-papillae absent; arm-spines roughened or thorny.
This family is represented only by Ophiothrix fragilis, which is perhaps the most abundant of all British Ophiuroids, and has been selected as the type for special description.
The back is covered with spinules, having, however, the triangular radial plates bare. This produces a contrast-effect, which suggested the name pentaphyllum, formerly used by some naturalists for the species. It occurs in swarms, and presents variations in colour nearly as marked as those of Ophiopholis. {500}Ophiopteron is probably a swimming Ophiuroid, as the lateral spines of each segment of the arm are connected by a web of skin.
Order III. Cladophiurae.
These, like the Streptophiurae, have the power of rolling the arms in a vertical plane, but the articulating surfaces of the vertebrae are well-developed and saddle-shaped. The dorsal surface of the disc and arms is covered with a thick skin with minute calcifications. Upper-arm plates wanting. Radial plates always present, though occasionally represented by lines of scales. {501}The order is divided into three families, two of which are represented in British waters.
Fam. 1. Astroschemidae.—Arms unbranched. Astronyx is comparatively common in the sea-lochs of Scotland. There are a series of pad-like ridges on the arms, representing the side-plates and bearing the spines. Astroschema.
Fam. 2. Trichasteridae.—Arms forked only at the distal ends. Trichaster, Astrocnida.
Fam. 3. Euryalidae.—Arms forked to their bases. Gorgonocephalus is occasionally taken in deep water off the north coast of Scotland. In it the arms repeatedly fork, so that a regular crown of interlacing arms is formed. The animal obviously clings to external objects with these, for it is often taken in fishermen's nets with its arms coiled around the meshes. The genital bursae are said to be represented by slits which open directly into the coelom. (Lyman describes the coelom as divided into ten compartments by radiating septa; it is possible—even probable—that these are really the bursae.) An allied species is common in the Bay of Fundy, being found in comparatively shallow water. Astrophyton (Fig. 222) is closely allied to Gorgonocephalus, differing only in trifling points. It is doubtful whether the separation of these two genera is justified.
Fossil Ophiuroidea.—The Ophiuroidea are rather sparsely represented among fossils, but in the Silurian and Devonian a series of very interesting forms occur which are intermediate in character between Starfish and Brittle Stars, and which were therefore in all probability closely allied to the common ancestors of modern Ophiuroids and Asteroids. Jaekel[469] has recently added largely to our knowledge of these primitive forms, and has described a number of new genera. Thus Eophiura from the Lower Silurian has an open ambulacral groove, and the vertebrae are represented by an alternating series of quadrate ossicles, each deeply grooved on its under surface for the reception of the tentacle, which was not yet (as in modern forms) enclosed in the vertebra. The lateral or adambulacral plates extended horizontally outwards, and each bore a series of spines at its outer edge.
A remarkable fact is that where the halves of the vertebrae (i.e. the ambulacral ossicles) diverge in order to form the {502}mouth-angles, no less than five or six vertebrae are thus affected, instead of only two as in modern forms. The actual "jaw," however, seems, as in modern forms, to consist only of the first adambulacral fused to the second ambulacral, so that instead of concluding with Jaekel that the "jaws" of modern forms result from the fusion of five or six vertebrae, a conclusion which would require that a number of tentacles had disappeared, we may suppose that the gaping "angles" of these old forms have, so to speak, healed up, except at their innermost portions.
In Bohemura, which belongs to a somewhat younger stratum, the structure is much the same, but the groove in the ambulacral ossicle for the tentacle has become converted into a canal, and the ambulacral groove itself has begun to be closed at the tip of the arm by the meeting of the adambulacrals.
In Sympterura, a Devonian form described by Bather,[470] the two ambulacral plates of each pair have thoroughly coalesced to form a vertebra, but there is still an open ventral groove, and no ventral plates.
In the Trias occurs the remarkable form Aspidura, which had short triangular arms, in which the tentacle pores were enormous and the ventral plates very small. The radial plates formed a continuous ring round the edge of the disc. Geocoma from the Jurassic is a still more typical Ophiuroid; it has long whip-like arms, and the dorsal skeleton of the disc is made of fifteen plates, ten radials, and five interradials. In the Jurassic the living genus Ophioglypha, appears.
The Cladophiurae are represented already in the Upper Silurian by Eucladia, in which, however, the arms branch not dichotomously, as they do in modern forms, but monopodially. There is a large single madreporite.
Onychaster, with unbranched arms, which occurs in the Carboniferous, is a representative of the Streptophiurae.
It will therefore be seen that the evolution of Ophiuroidea must have begun in the Lower Silurian epoch. The Streptophiurae are a few slightly modified survivors of the first Ophiuroids. By the time the Devonian period had commenced, the division of the group into Zygophiurae and Cladophiurae had been accomplished.
ECHINODERMATA (CONTINUED): ECHINOIDEA = SEA-URCHINS
CLASS III. ECHINOIDEA
The Sea-urchins or Echinoidea (Gr. ἐχῖνος, Hedgehog or Sea-urchin), which constitute the third class of the Eleutherozoa, have derived both their popular and scientific names from the covering of long spines with which they are provided. At first sight but little resemblance is to be discerned between them and the Starfish and Brittle Stars. They are devoid of any outgrowths that could be called arms; their outline is generally either circular or that of an equilateral pentagon, but as their height is almost always smaller than their diameter, they are never quite spherical; sometimes it is so small that the animals have the form of flattened discs.
All doubt as to the relationship of the Echinoidea to the Starfish is at once dispelled in the mind of any one who sees one of the common species alive. The surface is beset with delicate translucent tube-feet, terminated by suckers resembling those of Starfish, although capable of much more extension. The animal throws out these organs, which attach themselves by their suckers to the substratum and so pull the body along, whilst the spines are used to steady it and prevent it from overturning under the unbalanced pull of the tube-feet. When moving quickly the animal walks on its spines, the tube-feet being little used. The tube-feet are distributed over five bands, which run like meridians from one pole of the animal to the other. These bands are termed "radii," and they extend from the mouth, which is situated in the centre of the lower surface, up to the neighbourhood of the aboral pole. The radii must be compared to the ambulacral grooves on the {504}oral surface of the arms of Starfish, and hence in Urchins the aboral surfaces of the arms have, so to speak, been absorbed into the disc, so that the oral surfaces have become bent in the form of a semicircle. The radii are separated from one another by meridional bands called "interradii," which correspond to the interradial angles of the disc of a Starfish and to the sides of its arms. The small area enclosed between the upper terminations of the radii is called the "periproct," and this corresponds to the entire dorsal surface of the Starfish, including that of the arms.
One of the commonest species of British Sea-urchin is Echinus esculentus. In sheltered inlets, such as the Clyde, it is often left exposed by the receding tide, whilst everywhere on the coast in suitable localities it may be obtained by dredging at moderate depths on suitable ground. In the Clyde it is easy to observe the habits of the animal through the clear still water. It is then seen to frequent chiefly rocky ground, and to exhibit a liking for hiding itself in crevices. Often specimens will be seen clinging to the rock by some of their tube-feet, and, as it were, pawing the under surface of the water with the others. In the Clyde it feeds chiefly on the brown fronds of Laminaria, with which the rocks are covered. In more exposed situations, such as Plymouth Sound, it does not occur in shallower water than 18 to 20 fathoms. At this depth it occurs on a rocky ridge; but in 1899, after a south-west gale, all the specimens had disappeared from this ridge, showing at what a depth wave disturbance is felt.
A full-grown specimen is as large as a very large orange; its under surface is flattened, and it tapers somewhat towards the aboral pole. The outline is that of a pentagon with rounded angles. The spines in Echinus esculentus are short in comparison to the diameter of the body, and this is one of the characteristics of the species.
The animal is provided with a well-developed skeleton, consisting of a mail of plates fitting closely edge to edge, and carrying the spines. This cuirass bears the name "corona" (Fig. 227). It has two openings, an upper and a lower, which are both covered with flexible skin. The upper area is known as the "periproct" (Fig. 227, 2); it has in it small isolated plates, and the anus, situated at the end of a {505}small papilla, projects from it on one side of the centre. The lower area of flexible skin surrounds the mouth, and is called the "peristome" (Fig. 229), though it corresponds to considerably more than the peristome of Asteroidea. In the mouth the tips of the five white chisel-like teeth can be seen.
The plates forming the corona are, like all the elements of the skeleton of Echinodermata, products of the connective tissue which underlies the ectoderm, which in Echinoidea remains in a fully developed condition covering the plates, and does not, as in Ophiuroidea, dry up so as to form a mere cuticle. The ectoderm consists of the same elements as that of Asteroidea, viz. delicate tapering sense-cells with short sense-hairs, somewhat stouter supporting cells and glandular cells. It is everywhere underlaid by a plexus of nerve fibrils, which, in part, are to be regarded as the basal outgrowths of the sense-cells and partly as the outgrowths of a number of small bipolar ganglion-cells, found intermixed with the fibres.
Just as the muscular arm has been the determining factor in the structure of the Ophiuroidea, so the movable spine has been {506}the leading factor in the evolution of Echinoidea. The spines have cup-shaped basal ends, which are inserted on special projections of the plates of the skeleton called tubercles. The tubercle is much larger than the cup, and hence the spine has a great range of possible motion. The spines differ from those of Starfish and Brittle Stars in being connected with their tubercles by means of cylindrical sheaths of muscle fibres, by the contraction of which they can be moved in any direction. The muscles composing the sheath consist of an outer translucent and an inner white layer. The former are easily stimulated and soon relax; they cause the movements of the spines. The latter require stronger stimulation, but when aroused respond with a prolonged tetanus-like contraction, which causes the spines to stand up stiffly in one position; these muscles can be torn across sooner than forced to relax. Uexküll[471] has appropriately named them "block musculature." These sheaths, like everything else, are covered with ectoderm, which is, however, specially nervous, so that we may say that the muscular ring is covered by a nerve-ring from which stimuli are given off to the muscles.
The spines are, speaking generally, of two sizes, the larger being known as "primary spines" and the smaller as "secondary." In many Echinoidea these two varieties are very sharply contrasted, but in Echinus esculentus there is not such a great difference in length, and intermediate kinds occur. The forest of spines has an undergrowth of pedicellariae. All Echinoidea possess pedicellariae, which are much more highly developed than those of any Asteroid. With few exceptions all the pedicellariae of Echinoidea possess three jaws and a basal piece. This latter is, however, drawn out so as to form a slender rod, which articulates with a minute boss on a plate of the skeleton.
Of these pedicellariae there are in E. esculentus four varieties, viz. (1) "tridactyle" (Fig. 225, C; Fig. 226, B): large conspicuous pedicellariae with three pointed jaws, each armed with two rows of teeth on the edges. There is a flexible stalk, the basal rod reaching only half way up. These are scattered over the whole surface of the animal.
(2) "Gemmiform" (Fig. 225, A, B; Fig. 226, A), so called from the translucent, almost globular head. The appearance of {507}the head is due to the fact that there is on the outer surface of each jaw a sac-like gland developed as a pouch of the ectoderm. From it are given off two ducts which cross to the inner side of the blades and, uniting into one, run in a groove to near the tip. The gland secretes a poisonous fluid. The basal rod reaches up to the jaws, so that this form of pedicellaria has a stiff stalk. On the inner side of each blade, near the base, there is a slight elevation (Fig. 225, B, s), consisting of cells bearing long cilia; this is a sense-organ for perceiving mechanical stimuli. The gemmiform pedicellariae are particularly abundant on the upper surface of the animal.

Fig. 224.—View of the apical region of Echinus esculentus, showing spines and pedicellariae; drawn from the living specimen, × 3. a, Anus; g.p, genital pore; i, interradius; mp, madreporite; per, periproct; p.gemm, gemmiform pedicellaria; pod, podia; p.trid, tridactyle pedicellaria; p.trif, trifoliate pedicellaria; r, radius; t.t, pore for terminal tentacle of the radial water-vascular canal.
(3) "Trifoliate" (Fig. 225, E; Fig. 226, D): these are very small pedicellariae, in which the jaws are shaped like leaves with the broad end projecting outwards. They are scattered over the whole surface of the body.
(4) "Ophicephalous" (Fig. 225, D; Fig. 226, C): pedicellariae in which the jaws have broad rounded distal ends fringed with teeth; these ends bear a resemblance to a snake's head, whence the name. The bases are also broad and thin, with a strong median rib and a peculiar semicircular hoop beneath the spot where they articulate with one another. The three hoops of the three jaws work inside each other in such a way as to cause the jaws to have a strong grip and to be very difficult to dislocate from their mutual articulation.
The ophicephalous pedicellariae are in Echinus the most abundant of all; and they alone extend on to the peristome, where a special small variety of them is found.
A thorough investigation of the functions and reactions of the pedicellariae has quite recently been made by von Uexküll.[472] He showed, first of all, that there is a nervous centre in the stalk of each pedicellaria (see below), which causes the organ to incline towards a weak stimulus, but to bend away from a stronger stimulus. In the head there is an independent nervous centre, which regulates the opening and closing of the valves, and causes these to open on slight stimulus and close when a stronger one is applied. The amount of stimulus necessary to cause the pedicellariae to retreat varies with the kind of pedicellariae, being least with the tridactyle and most with the gemmiform, so that when a chemical stimulus, such as a drop of dilute ammonia, is applied to the skin, the tridactyle pedicellariae may be seen to flee from and the gemmiform to approach the point of stimulation. In a living Sea-urchin, if the attempt is made to seize the tridactyle pedicellariae they will evade the forceps, but the ophiocephalous are easy to catch.
The tridactyle pedicellariae open with the very slightest mechanical stimulus and close with rather greater mechanical stimuli or with exceedingly slight chemical ones. Uexküll calls them "Snap-pedicellariae," and their function is to seize and destroy the minute swimming larvae of various sessile parasitic {509}animals, which would otherwise settle on the delicate exposed ectoderm of the Sea-urchin.
The gemmiform pedicellariae are brought into action when a more serious danger threatens the Sea-urchin, such as an attack of a Starfish. The corrosive chemical influence, which it can be proved exudes not only from the stomach but even from the tube-feet of the Starfish, causes the gemmiform pedicellariae to approach and open widely. When the foe approaches so closely as to touch the sense-organs (Fig. 225, B, s) situated on the inner side of the valves of these pedicellariae, the blades close violently, wounding the aggressor and causing its juice to exude, thus producing a renewed and severe chemical stimulation which irritates the poison glands and causes the poison to exude. The virulence of the poison may be gauged from the fact that the bite of a single gemmiform pedicellaria caused a frog's heart to stop beating.

Fig. 225.—The pedicellariae of Echinus acutus, drawn from a living specimen. A, gemmiform pedicellaria, closed. B, gemmiform pedicellaria, open; g, poison gland; s, sense-organ, × 3. C, tridactyle pedicellaria, × 6. D, ophicephalous pedicellaria, × 9. E, trifoliate pedicellaria, × 12; a (in all figures), axial rod of the stalk. (After Uexküll.)
Prouho[473] has described a combat between a Sea-urchin and a Starfish. When the latter approached, the spines of the {510}Sea-urchin diverged widely (strong form of reaction to chemical stimulus), exposing the gemmiform pedicellariae. These at once seized the tube-feet of the enemy and the Starfish retreated, wrenching off the heads of these pedicellariae; then the Starfish returned to the attack and the same result followed, and this was repeated till all the pedicellariae were wrenched off, when the Starfish enwrapped its helpless victim with its stomach.
The minute trifoliate pedicellariae are brought into play by any prolonged general irritation of the skin, such as bright light or a rain of particles of grit or mud. They have the peculiarity that not all the blades close at once, so that an object may be held by two blades and smashed by the third. They may be seen in action if a shower of powdered chalk is poured on the animal, when they seize the particles and by breaking up any incipient lumps reduce the whole to an impalpable powder, which the cilia covering the skin speedily remove. In thus assisting in the removal of mechanical "dirt" they earn the name which Uexküll has bestowed on them, of "cleaning pedicellariae."

Fig. 226.—Views of a single blade of each kind of pedicellaria. A, blade of gemmiform pedicellaria of Echinus elegans; g, groove for duct of poison gland; B, blade of tridactyle pedicellaria of the same species; C, blade of ophicephalous pedicellaria of the same species; r, ring for clamping this blade to the other blades; D, blade of trifoliate pedicellaria of E. alexandri. (After Mortensen.)
The ophicephalous pedicellariae, with their powerful bull-dog grip, assist in holding small animals, such as Crustacea, till the tube-feet can reach them and convey them to the mouth.
The number and variety of the pedicellariae, then, is an eloquent testimony to the dangers to which the soft sensitive skins of the Sea-urchin and other Echinodermata are exposed, and afford confirmatory evidence in support of the view expressed above, that the method adopted to defend the skin was one of the great determining features which led to the division of the Asteroidea into different races.

Fig. 227.—Dried shell of Echinus esculentus, showing the arrangement of the plates of the corona. × 1. 1, The anus; 2, periproct, with irregular plates; 3, the madreporite; 4, one of the other genital plates; 5, an ocular plate; 6, an interambulacral plate; 7, an ambulacral plate; 8, pores for protrusion of the tube-feet; 9, tubercles of the primary spines, i.e. primary tubercles.
The corona consists of five radial or "ambulacral" bands of plates and five interradial, or as they are usually termed, "interambulacral" bands of plates—ten in all. Each of the ten consists of two vertical rows of plates throughout most of its extent, and each plate is studded with large bosses, or "primary tubercles" for the primary spines, smaller bosses called "secondary tubercles" for the secondary spines, and finally, minute elevations called "miliary tubercles" for the pedicellariae.

Fig. 228.—The so-called calyx and the periproct of Echinus esculentus. × 4. 1, Genital plates with genital pores; 2, ocular plates with pores for terminal tentacles of the radial water-vascular canals; 3, madreporite; 4, periproct with irregular plates; 5, anus. (After Chadwick.)
Even in the dried skeleton, however, the ambulacral plates can be discriminated from the interambulacral by the presence of pores to permit the passage of the tube-feet. These pores are arranged in pairs, and each pair corresponds to a single tube-foot, since the canal connecting the ampulla with the external portion of the tube-foot is double in the Echinoidea. In Echinus esculentus there are three pairs of such pores in each plate, in Strongylocentrotus droëbachiensis four pairs. The ambulacral plate is really made up of a series of "pore-plates," each carrying a single pair of pores, and these become united in threes in Echinus and fours in Strongylocentrotus, while in primitive forms like the Cidaridae they remain separate. Each ambulacral and interambulacral area ends at the edge of the periproct with a single plate. The plate terminating the ambulacral band is pierced by a single pore for the exit of the median tentacle, which, as in Asteroids, terminates the radial water-vascular canal. Thus the aboral end of the radius in an Echinoid corresponds to the tip of the arm in an Asteroid. The plate is termed "ocular," because the terminal tentacle has a mass of pigmented cells at its base; but no eye-cups can be seen, and there is no evidence that this spot is specially sensitive to light. Species which show special sensitiveness to light have often a large number of what we may perhaps term secondary eyes. The plate terminating the interambulacral {513}series is termed the "genital plate," because it is pierced by the duct of one of the five genital organs. One of the genital plates is also pierced by the madreporic pores. Some zoologists have separated the ocular and the genital plates under the name of "calyx" from the rest of the corona, under a mistaken idea that they are homologous with the plates of the body or calyx of a Crinoid.

Fig. 229.—The peristome of Echinus esculentus. × 2. 1, Tube-feet of the lower ends of the radii; 2, gill; 3, teeth; 4, buccal tube-foot; 5, smooth peristomial membrane. (After Kükenthal.)
The periproct (Fig. 228, 4) is covered with small plates and bears a few pedicellariae. The peristome (Fig. 229) is covered by flexible skin with abundant pedicellariae; it terminates in a thick lip surrounding the mouth, from which the tips of five white teeth are just seen projecting. There are ten short tube-feet projecting from the peristome—one pair in each radius—and each tube-foot terminates in an oval disc and is capable of little extension, and each has around its base a little plate. The presence of these tube-feet shows that in Echinus the {514}peristome extends outwards beyond the water-vascular ring, whereas in Asteroidea it is contained entirely within the ring. In the primitive Cidaridae (Fig. 235) the whole peristome down to the lip surrounding the mouth is covered with a series of ambulacral and interambulacral plates similar to those forming the corona, though smaller and not immovably united, and the series of tube-feet is continued on to it. It is thus evident that the peristome is merely part of the corona, which has become movable so as to permit of the extension of the teeth. In Echinus the peristome is continued in each interradius into two branched outgrowths called gills, the relation of which to the respiratory function will be described later. These gills (Fig. 229, 2) are situated in indentations of the edge of the corona called "gill-clefts" (Fig. 230, g).

Fig. 230.—The dried peristome of Echinus esculentus and the surrounding portions of the corona. × 1. amb, Ambulacral plate; b.t, buccal tube-foot; g, gill-cleft; inter, interambulacrum; per, peristome.
The most conspicuous plates in the peristome are those {515}surrounding the buccal tube-feet; besides these, however, there are in Echinus esculentus, and probably in most species, a large number of thinner irregularly-scattered plates (Fig. 230).
The term ambulacral plate, applied to the plate pierced by the pores for the tube-feet, conveys a misleading comparison with the ambulacral plate of an Asteroid. In Echinoids the ambulacral groove has become converted into a canal called the "epineural canal," and the ambulacral plates form the floor, not the roof, of this canal; they may perhaps correspond with the adambulacral plates of the Starfish, which one may imagine to have become continually approximated as the groove became narrower until they met.

Fig. 231.—Dissection of Echinus esculentus. × 1. The animal has been opened by a circumferential cut separating a small piece of the skeleton at the aboral end, which is turned outwards exposing the viscera on its inner surface. The other viscera are seen through the hole thus made. amp, Ampullae of the tube-feet; aur, auricle; b.v, so-called "dorsal blood-vessel"; comp, "compasses" of Aristotle's lantern, often termed "radii" by English authors; comp.elv, elevator muscles of the compasses; comp.ret, retractor muscles of the compasses; eph, epiphyses of the jaws in Aristotle's lantern; gon, gonad; g.rach, genital rachis; int, intestine; oe, oesophagus; prot, protractor of Aristotle's lantern; rect, rectum; ret, retractor of Aristotle's lantern; siph, siphon; st, stomach; stone.c, stone-canal.
The internal organs of the Urchin can best be examined by making a horizontal incision about one-third the distance from the mouth and pulling the two parts gently asunder. A large amount of fluid escapes from the exceedingly spacious coelomic cavity, the alimentary canal being comparatively narrow.
The alimentary canal commences with a short vertical tube which has been shown to be a stomodaeum; this is surrounded by the upper ends of the teeth and their supporting ossicles, which are collectively termed "Aristotle's lantern." The oesophagus leads into a baggy, flattened tube, the stomach, which runs horizontally round the animal, supported by strings of tissue from the coelomic wall, so that it hangs down in a series of festoons. Having encircled the animal, it bends directly back on itself and immediately opens into the intestine, which is also a flattened tube, which runs round the circumference of the animal, but in the opposite direction, the festoons of the second circle alternating with those of the first. The intestine opens into a short rectum which ascends vertically to open by the anus. The stomach is accompanied by a small cylindrical tube called the "siphon" (Fig. 231, siph), which opens into it at both ends; this represents merely a gutter which has been completely grooved off from the main intestine; it is lined by cilia, and its function is believed to be that of keeping a stream of fresh water flowing through the gut, so as to subserve respiration.
Echinus esculentus seems to feed chiefly on the brown fronds of Laminaria and the small animals found thereon, which it chews up with its teeth, but it may regale itself on the same diet as Brittle Stars, as Allen[474] has shown to be the case in Plymouth Sound. Dohrn[475] has described the Neapolitan Sphaerechinus granularis attacking and capturing Crustacea such as Squilla.
The water-vascular system presents several features of great interest. The ring-canal is situated at a considerable distance above the nerve-ring, and is separated from it by the whole of the jaws and teeth. It has five small interradial pouches on it, which apparently correspond to Tiedemann's bodies in an Asteroid. The stone-canal (Fig. 231) opens as {517}usual into the ring-canal, and is accompanied by the axial sinus and genital stolon. The name "stone-canal" is very unsuitable in this order, for there are no calcifications in its walls; it is a simple membranous tube of circular section. On reaching the upper wall of the test it expands into an ampulla, into which the numerous ciliated pore-canals traversing the madreporite open. The radial canals, starting from the ring-canal, pursue a downward course till they come into contact with the radial nerve-cords, and they then bend upwards and run along the centre of the ambulacral region, finally terminating in the small terminal tentacles. In the just metamorphosed Echinoid these are well-developed tube-feet, each with a well-developed sucker, in the centre of which is a conical sensory prominence, but as development proceeds they become enclosed in a circular outgrowth of the test, so that only the tip projects in the adult.
The long extensible tube-feet are connected by transverse canals with the radial canal. Instead of the pair of valves which in Asteroids prevent the reflux of liquid into the canal, there is a perforated diaphragm[476] with circular muscles, which by contraction close the opening in the diaphragm, while when they are relaxed fluid can return from the tube-foot. The ampulla is flattened, and is contracted by muscular fibres called "trabeculae" stretching across its cavity. These muscular strands are developed by the cells lining the ampulla. The external portion of the tube-foot, as in Asteroids, is provided with powerful longitudinal muscles, and there is the same alternate filling and emptying of the ampulla as the tube-foot is contracted and expanded. The tube-foot is connected by a double canal with the ampulla, the object of which is to assist in respiration. The cells lining it are ciliated, and produce a current up one side of the tube-foot and down the other, and the double canal leading to the ampulla separates these two currents and prevents them interfering with one another. Thus water is continually transported from the ampulla to the tube-foot, through the thin walls of which it absorbs oxygen, and it is then carried back to the ampulla, and transfers its oxygen to the fluid of the general body-cavity through the walls of the ampulla. The disc of the tube-foot is supported by a calcareous plate {518}(Fig. 232, oss), a circumstance which enabled Johannes Müller to recognise the Echinoid larva when the form of the adult was as yet unrecognisable. Below the edge of the disc there is a well-marked nerve-ring, from which two bundles of nerve-fibres go to the disc itself, in the edge of which there is an abundance of sense-cells.
The buccal tube-feet (Fig. 229, 4) are much shorter than the rest, and are provided with oval discs which are highly sensory. These feet are not used for seizing, but for tasting food; when a piece of food is placed near them they are thrown into the most violent agitation.

Fig. 232.—Diagrammatic transverse section of the radius of an Echinoid. amb.oss, Ambulacral ossicle; amp, ampulla of the tube-foot; ep, epineural canal; musc, muscles attaching spine to its boss; nerv, nervous ring in base of spine; n.r, radial nerve-cord; oss, ossicle in sucker of tube-foot; ped, tridactyle pedicellaria; perih, radial perihaemal canal; pod, tube-foot; wv.r, radial water-vascular canal.
The nervous system has the same form as in an Asteroid, viz. that of a ring surrounding the mouth and giving off radial nerve-cords (Fig. 232, n.r), one of which accompanies each water-vascular canal to the terminal tentacle, where it forms a nervous cushion in which pigmented cells are embedded.
A large band-like nerve is given off from the radial nerve-cord to each tube-foot. This pedal nerve, as it is called, contains bipolar neurons, and is really an extension of the nerve-cord itself. Beneath the sucker it branches out to form a sensory ring. From the base of the pedal nerve, branches are given off {519}which run to the ectoderm and enter into connexion with the plexus there. Romanes[477] scraped away the radial cords and found that the spines still converged when a point on the ectoderm was stimulated, but that, on the other hand, if definite locomotor movements were to be carried out, the presence of these cords was a necessity; hence he concluded that the superficial plexus sufficed for ordinary reflexes, but that for purposeful movements the central nervous system was necessary.
Von Uexküll[478] has made an exhaustive study of the physiology of the nervous system in the Echinoidea. He points out that all the organs controlled by the nervous system, spines, pedicellariae, tube-feet, and (see below) Aristotle's lantern, give two opposite reactions in response to the same stimulus according as it is strong or weak, bending away from the point of stimulation when it is strong and towards it when it is weak. This reversal of reaction can only be due to the action of the neuron in altering the effect of the stimulus on the muscles, and this Uexküll regards as its fundamental property. Thus in Preyer's[479] experiments with Starfish the strong form of stimulation is obtained by directly applying the stimulus to the radial cord or to the tube-feet, the weak form by stimulating the back, when of course the stimulus has to traverse a longer path before affecting the tube-feet, and is consequently weakened. Von Uexküll also introduces the conception of "tone" with regard to the nervous system. This term has been used to denote the amount of chronic contraction in a muscle, and it is to be distinguished from the fleeting contractions which cause movement. The more tone there is in a muscle the less responsive it is to stimuli tending to bring about movement. As applied to the nervous system "tone" denotes a condition when it is not receptive to small stimuli, but when it is maintaining a condition of tone in a muscle by which of course its own tone is measured. Tone in a neuron can therefore be measured by the produced tone in the muscle, and the one is to be discriminated from the other only by using stimulants, such as caffeine, which have no direct action on muscle. Tone can also be measured by the amount of stimulus necessary to irritate the neuron. When {520}muscles are stretched the tone is lowered, and this loss of tone extends to the neuron controlling the muscle, and vice versa. When the spines on being gently stimulated bend towards the point of stimulation, this is due to the contraction of the muscles on the side towards the point of stimulus, for if the superficial plexus of nerve-fibres be cut through so that the stimulus has to pursue a round-about course the spine will bend towards the direction from which the stimulus comes. The bending of the spines away from the stronger stimulus is likewise due to the muscles on the side towards the stimulus. It is caused by a sudden fall of tone in these muscles, which causes them to yield to the tone of the muscles on the opposite side, and this fall of tone is due to a fall of tone in the neurons, for it can be produced by chemicals, and the direct action of all chemicals applied to muscle is to raise tone.
In Arbacia this form of reaction cannot be produced; the spines respond to stimuli of all degrees of intensity by convergence towards the point of stimulation.
When a general skin-irritant like dilute acetic acid, or even strong light, is applied to the skin of a Sea-urchin the spines bend alternately to all points of the compass, or, in a word, rotate. This is due to the fact that the weight of the inclined spine stretches the muscles of one side and so renders them more open to the general stimulus; these muscles in consequence, contract, and so move the spine to a new position in which other muscles are stretched, and a similar result follows. A continuation of this process brings about rotation.
When a piece of glass rod or other light object is laid on the spines of a Sea-urchin, it naturally, by its weight, presses asunder the spines and stretches their muscles on one side, thus lowering the tone. If now the skin be stimulated at any point the piece of rod will be rolled by the spines towards the point of stimulation. This is caused by the fact that the muscles of the spines holding the rod are made more receptive by being stretched, and therefore they contract more than do the others in response to the stimulation, and so the rod is rolled onwards on to the next spines, which then act in the same manner. This passage of stimulus is entirely independent of direct nervous connexion between the bases of the spines, for it will traverse at right angles a crack going clean through the shell; it is merely the {521}result of the mechanical weight of the object and of the juxtaposition of the spines.
If the stimulation be too violent the first spines affected diverge wildly and strike their neighbours with vehemence, so arousing into activity the block musculature of these. This causes them to stand rigidly up, and so the path of the stimulus is barred.
Now the escape movements of the animal under strong stimulation which Romanes[480] alludes to are just an example of this handing on of stimulation from spine to spine, not by nervous connexion but by mechanical touch only; the object in this case is the substratum on which the animal lies, which is, so to speak, rolled towards the point of stimulation, or putting it otherwise, the animal is rolled away from it. Righting when upset is another example of the same phenomenon; the aboral spines are stretched by the weight of the animal, and the animal acts as if it were stimulated in the region of the periproct. When a Sea-urchin is in its normal position and is stimulated in the periproct (as for instance by a strong light), it would, according to this rule, tend to move downwards, which is of course impossible; but as the stimulus never affects all sides quite alike the result is that the Urchin rotates, turning itself ever away from the point of strongest stimulation. In the case of Strongylocentrotus lividus when living on limestone, as on the west coast of Ireland, this results in the animal excavating for itself holes in the rock, where it is safe from the action of the breakers.[481]
But it may be objected that no account is taken in the above description of the action of the "central nervous system," i.e. of the ring and the radial cords, and yet Romanes found that when they were removed the escape movements could not be carried out. The answer is that the central nervous system is a store-house of tone, not, as in higher animals, a controlling centre for co-ordinating the movements of the spines. When it is removed at first the escape movements can be carried out, but in a day or two all tone in the spine-muscles is lost, and then, since the tone of all is equally low, there is no tendency in those that are stretched to be more responsive than others, and hence the escape movements cannot be carried out. Sea-urchins kept in the tanks {522}of an aquarium are apt to lose the tone of their spines owing to the poisoning of the nervous system.
The central nervous system is, however, the system which controls the movements of the tube-feet. As we have seen, extensions of the radial nerves run to the tip of each podium. Tube-feet are chiefly used in ordinary progression; when this is quickened the spines come into play exclusively. The extent to which these two organs of locomotion are used varies from genus to genus. Thus Centrostephanus uses its spines a good deal, Echinus and Strongylocentrotus very little. The last-named genus sometimes walks on its tube-feet entirely without touching the ground with its spines.
The faculty of vision in its simplest form may be defined as sensitiveness to light and shade. Now strong light acts on all Sea-urchins as a general skin irritant. They fly from it towards the darkest corner, and then if it continues the spines rotate. A number of little violet spines on the aboral pole of Centrostephanus longispinosus are especially sensitive to light, and hence are almost constantly in rotation. This is due, according to Uexküll,[482] to a pigment of a purple colour, which can be extracted by means of alcohol and which is decomposed by light, the products of decomposition being supposed to irritate the nerves. Centrostephanus when exposed to light becomes darker in colour. This is due to the migration outwards of amoebocytes, which carry a pigment which acts as a screen in order to prevent the valuable visual purple being too rapidly decomposed. Not all Sea-urchins, in fact very few of those living in northern waters, give a reaction to shadow. C. longispinosus is one of the few; it reacts to a shadow by converging its spines towards it. A much larger number of species inhabiting tropical waters show this reaction. It is entirely stopped if the radial nerve-cords be removed, whereas the reaction to strong light continues. The reaction to shade is strongest after a long previous exposure to light, hence Uexküll has given the following explanation of it. The continued irritation due to light, having spread to all the spines, eventually reaches the radial cords and is there stored in the bipolar nerve-cells as tone. When the light-stimulus is interrupted some of {523}the stored tone spreads upwards to the spines, causing the weak form of spine reaction, and the spines converge.

Fig. 233.—To show character and distribution of the sphaeridia in Strongylocentrotus droëbachiensis. A, a portion of a radius, with sphaeridia, and the adjoining edge of the peristome. p, Pair of pores for a tube-foot; per, peristome; t, primary tubercle. B, an isolated sphaeridium. (After Lovén.)
It will be seen therefore that the so-called central nervous system of Echinus does not act in any sense as a brain, as indeed might have been guessed from the absence of any differentiation in it. As Uexküll points out, when an animal is covered all over with similar organs, such as spines and pedicellariae, capable of acting automatically, a brain is not needed. The object of a brain is to direct organs which are in a certain place to a danger which may come from any quarter, but in the Sea-urchin any spine is as good as any other spine, and such orientation is not needed. "In a dog the animal moves its legs, in a Sea-urchin the legs move the animal." What the Sea-urchin does need is a means to prevent its pedicellariae attacking its own organs with which they may come into contact. Thus it possesses an "autodermin," a chemical contained in the ectoderm which paralyses the muscles of the pedicellariae, as may be seen by offering to them a spine of the same animal. If, however, the spine be treated with boiling water, and then offered, it is viciously seized, showing that this substance can be dissolved out.
Just as in the case of the Starfish, when the nerve-ring is {524}cut through, the tube-feet in the various radii are no longer co-ordinated with one another.
Besides the tips of the tube-feet the Urchin possesses another kind of sense-organ, the sphaeridia (Fig. 233). These are minute glassy spheres of calcareous matter attached by connective tissue to equally minute bosses on the plates of the ambulacra, generally near the middle line. They are in fact diminutive spines, and like the latter are covered with a thick layer of ectoderm, beneath which is a particularly well-developed cushion of nerve-fibrils. Only the layer of muscles which connects a normal spine with its boss is wanting. Although definite experimental proof is lacking, the whole structure of the sphaeridia shows that they belong to the category of "balancing organs." As the animal sways from side to side climbing over uneven ground, the heavier head of the sphaeridia will incline more to one side or to another, and thus exercise a strain on different parts of the sheath, and in this way the animal learns its position with regard to the vertical.
Intervening between the radial nerve-cord and the radial vessel is a single radial perihaemal canal (Fig. 232, perih), representing the two parallel canals found in the same position in the Asteroid. The five perihaemal canals lead downwards to a space called the lantern-coelom, surrounding the oesophagus.[483] Since the skeleton of the corona is composed of plates immovably connected together, muscles corresponding to the ambulacral muscles of the Asteroids would be useless, and so the wall of the perihaemal canal remains thin and the side of it turned towards the general coelom develops no muscles, and that turned towards the nerve-cord no nerve-cells. Where, however, the radial nerve enters the nerve-ring, and on the ring itself, an inner layer of nerve-cells is developed from the lantern-coelom which represents the lower or oral portions of the radial perihaemal canals. These cells control the muscles moving the teeth. These canals are originally parts of the lantern-coelom, but in the adult they become closed off from it.

Fig. 234.—Echinus esculentus dissected in order to display Aristotle's lantern, × 2. The whole upper part of the shell has been cut away. 1, Upper growing end of tooth; 2, outer forked end of one "compass"; 3, muscle joining adjacent compasses and acting as elevator of these ossicles; 4, depressor of the compasses; 5, lower end of jaw; 6, retractor of the whole lantern; 7, protractor of the whole lantern; 8, auricle; 9, ampullae of the tube-feet; 10, interambulacral plate; 11, lower part of tooth; 12, water-vascular ring; 13, meeting-point of a pair of epiphyses; 14, so-called Polian vesicle, really equivalent to Tiedemann's body in an Asteroid; 15, oesophagus; 16, so-called ventral blood-vessel; 17, genital stolon; 18, stone-canal; 19, rectum; 20, aboral sinus. (Partly after Chadwick.)
In the outer wall of this space are developed the calcareous rods forming Aristotle's lantern. These are first: five teeth (Fig. 234, 11), chisel-shaped ossicles of peculiarly hard and close-set calcareous matter, the upper ends (1) pushing out projections of the upper wall of the lantern-coelom. These projections are the growing points of the teeth, whose lower ends pierce the {526}ectoderm and project into the lower end of the oesophagus. Each tooth is firmly fixed by a pair of ossicles inclined towards one another like the limbs of a V and meeting below. Each ossicle is called an "alveolus," and taken together they form a "jaw." Their upper ends are connected by a pair of ossicles called "epiphyses" (13). These two epiphyses meet in an arch above. The jaws and their contained teeth are situated interradially. Intervening between successive alveoli are radial pieces called "rotulae," which extend directly inwards towards the oesophagus. Above the rotulae are pieces termed "radii" or "compasses" (2), which are not firmly attached to the other pieces but lie loosely in the flexible roof of the lantern-coelom.
The uses of the various components of this structure can be made out from an inspection of the muscles which connect them together.
Overarching each radial perihaemal canal where it leaves the lantern is a bridge of calcareous matter called the "auricula" (Fig. 234, 8). This arises as two rods which meet each other in a pent-house over the canal. It is the only part of the skeleton which can be compared to the ambulacral ossicles of the Asteroidea, and like them it serves as the point of insertion for important muscles. Thus we find (1) protractor (Fig. 234, 7) muscles which arise from the upper ends of the alveoli and are inserted in the auricula; when these contract they tend to push the whole "lantern" outwards so as to expose the tips of the teeth. (2) The retractor muscles (Fig. 234, 6) extend from the auriculae to the lower ends of the jaws and restore the lantern when it has been extruded to its original position. (3) The comminator muscles connect adjacent jaws with one another: these on contraction approximate the pair of jaws into which they are inserted, and it will easily be seen that by the successive contraction of the five comminator muscles a rotating movement of the teeth would be produced which would cause them to exert an action something like that of an auger; by their simultaneous contraction the teeth are brought to a point. (4) The internal and external rotula muscles: these are small muscles which connect the outer side of the epiphysis with the rotula. There are two facets on the epiphysis, which permit it to rock to and fro on the rotula under the action of these muscles. This rocking action must greatly increase the cutting power of the {527}tooth. These muscles are controlled by the nerve-ring and the incipient portions of the radial nerves, which, as we have seen, have an inner layer of nerve-cells. If the nerve-ring be gently stimulated on one side the upper end of the lantern bends away from the spot, causing the lower end, i.e., the teeth, to move towards it; but a stronger stimulation produces the opposite effect, just as is the case with spines. But besides these masticatory muscles there are others which have nothing to do with moving the teeth. These muscles are attached to the rods called radii or compasses (Fig. 234, 2),[484] which lie in the upper wall of the lantern-coelom, and may be termed the compass muscles. There are two sets:—(1) The elevator muscles (Fig. 234, 3), which connect the inner ends of the compasses with one another. When these contract, the radii tend to bend upwards at the inner ends and thus raise the roof of the coelom. (2) The depressor muscles (Fig. 234, 4), which run downwards from the forked outer ends of the compasses to the auriculae. Uexküll[485] has shown that the function of these muscles and of the rods to which they are attached is respiratory. These muscles are also controlled by the nerve-ring. If this be stimulated by passing a pin-head into the oesophagus, the roof of the lantern cavity is raised by the contraction of the elevator muscles. This is followed by contraction of the depressor muscles lowering it; the same result may be brought about by placing the animal in water with excess of carbonic acid. The ten branched gills described on p. 514 are outgrowths of the lantern-coelom. When the roof of this cavity is depressed the fluid contents are driven out into the gills, which are thus expanded and then absorb oxygen from the surrounding sea water. When, on the other hand, the roof is raised the aerated water is sucked back into the lantern cavity, and the oxygen passes easily through the thin walls of the lantern into the fluid filling the main coelomic cavity. There are thus two independent respiratory mechanisms in the Sea-urchin, the one being the compass muscles, the other the cilia lining the interior of the tube-feet.
The function of excretion is performed, as in Asteroidea, by {528}the amoebocytes floating in the general coelomic cavity. These in part escape through the thin bases of the gills. In other parts of the body they seem not to succeed in reaching the exterior at all, but to degenerate and to form masses of pigment; the colour of the animal is largely due to these excrementitious substances.
The reproductive system, as in the two preceding orders, consists of a vertical pillar, the "genital stolon," and a circular "genital rachis" giving off interradial branches from which the genital organs bud. The genital stolon is developed from the wall of the general coelom near the upper end of the axial sinus; it attains a great development and ultimately completely surrounds the axial sinus, which then appears like the cavity of a glandular tube, the walls of which are constituted by the genital stolon. The compound structure consisting of stolon and axial sinus was actually described as a nephridium by the Sarasins[486] in the case of Asthenosoma. Its true nature, however, is shown when the upper end is examined; it is then seen to open into the stone-canal and to be in communication with the ampulla, into which the pore-canals open. Lying alongside the upper end of the axial sinus is the somewhat elongated "madreporic vesicle," or right hydrocoele, which was described by Sarasin as the accessory kidney (Nebenniere), since like the axial sinus it is partly enveloped by the genital stolon. Leipoldt,[487] however, showed clearly that it is a completely closed space.
The genital rachis springs from the upper end of the stolon, and as in Asteroids, it lies in the outer wall of a space called the "aboral sinus" (Fig. 234, 20) intervening between it and the test. In adult specimens it seems to degenerate. The genital organs are situated at the ends of five interradial branches of the rachis (Fig. 231, gon). Each is an immense tree-like structure consisting of branching tubes, which are lined by the sexual cells. So enormous do they become in the breeding season that they form an article of food among fishermen. The term esculentus is derived from this circumstance. Other species are regularly sold for food as Frutta di Mare (Fruit of the Sea) at Naples, and {529}as "sea eggs" in the West Indian Islands. One female Echinus esculentus will produce 20,000,000 eggs in a season.
The so-called blood system is more distinctly developed in Echinoidea than in Asteroidea and Ophiuroidea. There is an oral ring of lymphoid tissue surrounding the oesophagus below the water-vascular ring. From this are given off two strands, the so-called "dorsal" (Fig. 231, b.v), and "ventral" vessels (Fig. 234, 16), which run along the two opposite sides of the stomach or first coil of the alimentary canal. The position of these strands suggests that like the lacteals of the human intestine they are channels along which the products of digestion exude from the stomach. The dorsal strand is situated on the same side as the genital stolon, and from it branches are given off which ramify on the surface of the stolon, on account of which this organ, as in Asteroidea, was at one time regarded as a "heart," but the distinction of the stolon from the strands is easily made out. An aboral ring enclosing the genital rachis lies embedded in the septum dividing the aboral sinus (Fig. 234, 20) from the general coelom.
Classification of Echinoidea.
The Echinoidea are sharply divided into three main orders, which differ from each other profoundly in their habits and structure. These are: (1) The Endocyclica or Regular Urchins, of which the species just described may be taken as the type. (2) The Clypeastroidea or Cake-urchins, which are of extremely flattened form, and in which the periproct is shifted from the apical pole so that it is no longer surrounded by the genital plates, while some of the tube-feet of the dorsal surface are flattened so as to serve as gills. (3) The Spatangoidea or Heart-urchins, in which the outline is oval: the periproct is shifted, as in the Cake-urchins, and the dorsal tube-feet are similarly modified; but the Heart-urchins have totally lost Aristotle's lantern, whilst the Cake-urchins have retained it. This strongly-marked cleavage of the group was primarily due, as in all such cases, to the adoption of different habits by different members of the same group. Were we to term the three orders Rock-urchins, Sand-urchins, and Burrowing-urchins, it would not be entirely true, for secondary invasions of the other's territory on {530}the part of each order have undoubtedly taken place; but still the statement would remain roughly true, and would give a fair idea of the differences in habitat which have led to the differentiation of the group.
Order I. Endocyclica (Regular Urchins).
The principal variations concern (1) the peristome, (2) the periproct, (3) the corona, (4) Aristotle's lantern and its appendages, (5) the spines, (6) the pedicellariae, and lastly, (7) the tube-feet. We shall consider these points in order.
Peristome.—In the vast majority of species this region is covered only with flexible skin in which ten small plates are embedded, pierced by pores for the buccal tube-feet; besides these there are irregularly arranged thin plates. In the Cidaridae both the ambulacral and the interambulacral series of plates are continued on it; these plates differ from those of the corona in being movable on one another. In Echinothuriidae only the ambulacral series of plates is continued on to the peristome. In the case of both these families there are a considerable number of tube-feet within the region of the peristome which may be classed as buccal.
Periproct.—This area, which represents the whole dorsal surface of Asteroidea, is very large in the Cidaridae, where, as in Echinus, it is covered with leathery skin in which small plates are embedded. In the Saleniidae it is covered with a single large sur-anal plate, in the edge of which the anus is excavated; in the Arbaciidae it is covered with four valve-like plates; whilst in the remaining species its condition is similar to that described in the case of Echinus esculentus.
Corona.—In Echinothuriidae all the plates are separated by slips of membranous skin, so that the test is flexible. In all other families it is an unyielding cuirass. In the Cidaridae the pore-plates remain separate throughout life, and are therefore identical with the ambulacral plates. These are small and placed in two vertical rows, and so the ambulacra are exceedingly narrow. In Echinothuriidae there is some tendency to adhesion amongst the pore-plates; these are of different sizes, and usually one larger and one smaller adhere to one another. In all other species regular ambulacral plates are formed at least {531}in the lower part of the radii near the peristome by the adhesion of the pore-plates in groups of two, three, or more. Sometimes as many as nine pore-plates may thus adhere.
When adhesion takes place between the pore-plates it is of course preceded by crowding, and this interferes with their equal development. Some which extend so far horizontally as to meet their fellows of the opposite side of the radius are called primary plates; others which are small and wedged in between the larger ones are called demi-plates. Systems of classification have been built up (chiefly by palaeontologists) in which great stress has been laid on how the primaries and secondaries enter into the constitution of the compound plate, but it does not seem to the present author as if this were at all a satisfactory basis for classification. All the pore-plates are primarily equivalent, and the question as to which are interfered with in their growth so as to become secondary is trivial. The so-called Arbacioid type consists of one primary with a secondary on each side; the Diadematoid type of three primaries, with occasionally a secondary between the aboral and the middle primary; and finally the Triplechinoid type of two primaries, with one or more secondaries between them.
Aristotle's Lantern.—Under this head we may consider the auriculae and gills as well as the jaws and teeth. In Cidaridae external gills appear to be absent, but from the lantern coelom large radial pouches project upwards into the general coelom cavity. These pouches are supposed to be respiratory, and are termed internal gills or Stewart's organs.[488] They co-exist with external gills in Echinothuriidae and in Diadematidae, though in the last family they are present only in a vestigial form, two being found in each radius. The auricular arch both in Cidaridae and in Arbaciidae is composed of two pillars which do not meet, but in the last-named family they are based, as in Echinidae, generally on the ambulacral plates, whereas in Cidaridae they arise from the interambulacral plates (the ambulacral plates being here very narrow). The epiphyses are absent in Cidaridae and Arbaciidae, and are imperfect in Diadematidae.
Spines.—These organs are extraordinarily variable, and {532}usually differ very much in species of the same genus. In the vast majority of species there is a limited number of long spines called "primaries," amongst the bases of which a large number of much shorter "secondaries" are distributed. In Cidaridae the primaries are very long and thick and blunt at the ends, and the secondaries form small circles around their bases. The primaries in Cidaridae and the tips of the primaries in Arbaciidae and Echinothuriidae are covered with a special investment of extremely close, hard, calcareous matter very different from the loosely fenestrated material out of which the bodies of the spines of all species are composed. In Colobocentrotus and Heterocentrotus the primaries are very thick and triangular in section, whilst the secondaries on the aboral surface have expanded outer ends, which form a close-set pavement protecting the ectoderm from the shocks of the breakers. In Echinothuriidae the primaries are short and so delicate as to be termed silky.
Pedicellariae.—In Cidaridae only gemmiform and tridactyle pedicellariae are found. In the gemmiform the glands lie inside the grooved blades instead of outside as normally, and they are covered internally by ingrowths of calcareous matter from the edges. In Echinothuriidae only tridactyle and trifoliate are found in most species, but rudimentary gemmiform are found in one species and well-developed ophicephalous in another. In some species (Centrostephanus longispinosus) there are found gemmiform pedicellariae which have lost the jaws but retained the glands. These are termed "globiferae." Mortensen[489] uses minute details in the structure of the pedicellariae to discriminate species and even genera, but in this the present author is not prepared to follow him.
Tube-feet.—The tube-feet belonging to the aboral surface are pointed and devoid of a sucker in Diadematidae, Echinothuriidae, Arbaciidae, and Cidaridae;[490] in the last-named family those belonging to the oral surface have suckers, in the centre of which a pointed (sensory) prominence is to be noted.
The classification of the Endocyclica is by no means in a satisfactory condition, and different authorities have arrived at {533}widely different results. Agassiz,[491] for instance, places the genera Echinus (the common British form) and Strongylocentrotus (the commonest American form) in different families. Bell,[492] on the other hand, considers them to be closely allied. Bell's system, based as it is on the development of the peristome, seems to the present author the most justifiable, for the peristome is undoubtedly a differentiation of the corona, which has been brought about by the manner in which the animal breathes and masticates, two functions of prime importance. The periproct is also of importance, representing as it does the whole aboral surface of the Starfish, and so are to a less extent the arrangements of the spines and of the tube-feet. Proceeding in this way, living Endocyclica can be divided into six families, which are briefly described below.

Fig. 235.—Oral view of dried and cleaned test of Cidaris. p, Pores for tube-feet arranged in single series; per, peristome with both ambulacral and interambulacral plates; t, tubercle of a large interambulacral spine.
Fam. 1. Cidaridae.—Endocyclica with a large peristome and a large periproct. The peristome is covered with a regular series of both ambulacral and interambulacral plates, the former pierced by tube-feet. No special buccal tube-feet and no external gills. The periproct is large, and is covered with irregular plates (Fig. 236, A). The lantern coelom is provided with large Stewart's organs.
The auriculae are incomplete and consist only of pillars arising from the interambulacral plates. The ambulacral pore-plates remain disunited, and the pores are arranged in a single vertical series; hence the ambulacra are very narrow. The interambulacral plates each bear one large primary spine surrounded by several circles of secondaries. No ophicephalous or trifoliate pedicellariae are to be found, and the gland of the gemmiform pedicellaria is placed inside the concavity of the blade.

Fig. 236.—Figure showing periprocts of A, Cidaris; B, Echinus, × 1. amb, Ambulacral plate; g.o, genital opening; g.p, genital plate; inter, interambulacral plate; m.p, madreporite; oc, ocular pore.
The Cidaridae are in many respects the most primitive of the six families living. They are distributed all over the world, and chiefly inhabit deep water. No two naturalists agree as to how they are to be divided into genera. Mortensen,[493] who takes the structure of the pedicellariae as his principal guide, recognises fourteen genera. Others (as for instance Bell) have been inclined to attribute nearly all the living species to one polymorphic genus, Cidaris, finding all attempts to divide the genera from one another frustrated by the discovery of transitional forms. Goniocidaris (Fig. 237), however, distinguished by its comparatively broad poriferous zones, by bare places in the middle line of both radii and interradii, and by deep pits on the lines of suture of the plates, is by general consent distinct. This genus is confined to the Eastern Pacific, but from British waters three species of Cidaris have been recorded, only one of which, C. (Dorocidaris) papillata, is at all common. It is found in water from 100 to 500 fathoms in depth {535}off the western coast of Ireland and Scotland. It also occurs in the Mediterranean, and has been carefully examined and described when living by Prouho.[494] From his description it appears that locomotion is effected almost entirely by spines, and that the tube-feet of the lower parts of the radii have each in the centre of the disc a pointed sense-organ like those in the centre of the first tube-feet of the just metamorphosed Echinus, whilst those of the aboral surface have no suckers.
Fam. 2. Echinothuriidae.—Endocyclica with a large peristome and comparatively small periproct. The peristome has a regular series of ambulacral plates bearing pores for tube-feet, but no interambulacral plates. No specially modified buccal tube-feet, but external gills are present, and internal gills (Stewart's organs) also occur. The periproct is covered with numerous small plates. All the plates of the corona are separated by thin slips of flexible body wall. Numerous comparatively short primary spines on both ambulacral and interambulacral plates; these spines are covered on the tips with a layer of hard dense material.
This remarkable family is divided by Mortensen into ten genera, based as usual on the pedicellariae, but taking into account also the shape of the tip of hard material on the spines. Most authors refer the majority of the species to two genera, Phormosoma and Asthenosoma (Fig. 238), recognising also a genus Sperosoma for one or two aberrant species. Asthenosoma is distinguished by having wide interspaces of membrane between the plates, and by having ten longitudinal folds of the body-wall, two in each radius, in which powerful longitudinal muscles are developed projecting inwards in the radii. The organs of Stewart are very large. In Phormosoma, on the contrary, the interspaces of membrane are very narrow, and the longitudinal folds are thin and membranous and the organs of Stewart are vestigial. Asthenosoma hystrix and Phormosoma placenta have both been dredged in deep water off the Irish coast. A. urens, in which there are ectodermic poison-sacs at the bases of the spines, inhabits the Indian Ocean near Ceylon, and was thoroughly {537}described by the Sarasins,[495] who regarded its structure as a proof that Echinoidea were derived from Holothuroidea. Both palaeontology and embryology have, however, yielded strong evidence that Echinoidea were derived from Asteroidea, and hence there is ground for believing that Holothuroidea are descended from primitive Echinoidea and not vice versa. The Echinothuriidae may perhaps be regarded as showing the first steps in the change, and though possibly not closely related to the actual ancestors of the Holothuroidea, they at any rate show parallel modifications.

Fig. 239.—View of peristome of Asthenosoma hystrix. amb, Ambulacral plates on the lower edge of the corona; inter, lower plates of the interambulacral area. (From Wyville Thomson.)
Fam. 3. Saleniidae.—Endocyclica with a large peristome and periproct. The peristome is covered with thin, scattered, irregular plates. There are five pairs of special buccal tube-feet, each supported by a special plate, and there are external gills. The periproct is excavated in the side of a large central pentagonal plate. It is covered with fifteen or twenty plates.[496] The ambulacral plates are separate as in the Cidaridae, but occasionally adhere in pairs near the peristome. The interambulacral plates also, as in Cidaridae, each bear one large primary spine surrounded by a {538}circle of secondaries. A few deep-water forms belong to this family, the type genus Salenia (Fig. 240) being the best known. None occur in the British area. Superficially they resemble the Cidaridae, but in reality they are widely separated by the essentially modern character of the peristome.

Fig. 240.—Dried and cleaned shell of Salenia varispina, showing periproct covered by one large plate. × 4. (From Wyville Thomson.)
Fam. 4. Arbaciidae.—Endocyclica with a peristome on which, as in Saleniidae, there are only ten prominent plates perforated by the buccal tube-feet, and besides these thin irregular plates; external gills are present, and the auricles consist of incomplete arches springing from the ambulacral plates. The periproct is covered by four valve-like plates. The ambulacral pore-plates are separate near the periproct, but near the peristome unite on the "Arbacioid" pattern (v. p. 531) to form secondary plates. The interambulacral plates each carry several spines. No representatives of this remarkable family are known in British waters, but Arbacia is found both on the east coast of North America and in the Mediterranean. It is distinguished by its conical test. All the upper tube-feet are devoid of a sucker; only those on the oral surface are used for locomotion.
Uexküll has studied the Mediterranean species, and has shown that the spines converge no matter how strong the stimulus may be, and so are incapable of aiding in locomotion; also that the ectoderm is devoid of ciliation, and hence the faecal matter which falls on the surface of the animal is not, as in other genera, allowed to fall off by the divergence of the spines nor swept off by the action of the cilia. In its natural habitat the wash of the ripples on the shore cleanses the animal. In captivity it is liable to suffocate itself.
Fam. 5. Diadematidae.—Endocyclica with a peristome similar to that of the Arbaciidae and the Saleniidae. External gills present and ten buccal tube-feet. Periproct small, covered with numerous small plates. The auricles form complete arches arising from the ambulacral region. Aristotle's lantern is provided with rudimentary Stewart's organs. The ambulacral pore-plates are separated at the apex, but unite orally in {539}"Diadematoid" fashion (p. 531) to form compound plates. The interambulacral plates bear numerous primaries. The aboral tube-feet are pointed, having lost their suckers.
This family is represented (according to Agassiz) at the present day by seven genera, none of which are found in British waters, though one (Centrostephanus) enters the Mediterranean. C. longispinus[497] was investigated by Uexküll and found to be distinguished by its sensitiveness to light and shade, and by the quickness of its movements, which were mainly carried out by its long spines. The family resembles the Arbaciidae in the pointed aboral tube-feet, but in the complete auriculae it resembles the next family.
Fam. 6. Echinidae.—Endocyclica with peristome and periproct as in the preceding family. External gills and buccal tube-feet present, but Stewart's organs totally absent. Ambulacral plates combined on the "Triplechinoid" plan (p. 531) to form secondary plates. Interambulacral plates with numerous tubercles. All the tube-feet have suckers.
This family contains by far the larger number of living genera. It is divided into two sub-families, viz.:—
(a) Temnopleurinae.—Echinidae in which the plates of the corona dovetail into each other by means of pits and knobs along the line of suture. This sub-family does not occur in British waters; almost all the species are confined to the Indian and Pacific Oceans, but on the east coast of America it is represented by several genera, which however inhabit deep water, e.g. Trigonocidaris arbacina.
(b) Echininae.—Echinidae in which the plates meet each other in straight, simple sutures.
This sub-family is represented in British waters by three genera, viz. Echinus, Sphaerechinus, and Strongylocentrotus. Echinus is distinguished by having its pores arranged in arcs of three, owing to the fact that its pore-plates are united in threes to form secondary plates, whilst in the other two genera the ambulacral plates are composed of four or more pore-plates. Six species of Echinus have been recorded from British waters, viz. E. esculentus, E. acutus, E. miliaris, E. norvegicus, E. microstoma, and E. elegans. The validity of the last three is very doubtful. {540}Mortensen[498] regards E. norvegicus and E. microstoma as mere variations of E. acutus, and this is probably correct. E. esculentus has already been described; its most marked character is the forest of comparatively short, close-packed, reddish or white primary spines with which it is covered, between the bases of which the delicate secondaries are hard to detect. It is essentially a shallow-water species. E. acutus is distinguished by having much fewer and longer primaries and numerous delicate secondaries. It is an inhabitant of deeper water, being abundant at 100 fathoms, though stragglers are found in shallower water. At the depths at which it lives wave-disturbance can scarcely be felt, and hence the long primaries are not irritated.
E. elegans has spines intermediate in character between those of E. esculentus and those of E. acutus. Like the latter it is an inhabitant of the deeper water. It seems to the present author not at all improbable that further research might show that E. acutus, E. elegans, and E. esculentus are all members of continuous series of forms; certainly the larvae and early development of E. acutus and E. esculentus, the extreme members of the series, are strikingly similar.
E. miliaris differs somewhat widely from the other species and is closely allied to E. microtuberculatus of the Mediterranean, from which it is distinguished mainly by the greater thickness of the scattered plates on the peristome of the latter species. From the other British species it differs in its much smaller size and in the greenish hue of its primary spines, which are short and thick and possess purple tips. Its larva is markedly distinct from the larva of E. esculentus. E. miliaris is a littoral species, and is found in great numbers in some of the Scottish sea-lochs; when the tide recedes, under every stone of the gravelly beach several specimens will be found. It has a curious habit of "dressing" itself, i.e. of covering itself with fragments of dead shell, sea-weed, etc., which are held in position by the aboral tube-feet. This habit aids in concealing the animal, and has probably been developed on account of the dangers to which E. miliaris is exposed owing to its littoral habit of life.
Sphaerechinus differs from Echinus in the structure of the ambulacral plates, in which it agrees with Strongylocentrotus, but it is distinguished from this genus by the very deep {541}gill-clefts, or indentations of the edge of the corona from which the gills are extruded. Its most marked peculiarity, however, as shown by both Mortensen and Uexküll, consists in the highly developed character of its gemmiform pedicellariae, on the stalks of which are situated glands. When the head with its poison-glands is torn off, the secretion of these stalk-glands can envelop an enemy with a glutinous secretion, which impedes its movements. The blades on a slight mechanical stimulus divaricate very widely and become locked in this position, so that the enemy's body gets in well within their reach. The muscles of the poison-glands contract, but their ducts are bent by the act of opening, so that the secretion cannot escape. The sense-organs have stiff hairs, which penetrate the surface of the enemy and cause its juices to exude and so stimulate the blades to close, and at the same time permit the poison to be expelled. It will be remembered that the gemmiform pedicellariae of Echinus open in response to a chemical stimulus and close on a mechanical one being superadded; so that their responses are the direct opposite of what occurs in Sphaerechinus. S. granularis, a Mediterranean species with short red spines, just reaches the Channel Islands.
Strongylocentrotus has shallow gill-clefts and gemmiform pedicellariae, like those of Echinus, except that they have a muscular stalk. In the British area it is represented by two species, S. lividus, in which the primary spines are markedly longer than the secondaries and are of a brownish purple colour, and S. droëbachiensis, in which the primaries are little longer than the secondaries and are of a greenish brown colour. S. lividus occurs abundantly in the Mediterranean, and reaches the English Channel and the west coast of Ireland. In the last-named locality, where it is exposed to the full sweep of the Atlantic, it is said to excavate holes for itself in the limestone rocks, about ten inches in depth.[499] S. droëbachiensis, which has been recorded in the British area, chiefly from the west coast of Scotland, is one of the most abundant members of the fauna of the east coast of America. In the Gulf of St. Lawrence and in the branches of the Bay of Fundy it is found in thousands, and {542}is frequently left bare at low tide. It thus takes the place of E. miliaris in the British fauna. An allied if not identical species, S. purpuratus, is found in Puget Sound on the Pacific coast.
Other interesting genera of the Echininae are Echinometra, Colobocentrotus, and Heterocentrotus. All possess large, thick primaries, and all are elliptical in outline. In Echinometra the primaries are pointed, and the long axis of the body makes an oblique angle with the axis passing through mouth and madreporite. In Colobocentrotus and Heterocentrotus the axis passing through mouth and madreporite is the short axis of the ellipse, and the primary spines are very thick and triangular in section, whilst the expanded ends of the secondaries form a closely set armour between the bases of these. In Colobocentrotus the test is markedly flattened on the under side, and this flattened area is fringed with a circle of primaries; but in Heterocentrotus there are a few rows of primaries all over the test. These are tropical genera and are found on the outer side of coral reefs, and they require the cuirass of expanded secondaries to protect them against the waves.
Order II. Clypeastroidea (Cake-urchins).
The "Cake-urchins" have only one representative in the British area, and this is unsuitable for dissection on account of its small size. We shall therefore select as type the "Sand-dollar" Echinarachnius parma (Figs. 241, 242), which occurs abundantly in shallow water on the east coast of North America. As its popular name implies, this is an extremely flattened Sea-urchin of nearly circular outline, so as to suggest a resemblance to the silver dollar of North American currency. The peristome is exceedingly small, and is placed in the centre of the lower surface (Fig. 241), whilst the periproct is placed on one edge. The outline is not quite circular, for the periproct lies in a slight indentation of the edge; and this side is broader and of a lesser degree of curvature than the opposite one, so that a secondary bilateral symmetry is superimposed on the fundamental radial symmetry common to all Echinoderms. A line drawn so as to pass through the anus and the centre of the disc will divide the animal into two similar halves; the periproct of course lies in {543}an interradius and the axis of symmetry passes through the centre of one radius. We can thus distinguish an anterior group of three radii, or "trivium," from a posterior pair or "bivium." The madreporite lies in the left anterior interradius. The five genitals and five oculars surround a dorso-central plate, which covers the spot which in Endocyclica is occupied by the periproct.

Fig. 241.—Oral view of "Sand-dollar" (Echinarachnius parma), with spines. amb, Ambulacral furrow, × 1.
The whole test is covered with extremely short delicate spines, which form a velvety felt-work, and are all of approximately the same length; they are of a brownish purple colour. The spines on the dorsal surface are all ciliated, and these cilia cause a current of fresh sea-water to flow continually over the modified tube-feet. Pedicellariae are scattered amongst the bases of the {544}spines; they are of the tridactyle, the gemmiform, and the ophicephalous types, but they have only two jaws.

Fig. 242.—Aboral view of the "Sand-dollar" (Echinarachnius parma), with its spines. m.p, Madreporite; pod, small tube-foot with sucker; pod', flattened respiratory tube-foot. × 1.
The ambulacral areas on the upper surface of the test can be distinguished only by the flattened respiratory tube-feet (Fig. 242, pod'), which can be seen protruding from between the spines. Below these areas are clearly marked, for in the centre of each is a well-marked groove proceeding inwards to the peristome. This groove receives lateral branches on its course which traverse the adjacent interambulacral regions. The purpose of these grooves will be explained later. The interambulacral regions do not reach the peristome, which is entirely surrounded by the ambulacral areas. The ambulacral and interambulacral areas both consist of somewhat large hexagonal plates, except in the region of the respiratory tube-feet. Here the pore-plates are not united with one another. This region in each radius is termed {545}a "petal" (Fig. 243, A, p), for the respiratory tube-feet are arranged in two rows which diverge from their commencement at the "calyx" and slightly converge again towards the outer margin of the disc, and thus in a dried specimen the two rows of double pores outline an area having some resemblance in shape to the petal of a flower. Besides these double pores for the larger tube-feet there are numerous small single pores for the smaller tube-feet; these are found in all the plates, ambulacral and interambulacral, of the dorsal surface, but in the neighbourhood of the grooves only on the ventral side.

Fig. 243.—A, aboral, B, oral view of Echinarachnius parma after spines have been removed, amb (in A), Ambulacral plates, (in B), ambulacral furrows; g.p, genital pore; inter, interambulacral plate; p, petal; t.t, pore for terminal tentacle, × ½.
The sphaeridia are only present to the number of one in each radius. Each sphaeridium is enclosed in a pit situated near the edge of the peristome.
A remarkable feature in the skeleton of Echinarachnius which is characteristic in greater or lesser degree of all Clypeastroidea is the presence of vertical partitions of calcareous matter traversing the coelom and stretching from the upper to the lower surface of the test. These are found principally in the peripheral region of the animal; and there can be no doubt that they have originated as cellular bands traversing the coelom, for the formation of similar structures can be followed step by step in the Crinoidea. In the axis of these trabeculae, or folds of the coelomic wall, jelly is secreted, and into this the lime-producing {546}amoebocytes wander. In Echinarachnius these partitions are arranged in groups, each group radiating from a common centre.
The main peculiarities in the structure of Echinarachnius are comprehensible when the species is viewed from above in its normal environment. It is found in comparatively shallow water on a sandy bottom, and normally is nearly but not quite buried in the sand. It might thus be overturned by the force of the waves and currents, and it is protected against this fate by its flattened shape. This shape, however, necessitates some kind of support for the upper part of the test, and this is provided by the internal partitions.
In order to view the internal anatomy of the "Sand-dollar," it is necessary carefully to pick away the dorsal surface of the shell piece by piece. In this way the whole course of the alimentary canal is exposed; as in Echinus esculentus it can be seen to issue from the upper surface of Aristotle's lantern. It then bends sharply to the left, and makes a complete circle round the edge of the disc; this portion is the stomach, and is considerably inflated and accompanied by a "siphon." It then bends sharply back on itself, but only goes half way round; when it reaches the posterior interradius it ends in the anus (Fig. 244).
Aristotle's lantern is greatly simplified as compared with its condition in the Regular Urchins. Both rotulae and compasses are absent; the jaws are sharply bent on themselves, and their appearance gives one the impression that they have shared in the process of compression which the test as a whole has undergone, and have thus become bent. The teeth are nearly horizontal, and they actually articulate with the auriculae, which, as in Cidaridae, consist of disconnected pillars and spring from the plates of the interradius. Each pillar is fused with the adjacent one belonging to the next radius, so that the system which in Echinus consists of five radial arches here consists of five interradial pillars. Aristotle's lantern has lost its respiratory function and apparently its masticatory function as well, for the teeth are used as spades to shovel into the mouth the sand mixed with organic detritus and small organisms on which the animal lives.
The water-vascular system is highly modified. There are two sharply marked kinds of tube-feet—(a) the respiratory tube-feet, (b) the locomotor tube-feet. Both kinds are terminated by {547}suckers, but the first variety are much larger than the second; they possess a flattened lobed base, and are connected with the ampulla by a double canal. They issue only from the double pores which form the petal. The locomotor tube-feet are small and cylindrical; they are, as already mentioned, scattered over the whole upper surface of the test, penetrating both ambulacral and interambulacral plates, but all are connected by transverse canals with the radial canals of the water-vascular system. On the under surface they are confined to the neighbourhood of the ambulacral grooves, which have nothing to do with the ambulacral grooves of an Asteroid, but are due to secondary localisations of the tube-feet, which are here also connected in each radius with a single radial canal. The appearance of a living Echinarachnius covered with a veritable forest of short brown tube-feet is very striking.[500]

Fig. 244.—Dissection of Echinarachnius parma. × 1. The oesophagus has been cut through and moved to one side so as to expose Aristotle's lantern. The aboral part of the test has been removed. gon, Genital organ; int, intestine; musc, transverse muscle connecting jaws of adjacent interradii; rect, rectum; siph, siphon; st, stomach.
The condition of the water-vascular system is to be explained entirely by the peculiar environment of the animal. The demand for specialised respiratory organs is brought about by the habit of living half buried in the sand. Under these circumstances the strain of supplying the needful oxygen is thrown on the {548}dorsal tube-feet, and they become modified in order to fit them for this function. The locomotor tube-feet are very small and feeble compared with those of Echinus esculentus, but this is comprehensible when it is recollected how little resistance the yielding sand would offer to the pull of a powerful tube-foot like that of the Regular Urchins, for in order to move the creature through the sand a multitude of feeble pulls distributed all over its surface is necessary, and the locomotor tube-feet are exactly fitted, both as to size and number, for this object.
The principal points in which Clypeastroidea vary amongst themselves are (1) the nature of the internal skeleton, (2) the shape, and (3) the spines.
Internal Skeleton.—In Echinocyamus and its allies this consists in each interradius of two simple partitions radiating out towards the edge of the disc; in Laganum it consists of walls parallel to the edge of the disc; in Clypeaster, of isolated pillars.
Shape.—In Echinocyamus the outline is oval and the test comparatively high. In Clypeaster and its allies the outline is pentagonal, and the test is swollen up into a blunt elevation in the centre. In a large number of genera, however, the test is, as in Echinarachnius, extremely thin and flat, and the outline may be variously indented. A first indication of this process is seen in Echinarachnius itself, but in Rotula the edge is drawn out into finger-like processes which are all interradial. In Mellita these processes unite with one another distally so as to surround spaces called "lunules," which appear as perforations of the test.
The Classification of the Clypeastroidea adopted by Agassiz is based chiefly on the degree of development of the internal skeleton, and as this is of great physiological importance to the animals we shall follow it here; but since it was published the remarkable discovery has been made of Pygastrides, a type previously known only from fossils. We must therefore recognise two sub-orders:—
Sub-Order I. Protoclypeastroidea.
Anus on dorsal surface near apical pole. One species, Pygastrides relictus,[501] with no "petals," from deep water in the Caribbean Sea.
Sub-Order II. Euclypeastroidea.
Anus on under surface.
Fam. 1. Fibularidae.—The "petals" are short and imperfect, and the internal skeleton consists of two short outwardly-directed septa in each interradius. To this family the only British Clypeastroid, Echinocyamus pusillus, belongs. This animal never exceeds an inch in length, and has an oval outline. It inhabits shallow water, and is often found in the same ground as Echinus miliaris, but like all Clypeastroids it prefers a sandy bottom.
Fam. 2. Echinanthidae or Clypeastridae.—"Petals" well marked, internal skeleton consisting of isolated pillars. The largest Cake-urchins belong to this family, which is found chiefly in tropical waters. Clypeaster, the great Cake-urchin, with a deeply sunken peristome, belongs to this family.
Fam. 3. Laganidae.—Closely allied to the foregoing, but distinguished by the fact that the internal skeleton consists of walls parallel to the edge of the test. (Laganum, Arachnoides, Peronella.)
Fam. 4. Scutellidae.—This family includes about half the genera, and is sharply distinguished from all the rest by (1) the extremely flattened shape, (2) the indentation of the outline in the anal interradius and often elsewhere, (3) the branching of ambulacral furrows on the under surface. Echinarachnius, taken as the type in describing the anatomy of the Cake-urchins, is the best-known genus. Others are Mellita, with five perforations in the edge of the test; and Rotula, with the edge produced into a number of finger-like processes.
Order III. Spatangoidea (Heart-urchins).
As the type we may select Echinocardium cordatum, which occurs abundantly in the Clyde and on the west coast of Ireland. The animal is found buried in sand at a depth of about 8-10 inches from the surface. At this depth it lies in a burrow, the walls of which are kept from collapsing by the somewhat broadened tips to the spines. This burrow communicates with the surface by a narrow cylindrical opening similar to the opening of the burrows made by the Clams and other bivalves. A little practice, however, enables one to distinguish the burrow of the Heart-urchin from these.
The animal is about the size of a small potato, and is of light straw colour. Its outline is oval, and the test is about two-thirds as high as the shorter diameter. It is thus higher in proportion to its width than is the case with any living Cake-urchin. The highest point is behind the centre. The narrower end of the animal terminates in a vertical edge, in the upper part of which is a large periproct covered with a number of thin movable plates. The mouth is situated on the under surface, considerably nearer the front end of the test than the hinder end. It is entirely devoid of jaws or of teeth, and also of gills or of a movable peristome.
Aristotle's lantern has entirely disappeared, leaving as the only trace of its former presence a canal with membranous walls encircling the mouth, which has the form of a transverse slit, the posterior lip projecting considerably forward.
The ambulacral areas are easily distinguishable from the interambulacral areas by being comparatively bare of spines. On the upper surface they are distinctly grooved, the groove being especially deep in the case of the anterior one. On the lower surface they coalesce round the mouth, shutting out the interambulacral regions, and are here perforated by the large pores of the buccal tube-feet. Between the two posterior radii on the oral surface there is a space with specially arranged spines called the plastron or sternum. The interambulacral plates composing this region are very much lengthened, and interdigitate with one another at the sutures. To this lengthening is due the apparent forward shift of the mouth. The spines are very characteristic, and are very different from any which have as yet been described. They are the sole organs of locomotion. The primaries are long and curved, with flattened tips, admirably adapted to plough through the sand in which the animal lives. On the upper surface, mingled with the tube-feet, are a large number of small secondary spines. Between the two posterior petals there is a hoop-shaped band of very small black spines. These spines are ciliated, and draw a current of fresh sea-water over the respiratory tube-feet. Beneath the periproct there is a similar band called the "sub-anal fasciole"; this probably produces a current of water which sweeps away the material ejected from the anus.
The pedicellariae are of the trifoliate and gemmiform varieties. {551}The sphaeridia are situated in open pits, one or two in each, situated at the bases of the tube-feet nearest the mouth.
When the upper part of the test is picked away, the course of the alimentary canal is exposed (Fig. 247). It is very similar to the alimentary canal of Echinarachnius, except that from the first coil a large blind pouch, called the caecum, is given off.
The water-vascular system shows many characteristic features. The tube-feet are confined to two rows in each ambulacrum, the scattered smaller feet found in such abundance in Echinarachnius being entirely absent. There are four distinct varieties of tube-feet in Echinocardium, which are as follows:—(a) The respiratory tube-feet of the petals. These have, as in Echinarachnius, broad flat bases, but they have lost the sucker. (b) The prehensile tube-feet of the anterior ambulacrum. These are enormously long structures, measuring when expanded several times the length of the body. They end in discs, which are frayed out into fingers, so as to look like miniature sea-anemones. These tube-feet are comparatively few in number and are confined to the apical portion of the anterior ambulacrum. (c) The buccal tube-feet. These are short, thick, and pointed, and covered with a multitude of club-shaped processes. They are found on all the ambulacra in the neighbourhood of the mouth. (d) The degenerate tube-feet found in the portions of the ambulacra between the "floscelle" (see p. 553) and the petals. These are single and pointed, few in number, and issue from single pores in the test.
This extraordinary diversity in the tube-feet is fully explained {552}when the habits of the animal are known. The function of the respiratory tube-feet requires, of course, no special elucidation, but the peculiar anterior ambulacrum was a mystery till the feeding habits of the animal were observed by the late Dr. Robertson[502] of Cumbrae. He found that the animal protruded the long prehensile tube-feet through the opening of the burrow up to the surface of the sand. With their finger-like processes they then collected the surface film of the sand, which was impregnated with Diatoms and other small organisms. When a "handful," so to speak, of this nutritive material has been collected, the long tube-foot is withdrawn down the burrow and passed over the deeply grooved part of the ambulacrum to the buccal tube-feet, to which the food is given up. These last then push it into the mouth. Only one prehensile tube-foot is extended at a time.

Fig. 246.—Interior of test of Hemiaster philippi, showing the genital organs and their ducts (only three are developed in this species). Slightly enlarged. (From Wyville Thomson.)

Fig. 247.—Dissection of Echinocardium cordatum. × 1. The oral part of the test has been removed. caec, Blind pouch of the stomach; gon, genital organ; int, intestine; oe, oesophagus; rect, rectum; siph, siphon; st, stomach; w.v.r, water-vascular ring.
The stone-canal is very short and soon opens into the axial sinus; it is widely separated from the pore-canals which traverse the madreporite. Communication between the two is effected by the long axial sinus. There are only four genital organs.
Heart-urchins vary amongst themselves chiefly in the {553}following points, viz.:—(1) the shape and position of the peristome, (2) the characters of the "petals," and (3) the number and position of the fascioles.
Peristome.—In many genera this is pentagonal and central, and in these cases the interradii commence at the peristome with a single plate, which is often covered with a thick crowd of small spines and is termed a "bourrelet." The oral ends of the radii also often consist of a crowded series of narrow plates, looking something like a "petal," and termed a "phyllode." The five bourrelets and five phyllodes constitute a flower-like figure termed a "floscelle."
Ambulacra.—In Echinoneus all five are alike and are provided with similar tube-feet, which are respiratory but possess suckers. The ambulacra are not grooved, and the petaloid arrangement of the pores is hardly marked; but in Cassidulus, Pourtalesia, and many other genera the five petals are well marked, though they are all similar to one another.
Fascioles.—These structures are often entirely absent; the sub-anal one alone is present in Spatangus. In Eupatagus a peripetalous one is added. This surrounds all the "petals," and has obviously the function of sweeping fresh water over the respiratory tube-feet. In Echinocardium, as we have seen, there is an "internal fasciole" between the two anterior petals which has a similar function. In addition, this genus possesses an anal fasciole which surrounds the anus and sweeps away the faeces.

Fig. 248.—Young Echinoneus to show five equal radii scarcely petaloid. amb, Ambulacral area; g.p, genital pore; mad, madreporite. (After Agassiz.)
The Classification of the Spatangoidea is based mainly on the degree of development of the petals, that is to say, on the extent to which the burrowing habit has been developed. But weight is also laid on the shape of the peristome, the pentagonal form being more primitive. Seven families are recognised, which are as follows:—
Fam. 1. Echinonidae.—"Petals" hardly marked at all; peristome in the centre of the lower surface and pentagonal. Floscelle not developed.
One genus, Echinoneus (Fig. 248).
Fam. 2. Nucleolidae.—"Petals" distinct; peristome as in the foregoing family. No floscelle. Nucleolites, with the anus in a furrow. Anochanus, with a concave apical system serving as brood-pouch.
Fam. 3. Cassidulidae.—"Petals" usually distinct; peristome eccentric, but provided with a well-marked floscelle.
Echinolampas, with the anus on the under surface.
Neolampas, with the anus on a projecting papilla. One specimen of this genus has been dredged in the British area.
The three foregoing families probably use their tube-feet to walk with, and bury themselves only to a slight extent. They are often united as a sub-order, the Asternata, and distinguished from all the rest which possess an eccentric mouth and well-marked plastron. These families are then grouped together as Sternata. They are as follows:—
Fam. 4. Ananchytidae.[503]—Spatangoidea with elongated apical system, ambulacra all similar and not grooved. Petals feebly marked. Pourtalesia, with bottle-shaped posterior prolongation of the test. Platybrissus, with flattened test.
Fam. 5. Palaeostomatidae.—An aberrant family consisting of one genus, Palaeostoma. Petals grooved, with a peripetalous fasciole, but peristome central and pentagonal.
Fam. 6. Spatangidae.—Spatangoidea of more or less flattened shape, with well-marked petals and a sub-anal plastron as well as the ventral one. One fascicle at least, but a peripetalous one never present. The anterior ambulacrum grooved and different from the rest. This family is represented in British waters by two genera, Spatangus and Echinocardium. The former possesses only a sub-anal fasciole, and has specially long curved spines on the ventral plastron. It is represented by two species, S. purpureus and S. raschi, the latter being distinguished by a pointed lower lip. It is a deep-water species, found in 100 fathoms and over on the west coast. S. purpureus is fairly common in rather shallow water. From observations made on specimens kept in confinement it appears to burrow only so far as to leave the petals uncovered; hence there is no need of a peripetalous fasciole. Echinocardium is devoid of the thicker spines on the plastron, and has an internal fasciole and a perianal one as well as the sub-anal. As already mentioned, it is a deep burrower. It is represented by three species, E. cordatum, E. pennatifidum, and E. flavescens. The first, described as the type of the Spatangoidea, has a deeply grooved anterior ambulacrum. In the remaining two species this ambulacrum is not grooved. E. flavescens has only six or seven pairs of pores in the posterior {556}petals, E. pennatifidum twelve to fourteen. Both come from deeper water than E. cordatum.
Fam. 7. Brissidae.—Allied to the Spatangidae, but distinguished by sunken petals and a peripetalous fasciole.
Two genera are recorded from the British area, Schizaster and Brissopsis, but the first has only been found once in deep water; the second is common. Schizaster has the front petals three times as long as the hind ones, and no sub-anal fasciole. Brissopsis has the front and hind petals of about the same length, and a sub-anal fasciole. The only British species is called B. lyrifera, on account of the fiddle-shaped outline of the peripetalous fasciole.
Hemiaster (Fig. 250) in general resembles Schizaster, but the petals are equal in length, and the two posterior serve as brood-pouches for the young. This genus is mainly Antarctic.

Fig. 251.—Dried shell of Schizaster, showing peripetalous fasciole. ant.amb, Anterior ambulacrum; fasc, peripetalous fasciole; g.p, genital pores, × 1. (After Agassiz.)
Fossil Echinoidea.—Echinoidea are well represented in the geological record, and form a characteristic element in many fossil faunas. They appear in the Ordovician formation, but the first representatives of an existing family (Cidaridae) only appear in the Permian.
Space will only permit us to treat of the extinct members of the group very briefly. Leaving out of sight the representatives of families still living, the fossil Echinoidea may be divided into two great groups, viz.:—
(a) Palaeozoic forms, which in some points serve to connect the Endocyclica with the primitive Asteroidea.
(b) Mesozoic forms, which serve to connect the Clypeastroidea and Spatangoidea with the Endocyclica.

Fig. 252.—A, Bothriocidaris. × 1. B, Palaeoechinus. × 1. amb, Ambulacral plates; inter, interambulacral plates. (After Zittel.)
The Palaeozoic forms are often called Palaeoechinoidea, and they are above all distinguished by the fact that the number of vertical bands of plates composing the corona is variable, in a word, that the corona has not yet acquired a fixed definite constitution. One genus (Echinocystites) has the anus outside the apical system. It has four rows of pore-plates in each radius, and numerous rows of plates each with a single spine in the interradii. Another (Palaeodiscus) has been shown by Sollas[504] to be in many respects the missing link between Asteroidea and Echinoidea. Inside the plates of the corona there is a series of ambulacral plates like those of Asteroidea. The tube-feet in the oral portion of the radii seem to have issued between the (outer) ambulacral plates. No anus has been detected. All the rest are Endocyclic. The oldest known form, Bothriocidaris (Fig. 252, A), from the Ordovician, has only one row of interambulacral plates and two of ambulacral; no peristome is distinguishable from the corona. The Archaeocidaridae appear in the Devonian. They have narrow ambulacra of two rows of pore-plates as in the Cidaridae, but the interambulacra consist of many rows, the members of which overlap, and therefore were probably slightly movable, as in the Echinothuriidae; the primary tubercles are large, and there is only one on each plate. The Melonitidae (Fig. 252, B) appear in the Carboniferous. Each interambulacral plate, of which there may be five rows in each interradius, bears numerous small tubercles, and there may be four or more vertical rows of pore-plates, though in the genus figured, Palaeoechinus, there are only two. The Tiarechinidae are represented by one genus, Tiarechinus, with an enormous apical system, from the Triassic of the Tyrol. The interambulacra consist of one plate bordering the mouth, three, {558}side by side, forming the interradial area of the corona, and one large genital plate; the ambulacra, of two rows of pore-plates. This family consists of dwarfed forms which probably inhabited the land-locked seas and salt lagoons of the Triassic epoch.
When we recollect that some of the oldest Asteroidea known to us had very narrow arms and interradial areas edged by large square marginals, it does not require a very great effort to imagine how these marginals could be converted into the vertical rows of the interambulacra, and the pointed narrow arms becoming recurved, could have formed the ambulacra. The physiological advantage of this will be discussed in the chapter on development.
True Cidaridae occur in the Permian, and are abundant in all the younger formations. One Cretaceous genus, Tetracidaris, has four rows of interambulacral plates near the mouth, diminishing to two at the apex. This circumstance renders it probable that the Cidaridae are the direct descendants of the Archaeocidaridae. The Saleniidae, Echinothuriidae, and Diadematidae appear in the Jurassic, the Echinidae in the Cretaceous, and the Arbaciidae only in the Tertiary epoch.
Turning now to the Mesozoic forms with an excentric anus, there were a number of forms which have been grouped together as Holectypoidea which had auricles and teeth and gills, although these were only feebly developed, and in which the pore-plates remained separate. The periproct was a comparatively large area, and in Pygaster, as in the surviving form Pygastrides, it was in contact with the apical system, although outside it. Many of the genera were of considerable height in proportion to their length. In Conoclypeus and Discoidea the jaws and auricles were very weak. The Echinoconidae have only vestigial auricles, and on this account are often definitely grouped with the Spatangoidea, but they are closely allied to the Holectypoidea. They are Cretaceous forms of high conical shape (Galerites). In Hyboclypus (Fig. 253) all trace of the teeth has disappeared, {559}but the periproct is large and in contact with the apical system; these forms appeared in the Jurassic. The Collyritidae, also Jurassic, had a marginal anus. The apical system was so much elongated that two of the ocular plates are widely separated from the other three, two opposite interambulacra meeting between them. Unmistakable Spatangoidea (Spatangidae and Ananchytidae) appear in the Cretaceous, true Clypeastroidea (Fibularites) in the Cretaceous, the other families in the Tertiary.
Reviewing these facts, we see that from the Holectypoidea we can pass by insensible steps on the one hand into true Clypeastroidea, and on the other hand into true Spatangoidea. The Holectypoidea differed from Endocyclica only in the position of the anus, and the initial step in the backward shift of this organ is seen in Pygaster. One result follows from this conclusion, that the modification of the dorsal tube-feet into breathing organs, and the consequent appearance of petals which accompany the taking on of burrowing habits, were independently developed in the Clypeastroidea and Spatangoidea, since these features were absent in the more primitive members of both groups.
ECHINODERMATA (CONTINUED): HOLOTHUROIDEA = SEA-CUCUMBERS
CLASS IV. HOLOTHUROIDEA
This class of the Eleutherozoa comprises those sausage-shaped, leathery Echinodermata familiarly known as Sea-cucumbers. They are named Holothuroidea from ὁλοθούριον, an animal described by Aristotle, and believed to belong to this class.
The Holothuroidea resemble Echinoidea in the fact that the radial canals of the water-vascular system run backwards and upwards from the ring-canal over the surface of the body, terminating in small papillae near the anus, which, as in the Echinoidea Endocyclica, is situated at the upper pole of the body. There are, of course, no arms; and a further resemblance to Echinoidea is shown by the fact that the ambulacral grooves are represented by closed epineural canals, and that the ectoderm consists of long, slender, flagellated cells interspersed with gland-cells, underneath which is a plexus consisting of nerve-fibres and small bi-polar ganglion cells. There are, however, no spines or pedicellariae; and Holothuroidea differ not only from Echinoidea, but from all other Echinodermata, in the vestigial character of their skeleton, which consists merely of isolated nodules of calcium carbonate embedded in the skin. The body-wall is provided with transverse muscles running across the interradii, and also with powerful longitudinal muscles, running along the radii, by means of which worm-like contractions are carried out. Similar muscles, though much less developed, occur in the Echinothuriidae, and must have been present in many extinct Echinoidea in which the plates of the corona overlapped; and hence it is exceedingly probable that from some of these {561}early forms, as, for instance, Bothriocidaris, Holothuroidea may have been evolved. The muscular body-wall has indeed been as important a factor in the evolution and differentiation of the Holothuroidea as the muscular arm in that of Ophiuroidea, or the movable spine in the case of Echinoidea.
There are about 520 species of living Holothuroidea, and of these about twenty-one have been recorded from British waters. One of the best-known of the British species is Holothuria nigra (Fig. 254), commonly known as the "Cotton-spinner"; and this we shall take as a type for special description. The animal may attain a length of a foot when fully extended, and has a diameter of from 3 to 4 inches. It is of a very dark brown colour on one side, which in crawling it keeps uppermost, whilst on the lower side it is of a tawny yellow hue. Three of the radii (often termed the "trivium") are situated on the lower surface; two (termed the "bivium") on the upper surface. The podia are scattered fairly evenly over the whole surface without reference to the radii; below they are regular tube-feet provided with suckers, whilst on the upper surface they are pointed tentacles, employed only for sensory purposes.
If the animal be observed alive and in its natural surroundings, a ring of twenty large tentacles can be seen surrounding the mouth. These buccal tentacles are in every respect comparable with the buccal tube-feet of Ophiuroidea and Spatangoidea, and, like them, are employed in shovelling the muddy substratum on which the animal lies into the mouth.
Ludwig employs the term "feeler" for these buccal tentacles, {562}in order to distinguish them from the pointed podia scattered over the bivium. This procedure will be adopted here. In the Cotton-spinner the feelers, when extended, show a short smooth stem, from the apex of which springs a circle of short branches, which are in turn beset with a double row of branchlets, themselves branched. Such feelers are said to be shield-shaped.
A transverse section through the radius of a Sea-cucumber is, in general, like one through the radius of a Sea-urchin; the points of difference to be noted are: (a) In the Sea-cucumber, beneath the ectoderm, is a thick dermis with small plates scattered in it, instead of the whole dermis being calcified, as is the case in the Sea-urchin; (b) the ampulla of each podium is connected with the peripheral portion by one canal, not two, as in the case of the Sea-urchin; (c) there is a development of coelomic nervous tissue from the outer side of the perihaemal canal; (d) internal to the radial water-vascular canal are to be seen cross-sections of two great bands of longitudinal muscles, by the contraction of which the body is shortened. Lengthening is brought about by the contraction of transverse muscles, which are found on the inner side of the body-wall in each interradius; the five sets taken together act like circular muscles, or a rubber band, on the incompressible fluid in the body-cavity.
When the Sea-cucumber is opened by a cut along the left dorsal interradius, the spacious coelom is laid open, and lying in it is seen the alimentary canal. This tube is bent on itself, so that it has a form like ∽ (Fig. 255, B) running backwards to the posterior end of the body, then running forwards to near the anterior end, before it finally turns to run backwards to the anus. By taking cross-sections of the body at different levels, it can be shown that the alimentary canal makes a half-turn round the longitudinal axis (Fig. 255, A). It is suspended by bands of membrane, termed "mesenteries," to the body-wall, and of these there are three, the first of which (i.e. the one nearest the mouth) is attached to the mid-dorsal interradius (Fig. 255, A, M1), the next to the left dorsal interradius (M2), and the last to the right ventral interradius (M3).
The alimentary tube shows four regions, which are distinguished as follows:—(1) A short oesophagus with strongly-marked longitudinal folds in its walls; this is separated by a constriction from (2) the stomach, a very short region, {563}characterised by its strong musculature. Next follows (3) the intestine, a thin-walled tube comprising the middle limb and most of the descending and ascending limbs. This finally passes into (4) the wide terminal "rectum," or "cloaca," which is connected to the body-wall by muscular bands which traverse the coelom (Fig. 256, 10).
The cells lining the oesophagus resemble ectodermal cells; those lining the stomach are nearly all gland-cells, and obviously secrete the digestive juice. The powerful muscles of this portion of the gut produce a strong peristalsis which thoroughly mixes the juice with the food, and in the thin-walled intestine absorption of the digested material takes place. The extreme thinness of the intestinal wall is common to many animals (e.g. Sipunculus, Vol. II. p. 412) which swallow mud and sand for the sake of the organic matter which they contain.

Fig. 255.—A, diagrammatic cross-section of a Holothurian; B, diagrammatic longitudinal section. I-V, radii; Int.1-Int.3, the three limbs of the alimentary canal; M1-M3, the three mesenteries attaching the same to the body-wall. (After Ludwig.)
The rectum, or cloaca, is one of the most characteristic features in this and most other Sea-cucumbers. In addition to the passing of faeces, it is used to pump water in and out, and it thus serves as a breathing organ. This pumping is effected by alternate contractions of the radiating muscles attaching the cloaca to the body-wall, and of the circular muscles which immediately surround it. Two long branched tubes termed Respiratory trees (Fig. 256, 11) open into the cloaca, and into these the inspired water penetrates. The finer branches of these gills end in rounded thin-walled swellings termed "ampullae"; and when water is forced into these they become tense, and a considerable quantity diffuses through their walls, carrying {564}oxygen into the fluid which fills the coelom. If a Sea-cucumber be left in a limited quantity of water, it will sometimes direct the posterior end upwards until it reaches the surface of the liquid, and will pump air into the trees. Besides the trees, other much shorter tubes open into the cloaca, termed the Cuvierian organs. These tubes are really the modified basal branches of the trees. They are unbranched, and their peritoneum consists of cells which secrete a slime which swells up enormously on the addition of sea water. When the Cotton-spinner is strongly irritated, it contracts all the muscles of the body-wall, and these, acting on the incompressible fluid in the body-cavity, transmit the pressure to the thin rectum, which tears, and allows a portion of the viscera to be forced out. The first parts to be rejected are the Cuvierian organs, and the cells covering these absorb water, and their contained mucus splits up into a tangle of white threads, in which an enemy may be completely ensnared. A large lobster has been seen so enveloped with this "cotton" as to be completely incapable of motion. The origin of the name "Cotton-spinner" requires no further elucidation. Such self-mutilation, even when it involves not only the Cuvierian organs, but the trees and the whole of the intestine, is not necessarily fatal. If the animal be left alone, it can regenerate the whole of these organs.
The water-vascular system in its general features resembles that of the Echinoidea. We notice as its first striking peculiarity the modification of the stone-canal. This is often multiplied, as in the species (H. tubulosa) represented in Fig. 256, where there are five; but whether there is one or many, they do not reach the body-wall, but end each in a swelling projecting into and bathed by the coelomic fluid. These swellings are termed "internal madreporites." They are pierced by numerous fine ciliated canals, which lead into a space from which the stone-canal takes its origin. Both stone-canal and madreporite (especially the latter) are stiffened by the deposition of carbonate of lime. In the young Holothurian there is a single ciliated pore-canal opening to the exterior and leading into a thin-walled axial sinus, which, as Bury[505] has shown, is later converted into the internal madreporite; the pore-canal, which represents the external madreporite of other Echinoderms, disappearing at the same time.

Fig. 256.—Dissection of Holothuria tubulosa. × ½. 1, Feelers; 2, feeler-ampullae; 3, ring-canal; 4, Polian vesicle; 5, stone-canals; 6, radial canal; 7, one of a pair of longitudinal muscles; 8, genital tubes; 9, intestine; 10, radiating muscles of cloaca; 11, base of respiratory tree; 12, ventral blood-vessel; 13, plexus of dorsal blood-vessel. (After Ludwig.)
This extraordinary modification is the consequence of the habit of forcing water into the respiratory trees. The body-cavity is by this means kept tensely filled with fluid, and the stone-canal is enabled to draw on it for the supply to the water-vascular system, thus rendering the external madreporite supererogatory. A large-stalked sac—the Polian vesicle (Fig. 256, 4)—multiplied in many species, hangs down from the water-vascular ring and serves as a reservoir of fluid.
All the podia, including the feelers, have ampullae. In the feelers a semicircular valve is situated just where the external part passes into its long ampulla. When this valve is expanded, the feeler is moved about by the contraction of its muscles, but when it is contracted, the contents of the feeler can flow back into the ampulla, so that the feeler is reduced to an insignificant papilla (as in Fig. 254). The interior of the feeler is ciliated, and a current seems to flow up one side and down the other, so that this organ, like the dorsal tube-foot of a Cake-urchin or Heart-urchin, seems to assist in respiration.
The nervous system differs from that of Echinoidea in the absence of the pigment spot (or so-called eye) on the terminal podium of the radial water-vascular canal. Each podium receives a so-called nerve—really an extension of the radial nerve-cord with its ganglion-cells—and this ends in a plate of sensory epithelium in the sucker of the tube-foot or tip of the tentacle, or of each of its branches in the case of the feeler.
There is a coelomic nervous system developed from the radial perihaemal canals. The perihaemal ring is represented in Echinoidea by the lantern coelom, in Holothuroidea in all probability by the "buccal sinus," a space intervening between the water-vascular ring and the oesophagus. In the outer wall of this are developed ossicles, which constitute the calcareous ring found in all[506] Sea-cucumbers (Fig. 257, A and B). In this ring (Fig. 257, B) are to be distinguished radial and interradial pieces. The former are notched at their upper ends, and in all probability represent the auriculae of Echinoidea, as the radial nerve-cords pass out over the notches, whilst the interradial pieces probably represent a coalesced pair of jaws and their included tooth, since these ossicles develop from a single rudiment in the larval Echinoid.
The so-called blood system is in its main features similar to that of Echinoidea. It consists of a blood-ring surrounding the oesophagus inside the water-vascular ring, and sending branches along the stone-canal, and of dorsal and ventral strands accompanying the gut in its course. These are best marked in the region of the intestine, where absorption principally takes place; in the wall of the stomach they are represented by a delicate plexus which can hardly be traced into connexion with the blood-ring. The dorsal "vessel" is situated in a fold of peritoneum projecting from the intestinal wall; it gives off branches to the intestine, which unite on its surface to form a plexus. In the middle limb of the intestine these branches are grouped into tufts, and the fold of peritoneum between successive tufts becomes absorbed; through the holes so formed branches of the respiratory tree penetrate, so that the trees cannot be separated from the intestine without tearing the dorsal vessel (Fig. 256, 13).

Fig. 257.—Types of calcareous rings and of ossicles. A, calcareous ring of Phyllophorus rugosus, × 2; B, calcareous ring of Holothuria cinerascens; C, ossicle of Holothuria atra; D, ossicle of Holothuria fusco-rubra. r, Radial piece. (After Ludwig.)
The genital organs consist of a single group of branched tubes situated on the left side of the dorsal mesentery, which converge to open into a short genital duct, which leads to a pore situated in the mid-dorsal line, a short distance behind the feelers. From the common point of origin of the tubes, the "genital base," as it is called, a worm-shaped genital stolon[507] extends back along the genital duct towards the body-wall. There is no genital rachis.
Classification of Holothuroidea.
The class is in many points of structure exceedingly variable, but many striking variations in important organs occur in allied {568}species, and even in the same species, and hence are probably not of physiological importance. We shall therefore confine our attention mainly to those differences in structure which are correlated with differences in habits, and therefore of systematic importance. We shall consider in order (1) the feelers; (2) the method of protecting these; (3) the rest of the water-vascular system; (4) the gills; and (5) the skeleton.
Feelers.—These organs have been made the basis of the division of the Holothuroidea into orders, and as they are the means by which food is obtained, and are thus of first-class physiological importance, this procedure is fully justified. In three orders they have the shield-shaped ends described in the case of Holothuria nigra, but in another large order (Dendrochirota) they are much branched, and end in a mass of delicate twigs. In another order (Synaptida) they are feather-shaped, with two rows only of branches, whilst finally in Molpadiida they are simple finger-shaped processes with one or two lateral branches. The number of the feelers varies from ten to thirty.
In the Dendrochirota the entire anterior portion of the body can be introverted into the interior, so that in this way the crown of feelers can be effectively protected. The retractor muscles are modified portions of the longitudinal muscles of the body-wall, which traverse the body-cavity, and are inserted into the radial pieces of the calcareous ring. Similar muscles are found in the genus Molpadia and in many of the Synaptida. In Aspidochirota and Pelagothuria they are totally wanting, and here the feelers possess long ampullae which allow of the tentacles being individually contracted to very small dimensions. These ampullae seem to be present in nearly all cases in Molpadiida, and in Synaptida, although in the last-named order they are very feebly developed, and must be looked on as vestigial. In Dendrochirota, owing to the strongly developed retractors, they would be useless, and so are absent.
Water-Vascular System.—In Synaptida the radial canals are totally absent in the adult, and the only podia are the feelers, which spring directly from the ring-canal. The radial canals are present in Pelagothuria, but the feelers are still the only podia; in the Molpadiida there are only five small terminal tentacles round the anus in addition to the feelers. In the Elasipoda all the podia have pointed ends, but the dorsal podia {569}are few, long, and stiff, and often coalescent in places to form grotesque or remarkable appendages. In the remaining forms the podia of the trivium have always suckers, whilst those of the bivium may or may not be pointed. In Psolus the two dorsal radial canals and their podia are totally absent.
Respiratory Trees.—These are present in Aspidochirota, Dendrochirota, and Molpadiida, totally absent in the Synaptida and Pelagothuria, and doubtfully represented in a few Elasipoda by a single unbranched outgrowth of the gut.
Skeleton.—This consists, as explained above, of the scattered deposits in the skin and of the calcareous ring. As regards the first, their shape varies immensely, and yet one or two principal types characteristic of each of the main divisions can be defined. Thus the Synaptida are characterised by wheels, with spokes ending in a hub, and by anchors attached to a plate. The Elasipoda have simple St. Andrew's crosses, whilst the Aspidochirota are mainly characterised by "stools" (Fig. 257, C) and buckles (Fig. 257, D). The Dendrochirota have a bewildering variety of forms; the most characteristic, however, are a right-angled cross and a grating, very similar to the buckles of the Aspidochirota, except that in the former there are usually four holes placed cross-wise, whilst the buckle has generally two parallel rows of three holes. Since these ossicles are the only records we possess of the existence of fossil Holothuroidea, they have been studied with great care. The calcareous ring varies very much. The radials are always five (except in individuals where there are more than five radii), but the interradials are increased in the Synaptida, and in the other orders are in some cases diminished or occasionally suppressed altogether. The last is the case in nearly all Elasipoda; here the radials consist of a central horizontal piece with two diverging arms at each side. These arms, which can branch repeatedly, traverse the adjacent interradii, meeting those of the next radii, so that interradials are in most cases entirely absent. The Aspidochirota have usually a ring consisting of small squarish ossicles (Fig. 257, B). In the Molpadiida and Dendrochirota the radials are prolonged backwards into forked tails, which in some Dendrochirota are broken into a number of small pieces (Fig. 257, A), the lower parts of the interradialia being similarly divided.
The classification of the Holothuroidea is comparatively easy. {570}All authors recognise six divisions, and the only dispute is as to whether they are to be regarded as families or orders. Ludwig[508] divides the group into two orders, Paractinopoda and Actinopoda, but the first includes only those forms which have lost the radial canals, and this is only one step farther in a degeneration, intermediate stages of which can be traced in the other divisions. There is really no ground for placing the Paractinopoda in contrast to all the other divisions, and the only alternative is to regard the six main divisions as orders, since a class must be divided into orders. In the case of only one, however, is a further division into families practicable, and therefore each of the others will contain a single family.
Order I. Aspidochirota.
Holothuroidea with shield-shaped feelers provided with ampullae; with radial canals and numerous podia and with respiratory trees. Retractor muscles absent. Nearly a third (158) of the species of Holothuroidea belong to this order, but there are only six genera, and of these Holothuria includes no less than 109 species. The Aspidochirota seem for the most part to live on somewhat firm ground, the surface of which they are continually sweeping with their shield-shaped feelers, which brush the adherent organisms into the capacious mouth. Four species of Holothuria—viz. H. intestinalis, H. tremula, H. aspera, and H. nigra are recorded from British waters. The first-named is a northern form, distinguished by the fact that all its podia have suckers; it is found in the north of Scotland. H. tremula is intermediate in structure between H. intestinalis and H. nigra, and is found in deep water off our western coasts. H. aspera, remarkable for the radiating spines growing out from its ossicles, has been recorded only once from deep water. Of the other genera it is only necessary to mention Stichopus, remarkable for the square outline of its transverse section, and for the restriction of the ventral tube-feet to the radii; there is also a well-marked tapering of the anterior end, so that this genus may be said to have a neck. Stichopus is almost entirely confined to tropical waters, and some of its species, as also species of the ubiquitous genus Holothuria, as well as many other undetermined species, constitute the valuable "Trepang," which is a delicacy much prized {571}by the Chinese. The Trepang are caught in various parts of the Malay Archipelago. They are cooked in sea water to preserve them, dried in the sun, and boiled in fresh water repeatedly, till all the salt is extracted. They are then dried and sent to market, where they are used in making soup.
Order II. Elasipoda.
Holothuroidea with shield-shaped feelers, destitute of retractor muscles; all the podia have more or less pointed ends,[509] but there is a marked contrast between dorsal and ventral podia, and the ventral surface is flattened so as to constitute a creeping sole. No respiratory trees, at most a simple diverticulum of the intestine; frequently the primitive external madreporite is retained, and contains several pores.
A number of spherical sacs containing little spherical calcifications (otocysts) are attached to the nerve-ring in some genera. Can these be metamorphosed sphaeridia of Echinoid ancestors?
The first member of this remarkable order to be discovered was Elpidia, which was dredged in 1875 by the Swedish Arctic Expedition, and described by Théel.[510] The majority of the known members of the order were discovered by the dredging expedition of H.M.S. "Challenger." The species composing it are, with one exception, inhabitants of what may be termed the abysmal depths of the sea. The exception alluded to (Ilyodaemon maculatus) is confined to the belt between 100 and 150 fathoms in depth. The well-marked sole and the absence of suckers point to a life consisting of constant peregrinations over {572}the soft ooze forming the ocean floor. The ooze forms their food, and as their weight must to a certain extent immerse them in it, we can understand why the stiff, long dorsal podia have been specialised as respiratory organs, since there are no respiratory trees. These respiratory podia sometimes undergo extraordinary development; thus in Peniagone several very long ones cohere to form a huge vertical sail, whilst in Psychropotes one or two cohere to form a backwardly projecting tail. On the other hand, in Ilyodaemon (Fig. 258) the dorsal podia are numerous and slender.
Order III. Pelagothuriida.
Holothuroidea with shield-shaped feelers provided with long ampullae which project outwards, pushing the skin before them so as to form external appendages, connected at the base by a web. Calcifications absent. No retractor muscles. No respiratory trees. The external madreporite is retained, but all podia other than the feelers have disappeared, although the radial canals have been retained.
This order contains one species, Pelagothuria natans, which is the only free-swimming Holothuroid known, the muscular web connecting the freely projecting ampullae being the organ of locomotion.
Order IV. Dendrochirota.
Holothuroidea with long repeatedly branched feelers terminating in fine pointed twigs. No feeler-ampullae; but retractor muscles are present, which can introvert the anterior end of the body. Respiratory trees well developed. This order includes twelve genera and over 180 species, and, like the Aspidochirota, is of worldwide distribution. So far as can be safely generalised from the few species whose habits have been closely observed, it seems that this order is adapted to catch swimming prey—it is an order of fishers. The long branched tentacles are extended like the lines of an angler. Their surface is coated with adhesive slime, and before long becomes covered with small organisms which have come in contact with it. When a feeler has captured in this way a large enough haul, it is turned round and pushed into the mouth, which is closed on it. It is then forcibly pulled out, during which process the prey is, so to speak, stripped off it. {573}Four genera (Cucumaria, Thyone, Phyllophorus, and Psolus) and sixteen species have been recorded from British waters.
Cucumaria is remarkable for being the only genus of Holothuroidea in which the body is pentagonal in cross-section. In the majority of its species the tube-feet are confined to two rows along each radius, but in a few there are some scattered tube-feet in addition. There are only ten buccal tentacles. The species figured (C. crocea) is an Antarctic one which carries the young on the back. Thyone differs in being circular in cross-section and in having the tube-feet scattered evenly over the whole surface. In Phyllophorus (Fig. 260) the tentacles are more than fifteen, and are disposed in two circles, an inner of smaller and an outer of larger tentacles. The other podia are, as in Thyone, scattered.

Fig. 261.—Psolus ephippifer. × 3. A, with feelers retracted and brood-pouch closed; B, with feelers extended and some of the plates of the brood-pouch removed. (From Wyville Thomson.)
Psolus is a most extraordinary genus. There is a well-marked sole, to which the tube-feet are confined, whilst the dorsal radial canals, and consequently all the dorsal tube-feet, are absent. The dorsal ossicles are enlarged to form a complete mail of plates, recalling the corona of a Sea-urchin. The two British species are small, and found in comparatively deep water, but a fine large species is found in the Gulf of St. Lawrence, and {575}extends into brackish water up the estuary. The species figured (P. ephippifer) is an Antarctic one, which carries the eggs until development is complete in a dorsal brood-pouch.
Order V. Molpadiida.
Holothuroidea with simple, finger-shaped feelers, provided with ampullae; retractor muscles occasionally present; respiratory trees present. Besides the feelers, the only podia are five minute papillae terminating the radial canals in the neighbourhood of the anus.
This order includes six genera and about thirty species. Its peculiarities seem to be due to the fact that its members are burrowers, leading a life like an earthworm. Hence the absence of the tube-feet, and the small, almost vestigial character of the feelers. Trochostoma (Fig. 262) and Caudina are remarkable for the presence of a tail. This appendage is in reality only the narrow posterior end of the body, and is especially long in Caudina; and observations on a species found off the coast of Maine, U.S.A.,[511] have shown that the tail, like the siphon of a Mollusc, projects up from the burrow to the surface in order to maintain the respiratory current of water.
Order VI. Synaptida.
Holothuroidea with short bipinnate (i.e. feather-shaped) feelers, provided with only vestigial ampullae, and with well-developed retractor muscles. No other podia; radial canals absent in the {576}adult. Respiratory trees absent, and transverse muscles of adjacent interradii continuous, so as to form circular muscles. Otocysts attached to the nerve-ring as in Elasipoda.

Fig. 263.—Synapta digitata. A, animal viewed from the side, × 7⁄13; B, anterior end, with tentacles extended, × 2.
The members of this remarkable order, like those of the preceding one, are burrowers; but though their feelers are larger, the rest of their anatomy has undergone much more profound modification than that experienced by the Molpadiida. The loss of the radial canals, which must be practically functionless in Molpadiida, is not a great step, but the change in the mode of respiration is a greater modification. Respiration appears to be effected by diffusion through the body-wall, which is always comparatively thin. The circulation of the body-cavity fluid is assisted by a number of stalked, ciliated cups placed on the mesenteries near the line of their insertion on the body-wall. In dealing with Asteroidea it was pointed out that the ends of the tube-feet are the only places where numerous sense-hairs are to be found, and which, therefore, can be called sense-organs. This is true generally throughout Echinodermata. Now in Synaptida, where the tube-feet are lost, the surface of the body has scattered over it little sense-organs consisting of hillocks of ectoderm with an aggregation of sense-cells. These may be regarded as representing the discs of the missing tube-feet. One is involuntarily reminded by the ciliated cups and scattered sense-organs of the ciliated urns and sense-organs of the Sipunculidae, which lead a similar life; and taking into consideration the general superficial likeness in {577}appearance of the two groups, the epigram is almost justified that "if the Synaptida were not extremely careful they would become Gephyrea."
This order is represented in British waters by three species of the genus Synapta, which is remarkable for possessing, as ossicles, only the peculiar anchors attached to anchor plates. The present author has dug up the commonest species (S. inhaerens) from its burrows in the sand at low water in the Clyde. These animals seem to seek their food at the surface; the feather-shaped feelers are used to seize small algae and zoophytes, of which the food apparently consists. If seized, S. inhaerens readily amputates the posterior part of the body, whilst the head with its feelers immediately buries itself. The other genera of the order (except Anapta) are characterised by the possession of wheels with spokes as their characteristic ossicle, as the names Trochodota, Trochoderma, Acanthotrochus bear witness.
The only fossil remains of Holothuroidea consist of isolated ossicles—wheels, gratings, anchors, etc.—which first make their appearance in the Carboniferous limestone and tell us practically nothing of the evolution of the group. From a comparison with one another of the living families, certain conclusions can be drawn. The Aspidochirote feeler and the method of using it recall forcibly the shape and function of the buccal tube-feet of Spatangoidea. It is probably safe to assume that it is the primitive form from which the other forms of feeler have been derived. Secondly, the anal respiration and the curious internal madreporite have been developed in correlation with one another, and are like nothing found elsewhere among the Eleutherozoa. Hence we may with high probability assume a Protoholothuroid stock with shield-shaped feelers but devoid of respiratory trees, and with an external madreporite. From this stock the Elasipoda developed by migrating into deeper water, whilst the Pelagothuriida sprang from the same root by taking to swimming; the Aspidochirota constituting the main line. The Dendrochirota were developed from a stock with respiratory trees and internal madreporite—in a word, from Aspidochirota. From them the Synaptida and the Molpadiida have developed as offshoots at different periods through taking to a burrowing life. These relationships are shown by the following diagram:—
ECHINODERMATA (CONTINUED): PELMATOZOA—CRINOIDEA = SEA-LILIES—THECOIDEA—CARPOIDEA—CYSTOIDEA—BLASTOIDEA
SUB-PHYLUM II. PELMATOZOA
The Pelmatozoa differ from the Eleutherozoa in several important respects. They are fixed (at any rate in the young stage) by the centre of the aboral surface, and this portion of the body usually takes on the form of a stem supported by a definite series of ossicles, so that we can discriminate a "calyx"—the main part of the body—from the "stem." Further, the podia and the ambulacral grooves seem to be always covered with powerful cilia, which are employed in producing a current which sweeps small organisms to the mouth. The podia are never locomotor in function; their use is similar to that of the tentacles on the lophophore of Polyzoa and Brachiopoda.
The living Pelmatozoa are very few in number compared with the extinct forms. It may with justice be said that the group is nearly extinct; indeed, out of its five classes one alone, and that the most highly specialised class, survives till the present day. Now we have already seen that, in the case of the Eleutherozoa, if the annectant fossil types were taken into consideration, the definition of the classes would be difficult, so that it is not to be wondered at if the classes of the Pelmatozoa are also somewhat difficult to define; and it must be added that this difficulty is not only due to the fact that intermediate types occasionally occur, but also to our ignorance of the functions of many structures found in fossil types, speculations regarding which are to be received with caution. Bearing in mind, then, the provisional nature {580}of the classification, we may give the diagnoses of the principal divisions as follows:—
Class I. Crinoidea.—Pelmatozoa provided typically with a well-marked stem; calyx consisting of an aboral "patina" of two or three circles of plates, and a flexible "tegmen" or oral surface with small plates or none; radial canals supported by long branched arms, which are developed as direct prolongations of the uppermost circle of plates in the patina.
Class II. Thecoidea (Jaekel) = Edrioasteroidea (Bather).—Pelmatozoa without a stalk, fixed to the substratum by the whole aboral surface. The radial canals run out over the oral surface in grooves, which are closed by specially modified plates; but there are no arms of any kind.
Class III. Carpoidea (Jaekel).—Pelmatozoa with a well-developed stalk. The radial canals and their branches are devoid of a skeleton, and either produce no modifications at all on the skeleton of the calyx, or at most are supported by short horn-like outgrowths of some of its plates.
Class IV. Cystoidea.—Pelmatozoa which typically possess a well-developed stalk, a sac-like calyx contracted at the mouth and covered with plates, some of which are pierced with pores or slits; the radial canals, though they may for part of their course run over the surface in grooves, have their terminal portions supported by free unbranched arms ("fingers").
Class V. Blastoidea.—Pelmatozoa provided with a well-developed stalk and ovoid bud-like calyx. From the mouth the radial canals run backwards over the calyx, as in Echinoidea, but they give rise to numerous lateral branches, which are supported by free unbranched arms ("fingers"). Special respiratory organs occur on the interradial areas in the form of parallel folds called "hydrospires."
CLASS I. CRINOIDEA
This is the only class which has living representatives. There are twelve recent genera, of which eight retain the stalk throughout life; the remaining four lose it when adult, retaining only a stump, termed the "centro-dorsal," covered with fixing organs ("cirri"). The stalked forms are confined to considerable depths, and can only be obtained by deep dredging, whereas many of the {581}stalkless forms are comparatively common. We shall select as type for special description the common Feather-star, Antedon rosacea (bifida), which can be dredged in depths of ten fathoms off the south-west coast of England.

Fig. 264.—Oral view of Antedon rosacea. × 3. a, Arm; a.g, ambulacral groove; an, anus; c, calyx; m, mouth; p, pinnules.
The animal consists of a small flattened calyx, from which radiate out ten long delicate arms, each fringed with a double series of short branches called "pinnules." In the centre of the aboral surface can be seen the centre-dorsal plate (Fig. 265, c), a knob-like stump of the broken-off stem, covered with small whip-like outgrowths called "cirri," by means of which the animal is anchored to the substratum (Fig. 265, cir). When Antedon is disturbed it relaxes its hold, and swims by graceful muscular movements of the arms. These are arranged in five pairs, and the corresponding members (right and left) of all the pairs are bent and relaxed together. On coming to rest the animal reattaches itself by means of the cirri. These are composed of cylindrical ossicles joined to one another by muscles, and they can thus act as efficient grasping organs. In the centre of the oral surface, which is termed the "tegmen," and is soft, flexible, and without visible calcifications, is situated the mouth, surrounded by five short triangular flaps called "oral valves." In the intervals between these valves, grooves radiate from the mouth which bifurcate at the points of origin of each pair of arms, and are continued over their surfaces. These grooves correspond to the ambulacral grooves of Asteroidea, and to the epineural canals of the other classes of Eleutherozoa. At each side of each groove {582}are to be found a series of podia in the form of delicate finger-like processes, which serve only for respiration and for producing a current of water, their surfaces, like that of the grooves between them, being covered with powerful cilia. The anus is at the extremity of a little knob called the anal papilla, situated in one of the interradii (Fig. 264, an).
As in Ophiuroidea, the ectoderm cells have disappeared over the whole surface of the body, except the grooves and the podia, the only trace of their former existence being a cuticle with adherent nuclei. Pedicellariae are unknown in all Pelmatozoa; and spines have only been described from one fossil species of Crinoid. Beneath the cuticle is the dermis, having the composition described in the case of Asterias rubens; this on the aboral side of the calyx gives rise to the "patina," consisting of plates, in part movable on one another, in part immovably fused together. Those visible from the outside are (1) the centro-dorsal ossicle, from which the cirri spring; (2) five columns of ossicles termed radials (Fig. 266, R1, R2, R3); each column consists of three radials, extending from the centro-dorsal to the origin of a pair of arms. The uppermost radial in each column bears two facets for the articulation of these arms. Each arm is supported by a series of "brachial ossicles" (Br).

Fig. 265.—View of Antedon rosacea from aboral surface, × 4. c, Centro-dorsal; cir, cirrus; R1, R2, R3, the three radial plates of one column; syz, syzygy.
It is evident, both from the number of ambulacral grooves and of the columns of radials, that Antedon has only five radii, and each pair of arms must be regarded as having arisen by the bifurcation of a primitive arm. This is proved to be true by {583}a study of the development, and it can further be shown that the arms fork repeatedly; but in these further bifurcations one fork remains short, and forms a pinnule, whilst the other continues the arm. Thus the arm, instead of being a single axis, is really a series of axes—in a word, it is a "sympodium."
If in the case of any bifurcation the two forks were to develop equally, the number of arms in that ray would be doubled, and this actually happens in the case of other species of Antedon.
Digestive System.—The mouth leads through a short vertical oesophagus into an enlarged stomach, which lies horizontally curved around the axis of the calyx. The stomach is succeeded by a short intestine, which leads into the anal papilla. Both oesophagus and stomach are ciliated, and the food consists of minute organisms, swept into the mouth by the current produced by the cilia covering the ambulacral grooves and podia; the ten arms may indeed be compared to a net spread out in the water to catch swimming prey.
The water-vascular system consists of a ring closely surrounding the mouth, from which radial canals are given off which underlie the ambulacral grooves and bifurcate with them. The podia have no ampullae, but muscular strands traverse the cavities of the radial canals, and that of the ring-canal, and by their action water can be forced into the podia, which are thus extended. Numerous stone-canals hang down from the ring-canal, and open freely into the coelom; they do not, as in Holothuroidea (where the same arrangement occurs), end in sieve-like madreporites. The tegmen, i.e. the ventral surface of the calyx, is pierced by a number of isolated pores lined by ciliated cells, which suck in water. In the oldest Pelmatozoa there seems to have been a regular madreporite. In the larva of Antedon there is but one pore-canal, which, as in most Eleutherozoa, leads into a special section of the coelom, the "axial sinus," embedded in the body-wall, with which also the single stone-canal communicates; but later the division between the axial sinus and the rest of the coelom breaks down, and then the pore-canals and stone-canals become multiplied independently of each other (Fig. 266, m.p, p.c, and st.c).
Nervous System.—In the young stalked form the nervous system, as in other Echinoderms, consists of a ring round the {584}mouth, from which radial cords are given off which run under the ambulacral grooves (Fig. 266, nerv.rad.v).
The fibres of this nervous system are, as in Asteroidea, immediately beneath the bases of the ectoderm cells. A large band of fibres is given off to each podium, which is covered with minute elevations, each with pointed sense-hairs in the centre. As the animal grows, another nervous system makes its appearance, which is developed from the coelomic wall, the cells of certain tracts of which multiply and bud off ganglion cells from which the fibres grow out.

Fig. 266.—Diagrammatic longitudinal section through one arm and the opposite interradius of Antedon. ax, Central canal of centro-dorsal, with prolongation of genital stolon; B, rosette, consisting of coalesced basals; Br1, Br2, Br3, Br4, the first four brachial ossicles; chamb, chambered organ; coel.coe, coelomic canal of arm; gen.coe, genital canal of arm; gen.r, genital rachis; gen.st, genital stolon; m.p, madreporic pores; musc.long, longitudinal muscle; nerv.rad.d, dorsal radial nerve; nerv.rad.v, ventral radial nerve; p.c, pore-canal; pod, podium; R1-R3, 1st to 3rd radials; st.c, stone-canal; sub.coe, sub-tentacular canal of arm; w.v.r, radial water-vessel.
This "aboral nervous system," as it is called, has its centre in the "chambered organ" (Fig. 266, chamb), which is embedded in the centro-dorsal ossicle, and is roofed over by a plate called the "rosette." This represents the five coalesced "basals," a ring of plates which in other forms alternate with the lowest radials, and it intervenes between these and the centro-dorsal. The chambered organ consists of a ring of five vesicles, which have originated as pouches of the aboral coelom (Fig. 266, chamb). The walls of these vesicles develop nervous matter; from them radiate out five great cords, deeply embedded in the {585}plates of the patina. These cords rapidly fork, and one division of each of two adjacent cords enters the lowest radial. In the third radial all the cords are connected by a commissure which runs completely round the calyx. Each of the cords in the third radial forks again, and one branch of each cord enters each of the two arms connected with it, and the two branches entering an arm coalesce to form a single cord. In the arms, as in the calyx, the cords are deeply embedded in the ossicles, but branches extend to the ventral surface of the arms and here unite to form two longitudinal cords, one on each side of the groove. In the tegmen these cords are connected by an outer nerve-ring, branches from which join the ectodermal nerve-ring already described.
The researches first of W. B. Carpenter[512] and then of Marshall[513] have proved that it is the aboral nervous system which really controls the movements of the animal. If the chambered organ is destroyed by cautery, the whole movements of the animal are paralysed; but it will carry out its characteristic swimming movements just as well if the whole tegmen with the ambulacral nerve-ring and the whole of the alimentary canal are torn away. The commissure in the third radials co-ordinates the movements of the arms. If it is cut they move independently of one another. The position of the radial cords inside the ossicles is gradually acquired. At first they are gutter-like evaginations of the coelom; by upgrowth of their sides the gutters become canals, and are then surrounded by calcified tissue. The cirri have each a cord traversing them which originates from the chambered organ.
Coelom.—In the young stalked form the coelom consists of the water-vascular system ("hydrocoel"), and underlying it an oral coelom, separated from an aboral coelom by a horizontal mesentery. As the animal grows, this horizontal mesentery becomes largely absorbed, and the coelom becomes everywhere traversed by cellular cords (trabeculae), which are later calcified.
Both oral and aboral coelom become, like the hydrocoel, bent into hoops, and along the axis of the aboral coelom a cord of germ-cells is developed, which constitutes the "genital stolon." The chambered organ is developed from the aboral coelom, and {586}in the centre of its five chambers a median pocket grows down into the centro-dorsal, along the side of which is an extension of the genital stolon. In the arms the mesentery separating the extensions of the oral and aboral coelom persists; the oral extension consists of two parallel canals called "subtentacular" (Fig. 267, s.c), whilst the aboral space is termed the "coeliac" canal (Fig. 267, c.c). In the tip of the pinnule, that is to say at the extremity of a ramification of the arm, the coeliac and subtentacular canals communicate. As portions of the lining of both canals are ciliated, a circulation of the coelomic fluid is thus kept up. The genital stolon gives rise at the level of the remnant of the horizontal mesentery in the disc to a circular genital rachis, whence cords pass down the arms in the tissue separating subtentacular and coeliac canals (Fig. 267, g.r). Each cord is contained in a special tube, the "genital canal," which is probably developed in the same way as the aboral sinus of the Eleutherozoa, i.e. as a special sheltering outgrowth of the coelom (Fig. 267, g.c). In the pinnule the rachis swells out into a genital organ, from which a short duct is developed when the organ is mature. The eggs are large (3 mm. in diameter), and adhere for a considerable period of their development to the pinnules.

Fig. 267.—Diagrammatic transverse section of arm of Antedon. To compare it with the section of an Ophiuroid arm it is inverted from its natural position. br, Brachial ossicle; c.c, coeliac canal; c.p, covering plate; g.c, genital canal; g.r, genital rachis; n.r.d, dorsal nerve-cord; n.r.v, ventral nerve-cord; pod, podium; s.c, subtentacular canal; w.v.r, radial water-vessel.
The muscles of Antedon are of two kinds. Those of the water-vascular system are, as in Eleutherozoa, basal outgrowths of the cells forming the walls of the system. The muscles moving the joints of the arms appear to be modifications of connective-tissue cells. When the brachials are isolated their terminal faces, strikingly long, recall those of Ophiuroid vertebrae. There is a ventral groove for the coelomic canal. Above this groove the face is divided by ridges into four areas for attachment of the muscles. Dorsal to this is the pit for the strong ligament which binds the ossicles together; then comes the canal for the aboral nerve-cord, whilst dorsal to this is the pit for what is called the "dorsal elastic ligament." The theory underlying this name is that the muscles bend the arms ventrally, and the ligament by its elasticity restores them to their places; but there seems reason to believe that the "ligament" is really a dorsal muscle. It is particularly to be noted that similar muscles occur between the first and second radials, proving that the primary arm really begins with the first radial. The second and third radials, as also the first two ossicles and certain others of each arm, are closely united by calcified fibres, and this kind of union is called a "syzygy" (Fig. 265, syz). The cirri have all their ossicles united by muscular attachment, and can move rapidly.
The blood system (see pp. 449-451) forms a ring consisting of a network of strings round the oesophagus. This is termed the "labial plexus." From this cords can be traced to the wall of the stomach and to the surface of the genital stolon. The assertion that radial strands intervene between the ectodermic nerve-cord and the radial water-vascular canal, though usually made, does not appear to be justified, since what is termed the vessel appears to be a crevice formed by shrinkage in preservation.
The process of respiration is doubtless largely carried out by the podia, but it must be assisted by the constant instreaming of fresh sea-water through the pore-canals. The process of excretion has not been directly observed in Antedon, but structures called "sacculi" may be connected with this function. These are spherical masses of amoebocytes embedded in the tegmen. During life they are colourless, but after death they become coloured, showing that they secrete a peculiar compound. These sacculi abound in the disc, and a row of them is to be found at each side of the ambulacral groove in the arms. When, as in {588}tropical species, the groove is supported by side-plates, these are notched for the reception of the sacculi.
Turning now to survey the group Crinoidea as a whole, lack of space forces us to confine our attention mainly to the living forms. These differ amongst themselves chiefly in the following points: (1) the condition of the stem; (2) the structure of the calyx; (3) what is intimately connected with this, the method of branching of the arms; and (4) the length of the alimentary canal.
Condition of the Stem.—This is represented by a centro-dorsal stump in Antedon and most of its allies, but in Actinometra it becomes a flat plate, and in some species in old age all the cirri drop off. In Uintacrinus and Marsupites (fossil genera) there is no trace of cirri. In Pentacrinidae there is a long stem, pentagonal in cross-section, in which alternate ossicles carry whorls of cirri; in Rhizocrinidae the stem consists of compressed ossicles, elliptical in section, bearing cirri only at the rooting tip, whilst in Hyocrinus the stem is made up of cylindrical ossicles, cirri being apparently absent. Finally, in Holopus the stem is represented by an uncalcified leathery outgrowth from the calyx.
Skeleton of Calyx and Arm.—In living Crinoidea, with the doubtful exceptions of Holopus and Hyocrinus, the calyx is supposed to be built up originally of four whorls of plates, viz. "infra-basals," "basals," "radials," and "orals," the last named forming the skeleton of the oral valves round the mouth. In the two exceptions named there is no certain evidence of the existence of infra-basals. In living forms the infra-basals coalesce with the uppermost joint of the stem; the basals remain large and conspicuous, though they are fused into a ring in Rhizocrinidae, Atelecrinus, and Thaumatocrinus, whilst in Pentacrinidae this ring is nearly, and in Antedon and its allies completely, hidden when the calyx is viewed from the outside. In Hyocrinus the basals are represented by three ossicles. The lowest radials are an important element in the patina in every case, but the upper radials, the incipient portions of the arms, may be incorporated in the calyx (Pentacrinus, Antedon) or may be free (Rhizocrinidae and Hyocrinus); in Metacrinus there are five to eight radials in each column, all incorporated.
The oral plates are very large in Hyocrinus, Holopus, and {589}Thaumatocrinus, small in Rhizocrinus, vestigial or absent in Bathycrinus, and completely absorbed in Antedon. In addition to these main elements, in many species small accessory plates are developed (a) at the sides of the ambulacral groove, over which they can close down (many species of Antedon, Hyocrinus, Holopus, Rhizocrinidae, some species of Pentacrinidae); these are "covering plates," and correspond in function to the adambulacrals of Asteroidea; (b) supporting the sides of the groove and corresponding to the ambulacrals of Asteroidea;[514] these are "side-plates," and the covering plates articulate with them; (c) on the surface of the tegmen; these are the interradial plates, which in Thaumatocrinus alone among recent forms, but in many fossil forms, are continued into the patina, where they separate the radial plates.
Mode of Branching of the Arms.—All modern Crinoids have pinnules, and this, as has already been explained, is due to a suppressed dichotomy. The extent of the suppression determines the number of arms, which varies within the same genus.
Alimentary Canal.—In Hyocrinus there is no dilatation which could be called a stomach; in Actinometra the mouth is excentric, and the anal papilla occupies the centre of the tegmen. The intestine is elongated, and describes several turns round the papilla before ending in the anus.
The classification of Crinoidea cannot properly be considered without taking account of fossil forms, but to do so at all adequately is impossible on account of limitations of space. Less regret may be felt because the three specialists in this branch, viz. Bather in England, Springer in America, and Jaekel in Germany, come to fundamentally different conclusions on the subject. If we confine our attention to living forms we may, with P. H. Carpenter,[515] select the stem as the basis of classification. As the method of gaining food is the same in all cases, the Crinoidea have probably split on the method of attachment to the substratum. These families—it is impossible, in view of the greater range of variety in fossils, to dignify them with the name of orders—are as follow:—
Fam. 1. Hyocrinidae.—Stem long and persistent; cirri absent; stem ossicles cylindrical—ligaments uniting them not specialised. Arms (five) short, but with extremely long pinnules. Patina composed of long exposed basals and a ring of five spade-shaped radials. Five large persistent orals. Interradials and covering plates present. One species (Hyocrinus bethellianus, Figs. 268, 269) dredged up in the Southern Pacific Ocean.

Fig. 269.—Tegmen of Hyocrinus, viewed from above after removal of the arms, × 8. (From Wyville Thomson.)
Fam. 2. Rhizocrinidae.—Stem long and persistent; cirri confined to a few near the root or replaced by rooting branches of the stem. Stem-ossicles thin and pentagonal at the summit, but lower down compressed, elliptical in section, and united by two ligaments separated by a transverse ridge for articulation. Patina composed of exposed basals and a ring of five short radials. Orals large {591}or vestigial. Covering plates and interradials present. Two genera, both from great depths in the Atlantic—Rhizocrinus (Fig. 270), with five arms and well developed orals (attachment by branching root-cirri); and Bathycrinus, with ten arms and vestigial orals; attachment by root-like branches of stem (this is essentially the same as root-cirri).
Fam. 3. Pentacrinidae.—Stem consisting of ossicles which are pentagonal in section, united in pairs by syzygy, the upper one of each pair bearing a whorl of cirri and united by five bundles of fibres of petal-like section with the lower one of the pair above it. No rooting processes. Patina consists almost entirely of columns of radials, the basals being almost or completely hidden. Orals absent, but side-plates in the ambulacral grooves. Two recent genera, Pentacrinus (Isocrinus), with three radials in each column; Metacrinus, with five to eight radials in a column, but the third {592}radial bears a pinnule. Pentacrinus is found in both the Caribbean Sea and the Pacific Ocean; Metacrinus in the Pacific. It appears that the Pentacrinidae when young are attached by a foot-plate at the apex of the stem; but when adult, the stem is broken in two and the animals, like Antedon, swim by movements of the arms, dragging a large part of the stem after them, by which they effect temporary attachment. As in other stalked forms, the cavities of the chambered organ are prolonged into canals which traverse the stalk; but in this family there is the peculiarity that a repetition of the chambered organ is found opposite every whorl of cirri.
Fam. 4. Holopodidae.—Stem represented by a leathery noncalcified outgrowth from the base of the calyx; one circle of radials indistinguishably fused with the basals and with each other to form the walls of the calyx. Large oral plates, ten short arms. One genus, Holopus, in shallow water in the Caribbean Sea.

Fig. 272.—Arms and portion of stem of Pentacrinus maclearanus, slightly enlarged. In this species the basals can be seen. (From Wyville Thomson.)

Fig. 273.—Calyx of Actinocrinus, one of the Camerata, broken open to show structure. amb, Ambulacral groove enclosed in covering plates; B, basal; R1, R2, R3, the three radials of a column. (After Zittel.).
Fam. 5. Comatulidae.—Stem in the adult broken off, leaving only a stump, the centro-dorsal, covered with cirri. Six genera. Antedon (= Comatula) has already been described; many tropical species have numerous arms and often side-plates and covering plates. Actinometra is distinguished by its excentric mouth, and by the fact that the centro-dorsal is flat and has cirri only round its edges; Atelecrinus has an acorn-shaped centro-dorsal, and the basals are externally visible; Eudiocrinus differs from Antedon only in having five arms; Promachocrinus is a remarkable form, having ten radii (this is a unique feature in Crinoidea); finally, Thaumatocrinus has basals externally visible, large persistent orals and interradial plates, and in addition a short free appendage of several plates on the anal interradius. Antedon and Actinometra are almost world-wide. Six species of the first have been recorded from British waters, of which the commonest is Antedon rosacea; four others are distinguished by having longer cirri, and do not seem to be well defined; but A. eschrichtii, a northern form, is larger, and is distinguished by having long proximal pinnules. The other genera are rare, and occur in deep water.
When we turn to survey fossil Crinoidea, we are met with a bewildering variety of forms ranging from the Lower Cambrian to the present day. As already mentioned, there is no agreement amongst experts as to how they should be classified. Bather makes the fundamental cleavage depend on the possession of two whorls of plates in the base (Fig. 274), or of only one whorl. These two divisions he calls Dicyclica and Monocyclica respectively. He admits that in many forms allied to Dicyclica the infra-basals have disappeared; these he terms "pseudomonocyclic" forms, and believes that he is able to discriminate them from true Monocyclica.

Fig. 274.—Crotalocrinus pulcher. × 1. B, basal; Br, arm-fan of adhering branches; col, ossicle of stem; IB, infra-basal; R, radial. (After Zittel.)
The present author is utterly unable to believe that the Crinoidea diverged into two groups on what is a trifling point of meristic variation comparable to the varying number of rows of plates in the interradial areas of the older Echinoidea; and he is equally sceptical as to the validity of Jaekel's division of the group into Cladocrinoidea and Pentacrinoidea, leading to the view that organs like pinnules represent totally different structures in different groups. Wachsmuth and Springer adopt as bases of classification the extent to which the arms and their branches are incorporated in the disc, and they recognise three main divisions: Inadunata, in which the arms are completely free from the calyx; Articulata, in which the arms are partly incorporated but the tegmen remains flexible; and finally Camerata, in which the arms and their first branches are largely incorporated in the cup; the tegmen is converted into a rigid dome and the ambulacral grooves on it become closed, as does the mouth, by the meeting of overarching folds; the grooves remaining, of course, open in the distal portions of the arms (Fig. 273). This classification, founded as it is on physiological factors, seems to the present author more satisfactory. Speaking generally, the points in which fossil Crinoids may differ from living genera are: (1) the total absence or irregular nature of the branching in the arms, so that pinnules may be said to be absent; (2) the closure of the ambulacral grooves and mouth already alluded to, and (3) the adhesion of the arms in the same ray to produce net-like structures (Crotalocrinus, Fig. 274), or a fan-shaped structure (Petalocrinus); (4) the frequent presence of two rows of brachials in one arm (biserial structure); (5) the {596}development of an enormous anal tube, so large that in extreme cases (Eucalyptocrinus) the arms may be lodged in grooves of it.
CLASS II. THECOIDEA (EDRIOASTEROIDEA, Bather)
These remarkable Pelmatozoa are the most primitive known. They have sac-like or sometimes cushion-shaped or even disc-shaped bodies, covered with numerous irregular plates without any symmetry in their arrangement. There is no stem, but when they are fixed this is effected by an adhesion of the aboral pole. There are no arms, but on the upper surface is to be seen the impression of five ambulacral grooves radiating from a central mouth. These grooves are bordered by covering plates, which in the earliest form (Stromacystis) are seen to be slight modifications of the plates covering the upper surface of the body, but in the later genera (Fig. 275, Thecocystis) become specialised. The anus is situated on the side, as is also the madreporite. It has been suggested that Eleutherozoa were derived from this group; that individuals were occasionally overturned by the waves or currents, and in this way compelled to use their podia for locomotion. When Eleutherozoa, however, have a fixed stage in their development, they are fixed by the oral, not the aboral, surface, and hence can have no close affinity to Thecoidea. Thecoidea begin in the Middle Cambrian, but according to Jaekel impressions in the Lower Cambrian, referred to Medusae, may be casts of this group.
CLASS III. CARPOIDEA
Pelmatozoa with a well-developed stem; body bilaterally compressed; only two rays apparently developed. These are indicated only by grooves radiating from the mouth; but in {597}some cases slight horn-like outgrowths of some of the plates of the calyx may support prolongations of the grooves.
This group, which, like the foregoing, commences in the Cambrian, is perhaps more primitive than the Thecoidea in showing less influence of the water-vascular system on the skeleton; but in the presence of a differentiated stem and the development of only two rays, it is more differentiated. The anus is on one of the flat sides, covered with a flat plate acting as a valve. The members of this group were formerly confounded with Cystoidea, from which they differ in the absence of the characteristic pores. Trochocystis, the genus figured, is devoid of any horn-like outgrowths of the calyx.
CLASS IV. CYSTOIDEA
Pelmatozoa with respiratory organs in the form of "diplopores" or "pore-rhombs." In a great many cases there is a stalk, but in other cases this is atrophied, and the animal is attached by the base of the calyx. The radial canals run for a shorter or longer distance over the calyx, but the plates of the calyx themselves are not modified for them. Either they run in simple grooves, or they are protected by a special series of plates lying above the plates of the calyx. The terminal portions of the radial canals are in all cases free, supported by unbranched arms consisting usually of a double row of ossicles. These arms are termed "fingers."
It will be gathered from the description just given that the fingers and the respiratory organs distinguish Cystoidea from {598}the two foregoing classes. Formerly this class was a lumber-room in which were placed all primitive irregular Pelmatozoa. The labours of Jaekel[516] have, however, dispelled the mist which enveloped this group, and in his monograph all that can be extracted both from superficial examination and dissection of these fossils is contained. It seems possible to the present author that the class may eventually require to be divided into two, corresponding to the two main divisions which Jaekel recognises, viz. Dichoporita, with pectinated rhombs, and Diploporita, with diplopores.

Fig. 277.—Echinosphaerites aurantium. A, from above; B, from the side; C, neighbourhood of mouth, enlarged. amb, Ambulacral groove with side-plates and covering plate; mad, madreporite. The short parallel lines across the sutures are the "pore-rhombs." (After Zittel.)
The pore-rhombs of the Dichoporita (indicated in Fig. 277 by the small parallel lines crossing the boundaries of the plates) were, according to Jaekel, nothing but a series of folds of thin integument projecting into the interior, the outer opening of which in most cases adhered in the middle, leaving two pores connected by a groove. The inner boundaries of the folds are sometimes preserved, but in many cases they were entirely devoid of calcification, and so were lost. The radial vessels either branched a great deal, giving rise to a multitude of fingers, or, as in Echinosphaerites (Fig. 277), there were a few long fingers supporting a reduced number of radial canals. In some cases the calyx can be analysed into a regular series of cycles of plates, consisting of basals, orals, and three intervening whorls, thus including one more ring than the calyx of Crinoidea. Jaekel regards this as a primitive arrangement, believing that the irregularity seen in Echinosphaerites secondary. This is a doubtful hypothesis.
The diplopores of the Diploporita appear to consist of two canals traversing the body-wall, opening close together into a common pit externally, but diverging internally. Since in some cases, as in Aristocystis (Fig. 278), this common pit is proved to have been closed externally by a very delicate layer of calcification, it is probable that the pores represent in other cases the points of origin of finger-like gills similar to those of Asteroidea. Where they were closed by calcification this was so thin and porous that the diffusion through it sufficed for respiration. Jaekel regards the Diploporita as a group derived from Dichoporita, but this seems to be extremely doubtful.

Fig. 278.—Aristocystis. In the upper part of the calyx the heavy dots are "diplopores," seen owing to removal of the superficial layer. (After Zittel.)
CLASS V. BLASTOIDEA
Pelmatozoa with respiratory organs in the form of longitudinal calcified folds, termed "hydrospires," radiating from the mouth. Stem well developed; calyx regular, consisting of a whorl of basals surmounted by a whorl of forked radiais, in the clefts of which lay the recumbent radial water-vascular vessels, supported each on a special plate ("lancet plate"), and giving off two rows of branches supported by short fingers (Fig. 279). Side-plates and covering plates were also developed; five orals ("deltoids") completed the calyx. The anus was at the side, just beneath one of the orals.
The hydrospires, which are the great characteristic of the class, are seen in section in Fig. 279, B (hyd). They consist of a varying number of parallel folds on each side of each "pseudambulacrum," as the lancet plate with its adhering side-plates and covering plates has been termed. In the most primitive genus, Codaster, they appear to have opened directly to the exterior, and to have been placed at right angles to the lines of union of the radial and oral plates, just like the grooves of a pectinated rhomb. In more modified forms, such as Pentremites and Granatocrinus (Fig. 279), the outer openings were overarched {600}by the extension of the side-plates of the radial vessel, and the whole group of folds has a common opening near the mouth; indeed, in the highest form there is one common "spiracle" for the two groups of folds in an interradius, which in one interradius is confluent with the anus. The hydrospires, when they reach this form, irresistibly recall the genital bursae of Ophiuroidea (Fig. 214, p. 490), and very possibly served the same purpose.

Fig. 279.—Granatocrinus norwoodi. A, view of whole animal; B, section of radius; C, an isolated finger. hyd, Hydrospire; l, lancet plate; pinn, finger; p.p, covering plate; R and D both signify radial plate. (After Zittel.)
Reviewing the whole group of the Pelmatozoa, we see that in the Cambrian they begin with the extremely primitive Thecoidea and Carpoidea, together with some obscure forms which, combining a stem with pentamerous symmetry in the calyx, are supposed to be the forerunners of the Crinoidea. In the Lower Silurian or Ordovician the two groups of the Cystoidea make their appearance, possibly independently developed from either Carpoidea or primitive Crinoidea, which in this period are present in unmistakable form. In the Upper Silurian the Blastoidea appear, distinguishable from the most regular Cystoidea only by their hydrospires. It seems practically certain that they were developed from Cystoidea, and we follow Jaekel in believing that they arose from Dichoporita. The Carpoidea do not extend beyond the Ordovician, and by the end of the Carboniferous period Cystoidea and Blastoidea die out, leaving only the Crinoidea, which at that period were at their maximum development. From the Carboniferous to the present day the Crinoidea have continually decreased, leaving in recent seas, as sole representatives of the Pelmatozoa, only the few forms described at the beginning of this chapter.
ECHINODERMATA (CONTINUED): DEVELOPMENT AND PHYLOGENY
In Chapter XVI. it was stated that whilst a more or less perfectly developed radial symmetry was one of the characteristic features of the phylum Echinodermata when in the adult condition, yet in the immature or larval condition the members of the group have a strongly marked bilateral symmetry. In this feature larval Echinodermata resemble the other Phyla of the animal kingdom which have a well-developed coelom, such as Annelida, Mollusca, Vertebrata, etc. Since, then, the peculiar radial symmetry is gradually acquired during the growth of the Echinoderm, we may possibly discover by a close scrutiny of the life-history what is the nature and meaning of this departure from the ordinary type of structure among coelomate animals.
There are two kinds of development met with amongst Echinodermata, which may be roughly characterised as the "embryonic" and the "larval" type respectively, although neither description is exact. In developmental histories of the first type so much reserve material is laid up in the egg in the form of food-yolk that the young animal whilst in the bilateral stage requires little or no food. In some cases, however, as in Amphiura squamata, the mother pours out a nourishing exudation; but whether this is so or not, the parent in nearly every case carries the young about with her until they have reached the adult condition. In some Asteroidea, as for instance in the Antarctic species Asterias spirabilis (Fig. 280), the young become fixed to the everted lips of the mother; in Amphiura squamata, and some other Ophiuroidea the eggs remain in the genital bursae, which serve as nurseries; in some Spatangoidea, {602}as for instance in Hemiaster philippi (Figs. 250, 281), the eggs are carried in some of the deeply grooved petaloid ambulacra; whilst in Holothuroidea they may develop in the body-cavity (Phyllophorus urna), or they may adhere to the back of the mother (Cucumaria crocea, Fig. 259, p. 573), or they may be protected in special brood-pouches either on the ventral side of the parent (Cucumaria laevigata) or on the dorsal surface (Psolus ephippifer, Fig. 261).
The majority of these cases of embryonic development have been recorded from Arctic or Antarctic waters; it appears as if conditions there were not favourable to the larval type of development. In Pelmatozoa the development of Antedon rosacea alone is known, and that is of the embryonic type.

Fig. 280.—Oral view of Asterias spirabilis, slightly enlarged, showing embryos attached to the everted lips. emb, Embryos. (After Perrier.)
So far, however, as their mode of propagation is known, it may confidently be affirmed that the development of the majority of the species of Eleutherozoa is of the second or larval type. In this type there is little food-yolk in the egg, and the young animal or larva is forced from a very early period of development to seek its own living, and hence it is usually a considerable time (from a fortnight to two months) before the adult form is attained. When the embryos of different groups of Eleutherozoa are compared, there is no obvious agreement in structure between them; but the larvae of the four classes of Eleutherozoa exhibit with differences in detail a most remarkable fundamental similarity in type, and we are accordingly justified in regarding the larval development as primitive, and the embryonic type as derived from it and differently modified in each case.

Fig. 281.—Hemiaster philippi. Enlarged view of a single petal, showing the embryos in situ. (From Wyville Thomson.) The whole animal is shown in Fig. 250, p. 555.
In the typical larval development the eggs are fertilised after being laid, and they then undergo segmentation into a number of equal, or nearly equal, segments or "blastomeres." These arrange themselves in the form of a hollow sphere or "blastula," the cavity of which is called the "blastocoel" and afterwards becomes the primary body-cavity of the larva. This cavity contains an albuminous fluid, at the expense of which development appears to be carried on (Fig. 282, B). The cells forming the blastula acquire cilia, and the embryo begins to rotate within the egg-membrane, which it soon bursts, and, rising to the surface of the sea, begins its larval life. The blastula is therefore the first well-marked larval stage, and it is found in a more or less recognisable form in life-histories of members of every large group in the animal kingdom. Only in the case of Echinodermata and of forms still lower in the scale, however, does it appear as a larval stage. The free-swimming blastula stage is reached in from twelve to twenty-four hours. Soon the spherical form of the blastula is lost; one side becomes flattened and thickened, owing to a multiplication of cells, so that they become taller and narrower in shape. Shortly afterwards this thickened plate becomes buckled inwards, encroaching on the cavity of the blastocoel. The larva has now reached the second stage of its development; it has become a "gastrula" (Fig. 282, C). The plate of thickened cells has become {604}converted into a tube called the "archenteron" (Fig. 282, C, arch), which is the rudiment of both the alimentary canal and the coelom of the adult. This tube communicates with the exterior, in virtue of its mode of formation, by a single opening which is called the "blastopore," which becomes the anus of the later larva and adult. Whilst the gastrula stage is being acquired, the blastocoel or primary body-cavity is invaded by wandering cells budded from the wall of the archenteron (Fig. 282, A, B, C, mes). These cells, which are called "mesenchyme," are the formative cells of the skeleton, connective tissue, and wandering cells of the adult. When the larva has a skeleton they are formed very early, arising in the young blastula stage (Ophiuroidea) or in the stage immediately before the formation of the archenteron (Echinoidea, Fig. 282, A, B) and secreting the skeleton. When the larva is devoid of a skeleton (Asteroidea and Holothuroidea), the mesenchyme usually does not appear till the gastrula is fully formed.

Fig. 282.—Echinus esculentus. A, optical section of living blastula. B, section of preserved blastula. The network of strings in the interior is the result of the coagulation of the albuminous fluid. C, section of gastrula. arch, Archenteron; mes, mesenchyme cells, attached by protoplasmic strands to the wall of the embryo. × 150.
The gastrula stage is reached in twenty to thirty-six hours. Then one side of the larva becomes concave, and the cilia become restricted to a thick band surrounding this area. In this way is formed the rudiment of the longitudinal band of cilia, which is the organ of locomotion throughout the larval life. At the apex of the archenteron a thin-walled vesicle is formed, which soon becomes divided off from the rest. This vesicle, which almost immediately divides into two sacs, right and left, is the {605}rudiment of the "coelom" or secondary body-cavity of the larva; the remainder of the archenteron forms the definitive gut, and becomes divided by constriction into an oesophagus, a stomach, and an intestine, and at the same time bent into a shallower or deeper V-shape, the concavity of which is towards the concave side of the body. Within this area of the surface a new funnel-shaped depression makes its appearance. This is the "stomodaeum," the rudiment of the mouth of the larva, and it soon joins the apex of the larval oesophagus; the conjoined tubes henceforth bearing the name oesophagus since the ectodermal and endodermal parts become indistinguishably fused. Along the sides and floor of the oesophagus is formed a V-shaped ridge bearing strong cilia; this is the "adoral band of cilia" which sweeps the food (consisting of Diatoms, Infusoria, etc.) into the mouth. The larva is now known as a Dipleurula and appears in four modifications, each characteristic of a Class of Eleutherozoa. These differ from one another principally in the following points:—(a) The folding of the ciliated band; (b) the divisions of the coelomic sacs; (c) the development and fate of the praeoral lobe (i.e. the part of the body in front of the mouth); (d) the fate of the larval mouth. The types of Dipleurula are as follows:—

Fig. 283.—Bipinnaria of Luidia. a, Anus; a.b, adoral ciliated band; a.c.o.b, anterior median process; a.d.a, anterior dorsal process; a.v.a, prae-oral process; m, mouth; p.c.o.b, median dorsal process; p.d.a, posterior dorsal process; p.l.a, posterior lateral process; p.v.a, post-oral process. (After Garstang.)
(1) The Bipinnaria, the larva of Asteroidea. In this type there is a very long prae-oral lobe. The ciliated band runs along its edges, and is produced into a backwardly directed loop on its under surface. This loop soon becomes separated from the rest of the band as a distinct prae-oral loop, the rest forming a post-oral loop. Both loops are drawn {606}out into short tag-like processes, in which we may distinguish (following Mortensen's[517] notation) in the prae-oral loop an anterior median process (Fig. 283, a.c.o.b), and a pair of prae-oral processes (a.v.a). In the post-oral loop there is a median dorsal process (p.c.o.b) and paired anterior dorsal (a.d.a), posterior dorsal (p.d.a), posterior lateral (p.l.a), and post-oral (p.v.a) processes. At the apex of the prae-oral lobe between prae-oral and post-oral ciliated rings there is an ectodermic thickening, recalling the so-called apical plate of Annelid larvae.

Fig. 284.—A, Ophiopluteus of Ophiothrix fragilis. hy, Hydrocoel; l.p.c, left posterior coelom; oes, oesophagus; r.p.c, right posterior coelom; st, stomach. B, metamorphosis of Ophiopluteus of Ophiura sp. (After Johannes Müller.)
(2) The Ophiopluteus, the larva of the Ophiuroidea. In this type the prae-oral lobe remains small, and the primitive ciliated band is undivided. The processes into which it is drawn out are very long, and are supported by calcareous rods. Of these processes we may distinguish prae-oral, postero-dorsal, postero-lateral, and post-oral. The postero-lateral are always much longer than the rest, so that the larva when swimming appears to the naked eye as a tiny V. In the case of Ophiothrix fragilis (Fig. 284, A) the postero-lateral processes are many times longer than the rest of the body. The Ophiopluteus was the first Echinoderm larva to be recognised. It was discovered by Johannes Müller,[518] who also discovered the other three types of {607}Dipleurula. He named this one Pluteus (easel), from a fancied resemblance, when turned upside down, to a painter's easel. The same name was bestowed on the next type, to which it presents a superficial resemblance, and hence the distinguishing prefix "Ophio-" was added to the original name by Mortensen.
(3) The Echinopluteus, the larva of the Echinoidea. This type is strikingly like the preceding one in possessing a very small prae-oral lobe and in having the processes of the ciliated ring supported by calcareous rods, but a close inspection of these shows that they do not exactly correspond to those of the Ophiopluteus. Thus we have prae-oral, postero-dorsal, and post-oral processes (Fig. 285), but usually no postero-lateral process, and when it does occur it remains short. On the other hand, an antero-lateral process unrepresented in the Ophiopluteus is constantly present, and in its later stage the Echinopluteus develops, out of parts of the ciliated ring, horizontally-placed crescentic ridges of cilia, which are termed ciliated epaulettes (Fig. 285, a.cil.ep). There may even be, as in the larva of Echinus esculentus, a second posterior set of these (Fig. 285, p.cil.ep). In the older larva at the apex of the prae-oral lobe there is an ectodermic thickening, at the base of which are developed nerve-cells and nerve fibres constituting a larval brain (Fig. 285, ap).

Fig. 285.—Dorsal view of larva of Echinus esculentus. × 45. a.cil.ep, Anterior ciliated epaulette; ap, apical plate or larval brain; ech, rudiment of Sea-urchin; l.a.c, left anterior coelom; l.oes, larval oesophagus; l.p.c, r.p.c, as in Fig. 284; p.cil.ep, posterior ciliated epaulette; r.a.c, right anterior coelom.
(4) The Auricularia, the larva of the Holothuroidea. This type strikingly resembles the Bipinnaria in its external features. The prae-oral lobe is well developed, and has on its under surface a backwardly projecting loop of the ciliated band, which is not, {608}however, as in the Bipinnaria, separated from the rest of the band. The processes of the band are much more faintly marked than in the Bipinnaria, the anterior median, prae-oral, and median dorsal processes being absent; but a pair of intermediate dorsal processes are developed in the interspace between anterior and posterior dorsal.

Fig. 286.—Three views of metamorphosis of Auricularia of Synapta digitata. A, fully grown Auricularia; B and C, stages in the metamorphosis. hy, Hydrocoel; Int, intestine; l.p.c, left posterior coelom; O, fragments of ciliated band which are invaginated into the stomodaeum, and coalesce to form a ring round the mouth; oss, ossicle; pod, rudiment of feelers which here spring directly from the hydrocoel; r.p.c, right posterior coelom; st, stomach; w.v.r, rudiment of water-vascular radial canals; 1-5, corresponding pieces in the three figures of the longitudinal ciliated band. (After Bury.) × 40.
In the Bipinnaria, Ophiopluteus, and Echinopluteus the coelomic vesicle, after separation from the archenteron, divides into right and left halves. The left then sends out a short dorsal process, which, fusing with the ectoderm, acquires an opening to the exterior. This opening is the primary madreporic pore, and the process of the left coelomic sac, which is ciliated, is the pore-canal. In the Auricularia the pore and pore-canal are formed before the division of the coelom. In the Bipinnaria the right and left sacs subsequently fuse in the front part of the prae-oral lobe. In the first three types of larva the coelomic sac on each side then undergoes a segmentation into anterior and posterior portions. At the hinder end of the anterior sac on each side a swelling occurs. That on the left side is the "hydrocoel," or rudiment of the water-vascular system (Fig. 287, A3, l.hy); it {609}quickly assumes a crescentic form, and gives off five blunt outgrowths, which are the rudiments of the radial canals, and the terminal tentacles. It remains in connexion with the anterior coelom by a narrow neck, which later becomes the stone-canal. That on the right side separates completely from the right anterior coelom; it remains small, and forms the madreporic vesicle (Fig. 287, A3, r.hy) of the adult. In the Ophiopluteus and in the larva of Asterina gibbosa (v. infra) it occasionally takes on a form similar to that of the hydrocoel; from which circumstance, as well as from the similarity in its mode of origin, it is here regarded as a right hydrocoel, i.e. a rudimentary fellow of the organ which develops into the water-vascular system.

Fig. 287.—Diagrams of the mode of formation and division of the coelom in Echinodermata. a.c, Anterior coelom; coe, primitive coelomic rudiment; int, intestine; l.a.c, left anterior coelom; l.hy, left hydrocoel; l.p.c, left posterior coelom; oes, oesophagus; p.c, posterior coelom; r.a.c, right anterior coelom; r.hy, right hydrocoel; r.p.c, right posterior coelom; st, stomach; stom, stomodaeum.
In Auricularia (Fig. 287, D) the coelomic vesicle, after the pore-canal is formed, divides into an anterior and a posterior half. The posterior part then divides into right and left halves, {610}whilst the anterior sac divides into dorsal and ventral halves, connected by a narrow neck. The ventral half soon assumes the familiar feature of the hydrocoel (Fig. 287, D3, l.hy), whilst the dorsal half forms an insignificant swelling on the course of the conjoined stone- and pore-canals, which represents the left anterior coelom of the other types; neither right anterior coelom nor right hydrocoel being developed. The neck of communication between dorsal and ventral halves is, of course, the stone-canal.
The Dipleurula larva leads a free-swimming life for a period varying from two weeks to two months, and then undergoes metamorphosis into the adult form. The details of this process have been worked out in comparatively few cases; and the species in which they are most thoroughly known is the Asteroid Asterina gibbosa. The development of this species is intermediate in character between the embryonic and larval types. The eggs are larger than is usual among Asteroidea, and are filled with a bright orange yolk. The larva differs from the Bipinnaria in the absence of the characteristic ciliated bands and in the very early occlusion of the anus. There is, however, a band of cilia round the edge of the prae-oral lobe, which corresponds to portions of the prae-oral and post-oral bands combined of the Bipinnaria.[519]
The larva has a form which may be described as boot-shaped (Figs. 288, 289). The sole of the boot is the great prae-oral lobe, behind which is the mouth. The larva takes little or no food, and completes its metamorphosis in ten to twelve days. It does not swim at the surface, but creeps slowly over the bottom by the aid of the ciliated band mentioned above, while it can also attach itself, using the edges of the prae-oral lobe as a sucker.
After leading an existence of this kind for seven or eight days it fixes itself permanently by a disc-like prominence, which appears on the anterior surface of the prae-oral lobe within the area surrounded by the thickened rim which, as explained above, forms a margin to the prae-oral lobe. The larva then becomes divided by a constriction into a disc and a stalk, and the former is gradually converted into the body of the young Starfish, whilst {611}the latter continually diminishes in size, and eventually entirely disappears, when the young Starfish commences to walk about on its podia. The disc becomes bent downwards and to the left, so as to make nearly a right angle with the stalk, and the last vestige of the latter springs from the peristome of the Starfish inside the water-vascular ring (Figs. 289, B, C).

Fig. 288.—Fully grown larval stages of Asterina gibbosa. A, fully grown larva; B, left, and C, right view of a larva seven days old in the beginning of the metamorphosis. m, Mouth; 1-5, the five lobes of the hydrocoel; I.-V., the rudiments of the arms. (After Ludwig.) × 45.
The form of the Starfish is attained principally by the preponderant growth of the left hydrocoel and of the left posterior coelom. Both these sacs take on the form of hoops, which, by the meeting of their ends, are converted into rings. The hydrocoel has already grown out into five lobes, which are the rudiments of the radial water-vascular canals, and the tips of which become the terminal sensory tentacles (Figs. 288, 289, 1-5); but now the left posterior coelom grows out into five lobes also, forming a parallel but outer ring. These lobes (Figs. 288, 289, I.-V.) are the rudiments of the arms, which are at first quite independent of those of the radial canals, but gradually, when the larva has attained the age of nine days (Fig. 289, B, C), they become applied to the outgrowths of the hydrocoel. These by this time have developed each two pairs of branches, the rudiments of the first two pairs of tube-feet in each radius. The larval mouth vanishes, and a new mouth is formed on the left side in the centre of the hydrocoel ring, when the metamorphosis is complete. The adult anus is {612}formed about the same time. The primary pore-canal in Asterina as in all Dipleurulae opens into the anterior coelom; the stone-canal is formed from a ciliated groove running along the neck of communication between this and the hydrocoel. The constriction dividing the body into disc and stalk divides the anterior coelom (single in Asterina as in the older Bipinnaria) into two parts; the portion included in the disc forms the axial sinus of the adult. The lower end of the axial sinus expands and surrounds the adult mouth, forming the inner perihaemal ring; the outer perihaemal ring is formed by the juxtaposition of four wedge-shaped outgrowths from the left posterior coelom and one from the anterior coelom. From these the radial perihaemal canals subsequently grow out into the arms. The metamorphosis of Bipinnaria has been well worked out by Goto,[520] and it agrees in essential features with that of Asterina gibbosa; in fact, the differences which Goto maintains between the two types may be reasonably explained on the supposition of some stages having escaped the notice of this observer. The larva develops on the apex of the prae-oral lobe three papillae for occasional attachment,[521] and in the centre of these a cup-shaped disc for permanent fixation when the prae-oral lobe is converted into a stalk. When these papillae (Fig. 290, fix) have been developed the larva is known as a Brachiolaria.

Fig. 289.—Views of larvae of Asterina gibbosa in the course of metamorphosis. A, larva of eight days, from the right; B, left, and C, right view of larva of nine days; 1-5, lobes of hydrocoel; I-V, rudiments of arms. (After Ludwig.) × 45.

Fig. 290.—Brachiolaria fixing itself, × 60. Ast, rudiment of the body of the Starfish; fix, fixing processes. (After Johannes Müller.)
The metamorphoses of the other types of Dipleurula contain no fixed stage. They are what might be called "cataclysmal metamorphoses." That is to say, the outer form and habits of the larva are preserved till the last moment, whilst the organs of the adult are being gradually perfected; then in an hour or two all trace of larval structures disappears. The Ophiopluteus preserves the larval mouth, round which the hydrocoel grows; the long lateral ciliated processes are preserved till the animal has attained all the adult characters. Before this, however, it passes through what may be called an "Asteroid" stage in development, in which the ambulacral grooves are open. The Echinopluteus loses both larval mouth and anus. It develops the adult organs on the floor of a sac-like invagination of the ectoderm, situated on the left side within a loop of the ciliated band (Fig. 291, B, C). This invagination becomes completely closed. It is termed the "amniotic cavity," and its roof is termed the "amnion." On its floor are developed the primary tentacles, terminating the radial canals, as well as a number of spines. After taking on a creeping life and losing its larval appendages, the young Sea-urchin passes through an "Asteroid" condition, in which the arched dorsal surface, the future periproct, is greater in extent than the ventral, and the radial canals run horizontally out from the water-vascular ring and terminate in free movable podia (Fig. 291, C and D, pod), ending in suckers, in the centre of which are pointed sense-organs. These {614}podia become later enclosed in grooves in the corona, and are reduced to vestiges in the adult.

Fig. 291.—Four views of Echinopluteus from the left side, to show the metamorphosis. A, B, and C are taken from the development of Echinus miliaris. D is a young Echinus esculentus. The rudiment of the oral disc of the Echinus is seen beginning in B and larger in C. ad.stom, Adult stomodaeum; cil.ad, adoral ciliated band; cil.ep, ciliated epaulette; coe, coelom; d.sp, prismatic spine of dorsal surface (periproct) of adult; ech, rudiment of Echinus; int, intestine; l.oes, larval oesophagus; mp, madreporic pore; nerve circ, nerve-ring of adult; pod, first paired tube-feet; st, stomach; t, terminal tentacle of the radial band; v.sp, pointed ventral spine of adult. A, B, and D magnified 45 diameters; C, 60 diameters.
The Auricularia is the only type of Dipleurula in which larval mouth and anus are retained. For this reason it has been supposed that its median plane of symmetry remains the median plane of the adult. The researches of Bury[522] have shown that this is not so. As in other types of Dipleurula (with the possible exception of the Ophiopluteus) the adult position of the {615}mouth is on the left side of the larva, and in the commencement of the metamorphosis the mouth migrates into this position (Fig. 286, C). Then the rudimentary prae-oral lobe is rapidly absorbed, so that the mouth again acquires a terminal position. The hydrocoel (Fig. 286, A, hy) has by this time completely encircled the oesophagus, and from it grow out the radial canals which bud off the feelers[523] (buccal tentacles) into the larval stomodaeum. This, although it later flattens out to form the adult peristome, forms in these stages an almost closed sac, reminding us of the amniotic cavity in the Echinopluteus. The ciliated band breaks up into a number of pieces, which rearrange themselves so as to form a series of transverse rings of cilia; so that the free-swimming life can be carried on somewhat longer. The animal in this stage is called a "pupa" (Fig. 292); it eventually loses the rings, drops to the bottom, and develops tube-feet. From specimens which the author has seen, he has little doubt that in some cases the young animal passes through an "Echinoid" stage, for it possesses, besides the feelers, only median tube-feet, terminating the radial canals, and it is covered by a cuirass of plates, which recalls the Echinoid corona.[524]

Fig. 292.—"Pupa" of Synapta digitata. × 50. circ.cil, Ciliated rings; oss, calcareous ossicle; ot, otocysts; pod, feelers; w.v.r, radial water-vessel. (After Semon.)
Reviewing the development of the Eleutherozoa in the light of the facts so far presented, and using the same method of reasoning which is employed in the case of other groups of animals, we seem to be justified in concluding that the Echinodermata are descended from a simple free-swimming ancestor possessing the fundamental characters of the Dipleurula. These would include a longitudinal folded band of cilia as the principal organ of locomotion; a thickened plate of nervous epithelium at {616}the anterior end serving as combined sense-organ and brain; a V-shaped band of cilia projecting into the oesophagus as the organ of nutrition; a wide, shallow stomodaeum and an alimentary canal consisting of three well-marked divisions, viz. oesophagus, stomach, and intestine; and finally a secondary body-cavity or coelom, consisting of three divisions on each side, though possibly the most anterior pair were confluent in the prae-oral lobe. On the left side the anterior coelom opened to the exterior by a short ciliated canal. To the hypothetical group so defined which were certainly not Echinodermata the name Protocoelomata may be given.

Fig. 293.—Tornaria larva. a, Anus; a.c, anterior coelom; a.p, apical plate; g.s, rudiments of gill-sacs; m, mouth; m.c, middle or "collar" coelom; p, posterior ciliated band; p.c, posterior coelom; pr, longitudinal ciliated band. (After Morgan.)
Now amongst the lowest types of animal in which traces of Vertebrate structure can be detected, there is one group, the Hemichordata (Vol. VII. p. 3), in which there is a larva which strikingly recalls the Dipleurula. This larval form belongs to Balanoglossus and is called the Tornaria. It possesses a well-marked prae-oral lobe and a folded longitudinal ciliated band, which resembles that of Auricularia. Its peculiarity is that in addition there is a posterior ring of cilia (Fig. 293, p). The coelom is in five divisions:—a median anterior sac (a.c) opening to the exterior by a short ciliated canal on the left side; and paired middle divisions (m.c) and posterior divisions (p.c). At the apex of the prae-oral lobe there is a plate consisting of sensory epithelium, with nerve-fibres at its base, which acts as a brain. Tornaria undergoes metamorphosis, assumes a worm-like form, and takes on a burrowing life. The five divisions of {617}the coelom are retained, and it can be proved that the pore-canal, like the madreporite of Echinodermata, is used for taking in water. Further, there are two aberrant sessile members of the group (Cephalodiscus and Rhabdopleura), in which the middle divisions of the coelom which would correspond to the hydrocoels are produced into long arms, each with a double row of ciliated tentacles, which strikingly recall the radial canals and podia of the Pelmatozoa. Taking all these facts into consideration, it seems probable that Vertebrata and Echinodermata both arose from Protocoelomata.
When we turn to the developmental history of Echinodermata for light on the question as to how the bilaterally symmetrical ancestor became converted into the radially symmetrical Echinoderm, it seems probable that only in the development of the Asteroidea can we hope to find the solution of the problem. The abrupt changes of habits shown in the metamorphoses of the other types are clearly secondary phenomena. No species of animal could suddenly change its habits from swimming by means of cilia to walking with tube-feet. In the development, however, of Asterina gibbosa we get a hint of the way in which a free-swimming life could alternate with periods of temporary fixation, gradually passing into a condition in which the fixation was permanent. This period in the history of the race when ancestral Echinodermata were sessile would mark the point at which Eleutherozoa diverged from Pelmatozoa, and the former existence of a fixed ancestor explains the tendency first to asymmetry and later to radial symmetry. Bilateral symmetry is characteristic of most free-swimming animals which have to pursue a straight course through the water, but in fixed forms no disadvantage arises from want of symmetry. A radial disposition of organs is, however, valuable to them, since food must be sought and danger avoided from all points of the compass; and hence we can understand, when fixation became permanent, how one hydrocoel could grow larger than the other, and finally assume the form of a ring.
The last question which arises is the vexed one of the mutual relationships of the various Classes constituting the Phylum. Before attempting to seek for light on this problem from development, it will be necessary to sketch the life-history of Antedon rosacea, the only Pelmatozoon whose development is known.

Fig. 294.—Three views of the development of Antedon rosacea. A, free-swimming larva; B, longitudinal section of free-swimming larva; C, oral view of young fixed form. a.c, Anterior coelom; amb, ambulacral groove; ap, apical plate of sensory and nervous tissue; cil, ciliated ring; hy, hydrocoel; l.p.c, left posterior coelom; mad, primary pore-canal; pod, podia; r.p.c, right posterior coelom; stom, larval stomodaeum. (A and B after Bury; C after Perrier.)
The eggs are comparatively large and full of food-yolk, and they adhere for a considerable period to the pinnules. They pass through a large portion of the development within the egg-membrane. The blastula and gastrula are formed in the usual way, but the formation of the coelom is most remarkable (Fig. 287, E1, E2). The archenteron divides into anterior and posterior divisions. The posterior divides into right and left, posterior coelomic sacs, but before the division is complete a dorsal and a ventral tongue grow out from the anterior division and unite posteriorly, encircling the band of connexion between right and left posterior coelomic sacs like a ring. This band of connexion becomes solid and is absorbed, and pari passu the ring becomes converted, by the disappearance of its central opening, into a sac, which is the definitive gut (Fig. 287, E). The rest of the {619}anterior division divides into a thick-walled sac, the hydrocoel, on the left, and a median thin-walled anterior coelom, which sends a long extension into the anterior portion of the larva, which we may compare to the prae-oral lobe of the Bipinnaria. The anterior coelom communicates with the exterior by a short pore-canal, and later forms a connexion, the stone-canal, with the hydrocoel. At the apex of the prae-oral lobe there is formed a thickened patch of ectoderm, bearing stiff sensory hairs, and having at their bases nerve-fibres and ganglion cells. This larval brain corresponds to that of the Tornaria and Echinopluteus. Behind the brain there is a glandular pit, which is used for fixation, and recalls the similar organ in the Bipinnaria. A series of ciliated rings is then formed, and between the second and third of them an oval depression appears. This is the stomodaeum; but as the larva takes no food it does not communicate with the gut (Fig. 295, A, stom).
The larva next escapes from the egg-membrane and swims freely for a day or two, and then, like the Bipinnaria, fixes itself by the apex of the prae-oral lobe, which is converted into a stalk. The larval stomodaeum closes, and the oesophagus of the adult appears as a solid peg of cells abutting against it; round this peg the hydrocoel grows like a ring.
The closed stomodaeum and the underlying hydrocoel are now rotated backwards until they come to be at the end of the animal opposite the stalk (Fig. 295, C). The left posterior coelom, which has also, as in the Asteroid larva, assumed a hoop-like form, is carried along with them; but the right posterior coelom becomes shifted forwards and sends out five outgrowths into the stalk, which form the rudiments of the chambered organ, and a central one as a continuation of the genital stolon (Fig. 295, D, gen.st), the extension of the anterior coelom (Fig. 294, B) having disappeared.
Then the outer wall of the stomodaeum splits into five valves—the future oral valves. The radial canals appear as freely projecting tentacles, which issue in the intervals of these valves and soon acquire two pairs of lateral branches. The skeleton consists of five oral plates in the oral valves, of a ring of five basals, of three small under-basals, and of a series of "columnals," i.e. stem-ossicles, as rings embracing the stalk. The area of attachment is supported by a "foot-plate." The radial {620}plates next appear as a ring of small ossicles between the orals and basals, and simultaneously the arms make their appearance as five outgrowths supported by the first radials, and by the other radials when these appear. The free radial canals now become adherent to the arms, but these canals soon give off paired branches of unlimited growth, which are supported by bifurcations of the primitive arms, and in this way the ten arms of the adult are established. So far, then, as the water-vascular system is concerned, the apparent forking is not a true dichotomy, but results from the production of two opposite branches, whilst the main axis ceases to grow. The appearance of cirri marks the fusion of the uppermost stem-ossicles to form a centro-dorsal, and shortly afterwards the young Antedon snaps off its stem and swims away.

Fig. 295.—Four diagrams to explain the metamorphosis of the larva of Antedon rosacea. a.c, Anterior coelom; gen.st, genital stolon; l.hy, left hydrocoel; l.pc, left posterior coelom; mp, madreporic pore; r.p.c. right posterior coelom; stom, stomodaeum. (A, B, and C after Korschelt and Heider; D after Perrier.)
Now in reviewing this life-history we cannot fail to be struck with resemblances to the development of Asteroidea, and especially to that of Asterina gibbosa. The absence of a connexion between stomodaeum and gut is due to the embryonic mode of life. On the other hand, the presence of a long prae-oral lobe, containing an extension of the anterior coelom and having a fixing organ at its apex, can only be paralleled among Asteroidea. In broad outlines, then, up to the period of fixation the two developments are parallel, but after this point a {621}divergence takes place, which points clearly to the splitting of the Echinoderm stem into two main branches, corresponding with two different sets of habits. In the Eleutherozoan stock, represented by the development of the Asteroidea, the disc became flexed ventrally on the stalk, so that the mouth and podia were brought within reach of material drifting along the bottom, which the podia were employed to seize. As a consequence the base of the stalk was brought near the mouth, and so it came about that the hydrocoel, when it became a ring, encircled both. In the Pelmatozoan stock, on the other hand, the podia and mouth are rotated upwards and backwards from the stalk, which thus came to have an aboral position (Fig. 296, B). The podia are thus placed in a favourable position for capturing free-swimming organisms, which their cilia sweep toward them. It is worthy of note that a similar change of position of the mouth occurs in other groups of animals which have similar habits (Polyzoa Entoprocta, Tunicata).

Fig. 296—Figures to show the supposed connexion of Eleutherozoa and Pelmatozoa. A, common fixed ancestor of the two stocks, still bilaterally symmetrical; B, primitive Pelmatozoon; C, primitive Eleutherozoon. a, Anus; a.c, anterior coelom; l.p.c, l.p.c1, left posterior coelom; m, mouth; p, primary pore-canal; r.p.c, right posterior coelom.
The division therefore of the phylum must have occurred at an extremely remote epoch, before the hydrocoel was a closed ring, and before, therefore, radial symmetry was completely attained.
Turning now to the question of the origin of the classes of Eleutherozoa, we find that the study of development strongly reinforces the views gained from the study of adult anatomy. The Asteroidea are the most primitive group; only in their case {622}is the fixed stage retained, and both Ophiuroidea and Echinoidea pass through an Asteroid stage in development. The only serious competitors for the position are the Holothuroidea, which many have imagined to have been directly derived from Cystoidea (in the old sense; better Thecoidea). This view, though adopted by Semon,[525] Haeckel,[526] and Bather,[527] is open to many objections. The type of Holothuroid development referred to in these discussions is that of the extremely aberrant Synapta digitata, in which the radial canals are vestigial structures which disappear in the adult. In this species, where the feelers are multiplied, some originate in the larva directly from the water-vascular ring, and thus alternate with the canals. From this circumstance Semon drew the conclusion that the radial canals of Holothuroidea are not homologous with those of other Echinoderms, but this conclusion is contradicted by the development of more normal species, in which all the feelers spring from the radial canals. The meridional course of these canals, the closure of the ambulacral grooves, coupled with the retention of a nervous ectoderm, are all features found in Echinoidea. So is also a reduction in the number of the genital organs, on which Bell[528] laid such stress that he separated Holothuroidea from all other Echinoderms. But if in Spatangoidea a reduction to four and even three can take place (Fig. 246, p. 552), why should a reduction to two or one excite surprise? The primitive outer appearance of the Auricularia is counterbalanced by the development of the coelom, which is much modified, so that the primitive bilateral arrangement is obscured. If, then, Asteroidea are the most primitive Eleutherozoa, we may imagine that primitive Echinoidea were derived from Asteroidea through adaptation to life in crevices, where an upward bending of the radii was of advantage, in order to enable the animal to attach its podia above as well as below itself; and that Holothuroidea arose from primitive Echinoidea in which the plates of the corona were still movable, through a further adaptation to narrow crevices, where worm-like wriggling would be the most successful method of adapting themselves to their environment.
The final result, then, of all our inquiries leads us to a view of the mutual affinities of the classes of Echinoderms, which may be indicated in the following table:—
We shall hazard the prophecy that if ever pre-Cambrian Echinoderms are found, there will be amongst them small stalked forms which may be superficially classed with "Cystids," but which are in reality the fixed ancestors of Asteroidea. They should have an irregular skeleton, and be devoid of arms, which are secondary formations; but they should indicate, by the proximity of the mouth to the stalk and by the relation to the stalk of the grooves for the podia, that they have diverged from the Pelmatozoan stock, and are the ancestors of Eleutherozoa.
Every reference is to the page: words in italics are names of genera or species; figures in italics indicate that the reference relates to systematic position; figures in thick type refer to an illustration; f. = and in following page or pages; n. = note.
Abbott, 422
Abdomen (= third chamber of Monaxonic Radiolarian shell), 84
Abdominal cirrhi, 139 f.
Aboral, blood-ring, of Asterias rubens, 452;
of Ophiothrix fragilis, 490;
of Echinus esculentus, 529;
coelom, of Antedon rosacea, 585;
nervous system, of Antedon rosacea, 584;
sinus, of Asterias rubens, 449;
of Ophiothrix fragilis, 490;
of Echinus esculentus, 528, 529
Abortive nuclei, of Myxosporidiaceae, 107
Abyla, 307
Abylinae, 307
Abyssal, Radiolaria, 76;
Sponges, 239 f.
Acamptogorgia, 356
Acanella, 353;
A. simplex, 353
Acantharia (Actipylaea), 76, 78, 78, 80;
myophrisks in, 80;
Zooxanthella in, 80;
reproduction of, 86 n.;
skeleton of, 82
Acanthocephala, nutrition of, 38
Acanthoconia barrandei, 207, 207
Acanthocystis, 71;
budding of, 73;
habitat, 75
Acanthogorgia, 355
Acanthometrids (= Acantharia, q.v.), parasitic Amoebophrya in, 161
Acanthonida, 78
Acanthotrochus, 577
Acarnus, 223
Accumulative anabolism, growth, 9, 13, 15 f.;
in relation to brood-formation, 32 f.
Acephalinidae, 97
Acinetina = Suctoria, 158 f.
Acontia, 369
Acrasieae, 90 f.
Actine, 183
Actinelida, 78
Actinia, 381;
A. cari, 378;
A. equina, sense organ of larva of, 373;
A. mesembryanthemum, 379, 381;
longevity of, 375
Actiniaria, 377 f.;
viviparity of, 373;
symbiosis of, 377;
colours of, 379
Actiniidae, 381
Actiniina, 380
Actinobolus, 137;
tentacles and trichocysts of, 152
Actinocrinus, 594
Actinodendron, 384;
A. arboreum, 384;
A. glomeratum, 366;
Actinoloba (= Metridium), 371, 372, 381;
A. marginata, 378
Actinolophus, 70;
habitat of, 75
Actinomma, 77;
A. asteracanthion, skeleton of, 77
Actinomyxidiaceae, 98
A. sol, 71;
habitat of, 75
Actinopoda, 570
regeneration of, 35;
A. eichornii, 72
Actinostola (usually placed in Hertwig's family Paractidae, allied to Bunodidae, 382), 379
A. mussoides, 383
Actinozoa = Anthozoa, 326 f.
Actipylaea (= Acantharia), 76
active zygote of, 34
Adambulacral, ossicle, of Asterias rubens, 434;
of Ophiothrix fragilis, 479;
of Asteroidea compared to covering-plates of Crinoidea, 589;
spine, of Asterias rubens, 434
Adamsia, 377;
A. rondeletii, 378
Adaptation to fresh water, 176 f.
Adelea ovata, syngamy of, 101
Adelocodonic, 265
Adhesion of species, 174 n.
Adinidae, 130 n.
Adoral, band of cilia in Dipleurula larva, 605;
membranellae, 145;
trichocysts, 145;
wreath, 137 f.;
wreath of Vorticella, 156, 157
Aegina, 296
Aeginidae, 296
Aeginopsis, 296
Aeolis, nematocysts of, 248
Aequorea, 278
Aequoreidae, 278
Aerotaxy, 23
Aethalium septicum (= Fuligo varians), 92 f.
Afferent canal of contractile vacuole, of Stylonychia, 139, 140;
of Stentor, 156
Africa, Tropical, antimalarial measures in, 106;
Trypanosomic diseases of, 120 f.
Agalminae, 307
Agaricia, 403
Agaricoides, 350
on classification of Endocyclica, 533;
of Clypeastroidea, 548
Aggregate, formation, in Microgromia, 59;
social, of Vorticella, 158;
(rosette) of Treponema, 120
Aggregation of plasmodia into aethalium, 92 f.
Agitation, stimulus of, 19 f.
Aglaophenopsis (Plumulariidae, 279), 277
Aglaura, 294
Aglauridae, 294
Aiptasia couchii, 382
Air, presence of Flagellate spores in dust of, 118
Air-borne germs in relation to putrefaction, etc., 43
Alate, 185
Alcock, 268
Alcyonacea, 346 f.
colour, 337;
dimorphism, 333;
food, 339;
mesenteric filaments, 333;
nematocysts, 247;
phosphorescence, 338;
reproduction, 340;
skeleton, 334;
spicules, 334;
zooids, 330
Alcyoniidae, 349
nematocyst of, 247;
A. digitatum, 332, 338 f., 347, 349;
larva, 341;
A. glomeratum, 349;
A. palmatum, 340;
A. purpureum, 338
Aleurone, 37
Algae, related to holophytic colonial Flagellata, 109, 130
Algeria, dourine disease in, 119
Alicia mirabilis, 382
Aliciidae, 382
Alimentary canal, of Asterias rubens, 438;
of Ophiothrix fragilis, 485;
of Echinus esculentus, 516;
of Echinarachnius parma, 546 f.;
of Echinocardium cordatum, 551;
of Antedon rosacea, 583;
of Hyocrinus, 589;
of Actinometra, 589;
development of, in Eleutherozoa, 604, 605;
Allen, on food of Echinus esculentus in Plymouth Sound, 516
Allman, 246, 265, 267, 273, 274;
on Cystoflagellates, 135
Allogromidiaceae, 58;
Allopora, 287;
A. nobilis, 287
Alpheus, 351
Alternating modes of brood-formation in Sporozoa, 48
Alveolar, structure (fine) of cytoplasm, 6;
system (coarse) in relation to skeleton of Radiolaria, Dreyer's scheme of, 84
Alveolate ectoplasm, of pelagic Foraminifera, 61;
of Heliozoa, 71 f.;
of Actinosphaerium, 72 f.;
of Radiolaria, 79;
pedicellaria of Leptogonaster, 456
Alveole (= minute cavity in cytoplasm), 5 f.;
in Ciliata, 142;
in Stylonychia, 140;
(= large vacuole of Radiolaria, etc.), 76, 79, 84
Alveolina, 59
Alveolus, of Echinus esculentus, 526
Alveopora, 397
Amalthea (Fam. Corymorphidae, 273), 266
Ambulacral area, of Echinarachnius parma, 544;
of Echinocardium cordatum, 550
Ambulacral groove, of Asteroidea, 432;
representative in Ophiuroidea, 481;
representative in Echinoidea, 515;
of Pelmatozoa, 579;
of Antedon rosacea, 581, 582, 587;
of fossil Crinoidea, 595;
of Thecoidea, 596;
of Carpoidea, 596;
of young Ophiuroid, 613
Ambulacral ossicle, of Asteroidea, 432;
of Asterias rubens, 434;
of Ophiothrix fragilis, 481;
compared to auricula of Echinus esculentus, 526;
to inner plates of Palaeodiscus, 557;
to side-plates of Crinoidea, 589
Ambulacral plate, of Echinus esculentus, 511;
of Cidaridae, 533;
of Sphaerechinus, 539;
of Strongylocentrotus, 539;
of Echinarachnius parma, 544
Amines, 15
Ammodiscus, 59
Amnion, 613
Amniotic cavity, 613
Amoeba, 4 f.;
reactions of, 7 f.;
devouring
a plant cell, 9;
excretion of, 14 f.;
motion of, 17 f.;
respiration of, 17 f.;
taxies of, 22
Amoeba, 51;
habitat of, 57;
posterior disc or sucker, 53;
A. coli, habitat, 57;
A. limax, 5;
form of amoebulae of Acrasieae, 90;
A. polypodia in fission, 10;
A. proteus, 5;
brood-divisions of, 56 n.
Amoeboid, motion, 5;
stages of Acystosporidae, 97, 103 f.;
zoospores of Trichosphaerium, 54, 56;
gametes of Flagellata, 116 n.
of Didymium, 92;
of fever parasites, 104
Amphiaster (an aster in which the actines form a whorl at each extremity of the axis, which is straight), 222
Amphibia, hosts of Opalinidae, 111, 123
Amphiblastula, 226
Amphicaryoninae, 306
Amphidiscophora, 203 f.
Amphihelia, 399
Amphileptus, 137
Amphimonadidae, 111
Amphimonas, 111
Amphinema, 273
Amphiprion percula, 378
Amphiura, 497;
A. squamata (= elegans), 485 n., 497, 601
Amphiuridae, 497
Amphizonella, 51;
test of, 53
Amphoriscidae, 192
Ampulla, of Millepora, 259, 260;
of tube-foot of Asterias rubens, 441, 443;
synonym of axial sinus of Ophiothrix fragilis, 487;
of stone-canal of Echinus esculentus, 517;
of tube-foot of Echinus esculentus, 517;
of respiratory trees of Holothuria nigra, 563;
of podia of Holothuria nigra, 566;
of feelers of Aspidochirota, 568, 570;
of Molpadiida, 568;
of Synaptida, 568;
of Elasipoda, 571
Anabolic, 12 f.
Anabolism, 12 f.;
modes of, 15 f.
Anal, cirrhi, 139 f.;
papilla (tube) of Antedon rosacea, 581, 583;
of Eucalyptocrinus, 596
Anapta, 577
Anatomy, of a starfish (Asterias rubens), 432 f.;
of Ophiothrix fragilis, 479 f.;
of Echinus esculentus, 504 f.;
of Echinarachnius parma, 542 f.;
of Echinocardium cordatum, 549 f.;
of Holothuria nigra, 561 f.;
of Antedon rosacea, 581 f.
Anatriaene (a triaene of which the cladi or branches point backwards, in the same direction as the rhabdome or shaft), 224
Anchoring flagella, 114;
of Dallingeria, 112;
of Bodo saltans, 117
Ancistrum, 137
Anemonia, 381;
A. sulcata, 381
Animal-feeding Protista, 38
Animals, defined, 39 f.;
and plants, discussion on, 35 f.;
Anisochela (a chela of which the two ends are unequally developed), 222, 234
Anisonema, 110
Anisospores of Radiolaria, 76, 85;
of Collozoum inerme, 76
Anochanus, 554
Anopheles, intermediate host of malarial parasite, 103 f.;
enemies of, 106;
precautions against, 106
Antedon, 594;
external features, 581;
skeleton, 582;
alimentary canal, 583;
water-vascular system, 583;
nervous system, 583 f.;
coelom, 585;
genital organs, 586;
muscles, 587;
blood-system, 587;
development of, 617 f.;
A. eschrichtii, 594
Antennularia, 279;
A. antennina, 279;
A. ramosa, 279
Anterior, dorsal process, of ciliated band of Bipinnaria, 606;
of Auricularia, 608;
median process, of ciliated band of Bipinnaria, 606
Antero-lateral process, of ciliated band of Echinopluteus, 607
Anthea cereus, 381
Antheneidae, 471
Anthocaulus, 389
Anthocodia, 330
Anthomedusae, 262 f.
Anthophysa (Flagellata), 111, 112 f.;
(Siphonophora, Physophorinae), 308, 302
Anthoptilidae, 362
Anthoptilum grandiflorum, 362
Anthozoa, 326 f.;
commercial importance, 328
Antipatharia, 407 f.
Antipathella, 408;
A. gracilis, 408
Antipathes, 408;
A. ternatensis, 409
Antipathidea, 367, 371, 407 f.
Antiseptic properties of "aromatic" compounds, 36 n.
Anuncinataria, 203
Anus, of Ciliates, 143 f.;
of Stylonychia, 139 f.;
of Carchesium, 147;
of Vorticella, 156;
of Asterias rubens, 434;
of Echinus esculentus, 516;
of Echinarachnius parma, 546;
of Pygastrides relictus, 548;
of Euclypeastroidea, 549;
of Echinolampas, 554;
of Neolampas, 554;
of Holothuria nigra, 560;
of Antedon rosacea, 582;
of Dipleurula larva, 604;
of Asterina gibbosa, 611
Aphodal, 210
Aphrothoraca, 70
Aplanospore, 31
Aplysilla, 196
Aplysina, 225
Apocytial, condition, 32;
forms among Myxosporidiaceae, 107 f.;
Rhizopoda, 52
Apolemia, 308
Apoleminae, 307
Apopyle, 170
Aporosa, 397
Appendicularia, host of Gymnodinium pulvisculus, 132
Aquatic organisms, minute, distribution of, 47 n.
A. albida, 411;
A. americana, 411;
A. lloydii, 411
Arachnoides, 549
Arbaciidae, 530, 531, 532, 538, 558
Arbacioid type of ambulacral plate, 531, 538
Arcadomyaria, 324
A. vulgaris, 55
Archaeocyte, 171
Archaster, 467;
A. bifrons, 467
Archenteron, definition of, 604
Archer, on Protozoa, 45;
on Heliozoa, 71
Archicoel, 450
Arcuothrix, 52;
transition between pseudopodium and flagellum in, 47 n.
Arenaceous Foraminifera, 58 f.;
Carpenter on, 63 f.;
labyrinthine structure in, 66
Argas persica, 121
Aristotle, 166
Aristotle's lantern, of Echinus esculentus, 515, 516, 524, 525;
variations of, in Endocyclica, 531;
of Echinarachnius parma, 546, 547;
absent in Echinocardium cordatum, 550
Arm, of Asteroidea, 431, 432, 453;
of Ophiothrix fragilis, 479;
of Antedon rosacea, 581;
of Antedon (other species), 594;
of Hyocrinus, 590;
of Rhizocrinus, 591;
of Bathycrinus, 591;
of Pentacrinidae, 592;
of Holopus, 594;
of Eudiocrinus, 594;
of Inadunata, 595;
of Articulata, 595;
of Camerata, 595;
development of, in Asterina gibbosa, 611;
in Antedon rosacea, 620
Arm-spines, of Ophiothrix fragilis, 479;
of Ophiuroidea, 491;
of Ophiothrix, 492;
of Ophiacantha, 492;
of Ophiopteron 492
Aromatic compounds in relation to nutrition and antisepsis, 36
Arthropods, hosts of Gregarines, 97 f.
Articulata, 595
Ascitic dropsy, Leydenia associated with, 91
Ascon, 185
Asconema setubalense, 221
Asexual reproduction of Asteroidea, 459;
of Linckia, 459;
of Asterina wega, 459
Ashworth, 331 n.
Asiphonacea, 347
Asphyxia, its effect on contractile vacuole, 143
Aspidochirota, 568, 569, 570 f., 577, 578
Aspidosoma, 476
Aspirotrichaceae, 137, 148, 151, 153, 154
Assimilation, assimilative anabolism, growth, 9, 13, 15 f.
Association, in Gregarines, 95, 98 f.;
in Lankesteria ascidiae, 95 f.
Aster (= centrosome of mitotically dividing cell and peripheral rays), 25, 27;
(a polyaxonid spicule), 184
A. rubens, 432;
external features, 432;
pedicellariae, 433;
skeleton, 434;
coelom, 437;
alimentary canal, 438;
food, 439;
water-vascular system, 441;
nervous system, 444;
perihaemal spaces, 448;
blood-system, 449;
genital organs, 451;
A. glacialis, 473;
pedicellariae, 434;
A. hispida, 474;
A. muelleri, 473;
A. murrayi, 473;
A. ochracea, 474;
A. polaris, 474;
A. tenuispina, 453
Asterina, 454, 456, 459, 461, 463;
A. gibbosa, number of arms, 453;
eggs of, 463;
development of, 609, 610 f., 617;
A. wega, 459
Asternata, 554
Asteroid stage in the development of Ophiuroidea and Echinoidea, 613, 622
compared with Ophiuroidea, 477 f.;
compared with Echinoidea, 503, 558;
mesenchyme of larva of, 602;
development of, 605, 608, 609, 610 f.;
phylogeny of, 621
Asteropsis, 471
Asterosmilia, 401
Asthenosoma, 536;
A. urens, 536
Astraeopora, 390
Astrangia, 400;
Astrocnida, 501
Astrogonium, 472
Astroides, 404
Astronyx, 501
fossil, 475;
A. irregularis, 468;
movements, 468;
burrowing habits, 469
Astropectinidae, 454, 458, 459, 467, 470
Astropyle, 81
Astrorhiza, 59
Astrorhizidaceae, 59
Astroschema, 501;
vertebra, 481
Astroschemidae, 501
Astrosclera willeyana, 194, 194
Astroscleridae, 194
Astylus, 287
Athoria (usually placed in a subfamily Athoriinae of the Physonectidae, 307), 300
Atolla, 322;
A. bairdi, 322;
A. gigantea, 322;
A. valdiviae, 322
Atollidae, 322
Atoll, 390 f.
Atrophy, of oral apparatus of Ciliata during conjugation, 151
Attached, Foraminifera, 64;
Flagellata, 112 f.;
Ciliata, 152;
Attachment, temporary, of Stentor, 155;
permanent, of Rhizocrinus, 591;
of Bathycrinus, 591;
of young Pentacrinidae, 592;
of Thecoidea, 596;
of Cystoidea, 597;
— temporary, of larva of sterina gibbosa, 610;
of Brachiolaria larva, 612;
of larva of Antedon rosacea, 619
Aulactinium, 79;
A. actinastrum, 82
Aulena, 220
Aulocystis grayi, 208
A. aurita, 312
Aureliania heterocera, 383
Auricula, of Echinus esculentus, 526;
of Cidaridae, 531;
of Arbaciidae, 531;
of Echinarachnius parma, 546;
represented by radial pieces of calcareous ring of Holothuroidea, 566
Auricularia, 607;
Aurophore, 308
Autodermin, 523
Automatic processes, so-called, 12
Autotrophic, 37
Autozooids, 333
Axial filament, of Heliozoan pseudopodia, 49, 71, 72, 74;
of Actinophrys sol, 71;
of Actinosphaerium eichornii, 72;
of Radiolaria Acantharia, 80
Axial sinus, of Asterias rubens, 448;
of Ophiothrix fragilis, 487;
of Echinus esculentus, 517, 528;
of Echinocardium cordatum, 552;
of Holothuroid larva, 564;
of larva of Antedon rosacea, 583;
development of, in Asterina gibbosa, 609
Axifera, 353
A. erecta, 216
Axinellidae, 217
Axon, 444
Axoniderma, 216
Axopora, 262
Azoosporeae, 89
Babesia (= Piroplasma), 120
Bacteria, food of Myxomycetes, 92 f.;
nutrition of, 36
Bactronella, 193
Baker, H., on organisms of putrefaction, 43;
on Protozoa, 45;
on Noctiluca, 134 f.
Balanoglossus, larva and affinities of, 616
Balantidium, 137;
habitat of, 152
Balbiani, on Protozoa, 45;
on regeneration, 35 n.;
on "Pébrine" (Nosema bombycis), 107
Barbados, Miocene deposit of Radiolaria, 87
Barotaxy, 20
Barrier reef, 390 f.
Bary, A. de, on methods of culture of lower organisms, 44;
on nature of Myxomycetes, 91
Basal granules, of cilia, etc., 138 n., 141
—see also Blepharoplast
Basal plates, of Antedon rosacea, 584;
of Crinoidea, 588;
of Pentacrinidae, 591;
of Holopus, 592;
of Cystoidea, 598;
of Blastoidea, 599
Bastian, H. Charlton, on spontaneous generation, 44 n.
Bateson, 174
Bath sponge, 221
Bather, on Sympterura, 502;
on classification of Crinoidea, 589;
on phylogeny of Echinodermata, 622
Bathyactis, 404
Bathyanthus, 411
Bathypathes, 408
Bebryce, 356
Bell, F. J., 406 n.;
on classification of Ophiuroidea, 494;
of Endocyclica, 533
on relationships of Holothuroidea, 430 n., 622
Bell-animalcules, 155 f.;
feeding of, 145
—see also Carchesium, Epistylis, Peritrichaceae, Vorticella, Zoothamnium
Beloidea, 77
Beneden, E. van, on Sporozoa, 94;
on pelagic Anthozoa, 411
Bernard, F., 241 n.
Berthold, on protoplasmic movements, 16 n.
Bezzenberger, list of species of Opalina, 124 n.;
on parasitic Ciliata, 152 n.
Bicoeca, 111
Bicoecidae, 111
Bidder, 168, 172 n., 186 n., 235 n.
Biemma, 224
Bile, 13
Biloculine, 66
Binary sex (= syngamy with marked inequality between the pairing cells), 33 f.;
in Centropyxis, 57;
in Stylorhynchus, 99;
in Pterocephalus, 99;
in Coccidiaceae, 100 f.;
in Sarcocystis tenella, 108 n.;
in Volvocidae, 127 f.;
in Eudorina, 129;
in Peritrichaceae, 151 f.
Biomyxa, 58
Bionomics of Protistic life, 43
metamorphoses of, 612
Bipocillus (a microsclere consisting of a curved shaft, terminated by a cup-shaped expansion at each end, characteristic of the genus Iophon), 222
Birds, hosts of Acystosporidae, 103
Birth-pore, of brood-cavity of Suctoria, 161
Bisexual differentiation—see Binary sex
Bivium, of Echinarachnius parma, 543;
of Holothuria nigra, 561
Black Corals (= Gerardia, 406, and Antipatharia, 407)
Bladder, of Rotifers and Platyhelminthes, 14 n.
Blanchard, on Sporozoa, 94
Blastocoel, 603
Blastomere, 603
Blastostyles, 265
Blasts of Coccidiaceae, morphology of, 120;
of Acystosporidae, 104 f.
Blastula, definition of, 603
Blepharoplast, 19;
of Heliozoa, 72;
of Trypanosoma, 121;
of nuclear origin in Trypanosoma, 109 n.;
of T. noctuae, 120 f.;
of Ciliata, 141
Block-musculature, of spines of Echinus esculentus, 506
Blood, Acystosporidae and Haemosporidae parasitic in, 97, 102 f.;
Protomastigaceae parasitic in, in fever, 119 f.
Blood-corpuscle, entered by Haemosporidae and Acystosporidae, 102 f., 104 f.;
by Treponema and Trypanosoma, 120
Blood-system, of Asterias rubens, 449 f.;
of Echinus esculentus, 529;
of Holothuria nigra, 567;
of Antedon rosacea, 587
Bloody rain, 125
Blowflies, alleged spontaneous generation of, 42
B. caudatus, 116 n.;
B. saltans, 114, 116 n., 117 f.
Bodonidae, 111;
movements of, 114 n.
Body-cavity—see Coelom
Bohemura, 502
Bolina, 419;
B. infundibulum, 416
Bolinidae, 419
Bolinopsis, 419
Bolocera tuediae, 381
Borchgrevink, 310 n.
Borgert, on Dictyochidae, 87;
on fission in Aulacantha, 85;
on phaeodium in Radiolaria, 81 n.
Botanists, contributors to knowledge of Flagellates, 119
Botryoidea, 79
Bougainvillia, 263, 264, 266, 269;
B. ramosa, 269
Bougainvilliidae, 269
Boulenger, 294 n.
Bourne, G. C., 246, 337, 338 n., 347
Bourrelet, 553
Boveri, on functions of chromatin, 28 n.
Brachial ossicle, of Antedon rosacea, 582;
of fossil Crinoidea, 595
Brachionus, often found with Euglena viridis, 124
Brachyenemic, 405
Brachyeneminae, 405
Brady, classification of Foraminifera, 58 f.
Brain-Coral, 401
Branched, endoplasm, of Noctiluca, Trachelius, and Loxodes, 133, 153;
meganucleus, of Dendrosoma, 160;
theca, of Dinobryon, 112;
of Schizotricha, 152
Branching colony, of Carchesium, Epistylis, Zoothamnium, 158
Brandt, Karl, on commensals and parasites of Radiolaria, 87 n.;
on Radiolaria, 88
Breeding temperature of Protozoa, 47
Breeze Flies, intermediate hosts of Trypanosoma evansii, 119
Briareidae, 350
Brisinga, 474
Brissidae, 556
Brissopsis, 556;
B. lyrifera, 556
Brittle Star—see Ophiuroidea
Brood-cavity, in Suctoria, 160 f., 162
Brood-cell, 31
Brood-cyst, of Proteomyxa, 88 f.
Brood-division, brood-formation, 30 f.;
in Rhizopoda, 56 f.;
in Foraminifera, 67 f.;
in Radiolaria, 85 f.;
in Flagellata, 109, 115, 117 f.;
in Polytomeae, 115;
in Chlamydomonadidae, 115;
in Paramoeba eilhardii, 116 n.;
in Choanoflagellates, 122;
in Proterospongia, 122;
in Volvocidae, 127 f.;
in Ciliata, 147;
in Colpoda cucullus, 147, 153;
of male Peritrichaceae, 151 f., 157;
retarded, 31 f.
Brood-mother-cell, 31
Brood-pouch, of Pteraster, 465, 466;
of Hemiaster philippi, 556, 602;
of Anochanus, 554;
of Cucumaria laevigata, 602;
of Psolus ephippifer, 602
Brown colour of lakes or ponds often due to Dinoflagellates, 131
Browne, 273, 281 n., 291 n., 312
Bruce, Col. and Mrs., on Trypanosomic fever and sleeping sickness, 120
Bubbles of carbon dioxide formed in Arcella, etc., 53
Buccal sinus of Holothuria nigra, 566
Buccal tentacle—see Buccal tube-foot
Buccal tube-foot (and tentacle), of Ophiothrix fragilis, 486;
of Echinus esculentus, 518;
of Echinocardium cordatum, 551, 561;
of Holothuria nigra, 561;
of Spatangoidea, 577;
development of, in Holothuroid pupa, 615
Budding, in Trichosphaerium, 54;
in Rhizopoda, 56;
in Acanthocystis, 73;
in Acantharia, 86 n.;
in Spirochona, 147;
in Sponges, 177 f., 228, 229, 230;
in Hydrozoa, 250 f., 263, 275;
in Scyphistoma, 317;
in Alcyonaria, 340;
in Zoantharia, 371
Bud-fission in testaceous Rhizopoda, 55;
in Euglypha, 29
Buffon, on organisms of putrefaction, 43
Bulimina, 59
Bulk and surface of organism, ratio between, 14
Bunodactis gemmacea, 378
Bunodeopsis, 382
B. gemmacea, 382
Bunodidae, 382
Burrowing habits, of certain Asteroidea, 461;
of Astropecten, 469;
of Paxillosa, 469;
of Porcellanasteridae, 471;
of Strongylocentrotus lividus, 541;
of Echinarachnius parma, 546;
of Echinocardium cordatum, 549;
of Spatangus purpureus, 555;
of Molpadiida, 575;
of Synaptida, 576;
of Synapta inhaerens, 577
Burrowing Urchins, 529
Bury, on primary axial sinus of Holothuroid larva, 564;
on change in position of mouth during metamorphosis of Auricularia, 614
Bütschli, on protoplasm, 16 n.;
on paramylum, 95 n.;
on Protozoa, 46;
on Cystoflagellates, 135;
on classification of Ciliata, 137;
on Strombidium and Torquatella, 155 n.;
Caecum (diverticulum), of alimentary canal of Echinocardium cordatum, 551;
of alimentary canal of Elasipoda, 569, 571
Caenomorpha, 137, 141 n., 154;
C. uniserialis, 155
Cake-urchins = Clypeastroidea, q.v.
Calcaire Grossier, 70
Calcarea, 184 f.
Calcareous ooze, 114
Calcareous ring, of Holothuria nigra, 566;
of H. cinerascens, 567;
of Phyllophorus rugosus, 567
Calcaromma calcarea, 83
Calcituba, 59;
growth of, 64;
pylomes of, 64
Calices of Madreporaria, 371
Calicoblasts, 385
Calkins, on nucleus in Protozoa, 25 n.;
on Protozoa, 46;
on rhythm in life-cycle of Ciliata, 148 n.
Calliactis (family Sagartiidae, 381);
C. parasitica, 378
Callianira, 418
Callianiridae, 417
Calthrop, 184;
of Radiolaria, 83
Calymma, 420
Calymmidae, 420
Calyptoblastea, 275 f.
Calyx, of Echinus esculentus, 513;
of Echinarachnius parma, 545;
of Pelmatozoa, 579;
of Carpoidea, 580;
of Holopus, 592;
of fossil Crinoidea, 595
Camerata, 595
Campanularia, 280
Campanulariidae, 280
Campascus, 52;
test of, 55
Camptolithus, 346
Canalaria, 201
Canals, "feeding," afferent, or replenishing of contractile vacuole system in Ciliata, 14, 143, 146;
of Stylonychia, 139 f.;
of Stentor, 156
Cannopora, 283
Cannopylaea (= Phaeodaria), 76
Cannotidae, 278
Capillitium of Myxomycetes, 90 f., 92
Capnea sanguinea, 383
Capria, 321
Caravella, 308
Carbohydrates, formation of, 33
Carbon dioxide, attracts Paramecium, 23;
secreted by Arcella, etc., 53
Carchesium, 138;
Carinal ossicle of Asteroidea, 436
Carlgren, 378 n.
Carmarina, 295
Carpenter, P. H., on the classification of the Crinoidea, 589
Carpenter, W. B., classification of Foraminifera, 58;
on their true nature, 62;
on their structure, 63 f.;
on Arenacea, 65 f.;
on the nervous system of Antedon rosacea, 585
Carter, on Protozoa, 45;
on Sponges, 167, 180, 208, 237 n.;
on fossil Hydrozoa, 270 n.
Cash, on Rhizopoda, 58 n.
Cassidulidae, 554
Cassidulina, 59
Cassiopea, 324
Cassiopeidae, 324
Castellani, on Trypanosomic fever and sleeping sickness, 120
Catabolic, catabolism, 13 f., 24
Cataclysmal metamorphosis of Dipleurula, 613
Catallacta, 89
Catostylidae, 325
Cattle, Trypanosomic diseases of, 119 f.
Caudal cirrhi, 139 f.
Caudina, 575
Caullery and Mesnil, on Actinomyxidiaceae, 98 n.
C. obesa, 364
Cell, 3 f.;
definition of, 3;
-membrane of ovum of Sea-urchin, 7;
-wall, 3;
in Dinoflagellates, 130;
-boundary in Flagellates, 113;
Spencerian division, 31 f.;
collar-, of Choanoflagellates, 121, 122;
of Sponges (= choanocytes), 171, 176, 186
Cellular relationship explained, 10
Cellulose, 37;
cell-wall of holophytic Flagellates, 113;
in Dinoflagellates, 130
Central blood plexus—see Heart
Central capsule, 49, 76, 77, 82, 84;
its functions in regeneration, 35;
of Collozoum inerme, 76
Centrifugal force, stimulus of, 19 f.
Centripetal canals, 289
Centro-dorsal ossicle, of Crinoidea, 580;
of Antedon rosacea, 582;
of Atelecrinus, 594
Centrogenous (used of spicules = meeting in a common centre and growing outwards), 76
Centropyxis, 51;
test of, 55;
C. aculeata, reproduction of, 57
of Heliozoa, 72;
(= blepharoplast) in Flagellates, 115
C. longispinosus, 522, 532, 539
Cephalis (= uppermost chamber of monaxonic Radiolarian shells), 83
Cephalodiscus, 617
Cephalont of Gregarines, 98
Cephalopoda, erroneous reference of Foraminifera to, 62
Cephea, 325
Cepheidae, 324
C. fusca, 271
Ceratellidae, 271
Ceratium, 110;
habitat of, 131
C. dujardini, gametes of, 116 n.
Cereactis (family Actiniidae, 381);
C. aurantiaca, 378
Cerianthidea, 367, 373, 377, 409
nematocyst of, 247;
C. americanus, 411;
C. bathymetricus, 411;
C. lloydii, 411;
C. membranaceus, 370, 410, 411;
C. oligopodus, 411;
C. vogti, 411
Cestidae, 420
Cestus, 420;
C. pectenalis, 420;
Chaetetidae, 346
Chalarothoraca, 71
Chalk, Foraminifera, etc., in, 69 f.
Challengeridae (a family of Phaeogromia, 79);
Chambered organ, of Antedon rosacea, 584;
of Pentacrinidae, 592
Chambers, of Foraminiferal shell, 62
Chapman, on Foraminifera, 58 n., 70
Charistephane, 417
C. marsupialis, 319;
C. grandis, 319
Charybdeidae, 318
Cheilostomella, 59
Cheilostomellaceae, 59
Chela (a complex microsclere derived from a sigma and consisting of a curved shaft bearing recurved processes), 234
Chemical, reactions, of protoplasm and of vacuoles, 13;
substances in solution, 19, 22 f.;
rays of spectrum in relation to plant pigments, 36 n.
Chemiotaxy, 23;
its rôle in syngamy, 34;
of Coccidians, 100
Chirodropidae, 319
Chirodropus, 319
Chironephthya, 349;
C. variabilis, 338
Chiropsalmus, 319
Chitin, 37
Chlamydomonadidae, 111, 125, 126;
brood-division of active, 115
barotaxy of, 20;
conjugation of, 115 f.;
Dill on, 119 n.
Chlamydophora, 71
Chlamydophrys, 52;
C. stercorea, reproduction of, 57;
habitat of, 57 f.
Chloramoeba, 110
Chloromonadaceae, 110;
trichocysts in, 113 n.
Chlorophyll, 36 n.;
in Flagellates, 115 n.;
bodies of Euglenaceae, 124 f.
Chloroplasts (= chlorophyll bodies), of Eutreptia viridis, 124 f.
Choanocytes, 171, 176, 186, 200, 237
—see also Collar-cells
Choanoflagellata, Choanoflagellates (= Craspedomonadidae, 111), 121, 122 f.;
in relation to Sponges, 41, 171, 181
C. infundibulifera, 162
Choanosome, 170
Chondrilla, spicules of, 233
Chondrioderma, 90;
C. diffusum, 93
Chondrocladia, 216
Choristida, 212
Chromatin, 6 f.;
function of, in cell-division, 24 f.;
of ovum of Sea-urchin, 7;
of Radiolaria, 81;
Chromatophore, 13, 21, 36 f., 113, 115;
of Sphaerella, 126
—see also Chromoplastid, Chlorophyll, Plastid
Chromidia, 30;
of Rhizopoda, 51;
of Foraminifera, 67 f.
of Zooxanthella, 86
—see also Chromatophore
functions of, 28 f.
Chromulina, 110
Chrysamoeba, 110
Chrysaora, 312, 315, 316, 323;
Chrysogorgia, 355
Chrysogorgiidae, 355 (= Dasygorgiidae, 333)
Chrysomonadaceae, 110;
external plasmic layer of, 113;
Chun, 197 n., 300, 307 n., 308, 414 n.
Chytridieae, movements and affinities of, 114 n.;
Cidaridae, 530, 531, 532, 533, 558
C. (Dorocidaris) papillata, 534
Cienkowsky, on Monadineae (= Flagellates and Proteomyxa), 40, 89;
on Radiolaria, 88;
on Zooxanthella, 86;
on Cystoflagellates, 135
of Protozoa, 47;
paroral, 156 n.;
preoral, 139;
of Trichonymphidae, 123;
of Opalina, 123;
of Maupasia, 124;
of Ciliata, 141;
organs formed of combined, 138, 141, 413;
sensory, of Stylonychia, 138;
Schuberg, A., on, 141 n.
Ciliary motion, 18;
mechanism of, 18 n.
animal nutrition, 40;
conjugation, 149 f.;
contractile vacuole, 14 f., 143;
encystment, 147 f.;
feeding, 145;
fission, 147 f.;
form of body, 141;
galvanotaxy, 22;
infested by Suctorian parasites, 160 f.;
gut, 146;
mouth, 145;
nuclear apparatus, 144 f.;
parasitic, 152;
pharynx, 145;
pellicle, 141;
relations to Metazoa, 41;
rheotaxy, 21;
Suctoria allied to, 159;
thigmotaxy of, 20;
tubicolous, 152;
Zooxanthella symbiotic with, 125
Ciliated, buds of Suctoria, 159, 160 f., 162;
epaulette, 607
Cilioflagellata (= Dinoflagellata, given by misinterpretation of transverse flagellum), 130.
Cilium of Noctiluca, 133
C. barbata, 212
Cinclides, 369
Cinetochilum, 137
Ciocalypta, 225
Cirripathes, 408;
C. spiralis, 408
Cirrus, of Crinoidea, 430, 580;
of Pentacrinidae, 588, 591, 592;
of Rhizocrinus, 591;
of Comatulidae, 594;
of Actinometra, 594;
of Antedon, 594;
development of, in A. rosacea, 620;
of fossil Crinoidea, 595
Cladocarpus, 279
Cladocoryne, 272
Cladocrinoidea, 595
C. radiatum, 267
Cladonemidae, 270
Cladopathes, 408
Cladorhiza, 216
Cladotyle (a rhabdus on which one actine is branched, the other tylote or knobbed at the extremity), 222
Claparède and Lachmann on Protozoa, 45;
on Suctoria, 162
Clark—see James-Clark
Classification, of Protozoa, 48 f., 50;
of Rhizopoda, 51 f.;
of Foraminifera, 58 f.;
of Heliozoa, 70 f.;
of Radiolaria, 76 f.;
of Proteomyxa, 90;
of Sporozoa, 97;
of Flagellata, 109 f.;
of Protomastigaceae, 111;
of Volvocaceae, 111;
of Infusoria, 136;
of Ciliata, 137;
of Suctoria, 159;
of Sponges, 183 f.;
of Coelenterata, 249 f.;
of Ctenophora, 417 f.;
of Eleutherozoa, 430 f.;
of Asteroidea, 459 f.;
of Ophiuroidea, 491 f.;
of Echinoidea, 529 f.;
of Endocyclica, 532;
of Clypeastroidea, 548 f.;
of Spatangoidea, 552;
of Holothuroidea, 567 f.;
of Pelmatozoa, 580;
of Crinoidea, 589 f.
Clathria, 225
C. blanca, larva of, 227
Clathrinidae, 185 f.
Clathrissa, 223
C. wilsoni, 279
Clava, 272;
C. squamata, 263
Clavatellidae, 270
Clavidae, 272
C. viridis, 329, 337, 343 f., 344
Clavulariidae, 344
Clearing of tissues, physical explanation of, 11
Climacograptus, 282
Clionidae, 218
Cloaca of Holothuria nigra, 563
Clypeastridae (= Echinanthidae), 549
Clypeastroidea, 529, 542 f., 556, 559, 566
Clytia, 280;
Cnidocil, 248
Cnidopod, 248
Cnidosac, 300
Coalescence of individual Rhizopods during bud-fission, 55
relations to Trypanosoma, 120
Coccidiosis, 102
C. cuniculi, 102;
C. lacazei, syngamy of, 101;
Coccolithophora, 110
Coccolithophoridae, in Chalk, 70;
wall of, 114
Coccoseridae, 346
Cockroach, Lophomonas parasitic in gut of, 123
Codaster, 599
Codosiga, 111
Coelenterata, 243 f.;
definition, 245;
almost all immune from Gregarines, 99
Coeliac canal of Antedon rosacea, 586
Coelogorgia, 349
Coelogorgiidae, 349
Coelom (including body-cavity), 428;
of Asterias rubens, 437;
of arm of Ophiothrix fragilis, 480;
of Echinus esculentus, 516;
of Holothuria nigra, 562;
of Antedon rosacea, 585;
development of first rudiment in larva, 605;
subsequent development in Dipleurula, 608, 609;
in Asterina gibbosa, 611;
Coelomic nervous system, of Asterias rubens, 448;
of Ophiothrix fragilis, 488;
of Echinus esculentus, 524;
of Holothuria nigra, 566;
C. mitsukurii, 422
Coeloplanidae, 422
Coenocyte, 30
Coenograptus, 282
Coenopsammia, 404
Coenothecalia, 344
Cohn, Ferdinand, on cultures of Schizomycetes, etc., 44
Cold-blooded Vertebrates, as hosts of Haemosporidae, 102
Coleps, 137;
mail-like pellicle of, 141, 152;
C. hirtus, group feeding, 150
Collar, of Choanoflagellates, 121 f., 122;
of peristome of Vorticella, etc., 156
Collar-cells, in Choanoflagellates, 121 f., 122, 171, 237;
of Calcarea, 186;
—see also Choanocytes
Collencyte, 171
Colletocystophores, 320
Collida, 77 n.
Colloblasts, 414
Collodaria, 77
Colloidea, 77
Collosphaera, 77;
symbiotic Diatoms in, 86
Collosphaeridae, 85
Collozoidae, 85
Collozoum, 77;
C. inerme, 76
Collyritidae, 559
Colonial, cells, 31;
Protista, 31
Colony, 31;
of Collozoum inerme, 76;
-formation in Polycyttarian Radiolaria, 84 f.;
in Flagellata, 113;
of Choanoflagellates, 121, 122;
in Vorticellidae, 158;
of Volvocidae, 126 f.;
of Pandorina, 128 f.;
of Eudorina, 129
Colour, red, of lakes and ponds, often due to Dinoflagellata, 131
Coloured vegetal nutrition, 36 f.
Colouring matter of chromatophores of Flagellates, 115 n.
Colpidium, 137;
C. colpoda, diagram of conjugation, 149;
nuclear relations in conjugation, 151
Colpoda, 137;
C. cucullus, 153;
brood-fission in cyst, 147
Columnals, 619 (= Stem-ossicles, q.v.)
Columnaria, 344
Comatula = Antedon, q.v.
Comatulidae, 594
Comitalia, 201
Commensals, of Heliozoa, 73;
of Infusoria, 153 f.;
—see also Zoochlorella, Zooxanthella, and Symbiosis
Comminator muscles of Aristotle's lantern, 526
Commissure of radial cords of aboral nervous system of Antedon rosacea, 585
Compasses (or radii) of Aristotle's lantern, 526
Conant, 319
Conaria larva, 302
Conchophtheirus, 137
Conchula, 380
Confervaceae, related to green Flagellates, 48
Confervoid form of Hydrurus, 113
Conjugatae, syngamy of, compared to certain Chlamydomonads, 126
Conjugation, 33 f.;
of Trichosphaerium, 54, 56 f.;
exogamous, in Foraminifera, 68 f.;
of Sporozoa, 95 f.;
of Lankesteria, 95 f.;
of Monocystis, 96;
of Stylorhynchus, 99;
bisexual, of Sarcocystis tenella, 108 n.;
of Flagellates, 115;
of Bodo saltans, 117;
of Trypanosoma, 120;
by a fertilising tube in Chlamydomonas, 125;
of Volvocaceae, 127 f.;
of Volvox, 127 f.;
isogamous and endogamous, of Stephanosphaera, 128;
in Dinoflagellates, 131 n.;
of Noctiluca, 133;
of Ciliata, 148 f.;
of Paramecium caudatum, 148;
of Colpidium colpoda, diagram, 149;
of Peritrichaceae, 151 f., 157;
of Vorticella, 157;
of Suctoria, 161;
of meganucleus in Dendrocometes, 161, 162
—see also Syngamy, Fertilisation
Conoclypeus, 558
Constancy of type in Protista, 42 f.
Conte, 292 n.
Contractile vacuole, 5, 10, 14 f.;
of fresh-water and brackish Protozoa, accessory spaces and canals, 47;
of Rhizopods, 52;
of fresh-water Allogromidiaceae, 60;
of Microgromia socialis, 60;
of zoospore of Clathrulina, 74;
of Myxomycetes, 92;
of Cryptomonas, 112;
of Diplomita, 112;
of Oikomonas, 112;
of Tetramitus, 112;
of Trachelomonas, 112;
of Bodo saltans, 117;
of Choanoflagellates, 122;
absent from Opalinidae, 123;
of Euglenaceae, 125;
of Volvox, 126;
of Ciliata, 143 f.;
in fission, 147;
of Stylonychia, 139 f.
of Stentor, 156;
of Vorticella, 157;
muscular mechanism of, 14 f.
Contraction, of Amoeboid cell, 16 f.
Copepoda, infested by Epistylis, 158
—see also Cyclops
Coppinia, 280;
C. arcta, 280
Coprolites, Radiolaria in, 87
Copromyxa, 90
Organ-pipe, 343;
Flexible (= various Alcyonaria), 326;
Stony (= Madreporaria), 326, 384 f.;
Brain-, 401;
Black (= Gerardia, 406, and Antipatharia, 407);
-Reefs, 390 f.;
Reef-, 389 f.
commercial importance, 328
Corallimorphidae, 383
Corallimorphus, 383
C. boshuensis 352;
C. confusum, 352;
C. elatius, 352;
C. inutile, 352;
C. japonicum, 352;
C. johnsoni, 352;
C. konojoi, 352;
C. pusillum, 352;
C. reginae, 352;
C. stylasteroides 352;
C. sulcatum, 352
Corbula, 276
Cornulariidae, 344
Cornuspira, 59;
shell of, 64
Corona, of Echinus esculentus, 504, 511;
of Endocyclica, 530;
of Cidaridae, 530;
of Temnopleurinae, 539
Coronaster, 474
gastral cortex, 188
Corticata, 49 n.
Corydendrium (family Tiaridae, 273);
C. parasiticum, 269
Corymorpha, 263, 265, 266, 273;
C. nutans, 273
Corymorphidae, 273
C. viridis, 383
Coryne, 272
Corynidae, 272
Cosmiolithus, 346
Costia, 111;
C. necatrix, produces epidemics in fresh-water fish, 119
Cotte, 218 n.
Cotton-spinner, 564
Cotylorhiza, 325
in Flagellates, 116 f.
—see also Zygote
Covering-plates, of arms of Ophiuroidea, 491;
of arms of Crinoidea, 589;
of Pentacrinidae, 589;
of Thecoidea, 596;
of Blastoidea, 599
Crambessa, 325
Crambione, 325
C. cranium, 222
Craspedomonadidae, 111, 115 n., 121 f., 122;
transverse division in, 115 n.
—see also Choanoflagellata
Crescent (gametocyte of Laverania), 104 f.
Cretaceous firestone of Delitzet contains Peridinium, 132
C. (Henricia) sanguinolenta, 462, 463;
C. laeviuscula, 462
Cribriform organs, 470
Cricket, Mole-, Lophomonas parasitic in gut of, 123
development of, 617 f.
Cristellaria, 59
Crotalocrinus, 595;
C. pulcher, 595
Crustacea, small, rheotaxy of, 21
Cryptabacia, 404
Cryptogams, Higher, spermatozoa of, 38
Cryptoglena, shell of, 113
C. ramosa, 285
Cryptomonadaceae, 110
Cryptomonas, 110
Cryptozonate, 454
Crystals, in isospores of Collozoum inerme, 76;
proteid, 37
Ctenocella, 357
Ctenophora, 412 f.;
comb-plates of, 141
Ctenoplanidae, 421
Cucumaria, 573;
C. laevigata, 602
Cuénot, on Sporozoa, 94;
on reproduction of Monocystis, 96 n.
C. tetragona, 453
Culex, host of Haemoproteus or Proteosoma, 103;
intermediate host of a Trypanosoma, 120
Cultures, pure, 43
Cunanthidae, 296
Cunarcha, 296
Cunina, 296;
C. proboscidea, 296;
C. rhododactyla, 296
Cunoctantha, 296;
C. octonaria, 295
Cup (= theca), of Flagellates, 113;
of Salpingoeca, 122;
—see also Theca, Tube
Cupulita, 307;
C. sarsii, 304
electric, stimulus of, 19, 22;
in liquid, relation of protoplasmic movements to, 7, 19, 21
Cuticle, of Dinoflagellata, 130;
of Gregarines, 96;
of Noctiluca, 133
—see also Membrane, Pellicle
Cuticular shell of Flagellates, 113
Cuvierian organs of Holothuria nigra, 564
C. lamarcki, 324
Cyanaeidae, 324
Cyathaxoniidae, 394
Cyatholiths, 114
Cyathophyllidae, 394
Cyathophyllum, 394
Cycads, spermatozoa of, 38
Cyclidium, 137
Cyclocnemaria, 397
Cyclomyaria, 325
Cyclops, host of Choanophrya, 159;
of Rhyncheta and other Suctoria, 159 f., 162;
of Vorticellidæ, 158
Cycloseridae, 404
Cydippidea, 417
Cydippiform stage of Lobata and Cestoidea, 414
Cydonium milleri, 222
Cymbonectes, 306
Cymbonectinae, 306
Cyphoderia, 52
Cyrtoidea, 79
Cyst (a closed membrane distinct from the cytoplasm around a resting-cell or apocyte), 37, 39;
cellulose-, 37;
chitinous, 37;
growth of vegetal cell in, 37;
of Protozoa present in dust, 47;
of Centropyxis aculeata, 57;
of Chlamydophrys stercorea, 57;
of Amoeba coli, 57;
of Actinophrys sol, 72;
of Actinosphaerium, 73 f.;
brood-, of Paramoeba eilhardii, 116 n.;
of Bodo saltans, 117;
of Opalina, 123 f.;
of Volvocaceae, 128;
of Dinoflagellates, 131;
of Ciliata, 147;
of Colpoda cucullus, 147, 153;
temporary (hypnocyst) of Rhizopoda, 57;
of Proteomyxa, 88;
of Myxomycetes, 91;
-wall, of Acystosporidae, 104 f.
Cystiactis, 382
Cystid—see Cystoidea
Cystiphyllidae, 394
Cytogamy, 33 f.
Cytoplasm, 6;
of ovum of Sea-urchin, 7;
granular, nutritive, of muscle cell, 19;
in cell-division by mitosis, 26 f.;
during syngamy, 34
D. lactea, 312
Dactylopores, 257
Dactylozooids, 264;
of Hydractinia, 264;
of Millepora, 259;
of Siphonophora, 299
Dale, on chemiotaxy, 22
Dallinger, W. H., and Drysdale, C., on Protozoa, 44, 45;
on organisms of putrefaction, 44, 116 f.;
on life-histories of Flagellates, 116 f.
anchoring flagella of, 114;
D. drysdali, gametes of, 116 n.
Dangeard, on brood-division in active Chlamydomonadidae, 115;
on Flagellata, 119 n.
Dantec, Le, on protoplasmic movements, 16 n.;
on peptic digestion in Protozoa, 16
Darwinella, 221
Dasygorgiidae, 333 (= Chrysogorgiidae, 355)
Davenport on protoplasmic movements, 16 n., 19 n.
Dawydoff, 423
Dead men's fingers (= Alcyonium digitatum, 349)
Death, 11;
by diffluence, granular disintegration or solution, 14 f.;
by desolution, 15;
necessary, of colonial cells of Volvox, 128;
in Volvox and in Metazoa, compared, 130
Deep-sea deposits (Foraminifera), 70
Degen, on functions of contractile vacuole, 15 n.
Degeneration, senile, among Ciliata, 148
Deglutition in Podophrya trold, 159
—see also Ingestion of food
Deiopea, 419
Deiopeidae, 419
Delage, on protoplasm, 3 n.;
on syngamy, 34 n.;
on motion of flagella, 114 n.;
and Hérouard, on Protozoa, 46
Delap, M. J., 311 n.
Deltoid plate of Blastoidea, 599
Dendoryx, 224
Dendrite, 444
Dendrobrachia, 409
Dendrobrachiidae, 409
Dendrochirota, 568, 569, 572, 577, 578
Dendrocometes, 159, 160, 161 f.
Dendrograptidae, 281
Dendrograptus, 281
Dendrophyllia, 404
Depastrella, 321
Depastrum cyathiforme, 321
Depressor muscles of compasses of Echinus esculentus, 527
Dercitus bucklandi, 221
Dermal, gill—see Papula;
membrane, 170
Dermalia, 201
Dermasterias, 471
Desma (the megasclere which forms the characteristic skeletal network of the Lithistida, an irregular branched spicule), 215, 224
Desmacella, 224
Desmacidon, 222
Desmophyes, 307
Desmophyinae, 307
Desmothoraca, 71
Desolution of protoplasm, 11 f.
Deutomerite, 98
Development, of Sponges, 226;
of Scyphozoa, 316;
of Alcyonaria, 341;
of Zoantharia, 373;
of Echinodermata, 601 f.
Dextrin, 15
Diadematidae, 531, 532, 538 f., 558
Diadematoid type of ambulacral plate, 531, 539
Dialytinae, 192
Diancistra (a spicule resembling a stout sigma, but the inner margin of both hook and shaft thins out to a knife edge and is notched), 222
Diaphorodon, 59;
shell of, 60
Diaseris, 404;
asexual reproduction, 388
Diatomaceae, skeleton of, 84;
symbiotic with Collosphaera, 86
Diatomin, 86;
(?) in coloured Flagellates, 115 n.
Dictyoceratina, 220
Dictyocha, 110
Dictyochida (= Silicoflagellata, 110), 79;
in Phaeocystina, 86 f.
Dictyocystis, 137;
test of, 152
Dictyonalia, 201
Dictyonema, 281
Dictyonina, 202
Dictyostelium, 90
Dicyclica, 594
Dicymba, 308
Dicystidae, 97
Didinium, 137;
trichocysts of, 143
Didymium, 90;
D. difforme, 92
Didymograptus, 282
Diffluence, 14 f.
Difflugia, 52;
D. pyriformis, 55;
test of, 55
of reserves in brood-formation, 33;
in Carchesium, 147;
in Starfish, 440
Digestive system—see Alimentary Canal
Dill, on Chlamydomonas, etc., 119 n.
Dimorphism of chambered Foraminifera, 67 f.
Dinamoeba, 51;
test of, 53
undulating membranes of, 123
Dinoflagellata, 110, 113, 130, 131, 132;
plastids of, 40;
nutrition of, 113
Dinoflagellate condition of young Noctiluca, 134
Diphyidae, 306
Diphyopsinae, 307
Diplacanthid, 457
Dipleurula, definition, 605;
Diplocyathus, 277
Diplodal, 210
Diplodemia, 223
Diploëpora, 346
Diplomita, 111
Diprionidae, 282
Directives, 367
Disc, of Vorticellidae, 155, 158;
of Ophiothrix fragilis, 484
Discalia, 309
Discohexaster, 200
Discoidea, 77
Discoidea, 558
Discomedusae (= Ephyropsidae, 322 + Atollidae, 322 + Discophora, 323)
Discomorpha, 137
Discooctaster, 200
reproduction of, 69
Discosomatidae, 383
Diseases, produced by Coccidiidae, 102;
by Acystosporidae, 103 f.;
by Flagellates, 119 f.;
by Trypanosomes, 119 f.;
Protozoic organisms of, 43 f.
Dissogony, 419
Distomatidae, 110
Distribution of Protozoa, 47;
of Sponges, in space, 239 f.;
in time, 241
Disyringa dissimilis, 209, 214, 215
Diverticulum—see Caecum
Division, binary, 10;
reduction-, 75 n.
Dixon and Hartog on pepsin in Pelomyxa, 16
Dobie, 167
Doederlein, 193 n.
Doflein, 46;
on parasitic and morbitic Protozoa, 94 n.;
on syngamy of Cystoflagellates, 135
Dohrn, on carnivorous habits of Sphaerechinus, 516
Dolichosporidia (= Sarcosporidiaceae), 98, 108
Doramasia, 306;
D. picta, 303
Dorataspis, 78;
skeleton, 80
Dorocidaris—see Cidaris
Dorsal elastic ligament of Antedon rosacea, 587
Dorso-central plate of Echinarachnius parma, 543
Dourine, disease of horses and dogs, 119
Drepanidium (= Lankesterella), 97, 102
Dreyer, on genera and species of Radiolaria, 87 f.;
on skeleton of Radiolaria, 82 n.
Dropsy, ascitic, associated with Leydenia, 91
Drysdale and Dallinger, on organisms of putrefaction, 44 f., 116 f.
Dual force of dividing cell, 26 f.
Duboscq, Léger and, sexual process in Sarcocystis tenella, 108 n.
Duerden, 261, 369 n., 371, 373, 374, 389, 397 n., 400 n., 403, 405, 406
Dujardin, on sarcode (= protoplasm), 3;
on Protozoa, 45;
on true nature of Foraminifera, 62 f.;
on Sponges, 167
Dust, containing cysts of Protozoa, 47;
of Flagellata, 118
Dysentery, in Swiss cattle, caused by Coccidium, 102;
tropical, caused by Amoeba coli, 57
oral apparatus, 145;
shell, 141
Earthworm, Monocystis parasitic in, 95
Echinanthidae, 549
E. parma, 542 f., 543, 544, 545, 547;
shape, 542;
sphaeridia, 545;
internal skeleton, 545;
habits, 546;
alimentary canal, 546;
Aristotle's lantern, 546;
tube-feet, 547
Echinating, 217
Echininae, 539
Echinocardium, 549;
E. cordatum, 549 f., 551, 552;
habitat, 549;
shape, 550;
spines, 550;
sphaeridia, 551;
alimentary canal, 551;
tube-feet, 551;
habits, 552;
stone-canal, 552;
E. flavescens, 555;
E. pennatifidum, 555
Echinoconidae, 558
E. pusillus, 549
Echinocystites, 557
Echinodermata, 425 f.
Echinoid stage in the development of a Holothuroid, 615
compared with Holothuroidea, 560;
with Blastoidea, 580;
mesenchyme of larva, 604;
development of, 607, 608, 609, 613, 614;
phylogeny of, 622
Echinolampas, 554
Echinometra, 542
Echinomuricea, 356
Echinonidae, 553
Echinosphaerites, 598;
E. aurantium, 598
Echinothuriidae, 530, 531, 532, 535, 558, 560
E. esculentus, 504 f. 505, 507, 511-515;
locality, 504;
spines, 506;
pedicellariae, 506;
corona, 511;
periproct, 513;
peristome, 513;
alimentary canal, 516;
water-vascular system, 516 f.;
nervous system, 518 f.;
sphaeridia, 524;
perihaemal spaces, 524 f.;
genital system, 528;
blood-system, 529;
larva, 507;
E. acutus, 540;
pedicellariae, 509;
E. alexandri, pedicellaria, 510;
pedicellariae, 510;
E. microtuberculatus, 540;
Economic uses of Foraminifera, 69 f.
Ectocoele, 367
Ectoderm, 246
Ectoplasm (= ectosarc), 6, 46 f., 50;
of Amoeba, 5;
of Rhizopoda, 51 f.;
of Heliozoa, 71 f.;
of Radiolaria, 79 f. (see also Extracapsular protoplasm);
regeneration of, in Radiolaria, 35;
of Collozoum inerme, 76;
of Gregarines, 96 f.;
of Ciliata, 141 f.;
of Stylonychia, 140;
of Suctoria, 159;
of Trachelius ovum, 153;
of Vorticella, 156
Ectopleura, 268
Ectosarc—see Ectoplasm
Ectosome, 170
Ectyoninae, 217
Edwardsia, 328, 366, 368, 376;
E. allmani, 377;
E. carnea, 377;
E. goodsiri, 377;
E. tecta, 377;
Edwardsia stage of Zoantharia, 367
Edwardsiidae, 377
Edwardsiidea, 367, 371, 375, 395
Egg, fertilised, 31;
of Metazoa, 32 f.;
of bird, 32;
various meanings of, 34;
of affected Silkworm moths transmitters of pébrine, 107
Ehrenberg, on Protozoa, 45 f.;
on skeletons of Radiolaria, 87 f.;
on Ciliata, 146;
on Suctoria, 162
Eimer and Fickert, on classification of Foraminifera, 58 n.
Electric, currents, stimulus of, 19, 22;
shock, action on Amoeba, etc., 7
Eleutheria, the medusa of Clavatella, 265
Eleutheroblastea, 253
Eleutheroplea, 279
Eleutherozoa, 430, 560, 577, 579, 583;
development of, 602 f.;
larva of, 605;
Elevator muscles of compasses of Echinus esculentus, 527
Ellipsactinia, 283
Ellis, 167
Embryonic type of development, 601
of animal cells, 37;
growth during, 37;
of zygote, general in Protista, 34;
of Rhizopoda, 57;
temporary, of Rhizopoda, 57 (see also Hypnocyst);
of Heliozoa, 72 f.;
of Actinophrys, 72;
of Actinosphaerium, 73 f.;
of Myxomycete zoospores, 90 f.;
of Sporozoa, 96 f.;
of zygote of Sporozoa, 95 f.;
of Lankesteria, 95;
of Monocystis, 96;
of Coccidiidae, 97 f.;
of archespore or pansporoblast of Myxosporidiaceae, 107;
of zygote of Bodo saltans, 117;
of Opalina, 123 f.;
of oosperm of Volvocaceae, 127 f., 129 f.;
of Dinoflagellates, 131;
of Ciliata, 147;
Endocyclica, 529, 530 f., 556, 559
Endoderm, 246
Endogamy, in Amoeba coli, 57;
in Actinosphaerium, 73 f., 75 (diagram);
in Stephanosphaera, 128
Endogenous budding in Suctoria, 160 f., 162
Endoparasitic Suctoria, 86, 160 f.
Endoplasm (= endosarc, q.v.), 6
—see also Intracapsular protoplasm (Radiolaria)
Endoral, cilia, 139;
undulating membrane, 139
Endosarc (= endoplasm), 6;
of Gregarines, 95 f.;
branching, of Noctiluca, 110, 133;
of Loxodes and Trachelius, 144, 153;
of Ciliata, 143 f.;
of Stylonychia, 140;
of Suctoria, 161
Energy, changes of, in living organism, 8, 13;
sources of, 13 f.
Entocnemaria, 394
Entocoele, 367
Entosolenia, 66
Entz, Geza, on Choanoflagellates, 121 n.;
on structure of Vorticella, 157 n.
Eocene Foraminifera, 70
Eolis (= Aeolis), 248
Eophiura, 501
Eozoon, 70 n.
Epaulettes, 315
Epenthesis, 281
E. bütschliana, cytological study of, 162
E. fluviatilis, structure, etc., 174 f., 176, 177, 178, 179
Ephyra, 317
Ephyropsidae, 322
Epiactis (usually placed in the order Zoanthidea, 404);
E. marsupialis, 379;
E. prolifera, 379
Epibulia, 308
Epidemic, of pébrine among Silkworms, 107;
among Fish, due to Costia necatrix, 119;
to Myxosporidiaceae, 107;
to Icthyophtheirius, 152
—see also Diseases, Fever
Epigonactis fecunda, 379
Epimerite of Gregarines, 97, 98 f.
Epineural canal, of Ophiothrix fragilis, 481;
of Echinus esculentus, 515
Epiphysis, of jaw, of Echinus esculentus, 526;
of jaws of Diadematidae, 531;
absent in Cidaridae and Arbaciidae, 531
Epiphytic Protozoa, 48
Epiplasm (= cytoplasm of a brood-mother-cell remaining over unused in brood-formation), 96
Epistrelophyllum, 403
E. umbellaria, nematocysts of, 249
Epitheca, 386
Epizoanthus, 406;
on Hyalonema, 204;
E. glacialis, infested by Gregarines, 99;
E. incrustatus, 406;
E. paguriphilus, 406;
E. stellaris, 406
Epizoic, Protozoa, 48;
Ciliata, 158;
—see also Symbiosis
Equatorial plate (= the collective chromosomes at the equator of the spindle in mitosis), 25, 27
Equiangular, 185
E. glabra, 286;
E. ramosa, 286
Ersaea, 306;
E. picta, 303
Esperiopsis, 225
Euaster (a true aster in which the actines proceed from a centre, contrasting with the streptaster), 184
Eucalyptocrinus, 596
Eucharidae, 420
Eucharis, 420;
E. multicornis, 416, 418 f., 420
Enchlora, 417
Eucladia, 502
Euclypeastroidea, 549
Eudiocrinus, 594
Eudoxia, 306;
E. eschscholtzii, 303
Euglena, 110;
barotaxy of, 20;
nutrition of, 113;
E. viridis, 124
pellicle of, 113
Euglenoid motion, 124;
of Sporozoa, 50
Euglypha, 52;
in fission, 29;
Eunicea, 356;
Eunicella, 356;
E. cavolini, 356
Eupagurus prideauxii, 378, 381;
E. bernhardus, 378
Eupatagus, 553
Euphyllia, 401
Euplectella, 204;
E. aspergillum (Venus's Flower-Basket, 197);
E. imperialis, 206;
E. suberea, 202, 204, 205, 221
Euplexaura, 356
Euplokamis, 418
Euplotes, 138
Eurhamphaea, 419
Eurhamphaeidae, 419
Euryalidae, 501
Eurypylous, 210
Euspongia, 221
Eutreptia, 110;
E. viridis, 124
Evacuation of faeces by mouth in Noctiluca, 133
Excretion, 13 f.;
in Sponges, 172;
in Asterias rubens, 437;
in Echinus esculentus, 527, 528;
in Antedon rosacea, 587
Excretory, granules, 6;
of Ciliata, 144;
pore of contractile vacuole of Flagellates, 110;
of Trachelius ovum, 153
Exogametes of Trichosphaerium, 54
Exogamy, 34 n.;
in Rhizopoda, 56 f.;
in Foraminifera, 68 f.
Expansion of Amoeboid cell, 16 f.
Extracapsular protoplasm, of Phaeodaria, 76;
of Radiolaria, 79 f. (see also Ectoplasm)
of Echinoidea, 512
Eye-spot of coloured Protista, 21, 125 f.
Fascicularia, 348
Fasciole, of Echinocardium, 550, 555;
of Spatangoidea, 553;
of Spatangus, 553;
of Eupatagus, 553;
of Spatangidae, 555
Fats, fatty acids, 15;
formation of, 36
Fauré-Fremiet, on attachment of Peritrichaceae, 141 n.
Faurot, 368
Favosites, 344
Favositidae, 344
Feather-star, 581
Feeding, of Noctiluca, 133, 144;
of Peritrichaceae, 145
—see also Food
Feeler, of Holothuria nigra, 561 f., 566;
of Holothuroidea, 568;
Female gamete, 33;
of Pandorina, 128 f.;
of Acystosporidae, 104 f.;
—see also Megagamete, Oosphere
Ferment, required for germination, brood-formation, etc., 32 f.
—see also Zymase
Fermentation, organisms of, 43 f.
Fertilisation, 33 f.;
"chemical," 32 n.
Fertilised egg, 31
—see also Oosperm, Zygote
Fertilising tube of Chlamydomonas, 125
Fever, intermittent, malarial, 103 f.;
relapsing, 121;
remittent, 105;
Texas-, Tick, 120;
Trypanosomic, 119 f.
Fewkes, 268 n.
Fibularidae, 549
Fibularites, 559
Fickert, Eimer and, on classification of Foraminifera, 58 n.
F. ficus, 219
Filoplasmodieae, 90 f.
Filopodia, 47 n.
resemblance to Allogromidiaceae, 59
Finger, 580;
of Cystoidea, 597;
Firestone of Delitzet contains fossil Peridinium, 132
Fischer, on fixing reagents, 11;
on structure of flagellum, 114
Fish, rheotaxy of, 21;
epidemics of, due to Myxosporidiaceae, 107;
to Costia necatrix, 119;
to Ichthyophtheirius, 152
equal, 10;
Spencerian, 23;
multiple, 30 f. (see also Brood-division);
of Heliozoa, 72 f.;
of Radiolaria, 84 f.;
radial, in Volvocaceae, 110;
transverse, in Craspedomonadidae, 115 n.;
longitudinal and transverse, of Bodo saltans, 117;
of Opalina, 123;
of Euglenaceae, 124;
of Eutreptia viridis, 124;
of Noctiluca, 133;
of Ciliata, 147 f.;
of Stentor polymorphus, 156;
of Vorticellidae, 157 f.
—see also Bud-fission
Fixing protoplasm, 15
protandry of, 370
Flagella, flagellum, 17 f., 47;
of Protozoa, 47;
formed by altered pseudopodia in Microgromia, 60;
of Heliozoa, 73;
of sperms of Coccidiidae, 102;
of Acystosporidae, 105;
of Trichonymphidae, 114;
Delage on mechanism of, 114 n.;
of Bodo saltans, 117;
of Trypanosoma, 121;
of Euglenaceae, 124 f.;
of Maupasia, 124;
of Eutreptia viridis, 124;
of Sphaerella, 126;
of Peridinium, 131;
of Polykrikos, 132;
—see also Sarcoflagellum
Flagellar pit, in Flagellates, 110, 124 f.
Flagellata, 17 f., 40, 48 f., 50, 109 f.;
barotaxy of, 20;
galvanotaxy of, 22;
chemiotaxy of, 23;
of putrefying liquids, 44, 116 f.;
studied by botanists, 45;
as internal parasites, 48, 119 f.;
relations with Acystosporidae, 106;
shell of, 113;
stalk of, 113;
life-history of, 116 f.;
literature of, 119;
saprophytic, 119 f.
Flagellate stage, of Sarcodina, 56 f., 60, 109;
of Heliozoa, 74;
of Radiolaria, 85 f.
—see also Flagellula
Flagellated chamber, 170
Flagellula, 31;
of Didymium, 92
—see also Zoospores
Flagellum—see Flagella
Fleming, 168 n.
Flexible Corals, 326
Floricome, 203
Floscelle, of Echinocardium cordatum, 551;
of Cassidulidae, 554
Flowering plants, male cells of, 38
Flowers of tan (= Fuligo varians), 92 f.;
peptic ferment in, 16
Foam structure, 6
Folliculina, 137;
tube of, 152
Food, 35 f. (see also Ingestion);
of Higher Animals, 38;
absorption of, by Plants, 38;
in relation to life-cycle of Ciliata, 147 f.;
of Sponges, 237;
of Hydra, 256 and n.;
of Millepora, 261;
of Siphonophora, 304;
of Charybdea, 319;
of Alcyonium, 339;
of Zoantharia, 373;
of Asterias rubens, 439;
of Ophiothrix fragilis, 486;
of Ophiolepididae, 496;
of Echinus esculentus, 516;
of Echinarachnius parma, 546;
of Echinocardium cordatum, 552;
of Holothuria nigra, 561;
of Dendrochirota, 572;
of Synapta inhaerens, 577;
of Antedon rosacea, 583
Food-vacuole, of Actinosphaerium eichornii, 72;
of Ciliata, 145 f.;
of Carchesium, 146
Foot-plate, of young Pentacrinidae, 592;
of larva of Antedon rosacea, 619
Foraminifera, 40, 49, 50, 58 f.;
relations of, 49;
shell of, 49, 59 f., 60, 61, 63, 65;
habitat of, 59 f.;
literature of, 58 n.;
marine, 60 f.;
streaming of granules in, 17;
collection of, 62;
reproduction of, 67 f.;
economic uses of, 69 f.;
palaeontology of, 69 f.
Forbes, 338
Force, dual, of dividing-cell, 26 n.
Forcepia, 223
Forceps, 222
Forcipulate pedicellaria, 456, 473
Formative vacuole of contractile vacuole, in Flagellata, 110, 115;
in Ciliata, 143
Fossil, Foraminifera, 69 f.;
Radiolaria, 87 f.;
Dinoflagellata, 132;
Peridinium, 132;
Sponges, 192 f., 207 f., 215, 241;
Coelenterates, 270, 281 f., 343 f., 346, 393 f., 406;
Asteroidea, 475 f.;
Ophiuroidea, 501 f.;
Echinoidea, 556 f.;
Crinoidea, 594 f.;
Thecoidea, 596;
Carpoidea, 596 f.;
Cystoidea, 597 f.;
Blastoidea, 599 f.
Framboesia, 121 n.
France, epidemic of pébrine in, 107
Francé, on structure of funnel of Choanoflagellates, 115 n., 121 n.;
monograph of Choanoflagellates, 123, 182 n.;
on Polytomeae, 119 n.
Freetown, prophylaxis of malaria at, 106
Fringing reef, 390 f.
Frog's blood, Lankesterella in, 102
Fructification, of Mycetozoa, 90 f.;
of Acrasieae, 90;
Fry, E. and A., on Myxomycetes, 93 n.
Fuligo, 90;
F. varians, 92 f.;
pepsin in, 16
Fungacea, 402
Fungi, cell connexions in, 37 f.;
in relation to Protista, 40;
Gasteromycetous, 91
Fungia, 403;
asexual reproduction of, 388, 389;
F. crassitentaculata, 403
Fungiidae, 403
F. quadrangularis, 362
Funiculinidae, 362
Funnel, of Craspedomonadidae or Choanoflagellates, 111, 121, 122, 182;
of Phalausteridae, 111;
of choanocytes of Sponges, 171
Fusion of larval Sponges, 174
Fusion-nucleus of Ciliata, 150
—see also Reproduction, Syngamy, Zygotonucleus
Fusulina, 59
Galaxea, 400;
G. esperi, 400
Galeolaria, 307;
G. biloba, 304
Galeolarinae, 307
Galerites, 558
Gamble, 312 n.;
and Keeble, 175 n.
Gametes, 33 f.;
of Trichosphaerium, 54;
of certain Protomastigaceae, 116 n.;
of Volvocidae, 127 f.;
of Pandorina (of three sizes), 128, 129
Gametocyte of Acystosporidae, 104 f.
Gametogonium (= parent-cell of gametes), male, of Acystosporidae, 105
Gametonuclei (= nuclei capable of syngamous fusion), 34
Ganeria, 464
Gardiner, 345, 370, 375, 392 n., 404
Garveia, 270
Gasteromycetous fungi, 91
Gastral layer, 171
Gastralia, 201
Gastrozooids, of Millepora, 259, 260;
of Hydractinia, 264;
of Siphonophora, 299;
of Antipatharia, 408
Gastrula, definition of, 603
Gaule, misinterpretation of nature of Haemosporidae, 102
Gegenbaur, 302
G. varius, development, 172 f., 173, 174
Gemmantes, 400
Gemmaria, 405
Gemination = Budding, q.v.
Gemmiform, pedicellariae, of Echinus esculentus, 506;
of E. acutus, 509;
of E. elegans, 510;
of Cidaridae, 534;
of Echinarachnius parma, 544;
of Echinocardium cordatum, 550
Generation, spontaneous, 42 f.
Generations, alternation of, 44, 250
Genital base of Holothuria nigra, 567
Genital bursa, of Ophiothrix fragilis, 485
compared with hydrospires of Blastoidea, 600
Genital canal of Antedon rosacea, 586
Genital organs (including ducts), of Asterias rubens, 451 f.;
of Ophiothrix fragilis, 490;
of Ophiarachna, 491;
of Ophiuroidea, 494;
of Amphiura squamata, 494;
of Echinus esculentus, 528;
of Echinocardium cordatum, 552;
of Hemiaster philippi, 552;
of Holothuria nigra, 567;
of Antedon rosacea, 586
Genital plate, of Ophiothrix fragilis, 485;
of Echinus esculentus, 512, 513
Genital rachis, of Asterias rubens, 452;
of Ophiothrix fragilis, 490;
of Echinus esculentus, 528;
of Antedon rosacea, 586
Genital scale of Ophiothrix fragilis, 485
Genital stolon, of Asterias rubens, 451;
of Ophiothrix fragilis, 489;
of Echinus esculentus, 528;
of Holothuria nigra, 567;
of larva of A. rosacea, 619
Geodia, 211
Geographical distribution of Protozoa, 47
Geotaxy (= barotaxy), 20
Gephyrea, 577
Gerarde, 167
Gerardia savalia, 406
Gerbillus indicus infested by a Haemosporidian, 102 n.
Germinal spot (= nucleole of ovum), 7
Germinal vesicle (= nucleus of ovum), 7
Germination, 32;
of Myxosporidian spores, 107
Germ-plasm, 28 f.;
continuity of, 172
Germ theory, 44
Germs, invisible air-borne, 43
Gilchrist, 338
Gill-cleft, of Echinus, 514;
of Sphaerechinus, 540 f.;
of Strongylocentrotus, 541
Ginkgo, spermatozoa of, 38
G. scintillans, rate of fission of, 147 f.
Glauconite, 70
Globiceps, 272
Globiferae of Centrostephanus longispinosus, 532
-ooze, 61 f.;
Glossina morsitans, intermediate host of Trypanosoma brucei, 119;
G. palpalis, intermediate host of T. gambiense, 120
Glossograptus, 282
Glycerin, 15
Glycogen, of Ciliata, 144;
-vesicles of Pelomyxa palustris, 53
Gnat (Anopheles), intermediate host of Haemamoeba and Laverania, 103 f.;
(Culex) intermediate host of Haemoproteus, 103;
of Trypanosoma, 120
Golgi, on relation of Acystosporidian life-cycle and stages of intermittent fever, 103
Gonangium, 276
Goniaster, 471;
fossil, 475
Goniocidaris, 534;
G. canaliculata, 535
G. murbachii, 232, 290, 291, 292
Gonium, 111
Gonophore, of Gymnoblastea, 265;
of Calyptoblastea, 277;
of Stylasterina, 284;
of Siphonophora, 302
Gonozooids, of Siphonophora, 302;
of Antipatharia, 408
Gorgonacea, 350 f.
Gorgonella, 357;
spicule, 336
Gorgonia, 356;
G. cavolinii, 340;
G. flabellum, 357;
G. verrucosa, 356
Gosse, 273
on development of Bipinnaria, 612
Grammaria, 278
Granatocrinus, 599;
G. norwoodi, 600
Grant, 167
Grantiidae, 192
Grantiopsis, 191
Granular disintegration of Protista, 14 f.
Granules, in protoplasm, 6;
aleurone, 37;
basal, of cilia, etc., 138 n., 141 (see also Blepharoplast);
proteid, of Suctoria, 161
Graphiohexaster, 203
Graptolitoidea, 281
Grassi, on malarial parasites, 103
Gravity, stimulus of, 19 f.
Greasy film, outer clear layer of protoplasm behaves like, 17
Greeff, on Protozoa, 46
Green Flagellates, relations of, 48
Greensand, 70;
Cambridge, 208
Green water often due to Euglena viridis, 124
Greenwood, M., on peptic digestion in Protozoa, 16;
on feeding of Carchesium polypinum, 45 f., 146 f.
G. blattarum, 98
Gregarines, habitat, 99;
syngamy, 99
Gregory, 346
Grew, 166
Grey chalk, 61
Gromia, 52;
G. oviformis, 59 n.
—see also Allogromia
Grooves, longitudinal and transverse, of Dinoflagellata, 110, 130, 131, 132;
of Peridinium, 131;
of Polykrikos, 132;
oral, of Noctiluca, 133
Grosvenor, 249 n.
Growth, 19 f.;
Gruber, on regeneration in Protozoa, 35 n.;
on diffused nucleus in marine Ciliata, 144 n.;
on tubicolous marine Ciliata, 152
Gruppe, deposit of Radiolaria, 87
Guinea Coast, 106
Gullet (= pharynx) of Paramecium caudatum, 151
Gut, supposed, of Ciliata, 145
—see also Alimentary canal
Gutter, oral, of Vorticellidae, 156, 158
Gymnamoebae, 51 n.
Gymnasteridae, 471
Gymnoblastea, 262 f.
Gymnodinium, 110;
G. pulvisculus, parasitic in Appendicularia, 132
Gymnomyxa, 49 n.
Gymnophrys, 58
Gymnostomaceae, 137;
predaceous, trichocysts of, 143;
mouth and pharynx of, 145;
noteworthy members of, 152
Gyractis, 380
Häcker, on skeleton of Radiolaria, 82 n.
Haddon, 382
Haeckel, 168, 185, 192, 237, 308;
on Protista, 40 f.;
on Protozoa, 46;
on Heliozoa, 71;
on classification of Radiolaria, 76;
on functions of porocone in Radiolaria, 81;
on enumeration of Radiolaria, 87 f.;
on Myxobrachia, 83;
on phylogeny of Echinodermata, 622
H. malariae, parasite of quartan fever, 104 f.;
H. vivax, parasite of tertian fever, 104 f.
Haematochrome, 125
Haematococcus (= Sphaerella, 111), 125, 126
Haemoflagellates (= Trypanosoma, etc., q.v.), 119 n.
Haemoglobin, 103;
in water-vascular system of Ophiactis virens, 499
Haemogregarina, 97
Haemomenas (Ross's name for parasite of pernicious fever = Laverania, 97), 105
Haemoproteus, 97;
parasitic in birds, 103
Haimea hyalina, 342;
H. funebris, 342.
Haimeidae, 342
H. chrysanthellum, 380
Haleremita, 256
Halicalyx, 291
H. panicea, structure, etc., 168 f., 169, 170, 211
H. auricula, 320
Haliomma, 77
Haliphysema, 59
Halomitra, 404
Halteridium, 97;
regarded by Schaudinn as a state of Trypanosoma, 103 n., 120
Hamacantha, 223
Hamann, on supposed cavities in the body-wall of Asteroidea, 449;
on classification of Zygophiurae, 495 n.
Hanitsch, 168 n.
Hapalocarcinus, 402
Hardy, on structure of protoplasm and clearing, 11, 12 n.
Hartea elegans, 342
Hartog, on Protozoa, 1 f.;
on structure of protoplasm, living and dead, 11;
on function of contractile vacuole, 15 n.;
on intracellular digestion, 16;
on brood-division (multiple cell-division), 16 n.;
on dual force of dividing cell, 26 n.;
on syngamy, etc., 34 n.;
and Dixon, on pepsin in Pelomyxa, 16
Harvey, "omne vivum ex ovo," 42
Hauerina, 59
Heart, of Asterias rubens, 450
Heart-urchins = Spatangoidea, q.v.
Heat—see Temperature
Heat-rigor, 22
Heleopera, 52;
test of, 55
Heliaster, 474
Heliolites, 346
Heliolitidae, 346
Heliopora, 330, 334, 337, 345 f.
Helioporidae, 346
streaming of granules, 17;
regeneration, 35;
habitat, 48;
locomotion, 73;
various forms of, 74;
marine, 75;
distribution of, 75;
resemblance of Suctoria to, 159
Hemiaster, 556;
H. philippi, 552, 555, 602, 603
Hemichordata, 616
Henneguy, on protoplasm, 3 n.;
on syngamy, 34 n.
Henneguya, 98
Henricia—see Cribrella
Hérouard, Delage and, on Protozoa, 46
Herpetolitha, 404
Herpetomonas, 115
Hertwig, R., on Protozoa, 46;
on chromidia in Sarcodina, 52 n.;
on Heliozoa, 71;
on Radiolaria, 88;
Heteractinellida, 208
Heterastridium, 283
Heterocoela, 187 f.
Heterophrys, 71
Heteropidae, 192
Heterotrichaceae, 137, 153 f.;
fission of, 147
Hexactine (a triaxon in which all six actines are developed), 184
Hexactinellida, 194, 195, 197 f., 228, 240
Hexadella, 196
Hexamitus, 115
Hexaster (a hexactine with secondary or terminal rays = Carter's "rosette"), 203
Hexasterophora, 203 f.
Hickson, on interchange of cytoplasm in conjugating Infusoria, 149 n.;
on conjugation in Suctoria, 161 f.;
on Coelenterata, 243 f.;
on Millepora, 259 n.;
on Stylasterina, 286 n.;
on Alcyonaria, 329 n., 351 n., 352 n., 359 n.;
on Antipatharia, 408 n.;
on Ctenophora, 412 f.
Hieronymus on Chlamydomyxa, 90 n.
Hincks, 268 n.
Hippasterias, 471
Hippopodius, 307
Hippospongia, 221
Holectypoidea, 558
Holophytic, Algae and Fungi, zoospores of, 5;
nutrition, 37;
Flagellates, 113
Holopodidae, 592
Holothuria, 570;
H. nigra, 561 f.;
shape, 561;
feelers, 561;
body-wall, 562;
alimentary canal, 562;
respiratory trees, 563;
water-vascular system, 564;
nervous system, 566;
calcareous ring, 566;
blood system, 567;
genital organs, 567;
H. cinerascens, 567;
H. fusco-rubra, 567;
H. aspera, 570;
H. intestinalis, 570;
H. tremula, 570
Holothuroidea, 431, 537, 560 f., 583;
mesenchyme of larva, 604;
development of, 609, 614, 615;
phylogeny of, 622
Holotrypasta (= Porulosa), 76
Holozoic, 35 f.;
Flagellates, 113;
Dinoflagellates, 131
Holt, 311 n.;
on burrowing habits of Strongylocentrotus lividus, 541 n.
Homaxonic (= symmetrical about a centre along an indefinite number of equivalent axes), 76
Homocoela, 185 f.
Homoeonema, 294
Homostichanthus anemone, 383
Honey-bees, alleged spontaneous generation of, 42
Hormiphora, 418;
H. plumosa, 413
Human diseases, produced by Coccidiaceae, 102 f.;
by Amoeba histolytica, 57;
by Trypanosomids, 119 f.
Huxley, on Protozoa, 45;
first description of a living Radiolarian, 88;
on Cystoflagellates, 135
H. sieboldi, 206;
Hyalopus, 52;
H. dujardini, 59 n.
Hyalosphenia, 52;
H. lata, 55
Hyboclypus, 558;
H. gibberulus, 558
Hybocodon (Corymorphidae, 273), 265
Hydatina senta, often found with Euglena viridis, 124
nematocysts of, 247;
species of, 256;
specific gravity of, 13 n.;
host of Kerona and Trichodina, 158;
H. oligactis (= fusca), 253, 256;
H. pallida, 256 n.;
H. vulgaris, (= grisea), 253, 256;
nematocyst, 247
Hydractinia, 263, 265, 268, 270
Hydrallmania falcata, 278
Hydrichthys mirus, 268
Hydroceratinidae, 279
Hydrocladia, 276
Hydrocoel (including left hydrocoel), 428;
of Antedon rosacea, 585;
development in Asterina gibbosa, 611;
in Auricularia, 615;
in Antedon rosacea, 619
Hydroctena salenskii, 423, 424
Hydrolaridae, 273
Hydrorhiza, 262
Hydrospire, of Blastoidea, 580, 599;
of Codaster, 599;
of Pentremites, 599;
of Granatocrinus, 599 f.
Hydrotheca, 275
Hydrozoa, 249 f.;
hydrosome, 250;
life-history, 250;
sense-organs, 252
Hydrurus, 110;
theca of, 113
Hymedesmia, 222
Hymenaster, 466;
H. pellucidus, 465
Hymeniacidon, 224
Hymeraphia, 223
Hyocrinidae, 590
Hyocrinus, 588, 589, 590, 590;
H. bethellianus, 590
Hyperia (Amphipod), parasitic in Radiolaria, 87
Hyphalaster, 471;
H. moseri, 459
Hypnocyst, of Rhizopoda, 57;
of Proteomyxa, 88 f.;
of Pseudospora, 89;
of Myxomycetes, 90 f.
Hypolytus peregrinus, 262, 271 n.
Hypophare, 210
Hypostome, 250
Hypotrichaceae, 137, 138 f., 158 n.
Ianthella, 220
Ichthyophtheirius, 137;
noxious parasite of fish, 152
Iciligorgia, 351
Idioplasm, 29
Ilyanthus mitchellii, 380
I. maculatus, 571
Imperforate, Foraminifera, 58 f.;
Corals, 371
Inadunata, 595
Incurrent canal, 170
India, diseases of Trypanosomic origin, 119 f.
Induction shocks, action on Protozoa, 7, 22
Infero-marginal ossicle of Asteroidea, 436
Inflammation, 8
Infra-basal plate, of Crinoidea, 588;
of fossil Crinoidea, 594;
of larval Antedon rosacea, 619
Infundibulum, 415
Infusions, appearance of organisms in, 42 f.;
organisms of, 136
Infusoria, 40, 48, 50, 136 f.;
specific gravity of, 13 n.;
zygote does not encyst, 34.
Ingestion, of food, by Amoeba limax, 9;
by Choanoflagellates, 122;
by Dinoflagellates, 131;
by Carchesium, 146;
by Coleps, 150
—vacuole of, in Flagellates, 113;
in Oikomonas, 112;
in Choanoflagellates, 122
Inner perihaemal ring-canal, of Asterias rubens, 448;
development of, in Asterina gibbosa, 612
Inoculation of malarial fever in man through a mosquito, 105 f.
Insectivorous plants, 38
Insects, metamorphoses of, 44;
as hosts of Trichonymphidae, 123
Interambulacral area, of Echinarachnius parma, 544;
of Echinocardium cordatum, 550
Interambulacral plate, of Echinus esculentus, 511;
of Cidaridae, 533 f.;
of Echinarachnius parma, 544 f.
Interbrachial septa—see Interradial septa
Interchanges between cell and medium, 14
Intermediate dorsal process of ciliated band of Auricularia, 608
Intermediate (= supplemental) skeleton of Perforate Foraminiferal shell, 63, 66
Intermittent fever, malarial, produced by Acystosporidae, 103 f.
Internal budding of Suctoria, 160 f., 162;
of Ephydatia, 177
Internal gills—see Stewart's organs
Internal movements of protoplasm, 17
Interradial plates, of calcareous ring of Holothuria nigra, 566;
of Holothuroidea, 569;
of Synaptida, 569;
of Dendrochirota, 569;
of calyx of Crinoidea, 589;
of Thaumatocrinus, 589;
of Hyocrinus, 590;
of Rhizocrinidae, 591;
of corona of Echinoidea—see Interambulacral plate
Interradial septa, of Asterias rubens, 437;
of Heliasteridae, 474;
absent in Brisingidae, 475
Interradius, 428;
of Asterias rubens, 434;
of Echinus esculentus, 504;
of Holothuria nigra, 562
Interstitial growth, 10
Intestine, 415;
of Echinus esculentus, 516;
of Holothuria nigra, 563;
of Antedon rosacea, 583;
of Actinometra, 589;
of Dipleurula, 605;
of Protocoelomata, 616
Intracapsular protoplasm of Radiolaria, 80 f.
Intramolecular respiration, 14 n.
Intranuclear spindle of Euylypha, 29
Invertebrata, hosts of Gregarines, 97 f.
Iodine, 239
Iophon, 223
Iridogorgia, 355
Isaurus, 405
Ischadites, 207
Ischikawa, on syngamy of Cystoflagellates, 135;
on structure of Ephelota, 162
Isidella, 354
Isis, 353
Ismailia, prophylaxis of malaria at, 106
Isochela (a chela divisible by each of two planes into two equal parts, the two ends being equally developed), 222
Isocrinus—see Pentacrinus
Isogamy, 33 f.;
of Rhizopoda, 56 f.;
of Stephanosphaera, 128
—see also Syngamy
Isospores, 85;
of Radiolaria, 76;
of Collozoum inerme, 76
Jaekel, on Silurian Asteroidea and Ophiuroidea, 501;
on classification of Crinoidea, 589, 595;
on classification of Cystoidea, 598
James-Clark, on Protozoa, 46;
on Choanoflagellates, 121, 123;
on Sponges, 167
Jaw, of Ophiothrix fragilis, 482;
of fossil Ophiuroidea, 502;
of Echinus esculentus, 526
Jelly, forming theca in Flagellates, 113
Jennings, on protoplasmic movements, 4 n., 16 n.;
reaction of Protista to repellent stimuli, 20 n., 21 n.
Jensen, on density of living protoplasm, 13 n.;
on protoplasmic movements, 16 n.
Joblot, on organisms of putrefaction, 43;
on Protozoa, 45
Joenia, 111
Johnson, 352 n.
Jung, 253 n.
Jungersen, 359 n.
Karyogamy, 34 n.
—see also Syngamy of Ciliata
function of, 28 f.;
of micronuclei of Ciliata, 144 f.
—see also Mitosis
Karyolysus, 97
Karyosome, 24
Keeble, 175 n.
Keller, 233
Kemna, on stylopodium of Foraminifera, 60
Kent, Saville, on Choanoflagellates, 122 f., 182;
on Infusoria and Flagellates, 136 n.
Keroeides, 351
Kerona, 138;
K. polyporum, 158 n.
Kieselguhr, 87
Kirkpatrick, 215
Kishinouye, 313 n., 321 n., 333, 352
Klebs, on Flagellates, 119;
on Dinoflagellates, 130
Koch, von, on methods of cultivation of lower organisms, 44;
on malarial parasites, 103
Kölliker, on Sporozoa, 94 f.
Kophobelemnon, 362
Kophobelemnonidae, 362
Köppen, on Sticholonche and its parasite, Amoebophrya, 87 n.
Krukenberg, on pepsin in a Myxomycete, 16
Kükenthal, 363
Labbé, on Protozoa, 45;
monograph of Sporozoa, 102 n.
Labial plexus of Antedon rosacea, 587
Labyrinthine shell-wall of arenaceous Foraminifera, 59, 66
Labyrinthula, 90 f.
Lachmann, Claparède and, on Protozoa, 45;
on Suctoria, 162
encystment, 147
Lafoea, 280;
L. dumosa, 280
Lafoëina (Campanulariidae, 280), 277
fossil, 70
Lagenaceae, 59
Lagoon, 390 f.
Lamblia, 111;
L. intestinalis, conjugation, 116 n.
Lampetia, 418
Lancet-plate, 599
Lang, on syngamy, etc., 34 n.;
on Protozoa, 46;
on distinctions of pseudopodia, 47 n.
Lankester, on Protozoa, 45 f.;
on classification of Protozoa, 49 n.;
on Proteomyxa, 89;
on Sporozoa, 94;
on Haemosporidae, 102;
on pigment of Stentor coeruleus, 154 n.;
on Torquatella, 155 n.;
on chlorophyll of Ephydatia, 175;
on Limnocodium, 292
Lankesteria, 97;
L. ascidiae, life-cycle of, 95
Lantern-coelom of Echinus esculentus, 524;
represented by buccal sinus of Holothuria nigra, 566
Lanuginella pupa, 198
Lar, 273;
Larcoidea, 77
Larva, of sponges, 180, 226, 227;
"asexual," 228;
of Tubularia, 271;
of Stylasterina, 284;
of Trachomedusae, 290;
of Narcomedusae, 295;
of Velella, 302;
of Scyphozoa, 317;
of Alcyonaria, 341;
of Renilla, 360;
of Zoantharia, 373;
of Zoanthidae, 405;
of Cerianthidea, 411;
of Cribrella, 462;
of Luidia, 605;
of Asterina gibbosa, 463, 610, 611, 612;
of Ophiuroidea, 606;
of Echinus, 607;
of Synapta, 608;
of Antedon rosacea, 618, 619, 620
Larval brain, of Echinopluteus, 607;
of Antedon rosacea, 619
Larval type of development, 601
Lateral mouth-shields of Ophiothrix fragilis, 485
Lauterborn, on sapropelic organisms, 48;
on budding in Rhizopods, 56 n.
Laveran, on Sporozoa, 94;
on Acystosporidae, 102
Laverania, 97;
parasite of bilious or pernicious fever, 104 f.
Lebrunia, 382;
L. coralligens, 373
Lecqueureusia, 52;
L. spiralis, test of, 55
Lee, A. Bolles, on action of clearing reagents, 11 n.
Leech, host of Haemogregarina, for sexual process, 102
Leeuwenhoek, on organisms of putrefaction, 42 f.
Léger, on Protozoa, 45;
on Sporozoa, 94;
on sperms of Pterocephalus, 99 n.;
and Duboscq, on Sarcocystis tenella, 108 n.
Leidy, on Protozoa, 46
Leiopathidae, 409
Leiosella, 225
Leipoldt, on the madreporic vesicle of Echinoidea, 528
Lelapia australis, 192
Lembadion, 137;
caudal cilia of, 141 n.
Lembus, 137;
caudal cilia of, 141 n.
Lemnalia, 349
Lendenfeld, von, 218, 220, 220 n.
Lepidogorgia, 355
Leptobrachiidae, 325
Leptopenus, 404
Leptophyllia, 404
Lesser, Hertwig and, on Heliozoa, 71
Lesueuria, 419
Lesueuriidae, 419
Leucin, 15
Leuckart, 245
Leucocyte, 4 f.;
movements of, 7 f.
Leucophrys, 137
Leucosin, 115
Leucosolenia, 221;
collar-cell, 186;
larva, 227 f.;
spicule, 232;
Leucosoleniidae, 185 f.
Levander, on Caenomorpha, Metopus, etc., 154 n.
Leydenia, 90 f.
Liberation of sporozoites of Acystosporidian parasite in relation to fits of fever, 103
Lice, supposed spontaneous generation of, 42
Lichen compared to Radiolarian with symbiotic holophytic organisms, 86
Lichnophora, 138;
adoral wreath, 138 n.
Lieb, Calkins and, on rhythm in life-cycle of Ciliata, 148 n.
Life-cycle, life-history, of Trichosphaerium sieboldi, 54, 56;
of Polythalamic Foraminifera, 67 f.;
of Lankesteria ascidiae, 95 f.;
of Coccidium schubergi, 99 f., 101;
of malarial parasites, 103, 104 f.;
of Flagellata, 116 f.;
of Ciliata, 147 f.
Light, stimulus of, 19, 21 f.;
function of, in carbohydrate formation, 36;
effect on Euglena, 125
Lillie, on regeneration in Protozoa, 35 n.
Limicolous Protozoa, 48
Limit of growth, Herbert Spencer's, 23 f., 31
Limnocnida, 293;
L. tanganyicae, 293
Limnocodium, 293;
L. sowerbyi, 292
Linantha, 322
Lindström, 346
Linerges (allied to Atollidae, 322), 316
of ovum of Sea-urchin, 7
Linuche, 322
Lipochrome, 39
Liriantha appendiculata, 291, 295
L. rosacea, 289
Lissodendoryx, 224
Lissomyxilla, 225
Lister, A., on Myxomycetes, 93 n.
Lister, J. J., on Foraminifera—reproduction, 67 f.;
dimorphism, 67;
palaeontology, 70;
classification, 58 f.;
on Astrosclera, 194 n.
Lithobius forficatus, host of Coccidium schubergi, 99
Lithocercus, 78;
L. annularis, 82
Lithoninae, 193 f.
Lithostrotion, 394
Littoral Protozoa, 48
Lituaria, 364
Lituola, 59
Lituolidaceae, 59
Living beings, characters of, 16 f.;
criterion of, 11
Lobopodia, 47 n.
Locomotion, in Heliozoa, 73
Loeb, Jacques, on "chemical" fertilisation, 32 n.;
on polarity in regeneration, 229 f.
Lohmann, on Silicoflagellates, 114 n.
Longitudinal band of cilia of Dipleurula, 604;
of Tornaria, 616
Longitudinal fission of Eutreptia viridis, 124;
of Bodo saltans, 117 f.;
of Craspedomonadidae, 122
Longitudinal flagellum and groove in Dinoflagellata, 130, 131
Longitudinal section, of a young Asteroid, 445;
of a young Ophiuroid, 486;
of a Holothuroid, 563;
of Antedon, 584;
of free-swimming larva of Antedon, 618
Lophocalyx philippensis, 229
Lophoctenia, 418
Lophohelia, 399;
L. prolifera, 399
Lophophore, 579
Lophophyllum, 406 f.
Louse, host for sexual process, etc., of Haemosporidian, 102 n.
L. campanulata, 321
Lucernariidae, 320
Ludwig, on the blood-system of Asteroidea, 449;
on the axial sinus of Ophiuroidea, 487;
on the classification of Holothuroidea, 570
Lühe, figures of Lankesteria, 95
fossil, 475;
larva of L. ciliaris, 605
Luminosity or phosphorescence of sea, due to Cystoflagellata, 132, 134 f.;
to Dinoflagellata, 132
Lunule, 548
Lychnorhiza, 325
Lychnorhizidae, 325
Lytocarpus (Plumulariidae, 279), 277
Maas, 168, 189 n., 228 n., 230, 231 n., 232 n., 233, 324
MacBride, E. W., on Echinodermata, 425 f.
MacBride, Massee, on Myxomycetes, 93 n.
MacCallum, on malarial parasites, 103
M‘Dougall, on motile reaction of Protozoa, 19 n.
M‘Intosh, 370 n.
MacMunn, 169
Macrocnemic, 405
Macrocneminae, 405
Macrogonidia of Volvox, 126, 127
Macro-, prefix misused to mean "large," usually replaced here by "mega," q.v.
Madrepora, 368, 373, 387, 389, 395;
M. forma cervicornis, 395;
M. forma palmata, 395;
M. forma prolifera, 395
Madreporaria, 369, 371, 384 f.
Madrepores, 326 = Madreporaria, q.v.
Madreporic vesicle (or right hydrocoel) of Asterias rubens, 448;
of Ophiothrix fragilis, 490;
of Echinus esculentus, 528;
development in Dipleurula, 609
Madreporidae, 395
Madreporite, 428;
of Asterias rubens, 434;
of Ophiothrix fragilis, 487;
of Ophiuroidea, 493;
of Cladophiurae, 493;
of Echinus esculentus, 512, 517;
of Echinocardium cordatum, 562;
of Holothuria tubulosa, 564;
of Elasipoda, 571;
of Pelagothuriida, 572;
in older fossil Pelmatozoa, 583;
in Thecocystis sacculus, 596
Magosphaera, 89
Maidenhair tree, spermatozoa of, 38
Malarial fever produced by Acystosporidae, 103 f.
Mal de Caderas (= falling sickness of cattle), 119
Male gamete, 33;
motile in Lower Plants, Higher Cryptogams, Cycads, and Ginkgo, 38;
of Pandorina, 128 f.;
of Eudorina, 129;
of Peritrichaceae, 151
—see also Sperm, Spermatozoon
Malignant tumour, associated with Leydenia, 91
Mammals, syngamy in, 34;
contain Sarcosporidiaceae in muscles, 108
Man, host of Amoeba, 57;
of Coccidiaceae, 102 f.;
of Sarcocystis tenella, 108 n.;
of Trichomonas vaginalis, 119;
of Trypanosomes, 119 f.;
of the Ciliata Nyctotherus and Balantidium, 152
Manicina (Fam. Astraeidae, 399), 373;
Mann, on function of nucleus, 24 n.
Manson, on relation of Filarial disease to gnats or mosquitos, 103
Manson, the subject of inoculation experiments with malarial parasites, 106
Manubrium, 251
Margelis ramosa, 269
Marginal, anchors, 320;
cirrhi, 139 f.
Marginaster, 464
Marine, Foraminifera, 60 f.;
Heliozoa, 75
Marrow, red, of bones, habitat of resting states of malarial parasites, 106 n.
Marshall, on amphidiscs, 179
Marshall, on Pennatulacea, 359 n.;
on the physiology of the nervous system of Antedon rosacea, 585
Marsigli, 167
Marsupifer valdiviae, 379
Marsupites, 588
Maryna, 137;
M. socialis, tube, 152
Massee, on Myxomycetes, 93 n.
Mastigophora (Bütschli's name for Flagellata), 109
Maturation of schizont of Acystosporidian parasite in relation to fever-fit, 103
Maupas, on Protozoa, 45;
on life-cycle of Ciliata, 147 f.
Mayer, 312
M. labyrinthica, 370
Mechanical stimuli, 19 f.
Median dorsal process of ciliated band of Bipinnaria, 606
Medium gametes in Pandorina, 128 f.
Medusa, 250;
of Millepora, 259 f.;
of Gymnoblastea, 262 f.;
of Calyptoblastea, 277 f.;
in Trachomedusae, 288 f.;
in Narcomedusae, 295 f.;
fresh-water, 292 f.;
in Scyphozoa, 310 f.
Megagamete, 33;
see also Female gamete, Oosphere
Megalactis griffithsi, 384
Megalosphere, megalospheric, 67 f.
Meganucleus, 136, 139 f., 144, 149 f.;
degeneration of, in conjugation, 148 f.;
new formation of, in conjugation, 148, 151;
of Stylonychia mytilus, 139 f.;
of Carchesium, 146;
of Paramecium caudatum, 148, 151;
of Trachelius ovum, 153;
of Stentor, 154;
of S. polymorphus, 156;
conjugation of, in Dendrocometes, 161
Megazooid of Vorticella, 157
Megazoospores, 85
Meissner, on classification of Spatangoidea, 554 n.
Melanin, 103
M. chamaeleon, 338;
Melobesia, 422
Melonitidae, 557
Membrana reticularis, 199, 200
Membrane, undulating, of Flagellata, 110, 115, 123;
of Trypanosoma, 115;
of Trichonymphidae, 123;
of Ciliata, 137, 139 f., 145, 156 f.;
of Stylonychia mytilus, 139;
of Pleuronema, 145;
of Caenomorpha uniserialis, 155;
Membranella, 137, 139 f., 145;
of Stylonychia mytilus, 139 f.;
of Metopus sigmoides, 154;
of Caenomorpha uniserialis, 155;
of Vorticella, 156
Merozoite, 97;
of Coccidium schubergi, 99 f., 101;
of Haemosporidae, 102;
of Acystosporidae, 103, 104 f.
Mertensia, 417;
M. ovum, 417;
stage of Lobata and Cestoidea, 414
Mertensiidae, 417
Mesenchyme, 604
Mesenteric filaments, Alcyonaria, 331, 333;
Zoantharia, 369
Mesenteries, of Alcyonaria, 329, 334;
of Zoantharia, 329, 366 f., 368;
of Asterias rubens, 439;
of Holothuria nigra, 562;
Mesnil, on Sporozoa, 94;
Caullery and, on Actinomyxidiaceae, 98 n.
Mesogloea, 246;
of Alcyonaria, 330
Metabolic, metabolism, 13
Metacnemes, 367
Metallogorgia, 355
Metamorphosis, of Insects, 44;
of Dipleurula, 610 f.
Metaphytes, 41
—see also Plants, Higher
Metazoa, 40 f.;
rheotaxy of, 21;
origin of, from Protozoa, 40 f.;
flagellate sperms of, 109;
hosts of Polymastigidae, 111
—see also Animals, Higher
Method of study of the life-cycle, of organisms of putrefaction, etc., 44;
of Flagellata, 116;
of Ciliata, 147
Metopus, 137;
M. pyriformis, 154;
M. sigmoides, 154
Metridium, 381 (= Actinoloba, q.v.)
Metschnikoff, 167, 178, 237 n., 296
Microbes, 44
Microciona, 225
Microgamete, 33;
of certain Coccidiaceae, 101
—see also Sperm, Spermatozoon
Microgromia socialis, 59 f., 60
Microhydra, 256
Micronuclei, micronucleus, 136, 139, 144 f., 148 f., 151 f., 155, 157, 159, 160 f.;
of certain Flagellata, a blepharoplast, 109 n.;
relations of Trypanosomic blepharoplast to, 121;
of Stylonychia mytilus, 139;
of Paramecium caudatum, 148, 151;
in conjugation, 148 f.;
numerous, of Stentor, 154;
of Vorticella, 157;
of Suctoria, 160 f.;
of Podophrya, 160;
of Acineta jolyi, 160
Micropyle, 230
Microscleres, 176
Microsolena, 404
Microsphere, microspheric, 67 f.
Microzooid of Vorticella, 157
Microzoospores, 85
Miescher's tubes, 108
Migratory pairing nucleus, 149 f.;
of Peritrichaceae, 151 f.
Miliola (Quinqueloculina), 65
Miliolidaceae, 59
Milleporina, 257 f., 258, 260, 282;
Milleporina, 257
Mimicry among Gymnostomaceous Ciliata, 152 n.
Minchin, on Sporozoa, 94 f.;
on Sponges, 168, 172 n., 185, 186 n., 227 n., 232, 316 n.
Minnows prey on Anopheles, 106
Minous inermis, 268
Minyadidae, 328, 366, 377, 383
Miserly cells, 32 f.
Mithrodia, 464
Mithrodiidae, 464
functions of, 28 f.;
of micronuclei in Ciliata, 144
Mitrophanow, on trichocysts, 142 n.
Mitrophyes, 306
Miyajima, 273
Mnemia, 420
Mnemiidae, 420
Mnemiopsis, 420
Mnestra (position undetermined), 269
Mohl, von, on protoplasm, 3
Mole-cricket, host of Lophomonas, 123
Molluscs, hosts of Gregarines, 98
Molluscum contagiosum, 102
Molpadiida, 568, 569, 575, 576, 577, 578
Monacanthid, 457
Monadidae, 111
Monadineae, applied to Proteomyxa by Cienkowsky and Zopf, 89
Monads, a name for the lowest, simplest Flagellata, 109, 116 n.
M. dallingeri, gametes of, 116 n.
Monaxonic (= symmetrical about one single axis), 76
Moniliform meganucleus of Stentor, 156
Monobrachiidae, 274
Monobrachium, 274
Monocyclica, 594
Monocystis, 97 f.
Monograptus, 282
Monophyidae, 306
Monoprionidae, 282
Monopylaea (= Nassellaria), 76
Monorhaphis, 197
Monosiphonic, 275
Monotrypasta (= Osculosa), 76
Monoxenia darwinii, 342
Monstrous Foraminiferal shells, possible formation of, 69
Moore, 293
Mopsea, 353
Morgan, on regeneration, 35 n.
Morphological contrast of Animals and Plants, 38 f.
Mortensen, on classificatory value of pedicellariae, 532;
on classification of Cidaridae, 534;
of Echinothuriidae, 536
Moseley, 258, 333, 338, 345, 411
Mosquito (= gnat), 103 f.;
dappled-wing-, intermediate hosts of Acystosporidae, 103
Mosquito-netting, a prophylactic against malarial fever, 103
Moss-dwelling Protozoa, 48
Mosser, F., 418 n.
Motile organs, 17 f.
Motile reactions of Protozoa, 19 f.
Motility, 9
Motion, ciliary, 18;
gliding, of protoplasm, 47 n.
Moulting of cuticle or cell-wall in Dinoflagellata, 130;
of Dendrocometes, 161
Mouth, of Flagellata, 113;
absent from Opalinidae, 123;
of Maupasia, 124;
excreta expelled by, in Noctiluca, 133;
of Gymnostomaceae, 137, 143, 145, 152;
of Stylonychia mytilus, 139 f.;
of Dysteria, 145;
of Pleuronema, 145;
of P. chrysalis, 153;
of Paramecium caudatum, 148, 151;
trichocysts of, in Gymnostomaceae, 143;
of Trachelius ovum, 153
Mouth-angle of Ophiothrix fragilis, 482
Mouth-frame, of Asteroidea, 436, 483;
of Ophiothrix fragilis, 482;
of Ophiarachna incrassata, 484;
of Ophiacantha, 492;
of Ophioscolex, 492;
of Ophiothrix, 492
Mouth-papilla, of Ophiuroidea, 483, 492;
of Ophiocoma, 493
Movements, amoeboid, 5 f., 125 n.;
of Protista, 16 f.;
of Higher Plants, how produced, 38;
springing, of Bodo saltans, 114;
of Euglena, 124 f.;
euglenoid, 125 f.;
metabolic, 125 n.;
of Sporozoa, 125 n.;
of Stylonychia, 138;
springing, of tailed Ciliata, 141 n.;
of Halteria, 155;
of Suctorian tentacles, 159 f.
Muggiaea, 306;
M. atlantica, 304;
M. kochii, 303
Müller, J., on recognition of Echinoid larva, 518;
on the name Pluteus, 607
Müller, O. F., on Protozoa, 45
Multicilia, 109
Multinucleate Amoeba (Pelomyxa), 16;
Protozoa, regeneration of, 35
Multiple budding, in Suctoria, 160 f.
Multiple fission, 30 f.
—see also Brood-division
Muricea, 356
Muscle of Vorticella, 157
Muscle-cell, 19
Muscular contraction, physical explanation of, 19
—see also Myonemes
Mussa, 401
Mycetozoa (= Myxomycetes, q.v.), 50, 90 f.;
in relation to Fungi, 40;
studied by botanists, 45;
relations of, 49
of Trypanosoma, 120 f.
of Stylonychia, 140;
of Ciliata, 142;
of Vorticella, 157
Myophrisks, 80
Myriophrys, 71
Myriothelidae, 274
Myxaster, 466
Myxasteridae, 464
Myxidium, 98;
M. lieberkühnii, 107
Myxilla, 225
Myxobolus, 98;
spores of, 107
Myxobrachia, 83
Myxogasteres, Myxogastres, 90 f.
Myxoidea, 89
Myxomycetes, 90 f.;
rheotaxy of plasmodium in, 21
—see also Mycetozoa
Myxospongiae, 196
spores, 107
Nagana disease of hoofed quadrupeds, 119
Naked Protozoa, 51 n.
skeleton of, 83;
geological occurrence of, 88
Nassoidea, 78
Nausithoe, 322;
Scyphistoma of (= Spongicola fistularis), 317;
N. punctate, 322;
N. rubra, 322
Needham on spontaneous generation, 43
of Actinomyxidiaceae, 98;
of Myxobolus mülleri, 107;
of Epistylis, 249;
of Aeolis, 248;
of Hydra, 247;
of Siphonophora, 300;
of Scyphozoa, 312;
of Alcyonium, 247;
of Sarcophytum, 248;
of Cerianthus, 247
Nematodes parasitic in blood, 103
Nematophores, 277
Nemocera (= gnats or mosquitos), 103 n.
Neohelia, 399
Neolampas, 554
Nephthyidae, 349
Neresheimer, on neurophane fibrils in Ciliata, 143 n.
Nerve-ring, of Asterias rubens, 444, 447;
of Ophiothrix fragilis, 488;
of Echinus esculentus, 518, 521, 527;
of Antedon rosacea, 583;
outer, of A. rosacea, 585
Nervous fibrils in Ciliata, 143
Nervous system, in Animals, not in Plants, 39 f.;
of Asterias rubens, 444 f.;
of Ophiothrix fragilis, 488;
of Echinus esculentus, 518 f.;
of Holothuria nigra, 566;
of Antedon rosacea, 583 f.
Neuron, 444
Neurophane (= supposed nervous fibrils in Ciliata), 143 n.
Newts, Trichodina parasitic in, 158
Nitriles in relation to nutrition, 36
endosarc, 144;
N. miliaris, 133
Nosema, 98;
N. bombycis, 107;
organism of pébrine, 107
Nubecularia, 59
Nuclear apparatus, of Infusoria, 48, 136;
of diffused granules, in marine Ciliata, 144 n.;
of Suctoria, 159
—bipartition in Trichosphaerium, 54
—reduction of Actinosphaerium, 75 n.;
of Monocystis, 96;
of Acystosporidae, 104 f.;
of Myxosporidiaceae, 107;
of Flagellates, 116 n.
—divisions, in spores of Lankesteria, 95
—see also Mitosis, Karyokinesis
Nuclearia, 70
Nuclein mass (= karyosome), 24
Nucleinic acid, 7 n.
Nucleole, nucleolus, 7, 24, 25 f., 27;
of Sea-urchin ovum, 7;
of Sphaerella, 126
Nucleolidae, 554
Nudeolites, 554
Nucleoplasm, 6
Nucleoproteids, 12
Nucleus, 6;
of cell, 6 f.;
of Amoeba, 5 f.;
of A. polypodia, 10;
resting, function of, 24 n
—of Euglypha, 29;
of Paramecium caudatum, 148
—pairing state of, 34;
of Ciliata, 150 f.;
of Paramecium caudatum, 148
—of Rhizopods, 52;
of Pelomyxa, 52;
of Microgromia socialis, 60;
of Foraminifera, 62;
of mega- and microspheric forms of Foraminifera, 68 f.;
of Clathrulina, 74;
of Radiolaria, 76;
of Collozoum inerme, 76;
of Myxomycetes, 92;
of Sporozoa, 95 f.;
of Bodo saltans, 117;
kineto-, of Trypanosoma noctuae, 120, 121;
trophic, of T. noctuae, 120;
of Choanoflagellates, 122;
of Opalina, 123;
of Maupasia, 124;
of Sphaerella, 126;
of Volvox, 126;
of Noctiluca, 133
—fusion- or zygote-, 150;
of Paramecium caudatum, 148
—see also Meganucleus, Micronucleus, Gametonuclei, Nuclear apparatus
Nuda, 423
Nummulitaceae, 59
Nussbaum, on regeneration in Protozoa, 35 n.;
on Hydra, 254
Nutrition, 9;
animal and vegetal, 35 f.;
of Dinoflagellates, 130 f.—see Holozoic, Holophytic, Saprophytic
—of Alcyonaria, 339
Nutritive function of granular cytoplasm of muscle-cell, 19
Nuttall, history of discoveries on Acystosporidae, 103
Nyctotherus, 137;
habitat of, 152
Obelia, 280
Oceanapia, 223
Ocellus, 252
Octactine, 200
Octactinellida, 208
Octotremacis, 346
Ocular plate of Echinus esculentus, 512
Oculina, 399
Oculinidae, 399
Ocyroe, 420;
O. crystallina, 419
Ocyroidae, 420
Oecology of Protista, 43
Oesophagus, of Asterias rubens, 438;
of Echinus esculentus, 516;
of Holothuria nigra, 562;
of Antedon rosacea, 583;
of Dipleurula, 605
Ogilvie, M., 401 n.
Oikomonadidae, 111
Oil-drops, of Radiolaria, 79 f.;
luminous, 80
Oil-globules, 37;
of Ciliata, 144
Oleocyst, 305
Olindias, 291;
O. mülleri, 291
Olindioides, 291;
O. formosa, 291
Olynthus, 185
Omne vivum, ex ovo, 42;
ex vivo, 44
Onychaster, 203
Onychaster, 502
Oocyte (a cell which by unequal divisions or mere nuclear divisions becomes converted into an oosphere), 100
Oogamete of Coccidiaceae, 100 f.
Ookinete (active zygote) or oosperm, of Acystosporidae, 104 f.;
of Trypanosoma noctuae, 120
Oolitic limestones, nucleus of concretions of, 70
Oosperm, 34;
of Sporozoa, 96 f.;
of Volvox globator, 127 f.
Oosphere, 31;
formation of, in Metazoa, 75 n.;
of Coccidiaceae, 100 f.;
of Acystosporidae, 104 f.
Oospore (= zygotospore), from bisexual syngamy, 100;
of Volvox globator, 127 f.
Ooze, Globigerina, 61;
Radiolarian, 87
Oozooid, 358
Opalina, 111;
galvanotaxy, 22;
ciliiform flagella, 114;
species, 124 n.;
nuclei, 144 n.;
systematic position of, 144 n., 145 n.;
O. ranarum, 123
Opalinopsidae, 145 n.
Operculum of central capsule of Phaeodaria, 76, 82
mouth-frame, 492;
O. chelys, 499
Ophiactis, 498;
O. balli, 498;
Ophicephalous pedicellariae, of Echinus esculentus, 508;
of E. acutus, 509;
of E. elegans, 510;
absent in Cidaridae, 534;
of Echinarachnius parma, 544
Ophidiaster, 471
O. nigra, 499
Ophiocomidae, 499
Ophiodermatidae, 495 n.
Ophiogeron, 494
Ophioglypha—see Ophiura
O. umbella, skeleton, 493
Ophiolepididae, 495
Ophiomusium, 497;
O. pentagona, 494
O. aculeata, 499
metamorphosis of, 613
Ophiopsila, 499
Ophioscolex, mouth-frame, 492
mouth-frame, 492;
O. fragilis (pentaphyllum), 478, 479;
arm of, 479;
podia, 479;
mouth-frame, 482;
disc, 484;
genital bursa and respiratory movements, 485;
alimentary canal, 485;
water-vascular system, 486;
axial sinus, 487;
perihaemal spaces, 488;
nervous system, 488;
genital organs, 489
Ophiura (Ophioglypha), 496;
O. albida, 497;
Ophiuroidea (Brittle Stars), 431, 477 f., 561;
mesenchyme of larva, 604;
development of, 606, 606, 608, 613;
phylogeny, 622
Ophlitaspongia, 225
Ophryocystis, 97
Oplorhiza (Campanulariidae, 280), 277
Oractis, 377
Oral apparatus, of Ciliata, its atrophy and regeneration during conjugation, 151
Oral blood-ring, of Asterias rubens, 450;
of Ophiothrix fragilis, 488;
of Holothuria nigra, 567
Oral cleft or groove of Noctiluca, 132 f.
Oral coelom of Antedon rosacea, 585
Oral plates, of Crinoidea, 588;
of Thaumatocrinus, 589;
absent in adult Antedon, 589;
of Bathycrinus, 591;
of Cystoidea, 598;
of Blastoidea, 599;
of young Antedon rosacea, 619
Oral spots of Protomastigaceae, 110
Oral valves, of Antedon rosacea, 581;
of young A. rosacea, 619
Orbigny, A. d', on Foraminifera, 62
Orbitoides, 59
pylomes, 64;
dimorphism, 67;
monstrous shell, 69
O. universa, 68
Orbulinella, habitat, 75
Organ-pipe Coral, 343
Organella, 44 n.
Organic compounds, their function in nutrition, 35 f.
Organoid, 44 n.
Oriental sore, 121
Ornamentation of shell-wall in Foraminifera, 66
A. lobularis, 230
Osculosa (Monotrypasta), 76
Osculum, of Radiolaria, 76;
of Sponges, 169, 171, 174, 188, 189
Ossicles of Holothuroidea, 569
Ostium, 169
Otocysts, of Elasipoda, 571;
of Synaptida, 576
Outer perihaemal ring, of Asterias rubens, 448;
development of, in Asterina gibbosa, 612;
represented by lantern-coelom of Echinus esculentus, 524;
by buccal sinus of Holothuria nigra, 566
Ovary of gnat infected by Trypanosoma germs, 120
Ovoid gland of Ophiothrix fragilis, 489
Ovum of Sea-urchin, 7;
of Sarcocystis tenella, 108 n.;
of Volvox globator, 127 f.
—see also Oosphere, Oosperm, Egg
Owl, blood parasites of, 120
Oxyaster (an aster with a small centrum and oxeate actines), 222
Oxytricha, 138
Oxytylote (a rhabdus of which one actine is oxeate, the other tylote or knobbed, the latter directed towards the surface of the Sponge), 224
Pachychalina, 223
Pachymatisma, 215;
P. normani, 215
Pairing in Trichosphaerium, 54
in Lankesteria, 95
—see also Gametes, Syngamy
Pairing nuclei, state of, 34;
of Ciliata, 150;
of Paramecium, 148
Palaeaster, 476
Palaeocoma, 476
Palaeodiscus, 557
Palaeoechinoidea, 556
Palaeoechinus, 557
Palephyra, 322
Palmella state of Zooxanthella, 86
P. membranaceus, 464
Pamphagus, test of, 59 f.
Panceri, 339
Pansporoblast of Myxosporidiaceae, 107
Pantostomata, 109
Papula (including dermal gill), 432, 457;
compared to diplopore of Cystoidea, 599
Paractinopoda, 570
Paragaster, 187
Paragastric canals, 416
Paraglycogen (= paramylum), 95
Paralcyonium, 349
Paramecium, 137, 143 n., 151, 153;
specific gravity of, 13 n.;
thigmotaxy of, 20;
reaction to repellent stimuli, 21 n.;
thermotaxy of, 22;
galvanotaxy of, 22;
chemiotaxy of, 23;
contractile vacuoles of, 143 n., 151;
P. bursaria, 153;
P. caudatum, 151;
in conjugation, 148
nutrition of, 113;
formation and regeneration of chromatophores in, 115;
P. eilhardii, 116 n.;
reproduction of, 116 n.
Paramuricea, 356;
spicule, 336
Paramylum (= paraglycogen), 37, 95, 115;
in Gregarines, 95;
in Flagellates, 115;
in Ciliata, 144
Parangi, 121 n.
Parapyle, 81
Pararchaster, 466
Parasites, in relation to brood-formation, 33;
internal, belonging to Metazoa, nutrition of, 38;
of man, 57, 103 f., 108 n., 119 f., 152;
of Radiolaria, 86 f.;
of plants, 88 f.;
of Crucifers, 89;
of Crustacea, 89;
of earthworm, 95;
of centipedes, 99;
of Epizoanthus glacialis, 99;
of Lithobius forficatus, 99;
of cold-blooded Vertebrates, 102;
of rabbit, 102;
of birds, 103;
of fish, 107;
of silkworm, 107;
of sheep, 108 n.;
of Amphibia, 111, 123 f., 158;
of dog, 119;
of horse, 119;
of ox, 119;
of Rodents, 119;
of owl, 120 f.;
of cockroach, 123;
of mole-cricket, 123;
of Termites, 123;
of Metazoa, 152;
of Ruminants, 152;
of Heliozoa, 155;
of Raphidiophrys, 155;
of Hydra, 158;
of newts, 158;
of Ciliata, 159
Parasitic, Proteomyxa, 48, 88 f.;
Flagellata, 48, 111, 119 f., 123;
Rhizopoda, 57;
Suctoria, 161;
Hydrozoa, 268 f.
Parasmilia, 401
Parazoa, 181
Parazoanthus, 406;
P. anguicomus, 406;
P. separatus, 406;
P. tunicans, 406
Parenchymalia, 201
Parenchymula, 227
Parisis, 351
Parker, 371
Parkeria, 283
Paroral cilia, 139;
of Vorticella, 156 n.
Parthenogenesis, of malarial parasites, conjectured, 106 n.
Pasteur, on organisms of fermentation and putrefaction, 43;
on nature of pébrine (Nosema bombycis), 107
Patellina, 59;
reproduction of, 69
Patina, 580;
of Antedon rosacea, 582;
of Thaumatocrinus, 589;
of Hyocrinus, 590;
of Rhizocrinidae, 590;
of Pentacrinidae, 591
Paulinella, 52;
test of, 54;
pylome of, 54
Paxilla, 455;
evolution of, 466;
relation to granules of Valvata, 471
restriction of papulae to dorsal surface, 469
P. hastata, 370
Pébrine, 107
Pectinate pedicellariae, 456, 466
Pectyllidae, 294
Pectyllis, 294
Pedal laceration, 372
Pedal nerve, of Asterias rubens, 455;
of Ophiothrix fragilis, 488;
of Echinus esculentus, 518;
of Holothuria nigra, 566
Pedicellaria, of Asterias rubens, 432;
of A. glacialis, 434;
of Asteroidea, 456;
alveolate, 456;
pectinate, 456;
pincer-shaped, 456;
valvate, 456;
forcipulate, 433, 434, 456, 462;
representatives in Ophiuroidea, 492;
in Ophiothrix fragilis, 492;
of Echinus acutus, 509;
of E. esculentus, 506 f., 507;
function of, 508 f.;
of Endocyclica, 532;
of Cidaridae, 532;
of Centrostephanus longispinosus, 532;
of Echinarachnius parma, 544, 545;
of Echinocardium cordatum, 550;
absent in Pelmatozoa, 582
Pedicellaster, 474
Pegantha, 296
Peganthidae, 296
Pekelharing, 187 n., 234 n., 237
Pelagia, 311, 312, 315, 316, 323;
P. noctiluca, 311;
P. perla, 323;
Pelagic, Foraminifera, 61, 66, 69;
Radiolaria, 76;
Dinoflagellates, 131
Pelagiidae, 323
P. mirabilis, 274
Pelagohydridae, 274
P. natans, 572
Pellicle, of Protozoa, 46;
of Noctiluca, 133;
of Dysteria, 153;
of Vorticella, 157;
of Suctoria, 159;
of tentacles of Suctoria, 161
—see also Cuticle
phylogeny of, 621
Pelomyxa, 51;
P. palustris, 52 f.;
pepsin in, 16
Penard, on Heliozoa, 71;
on Rhizopoda, 58 n.
pylomes of, 64
Peniagone, 572
Pennaria, 272
Pennariidae, 272
Pennatula, 361;
P. grandis, 361;
P. naresi, 362;
Pennatulacea, 333, 335, 337, 358, 358, 359, 363 f.
Pentaceros, 472
Pentacerotidae, 457, 458, 459, 471
Pentachogon, 294
Pentacrinidae, 588, 589, 591 f.
Pentacrinoidea, 595
Pentacrinus (Isocrinus), 591 f.;
P. asteria, 592;
P. maclearanus, 593
Pentagonaster, 471;
P. japonicus, 472
Pentremites, 599
Pepsin, 16
Peptic, digestion, 16;
juice in Carchesium, 147
Peptones, 15
Perforate, Corals, 371;
Foraminifera, 58 f.
Peribranchial spaces of Asterias rubens, 449
Pericolpa, 322
Peridiniales, synonym of Dinoflagellata used by Schütt, 119, 132
Peridinium, 110;
fossil, 132;
P. divergens, 131
Perihaemal spaces (or canals), of Asterias rubens, 448;
of Ophiothrix fragilis, 481, 488;
of Echinus esculentus, 524;
of Holothuria nigra, 566
Peripatus, segmentation of, 32 n.
P. regina, 322
Periproct, of Echinus esculentus, 504, 507, 512, 513, 534;
of Endocyclica, 530, 534, 538;
of Arbaciidae, 530;
of Echinothuriidae, 535;
development of, in young Echinoid, 613
Peripylaea (= Spumellaria), 76
Peristalsis of intestine of Holothuria nigra, 563
Peristome, of Ciliata, 137 f.;
in fission, 147;
of Peritrichaceae, 155 f.;
of Vorticellidae, 155 f., 157;
of Asterias rubens, 434;
of Echinus esculentus, 505, 513, 514;
of Asteroidea, 514;
of Endocyclica, 530;
of Echinothuriidae, 535;
of Asthenosoma hystrix, 537;
of Saleniidae, 537;
of Arbaciidae, 538;
of Diadematidae, 538;
of Echinidae, 539;
of Echinocardium cordatum, 550;
of Spatangoidea, 553;
of Palaeostomatidae, 554
Peristomial area, 139;
of Caenomorpha and Metopus, 154;
of Bursaria, 155
Peristomial collar, 142, 156, 157
Peristomial plate, of Ophiothrix fragilis, 483;
of Endocyclica, 530;
of Saleniidae, 537;
of Arbaciidae, 538;
of Echinus microtuberculatus, 540
Peritoneum, of Asterias rubens, 437;
of Holothuria nigra, 567
Peritrichaceae, 138;
pellicle, 141;
myonemes, 142;
trichocysts, 143;
contractile vacuole and reservoir, 145;
pharynx, vestibule, mode of feeding, 145;
fission, 147;
conjugation, 151;
Peronella, 549
Peronium, 295
Perrier, on classification of Asteroidea, 461
Persian Tick, 121
Petal, of Echinarachnius parma, 545;
of Fibularidae, 549;
of Echinanthidae, 549;
of Echinocardium cordatum, 551
Petalocrinus, 595
Petasidae, 294
Petasus, 294
Peters, 414 n.
Petrostroma, 193;
P. schulzei, 193
Pfeiffer, on Sporozoa, 94
Phacella, 314
Phacotus, 111;
shell, 113
Phaeodaria (Cannopylaea, Tripylaea), 76, 79;
fission, 85
Phaeogromia, 79
Phaeosphaeria, 79
Phakellia, 224
Phalansteridae, 111
Phalansterium, 113
Phanerozonate, 454
Pharetronidae, 192
Pharynx, of Stylonychia mytilus, 139;
of Ciliata, 145;
of Gymnostomaceae, 145;
of Paramecium caudatum, 151;
of Caenomorpha uniserialis, 155;
of Peritrichaceae, 145;
of Carchesium polypinum, 146 f.;
Pharynx-tube of Euglenaceae, 124 f.
—see also Flagellar Pit
Pheronema, 204;
Phialidium temporarium, 281
Pholidaster, 474
Phoriospongia, 220
Phosphorescence, of Dinoflagellata, 132;
of Scyphozoa, 311;
of Alcyonaria, 338;
of Pennatulids, 361;
of Ctenophores, 414
Phosphorescent oil-drops in Radiolaria, 80
Photopathy, 21
Phototaxy, 21;
of Euglena, 125
Phycochromaceae, 39
Phycomycetes Zoosporeae related to Flagellata, 109
Phycomycetous Fungi, relations of, 48
Phylactocarp, 276
Phyllactidae, 382
Phyllangia, 400;
P. americana, 374
Phyllode, 553
Phyllograptus, 282
Phyllophorus, 573;
P. rugosus, 567;
P. urna, 574
Physiological contrast of Animals and Plants, 38
Physiology, of cell and protoplasm, 3 f.;
of Sponges, 234 f.;
of nervous system of Echinoidea, 519 f.
P. borealis, 304
Physophorae, 307
Physophorinae, 308
Pigment, of Stentor, 154;
-granules, of Phaeodaria, 76, 80 f.
Pincer-shaped pedicellariae, 456
Pineau, on spontaneous generation, 43
Pinnule, of Antedon rosacea, 581, 583;
of A. eschrichtii, 594;
of Hyocrinus, 590;
of Metacrinus, 592;
of fossil Crinoidea, 595
Pinulus (a pentactine triaxon in which the unpaired actine bears lateral spines and projects beyond the bounding surface), 204
Piroplasma, 120 f.
Placosmilia, 401
Placospongia, spicules of, 233
Plankton, Protozoa of, 48;
Foraminifera of, 61;
Radiolaria of, 75
Plant(s), definition, 39;
Animals and, discussion on, 35 f.;
Higher, movements of, 38;
insectivorous, 38;
-Protists, relations of, 48;
-cells, wall of, 37;
protoplasmic connexions of, 37
—see also Metaphytes
Planula, 341
Plasmodiophora, 89
Plasmodium, 30;
rheotaxy, 21;
of Proteomyxa, 88;
of Didymium, 92
Plasmodium, a generic name given to Acystosporid Coccidiaceae producing malarial fever, 103 f.—see Haemamoeba, Laverania
of Paulinella, 54 n.;
of Trachelomonas, 112
—see also Chromoplasts, Chromatophore, Chromoplastid
of Rhizopods, 56;
of Foraminifera, Discorbina and Patellina, 69;
temporary, in Actinophrys sol, 72;
of Myxomycetes, 90 f.
Plastron, of Echinocardium cordatum, 550;
of Sternata, 554
Plate, on Dendrocometes, 162
Plates, siliceous, of shell of Rhizopods, 29, 53 f.;
Platt, Julia B., on density of living protoplasm, 13 n.
Platybrissus, 554
Platyhelminthes, bladder of, 14 n.
Plectinia, 193
Plectoidea, 78
P. halli, 193
Plesiofungiidae, 403
Plesioporitidae, 404
Pleurobrachia, 418;
P. rhodopis, 418
Pleurobrachiidae, 418
Pleurocorallium, 352
Pleurocoralloides, 352
Pleurogorgia, 355
Pleuronema, 137;
Plexaura, 356
Plexauridae, 356
Plimmer and Rose Bradford, on Trypanosoma, 121
Plocamia, 223
Plumohalichondria, 225
Plumularia, 279;
P. echinulata, 276;
P. halecioides, 276;
P. profunda, 275;
P. setacea, 276
Pluteus, 607
Pneumatopyles, 309
Pneumotaxy, 23
Pocillon, 223
P. septata, 402
Pocilloporidae, 401
Podium, 428;
of Ophiothrix fragilis, 479 f., 487;
of Holothuria nigra, 561;
of Elasipoda, 571 f.;
of Molpadiida, 575;
of Antedon rosacea, 582
Podocoryne, 270
Podocorynidae, 270
Podoplast, 19 n.
—see also Blepharoplast
Podostoma, 52;
transition between pseudopodium and flagellum in, 47 n.
Poecillastra compressa, 213, 222
Polar body, in Heliozoan syngamy, 72, 74
Polar fields, 415
Polian vesicle, of Asteroidea, 458;
of Ctenodiscus, 458;
of Solasteridae, 463;
of Ophiothrix fragilis, 487;
of Echinus esculentus, 525;
of Holothuria nigra, 566
Polyaxon, 184
Polycanna, 278
Polycystineae (= Radiolarian skeletons), 87
Polycyttaria (= colonial Radiolaria), 76, 84 f.
Polygastrica (Ehrenberg's name for Ciliata), 146
Polykrikos, 110, 113 n., 131 f.
Polymastia, 224
Polymastigidae, 111
Polymitus form of male gametogonium of Acystosporidae liberating sperms, 104 f.
Polymorphina, 59
Polyoeca, 111;
stalk of, 113
Polyorchis, 278
Polyphyes, 307
Polyphyidae, 307
Polypodium, 257
alternation of generations in, 44, 250
Polysiphonic, 276
Polystomella, 59;
dimorphism and life-history of, 67 f.
Polythalamia (= Foraminifera with more than one chamber to shell), 64
Polytoma, 111;
brood-division of active, 115;
P. uvella, gametes of, 116 n.
Polytomeae, Francé on, 119 n.
Polytrema, 59;
shell substance of, 62
Polytremacis, 346
Entoprocta, 621
Pontosphaera, 110
P. pulvillus, 464
Poraniidae, 464
Porcellanaster, 471;
P. pacificus, 459;
P. caeruleus, 470
Porcellanasteridae, 455, 459, 470
Porcellanous Foraminifera, 58, 59, 62
Pore(s), in test of Foraminifera, 64;
in central capsule of Radiolaria, 76;
of contractile vacuole, 14;
of Ciliata, 143;
of Trachelius ovum, 153;
of embryonic cavity of Suctoria, not seen in Choanophrya, 161 n.;
of Sponges, 186, 187, 188, 189, 198
Pore-canal, of Asterias rubens, 441;
of Ophiothrix fragilis, 486, 487;
of Echinus esculentus, 517;
of Echinocardium cordatum, 552;
of a larval Holothuroid, 564;
of Elasipoda, 571;
of Antedon rosacea, 583;
of its larva, 619;
of Dipleurula, 608;
of larva of Asterina gibbosa, 612;
of Balanoglossus, 617
Pore-plate, of Echinus esculentus, 512;
of Strongylocentrotus droëbachiensis, 512;
of Endocyclica, 530 f.;
of petals of Echinarachnius parma, 544
Porifera, 163 f.;
definition, 180;
systematic position, 181
—see also Sponges
Porites, 368 f., 373, 387, 388, 397
Poritidae, 396
Porocone, 81
Porpita, 309
Porta, on reproduction of Radiolaria Acantharia, 86 n.
Porulosa (Holotrypasta), 76 f.
Portuguese Man-of-War, 300, 308
Post-abdomen = fourth chamber of Monaxonic Radiolarian shell, 84
Posterior dorsal process, of ciliated band, of Bipinnaria and Ophiopluteus, 606;
of Echinopluteus, 607;
of Auricularia, 608
Posterior lateral process, of ciliated band, of Bipinnaria, Ophiopluteus, and Ophiothrix fragilis, 606;
of Echinopluteus, 607
Posterior wreath, of Peritrichaceae, 138;
of Trichodina, 158
Post-oral process, of ciliated band, of Bipinnaria and Ophiopluteus, 606;
of Echinopluteus, 607
Potato, rest and germination of, 32
Poteriodendron, 111
Potts, 180
Pouchet (the elder), on spontaneous generation, 43
Pouchet, Georges, on Protozoa, 45
Pouchetia, 110
Pourtalesia, 554;
P. jeffreysi, 554
Pourtalesiidae, 554 n.
Prae-oral process, of ciliated band, of Bipinnaria and Ophiopluteus, 606;
of Echinopluteus, 607
—see also Pre-oral
Pratt, E. M., 339, 348 n., 399 n.
Prayinae, 306
Pre-Cambrian Echinodermata, 623
Pre-oral, cilia, 139;
ridge, 139;
undulating membrane, 139
—see also Prae-oral
Preyer, on the response of Asteroidea to stimuli, 446 f.;
on the intelligence of Ophiuroidea, 488 f.
Primary spine, of Echinus esculentus, 506;
of Cidaridae, 532;
of Arbaciidae, 532;
of Colobocentrotus, 532;
of Heterocentrotus, 532;
of Echinocardium cordatum, 550
Primnoa, 354;
P. lepadifera, 338
Pringsheim, on exogamy in Pandorina, 34 n.
Prionastraea (Astraeidae, 399), 375
Proboscis, of central capsule of Phaeodaria, 76
Progamic brood-division (= a brood-division to produce gametes), 96, 100
Proheliolites, 346
Promachocrinus, 594
Proper wall of Perforate Foraminiferal shell, 59, 63, 66
Prophylaxis, against mosquitos and malarial fever, 103, 106;
against pébrine in silkworms, 107
Prosodus, 210
Prosopyle, 170
Prostalia, 201
Protanthea, 377
Protantheidae, 377
Protaspidochirota, 578
Protechinoidea, 623
Proteid(s), 12;
digestion of, 15;
formation of, 36;
crystals, 37;
reserves in Flagellates, 110;
granules or spherules of Ciliata, 144;
of Suctoria, 161
Proteleia, 218
Proteomyxa, 48 f., 50, 88, 89 f.;
relations of, 40;
studied by botanists, 45;
parasitic in plant-cells, 48;
distinctions from Flagellates, 109
Proteoses, 15
Proterospongia, 111, 113, 122, 181 f.;
P. haeckeli, 182
Protista, 3 f.—see Protozoa
Protoclypeastroidea, 548
Protocnemes, 367
Protodendrochirota, 578
Protoholothuroid, 577
Protoholothuroidea, 578
Protohydra, 256
Protomastigaceae, 110 f., 112;
external plasmatic layer of, 113
Protomerite, of Gregarines, 97, 98
Protomyxa, 89
Protopelmatozoa, 623
Protophytes, 3 n.
Protoplasm, 3 f.;
structure of, 6;
refractive index of, 6, 11 n.;
movements of, 7;
specific gravity of, 13 n.;
of Protozoa, 46 f.;
of Radiolaria, 79 f.;
of Stylonychia, 140
Protospongia fenestrata, 207, 207
characters, 40;
literature, 45 f.;
geographical distribution, 47 f.;
habitat, 47;
classification, 50
Protractor muscles of Aristotle's lantern, 526
Protriaene (a triaene in which the cladi [branches] point forwards or in the opposite direction to the rhabdome or shaft), 224
Prouho, on transverse fission in Gonactinia, 371 n.;
on gemmiform pedicellariae of Echinoidea, 509;
on habits of Dorocidaris papillata, 535
Prunoidea, 77
Prunophracta, 78
Psammocora (Fungiidae, 403), 390
Pseudambulacrum, 599
Pseudomonocyclic, 594
Pseudonavicella, 96
Pseudophellia arctica, 379
Pseudopodia (um), 4 f., 17, 47, 49, 50 f.;
streaming of granules in, 17;
Lang's classification of, 47 n.;
transition to flagella, 47 n.;
of Foraminifera, 49, 50, 60, 61, 65;
of Lieberkühnia, 61;
of Allogromia, 65;
of Miliola, 65;
of Rotalia, 65;
of Squamulina, 65;
of Heliozoa, 49, 50, 71, 72 f.;
of Radiolaria, 49, 50, 79, 80;
of Lankesteria, 96 n.;
transitory, in Flagellates, 109;
of young Gellius varius, 173, 174
Pseudopodiospores, 68 f.
Pseudospora, 89;
P. lindstedtii, 89
Psilaster, 470;
P. acuminatus, 469
Psychropotes, 572
P. stellifer, 465;
P. militaris, 466
Pterocephalus, 97
Pteroeididae, 361
Puffballs, 91
Pulsatile vacuole, 14;
= Contractile vacuole, q.v.
Punjab, dourine disease in, 119
Pupa, of Holothuroidea, 615
Pure cultures, 43
Putrefaction, organisms of, 42 f., 116 f.
Pycnolithus, 346
P. relictus, 548
Pylome, 53;
of Rhizopods, 53 f.;
double, in monstrous Rhizopods, 55;
of Diaphorodon, 60;
of Foraminifera, 64;
of Radiolaria, 83;
of Sphaeropylida, 77 n.;
of Nassellaria, 83
Pyloric caeca (and duct), of Asterias rubens, 439;
of Porcellanaster pacificus, 459;
absent in Hyphalaster moseri, 459
Pyloric sac of Asterias rubens, 438
Pyramids, building-stone of, 70
of Sphaerella, 126
Pyrocystis, 110;
P. fusiformis, 132
Pyrsonympha, 111;
flagella of, 114
Pytheas, 223
Pythonaster, 464
Pythonasteridae, 464
Quadrula, 52;
Q. symmetrica, 55;
test of, 54
Quartan fever, a parasitic disease, 104 f.
Quartzites, Radiolarian, 87
Quasillina, 224
Quatrefages, de, 376
Quelch, 280
Quinqueloculine type, 67
Radial blood-strand (or vessel), of Asterias rubens, 450;
of Ophiothrix fragilis, 488
Radial canal, of water-vascular system, 428;
of Asterias rubens, 441;
of Ophiothrix fragilis, 480, 486;
of Echinus esculentus, 517;
of Echinarachnius parma, 547;
of Holothuria nigra, 560;
of Pelagothuria, 572;
of Carpoidea, 580;
of Thecoidea, 580;
of Crinoidea, 580;
of Antedon rosacea, 583;
development of, in Asterina gibbosa, 611;
in Holothuroidea, 615;
in Antedon rosacea, 619
Radial fission in Volvocaceae, 110 f.
Radial nerve-cord, of Asterias rubens, 444, 445, 446, 447;
of Ophiothrix fragilis, 488;
of Echinus esculentus, 518, 519, 522;
of Holothuria nigra, 566;
of oral system of Antedon rosacea, 584;
of coelomic system of A. rosacea, 584, 585
Radial ossicle—see Radial Plate
Radial perihaemal canal, of Asterias rubens, 448;
of Ophiothrix fragilis, 488;
of Echinus esculentus, 518, 524;
of Holothuria nigra, 566
Radial plate, of Ophiothrix fragilis, 484;
of Cladophiurae, 500;
of calcareous ring in Holothuria nigra, 566;
in Elasipoda, 569;
in Molpadiida, 569;
in Dendrochirota, 569;
of calyx in Antedon rosacea, 582, 584, 585;
in Crinoidea, 588;
in Holopus, 592;
in Blastoidea, 599;
development, in Antedon rosacea, 619 f.
streaming of granules, 17;
regeneration, 35;
animal nutrition of, 40;
relations of, 49;
freshwater (= Heliozoa), 71;
shells and skeletons of, 77, 78, 80, 82 f., 84, 85;
symbiosis of Diatoms and yellow cells (= Zooxanthella) in, 82, 86 f., 125;
Dreyer's scheme of skeletal forms in meshes of an alveolar system, 84;
habitat, 87;
census of, 87;
palaeontology, 87;
students of, 88
Radiolarian ooze, 87
Radiomyaria, 324
Radius, of Echinus esculentus, 503;
synonym of compass, 526;
of a Holothuroid, 562;
of Spatangoidea, 553;
of Antedon rosacea, 582;
of Promachocrinus, 594
Rain, bloody, 125
Rainey's tubes, 108
Ramulina, 59
Raspailia, 225
Rastrites, 282
Ratarula larva, 302
Ratio (Spencer's) between bulk and surface of organism, 14, 23 f., 31 f.
Reactions, chemical, of protoplasm and of vacuoles, 13;
motile, of Protozoa, 19 f.
Receptaculites, 207
Receptaculitidae, 207
Rectal caeca, of Asterias rubens, 439;
of Asteroidea, 459;
of Astropecten, 459;
of Asterias, 459;
of Asterina, 459;
of Echinasteridae, 459;
of Astropectinidae, 459
Rectum, of Asterias rubens, 439;
of Holothuria nigra, 563
Red colour of sea, due to Dinoflagellata, 132
Red Corals (= Coralliidae, 352)
Red marrow of bones, habitat of resting states of malarial parasites, 106 n.
Red snow, 125
Redi, on reproduction of Blowflies, 42
Reduction divisions, 75 n.;
of nucleus, 116 n.;
in Monocystis, 96;
in Coccidium, 100;
in Acystosporidae, 104 f.
Reef Corals, distribution of, 389
Reefs, Coral-, 390;
barrier-, 390 f.;
fringing, 390 f.;
atolls, 390 f.
Regeneration, 35;
of Stentor, 35;
of Thalassicolla nucleata, 79 n.;
of Infusoria after division, 145;
of oral apparatus of Ciliata during conjugation, 151
Regular Urchins, 529, 530 = Endocyclica, q.v.
Relapses of malarial fevers explained, 106
Relapsing fever, inoculated by Zambezian Tick, 121 n.
Relationship, cellular, explained, 10
R. reniformis, 363
Renilleae, 363
Renillidae, 363
Reophax, 59
Repellent stimuli, response to, 20 f.
of Actinophrys sol, 72;
of Radiolaria, 84 f.;
of Flagellata, 107;
of Choanoflagellates, 122;
of Opalina, 123 f.;
of Volvocidae, 126, 127 f., 129 f.
—see also Brood-division, Spore, Syngamy
Reproductive cells, 31
—see also Gametes, Brood-cell
Reproductive organs—see Genital organs
accumulation of, 9, 13, 32 f.;
digestion of, 16;
consumption of, in brood-formation, 24, 32 f.;
-products, of brood-mother-cell, 32
Reservoir of contractile vacuole, of Flagellates, 110, 115;
in Euglenaceae, 125;
of Vorticella, 157
intramolecular, 14 n.;
in Asteroidea, 432;
in Asterias rubens, 437;
in Astropecten, 469;
in Porcellanasteridae, 470;
in Ophiothrix fragilis, 485;
in Echinus esculentus, 517, 527;
in Echinarachnius parma, 544, 547;
in Echinocardium cordatum, 551;
in Holothuria nigra, 563, 564, 566;
in Molpadiida, 575;
in Synaptida, 576;
in Antedon rosacea, 582;
in Cystoidea, 597 f.;
in Blastoidea, 600
Respiratory tree, of Holothuria nigra, 563;
of Holothuroidea, 569;
of Aspidochirota, 569;
of Molpadiida, 569;
represented by caecum of Elasipoda, 569;
absent in Synaptida, 576;
in Pelagothuria, 572
Response, 8
Rest, of cell, 32;
vegetative and absolute contrasted, 37
—see also Cyst, Encystment, Hypnocyst
Resting state of malarial parasites, 106 n.
Reticulate, structure of protoplasm, 6;
endoplasm of Cystoflagellates, 110;
of Loxodes and Trachelius, 144, 153 f.
Retiograptus, 282
Retiolites, 282
Retiolitidae, 282
Retractor muscles, of Aristotle's lantern, 526;
of anterior part of body of Holothuroidea, 568;
Reusenapparat, 145
Rhabdocrepid, 215, 224 (Crepis is the term applied to the fundamental spicule by deposition of silica upon which a desma is formed. A desma of which the crepis is uniaxial is called a rhabdocrepid desma)
Rhabdopleura, 617
Rhabdosphaera, 110
Rhabdospheres, 114
Rheotaxy, 20 f.;
its rôle in syngamy of Mammals and Sauropsida, 34
Rhizocrinidae, 588, 589, 590 f.
formation of chromidia, 29 f.;
relations, 48 f.;
literature, 58
Rhizostoma, 325;
Rhizostomatidae, 325
Rhodactidae, 383
Rhodactis (Rhodactidae, 383);
R. sancti-thomae, 373
Rhodalia, 308
Rhodophysa (sub-family Physophorinae, 308), 300
Rhodophyton, 349
Rhodopsammia, 404
Rhopilema, 325;
R. esculenta, 312;
R. verrucosa, 312
Rhumbler, on Foraminifera, classification, 58 n.;
shell, 66 f.;
monstrous shells, 69 n.
Rhythm, of cell-life and reproduction, 30 f.;
of contractile vacuoles in Paramecium, 143 n.;
in life-cycle of Infusoria, 148 n.
Ricordea, 371
Riisea, 355
Ring-canal, of water-vascular system, 428;
of Asterias rubens, 441;
of Echinus esculentus, 517;
of Holothuria nigra, 566
Robertson, on the habits of Echinocardium cordatum, 552
Rock-urchins, 529
Rodents, hosts of Trypanosoma lewisii, 119
Roemer, 199 n.
Romanes, on physiology of Echinoidea, 519, 521
Rompel, on Spirochona, 144 n.
Rosette, -aggregate, of Treponema, 120;
of Antedon rosacea, 584
Ross, on intermediate host of malarial parasites, 103
Rotaliaceae, 59
Rotifers, bladder of, 14 n.;
rheotaxy of, 21;
distribution of, 48;
associated with Euglena viridis, 124;
formerly included under Infusoria, 136;
vibratile styles of, 141
Rotula of Echinus esculentus, 526
Rotula muscles of Aristotle's lantern, 526
Roule, 408
Royal Society, early publications on Protozoa, 45
Ruminants, infested by Trypanosoma evansii, 119;
parasitic Ciliata in paunch of, 152
Rumphius, 360
Sacculus, of Antedon rosacea, 587;
of other species of Antedon, 588
S. troglodytes, 378
Sagartiidae, 381
Sagittal, 185
plane, 414;
costae, 416 n.
Salenia, 538;
S. varispina, 538
Salivary gland of gnat in relation to malarial parasites, 105
Sand from sponges, a source of Foraminiferal tests, 62
Sand, René, on Suctoria, 162
Sand-dollar, 542
Sand-urchin, 529
Sanidaster (a modified euaster in which a slender rod-like axis bears spines at intervals along its length), 222
Sapropelic Protozoa, 48
Saprophyte, 33, 37, 90, 113, 119;
relation to brood-formation, 33
Saprophytic, nutrition, 33, 37;
Sarasin, C. F. and P. B., on the madreporic vesicle and axial sinus of Echinoidea, 528;
on the relationships of the Echinothuriidae and Holothuroidea, 537
Sarcocystis, 98;
S. tenella, 108 n.
Sarcode (Dujardin's term for protoplasm), 3 f.
Sarcodictyon, 344;
S. catenatum, 342
Sarcodictyum, 79
Sarcodina, relations of, 48 f., 49, 50 f.;
distinction from Flagellata, 109
Sarcoflagellum, 80
Sarcolemma of stalk-muscle of Vorticella, 157 n.
Sarcophyllum (Pennatulidae, 361), 360
Sarcophytum, 248, 330, 333, 347, 349
S. prolifera, 272;
S. siphonophora, 272
Sauropsida, egg of, 34
Schäfer, on mechanism of ciliary action, 18 n.
Schaudinn, on exogamy in Foraminifera and Trichosphaerium, 34 n.;
on Protozoa, 46;
Archiv für Protistenkunde, 46;
on chromidia in Sarcodina, 52 n.;
on Trichosphaerium sieboldi, 54, 56 f.;
on bud-fission in Rhizopoda, 55;
on syngamic processes of Rhizopoda, 57;
on reproduction in Foraminifera, 67, 69 n.;
on Heliozoa, 71;
on Sporozoa, 94;
on life-cycle of Coccidiidae, 99, 101;
on relations of Halteridium and Trypanosoma, 103 n., 116 n., 120;
on relations of Acystosporidae, 106;
on conjugation in Flagellates, 116 n.;
on Trypanosoma, 120;
on Treponema, 120;
Fauna Arctica, 199 n.;
on Haleremita, 257
Schaudinnia arctica, 200
Scheel, on brood-formation in Amoeba proteus, 56 n.
Schewiakoff, on protoplasmic granules, 6 n.;
on geographical distribution of fresh-water Protozoa, 47 n.
Schiemenz, on the way in which Starfish open bivalves, 440
Schizaster, 556
Schizogony, of Coccidiaceae, 99 f., 101 f.;
of Haemosporidae, 102;
of Acystosporidae, 104 f.
Schizogregarinidae, 97
Schizont, 99;
of Acystosporidae, 103, 104 f.
Schizopathes, 408
Schizophytes, relations of, 48
Schizotricha (Ciliata), 138;
S. socialis, branched tube of, 152;
S. dichotoma (Plumulariidae, 279), 276
Schlumberger, on dimorphism of Foraminifera, 67
Schneider, on Sporozoa, 94
Schrammen, 215 n.
Schröter, on Myxomycetes, 93 n.
Schuberg, on cilia and ciliary motion, 18 n., 141 n.
Schultze, Max, on Protozoa and on protoplasm, 46;
on structure of Foraminifera, 62
Schulze, F. E., on Heliozoa, 71;
on Sponges, 167, 197 n., 199, 200;
on Spongicola, 318
Schütt, on Dinoflagellata ("Peridiniales"), 119, 132
Scleroderm, 371
Sclerogorgiidae, 351
Sclerophytum, 330, 336, 348, 349;
S. querciforme, 348
Scopula, 141 n.
Scuta buccalia, of Ophiothrix fragilis, 485
Scutellidae, 549
S. scorpaenae, Zooxanthella symbiotic in, 125
Scyphistoma, 317
Scyphomedusae, 310 f.
Scyphozoa, 310 f.;
colour, 310;
food, 311;
phosphorescence, 311;
reproduction, 316;
size, 310;
structure, 312;
symbiosis, 311
Scytophorus, 380
Sea, luminosity or phosphorescence, produced by Cystoflagellata, 132, 134;
by Dinoflagellata, 132;
red colour of, due to Dinoflagellata, 132
Sea-cucumbers (= Holothuroidea), 561
Sea-fans (= species of Gorgonacea), 350
Sea-lilies (= Crinoidea), 580 f.
Sea-pansy, 364
Sea-urchins(= Echinoidea), 503;
ovum of, 7
Secondary body-cavity (= Coelom, q.v.)
Secondary spines, of Echinus esculentus, 506;
of Cidaridae, 532;
of Colobocentrotus, 532;
of Heterocentrotus, 532;
of Echinocardium cordatum, 550
Secretion, 13
Segmentation, 32 n.;
of schizont of Acystosporidae, 104;
of oosperm, 104 f.;
of zygotomeres, 104 f.;
of reproductive cells of Volvox, 126, 127;
telolecithal, 133 n.
Semaeostomata, 323
Semon, on the phylogeny of Echinodermata, 622
Semper's larva, 405
Senility in life-cycle of Ciliata, 148
Senn, on Flagellates, 119
Sense-organs of Metazoa, 40
Sensory cilia, 141
Septum, protoplasmic, in Dicystic Gregarines, 97, 98 f.;
calcareous, of Madreporaria, 370
Seriatopora, 401
Sertularia, 278;
S. abietina, 278
Sertulariidae, 278
Serumsporidium, 89
Sex, binary (= syngamy with marked inequality between the pairing-cells), 33 f.;
of Pterocephalus, 99;
of Coccidiaceae, 97 f., 99 f.;
of Haemosporidae, 102 f.;
of Sarcocystis tenella, 108 n.;
of Volvocaceae, 128 f.;
of Eudorina, 129;
of Peritrichaceae, 151 f.
Sex, ternary, of Pandorina, 128 f.
Sexual fusion of Halteridium, 103, 105
Sheath, tentacular, of Suctoria, 159
Sheep, host of Sarcocystis tenella, 108 n.
Shell, of Diatomaceae, 84;
of Foraminifera and of Rhizopoda—see Test;
of Radiolaria—see Skeleton;
cuticular, of Flagellates, 113—see Theca;
siliceous reticulate, of Silicoflagellates, 110;
-substance of Foraminifera, 62;
of Polytrema, 62
Shipley, 197 n.
Sickle-cells, sickle-germs, 48, 94 f., 97, 101;
of Lankesteria, 95;
of Sarcosporidiaceae, 108
Side-plates, of arms of Crinoidea, 589;
of Pentacrinidae, 591;
of Comatulidae, 594
S. sideraea, 403
Siebold, v., on Sporozoa, 94
Siedlecki, on Sporozoa, 94;
on reproduction of Lankesteria, 96 n.;
on life-cycle of Coccidiidae, 99
Sierra Leone, prophylaxis of malaria at, 106
Sigma (a slender rod-like spicule curved in the shape of the letter C), 220, 222
Sigmaspire (a slender rod-like spicule twisted through about a single revolution of a spiral, and consequently having the form of a C or an S according to the direction in which it is viewed), 222
Siliceous plates, of test of Rhizopods, 29, 53 f.
Siliceous skeleton of Heliozoa, 71, 74;
of Radiolaria, 76 f.;
of Silicoflagellata, 110;
of Sponges, 171 f., 175 f., 195 f.
Silicified cell-wall of some Dinoflagellates, 130
Silicoflagellata, 86, 110, 114
Silkworm infested by disease pébrine, due to Nosema bombycis, 107
Simpson, on life-cycle of Ciliata, 148 n.
Siphon, of Echinus esculentus, 516;
of Echinarachnius parma, 546, 547
Siphonia, 215
Siphonoglyph, 334, 334, 369, 410
Siphonogorgia, 349
Siphonogorgiidae, 349
Siphonophora, 297 f.;
dactylozooids, 299;
food, 304;
gastrozooids, 299;
gonozooids, 302;
hydrophyllia, 300;
life-history, 302;
nectocalyces, 298;
stolon, 301
Siphonozooids, 332;
of Pennatulacea, 359
Sipunculidae, 576
Sipunculus, 563
Skeleton, intermediate or supplemental of Perforate Foraminifera, 59, 63, 66;
of Raphidiophrys, 74;
of Radiolaria, 76 f., 77 f., 81 f., 84;
of Actinomma asteracanthion, 77;
of Acantharia, 76 f., 78, 80, 82;
of Xiphacantha, 78;
of Dorataspis, 80;
of Nassellaria, 76, 78, 82, 83;
of Lithocercus annularis, 82;
of Theoconus, 80;
of Phaeodaria, 76, 79, 82, 84, 85;
of Aulactinium actinastrum, 82;
of Challengeridae, 85;
of Pharyngella, 85;
of Haeckeliana, 85;
of Tuscarora, 85;
of Diatomaceae, 84;
gelatinous, of Volvox, 126;
—see also Shell, Test, Theca
—of Asterias rubens, 434 f.;
of disc of Ophiuroidea, 493;
internal, of Echinarachnius parma, 545, 548;
of Clypeastroidea, 548;
of Laganum, 548;
of Clypeaster, 548;
of Echinocyamus, 548;
of Echinanthidae, 549;
of Laganidae, 549;
of Holothuria nigra, 560;
of Holothuroidea, 569;
of Aspidochirota, 569;
of Dendrochirota, 569;
of Elasipoda, 569;
of Molpadiida, 569;
of Synaptida, 569;
calyx and arm in Crinoidea, 588
Sladen, on classification of Asteroidea, 460
Sleeping-sickness, 120
Snow, red, 125
fossil, 475;
S. endeca, 463;
S. papposus, 463
Solasteridae, 453, 455, 458, 462, 466
Solenocaulon, 350
Sollas, I. B. J., on Sponges, 163 f.
Sollas, W. J., on Sponges, 165 n., 168, 172 n., 176 n., 183 n., 207 n., 208, 212 n., 215 n., 216, 219 n., 233 n., 234 n., 238 n.;
on Palaeodiscus, 557
Solmaridae, 296
Soluble substances in greater or less concentration, effect on protoplasm, 7 f., 22 f.
Sore, Oriental, 121
Spallanzani, on origin of organisms of putrefaction, 43
Spanioplon, 223
Sparshall's discovery of Noctiluca, 135
Spatangoidea, 529, 549 f., 556, 559, 561, 577
S. purpureus, 555;
S. raschi, 555
Specialisation in Metazoa and Volvox compared, 129 f.
Specific gravity of living protoplasm, 13 n.
Spencer, Herbert, on limit of growth, 23
Spencer, W. B., on Hydroids, 271 n., 279
Spencerian, fission at limit of growth, 23;
rhythm, 30 f.
Sperm, Spermatozoon, 17, 31, 33 f.;
= spermogametes, 33;
penetration of ovum by, 34;
rheotaxy of, in Mammals and Sauropsida, 34;
of Sporozoa, 18;
of Acystosporidae, 104 f.;
of Sarcocystis tenella, 108 n.;
of bisexual Protozoa and most Metazoa comparable with Flagellata, 109;
of Volvox globator, 127 f.;
of Eudorina, 129
Spermatogone (= a brood-mother-cell, whose offspring are sperms) of Coccidiaceae, 100 f.;
of Volvox globator, 127 f.;
of Eudorina, 129;
of Acystosporidae, 104 f.
—see also Sperm
Sperosoma, 536
Sphaeractinia, 283
S. granularis, 541
Sphaerella, 111;
S. lacustris, 126;
S. nivalis, 125;
geological occurrence of, 88
Sphaeridium, of Strongylocentrotus, 523;
of Echinus esculentus, 524;
of Echinarachnius parma, 545;
of Echinocardium cordatum, 551
Sphaeronectes, 306
Sphaeronectinae, 306
Sphaerozoea, 77 n.
Sphaerozoidae, 85
Sphenopus, 404
Spheraster (an aster in which the centrum is large, with a diameter equal to or greater than one-third the length of the actines), 233
Sphere, 184
Spicatae, 362
Spicules, calcareous, of Coccolithophoridae, 114;
siliceous, of Heliozoa, 71, 74;
of Radiolaria, 83;
of Silicoflagellata, 110;
of Sponges, 170 f.;
composition, 170;
classification, 183;
development, 232;
Spindle, in cell-division by mitosis, 25 f.;
intranuclear, of Euglypha, 29
Spines, of pelagic Foraminiferal shell, 66, 69;
of Globigerina bulloides, 69;
of Asteroidea, 454;
of arms of Ophiothrix fragilis, 479;
of arms of Ophiuroidea, 491;
of Echinus esculentus, 505 f.;
of Endocyclica, 531 f.;
of Cidaridae, 532;
of Arbaciidae, 532;
of Echinothuriidae, 532;
of Colobocentrotus, 532;
of Heterocentrotus, 532;
of Echinarachnius parma, 543;
of Echinocardium cordatum, 550
Spiral, of stalk and stalk-muscle in Vorticella, 156 f.;
ridge on tentacles of Suctoria, 160 f., 162
Spiraster (a spire of one or more turns, produced on the outside into several spines), 222
Spirochaeta (= Treponema, 111), 120 f.;
"S." zeemannii, 120 f.;
S. obermeieri, 121
Spirochona, 138;
adoral wreath of, 138 n.;
bud-fission of, 147
supposed nervous fibrils in, 143
Spongelia, 225
Spongelidae, 220
Sponges (= Porifera), 163 f.;
spicules, 170, 171, 172, 177 f., 183, 184, 187 f., 198 f., 222, 224, 231 f., 232;
canal system, 170, 171, 191, 198, 210, 235 f.;
physiology, 234 f.;
distribution, in space, 239;
in time, 241;
history, 166;
nervous system, 39;
immune from Gregarines, 99;
relations to Protista, 41;
to Choanoflagellates, 122 f., 168, 181
Sponge-sand, a source of Foraminiferal tests, 62
Sponge-spicules, in arenaceous shell of Foraminifera, 64
—see also Spicules
Spongicola (= Nausithoe), 206, 318;
S. fistularis, 317;
(a Decapod Crustacean), 206;
S. venusta, 206
Spongidae, 220
Spongilla, 217, 225, 230, 232, 237, 238;
S. lacustris, spicule, 232
Spongioderma, 351
Spongocardium gilchristi, 215
Spongophare, 210
Spontaneous, generation, 42 f.;
rendered improbable by life-histories of Flagellates, 118;
—movements of Protista, 23
Sporange (= a sac containing spores), of Myxomycetes, 91 f.;
of Didymium, 92;
of Actinomyxidiaceae, 98
Spore, 31;
of Actinophrys, 72;
of Actinosphaerium, 73 f.;
of Acantharia, 86 n.;
of Myxomycetes, 90 f.;
of Didymium difforme, 92;
of Sporozoa, 94 f.;
of Gregarinidaceae, 97 f.;
of Lankesteria, 95;
of Gregarina blattarum, 98;
of Stylorhynchus, 100;
of Coccidiaceae, 97;
of Coccidiidae, 97;
of Coccidium schubergi, 101;
of malarial parasites, 104;
of Acystosporidae, 97;
of Myxobolus mülleri, 107;
of Actinomyxidiaceae, 98;
of Sarcosporidiaceae, 98, 108;
of Bodo saltans, 117;
of Flagellates highly resistant to heat, 118
—see also Oospore, Zoospores, Zygotospore
of Proteomyxa, 88;
of Bodo saltans, 117 f.
Sporoducts of Gregarina blattarum, 98 f.
Sporogony, 296
Sporont of Gregarines, 98 f.
Sporozoa, 31, 33, 40, 48, 50, 94 f.;
formation of chromidia, 29 f.;
habitat, 48;
relations, 48 f.;
Acystosporidae most primitive group of, 106;
distinction from Flagellata, 109
Sporozoite, 95 f.;
of Lankesteria, 95;
of Haemosporidae, 102;
of Acystosporidae, 104 f.
Sporulation, 31;
in Noctiluca, 133 f.;
= Brood-formation, q.v.
Springer, on classification of Crinoidea, 589
Springing movements, of Flagellates Dallingeria, 114, and Bodo saltans, 114, 117;
of tailed Ciliata, 141 n.;
of Pleuronema, 154
Spumellaria (Peripylaea), 76, 76 f., 77;
Spyroidea, 78
Stalk or stem, of Clathrulina, 74;
of Flagellates, 112 f.;
of Anthophysa, 112;
of Diplomita, 112;
of Choanoflagellates, 121, 122;
of Monosiga, 122;
of Peritrichaceae, 141 n.;
of Schizotricha socialis, 152;
of Vorticella, 157;
of Carchesium, 158;
of Epistylis, 158;
of Zoothamnium, 158;
of Crinoidea, 580;
of Actinometra, 588;
of Pentacrinidae, 588, 591, 592;
of larva of Asterina gibbosa, 610;
of Brachiolaria, 612;
of larva of Antedon rosacea, 619;
of ancestral Crinoidea, 600;
of ancestral Echinodermata, 621
in Zooxanthella, 86;
Starfish = Asteroidea, q.v.
Stationary pairing-nucleus, 150
Statoplea, 279
Statorhab in Geryonia, 252
Stauractin, 234
Steganophthalmata, 314
Stein, von, on Protozoa, 45 f.;
misinterpretation of parasitic Suctoria in Ciliata, 161;
on Suctoria, 162
Stelechotokea, 347
Stem—see Stalk
Stem-ossicles, of Pentacrinidae, 588, 591;
of larva of Antedon rosacea, 619
Stenoscyphidae, 321
Stenoscyphus inabai, 321
regeneration, 35;
supposed nervous fibrils, 143;
meganucleus, 144;
conjugation, 149;
attachment, 152;
S. coeruleus, 154;
S. igneus, 154;
S. polymorphus, young, and adult in fission, 156
Stentorin, blue, 154 n.
Stephalia, 308
S. superba, 307
Stephanophyinae, 307
Stephanophyllia, 404
Stephanoscyphus, 318;
S. mirabilis, 206
Stephoidea, 78 f.
Stereoplasm, 394
Stereosoma, 331, 334, 337, 344
Stereotaxy, 20
Sterilisation of colonial cells in Volvox, 129
Sternata, 554
Sternum = Plastron, q.v.
Sterraster (an aster with very numerous actines soldered together by subsequently deposited silica, which extends almost to their extremities), 224
Sterrula, 341
Stewart's organs, of Cidaridae, 531;
of Echinothuriidae, 531;
of Diadematidae, 538
Stichaster, 474
Sticholonche, 86;
host of Amoebophrya, 161
Stichopus, 570
Stichotricha, 138
Sticklebacks, prey on Anopheles, 106
Stimuli, 8 f.;
inducing responsive movements, 19 f.
Stolč, on Pelomyxa, 53 n.
Stolon, 301
Stolonifera, 342
Stomach, present in Metazoa, 38;
of gnats, seat of syngamy of Acystosporidae, 103, 104 f.;
of Asterias rubens, 438;
of Echinus esculentus, 516;
of Holothuria nigra, 562;
of Antedon rosacea, 583;
of Dipleurula, 605;
absent in Hyocrinus, 589
Stomatoca (Tiaridae, 273), 415
Stomatograptus, 282
Stomodaeum, of Anthozoa, 327;
of Ctenophora, 415;
of Echinus esculentus, 516;
of Dipleurula, 605;
of metamorphosing Auricularia, 615;
of larva of Antedon, 619
Stomolophus, 325
Stony Corals, 326
Strain-figure of cell dividing by mitosis, 25, 26, 27
Strained condition of cytoplasm during syngamy, 34
Streaming of granules in protoplasm, 17
Streptocaulus, 277
Streptophiurae, 491, 494, 500, 502
Strobila, 317
Stromacystis, 596
Stromatopora, 283
Stromatoporidae, 283
Strombidium (Torquatella), 137, 155 n.
Strongylocentrotus, 512, 522, 533, 540;
S. droëbachiensis, 512, 523, 541;
S. lividus, 541;
S. purpuratus, 542
Stryphnus ponderosus, 222
Studer, 340
S. abyssicola, 268;
S. minoi, 268;
S. spongicola, 268
Stylasteridae, 285
Stylasterina, 283
Stylatula darwinii (Virgulariidae, 362), 360
Style, 284
Stylocordyla stipitata, 216
Stylonychia, 138 f.;
meganucleus of, 144;
syngamy of, 100
Stylostichon, 225
Sub-costal canals, 415
Sub-tentacular canal of Antedon rosacea, 586
S. domuncula, 219
Suberogorgia, 351
Sucker(s), of Mesodinium, 152;
of Trachelius, 153 n.
Suction mechanism in Choanophrya, 159, 161
animal nutrition of, 40
Sugar, 15
Sulculus, 369
Sulcus, 369
Supero-marginal ossicle of Asteroidea, 436
Supplemental skeleton of Foraminifera, 63, 66
Surface, protoplasmic movements in relation to a, 20—see Thigmotaxy, Stereotaxy;
-tension in relation to protoplasmic movements, 17;
to penetration of ovum by sperm, 34
Surra disease, 119
Swarmers of Foraminifera, 67 f.;
of Dinoflagellates, 131
—see also Zoospores
Sycettidae, 187
S. carteri, 187;
S. coronatum, 187;
S. setosum, development, 188, 189, 231;
S. raphanus, development, 190, 226
Pelomyxa, 53;
Heliozoa, 73;
Acantharia, 80;
Ophrydium, 158;
Paramecium bursaria, 153;
Scyphidia scorpaenae, 125;
Stentor polymorphus, 154;
Vorticella sertulariae, 125;
Ephydatia fluviatilis, 175;
Convoluta, 73;
Millepora and Zooxanthellae, 261;
in Gymnoblastea, 268;
Lar and Sabella, 273;
Cannopora and Aulopora, 283;
in Scyphozoa, 311;
Alcyonaria and Zooxanthellae, 339;
Solenocaulon and Alpheus, 350;
Eunicella and Cirripede, 356;
Verrucella and Ophiurid, 357;
Pteroeides and Crab, 361;
Ptilosarcus and Hydroid, 361;
Zoantharia and Zooxanthellae, 373 f.;
Adamsia and hermit crabs, 377, 381;
Melia and Sea-anemone, 378;
Stoichactis and Amphiprion, 378;
Pocilloporidae and Hapalocarcinus, 402;
in Zoanthidae, 405
—see also Zoochlorella, Zooxanthella
Sympodium, 583
Sympodium, 344;
S. coralloides, 341
Sympterura, 502
Synalcyonacea, 342
Synapta, 577;
S. similis, 429;
S. digitata, 576;
its larva, 608;
its pupa, 615;
S. inhaerens, 577
Synapticula, 402;
of Eupsammiidae, 404
Syngamy (= Conjugation), 33 f.;
Rhizopoda, 56 f.;
Radiolaria, 85;
Flagellates, 115 f.;
Chlamydomonadidae, 115 f., 125;
between resting-cells, 115 f.;
by a fertilising tube, 125;
Volvocaceae, 128 f.;
Suctoria, 161
—Equal, exogamous, Trichosphaerium sieboldii, 54, 56;
Chlamydophrys stercorea, 57;
Foraminifera, 68 f.;
Actinophrys sol, 72;
Gregarines, 97 f.;
Lankesteria, 95;
Monocystis, 96;
Cercomonas dujardinii, 116 n.;
Lamblia, 116 n.;
Polytoma uvella, 116 n.;
Tetramitus rostratus, 116 n.;
Trichomonas, 116 n.;
Chlamydomonadidae, 125;
Dinoflagellates, 131;
Noctiluca, 133;
Ciliata, 148 f.
—Equal, endogamous, Amoeba coli, 57;
Actinosphaerium, 73 f.;
Stephanosphaera, 128
—Unequal (binary, bisexual), Centropyxis aculeata, 57;
Radiolaria (?), 85;
Pterocephalus, 99;
Adelea ovata, 101;
Sarcosporidiaceae, 108;
Dallingeria drysdali, 116 n.;
Monas dallingeri, 116 n.;
B. caudatus, 116 n.;
Halteridium, 120;
Chlamydomonadidae, 125;
Volvox, 127 f.;
Pandorina morum (ternary), 128, 129;
Eudorina, 129;
Vorticella, 157
Syphilis in relation to Treponema pallidum, 121
Syringolites, 344
Syringopora, 283, 329, 343, 344
Syzygy (= association) of Gregarines, 99;
Tail-like appendages, of certain Ciliata, 141 n.;
(spine) of Caenomorpha uniserialis, 154, 155
Tamoya punctata, 319
Tan-pits infested by Fuligo varians, 92
Tapeworms, nutrition of, 38;
alternation of generations in, 44
Tarsaster, 474
Tealia = Urticina, q.v.
Tedania, 223
Tegmen, of Crinoidea, 580;
of Hyocrinus, 590;
of Articulata, 595;
of Camerata, 595
Telestidae, 348
T. prolifera, 347;
Telolecithal (= segmentation limited to one region of the oosperm owing to excess of yolk), 133 n.
Telosporidia, 97 f.
Temnopleurinae, 539
Temperature, in relation to protoplasmic movements, 7;
to breeding, 47;
to fission of Ciliates, 148;
changes of, stimulus of, 19, 22;
maximum (Dallinger and Drysdale's experiments), 118
Tentacles, of Actinobolus and Ileonema, 152;
of Suctoria, 158 f., 160, 162;
of Hydrozoa, 251 f.;
of Scyphozoa, 311 f.;
of Anthozoa, 327;
of Alcyonaria, 331 f.;
of Zoantharia, 366 f.;
of Ctenophora, 414 f.;
of Holothuroidea, 561 f.;
of Pelmatozoa (= podia), 579 f.
Tentacle-scale, of Ophiothrix fragilis, 480
Tentacular (= transverse) plane, 414
Tentaculata, 417
Tentaculifera (= Suctoria), 159 f., 160, 162
Tentaculozooids, 265
Tentilla, 299
Termites, Trichonymphidae parasitic in, 123
Terrestrial Protozoa, 48
Tertian fever, a parasitic disease, 104 f.
Test, classificatory value of, 51 n.;
of Euglypha, 29;
of Trichosphaerium sieboldii, 54;
of Arcella vulgaris, 55;
of Diffugia pyriformis, 55;
of Hyalosphenia lata, 55;
of Quadrula symmetrica, 55;
of Foraminifera, 58 f., 61, 63, 65, 67, 68, 69;
of Allogromidiaceae, 60;
of Microgromia socialis, 60;
of Lieberkühnia, 61;
of marine Foraminifera, 62 f.;
of Discorbina, 63;
of Frondicularia, 63;
of Globigerina, 63;
of Lagena, 63;
of Nodosaria, 63;
of Nummulites, 63;
of Planorbulina, 63;
of Saccammina, 63;
of Spiroloculina, 63;
of Allogromia, 65;
of Miliola, 65;
of Rotalia, 65;
of Squamulina, 65;
of Biloculina, 67;
(gelatinous) of Nuclearia, 74;
of Chrysomonadaceae, 110, 113 f.;
(chitinous) of Tintinnidae, 152, 155
—see also Shell, Skeleton, Theca
gemmule, 230;
T. lyncurium, 218
T. casula, 212;
Tetracoralla, 394
Tetracrepid, 215—see Rhabdocrepid
Tetractinellida, 211 f., 218, 231
T. rostratus, gametes of, 116 n.
Texas fever, 120 f.
Textularia, 59;
Textulariaceae, 59
Thalassianthidae, 383
T. nucleata, regeneration, 79 n.
Thalassophysa, 77;
reproduction, 86 n.
Thamnograptus, 281
Thamnostylus, 270
Thaumactis, 382
Thaumantias, 278
Thaumantiidae, 278
Thaumatomastix, 110
Theca, of Flagellata, 112 f.;
of Dinobryon, 112;
(branched) of Rhipidodendron, 112;
(stalked) of Diplomita, 112;
of Salpingoeca, 122;
of Corals, 370
—see also Shell, Skeleton, Test
Thecamoebae, 51 n.
Thecidae, 346
Thecocarpus (Plumulariidae, 279), 276
Thélohan, on Sporozoa, 94
Thenea wyvillei, 212
Thermotaxy, 22
Thigmotaxy, 20
Thomson, Wyville, on Calcaromma, 83
Thorax = second chamber of Monaxonic Radiolarian shell, 84
Thyone, 573
Tiara, 273
Tiarechinidae, 557
Tiarechinus, 557
Tiaridae, 273
Tick, intermediate host of Karyolysus, 102;
of Piroplasma, 120;
of Treponema, 121 n.;
Persian, 120;
Zambezian, 121 n.
Tick fever, 120
Tiedemann's bodies, 442, 444, 458;
represented by so-called Polian vesicles of Echinus esculentus, 516
Tinctin bodies, 161
Tinerfe, 417
Tintinnidae, 155;
tests of, 152
Tintinnus, 137
Tissues (definition), 3
Tolerance, induced, of a higher temperature, 118
Tone, 519
Tooth, of Ophiothrix fragilis, 482;
of Echinus esculentus, 505, 524, 525;
of Echinarachnius parma, 546
Tooth-papilla, of Ophiothrix fragilis, 482;
of Ophiocoma, 493;
of Ophiocomidae, 499
Tornaria, 616
Torquatella typica (= Strombidium), 155 n.
Torus angularis, of Ophiothrix fragilis, 482
Toxa (= a bow-shaped spicule without spiral twist), 222
Toxaspire (a spiral rod in which the twist a little exceeds a single revolution. The pitch of the spiral being great the spicule appears bow-shaped), 222
Trabeculae, traversing ampullae of tube-feet of Echinus esculentus, 517;
traversing coelom of Echinarachnius parma, 545;
traversing coelom of Antedon rosacea, 585
Tracheae of Chondrophoridae, 309
T. ovum, 153;
endosarc of, 144
galvanotaxy of, 22
Trachomedusae, 288 f.
Trachynema, 294;
T. funerarium, 294
Trachynemidae, 294
Tragosia, 224
Transverse fission, of Flagellata, 109 f.;
of Polykrikos, 131;
of Ciliata, 147;
of Suctoria, 161
Transverse flagellum and groove, in Dinoflagellata, 110, 130 f.;
multiple, in Polykrikos, 132
Transverse (= tentacular) plane, 414
Transverse section, of the arm of an Asteroid, 443;
of the arm of an Ophiuroid, 480;
of the radius of an Echinoid, 518;
of the radius of a Holothuroid, 562;
of body of a Holothuroid, 563;
of arm of Antedon, 586
Trepang, 571
T. obermeieri, 121;
T. pallidum, 121;
T. zeemannii, 120
Triaxon, 184
Trichaster, 501
Trichasteridae, 501
Trichites (hair-like spicules often occurring in sheaves or clusters), 234
Trichocysts, 142 f.;
of Chloromonadaceae, 113 n.;
Mitrophanow on, 142 n.;
adoral, of Gymnostomaceae, 145
Trichodragmata (a sheaf of straight spicules of hair-like fineness), 222
Trichogorgia, 355
conjugation, 116 n.;
T. vaginalis, 119
Trichonympha, 111
flagella, 114
test, 53;
Trichostemma, 216
Trichostomata, 137
Tridactyle pedicellariae, of Echinus esculentus, 506, 507;
of E. acutus, 509;
of E. elegans, 510;
of Echinarachnius parma, 544
Trifoliate pedicellariae, of Echinus esculentus, 507, 508;
of E. acutus, 509;
of E. alexandri, 510;
of Echinocardium cordatum, 550;
absent in Cidaridae, 534
Trigonocidaris arbacina, 539
Tripedalia cystophora, 319
Tripedaliidae, 319
Triplechinoid type of ambulacral plate, 531, 539
Tripod, 83;
-shaped spicule of Radiolaria, 76
Tripolis, 87
Tripylaea, 76
Trivium, of Echinarachnius parma, 543;
of Holothuria nigra, 561
Trochammina, 59
Trochocyathus, 399;
T. hastatus, 398
Trochocystis, 597;
T. bohemicus, 597
Trochoderma, 577
Trochodota, 577
Trochosmilia, 401
Trochostoma, 575;
T. violaceum, 575
Trophodisc, 284
Trophozooid, 388
Tropical Africa, Trypanosomic diseases of, 119 f.
Trout, black-spotted, destroyed by Hydra, 256 n.
Trypanosoma, 111, 115 f., 119 f., 120;
podoplast or blepharoplast of, 19 n., 109 n.;
undulating membrane of, 115;
Halteridium, a supposed state of, 103 n., 120;
affinities to Acystosporidae, 106;
T. brucei infests hoofed quadrupeds, 119;
T. evansii causes Surra disease in Ruminants, 119;
T. gambiense, cause of sleeping-sickness, 120;
T. lewisii, infests Rodents, 119;
T. noctuae, 120;
conjugation in, 116 n.
Trypanosomoid character of blasts of Acystosporidae, 106
Tsetse Flies, intermediate hosts of Trypanosomes of Nagana and sleeping-sickness, 119 f.
Tube, of Phalansterium, 113;
of certain Ciliates, 152;
of Maryna socialis, 152;
of Schizotricha socialis, 152;
of Stentor, 154;
of Vorticellidae, 158;
fertilising in Chlamydomonas, 125
Tube-foot, 428;
of Asterias rubens, 441 f.;
of Echinus esculentus, 517 f.;
of Endocyclica, 532;
of Arbaciidae, 532;
of Cidaridae, 532;
of Diadematidae, 532;
of Echinothuriidae, 532;
of Echinocardium cordatum, 551;
of Echinarachnius parma, 545, 546, 547;
of Palaeodiscus, 557;
of Holothuria nigra, 561
Tubiporidae, 344
T. larynx, 263;
T. parasitica, 268
Tubulariidae, 271
Tumour, malignant, associated with Leydenia, 91
Tunicata, 621
Turbellaria, fresh-water, distribution of, 48;
symbiotic with Zoochlorella, 126
Turbinaria, 396
Turbinolia, 399
Turbinoliidae, 398
Tylostyle (a style in which a knob surrounds the origin), 224
Tylotoxea (a rhabdus of which one actine is tylote or knobbed, the other oxeate, the latter directed towards the surface of the Sponge), 224
Tyrosin, 15
Uexküll, on function of pedicellariae of Echinus, 508;
on physiology of nervous system of Echinoidea, 519;
on vision of Echinoidea, 522;
on respiratory function of Aristotle's lantern, 527;
on pedicellariae of Sphaerechinus, 541
Uintacrinus, 588
Ulmaridae, 324
Umbellula, 331, 359, 360, 363;
U. gracilis, 359
Umbellulidae, 362
Umbrella of Medusae, 251
Uncinataria, 203
Under arm-plate of Ophiuroidea, 491
Under basal-plate of Crinoidea—see Infra-basal plate
Undulating membrane, of Flagellata, 111, 115, 123;
of Trypanosoma, 121;
of Dinenympha, 123;
of Stylonychia mytilus, 139 f.;
of Ciliata Trichostomata, 137 f., 145;
of Glaucoma, 153;
of Pleuronema chrysalis, 153, 154;
of Caenomorpha uniserialis, 155;
Unequal fission in Spirochona, 147
Upper arm-plate, of Ophiuroidea, 491;
of Ophioteresis, 491;
absent in Cladophiurae, 491, 500
Urechinidae, 554 n.
Urine, 13
Ussov, 257 n.
Vacuole, 5 f.;
of Collozoum inerme, 76;
of Oikomonas, 112;
contractile or pulsatile, 14 f.—see Contractile vacuole;
formative, 14 f.—see Alveole, Food-vacuole, Formative vacuole, Ingestion, vacuole of
Valvate, pedicellariae, of Antheneidae, 456, 471
Vampyrella, 89
Vaney, 292 n.
Variation in character of Foraminiferal shell at different stages of growth, 66
Vegetative, growth, in coloured Flagellates, 115;
rest, 37
V. spirans, 304
Veley, Lilian, on Pelomyxa, 53 n.
Venus's Flower-basket (= Euplectella aspergillum), 197
Venus's girdle, 420
Veretilleae, 364
Veretillum, 364
Vermicles, of Gaule, a name for Lankesterella, 102
Verrucae, 331
Verrucella, 357;
V. guadaloupensis, 357
Vertebra, of Ophiuroidea, 481, 491;
of Zygophiurae, 491;
of Ophiothrix fragilis, 480;
of Ophiarachna, 481;
of Astroschema, 481;
of Gorgonocephalus, 491;
of Astrophyton, 491;
of fossil Ophiuroidea, 501, 502
Vertebrates, cold-blooded, hosts of Haemosporidae, 102
Verticilladeae, 363
Verworn, on general physiology and protoplasm, 3 n.;
on protoplasmic movements, 16 n., 17;
on regeneration, 35 n.;
of Thalassicolla nucleata, 79 n.
Vesicular nucleus, 25
Vestibule, of Peritrichaceae, 145;
of Carchesium, 146;
Vexillum, 421
Vibratile styles of Rotifers, 141
Villogorgia, 356
V. juncea, 360;
V. mirabilis, 362;
Virgulariidae, 362
Vision, of Asteroidea, 446;
of Echinoidea, 522
Vital forces, 12 f.;
processes, 11 f.
Voluntary muscles of Mammals infested by Sarcosporidiaceae, 108
Volvocaceae, 110, 111, 125 f.;
literature of, 119
Volvocidae, 111, 126 f., 127, 129;
theca, 113
Volvox, plasmic cell connexions of, 37 n.;
a true vegetable Protist, 130;
V. globator, 127 f.
V. sertulariae, symbiotic Zooxanthella in, 125
Vorticellidae, 157 f.;
fission, 158;
colonies, 158
Vosmaer, 187 n., 212, 234 n., 237
Wager, on Euglenaceae, 125
Wagner, 256 n.
Wallich, on Protozoa, 45
W. flemmingi, 206;
W. leuckarti, 206
Wasielewski, on Sporozoa, 94 n.
Water in protoplasm, 12
Water-Fleas, Vorticellidae found on, 158;
rheotaxy of (small Crustacea), 21
Water-vascular system, 428;
of Asterias rubens, 441;
of Asteroidea, 457;
of Ophiothrix fragilis, 486;
of Echinus esculentus, 516;
of Echinarachnius parma, 546;
of Echinocardium cordatum, 551;
of Holothuria nigra, 564;
of Holothuroidea, 568;
of Synaptida, 568;
of Molpadiida, 568;
of Elasipoda, 568;
of Psolus, 569;
of Antedon rosacea, 583;
of Carpoidea, 597
White Ants, hosts of Trichonymphidae, 123
White Man's Grave, 106
Wille, on Volvocaceae, 119
Williams, on density of living protoplasm, 13 n.
Williamson, on structure of Foraminifera, 62
Wilson, on protoplasm, 3 n.;
on syngamy, 34 n.
Woltereck, 302
Wolters, on reproduction of Monocystis, 96 n.
Woodcock, on association and conjugation in Gregarines, 99 n.;
on Haemoflagellates (= Trypanosomidae), 119 n.
Worms, Earth-, hosts of Monocystis, 95
Wreath, adoral, peristomial, of cilia or membranellae of Ciliata Trichostomata, 137 f.;
of Stylonychia mytilus, 139 f.;
of Metopus, 154;
of Caenomorpha uniserialis, 155;
posterior, of Vorticella, 156, 157
Wrightella, 351
Wrisburg, on organisms of putrefaction, 43
Würmchen, of Gaule, a name for Lankesterella, 102
Xenaster, 476
Xeniidae, 348
Xenospongia patelliformis, 216
Xiphigorgia, 357
Yaws, 121 n.
Yellow-cells (= Zooxanthella), 80, 86, 125, 261, 373, 396
Yolk-granules of ovum of Sea-urchin, 7
Young state of one pairing-nucleus essential, 34
Yvesia, 224
Zambezian Tick, infects man with Treponema, 121 n.
Zaphrentidae, 406
Zaphrentis, 407
Zederbauer, on syngamy in Dinoflagellates, 131 n.
Zittel, 241 n.
age, 375;
food, 373;
form, 366;
gonads, 369;
mesenteric filaments, 369;
reproduction, 371;
skeleton, 370;
stomodaeum, 369;
tentacles, 366
Zoanthidae, 404
Zoanthus, 405;
Z. macgillivrayi, 406;
Z. sulcatus, 406
Zonarial Radiolaria, 75
Zoochlorella, 111;
a Chlamydomonad, 126;
symbiotic, in Heliozoa, 73;
in Paramecium bursaria, 153;
in Stentor polymorphus, 154;
in Ophrydium, 158;
in Ephydatia, 175;
in Hydra viridis (= chlorophyll corpuscles), 256
Zooids of Volvox globator, 127
Zoosporeae, 89
Zoospores, of Algae and Fungi, possess contractile vacuole, 15;
of Lower Plants, 17 f.;
of Sarcodina, 49;
of Trichosphaerium, 54;
of Microgromia socialis, 60;
of Foraminifera, 67 f.;
of Clathrulina, 73;
of Radiolaria, 85 f.;
of Zooxanthella, 86;
of Acrasieae, 90;
of Didymium, 92;
of Paramoeba eilhardii, 116 n.
Zooxanthella, 110;
a Chrysomonad, 125;
in Vorticella sertulariae, 125;
in Millepora, 261;
in Zoantharia, 373 f.;
in Madrepores, 396
Zopf, on Monadineae (Flagellates and Proteomyxa), 40
Zoroaster, 474
Zostera, 422
Zygophiurae, 491, 494, 495 f., 502
Zygophylax, 280
Zygote, 37 f.;
Amoeba coli, 57;
Centropyxis aculeata, 57;
Chlamydophrys stercorea, 57;
Foraminifera, 69;
Actinophrys sol, 72;
Actinosphaerium, 75;
Bodo saltans, 117 f.;
Dinoflagellata, 131 n.;
Ciliata, 148 f.
—see also Coupled cell, Fertilised egg, Ookinete, Oosperm, Oospore, Zygotospore
Zygotoblasts of Acystosporidae, 104 f.
Zygotomeres (= naked spores of Acystosporidae), 104 f.
Zygotonucleus (= Fusion-nucleus, a nucleus formed by fusion of two gametonuclei), 33 f.
Zygotospore (= resting zygote), 97
Zykoff, 178
Zymase (= chemical ferment), 15;
in relation to brood-division, 32 f.
END OF VOL. I
Printed by R. & R. Clark, Limited, Edinburgh.
For detailed studies of protoplasm see Delage, Hérédité, 2nd ed. 1903; Henneguy, Leçons sur la Cellule, 1896; Verworn, General Physiology, English ed. 1899; Wilson, The Cell in Development and Inheritance, 2nd ed. 1900. All these books contain full bibliographies.
As we shall see later, it is by no means easy to separate sharply Protozoa and Protophyta, the lowest animals and the lowest plants; and therefore in our preliminary survey to designate lowly forms of life, not formed of the aggregation of differentiated cells, we shall employ the useful term "Protista," introduced by Haeckel to designate such beings at large, without reference to this difficult problem of separation into animals and plants (see also p. 35 f.).
The "micron," represented by the Greek letter µ, is 1⁄1000 mm., very nearly 1⁄25,000 of an inch, and is the unit of length commonly adopted for microscopic measurements.
A solid substratum is required, to which the lower surface adheres slightly: that movement is complicated by a sort of rolling over of the upper surface, constantly prolonging the front of the pseudopodium, while the material of the lower surface is brought up behind. H. S. Jennings, Contr. to the Study and Behaviour of the Lower Organisms, 1904, pt. vi. p. 129 f., "The Movements and Reactions of Amoeba."
If the protoplasm contains visible granules, as it usually does, within a clear external layer, we see that these stream constantly forwards along the central axis of each process as it forms, and backwards just within the clear layer all round, like a fountain playing in a bell-jar. This motion is most marked when a new pseudopodium is put forth, and ceases when it has attained full dimensions.
We use as a corresponding adjective the term "plasmic."
For the study of the structure of protoplasm under the microscope it is necessary to examine it in very thin layers, such as can for the most part be obtained only by mechanical methods (section-cutting, etc.). These methods, again, can only be applied to fixed specimens, for natural death is followed by rapid changes, and notably by softening, which makes the tissue less suitable for our methods. We further bring out and make obvious pre-existing differentiations of our specimens by various methods of staining with such dyes as logwood and cochineal and their derivatives, and coal-tar pigments (see also p. 11 n.).
In many Protista these granules have been shown by Schewiakoff, in Z. wiss. Zool. lvii. 1893, p. 32, to consist of a calcium phosphate, probably Ca3P2O8.
It is not always possible to tell how much of these structures represents what existed in life (see p. 11).
The chromatin and nucleoles are especially rich in phosphorus, probably in the combination nucleinic acid.
In chemical phrase the process is "exothermic."
The growth of crystals is a mere superficial deposit, and cannot at all be identified with protoplasmic growth.
A. Bolles Lee, in his Microtomist's Vade Mecum, 1st ed. (1885), pointed out that "Clearing reagents are liquids whose primary function is to make microscopic preparations transparent by penetrating amongst the highly refractive elements of which the tissues are composed, having an index of refraction not greatly inferior to that of the tissues to be cleared" (p. 213). We showed later ("The State in which Water exists in Live Protoplasm," in Rep. Brit. Ass. 1889, p. 645, and Journ. Roy. Micr. Soc. 1890, p. 441) that since the refractivity of living protoplasm is only 1.363-1.368, it follows that the water in the living protoplasm is in a state of perfect physical combination, like the water of a solution of gum [read a "mucilage"] or of a jelly. Now the phenomena of protoplasmic motions as studied in the Rhizopoda and in the vegetable cell, seem absolutely to preclude the jelly supposition, and for these cases we must admit that living protoplasm is a viscid liquid whose refractivity is probably the mean of the two constituents separated by death, the one solid, the other a watery solution: and death is for us essentially a process of precipitation (or better, "desolution"). For further work on these lines see Hardy in Journ. Physiol. vol. xxiv. 1899, p. 158, and Fischer, Fixirung u. Färbung, 1900.
In its original use "automatism" designates the continuous sequence and combination of actions, without external interference, performed by complex machines designed and made for specific ends by intelligent beings: thus we speak correctly of "automatic ball bearings" that tighten of themselves when they become loose; but even these cannot take up fresh steel and redeposit it, either to replace the worn parts or to strengthen a tube that is bending under a stress.
Proteids are organic compounds containing carbon, hydrogen, nitrogen, and oxygen, of which white of egg (albumen) is a familiar type. Nucleo-proteids are compounds of proteids with nucleinic acid, which in addition to the above elements contain phosphorus.
The specific gravity of living protoplasm has been estimated by determining the density of a solution of gum in which certain Infusoria float freely at any depth. It was found by the concurrent results of Julia B. Platt and Stephen R. Williams (see Amer. Natural. xxxiii. 1899, p. 31, xxxiv. 1900, p. 95) to be from 1.014 to 1.019, while the Metazoon Hydra was found to give a density of only 1.0095 to 1.0115. The difference of about 0.006, it is easy to show, is of the correct "order of magnitude," if we admit that the actual substance of the Hydra has about the same specific gravity as the Infusorian, while the density of the whole is lightened by the watery contents of the internal cavity, etc. Jensen obtained a much higher result for Paramecium, using a solution of the crystalloid substance, potassium carbonate; but it is almost certain that this would be readily absorbed by the organism, and so raise its density in the course of the experiment.
Energy may be derived from the mere splitting up of complex substances within the cell: when such a splitting involves the liberation of CO2 the process is (mis-)called "intramolecular respiration."
A similar organ, but with cellular walls, is the bladder of the Rotifers and certain Platyhelminthes, in connexion with their renal system (vol. ii. pp. 53, 199, and especially pp. 213-5).
In Rep. Brit. Ass. 1888, p. 714; Ann. Mag. Nat. Hist. (6), iii. 1889, p. 64. This view has been fully worked out, mainly on Ciliates, by Degen in Bot. Zeit. lxiii. Abt. 1. 1905.
See Hartog, "On Multiple Cell-division, as compared with Bi-partition as Herbert Spencer's limit of growth," in Rep. Brit. Ass. 1896, p. 833; "On a Peptic Zymase in Young Embryos," ibid. 1900, p. 786; "Some Problems of Reproduction," ii. Quart. Journ. Micr. Sci. xlvii. 1904, p. 583.
"On the Digestive Ferment of a large Protozoon." Rep. Brit. Ass. 1893, p. 801.
See for studies of the movements of Protoplasm, Berthold, Protoplasmamechanik (1886); Bütschli, Investigations on Microscopic Foams and on Protoplasm, English ed. 1894; Verworn, General Physiology, 1899; Le Dantec, La Matière Vivante, 1893?; and Jensen, "Unters. ueb. Protoplasmamechanik," in Arch. Ges. Phys. lxxxvii. 1901, p. 361; Davenport, Experimental Morphology, i. 1897; H. S. Jennings, Contr. etc. 1904.
The terms "expansion" and "contraction" refer only to the superficial area: it is very doubtful whether the volume alters during these changes.
For discussions on the mechanism of ciliary action, see Schäfer, Anat. Anz. xxiv. 1904, p. 497, xxvi. 1905, p. 517; Schuberg, Arch. Protist. vi. 1905, p. 85.
Like the line of most rapid growth in a circumnutating plant-stem.
A similar body lies at the centre to which the axial filaments of the radiating pseudopodia of the Heliozoa converge, and might be termed by parity a "podoplast"; but "centrosome" is a convenient general term to include all such bodies. It is clearly of nuclear origin in Trypanosoma (Fig. 39, p. 120).
See for development of this view W. M‘Dougall in Journ. Anal. Physiol. xxxi. 1897, pp. 410, 539. I put it forward in the first draft of this essay in 1894.
The best general account is to be found in Davenport, Experimental Morphology, 1897.
See Jennings in Woods Holl. Biol. Lect. 1899, p. 93.
It is not always easy to distinguish these two classes of phenomena.
Jennings, in his studies on Reactions to Stimuli in Unicellular Organisms (1899-1900), has shown that whatever be the nature of the repellent stimulus, chemical or mechanical or thermal, the reaction of Paramecium and many other Protista is always the same. It swims backward a short distance, turns towards the aboral surface, and then having thus reversed swims on again in the new direction, front foremost as before. Apparent "positive taxies" are often really negative ones; for if the Paramecium be placed in water containing CO2 it shows the reaction not on entering the part charged with this acid, but on passing away from it into purer water, so that it continually tends to turn back into the acid part, while within it or in the water at a distance not yet charged it swims about irregularly. It appears due to this that the individuals become aggregated together, as they excrete this gas into the water. If a repellent substance diffuse towards the hinder end of a Paramecium, the response, instead of carrying it away, brings it into the region of greater concentration, and may thus kill it.
"Galvanotaxis and Chemotaxis," Journ. of Physiol. vol. xxvi. 1900-1901, p. 291.
Let us take the case of a 1-centimetre cube, growing to the size of a 2-centimetre cube. The superficial area of the 1 cm. cube measures 6 square centimetres, and its bulk is 1 cubic centimetre. The superficial area of the 2-centimetre cube measures 24 square centimetres, while its volume measures 8 cubic centimetres. Thus the larger cube has only 3 cm. sq. of surface to every cubic cm. of volume, instead of 6; in other words, the ratio of surface to volume has been halved by growth. Three successive bipartitions of the larger cube will divide it into eight separate 1-centimetre cubes, each now possessing the original ratio of surface to volume.
The nucleus is regarded by some as equivalent to a central nervous organ for the cell; by others, such as G. Mann and Verworn, as the chief chemical centre of the cell, and notably the seat of the secretion of the zymases or ferments that play so important a part in its life-work; for it is found that a Protist deprived of its nucleus can execute its wonted movements, but can neither digest nor grow. This conclusion may appear to be rather sweeping and premature, but we have seen that the changes of surface tension are the direct antecedents of the motions of the cytoplasm, we know that such changes are induced by chemical changes; and thus the nucleus—if it be the central laboratory to which such changes are ultimately due—would really in a certain sense be a directive centre.
The term "resting" is very ill-chosen, for even superficial observation shows that the relative position and characters of the internal structures of such a nucleus are constantly changing with the vital activities and functions of the cell.
For a detailed study of the nucleus in Protista, see Calkins in Arch. Protistenk. vol. ii. 1903.
The "centriole" is a minute granule sometimes recognisable in the centre of the centrosphere, and undergoing fission in advance. But centrosomes are often found without a distinction into centrosphere and centriole, and there is much confusion in the use of the terms.
The origin of the centrosomes is a problem not yet certainly solved, if indeed it be susceptible of any universal solution. They are certainly absent in many plants; and, on the other hand, structures which correspond to them often appear in mitotic divisions of Protista. In some cases the centrosomes are undoubtedly of nuclear origin, and pass out through the nuclear wall into the cytoplasm.
Though the forces at work in the dividing cell are similar in their effects to such physical forces as magnetism, static electricity, and even capillarity, and models utilising such physical forces have been devised to represent the strain-figures of the cell, the cell forces are distinct from any known physical force. For discussions of the nature of the forces at work, with bibliographies, see Angel Gallardo, Interpretatión Dinámica de la División Celular, 1902; Rhumbler, in Arch. Entw. xvi. 1903, p. 476; Hartog, C.R. cxxxviii. 1904, p. 1525, and "On the Dual Force of the Dividing-cell," pt. i. Proc. Roy. Soc. 1905 B, lxxvi. p. 548.
See Th. Boveri, Ergebnisse ueb. d. Konstitution d. chromatischen Substanz des Zellkerns (1903), for the most recent defence of this view. He lays, however (p. 2), far more stress on the individuality of the segments themselves than on the actual chromatin material they contain.
The fact that it is by mitotic division that the undifferentiated germ-cells produce the "differentiated" tissue-cells of the body of the highest animals, is again irreconcilable with such theories, whose chief advocates have been A. Weismann and his disciples.
Temporary plastogamy is a process found in some Foraminifera, where two organisms unite by their cytoplasms so that there can be complete blending of these, while the nuclei remain distinct: they ultimately separate again. In the conjugation of the Infusoria, the union of the cytoplasms is a temporary plastogamy (see p. 148 f.).
One obvious effect of brood-formation is to augment rapidly the ratio of superficial area to bulk: after only three divisions (p. 23, note) the ratio is doubled; if the divisions be nine in succession so as to produce a brood of 512, the ratio is increased eightfold, on the supposition that the figure is preserved. However, the brood-mother-cell is usually spherical, while zoospores are mostly elongated, thus giving an additional increase to the surface, which we may correlate with that increased activity; so that they disseminate the species, spreading far and wide, and justifying the name of "spore" in its primitive sense (from the Greek σπείρω—I scatter [seed]).
This condition may be protracted in the segmentation of the egg of certain Higher Animals, such as Peripatus (Vol. V. p. 20). It is clearly only a secondary and derived condition.
The usual antecedent of change in the condition of the egg is "fertilisation"—its conjugation with the sperm; but this is not invariable; and a transitory sojourn of certain marine eggs in a liquid containing other substances than sea-water may induce the egg on its return to its native habitat to segment and develop. This has been mistermed "Chemical fertilisation," discovered within the last six years by Jacques Loeb, and already the subject of an enormous literature.
See Hartog in Rep. Brit. Ass. 1896, p. 933, 1900, p. 786.
Commonly called "fertilisation," or "sexual union," inadequate and misleading terms.
For details see Hartog, "Some Problems of Reproduction," Quart. Journ. Micr. Sci. xxxiii. p. 1, xlvii. p. 583; and Ann. Biol. vol. iv. (1895) 1897; E. B. Wilson, Yves Delage, and Henneguy (references on p. 3, note); and for a singularly clear and full treatment of the processes in Protozoa, Arnold Lang, Lehrb. d. Vergl. Anat. 2nd ed. Lief. 2, "Protozoa," 1900.
This phenomenon, which we have termed "exogamy," is common in Protophyta; it has been clearly demonstrated by Schaudinn in Foraminifera and the Lobose Rhizopod Trichosphaerium (p. 53 f. Fig. 9), and by Pringsheim in the Volvocine Pandorina (p. 128 f. Fig. 45). It is quite independent of the differentiation of binary sex.
Other modes of syngamy, such as karyogamy and plastogamy, we shall discuss below, pp. 69, 148; see also p. 30.
See Gruber in Biol. Centralb. iv. p. 710, v. p. 137 (1884-6), in Ber. Ges. Freiburg, i. ii. 1886-7; Verworn (reference on p. 16); F. R. Lillie in Journ. Morph. xii. 1896, p. 239; Nussbaum in Arch. mikr. Anat. xxvi. 1886, p. 485; Balbiani in Recueil Zool. Suisse, v. 1888, in Zool. Anz. 1891, pp. 312, 323, in Arch. Microgr. iv. v. 1892-3. For Higher Organisms especially see T. H. Morgan, Regeneration, 1901.
Whence the antiseptic powers of such aromatic alcohols as phenol and thymol, and acids as salicylic acid, etc., and their salts and esters.
The portion of the spectrum that is operative in "holophytic" nutrition is the red or less refrangible half, and notably those rays in the true red, which are absorbed by the green pigment chlorophyll, and so give a dark band in the red of its absorption spectrum. The more refrangible half of the spectrum, so active on silver salts, that it is usually said to consist of "chemical rays," is not only inoperative, but has a destructive action on the pigments themselves, and even on the protoplasm. Chlorophyll is present in all cases even when more or less modified or masked by the accompaniment of other pigments.
Similarly, threads unite the cells of the colonial plant—Flagellate Volvox, passing through the thick gelatinous cell-wall (pp. 126-127, Fig. 44).
Pigments soluble in the ordinary solvents of fats, such as ether, benzol, chloroform, etc.
We have ourselves had hard work to persuade intelligent men of fair general education, even belonging to a learned profession, that this is not the case.
Dr. H. Charlton Bastian has recently maintained a contrary thesis (The Nature and Origin of Living Matter, 1905), but has adduced no evidence likely to convince any one familiar with the continuous life-study of the lower organisms.
The terms "organoid," "organella," have been introduced to designate a definite portion of a Protist specialised for a definite function; the term "organ" being reserved for a similarly specialised group of cells or tissues in a Metazoon or Metaphyte. We do not consider that this distinction warrants the introduction of new words into the terminology of general Zoology, however convenient these may be in an essay on the particular question involved.
This has been especially the case with the Flagellata, the Proteomyxa, and the Mycetozoa.
Lang distinguishes "lobopodia," "filopodia," and "pseudopodia" according to their form,—blunt, thread-like, or anastomosing. In some cases the protoplasm shows a gliding motion as a whole without any distinct pseudopodium, as in Amoeba limax (Fig. 1, p. 5), and a pseudopodium may pass into a thin, active flagellum, which is, however, glutinous and serves for the capture of prey: such often occurs in the Lobosa Podostoma and Arcuothrix, which are possibly two names for one species or at least one genus; and in many cases a slender pseudopodium may be waved freely.
See Schewiakoff, "Ueb. d. Geograph. Verbreitung d. Süsswasserprotozoen," in Mém. Acad. St. Pétersb. ser. 7, xli. 1893, No. 8. His views apply to most minute aquatic organisms—Animal, Vegetable, or Protistic.
See E. R. Lankester, art. "Protozoa" in Encycl. Brit. 9th ed. (1885), reprinted with additions in "Zoological Articles." We cannot accept his primary division into Corticata and Gymnomyxa, which would split up the Flagellata and mark off the Gregarines from the other Sporozoa.
On this ground I have referred Paramoeba, Greeff, to the Cryptomonadineae.
Differences (1) from Foraminifera; (2) from Heliozoa; (3) from Proteomyxa and Sporozoa; (4) from Myxomycetes; (5) from many Foraminifera.
I have not followed the usual classification into Gymnamoebae and Thecamoebae, according to the absence or presence of a test (perforated by one or more openings) in the active state, as such a test occurs in isolated genera of Flagellata and Infusoria, and does not appear to have any great systematic importance.
The significance of chromidia in Sarcodina (first noted by Schaudinn in Foraminifera) was fully recognised and generalised by R. Hertwig in Arch. Protist. i. 1902, p. 1.
Stolč in Z. wiss. Zool. lxviii. 1900, p. 625. Lilian Veley, however, gives reasons for regarding them as of proteid composition, J. Linn. Soc. (Zool.) xxix. 1905, p. 374 f. They disappear when the Pelomyxa is starved or supplied with only proteid food.
This genus contains two sausage-shaped, blueish-green plastids, possibly symbiotic Cyanophyceous Algae.
See Lauterborn in Z. wiss. Zool. lix. 1895, pp. 167, 537.
C. Scheel has seen Amoeba proteus produce a brood of 500-600 young amoebulae, which he reared to full size (in Festschr. f. Kupffer, 1899).
Arb. Kais. Gesundheitsamte Berlin, xix. 1903.
Faune Rhizopodique du Bassin du Léman, 1902. See also Cash, The British Freshwater Rhizopoda and Heliozoa, vol. i., Ray Society, 1905.
Chapman, The Foraminifera, London, 1902; Lister, "Foraminifera" in Lankester's Treatise on Zoology, pt. i. fasc. 2, 1903.
Challenger Reports (Zool.), vol. ix. 1884.
In Lankester's Treat. Zool. pt. i. fasc. 1. For other classifications see Eimer and Fickert in Z. wiss. Zool. lxv. 1899; Rhumbler in Lang's Protozoa, 1901; and for a full synopsis of genera and species, "Systematische Zusammenstellung der recenten Reticulosae" (pt. i. only), in Arch. Prot. iii. 1903-4, p. 181.
The type of Dujardin's genus Gromia is G. oviformis = Hyalopus dujardinii, M. Sch., which is one of the Filosa.
This convenient name is due to my friend Dr. A. Kemna of Antwerp.
The name Foraminifera was used to express the fact that the chambers communicated by pores, not by a tubular siphon as in Nautiloidea and Ammonoidea (Vol. III. pp. 393, 396).
Which probably accounts for the earlier failure of Lister and of Schaudinn himself to note their conjugation.
Rhumbler, "Die Doppelschalen v. Orbitolites u. and. Foraminiferen," in Arch. Protist. i. 1902, p. 193.
The alleged Archaean genus Eozoon, founded by Carpenter and Dawson on structures found in the Lower Laurentian serpentines (ophicalcites), and referred to the close proximity of Nummulites, has been claimed as of purely mineral structure by the petrologists; and recent biologists have admitted this claim.
Possibly composed of the same proteid, "acanthin," that forms spicules of greater permanence in the Acantharia among the Radiolaria (p. 75 f. Figs. 24, 25, A).
Such divisions into functional and abortive sister nuclei are termed "reducing divisions," and are not infrequent in the formation of pairing-cells, especially oospheres of Metazoa, where the process is termed the maturation of the ovum.
Besides these genera enumerated by Schaudinn, we include Dimorpha Gruber (Fig. 37 5, p. 112), Mastigophrys Frenzel, Ciliophrys Cienk., and Actinomonas usually referred to Flagellates.
K. Brandt, in Arch. Prot. i. 1902, p. 59, regards the presence of spicules as not even of generic moment, and subdivides the Collodaria into two families—Collida (solitary), and Sphaerozoea, colonial, i.e. with numerous central capsules.
Dreyer adds an additional order—Sphaeropylida, distinguished by a basal (or a basal and an apical) pylome.
Verworn has shown that Thalassicolla nucleata can, when the exoplasm is removed from the central capsule, regenerate it completely. First a delicate exoplasm gives off numerous fine radiating pseudopodia, and the jelly is re-formed at their bases, and carries them farther out from the central capsule. See General Physiology (Engl. ed. 1899), p. 379.
The pigment is singularly resistant and insoluble, and shows no proteid reaction. Borgert states that it appears to be formed in the oral part of the endoplasm, and to pass through the astropyle into the ectoplasm, where it accumulates. It is probably a product of excretion, and may serve, by its retention, indirectly to augment the surface. See Borgert, "Ueb. die Fortpflanzung der tripyleen Radiolarien" in Zool. Jahrb. Anat. xiv. 1900, p. 203.
Dreyer has shown that in many cases it may be explained by geometrical considerations. V. Häcker has written a most valuable account of the Biological relations of the skeleton of Radiolaria in Jen. Zeitschr. xxxix. 1904, p. 297.
Zool. Jahrb. Anat. xiv. 1900, p. 203.
Porta has described reproduction by spores and by budding in Acantharia, Rend. R. Ist. Lomb. xxxiv. 1901 (ex Journ. R. Micr. Soc. 1903, p. 45). In Thalassophysa and its allies zoospore reproduction appears to be replaced by a process in which the central capsule loses its membrane, elongates, becomes multinuclear, and ultimately breaks up into the nucleate portions, each annexing an envelope of ectoplasm to become a new individual (see Arch. Prot. vol. i. 1902).
Brandt, "Die Koloniebildenden Radiolarien," in Fauna u. Flora des Golfes v. Neapel, xiii. 1885, gives a full account of the Zooxanthellae and Diatoms, and notes the parasitism of Hyperia.
See Köppen in Zool. Anz. xvii. 1894, p. 417. For Sticholonche, see R. Hertwig in Jena. Zeitsch. xi. 1877, p. 324; and Korotneff in Zeitsch. wiss. Zool. li. 1891, p. 613. Borgert's paper on Dictyochidae is in the same volume, p. 629.
Most of Haeckel's Monera, described as non-nucleate, belong here. Several have been proved to be nucleate, and to be rightly placed here; and all require renewed study.
Even the Acystosporidiae have sickle-germs (blasts) in the insect host.
See Zopf, Beitr. Nied. Org. ii. 1892, p. 36, iv. 1894, p. 60, for the doubtful genus Chlamydomyxa; Hieronymus, abstracted by Jenkinson, in Quart. J. Micr. Sci. xiii. 1899; Penard, Arch. Protist. iv. 1904, p. 296.
The name "aethalium" is now always used in this sense.
The group was monographed by Schröter in Engler and Prantl's Pflanzenfamilien, I. Teil, Abt. 1, 1897. See also A. Lister's Monograph of the Mycetozoa, 1894; Massee, Monog. of the Myxogastres, 1893; Sir Edward and Agnes Fry, The Mycetozoa, 1899; and Massee MacBride, The North American Slime Moulds, 1899.
Several monographs of the group have been published recently dealing with the group from a systematic point of view, including their relation to their hosts. Wasielewski, "Sporozoenkunde" (1896); Labbé, "Sporozoa" (in Tierreich, 1899). Doflein's "Protozoen als Parasiten und Krankheitserreger" (1901) contains most valuable information of the diseases produced by these and other Protozoic hosts. Minchin's Monograph in Lankester's Treatise on Zoology, pt. i. fasc. 2 (1903), is a full account of the class, and admirable in every way.
For its reactions see Bütschli, Arch. Protist. vii. 1906, p. 197.
The cuticle in the allied genus Lankesteria, which is the form we figure on p. 95, is perforated by a terminal pore, through which the clear plasma of the sarcocyte may protrude as a pseudopodium.
This account is taken from Cuénot (in Arch. de Biol. 1900, p. 49), which confirms Siedlecki's account of the process in the allied genus Lankesteria in Bull. Acad. Cracow, 1899. Wolters's previous description, assimilating the processes to those of Actinophrys, is by these authors explained as the result of imperfect preservation of his material.
See Caullery and Mesnil, "Rech. sur les Actinomyxidies," Arch. Bot. vi. 1905, p. 272 f.
Léger, Arch. Zool. Exp. sér. 3, x. and sér. 4, v. (1902-3); for a full discussion of the relations of association and conjugation in Gregarines, see Woodcock in Quart. Journ. Micr. Sci. l. 1906, p. 61 f.
A Lithobius is figured in Vol. V. p. 45.
The schizont forms of some species, before the invariable alternation of schizogony and sporogony had been made out clearly, were regarded as "monogenic" genera, under the names of Eimeria, A. Schn., and Pfeifferella, Labbé; while those in which the formation of spores containing sickles had been clearly seen were termed "digenic." Labbé's monograph, "Die Sporozoen," in the Tierreich, is unfortunately written from this point of view, which had already become doubtful, and is now demonstrated to be erroneous, chiefly by the labours of Schaudinn and Siedlecki.
A species has been described, however, in the blood of the Indian Gerbille (Gerbillus indicus), completing the sexual process in the Louse of its host. A figure of G. aegyptius will be found in Vol. X. (1902) p. 475.
There is no difference between a mosquito (little fly) and a gnat, both names are applied indiscriminately to thin-bodied Diptera of the group Nemocera which attack man; only the females bite (see Vol. VI. pp. 466-468).
In Quart. Journ. Micr. Sci. xliv. 1901, p. 429.
It would seem that resting-cells, i.e. the crescents and corresponding spheres, of Laverania and Haemamoeba may linger during months of apparent health in the spleen and red marrow of the bones; and that these by parthenogenesis produce sporozoites and determine relapses when, owing to a lowering of the general health, conditions favourable to new sporulation occur.
Léger and Duboscq have found that Sarcocystis tenella, a parasite common in the muscles of the sheep (and rarely found in man), has a conjugation and sexual process recalling that of Stylorhynchus, save that the sperms are much smaller than the ova (C.R. 1902, i. p. 1148).
The alleged micronucleus of certain forms appears to be merely a "blepharoplast" (see p. 19); even when of nuclear origin, as in Trypanosoma, it has no function in reproduction like the micronucleus of Infusoria (see pp. 115, 120 f.).
I.e. resembling the thread-like water Algae.
Trichocysts (see p. 142) occur in some Chloromonadaceae; and the Dinoflagellate Polykrikos possesses true nematocysts (see p. 131).
For a full monograph of this family see H. Lohmann, in Arch. f. Protistenkunde, vol. i. 1902, p. 89.
Delage has well explained the action of the single anterior flagellum which waves in a continuous spiral like a loaded string whirled round one's head; it thus induces a movement of the water, beyond its actual range, backwards and outwards, maintained by a constant influx from behind, which carries the cell onward at the same time that it necessarily rotates round its axis. If there is a pair of symmetrically placed flagella they co-operate like the arms of a swimmer; when the second flagellum is unilateral the motion is most erratic, as seen in the Bodonidae (and the zoospores of many Chytridieae, which have most of the characters of the Flagellates, though habitually removed to the Fungi).
The colouring matter is chlorophyll or some allied colouring matter. In the yellow and brown forms the additional pigment is termed loosely "diatomin," but its identity with that of Diatoms is in no case proved.
Notably in the Craspedomonadidae, where transverse division also occurs. See Raoul Francé, Die Craspedomonadineen (Buda-Pesth, 1897).
And also in the "Monads," described by Dallinger and Drysdale, see above.
In Cercomonas dujardinii, Polytoma uvella, and Tetramitus rostratus the gametes resemble the ordinary forms and are isogamous. In Monas dallingeri and Bodo caudatus conjugation takes place between one of the ordinary form and size and another similar but smaller. In Dallingeria drysdali the one has the ordinary size and form, the other is equal in size, but has only one flagellum, not three; in Bodo saltans they are unequal, the larger gamete arising in the ordinary way by longitudinal fission, the smaller by transverse division. Doubt has been thrown on the validity of our authors' results by subsequent observers abroad; but I can find no evidence that these have even attempted to repeat the English observations under the same severely critical conditions, and therefore consider the attacks so far unjustified. Schaudinn has observed conjugation between Trichomonas individuals which have lost their flagella and become amoeboid; also in Lamblia intestinalis and in Trypanosoma (Halteridium?) noctuae (Fig. 39) "Reduction-divisions" (see p. 75, note 1) of the nuclei take place before fusion, and the nuclear phenomena are described as "complicated" (Arb. Kais. Gesundheitsamte, xx. 1904, p. 387). Paramoeba eilhardii in its adult state is colourless, amoeboid, multiciliate. It forms a brood cyst, from which are liberated flagellate zoospores, with a chromatophore, which reproduce by longitudinal fission in this state. They may also conjugate.
In P.R.S. xxvii. 1878, p. 332.
In Z. wiss. Zool. lv. 1893, p. 353.
1. Teil, Abt. 1. a, 1900.
In the Chlorophyceae, 1. Teil, Abt. 2, 1897.
1. Teil, Abt. 1. b, 1896.
Besides the above, Dangeard, in various papers in his periodical Le Botaniste, has treated of most of the groups, and Raoul Francé has monographed the Polytomeae in the Jahrb. wiss. Bot. xxvi. 1894, p. 295, and Dill the genus Chlamydomonas, etc., its closest allies, in op. cit. xxviii. 1895, p. 323.
For a detailed abstract of our knowledge of Trypanosoma and its allies up to Feb. 1, 1906, see Woodcock, "The Haemoflagellates," in Quart. Journ. Micr. Sci. 1. 1906, p. 151.
Doubts still subsist as to the interpretation of Schaudinn's observations.
Quart. Journ. Micr. Sci. xlvi. 1902.
A Zambezian Tick infects man with a Treponema, producing relapsing-fever; another species is found in the tropical disease "framboesia" ("yaws" or "parangi").
Stated by Geza Entz and Raoul Francé to be due to the spiral twisting of a plasmic membrane, and to be like a cone formed by twisting paper, with the free edges overlapping.
Discovered by Leidy. For the most recent description of this group see Grassi and Sandias in Quart. Journ. Micr. Sci. xxxix. (figures) and xl. p. 1 (text), 1897.
Bezzenberger has given an analytical table of the eleven known species of the genus Opalina in Arch. Protist. iii. 1903, p. 138.
Such movements, permissible by the perfectly flexible but firm pellicle, are termed "metabolic" or "euglenoid" in contradistinction to "amoeboid." They also occur in many Sporozoa.
Within which is often harboured the Rotifer, Proales parasita, Vol. II. p. 227.
In the Adinidae there is no groove; the two lashes arise close together, and the one is coiled round the base of the other.
In Unt. Inst. Tübingen, i. 1883, p. 233.
Conjugation of adults has been observed by Zederbauer (Ber. Deutsch. Ges. xxii. 1904). A short connecting tube is formed by the meeting of outgrowths from either mate; their protoplasmic contents meet and fuse herein to form a spherical resting-spore, as in the Conjugate Algae.
According to Bergh, Polykrikos has as many nuclei as grooves, each accompanied by one or more "micronuclei." Possibly these latter bodies are merely blepharoplasts, in connexion with the transverse flagella.
Engler and Prantl's Pflanzenfamilien, 1. Teil, Abt. 1, 1896.
The luminous genus, Pyrocystis (Fig. 47), regarded as a Cystoflagellate by Wyville Thomson, has a cellulose wall, no mouth, and in the zoospore state has the two flagella in longitudinal and transverse grooves of the Dinoflagellata.
This process has the character of telolecithal segmentation in a Metazoan egg.
See Doflein, in Zool. Jahrb. Anat. xiv. 1900, p. 1.
London, 1753, 402-403.
On this account Hickson has termed the group "Heterokaryota" in Lankester's Treat. Zool. i. fasc. 1, 1903.
See Baker, Employment for the Microscope, ed. 2, 1758.
Saville Kent's valuable Manual of the Infusoria (1880-1882), which gives figures of every genus and descriptions of every species known at that date, includes the Flagellates in its scope.
Orders 1 and 2 constitute together the Holotricha of Stein; Bütschli regards 3 to 6 as sections of Spirotrocha.
Dextrorse in all but Lichnophora and Spirochona.
Each membranella is a transversely elongated oval in reality, and below it is a double row of basal granules, corresponding to the individual cilia that constitute it. Similarly, the undulating membranes have a single row of basal granules.
Tail-like appendages are found in Scaphiodon and in Dysteria and its allies (Gymnostomaceae), Urocentrum (Aspirotrichaceae), Discomorpha and Caenomorpha (Heterotrichaceae). In the first two and last two cases they are prolongations of the body; in the third an aggregate of cilia. One or more long caudal setiform cilia are present in the genera Lembadion, Pleuronema, Cyclidium, Lembus, Cinetochilum, Ancistrum, and Uronema; all these are addicted to making springing darts. Tufts of cilia of exceptional character often serve for temporary attachment. The stalk (or at least its external tube) of the Peritrichaceae appears to be the chitinous excretion of a zone of such cilia. Fauré-Fremiet terms such a zone or annular brush a "scopula" ("Struct. de l'app. fixateur chez les Vorticellides," Arch. Protist. vi. 1905, p. 207). For a discussion of the finer structure of the cilia in Ciliata, and the mechanism of their action, see Schuberg, Arch. Protist. vi. 1905, p. 61.
See Mitrophanow "Sur les Trichocystes ... du Paramoecium," Arch. Protist. v. 1904, p. 78.
The "neurophane" fibrils of Neresheimer, Arch. Protist. ii. 1903, p. 305 f.
Sometimes the number of afferent canals is limited to five (Paramecium), or even one. There may be one or more contractile vacuoles, and in the latter case the different ones have an independent rhythm.
It is from such conclusive cases that the universal character of a discharge to the surface has been inferred in the rest of Protista possessing this organ.
Gruber (Ber. Ges. Freib. 1888) has shown that in several marine Ciliata the meganucleus is represented by an enormous number of minute granules disseminated through the endosarc, which, on the approach of fission, unite into a single meganucleus. As an adjacent micronucleus makes its appearance at this stage, he infers that the micronucleus must be also resolved in the intermediate life of the cell into granules too small for recognition under the highest magnification attainable, and that they must then coalesce.
In the peculiar Peritrichan Spirochona the division of the meganucleus is a much more complex process than usual, and recalls that of the undifferentiated nuclei of many Rhizopods (see Rompel in Z. wiss. Zool. lviii. 1894, p. 618). Opalina has neither mouth nor anus, nor contractile vacuole, but a large number of similar nuclei, that divide by a true mitotic process, like micronuclei. We have referred it (pp. 114, 123) to the Flagellates, next to the Trichonymphidae.
Save the Opalinopsidae, which are usually termed "Opalinidae"; but which cannot retain the latter name on the removal of the genus Opalina to the Flagellates.
Phil. Trans. clxxxv. 1895, pp. 355 f.
Arch. Zool. Exp. (2) vi. vii. 1888-1889.
Calkins has recently found that the vitality within a cycle is rhythmical, with alternations of more and of less frequent fissions, under the same set of conditions; and that minute doses of beef-tea or various mineral salts will not only keep up the higher rate, but even stave off senescence. Minute doses of alcohol will keep up the higher rate, but not avert senescence. He considers that Maupas' generalisations are in most respects too sweeping (Arch. Entw. xv. 1902, p. 139). But Dr. James Y. Simpson informs me that the possibility of stimulative regeneration has been found to be limited. See also Calkins and Lieb, Arch. Prot. i. 1902, p. 355.
As inferred by Hickson from the prolongation of the union.
When there are at the outset two or more micronuclei all undergo the first two fissions, but only one undergoes the third.
Zeitschr. wiss. Zool. xxxiii. 1880, p. 439.
Bezzenberger has given a key to the species of these two genera in Arch. Prot. iii. 1903, pp. 149, 157.
We note that Lacrymaria is prolonged in front into a long, slender flexible "neck," with the mouth terminal. This swan-like conformation is "mimicked" by Dileptus and Lionotus, where the neck, like the prostomium of worms, is a mere extension of the front of the body above and beyond the mouth; all three swim with peculiar grace. Trachelius (Fig. 56) has a distinct cup-shaped sucker behind the mouth, and is remarkable, like Loxodes, for the branching disposition of its endosarc.
The pigment of this species has been examined and described by Lankester under the name of "blue stentorin" (Quart. Journ. Micr. Sci. xii. 1873).
For a full account of Caenomorpha, Metopus, and allied forms, see Levander, Beitr. z. Kenntn. einiger Ciliaten, Dissert. Helsingfors, 1894.
Torquatella typica, described by Lankester as possessing a continuous undulating membrane for its peristomial wreath, is identified by Bütschli as a Strombidium, possessing exceptionally large membranellae.
Outside the principal wreath is another of fine cilia ("paroral"), standing out at an angle.
Covered with a rather lax structureless membrane (sarcolemma), which is spirally wrinkled when the muscle contracts. I am unable to verify Geza Entz's observations, adopted by Calkins and Delage.
Of the composition of cellulose (Halliburton, in Quart. Journ. Micr. Sci. xxv. 1885, p. 445).
As does the Hypotrichan Kerona polyporum.
Permanently ciliate in Hypocoma and Suctorella.
In this case the débris of the live prey torn up by the Cyclops on which they live.
The spiral ridge figured by Hertwig (Fig. 61, 1. c) is probably an incorrect representation of this structure, exceedingly minute in all genera but Choanophrya.
In Choanophrya I have failed to find any pore, and believe the bud-formation to be strictly endogenous.
See Quart. Journ. Micr. Sc. xlv. 1902, p. 325.
In Journ. Coll. Sc. Japan, x. 1896.
Étude monographique sur le groupe des Tentaculifères, Ann. Soc. Belge Micr. xxiv.-xxvi. 1901.
To Professor W. J. Sollas, Sc.D., F.R.S., who undertook to write the chapters on Porifera when the work was first planned, the Author and the Editors are indebted for his kind assistance in reading and criticising this article.
Rarities belonging to the Royal Society preserved at Gresham College, 1686.
Gerarde's Herbal, enlarged and revised by Thomas Johnson, 1636, p. 1587.
Phil. Trans. lv. p. 280.
Histoire Phys. de la Mer, 1725.
Mem. Boston Soc. i. 1867, p. 305.
Zeitschr. wiss. Zool. xxxi. 1878, p. 262.
Ann. Mag. Nat. Hist. (5) xiii. 1884, p. 381.
Quart. Journ. Micr. Sci. xxiv. 1884, p. 612.
The name was coined by Dr. Fleming from χάλιξ "silex" and χόνδρος "cartilage," and as these roots could only give Chalic-chondria it is not surprising that those who have not referred to Dr. Fleming's statements give the derivation as ἅλς "sea" and χόνδρος.
Monograph of British Sponges, vol. iii. pl. xxxix.-xl. For revision of nomenclature in this Monograph, see Hanitsch, Tr. Liverp. Biol. Soc. viii. 1894, p. 173.
Journ. Physiol. ix. 1888, p. 1.
Sollas, Ann. Mag. Nat. Hist. (4) xx. 1877, p. 285; Bütschli, Zeitschr. f. wiss. Zool. xix. 1901, p. 236.
Minchin, "Sponges" in Treatise on Zoology, edited by E. Ray Lankester, p. 87. See also Bidder, Proc. Roy. Soc. li. 1892, p. 474.
Zool. Jahrb. Anat. vii. 1894.
Materials for the Study of Variation, 1894, p. 30.
Arch. de Zool. Exp. (2) x. 1892, pp. 345-498. On the general subject of adhesion of species, see Bowerbank, Brit. Ass. Rep. 1857, p. 11, who quotes Grant as the first to observe the phenomenon.
Quart. Journ. Micr. Sci. xxii. 1882, p. 229.
But see Gamble and Keeble, Quart. Journ. Micr. Sci. xlvii. 1904, p. 363, who show that various green animals really owe their colour to "algae," though the infection with the "alga" is difficult to detect because it takes place by means of a colourless cell. See also Zoochlorella, on p. 126.
Sollas, Tr. Dublin Soc. (2) iii. 1884, p. 87.
Arch. Naturg. lix. 1893, p. 246.
Weltner, Blatt. Aquar. Fr. vii. 1896, p. 277, and "Spongillidenstudien," Arch. Naturg. ii. 1893, p. 271.
Evans, Quart. Journ. Micr. Sci. xliv. 1900, p. 72.
Ann. Mag. Nat. Hist. (2), x. 1882, p. 365.
P. Ac. Philad. 1887, pp. 158-278.
Evans, Quart. Journ. Micr. Sci. xlii. 1899, p. 363.
Francé, Organismus der Craspedomonaden, Budapest, 1897, p. 217.
Sollas, Encyclopædia Britannica, art. "Sponges," 1887.
Sollas, Ann. Mag. Nat. Hist. (5) iii. 1879, p. 23; Challenger Report, vol. xxv. pt. lxiii. 1888, p. lii.
Minchin, Lankester's Treatise on Zoology, pt. ii. 1900.
Minchin, loc. cit. p. 110.
Bidder, Quart. Journ. Micr. Sci. xxxii. 1891, p. 631, and Minchin, Quart. Journ. Micr. Sci. xxxiii. 1892, p. 266.
Minchin, Lankester's Treatise on Zoology, p. 30.
Vosmaer and Pekelharing, Verh. Ak. Amsterdam, (2) vi. 3, 1898, p. 1.
Dendy, Quart. Journ. Micr. Sci. xxxv. 1894, p. 230.
Maas, Zeitschr. wiss. Zool. lxvii. 1899-1900, p. 215.
"Die Kalkschwämme," 1871.
Dendy. loc. cit. p. 159.
Quart. Journ. Micr. Sci. xxxvi. 1894, p. 127.
Doederlein, Zool. Jahrb. Abth. Anat. x. 1896, p. 15, pl. ii. and iii.
Hinde, Quart. Journ. Geol. Soc. lvi. 1900, p. 50.
Hinde, Tr. R. Micr. Soc. 1904, p. 3.
Počta, Bull. Acad. Bohème, 1903.
J. J. Lister in Willey's Zoological Results, pt. iv. 1900, p. 459.
Mém. Soc. Zool. France, 1896, p. 119.
Arch. Zool. Exp. (3) iii. 1895, p. 561, pl. xxiii.
F. E. Schulze, Challenger Monograph, xxi.
Chun, "Aus den Tiefen des Weltmeeres," 1900, p. 481.
J. Coll. Japan, xv. 1901, pp. 128, 147, 190.
Fauna Arctica (Roemer and Schaudinn), i. 1900, p. 84; and Sitzb. Akad. Berlin, 1899, p. 98.
Sollas, Quart. Journ. Geol. Soc. 1880, p. 362.
Quart. Journ. Geol. Soc. xl. 1884, p. 795.
"Monograph British Fossil Sponges," Palaeont. Soc. xl. and xli. 1887 and 1888.
Sollas, Challenger Monograph, xxv. 1888.
Marine Investigations in South Africa, i. 1902, p. 224.
Cf. Sollas, Encyclopædia Britannica, 1887, art. "Sponges," and Schrammen, Mitth. Mus. Hildesheim, 14, 1901.
Sollas, Quart. Journ. Geol. Soc. xxxiii. 1877, p. 790.
Ridley and Dendy, Challenger Monograph, lix. 1887.
Quart. J. Micr. Sci. xli. 1901, p. 477.
Loisel, J. de l'Anat. et Phys. xxxiv. 1898, p. 1.
R. v. Lendenfeld, Acta Ac. German. lxix. 1896, p. 22.
Challenger Report, lix. 1887, p. 214.
Topsent, Zoologie Descriptive, i.; also Cotte, C. R. Soc. Biol. Paris, 1902, pp. 638-639.
Topsent, Arch. Zool. Exp. (3) viii. 1900, p. 36.
Sollas, Challenger Monograph, xxv. pt. lxiii. 1888, p. lxxxix.
Topsent, Arch. Zool. Exp. (3) viii. 1900, p. 226. For an account of certain very remarkable structures termed diaphragms in Cliona mucronata and C. ensifera, see Sollas, Ann. Mag. Nat. Hist. (5) i. 1878, p. 54.
R. von Lendenfeld, Monograph of Horny Sponges, 1889, p. 831.
Cf. Minchin in E. Ray Lankester's Treatise, p. 77.
Maas, Zool. Centralbl. v. 1898, p. 581.
Arch. Zool. Exp. viii. 1879, p. 59.
"Biological Lectures, Wood's Holl," 1894, p. 43.
F. E. Schulze, Zool. Anz. ii. 1879, p. 636.
Maas, Zeitschr. wiss. Zool. lxx. 1901, p. 263.
Maas, loc. cit. p. 284.
J. Coll. Japan, xv. 1901, p. 180.
Perkins, Johns Hopkins Univ. Circ. xxi. 1902, p. 87.
For details of this interesting process see Minchin, Quart. J. Micr. Sci. xl. 1898, p. 469.
Maas, Zeitschr. wiss. Zool. lxvii. 1900, p. 225.
Maas, SB. Ak. München, xxx. 1900, p. 553, and Zeitschr. wiss. Zool. lxx. 1901, p. 265; see also Sollas, Ann. Mag. Nat. Hist. (5) ix. 1880, p. 401.
Sollas, Challenger Monograph, xxv. 1888, p. xlv.
Sollas, ibid. pp. 13 and 34, pl. v.
Zeitschr. wiss. Zool. lii. 1891, p. 294.
I. Sollas, P. Zool. Soc. London, ii. 1902, p. 215.
Sollas, Ann. Mag. Nat. Hist. (5) ix. 1880, p. 402.
Bowerbank, and also Vosmaer and Pekelharing, Verh. Ak. Amsterdam (2) vi. 3, 1898.
J. Coll. Japan, xv. 1901, p. 193.
Vosmaer and Pekelharing, Verh. Ak. Amsterdam, 1898.
See Bidder, P. Camb. Soc. vi. 1888, p. 183; Sollas, Challenger Monograph, xxv. 1883, pp. xviii.-xxi.; and Vosmaer and Pekelharing, loc. cit.
Carter and Lieberkühn in 1856, Haeckel in 1872, Metschnikoff in 1879, and many later workers.
Die Kalkschwämme, 1872, i. p. 372.
J. Anat. Physiol. 1898, pp. 1, 6, 234.
Mém. Ac. St. Pétersb. (7) xxvi. 1878, p. 10.
Sollas, Challenger Report, xxv. pt. lxiii. p. lxxxviii.
Vergl. Physiologie d. niederen Thiere, 1903, p. 441.
For further details see Zittel, Lehrbuch der Palaeontologie, and Felix Bernard, Eléments de Palaeontologie, 1894.
For further details see Sollas, "The Formation of Flints," in The Age of the Earth, 1905, p. 131.
Willey's Zool. Results, pt. ii. 1899, p. 127.
Murbach, Archiv f. Naturg. lx. Bd. i. 1894, p. 217.
G. H. Grosvenor, Proc. Roy. Soc. lxxii. 1903, p. 462.
H. Jung, Morph. Jahrb. viii. 1881, p. 339.
Verh. Ver. Rheinland, xlix. 1893, pp. 13, 14, 40, 41.
For an account of the development and of the chitinous membrane see A. Brauer, Zeitschr. f. wiss. Zool. lii. 1891, p. 9.
Trembley, Mémoires pour servir à l'Histoire d'un genre de Polypes d'eau douce, 1744.
G. Wagner, Quart. Journ. Micr. Sci. xlviii. 1905, p. 589.
Hydra pallida, Beardsley, has been found to be very destructive to the fry of the Black-spotted Trout in Colorado, U.S. Fish. Rep. Bull. 1902, p. 158.
For figures of Protohydra see Chun, Bronn's Thier-Reich, "Coelenterata," 1894, Bd. ii. pl. ii.
Sitzber. Ges. naturf. Freunde Berlin, ix. 1894, p. 226.
M. Ussov, Morph. Jahrb. xii. 1887, p. 137.
This organism is usually described as a fungus (Achlya), but it is probably a green Alga. See J. E. Duerden, Bull. Amer. Mus. Nat. Hist. xvi. 1902, p. 323.
Bibl. Univ. de Genève, Arch. des Sciences, v. 1859, p. 80.
Phil. Trans. cxlvii. 1876, p. 117.
S. J. Hickson, Willey's Zool. Results, pt. ii. 1899, p. 127.
Quart. Journ. Micr. Sci. xlii. 1899, p. 341.
"Gymnoblastic Hydroids," Ray Society, 1871, p. 359.
Hincks, British Hydroid Zoophytes, 1868, p. 74.
Ann. Mag. Nat. Hist. (6) x. 1892, p. 207.
Fewkes, Bull. Mus. Comp. Zool. xiii. 1887, p. 224.
Hartlaub, Wiss. Meeresunt. deutsch. Meere in Kiel N.F.I. 1894, p. 1.
Carter, Ann. Mag. Nat. Hist. (4) xix. 1877, p. 44; (5) i. 1878, p. 298.
Spencer, Trans. Roy. Soc. Vict. 1892, p. 8.
Journ. Coll. Sci. Tokyo, xiii. 1900, p. 235 (with a beautiful coloured illustration).
Proc. Zool. Soc. 1897, p. 818.
Zeitschr. f. wiss. Zool. lxiii. 1898, p. 489.
Quart. Journ. Micr. Sci. xlvi. 1902, p. 1.
Zool. Zentralbl. x. 1903, p. 27.
For a discussion of the origin of the polysiphonic stem in Calyptoblastea see Nutting, "American Hydroids," Smithsonian Institution Special Bulletin, pt. i. 1900, p. 4.
Loc. cit. p. 33.
The term "sarcostyle" is usually applied to the dactylozooid of the Calyptoblastea.
Trans. Roy. Soc. Victoria, 1890, p. 121.
See C. C. Nutting, Proc. U.S. National Museum, xxi. 1899, p. 747.
E. T. Browne, Bergens Museums Aarbog, 1903, iv. p. 18.
Cf. Schepotieff, Neues Jahrb. f. Mineralogie, 1905, ii. pp. 79-98.
S. J. Hickson and H. England, Siboga Exped. viii. 1904, p. 26.
"Life-History of the Hydromedusae," Mem. Boston Soc. iii. 1885, p. 359.
Journ. Morph. xi. 1895, p. 493.
H. F. Perkins, Proc. Acad. Nat. Sci. Phil. Nov. 1902, p. 773.
E. T. Browne, Proc. Zool. Soc. 1896, p. 495.
Mark Anniversary Volume, New York, 1903, p. 1.
C. Vaney et A. Conte, Zool. Anz. xxiv. 1901, p. 533.
S. Goto, l.c.
G. H. Fowler, Quart. Journ. Micr. Sci. xxx. 1890, p. 507.
Limnocnida has recently been discovered by Budgett in the river Niger. See Browne, Ann. Nat. Hist. xvii. 1906, p. 304.
"The Tanganyika Problem," 1903, p. 298.
Cf. Boulenger, Presidential Address to Section D of the British Association (Cape Town, 1905).
C. Hartlaub, Verhandl. Deutsch. Zool. Ges. 1896, p. 3.
Abh. Senckenb. Ges. xvi. 1891, p. 44.
This gas is frequently called air. The gas contained in the pneumatophore of Physalia was analysed by Schloessing and Richard, C. R. cxxii. 1896, p. 615, and found to consist of CO2, 1.7 parts, O 15.1, nitrogen and argon, 83.2.
The chemical composition of the substance here called "chitin" has not been accurately determined. An analysis of two specimens of Velella bladders gave 9.71 and 10.35 per cent of nitrogen, which is higher than that of chitin and nearer to that of mucin.
Zool. Jahrb. Suppl. 1904, p. 347.
Johns Hopkins Univ. Circ. x. 1891, p. 91.
C. Chun, Abh. Senck. Nat. Ges. Frankfort, xvi. 1891.
Brooks and Conklin, Johns Hopkins Univ. Circ. x. 1891, No. 88.
C. E. Borchgrevink, "First on the Antarctic Continent," 1901, p. 227.
M. J. Delap, Irish Naturalist, x. 1901, p. 27.
E. W. L. Holt, Report on the Sea and Inland Fisheries of Ireland for 1902, pt. ii. 1903, p. xvi.
F. W. Gamble. See E. T. Browne, Proc. Roy. Irish Acad. 1900, p. 735.
Bull. Mus. Comp. Zool. xxxii. 1, 1898.
ἀκαλήφη = a nettle.
Biometrika, i. 1901, p. 90.
K. Kishinouye, Zool. Jahrb. Syst. xii. 1899, p. 206.
For the discussion of this relationship the reader is referred to Goette, Zeitschr. wiss. Zool. lxiii. 1897, p. 360, and Carlgren, Zool. Anz. xxii. 1899, p. 31.
See note [347], above.
E. A. Minchin, Proc. Zool. Soc. 1889, p. 583.
For good illustrations of this see Sir J. Dalyell, "Rare and Remarkable Animals of Scotland," vol. i. 1847, pll. 13, 14, 18, 19, 20.
Archiv. Mikr. Anat. xiii. 1877, p. 795.
Agassiz and Mayer, Mem. Mus. Comp. Zool. xxvi. 3, 1902, p. 153.
F. S. Conant, Mem. Johns Hopkins Univ. iv. 1, 1898.
Kishinouye, Journ. Coll. Sci. Tokyo, xvii. 7, 1902.
A discussion of the classification of this order occurs in Vanhöffen, "Acrasped. Med. d. deutschen Tiefsee Expedition," iii. 1902, p. 49.
The Periphyllidae constitute Haeckel's order Peromedusae.
A stage in development before the formation of the sub-umbrellar cavity, but subsequent to the formation of the first tentacles, is regarded as homologous with the Scyphistoma stage of other Scyphozoa.
"Siboga" Exped. Mon. xi. 1903.
Proc. Roy. Irish Acad. 3rd ser. v. 1900, p. 735.
Cf. Darwin, Voyage of the Beagle, chap. v.
Hickson, K. Akad. Wet. Amsterdam, 1905.
J. H. Ashworth, Proc. Roy. Soc. lxiii. 1898, p. 443.
Quart. Journ. Micr. Sci. xli. 1899, p. 521.
Quoted by Hickson, Marine Investigations, S. Africa, iii. 1904, p. 215.
G. C. Bourne, Phil. Trans. Roy. Soc. clxxxvi. 1895, B. p. 464.
Quoted by Marshall, Oban Pennatulida, 1882, p. 49.
Quart. Journ. Micr. Sci. xlix. 1905, p. 327.
Corallium nobile appears to be the exception to this rule, as it is stated that colonies and even individual zooids are occasionally hermaphrodite. Lacaze Duthiers, "Hist. Nat. du Corail," 1864, p. 127.
G. Lindström, Handl. k. Svensk. Vet. Akad. xxxii. 1899.
J. W. Gregory, Proc. Roy. Soc. lxvi. 1899, p. 291.
G. C. Bourne, Lankester's Treatise on Zoology, pt. ii. 1900, "Anthozoa," p. 26.
E. M. Pratt, Fauna and Geogr. Maldive Archip. ii. pt. i. 1903, p. 516.
Zool. Anz. xxix. 1905, p. 263.
S. J. Hickson, Fauna and Geog. Maldive Archip. ii. pt. i. 1903, p. 495.
Hickson, K. Akad. Wet. Amsterdam, 1905.
Journ. Imp. Fish. Bureau, Tokyo, xiv. 1, 1904.
Kitahara, Journ. Imp. Fish. Bureau, Tokyo, xiii. 3, 1904.
Johnson, Proc. Zool. Soc. 1899, p. 57.
Hickson, Nature, lxxiii. 1905, p. 5.
Moroff, Zool. Jahrb. Syst. xvii. 1902, p. 404.
Ridley, Proc. Zool. Soc. 1882, p. 231.
For a revision of this family, see Versluys, Siboga Expeditie, xii. 1902.
Jungersen (Danish Ingolf Expedition, Pennatulida, 1904) has shown that this is the correct nomenclature of the regions of the rachis. Nearly all other authors describe the dorsal side as ventral and the ventral as dorsal.
S. J. Hickson, Report British Association (Southport Meeting), 1903, p. 688.
Marshall, Trans. Roy. Soc. Edinb. xxxii. 1883, p. 143.
Rumphius, Amboinsche Rariteitkamer, 1741, p. 64.
Darwin, Naturalist's Voyage round the World, 1845, p. 99.
To be described in the forthcoming Report on the Pennatulidae of the "Siboga" Expedition.
Zool. Anz. xxv. 1902, p. 302.
Faurot, Arch. Zool. Expér. 3rd ser. iii. 1895, p. 71.
Duerden, Mem. Acad. Washington, 3rd Ser. viii. 1902.
Duerden, l.c. p. 436.
M‘Intosh, "The Marine Invertebrates and Fishes of St. Andrews," 1875, pp. 37, 38.
M‘Intosh, "The Resources of the Sea," 1899, pp. 10, 129.
H. Prouho, Arch. Zool. Expér. 2nd ser. ix. 1891, p. 247.
Duerden, Mem. Acad. Washington, viii. 1902, p. 437.
Ashworth and Annandale, Proc. Roy. Soc. Edinb. xxv. 1904, p. 11.
For recent experiments on this case, see a forthcoming paper by J. E. Duerden (P.Z.S.).
Saville Kent, "Great Barrier Reef," London, 1893, p. 145.
O. Carlgren, Biolog. Centralbl. xxi. 1901, p. 480.
Saville Kent, "The Great Barrier Reef," 1893, p. 144.
A. C. Haddon, Trans. Roy. Dubl. Soc. iv. 1889, p. 325.
G. H. Fowler, Quart. Journ. Micr. Sci. xxix. 1888, p. 143.
For a general account of the Madreporarian skeleton, cf. Ogilvie, Phil. Trans. Roy. Soc. clxxxvii. B. 1896.
H. M. Bernard, Ann. Mag. Nat. Hist. (7) xiii. 1904, p. 1.
"Report on the Results of Dredging on the Macclesfield Bank," Admiralty Report, 1894.
C. Darwin, Coral Reefs, 3rd edition, 1889, p. 125.
For the details of these borings, see "The Atoll of Funafuti," Royal Society of London, 1904.
For further information, see J. Stanley Gardiner, The Fauna and Geography of the Maldive and Laccadive-Archipelagoes, vol. i. pt. ii. 1902, p. 172.
Saville Kent, "Great Barrier Reef," 1893, p. 185.
Duerden, Mem. Ac. Washington, viii. 1902, p. 550.
H. M. Bernard, Journ. Linn. Soc. Zool. xxvi. 1897, p. 495.
E. M. Pratt, Willey's Zoological Results, pt. v. 1900, p. 591.
G. H. Fowler, Quart. Journ. Micr. Sci. xxx. 1890, p. 410.
J. E. Duerden, Mem. Ac. Washington, viii. 1902, p. 553.
M. Ogilvie, Trans. Roy. Soc. clxxxvii. B. 1896.
"The Coral Siderastraea," Carnegie Inst. No. 20, Washington, 1904.
The reader is referred to the excellent photographs of living Fungias in Saville Kent's "Great Barrier Reef," 1893, pl. xxiv. p. 160.
Trans. Roy. Soc. Dubl. (2) vi. 1898, p. 331.
F. J. Bell, Trans. Zool. Soc. xiii. pt. ii. 1891, p. 87.
J. E. Duerden, Ann. Mag. Nat. Hist. (7) ix. 1902, p. 381.
Hickson, Nature, lxxiii. 1905, p. 5.
L. Roule, Bull. Mus. Océanogr. Monaco, 1904, p. 3.
E. van Beneden, Les Anthozoaires de la Plankton Expédition, Kiel, 1898.
A. W. Peters, Journ. Exper. Zool. ii. (1) 1905, p. 103.
Cnidoblasts are stated by Chun to occur on the tentacles of Euchlora; and batteries of "nettle cells" by Abbott on the tentacles of Coeloplana.
The two costae that are seen in the middle when the Ctenophore is viewed in the transverse plane, as in Figs. 180 and 181, and the corresponding costae on the opposite side are called the "transverse" costae; the other four are called the "sagittal" costae.
F. Mosser, "Ctenophoren de Siboga Expedition," Leiden, 1903.
H. B. Bigelow, Bull. Mus. Comp. Zool. xxxix. 1904, p. 267.
Quart. Journ. Micr. Sci. xxxix. 1897, p. 323.
Annot. Zoolog. Japon. iv. pt. iv. 1902, p. 103.
Abbott, l.c. p. 106.
Zool. Anz. xxvii. 1904, p. 223.
The name seems first to have been used by Klein in 1734, "Naturalis dispositio Echinodermatum" (Danzig). Leuckart about 1850 first established Echinodermata as a primary division of the animal kingdom.
In the Synaptidae the radial canals although present in the young are lost in the adult (Ludwig, 1892, in Bronn's Thier-Reich, Bd. ii. Abt. 3, Buch i. p. 460).
Ludwig, loc. cit. p. 357.
This classification is substantially that suggested by Jeffrey Bell, Catalogue of British Echinoderms in the British Museum, 1892, except that Bell separates Holothuroidea from all others. Reasons will be given later for regarding Holothuroidea as modified Echinoidea.
Gr. ἀστήρ, a "star"; εἶδος, "form." Linnaeus established the genus Asterias in 1766. Johannes Müller in 1842 used the name "Asteriden," and in System der Asteriden, 1842, by Müller and Troschel, the foundation of our knowledge of the group was laid.
Uexküll, "Die Physiologie der Pedicellarien," Zeitschr. f. Biol. xxxvii. 1899, p. 356.
Durham, "Wandering Cells in Echinodermata," Quart. J. Micr. Sci. xxxiii. 1891, pp. 81 et seq.
Starfish are most destructive on oyster-beds, and hence possess considerable negative economic value.
Mitth. des deutschen Seefischervereins, xii. 1896, p. 102, and J. Mar. Biol. Ass. iv. 1895-97, p. 266.
Romanes, "Jellyfish, Starfish, and Sea Urchins," Intern. Scientific Series, 1885, pp. 320, 321; Preyer, "Bewegungen von Stelleriden," Mitth. Zool. Stat. Neapel, vii. 1886-87, p. 22.
Preyer, loc. cit. p. 49.
Bronn's Thier-Reich, Bd. ii. Abt. 3, Buch ii. Seesterne, p. 617.
Beiträge zur Histologie der Echinodermen, Jena, 1889. Such spaces are always to be seen in Asterina gibbosa when preserved with corrosive sublimate or other acid reagents, but are absent when it is preserved with osmic acid and Mueller's fluid. Though corrosive sublimate is usually regarded as a neutral salt, its aqueous solution decomposes with the production of a certain amount of free hydrochloric acid.
"Beiträge zur Anatomie der Asteriden," Zeitschr. wiss. Zool. xxx. 1877, pp. 122 et seq.
"Cont. à l'Étude anat. des Astérides," Arch. Zool. Exp. (2) v. bis, 1887, p. 104.
The analogy of Echinoidea (see p. 529) might suggest that, like the lacteals in man, these strands were channels along which the products of digestion diffused outward. No connexion, however, between the oral ring and the alimentary canal has been made out, nor do there appear to be such strands developed in the proximity of the wall of the digestive tube. A connexion between the aboral ring and the rectum through a mesenteric cord has been asserted, but this is doubtful.
"Die Echinodermen des Golfes von Neapel," Fauna u. Flora G. von Neapel, xxiv. Monogr. 1897, pp. 349-351.
Ludwig, "Die Echinodermen des Golfes von Neapel," pp. 68, 69.
Ludwig, "Scientific Results of the Expedition of the 'Albatross' to the Tropical Pacific"—"Asteroidea," 1905, pp. 91, 103.
Rés. sci. Expéd. Travailleur et Talisman, "Échinodermes," 1894, pp. 10-15.
This fact was discovered by Dr. E. J. Allen, Director of the Plymouth Biological Station, who pointed it out to the author during the latter's sojourn at the station in 1899.
This figure does not show the animal's attitude during forward progression quite correctly. The tips of the two anterior arms should be bent outwards, not inwards as in the figure.
In the more primitive Ophiuroidea (Streptophiurae) it persists all over the body; in Cladophiurae it is found on the central part of the disc.
How far this form of respiratory mechanism is distributed amongst Ophiurids it is impossible to say. It was first observed by me in the case of Ophiothrix fragilis at Plymouth in 1905, but since then I have found it in Ophiura ciliaris and in Amphiura squamata.
"Neue Beitr. zur Anat. d. Ophiuriden," Zeitschr. wiss. Zool. xxxiv. 1880, p. 340.
"Bewegungen d. Seesternen," Mitth. Zool. Stat. Neapel, vii. 1886-87, p. 123.
Bell, "Contribution to the Classification of Ophiuroids," Proc. Zool. Soc. 1892, p. 175.
Hamann, Bronn's Thier-Reich, Bd. ii. Abt. 3, Ophiuroidea, 1900, p. 910 f., discriminates a family Ophiodermatidae, but gives no character by which it can be distinguished from Ophiolepididae.
Forbes, "A History of British Starfishes and other animals of the class Echinodermata," 1841, p. 23.
Simroth, "Anatomie und Schizogonie der Ophiactis virens," Zeitschr. wiss. Zool. xxvii. 1876, p. 452.
Cuénot, "Études Morphologiques sur les Echinodermes," Arch. Biologie, xi. 1891, pp. 568 et seq.
This type of mouth-frame is represented in Fig. 215, A, by a figure of Ophioscolex, which belongs to the Streptophiurae.
"Asteriden und Ophiuriden aus dem Silur Böhmens," Zeitschr. der deutschen geol. Ges. lv. 1903, pp. 106-113 (Protokolle).
Geol. Magazine, No. 490, April 1905, pp. 161-168.
"Die Physiologie des Seeigelstachels," Zeitschr. für Biol. xxxix. 1900, pp. 73 et seq.
Uexküll, "Die Physiologie der Pedicellarien," Zeitschr. für Biol. xxxvii. 1899, p. 334.
"Du rôle des pédicellaires gemmiformes des Oursins," Compt. Rend. Acad. de Paris, cxi. 1890, pp. 62-64.
"The Fauna and Bottom Deposits near the thirty-fathom line from the Eddystone grounds to Startpoint," Journ. Marine Biol. Ass. v. 1899, p. 472.
"Mitth. über die zool. Stat. v. Neapel," Zeitschr. wiss. Zool. xxv. 1875, p. 471.
Cuénot, "Études Morphologiques sur les Échinodermes," Arch. Biol. xi. 1891, p. 544.
"Jellyfish, Starfish, and Sea-urchins," Intern. Sci. Series, 1885, p. 302 et seq.
"Die Physiologie des Seeigelstachels," Zeitschr. für Biol. xxxix. p. 73.
"Bewegungen von Stelleriden," Mitth. Zool. Stat. Neapel, vii. 1886-7, p. 22.
Loc. cit.
"Die Wirkung von Licht und Schatten auf die Seeigel," Zeitschr. für Biol. xl. 1900, p. 447.
In the aberrant genus Asthenosoma, where there are internal radial muscles, there is also an internal series of nerve-cells on the radial cord.
We prefer the term "compasses," to avoid confusion with the other meanings of the word "radius."
"Ueber die Function der Polischen Blasen am Kauapparat der regulären Seeigel," Mitth. Zool. Stat. Neapel, xii. 1897, p. 464.
Ergebnisse naturwissenschaftlicher Forschungen auf Ceylon, 1887-1888, Bd. i. Heft 3, pp. 105 et seq.
"Das angebliche Excretionsorgan der Seeigel," Zeitschr. wiss. Zool. lv. 1893, p. 585.
In this case the fluid flows from the lantern coelom into Stewart's organs and vice versa. Oxygen must be absorbed through the peristome. The Cidaridae are not as sensitive to want of oxygen as the other families (Uexküll, loc. cit.).
Danish Ingolf Expedition, "Echinoidea," pt. i. 1903.
Prouho, "Recherches sur le Dorocidaris papillata et quelques autres Échinides de la Méditerranée," Arch. Zool. Exp. (2) v. 1887, p. 308.
"Revision of the Echini," Illustrated Catalogue of Museum of Comp. Zool. Harvard, No. 7, 1874, p. 423.
British Museum Catalogue, "British Echinoderms," 1892, p. 30.
Loc. cit.
Loc. cit.
This account of the periproct is different from that ordinarily given. It is based on the most recent examination of this family—Agassiz, "Panamic Deep-sea Echini," Mem. Mus. Comp. Zool. xxxi. 1904, p. 36.
"Der Schatten als Reiz für Centrostephanus longispinus," Zeitschr. für Biol. xxxiv. 1896, p. 319.
Mr. E. W. L. Holt, Scientific Adviser to the Irish Board of Fisheries, casts doubt (in litt.) on much of this supposed excavation. While disclaiming any novelty in this observation, he points out that in many cases one side of the cavity is formed by calcareous algae, and it seems as if the animal wanders into a crevice, in which it is imprisoned by the growth of this plant.
These statements are based on the author's observations of the animal in the Bay of Fundy in 1900.
Lovén, "On a recent Form of Echinoconidae," Bih. Svenska Akad. Hand. xiii. Af. 4, No. 10, 1889.
Details were given to the author in conversation with Dr. Robertson in 1896.
This family includes three families discriminated by Meissner (Bronn's Thier-Reich, vol. ii. Abt. 3, Buch iv. "Die Seeigel," 1904, p. 402), viz.: Ananchytidae, Pourtalesiidae, and Urechinidae. They only differ in the pores for the tube-feet, which are paired in the first, slit-like and single in the second, and single in the third.
"On Silurian Echinoidea and Ophiuroidea," Quart. Journ. Geol. Soc. lv. 1899, pp. 701 et seq.
"The Metamorphosis of Echinoderms," Quart. J. Micr. Sci. xxxviii. 1896, p. 53.
Pelagothuria is said to have no calcifications.
Hjalmar Théel, "On a singular Case of Hermaphroditism in Holothurids," Bih. Svenska Vet. Akad. Hand. xxvii. Af. 4, No. 6, 1901.
Bronn's Thier-Reich, vol. ii. Abt. 3, Buch i. "Die Seewalzen," 1891, pp. 327 et seq.
Genuine suckers appear never to be developed; but the ends of the ventral podia are sometimes rounded, sometimes slightly flattened.
"Mémoire sur l'Elpidia nouveau genre d'Holothuries," K. Sv. Vet. Akad. xiv. No. 8, 1877.
Gerould, "The Anatomy and Histology of Caudina armata," Bull. Mus. Comp. Zool. No. 56, 1895, p. 124.
"Structure, Physiology, and Development of Antedon rosaceus," Phil. Trans. Roy. Soc. 1866, pp. 671-756.
"On the Nervous System of Antedon rosaceus," Q.J.M.S. xxiv. 1884, p. 507.
Bather calls the side-plates "adambulacral." The name is unfortunate, as it suggests that the side-plates correspond to the adambulacrals of Asteroidea; but when the groove is closed the position and function of adambulacrals and covering plates is the same (Lankester's Treatise on Zoology, iii. "Echinodermata," 1900).
Challenger Reports (Stalked Crinoids), vol. xi. pt. xxxii. 1884.
Stammesgeschichte der Pelmatozoen, pt. i. 1899.
"Die Echinodermenlarven der Plankton Expedition," Ergebn. Plankton Exp. Bd. ii. J, 1898.
"Über die Larvenzustande und die Metamorphose der Ophiuren und Seeigel," Abh. K. Akad. wiss. Berlin, 1846, and other papers in the same publication in subsequent years.
This is clearly seen by comparing the larva of Asterina gibbosa with a young Bipinnaria in which the longitudinal band is as yet undivided. The shape of the prae-oral lobe is practically the same in both.
"The Fate of the Body-cavities in the Metamorphosis of Asterias pallida," J. Coll. Japan, x. 1898, p. 239.
Yves Delage, "Elevage des Larves parthénogénétiques d'Asterias glacialis," Arch. Zool. Exp. (4) ii. 1904, p. 27.
"The Metamorphosis of Echinoderms," Quart. Journ. Micr. Sc. xxxviii. 1895, p. 45.
In the type figured (larva of Synapta digitata) the feelers are budded off directly from the ring-canal and alternate with the rudiments of the radial canal.
Observed in Plymouth, 1905.
"Die Entwicklung der Synapta digitata," Jen. Zeitschr. xxii. 1888, p. 175.
"Die Cambrische Stammgruppe der Echinodermen," Jen. Zeitschr. xxx. 1895.
Lankester's Treatise on Zoology, "Echinodermata," pt. iii. 1900, p. 33.
Brit. Mus. Cat. "British Echinodermata," 1892, p. 14.